User login
Treatment augmentation strategies for OCD: A review of 8 studies
Obsessive-compulsive disorder (OCD) is a chronic, debilitating neuropsychiatric disorder that affects 1% to 3% of the population worldwide.1,2 Together, serotonin reuptake inhibitors (SRIs) and cognitive-behavior therapy (CBT) are considered the first-line treatment for OCD.3 In children and adults, CBT is considered at least as effective as pharmacotherapy.4 Despite being an effective treatment, CBT continues to have barriers to its widespread use, including limited availability of trained CBT therapists, delayed clinical response, and high costs.5
Only approximately one-half of patients with OCD respond to SRI therapy, and a considerable percentage (30% to 40%) show significant residual symptoms even after multiple trials of SRIs.6-8 In addition, SRIs may have adverse effects (eg, sexual dysfunction, gastrointestinal symptoms) that impair patient adherence to these medications.9 Therefore, finding better treatment options is important for managing patients with OCD.
Augmentation strategies are recommended for patients who show partial response to SRI treatment or poor response to multiple SRIs. Augmentation typically includes incorporating additional medications with the primary drug with the goal of boosting the therapeutic efficacy of the primary drug. Typically, these additional medications have different mechanisms of action. However, there are no large-scale randomized controlled trials (RCTs) to inform treatment augmentation after first-line treatments for OCD produce suboptimal outcomes. The available evidence is predominantly based on small-scale RCTs, open-label trials, and case series.
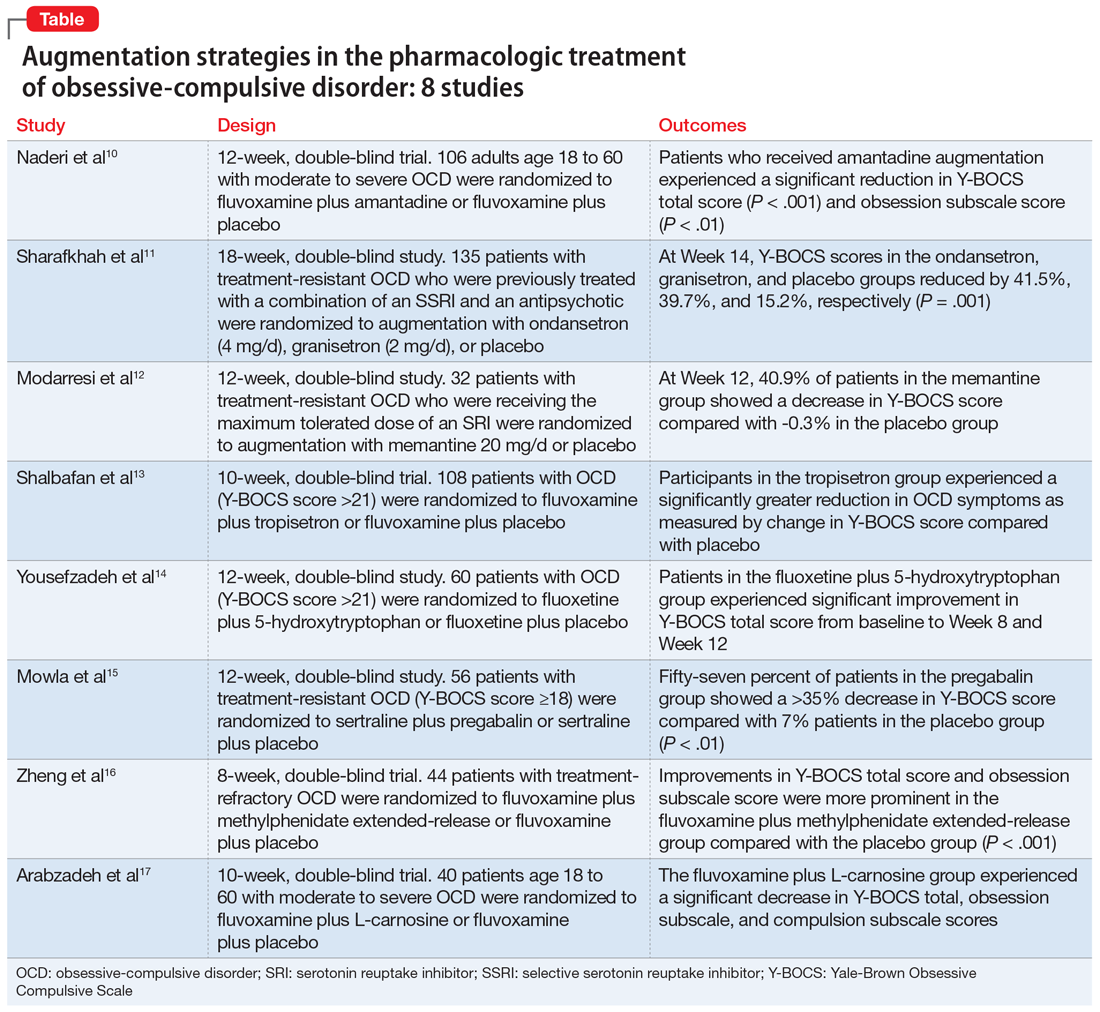
In this article, we review the evidence for treatment augmentation strategies for OCD and summarize 8 studies that show promising results (Table10-17). We focus only on pharmacologic agents and do not include other biological interventions, such as repetitive transcranial magnetic stimulation over supplementary motor area, ablative neurosurgery, or deep brain stimulation.
Continue to: Reference 1...
1. Naderi S, Faghih H , Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessivecompulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/ pcn.12803
Numerous studies support the role of glutamate dysregulation in the pathophysiology of OCD. Cortico-striato-thalamo-cortical (CSTC) abnormalities play a major role in the pathophysiology of OCD as suggested by neuroimaging research studies that indicate glutamate is the fundamental neurotransmitter of the CSTC circuit. Dysregulation of glutamatergic signaling within this circuit has been linked to OCD. Patients with OCD have been found to have an increase of glutamate in the CSF. As a result, medications that affect glutamate levels can be used to treat patients with OCD who do not respond to first-line agents. In patients already taking SRIs, augmentation of glutamate-modulating medications can reduce OCD symptoms. As an uncompetitive antagonist of the N-methyl-
Naderi et al10 evaluated amantadine as augmentative therapy to fluvoxamine for treating patients with moderate to severe OCD.
Study design
- This 12-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of amantadine as an augmentative agent to fluvoxamine in 106 patients age 18 to 60 with moderate to severe OCD.
- Participants met DSM-5 criteria for OCD and had a Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score >21. Participants were excluded if they had any substance dependence; an IQ <70; any other Axis I mental disorder; any serious cardiac, renal, or hepatic disease; had received psychotropic medications during the last 6 weeks, were pregnant or breastfeeding, or had rising liver transaminases to 3 times the upper limit of normal or higher.
- Participants received fluvoxamine 100 mg twice daily plus amantadine 100 mg/d, or fluvoxamine 100 mg twice daily plus placebo. All patients received fluvoxamine 100 mg/d for 28 days followed by 200 mg/d for the remainder of the trial.
- The primary outcome measure was difference in Y-BOCS total scores between the amantadine and placebo groups. The secondary outcome was the difference in Y-BOCS obsession and compulsion subscale scores.
Outcomes
- Patients who received amantadine augmentation experienced a significant reduction in Y-BOCS total score (P < .001) and obsession subscale score (P < .01).
- The amantadine group showed good tolerability and safety. There were no clinically significant adverse effects.
- Amantadine is an effective adjuvant to fluvoxamine for reducing OCD symptoms.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
2. Sharafkhah M, Aghakarim Alamdar M, Massoudifar A, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222- 233. doi:10.1097/YIC.0000000000000267
Although selective serotonin reuptake inhibitors (SSRIs) are considered a first-line treatment when teamed with CBT and antipsychotic augmentation, symptom resolution is not always achieved, and treatment resistance is a common problem. Sharafkhah et al11 compared the efficacy of ondansetron and granisetron augmentation specifically for patients with treatment-resistant OCD.
Study Design
- In this 18-week, randomized, double-blind, placebo-controlled study, 135 patients with treatment-resistant OCD who were previously treated with a combination of an SSRI and an antipsychotic received augmentation with ondansetron (n = 45, 4 mg/d), granisetron (n = 45, 2 mg/d), or placebo.
- Patients were rated using Y-BOCS every 2 weeks during phase I (intervention period), which lasted 14 weeks. After completing the intervention, patients were followed for 4 more weeks during phase II (discontinuation period).
- The aim of this study was to determine the safety, efficacy, and tolerability of ondansetron vs granisetron as augmentation for patients with treatment-resistant OCD. A secondary aim was to determine the rate of relapse of OCD symptoms after discontinuing ondansetron as compared with granisetron at 4 weeks after intervention.
Outcomes
- At Week 14, the reductions in Y-BOCS scores in the ondansetron, granisetron, and placebo groups were 41.5%, 39.7%, and 15.2%, respectively (P = .001). The reduction in Y-BOCS score in the ondansetron and granisetron groups was significantly greater than placebo at all phase I visits.
- Complete response was higher in the ondansetron group compared with the granisetron group (P = .041).
- Y-BOCS scores increased in both the ondansetron and granisetron groups during the discontinuation phase, but OCD symptoms were not significantly exacerbated.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
3. Modarresi A, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitorrefractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-120268
Increased glutamate levels in CSF, glutamatergic overactivity, and polymorphisms of genes coding the NMDA receptor have been shown to contribute to the occurrence of OCD. Memantine is a noncompetitive antagonist of the NMDA receptor. Various control trials have shown augmentation with memantine 5 mg/d to 20 mg/d significantly reduced symptom severity in patients with moderate to severe OCD. Modarresi et al12 evaluated memantine as a treatment option for patients with severe OCD who did not respond to SRI monotherapy.
Study design
- This 12-week, double-blind, randomized, placebo-controlled trial evaluated the efficacy of memantine augmentation in 32 patients age 18 to 40 who met DSM-5 criteria for OCD, had a Y-BOCS score ≥24, and no psychiatric comorbidity. Participants had not responded to ≥3 adequate trials (minimum 3 months) of SRI therapy, 1 of which was clomipramine.
- Individuals were excluded if they were undergoing CBT; had an additional anxiety disorder, mood disorder, or current drug or alcohol use disorder, or any systemic disorder; had a history of seizures; were pregnant or breastfeeding; or had a history of memantine use.
- Participants already receiving the maximum tolerated dose of an SRI received augmentation with memantine 20 mg/d or placebo.
- The primary outcome measure was change in Y-BOCS score from baseline. The secondary outcome was the number of individuals who achieved treatment response (defined as ≥35% reduction in Y-BOCS score).
Continue to: Outcomes...
Outcomes
- There was a statistically significant difference in Y-BOCS score in patients treated with memantine at Week 8 and Week 12 vs those who received placebo. By Week 8, 17.2% of patients in the memantine group showed a decrease in Y-BOCS score, compared with -0.8% patients in the placebo group. The difference became more significant by Week 12, with 40.9% in the memantine group showing a decrease in Y-BOCS score vs -0.3% in the placebo group. This resulted in 73.3% of patients achieving treatment response.
- Eight weeks of memantine augmentation was necessary to observe a significant improvement in OCD symptoms, and 12 weeks was needed for treatment response.
- The mean Y-BOCS total score decreased significantly in the memantine group from Week 4 to Week 8 (16.8%) and again from Week 8 to Week 12 (28.5%).
- The memantine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- Memantine augmentation in patients with severe OCD who do not respond to an SRI is effective and well-tolerated.
4. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407- 1414. doi:10.1177/0269881119878177
Studies have demonstrated the involvement of the amygdala, medial and lateral orbitofrontal cortex, and dorsal anterior cingulate cortex in OCD. Additionally, studies have also investigated the role of serotonin, dopamine, and glutamate system dysregulation in the pathology of OCD.
The 5-HT3 receptors are ligand-gated ion channels found in the prefrontal cortex, amygdala, and hippocampus. Studies of 5-HT3 receptor antagonists such as ondansetron and granisetron have shown beneficial results in augmentation with SSRIs for patients with OCD.11 Tropisetron, a 5-HT3 receptor antagonist, is highly lipophilic and able to cross the blood brain barrier. It also has dopamine-inhibiting properties that could have benefits in OCD management. Shalbafan et al13 evaluated the efficacy of tropisetron augmentation to fluvoxamine for patients with OCD.
Study design
- In a 10-week, randomized, double-blind, placebo-controlled, parallel-group trial, 108 individuals age 18 to 60 who met DSM-5 criteria for OCD and had a Y-BOCS score >21 received fluvoxamine plus tropisetron or fluvoxamine plus placebo. A total of 48 (44.4%) participants in each group completed the trial. Participants were evaluated using the Y-BOCS scale at baseline and at Week 4 and Week 10.
- The primary outcome was decrease in total Y-BOCS score from baseline to Week 10. The secondary outcome was the difference in change in Y-BOCS obsession and compulsion subscale scores between the groups.
Outcomes
- The Y-BOCS total score was not significantly different between the 2 groups (P = .975). Repeated measures analysis of variance determined a significant effect for time in both tropisetron and placebo groups (Greenhouse-Geisser F [2.72–2303.84] = 152.25, P < .001; and Greenhouse-Geisser F [1.37–1736.81] = 75.57, P < .001, respectively). At Week 10, 35 participants in the tropisetron group and 19 participants in the placebo group were complete responders.
- The baseline Y-BOCS obsession and compulsion subscales did not significantly differ between treatment groups.
Conclusion
- Compared with participants in the placebo group, those in the tropisetron group experienced a significantly greater reduction in OCD symptoms as measured by Y-BOCS score. More participants in the tropisetron group experienced complete response and remission.
- This study demonstrated that compared with placebo, when administered as augmentation with fluvoxamine, tropisetron can have beneficial effects for patients with OCD.
Continue to: Reference 5...
5. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a doubleblind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254- 262. doi:10.1097/YIC.0000000000000321
Nutraceuticals such as glycine, milk thistle, myoinositol, and serotonin (5-hydroxytryptophan) have been proposed as augmentation options for OCD. Yousefzadeh et al14 investigated the effectiveness of using 5-hydroxytryptophan in treating OCD.
Study design
- In a 12-week, randomized, double-blind study, 60 patients who met DSM-5 criteria for moderate to severe OCD (Y-BOCS score >21) were randomly assigned to receive fluoxetine plus 5-hydroxytryptophan 100 mg twice daily or fluoxetine plus placebo.
- All patients were administered fluoxetine 20 mg/d for the first 4 weeks of the study followed by fluoxetine 60 mg/d for the remainder of the trial.
- Symptoms were assessed using the Y-BOCS at baseline, Week 4, Week 8, and Week 12.
- The primary outcome measure was the difference between the 2 groups in change in Y-BOCS total score from baseline to the end of the trial. Secondary outcome measures were the differences in the Y-BOCS obsession and compulsion subscale scores from baseline to Week 12.
Outcomes
- Compared to the placebo group, the 5-hydroxytryptophan group experienced a statistically significant greater improvement in Y-BOCS total score from baseline to Week 8 (P = .002) and Week 12 (P < .001).
- General linear model repeated measure showed significant effects for time × treatment interaction on Y-BOCS total (F = 12.07, df = 2.29, P < .001), obsession subscale (F = 8.25, df = 1.91, P = .001), and compulsion subscale scores (F = 6.64, df = 2.01, P = .002).
- The 5-hydroxytryptophan group demonstrated higher partial and complete treatment response rates (P = .032 and P = .001, respectively) as determined by change in Y-BOCS total score.
- The 5-hydroxytryptophan group showed a significant improvement from baseline to Week 12 in Y-BOCS obsession subscale score (5.23 ± 2.33 vs 3.53 ± 2.13, P = .009).
- There was a significant change from baseline to the end of the trial in the Y-BOCS compulsion subscale score (3.88 ± 2.04 vs 2.30 ± 1.37, P = .002).
Conclusion
- This trial demonstrated the potential benefits of 5-hydroxytryptophan in combination with fluoxetine for patients with OCD.
6. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
Glutamatergic dysfunction has been identified as a potential cause of OCD. Studies have found elevated levels of glutamatergic transmission in the cortical-striatal-thalamic circuit of the brain and elevated glutamate concentration in the CSF in patients with OCD. Pregabalin has multiple mechanisms of action that inhibit the release of glutamate. Mowla et al15 evaluated pregabalin as an augmentation treatment for resistant OCD.
Study design
- This 12-week, double-blind, placebo-controlled clinical trial evaluated the efficacy of adjunctive pregabalin in 56 patients who met DSM-5 criteria for OCD and had not responded to ≥12 weeks of treatment with an adequate and stable dose of sertraline (baseline Y-BOCS score ≥18).
- Individuals who had other major psychiatric disorders, major medical problems, were pregnant, or had past substance or alcohol abuse were excluded.
- Participants were randomly assigned to receive sertraline plus pregabalin (n = 28) or sertraline plus placebo (n = 28). Mean sertraline dosage was 256.5 mg/d; range was 100 mg/d to 300 mg/d. Pregabalin was started at 75 mg/d and increased by 75 mg increments weekly. The mean dosage was 185.9 mg/d; range was 75 mg/d to 225 mg/d.
- The primary outcome measure was change in Y-BOCS score. A decrease >35% in Y-BOCS score was considered a significant response rate.
Outcomes
- There was a statistically significant decrease in Y-BOCS score in patients who received pregabalin. In the pregabalin group, 57.14% of patients (n = 16) showed a >35% decrease in Y-BOCS score compared with 7.14% of patients (n = 2) in the placebo group (P < .01).
- The pregabalin group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- In patients with treatment-resistant OCD who did not respond to sertraline monotherapy, augmentation with pregabalin significantly decreases Y-BOCS scores compared with placebo.
Continue to: Reference 7...
7. Zheng H, Jia F, Han H, et al. Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebocontrolled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro. 2018.12.010
Recent evidence suggests dysregulation of serotonin and dopamine in patients with OCD. Methylphenidate is a dopamine and norepinephrine inhibitor and releaser. A limited number of studies have suggested stimulants might be useful for OCD patients. Zheng et al16 conducted a pilot trial to determine whether methylphenidate augmentation may be of benefit in the management of outpatients with OCD.
Study design
- In an 8-week, double-blind, randomized, placebo-controlled trial, 44 patients (29 [66%] men, with a mean [SD] age of 24.7 [6]) with treatment-refractory OCD were randomized to receive fluvoxamine 250 mg/d plus methylphenidate extended-release (MPH-ER) 36 mg/d or fluvoxamine 250 mg/d plus placebo. The MPH-ER dose was 18 mg/d for the first 4 weeks and 36 mg/d for the rest of the trial.
- Biweekly assessments consisted of scores on the Y-BOCS, Hamilton Depression Rating Scale (HDRS), and Hamilton Anxiety Rating Scale (HAM-A).
- The primary outcomes were improvement in Y-BOCS score and the clinical response rate. Secondary outcomes included a change in score on the Y-BOCS subscales, HARS, and HAM-A. Data were analyzed with the intention-to-treat sample.
Outcomes
- Forty-one patients finished the trial. The baseline Y-BOCS total scores and subscale scores did not differ significantly between the 2 groups.
- Improvements in Y-BOCS total score and obsession subscale score were more prominent in the fluvoxamine plus MPH-ER group compared with the placebo group (P < .001).
- HDRS score decreased in both the placebo and MPH-ER groups. HAM-A scores decreased significantly in the MPH-ER plus fluvoxamine group compared with the placebo group.
Conclusion
- This study demonstrated that the combination of fluvoxamine and MPH-ER produces a higher and faster response rate than fluvoxamine plus placebo in patients with OCD.
8. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Glutamate dysregulation is implicated in the pathogenesis of OCD. Glutamate-modulating agents have been used to treat OCD. Studies have shown L-carnosine has a neuroprotective role via its modulatory effect on glutamate. Arabzadeh et al17 evaluated the efficacy of L-carnosine as an adjuvant to fluvoxamine for treating OCD.
Study design
- This 10-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy of adjunctive L-carnosine in 40 patients age 18 to 60 who met DSM-5 criteria for OCD and had moderate to severe OCD (Y-BOCS score ≥21).
- Individuals with any other DSM-5 major psychiatric disorders, serious medical or neurologic illness, substance dependence (other than caffeine or nicotine), mental retardation (based on clinical judgment), were pregnant or breastfeeding, had any contraindication for the use of L‐carnosine or fluvoxamine, or received any psychotropic drugs in the previous 6 weeks were excluded.
- Participants received fluvoxamine 100 mg/d for the first 4 weeks and 200 mg/d for the next 6 weeks plus either L-carnosine 500 mg twice daily or placebo. This dosage of L-carnosine was chosen because previously it had been tolerated and effective.
- The primary outcome measure was difference in Y-BOCS total scores. Secondary outcomes were differences in Y-BOCS obsession and compulsion subscale scores and differences in change in score on Y-BOCS total and subscale scores from baseline.
Outcomes
- The L-carnosine group experienced a significant decrease in Y-BOCS total score (P < .001), obsession subscale score (P < .01), and compulsion subscale score (P < .01).
- The group that received fluvoxamine plus L-carnosine also experienced a more complete response (P = .03).
- The L-carnosine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- L-carnosine significantly reduces OCD symptoms when used as an adjuvant to fluvoxamine.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
3. Eddy KT, Dutra L, Bradley, R, et al. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24(8):1011-1030.
4. Franklin ME, Foa EB. Treatment of obsessive compulsive disorder. Annu Rev Clin Psychol. 2011;7:229-243.
5. Koran LM, Hanna GL, Hollander E, et al. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(7 Suppl):5-53.
6. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37(3):375-391.
7. Pallanti S, Hollander E, Bienstock C, et al. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002;5(2):181-191.
8. Atmaca M. Treatment-refractory obsessive compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:127-133.
9. Barth M, Kriston L, Klostermann S, et al. Efficacy of selective serotonin reuptake inhibitors and adverse events: meta-regression and mediation analysis of placebo-controlled trials. Br J Psychiatry. 2016;208(2):114-119.
10. NaderiS, Faghih H, Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/pcn.12803
11. SharafkhahM, Aghakarim Alamdar M, MassoudifarA, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222-233. doi:10.1097/YIC.0000000000000267
12. ModarresiA, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-12026
13. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407-1414. doi:10.1177/0269881119878177
14. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254-262. doi:10.1097/YIC.0000000000000321
15. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
16. Zheng H, Jia F, Han H, et al.Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro.2018.12.010
17. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Obsessive-compulsive disorder (OCD) is a chronic, debilitating neuropsychiatric disorder that affects 1% to 3% of the population worldwide.1,2 Together, serotonin reuptake inhibitors (SRIs) and cognitive-behavior therapy (CBT) are considered the first-line treatment for OCD.3 In children and adults, CBT is considered at least as effective as pharmacotherapy.4 Despite being an effective treatment, CBT continues to have barriers to its widespread use, including limited availability of trained CBT therapists, delayed clinical response, and high costs.5
Only approximately one-half of patients with OCD respond to SRI therapy, and a considerable percentage (30% to 40%) show significant residual symptoms even after multiple trials of SRIs.6-8 In addition, SRIs may have adverse effects (eg, sexual dysfunction, gastrointestinal symptoms) that impair patient adherence to these medications.9 Therefore, finding better treatment options is important for managing patients with OCD.
Augmentation strategies are recommended for patients who show partial response to SRI treatment or poor response to multiple SRIs. Augmentation typically includes incorporating additional medications with the primary drug with the goal of boosting the therapeutic efficacy of the primary drug. Typically, these additional medications have different mechanisms of action. However, there are no large-scale randomized controlled trials (RCTs) to inform treatment augmentation after first-line treatments for OCD produce suboptimal outcomes. The available evidence is predominantly based on small-scale RCTs, open-label trials, and case series.
In this article, we review the evidence for treatment augmentation strategies for OCD and summarize 8 studies that show promising results (Table10-17). We focus only on pharmacologic agents and do not include other biological interventions, such as repetitive transcranial magnetic stimulation over supplementary motor area, ablative neurosurgery, or deep brain stimulation.
Continue to: Reference 1...
1. Naderi S, Faghih H , Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessivecompulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/ pcn.12803
Numerous studies support the role of glutamate dysregulation in the pathophysiology of OCD. Cortico-striato-thalamo-cortical (CSTC) abnormalities play a major role in the pathophysiology of OCD as suggested by neuroimaging research studies that indicate glutamate is the fundamental neurotransmitter of the CSTC circuit. Dysregulation of glutamatergic signaling within this circuit has been linked to OCD. Patients with OCD have been found to have an increase of glutamate in the CSF. As a result, medications that affect glutamate levels can be used to treat patients with OCD who do not respond to first-line agents. In patients already taking SRIs, augmentation of glutamate-modulating medications can reduce OCD symptoms. As an uncompetitive antagonist of the N-methyl-
Naderi et al10 evaluated amantadine as augmentative therapy to fluvoxamine for treating patients with moderate to severe OCD.
Study design
- This 12-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of amantadine as an augmentative agent to fluvoxamine in 106 patients age 18 to 60 with moderate to severe OCD.
- Participants met DSM-5 criteria for OCD and had a Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score >21. Participants were excluded if they had any substance dependence; an IQ <70; any other Axis I mental disorder; any serious cardiac, renal, or hepatic disease; had received psychotropic medications during the last 6 weeks, were pregnant or breastfeeding, or had rising liver transaminases to 3 times the upper limit of normal or higher.
- Participants received fluvoxamine 100 mg twice daily plus amantadine 100 mg/d, or fluvoxamine 100 mg twice daily plus placebo. All patients received fluvoxamine 100 mg/d for 28 days followed by 200 mg/d for the remainder of the trial.
- The primary outcome measure was difference in Y-BOCS total scores between the amantadine and placebo groups. The secondary outcome was the difference in Y-BOCS obsession and compulsion subscale scores.
Outcomes
- Patients who received amantadine augmentation experienced a significant reduction in Y-BOCS total score (P < .001) and obsession subscale score (P < .01).
- The amantadine group showed good tolerability and safety. There were no clinically significant adverse effects.
- Amantadine is an effective adjuvant to fluvoxamine for reducing OCD symptoms.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
2. Sharafkhah M, Aghakarim Alamdar M, Massoudifar A, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222- 233. doi:10.1097/YIC.0000000000000267
Although selective serotonin reuptake inhibitors (SSRIs) are considered a first-line treatment when teamed with CBT and antipsychotic augmentation, symptom resolution is not always achieved, and treatment resistance is a common problem. Sharafkhah et al11 compared the efficacy of ondansetron and granisetron augmentation specifically for patients with treatment-resistant OCD.
Study Design
- In this 18-week, randomized, double-blind, placebo-controlled study, 135 patients with treatment-resistant OCD who were previously treated with a combination of an SSRI and an antipsychotic received augmentation with ondansetron (n = 45, 4 mg/d), granisetron (n = 45, 2 mg/d), or placebo.
- Patients were rated using Y-BOCS every 2 weeks during phase I (intervention period), which lasted 14 weeks. After completing the intervention, patients were followed for 4 more weeks during phase II (discontinuation period).
- The aim of this study was to determine the safety, efficacy, and tolerability of ondansetron vs granisetron as augmentation for patients with treatment-resistant OCD. A secondary aim was to determine the rate of relapse of OCD symptoms after discontinuing ondansetron as compared with granisetron at 4 weeks after intervention.
Outcomes
- At Week 14, the reductions in Y-BOCS scores in the ondansetron, granisetron, and placebo groups were 41.5%, 39.7%, and 15.2%, respectively (P = .001). The reduction in Y-BOCS score in the ondansetron and granisetron groups was significantly greater than placebo at all phase I visits.
- Complete response was higher in the ondansetron group compared with the granisetron group (P = .041).
- Y-BOCS scores increased in both the ondansetron and granisetron groups during the discontinuation phase, but OCD symptoms were not significantly exacerbated.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
3. Modarresi A, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitorrefractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-120268
Increased glutamate levels in CSF, glutamatergic overactivity, and polymorphisms of genes coding the NMDA receptor have been shown to contribute to the occurrence of OCD. Memantine is a noncompetitive antagonist of the NMDA receptor. Various control trials have shown augmentation with memantine 5 mg/d to 20 mg/d significantly reduced symptom severity in patients with moderate to severe OCD. Modarresi et al12 evaluated memantine as a treatment option for patients with severe OCD who did not respond to SRI monotherapy.
Study design
- This 12-week, double-blind, randomized, placebo-controlled trial evaluated the efficacy of memantine augmentation in 32 patients age 18 to 40 who met DSM-5 criteria for OCD, had a Y-BOCS score ≥24, and no psychiatric comorbidity. Participants had not responded to ≥3 adequate trials (minimum 3 months) of SRI therapy, 1 of which was clomipramine.
- Individuals were excluded if they were undergoing CBT; had an additional anxiety disorder, mood disorder, or current drug or alcohol use disorder, or any systemic disorder; had a history of seizures; were pregnant or breastfeeding; or had a history of memantine use.
- Participants already receiving the maximum tolerated dose of an SRI received augmentation with memantine 20 mg/d or placebo.
- The primary outcome measure was change in Y-BOCS score from baseline. The secondary outcome was the number of individuals who achieved treatment response (defined as ≥35% reduction in Y-BOCS score).
Continue to: Outcomes...
Outcomes
- There was a statistically significant difference in Y-BOCS score in patients treated with memantine at Week 8 and Week 12 vs those who received placebo. By Week 8, 17.2% of patients in the memantine group showed a decrease in Y-BOCS score, compared with -0.8% patients in the placebo group. The difference became more significant by Week 12, with 40.9% in the memantine group showing a decrease in Y-BOCS score vs -0.3% in the placebo group. This resulted in 73.3% of patients achieving treatment response.
- Eight weeks of memantine augmentation was necessary to observe a significant improvement in OCD symptoms, and 12 weeks was needed for treatment response.
- The mean Y-BOCS total score decreased significantly in the memantine group from Week 4 to Week 8 (16.8%) and again from Week 8 to Week 12 (28.5%).
- The memantine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- Memantine augmentation in patients with severe OCD who do not respond to an SRI is effective and well-tolerated.
4. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407- 1414. doi:10.1177/0269881119878177
Studies have demonstrated the involvement of the amygdala, medial and lateral orbitofrontal cortex, and dorsal anterior cingulate cortex in OCD. Additionally, studies have also investigated the role of serotonin, dopamine, and glutamate system dysregulation in the pathology of OCD.
The 5-HT3 receptors are ligand-gated ion channels found in the prefrontal cortex, amygdala, and hippocampus. Studies of 5-HT3 receptor antagonists such as ondansetron and granisetron have shown beneficial results in augmentation with SSRIs for patients with OCD.11 Tropisetron, a 5-HT3 receptor antagonist, is highly lipophilic and able to cross the blood brain barrier. It also has dopamine-inhibiting properties that could have benefits in OCD management. Shalbafan et al13 evaluated the efficacy of tropisetron augmentation to fluvoxamine for patients with OCD.
Study design
- In a 10-week, randomized, double-blind, placebo-controlled, parallel-group trial, 108 individuals age 18 to 60 who met DSM-5 criteria for OCD and had a Y-BOCS score >21 received fluvoxamine plus tropisetron or fluvoxamine plus placebo. A total of 48 (44.4%) participants in each group completed the trial. Participants were evaluated using the Y-BOCS scale at baseline and at Week 4 and Week 10.
- The primary outcome was decrease in total Y-BOCS score from baseline to Week 10. The secondary outcome was the difference in change in Y-BOCS obsession and compulsion subscale scores between the groups.
Outcomes
- The Y-BOCS total score was not significantly different between the 2 groups (P = .975). Repeated measures analysis of variance determined a significant effect for time in both tropisetron and placebo groups (Greenhouse-Geisser F [2.72–2303.84] = 152.25, P < .001; and Greenhouse-Geisser F [1.37–1736.81] = 75.57, P < .001, respectively). At Week 10, 35 participants in the tropisetron group and 19 participants in the placebo group were complete responders.
- The baseline Y-BOCS obsession and compulsion subscales did not significantly differ between treatment groups.
Conclusion
- Compared with participants in the placebo group, those in the tropisetron group experienced a significantly greater reduction in OCD symptoms as measured by Y-BOCS score. More participants in the tropisetron group experienced complete response and remission.
- This study demonstrated that compared with placebo, when administered as augmentation with fluvoxamine, tropisetron can have beneficial effects for patients with OCD.
Continue to: Reference 5...
5. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a doubleblind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254- 262. doi:10.1097/YIC.0000000000000321
Nutraceuticals such as glycine, milk thistle, myoinositol, and serotonin (5-hydroxytryptophan) have been proposed as augmentation options for OCD. Yousefzadeh et al14 investigated the effectiveness of using 5-hydroxytryptophan in treating OCD.
Study design
- In a 12-week, randomized, double-blind study, 60 patients who met DSM-5 criteria for moderate to severe OCD (Y-BOCS score >21) were randomly assigned to receive fluoxetine plus 5-hydroxytryptophan 100 mg twice daily or fluoxetine plus placebo.
- All patients were administered fluoxetine 20 mg/d for the first 4 weeks of the study followed by fluoxetine 60 mg/d for the remainder of the trial.
- Symptoms were assessed using the Y-BOCS at baseline, Week 4, Week 8, and Week 12.
- The primary outcome measure was the difference between the 2 groups in change in Y-BOCS total score from baseline to the end of the trial. Secondary outcome measures were the differences in the Y-BOCS obsession and compulsion subscale scores from baseline to Week 12.
Outcomes
- Compared to the placebo group, the 5-hydroxytryptophan group experienced a statistically significant greater improvement in Y-BOCS total score from baseline to Week 8 (P = .002) and Week 12 (P < .001).
- General linear model repeated measure showed significant effects for time × treatment interaction on Y-BOCS total (F = 12.07, df = 2.29, P < .001), obsession subscale (F = 8.25, df = 1.91, P = .001), and compulsion subscale scores (F = 6.64, df = 2.01, P = .002).
- The 5-hydroxytryptophan group demonstrated higher partial and complete treatment response rates (P = .032 and P = .001, respectively) as determined by change in Y-BOCS total score.
- The 5-hydroxytryptophan group showed a significant improvement from baseline to Week 12 in Y-BOCS obsession subscale score (5.23 ± 2.33 vs 3.53 ± 2.13, P = .009).
- There was a significant change from baseline to the end of the trial in the Y-BOCS compulsion subscale score (3.88 ± 2.04 vs 2.30 ± 1.37, P = .002).
Conclusion
- This trial demonstrated the potential benefits of 5-hydroxytryptophan in combination with fluoxetine for patients with OCD.
6. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
Glutamatergic dysfunction has been identified as a potential cause of OCD. Studies have found elevated levels of glutamatergic transmission in the cortical-striatal-thalamic circuit of the brain and elevated glutamate concentration in the CSF in patients with OCD. Pregabalin has multiple mechanisms of action that inhibit the release of glutamate. Mowla et al15 evaluated pregabalin as an augmentation treatment for resistant OCD.
Study design
- This 12-week, double-blind, placebo-controlled clinical trial evaluated the efficacy of adjunctive pregabalin in 56 patients who met DSM-5 criteria for OCD and had not responded to ≥12 weeks of treatment with an adequate and stable dose of sertraline (baseline Y-BOCS score ≥18).
- Individuals who had other major psychiatric disorders, major medical problems, were pregnant, or had past substance or alcohol abuse were excluded.
- Participants were randomly assigned to receive sertraline plus pregabalin (n = 28) or sertraline plus placebo (n = 28). Mean sertraline dosage was 256.5 mg/d; range was 100 mg/d to 300 mg/d. Pregabalin was started at 75 mg/d and increased by 75 mg increments weekly. The mean dosage was 185.9 mg/d; range was 75 mg/d to 225 mg/d.
- The primary outcome measure was change in Y-BOCS score. A decrease >35% in Y-BOCS score was considered a significant response rate.
Outcomes
- There was a statistically significant decrease in Y-BOCS score in patients who received pregabalin. In the pregabalin group, 57.14% of patients (n = 16) showed a >35% decrease in Y-BOCS score compared with 7.14% of patients (n = 2) in the placebo group (P < .01).
- The pregabalin group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- In patients with treatment-resistant OCD who did not respond to sertraline monotherapy, augmentation with pregabalin significantly decreases Y-BOCS scores compared with placebo.
Continue to: Reference 7...
7. Zheng H, Jia F, Han H, et al. Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebocontrolled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro. 2018.12.010
Recent evidence suggests dysregulation of serotonin and dopamine in patients with OCD. Methylphenidate is a dopamine and norepinephrine inhibitor and releaser. A limited number of studies have suggested stimulants might be useful for OCD patients. Zheng et al16 conducted a pilot trial to determine whether methylphenidate augmentation may be of benefit in the management of outpatients with OCD.
Study design
- In an 8-week, double-blind, randomized, placebo-controlled trial, 44 patients (29 [66%] men, with a mean [SD] age of 24.7 [6]) with treatment-refractory OCD were randomized to receive fluvoxamine 250 mg/d plus methylphenidate extended-release (MPH-ER) 36 mg/d or fluvoxamine 250 mg/d plus placebo. The MPH-ER dose was 18 mg/d for the first 4 weeks and 36 mg/d for the rest of the trial.
- Biweekly assessments consisted of scores on the Y-BOCS, Hamilton Depression Rating Scale (HDRS), and Hamilton Anxiety Rating Scale (HAM-A).
- The primary outcomes were improvement in Y-BOCS score and the clinical response rate. Secondary outcomes included a change in score on the Y-BOCS subscales, HARS, and HAM-A. Data were analyzed with the intention-to-treat sample.
Outcomes
- Forty-one patients finished the trial. The baseline Y-BOCS total scores and subscale scores did not differ significantly between the 2 groups.
- Improvements in Y-BOCS total score and obsession subscale score were more prominent in the fluvoxamine plus MPH-ER group compared with the placebo group (P < .001).
- HDRS score decreased in both the placebo and MPH-ER groups. HAM-A scores decreased significantly in the MPH-ER plus fluvoxamine group compared with the placebo group.
Conclusion
- This study demonstrated that the combination of fluvoxamine and MPH-ER produces a higher and faster response rate than fluvoxamine plus placebo in patients with OCD.
8. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Glutamate dysregulation is implicated in the pathogenesis of OCD. Glutamate-modulating agents have been used to treat OCD. Studies have shown L-carnosine has a neuroprotective role via its modulatory effect on glutamate. Arabzadeh et al17 evaluated the efficacy of L-carnosine as an adjuvant to fluvoxamine for treating OCD.
Study design
- This 10-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy of adjunctive L-carnosine in 40 patients age 18 to 60 who met DSM-5 criteria for OCD and had moderate to severe OCD (Y-BOCS score ≥21).
- Individuals with any other DSM-5 major psychiatric disorders, serious medical or neurologic illness, substance dependence (other than caffeine or nicotine), mental retardation (based on clinical judgment), were pregnant or breastfeeding, had any contraindication for the use of L‐carnosine or fluvoxamine, or received any psychotropic drugs in the previous 6 weeks were excluded.
- Participants received fluvoxamine 100 mg/d for the first 4 weeks and 200 mg/d for the next 6 weeks plus either L-carnosine 500 mg twice daily or placebo. This dosage of L-carnosine was chosen because previously it had been tolerated and effective.
- The primary outcome measure was difference in Y-BOCS total scores. Secondary outcomes were differences in Y-BOCS obsession and compulsion subscale scores and differences in change in score on Y-BOCS total and subscale scores from baseline.
Outcomes
- The L-carnosine group experienced a significant decrease in Y-BOCS total score (P < .001), obsession subscale score (P < .01), and compulsion subscale score (P < .01).
- The group that received fluvoxamine plus L-carnosine also experienced a more complete response (P = .03).
- The L-carnosine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- L-carnosine significantly reduces OCD symptoms when used as an adjuvant to fluvoxamine.
Obsessive-compulsive disorder (OCD) is a chronic, debilitating neuropsychiatric disorder that affects 1% to 3% of the population worldwide.1,2 Together, serotonin reuptake inhibitors (SRIs) and cognitive-behavior therapy (CBT) are considered the first-line treatment for OCD.3 In children and adults, CBT is considered at least as effective as pharmacotherapy.4 Despite being an effective treatment, CBT continues to have barriers to its widespread use, including limited availability of trained CBT therapists, delayed clinical response, and high costs.5
Only approximately one-half of patients with OCD respond to SRI therapy, and a considerable percentage (30% to 40%) show significant residual symptoms even after multiple trials of SRIs.6-8 In addition, SRIs may have adverse effects (eg, sexual dysfunction, gastrointestinal symptoms) that impair patient adherence to these medications.9 Therefore, finding better treatment options is important for managing patients with OCD.
Augmentation strategies are recommended for patients who show partial response to SRI treatment or poor response to multiple SRIs. Augmentation typically includes incorporating additional medications with the primary drug with the goal of boosting the therapeutic efficacy of the primary drug. Typically, these additional medications have different mechanisms of action. However, there are no large-scale randomized controlled trials (RCTs) to inform treatment augmentation after first-line treatments for OCD produce suboptimal outcomes. The available evidence is predominantly based on small-scale RCTs, open-label trials, and case series.
In this article, we review the evidence for treatment augmentation strategies for OCD and summarize 8 studies that show promising results (Table10-17). We focus only on pharmacologic agents and do not include other biological interventions, such as repetitive transcranial magnetic stimulation over supplementary motor area, ablative neurosurgery, or deep brain stimulation.
Continue to: Reference 1...
1. Naderi S, Faghih H , Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessivecompulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/ pcn.12803
Numerous studies support the role of glutamate dysregulation in the pathophysiology of OCD. Cortico-striato-thalamo-cortical (CSTC) abnormalities play a major role in the pathophysiology of OCD as suggested by neuroimaging research studies that indicate glutamate is the fundamental neurotransmitter of the CSTC circuit. Dysregulation of glutamatergic signaling within this circuit has been linked to OCD. Patients with OCD have been found to have an increase of glutamate in the CSF. As a result, medications that affect glutamate levels can be used to treat patients with OCD who do not respond to first-line agents. In patients already taking SRIs, augmentation of glutamate-modulating medications can reduce OCD symptoms. As an uncompetitive antagonist of the N-methyl-
Naderi et al10 evaluated amantadine as augmentative therapy to fluvoxamine for treating patients with moderate to severe OCD.
Study design
- This 12-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of amantadine as an augmentative agent to fluvoxamine in 106 patients age 18 to 60 with moderate to severe OCD.
- Participants met DSM-5 criteria for OCD and had a Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score >21. Participants were excluded if they had any substance dependence; an IQ <70; any other Axis I mental disorder; any serious cardiac, renal, or hepatic disease; had received psychotropic medications during the last 6 weeks, were pregnant or breastfeeding, or had rising liver transaminases to 3 times the upper limit of normal or higher.
- Participants received fluvoxamine 100 mg twice daily plus amantadine 100 mg/d, or fluvoxamine 100 mg twice daily plus placebo. All patients received fluvoxamine 100 mg/d for 28 days followed by 200 mg/d for the remainder of the trial.
- The primary outcome measure was difference in Y-BOCS total scores between the amantadine and placebo groups. The secondary outcome was the difference in Y-BOCS obsession and compulsion subscale scores.
Outcomes
- Patients who received amantadine augmentation experienced a significant reduction in Y-BOCS total score (P < .001) and obsession subscale score (P < .01).
- The amantadine group showed good tolerability and safety. There were no clinically significant adverse effects.
- Amantadine is an effective adjuvant to fluvoxamine for reducing OCD symptoms.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
2. Sharafkhah M, Aghakarim Alamdar M, Massoudifar A, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222- 233. doi:10.1097/YIC.0000000000000267
Although selective serotonin reuptake inhibitors (SSRIs) are considered a first-line treatment when teamed with CBT and antipsychotic augmentation, symptom resolution is not always achieved, and treatment resistance is a common problem. Sharafkhah et al11 compared the efficacy of ondansetron and granisetron augmentation specifically for patients with treatment-resistant OCD.
Study Design
- In this 18-week, randomized, double-blind, placebo-controlled study, 135 patients with treatment-resistant OCD who were previously treated with a combination of an SSRI and an antipsychotic received augmentation with ondansetron (n = 45, 4 mg/d), granisetron (n = 45, 2 mg/d), or placebo.
- Patients were rated using Y-BOCS every 2 weeks during phase I (intervention period), which lasted 14 weeks. After completing the intervention, patients were followed for 4 more weeks during phase II (discontinuation period).
- The aim of this study was to determine the safety, efficacy, and tolerability of ondansetron vs granisetron as augmentation for patients with treatment-resistant OCD. A secondary aim was to determine the rate of relapse of OCD symptoms after discontinuing ondansetron as compared with granisetron at 4 weeks after intervention.
Outcomes
- At Week 14, the reductions in Y-BOCS scores in the ondansetron, granisetron, and placebo groups were 41.5%, 39.7%, and 15.2%, respectively (P = .001). The reduction in Y-BOCS score in the ondansetron and granisetron groups was significantly greater than placebo at all phase I visits.
- Complete response was higher in the ondansetron group compared with the granisetron group (P = .041).
- Y-BOCS scores increased in both the ondansetron and granisetron groups during the discontinuation phase, but OCD symptoms were not significantly exacerbated.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
3. Modarresi A, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitorrefractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-120268
Increased glutamate levels in CSF, glutamatergic overactivity, and polymorphisms of genes coding the NMDA receptor have been shown to contribute to the occurrence of OCD. Memantine is a noncompetitive antagonist of the NMDA receptor. Various control trials have shown augmentation with memantine 5 mg/d to 20 mg/d significantly reduced symptom severity in patients with moderate to severe OCD. Modarresi et al12 evaluated memantine as a treatment option for patients with severe OCD who did not respond to SRI monotherapy.
Study design
- This 12-week, double-blind, randomized, placebo-controlled trial evaluated the efficacy of memantine augmentation in 32 patients age 18 to 40 who met DSM-5 criteria for OCD, had a Y-BOCS score ≥24, and no psychiatric comorbidity. Participants had not responded to ≥3 adequate trials (minimum 3 months) of SRI therapy, 1 of which was clomipramine.
- Individuals were excluded if they were undergoing CBT; had an additional anxiety disorder, mood disorder, or current drug or alcohol use disorder, or any systemic disorder; had a history of seizures; were pregnant or breastfeeding; or had a history of memantine use.
- Participants already receiving the maximum tolerated dose of an SRI received augmentation with memantine 20 mg/d or placebo.
- The primary outcome measure was change in Y-BOCS score from baseline. The secondary outcome was the number of individuals who achieved treatment response (defined as ≥35% reduction in Y-BOCS score).
Continue to: Outcomes...
Outcomes
- There was a statistically significant difference in Y-BOCS score in patients treated with memantine at Week 8 and Week 12 vs those who received placebo. By Week 8, 17.2% of patients in the memantine group showed a decrease in Y-BOCS score, compared with -0.8% patients in the placebo group. The difference became more significant by Week 12, with 40.9% in the memantine group showing a decrease in Y-BOCS score vs -0.3% in the placebo group. This resulted in 73.3% of patients achieving treatment response.
- Eight weeks of memantine augmentation was necessary to observe a significant improvement in OCD symptoms, and 12 weeks was needed for treatment response.
- The mean Y-BOCS total score decreased significantly in the memantine group from Week 4 to Week 8 (16.8%) and again from Week 8 to Week 12 (28.5%).
- The memantine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- Memantine augmentation in patients with severe OCD who do not respond to an SRI is effective and well-tolerated.
4. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407- 1414. doi:10.1177/0269881119878177
Studies have demonstrated the involvement of the amygdala, medial and lateral orbitofrontal cortex, and dorsal anterior cingulate cortex in OCD. Additionally, studies have also investigated the role of serotonin, dopamine, and glutamate system dysregulation in the pathology of OCD.
The 5-HT3 receptors are ligand-gated ion channels found in the prefrontal cortex, amygdala, and hippocampus. Studies of 5-HT3 receptor antagonists such as ondansetron and granisetron have shown beneficial results in augmentation with SSRIs for patients with OCD.11 Tropisetron, a 5-HT3 receptor antagonist, is highly lipophilic and able to cross the blood brain barrier. It also has dopamine-inhibiting properties that could have benefits in OCD management. Shalbafan et al13 evaluated the efficacy of tropisetron augmentation to fluvoxamine for patients with OCD.
Study design
- In a 10-week, randomized, double-blind, placebo-controlled, parallel-group trial, 108 individuals age 18 to 60 who met DSM-5 criteria for OCD and had a Y-BOCS score >21 received fluvoxamine plus tropisetron or fluvoxamine plus placebo. A total of 48 (44.4%) participants in each group completed the trial. Participants were evaluated using the Y-BOCS scale at baseline and at Week 4 and Week 10.
- The primary outcome was decrease in total Y-BOCS score from baseline to Week 10. The secondary outcome was the difference in change in Y-BOCS obsession and compulsion subscale scores between the groups.
Outcomes
- The Y-BOCS total score was not significantly different between the 2 groups (P = .975). Repeated measures analysis of variance determined a significant effect for time in both tropisetron and placebo groups (Greenhouse-Geisser F [2.72–2303.84] = 152.25, P < .001; and Greenhouse-Geisser F [1.37–1736.81] = 75.57, P < .001, respectively). At Week 10, 35 participants in the tropisetron group and 19 participants in the placebo group were complete responders.
- The baseline Y-BOCS obsession and compulsion subscales did not significantly differ between treatment groups.
Conclusion
- Compared with participants in the placebo group, those in the tropisetron group experienced a significantly greater reduction in OCD symptoms as measured by Y-BOCS score. More participants in the tropisetron group experienced complete response and remission.
- This study demonstrated that compared with placebo, when administered as augmentation with fluvoxamine, tropisetron can have beneficial effects for patients with OCD.
Continue to: Reference 5...
5. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a doubleblind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254- 262. doi:10.1097/YIC.0000000000000321
Nutraceuticals such as glycine, milk thistle, myoinositol, and serotonin (5-hydroxytryptophan) have been proposed as augmentation options for OCD. Yousefzadeh et al14 investigated the effectiveness of using 5-hydroxytryptophan in treating OCD.
Study design
- In a 12-week, randomized, double-blind study, 60 patients who met DSM-5 criteria for moderate to severe OCD (Y-BOCS score >21) were randomly assigned to receive fluoxetine plus 5-hydroxytryptophan 100 mg twice daily or fluoxetine plus placebo.
- All patients were administered fluoxetine 20 mg/d for the first 4 weeks of the study followed by fluoxetine 60 mg/d for the remainder of the trial.
- Symptoms were assessed using the Y-BOCS at baseline, Week 4, Week 8, and Week 12.
- The primary outcome measure was the difference between the 2 groups in change in Y-BOCS total score from baseline to the end of the trial. Secondary outcome measures were the differences in the Y-BOCS obsession and compulsion subscale scores from baseline to Week 12.
Outcomes
- Compared to the placebo group, the 5-hydroxytryptophan group experienced a statistically significant greater improvement in Y-BOCS total score from baseline to Week 8 (P = .002) and Week 12 (P < .001).
- General linear model repeated measure showed significant effects for time × treatment interaction on Y-BOCS total (F = 12.07, df = 2.29, P < .001), obsession subscale (F = 8.25, df = 1.91, P = .001), and compulsion subscale scores (F = 6.64, df = 2.01, P = .002).
- The 5-hydroxytryptophan group demonstrated higher partial and complete treatment response rates (P = .032 and P = .001, respectively) as determined by change in Y-BOCS total score.
- The 5-hydroxytryptophan group showed a significant improvement from baseline to Week 12 in Y-BOCS obsession subscale score (5.23 ± 2.33 vs 3.53 ± 2.13, P = .009).
- There was a significant change from baseline to the end of the trial in the Y-BOCS compulsion subscale score (3.88 ± 2.04 vs 2.30 ± 1.37, P = .002).
Conclusion
- This trial demonstrated the potential benefits of 5-hydroxytryptophan in combination with fluoxetine for patients with OCD.
6. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
Glutamatergic dysfunction has been identified as a potential cause of OCD. Studies have found elevated levels of glutamatergic transmission in the cortical-striatal-thalamic circuit of the brain and elevated glutamate concentration in the CSF in patients with OCD. Pregabalin has multiple mechanisms of action that inhibit the release of glutamate. Mowla et al15 evaluated pregabalin as an augmentation treatment for resistant OCD.
Study design
- This 12-week, double-blind, placebo-controlled clinical trial evaluated the efficacy of adjunctive pregabalin in 56 patients who met DSM-5 criteria for OCD and had not responded to ≥12 weeks of treatment with an adequate and stable dose of sertraline (baseline Y-BOCS score ≥18).
- Individuals who had other major psychiatric disorders, major medical problems, were pregnant, or had past substance or alcohol abuse were excluded.
- Participants were randomly assigned to receive sertraline plus pregabalin (n = 28) or sertraline plus placebo (n = 28). Mean sertraline dosage was 256.5 mg/d; range was 100 mg/d to 300 mg/d. Pregabalin was started at 75 mg/d and increased by 75 mg increments weekly. The mean dosage was 185.9 mg/d; range was 75 mg/d to 225 mg/d.
- The primary outcome measure was change in Y-BOCS score. A decrease >35% in Y-BOCS score was considered a significant response rate.
Outcomes
- There was a statistically significant decrease in Y-BOCS score in patients who received pregabalin. In the pregabalin group, 57.14% of patients (n = 16) showed a >35% decrease in Y-BOCS score compared with 7.14% of patients (n = 2) in the placebo group (P < .01).
- The pregabalin group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- In patients with treatment-resistant OCD who did not respond to sertraline monotherapy, augmentation with pregabalin significantly decreases Y-BOCS scores compared with placebo.
Continue to: Reference 7...
7. Zheng H, Jia F, Han H, et al. Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebocontrolled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro. 2018.12.010
Recent evidence suggests dysregulation of serotonin and dopamine in patients with OCD. Methylphenidate is a dopamine and norepinephrine inhibitor and releaser. A limited number of studies have suggested stimulants might be useful for OCD patients. Zheng et al16 conducted a pilot trial to determine whether methylphenidate augmentation may be of benefit in the management of outpatients with OCD.
Study design
- In an 8-week, double-blind, randomized, placebo-controlled trial, 44 patients (29 [66%] men, with a mean [SD] age of 24.7 [6]) with treatment-refractory OCD were randomized to receive fluvoxamine 250 mg/d plus methylphenidate extended-release (MPH-ER) 36 mg/d or fluvoxamine 250 mg/d plus placebo. The MPH-ER dose was 18 mg/d for the first 4 weeks and 36 mg/d for the rest of the trial.
- Biweekly assessments consisted of scores on the Y-BOCS, Hamilton Depression Rating Scale (HDRS), and Hamilton Anxiety Rating Scale (HAM-A).
- The primary outcomes were improvement in Y-BOCS score and the clinical response rate. Secondary outcomes included a change in score on the Y-BOCS subscales, HARS, and HAM-A. Data were analyzed with the intention-to-treat sample.
Outcomes
- Forty-one patients finished the trial. The baseline Y-BOCS total scores and subscale scores did not differ significantly between the 2 groups.
- Improvements in Y-BOCS total score and obsession subscale score were more prominent in the fluvoxamine plus MPH-ER group compared with the placebo group (P < .001).
- HDRS score decreased in both the placebo and MPH-ER groups. HAM-A scores decreased significantly in the MPH-ER plus fluvoxamine group compared with the placebo group.
Conclusion
- This study demonstrated that the combination of fluvoxamine and MPH-ER produces a higher and faster response rate than fluvoxamine plus placebo in patients with OCD.
8. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Glutamate dysregulation is implicated in the pathogenesis of OCD. Glutamate-modulating agents have been used to treat OCD. Studies have shown L-carnosine has a neuroprotective role via its modulatory effect on glutamate. Arabzadeh et al17 evaluated the efficacy of L-carnosine as an adjuvant to fluvoxamine for treating OCD.
Study design
- This 10-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy of adjunctive L-carnosine in 40 patients age 18 to 60 who met DSM-5 criteria for OCD and had moderate to severe OCD (Y-BOCS score ≥21).
- Individuals with any other DSM-5 major psychiatric disorders, serious medical or neurologic illness, substance dependence (other than caffeine or nicotine), mental retardation (based on clinical judgment), were pregnant or breastfeeding, had any contraindication for the use of L‐carnosine or fluvoxamine, or received any psychotropic drugs in the previous 6 weeks were excluded.
- Participants received fluvoxamine 100 mg/d for the first 4 weeks and 200 mg/d for the next 6 weeks plus either L-carnosine 500 mg twice daily or placebo. This dosage of L-carnosine was chosen because previously it had been tolerated and effective.
- The primary outcome measure was difference in Y-BOCS total scores. Secondary outcomes were differences in Y-BOCS obsession and compulsion subscale scores and differences in change in score on Y-BOCS total and subscale scores from baseline.
Outcomes
- The L-carnosine group experienced a significant decrease in Y-BOCS total score (P < .001), obsession subscale score (P < .01), and compulsion subscale score (P < .01).
- The group that received fluvoxamine plus L-carnosine also experienced a more complete response (P = .03).
- The L-carnosine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- L-carnosine significantly reduces OCD symptoms when used as an adjuvant to fluvoxamine.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
3. Eddy KT, Dutra L, Bradley, R, et al. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24(8):1011-1030.
4. Franklin ME, Foa EB. Treatment of obsessive compulsive disorder. Annu Rev Clin Psychol. 2011;7:229-243.
5. Koran LM, Hanna GL, Hollander E, et al. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(7 Suppl):5-53.
6. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37(3):375-391.
7. Pallanti S, Hollander E, Bienstock C, et al. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002;5(2):181-191.
8. Atmaca M. Treatment-refractory obsessive compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:127-133.
9. Barth M, Kriston L, Klostermann S, et al. Efficacy of selective serotonin reuptake inhibitors and adverse events: meta-regression and mediation analysis of placebo-controlled trials. Br J Psychiatry. 2016;208(2):114-119.
10. NaderiS, Faghih H, Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/pcn.12803
11. SharafkhahM, Aghakarim Alamdar M, MassoudifarA, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222-233. doi:10.1097/YIC.0000000000000267
12. ModarresiA, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-12026
13. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407-1414. doi:10.1177/0269881119878177
14. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254-262. doi:10.1097/YIC.0000000000000321
15. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
16. Zheng H, Jia F, Han H, et al.Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro.2018.12.010
17. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
3. Eddy KT, Dutra L, Bradley, R, et al. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24(8):1011-1030.
4. Franklin ME, Foa EB. Treatment of obsessive compulsive disorder. Annu Rev Clin Psychol. 2011;7:229-243.
5. Koran LM, Hanna GL, Hollander E, et al. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(7 Suppl):5-53.
6. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37(3):375-391.
7. Pallanti S, Hollander E, Bienstock C, et al. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002;5(2):181-191.
8. Atmaca M. Treatment-refractory obsessive compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:127-133.
9. Barth M, Kriston L, Klostermann S, et al. Efficacy of selective serotonin reuptake inhibitors and adverse events: meta-regression and mediation analysis of placebo-controlled trials. Br J Psychiatry. 2016;208(2):114-119.
10. NaderiS, Faghih H, Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/pcn.12803
11. SharafkhahM, Aghakarim Alamdar M, MassoudifarA, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222-233. doi:10.1097/YIC.0000000000000267
12. ModarresiA, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-12026
13. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407-1414. doi:10.1177/0269881119878177
14. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254-262. doi:10.1097/YIC.0000000000000321
15. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
16. Zheng H, Jia F, Han H, et al.Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro.2018.12.010
17. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Psychoses: The 5 comorbidity-defined subtypes
How can we treat psychosis if we don’t know what we are treating? Over the years, attempts at defining psychosis subtypes have met with dead ends. However, recent research supports a new approach that offers a rational classification model organized according to 5 specific comorbid anxiety and depressive disorder diagnoses.
Anxiety and depressive symptoms are not just the result of psychotic despair. They are specific diagnoses, they precede psychosis onset, they help define psychotic syndromes, and they can point to much more effective treatment approaches. Most of the psychotic diagnoses in this schema are already recognized or posited. And, just as patients who do not have psychotic illness can have more than 1 anxiety or depressive disorder, patients with psychosis can present with a mixed picture that reflects more than 1 contributing comorbidity. Research further suggests that each of the 5 psychosis comorbidity diagnoses may involve some similar underlying factors that facilitate the formation of psychosis.
This article describes the basics of 5 psychosis subtypes, and provides initial guidelines to diagnosis, symptomatology, and treatment. Though clinical experience and existing research support the clinical presence and treatment value of this classification model, further verification will require considerably more controlled studies. An eventual validation of this approach could largely supplant ill-defined diagnoses of “schizophrenia” and other functional psychoses.
Recognizing the comorbidities in the context of their corresponding psychoses entails learning new interviewing skills and devoting more time to both initial and subsequent diagnosis and treatment. In our recently published book,1 we provide extensive details on the approach we describe in this article, including case examples, new interview tools to simplify the diagnostic journey, and novel treatment approaches.
Psychosis-proneness underlies functional psychoses
Functional (idiopathic) schizophrenia and psychotic disorders have long been difficult to separate, and many categorizations have been discarded. Despite clinical dissimilarities, today we too often casually lump psychoses together as schizophrenia.2,3 Eugen Bleuler first suggested the existence of a “group of schizophrenias.”4 It is possible that his group encompasses our 5 psychoses from 5 inbuilt emotional instincts,5 each corresponding to a specific anxiety or depressive subtype.
The 5 anxiety and depressive subtypes noted in this article are common, but psychosis is not. Considerable research suggests that certain global “psychotogenic” factors create susceptibility to all psychoses.6,7 While many genetic, neuroanatomical, experiential, and other factors have been reported, the most important may be “hypofrontality” (genetically reduced frontal lobe function, size, or neuronal activity) and dopaminergic hyperfunction (genetically increased dopamine activity).5-7
An evolutionary perspective
One evolutionary theory of psychopathology starts with the subtypes of depression and anxiety. For example, major depressive disorder and generalized anxiety disorder may encompass 5 commonplace and more specific anxiety and depressive subtypes. Consideration of the emotional, cognitive, and functional aspects of those subtypes suggests that they may have once been advantageous for primeval human herds. Those primeval altruistic instincts may have helped survival, reproduction, and preservation of kin group DNA.5
More than any other species, humans can draw upon consciousness and culture to rationally overcome the influences of unconscious instincts. But those instincts can then emerge from the deep, and painfully encourage obedience to their guidance. In nonpsychotic anxiety and depressive disorders, the specific messages are experienced as specific anxiety and depressive symptoms.5 In psychotic disorders, the messages can emerge as unreasoned and frightful fears, perceptions, beliefs, and behaviors. With newer research, clinical observation, and an evolutionary perspective, a novel and counterintuitive approach may improve our ability to help patients.8
Continue to: Five affective comorbidities evolved from primeval altruistic instincts...
Five affective comorbidities evolved from primeval altruistic instincts
Melancholic depression5
Melancholic depression is often triggered by serious illness, group exclusion, pronounced loss, or purposelessness. We hear patients talk painfully about illness, guilt, and death. Indeed, some increased risk of death, especially from infectious disease, may result from hypercortisolemia (documented by the dexamethasone suppression test). Hypercortisolemic death also occurs in salmon after spawning, and in male marsupial mice after mating. The tragic passing of an individual saves scarce resources for the remainder of the herd.
Obsessive-compulsive disorder5
Factor-analytic studies suggest 4 main obsessive-compulsive disorder (OCD) subtypes: cleanliness, hoarding, intrusive thoughts, and organizing. Obsessive-compulsive traits can help maintain a safe and efficient environment in humans and other species, but OCD is dysfunctional.
Panic anxiety5
Panic anxiety is triggered by real, symbolic, or emotional separation from home and family. In toddlers, separation anxiety can reduce the odds of getting lost and hurt.
Social anxiety5
Social anxiety includes fear of self-embarrassment, exposure as a pretender to higher social rank, and thus often a reluctant avoidance of increased social rank. While consciousness and cultural encouragement can overcome that hesitation and thus lead to greater success, social anxiety activation can still cause painful anxiety. The social hierarchies of many species include comparable biological influences, and help preserve group DNA by reducing hierarchical infighting.
Atypical depression and bipolar I mania5
Atypical depression includes increased rejection sensitivity, resulting in inoffensive behavior to avoid social rejection. This reduces risk of isolation from the group, and improves group harmony. Unlike the 4 other syndromes, atypical depression and bipolar I mania may reflect 2 separate seasonal mood phases. Atypical depression (including seasonal affective disorder) often worsens with shortened winter daylight hours, akin to hibernation. Initial bipolar I mania is more common with springtime daylight, with symptoms not unlike exaggerated hibernation awakening.9
Primeval biological altruism has great evolutionary value in many species, and even somewhat in modern humans. But it is quite different from modern rational altruism. Although we sometimes override our instincts, they respond with messages experienced as emotional pain—they still tell us to follow instructions for primeval herd survival. In an earlier book, I (JPK) provide a lengthier description of the evidence for this evolutionary psychopathology theory, including interplay of the 5 instincts with psychotogenic factors.5
Continue to: Five comorbidity psychoses from 5 primeval instincts.....
Five comorbidity psychoses from 5 primeval instincts
The 5 affective comorbidities described above contribute to the presence, subtype, and treatment approaches of 5 corresponding psychoses. Ordinary panic attacks might occur when feeling trapped or separated from home, so people want to flee to safety. Nonhuman species with limited consciousness and language are unlikely to think “time to head for safety.” Instead, instincts encourage flight from danger through internally generated perceptions of threat. Likewise, people with psychosis and panic, without sufficient conscious modulation, may experience sensory perceptions of actual danger when feeling symbolically trapped.1,10
One pilot study carefully examined the prevalence of these 5 comorbidities in an unselected group of psychotic patients.10 At least 85% met criteria for ≥1 of the 5 subtypes.10 Moreover, organic psychoses related to physical illness, substances, and iatrogenesis may also predict future episodes of functional psychoses.1
Using statistical analysis of psychosis rating scales, 2 studies took a “transdiagnostic” look at psychoses, and each found 5 psychosis subtypes and a generalized psychosis susceptibility factor.11,12 Replication of that transdiagnostic approach, newly including psychosis symptoms and our 5 specific comorbidities, might well find that the 5 subtype models resemble each other.11,12
Our proposed 5 comorbidity subtypes are1:
Delusional depression (melancholic depression). Most common in geriatric patients, this psychosis can also occur at younger ages. Prodromal melancholic depression can include guilt and hopelessness, and is acute, rather than the chronic course of our other 4 syndromes. Subsequent delusional depression includes delusions of bodily decay, illness, or death, as well as overwhelming guilt, shame, and remorse. The classic vegetative symptoms of depression continue. In addition to infectious disease issues, high suicide risk makes hospitalization imperative.
Obsessive-compulsive schizophrenia. Just as OCD has an early age of onset, obsessive-compulsive schizophrenia begins earlier than other psychoses. Despite preserved cognition, some nonpsychotic patients with OCD have diminished symptom insight. OCD may be comorbid with schizophrenia in 12% of cases, typically preceding psychosis onset. Obsessive-compulsive schizophrenia symptoms may include highly exaggerated doubt or ambivalence; contamination concerns; eccentric, ritualistic, motor stereotypy, checking, disorganized, and other behaviors; and paranoia.
Schizophrenia with voices (panic anxiety). Classic paranoid schizophrenia with voices appears to be the most similar to a “panic psychosis.” Patients with nonpsychotic panic anxiety have increased paranoid ideation and ideas of reference as measured on the Symptom Checklist-90. Schizophrenia is highly comorbid with panic anxiety, estimated at 45% in the Epidemiologic Catchment Area study.13 These are likely underestimates: cognitive impairment hinders reporting, and psychotic panic is masked as auditory hallucinations. A pilot study of schizophrenia with voices using a carbon dioxide panic induction challenge found that 100% had panic anxiety.14 That study and another found that virtually all participants reported voices concurrent with panic using our Panic and Schizophrenia Interview (PaSI) (Box 1). Panic onset precedes schizophrenia onset, and panic may reappear if antipsychotic medications sufficiently control voices: “voices without the voices,” say some.
Box 1
Let’s talk for a minute about your voices.
[IDENTIFYING PAROXYSMAL MOMENTS OF VOICE ONSET]
Do you hear voices at every single moment, or are they sometimes silent? Think about those times when you are not actually hearing any voices.
Now, there may be reasons why the voices start talking when they do, but let’s leave that aside for now.
So, whenever the voices do begin speaking—and for whatever reason they do—is it all of a sudden, or do they start very softly and then very gradually get louder?
If your voices are nearly always there, then are there times when the voices suddenly come back, get louder, get more insistent, or just get more obvious to you?
[Focus patient on sudden moment of voice onset, intensification, or awareness]
Let’s talk about that sudden moment when the voices begin (or intensify, or become obvious), even if you know the reason why they start.
I’m going to ask you about some symptoms that you might have at that same sudden moment when the voices start (or intensify, or become obvious). If you have any of these symptoms at the other times, they do not count for now.
So, when I ask about each symptom, tell me whether it comes on at the same sudden moments as the voices, and also if it used to come on with the voices in the past.
For each sudden symptom, just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same sudden moment that the voices come on”]
- Sudden anxiety, fear, or panic on the inside?
- Sudden anger or rage on the inside? [ANGER QUERY]
- Sudden heart racing? Heart pounding?
- Sudden chest pain? Chest pressure?
- Sudden sweating?
- Sudden trembling or shaking?
- Sudden shortness of breath, or like you can’t catch your breath?
- Sudden choking or a lump in your throat?
- Sudden nausea or queasiness?
- Sudden dizziness, lightheadedness, or faintness?
- Sudden feeling of detachment, sort of like you are in a glass box?
- Sudden fear of losing control? Fear of going crazy?
- Sudden fear afraid of dying? Afraid of having a heart attack?
- Sudden numbness or tingling, especially in your hands or face?
- Sudden feeling of heat, or cold?
- Sudden itching in your teeth? [VALIDITY CHECK]
- Sudden fear that people want to hurt you? [EXCESS FEAR QUERY]
- Sudden voices? [VOICES QUERY]
[PAST & PRODROMAL PANIC HISTORY]
At what age did you first see a therapist or psychiatrist?
At what age were you first hospitalized for an emotional problem?
At what age did you first start hearing voices?
At what age did you first start having strong fears of other people?
Before you ever heard voices, did you ever have any of the other sudden symptoms like the ones we just talked about?
Did those episodes back then feel sort of like your voices or sudden fears do now, except that there were no voices or sudden fears of people back then?
At what age did those sudden anxiety (or panic or rage) episodes begin?
Back then, was there MORE (M) sudden anxiety, or the SAME (S) sudden anxiety, or LESS (L) sudden anxiety than with your sudden voices now?
[PAST & PRODROMAL PANIC SYMPTOMS]
Now let’s talk about some symptoms that you might have had at those same sudden anxiety moments, in the time before you ever heard any voices. So, for each sudden symptom just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same moment the sudden anxiety came on—but only during the time before you ever heard sudden voices”]
[Ask about the same 18 panic-related symptoms listed above]
[PHOBIA-RELATED PANIC AND VOICES]
Have you ever been afraid to go into a (car, bus, plane, train, subway, elevator, mall, tunnel, bridge, heights, small place, CAT scan or MRI, being alone, crowds)?
[If yes or maybe: Ask about panic symptoms in phobic situations]
Now let’s talk about some symptoms that you might have had at some of those times you were afraid. So, for each symptom just say “YES” or “NO” or “MAYBE.”
[Ask about the same 18 panic-related symptoms listed above]
At what age did you last have sudden anxiety without voices?
Has medication ever completely stopped your voices? Somewhat?
If so, did those other sudden symptoms still happen sometimes?
Thank you for your help, and for answering all of these questions!
Persecutory delusional disorder (social anxiety). Some “schizophrenia” without voices may be misdiagnosis of persecutory (paranoid) delusional disorder (PDD). Therefore, the reported population prevalence (0.02%) may be underestimated. Social anxiety is highly comorbid with “schizophrenia” (15%).16 Case reports and clinical experience suggest that PDD is commonly preceded by social anxiety.17 Some nonpsychotic social anxiety symptoms closely resemble the PDD psychotic ideas of reference (a perception that low social rank attracts critical scrutiny by authorities). Patients with PDD may remain relatively functional, with few negative symptoms, despite pronounced paranoia. Outward manifestation of paranoia may be limited, unless quite intense. The typical age of onset (40 years) is later than that of schizophrenia, and symptoms can last a long time.18
Continue to: Bipolar 1 mania with delusions...
Bipolar I mania with delusions (atypical depression). Atypical depression is the most common depression in bipolar I disorder. Often more pronounced in winter, it may intensify at any time of year. Long ago, hypersomnia, lethargy, inactivity, inoffensiveness, and craving high-calorie food may have been conducive to hibernation.
Bipolar I mania includes delusions of special accomplishments or abilities, energetically focused on a grandiose mission to help everyone. These intense symptoms may be related to reduced frontal lobe modulation. In some milder form, bipolar I mania may once have encouraged hibernation awakening. Indeed, initial bipolar I mania episodes are more common in spring, as is the spring cleaning that helps us prepare for summer.
Recognizing affective trees in a psychotic forest
Though long observed, comorbid affective symptoms have generally been considered a hodgepodge of distress caused by painful psychotic illness. But the affective symptoms precede psychosis onset, can be masked during acute psychosis, and will revert to ordinary form if psychosis abates.11-13
Rather than affective symptoms being a consequence of psychosis, it may well be the other way around. Affective disorders could be important causal and differentiating components of psychotic disorders.11-13 Research and clinical experience suggest that adjunctive treatment of the comorbidities with correct medication can greatly enhance outcome.
Diagnostic approaches
Because interviews of patients with psychosis are often complicated by confusion, irritability, paranoid evasiveness, cognitive impairment, and medication, nuanced diagnosis is difficult. Interviews should explore psychotic syndromes and subtypes that correlate with comorbidity psychoses, including pre-psychotic anxiety and depressive diagnoses that are chronic (though unlike our 4 other diagnoses, melancholic depression is not chronic).
Establishing pre-psychotic diagnosis of chronic syndromes suggests that they are still present, even if they are difficult to assess during psychosis. Re-interview after some improvement allows for a significantly better diagnosis. Just as in nonpsychotic affective disorders, multiple comorbidities are common, and can lead to a mixed psychotic diagnosis and treatment plan.1
Structured interview tools can assist diagnosis. The PaSI (Box 1,15) elicits past, present, and detailed history of DSM panic, and has been validated in a small pilot randomized controlled trial. The PaSI focuses patient attention on paroxysmal onset voices, and then evaluates the presence of concurrent DSM panic symptoms. If voices are mostly psychotic panic, they may well be a proxy for panic. Ultimately, diagnosis of 5 comorbidities and associated psychotic symptoms may allow simpler categorization into 1 (or more) of the 5 psychosis subtypes.
Continue to: Treatment by comorbidity subtype...
Treatment by comorbidity subtype
Treatment of psychosis generally begins with antipsychotics. Nominal psychotherapy (presence of a professionally detached, compassionate clinician) improves compliance and leads to supportive therapy. Cognitive-behavioral therapy and dialectical behavior therapy may help later, with limited interpersonal approaches further on for some patients.
The suggested approaches to pharmacotherapy noted here draw on research and clinical experience.1,14,19-21 All medications used to treat comorbidities noted here are approved or generally accepted for that diagnosis. Estimated doses are similar to those for comorbidities when patients are nonpsychotic, and vary among patients. Doses, dosing schedules, and titration are extremely important for full benefit. Always consider compliance issues, suicidality, possible adverse effects, and potential drug/drug interactions. Although the medications we suggest using to treat the comorbidities may appear to also benefit psychosis, only antipsychotics are approved for psychosis per se.
Delusional depression. Antipsychotic + antidepressant. Tricyclic antidepressants are possibly most effective, but increase the risk of overdose and dangerous falls among fragile patients. Electroconvulsive therapy is sometimes used.
Obsessive-compulsive schizophrenia. Antipsychotic + selective serotonin reuptake inhibitor (SSRI). Consider aripiprazole (
Schizophrenia with voices. Antipsychotic + clonazepam. Concurrent usage may stabilize psychosis more rapidly, and with a lower antipsychotic dose.23 Titrate a fixed dose of clonazepam every 12 hours (avoid as-needed doses), starting low (ie, 0.5 mg) to limit initial drowsiness (which typically diminishes in 3 to 10 days). Titrate to full voice and panic cessation (1 to 2.5 mg every 12 hours).14 Exercise caution about excessive drowsiness, as well as outpatient compliance and abuse. Besides alprazolam, other antipanic medications have little incidental benefit for psychosis.
Persecutory delusional disorder. Antipsychotic + SSRI. Aripiprazole (consider long-acting injectable for compliance) also enhances the benefits of fluoxetine for social anxiety. Long half-life fluoxetine (20 mg/d) improves compliance and near-term outcomes.
Bipolar I mania: mania with delusions. Consider olanzapine for acute phase, then add other antimanic medication (commonly lithium or valproic acid), check blood level, and then taper olanzapine some weeks later. Importantly, lamotrigine is not effective for bipolar I mania. Consider suicide risk, medical conditions, and outpatient compliance. Comorbid panic anxiety is also common in bipolar I mania, often presenting as nonthreatening voices.
Seasonality: Following research that bipolar I mania is more common in spring and summer, studies have shown beneficial clinical augmentation from dark therapy as provided by reduced light exposure, blue-blocking glasses, and exogenous melatonin (a darkness-signaling hormone).24
Bipolar I mania atypical depression (significant current or historical symptoms). SSRI + booster medication. An SSRI (ie, escitalopram, 10 mg/d) is best started several weeks after full bipolar I mania resolution, while also continuing long-term antimanic medication. Booster medications (ie, buspirone 15 mg every 12 hours; lithium 300 mg/d; or trazodone 50 mg every 12 hours) can enhance SSRI benefits. Meta-analysis suggests SSRIs may have limited risk of inducing bipolar I mania.25 Although not yet specifically tested for atypical depression, lamotrigine may be effective, and may be safer still.25 However, lamotrigine requires very gradual dose titration to prevent a potentially dangerous rash, including after periods of outpatient noncompliance.
Seasonality: Atypical depression is often worse in winter (seasonal affective disorder). Light therapy can produce some clinically helpful benefits year-round.
To illustrate this new approach to psychosis diagnosis and treatment, our book
Box 2
Ms. B, a studious 19-year-old, has been very shy since childhood, with few friends. Meeting new people always gave her gradually increasing anxiety, thinking that she would embarrass herself in their eyes. She had that same anxiety, along with sweating and tachycardia, when she couldn’t avoid speaking in front of class. Sometimes, while walking down the street she would think that strangers were casting a disdainful eye on her, though she knew that wasn’t true. Another anxiety started when she was 16. While looking for paper in a small supply closet, she suddenly felt panicky. With a racing heart and short of breath, she desperately fled the closet. These episodes continued, sometimes for no apparent reason, and nearly always unnoticed by others.
At age 17, she began to believe that those strangers on the street were looking down on her with evil intent, and even following her around. She became afraid to walk around town. A few months later, she also started to hear angry and critical voices at sudden moments. Although the paroxysmal voices always coincided with her panicky symptoms, the threatening voices now felt more important to her than the panic itself. Nonpsychotic panics had stopped. Mostly a recluse, she saw less of her family, left her job, and stopped going to the movies.
After a family dinner, she was detached, scared, and quieter than usual. She sought help from her primary care physician, who referred her to a psychiatrist. A thorough history from Ms. B and her family revealed her disturbing fears, as well as her history of social anxiety. Interviewing for panic was prompted by her mother’s recollection of the supply closet story.
In view of Ms. B’s cooperativeness and supportive family, outpatient treatment of her recent-onset psychosis began with aripiprazole, 10 mg/d, and clonazepam, 0.5 mg every 12 hours. Clonazepam was gradually increased until voices (and panic) ceased. She was then able to describe how earlier panics had felt just like voices, but without the voices. The fears of strangers continued. Escitalopram, 20 mg/d, was added for social anxiety (aripiprazole enhances the benefits of selective serotonin reuptake inhibitors).
One month later, her fears of strangers diminished, and she felt more comfortable around people than ever before. On the same medications, and in psychotherapy over the next year, she began to increase her social network while making plans to start college.
Larger studies are needed
Current research supports the concept of a 5-diagnosis classification of psychoses, which may correlate with our comorbid anxiety and depression model. Larger diagnostic and treatment studies would invaluably examine existing research and clinical experience, and potentially encourage more clinically useful diagnoses, specific treatments, and improved outcomes.
Bottom Line
New insights from evolutionary psychopathology, clinical research and observation, psychotogenesis, genetics, and epidemiology suggest that most functional psychoses may fall into 1 of 5 comorbidity-defined subtypes, for which specific treatments can lead to much improved outcomes.
1. Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
2. Gaebel W, Zielasek J. Focus on psychosis. Dialogues Clin Neuroscience. 2015;17(1):9-18.
3. Guloksuz S, Van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychological Medicine. 2018;48(2):229-244.
4. Bleuler E. Dementia Praecox or the Group of Schizophrenias. International Universities Press; 1950.
5. Kahn JP. Angst: Origins of Depression and Anxiety. Oxford University Press; 2013.
6. Howes OD, McCutcheon R, Owen MJ, et al. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81(1):9-20.
7. Mubarik A, Tohid H. Frontal lobe alterations in schizophrenia: a review. Trends Psychiatry Psychother. 2016;38(4):198-206.
8. Murray RM, Bhavsar V, Tripoli G, et al. 30 Years on: How the neurodevelopmental hypothesis of schizophrenia morphed into the developmental risk factor model of psychosis. Schizophr Bull. 2017;43(6):1190-1196.
9. Bauer M, Glenn T, Alda M, et al. Solar insolation in springtime influences age of onset of bipolar I disorder. Acta Psychiatr Scand. 2017;136(6):571-582.
10. Kahn JP, Bombassaro T, Veras AB. Comorbid schizophrenia and panic anxiety: panic psychosis revisited. Psychiatr Ann. 2018;48(12):561-565.
11. Bebbington P, Freeman D. Transdiagnostic extension of delusions: schizophrenia and beyond. Schizophr Bull. 2017;43(2):273-282.
12. Catalan A, Simons CJP, Bustamante S, et al. Data gathering bias: trait vulnerability to psychotic symptoms? PLoS One. 2015;10(7):e0132442. doi:10.1371/journal.pone.0132442
13. Goodwin R, Lyons JS, McNally RJ. Panic attacks in schizophrenia. Schizophr Res. 2002;58(2-3):213-220.
14. Kahn JP, Puertollano MA, Schane MD, et al. Adjunctive alprazolam for schizophrenia with panic anxiety: clinical observation and pathogenetic implications. Am J Psychiatry. 1988;145(6):742-744.
15. Kahn JP. Chapter 4: Paranoid schizophrenia with voices and panic anxiety. In: Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
16. Achim AM, Maziade M, Raymond E, et al. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull. 2011;37(4):811-821.
17. Veras AB, Souza TG, Ricci TG, et al. Paranoid delusional disorder follows social anxiety disorder in a long-term case series: evolutionary perspective. J Nerv Ment Dis. 2015;203(6):477-479.
18. McIntyre JC, Wickham S, Barr B, et al. Social identity and psychosis: associations and psychological mechanisms. Schizophr Bull. 2018;44(3):681-690.
19. Barbee JG, Mancuso DM, Freed CR. Alprazolam as a neuroleptic adjunct in the emergency treatment of schizophrenia. Am J Psychiatry. 1992;149(4):506-510.
20. Nardi AE, Machado S, Almada LF. Clonazepam for the treatment of panic disorder. Curr Drug Targets. 2013;14(3):353-364.
21. Poyurovsky M. Schizo-Obsessive Disorder. Cambridge University Press; 2013.
22. Reznik I, Sirota P. Obsessive and compulsive symptoms in schizophrenia: a randomized controlled trial with fluvoxamine and neuroleptics. J Clin Psychopharmacol. 2000;20(4):410-416.
23. Bodkin JA. Emerging uses for high-potency benzodiazepines in psychotic disorders. J Clin Psychiatry. 1990;51 Suppl:41-53.
24. Gottlieb JF, Benedetti F, Geoffroy PA, et al. The chronotherapeutic treatment of bipolar disorders: a systematic review and practice recommendations from the ISBD task force on chronotherapy and chronobiology. Bipolar Disord. 2019;21(8):741-773.
25. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.
How can we treat psychosis if we don’t know what we are treating? Over the years, attempts at defining psychosis subtypes have met with dead ends. However, recent research supports a new approach that offers a rational classification model organized according to 5 specific comorbid anxiety and depressive disorder diagnoses.
Anxiety and depressive symptoms are not just the result of psychotic despair. They are specific diagnoses, they precede psychosis onset, they help define psychotic syndromes, and they can point to much more effective treatment approaches. Most of the psychotic diagnoses in this schema are already recognized or posited. And, just as patients who do not have psychotic illness can have more than 1 anxiety or depressive disorder, patients with psychosis can present with a mixed picture that reflects more than 1 contributing comorbidity. Research further suggests that each of the 5 psychosis comorbidity diagnoses may involve some similar underlying factors that facilitate the formation of psychosis.
This article describes the basics of 5 psychosis subtypes, and provides initial guidelines to diagnosis, symptomatology, and treatment. Though clinical experience and existing research support the clinical presence and treatment value of this classification model, further verification will require considerably more controlled studies. An eventual validation of this approach could largely supplant ill-defined diagnoses of “schizophrenia” and other functional psychoses.
Recognizing the comorbidities in the context of their corresponding psychoses entails learning new interviewing skills and devoting more time to both initial and subsequent diagnosis and treatment. In our recently published book,1 we provide extensive details on the approach we describe in this article, including case examples, new interview tools to simplify the diagnostic journey, and novel treatment approaches.
Psychosis-proneness underlies functional psychoses
Functional (idiopathic) schizophrenia and psychotic disorders have long been difficult to separate, and many categorizations have been discarded. Despite clinical dissimilarities, today we too often casually lump psychoses together as schizophrenia.2,3 Eugen Bleuler first suggested the existence of a “group of schizophrenias.”4 It is possible that his group encompasses our 5 psychoses from 5 inbuilt emotional instincts,5 each corresponding to a specific anxiety or depressive subtype.
The 5 anxiety and depressive subtypes noted in this article are common, but psychosis is not. Considerable research suggests that certain global “psychotogenic” factors create susceptibility to all psychoses.6,7 While many genetic, neuroanatomical, experiential, and other factors have been reported, the most important may be “hypofrontality” (genetically reduced frontal lobe function, size, or neuronal activity) and dopaminergic hyperfunction (genetically increased dopamine activity).5-7
An evolutionary perspective
One evolutionary theory of psychopathology starts with the subtypes of depression and anxiety. For example, major depressive disorder and generalized anxiety disorder may encompass 5 commonplace and more specific anxiety and depressive subtypes. Consideration of the emotional, cognitive, and functional aspects of those subtypes suggests that they may have once been advantageous for primeval human herds. Those primeval altruistic instincts may have helped survival, reproduction, and preservation of kin group DNA.5
More than any other species, humans can draw upon consciousness and culture to rationally overcome the influences of unconscious instincts. But those instincts can then emerge from the deep, and painfully encourage obedience to their guidance. In nonpsychotic anxiety and depressive disorders, the specific messages are experienced as specific anxiety and depressive symptoms.5 In psychotic disorders, the messages can emerge as unreasoned and frightful fears, perceptions, beliefs, and behaviors. With newer research, clinical observation, and an evolutionary perspective, a novel and counterintuitive approach may improve our ability to help patients.8
Continue to: Five affective comorbidities evolved from primeval altruistic instincts...
Five affective comorbidities evolved from primeval altruistic instincts
Melancholic depression5
Melancholic depression is often triggered by serious illness, group exclusion, pronounced loss, or purposelessness. We hear patients talk painfully about illness, guilt, and death. Indeed, some increased risk of death, especially from infectious disease, may result from hypercortisolemia (documented by the dexamethasone suppression test). Hypercortisolemic death also occurs in salmon after spawning, and in male marsupial mice after mating. The tragic passing of an individual saves scarce resources for the remainder of the herd.
Obsessive-compulsive disorder5
Factor-analytic studies suggest 4 main obsessive-compulsive disorder (OCD) subtypes: cleanliness, hoarding, intrusive thoughts, and organizing. Obsessive-compulsive traits can help maintain a safe and efficient environment in humans and other species, but OCD is dysfunctional.
Panic anxiety5
Panic anxiety is triggered by real, symbolic, or emotional separation from home and family. In toddlers, separation anxiety can reduce the odds of getting lost and hurt.
Social anxiety5
Social anxiety includes fear of self-embarrassment, exposure as a pretender to higher social rank, and thus often a reluctant avoidance of increased social rank. While consciousness and cultural encouragement can overcome that hesitation and thus lead to greater success, social anxiety activation can still cause painful anxiety. The social hierarchies of many species include comparable biological influences, and help preserve group DNA by reducing hierarchical infighting.
Atypical depression and bipolar I mania5
Atypical depression includes increased rejection sensitivity, resulting in inoffensive behavior to avoid social rejection. This reduces risk of isolation from the group, and improves group harmony. Unlike the 4 other syndromes, atypical depression and bipolar I mania may reflect 2 separate seasonal mood phases. Atypical depression (including seasonal affective disorder) often worsens with shortened winter daylight hours, akin to hibernation. Initial bipolar I mania is more common with springtime daylight, with symptoms not unlike exaggerated hibernation awakening.9
Primeval biological altruism has great evolutionary value in many species, and even somewhat in modern humans. But it is quite different from modern rational altruism. Although we sometimes override our instincts, they respond with messages experienced as emotional pain—they still tell us to follow instructions for primeval herd survival. In an earlier book, I (JPK) provide a lengthier description of the evidence for this evolutionary psychopathology theory, including interplay of the 5 instincts with psychotogenic factors.5
Continue to: Five comorbidity psychoses from 5 primeval instincts.....
Five comorbidity psychoses from 5 primeval instincts
The 5 affective comorbidities described above contribute to the presence, subtype, and treatment approaches of 5 corresponding psychoses. Ordinary panic attacks might occur when feeling trapped or separated from home, so people want to flee to safety. Nonhuman species with limited consciousness and language are unlikely to think “time to head for safety.” Instead, instincts encourage flight from danger through internally generated perceptions of threat. Likewise, people with psychosis and panic, without sufficient conscious modulation, may experience sensory perceptions of actual danger when feeling symbolically trapped.1,10
One pilot study carefully examined the prevalence of these 5 comorbidities in an unselected group of psychotic patients.10 At least 85% met criteria for ≥1 of the 5 subtypes.10 Moreover, organic psychoses related to physical illness, substances, and iatrogenesis may also predict future episodes of functional psychoses.1
Using statistical analysis of psychosis rating scales, 2 studies took a “transdiagnostic” look at psychoses, and each found 5 psychosis subtypes and a generalized psychosis susceptibility factor.11,12 Replication of that transdiagnostic approach, newly including psychosis symptoms and our 5 specific comorbidities, might well find that the 5 subtype models resemble each other.11,12
Our proposed 5 comorbidity subtypes are1:
Delusional depression (melancholic depression). Most common in geriatric patients, this psychosis can also occur at younger ages. Prodromal melancholic depression can include guilt and hopelessness, and is acute, rather than the chronic course of our other 4 syndromes. Subsequent delusional depression includes delusions of bodily decay, illness, or death, as well as overwhelming guilt, shame, and remorse. The classic vegetative symptoms of depression continue. In addition to infectious disease issues, high suicide risk makes hospitalization imperative.
Obsessive-compulsive schizophrenia. Just as OCD has an early age of onset, obsessive-compulsive schizophrenia begins earlier than other psychoses. Despite preserved cognition, some nonpsychotic patients with OCD have diminished symptom insight. OCD may be comorbid with schizophrenia in 12% of cases, typically preceding psychosis onset. Obsessive-compulsive schizophrenia symptoms may include highly exaggerated doubt or ambivalence; contamination concerns; eccentric, ritualistic, motor stereotypy, checking, disorganized, and other behaviors; and paranoia.
Schizophrenia with voices (panic anxiety). Classic paranoid schizophrenia with voices appears to be the most similar to a “panic psychosis.” Patients with nonpsychotic panic anxiety have increased paranoid ideation and ideas of reference as measured on the Symptom Checklist-90. Schizophrenia is highly comorbid with panic anxiety, estimated at 45% in the Epidemiologic Catchment Area study.13 These are likely underestimates: cognitive impairment hinders reporting, and psychotic panic is masked as auditory hallucinations. A pilot study of schizophrenia with voices using a carbon dioxide panic induction challenge found that 100% had panic anxiety.14 That study and another found that virtually all participants reported voices concurrent with panic using our Panic and Schizophrenia Interview (PaSI) (Box 1). Panic onset precedes schizophrenia onset, and panic may reappear if antipsychotic medications sufficiently control voices: “voices without the voices,” say some.
Box 1
Let’s talk for a minute about your voices.
[IDENTIFYING PAROXYSMAL MOMENTS OF VOICE ONSET]
Do you hear voices at every single moment, or are they sometimes silent? Think about those times when you are not actually hearing any voices.
Now, there may be reasons why the voices start talking when they do, but let’s leave that aside for now.
So, whenever the voices do begin speaking—and for whatever reason they do—is it all of a sudden, or do they start very softly and then very gradually get louder?
If your voices are nearly always there, then are there times when the voices suddenly come back, get louder, get more insistent, or just get more obvious to you?
[Focus patient on sudden moment of voice onset, intensification, or awareness]
Let’s talk about that sudden moment when the voices begin (or intensify, or become obvious), even if you know the reason why they start.
I’m going to ask you about some symptoms that you might have at that same sudden moment when the voices start (or intensify, or become obvious). If you have any of these symptoms at the other times, they do not count for now.
So, when I ask about each symptom, tell me whether it comes on at the same sudden moments as the voices, and also if it used to come on with the voices in the past.
For each sudden symptom, just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same sudden moment that the voices come on”]
- Sudden anxiety, fear, or panic on the inside?
- Sudden anger or rage on the inside? [ANGER QUERY]
- Sudden heart racing? Heart pounding?
- Sudden chest pain? Chest pressure?
- Sudden sweating?
- Sudden trembling or shaking?
- Sudden shortness of breath, or like you can’t catch your breath?
- Sudden choking or a lump in your throat?
- Sudden nausea or queasiness?
- Sudden dizziness, lightheadedness, or faintness?
- Sudden feeling of detachment, sort of like you are in a glass box?
- Sudden fear of losing control? Fear of going crazy?
- Sudden fear afraid of dying? Afraid of having a heart attack?
- Sudden numbness or tingling, especially in your hands or face?
- Sudden feeling of heat, or cold?
- Sudden itching in your teeth? [VALIDITY CHECK]
- Sudden fear that people want to hurt you? [EXCESS FEAR QUERY]
- Sudden voices? [VOICES QUERY]
[PAST & PRODROMAL PANIC HISTORY]
At what age did you first see a therapist or psychiatrist?
At what age were you first hospitalized for an emotional problem?
At what age did you first start hearing voices?
At what age did you first start having strong fears of other people?
Before you ever heard voices, did you ever have any of the other sudden symptoms like the ones we just talked about?
Did those episodes back then feel sort of like your voices or sudden fears do now, except that there were no voices or sudden fears of people back then?
At what age did those sudden anxiety (or panic or rage) episodes begin?
Back then, was there MORE (M) sudden anxiety, or the SAME (S) sudden anxiety, or LESS (L) sudden anxiety than with your sudden voices now?
[PAST & PRODROMAL PANIC SYMPTOMS]
Now let’s talk about some symptoms that you might have had at those same sudden anxiety moments, in the time before you ever heard any voices. So, for each sudden symptom just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same moment the sudden anxiety came on—but only during the time before you ever heard sudden voices”]
[Ask about the same 18 panic-related symptoms listed above]
[PHOBIA-RELATED PANIC AND VOICES]
Have you ever been afraid to go into a (car, bus, plane, train, subway, elevator, mall, tunnel, bridge, heights, small place, CAT scan or MRI, being alone, crowds)?
[If yes or maybe: Ask about panic symptoms in phobic situations]
Now let’s talk about some symptoms that you might have had at some of those times you were afraid. So, for each symptom just say “YES” or “NO” or “MAYBE.”
[Ask about the same 18 panic-related symptoms listed above]
At what age did you last have sudden anxiety without voices?
Has medication ever completely stopped your voices? Somewhat?
If so, did those other sudden symptoms still happen sometimes?
Thank you for your help, and for answering all of these questions!
Persecutory delusional disorder (social anxiety). Some “schizophrenia” without voices may be misdiagnosis of persecutory (paranoid) delusional disorder (PDD). Therefore, the reported population prevalence (0.02%) may be underestimated. Social anxiety is highly comorbid with “schizophrenia” (15%).16 Case reports and clinical experience suggest that PDD is commonly preceded by social anxiety.17 Some nonpsychotic social anxiety symptoms closely resemble the PDD psychotic ideas of reference (a perception that low social rank attracts critical scrutiny by authorities). Patients with PDD may remain relatively functional, with few negative symptoms, despite pronounced paranoia. Outward manifestation of paranoia may be limited, unless quite intense. The typical age of onset (40 years) is later than that of schizophrenia, and symptoms can last a long time.18
Continue to: Bipolar 1 mania with delusions...
Bipolar I mania with delusions (atypical depression). Atypical depression is the most common depression in bipolar I disorder. Often more pronounced in winter, it may intensify at any time of year. Long ago, hypersomnia, lethargy, inactivity, inoffensiveness, and craving high-calorie food may have been conducive to hibernation.
Bipolar I mania includes delusions of special accomplishments or abilities, energetically focused on a grandiose mission to help everyone. These intense symptoms may be related to reduced frontal lobe modulation. In some milder form, bipolar I mania may once have encouraged hibernation awakening. Indeed, initial bipolar I mania episodes are more common in spring, as is the spring cleaning that helps us prepare for summer.
Recognizing affective trees in a psychotic forest
Though long observed, comorbid affective symptoms have generally been considered a hodgepodge of distress caused by painful psychotic illness. But the affective symptoms precede psychosis onset, can be masked during acute psychosis, and will revert to ordinary form if psychosis abates.11-13
Rather than affective symptoms being a consequence of psychosis, it may well be the other way around. Affective disorders could be important causal and differentiating components of psychotic disorders.11-13 Research and clinical experience suggest that adjunctive treatment of the comorbidities with correct medication can greatly enhance outcome.
Diagnostic approaches
Because interviews of patients with psychosis are often complicated by confusion, irritability, paranoid evasiveness, cognitive impairment, and medication, nuanced diagnosis is difficult. Interviews should explore psychotic syndromes and subtypes that correlate with comorbidity psychoses, including pre-psychotic anxiety and depressive diagnoses that are chronic (though unlike our 4 other diagnoses, melancholic depression is not chronic).
Establishing pre-psychotic diagnosis of chronic syndromes suggests that they are still present, even if they are difficult to assess during psychosis. Re-interview after some improvement allows for a significantly better diagnosis. Just as in nonpsychotic affective disorders, multiple comorbidities are common, and can lead to a mixed psychotic diagnosis and treatment plan.1
Structured interview tools can assist diagnosis. The PaSI (Box 1,15) elicits past, present, and detailed history of DSM panic, and has been validated in a small pilot randomized controlled trial. The PaSI focuses patient attention on paroxysmal onset voices, and then evaluates the presence of concurrent DSM panic symptoms. If voices are mostly psychotic panic, they may well be a proxy for panic. Ultimately, diagnosis of 5 comorbidities and associated psychotic symptoms may allow simpler categorization into 1 (or more) of the 5 psychosis subtypes.
Continue to: Treatment by comorbidity subtype...
Treatment by comorbidity subtype
Treatment of psychosis generally begins with antipsychotics. Nominal psychotherapy (presence of a professionally detached, compassionate clinician) improves compliance and leads to supportive therapy. Cognitive-behavioral therapy and dialectical behavior therapy may help later, with limited interpersonal approaches further on for some patients.
The suggested approaches to pharmacotherapy noted here draw on research and clinical experience.1,14,19-21 All medications used to treat comorbidities noted here are approved or generally accepted for that diagnosis. Estimated doses are similar to those for comorbidities when patients are nonpsychotic, and vary among patients. Doses, dosing schedules, and titration are extremely important for full benefit. Always consider compliance issues, suicidality, possible adverse effects, and potential drug/drug interactions. Although the medications we suggest using to treat the comorbidities may appear to also benefit psychosis, only antipsychotics are approved for psychosis per se.
Delusional depression. Antipsychotic + antidepressant. Tricyclic antidepressants are possibly most effective, but increase the risk of overdose and dangerous falls among fragile patients. Electroconvulsive therapy is sometimes used.
Obsessive-compulsive schizophrenia. Antipsychotic + selective serotonin reuptake inhibitor (SSRI). Consider aripiprazole (
Schizophrenia with voices. Antipsychotic + clonazepam. Concurrent usage may stabilize psychosis more rapidly, and with a lower antipsychotic dose.23 Titrate a fixed dose of clonazepam every 12 hours (avoid as-needed doses), starting low (ie, 0.5 mg) to limit initial drowsiness (which typically diminishes in 3 to 10 days). Titrate to full voice and panic cessation (1 to 2.5 mg every 12 hours).14 Exercise caution about excessive drowsiness, as well as outpatient compliance and abuse. Besides alprazolam, other antipanic medications have little incidental benefit for psychosis.
Persecutory delusional disorder. Antipsychotic + SSRI. Aripiprazole (consider long-acting injectable for compliance) also enhances the benefits of fluoxetine for social anxiety. Long half-life fluoxetine (20 mg/d) improves compliance and near-term outcomes.
Bipolar I mania: mania with delusions. Consider olanzapine for acute phase, then add other antimanic medication (commonly lithium or valproic acid), check blood level, and then taper olanzapine some weeks later. Importantly, lamotrigine is not effective for bipolar I mania. Consider suicide risk, medical conditions, and outpatient compliance. Comorbid panic anxiety is also common in bipolar I mania, often presenting as nonthreatening voices.
Seasonality: Following research that bipolar I mania is more common in spring and summer, studies have shown beneficial clinical augmentation from dark therapy as provided by reduced light exposure, blue-blocking glasses, and exogenous melatonin (a darkness-signaling hormone).24
Bipolar I mania atypical depression (significant current or historical symptoms). SSRI + booster medication. An SSRI (ie, escitalopram, 10 mg/d) is best started several weeks after full bipolar I mania resolution, while also continuing long-term antimanic medication. Booster medications (ie, buspirone 15 mg every 12 hours; lithium 300 mg/d; or trazodone 50 mg every 12 hours) can enhance SSRI benefits. Meta-analysis suggests SSRIs may have limited risk of inducing bipolar I mania.25 Although not yet specifically tested for atypical depression, lamotrigine may be effective, and may be safer still.25 However, lamotrigine requires very gradual dose titration to prevent a potentially dangerous rash, including after periods of outpatient noncompliance.
Seasonality: Atypical depression is often worse in winter (seasonal affective disorder). Light therapy can produce some clinically helpful benefits year-round.
To illustrate this new approach to psychosis diagnosis and treatment, our book
Box 2
Ms. B, a studious 19-year-old, has been very shy since childhood, with few friends. Meeting new people always gave her gradually increasing anxiety, thinking that she would embarrass herself in their eyes. She had that same anxiety, along with sweating and tachycardia, when she couldn’t avoid speaking in front of class. Sometimes, while walking down the street she would think that strangers were casting a disdainful eye on her, though she knew that wasn’t true. Another anxiety started when she was 16. While looking for paper in a small supply closet, she suddenly felt panicky. With a racing heart and short of breath, she desperately fled the closet. These episodes continued, sometimes for no apparent reason, and nearly always unnoticed by others.
At age 17, she began to believe that those strangers on the street were looking down on her with evil intent, and even following her around. She became afraid to walk around town. A few months later, she also started to hear angry and critical voices at sudden moments. Although the paroxysmal voices always coincided with her panicky symptoms, the threatening voices now felt more important to her than the panic itself. Nonpsychotic panics had stopped. Mostly a recluse, she saw less of her family, left her job, and stopped going to the movies.
After a family dinner, she was detached, scared, and quieter than usual. She sought help from her primary care physician, who referred her to a psychiatrist. A thorough history from Ms. B and her family revealed her disturbing fears, as well as her history of social anxiety. Interviewing for panic was prompted by her mother’s recollection of the supply closet story.
In view of Ms. B’s cooperativeness and supportive family, outpatient treatment of her recent-onset psychosis began with aripiprazole, 10 mg/d, and clonazepam, 0.5 mg every 12 hours. Clonazepam was gradually increased until voices (and panic) ceased. She was then able to describe how earlier panics had felt just like voices, but without the voices. The fears of strangers continued. Escitalopram, 20 mg/d, was added for social anxiety (aripiprazole enhances the benefits of selective serotonin reuptake inhibitors).
One month later, her fears of strangers diminished, and she felt more comfortable around people than ever before. On the same medications, and in psychotherapy over the next year, she began to increase her social network while making plans to start college.
Larger studies are needed
Current research supports the concept of a 5-diagnosis classification of psychoses, which may correlate with our comorbid anxiety and depression model. Larger diagnostic and treatment studies would invaluably examine existing research and clinical experience, and potentially encourage more clinically useful diagnoses, specific treatments, and improved outcomes.
Bottom Line
New insights from evolutionary psychopathology, clinical research and observation, psychotogenesis, genetics, and epidemiology suggest that most functional psychoses may fall into 1 of 5 comorbidity-defined subtypes, for which specific treatments can lead to much improved outcomes.
How can we treat psychosis if we don’t know what we are treating? Over the years, attempts at defining psychosis subtypes have met with dead ends. However, recent research supports a new approach that offers a rational classification model organized according to 5 specific comorbid anxiety and depressive disorder diagnoses.
Anxiety and depressive symptoms are not just the result of psychotic despair. They are specific diagnoses, they precede psychosis onset, they help define psychotic syndromes, and they can point to much more effective treatment approaches. Most of the psychotic diagnoses in this schema are already recognized or posited. And, just as patients who do not have psychotic illness can have more than 1 anxiety or depressive disorder, patients with psychosis can present with a mixed picture that reflects more than 1 contributing comorbidity. Research further suggests that each of the 5 psychosis comorbidity diagnoses may involve some similar underlying factors that facilitate the formation of psychosis.
This article describes the basics of 5 psychosis subtypes, and provides initial guidelines to diagnosis, symptomatology, and treatment. Though clinical experience and existing research support the clinical presence and treatment value of this classification model, further verification will require considerably more controlled studies. An eventual validation of this approach could largely supplant ill-defined diagnoses of “schizophrenia” and other functional psychoses.
Recognizing the comorbidities in the context of their corresponding psychoses entails learning new interviewing skills and devoting more time to both initial and subsequent diagnosis and treatment. In our recently published book,1 we provide extensive details on the approach we describe in this article, including case examples, new interview tools to simplify the diagnostic journey, and novel treatment approaches.
Psychosis-proneness underlies functional psychoses
Functional (idiopathic) schizophrenia and psychotic disorders have long been difficult to separate, and many categorizations have been discarded. Despite clinical dissimilarities, today we too often casually lump psychoses together as schizophrenia.2,3 Eugen Bleuler first suggested the existence of a “group of schizophrenias.”4 It is possible that his group encompasses our 5 psychoses from 5 inbuilt emotional instincts,5 each corresponding to a specific anxiety or depressive subtype.
The 5 anxiety and depressive subtypes noted in this article are common, but psychosis is not. Considerable research suggests that certain global “psychotogenic” factors create susceptibility to all psychoses.6,7 While many genetic, neuroanatomical, experiential, and other factors have been reported, the most important may be “hypofrontality” (genetically reduced frontal lobe function, size, or neuronal activity) and dopaminergic hyperfunction (genetically increased dopamine activity).5-7
An evolutionary perspective
One evolutionary theory of psychopathology starts with the subtypes of depression and anxiety. For example, major depressive disorder and generalized anxiety disorder may encompass 5 commonplace and more specific anxiety and depressive subtypes. Consideration of the emotional, cognitive, and functional aspects of those subtypes suggests that they may have once been advantageous for primeval human herds. Those primeval altruistic instincts may have helped survival, reproduction, and preservation of kin group DNA.5
More than any other species, humans can draw upon consciousness and culture to rationally overcome the influences of unconscious instincts. But those instincts can then emerge from the deep, and painfully encourage obedience to their guidance. In nonpsychotic anxiety and depressive disorders, the specific messages are experienced as specific anxiety and depressive symptoms.5 In psychotic disorders, the messages can emerge as unreasoned and frightful fears, perceptions, beliefs, and behaviors. With newer research, clinical observation, and an evolutionary perspective, a novel and counterintuitive approach may improve our ability to help patients.8
Continue to: Five affective comorbidities evolved from primeval altruistic instincts...
Five affective comorbidities evolved from primeval altruistic instincts
Melancholic depression5
Melancholic depression is often triggered by serious illness, group exclusion, pronounced loss, or purposelessness. We hear patients talk painfully about illness, guilt, and death. Indeed, some increased risk of death, especially from infectious disease, may result from hypercortisolemia (documented by the dexamethasone suppression test). Hypercortisolemic death also occurs in salmon after spawning, and in male marsupial mice after mating. The tragic passing of an individual saves scarce resources for the remainder of the herd.
Obsessive-compulsive disorder5
Factor-analytic studies suggest 4 main obsessive-compulsive disorder (OCD) subtypes: cleanliness, hoarding, intrusive thoughts, and organizing. Obsessive-compulsive traits can help maintain a safe and efficient environment in humans and other species, but OCD is dysfunctional.
Panic anxiety5
Panic anxiety is triggered by real, symbolic, or emotional separation from home and family. In toddlers, separation anxiety can reduce the odds of getting lost and hurt.
Social anxiety5
Social anxiety includes fear of self-embarrassment, exposure as a pretender to higher social rank, and thus often a reluctant avoidance of increased social rank. While consciousness and cultural encouragement can overcome that hesitation and thus lead to greater success, social anxiety activation can still cause painful anxiety. The social hierarchies of many species include comparable biological influences, and help preserve group DNA by reducing hierarchical infighting.
Atypical depression and bipolar I mania5
Atypical depression includes increased rejection sensitivity, resulting in inoffensive behavior to avoid social rejection. This reduces risk of isolation from the group, and improves group harmony. Unlike the 4 other syndromes, atypical depression and bipolar I mania may reflect 2 separate seasonal mood phases. Atypical depression (including seasonal affective disorder) often worsens with shortened winter daylight hours, akin to hibernation. Initial bipolar I mania is more common with springtime daylight, with symptoms not unlike exaggerated hibernation awakening.9
Primeval biological altruism has great evolutionary value in many species, and even somewhat in modern humans. But it is quite different from modern rational altruism. Although we sometimes override our instincts, they respond with messages experienced as emotional pain—they still tell us to follow instructions for primeval herd survival. In an earlier book, I (JPK) provide a lengthier description of the evidence for this evolutionary psychopathology theory, including interplay of the 5 instincts with psychotogenic factors.5
Continue to: Five comorbidity psychoses from 5 primeval instincts.....
Five comorbidity psychoses from 5 primeval instincts
The 5 affective comorbidities described above contribute to the presence, subtype, and treatment approaches of 5 corresponding psychoses. Ordinary panic attacks might occur when feeling trapped or separated from home, so people want to flee to safety. Nonhuman species with limited consciousness and language are unlikely to think “time to head for safety.” Instead, instincts encourage flight from danger through internally generated perceptions of threat. Likewise, people with psychosis and panic, without sufficient conscious modulation, may experience sensory perceptions of actual danger when feeling symbolically trapped.1,10
One pilot study carefully examined the prevalence of these 5 comorbidities in an unselected group of psychotic patients.10 At least 85% met criteria for ≥1 of the 5 subtypes.10 Moreover, organic psychoses related to physical illness, substances, and iatrogenesis may also predict future episodes of functional psychoses.1
Using statistical analysis of psychosis rating scales, 2 studies took a “transdiagnostic” look at psychoses, and each found 5 psychosis subtypes and a generalized psychosis susceptibility factor.11,12 Replication of that transdiagnostic approach, newly including psychosis symptoms and our 5 specific comorbidities, might well find that the 5 subtype models resemble each other.11,12
Our proposed 5 comorbidity subtypes are1:
Delusional depression (melancholic depression). Most common in geriatric patients, this psychosis can also occur at younger ages. Prodromal melancholic depression can include guilt and hopelessness, and is acute, rather than the chronic course of our other 4 syndromes. Subsequent delusional depression includes delusions of bodily decay, illness, or death, as well as overwhelming guilt, shame, and remorse. The classic vegetative symptoms of depression continue. In addition to infectious disease issues, high suicide risk makes hospitalization imperative.
Obsessive-compulsive schizophrenia. Just as OCD has an early age of onset, obsessive-compulsive schizophrenia begins earlier than other psychoses. Despite preserved cognition, some nonpsychotic patients with OCD have diminished symptom insight. OCD may be comorbid with schizophrenia in 12% of cases, typically preceding psychosis onset. Obsessive-compulsive schizophrenia symptoms may include highly exaggerated doubt or ambivalence; contamination concerns; eccentric, ritualistic, motor stereotypy, checking, disorganized, and other behaviors; and paranoia.
Schizophrenia with voices (panic anxiety). Classic paranoid schizophrenia with voices appears to be the most similar to a “panic psychosis.” Patients with nonpsychotic panic anxiety have increased paranoid ideation and ideas of reference as measured on the Symptom Checklist-90. Schizophrenia is highly comorbid with panic anxiety, estimated at 45% in the Epidemiologic Catchment Area study.13 These are likely underestimates: cognitive impairment hinders reporting, and psychotic panic is masked as auditory hallucinations. A pilot study of schizophrenia with voices using a carbon dioxide panic induction challenge found that 100% had panic anxiety.14 That study and another found that virtually all participants reported voices concurrent with panic using our Panic and Schizophrenia Interview (PaSI) (Box 1). Panic onset precedes schizophrenia onset, and panic may reappear if antipsychotic medications sufficiently control voices: “voices without the voices,” say some.
Box 1
Let’s talk for a minute about your voices.
[IDENTIFYING PAROXYSMAL MOMENTS OF VOICE ONSET]
Do you hear voices at every single moment, or are they sometimes silent? Think about those times when you are not actually hearing any voices.
Now, there may be reasons why the voices start talking when they do, but let’s leave that aside for now.
So, whenever the voices do begin speaking—and for whatever reason they do—is it all of a sudden, or do they start very softly and then very gradually get louder?
If your voices are nearly always there, then are there times when the voices suddenly come back, get louder, get more insistent, or just get more obvious to you?
[Focus patient on sudden moment of voice onset, intensification, or awareness]
Let’s talk about that sudden moment when the voices begin (or intensify, or become obvious), even if you know the reason why they start.
I’m going to ask you about some symptoms that you might have at that same sudden moment when the voices start (or intensify, or become obvious). If you have any of these symptoms at the other times, they do not count for now.
So, when I ask about each symptom, tell me whether it comes on at the same sudden moments as the voices, and also if it used to come on with the voices in the past.
For each sudden symptom, just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same sudden moment that the voices come on”]
- Sudden anxiety, fear, or panic on the inside?
- Sudden anger or rage on the inside? [ANGER QUERY]
- Sudden heart racing? Heart pounding?
- Sudden chest pain? Chest pressure?
- Sudden sweating?
- Sudden trembling or shaking?
- Sudden shortness of breath, or like you can’t catch your breath?
- Sudden choking or a lump in your throat?
- Sudden nausea or queasiness?
- Sudden dizziness, lightheadedness, or faintness?
- Sudden feeling of detachment, sort of like you are in a glass box?
- Sudden fear of losing control? Fear of going crazy?
- Sudden fear afraid of dying? Afraid of having a heart attack?
- Sudden numbness or tingling, especially in your hands or face?
- Sudden feeling of heat, or cold?
- Sudden itching in your teeth? [VALIDITY CHECK]
- Sudden fear that people want to hurt you? [EXCESS FEAR QUERY]
- Sudden voices? [VOICES QUERY]
[PAST & PRODROMAL PANIC HISTORY]
At what age did you first see a therapist or psychiatrist?
At what age were you first hospitalized for an emotional problem?
At what age did you first start hearing voices?
At what age did you first start having strong fears of other people?
Before you ever heard voices, did you ever have any of the other sudden symptoms like the ones we just talked about?
Did those episodes back then feel sort of like your voices or sudden fears do now, except that there were no voices or sudden fears of people back then?
At what age did those sudden anxiety (or panic or rage) episodes begin?
Back then, was there MORE (M) sudden anxiety, or the SAME (S) sudden anxiety, or LESS (L) sudden anxiety than with your sudden voices now?
[PAST & PRODROMAL PANIC SYMPTOMS]
Now let’s talk about some symptoms that you might have had at those same sudden anxiety moments, in the time before you ever heard any voices. So, for each sudden symptom just say “YES” or “NO” or “SOMETIMES.”
[Begin each query with: “At the same moment the sudden anxiety came on—but only during the time before you ever heard sudden voices”]
[Ask about the same 18 panic-related symptoms listed above]
[PHOBIA-RELATED PANIC AND VOICES]
Have you ever been afraid to go into a (car, bus, plane, train, subway, elevator, mall, tunnel, bridge, heights, small place, CAT scan or MRI, being alone, crowds)?
[If yes or maybe: Ask about panic symptoms in phobic situations]
Now let’s talk about some symptoms that you might have had at some of those times you were afraid. So, for each symptom just say “YES” or “NO” or “MAYBE.”
[Ask about the same 18 panic-related symptoms listed above]
At what age did you last have sudden anxiety without voices?
Has medication ever completely stopped your voices? Somewhat?
If so, did those other sudden symptoms still happen sometimes?
Thank you for your help, and for answering all of these questions!
Persecutory delusional disorder (social anxiety). Some “schizophrenia” without voices may be misdiagnosis of persecutory (paranoid) delusional disorder (PDD). Therefore, the reported population prevalence (0.02%) may be underestimated. Social anxiety is highly comorbid with “schizophrenia” (15%).16 Case reports and clinical experience suggest that PDD is commonly preceded by social anxiety.17 Some nonpsychotic social anxiety symptoms closely resemble the PDD psychotic ideas of reference (a perception that low social rank attracts critical scrutiny by authorities). Patients with PDD may remain relatively functional, with few negative symptoms, despite pronounced paranoia. Outward manifestation of paranoia may be limited, unless quite intense. The typical age of onset (40 years) is later than that of schizophrenia, and symptoms can last a long time.18
Continue to: Bipolar 1 mania with delusions...
Bipolar I mania with delusions (atypical depression). Atypical depression is the most common depression in bipolar I disorder. Often more pronounced in winter, it may intensify at any time of year. Long ago, hypersomnia, lethargy, inactivity, inoffensiveness, and craving high-calorie food may have been conducive to hibernation.
Bipolar I mania includes delusions of special accomplishments or abilities, energetically focused on a grandiose mission to help everyone. These intense symptoms may be related to reduced frontal lobe modulation. In some milder form, bipolar I mania may once have encouraged hibernation awakening. Indeed, initial bipolar I mania episodes are more common in spring, as is the spring cleaning that helps us prepare for summer.
Recognizing affective trees in a psychotic forest
Though long observed, comorbid affective symptoms have generally been considered a hodgepodge of distress caused by painful psychotic illness. But the affective symptoms precede psychosis onset, can be masked during acute psychosis, and will revert to ordinary form if psychosis abates.11-13
Rather than affective symptoms being a consequence of psychosis, it may well be the other way around. Affective disorders could be important causal and differentiating components of psychotic disorders.11-13 Research and clinical experience suggest that adjunctive treatment of the comorbidities with correct medication can greatly enhance outcome.
Diagnostic approaches
Because interviews of patients with psychosis are often complicated by confusion, irritability, paranoid evasiveness, cognitive impairment, and medication, nuanced diagnosis is difficult. Interviews should explore psychotic syndromes and subtypes that correlate with comorbidity psychoses, including pre-psychotic anxiety and depressive diagnoses that are chronic (though unlike our 4 other diagnoses, melancholic depression is not chronic).
Establishing pre-psychotic diagnosis of chronic syndromes suggests that they are still present, even if they are difficult to assess during psychosis. Re-interview after some improvement allows for a significantly better diagnosis. Just as in nonpsychotic affective disorders, multiple comorbidities are common, and can lead to a mixed psychotic diagnosis and treatment plan.1
Structured interview tools can assist diagnosis. The PaSI (Box 1,15) elicits past, present, and detailed history of DSM panic, and has been validated in a small pilot randomized controlled trial. The PaSI focuses patient attention on paroxysmal onset voices, and then evaluates the presence of concurrent DSM panic symptoms. If voices are mostly psychotic panic, they may well be a proxy for panic. Ultimately, diagnosis of 5 comorbidities and associated psychotic symptoms may allow simpler categorization into 1 (or more) of the 5 psychosis subtypes.
Continue to: Treatment by comorbidity subtype...
Treatment by comorbidity subtype
Treatment of psychosis generally begins with antipsychotics. Nominal psychotherapy (presence of a professionally detached, compassionate clinician) improves compliance and leads to supportive therapy. Cognitive-behavioral therapy and dialectical behavior therapy may help later, with limited interpersonal approaches further on for some patients.
The suggested approaches to pharmacotherapy noted here draw on research and clinical experience.1,14,19-21 All medications used to treat comorbidities noted here are approved or generally accepted for that diagnosis. Estimated doses are similar to those for comorbidities when patients are nonpsychotic, and vary among patients. Doses, dosing schedules, and titration are extremely important for full benefit. Always consider compliance issues, suicidality, possible adverse effects, and potential drug/drug interactions. Although the medications we suggest using to treat the comorbidities may appear to also benefit psychosis, only antipsychotics are approved for psychosis per se.
Delusional depression. Antipsychotic + antidepressant. Tricyclic antidepressants are possibly most effective, but increase the risk of overdose and dangerous falls among fragile patients. Electroconvulsive therapy is sometimes used.
Obsessive-compulsive schizophrenia. Antipsychotic + selective serotonin reuptake inhibitor (SSRI). Consider aripiprazole (
Schizophrenia with voices. Antipsychotic + clonazepam. Concurrent usage may stabilize psychosis more rapidly, and with a lower antipsychotic dose.23 Titrate a fixed dose of clonazepam every 12 hours (avoid as-needed doses), starting low (ie, 0.5 mg) to limit initial drowsiness (which typically diminishes in 3 to 10 days). Titrate to full voice and panic cessation (1 to 2.5 mg every 12 hours).14 Exercise caution about excessive drowsiness, as well as outpatient compliance and abuse. Besides alprazolam, other antipanic medications have little incidental benefit for psychosis.
Persecutory delusional disorder. Antipsychotic + SSRI. Aripiprazole (consider long-acting injectable for compliance) also enhances the benefits of fluoxetine for social anxiety. Long half-life fluoxetine (20 mg/d) improves compliance and near-term outcomes.
Bipolar I mania: mania with delusions. Consider olanzapine for acute phase, then add other antimanic medication (commonly lithium or valproic acid), check blood level, and then taper olanzapine some weeks later. Importantly, lamotrigine is not effective for bipolar I mania. Consider suicide risk, medical conditions, and outpatient compliance. Comorbid panic anxiety is also common in bipolar I mania, often presenting as nonthreatening voices.
Seasonality: Following research that bipolar I mania is more common in spring and summer, studies have shown beneficial clinical augmentation from dark therapy as provided by reduced light exposure, blue-blocking glasses, and exogenous melatonin (a darkness-signaling hormone).24
Bipolar I mania atypical depression (significant current or historical symptoms). SSRI + booster medication. An SSRI (ie, escitalopram, 10 mg/d) is best started several weeks after full bipolar I mania resolution, while also continuing long-term antimanic medication. Booster medications (ie, buspirone 15 mg every 12 hours; lithium 300 mg/d; or trazodone 50 mg every 12 hours) can enhance SSRI benefits. Meta-analysis suggests SSRIs may have limited risk of inducing bipolar I mania.25 Although not yet specifically tested for atypical depression, lamotrigine may be effective, and may be safer still.25 However, lamotrigine requires very gradual dose titration to prevent a potentially dangerous rash, including after periods of outpatient noncompliance.
Seasonality: Atypical depression is often worse in winter (seasonal affective disorder). Light therapy can produce some clinically helpful benefits year-round.
To illustrate this new approach to psychosis diagnosis and treatment, our book
Box 2
Ms. B, a studious 19-year-old, has been very shy since childhood, with few friends. Meeting new people always gave her gradually increasing anxiety, thinking that she would embarrass herself in their eyes. She had that same anxiety, along with sweating and tachycardia, when she couldn’t avoid speaking in front of class. Sometimes, while walking down the street she would think that strangers were casting a disdainful eye on her, though she knew that wasn’t true. Another anxiety started when she was 16. While looking for paper in a small supply closet, she suddenly felt panicky. With a racing heart and short of breath, she desperately fled the closet. These episodes continued, sometimes for no apparent reason, and nearly always unnoticed by others.
At age 17, she began to believe that those strangers on the street were looking down on her with evil intent, and even following her around. She became afraid to walk around town. A few months later, she also started to hear angry and critical voices at sudden moments. Although the paroxysmal voices always coincided with her panicky symptoms, the threatening voices now felt more important to her than the panic itself. Nonpsychotic panics had stopped. Mostly a recluse, she saw less of her family, left her job, and stopped going to the movies.
After a family dinner, she was detached, scared, and quieter than usual. She sought help from her primary care physician, who referred her to a psychiatrist. A thorough history from Ms. B and her family revealed her disturbing fears, as well as her history of social anxiety. Interviewing for panic was prompted by her mother’s recollection of the supply closet story.
In view of Ms. B’s cooperativeness and supportive family, outpatient treatment of her recent-onset psychosis began with aripiprazole, 10 mg/d, and clonazepam, 0.5 mg every 12 hours. Clonazepam was gradually increased until voices (and panic) ceased. She was then able to describe how earlier panics had felt just like voices, but without the voices. The fears of strangers continued. Escitalopram, 20 mg/d, was added for social anxiety (aripiprazole enhances the benefits of selective serotonin reuptake inhibitors).
One month later, her fears of strangers diminished, and she felt more comfortable around people than ever before. On the same medications, and in psychotherapy over the next year, she began to increase her social network while making plans to start college.
Larger studies are needed
Current research supports the concept of a 5-diagnosis classification of psychoses, which may correlate with our comorbid anxiety and depression model. Larger diagnostic and treatment studies would invaluably examine existing research and clinical experience, and potentially encourage more clinically useful diagnoses, specific treatments, and improved outcomes.
Bottom Line
New insights from evolutionary psychopathology, clinical research and observation, psychotogenesis, genetics, and epidemiology suggest that most functional psychoses may fall into 1 of 5 comorbidity-defined subtypes, for which specific treatments can lead to much improved outcomes.
1. Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
2. Gaebel W, Zielasek J. Focus on psychosis. Dialogues Clin Neuroscience. 2015;17(1):9-18.
3. Guloksuz S, Van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychological Medicine. 2018;48(2):229-244.
4. Bleuler E. Dementia Praecox or the Group of Schizophrenias. International Universities Press; 1950.
5. Kahn JP. Angst: Origins of Depression and Anxiety. Oxford University Press; 2013.
6. Howes OD, McCutcheon R, Owen MJ, et al. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81(1):9-20.
7. Mubarik A, Tohid H. Frontal lobe alterations in schizophrenia: a review. Trends Psychiatry Psychother. 2016;38(4):198-206.
8. Murray RM, Bhavsar V, Tripoli G, et al. 30 Years on: How the neurodevelopmental hypothesis of schizophrenia morphed into the developmental risk factor model of psychosis. Schizophr Bull. 2017;43(6):1190-1196.
9. Bauer M, Glenn T, Alda M, et al. Solar insolation in springtime influences age of onset of bipolar I disorder. Acta Psychiatr Scand. 2017;136(6):571-582.
10. Kahn JP, Bombassaro T, Veras AB. Comorbid schizophrenia and panic anxiety: panic psychosis revisited. Psychiatr Ann. 2018;48(12):561-565.
11. Bebbington P, Freeman D. Transdiagnostic extension of delusions: schizophrenia and beyond. Schizophr Bull. 2017;43(2):273-282.
12. Catalan A, Simons CJP, Bustamante S, et al. Data gathering bias: trait vulnerability to psychotic symptoms? PLoS One. 2015;10(7):e0132442. doi:10.1371/journal.pone.0132442
13. Goodwin R, Lyons JS, McNally RJ. Panic attacks in schizophrenia. Schizophr Res. 2002;58(2-3):213-220.
14. Kahn JP, Puertollano MA, Schane MD, et al. Adjunctive alprazolam for schizophrenia with panic anxiety: clinical observation and pathogenetic implications. Am J Psychiatry. 1988;145(6):742-744.
15. Kahn JP. Chapter 4: Paranoid schizophrenia with voices and panic anxiety. In: Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
16. Achim AM, Maziade M, Raymond E, et al. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull. 2011;37(4):811-821.
17. Veras AB, Souza TG, Ricci TG, et al. Paranoid delusional disorder follows social anxiety disorder in a long-term case series: evolutionary perspective. J Nerv Ment Dis. 2015;203(6):477-479.
18. McIntyre JC, Wickham S, Barr B, et al. Social identity and psychosis: associations and psychological mechanisms. Schizophr Bull. 2018;44(3):681-690.
19. Barbee JG, Mancuso DM, Freed CR. Alprazolam as a neuroleptic adjunct in the emergency treatment of schizophrenia. Am J Psychiatry. 1992;149(4):506-510.
20. Nardi AE, Machado S, Almada LF. Clonazepam for the treatment of panic disorder. Curr Drug Targets. 2013;14(3):353-364.
21. Poyurovsky M. Schizo-Obsessive Disorder. Cambridge University Press; 2013.
22. Reznik I, Sirota P. Obsessive and compulsive symptoms in schizophrenia: a randomized controlled trial with fluvoxamine and neuroleptics. J Clin Psychopharmacol. 2000;20(4):410-416.
23. Bodkin JA. Emerging uses for high-potency benzodiazepines in psychotic disorders. J Clin Psychiatry. 1990;51 Suppl:41-53.
24. Gottlieb JF, Benedetti F, Geoffroy PA, et al. The chronotherapeutic treatment of bipolar disorders: a systematic review and practice recommendations from the ISBD task force on chronotherapy and chronobiology. Bipolar Disord. 2019;21(8):741-773.
25. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.
1. Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
2. Gaebel W, Zielasek J. Focus on psychosis. Dialogues Clin Neuroscience. 2015;17(1):9-18.
3. Guloksuz S, Van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychological Medicine. 2018;48(2):229-244.
4. Bleuler E. Dementia Praecox or the Group of Schizophrenias. International Universities Press; 1950.
5. Kahn JP. Angst: Origins of Depression and Anxiety. Oxford University Press; 2013.
6. Howes OD, McCutcheon R, Owen MJ, et al. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81(1):9-20.
7. Mubarik A, Tohid H. Frontal lobe alterations in schizophrenia: a review. Trends Psychiatry Psychother. 2016;38(4):198-206.
8. Murray RM, Bhavsar V, Tripoli G, et al. 30 Years on: How the neurodevelopmental hypothesis of schizophrenia morphed into the developmental risk factor model of psychosis. Schizophr Bull. 2017;43(6):1190-1196.
9. Bauer M, Glenn T, Alda M, et al. Solar insolation in springtime influences age of onset of bipolar I disorder. Acta Psychiatr Scand. 2017;136(6):571-582.
10. Kahn JP, Bombassaro T, Veras AB. Comorbid schizophrenia and panic anxiety: panic psychosis revisited. Psychiatr Ann. 2018;48(12):561-565.
11. Bebbington P, Freeman D. Transdiagnostic extension of delusions: schizophrenia and beyond. Schizophr Bull. 2017;43(2):273-282.
12. Catalan A, Simons CJP, Bustamante S, et al. Data gathering bias: trait vulnerability to psychotic symptoms? PLoS One. 2015;10(7):e0132442. doi:10.1371/journal.pone.0132442
13. Goodwin R, Lyons JS, McNally RJ. Panic attacks in schizophrenia. Schizophr Res. 2002;58(2-3):213-220.
14. Kahn JP, Puertollano MA, Schane MD, et al. Adjunctive alprazolam for schizophrenia with panic anxiety: clinical observation and pathogenetic implications. Am J Psychiatry. 1988;145(6):742-744.
15. Kahn JP. Chapter 4: Paranoid schizophrenia with voices and panic anxiety. In: Veras AB, Kahn JP, eds. Psychotic Disorders: Comorbidity Detection Promotes Improved Diagnosis and Treatment. Elsevier; 2021.
16. Achim AM, Maziade M, Raymond E, et al. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull. 2011;37(4):811-821.
17. Veras AB, Souza TG, Ricci TG, et al. Paranoid delusional disorder follows social anxiety disorder in a long-term case series: evolutionary perspective. J Nerv Ment Dis. 2015;203(6):477-479.
18. McIntyre JC, Wickham S, Barr B, et al. Social identity and psychosis: associations and psychological mechanisms. Schizophr Bull. 2018;44(3):681-690.
19. Barbee JG, Mancuso DM, Freed CR. Alprazolam as a neuroleptic adjunct in the emergency treatment of schizophrenia. Am J Psychiatry. 1992;149(4):506-510.
20. Nardi AE, Machado S, Almada LF. Clonazepam for the treatment of panic disorder. Curr Drug Targets. 2013;14(3):353-364.
21. Poyurovsky M. Schizo-Obsessive Disorder. Cambridge University Press; 2013.
22. Reznik I, Sirota P. Obsessive and compulsive symptoms in schizophrenia: a randomized controlled trial with fluvoxamine and neuroleptics. J Clin Psychopharmacol. 2000;20(4):410-416.
23. Bodkin JA. Emerging uses for high-potency benzodiazepines in psychotic disorders. J Clin Psychiatry. 1990;51 Suppl:41-53.
24. Gottlieb JF, Benedetti F, Geoffroy PA, et al. The chronotherapeutic treatment of bipolar disorders: a systematic review and practice recommendations from the ISBD task force on chronotherapy and chronobiology. Bipolar Disord. 2019;21(8):741-773.
25. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262.
Autism spectrum disorder: Keys to early detection and accurate diagnosis
FIRST OF 2 PARTS
Autism spectrum disorder (ASD) is a complex, heterogenous neurodevelopmental disorder with genetic and environmental underpinnings, and an onset early in life.1-9 It affects social communication, cognition, and sensory-motor domains, and manifests as deficits in social reciprocity, repetitive behavior, restricted range of interests, and sensory sensitivities.6,10-14 In recent years, the prevalence of ASD has been increasing.3,6,10 A large percentage of individuals with ASD experience significant social deficits in adulthood,10 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.15,16 Interventions in early childhood can result in significant and lasting changes in outcome and in functioning of individuals with ASD.
This article provides an update on various aspects of ASD diagnosis, with the goal of equipping clinicians with knowledge to help make an accurate ASD diagnosis at an early stage. Part 1 focuses on early detection and diagnosis, while Part 2 will describe treatment strategies.
Benefits of early detection
Substantial research has established that early intervention confers substantial benefits for outcomes among children with ASD.2,3,5,6,9,13,14,16-22 Earlier age of intervention correlates with greater developmental gain and symptom reduction.21,23 The atypical neural development responsible for ASD likely occurs much earlier than the behavioral manifestations of this disorder, which implies that there is a crucial period to intervene before behavioral features emerge.1 This necessitates early recognition of ASD,9,17 and the need for further research to find novel ways to detect ASD earlier.
In the United States, children with ASD are diagnosed with the disorder on average between age 3 and 4 years.6,24 However, evidence suggests there may be a prodromal phase for ASD during the first several months of life, wherein infants and toddlers exhibit developmentally inadequate communication and social skills and/or unusual behaviors.18 Behavioral signs suggestive of ASD may be evident as early as infancy, and commonly earlier than age 18 months.1,17,19 Problems with sleeping and eating may be evident in early childhood.19 Deficits in joint attention may be evident as early as age 6 months to 8 months. Research suggests that a diagnosis of ASD by trained, expert professionals is likely to be accurate at the age of 2, and even as early as 18 months.6,24
In a prospective study, Anderson et al25 found that 9% of children who were diagnosed with ASD at age 2 no longer met the diagnostic criteria for ASD by adulthood.6 Those who no longer met ASD criteria were more likely to have received early intervention, had a verbal IQ ≥70, and had experienced a larger decrease in repetitive behaviors between ages 2 and 3, compared with other youth in this study who had a verbal IQ ≥70. One of the limitations of this study was a small sample size (85 participants); larger, randomized studies are needed to replicate these findings.25
Continue to: Characteristics of ASD...
Characteristics of ASD
Table 16,8,10,13,15,26-29 outlines various characteristics of ASD, which may manifest in varying degrees among children with the condition.
Speech/language. Speech helps to facilitate bonding between parents and an infant by offering a soothing, pleasurable, and reinforcing experience.30 More than 50% of children with ASD have language delays or deficits that persist throughout adulthood.13 The extent of these language deficits varies; in general, the more severe the speech/language deficits, the more severe the long-term symptoms.13 Language deficits in young children with ASD tend to be of both the expressive and receptive type, with onset in infancy, which suggests that neural processes predate the emergence of behavioral symptoms of ASD, and also that early language deficits/delays could be a marker for or indicator of future risk of ASD.13 Individuals with ASD also have been noted to have limitations in orienting or attending to human voices.13,30
Facial recognition. Evidence has linked ASD with deficits in facial recognition that emerge in the first few months of life.2 Earlier studies have found that lack of attention to others’ faces was the strongest distinguishing factor between 1-year-olds with ASD and typically developing 1-year-olds.2,31 A recent study that used EEG to compare facial emotion recognition in boys with ASD vs typically developing boys found that boys with ASD exhibited significantly lower sensitivity to angry and fearful faces.27
Other features. A 2020 study (N = 37) found that compared with typically developing children, those with ASD show less “interactional synchrony’’ (a dynamic process in which the timing of children and caregivers’ behaviors [specifically, vocalizations and movements] become mutually coordinated) with both familiar and unfamiliar adults.32 These researchers concluded that impairment in interactional synchrony may be linked to social communication deficits in ASD.32
A recent study (N = 98) evaluated “sluggish cognitive tempo” in 3 groups of children: children with attention-deficit/hyperactivity disorder (ADHD), children with ASD, and children with both ADHD and ASD.33 It found that children with ASD exhibited sluggish cognitive tempo at levels similar to those of the other 2 groups, and indicated that sluggish cognitive tempo may be linked with “social and global impairment above and beyond” the impairment associated with ASD.
Understanding early aberrations in neurobiologic processes in ASD can help develop biomarkers for early recognition of ASD, as well as guide the development of targeted interventions and treatments (Box1-3,7-9,12,13,30,35-39).
Box
Compared with individuals who do not have autism spectrum disorder (ASD), individuals with ASD exhibit anatomical differences in the brain that can be seen on MRI.9,35 Brain regions affected in ASD include the frontal gyrus, temporal gyrus, cingulate gyrus, postcentral gyrus, precuneus, caudate, and hippocampus.9 Some studies have found anomalous structural neural characteristics in infants, such as in the uncinate fasciculus, that correlated with later joint attention challenges, while others have found aberrations in the corpus callosum(responsible for transfer of procedural learning between the hemispheres, and oculomotor response)and internal capsule (responsible for sensorimotor function, as well as other functions) in children with ASD.12
Widespread white matter anomalies have been noted in ASD.12,35,36 In a 2-year longitudinal study that used diffusion tensor imaging, Li et al35 found that preschool children with ASD experience overgrowth of the uncinate fasciculus, which is one of the brain regions implicated in socioemotional processing, and concluded that this overgrowth correlated with ASD severity.35 Andrews et al37 used diffusion-weighted MRI to examine white matter in 127 preschool children. They found that compared with typically developing children, children with ASD exhibited altered white matter microstructure.37
Research suggests that developing representations of the reward value of social stimuli may be challenging for children with ASD.2 Abrams et al30 used resting-state functional brain MRI to evaluate children with typical development and children with highfunctioning, “verbally fluent” ASD. They found that the children with ASD exhibited lower functional connectivity between voice-specific left hemisphere posterior superior temporal sulcus and areas representing the reward circuitry.30 This study also found that children with ASD had underconnectivity between the right hemisphere posterior superior temporal sulcus (which deals with speech prosody) and areas known for emotion-linked associative learning, the orbitofrontal cortex and amygdala.30 These findings are thought to align with the social motivation theory of ASD.13,30,38
The extent of underconnectivity between these systems was found to determine the severity of communication challenges in high-functioning children with ASD.30 One MRI study observed lower gray matter volume in the voice-selective bilateral superior temporal sulcus in children age approximately 9 to 11 years with ASD.39
Neural systems responsible for facial recognition (particularly the right fusiform gyrus and other brain areas) have been shown to exist or begin “very early in life,” which suggests that impaired face recognition may be an early marker of ASD.2 In addition to problems with visual scanning, preferential attention to (and visual sensitivity to) biological motion is a forerunner for the development of social interactions in infants, specifically in regard to being able to detect and recognize emotion, which is considered vital for attachment.7,8 Impaired biological motion perception has been found in very young children with ASD.7,8 This presents an important avenue/potential biomarker for further research to better understand neurobiologic processes underlying atypical development at an earlier age.3,8
Early neural biomarkers for ASD
Nonlinear EEG values may serve as an early neurobiomarker for detecting ASD in young children.1 Because it is relatively inexpensive and convenient, EEG may be highly useful for detecting ASD.1 A study that compared EEG results of 99 infants who had siblings with ASD and 89 low-risk controls from age 3 months to 36 months found that nonlinear EEG measurements predicted with high accuracy later diagnosis of ASD, and were strongly correlated with later Autism Diagnostic Observation Schedule scores.1
Continue to: A complex differential diagnosis...
A complex differential diagnosis
The differential diagnosis of ASD warrants careful attention and consideration to rule out other developmental and psychiatric conditions.
Intellectual disability (ID). DSM-5 diagnostic criteria for ASD necessitate that disturbances are not better explained by ID or global developmental delay and that deficits should exceed impairment consistent with the level of intellectual disability.28 Still, ASD is often overdiagnosed in children with ID.28 Research suggests phenotypic and genetic overlap between ID and ASD.28 Social functioning is often impaired in patients with ID; the greater the severity of ID, the greater the degree of social deficits.28 In approximately 30% of cases, ASD and ID are comorbid.6 This overlap and comorbidity can pose a challenge, particularly due to the inherent complexities involved in assessment and differentiation.28 When ID is present in ASD, there is a greater degree of social-communication deficits.6 It may be difficult to assess for ASD symptoms in children with severe ID.28 Although there is no minimum age or developmental level below which ASD should not be diagnosed, some studies have started to use minimum criteria for diagnosis, such as a nonverbal mental age of 18 months.28,40 Commonly used tests for ASD have much lower specificity when used for children with nonverbal age <15 months.28 It would make sense, then, that the presence of ID might significantly affect the results of these diagnostic tests.28
Other conditions that need to be ruled out include language disorders, hearing loss, rare genetic neurodevelopmental disorders (eg, Fragile X syndrome,3 Rett syndrome6), childhood-onset schizophrenia, obsessive-compulsive disorder, attachment disorders, and other conditions.18 ASD may be overdiagnosed in children with genetic disorders such as Angelman syndrome.41 In a systematic review, Moss and Howlin42 recommended caution when evaluating ASD-like behavioral symptoms in children with genetic syndromes and severe ID. On the other hand, some research has observed that individuals with Fragile X syndrome may exhibit symptoms that meet criteria for ASD.6,43 McDuffie et al43 used the Autism Diagnostic Interview-Revised (ADI-R) to compare boys with Fragile X syndrome who also met criteria for ASD with boys with nonsyndromic ASD. Those in the former group had lesser impairment in social smiling, offering, showing, and nonverbal gestures, but had more complex mannerisms, compared with boys in the latter group.43
Milder manifestations of ASD may be more challenging to diagnose,1 particularly in children age <3 and those with above-average cognition.6 Generally, in the case of a patient with ASD, parents find that the child did not have a period of typical development, or unusual behaviors were evident early on.17
ASD can be comorbid with ADHD. The presence of ADHD may mask or delay the diagnosis of ASD in children.6 In children with both ASD and ADHD, studies have found greater reduction in social and adaptive functioning compared with children with ADHD alone.44
Table 26,10,15,17,31,43 highlights some of the features that can be used to distinguish ASD from other conditions.
Continue to: Screening and diagnosis...
Screening and diagnosis
A medical workup is the first step to rule out other potential conditions that could be masquerading as ASD.17 Obtain a comprehensive history from parents/caregivers, particularly regarding social, behavioral, movement, sensory, and developmental aspects. In addition, audiologic testing is an essential step. Consider genetic testing, particularly if any dysmorphic features and/or ID are present, both of which confer additional risk for a genetic syndrome.6 A physical exam to detect any neurologic anomalies, organ dysfunction, and body dysmorphic features should be conducted.6
The Modified Checklist for Autism in Toddlers–Revised (MCHAT-R) is a commonly used, validated parental screening survey for ASD.5,6 Research has shown that this survey has <50% specificity.5A recent American Academy of Pediatrics Clinical Report recommended universal screening for ASD at pediatric visits at age 18 months and at 24 months, in addition to developmental screening for all children at routine pediatric visits at age 9, 18, and 30 months.6,19
Screening tools such as the Modified Checklist for Autism in Toddlers with Follow-Up (M-CHAT/F) can be integrated into routine primary health care. In a large (N = 25,999) study, Guthrie et al45 used M-CHAT/F to conduct universal, primary care–based screening in young children. They found that the positive predictive value of M-CHAT/F was lower among girls, children of color, and those from lower-income households. There is a need for development of screening tools with higher accuracy and sensitivity for identifying young children with ASD regardless of their ethnic or socioeconomic background, and also for children older than 30 months.5,6,45
Definitive diagnosis of ASD is ideally done by a multidisciplinary team46 using established gold standard measures such as the ADOS (Autism Diagnostic Observation Schedule) and ADI-R.47 Such multidisciplinary teams usually include a child psychiatrist, child psychologist, speech therapist, occupational therapist, school educator, and developmental pediatrician. However, because there are long wait times to receive this type of diagnosis in the United States,6 in the interest of not missing the critical window of early intervention, physicians who suspect a patient may have ASD should refer the child and family for appropriate educational and behavioral interventions as early as possible, rather than waiting for definitive testing.6
ADI-R has limitations in distinguishing ASD from other conditions, especially in very young children, and particularly in distinguishing ASD from childhood-onset schizophrenia.47 Similarly, ADOS, which is a semi-structured, standardized, observation assessment tool, also has limitations, including generating false-positive results, which can make it difficult to distinguish children and adolescents with developmental disabilities from those with ASD.47 However, in combination, these 2 tools are generally efficacious.47 Further research is warranted to develop and fine-tune definitive diagnostic tools with greater sensitivity and specificity.
A newer measure—the Autism Parent Screen for Infants (APSI) questionnaire—has been shown to be effective in detecting early signs predictive of ASD in high-risk infants (eg, siblings of children with ASD), and has potential as an early screening tool.48,49
Part 2 of this article will review nonpharmacologic and pharmacologic treatments for patients with ASD.
1. Bosl WJ, Tager-Flusberg H, Nelson CA. EEG analytics for early detection of autism spectrum disorder: a data-driven approach. Sci Rep. 2018;8(1):6828. doi:10.1038/s41598-018-24318-x
2. Dawson G, Carver L, Meltzoff AN, et al. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700-717. doi:10.1111/1467-8624.00433
3. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.5
4. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
5. Hicks SD, Carpenter RL, Wagner KE, et al. Saliva microRNA differentiates children with autism from peers with typical and atypical development. J Am Acad Child Adolesc Psychiatry. 2020;59(2):296-308.
6. Hyman SL, Levy SE, Myers SM, et al; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
7. Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proc Natl Acad Sci U S A. 2010;107(49):21223-1228. doi:10.1073/pnas.1010412107
8. Klin A, Lin DJ, Gorrindo P, et al. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257-261. doi:10.1038/nature07868
9. Chen T, Chen Y, Yuan M, et al. Towards developing a practical artificial intelligence tool for diagnosing and evaluating autism spectrum disorder: a study using multicenter ABIDE II datasets. JMIR Med Inform. 2020;8(5):e15767. doi:10.2196/15767
10. Maglione MA, Gans D, Das L, et al; Technical Expert Panel, & HRSA Autism Intervention Research – Behavioral (AIR‐B) Network. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2), S169-S178.
11. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):5170-526. doi:10.1002/aur.2070
12. Shukla DK, Keehn B, Lincoln AJ, et al. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1269-1278.e12782. doi:10.1016/j.jaac.2010.08.018
13. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi: 10.3389/fnins.2016.00393
14. Zwaigenbaum L, Brian JA, Ip A. Early detection for autism spectrum disorder in young children. Paediatr Child Health. 2019;24(7):424-443. doi:10.1093/pch/pxz119
15. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
16. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
17. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry, 2014;53(2):237-257.
18. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
19. Lipkin PH, Macias MM; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Promoting optimal development: identifying infants and young children with developmental disorders through developmental surveillance and screening. Pediatrics. 2020;145(1)e20193449. doi:10.1542/peds.2019-3449
20. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
21. Rogers SJ, Estes A, Lord C, et al. Effects of a brief early start Denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
22. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
23. Mundy P. A review of joint attention and social-cognitive brain systems in typical development and autism spectrum disorder. Eur J Neurosci. 2018;47(6):497-514.
24. Zwaigenbaum L, Bryson SE, Brian J, et al. Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Res. 2016;9(7):790-800. doi:10.1002/aur.1585
25. Anderson DK, Liang JW, Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J Child Psychol Psychiatry. 2014;55(5):485-494. doi:10.1111/jcpp.12178
26. Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65(8):946-954. doi:10.1001/archpsyc.65.8.946
27. Van der Donck S, Dzhelyova M, Vettori S, et al. Rapid neural categorization of angry and fearful faces is specifically impaired in boys with autism spectrum disorder. J Child Psychol Psychiatry. 2020;61(9):1019-1029. doi:10.1111/jcpp.13201
28. Thurm A, Farmer C, Salzman E, et al. State of the field: differentiating intellectual disability from autism spectrum disorder. Front Psychiatry. 2019;10:526. doi:10.3389/fpsyt.2019.00526
29. Kuno-Fujita A, Iwabuchi T, Wakusawa K, et al. Sensory processing patterns and fusiform activity during face processing in autism spectrum disorder. Autism Res. 2020;13(5):741-750. doi: 10.1002/aur.2283
30. Abrams DA, Lynch CJ, Cheng KM, et al. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci U S A. 2013;110(29):12060-12065. doi:10.1073/pnas.1302982110
31. Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24(3):247-257.
32. Zampella CJ, Csumitta KD, Simon E, et al. Interactional synchrony and its association with social and communication ability in children with and without autism spectrum disorder. J Autism Dev Disord. 2020;50(9):3195-3206. doi:10.1007/s10803-020-04412-8
33. McFayden T, Jarrett MA, White SW, et al. Sluggish cognitive tempo in autism spectrum disorder, ADHD, and their comorbidity: implications for impairment. J Clin Child Adolesc Psychol. 2020:1-8. doi:10.1080/15374416.2020.1716365
34. Baribeau DA, Vigod S, Pullenayegum E, et al. Repetitive behavior severity as an early indicator of risk for elevated anxiety symptoms in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2020;59(7):890-899.e3. doi:10.1016/j.jaac.2019.08.478
35. Li Y, Zhou Z, Chang C, et al. Anomalies in uncinate fasciculus development and social defects in preschoolers with autism spectrum disorder. BMC Psychiatry. 2019;19(1):399. doi:10.1186/s12888-019-2391-1
36. Payabvash S, Palacios EM, Owen JP, et al. White matter connectome edge density in children with autism spectrum disorders: potential imaging biomarkers using machine-learning models. Brain Connect. 2019;9(2):209-220. doi:10.1089/brain.2018.0658
37. Andrews DS, Lee JK, Solomon M, et al. A diffusion-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-aged children. J Neurodev Disord. 2019;11(1):32. doi:10.1186/s11689-019-9291-z
38. Chevallier C, Kohls G, Troiani V, et al. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231-239. doi:10.1016/j.tics.2012.02.007
39. Boddaert N, Chabane N, Gervais H, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23(1):364-369. doi:10.1016/j.neuroimage.2004.06.016
40. Lord C, Petkova E, Hus V, et al. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch Gen Psychiatry. 2012;69(3):306-313. doi:10.1001/archgenpsychiatry.2011.148
41. Trillingsgaard A, ØStergaard JR. Autism in Angelman syndrome: an exploration of comorbidity. Autism. 2004;8(2):163-174.
42. Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res. 2009;53(10):852-873. doi:10.1111/j.1365-2788.2009.01197.x
43. McDuffie A, Thurman AJ, Hagerman RJ, et al. Symptoms of autism in males with Fragile X syndrome: a comparison to nonsyndromic ASD using current ADI-R scores. J Autism Dev Disord. 2015;45(7):1925-1937. doi:10.1007/s10803-013-2013-6
44. Ashwood KL, Tye C, Azadi B, et al. Brief report: adaptive functioning in children with ASD, ADHD and ASD + ADHD. J Autism Dev Disord. 2015;45(7):2235-4222. doi:10.1007/s10803-014-2352-y
45. Guthrie W, Wallis K, Bennett A, et al. Accuracy of autism screening in a large pediatric network. Pediatrics. 2019;144(4): e20183963. doi:10.1542/peds.2018-3963
46. Brian JA, Zwaigenbaum L, Ip A. Standards of diagnostic assessment for autism spectrum disorder. Paediatr Child Health. 2019;24(7):444-460. doi:10.1093/pch/pxz117
47. Frigaux A, Evrard R, Lighezzolo-Alnot J. ADI-R and ADOS and the differential diagnosis of autism spectrum disorders: interests, limits and openings. Encephale. 2019;45(5):441-448. doi:10.1016/j.encep.2019.07.002
48. Sacrey LR, Zwaigenbaum L, Bryson S, et al. Screening for behavioral signs of autism spectrum disorder in 9-month-old infant siblings. J Autism Dev Disord. 2021;51(3):839-848. doi:10.1007/s10803-020-04371-0
49. Sacrey LR, Bryson S, Zwaigenbaum L, et al. The autism parent screen for infants: predicting risk of autism spectrum disorder based on parent-reported behavior observed at 6-24 months of age. Autism. 2018;22(3):322-334
FIRST OF 2 PARTS
Autism spectrum disorder (ASD) is a complex, heterogenous neurodevelopmental disorder with genetic and environmental underpinnings, and an onset early in life.1-9 It affects social communication, cognition, and sensory-motor domains, and manifests as deficits in social reciprocity, repetitive behavior, restricted range of interests, and sensory sensitivities.6,10-14 In recent years, the prevalence of ASD has been increasing.3,6,10 A large percentage of individuals with ASD experience significant social deficits in adulthood,10 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.15,16 Interventions in early childhood can result in significant and lasting changes in outcome and in functioning of individuals with ASD.
This article provides an update on various aspects of ASD diagnosis, with the goal of equipping clinicians with knowledge to help make an accurate ASD diagnosis at an early stage. Part 1 focuses on early detection and diagnosis, while Part 2 will describe treatment strategies.
Benefits of early detection
Substantial research has established that early intervention confers substantial benefits for outcomes among children with ASD.2,3,5,6,9,13,14,16-22 Earlier age of intervention correlates with greater developmental gain and symptom reduction.21,23 The atypical neural development responsible for ASD likely occurs much earlier than the behavioral manifestations of this disorder, which implies that there is a crucial period to intervene before behavioral features emerge.1 This necessitates early recognition of ASD,9,17 and the need for further research to find novel ways to detect ASD earlier.
In the United States, children with ASD are diagnosed with the disorder on average between age 3 and 4 years.6,24 However, evidence suggests there may be a prodromal phase for ASD during the first several months of life, wherein infants and toddlers exhibit developmentally inadequate communication and social skills and/or unusual behaviors.18 Behavioral signs suggestive of ASD may be evident as early as infancy, and commonly earlier than age 18 months.1,17,19 Problems with sleeping and eating may be evident in early childhood.19 Deficits in joint attention may be evident as early as age 6 months to 8 months. Research suggests that a diagnosis of ASD by trained, expert professionals is likely to be accurate at the age of 2, and even as early as 18 months.6,24
In a prospective study, Anderson et al25 found that 9% of children who were diagnosed with ASD at age 2 no longer met the diagnostic criteria for ASD by adulthood.6 Those who no longer met ASD criteria were more likely to have received early intervention, had a verbal IQ ≥70, and had experienced a larger decrease in repetitive behaviors between ages 2 and 3, compared with other youth in this study who had a verbal IQ ≥70. One of the limitations of this study was a small sample size (85 participants); larger, randomized studies are needed to replicate these findings.25
Continue to: Characteristics of ASD...
Characteristics of ASD
Table 16,8,10,13,15,26-29 outlines various characteristics of ASD, which may manifest in varying degrees among children with the condition.
Speech/language. Speech helps to facilitate bonding between parents and an infant by offering a soothing, pleasurable, and reinforcing experience.30 More than 50% of children with ASD have language delays or deficits that persist throughout adulthood.13 The extent of these language deficits varies; in general, the more severe the speech/language deficits, the more severe the long-term symptoms.13 Language deficits in young children with ASD tend to be of both the expressive and receptive type, with onset in infancy, which suggests that neural processes predate the emergence of behavioral symptoms of ASD, and also that early language deficits/delays could be a marker for or indicator of future risk of ASD.13 Individuals with ASD also have been noted to have limitations in orienting or attending to human voices.13,30
Facial recognition. Evidence has linked ASD with deficits in facial recognition that emerge in the first few months of life.2 Earlier studies have found that lack of attention to others’ faces was the strongest distinguishing factor between 1-year-olds with ASD and typically developing 1-year-olds.2,31 A recent study that used EEG to compare facial emotion recognition in boys with ASD vs typically developing boys found that boys with ASD exhibited significantly lower sensitivity to angry and fearful faces.27
Other features. A 2020 study (N = 37) found that compared with typically developing children, those with ASD show less “interactional synchrony’’ (a dynamic process in which the timing of children and caregivers’ behaviors [specifically, vocalizations and movements] become mutually coordinated) with both familiar and unfamiliar adults.32 These researchers concluded that impairment in interactional synchrony may be linked to social communication deficits in ASD.32
A recent study (N = 98) evaluated “sluggish cognitive tempo” in 3 groups of children: children with attention-deficit/hyperactivity disorder (ADHD), children with ASD, and children with both ADHD and ASD.33 It found that children with ASD exhibited sluggish cognitive tempo at levels similar to those of the other 2 groups, and indicated that sluggish cognitive tempo may be linked with “social and global impairment above and beyond” the impairment associated with ASD.
Understanding early aberrations in neurobiologic processes in ASD can help develop biomarkers for early recognition of ASD, as well as guide the development of targeted interventions and treatments (Box1-3,7-9,12,13,30,35-39).
Box
Compared with individuals who do not have autism spectrum disorder (ASD), individuals with ASD exhibit anatomical differences in the brain that can be seen on MRI.9,35 Brain regions affected in ASD include the frontal gyrus, temporal gyrus, cingulate gyrus, postcentral gyrus, precuneus, caudate, and hippocampus.9 Some studies have found anomalous structural neural characteristics in infants, such as in the uncinate fasciculus, that correlated with later joint attention challenges, while others have found aberrations in the corpus callosum(responsible for transfer of procedural learning between the hemispheres, and oculomotor response)and internal capsule (responsible for sensorimotor function, as well as other functions) in children with ASD.12
Widespread white matter anomalies have been noted in ASD.12,35,36 In a 2-year longitudinal study that used diffusion tensor imaging, Li et al35 found that preschool children with ASD experience overgrowth of the uncinate fasciculus, which is one of the brain regions implicated in socioemotional processing, and concluded that this overgrowth correlated with ASD severity.35 Andrews et al37 used diffusion-weighted MRI to examine white matter in 127 preschool children. They found that compared with typically developing children, children with ASD exhibited altered white matter microstructure.37
Research suggests that developing representations of the reward value of social stimuli may be challenging for children with ASD.2 Abrams et al30 used resting-state functional brain MRI to evaluate children with typical development and children with highfunctioning, “verbally fluent” ASD. They found that the children with ASD exhibited lower functional connectivity between voice-specific left hemisphere posterior superior temporal sulcus and areas representing the reward circuitry.30 This study also found that children with ASD had underconnectivity between the right hemisphere posterior superior temporal sulcus (which deals with speech prosody) and areas known for emotion-linked associative learning, the orbitofrontal cortex and amygdala.30 These findings are thought to align with the social motivation theory of ASD.13,30,38
The extent of underconnectivity between these systems was found to determine the severity of communication challenges in high-functioning children with ASD.30 One MRI study observed lower gray matter volume in the voice-selective bilateral superior temporal sulcus in children age approximately 9 to 11 years with ASD.39
Neural systems responsible for facial recognition (particularly the right fusiform gyrus and other brain areas) have been shown to exist or begin “very early in life,” which suggests that impaired face recognition may be an early marker of ASD.2 In addition to problems with visual scanning, preferential attention to (and visual sensitivity to) biological motion is a forerunner for the development of social interactions in infants, specifically in regard to being able to detect and recognize emotion, which is considered vital for attachment.7,8 Impaired biological motion perception has been found in very young children with ASD.7,8 This presents an important avenue/potential biomarker for further research to better understand neurobiologic processes underlying atypical development at an earlier age.3,8
Early neural biomarkers for ASD
Nonlinear EEG values may serve as an early neurobiomarker for detecting ASD in young children.1 Because it is relatively inexpensive and convenient, EEG may be highly useful for detecting ASD.1 A study that compared EEG results of 99 infants who had siblings with ASD and 89 low-risk controls from age 3 months to 36 months found that nonlinear EEG measurements predicted with high accuracy later diagnosis of ASD, and were strongly correlated with later Autism Diagnostic Observation Schedule scores.1
Continue to: A complex differential diagnosis...
A complex differential diagnosis
The differential diagnosis of ASD warrants careful attention and consideration to rule out other developmental and psychiatric conditions.
Intellectual disability (ID). DSM-5 diagnostic criteria for ASD necessitate that disturbances are not better explained by ID or global developmental delay and that deficits should exceed impairment consistent with the level of intellectual disability.28 Still, ASD is often overdiagnosed in children with ID.28 Research suggests phenotypic and genetic overlap between ID and ASD.28 Social functioning is often impaired in patients with ID; the greater the severity of ID, the greater the degree of social deficits.28 In approximately 30% of cases, ASD and ID are comorbid.6 This overlap and comorbidity can pose a challenge, particularly due to the inherent complexities involved in assessment and differentiation.28 When ID is present in ASD, there is a greater degree of social-communication deficits.6 It may be difficult to assess for ASD symptoms in children with severe ID.28 Although there is no minimum age or developmental level below which ASD should not be diagnosed, some studies have started to use minimum criteria for diagnosis, such as a nonverbal mental age of 18 months.28,40 Commonly used tests for ASD have much lower specificity when used for children with nonverbal age <15 months.28 It would make sense, then, that the presence of ID might significantly affect the results of these diagnostic tests.28
Other conditions that need to be ruled out include language disorders, hearing loss, rare genetic neurodevelopmental disorders (eg, Fragile X syndrome,3 Rett syndrome6), childhood-onset schizophrenia, obsessive-compulsive disorder, attachment disorders, and other conditions.18 ASD may be overdiagnosed in children with genetic disorders such as Angelman syndrome.41 In a systematic review, Moss and Howlin42 recommended caution when evaluating ASD-like behavioral symptoms in children with genetic syndromes and severe ID. On the other hand, some research has observed that individuals with Fragile X syndrome may exhibit symptoms that meet criteria for ASD.6,43 McDuffie et al43 used the Autism Diagnostic Interview-Revised (ADI-R) to compare boys with Fragile X syndrome who also met criteria for ASD with boys with nonsyndromic ASD. Those in the former group had lesser impairment in social smiling, offering, showing, and nonverbal gestures, but had more complex mannerisms, compared with boys in the latter group.43
Milder manifestations of ASD may be more challenging to diagnose,1 particularly in children age <3 and those with above-average cognition.6 Generally, in the case of a patient with ASD, parents find that the child did not have a period of typical development, or unusual behaviors were evident early on.17
ASD can be comorbid with ADHD. The presence of ADHD may mask or delay the diagnosis of ASD in children.6 In children with both ASD and ADHD, studies have found greater reduction in social and adaptive functioning compared with children with ADHD alone.44
Table 26,10,15,17,31,43 highlights some of the features that can be used to distinguish ASD from other conditions.
Continue to: Screening and diagnosis...
Screening and diagnosis
A medical workup is the first step to rule out other potential conditions that could be masquerading as ASD.17 Obtain a comprehensive history from parents/caregivers, particularly regarding social, behavioral, movement, sensory, and developmental aspects. In addition, audiologic testing is an essential step. Consider genetic testing, particularly if any dysmorphic features and/or ID are present, both of which confer additional risk for a genetic syndrome.6 A physical exam to detect any neurologic anomalies, organ dysfunction, and body dysmorphic features should be conducted.6
The Modified Checklist for Autism in Toddlers–Revised (MCHAT-R) is a commonly used, validated parental screening survey for ASD.5,6 Research has shown that this survey has <50% specificity.5A recent American Academy of Pediatrics Clinical Report recommended universal screening for ASD at pediatric visits at age 18 months and at 24 months, in addition to developmental screening for all children at routine pediatric visits at age 9, 18, and 30 months.6,19
Screening tools such as the Modified Checklist for Autism in Toddlers with Follow-Up (M-CHAT/F) can be integrated into routine primary health care. In a large (N = 25,999) study, Guthrie et al45 used M-CHAT/F to conduct universal, primary care–based screening in young children. They found that the positive predictive value of M-CHAT/F was lower among girls, children of color, and those from lower-income households. There is a need for development of screening tools with higher accuracy and sensitivity for identifying young children with ASD regardless of their ethnic or socioeconomic background, and also for children older than 30 months.5,6,45
Definitive diagnosis of ASD is ideally done by a multidisciplinary team46 using established gold standard measures such as the ADOS (Autism Diagnostic Observation Schedule) and ADI-R.47 Such multidisciplinary teams usually include a child psychiatrist, child psychologist, speech therapist, occupational therapist, school educator, and developmental pediatrician. However, because there are long wait times to receive this type of diagnosis in the United States,6 in the interest of not missing the critical window of early intervention, physicians who suspect a patient may have ASD should refer the child and family for appropriate educational and behavioral interventions as early as possible, rather than waiting for definitive testing.6
ADI-R has limitations in distinguishing ASD from other conditions, especially in very young children, and particularly in distinguishing ASD from childhood-onset schizophrenia.47 Similarly, ADOS, which is a semi-structured, standardized, observation assessment tool, also has limitations, including generating false-positive results, which can make it difficult to distinguish children and adolescents with developmental disabilities from those with ASD.47 However, in combination, these 2 tools are generally efficacious.47 Further research is warranted to develop and fine-tune definitive diagnostic tools with greater sensitivity and specificity.
A newer measure—the Autism Parent Screen for Infants (APSI) questionnaire—has been shown to be effective in detecting early signs predictive of ASD in high-risk infants (eg, siblings of children with ASD), and has potential as an early screening tool.48,49
Part 2 of this article will review nonpharmacologic and pharmacologic treatments for patients with ASD.
FIRST OF 2 PARTS
Autism spectrum disorder (ASD) is a complex, heterogenous neurodevelopmental disorder with genetic and environmental underpinnings, and an onset early in life.1-9 It affects social communication, cognition, and sensory-motor domains, and manifests as deficits in social reciprocity, repetitive behavior, restricted range of interests, and sensory sensitivities.6,10-14 In recent years, the prevalence of ASD has been increasing.3,6,10 A large percentage of individuals with ASD experience significant social deficits in adulthood,10 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.15,16 Interventions in early childhood can result in significant and lasting changes in outcome and in functioning of individuals with ASD.
This article provides an update on various aspects of ASD diagnosis, with the goal of equipping clinicians with knowledge to help make an accurate ASD diagnosis at an early stage. Part 1 focuses on early detection and diagnosis, while Part 2 will describe treatment strategies.
Benefits of early detection
Substantial research has established that early intervention confers substantial benefits for outcomes among children with ASD.2,3,5,6,9,13,14,16-22 Earlier age of intervention correlates with greater developmental gain and symptom reduction.21,23 The atypical neural development responsible for ASD likely occurs much earlier than the behavioral manifestations of this disorder, which implies that there is a crucial period to intervene before behavioral features emerge.1 This necessitates early recognition of ASD,9,17 and the need for further research to find novel ways to detect ASD earlier.
In the United States, children with ASD are diagnosed with the disorder on average between age 3 and 4 years.6,24 However, evidence suggests there may be a prodromal phase for ASD during the first several months of life, wherein infants and toddlers exhibit developmentally inadequate communication and social skills and/or unusual behaviors.18 Behavioral signs suggestive of ASD may be evident as early as infancy, and commonly earlier than age 18 months.1,17,19 Problems with sleeping and eating may be evident in early childhood.19 Deficits in joint attention may be evident as early as age 6 months to 8 months. Research suggests that a diagnosis of ASD by trained, expert professionals is likely to be accurate at the age of 2, and even as early as 18 months.6,24
In a prospective study, Anderson et al25 found that 9% of children who were diagnosed with ASD at age 2 no longer met the diagnostic criteria for ASD by adulthood.6 Those who no longer met ASD criteria were more likely to have received early intervention, had a verbal IQ ≥70, and had experienced a larger decrease in repetitive behaviors between ages 2 and 3, compared with other youth in this study who had a verbal IQ ≥70. One of the limitations of this study was a small sample size (85 participants); larger, randomized studies are needed to replicate these findings.25
Continue to: Characteristics of ASD...
Characteristics of ASD
Table 16,8,10,13,15,26-29 outlines various characteristics of ASD, which may manifest in varying degrees among children with the condition.
Speech/language. Speech helps to facilitate bonding between parents and an infant by offering a soothing, pleasurable, and reinforcing experience.30 More than 50% of children with ASD have language delays or deficits that persist throughout adulthood.13 The extent of these language deficits varies; in general, the more severe the speech/language deficits, the more severe the long-term symptoms.13 Language deficits in young children with ASD tend to be of both the expressive and receptive type, with onset in infancy, which suggests that neural processes predate the emergence of behavioral symptoms of ASD, and also that early language deficits/delays could be a marker for or indicator of future risk of ASD.13 Individuals with ASD also have been noted to have limitations in orienting or attending to human voices.13,30
Facial recognition. Evidence has linked ASD with deficits in facial recognition that emerge in the first few months of life.2 Earlier studies have found that lack of attention to others’ faces was the strongest distinguishing factor between 1-year-olds with ASD and typically developing 1-year-olds.2,31 A recent study that used EEG to compare facial emotion recognition in boys with ASD vs typically developing boys found that boys with ASD exhibited significantly lower sensitivity to angry and fearful faces.27
Other features. A 2020 study (N = 37) found that compared with typically developing children, those with ASD show less “interactional synchrony’’ (a dynamic process in which the timing of children and caregivers’ behaviors [specifically, vocalizations and movements] become mutually coordinated) with both familiar and unfamiliar adults.32 These researchers concluded that impairment in interactional synchrony may be linked to social communication deficits in ASD.32
A recent study (N = 98) evaluated “sluggish cognitive tempo” in 3 groups of children: children with attention-deficit/hyperactivity disorder (ADHD), children with ASD, and children with both ADHD and ASD.33 It found that children with ASD exhibited sluggish cognitive tempo at levels similar to those of the other 2 groups, and indicated that sluggish cognitive tempo may be linked with “social and global impairment above and beyond” the impairment associated with ASD.
Understanding early aberrations in neurobiologic processes in ASD can help develop biomarkers for early recognition of ASD, as well as guide the development of targeted interventions and treatments (Box1-3,7-9,12,13,30,35-39).
Box
Compared with individuals who do not have autism spectrum disorder (ASD), individuals with ASD exhibit anatomical differences in the brain that can be seen on MRI.9,35 Brain regions affected in ASD include the frontal gyrus, temporal gyrus, cingulate gyrus, postcentral gyrus, precuneus, caudate, and hippocampus.9 Some studies have found anomalous structural neural characteristics in infants, such as in the uncinate fasciculus, that correlated with later joint attention challenges, while others have found aberrations in the corpus callosum(responsible for transfer of procedural learning between the hemispheres, and oculomotor response)and internal capsule (responsible for sensorimotor function, as well as other functions) in children with ASD.12
Widespread white matter anomalies have been noted in ASD.12,35,36 In a 2-year longitudinal study that used diffusion tensor imaging, Li et al35 found that preschool children with ASD experience overgrowth of the uncinate fasciculus, which is one of the brain regions implicated in socioemotional processing, and concluded that this overgrowth correlated with ASD severity.35 Andrews et al37 used diffusion-weighted MRI to examine white matter in 127 preschool children. They found that compared with typically developing children, children with ASD exhibited altered white matter microstructure.37
Research suggests that developing representations of the reward value of social stimuli may be challenging for children with ASD.2 Abrams et al30 used resting-state functional brain MRI to evaluate children with typical development and children with highfunctioning, “verbally fluent” ASD. They found that the children with ASD exhibited lower functional connectivity between voice-specific left hemisphere posterior superior temporal sulcus and areas representing the reward circuitry.30 This study also found that children with ASD had underconnectivity between the right hemisphere posterior superior temporal sulcus (which deals with speech prosody) and areas known for emotion-linked associative learning, the orbitofrontal cortex and amygdala.30 These findings are thought to align with the social motivation theory of ASD.13,30,38
The extent of underconnectivity between these systems was found to determine the severity of communication challenges in high-functioning children with ASD.30 One MRI study observed lower gray matter volume in the voice-selective bilateral superior temporal sulcus in children age approximately 9 to 11 years with ASD.39
Neural systems responsible for facial recognition (particularly the right fusiform gyrus and other brain areas) have been shown to exist or begin “very early in life,” which suggests that impaired face recognition may be an early marker of ASD.2 In addition to problems with visual scanning, preferential attention to (and visual sensitivity to) biological motion is a forerunner for the development of social interactions in infants, specifically in regard to being able to detect and recognize emotion, which is considered vital for attachment.7,8 Impaired biological motion perception has been found in very young children with ASD.7,8 This presents an important avenue/potential biomarker for further research to better understand neurobiologic processes underlying atypical development at an earlier age.3,8
Early neural biomarkers for ASD
Nonlinear EEG values may serve as an early neurobiomarker for detecting ASD in young children.1 Because it is relatively inexpensive and convenient, EEG may be highly useful for detecting ASD.1 A study that compared EEG results of 99 infants who had siblings with ASD and 89 low-risk controls from age 3 months to 36 months found that nonlinear EEG measurements predicted with high accuracy later diagnosis of ASD, and were strongly correlated with later Autism Diagnostic Observation Schedule scores.1
Continue to: A complex differential diagnosis...
A complex differential diagnosis
The differential diagnosis of ASD warrants careful attention and consideration to rule out other developmental and psychiatric conditions.
Intellectual disability (ID). DSM-5 diagnostic criteria for ASD necessitate that disturbances are not better explained by ID or global developmental delay and that deficits should exceed impairment consistent with the level of intellectual disability.28 Still, ASD is often overdiagnosed in children with ID.28 Research suggests phenotypic and genetic overlap between ID and ASD.28 Social functioning is often impaired in patients with ID; the greater the severity of ID, the greater the degree of social deficits.28 In approximately 30% of cases, ASD and ID are comorbid.6 This overlap and comorbidity can pose a challenge, particularly due to the inherent complexities involved in assessment and differentiation.28 When ID is present in ASD, there is a greater degree of social-communication deficits.6 It may be difficult to assess for ASD symptoms in children with severe ID.28 Although there is no minimum age or developmental level below which ASD should not be diagnosed, some studies have started to use minimum criteria for diagnosis, such as a nonverbal mental age of 18 months.28,40 Commonly used tests for ASD have much lower specificity when used for children with nonverbal age <15 months.28 It would make sense, then, that the presence of ID might significantly affect the results of these diagnostic tests.28
Other conditions that need to be ruled out include language disorders, hearing loss, rare genetic neurodevelopmental disorders (eg, Fragile X syndrome,3 Rett syndrome6), childhood-onset schizophrenia, obsessive-compulsive disorder, attachment disorders, and other conditions.18 ASD may be overdiagnosed in children with genetic disorders such as Angelman syndrome.41 In a systematic review, Moss and Howlin42 recommended caution when evaluating ASD-like behavioral symptoms in children with genetic syndromes and severe ID. On the other hand, some research has observed that individuals with Fragile X syndrome may exhibit symptoms that meet criteria for ASD.6,43 McDuffie et al43 used the Autism Diagnostic Interview-Revised (ADI-R) to compare boys with Fragile X syndrome who also met criteria for ASD with boys with nonsyndromic ASD. Those in the former group had lesser impairment in social smiling, offering, showing, and nonverbal gestures, but had more complex mannerisms, compared with boys in the latter group.43
Milder manifestations of ASD may be more challenging to diagnose,1 particularly in children age <3 and those with above-average cognition.6 Generally, in the case of a patient with ASD, parents find that the child did not have a period of typical development, or unusual behaviors were evident early on.17
ASD can be comorbid with ADHD. The presence of ADHD may mask or delay the diagnosis of ASD in children.6 In children with both ASD and ADHD, studies have found greater reduction in social and adaptive functioning compared with children with ADHD alone.44
Table 26,10,15,17,31,43 highlights some of the features that can be used to distinguish ASD from other conditions.
Continue to: Screening and diagnosis...
Screening and diagnosis
A medical workup is the first step to rule out other potential conditions that could be masquerading as ASD.17 Obtain a comprehensive history from parents/caregivers, particularly regarding social, behavioral, movement, sensory, and developmental aspects. In addition, audiologic testing is an essential step. Consider genetic testing, particularly if any dysmorphic features and/or ID are present, both of which confer additional risk for a genetic syndrome.6 A physical exam to detect any neurologic anomalies, organ dysfunction, and body dysmorphic features should be conducted.6
The Modified Checklist for Autism in Toddlers–Revised (MCHAT-R) is a commonly used, validated parental screening survey for ASD.5,6 Research has shown that this survey has <50% specificity.5A recent American Academy of Pediatrics Clinical Report recommended universal screening for ASD at pediatric visits at age 18 months and at 24 months, in addition to developmental screening for all children at routine pediatric visits at age 9, 18, and 30 months.6,19
Screening tools such as the Modified Checklist for Autism in Toddlers with Follow-Up (M-CHAT/F) can be integrated into routine primary health care. In a large (N = 25,999) study, Guthrie et al45 used M-CHAT/F to conduct universal, primary care–based screening in young children. They found that the positive predictive value of M-CHAT/F was lower among girls, children of color, and those from lower-income households. There is a need for development of screening tools with higher accuracy and sensitivity for identifying young children with ASD regardless of their ethnic or socioeconomic background, and also for children older than 30 months.5,6,45
Definitive diagnosis of ASD is ideally done by a multidisciplinary team46 using established gold standard measures such as the ADOS (Autism Diagnostic Observation Schedule) and ADI-R.47 Such multidisciplinary teams usually include a child psychiatrist, child psychologist, speech therapist, occupational therapist, school educator, and developmental pediatrician. However, because there are long wait times to receive this type of diagnosis in the United States,6 in the interest of not missing the critical window of early intervention, physicians who suspect a patient may have ASD should refer the child and family for appropriate educational and behavioral interventions as early as possible, rather than waiting for definitive testing.6
ADI-R has limitations in distinguishing ASD from other conditions, especially in very young children, and particularly in distinguishing ASD from childhood-onset schizophrenia.47 Similarly, ADOS, which is a semi-structured, standardized, observation assessment tool, also has limitations, including generating false-positive results, which can make it difficult to distinguish children and adolescents with developmental disabilities from those with ASD.47 However, in combination, these 2 tools are generally efficacious.47 Further research is warranted to develop and fine-tune definitive diagnostic tools with greater sensitivity and specificity.
A newer measure—the Autism Parent Screen for Infants (APSI) questionnaire—has been shown to be effective in detecting early signs predictive of ASD in high-risk infants (eg, siblings of children with ASD), and has potential as an early screening tool.48,49
Part 2 of this article will review nonpharmacologic and pharmacologic treatments for patients with ASD.
1. Bosl WJ, Tager-Flusberg H, Nelson CA. EEG analytics for early detection of autism spectrum disorder: a data-driven approach. Sci Rep. 2018;8(1):6828. doi:10.1038/s41598-018-24318-x
2. Dawson G, Carver L, Meltzoff AN, et al. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700-717. doi:10.1111/1467-8624.00433
3. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.5
4. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
5. Hicks SD, Carpenter RL, Wagner KE, et al. Saliva microRNA differentiates children with autism from peers with typical and atypical development. J Am Acad Child Adolesc Psychiatry. 2020;59(2):296-308.
6. Hyman SL, Levy SE, Myers SM, et al; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
7. Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proc Natl Acad Sci U S A. 2010;107(49):21223-1228. doi:10.1073/pnas.1010412107
8. Klin A, Lin DJ, Gorrindo P, et al. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257-261. doi:10.1038/nature07868
9. Chen T, Chen Y, Yuan M, et al. Towards developing a practical artificial intelligence tool for diagnosing and evaluating autism spectrum disorder: a study using multicenter ABIDE II datasets. JMIR Med Inform. 2020;8(5):e15767. doi:10.2196/15767
10. Maglione MA, Gans D, Das L, et al; Technical Expert Panel, & HRSA Autism Intervention Research – Behavioral (AIR‐B) Network. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2), S169-S178.
11. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):5170-526. doi:10.1002/aur.2070
12. Shukla DK, Keehn B, Lincoln AJ, et al. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1269-1278.e12782. doi:10.1016/j.jaac.2010.08.018
13. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi: 10.3389/fnins.2016.00393
14. Zwaigenbaum L, Brian JA, Ip A. Early detection for autism spectrum disorder in young children. Paediatr Child Health. 2019;24(7):424-443. doi:10.1093/pch/pxz119
15. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
16. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
17. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry, 2014;53(2):237-257.
18. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
19. Lipkin PH, Macias MM; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Promoting optimal development: identifying infants and young children with developmental disorders through developmental surveillance and screening. Pediatrics. 2020;145(1)e20193449. doi:10.1542/peds.2019-3449
20. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
21. Rogers SJ, Estes A, Lord C, et al. Effects of a brief early start Denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
22. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
23. Mundy P. A review of joint attention and social-cognitive brain systems in typical development and autism spectrum disorder. Eur J Neurosci. 2018;47(6):497-514.
24. Zwaigenbaum L, Bryson SE, Brian J, et al. Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Res. 2016;9(7):790-800. doi:10.1002/aur.1585
25. Anderson DK, Liang JW, Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J Child Psychol Psychiatry. 2014;55(5):485-494. doi:10.1111/jcpp.12178
26. Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65(8):946-954. doi:10.1001/archpsyc.65.8.946
27. Van der Donck S, Dzhelyova M, Vettori S, et al. Rapid neural categorization of angry and fearful faces is specifically impaired in boys with autism spectrum disorder. J Child Psychol Psychiatry. 2020;61(9):1019-1029. doi:10.1111/jcpp.13201
28. Thurm A, Farmer C, Salzman E, et al. State of the field: differentiating intellectual disability from autism spectrum disorder. Front Psychiatry. 2019;10:526. doi:10.3389/fpsyt.2019.00526
29. Kuno-Fujita A, Iwabuchi T, Wakusawa K, et al. Sensory processing patterns and fusiform activity during face processing in autism spectrum disorder. Autism Res. 2020;13(5):741-750. doi: 10.1002/aur.2283
30. Abrams DA, Lynch CJ, Cheng KM, et al. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci U S A. 2013;110(29):12060-12065. doi:10.1073/pnas.1302982110
31. Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24(3):247-257.
32. Zampella CJ, Csumitta KD, Simon E, et al. Interactional synchrony and its association with social and communication ability in children with and without autism spectrum disorder. J Autism Dev Disord. 2020;50(9):3195-3206. doi:10.1007/s10803-020-04412-8
33. McFayden T, Jarrett MA, White SW, et al. Sluggish cognitive tempo in autism spectrum disorder, ADHD, and their comorbidity: implications for impairment. J Clin Child Adolesc Psychol. 2020:1-8. doi:10.1080/15374416.2020.1716365
34. Baribeau DA, Vigod S, Pullenayegum E, et al. Repetitive behavior severity as an early indicator of risk for elevated anxiety symptoms in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2020;59(7):890-899.e3. doi:10.1016/j.jaac.2019.08.478
35. Li Y, Zhou Z, Chang C, et al. Anomalies in uncinate fasciculus development and social defects in preschoolers with autism spectrum disorder. BMC Psychiatry. 2019;19(1):399. doi:10.1186/s12888-019-2391-1
36. Payabvash S, Palacios EM, Owen JP, et al. White matter connectome edge density in children with autism spectrum disorders: potential imaging biomarkers using machine-learning models. Brain Connect. 2019;9(2):209-220. doi:10.1089/brain.2018.0658
37. Andrews DS, Lee JK, Solomon M, et al. A diffusion-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-aged children. J Neurodev Disord. 2019;11(1):32. doi:10.1186/s11689-019-9291-z
38. Chevallier C, Kohls G, Troiani V, et al. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231-239. doi:10.1016/j.tics.2012.02.007
39. Boddaert N, Chabane N, Gervais H, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23(1):364-369. doi:10.1016/j.neuroimage.2004.06.016
40. Lord C, Petkova E, Hus V, et al. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch Gen Psychiatry. 2012;69(3):306-313. doi:10.1001/archgenpsychiatry.2011.148
41. Trillingsgaard A, ØStergaard JR. Autism in Angelman syndrome: an exploration of comorbidity. Autism. 2004;8(2):163-174.
42. Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res. 2009;53(10):852-873. doi:10.1111/j.1365-2788.2009.01197.x
43. McDuffie A, Thurman AJ, Hagerman RJ, et al. Symptoms of autism in males with Fragile X syndrome: a comparison to nonsyndromic ASD using current ADI-R scores. J Autism Dev Disord. 2015;45(7):1925-1937. doi:10.1007/s10803-013-2013-6
44. Ashwood KL, Tye C, Azadi B, et al. Brief report: adaptive functioning in children with ASD, ADHD and ASD + ADHD. J Autism Dev Disord. 2015;45(7):2235-4222. doi:10.1007/s10803-014-2352-y
45. Guthrie W, Wallis K, Bennett A, et al. Accuracy of autism screening in a large pediatric network. Pediatrics. 2019;144(4): e20183963. doi:10.1542/peds.2018-3963
46. Brian JA, Zwaigenbaum L, Ip A. Standards of diagnostic assessment for autism spectrum disorder. Paediatr Child Health. 2019;24(7):444-460. doi:10.1093/pch/pxz117
47. Frigaux A, Evrard R, Lighezzolo-Alnot J. ADI-R and ADOS and the differential diagnosis of autism spectrum disorders: interests, limits and openings. Encephale. 2019;45(5):441-448. doi:10.1016/j.encep.2019.07.002
48. Sacrey LR, Zwaigenbaum L, Bryson S, et al. Screening for behavioral signs of autism spectrum disorder in 9-month-old infant siblings. J Autism Dev Disord. 2021;51(3):839-848. doi:10.1007/s10803-020-04371-0
49. Sacrey LR, Bryson S, Zwaigenbaum L, et al. The autism parent screen for infants: predicting risk of autism spectrum disorder based on parent-reported behavior observed at 6-24 months of age. Autism. 2018;22(3):322-334
1. Bosl WJ, Tager-Flusberg H, Nelson CA. EEG analytics for early detection of autism spectrum disorder: a data-driven approach. Sci Rep. 2018;8(1):6828. doi:10.1038/s41598-018-24318-x
2. Dawson G, Carver L, Meltzoff AN, et al. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700-717. doi:10.1111/1467-8624.00433
3. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.5
4. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
5. Hicks SD, Carpenter RL, Wagner KE, et al. Saliva microRNA differentiates children with autism from peers with typical and atypical development. J Am Acad Child Adolesc Psychiatry. 2020;59(2):296-308.
6. Hyman SL, Levy SE, Myers SM, et al; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
7. Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proc Natl Acad Sci U S A. 2010;107(49):21223-1228. doi:10.1073/pnas.1010412107
8. Klin A, Lin DJ, Gorrindo P, et al. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257-261. doi:10.1038/nature07868
9. Chen T, Chen Y, Yuan M, et al. Towards developing a practical artificial intelligence tool for diagnosing and evaluating autism spectrum disorder: a study using multicenter ABIDE II datasets. JMIR Med Inform. 2020;8(5):e15767. doi:10.2196/15767
10. Maglione MA, Gans D, Das L, et al; Technical Expert Panel, & HRSA Autism Intervention Research – Behavioral (AIR‐B) Network. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2), S169-S178.
11. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):5170-526. doi:10.1002/aur.2070
12. Shukla DK, Keehn B, Lincoln AJ, et al. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1269-1278.e12782. doi:10.1016/j.jaac.2010.08.018
13. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi: 10.3389/fnins.2016.00393
14. Zwaigenbaum L, Brian JA, Ip A. Early detection for autism spectrum disorder in young children. Paediatr Child Health. 2019;24(7):424-443. doi:10.1093/pch/pxz119
15. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
16. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
17. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry, 2014;53(2):237-257.
18. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
19. Lipkin PH, Macias MM; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Promoting optimal development: identifying infants and young children with developmental disorders through developmental surveillance and screening. Pediatrics. 2020;145(1)e20193449. doi:10.1542/peds.2019-3449
20. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
21. Rogers SJ, Estes A, Lord C, et al. Effects of a brief early start Denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
22. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
23. Mundy P. A review of joint attention and social-cognitive brain systems in typical development and autism spectrum disorder. Eur J Neurosci. 2018;47(6):497-514.
24. Zwaigenbaum L, Bryson SE, Brian J, et al. Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Res. 2016;9(7):790-800. doi:10.1002/aur.1585
25. Anderson DK, Liang JW, Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J Child Psychol Psychiatry. 2014;55(5):485-494. doi:10.1111/jcpp.12178
26. Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65(8):946-954. doi:10.1001/archpsyc.65.8.946
27. Van der Donck S, Dzhelyova M, Vettori S, et al. Rapid neural categorization of angry and fearful faces is specifically impaired in boys with autism spectrum disorder. J Child Psychol Psychiatry. 2020;61(9):1019-1029. doi:10.1111/jcpp.13201
28. Thurm A, Farmer C, Salzman E, et al. State of the field: differentiating intellectual disability from autism spectrum disorder. Front Psychiatry. 2019;10:526. doi:10.3389/fpsyt.2019.00526
29. Kuno-Fujita A, Iwabuchi T, Wakusawa K, et al. Sensory processing patterns and fusiform activity during face processing in autism spectrum disorder. Autism Res. 2020;13(5):741-750. doi: 10.1002/aur.2283
30. Abrams DA, Lynch CJ, Cheng KM, et al. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci U S A. 2013;110(29):12060-12065. doi:10.1073/pnas.1302982110
31. Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24(3):247-257.
32. Zampella CJ, Csumitta KD, Simon E, et al. Interactional synchrony and its association with social and communication ability in children with and without autism spectrum disorder. J Autism Dev Disord. 2020;50(9):3195-3206. doi:10.1007/s10803-020-04412-8
33. McFayden T, Jarrett MA, White SW, et al. Sluggish cognitive tempo in autism spectrum disorder, ADHD, and their comorbidity: implications for impairment. J Clin Child Adolesc Psychol. 2020:1-8. doi:10.1080/15374416.2020.1716365
34. Baribeau DA, Vigod S, Pullenayegum E, et al. Repetitive behavior severity as an early indicator of risk for elevated anxiety symptoms in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2020;59(7):890-899.e3. doi:10.1016/j.jaac.2019.08.478
35. Li Y, Zhou Z, Chang C, et al. Anomalies in uncinate fasciculus development and social defects in preschoolers with autism spectrum disorder. BMC Psychiatry. 2019;19(1):399. doi:10.1186/s12888-019-2391-1
36. Payabvash S, Palacios EM, Owen JP, et al. White matter connectome edge density in children with autism spectrum disorders: potential imaging biomarkers using machine-learning models. Brain Connect. 2019;9(2):209-220. doi:10.1089/brain.2018.0658
37. Andrews DS, Lee JK, Solomon M, et al. A diffusion-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-aged children. J Neurodev Disord. 2019;11(1):32. doi:10.1186/s11689-019-9291-z
38. Chevallier C, Kohls G, Troiani V, et al. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231-239. doi:10.1016/j.tics.2012.02.007
39. Boddaert N, Chabane N, Gervais H, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23(1):364-369. doi:10.1016/j.neuroimage.2004.06.016
40. Lord C, Petkova E, Hus V, et al. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch Gen Psychiatry. 2012;69(3):306-313. doi:10.1001/archgenpsychiatry.2011.148
41. Trillingsgaard A, ØStergaard JR. Autism in Angelman syndrome: an exploration of comorbidity. Autism. 2004;8(2):163-174.
42. Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res. 2009;53(10):852-873. doi:10.1111/j.1365-2788.2009.01197.x
43. McDuffie A, Thurman AJ, Hagerman RJ, et al. Symptoms of autism in males with Fragile X syndrome: a comparison to nonsyndromic ASD using current ADI-R scores. J Autism Dev Disord. 2015;45(7):1925-1937. doi:10.1007/s10803-013-2013-6
44. Ashwood KL, Tye C, Azadi B, et al. Brief report: adaptive functioning in children with ASD, ADHD and ASD + ADHD. J Autism Dev Disord. 2015;45(7):2235-4222. doi:10.1007/s10803-014-2352-y
45. Guthrie W, Wallis K, Bennett A, et al. Accuracy of autism screening in a large pediatric network. Pediatrics. 2019;144(4): e20183963. doi:10.1542/peds.2018-3963
46. Brian JA, Zwaigenbaum L, Ip A. Standards of diagnostic assessment for autism spectrum disorder. Paediatr Child Health. 2019;24(7):444-460. doi:10.1093/pch/pxz117
47. Frigaux A, Evrard R, Lighezzolo-Alnot J. ADI-R and ADOS and the differential diagnosis of autism spectrum disorders: interests, limits and openings. Encephale. 2019;45(5):441-448. doi:10.1016/j.encep.2019.07.002
48. Sacrey LR, Zwaigenbaum L, Bryson S, et al. Screening for behavioral signs of autism spectrum disorder in 9-month-old infant siblings. J Autism Dev Disord. 2021;51(3):839-848. doi:10.1007/s10803-020-04371-0
49. Sacrey LR, Bryson S, Zwaigenbaum L, et al. The autism parent screen for infants: predicting risk of autism spectrum disorder based on parent-reported behavior observed at 6-24 months of age. Autism. 2018;22(3):322-334
Assessing imminent suicide risk: What about future planning?
A patient who has the ability to plan for their future can be reassuring for a clinician who is conducting an imminent suicide risk evaluation. However, that patient may report future plans even as they are contemplating suicide. Therefore, this variable should not be simplified categorically to the mere presence or absence of future plans. Such plans, and the process by which they are produced, should be examined more closely. In this article, we explore the relationship between a patient’s intent to die by suicide in the near future and their ability to maintain future planning. We also use case examples to highlight certain characteristics that may allow future planning to be integrated more reliably into the assessment of imminent risk of suicide.
An inherent challenge
Suicide risk assessment can be challenging due to the numerous factors that can contribute to a patient’s suicidal intent.1 Some individuals don’t seek help when they develop suicidal thoughts, and even among those who do, recognizing who may be at greater risk is not an easy task. Sometimes, this leads to inadequate interventions and a subsequent failure to ensure safety, or to an overreaction and unnecessary hospitalization.
A common difficulty is a patient’s unwillingness to cooperate with the examination.2 Some patients do not present voluntarily, while others may seek help but then conceal suicidal intent. In a sample of 66 psychotherapy patients who reported concealing suicidal ideation from their therapist and provided short essay responses explaining their motives for doing so, approximately 70% said fear of involuntary hospitalization was their motive to hide those thoughts from their doctor.3 Other reasons for concealment are shame, stigma, embarrassment, fear of rejection, and loss of autonomy.3-5 Moreover, higher levels of suicidal ideation are associated with treatment avoidance.6 Therefore, it is important to improve suicide predictability independent of the patient report. In a survey of 1,150 emergency physicians in Australasia, Canada, the United Kingdom, and the United States, the need for evidence-based guidelines on when to hospitalize a patient at risk for suicide was ranked as the 7th-highest priority.7 There are limitations to using suicide risk assessment scales,8,9 because scales designed to have high sensitivity are less specific, and those with high specificity fail to identify individuals at high risk.9,10 Most of the research conducted in this area has focused on the risk of suicide in 2 to 6 months, and not on imminent risk.11
What is ‘imminent’ risk?
There is no specific time definition for “imminent risk,” but the Lifeline Standards, Trainings, and Practices Subcommittee, a group of national and international experts in suicide prevention, defines imminent risk of suicide as the belief that there is a “close temporal connection between the person’s current risk status and actions that could lead to his/her suicide.”12 Practically, suicide could be considered imminent when it occurs within a few days of the evaluation. However, suicide may take place within a few days of an evaluation due to new life events or impulsive actions, which may explain why imminent risk of suicide can be difficult to define and predict. In clinical practice, there is little evidence-based knowledge about estimating imminent risk. Recent studies have explored certain aspects of a patient’s history in the attempt to improve imminent risk predictability.13 In light of the complexity of this matter and the lack of widely validated tools, clinicians are encouraged to share their experience with other clinicians while the efforts to advance evidence-based knowledge and tools continue.
The function of future planning
Future planning is a mental process embedded in several crucial executive functions. It operates on a daily basis to organize, prioritize, and carry out tasks to achieve day-to-day and more distant future goals. Some research has found that a decreased ability to generate positive future thoughts is linked to increased suicide risk in the long term.14-17 Positive future planning can be affected by even minor fluctuations in mood because the additional processing capacity needed during these mood changes may limit one’s ability to generate positive future thoughts.18 Patients experiencing mood episodes are known to experience cognitive dysfunctions.19-21 However, additional measurable cognitive changes have been detected in patients who are suicidal. For example, in a small study (N = 33) of patients with depression, those who were experiencing suicidal thoughts underperformed on several measures of executive functioning compared to patients with no suicidal ideation.22
However, when addressing imminent rather than future suicide risk, even neutral future plans—such as day-to-day plans or those addressing barriers to treatment—can be a meaningful indicator of the investment in one’s future beyond a potential near-term suicide, and therefore can be explored to further inform the risk evaluation. Significant mental resources can be consumed due to the level of distress associated with contemplating suicide, and therefore patients may have a reduced capacity for day-to-day planning. Thus, serious suicide contemplation is less likely in the presence of typical future planning.
Continue to: Characteristics of future planning...
Characteristics of future planning
Some patients may pretend to engage in future planning to indicate the absence of suicidal intent. This necessitates a more nuanced assessment of future plans beyond whether they exist or not by examining the genuineness of such plans, and the authenticity of the process by which they are produced. The Table lists 3 characteristics of future plans/future planning that, based on our clinical experience, can be helpful to evaluate during an imminent suicide risk evaluation. These are described in the following case examples.
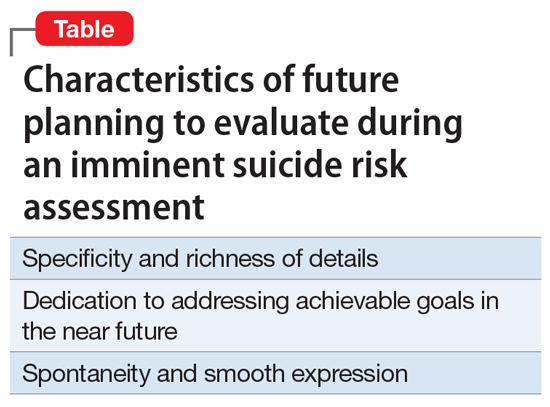
Specificity and richness of details
CASE 1
Mr. A, a college student, presents to the emergency department (ED) complaining of depression and suicidal thoughts that he is able to dismiss. He would like to avoid starting a medication because he has finals in 2 weeks and is worried about adverse effects. He learned about cognitive-behavioral therapy and is interested in getting a referral to a specific office because it is located within a walking distance from campus and easy for him to access because he does not own a car.
The volume of details expressed in a patient’s future plans is important. The more detailed these plans are, the more likely the patient is invested in them. Attendance to the details, especially when addressing expected barriers to treatment, such as transportation, can be evidence of genuine future planning and subsequently of low imminent suicide risk. Spreng et al23 found that autobiographic plans that are more specific and richer in detail recruit additional brain regions that are not recruited in plans that are sparsely detailed or constructed from more generalized representations.
CASE 2
An ambulance transports Ms. B, age 42, from a primary care clinic to the ED because she has been having suicidal thoughts, with a plan to hang herself, for the past 2 days. During the evaluation, Ms. B denies having further suicidal thoughts and declines inpatient admission. She claims that she cannot be away from her children because she is their primary caretaker. Collateral information reveals that Ms. B’s mother has been caring for her children for the last 2 weeks because Ms. B has been too depressed to do so. She continues to refuse admission and is in tears while trying to explain how her absence due to inpatient treatment will be detrimental to her children. Eventually, she angrily accuses the clinician of abusing her children by forcing her to be hospitalized.
In an effort to conceal suicidal intent, patients may present obligations or excuses that would be an obstacle to psychiatric hospitalization. This might give a false perception of intact future planning. However, in these cases, patients often fail to volunteer details about their future plans or show evidence for actual attendance to their obligations. Due to the lack of tangible details to explain the negative effects of inpatient treatment, patients may compensate by using an exaggerated emotional response, with a strong emotional attachment to the obligation and severe distress over their potential inability to fulfill it due to a psychiatric hospitalization. This may contribute to concealing suicidal intent in a different way. A patient may be distressed by the prospect of losing their autonomy or ability to attempt suicide if hospitalized, and they may employ a false excuse as a substitute for the actual reason underlying their distress. A clinician may be falsely reassured if they do not accurately perceive the true cause of the emotional distress. Upon deeper exploration, the expressed emotional attachment is often found to be superficial and has little substantive support.
Continue to: Dedication to addressing acheivable goals in the near future...
Dedication to addressing achievable goals in the near future
CASE 3
Ms. C, age 15, survived a suicide attempt via a medication overdose. She says that she regrets what she did and is not planning to attempt suicide again. Ms. C says she no longer wants to die because in the future she wants to help people by becoming a nurse. She adds that there is a lot waiting for her because she wants to travel all over the world.
Ms. D, age 15, also survived a suicide attempt via a medication overdose. She also says that she regrets what she did and is not planning to attempt suicide again. Ms. D asks whether the physician would be willing to contact the school on her behalf to explain why she had to miss class and to ask for accommodations at school to assist with her panic attacks.
Future planning that involves a patient generating new plans to address current circumstances or the near future may be more reliable than future planning in which a patient repeats their previously constructed plans for the distant future. Eliciting more distant plans, such as a career or family-oriented decisions, indicates the ability to access these “memorized” plans rather than the ability to generate future plans.
Plans that address the distant future, such as those expressed by Ms. C, may have stronger neurologic imprints as a result of repeated memorization and modifications over the years, which may allow a patient to access these plans even while under the stress associated with suicidal thinking. On the other hand, plans that address the near future, such as those expressed by Ms. D, are likely generated in response to current circumstances, which indicates the presence of adequate mental capacity to attend to the current situation, and hence, less preoccupation with suicidal thinking. There might be a neurologic basis for this: some evidence suggests that executive frontoparietal control is recruited in achievable, near-future planning, whereas abstract, difficult-to-achieve, more distant planning fails to engage these additional brain regions.23,24
Spontaneity and smooth expression
CASE 4
Mr. E, age 48, reassures his psychiatrist that he has no intent to act on his suicidal thoughts. When he is offered treatment options, he explains that he would like to start pharmacologic treatment because he only has a few weeks left before he relocates for a new job. The clinician discusses starting a specific medication, and Mr. E expresses interest unless the medication will interfere with his future position as a machine operator. Later, he declines social work assistance to establish care in his new location, preferring to first get the new health care insurance.
A smooth and noncalculated flow of future plans in a patient’s speech allows their plans to be more believable. Plans that naturally flow in response to a verbal exchange and without direct inquiry from the clinician are less likely to be confabulated. This leaves clinicians with the burden of improving the skill of subtly eliciting a patient’s future plans while avoiding directly asking about them. Directly inquiring about such plans may easily tip off the patient that their future planning is under investigation, which may result in misleading responses.
Although future plans that are expressed abruptly, without introductory verbal exchange, or are explicitly linked to why the patient doesn’t intend to kill themselves, can be genuine, the clinician may need to be skeptical about their significance during the risk evaluation. While facing such challenges, clinicians could attempt to shift the patient’s attention away from a safety and disposition-focused conversation toward a less goal-directed verbal exchange during which other opportunities for smooth expression of future plans may emerge. For example, if a patient suddenly discusses how much they care about X in attempt to emphasize why they are not contemplating suicide, the clinician may respond by gently asking the patient to talk more about X.
Continue to: Adopt a more nuanced approach...
Adopt a more nuanced approach
Assessment of the imminent risk of suicide is complicated and not well researched. A patient’s future planning can be used to better inform the evaluation. A patient may have a limited ability to generate future plans while contemplating suicide. Future plans that are specific, rich in details, achievable, dedicated to addressing the near future, and expressed smoothly and in a noncalculated fashion may be more reliable than other types of plans. The process of future planning may indicate low imminent suicide risk when it leads the patient to generate new plans to address current circumstances or the near future. When evaluating a patient’s imminent suicide risk, clinicians should consider abandoning a binary “is there future planning or not” approach and adopting a more complex, nuanced understanding to appropriately utilize this important factor in the risk assessment.
Bottom Line
A patient’s ability to plan for the future should be explored during an assessment of imminent suicide risk. Future plans that are specific, rich in details, achievable, dedicated to addressing the near future, and expressed smoothly and in a noncalculated fashion may be more reliable than other types of plans.
1. Gilbert AM, Garno JL, Braga RJ, et al. Clinical and cognitive correlates of suicide attempts in bipolar disorder: is suicide predictable? J Clin Psychiatry. 2011;72(8):1027-1033.
2. Obegi JH. Your patient refuses a suicide risk assessment. Now what? Current Psychiatry. 2021;20(4):45.
3. Blanchard M, Farber BA. “It is never okay to talk about suicide”: Patients’ reasons for concealing suicidal ideation in psychotherapy. Psychother Res. 2020;30(1):124-136.
4. Richards JE, Whiteside U, Ludman EJ, et al. Understanding why patients may not report suicidal ideation at a health care visit prior to a suicide attempt: a qualitative study. Psychiatr Serv. 2019;70(1):40-45.
5. Fulginiti A, Frey LM. Exploring suicide-related disclosure motivation and the impact on mechanisms linked to suicide. Death Stud. 2019;43(9):562-569.
6. Wilson CJ, Deane FP, Marshall KL, et al. Adolescents’ suicidal thinking and reluctance to consult general medical practitioners. J Youth Adolesc. 2010;39(4):343-356.
7. Eagles D, Stiell IG, Clement CM, et al. International survey of emergency physicians’ priorities for clinical decision rules. Acad Emerg Med. 2008;15(2):177-182.
8. Swedish Council on Health Technology Assessment (SBU): SBU Systematic Review Summaries. Instruments for Suicide Risk Assessment. Summary and Conclusions. SBU Yellow Report No. 242. 2015. Accessed January 6, 2021. https://www.ncbi.nlm.nih.gov/books/NBK350492/
9. Runeson B, Odeberg J, Pettersson A, et al. Instruments for the assessment of suicide risk: a systematic review evaluating the certainty of the evidence. PLoS One. 2017;12(7):e0180292. doi:10.1371/journal.pone.0180292
10. Steeg S, Quinlivan L, Nowland R, et al. Accuracy of risk scales for predicting repeat self-harm and suicide: a multicentre, population-level cohort study using routine clinical data. BMC Psychiatry. 2018;18(1):113.
11. Nock MK, Banaji MR. Prediction of suicide ideation and attempts among adolescents using a brief performance-based test. J Consult Clin Psychol. 2007;75(5):707-715.
12. Draper J, Murphy G, Vega E, et al. Helping callers to the National Suicide Prevention Lifeline who are at imminent risk of suicide: the importance of active engagement, active rescue, and collaboration between crisis and emergency services. Suicide Life Threat Behav. 2015;45(3):261-270.
13. Glenn CR, Nock MK. Improving the short-term prediction of suicidal behavior. Am J Prev Med. 2014;47(3 Suppl 2):S176-S180.
14. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
15. MacLeod AK, Tata P, Evans K, et al. Recovery of positive future thinking within a high-risk parasuicide group: results from a pilot randomized controlled trial. Br J Clin Psychol. 1998;37(4):371-379.
16. MacLeod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2005;44(Pt 4):495-504.
17. O’Connor RC, Smyth R, Williams JM. Intrapersonal positive future thinking predicts repeat suicide attempts in hospital-treated suicide attempters. J Consult Clin Psychol. 2015;83(1):169-176.
18. O’Connor RC, Williams JMG. The relationship between positive future thinking, brooding, defeat and entrapment. Personality and Individual Differences. 2014;70:29-34.
19. Castaneda AE, Tuulio-Henriksson A, Marttunen M, et al. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008;106(1-2):1-27.
20. Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200-206.
21. Buoli M, Caldiroli A, Caletti E, et al. The impact of mood episodes and duration of illness on cognition in bipolar disorder. Compr Psychiatry. 2014;55(7):1561-1566.
22. Marzuk PM, Hartwell N, Leon AC, et al. Executive functioning in depressed patients with suicidal ideation. Acta Psychiatr Scand. 2005;112(4):294-301.
23. Spreng RN, Gerlach KD, Turner GR, et al. Autobiographical planning and the brain: activation and its modulation by qualitative features. J Cogn Neurosci. 2015;27(11):2147-2157.
24. Spreng RN, Sepulcre J, Turner GR, et al. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25(1):74-86.
A patient who has the ability to plan for their future can be reassuring for a clinician who is conducting an imminent suicide risk evaluation. However, that patient may report future plans even as they are contemplating suicide. Therefore, this variable should not be simplified categorically to the mere presence or absence of future plans. Such plans, and the process by which they are produced, should be examined more closely. In this article, we explore the relationship between a patient’s intent to die by suicide in the near future and their ability to maintain future planning. We also use case examples to highlight certain characteristics that may allow future planning to be integrated more reliably into the assessment of imminent risk of suicide.
An inherent challenge
Suicide risk assessment can be challenging due to the numerous factors that can contribute to a patient’s suicidal intent.1 Some individuals don’t seek help when they develop suicidal thoughts, and even among those who do, recognizing who may be at greater risk is not an easy task. Sometimes, this leads to inadequate interventions and a subsequent failure to ensure safety, or to an overreaction and unnecessary hospitalization.
A common difficulty is a patient’s unwillingness to cooperate with the examination.2 Some patients do not present voluntarily, while others may seek help but then conceal suicidal intent. In a sample of 66 psychotherapy patients who reported concealing suicidal ideation from their therapist and provided short essay responses explaining their motives for doing so, approximately 70% said fear of involuntary hospitalization was their motive to hide those thoughts from their doctor.3 Other reasons for concealment are shame, stigma, embarrassment, fear of rejection, and loss of autonomy.3-5 Moreover, higher levels of suicidal ideation are associated with treatment avoidance.6 Therefore, it is important to improve suicide predictability independent of the patient report. In a survey of 1,150 emergency physicians in Australasia, Canada, the United Kingdom, and the United States, the need for evidence-based guidelines on when to hospitalize a patient at risk for suicide was ranked as the 7th-highest priority.7 There are limitations to using suicide risk assessment scales,8,9 because scales designed to have high sensitivity are less specific, and those with high specificity fail to identify individuals at high risk.9,10 Most of the research conducted in this area has focused on the risk of suicide in 2 to 6 months, and not on imminent risk.11
What is ‘imminent’ risk?
There is no specific time definition for “imminent risk,” but the Lifeline Standards, Trainings, and Practices Subcommittee, a group of national and international experts in suicide prevention, defines imminent risk of suicide as the belief that there is a “close temporal connection between the person’s current risk status and actions that could lead to his/her suicide.”12 Practically, suicide could be considered imminent when it occurs within a few days of the evaluation. However, suicide may take place within a few days of an evaluation due to new life events or impulsive actions, which may explain why imminent risk of suicide can be difficult to define and predict. In clinical practice, there is little evidence-based knowledge about estimating imminent risk. Recent studies have explored certain aspects of a patient’s history in the attempt to improve imminent risk predictability.13 In light of the complexity of this matter and the lack of widely validated tools, clinicians are encouraged to share their experience with other clinicians while the efforts to advance evidence-based knowledge and tools continue.
The function of future planning
Future planning is a mental process embedded in several crucial executive functions. It operates on a daily basis to organize, prioritize, and carry out tasks to achieve day-to-day and more distant future goals. Some research has found that a decreased ability to generate positive future thoughts is linked to increased suicide risk in the long term.14-17 Positive future planning can be affected by even minor fluctuations in mood because the additional processing capacity needed during these mood changes may limit one’s ability to generate positive future thoughts.18 Patients experiencing mood episodes are known to experience cognitive dysfunctions.19-21 However, additional measurable cognitive changes have been detected in patients who are suicidal. For example, in a small study (N = 33) of patients with depression, those who were experiencing suicidal thoughts underperformed on several measures of executive functioning compared to patients with no suicidal ideation.22
However, when addressing imminent rather than future suicide risk, even neutral future plans—such as day-to-day plans or those addressing barriers to treatment—can be a meaningful indicator of the investment in one’s future beyond a potential near-term suicide, and therefore can be explored to further inform the risk evaluation. Significant mental resources can be consumed due to the level of distress associated with contemplating suicide, and therefore patients may have a reduced capacity for day-to-day planning. Thus, serious suicide contemplation is less likely in the presence of typical future planning.
Continue to: Characteristics of future planning...
Characteristics of future planning
Some patients may pretend to engage in future planning to indicate the absence of suicidal intent. This necessitates a more nuanced assessment of future plans beyond whether they exist or not by examining the genuineness of such plans, and the authenticity of the process by which they are produced. The Table lists 3 characteristics of future plans/future planning that, based on our clinical experience, can be helpful to evaluate during an imminent suicide risk evaluation. These are described in the following case examples.

