User login
Prescriber adherence to antiemetic guidelines with the new agent trifluridine-tipiracil
Cancer drugs are becoming available at an unprecedented rate. In 2015 alone, the US Food and Drug Administration (FDA) approved 18 new agents.1 Although many of those agents have adverse event profiles that are more favorable than those seen with conventional chemotherapy, nausea and vomiting still occur. In fact, nausea and vomiting continue to be ranked as among the most common and distressing of cancer symptoms.2,3 In a 2004 study, Grunberg and colleagues reported that as many as 75% of health care providers misjudge the risk for chemotherapy-induced nausea and vomiting (CINV), even when prescribing cancer drugs that have been available for years,4 thus amplifying concerns that such risk assessment might be even worse when new cancer agents are prescribed for the first time.
In this study, we hypothesized that patients prescribed a new cancer drug, trifluridine-tipiracil, would be at risk for CINV because of poor guideline adherence on the part of health care providers. The correct matching of antiemetics to chemotherapy is important. Inadequate antiemetic prophylaxis predisposes to nausea and vomiting with dehydration and metabolic and electrolyte derangements – complications that can occur in up to one-third of patients who receive moderately or highly emetogenic chemotherapy and who have been reported to achieve poor symptom control.4 Over-prophylaxis also has drawbacks. For example, antiemetics are expensive and, at times, they can induce their own adverse events, such as lethargy, dyskinesia, constipation, headaches, hiccups, fatigue, and even cardiac arrhythmias.5 The best approach is to appropriately match the antiemetic to the chemotherapy. Indeed, adherence to evidence-based guidelines has yielded success in symptom control, but the guidelines work on the assumption that the emetogenic potential of new chemotherapy agents has been accurately determined and then disseminated to and acted upon by health care providers.6,7 To our knowledge, no previous studies have tested that assumption, as we do in the present study.
Trifluridine-tipiracil was selected as the focus of this project and as illustrative of other newly approved chemotherapy agents for two reasons. First, it became available for routine prescribing in pretreated patients with metastatic colorectal cancer in the United States in September 2015.1 That timing allowed us to analyze much of the early prescribing period, both during the 9 months before approval, when the drug was available on a compassionate-use basis at our institution, and the 3 months after approval. Second, trifluridine-tipiracil has classifiably low emetogenic potential, and mismatching of antiemetics tends to occur more often with low emetogenic chemotherapy.9 Trifluridine-tipiracil and placebo patients manifest rates of nausea at 48% and 24%, respectively, and rates of vomiting at 28% and 14%, respectively.8
Hence, the goal of this study was to explore whether a guideline-based prophylactic antiemetic regimen was appropriately matched to the new chemotherapy agent, trifluridine-tipiracil, to report whether such symptoms of nausea and vomiting are kept at bay, and to identify a potentially vulnerable interval – immediately after drug approval – when cancer patients may be at risk for CINV because of poor adherence to antiemetic guideline prescribing practices by health care providers.
Methods
Overview
The Mayo Clinic Institutional Review Board approved this study. We obtained the identifying information of all patients treated with trifluridine-tipiracil at our institution from the Mayo Clinic Specialty Pharmacy, which uses an electronic prescribing system that contributed to the comprehensiveness of the data set. Patients included those who had participated in a colorectal cancer compassionate-use program before the September 2015 approval of the drug and those who received the drug shortly after its approval. In essence, this retrospective, single-institution study included every patient who received trifluridine-tipiracil for metastatic colorectal cancer in 2015 (January through December); this approach enabled us to systematically report on early first-cycle prescribing practices 9 months before and 3 months after the drug’s approval in September of 2015.
Determination of guideline adherence
This project relied on the National Comprehensive Cancer Network (NCCN) Guidelines (v1.2015, behind paywall) because they had been updated in 2015 (and hence coincided with this project’s study dates) to incorporate recommendations specific to oral chemotherapy and because they seemed concordant with other guidelines.10,11
Antiemetic prophylaxis for a specific patient was deemed guideline adherent if a version of the recommended NCCN antiemetic regimen had been prescribed during the first cycle of chemotherapy. This regimen consisted of metoclopramide, prochlorperazine, haloperidol, or a 5-hydroxytryptamine receptor antagonist. In contrast, if a patient had been prescribed a more aggressive or less aggressive regimen, such prescribing practices were deemed non–guideline adherent/aggressive (received more prophylaxis than called for) or non–guideline adherent/less aggressive (including no antiemetics), respectively. Again, medical record prescribing determined adherence.
Data reporting
The primary goal of this study was to report the percentage of patients who had been prescribed a first-cycle antiemetic prophylaxis regimen concordant with NCCN guidelines. Secondary goals included reporting the incidence of nausea and vomiting, the use of rescue antiemetics other than those prescribed up front, the need for an unplanned medical encounter to address nausea and vomiting, and change in antiemetic prescribing before the second chemotherapy cycle. Confidence intervals were calculated with JMP Pro 10.0.0. This study was too limited in sample size to assess sex-based differences in outcomes.
Results
Demographics
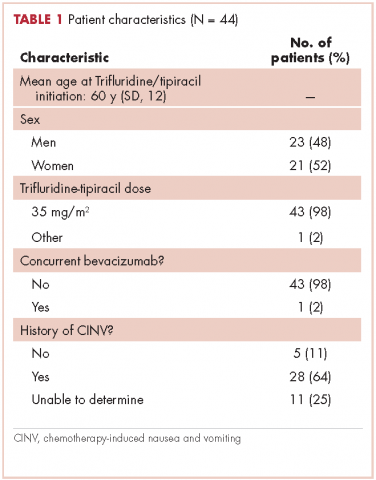
This report focuses on 44 patients who received first-cycle trifluridine-tipiracil during the first calendar year of the drug’s FDA approval. All patients had metastatic colorectal cancer and had previous exposures to other chemotherapy agents (Table 1). Of note, 28 patients (64%) had experienced CINV before starting trifluridine-tipiracil and all these patients had been heavily pretreated with multiple lines of chemotherapy.
Guideline adherence
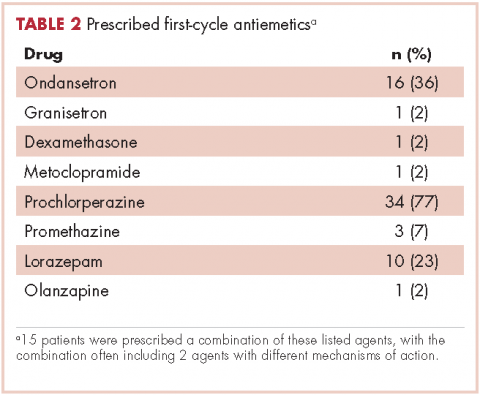
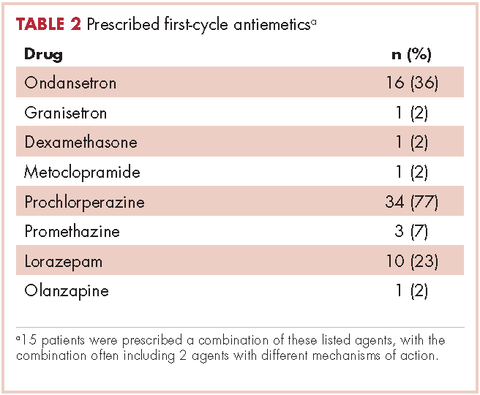
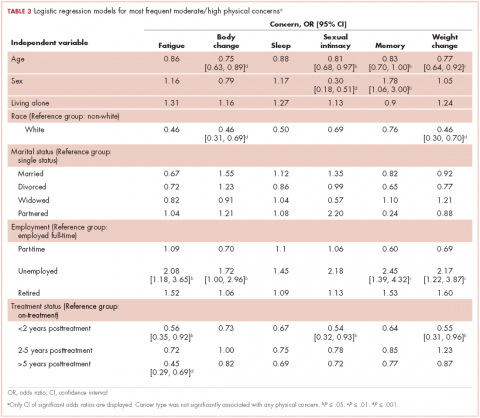
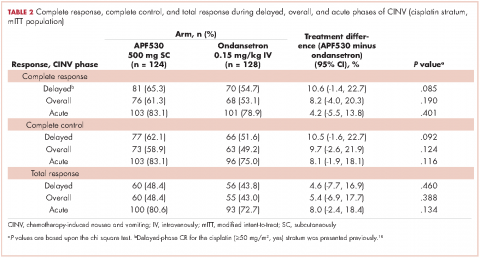
Patients were most commonly prescribed prochlorperazine and ondansetron prophylaxis for CINV before the first chemotherapy cycle of trifluridine-tipiracil (Table 2): 15 patients were prescribed combination antiemetic therapy, typically two of the most commonly prescribed single agents with different mechanisms of action. Twenty-five patients (57%; 95% confidence interval (CI): 42%, 70%) were prescribed antiemetics in a manner consistent with guidelines; 15 (34%; 95% CI: 22%, 49%) were prescribed antiemetics in a non–guideline-adherent/more aggressive manner (received more prophylaxis than called for); and 4 (9%; 95% CI: 4%, 21%) were prescribed them in a non–guideline-adherent/less aggressive manner.
Clinical outcomes based on guideline adherence
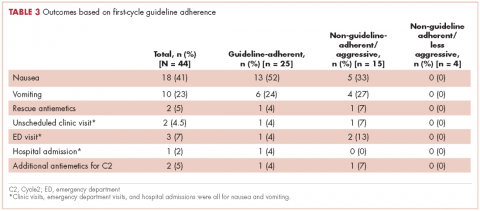
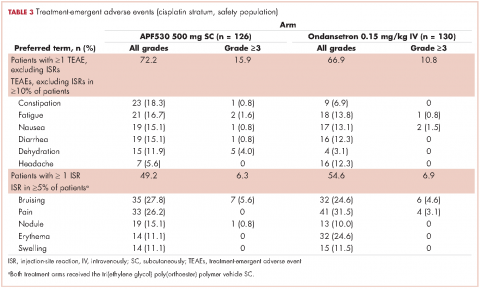
In guideline-adherent patients, first-cycle nausea and vomiting occurred in 13 patients (52%) and 6 patients (24%), respectively, with 1 patient requiring an unscheduled clinic visit and another an emergency department visit and hospital admission – all for nausea and vomiting (Table 3). In non–guideline-adherent/more aggressive patients, those symptoms occurred in 5 patients (33%, nausea) and 4 patients (27%, vomiting), with 1 patient requiring a clinic visit and emergency department visit and another an emergency department visit – again, all for nausea and vomiting. In non–guideline-adherent/less aggressive patients, no nausea or vomiting was reported.
Discussion
This study examined adherence to antiemetic guidelines in the setting of a soon-to-be-approved or newly approved antineoplastic agent. As hypothesized, a substantial proportion of patients (43% in this study) were prescribed antiemetics in a nonadherent manner with respect to guidelines, thus identifying the period shortly before and after FDA approval as a particularly vulnerable interval with respect to antiemetic guideline adherence. It is possible that our institution’s practice of testing novel chemotherapy agents for the treatment of colorectal cancer prompted a heightened awareness of potential adverse events, leading to greater guideline adherence than might have occurred in other settings and resulting in judicious straying from guideline adherence only when appropriate.12-14 Thus, these high rates of poor adherence may in fact represent an underestimate of what one might see in other clinical practices; and, similarly, these rates of symptom control might also be more favorable than those one might see in other clinical practices. To our knowledge, antiemetic prescribing practices with newer chemotherapy agents have not been explored before now, and our data underscore a clear need to do so – particularly during this limited interval when health care providers begin to prescribe new chemotherapy agents for the first time.
It is worth noting that despite the high rates of guideline nonadherence, rates of nausea and vomiting seemed to be comparable in patients prescribed antiemetics in a guideline-adherent manner and those prescribed antiemetics in a non–guideline-adherent/aggressive manner.A small number of patients in both the guideline-adherent and non–guideline-adherent/aggressive groups required rescue medications, unscheduled medical visits for nausea and vomiting, and additional antiemetics during the second cycle of chemotherapy. Of note,none of those interventions occurred in patients who were prescribed antiemetics in a non–guideline-adherent/less aggressive manner. These findings might reflect the fact that the patients had proven themselves to be at risk for nausea and vomiting with previous chemotherapy. Before they became candidates for trifluridine-tipiracil, patients had been heavily pretreated with other chemotherapy agents, most had experienced CINV, and many were therefore highly predisposed to nausea and vomiting. These observations underscore the fact that guidelines – even those that are well accepted and widely used – should be implemented in concert with good clinical judgment.10,11 This study has shortcomings, most notably its small sample size. However, had we extended our study beyond 3 months of the FDA approval to include more patients, our findings would have reflected more experienced prescribing practices and we thereby would have deviated from our primary goal of assessing antiemetic prescribing practices with only recently-approved and available chemotherapy agents. In this context, this limited sample size aptly serves a primary role of capturing outcomes within a fleeting but critical interval of new drug availability.In summary, this study found a notable rate of poor guideline adherence when prescribing antiemetics for trifluridine-tipiracil, a new chemotherapy agent of low emetogenic potential. Although the resultant rates of nausea and vomiting suggest that good clinical judgment might have influenced whether or not guidelines were adhered to, these findings nonetheless underscore the need to assess adherence to antiemetic guidelines when new chemotherapy drugs become available and potentially to put in place institutional infrastructure rapidly to promote improved adherence. Such an assessment should be deliberate, formalized, and prompt within individual oncology clinics and cancer centers after a new cancer drug becomes available. In conjunction with clinical judgment, such measures might lead to improved symptom control.
Acknowledgment
This paper is based on a poster that was presented at the 2016 Palliative Care in Oncology Symposium, on September 10, 2016: Adherence to antiemetic guidelines with a newly approved chemotherapy agent, trifluridine-tipiracil (TAS-102): a single-institution study. Daniel Childs and Aminah Jatoi, Mayo Clinic, Rochester, MN. http://meetinglibrary.asco.org/record/136444/abstract. J Clin Oncol. 2016;34(suppl 26S):abstract 221.
1. CenterWatch. FDA website. FDA approved drugs for oncology: drugs approved for 2015. https://www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/12/oncology. Last updated April 2017. Accessed June 4, 2016.
2. Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374:1356-1367.
3. Kottschade L, Novotny P, Lyss A, et al. Chemotherapy-induced nausea and vomiting: incidence and characteristics of persistent symptoms and future directions NCCTG N08C3. Support Care Cancer. 2016;24:2661-2667.
4. Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261-2268.
5. Navari RM. The safety of antiemetic medications for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin Drug Saf. 2016; 15:343-356.
6. Gilmore JW, Peacock NW, Gu A, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract. 2014;10:68-74.
7. Mertens WC, Higby DJ, Brown D, et al. Improving the care of patients with regard to chemotherapy-induced nausea and emesis: the effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol. 2003;21:1373-1378.
8. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919.
9. Schwartzberg L, Morrow G, Balu S, et al. Chemotherapy-induced nausea and vomiting and antiemetic prophylaxis with palonosetron versus other 5-HT3 receptor antagonists in patients with cancer treated with low emetogenic chemotherapy in a hospital outpatient setting in the United States. Curr Med Res Opin. 2011;27:1613-1622.
10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines on Antiemesis, Version1,2015 [behind paywall]. https://www.nccn.org. Last update not known. Accessed June 4, 2016.
11. Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21:v232-v243.
12. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomized, placebo-controlled, phase 3 study. Lancet. 2013;381:303-312.
13. Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383-1393.
14. Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24:3347-3353.
Cancer drugs are becoming available at an unprecedented rate. In 2015 alone, the US Food and Drug Administration (FDA) approved 18 new agents.1 Although many of those agents have adverse event profiles that are more favorable than those seen with conventional chemotherapy, nausea and vomiting still occur. In fact, nausea and vomiting continue to be ranked as among the most common and distressing of cancer symptoms.2,3 In a 2004 study, Grunberg and colleagues reported that as many as 75% of health care providers misjudge the risk for chemotherapy-induced nausea and vomiting (CINV), even when prescribing cancer drugs that have been available for years,4 thus amplifying concerns that such risk assessment might be even worse when new cancer agents are prescribed for the first time.
In this study, we hypothesized that patients prescribed a new cancer drug, trifluridine-tipiracil, would be at risk for CINV because of poor guideline adherence on the part of health care providers. The correct matching of antiemetics to chemotherapy is important. Inadequate antiemetic prophylaxis predisposes to nausea and vomiting with dehydration and metabolic and electrolyte derangements – complications that can occur in up to one-third of patients who receive moderately or highly emetogenic chemotherapy and who have been reported to achieve poor symptom control.4 Over-prophylaxis also has drawbacks. For example, antiemetics are expensive and, at times, they can induce their own adverse events, such as lethargy, dyskinesia, constipation, headaches, hiccups, fatigue, and even cardiac arrhythmias.5 The best approach is to appropriately match the antiemetic to the chemotherapy. Indeed, adherence to evidence-based guidelines has yielded success in symptom control, but the guidelines work on the assumption that the emetogenic potential of new chemotherapy agents has been accurately determined and then disseminated to and acted upon by health care providers.6,7 To our knowledge, no previous studies have tested that assumption, as we do in the present study.
Trifluridine-tipiracil was selected as the focus of this project and as illustrative of other newly approved chemotherapy agents for two reasons. First, it became available for routine prescribing in pretreated patients with metastatic colorectal cancer in the United States in September 2015.1 That timing allowed us to analyze much of the early prescribing period, both during the 9 months before approval, when the drug was available on a compassionate-use basis at our institution, and the 3 months after approval. Second, trifluridine-tipiracil has classifiably low emetogenic potential, and mismatching of antiemetics tends to occur more often with low emetogenic chemotherapy.9 Trifluridine-tipiracil and placebo patients manifest rates of nausea at 48% and 24%, respectively, and rates of vomiting at 28% and 14%, respectively.8
Hence, the goal of this study was to explore whether a guideline-based prophylactic antiemetic regimen was appropriately matched to the new chemotherapy agent, trifluridine-tipiracil, to report whether such symptoms of nausea and vomiting are kept at bay, and to identify a potentially vulnerable interval – immediately after drug approval – when cancer patients may be at risk for CINV because of poor adherence to antiemetic guideline prescribing practices by health care providers.
Methods
Overview
The Mayo Clinic Institutional Review Board approved this study. We obtained the identifying information of all patients treated with trifluridine-tipiracil at our institution from the Mayo Clinic Specialty Pharmacy, which uses an electronic prescribing system that contributed to the comprehensiveness of the data set. Patients included those who had participated in a colorectal cancer compassionate-use program before the September 2015 approval of the drug and those who received the drug shortly after its approval. In essence, this retrospective, single-institution study included every patient who received trifluridine-tipiracil for metastatic colorectal cancer in 2015 (January through December); this approach enabled us to systematically report on early first-cycle prescribing practices 9 months before and 3 months after the drug’s approval in September of 2015.
Determination of guideline adherence
This project relied on the National Comprehensive Cancer Network (NCCN) Guidelines (v1.2015, behind paywall) because they had been updated in 2015 (and hence coincided with this project’s study dates) to incorporate recommendations specific to oral chemotherapy and because they seemed concordant with other guidelines.10,11
Antiemetic prophylaxis for a specific patient was deemed guideline adherent if a version of the recommended NCCN antiemetic regimen had been prescribed during the first cycle of chemotherapy. This regimen consisted of metoclopramide, prochlorperazine, haloperidol, or a 5-hydroxytryptamine receptor antagonist. In contrast, if a patient had been prescribed a more aggressive or less aggressive regimen, such prescribing practices were deemed non–guideline adherent/aggressive (received more prophylaxis than called for) or non–guideline adherent/less aggressive (including no antiemetics), respectively. Again, medical record prescribing determined adherence.
Data reporting
The primary goal of this study was to report the percentage of patients who had been prescribed a first-cycle antiemetic prophylaxis regimen concordant with NCCN guidelines. Secondary goals included reporting the incidence of nausea and vomiting, the use of rescue antiemetics other than those prescribed up front, the need for an unplanned medical encounter to address nausea and vomiting, and change in antiemetic prescribing before the second chemotherapy cycle. Confidence intervals were calculated with JMP Pro 10.0.0. This study was too limited in sample size to assess sex-based differences in outcomes.
Results
Demographics
This report focuses on 44 patients who received first-cycle trifluridine-tipiracil during the first calendar year of the drug’s FDA approval. All patients had metastatic colorectal cancer and had previous exposures to other chemotherapy agents (Table 1). Of note, 28 patients (64%) had experienced CINV before starting trifluridine-tipiracil and all these patients had been heavily pretreated with multiple lines of chemotherapy.
Guideline adherence
Patients were most commonly prescribed prochlorperazine and ondansetron prophylaxis for CINV before the first chemotherapy cycle of trifluridine-tipiracil (Table 2): 15 patients were prescribed combination antiemetic therapy, typically two of the most commonly prescribed single agents with different mechanisms of action. Twenty-five patients (57%; 95% confidence interval (CI): 42%, 70%) were prescribed antiemetics in a manner consistent with guidelines; 15 (34%; 95% CI: 22%, 49%) were prescribed antiemetics in a non–guideline-adherent/more aggressive manner (received more prophylaxis than called for); and 4 (9%; 95% CI: 4%, 21%) were prescribed them in a non–guideline-adherent/less aggressive manner.
Clinical outcomes based on guideline adherence
In guideline-adherent patients, first-cycle nausea and vomiting occurred in 13 patients (52%) and 6 patients (24%), respectively, with 1 patient requiring an unscheduled clinic visit and another an emergency department visit and hospital admission – all for nausea and vomiting (Table 3). In non–guideline-adherent/more aggressive patients, those symptoms occurred in 5 patients (33%, nausea) and 4 patients (27%, vomiting), with 1 patient requiring a clinic visit and emergency department visit and another an emergency department visit – again, all for nausea and vomiting. In non–guideline-adherent/less aggressive patients, no nausea or vomiting was reported.
Discussion
This study examined adherence to antiemetic guidelines in the setting of a soon-to-be-approved or newly approved antineoplastic agent. As hypothesized, a substantial proportion of patients (43% in this study) were prescribed antiemetics in a nonadherent manner with respect to guidelines, thus identifying the period shortly before and after FDA approval as a particularly vulnerable interval with respect to antiemetic guideline adherence. It is possible that our institution’s practice of testing novel chemotherapy agents for the treatment of colorectal cancer prompted a heightened awareness of potential adverse events, leading to greater guideline adherence than might have occurred in other settings and resulting in judicious straying from guideline adherence only when appropriate.12-14 Thus, these high rates of poor adherence may in fact represent an underestimate of what one might see in other clinical practices; and, similarly, these rates of symptom control might also be more favorable than those one might see in other clinical practices. To our knowledge, antiemetic prescribing practices with newer chemotherapy agents have not been explored before now, and our data underscore a clear need to do so – particularly during this limited interval when health care providers begin to prescribe new chemotherapy agents for the first time.
It is worth noting that despite the high rates of guideline nonadherence, rates of nausea and vomiting seemed to be comparable in patients prescribed antiemetics in a guideline-adherent manner and those prescribed antiemetics in a non–guideline-adherent/aggressive manner.A small number of patients in both the guideline-adherent and non–guideline-adherent/aggressive groups required rescue medications, unscheduled medical visits for nausea and vomiting, and additional antiemetics during the second cycle of chemotherapy. Of note,none of those interventions occurred in patients who were prescribed antiemetics in a non–guideline-adherent/less aggressive manner. These findings might reflect the fact that the patients had proven themselves to be at risk for nausea and vomiting with previous chemotherapy. Before they became candidates for trifluridine-tipiracil, patients had been heavily pretreated with other chemotherapy agents, most had experienced CINV, and many were therefore highly predisposed to nausea and vomiting. These observations underscore the fact that guidelines – even those that are well accepted and widely used – should be implemented in concert with good clinical judgment.10,11 This study has shortcomings, most notably its small sample size. However, had we extended our study beyond 3 months of the FDA approval to include more patients, our findings would have reflected more experienced prescribing practices and we thereby would have deviated from our primary goal of assessing antiemetic prescribing practices with only recently-approved and available chemotherapy agents. In this context, this limited sample size aptly serves a primary role of capturing outcomes within a fleeting but critical interval of new drug availability.In summary, this study found a notable rate of poor guideline adherence when prescribing antiemetics for trifluridine-tipiracil, a new chemotherapy agent of low emetogenic potential. Although the resultant rates of nausea and vomiting suggest that good clinical judgment might have influenced whether or not guidelines were adhered to, these findings nonetheless underscore the need to assess adherence to antiemetic guidelines when new chemotherapy drugs become available and potentially to put in place institutional infrastructure rapidly to promote improved adherence. Such an assessment should be deliberate, formalized, and prompt within individual oncology clinics and cancer centers after a new cancer drug becomes available. In conjunction with clinical judgment, such measures might lead to improved symptom control.
Acknowledgment
This paper is based on a poster that was presented at the 2016 Palliative Care in Oncology Symposium, on September 10, 2016: Adherence to antiemetic guidelines with a newly approved chemotherapy agent, trifluridine-tipiracil (TAS-102): a single-institution study. Daniel Childs and Aminah Jatoi, Mayo Clinic, Rochester, MN. http://meetinglibrary.asco.org/record/136444/abstract. J Clin Oncol. 2016;34(suppl 26S):abstract 221.
Cancer drugs are becoming available at an unprecedented rate. In 2015 alone, the US Food and Drug Administration (FDA) approved 18 new agents.1 Although many of those agents have adverse event profiles that are more favorable than those seen with conventional chemotherapy, nausea and vomiting still occur. In fact, nausea and vomiting continue to be ranked as among the most common and distressing of cancer symptoms.2,3 In a 2004 study, Grunberg and colleagues reported that as many as 75% of health care providers misjudge the risk for chemotherapy-induced nausea and vomiting (CINV), even when prescribing cancer drugs that have been available for years,4 thus amplifying concerns that such risk assessment might be even worse when new cancer agents are prescribed for the first time.
In this study, we hypothesized that patients prescribed a new cancer drug, trifluridine-tipiracil, would be at risk for CINV because of poor guideline adherence on the part of health care providers. The correct matching of antiemetics to chemotherapy is important. Inadequate antiemetic prophylaxis predisposes to nausea and vomiting with dehydration and metabolic and electrolyte derangements – complications that can occur in up to one-third of patients who receive moderately or highly emetogenic chemotherapy and who have been reported to achieve poor symptom control.4 Over-prophylaxis also has drawbacks. For example, antiemetics are expensive and, at times, they can induce their own adverse events, such as lethargy, dyskinesia, constipation, headaches, hiccups, fatigue, and even cardiac arrhythmias.5 The best approach is to appropriately match the antiemetic to the chemotherapy. Indeed, adherence to evidence-based guidelines has yielded success in symptom control, but the guidelines work on the assumption that the emetogenic potential of new chemotherapy agents has been accurately determined and then disseminated to and acted upon by health care providers.6,7 To our knowledge, no previous studies have tested that assumption, as we do in the present study.
Trifluridine-tipiracil was selected as the focus of this project and as illustrative of other newly approved chemotherapy agents for two reasons. First, it became available for routine prescribing in pretreated patients with metastatic colorectal cancer in the United States in September 2015.1 That timing allowed us to analyze much of the early prescribing period, both during the 9 months before approval, when the drug was available on a compassionate-use basis at our institution, and the 3 months after approval. Second, trifluridine-tipiracil has classifiably low emetogenic potential, and mismatching of antiemetics tends to occur more often with low emetogenic chemotherapy.9 Trifluridine-tipiracil and placebo patients manifest rates of nausea at 48% and 24%, respectively, and rates of vomiting at 28% and 14%, respectively.8
Hence, the goal of this study was to explore whether a guideline-based prophylactic antiemetic regimen was appropriately matched to the new chemotherapy agent, trifluridine-tipiracil, to report whether such symptoms of nausea and vomiting are kept at bay, and to identify a potentially vulnerable interval – immediately after drug approval – when cancer patients may be at risk for CINV because of poor adherence to antiemetic guideline prescribing practices by health care providers.
Methods
Overview
The Mayo Clinic Institutional Review Board approved this study. We obtained the identifying information of all patients treated with trifluridine-tipiracil at our institution from the Mayo Clinic Specialty Pharmacy, which uses an electronic prescribing system that contributed to the comprehensiveness of the data set. Patients included those who had participated in a colorectal cancer compassionate-use program before the September 2015 approval of the drug and those who received the drug shortly after its approval. In essence, this retrospective, single-institution study included every patient who received trifluridine-tipiracil for metastatic colorectal cancer in 2015 (January through December); this approach enabled us to systematically report on early first-cycle prescribing practices 9 months before and 3 months after the drug’s approval in September of 2015.
Determination of guideline adherence
This project relied on the National Comprehensive Cancer Network (NCCN) Guidelines (v1.2015, behind paywall) because they had been updated in 2015 (and hence coincided with this project’s study dates) to incorporate recommendations specific to oral chemotherapy and because they seemed concordant with other guidelines.10,11
Antiemetic prophylaxis for a specific patient was deemed guideline adherent if a version of the recommended NCCN antiemetic regimen had been prescribed during the first cycle of chemotherapy. This regimen consisted of metoclopramide, prochlorperazine, haloperidol, or a 5-hydroxytryptamine receptor antagonist. In contrast, if a patient had been prescribed a more aggressive or less aggressive regimen, such prescribing practices were deemed non–guideline adherent/aggressive (received more prophylaxis than called for) or non–guideline adherent/less aggressive (including no antiemetics), respectively. Again, medical record prescribing determined adherence.
Data reporting
The primary goal of this study was to report the percentage of patients who had been prescribed a first-cycle antiemetic prophylaxis regimen concordant with NCCN guidelines. Secondary goals included reporting the incidence of nausea and vomiting, the use of rescue antiemetics other than those prescribed up front, the need for an unplanned medical encounter to address nausea and vomiting, and change in antiemetic prescribing before the second chemotherapy cycle. Confidence intervals were calculated with JMP Pro 10.0.0. This study was too limited in sample size to assess sex-based differences in outcomes.
Results
Demographics
This report focuses on 44 patients who received first-cycle trifluridine-tipiracil during the first calendar year of the drug’s FDA approval. All patients had metastatic colorectal cancer and had previous exposures to other chemotherapy agents (Table 1). Of note, 28 patients (64%) had experienced CINV before starting trifluridine-tipiracil and all these patients had been heavily pretreated with multiple lines of chemotherapy.
Guideline adherence
Patients were most commonly prescribed prochlorperazine and ondansetron prophylaxis for CINV before the first chemotherapy cycle of trifluridine-tipiracil (Table 2): 15 patients were prescribed combination antiemetic therapy, typically two of the most commonly prescribed single agents with different mechanisms of action. Twenty-five patients (57%; 95% confidence interval (CI): 42%, 70%) were prescribed antiemetics in a manner consistent with guidelines; 15 (34%; 95% CI: 22%, 49%) were prescribed antiemetics in a non–guideline-adherent/more aggressive manner (received more prophylaxis than called for); and 4 (9%; 95% CI: 4%, 21%) were prescribed them in a non–guideline-adherent/less aggressive manner.
Clinical outcomes based on guideline adherence
In guideline-adherent patients, first-cycle nausea and vomiting occurred in 13 patients (52%) and 6 patients (24%), respectively, with 1 patient requiring an unscheduled clinic visit and another an emergency department visit and hospital admission – all for nausea and vomiting (Table 3). In non–guideline-adherent/more aggressive patients, those symptoms occurred in 5 patients (33%, nausea) and 4 patients (27%, vomiting), with 1 patient requiring a clinic visit and emergency department visit and another an emergency department visit – again, all for nausea and vomiting. In non–guideline-adherent/less aggressive patients, no nausea or vomiting was reported.
Discussion
This study examined adherence to antiemetic guidelines in the setting of a soon-to-be-approved or newly approved antineoplastic agent. As hypothesized, a substantial proportion of patients (43% in this study) were prescribed antiemetics in a nonadherent manner with respect to guidelines, thus identifying the period shortly before and after FDA approval as a particularly vulnerable interval with respect to antiemetic guideline adherence. It is possible that our institution’s practice of testing novel chemotherapy agents for the treatment of colorectal cancer prompted a heightened awareness of potential adverse events, leading to greater guideline adherence than might have occurred in other settings and resulting in judicious straying from guideline adherence only when appropriate.12-14 Thus, these high rates of poor adherence may in fact represent an underestimate of what one might see in other clinical practices; and, similarly, these rates of symptom control might also be more favorable than those one might see in other clinical practices. To our knowledge, antiemetic prescribing practices with newer chemotherapy agents have not been explored before now, and our data underscore a clear need to do so – particularly during this limited interval when health care providers begin to prescribe new chemotherapy agents for the first time.
It is worth noting that despite the high rates of guideline nonadherence, rates of nausea and vomiting seemed to be comparable in patients prescribed antiemetics in a guideline-adherent manner and those prescribed antiemetics in a non–guideline-adherent/aggressive manner.A small number of patients in both the guideline-adherent and non–guideline-adherent/aggressive groups required rescue medications, unscheduled medical visits for nausea and vomiting, and additional antiemetics during the second cycle of chemotherapy. Of note,none of those interventions occurred in patients who were prescribed antiemetics in a non–guideline-adherent/less aggressive manner. These findings might reflect the fact that the patients had proven themselves to be at risk for nausea and vomiting with previous chemotherapy. Before they became candidates for trifluridine-tipiracil, patients had been heavily pretreated with other chemotherapy agents, most had experienced CINV, and many were therefore highly predisposed to nausea and vomiting. These observations underscore the fact that guidelines – even those that are well accepted and widely used – should be implemented in concert with good clinical judgment.10,11 This study has shortcomings, most notably its small sample size. However, had we extended our study beyond 3 months of the FDA approval to include more patients, our findings would have reflected more experienced prescribing practices and we thereby would have deviated from our primary goal of assessing antiemetic prescribing practices with only recently-approved and available chemotherapy agents. In this context, this limited sample size aptly serves a primary role of capturing outcomes within a fleeting but critical interval of new drug availability.In summary, this study found a notable rate of poor guideline adherence when prescribing antiemetics for trifluridine-tipiracil, a new chemotherapy agent of low emetogenic potential. Although the resultant rates of nausea and vomiting suggest that good clinical judgment might have influenced whether or not guidelines were adhered to, these findings nonetheless underscore the need to assess adherence to antiemetic guidelines when new chemotherapy drugs become available and potentially to put in place institutional infrastructure rapidly to promote improved adherence. Such an assessment should be deliberate, formalized, and prompt within individual oncology clinics and cancer centers after a new cancer drug becomes available. In conjunction with clinical judgment, such measures might lead to improved symptom control.
Acknowledgment
This paper is based on a poster that was presented at the 2016 Palliative Care in Oncology Symposium, on September 10, 2016: Adherence to antiemetic guidelines with a newly approved chemotherapy agent, trifluridine-tipiracil (TAS-102): a single-institution study. Daniel Childs and Aminah Jatoi, Mayo Clinic, Rochester, MN. http://meetinglibrary.asco.org/record/136444/abstract. J Clin Oncol. 2016;34(suppl 26S):abstract 221.
1. CenterWatch. FDA website. FDA approved drugs for oncology: drugs approved for 2015. https://www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/12/oncology. Last updated April 2017. Accessed June 4, 2016.
2. Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374:1356-1367.
3. Kottschade L, Novotny P, Lyss A, et al. Chemotherapy-induced nausea and vomiting: incidence and characteristics of persistent symptoms and future directions NCCTG N08C3. Support Care Cancer. 2016;24:2661-2667.
4. Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261-2268.
5. Navari RM. The safety of antiemetic medications for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin Drug Saf. 2016; 15:343-356.
6. Gilmore JW, Peacock NW, Gu A, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract. 2014;10:68-74.
7. Mertens WC, Higby DJ, Brown D, et al. Improving the care of patients with regard to chemotherapy-induced nausea and emesis: the effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol. 2003;21:1373-1378.
8. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919.
9. Schwartzberg L, Morrow G, Balu S, et al. Chemotherapy-induced nausea and vomiting and antiemetic prophylaxis with palonosetron versus other 5-HT3 receptor antagonists in patients with cancer treated with low emetogenic chemotherapy in a hospital outpatient setting in the United States. Curr Med Res Opin. 2011;27:1613-1622.
10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines on Antiemesis, Version1,2015 [behind paywall]. https://www.nccn.org. Last update not known. Accessed June 4, 2016.
11. Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21:v232-v243.
12. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomized, placebo-controlled, phase 3 study. Lancet. 2013;381:303-312.
13. Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383-1393.
14. Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24:3347-3353.
1. CenterWatch. FDA website. FDA approved drugs for oncology: drugs approved for 2015. https://www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/12/oncology. Last updated April 2017. Accessed June 4, 2016.
2. Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374:1356-1367.
3. Kottschade L, Novotny P, Lyss A, et al. Chemotherapy-induced nausea and vomiting: incidence and characteristics of persistent symptoms and future directions NCCTG N08C3. Support Care Cancer. 2016;24:2661-2667.
4. Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261-2268.
5. Navari RM. The safety of antiemetic medications for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin Drug Saf. 2016; 15:343-356.
6. Gilmore JW, Peacock NW, Gu A, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract. 2014;10:68-74.
7. Mertens WC, Higby DJ, Brown D, et al. Improving the care of patients with regard to chemotherapy-induced nausea and emesis: the effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol. 2003;21:1373-1378.
8. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919.
9. Schwartzberg L, Morrow G, Balu S, et al. Chemotherapy-induced nausea and vomiting and antiemetic prophylaxis with palonosetron versus other 5-HT3 receptor antagonists in patients with cancer treated with low emetogenic chemotherapy in a hospital outpatient setting in the United States. Curr Med Res Opin. 2011;27:1613-1622.
10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines on Antiemesis, Version1,2015 [behind paywall]. https://www.nccn.org. Last update not known. Accessed June 4, 2016.
11. Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21:v232-v243.
12. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomized, placebo-controlled, phase 3 study. Lancet. 2013;381:303-312.
13. Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383-1393.
14. Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24:3347-3353.
Physician attitudes and prevalence of molecular testing in lung cancer
Lung cancer is the leading cause of cancer death in the United States. It is estimated that there will be 222,500 new cases of lung cancer and 155,870 deaths from lung cancer in 2017. Non–small-cell lung carcinoma (NSCLC) accounts for 80%-85% of lung cancers, with adenocarcinoma being the most common histologic subtype. Other less common subtypes include squamous-cell carcinoma, large-cell carcinoma, and NSCLC that cannot be further classified.1 Nearly 70% of patients present with locally advanced or metastatic disease at the time of diagnosis and are not candidates for surgical resection.2 For that group of patients, the mainstay of treatment is platinum-based chemotherapy with or without radiation therapy. Patients who are chemotherapy naive often experience a modest response, however; durable remission is short lived, and the 5-year survival rate remains staggeringly low.3 Improved understanding of the molecular pathways that drive malignancy in NSCLC has led to the development of drugs that target specific molecular pathways.4 By definition, these driver mutations facilitate oncogenesis by conferring a selective advantage during clonal evolution.5 Moreover, agents targeting these pathways are extremely active and induce durable responses in many patients.6,7,8
Predictive biomarkers in NSCLC include anaplastic lymphoma kinase (ALK) fusion oncogene and sensitizing epidermal growth factor receptor (EGFR) mutations. Mutations in the EGFR tyrosine kinase are observed in about 15%-20% of NSCLC adenocarcinomas in the United States and upward of 60% in Asian populations. They are also found more frequently in nonsmokers and women.6 The two most prevalent mutations in the EGFR tyrosine kinase domain are in-frame deletions of exon 19 and L858R substitution in exon 21, representing about 45% and 40% of mutations, respectively.9 Both mutations result in activation of the tyrosine kinase domain, and both are associated with sensitivity to the small-molecule tyrosine kinase inhibitors (TKIs), such as erlotinib, gefitinib, and afatinib.10 Other drug-sensitive mutations include point mutations at exon 21 (L861Q) and exon 18 (G719X).11 Targeted therapy produces durable responses in the majority of patients.12,13,14 Unfortunately, most patients develop acquired resistance to these therapies, which leads to disease progression.4,15-17
ALK gene rearrangements, although less prevalent, are another important molecular target in NSCLC and are seen in 2%-7% of cases in the United States.7 As with EGFR mutations, these mutations are more prevalent in nonsmokers, and they are found more commonly in younger patients and in men.8
Identification of driver mutations early in the course of disease and acquired resistance mutations later are crucial for the optimal management of advanced NSCLC. DNA analysis using polymerase chain reaction (PCR) and next-generation sequencing is the preferred method for testing for EGFR mutations, and ALK rearrangements are generally tested either by flourescence in situ hybridization (FISH) or immunohistochemistry.18,19 Newer blood-based assays have shown great promise, and clinicians may soon have the ability to monitor subtle genetic changes, identify resistance patterns, and change therapy when acquired resistance occurs.20
The American College of Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology have proposed guidelines for molecular testing in lung cancer. It is recommended that all advanced squamous and nonsquamous cell lung cancers with an adenocarcinoma component should be tested for EGFR and ALK mutations independent of age, sex, ethnicity, or smoking history. In the setting of smaller lung cancer specimens (eg, from biopsies, cytology) where an adenocarcinoma component cannot be completely excluded, EGFR and ALK testing may be performed in cases showing squamous or small cell histology but clinical criteria (eg, young age, lack of smoking history) may be useful in selecting a subset of these samples for testing. Samples obtained through surgical resection, open biopsy, endoscopy, transthoracic needle biopsy, fine-needle aspiration, and thoracentesis are all considered suitable for testing, but large biopsy samples are generally preferred over small biopsy samples, cell-blocks, and cytology samples.21 Despite this recommendation, not all patients who are eligible for mutation analysis are tested. At our institution, preliminary observations suggested that the percentage of patients being tested and the prevalence of driver mutations were significantly lower compared with published data. The purpose of this study was to evaluate physician attitudes about molecular testing, and to determine the rate of testing, the effect of biopsy sample size on rate of testing, and the prevalence of driver mutations at our institution.
Methods
In this retrospective clinical study, we identified 206 cases of advanced nsNSCLC from the tumor registry (February 2011-February 2013). Registry data was obtained from three hospitals within our health network – two academic tertiary care centers, and one community-based hospital. The other hospitals in the network were excluded because their EHR systems were not integrated with the rest of the hospitals and/or there was a lack of registry data. The testing rates for driver mutations, prevalence of driver mutations, and the tissue procurement techniques were obtained from individual chart review. Surgical specimens, core biopsy samples, and large volume thoracentesis specimens were categorized as large biopsy samples, and samples obtained by fine-needle aspiration, bronchial washing, and bronchial brushing were considered small biopsy samples. We used a chi-square analysis to compare mutation testing rates between the large and small biopsy sample groups. The prevalence of driver mutations was determined, excluding unknown or inadequate samples.
EGFR analysis had been conducted at Integrated Oncology, using formalin-fixed, paraffin-embedded tissue. Genomic DNA was isolated, and EGFR mutation analysis was performed using SNaPShot multiplex PCR, primer extension assay for exons 18-21; samples with >4mm2 and ≥50% tumor content were preferred. Macrodissection was used to enrich for tumor cells when samples had lower tumor cellularity and content. ALK rearrangements were tested in the hospital using the Vysis ALK Break Apart FISH probe kit (Abott Molecular Inc, Des Plaines, IL).
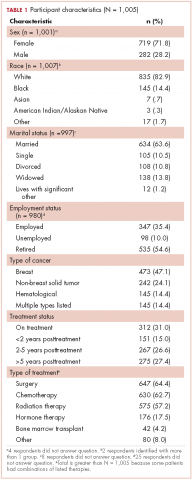
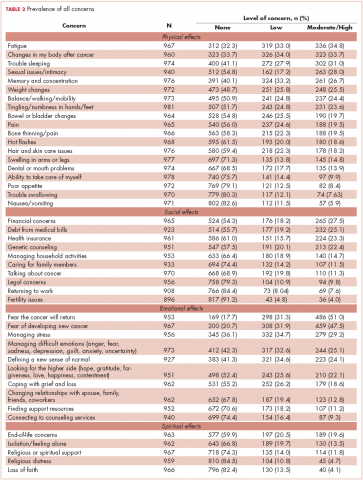
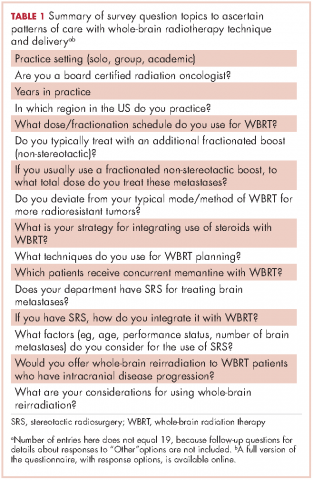
We conducted a web-based, 20-question survey about molecular profiling among 110 practitioners to gauge their knowledge and opinions about molecular testing. The practitioners included medical oncologists, thoracic surgeons, pulmonologists, and interventional radiologists. Each received an initial e-mail informing them of the study, inviting them to complete survey, and providing a link to it, and two reminder e-mails at biweekly intervals to maximize survey participation and responses. The questions were aimed at understanding the challenges surrounding molecular testing within our network. Apart from the questions gathering demographic information about the respondents, the questions were intended to highlight the disparities between guideline recommendations and physician practices; to gauge the perceived importance of molecular evaluation; to identify individual, subspecialty, and hospital-based challenges; and to assess physician attitudes toward alternatives to traditional tissue-based testing (Table 1, p. e150). Nineteen of the questions were structured as single or best answer, whereas Question 9, which was aimed at identifying system-based challenges, allowed for multiple answer selections.
Results
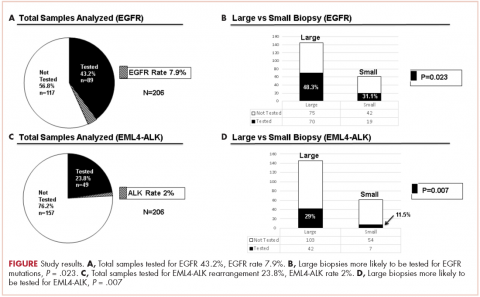
There were a total of 206 cases of advanced stage IIIb or IV nsNSCLC identified at three hospitals during 2011-2013. Of those 206 cases, 161 (78.2%) were recorded at the two large academic medical centers, and 45 (21.9%) were recorded at the smaller community-based hospital. Of the total, there were 145 (70.4%) large biopsy specimens and 61 (29.6%) small biopsy specimens. We found that 89 of the 206 cases (43.2 %) had been tested for EGFR mutations, and 49 (23.8%) had been tested for ALK rearrangements (Figure, A and C). In all, 70 (48.3%) large-sample biopsies and 19 (31.1%) small-sample biopsies were submitted for EGFR analysis (Figure, B), and 42 (29%) large-sample biopsies and 7 (11.5%) small-sample biopsies were tested for ALK rearrangements (Figure, D). Large-sample biopsies were more likely to be analyzed for EGFR mutations and ALK rearrangements, with the results reaching statistical significance (P = .023 and P = .007, respectively). Across all samples, a total of 7 EGFR mutations and 1 ALK rearrangement were identified, yielding a prevalence of 7.9% and 2% respectively (Figure, A and C).
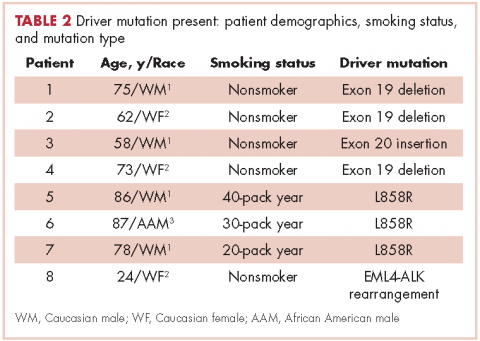
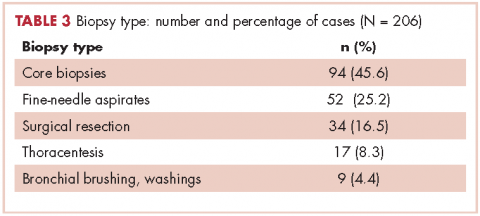
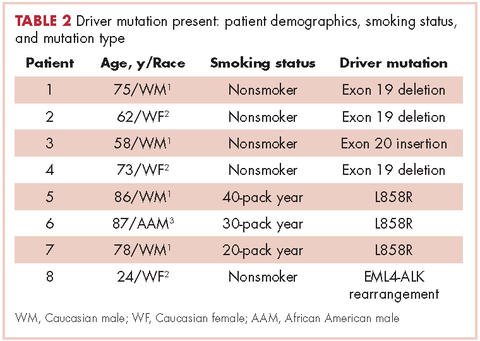
Table 2 shows the demographics, smoking status and type of driver mutation identified. Core biopsies were obtained in 45.6% of the cases and fine-needle aspiration biopsies were obtained in 25.2% of the cases with surgical resections, with thoracentesis and bronchial washings comprising the rest of the biopsies (Table 3).
The average age at diagnosis of the patients in the cases that were analyzed was 69.3 years. Most of the patients (83.9%) identified as white, 3.8% were African American, and 12.6% were in the Unknown category. Of the total number of patients, 11 were identified as never-smokers (5.3%), 50 (24.3%) had a 1-15 pack-year smoking history, 104 (50.5%) had a 16-45 pack-year smoking history, and 41 (19.9%) had a >45 pack-year smoking history.
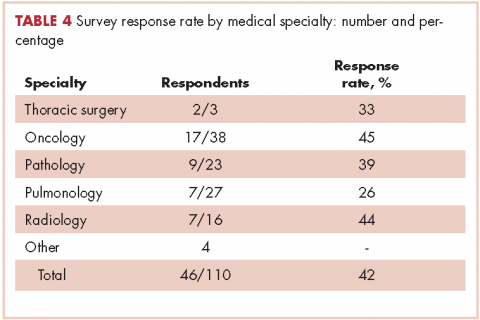
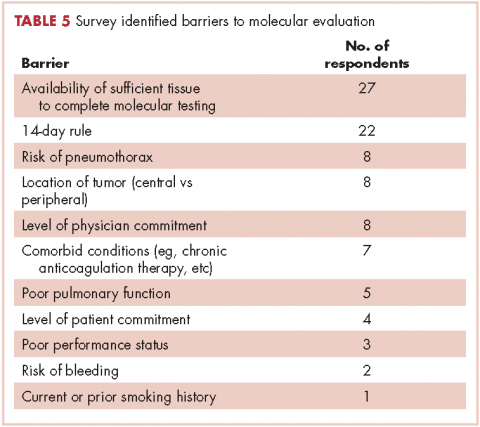
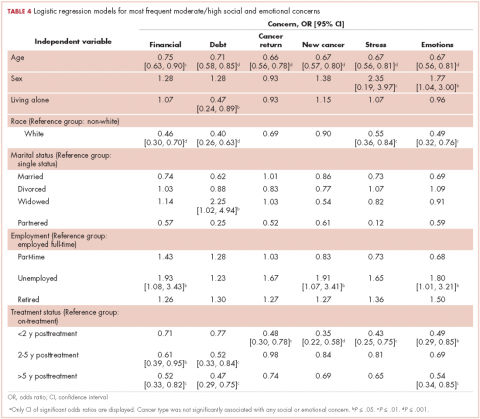
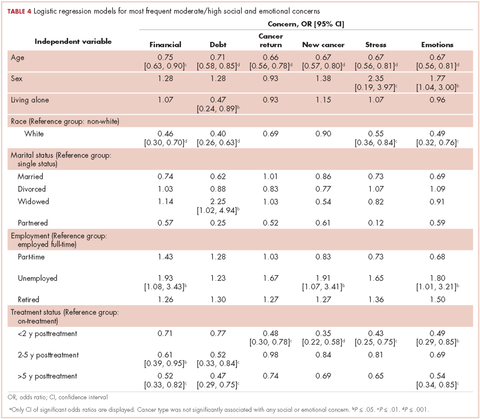
In regard to the survey, 46 of the 110 physicians asked to participate in the survey responded, representing a response rate of 41.8% (range across medical specialties, 26%-45%, Table 4). Of those respondents, 38 (82.6%) indicated they believed molecular evaluation was a very important aspect of NSCLC care, with the remainder indicating it was somewhat important. 91.4% of the respondents who routinely ordered molecular testing agreed that stage IIIb or IV nsNSCLC should undergo molecular evaluation.
The top barriers to molecular evaluation identified through this survey were the availability of sufficient tissue to complete molecular testing and the Center for Medicare and Medicaid Services’s (CMS’s) 14-day rule that requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing (Table 5).
Discussion
The treatment of advanced nsNSCLC has evolved significantly over the past decade. Molecular profiling is now an essential part of initial evaluation, and larger-sample biopsies are needed to ensure accurate evaluation and appropriate treatment. The detection of EGFR and EML4-ALK driver mutations are associated with increased response to tyrosine kinase inhibitors and are associated with improvement in progression-free survival, patient quality of life, and even overall survival in some studies.12,22,23,24 Early identification of these driver mutations is crucial, however, preliminary observation in our network suggested that a large percentage of patients with advanced nsNSCLC in were not being appropriately evaluated for those mutations. To evaluate our molecular profiling rates, we conducted a retrospective study and reviewed 3 years of registry data at 3 hospitals within our health system. Two of the hospitals included in our analysis were large tertiary academic centers, and one was a community hospital. Our findings confirmed that a large percentage of our patients who are eligible for molecular evaluation are not tested: 56.7% of cases were not tested for EGFR mutations, and 76.2% of cases were not tested for ALK rearrangements.
In a similar study, the Association for Community Cancer Centers conducted a project aimed at understanding the landscape and current challenges for molecular profiling in NSCLC. Eight institutions participated in the study, and baseline testing rates were analyzed. The findings demonstrated that high-volume institutions (treating >100 lung cancer patients a year tested 62% and 60% of advanced lung cancer patients for EGFR and EML4-ALK, respectively, and low-volume institutions (treating <100 lung cancer patients a year tested 52% and 47% for EGFR and EML4-ALK, respectively.25,26 In a recent international physician self-reported survey, Spicer and colleagues found that EGFR testing was requested before first-line therapy in patients with stage IIIB or IV disease in 81% of cases, and mutation results were available before start of therapy in 77% of the cases.27 Those percentages are relatively low, given that current guidelines recommend that molecular testing should be done for all patients with stage IIIB or IV nsNSCLC. This highlights the need for objective performance feedback so oncologists can make the necessary practice changes so that molecular testing is done before the start of therapy to ensure high-quality cancer care that will translate into better, cost-effective outcomes and improved patient quality of life.
Our study findings showed that the prevalence of EGFR and ALK mutations is substantially lower among the patients we treat in our network compared with other published data on prevalence. The reason for those low rates is not clear, but it is likely multifactorial. First, Western Pennsylvania, the region our network serves, has a large proportion of older adults – 17.3% of the population is older than 65 years (national average, 14.5%) and advanced age might have contributed to the lower EGFR and ALK rates measured in our study.28 Second, the smoking rate in Pennsylvania is higher than the national average, 20%-24% compared with 18%, respectively.29 Third, the air quality in Western Pennsylvania has historically been very poor as a result of the large steel and coal mining industries. Even though the air quality has improved in recent decades, the American Lung Association’s 2017 State of the Air report ranked Pittsburgh and surrounding areas in Western Pennsylvania among the top 25 most air polluted areas in the United States.30 It is not certain whether air pollution and air quality have any impact on driver mutation rates, but the correlation with smoking, ethnicity, and geographic distribution highlight the need for further epidemiologic studies.
Biopsy sufficiency – getting an adequate amount of sample tissue during biopsy – is a known challenge to molecular profiling, and we found that biopsy sample size had an impact on the testing rates in a large percentage of our cases. To fully understand the impact of biopsy sufficiency, we conducted a subset analysis and compared the testing rates between our large and small biopsy samples. Our analysis showed that larger-sample biopsies were more likely to be tested for mutations than were smaller-sample biopsies (EGFR: P = .023; ALK: P = .007).
Those results suggest that larger-sample biopsies should be encouraged, but procedural risks, tumor location, and patient age and wishes need to be considered before tissue acquisition.21 Furthermore, clinicians who are responsible for tissue procurement need to be properly educated on the tissue sample requirements and the impact these results have on treatment decisions.31 Our institution, like many others, has adopted rapid onsite evaluation (ROSE) of biopsy samples, whereby a trained cytopathologist reviews sample adequacy at the time of tissue procurement. Although there is scant data directly comparing molecular testing success rates with and without the ROSE protocol, a meta-analysis conducted by Schmidt and colleagues concluded that ROSE improved the adequacy rate of fine-needle aspiration cytology by 12%.32,33 Given that molecular profiling depends on both the absolute and relative amount of tumor cells present in the sample, the ROSE protocol likely enhances the procedural success rate and reduces the need for repeat and subsequent biopsies.
It is interesting to note that our data also demonstrated that we are obtaining large-sample biopsies in most of our patients (about 70%). However, we are still failing to test more than half of our cases for driver mutations (Figure, A and C). This strongly suggests there are additional factors beyond tissue adequacy that are contributing to our high failure rate. It is essential to understand the dynamics and system practices that influence testing rates if we are to improve the care and outcomes of our cancer patients. To better understand those barriers, we surveyed 110 practitioners (including medical oncologists, pulmonologists, thoracic surgeons, and interventional radiologists) about the molecular profiling process and their responses highlighted several important areas that deserve special attention (Tables 1, 4, 5).
In our institution, testing initiation is primarily the responsibility of the treating medical oncologist. This presents a challenge because there is often a significant delay between tissue acquisition, histologic confirmation, and oncologic review. Many institutions have adopted pathology-driven reflex testing to help overcome such delays. Automatic testing after pathologic confirmation streamlines the process, increases testing rates, and eliminates unnecessary delay between the time of diagnosis and the time of test ordering.34 It also allows for the molecular and histologic diagnosis to be integrated into a single pathology report before therapy is initiated.
