User login
Grand Rounds: Man, 60, With Abdominal Pain
A 60-year-old white man with a history of hyperlipidemia, hypertension, and anxiety presented with complaints of abdominal pain, localized to an area left of the umbilicus. He described the pain as constant and rated it 6 on a scale of 1 to 10. He said the pain had been present for longer than three weeks.
The man said he had been seen by another health care provider shortly after the pain began, but he did not think the provider took his complaint seriously. At that visit, antacids were prescribed, blood work was ordered, and the man was told to return if there was no improvement. He felt that because he was being treated for anxiety, the provider believed he was just imagining the pain.
At the current visit, the review of systems revealed additional complaints of shakiness and nausea without vomiting, with other findings unremarkable. The persistent pain did not seem related to eating, and the patient had no history of any surgeries that might help explain his current complaints. He had smoked a pack of cigarettes daily for 40 years and had a history of heavy alcohol use, although he denied having consumed any alcohol during the previous five years.
His prescribed medications included gemfibrozil 600 mg per day, hydrochlorothiazide 25 mg each morning, and diazepam 5 mg twice daily, with an OTC antacid.
The patient’s recent laboratory results were normal; they included a complete blood count, comprehensive metabolic panel, liver enzyme levels, and a serum amylase level. The patient weighed 280 lb and his height was 5’10”; his BMI was 40. His temperature was 97.7°F, with a regular heart rate of 88 beats/min; blood pressure, 140/90 mm Hg; and respiratory rate, 18 breaths/min.
The patient did not appear to be in acute distress. A bruit was heard in the indicated area of pain. No mass was palpated, and the width of his aorta could not be determined because of his obesity. His physical exam was otherwise normal.
Abdominal ultrasonography (US) revealed a 5.5-cm abdominal aortic aneurysm (AAA), and the man was referred for immediate surgery. The aneurysm was repaired in an open abdominal procedure with a polyester prosthetic graft. The surgery was successful.
Discussion
AAA is a permanent bulging area of the aorta that exceeds 3.0 cm in diameter (see Figure 1). It is a potentially life-threatening condition due to the possibility of rupture. Often an aneurysm is asymptomatic until it ruptures, making this a difficult illness to diagnose.1
Each year, an estimated 10,000 deaths result from a ruptured AAA, making this condition the 14th leading cause of death in the United States.2,3 Incidence of AAA appears to have increased over the past two decades. Causes for this may include the aging of the US population, an increase in the number of smokers, and a trend toward diets that are higher in fat.
Prognosis among patients with AAA can be improved with increased awareness of the disease among health care providers, earlier detection of AAAs at risk for rupture, and timely, effective interventions.
Symptomatology
In about one-third of patients with a ruptured AAA, a clinical triad of symptoms is present: abdominal and/or back pain, a pulsatile abdominal mass, and hypotension.4,5 In these cases, according to the American College of Cardiology/American Heart Association (ACC/AHA),4 immediate surgical evaluation is indicated.
Prior to the rupture of an AAA, the patient may feel a pulsing sensation in the abdomen or may experience no symptoms at all. Some patients report vague complaints, such as back, flank, groin, or abdominal pain. Syncope may be the chief complaint as the aneurysm expands, so it is important for primary care providers to be alert to progressive symptoms, including this signal that an aneurysm may exist and may be expanding.6
Pain may also be abrupt and severe in the lower abdomen and back, including tenderness in the area over the aneurysm. Shock can develop rapidly and symptoms such as cyanosis, mottling, altered mental status, tachycardia, and hypotension may be present.1,4
Since symptoms may be vague, the differential diagnosis can be broad (see Table 14,7,8), necessitating a detailed patient history and a careful physical examination. In an elderly patient, low back pain should be evaluated for AAA.9 In addition, acute abdominal pain in a patient older than 50 should be presumed to be a ruptured AAA.8
Risk Factors
A clinician should be familiar with the risk factors for AAA so that diagnosis can be made before a rupture occurs. Male gender and age greater than 65 are important risk factors for AAA, but one of the most important environmental risks is cigarette smoking.9,10 Current smokers are more than seven times more likely than nonsmokers to have an aneurysm.10 Atherosclerosis, which weakens the wall of the aorta, is also believed to contribute to the risk for AAA.11
Other contributing factors include hypertension, chronic obstructive pulmonary disease, hyperlipidemia, and family history. Chronic infection, inflammatory illnesses, and connective tissue disorders (eg, Marfan syndrome) can also increase the risk for aneurysm. Less frequent causes of AAA are trauma and infectious diseases, such as syphilis.1,12
In 85% of patients with femoral aneurysms, AAA has been found to coexist, as it has in 62% of patients with popliteal aneurysms. Patients previously diagnosed with these conditions should be screened for AAA.4,13,14
Diagnosis
An abdominal bruit or a pulsating mass may be found on palpation, but the sensitivity for detection of AAA is related to its size. An aneurysm greater than 5.0 cm has an 82% chance of detection by palpation.15 To assess for the presence of an abdominal aneurysm, the examiner should press the midline between the xiphoid and umbilicus bimanually, firmly but gently.12 There is no evidence to suggest that palpating the abdomen can cause an aneurysm to rupture.
The most useful tests for diagnosis of AAA are US, CT, and MRI.6 US is the simplest and least costly of these diagnostic procedures; it is noninvasive and has a sensitivity of 95% and specificity of nearly 100%. Bedside US can provide a rapid diagnosis in an unstable patient.16
CT is nearly 100% effective in diagnosing AAA and is usually used to help decide on appropriate treatment, as it can determine the size and shape of the aneurysm.17 However, CT should not be used for unstable patients.
MRI is useful in diagnosing AAA, but it is expensive, and inappropriate for unstable patients. Currently, conventional aortography is rarely used for preoperative assessment but may still be used for placement of endovascular devices or in patients with renal complications.1,12
Screening Recommendations
The US Preventive Services Task Force (USPSTF) recommends that all men ages 65 to 74 who have a lifelong history of smoking at least 100 cigarettes should be screened for AAA with abdominal US.3,18 Screening is not recommended for those younger than 65 who have never smoked, but this decision must be individualized to the patient, with other risk factors considered.
The ACC/AHA4 advises that men whose parents or siblings have a history of AAA and who are older than 60 should undergo physical examination and screening US for AAA. In addition, patients with a small AAA should receive US surveillance until the aneurysm reaches 5.5 cm in diameter; survival has not been shown to improve if an AAA is repaired before it reaches this size.1,2,19 In consideration of increased comorbidities and decreased life expectancy, screening is not recommended for men older than 75, but this too should be determined individually.3
Screening for women is not recommended by the USPSTF.3,18 The document states that the prevalence of large AAAs in women is low and that screening may lead to an increased number of unnecessary surgeries with associated morbidity and mortality. Clinical judgment must be used in making this decision, however, as several studies have shown that women have an AAA rupture rate that is three times higher than that in men; they also have an increased in-hospital mortality rate when rupture does occur. Thus, women are less likely to experience AAA but have a worse prognosis when AAA does develop.20-22
Management
The size of an AAA is the most important predictor of rupture. According to the ACC/AHA,4 the associated risk for rupture is about 20% for aneurysms that measure 5.0 cm in diameter, 40% for those measuring at least 6.0 cm, and at least 50% for aneurysms exceeding 7.0 cm.4,23,24 Regarding surveillance of known aneurysms, it is recommended that a patient with an aneurysm smaller than 3.0 cm in diameter requires no further testing. If an AAA measures 3.0 to 4.0 cm, US should be performed yearly; if it is 4.0 to 4.9 cm, US should be performed every six months.4,25
If an identified AAA is larger than 4.5 cm, or if any segment of the aorta is more than 1.5 times the diameter of an adjacent section, referral to a vascular surgeon for further evaluation is indicated. The vascular surgeon should be consulted immediately regarding a symptomatic patient with an AAA, or one with an aneurysm that measures 5.5 cm or larger, as the risk for rupture is high.4,26
Preventing rupture of an AAA is the primary aim in management. Beta-blockers may be used to reduce systolic hypertension in cardiac patients, thus slowing the rate of expansion in those with aortic aneurysms. Patients with a known AAA should undergo frequent monitoring for blood pressure and lipid levels and be advised to stop smoking. Smoking cessation interventions such as behavior modification, nicotine replacement, or bupropion should be offered.27,28
There is evidence that statin use may reduce the size of aneurysms, even in patients without hypercholesterolemia, possibly due to statins’ anti-inflammatory properties.22,29 ACE inhibitors may also be beneficial in reducing AAA growth and in lowering blood pressure. Antiplatelet medications are important in general cardiovascular risk reduction in the patient with AAA. Aspirin is the drug of choice.27,29
Surgical Repair
AAAs are usually repaired by one of two types of surgery: endovascular repair (EVR) or open surgery. Open surgical repair, the more traditional method, involves an incision into the abdomen from the breastbone to below the navel. The weakened area is replaced with a graft made of synthetic material. Open repair of an intact AAA, performed under general anesthesia, takes from three to six hours, and the patient must be hospitalized for five to eight days.30
In EVR, the patient is given epidural anesthesia and an incision is made in the right groin, allowing a synthetic stent graft to be threaded by way of a catheter through the femoral artery to repair the lesion (see Figure 2). EVR generally takes two to five hours, followed by a two- to five-day hospital stay. EVR is usually recommended for patients who are at high risk for complications from open operations because of severe cardiopulmonary disease or other risk factors, such as advanced age, morbid obesity, or a history of multiple abdominal operations.1,2,4,19
Prognosis
Patients with a ruptured AAA have a survival rate of less than 50%, with most deaths occurring before surgical repair has been attempted.3,31 In patients with kidney failure resulting from AAA (whether ruptured or unruptured, an AAA can disrupt renal blood flow), the chance for survival is poor. By contrast, the risk for death during surgical graft repair of an AAA is only about 2% to 8%.1,12
In a systematic review, EVR was associated with a lower 30-day mortality rate compared with open surgical repair (1.6% vs 4.7%, respectively), but this reduction did not persist over two years’ follow-up; neither did EVR improve overall survival or quality of life, compared with open surgery.1 Additionally, EVR requires periodic imaging throughout the patient’s life, which is associated with more reinterventions.1,19
Patient Education
Clinicians should encourage all patients to stop smoking, follow a low-cholesterol diet, control hypertension, and exercise regularly to lower the risk for AAAs. Screening recommendations should be explained to patients at risk, as should the signs and symptoms of an aneurysm. These patients should be instructed to call their health care provider immediately if they suspect a problem.
Conclusion
The incidence of AAA is increasing, and primary care providers must be prepared to act promptly in any case of suspected AAA to ensure a safe outcome. For aneurysms measuring greater than 5.5 cm in diameter, open or endovascular surgical repair should be considered. Patients with smaller aneurysms or contraindications for surgery should receive careful medical management and education to reduce the risks of AAA expansion leading to possible rupture.
1. Wilt TJ, Lederle FA, MacDonald R, et al; Agency for Healthcare Research and Quality. Comparison of Endovascular and Open Surgical Repairs for Abdominal Aortic Aneurysm. Rockville, MD: Agency for Healthcare Research and Quality; 2006. AHRQ publication 06-E107. Evidence Report/Technology Assessment 144. www.ahrq.gov/CLINIC/tp/aaareptp.htm. Accessed June 23, 2009.
2. Birkmeyer JD, Upchurch GR Jr. Evidence-based screening and management of abdominal aortic aneurysm. Ann Intern Med. 2007;146(10):749-750.
3. Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med. 2005;142(3):203-211.
4. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47(6):1239-1312.
5. Kiell CS, Ernst CB. Advances in management of abdominal aortic aneurysm. Adv Surg. 1993;26:73–98.
6. O’Connor RE. Aneurysm, abdominal. http://emedicine.medscape.com/article/756735-overview. Accessed June 23, 2009.
7. Lederle FA, Parenti CM, Chute EP. Ruptured abdominal aortic aneurysm: the internist as diagnostician. Am J Med. 1994;96:163-167.
8. Cartwright SL, Knudson MP. Evaluation of acute abdominal pain in adults. Am Fam Physician. 2008;77(7): 971-978.
9. Lyon C, Clark DC. Diagnosis of acute abdominal pain in older patients. Am Fam Physician. 2006;74(9):1537-1544.
10. Wilmink TB, Quick CR, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg. 1999;30(6):1099-1105.
11. Palazzuoli P, Gallotta M, Guerrieri G, et al. Prevalence of risk factors, coronary and systemic atherosclerosis in abdominal aortic aneurysm: comparison with high cardiovascular risk population. Vasc Health Risk Manag. 2008;4(4):877-883.
12. Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365(9470):1577-1589.
13. Graham LM, Zelenock GB, Whitehouse WM Jr, et al. Clinical significance of arteriosclerotic femoral artery aneurysms. Arch Surg. 1980;115(4):502–507.
14. Whitehouse WM Jr, Wakefield TW, Graham LM, et al. Limb-threatening potential of arteriosclerotic popliteal artery aneurysms. Surgery. 1983;93(5):694–699.
15. Fink HA, Lederle FA, Roth CS, et al. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med. 2000;160:833-836.
16. Bentz S, Jones J. Accuracy of emergency department ultrasound scanning in detecting abdominal aortic aneurysm. Emerg Med J. 2006;23(10):803-804.
17. Kvilekval KH, Best IM, Mason RA, et al. The value of computed tomography in the management of symptomatic abdominal aortic aneurysm. J Vasc Surg. 1990;12(1):28-33.
18. US Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med. 2005;142(3):198-202.
19. Lederle FA, Kane RL, MacDonald R, Wilt TJ. Systematic review: repair of unruptured abdominal aortic aneurysm. Ann Intern Med. 2007;146(10):735-741.
20. McPhee JT, Hill JS, Elami MH. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001-2004. J Vasc Surg. 2007;45(5):891-899.
21. Mofidi R, Goldie VJ, Kelman J, et al. Influence of sex on expansion rate of abdominal aortic aneurysms. Br J Surg. 2007;94(3):310-314.
22. Norman PE, Powell JT. Abdominal aortic aneurysm: the prognosis in women is worse than in men. Circulation. 2007;115(22):2865-2869.
23. Englund R, Hudson P, Hanel K, Stanton A. Expansion rates of small abdominal aortic aneurysms. Aust N Z J Surg. 1998;68(1):21–24.
24. Conway KP, Byrne J, Townsend M, Lane IF. Prognosis of patients turned down for conventional abdominal aortic aneurysm repair in the endovascular and sonographic era: Szilagyi revisited? J Vasc Surg. 2001;33(4):752–757.
25. Cook TA, Galland RB. A prospective study to define the optimum rescreening interval for small abdominal aortic aneurysm. Cardiovasc Surg. 1996;4(4):441–444.
26. Kent KC, Zwolak RM, Jaff MR, et al; Society for Vascular Surgery; American Association of Vascular Surgery; Society for Vascular Medicine and Biology. Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg. 2004;39(1):267-269.
27. Golledge J, Powell JT. Medical management of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2007;4(3):267-273.
28. Sule S, Aronow WS. Management of abdominal aortic aneurysms. Compr Ther. 2009;35(1):3-8.
29. Powell JT. Non-operative or medical management of abdominal aortic aneurysm. Scand J Surg. 2008;97(2): 121-124.
30. Huber TS, Wang JG, Derrow AE, et al. Experience in the United States with intact abdominal aortic aneurysm repair. J Vasc Surg. 2001;33(2):304-310.
31. Adam DJ, Mohan IV, Stuart WP, et al. Community and hospital outcome from ruptured abdominal aortic aneurysm within the catchment area of a regional vascular surgical service. J Vasc Surg. 1999;30(5):922-928.
A 60-year-old white man with a history of hyperlipidemia, hypertension, and anxiety presented with complaints of abdominal pain, localized to an area left of the umbilicus. He described the pain as constant and rated it 6 on a scale of 1 to 10. He said the pain had been present for longer than three weeks.
The man said he had been seen by another health care provider shortly after the pain began, but he did not think the provider took his complaint seriously. At that visit, antacids were prescribed, blood work was ordered, and the man was told to return if there was no improvement. He felt that because he was being treated for anxiety, the provider believed he was just imagining the pain.
At the current visit, the review of systems revealed additional complaints of shakiness and nausea without vomiting, with other findings unremarkable. The persistent pain did not seem related to eating, and the patient had no history of any surgeries that might help explain his current complaints. He had smoked a pack of cigarettes daily for 40 years and had a history of heavy alcohol use, although he denied having consumed any alcohol during the previous five years.
His prescribed medications included gemfibrozil 600 mg per day, hydrochlorothiazide 25 mg each morning, and diazepam 5 mg twice daily, with an OTC antacid.
The patient’s recent laboratory results were normal; they included a complete blood count, comprehensive metabolic panel, liver enzyme levels, and a serum amylase level. The patient weighed 280 lb and his height was 5’10”; his BMI was 40. His temperature was 97.7°F, with a regular heart rate of 88 beats/min; blood pressure, 140/90 mm Hg; and respiratory rate, 18 breaths/min.
The patient did not appear to be in acute distress. A bruit was heard in the indicated area of pain. No mass was palpated, and the width of his aorta could not be determined because of his obesity. His physical exam was otherwise normal.
Abdominal ultrasonography (US) revealed a 5.5-cm abdominal aortic aneurysm (AAA), and the man was referred for immediate surgery. The aneurysm was repaired in an open abdominal procedure with a polyester prosthetic graft. The surgery was successful.
Discussion
AAA is a permanent bulging area of the aorta that exceeds 3.0 cm in diameter (see Figure 1). It is a potentially life-threatening condition due to the possibility of rupture. Often an aneurysm is asymptomatic until it ruptures, making this a difficult illness to diagnose.1
Each year, an estimated 10,000 deaths result from a ruptured AAA, making this condition the 14th leading cause of death in the United States.2,3 Incidence of AAA appears to have increased over the past two decades. Causes for this may include the aging of the US population, an increase in the number of smokers, and a trend toward diets that are higher in fat.
Prognosis among patients with AAA can be improved with increased awareness of the disease among health care providers, earlier detection of AAAs at risk for rupture, and timely, effective interventions.
Symptomatology
In about one-third of patients with a ruptured AAA, a clinical triad of symptoms is present: abdominal and/or back pain, a pulsatile abdominal mass, and hypotension.4,5 In these cases, according to the American College of Cardiology/American Heart Association (ACC/AHA),4 immediate surgical evaluation is indicated.
Prior to the rupture of an AAA, the patient may feel a pulsing sensation in the abdomen or may experience no symptoms at all. Some patients report vague complaints, such as back, flank, groin, or abdominal pain. Syncope may be the chief complaint as the aneurysm expands, so it is important for primary care providers to be alert to progressive symptoms, including this signal that an aneurysm may exist and may be expanding.6
Pain may also be abrupt and severe in the lower abdomen and back, including tenderness in the area over the aneurysm. Shock can develop rapidly and symptoms such as cyanosis, mottling, altered mental status, tachycardia, and hypotension may be present.1,4
Since symptoms may be vague, the differential diagnosis can be broad (see Table 14,7,8), necessitating a detailed patient history and a careful physical examination. In an elderly patient, low back pain should be evaluated for AAA.9 In addition, acute abdominal pain in a patient older than 50 should be presumed to be a ruptured AAA.8
Risk Factors
A clinician should be familiar with the risk factors for AAA so that diagnosis can be made before a rupture occurs. Male gender and age greater than 65 are important risk factors for AAA, but one of the most important environmental risks is cigarette smoking.9,10 Current smokers are more than seven times more likely than nonsmokers to have an aneurysm.10 Atherosclerosis, which weakens the wall of the aorta, is also believed to contribute to the risk for AAA.11
Other contributing factors include hypertension, chronic obstructive pulmonary disease, hyperlipidemia, and family history. Chronic infection, inflammatory illnesses, and connective tissue disorders (eg, Marfan syndrome) can also increase the risk for aneurysm. Less frequent causes of AAA are trauma and infectious diseases, such as syphilis.1,12
In 85% of patients with femoral aneurysms, AAA has been found to coexist, as it has in 62% of patients with popliteal aneurysms. Patients previously diagnosed with these conditions should be screened for AAA.4,13,14
Diagnosis
An abdominal bruit or a pulsating mass may be found on palpation, but the sensitivity for detection of AAA is related to its size. An aneurysm greater than 5.0 cm has an 82% chance of detection by palpation.15 To assess for the presence of an abdominal aneurysm, the examiner should press the midline between the xiphoid and umbilicus bimanually, firmly but gently.12 There is no evidence to suggest that palpating the abdomen can cause an aneurysm to rupture.
The most useful tests for diagnosis of AAA are US, CT, and MRI.6 US is the simplest and least costly of these diagnostic procedures; it is noninvasive and has a sensitivity of 95% and specificity of nearly 100%. Bedside US can provide a rapid diagnosis in an unstable patient.16
CT is nearly 100% effective in diagnosing AAA and is usually used to help decide on appropriate treatment, as it can determine the size and shape of the aneurysm.17 However, CT should not be used for unstable patients.
MRI is useful in diagnosing AAA, but it is expensive, and inappropriate for unstable patients. Currently, conventional aortography is rarely used for preoperative assessment but may still be used for placement of endovascular devices or in patients with renal complications.1,12
Screening Recommendations
The US Preventive Services Task Force (USPSTF) recommends that all men ages 65 to 74 who have a lifelong history of smoking at least 100 cigarettes should be screened for AAA with abdominal US.3,18 Screening is not recommended for those younger than 65 who have never smoked, but this decision must be individualized to the patient, with other risk factors considered.