Specificity and richness of details
CASE 1
Mr. A, a college student, presents to the emergency department (ED) complaining of depression and suicidal thoughts that he is able to dismiss. He would like to avoid starting a medication because he has finals in 2 weeks and is worried about adverse effects. He learned about cognitive-behavioral therapy and is interested in getting a referral to a specific office because it is located within a walking distance from campus and easy for him to access because he does not own a car.
The volume of details expressed in a patient’s future plans is important. The more detailed these plans are, the more likely the patient is invested in them. Attendance to the details, especially when addressing expected barriers to treatment, such as transportation, can be evidence of genuine future planning and subsequently of low imminent suicide risk. Spreng et al23 found that autobiographic plans that are more specific and richer in detail recruit additional brain regions that are not recruited in plans that are sparsely detailed or constructed from more generalized representations.
CASE 2
An ambulance transports Ms. B, age 42, from a primary care clinic to the ED because she has been having suicidal thoughts, with a plan to hang herself, for the past 2 days. During the evaluation, Ms. B denies having further suicidal thoughts and declines inpatient admission. She claims that she cannot be away from her children because she is their primary caretaker. Collateral information reveals that Ms. B’s mother has been caring for her children for the last 2 weeks because Ms. B has been too depressed to do so. She continues to refuse admission and is in tears while trying to explain how her absence due to inpatient treatment will be detrimental to her children. Eventually, she angrily accuses the clinician of abusing her children by forcing her to be hospitalized.
In an effort to conceal suicidal intent, patients may present obligations or excuses that would be an obstacle to psychiatric hospitalization. This might give a false perception of intact future planning. However, in these cases, patients often fail to volunteer details about their future plans or show evidence for actual attendance to their obligations. Due to the lack of tangible details to explain the negative effects of inpatient treatment, patients may compensate by using an exaggerated emotional response, with a strong emotional attachment to the obligation and severe distress over their potential inability to fulfill it due to a psychiatric hospitalization. This may contribute to concealing suicidal intent in a different way. A patient may be distressed by the prospect of losing their autonomy or ability to attempt suicide if hospitalized, and they may employ a false excuse as a substitute for the actual reason underlying their distress. A clinician may be falsely reassured if they do not accurately perceive the true cause of the emotional distress. Upon deeper exploration, the expressed emotional attachment is often found to be superficial and has little substantive support.
Continue to: Dedication to addressing acheivable goals in the near future...
Dedication to addressing achievable goals in the near future
CASE 3
Ms. C, age 15, survived a suicide attempt via a medication overdose. She says that she regrets what she did and is not planning to attempt suicide again. Ms. C says she no longer wants to die because in the future she wants to help people by becoming a nurse. She adds that there is a lot waiting for her because she wants to travel all over the world.
Ms. D, age 15, also survived a suicide attempt via a medication overdose. She also says that she regrets what she did and is not planning to attempt suicide again. Ms. D asks whether the physician would be willing to contact the school on her behalf to explain why she had to miss class and to ask for accommodations at school to assist with her panic attacks.
Future planning that involves a patient generating new plans to address current circumstances or the near future may be more reliable than future planning in which a patient repeats their previously constructed plans for the distant future. Eliciting more distant plans, such as a career or family-oriented decisions, indicates the ability to access these “memorized” plans rather than the ability to generate future plans.
Plans that address the distant future, such as those expressed by Ms. C, may have stronger neurologic imprints as a result of repeated memorization and modifications over the years, which may allow a patient to access these plans even while under the stress associated with suicidal thinking. On the other hand, plans that address the near future, such as those expressed by Ms. D, are likely generated in response to current circumstances, which indicates the presence of adequate mental capacity to attend to the current situation, and hence, less preoccupation with suicidal thinking. There might be a neurologic basis for this: some evidence suggests that executive frontoparietal control is recruited in achievable, near-future planning, whereas abstract, difficult-to-achieve, more distant planning fails to engage these additional brain regions.23,24
Spontaneity and smooth expression
CASE 4
Mr. E, age 48, reassures his psychiatrist that he has no intent to act on his suicidal thoughts. When he is offered treatment options, he explains that he would like to start pharmacologic treatment because he only has a few weeks left before he relocates for a new job. The clinician discusses starting a specific medication, and Mr. E expresses interest unless the medication will interfere with his future position as a machine operator. Later, he declines social work assistance to establish care in his new location, preferring to first get the new health care insurance.
A smooth and noncalculated flow of future plans in a patient’s speech allows their plans to be more believable. Plans that naturally flow in response to a verbal exchange and without direct inquiry from the clinician are less likely to be confabulated. This leaves clinicians with the burden of improving the skill of subtly eliciting a patient’s future plans while avoiding directly asking about them. Directly inquiring about such plans may easily tip off the patient that their future planning is under investigation, which may result in misleading responses.
Although future plans that are expressed abruptly, without introductory verbal exchange, or are explicitly linked to why the patient doesn’t intend to kill themselves, can be genuine, the clinician may need to be skeptical about their significance during the risk evaluation. While facing such challenges, clinicians could attempt to shift the patient’s attention away from a safety and disposition-focused conversation toward a less goal-directed verbal exchange during which other opportunities for smooth expression of future plans may emerge. For example, if a patient suddenly discusses how much they care about X in attempt to emphasize why they are not contemplating suicide, the clinician may respond by gently asking the patient to talk more about X.
Continue to: Adopt a more nuanced approach...
Adopt a more nuanced approach
Assessment of the imminent risk of suicide is complicated and not well researched. A patient’s future planning can be used to better inform the evaluation. A patient may have a limited ability to generate future plans while contemplating suicide. Future plans that are specific, rich in details, achievable, dedicated to addressing the near future, and expressed smoothly and in a noncalculated fashion may be more reliable than other types of plans. The process of future planning may indicate low imminent suicide risk when it leads the patient to generate new plans to address current circumstances or the near future. When evaluating a patient’s imminent suicide risk, clinicians should consider abandoning a binary “is there future planning or not” approach and adopting a more complex, nuanced understanding to appropriately utilize this important factor in the risk assessment.
Bottom Line
A patient’s ability to plan for the future should be explored during an assessment of imminent suicide risk. Future plans that are specific, rich in details, achievable, dedicated to addressing the near future, and expressed smoothly and in a noncalculated fashion may be more reliable than other types of plans.
A patient who has the ability to plan for their future can be reassuring for a clinician who is conducting an imminent suicide risk evaluation. However, that patient may report future plans even as they are contemplating suicide. Therefore, this variable should not be simplified categorically to the mere presence or absence of future plans. Such plans, and the process by which they are produced, should be examined more closely. In this article, we explore the relationship between a patient’s intent to die by suicide in the near future and their ability to maintain future planning. We also use case examples to highlight certain characteristics that may allow future planning to be integrated more reliably into the assessment of imminent risk of suicide.
An inherent challenge
Suicide risk assessment can be challenging due to the numerous factors that can contribute to a patient’s suicidal intent.1 Some individuals don’t seek help when they develop suicidal thoughts, and even among those who do, recognizing who may be at greater risk is not an easy task. Sometimes, this leads to inadequate interventions and a subsequent failure to ensure safety, or to an overreaction and unnecessary hospitalization.
A common difficulty is a patient’s unwillingness to cooperate with the examination.2 Some patients do not present voluntarily, while others may seek help but then conceal suicidal intent. In a sample of 66 psychotherapy patients who reported concealing suicidal ideation from their therapist and provided short essay responses explaining their motives for doing so, approximately 70% said fear of involuntary hospitalization was their motive to hide those thoughts from their doctor.3 Other reasons for concealment are shame, stigma, embarrassment, fear of rejection, and loss of autonomy.3-5 Moreover, higher levels of suicidal ideation are associated with treatment avoidance.6 Therefore, it is important to improve suicide predictability independent of the patient report. In a survey of 1,150 emergency physicians in Australasia, Canada, the United Kingdom, and the United States, the need for evidence-based guidelines on when to hospitalize a patient at risk for suicide was ranked as the 7th-highest priority.7 There are limitations to using suicide risk assessment scales,8,9 because scales designed to have high sensitivity are less specific, and those with high specificity fail to identify individuals at high risk.9,10 Most of the research conducted in this area has focused on the risk of suicide in 2 to 6 months, and not on imminent risk.11
What is ‘imminent’ risk?
There is no specific time definition for “imminent risk,” but the Lifeline Standards, Trainings, and Practices Subcommittee, a group of national and international experts in suicide prevention, defines imminent risk of suicide as the belief that there is a “close temporal connection between the person’s current risk status and actions that could lead to his/her suicide.”12 Practically, suicide could be considered imminent when it occurs within a few days of the evaluation. However, suicide may take place within a few days of an evaluation due to new life events or impulsive actions, which may explain why imminent risk of suicide can be difficult to define and predict. In clinical practice, there is little evidence-based knowledge about estimating imminent risk. Recent studies have explored certain aspects of a patient’s history in the attempt to improve imminent risk predictability.13 In light of the complexity of this matter and the lack of widely validated tools, clinicians are encouraged to share their experience with other clinicians while the efforts to advance evidence-based knowledge and tools continue.
The function of future planning
Future planning is a mental process embedded in several crucial executive functions. It operates on a daily basis to organize, prioritize, and carry out tasks to achieve day-to-day and more distant future goals. Some research has found that a decreased ability to generate positive future thoughts is linked to increased suicide risk in the long term.14-17 Positive future planning can be affected by even minor fluctuations in mood because the additional processing capacity needed during these mood changes may limit one’s ability to generate positive future thoughts.18 Patients experiencing mood episodes are known to experience cognitive dysfunctions.19-21 However, additional measurable cognitive changes have been detected in patients who are suicidal. For example, in a small study (N = 33) of patients with depression, those who were experiencing suicidal thoughts underperformed on several measures of executive functioning compared to patients with no suicidal ideation.22
However, when addressing imminent rather than future suicide risk, even neutral future plans—such as day-to-day plans or those addressing barriers to treatment—can be a meaningful indicator of the investment in one’s future beyond a potential near-term suicide, and therefore can be explored to further inform the risk evaluation. Significant mental resources can be consumed due to the level of distress associated with contemplating suicide, and therefore patients may have a reduced capacity for day-to-day planning. Thus, serious suicide contemplation is less likely in the presence of typical future planning.
Continue to: Characteristics of future planning...
Characteristics of future planning
Some patients may pretend to engage in future planning to indicate the absence of suicidal intent. This necessitates a more nuanced assessment of future plans beyond whether they exist or not by examining the genuineness of such plans, and the authenticity of the process by which they are produced. The Table lists 3 characteristics of future plans/future planning that, based on our clinical experience, can be helpful to evaluate during an imminent suicide risk evaluation. These are described in the following case examples.