Another barrier to timely testing according to the respondents, was the CMS’s 14-day rule. The 14-day rule requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing and was frequently identified as a cause for significant delay in testing and having an impact on first-line treatment decisions.35,36
Often clinicians will choose to defer testing until this time has elapsed to reduce the financial burden placed on the hospital but by that time, they might well have initiated treatment without knowing if the patient has a mutation. This is a significant challenge identified by many of our oncologists, and is a limitation to our analysis above as it is unclear what percentage of patients received follow up testing once care was established at an outside facility and once the 14-day time period had elapsed.
The data from our institution suggests there is discordance between physician attitudes and molecular testing practices. However, there are several limitations in our study. First, most of the survey respondents agreed that molecular testing is an important aspect of treating advanced lung cancer patients, but the retrospective nature of the study made it difficult to identify why testing was deferred or never conducted. Second, the absence of a centralized reporting system for molecular testing results at our institution, may have resulted in an overestimation of our testing failure rate in cases where results were not integrated our electronic medical record.
Third, the low survey response rate only allowed us to make generalizations regarding the conclusions, although it does provide a framework for future process improvements.
We believe the poor testing rates observed in our study are not isolated to our institution and reflect a significant challenge within the broader oncology community.27 A system of best practices is essential for capturing this subset of patients who are never tested. There is agreement among oncologists that improving our current testing rates will require a multidisciplinary approach, a refined process for molecular evaluation, a push toward reflex testing, and standardization of biopsy techniques and tissue handling procedures. In our institution, we have initiated a Lean Six Sigma and PDSA (plan, do, study, act) initiative to improve our current molecular testing process. In addition, because obtaining larger-sample biopsies or additional biopsies is often not feasible for many of our advanced cancer patients, we have started using whole blood circulating tumor cells (CTC) and plasma ctDNA (cell-free circulating DNA) for molecular testing. Recent studies have shown high concordance (89%) between tissue biopsies and blood-based mutation testing, which will likely have a positive impact on the cancer care of our patients and help to capture a subset of patients who are not candidates for traditional biopsies.37
Conclusions
Despite current guidelines for testing driver mutations in advanced nsNSCLC, a large segment of our patients are not being tested for those genetic aberrations. There are several barriers that continue to thwart the recommendation, including failure to integrate driver mutation testing into routine pathology practice (ie, reflex testing), insufficient tissue obtained from biopsy, and difficulty in obtaining tissue because of tumor location or risk of complications from the biopsy procedure. More important, these trends are not isolated to our institution and reflect a significant challenge within the oncology community. Our data show that for the purpose of driver mutation testing, larger-sample biopsies, such as surgical/core biopsies, are better than small-sample biopsies, such as needle aspiration. We have also demonstrated that the prevalence of driver mutations is lower in Western Pennsylvania, which is served by our network, than elsewhere in the United States.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
3. Kim TE, Murren JR. Therapy for stage IIIB and stage IV non-small cell lung cancer. Clin Chest Med. 2002;23(1):209-224.
4. Black RC, Khurshid H. NSCLC: An update of driver mutations, their role in pathogenesis and clinical significance. R I Med J (2013). 2015;98(10):25-28.
5. Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306-313.
6. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327-3334.
7. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866-2874.
8. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246.
9. Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(suppl 1):S24-31.
10. Langer CJ. Epidermal growth factor receptor inhibition in mutation-positive non-small-cell lung cancer: is afatinib better or simply newer? J Clin Oncol. 2013;31(27):3303-3306.
11. Riely GJ, Politi KA, Miller VA, et al. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12(24):7232-7241.
12. Shi Y, Siu-Kie JA, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154-162.
13. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957.
14. Khozin S, Blumenthal GM, Jiang X, et al. US Food and Drug Administration approval summary: Erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist. 2014;19(7):774-779.
15. Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12(2):220-229.
16. Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73.
17. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240-2247.
18. Ellison G, Zhu G, Moulis A, Dearden S, et al. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol. 2013;66(2):79-89.
19. Alì G, Proietti A, Pelliccioni S, et al. ALK rearrangement in a large series of consecutive non-small cell lung cancers: comparison between a new immunohistochemical approach and fluorescence in situ hybridization for the screening of patients eligible for crizotinib treatment. Arch Pathol Lab Med. 2014;138(11):1449-1158.
20. Crowley E, Di Nicolantonio F, Loupakis F, et al. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10(8):472-484.
21. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823-859.
22. Kwak EL, Bany YJ, Cambridge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693-1703.
23. Shaw A, Yeap BY, Kenudson MM, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247-4253.
24. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380-2388.
25. Association of Community Cancer Centers. Molecular Testing in the Community Setting. In: Molecular testing: resources and tools for the multidisciplinary team. http://accc-cancer.org/resources/molecularTesting-Overview.asp. Accessed November 15, 2015.
26. Association of Community Cancer Centers. Molecular testing: ACCC peer-to-peer webinars. The tissue issue: sampling and testing with Gail Probst, RN, MS, AOCN. https://www.youtube.com/watch?v=lapmni938Mc&feature=youtu.be. Published September 14, 2015. Accessed November 2015.
27. Spicer J S, Tischer B, Peters M. EGFR mutation testing and oncologist treatment choice in advanced NSCLC: global trends and differences. Ann Oncol. 2015;26(suppl 1):i60.
28. West L, Cole S, Goodkind D. US Census Bureau, 65+ in the United States: 2010, U.S. Government Printing Office, Washington, DC, 2014
29. Centers for Disease Control and Prevention. State tobacco activities tracking and evaluation system. Current cigarette use among adults (Behavior Risk Factor Surveillance System) 2015. https://www.cdc.gov/statesystem/cigaretteuseadult.html. Last updated September 16, 2016. Accessed May 26, 2017.
30. The American Lung Association. State of the Air 2017. http://www.lung.org/assets/documents/healthy-air/state-of-the-air/state-of-the-air-2017.pdf. Published 2017. Accessed May 26, 2017.
31. Gaga M, Powell CA, Schraufnagel DE, Schönfeld N, et al. An official American Thoracic Society/European Respiratory Society statement: the role of the pulmonologist in the diagnosis and management of lung cancer. Am J Respir Crit Care Med. 2013;188(4):503-507.
32. Ferguson PE, Sales CM, Hodges DC, et al. Effects of a multidisciplinary approach to improve volume of diagnostic material in CT-guided lung biopsies. PLoS One. 2015 Oct 19;10(10).
33. Schmidt RL, Witt BL, Lopez-Calderon LE, et al. The influence of rapid onsite evaluation on the adequacy rate of fine-needle aspiration cytology: a systematic review and meta-analysis. Am J Clin Pathol. 2013;139(3):300-309.
34. Cengiz Inal, Yilmaz E, Chenget H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: Experience from a community-based academic center. J Clin Oncol. 2014;32(suppl):5s. [abstract 8098].
35. Grzegorz K, Leighl, M. Challenges in NSCLC molecular testing barriers to implementation. Oncology Exchange. 2012;11(4):8-10.
36. Lynch JA, Khoury MJ, Ann Borzecket A, et al. Utilization of epidermal growth factor receptor (EGFR) testing in the United States: a case study of T3 translational research. Genet Med. 2013;15(8):630-638.
37. Reck M. Investigating the utility of circulating-free tumour-derived DNA (ctDNA) in plasma for the detection of epidermal growth factor receptor (EGFR) mutation status in European and Japanese patients (pts) with advanced non-small-cell lung cancer (NSCLC): ASSESS study. Presented at the European Lung Cancer Conference (ELCC) Annual Meeting, Geneva; 15-18 April 2015.
Lung cancer is the leading cause of cancer death in the United States. It is estimated that there will be 222,500 new cases of lung cancer and 155,870 deaths from lung cancer in 2017. Non–small-cell lung carcinoma (NSCLC) accounts for 80%-85% of lung cancers, with adenocarcinoma being the most common histologic subtype. Other less common subtypes include squamous-cell carcinoma, large-cell carcinoma, and NSCLC that cannot be further classified.1 Nearly 70% of patients present with locally advanced or metastatic disease at the time of diagnosis and are not candidates for surgical resection.2 For that group of patients, the mainstay of treatment is platinum-based chemotherapy with or without radiation therapy. Patients who are chemotherapy naive often experience a modest response, however; durable remission is short lived, and the 5-year survival rate remains staggeringly low.3 Improved understanding of the molecular pathways that drive malignancy in NSCLC has led to the development of drugs that target specific molecular pathways.4 By definition, these driver mutations facilitate oncogenesis by conferring a selective advantage during clonal evolution.5 Moreover, agents targeting these pathways are extremely active and induce durable responses in many patients.6,7,8
Predictive biomarkers in NSCLC include anaplastic lymphoma kinase (ALK) fusion oncogene and sensitizing epidermal growth factor receptor (EGFR) mutations. Mutations in the EGFR tyrosine kinase are observed in about 15%-20% of NSCLC adenocarcinomas in the United States and upward of 60% in Asian populations. They are also found more frequently in nonsmokers and women.6 The two most prevalent mutations in the EGFR tyrosine kinase domain are in-frame deletions of exon 19 and L858R substitution in exon 21, representing about 45% and 40% of mutations, respectively.9 Both mutations result in activation of the tyrosine kinase domain, and both are associated with sensitivity to the small-molecule tyrosine kinase inhibitors (TKIs), such as erlotinib, gefitinib, and afatinib.10 Other drug-sensitive mutations include point mutations at exon 21 (L861Q) and exon 18 (G719X).11 Targeted therapy produces durable responses in the majority of patients.12,13,14 Unfortunately, most patients develop acquired resistance to these therapies, which leads to disease progression.4,15-17
ALK gene rearrangements, although less prevalent, are another important molecular target in NSCLC and are seen in 2%-7% of cases in the United States.7 As with EGFR mutations, these mutations are more prevalent in nonsmokers, and they are found more commonly in younger patients and in men.8
Identification of driver mutations early in the course of disease and acquired resistance mutations later are crucial for the optimal management of advanced NSCLC. DNA analysis using polymerase chain reaction (PCR) and next-generation sequencing is the preferred method for testing for EGFR mutations, and ALK rearrangements are generally tested either by flourescence in situ hybridization (FISH) or immunohistochemistry.18,19 Newer blood-based assays have shown great promise, and clinicians may soon have the ability to monitor subtle genetic changes, identify resistance patterns, and change therapy when acquired resistance occurs.20
The American College of Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology have proposed guidelines for molecular testing in lung cancer. It is recommended that all advanced squamous and nonsquamous cell lung cancers with an adenocarcinoma component should be tested for EGFR and ALK mutations independent of age, sex, ethnicity, or smoking history. In the setting of smaller lung cancer specimens (eg, from biopsies, cytology) where an adenocarcinoma component cannot be completely excluded, EGFR and ALK testing may be performed in cases showing squamous or small cell histology but clinical criteria (eg, young age, lack of smoking history) may be useful in selecting a subset of these samples for testing. Samples obtained through surgical resection, open biopsy, endoscopy, transthoracic needle biopsy, fine-needle aspiration, and thoracentesis are all considered suitable for testing, but large biopsy samples are generally preferred over small biopsy samples, cell-blocks, and cytology samples.21 Despite this recommendation, not all patients who are eligible for mutation analysis are tested. At our institution, preliminary observations suggested that the percentage of patients being tested and the prevalence of driver mutations were significantly lower compared with published data. The purpose of this study was to evaluate physician attitudes about molecular testing, and to determine the rate of testing, the effect of biopsy sample size on rate of testing, and the prevalence of driver mutations at our institution.
Methods
In this retrospective clinical study, we identified 206 cases of advanced nsNSCLC from the tumor registry (February 2011-February 2013). Registry data was obtained from three hospitals within our health network – two academic tertiary care centers, and one community-based hospital. The other hospitals in the network were excluded because their EHR systems were not integrated with the rest of the hospitals and/or there was a lack of registry data. The testing rates for driver mutations, prevalence of driver mutations, and the tissue procurement techniques were obtained from individual chart review. Surgical specimens, core biopsy samples, and large volume thoracentesis specimens were categorized as large biopsy samples, and samples obtained by fine-needle aspiration, bronchial washing, and bronchial brushing were considered small biopsy samples. We used a chi-square analysis to compare mutation testing rates between the large and small biopsy sample groups. The prevalence of driver mutations was determined, excluding unknown or inadequate samples.
EGFR analysis had been conducted at Integrated Oncology, using formalin-fixed, paraffin-embedded tissue. Genomic DNA was isolated, and EGFR mutation analysis was performed using SNaPShot multiplex PCR, primer extension assay for exons 18-21; samples with >4mm2 and ≥50% tumor content were preferred. Macrodissection was used to enrich for tumor cells when samples had lower tumor cellularity and content. ALK rearrangements were tested in the hospital using the Vysis ALK Break Apart FISH probe kit (Abott Molecular Inc, Des Plaines, IL).
We conducted a web-based, 20-question survey about molecular profiling among 110 practitioners to gauge their knowledge and opinions about molecular testing. The practitioners included medical oncologists, thoracic surgeons, pulmonologists, and interventional radiologists. Each received an initial e-mail informing them of the study, inviting them to complete survey, and providing a link to it, and two reminder e-mails at biweekly intervals to maximize survey participation and responses. The questions were aimed at understanding the challenges surrounding molecular testing within our network. Apart from the questions gathering demographic information about the respondents, the questions were intended to highlight the disparities between guideline recommendations and physician practices; to gauge the perceived importance of molecular evaluation; to identify individual, subspecialty, and hospital-based challenges; and to assess physician attitudes toward alternatives to traditional tissue-based testing (Table 1, p. e150). Nineteen of the questions were structured as single or best answer, whereas Question 9, which was aimed at identifying system-based challenges, allowed for multiple answer selections.
Results
There were a total of 206 cases of advanced stage IIIb or IV nsNSCLC identified at three hospitals during 2011-2013. Of those 206 cases, 161 (78.2%) were recorded at the two large academic medical centers, and 45 (21.9%) were recorded at the smaller community-based hospital. Of the total, there were 145 (70.4%) large biopsy specimens and 61 (29.6%) small biopsy specimens. We found that 89 of the 206 cases (43.2 %) had been tested for EGFR mutations, and 49 (23.8%) had been tested for ALK rearrangements (Figure, A and C). In all, 70 (48.3%) large-sample biopsies and 19 (31.1%) small-sample biopsies were submitted for EGFR analysis (Figure, B), and 42 (29%) large-sample biopsies and 7 (11.5%) small-sample biopsies were tested for ALK rearrangements (Figure, D). Large-sample biopsies were more likely to be analyzed for EGFR mutations and ALK rearrangements, with the results reaching statistical significance (P = .023 and P = .007, respectively). Across all samples, a total of 7 EGFR mutations and 1 ALK rearrangement were identified, yielding a prevalence of 7.9% and 2% respectively (Figure, A and C).
Table 2 shows the demographics, smoking status and type of driver mutation identified. Core biopsies were obtained in 45.6% of the cases and fine-needle aspiration biopsies were obtained in 25.2% of the cases with surgical resections, with thoracentesis and bronchial washings comprising the rest of the biopsies (Table 3).
The average age at diagnosis of the patients in the cases that were analyzed was 69.3 years. Most of the patients (83.9%) identified as white, 3.8% were African American, and 12.6% were in the Unknown category. Of the total number of patients, 11 were identified as never-smokers (5.3%), 50 (24.3%) had a 1-15 pack-year smoking history, 104 (50.5%) had a 16-45 pack-year smoking history, and 41 (19.9%) had a >45 pack-year smoking history.
In regard to the survey, 46 of the 110 physicians asked to participate in the survey responded, representing a response rate of 41.8% (range across medical specialties, 26%-45%, Table 4). Of those respondents, 38 (82.6%) indicated they believed molecular evaluation was a very important aspect of NSCLC care, with the remainder indicating it was somewhat important. 91.4% of the respondents who routinely ordered molecular testing agreed that stage IIIb or IV nsNSCLC should undergo molecular evaluation.
The top barriers to molecular evaluation identified through this survey were the availability of sufficient tissue to complete molecular testing and the Center for Medicare and Medicaid Services’s (CMS’s) 14-day rule that requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing (Table 5).
Discussion
The treatment of advanced nsNSCLC has evolved significantly over the past decade. Molecular profiling is now an essential part of initial evaluation, and larger-sample biopsies are needed to ensure accurate evaluation and appropriate treatment. The detection of EGFR and EML4-ALK driver mutations are associated with increased response to tyrosine kinase inhibitors and are associated with improvement in progression-free survival, patient quality of life, and even overall survival in some studies.12,22,23,24 Early identification of these driver mutations is crucial, however, preliminary observation in our network suggested that a large percentage of patients with advanced nsNSCLC in were not being appropriately evaluated for those mutations. To evaluate our molecular profiling rates, we conducted a retrospective study and reviewed 3 years of registry data at 3 hospitals within our health system. Two of the hospitals included in our analysis were large tertiary academic centers, and one was a community hospital. Our findings confirmed that a large percentage of our patients who are eligible for molecular evaluation are not tested: 56.7% of cases were not tested for EGFR mutations, and 76.2% of cases were not tested for ALK rearrangements.
In a similar study, the Association for Community Cancer Centers conducted a project aimed at understanding the landscape and current challenges for molecular profiling in NSCLC. Eight institutions participated in the study, and baseline testing rates were analyzed. The findings demonstrated that high-volume institutions (treating >100 lung cancer patients a year tested 62% and 60% of advanced lung cancer patients for EGFR and EML4-ALK, respectively, and low-volume institutions (treating <100 lung cancer patients a year tested 52% and 47% for EGFR and EML4-ALK, respectively.25,26 In a recent international physician self-reported survey, Spicer and colleagues found that EGFR testing was requested before first-line therapy in patients with stage IIIB or IV disease in 81% of cases, and mutation results were available before start of therapy in 77% of the cases.27 Those percentages are relatively low, given that current guidelines recommend that molecular testing should be done for all patients with stage IIIB or IV nsNSCLC. This highlights the need for objective performance feedback so oncologists can make the necessary practice changes so that molecular testing is done before the start of therapy to ensure high-quality cancer care that will translate into better, cost-effective outcomes and improved patient quality of life.
Our study findings showed that the prevalence of EGFR and ALK mutations is substantially lower among the patients we treat in our network compared with other published data on prevalence. The reason for those low rates is not clear, but it is likely multifactorial. First, Western Pennsylvania, the region our network serves, has a large proportion of older adults – 17.3% of the population is older than 65 years (national average, 14.5%) and advanced age might have contributed to the lower EGFR and ALK rates measured in our study.28 Second, the smoking rate in Pennsylvania is higher than the national average, 20%-24% compared with 18%, respectively.29 Third, the air quality in Western Pennsylvania has historically been very poor as a result of the large steel and coal mining industries. Even though the air quality has improved in recent decades, the American Lung Association’s 2017 State of the Air report ranked Pittsburgh and surrounding areas in Western Pennsylvania among the top 25 most air polluted areas in the United States.30 It is not certain whether air pollution and air quality have any impact on driver mutation rates, but the correlation with smoking, ethnicity, and geographic distribution highlight the need for further epidemiologic studies.
Biopsy sufficiency – getting an adequate amount of sample tissue during biopsy – is a known challenge to molecular profiling, and we found that biopsy sample size had an impact on the testing rates in a large percentage of our cases. To fully understand the impact of biopsy sufficiency, we conducted a subset analysis and compared the testing rates between our large and small biopsy samples. Our analysis showed that larger-sample biopsies were more likely to be tested for mutations than were smaller-sample biopsies (EGFR: P = .023; ALK: P = .007).
Those results suggest that larger-sample biopsies should be encouraged, but procedural risks, tumor location, and patient age and wishes need to be considered before tissue acquisition.21 Furthermore, clinicians who are responsible for tissue procurement need to be properly educated on the tissue sample requirements and the impact these results have on treatment decisions.31 Our institution, like many others, has adopted rapid onsite evaluation (ROSE) of biopsy samples, whereby a trained cytopathologist reviews sample adequacy at the time of tissue procurement. Although there is scant data directly comparing molecular testing success rates with and without the ROSE protocol, a meta-analysis conducted by Schmidt and colleagues concluded that ROSE improved the adequacy rate of fine-needle aspiration cytology by 12%.32,33 Given that molecular profiling depends on both the absolute and relative amount of tumor cells present in the sample, the ROSE protocol likely enhances the procedural success rate and reduces the need for repeat and subsequent biopsies.
It is interesting to note that our data also demonstrated that we are obtaining large-sample biopsies in most of our patients (about 70%). However, we are still failing to test more than half of our cases for driver mutations (Figure, A and C). This strongly suggests there are additional factors beyond tissue adequacy that are contributing to our high failure rate. It is essential to understand the dynamics and system practices that influence testing rates if we are to improve the care and outcomes of our cancer patients. To better understand those barriers, we surveyed 110 practitioners (including medical oncologists, pulmonologists, thoracic surgeons, and interventional radiologists) about the molecular profiling process and their responses highlighted several important areas that deserve special attention (Tables 1, 4, 5).
In our institution, testing initiation is primarily the responsibility of the treating medical oncologist. This presents a challenge because there is often a significant delay between tissue acquisition, histologic confirmation, and oncologic review. Many institutions have adopted pathology-driven reflex testing to help overcome such delays. Automatic testing after pathologic confirmation streamlines the process, increases testing rates, and eliminates unnecessary delay between the time of diagnosis and the time of test ordering.34 It also allows for the molecular and histologic diagnosis to be integrated into a single pathology report before therapy is initiated.
Another barrier to timely testing according to the respondents, was the CMS’s 14-day rule. The 14-day rule requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing and was frequently identified as a cause for significant delay in testing and having an impact on first-line treatment decisions.35,36
Often clinicians will choose to defer testing until this time has elapsed to reduce the financial burden placed on the hospital but by that time, they might well have initiated treatment without knowing if the patient has a mutation. This is a significant challenge identified by many of our oncologists, and is a limitation to our analysis above as it is unclear what percentage of patients received follow up testing once care was established at an outside facility and once the 14-day time period had elapsed.
The data from our institution suggests there is discordance between physician attitudes and molecular testing practices. However, there are several limitations in our study. First, most of the survey respondents agreed that molecular testing is an important aspect of treating advanced lung cancer patients, but the retrospective nature of the study made it difficult to identify why testing was deferred or never conducted. Second, the absence of a centralized reporting system for molecular testing results at our institution, may have resulted in an overestimation of our testing failure rate in cases where results were not integrated our electronic medical record.
Third, the low survey response rate only allowed us to make generalizations regarding the conclusions, although it does provide a framework for future process improvements.
We believe the poor testing rates observed in our study are not isolated to our institution and reflect a significant challenge within the broader oncology community.27 A system of best practices is essential for capturing this subset of patients who are never tested. There is agreement among oncologists that improving our current testing rates will require a multidisciplinary approach, a refined process for molecular evaluation, a push toward reflex testing, and standardization of biopsy techniques and tissue handling procedures. In our institution, we have initiated a Lean Six Sigma and PDSA (plan, do, study, act) initiative to improve our current molecular testing process. In addition, because obtaining larger-sample biopsies or additional biopsies is often not feasible for many of our advanced cancer patients, we have started using whole blood circulating tumor cells (CTC) and plasma ctDNA (cell-free circulating DNA) for molecular testing. Recent studies have shown high concordance (89%) between tissue biopsies and blood-based mutation testing, which will likely have a positive impact on the cancer care of our patients and help to capture a subset of patients who are not candidates for traditional biopsies.37
Conclusions
Despite current guidelines for testing driver mutations in advanced nsNSCLC, a large segment of our patients are not being tested for those genetic aberrations. There are several barriers that continue to thwart the recommendation, including failure to integrate driver mutation testing into routine pathology practice (ie, reflex testing), insufficient tissue obtained from biopsy, and difficulty in obtaining tissue because of tumor location or risk of complications from the biopsy procedure. More important, these trends are not isolated to our institution and reflect a significant challenge within the oncology community. Our data show that for the purpose of driver mutation testing, larger-sample biopsies, such as surgical/core biopsies, are better than small-sample biopsies, such as needle aspiration. We have also demonstrated that the prevalence of driver mutations is lower in Western Pennsylvania, which is served by our network, than elsewhere in the United States.
Lung cancer is the leading cause of cancer death in the United States. It is estimated that there will be 222,500 new cases of lung cancer and 155,870 deaths from lung cancer in 2017. Non–small-cell lung carcinoma (NSCLC) accounts for 80%-85% of lung cancers, with adenocarcinoma being the most common histologic subtype. Other less common subtypes include squamous-cell carcinoma, large-cell carcinoma, and NSCLC that cannot be further classified.1 Nearly 70% of patients present with locally advanced or metastatic disease at the time of diagnosis and are not candidates for surgical resection.2 For that group of patients, the mainstay of treatment is platinum-based chemotherapy with or without radiation therapy. Patients who are chemotherapy naive often experience a modest response, however; durable remission is short lived, and the 5-year survival rate remains staggeringly low.3 Improved understanding of the molecular pathways that drive malignancy in NSCLC has led to the development of drugs that target specific molecular pathways.4 By definition, these driver mutations facilitate oncogenesis by conferring a selective advantage during clonal evolution.5 Moreover, agents targeting these pathways are extremely active and induce durable responses in many patients.6,7,8
Predictive biomarkers in NSCLC include anaplastic lymphoma kinase (ALK) fusion oncogene and sensitizing epidermal growth factor receptor (EGFR) mutations. Mutations in the EGFR tyrosine kinase are observed in about 15%-20% of NSCLC adenocarcinomas in the United States and upward of 60% in Asian populations. They are also found more frequently in nonsmokers and women.6 The two most prevalent mutations in the EGFR tyrosine kinase domain are in-frame deletions of exon 19 and L858R substitution in exon 21, representing about 45% and 40% of mutations, respectively.9 Both mutations result in activation of the tyrosine kinase domain, and both are associated with sensitivity to the small-molecule tyrosine kinase inhibitors (TKIs), such as erlotinib, gefitinib, and afatinib.10 Other drug-sensitive mutations include point mutations at exon 21 (L861Q) and exon 18 (G719X).11 Targeted therapy produces durable responses in the majority of patients.12,13,14 Unfortunately, most patients develop acquired resistance to these therapies, which leads to disease progression.4,15-17
ALK gene rearrangements, although less prevalent, are another important molecular target in NSCLC and are seen in 2%-7% of cases in the United States.7 As with EGFR mutations, these mutations are more prevalent in nonsmokers, and they are found more commonly in younger patients and in men.8
Identification of driver mutations early in the course of disease and acquired resistance mutations later are crucial for the optimal management of advanced NSCLC. DNA analysis using polymerase chain reaction (PCR) and next-generation sequencing is the preferred method for testing for EGFR mutations, and ALK rearrangements are generally tested either by flourescence in situ hybridization (FISH) or immunohistochemistry.18,19 Newer blood-based assays have shown great promise, and clinicians may soon have the ability to monitor subtle genetic changes, identify resistance patterns, and change therapy when acquired resistance occurs.20
The American College of Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology have proposed guidelines for molecular testing in lung cancer. It is recommended that all advanced squamous and nonsquamous cell lung cancers with an adenocarcinoma component should be tested for EGFR and ALK mutations independent of age, sex, ethnicity, or smoking history. In the setting of smaller lung cancer specimens (eg, from biopsies, cytology) where an adenocarcinoma component cannot be completely excluded, EGFR and ALK testing may be performed in cases showing squamous or small cell histology but clinical criteria (eg, young age, lack of smoking history) may be useful in selecting a subset of these samples for testing. Samples obtained through surgical resection, open biopsy, endoscopy, transthoracic needle biopsy, fine-needle aspiration, and thoracentesis are all considered suitable for testing, but large biopsy samples are generally preferred over small biopsy samples, cell-blocks, and cytology samples.21 Despite this recommendation, not all patients who are eligible for mutation analysis are tested. At our institution, preliminary observations suggested that the percentage of patients being tested and the prevalence of driver mutations were significantly lower compared with published data. The purpose of this study was to evaluate physician attitudes about molecular testing, and to determine the rate of testing, the effect of biopsy sample size on rate of testing, and the prevalence of driver mutations at our institution.
Methods
In this retrospective clinical study, we identified 206 cases of advanced nsNSCLC from the tumor registry (February 2011-February 2013). Registry data was obtained from three hospitals within our health network – two academic tertiary care centers, and one community-based hospital. The other hospitals in the network were excluded because their EHR systems were not integrated with the rest of the hospitals and/or there was a lack of registry data. The testing rates for driver mutations, prevalence of driver mutations, and the tissue procurement techniques were obtained from individual chart review. Surgical specimens, core biopsy samples, and large volume thoracentesis specimens were categorized as large biopsy samples, and samples obtained by fine-needle aspiration, bronchial washing, and bronchial brushing were considered small biopsy samples. We used a chi-square analysis to compare mutation testing rates between the large and small biopsy sample groups. The prevalence of driver mutations was determined, excluding unknown or inadequate samples.
EGFR analysis had been conducted at Integrated Oncology, using formalin-fixed, paraffin-embedded tissue. Genomic DNA was isolated, and EGFR mutation analysis was performed using SNaPShot multiplex PCR, primer extension assay for exons 18-21; samples with >4mm2 and ≥50% tumor content were preferred. Macrodissection was used to enrich for tumor cells when samples had lower tumor cellularity and content. ALK rearrangements were tested in the hospital using the Vysis ALK Break Apart FISH probe kit (Abott Molecular Inc, Des Plaines, IL).
We conducted a web-based, 20-question survey about molecular profiling among 110 practitioners to gauge their knowledge and opinions about molecular testing. The practitioners included medical oncologists, thoracic surgeons, pulmonologists, and interventional radiologists. Each received an initial e-mail informing them of the study, inviting them to complete survey, and providing a link to it, and two reminder e-mails at biweekly intervals to maximize survey participation and responses. The questions were aimed at understanding the challenges surrounding molecular testing within our network. Apart from the questions gathering demographic information about the respondents, the questions were intended to highlight the disparities between guideline recommendations and physician practices; to gauge the perceived importance of molecular evaluation; to identify individual, subspecialty, and hospital-based challenges; and to assess physician attitudes toward alternatives to traditional tissue-based testing (Table 1, p. e150). Nineteen of the questions were structured as single or best answer, whereas Question 9, which was aimed at identifying system-based challenges, allowed for multiple answer selections.
Results
There were a total of 206 cases of advanced stage IIIb or IV nsNSCLC identified at three hospitals during 2011-2013. Of those 206 cases, 161 (78.2%) were recorded at the two large academic medical centers, and 45 (21.9%) were recorded at the smaller community-based hospital. Of the total, there were 145 (70.4%) large biopsy specimens and 61 (29.6%) small biopsy specimens. We found that 89 of the 206 cases (43.2 %) had been tested for EGFR mutations, and 49 (23.8%) had been tested for ALK rearrangements (Figure, A and C). In all, 70 (48.3%) large-sample biopsies and 19 (31.1%) small-sample biopsies were submitted for EGFR analysis (Figure, B), and 42 (29%) large-sample biopsies and 7 (11.5%) small-sample biopsies were tested for ALK rearrangements (Figure, D). Large-sample biopsies were more likely to be analyzed for EGFR mutations and ALK rearrangements, with the results reaching statistical significance (P = .023 and P = .007, respectively). Across all samples, a total of 7 EGFR mutations and 1 ALK rearrangement were identified, yielding a prevalence of 7.9% and 2% respectively (Figure, A and C).
Table 2 shows the demographics, smoking status and type of driver mutation identified. Core biopsies were obtained in 45.6% of the cases and fine-needle aspiration biopsies were obtained in 25.2% of the cases with surgical resections, with thoracentesis and bronchial washings comprising the rest of the biopsies (Table 3).
The average age at diagnosis of the patients in the cases that were analyzed was 69.3 years. Most of the patients (83.9%) identified as white, 3.8% were African American, and 12.6% were in the Unknown category. Of the total number of patients, 11 were identified as never-smokers (5.3%), 50 (24.3%) had a 1-15 pack-year smoking history, 104 (50.5%) had a 16-45 pack-year smoking history, and 41 (19.9%) had a >45 pack-year smoking history.
In regard to the survey, 46 of the 110 physicians asked to participate in the survey responded, representing a response rate of 41.8% (range across medical specialties, 26%-45%, Table 4). Of those respondents, 38 (82.6%) indicated they believed molecular evaluation was a very important aspect of NSCLC care, with the remainder indicating it was somewhat important. 91.4% of the respondents who routinely ordered molecular testing agreed that stage IIIb or IV nsNSCLC should undergo molecular evaluation.
The top barriers to molecular evaluation identified through this survey were the availability of sufficient tissue to complete molecular testing and the Center for Medicare and Medicaid Services’s (CMS’s) 14-day rule that requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing (Table 5).
Discussion
The treatment of advanced nsNSCLC has evolved significantly over the past decade. Molecular profiling is now an essential part of initial evaluation, and larger-sample biopsies are needed to ensure accurate evaluation and appropriate treatment. The detection of EGFR and EML4-ALK driver mutations are associated with increased response to tyrosine kinase inhibitors and are associated with improvement in progression-free survival, patient quality of life, and even overall survival in some studies.12,22,23,24 Early identification of these driver mutations is crucial, however, preliminary observation in our network suggested that a large percentage of patients with advanced nsNSCLC in were not being appropriately evaluated for those mutations. To evaluate our molecular profiling rates, we conducted a retrospective study and reviewed 3 years of registry data at 3 hospitals within our health system. Two of the hospitals included in our analysis were large tertiary academic centers, and one was a community hospital. Our findings confirmed that a large percentage of our patients who are eligible for molecular evaluation are not tested: 56.7% of cases were not tested for EGFR mutations, and 76.2% of cases were not tested for ALK rearrangements.
In a similar study, the Association for Community Cancer Centers conducted a project aimed at understanding the landscape and current challenges for molecular profiling in NSCLC. Eight institutions participated in the study, and baseline testing rates were analyzed. The findings demonstrated that high-volume institutions (treating >100 lung cancer patients a year tested 62% and 60% of advanced lung cancer patients for EGFR and EML4-ALK, respectively, and low-volume institutions (treating <100 lung cancer patients a year tested 52% and 47% for EGFR and EML4-ALK, respectively.25,26 In a recent international physician self-reported survey, Spicer and colleagues found that EGFR testing was requested before first-line therapy in patients with stage IIIB or IV disease in 81% of cases, and mutation results were available before start of therapy in 77% of the cases.27 Those percentages are relatively low, given that current guidelines recommend that molecular testing should be done for all patients with stage IIIB or IV nsNSCLC. This highlights the need for objective performance feedback so oncologists can make the necessary practice changes so that molecular testing is done before the start of therapy to ensure high-quality cancer care that will translate into better, cost-effective outcomes and improved patient quality of life.
Our study findings showed that the prevalence of EGFR and ALK mutations is substantially lower among the patients we treat in our network compared with other published data on prevalence. The reason for those low rates is not clear, but it is likely multifactorial. First, Western Pennsylvania, the region our network serves, has a large proportion of older adults – 17.3% of the population is older than 65 years (national average, 14.5%) and advanced age might have contributed to the lower EGFR and ALK rates measured in our study.28 Second, the smoking rate in Pennsylvania is higher than the national average, 20%-24% compared with 18%, respectively.29 Third, the air quality in Western Pennsylvania has historically been very poor as a result of the large steel and coal mining industries. Even though the air quality has improved in recent decades, the American Lung Association’s 2017 State of the Air report ranked Pittsburgh and surrounding areas in Western Pennsylvania among the top 25 most air polluted areas in the United States.30 It is not certain whether air pollution and air quality have any impact on driver mutation rates, but the correlation with smoking, ethnicity, and geographic distribution highlight the need for further epidemiologic studies.
Biopsy sufficiency – getting an adequate amount of sample tissue during biopsy – is a known challenge to molecular profiling, and we found that biopsy sample size had an impact on the testing rates in a large percentage of our cases. To fully understand the impact of biopsy sufficiency, we conducted a subset analysis and compared the testing rates between our large and small biopsy samples. Our analysis showed that larger-sample biopsies were more likely to be tested for mutations than were smaller-sample biopsies (EGFR: P = .023; ALK: P = .007).
Those results suggest that larger-sample biopsies should be encouraged, but procedural risks, tumor location, and patient age and wishes need to be considered before tissue acquisition.21 Furthermore, clinicians who are responsible for tissue procurement need to be properly educated on the tissue sample requirements and the impact these results have on treatment decisions.31 Our institution, like many others, has adopted rapid onsite evaluation (ROSE) of biopsy samples, whereby a trained cytopathologist reviews sample adequacy at the time of tissue procurement. Although there is scant data directly comparing molecular testing success rates with and without the ROSE protocol, a meta-analysis conducted by Schmidt and colleagues concluded that ROSE improved the adequacy rate of fine-needle aspiration cytology by 12%.32,33 Given that molecular profiling depends on both the absolute and relative amount of tumor cells present in the sample, the ROSE protocol likely enhances the procedural success rate and reduces the need for repeat and subsequent biopsies.
It is interesting to note that our data also demonstrated that we are obtaining large-sample biopsies in most of our patients (about 70%). However, we are still failing to test more than half of our cases for driver mutations (Figure, A and C). This strongly suggests there are additional factors beyond tissue adequacy that are contributing to our high failure rate. It is essential to understand the dynamics and system practices that influence testing rates if we are to improve the care and outcomes of our cancer patients. To better understand those barriers, we surveyed 110 practitioners (including medical oncologists, pulmonologists, thoracic surgeons, and interventional radiologists) about the molecular profiling process and their responses highlighted several important areas that deserve special attention (Tables 1, 4, 5).
In our institution, testing initiation is primarily the responsibility of the treating medical oncologist. This presents a challenge because there is often a significant delay between tissue acquisition, histologic confirmation, and oncologic review. Many institutions have adopted pathology-driven reflex testing to help overcome such delays. Automatic testing after pathologic confirmation streamlines the process, increases testing rates, and eliminates unnecessary delay between the time of diagnosis and the time of test ordering.34 It also allows for the molecular and histologic diagnosis to be integrated into a single pathology report before therapy is initiated.
Another barrier to timely testing according to the respondents, was the CMS’s 14-day rule. The 14-day rule requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing and was frequently identified as a cause for significant delay in testing and having an impact on first-line treatment decisions.35,36
Often clinicians will choose to defer testing until this time has elapsed to reduce the financial burden placed on the hospital but by that time, they might well have initiated treatment without knowing if the patient has a mutation. This is a significant challenge identified by many of our oncologists, and is a limitation to our analysis above as it is unclear what percentage of patients received follow up testing once care was established at an outside facility and once the 14-day time period had elapsed.
The data from our institution suggests there is discordance between physician attitudes and molecular testing practices. However, there are several limitations in our study. First, most of the survey respondents agreed that molecular testing is an important aspect of treating advanced lung cancer patients, but the retrospective nature of the study made it difficult to identify why testing was deferred or never conducted. Second, the absence of a centralized reporting system for molecular testing results at our institution, may have resulted in an overestimation of our testing failure rate in cases where results were not integrated our electronic medical record.
Third, the low survey response rate only allowed us to make generalizations regarding the conclusions, although it does provide a framework for future process improvements.
We believe the poor testing rates observed in our study are not isolated to our institution and reflect a significant challenge within the broader oncology community.27 A system of best practices is essential for capturing this subset of patients who are never tested. There is agreement among oncologists that improving our current testing rates will require a multidisciplinary approach, a refined process for molecular evaluation, a push toward reflex testing, and standardization of biopsy techniques and tissue handling procedures. In our institution, we have initiated a Lean Six Sigma and PDSA (plan, do, study, act) initiative to improve our current molecular testing process. In addition, because obtaining larger-sample biopsies or additional biopsies is often not feasible for many of our advanced cancer patients, we have started using whole blood circulating tumor cells (CTC) and plasma ctDNA (cell-free circulating DNA) for molecular testing. Recent studies have shown high concordance (89%) between tissue biopsies and blood-based mutation testing, which will likely have a positive impact on the cancer care of our patients and help to capture a subset of patients who are not candidates for traditional biopsies.37
Conclusions
Despite current guidelines for testing driver mutations in advanced nsNSCLC, a large segment of our patients are not being tested for those genetic aberrations. There are several barriers that continue to thwart the recommendation, including failure to integrate driver mutation testing into routine pathology practice (ie, reflex testing), insufficient tissue obtained from biopsy, and difficulty in obtaining tissue because of tumor location or risk of complications from the biopsy procedure. More important, these trends are not isolated to our institution and reflect a significant challenge within the oncology community. Our data show that for the purpose of driver mutation testing, larger-sample biopsies, such as surgical/core biopsies, are better than small-sample biopsies, such as needle aspiration. We have also demonstrated that the prevalence of driver mutations is lower in Western Pennsylvania, which is served by our network, than elsewhere in the United States.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
3. Kim TE, Murren JR. Therapy for stage IIIB and stage IV non-small cell lung cancer. Clin Chest Med. 2002;23(1):209-224.
4. Black RC, Khurshid H. NSCLC: An update of driver mutations, their role in pathogenesis and clinical significance. R I Med J (2013). 2015;98(10):25-28.
5. Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306-313.
6. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327-3334.
7. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866-2874.
8. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246.
9. Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(suppl 1):S24-31.
10. Langer CJ. Epidermal growth factor receptor inhibition in mutation-positive non-small-cell lung cancer: is afatinib better or simply newer? J Clin Oncol. 2013;31(27):3303-3306.
11. Riely GJ, Politi KA, Miller VA, et al. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12(24):7232-7241.
12. Shi Y, Siu-Kie JA, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154-162.
13. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957.
14. Khozin S, Blumenthal GM, Jiang X, et al. US Food and Drug Administration approval summary: Erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist. 2014;19(7):774-779.
15. Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12(2):220-229.
16. Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73.
17. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240-2247.
18. Ellison G, Zhu G, Moulis A, Dearden S, et al. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol. 2013;66(2):79-89.
19. Alì G, Proietti A, Pelliccioni S, et al. ALK rearrangement in a large series of consecutive non-small cell lung cancers: comparison between a new immunohistochemical approach and fluorescence in situ hybridization for the screening of patients eligible for crizotinib treatment. Arch Pathol Lab Med. 2014;138(11):1449-1158.
20. Crowley E, Di Nicolantonio F, Loupakis F, et al. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10(8):472-484.
21. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823-859.
22. Kwak EL, Bany YJ, Cambridge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693-1703.
23. Shaw A, Yeap BY, Kenudson MM, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247-4253.
24. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380-2388.
25. Association of Community Cancer Centers. Molecular Testing in the Community Setting. In: Molecular testing: resources and tools for the multidisciplinary team. http://accc-cancer.org/resources/molecularTesting-Overview.asp. Accessed November 15, 2015.
26. Association of Community Cancer Centers. Molecular testing: ACCC peer-to-peer webinars. The tissue issue: sampling and testing with Gail Probst, RN, MS, AOCN. https://www.youtube.com/watch?v=lapmni938Mc&feature=youtu.be. Published September 14, 2015. Accessed November 2015.
27. Spicer J S, Tischer B, Peters M. EGFR mutation testing and oncologist treatment choice in advanced NSCLC: global trends and differences. Ann Oncol. 2015;26(suppl 1):i60.
28. West L, Cole S, Goodkind D. US Census Bureau, 65+ in the United States: 2010, U.S. Government Printing Office, Washington, DC, 2014
29. Centers for Disease Control and Prevention. State tobacco activities tracking and evaluation system. Current cigarette use among adults (Behavior Risk Factor Surveillance System) 2015. https://www.cdc.gov/statesystem/cigaretteuseadult.html. Last updated September 16, 2016. Accessed May 26, 2017.
30. The American Lung Association. State of the Air 2017. http://www.lung.org/assets/documents/healthy-air/state-of-the-air/state-of-the-air-2017.pdf. Published 2017. Accessed May 26, 2017.
31. Gaga M, Powell CA, Schraufnagel DE, Schönfeld N, et al. An official American Thoracic Society/European Respiratory Society statement: the role of the pulmonologist in the diagnosis and management of lung cancer. Am J Respir Crit Care Med. 2013;188(4):503-507.
32. Ferguson PE, Sales CM, Hodges DC, et al. Effects of a multidisciplinary approach to improve volume of diagnostic material in CT-guided lung biopsies. PLoS One. 2015 Oct 19;10(10).
33. Schmidt RL, Witt BL, Lopez-Calderon LE, et al. The influence of rapid onsite evaluation on the adequacy rate of fine-needle aspiration cytology: a systematic review and meta-analysis. Am J Clin Pathol. 2013;139(3):300-309.
34. Cengiz Inal, Yilmaz E, Chenget H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: Experience from a community-based academic center. J Clin Oncol. 2014;32(suppl):5s. [abstract 8098].
35. Grzegorz K, Leighl, M. Challenges in NSCLC molecular testing barriers to implementation. Oncology Exchange. 2012;11(4):8-10.
36. Lynch JA, Khoury MJ, Ann Borzecket A, et al. Utilization of epidermal growth factor receptor (EGFR) testing in the United States: a case study of T3 translational research. Genet Med. 2013;15(8):630-638.
37. Reck M. Investigating the utility of circulating-free tumour-derived DNA (ctDNA) in plasma for the detection of epidermal growth factor receptor (EGFR) mutation status in European and Japanese patients (pts) with advanced non-small-cell lung cancer (NSCLC): ASSESS study. Presented at the European Lung Cancer Conference (ELCC) Annual Meeting, Geneva; 15-18 April 2015.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
3. Kim TE, Murren JR. Therapy for stage IIIB and stage IV non-small cell lung cancer. Clin Chest Med. 2002;23(1):209-224.
4. Black RC, Khurshid H. NSCLC: An update of driver mutations, their role in pathogenesis and clinical significance. R I Med J (2013). 2015;98(10):25-28.
5. Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306-313.
6. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327-3334.
7. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866-2874.
8. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246.
9. Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(suppl 1):S24-31.
10. Langer CJ. Epidermal growth factor receptor inhibition in mutation-positive non-small-cell lung cancer: is afatinib better or simply newer? J Clin Oncol. 2013;31(27):3303-3306.
11. Riely GJ, Politi KA, Miller VA, et al. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12(24):7232-7241.
12. Shi Y, Siu-Kie JA, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154-162.
13. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957.
14. Khozin S, Blumenthal GM, Jiang X, et al. US Food and Drug Administration approval summary: Erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist. 2014;19(7):774-779.
15. Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12(2):220-229.
16. Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73.
17. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240-2247.
18. Ellison G, Zhu G, Moulis A, Dearden S, et al. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol. 2013;66(2):79-89.
19. Alì G, Proietti A, Pelliccioni S, et al. ALK rearrangement in a large series of consecutive non-small cell lung cancers: comparison between a new immunohistochemical approach and fluorescence in situ hybridization for the screening of patients eligible for crizotinib treatment. Arch Pathol Lab Med. 2014;138(11):1449-1158.
20. Crowley E, Di Nicolantonio F, Loupakis F, et al. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10(8):472-484.
21. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823-859.
22. Kwak EL, Bany YJ, Cambridge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693-1703.
23. Shaw A, Yeap BY, Kenudson MM, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247-4253.
24. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380-2388.
25. Association of Community Cancer Centers. Molecular Testing in the Community Setting. In: Molecular testing: resources and tools for the multidisciplinary team. http://accc-cancer.org/resources/molecularTesting-Overview.asp. Accessed November 15, 2015.
26. Association of Community Cancer Centers. Molecular testing: ACCC peer-to-peer webinars. The tissue issue: sampling and testing with Gail Probst, RN, MS, AOCN. https://www.youtube.com/watch?v=lapmni938Mc&feature=youtu.be. Published September 14, 2015. Accessed November 2015.
27. Spicer J S, Tischer B, Peters M. EGFR mutation testing and oncologist treatment choice in advanced NSCLC: global trends and differences. Ann Oncol. 2015;26(suppl 1):i60.
28. West L, Cole S, Goodkind D. US Census Bureau, 65+ in the United States: 2010, U.S. Government Printing Office, Washington, DC, 2014
29. Centers for Disease Control and Prevention. State tobacco activities tracking and evaluation system. Current cigarette use among adults (Behavior Risk Factor Surveillance System) 2015. https://www.cdc.gov/statesystem/cigaretteuseadult.html. Last updated September 16, 2016. Accessed May 26, 2017.
30. The American Lung Association. State of the Air 2017. http://www.lung.org/assets/documents/healthy-air/state-of-the-air/state-of-the-air-2017.pdf. Published 2017. Accessed May 26, 2017.
31. Gaga M, Powell CA, Schraufnagel DE, Schönfeld N, et al. An official American Thoracic Society/European Respiratory Society statement: the role of the pulmonologist in the diagnosis and management of lung cancer. Am J Respir Crit Care Med. 2013;188(4):503-507.
32. Ferguson PE, Sales CM, Hodges DC, et al. Effects of a multidisciplinary approach to improve volume of diagnostic material in CT-guided lung biopsies. PLoS One. 2015 Oct 19;10(10).
33. Schmidt RL, Witt BL, Lopez-Calderon LE, et al. The influence of rapid onsite evaluation on the adequacy rate of fine-needle aspiration cytology: a systematic review and meta-analysis. Am J Clin Pathol. 2013;139(3):300-309.
34. Cengiz Inal, Yilmaz E, Chenget H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: Experience from a community-based academic center. J Clin Oncol. 2014;32(suppl):5s. [abstract 8098].
35. Grzegorz K, Leighl, M. Challenges in NSCLC molecular testing barriers to implementation. Oncology Exchange. 2012;11(4):8-10.
36. Lynch JA, Khoury MJ, Ann Borzecket A, et al. Utilization of epidermal growth factor receptor (EGFR) testing in the United States: a case study of T3 translational research. Genet Med. 2013;15(8):630-638.
37. Reck M. Investigating the utility of circulating-free tumour-derived DNA (ctDNA) in plasma for the detection of epidermal growth factor receptor (EGFR) mutation status in European and Japanese patients (pts) with advanced non-small-cell lung cancer (NSCLC): ASSESS study. Presented at the European Lung Cancer Conference (ELCC) Annual Meeting, Geneva; 15-18 April 2015.
Comprehensive assessment of cancer survivors’ concerns to inform program development
Complex cancer treatments, limited personnel resources, and a growing number of cancer survivors are challenging cancer health care professionals’ abilities to provide comprehensive care. Cancer survivors have a range of needs that extend over the cancer care trajectory and that represent physical, psychological, social, and spiritual domains. Numerous studies have explored supportive care needs and recent systematic reviews have highlighted the supportive care needs related to cancer1 and to specific cancer types, including prostate cancer,2 breast cancer,3 gynecologic cancer,4 hematological cancer,5 and lung cancer.6 However, reviews are limited in that they do not always assess needs across the cancer trajectory or identify demographic or clinical variables that are associated with needs. These data are needed to focus survivorship program development in cancer centers in order to target populations most likely at risk for unmet needs, identify what salient concerns to address, and to appropriately schedule supportive care programs.
The importance of assessing the patient’s subjective view of his/her needs or concerns is well acknowledged as being fundamental to patient-centered care.7 Clinicians routinely assess needs in practice using a variety of screening tools. However, there needs to be a broader assessment of concerns and needs in a population of survivors with mixed cancer diagnoses, along with their appraisal of how well their needs were addressed by their health care team, to provide an overall identification of gaps in supportive care. The primary purpose of the present study was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs; ascertain survivors’ perceived importance of those needs and the extent to which our institution, the University Hospitals Seidman Cancer Center, was attentive to those needs; and to identify who might be at risk for having greater concerns. The overall goal was to use the data to inform survivorship and supportive care program development.
Methods
Design, sample and setting
We used a cross-sectional design. Surveys were mailed once to a convenience sample of 2,750 adult patients who had been seen in follow-up during the previous 2 years (2010-2011) at all clinical sites of University Hospitals Seidman Cancer Center, a Midwestern National Cancer Institute-designated Comprehensive Cancer Center. Patients who had a noncancer diagnosis were excluded. The distribution list was screened for deceased individuals and those patients who had multiple visits during the time period. The project was reviewed and approved as nonresearch by the Case Western Reserve University Cancer Institutional Review Board.
Survey
An interdisciplinary team of clinicians, administrators, and researchers adapted the Mayo Clinic Cancer Center’s Cancer Survivors Survey of Needs8 to create a comprehensive survey for the cancer center. Input regarding the scope of the survey was sought from the Patient and Family Advisory Council of the cancer center. The survey, which was formatted for scanning purposes, consisted of 33 questions that were compiled into 4 sections. Sections 1 and 2 focused on demographic and treatment-related information, including use of community and hospital support services and preferences for follow-up care. In section 3, a quality-of-life framework was used to assess physical, social, emotional, and spiritual needs. Respondents were asked to rate their current level of concern for 19 physical effects, 10 social effects, 10 emotional effects, and 5 spiritual effects on a scale ranging from 0 (no concern) to 5 (extreme concern). In section 4, respondents were asked to indicate the importance of the cancer team addressing their physical, social, emotional, and spiritual needs. This was followed by their rating of the cancer team’s attention to their needs as Poor, Fair, Good, Excellent, or They did not ask about my needs. Respondents were asked about preferences for learning about physical, social, emotional, and spiritual effects. In addition to the 33 questions, there were 6 open-ended questions in which respondents were encouraged to share additional information about their needs, sources of support, and other concerns.
Procedures
Eligible respondents were mailed a cover letter explaining the survey from both the director and president of the cancer center, a survey, and a postage-paid return envelope. The option to respond to the survey by a telephone call to the director of the Office of Cancer Survivorship was offered in the cover letter.
Data analysis
Returned surveys were scanned into a Teleform database, verified, and exported into an SPSS data file. Data quality was checked by running frequency analyses and summarizing variables. Time-since-treatment responses were collapsed into 4 categories: on treatment, up to 2 years posttreatment, 2-5 years posttreatment, and more than 5 years posttreatment. Descriptive statistics were used to summarize demographic and medical characteristics of the respondents and to calculate the mean score for each concern for the total sample and then for each category of time since treatment. Because of the large number of respondents with breast cancer, the respondents were stratified into two groups, one of breast cancer the other of nonbreast cancer respondents. Then, the Mann-Whitney test was performed for each concern to examine differences between respondents with and without breast cancer.
To identify the most prevalent concerns, ratings for concerns were recoded into no concern (rated as 0), low concern (1 or 2), and moderate/high concern (3, 4, or 5). Since our interest was in the moderate and high concerns, the responses were dichotomized into moderate/high concerns and all other levels. Logistic regression models were then used to identify associations between a set of survivor characteristics or covariates (age, sex, living status, marital status, employment status, cancer type, and time since treatment) with the 12 most highly rated moderate/high concerns. All the analyses were performed using statistical software SPSS 20 and Stata 13.0
Results
Respondents
A total of 1,005 surveys were returned for a 37% response rate. Forty-two patients responded by telephone. The mean age of respondents was 64.9 years (range, 22-98; SD, 12.8). The typical respondent was female, white, and married (Table 1). Twenty-four percent of the respondents (n = 240) reported living alone. Although about 47% of respondents (n = 473) reported a breast cancer diagnosis, more than 17 cancers were identified, and 14% of respondents (n = 145) listed multiple diagnoses. About a third of respondents were receiving treatment when they completed the survey.
Just under half of the respondents (n = 498) reported using community resources for support and information about cancer, and 29.5% (n = 296) sought information on the internet during their cancer experience. The most commonly used community resources were The Gathering Place, a local organization offering free supportive programs and services to individuals with cancer and their families (n = 167), and the American Cancer Society (n = 138). Of the 496 respondents who reported accessing hospital resources, most (n = 322) said they used information that their health care team recommended. Other supportive options were used to a lesser degree: support groups (n = 92), chemotherapy and radiation therapy classes (n = 129), and supportive/educational programs offered by the cancer center (n = 27). Most of the respondents (n = 822, 88.6%) preferred to have their follow-up care remain with their cancer care team 1 year after treatments are completed. Almost two-thirds of respondents (n = 601, 64%) cited being seen at the cancer center for follow-up care as the most important factor in considering follow-up care.
Concerns In determining whether the large proportion of respondents with breast cancer skewed the study results, it was determined that median scores differed significantly in only four concerns. Compared with respondents without breast cancer, respondents with breast cancer were more likely to have significantly lower scores for concerns related to fatigue (P <.001) and sexual issues/intimacy (P = .001). Respondents with breast cancer were more likely to have significantly higher scores than respondents without breast cancer for concerns related to genetic counseling (P = .001) and fear of developing a new cancer (P = .010).
Fears of the cancer returning and developing a new cancer were the two most prevalent concerns, identified by 51% (n = 486) and 47.5% (n = 459), respectively (Table 2). Physical concerns, rated as moderate/high concerns by at least 25% of the sample, were fatigue (n = 336, 34.8%), changes in [the] body after cancer (n = 323, 33.7%), trouble sleeping (n = 302, 31.0%), sexual issues/intimacy (n = 263, 28.0%), memory and concentration (n = 261, 26.7%), and weight changes (n = 248, 25.5%). The most prevalent moderate/high social concerns were related to finances (n = 265, 27.5%) and debt from medical bills (n = 232, 25.1%). Managing stress (n = 279, 29.2%) and difficult emotions (n = 244, 25.1%) were prevalent moderate/high emotional concerns. Spiritual concerns were less often rated as moderate/high concerns. Having a breast cancer diagnosis was not significantly related to the number of reported moderate to high concerns (P = 1.00).
Variables associated with the 12 most frequent moderate/high concerns are shown in Tables 3 and 4. Age was associated with the most moderate/high concerns. With every decade of age, the odds of having the following moderate/high concerns decreased: bodily changes after cancer (odds ratio [OR], 0.75), sexual intimacy (OR, 0.81), memory and concentration (OR, 0.83), weight changes (OR, 0.77), financial (OR, 0.75), debt (OR, 0.71), cancer returning (OR, 0.66), developing a new cancer (OR, 0.67), managing stress (OR, 0.67), and managing difficult emotions (OR, 0.67).