The ACC/AHA4 advises that men whose parents or siblings have a history of AAA and who are older than 60 should undergo physical examination and screening US for AAA. In addition, patients with a small AAA should receive US surveillance until the aneurysm reaches 5.5 cm in diameter; survival has not been shown to improve if an AAA is repaired before it reaches this size.1,2,19 In consideration of increased comorbidities and decreased life expectancy, screening is not recommended for men older than 75, but this too should be determined individually.3
Screening for women is not recommended by the USPSTF.3,18 The document states that the prevalence of large AAAs in women is low and that screening may lead to an increased number of unnecessary surgeries with associated morbidity and mortality. Clinical judgment must be used in making this decision, however, as several studies have shown that women have an AAA rupture rate that is three times higher than that in men; they also have an increased in-hospital mortality rate when rupture does occur. Thus, women are less likely to experience AAA but have a worse prognosis when AAA does develop.20-22
Management
The size of an AAA is the most important predictor of rupture. According to the ACC/AHA,4 the associated risk for rupture is about 20% for aneurysms that measure 5.0 cm in diameter, 40% for those measuring at least 6.0 cm, and at least 50% for aneurysms exceeding 7.0 cm.4,23,24 Regarding surveillance of known aneurysms, it is recommended that a patient with an aneurysm smaller than 3.0 cm in diameter requires no further testing. If an AAA measures 3.0 to 4.0 cm, US should be performed yearly; if it is 4.0 to 4.9 cm, US should be performed every six months.4,25
If an identified AAA is larger than 4.5 cm, or if any segment of the aorta is more than 1.5 times the diameter of an adjacent section, referral to a vascular surgeon for further evaluation is indicated. The vascular surgeon should be consulted immediately regarding a symptomatic patient with an AAA, or one with an aneurysm that measures 5.5 cm or larger, as the risk for rupture is high.4,26
Preventing rupture of an AAA is the primary aim in management. Beta-blockers may be used to reduce systolic hypertension in cardiac patients, thus slowing the rate of expansion in those with aortic aneurysms. Patients with a known AAA should undergo frequent monitoring for blood pressure and lipid levels and be advised to stop smoking. Smoking cessation interventions such as behavior modification, nicotine replacement, or bupropion should be offered.27,28
There is evidence that statin use may reduce the size of aneurysms, even in patients without hypercholesterolemia, possibly due to statins’ anti-inflammatory properties.22,29 ACE inhibitors may also be beneficial in reducing AAA growth and in lowering blood pressure. Antiplatelet medications are important in general cardiovascular risk reduction in the patient with AAA. Aspirin is the drug of choice.27,29
Surgical Repair
AAAs are usually repaired by one of two types of surgery: endovascular repair (EVR) or open surgery. Open surgical repair, the more traditional method, involves an incision into the abdomen from the breastbone to below the navel. The weakened area is replaced with a graft made of synthetic material. Open repair of an intact AAA, performed under general anesthesia, takes from three to six hours, and the patient must be hospitalized for five to eight days.30
In EVR, the patient is given epidural anesthesia and an incision is made in the right groin, allowing a synthetic stent graft to be threaded by way of a catheter through the femoral artery to repair the lesion (see Figure 2). EVR generally takes two to five hours, followed by a two- to five-day hospital stay. EVR is usually recommended for patients who are at high risk for complications from open operations because of severe cardiopulmonary disease or other risk factors, such as advanced age, morbid obesity, or a history of multiple abdominal operations.1,2,4,19
Prognosis
Patients with a ruptured AAA have a survival rate of less than 50%, with most deaths occurring before surgical repair has been attempted.3,31 In patients with kidney failure resulting from AAA (whether ruptured or unruptured, an AAA can disrupt renal blood flow), the chance for survival is poor. By contrast, the risk for death during surgical graft repair of an AAA is only about 2% to 8%.1,12
In a systematic review, EVR was associated with a lower 30-day mortality rate compared with open surgical repair (1.6% vs 4.7%, respectively), but this reduction did not persist over two years’ follow-up; neither did EVR improve overall survival or quality of life, compared with open surgery.1 Additionally, EVR requires periodic imaging throughout the patient’s life, which is associated with more reinterventions.1,19
Patient Education
Clinicians should encourage all patients to stop smoking, follow a low-cholesterol diet, control hypertension, and exercise regularly to lower the risk for AAAs. Screening recommendations should be explained to patients at risk, as should the signs and symptoms of an aneurysm. These patients should be instructed to call their health care provider immediately if they suspect a problem.
Conclusion
The incidence of AAA is increasing, and primary care providers must be prepared to act promptly in any case of suspected AAA to ensure a safe outcome. For aneurysms measuring greater than 5.5 cm in diameter, open or endovascular surgical repair should be considered. Patients with smaller aneurysms or contraindications for surgery should receive careful medical management and education to reduce the risks of AAA expansion leading to possible rupture.
A 60-year-old white man with a history of hyperlipidemia, hypertension, and anxiety presented with complaints of abdominal pain, localized to an area left of the umbilicus. He described the pain as constant and rated it 6 on a scale of 1 to 10. He said the pain had been present for longer than three weeks.
The man said he had been seen by another health care provider shortly after the pain began, but he did not think the provider took his complaint seriously. At that visit, antacids were prescribed, blood work was ordered, and the man was told to return if there was no improvement. He felt that because he was being treated for anxiety, the provider believed he was just imagining the pain.
At the current visit, the review of systems revealed additional complaints of shakiness and nausea without vomiting, with other findings unremarkable. The persistent pain did not seem related to eating, and the patient had no history of any surgeries that might help explain his current complaints. He had smoked a pack of cigarettes daily for 40 years and had a history of heavy alcohol use, although he denied having consumed any alcohol during the previous five years.
His prescribed medications included gemfibrozil 600 mg per day, hydrochlorothiazide 25 mg each morning, and diazepam 5 mg twice daily, with an OTC antacid.
The patient’s recent laboratory results were normal; they included a complete blood count, comprehensive metabolic panel, liver enzyme levels, and a serum amylase level. The patient weighed 280 lb and his height was 5’10”; his BMI was 40. His temperature was 97.7°F, with a regular heart rate of 88 beats/min; blood pressure, 140/90 mm Hg; and respiratory rate, 18 breaths/min.
The patient did not appear to be in acute distress. A bruit was heard in the indicated area of pain. No mass was palpated, and the width of his aorta could not be determined because of his obesity. His physical exam was otherwise normal.
Abdominal ultrasonography (US) revealed a 5.5-cm abdominal aortic aneurysm (AAA), and the man was referred for immediate surgery. The aneurysm was repaired in an open abdominal procedure with a polyester prosthetic graft. The surgery was successful.
Discussion
AAA is a permanent bulging area of the aorta that exceeds 3.0 cm in diameter (see Figure 1). It is a potentially life-threatening condition due to the possibility of rupture. Often an aneurysm is asymptomatic until it ruptures, making this a difficult illness to diagnose.1
Each year, an estimated 10,000 deaths result from a ruptured AAA, making this condition the 14th leading cause of death in the United States.2,3 Incidence of AAA appears to have increased over the past two decades. Causes for this may include the aging of the US population, an increase in the number of smokers, and a trend toward diets that are higher in fat.
Prognosis among patients with AAA can be improved with increased awareness of the disease among health care providers, earlier detection of AAAs at risk for rupture, and timely, effective interventions.
Symptomatology
In about one-third of patients with a ruptured AAA, a clinical triad of symptoms is present: abdominal and/or back pain, a pulsatile abdominal mass, and hypotension.4,5 In these cases, according to the American College of Cardiology/American Heart Association (ACC/AHA),4 immediate surgical evaluation is indicated.
Prior to the rupture of an AAA, the patient may feel a pulsing sensation in the abdomen or may experience no symptoms at all. Some patients report vague complaints, such as back, flank, groin, or abdominal pain. Syncope may be the chief complaint as the aneurysm expands, so it is important for primary care providers to be alert to progressive symptoms, including this signal that an aneurysm may exist and may be expanding.6
Pain may also be abrupt and severe in the lower abdomen and back, including tenderness in the area over the aneurysm. Shock can develop rapidly and symptoms such as cyanosis, mottling, altered mental status, tachycardia, and hypotension may be present.1,4
Since symptoms may be vague, the differential diagnosis can be broad (see Table 14,7,8), necessitating a detailed patient history and a careful physical examination. In an elderly patient, low back pain should be evaluated for AAA.9 In addition, acute abdominal pain in a patient older than 50 should be presumed to be a ruptured AAA.8
Risk Factors
A clinician should be familiar with the risk factors for AAA so that diagnosis can be made before a rupture occurs. Male gender and age greater than 65 are important risk factors for AAA, but one of the most important environmental risks is cigarette smoking.9,10 Current smokers are more than seven times more likely than nonsmokers to have an aneurysm.10 Atherosclerosis, which weakens the wall of the aorta, is also believed to contribute to the risk for AAA.11
Other contributing factors include hypertension, chronic obstructive pulmonary disease, hyperlipidemia, and family history. Chronic infection, inflammatory illnesses, and connective tissue disorders (eg, Marfan syndrome) can also increase the risk for aneurysm. Less frequent causes of AAA are trauma and infectious diseases, such as syphilis.1,12
In 85% of patients with femoral aneurysms, AAA has been found to coexist, as it has in 62% of patients with popliteal aneurysms. Patients previously diagnosed with these conditions should be screened for AAA.4,13,14
Diagnosis
An abdominal bruit or a pulsating mass may be found on palpation, but the sensitivity for detection of AAA is related to its size. An aneurysm greater than 5.0 cm has an 82% chance of detection by palpation.15 To assess for the presence of an abdominal aneurysm, the examiner should press the midline between the xiphoid and umbilicus bimanually, firmly but gently.12 There is no evidence to suggest that palpating the abdomen can cause an aneurysm to rupture.
The most useful tests for diagnosis of AAA are US, CT, and MRI.6 US is the simplest and least costly of these diagnostic procedures; it is noninvasive and has a sensitivity of 95% and specificity of nearly 100%. Bedside US can provide a rapid diagnosis in an unstable patient.16
CT is nearly 100% effective in diagnosing AAA and is usually used to help decide on appropriate treatment, as it can determine the size and shape of the aneurysm.17 However, CT should not be used for unstable patients.
MRI is useful in diagnosing AAA, but it is expensive, and inappropriate for unstable patients. Currently, conventional aortography is rarely used for preoperative assessment but may still be used for placement of endovascular devices or in patients with renal complications.1,12
Screening Recommendations
The US Preventive Services Task Force (USPSTF) recommends that all men ages 65 to 74 who have a lifelong history of smoking at least 100 cigarettes should be screened for AAA with abdominal US.3,18 Screening is not recommended for those younger than 65 who have never smoked, but this decision must be individualized to the patient, with other risk factors considered.
The ACC/AHA4 advises that men whose parents or siblings have a history of AAA and who are older than 60 should undergo physical examination and screening US for AAA. In addition, patients with a small AAA should receive US surveillance until the aneurysm reaches 5.5 cm in diameter; survival has not been shown to improve if an AAA is repaired before it reaches this size.1,2,19 In consideration of increased comorbidities and decreased life expectancy, screening is not recommended for men older than 75, but this too should be determined individually.3
Screening for women is not recommended by the USPSTF.3,18 The document states that the prevalence of large AAAs in women is low and that screening may lead to an increased number of unnecessary surgeries with associated morbidity and mortality. Clinical judgment must be used in making this decision, however, as several studies have shown that women have an AAA rupture rate that is three times higher than that in men; they also have an increased in-hospital mortality rate when rupture does occur. Thus, women are less likely to experience AAA but have a worse prognosis when AAA does develop.20-22
Management
The size of an AAA is the most important predictor of rupture. According to the ACC/AHA,4 the associated risk for rupture is about 20% for aneurysms that measure 5.0 cm in diameter, 40% for those measuring at least 6.0 cm, and at least 50% for aneurysms exceeding 7.0 cm.4,23,24 Regarding surveillance of known aneurysms, it is recommended that a patient with an aneurysm smaller than 3.0 cm in diameter requires no further testing. If an AAA measures 3.0 to 4.0 cm, US should be performed yearly; if it is 4.0 to 4.9 cm, US should be performed every six months.4,25
If an identified AAA is larger than 4.5 cm, or if any segment of the aorta is more than 1.5 times the diameter of an adjacent section, referral to a vascular surgeon for further evaluation is indicated. The vascular surgeon should be consulted immediately regarding a symptomatic patient with an AAA, or one with an aneurysm that measures 5.5 cm or larger, as the risk for rupture is high.4,26
Preventing rupture of an AAA is the primary aim in management. Beta-blockers may be used to reduce systolic hypertension in cardiac patients, thus slowing the rate of expansion in those with aortic aneurysms. Patients with a known AAA should undergo frequent monitoring for blood pressure and lipid levels and be advised to stop smoking. Smoking cessation interventions such as behavior modification, nicotine replacement, or bupropion should be offered.27,28
There is evidence that statin use may reduce the size of aneurysms, even in patients without hypercholesterolemia, possibly due to statins’ anti-inflammatory properties.22,29 ACE inhibitors may also be beneficial in reducing AAA growth and in lowering blood pressure. Antiplatelet medications are important in general cardiovascular risk reduction in the patient with AAA. Aspirin is the drug of choice.27,29
Surgical Repair
AAAs are usually repaired by one of two types of surgery: endovascular repair (EVR) or open surgery. Open surgical repair, the more traditional method, involves an incision into the abdomen from the breastbone to below the navel. The weakened area is replaced with a graft made of synthetic material. Open repair of an intact AAA, performed under general anesthesia, takes from three to six hours, and the patient must be hospitalized for five to eight days.30
In EVR, the patient is given epidural anesthesia and an incision is made in the right groin, allowing a synthetic stent graft to be threaded by way of a catheter through the femoral artery to repair the lesion (see Figure 2). EVR generally takes two to five hours, followed by a two- to five-day hospital stay. EVR is usually recommended for patients who are at high risk for complications from open operations because of severe cardiopulmonary disease or other risk factors, such as advanced age, morbid obesity, or a history of multiple abdominal operations.1,2,4,19
Prognosis
Patients with a ruptured AAA have a survival rate of less than 50%, with most deaths occurring before surgical repair has been attempted.3,31 In patients with kidney failure resulting from AAA (whether ruptured or unruptured, an AAA can disrupt renal blood flow), the chance for survival is poor. By contrast, the risk for death during surgical graft repair of an AAA is only about 2% to 8%.1,12
In a systematic review, EVR was associated with a lower 30-day mortality rate compared with open surgical repair (1.6% vs 4.7%, respectively), but this reduction did not persist over two years’ follow-up; neither did EVR improve overall survival or quality of life, compared with open surgery.1 Additionally, EVR requires periodic imaging throughout the patient’s life, which is associated with more reinterventions.1,19
Patient Education
Clinicians should encourage all patients to stop smoking, follow a low-cholesterol diet, control hypertension, and exercise regularly to lower the risk for AAAs. Screening recommendations should be explained to patients at risk, as should the signs and symptoms of an aneurysm. These patients should be instructed to call their health care provider immediately if they suspect a problem.
Conclusion
The incidence of AAA is increasing, and primary care providers must be prepared to act promptly in any case of suspected AAA to ensure a safe outcome. For aneurysms measuring greater than 5.5 cm in diameter, open or endovascular surgical repair should be considered. Patients with smaller aneurysms or contraindications for surgery should receive careful medical management and education to reduce the risks of AAA expansion leading to possible rupture.
1. Wilt TJ, Lederle FA, MacDonald R, et al; Agency for Healthcare Research and Quality. Comparison of Endovascular and Open Surgical Repairs for Abdominal Aortic Aneurysm. Rockville, MD: Agency for Healthcare Research and Quality; 2006. AHRQ publication 06-E107. Evidence Report/Technology Assessment 144. www.ahrq.gov/CLINIC/tp/aaareptp.htm. Accessed June 23, 2009.
2. Birkmeyer JD, Upchurch GR Jr. Evidence-based screening and management of abdominal aortic aneurysm. Ann Intern Med. 2007;146(10):749-750.
3. Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med. 2005;142(3):203-211.
4. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47(6):1239-1312.
5. Kiell CS, Ernst CB. Advances in management of abdominal aortic aneurysm. Adv Surg. 1993;26:73–98.
6. O’Connor RE. Aneurysm, abdominal. http://emedicine.medscape.com/article/756735-overview. Accessed June 23, 2009.
7. Lederle FA, Parenti CM, Chute EP. Ruptured abdominal aortic aneurysm: the internist as diagnostician. Am J Med. 1994;96:163-167.
8. Cartwright SL, Knudson MP. Evaluation of acute abdominal pain in adults. Am Fam Physician. 2008;77(7): 971-978.
9. Lyon C, Clark DC. Diagnosis of acute abdominal pain in older patients. Am Fam Physician. 2006;74(9):1537-1544.
10. Wilmink TB, Quick CR, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg. 1999;30(6):1099-1105.
11. Palazzuoli P, Gallotta M, Guerrieri G, et al. Prevalence of risk factors, coronary and systemic atherosclerosis in abdominal aortic aneurysm: comparison with high cardiovascular risk population. Vasc Health Risk Manag. 2008;4(4):877-883.
12. Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365(9470):1577-1589.
13. Graham LM, Zelenock GB, Whitehouse WM Jr, et al. Clinical significance of arteriosclerotic femoral artery aneurysms. Arch Surg. 1980;115(4):502–507.
14. Whitehouse WM Jr, Wakefield TW, Graham LM, et al. Limb-threatening potential of arteriosclerotic popliteal artery aneurysms. Surgery. 1983;93(5):694–699.
15. Fink HA, Lederle FA, Roth CS, et al. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med. 2000;160:833-836.
16. Bentz S, Jones J. Accuracy of emergency department ultrasound scanning in detecting abdominal aortic aneurysm. Emerg Med J. 2006;23(10):803-804.
17. Kvilekval KH, Best IM, Mason RA, et al. The value of computed tomography in the management of symptomatic abdominal aortic aneurysm. J Vasc Surg. 1990;12(1):28-33.
18. US Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med. 2005;142(3):198-202.
19. Lederle FA, Kane RL, MacDonald R, Wilt TJ. Systematic review: repair of unruptured abdominal aortic aneurysm. Ann Intern Med. 2007;146(10):735-741.
20. McPhee JT, Hill JS, Elami MH. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001-2004. J Vasc Surg. 2007;45(5):891-899.
21. Mofidi R, Goldie VJ, Kelman J, et al. Influence of sex on expansion rate of abdominal aortic aneurysms. Br J Surg. 2007;94(3):310-314.
22. Norman PE, Powell JT. Abdominal aortic aneurysm: the prognosis in women is worse than in men. Circulation. 2007;115(22):2865-2869.
23. Englund R, Hudson P, Hanel K, Stanton A. Expansion rates of small abdominal aortic aneurysms. Aust N Z J Surg. 1998;68(1):21–24.
24. Conway KP, Byrne J, Townsend M, Lane IF. Prognosis of patients turned down for conventional abdominal aortic aneurysm repair in the endovascular and sonographic era: Szilagyi revisited? J Vasc Surg. 2001;33(4):752–757.
25. Cook TA, Galland RB. A prospective study to define the optimum rescreening interval for small abdominal aortic aneurysm. Cardiovasc Surg. 1996;4(4):441–444.
26. Kent KC, Zwolak RM, Jaff MR, et al; Society for Vascular Surgery; American Association of Vascular Surgery; Society for Vascular Medicine and Biology. Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg. 2004;39(1):267-269.
27. Golledge J, Powell JT. Medical management of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2007;4(3):267-273.
28. Sule S, Aronow WS. Management of abdominal aortic aneurysms. Compr Ther. 2009;35(1):3-8.
29. Powell JT. Non-operative or medical management of abdominal aortic aneurysm. Scand J Surg. 2008;97(2): 121-124.
30. Huber TS, Wang JG, Derrow AE, et al. Experience in the United States with intact abdominal aortic aneurysm repair. J Vasc Surg. 2001;33(2):304-310.
31. Adam DJ, Mohan IV, Stuart WP, et al. Community and hospital outcome from ruptured abdominal aortic aneurysm within the catchment area of a regional vascular surgical service. J Vasc Surg. 1999;30(5):922-928.
1. Wilt TJ, Lederle FA, MacDonald R, et al; Agency for Healthcare Research and Quality. Comparison of Endovascular and Open Surgical Repairs for Abdominal Aortic Aneurysm. Rockville, MD: Agency for Healthcare Research and Quality; 2006. AHRQ publication 06-E107. Evidence Report/Technology Assessment 144. www.ahrq.gov/CLINIC/tp/aaareptp.htm. Accessed June 23, 2009.
2. Birkmeyer JD, Upchurch GR Jr. Evidence-based screening and management of abdominal aortic aneurysm. Ann Intern Med. 2007;146(10):749-750.
3. Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med. 2005;142(3):203-211.
4. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47(6):1239-1312.
5. Kiell CS, Ernst CB. Advances in management of abdominal aortic aneurysm. Adv Surg. 1993;26:73–98.
6. O’Connor RE. Aneurysm, abdominal. http://emedicine.medscape.com/article/756735-overview. Accessed June 23, 2009.
7. Lederle FA, Parenti CM, Chute EP. Ruptured abdominal aortic aneurysm: the internist as diagnostician. Am J Med. 1994;96:163-167.
8. Cartwright SL, Knudson MP. Evaluation of acute abdominal pain in adults. Am Fam Physician. 2008;77(7): 971-978.
9. Lyon C, Clark DC. Diagnosis of acute abdominal pain in older patients. Am Fam Physician. 2006;74(9):1537-1544.
10. Wilmink TB, Quick CR, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg. 1999;30(6):1099-1105.
11. Palazzuoli P, Gallotta M, Guerrieri G, et al. Prevalence of risk factors, coronary and systemic atherosclerosis in abdominal aortic aneurysm: comparison with high cardiovascular risk population. Vasc Health Risk Manag. 2008;4(4):877-883.
12. Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365(9470):1577-1589.
13. Graham LM, Zelenock GB, Whitehouse WM Jr, et al. Clinical significance of arteriosclerotic femoral artery aneurysms. Arch Surg. 1980;115(4):502–507.
14. Whitehouse WM Jr, Wakefield TW, Graham LM, et al. Limb-threatening potential of arteriosclerotic popliteal artery aneurysms. Surgery. 1983;93(5):694–699.
15. Fink HA, Lederle FA, Roth CS, et al. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med. 2000;160:833-836.
16. Bentz S, Jones J. Accuracy of emergency department ultrasound scanning in detecting abdominal aortic aneurysm. Emerg Med J. 2006;23(10):803-804.