Specificity and richness of details
CASE 1
Mr. A, a college student, presents to the emergency department (ED) complaining of depression and suicidal thoughts that he is able to dismiss. He would like to avoid starting a medication because he has finals in 2 weeks and is worried about adverse effects. He learned about cognitive-behavioral therapy and is interested in getting a referral to a specific office because it is located within a walking distance from campus and easy for him to access because he does not own a car.
The volume of details expressed in a patient’s future plans is important. The more detailed these plans are, the more likely the patient is invested in them. Attendance to the details, especially when addressing expected barriers to treatment, such as transportation, can be evidence of genuine future planning and subsequently of low imminent suicide risk. Spreng et al23 found that autobiographic plans that are more specific and richer in detail recruit additional brain regions that are not recruited in plans that are sparsely detailed or constructed from more generalized representations.
CASE 2
An ambulance transports Ms. B, age 42, from a primary care clinic to the ED because she has been having suicidal thoughts, with a plan to hang herself, for the past 2 days. During the evaluation, Ms. B denies having further suicidal thoughts and declines inpatient admission. She claims that she cannot be away from her children because she is their primary caretaker. Collateral information reveals that Ms. B’s mother has been caring for her children for the last 2 weeks because Ms. B has been too depressed to do so. She continues to refuse admission and is in tears while trying to explain how her absence due to inpatient treatment will be detrimental to her children. Eventually, she angrily accuses the clinician of abusing her children by forcing her to be hospitalized.
In an effort to conceal suicidal intent, patients may present obligations or excuses that would be an obstacle to psychiatric hospitalization. This might give a false perception of intact future planning. However, in these cases, patients often fail to volunteer details about their future plans or show evidence for actual attendance to their obligations. Due to the lack of tangible details to explain the negative effects of inpatient treatment, patients may compensate by using an exaggerated emotional response, with a strong emotional attachment to the obligation and severe distress over their potential inability to fulfill it due to a psychiatric hospitalization. This may contribute to concealing suicidal intent in a different way. A patient may be distressed by the prospect of losing their autonomy or ability to attempt suicide if hospitalized, and they may employ a false excuse as a substitute for the actual reason underlying their distress. A clinician may be falsely reassured if they do not accurately perceive the true cause of the emotional distress. Upon deeper exploration, the expressed emotional attachment is often found to be superficial and has little substantive support.
Continue to: Dedication to addressing acheivable goals in the near future...
Dedication to addressing achievable goals in the near future
CASE 3
Ms. C, age 15, survived a suicide attempt via a medication overdose. She says that she regrets what she did and is not planning to attempt suicide again. Ms. C says she no longer wants to die because in the future she wants to help people by becoming a nurse. She adds that there is a lot waiting for her because she wants to travel all over the world.
Ms. D, age 15, also survived a suicide attempt via a medication overdose. She also says that she regrets what she did and is not planning to attempt suicide again. Ms. D asks whether the physician would be willing to contact the school on her behalf to explain why she had to miss class and to ask for accommodations at school to assist with her panic attacks.
Future planning that involves a patient generating new plans to address current circumstances or the near future may be more reliable than future planning in which a patient repeats their previously constructed plans for the distant future. Eliciting more distant plans, such as a career or family-oriented decisions, indicates the ability to access these “memorized” plans rather than the ability to generate future plans.
Plans that address the distant future, such as those expressed by Ms. C, may have stronger neurologic imprints as a result of repeated memorization and modifications over the years, which may allow a patient to access these plans even while under the stress associated with suicidal thinking. On the other hand, plans that address the near future, such as those expressed by Ms. D, are likely generated in response to current circumstances, which indicates the presence of adequate mental capacity to attend to the current situation, and hence, less preoccupation with suicidal thinking. There might be a neurologic basis for this: some evidence suggests that executive frontoparietal control is recruited in achievable, near-future planning, whereas abstract, difficult-to-achieve, more distant planning fails to engage these additional brain regions.23,24
Spontaneity and smooth expression
CASE 4
Mr. E, age 48, reassures his psychiatrist that he has no intent to act on his suicidal thoughts. When he is offered treatment options, he explains that he would like to start pharmacologic treatment because he only has a few weeks left before he relocates for a new job. The clinician discusses starting a specific medication, and Mr. E expresses interest unless the medication will interfere with his future position as a machine operator. Later, he declines social work assistance to establish care in his new location, preferring to first get the new health care insurance.
A smooth and noncalculated flow of future plans in a patient’s speech allows their plans to be more believable. Plans that naturally flow in response to a verbal exchange and without direct inquiry from the clinician are less likely to be confabulated. This leaves clinicians with the burden of improving the skill of subtly eliciting a patient’s future plans while avoiding directly asking about them. Directly inquiring about such plans may easily tip off the patient that their future planning is under investigation, which may result in misleading responses.
Although future plans that are expressed abruptly, without introductory verbal exchange, or are explicitly linked to why the patient doesn’t intend to kill themselves, can be genuine, the clinician may need to be skeptical about their significance during the risk evaluation. While facing such challenges, clinicians could attempt to shift the patient’s attention away from a safety and disposition-focused conversation toward a less goal-directed verbal exchange during which other opportunities for smooth expression of future plans may emerge. For example, if a patient suddenly discusses how much they care about X in attempt to emphasize why they are not contemplating suicide, the clinician may respond by gently asking the patient to talk more about X.
Continue to: Adopt a more nuanced approach...
Adopt a more nuanced approach
Assessment of the imminent risk of suicide is complicated and not well researched. A patient’s future planning can be used to better inform the evaluation. A patient may have a limited ability to generate future plans while contemplating suicide. Future plans that are specific, rich in details, achievable, dedicated to addressing the near future, and expressed smoothly and in a noncalculated fashion may be more reliable than other types of plans. The process of future planning may indicate low imminent suicide risk when it leads the patient to generate new plans to address current circumstances or the near future. When evaluating a patient’s imminent suicide risk, clinicians should consider abandoning a binary “is there future planning or not” approach and adopting a more complex, nuanced understanding to appropriately utilize this important factor in the risk assessment.
Bottom Line
A patient’s ability to plan for the future should be explored during an assessment of imminent suicide risk. Future plans that are specific, rich in details, achievable, dedicated to addressing the near future, and expressed smoothly and in a noncalculated fashion may be more reliable than other types of plans.
1. Gilbert AM, Garno JL, Braga RJ, et al. Clinical and cognitive correlates of suicide attempts in bipolar disorder: is suicide predictable? J Clin Psychiatry. 2011;72(8):1027-1033.
2. Obegi JH. Your patient refuses a suicide risk assessment. Now what? Current Psychiatry. 2021;20(4):45.
3. Blanchard M, Farber BA. “It is never okay to talk about suicide”: Patients’ reasons for concealing suicidal ideation in psychotherapy. Psychother Res. 2020;30(1):124-136.
4. Richards JE, Whiteside U, Ludman EJ, et al. Understanding why patients may not report suicidal ideation at a health care visit prior to a suicide attempt: a qualitative study. Psychiatr Serv. 2019;70(1):40-45.
5. Fulginiti A, Frey LM. Exploring suicide-related disclosure motivation and the impact on mechanisms linked to suicide. Death Stud. 2019;43(9):562-569.
6. Wilson CJ, Deane FP, Marshall KL, et al. Adolescents’ suicidal thinking and reluctance to consult general medical practitioners. J Youth Adolesc. 2010;39(4):343-356.
7. Eagles D, Stiell IG, Clement CM, et al. International survey of emergency physicians’ priorities for clinical decision rules. Acad Emerg Med. 2008;15(2):177-182.
8. Swedish Council on Health Technology Assessment (SBU): SBU Systematic Review Summaries. Instruments for Suicide Risk Assessment. Summary and Conclusions. SBU Yellow Report No. 242. 2015. Accessed January 6, 2021. https://www.ncbi.nlm.nih.gov/books/NBK350492/
9. Runeson B, Odeberg J, Pettersson A, et al. Instruments for the assessment of suicide risk: a systematic review evaluating the certainty of the evidence. PLoS One. 2017;12(7):e0180292. doi:10.1371/journal.pone.0180292
10. Steeg S, Quinlivan L, Nowland R, et al. Accuracy of risk scales for predicting repeat self-harm and suicide: a multicentre, population-level cohort study using routine clinical data. BMC Psychiatry. 2018;18(1):113.
11. Nock MK, Banaji MR. Prediction of suicide ideation and attempts among adolescents using a brief performance-based test. J Consult Clin Psychol. 2007;75(5):707-715.
12. Draper J, Murphy G, Vega E, et al. Helping callers to the National Suicide Prevention Lifeline who are at imminent risk of suicide: the importance of active engagement, active rescue, and collaboration between crisis and emergency services. Suicide Life Threat Behav. 2015;45(3):261-270.
13. Glenn CR, Nock MK. Improving the short-term prediction of suicidal behavior. Am J Prev Med. 2014;47(3 Suppl 2):S176-S180.
14. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
15. MacLeod AK, Tata P, Evans K, et al. Recovery of positive future thinking within a high-risk parasuicide group: results from a pilot randomized controlled trial. Br J Clin Psychol. 1998;37(4):371-379.
16. MacLeod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2005;44(Pt 4):495-504.
17. O’Connor RC, Smyth R, Williams JM. Intrapersonal positive future thinking predicts repeat suicide attempts in hospital-treated suicide attempters. J Consult Clin Psychol. 2015;83(1):169-176.
18. O’Connor RC, Williams JMG. The relationship between positive future thinking, brooding, defeat and entrapment. Personality and Individual Differences. 2014;70:29-34.
19. Castaneda AE, Tuulio-Henriksson A, Marttunen M, et al. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008;106(1-2):1-27.
20. Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200-206.
21. Buoli M, Caldiroli A, Caletti E, et al. The impact of mood episodes and duration of illness on cognition in bipolar disorder. Compr Psychiatry. 2014;55(7):1561-1566.
22. Marzuk PM, Hartwell N, Leon AC, et al. Executive functioning in depressed patients with suicidal ideation. Acta Psychiatr Scand. 2005;112(4):294-301.
23. Spreng RN, Gerlach KD, Turner GR, et al. Autobiographical planning and the brain: activation and its modulation by qualitative features. J Cogn Neurosci. 2015;27(11):2147-2157.
24. Spreng RN, Sepulcre J, Turner GR, et al. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25(1):74-86.
1. Gilbert AM, Garno JL, Braga RJ, et al. Clinical and cognitive correlates of suicide attempts in bipolar disorder: is suicide predictable? J Clin Psychiatry. 2011;72(8):1027-1033.
2. Obegi JH. Your patient refuses a suicide risk assessment. Now what? Current Psychiatry. 2021;20(4):45.
3. Blanchard M, Farber BA. “It is never okay to talk about suicide”: Patients’ reasons for concealing suicidal ideation in psychotherapy. Psychother Res. 2020;30(1):124-136.
4. Richards JE, Whiteside U, Ludman EJ, et al. Understanding why patients may not report suicidal ideation at a health care visit prior to a suicide attempt: a qualitative study. Psychiatr Serv. 2019;70(1):40-45.
5. Fulginiti A, Frey LM. Exploring suicide-related disclosure motivation and the impact on mechanisms linked to suicide. Death Stud. 2019;43(9):562-569.
6. Wilson CJ, Deane FP, Marshall KL, et al. Adolescents’ suicidal thinking and reluctance to consult general medical practitioners. J Youth Adolesc. 2010;39(4):343-356.
7. Eagles D, Stiell IG, Clement CM, et al. International survey of emergency physicians’ priorities for clinical decision rules. Acad Emerg Med. 2008;15(2):177-182.
8. Swedish Council on Health Technology Assessment (SBU): SBU Systematic Review Summaries. Instruments for Suicide Risk Assessment. Summary and Conclusions. SBU Yellow Report No. 242. 2015. Accessed January 6, 2021. https://www.ncbi.nlm.nih.gov/books/NBK350492/
9. Runeson B, Odeberg J, Pettersson A, et al. Instruments for the assessment of suicide risk: a systematic review evaluating the certainty of the evidence. PLoS One. 2017;12(7):e0180292. doi:10.1371/journal.pone.0180292
10. Steeg S, Quinlivan L, Nowland R, et al. Accuracy of risk scales for predicting repeat self-harm and suicide: a multicentre, population-level cohort study using routine clinical data. BMC Psychiatry. 2018;18(1):113.
11. Nock MK, Banaji MR. Prediction of suicide ideation and attempts among adolescents using a brief performance-based test. J Consult Clin Psychol. 2007;75(5):707-715.
12. Draper J, Murphy G, Vega E, et al. Helping callers to the National Suicide Prevention Lifeline who are at imminent risk of suicide: the importance of active engagement, active rescue, and collaboration between crisis and emergency services. Suicide Life Threat Behav. 2015;45(3):261-270.
13. Glenn CR, Nock MK. Improving the short-term prediction of suicidal behavior. Am J Prev Med. 2014;47(3 Suppl 2):S176-S180.
14. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
15. MacLeod AK, Tata P, Evans K, et al. Recovery of positive future thinking within a high-risk parasuicide group: results from a pilot randomized controlled trial. Br J Clin Psychol. 1998;37(4):371-379.
16. MacLeod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2005;44(Pt 4):495-504.
17. O’Connor RC, Smyth R, Williams JM. Intrapersonal positive future thinking predicts repeat suicide attempts in hospital-treated suicide attempters. J Consult Clin Psychol. 2015;83(1):169-176.
18. O’Connor RC, Williams JMG. The relationship between positive future thinking, brooding, defeat and entrapment. Personality and Individual Differences. 2014;70:29-34.
19. Castaneda AE, Tuulio-Henriksson A, Marttunen M, et al. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008;106(1-2):1-27.
20. Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200-206.
21. Buoli M, Caldiroli A, Caletti E, et al. The impact of mood episodes and duration of illness on cognition in bipolar disorder. Compr Psychiatry. 2014;55(7):1561-1566.
22. Marzuk PM, Hartwell N, Leon AC, et al. Executive functioning in depressed patients with suicidal ideation. Acta Psychiatr Scand. 2005;112(4):294-301.
23. Spreng RN, Gerlach KD, Turner GR, et al. Autobiographical planning and the brain: activation and its modulation by qualitative features. J Cogn Neurosci. 2015;27(11):2147-2157.
24. Spreng RN, Sepulcre J, Turner GR, et al. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25(1):74-86.
Borderline personality disorder: 6 studies of psychosocial interventions
SECOND OF 2 PARTS
Borderline personality disorder (BPD) is associated with serious impairment in psychosocial functioning.1 It is characterized by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior that often results in problems in relationships. As a result, patients with BPD tend to utilize more mental health services than patients with other personality disorders or major depressive disorder.2
Some clinicians believe BPD is difficult to treat. While historically there has been little consensus on the best treatments for this disorder, current options include both pharmacologic and psychological interventions. In Part 1 of this 2-part article, we focused on 6 studies that evaluated biological interventions.3 Here in Part 2, we focus on findings from 6 recent randomized controlled trials (RCTs) of psychosocial interventions for BPD
1. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi: 10.4088/JCP.16m11153
Research has shown that BPD is a treatable illness with a more favorable prognosis than previously believed. Despite this, patients often experience difficulty accessing the most up-to-date information on BPD, which can impede their treatment. A 2008 study by Zanarini et al10 of younger female patients with BPD demonstrated that immediate, in-person psychoeducation improved impulsivity and relationships. Widespread implementation of this program proved problematic, however, due to cost and personnel constraints. To resolve this issue, researchers developed an internet-based version of the program. In a 2018 follow-up study, Zanarini et al4 examined the effect of this internet-based psychoeducation program on symptoms of BPD.
Continue to: Study design...
Study design
- Women (age 18 to 30) who met DSM-IV and Diagnostic Interview for Borderlines–Revised criteria for BPD were randomized to an internet-based psychoeducation treatment group (n = 40) or a control group (n = 40).
- Ten outcomes concerning symptom severity and psychosocial functioning were assessed during weeks 1 to 12 (acute phase) and at months 6, 9, and 12 (maintenance phase) using the self-report version of the Zanarini Rating Scale for BPD (ZAN-BPD), the Borderline Evaluation of Severity over Time, the Sheehan Disability Scale, the Clinically Useful Depression Outcome Scale, the Clinically Useful Anxiety Outcome Scale, and Weissman’s Social Adjustment Scale (SAS).
Outcomes
- In the acute phase, treatment group participants experienced statistically significant improvements in all 10 outcomes. Control group participants demonstrated similar results, achieving statistically significant improvements in 7 of 10 outcomes.
- Compared to the control group, the treatment group experienced a more significant reduction in impulsivity and improvement in psychosocial functioning as measured by the ZAN-BPD and SAS.
- In the maintenance phase, treatment group participants achieved statistically significant improvements in 9 of 10 outcomes, whereas control group participants demonstrated statistically significant improvements in only 3 of 10 outcomes.
- Compared to the control group, the treatment group also demonstrated a significantly greater improvement in all 4 sector scores and the total score of the ZAN-BP
Conclusions/limitations
- In patients with BPD, internet-based psychoeducation reduced symptom severity and improved psychosocial functioning, with effects lasting 1 year. Treatment group participants experienced clinically significant improvements in all outcomes measured during the acute phase of the study; most improvements were maintained over 1 year.
- While the control group initially saw similar improvements in most measurements, these improvements were not maintained as effectively over 1 year.
- Limitations include a female-only population, the restricted age range of participants, and recruitment exclusively from volunteers.
2. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148.
Standard dialectical behavior therapy (DBT) is an effective treatment for BPD; however, access is often limited by shortages of clinicians and resources. Therefore, it has become increasingly common for clinical settings to offer patients only the skills training component of DBT, which requires fewer resources. While several clinical trials examining brief DBT skills–only treatment for BPD have shown promising results, it is unclear how effective this intervention is at reducing suicidal or nonsuicidal self-injury (NSSI) episodes. McMain et al5 explored the effectiveness of brief DBT skills–only adjunctive treatment on the rates of suicidal and NSSI episodes in patients with BPD.
Study design
- In this 2-arm, single-blind, prospective controlled trial, 84 adults who met DSM-IV criteria for BPD were randomized to a 20-week DBT skills training group (DBT group) or an active waitlist (WL group). No restrictions on additional psychosocial or pharmacologic treatments were imposed on either group.
- The primary outcome was the frequency of suicidal and NSSI episodes as measured by the Lifetime Suicide Attempt Self-Injury Interview and the Deliberate Self-Harm Inventory (DSHI). Measurements occurred at baseline, 10 weeks, 20 weeks, and 3 months posttreatment (32 weeks).
- Secondary outcomes included changes in health care utilization, BPD symptoms, and coping. These were assessed using the Treatment History Interview-2, Borderline Symptom List-23, State-Trait Anger Expression Inventory, Symptom Checklist-90-revised (SCL-90-R), Barratt Impulsiveness Scale-11, Beck Depression Inventory (BDI)-II, Social Adjustment Scale Self-report, Difficulties in Emotion Regulation Scale, Distress Tolerance Scale, and Kentucky Inventory of Mindfulness Scale.
Outcomes
- At Week 32, compared to the WL group, the DBT group showed statistically significant greater reductions in the frequency of suicidal and NSSI episodes as measured by the LSASI but not by the DSHI. The DBT group experienced statistically significant improvements in distress tolerance and emotion regulation over the WL group at all points, but no difference on mindfulness. The DBT group achieved greater reductions in anger over time as compared to the WL group.
- At Week 20, compared to the WL group, the DBT group showed significant improvements in social adjustment, symptom distress, and borderline symptoms. There were no significant group differences on impulsivity. Between-group differences in the number of hospital admissions favored the DBT group at 10 and 20 weeks, but not at 32 weeks. There was no statistically significant difference between the groups with respect to the number of emergency department visits.
- Analyses of group differences in clinical improvement as measured by the SCL-90-R revealed statistically reliable and clinically significant changes in the DBT group over the WL group at 20 weeks, but not at 32 weeks.
Conclusions/limitations
- Brief DBT skills training reduced suicidal and NSSI episodes in patients with BPD. Participants in the DBT group also demonstrated greater improvement in anger, distress tolerance, and emotion regulation compared to the control group. These results were evident 3 months after treatment. However, any gains in health care utilization, social adjustment, symptom distress, and borderline symptoms diminished or did not differ from waitlist participants at Week 32. At that time, participants in the DBT group demonstrated a similar level of symptomatology as the WL group.
- Limitations include the use of an active waitlist control group, allowance of concurrent treatments, the absence of an active therapeutic comparator group, use of self-report measures, use of an instrument with unknown psychometric properties, and a relatively short 3-month follow-up period.
3. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156.
Psychotherapeutic options for treating BPD include DBT, mentalization-based treatment, schema-focused therapy, transference-based psychotherapy, and systems training for emotional predictability and problem solving. More recently, interpersonal therapy also has been adapted for BPD (IPT-BPD). However, thus far no trials have investigated the long-term effects of this therapy on BPD. In 2010, Bellino et al11 published a 32-week RCT examining the effect of IPT-BPD on BPD. They concluded that IPT-BPD plus fluoxetine was superior to fluoxetine alone in improving symptoms and quality of life. The present study by Bozzatello et al6 examined whether the benefits of IPT-BPD plus fluoxetine demonstrated in the 2010 study persisted over a 24-month follow-up.
Continue to: Study design...
Study design
- In the 2010 study by Bellino et al,11 55 outpatients who met DSM-IV criteria for BPD were randomized to receive IPT-BPD plus fluoxetine (combined therapy) or fluoxetine alone for 32 weeks. Forty-four participants completed a 24-month follow-up study (n = 22 for IPT-BPD plus fluoxetine, n = 22 for fluoxetine only).
- Clinical assessments were performed at 6, 12, and 24 months, and used the same instruments as the original study, including the Clinical Global Impression Scale–Severity item, Hamilton Depression Rating Scale, Hamilton Anxiety Rating Scale (HARS), Social and Occupational Functioning Assessment, Satisfaction Profile (SAT-P), and the Borderline Personality Disorder Severity Index (BPDSI).
Outcomes
- While the original study demonstrated that combined therapy had a clinically significant effect over fluoxetine alone on both HARS score and the BPDSI item “affective instability” at 32 weeks, this advantage was maintained only at the 6-month assessment.
- The improvements that the combined therapy provided over fluoxetine monotherapy on the BPDSI items of “impulsivity” and “interpersonal relationships” as well as the SAT-P factors of social and psychological functioning at 32 weeks were preserved at 24 months. No additional improvements were seen.
Conclusions/limitations
- The improvements in impulsivity, interpersonal functioning, social functioning, and psychological functioning at 32 weeks seen with IPT-BPD plus fluoxetine compared with fluoxetine alone persisted for 2 years after completing therapy; no further improvements were seen.
- The improvements to anxiety and affective instability that combined therapy demonstrated over fluoxetine monotherapy at 32 weeks were not maintained at 24 months.
- Limitations include a small sample size, exclusion of psychiatric comorbidities, and a lack of assessment of session or medication adherence.
4. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63.
While many studies have demonstrated the benefits of psychotherapy for treating personality disorders, there is limited research of how different levels of psychotherapy may impact treatment outcomes. An RCT called the Ullevål Personality Project (UPP)12 compared an intensive combined treatment program (CP) with outpatient individual psychotherapy (OIP) in patients with personality disorders. The CP program consisted of short-term day-hospital treatment followed by outpatient combined group and individual psychotherapy. The outcomes this RCT evaluated included suicide attempts, suicidal thoughts, self-injury, psychosocial functioning, symptom distress, and interpersonal and personality problems. A 6-year follow-up concluded there were no differences in outcomes between the 2 treatment groups. However, in this RCT, Antonsen et al7 examined whether CP produced statistically significant benefits over OIP in a subset of patients with BPD.
Study design
- In the UPP trial,12 117 patients who met DSM-IV criteria for personality disorders (excluding antisocial and schizotypal personality disorder) were randomized to receive 18 weeks of day hospital psychotherapy followed by CP or OIP. Fifty-two participants in the UPP were diagnosed with BPD, and 34 of these participants completed the 6-year follow-up investigation.
- Symptom distress, psychosocial functioning, interpersonal problems, quality of life, personality functioning, and self-harm/suicidal thoughts/suicide attempts were assessed at baseline, 8 months, 18 months, 3 years, and 6 years using the SCL-90-R Global Severity Index (GSI), BDI, Global Assessment of Functioning (GAF), Work and Social Adjustment Scale (WSAS), Quality of Life 10-point scale (QOL), Circumplex of Interpersonal Problems (CIP), and the 60-item short form of the Severity Indices of Personality Problems (SIPP-118) questionnaire.
Outcomes
- Compared to the OIP group, the CP group demonstrated statistically significant reductions in symptom distress at Year 6 as measured by the SCL-90-R GSI. Between Years 3 and 6, the CP group continued to show improvements in psychosocial functioning as demonstrated by improvements in GAF and WSAS scores. The OIP group’s scores worsened during this time. Compared to the OIP group, participants in the CP group also had significantly better outcomes on the SIPP-118 domains of self-control and identity integration.
- There were no significant differences between groups on the proportion of participants who engaged in self-harm or experienced suicidal thoughts or attempts. There were no significant differences in outcomes between the treatment groups on the CIP, BDI, or QOL.
- Participants in CP group tended to use fewer psychotropic medications than those in the OIP group over time, but this difference was not statistically significant. The 2 groups did not differ in use of health care services over the last year.
- Avoidant personality disorder (AVPD) did not have a significant moderator effect on GAF score. Comorbid AVPD was a negative predictor of GAF score, independent of the group.
Conclusions/limitations
- Both groups experienced a remission rate of 90% at 6-year follow-up. Compared with the OIP group, participants in the CP group experienced significantly greater reductions in symptom distress and improvements in self-control and identity integration at 6 years. Between Years 3 and 6, participants in the CP group experienced significant improvements in psychosocial functioning compared with OIP group participants. The 2 groups did not differ on other outcomes, including the CIP, BDI, QOL, suicidal thoughts, suicidal attempts, self-harm, and health care utilization.
- Despite statistically significant differences in GAF scores favoring the CP group over the OIP group during Years 3 to 6, GAF scores did not differ significantly in the final year, which suggests that symptomatic remission does not equal functional improvement.
- Limitations include a lack of control for intensity or length of treatment in statistical analyses, small sample size, lack of correction for multiple testing, lack of an a priori power analysis, missing data and potential violation of the missing at random assumption, use of therapists’ preferred treatment method/practice, and a lack of control for other treatments.
5. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299.
The efficacy of various psychotherapies for symptoms of BPD has been well established. However, there is limited evidence that these effects persist over time. In 2009, Bateman et al13 conducted an 18-month RCT comparing the effectiveness of outpatient mentalization-based treatment (MBT) against structured clinical management (SCM) for patients with BPD. Both groups experienced substantial improvements, but patients assigned to MBT demonstrated greater improvement in clinically significant problems, including suicide attempts and hospitalizations. In a 2021 follow-up to this study, Bateman et al8 investigated whether the MBT group’s gains in the primary outcomes (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months), social functioning, vocational engagement, and mental health service usage were maintained throughout an 8-year follow-up period.
Continue to: Study design...
Study design
- In the 2009 trial, Bateman et al13 randomized adult participants who met DSM-IV criteria for BPD and had a suicide attempt or episode of life-threatening self-harm in the past 6 months to receive 18 months of MBT or SCM. The primary outcome was crisis events, defined as a composite of suicidal and severe self-injurious behaviors and hospitalizations. The 2021 Bateman et al8 study expanded this investigation by collecting additional data on a yearly basis for 8 years.
- Of the 134 original participants, 98 agreed to complete the follow-up. Due to attrition, the follow-up period was limited to 8 years. At each yearly visit, researchers collected information on the primary outcome, the absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months.
- Secondary measures were collected mainly through a modified version of the Client Service Receipt Inventory and included critical incidents, psychiatric and medical hospital and community services, employment and other personally meaningful activity, psychoactive medication, and other mental health treatments.
Outcomes
- The number of participants who met diagnostic criteria for BPD at the 1-year follow-up was significantly lower in the MBT group compared with the SCM group. To improve participant retention, this outcome was not evaluated at later visits.
- The number of participants who achieved the primary recovery criteria of the original trial (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months) and remained well throughout the entire follow-up period was significantly higher in the MBT group compared with the SCM group. The average number of years during which participants failed to meet recovery criteria was significantly greater in the SCM group compared with the MBT group.
- When controlling for age, treatment group was a significant predictor of recovery during the follow-up period. Overall, significantly fewer participants in the MBT group experienced critical incidents during the follow-up period.
- The SCM group used crisis mental health services for a significantly greater number of follow-up years than the MBT group, although the likelihood of ever using crisis services did not statistically differ between the groups. Both groups had similar use of outpatient mental health services, primary care services, and nonmental health medical services. Compared to the SCM group, the MBT group had significantly fewer professional support service visits and significantly fewer outpatient psychiatrist visits.
- MBT group participants spent more time in education, were less likely to be unemployed, and were less likely to use social care interventions than SCM group participants. Although those in the MBT group spent more months engaged in purposeful activity, there was no significant difference between the groups in the proportion of participants who did not engage in purposeful activity.
- The MBT group spent fewer months receiving psychotherapeutic medication compared with the SCM group. The variables that yielded significant 2-way interactions were eating disorder, substance use disorder, and physical abuse, suggesting greater benefit from MBT with these concurrent diagnoses. Younger age was associated with better outcomes.
Conclusions/limitations
- This study demonstrated that patients with BPD significantly benefited from specialized therapies such as MBT.
- At the 1 follow-up visit, the number of participants who met diagnostic criteria for BPD was significantly lower in the MBT group compared with the SCM group.
- The number of participants who had achieved the primary recovery criteria and remained well during the 8-year follow-up period was significantly higher in the MBT group compared to the SCM group.
- Limitations include increasing attrition over time, possible allegiance effects and unmasking of research assistants, lack of self-report questionnaires, and the potentially erroneous conclusion that increased use of services equates to poorer treatment response and greater need for support.
6. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771.
Fewer than 1 in 4 patients with BPD have access to effective psychotherapies. The use of internet-based self-management interventions (SMIs) developed from evidence-based psychotherapies can help close this treatment gap. Although the efficacy of SMIs for several mental disorders has been demonstrated in multiple meta-analyses, results for BPD are mixed. In this study, Klein et al9 examined the effectiveness and safety of the adjunctive use of an SMI based on schema therapy in addition to care as usual (CAU) in patients with BPD.
Study design
- In a 12-month, rater-blind, controlled parallel group trial, adults who had a total BPDSI score ≥15 and either a diagnosis of BPD according to DSM-IV criteria or a probable diagnosis of BPD (if they had also received a BPD diagnosis from their treating physician) were randomized to an internet-based SMI based on schema therapy called priovi (n = 103) or CAU (n = 101). Participants could complete the SMI content in approximately 6 months but were recommended to use the intervention for the entire year.
- Participation in psychotherapy and psychiatric treatment, including pharmacotherapy, was permitted. At baseline, 74% of participants were receiving psychotherapy and 88% were receiving psychiatric treatment.
- The primary outcome was change in BPDSI score at 12 months. The primary safety outcome was the number of serious adverse events at 12 months. Secondary outcomes included BPD severity, depressive symptoms, anxiety symptoms, quality of life, uncontrolled internet use, negative treatment effects, and satisfaction with the intervention. Most assessments were measured at baseline, 3 months, 6 months, 9 months, and 12 months.
Outcomes
- Large reductions in the severity of BPD symptoms as measured by change in BPDSI score was observed in both groups. Although the average reduction in BPDSI score was greater in the SMI group, this difference was not statistically significant from the CAU group.
- There was no statistically significant difference in the number of serious adverse events between groups at any time.
Conclusions/limitations
- Treatment with SMI did not result in improved outcomes over CAU. Although the average reduction in BPDSI score was greater in the SMI group compared to the CAU group, this difference was not statistically significant.
- The authors cautioned that the smaller-than-expected between-groups effect size must be interpreted against the background of an unexpectedly large effect in the CAU group. In fact, the CAU group pre/post effect was comparable to the pre/post effect of intensive specialized DBT treatment groups in previous RCTs.
- The authors also suggested that the high percentage of participants who received psychotherapy did not allow for an additional benefit from SMI.
- Limitations include recruitment method, lack of systematic assessment of accidental unblinding, high exclusion rate due to failure of the participant or treating clinician to provide confirmation of diagnosis, and the use of a serious adverse events assessment that is not psychometrically validated.
Bottom Line
Evidence from randomized controlled trials suggests that internet-based psychoeducation, brief dialectical behavior therapy skills-only treatment, interpersonal therapy, a program that combines day treatment with individual and group psychotherapy, and mentalization-based treatment can improve symptoms and quality of life for patients with borderline personality disorder.
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159(2):276-83.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158(2):295-302.
3. Saeed SA, Kallis AC. Borderline personality disorder: 6 studies of biological interventions. Current Psychiatry. 2021;20(11):26-30,34-36.
4. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi:10.4088/JCP.16m11153
5. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148. doi:10.1111/acps.12664
6. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156. doi:10.1016/j.psychres.2016.04.014
7. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63. doi:10.1080/10503307.2015.1072283
8. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299. doi:10.1037/per0000422
9. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771. doi:10.1136/bmjopen-2020-047771
10. Zanarini MC, Frankenburg FR. A preliminary, randomized trial of psychoeducation for women with borderline personality disorder. J Pers Disord. 2008;22(3):284-290.
11. Bellino S, Rinaldi C, Bogetto F. Adaptation of interpersonal psychotherapy to borderline personality disorder: a comparison of combined therapy and single pharmacotherapy. Can J Psychiatry. 2010;55(2):74-81.
12. Arnevik E, Wilberg T, Urnes Ø, et al. Psychotherapy for personality disorders: short-term day hospital psychotherapy versus outpatient individual therapy – a randomized controlled study. Eur Psychiatry. 2009,24(2):71-78. doi:10.1016/j.eurpsy.2008.09.004
13. Bateman A, Fonagy P. Randomized controlled trial of outpatient mentalization-based treatment versus structured clinical management for borderline personality disorder. Am J Psychiatry. 2009;166(12):1355-1364.
SECOND OF 2 PARTS
Borderline personality disorder (BPD) is associated with serious impairment in psychosocial functioning.1 It is characterized by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior that often results in problems in relationships. As a result, patients with BPD tend to utilize more mental health services than patients with other personality disorders or major depressive disorder.2
Some clinicians believe BPD is difficult to treat. While historically there has been little consensus on the best treatments for this disorder, current options include both pharmacologic and psychological interventions. In Part 1 of this 2-part article, we focused on 6 studies that evaluated biological interventions.3 Here in Part 2, we focus on findings from 6 recent randomized controlled trials (RCTs) of psychosocial interventions for BPD
1. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi: 10.4088/JCP.16m11153
Research has shown that BPD is a treatable illness with a more favorable prognosis than previously believed. Despite this, patients often experience difficulty accessing the most up-to-date information on BPD, which can impede their treatment. A 2008 study by Zanarini et al10 of younger female patients with BPD demonstrated that immediate, in-person psychoeducation improved impulsivity and relationships. Widespread implementation of this program proved problematic, however, due to cost and personnel constraints. To resolve this issue, researchers developed an internet-based version of the program. In a 2018 follow-up study, Zanarini et al4 examined the effect of this internet-based psychoeducation program on symptoms of BPD.
Continue to: Study design...
Study design
- Women (age 18 to 30) who met DSM-IV and Diagnostic Interview for Borderlines–Revised criteria for BPD were randomized to an internet-based psychoeducation treatment group (n = 40) or a control group (n = 40).
- Ten outcomes concerning symptom severity and psychosocial functioning were assessed during weeks 1 to 12 (acute phase) and at months 6, 9, and 12 (maintenance phase) using the self-report version of the Zanarini Rating Scale for BPD (ZAN-BPD), the Borderline Evaluation of Severity over Time, the Sheehan Disability Scale, the Clinically Useful Depression Outcome Scale, the Clinically Useful Anxiety Outcome Scale, and Weissman’s Social Adjustment Scale (SAS).
Outcomes
- In the acute phase, treatment group participants experienced statistically significant improvements in all 10 outcomes. Control group participants demonstrated similar results, achieving statistically significant improvements in 7 of 10 outcomes.
- Compared to the control group, the treatment group experienced a more significant reduction in impulsivity and improvement in psychosocial functioning as measured by the ZAN-BPD and SAS.
- In the maintenance phase, treatment group participants achieved statistically significant improvements in 9 of 10 outcomes, whereas control group participants demonstrated statistically significant improvements in only 3 of 10 outcomes.
- Compared to the control group, the treatment group also demonstrated a significantly greater improvement in all 4 sector scores and the total score of the ZAN-BP
Conclusions/limitations
- In patients with BPD, internet-based psychoeducation reduced symptom severity and improved psychosocial functioning, with effects lasting 1 year. Treatment group participants experienced clinically significant improvements in all outcomes measured during the acute phase of the study; most improvements were maintained over 1 year.
- While the control group initially saw similar improvements in most measurements, these improvements were not maintained as effectively over 1 year.
- Limitations include a female-only population, the restricted age range of participants, and recruitment exclusively from volunteers.
2. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148.
Standard dialectical behavior therapy (DBT) is an effective treatment for BPD; however, access is often limited by shortages of clinicians and resources. Therefore, it has become increasingly common for clinical settings to offer patients only the skills training component of DBT, which requires fewer resources. While several clinical trials examining brief DBT skills–only treatment for BPD have shown promising results, it is unclear how effective this intervention is at reducing suicidal or nonsuicidal self-injury (NSSI) episodes. McMain et al5 explored the effectiveness of brief DBT skills–only adjunctive treatment on the rates of suicidal and NSSI episodes in patients with BPD.
Study design
- In this 2-arm, single-blind, prospective controlled trial, 84 adults who met DSM-IV criteria for BPD were randomized to a 20-week DBT skills training group (DBT group) or an active waitlist (WL group). No restrictions on additional psychosocial or pharmacologic treatments were imposed on either group.
- The primary outcome was the frequency of suicidal and NSSI episodes as measured by the Lifetime Suicide Attempt Self-Injury Interview and the Deliberate Self-Harm Inventory (DSHI). Measurements occurred at baseline, 10 weeks, 20 weeks, and 3 months posttreatment (32 weeks).
- Secondary outcomes included changes in health care utilization, BPD symptoms, and coping. These were assessed using the Treatment History Interview-2, Borderline Symptom List-23, State-Trait Anger Expression Inventory, Symptom Checklist-90-revised (SCL-90-R), Barratt Impulsiveness Scale-11, Beck Depression Inventory (BDI)-II, Social Adjustment Scale Self-report, Difficulties in Emotion Regulation Scale, Distress Tolerance Scale, and Kentucky Inventory of Mindfulness Scale.
Outcomes
- At Week 32, compared to the WL group, the DBT group showed statistically significant greater reductions in the frequency of suicidal and NSSI episodes as measured by the LSASI but not by the DSHI. The DBT group experienced statistically significant improvements in distress tolerance and emotion regulation over the WL group at all points, but no difference on mindfulness. The DBT group achieved greater reductions in anger over time as compared to the WL group.
- At Week 20, compared to the WL group, the DBT group showed significant improvements in social adjustment, symptom distress, and borderline symptoms. There were no significant group differences on impulsivity. Between-group differences in the number of hospital admissions favored the DBT group at 10 and 20 weeks, but not at 32 weeks. There was no statistically significant difference between the groups with respect to the number of emergency department visits.
- Analyses of group differences in clinical improvement as measured by the SCL-90-R revealed statistically reliable and clinically significant changes in the DBT group over the WL group at 20 weeks, but not at 32 weeks.
Conclusions/limitations
- Brief DBT skills training reduced suicidal and NSSI episodes in patients with BPD. Participants in the DBT group also demonstrated greater improvement in anger, distress tolerance, and emotion regulation compared to the control group. These results were evident 3 months after treatment. However, any gains in health care utilization, social adjustment, symptom distress, and borderline symptoms diminished or did not differ from waitlist participants at Week 32. At that time, participants in the DBT group demonstrated a similar level of symptomatology as the WL group.
- Limitations include the use of an active waitlist control group, allowance of concurrent treatments, the absence of an active therapeutic comparator group, use of self-report measures, use of an instrument with unknown psychometric properties, and a relatively short 3-month follow-up period.
3. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156.
Psychotherapeutic options for treating BPD include DBT, mentalization-based treatment, schema-focused therapy, transference-based psychotherapy, and systems training for emotional predictability and problem solving. More recently, interpersonal therapy also has been adapted for BPD (IPT-BPD). However, thus far no trials have investigated the long-term effects of this therapy on BPD. In 2010, Bellino et al11 published a 32-week RCT examining the effect of IPT-BPD on BPD. They concluded that IPT-BPD plus fluoxetine was superior to fluoxetine alone in improving symptoms and quality of life. The present study by Bozzatello et al6 examined whether the benefits of IPT-BPD plus fluoxetine demonstrated in the 2010 study persisted over a 24-month follow-up.
Continue to: Study design...
Study design
- In the 2010 study by Bellino et al,11 55 outpatients who met DSM-IV criteria for BPD were randomized to receive IPT-BPD plus fluoxetine (combined therapy) or fluoxetine alone for 32 weeks. Forty-four participants completed a 24-month follow-up study (n = 22 for IPT-BPD plus fluoxetine, n = 22 for fluoxetine only).
- Clinical assessments were performed at 6, 12, and 24 months, and used the same instruments as the original study, including the Clinical Global Impression Scale–Severity item, Hamilton Depression Rating Scale, Hamilton Anxiety Rating Scale (HARS), Social and Occupational Functioning Assessment, Satisfaction Profile (SAT-P), and the Borderline Personality Disorder Severity Index (BPDSI).
Outcomes
- While the original study demonstrated that combined therapy had a clinically significant effect over fluoxetine alone on both HARS score and the BPDSI item “affective instability” at 32 weeks, this advantage was maintained only at the 6-month assessment.
- The improvements that the combined therapy provided over fluoxetine monotherapy on the BPDSI items of “impulsivity” and “interpersonal relationships” as well as the SAT-P factors of social and psychological functioning at 32 weeks were preserved at 24 months. No additional improvements were seen.
Conclusions/limitations
- The improvements in impulsivity, interpersonal functioning, social functioning, and psychological functioning at 32 weeks seen with IPT-BPD plus fluoxetine compared with fluoxetine alone persisted for 2 years after completing therapy; no further improvements were seen.
- The improvements to anxiety and affective instability that combined therapy demonstrated over fluoxetine monotherapy at 32 weeks were not maintained at 24 months.
- Limitations include a small sample size, exclusion of psychiatric comorbidities, and a lack of assessment of session or medication adherence.
4. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63.
While many studies have demonstrated the benefits of psychotherapy for treating personality disorders, there is limited research of how different levels of psychotherapy may impact treatment outcomes. An RCT called the Ullevål Personality Project (UPP)12 compared an intensive combined treatment program (CP) with outpatient individual psychotherapy (OIP) in patients with personality disorders. The CP program consisted of short-term day-hospital treatment followed by outpatient combined group and individual psychotherapy. The outcomes this RCT evaluated included suicide attempts, suicidal thoughts, self-injury, psychosocial functioning, symptom distress, and interpersonal and personality problems. A 6-year follow-up concluded there were no differences in outcomes between the 2 treatment groups. However, in this RCT, Antonsen et al7 examined whether CP produced statistically significant benefits over OIP in a subset of patients with BPD.
Study design
- In the UPP trial,12 117 patients who met DSM-IV criteria for personality disorders (excluding antisocial and schizotypal personality disorder) were randomized to receive 18 weeks of day hospital psychotherapy followed by CP or OIP. Fifty-two participants in the UPP were diagnosed with BPD, and 34 of these participants completed the 6-year follow-up investigation.
- Symptom distress, psychosocial functioning, interpersonal problems, quality of life, personality functioning, and self-harm/suicidal thoughts/suicide attempts were assessed at baseline, 8 months, 18 months, 3 years, and 6 years using the SCL-90-R Global Severity Index (GSI), BDI, Global Assessment of Functioning (GAF), Work and Social Adjustment Scale (WSAS), Quality of Life 10-point scale (QOL), Circumplex of Interpersonal Problems (CIP), and the 60-item short form of the Severity Indices of Personality Problems (SIPP-118) questionnaire.
Outcomes
- Compared to the OIP group, the CP group demonstrated statistically significant reductions in symptom distress at Year 6 as measured by the SCL-90-R GSI. Between Years 3 and 6, the CP group continued to show improvements in psychosocial functioning as demonstrated by improvements in GAF and WSAS scores. The OIP group’s scores worsened during this time. Compared to the OIP group, participants in the CP group also had significantly better outcomes on the SIPP-118 domains of self-control and identity integration.
- There were no significant differences between groups on the proportion of participants who engaged in self-harm or experienced suicidal thoughts or attempts. There were no significant differences in outcomes between the treatment groups on the CIP, BDI, or QOL.
- Participants in CP group tended to use fewer psychotropic medications than those in the OIP group over time, but this difference was not statistically significant. The 2 groups did not differ in use of health care services over the last year.
- Avoidant personality disorder (AVPD) did not have a significant moderator effect on GAF score. Comorbid AVPD was a negative predictor of GAF score, independent of the group.
Conclusions/limitations
- Both groups experienced a remission rate of 90% at 6-year follow-up. Compared with the OIP group, participants in the CP group experienced significantly greater reductions in symptom distress and improvements in self-control and identity integration at 6 years. Between Years 3 and 6, participants in the CP group experienced significant improvements in psychosocial functioning compared with OIP group participants. The 2 groups did not differ on other outcomes, including the CIP, BDI, QOL, suicidal thoughts, suicidal attempts, self-harm, and health care utilization.
- Despite statistically significant differences in GAF scores favoring the CP group over the OIP group during Years 3 to 6, GAF scores did not differ significantly in the final year, which suggests that symptomatic remission does not equal functional improvement.
- Limitations include a lack of control for intensity or length of treatment in statistical analyses, small sample size, lack of correction for multiple testing, lack of an a priori power analysis, missing data and potential violation of the missing at random assumption, use of therapists’ preferred treatment method/practice, and a lack of control for other treatments.
5. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299.
The efficacy of various psychotherapies for symptoms of BPD has been well established. However, there is limited evidence that these effects persist over time. In 2009, Bateman et al13 conducted an 18-month RCT comparing the effectiveness of outpatient mentalization-based treatment (MBT) against structured clinical management (SCM) for patients with BPD. Both groups experienced substantial improvements, but patients assigned to MBT demonstrated greater improvement in clinically significant problems, including suicide attempts and hospitalizations. In a 2021 follow-up to this study, Bateman et al8 investigated whether the MBT group’s gains in the primary outcomes (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months), social functioning, vocational engagement, and mental health service usage were maintained throughout an 8-year follow-up period.
Continue to: Study design...
Study design
- In the 2009 trial, Bateman et al13 randomized adult participants who met DSM-IV criteria for BPD and had a suicide attempt or episode of life-threatening self-harm in the past 6 months to receive 18 months of MBT or SCM. The primary outcome was crisis events, defined as a composite of suicidal and severe self-injurious behaviors and hospitalizations. The 2021 Bateman et al8 study expanded this investigation by collecting additional data on a yearly basis for 8 years.
- Of the 134 original participants, 98 agreed to complete the follow-up. Due to attrition, the follow-up period was limited to 8 years. At each yearly visit, researchers collected information on the primary outcome, the absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months.
- Secondary measures were collected mainly through a modified version of the Client Service Receipt Inventory and included critical incidents, psychiatric and medical hospital and community services, employment and other personally meaningful activity, psychoactive medication, and other mental health treatments.
Outcomes
- The number of participants who met diagnostic criteria for BPD at the 1-year follow-up was significantly lower in the MBT group compared with the SCM group. To improve participant retention, this outcome was not evaluated at later visits.
- The number of participants who achieved the primary recovery criteria of the original trial (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months) and remained well throughout the entire follow-up period was significantly higher in the MBT group compared with the SCM group. The average number of years during which participants failed to meet recovery criteria was significantly greater in the SCM group compared with the MBT group.
- When controlling for age, treatment group was a significant predictor of recovery during the follow-up period. Overall, significantly fewer participants in the MBT group experienced critical incidents during the follow-up period.
- The SCM group used crisis mental health services for a significantly greater number of follow-up years than the MBT group, although the likelihood of ever using crisis services did not statistically differ between the groups. Both groups had similar use of outpatient mental health services, primary care services, and nonmental health medical services. Compared to the SCM group, the MBT group had significantly fewer professional support service visits and significantly fewer outpatient psychiatrist visits.
- MBT group participants spent more time in education, were less likely to be unemployed, and were less likely to use social care interventions than SCM group participants. Although those in the MBT group spent more months engaged in purposeful activity, there was no significant difference between the groups in the proportion of participants who did not engage in purposeful activity.
- The MBT group spent fewer months receiving psychotherapeutic medication compared with the SCM group. The variables that yielded significant 2-way interactions were eating disorder, substance use disorder, and physical abuse, suggesting greater benefit from MBT with these concurrent diagnoses. Younger age was associated with better outcomes.
Conclusions/limitations
- This study demonstrated that patients with BPD significantly benefited from specialized therapies such as MBT.
- At the 1 follow-up visit, the number of participants who met diagnostic criteria for BPD was significantly lower in the MBT group compared with the SCM group.
- The number of participants who had achieved the primary recovery criteria and remained well during the 8-year follow-up period was significantly higher in the MBT group compared to the SCM group.
- Limitations include increasing attrition over time, possible allegiance effects and unmasking of research assistants, lack of self-report questionnaires, and the potentially erroneous conclusion that increased use of services equates to poorer treatment response and greater need for support.
6. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771.
Fewer than 1 in 4 patients with BPD have access to effective psychotherapies. The use of internet-based self-management interventions (SMIs) developed from evidence-based psychotherapies can help close this treatment gap. Although the efficacy of SMIs for several mental disorders has been demonstrated in multiple meta-analyses, results for BPD are mixed. In this study, Klein et al9 examined the effectiveness and safety of the adjunctive use of an SMI based on schema therapy in addition to care as usual (CAU) in patients with BPD.
Study design
- In a 12-month, rater-blind, controlled parallel group trial, adults who had a total BPDSI score ≥15 and either a diagnosis of BPD according to DSM-IV criteria or a probable diagnosis of BPD (if they had also received a BPD diagnosis from their treating physician) were randomized to an internet-based SMI based on schema therapy called priovi (n = 103) or CAU (n = 101). Participants could complete the SMI content in approximately 6 months but were recommended to use the intervention for the entire year.
- Participation in psychotherapy and psychiatric treatment, including pharmacotherapy, was permitted. At baseline, 74% of participants were receiving psychotherapy and 88% were receiving psychiatric treatment.
- The primary outcome was change in BPDSI score at 12 months. The primary safety outcome was the number of serious adverse events at 12 months. Secondary outcomes included BPD severity, depressive symptoms, anxiety symptoms, quality of life, uncontrolled internet use, negative treatment effects, and satisfaction with the intervention. Most assessments were measured at baseline, 3 months, 6 months, 9 months, and 12 months.
Outcomes
- Large reductions in the severity of BPD symptoms as measured by change in BPDSI score was observed in both groups. Although the average reduction in BPDSI score was greater in the SMI group, this difference was not statistically significant from the CAU group.
- There was no statistically significant difference in the number of serious adverse events between groups at any time.
Conclusions/limitations
- Treatment with SMI did not result in improved outcomes over CAU. Although the average reduction in BPDSI score was greater in the SMI group compared to the CAU group, this difference was not statistically significant.
- The authors cautioned that the smaller-than-expected between-groups effect size must be interpreted against the background of an unexpectedly large effect in the CAU group. In fact, the CAU group pre/post effect was comparable to the pre/post effect of intensive specialized DBT treatment groups in previous RCTs.
- The authors also suggested that the high percentage of participants who received psychotherapy did not allow for an additional benefit from SMI.
- Limitations include recruitment method, lack of systematic assessment of accidental unblinding, high exclusion rate due to failure of the participant or treating clinician to provide confirmation of diagnosis, and the use of a serious adverse events assessment that is not psychometrically validated.
Bottom Line
Evidence from randomized controlled trials suggests that internet-based psychoeducation, brief dialectical behavior therapy skills-only treatment, interpersonal therapy, a program that combines day treatment with individual and group psychotherapy, and mentalization-based treatment can improve symptoms and quality of life for patients with borderline personality disorder.
SECOND OF 2 PARTS
Borderline personality disorder (BPD) is associated with serious impairment in psychosocial functioning.1 It is characterized by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior that often results in problems in relationships. As a result, patients with BPD tend to utilize more mental health services than patients with other personality disorders or major depressive disorder.2
Some clinicians believe BPD is difficult to treat. While historically there has been little consensus on the best treatments for this disorder, current options include both pharmacologic and psychological interventions. In Part 1 of this 2-part article, we focused on 6 studies that evaluated biological interventions.3 Here in Part 2, we focus on findings from 6 recent randomized controlled trials (RCTs) of psychosocial interventions for BPD
1. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi: 10.4088/JCP.16m11153
Research has shown that BPD is a treatable illness with a more favorable prognosis than previously believed. Despite this, patients often experience difficulty accessing the most up-to-date information on BPD, which can impede their treatment. A 2008 study by Zanarini et al10 of younger female patients with BPD demonstrated that immediate, in-person psychoeducation improved impulsivity and relationships. Widespread implementation of this program proved problematic, however, due to cost and personnel constraints. To resolve this issue, researchers developed an internet-based version of the program. In a 2018 follow-up study, Zanarini et al4 examined the effect of this internet-based psychoeducation program on symptoms of BPD.
Continue to: Study design...
Study design
- Women (age 18 to 30) who met DSM-IV and Diagnostic Interview for Borderlines–Revised criteria for BPD were randomized to an internet-based psychoeducation treatment group (n = 40) or a control group (n = 40).
- Ten outcomes concerning symptom severity and psychosocial functioning were assessed during weeks 1 to 12 (acute phase) and at months 6, 9, and 12 (maintenance phase) using the self-report version of the Zanarini Rating Scale for BPD (ZAN-BPD), the Borderline Evaluation of Severity over Time, the Sheehan Disability Scale, the Clinically Useful Depression Outcome Scale, the Clinically Useful Anxiety Outcome Scale, and Weissman’s Social Adjustment Scale (SAS).
Outcomes
- In the acute phase, treatment group participants experienced statistically significant improvements in all 10 outcomes. Control group participants demonstrated similar results, achieving statistically significant improvements in 7 of 10 outcomes.
- Compared to the control group, the treatment group experienced a more significant reduction in impulsivity and improvement in psychosocial functioning as measured by the ZAN-BPD and SAS.
- In the maintenance phase, treatment group participants achieved statistically significant improvements in 9 of 10 outcomes, whereas control group participants demonstrated statistically significant improvements in only 3 of 10 outcomes.
- Compared to the control group, the treatment group also demonstrated a significantly greater improvement in all 4 sector scores and the total score of the ZAN-BP
Conclusions/limitations
- In patients with BPD, internet-based psychoeducation reduced symptom severity and improved psychosocial functioning, with effects lasting 1 year. Treatment group participants experienced clinically significant improvements in all outcomes measured during the acute phase of the study; most improvements were maintained over 1 year.
- While the control group initially saw similar improvements in most measurements, these improvements were not maintained as effectively over 1 year.
- Limitations include a female-only population, the restricted age range of participants, and recruitment exclusively from volunteers.
2. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148.
Standard dialectical behavior therapy (DBT) is an effective treatment for BPD; however, access is often limited by shortages of clinicians and resources. Therefore, it has become increasingly common for clinical settings to offer patients only the skills training component of DBT, which requires fewer resources. While several clinical trials examining brief DBT skills–only treatment for BPD have shown promising results, it is unclear how effective this intervention is at reducing suicidal or nonsuicidal self-injury (NSSI) episodes. McMain et al5 explored the effectiveness of brief DBT skills–only adjunctive treatment on the rates of suicidal and NSSI episodes in patients with BPD.
Study design
- In this 2-arm, single-blind, prospective controlled trial, 84 adults who met DSM-IV criteria for BPD were randomized to a 20-week DBT skills training group (DBT group) or an active waitlist (WL group). No restrictions on additional psychosocial or pharmacologic treatments were imposed on either group.
- The primary outcome was the frequency of suicidal and NSSI episodes as measured by the Lifetime Suicide Attempt Self-Injury Interview and the Deliberate Self-Harm Inventory (DSHI). Measurements occurred at baseline, 10 weeks, 20 weeks, and 3 months posttreatment (32 weeks).
- Secondary outcomes included changes in health care utilization, BPD symptoms, and coping. These were assessed using the Treatment History Interview-2, Borderline Symptom List-23, State-Trait Anger Expression Inventory, Symptom Checklist-90-revised (SCL-90-R), Barratt Impulsiveness Scale-11, Beck Depression Inventory (BDI)-II, Social Adjustment Scale Self-report, Difficulties in Emotion Regulation Scale, Distress Tolerance Scale, and Kentucky Inventory of Mindfulness Scale.
Outcomes
- At Week 32, compared to the WL group, the DBT group showed statistically significant greater reductions in the frequency of suicidal and NSSI episodes as measured by the LSASI but not by the DSHI. The DBT group experienced statistically significant improvements in distress tolerance and emotion regulation over the WL group at all points, but no difference on mindfulness. The DBT group achieved greater reductions in anger over time as compared to the WL group.
- At Week 20, compared to the WL group, the DBT group showed significant improvements in social adjustment, symptom distress, and borderline symptoms. There were no significant group differences on impulsivity. Between-group differences in the number of hospital admissions favored the DBT group at 10 and 20 weeks, but not at 32 weeks. There was no statistically significant difference between the groups with respect to the number of emergency department visits.
- Analyses of group differences in clinical improvement as measured by the SCL-90-R revealed statistically reliable and clinically significant changes in the DBT group over the WL group at 20 weeks, but not at 32 weeks.
Conclusions/limitations
- Brief DBT skills training reduced suicidal and NSSI episodes in patients with BPD. Participants in the DBT group also demonstrated greater improvement in anger, distress tolerance, and emotion regulation compared to the control group. These results were evident 3 months after treatment. However, any gains in health care utilization, social adjustment, symptom distress, and borderline symptoms diminished or did not differ from waitlist participants at Week 32. At that time, participants in the DBT group demonstrated a similar level of symptomatology as the WL group.
- Limitations include the use of an active waitlist control group, allowance of concurrent treatments, the absence of an active therapeutic comparator group, use of self-report measures, use of an instrument with unknown psychometric properties, and a relatively short 3-month follow-up period.
3. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156.
Psychotherapeutic options for treating BPD include DBT, mentalization-based treatment, schema-focused therapy, transference-based psychotherapy, and systems training for emotional predictability and problem solving. More recently, interpersonal therapy also has been adapted for BPD (IPT-BPD). However, thus far no trials have investigated the long-term effects of this therapy on BPD. In 2010, Bellino et al11 published a 32-week RCT examining the effect of IPT-BPD on BPD. They concluded that IPT-BPD plus fluoxetine was superior to fluoxetine alone in improving symptoms and quality of life. The present study by Bozzatello et al6 examined whether the benefits of IPT-BPD plus fluoxetine demonstrated in the 2010 study persisted over a 24-month follow-up.
Continue to: Study design...
Study design
- In the 2010 study by Bellino et al,11 55 outpatients who met DSM-IV criteria for BPD were randomized to receive IPT-BPD plus fluoxetine (combined therapy) or fluoxetine alone for 32 weeks. Forty-four participants completed a 24-month follow-up study (n = 22 for IPT-BPD plus fluoxetine, n = 22 for fluoxetine only).
- Clinical assessments were performed at 6, 12, and 24 months, and used the same instruments as the original study, including the Clinical Global Impression Scale–Severity item, Hamilton Depression Rating Scale, Hamilton Anxiety Rating Scale (HARS), Social and Occupational Functioning Assessment, Satisfaction Profile (SAT-P), and the Borderline Personality Disorder Severity Index (BPDSI).
Outcomes
- While the original study demonstrated that combined therapy had a clinically significant effect over fluoxetine alone on both HARS score and the BPDSI item “affective instability” at 32 weeks, this advantage was maintained only at the 6-month assessment.
- The improvements that the combined therapy provided over fluoxetine monotherapy on the BPDSI items of “impulsivity” and “interpersonal relationships” as well as the SAT-P factors of social and psychological functioning at 32 weeks were preserved at 24 months. No additional improvements were seen.
Conclusions/limitations
- The improvements in impulsivity, interpersonal functioning, social functioning, and psychological functioning at 32 weeks seen with IPT-BPD plus fluoxetine compared with fluoxetine alone persisted for 2 years after completing therapy; no further improvements were seen.
- The improvements to anxiety and affective instability that combined therapy demonstrated over fluoxetine monotherapy at 32 weeks were not maintained at 24 months.
- Limitations include a small sample size, exclusion of psychiatric comorbidities, and a lack of assessment of session or medication adherence.
4. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63.
While many studies have demonstrated the benefits of psychotherapy for treating personality disorders, there is limited research of how different levels of psychotherapy may impact treatment outcomes. An RCT called the Ullevål Personality Project (UPP)12 compared an intensive combined treatment program (CP) with outpatient individual psychotherapy (OIP) in patients with personality disorders. The CP program consisted of short-term day-hospital treatment followed by outpatient combined group and individual psychotherapy. The outcomes this RCT evaluated included suicide attempts, suicidal thoughts, self-injury, psychosocial functioning, symptom distress, and interpersonal and personality problems. A 6-year follow-up concluded there were no differences in outcomes between the 2 treatment groups. However, in this RCT, Antonsen et al7 examined whether CP produced statistically significant benefits over OIP in a subset of patients with BPD.
Study design
- In the UPP trial,12 117 patients who met DSM-IV criteria for personality disorders (excluding antisocial and schizotypal personality disorder) were randomized to receive 18 weeks of day hospital psychotherapy followed by CP or OIP. Fifty-two participants in the UPP were diagnosed with BPD, and 34 of these participants completed the 6-year follow-up investigation.
- Symptom distress, psychosocial functioning, interpersonal problems, quality of life, personality functioning, and self-harm/suicidal thoughts/suicide attempts were assessed at baseline, 8 months, 18 months, 3 years, and 6 years using the SCL-90-R Global Severity Index (GSI), BDI, Global Assessment of Functioning (GAF), Work and Social Adjustment Scale (WSAS), Quality of Life 10-point scale (QOL), Circumplex of Interpersonal Problems (CIP), and the 60-item short form of the Severity Indices of Personality Problems (SIPP-118) questionnaire.
Outcomes
- Compared to the OIP group, the CP group demonstrated statistically significant reductions in symptom distress at Year 6 as measured by the SCL-90-R GSI. Between Years 3 and 6, the CP group continued to show improvements in psychosocial functioning as demonstrated by improvements in GAF and WSAS scores. The OIP group’s scores worsened during this time. Compared to the OIP group, participants in the CP group also had significantly better outcomes on the SIPP-118 domains of self-control and identity integration.
- There were no significant differences between groups on the proportion of participants who engaged in self-harm or experienced suicidal thoughts or attempts. There were no significant differences in outcomes between the treatment groups on the CIP, BDI, or QOL.
- Participants in CP group tended to use fewer psychotropic medications than those in the OIP group over time, but this difference was not statistically significant. The 2 groups did not differ in use of health care services over the last year.
- Avoidant personality disorder (AVPD) did not have a significant moderator effect on GAF score. Comorbid AVPD was a negative predictor of GAF score, independent of the group.
Conclusions/limitations
- Both groups experienced a remission rate of 90% at 6-year follow-up. Compared with the OIP group, participants in the CP group experienced significantly greater reductions in symptom distress and improvements in self-control and identity integration at 6 years. Between Years 3 and 6, participants in the CP group experienced significant improvements in psychosocial functioning compared with OIP group participants. The 2 groups did not differ on other outcomes, including the CIP, BDI, QOL, suicidal thoughts, suicidal attempts, self-harm, and health care utilization.
- Despite statistically significant differences in GAF scores favoring the CP group over the OIP group during Years 3 to 6, GAF scores did not differ significantly in the final year, which suggests that symptomatic remission does not equal functional improvement.
- Limitations include a lack of control for intensity or length of treatment in statistical analyses, small sample size, lack of correction for multiple testing, lack of an a priori power analysis, missing data and potential violation of the missing at random assumption, use of therapists’ preferred treatment method/practice, and a lack of control for other treatments.
5. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299.
The efficacy of various psychotherapies for symptoms of BPD has been well established. However, there is limited evidence that these effects persist over time. In 2009, Bateman et al13 conducted an 18-month RCT comparing the effectiveness of outpatient mentalization-based treatment (MBT) against structured clinical management (SCM) for patients with BPD. Both groups experienced substantial improvements, but patients assigned to MBT demonstrated greater improvement in clinically significant problems, including suicide attempts and hospitalizations. In a 2021 follow-up to this study, Bateman et al8 investigated whether the MBT group’s gains in the primary outcomes (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months), social functioning, vocational engagement, and mental health service usage were maintained throughout an 8-year follow-up period.
Continue to: Study design...
Study design
- In the 2009 trial, Bateman et al13 randomized adult participants who met DSM-IV criteria for BPD and had a suicide attempt or episode of life-threatening self-harm in the past 6 months to receive 18 months of MBT or SCM. The primary outcome was crisis events, defined as a composite of suicidal and severe self-injurious behaviors and hospitalizations. The 2021 Bateman et al8 study expanded this investigation by collecting additional data on a yearly basis for 8 years.
- Of the 134 original participants, 98 agreed to complete the follow-up. Due to attrition, the follow-up period was limited to 8 years. At each yearly visit, researchers collected information on the primary outcome, the absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months.
- Secondary measures were collected mainly through a modified version of the Client Service Receipt Inventory and included critical incidents, psychiatric and medical hospital and community services, employment and other personally meaningful activity, psychoactive medication, and other mental health treatments.
Outcomes
- The number of participants who met diagnostic criteria for BPD at the 1-year follow-up was significantly lower in the MBT group compared with the SCM group. To improve participant retention, this outcome was not evaluated at later visits.
- The number of participants who achieved the primary recovery criteria of the original trial (absence of severe self-harm, suicide attempts, and inpatient admissions in the previous 12 months) and remained well throughout the entire follow-up period was significantly higher in the MBT group compared with the SCM group. The average number of years during which participants failed to meet recovery criteria was significantly greater in the SCM group compared with the MBT group.
- When controlling for age, treatment group was a significant predictor of recovery during the follow-up period. Overall, significantly fewer participants in the MBT group experienced critical incidents during the follow-up period.
- The SCM group used crisis mental health services for a significantly greater number of follow-up years than the MBT group, although the likelihood of ever using crisis services did not statistically differ between the groups. Both groups had similar use of outpatient mental health services, primary care services, and nonmental health medical services. Compared to the SCM group, the MBT group had significantly fewer professional support service visits and significantly fewer outpatient psychiatrist visits.
- MBT group participants spent more time in education, were less likely to be unemployed, and were less likely to use social care interventions than SCM group participants. Although those in the MBT group spent more months engaged in purposeful activity, there was no significant difference between the groups in the proportion of participants who did not engage in purposeful activity.
- The MBT group spent fewer months receiving psychotherapeutic medication compared with the SCM group. The variables that yielded significant 2-way interactions were eating disorder, substance use disorder, and physical abuse, suggesting greater benefit from MBT with these concurrent diagnoses. Younger age was associated with better outcomes.
Conclusions/limitations
- This study demonstrated that patients with BPD significantly benefited from specialized therapies such as MBT.
- At the 1 follow-up visit, the number of participants who met diagnostic criteria for BPD was significantly lower in the MBT group compared with the SCM group.
- The number of participants who had achieved the primary recovery criteria and remained well during the 8-year follow-up period was significantly higher in the MBT group compared to the SCM group.
- Limitations include increasing attrition over time, possible allegiance effects and unmasking of research assistants, lack of self-report questionnaires, and the potentially erroneous conclusion that increased use of services equates to poorer treatment response and greater need for support.
6. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771.
Fewer than 1 in 4 patients with BPD have access to effective psychotherapies. The use of internet-based self-management interventions (SMIs) developed from evidence-based psychotherapies can help close this treatment gap. Although the efficacy of SMIs for several mental disorders has been demonstrated in multiple meta-analyses, results for BPD are mixed. In this study, Klein et al9 examined the effectiveness and safety of the adjunctive use of an SMI based on schema therapy in addition to care as usual (CAU) in patients with BPD.
Study design
- In a 12-month, rater-blind, controlled parallel group trial, adults who had a total BPDSI score ≥15 and either a diagnosis of BPD according to DSM-IV criteria or a probable diagnosis of BPD (if they had also received a BPD diagnosis from their treating physician) were randomized to an internet-based SMI based on schema therapy called priovi (n = 103) or CAU (n = 101). Participants could complete the SMI content in approximately 6 months but were recommended to use the intervention for the entire year.
- Participation in psychotherapy and psychiatric treatment, including pharmacotherapy, was permitted. At baseline, 74% of participants were receiving psychotherapy and 88% were receiving psychiatric treatment.
- The primary outcome was change in BPDSI score at 12 months. The primary safety outcome was the number of serious adverse events at 12 months. Secondary outcomes included BPD severity, depressive symptoms, anxiety symptoms, quality of life, uncontrolled internet use, negative treatment effects, and satisfaction with the intervention. Most assessments were measured at baseline, 3 months, 6 months, 9 months, and 12 months.
Outcomes
- Large reductions in the severity of BPD symptoms as measured by change in BPDSI score was observed in both groups. Although the average reduction in BPDSI score was greater in the SMI group, this difference was not statistically significant from the CAU group.
- There was no statistically significant difference in the number of serious adverse events between groups at any time.
Conclusions/limitations
- Treatment with SMI did not result in improved outcomes over CAU. Although the average reduction in BPDSI score was greater in the SMI group compared to the CAU group, this difference was not statistically significant.
- The authors cautioned that the smaller-than-expected between-groups effect size must be interpreted against the background of an unexpectedly large effect in the CAU group. In fact, the CAU group pre/post effect was comparable to the pre/post effect of intensive specialized DBT treatment groups in previous RCTs.
- The authors also suggested that the high percentage of participants who received psychotherapy did not allow for an additional benefit from SMI.
- Limitations include recruitment method, lack of systematic assessment of accidental unblinding, high exclusion rate due to failure of the participant or treating clinician to provide confirmation of diagnosis, and the use of a serious adverse events assessment that is not psychometrically validated.
Bottom Line
Evidence from randomized controlled trials suggests that internet-based psychoeducation, brief dialectical behavior therapy skills-only treatment, interpersonal therapy, a program that combines day treatment with individual and group psychotherapy, and mentalization-based treatment can improve symptoms and quality of life for patients with borderline personality disorder.
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159(2):276-83.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158(2):295-302.
3. Saeed SA, Kallis AC. Borderline personality disorder: 6 studies of biological interventions. Current Psychiatry. 2021;20(11):26-30,34-36.
4. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi:10.4088/JCP.16m11153
5. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148. doi:10.1111/acps.12664
6. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156. doi:10.1016/j.psychres.2016.04.014
7. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63. doi:10.1080/10503307.2015.1072283
8. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299. doi:10.1037/per0000422
9. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771. doi:10.1136/bmjopen-2020-047771
10. Zanarini MC, Frankenburg FR. A preliminary, randomized trial of psychoeducation for women with borderline personality disorder. J Pers Disord. 2008;22(3):284-290.
11. Bellino S, Rinaldi C, Bogetto F. Adaptation of interpersonal psychotherapy to borderline personality disorder: a comparison of combined therapy and single pharmacotherapy. Can J Psychiatry. 2010;55(2):74-81.
12. Arnevik E, Wilberg T, Urnes Ø, et al. Psychotherapy for personality disorders: short-term day hospital psychotherapy versus outpatient individual therapy – a randomized controlled study. Eur Psychiatry. 2009,24(2):71-78. doi:10.1016/j.eurpsy.2008.09.004
13. Bateman A, Fonagy P. Randomized controlled trial of outpatient mentalization-based treatment versus structured clinical management for borderline personality disorder. Am J Psychiatry. 2009;166(12):1355-1364.
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159(2):276-83.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158(2):295-302.
3. Saeed SA, Kallis AC. Borderline personality disorder: 6 studies of biological interventions. Current Psychiatry. 2021;20(11):26-30,34-36.
4. Zanarini MC, Conkey LC, Temes CM, et al. Randomized controlled trial of web-based psychoeducation for women with borderline personality disorder. J Clin Psychiatry. 2018;79(3):16m11153. doi:10.4088/JCP.16m11153
5. McMain SF, Guimond T, Barnhart R, et al. A randomized trial of brief dialectical behaviour therapy skills training in suicidal patients suffering from borderline disorder. Acta Psychiatr Scand. 2017;135(2):138-148. doi:10.1111/acps.12664
6. Bozzatello P, Bellino S. Combined therapy with interpersonal psychotherapy adapted for borderline personality disorder: a two-years follow-up. Psychiatry Res. 2016;240:151-156. doi:10.1016/j.psychres.2016.04.014
7. Antonsen BT, Kvarstein EH, Urnes Ø, et al. Favourable outcome of long-term combined psychotherapy for patients with borderline personality disorder: six-year follow-up of a randomized study. Psychother Res. 2017;27(1):51-63. doi:10.1080/10503307.2015.1072283
8. Bateman A, Constantinou MP, Fonagy P, et al. Eight-year prospective follow-up of mentalization-based treatment versus structured clinical management for people with borderline personality disorder. Personal Disord. 2021;12(4):291-299. doi:10.1037/per0000422
9. Klein JP, Hauer-von Mauschwitz A, Berger T, et al. Effectiveness and safety of the adjunctive use of an internet-based self-management intervention for borderline personality disorder in addition to care as usual: results from a randomised controlled trial. BMJ Open. 2021;11(9):e047771. doi:10.1136/bmjopen-2020-047771
10. Zanarini MC, Frankenburg FR. A preliminary, randomized trial of psychoeducation for women with borderline personality disorder. J Pers Disord. 2008;22(3):284-290.
11. Bellino S, Rinaldi C, Bogetto F. Adaptation of interpersonal psychotherapy to borderline personality disorder: a comparison of combined therapy and single pharmacotherapy. Can J Psychiatry. 2010;55(2):74-81.
12. Arnevik E, Wilberg T, Urnes Ø, et al. Psychotherapy for personality disorders: short-term day hospital psychotherapy versus outpatient individual therapy – a randomized controlled study. Eur Psychiatry. 2009,24(2):71-78. doi:10.1016/j.eurpsy.2008.09.004
13. Bateman A, Fonagy P. Randomized controlled trial of outpatient mentalization-based treatment versus structured clinical management for borderline personality disorder. Am J Psychiatry. 2009;166(12):1355-1364.
Psychiatric partial hospitalization programs: What you need to know
Psychiatric partial hospitalization programs (PHPs), previously known as “day hospitals,” serve to bridge the gap between inpatient and outpatient facilities by providing intensive, highly structured outpatient behavioral health services (typically several hours of psychotherapy each weekday for most days of the week). The concept of PHPs has existed since at least the 1950s, but such programs started to become more common in the United States as the result of legislation passed in 1963 (Box1-3). In this article, I provide a brief introductory review of PHPs, while acknowledging that most research on PHPs was conducted years ago and is rather limited.
Box
The concept of partial hospitalization programs (PHPs) was developed before the 1950s.1 However, in the United States, PHPs did not take hold until Congress passed the Community Mental Health Act of 1963, which required that PHPs must be a core component of Community Mental Health Centers (CMHCs). The Omnibus Budget Reconciliation Acts of 1987 and 1990 required Medicare to pay for PHPs affiliated with or based in CMHCs and psychiatric hospitals, which resulted in a proliferation of PHPs across the country. The number of CMHCbased PHPs grew from 296 in 1993 to 769 in 1997.2 By 2016, more than one-third (38.7%) of all metropolitan hospitals and 11.4% of nonmetropolitan hospitals in the United States provided PHP services.3 This growth was also partially the result of private health insurance companies and the managed care industry clamping down on inpatient hospital stays and approving PHP care to reduce costs. In recent years, freestanding PHPs that are not affiliated with a CMHC or hospital have opened to serve high-functioning patients who do not want inpatient hospitalization or the stigma associated with it.
PHPs: What they are, and how they work
The term “partial hospitalization” is fraught with confusion because initially it was used to contrast such services from full hospitalization. Historically, it was used to describe services for patients who had been discharged home from a state hospital and attended a program on the hospital grounds during the day as outpatients. In reality, today’s PHPs are “day treatment” programs, but the terminology has stuck.
PHPs are neither an inpatient service nor a strict outpatient service, but rather a midground along the continuum of treatment intensity between the 2 traditional types of psychiatric services for patients with a range of mental illness of varying severity. The Association for Ambulatory Behavioral Healthcare, which has set standards and guidelines for PHPs, defines a PHP as “an ambulatory treatment program that includes the major diagnostic, medical, psychiatric, psychosocial, and prevocational treatment modalities designed for patients with serious mental disorders who require coordinated intensive, comprehensive, and multidisciplinary treatment not provided in an outpatient clinical setting.”4 PHPs can render acute care as an alternative to inpatient treatment, provide transitional stabilization treatment between an inpatient stay and traditional outpatient treatment (once a week or less frequent), and function as a supplement to traditional outpatient treatment.
Medicare has established criteria that PHPs must meet to qualify for reimbursement5; these criteria are now widely accepted as standards of care by the insurance industry. To meet the Medicare criteria, PHP treatment must be active and structured to provide an individualized treatment plan that incorporates coordination of services to meet the particular needs of the patient.5 It must include a multidisciplinary team approach to patient care under the direction of a physician, and the treatment goals must be measurable, functional, time-framed, medically necessary, and directly related to the reason for admission.5 The physician must certify the medical necessity for admission by documenting that the patient has a diagnosis of an acute Axis I mental disorder, a level of functioning that includes severe impairments in multiple areas of daily life, and a “reasonable expectation” that the disorder and level of functioning will improve as a result of the treatment.5
The Joint Commission (formerly JCAHO) lumps day treatment, intensive outpatient, partial hospitalization, and adult day care services into a single category of an ambulatory health care environment offering an organized day or night program of assessment, treatment, care, services, habilitation, or rehabilitation for individuals who do not require 24-hour care.6 For behavioral health, this may be a structured, ongoing program that typically meets 2 to 5 times a week for 2 to 5 hours per day.6
Most PHPs for adult patients provide services during the day 5 days per week and average 5 to 6 hours of programming per day. Night or evening programming may be a good option for patients who work during the day. Typically, treatment is provided in a group therapy format, with individual therapy at least once a week. Group therapy may include cognitive-behavioral therapy, coping with grief and loss, trauma recovery, conflict resolution, stress management, anger control, and behavioral modification. Family therapy is provided as needed, but usually is mandatory for children and adolescents. Many PHPs offer intensive outpatient programs (IOPs) as a step down to further facilitate a patient’s adjustment to psychosocial and family functioning while returning to work on a part-time basis. IOPs typically provide 3 to 4 hours of service per day 3 days per week. While not evidence-based, the typical duration of PHP treatment is 4 to 6 weeks, followed by an additional 2 to 4 weeks of IOP.
Continue to: Advantages and disadvantages...
Advantages and disadvantages
Based on a qualitative literature review, Neffinger1 outlined potential advantages and disadvantages of PHPs vs inpatient hospitalization (Table1). While these have not been empirically studied, they may be useful to consider when determining if a PHP would be beneficial for a specific patient.
Which factors are most therapeutic?
Research on which factors of PHPs are of therapeutic value is quite limited and primarily consists of surveys of small numbers of participants. Hoge et al7 explored the active therapeutic factors responsible for change in the Connecticut Mental Health Center PHP by comparing responses of 20 patients with those of their clinicians. Ninety-five percent of patients rated structure as the top therapeutic factor, followed by interpersonal contact, medication, and altruism. Other factors that were rated as important by 40% or fewer participants were catharsis, learning, mobilization of family support, connection to community, universality, patient autonomy, successful completion, security, feedback on behavior, and practice at home. In a British study8 that used a similar method, patients reported that counseling was the most helpful aspect of treatment, followed by medical treatment, while staff picked groups followed by a planned approach.
Evidence supports PHPs’ effectiveness
Some research has suggested that PHPs can be effective, both clinically and in terms of cost. Marshall et al9 conducted a systematic review of the effectiveness of an acute day hospital vs inpatient admission vs outpatient care that included 9 trials. They concluded that psychiatric inpatient admissions could be reduced by at least 23% if patients were diverted to an acute day hospital. They also found some evidence that day treatment programs may be superior to outpatient care in improving symptoms in nonpsychotic patients who are refractory to outpatient treatment. In a systematic review of 18 studies, high rates of satisfaction with PHP services suggested PHPs have an advantage over inpatient treatment within 1 year of discharge; patients and families were more satisfied with PHPs.10 In a study of 197 urban, socioeconomically disadvantaged, severely ill patients, Sledge et al11 compared the clinical outcomes of those who participated in day hospital programs with those of patients who received inpatient care. They found that while overall the 2 approaches produced similar outcomes, there were slightly more positive effects for day hospital programs in measures of symptoms, overall functioning, and social functioning. In terms of cost effectiveness, a 1978 study found that even after correcting for differences in treatment days between inpatient and PHP services, there was a significant financial advantage with PHP (costs were one-third less), primarily because of lower costs per day.12 In another study, PHP cost savings were 20%, and potential savings were higher for nonpsychotic patients.13
Are PHPs appropriate for children and adolescents?
Studies of PHPs for adolescents found that patients made gains in peer relationships, behavioral and academic performance, and control of their emotions.14,15 A review of PHPs’ effectiveness for children suggested that 66% to 99% of treated patients demonstrated improvement and successful return to community-based schools, and family functioning was a major factor in improvement.14 In a follow-up study that surveyed patients via telephone >1 year after discharge from a PHP, almost 80% of the children and adolescents were either “doing OK” or were “well-adjusted.”14 Only 22.5% required inpatient or residential treatment; the majority were doing well in school, with only 7% failing.14 In addition, 60% of parents reported satisfaction with treatment, and 85% reported functional improvement in their children.
Factors that predict PHP success or failure
In an analysis of a day treatment program that provided 4 months of intensive psychodynamic, group-oriented milieu treatment for patients with long-standing personality disorders, Rosie et al16 found 3 factors that contributed to the success of the PHP:
- optimal treatment-patient matching
- judicious use of authority in milieu therapy
- maintaining close relationships with referral sources.
In a study that compared 58 patients who completed an Ottawa hospital PHP and 44 who did not complete the program, psychological mindedness and chronicity of psychiatric illness were found to predict completion.17 However, a study of 59 females with anorexia nervosa who were transferred from inpatient care to an eating disorder day hospital program found that a longer duration of illness, amenorrhea, and a lower body mass index were associated with PHP treatment failure and inpatient rehospitalization.18 One study found that for individuals who were referred to a PHP from inpatient care, suicidal ideation and greater psychotic symptoms were associated with acute inpatient rehospitalization.19 Other factors associated with PHP nonattendance and treatment failure include limited personal and economic resources, high rates of substance abuse disorders, multiple admissions, and disability.20 In a study of 103 alcohol-dependent patients who completed IOP treatment, 64% were abstinent at 6 months follow-up; relapse was associated with a longer duration of alcohol dependence and a higher number of prior treatments, while favorable outcomes were associated with a lower degree of depression, anxiety, and craving.21 Patients with cocaine dependence who completed an IOP showed significant improvements in addiction scores and had more favorable outcomes in employment status and psychological problems if they stayed longer in treatment.22
Bottom Line
Psychiatric partial hospitalization programs (PHPs) provide a transition from inpatient hospitalization to outpatient treatment for patients who need further stabilization, or serve as an alternative to inpatient treatment for patients who don’t need or want inpatient hospitalization. PHPs can be as effective as inpatient treatment for all but the most seriously ill patients, and are more cost-effective than inpatient treatment.
1. Neffinger GG. Partial hospitalization: an overview. J Community Psychol. 1981;9(3):262-269.
2. Leung MY, Drozd EM, Healy DA, et al. Impacts associated with the Medicare Psychiatric PPS: a study of partial hospitalization programs. February 2009. Accessed January 8, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Reports/downloads/Leung_PHP_PPS_2010.pdf
3. Williams T, Borders TF, Jasinski L. Partial Psychiatric Hospitalization Program Availability in Non-Metropolitan and Non-Metropolitan Hospitals Nationally. Rural and Underserved Health Research Center; 2019.
4. Rosser J, Michael S, eds. Standards and guidelines for partial hospital programs and intensive outpatient programs. Association for Ambulatory Behavioral Healthcare. 2018. Accessed January 8, 2022. https://aabh.org/wp-content/uploads/2021/05/2021-SandG-Final.pdf
5. CMS Manual System PUB. 100-02 Medicare Benefit Policy: Partial Hospitalization Services, Department of Health and Human Services. Centers for Medicare & Medicaid Services; 2004:6-9.
6. Joint Commission. Guide to Joint Commission Behavioral Healthcare Accreditation. Joint Commission; 2007:36.
7. Hoge MA, Farrell SP, Munchel ME, et al. Therapeutic factors in partial hospitalization. Psychiatry. 1988;51(2):199-210.
8. Ricketts T, Kirshbaum MN. Helpfulness of mental health day care: client and staff views. J Adv Nurs. 1994;20(2):297-306.
9. Marshall M, Crowther R, Almaraz-Serrano A, et al. Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) acute day hospital versus admission; (2) vocational rehabilitation; (3) day hospital versus outpatient care. Health Technol Assess. 2001;5(21):1-75.
10. Horvitz-Lennon M, Normand SL, Gaccione P, et al. Partial versus full hospitalization for adults in psychiatric distress: a systematic review of the published literature (1957-1997). Am J Psychiatry. 2001;158(5):676-685.
11. Sledge WH, Tebes J, Rakfeldt J, et al. Day hospital/crisis respite care versus inpatient care, Part I: Clinical outcomes. Am J Psychiatry. 1996;153(8):1065-1073.
12. Fink EB, Longabaugh R, Stout R. The paradoxical underutilization of partial hospitalization. Am J Psychiatry. 1978;135(6):713-716.
13. Sledge WH, Tebes J, Wolff N, et al. Day hospital/crisis respite care versus inpatient care, Part II: service utilization and costs. Am J Psychiatry. 1996;153(8):1074-1083.
14. Kiser LJ. Treatment-effectiveness research in child and adolescent partial hospitalization. Psychiatr Hosp. 1991;22(2):51-8.
15. Milin R, Coupland K, Walker S, et al. Outcome and follow-up study of an adolescent psychiatric day treatment school program. J Am Acad Child Adolesc Psychiatry. 2000;39(3):320-328.
16. Rosie JS, Azim HF, Piper WE, et al. Effective psychiatric day treatment: historical lessons. Psychiatr Serv. 1995;46(10):1019-1026.
17. Tasca GA, Balfour L, Bissada H, et al. Treatment completion and outcome in a partial hospitalization program: interactions among patient variables. Psychotherapy Res. 1999;9(2):232-247.
18. Howard WT, Evans KK, Quintero-Howard CV, et al. Predictors of success or failure of transition to day hospital treatment for inpatients with anorexia nervosa. Am J Psychiatry. 1999;156(11):1697-1702.
19. Beard C, Hearon BA, Lee J, et al. When partial hospitalization fails: risk factors for inpatient hospitalization. J Nerv Ment Dis. 2016;204(6):431-436.
20. Lieberman PB, Guggenheim FG. Reasons for patient nonattendance during acute partial hospitalization. Psychiatr Serv. 2016;67(6):684-687.
21. Bottlender M, Soyka M. Efficacy of an intensive outpatient rehabilitation program in alcoholism: predictors of outcome 6 months after treatment. Eur Addict Res. 2005;11(3):132-137.
22. Gottheil E, Weinstein SP, Sterling RC, et al. A randomized controlled study of the effectiveness of intensive outpatient treatment for cocaine dependence. Psychiatr Serv. 1998;49(6):782-787.
Psychiatric partial hospitalization programs (PHPs), previously known as “day hospitals,” serve to bridge the gap between inpatient and outpatient facilities by providing intensive, highly structured outpatient behavioral health services (typically several hours of psychotherapy each weekday for most days of the week). The concept of PHPs has existed since at least the 1950s, but such programs started to become more common in the United States as the result of legislation passed in 1963 (Box1-3). In this article, I provide a brief introductory review of PHPs, while acknowledging that most research on PHPs was conducted years ago and is rather limited.
Box
The concept of partial hospitalization programs (PHPs) was developed before the 1950s.1 However, in the United States, PHPs did not take hold until Congress passed the Community Mental Health Act of 1963, which required that PHPs must be a core component of Community Mental Health Centers (CMHCs). The Omnibus Budget Reconciliation Acts of 1987 and 1990 required Medicare to pay for PHPs affiliated with or based in CMHCs and psychiatric hospitals, which resulted in a proliferation of PHPs across the country. The number of CMHCbased PHPs grew from 296 in 1993 to 769 in 1997.2 By 2016, more than one-third (38.7%) of all metropolitan hospitals and 11.4% of nonmetropolitan hospitals in the United States provided PHP services.3 This growth was also partially the result of private health insurance companies and the managed care industry clamping down on inpatient hospital stays and approving PHP care to reduce costs. In recent years, freestanding PHPs that are not affiliated with a CMHC or hospital have opened to serve high-functioning patients who do not want inpatient hospitalization or the stigma associated with it.
PHPs: What they are, and how they work
The term “partial hospitalization” is fraught with confusion because initially it was used to contrast such services from full hospitalization. Historically, it was used to describe services for patients who had been discharged home from a state hospital and attended a program on the hospital grounds during the day as outpatients. In reality, today’s PHPs are “day treatment” programs, but the terminology has stuck.
PHPs are neither an inpatient service nor a strict outpatient service, but rather a midground along the continuum of treatment intensity between the 2 traditional types of psychiatric services for patients with a range of mental illness of varying severity. The Association for Ambulatory Behavioral Healthcare, which has set standards and guidelines for PHPs, defines a PHP as “an ambulatory treatment program that includes the major diagnostic, medical, psychiatric, psychosocial, and prevocational treatment modalities designed for patients with serious mental disorders who require coordinated intensive, comprehensive, and multidisciplinary treatment not provided in an outpatient clinical setting.”4 PHPs can render acute care as an alternative to inpatient treatment, provide transitional stabilization treatment between an inpatient stay and traditional outpatient treatment (once a week or less frequent), and function as a supplement to traditional outpatient treatment.
Medicare has established criteria that PHPs must meet to qualify for reimbursement5; these criteria are now widely accepted as standards of care by the insurance industry. To meet the Medicare criteria, PHP treatment must be active and structured to provide an individualized treatment plan that incorporates coordination of services to meet the particular needs of the patient.5 It must include a multidisciplinary team approach to patient care under the direction of a physician, and the treatment goals must be measurable, functional, time-framed, medically necessary, and directly related to the reason for admission.5 The physician must certify the medical necessity for admission by documenting that the patient has a diagnosis of an acute Axis I mental disorder, a level of functioning that includes severe impairments in multiple areas of daily life, and a “reasonable expectation” that the disorder and level of functioning will improve as a result of the treatment.5
The Joint Commission (formerly JCAHO) lumps day treatment, intensive outpatient, partial hospitalization, and adult day care services into a single category of an ambulatory health care environment offering an organized day or night program of assessment, treatment, care, services, habilitation, or rehabilitation for individuals who do not require 24-hour care.6 For behavioral health, this may be a structured, ongoing program that typically meets 2 to 5 times a week for 2 to 5 hours per day.6
Most PHPs for adult patients provide services during the day 5 days per week and average 5 to 6 hours of programming per day. Night or evening programming may be a good option for patients who work during the day. Typically, treatment is provided in a group therapy format, with individual therapy at least once a week. Group therapy may include cognitive-behavioral therapy, coping with grief and loss, trauma recovery, conflict resolution, stress management, anger control, and behavioral modification. Family therapy is provided as needed, but usually is mandatory for children and adolescents. Many PHPs offer intensive outpatient programs (IOPs) as a step down to further facilitate a patient’s adjustment to psychosocial and family functioning while returning to work on a part-time basis. IOPs typically provide 3 to 4 hours of service per day 3 days per week. While not evidence-based, the typical duration of PHP treatment is 4 to 6 weeks, followed by an additional 2 to 4 weeks of IOP.
Continue to: Advantages and disadvantages...
Advantages and disadvantages
Based on a qualitative literature review, Neffinger1 outlined potential advantages and disadvantages of PHPs vs inpatient hospitalization (Table1). While these have not been empirically studied, they may be useful to consider when determining if a PHP would be beneficial for a specific patient.
Which factors are most therapeutic?
Research on which factors of PHPs are of therapeutic value is quite limited and primarily consists of surveys of small numbers of participants. Hoge et al7 explored the active therapeutic factors responsible for change in the Connecticut Mental Health Center PHP by comparing responses of 20 patients with those of their clinicians. Ninety-five percent of patients rated structure as the top therapeutic factor, followed by interpersonal contact, medication, and altruism. Other factors that were rated as important by 40% or fewer participants were catharsis, learning, mobilization of family support, connection to community, universality, patient autonomy, successful completion, security, feedback on behavior, and practice at home. In a British study8 that used a similar method, patients reported that counseling was the most helpful aspect of treatment, followed by medical treatment, while staff picked groups followed by a planned approach.
Evidence supports PHPs’ effectiveness
Some research has suggested that PHPs can be effective, both clinically and in terms of cost. Marshall et al9 conducted a systematic review of the effectiveness of an acute day hospital vs inpatient admission vs outpatient care that included 9 trials. They concluded that psychiatric inpatient admissions could be reduced by at least 23% if patients were diverted to an acute day hospital. They also found some evidence that day treatment programs may be superior to outpatient care in improving symptoms in nonpsychotic patients who are refractory to outpatient treatment. In a systematic review of 18 studies, high rates of satisfaction with PHP services suggested PHPs have an advantage over inpatient treatment within 1 year of discharge; patients and families were more satisfied with PHPs.10 In a study of 197 urban, socioeconomically disadvantaged, severely ill patients, Sledge et al11 compared the clinical outcomes of those who participated in day hospital programs with those of patients who received inpatient care. They found that while overall the 2 approaches produced similar outcomes, there were slightly more positive effects for day hospital programs in measures of symptoms, overall functioning, and social functioning. In terms of cost effectiveness, a 1978 study found that even after correcting for differences in treatment days between inpatient and PHP services, there was a significant financial advantage with PHP (costs were one-third less), primarily because of lower costs per day.12 In another study, PHP cost savings were 20%, and potential savings were higher for nonpsychotic patients.13
Are PHPs appropriate for children and adolescents?
Studies of PHPs for adolescents found that patients made gains in peer relationships, behavioral and academic performance, and control of their emotions.14,15 A review of PHPs’ effectiveness for children suggested that 66% to 99% of treated patients demonstrated improvement and successful return to community-based schools, and family functioning was a major factor in improvement.14 In a follow-up study that surveyed patients via telephone >1 year after discharge from a PHP, almost 80% of the children and adolescents were either “doing OK” or were “well-adjusted.”14 Only 22.5% required inpatient or residential treatment; the majority were doing well in school, with only 7% failing.14 In addition, 60% of parents reported satisfaction with treatment, and 85% reported functional improvement in their children.
Factors that predict PHP success or failure
In an analysis of a day treatment program that provided 4 months of intensive psychodynamic, group-oriented milieu treatment for patients with long-standing personality disorders, Rosie et al16 found 3 factors that contributed to the success of the PHP:
- optimal treatment-patient matching
- judicious use of authority in milieu therapy
- maintaining close relationships with referral sources.
In a study that compared 58 patients who completed an Ottawa hospital PHP and 44 who did not complete the program, psychological mindedness and chronicity of psychiatric illness were found to predict completion.17 However, a study of 59 females with anorexia nervosa who were transferred from inpatient care to an eating disorder day hospital program found that a longer duration of illness, amenorrhea, and a lower body mass index were associated with PHP treatment failure and inpatient rehospitalization.18 One study found that for individuals who were referred to a PHP from inpatient care, suicidal ideation and greater psychotic symptoms were associated with acute inpatient rehospitalization.19 Other factors associated with PHP nonattendance and treatment failure include limited personal and economic resources, high rates of substance abuse disorders, multiple admissions, and disability.20 In a study of 103 alcohol-dependent patients who completed IOP treatment, 64% were abstinent at 6 months follow-up; relapse was associated with a longer duration of alcohol dependence and a higher number of prior treatments, while favorable outcomes were associated with a lower degree of depression, anxiety, and craving.21 Patients with cocaine dependence who completed an IOP showed significant improvements in addiction scores and had more favorable outcomes in employment status and psychological problems if they stayed longer in treatment.22
Bottom Line
Psychiatric partial hospitalization programs (PHPs) provide a transition from inpatient hospitalization to outpatient treatment for patients who need further stabilization, or serve as an alternative to inpatient treatment for patients who don’t need or want inpatient hospitalization. PHPs can be as effective as inpatient treatment for all but the most seriously ill patients, and are more cost-effective than inpatient treatment.
Psychiatric partial hospitalization programs (PHPs), previously known as “day hospitals,” serve to bridge the gap between inpatient and outpatient facilities by providing intensive, highly structured outpatient behavioral health services (typically several hours of psychotherapy each weekday for most days of the week). The concept of PHPs has existed since at least the 1950s, but such programs started to become more common in the United States as the result of legislation passed in 1963 (Box1-3). In this article, I provide a brief introductory review of PHPs, while acknowledging that most research on PHPs was conducted years ago and is rather limited.
Box
The concept of partial hospitalization programs (PHPs) was developed before the 1950s.1 However, in the United States, PHPs did not take hold until Congress passed the Community Mental Health Act of 1963, which required that PHPs must be a core component of Community Mental Health Centers (CMHCs). The Omnibus Budget Reconciliation Acts of 1987 and 1990 required Medicare to pay for PHPs affiliated with or based in CMHCs and psychiatric hospitals, which resulted in a proliferation of PHPs across the country. The number of CMHCbased PHPs grew from 296 in 1993 to 769 in 1997.2 By 2016, more than one-third (38.7%) of all metropolitan hospitals and 11.4% of nonmetropolitan hospitals in the United States provided PHP services.3 This growth was also partially the result of private health insurance companies and the managed care industry clamping down on inpatient hospital stays and approving PHP care to reduce costs. In recent years, freestanding PHPs that are not affiliated with a CMHC or hospital have opened to serve high-functioning patients who do not want inpatient hospitalization or the stigma associated with it.
PHPs: What they are, and how they work
The term “partial hospitalization” is fraught with confusion because initially it was used to contrast such services from full hospitalization. Historically, it was used to describe services for patients who had been discharged home from a state hospital and attended a program on the hospital grounds during the day as outpatients. In reality, today’s PHPs are “day treatment” programs, but the terminology has stuck.
PHPs are neither an inpatient service nor a strict outpatient service, but rather a midground along the continuum of treatment intensity between the 2 traditional types of psychiatric services for patients with a range of mental illness of varying severity. The Association for Ambulatory Behavioral Healthcare, which has set standards and guidelines for PHPs, defines a PHP as “an ambulatory treatment program that includes the major diagnostic, medical, psychiatric, psychosocial, and prevocational treatment modalities designed for patients with serious mental disorders who require coordinated intensive, comprehensive, and multidisciplinary treatment not provided in an outpatient clinical setting.”4 PHPs can render acute care as an alternative to inpatient treatment, provide transitional stabilization treatment between an inpatient stay and traditional outpatient treatment (once a week or less frequent), and function as a supplement to traditional outpatient treatment.
Medicare has established criteria that PHPs must meet to qualify for reimbursement5; these criteria are now widely accepted as standards of care by the insurance industry. To meet the Medicare criteria, PHP treatment must be active and structured to provide an individualized treatment plan that incorporates coordination of services to meet the particular needs of the patient.5 It must include a multidisciplinary team approach to patient care under the direction of a physician, and the treatment goals must be measurable, functional, time-framed, medically necessary, and directly related to the reason for admission.5 The physician must certify the medical necessity for admission by documenting that the patient has a diagnosis of an acute Axis I mental disorder, a level of functioning that includes severe impairments in multiple areas of daily life, and a “reasonable expectation” that the disorder and level of functioning will improve as a result of the treatment.5
The Joint Commission (formerly JCAHO) lumps day treatment, intensive outpatient, partial hospitalization, and adult day care services into a single category of an ambulatory health care environment offering an organized day or night program of assessment, treatment, care, services, habilitation, or rehabilitation for individuals who do not require 24-hour care.6 For behavioral health, this may be a structured, ongoing program that typically meets 2 to 5 times a week for 2 to 5 hours per day.6
Most PHPs for adult patients provide services during the day 5 days per week and average 5 to 6 hours of programming per day. Night or evening programming may be a good option for patients who work during the day. Typically, treatment is provided in a group therapy format, with individual therapy at least once a week. Group therapy may include cognitive-behavioral therapy, coping with grief and loss, trauma recovery, conflict resolution, stress management, anger control, and behavioral modification. Family therapy is provided as needed, but usually is mandatory for children and adolescents. Many PHPs offer intensive outpatient programs (IOPs) as a step down to further facilitate a patient’s adjustment to psychosocial and family functioning while returning to work on a part-time basis. IOPs typically provide 3 to 4 hours of service per day 3 days per week. While not evidence-based, the typical duration of PHP treatment is 4 to 6 weeks, followed by an additional 2 to 4 weeks of IOP.
Continue to: Advantages and disadvantages...
Advantages and disadvantages
Based on a qualitative literature review, Neffinger1 outlined potential advantages and disadvantages of PHPs vs inpatient hospitalization (Table1). While these have not been empirically studied, they may be useful to consider when determining if a PHP would be beneficial for a specific patient.
Which factors are most therapeutic?
Research on which factors of PHPs are of therapeutic value is quite limited and primarily consists of surveys of small numbers of participants. Hoge et al7 explored the active therapeutic factors responsible for change in the Connecticut Mental Health Center PHP by comparing responses of 20 patients with those of their clinicians. Ninety-five percent of patients rated structure as the top therapeutic factor, followed by interpersonal contact, medication, and altruism. Other factors that were rated as important by 40% or fewer participants were catharsis, learning, mobilization of family support, connection to community, universality, patient autonomy, successful completion, security, feedback on behavior, and practice at home. In a British study8 that used a similar method, patients reported that counseling was the most helpful aspect of treatment, followed by medical treatment, while staff picked groups followed by a planned approach.
Evidence supports PHPs’ effectiveness
Some research has suggested that PHPs can be effective, both clinically and in terms of cost. Marshall et al9 conducted a systematic review of the effectiveness of an acute day hospital vs inpatient admission vs outpatient care that included 9 trials. They concluded that psychiatric inpatient admissions could be reduced by at least 23% if patients were diverted to an acute day hospital. They also found some evidence that day treatment programs may be superior to outpatient care in improving symptoms in nonpsychotic patients who are refractory to outpatient treatment. In a systematic review of 18 studies, high rates of satisfaction with PHP services suggested PHPs have an advantage over inpatient treatment within 1 year of discharge; patients and families were more satisfied with PHPs.10 In a study of 197 urban, socioeconomically disadvantaged, severely ill patients, Sledge et al11 compared the clinical outcomes of those who participated in day hospital programs with those of patients who received inpatient care. They found that while overall the 2 approaches produced similar outcomes, there were slightly more positive effects for day hospital programs in measures of symptoms, overall functioning, and social functioning. In terms of cost effectiveness, a 1978 study found that even after correcting for differences in treatment days between inpatient and PHP services, there was a significant financial advantage with PHP (costs were one-third less), primarily because of lower costs per day.12 In another study, PHP cost savings were 20%, and potential savings were higher for nonpsychotic patients.13
Are PHPs appropriate for children and adolescents?
Studies of PHPs for adolescents found that patients made gains in peer relationships, behavioral and academic performance, and control of their emotions.14,15 A review of PHPs’ effectiveness for children suggested that 66% to 99% of treated patients demonstrated improvement and successful return to community-based schools, and family functioning was a major factor in improvement.14 In a follow-up study that surveyed patients via telephone >1 year after discharge from a PHP, almost 80% of the children and adolescents were either “doing OK” or were “well-adjusted.”14 Only 22.5% required inpatient or residential treatment; the majority were doing well in school, with only 7% failing.14 In addition, 60% of parents reported satisfaction with treatment, and 85% reported functional improvement in their children.
Factors that predict PHP success or failure
In an analysis of a day treatment program that provided 4 months of intensive psychodynamic, group-oriented milieu treatment for patients with long-standing personality disorders, Rosie et al16 found 3 factors that contributed to the success of the PHP:
- optimal treatment-patient matching
- judicious use of authority in milieu therapy
- maintaining close relationships with referral sources.
In a study that compared 58 patients who completed an Ottawa hospital PHP and 44 who did not complete the program, psychological mindedness and chronicity of psychiatric illness were found to predict completion.17 However, a study of 59 females with anorexia nervosa who were transferred from inpatient care to an eating disorder day hospital program found that a longer duration of illness, amenorrhea, and a lower body mass index were associated with PHP treatment failure and inpatient rehospitalization.18 One study found that for individuals who were referred to a PHP from inpatient care, suicidal ideation and greater psychotic symptoms were associated with acute inpatient rehospitalization.19 Other factors associated with PHP nonattendance and treatment failure include limited personal and economic resources, high rates of substance abuse disorders, multiple admissions, and disability.20 In a study of 103 alcohol-dependent patients who completed IOP treatment, 64% were abstinent at 6 months follow-up; relapse was associated with a longer duration of alcohol dependence and a higher number of prior treatments, while favorable outcomes were associated with a lower degree of depression, anxiety, and craving.21 Patients with cocaine dependence who completed an IOP showed significant improvements in addiction scores and had more favorable outcomes in employment status and psychological problems if they stayed longer in treatment.22
Bottom Line
Psychiatric partial hospitalization programs (PHPs) provide a transition from inpatient hospitalization to outpatient treatment for patients who need further stabilization, or serve as an alternative to inpatient treatment for patients who don’t need or want inpatient hospitalization. PHPs can be as effective as inpatient treatment for all but the most seriously ill patients, and are more cost-effective than inpatient treatment.
1. Neffinger GG. Partial hospitalization: an overview. J Community Psychol. 1981;9(3):262-269.
2. Leung MY, Drozd EM, Healy DA, et al. Impacts associated with the Medicare Psychiatric PPS: a study of partial hospitalization programs. February 2009. Accessed January 8, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Reports/downloads/Leung_PHP_PPS_2010.pdf
3. Williams T, Borders TF, Jasinski L. Partial Psychiatric Hospitalization Program Availability in Non-Metropolitan and Non-Metropolitan Hospitals Nationally. Rural and Underserved Health Research Center; 2019.
4. Rosser J, Michael S, eds. Standards and guidelines for partial hospital programs and intensive outpatient programs. Association for Ambulatory Behavioral Healthcare. 2018. Accessed January 8, 2022. https://aabh.org/wp-content/uploads/2021/05/2021-SandG-Final.pdf
5. CMS Manual System PUB. 100-02 Medicare Benefit Policy: Partial Hospitalization Services, Department of Health and Human Services. Centers for Medicare & Medicaid Services; 2004:6-9.
6. Joint Commission. Guide to Joint Commission Behavioral Healthcare Accreditation. Joint Commission; 2007:36.
7. Hoge MA, Farrell SP, Munchel ME, et al. Therapeutic factors in partial hospitalization. Psychiatry. 1988;51(2):199-210.
8. Ricketts T, Kirshbaum MN. Helpfulness of mental health day care: client and staff views. J Adv Nurs. 1994;20(2):297-306.
9. Marshall M, Crowther R, Almaraz-Serrano A, et al. Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) acute day hospital versus admission; (2) vocational rehabilitation; (3) day hospital versus outpatient care. Health Technol Assess. 2001;5(21):1-75.
10. Horvitz-Lennon M, Normand SL, Gaccione P, et al. Partial versus full hospitalization for adults in psychiatric distress: a systematic review of the published literature (1957-1997). Am J Psychiatry. 2001;158(5):676-685.
11. Sledge WH, Tebes J, Rakfeldt J, et al. Day hospital/crisis respite care versus inpatient care, Part I: Clinical outcomes. Am J Psychiatry. 1996;153(8):1065-1073.
12. Fink EB, Longabaugh R, Stout R. The paradoxical underutilization of partial hospitalization. Am J Psychiatry. 1978;135(6):713-716.
13. Sledge WH, Tebes J, Wolff N, et al. Day hospital/crisis respite care versus inpatient care, Part II: service utilization and costs. Am J Psychiatry. 1996;153(8):1074-1083.
14. Kiser LJ. Treatment-effectiveness research in child and adolescent partial hospitalization. Psychiatr Hosp. 1991;22(2):51-8.
15. Milin R, Coupland K, Walker S, et al. Outcome and follow-up study of an adolescent psychiatric day treatment school program. J Am Acad Child Adolesc Psychiatry. 2000;39(3):320-328.
16. Rosie JS, Azim HF, Piper WE, et al. Effective psychiatric day treatment: historical lessons. Psychiatr Serv. 1995;46(10):1019-1026.
17. Tasca GA, Balfour L, Bissada H, et al. Treatment completion and outcome in a partial hospitalization program: interactions among patient variables. Psychotherapy Res. 1999;9(2):232-247.
18. Howard WT, Evans KK, Quintero-Howard CV, et al. Predictors of success or failure of transition to day hospital treatment for inpatients with anorexia nervosa. Am J Psychiatry. 1999;156(11):1697-1702.
19. Beard C, Hearon BA, Lee J, et al. When partial hospitalization fails: risk factors for inpatient hospitalization. J Nerv Ment Dis. 2016;204(6):431-436.
20. Lieberman PB, Guggenheim FG. Reasons for patient nonattendance during acute partial hospitalization. Psychiatr Serv. 2016;67(6):684-687.
21. Bottlender M, Soyka M. Efficacy of an intensive outpatient rehabilitation program in alcoholism: predictors of outcome 6 months after treatment. Eur Addict Res. 2005;11(3):132-137.
22. Gottheil E, Weinstein SP, Sterling RC, et al. A randomized controlled study of the effectiveness of intensive outpatient treatment for cocaine dependence. Psychiatr Serv. 1998;49(6):782-787.
1. Neffinger GG. Partial hospitalization: an overview. J Community Psychol. 1981;9(3):262-269.
2. Leung MY, Drozd EM, Healy DA, et al. Impacts associated with the Medicare Psychiatric PPS: a study of partial hospitalization programs. February 2009. Accessed January 8, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Reports/downloads/Leung_PHP_PPS_2010.pdf
3. Williams T, Borders TF, Jasinski L. Partial Psychiatric Hospitalization Program Availability in Non-Metropolitan and Non-Metropolitan Hospitals Nationally. Rural and Underserved Health Research Center; 2019.
4. Rosser J, Michael S, eds. Standards and guidelines for partial hospital programs and intensive outpatient programs. Association for Ambulatory Behavioral Healthcare. 2018. Accessed January 8, 2022. https://aabh.org/wp-content/uploads/2021/05/2021-SandG-Final.pdf
5. CMS Manual System PUB. 100-02 Medicare Benefit Policy: Partial Hospitalization Services, Department of Health and Human Services. Centers for Medicare & Medicaid Services; 2004:6-9.
6. Joint Commission. Guide to Joint Commission Behavioral Healthcare Accreditation. Joint Commission; 2007:36.
7. Hoge MA, Farrell SP, Munchel ME, et al. Therapeutic factors in partial hospitalization. Psychiatry. 1988;51(2):199-210.
8. Ricketts T, Kirshbaum MN. Helpfulness of mental health day care: client and staff views. J Adv Nurs. 1994;20(2):297-306.
9. Marshall M, Crowther R, Almaraz-Serrano A, et al. Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) acute day hospital versus admission; (2) vocational rehabilitation; (3) day hospital versus outpatient care. Health Technol Assess. 2001;5(21):1-75.
10. Horvitz-Lennon M, Normand SL, Gaccione P, et al. Partial versus full hospitalization for adults in psychiatric distress: a systematic review of the published literature (1957-1997). Am J Psychiatry. 2001;158(5):676-685.
11. Sledge WH, Tebes J, Rakfeldt J, et al. Day hospital/crisis respite care versus inpatient care, Part I: Clinical outcomes. Am J Psychiatry. 1996;153(8):1065-1073.
12. Fink EB, Longabaugh R, Stout R. The paradoxical underutilization of partial hospitalization. Am J Psychiatry. 1978;135(6):713-716.
13. Sledge WH, Tebes J, Wolff N, et al. Day hospital/crisis respite care versus inpatient care, Part II: service utilization and costs. Am J Psychiatry. 1996;153(8):1074-1083.
14. Kiser LJ. Treatment-effectiveness research in child and adolescent partial hospitalization. Psychiatr Hosp. 1991;22(2):51-8.
15. Milin R, Coupland K, Walker S, et al. Outcome and follow-up study of an adolescent psychiatric day treatment school program. J Am Acad Child Adolesc Psychiatry. 2000;39(3):320-328.
16. Rosie JS, Azim HF, Piper WE, et al. Effective psychiatric day treatment: historical lessons. Psychiatr Serv. 1995;46(10):1019-1026.
17. Tasca GA, Balfour L, Bissada H, et al. Treatment completion and outcome in a partial hospitalization program: interactions among patient variables. Psychotherapy Res. 1999;9(2):232-247.
18. Howard WT, Evans KK, Quintero-Howard CV, et al. Predictors of success or failure of transition to day hospital treatment for inpatients with anorexia nervosa. Am J Psychiatry. 1999;156(11):1697-1702.
19. Beard C, Hearon BA, Lee J, et al. When partial hospitalization fails: risk factors for inpatient hospitalization. J Nerv Ment Dis. 2016;204(6):431-436.
20. Lieberman PB, Guggenheim FG. Reasons for patient nonattendance during acute partial hospitalization. Psychiatr Serv. 2016;67(6):684-687.
21. Bottlender M, Soyka M. Efficacy of an intensive outpatient rehabilitation program in alcoholism: predictors of outcome 6 months after treatment. Eur Addict Res. 2005;11(3):132-137.
22. Gottheil E, Weinstein SP, Sterling RC, et al. A randomized controlled study of the effectiveness of intensive outpatient treatment for cocaine dependence. Psychiatr Serv. 1998;49(6):782-787.
Honor thy parents? Understanding parricide and associated spree killings
Mr. B, age 37, presents to a community mental health center for an appointment following a recent emergency department visit. He is diagnosed with schizophrenia, and has been treated for approximately 1 year. Six months ago, Mr. B stopped taking his antipsychotic due to its adverse effects. Despite compliance with another agent, he has become increasingly disorganized and paranoid.
He now believes that his mother, with whom he has lived all his life and who serves as his guardian, is poisoning his food and trying to kill him. She is an employee at a local grocery store, and Mr. B has expressed concern that her coworkers are assisting her in the plot to kill him.
Following a home visit, Mr. B’s case manager indicates that the patient showed them the collection of weapons he is amassing to “defend” himself. This leads to a concern for the safety of the patient, his mother, and others.
Although parricide—killing one’s parent—is a relatively rare event, its sensationalistic nature has long captured the attention of headline writers and the general public. This article discusses the diagnostic and demographic factors that may be seen among individuals who kill their parents, with an emphasis on those who commit matricide (murder of one’s mother) and associated spree killings, where an individual kills multiple people within a single brief but contiguous time period. Understanding these characteristics can help clinicians identify and more safely manage patients who may be at risk of harming their parents in addition to others.
Characteristics of perpetrators of parricide
Worldwide, approximately 2% to 4% of homicides involve parricide, or killing one’s parent.1,2 Most offenders are men in early adulthood, though a proportion are adolescents and some are women.1,3 They are often single, unemployed, and live with the parent prior to the killing.1 Patricide occurs more frequently than matricide.4 In the United States, approximately 150 fathers and 100 mothers are killed by their child each year.5
In a study of all homicides in England and Wales between 1997 and 2014, two-thirds of parricide offenders had previously been diagnosed with a mental disorder.1 One-third were diagnosed with schizophrenia.1 In a Canadian study focusing on 43 adult perpetrators found not criminally responsible,6 most were experiencing psychotic symptoms at the time of parricide; symptoms of a personality disorder were the second-most prevalent symptoms. Similarly, Bourget et al4 studied Canadian coroner records for 64 parents killed by their children. Of the children involved in those parricides, 15% attempted suicide after the killing. Two-thirds of the male offenders evidenced delusional thinking, and/or excessive violence (overkill) was common. Some cases (16%) followed an argument, and some of those perpetrators were intoxicated or psychotic. From our clinical experience, when there are identifiable nonpsychotic triggers, they often can be small things such as an argument over food, smoking, or video games. Often, the perpetrator was financially dependent on their parents and were “trapped in a difficult/hostile/dependence/love relationship” with that parent.6 Adolescent males who kill their parents may not have psychosis7; however, they may be victims of longstanding serious abuse at the hands of their parents. These perpetrators often express relief rather than remorse after committing murder.
Three categories to classify the perpetrators of parricide have been proposed: severely abused, severely mentally ill, and “dangerously antisocial.”3 While severe mental illness was most common in adult defendants, severe abuse was most common in adolescent offenders. There may be significant commonalities between adolescent and adult perpetrators. A more recent latent class analysis by Bojanic et al1 indicated 3 unique types of parricide offenders (Table 1).
Continue to: Matricide: A closer look...
Matricide: A closer look
Though multiple studies have found a higher rate of psychosis among perpetrators of matricide, it is important to note that most people with psychotic disorders would never kill their mother. These events, however, tend to grab headlines and may be highly focused upon by the general population. In addition, matricide may be part of a larger crime that draws additional attention. For example, the 1966 University of Texas Bell Tower shooter and the 2012 Sandy Hook Elementary school shooter both killed their mothers before engaging in mass homicide. Often in cases of matricide, a longer-term dysfunctional relationship existed between the mother and the child. The mother is frequently described as controlling and intrusive and the (often adult) child as overly dependent, while the father may be absent or ineffectual. Hostility and mutual dependence are usual hallmarks of these relationships.8
However, in some cases where an individual with a psychotic disorder kills their mother, there may have been a traditional nurturing relationship.8 Alternative motivations unrelated to psychosis must be considered, including crimes motivated by money/inheritance or those perpetrated out of nonpsychotic anger. Green8 described motive categories for matricide that include paranoid and persecutory, altruistic, and other. In the “paranoid and persecutory” group, delusional beliefs about the mother occur; for example, the perpetrator may believe their mother was the devil. Sexual elements are found in some cases.8 Alternatively, the “altruistic” group demonstrated rather selfless reasons for killing, such as altruistic infanticide cases, or killing out of love.9 The altruistic matricide perpetrator may believe their mother is unwell, which may be a delusion or actually true. Finally, the “other” category contains cases related to jealousy, rage, and impulsivity.
In a study of 15 matricidal men in New York conducted by Campion et al,10 individuals seen by forensic psychiatric services for the crime of matricide included those with schizophrenia, substance-induced psychosis, and impulse control disorders. The authors noted there was often “a serious chronic derangement in the relationships of most matricidal men and their mothers.” Psychometric testing in these cases indicated feelings of dependency, weakness, and difficulty accepting an adult role separate from their mother. Some had conceptualized their mothers as a threat to their masculinity, while others had become enraged at their mothers.
Prevention requires addressing underlying issues
As described above, several factors are common among individuals who commit parricide, and these can be used to develop prevention strategies that focus on addressing underlying issues (Table 2). It is important to consider the relationship dynamics between the potential victim and perpetrator, as well as the motive, rather than focusing solely on mental illness or substance misuse.2
Spree killings that start as parricide
Although spree killing is a relatively rare event, a subset of spree killings involve parricide. One infamous recent event occurred in 2012 at Sandy Hook Elementary School, where the gunman killed his mother before going to the school and killing 26 additional people, many of whom were children.11,12 Because such events are rare, and because in these cases there is a high likelihood that the perpetrator is deceased (eg, died by suicide or killed by the police), much remains unknown about specific motivations and causative factors.
Information is often pieced together from postmortem reviews, which can be hampered by hindsight/recall bias and lack of contemporaneous documentation. Even worse, when these events occur, they may lead to a bias that all parricides or mass murders follow the pattern of the most recent case. This can result in overgeneralization of an individual’s history as being actionable risk violence factors for all potential parricide cases both by the public (eg, “My sister’s son has autism, and the Sandy Hook shooter was reported to have autism—should I be worried for my sister?”) and professionals (eg, “Will I be blamed for the next Sandy Hook by not taking more aggressive action even though I am not sure it is clinically warranted?”).
To identify trends for individuals committing parricide who engage in mass murder events (such as spree killing), we reviewed the 2000-2019 FBI active shooter list.12 Of the 333 events identified, 46 could be classified as domestic violence situations (eg, the perpetrator was in a romantic or familial relationship/former relationship and engaged in an active shooting incident involving at least 1 person from that relationship). We classified 11 of those 46 cases as parricide. Ten of those 11 parricides involved a child killing a parent (Table 3), and the other involved a grandchild killing a grandfather who served as their primary caregiver. Of the 11 incidents, mothers were involved (killed or wounded) in 4, and father figures (including the grandfather serving as a father and a stepfather) were killed in 9, with 2 incidents involving both parents. In 4 of the 11 parricides, other family members were killed in addition to the parent (including siblings, grandparents, or extended family). When considering spree shooters who committed parricide, 4 alleged perpetrators died by suicide, 1 was killed at the scene, and the rest were apprehended. The most common active shooting site endangering the public was an educational location (5), followed by commerce locations (4), with 2 involving open spaces. Eight of the 11 parricides occurred before the event was designated as an active shooting. The mean age for a parricide plus spree shooter was 23, once the oldest (age 61) and youngest (age 14) were removed from the calculation. The majority of the cases fell into the age range of 16 to 25 (n = 6), followed by 3 individuals who were age 26 to 31 (n = 3). All suspected individuals were male.
It is difficult to ascertain the existence of prior mental health care because perpetrators’ medical records may be confidential, juvenile court records may be sealed, and there may not even be a trial due to death or suicide (leading to limited forensic psychiatry examination or public testimony). Among those apprehended, many individuals raise some form of mental health defense, but the validity of their diagnosis may be undermined by concerns of possible malingering, especially in cases where the individual did not have a history of psychiatric treatment prior to the event.11 In summary, based on FBI data,12 spree shooters who committed parricide were usually male, in their late adolescence or early 20s, and more frequently targeted father figures. They often committed the parricide first and at a different location from later “active” shootings. Police were usually not aware of the parricide until after the spree is over.
Continue to: Parricide and society...
Parricide and society
For centuries, mothers and fathers have been honored and revered. Therefore, it is not surprising that killing of one’s mother or father attracts a great deal of macabre interest. Examples of parricide are present throughout popular culture, in mythology, comic books, movies, and television. As all psychiatrists know, Oedipus killed his father and married his mother. Other popular culture examples include: Grant Morrison’s Arkham Asylum: A Serious House on Serious Earth, Alfred Hitchcock’s Psycho, Oliver Stone’s Natural Born Killers, Peter Jackson’s Heavenly Creatures, The Affair drama series, and Star Wars: The Force Awakens.13,14
CASE CONTINUED
In Mr. B’s case, it is imperative for the treatment team to inquire about his history of violence, paying particular attention to prior violent acts towards his mother. His clinicians should consider hospitalization with the guardian’s consent if the danger appears imminent, especially considering the presence of weapons at home. They should attempt to stabilize Mr. B on effective, tolerable medications to ameliorate his psychosis, and to refer him for long-term psychotherapy to address difficult dynamic issues in the family relationship and encourage compliance with treatment. These steps may help avert a tragedy.
Bottom Line
Individuals who commit parricide often have a history of psychosis, a mood disorder, childhood abuse, and/or difficult relationship dynamics with the parent they kill. Some go on a spree killing in the community. Through careful consideration of individual risk factors, psychiatrists may help prevent some cases of parent murder by a child and possibly more tragedy in the community.
1. Bojanié L, Flynn S, Gianatsi M, et al. The typology of parricide and the role of mental illness: data-driven approach. Aggress Behav. 2020;46(6):516-522.
2. Pinals DS. Parricide. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:113-138.
3. Heide KM. Matricide and stepmatricide victims and offenders: an empirical analysis of US arrest data. Behav Sci Law. 2013;31(2):203-214.
4. Bourget D, Gagné P, Labelle ME. Parricide: a comparative study of matricide versus patricide. J Am Acad Psychiatry Law. 2007;35(3):306-312.
5. Heide KM, Frei A. Matricide: a critique of the literature. Trauma Violence Abuse. 2010;11(1):3-17.
6. Marleau JD, Auclair N, Millaud F. Comparison of factors associated with parricide in adults and adolescents. J Fam Viol. 2006;21:321-325.
7. West SG, Feldsher M. Parricide: characteristics of sons and daughters who kill their parents. Current Psychiatry. 2010;9(11):20-38.
8. Green CM. Matricide by sons. Med Sci Law. 1981;21(3):207-214.
9. Friedman SH. Conclusions. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:161-164.
10. Campion J, Cravens JM, Rotholc A, et al. A study of 15 matricidal men. Am J Psychiatry. 1985;142(3):312-317.
11. Hall RCW, Friedman SH, Sorrentino R, et al. The myth of school shooters and psychotropic medications. Behav Sci Law. 2019;37(5):540-558.
12. Department of Justice Federal Bureau of Investigation. Active Shooter Incidents: 20-Year Review, 2000-2019. June 1, 2021. Accessed October 12, 2021. https://www.fbi.gov/file-repository/active-shooter-incidents-20-year-review-2000-2019-060121.pdf/view
13. Friedman SH, Hall RCW. Star Wars: The Force Awakens, forensic teaching about matricide. J Am Acad Psychiatry Law. 2017;45(1):128-130.
14. Friedman SH, Hall RCW. Deadly and dysfunctional family dynamics: when fiction mirrors fact. In: Packer S, Fredrick DR, eds. Welcome to Arkham Asylum: Essays on Psychiatry and the Gotham City Institution. McFarland; 2019:65-75.
Mr. B, age 37, presents to a community mental health center for an appointment following a recent emergency department visit. He is diagnosed with schizophrenia, and has been treated for approximately 1 year. Six months ago, Mr. B stopped taking his antipsychotic due to its adverse effects. Despite compliance with another agent, he has become increasingly disorganized and paranoid.
He now believes that his mother, with whom he has lived all his life and who serves as his guardian, is poisoning his food and trying to kill him. She is an employee at a local grocery store, and Mr. B has expressed concern that her coworkers are assisting her in the plot to kill him.
Following a home visit, Mr. B’s case manager indicates that the patient showed them the collection of weapons he is amassing to “defend” himself. This leads to a concern for the safety of the patient, his mother, and others.
Although parricide—killing one’s parent—is a relatively rare event, its sensationalistic nature has long captured the attention of headline writers and the general public. This article discusses the diagnostic and demographic factors that may be seen among individuals who kill their parents, with an emphasis on those who commit matricide (murder of one’s mother) and associated spree killings, where an individual kills multiple people within a single brief but contiguous time period. Understanding these characteristics can help clinicians identify and more safely manage patients who may be at risk of harming their parents in addition to others.
Characteristics of perpetrators of parricide
Worldwide, approximately 2% to 4% of homicides involve parricide, or killing one’s parent.1,2 Most offenders are men in early adulthood, though a proportion are adolescents and some are women.1,3 They are often single, unemployed, and live with the parent prior to the killing.1 Patricide occurs more frequently than matricide.4 In the United States, approximately 150 fathers and 100 mothers are killed by their child each year.5
In a study of all homicides in England and Wales between 1997 and 2014, two-thirds of parricide offenders had previously been diagnosed with a mental disorder.1 One-third were diagnosed with schizophrenia.1 In a Canadian study focusing on 43 adult perpetrators found not criminally responsible,6 most were experiencing psychotic symptoms at the time of parricide; symptoms of a personality disorder were the second-most prevalent symptoms. Similarly, Bourget et al4 studied Canadian coroner records for 64 parents killed by their children. Of the children involved in those parricides, 15% attempted suicide after the killing. Two-thirds of the male offenders evidenced delusional thinking, and/or excessive violence (overkill) was common. Some cases (16%) followed an argument, and some of those perpetrators were intoxicated or psychotic. From our clinical experience, when there are identifiable nonpsychotic triggers, they often can be small things such as an argument over food, smoking, or video games. Often, the perpetrator was financially dependent on their parents and were “trapped in a difficult/hostile/dependence/love relationship” with that parent.6 Adolescent males who kill their parents may not have psychosis7; however, they may be victims of longstanding serious abuse at the hands of their parents. These perpetrators often express relief rather than remorse after committing murder.
Three categories to classify the perpetrators of parricide have been proposed: severely abused, severely mentally ill, and “dangerously antisocial.”3 While severe mental illness was most common in adult defendants, severe abuse was most common in adolescent offenders. There may be significant commonalities between adolescent and adult perpetrators. A more recent latent class analysis by Bojanic et al1 indicated 3 unique types of parricide offenders (Table 1).
Continue to: Matricide: A closer look...
Matricide: A closer look
Though multiple studies have found a higher rate of psychosis among perpetrators of matricide, it is important to note that most people with psychotic disorders would never kill their mother. These events, however, tend to grab headlines and may be highly focused upon by the general population. In addition, matricide may be part of a larger crime that draws additional attention. For example, the 1966 University of Texas Bell Tower shooter and the 2012 Sandy Hook Elementary school shooter both killed their mothers before engaging in mass homicide. Often in cases of matricide, a longer-term dysfunctional relationship existed between the mother and the child. The mother is frequently described as controlling and intrusive and the (often adult) child as overly dependent, while the father may be absent or ineffectual. Hostility and mutual dependence are usual hallmarks of these relationships.8
However, in some cases where an individual with a psychotic disorder kills their mother, there may have been a traditional nurturing relationship.8 Alternative motivations unrelated to psychosis must be considered, including crimes motivated by money/inheritance or those perpetrated out of nonpsychotic anger. Green8 described motive categories for matricide that include paranoid and persecutory, altruistic, and other. In the “paranoid and persecutory” group, delusional beliefs about the mother occur; for example, the perpetrator may believe their mother was the devil. Sexual elements are found in some cases.8 Alternatively, the “altruistic” group demonstrated rather selfless reasons for killing, such as altruistic infanticide cases, or killing out of love.9 The altruistic matricide perpetrator may believe their mother is unwell, which may be a delusion or actually true. Finally, the “other” category contains cases related to jealousy, rage, and impulsivity.
In a study of 15 matricidal men in New York conducted by Campion et al,10 individuals seen by forensic psychiatric services for the crime of matricide included those with schizophrenia, substance-induced psychosis, and impulse control disorders. The authors noted there was often “a serious chronic derangement in the relationships of most matricidal men and their mothers.” Psychometric testing in these cases indicated feelings of dependency, weakness, and difficulty accepting an adult role separate from their mother. Some had conceptualized their mothers as a threat to their masculinity, while others had become enraged at their mothers.
Prevention requires addressing underlying issues
As described above, several factors are common among individuals who commit parricide, and these can be used to develop prevention strategies that focus on addressing underlying issues (Table 2). It is important to consider the relationship dynamics between the potential victim and perpetrator, as well as the motive, rather than focusing solely on mental illness or substance misuse.2
Spree killings that start as parricide
Although spree killing is a relatively rare event, a subset of spree killings involve parricide. One infamous recent event occurred in 2012 at Sandy Hook Elementary School, where the gunman killed his mother before going to the school and killing 26 additional people, many of whom were children.11,12 Because such events are rare, and because in these cases there is a high likelihood that the perpetrator is deceased (eg, died by suicide or killed by the police), much remains unknown about specific motivations and causative factors.
Information is often pieced together from postmortem reviews, which can be hampered by hindsight/recall bias and lack of contemporaneous documentation. Even worse, when these events occur, they may lead to a bias that all parricides or mass murders follow the pattern of the most recent case. This can result in overgeneralization of an individual’s history as being actionable risk violence factors for all potential parricide cases both by the public (eg, “My sister’s son has autism, and the Sandy Hook shooter was reported to have autism—should I be worried for my sister?”) and professionals (eg, “Will I be blamed for the next Sandy Hook by not taking more aggressive action even though I am not sure it is clinically warranted?”).
To identify trends for individuals committing parricide who engage in mass murder events (such as spree killing), we reviewed the 2000-2019 FBI active shooter list.12 Of the 333 events identified, 46 could be classified as domestic violence situations (eg, the perpetrator was in a romantic or familial relationship/former relationship and engaged in an active shooting incident involving at least 1 person from that relationship). We classified 11 of those 46 cases as parricide. Ten of those 11 parricides involved a child killing a parent (Table 3), and the other involved a grandchild killing a grandfather who served as their primary caregiver. Of the 11 incidents, mothers were involved (killed or wounded) in 4, and father figures (including the grandfather serving as a father and a stepfather) were killed in 9, with 2 incidents involving both parents. In 4 of the 11 parricides, other family members were killed in addition to the parent (including siblings, grandparents, or extended family). When considering spree shooters who committed parricide, 4 alleged perpetrators died by suicide, 1 was killed at the scene, and the rest were apprehended. The most common active shooting site endangering the public was an educational location (5), followed by commerce locations (4), with 2 involving open spaces. Eight of the 11 parricides occurred before the event was designated as an active shooting. The mean age for a parricide plus spree shooter was 23, once the oldest (age 61) and youngest (age 14) were removed from the calculation. The majority of the cases fell into the age range of 16 to 25 (n = 6), followed by 3 individuals who were age 26 to 31 (n = 3). All suspected individuals were male.
It is difficult to ascertain the existence of prior mental health care because perpetrators’ medical records may be confidential, juvenile court records may be sealed, and there may not even be a trial due to death or suicide (leading to limited forensic psychiatry examination or public testimony). Among those apprehended, many individuals raise some form of mental health defense, but the validity of their diagnosis may be undermined by concerns of possible malingering, especially in cases where the individual did not have a history of psychiatric treatment prior to the event.11 In summary, based on FBI data,12 spree shooters who committed parricide were usually male, in their late adolescence or early 20s, and more frequently targeted father figures. They often committed the parricide first and at a different location from later “active” shootings. Police were usually not aware of the parricide until after the spree is over.
Continue to: Parricide and society...
Parricide and society
For centuries, mothers and fathers have been honored and revered. Therefore, it is not surprising that killing of one’s mother or father attracts a great deal of macabre interest. Examples of parricide are present throughout popular culture, in mythology, comic books, movies, and television. As all psychiatrists know, Oedipus killed his father and married his mother. Other popular culture examples include: Grant Morrison’s Arkham Asylum: A Serious House on Serious Earth, Alfred Hitchcock’s Psycho, Oliver Stone’s Natural Born Killers, Peter Jackson’s Heavenly Creatures, The Affair drama series, and Star Wars: The Force Awakens.13,14
CASE CONTINUED
In Mr. B’s case, it is imperative for the treatment team to inquire about his history of violence, paying particular attention to prior violent acts towards his mother. His clinicians should consider hospitalization with the guardian’s consent if the danger appears imminent, especially considering the presence of weapons at home. They should attempt to stabilize Mr. B on effective, tolerable medications to ameliorate his psychosis, and to refer him for long-term psychotherapy to address difficult dynamic issues in the family relationship and encourage compliance with treatment. These steps may help avert a tragedy.
Bottom Line
Individuals who commit parricide often have a history of psychosis, a mood disorder, childhood abuse, and/or difficult relationship dynamics with the parent they kill. Some go on a spree killing in the community. Through careful consideration of individual risk factors, psychiatrists may help prevent some cases of parent murder by a child and possibly more tragedy in the community.
Mr. B, age 37, presents to a community mental health center for an appointment following a recent emergency department visit. He is diagnosed with schizophrenia, and has been treated for approximately 1 year. Six months ago, Mr. B stopped taking his antipsychotic due to its adverse effects. Despite compliance with another agent, he has become increasingly disorganized and paranoid.
He now believes that his mother, with whom he has lived all his life and who serves as his guardian, is poisoning his food and trying to kill him. She is an employee at a local grocery store, and Mr. B has expressed concern that her coworkers are assisting her in the plot to kill him.
Following a home visit, Mr. B’s case manager indicates that the patient showed them the collection of weapons he is amassing to “defend” himself. This leads to a concern for the safety of the patient, his mother, and others.
Although parricide—killing one’s parent—is a relatively rare event, its sensationalistic nature has long captured the attention of headline writers and the general public. This article discusses the diagnostic and demographic factors that may be seen among individuals who kill their parents, with an emphasis on those who commit matricide (murder of one’s mother) and associated spree killings, where an individual kills multiple people within a single brief but contiguous time period. Understanding these characteristics can help clinicians identify and more safely manage patients who may be at risk of harming their parents in addition to others.
Characteristics of perpetrators of parricide
Worldwide, approximately 2% to 4% of homicides involve parricide, or killing one’s parent.1,2 Most offenders are men in early adulthood, though a proportion are adolescents and some are women.1,3 They are often single, unemployed, and live with the parent prior to the killing.1 Patricide occurs more frequently than matricide.4 In the United States, approximately 150 fathers and 100 mothers are killed by their child each year.5
In a study of all homicides in England and Wales between 1997 and 2014, two-thirds of parricide offenders had previously been diagnosed with a mental disorder.1 One-third were diagnosed with schizophrenia.1 In a Canadian study focusing on 43 adult perpetrators found not criminally responsible,6 most were experiencing psychotic symptoms at the time of parricide; symptoms of a personality disorder were the second-most prevalent symptoms. Similarly, Bourget et al4 studied Canadian coroner records for 64 parents killed by their children. Of the children involved in those parricides, 15% attempted suicide after the killing. Two-thirds of the male offenders evidenced delusional thinking, and/or excessive violence (overkill) was common. Some cases (16%) followed an argument, and some of those perpetrators were intoxicated or psychotic. From our clinical experience, when there are identifiable nonpsychotic triggers, they often can be small things such as an argument over food, smoking, or video games. Often, the perpetrator was financially dependent on their parents and were “trapped in a difficult/hostile/dependence/love relationship” with that parent.6 Adolescent males who kill their parents may not have psychosis7; however, they may be victims of longstanding serious abuse at the hands of their parents. These perpetrators often express relief rather than remorse after committing murder.
Three categories to classify the perpetrators of parricide have been proposed: severely abused, severely mentally ill, and “dangerously antisocial.”3 While severe mental illness was most common in adult defendants, severe abuse was most common in adolescent offenders. There may be significant commonalities between adolescent and adult perpetrators. A more recent latent class analysis by Bojanic et al1 indicated 3 unique types of parricide offenders (Table 1).
Continue to: Matricide: A closer look...
Matricide: A closer look
Though multiple studies have found a higher rate of psychosis among perpetrators of matricide, it is important to note that most people with psychotic disorders would never kill their mother. These events, however, tend to grab headlines and may be highly focused upon by the general population. In addition, matricide may be part of a larger crime that draws additional attention. For example, the 1966 University of Texas Bell Tower shooter and the 2012 Sandy Hook Elementary school shooter both killed their mothers before engaging in mass homicide. Often in cases of matricide, a longer-term dysfunctional relationship existed between the mother and the child. The mother is frequently described as controlling and intrusive and the (often adult) child as overly dependent, while the father may be absent or ineffectual. Hostility and mutual dependence are usual hallmarks of these relationships.8
However, in some cases where an individual with a psychotic disorder kills their mother, there may have been a traditional nurturing relationship.8 Alternative motivations unrelated to psychosis must be considered, including crimes motivated by money/inheritance or those perpetrated out of nonpsychotic anger. Green8 described motive categories for matricide that include paranoid and persecutory, altruistic, and other. In the “paranoid and persecutory” group, delusional beliefs about the mother occur; for example, the perpetrator may believe their mother was the devil. Sexual elements are found in some cases.8 Alternatively, the “altruistic” group demonstrated rather selfless reasons for killing, such as altruistic infanticide cases, or killing out of love.9 The altruistic matricide perpetrator may believe their mother is unwell, which may be a delusion or actually true. Finally, the “other” category contains cases related to jealousy, rage, and impulsivity.
In a study of 15 matricidal men in New York conducted by Campion et al,10 individuals seen by forensic psychiatric services for the crime of matricide included those with schizophrenia, substance-induced psychosis, and impulse control disorders. The authors noted there was often “a serious chronic derangement in the relationships of most matricidal men and their mothers.” Psychometric testing in these cases indicated feelings of dependency, weakness, and difficulty accepting an adult role separate from their mother. Some had conceptualized their mothers as a threat to their masculinity, while others had become enraged at their mothers.
Prevention requires addressing underlying issues
As described above, several factors are common among individuals who commit parricide, and these can be used to develop prevention strategies that focus on addressing underlying issues (Table 2). It is important to consider the relationship dynamics between the potential victim and perpetrator, as well as the motive, rather than focusing solely on mental illness or substance misuse.2
Spree killings that start as parricide
Although spree killing is a relatively rare event, a subset of spree killings involve parricide. One infamous recent event occurred in 2012 at Sandy Hook Elementary School, where the gunman killed his mother before going to the school and killing 26 additional people, many of whom were children.11,12 Because such events are rare, and because in these cases there is a high likelihood that the perpetrator is deceased (eg, died by suicide or killed by the police), much remains unknown about specific motivations and causative factors.
Information is often pieced together from postmortem reviews, which can be hampered by hindsight/recall bias and lack of contemporaneous documentation. Even worse, when these events occur, they may lead to a bias that all parricides or mass murders follow the pattern of the most recent case. This can result in overgeneralization of an individual’s history as being actionable risk violence factors for all potential parricide cases both by the public (eg, “My sister’s son has autism, and the Sandy Hook shooter was reported to have autism—should I be worried for my sister?”) and professionals (eg, “Will I be blamed for the next Sandy Hook by not taking more aggressive action even though I am not sure it is clinically warranted?”).
To identify trends for individuals committing parricide who engage in mass murder events (such as spree killing), we reviewed the 2000-2019 FBI active shooter list.12 Of the 333 events identified, 46 could be classified as domestic violence situations (eg, the perpetrator was in a romantic or familial relationship/former relationship and engaged in an active shooting incident involving at least 1 person from that relationship). We classified 11 of those 46 cases as parricide. Ten of those 11 parricides involved a child killing a parent (Table 3), and the other involved a grandchild killing a grandfather who served as their primary caregiver. Of the 11 incidents, mothers were involved (killed or wounded) in 4, and father figures (including the grandfather serving as a father and a stepfather) were killed in 9, with 2 incidents involving both parents. In 4 of the 11 parricides, other family members were killed in addition to the parent (including siblings, grandparents, or extended family). When considering spree shooters who committed parricide, 4 alleged perpetrators died by suicide, 1 was killed at the scene, and the rest were apprehended. The most common active shooting site endangering the public was an educational location (5), followed by commerce locations (4), with 2 involving open spaces. Eight of the 11 parricides occurred before the event was designated as an active shooting. The mean age for a parricide plus spree shooter was 23, once the oldest (age 61) and youngest (age 14) were removed from the calculation. The majority of the cases fell into the age range of 16 to 25 (n = 6), followed by 3 individuals who were age 26 to 31 (n = 3). All suspected individuals were male.
It is difficult to ascertain the existence of prior mental health care because perpetrators’ medical records may be confidential, juvenile court records may be sealed, and there may not even be a trial due to death or suicide (leading to limited forensic psychiatry examination or public testimony). Among those apprehended, many individuals raise some form of mental health defense, but the validity of their diagnosis may be undermined by concerns of possible malingering, especially in cases where the individual did not have a history of psychiatric treatment prior to the event.11 In summary, based on FBI data,12 spree shooters who committed parricide were usually male, in their late adolescence or early 20s, and more frequently targeted father figures. They often committed the parricide first and at a different location from later “active” shootings. Police were usually not aware of the parricide until after the spree is over.
Continue to: Parricide and society...
Parricide and society
For centuries, mothers and fathers have been honored and revered. Therefore, it is not surprising that killing of one’s mother or father attracts a great deal of macabre interest. Examples of parricide are present throughout popular culture, in mythology, comic books, movies, and television. As all psychiatrists know, Oedipus killed his father and married his mother. Other popular culture examples include: Grant Morrison’s Arkham Asylum: A Serious House on Serious Earth, Alfred Hitchcock’s Psycho, Oliver Stone’s Natural Born Killers, Peter Jackson’s Heavenly Creatures, The Affair drama series, and Star Wars: The Force Awakens.13,14
CASE CONTINUED
In Mr. B’s case, it is imperative for the treatment team to inquire about his history of violence, paying particular attention to prior violent acts towards his mother. His clinicians should consider hospitalization with the guardian’s consent if the danger appears imminent, especially considering the presence of weapons at home. They should attempt to stabilize Mr. B on effective, tolerable medications to ameliorate his psychosis, and to refer him for long-term psychotherapy to address difficult dynamic issues in the family relationship and encourage compliance with treatment. These steps may help avert a tragedy.
Bottom Line
Individuals who commit parricide often have a history of psychosis, a mood disorder, childhood abuse, and/or difficult relationship dynamics with the parent they kill. Some go on a spree killing in the community. Through careful consideration of individual risk factors, psychiatrists may help prevent some cases of parent murder by a child and possibly more tragedy in the community.
1. Bojanié L, Flynn S, Gianatsi M, et al. The typology of parricide and the role of mental illness: data-driven approach. Aggress Behav. 2020;46(6):516-522.
2. Pinals DS. Parricide. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:113-138.
3. Heide KM. Matricide and stepmatricide victims and offenders: an empirical analysis of US arrest data. Behav Sci Law. 2013;31(2):203-214.
4. Bourget D, Gagné P, Labelle ME. Parricide: a comparative study of matricide versus patricide. J Am Acad Psychiatry Law. 2007;35(3):306-312.
5. Heide KM, Frei A. Matricide: a critique of the literature. Trauma Violence Abuse. 2010;11(1):3-17.
6. Marleau JD, Auclair N, Millaud F. Comparison of factors associated with parricide in adults and adolescents. J Fam Viol. 2006;21:321-325.
7. West SG, Feldsher M. Parricide: characteristics of sons and daughters who kill their parents. Current Psychiatry. 2010;9(11):20-38.
8. Green CM. Matricide by sons. Med Sci Law. 1981;21(3):207-214.
9. Friedman SH. Conclusions. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:161-164.
10. Campion J, Cravens JM, Rotholc A, et al. A study of 15 matricidal men. Am J Psychiatry. 1985;142(3):312-317.
11. Hall RCW, Friedman SH, Sorrentino R, et al. The myth of school shooters and psychotropic medications. Behav Sci Law. 2019;37(5):540-558.
12. Department of Justice Federal Bureau of Investigation. Active Shooter Incidents: 20-Year Review, 2000-2019. June 1, 2021. Accessed October 12, 2021. https://www.fbi.gov/file-repository/active-shooter-incidents-20-year-review-2000-2019-060121.pdf/view
13. Friedman SH, Hall RCW. Star Wars: The Force Awakens, forensic teaching about matricide. J Am Acad Psychiatry Law. 2017;45(1):128-130.
14. Friedman SH, Hall RCW. Deadly and dysfunctional family dynamics: when fiction mirrors fact. In: Packer S, Fredrick DR, eds. Welcome to Arkham Asylum: Essays on Psychiatry and the Gotham City Institution. McFarland; 2019:65-75.
1. Bojanié L, Flynn S, Gianatsi M, et al. The typology of parricide and the role of mental illness: data-driven approach. Aggress Behav. 2020;46(6):516-522.
2. Pinals DS. Parricide. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:113-138.
3. Heide KM. Matricide and stepmatricide victims and offenders: an empirical analysis of US arrest data. Behav Sci Law. 2013;31(2):203-214.
4. Bourget D, Gagné P, Labelle ME. Parricide: a comparative study of matricide versus patricide. J Am Acad Psychiatry Law. 2007;35(3):306-312.
5. Heide KM, Frei A. Matricide: a critique of the literature. Trauma Violence Abuse. 2010;11(1):3-17.
6. Marleau JD, Auclair N, Millaud F. Comparison of factors associated with parricide in adults and adolescents. J Fam Viol. 2006;21:321-325.
7. West SG, Feldsher M. Parricide: characteristics of sons and daughters who kill their parents. Current Psychiatry. 2010;9(11):20-38.
8. Green CM. Matricide by sons. Med Sci Law. 1981;21(3):207-214.
9. Friedman SH. Conclusions. In Friedman SH, ed. Family Murder: Pathologies of Love and Hate. American Psychiatric Publishing; 2019:161-164.
10. Campion J, Cravens JM, Rotholc A, et al. A study of 15 matricidal men. Am J Psychiatry. 1985;142(3):312-317.
11. Hall RCW, Friedman SH, Sorrentino R, et al. The myth of school shooters and psychotropic medications. Behav Sci Law. 2019;37(5):540-558.
12. Department of Justice Federal Bureau of Investigation. Active Shooter Incidents: 20-Year Review, 2000-2019. June 1, 2021. Accessed October 12, 2021. https://www.fbi.gov/file-repository/active-shooter-incidents-20-year-review-2000-2019-060121.pdf/view
13. Friedman SH, Hall RCW. Star Wars: The Force Awakens, forensic teaching about matricide. J Am Acad Psychiatry Law. 2017;45(1):128-130.
14. Friedman SH, Hall RCW. Deadly and dysfunctional family dynamics: when fiction mirrors fact. In: Packer S, Fredrick DR, eds. Welcome to Arkham Asylum: Essays on Psychiatry and the Gotham City Institution. McFarland; 2019:65-75.
Too close for comfort: When the psychiatrist is stalked
Dr. A has been treating Ms. W, a graduate student, for depression. Ms. W made subtle comments expressing her interest in pursuing a romantic relationship with her psychiatrist. Dr. A gently redirected her, and she seemed to respond appropriately. However, over the past 2 weeks, Dr. A has seen Ms. W at a local park and at the grocery store. Today, Dr. A is startled to see Ms. W at her weekly yoga class. Dr. A plans to ask her supervisor for advice.
Dr. M is a child psychiatrist who spoke at his local school board meeting in support of masking requirements for students during COVID-19. During the discussion, Dr. M shared that, as a psychiatrist, he does not believe it is especially distressing for students to wear masks, and that doing so is a necessary public health measure. On leaving, other parents shouted, “We know who you are and where you live!” The next day, his integrated clinic started receiving threatening and harassing messages, including threats to kill him or his staff if they take part in vaccinating children against COVID-19.
Because of their work, mental health professionals—like other health care professionals—face an elevated risk of being harassed or stalked. Stalking often includes online harassment and may escalate to serious physical violence. Stalking is criminal behavior by a patient and should not be constructed as a “failure to manage transference.” This article explores basic strategies to reduce the risk of harassment and stalking, describes how to recognize early behaviors, and outlines basic steps health care professionals and their employers can take to respond to stalking and harassing behaviors.
Although this article is intended for psychiatrists, it is important to note that all health professionals have significant risk for experiencing stalking or harassment. This is due in part, but not exclusively, to our clinical work. Estimates of how many health professionals experience stalking vary substantially depending upon the study, and differences in methodologies limit easy comparison or extrapolation. More thorough reviews have reported ranges from 2% to 70% among physicians; psychiatrists and other mental health professionals appear to be at greater risk than those in other specialties and the general population.1-3 Physicians who are active on social media may also be at elevated risk.4 Unexpected communications from patients and their family members—especially those with threatening, harassing, or sexualized tones, or involving contact outside of a work setting—can be distressing. These behaviors represent potential harbingers of more dangerous behavior, including physical assault, sexual assault, or homicide. Despite their elevated risk, many psychiatrists are unaware of how to prevent or respond to stalking or harassment.
Recognizing harassment and stalking
Repeated and unwanted contact or communication, regardless of intent, may constitute stalking. Legal definitions vary by jurisdiction and may not align with subjective experiences or understanding of what constitutes stalking.5 At its essence, stalking is repeated harassing behaviors likely to provoke fear in the targeted person. FOUR is a helpful mnemonic when conceptualizing the attributes of stalking: Fixated, Obsessive, Unwanted, and Repetitive.6Table 1 lists examples of common stalking behaviors. Stalking and harassing behavior may be from a known source (eg, a patient, coworker, or paramour), a masked source (ie, someone known to the target but who conceals or obscures their identity), or from otherwise unknown persons. Behaviors that persist after the person engaging in the behaviors has clearly been informed that they are unwanted or inappropriate are especially concerning. Stalking may escalate to include physical or sexual assault and, in some cases, homicide.