Female sex was associated with lower odds of having a concern about sexual intimacy (OR, 0.30) and increased odds of having concerns related to memory and concentration (OR, 1.78), managing stress (OR, 2.35), and managing difficult emotions (OR, 1.77). Race was another demographic characteristic statistically associated with numerous moderate/high concerns. Survivors who identified white, were more likely than other people of other races to have fewer moderate/high concerns regarding bodily changes after cancer (OR, 0.46), weight change (OR, 0.46), finances (OR, 0.46), debt (OR, 0.40), managing stress (OR, 0.55), and managing difficult emotions (OR, 0.49). The odds of having a moderate/high concern regarding debt was 2.25 times higher given widowed marital status compared with those survivors who were single. Unemployment status, when compared with full-time employment, was significantly associated with increased odds of having moderate/high concerns related to fatigue (OR, 2.08), bodily changes after cancer (OR, 1.72), memory and concentration (OR, 2.45), weight changes (OR, 2.17), finances (OR, 1.93), developing a new cancer (OR, 1.91), and managing difficult emotions (OR, 1.80).
As expected, respondents who had completed treatment were less likely to have many of the moderate/high concerns as those still undergoing treatment. Survivors who were up to 2 years posttreatment were significantly more likely than those survivors receiving treatment to have fewer moderate/high concerns regarding fatigue (OR, 0.56), sexual intimacy (OR, 0.54), weight change (OR, 0.55), fears of the cancer returning (OR, 0.48), developing a new cancer (OR, 0.35), managing stress (OR, 0.43), and managing difficult emotions (OR, 0.49).
However, those improved odds were not sustained over the cancer trajectory. Compared with survivors who were receiving treatment, survivors who were between 2-5 years posttreatment did not have significantly reduced odds for moderate/high concerns related to fatigue, sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress, and managing difficult emotions. They did have significantly reduced odds for having concerns only related to finances (OR, 0.61) and debt (OR, 0.52).
Long-term survivors, who were beyond 5 years posttreatment, had significantly reduced odds for having moderate/high concerns related to fatigue (OR, 0.45), finances (OR, 0.52), debt (OR, 0.47), and managing difficult emotions (OR, 0.54), compared with survivors receiving treatment. Moderate/high concerns related to sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress did not have improved odds for these long-term survivors.
Attention to needs
The health care teams were rated highly for their attention to the patients’ physical needs. Most respondents (n = 845, 92.4%) viewed the health care team’s attention their physical needs as important and 763 (77.6%) survivors rated the team’s attention to these needs as excellent. The importance of addressing emotional needs was affirmed by 723 (78.5%) respondents, and although 454 (46.8%) viewed the team’s attention to these needs as excellent, 119 (12.3%) reported that the health care team did not ask about emotional needs. In addition, 566 respondents (60%) viewed having the health care team address their social needs as important, and most (n = 715, 74.2%) rated the team’s attention to social needs as good or excellent. Yet, 162 (16.8%) respondents reported that team did not ask about their social needs. The health care team’s addressing of spiritual needs was viewed as important by 346 (37.5%) respondents and ratings for how well the team attended to spiritual needs were: 148 (15.6%) poor or fair, 204 (21.5%) good, and 150 (15.8%) excellent. However, 448 (47.2%) respondents reported that the health care team did not ask about their spiritual needs.
Discussion
The primary purpose of this project was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs and to assess the perceived importance of these needs and the extent to which the cancer center staff were attentive to those needs. The overall goal of this assessment was to inform the development of survivorship and supportive care programs by highlighting common concerns, demographic and medical factors associated with specific concerns, and timing of moderate/high level concerns along the cancer trajectory. There were 3 main findings.
First, the results support the need for enhancing supportive care services to meet emotional concerns of survivors beyond the treatment phase. Similar to other studies,8,9 emotional concerns ranked higher than all other concerns in this study with about 50% of the sample rating “fear the cancer will return” and “fear of developing a new cancer” as moderate/high concern. Although the odds of not having these emotional concerns improved up to 2 years posttreatment, these concerns are likely to resurface, as odds for survivors beyond 2 years were not significantly different from those receiving treatment. A recent systematic review reported that fear of cancer recurrence is experienced by about 73% of cancer survivors, with 49% reporting a moderate to high degree.10 It can have a chronic, stable trajectory for some survivors and is strongly associated with higher levels of anxiety, distress, and depression, and less global, emotional/mental, physical, role, social, and cognitive quality of life.10 In this sample, managing stress and difficult emotions were also rated as moderate/high concerns by at least 25% of the sample.
Second, the findings identified patients at risk for cancer-related concerns throughout the cancer trajectory. As demonstrated in other studies, younger age was associated with greater odds of having multiple greater moderate/high concerns.11-13 Unemployment was the second most common demographic factor associated with multiple moderate/high concerns related to physical symptoms, finances and emotions. Similarly, identifying as black, Asian, American Indian/Alaskan Native, or other was also associated with greater odds of having numerous physical, financial, and emotional concerns. Women had greater concerns related to memory, sexual intimacy, coping with difficult emotions, and stress.
Third, the results helped to identify gaps in supportive care at our cancer center. Although spiritual concerns were not prevalent as being moderate/high, they were still viewed by about a third of survivors as being an important area for the health care team to address. Yet, consistent with other need assessments, spiritual concerns in this study were least often addressed by staff.1 Assessment of spiritual care needs, screening for spiritual distress, and providing spiritual care are essential components of a clinician-patient relationship that supports healing.14 The importance of attending to spiritual care needs was underscored by a recent systematic review that found a positive association between overall spiritual well-being and quality of life in patients with cancer, with the meaning/peace factor consistently and positively associated with physical and mental health.15 Another identified gap was the health care team’s lack of attention to the patient’s social needs, which included concerns related to finances and debt from medical bills. In all, 46% of the respondents reported having financial concerns, with the odds of having moderate/high financial concerns being greatest during treatment to 2 years posttreatment. Attention to the financial burden of cancer patients is critical because the magnitude of cancer-related financial concerns is a significant, strong predictor of quality of life and adverse psychological issues such as depression, anxiety, and distress.16,17
There were several program implications based on the results. A periodic audit of the concerns of survivors and their views on how well their needs were being met was a relatively low cost endeavor. Although the findings were consistent with the literature, the results, when shared with administrators and clinicians, were instrumental in effecting change because they represented the concerns of survivors at the cancer center. Another program directive, based on the results, was to extend the routine screening of patients’ needs during treatment to posttreatment survivorship. Patients who are young, unemployed, do not identify as white, and female warrant more thorough assessment of needs and concerns along the cancer trajectory. Integral to these screenings is the need for patient-centered communication, with discussion of how cancer is affecting the different domains of quality of life within the context of the patient’s life. Lastly, the results clearly indicated the need for additional training of health care providers on how to assess and address spiritual well-being in cancer survivors.
There were limitations to this study, including use of a nonvalidated survey and cross-sectional approach that limited our ability to explore how concerns might change over the trajectory. Also, it was not possible to clarify medical information of the respondents, such as cancer stage. Although the response rate of this study was not high, we are confident in the results because of the large sample size and the finding that the large proportion of respondents with breast cancer was not influential. Despite these limitations, this needs assessment of cancer survivors over the trajectory of care provided insight into the scope of their concerns, identified vulnerable groups of survivors, and highlighted gaps in addressing those concerns. A quality- of-life framework for assessing needs assured a comprehensive focus and generated practice changes to strengthen holistic, comprehensive oncology care.
1. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
2. Paterson C, Robertson A, Smith A, Nabi G. Identifying the unmet supportive care needs of men living with and beyond prostate cancer: A systematic review. Eur J Oncol Nurs. 2015;19:405-418.
3. Fiszer C, Dolbeault S, Sultan S, Bredart A. Prevalence, intensity, and predictors of the supportive care needs of women diagnosed with breast cancer: A systematic review. Psychooncology. 2014;23:361-374.
4. Maguire R, Kotronoulas G, Simpson M, Paterson C. A systematic review of the supportive care needs of women living with and beyond cervical cancer. Gynecol Oncol. 2015;136:478-490.
5. Hall A, Lynagh M, Bryant J, Sanson-Fisher R. Supportive care needs of hematological cancer survivors: A critical review of the literature. Crit Rev Oncol Hematol. 2013;88:102-116.
6. Maguire R, Papadopoulou C, Kotronoulas G, Simpson MF, McPhelim J, Irvine L. A systematic review of supportive care needs of people living with lung cancer. Eur J Oncol Nurs. 2013;17:449-464.
7. Adler NE, Page EK. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academies Press; Institute of Medicine, 2008.
8. Ness S, Kokal J, Fee-Schroeder K, Novotny P, Satele D, Barton D. Concerns across the survivorship trajectory: results from a survey of cancer survivors. Oncol Nurs Forum. 2013;40:35-42.
9. Swash B, Hulbert-Williams N, Bramwell R. Unmet psychosocial needs in haematological cancer: A systematic review. Support Care Cancer. 2014;22:1131-1141.
10. Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: A systematic review of quantitative studies. J Cancer Surviv. 2013;7:300-322.
11. Choi KH, Park JH, Park JH, Park JS. Psychosocial needs of cancer patients and related factors: A multi-center, cross-sectional study in Korea. Psychooncology. 2013;22:1073-1080.
12. Pauwels EE, Charlier C, De Bourdeaudhuij I, Lechner L, Van Hoof E. Care needs after primary breast cancer treatment. Survivors’ associated sociodemographic and medical characteristics. Psychooncology. 2013;22:125-132.
13. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
14. Puchalski CM, Blatt B, Kogan M, Butler A. Spirituality and health: The development of a field. Academic Medicine. 2014;89:10-16.
15. Bai M, Lazenby M. A systematic review of associations between spiritual well-being and quality of life at the scale and factor levels in studies among patients with cancer. J Palliat Med. 2015;18:286-298.
16. Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Pract. 2014;10:332-338.
17. Sharp L, Carsin AE, Timmons A. Associations between cancer-related financial stress and strain and psychological wellbeing among individuals living with cancer. Psychooncology. 2013;22:745-755.
Complex cancer treatments, limited personnel resources, and a growing number of cancer survivors are challenging cancer health care professionals’ abilities to provide comprehensive care. Cancer survivors have a range of needs that extend over the cancer care trajectory and that represent physical, psychological, social, and spiritual domains. Numerous studies have explored supportive care needs and recent systematic reviews have highlighted the supportive care needs related to cancer1 and to specific cancer types, including prostate cancer,2 breast cancer,3 gynecologic cancer,4 hematological cancer,5 and lung cancer.6 However, reviews are limited in that they do not always assess needs across the cancer trajectory or identify demographic or clinical variables that are associated with needs. These data are needed to focus survivorship program development in cancer centers in order to target populations most likely at risk for unmet needs, identify what salient concerns to address, and to appropriately schedule supportive care programs.
The importance of assessing the patient’s subjective view of his/her needs or concerns is well acknowledged as being fundamental to patient-centered care.7 Clinicians routinely assess needs in practice using a variety of screening tools. However, there needs to be a broader assessment of concerns and needs in a population of survivors with mixed cancer diagnoses, along with their appraisal of how well their needs were addressed by their health care team, to provide an overall identification of gaps in supportive care. The primary purpose of the present study was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs; ascertain survivors’ perceived importance of those needs and the extent to which our institution, the University Hospitals Seidman Cancer Center, was attentive to those needs; and to identify who might be at risk for having greater concerns. The overall goal was to use the data to inform survivorship and supportive care program development.
Methods
Design, sample and setting
We used a cross-sectional design. Surveys were mailed once to a convenience sample of 2,750 adult patients who had been seen in follow-up during the previous 2 years (2010-2011) at all clinical sites of University Hospitals Seidman Cancer Center, a Midwestern National Cancer Institute-designated Comprehensive Cancer Center. Patients who had a noncancer diagnosis were excluded. The distribution list was screened for deceased individuals and those patients who had multiple visits during the time period. The project was reviewed and approved as nonresearch by the Case Western Reserve University Cancer Institutional Review Board.
Survey
An interdisciplinary team of clinicians, administrators, and researchers adapted the Mayo Clinic Cancer Center’s Cancer Survivors Survey of Needs8 to create a comprehensive survey for the cancer center. Input regarding the scope of the survey was sought from the Patient and Family Advisory Council of the cancer center. The survey, which was formatted for scanning purposes, consisted of 33 questions that were compiled into 4 sections. Sections 1 and 2 focused on demographic and treatment-related information, including use of community and hospital support services and preferences for follow-up care. In section 3, a quality-of-life framework was used to assess physical, social, emotional, and spiritual needs. Respondents were asked to rate their current level of concern for 19 physical effects, 10 social effects, 10 emotional effects, and 5 spiritual effects on a scale ranging from 0 (no concern) to 5 (extreme concern). In section 4, respondents were asked to indicate the importance of the cancer team addressing their physical, social, emotional, and spiritual needs. This was followed by their rating of the cancer team’s attention to their needs as Poor, Fair, Good, Excellent, or They did not ask about my needs. Respondents were asked about preferences for learning about physical, social, emotional, and spiritual effects. In addition to the 33 questions, there were 6 open-ended questions in which respondents were encouraged to share additional information about their needs, sources of support, and other concerns.
Procedures
Eligible respondents were mailed a cover letter explaining the survey from both the director and president of the cancer center, a survey, and a postage-paid return envelope. The option to respond to the survey by a telephone call to the director of the Office of Cancer Survivorship was offered in the cover letter.
Data analysis
Returned surveys were scanned into a Teleform database, verified, and exported into an SPSS data file. Data quality was checked by running frequency analyses and summarizing variables. Time-since-treatment responses were collapsed into 4 categories: on treatment, up to 2 years posttreatment, 2-5 years posttreatment, and more than 5 years posttreatment. Descriptive statistics were used to summarize demographic and medical characteristics of the respondents and to calculate the mean score for each concern for the total sample and then for each category of time since treatment. Because of the large number of respondents with breast cancer, the respondents were stratified into two groups, one of breast cancer the other of nonbreast cancer respondents. Then, the Mann-Whitney test was performed for each concern to examine differences between respondents with and without breast cancer.
To identify the most prevalent concerns, ratings for concerns were recoded into no concern (rated as 0), low concern (1 or 2), and moderate/high concern (3, 4, or 5). Since our interest was in the moderate and high concerns, the responses were dichotomized into moderate/high concerns and all other levels. Logistic regression models were then used to identify associations between a set of survivor characteristics or covariates (age, sex, living status, marital status, employment status, cancer type, and time since treatment) with the 12 most highly rated moderate/high concerns. All the analyses were performed using statistical software SPSS 20 and Stata 13.0
Results
Respondents
A total of 1,005 surveys were returned for a 37% response rate. Forty-two patients responded by telephone. The mean age of respondents was 64.9 years (range, 22-98; SD, 12.8). The typical respondent was female, white, and married (Table 1). Twenty-four percent of the respondents (n = 240) reported living alone. Although about 47% of respondents (n = 473) reported a breast cancer diagnosis, more than 17 cancers were identified, and 14% of respondents (n = 145) listed multiple diagnoses. About a third of respondents were receiving treatment when they completed the survey.
Just under half of the respondents (n = 498) reported using community resources for support and information about cancer, and 29.5% (n = 296) sought information on the internet during their cancer experience. The most commonly used community resources were The Gathering Place, a local organization offering free supportive programs and services to individuals with cancer and their families (n = 167), and the American Cancer Society (n = 138). Of the 496 respondents who reported accessing hospital resources, most (n = 322) said they used information that their health care team recommended. Other supportive options were used to a lesser degree: support groups (n = 92), chemotherapy and radiation therapy classes (n = 129), and supportive/educational programs offered by the cancer center (n = 27). Most of the respondents (n = 822, 88.6%) preferred to have their follow-up care remain with their cancer care team 1 year after treatments are completed. Almost two-thirds of respondents (n = 601, 64%) cited being seen at the cancer center for follow-up care as the most important factor in considering follow-up care.
Concerns In determining whether the large proportion of respondents with breast cancer skewed the study results, it was determined that median scores differed significantly in only four concerns. Compared with respondents without breast cancer, respondents with breast cancer were more likely to have significantly lower scores for concerns related to fatigue (P <.001) and sexual issues/intimacy (P = .001). Respondents with breast cancer were more likely to have significantly higher scores than respondents without breast cancer for concerns related to genetic counseling (P = .001) and fear of developing a new cancer (P = .010).
Fears of the cancer returning and developing a new cancer were the two most prevalent concerns, identified by 51% (n = 486) and 47.5% (n = 459), respectively (Table 2). Physical concerns, rated as moderate/high concerns by at least 25% of the sample, were fatigue (n = 336, 34.8%), changes in [the] body after cancer (n = 323, 33.7%), trouble sleeping (n = 302, 31.0%), sexual issues/intimacy (n = 263, 28.0%), memory and concentration (n = 261, 26.7%), and weight changes (n = 248, 25.5%). The most prevalent moderate/high social concerns were related to finances (n = 265, 27.5%) and debt from medical bills (n = 232, 25.1%). Managing stress (n = 279, 29.2%) and difficult emotions (n = 244, 25.1%) were prevalent moderate/high emotional concerns. Spiritual concerns were less often rated as moderate/high concerns. Having a breast cancer diagnosis was not significantly related to the number of reported moderate to high concerns (P = 1.00).
Variables associated with the 12 most frequent moderate/high concerns are shown in Tables 3 and 4. Age was associated with the most moderate/high concerns. With every decade of age, the odds of having the following moderate/high concerns decreased: bodily changes after cancer (odds ratio [OR], 0.75), sexual intimacy (OR, 0.81), memory and concentration (OR, 0.83), weight changes (OR, 0.77), financial (OR, 0.75), debt (OR, 0.71), cancer returning (OR, 0.66), developing a new cancer (OR, 0.67), managing stress (OR, 0.67), and managing difficult emotions (OR, 0.67).
Female sex was associated with lower odds of having a concern about sexual intimacy (OR, 0.30) and increased odds of having concerns related to memory and concentration (OR, 1.78), managing stress (OR, 2.35), and managing difficult emotions (OR, 1.77). Race was another demographic characteristic statistically associated with numerous moderate/high concerns. Survivors who identified white, were more likely than other people of other races to have fewer moderate/high concerns regarding bodily changes after cancer (OR, 0.46), weight change (OR, 0.46), finances (OR, 0.46), debt (OR, 0.40), managing stress (OR, 0.55), and managing difficult emotions (OR, 0.49). The odds of having a moderate/high concern regarding debt was 2.25 times higher given widowed marital status compared with those survivors who were single. Unemployment status, when compared with full-time employment, was significantly associated with increased odds of having moderate/high concerns related to fatigue (OR, 2.08), bodily changes after cancer (OR, 1.72), memory and concentration (OR, 2.45), weight changes (OR, 2.17), finances (OR, 1.93), developing a new cancer (OR, 1.91), and managing difficult emotions (OR, 1.80).
As expected, respondents who had completed treatment were less likely to have many of the moderate/high concerns as those still undergoing treatment. Survivors who were up to 2 years posttreatment were significantly more likely than those survivors receiving treatment to have fewer moderate/high concerns regarding fatigue (OR, 0.56), sexual intimacy (OR, 0.54), weight change (OR, 0.55), fears of the cancer returning (OR, 0.48), developing a new cancer (OR, 0.35), managing stress (OR, 0.43), and managing difficult emotions (OR, 0.49).
However, those improved odds were not sustained over the cancer trajectory. Compared with survivors who were receiving treatment, survivors who were between 2-5 years posttreatment did not have significantly reduced odds for moderate/high concerns related to fatigue, sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress, and managing difficult emotions. They did have significantly reduced odds for having concerns only related to finances (OR, 0.61) and debt (OR, 0.52).
Long-term survivors, who were beyond 5 years posttreatment, had significantly reduced odds for having moderate/high concerns related to fatigue (OR, 0.45), finances (OR, 0.52), debt (OR, 0.47), and managing difficult emotions (OR, 0.54), compared with survivors receiving treatment. Moderate/high concerns related to sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress did not have improved odds for these long-term survivors.
Attention to needs
The health care teams were rated highly for their attention to the patients’ physical needs. Most respondents (n = 845, 92.4%) viewed the health care team’s attention their physical needs as important and 763 (77.6%) survivors rated the team’s attention to these needs as excellent. The importance of addressing emotional needs was affirmed by 723 (78.5%) respondents, and although 454 (46.8%) viewed the team’s attention to these needs as excellent, 119 (12.3%) reported that the health care team did not ask about emotional needs. In addition, 566 respondents (60%) viewed having the health care team address their social needs as important, and most (n = 715, 74.2%) rated the team’s attention to social needs as good or excellent. Yet, 162 (16.8%) respondents reported that team did not ask about their social needs. The health care team’s addressing of spiritual needs was viewed as important by 346 (37.5%) respondents and ratings for how well the team attended to spiritual needs were: 148 (15.6%) poor or fair, 204 (21.5%) good, and 150 (15.8%) excellent. However, 448 (47.2%) respondents reported that the health care team did not ask about their spiritual needs.
Discussion
The primary purpose of this project was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs and to assess the perceived importance of these needs and the extent to which the cancer center staff were attentive to those needs. The overall goal of this assessment was to inform the development of survivorship and supportive care programs by highlighting common concerns, demographic and medical factors associated with specific concerns, and timing of moderate/high level concerns along the cancer trajectory. There were 3 main findings.
First, the results support the need for enhancing supportive care services to meet emotional concerns of survivors beyond the treatment phase. Similar to other studies,8,9 emotional concerns ranked higher than all other concerns in this study with about 50% of the sample rating “fear the cancer will return” and “fear of developing a new cancer” as moderate/high concern. Although the odds of not having these emotional concerns improved up to 2 years posttreatment, these concerns are likely to resurface, as odds for survivors beyond 2 years were not significantly different from those receiving treatment. A recent systematic review reported that fear of cancer recurrence is experienced by about 73% of cancer survivors, with 49% reporting a moderate to high degree.10 It can have a chronic, stable trajectory for some survivors and is strongly associated with higher levels of anxiety, distress, and depression, and less global, emotional/mental, physical, role, social, and cognitive quality of life.10 In this sample, managing stress and difficult emotions were also rated as moderate/high concerns by at least 25% of the sample.
Second, the findings identified patients at risk for cancer-related concerns throughout the cancer trajectory. As demonstrated in other studies, younger age was associated with greater odds of having multiple greater moderate/high concerns.11-13 Unemployment was the second most common demographic factor associated with multiple moderate/high concerns related to physical symptoms, finances and emotions. Similarly, identifying as black, Asian, American Indian/Alaskan Native, or other was also associated with greater odds of having numerous physical, financial, and emotional concerns. Women had greater concerns related to memory, sexual intimacy, coping with difficult emotions, and stress.
Third, the results helped to identify gaps in supportive care at our cancer center. Although spiritual concerns were not prevalent as being moderate/high, they were still viewed by about a third of survivors as being an important area for the health care team to address. Yet, consistent with other need assessments, spiritual concerns in this study were least often addressed by staff.1 Assessment of spiritual care needs, screening for spiritual distress, and providing spiritual care are essential components of a clinician-patient relationship that supports healing.14 The importance of attending to spiritual care needs was underscored by a recent systematic review that found a positive association between overall spiritual well-being and quality of life in patients with cancer, with the meaning/peace factor consistently and positively associated with physical and mental health.15 Another identified gap was the health care team’s lack of attention to the patient’s social needs, which included concerns related to finances and debt from medical bills. In all, 46% of the respondents reported having financial concerns, with the odds of having moderate/high financial concerns being greatest during treatment to 2 years posttreatment. Attention to the financial burden of cancer patients is critical because the magnitude of cancer-related financial concerns is a significant, strong predictor of quality of life and adverse psychological issues such as depression, anxiety, and distress.16,17
There were several program implications based on the results. A periodic audit of the concerns of survivors and their views on how well their needs were being met was a relatively low cost endeavor. Although the findings were consistent with the literature, the results, when shared with administrators and clinicians, were instrumental in effecting change because they represented the concerns of survivors at the cancer center. Another program directive, based on the results, was to extend the routine screening of patients’ needs during treatment to posttreatment survivorship. Patients who are young, unemployed, do not identify as white, and female warrant more thorough assessment of needs and concerns along the cancer trajectory. Integral to these screenings is the need for patient-centered communication, with discussion of how cancer is affecting the different domains of quality of life within the context of the patient’s life. Lastly, the results clearly indicated the need for additional training of health care providers on how to assess and address spiritual well-being in cancer survivors.
There were limitations to this study, including use of a nonvalidated survey and cross-sectional approach that limited our ability to explore how concerns might change over the trajectory. Also, it was not possible to clarify medical information of the respondents, such as cancer stage. Although the response rate of this study was not high, we are confident in the results because of the large sample size and the finding that the large proportion of respondents with breast cancer was not influential. Despite these limitations, this needs assessment of cancer survivors over the trajectory of care provided insight into the scope of their concerns, identified vulnerable groups of survivors, and highlighted gaps in addressing those concerns. A quality- of-life framework for assessing needs assured a comprehensive focus and generated practice changes to strengthen holistic, comprehensive oncology care.
Complex cancer treatments, limited personnel resources, and a growing number of cancer survivors are challenging cancer health care professionals’ abilities to provide comprehensive care. Cancer survivors have a range of needs that extend over the cancer care trajectory and that represent physical, psychological, social, and spiritual domains. Numerous studies have explored supportive care needs and recent systematic reviews have highlighted the supportive care needs related to cancer1 and to specific cancer types, including prostate cancer,2 breast cancer,3 gynecologic cancer,4 hematological cancer,5 and lung cancer.6 However, reviews are limited in that they do not always assess needs across the cancer trajectory or identify demographic or clinical variables that are associated with needs. These data are needed to focus survivorship program development in cancer centers in order to target populations most likely at risk for unmet needs, identify what salient concerns to address, and to appropriately schedule supportive care programs.
The importance of assessing the patient’s subjective view of his/her needs or concerns is well acknowledged as being fundamental to patient-centered care.7 Clinicians routinely assess needs in practice using a variety of screening tools. However, there needs to be a broader assessment of concerns and needs in a population of survivors with mixed cancer diagnoses, along with their appraisal of how well their needs were addressed by their health care team, to provide an overall identification of gaps in supportive care. The primary purpose of the present study was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs; ascertain survivors’ perceived importance of those needs and the extent to which our institution, the University Hospitals Seidman Cancer Center, was attentive to those needs; and to identify who might be at risk for having greater concerns. The overall goal was to use the data to inform survivorship and supportive care program development.
Methods
Design, sample and setting
We used a cross-sectional design. Surveys were mailed once to a convenience sample of 2,750 adult patients who had been seen in follow-up during the previous 2 years (2010-2011) at all clinical sites of University Hospitals Seidman Cancer Center, a Midwestern National Cancer Institute-designated Comprehensive Cancer Center. Patients who had a noncancer diagnosis were excluded. The distribution list was screened for deceased individuals and those patients who had multiple visits during the time period. The project was reviewed and approved as nonresearch by the Case Western Reserve University Cancer Institutional Review Board.
Survey
An interdisciplinary team of clinicians, administrators, and researchers adapted the Mayo Clinic Cancer Center’s Cancer Survivors Survey of Needs8 to create a comprehensive survey for the cancer center. Input regarding the scope of the survey was sought from the Patient and Family Advisory Council of the cancer center. The survey, which was formatted for scanning purposes, consisted of 33 questions that were compiled into 4 sections. Sections 1 and 2 focused on demographic and treatment-related information, including use of community and hospital support services and preferences for follow-up care. In section 3, a quality-of-life framework was used to assess physical, social, emotional, and spiritual needs. Respondents were asked to rate their current level of concern for 19 physical effects, 10 social effects, 10 emotional effects, and 5 spiritual effects on a scale ranging from 0 (no concern) to 5 (extreme concern). In section 4, respondents were asked to indicate the importance of the cancer team addressing their physical, social, emotional, and spiritual needs. This was followed by their rating of the cancer team’s attention to their needs as Poor, Fair, Good, Excellent, or They did not ask about my needs. Respondents were asked about preferences for learning about physical, social, emotional, and spiritual effects. In addition to the 33 questions, there were 6 open-ended questions in which respondents were encouraged to share additional information about their needs, sources of support, and other concerns.
Procedures
Eligible respondents were mailed a cover letter explaining the survey from both the director and president of the cancer center, a survey, and a postage-paid return envelope. The option to respond to the survey by a telephone call to the director of the Office of Cancer Survivorship was offered in the cover letter.
Data analysis
Returned surveys were scanned into a Teleform database, verified, and exported into an SPSS data file. Data quality was checked by running frequency analyses and summarizing variables. Time-since-treatment responses were collapsed into 4 categories: on treatment, up to 2 years posttreatment, 2-5 years posttreatment, and more than 5 years posttreatment. Descriptive statistics were used to summarize demographic and medical characteristics of the respondents and to calculate the mean score for each concern for the total sample and then for each category of time since treatment. Because of the large number of respondents with breast cancer, the respondents were stratified into two groups, one of breast cancer the other of nonbreast cancer respondents. Then, the Mann-Whitney test was performed for each concern to examine differences between respondents with and without breast cancer.
To identify the most prevalent concerns, ratings for concerns were recoded into no concern (rated as 0), low concern (1 or 2), and moderate/high concern (3, 4, or 5). Since our interest was in the moderate and high concerns, the responses were dichotomized into moderate/high concerns and all other levels. Logistic regression models were then used to identify associations between a set of survivor characteristics or covariates (age, sex, living status, marital status, employment status, cancer type, and time since treatment) with the 12 most highly rated moderate/high concerns. All the analyses were performed using statistical software SPSS 20 and Stata 13.0
Results
Respondents
A total of 1,005 surveys were returned for a 37% response rate. Forty-two patients responded by telephone. The mean age of respondents was 64.9 years (range, 22-98; SD, 12.8). The typical respondent was female, white, and married (Table 1). Twenty-four percent of the respondents (n = 240) reported living alone. Although about 47% of respondents (n = 473) reported a breast cancer diagnosis, more than 17 cancers were identified, and 14% of respondents (n = 145) listed multiple diagnoses. About a third of respondents were receiving treatment when they completed the survey.
Just under half of the respondents (n = 498) reported using community resources for support and information about cancer, and 29.5% (n = 296) sought information on the internet during their cancer experience. The most commonly used community resources were The Gathering Place, a local organization offering free supportive programs and services to individuals with cancer and their families (n = 167), and the American Cancer Society (n = 138). Of the 496 respondents who reported accessing hospital resources, most (n = 322) said they used information that their health care team recommended. Other supportive options were used to a lesser degree: support groups (n = 92), chemotherapy and radiation therapy classes (n = 129), and supportive/educational programs offered by the cancer center (n = 27). Most of the respondents (n = 822, 88.6%) preferred to have their follow-up care remain with their cancer care team 1 year after treatments are completed. Almost two-thirds of respondents (n = 601, 64%) cited being seen at the cancer center for follow-up care as the most important factor in considering follow-up care.
Concerns In determining whether the large proportion of respondents with breast cancer skewed the study results, it was determined that median scores differed significantly in only four concerns. Compared with respondents without breast cancer, respondents with breast cancer were more likely to have significantly lower scores for concerns related to fatigue (P <.001) and sexual issues/intimacy (P = .001). Respondents with breast cancer were more likely to have significantly higher scores than respondents without breast cancer for concerns related to genetic counseling (P = .001) and fear of developing a new cancer (P = .010).
Fears of the cancer returning and developing a new cancer were the two most prevalent concerns, identified by 51% (n = 486) and 47.5% (n = 459), respectively (Table 2). Physical concerns, rated as moderate/high concerns by at least 25% of the sample, were fatigue (n = 336, 34.8%), changes in [the] body after cancer (n = 323, 33.7%), trouble sleeping (n = 302, 31.0%), sexual issues/intimacy (n = 263, 28.0%), memory and concentration (n = 261, 26.7%), and weight changes (n = 248, 25.5%). The most prevalent moderate/high social concerns were related to finances (n = 265, 27.5%) and debt from medical bills (n = 232, 25.1%). Managing stress (n = 279, 29.2%) and difficult emotions (n = 244, 25.1%) were prevalent moderate/high emotional concerns. Spiritual concerns were less often rated as moderate/high concerns. Having a breast cancer diagnosis was not significantly related to the number of reported moderate to high concerns (P = 1.00).
Variables associated with the 12 most frequent moderate/high concerns are shown in Tables 3 and 4. Age was associated with the most moderate/high concerns. With every decade of age, the odds of having the following moderate/high concerns decreased: bodily changes after cancer (odds ratio [OR], 0.75), sexual intimacy (OR, 0.81), memory and concentration (OR, 0.83), weight changes (OR, 0.77), financial (OR, 0.75), debt (OR, 0.71), cancer returning (OR, 0.66), developing a new cancer (OR, 0.67), managing stress (OR, 0.67), and managing difficult emotions (OR, 0.67).
Female sex was associated with lower odds of having a concern about sexual intimacy (OR, 0.30) and increased odds of having concerns related to memory and concentration (OR, 1.78), managing stress (OR, 2.35), and managing difficult emotions (OR, 1.77). Race was another demographic characteristic statistically associated with numerous moderate/high concerns. Survivors who identified white, were more likely than other people of other races to have fewer moderate/high concerns regarding bodily changes after cancer (OR, 0.46), weight change (OR, 0.46), finances (OR, 0.46), debt (OR, 0.40), managing stress (OR, 0.55), and managing difficult emotions (OR, 0.49). The odds of having a moderate/high concern regarding debt was 2.25 times higher given widowed marital status compared with those survivors who were single. Unemployment status, when compared with full-time employment, was significantly associated with increased odds of having moderate/high concerns related to fatigue (OR, 2.08), bodily changes after cancer (OR, 1.72), memory and concentration (OR, 2.45), weight changes (OR, 2.17), finances (OR, 1.93), developing a new cancer (OR, 1.91), and managing difficult emotions (OR, 1.80).
As expected, respondents who had completed treatment were less likely to have many of the moderate/high concerns as those still undergoing treatment. Survivors who were up to 2 years posttreatment were significantly more likely than those survivors receiving treatment to have fewer moderate/high concerns regarding fatigue (OR, 0.56), sexual intimacy (OR, 0.54), weight change (OR, 0.55), fears of the cancer returning (OR, 0.48), developing a new cancer (OR, 0.35), managing stress (OR, 0.43), and managing difficult emotions (OR, 0.49).
However, those improved odds were not sustained over the cancer trajectory. Compared with survivors who were receiving treatment, survivors who were between 2-5 years posttreatment did not have significantly reduced odds for moderate/high concerns related to fatigue, sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress, and managing difficult emotions. They did have significantly reduced odds for having concerns only related to finances (OR, 0.61) and debt (OR, 0.52).
Long-term survivors, who were beyond 5 years posttreatment, had significantly reduced odds for having moderate/high concerns related to fatigue (OR, 0.45), finances (OR, 0.52), debt (OR, 0.47), and managing difficult emotions (OR, 0.54), compared with survivors receiving treatment. Moderate/high concerns related to sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress did not have improved odds for these long-term survivors.
Attention to needs
The health care teams were rated highly for their attention to the patients’ physical needs. Most respondents (n = 845, 92.4%) viewed the health care team’s attention their physical needs as important and 763 (77.6%) survivors rated the team’s attention to these needs as excellent. The importance of addressing emotional needs was affirmed by 723 (78.5%) respondents, and although 454 (46.8%) viewed the team’s attention to these needs as excellent, 119 (12.3%) reported that the health care team did not ask about emotional needs. In addition, 566 respondents (60%) viewed having the health care team address their social needs as important, and most (n = 715, 74.2%) rated the team’s attention to social needs as good or excellent. Yet, 162 (16.8%) respondents reported that team did not ask about their social needs. The health care team’s addressing of spiritual needs was viewed as important by 346 (37.5%) respondents and ratings for how well the team attended to spiritual needs were: 148 (15.6%) poor or fair, 204 (21.5%) good, and 150 (15.8%) excellent. However, 448 (47.2%) respondents reported that the health care team did not ask about their spiritual needs.
Discussion
The primary purpose of this project was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs and to assess the perceived importance of these needs and the extent to which the cancer center staff were attentive to those needs. The overall goal of this assessment was to inform the development of survivorship and supportive care programs by highlighting common concerns, demographic and medical factors associated with specific concerns, and timing of moderate/high level concerns along the cancer trajectory. There were 3 main findings.
First, the results support the need for enhancing supportive care services to meet emotional concerns of survivors beyond the treatment phase. Similar to other studies,8,9 emotional concerns ranked higher than all other concerns in this study with about 50% of the sample rating “fear the cancer will return” and “fear of developing a new cancer” as moderate/high concern. Although the odds of not having these emotional concerns improved up to 2 years posttreatment, these concerns are likely to resurface, as odds for survivors beyond 2 years were not significantly different from those receiving treatment. A recent systematic review reported that fear of cancer recurrence is experienced by about 73% of cancer survivors, with 49% reporting a moderate to high degree.10 It can have a chronic, stable trajectory for some survivors and is strongly associated with higher levels of anxiety, distress, and depression, and less global, emotional/mental, physical, role, social, and cognitive quality of life.10 In this sample, managing stress and difficult emotions were also rated as moderate/high concerns by at least 25% of the sample.
Second, the findings identified patients at risk for cancer-related concerns throughout the cancer trajectory. As demonstrated in other studies, younger age was associated with greater odds of having multiple greater moderate/high concerns.11-13 Unemployment was the second most common demographic factor associated with multiple moderate/high concerns related to physical symptoms, finances and emotions. Similarly, identifying as black, Asian, American Indian/Alaskan Native, or other was also associated with greater odds of having numerous physical, financial, and emotional concerns. Women had greater concerns related to memory, sexual intimacy, coping with difficult emotions, and stress.
Third, the results helped to identify gaps in supportive care at our cancer center. Although spiritual concerns were not prevalent as being moderate/high, they were still viewed by about a third of survivors as being an important area for the health care team to address. Yet, consistent with other need assessments, spiritual concerns in this study were least often addressed by staff.1 Assessment of spiritual care needs, screening for spiritual distress, and providing spiritual care are essential components of a clinician-patient relationship that supports healing.14 The importance of attending to spiritual care needs was underscored by a recent systematic review that found a positive association between overall spiritual well-being and quality of life in patients with cancer, with the meaning/peace factor consistently and positively associated with physical and mental health.15 Another identified gap was the health care team’s lack of attention to the patient’s social needs, which included concerns related to finances and debt from medical bills. In all, 46% of the respondents reported having financial concerns, with the odds of having moderate/high financial concerns being greatest during treatment to 2 years posttreatment. Attention to the financial burden of cancer patients is critical because the magnitude of cancer-related financial concerns is a significant, strong predictor of quality of life and adverse psychological issues such as depression, anxiety, and distress.16,17
There were several program implications based on the results. A periodic audit of the concerns of survivors and their views on how well their needs were being met was a relatively low cost endeavor. Although the findings were consistent with the literature, the results, when shared with administrators and clinicians, were instrumental in effecting change because they represented the concerns of survivors at the cancer center. Another program directive, based on the results, was to extend the routine screening of patients’ needs during treatment to posttreatment survivorship. Patients who are young, unemployed, do not identify as white, and female warrant more thorough assessment of needs and concerns along the cancer trajectory. Integral to these screenings is the need for patient-centered communication, with discussion of how cancer is affecting the different domains of quality of life within the context of the patient’s life. Lastly, the results clearly indicated the need for additional training of health care providers on how to assess and address spiritual well-being in cancer survivors.
There were limitations to this study, including use of a nonvalidated survey and cross-sectional approach that limited our ability to explore how concerns might change over the trajectory. Also, it was not possible to clarify medical information of the respondents, such as cancer stage. Although the response rate of this study was not high, we are confident in the results because of the large sample size and the finding that the large proportion of respondents with breast cancer was not influential. Despite these limitations, this needs assessment of cancer survivors over the trajectory of care provided insight into the scope of their concerns, identified vulnerable groups of survivors, and highlighted gaps in addressing those concerns. A quality- of-life framework for assessing needs assured a comprehensive focus and generated practice changes to strengthen holistic, comprehensive oncology care.
1. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
2. Paterson C, Robertson A, Smith A, Nabi G. Identifying the unmet supportive care needs of men living with and beyond prostate cancer: A systematic review. Eur J Oncol Nurs. 2015;19:405-418.
3. Fiszer C, Dolbeault S, Sultan S, Bredart A. Prevalence, intensity, and predictors of the supportive care needs of women diagnosed with breast cancer: A systematic review. Psychooncology. 2014;23:361-374.
4. Maguire R, Kotronoulas G, Simpson M, Paterson C. A systematic review of the supportive care needs of women living with and beyond cervical cancer. Gynecol Oncol. 2015;136:478-490.
5. Hall A, Lynagh M, Bryant J, Sanson-Fisher R. Supportive care needs of hematological cancer survivors: A critical review of the literature. Crit Rev Oncol Hematol. 2013;88:102-116.
6. Maguire R, Papadopoulou C, Kotronoulas G, Simpson MF, McPhelim J, Irvine L. A systematic review of supportive care needs of people living with lung cancer. Eur J Oncol Nurs. 2013;17:449-464.
7. Adler NE, Page EK. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academies Press; Institute of Medicine, 2008.
8. Ness S, Kokal J, Fee-Schroeder K, Novotny P, Satele D, Barton D. Concerns across the survivorship trajectory: results from a survey of cancer survivors. Oncol Nurs Forum. 2013;40:35-42.
9. Swash B, Hulbert-Williams N, Bramwell R. Unmet psychosocial needs in haematological cancer: A systematic review. Support Care Cancer. 2014;22:1131-1141.
10. Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: A systematic review of quantitative studies. J Cancer Surviv. 2013;7:300-322.
11. Choi KH, Park JH, Park JH, Park JS. Psychosocial needs of cancer patients and related factors: A multi-center, cross-sectional study in Korea. Psychooncology. 2013;22:1073-1080.
12. Pauwels EE, Charlier C, De Bourdeaudhuij I, Lechner L, Van Hoof E. Care needs after primary breast cancer treatment. Survivors’ associated sociodemographic and medical characteristics. Psychooncology. 2013;22:125-132.
13. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
14. Puchalski CM, Blatt B, Kogan M, Butler A. Spirituality and health: The development of a field. Academic Medicine. 2014;89:10-16.
15. Bai M, Lazenby M. A systematic review of associations between spiritual well-being and quality of life at the scale and factor levels in studies among patients with cancer. J Palliat Med. 2015;18:286-298.
16. Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Pract. 2014;10:332-338.
17. Sharp L, Carsin AE, Timmons A. Associations between cancer-related financial stress and strain and psychological wellbeing among individuals living with cancer. Psychooncology. 2013;22:745-755.
1. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
2. Paterson C, Robertson A, Smith A, Nabi G. Identifying the unmet supportive care needs of men living with and beyond prostate cancer: A systematic review. Eur J Oncol Nurs. 2015;19:405-418.
3. Fiszer C, Dolbeault S, Sultan S, Bredart A. Prevalence, intensity, and predictors of the supportive care needs of women diagnosed with breast cancer: A systematic review. Psychooncology. 2014;23:361-374.
4. Maguire R, Kotronoulas G, Simpson M, Paterson C. A systematic review of the supportive care needs of women living with and beyond cervical cancer. Gynecol Oncol. 2015;136:478-490.
5. Hall A, Lynagh M, Bryant J, Sanson-Fisher R. Supportive care needs of hematological cancer survivors: A critical review of the literature. Crit Rev Oncol Hematol. 2013;88:102-116.
6. Maguire R, Papadopoulou C, Kotronoulas G, Simpson MF, McPhelim J, Irvine L. A systematic review of supportive care needs of people living with lung cancer. Eur J Oncol Nurs. 2013;17:449-464.
7. Adler NE, Page EK. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academies Press; Institute of Medicine, 2008.
8. Ness S, Kokal J, Fee-Schroeder K, Novotny P, Satele D, Barton D. Concerns across the survivorship trajectory: results from a survey of cancer survivors. Oncol Nurs Forum. 2013;40:35-42.
9. Swash B, Hulbert-Williams N, Bramwell R. Unmet psychosocial needs in haematological cancer: A systematic review. Support Care Cancer. 2014;22:1131-1141.
10. Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: A systematic review of quantitative studies. J Cancer Surviv. 2013;7:300-322.
11. Choi KH, Park JH, Park JH, Park JS. Psychosocial needs of cancer patients and related factors: A multi-center, cross-sectional study in Korea. Psychooncology. 2013;22:1073-1080.
12. Pauwels EE, Charlier C, De Bourdeaudhuij I, Lechner L, Van Hoof E. Care needs after primary breast cancer treatment. Survivors’ associated sociodemographic and medical characteristics. Psychooncology. 2013;22:125-132.
13. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
14. Puchalski CM, Blatt B, Kogan M, Butler A. Spirituality and health: The development of a field. Academic Medicine. 2014;89:10-16.
15. Bai M, Lazenby M. A systematic review of associations between spiritual well-being and quality of life at the scale and factor levels in studies among patients with cancer. J Palliat Med. 2015;18:286-298.
16. Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Pract. 2014;10:332-338.
17. Sharp L, Carsin AE, Timmons A. Associations between cancer-related financial stress and strain and psychological wellbeing among individuals living with cancer. Psychooncology. 2013;22:745-755.
Emergency department use by recently diagnosed cancer patients in California
In 2017 there will be nearly 1.7 million new cancer cases diagnosed, and over 600,000 cancer deaths in the Unites States.1 A 2013 Institute of Medicine report highlighted problems with the current quality of cancer care, including high costs and fragmentation of care.2 Other national reports have called for improvements in the overall quality of care and for reducing costly and possibly avoidable use of health services such as emergency department (ED) visits.3-6 Reduction of avoidable ED visits is often cited as a pathway to reduce costs by avoiding unnecessary tests and treatments that occur in the ED and subsequent hospital admissions.7,8
ED crowding, long waits, and unpredictable treatment environments can also make an ED visit an unpleasant experience for the patient. ED visits during cancer treatment can be particularly troubling and present health concerns for patients who are immunocompromised. In particular, cancer patients in the ED have been found to experience delays in the administration of analgesics, antiemetics, or antibiotics.9
Few studies have examined ED use or its associated predictors among cancer patients. Reports to date that have described ED use have focused on different cancers, which makes comparisons across studies difficult.10 Moreover, the time frames of interest and the type of event after which ED use is evaluated (ie, diagnosis or treatment) are inconsistent in the existing literature.10 Some studies quantifying ED use excluded patients admitted to the hospital after an ED visit.11,12 Taken together, these studies do not provide a clear overview of the extent of ED use by cancer patients or the amount of cancer-related care provided by EDs.
Patterns of ED use among cancer patients derived from large and generalizable samples may help inform providers about true risk factors for ED use. In addition, prioritizing new interventions and focusing future research on groups of patients who are at higher risk for preventable ED use could also improve overall care. To address these issues, accurate estimates of ED use among cancer patients are required.
To our knowledge, this is the first study to describe ED use across a range of cancers in a large population-based sample and to consider the timing of ED visits in relation to initial diagnosis. The findings could provide benchmark comparison data to inform future efforts to identify the subset of possibly preventable ED visits and to design interventions to address preventable ED use.
Material and methods
Data source
California’s Office of Statewide Health Planning and Development (OSHPD) manages the patient discharge dataset (PDD) and the emergency department use (EDU) dataset, providing a high-quality source of information on inpatient and ED use in the state.13 A principal diagnosis and up to 24 secondary diagnoses are recorded in OSHPD datasets. The EDU dataset was used to identify treat-and-release ED visits, and the PDD was used to identify hospitalizations initiated in the ED. The California Cancer Registry (CCR) obtains demographic and diagnosis information for every new invasive cancer diagnosed in California, and data collected by the registry are considered to be complete.14 CCR-OSPHD-linked data provide high-quality health care use information for cancer patients in California.15,16 Using an encrypted version of the social security number called the record linkage number (RLN), we linked the CCR records to the corresponding OSHPD files from 2009-2010.
Institutional review board approval for this study was obtained from the University of California, Davis, Human Subjects Committee and the State of California Committee for the Protection of Human Subjects.
Analysis
ED visits. Visits were included if they occurred on or up to 365 days after the date of cancer diagnosis recorded in the CCR. The visits were coded in mutually exclusive groups as occurring within 30, 31-180, and 181-365 days of diagnosis. Subsequently, we flagged each person as having any ED visit (Yes/No) within 180 days and within 365 days of diagnosis, and we tallied the total number of visits occurring within these time frames for each person.
Cancer type. We used relevant site and histology codes to classify cancer type into 24 mutually exclusive categories using the Surveillance Epidemiology and End Result‘s International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) Recode Definitions17-19 (Suppl Figure 1).
Individual-level variables. Sociodemographic information for each person was collected from the CCR including gender, age, race/ethnicity, marital status, health insurance status, rural residence, survival time in months, neighborhood socio-economic (SES) status based on the Yang index, and the American Joint Committee on Cancer (AJCC) stage.20,21
Data analysis
Demographic information was analyzed for the cohort using descriptive statistics (frequencies, proportions, means, standard deviations, and ranges) and evaluated for correlations. Fewer than 20 observations had missing data and we removed those observations from our analyses on an item-specific basis.
We tabulated ED visits by cancer type and time from diagnosis and then collapsed visit-level data by RLN to determine the number of ED visits for each person in the sample. The number of days from diagnosis to first ED visit was also tabulated. The cohort was stratified by cancer type and cumulative rates of ED visits were tabulated for individuals with ED visits within 0-180 and 0-365 days from diagnosis. To test the robustness of the findings adjusting for confounding factors known to impact ED use, we used logistic regression to model any ED use (Yes/No) as a function of age, gender, race/ethnicity, cancer stage, insurance status, marital status, urban residence, and Yang SES. After model estimation, we used the method of recycled predictions controlling for the confounding variables to compute the marginal probabilities of ED use by cancer type.22 To adjust for the possible impact of survival on ED use, we performed sensitivity analyses and estimated predicted probabilities adjusting for survival. Separate analyses were performed first adjusting for whether the patient died during the course of each month after diagnosis and then adjusting for whether or not the patient died within 180 days of diagnosis. All analyses were conducted using Stata 13.1.23
Results
The CCR identified 222,087 adults with a new primary cancer diagnosis in 2009-2010. After excluding those with Stage 0 cancer (n = 21,154) and nonmelanoma skin cancer (n = 1,031), for whom data are inconsistently collected by CCR, a total of 199,872 individuals were included in the analytic sample. Of those patients (Table 1), most were white non-Hispanic (62%), women (51%), holders of private insurance (53%), married (56%), and urban residents (86%). Most were older than 50 years and had either Stage I or Stage II cancer. The most common cancer types were breast (17%), prostate (16%), lung (11%), and colon (9%; results not shown). In unadjusted comparisons, the incidence of ED use was significantly higher among those who were older, of non-Hispanic black race/ethnicity, uninsured, in the lowest SES group, widowed, or diagnosed with Stage IV cancer (Table 1).
ED visits
Within 365 days after initial cancer diagnosis, 87,025 cancer patients made a total of 197,886 ED visits (not shown in tables). Of those visits, 68% (n = 134,556) occurred within 180 days of diagnosis, with 22% (n = 43,535) occurring within the first 30 days and 46% (n = 91,027) occurring within 31-180 days after diagnosis (Figure). Given that most of the visits occurred within 180 days of diagnosis, we used that time frame in subsequent analyses. Among all ED visits within 180 days of diagnosis (Table 2), the largest proportions of visits were made by those with lung cancer (16%), breast cancer (11%), and colon cancer (10%).
About 51% of visits resulted in admission to the hospital and 45% in discharge (Table 2). For some cancers (lung, colon, non-Hodgkin lymphoma, pancreatic, digestive, liver, stomach, leukemia, and myeloma) most of the visits resulted in admission to the hospital (Table 2). Among visits resulting in admission, the top three principal diagnoses were: septicemia (8%), cardiovascular problems (7%), and complications from surgery (5%) (not shown in tables). Among visits resulting in a discharge home, the three top principal diagnoses were abdominal pain (7%), cardiovascular problems (6%), and urinary, kidney, and bladder complaints other than a urinary tract infection (5%) (results not shown).
Individuals
The cumulative incidence of at least one ED visit was 35% (n = 70,813) within 180 days after diagnosis (Table 3). Visit rates varied by cancer type: individuals with pancreatic (62%), brain (60%), and lung (55%) cancers had the highest cumulative incidences of ED use within 180 days of diagnosis (Table 3). Those with melanoma (14%), prostate (17%), and eye (18%) cancers had the lowest cumulative incidences of ED visits (Table 3).
Recycled predictions from logistic regression models, accounting for potential confounding factors, yielded substantively similar results for the cumulative incidence of ED use across cancer types (Table 4). Results did not differ substantially after accounting for survival. Differences in the predicted probability of an ED visits adjusting for death within 180 days of diagnosis were noted to be 2% or greater from estimates reported in Table 4 for only four cancers. Estimates of having any ED visits for those with lung cancer decreased from 46% to 44% (95% CI: 43.0-44.4%), pancreatic cancer from 53% to 49% (95% CI: 48-51%), liver cancer from 51% to 47% (95% CI: 49-53%), and those with eye cancers increased from 20% to 22% (95% CI: 18-26%) (not shown in tables).
For patients with certain cancers (eg, lung, pancreas, leukemia) the proportion of individuals with an initial ED visit was highest in the first 30 days after diagnosis (Table 3). For individuals with other cancers (eg, breast, prostate, melanoma) the proportion of individuals with an initial ED visit increased by more than 5% during the 31-181–day time period. Those with the remaining cancers had less than 5% change in cumulative ED use between the two time periods.
The number of visits per person ranged from 0-44 during the first 180 days after diagnosis (results not shown in tables). Of all patients diagnosed with cancer, 20% (n = 39,429) had one ED visit, 8% (n = 16,238) had two visits, and 7% (n = 14,760) had three or more visits. Of those patients having at least one ED visit within 180 days of diagnosis, 44% (n = 31,080) had two or more visits and 21% (n = 14,760) had 3 or more visits.
Discussion
This study extends previous research by describing ED use for more than 20 cancer types by time from diagnosis in a large, heterogeneous and population-based sample of recently diagnosed adults in California. We found that 16% of newly diagnosed individuals with cancer used the ED within 30 days of diagnosis, 35% within 6 months of diagnosis, and 44% within 1 year of diagnosis. These findings suggest that ED use by cancer patients is more than double that of the US general population and is higher than previously estimated for cancer patients.10,24 In 2010, about 21% of the US population visited the ED, compared with 44% of cancer patients in the same time period.24 Although persons with greater medical need, such as those with cancer, inevitably require more health services, new approaches are needed to explore the extent to which some of these visits by cancer patients could be prevented by providing care in other settings.
Few studies have examined ED use by cancer patients, but previous findings suggest that 1%-12% of cancer patients use the ED within 30 days of diagnosis, and 15%-25% use the ED within a year of diagnosis.10,25,26 One study did report higher rates of ED use by cancer patients, but attributed the increased use to changes in Medicaid copayments.27 The finding that ED use is higher among cancer patients than previously considered is important for several reasons. First, high rates of ED use may reflect excessive fragmentation in cancer care, or patients’ inability to access providers when acute concerns arise. Furthermore, providers and policymakers may be particularly interested in populations with high ED use because reducing potentially preventable ED use is often cited as one of the goals of care coordination and alternative health care model programs.2,28,29
The number of newly diagnosed cancer patients with multiple ED visits is also substantial. We found 15% of recently diagnosed cancer patients had two or more ED visits within 180 days of diagnosis, compared with 8% of the general US population having two or more ED visits in all of 2010.24 Among cancer patients with at least one ED visit, 44% visited more than once. Repeat visits may represent worsening health status, continued unmet health needs, or new complications that might have been prevented or treated in other health care settings. In addition, there may be opportunities to identify cancer patients at risk for multiple visits at their initial ED visit. A better understanding of the reasons for ED visits and the factors driving unmet need – such as inadequate patient education, limited access to specialty services, or failure to admit a patient to resolve a problem appropriately (eg, pain, infection) – may help to identify which visits are potentially preventable. Ultimately, failure to adequately describe the number of cancer patients that visit the ED and the number of times they visit may result in a lost opportunity for improvement in care, the patient experience and cost reduction in cancer care.
The distinction between cancer types that account for the most ED visits and cancer types with the highest cumulative incidences of ED use is informative. For instance, lung cancer patients accounted for the largest number of ED visits and over half of those with lung cancer visited the ED within 180 days of diagnosis. However, although more than 60% of individuals with pancreatic cancer visited an ED within 180 days of diagnosis; they accounted for only 5% of all ED visits by cancer patients during the same time period. This in part reflects the relative frequency of these cancers. However, prostate cancer, which has a high incidence rate, represents about 8% of all ED visits by cancer patients, yet only 17% of all prostate cancer patients visit the ED within 180 days of diagnosis.
One approach to reduce the absolute number of ED visits by cancer patients would be to target the most frequent users of the ED such as lung, breast, prostate, and colon cancer patients. These cancers are the most common in the general population, so proportionate reduction in ED visits in these groups would have a large overall impact on ED use. Alternatively, patients with cancers that have high rates of ED use could be targeted with interventions to better address their needs. Additional studies of ED use among cancer patients, including understudied cancers, are needed to determine whether care provided in the ED could be provided in alternate clinical settings. Such research can also support training of emergency department staff to manage the full range of cancer-related conditions presenting to the ED.