17. Kvilekval KH, Best IM, Mason RA, et al. The value of computed tomography in the management of symptomatic abdominal aortic aneurysm. J Vasc Surg. 1990;12(1):28-33.
18. US Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med. 2005;142(3):198-202.
19. Lederle FA, Kane RL, MacDonald R, Wilt TJ. Systematic review: repair of unruptured abdominal aortic aneurysm. Ann Intern Med. 2007;146(10):735-741.
20. McPhee JT, Hill JS, Elami MH. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001-2004. J Vasc Surg. 2007;45(5):891-899.
21. Mofidi R, Goldie VJ, Kelman J, et al. Influence of sex on expansion rate of abdominal aortic aneurysms. Br J Surg. 2007;94(3):310-314.
22. Norman PE, Powell JT. Abdominal aortic aneurysm: the prognosis in women is worse than in men. Circulation. 2007;115(22):2865-2869.
23. Englund R, Hudson P, Hanel K, Stanton A. Expansion rates of small abdominal aortic aneurysms. Aust N Z J Surg. 1998;68(1):21–24.
24. Conway KP, Byrne J, Townsend M, Lane IF. Prognosis of patients turned down for conventional abdominal aortic aneurysm repair in the endovascular and sonographic era: Szilagyi revisited? J Vasc Surg. 2001;33(4):752–757.
25. Cook TA, Galland RB. A prospective study to define the optimum rescreening interval for small abdominal aortic aneurysm. Cardiovasc Surg. 1996;4(4):441–444.
26. Kent KC, Zwolak RM, Jaff MR, et al; Society for Vascular Surgery; American Association of Vascular Surgery; Society for Vascular Medicine and Biology. Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg. 2004;39(1):267-269.
27. Golledge J, Powell JT. Medical management of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2007;4(3):267-273.
28. Sule S, Aronow WS. Management of abdominal aortic aneurysms. Compr Ther. 2009;35(1):3-8.
29. Powell JT. Non-operative or medical management of abdominal aortic aneurysm. Scand J Surg. 2008;97(2): 121-124.
30. Huber TS, Wang JG, Derrow AE, et al. Experience in the United States with intact abdominal aortic aneurysm repair. J Vasc Surg. 2001;33(2):304-310.
31. Adam DJ, Mohan IV, Stuart WP, et al. Community and hospital outcome from ruptured abdominal aortic aneurysm within the catchment area of a regional vascular surgical service. J Vasc Surg. 1999;30(5):922-928.
Grand Rounds: Boy, 10, With Knee Pain
A 10-year-old boy first complained of right knee pain two months prior to presentation. There was no traumatic event to explain the pain and no prior viral or bacterial illness. Radiographs taken earlier at another facility were initially pronounced normal. One month later, repeat x-rays showed a possible hairline fracture, and MRI was ordered. MRI documented a destructive lesion in the right distal femur with a soft-tissue mass that was worrisome for primary bone malignancy.
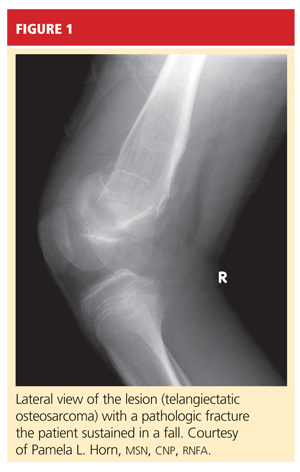
The boy was placed on weight-bearing restrictions and was given a wheelchair. Unfortunately, he fell from the wheelchair and sustained a pathologic fracture through the lesion (see Figure 1). He was transported to the hospital and admitted. A biopsy was performed with a closed reduction, as the fracture was maligned. The patient was placed in a long leg cast with a pelvic band.
His history was previously unremarkable. He was taking no medications and had experienced no recent illnesses. His surgical/medical history was positive for a tonsillectomy at an early age and a fracture of the right proximal femur at age 2. On examination, he was noted to be talkative with his family but guarded during conversations with staff.
His physical exam was positive for pain at the right distal femur and knee with palpation; otherwise, all other systems were unremarkable. The patient was in too much pain to range the knee and had been placed in a long posterior leg splint (prior to surgery and application of the cast). Distally, his right lower extremity motor and sensory function were intact.
The patient’s vital signs were within normal limits, and results from his blood chemistries and alkaline phosphatase and C-reactive protein levels were unremarkable. Findings on the complete blood cell count were slightly abnormal: Hemoglobin was 11 g and the hematocrit, 33% (both within normal limits); however, in the differential there was an elevation in segmented neutrophils (72%, compared with a reference range of 31% to 61%), with Döhle bodies present—possibly signifying acute and/or chronic systemic infection or malignancy. The lymphocyte count represented 11% of the total white blood cell count (range, 28% to 48%), and platelets were 82 x 103/mL (normal range, 150 to 350 x 103/mL). The patient’s erythrocyte sedimentation rate was 44 mm/h (normal range, 0 to 20).
Result from pathology were positive for osteosarcoma, telangiectatic type. The patient underwent a nuclear medicine bone scan that showed no metastases, and chest CT was negative for pulmonary lesions as well. After a psychology consult, the boy was gently told about his condition.
Treatment then proceeded, including surgical placement of a double-lumen chest catheter for delivery of neoadjuvant and adjuvant chemotherapy. Doxorubicin, cisplatin, and methotrexate were used because the boy was enrolled in an international cooperative trial through the Children’s Oncology Group for treatment of localized osteosarcoma.
Discussion
Osteosarcoma (OS) is the most common primary bone malignancy.1,2 Approximately 5% of all pediatric patients with tumors present with this diagnosis, and about 400 new cases are diagnosed in the United States each year.1 Most osteosarcomas develop in the bones of the lower extremities and in the humerus, affecting males more often than females.1-3 This kind of malignancy is frequently seen during the adolescent growth spurt, but it can affect patients of any age.1,2 Patients usually present with pain or functional limitation in gait or daily activities or both.1-3
The telangiectatic subtype of OS is a rare, aggressive variant that represents 2% to 12% of all cases of OS.4-6 Telangiectatic OS (TOS) is characterized by multiple aneurysmally dilated, blood-filled cavities with high-grade sarcomatous cells seen in the peripheral rim and septae.3,7,8 This process can cause the lesion to resemble an aneurysmal bone cyst, explaining why some cases of TOS are misdiagnosed—with delayed time to treatment and increased morbidity and mortality.3,5 Generally, TOS patients are more likely than other OS patients to have tumors of femoral location, larger lesions, and normal alkaline phosphatase values. Many have pathologic fractures on presentation.7
The medical literature chronicles a long debate regarding the difference in mortality between patients with OS and those with TOS. It was once believed that patients with TOS were at higher risk for recurrence (especially those with a pathologic fracture) and mortality. However, in recent studies examining newer neoadjuvant and adjuvant chemotherapies, mortality rates for the two conditions are similar and certainly lower than they were many years ago.7,8 In one study, a better histologic response was reported to neoadjuvant chemotherapy in patients with TOS than with OS.7
Diagnosis
The first diagnostic tool used for patients with suspected OS or TOS is a plain radiographic film. A TOS lesion is lytic, with no areas of sclerosis, and almost always involves the long bones. It is poorly defined, destroying the cortex with formation of periosteal bone and invading the soft tissue. An initial pattern of parallel striations is highly suggestive of TOS.5
MRI and CT often reveal thick nodular tissue in a largely hemorrhagic and/or necrotic osseous lesion, with an associated soft-tissue mass that allows distinction from an aneurysmal bone cyst.3 Next, patients generally undergo a nuclear medicine bone scan and CT of the chest to observe for signs of metastases. Chest CT is commonly repeated on a regular basis during and after treatment.9
Pathologic evaluation, the final step to diagnosis, is very important, especially in the effort to differentiate TOS from an aneurysmal bone cyst. The typical gross findings for a TOS tumor include a dominant cystic cavity–like architecture, with a pushing peripheral margin that frequently expands and erodes the adjacent cortex and extends into the surrounding tissue. There is usually no area of intramural bone tissue.
Microscopically, the cystic areas contain clots and fragments of tumor that are often lined with a layer of neoplasm. The blood-filled telangiectatic spaces form in these areas. The spaces are irregularly shaped and typically traversed by septae composed in part of neoplastic cells. Osteoid formation through these cells can appear as a fine, ice-like material between tumor cells.4,7
Treatment
The main goals of treatment are to limit the anatomical extent of the disease, decrease the possibility of recurrence, and restore the highest possible level of function.2 Initial treatment of any OS or TOS consists of aggressive, immediate chemotherapy prior to and after any surgical intervention.1 (Chemotherapy will not be discussed in further detail here.) Surgical treatments for patients younger than 14 include amputation (above the lesion with wide margins), an expanding prosthesis, or rotationplasty. The location and extent of the tumor, the patient’s age, and his or her desired lifestyle will all have an impact on the choice of surgery.10
Historic data demonstrate that patients who undergo amputation alone almost always develop metastatic disease.1 Other data show that only 10% of patients with OS have been cured by chemotherapy alone. Yet when medical treatment is combined with surgical treatment, the overall expected cure rate can be as high as 65%.2
Discussing amputation with a young patient and the family can be emotionally difficult. If functional levels are to be restored, above-knee amputation (AKA) is the least favored surgical method. Compared with healthy individuals, patients who undergo AKA will walk 43% less quickly and will expend much more energy. These patients frequently have an inefficient gait and, given their limited reserve, they may lose the ability to walk altogether.2
Reconstructive surgical options include limb-salvage procedures; since the late 1980s, these have become the standard of care for OS at all sites.11 One such option includes removal of the lesion (eg, a distal femoral or proximal tibial lesion) with acceptable margins and replacement of the lost bone with an allograft or with a metallic prosthesis and knee joint (called arthroplasty). This endoprosthesis expands as the child grows (by way of a minor surgical procedure or a magnetic spring) so there is no apparent discrepancy between limb lengths, and the patient’s appearance is as normal and socially acceptable as possible.1,2
Because the case patient developed a pathologic fracture through his TOS tumor, he was not a candidate for endoprosthesis. His options were AKA or rotationplasty.
This procedure was first described in 195012 for treatment of proximal focal femoral deficiency. It is considered an alternative for skeletally immature individuals for whom the goal is to preserve function.
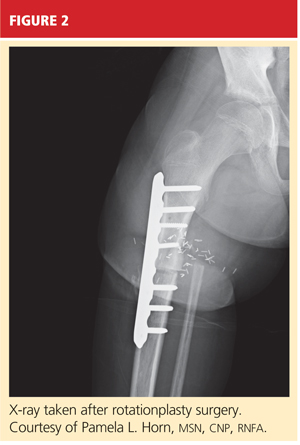
When AKA is indicated, the lower limb can be salvaged to allow functioning similar to that of a patient with a below-knee amputation (BKA). During rotationplasty, all but the most proximal aspect of the femur is resected. The tibia is externally rotated on the axis of the neurovascular bundle, then an arthrodesis of the proximal portion of the femur and the tibial plateau is performed (see Figure 2).
The end result is an extremity with the appearance, dimensions, and functional potential of a BKA. The ankle is rotated 180° so that it can serve as the new knee joint, and the attached foot, now pointing in the opposite direction, acts as the residual limb for fitting a prosthesis.2 This procedure is favored in patients with an extensive soft-tissue mass, intra-articular extension of the tumor, and/or pathologic fractures. It can also help prevent phantom pain.13
The Case Patient
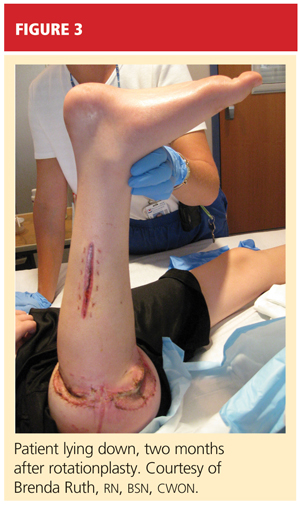
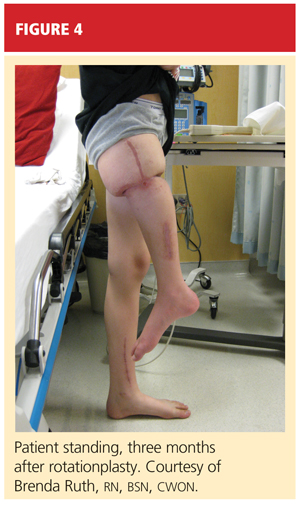
After psychological evaluation of the patient and extensive family discussion, he underwent successful rotationplasty. The day after his surgery, however, he developed compartment syndrome and was required to undergo fasciotomies of the calf and proximal thigh. His wounds were treated, a skin graft was performed to close the proximal thigh wound, and his calf wounds were sutured closed (see Figures 3 and 4). His hip range of motion is excellent, and his ankle range of motion continues to improve with physical therapy.
At this writing, the patient was scheduled for his first prosthetic fitting, and he had nearly completed his chemotherapy. His outlook is very promising.
Conclusion
TOS is a rare, aggressive subtype of OS but the most common primary malignant bone tumor of childhood. In the past, outcomes in patients treated with surgery alone were poor. With the advent of chemotherapy and the combination of medical and surgical treatment, TOS-associated mortality has continued to decline. There is no significant difference in outcomes among the available surgical options, but limb-salvage surgical procedures usually offer patients much better function and quality of life. The most important consideration is early diagnosis followed by immediate treatment.
1. Siegel HJ, Pressey JG. Current concepts on the surgical and medical management of osteosarcoma. Expert Rev Anticancer Ther. 2008;8(8):1257-1269.
2. Marulanda GA, Henderson ER, Johnson DA, et al. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15(1):13-20.
3. Murphey MD, wan Jaovisidha S, Temple HT, et al. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology. 2003;229(2):545-553.
4. Mervak TR, Unni KK, Pritchard DJ, McLeod RA. Telangiectatic osteosarcoma. Clin Orthop Relat Res. 1991 Sep;270:135-139.
5. Vanel D, Tcheng S, Contesso G, et al. The radiological appearances of telangiectatic osteosarcoma: a study of 14 cases. Skeletal Radiol. 1987;16(3):196-200.
6. Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23(34):8845-8852.
7. Bacci G, Ferrari S, Ruggieri P, et al. Telangiectatic osteosarcoma of the extremity: neoadjuvant chemotherapy in 24 cases. Acta Orthop Scand. 2001;72(2):167-172.
8. Weiss A, Khoury JD, Hoffer FA, et al. Telangiectatic osteosarcoma: the St. Jude Children’s Research Hospital’s experience. Cancer. 2007;109(8):1627-1637.
9. Agarwal M, Anchan C, Shah M, et al. Limb salvage surgery for osteosarcoma: effective low-cost treatment. Clin Orthop Relat Res. 2007;459:82-91.
10. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb: amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
11. Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68(9):1331-1337.
12. Van Nes CP. Rotation-plasty for congenital defects of the femur: making use of the shortened limb to control the knee joint of a prosthesis. J Bone Joint Surg. 1950;32B:12-16.
13. Sawamura C, Hornicek FJ, Gebhardt MC. Complications and risk factors for failure of rotationplasty: review of 25 patients. Clin Orthop Relat Res. 2008;466(6):1302-1308.
A 10-year-old boy first complained of right knee pain two months prior to presentation. There was no traumatic event to explain the pain and no prior viral or bacterial illness. Radiographs taken earlier at another facility were initially pronounced normal. One month later, repeat x-rays showed a possible hairline fracture, and MRI was ordered. MRI documented a destructive lesion in the right distal femur with a soft-tissue mass that was worrisome for primary bone malignancy.
The boy was placed on weight-bearing restrictions and was given a wheelchair. Unfortunately, he fell from the wheelchair and sustained a pathologic fracture through the lesion (see Figure 1). He was transported to the hospital and admitted. A biopsy was performed with a closed reduction, as the fracture was maligned. The patient was placed in a long leg cast with a pelvic band.

His history was previously unremarkable. He was taking no medications and had experienced no recent illnesses. His surgical/medical history was positive for a tonsillectomy at an early age and a fracture of the right proximal femur at age 2. On examination, he was noted to be talkative with his family but guarded during conversations with staff.
His physical exam was positive for pain at the right distal femur and knee with palpation; otherwise, all other systems were unremarkable. The patient was in too much pain to range the knee and had been placed in a long posterior leg splint (prior to surgery and application of the cast). Distally, his right lower extremity motor and sensory function were intact.
The patient’s vital signs were within normal limits, and results from his blood chemistries and alkaline phosphatase and C-reactive protein levels were unremarkable. Findings on the complete blood cell count were slightly abnormal: Hemoglobin was 11 g and the hematocrit, 33% (both within normal limits); however, in the differential there was an elevation in segmented neutrophils (72%, compared with a reference range of 31% to 61%), with Döhle bodies present—possibly signifying acute and/or chronic systemic infection or malignancy. The lymphocyte count represented 11% of the total white blood cell count (range, 28% to 48%), and platelets were 82 x 103/mL (normal range, 150 to 350 x 103/mL). The patient’s erythrocyte sedimentation rate was 44 mm/h (normal range, 0 to 20).
Result from pathology were positive for osteosarcoma, telangiectatic type. The patient underwent a nuclear medicine bone scan that showed no metastases, and chest CT was negative for pulmonary lesions as well. After a psychology consult, the boy was gently told about his condition.
Treatment then proceeded, including surgical placement of a double-lumen chest catheter for delivery of neoadjuvant and adjuvant chemotherapy. Doxorubicin, cisplatin, and methotrexate were used because the boy was enrolled in an international cooperative trial through the Children’s Oncology Group for treatment of localized osteosarcoma.
Discussion
Osteosarcoma (OS) is the most common primary bone malignancy.1,2 Approximately 5% of all pediatric patients with tumors present with this diagnosis, and about 400 new cases are diagnosed in the United States each year.1 Most osteosarcomas develop in the bones of the lower extremities and in the humerus, affecting males more often than females.1-3 This kind of malignancy is frequently seen during the adolescent growth spurt, but it can affect patients of any age.1,2 Patients usually present with pain or functional limitation in gait or daily activities or both.1-3
The telangiectatic subtype of OS is a rare, aggressive variant that represents 2% to 12% of all cases of OS.4-6 Telangiectatic OS (TOS) is characterized by multiple aneurysmally dilated, blood-filled cavities with high-grade sarcomatous cells seen in the peripheral rim and septae.3,7,8 This process can cause the lesion to resemble an aneurysmal bone cyst, explaining why some cases of TOS are misdiagnosed—with delayed time to treatment and increased morbidity and mortality.3,5 Generally, TOS patients are more likely than other OS patients to have tumors of femoral location, larger lesions, and normal alkaline phosphatase values. Many have pathologic fractures on presentation.7
The medical literature chronicles a long debate regarding the difference in mortality between patients with OS and those with TOS. It was once believed that patients with TOS were at higher risk for recurrence (especially those with a pathologic fracture) and mortality. However, in recent studies examining newer neoadjuvant and adjuvant chemotherapies, mortality rates for the two conditions are similar and certainly lower than they were many years ago.7,8 In one study, a better histologic response was reported to neoadjuvant chemotherapy in patients with TOS than with OS.7
Diagnosis
The first diagnostic tool used for patients with suspected OS or TOS is a plain radiographic film. A TOS lesion is lytic, with no areas of sclerosis, and almost always involves the long bones. It is poorly defined, destroying the cortex with formation of periosteal bone and invading the soft tissue. An initial pattern of parallel striations is highly suggestive of TOS.5
MRI and CT often reveal thick nodular tissue in a largely hemorrhagic and/or necrotic osseous lesion, with an associated soft-tissue mass that allows distinction from an aneurysmal bone cyst.3 Next, patients generally undergo a nuclear medicine bone scan and CT of the chest to observe for signs of metastases. Chest CT is commonly repeated on a regular basis during and after treatment.9
Pathologic evaluation, the final step to diagnosis, is very important, especially in the effort to differentiate TOS from an aneurysmal bone cyst. The typical gross findings for a TOS tumor include a dominant cystic cavity–like architecture, with a pushing peripheral margin that frequently expands and erodes the adjacent cortex and extends into the surrounding tissue. There is usually no area of intramural bone tissue.
Microscopically, the cystic areas contain clots and fragments of tumor that are often lined with a layer of neoplasm. The blood-filled telangiectatic spaces form in these areas. The spaces are irregularly shaped and typically traversed by septae composed in part of neoplastic cells. Osteoid formation through these cells can appear as a fine, ice-like material between tumor cells.4,7
Treatment
The main goals of treatment are to limit the anatomical extent of the disease, decrease the possibility of recurrence, and restore the highest possible level of function.2 Initial treatment of any OS or TOS consists of aggressive, immediate chemotherapy prior to and after any surgical intervention.1 (Chemotherapy will not be discussed in further detail here.) Surgical treatments for patients younger than 14 include amputation (above the lesion with wide margins), an expanding prosthesis, or rotationplasty. The location and extent of the tumor, the patient’s age, and his or her desired lifestyle will all have an impact on the choice of surgery.10
Historic data demonstrate that patients who undergo amputation alone almost always develop metastatic disease.1 Other data show that only 10% of patients with OS have been cured by chemotherapy alone. Yet when medical treatment is combined with surgical treatment, the overall expected cure rate can be as high as 65%.2
Discussing amputation with a young patient and the family can be emotionally difficult. If functional levels are to be restored, above-knee amputation (AKA) is the least favored surgical method. Compared with healthy individuals, patients who undergo AKA will walk 43% less quickly and will expend much more energy. These patients frequently have an inefficient gait and, given their limited reserve, they may lose the ability to walk altogether.2
Reconstructive surgical options include limb-salvage procedures; since the late 1980s, these have become the standard of care for OS at all sites.11 One such option includes removal of the lesion (eg, a distal femoral or proximal tibial lesion) with acceptable margins and replacement of the lost bone with an allograft or with a metallic prosthesis and knee joint (called arthroplasty). This endoprosthesis expands as the child grows (by way of a minor surgical procedure or a magnetic spring) so there is no apparent discrepancy between limb lengths, and the patient’s appearance is as normal and socially acceptable as possible.1,2
Because the case patient developed a pathologic fracture through his TOS tumor, he was not a candidate for endoprosthesis. His options were AKA or rotationplasty.