Stalking duration can vary substantially, as can the factors that lead to the cessation of the behavior. Indicators of increased risk for physical violence include unwanted physical presence/following of the target (“approach behaviors”), having a prior violent intimate relationship, property destruction, explicit threats, and having a prior intimate relationship with the target.7
Stalking contact or communication may be unwanted because of the content (eg, sexualized or threatening tone), location (eg, at a professional’s home), or means (eg, through social media). Stalking behaviors are not appropriate in any relationship, including a clinical relationship. They should not be treated as a “failure to manage transference” or in other victim-blaming ways.
There are multiple typologies for stalking behavior. Common motivations for stalking health professionals include resentment or grievance, misjudgment of social boundaries, and delusional fixation, including erotomania.8 Associated psychopathologies vary significantly and, while some may be more amenable to psychiatric treatment than others, psychiatrists should not feel compelled to treat patients who repeatedly violate boundaries, regardless of intent or comorbidity.
Patients are not the exclusive perpetrators of stalking; a recent study found that 4% of physicians surveyed reported current or recent stalking by a current or former intimate partner.9 When a person who is a victim of intimate partner violence is also stalked as part of the abuse, homicide risk increases.10 Workplace homicides of health care professionals are most likely to be committed by a current or former partner or other personal acquaintance, not by a patient.11 Workplace harassment and stalking of health care professionals is especially concerning because this behavior can escalate and endanger coworkers or clients.
Continue to: Risk awareness: Recognize your exposure...
Risk awareness: Recognize your exposure
About 80% of stalking involves some form of technology—often telephone calls but also online or other “cyber” elements.12 One recent survey found the rate of online harassment, including threats of physical and sexual violence, was >20% among physicians who were active on social media.4 Health professionals may be at greater risk of having patients find their personal information simply because patients routinely search online for information about new clinicians. Personal information about a clinician may be readily visible among professional information in search results, or a curious patient may simply scroll further down in the results. For a potential stalker, clicking on a search result linking to a personal social media page may be far easier than finding a home address and going in person—but the action may be just as distressing or risky for the clinician.13 Additionally, items visible in a clinician’s office—or visible in the background of those providing telehealth services from their home—may inadvertently reveal personal information about the clinician, their home, or their family.
Psychiatrists are often in a special position in relation to patients and times of crises. They may be involved in involuntary commitment—or declining an admission when a patient or family wishes it. They may be present at the time of the revelation of a serious diagnosis, abuse, injury, or death. They may be a mandated reporter of child or elder abuse.2 Additionally, physicians may be engaged in discourse on politically charged public health topics.14 These factors may increase their risk of being stalked.
Conducting an online visibility self-assessment can be a useful way to learn what information others can find. Table 2 outlines the steps for completing this exercise. Searching multiple iterations of your current and former names (with and without degrees, titles, and cities) will yield differing results in various search engines. After establishing a baseline of what information is available online, it can be helpful to periodically repeat this exercise, and to set up automated alerts for your name, number(s), email(s), and address(es).