Another approach to identifying potentially avoidable ED visits is to explore visits that result in admission to the hospital compared with those that result in discharge from the ED. In some circumstances, visits that result in discharge home may not have been true medical emergencies, and therefore might have been preventable. It is also true that even an acute problem requiring admission may have been preventable with timely outpatient management. While we found that 45% of visits ended in discharge home, over half of cancer patients who visit the ED are admitted to the hospital. This is higher than admission rates from the ED for the U.S. population overall (11%-15%),30-32and even higher than the estimated rate of individuals with chronic conditions, such as diabetes (42%), who visit the ED and then are subsequently admitted to the hospital.33
Relatively high rates of admissions may indicate that cancer patients seeking care in the ED require increased medical attention; however, it is possible that other explanations exist. For instance, ED physicians may be uncomfortable with complex cancer cases and may admit patients to be evaluated by a specialist. As such, it is possible that some of these admissions could have been appropriate for outpatient follow up. It is also possible that patients are referred to the ED for admission to the hospital. In these situations the ED visit may be entirely preventable through a direct admission process, although such processes are not available at all institutions and may vary by the admitting provider. For instance, if a hospitalist is overseeing the hospital stay, they may prefer the admission to occur through the ED, whereas a primary care provider or oncologist may be more likely to facilitate a direct admission. Future research could address the extent to which admissions from the ED may be avoidable by examining reasons for and length of admission following an ED visit. While this study found top reasons for admission (principal diagnosis) to be septicemia, cardiovascular complaints and complications from surgery, cumulatively these diagnosis accounted for less than 20% of admissions from the ED. Examining frequent diagnoses by cancer type will also provide insight into potentially avoidable ED use, which may vary by disease course and treatment regimen.
The distribution of days from diagnosis to the first ED visit also varied by cancer type. This variation is likely attributable, at least in part, to differences in condition-specific treatment regimens, severity of illness, and stage at diagnosis. For example, patients with ED visits within 30 days of diagnosis may be those with advanced stage cancers who are at higher risk of complications, or they may be visiting the ED for post-surgical problems. Likewise, individuals who incur visits during later time periods may be undergoing longer treatment regimens. Further research is warranted to explore site-specific predictors of ED use and high-risk periods, accounting for cancer treatment and the timing of treatments.
In summary, ED use among cancer patients is substantial and higher than previously reported. Most ED visits occur within the first 180 days after diagnosis, suggesting focus on the first 30 days after hospital discharge may be misguided. Time frames for ED measurement in future research should be selected with careful attention to cancer-specific periods within which most ED use occurs and the outcomes of interest. Furthermore, better models identifying cancer-specific predictors of ED use, which account for treatment and comorbidities, will facilitate the development of interventions focused on high-risk segments of this population. Research is needed to explore cancer-specific reasons for ED visits and which ED admission diagnoses may be potentially preventable.
Limitations
The limitations of this study include those common to use of administrative and registry data and the CCR and OSHPD data in particular. While CCR data are known to be complete with respect to demographic and cancer information, treatment data is less robust and specific treatment dates are not available.14,34 As a result, we were unable to analyze ED use in relation to receipt of outpatient treatment. As we included all ED visits on or up to a year after the day of diagnosis, it is possible that our analysis includes diagnoses that occurred in conjunction with an ED visits. However, it is unlikely a reporting hospital would report a cancer diagnosis to the CCR without a corresponding hospital admission. Therefore, we assume such cases to be rare.
Lastly, California had lower prevalence of health insurance coverage and higher market penetration by health maintenance organizations, relative to the national average, which may limit the generalizability of the results to other states.35 At the same time, CCR-OSHPD linked data offer the advantage of providing complete data to enumerate ED visits among patients whether they were discharged home or subsequently admitted to hospital.
1. American Cancer Society. Facts & Figures 2017. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. Published 2017. Accessed March 16, 2017.
2. Levit L, Balogh E, Nass S, Ganz PA. Delivering high-quality cancer care: charting a new course for a system in crisis. https://www.nap.edu/read/18359/chapter/1. Published 2013. Accessed March 16, 2017.
3. Readmissions Reduction Program (HRRP). CMS website. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. Updated April 8, 2016. Accessed March 16, 2017.
4. Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists: challenges to assuring access to oncology services. J Clin Oncol. 2007;3(2):79-86.
5. Guadagnolo B, Dohan D, Raich P. Metrics for evaluating patient navigation during cancer diagnosis and treatment: crafting a policy-relevant research agenda for patient navigation in cancer care. Cancer. 2011;117(15 Suppl):3565-3574.
6. Medicare Patient Access to Cancer Treatment Act of 2013, H.R.2869, 113th Cong.(2013). https://www.congress.gov/bill/113th-congress/house-bill/2869/text?format=txt. Introduced July 31, 2013; latest action, referred to the Subcommittee on Health, August 2, 2013. Accessed March 16, 2017.
7. Smulowitz P, Honigman L, Landon B. A novel approach to identifying targets for cost reduction in the emergency department. Ann Emerg Med. 2013;61(3):293-300.
8. Agrawal S, Conway P. Integrating emergency care into a patient- and outcome-centered health care system. Ann Emerg Med. 2013;61(3):301-302.
9. Swenson K, Rose M, Ritz L, Murray CL, Adlis S. Recognition and evaluation of oncology-related symptoms in the emergency department. Ann Emerg Med.1995;26(1):12-17.
10. Lash R, Bell J, Reed S, et al. A systematic review of emergency department use among cancer patients. Cancer Nurs. 2017;40(2):135-144.
11. Sanoff H, Carpenter W, Freburger J, et al. Comparison of adverse events during 5-fluorouracil versus 5-fluorouracil/oxaliplatin adjuvant chemotherapy for stage III colon cancer: a population-based analysis. Cancer. 2012;118(17):4309-4320.
12. Hansen D, Fox J, Gross C, Bruun J. Hospital readmissions and emergency department visits following laparoscopic and open colon resection for cancer. Dis Colon Rectum. 2013;56(9):1053-1061.
13. Office of Statewide Health Planning and Development. Hospital Data Products. http://www.oshpd.ca.gov/HID/DataFlow/HospData.html. Last updated September 6, 2016. Accessed March 16, 2017.
14. California Cancer Registry. Overview. http://www.ccrcal.org/Inside_CCR/About_Us.shtml. Published 2009. Accessed April 15, 2015.
15. Patel M, Ma Y, Mitchell B, Rhoads K. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24(2):344-349.
16. Parikh-Patel A, White R, Allen M, Cress R. Risk of cancer among rheumatoid arthritis patients in California. Cancer Causes Control. 2009;20(6):1001-1010.
17. National Cancer Institute: Surveillance, Epidemiology and End Results Program. Site recode ICD-O-3/WHO 2008 definition. https://seer.cancer.gov/siterecode/icdo3_dwhoheme/. Published 2008. Accessed March 16, 2017.
18. Washington State Cancer Registry. Cancer Codes Used in Reports. https://fortress. wa.gov/doh/wscr/WSCR/CancerCode.mvc/CancerCode. Data updated, January 2016; report updated, March 2016. Accessed March 16, 2017.
19. National Cancer Institute: Surveillance, Epidemiology and End Results Program. ICD-O-3 SEER Site/Histology Validation List. https://seer.cancer.gov/icd-o-3/. Published 2012, updated September 2015. Accessed March 16, 2017.
20. American Joint Committee on Cancer. What is Cancer Staging? https://cancerstaging.org/references-tools/Pages/What-is-Cancer-Staging.aspx. Published 2010. Accessed March 16, 2017.
21. Yang J S, Harrati A, Clarke C, Keegan T, Gomez S. Cancer Prevention Institute of California. Developing an area based socioeconomic measures from American Community Survey data. http://www.cpic.org/files/PDF/Research_Files/Reports/CPIC_ACS_SES_Index_Documentation_3-102014.pdf. Published March 10, 2014. Accessed March 16, 2017.
22. Basu A, Rathouz P. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics. 2005;6(1):93-109.
23. Stata Statistical Software [computer program]. Version 13 College Station, TX: StataCorp LP. 2013.
24. National Center for Health Statistics. Health, United States, 2013 – with a special feature on prescription drugs. https://www.cdc.gov/nchs/data/hus/hus13.pdf. Updated May 2014. Accessed March 16, 2017.
25. Goyal R, Wheeler S, Kohler R, et al. Health care utilization from chemotherapy-related adverse events among low-income breast cancer patients: effect of enrollment in a medical home program. N C Med J. 2014;75(4):231-238.
26. Hassett M, O’Malley A, Pakes J, Newhouse J, Earle C. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst. 2006;98(16):1108-1117.
27. Subramanian S. Impact of Medicaid copayments on patients with cancer: lessons for Medicaid expansion under health reform. Med Care. 2011;49(9):842-847.
28. Coyle Y, Miller A, Paulson R. Model for the cost-efficient delivery of continuous quality cancer care: a hospital and private-practice collaboration. Proc (Bayl Univ Med Cent). 2013;26(2):95-99.
29. Agency for Healthcare Research and Quality. 2011 National Healthcare Disparities Report. https://archive.ahrq.gov/research/findings/nhqrdr/nhdr11/index.html. Last reviewed October 2014. Accessed March 16, 2017.
30. Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Emergency Department Sample (NEDS) 2010. https://www.hcup-us.ahrq.gov/db/nation/neds/NEDS_Introduction_2010.jsp. Issued November 2012, updated November 2015. Accessed March 16, 2017.
31. Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Emergency Department Sample (NEDS) 2013. https://www.hcup-us.ahrq.gov/db/nation/neds/NEDS_Introduction_2013.jsp. Published November 2015. Accessed March 16, 2017.
32. Weiss AJ, Wier LM, Stocks C, Blanchard J. Overview of emergency department visits in the United States, 2011. Statistical Brief #174. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb174-Emergency-Department-Visits-Overview.pdf. Published June 2014. Accessed March 16, 2017.
33. Washington R, Andrews R, Mutter, R. Emergency department visits for adults with diabetes, 2010. Statistical Brief #167. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb167.jsp. Published November 2013. Accessed March 16, 2017.
34. Penberthy L, Petkov V, McClish D, et al. The value of billing data from oncology practice to supplement treatment information for cancer surveillance. Journal of registry management. 2014;41(2):57-64.
35. National Center for Health Statistics. Health, United States, 2014 – with a special feature on adults aged 55-64. https://www.cdc.gov/nchs/data/hus/hus14.pdf. Published May 2015. Accessed March 16, 2017.
In 2017 there will be nearly 1.7 million new cancer cases diagnosed, and over 600,000 cancer deaths in the Unites States.1 A 2013 Institute of Medicine report highlighted problems with the current quality of cancer care, including high costs and fragmentation of care.2 Other national reports have called for improvements in the overall quality of care and for reducing costly and possibly avoidable use of health services such as emergency department (ED) visits.3-6 Reduction of avoidable ED visits is often cited as a pathway to reduce costs by avoiding unnecessary tests and treatments that occur in the ED and subsequent hospital admissions.7,8
ED crowding, long waits, and unpredictable treatment environments can also make an ED visit an unpleasant experience for the patient. ED visits during cancer treatment can be particularly troubling and present health concerns for patients who are immunocompromised. In particular, cancer patients in the ED have been found to experience delays in the administration of analgesics, antiemetics, or antibiotics.9
Few studies have examined ED use or its associated predictors among cancer patients. Reports to date that have described ED use have focused on different cancers, which makes comparisons across studies difficult.10 Moreover, the time frames of interest and the type of event after which ED use is evaluated (ie, diagnosis or treatment) are inconsistent in the existing literature.10 Some studies quantifying ED use excluded patients admitted to the hospital after an ED visit.11,12 Taken together, these studies do not provide a clear overview of the extent of ED use by cancer patients or the amount of cancer-related care provided by EDs.
Patterns of ED use among cancer patients derived from large and generalizable samples may help inform providers about true risk factors for ED use. In addition, prioritizing new interventions and focusing future research on groups of patients who are at higher risk for preventable ED use could also improve overall care. To address these issues, accurate estimates of ED use among cancer patients are required.
To our knowledge, this is the first study to describe ED use across a range of cancers in a large population-based sample and to consider the timing of ED visits in relation to initial diagnosis. The findings could provide benchmark comparison data to inform future efforts to identify the subset of possibly preventable ED visits and to design interventions to address preventable ED use.
Material and methods
Data source
California’s Office of Statewide Health Planning and Development (OSHPD) manages the patient discharge dataset (PDD) and the emergency department use (EDU) dataset, providing a high-quality source of information on inpatient and ED use in the state.13 A principal diagnosis and up to 24 secondary diagnoses are recorded in OSHPD datasets. The EDU dataset was used to identify treat-and-release ED visits, and the PDD was used to identify hospitalizations initiated in the ED. The California Cancer Registry (CCR) obtains demographic and diagnosis information for every new invasive cancer diagnosed in California, and data collected by the registry are considered to be complete.14 CCR-OSPHD-linked data provide high-quality health care use information for cancer patients in California.15,16 Using an encrypted version of the social security number called the record linkage number (RLN), we linked the CCR records to the corresponding OSHPD files from 2009-2010.
Institutional review board approval for this study was obtained from the University of California, Davis, Human Subjects Committee and the State of California Committee for the Protection of Human Subjects.
Analysis
ED visits. Visits were included if they occurred on or up to 365 days after the date of cancer diagnosis recorded in the CCR. The visits were coded in mutually exclusive groups as occurring within 30, 31-180, and 181-365 days of diagnosis. Subsequently, we flagged each person as having any ED visit (Yes/No) within 180 days and within 365 days of diagnosis, and we tallied the total number of visits occurring within these time frames for each person.
Cancer type. We used relevant site and histology codes to classify cancer type into 24 mutually exclusive categories using the Surveillance Epidemiology and End Result‘s International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) Recode Definitions17-19 (Suppl Figure 1).
Individual-level variables. Sociodemographic information for each person was collected from the CCR including gender, age, race/ethnicity, marital status, health insurance status, rural residence, survival time in months, neighborhood socio-economic (SES) status based on the Yang index, and the American Joint Committee on Cancer (AJCC) stage.20,21
Data analysis
Demographic information was analyzed for the cohort using descriptive statistics (frequencies, proportions, means, standard deviations, and ranges) and evaluated for correlations. Fewer than 20 observations had missing data and we removed those observations from our analyses on an item-specific basis.
We tabulated ED visits by cancer type and time from diagnosis and then collapsed visit-level data by RLN to determine the number of ED visits for each person in the sample. The number of days from diagnosis to first ED visit was also tabulated. The cohort was stratified by cancer type and cumulative rates of ED visits were tabulated for individuals with ED visits within 0-180 and 0-365 days from diagnosis. To test the robustness of the findings adjusting for confounding factors known to impact ED use, we used logistic regression to model any ED use (Yes/No) as a function of age, gender, race/ethnicity, cancer stage, insurance status, marital status, urban residence, and Yang SES. After model estimation, we used the method of recycled predictions controlling for the confounding variables to compute the marginal probabilities of ED use by cancer type.22 To adjust for the possible impact of survival on ED use, we performed sensitivity analyses and estimated predicted probabilities adjusting for survival. Separate analyses were performed first adjusting for whether the patient died during the course of each month after diagnosis and then adjusting for whether or not the patient died within 180 days of diagnosis. All analyses were conducted using Stata 13.1.23
Results
The CCR identified 222,087 adults with a new primary cancer diagnosis in 2009-2010. After excluding those with Stage 0 cancer (n = 21,154) and nonmelanoma skin cancer (n = 1,031), for whom data are inconsistently collected by CCR, a total of 199,872 individuals were included in the analytic sample. Of those patients (Table 1), most were white non-Hispanic (62%), women (51%), holders of private insurance (53%), married (56%), and urban residents (86%). Most were older than 50 years and had either Stage I or Stage II cancer. The most common cancer types were breast (17%), prostate (16%), lung (11%), and colon (9%; results not shown). In unadjusted comparisons, the incidence of ED use was significantly higher among those who were older, of non-Hispanic black race/ethnicity, uninsured, in the lowest SES group, widowed, or diagnosed with Stage IV cancer (Table 1).
ED visits
Within 365 days after initial cancer diagnosis, 87,025 cancer patients made a total of 197,886 ED visits (not shown in tables). Of those visits, 68% (n = 134,556) occurred within 180 days of diagnosis, with 22% (n = 43,535) occurring within the first 30 days and 46% (n = 91,027) occurring within 31-180 days after diagnosis (Figure). Given that most of the visits occurred within 180 days of diagnosis, we used that time frame in subsequent analyses. Among all ED visits within 180 days of diagnosis (Table 2), the largest proportions of visits were made by those with lung cancer (16%), breast cancer (11%), and colon cancer (10%).
About 51% of visits resulted in admission to the hospital and 45% in discharge (Table 2). For some cancers (lung, colon, non-Hodgkin lymphoma, pancreatic, digestive, liver, stomach, leukemia, and myeloma) most of the visits resulted in admission to the hospital (Table 2). Among visits resulting in admission, the top three principal diagnoses were: septicemia (8%), cardiovascular problems (7%), and complications from surgery (5%) (not shown in tables). Among visits resulting in a discharge home, the three top principal diagnoses were abdominal pain (7%), cardiovascular problems (6%), and urinary, kidney, and bladder complaints other than a urinary tract infection (5%) (results not shown).
Individuals
The cumulative incidence of at least one ED visit was 35% (n = 70,813) within 180 days after diagnosis (Table 3). Visit rates varied by cancer type: individuals with pancreatic (62%), brain (60%), and lung (55%) cancers had the highest cumulative incidences of ED use within 180 days of diagnosis (Table 3). Those with melanoma (14%), prostate (17%), and eye (18%) cancers had the lowest cumulative incidences of ED visits (Table 3).
Recycled predictions from logistic regression models, accounting for potential confounding factors, yielded substantively similar results for the cumulative incidence of ED use across cancer types (Table 4). Results did not differ substantially after accounting for survival. Differences in the predicted probability of an ED visits adjusting for death within 180 days of diagnosis were noted to be 2% or greater from estimates reported in Table 4 for only four cancers. Estimates of having any ED visits for those with lung cancer decreased from 46% to 44% (95% CI: 43.0-44.4%), pancreatic cancer from 53% to 49% (95% CI: 48-51%), liver cancer from 51% to 47% (95% CI: 49-53%), and those with eye cancers increased from 20% to 22% (95% CI: 18-26%) (not shown in tables).
For patients with certain cancers (eg, lung, pancreas, leukemia) the proportion of individuals with an initial ED visit was highest in the first 30 days after diagnosis (Table 3). For individuals with other cancers (eg, breast, prostate, melanoma) the proportion of individuals with an initial ED visit increased by more than 5% during the 31-181–day time period. Those with the remaining cancers had less than 5% change in cumulative ED use between the two time periods.
The number of visits per person ranged from 0-44 during the first 180 days after diagnosis (results not shown in tables). Of all patients diagnosed with cancer, 20% (n = 39,429) had one ED visit, 8% (n = 16,238) had two visits, and 7% (n = 14,760) had three or more visits. Of those patients having at least one ED visit within 180 days of diagnosis, 44% (n = 31,080) had two or more visits and 21% (n = 14,760) had 3 or more visits.
Discussion
This study extends previous research by describing ED use for more than 20 cancer types by time from diagnosis in a large, heterogeneous and population-based sample of recently diagnosed adults in California. We found that 16% of newly diagnosed individuals with cancer used the ED within 30 days of diagnosis, 35% within 6 months of diagnosis, and 44% within 1 year of diagnosis. These findings suggest that ED use by cancer patients is more than double that of the US general population and is higher than previously estimated for cancer patients.10,24 In 2010, about 21% of the US population visited the ED, compared with 44% of cancer patients in the same time period.24 Although persons with greater medical need, such as those with cancer, inevitably require more health services, new approaches are needed to explore the extent to which some of these visits by cancer patients could be prevented by providing care in other settings.
Few studies have examined ED use by cancer patients, but previous findings suggest that 1%-12% of cancer patients use the ED within 30 days of diagnosis, and 15%-25% use the ED within a year of diagnosis.10,25,26 One study did report higher rates of ED use by cancer patients, but attributed the increased use to changes in Medicaid copayments.27 The finding that ED use is higher among cancer patients than previously considered is important for several reasons. First, high rates of ED use may reflect excessive fragmentation in cancer care, or patients’ inability to access providers when acute concerns arise. Furthermore, providers and policymakers may be particularly interested in populations with high ED use because reducing potentially preventable ED use is often cited as one of the goals of care coordination and alternative health care model programs.2,28,29
The number of newly diagnosed cancer patients with multiple ED visits is also substantial. We found 15% of recently diagnosed cancer patients had two or more ED visits within 180 days of diagnosis, compared with 8% of the general US population having two or more ED visits in all of 2010.24 Among cancer patients with at least one ED visit, 44% visited more than once. Repeat visits may represent worsening health status, continued unmet health needs, or new complications that might have been prevented or treated in other health care settings. In addition, there may be opportunities to identify cancer patients at risk for multiple visits at their initial ED visit. A better understanding of the reasons for ED visits and the factors driving unmet need – such as inadequate patient education, limited access to specialty services, or failure to admit a patient to resolve a problem appropriately (eg, pain, infection) – may help to identify which visits are potentially preventable. Ultimately, failure to adequately describe the number of cancer patients that visit the ED and the number of times they visit may result in a lost opportunity for improvement in care, the patient experience and cost reduction in cancer care.
The distinction between cancer types that account for the most ED visits and cancer types with the highest cumulative incidences of ED use is informative. For instance, lung cancer patients accounted for the largest number of ED visits and over half of those with lung cancer visited the ED within 180 days of diagnosis. However, although more than 60% of individuals with pancreatic cancer visited an ED within 180 days of diagnosis; they accounted for only 5% of all ED visits by cancer patients during the same time period. This in part reflects the relative frequency of these cancers. However, prostate cancer, which has a high incidence rate, represents about 8% of all ED visits by cancer patients, yet only 17% of all prostate cancer patients visit the ED within 180 days of diagnosis.
One approach to reduce the absolute number of ED visits by cancer patients would be to target the most frequent users of the ED such as lung, breast, prostate, and colon cancer patients. These cancers are the most common in the general population, so proportionate reduction in ED visits in these groups would have a large overall impact on ED use. Alternatively, patients with cancers that have high rates of ED use could be targeted with interventions to better address their needs. Additional studies of ED use among cancer patients, including understudied cancers, are needed to determine whether care provided in the ED could be provided in alternate clinical settings. Such research can also support training of emergency department staff to manage the full range of cancer-related conditions presenting to the ED.
Another approach to identifying potentially avoidable ED visits is to explore visits that result in admission to the hospital compared with those that result in discharge from the ED. In some circumstances, visits that result in discharge home may not have been true medical emergencies, and therefore might have been preventable. It is also true that even an acute problem requiring admission may have been preventable with timely outpatient management. While we found that 45% of visits ended in discharge home, over half of cancer patients who visit the ED are admitted to the hospital. This is higher than admission rates from the ED for the U.S. population overall (11%-15%),30-32and even higher than the estimated rate of individuals with chronic conditions, such as diabetes (42%), who visit the ED and then are subsequently admitted to the hospital.33
Relatively high rates of admissions may indicate that cancer patients seeking care in the ED require increased medical attention; however, it is possible that other explanations exist. For instance, ED physicians may be uncomfortable with complex cancer cases and may admit patients to be evaluated by a specialist. As such, it is possible that some of these admissions could have been appropriate for outpatient follow up. It is also possible that patients are referred to the ED for admission to the hospital. In these situations the ED visit may be entirely preventable through a direct admission process, although such processes are not available at all institutions and may vary by the admitting provider. For instance, if a hospitalist is overseeing the hospital stay, they may prefer the admission to occur through the ED, whereas a primary care provider or oncologist may be more likely to facilitate a direct admission. Future research could address the extent to which admissions from the ED may be avoidable by examining reasons for and length of admission following an ED visit. While this study found top reasons for admission (principal diagnosis) to be septicemia, cardiovascular complaints and complications from surgery, cumulatively these diagnosis accounted for less than 20% of admissions from the ED. Examining frequent diagnoses by cancer type will also provide insight into potentially avoidable ED use, which may vary by disease course and treatment regimen.
The distribution of days from diagnosis to the first ED visit also varied by cancer type. This variation is likely attributable, at least in part, to differences in condition-specific treatment regimens, severity of illness, and stage at diagnosis. For example, patients with ED visits within 30 days of diagnosis may be those with advanced stage cancers who are at higher risk of complications, or they may be visiting the ED for post-surgical problems. Likewise, individuals who incur visits during later time periods may be undergoing longer treatment regimens. Further research is warranted to explore site-specific predictors of ED use and high-risk periods, accounting for cancer treatment and the timing of treatments.
In summary, ED use among cancer patients is substantial and higher than previously reported. Most ED visits occur within the first 180 days after diagnosis, suggesting focus on the first 30 days after hospital discharge may be misguided. Time frames for ED measurement in future research should be selected with careful attention to cancer-specific periods within which most ED use occurs and the outcomes of interest. Furthermore, better models identifying cancer-specific predictors of ED use, which account for treatment and comorbidities, will facilitate the development of interventions focused on high-risk segments of this population. Research is needed to explore cancer-specific reasons for ED visits and which ED admission diagnoses may be potentially preventable.
Limitations
The limitations of this study include those common to use of administrative and registry data and the CCR and OSHPD data in particular. While CCR data are known to be complete with respect to demographic and cancer information, treatment data is less robust and specific treatment dates are not available.14,34 As a result, we were unable to analyze ED use in relation to receipt of outpatient treatment. As we included all ED visits on or up to a year after the day of diagnosis, it is possible that our analysis includes diagnoses that occurred in conjunction with an ED visits. However, it is unlikely a reporting hospital would report a cancer diagnosis to the CCR without a corresponding hospital admission. Therefore, we assume such cases to be rare.
Lastly, California had lower prevalence of health insurance coverage and higher market penetration by health maintenance organizations, relative to the national average, which may limit the generalizability of the results to other states.35 At the same time, CCR-OSHPD linked data offer the advantage of providing complete data to enumerate ED visits among patients whether they were discharged home or subsequently admitted to hospital.
In 2017 there will be nearly 1.7 million new cancer cases diagnosed, and over 600,000 cancer deaths in the Unites States.1 A 2013 Institute of Medicine report highlighted problems with the current quality of cancer care, including high costs and fragmentation of care.2 Other national reports have called for improvements in the overall quality of care and for reducing costly and possibly avoidable use of health services such as emergency department (ED) visits.3-6 Reduction of avoidable ED visits is often cited as a pathway to reduce costs by avoiding unnecessary tests and treatments that occur in the ED and subsequent hospital admissions.7,8
ED crowding, long waits, and unpredictable treatment environments can also make an ED visit an unpleasant experience for the patient. ED visits during cancer treatment can be particularly troubling and present health concerns for patients who are immunocompromised. In particular, cancer patients in the ED have been found to experience delays in the administration of analgesics, antiemetics, or antibiotics.9
Few studies have examined ED use or its associated predictors among cancer patients. Reports to date that have described ED use have focused on different cancers, which makes comparisons across studies difficult.10 Moreover, the time frames of interest and the type of event after which ED use is evaluated (ie, diagnosis or treatment) are inconsistent in the existing literature.10 Some studies quantifying ED use excluded patients admitted to the hospital after an ED visit.11,12 Taken together, these studies do not provide a clear overview of the extent of ED use by cancer patients or the amount of cancer-related care provided by EDs.
Patterns of ED use among cancer patients derived from large and generalizable samples may help inform providers about true risk factors for ED use. In addition, prioritizing new interventions and focusing future research on groups of patients who are at higher risk for preventable ED use could also improve overall care. To address these issues, accurate estimates of ED use among cancer patients are required.
To our knowledge, this is the first study to describe ED use across a range of cancers in a large population-based sample and to consider the timing of ED visits in relation to initial diagnosis. The findings could provide benchmark comparison data to inform future efforts to identify the subset of possibly preventable ED visits and to design interventions to address preventable ED use.
Material and methods
Data source
California’s Office of Statewide Health Planning and Development (OSHPD) manages the patient discharge dataset (PDD) and the emergency department use (EDU) dataset, providing a high-quality source of information on inpatient and ED use in the state.13 A principal diagnosis and up to 24 secondary diagnoses are recorded in OSHPD datasets. The EDU dataset was used to identify treat-and-release ED visits, and the PDD was used to identify hospitalizations initiated in the ED. The California Cancer Registry (CCR) obtains demographic and diagnosis information for every new invasive cancer diagnosed in California, and data collected by the registry are considered to be complete.14 CCR-OSPHD-linked data provide high-quality health care use information for cancer patients in California.15,16 Using an encrypted version of the social security number called the record linkage number (RLN), we linked the CCR records to the corresponding OSHPD files from 2009-2010.
Institutional review board approval for this study was obtained from the University of California, Davis, Human Subjects Committee and the State of California Committee for the Protection of Human Subjects.
Analysis
ED visits. Visits were included if they occurred on or up to 365 days after the date of cancer diagnosis recorded in the CCR. The visits were coded in mutually exclusive groups as occurring within 30, 31-180, and 181-365 days of diagnosis. Subsequently, we flagged each person as having any ED visit (Yes/No) within 180 days and within 365 days of diagnosis, and we tallied the total number of visits occurring within these time frames for each person.
Cancer type. We used relevant site and histology codes to classify cancer type into 24 mutually exclusive categories using the Surveillance Epidemiology and End Result‘s International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) Recode Definitions17-19 (Suppl Figure 1).
Individual-level variables. Sociodemographic information for each person was collected from the CCR including gender, age, race/ethnicity, marital status, health insurance status, rural residence, survival time in months, neighborhood socio-economic (SES) status based on the Yang index, and the American Joint Committee on Cancer (AJCC) stage.20,21
Data analysis
Demographic information was analyzed for the cohort using descriptive statistics (frequencies, proportions, means, standard deviations, and ranges) and evaluated for correlations. Fewer than 20 observations had missing data and we removed those observations from our analyses on an item-specific basis.
We tabulated ED visits by cancer type and time from diagnosis and then collapsed visit-level data by RLN to determine the number of ED visits for each person in the sample. The number of days from diagnosis to first ED visit was also tabulated. The cohort was stratified by cancer type and cumulative rates of ED visits were tabulated for individuals with ED visits within 0-180 and 0-365 days from diagnosis. To test the robustness of the findings adjusting for confounding factors known to impact ED use, we used logistic regression to model any ED use (Yes/No) as a function of age, gender, race/ethnicity, cancer stage, insurance status, marital status, urban residence, and Yang SES. After model estimation, we used the method of recycled predictions controlling for the confounding variables to compute the marginal probabilities of ED use by cancer type.22 To adjust for the possible impact of survival on ED use, we performed sensitivity analyses and estimated predicted probabilities adjusting for survival. Separate analyses were performed first adjusting for whether the patient died during the course of each month after diagnosis and then adjusting for whether or not the patient died within 180 days of diagnosis. All analyses were conducted using Stata 13.1.23
Results
The CCR identified 222,087 adults with a new primary cancer diagnosis in 2009-2010. After excluding those with Stage 0 cancer (n = 21,154) and nonmelanoma skin cancer (n = 1,031), for whom data are inconsistently collected by CCR, a total of 199,872 individuals were included in the analytic sample. Of those patients (Table 1), most were white non-Hispanic (62%), women (51%), holders of private insurance (53%), married (56%), and urban residents (86%). Most were older than 50 years and had either Stage I or Stage II cancer. The most common cancer types were breast (17%), prostate (16%), lung (11%), and colon (9%; results not shown). In unadjusted comparisons, the incidence of ED use was significantly higher among those who were older, of non-Hispanic black race/ethnicity, uninsured, in the lowest SES group, widowed, or diagnosed with Stage IV cancer (Table 1).
ED visits
Within 365 days after initial cancer diagnosis, 87,025 cancer patients made a total of 197,886 ED visits (not shown in tables). Of those visits, 68% (n = 134,556) occurred within 180 days of diagnosis, with 22% (n = 43,535) occurring within the first 30 days and 46% (n = 91,027) occurring within 31-180 days after diagnosis (Figure). Given that most of the visits occurred within 180 days of diagnosis, we used that time frame in subsequent analyses. Among all ED visits within 180 days of diagnosis (Table 2), the largest proportions of visits were made by those with lung cancer (16%), breast cancer (11%), and colon cancer (10%).
About 51% of visits resulted in admission to the hospital and 45% in discharge (Table 2). For some cancers (lung, colon, non-Hodgkin lymphoma, pancreatic, digestive, liver, stomach, leukemia, and myeloma) most of the visits resulted in admission to the hospital (Table 2). Among visits resulting in admission, the top three principal diagnoses were: septicemia (8%), cardiovascular problems (7%), and complications from surgery (5%) (not shown in tables). Among visits resulting in a discharge home, the three top principal diagnoses were abdominal pain (7%), cardiovascular problems (6%), and urinary, kidney, and bladder complaints other than a urinary tract infection (5%) (results not shown).
Individuals
The cumulative incidence of at least one ED visit was 35% (n = 70,813) within 180 days after diagnosis (Table 3). Visit rates varied by cancer type: individuals with pancreatic (62%), brain (60%), and lung (55%) cancers had the highest cumulative incidences of ED use within 180 days of diagnosis (Table 3). Those with melanoma (14%), prostate (17%), and eye (18%) cancers had the lowest cumulative incidences of ED visits (Table 3).
Recycled predictions from logistic regression models, accounting for potential confounding factors, yielded substantively similar results for the cumulative incidence of ED use across cancer types (Table 4). Results did not differ substantially after accounting for survival. Differences in the predicted probability of an ED visits adjusting for death within 180 days of diagnosis were noted to be 2% or greater from estimates reported in Table 4 for only four cancers. Estimates of having any ED visits for those with lung cancer decreased from 46% to 44% (95% CI: 43.0-44.4%), pancreatic cancer from 53% to 49% (95% CI: 48-51%), liver cancer from 51% to 47% (95% CI: 49-53%), and those with eye cancers increased from 20% to 22% (95% CI: 18-26%) (not shown in tables).
For patients with certain cancers (eg, lung, pancreas, leukemia) the proportion of individuals with an initial ED visit was highest in the first 30 days after diagnosis (Table 3). For individuals with other cancers (eg, breast, prostate, melanoma) the proportion of individuals with an initial ED visit increased by more than 5% during the 31-181–day time period. Those with the remaining cancers had less than 5% change in cumulative ED use between the two time periods.
The number of visits per person ranged from 0-44 during the first 180 days after diagnosis (results not shown in tables). Of all patients diagnosed with cancer, 20% (n = 39,429) had one ED visit, 8% (n = 16,238) had two visits, and 7% (n = 14,760) had three or more visits. Of those patients having at least one ED visit within 180 days of diagnosis, 44% (n = 31,080) had two or more visits and 21% (n = 14,760) had 3 or more visits.
Discussion
This study extends previous research by describing ED use for more than 20 cancer types by time from diagnosis in a large, heterogeneous and population-based sample of recently diagnosed adults in California. We found that 16% of newly diagnosed individuals with cancer used the ED within 30 days of diagnosis, 35% within 6 months of diagnosis, and 44% within 1 year of diagnosis. These findings suggest that ED use by cancer patients is more than double that of the US general population and is higher than previously estimated for cancer patients.10,24 In 2010, about 21% of the US population visited the ED, compared with 44% of cancer patients in the same time period.24 Although persons with greater medical need, such as those with cancer, inevitably require more health services, new approaches are needed to explore the extent to which some of these visits by cancer patients could be prevented by providing care in other settings.
Few studies have examined ED use by cancer patients, but previous findings suggest that 1%-12% of cancer patients use the ED within 30 days of diagnosis, and 15%-25% use the ED within a year of diagnosis.10,25,26 One study did report higher rates of ED use by cancer patients, but attributed the increased use to changes in Medicaid copayments.27 The finding that ED use is higher among cancer patients than previously considered is important for several reasons. First, high rates of ED use may reflect excessive fragmentation in cancer care, or patients’ inability to access providers when acute concerns arise. Furthermore, providers and policymakers may be particularly interested in populations with high ED use because reducing potentially preventable ED use is often cited as one of the goals of care coordination and alternative health care model programs.2,28,29
The number of newly diagnosed cancer patients with multiple ED visits is also substantial. We found 15% of recently diagnosed cancer patients had two or more ED visits within 180 days of diagnosis, compared with 8% of the general US population having two or more ED visits in all of 2010.24 Among cancer patients with at least one ED visit, 44% visited more than once. Repeat visits may represent worsening health status, continued unmet health needs, or new complications that might have been prevented or treated in other health care settings. In addition, there may be opportunities to identify cancer patients at risk for multiple visits at their initial ED visit. A better understanding of the reasons for ED visits and the factors driving unmet need – such as inadequate patient education, limited access to specialty services, or failure to admit a patient to resolve a problem appropriately (eg, pain, infection) – may help to identify which visits are potentially preventable. Ultimately, failure to adequately describe the number of cancer patients that visit the ED and the number of times they visit may result in a lost opportunity for improvement in care, the patient experience and cost reduction in cancer care.
The distinction between cancer types that account for the most ED visits and cancer types with the highest cumulative incidences of ED use is informative. For instance, lung cancer patients accounted for the largest number of ED visits and over half of those with lung cancer visited the ED within 180 days of diagnosis. However, although more than 60% of individuals with pancreatic cancer visited an ED within 180 days of diagnosis; they accounted for only 5% of all ED visits by cancer patients during the same time period. This in part reflects the relative frequency of these cancers. However, prostate cancer, which has a high incidence rate, represents about 8% of all ED visits by cancer patients, yet only 17% of all prostate cancer patients visit the ED within 180 days of diagnosis.
One approach to reduce the absolute number of ED visits by cancer patients would be to target the most frequent users of the ED such as lung, breast, prostate, and colon cancer patients. These cancers are the most common in the general population, so proportionate reduction in ED visits in these groups would have a large overall impact on ED use. Alternatively, patients with cancers that have high rates of ED use could be targeted with interventions to better address their needs. Additional studies of ED use among cancer patients, including understudied cancers, are needed to determine whether care provided in the ED could be provided in alternate clinical settings. Such research can also support training of emergency department staff to manage the full range of cancer-related conditions presenting to the ED.
Another approach to identifying potentially avoidable ED visits is to explore visits that result in admission to the hospital compared with those that result in discharge from the ED. In some circumstances, visits that result in discharge home may not have been true medical emergencies, and therefore might have been preventable. It is also true that even an acute problem requiring admission may have been preventable with timely outpatient management. While we found that 45% of visits ended in discharge home, over half of cancer patients who visit the ED are admitted to the hospital. This is higher than admission rates from the ED for the U.S. population overall (11%-15%),30-32and even higher than the estimated rate of individuals with chronic conditions, such as diabetes (42%), who visit the ED and then are subsequently admitted to the hospital.33
Relatively high rates of admissions may indicate that cancer patients seeking care in the ED require increased medical attention; however, it is possible that other explanations exist. For instance, ED physicians may be uncomfortable with complex cancer cases and may admit patients to be evaluated by a specialist. As such, it is possible that some of these admissions could have been appropriate for outpatient follow up. It is also possible that patients are referred to the ED for admission to the hospital. In these situations the ED visit may be entirely preventable through a direct admission process, although such processes are not available at all institutions and may vary by the admitting provider. For instance, if a hospitalist is overseeing the hospital stay, they may prefer the admission to occur through the ED, whereas a primary care provider or oncologist may be more likely to facilitate a direct admission. Future research could address the extent to which admissions from the ED may be avoidable by examining reasons for and length of admission following an ED visit. While this study found top reasons for admission (principal diagnosis) to be septicemia, cardiovascular complaints and complications from surgery, cumulatively these diagnosis accounted for less than 20% of admissions from the ED. Examining frequent diagnoses by cancer type will also provide insight into potentially avoidable ED use, which may vary by disease course and treatment regimen.
The distribution of days from diagnosis to the first ED visit also varied by cancer type. This variation is likely attributable, at least in part, to differences in condition-specific treatment regimens, severity of illness, and stage at diagnosis. For example, patients with ED visits within 30 days of diagnosis may be those with advanced stage cancers who are at higher risk of complications, or they may be visiting the ED for post-surgical problems. Likewise, individuals who incur visits during later time periods may be undergoing longer treatment regimens. Further research is warranted to explore site-specific predictors of ED use and high-risk periods, accounting for cancer treatment and the timing of treatments.
In summary, ED use among cancer patients is substantial and higher than previously reported. Most ED visits occur within the first 180 days after diagnosis, suggesting focus on the first 30 days after hospital discharge may be misguided. Time frames for ED measurement in future research should be selected with careful attention to cancer-specific periods within which most ED use occurs and the outcomes of interest. Furthermore, better models identifying cancer-specific predictors of ED use, which account for treatment and comorbidities, will facilitate the development of interventions focused on high-risk segments of this population. Research is needed to explore cancer-specific reasons for ED visits and which ED admission diagnoses may be potentially preventable.
Limitations
The limitations of this study include those common to use of administrative and registry data and the CCR and OSHPD data in particular. While CCR data are known to be complete with respect to demographic and cancer information, treatment data is less robust and specific treatment dates are not available.14,34 As a result, we were unable to analyze ED use in relation to receipt of outpatient treatment. As we included all ED visits on or up to a year after the day of diagnosis, it is possible that our analysis includes diagnoses that occurred in conjunction with an ED visits. However, it is unlikely a reporting hospital would report a cancer diagnosis to the CCR without a corresponding hospital admission. Therefore, we assume such cases to be rare.
Lastly, California had lower prevalence of health insurance coverage and higher market penetration by health maintenance organizations, relative to the national average, which may limit the generalizability of the results to other states.35 At the same time, CCR-OSHPD linked data offer the advantage of providing complete data to enumerate ED visits among patients whether they were discharged home or subsequently admitted to hospital.
1. American Cancer Society. Facts & Figures 2017. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. Published 2017. Accessed March 16, 2017.
2. Levit L, Balogh E, Nass S, Ganz PA. Delivering high-quality cancer care: charting a new course for a system in crisis. https://www.nap.edu/read/18359/chapter/1. Published 2013. Accessed March 16, 2017.
3. Readmissions Reduction Program (HRRP). CMS website. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. Updated April 8, 2016. Accessed March 16, 2017.
4. Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists: challenges to assuring access to oncology services. J Clin Oncol. 2007;3(2):79-86.
5. Guadagnolo B, Dohan D, Raich P. Metrics for evaluating patient navigation during cancer diagnosis and treatment: crafting a policy-relevant research agenda for patient navigation in cancer care. Cancer. 2011;117(15 Suppl):3565-3574.
6. Medicare Patient Access to Cancer Treatment Act of 2013, H.R.2869, 113th Cong.(2013). https://www.congress.gov/bill/113th-congress/house-bill/2869/text?format=txt. Introduced July 31, 2013; latest action, referred to the Subcommittee on Health, August 2, 2013. Accessed March 16, 2017.
7. Smulowitz P, Honigman L, Landon B. A novel approach to identifying targets for cost reduction in the emergency department. Ann Emerg Med. 2013;61(3):293-300.
8. Agrawal S, Conway P. Integrating emergency care into a patient- and outcome-centered health care system. Ann Emerg Med. 2013;61(3):301-302.
9. Swenson K, Rose M, Ritz L, Murray CL, Adlis S. Recognition and evaluation of oncology-related symptoms in the emergency department. Ann Emerg Med.1995;26(1):12-17.
10. Lash R, Bell J, Reed S, et al. A systematic review of emergency department use among cancer patients. Cancer Nurs. 2017;40(2):135-144.
11. Sanoff H, Carpenter W, Freburger J, et al. Comparison of adverse events during 5-fluorouracil versus 5-fluorouracil/oxaliplatin adjuvant chemotherapy for stage III colon cancer: a population-based analysis. Cancer. 2012;118(17):4309-4320.
12. Hansen D, Fox J, Gross C, Bruun J. Hospital readmissions and emergency department visits following laparoscopic and open colon resection for cancer. Dis Colon Rectum. 2013;56(9):1053-1061.
13. Office of Statewide Health Planning and Development. Hospital Data Products. http://www.oshpd.ca.gov/HID/DataFlow/HospData.html. Last updated September 6, 2016. Accessed March 16, 2017.
14. California Cancer Registry. Overview. http://www.ccrcal.org/Inside_CCR/About_Us.shtml. Published 2009. Accessed April 15, 2015.
15. Patel M, Ma Y, Mitchell B, Rhoads K. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24(2):344-349.
16. Parikh-Patel A, White R, Allen M, Cress R. Risk of cancer among rheumatoid arthritis patients in California. Cancer Causes Control. 2009;20(6):1001-1010.
17. National Cancer Institute: Surveillance, Epidemiology and End Results Program. Site recode ICD-O-3/WHO 2008 definition. https://seer.cancer.gov/siterecode/icdo3_dwhoheme/. Published 2008. Accessed March 16, 2017.
18. Washington State Cancer Registry. Cancer Codes Used in Reports. https://fortress. wa.gov/doh/wscr/WSCR/CancerCode.mvc/CancerCode. Data updated, January 2016; report updated, March 2016. Accessed March 16, 2017.
19. National Cancer Institute: Surveillance, Epidemiology and End Results Program. ICD-O-3 SEER Site/Histology Validation List. https://seer.cancer.gov/icd-o-3/. Published 2012, updated September 2015. Accessed March 16, 2017.
20. American Joint Committee on Cancer. What is Cancer Staging? https://cancerstaging.org/references-tools/Pages/What-is-Cancer-Staging.aspx. Published 2010. Accessed March 16, 2017.
21. Yang J S, Harrati A, Clarke C, Keegan T, Gomez S. Cancer Prevention Institute of California. Developing an area based socioeconomic measures from American Community Survey data. http://www.cpic.org/files/PDF/Research_Files/Reports/CPIC_ACS_SES_Index_Documentation_3-102014.pdf. Published March 10, 2014. Accessed March 16, 2017.
22. Basu A, Rathouz P. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics. 2005;6(1):93-109.
23. Stata Statistical Software [computer program]. Version 13 College Station, TX: StataCorp LP. 2013.
24. National Center for Health Statistics. Health, United States, 2013 – with a special feature on prescription drugs. https://www.cdc.gov/nchs/data/hus/hus13.pdf. Updated May 2014. Accessed March 16, 2017.
25. Goyal R, Wheeler S, Kohler R, et al. Health care utilization from chemotherapy-related adverse events among low-income breast cancer patients: effect of enrollment in a medical home program. N C Med J. 2014;75(4):231-238.
26. Hassett M, O’Malley A, Pakes J, Newhouse J, Earle C. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst. 2006;98(16):1108-1117.
27. Subramanian S. Impact of Medicaid copayments on patients with cancer: lessons for Medicaid expansion under health reform. Med Care. 2011;49(9):842-847.
28. Coyle Y, Miller A, Paulson R. Model for the cost-efficient delivery of continuous quality cancer care: a hospital and private-practice collaboration. Proc (Bayl Univ Med Cent). 2013;26(2):95-99.
29. Agency for Healthcare Research and Quality. 2011 National Healthcare Disparities Report. https://archive.ahrq.gov/research/findings/nhqrdr/nhdr11/index.html. Last reviewed October 2014. Accessed March 16, 2017.
30. Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Emergency Department Sample (NEDS) 2010. https://www.hcup-us.ahrq.gov/db/nation/neds/NEDS_Introduction_2010.jsp. Issued November 2012, updated November 2015. Accessed March 16, 2017.
31. Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Emergency Department Sample (NEDS) 2013. https://www.hcup-us.ahrq.gov/db/nation/neds/NEDS_Introduction_2013.jsp. Published November 2015. Accessed March 16, 2017.
32. Weiss AJ, Wier LM, Stocks C, Blanchard J. Overview of emergency department visits in the United States, 2011. Statistical Brief #174. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb174-Emergency-Department-Visits-Overview.pdf. Published June 2014. Accessed March 16, 2017.
33. Washington R, Andrews R, Mutter, R. Emergency department visits for adults with diabetes, 2010. Statistical Brief #167. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb167.jsp. Published November 2013. Accessed March 16, 2017.
34. Penberthy L, Petkov V, McClish D, et al. The value of billing data from oncology practice to supplement treatment information for cancer surveillance. Journal of registry management. 2014;41(2):57-64.
35. National Center for Health Statistics. Health, United States, 2014 – with a special feature on adults aged 55-64. https://www.cdc.gov/nchs/data/hus/hus14.pdf. Published May 2015. Accessed March 16, 2017.
1. American Cancer Society. Facts & Figures 2017. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. Published 2017. Accessed March 16, 2017.
2. Levit L, Balogh E, Nass S, Ganz PA. Delivering high-quality cancer care: charting a new course for a system in crisis. https://www.nap.edu/read/18359/chapter/1. Published 2013. Accessed March 16, 2017.
3. Readmissions Reduction Program (HRRP). CMS website. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. Updated April 8, 2016. Accessed March 16, 2017.
4. Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists: challenges to assuring access to oncology services. J Clin Oncol. 2007;3(2):79-86.
5. Guadagnolo B, Dohan D, Raich P. Metrics for evaluating patient navigation during cancer diagnosis and treatment: crafting a policy-relevant research agenda for patient navigation in cancer care. Cancer. 2011;117(15 Suppl):3565-3574.
6. Medicare Patient Access to Cancer Treatment Act of 2013, H.R.2869, 113th Cong.(2013). https://www.congress.gov/bill/113th-congress/house-bill/2869/text?format=txt. Introduced July 31, 2013; latest action, referred to the Subcommittee on Health, August 2, 2013. Accessed March 16, 2017.
7. Smulowitz P, Honigman L, Landon B. A novel approach to identifying targets for cost reduction in the emergency department. Ann Emerg Med. 2013;61(3):293-300.
8. Agrawal S, Conway P. Integrating emergency care into a patient- and outcome-centered health care system. Ann Emerg Med. 2013;61(3):301-302.
9. Swenson K, Rose M, Ritz L, Murray CL, Adlis S. Recognition and evaluation of oncology-related symptoms in the emergency department. Ann Emerg Med.1995;26(1):12-17.
10. Lash R, Bell J, Reed S, et al. A systematic review of emergency department use among cancer patients. Cancer Nurs. 2017;40(2):135-144.
11. Sanoff H, Carpenter W, Freburger J, et al. Comparison of adverse events during 5-fluorouracil versus 5-fluorouracil/oxaliplatin adjuvant chemotherapy for stage III colon cancer: a population-based analysis. Cancer. 2012;118(17):4309-4320.
12. Hansen D, Fox J, Gross C, Bruun J. Hospital readmissions and emergency department visits following laparoscopic and open colon resection for cancer. Dis Colon Rectum. 2013;56(9):1053-1061.
13. Office of Statewide Health Planning and Development. Hospital Data Products. http://www.oshpd.ca.gov/HID/DataFlow/HospData.html. Last updated September 6, 2016. Accessed March 16, 2017.
14. California Cancer Registry. Overview. http://www.ccrcal.org/Inside_CCR/About_Us.shtml. Published 2009. Accessed April 15, 2015.
15. Patel M, Ma Y, Mitchell B, Rhoads K. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24(2):344-349.
16. Parikh-Patel A, White R, Allen M, Cress R. Risk of cancer among rheumatoid arthritis patients in California. Cancer Causes Control. 2009;20(6):1001-1010.
17. National Cancer Institute: Surveillance, Epidemiology and End Results Program. Site recode ICD-O-3/WHO 2008 definition. https://seer.cancer.gov/siterecode/icdo3_dwhoheme/. Published 2008. Accessed March 16, 2017.
18. Washington State Cancer Registry. Cancer Codes Used in Reports. https://fortress. wa.gov/doh/wscr/WSCR/CancerCode.mvc/CancerCode. Data updated, January 2016; report updated, March 2016. Accessed March 16, 2017.
19. National Cancer Institute: Surveillance, Epidemiology and End Results Program. ICD-O-3 SEER Site/Histology Validation List. https://seer.cancer.gov/icd-o-3/. Published 2012, updated September 2015. Accessed March 16, 2017.
20. American Joint Committee on Cancer. What is Cancer Staging? https://cancerstaging.org/references-tools/Pages/What-is-Cancer-Staging.aspx. Published 2010. Accessed March 16, 2017.
21. Yang J S, Harrati A, Clarke C, Keegan T, Gomez S. Cancer Prevention Institute of California. Developing an area based socioeconomic measures from American Community Survey data. http://www.cpic.org/files/PDF/Research_Files/Reports/CPIC_ACS_SES_Index_Documentation_3-102014.pdf. Published March 10, 2014. Accessed March 16, 2017.
22. Basu A, Rathouz P. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics. 2005;6(1):93-109.
23. Stata Statistical Software [computer program]. Version 13 College Station, TX: StataCorp LP. 2013.
24. National Center for Health Statistics. Health, United States, 2013 – with a special feature on prescription drugs. https://www.cdc.gov/nchs/data/hus/hus13.pdf. Updated May 2014. Accessed March 16, 2017.
25. Goyal R, Wheeler S, Kohler R, et al. Health care utilization from chemotherapy-related adverse events among low-income breast cancer patients: effect of enrollment in a medical home program. N C Med J. 2014;75(4):231-238.
26. Hassett M, O’Malley A, Pakes J, Newhouse J, Earle C. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst. 2006;98(16):1108-1117.
27. Subramanian S. Impact of Medicaid copayments on patients with cancer: lessons for Medicaid expansion under health reform. Med Care. 2011;49(9):842-847.
28. Coyle Y, Miller A, Paulson R. Model for the cost-efficient delivery of continuous quality cancer care: a hospital and private-practice collaboration. Proc (Bayl Univ Med Cent). 2013;26(2):95-99.
29. Agency for Healthcare Research and Quality. 2011 National Healthcare Disparities Report. https://archive.ahrq.gov/research/findings/nhqrdr/nhdr11/index.html. Last reviewed October 2014. Accessed March 16, 2017.
30. Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Emergency Department Sample (NEDS) 2010. https://www.hcup-us.ahrq.gov/db/nation/neds/NEDS_Introduction_2010.jsp. Issued November 2012, updated November 2015. Accessed March 16, 2017.
31. Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Emergency Department Sample (NEDS) 2013. https://www.hcup-us.ahrq.gov/db/nation/neds/NEDS_Introduction_2013.jsp. Published November 2015. Accessed March 16, 2017.
32. Weiss AJ, Wier LM, Stocks C, Blanchard J. Overview of emergency department visits in the United States, 2011. Statistical Brief #174. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb174-Emergency-Department-Visits-Overview.pdf. Published June 2014. Accessed March 16, 2017.
33. Washington R, Andrews R, Mutter, R. Emergency department visits for adults with diabetes, 2010. Statistical Brief #167. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb167.jsp. Published November 2013. Accessed March 16, 2017.
34. Penberthy L, Petkov V, McClish D, et al. The value of billing data from oncology practice to supplement treatment information for cancer surveillance. Journal of registry management. 2014;41(2):57-64.
35. National Center for Health Statistics. Health, United States, 2014 – with a special feature on adults aged 55-64. https://www.cdc.gov/nchs/data/hus/hus14.pdf. Published May 2015. Accessed March 16, 2017.
APF530 for nausea and vomiting prevention following cisplatin: phase 3 MAGIC trial analysis
Despite available antiemetic therapies, chemotherapy-induced nausea and vomiting (CINV) following highly emetogenic chemotherapy (HEC), particularly in the delayed phase (>24-120 h after chemotherapy), continues to impair patient quality of life and chemotherapy compliance.1 Cisplatin-based chemotherapy, classified as HEC at any dose,2 is widely used to treat cancers such as non–small-cell and small
5-hydroxytryptamine type 3 (5-HT3) receptor antagonists (RAs; eg, granisetron, ondansetron, dolasetron, and palonosetron) have been the cornerstone of CINV therapy for decades and remain an integral part of contemporary antiemetic treatment regimens. Most current antiemetic guidelines for HEC recommend a 3-drug regimen, comprising a 5-HT3 RA, a neurokinin 1 (NK-1) RA, and a corticosteroid (dexamethasone).2,6,7 A regimen of olanzapine (antipsychotic), palonosetron (5-HT3 RA), and dexamethasone (corticosteroid) has been recommended as an alternative option. Recently, the oral fixed-dose combination of netupitant and palonosetron (NEPA) was approved and has shown efficacy in the cisplatin setting.8,9 However, the administration of oral medication to patients experiencing CINV and those with head and neck cancer may be difficult.10 Alternative antiemetic treatments that provide CINV control into the delayed phase and with a convenient route of administration, are needed.
APF530 is a novel extended-release granisetron formulation that provides sustained release of therapeutic concentrations for ≥5 days. The Biochronomer tri(ethylene glycol) poly(orthoester) (TEG-POE) vehicle releases granisetron slowly by polymer hydrolysis after it has been injected subcutaneously (SC) into the abdomen or upper arm.11,12 In 2016, the US Food and Drug Administration approved APF530 in combination with other antiemetics for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic chemotherapy (MEC) or anthracycline plus cyclophosphamide (AC) combination chemotherapy regimens based on data from 2 pivotal phase 3 trials.13
A phase 3 trial demonstrated noninferiority of APF530 (500 mg, SC) to palonosetron (0.25 mg, intravenously [IV]), each with dexamethasone (corticosteroid), in the control of acute-phase CINV after MEC or HEC, and delayed-phase CINV after MEC (classified by Hesketh criteria).14,15 Furthermore, APF530 provided sustained CINV control over multiple cycles of chemotherapy.16 Numerically higher complete response (CR: no emesis, no rescue medication use) rates were observed with APF530, compared with palonosetron, in the delayed phase after HEC (APF530 500 mg, 67.1%; palonosetron 0.25 mg, 64.3%).15
A reanalysis of study endpoints by newer emetogenicity classification guidelines from the American Society of Clinical Oncology (ASCO)7 maintained overall study conclusions.17 Notably, the numerically higher CR rates with APF530 in the delayed phase following HEC were enhanced (APF530 500 mg, 55.8%; palonosetron 0.25 mg, 50.5%), suggesting a need for further examination in this setting. The subsequent APF530 phase 3 MAGIC trial (Modified Absorption of Granisetron In the prevention of CINV; NCT02106494), compared APF530 (500 mg, SC) with ondansetron (0.15 mg/kg, IV), each with fosaprepitant (NK-1 RA) and dexamethasone in patients receiving HEC. The primary endpoint was met: the APF530 regimen demonstrated superior delayed-phase CR compared with the ondansetron regimen (64.7% vs 56.6%; 95% confidence interval [CI]:
1.7-14.4; P = .014; 8.0% absolute improvement).18
APF530 also demonstrated a significant benefit over ondansetron for other endpoints including nausea control, rescue medication use, and satisfaction with antiemetic therapy.18 APF530 is the first and only 5-HT3 RA to demonstrate superiority over another in a phase 3 efficacy trial using a guideline-recommended 3-drug regimen for both arms.