This procedure was first described in 195012 for treatment of proximal focal femoral deficiency. It is considered an alternative for skeletally immature individuals for whom the goal is to preserve function.
When AKA is indicated, the lower limb can be salvaged to allow functioning similar to that of a patient with a below-knee amputation (BKA). During rotationplasty, all but the most proximal aspect of the femur is resected. The tibia is externally rotated on the axis of the neurovascular bundle, then an arthrodesis of the proximal portion of the femur and the tibial plateau is performed (see Figure 2).

The end result is an extremity with the appearance, dimensions, and functional potential of a BKA. The ankle is rotated 180° so that it can serve as the new knee joint, and the attached foot, now pointing in the opposite direction, acts as the residual limb for fitting a prosthesis.2 This procedure is favored in patients with an extensive soft-tissue mass, intra-articular extension of the tumor, and/or pathologic fractures. It can also help prevent phantom pain.13

The Case Patient
After psychological evaluation of the patient and extensive family discussion, he underwent successful rotationplasty. The day after his surgery, however, he developed compartment syndrome and was required to undergo fasciotomies of the calf and proximal thigh. His wounds were treated, a skin graft was performed to close the proximal thigh wound, and his calf wounds were sutured closed (see Figures 3 and 4). His hip range of motion is excellent, and his ankle range of motion continues to improve with physical therapy.

At this writing, the patient was scheduled for his first prosthetic fitting, and he had nearly completed his chemotherapy. His outlook is very promising.
Conclusion
TOS is a rare, aggressive subtype of OS but the most common primary malignant bone tumor of childhood. In the past, outcomes in patients treated with surgery alone were poor. With the advent of chemotherapy and the combination of medical and surgical treatment, TOS-associated mortality has continued to decline. There is no significant difference in outcomes among the available surgical options, but limb-salvage surgical procedures usually offer patients much better function and quality of life. The most important consideration is early diagnosis followed by immediate treatment.
A 10-year-old boy first complained of right knee pain two months prior to presentation. There was no traumatic event to explain the pain and no prior viral or bacterial illness. Radiographs taken earlier at another facility were initially pronounced normal. One month later, repeat x-rays showed a possible hairline fracture, and MRI was ordered. MRI documented a destructive lesion in the right distal femur with a soft-tissue mass that was worrisome for primary bone malignancy.
The boy was placed on weight-bearing restrictions and was given a wheelchair. Unfortunately, he fell from the wheelchair and sustained a pathologic fracture through the lesion (see Figure 1). He was transported to the hospital and admitted. A biopsy was performed with a closed reduction, as the fracture was maligned. The patient was placed in a long leg cast with a pelvic band.

His history was previously unremarkable. He was taking no medications and had experienced no recent illnesses. His surgical/medical history was positive for a tonsillectomy at an early age and a fracture of the right proximal femur at age 2. On examination, he was noted to be talkative with his family but guarded during conversations with staff.
His physical exam was positive for pain at the right distal femur and knee with palpation; otherwise, all other systems were unremarkable. The patient was in too much pain to range the knee and had been placed in a long posterior leg splint (prior to surgery and application of the cast). Distally, his right lower extremity motor and sensory function were intact.
The patient’s vital signs were within normal limits, and results from his blood chemistries and alkaline phosphatase and C-reactive protein levels were unremarkable. Findings on the complete blood cell count were slightly abnormal: Hemoglobin was 11 g and the hematocrit, 33% (both within normal limits); however, in the differential there was an elevation in segmented neutrophils (72%, compared with a reference range of 31% to 61%), with Döhle bodies present—possibly signifying acute and/or chronic systemic infection or malignancy. The lymphocyte count represented 11% of the total white blood cell count (range, 28% to 48%), and platelets were 82 x 103/mL (normal range, 150 to 350 x 103/mL). The patient’s erythrocyte sedimentation rate was 44 mm/h (normal range, 0 to 20).
Result from pathology were positive for osteosarcoma, telangiectatic type. The patient underwent a nuclear medicine bone scan that showed no metastases, and chest CT was negative for pulmonary lesions as well. After a psychology consult, the boy was gently told about his condition.
Treatment then proceeded, including surgical placement of a double-lumen chest catheter for delivery of neoadjuvant and adjuvant chemotherapy. Doxorubicin, cisplatin, and methotrexate were used because the boy was enrolled in an international cooperative trial through the Children’s Oncology Group for treatment of localized osteosarcoma.
Discussion
Osteosarcoma (OS) is the most common primary bone malignancy.1,2 Approximately 5% of all pediatric patients with tumors present with this diagnosis, and about 400 new cases are diagnosed in the United States each year.1 Most osteosarcomas develop in the bones of the lower extremities and in the humerus, affecting males more often than females.1-3 This kind of malignancy is frequently seen during the adolescent growth spurt, but it can affect patients of any age.1,2 Patients usually present with pain or functional limitation in gait or daily activities or both.1-3
The telangiectatic subtype of OS is a rare, aggressive variant that represents 2% to 12% of all cases of OS.4-6 Telangiectatic OS (TOS) is characterized by multiple aneurysmally dilated, blood-filled cavities with high-grade sarcomatous cells seen in the peripheral rim and septae.3,7,8 This process can cause the lesion to resemble an aneurysmal bone cyst, explaining why some cases of TOS are misdiagnosed—with delayed time to treatment and increased morbidity and mortality.3,5 Generally, TOS patients are more likely than other OS patients to have tumors of femoral location, larger lesions, and normal alkaline phosphatase values. Many have pathologic fractures on presentation.7
The medical literature chronicles a long debate regarding the difference in mortality between patients with OS and those with TOS. It was once believed that patients with TOS were at higher risk for recurrence (especially those with a pathologic fracture) and mortality. However, in recent studies examining newer neoadjuvant and adjuvant chemotherapies, mortality rates for the two conditions are similar and certainly lower than they were many years ago.7,8 In one study, a better histologic response was reported to neoadjuvant chemotherapy in patients with TOS than with OS.7
Diagnosis
The first diagnostic tool used for patients with suspected OS or TOS is a plain radiographic film. A TOS lesion is lytic, with no areas of sclerosis, and almost always involves the long bones. It is poorly defined, destroying the cortex with formation of periosteal bone and invading the soft tissue. An initial pattern of parallel striations is highly suggestive of TOS.5
MRI and CT often reveal thick nodular tissue in a largely hemorrhagic and/or necrotic osseous lesion, with an associated soft-tissue mass that allows distinction from an aneurysmal bone cyst.3 Next, patients generally undergo a nuclear medicine bone scan and CT of the chest to observe for signs of metastases. Chest CT is commonly repeated on a regular basis during and after treatment.9
Pathologic evaluation, the final step to diagnosis, is very important, especially in the effort to differentiate TOS from an aneurysmal bone cyst. The typical gross findings for a TOS tumor include a dominant cystic cavity–like architecture, with a pushing peripheral margin that frequently expands and erodes the adjacent cortex and extends into the surrounding tissue. There is usually no area of intramural bone tissue.
Microscopically, the cystic areas contain clots and fragments of tumor that are often lined with a layer of neoplasm. The blood-filled telangiectatic spaces form in these areas. The spaces are irregularly shaped and typically traversed by septae composed in part of neoplastic cells. Osteoid formation through these cells can appear as a fine, ice-like material between tumor cells.4,7
Treatment
The main goals of treatment are to limit the anatomical extent of the disease, decrease the possibility of recurrence, and restore the highest possible level of function.2 Initial treatment of any OS or TOS consists of aggressive, immediate chemotherapy prior to and after any surgical intervention.1 (Chemotherapy will not be discussed in further detail here.) Surgical treatments for patients younger than 14 include amputation (above the lesion with wide margins), an expanding prosthesis, or rotationplasty. The location and extent of the tumor, the patient’s age, and his or her desired lifestyle will all have an impact on the choice of surgery.10
Historic data demonstrate that patients who undergo amputation alone almost always develop metastatic disease.1 Other data show that only 10% of patients with OS have been cured by chemotherapy alone. Yet when medical treatment is combined with surgical treatment, the overall expected cure rate can be as high as 65%.2
Discussing amputation with a young patient and the family can be emotionally difficult. If functional levels are to be restored, above-knee amputation (AKA) is the least favored surgical method. Compared with healthy individuals, patients who undergo AKA will walk 43% less quickly and will expend much more energy. These patients frequently have an inefficient gait and, given their limited reserve, they may lose the ability to walk altogether.2
Reconstructive surgical options include limb-salvage procedures; since the late 1980s, these have become the standard of care for OS at all sites.11 One such option includes removal of the lesion (eg, a distal femoral or proximal tibial lesion) with acceptable margins and replacement of the lost bone with an allograft or with a metallic prosthesis and knee joint (called arthroplasty). This endoprosthesis expands as the child grows (by way of a minor surgical procedure or a magnetic spring) so there is no apparent discrepancy between limb lengths, and the patient’s appearance is as normal and socially acceptable as possible.1,2
Because the case patient developed a pathologic fracture through his TOS tumor, he was not a candidate for endoprosthesis. His options were AKA or rotationplasty.
This procedure was first described in 195012 for treatment of proximal focal femoral deficiency. It is considered an alternative for skeletally immature individuals for whom the goal is to preserve function.
When AKA is indicated, the lower limb can be salvaged to allow functioning similar to that of a patient with a below-knee amputation (BKA). During rotationplasty, all but the most proximal aspect of the femur is resected. The tibia is externally rotated on the axis of the neurovascular bundle, then an arthrodesis of the proximal portion of the femur and the tibial plateau is performed (see Figure 2).

The end result is an extremity with the appearance, dimensions, and functional potential of a BKA. The ankle is rotated 180° so that it can serve as the new knee joint, and the attached foot, now pointing in the opposite direction, acts as the residual limb for fitting a prosthesis.2 This procedure is favored in patients with an extensive soft-tissue mass, intra-articular extension of the tumor, and/or pathologic fractures. It can also help prevent phantom pain.13

The Case Patient
After psychological evaluation of the patient and extensive family discussion, he underwent successful rotationplasty. The day after his surgery, however, he developed compartment syndrome and was required to undergo fasciotomies of the calf and proximal thigh. His wounds were treated, a skin graft was performed to close the proximal thigh wound, and his calf wounds were sutured closed (see Figures 3 and 4). His hip range of motion is excellent, and his ankle range of motion continues to improve with physical therapy.

At this writing, the patient was scheduled for his first prosthetic fitting, and he had nearly completed his chemotherapy. His outlook is very promising.
Conclusion
TOS is a rare, aggressive subtype of OS but the most common primary malignant bone tumor of childhood. In the past, outcomes in patients treated with surgery alone were poor. With the advent of chemotherapy and the combination of medical and surgical treatment, TOS-associated mortality has continued to decline. There is no significant difference in outcomes among the available surgical options, but limb-salvage surgical procedures usually offer patients much better function and quality of life. The most important consideration is early diagnosis followed by immediate treatment.
1. Siegel HJ, Pressey JG. Current concepts on the surgical and medical management of osteosarcoma. Expert Rev Anticancer Ther. 2008;8(8):1257-1269.
2. Marulanda GA, Henderson ER, Johnson DA, et al. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15(1):13-20.
3. Murphey MD, wan Jaovisidha S, Temple HT, et al. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology. 2003;229(2):545-553.
4. Mervak TR, Unni KK, Pritchard DJ, McLeod RA. Telangiectatic osteosarcoma. Clin Orthop Relat Res. 1991 Sep;270:135-139.
5. Vanel D, Tcheng S, Contesso G, et al. The radiological appearances of telangiectatic osteosarcoma: a study of 14 cases. Skeletal Radiol. 1987;16(3):196-200.
6. Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23(34):8845-8852.
7. Bacci G, Ferrari S, Ruggieri P, et al. Telangiectatic osteosarcoma of the extremity: neoadjuvant chemotherapy in 24 cases. Acta Orthop Scand. 2001;72(2):167-172.
8. Weiss A, Khoury JD, Hoffer FA, et al. Telangiectatic osteosarcoma: the St. Jude Children’s Research Hospital’s experience. Cancer. 2007;109(8):1627-1637.
9. Agarwal M, Anchan C, Shah M, et al. Limb salvage surgery for osteosarcoma: effective low-cost treatment. Clin Orthop Relat Res. 2007;459:82-91.
10. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb: amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
11. Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68(9):1331-1337.
12. Van Nes CP. Rotation-plasty for congenital defects of the femur: making use of the shortened limb to control the knee joint of a prosthesis. J Bone Joint Surg. 1950;32B:12-16.
13. Sawamura C, Hornicek FJ, Gebhardt MC. Complications and risk factors for failure of rotationplasty: review of 25 patients. Clin Orthop Relat Res. 2008;466(6):1302-1308.
1. Siegel HJ, Pressey JG. Current concepts on the surgical and medical management of osteosarcoma. Expert Rev Anticancer Ther. 2008;8(8):1257-1269.
2. Marulanda GA, Henderson ER, Johnson DA, et al. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15(1):13-20.
3. Murphey MD, wan Jaovisidha S, Temple HT, et al. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology. 2003;229(2):545-553.
4. Mervak TR, Unni KK, Pritchard DJ, McLeod RA. Telangiectatic osteosarcoma. Clin Orthop Relat Res. 1991 Sep;270:135-139.
5. Vanel D, Tcheng S, Contesso G, et al. The radiological appearances of telangiectatic osteosarcoma: a study of 14 cases. Skeletal Radiol. 1987;16(3):196-200.
6. Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23(34):8845-8852.
7. Bacci G, Ferrari S, Ruggieri P, et al. Telangiectatic osteosarcoma of the extremity: neoadjuvant chemotherapy in 24 cases. Acta Orthop Scand. 2001;72(2):167-172.
8. Weiss A, Khoury JD, Hoffer FA, et al. Telangiectatic osteosarcoma: the St. Jude Children’s Research Hospital’s experience. Cancer. 2007;109(8):1627-1637.
9. Agarwal M, Anchan C, Shah M, et al. Limb salvage surgery for osteosarcoma: effective low-cost treatment. Clin Orthop Relat Res. 2007;459:82-91.
10. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb: amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
11. Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68(9):1331-1337.
12. Van Nes CP. Rotation-plasty for congenital defects of the femur: making use of the shortened limb to control the knee joint of a prosthesis. J Bone Joint Surg. 1950;32B:12-16.
13. Sawamura C, Hornicek FJ, Gebhardt MC. Complications and risk factors for failure of rotationplasty: review of 25 patients. Clin Orthop Relat Res. 2008;466(6):1302-1308.
Grand Rounds: Man, 82, With New-Onset Headaches
An 82-year-old man presented to his primary care provider complaining of headaches for the past week. At the time of presentation, he reported persistent, nonthrobbing pain behind his right eye. Previously, he had experienced pain on the top and right side of his head.
The patient denied any recent visual changes. His last eye examination had taken place four weeks earlier. He was prescribed new eyeglasses, but he had not yet filled the prescription. He denied having symptoms of transient ischemic attack or stroke. He denied any nasal drainage, fever, or chills and reported no prior history of headaches. For the current headache, he had been taking acetaminophen intermittently and said it provided some relief.
The patient’s prior diagnoses included type 2 diabetes, hypertension, dyslipidemia, gout, metabolic syndrome, osteoarthritis, leg edema, and atrial fibrillation. His current medications were allopurinol, diltiazem, glipizide, hydrochlorothiazide, rosiglitazone, valsartan, vardenafil, and warfarin.
His most recent international normalized ratio (INR), measured five days earlier, was 3.34. Fifteen days earlier, however, his INR had been measured at 4.6.
The patient described himself as active, riding his bicycle 50 miles each week. He denied using tobacco but admitted to having “a couple of cocktails” before dinner each evening. He was a widower who lived alone. He owned an advertising company and was involved in its day-to-day operation.
On examination, the patient was alert and oriented. He had an irregularly irregular heart rate with a controlled ventricular response. Cranial nerves II through XII were intact. No papilledema was noted.
The patient was given a diagnosis of headaches of unknown etiology. He was told that he could continue using acetaminophen and was scheduled for head CT with and without contrast the following day.
CT revealed a 2.3-cm, right-sided subacute (mixed-density) subdural hematoma (SDH) with midline shift of 1.8 cm (see Figure 1). The patient’s provider was notified of the CT results, and the patient was sent directly from radiology to the emergency department. His INR was 2.7. The patient was given a partial dose of recombinant factor VIIa (rFVIIa), then emergently transferred to another facility for neurosurgical care.
Upon his arrival there, the patient was noted to be drowsy but oriented, without any focal neurologic deficits. The dose of rFVIIa was completed, and he was given 5 mg of vitamin K. He underwent an emergency craniotomy for clot evacuation. Intraoperatively, his INR was measured at 1.5, and he was given two units of fresh frozen plasma (FFP) to further reverse his coagulopathy.
Repeat head CT the following morning revealed nearly complete removal of the clot, with reexpansion of the brain (see Figure 2). The patient’s INR was 1.1. Additional doses of FFP or rFVIIa were deemed unnecessary. The patient recovered and was discharged from the hospital four days after his surgery. When he was seen at the clinic one month later, he had no neurologic deficits. Head CT was found stable with only a thin rim of residual subdural fluid noted (see Figure 3). He was followed as an outpatient with serial head CTs until all the subdural fluid completely resolved. At that time, he was allowed to restart warfarin.
Discussion
Use of anticoagulation therapy will become increasingly common as our population ages. While anticoagulants are important for preventing thromboembolic events that may result from use of mechanical heart valves, atrial fibrillation, and other conditions, their use is not without risk. The most significant and potentially lethal complication is hemorrhage.
Warfarin-Associated Hemorrhage
In patients who take warfarin, hemorrhage can occur in a variety of areas—most commonly, cerebral and gastrointestinal sites, the nose, the airways, the urinary tract, muscle, and skin.1,2 The site of hemorrhage that carries the highest risk of mortality and morbidity is cerebral.3-5 Among anticoagulated patients experiencing intracranial hemorrhage, a fourfold to fivefold increase in mortality has been reported.6 Among study patients who experienced intracranial hemorrhages while taking warfarin, only 14% were able to return to living independently.4
Excessive Anticoagulation
Recent studies have led to the conclusion that excessive anticoagulation, not anticoagulation targeting specific therapeutic levels, is associated with major bleeding events.7,8 In a review of 2,460 patients from 2000 to 2003 at Brigham and Women’s Hospital in Boston, Fanikos et al8 found that 83% of major bleeding events occurred in patients with an INR exceeding 3.0.
In addition, excessive anticoagulation has been associated with increased morbidity and mortality.5,9,10 Pieracci et al9 found that among patients who experienced a traumatic intracranial hemorrhage with an INR exceeding 3.5, the mortality rate was nearly 75%.
Intracranial Hemorrhage
Subdural hematoma is one of the most common types of intracranial hemorrhage. SDHs are classified based on radiographic findings and age. Acute SDHs are those less than three days old, subacute (mixed-density) SDHs are three to 20 days old, and chronic SDHs (CSDHs) are at least 21 days old.
Acute hemorrhages are more dense and appear white on CT, whereas CSDHs are hypodense and appear darker than the brain parenchyma. Subacute SDHs may have features of both acute SDHs and CSDHs or may appear isodense. While acute SDHs are often associated with trauma and are readily diagnosed, chronic and subacute SDHs present a greater diagnostic challenge. Clinically, subacute SDHs act like CSDHs and are treated similarly.11 For the purposes of this discussion, the case patient’s SDH will be considered a form of CSDH.
Pathophysiology of Chronic Subdural Hematomas
Chronic subdural hematomas form in a number of ways. Major causes are related to brain atrophy resulting from advanced age, alcoholism, brain injury, stroke, or other conditions.11 Atrophy of the brain causes the size of the subdural space to increase. This increased space causes the bridging veins between the cortical surface of the brain and the dura to become stretched and easily torn. As a result, seemingly minor trauma can easily lead to hemorrhage.
Over time, these small, acute hemorrhages in the subdural space may liquefy into CSDHs. Bleeding triggers an inflammatory response, and gradually, blood begins to break down, as with any bruise. Unlike most blood clots, however, blood in the subdural space is affected by fluid dynamics, fibrinolysis, and the formation of neomembranes.11,12 As a result, the blood may not be completely reabsorbed and may actually expand, causing patients to experience symptoms.
Potentially, SDHs can also be caused by subdural hygromas, low intracranial pressure, dehydration, or overdrainage of cerebrospinal fluid during lumbar puncture, spinal anesthesia, or shunting.13
Epidemiology
The annual incidence of CSDH is one to two cases per 100,000 persons. Incidence increases to seven cases per 100,000 among persons older than 70.13 The mortality rate for SDH is 31% to 36%.14,15 The mortality rate for CSDH is approximately 6%. For patients older than 60, the rate increases to 8.8%.16 Rates of morbidity (ie, severe disability or persistent vegetative state) associated with CSDHs have been reported at about 10%.16,17
Men are affected more commonly than are women (accounting for 61% to 70% of cases), and median ages between 71 and 78 have been reported.4,12,18,19
The risk factors for CSDH are listed in Table 1.4,10 SDHs frequently occur in the context of trauma, but they can occur spontaneously, especially in coagulopathic patients. Among patients with CSDHs who are taking warfarin, 45.5% to 52% deny recent experiences of trauma.4,14
Signs and Symptoms of Chronic Subdural Hematomas
The clinical onset of CSDH is insidious. Possible presenting symptoms are listed in Table 2.14,18,20,21 Frequently, the neurologic examination fails to reveal any focal deficits. Many of the symptoms are vague and nonspecific and may mimic those of other conditions that are common in the elderly, thus making diagnosis difficult. Despite clinical suspicion, the definitive diagnosis of SDH is based on CT results.
Reversing Warfarin-Induced Coagulopathy
In all patients with intracranial hemorrhages who are taking warfarin, the coagulopathy must be reversed. The agents commonly used to reverse the effects of warfarin include vitamin K, FFP, and rFVIIa.9,22-24 The choice of agents depends on the timing of intervention.