Basic mitigation strategies
In the modern era, being invisible online is impractical and likely impossible—especially for a health care professional. Instead, it may be prudent to limit your public visibility to professional portals (eg, LinkedIn or Doximity) and maximize privacy settings on other platforms. Another basic strategy is to avoid providing personal contact information (your home address, phone number, or personal email) for professional purposes, such as licensing and credentialing, conference submissions, or journal publications. Be aware that driving a visually distinct vehicle—one with vanity plates or distinct bumper stickers, or an exotic sportscar—can make it easier to be recognized and located. A personally recorded voicemail greeting (vs one recorded by, for example, an office manager) may be inappropriately reinforcing for some stalkers.
Workplaces should have an established safety policy that addresses stalking and harassment of employees. Similarly, patients and others should receive clear education on how to contact different staff, including physicians, with consideration of how and when to use electronic health information portals, office numbers, and emails. Workplaces should not disclose staff schedules. For example, a receptionist should say “I’ll have Dr. Diaz return your call when she can” instead of “Dr. Diaz is not in until tomorrow.” Avoid unnecessary location/name signals (eg, a parking spot labeled “Dr. Diaz”). Consider creating alert words or phrases for staff to use to signal they are concerned about their immediate safety—and provide education and training, including drills, to test emergency responses when the words/phrases are used. Leaders and managers should nurture a workplace culture where people are comfortable seeking support if they feel they may be the target of harassment or stalking. Many larger health care organizations have threat management programs, which can play a critical role in preventing, investigating, and responding to stalking of employees. Increasingly, threat management teams are being identified as a best practice in health care settings.15Table 3 summarizes measures to mitigate risk.
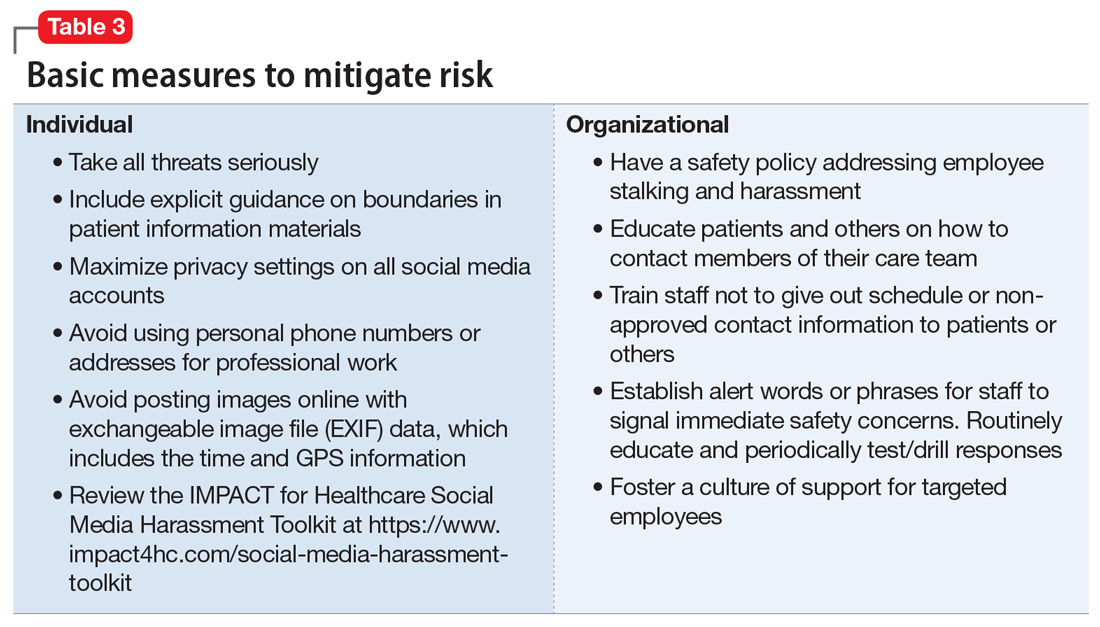
What to do when harassment or stalking occurs
Consulting with subject matter experts is essential. Approach behaviors, stalking patterns, and immediate circumstances vary highly, and so too must responses. A socially inept approach outside of the work setting by a patient may be effectively responded to with a firm explanation of why the behavior was inappropriate and a reiteration of limits. More persistent or serious threats may require taking actions for immediate safety, calling law enforcement or security (who may have the expertise to assist appropriately), or even run/hide/fight measures. Others to notify early on include human resources, supervisors, front desk staff, and coworkers. Although no single measure is always indicated and no single measure will always be effective, consultation with a specialist is always advisable.
Attempting to assess your own risk may be subject to bias and error, even for an experienced forensic psychiatrist. Risk assessment in stalking and harassment cases is complex, nuanced, and beyond the scope of this article; engagement with specialized threat programs or subject matter experts is advisable.15,16 If your medical center or area has police or security officers, engage them early. Risk management, insurers, and legal can also be helpful to consult. Attorneys specializing in harassment, stalking, and domestic violence may be helpful in extreme situations.17Table 417,18 highlights steps to take.
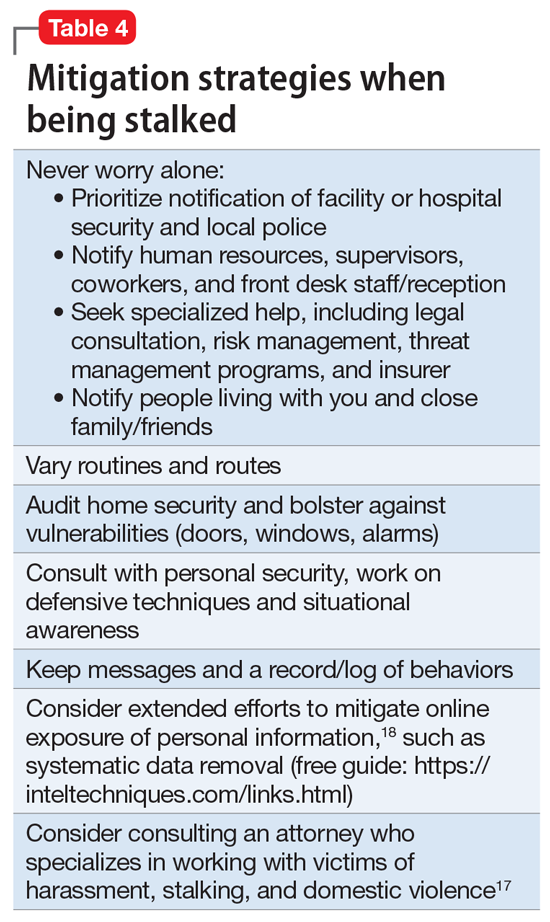
While effective interventions to stop or redirect stalking behavior may vary, some initial considerations include changing established routines (eg, your parking location or daily/weekly patterns such as gym, class, etc.) and letting family and others you live with know what is occurring. Consider implementing and bolstering personal, work, and home security; honing situational awareness skills; and learning advanced situational awareness and self-defense techniques.
Continue to: Clinical documentation and termination of care...
Clinical documentation and termination of care
Repeated and unwanted contact behaviors by a patient may be considered grounds for termination of care by the targeted clinician. Termination may occur through a direct conversation, followed by a mailed letter explaining that the patient’s inappropriate behaviors are the basis for termination. The letter should outline steps for establishing care with another psychiatrist and signing a release to facilitate transfer of records to the next psychiatrist. Ensure that the patient has access to a reasonable supply of medications or refills according to jurisdictional standards for transfer or termination of care.19 While these are common legal standards for termination of care in the United States, clinicians would be well served by appropriate consultation to verify the most appropriate standards for their location.
Documentation of a patient’s behavior should be factual and clear. Under the 21st Century Cures Act, patients often have access to their own electronic records.20 Therefore, clinicians should avoid documenting personal security measures or other information that is not clinically relevant. Communications with legal or risk management should not be documented unless otherwise advised, because such communications may be privileged and may not be clinically relevant.
In some circumstances, continuing to treat a patient who has stalked a member of the current treatment team may be appropriate or necessary. For example, a patient may respond appropriately to redirection after an initial approach behavior and continue to make clinical progress, or may be in a forensic specialty setting with appropriate operational support to continue with treatment.
Ethical dilemmas may arise in underserved areas where there are limited options for psychiatric care and in communicating the reasons for termination to a new clinician. Consultation may help to address these issues. However, as noted before, clinicians should be permitted to discontinue and transfer treatment and should not be compelled to continue to treat a patient who has threatened or harassed them.
Organizational and employer considerations
Victims of stalking have reported that they appreciated explicit support from their supervisor, regular meetings, and measures to reduce potential stalking or violence in the workplace; unsurprisingly, victim blaming and leaving the employee to address the situation on their own were labeled experienced as negative.2 Employers may consider implementing physical security, access controls and panic alarms, and enhancing coworkers’ situational awareness.21 Explicit policies about and attention to reducing workplace violence, including stalking, are always beneficial—and in some settings such policies may be a regulatory requirement.22 Large health care organizations may benefit from developing specialized threat management programs to assist with the evaluation and mitigation of stalking and other workplace violence risks.15,23
Self-care considerations
The impact of stalking can include psychological distress, disruption of work and personal relationships, and false allegations of impropriety. Stalking can make targets feel isolated, violated, and fearful, which makes it challenging to reach out to others for support and safety. It takes time to regain a sense of safety and to find a “new normal,” particularly while experiencing and responding to stalking behavior. Notifying close personal contacts such as family and coworkers about what is occurring (without sharing protected health information) can be helpful for recovery and important for the clinician’s safety. Reaching out for organizational and legal supports is also prudent. It is also important to allow time for, and patience with, a targeted individual’s normal responses, such as decreased work performance, sleep/appetite changes, and hypervigilance, without pathologizing these common stress reactions. Further review of appropriate resources by impacted clinicians is advisable.24-26
1. Nelsen AJ, Johnson RS, Ostermeyer B, et al. The prevalence of physicians who have been stalked: a systematic review. J Am Acad Psychiatry Law. 2015;43(2):177-182.
2. Jutasi C, McEwan TE. Stalking of professionals: a scoping review. Journal of Threat Assessment and Management. 2021;8(3):94-124.
3. Pathé MT, Meloy JR. Commentary: Stalking by patients—psychiatrists’ tales of anger, lust and ignorance. J Am Acad Psychiatry Law. 2013;41(2):200-205.
4. Pendergrast TR, Jain S, Trueger NS, et al. Prevalence of personal attacks and sexual harassment of physicians on social media. JAMA Intern Med. 2021;181(4):550-552.
5. Owens JG. Why definitions matter: stalking victimization in the United States. J Interpers Violence. 2016;31(12):2196-2226.
6. College of Policing. Stalking or harassment. May 2019. Accessed March 8, 2020. https://library.college.police.uk/docs/college-of-policing/Stalking_or_harassment_guidance_200519.pdf
7. McEwan TE, Daffern M, MacKenzie RD, et al. Risk factors for stalking violence, persistence, and recurrence. Journal of Forensic Psychiatry & Psychology. 2017;28(1):3856.
8. Pathé MT, Mullen PE, Purcell R. Patients who stalk doctors: their motives and management. Med J Australia. 2002;176(7):335-338.
9. Reibling ET, Distelberg B, Guptill M, et al. Intimate partner violence experienced by physicians. J Prim Care Community Health. 2020;11:2150132720965077.
10. Matias A, Gonçalves M, Soeiro C, et al. Intimate partner homicide: a meta-analysis of risk factors. Aggression and Violent Behavior. 2019;50:101358.
11. US Bureau of Labor Statistics. Fact sheet. Workplace violence in healthcare, 2018. April 2020. Accessed November 24, 2021. https://www.bls.gov/iif/oshwc/cfoi/workplace-violence-healthcare-2018.htm
12. Truman JL, Morgan RE. Stalking victimization, 2016. Bureau of Justice Statistics, Office of Justice Programs, U.S. Department of Justice. Report No.: NCJ 253526. April 2021. Accessed November 24, 2021. https://bjs.ojp.gov/library/publications/stalking-victimization-2016
13. Reyns BW, Henson B, Fisher BS. Being pursued online: applying cyberlifestyle–routine activities theory to cyberstalking victimization. Criminal Justice and Behavior. 2011;38(11):1149-1169.
14. Stea JN. When promoting knowledge makes you a target. Scientific American Blog Network. March 16, 2020. Accessed November 24, 2021. https://blogs.scientificamerican.com/observations/when-promoting-knowledge-makes-you-a-target/
15. Henkel SJ. Threat assessment strategies to mitigate violence in healthcare. IAHSS Foundation. IAHSS-F RS-19-02. November 2019. Accessed November 24, 2021. https://iahssf.org/assets/IAHSS-Foundation-Threat-Assessment-Strategies-to-Mitigate-Violence-in-Healthcare.pdf
16. McEwan TE. Stalking threat and risk assessment. In: Reid Meloy J, Hoffman J (eds). International Handbook of Threat Assessment. 2nd ed. Oxford University Press; 2021:210-234.
17. Goldberg C. Nobody’s Victim: Fighting Psychos, Stalkers, Pervs, and Trolls. Plume; 2019.
18. Bazzell M. Extreme Privacy: What It Takes to Disappear. 2nd ed. Independently published; 2020.
19. Simon RI, Shuman DW. The doctor-patient relationship. Focus. 2007;5(4):423-431.
20. Department of Health and Human Services. 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program Final Rule (To be codified at 45 CFR 170 and 171). Federal Register. 2020;85(85):25642-25961.
21. Sheridan L, North AC, Scott AJ. Stalking in the workplace. Journal of Threat Assessment and Management. 2019;6(2):61-75.
22. The Joint Commission. Workplace Violence Prevention Standards. R3 Report: Requirement, Rationale, Reference. Issue 30. June 18, 2021. Accessed November 24, 2021. https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/wpvp-r3-30_revised_06302021.pdf
23. Terry LP. Threat assessment teams. J Healthc Prot Manage. 2015;31(2):23-35.
24. Pathé M. Surviving Stalking. Cambridge University Press; 2002.
25. Noffsinger S. What stalking victims need to restore their mental and somatic health. Current Psychiatry. 2015;14(6):43-47.
26. Mullen P, Whyte S, McIvor R; Psychiatrists’ Support Service, Royal College of Psychiatry. PSS Information Guide: Stalking. Report No. 11. 2017. Accessed November 24, 2021. https://www.rcpsych.ac.uk/docs/default-source/members/supporting-you/pss/pss-guide-11-stalking.pdf?sfvrsn=2f1c7253_2
Dr. A has been treating Ms. W, a graduate student, for depression. Ms. W made subtle comments expressing her interest in pursuing a romantic relationship with her psychiatrist. Dr. A gently redirected her, and she seemed to respond appropriately. However, over the past 2 weeks, Dr. A has seen Ms. W at a local park and at the grocery store. Today, Dr. A is startled to see Ms. W at her weekly yoga class. Dr. A plans to ask her supervisor for advice.
Dr. M is a child psychiatrist who spoke at his local school board meeting in support of masking requirements for students during COVID-19. During the discussion, Dr. M shared that, as a psychiatrist, he does not believe it is especially distressing for students to wear masks, and that doing so is a necessary public health measure. On leaving, other parents shouted, “We know who you are and where you live!” The next day, his integrated clinic started receiving threatening and harassing messages, including threats to kill him or his staff if they take part in vaccinating children against COVID-19.
Because of their work, mental health professionals—like other health care professionals—face an elevated risk of being harassed or stalked. Stalking often includes online harassment and may escalate to serious physical violence. Stalking is criminal behavior by a patient and should not be constructed as a “failure to manage transference.” This article explores basic strategies to reduce the risk of harassment and stalking, describes how to recognize early behaviors, and outlines basic steps health care professionals and their employers can take to respond to stalking and harassing behaviors.
Although this article is intended for psychiatrists, it is important to note that all health professionals have significant risk for experiencing stalking or harassment. This is due in part, but not exclusively, to our clinical work. Estimates of how many health professionals experience stalking vary substantially depending upon the study, and differences in methodologies limit easy comparison or extrapolation. More thorough reviews have reported ranges from 2% to 70% among physicians; psychiatrists and other mental health professionals appear to be at greater risk than those in other specialties and the general population.1-3 Physicians who are active on social media may also be at elevated risk.4 Unexpected communications from patients and their family members—especially those with threatening, harassing, or sexualized tones, or involving contact outside of a work setting—can be distressing. These behaviors represent potential harbingers of more dangerous behavior, including physical assault, sexual assault, or homicide. Despite their elevated risk, many psychiatrists are unaware of how to prevent or respond to stalking or harassment.
Recognizing harassment and stalking
Repeated and unwanted contact or communication, regardless of intent, may constitute stalking. Legal definitions vary by jurisdiction and may not align with subjective experiences or understanding of what constitutes stalking.5 At its essence, stalking is repeated harassing behaviors likely to provoke fear in the targeted person. FOUR is a helpful mnemonic when conceptualizing the attributes of stalking: Fixated, Obsessive, Unwanted, and Repetitive.6Table 1 lists examples of common stalking behaviors. Stalking and harassing behavior may be from a known source (eg, a patient, coworker, or paramour), a masked source (ie, someone known to the target but who conceals or obscures their identity), or from otherwise unknown persons. Behaviors that persist after the person engaging in the behaviors has clearly been informed that they are unwanted or inappropriate are especially concerning. Stalking may escalate to include physical or sexual assault and, in some cases, homicide.