A prespecified MAGIC trial analysis of the primary endpoint by intent to receive cisplatin (≥50 mg/m2, Yes/No) demonstrated a pronounced treatment benefit in terms of delayed-phase CR rates with the APF530 regimen among patients in the cisplatin (≥50 mg/m2, Yes) stratum (CR: 65.3% vs 54.7%; 95% CI: -1.4-22.7; 10.6% absolute improvement).18 These results are compelling, since cisplatin represents a particularly emetogenic class of chemotherapy; a more in-depth analysis of additional MAGIC trial endpoints for these patients would be of clinical interest, and is presented here. Efficacy endpoints in this analysis include CR in the overall and acute phases, complete control (CC) and total response (TR) rates, rescue medication use, nausea frequency, and safety.
Methods
Study design and patients
The MAGIC trial was a prospective, randomized, multicenter, placebo-controlled, double-blind, double-dummy phase 3 trial conducted at 77 centers across the United States. The study protocol was reviewed and approved by the institutional review board at each participating center, and conducted according to the Declaration of Helsinki. The study design, previously presented in detail,18 is reviewed briefly here.
Eligible men and women were 18-80 years of age with histologically or cytologically confirmed malignancy (cancer type information was not captured) and were entering the first cycle of their single-day HEC treatment (defined by ASCO 2011 emetogenicity criteria).7 Patients had Eastern Cooperative Oncology Group Performance Status (ECOG-PS) of 0 or 1, no history or presence of significant cardiac disease or QT interval prolongation, and adequate bone marrow, kidney, and liver function. All patients provided written informed consent.
Procedures
Patients were stratified by planned receipt of the cisplatin regimen ≥50 mg/m2 (Yes/No), randomized 1:1 to receive APF530 500 mg SC (granisetron 10 mg) or ondansetron 0.15 mg/kg IV (up to a maximum of 16 mg as a single dose) on day 1 (Figure 1). The APF530 arm received the ondansetron saline placebo, and the ondansetron arm received the APF530 SC placebo containing the TEG-POE vehicle. All patients were scheduled to receive fosaprepitant 150 mg IV and dexamethasone 12 mg IV on day 1, then oral dexamethasone 8 mg once daily on day 2 and 8 mg twice daily on days 3 and 4. Rescue medication was allowed at the investigator’s discretion.
Outcomes
The primary objective of the trial was to demonstrate the superiority of APF530 500 mg SC compared with ondansetron 0.15 mg/kg IV, as part of the current guideline-recommended 3-drug regimen, in preventing delayed-phase CINV after HEC. The primary endpoint was delayed-phase (24-120 h) CR (no emetic episodes [vomit or retch] and no rescue medication use). In addition, a prespecified analysis of delayed-phase CR by randomization strata (planned use of cisplatin) was performed.
Secondary and other endpoints included overall-phase CR
(0-120 h); delayed-, overall-, and acute-phase complete control (CC: CR and no more than mild nausea); delayed-, overall-, and acute-phase total response (TR; CR and no nausea); and rescue medication use. A post hoc analysis of nausea severity was also conducted. Safety assessments included treatment-emergent adverse events (TEAEs), injection-site reactions (ISRs), laboratory parameters, and vital signs. TEAEs were assessed by type, duration, severity, and relationship to study drug. ISR timing and severity were captured in patient diaries.
Statistical analysis
All efficacy analyses were conducted using the modified intent-to-treat population (mITT; randomized patients who received study drug and a HEC regimen and had post-baseline efficacy data). Safety assessments were performed on the safety population (randomized patients who received study drug).
This analysis conducted on the subgroup of patients with intent to receive cisplatin (cisplatin randomization stratum, ≥50 mg/m2, Yes) was exploratory and was not powered to detect treatment differences. Preplanned analyses compared CR, CC, and TR rates across treatment arms using 95% CIs.
Post hoc analyses of time to first rescue medication use, proportion of patients with rescue medication use, and less frequent nausea were performed. All P values were calculated using the Cochran-Mantel-Haenszel chi square test. Rescue medication use results were based on observed data, without imputation for missing results (ie, calculated from the number of patients with a response). Further analyses of efficacy endpoints CR, CC, and TR in the subset of female patients in the cisplatin randomization stratum were performed. Safety assessments were summarized descriptively.
Results
A total of 942 patients were randomized across 77 US centers during March 31, 2014 and May 15, 2015 (471 APF530, 471 ondansetron). Among those, 264 had intent to receive cisplatin and were included in the cisplatin randomization stratum (≥50 mg/m2, Yes) (Figure 2). A total of 256 patients in the cisplatin stratum received study drug and were included in the safety population (126 APF530, 130 ondansetron); 252 patients were included in the mITT population (124 APF530, 128 ondansetron).
Baseline demographics were generally balanced between treatment arms (Table 1). The proportion of female patients was 41.1% (51/124) and 48.4% (62/128) in the APF530 and ondansetron arms, respectively. The majority of patients had an ECOG PS of 0 (57.3% [71/124] APF530; 60.2% [77/128] ondansetron). The most common cisplatin-based chemotherapy regimen in both treatment arms was cisplatin and gemcitabine (25.0% [31/124] APF530; 28.9% [37/128] ondansetron) (Suppl Table 1). Two patients in the APF530 arm and 3 patients in the ondansetron arm either received a lower cisplatin dose (<50 mg/m2) or did not go on to receive cisplatin as intended at randomization (Suppl Table 1). As previously reported, in the cisplatin stratum (Table 2), delayed-phase CR was numerically higher in the APF530 arm versus the ondansetron arm, with a corresponding treatment difference of 10.6% (65.3% [81/124] APF530; 54.7% [70/128] ondansetron; 95% CI [-1.4, 22.7]; P = .085). Although the CI contains 0, the result is consistent with the significant benefit observed in the overall study population (64.7% [291/450] APF530; 56.6% [256/452] ondansetron; 95% CI [1.7, 14.4];
P = .014).18 This more in-depth analysis found similar trends favoring the APF530 over the ondansetron regimen across overall- and acute-phase CR (Table 2).
A significantly greater proportion of patients in the APF530 arm, compared with the ondansetron arm, reported no rescue medication use during the delayed phase (74.4% [90/121] APF530; 62.6% [77/123] ondansetron; P = .048). Trends in favor of APF530 were observed in the overall phase (71.1% [86/121] APF530; 61.8% [76/123] ondansetron; P = .125) and acute phase (86.9% [106/122] APF530; 81.9% [104/127] ondansetron; P = .278). Time to first rescue medication use was consistently longer in the APF530 arm, compared with the ondansetron arm, although not statistically significantly (P = .150) (Figure 3).
The APF530 regimen was generally well tolerated in the cisplatin subgroup, and no new safety signals were identified (Table 3). Most patients experienced at least one TEAE. Excluding ISRs, TEAE incidences were 72.2% and 66.9% in the APF530 and ondansetron arms, respectively; most common were constipation, fatigue, nausea, diarrhea, dehydration, and headache. Excluding ISRs, the most common treatment-related TEAEs in the APF530 and ondansetron arms were constipation (2.4% and 2.3%, respectively and headache (3.2% and 4.6%).
Discussion
The MAGIC trial is the first phase 3 efficacy trial in the prevention of CINV in patients receiving HEC using the current guideline-recommended 3-drug antiemetic regimen in both treatment arms.18 Ondansetron was chosen as the appropriate 5-HT3 RA comparator because no other 5-HT3 RA has shown superiority to ondansetron in delayed-phase CINV following HEC. Furthermore, ondansetron is indicated for prevention of nausea and vomiting associated with initial and repeat courses of chemotherapy, including high-dose cisplatin.19 The MAGIC trial primary endpoint was met for the overall study population; in the context of a 3-drug regimen, APF530 demonstrated superior control of delayed-phase CINV following HEC compared with standard-of-care ondansetron.18 As reported previously, significant benefits were also observed with the APF530 regimen over the ondansetron regimen in terms of rescue medication use, patient satisfaction with antiemetic therapy, and nausea frequency in the overall study population.18
Cisplatin is generally regarded as one of the most emetogenic chemotherapeutic agents. For this reason, cisplatin is often evaluated separately in clinical trials, and was a stratification factor in the MAGIC trial. Consistent with the previously reported significant results,18 trends in the cisplatin stratum analysis favored the APF530 regimen, compared with the ondansetron regimen, in delayed- and overall-phase CR (treatment difference: 10.6%).18 Numerical trends presented here favoring the APF530 regimen over the ondansetron regimen were observed in CC and TR, two more stringent measures of CINV control that account for incidence of nausea. Furthermore, among women in the cisplatin stratum, a population at increased risk for CINV, the numerically higher CR, CC, and TR persisted in the APF530 arm, compared with the ondansetron arm.
The APF530 regimen was generally well tolerated in the cisplatin stratum, and no new safety signals were identified. The most common TEAEs were ISRs, mostly mild or moderate and resolving by study end. The double-dummy design resulted in ISRs in the ondansetron arm due to TEG-POE vehicle as the dummy APF530 injection. Transient ISRs have been observed with other agents administered SC, and are expected.20,21 Excluding ISRs, TEAEs were generally consistent with those observed for the 5-HT3 RA class.22
This analysis of patients randomized to receive cisplatin-based HEC in the MAGIC trial is exploratory and was not sufficiently powered to detect between-arm differences. Five total patients did not go on to receive cisplatin ≥50 mg/m2 as intended at randomization (2 APF530, 3 ondansetron); however, this is not uncommon in large clinical trials and represents less than 2% of patients in this analysis.
Recent phase 3 studies in patients receiving cisplatin-based HEC showed significant improvement in CINV prevention with the current guideline-recommended 3-drug regimen over the traditional 2-drug regimen (5-HT3 RA + dexamethasone).8,23 Results presented here, in a similar population receiving cisplatin-based HEC, suggest that in the context of a 3-drug antiemetic regimen in both treatment arms, APF530 provides additional benefit in CINV prevention compared with the standard of care, ondansetron. Furthermore, a recent phase 3 trial in patients receiving cisplatin or AC-based HEC demonstrated significant improvement in nausea when olanzapine was added to a traditional 3-drug regimen of a 5-HT3 RA, NK-1 RA, and dexamethasone.24 These compelling data support the addition of olanzapine as a fourth agent to the CINV treatment regimen to provide further control of nausea, which has been one of the more difficult components of CINV to control to date.
APF530 is the only 5-HT3 RA to demonstrate superiority over another as part of the guideline-recommended regimen in a 3-drug versus 3-drug phase 3 efficacy trial examining antiemetic efficacy following HEC. Results from the MAGIC trial, this exploratory analysis, and previous studies in MEC and HEC provide clinically meaningful benefits in preventing both acute- and delayed-phase CINV following guideline-specified MEC or HEC regimens. Consequently, APF530 was approved for use in combination with other antiemetics for prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of MEC or AC combination chemotherapy regimens.13 Both the superior control of delayed-phase CINV following HEC demonstrated by the MAGIC trial18 and the consistent trends in the cisplatin stratum indicate a particular benefit for high-risk patients receiving high doses of cisplatin.
Acknowledgments
Joanna K Sandilos Rega, PhD, of SciStrategy Communications provided medical writing assistance, supported by Heron Therapeutics Inc, the maker of the study drug.
1. Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK. Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer. 2012;20:107-117.
2. NCCN clinical practice guidelines in oncology: antiemesis—version 1.2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#antiemesis. Accessed March 1, 2017.
3. Platinol (cisplatin for injection, USP) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018057s079lbl.pdf. Updated May 2010. Accessed September 12, 2016.
4. Pollera CF, Giannarelli D. Prognostic factors influencing cisplatin-induced emesis. Definition and validation of a predictive logistic model. Cancer. 1989;64:1117-1122.
5. Roila F, Boschetti E, Tonato M, et al. Predictive factors of delayed emesis in cisplatin-treated patients and antiemetic activity and tolerability of metoclopramide or dexamethasone. A randomized single-blind study. Am J Clin Oncol. 1991;14:238-242.
6. Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119-v133.
7. Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189-4198.
8. Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol. 2014;25:1340-1346.
9. Akynzeo (netupitant and palonosetron capsules) [prescribing information]. Woodcliff, NJ: Eisai; 2015. http://www.akynzeo.com. Revised April 2015. Accessed September 12, 2016.
10. Tsukahara K, Nakamura K, Motohashi R, et al. Antiemetic therapy of fosaprepitant, palonosetron, and dexamethasone combined with cisplatin-based chemotherapy for head and neck carcinomas. Acta Otolaryngol. 2014;134:1198-1204.
11. Ottoboni. Biochronomer technology and the development of APF530, a sustained release formulation of granisetron. J Exp Pharmacol. 2014;6:15-21.
12. Gabrail N, Yanagihara R, Spaczynski M, et al. Pharmacokinetics, safety, and efficacy of APF530 (extended-release granisetron) in patients receiving moderately or highly emetogenic chemotherapy: results of two phase II trials. Cancer Manag Res. 2015;7:83-92.
13. Sustol (granisetron) extended-release injection, for subcutaneous use [prescribing information]. Redwood City, CA; Heron Therapeutics; 2016. http://sustol.com/hcp/healthcare-professionals. Updated August 2016. Accessed September 12, 2016.
14. Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997;15:103-109.
15. Raftopoulos H, Cooper W, O’Boyle E, Gabrail N, Boccia R, Gralla RJ. Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double-blind, noninferiority phase 3 trial. Support Care Cancer. 2015;23:723-732.
16. Boccia RV, Cooper W, O’Boyle E. Sustained antiemetic responses with APF530 (sustained-release granisetron) during multiple cycles of emetogenic chemotherapy. J Community Support Oncol. 2015;13:38-46.
17. Raftopoulos H, Boccia R, Cooper W, O’Boyle E, Gralla RJ. Slow-release granisetron (APF530) versus palonosetron for chemotherapy-induced nausea/vomiting: analysis by American Society of Clinical Oncology emetogenicity criteria. Future Oncol. 2015;11:2541-2551.
18. Schnadig ID, Agajanian R, Dakhil C, et al. APF530 (Granisetron injection extended-release) in a three-drug regimen for delayed CINV in highly emetogenic chemotherapy. Future Oncol. 2016;12:1469-1481.
19. Zofran (ondansetron hydrochloride) injection for intravenous use. Research Triangle Park, NC: GlaxoSmithKline; 2014. http://www.pharma.us.novartis.com/product/pi/pdf/zofran_inj.pdf. Revised September 2014. Accessed September 12, 2016.
20. Eligard (luprolide acetate) kit for subcutaneous use [prescribing information]. Fort Collins, CO: Tolmar Pharmaceuticals Inc; 2017. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b78d1919-9dee-44fa-90f9-e0a26d32481d. Revised January 2017. Accessed March 1, 2017.
21. Sandostatin LAR Depot (octreotide acetate for injectable suspension) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016. https://www.pharma.us.novartis.com/product/pi/pdf/sandostatin_lar.pdf. Revised July 2016. Accessed September 12, 2016.
22. Navari RM. Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs. 2013;73:249-262.
23. Rapoport BL, Chasen MR, Gridelli C, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol. 2015;16:1079-1089.
24. Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375:134-142.
Despite available antiemetic therapies, chemotherapy-induced nausea and vomiting (CINV) following highly emetogenic chemotherapy (HEC), particularly in the delayed phase (>24-120 h after chemotherapy), continues to impair patient quality of life and chemotherapy compliance.1 Cisplatin-based chemotherapy, classified as HEC at any dose,2 is widely used to treat cancers such as non–small-cell and small
5-hydroxytryptamine type 3 (5-HT3) receptor antagonists (RAs; eg, granisetron, ondansetron, dolasetron, and palonosetron) have been the cornerstone of CINV therapy for decades and remain an integral part of contemporary antiemetic treatment regimens. Most current antiemetic guidelines for HEC recommend a 3-drug regimen, comprising a 5-HT3 RA, a neurokinin 1 (NK-1) RA, and a corticosteroid (dexamethasone).2,6,7 A regimen of olanzapine (antipsychotic), palonosetron (5-HT3 RA), and dexamethasone (corticosteroid) has been recommended as an alternative option. Recently, the oral fixed-dose combination of netupitant and palonosetron (NEPA) was approved and has shown efficacy in the cisplatin setting.8,9 However, the administration of oral medication to patients experiencing CINV and those with head and neck cancer may be difficult.10 Alternative antiemetic treatments that provide CINV control into the delayed phase and with a convenient route of administration, are needed.
APF530 is a novel extended-release granisetron formulation that provides sustained release of therapeutic concentrations for ≥5 days. The Biochronomer tri(ethylene glycol) poly(orthoester) (TEG-POE) vehicle releases granisetron slowly by polymer hydrolysis after it has been injected subcutaneously (SC) into the abdomen or upper arm.11,12 In 2016, the US Food and Drug Administration approved APF530 in combination with other antiemetics for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic chemotherapy (MEC) or anthracycline plus cyclophosphamide (AC) combination chemotherapy regimens based on data from 2 pivotal phase 3 trials.13
A phase 3 trial demonstrated noninferiority of APF530 (500 mg, SC) to palonosetron (0.25 mg, intravenously [IV]), each with dexamethasone (corticosteroid), in the control of acute-phase CINV after MEC or HEC, and delayed-phase CINV after MEC (classified by Hesketh criteria).14,15 Furthermore, APF530 provided sustained CINV control over multiple cycles of chemotherapy.16 Numerically higher complete response (CR: no emesis, no rescue medication use) rates were observed with APF530, compared with palonosetron, in the delayed phase after HEC (APF530 500 mg, 67.1%; palonosetron 0.25 mg, 64.3%).15
A reanalysis of study endpoints by newer emetogenicity classification guidelines from the American Society of Clinical Oncology (ASCO)7 maintained overall study conclusions.17 Notably, the numerically higher CR rates with APF530 in the delayed phase following HEC were enhanced (APF530 500 mg, 55.8%; palonosetron 0.25 mg, 50.5%), suggesting a need for further examination in this setting. The subsequent APF530 phase 3 MAGIC trial (Modified Absorption of Granisetron In the prevention of CINV; NCT02106494), compared APF530 (500 mg, SC) with ondansetron (0.15 mg/kg, IV), each with fosaprepitant (NK-1 RA) and dexamethasone in patients receiving HEC. The primary endpoint was met: the APF530 regimen demonstrated superior delayed-phase CR compared with the ondansetron regimen (64.7% vs 56.6%; 95% confidence interval [CI]:
1.7-14.4; P = .014; 8.0% absolute improvement).18
APF530 also demonstrated a significant benefit over ondansetron for other endpoints including nausea control, rescue medication use, and satisfaction with antiemetic therapy.18 APF530 is the first and only 5-HT3 RA to demonstrate superiority over another in a phase 3 efficacy trial using a guideline-recommended 3-drug regimen for both arms.
A prespecified MAGIC trial analysis of the primary endpoint by intent to receive cisplatin (≥50 mg/m2, Yes/No) demonstrated a pronounced treatment benefit in terms of delayed-phase CR rates with the APF530 regimen among patients in the cisplatin (≥50 mg/m2, Yes) stratum (CR: 65.3% vs 54.7%; 95% CI: -1.4-22.7; 10.6% absolute improvement).18 These results are compelling, since cisplatin represents a particularly emetogenic class of chemotherapy; a more in-depth analysis of additional MAGIC trial endpoints for these patients would be of clinical interest, and is presented here. Efficacy endpoints in this analysis include CR in the overall and acute phases, complete control (CC) and total response (TR) rates, rescue medication use, nausea frequency, and safety.
Methods
Study design and patients
The MAGIC trial was a prospective, randomized, multicenter, placebo-controlled, double-blind, double-dummy phase 3 trial conducted at 77 centers across the United States. The study protocol was reviewed and approved by the institutional review board at each participating center, and conducted according to the Declaration of Helsinki. The study design, previously presented in detail,18 is reviewed briefly here.
Eligible men and women were 18-80 years of age with histologically or cytologically confirmed malignancy (cancer type information was not captured) and were entering the first cycle of their single-day HEC treatment (defined by ASCO 2011 emetogenicity criteria).7 Patients had Eastern Cooperative Oncology Group Performance Status (ECOG-PS) of 0 or 1, no history or presence of significant cardiac disease or QT interval prolongation, and adequate bone marrow, kidney, and liver function. All patients provided written informed consent.
Procedures
Patients were stratified by planned receipt of the cisplatin regimen ≥50 mg/m2 (Yes/No), randomized 1:1 to receive APF530 500 mg SC (granisetron 10 mg) or ondansetron 0.15 mg/kg IV (up to a maximum of 16 mg as a single dose) on day 1 (Figure 1). The APF530 arm received the ondansetron saline placebo, and the ondansetron arm received the APF530 SC placebo containing the TEG-POE vehicle. All patients were scheduled to receive fosaprepitant 150 mg IV and dexamethasone 12 mg IV on day 1, then oral dexamethasone 8 mg once daily on day 2 and 8 mg twice daily on days 3 and 4. Rescue medication was allowed at the investigator’s discretion.
Outcomes
The primary objective of the trial was to demonstrate the superiority of APF530 500 mg SC compared with ondansetron 0.15 mg/kg IV, as part of the current guideline-recommended 3-drug regimen, in preventing delayed-phase CINV after HEC. The primary endpoint was delayed-phase (24-120 h) CR (no emetic episodes [vomit or retch] and no rescue medication use). In addition, a prespecified analysis of delayed-phase CR by randomization strata (planned use of cisplatin) was performed.
Secondary and other endpoints included overall-phase CR
(0-120 h); delayed-, overall-, and acute-phase complete control (CC: CR and no more than mild nausea); delayed-, overall-, and acute-phase total response (TR; CR and no nausea); and rescue medication use. A post hoc analysis of nausea severity was also conducted. Safety assessments included treatment-emergent adverse events (TEAEs), injection-site reactions (ISRs), laboratory parameters, and vital signs. TEAEs were assessed by type, duration, severity, and relationship to study drug. ISR timing and severity were captured in patient diaries.
Statistical analysis
All efficacy analyses were conducted using the modified intent-to-treat population (mITT; randomized patients who received study drug and a HEC regimen and had post-baseline efficacy data). Safety assessments were performed on the safety population (randomized patients who received study drug).
This analysis conducted on the subgroup of patients with intent to receive cisplatin (cisplatin randomization stratum, ≥50 mg/m2, Yes) was exploratory and was not powered to detect treatment differences. Preplanned analyses compared CR, CC, and TR rates across treatment arms using 95% CIs.
Post hoc analyses of time to first rescue medication use, proportion of patients with rescue medication use, and less frequent nausea were performed. All P values were calculated using the Cochran-Mantel-Haenszel chi square test. Rescue medication use results were based on observed data, without imputation for missing results (ie, calculated from the number of patients with a response). Further analyses of efficacy endpoints CR, CC, and TR in the subset of female patients in the cisplatin randomization stratum were performed. Safety assessments were summarized descriptively.
Results
A total of 942 patients were randomized across 77 US centers during March 31, 2014 and May 15, 2015 (471 APF530, 471 ondansetron). Among those, 264 had intent to receive cisplatin and were included in the cisplatin randomization stratum (≥50 mg/m2, Yes) (Figure 2). A total of 256 patients in the cisplatin stratum received study drug and were included in the safety population (126 APF530, 130 ondansetron); 252 patients were included in the mITT population (124 APF530, 128 ondansetron).
Baseline demographics were generally balanced between treatment arms (Table 1). The proportion of female patients was 41.1% (51/124) and 48.4% (62/128) in the APF530 and ondansetron arms, respectively. The majority of patients had an ECOG PS of 0 (57.3% [71/124] APF530; 60.2% [77/128] ondansetron). The most common cisplatin-based chemotherapy regimen in both treatment arms was cisplatin and gemcitabine (25.0% [31/124] APF530; 28.9% [37/128] ondansetron) (Suppl Table 1). Two patients in the APF530 arm and 3 patients in the ondansetron arm either received a lower cisplatin dose (<50 mg/m2) or did not go on to receive cisplatin as intended at randomization (Suppl Table 1). As previously reported, in the cisplatin stratum (Table 2), delayed-phase CR was numerically higher in the APF530 arm versus the ondansetron arm, with a corresponding treatment difference of 10.6% (65.3% [81/124] APF530; 54.7% [70/128] ondansetron; 95% CI [-1.4, 22.7]; P = .085). Although the CI contains 0, the result is consistent with the significant benefit observed in the overall study population (64.7% [291/450] APF530; 56.6% [256/452] ondansetron; 95% CI [1.7, 14.4];
P = .014).18 This more in-depth analysis found similar trends favoring the APF530 over the ondansetron regimen across overall- and acute-phase CR (Table 2).
A significantly greater proportion of patients in the APF530 arm, compared with the ondansetron arm, reported no rescue medication use during the delayed phase (74.4% [90/121] APF530; 62.6% [77/123] ondansetron; P = .048). Trends in favor of APF530 were observed in the overall phase (71.1% [86/121] APF530; 61.8% [76/123] ondansetron; P = .125) and acute phase (86.9% [106/122] APF530; 81.9% [104/127] ondansetron; P = .278). Time to first rescue medication use was consistently longer in the APF530 arm, compared with the ondansetron arm, although not statistically significantly (P = .150) (Figure 3).
The APF530 regimen was generally well tolerated in the cisplatin subgroup, and no new safety signals were identified (Table 3). Most patients experienced at least one TEAE. Excluding ISRs, TEAE incidences were 72.2% and 66.9% in the APF530 and ondansetron arms, respectively; most common were constipation, fatigue, nausea, diarrhea, dehydration, and headache. Excluding ISRs, the most common treatment-related TEAEs in the APF530 and ondansetron arms were constipation (2.4% and 2.3%, respectively and headache (3.2% and 4.6%).
Discussion
The MAGIC trial is the first phase 3 efficacy trial in the prevention of CINV in patients receiving HEC using the current guideline-recommended 3-drug antiemetic regimen in both treatment arms.18 Ondansetron was chosen as the appropriate 5-HT3 RA comparator because no other 5-HT3 RA has shown superiority to ondansetron in delayed-phase CINV following HEC. Furthermore, ondansetron is indicated for prevention of nausea and vomiting associated with initial and repeat courses of chemotherapy, including high-dose cisplatin.19 The MAGIC trial primary endpoint was met for the overall study population; in the context of a 3-drug regimen, APF530 demonstrated superior control of delayed-phase CINV following HEC compared with standard-of-care ondansetron.18 As reported previously, significant benefits were also observed with the APF530 regimen over the ondansetron regimen in terms of rescue medication use, patient satisfaction with antiemetic therapy, and nausea frequency in the overall study population.18
Cisplatin is generally regarded as one of the most emetogenic chemotherapeutic agents. For this reason, cisplatin is often evaluated separately in clinical trials, and was a stratification factor in the MAGIC trial. Consistent with the previously reported significant results,18 trends in the cisplatin stratum analysis favored the APF530 regimen, compared with the ondansetron regimen, in delayed- and overall-phase CR (treatment difference: 10.6%).18 Numerical trends presented here favoring the APF530 regimen over the ondansetron regimen were observed in CC and TR, two more stringent measures of CINV control that account for incidence of nausea. Furthermore, among women in the cisplatin stratum, a population at increased risk for CINV, the numerically higher CR, CC, and TR persisted in the APF530 arm, compared with the ondansetron arm.
The APF530 regimen was generally well tolerated in the cisplatin stratum, and no new safety signals were identified. The most common TEAEs were ISRs, mostly mild or moderate and resolving by study end. The double-dummy design resulted in ISRs in the ondansetron arm due to TEG-POE vehicle as the dummy APF530 injection. Transient ISRs have been observed with other agents administered SC, and are expected.20,21 Excluding ISRs, TEAEs were generally consistent with those observed for the 5-HT3 RA class.22
This analysis of patients randomized to receive cisplatin-based HEC in the MAGIC trial is exploratory and was not sufficiently powered to detect between-arm differences. Five total patients did not go on to receive cisplatin ≥50 mg/m2 as intended at randomization (2 APF530, 3 ondansetron); however, this is not uncommon in large clinical trials and represents less than 2% of patients in this analysis.
Recent phase 3 studies in patients receiving cisplatin-based HEC showed significant improvement in CINV prevention with the current guideline-recommended 3-drug regimen over the traditional 2-drug regimen (5-HT3 RA + dexamethasone).8,23 Results presented here, in a similar population receiving cisplatin-based HEC, suggest that in the context of a 3-drug antiemetic regimen in both treatment arms, APF530 provides additional benefit in CINV prevention compared with the standard of care, ondansetron. Furthermore, a recent phase 3 trial in patients receiving cisplatin or AC-based HEC demonstrated significant improvement in nausea when olanzapine was added to a traditional 3-drug regimen of a 5-HT3 RA, NK-1 RA, and dexamethasone.24 These compelling data support the addition of olanzapine as a fourth agent to the CINV treatment regimen to provide further control of nausea, which has been one of the more difficult components of CINV to control to date.
APF530 is the only 5-HT3 RA to demonstrate superiority over another as part of the guideline-recommended regimen in a 3-drug versus 3-drug phase 3 efficacy trial examining antiemetic efficacy following HEC. Results from the MAGIC trial, this exploratory analysis, and previous studies in MEC and HEC provide clinically meaningful benefits in preventing both acute- and delayed-phase CINV following guideline-specified MEC or HEC regimens. Consequently, APF530 was approved for use in combination with other antiemetics for prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of MEC or AC combination chemotherapy regimens.13 Both the superior control of delayed-phase CINV following HEC demonstrated by the MAGIC trial18 and the consistent trends in the cisplatin stratum indicate a particular benefit for high-risk patients receiving high doses of cisplatin.
Acknowledgments
Joanna K Sandilos Rega, PhD, of SciStrategy Communications provided medical writing assistance, supported by Heron Therapeutics Inc, the maker of the study drug.
Despite available antiemetic therapies, chemotherapy-induced nausea and vomiting (CINV) following highly emetogenic chemotherapy (HEC), particularly in the delayed phase (>24-120 h after chemotherapy), continues to impair patient quality of life and chemotherapy compliance.1 Cisplatin-based chemotherapy, classified as HEC at any dose,2 is widely used to treat cancers such as non–small-cell and small
5-hydroxytryptamine type 3 (5-HT3) receptor antagonists (RAs; eg, granisetron, ondansetron, dolasetron, and palonosetron) have been the cornerstone of CINV therapy for decades and remain an integral part of contemporary antiemetic treatment regimens. Most current antiemetic guidelines for HEC recommend a 3-drug regimen, comprising a 5-HT3 RA, a neurokinin 1 (NK-1) RA, and a corticosteroid (dexamethasone).2,6,7 A regimen of olanzapine (antipsychotic), palonosetron (5-HT3 RA), and dexamethasone (corticosteroid) has been recommended as an alternative option. Recently, the oral fixed-dose combination of netupitant and palonosetron (NEPA) was approved and has shown efficacy in the cisplatin setting.8,9 However, the administration of oral medication to patients experiencing CINV and those with head and neck cancer may be difficult.10 Alternative antiemetic treatments that provide CINV control into the delayed phase and with a convenient route of administration, are needed.
APF530 is a novel extended-release granisetron formulation that provides sustained release of therapeutic concentrations for ≥5 days. The Biochronomer tri(ethylene glycol) poly(orthoester) (TEG-POE) vehicle releases granisetron slowly by polymer hydrolysis after it has been injected subcutaneously (SC) into the abdomen or upper arm.11,12 In 2016, the US Food and Drug Administration approved APF530 in combination with other antiemetics for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic chemotherapy (MEC) or anthracycline plus cyclophosphamide (AC) combination chemotherapy regimens based on data from 2 pivotal phase 3 trials.13
A phase 3 trial demonstrated noninferiority of APF530 (500 mg, SC) to palonosetron (0.25 mg, intravenously [IV]), each with dexamethasone (corticosteroid), in the control of acute-phase CINV after MEC or HEC, and delayed-phase CINV after MEC (classified by Hesketh criteria).14,15 Furthermore, APF530 provided sustained CINV control over multiple cycles of chemotherapy.16 Numerically higher complete response (CR: no emesis, no rescue medication use) rates were observed with APF530, compared with palonosetron, in the delayed phase after HEC (APF530 500 mg, 67.1%; palonosetron 0.25 mg, 64.3%).15
A reanalysis of study endpoints by newer emetogenicity classification guidelines from the American Society of Clinical Oncology (ASCO)7 maintained overall study conclusions.17 Notably, the numerically higher CR rates with APF530 in the delayed phase following HEC were enhanced (APF530 500 mg, 55.8%; palonosetron 0.25 mg, 50.5%), suggesting a need for further examination in this setting. The subsequent APF530 phase 3 MAGIC trial (Modified Absorption of Granisetron In the prevention of CINV; NCT02106494), compared APF530 (500 mg, SC) with ondansetron (0.15 mg/kg, IV), each with fosaprepitant (NK-1 RA) and dexamethasone in patients receiving HEC. The primary endpoint was met: the APF530 regimen demonstrated superior delayed-phase CR compared with the ondansetron regimen (64.7% vs 56.6%; 95% confidence interval [CI]:
1.7-14.4; P = .014; 8.0% absolute improvement).18
APF530 also demonstrated a significant benefit over ondansetron for other endpoints including nausea control, rescue medication use, and satisfaction with antiemetic therapy.18 APF530 is the first and only 5-HT3 RA to demonstrate superiority over another in a phase 3 efficacy trial using a guideline-recommended 3-drug regimen for both arms.
A prespecified MAGIC trial analysis of the primary endpoint by intent to receive cisplatin (≥50 mg/m2, Yes/No) demonstrated a pronounced treatment benefit in terms of delayed-phase CR rates with the APF530 regimen among patients in the cisplatin (≥50 mg/m2, Yes) stratum (CR: 65.3% vs 54.7%; 95% CI: -1.4-22.7; 10.6% absolute improvement).18 These results are compelling, since cisplatin represents a particularly emetogenic class of chemotherapy; a more in-depth analysis of additional MAGIC trial endpoints for these patients would be of clinical interest, and is presented here. Efficacy endpoints in this analysis include CR in the overall and acute phases, complete control (CC) and total response (TR) rates, rescue medication use, nausea frequency, and safety.
Methods
Study design and patients
The MAGIC trial was a prospective, randomized, multicenter, placebo-controlled, double-blind, double-dummy phase 3 trial conducted at 77 centers across the United States. The study protocol was reviewed and approved by the institutional review board at each participating center, and conducted according to the Declaration of Helsinki. The study design, previously presented in detail,18 is reviewed briefly here.
Eligible men and women were 18-80 years of age with histologically or cytologically confirmed malignancy (cancer type information was not captured) and were entering the first cycle of their single-day HEC treatment (defined by ASCO 2011 emetogenicity criteria).7 Patients had Eastern Cooperative Oncology Group Performance Status (ECOG-PS) of 0 or 1, no history or presence of significant cardiac disease or QT interval prolongation, and adequate bone marrow, kidney, and liver function. All patients provided written informed consent.
Procedures
Patients were stratified by planned receipt of the cisplatin regimen ≥50 mg/m2 (Yes/No), randomized 1:1 to receive APF530 500 mg SC (granisetron 10 mg) or ondansetron 0.15 mg/kg IV (up to a maximum of 16 mg as a single dose) on day 1 (Figure 1). The APF530 arm received the ondansetron saline placebo, and the ondansetron arm received the APF530 SC placebo containing the TEG-POE vehicle. All patients were scheduled to receive fosaprepitant 150 mg IV and dexamethasone 12 mg IV on day 1, then oral dexamethasone 8 mg once daily on day 2 and 8 mg twice daily on days 3 and 4. Rescue medication was allowed at the investigator’s discretion.
Outcomes
The primary objective of the trial was to demonstrate the superiority of APF530 500 mg SC compared with ondansetron 0.15 mg/kg IV, as part of the current guideline-recommended 3-drug regimen, in preventing delayed-phase CINV after HEC. The primary endpoint was delayed-phase (24-120 h) CR (no emetic episodes [vomit or retch] and no rescue medication use). In addition, a prespecified analysis of delayed-phase CR by randomization strata (planned use of cisplatin) was performed.
Secondary and other endpoints included overall-phase CR
(0-120 h); delayed-, overall-, and acute-phase complete control (CC: CR and no more than mild nausea); delayed-, overall-, and acute-phase total response (TR; CR and no nausea); and rescue medication use. A post hoc analysis of nausea severity was also conducted. Safety assessments included treatment-emergent adverse events (TEAEs), injection-site reactions (ISRs), laboratory parameters, and vital signs. TEAEs were assessed by type, duration, severity, and relationship to study drug. ISR timing and severity were captured in patient diaries.
Statistical analysis
All efficacy analyses were conducted using the modified intent-to-treat population (mITT; randomized patients who received study drug and a HEC regimen and had post-baseline efficacy data). Safety assessments were performed on the safety population (randomized patients who received study drug).
This analysis conducted on the subgroup of patients with intent to receive cisplatin (cisplatin randomization stratum, ≥50 mg/m2, Yes) was exploratory and was not powered to detect treatment differences. Preplanned analyses compared CR, CC, and TR rates across treatment arms using 95% CIs.
Post hoc analyses of time to first rescue medication use, proportion of patients with rescue medication use, and less frequent nausea were performed. All P values were calculated using the Cochran-Mantel-Haenszel chi square test. Rescue medication use results were based on observed data, without imputation for missing results (ie, calculated from the number of patients with a response). Further analyses of efficacy endpoints CR, CC, and TR in the subset of female patients in the cisplatin randomization stratum were performed. Safety assessments were summarized descriptively.
Results
A total of 942 patients were randomized across 77 US centers during March 31, 2014 and May 15, 2015 (471 APF530, 471 ondansetron). Among those, 264 had intent to receive cisplatin and were included in the cisplatin randomization stratum (≥50 mg/m2, Yes) (Figure 2). A total of 256 patients in the cisplatin stratum received study drug and were included in the safety population (126 APF530, 130 ondansetron); 252 patients were included in the mITT population (124 APF530, 128 ondansetron).
Baseline demographics were generally balanced between treatment arms (Table 1). The proportion of female patients was 41.1% (51/124) and 48.4% (62/128) in the APF530 and ondansetron arms, respectively. The majority of patients had an ECOG PS of 0 (57.3% [71/124] APF530; 60.2% [77/128] ondansetron). The most common cisplatin-based chemotherapy regimen in both treatment arms was cisplatin and gemcitabine (25.0% [31/124] APF530; 28.9% [37/128] ondansetron) (Suppl Table 1). Two patients in the APF530 arm and 3 patients in the ondansetron arm either received a lower cisplatin dose (<50 mg/m2) or did not go on to receive cisplatin as intended at randomization (Suppl Table 1). As previously reported, in the cisplatin stratum (Table 2), delayed-phase CR was numerically higher in the APF530 arm versus the ondansetron arm, with a corresponding treatment difference of 10.6% (65.3% [81/124] APF530; 54.7% [70/128] ondansetron; 95% CI [-1.4, 22.7]; P = .085). Although the CI contains 0, the result is consistent with the significant benefit observed in the overall study population (64.7% [291/450] APF530; 56.6% [256/452] ondansetron; 95% CI [1.7, 14.4];
P = .014).18 This more in-depth analysis found similar trends favoring the APF530 over the ondansetron regimen across overall- and acute-phase CR (Table 2).
A significantly greater proportion of patients in the APF530 arm, compared with the ondansetron arm, reported no rescue medication use during the delayed phase (74.4% [90/121] APF530; 62.6% [77/123] ondansetron; P = .048). Trends in favor of APF530 were observed in the overall phase (71.1% [86/121] APF530; 61.8% [76/123] ondansetron; P = .125) and acute phase (86.9% [106/122] APF530; 81.9% [104/127] ondansetron; P = .278). Time to first rescue medication use was consistently longer in the APF530 arm, compared with the ondansetron arm, although not statistically significantly (P = .150) (Figure 3).
The APF530 regimen was generally well tolerated in the cisplatin subgroup, and no new safety signals were identified (Table 3). Most patients experienced at least one TEAE. Excluding ISRs, TEAE incidences were 72.2% and 66.9% in the APF530 and ondansetron arms, respectively; most common were constipation, fatigue, nausea, diarrhea, dehydration, and headache. Excluding ISRs, the most common treatment-related TEAEs in the APF530 and ondansetron arms were constipation (2.4% and 2.3%, respectively and headache (3.2% and 4.6%).
Discussion
The MAGIC trial is the first phase 3 efficacy trial in the prevention of CINV in patients receiving HEC using the current guideline-recommended 3-drug antiemetic regimen in both treatment arms.18 Ondansetron was chosen as the appropriate 5-HT3 RA comparator because no other 5-HT3 RA has shown superiority to ondansetron in delayed-phase CINV following HEC. Furthermore, ondansetron is indicated for prevention of nausea and vomiting associated with initial and repeat courses of chemotherapy, including high-dose cisplatin.19 The MAGIC trial primary endpoint was met for the overall study population; in the context of a 3-drug regimen, APF530 demonstrated superior control of delayed-phase CINV following HEC compared with standard-of-care ondansetron.18 As reported previously, significant benefits were also observed with the APF530 regimen over the ondansetron regimen in terms of rescue medication use, patient satisfaction with antiemetic therapy, and nausea frequency in the overall study population.18
Cisplatin is generally regarded as one of the most emetogenic chemotherapeutic agents. For this reason, cisplatin is often evaluated separately in clinical trials, and was a stratification factor in the MAGIC trial. Consistent with the previously reported significant results,18 trends in the cisplatin stratum analysis favored the APF530 regimen, compared with the ondansetron regimen, in delayed- and overall-phase CR (treatment difference: 10.6%).18 Numerical trends presented here favoring the APF530 regimen over the ondansetron regimen were observed in CC and TR, two more stringent measures of CINV control that account for incidence of nausea. Furthermore, among women in the cisplatin stratum, a population at increased risk for CINV, the numerically higher CR, CC, and TR persisted in the APF530 arm, compared with the ondansetron arm.
The APF530 regimen was generally well tolerated in the cisplatin stratum, and no new safety signals were identified. The most common TEAEs were ISRs, mostly mild or moderate and resolving by study end. The double-dummy design resulted in ISRs in the ondansetron arm due to TEG-POE vehicle as the dummy APF530 injection. Transient ISRs have been observed with other agents administered SC, and are expected.20,21 Excluding ISRs, TEAEs were generally consistent with those observed for the 5-HT3 RA class.22
This analysis of patients randomized to receive cisplatin-based HEC in the MAGIC trial is exploratory and was not sufficiently powered to detect between-arm differences. Five total patients did not go on to receive cisplatin ≥50 mg/m2 as intended at randomization (2 APF530, 3 ondansetron); however, this is not uncommon in large clinical trials and represents less than 2% of patients in this analysis.
Recent phase 3 studies in patients receiving cisplatin-based HEC showed significant improvement in CINV prevention with the current guideline-recommended 3-drug regimen over the traditional 2-drug regimen (5-HT3 RA + dexamethasone).8,23 Results presented here, in a similar population receiving cisplatin-based HEC, suggest that in the context of a 3-drug antiemetic regimen in both treatment arms, APF530 provides additional benefit in CINV prevention compared with the standard of care, ondansetron. Furthermore, a recent phase 3 trial in patients receiving cisplatin or AC-based HEC demonstrated significant improvement in nausea when olanzapine was added to a traditional 3-drug regimen of a 5-HT3 RA, NK-1 RA, and dexamethasone.24 These compelling data support the addition of olanzapine as a fourth agent to the CINV treatment regimen to provide further control of nausea, which has been one of the more difficult components of CINV to control to date.
APF530 is the only 5-HT3 RA to demonstrate superiority over another as part of the guideline-recommended regimen in a 3-drug versus 3-drug phase 3 efficacy trial examining antiemetic efficacy following HEC. Results from the MAGIC trial, this exploratory analysis, and previous studies in MEC and HEC provide clinically meaningful benefits in preventing both acute- and delayed-phase CINV following guideline-specified MEC or HEC regimens. Consequently, APF530 was approved for use in combination with other antiemetics for prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of MEC or AC combination chemotherapy regimens.13 Both the superior control of delayed-phase CINV following HEC demonstrated by the MAGIC trial18 and the consistent trends in the cisplatin stratum indicate a particular benefit for high-risk patients receiving high doses of cisplatin.
Acknowledgments
Joanna K Sandilos Rega, PhD, of SciStrategy Communications provided medical writing assistance, supported by Heron Therapeutics Inc, the maker of the study drug.
1. Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK. Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer. 2012;20:107-117.
2. NCCN clinical practice guidelines in oncology: antiemesis—version 1.2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#antiemesis. Accessed March 1, 2017.
3. Platinol (cisplatin for injection, USP) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018057s079lbl.pdf. Updated May 2010. Accessed September 12, 2016.
4. Pollera CF, Giannarelli D. Prognostic factors influencing cisplatin-induced emesis. Definition and validation of a predictive logistic model. Cancer. 1989;64:1117-1122.
5. Roila F, Boschetti E, Tonato M, et al. Predictive factors of delayed emesis in cisplatin-treated patients and antiemetic activity and tolerability of metoclopramide or dexamethasone. A randomized single-blind study. Am J Clin Oncol. 1991;14:238-242.
6. Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119-v133.
7. Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189-4198.
8. Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol. 2014;25:1340-1346.
9. Akynzeo (netupitant and palonosetron capsules) [prescribing information]. Woodcliff, NJ: Eisai; 2015. http://www.akynzeo.com. Revised April 2015. Accessed September 12, 2016.
10. Tsukahara K, Nakamura K, Motohashi R, et al. Antiemetic therapy of fosaprepitant, palonosetron, and dexamethasone combined with cisplatin-based chemotherapy for head and neck carcinomas. Acta Otolaryngol. 2014;134:1198-1204.
11. Ottoboni. Biochronomer technology and the development of APF530, a sustained release formulation of granisetron. J Exp Pharmacol. 2014;6:15-21.
12. Gabrail N, Yanagihara R, Spaczynski M, et al. Pharmacokinetics, safety, and efficacy of APF530 (extended-release granisetron) in patients receiving moderately or highly emetogenic chemotherapy: results of two phase II trials. Cancer Manag Res. 2015;7:83-92.
13. Sustol (granisetron) extended-release injection, for subcutaneous use [prescribing information]. Redwood City, CA; Heron Therapeutics; 2016. http://sustol.com/hcp/healthcare-professionals. Updated August 2016. Accessed September 12, 2016.
14. Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997;15:103-109.
15. Raftopoulos H, Cooper W, O’Boyle E, Gabrail N, Boccia R, Gralla RJ. Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double-blind, noninferiority phase 3 trial. Support Care Cancer. 2015;23:723-732.
16. Boccia RV, Cooper W, O’Boyle E. Sustained antiemetic responses with APF530 (sustained-release granisetron) during multiple cycles of emetogenic chemotherapy. J Community Support Oncol. 2015;13:38-46.
17. Raftopoulos H, Boccia R, Cooper W, O’Boyle E, Gralla RJ. Slow-release granisetron (APF530) versus palonosetron for chemotherapy-induced nausea/vomiting: analysis by American Society of Clinical Oncology emetogenicity criteria. Future Oncol. 2015;11:2541-2551.
18. Schnadig ID, Agajanian R, Dakhil C, et al. APF530 (Granisetron injection extended-release) in a three-drug regimen for delayed CINV in highly emetogenic chemotherapy. Future Oncol. 2016;12:1469-1481.
19. Zofran (ondansetron hydrochloride) injection for intravenous use. Research Triangle Park, NC: GlaxoSmithKline; 2014. http://www.pharma.us.novartis.com/product/pi/pdf/zofran_inj.pdf. Revised September 2014. Accessed September 12, 2016.
20. Eligard (luprolide acetate) kit for subcutaneous use [prescribing information]. Fort Collins, CO: Tolmar Pharmaceuticals Inc; 2017. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b78d1919-9dee-44fa-90f9-e0a26d32481d. Revised January 2017. Accessed March 1, 2017.
21. Sandostatin LAR Depot (octreotide acetate for injectable suspension) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016. https://www.pharma.us.novartis.com/product/pi/pdf/sandostatin_lar.pdf. Revised July 2016. Accessed September 12, 2016.
22. Navari RM. Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs. 2013;73:249-262.
23. Rapoport BL, Chasen MR, Gridelli C, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol. 2015;16:1079-1089.
24. Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375:134-142.
1. Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK. Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer. 2012;20:107-117.
2. NCCN clinical practice guidelines in oncology: antiemesis—version 1.2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#antiemesis. Accessed March 1, 2017.
3. Platinol (cisplatin for injection, USP) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018057s079lbl.pdf. Updated May 2010. Accessed September 12, 2016.
4. Pollera CF, Giannarelli D. Prognostic factors influencing cisplatin-induced emesis. Definition and validation of a predictive logistic model. Cancer. 1989;64:1117-1122.
5. Roila F, Boschetti E, Tonato M, et al. Predictive factors of delayed emesis in cisplatin-treated patients and antiemetic activity and tolerability of metoclopramide or dexamethasone. A randomized single-blind study. Am J Clin Oncol. 1991;14:238-242.
6. Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119-v133.
7. Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189-4198.
8. Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol. 2014;25:1340-1346.
9. Akynzeo (netupitant and palonosetron capsules) [prescribing information]. Woodcliff, NJ: Eisai; 2015. http://www.akynzeo.com. Revised April 2015. Accessed September 12, 2016.
10. Tsukahara K, Nakamura K, Motohashi R, et al. Antiemetic therapy of fosaprepitant, palonosetron, and dexamethasone combined with cisplatin-based chemotherapy for head and neck carcinomas. Acta Otolaryngol. 2014;134:1198-1204.
11. Ottoboni. Biochronomer technology and the development of APF530, a sustained release formulation of granisetron. J Exp Pharmacol. 2014;6:15-21.
12. Gabrail N, Yanagihara R, Spaczynski M, et al. Pharmacokinetics, safety, and efficacy of APF530 (extended-release granisetron) in patients receiving moderately or highly emetogenic chemotherapy: results of two phase II trials. Cancer Manag Res. 2015;7:83-92.
13. Sustol (granisetron) extended-release injection, for subcutaneous use [prescribing information]. Redwood City, CA; Heron Therapeutics; 2016. http://sustol.com/hcp/healthcare-professionals. Updated August 2016. Accessed September 12, 2016.
14. Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997;15:103-109.
15. Raftopoulos H, Cooper W, O’Boyle E, Gabrail N, Boccia R, Gralla RJ. Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double-blind, noninferiority phase 3 trial. Support Care Cancer. 2015;23:723-732.
16. Boccia RV, Cooper W, O’Boyle E. Sustained antiemetic responses with APF530 (sustained-release granisetron) during multiple cycles of emetogenic chemotherapy. J Community Support Oncol. 2015;13:38-46.
17. Raftopoulos H, Boccia R, Cooper W, O’Boyle E, Gralla RJ. Slow-release granisetron (APF530) versus palonosetron for chemotherapy-induced nausea/vomiting: analysis by American Society of Clinical Oncology emetogenicity criteria. Future Oncol. 2015;11:2541-2551.
18. Schnadig ID, Agajanian R, Dakhil C, et al. APF530 (Granisetron injection extended-release) in a three-drug regimen for delayed CINV in highly emetogenic chemotherapy. Future Oncol. 2016;12:1469-1481.
19. Zofran (ondansetron hydrochloride) injection for intravenous use. Research Triangle Park, NC: GlaxoSmithKline; 2014. http://www.pharma.us.novartis.com/product/pi/pdf/zofran_inj.pdf. Revised September 2014. Accessed September 12, 2016.
20. Eligard (luprolide acetate) kit for subcutaneous use [prescribing information]. Fort Collins, CO: Tolmar Pharmaceuticals Inc; 2017. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b78d1919-9dee-44fa-90f9-e0a26d32481d. Revised January 2017. Accessed March 1, 2017.
21. Sandostatin LAR Depot (octreotide acetate for injectable suspension) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016. https://www.pharma.us.novartis.com/product/pi/pdf/sandostatin_lar.pdf. Revised July 2016. Accessed September 12, 2016.
22. Navari RM. Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs. 2013;73:249-262.
23. Rapoport BL, Chasen MR, Gridelli C, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol. 2015;16:1079-1089.
24. Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375:134-142.
Patterns of care with regard to whole-brain radiotherapy technique and delivery among academic centers in the United States
Despite the recent advances in systemic therapy, metastatic spread to the brain continues to be the most common neurologic complication of many cancers. The clinical incidence of brain metastases varies with primary cancer diagnosis, with estimates ranging from 1.2%-19.8%.1,2 Metastatic spread to the brain is even more prevalent at autopsy, with evidence of intracranial tumor being found in 26% of patients in some series.3 It is possible that the clinical incidence of metastatic disease to the brain will continue to increase as newer therapeutic agents improve survival and imaging techniques continue to improve.
The management of brain metastases has changed rapidly as technological improvements have made treatment increasingly safe and efficacious. Traditionally, treatment consisted of radiotherapy to the whole brain, with or without surgical resection.4,5 More recently, stereotactic radiosurgery (SRS) has been adopted on the basis of evidence that it is safe and efficacious alone or in combination with radiotherapy to the whole brain.6 Further evidence is emerging that neurocognitive outcomes are improved when whole-brain radiotherapy (WBRT) is omitted, which possibly contributes to improved patient quality of life.7 Taking into account this and other data, the American Society for Radiation Oncology’s Choosing Wisely campaign now recommends not routinely adding WBRT to radiosurgery in patients with limited brain metastases.8
Despite this recommendation, many patients continue to benefit from WBRT, and it remains a common treatment in radiation oncology clinics across the US for several reasons. Many patients present with multiple brain metastases and are ineligible for radiosurgery. Even for technically eligible patients, WBRT has been shown to improve local control and decrease the rate of distant brain failure over radiosurgery alone.6 With higher rates of subsequent failures, patients receiving radiosurgery alone must adhere to more rigorous follow-up and imaging schedules, which can be difficult for many rural patients who have to travel long distances to centers. Furthermore, there is some suggestion that this decreased failure rate may result in improved survival in highly selected patients with excellent disease and performance status.9 Controversies exist, however, and strong institutional biases persist, contributing to significant differences in practice. We surveyed academic radiation oncologists and in an effort to identify and describe practice patterns in the delivery of WBRT at academic centers.
Methods
We conducted a thorough review of available literature on radiation for brain metastases and based on our findings, devised a survey 19 questions to ascertain practice patterns and treatment delivery among US academic physicians (Table 1). After obtaining institutional review board approval to do the study, we sent the survey to program coordinators at radiation oncology programs that are accredited by the Accreditation Council for Graduate Medical Education. We instructed coordinators to e-mail the survey to their practicing resident and attending physicians. The surveys were created using SurveyMonkey software. We obtained informed consent from the providers. A total of 3 follow-up e-mails were sent to each recipient of the survey to solicit responses, similar to the Dillman Total Design Survey Method.10
SPSS version 22.0 was used to analyze the data in an exploratory fashion. Statistical methods were used to assess the association of demographic data with SRS and WBRT delivery and treatment technique items when the analyses involved percentages that included the Pearson chi-square statistic and the chi-square test for linear trend. When the analysis focused on ranking data, the Kruskal-Wallis test, Mann-Whitney U test, the Jonckheere-Terpstra and the Kendall tau-b rank correlation were used as appropriate. If there were small sample sizes within some groups, then exact significant levels were assessed. Statistical significance was set by convention at P < .05.
Results
We received 95 responses of which 87 were considered complete for analysis. Forty-seven percent of the 87 respondents were not board-certified, and the remainder had passed their radiobiology and physics boards exams. A majority of respondents (70%, 61 of 87) were physicians who had been in practice for ≤5 years. Fifty-four percent of respondents were located in the Northeast US, 22% in the South, 14% in the West, and 10% in the Midwest and Hawaii (Table 2).
We used the chi-square test for linear trends to assess for a relationship between years of practice and whether respondents deviated from their typical method of WBRT therapy when treating more radioresistant tumors (melanoma, renal cell carcinoma). Respondents were classified by years in practice: 0-5, 6-10, 11-20, and >21 years. The results showed a linear association, with those in practice for longer periods more likely to use SRS alone, P = .027 (Figure 1).
Discussion
The incidence of brain metastases is increasing because of improvements in diagnostic imaging techniques and advancements in systemic therapy control of extracranial disease but not of intracranial disease or metastasis, because therapies do not cross the blood-brain barrier.11,12 Brain metastases are the most common type of brain tumor. Given that most chemotherapeutic agents cannot cross the blood-brain barrier, radiotherapy is considered a means of treatment and of controlling brain metastases. Early data from the 1950s13 and 1960s14 have suggested clinical improvement with brain radiation, making radiotherapy the cornerstone for treatment of brain metastases.
The Radiation Therapy Oncology Group (RTOG) has evaluated several fractionation schedules, with 5 schemas evaluated by the RTOG 6901 and 7361 studies: 30 Gy in 10 fractions, 30 Gy in 15 fractions, 40 Gy in 15 fractions, 40 Gy in 20 fractions, and 20 Gy in 5 fractions. The combined results from these two trials showed that outcomes were similar for patients treated with a shorter regimen than for those treated with a more protracted schedule. In our study, respondents reported that they most frequently treated brain metastases to a total dose of 30 Gy in 10 fractions. Given the results of the aforementioned RTOG trials and practice patterns among academic physicians, we recommend all practitioners consider a shorter hypofractioned course when treating brain metastases with WBRT. This will also reduce delays for patients who are likely to benefit greatly from earlier enrollment into hospice care, because protracted radiation schedules typically are not covered while a patient is in hospice.
Pharmacologic management for patients with brain metastases is important for symptomatic improvement. Glucocorticoids are important for palliation of symptoms from edema and increased intracranial pressure.15 However, steroids have a multitude of side effects and their use in asymptomatic patients is unnecessary. Improvements in imaging and detection11 have allowed us to find smaller and asymptomatic brain tumors. In our survey, it was promising to see a change in former practice patterns, with only 8% of academic practitioners regularly prescribing steroids to all of their patients receiving whole-brain radiation.