Vitamin K is commonly given to patients either intravenously or orally in combination with FFP and/or rFVIIa to promote the reversal of warfarin-induced coagulopathy. Vitamin K is seldom used alone, as its effects may not be seen for 24 hours or longer, and may not completely reverse the effects of warfarin.25
Another frequently used product is FFP. Unfortunately, FFP has been associated with complications such as fluid overload, infectious disease transmission, and anaphylaxis. Additionally, FFP too reverses coagulopathy very slowly. Boulis et al26 found that in patients given FFP with single-dose vitamin K, INR reduction averaged 0.18/hour. At this rate, it would take approximately 11 hours to correct an INR of 3.0 to the desired target of 1.0.
In contrast, rFVIIa, used off-label, has proved highly effective in rapidly reversing coagulopathy and allowing patients to safely undergo immediate surgical treatment.23,24 To its disadvantage, rFVIIa increases the risk of thromboembolism and is significantly more expensive than FFP. Compared with $105 for one unit of FFP, the cost of an 80-mcg/kg dose of rFVIIa for a patient weighing 80 kg is about $6,400.27
Factors Predicting Outcome for Subdural Hematomas
A number of factors determine post-SDH outcome. Rozzelle et al14 found that a Glasgow Coma Scale score below 7, age greater than 80, more acute hemorrhages, and hemorrhages requiring craniotomy rather than burr-hole drainage were associated with significantly higher mortality rates than when these factors were absent.
Other studies have revealed that patients with poor clinical status and larger hematomas with more midline shift are also prone to higher mortality rates.20,28 Merlicco et al29 found that younger, nonalcoholic patients without severe trauma whose hematomas were under high pressure had better chances for full recovery than other patients.
Patient Outcome
This case study illustrates the importance of patient education. The patient described here was aware of his excessive anticoagulation and told his provider that he was concerned about bleeding in the brain. Because the patient had been educated about the potential risks of warfarin therapy, he was able to alert his provider when he experienced symptoms of a possible complication. As a result, his condition was quickly diagnosed and treated, with an excellent outcome.
Conclusion
Intracranial hemorrhage is a serious and potentially life-threatening complication of warfarin therapy. CSDHs in particular are a significant cause of mortality and morbidity in older patients. The risk of death or disability increases in patients who are undergoing anticoagulation therapy. In addition, patients with an INR elevated above therapeutic levels face a significantly higher risk for major bleeding events. For this reason, it is important that anticoagulation be tightly controlled within the therapeutic range. It is equally important to educate patients and their families about anticoagulation’s potential risks and complications.
Making the diagnosis of CSDH can be difficult because its symptoms are so often nonspecific and a concomitant illness may be present. Thus, providers must maintain a low threshold for evaluating even minor patient complaints that may signal a complication of warfarin therapy. All too often, minor signs and symptoms go unrecognized, sometimes leading to devastating consequences.
Although many factors predict outcomes for CSDHs, the most important can be controlled by patients and their providers. If patients are well educated and providers listen to their patients, then early diagnosis of SDH can lead to early intervention and improved outcomes.
1. Pullicino P, Thompson JL. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2003;348(3): 256-257.
2. Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347(13):969-974.
3. DeSilvey DL. Clinical trials: advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Am J Geriatr Cardiol. 2005;14(2):98-99.
4. Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med. 2004;141(10):745-752.
5. Koo S, Kucher N, Nguyen PL, et al. The effect of excessive anticoagulation on mortality and morbidity in hospitalized patients with anticoagulant-related major hemorrhage. Arch Intern Med. 2004;164(14):1557-1560.
6. Mina AA, Knipfer JF, Park DY, et al. Intracranial complications of preinjury anticoagulation in trauma patients with head injury. J Trauma. 2002;53(4):668-672.
7. Pieracci FM, Eachempati SR, Shou J, et al. Degree of anticoagulation, but not warfarin use itself, predicts adverse outcomes after traumatic brain injury in elderly trauma patients. J Trauma. 2007;63(3):525-530.
8. Fanikos J, Grasso-Correnti N, Shah R, et al. Major bleeding complications in a specialized anticoagulation service. Am J Cardiol. 2005;96(4):595-598.
9. Pieracci FM, Eachempati SR, Shou J, et al. Use of long-term anticoagulation is associated with traumatic intracranial hemorrhage and subsequent mortality in elderly patients hospitalized after falls: analysis of the New York State Administrative Database. J Trauma. 2007;63(3):519-524.
10. Franko J, Kish KJ, O’Connell BG, et al. Advanced age and preinjury warfarin anticoagulation increase the risk of mortality after head trauma. J Trauma. 2006; 61(1):107-110.
11. Drapkin AJ. Chronic subdural hematoma: pathophysiological basis for treatment. Br J Neurosurg. 1991; 5(5):467-473.
12. Yamamoto H, Hirashima Y, Hamada H, et al. Independent predictors of recurrence of chronic subdural hematoma: results of multivariate analysis performed using a logistic regression model. J Neurosurg. 2003;98(6):1217-1221.
13. Iantosca MR, Simon RH. Chronic subdural hematoma in adult and elderly patients. Neurosurg Clin N Am. 2000;11(3):447-454.
14. Rozzelle CJ, Wofford JL, Branch CL. Predictors of hospital mortality in older patients with subdural hematoma. J Am Geriatr Soc. 1995;43(3):240-244.
15. Wintzen AR, Tijssen JG. Subdural hematoma and oral anticoagulant therapy. Arch Neurol. 1982;39(2): 69-72.
16. Ramachandran R, Hegde T. Chronic subdural hematomas: causes of morbidity and mortality. Surg Neurol. 2007;67(4):367-372.
17. Amirjamshidi A, Eftekhar B, Abouzari M, Rashidi A. The relationship between Glasgow coma/outcome scores and abnormal CT scan findings in chronic subdural hematoma. Clin Neurol Neurosurg. 2007;109(2): 152-157.
18. Lee JY, Ebel H, Ernestus RI, Klug N. Various surgical treatments of chronic subdural hematoma and outcome in 172 patients: is membranectomy necessary? Surg Neurol. 2004;61(6):523-527.
19. Gelabert-González M, Iglesias-Pais M, García-Allut A, Martínez-Rumbo R. Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005;107(3):223-229.
20. Mattle H, Kohler S, Huber P, et al. Anticoagulation-related intracranial extracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 1989;52(7):829-837.
21. Sambasivan M. An overview of chronic subdural hematoma: experience with 2300 cases. Surg Neurol. 1997;47(5):418-422.
22. Lin J, Hanigan WC, Tarantino M, Wang J. The use of recombinant activated factor VII to reverse warfarin-induced anticoagulation in patients with hemorrhages in the central nervous system: preliminary findings. J Neurosurg. 2003;98(4):737-740.
23. Freeman WD, Brott TG, Barrett KM, et al. Recombinant factor VIIa for rapid reversal of warfarin anticoagulation in acute intracranial hemorrhage. Mayo Clin Proc. 2004;79(12):1495-1500.
24. Dager WE, King JH, Regalia RC, et al. Reversal of elevated international normalized ratios and bleeding with low-dose recombinant activated factor VII in patients receiving warfarin. Pharmacotherapy. 2006;26(8): 1091-1098.
25. Denas G, Marzot F, Offelli P, et al. Effectiveness and safety of a management protocol to correct over-anticoagulation with oral vitamin K: a retrospective study of 1,043 cases. J Thromb Thrombolysis. 2008 Mar 13; [Epub ahead of print].
26. Boulis NM, Bobek MP, Schmaier A, Hoff JT. Use of factor IX complex in warfarin-related intracranial hemorrhage. Neurosurgery. 1999;45(5):1113-1118.
27. Kissela BM, Eckman MH. Cost effectiveness of recombinant factor VIIa for treatment of intracerebral hemorrhage. BMC Neurol. 2008;8:17.
28. Ernestus RI, Beldzinski P, Lanfermann H, Klug N. Chronic subdural hematoma: surgical treatment and outcome in 104 patients. Surg Neurol. 1997;48(3): 220-225.
29. Merlicco G, Pierangeli E, di Padova PL. Chronic subdural hematomas in adults: prognostic factors: analysis of 70 cases. Neurosurg Rev. 1995;18(4):247-251.
An 82-year-old man presented to his primary care provider complaining of headaches for the past week. At the time of presentation, he reported persistent, nonthrobbing pain behind his right eye. Previously, he had experienced pain on the top and right side of his head.
The patient denied any recent visual changes. His last eye examination had taken place four weeks earlier. He was prescribed new eyeglasses, but he had not yet filled the prescription. He denied having symptoms of transient ischemic attack or stroke. He denied any nasal drainage, fever, or chills and reported no prior history of headaches. For the current headache, he had been taking acetaminophen intermittently and said it provided some relief.
The patient’s prior diagnoses included type 2 diabetes, hypertension, dyslipidemia, gout, metabolic syndrome, osteoarthritis, leg edema, and atrial fibrillation. His current medications were allopurinol, diltiazem, glipizide, hydrochlorothiazide, rosiglitazone, valsartan, vardenafil, and warfarin.
His most recent international normalized ratio (INR), measured five days earlier, was 3.34. Fifteen days earlier, however, his INR had been measured at 4.6.
The patient described himself as active, riding his bicycle 50 miles each week. He denied using tobacco but admitted to having “a couple of cocktails” before dinner each evening. He was a widower who lived alone. He owned an advertising company and was involved in its day-to-day operation.
On examination, the patient was alert and oriented. He had an irregularly irregular heart rate with a controlled ventricular response. Cranial nerves II through XII were intact. No papilledema was noted.
The patient was given a diagnosis of headaches of unknown etiology. He was told that he could continue using acetaminophen and was scheduled for head CT with and without contrast the following day.
CT revealed a 2.3-cm, right-sided subacute (mixed-density) subdural hematoma (SDH) with midline shift of 1.8 cm (see Figure 1). The patient’s provider was notified of the CT results, and the patient was sent directly from radiology to the emergency department. His INR was 2.7. The patient was given a partial dose of recombinant factor VIIa (rFVIIa), then emergently transferred to another facility for neurosurgical care.
Upon his arrival there, the patient was noted to be drowsy but oriented, without any focal neurologic deficits. The dose of rFVIIa was completed, and he was given 5 mg of vitamin K. He underwent an emergency craniotomy for clot evacuation. Intraoperatively, his INR was measured at 1.5, and he was given two units of fresh frozen plasma (FFP) to further reverse his coagulopathy.
Repeat head CT the following morning revealed nearly complete removal of the clot, with reexpansion of the brain (see Figure 2). The patient’s INR was 1.1. Additional doses of FFP or rFVIIa were deemed unnecessary. The patient recovered and was discharged from the hospital four days after his surgery. When he was seen at the clinic one month later, he had no neurologic deficits. Head CT was found stable with only a thin rim of residual subdural fluid noted (see Figure 3). He was followed as an outpatient with serial head CTs until all the subdural fluid completely resolved. At that time, he was allowed to restart warfarin.
Discussion
Use of anticoagulation therapy will become increasingly common as our population ages. While anticoagulants are important for preventing thromboembolic events that may result from use of mechanical heart valves, atrial fibrillation, and other conditions, their use is not without risk. The most significant and potentially lethal complication is hemorrhage.
Warfarin-Associated Hemorrhage
In patients who take warfarin, hemorrhage can occur in a variety of areas—most commonly, cerebral and gastrointestinal sites, the nose, the airways, the urinary tract, muscle, and skin.1,2 The site of hemorrhage that carries the highest risk of mortality and morbidity is cerebral.3-5 Among anticoagulated patients experiencing intracranial hemorrhage, a fourfold to fivefold increase in mortality has been reported.6 Among study patients who experienced intracranial hemorrhages while taking warfarin, only 14% were able to return to living independently.4
Excessive Anticoagulation
Recent studies have led to the conclusion that excessive anticoagulation, not anticoagulation targeting specific therapeutic levels, is associated with major bleeding events.7,8 In a review of 2,460 patients from 2000 to 2003 at Brigham and Women’s Hospital in Boston, Fanikos et al8 found that 83% of major bleeding events occurred in patients with an INR exceeding 3.0.
In addition, excessive anticoagulation has been associated with increased morbidity and mortality.5,9,10 Pieracci et al9 found that among patients who experienced a traumatic intracranial hemorrhage with an INR exceeding 3.5, the mortality rate was nearly 75%.
Intracranial Hemorrhage
Subdural hematoma is one of the most common types of intracranial hemorrhage. SDHs are classified based on radiographic findings and age. Acute SDHs are those less than three days old, subacute (mixed-density) SDHs are three to 20 days old, and chronic SDHs (CSDHs) are at least 21 days old.
Acute hemorrhages are more dense and appear white on CT, whereas CSDHs are hypodense and appear darker than the brain parenchyma. Subacute SDHs may have features of both acute SDHs and CSDHs or may appear isodense. While acute SDHs are often associated with trauma and are readily diagnosed, chronic and subacute SDHs present a greater diagnostic challenge. Clinically, subacute SDHs act like CSDHs and are treated similarly.11 For the purposes of this discussion, the case patient’s SDH will be considered a form of CSDH.
Pathophysiology of Chronic Subdural Hematomas
Chronic subdural hematomas form in a number of ways. Major causes are related to brain atrophy resulting from advanced age, alcoholism, brain injury, stroke, or other conditions.11 Atrophy of the brain causes the size of the subdural space to increase. This increased space causes the bridging veins between the cortical surface of the brain and the dura to become stretched and easily torn. As a result, seemingly minor trauma can easily lead to hemorrhage.
Over time, these small, acute hemorrhages in the subdural space may liquefy into CSDHs. Bleeding triggers an inflammatory response, and gradually, blood begins to break down, as with any bruise. Unlike most blood clots, however, blood in the subdural space is affected by fluid dynamics, fibrinolysis, and the formation of neomembranes.11,12 As a result, the blood may not be completely reabsorbed and may actually expand, causing patients to experience symptoms.
Potentially, SDHs can also be caused by subdural hygromas, low intracranial pressure, dehydration, or overdrainage of cerebrospinal fluid during lumbar puncture, spinal anesthesia, or shunting.13
Epidemiology
The annual incidence of CSDH is one to two cases per 100,000 persons. Incidence increases to seven cases per 100,000 among persons older than 70.13 The mortality rate for SDH is 31% to 36%.14,15 The mortality rate for CSDH is approximately 6%. For patients older than 60, the rate increases to 8.8%.16 Rates of morbidity (ie, severe disability or persistent vegetative state) associated with CSDHs have been reported at about 10%.16,17
Men are affected more commonly than are women (accounting for 61% to 70% of cases), and median ages between 71 and 78 have been reported.4,12,18,19
The risk factors for CSDH are listed in Table 1.4,10 SDHs frequently occur in the context of trauma, but they can occur spontaneously, especially in coagulopathic patients. Among patients with CSDHs who are taking warfarin, 45.5% to 52% deny recent experiences of trauma.4,14
Signs and Symptoms of Chronic Subdural Hematomas
The clinical onset of CSDH is insidious. Possible presenting symptoms are listed in Table 2.14,18,20,21 Frequently, the neurologic examination fails to reveal any focal deficits. Many of the symptoms are vague and nonspecific and may mimic those of other conditions that are common in the elderly, thus making diagnosis difficult. Despite clinical suspicion, the definitive diagnosis of SDH is based on CT results.
Reversing Warfarin-Induced Coagulopathy
In all patients with intracranial hemorrhages who are taking warfarin, the coagulopathy must be reversed. The agents commonly used to reverse the effects of warfarin include vitamin K, FFP, and rFVIIa.9,22-24 The choice of agents depends on the timing of intervention.
Vitamin K is commonly given to patients either intravenously or orally in combination with FFP and/or rFVIIa to promote the reversal of warfarin-induced coagulopathy. Vitamin K is seldom used alone, as its effects may not be seen for 24 hours or longer, and may not completely reverse the effects of warfarin.25
Another frequently used product is FFP. Unfortunately, FFP has been associated with complications such as fluid overload, infectious disease transmission, and anaphylaxis. Additionally, FFP too reverses coagulopathy very slowly. Boulis et al26 found that in patients given FFP with single-dose vitamin K, INR reduction averaged 0.18/hour. At this rate, it would take approximately 11 hours to correct an INR of 3.0 to the desired target of 1.0.
In contrast, rFVIIa, used off-label, has proved highly effective in rapidly reversing coagulopathy and allowing patients to safely undergo immediate surgical treatment.23,24 To its disadvantage, rFVIIa increases the risk of thromboembolism and is significantly more expensive than FFP. Compared with $105 for one unit of FFP, the cost of an 80-mcg/kg dose of rFVIIa for a patient weighing 80 kg is about $6,400.27
Factors Predicting Outcome for Subdural Hematomas
A number of factors determine post-SDH outcome. Rozzelle et al14 found that a Glasgow Coma Scale score below 7, age greater than 80, more acute hemorrhages, and hemorrhages requiring craniotomy rather than burr-hole drainage were associated with significantly higher mortality rates than when these factors were absent.
Other studies have revealed that patients with poor clinical status and larger hematomas with more midline shift are also prone to higher mortality rates.20,28 Merlicco et al29 found that younger, nonalcoholic patients without severe trauma whose hematomas were under high pressure had better chances for full recovery than other patients.
Patient Outcome
This case study illustrates the importance of patient education. The patient described here was aware of his excessive anticoagulation and told his provider that he was concerned about bleeding in the brain. Because the patient had been educated about the potential risks of warfarin therapy, he was able to alert his provider when he experienced symptoms of a possible complication. As a result, his condition was quickly diagnosed and treated, with an excellent outcome.
Conclusion
Intracranial hemorrhage is a serious and potentially life-threatening complication of warfarin therapy. CSDHs in particular are a significant cause of mortality and morbidity in older patients. The risk of death or disability increases in patients who are undergoing anticoagulation therapy. In addition, patients with an INR elevated above therapeutic levels face a significantly higher risk for major bleeding events. For this reason, it is important that anticoagulation be tightly controlled within the therapeutic range. It is equally important to educate patients and their families about anticoagulation’s potential risks and complications.
Making the diagnosis of CSDH can be difficult because its symptoms are so often nonspecific and a concomitant illness may be present. Thus, providers must maintain a low threshold for evaluating even minor patient complaints that may signal a complication of warfarin therapy. All too often, minor signs and symptoms go unrecognized, sometimes leading to devastating consequences.
Although many factors predict outcomes for CSDHs, the most important can be controlled by patients and their providers. If patients are well educated and providers listen to their patients, then early diagnosis of SDH can lead to early intervention and improved outcomes.
An 82-year-old man presented to his primary care provider complaining of headaches for the past week. At the time of presentation, he reported persistent, nonthrobbing pain behind his right eye. Previously, he had experienced pain on the top and right side of his head.
The patient denied any recent visual changes. His last eye examination had taken place four weeks earlier. He was prescribed new eyeglasses, but he had not yet filled the prescription. He denied having symptoms of transient ischemic attack or stroke. He denied any nasal drainage, fever, or chills and reported no prior history of headaches. For the current headache, he had been taking acetaminophen intermittently and said it provided some relief.
The patient’s prior diagnoses included type 2 diabetes, hypertension, dyslipidemia, gout, metabolic syndrome, osteoarthritis, leg edema, and atrial fibrillation. His current medications were allopurinol, diltiazem, glipizide, hydrochlorothiazide, rosiglitazone, valsartan, vardenafil, and warfarin.
His most recent international normalized ratio (INR), measured five days earlier, was 3.34. Fifteen days earlier, however, his INR had been measured at 4.6.
The patient described himself as active, riding his bicycle 50 miles each week. He denied using tobacco but admitted to having “a couple of cocktails” before dinner each evening. He was a widower who lived alone. He owned an advertising company and was involved in its day-to-day operation.
On examination, the patient was alert and oriented. He had an irregularly irregular heart rate with a controlled ventricular response. Cranial nerves II through XII were intact. No papilledema was noted.
The patient was given a diagnosis of headaches of unknown etiology. He was told that he could continue using acetaminophen and was scheduled for head CT with and without contrast the following day.
CT revealed a 2.3-cm, right-sided subacute (mixed-density) subdural hematoma (SDH) with midline shift of 1.8 cm (see Figure 1). The patient’s provider was notified of the CT results, and the patient was sent directly from radiology to the emergency department. His INR was 2.7. The patient was given a partial dose of recombinant factor VIIa (rFVIIa), then emergently transferred to another facility for neurosurgical care.
Upon his arrival there, the patient was noted to be drowsy but oriented, without any focal neurologic deficits. The dose of rFVIIa was completed, and he was given 5 mg of vitamin K. He underwent an emergency craniotomy for clot evacuation. Intraoperatively, his INR was measured at 1.5, and he was given two units of fresh frozen plasma (FFP) to further reverse his coagulopathy.
Repeat head CT the following morning revealed nearly complete removal of the clot, with reexpansion of the brain (see Figure 2). The patient’s INR was 1.1. Additional doses of FFP or rFVIIa were deemed unnecessary. The patient recovered and was discharged from the hospital four days after his surgery. When he was seen at the clinic one month later, he had no neurologic deficits. Head CT was found stable with only a thin rim of residual subdural fluid noted (see Figure 3). He was followed as an outpatient with serial head CTs until all the subdural fluid completely resolved. At that time, he was allowed to restart warfarin.
Discussion
Use of anticoagulation therapy will become increasingly common as our population ages. While anticoagulants are important for preventing thromboembolic events that may result from use of mechanical heart valves, atrial fibrillation, and other conditions, their use is not without risk. The most significant and potentially lethal complication is hemorrhage.
Warfarin-Associated Hemorrhage
In patients who take warfarin, hemorrhage can occur in a variety of areas—most commonly, cerebral and gastrointestinal sites, the nose, the airways, the urinary tract, muscle, and skin.1,2 The site of hemorrhage that carries the highest risk of mortality and morbidity is cerebral.3-5 Among anticoagulated patients experiencing intracranial hemorrhage, a fourfold to fivefold increase in mortality has been reported.6 Among study patients who experienced intracranial hemorrhages while taking warfarin, only 14% were able to return to living independently.4
Excessive Anticoagulation
Recent studies have led to the conclusion that excessive anticoagulation, not anticoagulation targeting specific therapeutic levels, is associated with major bleeding events.7,8 In a review of 2,460 patients from 2000 to 2003 at Brigham and Women’s Hospital in Boston, Fanikos et al8 found that 83% of major bleeding events occurred in patients with an INR exceeding 3.0.