Stalking duration can vary substantially, as can the factors that lead to the cessation of the behavior. Indicators of increased risk for physical violence include unwanted physical presence/following of the target (“approach behaviors”), having a prior violent intimate relationship, property destruction, explicit threats, and having a prior intimate relationship with the target.7
Stalking contact or communication may be unwanted because of the content (eg, sexualized or threatening tone), location (eg, at a professional’s home), or means (eg, through social media). Stalking behaviors are not appropriate in any relationship, including a clinical relationship. They should not be treated as a “failure to manage transference” or in other victim-blaming ways.
There are multiple typologies for stalking behavior. Common motivations for stalking health professionals include resentment or grievance, misjudgment of social boundaries, and delusional fixation, including erotomania.8 Associated psychopathologies vary significantly and, while some may be more amenable to psychiatric treatment than others, psychiatrists should not feel compelled to treat patients who repeatedly violate boundaries, regardless of intent or comorbidity.
Patients are not the exclusive perpetrators of stalking; a recent study found that 4% of physicians surveyed reported current or recent stalking by a current or former intimate partner.9 When a person who is a victim of intimate partner violence is also stalked as part of the abuse, homicide risk increases.10 Workplace homicides of health care professionals are most likely to be committed by a current or former partner or other personal acquaintance, not by a patient.11 Workplace harassment and stalking of health care professionals is especially concerning because this behavior can escalate and endanger coworkers or clients.
Continue to: Risk awareness: Recognize your exposure...
Risk awareness: Recognize your exposure
About 80% of stalking involves some form of technology—often telephone calls but also online or other “cyber” elements.12 One recent survey found the rate of online harassment, including threats of physical and sexual violence, was >20% among physicians who were active on social media.4 Health professionals may be at greater risk of having patients find their personal information simply because patients routinely search online for information about new clinicians. Personal information about a clinician may be readily visible among professional information in search results, or a curious patient may simply scroll further down in the results. For a potential stalker, clicking on a search result linking to a personal social media page may be far easier than finding a home address and going in person—but the action may be just as distressing or risky for the clinician.13 Additionally, items visible in a clinician’s office—or visible in the background of those providing telehealth services from their home—may inadvertently reveal personal information about the clinician, their home, or their family.
Psychiatrists are often in a special position in relation to patients and times of crises. They may be involved in involuntary commitment—or declining an admission when a patient or family wishes it. They may be present at the time of the revelation of a serious diagnosis, abuse, injury, or death. They may be a mandated reporter of child or elder abuse.2 Additionally, physicians may be engaged in discourse on politically charged public health topics.14 These factors may increase their risk of being stalked.
Conducting an online visibility self-assessment can be a useful way to learn what information others can find. Table 2 outlines the steps for completing this exercise. Searching multiple iterations of your current and former names (with and without degrees, titles, and cities) will yield differing results in various search engines. After establishing a baseline of what information is available online, it can be helpful to periodically repeat this exercise, and to set up automated alerts for your name, number(s), email(s), and address(es).