Diminished cognitive function and short-term memory loss are troublesome side effects of WBRT. As cancer patients live longer, such cognitive dysfunction will become more than just a nuisance. The RTOG has investigated the use of prophylactic memantine for patients receiving whole-brain radiation to determine if it would aid in the preservation of cognition. It found that patients who received memantine did better and had delayed time to cognitive decline and a reduced rate of memory decline, executive function, and processing speed.16 In our study, about a third of practitioners prescribed memantine and it was reserved for patients who had an otherwise favorable prognosis.
The RTOG has also investigated adjusting treatment technique for patients who receive WBRT. RTOG 0933 was a phase 2 trial that evaluated hippocampal avoidance during deliverance of WBRT with intensity-modulated radiation therapy (IMRT). Results showed that avoiding the hippocampus during WBRT was associated with improved memory preservation and patient quality of life.17 In a survey of practicing radiation oncologists in the US, most reported that they did not use memantine or IMRT for hippocampal sparing when delivering whole-brain radiation.18 Given the positive results of RTOG 0933 and 0614, the NRG Oncology research organization is conducting a phase 3 randomized trial that compares memantine use for patients receiving whole-brain radiation with or without hippocampal sparing to determine if patients will have reduced cognitive decline. All patients receiving WBRT should be considered for enrolment on this trial if they are eligible.
The delivery of brain radiation has continued to change, especially with the introduction of SRS. Recent publication of a meta-analysis of three phase 3 trials evaluating SRS with or without WBRT for 1-4 brain metastases showed that patients aged 50 years or younger experienced a survival benefit with SRS, and the omission of whole-brain radiation did not affect distant brain relapse rates. 19 The authors recommended that for this population, SRS alone is the preferred treatment. In our study, physicians who had been in practice for a longer time were more likely to treat using SRS alone. The results showed a linear association, with those in practice for a longer time being more likely to use SRS alone compared with those practicing for a shorter time (P = .027). Accordingly, 67% of respondents (8 of 12) who had been in practice for 11 or more years used SRS alone, whereas 24% (14 of 58) who had practiced for 0-5 years and 42% (5 of 12) who had practice from 6-10 years used SRS alone (Figure 1). When treating with SRS, younger practitioners placed more importance on the status of extracranial disease, whereas older practitioners placed more importance on tumor histopathology.
The use of repeat whole-brain reirradiation is more controversial among practitioners.20-22 Son and colleagues evaluated patients who needed whole-brain reirradiation after intracranial disease progression.22 The authors noted that patients with stable extracranial disease benefited from reirradiation. In our study, we found that when considering whole-brain reirradiation, older practitioners placed more importance on tumor histology than other factors.
As far as we know, this is the first study evaluating the practices and patterns of care with regard to the delivery of brain radiation in academic centers in the US. We found that time in practice was the most significant predictor of treatment technique and delivery. We also found that older practitioners place more importance on tumor histopathology compared with younger practitioners. A limitation of this study is that we had contact information only for program coordinators at ACGME-accredited programs. As such, we were not able to assess practice patterns among community practitioners. In addition, it seemed that residents and junior faculty were more likely to respond to this survey, likely because of the dissemination pattern. Given the evolution and diversity of treatment regimens for brain metastases, we believe that patients with brain metastases should be managed individually using a multidisciplinary approach.
1. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865-2872.
2. Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698-2705.
3. Takakura K. Metastatic tumors of the central nervous system. Tokyo: Igaku-Shoin; 1982.
4. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485-1489.
5. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. New Engl J Med. 1990;322(8):494-500.
6. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2015;91(4):710-717.
7. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
8. Choosing Wisely [ASTRO]. Don’t routinely add adjuvant whole-brain radiation therapy to stereotactic radiosurgery for limited brain metastases. http://www.choosingwisely.org/clinician-lists/american-society-radiation-oncology-adjunct-whole-brain-radiation-therapy/. Updated June 21, 2016. Accessed November 10, 2016.
9. Aoyama H, Tago M, Shirato H, Japanese Radiation Oncology Study Group I. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA Oncol. 2015;1(4):457-464.
10. Hoddinott SN, Bass MJ. The Dillman total design survey method. Can Fam Physician. 1986;32:2366-2368.
11. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48-54.
12. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5-14.
13. Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7(4):682-689.
14. Nieder C, Niewald M, Schnabel K. Treatment of brain metastases from hypernephroma. Urol Int. 1996;57(1):17-20.
15. Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103-114.
16. Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429-1437.
17. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810-3816.
18. Slade AN, Stanic S. The impact of RTOG 0614 and RTOG 0933 trials in routine clinical practice: The US Survey of Utilization of Memantine and IMRT planning for hippocampus sparing in patients receiving whole-brain radiotherapy for brain metastases. Contemp Clin Trials. 2016;47:74-77.
19. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. International journal of radiation oncology, biology, physics. 2015;91(4):710-717.
20. Hazuka MB, Kinzie JJ. Brain metastases: results and effects of re-irradiation. Int J Radiat Oncol Biol Phys. 1988;15(2):433-437.
21. Sadikov E, Bezjak A, Yi QL, et al. Value of whole-brain re-irradiation for brain metastases — single centre experience. Clin Oncol (R Coll Radiol). 2007;19(7):532-538.
22. Son CH, Jimenez R, Niemierko A, Loeffler JS, Oh KS, Shih HA. outcomes after whole-brain reirradiation in patients with brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(2):e167-e172.
Despite the recent advances in systemic therapy, metastatic spread to the brain continues to be the most common neurologic complication of many cancers. The clinical incidence of brain metastases varies with primary cancer diagnosis, with estimates ranging from 1.2%-19.8%.1,2 Metastatic spread to the brain is even more prevalent at autopsy, with evidence of intracranial tumor being found in 26% of patients in some series.3 It is possible that the clinical incidence of metastatic disease to the brain will continue to increase as newer therapeutic agents improve survival and imaging techniques continue to improve.
The management of brain metastases has changed rapidly as technological improvements have made treatment increasingly safe and efficacious. Traditionally, treatment consisted of radiotherapy to the whole brain, with or without surgical resection.4,5 More recently, stereotactic radiosurgery (SRS) has been adopted on the basis of evidence that it is safe and efficacious alone or in combination with radiotherapy to the whole brain.6 Further evidence is emerging that neurocognitive outcomes are improved when whole-brain radiotherapy (WBRT) is omitted, which possibly contributes to improved patient quality of life.7 Taking into account this and other data, the American Society for Radiation Oncology’s Choosing Wisely campaign now recommends not routinely adding WBRT to radiosurgery in patients with limited brain metastases.8
Despite this recommendation, many patients continue to benefit from WBRT, and it remains a common treatment in radiation oncology clinics across the US for several reasons. Many patients present with multiple brain metastases and are ineligible for radiosurgery. Even for technically eligible patients, WBRT has been shown to improve local control and decrease the rate of distant brain failure over radiosurgery alone.6 With higher rates of subsequent failures, patients receiving radiosurgery alone must adhere to more rigorous follow-up and imaging schedules, which can be difficult for many rural patients who have to travel long distances to centers. Furthermore, there is some suggestion that this decreased failure rate may result in improved survival in highly selected patients with excellent disease and performance status.9 Controversies exist, however, and strong institutional biases persist, contributing to significant differences in practice. We surveyed academic radiation oncologists and in an effort to identify and describe practice patterns in the delivery of WBRT at academic centers.
Methods
We conducted a thorough review of available literature on radiation for brain metastases and based on our findings, devised a survey 19 questions to ascertain practice patterns and treatment delivery among US academic physicians (Table 1). After obtaining institutional review board approval to do the study, we sent the survey to program coordinators at radiation oncology programs that are accredited by the Accreditation Council for Graduate Medical Education. We instructed coordinators to e-mail the survey to their practicing resident and attending physicians. The surveys were created using SurveyMonkey software. We obtained informed consent from the providers. A total of 3 follow-up e-mails were sent to each recipient of the survey to solicit responses, similar to the Dillman Total Design Survey Method.10
SPSS version 22.0 was used to analyze the data in an exploratory fashion. Statistical methods were used to assess the association of demographic data with SRS and WBRT delivery and treatment technique items when the analyses involved percentages that included the Pearson chi-square statistic and the chi-square test for linear trend. When the analysis focused on ranking data, the Kruskal-Wallis test, Mann-Whitney U test, the Jonckheere-Terpstra and the Kendall tau-b rank correlation were used as appropriate. If there were small sample sizes within some groups, then exact significant levels were assessed. Statistical significance was set by convention at P < .05.
Results
We received 95 responses of which 87 were considered complete for analysis. Forty-seven percent of the 87 respondents were not board-certified, and the remainder had passed their radiobiology and physics boards exams. A majority of respondents (70%, 61 of 87) were physicians who had been in practice for ≤5 years. Fifty-four percent of respondents were located in the Northeast US, 22% in the South, 14% in the West, and 10% in the Midwest and Hawaii (Table 2).
We used the chi-square test for linear trends to assess for a relationship between years of practice and whether respondents deviated from their typical method of WBRT therapy when treating more radioresistant tumors (melanoma, renal cell carcinoma). Respondents were classified by years in practice: 0-5, 6-10, 11-20, and >21 years. The results showed a linear association, with those in practice for longer periods more likely to use SRS alone, P = .027 (Figure 1).
Discussion
The incidence of brain metastases is increasing because of improvements in diagnostic imaging techniques and advancements in systemic therapy control of extracranial disease but not of intracranial disease or metastasis, because therapies do not cross the blood-brain barrier.11,12 Brain metastases are the most common type of brain tumor. Given that most chemotherapeutic agents cannot cross the blood-brain barrier, radiotherapy is considered a means of treatment and of controlling brain metastases. Early data from the 1950s13 and 1960s14 have suggested clinical improvement with brain radiation, making radiotherapy the cornerstone for treatment of brain metastases.
The Radiation Therapy Oncology Group (RTOG) has evaluated several fractionation schedules, with 5 schemas evaluated by the RTOG 6901 and 7361 studies: 30 Gy in 10 fractions, 30 Gy in 15 fractions, 40 Gy in 15 fractions, 40 Gy in 20 fractions, and 20 Gy in 5 fractions. The combined results from these two trials showed that outcomes were similar for patients treated with a shorter regimen than for those treated with a more protracted schedule. In our study, respondents reported that they most frequently treated brain metastases to a total dose of 30 Gy in 10 fractions. Given the results of the aforementioned RTOG trials and practice patterns among academic physicians, we recommend all practitioners consider a shorter hypofractioned course when treating brain metastases with WBRT. This will also reduce delays for patients who are likely to benefit greatly from earlier enrollment into hospice care, because protracted radiation schedules typically are not covered while a patient is in hospice.
Pharmacologic management for patients with brain metastases is important for symptomatic improvement. Glucocorticoids are important for palliation of symptoms from edema and increased intracranial pressure.15 However, steroids have a multitude of side effects and their use in asymptomatic patients is unnecessary. Improvements in imaging and detection11 have allowed us to find smaller and asymptomatic brain tumors. In our survey, it was promising to see a change in former practice patterns, with only 8% of academic practitioners regularly prescribing steroids to all of their patients receiving whole-brain radiation.
Diminished cognitive function and short-term memory loss are troublesome side effects of WBRT. As cancer patients live longer, such cognitive dysfunction will become more than just a nuisance. The RTOG has investigated the use of prophylactic memantine for patients receiving whole-brain radiation to determine if it would aid in the preservation of cognition. It found that patients who received memantine did better and had delayed time to cognitive decline and a reduced rate of memory decline, executive function, and processing speed.16 In our study, about a third of practitioners prescribed memantine and it was reserved for patients who had an otherwise favorable prognosis.
The RTOG has also investigated adjusting treatment technique for patients who receive WBRT. RTOG 0933 was a phase 2 trial that evaluated hippocampal avoidance during deliverance of WBRT with intensity-modulated radiation therapy (IMRT). Results showed that avoiding the hippocampus during WBRT was associated with improved memory preservation and patient quality of life.17 In a survey of practicing radiation oncologists in the US, most reported that they did not use memantine or IMRT for hippocampal sparing when delivering whole-brain radiation.18 Given the positive results of RTOG 0933 and 0614, the NRG Oncology research organization is conducting a phase 3 randomized trial that compares memantine use for patients receiving whole-brain radiation with or without hippocampal sparing to determine if patients will have reduced cognitive decline. All patients receiving WBRT should be considered for enrolment on this trial if they are eligible.
The delivery of brain radiation has continued to change, especially with the introduction of SRS. Recent publication of a meta-analysis of three phase 3 trials evaluating SRS with or without WBRT for 1-4 brain metastases showed that patients aged 50 years or younger experienced a survival benefit with SRS, and the omission of whole-brain radiation did not affect distant brain relapse rates. 19 The authors recommended that for this population, SRS alone is the preferred treatment. In our study, physicians who had been in practice for a longer time were more likely to treat using SRS alone. The results showed a linear association, with those in practice for a longer time being more likely to use SRS alone compared with those practicing for a shorter time (P = .027). Accordingly, 67% of respondents (8 of 12) who had been in practice for 11 or more years used SRS alone, whereas 24% (14 of 58) who had practiced for 0-5 years and 42% (5 of 12) who had practice from 6-10 years used SRS alone (Figure 1). When treating with SRS, younger practitioners placed more importance on the status of extracranial disease, whereas older practitioners placed more importance on tumor histopathology.
The use of repeat whole-brain reirradiation is more controversial among practitioners.20-22 Son and colleagues evaluated patients who needed whole-brain reirradiation after intracranial disease progression.22 The authors noted that patients with stable extracranial disease benefited from reirradiation. In our study, we found that when considering whole-brain reirradiation, older practitioners placed more importance on tumor histology than other factors.
As far as we know, this is the first study evaluating the practices and patterns of care with regard to the delivery of brain radiation in academic centers in the US. We found that time in practice was the most significant predictor of treatment technique and delivery. We also found that older practitioners place more importance on tumor histopathology compared with younger practitioners. A limitation of this study is that we had contact information only for program coordinators at ACGME-accredited programs. As such, we were not able to assess practice patterns among community practitioners. In addition, it seemed that residents and junior faculty were more likely to respond to this survey, likely because of the dissemination pattern. Given the evolution and diversity of treatment regimens for brain metastases, we believe that patients with brain metastases should be managed individually using a multidisciplinary approach.
Despite the recent advances in systemic therapy, metastatic spread to the brain continues to be the most common neurologic complication of many cancers. The clinical incidence of brain metastases varies with primary cancer diagnosis, with estimates ranging from 1.2%-19.8%.1,2 Metastatic spread to the brain is even more prevalent at autopsy, with evidence of intracranial tumor being found in 26% of patients in some series.3 It is possible that the clinical incidence of metastatic disease to the brain will continue to increase as newer therapeutic agents improve survival and imaging techniques continue to improve.
The management of brain metastases has changed rapidly as technological improvements have made treatment increasingly safe and efficacious. Traditionally, treatment consisted of radiotherapy to the whole brain, with or without surgical resection.4,5 More recently, stereotactic radiosurgery (SRS) has been adopted on the basis of evidence that it is safe and efficacious alone or in combination with radiotherapy to the whole brain.6 Further evidence is emerging that neurocognitive outcomes are improved when whole-brain radiotherapy (WBRT) is omitted, which possibly contributes to improved patient quality of life.7 Taking into account this and other data, the American Society for Radiation Oncology’s Choosing Wisely campaign now recommends not routinely adding WBRT to radiosurgery in patients with limited brain metastases.8
Despite this recommendation, many patients continue to benefit from WBRT, and it remains a common treatment in radiation oncology clinics across the US for several reasons. Many patients present with multiple brain metastases and are ineligible for radiosurgery. Even for technically eligible patients, WBRT has been shown to improve local control and decrease the rate of distant brain failure over radiosurgery alone.6 With higher rates of subsequent failures, patients receiving radiosurgery alone must adhere to more rigorous follow-up and imaging schedules, which can be difficult for many rural patients who have to travel long distances to centers. Furthermore, there is some suggestion that this decreased failure rate may result in improved survival in highly selected patients with excellent disease and performance status.9 Controversies exist, however, and strong institutional biases persist, contributing to significant differences in practice. We surveyed academic radiation oncologists and in an effort to identify and describe practice patterns in the delivery of WBRT at academic centers.
Methods
We conducted a thorough review of available literature on radiation for brain metastases and based on our findings, devised a survey 19 questions to ascertain practice patterns and treatment delivery among US academic physicians (Table 1). After obtaining institutional review board approval to do the study, we sent the survey to program coordinators at radiation oncology programs that are accredited by the Accreditation Council for Graduate Medical Education. We instructed coordinators to e-mail the survey to their practicing resident and attending physicians. The surveys were created using SurveyMonkey software. We obtained informed consent from the providers. A total of 3 follow-up e-mails were sent to each recipient of the survey to solicit responses, similar to the Dillman Total Design Survey Method.10
SPSS version 22.0 was used to analyze the data in an exploratory fashion. Statistical methods were used to assess the association of demographic data with SRS and WBRT delivery and treatment technique items when the analyses involved percentages that included the Pearson chi-square statistic and the chi-square test for linear trend. When the analysis focused on ranking data, the Kruskal-Wallis test, Mann-Whitney U test, the Jonckheere-Terpstra and the Kendall tau-b rank correlation were used as appropriate. If there were small sample sizes within some groups, then exact significant levels were assessed. Statistical significance was set by convention at P < .05.
Results
We received 95 responses of which 87 were considered complete for analysis. Forty-seven percent of the 87 respondents were not board-certified, and the remainder had passed their radiobiology and physics boards exams. A majority of respondents (70%, 61 of 87) were physicians who had been in practice for ≤5 years. Fifty-four percent of respondents were located in the Northeast US, 22% in the South, 14% in the West, and 10% in the Midwest and Hawaii (Table 2).
We used the chi-square test for linear trends to assess for a relationship between years of practice and whether respondents deviated from their typical method of WBRT therapy when treating more radioresistant tumors (melanoma, renal cell carcinoma). Respondents were classified by years in practice: 0-5, 6-10, 11-20, and >21 years. The results showed a linear association, with those in practice for longer periods more likely to use SRS alone, P = .027 (Figure 1).
Discussion
The incidence of brain metastases is increasing because of improvements in diagnostic imaging techniques and advancements in systemic therapy control of extracranial disease but not of intracranial disease or metastasis, because therapies do not cross the blood-brain barrier.11,12 Brain metastases are the most common type of brain tumor. Given that most chemotherapeutic agents cannot cross the blood-brain barrier, radiotherapy is considered a means of treatment and of controlling brain metastases. Early data from the 1950s13 and 1960s14 have suggested clinical improvement with brain radiation, making radiotherapy the cornerstone for treatment of brain metastases.
The Radiation Therapy Oncology Group (RTOG) has evaluated several fractionation schedules, with 5 schemas evaluated by the RTOG 6901 and 7361 studies: 30 Gy in 10 fractions, 30 Gy in 15 fractions, 40 Gy in 15 fractions, 40 Gy in 20 fractions, and 20 Gy in 5 fractions. The combined results from these two trials showed that outcomes were similar for patients treated with a shorter regimen than for those treated with a more protracted schedule. In our study, respondents reported that they most frequently treated brain metastases to a total dose of 30 Gy in 10 fractions. Given the results of the aforementioned RTOG trials and practice patterns among academic physicians, we recommend all practitioners consider a shorter hypofractioned course when treating brain metastases with WBRT. This will also reduce delays for patients who are likely to benefit greatly from earlier enrollment into hospice care, because protracted radiation schedules typically are not covered while a patient is in hospice.
Pharmacologic management for patients with brain metastases is important for symptomatic improvement. Glucocorticoids are important for palliation of symptoms from edema and increased intracranial pressure.15 However, steroids have a multitude of side effects and their use in asymptomatic patients is unnecessary. Improvements in imaging and detection11 have allowed us to find smaller and asymptomatic brain tumors. In our survey, it was promising to see a change in former practice patterns, with only 8% of academic practitioners regularly prescribing steroids to all of their patients receiving whole-brain radiation.
Diminished cognitive function and short-term memory loss are troublesome side effects of WBRT. As cancer patients live longer, such cognitive dysfunction will become more than just a nuisance. The RTOG has investigated the use of prophylactic memantine for patients receiving whole-brain radiation to determine if it would aid in the preservation of cognition. It found that patients who received memantine did better and had delayed time to cognitive decline and a reduced rate of memory decline, executive function, and processing speed.16 In our study, about a third of practitioners prescribed memantine and it was reserved for patients who had an otherwise favorable prognosis.
The RTOG has also investigated adjusting treatment technique for patients who receive WBRT. RTOG 0933 was a phase 2 trial that evaluated hippocampal avoidance during deliverance of WBRT with intensity-modulated radiation therapy (IMRT). Results showed that avoiding the hippocampus during WBRT was associated with improved memory preservation and patient quality of life.17 In a survey of practicing radiation oncologists in the US, most reported that they did not use memantine or IMRT for hippocampal sparing when delivering whole-brain radiation.18 Given the positive results of RTOG 0933 and 0614, the NRG Oncology research organization is conducting a phase 3 randomized trial that compares memantine use for patients receiving whole-brain radiation with or without hippocampal sparing to determine if patients will have reduced cognitive decline. All patients receiving WBRT should be considered for enrolment on this trial if they are eligible.
The delivery of brain radiation has continued to change, especially with the introduction of SRS. Recent publication of a meta-analysis of three phase 3 trials evaluating SRS with or without WBRT for 1-4 brain metastases showed that patients aged 50 years or younger experienced a survival benefit with SRS, and the omission of whole-brain radiation did not affect distant brain relapse rates. 19 The authors recommended that for this population, SRS alone is the preferred treatment. In our study, physicians who had been in practice for a longer time were more likely to treat using SRS alone. The results showed a linear association, with those in practice for a longer time being more likely to use SRS alone compared with those practicing for a shorter time (P = .027). Accordingly, 67% of respondents (8 of 12) who had been in practice for 11 or more years used SRS alone, whereas 24% (14 of 58) who had practiced for 0-5 years and 42% (5 of 12) who had practice from 6-10 years used SRS alone (Figure 1). When treating with SRS, younger practitioners placed more importance on the status of extracranial disease, whereas older practitioners placed more importance on tumor histopathology.
The use of repeat whole-brain reirradiation is more controversial among practitioners.20-22 Son and colleagues evaluated patients who needed whole-brain reirradiation after intracranial disease progression.22 The authors noted that patients with stable extracranial disease benefited from reirradiation. In our study, we found that when considering whole-brain reirradiation, older practitioners placed more importance on tumor histology than other factors.
As far as we know, this is the first study evaluating the practices and patterns of care with regard to the delivery of brain radiation in academic centers in the US. We found that time in practice was the most significant predictor of treatment technique and delivery. We also found that older practitioners place more importance on tumor histopathology compared with younger practitioners. A limitation of this study is that we had contact information only for program coordinators at ACGME-accredited programs. As such, we were not able to assess practice patterns among community practitioners. In addition, it seemed that residents and junior faculty were more likely to respond to this survey, likely because of the dissemination pattern. Given the evolution and diversity of treatment regimens for brain metastases, we believe that patients with brain metastases should be managed individually using a multidisciplinary approach.
1. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865-2872.
2. Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698-2705.
3. Takakura K. Metastatic tumors of the central nervous system. Tokyo: Igaku-Shoin; 1982.
4. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485-1489.
5. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. New Engl J Med. 1990;322(8):494-500.
6. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2015;91(4):710-717.
7. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
8. Choosing Wisely [ASTRO]. Don’t routinely add adjuvant whole-brain radiation therapy to stereotactic radiosurgery for limited brain metastases. http://www.choosingwisely.org/clinician-lists/american-society-radiation-oncology-adjunct-whole-brain-radiation-therapy/. Updated June 21, 2016. Accessed November 10, 2016.
9. Aoyama H, Tago M, Shirato H, Japanese Radiation Oncology Study Group I. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA Oncol. 2015;1(4):457-464.
10. Hoddinott SN, Bass MJ. The Dillman total design survey method. Can Fam Physician. 1986;32:2366-2368.
11. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48-54.
12. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5-14.
13. Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7(4):682-689.
14. Nieder C, Niewald M, Schnabel K. Treatment of brain metastases from hypernephroma. Urol Int. 1996;57(1):17-20.
15. Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103-114.
16. Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429-1437.
17. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810-3816.
18. Slade AN, Stanic S. The impact of RTOG 0614 and RTOG 0933 trials in routine clinical practice: The US Survey of Utilization of Memantine and IMRT planning for hippocampus sparing in patients receiving whole-brain radiotherapy for brain metastases. Contemp Clin Trials. 2016;47:74-77.
19. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. International journal of radiation oncology, biology, physics. 2015;91(4):710-717.
20. Hazuka MB, Kinzie JJ. Brain metastases: results and effects of re-irradiation. Int J Radiat Oncol Biol Phys. 1988;15(2):433-437.
21. Sadikov E, Bezjak A, Yi QL, et al. Value of whole-brain re-irradiation for brain metastases — single centre experience. Clin Oncol (R Coll Radiol). 2007;19(7):532-538.
22. Son CH, Jimenez R, Niemierko A, Loeffler JS, Oh KS, Shih HA. outcomes after whole-brain reirradiation in patients with brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(2):e167-e172.
1. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865-2872.
2. Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698-2705.
3. Takakura K. Metastatic tumors of the central nervous system. Tokyo: Igaku-Shoin; 1982.
4. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485-1489.
5. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. New Engl J Med. 1990;322(8):494-500.
6. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2015;91(4):710-717.
7. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
8. Choosing Wisely [ASTRO]. Don’t routinely add adjuvant whole-brain radiation therapy to stereotactic radiosurgery for limited brain metastases. http://www.choosingwisely.org/clinician-lists/american-society-radiation-oncology-adjunct-whole-brain-radiation-therapy/. Updated June 21, 2016. Accessed November 10, 2016.
9. Aoyama H, Tago M, Shirato H, Japanese Radiation Oncology Study Group I. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA Oncol. 2015;1(4):457-464.
10. Hoddinott SN, Bass MJ. The Dillman total design survey method. Can Fam Physician. 1986;32:2366-2368.
11. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48-54.
12. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5-14.
13. Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7(4):682-689.
14. Nieder C, Niewald M, Schnabel K. Treatment of brain metastases from hypernephroma. Urol Int. 1996;57(1):17-20.
15. Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103-114.
16. Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429-1437.
17. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810-3816.
18. Slade AN, Stanic S. The impact of RTOG 0614 and RTOG 0933 trials in routine clinical practice: The US Survey of Utilization of Memantine and IMRT planning for hippocampus sparing in patients receiving whole-brain radiotherapy for brain metastases. Contemp Clin Trials. 2016;47:74-77.
19. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. International journal of radiation oncology, biology, physics. 2015;91(4):710-717.
20. Hazuka MB, Kinzie JJ. Brain metastases: results and effects of re-irradiation. Int J Radiat Oncol Biol Phys. 1988;15(2):433-437.
21. Sadikov E, Bezjak A, Yi QL, et al. Value of whole-brain re-irradiation for brain metastases — single centre experience. Clin Oncol (R Coll Radiol). 2007;19(7):532-538.
22. Son CH, Jimenez R, Niemierko A, Loeffler JS, Oh KS, Shih HA. outcomes after whole-brain reirradiation in patients with brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(2):e167-e172.
Point of prostate cancer diagnosis experiences and needs of black men: the Florida CaPCaS study
As of 2016, Florida ranks second among all states in the United States in estimated new cases of prostate cancer and second in estimated deaths from prostate cancer.1 Disparities in diagnosis, mortality rates, and access to cancer care also continue to be a major problem in Florida, especially for black men. For example, black men were the only racial/ethnic group that did not meet the Healthy People (HP) 2010 objective to reduce the prostate cancer death rate to 28.2 per 100,000 men and that has not met the HP 2020 objective to reduce the prostate cancer death rate to 21.2 per 100,000 men (Table 1). Based on the 2013 prostate cancer mortality rates for Florida,2 the death rate for black men is almost twice the HP 2020 goal (37.49 per 100,000).
A diagnosis of prostate cancer is a life changing event for a man. In particular, there is limited research on the experiences and coping mechanisms of black men at diagnosis. This limited body of research indicates that black men’s reactions to their initial diagnoses varied, from being shocked when notified of their initial diagnosis of prostate cancer,3 to perceiving that they had received a “death sentence”.4 In regard to having to make decisions about their treatment options, some black men indicated that the information about treatment that they received from physicians decreased their anxiety,5 whereas others noted that they had not been given adequate information by a physician to make a decision.6 Patients have also reported that they felt as though they were not knowledgeable enough to ask questions concerning treatment options and preferred for the physician to make the treatment choice for them.6 Decisional regret is now a common observation among men who are not involved in making decisions about their treatment.3
According to the American Cancer Society, about 30,000 black men were diagnosed with prostate cancer in 2016.7 It is important to understand these men’s needs and help them cope effectively as they navigate the survivorship continuum. In line with our research program’s goal of ensuring quality cancer care for black men, the primary objective of this study was to explore the experiences and needs of black men at the point of prostate cancer diagnosis (PPCD). Specifically, we developed an interpretative framework for black men’s experiences at the PPCD, focusing on United States or native-born black men (NBBM) and Caribbean-born black men (CBBM). African-born black men were not included in this study because of the low sample size for that ethnicity. This study is part of a large-scale study that focuses on developing a model of prostate cancer care and survivorship (CaPCaS model) using grounded theory to study black, ethnically diverse prostate cancer survivors.
Methods
The study aims to close the prostate health disparity gap for black men in Florida through community engaged research in partnership with survivors of prostate cancer and their advocates. The current study was a prospective, grounded theory study that involved one-on-one, in-depth interviews with 31 prostate cancer patients about their care and survivorship experiences. Specifically, 17 NBBM and 14 CBBM were enrolled in the project. Appropriate human subjects review and approval were obtained from the University of Florida, the Florida Department of Health, and the Department of Defense.
Research design
This is a qualitative research study. Based on the principles of community engaged research and using a rigorous qualitative research methodology, we recruited NBBM and CBBM with a personal history of prostate cancer. Guided by open-ended questions developed by the team, one-on-one in-depth interviews were conducted with each participant in their home or at a convenient location in the community. Our primary focus was on the participants’ care and survivorship experiences, with primary focus on their prostate cancer diagnosis. Qualitative research was our methodology of choice because little is known about the PPCD experiences of black men.8 With qualitative research, we were able to get our participants to “relive” their experiences in the presence of a culturally competent, well-trained interviewer and elicit the information about their care and survivorship experiences based on their interpretation. In addition, we were able to capture the dynamic processes associated with their experiences, documenting sequential patterns and change through both verbal and nonverbal communications, because the participants were interviewed twice.
Research population and recruitment
The study setting was Florida. The inclusion criteria were: black men, personal history of prostate cancer, ability to complete two separate interviews with each one expected to last 2-3 hours, and flexibility to meet interviewers at a convenient community site for the interviews. Participants were identified through the Florida Cancer Data System (FCDS)9 database. At the time of the study, the most recent FCDS database was for 2010. The FCDS has collected the number of new cancer cancers diagnosed in the state of Florida annually since 1981. It is a comprehensive incidence-only registry and does not extract data on patients with a death certificate. All investigators are bounded by the confidential pledge required for the use of the FCDS data.
We used the Florida Department of Health’s (DoH’s) Bureau of Epidemiology standard procedure for the FCDS9 to recruit participants. Our recruitment strategies included: initial patient contact by written correspondence; second mailing that included a telephone opt-out card after 3 weeks for nonrespondents (the telephone opt-out card explained to the patient that if no response was received, the study investigator would attempt a telephone call to introduce the study); and a telephone call by a study staff to introduce the study for nonrespondents. As per the Florida DoH standard procedure, we did not disclose on the cover of the study mailings that the patient was being contacted for a study specific to cancer. Efforts to recruit a patient stopped immediately if a patient indicated that he did not wish to participate. All of the study staff making participant contact were extensively trained to provide a clear and accurate description of cancer registration in Florida. In addition, to assist the study staff in providing clear and accurate responses, responses to frequently asked questions were made available to the study staff. During the participant recruitment phase, anyone who seemed to be upset when contacted was reported immediately (within 24 hours) to the DoH cancer epidemiologist. In addition, the name of anyone who stated that he did not wish to be contacted again was given to the DoH so that the person would not be re-contacted.
Prescreening of participants for eligibility
All eligible participants who agreed to participate in the study comprised the pool of potential study participants. For those who agreed to participate, the following information was obtained by telephone interview using REDCap software:10 name and contact information, country of birth, age, marital status, and education level. The demographic information facilitated a purposeful systematic selection of black men of diverse age groups (younger than 50 years or older than 50 years), marital status (single, including divorced or separated, or married/in a relationship), and educational level (college degree or not college educated). An incentive of a $5 gift card was provided to all the men who participated in the screening phase. Using systematic sampling to ensure demographically diverse participants, 40 participants (20 NBBM, 20 CBBM) were selected from the initial pool of participants to participate in the study.
Data collection
The data collection was conducted by a trained Community Health Worker (CHW) using semi-structured interview process. The interview guide was constructed by the research team and the study community advisory board members to ensure language appropriateness, understanding and cultural sensitivity. For this study, the interview questions focused on participants’ background information and diagnosis history, including: participants’ personal story of diagnosis, feelings, emotions, reactions, regrets and level of personal/family/physician involvement in diagnosis. For the CBBM, we also obtained information on the age at which they immigrated to the United States. The CHW interviewer was trained to question participants and encourage them to elaborate on areas of importance to their experience.
A total time of about 5-6 hours was scheduled for the data collection per participant, which is sufficient for gathering in-depth perspectives. We scheduled two interviews lasting not more than 3 hours at a time so as not to create burden for study participants. Participants had the choice to have the interviews completed in a single session or spread out over 2 days. The interviews were audio-recorded to provide ease of transcription and back-up of data. At the end of the interviews, participants were compensated for participating in the study.
Data management and analyses
The study dataset included interview transcripts and field notes of the CHW interviewer describing his insights about the interviews. The data analyses included preparing and verifying the narrative data, coding data, and developing an interpretative framework for black men’s experiences at the PPCD. Interviews were transcribed verbatim by a professional transcription service that has policies in place for protected health information. Each transcript was then verified for accuracy by the CHW interviewer. The interview transcripts were imported directly into NVivo 11, a computer-assisted data analysis software that allows coding and modeling of complex narrative data. The data coding was conducted by our interdisciplinary team of clinicians, behavioral scientists, and social scientists. It is important to note that the NVivo 11 software was not used to analyze the data per se. However, it provided a sophisticated and systematic way to manage the following tasks for the analyses: organizing large quantities of narrative data, coding text, retrieving text by codes, querying the data, comparing sets of data interpretation between NBBM and CBBM; and developing analytic models. The study team members coded the data in weekly team meetings. The coding consisted of reading the data and identifying major themes, then assigning labels to and defining emerging categories.
Two levels of coding were used. The first, open coding, refers to an approach to data with no preconceived ideas about what will be found; and the second, focused or axial coding, refers to reviewing data for the purpose of more richly coding on a particular theme.11 We used dimensional analysis to ensure that each emerging concept was carefully defined. The study team went back and forth between the data and the emerging analytic framework, using constant comparison of new data with already coded data and new categories with previously analyzed text.12
To ensure trustworthiness and credibility,13 the study team maintained an audit trail that documented how and when analytic decisions were made. In addition, peer debriefing was conducted to ensure credibility, including the presentation of findings to the study community advisory board members as part of the community engaged research approach.
Results
Description of participants
The FCDS provided a database of 1,813 participants identified as black men diagnosed with prostate cancer in 2010. Because the FCDS does not extract data on patients with a death certificate, we found out during the pre-screening phase that a few of the men were deceased. In addition, there were a significant number of incorrect addresses. We obtained a total of 212 completed responses by phone during the prescreening phase. The majority of the participants were aged 60-69 years (48.2%), had a high school diploma only (26.1%), and were currently married (65.3%). Relative to ethnicity, 67% of participants classified themselves NBBM, 24% as CBBM, 3.5% as black men born in Africa, and 5.5% as Other/Don’t know/Refused. For the CBBM, the most common countries of birth were Jamaica, Haiti, and Guyana, respectively.
In all, 40 participants (20 NBBM and 20 CBBM) were selected from the 212 participants to participate in the study. Selection was conducted systematically to ensure representation in terms of age, marital status, education, and geographical location. Data saturation was achieved with 17 NBBM and 14 CBBM, after which we ended data collection (Table 2). Data saturation is the standard for deciding that we are not finding anything different from the interviews first coded and last coded. Although we were specifically looking for differences between the two groups (NBBM and CBBM), no between-group differences emerged. Each man’s experience was unique to him with some common themes emerging described hereinafter (Figure).
Moderating factors and experiences at PPCD
Some of the moderating factors that the study participants identified as affecting their reactions to the PPCD included health literacy, insurance status, spirituality, mistrust, prior experience with cancer, perceived susceptibility to cancer, and delay in diagnosis (Table 3). Health literacy, defined as personal, cognitive, and social skills that determine the ability of individuals to gain access to, understand, and use information to promote and maintain good health, was one of the moderating factors found in this study.13 Some of the black men came to the PPCD with a low level of health literacy, which had an impact on their understanding of the treatment options. For example, in the interview, participant 798 (NBBM) was confused about what tests had been done and was not able to accurately describe the treatments offered to him. Participant 1263 (CBBM) struggled to express the purpose and procedures associated with diagnostic biopsy. However, there were participants with a high level of health literacy (eg, participant 449 [NBBM]), who decided to research the disease.
Another factor to consider is the insurance status of participants at the PPCD. The majority of the participants had good insurance coverage, but some were affected by poor insurance coverage. Participant 1881(NBBM) made his treatment decision primarily on the basis of the pending lapse of his insurance coverage rather than the best clinical option for him. Participant 1979 (CBBM) described both his confusion on the screening tests and the impact of not having insurance coverage. Upon obtaining insurance coverage, he sought treatment for his prostate cancer with an urgency that he did not experience when he was first diagnosed when uninsured.
The spirituality of black men was another moderating factor at the PPCD. Participant 827 (NBBM) noted that he was unaffected when he received his diagnosis because he was a true believer. Some of the black men also came to the PPCD with lack of trust in the physician and/or the health care system and perceived a sense of contempt from the physician. Participant 1594 (NBBM) described mistrust based on the history of medical exploitation of black men as well as a perception of current discriminatory practices.
Another important PPCD status to note for black men is prior experience with cancer, including prior personal cancer history and/or prior cancer history of a family member. Participant 2024 (CBBM) described the meaning of cancer to him, while participant 798 (NBBM) echoed the despair of the cancer diagnosis based on experience with other cancers in the family. Sometimes there were multiple cancers in the family or even among the significant others of the participant, as was the case with Participant 1936 (CBBM).
Of greatest concern were men who delayed their diagnosis or treatment, perhaps resulting in their cancer being at a more advanced stage when they eventually did return for care. Finally, some of the men came to the PPCD appointment with a low expectation of receiving a diagnosis of prostate cancer, whereas others came to the PPCD fearful of the results of their testing.
In describing their experiences, participants expressed both positive and negative experiences: on the positive side, they found the information provided by the physician to be helpful; but on the negative side, the sterile or medically focused encounter was perceived as a lack empathy on the part of the physician.
Cognitive, emotional, and behavioral coping experiences
As expected, there were ranges of emotions, including shock, disbelief and denial (Table 4). Some of the men questioned why this (the cancer) was happening to them when they had done “nothing” to deserve it. Doing nothing in this case meant that they had lived a healthy lifestyle with no obvious apparent cause to have the cancer. Fear and cancer fatalism were experienced by a significant number of the men, with their thoughts immediately turning to death and dying. This was especially the case for men who had lost a loved one to cancer. Conversely, some of the men wanted immediate resolution, focusing instead on ways to beat the cancer and with a strong will to live.
Reliance on faith was a big part of coping at the PPCD. Some of the men drew strength from their faith to get them through their cancer journey. Others found a way to accept the diagnosis – one participant accepted the diagnosis and the fact that this could mean dying (after living a good life), whereas another participant accepted the diagnosis with the hope that he would find a cure. Hope was more realistic with the knowledge that other men had survived prostate cancer.
Reflecting back on their experiences, the men also identified clear needs at the PPCD. One of the needs they identified was having a physician they were comfortable with to discuss their diagnosis. Another need was for a second opinion. Participant 1594 (NBBM) advised that it was important for black men to take control by requesting a second opinion. Participant 2039 (NBBM) described a feeling of navigating blindly and trying to find answers that would be helpful to him in his cancer journey. However, his experience with a second opinion was not helpful because the second physician was at the same clinic as his primary physician. His recommendation was to get a second opinion at a different clinic or center. Another important need was emotional support at the PPCD. Participant 2024 (CBBM) made a strong case for emotional support, especially for men who are not accompanied during diagnosis. In addition, Participant 2024’s (CBBM) reflections underscore the fact that the PPCD may not be an ideal place or time to discuss treatment options. With the range of emotions that the men go through at the PPCD, it is difficult to comprehend any follow-up discussions after hearing the words “you have prostate cancer.” Participant 2024 (CBBM) also strongly expressed that men need time to deal with the diagnosis at the PPCD.
Discussion
The primary goal of this study was to develop an interpretative framework of black men’s experiences at the PPCD. The Figure provides a pictorial summary of the framework. Study results indicated that black men come to the PPCD with different emotions and different experiences. Although the majority of the men were NBBM, there is a significantly increasing number of foreign-born black men receiving a diagnosis of prostate cancer in the United States. Given that black men carry a disproportionate burden of the disease, with a significantly higher incidence compared with any other racial group, it is important that tailored services are provided to black men at the PPCD.
We also found that black men came to the PPCD diverse in terms of their ethnicity, health literacy, spirituality, trust in health care system/physician, prior experience with cancer, perceived susceptibility to cancer, delayed time for diagnosis, and fear of diagnosis. Of importance for physicians is that the black race is not homogeneous. There is a significant number of foreign-born blacks at the PPCD, and they often have different cultural beliefs and values compared with NBBM. In addition, some of the foreign-born black men may not have English proficiency and may need a medical interpreter during the PPCD consultation. In addition, a patient’s pre-existing lack of trust in the health care system may have a negative impact on the PPCD consultation. It is thus important that the physician takes the time to instill trust and make the men comfortable during the PPCD consultation.
For some of the men who had fear of a prostate cancer diagnosis and/or prior experience with cancer, cancer fatalism was experienced at the PPCD. Cancer fatalism, defined as an individual’s belief that death is bound to happen when diagnosed with cancer, has been documented as a major barrier to cancer detection and control.15 For example, fatalistic perspectives have been reported to affect cervical cancer,16 breast cancer,17,18 colorectal cancer,19 and prostate cancer20,21 among blacks. It is thus important to effectively address fatalistic beliefs when a man is diagnosed with prostate cancer.
Other emotions at the PPCD that may affect effective treatment decision making also need to be addressed immediately. For example, the emotions of fear, denial, and feeling overwhelmed are potential barriers to timely treatment decision making. Psycho-oncology interventions to appropriately deal with these emotions at PPCD or right after the diagnosis may be crucial for the men. In particular, a group-based psychosocial intervention focusing on: provision of education about treatment options for prostate cancer and their acute and late effects; negotiating treatment and treatment side effects; enhancing communication with treatment providers; managing distress; and engaging positive family- and community-based social support to optimize emotional, behavioral, social, and physical outcomes in black men with prostate cancer.
In addition to having physicians make them comfortable at PPCD, the PPCD needs expressed by participants included having time to come to terms with the diagnosis and receiving psycho-oncology/emotional support. Anyone who has just received a diagnosis of cancer cannot be expected to immediately continue to function as he did before the PPCD. This is especially difficult for men who are alone at the PPCD. Nevertheless, it is expected that they will listen attentively and understand subsequent consultation by the physician, then leave the consultation room almost immediately, and be able drive home or back to work right after the diagnosis. There seems to be a support gap that needs to be closed at the PPCD. Providing the men with immediate support to cope with the diagnosis may make a significant difference in effective treatment choices and eliminating treatment decisional regrets.
Methodological rigor was established through purposeful sampling, extended time with participants, standardized procedures for data collection, management and analysis, multidisciplinary interpretation, and validation of results with the community advisory board. Because the research participants were purposefully selected from a statewide database of black men diagnosed with CaP, generalizability of findings to the two target groups of NBBM and CBBM can be assumed, with the caveat that men with different experiences may have chosen not to respond to recruitment efforts or refused participation. Black men who were not sufficiently fluent in English to be interviewed were also excluded and are not represented in these findings. Black men of other nativity (including African-born black men) and residing outside of Florida were also not represented.
In conclusion, the PPCD interpretative framework developed in this study, describes the status of black men at the PPCD, their experiences during the PPCD, and their needs at the PPCD. The framework provides information that can be used by physicians to prepare for their PPCD consultation with black men as well as develop a support system for black men at the PPCD.
Acknowledgments
The authors thank the men who participated in the CaPCaS study. They also thank the CaPCaS project community advisory board chairs (Mr Jim West, Dr Angela Adams, and Prince Oladapo Odedina) and all the CaPCaS project community advisory board members for their effort throughout the project. Finally, they rxecognize the effort of additional CaPCaS scientific team, especially the primary interviewer, Mr Kenneth Stokes. Weekly meeting support for this study was provided by the University of Florida MiCaRT Center, which is funded by the NIH-National Cancer Institute Award # 1P20CA192990-02. REDCaP software was supported by the UF Clinical and Translational Science Institute, which is funded in part by the NIH Clinical and Translational Science Award program (grants UL1TR001427, KL2TR001429 and TL1TR001428).
1. American Cancer Society. Cancer facts & figures 2016. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/. Published 2016. Accessed January 10, 2017.
2. Florida Cancer Data System. Florida Statewide Population-Based Cancer Registry. https://fcds.med.miami.edu/scripts/fcdspubrates/production/doSelection.aspx?election=map. Processed February 16, 2016.
3. Sinfield P, Baker R, Camosso-Stefinovic J, et al. Men’s and carers’ experiences of care for prostate cancer: a narrative literature review. Health Expect. 2009;12:301-312.
4. Maliski SL, Connor SE, Williams L, Litwin MS. Faith among low-income, African American/ black men treated for prostate cancer. Cancer Nurs. 2010;33(6):470-478.
5. Jones RA, Wenzel J, Hinton I, et el. Exploring cancer support needs for older African-American men with prostate cancer. Support Care Cancer. 2011;19(9):1411-1419.
6. Sinfield P, Baker R, Agarwal S, Tarrant C. Patient-centred care: what are the experiences of prostate cancer patients and their partners? Patient Educ Couns. 2008;73(1):91-96.
7. American Cancer Society. Cancer facts & figures for African Americans 2016-2018. http://www.cancer.org/research/cancerfactsstatistics/cancer-facts-figures-for-african-americans. Published 2016. Accessed January 10, 2017.
8. Patton MQ. Qualitative research & evaluation methods. 4th ed. Thousand Oaks, CA: Sage Publications; 2001.
9. Florida Department of Health, Bureau of Epidemiology. Procedure guide for studies that utilize patient identifiable data from the Florida Cancer Data System. http://www.fcds.med.miami.edu/downloads/datarequest/Procedure%20Guide_Revised%20
October%202007.pdf. Accessed July 24, 2010.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
11. Strauss A. Qualitative analysis for social scientists. New York, NY: Cambridge University Press; 1987.
12. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Chicago, IL: Aldine; 1967.
13. Miles MB, Huberman AM. Qualitative data analysis: an expanded sourcebook. 2nd ed. Thousand Oaks, CA: Sage Publications; 1994.
14. Nutbeam, D. Health literacy as a public health goal: a challenge for contemporary health education and communication strategies into the 21st century. Health Promot Int. 2000;15(3):259-267.
15. Powe BD, Finnie R. Cancer fatalism: the state of the science. Cancer Nurs. 2003;26:454-467.
16. Powe BD. Fatalism among elderly African Americans: effects on colorectal cancer screening. Cancer Nurs. 1995;18:285-392.
17. Powe BD. Cancer fatalism among elderly Caucasians and African Americans. Oncol Nurs Forum. 1995;22(9):1355-1359.
18. Thoresen CE. Spirituality, health, and science: the coming revival? In: Roth RS, Kurpius SR, eds. The emerging role of counseling psychology in health care. New York, NY: WW Norton; 1998.
19. Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56:267-283.
21. Odedina FT, Yu D, Akinremi TO, Reams RR, Freedman ML, Kumar N. Prostate cancer cognitive-behavioral factors in a West African population. J Immigr Minor Health. 2009;11(4):258-267.
22. Odedina FT, Scrivens JJ Jr, Larose-Pierre M, et al. Modifiable prostate cancer risk reduction and early detection behaviors in black men. Am J Health Behav. 2011;35(4):470-484.
As of 2016, Florida ranks second among all states in the United States in estimated new cases of prostate cancer and second in estimated deaths from prostate cancer.1 Disparities in diagnosis, mortality rates, and access to cancer care also continue to be a major problem in Florida, especially for black men. For example, black men were the only racial/ethnic group that did not meet the Healthy People (HP) 2010 objective to reduce the prostate cancer death rate to 28.2 per 100,000 men and that has not met the HP 2020 objective to reduce the prostate cancer death rate to 21.2 per 100,000 men (Table 1). Based on the 2013 prostate cancer mortality rates for Florida,2 the death rate for black men is almost twice the HP 2020 goal (37.49 per 100,000).
A diagnosis of prostate cancer is a life changing event for a man. In particular, there is limited research on the experiences and coping mechanisms of black men at diagnosis. This limited body of research indicates that black men’s reactions to their initial diagnoses varied, from being shocked when notified of their initial diagnosis of prostate cancer,3 to perceiving that they had received a “death sentence”.4 In regard to having to make decisions about their treatment options, some black men indicated that the information about treatment that they received from physicians decreased their anxiety,5 whereas others noted that they had not been given adequate information by a physician to make a decision.6 Patients have also reported that they felt as though they were not knowledgeable enough to ask questions concerning treatment options and preferred for the physician to make the treatment choice for them.6 Decisional regret is now a common observation among men who are not involved in making decisions about their treatment.3
According to the American Cancer Society, about 30,000 black men were diagnosed with prostate cancer in 2016.7 It is important to understand these men’s needs and help them cope effectively as they navigate the survivorship continuum. In line with our research program’s goal of ensuring quality cancer care for black men, the primary objective of this study was to explore the experiences and needs of black men at the point of prostate cancer diagnosis (PPCD). Specifically, we developed an interpretative framework for black men’s experiences at the PPCD, focusing on United States or native-born black men (NBBM) and Caribbean-born black men (CBBM). African-born black men were not included in this study because of the low sample size for that ethnicity. This study is part of a large-scale study that focuses on developing a model of prostate cancer care and survivorship (CaPCaS model) using grounded theory to study black, ethnically diverse prostate cancer survivors.
Methods
The study aims to close the prostate health disparity gap for black men in Florida through community engaged research in partnership with survivors of prostate cancer and their advocates. The current study was a prospective, grounded theory study that involved one-on-one, in-depth interviews with 31 prostate cancer patients about their care and survivorship experiences. Specifically, 17 NBBM and 14 CBBM were enrolled in the project. Appropriate human subjects review and approval were obtained from the University of Florida, the Florida Department of Health, and the Department of Defense.
Research design
This is a qualitative research study. Based on the principles of community engaged research and using a rigorous qualitative research methodology, we recruited NBBM and CBBM with a personal history of prostate cancer. Guided by open-ended questions developed by the team, one-on-one in-depth interviews were conducted with each participant in their home or at a convenient location in the community. Our primary focus was on the participants’ care and survivorship experiences, with primary focus on their prostate cancer diagnosis. Qualitative research was our methodology of choice because little is known about the PPCD experiences of black men.8 With qualitative research, we were able to get our participants to “relive” their experiences in the presence of a culturally competent, well-trained interviewer and elicit the information about their care and survivorship experiences based on their interpretation. In addition, we were able to capture the dynamic processes associated with their experiences, documenting sequential patterns and change through both verbal and nonverbal communications, because the participants were interviewed twice.
Research population and recruitment
The study setting was Florida. The inclusion criteria were: black men, personal history of prostate cancer, ability to complete two separate interviews with each one expected to last 2-3 hours, and flexibility to meet interviewers at a convenient community site for the interviews. Participants were identified through the Florida Cancer Data System (FCDS)9 database. At the time of the study, the most recent FCDS database was for 2010. The FCDS has collected the number of new cancer cancers diagnosed in the state of Florida annually since 1981. It is a comprehensive incidence-only registry and does not extract data on patients with a death certificate. All investigators are bounded by the confidential pledge required for the use of the FCDS data.
We used the Florida Department of Health’s (DoH’s) Bureau of Epidemiology standard procedure for the FCDS9 to recruit participants. Our recruitment strategies included: initial patient contact by written correspondence; second mailing that included a telephone opt-out card after 3 weeks for nonrespondents (the telephone opt-out card explained to the patient that if no response was received, the study investigator would attempt a telephone call to introduce the study); and a telephone call by a study staff to introduce the study for nonrespondents. As per the Florida DoH standard procedure, we did not disclose on the cover of the study mailings that the patient was being contacted for a study specific to cancer. Efforts to recruit a patient stopped immediately if a patient indicated that he did not wish to participate. All of the study staff making participant contact were extensively trained to provide a clear and accurate description of cancer registration in Florida. In addition, to assist the study staff in providing clear and accurate responses, responses to frequently asked questions were made available to the study staff. During the participant recruitment phase, anyone who seemed to be upset when contacted was reported immediately (within 24 hours) to the DoH cancer epidemiologist. In addition, the name of anyone who stated that he did not wish to be contacted again was given to the DoH so that the person would not be re-contacted.
Prescreening of participants for eligibility
All eligible participants who agreed to participate in the study comprised the pool of potential study participants. For those who agreed to participate, the following information was obtained by telephone interview using REDCap software:10 name and contact information, country of birth, age, marital status, and education level. The demographic information facilitated a purposeful systematic selection of black men of diverse age groups (younger than 50 years or older than 50 years), marital status (single, including divorced or separated, or married/in a relationship), and educational level (college degree or not college educated). An incentive of a $5 gift card was provided to all the men who participated in the screening phase. Using systematic sampling to ensure demographically diverse participants, 40 participants (20 NBBM, 20 CBBM) were selected from the initial pool of participants to participate in the study.
Data collection
The data collection was conducted by a trained Community Health Worker (CHW) using semi-structured interview process. The interview guide was constructed by the research team and the study community advisory board members to ensure language appropriateness, understanding and cultural sensitivity. For this study, the interview questions focused on participants’ background information and diagnosis history, including: participants’ personal story of diagnosis, feelings, emotions, reactions, regrets and level of personal/family/physician involvement in diagnosis. For the CBBM, we also obtained information on the age at which they immigrated to the United States. The CHW interviewer was trained to question participants and encourage them to elaborate on areas of importance to their experience.
A total time of about 5-6 hours was scheduled for the data collection per participant, which is sufficient for gathering in-depth perspectives. We scheduled two interviews lasting not more than 3 hours at a time so as not to create burden for study participants. Participants had the choice to have the interviews completed in a single session or spread out over 2 days. The interviews were audio-recorded to provide ease of transcription and back-up of data. At the end of the interviews, participants were compensated for participating in the study.
Data management and analyses
The study dataset included interview transcripts and field notes of the CHW interviewer describing his insights about the interviews. The data analyses included preparing and verifying the narrative data, coding data, and developing an interpretative framework for black men’s experiences at the PPCD. Interviews were transcribed verbatim by a professional transcription service that has policies in place for protected health information. Each transcript was then verified for accuracy by the CHW interviewer. The interview transcripts were imported directly into NVivo 11, a computer-assisted data analysis software that allows coding and modeling of complex narrative data. The data coding was conducted by our interdisciplinary team of clinicians, behavioral scientists, and social scientists. It is important to note that the NVivo 11 software was not used to analyze the data per se. However, it provided a sophisticated and systematic way to manage the following tasks for the analyses: organizing large quantities of narrative data, coding text, retrieving text by codes, querying the data, comparing sets of data interpretation between NBBM and CBBM; and developing analytic models. The study team members coded the data in weekly team meetings. The coding consisted of reading the data and identifying major themes, then assigning labels to and defining emerging categories.
Two levels of coding were used. The first, open coding, refers to an approach to data with no preconceived ideas about what will be found; and the second, focused or axial coding, refers to reviewing data for the purpose of more richly coding on a particular theme.11 We used dimensional analysis to ensure that each emerging concept was carefully defined. The study team went back and forth between the data and the emerging analytic framework, using constant comparison of new data with already coded data and new categories with previously analyzed text.12
To ensure trustworthiness and credibility,13 the study team maintained an audit trail that documented how and when analytic decisions were made. In addition, peer debriefing was conducted to ensure credibility, including the presentation of findings to the study community advisory board members as part of the community engaged research approach.
Results
Description of participants
The FCDS provided a database of 1,813 participants identified as black men diagnosed with prostate cancer in 2010. Because the FCDS does not extract data on patients with a death certificate, we found out during the pre-screening phase that a few of the men were deceased. In addition, there were a significant number of incorrect addresses. We obtained a total of 212 completed responses by phone during the prescreening phase. The majority of the participants were aged 60-69 years (48.2%), had a high school diploma only (26.1%), and were currently married (65.3%). Relative to ethnicity, 67% of participants classified themselves NBBM, 24% as CBBM, 3.5% as black men born in Africa, and 5.5% as Other/Don’t know/Refused. For the CBBM, the most common countries of birth were Jamaica, Haiti, and Guyana, respectively.
In all, 40 participants (20 NBBM and 20 CBBM) were selected from the 212 participants to participate in the study. Selection was conducted systematically to ensure representation in terms of age, marital status, education, and geographical location. Data saturation was achieved with 17 NBBM and 14 CBBM, after which we ended data collection (Table 2). Data saturation is the standard for deciding that we are not finding anything different from the interviews first coded and last coded. Although we were specifically looking for differences between the two groups (NBBM and CBBM), no between-group differences emerged. Each man’s experience was unique to him with some common themes emerging described hereinafter (Figure).