In addition, excessive anticoagulation has been associated with increased morbidity and mortality.5,9,10 Pieracci et al9 found that among patients who experienced a traumatic intracranial hemorrhage with an INR exceeding 3.5, the mortality rate was nearly 75%.
Intracranial Hemorrhage
Subdural hematoma is one of the most common types of intracranial hemorrhage. SDHs are classified based on radiographic findings and age. Acute SDHs are those less than three days old, subacute (mixed-density) SDHs are three to 20 days old, and chronic SDHs (CSDHs) are at least 21 days old.
Acute hemorrhages are more dense and appear white on CT, whereas CSDHs are hypodense and appear darker than the brain parenchyma. Subacute SDHs may have features of both acute SDHs and CSDHs or may appear isodense. While acute SDHs are often associated with trauma and are readily diagnosed, chronic and subacute SDHs present a greater diagnostic challenge. Clinically, subacute SDHs act like CSDHs and are treated similarly.11 For the purposes of this discussion, the case patient’s SDH will be considered a form of CSDH.
Pathophysiology of Chronic Subdural Hematomas
Chronic subdural hematomas form in a number of ways. Major causes are related to brain atrophy resulting from advanced age, alcoholism, brain injury, stroke, or other conditions.11 Atrophy of the brain causes the size of the subdural space to increase. This increased space causes the bridging veins between the cortical surface of the brain and the dura to become stretched and easily torn. As a result, seemingly minor trauma can easily lead to hemorrhage.
Over time, these small, acute hemorrhages in the subdural space may liquefy into CSDHs. Bleeding triggers an inflammatory response, and gradually, blood begins to break down, as with any bruise. Unlike most blood clots, however, blood in the subdural space is affected by fluid dynamics, fibrinolysis, and the formation of neomembranes.11,12 As a result, the blood may not be completely reabsorbed and may actually expand, causing patients to experience symptoms.
Potentially, SDHs can also be caused by subdural hygromas, low intracranial pressure, dehydration, or overdrainage of cerebrospinal fluid during lumbar puncture, spinal anesthesia, or shunting.13
Epidemiology
The annual incidence of CSDH is one to two cases per 100,000 persons. Incidence increases to seven cases per 100,000 among persons older than 70.13 The mortality rate for SDH is 31% to 36%.14,15 The mortality rate for CSDH is approximately 6%. For patients older than 60, the rate increases to 8.8%.16 Rates of morbidity (ie, severe disability or persistent vegetative state) associated with CSDHs have been reported at about 10%.16,17
Men are affected more commonly than are women (accounting for 61% to 70% of cases), and median ages between 71 and 78 have been reported.4,12,18,19
The risk factors for CSDH are listed in Table 1.4,10 SDHs frequently occur in the context of trauma, but they can occur spontaneously, especially in coagulopathic patients. Among patients with CSDHs who are taking warfarin, 45.5% to 52% deny recent experiences of trauma.4,14
Signs and Symptoms of Chronic Subdural Hematomas
The clinical onset of CSDH is insidious. Possible presenting symptoms are listed in Table 2.14,18,20,21 Frequently, the neurologic examination fails to reveal any focal deficits. Many of the symptoms are vague and nonspecific and may mimic those of other conditions that are common in the elderly, thus making diagnosis difficult. Despite clinical suspicion, the definitive diagnosis of SDH is based on CT results.
Reversing Warfarin-Induced Coagulopathy
In all patients with intracranial hemorrhages who are taking warfarin, the coagulopathy must be reversed. The agents commonly used to reverse the effects of warfarin include vitamin K, FFP, and rFVIIa.9,22-24 The choice of agents depends on the timing of intervention.
Vitamin K is commonly given to patients either intravenously or orally in combination with FFP and/or rFVIIa to promote the reversal of warfarin-induced coagulopathy. Vitamin K is seldom used alone, as its effects may not be seen for 24 hours or longer, and may not completely reverse the effects of warfarin.25
Another frequently used product is FFP. Unfortunately, FFP has been associated with complications such as fluid overload, infectious disease transmission, and anaphylaxis. Additionally, FFP too reverses coagulopathy very slowly. Boulis et al26 found that in patients given FFP with single-dose vitamin K, INR reduction averaged 0.18/hour. At this rate, it would take approximately 11 hours to correct an INR of 3.0 to the desired target of 1.0.
In contrast, rFVIIa, used off-label, has proved highly effective in rapidly reversing coagulopathy and allowing patients to safely undergo immediate surgical treatment.23,24 To its disadvantage, rFVIIa increases the risk of thromboembolism and is significantly more expensive than FFP. Compared with $105 for one unit of FFP, the cost of an 80-mcg/kg dose of rFVIIa for a patient weighing 80 kg is about $6,400.27
Factors Predicting Outcome for Subdural Hematomas
A number of factors determine post-SDH outcome. Rozzelle et al14 found that a Glasgow Coma Scale score below 7, age greater than 80, more acute hemorrhages, and hemorrhages requiring craniotomy rather than burr-hole drainage were associated with significantly higher mortality rates than when these factors were absent.
Other studies have revealed that patients with poor clinical status and larger hematomas with more midline shift are also prone to higher mortality rates.20,28 Merlicco et al29 found that younger, nonalcoholic patients without severe trauma whose hematomas were under high pressure had better chances for full recovery than other patients.
Patient Outcome
This case study illustrates the importance of patient education. The patient described here was aware of his excessive anticoagulation and told his provider that he was concerned about bleeding in the brain. Because the patient had been educated about the potential risks of warfarin therapy, he was able to alert his provider when he experienced symptoms of a possible complication. As a result, his condition was quickly diagnosed and treated, with an excellent outcome.
Conclusion
Intracranial hemorrhage is a serious and potentially life-threatening complication of warfarin therapy. CSDHs in particular are a significant cause of mortality and morbidity in older patients. The risk of death or disability increases in patients who are undergoing anticoagulation therapy. In addition, patients with an INR elevated above therapeutic levels face a significantly higher risk for major bleeding events. For this reason, it is important that anticoagulation be tightly controlled within the therapeutic range. It is equally important to educate patients and their families about anticoagulation’s potential risks and complications.
Making the diagnosis of CSDH can be difficult because its symptoms are so often nonspecific and a concomitant illness may be present. Thus, providers must maintain a low threshold for evaluating even minor patient complaints that may signal a complication of warfarin therapy. All too often, minor signs and symptoms go unrecognized, sometimes leading to devastating consequences.
Although many factors predict outcomes for CSDHs, the most important can be controlled by patients and their providers. If patients are well educated and providers listen to their patients, then early diagnosis of SDH can lead to early intervention and improved outcomes.
1. Pullicino P, Thompson JL. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2003;348(3): 256-257.
2. Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347(13):969-974.
3. DeSilvey DL. Clinical trials: advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Am J Geriatr Cardiol. 2005;14(2):98-99.
4. Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med. 2004;141(10):745-752.
5. Koo S, Kucher N, Nguyen PL, et al. The effect of excessive anticoagulation on mortality and morbidity in hospitalized patients with anticoagulant-related major hemorrhage. Arch Intern Med. 2004;164(14):1557-1560.
6. Mina AA, Knipfer JF, Park DY, et al. Intracranial complications of preinjury anticoagulation in trauma patients with head injury. J Trauma. 2002;53(4):668-672.
7. Pieracci FM, Eachempati SR, Shou J, et al. Degree of anticoagulation, but not warfarin use itself, predicts adverse outcomes after traumatic brain injury in elderly trauma patients. J Trauma. 2007;63(3):525-530.
8. Fanikos J, Grasso-Correnti N, Shah R, et al. Major bleeding complications in a specialized anticoagulation service. Am J Cardiol. 2005;96(4):595-598.
9. Pieracci FM, Eachempati SR, Shou J, et al. Use of long-term anticoagulation is associated with traumatic intracranial hemorrhage and subsequent mortality in elderly patients hospitalized after falls: analysis of the New York State Administrative Database. J Trauma. 2007;63(3):519-524.
10. Franko J, Kish KJ, O’Connell BG, et al. Advanced age and preinjury warfarin anticoagulation increase the risk of mortality after head trauma. J Trauma. 2006; 61(1):107-110.
11. Drapkin AJ. Chronic subdural hematoma: pathophysiological basis for treatment. Br J Neurosurg. 1991; 5(5):467-473.
12. Yamamoto H, Hirashima Y, Hamada H, et al. Independent predictors of recurrence of chronic subdural hematoma: results of multivariate analysis performed using a logistic regression model. J Neurosurg. 2003;98(6):1217-1221.
13. Iantosca MR, Simon RH. Chronic subdural hematoma in adult and elderly patients. Neurosurg Clin N Am. 2000;11(3):447-454.
14. Rozzelle CJ, Wofford JL, Branch CL. Predictors of hospital mortality in older patients with subdural hematoma. J Am Geriatr Soc. 1995;43(3):240-244.
15. Wintzen AR, Tijssen JG. Subdural hematoma and oral anticoagulant therapy. Arch Neurol. 1982;39(2): 69-72.
16. Ramachandran R, Hegde T. Chronic subdural hematomas: causes of morbidity and mortality. Surg Neurol. 2007;67(4):367-372.
17. Amirjamshidi A, Eftekhar B, Abouzari M, Rashidi A. The relationship between Glasgow coma/outcome scores and abnormal CT scan findings in chronic subdural hematoma. Clin Neurol Neurosurg. 2007;109(2): 152-157.
18. Lee JY, Ebel H, Ernestus RI, Klug N. Various surgical treatments of chronic subdural hematoma and outcome in 172 patients: is membranectomy necessary? Surg Neurol. 2004;61(6):523-527.
19. Gelabert-González M, Iglesias-Pais M, García-Allut A, Martínez-Rumbo R. Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005;107(3):223-229.
20. Mattle H, Kohler S, Huber P, et al. Anticoagulation-related intracranial extracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 1989;52(7):829-837.
21. Sambasivan M. An overview of chronic subdural hematoma: experience with 2300 cases. Surg Neurol. 1997;47(5):418-422.
22. Lin J, Hanigan WC, Tarantino M, Wang J. The use of recombinant activated factor VII to reverse warfarin-induced anticoagulation in patients with hemorrhages in the central nervous system: preliminary findings. J Neurosurg. 2003;98(4):737-740.
23. Freeman WD, Brott TG, Barrett KM, et al. Recombinant factor VIIa for rapid reversal of warfarin anticoagulation in acute intracranial hemorrhage. Mayo Clin Proc. 2004;79(12):1495-1500.
24. Dager WE, King JH, Regalia RC, et al. Reversal of elevated international normalized ratios and bleeding with low-dose recombinant activated factor VII in patients receiving warfarin. Pharmacotherapy. 2006;26(8): 1091-1098.
25. Denas G, Marzot F, Offelli P, et al. Effectiveness and safety of a management protocol to correct over-anticoagulation with oral vitamin K: a retrospective study of 1,043 cases. J Thromb Thrombolysis. 2008 Mar 13; [Epub ahead of print].
26. Boulis NM, Bobek MP, Schmaier A, Hoff JT. Use of factor IX complex in warfarin-related intracranial hemorrhage. Neurosurgery. 1999;45(5):1113-1118.
27. Kissela BM, Eckman MH. Cost effectiveness of recombinant factor VIIa for treatment of intracerebral hemorrhage. BMC Neurol. 2008;8:17.
28. Ernestus RI, Beldzinski P, Lanfermann H, Klug N. Chronic subdural hematoma: surgical treatment and outcome in 104 patients. Surg Neurol. 1997;48(3): 220-225.
29. Merlicco G, Pierangeli E, di Padova PL. Chronic subdural hematomas in adults: prognostic factors: analysis of 70 cases. Neurosurg Rev. 1995;18(4):247-251.
1. Pullicino P, Thompson JL. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2003;348(3): 256-257.
2. Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347(13):969-974.
3. DeSilvey DL. Clinical trials: advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Am J Geriatr Cardiol. 2005;14(2):98-99.
4. Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med. 2004;141(10):745-752.
5. Koo S, Kucher N, Nguyen PL, et al. The effect of excessive anticoagulation on mortality and morbidity in hospitalized patients with anticoagulant-related major hemorrhage. Arch Intern Med. 2004;164(14):1557-1560.
6. Mina AA, Knipfer JF, Park DY, et al. Intracranial complications of preinjury anticoagulation in trauma patients with head injury. J Trauma. 2002;53(4):668-672.
7. Pieracci FM, Eachempati SR, Shou J, et al. Degree of anticoagulation, but not warfarin use itself, predicts adverse outcomes after traumatic brain injury in elderly trauma patients. J Trauma. 2007;63(3):525-530.
8. Fanikos J, Grasso-Correnti N, Shah R, et al. Major bleeding complications in a specialized anticoagulation service. Am J Cardiol. 2005;96(4):595-598.
9. Pieracci FM, Eachempati SR, Shou J, et al. Use of long-term anticoagulation is associated with traumatic intracranial hemorrhage and subsequent mortality in elderly patients hospitalized after falls: analysis of the New York State Administrative Database. J Trauma. 2007;63(3):519-524.
10. Franko J, Kish KJ, O’Connell BG, et al. Advanced age and preinjury warfarin anticoagulation increase the risk of mortality after head trauma. J Trauma. 2006; 61(1):107-110.
11. Drapkin AJ. Chronic subdural hematoma: pathophysiological basis for treatment. Br J Neurosurg. 1991; 5(5):467-473.
12. Yamamoto H, Hirashima Y, Hamada H, et al. Independent predictors of recurrence of chronic subdural hematoma: results of multivariate analysis performed using a logistic regression model. J Neurosurg. 2003;98(6):1217-1221.
13. Iantosca MR, Simon RH. Chronic subdural hematoma in adult and elderly patients. Neurosurg Clin N Am. 2000;11(3):447-454.
14. Rozzelle CJ, Wofford JL, Branch CL. Predictors of hospital mortality in older patients with subdural hematoma. J Am Geriatr Soc. 1995;43(3):240-244.
15. Wintzen AR, Tijssen JG. Subdural hematoma and oral anticoagulant therapy. Arch Neurol. 1982;39(2): 69-72.
16. Ramachandran R, Hegde T. Chronic subdural hematomas: causes of morbidity and mortality. Surg Neurol. 2007;67(4):367-372.
17. Amirjamshidi A, Eftekhar B, Abouzari M, Rashidi A. The relationship between Glasgow coma/outcome scores and abnormal CT scan findings in chronic subdural hematoma. Clin Neurol Neurosurg. 2007;109(2): 152-157.
18. Lee JY, Ebel H, Ernestus RI, Klug N. Various surgical treatments of chronic subdural hematoma and outcome in 172 patients: is membranectomy necessary? Surg Neurol. 2004;61(6):523-527.
19. Gelabert-González M, Iglesias-Pais M, García-Allut A, Martínez-Rumbo R. Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005;107(3):223-229.
20. Mattle H, Kohler S, Huber P, et al. Anticoagulation-related intracranial extracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 1989;52(7):829-837.
21. Sambasivan M. An overview of chronic subdural hematoma: experience with 2300 cases. Surg Neurol. 1997;47(5):418-422.
22. Lin J, Hanigan WC, Tarantino M, Wang J. The use of recombinant activated factor VII to reverse warfarin-induced anticoagulation in patients with hemorrhages in the central nervous system: preliminary findings. J Neurosurg. 2003;98(4):737-740.
23. Freeman WD, Brott TG, Barrett KM, et al. Recombinant factor VIIa for rapid reversal of warfarin anticoagulation in acute intracranial hemorrhage. Mayo Clin Proc. 2004;79(12):1495-1500.
24. Dager WE, King JH, Regalia RC, et al. Reversal of elevated international normalized ratios and bleeding with low-dose recombinant activated factor VII in patients receiving warfarin. Pharmacotherapy. 2006;26(8): 1091-1098.
25. Denas G, Marzot F, Offelli P, et al. Effectiveness and safety of a management protocol to correct over-anticoagulation with oral vitamin K: a retrospective study of 1,043 cases. J Thromb Thrombolysis. 2008 Mar 13; [Epub ahead of print].
26. Boulis NM, Bobek MP, Schmaier A, Hoff JT. Use of factor IX complex in warfarin-related intracranial hemorrhage. Neurosurgery. 1999;45(5):1113-1118.
27. Kissela BM, Eckman MH. Cost effectiveness of recombinant factor VIIa for treatment of intracerebral hemorrhage. BMC Neurol. 2008;8:17.
28. Ernestus RI, Beldzinski P, Lanfermann H, Klug N. Chronic subdural hematoma: surgical treatment and outcome in 104 patients. Surg Neurol. 1997;48(3): 220-225.
29. Merlicco G, Pierangeli E, di Padova PL. Chronic subdural hematomas in adults: prognostic factors: analysis of 70 cases. Neurosurg Rev. 1995;18(4):247-251.
Man, 48, With Excruciating Leg Pain
A 48-year-old black man, on hemodialysis since August 2002, presented to his primary care provider (PCP) in July 2006 with excruciating leg pain. According to the patient, the leg pain had worsened during the previous six months and was so severe that he was barely able to walk without pain. He was a full-time night security guard and reported walking three to five miles each night.
The man was undergoing hemodialysis three times per week, necessitated by nephritic range proteinuria. He had a questionable history of diabetes but a known diagnosis of hypertension. Definitive diagnosis through kidney biopsy was not obtained because of the associated risk, the patient's obesity, and his aversion to the procedure.
The patient had recently been hospitalized with shortness of breath and fluid overload. Intensive dialysis allowed a significant drop in his dialysis target weight. He was readmitted a few days later with chills, fever, cough, and shortness of breath. He was diagnosed with bilateral pulmonary emboli. The patient said his hypercoagulation work-up was negative, but he was started on warfarin before discharge.
On current presentation, he had swollen, tender legs and multiple excoriations over the calves, explained by the patient's admitted scratching. His skin was shiny and tight. He was still taking warfarin, with an international normalized ratio of 2.1. The patient denied shortness of breath, pruritus (any more than expected with renal disease), or increased fluid.
In addition to warfarin, he was taking esomeprazole 40 mg/d, extended-release metoprolol 25 mg bid, cinacalcet 90 mg/d, sevelamer 4,000 mg and lanthanum 5,000 mg before every meal, mometasone furoate as needed, hydroxyzine 25 mg every four hours as needed, miconazole powder applied to the feet as needed, and a daily prescription multivitamin complex.
Laboratory tests included normal findings (for a dialysis patient) on the complete blood count; blood urea nitrogen, 101 mg/dL (reference range, 7 to 20 mg/dL); serum creatinine, 16.6 mg/dL (0.8 to 1.4 mg/dL); Kt/V (a measure of adequacy of dialysis), 1.37 (acceptable); calcium, 9.6 mg/dL (8.2 to 10.2 mg/dL); serum phosphorus, 5.6 mg/dL (2.4 to 4.1 mg/dL); intact parathyroid hormone, 359 ng/L (10 to 65 ng/L).
The patient's PCP prescribed oxycodone for the pain and referred him to the vascular clinic for evaluation of his legs. A lower leg duplex scan with ankle/brachial indices performed on July 18 showed significant bilateral peripheral vascular disease. Subsequent magnetic resonance angiography (MRA) showed a questionable adrenal gland mass. Abdominal CT with and without contrast yielded negative results for the adrenal mass but showed a cyst in the right kidney. Although cysts are commonly found in dialysis patients, the vascular surgeon elected to evaluate the cyst with an MRI with gadolinium; the mass was found to be hemorrhagic.
Further vascular work-up continued, including MRI with gadolinium on September 26, 2006, which revealed two-vessel runoff in the right foot and three-vessel runoff in the left foot. According to the vascular consult, there was no area to bypass. The patient was sent back to his PCP. At this point, he was taking oxycodone four times per day and continuing to work full-time as a night security guard.
The patient was then sent to neurology for evaluation. By this time, the severity of his leg pain had increased 90%, with worsening swelling and persistent shininess (see figure). The neurologist was unable to obtain electromyograms due to the severity of the patient's pain and lower extremity swelling. No definitive diagnosis could be made.
About one year later, the man's attending nephrology group received copies of the work-up that the PCP sent to the dialysis center. It was apparent that neither the patient's PCP nor the vascular, radiology, or neurology consultants had seen the FDA warning released in June 20061 regarding the use of gadolinium in patients with renal disease. What had started out as a peripheral neuropathy (either renal or diabetic in etiology) was now a full-blown case of nephrogenic systemic fibrosis (NSF).
Open biopsy performed on October 29, 2007, confirmed the presence of gadolinium in the patient's epidermis. He became the first documented case of NSF in the Washington, DC area.
Discussion
In the late 1990s, several reports of an unknown sclerosing dermopathy in patients with chronic kidney disease began to emerge. In 2000, the new entity was named nephrogenic systemic fibrosis, with a disease course demonstrating systemic involvement that affected multiple organ systems and often resulted in severe joint limitations. A Web-based reporting system for this newly described disease, created by Shawn Cowper, MD, of Yale University,2 made it possible to investigate associated epidemiologic factors.
Neither gender, race, nor age appeared relevant. However, all patients had renal disease—acute, chronic, or transient—and more than 90% of patients were dialysis dependent. Factors since recognized to confirm a diagnosis of NSF are severe renal impairment (ie, glomerular filtration rate [GFR] < 30 mL/min/1.73 m2),3 CD34+ dendritic cells found on deep biopsy,4 and the following clinical manifestations:
• Skin. Burning or itching, reddened or darkened patches; possible skin swelling, hardening, and/or tightening.
• Eyes. Yellow raised spots in the whites of the eyes.