Basic mitigation strategies
In the modern era, being invisible online is impractical and likely impossible—especially for a health care professional. Instead, it may be prudent to limit your public visibility to professional portals (eg, LinkedIn or Doximity) and maximize privacy settings on other platforms. Another basic strategy is to avoid providing personal contact information (your home address, phone number, or personal email) for professional purposes, such as licensing and credentialing, conference submissions, or journal publications. Be aware that driving a visually distinct vehicle—one with vanity plates or distinct bumper stickers, or an exotic sportscar—can make it easier to be recognized and located. A personally recorded voicemail greeting (vs one recorded by, for example, an office manager) may be inappropriately reinforcing for some stalkers.
Workplaces should have an established safety policy that addresses stalking and harassment of employees. Similarly, patients and others should receive clear education on how to contact different staff, including physicians, with consideration of how and when to use electronic health information portals, office numbers, and emails. Workplaces should not disclose staff schedules. For example, a receptionist should say “I’ll have Dr. Diaz return your call when she can” instead of “Dr. Diaz is not in until tomorrow.” Avoid unnecessary location/name signals (eg, a parking spot labeled “Dr. Diaz”). Consider creating alert words or phrases for staff to use to signal they are concerned about their immediate safety—and provide education and training, including drills, to test emergency responses when the words/phrases are used. Leaders and managers should nurture a workplace culture where people are comfortable seeking support if they feel they may be the target of harassment or stalking. Many larger health care organizations have threat management programs, which can play a critical role in preventing, investigating, and responding to stalking of employees. Increasingly, threat management teams are being identified as a best practice in health care settings.15Table 3 summarizes measures to mitigate risk.

What to do when harassment or stalking occurs
Consulting with subject matter experts is essential. Approach behaviors, stalking patterns, and immediate circumstances vary highly, and so too must responses. A socially inept approach outside of the work setting by a patient may be effectively responded to with a firm explanation of why the behavior was inappropriate and a reiteration of limits. More persistent or serious threats may require taking actions for immediate safety, calling law enforcement or security (who may have the expertise to assist appropriately), or even run/hide/fight measures. Others to notify early on include human resources, supervisors, front desk staff, and coworkers. Although no single measure is always indicated and no single measure will always be effective, consultation with a specialist is always advisable.
Attempting to assess your own risk may be subject to bias and error, even for an experienced forensic psychiatrist. Risk assessment in stalking and harassment cases is complex, nuanced, and beyond the scope of this article; engagement with specialized threat programs or subject matter experts is advisable.15,16 If your medical center or area has police or security officers, engage them early. Risk management, insurers, and legal can also be helpful to consult. Attorneys specializing in harassment, stalking, and domestic violence may be helpful in extreme situations.17Table 417,18 highlights steps to take.

While effective interventions to stop or redirect stalking behavior may vary, some initial considerations include changing established routines (eg, your parking location or daily/weekly patterns such as gym, class, etc.) and letting family and others you live with know what is occurring. Consider implementing and bolstering personal, work, and home security; honing situational awareness skills; and learning advanced situational awareness and self-defense techniques.
Continue to: Clinical documentation and termination of care...
Clinical documentation and termination of care
Repeated and unwanted contact behaviors by a patient may be considered grounds for termination of care by the targeted clinician. Termination may occur through a direct conversation, followed by a mailed letter explaining that the patient’s inappropriate behaviors are the basis for termination. The letter should outline steps for establishing care with another psychiatrist and signing a release to facilitate transfer of records to the next psychiatrist. Ensure that the patient has access to a reasonable supply of medications or refills according to jurisdictional standards for transfer or termination of care.19 While these are common legal standards for termination of care in the United States, clinicians would be well served by appropriate consultation to verify the most appropriate standards for their location.
Documentation of a patient’s behavior should be factual and clear. Under the 21st Century Cures Act, patients often have access to their own electronic records.20 Therefore, clinicians should avoid documenting personal security measures or other information that is not clinically relevant. Communications with legal or risk management should not be documented unless otherwise advised, because such communications may be privileged and may not be clinically relevant.
In some circumstances, continuing to treat a patient who has stalked a member of the current treatment team may be appropriate or necessary. For example, a patient may respond appropriately to redirection after an initial approach behavior and continue to make clinical progress, or may be in a forensic specialty setting with appropriate operational support to continue with treatment.
Ethical dilemmas may arise in underserved areas where there are limited options for psychiatric care and in communicating the reasons for termination to a new clinician. Consultation may help to address these issues. However, as noted before, clinicians should be permitted to discontinue and transfer treatment and should not be compelled to continue to treat a patient who has threatened or harassed them.
Organizational and employer considerations
Victims of stalking have reported that they appreciated explicit support from their supervisor, regular meetings, and measures to reduce potential stalking or violence in the workplace; unsurprisingly, victim blaming and leaving the employee to address the situation on their own were labeled experienced as negative.2 Employers may consider implementing physical security, access controls and panic alarms, and enhancing coworkers’ situational awareness.21 Explicit policies about and attention to reducing workplace violence, including stalking, are always beneficial—and in some settings such policies may be a regulatory requirement.22 Large health care organizations may benefit from developing specialized threat management programs to assist with the evaluation and mitigation of stalking and other workplace violence risks.15,23
Self-care considerations
The impact of stalking can include psychological distress, disruption of work and personal relationships, and false allegations of impropriety. Stalking can make targets feel isolated, violated, and fearful, which makes it challenging to reach out to others for support and safety. It takes time to regain a sense of safety and to find a “new normal,” particularly while experiencing and responding to stalking behavior. Notifying close personal contacts such as family and coworkers about what is occurring (without sharing protected health information) can be helpful for recovery and important for the clinician’s safety. Reaching out for organizational and legal supports is also prudent. It is also important to allow time for, and patience with, a targeted individual’s normal responses, such as decreased work performance, sleep/appetite changes, and hypervigilance, without pathologizing these common stress reactions. Further review of appropriate resources by impacted clinicians is advisable.24-26
Dr. A has been treating Ms. W, a graduate student, for depression. Ms. W made subtle comments expressing her interest in pursuing a romantic relationship with her psychiatrist. Dr. A gently redirected her, and she seemed to respond appropriately. However, over the past 2 weeks, Dr. A has seen Ms. W at a local park and at the grocery store. Today, Dr. A is startled to see Ms. W at her weekly yoga class. Dr. A plans to ask her supervisor for advice.
Dr. M is a child psychiatrist who spoke at his local school board meeting in support of masking requirements for students during COVID-19. During the discussion, Dr. M shared that, as a psychiatrist, he does not believe it is especially distressing for students to wear masks, and that doing so is a necessary public health measure. On leaving, other parents shouted, “We know who you are and where you live!” The next day, his integrated clinic started receiving threatening and harassing messages, including threats to kill him or his staff if they take part in vaccinating children against COVID-19.
Because of their work, mental health professionals—like other health care professionals—face an elevated risk of being harassed or stalked. Stalking often includes online harassment and may escalate to serious physical violence. Stalking is criminal behavior by a patient and should not be constructed as a “failure to manage transference.” This article explores basic strategies to reduce the risk of harassment and stalking, describes how to recognize early behaviors, and outlines basic steps health care professionals and their employers can take to respond to stalking and harassing behaviors.
Although this article is intended for psychiatrists, it is important to note that all health professionals have significant risk for experiencing stalking or harassment. This is due in part, but not exclusively, to our clinical work. Estimates of how many health professionals experience stalking vary substantially depending upon the study, and differences in methodologies limit easy comparison or extrapolation. More thorough reviews have reported ranges from 2% to 70% among physicians; psychiatrists and other mental health professionals appear to be at greater risk than those in other specialties and the general population.1-3 Physicians who are active on social media may also be at elevated risk.4 Unexpected communications from patients and their family members—especially those with threatening, harassing, or sexualized tones, or involving contact outside of a work setting—can be distressing. These behaviors represent potential harbingers of more dangerous behavior, including physical assault, sexual assault, or homicide. Despite their elevated risk, many psychiatrists are unaware of how to prevent or respond to stalking or harassment.
Recognizing harassment and stalking
Repeated and unwanted contact or communication, regardless of intent, may constitute stalking. Legal definitions vary by jurisdiction and may not align with subjective experiences or understanding of what constitutes stalking.5 At its essence, stalking is repeated harassing behaviors likely to provoke fear in the targeted person. FOUR is a helpful mnemonic when conceptualizing the attributes of stalking: Fixated, Obsessive, Unwanted, and Repetitive.6Table 1 lists examples of common stalking behaviors. Stalking and harassing behavior may be from a known source (eg, a patient, coworker, or paramour), a masked source (ie, someone known to the target but who conceals or obscures their identity), or from otherwise unknown persons. Behaviors that persist after the person engaging in the behaviors has clearly been informed that they are unwanted or inappropriate are especially concerning. Stalking may escalate to include physical or sexual assault and, in some cases, homicide.

Stalking duration can vary substantially, as can the factors that lead to the cessation of the behavior. Indicators of increased risk for physical violence include unwanted physical presence/following of the target (“approach behaviors”), having a prior violent intimate relationship, property destruction, explicit threats, and having a prior intimate relationship with the target.7
Stalking contact or communication may be unwanted because of the content (eg, sexualized or threatening tone), location (eg, at a professional’s home), or means (eg, through social media). Stalking behaviors are not appropriate in any relationship, including a clinical relationship. They should not be treated as a “failure to manage transference” or in other victim-blaming ways.
There are multiple typologies for stalking behavior. Common motivations for stalking health professionals include resentment or grievance, misjudgment of social boundaries, and delusional fixation, including erotomania.8 Associated psychopathologies vary significantly and, while some may be more amenable to psychiatric treatment than others, psychiatrists should not feel compelled to treat patients who repeatedly violate boundaries, regardless of intent or comorbidity.
Patients are not the exclusive perpetrators of stalking; a recent study found that 4% of physicians surveyed reported current or recent stalking by a current or former intimate partner.9 When a person who is a victim of intimate partner violence is also stalked as part of the abuse, homicide risk increases.10 Workplace homicides of health care professionals are most likely to be committed by a current or former partner or other personal acquaintance, not by a patient.11 Workplace harassment and stalking of health care professionals is especially concerning because this behavior can escalate and endanger coworkers or clients.
Continue to: Risk awareness: Recognize your exposure...
Risk awareness: Recognize your exposure
About 80% of stalking involves some form of technology—often telephone calls but also online or other “cyber” elements.12 One recent survey found the rate of online harassment, including threats of physical and sexual violence, was >20% among physicians who were active on social media.4 Health professionals may be at greater risk of having patients find their personal information simply because patients routinely search online for information about new clinicians. Personal information about a clinician may be readily visible among professional information in search results, or a curious patient may simply scroll further down in the results. For a potential stalker, clicking on a search result linking to a personal social media page may be far easier than finding a home address and going in person—but the action may be just as distressing or risky for the clinician.13 Additionally, items visible in a clinician’s office—or visible in the background of those providing telehealth services from their home—may inadvertently reveal personal information about the clinician, their home, or their family.
Psychiatrists are often in a special position in relation to patients and times of crises. They may be involved in involuntary commitment—or declining an admission when a patient or family wishes it. They may be present at the time of the revelation of a serious diagnosis, abuse, injury, or death. They may be a mandated reporter of child or elder abuse.2 Additionally, physicians may be engaged in discourse on politically charged public health topics.14 These factors may increase their risk of being stalked.
Conducting an online visibility self-assessment can be a useful way to learn what information others can find. Table 2 outlines the steps for completing this exercise. Searching multiple iterations of your current and former names (with and without degrees, titles, and cities) will yield differing results in various search engines. After establishing a baseline of what information is available online, it can be helpful to periodically repeat this exercise, and to set up automated alerts for your name, number(s), email(s), and address(es).

Basic mitigation strategies
In the modern era, being invisible online is impractical and likely impossible—especially for a health care professional. Instead, it may be prudent to limit your public visibility to professional portals (eg, LinkedIn or Doximity) and maximize privacy settings on other platforms. Another basic strategy is to avoid providing personal contact information (your home address, phone number, or personal email) for professional purposes, such as licensing and credentialing, conference submissions, or journal publications. Be aware that driving a visually distinct vehicle—one with vanity plates or distinct bumper stickers, or an exotic sportscar—can make it easier to be recognized and located. A personally recorded voicemail greeting (vs one recorded by, for example, an office manager) may be inappropriately reinforcing for some stalkers.
Workplaces should have an established safety policy that addresses stalking and harassment of employees. Similarly, patients and others should receive clear education on how to contact different staff, including physicians, with consideration of how and when to use electronic health information portals, office numbers, and emails. Workplaces should not disclose staff schedules. For example, a receptionist should say “I’ll have Dr. Diaz return your call when she can” instead of “Dr. Diaz is not in until tomorrow.” Avoid unnecessary location/name signals (eg, a parking spot labeled “Dr. Diaz”). Consider creating alert words or phrases for staff to use to signal they are concerned about their immediate safety—and provide education and training, including drills, to test emergency responses when the words/phrases are used. Leaders and managers should nurture a workplace culture where people are comfortable seeking support if they feel they may be the target of harassment or stalking. Many larger health care organizations have threat management programs, which can play a critical role in preventing, investigating, and responding to stalking of employees. Increasingly, threat management teams are being identified as a best practice in health care settings.15Table 3 summarizes measures to mitigate risk.

What to do when harassment or stalking occurs
Consulting with subject matter experts is essential. Approach behaviors, stalking patterns, and immediate circumstances vary highly, and so too must responses. A socially inept approach outside of the work setting by a patient may be effectively responded to with a firm explanation of why the behavior was inappropriate and a reiteration of limits. More persistent or serious threats may require taking actions for immediate safety, calling law enforcement or security (who may have the expertise to assist appropriately), or even run/hide/fight measures. Others to notify early on include human resources, supervisors, front desk staff, and coworkers. Although no single measure is always indicated and no single measure will always be effective, consultation with a specialist is always advisable.
Attempting to assess your own risk may be subject to bias and error, even for an experienced forensic psychiatrist. Risk assessment in stalking and harassment cases is complex, nuanced, and beyond the scope of this article; engagement with specialized threat programs or subject matter experts is advisable.15,16 If your medical center or area has police or security officers, engage them early. Risk management, insurers, and legal can also be helpful to consult. Attorneys specializing in harassment, stalking, and domestic violence may be helpful in extreme situations.17Table 417,18 highlights steps to take.

While effective interventions to stop or redirect stalking behavior may vary, some initial considerations include changing established routines (eg, your parking location or daily/weekly patterns such as gym, class, etc.) and letting family and others you live with know what is occurring. Consider implementing and bolstering personal, work, and home security; honing situational awareness skills; and learning advanced situational awareness and self-defense techniques.
Continue to: Clinical documentation and termination of care...
Clinical documentation and termination of care
Repeated and unwanted contact behaviors by a patient may be considered grounds for termination of care by the targeted clinician. Termination may occur through a direct conversation, followed by a mailed letter explaining that the patient’s inappropriate behaviors are the basis for termination. The letter should outline steps for establishing care with another psychiatrist and signing a release to facilitate transfer of records to the next psychiatrist. Ensure that the patient has access to a reasonable supply of medications or refills according to jurisdictional standards for transfer or termination of care.19 While these are common legal standards for termination of care in the United States, clinicians would be well served by appropriate consultation to verify the most appropriate standards for their location.
Documentation of a patient’s behavior should be factual and clear. Under the 21st Century Cures Act, patients often have access to their own electronic records.20 Therefore, clinicians should avoid documenting personal security measures or other information that is not clinically relevant. Communications with legal or risk management should not be documented unless otherwise advised, because such communications may be privileged and may not be clinically relevant.
In some circumstances, continuing to treat a patient who has stalked a member of the current treatment team may be appropriate or necessary. For example, a patient may respond appropriately to redirection after an initial approach behavior and continue to make clinical progress, or may be in a forensic specialty setting with appropriate operational support to continue with treatment.
Ethical dilemmas may arise in underserved areas where there are limited options for psychiatric care and in communicating the reasons for termination to a new clinician. Consultation may help to address these issues. However, as noted before, clinicians should be permitted to discontinue and transfer treatment and should not be compelled to continue to treat a patient who has threatened or harassed them.
Organizational and employer considerations
Victims of stalking have reported that they appreciated explicit support from their supervisor, regular meetings, and measures to reduce potential stalking or violence in the workplace; unsurprisingly, victim blaming and leaving the employee to address the situation on their own were labeled experienced as negative.2 Employers may consider implementing physical security, access controls and panic alarms, and enhancing coworkers’ situational awareness.21 Explicit policies about and attention to reducing workplace violence, including stalking, are always beneficial—and in some settings such policies may be a regulatory requirement.22 Large health care organizations may benefit from developing specialized threat management programs to assist with the evaluation and mitigation of stalking and other workplace violence risks.15,23
Self-care considerations
The impact of stalking can include psychological distress, disruption of work and personal relationships, and false allegations of impropriety. Stalking can make targets feel isolated, violated, and fearful, which makes it challenging to reach out to others for support and safety. It takes time to regain a sense of safety and to find a “new normal,” particularly while experiencing and responding to stalking behavior. Notifying close personal contacts such as family and coworkers about what is occurring (without sharing protected health information) can be helpful for recovery and important for the clinician’s safety. Reaching out for organizational and legal supports is also prudent. It is also important to allow time for, and patience with, a targeted individual’s normal responses, such as decreased work performance, sleep/appetite changes, and hypervigilance, without pathologizing these common stress reactions. Further review of appropriate resources by impacted clinicians is advisable.24-26
1. Nelsen AJ, Johnson RS, Ostermeyer B, et al. The prevalence of physicians who have been stalked: a systematic review. J Am Acad Psychiatry Law. 2015;43(2):177-182.
2. Jutasi C, McEwan TE. Stalking of professionals: a scoping review. Journal of Threat Assessment and Management. 2021;8(3):94-124.
3. Pathé MT, Meloy JR. Commentary: Stalking by patients—psychiatrists’ tales of anger, lust and ignorance. J Am Acad Psychiatry Law. 2013;41(2):200-205.
4. Pendergrast TR, Jain S, Trueger NS, et al. Prevalence of personal attacks and sexual harassment of physicians on social media. JAMA Intern Med. 2021;181(4):550-552.
5. Owens JG. Why definitions matter: stalking victimization in the United States. J Interpers Violence. 2016;31(12):2196-2226.
6. College of Policing. Stalking or harassment. May 2019. Accessed March 8, 2020. https://library.college.police.uk/docs/college-of-policing/Stalking_or_harassment_guidance_200519.pdf
7. McEwan TE, Daffern M, MacKenzie RD, et al. Risk factors for stalking violence, persistence, and recurrence. Journal of Forensic Psychiatry & Psychology. 2017;28(1):3856.
8. Pathé MT, Mullen PE, Purcell R. Patients who stalk doctors: their motives and management. Med J Australia. 2002;176(7):335-338.
9. Reibling ET, Distelberg B, Guptill M, et al. Intimate partner violence experienced by physicians. J Prim Care Community Health. 2020;11:2150132720965077.
10. Matias A, Gonçalves M, Soeiro C, et al. Intimate partner homicide: a meta-analysis of risk factors. Aggression and Violent Behavior. 2019;50:101358.
11. US Bureau of Labor Statistics. Fact sheet. Workplace violence in healthcare, 2018. April 2020. Accessed November 24, 2021. https://www.bls.gov/iif/oshwc/cfoi/workplace-violence-healthcare-2018.htm
12. Truman JL, Morgan RE. Stalking victimization, 2016. Bureau of Justice Statistics, Office of Justice Programs, U.S. Department of Justice. Report No.: NCJ 253526. April 2021. Accessed November 24, 2021. https://bjs.ojp.gov/library/publications/stalking-victimization-2016
13. Reyns BW, Henson B, Fisher BS. Being pursued online: applying cyberlifestyle–routine activities theory to cyberstalking victimization. Criminal Justice and Behavior. 2011;38(11):1149-1169.
14. Stea JN. When promoting knowledge makes you a target. Scientific American Blog Network. March 16, 2020. Accessed November 24, 2021. https://blogs.scientificamerican.com/observations/when-promoting-knowledge-makes-you-a-target/
15. Henkel SJ. Threat assessment strategies to mitigate violence in healthcare. IAHSS Foundation. IAHSS-F RS-19-02. November 2019. Accessed November 24, 2021. https://iahssf.org/assets/IAHSS-Foundation-Threat-Assessment-Strategies-to-Mitigate-Violence-in-Healthcare.pdf
16. McEwan TE. Stalking threat and risk assessment. In: Reid Meloy J, Hoffman J (eds). International Handbook of Threat Assessment. 2nd ed. Oxford University Press; 2021:210-234.
17. Goldberg C. Nobody’s Victim: Fighting Psychos, Stalkers, Pervs, and Trolls. Plume; 2019.
18. Bazzell M. Extreme Privacy: What It Takes to Disappear. 2nd ed. Independently published; 2020.
19. Simon RI, Shuman DW. The doctor-patient relationship. Focus. 2007;5(4):423-431.
20. Department of Health and Human Services. 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program Final Rule (To be codified at 45 CFR 170 and 171). Federal Register. 2020;85(85):25642-25961.
21. Sheridan L, North AC, Scott AJ. Stalking in the workplace. Journal of Threat Assessment and Management. 2019;6(2):61-75.
22. The Joint Commission. Workplace Violence Prevention Standards. R3 Report: Requirement, Rationale, Reference. Issue 30. June 18, 2021. Accessed November 24, 2021. https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/wpvp-r3-30_revised_06302021.pdf
23. Terry LP. Threat assessment teams. J Healthc Prot Manage. 2015;31(2):23-35.
24. Pathé M. Surviving Stalking. Cambridge University Press; 2002.
25. Noffsinger S. What stalking victims need to restore their mental and somatic health. Current Psychiatry. 2015;14(6):43-47.
26. Mullen P, Whyte S, McIvor R; Psychiatrists’ Support Service, Royal College of Psychiatry. PSS Information Guide: Stalking. Report No. 11. 2017. Accessed November 24, 2021. https://www.rcpsych.ac.uk/docs/default-source/members/supporting-you/pss/pss-guide-11-stalking.pdf?sfvrsn=2f1c7253_2
1. Nelsen AJ, Johnson RS, Ostermeyer B, et al. The prevalence of physicians who have been stalked: a systematic review. J Am Acad Psychiatry Law. 2015;43(2):177-182.
2. Jutasi C, McEwan TE. Stalking of professionals: a scoping review. Journal of Threat Assessment and Management. 2021;8(3):94-124.
3. Pathé MT, Meloy JR. Commentary: Stalking by patients—psychiatrists’ tales of anger, lust and ignorance. J Am Acad Psychiatry Law. 2013;41(2):200-205.
4. Pendergrast TR, Jain S, Trueger NS, et al. Prevalence of personal attacks and sexual harassment of physicians on social media. JAMA Intern Med. 2021;181(4):550-552.
5. Owens JG. Why definitions matter: stalking victimization in the United States. J Interpers Violence. 2016;31(12):2196-2226.
6. College of Policing. Stalking or harassment. May 2019. Accessed March 8, 2020. https://library.college.police.uk/docs/college-of-policing/Stalking_or_harassment_guidance_200519.pdf
7. McEwan TE, Daffern M, MacKenzie RD, et al. Risk factors for stalking violence, persistence, and recurrence. Journal of Forensic Psychiatry & Psychology. 2017;28(1):3856.
8. Pathé MT, Mullen PE, Purcell R. Patients who stalk doctors: their motives and management. Med J Australia. 2002;176(7):335-338.
9. Reibling ET, Distelberg B, Guptill M, et al. Intimate partner violence experienced by physicians. J Prim Care Community Health. 2020;11:2150132720965077.
10. Matias A, Gonçalves M, Soeiro C, et al. Intimate partner homicide: a meta-analysis of risk factors. Aggression and Violent Behavior. 2019;50:101358.
11. US Bureau of Labor Statistics. Fact sheet. Workplace violence in healthcare, 2018. April 2020. Accessed November 24, 2021. https://www.bls.gov/iif/oshwc/cfoi/workplace-violence-healthcare-2018.htm
12. Truman JL, Morgan RE. Stalking victimization, 2016. Bureau of Justice Statistics, Office of Justice Programs, U.S. Department of Justice. Report No.: NCJ 253526. April 2021. Accessed November 24, 2021. https://bjs.ojp.gov/library/publications/stalking-victimization-2016
13. Reyns BW, Henson B, Fisher BS. Being pursued online: applying cyberlifestyle–routine activities theory to cyberstalking victimization. Criminal Justice and Behavior. 2011;38(11):1149-1169.
14. Stea JN. When promoting knowledge makes you a target. Scientific American Blog Network. March 16, 2020. Accessed November 24, 2021. https://blogs.scientificamerican.com/observations/when-promoting-knowledge-makes-you-a-target/
15. Henkel SJ. Threat assessment strategies to mitigate violence in healthcare. IAHSS Foundation. IAHSS-F RS-19-02. November 2019. Accessed November 24, 2021. https://iahssf.org/assets/IAHSS-Foundation-Threat-Assessment-Strategies-to-Mitigate-Violence-in-Healthcare.pdf
16. McEwan TE. Stalking threat and risk assessment. In: Reid Meloy J, Hoffman J (eds). International Handbook of Threat Assessment. 2nd ed. Oxford University Press; 2021:210-234.
17. Goldberg C. Nobody’s Victim: Fighting Psychos, Stalkers, Pervs, and Trolls. Plume; 2019.
18. Bazzell M. Extreme Privacy: What It Takes to Disappear. 2nd ed. Independently published; 2020.
19. Simon RI, Shuman DW. The doctor-patient relationship. Focus. 2007;5(4):423-431.
20. Department of Health and Human Services. 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program Final Rule (To be codified at 45 CFR 170 and 171). Federal Register. 2020;85(85):25642-25961.
21. Sheridan L, North AC, Scott AJ. Stalking in the workplace. Journal of Threat Assessment and Management. 2019;6(2):61-75.
22. The Joint Commission. Workplace Violence Prevention Standards. R3 Report: Requirement, Rationale, Reference. Issue 30. June 18, 2021. Accessed November 24, 2021. https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/wpvp-r3-30_revised_06302021.pdf
23. Terry LP. Threat assessment teams. J Healthc Prot Manage. 2015;31(2):23-35.
24. Pathé M. Surviving Stalking. Cambridge University Press; 2002.
25. Noffsinger S. What stalking victims need to restore their mental and somatic health. Current Psychiatry. 2015;14(6):43-47.
26. Mullen P, Whyte S, McIvor R; Psychiatrists’ Support Service, Royal College of Psychiatry. PSS Information Guide: Stalking. Report No. 11. 2017. Accessed November 24, 2021. https://www.rcpsych.ac.uk/docs/default-source/members/supporting-you/pss/pss-guide-11-stalking.pdf?sfvrsn=2f1c7253_2
Pediatric insomnia: Treatment
Children and adolescents who do not receive sufficient sleep can experience worsening inattention, daytime fatigue, and cognitive and behavioral difficulties. Assessment and treatment of insomnia and other sleep difficulties in young patients is critical as poor sleep increases their risk for depression, self-harm, and suicide.
In Part 1 of this article (Pediatric insomnia: Assessment and diagnosis,
Psychotherapeutic interventions
Regardless of the source of a child’s insomnia or co-occurring disorders, healthy sleep practices are the first line behavioral treatment, including for youth with attention-deficit/hyperactivity disorder (ADHD), anxiety disorders, obsessive-compulsive disorder, and depressive disorders.
Healthy sleep practices/sleep hygiene
Developmentally appropriate bedtimes and routines (Table). Helping children establish a regular, consistent bedtime is key in promoting healthy sleep. Ideally, the bedtime routine involves 3 to 4 activities each night in the same order, and these activities should be relaxing and soothing (eg, taking a bath, putting on pajamas, reading books). Setting age-appropriate bedtimes also is important. If an older child is asked to go to bed at 8 pm but cannot fall asleep for an hour, they may not have insomnia but instead a developmentally inappropriate bedtime. Several studies found that children younger than age 10 should go to bed no later than 9 pm. Bedtimes later than 9 pm for young children are correlated with shorter sleep duration.1