Moderating factors and experiences at PPCD
Some of the moderating factors that the study participants identified as affecting their reactions to the PPCD included health literacy, insurance status, spirituality, mistrust, prior experience with cancer, perceived susceptibility to cancer, and delay in diagnosis (Table 3). Health literacy, defined as personal, cognitive, and social skills that determine the ability of individuals to gain access to, understand, and use information to promote and maintain good health, was one of the moderating factors found in this study.13 Some of the black men came to the PPCD with a low level of health literacy, which had an impact on their understanding of the treatment options. For example, in the interview, participant 798 (NBBM) was confused about what tests had been done and was not able to accurately describe the treatments offered to him. Participant 1263 (CBBM) struggled to express the purpose and procedures associated with diagnostic biopsy. However, there were participants with a high level of health literacy (eg, participant 449 [NBBM]), who decided to research the disease.
Another factor to consider is the insurance status of participants at the PPCD. The majority of the participants had good insurance coverage, but some were affected by poor insurance coverage. Participant 1881(NBBM) made his treatment decision primarily on the basis of the pending lapse of his insurance coverage rather than the best clinical option for him. Participant 1979 (CBBM) described both his confusion on the screening tests and the impact of not having insurance coverage. Upon obtaining insurance coverage, he sought treatment for his prostate cancer with an urgency that he did not experience when he was first diagnosed when uninsured.
The spirituality of black men was another moderating factor at the PPCD. Participant 827 (NBBM) noted that he was unaffected when he received his diagnosis because he was a true believer. Some of the black men also came to the PPCD with lack of trust in the physician and/or the health care system and perceived a sense of contempt from the physician. Participant 1594 (NBBM) described mistrust based on the history of medical exploitation of black men as well as a perception of current discriminatory practices.
Another important PPCD status to note for black men is prior experience with cancer, including prior personal cancer history and/or prior cancer history of a family member. Participant 2024 (CBBM) described the meaning of cancer to him, while participant 798 (NBBM) echoed the despair of the cancer diagnosis based on experience with other cancers in the family. Sometimes there were multiple cancers in the family or even among the significant others of the participant, as was the case with Participant 1936 (CBBM).
Of greatest concern were men who delayed their diagnosis or treatment, perhaps resulting in their cancer being at a more advanced stage when they eventually did return for care. Finally, some of the men came to the PPCD appointment with a low expectation of receiving a diagnosis of prostate cancer, whereas others came to the PPCD fearful of the results of their testing.
In describing their experiences, participants expressed both positive and negative experiences: on the positive side, they found the information provided by the physician to be helpful; but on the negative side, the sterile or medically focused encounter was perceived as a lack empathy on the part of the physician.
Cognitive, emotional, and behavioral coping experiences
As expected, there were ranges of emotions, including shock, disbelief and denial (Table 4). Some of the men questioned why this (the cancer) was happening to them when they had done “nothing” to deserve it. Doing nothing in this case meant that they had lived a healthy lifestyle with no obvious apparent cause to have the cancer. Fear and cancer fatalism were experienced by a significant number of the men, with their thoughts immediately turning to death and dying. This was especially the case for men who had lost a loved one to cancer. Conversely, some of the men wanted immediate resolution, focusing instead on ways to beat the cancer and with a strong will to live.
Reliance on faith was a big part of coping at the PPCD. Some of the men drew strength from their faith to get them through their cancer journey. Others found a way to accept the diagnosis – one participant accepted the diagnosis and the fact that this could mean dying (after living a good life), whereas another participant accepted the diagnosis with the hope that he would find a cure. Hope was more realistic with the knowledge that other men had survived prostate cancer.
Reflecting back on their experiences, the men also identified clear needs at the PPCD. One of the needs they identified was having a physician they were comfortable with to discuss their diagnosis. Another need was for a second opinion. Participant 1594 (NBBM) advised that it was important for black men to take control by requesting a second opinion. Participant 2039 (NBBM) described a feeling of navigating blindly and trying to find answers that would be helpful to him in his cancer journey. However, his experience with a second opinion was not helpful because the second physician was at the same clinic as his primary physician. His recommendation was to get a second opinion at a different clinic or center. Another important need was emotional support at the PPCD. Participant 2024 (CBBM) made a strong case for emotional support, especially for men who are not accompanied during diagnosis. In addition, Participant 2024’s (CBBM) reflections underscore the fact that the PPCD may not be an ideal place or time to discuss treatment options. With the range of emotions that the men go through at the PPCD, it is difficult to comprehend any follow-up discussions after hearing the words “you have prostate cancer.” Participant 2024 (CBBM) also strongly expressed that men need time to deal with the diagnosis at the PPCD.
Discussion
The primary goal of this study was to develop an interpretative framework of black men’s experiences at the PPCD. The Figure provides a pictorial summary of the framework. Study results indicated that black men come to the PPCD with different emotions and different experiences. Although the majority of the men were NBBM, there is a significantly increasing number of foreign-born black men receiving a diagnosis of prostate cancer in the United States. Given that black men carry a disproportionate burden of the disease, with a significantly higher incidence compared with any other racial group, it is important that tailored services are provided to black men at the PPCD.
We also found that black men came to the PPCD diverse in terms of their ethnicity, health literacy, spirituality, trust in health care system/physician, prior experience with cancer, perceived susceptibility to cancer, delayed time for diagnosis, and fear of diagnosis. Of importance for physicians is that the black race is not homogeneous. There is a significant number of foreign-born blacks at the PPCD, and they often have different cultural beliefs and values compared with NBBM. In addition, some of the foreign-born black men may not have English proficiency and may need a medical interpreter during the PPCD consultation. In addition, a patient’s pre-existing lack of trust in the health care system may have a negative impact on the PPCD consultation. It is thus important that the physician takes the time to instill trust and make the men comfortable during the PPCD consultation.
For some of the men who had fear of a prostate cancer diagnosis and/or prior experience with cancer, cancer fatalism was experienced at the PPCD. Cancer fatalism, defined as an individual’s belief that death is bound to happen when diagnosed with cancer, has been documented as a major barrier to cancer detection and control.15 For example, fatalistic perspectives have been reported to affect cervical cancer,16 breast cancer,17,18 colorectal cancer,19 and prostate cancer20,21 among blacks. It is thus important to effectively address fatalistic beliefs when a man is diagnosed with prostate cancer.
Other emotions at the PPCD that may affect effective treatment decision making also need to be addressed immediately. For example, the emotions of fear, denial, and feeling overwhelmed are potential barriers to timely treatment decision making. Psycho-oncology interventions to appropriately deal with these emotions at PPCD or right after the diagnosis may be crucial for the men. In particular, a group-based psychosocial intervention focusing on: provision of education about treatment options for prostate cancer and their acute and late effects; negotiating treatment and treatment side effects; enhancing communication with treatment providers; managing distress; and engaging positive family- and community-based social support to optimize emotional, behavioral, social, and physical outcomes in black men with prostate cancer.
In addition to having physicians make them comfortable at PPCD, the PPCD needs expressed by participants included having time to come to terms with the diagnosis and receiving psycho-oncology/emotional support. Anyone who has just received a diagnosis of cancer cannot be expected to immediately continue to function as he did before the PPCD. This is especially difficult for men who are alone at the PPCD. Nevertheless, it is expected that they will listen attentively and understand subsequent consultation by the physician, then leave the consultation room almost immediately, and be able drive home or back to work right after the diagnosis. There seems to be a support gap that needs to be closed at the PPCD. Providing the men with immediate support to cope with the diagnosis may make a significant difference in effective treatment choices and eliminating treatment decisional regrets.
Methodological rigor was established through purposeful sampling, extended time with participants, standardized procedures for data collection, management and analysis, multidisciplinary interpretation, and validation of results with the community advisory board. Because the research participants were purposefully selected from a statewide database of black men diagnosed with CaP, generalizability of findings to the two target groups of NBBM and CBBM can be assumed, with the caveat that men with different experiences may have chosen not to respond to recruitment efforts or refused participation. Black men who were not sufficiently fluent in English to be interviewed were also excluded and are not represented in these findings. Black men of other nativity (including African-born black men) and residing outside of Florida were also not represented.
In conclusion, the PPCD interpretative framework developed in this study, describes the status of black men at the PPCD, their experiences during the PPCD, and their needs at the PPCD. The framework provides information that can be used by physicians to prepare for their PPCD consultation with black men as well as develop a support system for black men at the PPCD.
Acknowledgments
The authors thank the men who participated in the CaPCaS study. They also thank the CaPCaS project community advisory board chairs (Mr Jim West, Dr Angela Adams, and Prince Oladapo Odedina) and all the CaPCaS project community advisory board members for their effort throughout the project. Finally, they rxecognize the effort of additional CaPCaS scientific team, especially the primary interviewer, Mr Kenneth Stokes. Weekly meeting support for this study was provided by the University of Florida MiCaRT Center, which is funded by the NIH-National Cancer Institute Award # 1P20CA192990-02. REDCaP software was supported by the UF Clinical and Translational Science Institute, which is funded in part by the NIH Clinical and Translational Science Award program (grants UL1TR001427, KL2TR001429 and TL1TR001428).
As of 2016, Florida ranks second among all states in the United States in estimated new cases of prostate cancer and second in estimated deaths from prostate cancer.1 Disparities in diagnosis, mortality rates, and access to cancer care also continue to be a major problem in Florida, especially for black men. For example, black men were the only racial/ethnic group that did not meet the Healthy People (HP) 2010 objective to reduce the prostate cancer death rate to 28.2 per 100,000 men and that has not met the HP 2020 objective to reduce the prostate cancer death rate to 21.2 per 100,000 men (Table 1). Based on the 2013 prostate cancer mortality rates for Florida,2 the death rate for black men is almost twice the HP 2020 goal (37.49 per 100,000).
A diagnosis of prostate cancer is a life changing event for a man. In particular, there is limited research on the experiences and coping mechanisms of black men at diagnosis. This limited body of research indicates that black men’s reactions to their initial diagnoses varied, from being shocked when notified of their initial diagnosis of prostate cancer,3 to perceiving that they had received a “death sentence”.4 In regard to having to make decisions about their treatment options, some black men indicated that the information about treatment that they received from physicians decreased their anxiety,5 whereas others noted that they had not been given adequate information by a physician to make a decision.6 Patients have also reported that they felt as though they were not knowledgeable enough to ask questions concerning treatment options and preferred for the physician to make the treatment choice for them.6 Decisional regret is now a common observation among men who are not involved in making decisions about their treatment.3
According to the American Cancer Society, about 30,000 black men were diagnosed with prostate cancer in 2016.7 It is important to understand these men’s needs and help them cope effectively as they navigate the survivorship continuum. In line with our research program’s goal of ensuring quality cancer care for black men, the primary objective of this study was to explore the experiences and needs of black men at the point of prostate cancer diagnosis (PPCD). Specifically, we developed an interpretative framework for black men’s experiences at the PPCD, focusing on United States or native-born black men (NBBM) and Caribbean-born black men (CBBM). African-born black men were not included in this study because of the low sample size for that ethnicity. This study is part of a large-scale study that focuses on developing a model of prostate cancer care and survivorship (CaPCaS model) using grounded theory to study black, ethnically diverse prostate cancer survivors.
Methods
The study aims to close the prostate health disparity gap for black men in Florida through community engaged research in partnership with survivors of prostate cancer and their advocates. The current study was a prospective, grounded theory study that involved one-on-one, in-depth interviews with 31 prostate cancer patients about their care and survivorship experiences. Specifically, 17 NBBM and 14 CBBM were enrolled in the project. Appropriate human subjects review and approval were obtained from the University of Florida, the Florida Department of Health, and the Department of Defense.
Research design
This is a qualitative research study. Based on the principles of community engaged research and using a rigorous qualitative research methodology, we recruited NBBM and CBBM with a personal history of prostate cancer. Guided by open-ended questions developed by the team, one-on-one in-depth interviews were conducted with each participant in their home or at a convenient location in the community. Our primary focus was on the participants’ care and survivorship experiences, with primary focus on their prostate cancer diagnosis. Qualitative research was our methodology of choice because little is known about the PPCD experiences of black men.8 With qualitative research, we were able to get our participants to “relive” their experiences in the presence of a culturally competent, well-trained interviewer and elicit the information about their care and survivorship experiences based on their interpretation. In addition, we were able to capture the dynamic processes associated with their experiences, documenting sequential patterns and change through both verbal and nonverbal communications, because the participants were interviewed twice.
Research population and recruitment
The study setting was Florida. The inclusion criteria were: black men, personal history of prostate cancer, ability to complete two separate interviews with each one expected to last 2-3 hours, and flexibility to meet interviewers at a convenient community site for the interviews. Participants were identified through the Florida Cancer Data System (FCDS)9 database. At the time of the study, the most recent FCDS database was for 2010. The FCDS has collected the number of new cancer cancers diagnosed in the state of Florida annually since 1981. It is a comprehensive incidence-only registry and does not extract data on patients with a death certificate. All investigators are bounded by the confidential pledge required for the use of the FCDS data.
We used the Florida Department of Health’s (DoH’s) Bureau of Epidemiology standard procedure for the FCDS9 to recruit participants. Our recruitment strategies included: initial patient contact by written correspondence; second mailing that included a telephone opt-out card after 3 weeks for nonrespondents (the telephone opt-out card explained to the patient that if no response was received, the study investigator would attempt a telephone call to introduce the study); and a telephone call by a study staff to introduce the study for nonrespondents. As per the Florida DoH standard procedure, we did not disclose on the cover of the study mailings that the patient was being contacted for a study specific to cancer. Efforts to recruit a patient stopped immediately if a patient indicated that he did not wish to participate. All of the study staff making participant contact were extensively trained to provide a clear and accurate description of cancer registration in Florida. In addition, to assist the study staff in providing clear and accurate responses, responses to frequently asked questions were made available to the study staff. During the participant recruitment phase, anyone who seemed to be upset when contacted was reported immediately (within 24 hours) to the DoH cancer epidemiologist. In addition, the name of anyone who stated that he did not wish to be contacted again was given to the DoH so that the person would not be re-contacted.
Prescreening of participants for eligibility
All eligible participants who agreed to participate in the study comprised the pool of potential study participants. For those who agreed to participate, the following information was obtained by telephone interview using REDCap software:10 name and contact information, country of birth, age, marital status, and education level. The demographic information facilitated a purposeful systematic selection of black men of diverse age groups (younger than 50 years or older than 50 years), marital status (single, including divorced or separated, or married/in a relationship), and educational level (college degree or not college educated). An incentive of a $5 gift card was provided to all the men who participated in the screening phase. Using systematic sampling to ensure demographically diverse participants, 40 participants (20 NBBM, 20 CBBM) were selected from the initial pool of participants to participate in the study.
Data collection
The data collection was conducted by a trained Community Health Worker (CHW) using semi-structured interview process. The interview guide was constructed by the research team and the study community advisory board members to ensure language appropriateness, understanding and cultural sensitivity. For this study, the interview questions focused on participants’ background information and diagnosis history, including: participants’ personal story of diagnosis, feelings, emotions, reactions, regrets and level of personal/family/physician involvement in diagnosis. For the CBBM, we also obtained information on the age at which they immigrated to the United States. The CHW interviewer was trained to question participants and encourage them to elaborate on areas of importance to their experience.
A total time of about 5-6 hours was scheduled for the data collection per participant, which is sufficient for gathering in-depth perspectives. We scheduled two interviews lasting not more than 3 hours at a time so as not to create burden for study participants. Participants had the choice to have the interviews completed in a single session or spread out over 2 days. The interviews were audio-recorded to provide ease of transcription and back-up of data. At the end of the interviews, participants were compensated for participating in the study.
Data management and analyses
The study dataset included interview transcripts and field notes of the CHW interviewer describing his insights about the interviews. The data analyses included preparing and verifying the narrative data, coding data, and developing an interpretative framework for black men’s experiences at the PPCD. Interviews were transcribed verbatim by a professional transcription service that has policies in place for protected health information. Each transcript was then verified for accuracy by the CHW interviewer. The interview transcripts were imported directly into NVivo 11, a computer-assisted data analysis software that allows coding and modeling of complex narrative data. The data coding was conducted by our interdisciplinary team of clinicians, behavioral scientists, and social scientists. It is important to note that the NVivo 11 software was not used to analyze the data per se. However, it provided a sophisticated and systematic way to manage the following tasks for the analyses: organizing large quantities of narrative data, coding text, retrieving text by codes, querying the data, comparing sets of data interpretation between NBBM and CBBM; and developing analytic models. The study team members coded the data in weekly team meetings. The coding consisted of reading the data and identifying major themes, then assigning labels to and defining emerging categories.
Two levels of coding were used. The first, open coding, refers to an approach to data with no preconceived ideas about what will be found; and the second, focused or axial coding, refers to reviewing data for the purpose of more richly coding on a particular theme.11 We used dimensional analysis to ensure that each emerging concept was carefully defined. The study team went back and forth between the data and the emerging analytic framework, using constant comparison of new data with already coded data and new categories with previously analyzed text.12
To ensure trustworthiness and credibility,13 the study team maintained an audit trail that documented how and when analytic decisions were made. In addition, peer debriefing was conducted to ensure credibility, including the presentation of findings to the study community advisory board members as part of the community engaged research approach.
Results
Description of participants
The FCDS provided a database of 1,813 participants identified as black men diagnosed with prostate cancer in 2010. Because the FCDS does not extract data on patients with a death certificate, we found out during the pre-screening phase that a few of the men were deceased. In addition, there were a significant number of incorrect addresses. We obtained a total of 212 completed responses by phone during the prescreening phase. The majority of the participants were aged 60-69 years (48.2%), had a high school diploma only (26.1%), and were currently married (65.3%). Relative to ethnicity, 67% of participants classified themselves NBBM, 24% as CBBM, 3.5% as black men born in Africa, and 5.5% as Other/Don’t know/Refused. For the CBBM, the most common countries of birth were Jamaica, Haiti, and Guyana, respectively.
In all, 40 participants (20 NBBM and 20 CBBM) were selected from the 212 participants to participate in the study. Selection was conducted systematically to ensure representation in terms of age, marital status, education, and geographical location. Data saturation was achieved with 17 NBBM and 14 CBBM, after which we ended data collection (Table 2). Data saturation is the standard for deciding that we are not finding anything different from the interviews first coded and last coded. Although we were specifically looking for differences between the two groups (NBBM and CBBM), no between-group differences emerged. Each man’s experience was unique to him with some common themes emerging described hereinafter (Figure).
Moderating factors and experiences at PPCD
Some of the moderating factors that the study participants identified as affecting their reactions to the PPCD included health literacy, insurance status, spirituality, mistrust, prior experience with cancer, perceived susceptibility to cancer, and delay in diagnosis (Table 3). Health literacy, defined as personal, cognitive, and social skills that determine the ability of individuals to gain access to, understand, and use information to promote and maintain good health, was one of the moderating factors found in this study.13 Some of the black men came to the PPCD with a low level of health literacy, which had an impact on their understanding of the treatment options. For example, in the interview, participant 798 (NBBM) was confused about what tests had been done and was not able to accurately describe the treatments offered to him. Participant 1263 (CBBM) struggled to express the purpose and procedures associated with diagnostic biopsy. However, there were participants with a high level of health literacy (eg, participant 449 [NBBM]), who decided to research the disease.
Another factor to consider is the insurance status of participants at the PPCD. The majority of the participants had good insurance coverage, but some were affected by poor insurance coverage. Participant 1881(NBBM) made his treatment decision primarily on the basis of the pending lapse of his insurance coverage rather than the best clinical option for him. Participant 1979 (CBBM) described both his confusion on the screening tests and the impact of not having insurance coverage. Upon obtaining insurance coverage, he sought treatment for his prostate cancer with an urgency that he did not experience when he was first diagnosed when uninsured.
The spirituality of black men was another moderating factor at the PPCD. Participant 827 (NBBM) noted that he was unaffected when he received his diagnosis because he was a true believer. Some of the black men also came to the PPCD with lack of trust in the physician and/or the health care system and perceived a sense of contempt from the physician. Participant 1594 (NBBM) described mistrust based on the history of medical exploitation of black men as well as a perception of current discriminatory practices.
Another important PPCD status to note for black men is prior experience with cancer, including prior personal cancer history and/or prior cancer history of a family member. Participant 2024 (CBBM) described the meaning of cancer to him, while participant 798 (NBBM) echoed the despair of the cancer diagnosis based on experience with other cancers in the family. Sometimes there were multiple cancers in the family or even among the significant others of the participant, as was the case with Participant 1936 (CBBM).
Of greatest concern were men who delayed their diagnosis or treatment, perhaps resulting in their cancer being at a more advanced stage when they eventually did return for care. Finally, some of the men came to the PPCD appointment with a low expectation of receiving a diagnosis of prostate cancer, whereas others came to the PPCD fearful of the results of their testing.
In describing their experiences, participants expressed both positive and negative experiences: on the positive side, they found the information provided by the physician to be helpful; but on the negative side, the sterile or medically focused encounter was perceived as a lack empathy on the part of the physician.
Cognitive, emotional, and behavioral coping experiences
As expected, there were ranges of emotions, including shock, disbelief and denial (Table 4). Some of the men questioned why this (the cancer) was happening to them when they had done “nothing” to deserve it. Doing nothing in this case meant that they had lived a healthy lifestyle with no obvious apparent cause to have the cancer. Fear and cancer fatalism were experienced by a significant number of the men, with their thoughts immediately turning to death and dying. This was especially the case for men who had lost a loved one to cancer. Conversely, some of the men wanted immediate resolution, focusing instead on ways to beat the cancer and with a strong will to live.
Reliance on faith was a big part of coping at the PPCD. Some of the men drew strength from their faith to get them through their cancer journey. Others found a way to accept the diagnosis – one participant accepted the diagnosis and the fact that this could mean dying (after living a good life), whereas another participant accepted the diagnosis with the hope that he would find a cure. Hope was more realistic with the knowledge that other men had survived prostate cancer.
Reflecting back on their experiences, the men also identified clear needs at the PPCD. One of the needs they identified was having a physician they were comfortable with to discuss their diagnosis. Another need was for a second opinion. Participant 1594 (NBBM) advised that it was important for black men to take control by requesting a second opinion. Participant 2039 (NBBM) described a feeling of navigating blindly and trying to find answers that would be helpful to him in his cancer journey. However, his experience with a second opinion was not helpful because the second physician was at the same clinic as his primary physician. His recommendation was to get a second opinion at a different clinic or center. Another important need was emotional support at the PPCD. Participant 2024 (CBBM) made a strong case for emotional support, especially for men who are not accompanied during diagnosis. In addition, Participant 2024’s (CBBM) reflections underscore the fact that the PPCD may not be an ideal place or time to discuss treatment options. With the range of emotions that the men go through at the PPCD, it is difficult to comprehend any follow-up discussions after hearing the words “you have prostate cancer.” Participant 2024 (CBBM) also strongly expressed that men need time to deal with the diagnosis at the PPCD.
Discussion
The primary goal of this study was to develop an interpretative framework of black men’s experiences at the PPCD. The Figure provides a pictorial summary of the framework. Study results indicated that black men come to the PPCD with different emotions and different experiences. Although the majority of the men were NBBM, there is a significantly increasing number of foreign-born black men receiving a diagnosis of prostate cancer in the United States. Given that black men carry a disproportionate burden of the disease, with a significantly higher incidence compared with any other racial group, it is important that tailored services are provided to black men at the PPCD.
We also found that black men came to the PPCD diverse in terms of their ethnicity, health literacy, spirituality, trust in health care system/physician, prior experience with cancer, perceived susceptibility to cancer, delayed time for diagnosis, and fear of diagnosis. Of importance for physicians is that the black race is not homogeneous. There is a significant number of foreign-born blacks at the PPCD, and they often have different cultural beliefs and values compared with NBBM. In addition, some of the foreign-born black men may not have English proficiency and may need a medical interpreter during the PPCD consultation. In addition, a patient’s pre-existing lack of trust in the health care system may have a negative impact on the PPCD consultation. It is thus important that the physician takes the time to instill trust and make the men comfortable during the PPCD consultation.
For some of the men who had fear of a prostate cancer diagnosis and/or prior experience with cancer, cancer fatalism was experienced at the PPCD. Cancer fatalism, defined as an individual’s belief that death is bound to happen when diagnosed with cancer, has been documented as a major barrier to cancer detection and control.15 For example, fatalistic perspectives have been reported to affect cervical cancer,16 breast cancer,17,18 colorectal cancer,19 and prostate cancer20,21 among blacks. It is thus important to effectively address fatalistic beliefs when a man is diagnosed with prostate cancer.
Other emotions at the PPCD that may affect effective treatment decision making also need to be addressed immediately. For example, the emotions of fear, denial, and feeling overwhelmed are potential barriers to timely treatment decision making. Psycho-oncology interventions to appropriately deal with these emotions at PPCD or right after the diagnosis may be crucial for the men. In particular, a group-based psychosocial intervention focusing on: provision of education about treatment options for prostate cancer and their acute and late effects; negotiating treatment and treatment side effects; enhancing communication with treatment providers; managing distress; and engaging positive family- and community-based social support to optimize emotional, behavioral, social, and physical outcomes in black men with prostate cancer.
In addition to having physicians make them comfortable at PPCD, the PPCD needs expressed by participants included having time to come to terms with the diagnosis and receiving psycho-oncology/emotional support. Anyone who has just received a diagnosis of cancer cannot be expected to immediately continue to function as he did before the PPCD. This is especially difficult for men who are alone at the PPCD. Nevertheless, it is expected that they will listen attentively and understand subsequent consultation by the physician, then leave the consultation room almost immediately, and be able drive home or back to work right after the diagnosis. There seems to be a support gap that needs to be closed at the PPCD. Providing the men with immediate support to cope with the diagnosis may make a significant difference in effective treatment choices and eliminating treatment decisional regrets.
Methodological rigor was established through purposeful sampling, extended time with participants, standardized procedures for data collection, management and analysis, multidisciplinary interpretation, and validation of results with the community advisory board. Because the research participants were purposefully selected from a statewide database of black men diagnosed with CaP, generalizability of findings to the two target groups of NBBM and CBBM can be assumed, with the caveat that men with different experiences may have chosen not to respond to recruitment efforts or refused participation. Black men who were not sufficiently fluent in English to be interviewed were also excluded and are not represented in these findings. Black men of other nativity (including African-born black men) and residing outside of Florida were also not represented.
In conclusion, the PPCD interpretative framework developed in this study, describes the status of black men at the PPCD, their experiences during the PPCD, and their needs at the PPCD. The framework provides information that can be used by physicians to prepare for their PPCD consultation with black men as well as develop a support system for black men at the PPCD.
Acknowledgments
The authors thank the men who participated in the CaPCaS study. They also thank the CaPCaS project community advisory board chairs (Mr Jim West, Dr Angela Adams, and Prince Oladapo Odedina) and all the CaPCaS project community advisory board members for their effort throughout the project. Finally, they rxecognize the effort of additional CaPCaS scientific team, especially the primary interviewer, Mr Kenneth Stokes. Weekly meeting support for this study was provided by the University of Florida MiCaRT Center, which is funded by the NIH-National Cancer Institute Award # 1P20CA192990-02. REDCaP software was supported by the UF Clinical and Translational Science Institute, which is funded in part by the NIH Clinical and Translational Science Award program (grants UL1TR001427, KL2TR001429 and TL1TR001428).
1. American Cancer Society. Cancer facts & figures 2016. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/. Published 2016. Accessed January 10, 2017.
2. Florida Cancer Data System. Florida Statewide Population-Based Cancer Registry. https://fcds.med.miami.edu/scripts/fcdspubrates/production/doSelection.aspx?election=map. Processed February 16, 2016.
3. Sinfield P, Baker R, Camosso-Stefinovic J, et al. Men’s and carers’ experiences of care for prostate cancer: a narrative literature review. Health Expect. 2009;12:301-312.
4. Maliski SL, Connor SE, Williams L, Litwin MS. Faith among low-income, African American/ black men treated for prostate cancer. Cancer Nurs. 2010;33(6):470-478.
5. Jones RA, Wenzel J, Hinton I, et el. Exploring cancer support needs for older African-American men with prostate cancer. Support Care Cancer. 2011;19(9):1411-1419.
6. Sinfield P, Baker R, Agarwal S, Tarrant C. Patient-centred care: what are the experiences of prostate cancer patients and their partners? Patient Educ Couns. 2008;73(1):91-96.
7. American Cancer Society. Cancer facts & figures for African Americans 2016-2018. http://www.cancer.org/research/cancerfactsstatistics/cancer-facts-figures-for-african-americans. Published 2016. Accessed January 10, 2017.
8. Patton MQ. Qualitative research & evaluation methods. 4th ed. Thousand Oaks, CA: Sage Publications; 2001.
9. Florida Department of Health, Bureau of Epidemiology. Procedure guide for studies that utilize patient identifiable data from the Florida Cancer Data System. http://www.fcds.med.miami.edu/downloads/datarequest/Procedure%20Guide_Revised%20
October%202007.pdf. Accessed July 24, 2010.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
11. Strauss A. Qualitative analysis for social scientists. New York, NY: Cambridge University Press; 1987.
12. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Chicago, IL: Aldine; 1967.
13. Miles MB, Huberman AM. Qualitative data analysis: an expanded sourcebook. 2nd ed. Thousand Oaks, CA: Sage Publications; 1994.
14. Nutbeam, D. Health literacy as a public health goal: a challenge for contemporary health education and communication strategies into the 21st century. Health Promot Int. 2000;15(3):259-267.
15. Powe BD, Finnie R. Cancer fatalism: the state of the science. Cancer Nurs. 2003;26:454-467.
16. Powe BD. Fatalism among elderly African Americans: effects on colorectal cancer screening. Cancer Nurs. 1995;18:285-392.
17. Powe BD. Cancer fatalism among elderly Caucasians and African Americans. Oncol Nurs Forum. 1995;22(9):1355-1359.
18. Thoresen CE. Spirituality, health, and science: the coming revival? In: Roth RS, Kurpius SR, eds. The emerging role of counseling psychology in health care. New York, NY: WW Norton; 1998.
19. Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56:267-283.
21. Odedina FT, Yu D, Akinremi TO, Reams RR, Freedman ML, Kumar N. Prostate cancer cognitive-behavioral factors in a West African population. J Immigr Minor Health. 2009;11(4):258-267.
22. Odedina FT, Scrivens JJ Jr, Larose-Pierre M, et al. Modifiable prostate cancer risk reduction and early detection behaviors in black men. Am J Health Behav. 2011;35(4):470-484.
1. American Cancer Society. Cancer facts & figures 2016. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/. Published 2016. Accessed January 10, 2017.
2. Florida Cancer Data System. Florida Statewide Population-Based Cancer Registry. https://fcds.med.miami.edu/scripts/fcdspubrates/production/doSelection.aspx?election=map. Processed February 16, 2016.
3. Sinfield P, Baker R, Camosso-Stefinovic J, et al. Men’s and carers’ experiences of care for prostate cancer: a narrative literature review. Health Expect. 2009;12:301-312.
4. Maliski SL, Connor SE, Williams L, Litwin MS. Faith among low-income, African American/ black men treated for prostate cancer. Cancer Nurs. 2010;33(6):470-478.
5. Jones RA, Wenzel J, Hinton I, et el. Exploring cancer support needs for older African-American men with prostate cancer. Support Care Cancer. 2011;19(9):1411-1419.
6. Sinfield P, Baker R, Agarwal S, Tarrant C. Patient-centred care: what are the experiences of prostate cancer patients and their partners? Patient Educ Couns. 2008;73(1):91-96.
7. American Cancer Society. Cancer facts & figures for African Americans 2016-2018. http://www.cancer.org/research/cancerfactsstatistics/cancer-facts-figures-for-african-americans. Published 2016. Accessed January 10, 2017.
8. Patton MQ. Qualitative research & evaluation methods. 4th ed. Thousand Oaks, CA: Sage Publications; 2001.
9. Florida Department of Health, Bureau of Epidemiology. Procedure guide for studies that utilize patient identifiable data from the Florida Cancer Data System. http://www.fcds.med.miami.edu/downloads/datarequest/Procedure%20Guide_Revised%20
October%202007.pdf. Accessed July 24, 2010.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
11. Strauss A. Qualitative analysis for social scientists. New York, NY: Cambridge University Press; 1987.
12. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Chicago, IL: Aldine; 1967.
13. Miles MB, Huberman AM. Qualitative data analysis: an expanded sourcebook. 2nd ed. Thousand Oaks, CA: Sage Publications; 1994.
14. Nutbeam, D. Health literacy as a public health goal: a challenge for contemporary health education and communication strategies into the 21st century. Health Promot Int. 2000;15(3):259-267.
15. Powe BD, Finnie R. Cancer fatalism: the state of the science. Cancer Nurs. 2003;26:454-467.
16. Powe BD. Fatalism among elderly African Americans: effects on colorectal cancer screening. Cancer Nurs. 1995;18:285-392.
17. Powe BD. Cancer fatalism among elderly Caucasians and African Americans. Oncol Nurs Forum. 1995;22(9):1355-1359.
18. Thoresen CE. Spirituality, health, and science: the coming revival? In: Roth RS, Kurpius SR, eds. The emerging role of counseling psychology in health care. New York, NY: WW Norton; 1998.
19. Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56:267-283.
21. Odedina FT, Yu D, Akinremi TO, Reams RR, Freedman ML, Kumar N. Prostate cancer cognitive-behavioral factors in a West African population. J Immigr Minor Health. 2009;11(4):258-267.
22. Odedina FT, Scrivens JJ Jr, Larose-Pierre M, et al. Modifiable prostate cancer risk reduction and early detection behaviors in black men. Am J Health Behav. 2011;35(4):470-484.
Posttreatment survivorship care needs of Spanish-speaking Latinas with breast cancer
After treatment, cancer patients transition to a survivorship phase, often with little information or support. Cancer survivors are at increased risk of recurrence, secondary cancers, comorbid conditions, and late treatment effects.1,2 However, many remain unaware of these risks and the options for managing them3 and face numerous unmet medical, psychosocial, and informational needs that can be addressed through survivorship care programs.4 Anxiety may increase as they lose their treatment team’s support while attempting to reestablish their lives.2 Patients need to know the long-term risks of cancer treatments, probabilities of recurrence and second cancers, effectiveness of surveillance and interventions for managing late effects and psychosocial concerns, and benefits of healthy lifestyles.2
Due to sociocultural and economic factors, Spanish-speaking Latina breast cancer survivors (SSBCS) suffer worse posttreatment health-related quality of life and more pain, fatigue, depressive symptoms, body image issues, and distress than their white counterparts.5-7 However, they are less likely to receive necessary cancer treatment, symptom management, and surveillance. For example, compared with whites, Latina breast cancer survivors receive less guideline-adherent treatment8 and follow-up care, including survivorship information.3,9 SSBCS, in particular have less access to survivorship information.10 Consequently, SSBCS are more likely to report unmet symptom management needs.11
Several breast cancer survivorship program trials have included Latinas,12,13 but their effectiveness has been demonstrated only for depressive symptoms or health worry. A comprehensive assessment of the posttreatment needs of SSBCS would provide a foundation for designing tailored survivorship interventions for this vulnerable group. This study aimed to identify the symptom management, psychosocial, and informational needs of SSBCS during the transition to survivorship from the perspectives of SSBCS and their cancer support providers and cancer physicians.
Methods
We sampled respondents within a 5-county area in Northern California to obtain multiple perspectives of the survivorship care needs of SSBCS using structured and in-depth methods: a telephone survey of SSBCS; semistructured interviews with SSBCS; semistructured interviews with cancer support providers serving SSBCS; and semistructured interviews with physicians providing cancer care for SSBCS. The study protocol was approved by the University of California San Francisco Committee on Human Research.
Sample and procedures
Structured telephone survey with SSBCS. The sample was drawn evenly from San Francisco General Hospital-University of California San Francisco primary care practices and SSBCS from a previous study who agreed to be re-contacted.14 The inclusion criteria were: completed active treatment (except adjuvant hormonal therapy) for nonmetastatic breast cancer within 10 years; living in one of the five counties; primarily Spanish-speaking; and self-identified as Latina. The exclusion criteria were: previous cancer except nonmelanoma skin cancer; terminal illness; or metastatic breast cancer. Study staff mailed potential participants a bilingual letter and information sheet, and bilingual opt-out postcard (6th grade reading level assessed by Flesch-Kincaid grade level statistic). Female bilingual-bicultural research associates conducted interviews of 20-30 minutes in Spanish after obtaining verbal consent. Participants were mailed $20. Surveys were conducted during March-November 2014.
Semistructured in-person interviews with SSBCS. Four community-based organizations (CBOs) in the targeted area providing cancer support services to Latinos agreed to recruit SSBCS for interviews. Inclusion criteria were identical to the survey. Patient navigators or support providers from CBOs contacted women by phone or in-person to invite them to an interview to assess their cancer survivorship needs. Women could choose a focus group or individual interview. With permission, names and contact information were given to study interviewers who called, explained the study, screened for eligibility, and scheduled an interview.
Recruitment was stratified by age (under or over age 50). We sampled women until saturation was achieved (no new themes emerged). Focus groups (90 minutes) were conducted at the CBOs. Individual interviews (45 minutes) were conducted in participants’ homes. Written informed consent was obtained. Participants were paid $50. Interviews were conducted during August-November, 2014, audiotaped, and transcribed.
Semistructured in-person interviews with cancer support providers and physicians. Investigators invited five cancer support providers (three patient navigators from three county hospitals, and two CBO directors of cancer psychosocial support services) and four physicians (three oncologists and one breast cancer surgeon from three county hospitals) to an in-person interview to identify SSBCS’ survivorship care needs. All agreed to participate. No further candidates were approached because saturation was achieved. We obtained written informed consent and 30-minute interviews were conducted in participants’ offices during August-October, 2014. Interviews were audiotaped and transcribed. Participants were paid $50.
Ethical approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Measures
Structured telephone survey. Based on cancer survivor needs assessments,15 we assessed: physical and emotional symptoms; problems with sleep and memory/concentration; concerns about mortality, family, social isolation, intimacy, appearance; and healthy lifestyles. Items were adapted and translated into Spanish if needed, using forward/backward translation with team reconciliation. These questions used the introduction, “Now I am going to ask you if you have had any problems because of your cancer. In the past month, how much have you been bothered by …” with responses rated on a scale of 1-5 (1, Not at all; 5, A lot). For example, we asked, “In the past month, how much have you been bothered by fatigue?”
Regarding healthy lifestyles, we used the introduction, “Here are some changes women sometimes want to make after cancer. Would you like help with …?” For example, we asked, “Would you like help with getting more exercise?” We asked if they wanted help getting more exercise, eating healthier, managing stress, and doing meditation or yoga (Yes/No).
Semistructured interview guide for SSBCS. Participants were asked about their emotional and physical concerns when treatment ended, current cancer needs, symptoms or late effects, and issues related to relationships, family, employment, insurance, financial hardships, barriers to follow-up care, health behaviors, and survivorship program content. Sample questions are, “Have you had any symptoms or side effects related to your cancer or treatment?” and “What kinds of information do you feel you need now about your cancer or treatment?” A brief questionnaire assessed demographics.
Semistructured interview guide for cancer support providers and physicians. Support providers and physicians were asked about informational, psychosocial, and symptom management needs of SSBCS and recommended self-management content and formats. Sample questions are, “What kinds of information and support do you wish was available to help Spanish-speaking women take care of their health after treatment ends?” and “What do you think are the most pressing emotional needs of Spanish-speaking women after breast cancer treatment ends?” A brief questionnaire assessed demographics.
Analysis
Frequencies are reported for survey items. For questions about symptoms/concerns, we report the frequency of responding that they were bothered Somewhat/Quite a bit/A lot. For healthy lifestyles, we present the frequency answering Yes.
Verbatim semistructured interview transcripts were verified against audiotapes. Using QSR NVIVO software, transcripts were coded independently by two bilingual-bicultural investigators using a constant comparative method to generate coding categories for cancer survivorship needs.16 Coders started with themes specified by the interview guides, expanded them to represent the data, and discussed and reconciled coding discrepancies. Coding was compared by type of interview participant (survivor, support provider, or physician). Triangulation of survey and semistructured interviews occurred through team discussions to verify codes, themes, and implications for interventions.
Results
Telephone survey of SSBCS
Of the telephone survey sampling frame (N = 231), 118 individuals (51%) completed the interview, 37 (16%) were ineligible, 31 (13%) could not be reached, 22 (10%) had incorrect contact information, 19 (8%) refused to participate, and 4 (2%) were deceased. Mean age of the participants was 54.9 years (SD, 12.3); all were foreign-born, with more than half of Mexican origin; and most had less than a high-school education (Table 1). All had completed active treatment, and most (68%) were within 2 years of diagnosis.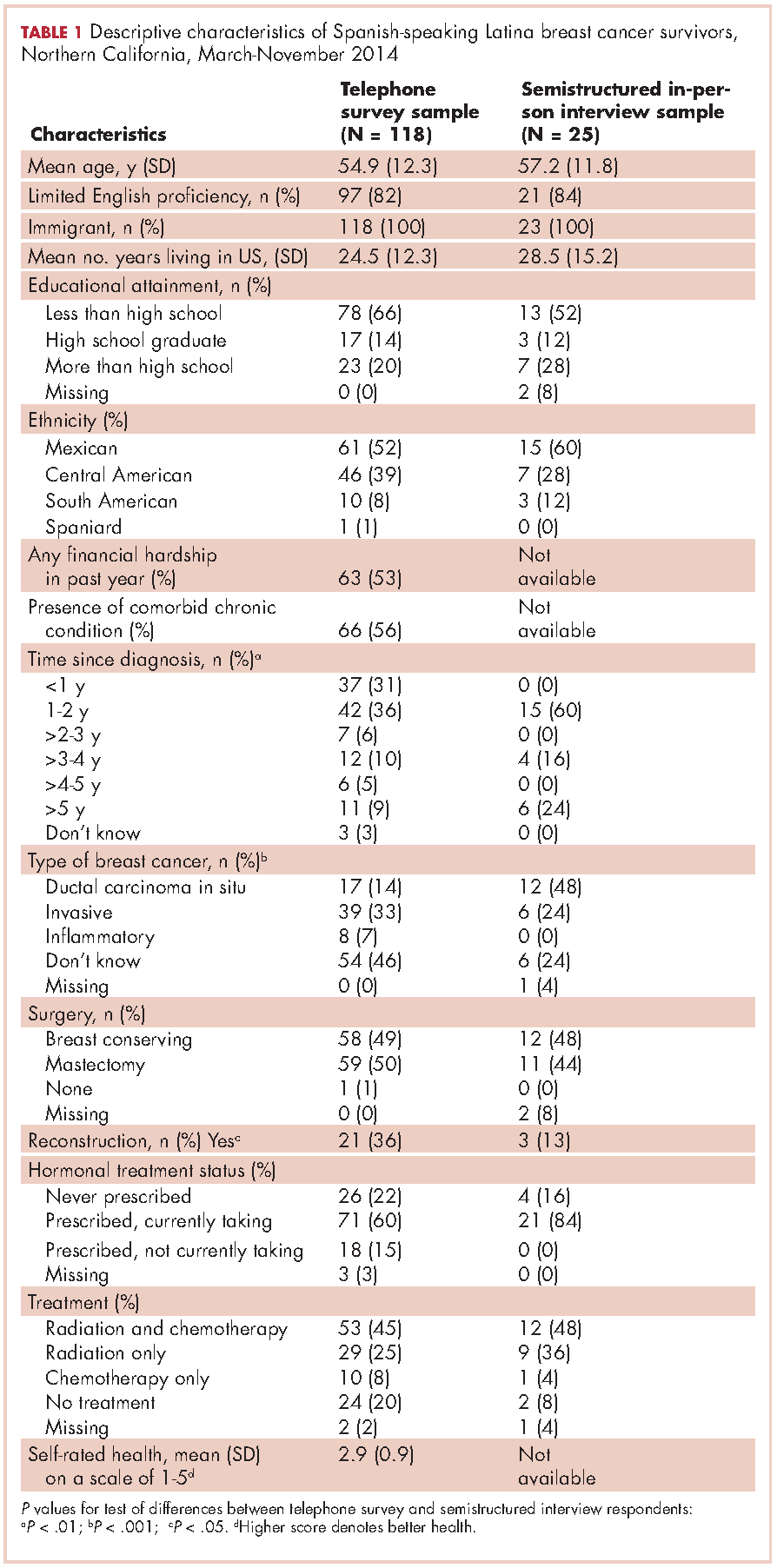
For symptom management needs (Table 2), the most prevalent (bothersome) symptoms (reported by more than 30%, in rank order) were joint pain, sleep problems, fatigue, hot flashes, numbness/tingling of extremities, and memory. Next most prevalent (reported by 20%-30%) were vaginal dryness, dry/itchy skin, dry nose/mouth, inability to concentrate, constipation, changes in urination, and shortness of breath.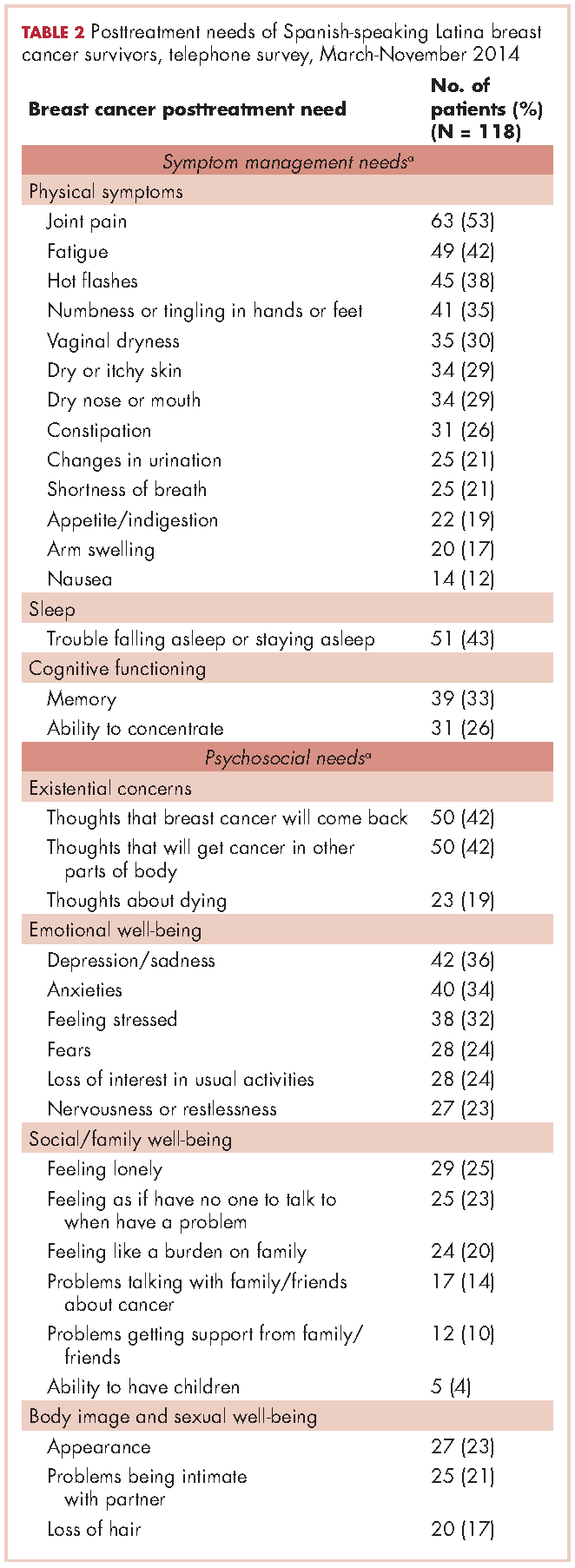

For psychosocial needs, fears of recurrence or new cancers were reported by 42%. Emotional symptoms reported by more than 30% were depression/sadness, anxieties, and feeling stressed. Next most prevalent (20%-30%) were fears, loss of interest in usual activities, and nervousness/restlessness. Social well-being concerns reported by 20%-30% of survivors were loneliness, having no one to talk to, and being a burden to their families. Body image and sexual problems reported by 20%-30% of survivors included appearance and problems being intimate with partners.
Regarding lifestyle, most of the participants said they wanted help with eating a healthier diet (74%), getting more exercise (69%), managing stress (63%), and doing yoga or meditation (55%).
Semistructured interviews
Twenty-five SSBCS completed semistructured interviews, 10 in individual interviews and 15 in one of two focus groups (one of 9 women older than 50 years; one of 6 women younger than 50). The telephone survey respondents were similar to semistructured interview respondents on all sociodemographic characteristics, but differed slightly on some clinical characteristics (Table 1). The telephone survey women had been more recently diagnosed (P < .01), were less likely to have ductal carcinoma in situ (P < .001), and more likely to have had reconstructive surgery
(P < .05).
Five cancer support providers and 4 physicians were interviewed. All support providers were Spanish-speaking Latinas with at least some college education. Cancer physicians were board certified. Two were men; two were white and two Asian; one was a breast surgeon and three were hematologists/oncologists; three spoke Spanish poorly/not at all and one spoke it fairly well.
Seven themes emerged from interviews: unmet physical symptom management needs; social support often ends when treatment ends; challenges resuming roles; sense of abandonment by health care system when treatment ends; need for formal transition from active treatment to follow-up care; fear of recurrence especially when obtaining follow-up care; and desire for information on late effects of initial treatments and side effects of hormonal treatments. We summarize results according to these themes.
Unmet physical symptom management needs. The main physical symptoms reported by survivors and physicians in interviews were arthralgia, menopausal symptoms, and neuropathy. Fatigue was reported only by survivors. Many survivors and several support providers expressed that symptoms were poorly managed and often ignored. One stated,
I have a lot of pain where I had surgery, it burns. I worry a lot about my arm because I have sacs of fluid. My doctor only says, ‘They will dissolve over the years.’ So, I don’t feel any support. (FocGrp1#6)
Survivors reported side effects of hormonal therapies, and felt that physicians downplayed these to prevent them from discontinuing medications.
Social support from family and friends often ends when treatment ends. Many survivors described a loss of support from family and friends who expected them to get back to “normal” once treatment ended. One said,
My sisters have told me to my face that there’s nothing wrong with me. So now when people ask me, I say, ‘I’m fine, thank God, I have nothing,’ even though I’m dying of pain and have all these pills to take. (Survivor#1025)
A support provider related,
The client was telling me that as she was getting closer to finishing her treatment, her husband was upset because he felt like all she was doing was focusing on the cancer. I think caregivers, family, spouses, and children out of their own sort of selfishness want this person to be well. (SuppProv#104)
A few survivors said that family bonds were strengthened after cancer and several reported lacking support because their families were in their home countries.
Challenges resuming roles, especially returning to work. Survivors, support providers, and physicians described challenges and few resources as women transition back to their normal roles. Survivors questioned their ability to return to work due to physically demanding occupations. One stated,
I would like information on how to take care of myself, how working can affect this side if I don’t take care of it. I clean houses and I need both hands. (Survivor#3012)
Survivors described how changes in memory affected daily chores and work performance. Support providers and physicians described the need for resources to aid with return to work and household responsibilities. One physician noted,
There are usually questions about how to go ahead and live their lives from that point forward. It’s a sort of reverse shock: going back to life as they know it. (Physician#004)
Support providers and physicians mentioned that women needed help with resuming intimate partner relations.
Sense of abandonment by health care system once active treatment ends. Survivors, support providers, and physicians reported a loss of support and sense of abandonment by the patients’ oncology team at the end of active treatment. One survivor stated,
Once they tell you to stop the pills, ‘You’re cured, there’s nothing wrong with you,’ the truth is that one feels, ‘Now what do I do? I have no one to help me.’ I felt very abandoned. (FocGrp1#5)
A provider said,
The support system falls apart once women complete treatment. They lose their entire support system at the medical level. They no longer have nurses checking in about symptoms and addressing anything that’s come up. They won’t have access to doctors unless they’re doing their screening. (SuppProv#101)
An oncologist, noting that this loss of support occurs when women face pressures of transitioning back to work or family obligations, commented,
So here’s a woman whose marriage is in turmoil, whose husband may even have left her during this, and now her clinic is leaving her and she’s on her own … that must be scary as hell because there’s nobody out there to support her. (Physician#002)
Need for formal transition from active treatment to follow-up care. Two themes emerged about transitioning from active treatment: transferring care from oncologists to primary care physicians (PCP); and issues of follow-up care (with oncologists or PCPs). Survivors felt lost in transitioning from specialty to primary care, or expressed apprehension seeing a PCP rather than a cancer specialist. One stated,
I have my doctor but she is not a specialist. She does what I tell her to and orders a mammogram every year. But, I don’t go to the oncologist anymore, and so I worry. With the specialists, I feel protected. (FocGrp1#5)
Physicians acknowledged the lack of a formal transition to primary care such as a survivorship care program.
Follow-up care issues were common. Physicians stressed that women needed to know how often to return for follow-up once active treatment ends and about recommended examinations and tests, especially when receiving hormonal therapy. Physicians indicated the need for patient education materials specific to patients’ treatments, for example, elevated risk of heart disease with certain chemotherapy agents. An oncologist expressed concern that PCPs are not prepared adequately about late effects and hormonal treatment side effects, and suggested providing summary notes for PCPs detailing these.
Survivors identified several barriers to follow-up care: lacking information on which symptoms merited a call to physicians; financial burden/limited health insurance; lacking appointment reminders; fear of examinations; and limited English proficiency. A survivor stated,
If you have insurance, you can make your appointment, see the doctor, and have your mammogram. I stopped taking my pills because I didn’t have insurance. I tried to get them again but they told me they would cost me a thousand dollars. (FocGrp1#5)
One oncologist suggested scheduling a follow-up appointment before patients leave treatment and calling patients who miss appointments.
Facilitators of regular follow-up care identified by survivors were physicians informing them about symptom monitoring and reporting, having a clinic contact person/navigator, being given a follow-up appointment, being assertive about one’s care, and physicians’ reinforcement of adherence to hormonal treatment and follow-up. According to support providers, a key facilitator was having a clinic contact person/navigator. Once treatment ended, support providers often served as the liaison between the patient and the physician, making them the first point of contact for symptom reporting.
Fear of recurrence especially when obtaining follow-up care. Fear of recurrence dominated survivor interviews. This fear was heightened at the time of follow-up examinations or when they experienced unusual pain. A survivor commented,
Every time I’m due for my mammogram, I can’t sleep, worrying. I lose sleep until I get the letter with my results. Then I feel at peace again. (FocGrp1#9)
Support providers discussed the need to provide reassurance to SSBCS to help them cope with fears of recurrence. Physicians expressed challenges in allaying fears of recurrence among SSBCS, requiring a lot of time when recommending follow-up mammograms.
Desire for information on late effects of treatments and side effects of hormonal therapies. All survivors expressed receiving insufficient information on potential symptoms and side effects. One stated,
Doctors only have five minutes. There has never been someone who gave me guidance like, ‘From now on you have to do this or you might get these symptoms now or in the future. (Survivor#6019)
They indicated uncertainty about what symptoms were “normal” and when symptoms merited a call to the physician. Several survivors reported being unaware that fatigue, arthralgia and neuropathy were side effects of breast cancer treatments until they reported these to physicians.
Physicians stressed the importance of women knowing about the elevated risk of future cancers, symptoms of recurrence, and seeking follow-up care if they experience symptoms that are out of the ordinary. Support providers felt that it was important to provide SSBCS with information on signs of recurrence and when to report these. However, providers expressed concern that giving women too much information might elevate their anxiety. A physician suggested,
It’s probably better to have a symptom list that’s short and relevant for the most common and catastrophic things, same thing with side effects … short to avoid overwhelming the patient. (Physician#001)
Hormonal treatments were of special concern. Survivors expressed a need for information on hormonal treatments and support providers stressed that this information is needed in simple Spanish. Several survivors indicated they stopped taking hormonal treatments due to side effects. One woman experienced severe headaches and heart palpitations, stopped taking the hormonal medication, felt better, and did not inform her physician until her next appointment. A support provider stated,
What I hear from a lot of women is that if side effects are too uncomfortable, they just stop it (hormonal treatment) without saying anything to the doctor. So more information about why they have to take it and that there is a good chance of recurrence is really important. (SuppProv#101)
Likewise, physicians indicated that SSBCS’ lack of information on hormonal treatments often resulted in nonadherence, emphasizing the need to reinforce adherence to prevent recurrence.
Conceptual framework of interventions
Based on triangulation of survey and interview results, we compiled a conceptual framework that includes needs identified, suggested components of a survivorship care intervention to address these needs, potential mediators by which such interventions could improve outcomes, and relevant outcomes (Figure). Survivorship care needs fell into four categories: symptom management, psychosocial, sense of abandonment by health care team, and healthy lifestyles. Survivorship care programs would provide skills training in symptom and stress management, and communicating with providers, family, friends, and coworkers. Mediators include increased self-efficacy, knowledge and perceived social support, ultimately leading to reduced distress (anxiety and depressive symptoms) and stress, and improved health-related quality of life.
Conclusions
Our study aimed to identify the most critical needs of SSBCS in the posttreatment survivorship phase to facilitate the design of survivorship interventions for this vulnerable group. SSBCS, cancer support providers, and cancer physicians reported substantial symptom management, psychosocial, and informational needs among this population. Results from surveys and open-ended interviews were remarkably consistent. Survivors, physicians, and support providers viewed transition out of active treatment as a time of increased psychosocial need and heightened vulnerability.