• Bones, joints, muscles. Joint stiffness; limited range of motion in the arms, hands, legs, or feet; pain deep in the hip bone or ribs; and/or muscle weakness.3
Theories abounded on the cause of NSF. While the presence of renal disease is a requirement, dialysis did not seem to be.5 Ten percent of NSF cases are patients who have never been dialyzed, and thousands of dialysis patients never develop NSF. Neither was any temporal correlation to dialysis found: While some patients developed NSF soon after starting dialysis, many had been on dialysis for years before NSF occurred. No association was found between NSF and the type of dialysis (inpatient, outpatient, hemodialysis, or peritoneal dialysis), the filter, manufacturer, dialysate, technique, or dialysis unit.2
Authors of a retrospective study involving two large tissue repositories looked for cases of NSF before 1997, but none were found.6 If dialysis was not causing NSF, and the disease did not appear to have existed before 1997, what renal toxin had been introduced in the 1990s to explain it?
One early suspicion involved erythropoietin (EPO), used to treat anemia in patients with kidney disease. Skin changes had been reported in some patients after initiation of treatment with EPO, and the NSF patients received a significantly higher mean dose of EPO than controls received.7
Ninety percent of patients with NSF had fistula reconstruction or dialysis catheter placement, but these are common in renal disease patients.8 Forty-eight percent of patients had had liver or kidney transplants, and 12% had hypercoagulable states. Most patients with NSF had never received ACE inhibitors. Were the protective antifibrogenic properties of these agents missing?
Mystery Solved
In a triumph for the Internet and its capacity to disseminate information around the world, a breakthrough came in 2006 from a small town in Austria. Grobner9 described nine patients who had received gadodiamide (Omniscan™)–enhanced MRA, five of whom developed NSF. Upon release of this report, researchers reexamined the original cases and detected a clear correlation between gadolinium and NSF. Because the contrast dose given for MRA can be as much as three times that required for routine MRI, the absence of NSF cases before 1997 suddenly made sense.
In May 2006, researchers for the Danish Medicines Agency reported 13 cases of NSF in patients injected with gadodiamide.10 Within months, 28 biopsy-proven cases were reported in St. Louis, six in Texas, and 13 at the University of Wisconsin—all involving patients exposed to gadolinium.11-13 It was apparent that NSF was iatrogenic and could be controlled.
What We Have Learned Since
In subsequent research, it has been found that more than 90% of reported cases of NSF occurred following exposure to gadodiamide—although gadodiamide accounts for only 15% of all gadolinium injections worldwide,14 and this number is decreasing as more cases are reported. The correlation between gadodiamide and NSF is so strong that its manufacturer, GE Healthcare, sent practitioners a letter in June 2006 warning of NSF as an adverse effect of gadolinium exposure.15 Two days later, the FDA issued an advisory on gadolinium-enhanced imaging procedures, recommending prompt hemodialysis after gadolinium exposure and reminding radiologists and nephrologists that gadolinium is not FDA approved for MRA.1
Although the 44% incidence rate of NSF reported by Grobner9 has never been replicated, a retrospective review of all known NSF cases affirmed that more than 90% of patients had been exposed to gadolinium.14 Two 2007 reports published in the Journal of the American Academy of Dermatology demonstrated that gadolinium was detectable in the tissues of patients with NSF.16,17
In Europe, in response to the May 2006 report from the Danish Medicines Agency,10 the European Society of Urogenital Radiology revised its guidelines with a directive that gadodiamide not be administered in any patients who had reduced kidney function or were undergoing dialysis.18 Shortly thereafter, the European Committee for Medicinal Products for Human Use issued a contraindication for gadodiamide use in patients with severe renal impairment and advised that these patients not be given gadolinium unless there was no other choice.19 A contraindication was also issued for gadodiamide use in patients with previous or anticipated liver transplantation.
The American College of Radiology guidelines published in 200720 stated that patients with any level of renal disease should not receive gadodiamide.
In March 2007, GE Healthcare published a paper on NSF, reiterating the safety of gadodiamide while acknowledging that 120 more cases had been reported to them ("usually associated with exposure at high doses").21 The FDA upholds an alert regarding use of all gadolinium-based contrast agents for patients with acute or chronic severe renal insufficiency,3 while stopping short of a ban on gadodiamide in such patients.
How Common Is NSF?
In a 2007 study conducted at the University of Wisconsin, Sadowski et al13 reported 13 cases of gadolinium-induced NSF, 11 involving patients with a GFR below 30 mL/min/1.73 m2 but two with a GFR between 30 and 60 mL/min/1.73 m2 (ie, with renal insufficiency, although the authors noted that renal insufficiency was acute in these two patients). The incidence of NSF was 4.6% among hospitalized patients with a GFR be-low 60 mL/min/1.73 m2 who underwent gadolinium-enhanced MRI at the university hospital's radiology department. A reexamination of the charts of the patients with a GFR between 30 and 60 mL/min/1.73 m2 revealed that these patients had levels below 30 mL/min/1.73 m2 when their gadolinium exposure took place.
In an outpatient population–based calculation performed by Deo et al,22 a 2.4% chance of NSF was determined for each gadolinium exposure. Incidence of NSF was calculated at 4.3 cases per 1,000 patient-years in this population, making NSF as common as contrast-induced nephropathy. Nearly 5% of patients with NSF have an exceedingly rapid and fulminant disease course that may result in death. NSF, of itself, is not a cause of death but may contribute to death by restricting effective ventilation or by restricting mobility to the point of causing an accidental fall that may be further exacerbated by fractures and clotting complications. NSF survivors may experience disabling systemic symptoms. Full recovery occurs only in patients who recover renal function, either naturally or by kidney transplantation.4
Why Is NSF More Common With Gadodiamide?
As of June 2008, five gadolinium-based contrast agents were FDA approved for use with MRI (none with MRA)3: gadobenate (MultiHance®), gadodiamide (Omniscan), gadopentetate (Magnevist®), gadoteridol (ProHance®), and gadoversetamide (Opti-MARK®). More than 90% of NSF cases are associated with gadodiamide. Because this agent is the least stable thermodynamically, it may be more likely than the others to transmetallate.14 All gadolinium chelates are excreted by the kidney, and the decreased renal clearances associated with renal impairment may expose patients to prolonged gadolinium transmetallation, allowing the agent to accumulate in bone and other tissue.
Gadoterate (Dotarem®), a cyclic gadolinium-based agent that is available in Europe but not the US, is considered more stable than other agents. It has been suggested that such agents may be safer choices for patients with decreased renal function.14,19
Strategies to Prevent NSF
In the US and Europe, only a physician who has consulted with a radiologist can write an order for gadolinium use in a patient with a GFR below 30 mL/min/1.73 m2.18,20 European guidelines do not allow use of gadodiamide in such patients.
Although the actual population-based occurrence of NSF is low, the nature of the disease calls for an effort to limit vulnerable patients' exposure to gadolinium (see box). Outside of withholding imaging procedures, the only currently known strategies to reduce the incidence of NSF are to use a more stable, nonchelating gadolinium14 and to remove the gadolinium as soon as possible.3,24
It has been recommended that patients with renal disease who are presently undergoing dialysis be dialyzed within two to three hours of gadolinium exposure, then again within 24 and 48 hours, provided it is clinically safe.20,24 This has been shown to remove 99% of the gadolinium.23
Since peritoneal dialysis clears gadolinium poorly, hemodialysis is recommended for peritoneal dialysis patients after gadolinium exposure, following the regimen outlined above.20
No consensus has been reached regarding the patient with a GFR between 30 and 60 mL/min/1.73 m2, nor for the patient with a lower GFR and no access for dialysis to be administered. Placement of a catheter for two days' dialysis incurs both surgical and renal risks for these patients.8
Patient Outcome
The only known cure for NSF is kidney transplantation, which is associated with a complete cure rate of 40%.4,25 Nevertheless, while this manuscript was in preparation, the patient presented in this case study underwent kidney transplantation. On day 8 postsurgery, he was no longer taking oxycodone, his skin condition was clearing up, and he was feeling considerably better. His health care providers hope for further regression from his disease.
Conclusion
NSF is just one example of iatrogenic conditions that can occur in any hospital, office, or clinic. Health care providers cannot be too vigilant in keeping abreast of warnings from the FDA and other agencies. In this case, several clinicians overlooked a recent, urgent public health advisory, with significant consequences.
1. US Food and Drug Administration. Public health advisory: gadolinium-containing contrast agents for magnetic resonance imaging (MRI): Omniscan, OptiMARK, Magnevist, ProHance, and MultiHance. www.fda.gov/cder/drug/advisory/gadolinium_agents.htm. Accessed July 24, 2008.
2. Cowper SE, Su L, Bhawan J, et al. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23(5):383-393.
3. US Food and Drug Administration. Information for healthcare professionals: gadolinium-based contrast agents for magnetic resonance imaging (marketed as Magnevist, MultiHance, Omniscan, OptiMARK, ProHance). Last updated June 4, 2008. www.fda.gov/cder/drug/InfoSheets/HCP/gcca_200705.htm. Accessed July 24, 2008.
4. International Center for Nephrogenic Fibrosing Dermopathy Research. www.icnfdr.org. Accessed July 24, 2008.
5. DeHoratius DM, Cowper SE. Nephrogenic systemic fibrosis: an emerging threat among renal patients. Semin Dial. 2006;19(3):191-194.
6. Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol. 2006;18(6):614-617.
7. Swaminathan S, Ahmed I, McCarthy JT, et al. Nephrogenic fibrosing dermopathy and high-dose erythropoietin therapy. Ann Intern Med. 2006;145(3):234-235.
8. Miskulin D, Gul A, Rudnick MR, Cowper SE. Nephrogenic systemic fibrosis/nephrogenic fibrosing dermopathy in advanced renal failure. www.uptodate.com/patients/content/topic.do?topicKey=dialysis/48700. Accessed July 24, 2008.
9. Grobner T. Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
10. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
11. Centers for Disease Control and Prevention. Nephrogenic fibrosing dermopathy associated with exposure to gadolinium-containing contrast agents—St. Louis, Missouri, 2002-2006. MMWR Morb Mortal Wkly Rep. 2007;56(7):137-141.
12. Khurana A, Runge VM, Narayanan M, et al. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (Omniscan). Invest Radiol. 2007;42(2):139-145.
13. Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243(1):148-157.
14. Morcos SK. Nephrogenic systemic fibrosis following the administration of extracellular gadolinium based contrast agents: is the stability of the contrast agent molecule an important factor in the pathogenesis of this condition? Br J Radiol. 2007;80(950):73-76.
15. GE Healthcare. Omniscan safety update. http://md.gehealthcare.com/omniscan/safety/index.html. Accessed July 24, 2008.
16. Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol. 2007;56(1):27-30.
17. High WA, Ayers RA, Chandler J, et al. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;56(1):21-26.
18. Thomsen H; European Society of Urogenital Radiology. European Society of Urogenital Radiology guidelines on contrast media application. Curr Opin Urol. 2007;17(1):70-76.
19. Bongartz G. Imaging in the time of NFD/NSF: do we have to change our routines concerning renal insufficiency? MAGMA. 2007;20(2):57-62.
20. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
21. GE Healthcare Paper on Nephrogenic Systemic Fibrosis (March 2007). http://md.gehealthcare.com/omniscan/GE% 20Healthcare%20Paper%20On%20Nephrogenic%20 Systemic%20Fibrosis.pdf. Accessed July 24, 2008.
22. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267.
23. Okada S, Katagiri K, Kumazaki T, Yokoyama H. Safety of gadolinium contrast agent in hemodialysis patients. Acta Radiol. 2001;42(3):339-341.
24. Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242(3):647-649.
25. Cowper SE. Nephrogenic systemic fibrosis: the nosological and conceptual evolution of nephrogenic fibrosing dermopathy. Am J Kidney Dis. 2005;46(4):763-765.
A 48-year-old black man, on hemodialysis since August 2002, presented to his primary care provider (PCP) in July 2006 with excruciating leg pain. According to the patient, the leg pain had worsened during the previous six months and was so severe that he was barely able to walk without pain. He was a full-time night security guard and reported walking three to five miles each night.
The man was undergoing hemodialysis three times per week, necessitated by nephritic range proteinuria. He had a questionable history of diabetes but a known diagnosis of hypertension. Definitive diagnosis through kidney biopsy was not obtained because of the associated risk, the patient's obesity, and his aversion to the procedure.
The patient had recently been hospitalized with shortness of breath and fluid overload. Intensive dialysis allowed a significant drop in his dialysis target weight. He was readmitted a few days later with chills, fever, cough, and shortness of breath. He was diagnosed with bilateral pulmonary emboli. The patient said his hypercoagulation work-up was negative, but he was started on warfarin before discharge.
On current presentation, he had swollen, tender legs and multiple excoriations over the calves, explained by the patient's admitted scratching. His skin was shiny and tight. He was still taking warfarin, with an international normalized ratio of 2.1. The patient denied shortness of breath, pruritus (any more than expected with renal disease), or increased fluid.
In addition to warfarin, he was taking esomeprazole 40 mg/d, extended-release metoprolol 25 mg bid, cinacalcet 90 mg/d, sevelamer 4,000 mg and lanthanum 5,000 mg before every meal, mometasone furoate as needed, hydroxyzine 25 mg every four hours as needed, miconazole powder applied to the feet as needed, and a daily prescription multivitamin complex.
Laboratory tests included normal findings (for a dialysis patient) on the complete blood count; blood urea nitrogen, 101 mg/dL (reference range, 7 to 20 mg/dL); serum creatinine, 16.6 mg/dL (0.8 to 1.4 mg/dL); Kt/V (a measure of adequacy of dialysis), 1.37 (acceptable); calcium, 9.6 mg/dL (8.2 to 10.2 mg/dL); serum phosphorus, 5.6 mg/dL (2.4 to 4.1 mg/dL); intact parathyroid hormone, 359 ng/L (10 to 65 ng/L).
The patient's PCP prescribed oxycodone for the pain and referred him to the vascular clinic for evaluation of his legs. A lower leg duplex scan with ankle/brachial indices performed on July 18 showed significant bilateral peripheral vascular disease. Subsequent magnetic resonance angiography (MRA) showed a questionable adrenal gland mass. Abdominal CT with and without contrast yielded negative results for the adrenal mass but showed a cyst in the right kidney. Although cysts are commonly found in dialysis patients, the vascular surgeon elected to evaluate the cyst with an MRI with gadolinium; the mass was found to be hemorrhagic.
Further vascular work-up continued, including MRI with gadolinium on September 26, 2006, which revealed two-vessel runoff in the right foot and three-vessel runoff in the left foot. According to the vascular consult, there was no area to bypass. The patient was sent back to his PCP. At this point, he was taking oxycodone four times per day and continuing to work full-time as a night security guard.
The patient was then sent to neurology for evaluation. By this time, the severity of his leg pain had increased 90%, with worsening swelling and persistent shininess (see figure). The neurologist was unable to obtain electromyograms due to the severity of the patient's pain and lower extremity swelling. No definitive diagnosis could be made.
About one year later, the man's attending nephrology group received copies of the work-up that the PCP sent to the dialysis center. It was apparent that neither the patient's PCP nor the vascular, radiology, or neurology consultants had seen the FDA warning released in June 20061 regarding the use of gadolinium in patients with renal disease. What had started out as a peripheral neuropathy (either renal or diabetic in etiology) was now a full-blown case of nephrogenic systemic fibrosis (NSF).
Open biopsy performed on October 29, 2007, confirmed the presence of gadolinium in the patient's epidermis. He became the first documented case of NSF in the Washington, DC area.
Discussion
In the late 1990s, several reports of an unknown sclerosing dermopathy in patients with chronic kidney disease began to emerge. In 2000, the new entity was named nephrogenic systemic fibrosis, with a disease course demonstrating systemic involvement that affected multiple organ systems and often resulted in severe joint limitations. A Web-based reporting system for this newly described disease, created by Shawn Cowper, MD, of Yale University,2 made it possible to investigate associated epidemiologic factors.
Neither gender, race, nor age appeared relevant. However, all patients had renal disease—acute, chronic, or transient—and more than 90% of patients were dialysis dependent. Factors since recognized to confirm a diagnosis of NSF are severe renal impairment (ie, glomerular filtration rate [GFR] < 30 mL/min/1.73 m2),3 CD34+ dendritic cells found on deep biopsy,4 and the following clinical manifestations:
• Skin. Burning or itching, reddened or darkened patches; possible skin swelling, hardening, and/or tightening.
• Eyes. Yellow raised spots in the whites of the eyes.
• Bones, joints, muscles. Joint stiffness; limited range of motion in the arms, hands, legs, or feet; pain deep in the hip bone or ribs; and/or muscle weakness.3
Theories abounded on the cause of NSF. While the presence of renal disease is a requirement, dialysis did not seem to be.5 Ten percent of NSF cases are patients who have never been dialyzed, and thousands of dialysis patients never develop NSF. Neither was any temporal correlation to dialysis found: While some patients developed NSF soon after starting dialysis, many had been on dialysis for years before NSF occurred. No association was found between NSF and the type of dialysis (inpatient, outpatient, hemodialysis, or peritoneal dialysis), the filter, manufacturer, dialysate, technique, or dialysis unit.2
Authors of a retrospective study involving two large tissue repositories looked for cases of NSF before 1997, but none were found.6 If dialysis was not causing NSF, and the disease did not appear to have existed before 1997, what renal toxin had been introduced in the 1990s to explain it?
One early suspicion involved erythropoietin (EPO), used to treat anemia in patients with kidney disease. Skin changes had been reported in some patients after initiation of treatment with EPO, and the NSF patients received a significantly higher mean dose of EPO than controls received.7
Ninety percent of patients with NSF had fistula reconstruction or dialysis catheter placement, but these are common in renal disease patients.8 Forty-eight percent of patients had had liver or kidney transplants, and 12% had hypercoagulable states. Most patients with NSF had never received ACE inhibitors. Were the protective antifibrogenic properties of these agents missing?
Mystery Solved
In a triumph for the Internet and its capacity to disseminate information around the world, a breakthrough came in 2006 from a small town in Austria. Grobner9 described nine patients who had received gadodiamide (Omniscan™)–enhanced MRA, five of whom developed NSF. Upon release of this report, researchers reexamined the original cases and detected a clear correlation between gadolinium and NSF. Because the contrast dose given for MRA can be as much as three times that required for routine MRI, the absence of NSF cases before 1997 suddenly made sense.
In May 2006, researchers for the Danish Medicines Agency reported 13 cases of NSF in patients injected with gadodiamide.10 Within months, 28 biopsy-proven cases were reported in St. Louis, six in Texas, and 13 at the University of Wisconsin—all involving patients exposed to gadolinium.11-13 It was apparent that NSF was iatrogenic and could be controlled.
What We Have Learned Since
In subsequent research, it has been found that more than 90% of reported cases of NSF occurred following exposure to gadodiamide—although gadodiamide accounts for only 15% of all gadolinium injections worldwide,14 and this number is decreasing as more cases are reported. The correlation between gadodiamide and NSF is so strong that its manufacturer, GE Healthcare, sent practitioners a letter in June 2006 warning of NSF as an adverse effect of gadolinium exposure.15 Two days later, the FDA issued an advisory on gadolinium-enhanced imaging procedures, recommending prompt hemodialysis after gadolinium exposure and reminding radiologists and nephrologists that gadolinium is not FDA approved for MRA.1
Although the 44% incidence rate of NSF reported by Grobner9 has never been replicated, a retrospective review of all known NSF cases affirmed that more than 90% of patients had been exposed to gadolinium.14 Two 2007 reports published in the Journal of the American Academy of Dermatology demonstrated that gadolinium was detectable in the tissues of patients with NSF.16,17
In Europe, in response to the May 2006 report from the Danish Medicines Agency,10 the European Society of Urogenital Radiology revised its guidelines with a directive that gadodiamide not be administered in any patients who had reduced kidney function or were undergoing dialysis.18 Shortly thereafter, the European Committee for Medicinal Products for Human Use issued a contraindication for gadodiamide use in patients with severe renal impairment and advised that these patients not be given gadolinium unless there was no other choice.19 A contraindication was also issued for gadodiamide use in patients with previous or anticipated liver transplantation.
The American College of Radiology guidelines published in 200720 stated that patients with any level of renal disease should not receive gadodiamide.
In March 2007, GE Healthcare published a paper on NSF, reiterating the safety of gadodiamide while acknowledging that 120 more cases had been reported to them ("usually associated with exposure at high doses").21 The FDA upholds an alert regarding use of all gadolinium-based contrast agents for patients with acute or chronic severe renal insufficiency,3 while stopping short of a ban on gadodiamide in such patients.
How Common Is NSF?
In a 2007 study conducted at the University of Wisconsin, Sadowski et al13 reported 13 cases of gadolinium-induced NSF, 11 involving patients with a GFR below 30 mL/min/1.73 m2 but two with a GFR between 30 and 60 mL/min/1.73 m2 (ie, with renal insufficiency, although the authors noted that renal insufficiency was acute in these two patients). The incidence of NSF was 4.6% among hospitalized patients with a GFR be-low 60 mL/min/1.73 m2 who underwent gadolinium-enhanced MRI at the university hospital's radiology department. A reexamination of the charts of the patients with a GFR between 30 and 60 mL/min/1.73 m2 revealed that these patients had levels below 30 mL/min/1.73 m2 when their gadolinium exposure took place.
In an outpatient population–based calculation performed by Deo et al,22 a 2.4% chance of NSF was determined for each gadolinium exposure. Incidence of NSF was calculated at 4.3 cases per 1,000 patient-years in this population, making NSF as common as contrast-induced nephropathy. Nearly 5% of patients with NSF have an exceedingly rapid and fulminant disease course that may result in death. NSF, of itself, is not a cause of death but may contribute to death by restricting effective ventilation or by restricting mobility to the point of causing an accidental fall that may be further exacerbated by fractures and clotting complications. NSF survivors may experience disabling systemic symptoms. Full recovery occurs only in patients who recover renal function, either naturally or by kidney transplantation.4
Why Is NSF More Common With Gadodiamide?
As of June 2008, five gadolinium-based contrast agents were FDA approved for use with MRI (none with MRA)3: gadobenate (MultiHance®), gadodiamide (Omniscan), gadopentetate (Magnevist®), gadoteridol (ProHance®), and gadoversetamide (Opti-MARK®). More than 90% of NSF cases are associated with gadodiamide. Because this agent is the least stable thermodynamically, it may be more likely than the others to transmetallate.14 All gadolinium chelates are excreted by the kidney, and the decreased renal clearances associated with renal impairment may expose patients to prolonged gadolinium transmetallation, allowing the agent to accumulate in bone and other tissue.