Consistent sleep schedules. Another important aspect of healthy sleep is working with parents to enforce a consistent bedtime and wake-up time, including weekdays and weekends. Ideally, bedtime on weekdays and weekends should not vary by more than 1 hour. Helping children wake up at the same time each day helps to set and regulate their circadian rhythm. Keeping these schedules consistent on vacations and school holidays also is helpful. For adolescents, the weekday/weekend bedtimes can vary by up to 2 hours because adolescents have a delayed circadian rhythm and wake-up times for high school can be early.
Environmental factors. An important piece of parental education is stimulus control and the ingredients of healthy sleep. Healthy sleep ingredients include a dark, quiet, consistent, and cool bedroom; a comfortable bed, the child feeling safe, and limited environmental stimuli.
Continue to: Cognitive-behavioral therapy for insomnia...
Cognitive-behavioral therapy for insomnia
Relaxation. Pediatric patients can be taught relaxation, mindfulness, meditation, and progressive muscle relaxation techniques to help lower overall stress. This can be especially helpful for youth with sleep disorders or anxiety. Guided relaxation apps are popular among children and teens, and various apps offer soothing sounds, deep breathing, progressive muscle relaxation, and guided imagery. This can be taught in psychotherapy sessions and used at home to promote gains in between sessions.
Stimulus control. Stimulus control involves using the bed exclusively for sleep and avoiding nonsleep activities in bed (eg, reading, watching television, using a computer, worrying). These activities promote wakefulness and insomnia. This may mean the child does not get into bed until they cannot keep their eyes open, even if that delays bedtime. If the child is still awake within 15 to 20 minutes, they should be encouraged to get out of bed and engage in a nonstimulating activity such as meditation, reading, or sitting quietly in the dark or low light. This recommendation can run counter to parents’ intuition that children with sleep problems should go to bed earlier. Using the bed only for sleep conditions the child to falling asleep or being asleep when in bed.
Sleep restriction. Sleep restriction involves restricting sleep to a set number of hours in order to increase their sleep efficiency (time slept in bed divided by total time spent in bed x 100). Restricting sleep to 6 to 7 hours increases sleep efficiency, consolidates sleep, and extinguishes the association of being awake in bed. For older adolescents, sleep restriction may help to limit their time in bed to either falling asleep or being asleep. This is intended to be used as a short-term strategy and only after other sleep hygiene measures (bedtime routine, environmental factors, etc) have been put into place for several weeks. While this strategy sounds unappealing to most individuals with insomnia, it can lead to lasting change due to the use of behavioral conditioning in bed. Because sleep restriction can lead to significant daytime sleepiness and impairment during the day, sleep should not be restricted to <6 hours a day for children and adolescents. Once the adolescent is sleeping more consistently and sleep efficiency reaches 85% or higher, time in bed for sleep is increased.2
Cognitive restructuring. Some children and adolescents develop maladaptive thoughts about sleep that further promote insomnia. These thoughts might include “I will never get to sleep,” “I am going to have a terrible day if I cannot fall asleep,” or “I will fail my test tomorrow if I am unable to sleep.” Such maladaptive thoughts are often untrue but promote wakefulness in the early or middle part of the night. Cognitive restructuring involves helping the child identify each problematic thought, challenge how accurate each thought is with evidence, and replace the problematic thought with a more helpful thought. For instance, an adolescent can recognize that even if they have a sleepless night, their catastrophic outcome (eg, “I will not be able to function”) is likely untrue. A psychologist can help review evidence for this, including previous times when the adolescent has not slept well and managed to get through the next day.
When is pharmacologic treatment needed?
Pharmacologic treatment may be indicated if a child does not show significant improvement following behavioral intervention (Figure). However, it is critical to exclude other primary causes of dyssomnia (eg, obstructive sleep apnea, iron deficiency anemia) before pursuing pharmacotherapy, because pharmacotherapy could mask an underlying disorder. Moreover, while there is relatively limited evidence for psychopharmacologic interventions for sleep difficulties in children and adolescents, a large survey of child and adolescent psychiatrists (N = 1,273) suggested that medications were considered for one-quarter of pediatric patients with insomnia.3 Further, patients with specific comorbidities such as neurodevelopmental disorders may be more likely to be prescribed soporifics.4

Continue to: What is the evidence for pharmacotherapy?...
What is the evidence for pharmacotherapy?
Antihistamines. Histamine antagonists—which promote sleep by blocking the wakefulness-promoting and circadian-related effects of histamine—are the most commonly used medications to treat pediatric insomnia, despite a dearth of data from prospective trials.5,6 In 1 small study, Russo et al7 found diphenhydramine, 1 mg/kg at bedtime, reduced sleep latency and nighttime awakenings, and increased sleep duration in patients ages 2 to 12; similar effects have been observed in pediatric burn patients.8 There are some limited data for other H1 antagonists (eg, hydroxyzine) in pediatric insomnia.9-11
Alpha-2 agonists increase rapid eye movement sleep via dose-dependent downregulation of noradrenergic signaling12 and thus have been commonly prescribed for insomnia in children and adolescents. In fact, the nonselective alpha-2 agonist clonidine is among the most prescribed medications for youth with insomnia, and may be efficacious in youth with neurodevelopmental disorders and ADHD.13 In small retrospective studies, clonidine decreased sleep latency and nighttime awakenings in addition to increasing sleep duration.14 Also, clonidine was well tolerated but associated with daytime somnolence. Guanfacine—a selective alpha-2 agonist—is also commonly prescribed for insomnia in youth, although results of trials have been equivocal.15 Given the more rapid absorption and shorter Tmax of clonidine relative to guanfacine, the former may be preferred as a soporific.
Melatonin and melatonin agonists. The primary regulator of the sleep-wake cycle is melatonin, an endogenous hormone produced by the pineal gland in response to changes in retinal light perception. Exogenous melatonin supplementation may be the preferred initial pharmacotherapy for sleep-onset insomnia due to its chronobiotic properties.16 In clinical studies, both immediate-release17,18 and extended-release19 melatonin reduced sleep-onset latency and increased total sleep duration in pediatric patients, although the increase in total duration of sleep was greater with extended-release preparations. Additionally, tolerability data for melatonin in pediatric patients are encouraging. A 2-year randomized trial of prolonged-release melatonin for insomnia in pediatric patients found no adverse effects with regard to growth, body mass index, or pubertal development.20 Additionally, significant improvements in sleep quality, sleep patterns, and caregiver satisfaction were maintained throughout the trial, and no withdrawal symptoms were observed upon discontinuation.
Melatonin may have a particularly important role in circadian rhythm sleep disorders. In this regard, low-dose melatonin (0.5 mg), when timed relative to the endogenous dim light melatonin onset (DLMO), is more effective in shifting sleep phase than higher doses, which suggests that timing may have greater impact than dosage.21 Data regarding melatonin administration with respect to DLMO suggest that the optimal administration time is 4 to 6 hours before a child’s preferred bedtime, and doses of 0.5 to 1 mg have been effective when given in this window.22 Variation across studies has contributed to a lack of consensus regarding pediatric melatonin dosing. For example, .05 mg/kg may be a minimal effective dose when given 1 to 2 hours before bedtime18; however, in surveys doses vary considerably, with typical doses of 2.5 to 3 mg for prepubertal children and 5 mg for adolescents.5 Of note, in patients with decreased cytochrome P450 (CYP) 1A2 activity, lack of diurnal variation in melatonin serum concentration may decrease the effectiveness of melatonin.16Ramelteon is a potent agonist of the melatonin MT1 and MT2 receptors, with a significantly higher binding affinity than melatonin in vitro. In case reports, ramelteon was well-tolerated, improved delayed sleep onset, and decreased nighttime awakenings.23
Zolpidem, eszopiclone and zaleplon. Studies of selective GABAergic modulators and benzodiazepines have not produced positive results in prospective trials of youth with insomnia. Zolpidem was studied in children and adolescents (N = 201) with ADHD; although sleep latency did not differ between zolpidem and placebo, some significant improvements were observed in adolescents.24 Zolpidem was generally well tolerated, with approximately 10% of youth discontinuing due to adverse effects. Additionally, eszopiclone—which has a longer duration of action compared with zolpidem—has been studied in children and adolescents with ADHD (N = 486) who were also evaluated with a sleep study. No differences were observed between placebo and eszopiclone in terms of sleep latency and approximately 10% of patients discontinued treatment as a result of adverse events.25 We were unable to locate any prospective trials of zaleplon or benzodiazepine receptor agonists for insomnia in youth, although some reports suggest that clonazepam may have a possible role for specific parasomnias.26,27Dual orexin receptor antagonists. Suvorexant, an antagonist of the wakefulness-promoting neuropeptide orexin, improved subjective sleep quality in a prospective trial of adolescents with insomnia (N = 30), although dropout was high (44%).28 Of those patients, reasons for discontinuation included loss to follow-up, lack of effectiveness, and abnormal dreams. We were unable to locate any trials of lemborexant in pediatric patients.
Atypical antidepressants. Trazodone is commonly prescribed for insomnia in pediatric patients with comorbid mood or anxiety disorders. In open-label studies of children and toddlers, trazodone may be well-tolerated and improve sleep.29 Additionally, development of a physiologically based pharmacokinetic model to inform trazodone dosing for youth with insomnia is underway.30 Some studies in adolescents with depression suggest that caution should be used when combining trazodone with medications that inhibit CYP2D6. In the Treatment of SSRI-Resistant Depression in Adolescents study, none of the patients who were treated with trazodone (vs other soporifics) improved.31 This may relate to CYP2D6 interactions and accumulation of methyl-chloro-piperazine (mCPP), a trazodone metabolite that is associated with dysphoria, irritability, and depression.31 This finding has been replicated in a separate cohort of depressed adolescents.32
Because of its antihistaminergic effects, mirtazapine has been used to treat insomnia in adults. One open-label study of mirtazapine in children and young adults ages 3 to 23 with neurodevelopmental disorders suggested that mirtazapine improved behavioral symptoms and insomnia, and was associated with few treatment-limiting adverse effects.33
Tricyclic antidepressants. In a retrospective study of youth with insomnia who failed behavioral interventions and melatonin (N = 29), doxepin, a potent H1 antagonist, improved subjective sleep in one-half of patients.34
Continue to: Consultation with pediatric sleep medicine specialists...
Consultation with pediatric sleep medicine specialists
It often will behoove the psychiatric clinician to review their concerns with a behavioral sleep medicine specialist or a psychologist with specific expertise in the psychotherapeutic treatment of sleep who can provide important guidance regarding the key aspects of treatment. When discussing a particular patient’s presentation with the pediatric behavioral sleep psychologist/specialist, consider the following questions:
- Is the child’s sleep disorder the primary problem, or is the child’s insomnia secondary to another diagnosis (psychiatric or nonpsychiatric)?
- What are the primary sleep-related problems the child/family presents with? How long have the symptoms been present?
- Is the sleep disorder interfering with the child’s functioning, either academically or socially? Does the child’s sleep problem interfere with other family members’ sleep?
- Does the child have a comorbid psychological conditions such as ADHD, depression, or anxiety, and/or is undergoing treatment for these disorders, which may play a role in the sleep problem?
- Is a sleep study warranted?
A sleep study, also known as polysomnography (PSG), is a diagnostic test in which physiologic parameters are continuously recorded during a period of sleep via electroencephalography, electromyography, electrooculogram, electrocardiogram, airflow sensors, pulse oximeter, body position monitors, and video. In 2012, the American Academy of Sleep Medicine published evidenced-based practice parameters for respiratory and nonrespiratory indications for PSG.35 It is most commonly indicated to rule out obstructive sleep apnea in pediatric patients who exhibit snoring, gasping, irregular breathing, witnessed apneic events, unusual head positioning, or other signs of obstructive breathing during sleep. Nonrespiratory indications for PSG include children suspected of having periodic limb movement disorder and suspected narcolepsy. Children with frequent parasomnias, epilepsy, or nocturnal enuresis should be clinically screened for presence of comorbid sleep disorders, and PSG would be indicated if there are concerns about a possible sleep-disordered breathing disorder. PSG is also recommended for children with hypersomnia, to differentiate a parasomnia from sleep-related epilepsy, and for children suspected of having restless leg syndrome.36 PSG is not typically indicated in the initial evaluation of insomnia (unless there is evidence of a comorbid sleep disorder), circadian rhythm disorders (ie, delayed sleep phase syndrome), or for evaluation of children with sleep-related bruxism.3 Special considerations for PSG in children include ensuring a parent or guardian is always with the child, providing developmentally appropriate sleeping arrangements, arranging family tours of the sleep lab prior to the study, and accommodating for earlier bedtimes.37
Bottom Line
Techniques to promote healthy sleep in pediatric patients include behavioral interventions such as setting a developmentally appropriate bedtime and a consistent wake time, establishing bedtime routines, and encouraging relaxation/ wind-down period before bed. Cognitive-behavioral therapy for insomnia (CBT-I) may include cognitive restructuring of anxious thoughts, relaxation training, stimulus control, and sleep restriction. Use of medications may be indicated for children and teens who have not responded to CBT-I or soporific dosing of melatonin.
1. Mindell JA, Li AM, Sadeh A, et al. Bedtime routines for young children: a dose-dependent association with sleep outcomes. Sleep. 2015;38(5):717-722.
2. Kansagra S. Sleep disorders in adolescents. Pediatrics. 2020;145(Suppl 2):S204-S209.
3. Owens JA, Mindell JA. Pediatric insomnia. Pediatr Clin North Am. 2011;58(3):555-569.
4. Bruni O, Angriman M, Melegari MG, et al. Pharmacotherapeutic management of sleep disorders in children with neurodevelopmental disorders. Expert Opin Pharmacother. 2019;20(18):2257-2271.
5. Owens JA, Rosen CL, Mindell JA, et al. Use of pharmacotherapy for insomnia in child psychiatry practice: a national survey. Sleep Med. 2010;11(7):692-700.
6. Schnoes CJ, Kuhn BR, Workman EF, et al. Pediatric prescribing practices for clonidine and other pharmacologic agents for children with sleep disturbance. Clin Pediatr (Phila). 2006;45(3):229-238.
7. Russo RM, Gururaj VJ, Allen JE. The effectiveness of diphenhydramine HCI in pediatric sleep disorders. J Clin Pharmacol. 1976;16(5-6):284-288.
8. Yangzom N, Gottschlich MM, Ossege J, et al. The effect of diphenhydramine on sleep in pediatric burn patients: a secondary analysis. J Burn Care Res. 2015;36(2):266-271.
9. Ghanizadeh A, Zare S. A preliminary randomised double-blind placebo-controlled clinical trial of hydroxyzine for treating sleep bruxism in children. J Oral Rehabil. 2013;40(6):413-417.
10. Bektas O, Arıca B, Teber S, et al. Chloral hydrate and/or hydroxyzine for sedation in pediatric EEG recording. Brain Dev. 2014;36(2):130-136.
11. Ottaviano S, Giannotti F, Cortesi F. The effect of niaprazine on some common sleep disorders in children. A double-blind clinical trial by means of continuous home-videorecorded sleep. Childs Nerv Syst. 1991;7(6):332-335.
12. Nguyen M, Tharani S, Rahmani M, et al. A review of the use of clonidine as a sleep aid in the child and adolescent population. Clin Pediatr (Phila). 2014;53(3):211-216.
13. Prince JB, Wilens TE, Biederman J, et al. Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder: a systematic chart review of 62 cases. J Am Acad Child Adolesc Psychiatry. 1996;35(5):599-605.

14. Ingrassia A, Turk J. The use of clonidine for severe and intractable sleep problems in children with neurodevelopmental disorders--a case series. Eur Child Adolesc Psychiatry. 2005;14(1):34-40.
15. Politte LC, Scahill L, Figueroa J, et al. A randomized, placebo-controlled trial of extended-release guanfacine in children with autism spectrum disorder and ADHD symptoms: an analysis of secondary outcome measures. Neuropsychopharmacology. 2018;43(8):1772-1778.
16. Bruni O, Alonso-Alconada D, Besag F, et al. Current role of melatonin in pediatric neurology: clinical recommendations. Eur J Paediatr Neurol. 2015;19(2):122-1233.
17. Jain SV, Horn PS, Simakajornboon N, et al. Melatonin improves sleep in children with epilepsy: a randomized, double-blind, crossover study. Sleep Med. 2015;16(5):637-644.
18. van Geijlswijk IM, van der Heijden KB, Egberts AC, et al. Dose finding of melatonin for chronic idiopathic childhood sleep onset insomnia: an RCT. Psychopharmacology (Berl). 2010;212(3):379-391.
19. Gringras P, Nir T, Breddy J, et al. Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2017;56(11):948-957.e4.
20. Malow BA, Findling RL, Schroder CM, et al. Sleep, growth, and puberty after two years of prolonged-release melatonin in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2021;60(2):252-261.e3.
21. Burgess HJ, Emens JS. Drugs used in circadian sleep-wake rhythm disturbances. Sleep Med Clin. 2020;15(2):301-310.
22. Arns M, Kooij JJS, Coogan AN. Review: identification and management of circadian rhythm sleep disorders as a transdiagnostic feature in child and adolescent psychiatry. J Am Acad Child Adolesc Psychiatry. 2021;60(9):1085-1095.
23. Kawabe K, Horiuchi F, Oka Y, et al. The melatonin receptor agonist ramelteon effectively treats insomnia and behavioral symptoms in autistic disorder. Case Rep Psychiatry. 2014;2014:561071.
24. Blumer JL, Findling RL, Shih WJ, et al. Controlled clinical trial of zolpidem for the treatment of insomnia associated with attention-deficit/hyperactivity disorder in children 6 to 17 years of age. Pediatrics. 2009;123(5):e770-e776.
25. Sangal RB, Blumer JL, Lankford DA, et al. Eszopiclone for insomnia associated with attention-deficit/hyperactivity disorder. Pediatrics. 2014;134(4):e1095-e1103.
26. Arens R, Wright B, Elliott J, et al. Periodic limb movement in sleep in children with Williams syndrome. J Pediatr. 1998;133(5):670-674.
27. Thirumalai SS, Shubin RA, Robinson R. Rapid eye movement sleep behavior disorder in children with autism. J Child Neurol. 2002;17(3):173-178.
28. Kawabe K, Horiuchi F, Ochi M, et al. Suvorexant for the treatment of insomnia in adolescents. J Child Adolesc Psychopharmacol. 2017;27(9):792-795.
29. Pranzatelli MR, Tate ED, Dukart WS, et al. Sleep disturbance and rage attacks in opsoclonus-myoclonus syndrome: Response to trazodone. J Pediatr. 2005;147(3):372-378.
30. Oggianu L, Ke AB, Chetty M, et al. Estimation of an appropriate dose of trazodone for pediatric insomnia and the potential for a trazodone-atomoxetine interaction. CPT Pharmacometrics Syst Pharmacol. 2020;9(2):77-86.
31. Shamseddeen W, Clarke G, Keller MB, et al. Adjunctive sleep medications and depression outcome in the treatment of serotonin-selective reuptake inhibitor resistant depression in adolescents study. J Child Adolesc Psychopharmacol. 2012;22(1):29-36.
32. Sultan MA, Courtney DB. Adjunctive trazodone and depression outcome in adolescents treated with serotonin re-uptake inhibitors. J Can Acad Child Adolesc Psychiatry. 2017;26(3):233-240.
33. Posey DJ, Guenin KD, Kohn AE, et al. A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2001;11(3):267-277.
34. Shah YD, Stringel V, Pavkovic I, et al. Doxepin in children and adolescents with symptoms of insomnia: a single-center experience. J Clin Sleep Med. 2020;16(5):743-747.
35. Aurora RN, Lamm CI, Zak RS, et al. Practice parameters for the non-respiratory indications for polysomnography and multiple sleep latency testing for children. Sleep. 2012;35(11):1467-1473.
36. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24.
37. Beck SE, Marcus CL. Pediatric polysomnography. Sleep Med Clin. 2009;4(3):393-406.
Children and adolescents who do not receive sufficient sleep can experience worsening inattention, daytime fatigue, and cognitive and behavioral difficulties. Assessment and treatment of insomnia and other sleep difficulties in young patients is critical as poor sleep increases their risk for depression, self-harm, and suicide.
In Part 1 of this article (Pediatric insomnia: Assessment and diagnosis,
Psychotherapeutic interventions
Regardless of the source of a child’s insomnia or co-occurring disorders, healthy sleep practices are the first line behavioral treatment, including for youth with attention-deficit/hyperactivity disorder (ADHD), anxiety disorders, obsessive-compulsive disorder, and depressive disorders.
Healthy sleep practices/sleep hygiene
Developmentally appropriate bedtimes and routines (Table). Helping children establish a regular, consistent bedtime is key in promoting healthy sleep. Ideally, the bedtime routine involves 3 to 4 activities each night in the same order, and these activities should be relaxing and soothing (eg, taking a bath, putting on pajamas, reading books). Setting age-appropriate bedtimes also is important. If an older child is asked to go to bed at 8 pm but cannot fall asleep for an hour, they may not have insomnia but instead a developmentally inappropriate bedtime. Several studies found that children younger than age 10 should go to bed no later than 9 pm. Bedtimes later than 9 pm for young children are correlated with shorter sleep duration.1

Consistent sleep schedules. Another important aspect of healthy sleep is working with parents to enforce a consistent bedtime and wake-up time, including weekdays and weekends. Ideally, bedtime on weekdays and weekends should not vary by more than 1 hour. Helping children wake up at the same time each day helps to set and regulate their circadian rhythm. Keeping these schedules consistent on vacations and school holidays also is helpful. For adolescents, the weekday/weekend bedtimes can vary by up to 2 hours because adolescents have a delayed circadian rhythm and wake-up times for high school can be early.
Environmental factors. An important piece of parental education is stimulus control and the ingredients of healthy sleep. Healthy sleep ingredients include a dark, quiet, consistent, and cool bedroom; a comfortable bed, the child feeling safe, and limited environmental stimuli.
Continue to: Cognitive-behavioral therapy for insomnia...
Cognitive-behavioral therapy for insomnia
Relaxation. Pediatric patients can be taught relaxation, mindfulness, meditation, and progressive muscle relaxation techniques to help lower overall stress. This can be especially helpful for youth with sleep disorders or anxiety. Guided relaxation apps are popular among children and teens, and various apps offer soothing sounds, deep breathing, progressive muscle relaxation, and guided imagery. This can be taught in psychotherapy sessions and used at home to promote gains in between sessions.
Stimulus control. Stimulus control involves using the bed exclusively for sleep and avoiding nonsleep activities in bed (eg, reading, watching television, using a computer, worrying). These activities promote wakefulness and insomnia. This may mean the child does not get into bed until they cannot keep their eyes open, even if that delays bedtime. If the child is still awake within 15 to 20 minutes, they should be encouraged to get out of bed and engage in a nonstimulating activity such as meditation, reading, or sitting quietly in the dark or low light. This recommendation can run counter to parents’ intuition that children with sleep problems should go to bed earlier. Using the bed only for sleep conditions the child to falling asleep or being asleep when in bed.
Sleep restriction. Sleep restriction involves restricting sleep to a set number of hours in order to increase their sleep efficiency (time slept in bed divided by total time spent in bed x 100). Restricting sleep to 6 to 7 hours increases sleep efficiency, consolidates sleep, and extinguishes the association of being awake in bed. For older adolescents, sleep restriction may help to limit their time in bed to either falling asleep or being asleep. This is intended to be used as a short-term strategy and only after other sleep hygiene measures (bedtime routine, environmental factors, etc) have been put into place for several weeks. While this strategy sounds unappealing to most individuals with insomnia, it can lead to lasting change due to the use of behavioral conditioning in bed. Because sleep restriction can lead to significant daytime sleepiness and impairment during the day, sleep should not be restricted to <6 hours a day for children and adolescents. Once the adolescent is sleeping more consistently and sleep efficiency reaches 85% or higher, time in bed for sleep is increased.2
Cognitive restructuring. Some children and adolescents develop maladaptive thoughts about sleep that further promote insomnia. These thoughts might include “I will never get to sleep,” “I am going to have a terrible day if I cannot fall asleep,” or “I will fail my test tomorrow if I am unable to sleep.” Such maladaptive thoughts are often untrue but promote wakefulness in the early or middle part of the night. Cognitive restructuring involves helping the child identify each problematic thought, challenge how accurate each thought is with evidence, and replace the problematic thought with a more helpful thought. For instance, an adolescent can recognize that even if they have a sleepless night, their catastrophic outcome (eg, “I will not be able to function”) is likely untrue. A psychologist can help review evidence for this, including previous times when the adolescent has not slept well and managed to get through the next day.
When is pharmacologic treatment needed?
Pharmacologic treatment may be indicated if a child does not show significant improvement following behavioral intervention (Figure). However, it is critical to exclude other primary causes of dyssomnia (eg, obstructive sleep apnea, iron deficiency anemia) before pursuing pharmacotherapy, because pharmacotherapy could mask an underlying disorder. Moreover, while there is relatively limited evidence for psychopharmacologic interventions for sleep difficulties in children and adolescents, a large survey of child and adolescent psychiatrists (N = 1,273) suggested that medications were considered for one-quarter of pediatric patients with insomnia.3 Further, patients with specific comorbidities such as neurodevelopmental disorders may be more likely to be prescribed soporifics.4

Continue to: What is the evidence for pharmacotherapy?...
What is the evidence for pharmacotherapy?
Antihistamines. Histamine antagonists—which promote sleep by blocking the wakefulness-promoting and circadian-related effects of histamine—are the most commonly used medications to treat pediatric insomnia, despite a dearth of data from prospective trials.5,6 In 1 small study, Russo et al7 found diphenhydramine, 1 mg/kg at bedtime, reduced sleep latency and nighttime awakenings, and increased sleep duration in patients ages 2 to 12; similar effects have been observed in pediatric burn patients.8 There are some limited data for other H1 antagonists (eg, hydroxyzine) in pediatric insomnia.9-11
Alpha-2 agonists increase rapid eye movement sleep via dose-dependent downregulation of noradrenergic signaling12 and thus have been commonly prescribed for insomnia in children and adolescents. In fact, the nonselective alpha-2 agonist clonidine is among the most prescribed medications for youth with insomnia, and may be efficacious in youth with neurodevelopmental disorders and ADHD.13 In small retrospective studies, clonidine decreased sleep latency and nighttime awakenings in addition to increasing sleep duration.14 Also, clonidine was well tolerated but associated with daytime somnolence. Guanfacine—a selective alpha-2 agonist—is also commonly prescribed for insomnia in youth, although results of trials have been equivocal.15 Given the more rapid absorption and shorter Tmax of clonidine relative to guanfacine, the former may be preferred as a soporific.
Melatonin and melatonin agonists. The primary regulator of the sleep-wake cycle is melatonin, an endogenous hormone produced by the pineal gland in response to changes in retinal light perception. Exogenous melatonin supplementation may be the preferred initial pharmacotherapy for sleep-onset insomnia due to its chronobiotic properties.16 In clinical studies, both immediate-release17,18 and extended-release19 melatonin reduced sleep-onset latency and increased total sleep duration in pediatric patients, although the increase in total duration of sleep was greater with extended-release preparations. Additionally, tolerability data for melatonin in pediatric patients are encouraging. A 2-year randomized trial of prolonged-release melatonin for insomnia in pediatric patients found no adverse effects with regard to growth, body mass index, or pubertal development.20 Additionally, significant improvements in sleep quality, sleep patterns, and caregiver satisfaction were maintained throughout the trial, and no withdrawal symptoms were observed upon discontinuation.
Melatonin may have a particularly important role in circadian rhythm sleep disorders. In this regard, low-dose melatonin (0.5 mg), when timed relative to the endogenous dim light melatonin onset (DLMO), is more effective in shifting sleep phase than higher doses, which suggests that timing may have greater impact than dosage.21 Data regarding melatonin administration with respect to DLMO suggest that the optimal administration time is 4 to 6 hours before a child’s preferred bedtime, and doses of 0.5 to 1 mg have been effective when given in this window.22 Variation across studies has contributed to a lack of consensus regarding pediatric melatonin dosing. For example, .05 mg/kg may be a minimal effective dose when given 1 to 2 hours before bedtime18; however, in surveys doses vary considerably, with typical doses of 2.5 to 3 mg for prepubertal children and 5 mg for adolescents.5 Of note, in patients with decreased cytochrome P450 (CYP) 1A2 activity, lack of diurnal variation in melatonin serum concentration may decrease the effectiveness of melatonin.16Ramelteon is a potent agonist of the melatonin MT1 and MT2 receptors, with a significantly higher binding affinity than melatonin in vitro. In case reports, ramelteon was well-tolerated, improved delayed sleep onset, and decreased nighttime awakenings.23
Zolpidem, eszopiclone and zaleplon. Studies of selective GABAergic modulators and benzodiazepines have not produced positive results in prospective trials of youth with insomnia. Zolpidem was studied in children and adolescents (N = 201) with ADHD; although sleep latency did not differ between zolpidem and placebo, some significant improvements were observed in adolescents.24 Zolpidem was generally well tolerated, with approximately 10% of youth discontinuing due to adverse effects. Additionally, eszopiclone—which has a longer duration of action compared with zolpidem—has been studied in children and adolescents with ADHD (N = 486) who were also evaluated with a sleep study. No differences were observed between placebo and eszopiclone in terms of sleep latency and approximately 10% of patients discontinued treatment as a result of adverse events.25 We were unable to locate any prospective trials of zaleplon or benzodiazepine receptor agonists for insomnia in youth, although some reports suggest that clonazepam may have a possible role for specific parasomnias.26,27Dual orexin receptor antagonists. Suvorexant, an antagonist of the wakefulness-promoting neuropeptide orexin, improved subjective sleep quality in a prospective trial of adolescents with insomnia (N = 30), although dropout was high (44%).28 Of those patients, reasons for discontinuation included loss to follow-up, lack of effectiveness, and abnormal dreams. We were unable to locate any trials of lemborexant in pediatric patients.
Atypical antidepressants. Trazodone is commonly prescribed for insomnia in pediatric patients with comorbid mood or anxiety disorders. In open-label studies of children and toddlers, trazodone may be well-tolerated and improve sleep.29 Additionally, development of a physiologically based pharmacokinetic model to inform trazodone dosing for youth with insomnia is underway.30 Some studies in adolescents with depression suggest that caution should be used when combining trazodone with medications that inhibit CYP2D6. In the Treatment of SSRI-Resistant Depression in Adolescents study, none of the patients who were treated with trazodone (vs other soporifics) improved.31 This may relate to CYP2D6 interactions and accumulation of methyl-chloro-piperazine (mCPP), a trazodone metabolite that is associated with dysphoria, irritability, and depression.31 This finding has been replicated in a separate cohort of depressed adolescents.32
Because of its antihistaminergic effects, mirtazapine has been used to treat insomnia in adults. One open-label study of mirtazapine in children and young adults ages 3 to 23 with neurodevelopmental disorders suggested that mirtazapine improved behavioral symptoms and insomnia, and was associated with few treatment-limiting adverse effects.33
Tricyclic antidepressants. In a retrospective study of youth with insomnia who failed behavioral interventions and melatonin (N = 29), doxepin, a potent H1 antagonist, improved subjective sleep in one-half of patients.34
Continue to: Consultation with pediatric sleep medicine specialists...
Consultation with pediatric sleep medicine specialists
It often will behoove the psychiatric clinician to review their concerns with a behavioral sleep medicine specialist or a psychologist with specific expertise in the psychotherapeutic treatment of sleep who can provide important guidance regarding the key aspects of treatment. When discussing a particular patient’s presentation with the pediatric behavioral sleep psychologist/specialist, consider the following questions:
- Is the child’s sleep disorder the primary problem, or is the child’s insomnia secondary to another diagnosis (psychiatric or nonpsychiatric)?
- What are the primary sleep-related problems the child/family presents with? How long have the symptoms been present?
- Is the sleep disorder interfering with the child’s functioning, either academically or socially? Does the child’s sleep problem interfere with other family members’ sleep?
- Does the child have a comorbid psychological conditions such as ADHD, depression, or anxiety, and/or is undergoing treatment for these disorders, which may play a role in the sleep problem?
- Is a sleep study warranted?
A sleep study, also known as polysomnography (PSG), is a diagnostic test in which physiologic parameters are continuously recorded during a period of sleep via electroencephalography, electromyography, electrooculogram, electrocardiogram, airflow sensors, pulse oximeter, body position monitors, and video. In 2012, the American Academy of Sleep Medicine published evidenced-based practice parameters for respiratory and nonrespiratory indications for PSG.35 It is most commonly indicated to rule out obstructive sleep apnea in pediatric patients who exhibit snoring, gasping, irregular breathing, witnessed apneic events, unusual head positioning, or other signs of obstructive breathing during sleep. Nonrespiratory indications for PSG include children suspected of having periodic limb movement disorder and suspected narcolepsy. Children with frequent parasomnias, epilepsy, or nocturnal enuresis should be clinically screened for presence of comorbid sleep disorders, and PSG would be indicated if there are concerns about a possible sleep-disordered breathing disorder. PSG is also recommended for children with hypersomnia, to differentiate a parasomnia from sleep-related epilepsy, and for children suspected of having restless leg syndrome.36 PSG is not typically indicated in the initial evaluation of insomnia (unless there is evidence of a comorbid sleep disorder), circadian rhythm disorders (ie, delayed sleep phase syndrome), or for evaluation of children with sleep-related bruxism.3 Special considerations for PSG in children include ensuring a parent or guardian is always with the child, providing developmentally appropriate sleeping arrangements, arranging family tours of the sleep lab prior to the study, and accommodating for earlier bedtimes.37
Bottom Line
Techniques to promote healthy sleep in pediatric patients include behavioral interventions such as setting a developmentally appropriate bedtime and a consistent wake time, establishing bedtime routines, and encouraging relaxation/ wind-down period before bed. Cognitive-behavioral therapy for insomnia (CBT-I) may include cognitive restructuring of anxious thoughts, relaxation training, stimulus control, and sleep restriction. Use of medications may be indicated for children and teens who have not responded to CBT-I or soporific dosing of melatonin.
Children and adolescents who do not receive sufficient sleep can experience worsening inattention, daytime fatigue, and cognitive and behavioral difficulties. Assessment and treatment of insomnia and other sleep difficulties in young patients is critical as poor sleep increases their risk for depression, self-harm, and suicide.
In Part 1 of this article (Pediatric insomnia: Assessment and diagnosis,
Psychotherapeutic interventions
Regardless of the source of a child’s insomnia or co-occurring disorders, healthy sleep practices are the first line behavioral treatment, including for youth with attention-deficit/hyperactivity disorder (ADHD), anxiety disorders, obsessive-compulsive disorder, and depressive disorders.
Healthy sleep practices/sleep hygiene
Developmentally appropriate bedtimes and routines (Table). Helping children establish a regular, consistent bedtime is key in promoting healthy sleep. Ideally, the bedtime routine involves 3 to 4 activities each night in the same order, and these activities should be relaxing and soothing (eg, taking a bath, putting on pajamas, reading books). Setting age-appropriate bedtimes also is important. If an older child is asked to go to bed at 8 pm but cannot fall asleep for an hour, they may not have insomnia but instead a developmentally inappropriate bedtime. Several studies found that children younger than age 10 should go to bed no later than 9 pm. Bedtimes later than 9 pm for young children are correlated with shorter sleep duration.1

Consistent sleep schedules. Another important aspect of healthy sleep is working with parents to enforce a consistent bedtime and wake-up time, including weekdays and weekends. Ideally, bedtime on weekdays and weekends should not vary by more than 1 hour. Helping children wake up at the same time each day helps to set and regulate their circadian rhythm. Keeping these schedules consistent on vacations and school holidays also is helpful. For adolescents, the weekday/weekend bedtimes can vary by up to 2 hours because adolescents have a delayed circadian rhythm and wake-up times for high school can be early.
Environmental factors. An important piece of parental education is stimulus control and the ingredients of healthy sleep. Healthy sleep ingredients include a dark, quiet, consistent, and cool bedroom; a comfortable bed, the child feeling safe, and limited environmental stimuli.
Continue to: Cognitive-behavioral therapy for insomnia...
Cognitive-behavioral therapy for insomnia
Relaxation. Pediatric patients can be taught relaxation, mindfulness, meditation, and progressive muscle relaxation techniques to help lower overall stress. This can be especially helpful for youth with sleep disorders or anxiety. Guided relaxation apps are popular among children and teens, and various apps offer soothing sounds, deep breathing, progressive muscle relaxation, and guided imagery. This can be taught in psychotherapy sessions and used at home to promote gains in between sessions.
Stimulus control. Stimulus control involves using the bed exclusively for sleep and avoiding nonsleep activities in bed (eg, reading, watching television, using a computer, worrying). These activities promote wakefulness and insomnia. This may mean the child does not get into bed until they cannot keep their eyes open, even if that delays bedtime. If the child is still awake within 15 to 20 minutes, they should be encouraged to get out of bed and engage in a nonstimulating activity such as meditation, reading, or sitting quietly in the dark or low light. This recommendation can run counter to parents’ intuition that children with sleep problems should go to bed earlier. Using the bed only for sleep conditions the child to falling asleep or being asleep when in bed.
Sleep restriction. Sleep restriction involves restricting sleep to a set number of hours in order to increase their sleep efficiency (time slept in bed divided by total time spent in bed x 100). Restricting sleep to 6 to 7 hours increases sleep efficiency, consolidates sleep, and extinguishes the association of being awake in bed. For older adolescents, sleep restriction may help to limit their time in bed to either falling asleep or being asleep. This is intended to be used as a short-term strategy and only after other sleep hygiene measures (bedtime routine, environmental factors, etc) have been put into place for several weeks. While this strategy sounds unappealing to most individuals with insomnia, it can lead to lasting change due to the use of behavioral conditioning in bed. Because sleep restriction can lead to significant daytime sleepiness and impairment during the day, sleep should not be restricted to <6 hours a day for children and adolescents. Once the adolescent is sleeping more consistently and sleep efficiency reaches 85% or higher, time in bed for sleep is increased.2
Cognitive restructuring. Some children and adolescents develop maladaptive thoughts about sleep that further promote insomnia. These thoughts might include “I will never get to sleep,” “I am going to have a terrible day if I cannot fall asleep,” or “I will fail my test tomorrow if I am unable to sleep.” Such maladaptive thoughts are often untrue but promote wakefulness in the early or middle part of the night. Cognitive restructuring involves helping the child identify each problematic thought, challenge how accurate each thought is with evidence, and replace the problematic thought with a more helpful thought. For instance, an adolescent can recognize that even if they have a sleepless night, their catastrophic outcome (eg, “I will not be able to function”) is likely untrue. A psychologist can help review evidence for this, including previous times when the adolescent has not slept well and managed to get through the next day.
When is pharmacologic treatment needed?
Pharmacologic treatment may be indicated if a child does not show significant improvement following behavioral intervention (Figure). However, it is critical to exclude other primary causes of dyssomnia (eg, obstructive sleep apnea, iron deficiency anemia) before pursuing pharmacotherapy, because pharmacotherapy could mask an underlying disorder. Moreover, while there is relatively limited evidence for psychopharmacologic interventions for sleep difficulties in children and adolescents, a large survey of child and adolescent psychiatrists (N = 1,273) suggested that medications were considered for one-quarter of pediatric patients with insomnia.3 Further, patients with specific comorbidities such as neurodevelopmental disorders may be more likely to be prescribed soporifics.4

Continue to: What is the evidence for pharmacotherapy?...
What is the evidence for pharmacotherapy?
Antihistamines. Histamine antagonists—which promote sleep by blocking the wakefulness-promoting and circadian-related effects of histamine—are the most commonly used medications to treat pediatric insomnia, despite a dearth of data from prospective trials.5,6 In 1 small study, Russo et al7 found diphenhydramine, 1 mg/kg at bedtime, reduced sleep latency and nighttime awakenings, and increased sleep duration in patients ages 2 to 12; similar effects have been observed in pediatric burn patients.8 There are some limited data for other H1 antagonists (eg, hydroxyzine) in pediatric insomnia.9-11
Alpha-2 agonists increase rapid eye movement sleep via dose-dependent downregulation of noradrenergic signaling12 and thus have been commonly prescribed for insomnia in children and adolescents. In fact, the nonselective alpha-2 agonist clonidine is among the most prescribed medications for youth with insomnia, and may be efficacious in youth with neurodevelopmental disorders and ADHD.13 In small retrospective studies, clonidine decreased sleep latency and nighttime awakenings in addition to increasing sleep duration.14 Also, clonidine was well tolerated but associated with daytime somnolence. Guanfacine—a selective alpha-2 agonist—is also commonly prescribed for insomnia in youth, although results of trials have been equivocal.15 Given the more rapid absorption and shorter Tmax of clonidine relative to guanfacine, the former may be preferred as a soporific.
Melatonin and melatonin agonists. The primary regulator of the sleep-wake cycle is melatonin, an endogenous hormone produced by the pineal gland in response to changes in retinal light perception. Exogenous melatonin supplementation may be the preferred initial pharmacotherapy for sleep-onset insomnia due to its chronobiotic properties.16 In clinical studies, both immediate-release17,18 and extended-release19 melatonin reduced sleep-onset latency and increased total sleep duration in pediatric patients, although the increase in total duration of sleep was greater with extended-release preparations. Additionally, tolerability data for melatonin in pediatric patients are encouraging. A 2-year randomized trial of prolonged-release melatonin for insomnia in pediatric patients found no adverse effects with regard to growth, body mass index, or pubertal development.20 Additionally, significant improvements in sleep quality, sleep patterns, and caregiver satisfaction were maintained throughout the trial, and no withdrawal symptoms were observed upon discontinuation.
Melatonin may have a particularly important role in circadian rhythm sleep disorders. In this regard, low-dose melatonin (0.5 mg), when timed relative to the endogenous dim light melatonin onset (DLMO), is more effective in shifting sleep phase than higher doses, which suggests that timing may have greater impact than dosage.21 Data regarding melatonin administration with respect to DLMO suggest that the optimal administration time is 4 to 6 hours before a child’s preferred bedtime, and doses of 0.5 to 1 mg have been effective when given in this window.22 Variation across studies has contributed to a lack of consensus regarding pediatric melatonin dosing. For example, .05 mg/kg may be a minimal effective dose when given 1 to 2 hours before bedtime18; however, in surveys doses vary considerably, with typical doses of 2.5 to 3 mg for prepubertal children and 5 mg for adolescents.5 Of note, in patients with decreased cytochrome P450 (CYP) 1A2 activity, lack of diurnal variation in melatonin serum concentration may decrease the effectiveness of melatonin.16Ramelteon is a potent agonist of the melatonin MT1 and MT2 receptors, with a significantly higher binding affinity than melatonin in vitro. In case reports, ramelteon was well-tolerated, improved delayed sleep onset, and decreased nighttime awakenings.23
Zolpidem, eszopiclone and zaleplon. Studies of selective GABAergic modulators and benzodiazepines have not produced positive results in prospective trials of youth with insomnia. Zolpidem was studied in children and adolescents (N = 201) with ADHD; although sleep latency did not differ between zolpidem and placebo, some significant improvements were observed in adolescents.24 Zolpidem was generally well tolerated, with approximately 10% of youth discontinuing due to adverse effects. Additionally, eszopiclone—which has a longer duration of action compared with zolpidem—has been studied in children and adolescents with ADHD (N = 486) who were also evaluated with a sleep study. No differences were observed between placebo and eszopiclone in terms of sleep latency and approximately 10% of patients discontinued treatment as a result of adverse events.25 We were unable to locate any prospective trials of zaleplon or benzodiazepine receptor agonists for insomnia in youth, although some reports suggest that clonazepam may have a possible role for specific parasomnias.26,27Dual orexin receptor antagonists. Suvorexant, an antagonist of the wakefulness-promoting neuropeptide orexin, improved subjective sleep quality in a prospective trial of adolescents with insomnia (N = 30), although dropout was high (44%).28 Of those patients, reasons for discontinuation included loss to follow-up, lack of effectiveness, and abnormal dreams. We were unable to locate any trials of lemborexant in pediatric patients.
Atypical antidepressants. Trazodone is commonly prescribed for insomnia in pediatric patients with comorbid mood or anxiety disorders. In open-label studies of children and toddlers, trazodone may be well-tolerated and improve sleep.29 Additionally, development of a physiologically based pharmacokinetic model to inform trazodone dosing for youth with insomnia is underway.30 Some studies in adolescents with depression suggest that caution should be used when combining trazodone with medications that inhibit CYP2D6. In the Treatment of SSRI-Resistant Depression in Adolescents study, none of the patients who were treated with trazodone (vs other soporifics) improved.31 This may relate to CYP2D6 interactions and accumulation of methyl-chloro-piperazine (mCPP), a trazodone metabolite that is associated with dysphoria, irritability, and depression.31 This finding has been replicated in a separate cohort of depressed adolescents.32
Because of its antihistaminergic effects, mirtazapine has been used to treat insomnia in adults. One open-label study of mirtazapine in children and young adults ages 3 to 23 with neurodevelopmental disorders suggested that mirtazapine improved behavioral symptoms and insomnia, and was associated with few treatment-limiting adverse effects.33
Tricyclic antidepressants. In a retrospective study of youth with insomnia who failed behavioral interventions and melatonin (N = 29), doxepin, a potent H1 antagonist, improved subjective sleep in one-half of patients.34
Continue to: Consultation with pediatric sleep medicine specialists...
Consultation with pediatric sleep medicine specialists
It often will behoove the psychiatric clinician to review their concerns with a behavioral sleep medicine specialist or a psychologist with specific expertise in the psychotherapeutic treatment of sleep who can provide important guidance regarding the key aspects of treatment. When discussing a particular patient’s presentation with the pediatric behavioral sleep psychologist/specialist, consider the following questions:
- Is the child’s sleep disorder the primary problem, or is the child’s insomnia secondary to another diagnosis (psychiatric or nonpsychiatric)?
- What are the primary sleep-related problems the child/family presents with? How long have the symptoms been present?
- Is the sleep disorder interfering with the child’s functioning, either academically or socially? Does the child’s sleep problem interfere with other family members’ sleep?
- Does the child have a comorbid psychological conditions such as ADHD, depression, or anxiety, and/or is undergoing treatment for these disorders, which may play a role in the sleep problem?
- Is a sleep study warranted?
A sleep study, also known as polysomnography (PSG), is a diagnostic test in which physiologic parameters are continuously recorded during a period of sleep via electroencephalography, electromyography, electrooculogram, electrocardiogram, airflow sensors, pulse oximeter, body position monitors, and video. In 2012, the American Academy of Sleep Medicine published evidenced-based practice parameters for respiratory and nonrespiratory indications for PSG.35 It is most commonly indicated to rule out obstructive sleep apnea in pediatric patients who exhibit snoring, gasping, irregular breathing, witnessed apneic events, unusual head positioning, or other signs of obstructive breathing during sleep. Nonrespiratory indications for PSG include children suspected of having periodic limb movement disorder and suspected narcolepsy. Children with frequent parasomnias, epilepsy, or nocturnal enuresis should be clinically screened for presence of comorbid sleep disorders, and PSG would be indicated if there are concerns about a possible sleep-disordered breathing disorder. PSG is also recommended for children with hypersomnia, to differentiate a parasomnia from sleep-related epilepsy, and for children suspected of having restless leg syndrome.36 PSG is not typically indicated in the initial evaluation of insomnia (unless there is evidence of a comorbid sleep disorder), circadian rhythm disorders (ie, delayed sleep phase syndrome), or for evaluation of children with sleep-related bruxism.3 Special considerations for PSG in children include ensuring a parent or guardian is always with the child, providing developmentally appropriate sleeping arrangements, arranging family tours of the sleep lab prior to the study, and accommodating for earlier bedtimes.37
Bottom Line
Techniques to promote healthy sleep in pediatric patients include behavioral interventions such as setting a developmentally appropriate bedtime and a consistent wake time, establishing bedtime routines, and encouraging relaxation/ wind-down period before bed. Cognitive-behavioral therapy for insomnia (CBT-I) may include cognitive restructuring of anxious thoughts, relaxation training, stimulus control, and sleep restriction. Use of medications may be indicated for children and teens who have not responded to CBT-I or soporific dosing of melatonin.
1. Mindell JA, Li AM, Sadeh A, et al. Bedtime routines for young children: a dose-dependent association with sleep outcomes. Sleep. 2015;38(5):717-722.
2. Kansagra S. Sleep disorders in adolescents. Pediatrics. 2020;145(Suppl 2):S204-S209.
3. Owens JA, Mindell JA. Pediatric insomnia. Pediatr Clin North Am. 2011;58(3):555-569.
4. Bruni O, Angriman M, Melegari MG, et al. Pharmacotherapeutic management of sleep disorders in children with neurodevelopmental disorders. Expert Opin Pharmacother. 2019;20(18):2257-2271.
5. Owens JA, Rosen CL, Mindell JA, et al. Use of pharmacotherapy for insomnia in child psychiatry practice: a national survey. Sleep Med. 2010;11(7):692-700.
6. Schnoes CJ, Kuhn BR, Workman EF, et al. Pediatric prescribing practices for clonidine and other pharmacologic agents for children with sleep disturbance. Clin Pediatr (Phila). 2006;45(3):229-238.
7. Russo RM, Gururaj VJ, Allen JE. The effectiveness of diphenhydramine HCI in pediatric sleep disorders. J Clin Pharmacol. 1976;16(5-6):284-288.
8. Yangzom N, Gottschlich MM, Ossege J, et al. The effect of diphenhydramine on sleep in pediatric burn patients: a secondary analysis. J Burn Care Res. 2015;36(2):266-271.
9. Ghanizadeh A, Zare S. A preliminary randomised double-blind placebo-controlled clinical trial of hydroxyzine for treating sleep bruxism in children. J Oral Rehabil. 2013;40(6):413-417.
10. Bektas O, Arıca B, Teber S, et al. Chloral hydrate and/or hydroxyzine for sedation in pediatric EEG recording. Brain Dev. 2014;36(2):130-136.
11. Ottaviano S, Giannotti F, Cortesi F. The effect of niaprazine on some common sleep disorders in children. A double-blind clinical trial by means of continuous home-videorecorded sleep. Childs Nerv Syst. 1991;7(6):332-335.
12. Nguyen M, Tharani S, Rahmani M, et al. A review of the use of clonidine as a sleep aid in the child and adolescent population. Clin Pediatr (Phila). 2014;53(3):211-216.
13. Prince JB, Wilens TE, Biederman J, et al. Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder: a systematic chart review of 62 cases. J Am Acad Child Adolesc Psychiatry. 1996;35(5):599-605.

14. Ingrassia A, Turk J. The use of clonidine for severe and intractable sleep problems in children with neurodevelopmental disorders--a case series. Eur Child Adolesc Psychiatry. 2005;14(1):34-40.
15. Politte LC, Scahill L, Figueroa J, et al. A randomized, placebo-controlled trial of extended-release guanfacine in children with autism spectrum disorder and ADHD symptoms: an analysis of secondary outcome measures. Neuropsychopharmacology. 2018;43(8):1772-1778.
16. Bruni O, Alonso-Alconada D, Besag F, et al. Current role of melatonin in pediatric neurology: clinical recommendations. Eur J Paediatr Neurol. 2015;19(2):122-1233.
17. Jain SV, Horn PS, Simakajornboon N, et al. Melatonin improves sleep in children with epilepsy: a randomized, double-blind, crossover study. Sleep Med. 2015;16(5):637-644.
18. van Geijlswijk IM, van der Heijden KB, Egberts AC, et al. Dose finding of melatonin for chronic idiopathic childhood sleep onset insomnia: an RCT. Psychopharmacology (Berl). 2010;212(3):379-391.
19. Gringras P, Nir T, Breddy J, et al. Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2017;56(11):948-957.e4.
20. Malow BA, Findling RL, Schroder CM, et al. Sleep, growth, and puberty after two years of prolonged-release melatonin in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2021;60(2):252-261.e3.
21. Burgess HJ, Emens JS. Drugs used in circadian sleep-wake rhythm disturbances. Sleep Med Clin. 2020;15(2):301-310.
22. Arns M, Kooij JJS, Coogan AN. Review: identification and management of circadian rhythm sleep disorders as a transdiagnostic feature in child and adolescent psychiatry. J Am Acad Child Adolesc Psychiatry. 2021;60(9):1085-1095.
23. Kawabe K, Horiuchi F, Oka Y, et al. The melatonin receptor agonist ramelteon effectively treats insomnia and behavioral symptoms in autistic disorder. Case Rep Psychiatry. 2014;2014:561071.
24. Blumer JL, Findling RL, Shih WJ, et al. Controlled clinical trial of zolpidem for the treatment of insomnia associated with attention-deficit/hyperactivity disorder in children 6 to 17 years of age. Pediatrics. 2009;123(5):e770-e776.
25. Sangal RB, Blumer JL, Lankford DA, et al. Eszopiclone for insomnia associated with attention-deficit/hyperactivity disorder. Pediatrics. 2014;134(4):e1095-e1103.
26. Arens R, Wright B, Elliott J, et al. Periodic limb movement in sleep in children with Williams syndrome. J Pediatr. 1998;133(5):670-674.
27. Thirumalai SS, Shubin RA, Robinson R. Rapid eye movement sleep behavior disorder in children with autism. J Child Neurol. 2002;17(3):173-178.
28. Kawabe K, Horiuchi F, Ochi M, et al. Suvorexant for the treatment of insomnia in adolescents. J Child Adolesc Psychopharmacol. 2017;27(9):792-795.
29. Pranzatelli MR, Tate ED, Dukart WS, et al. Sleep disturbance and rage attacks in opsoclonus-myoclonus syndrome: Response to trazodone. J Pediatr. 2005;147(3):372-378.
30. Oggianu L, Ke AB, Chetty M, et al. Estimation of an appropriate dose of trazodone for pediatric insomnia and the potential for a trazodone-atomoxetine interaction. CPT Pharmacometrics Syst Pharmacol. 2020;9(2):77-86.
31. Shamseddeen W, Clarke G, Keller MB, et al. Adjunctive sleep medications and depression outcome in the treatment of serotonin-selective reuptake inhibitor resistant depression in adolescents study. J Child Adolesc Psychopharmacol. 2012;22(1):29-36.
32. Sultan MA, Courtney DB. Adjunctive trazodone and depression outcome in adolescents treated with serotonin re-uptake inhibitors. J Can Acad Child Adolesc Psychiatry. 2017;26(3):233-240.
33. Posey DJ, Guenin KD, Kohn AE, et al. A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2001;11(3):267-277.
34. Shah YD, Stringel V, Pavkovic I, et al. Doxepin in children and adolescents with symptoms of insomnia: a single-center experience. J Clin Sleep Med. 2020;16(5):743-747.
35. Aurora RN, Lamm CI, Zak RS, et al. Practice parameters for the non-respiratory indications for polysomnography and multiple sleep latency testing for children. Sleep. 2012;35(11):1467-1473.
36. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24.
37. Beck SE, Marcus CL. Pediatric polysomnography. Sleep Med Clin. 2009;4(3):393-406.
1. Mindell JA, Li AM, Sadeh A, et al. Bedtime routines for young children: a dose-dependent association with sleep outcomes. Sleep. 2015;38(5):717-722.
2. Kansagra S. Sleep disorders in adolescents. Pediatrics. 2020;145(Suppl 2):S204-S209.
3. Owens JA, Mindell JA. Pediatric insomnia. Pediatr Clin North Am. 2011;58(3):555-569.
4. Bruni O, Angriman M, Melegari MG, et al. Pharmacotherapeutic management of sleep disorders in children with neurodevelopmental disorders. Expert Opin Pharmacother. 2019;20(18):2257-2271.
5. Owens JA, Rosen CL, Mindell JA, et al. Use of pharmacotherapy for insomnia in child psychiatry practice: a national survey. Sleep Med. 2010;11(7):692-700.
6. Schnoes CJ, Kuhn BR, Workman EF, et al. Pediatric prescribing practices for clonidine and other pharmacologic agents for children with sleep disturbance. Clin Pediatr (Phila). 2006;45(3):229-238.
7. Russo RM, Gururaj VJ, Allen JE. The effectiveness of diphenhydramine HCI in pediatric sleep disorders. J Clin Pharmacol. 1976;16(5-6):284-288.
8. Yangzom N, Gottschlich MM, Ossege J, et al. The effect of diphenhydramine on sleep in pediatric burn patients: a secondary analysis. J Burn Care Res. 2015;36(2):266-271.
9. Ghanizadeh A, Zare S. A preliminary randomised double-blind placebo-controlled clinical trial of hydroxyzine for treating sleep bruxism in children. J Oral Rehabil. 2013;40(6):413-417.
10. Bektas O, Arıca B, Teber S, et al. Chloral hydrate and/or hydroxyzine for sedation in pediatric EEG recording. Brain Dev. 2014;36(2):130-136.
11. Ottaviano S, Giannotti F, Cortesi F. The effect of niaprazine on some common sleep disorders in children. A double-blind clinical trial by means of continuous home-videorecorded sleep. Childs Nerv Syst. 1991;7(6):332-335.
12. Nguyen M, Tharani S, Rahmani M, et al. A review of the use of clonidine as a sleep aid in the child and adolescent population. Clin Pediatr (Phila). 2014;53(3):211-216.
13. Prince JB, Wilens TE, Biederman J, et al. Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder: a systematic chart review of 62 cases. J Am Acad Child Adolesc Psychiatry. 1996;35(5):599-605.

14. Ingrassia A, Turk J. The use of clonidine for severe and intractable sleep problems in children with neurodevelopmental disorders--a case series. Eur Child Adolesc Psychiatry. 2005;14(1):34-40.
15. Politte LC, Scahill L, Figueroa J, et al. A randomized, placebo-controlled trial of extended-release guanfacine in children with autism spectrum disorder and ADHD symptoms: an analysis of secondary outcome measures. Neuropsychopharmacology. 2018;43(8):1772-1778.
16. Bruni O, Alonso-Alconada D, Besag F, et al. Current role of melatonin in pediatric neurology: clinical recommendations. Eur J Paediatr Neurol. 2015;19(2):122-1233.
17. Jain SV, Horn PS, Simakajornboon N, et al. Melatonin improves sleep in children with epilepsy: a randomized, double-blind, crossover study. Sleep Med. 2015;16(5):637-644.
18. van Geijlswijk IM, van der Heijden KB, Egberts AC, et al. Dose finding of melatonin for chronic idiopathic childhood sleep onset insomnia: an RCT. Psychopharmacology (Berl). 2010;212(3):379-391.
19. Gringras P, Nir T, Breddy J, et al. Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2017;56(11):948-957.e4.
20. Malow BA, Findling RL, Schroder CM, et al. Sleep, growth, and puberty after two years of prolonged-release melatonin in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2021;60(2):252-261.e3.
21. Burgess HJ, Emens JS. Drugs used in circadian sleep-wake rhythm disturbances. Sleep Med Clin. 2020;15(2):301-310.
22. Arns M, Kooij JJS, Coogan AN. Review: identification and management of circadian rhythm sleep disorders as a transdiagnostic feature in child and adolescent psychiatry. J Am Acad Child Adolesc Psychiatry. 2021;60(9):1085-1095.
23. Kawabe K, Horiuchi F, Oka Y, et al. The melatonin receptor agonist ramelteon effectively treats insomnia and behavioral symptoms in autistic disorder. Case Rep Psychiatry. 2014;2014:561071.
24. Blumer JL, Findling RL, Shih WJ, et al. Controlled clinical trial of zolpidem for the treatment of insomnia associated with attention-deficit/hyperactivity disorder in children 6 to 17 years of age. Pediatrics. 2009;123(5):e770-e776.
25. Sangal RB, Blumer JL, Lankford DA, et al. Eszopiclone for insomnia associated with attention-deficit/hyperactivity disorder. Pediatrics. 2014;134(4):e1095-e1103.
26. Arens R, Wright B, Elliott J, et al. Periodic limb movement in sleep in children with Williams syndrome. J Pediatr. 1998;133(5):670-674.
27. Thirumalai SS, Shubin RA, Robinson R. Rapid eye movement sleep behavior disorder in children with autism. J Child Neurol. 2002;17(3):173-178.
28. Kawabe K, Horiuchi F, Ochi M, et al. Suvorexant for the treatment of insomnia in adolescents. J Child Adolesc Psychopharmacol. 2017;27(9):792-795.
29. Pranzatelli MR, Tate ED, Dukart WS, et al. Sleep disturbance and rage attacks in opsoclonus-myoclonus syndrome: Response to trazodone. J Pediatr. 2005;147(3):372-378.
30. Oggianu L, Ke AB, Chetty M, et al. Estimation of an appropriate dose of trazodone for pediatric insomnia and the potential for a trazodone-atomoxetine interaction. CPT Pharmacometrics Syst Pharmacol. 2020;9(2):77-86.
31. Shamseddeen W, Clarke G, Keller MB, et al. Adjunctive sleep medications and depression outcome in the treatment of serotonin-selective reuptake inhibitor resistant depression in adolescents study. J Child Adolesc Psychopharmacol. 2012;22(1):29-36.
32. Sultan MA, Courtney DB. Adjunctive trazodone and depression outcome in adolescents treated with serotonin re-uptake inhibitors. J Can Acad Child Adolesc Psychiatry. 2017;26(3):233-240.
33. Posey DJ, Guenin KD, Kohn AE, et al. A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2001;11(3):267-277.
34. Shah YD, Stringel V, Pavkovic I, et al. Doxepin in children and adolescents with symptoms of insomnia: a single-center experience. J Clin Sleep Med. 2020;16(5):743-747.
35. Aurora RN, Lamm CI, Zak RS, et al. Practice parameters for the non-respiratory indications for polysomnography and multiple sleep latency testing for children. Sleep. 2012;35(11):1467-1473.
36. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24.
37. Beck SE, Marcus CL. Pediatric polysomnography. Sleep Med Clin. 2009;4(3):393-406.