Gadoterate (Dotarem®), a cyclic gadolinium-based agent that is available in Europe but not the US, is considered more stable than other agents. It has been suggested that such agents may be safer choices for patients with decreased renal function.14,19
Strategies to Prevent NSF
In the US and Europe, only a physician who has consulted with a radiologist can write an order for gadolinium use in a patient with a GFR below 30 mL/min/1.73 m2.18,20 European guidelines do not allow use of gadodiamide in such patients.
Although the actual population-based occurrence of NSF is low, the nature of the disease calls for an effort to limit vulnerable patients' exposure to gadolinium (see box). Outside of withholding imaging procedures, the only currently known strategies to reduce the incidence of NSF are to use a more stable, nonchelating gadolinium14 and to remove the gadolinium as soon as possible.3,24
It has been recommended that patients with renal disease who are presently undergoing dialysis be dialyzed within two to three hours of gadolinium exposure, then again within 24 and 48 hours, provided it is clinically safe.20,24 This has been shown to remove 99% of the gadolinium.23
Since peritoneal dialysis clears gadolinium poorly, hemodialysis is recommended for peritoneal dialysis patients after gadolinium exposure, following the regimen outlined above.20
No consensus has been reached regarding the patient with a GFR between 30 and 60 mL/min/1.73 m2, nor for the patient with a lower GFR and no access for dialysis to be administered. Placement of a catheter for two days' dialysis incurs both surgical and renal risks for these patients.8
Patient Outcome
The only known cure for NSF is kidney transplantation, which is associated with a complete cure rate of 40%.4,25 Nevertheless, while this manuscript was in preparation, the patient presented in this case study underwent kidney transplantation. On day 8 postsurgery, he was no longer taking oxycodone, his skin condition was clearing up, and he was feeling considerably better. His health care providers hope for further regression from his disease.
Conclusion
NSF is just one example of iatrogenic conditions that can occur in any hospital, office, or clinic. Health care providers cannot be too vigilant in keeping abreast of warnings from the FDA and other agencies. In this case, several clinicians overlooked a recent, urgent public health advisory, with significant consequences.
A 48-year-old black man, on hemodialysis since August 2002, presented to his primary care provider (PCP) in July 2006 with excruciating leg pain. According to the patient, the leg pain had worsened during the previous six months and was so severe that he was barely able to walk without pain. He was a full-time night security guard and reported walking three to five miles each night.
The man was undergoing hemodialysis three times per week, necessitated by nephritic range proteinuria. He had a questionable history of diabetes but a known diagnosis of hypertension. Definitive diagnosis through kidney biopsy was not obtained because of the associated risk, the patient's obesity, and his aversion to the procedure.
The patient had recently been hospitalized with shortness of breath and fluid overload. Intensive dialysis allowed a significant drop in his dialysis target weight. He was readmitted a few days later with chills, fever, cough, and shortness of breath. He was diagnosed with bilateral pulmonary emboli. The patient said his hypercoagulation work-up was negative, but he was started on warfarin before discharge.
On current presentation, he had swollen, tender legs and multiple excoriations over the calves, explained by the patient's admitted scratching. His skin was shiny and tight. He was still taking warfarin, with an international normalized ratio of 2.1. The patient denied shortness of breath, pruritus (any more than expected with renal disease), or increased fluid.
In addition to warfarin, he was taking esomeprazole 40 mg/d, extended-release metoprolol 25 mg bid, cinacalcet 90 mg/d, sevelamer 4,000 mg and lanthanum 5,000 mg before every meal, mometasone furoate as needed, hydroxyzine 25 mg every four hours as needed, miconazole powder applied to the feet as needed, and a daily prescription multivitamin complex.
Laboratory tests included normal findings (for a dialysis patient) on the complete blood count; blood urea nitrogen, 101 mg/dL (reference range, 7 to 20 mg/dL); serum creatinine, 16.6 mg/dL (0.8 to 1.4 mg/dL); Kt/V (a measure of adequacy of dialysis), 1.37 (acceptable); calcium, 9.6 mg/dL (8.2 to 10.2 mg/dL); serum phosphorus, 5.6 mg/dL (2.4 to 4.1 mg/dL); intact parathyroid hormone, 359 ng/L (10 to 65 ng/L).
The patient's PCP prescribed oxycodone for the pain and referred him to the vascular clinic for evaluation of his legs. A lower leg duplex scan with ankle/brachial indices performed on July 18 showed significant bilateral peripheral vascular disease. Subsequent magnetic resonance angiography (MRA) showed a questionable adrenal gland mass. Abdominal CT with and without contrast yielded negative results for the adrenal mass but showed a cyst in the right kidney. Although cysts are commonly found in dialysis patients, the vascular surgeon elected to evaluate the cyst with an MRI with gadolinium; the mass was found to be hemorrhagic.
Further vascular work-up continued, including MRI with gadolinium on September 26, 2006, which revealed two-vessel runoff in the right foot and three-vessel runoff in the left foot. According to the vascular consult, there was no area to bypass. The patient was sent back to his PCP. At this point, he was taking oxycodone four times per day and continuing to work full-time as a night security guard.
The patient was then sent to neurology for evaluation. By this time, the severity of his leg pain had increased 90%, with worsening swelling and persistent shininess (see figure). The neurologist was unable to obtain electromyograms due to the severity of the patient's pain and lower extremity swelling. No definitive diagnosis could be made.
About one year later, the man's attending nephrology group received copies of the work-up that the PCP sent to the dialysis center. It was apparent that neither the patient's PCP nor the vascular, radiology, or neurology consultants had seen the FDA warning released in June 20061 regarding the use of gadolinium in patients with renal disease. What had started out as a peripheral neuropathy (either renal or diabetic in etiology) was now a full-blown case of nephrogenic systemic fibrosis (NSF).
Open biopsy performed on October 29, 2007, confirmed the presence of gadolinium in the patient's epidermis. He became the first documented case of NSF in the Washington, DC area.
Discussion
In the late 1990s, several reports of an unknown sclerosing dermopathy in patients with chronic kidney disease began to emerge. In 2000, the new entity was named nephrogenic systemic fibrosis, with a disease course demonstrating systemic involvement that affected multiple organ systems and often resulted in severe joint limitations. A Web-based reporting system for this newly described disease, created by Shawn Cowper, MD, of Yale University,2 made it possible to investigate associated epidemiologic factors.
Neither gender, race, nor age appeared relevant. However, all patients had renal disease—acute, chronic, or transient—and more than 90% of patients were dialysis dependent. Factors since recognized to confirm a diagnosis of NSF are severe renal impairment (ie, glomerular filtration rate [GFR] < 30 mL/min/1.73 m2),3 CD34+ dendritic cells found on deep biopsy,4 and the following clinical manifestations:
• Skin. Burning or itching, reddened or darkened patches; possible skin swelling, hardening, and/or tightening.
• Eyes. Yellow raised spots in the whites of the eyes.
• Bones, joints, muscles. Joint stiffness; limited range of motion in the arms, hands, legs, or feet; pain deep in the hip bone or ribs; and/or muscle weakness.3
Theories abounded on the cause of NSF. While the presence of renal disease is a requirement, dialysis did not seem to be.5 Ten percent of NSF cases are patients who have never been dialyzed, and thousands of dialysis patients never develop NSF. Neither was any temporal correlation to dialysis found: While some patients developed NSF soon after starting dialysis, many had been on dialysis for years before NSF occurred. No association was found between NSF and the type of dialysis (inpatient, outpatient, hemodialysis, or peritoneal dialysis), the filter, manufacturer, dialysate, technique, or dialysis unit.2
Authors of a retrospective study involving two large tissue repositories looked for cases of NSF before 1997, but none were found.6 If dialysis was not causing NSF, and the disease did not appear to have existed before 1997, what renal toxin had been introduced in the 1990s to explain it?
One early suspicion involved erythropoietin (EPO), used to treat anemia in patients with kidney disease. Skin changes had been reported in some patients after initiation of treatment with EPO, and the NSF patients received a significantly higher mean dose of EPO than controls received.7
Ninety percent of patients with NSF had fistula reconstruction or dialysis catheter placement, but these are common in renal disease patients.8 Forty-eight percent of patients had had liver or kidney transplants, and 12% had hypercoagulable states. Most patients with NSF had never received ACE inhibitors. Were the protective antifibrogenic properties of these agents missing?
Mystery Solved
In a triumph for the Internet and its capacity to disseminate information around the world, a breakthrough came in 2006 from a small town in Austria. Grobner9 described nine patients who had received gadodiamide (Omniscan™)–enhanced MRA, five of whom developed NSF. Upon release of this report, researchers reexamined the original cases and detected a clear correlation between gadolinium and NSF. Because the contrast dose given for MRA can be as much as three times that required for routine MRI, the absence of NSF cases before 1997 suddenly made sense.
In May 2006, researchers for the Danish Medicines Agency reported 13 cases of NSF in patients injected with gadodiamide.10 Within months, 28 biopsy-proven cases were reported in St. Louis, six in Texas, and 13 at the University of Wisconsin—all involving patients exposed to gadolinium.11-13 It was apparent that NSF was iatrogenic and could be controlled.
What We Have Learned Since
In subsequent research, it has been found that more than 90% of reported cases of NSF occurred following exposure to gadodiamide—although gadodiamide accounts for only 15% of all gadolinium injections worldwide,14 and this number is decreasing as more cases are reported. The correlation between gadodiamide and NSF is so strong that its manufacturer, GE Healthcare, sent practitioners a letter in June 2006 warning of NSF as an adverse effect of gadolinium exposure.15 Two days later, the FDA issued an advisory on gadolinium-enhanced imaging procedures, recommending prompt hemodialysis after gadolinium exposure and reminding radiologists and nephrologists that gadolinium is not FDA approved for MRA.1
Although the 44% incidence rate of NSF reported by Grobner9 has never been replicated, a retrospective review of all known NSF cases affirmed that more than 90% of patients had been exposed to gadolinium.14 Two 2007 reports published in the Journal of the American Academy of Dermatology demonstrated that gadolinium was detectable in the tissues of patients with NSF.16,17
In Europe, in response to the May 2006 report from the Danish Medicines Agency,10 the European Society of Urogenital Radiology revised its guidelines with a directive that gadodiamide not be administered in any patients who had reduced kidney function or were undergoing dialysis.18 Shortly thereafter, the European Committee for Medicinal Products for Human Use issued a contraindication for gadodiamide use in patients with severe renal impairment and advised that these patients not be given gadolinium unless there was no other choice.19 A contraindication was also issued for gadodiamide use in patients with previous or anticipated liver transplantation.
The American College of Radiology guidelines published in 200720 stated that patients with any level of renal disease should not receive gadodiamide.
In March 2007, GE Healthcare published a paper on NSF, reiterating the safety of gadodiamide while acknowledging that 120 more cases had been reported to them ("usually associated with exposure at high doses").21 The FDA upholds an alert regarding use of all gadolinium-based contrast agents for patients with acute or chronic severe renal insufficiency,3 while stopping short of a ban on gadodiamide in such patients.
How Common Is NSF?
In a 2007 study conducted at the University of Wisconsin, Sadowski et al13 reported 13 cases of gadolinium-induced NSF, 11 involving patients with a GFR below 30 mL/min/1.73 m2 but two with a GFR between 30 and 60 mL/min/1.73 m2 (ie, with renal insufficiency, although the authors noted that renal insufficiency was acute in these two patients). The incidence of NSF was 4.6% among hospitalized patients with a GFR be-low 60 mL/min/1.73 m2 who underwent gadolinium-enhanced MRI at the university hospital's radiology department. A reexamination of the charts of the patients with a GFR between 30 and 60 mL/min/1.73 m2 revealed that these patients had levels below 30 mL/min/1.73 m2 when their gadolinium exposure took place.
In an outpatient population–based calculation performed by Deo et al,22 a 2.4% chance of NSF was determined for each gadolinium exposure. Incidence of NSF was calculated at 4.3 cases per 1,000 patient-years in this population, making NSF as common as contrast-induced nephropathy. Nearly 5% of patients with NSF have an exceedingly rapid and fulminant disease course that may result in death. NSF, of itself, is not a cause of death but may contribute to death by restricting effective ventilation or by restricting mobility to the point of causing an accidental fall that may be further exacerbated by fractures and clotting complications. NSF survivors may experience disabling systemic symptoms. Full recovery occurs only in patients who recover renal function, either naturally or by kidney transplantation.4
Why Is NSF More Common With Gadodiamide?
As of June 2008, five gadolinium-based contrast agents were FDA approved for use with MRI (none with MRA)3: gadobenate (MultiHance®), gadodiamide (Omniscan), gadopentetate (Magnevist®), gadoteridol (ProHance®), and gadoversetamide (Opti-MARK®). More than 90% of NSF cases are associated with gadodiamide. Because this agent is the least stable thermodynamically, it may be more likely than the others to transmetallate.14 All gadolinium chelates are excreted by the kidney, and the decreased renal clearances associated with renal impairment may expose patients to prolonged gadolinium transmetallation, allowing the agent to accumulate in bone and other tissue.
Gadoterate (Dotarem®), a cyclic gadolinium-based agent that is available in Europe but not the US, is considered more stable than other agents. It has been suggested that such agents may be safer choices for patients with decreased renal function.14,19
Strategies to Prevent NSF
In the US and Europe, only a physician who has consulted with a radiologist can write an order for gadolinium use in a patient with a GFR below 30 mL/min/1.73 m2.18,20 European guidelines do not allow use of gadodiamide in such patients.
Although the actual population-based occurrence of NSF is low, the nature of the disease calls for an effort to limit vulnerable patients' exposure to gadolinium (see box). Outside of withholding imaging procedures, the only currently known strategies to reduce the incidence of NSF are to use a more stable, nonchelating gadolinium14 and to remove the gadolinium as soon as possible.3,24
It has been recommended that patients with renal disease who are presently undergoing dialysis be dialyzed within two to three hours of gadolinium exposure, then again within 24 and 48 hours, provided it is clinically safe.20,24 This has been shown to remove 99% of the gadolinium.23
Since peritoneal dialysis clears gadolinium poorly, hemodialysis is recommended for peritoneal dialysis patients after gadolinium exposure, following the regimen outlined above.20
No consensus has been reached regarding the patient with a GFR between 30 and 60 mL/min/1.73 m2, nor for the patient with a lower GFR and no access for dialysis to be administered. Placement of a catheter for two days' dialysis incurs both surgical and renal risks for these patients.8
Patient Outcome
The only known cure for NSF is kidney transplantation, which is associated with a complete cure rate of 40%.4,25 Nevertheless, while this manuscript was in preparation, the patient presented in this case study underwent kidney transplantation. On day 8 postsurgery, he was no longer taking oxycodone, his skin condition was clearing up, and he was feeling considerably better. His health care providers hope for further regression from his disease.
Conclusion
NSF is just one example of iatrogenic conditions that can occur in any hospital, office, or clinic. Health care providers cannot be too vigilant in keeping abreast of warnings from the FDA and other agencies. In this case, several clinicians overlooked a recent, urgent public health advisory, with significant consequences.
1. US Food and Drug Administration. Public health advisory: gadolinium-containing contrast agents for magnetic resonance imaging (MRI): Omniscan, OptiMARK, Magnevist, ProHance, and MultiHance. www.fda.gov/cder/drug/advisory/gadolinium_agents.htm. Accessed July 24, 2008.
2. Cowper SE, Su L, Bhawan J, et al. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23(5):383-393.
3. US Food and Drug Administration. Information for healthcare professionals: gadolinium-based contrast agents for magnetic resonance imaging (marketed as Magnevist, MultiHance, Omniscan, OptiMARK, ProHance). Last updated June 4, 2008. www.fda.gov/cder/drug/InfoSheets/HCP/gcca_200705.htm. Accessed July 24, 2008.
4. International Center for Nephrogenic Fibrosing Dermopathy Research. www.icnfdr.org. Accessed July 24, 2008.
5. DeHoratius DM, Cowper SE. Nephrogenic systemic fibrosis: an emerging threat among renal patients. Semin Dial. 2006;19(3):191-194.
6. Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol. 2006;18(6):614-617.
7. Swaminathan S, Ahmed I, McCarthy JT, et al. Nephrogenic fibrosing dermopathy and high-dose erythropoietin therapy. Ann Intern Med. 2006;145(3):234-235.
8. Miskulin D, Gul A, Rudnick MR, Cowper SE. Nephrogenic systemic fibrosis/nephrogenic fibrosing dermopathy in advanced renal failure. www.uptodate.com/patients/content/topic.do?topicKey=dialysis/48700. Accessed July 24, 2008.
9. Grobner T. Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
10. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
11. Centers for Disease Control and Prevention. Nephrogenic fibrosing dermopathy associated with exposure to gadolinium-containing contrast agents—St. Louis, Missouri, 2002-2006. MMWR Morb Mortal Wkly Rep. 2007;56(7):137-141.
12. Khurana A, Runge VM, Narayanan M, et al. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (Omniscan). Invest Radiol. 2007;42(2):139-145.
13. Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243(1):148-157.
14. Morcos SK. Nephrogenic systemic fibrosis following the administration of extracellular gadolinium based contrast agents: is the stability of the contrast agent molecule an important factor in the pathogenesis of this condition? Br J Radiol. 2007;80(950):73-76.
15. GE Healthcare. Omniscan safety update. http://md.gehealthcare.com/omniscan/safety/index.html. Accessed July 24, 2008.
16. Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol. 2007;56(1):27-30.
17. High WA, Ayers RA, Chandler J, et al. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;56(1):21-26.
18. Thomsen H; European Society of Urogenital Radiology. European Society of Urogenital Radiology guidelines on contrast media application. Curr Opin Urol. 2007;17(1):70-76.
19. Bongartz G. Imaging in the time of NFD/NSF: do we have to change our routines concerning renal insufficiency? MAGMA. 2007;20(2):57-62.
20. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
21. GE Healthcare Paper on Nephrogenic Systemic Fibrosis (March 2007). http://md.gehealthcare.com/omniscan/GE% 20Healthcare%20Paper%20On%20Nephrogenic%20 Systemic%20Fibrosis.pdf. Accessed July 24, 2008.
22. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267.
23. Okada S, Katagiri K, Kumazaki T, Yokoyama H. Safety of gadolinium contrast agent in hemodialysis patients. Acta Radiol. 2001;42(3):339-341.
24. Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242(3):647-649.
25. Cowper SE. Nephrogenic systemic fibrosis: the nosological and conceptual evolution of nephrogenic fibrosing dermopathy. Am J Kidney Dis. 2005;46(4):763-765.
1. US Food and Drug Administration. Public health advisory: gadolinium-containing contrast agents for magnetic resonance imaging (MRI): Omniscan, OptiMARK, Magnevist, ProHance, and MultiHance. www.fda.gov/cder/drug/advisory/gadolinium_agents.htm. Accessed July 24, 2008.
2. Cowper SE, Su L, Bhawan J, et al. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23(5):383-393.
3. US Food and Drug Administration. Information for healthcare professionals: gadolinium-based contrast agents for magnetic resonance imaging (marketed as Magnevist, MultiHance, Omniscan, OptiMARK, ProHance). Last updated June 4, 2008. www.fda.gov/cder/drug/InfoSheets/HCP/gcca_200705.htm. Accessed July 24, 2008.
4. International Center for Nephrogenic Fibrosing Dermopathy Research. www.icnfdr.org. Accessed July 24, 2008.
5. DeHoratius DM, Cowper SE. Nephrogenic systemic fibrosis: an emerging threat among renal patients. Semin Dial. 2006;19(3):191-194.
6. Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol. 2006;18(6):614-617.
7. Swaminathan S, Ahmed I, McCarthy JT, et al. Nephrogenic fibrosing dermopathy and high-dose erythropoietin therapy. Ann Intern Med. 2006;145(3):234-235.
8. Miskulin D, Gul A, Rudnick MR, Cowper SE. Nephrogenic systemic fibrosis/nephrogenic fibrosing dermopathy in advanced renal failure. www.uptodate.com/patients/content/topic.do?topicKey=dialysis/48700. Accessed July 24, 2008.
9. Grobner T. Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
10. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
11. Centers for Disease Control and Prevention. Nephrogenic fibrosing dermopathy associated with exposure to gadolinium-containing contrast agents—St. Louis, Missouri, 2002-2006. MMWR Morb Mortal Wkly Rep. 2007;56(7):137-141.
12. Khurana A, Runge VM, Narayanan M, et al. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (Omniscan). Invest Radiol. 2007;42(2):139-145.
13. Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243(1):148-157.
14. Morcos SK. Nephrogenic systemic fibrosis following the administration of extracellular gadolinium based contrast agents: is the stability of the contrast agent molecule an important factor in the pathogenesis of this condition? Br J Radiol. 2007;80(950):73-76.
15. GE Healthcare. Omniscan safety update. http://md.gehealthcare.com/omniscan/safety/index.html. Accessed July 24, 2008.
16. Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol. 2007;56(1):27-30.
17. High WA, Ayers RA, Chandler J, et al. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;56(1):21-26.
18. Thomsen H; European Society of Urogenital Radiology. European Society of Urogenital Radiology guidelines on contrast media application. Curr Opin Urol. 2007;17(1):70-76.
19. Bongartz G. Imaging in the time of NFD/NSF: do we have to change our routines concerning renal insufficiency? MAGMA. 2007;20(2):57-62.
20. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
21. GE Healthcare Paper on Nephrogenic Systemic Fibrosis (March 2007). http://md.gehealthcare.com/omniscan/GE% 20Healthcare%20Paper%20On%20Nephrogenic%20 Systemic%20Fibrosis.pdf. Accessed July 24, 2008.
22. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267.
23. Okada S, Katagiri K, Kumazaki T, Yokoyama H. Safety of gadolinium contrast agent in hemodialysis patients. Acta Radiol. 2001;42(3):339-341.
24. Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242(3):647-649.
25. Cowper SE. Nephrogenic systemic fibrosis: the nosological and conceptual evolution of nephrogenic fibrosing dermopathy. Am J Kidney Dis. 2005;46(4):763-765.