Our findings are consistent with needs assessments conducted in other breast cancer survivors. Similar to a study of rural white women with breast cancer, fear of recurrence was among the most common psychosocial concerns.17 Results of two studies that included white, African American and Latina breast cancer survivors were consistent with ours in finding that pain and fatigue were among the most persistent symptoms; in both studies, Latinas were more likely to report pain and a higher number of symptoms.7,18 The prevalence of sleep problems in our sample was identical to that reported in a sample of African American breast cancer survivors.19 Our findings of a high need for symptom management information and support, social support from family and friends, and self-management resources were similar to studies of other vulnerable breast cancer survivors.18,20
Our results suggest that it is critical for health care professionals to provide assistance with managing side effects and information to alleviate fears, and reinforce behaviors of symptom monitoring and reporting, and adherence to follow-up care and hormonal therapies. Yet this information is not being conveyed effectively and is complicated by the need to balance women’s need for information with minimizing anxiety when providing such information.
A limitation of our study is that most of our sample was Mexican origin and may not reflect experiences of Spanish-speaking Latinas of other national origin groups or outside of Northern California. Another limitation is the lack of an English-speaking comparison group, which would have permitted the identification of similarities and differences across language groups. Finally, we did not interview radiation oncologists who may have had opinions that are not represented here.
Survivorship care programs offer great promise for meeting patients’ informational and symptom management needs and improving well-being and communication with clinicians.21 Due to limited access to survivorship care information, financial hardships, and pressures from their families to resume their social roles, concerted efforts are needed to develop appropriate survivorship programs for SSBCS.22 Unique language, cultural and socioeconomic factors of Spanish-speaking Latinas require tailoring of cancer survivorship programs to best meet their needs.23 These programs need to provide psychosocial stress and symptom management assistance, simple information on recommended follow-up care, and healthy lifestyle and role reintegration strategies that account for their unique sociocultural contexts.
1. Danese MD, O’Malley C, Lindquist K, Gleeson M, Griffiths RI. An observational study of the prevalence and incidence of comorbid conditions in older women with breast cancer. Ann Oncol. 2012;23(7):1756-1765.
2. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academy of Sciences; 2006.
3. Beckjord EB, Arora NK, McLaughlin W, Oakley-Girvan I, Hamilton AS, Hesse BW. Health-related information needs in a large and diverse sample of adult cancer survivors: implications for cancer care. J Cancer Surviv. 2008;2(3):179-189.
4. Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on posttreatment cancer care: qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25(16):2270-2273.
5. Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16(3):413-428.
6. Clauser SB, Arora NK, Bellizzi KM, Haffer SC, Topor M, Hays RD. Disparities in HRQOL of cancer survivors and non-cancer managed care enrollees. Health Care Financ Rev. 2008;29(4):23-40.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Posttreatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-256.
8. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357-1362.
9. Arora NK, Reeve BB, Hays RD, Clauser SB, Oakley-Girvan I. Assessment of quality of cancer-related follow-up care from the cancer survivor’s perspective. J Clin Oncol. 2011;29(10):1280-1289.
10. Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008;113(5):1058-1067.
11. Yoon J, Malin JL, Tisnado DM, et al. Symptom management after breast cancer treatment: is it influenced by patient characteristics? Breast Cancer Res Treat. 2008;108(1):69-77.
12. Ashing K, Rosales M. A telephonic-based trial to reduce depressive symptoms among Latina breast cancer survivors. Psychooncology. 2014;23(5):507-515.
13. Hershman DL, Greenlee H, Awad D, et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat. 2013;138(3):795-806.
14. Napoles AM, Ortiz C, Santoyo-Olsson J, et al. Nuevo Amanecer: results of a randomized controlled trial of a community-based, peer-delivered stress management intervention to improve quality of life in Latinas with breast cancer. Am J Public Health. 2015;105(suppl 3):e55-63.
15. Rechis R, Reynolds KA, Beckjord EB, Nutt S, Burns RM, Schaefer JS. ‘I learned to live with it’ is not good enough: challenges reported by posttreatment cancer survivors in the Livestrong surveys. Austin, TX: Livestrong;2011.
16. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Hawthorne: Aldine Publishing Company; 1967.
17. Befort CA, Klemp J. Sequelae of breast cancer and the influence of menopausal status at diagnosis among rural breast cancer survivors. J Womens Health (Larchmt). 2011;20(9):1307-1313.
18. Fu OS, Crew KD, Jacobson JS, et al. Ethnicity and persistent symptom burden in breast cancer survivors. J Cancer Surviv. 2009;3(4):241-250.
19. Taylor TR, Huntley ED, Makambi K, et al. Understanding sleep disturbances in African-American breast cancer survivors: a pilot study. Psychooncology. 2012;21(8):896-902.
20. Adams N, Gisiger-Camata S, Hardy CM, Thomas TF, Jukkala A, Meneses K. Evaluating survivorship experiences and needs among rural African American breast cancer survivors. J Cancer Educ. October 24, 2015 [Epub ahead of print].
21. Blinder VS, Patil S, Thind A, et al. Return to work in low-income Latina and non-Latina white breast cancer survivors: a 3-year longitudinal study. Cancer. 2012;118(6):1664-1674.
22. Lopez-Class M, Perret-Gentil M, Kreling B, Caicedo L, Mandelblatt J, Graves KD. Quality of life among immigrant Latina breast cancer survivors: realities of culture and enhancing cancer care. J Cancer Educ. 2011;26(4):724-733.
23. Napoles-Springer AM, Ortiz C, O’Brien H, Diaz-Mendez M. Developing a culturally competent peer support intervention for Spanish-speaking Latinas with breast cancer. J Immigr Minor Health. 2009;11(4):268-280
After treatment, cancer patients transition to a survivorship phase, often with little information or support. Cancer survivors are at increased risk of recurrence, secondary cancers, comorbid conditions, and late treatment effects.1,2 However, many remain unaware of these risks and the options for managing them3 and face numerous unmet medical, psychosocial, and informational needs that can be addressed through survivorship care programs.4 Anxiety may increase as they lose their treatment team’s support while attempting to reestablish their lives.2 Patients need to know the long-term risks of cancer treatments, probabilities of recurrence and second cancers, effectiveness of surveillance and interventions for managing late effects and psychosocial concerns, and benefits of healthy lifestyles.2
Due to sociocultural and economic factors, Spanish-speaking Latina breast cancer survivors (SSBCS) suffer worse posttreatment health-related quality of life and more pain, fatigue, depressive symptoms, body image issues, and distress than their white counterparts.5-7 However, they are less likely to receive necessary cancer treatment, symptom management, and surveillance. For example, compared with whites, Latina breast cancer survivors receive less guideline-adherent treatment8 and follow-up care, including survivorship information.3,9 SSBCS, in particular have less access to survivorship information.10 Consequently, SSBCS are more likely to report unmet symptom management needs.11
Several breast cancer survivorship program trials have included Latinas,12,13 but their effectiveness has been demonstrated only for depressive symptoms or health worry. A comprehensive assessment of the posttreatment needs of SSBCS would provide a foundation for designing tailored survivorship interventions for this vulnerable group. This study aimed to identify the symptom management, psychosocial, and informational needs of SSBCS during the transition to survivorship from the perspectives of SSBCS and their cancer support providers and cancer physicians.
Methods
We sampled respondents within a 5-county area in Northern California to obtain multiple perspectives of the survivorship care needs of SSBCS using structured and in-depth methods: a telephone survey of SSBCS; semistructured interviews with SSBCS; semistructured interviews with cancer support providers serving SSBCS; and semistructured interviews with physicians providing cancer care for SSBCS. The study protocol was approved by the University of California San Francisco Committee on Human Research.
Sample and procedures
Structured telephone survey with SSBCS. The sample was drawn evenly from San Francisco General Hospital-University of California San Francisco primary care practices and SSBCS from a previous study who agreed to be re-contacted.14 The inclusion criteria were: completed active treatment (except adjuvant hormonal therapy) for nonmetastatic breast cancer within 10 years; living in one of the five counties; primarily Spanish-speaking; and self-identified as Latina. The exclusion criteria were: previous cancer except nonmelanoma skin cancer; terminal illness; or metastatic breast cancer. Study staff mailed potential participants a bilingual letter and information sheet, and bilingual opt-out postcard (6th grade reading level assessed by Flesch-Kincaid grade level statistic). Female bilingual-bicultural research associates conducted interviews of 20-30 minutes in Spanish after obtaining verbal consent. Participants were mailed $20. Surveys were conducted during March-November 2014.
Semistructured in-person interviews with SSBCS. Four community-based organizations (CBOs) in the targeted area providing cancer support services to Latinos agreed to recruit SSBCS for interviews. Inclusion criteria were identical to the survey. Patient navigators or support providers from CBOs contacted women by phone or in-person to invite them to an interview to assess their cancer survivorship needs. Women could choose a focus group or individual interview. With permission, names and contact information were given to study interviewers who called, explained the study, screened for eligibility, and scheduled an interview.
Recruitment was stratified by age (under or over age 50). We sampled women until saturation was achieved (no new themes emerged). Focus groups (90 minutes) were conducted at the CBOs. Individual interviews (45 minutes) were conducted in participants’ homes. Written informed consent was obtained. Participants were paid $50. Interviews were conducted during August-November, 2014, audiotaped, and transcribed.
Semistructured in-person interviews with cancer support providers and physicians. Investigators invited five cancer support providers (three patient navigators from three county hospitals, and two CBO directors of cancer psychosocial support services) and four physicians (three oncologists and one breast cancer surgeon from three county hospitals) to an in-person interview to identify SSBCS’ survivorship care needs. All agreed to participate. No further candidates were approached because saturation was achieved. We obtained written informed consent and 30-minute interviews were conducted in participants’ offices during August-October, 2014. Interviews were audiotaped and transcribed. Participants were paid $50.
Ethical approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Measures
Structured telephone survey. Based on cancer survivor needs assessments,15 we assessed: physical and emotional symptoms; problems with sleep and memory/concentration; concerns about mortality, family, social isolation, intimacy, appearance; and healthy lifestyles. Items were adapted and translated into Spanish if needed, using forward/backward translation with team reconciliation. These questions used the introduction, “Now I am going to ask you if you have had any problems because of your cancer. In the past month, how much have you been bothered by …” with responses rated on a scale of 1-5 (1, Not at all; 5, A lot). For example, we asked, “In the past month, how much have you been bothered by fatigue?”
Regarding healthy lifestyles, we used the introduction, “Here are some changes women sometimes want to make after cancer. Would you like help with …?” For example, we asked, “Would you like help with getting more exercise?” We asked if they wanted help getting more exercise, eating healthier, managing stress, and doing meditation or yoga (Yes/No).
Semistructured interview guide for SSBCS. Participants were asked about their emotional and physical concerns when treatment ended, current cancer needs, symptoms or late effects, and issues related to relationships, family, employment, insurance, financial hardships, barriers to follow-up care, health behaviors, and survivorship program content. Sample questions are, “Have you had any symptoms or side effects related to your cancer or treatment?” and “What kinds of information do you feel you need now about your cancer or treatment?” A brief questionnaire assessed demographics.
Semistructured interview guide for cancer support providers and physicians. Support providers and physicians were asked about informational, psychosocial, and symptom management needs of SSBCS and recommended self-management content and formats. Sample questions are, “What kinds of information and support do you wish was available to help Spanish-speaking women take care of their health after treatment ends?” and “What do you think are the most pressing emotional needs of Spanish-speaking women after breast cancer treatment ends?” A brief questionnaire assessed demographics.
Analysis
Frequencies are reported for survey items. For questions about symptoms/concerns, we report the frequency of responding that they were bothered Somewhat/Quite a bit/A lot. For healthy lifestyles, we present the frequency answering Yes.
Verbatim semistructured interview transcripts were verified against audiotapes. Using QSR NVIVO software, transcripts were coded independently by two bilingual-bicultural investigators using a constant comparative method to generate coding categories for cancer survivorship needs.16 Coders started with themes specified by the interview guides, expanded them to represent the data, and discussed and reconciled coding discrepancies. Coding was compared by type of interview participant (survivor, support provider, or physician). Triangulation of survey and semistructured interviews occurred through team discussions to verify codes, themes, and implications for interventions.
Results
Telephone survey of SSBCS
Of the telephone survey sampling frame (N = 231), 118 individuals (51%) completed the interview, 37 (16%) were ineligible, 31 (13%) could not be reached, 22 (10%) had incorrect contact information, 19 (8%) refused to participate, and 4 (2%) were deceased. Mean age of the participants was 54.9 years (SD, 12.3); all were foreign-born, with more than half of Mexican origin; and most had less than a high-school education (Table 1). All had completed active treatment, and most (68%) were within 2 years of diagnosis.
For symptom management needs (Table 2), the most prevalent (bothersome) symptoms (reported by more than 30%, in rank order) were joint pain, sleep problems, fatigue, hot flashes, numbness/tingling of extremities, and memory. Next most prevalent (reported by 20%-30%) were vaginal dryness, dry/itchy skin, dry nose/mouth, inability to concentrate, constipation, changes in urination, and shortness of breath.

For psychosocial needs, fears of recurrence or new cancers were reported by 42%. Emotional symptoms reported by more than 30% were depression/sadness, anxieties, and feeling stressed. Next most prevalent (20%-30%) were fears, loss of interest in usual activities, and nervousness/restlessness. Social well-being concerns reported by 20%-30% of survivors were loneliness, having no one to talk to, and being a burden to their families. Body image and sexual problems reported by 20%-30% of survivors included appearance and problems being intimate with partners.
Regarding lifestyle, most of the participants said they wanted help with eating a healthier diet (74%), getting more exercise (69%), managing stress (63%), and doing yoga or meditation (55%).
Semistructured interviews
Twenty-five SSBCS completed semistructured interviews, 10 in individual interviews and 15 in one of two focus groups (one of 9 women older than 50 years; one of 6 women younger than 50). The telephone survey respondents were similar to semistructured interview respondents on all sociodemographic characteristics, but differed slightly on some clinical characteristics (Table 1). The telephone survey women had been more recently diagnosed (P < .01), were less likely to have ductal carcinoma in situ (P < .001), and more likely to have had reconstructive surgery
(P < .05).
Five cancer support providers and 4 physicians were interviewed. All support providers were Spanish-speaking Latinas with at least some college education. Cancer physicians were board certified. Two were men; two were white and two Asian; one was a breast surgeon and three were hematologists/oncologists; three spoke Spanish poorly/not at all and one spoke it fairly well.
Seven themes emerged from interviews: unmet physical symptom management needs; social support often ends when treatment ends; challenges resuming roles; sense of abandonment by health care system when treatment ends; need for formal transition from active treatment to follow-up care; fear of recurrence especially when obtaining follow-up care; and desire for information on late effects of initial treatments and side effects of hormonal treatments. We summarize results according to these themes.
Unmet physical symptom management needs. The main physical symptoms reported by survivors and physicians in interviews were arthralgia, menopausal symptoms, and neuropathy. Fatigue was reported only by survivors. Many survivors and several support providers expressed that symptoms were poorly managed and often ignored. One stated,
I have a lot of pain where I had surgery, it burns. I worry a lot about my arm because I have sacs of fluid. My doctor only says, ‘They will dissolve over the years.’ So, I don’t feel any support. (FocGrp1#6)
Survivors reported side effects of hormonal therapies, and felt that physicians downplayed these to prevent them from discontinuing medications.
Social support from family and friends often ends when treatment ends. Many survivors described a loss of support from family and friends who expected them to get back to “normal” once treatment ended. One said,
My sisters have told me to my face that there’s nothing wrong with me. So now when people ask me, I say, ‘I’m fine, thank God, I have nothing,’ even though I’m dying of pain and have all these pills to take. (Survivor#1025)
A support provider related,
The client was telling me that as she was getting closer to finishing her treatment, her husband was upset because he felt like all she was doing was focusing on the cancer. I think caregivers, family, spouses, and children out of their own sort of selfishness want this person to be well. (SuppProv#104)
A few survivors said that family bonds were strengthened after cancer and several reported lacking support because their families were in their home countries.
Challenges resuming roles, especially returning to work. Survivors, support providers, and physicians described challenges and few resources as women transition back to their normal roles. Survivors questioned their ability to return to work due to physically demanding occupations. One stated,
I would like information on how to take care of myself, how working can affect this side if I don’t take care of it. I clean houses and I need both hands. (Survivor#3012)
Survivors described how changes in memory affected daily chores and work performance. Support providers and physicians described the need for resources to aid with return to work and household responsibilities. One physician noted,
There are usually questions about how to go ahead and live their lives from that point forward. It’s a sort of reverse shock: going back to life as they know it. (Physician#004)
Support providers and physicians mentioned that women needed help with resuming intimate partner relations.
Sense of abandonment by health care system once active treatment ends. Survivors, support providers, and physicians reported a loss of support and sense of abandonment by the patients’ oncology team at the end of active treatment. One survivor stated,
Once they tell you to stop the pills, ‘You’re cured, there’s nothing wrong with you,’ the truth is that one feels, ‘Now what do I do? I have no one to help me.’ I felt very abandoned. (FocGrp1#5)
A provider said,
The support system falls apart once women complete treatment. They lose their entire support system at the medical level. They no longer have nurses checking in about symptoms and addressing anything that’s come up. They won’t have access to doctors unless they’re doing their screening. (SuppProv#101)
An oncologist, noting that this loss of support occurs when women face pressures of transitioning back to work or family obligations, commented,
So here’s a woman whose marriage is in turmoil, whose husband may even have left her during this, and now her clinic is leaving her and she’s on her own … that must be scary as hell because there’s nobody out there to support her. (Physician#002)
Need for formal transition from active treatment to follow-up care. Two themes emerged about transitioning from active treatment: transferring care from oncologists to primary care physicians (PCP); and issues of follow-up care (with oncologists or PCPs). Survivors felt lost in transitioning from specialty to primary care, or expressed apprehension seeing a PCP rather than a cancer specialist. One stated,
I have my doctor but she is not a specialist. She does what I tell her to and orders a mammogram every year. But, I don’t go to the oncologist anymore, and so I worry. With the specialists, I feel protected. (FocGrp1#5)
Physicians acknowledged the lack of a formal transition to primary care such as a survivorship care program.
Follow-up care issues were common. Physicians stressed that women needed to know how often to return for follow-up once active treatment ends and about recommended examinations and tests, especially when receiving hormonal therapy. Physicians indicated the need for patient education materials specific to patients’ treatments, for example, elevated risk of heart disease with certain chemotherapy agents. An oncologist expressed concern that PCPs are not prepared adequately about late effects and hormonal treatment side effects, and suggested providing summary notes for PCPs detailing these.
Survivors identified several barriers to follow-up care: lacking information on which symptoms merited a call to physicians; financial burden/limited health insurance; lacking appointment reminders; fear of examinations; and limited English proficiency. A survivor stated,
If you have insurance, you can make your appointment, see the doctor, and have your mammogram. I stopped taking my pills because I didn’t have insurance. I tried to get them again but they told me they would cost me a thousand dollars. (FocGrp1#5)
One oncologist suggested scheduling a follow-up appointment before patients leave treatment and calling patients who miss appointments.
Facilitators of regular follow-up care identified by survivors were physicians informing them about symptom monitoring and reporting, having a clinic contact person/navigator, being given a follow-up appointment, being assertive about one’s care, and physicians’ reinforcement of adherence to hormonal treatment and follow-up. According to support providers, a key facilitator was having a clinic contact person/navigator. Once treatment ended, support providers often served as the liaison between the patient and the physician, making them the first point of contact for symptom reporting.
Fear of recurrence especially when obtaining follow-up care. Fear of recurrence dominated survivor interviews. This fear was heightened at the time of follow-up examinations or when they experienced unusual pain. A survivor commented,
Every time I’m due for my mammogram, I can’t sleep, worrying. I lose sleep until I get the letter with my results. Then I feel at peace again. (FocGrp1#9)
Support providers discussed the need to provide reassurance to SSBCS to help them cope with fears of recurrence. Physicians expressed challenges in allaying fears of recurrence among SSBCS, requiring a lot of time when recommending follow-up mammograms.
Desire for information on late effects of treatments and side effects of hormonal therapies. All survivors expressed receiving insufficient information on potential symptoms and side effects. One stated,
Doctors only have five minutes. There has never been someone who gave me guidance like, ‘From now on you have to do this or you might get these symptoms now or in the future. (Survivor#6019)
They indicated uncertainty about what symptoms were “normal” and when symptoms merited a call to the physician. Several survivors reported being unaware that fatigue, arthralgia and neuropathy were side effects of breast cancer treatments until they reported these to physicians.
Physicians stressed the importance of women knowing about the elevated risk of future cancers, symptoms of recurrence, and seeking follow-up care if they experience symptoms that are out of the ordinary. Support providers felt that it was important to provide SSBCS with information on signs of recurrence and when to report these. However, providers expressed concern that giving women too much information might elevate their anxiety. A physician suggested,
It’s probably better to have a symptom list that’s short and relevant for the most common and catastrophic things, same thing with side effects … short to avoid overwhelming the patient. (Physician#001)
Hormonal treatments were of special concern. Survivors expressed a need for information on hormonal treatments and support providers stressed that this information is needed in simple Spanish. Several survivors indicated they stopped taking hormonal treatments due to side effects. One woman experienced severe headaches and heart palpitations, stopped taking the hormonal medication, felt better, and did not inform her physician until her next appointment. A support provider stated,
What I hear from a lot of women is that if side effects are too uncomfortable, they just stop it (hormonal treatment) without saying anything to the doctor. So more information about why they have to take it and that there is a good chance of recurrence is really important. (SuppProv#101)
Likewise, physicians indicated that SSBCS’ lack of information on hormonal treatments often resulted in nonadherence, emphasizing the need to reinforce adherence to prevent recurrence.
Conceptual framework of interventions
Based on triangulation of survey and interview results, we compiled a conceptual framework that includes needs identified, suggested components of a survivorship care intervention to address these needs, potential mediators by which such interventions could improve outcomes, and relevant outcomes (Figure). Survivorship care needs fell into four categories: symptom management, psychosocial, sense of abandonment by health care team, and healthy lifestyles. Survivorship care programs would provide skills training in symptom and stress management, and communicating with providers, family, friends, and coworkers. Mediators include increased self-efficacy, knowledge and perceived social support, ultimately leading to reduced distress (anxiety and depressive symptoms) and stress, and improved health-related quality of life.
Conclusions
Our study aimed to identify the most critical needs of SSBCS in the posttreatment survivorship phase to facilitate the design of survivorship interventions for this vulnerable group. SSBCS, cancer support providers, and cancer physicians reported substantial symptom management, psychosocial, and informational needs among this population. Results from surveys and open-ended interviews were remarkably consistent. Survivors, physicians, and support providers viewed transition out of active treatment as a time of increased psychosocial need and heightened vulnerability.
Our findings are consistent with needs assessments conducted in other breast cancer survivors. Similar to a study of rural white women with breast cancer, fear of recurrence was among the most common psychosocial concerns.17 Results of two studies that included white, African American and Latina breast cancer survivors were consistent with ours in finding that pain and fatigue were among the most persistent symptoms; in both studies, Latinas were more likely to report pain and a higher number of symptoms.7,18 The prevalence of sleep problems in our sample was identical to that reported in a sample of African American breast cancer survivors.19 Our findings of a high need for symptom management information and support, social support from family and friends, and self-management resources were similar to studies of other vulnerable breast cancer survivors.18,20
Our results suggest that it is critical for health care professionals to provide assistance with managing side effects and information to alleviate fears, and reinforce behaviors of symptom monitoring and reporting, and adherence to follow-up care and hormonal therapies. Yet this information is not being conveyed effectively and is complicated by the need to balance women’s need for information with minimizing anxiety when providing such information.
A limitation of our study is that most of our sample was Mexican origin and may not reflect experiences of Spanish-speaking Latinas of other national origin groups or outside of Northern California. Another limitation is the lack of an English-speaking comparison group, which would have permitted the identification of similarities and differences across language groups. Finally, we did not interview radiation oncologists who may have had opinions that are not represented here.
Survivorship care programs offer great promise for meeting patients’ informational and symptom management needs and improving well-being and communication with clinicians.21 Due to limited access to survivorship care information, financial hardships, and pressures from their families to resume their social roles, concerted efforts are needed to develop appropriate survivorship programs for SSBCS.22 Unique language, cultural and socioeconomic factors of Spanish-speaking Latinas require tailoring of cancer survivorship programs to best meet their needs.23 These programs need to provide psychosocial stress and symptom management assistance, simple information on recommended follow-up care, and healthy lifestyle and role reintegration strategies that account for their unique sociocultural contexts.
After treatment, cancer patients transition to a survivorship phase, often with little information or support. Cancer survivors are at increased risk of recurrence, secondary cancers, comorbid conditions, and late treatment effects.1,2 However, many remain unaware of these risks and the options for managing them3 and face numerous unmet medical, psychosocial, and informational needs that can be addressed through survivorship care programs.4 Anxiety may increase as they lose their treatment team’s support while attempting to reestablish their lives.2 Patients need to know the long-term risks of cancer treatments, probabilities of recurrence and second cancers, effectiveness of surveillance and interventions for managing late effects and psychosocial concerns, and benefits of healthy lifestyles.2
Due to sociocultural and economic factors, Spanish-speaking Latina breast cancer survivors (SSBCS) suffer worse posttreatment health-related quality of life and more pain, fatigue, depressive symptoms, body image issues, and distress than their white counterparts.5-7 However, they are less likely to receive necessary cancer treatment, symptom management, and surveillance. For example, compared with whites, Latina breast cancer survivors receive less guideline-adherent treatment8 and follow-up care, including survivorship information.3,9 SSBCS, in particular have less access to survivorship information.10 Consequently, SSBCS are more likely to report unmet symptom management needs.11
Several breast cancer survivorship program trials have included Latinas,12,13 but their effectiveness has been demonstrated only for depressive symptoms or health worry. A comprehensive assessment of the posttreatment needs of SSBCS would provide a foundation for designing tailored survivorship interventions for this vulnerable group. This study aimed to identify the symptom management, psychosocial, and informational needs of SSBCS during the transition to survivorship from the perspectives of SSBCS and their cancer support providers and cancer physicians.
Methods
We sampled respondents within a 5-county area in Northern California to obtain multiple perspectives of the survivorship care needs of SSBCS using structured and in-depth methods: a telephone survey of SSBCS; semistructured interviews with SSBCS; semistructured interviews with cancer support providers serving SSBCS; and semistructured interviews with physicians providing cancer care for SSBCS. The study protocol was approved by the University of California San Francisco Committee on Human Research.
Sample and procedures
Structured telephone survey with SSBCS. The sample was drawn evenly from San Francisco General Hospital-University of California San Francisco primary care practices and SSBCS from a previous study who agreed to be re-contacted.14 The inclusion criteria were: completed active treatment (except adjuvant hormonal therapy) for nonmetastatic breast cancer within 10 years; living in one of the five counties; primarily Spanish-speaking; and self-identified as Latina. The exclusion criteria were: previous cancer except nonmelanoma skin cancer; terminal illness; or metastatic breast cancer. Study staff mailed potential participants a bilingual letter and information sheet, and bilingual opt-out postcard (6th grade reading level assessed by Flesch-Kincaid grade level statistic). Female bilingual-bicultural research associates conducted interviews of 20-30 minutes in Spanish after obtaining verbal consent. Participants were mailed $20. Surveys were conducted during March-November 2014.
Semistructured in-person interviews with SSBCS. Four community-based organizations (CBOs) in the targeted area providing cancer support services to Latinos agreed to recruit SSBCS for interviews. Inclusion criteria were identical to the survey. Patient navigators or support providers from CBOs contacted women by phone or in-person to invite them to an interview to assess their cancer survivorship needs. Women could choose a focus group or individual interview. With permission, names and contact information were given to study interviewers who called, explained the study, screened for eligibility, and scheduled an interview.
Recruitment was stratified by age (under or over age 50). We sampled women until saturation was achieved (no new themes emerged). Focus groups (90 minutes) were conducted at the CBOs. Individual interviews (45 minutes) were conducted in participants’ homes. Written informed consent was obtained. Participants were paid $50. Interviews were conducted during August-November, 2014, audiotaped, and transcribed.
Semistructured in-person interviews with cancer support providers and physicians. Investigators invited five cancer support providers (three patient navigators from three county hospitals, and two CBO directors of cancer psychosocial support services) and four physicians (three oncologists and one breast cancer surgeon from three county hospitals) to an in-person interview to identify SSBCS’ survivorship care needs. All agreed to participate. No further candidates were approached because saturation was achieved. We obtained written informed consent and 30-minute interviews were conducted in participants’ offices during August-October, 2014. Interviews were audiotaped and transcribed. Participants were paid $50.
Ethical approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Measures
Structured telephone survey. Based on cancer survivor needs assessments,15 we assessed: physical and emotional symptoms; problems with sleep and memory/concentration; concerns about mortality, family, social isolation, intimacy, appearance; and healthy lifestyles. Items were adapted and translated into Spanish if needed, using forward/backward translation with team reconciliation. These questions used the introduction, “Now I am going to ask you if you have had any problems because of your cancer. In the past month, how much have you been bothered by …” with responses rated on a scale of 1-5 (1, Not at all; 5, A lot). For example, we asked, “In the past month, how much have you been bothered by fatigue?”
Regarding healthy lifestyles, we used the introduction, “Here are some changes women sometimes want to make after cancer. Would you like help with …?” For example, we asked, “Would you like help with getting more exercise?” We asked if they wanted help getting more exercise, eating healthier, managing stress, and doing meditation or yoga (Yes/No).
Semistructured interview guide for SSBCS. Participants were asked about their emotional and physical concerns when treatment ended, current cancer needs, symptoms or late effects, and issues related to relationships, family, employment, insurance, financial hardships, barriers to follow-up care, health behaviors, and survivorship program content. Sample questions are, “Have you had any symptoms or side effects related to your cancer or treatment?” and “What kinds of information do you feel you need now about your cancer or treatment?” A brief questionnaire assessed demographics.
Semistructured interview guide for cancer support providers and physicians. Support providers and physicians were asked about informational, psychosocial, and symptom management needs of SSBCS and recommended self-management content and formats. Sample questions are, “What kinds of information and support do you wish was available to help Spanish-speaking women take care of their health after treatment ends?” and “What do you think are the most pressing emotional needs of Spanish-speaking women after breast cancer treatment ends?” A brief questionnaire assessed demographics.
Analysis
Frequencies are reported for survey items. For questions about symptoms/concerns, we report the frequency of responding that they were bothered Somewhat/Quite a bit/A lot. For healthy lifestyles, we present the frequency answering Yes.
Verbatim semistructured interview transcripts were verified against audiotapes. Using QSR NVIVO software, transcripts were coded independently by two bilingual-bicultural investigators using a constant comparative method to generate coding categories for cancer survivorship needs.16 Coders started with themes specified by the interview guides, expanded them to represent the data, and discussed and reconciled coding discrepancies. Coding was compared by type of interview participant (survivor, support provider, or physician). Triangulation of survey and semistructured interviews occurred through team discussions to verify codes, themes, and implications for interventions.
Results
Telephone survey of SSBCS
Of the telephone survey sampling frame (N = 231), 118 individuals (51%) completed the interview, 37 (16%) were ineligible, 31 (13%) could not be reached, 22 (10%) had incorrect contact information, 19 (8%) refused to participate, and 4 (2%) were deceased. Mean age of the participants was 54.9 years (SD, 12.3); all were foreign-born, with more than half of Mexican origin; and most had less than a high-school education (Table 1). All had completed active treatment, and most (68%) were within 2 years of diagnosis.
For symptom management needs (Table 2), the most prevalent (bothersome) symptoms (reported by more than 30%, in rank order) were joint pain, sleep problems, fatigue, hot flashes, numbness/tingling of extremities, and memory. Next most prevalent (reported by 20%-30%) were vaginal dryness, dry/itchy skin, dry nose/mouth, inability to concentrate, constipation, changes in urination, and shortness of breath.

For psychosocial needs, fears of recurrence or new cancers were reported by 42%. Emotional symptoms reported by more than 30% were depression/sadness, anxieties, and feeling stressed. Next most prevalent (20%-30%) were fears, loss of interest in usual activities, and nervousness/restlessness. Social well-being concerns reported by 20%-30% of survivors were loneliness, having no one to talk to, and being a burden to their families. Body image and sexual problems reported by 20%-30% of survivors included appearance and problems being intimate with partners.
Regarding lifestyle, most of the participants said they wanted help with eating a healthier diet (74%), getting more exercise (69%), managing stress (63%), and doing yoga or meditation (55%).
Semistructured interviews
Twenty-five SSBCS completed semistructured interviews, 10 in individual interviews and 15 in one of two focus groups (one of 9 women older than 50 years; one of 6 women younger than 50). The telephone survey respondents were similar to semistructured interview respondents on all sociodemographic characteristics, but differed slightly on some clinical characteristics (Table 1). The telephone survey women had been more recently diagnosed (P < .01), were less likely to have ductal carcinoma in situ (P < .001), and more likely to have had reconstructive surgery
(P < .05).
Five cancer support providers and 4 physicians were interviewed. All support providers were Spanish-speaking Latinas with at least some college education. Cancer physicians were board certified. Two were men; two were white and two Asian; one was a breast surgeon and three were hematologists/oncologists; three spoke Spanish poorly/not at all and one spoke it fairly well.
Seven themes emerged from interviews: unmet physical symptom management needs; social support often ends when treatment ends; challenges resuming roles; sense of abandonment by health care system when treatment ends; need for formal transition from active treatment to follow-up care; fear of recurrence especially when obtaining follow-up care; and desire for information on late effects of initial treatments and side effects of hormonal treatments. We summarize results according to these themes.
Unmet physical symptom management needs. The main physical symptoms reported by survivors and physicians in interviews were arthralgia, menopausal symptoms, and neuropathy. Fatigue was reported only by survivors. Many survivors and several support providers expressed that symptoms were poorly managed and often ignored. One stated,
I have a lot of pain where I had surgery, it burns. I worry a lot about my arm because I have sacs of fluid. My doctor only says, ‘They will dissolve over the years.’ So, I don’t feel any support. (FocGrp1#6)
Survivors reported side effects of hormonal therapies, and felt that physicians downplayed these to prevent them from discontinuing medications.
Social support from family and friends often ends when treatment ends. Many survivors described a loss of support from family and friends who expected them to get back to “normal” once treatment ended. One said,
My sisters have told me to my face that there’s nothing wrong with me. So now when people ask me, I say, ‘I’m fine, thank God, I have nothing,’ even though I’m dying of pain and have all these pills to take. (Survivor#1025)
A support provider related,
The client was telling me that as she was getting closer to finishing her treatment, her husband was upset because he felt like all she was doing was focusing on the cancer. I think caregivers, family, spouses, and children out of their own sort of selfishness want this person to be well. (SuppProv#104)
A few survivors said that family bonds were strengthened after cancer and several reported lacking support because their families were in their home countries.
Challenges resuming roles, especially returning to work. Survivors, support providers, and physicians described challenges and few resources as women transition back to their normal roles. Survivors questioned their ability to return to work due to physically demanding occupations. One stated,
I would like information on how to take care of myself, how working can affect this side if I don’t take care of it. I clean houses and I need both hands. (Survivor#3012)
Survivors described how changes in memory affected daily chores and work performance. Support providers and physicians described the need for resources to aid with return to work and household responsibilities. One physician noted,
There are usually questions about how to go ahead and live their lives from that point forward. It’s a sort of reverse shock: going back to life as they know it. (Physician#004)
Support providers and physicians mentioned that women needed help with resuming intimate partner relations.
Sense of abandonment by health care system once active treatment ends. Survivors, support providers, and physicians reported a loss of support and sense of abandonment by the patients’ oncology team at the end of active treatment. One survivor stated,
Once they tell you to stop the pills, ‘You’re cured, there’s nothing wrong with you,’ the truth is that one feels, ‘Now what do I do? I have no one to help me.’ I felt very abandoned. (FocGrp1#5)
A provider said,
The support system falls apart once women complete treatment. They lose their entire support system at the medical level. They no longer have nurses checking in about symptoms and addressing anything that’s come up. They won’t have access to doctors unless they’re doing their screening. (SuppProv#101)
An oncologist, noting that this loss of support occurs when women face pressures of transitioning back to work or family obligations, commented,
So here’s a woman whose marriage is in turmoil, whose husband may even have left her during this, and now her clinic is leaving her and she’s on her own … that must be scary as hell because there’s nobody out there to support her. (Physician#002)
Need for formal transition from active treatment to follow-up care. Two themes emerged about transitioning from active treatment: transferring care from oncologists to primary care physicians (PCP); and issues of follow-up care (with oncologists or PCPs). Survivors felt lost in transitioning from specialty to primary care, or expressed apprehension seeing a PCP rather than a cancer specialist. One stated,
I have my doctor but she is not a specialist. She does what I tell her to and orders a mammogram every year. But, I don’t go to the oncologist anymore, and so I worry. With the specialists, I feel protected. (FocGrp1#5)
Physicians acknowledged the lack of a formal transition to primary care such as a survivorship care program.
Follow-up care issues were common. Physicians stressed that women needed to know how often to return for follow-up once active treatment ends and about recommended examinations and tests, especially when receiving hormonal therapy. Physicians indicated the need for patient education materials specific to patients’ treatments, for example, elevated risk of heart disease with certain chemotherapy agents. An oncologist expressed concern that PCPs are not prepared adequately about late effects and hormonal treatment side effects, and suggested providing summary notes for PCPs detailing these.
Survivors identified several barriers to follow-up care: lacking information on which symptoms merited a call to physicians; financial burden/limited health insurance; lacking appointment reminders; fear of examinations; and limited English proficiency. A survivor stated,
If you have insurance, you can make your appointment, see the doctor, and have your mammogram. I stopped taking my pills because I didn’t have insurance. I tried to get them again but they told me they would cost me a thousand dollars. (FocGrp1#5)
One oncologist suggested scheduling a follow-up appointment before patients leave treatment and calling patients who miss appointments.
Facilitators of regular follow-up care identified by survivors were physicians informing them about symptom monitoring and reporting, having a clinic contact person/navigator, being given a follow-up appointment, being assertive about one’s care, and physicians’ reinforcement of adherence to hormonal treatment and follow-up. According to support providers, a key facilitator was having a clinic contact person/navigator. Once treatment ended, support providers often served as the liaison between the patient and the physician, making them the first point of contact for symptom reporting.
Fear of recurrence especially when obtaining follow-up care. Fear of recurrence dominated survivor interviews. This fear was heightened at the time of follow-up examinations or when they experienced unusual pain. A survivor commented,
Every time I’m due for my mammogram, I can’t sleep, worrying. I lose sleep until I get the letter with my results. Then I feel at peace again. (FocGrp1#9)
Support providers discussed the need to provide reassurance to SSBCS to help them cope with fears of recurrence. Physicians expressed challenges in allaying fears of recurrence among SSBCS, requiring a lot of time when recommending follow-up mammograms.
Desire for information on late effects of treatments and side effects of hormonal therapies. All survivors expressed receiving insufficient information on potential symptoms and side effects. One stated,
Doctors only have five minutes. There has never been someone who gave me guidance like, ‘From now on you have to do this or you might get these symptoms now or in the future. (Survivor#6019)
They indicated uncertainty about what symptoms were “normal” and when symptoms merited a call to the physician. Several survivors reported being unaware that fatigue, arthralgia and neuropathy were side effects of breast cancer treatments until they reported these to physicians.
Physicians stressed the importance of women knowing about the elevated risk of future cancers, symptoms of recurrence, and seeking follow-up care if they experience symptoms that are out of the ordinary. Support providers felt that it was important to provide SSBCS with information on signs of recurrence and when to report these. However, providers expressed concern that giving women too much information might elevate their anxiety. A physician suggested,
It’s probably better to have a symptom list that’s short and relevant for the most common and catastrophic things, same thing with side effects … short to avoid overwhelming the patient. (Physician#001)
Hormonal treatments were of special concern. Survivors expressed a need for information on hormonal treatments and support providers stressed that this information is needed in simple Spanish. Several survivors indicated they stopped taking hormonal treatments due to side effects. One woman experienced severe headaches and heart palpitations, stopped taking the hormonal medication, felt better, and did not inform her physician until her next appointment. A support provider stated,
What I hear from a lot of women is that if side effects are too uncomfortable, they just stop it (hormonal treatment) without saying anything to the doctor. So more information about why they have to take it and that there is a good chance of recurrence is really important. (SuppProv#101)
Likewise, physicians indicated that SSBCS’ lack of information on hormonal treatments often resulted in nonadherence, emphasizing the need to reinforce adherence to prevent recurrence.
Conceptual framework of interventions
Based on triangulation of survey and interview results, we compiled a conceptual framework that includes needs identified, suggested components of a survivorship care intervention to address these needs, potential mediators by which such interventions could improve outcomes, and relevant outcomes (Figure). Survivorship care needs fell into four categories: symptom management, psychosocial, sense of abandonment by health care team, and healthy lifestyles. Survivorship care programs would provide skills training in symptom and stress management, and communicating with providers, family, friends, and coworkers. Mediators include increased self-efficacy, knowledge and perceived social support, ultimately leading to reduced distress (anxiety and depressive symptoms) and stress, and improved health-related quality of life.
Conclusions
Our study aimed to identify the most critical needs of SSBCS in the posttreatment survivorship phase to facilitate the design of survivorship interventions for this vulnerable group. SSBCS, cancer support providers, and cancer physicians reported substantial symptom management, psychosocial, and informational needs among this population. Results from surveys and open-ended interviews were remarkably consistent. Survivors, physicians, and support providers viewed transition out of active treatment as a time of increased psychosocial need and heightened vulnerability.
Our findings are consistent with needs assessments conducted in other breast cancer survivors. Similar to a study of rural white women with breast cancer, fear of recurrence was among the most common psychosocial concerns.17 Results of two studies that included white, African American and Latina breast cancer survivors were consistent with ours in finding that pain and fatigue were among the most persistent symptoms; in both studies, Latinas were more likely to report pain and a higher number of symptoms.7,18 The prevalence of sleep problems in our sample was identical to that reported in a sample of African American breast cancer survivors.19 Our findings of a high need for symptom management information and support, social support from family and friends, and self-management resources were similar to studies of other vulnerable breast cancer survivors.18,20
Our results suggest that it is critical for health care professionals to provide assistance with managing side effects and information to alleviate fears, and reinforce behaviors of symptom monitoring and reporting, and adherence to follow-up care and hormonal therapies. Yet this information is not being conveyed effectively and is complicated by the need to balance women’s need for information with minimizing anxiety when providing such information.
A limitation of our study is that most of our sample was Mexican origin and may not reflect experiences of Spanish-speaking Latinas of other national origin groups or outside of Northern California. Another limitation is the lack of an English-speaking comparison group, which would have permitted the identification of similarities and differences across language groups. Finally, we did not interview radiation oncologists who may have had opinions that are not represented here.
Survivorship care programs offer great promise for meeting patients’ informational and symptom management needs and improving well-being and communication with clinicians.21 Due to limited access to survivorship care information, financial hardships, and pressures from their families to resume their social roles, concerted efforts are needed to develop appropriate survivorship programs for SSBCS.22 Unique language, cultural and socioeconomic factors of Spanish-speaking Latinas require tailoring of cancer survivorship programs to best meet their needs.23 These programs need to provide psychosocial stress and symptom management assistance, simple information on recommended follow-up care, and healthy lifestyle and role reintegration strategies that account for their unique sociocultural contexts.
1. Danese MD, O’Malley C, Lindquist K, Gleeson M, Griffiths RI. An observational study of the prevalence and incidence of comorbid conditions in older women with breast cancer. Ann Oncol. 2012;23(7):1756-1765.
2. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academy of Sciences; 2006.
3. Beckjord EB, Arora NK, McLaughlin W, Oakley-Girvan I, Hamilton AS, Hesse BW. Health-related information needs in a large and diverse sample of adult cancer survivors: implications for cancer care. J Cancer Surviv. 2008;2(3):179-189.
4. Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on posttreatment cancer care: qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25(16):2270-2273.
5. Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16(3):413-428.
6. Clauser SB, Arora NK, Bellizzi KM, Haffer SC, Topor M, Hays RD. Disparities in HRQOL of cancer survivors and non-cancer managed care enrollees. Health Care Financ Rev. 2008;29(4):23-40.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Posttreatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-256.
8. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357-1362.
9. Arora NK, Reeve BB, Hays RD, Clauser SB, Oakley-Girvan I. Assessment of quality of cancer-related follow-up care from the cancer survivor’s perspective. J Clin Oncol. 2011;29(10):1280-1289.
10. Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008;113(5):1058-1067.
11. Yoon J, Malin JL, Tisnado DM, et al. Symptom management after breast cancer treatment: is it influenced by patient characteristics? Breast Cancer Res Treat. 2008;108(1):69-77.
12. Ashing K, Rosales M. A telephonic-based trial to reduce depressive symptoms among Latina breast cancer survivors. Psychooncology. 2014;23(5):507-515.
13. Hershman DL, Greenlee H, Awad D, et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat. 2013;138(3):795-806.
14. Napoles AM, Ortiz C, Santoyo-Olsson J, et al. Nuevo Amanecer: results of a randomized controlled trial of a community-based, peer-delivered stress management intervention to improve quality of life in Latinas with breast cancer. Am J Public Health. 2015;105(suppl 3):e55-63.
15. Rechis R, Reynolds KA, Beckjord EB, Nutt S, Burns RM, Schaefer JS. ‘I learned to live with it’ is not good enough: challenges reported by posttreatment cancer survivors in the Livestrong surveys. Austin, TX: Livestrong;2011.
16. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Hawthorne: Aldine Publishing Company; 1967.
17. Befort CA, Klemp J. Sequelae of breast cancer and the influence of menopausal status at diagnosis among rural breast cancer survivors. J Womens Health (Larchmt). 2011;20(9):1307-1313.
18. Fu OS, Crew KD, Jacobson JS, et al. Ethnicity and persistent symptom burden in breast cancer survivors. J Cancer Surviv. 2009;3(4):241-250.
19. Taylor TR, Huntley ED, Makambi K, et al. Understanding sleep disturbances in African-American breast cancer survivors: a pilot study. Psychooncology. 2012;21(8):896-902.
20. Adams N, Gisiger-Camata S, Hardy CM, Thomas TF, Jukkala A, Meneses K. Evaluating survivorship experiences and needs among rural African American breast cancer survivors. J Cancer Educ. October 24, 2015 [Epub ahead of print].
21. Blinder VS, Patil S, Thind A, et al. Return to work in low-income Latina and non-Latina white breast cancer survivors: a 3-year longitudinal study. Cancer. 2012;118(6):1664-1674.
22. Lopez-Class M, Perret-Gentil M, Kreling B, Caicedo L, Mandelblatt J, Graves KD. Quality of life among immigrant Latina breast cancer survivors: realities of culture and enhancing cancer care. J Cancer Educ. 2011;26(4):724-733.
23. Napoles-Springer AM, Ortiz C, O’Brien H, Diaz-Mendez M. Developing a culturally competent peer support intervention for Spanish-speaking Latinas with breast cancer. J Immigr Minor Health. 2009;11(4):268-280
1. Danese MD, O’Malley C, Lindquist K, Gleeson M, Griffiths RI. An observational study of the prevalence and incidence of comorbid conditions in older women with breast cancer. Ann Oncol. 2012;23(7):1756-1765.
2. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academy of Sciences; 2006.
3. Beckjord EB, Arora NK, McLaughlin W, Oakley-Girvan I, Hamilton AS, Hesse BW. Health-related information needs in a large and diverse sample of adult cancer survivors: implications for cancer care. J Cancer Surviv. 2008;2(3):179-189.
4. Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on posttreatment cancer care: qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25(16):2270-2273.
5. Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16(3):413-428.
6. Clauser SB, Arora NK, Bellizzi KM, Haffer SC, Topor M, Hays RD. Disparities in HRQOL of cancer survivors and non-cancer managed care enrollees. Health Care Financ Rev. 2008;29(4):23-40.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Posttreatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-256.
8. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357-1362.
9. Arora NK, Reeve BB, Hays RD, Clauser SB, Oakley-Girvan I. Assessment of quality of cancer-related follow-up care from the cancer survivor’s perspective. J Clin Oncol. 2011;29(10):1280-1289.
10. Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008;113(5):1058-1067.
11. Yoon J, Malin JL, Tisnado DM, et al. Symptom management after breast cancer treatment: is it influenced by patient characteristics? Breast Cancer Res Treat. 2008;108(1):69-77.
12. Ashing K, Rosales M. A telephonic-based trial to reduce depressive symptoms among Latina breast cancer survivors. Psychooncology. 2014;23(5):507-515.
13. Hershman DL, Greenlee H, Awad D, et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat. 2013;138(3):795-806.
14. Napoles AM, Ortiz C, Santoyo-Olsson J, et al. Nuevo Amanecer: results of a randomized controlled trial of a community-based, peer-delivered stress management intervention to improve quality of life in Latinas with breast cancer. Am J Public Health. 2015;105(suppl 3):e55-63.
15. Rechis R, Reynolds KA, Beckjord EB, Nutt S, Burns RM, Schaefer JS. ‘I learned to live with it’ is not good enough: challenges reported by posttreatment cancer survivors in the Livestrong surveys. Austin, TX: Livestrong;2011.
16. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Hawthorne: Aldine Publishing Company; 1967.
17. Befort CA, Klemp J. Sequelae of breast cancer and the influence of menopausal status at diagnosis among rural breast cancer survivors. J Womens Health (Larchmt). 2011;20(9):1307-1313.
18. Fu OS, Crew KD, Jacobson JS, et al. Ethnicity and persistent symptom burden in breast cancer survivors. J Cancer Surviv. 2009;3(4):241-250.
19. Taylor TR, Huntley ED, Makambi K, et al. Understanding sleep disturbances in African-American breast cancer survivors: a pilot study. Psychooncology. 2012;21(8):896-902.
20. Adams N, Gisiger-Camata S, Hardy CM, Thomas TF, Jukkala A, Meneses K. Evaluating survivorship experiences and needs among rural African American breast cancer survivors. J Cancer Educ. October 24, 2015 [Epub ahead of print].
21. Blinder VS, Patil S, Thind A, et al. Return to work in low-income Latina and non-Latina white breast cancer survivors: a 3-year longitudinal study. Cancer. 2012;118(6):1664-1674.
22. Lopez-Class M, Perret-Gentil M, Kreling B, Caicedo L, Mandelblatt J, Graves KD. Quality of life among immigrant Latina breast cancer survivors: realities of culture and enhancing cancer care. J Cancer Educ. 2011;26(4):724-733.
23. Napoles-Springer AM, Ortiz C, O’Brien H, Diaz-Mendez M. Developing a culturally competent peer support intervention for Spanish-speaking Latinas with breast cancer. J Immigr Minor Health. 2009;11(4):268-280
Gap analysis: a strategy to improve the quality of care of head and neck cancer patients
In the United States, there will be an estimated 49,670 new cases of head and neck cancer for 2017.1 Head and neck cancer (HNC) is a term used to describe a range of tumors that originate in the area of the body spanning from the lower neck to the upper nasal cavity.2 Specifically, they are malignancies arising in the mouth, larynx, nasal cavity, sinuses, tongue, lips, and numerous glands such as the thyroid and salivary.2 To clarify, HNC, despite the encompassing name, does not include growths of the bones, teeth, skin, brain parenchyma, and eye; therefore, such tumors will not be addressed in this article.
Patients with HNC often experience fragmented and uncoordinated care that leads to delays in cancer treatment, severe distress in patients and families, and dissatisfaction with care. Literature reports that these patients face numerous stressors including aggressive cancer treatments, severe symptoms, body image concerns, loss of speech, difficulty swallowing, nutritional issues, and respiratory problems that affect their quality of life and ability to function on a day-to-day basis.3,4In addition, patients with HNC and their families are challenged to navigate the health care system and to overcome the difficulties of accessing services within the context of financial constraints. A multidisciplinary team (MDT) approach is the standard of care for HNC patients, as demonstrated in studies reporting better 5-year survival outcomes, increased completion of adjuvant therapy, and higher compliance with speech-language pathologist (SLP) recommendations.5, 6 Furthermore, a recent systematic review of cancer teams concluded that the MDT approach leads to improved clinical outcomes and enhanced communication between the patient and the team.7
The Institute of Medicine (IOM) stated in its 2013 report on cancer care that a high-quality care delivery system requires continuing measurement of cancer care and strategies to carry out performance improvement.8 Following the IOM premise, the cancer center at an academic medical center in Philadelphia made efforts to improve patient access to multidisciplinary services, first, by creating a multidisciplinary Cancer Appetite and Rehabilitation (CARE)clinic to address the symptoms and nutritional needs of HNC patients,9 and second, by using a gap analysis to conduct an assessment of the cancer care services provided to this cancer population. The need to conduct this assessment was generated by the desire to improve access to multidisciplinary care, with the goal of meeting standard benchmarks for completion of treatment while increasing the use of ancillary services. This article describes the process of conducting a gap analysis of cancer services for HNC patients, and includes discussion of the findings, recommendations for improving care, a description of the quality improvement interventions, and a report of the outcomes based on an interval re-assessment 18 months later.
Methods
Methods included a gap analysis, implementation of quality improvement recommendations, and re-assessment of indicators (Figure). A gap analysis “identifies differences between desired and actual practice conditions, including service delivery and quality patient outcomes as measured against evidence-based benchmarks while incorporating key stakeholder concerns and expectations.”10 The gap analysis of cancer care services offered to HNC patients was achieved through the step-by-step process described hereinafter. The implementation of quality improvement recommendations was accomplished by establishing two task force committees focused respectively on education and transitions in care coordination. Re-assessment of indicators related to timeliness of delivery of cancer treatments and collection of additional baseline data regarding supportive services.
Gap analysis
Identification of the scope of the problem. Members of the HNC multidisciplinary team raised concerns about unintended breaches in care for HNC patients that resulted not only in delay of the patients’ cancer treatments, but also in unnecessary distress for the patients and their families. As a result, the HNC team decided to conduct a gap analysis to identify the barriers in care for HNC patients, and by doing this, to determine possible solutions.
Identification of best practice care indicators. The indicators of best practice care (benchmarks) for HNC patients were identified after exhaustive review of the literature11-22(Table 1). For this gap analysis, the indicators focused on waiting time to treatment (surgery, chemotherapy, radiation therapy) and to supportive care interventions (nutrition, speech and language pathology) as follows:
- 9.2Futura StdInitial ear-nose-throat (ENT) visit to surgery: <30 days
- Biopsy to start radiation therapy (RT) for nonsurgical patients: 40 days
- Surgery to RT start: 42 days
- Surgery to nutrition consultation (outpatient), start RT to nutrition: Pretreatment
- Surgery to outpatient SLP, initial ENT visit to SLP referral, surgery to SLP referral, RT start to outpatient SLP start: Pretreatment
Measure gaps against benchmarks. To Gap analysis of measure gaps against benchmarks, the authors used the Agency for Health Care Research and Quality tool that provides a systematic method to compare current practice with best practices and determine the barriers to best practice and the feasibility of implementing best practices by the institution23 (Table 1). For this project, a process map of waiting time to treatment and supportive care interventions was created, so that real-world conditions could be measured against benchmarks.
Process map. The authors identified 67 newly diagnosed HNC patients during January-July 2014 from the surgery, radiation therapy, and nutrition departments, but only 33 patients were able to be tracked from their initial visit at the cancer center until the completion of their treatment through the electronic medical record (EMR) system. Their information was compiled in a spreadsheet based on the EMR information. Data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
To map the typical patient process, the patients were split into two groups: surgical (n = 22) and nonsurgical (n = 11). Surgical patients underwent surgery as their primary treatment and received adjuvant radiation therapy or concurrent chemotherapy. Nonsurgical patients did not require surgery other than biopsy as a part of their treatment. Most of the nonsurgical patients received chemotherapy, and 1 patient received palliative radiation therapy.
SWOT analysis. The SWOT analysis is used to chart institution performance in relation to benchmarks while describing stakeholders’ perceptions.24The stakeholder perspective for this project focused on the views of the health care providers from all disciplines regarding the quality of care provided to the HNC population. In addition, a patient survey was conducted to assess their perception of the care they received.
Clinician survey. We surveyed 25 clinicians, including physicians, advanced practice providers, nurses, and allied health professionals, from the surgical (n = 3), hospitalization (n = 6), radiation (n = 3), chemotherapy (n = 3) and supportive services teams (n = 10). The survey was conducted face to face and included 7 open-ended questions designed to gain insight about problems encountered with coordination of care, suggestions to improve coordination of care, factors in treatment delays, suggestions to decrease treatment delays, factors in excellent patient outcomes, rate overall patient care, and suggestions for improvement of service. Initial survey responses were filtered by recurring themes in each question among the different patient service teams.
Patient satisfaction survey. The sample of patients was obtained from the surgery, radiation therapy, and nutrition departments during January-July 2014. Sixty-seven initial patients were identified but only 43 were eligible for interview because they had a listed phone number. A six-question nonvalidated survey was developed by the authors to measure patient satisfaction with the scheduling process, waiting time, information provided about treatment and their medical status, emotional support, the coordination of care, and the payment process. Satisfaction was rated on a scale from 1 to 5 (1 = Poor, 2 = Fair, 3 = Satisfactory, 5 = Great).
Analysis and final report. See Results section.
Quality improvement implementation. The transitions and the education committees were created to address the gaps identified during the analysis. The transitions committee developed strategies to improve the coordination of care of HNC patients throughout their cancer treatment and the education committee elaborated new ways to enhance patient education while meeting treatment timeline standards. The implementation of the interventions was developed by the inpatient and outpatient MTD teams caring for the HNC population.
Re-assessment of indicators. During January-December 2015, a total of 58 patients diagnosed with HNC were identified. Of those, 40 patients with recurrent disease were eliminated, leaving 18 patients (10 surgical, 8 nonsurgical). Similar to the initial assessment for the gap analysis, data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
Results
Most of the patients were men (70%), white (70%), and 60% were within the 50-69 years age range at the time of diagnosis.
Clinician survey
The clinicians were surveyed and their responses analyzed by two people, the project leader and the project assistant. The most commonly identified weaknesses in care that the clinicians identified were delayed access to dental referrals, insufficient preoperative patient education, and inefficient discharge planning and/or home care coordination. Dental referrals were identified as a major cause of delay in starting radiation therapy because of scheduling issues, a lack of patient motivation, limited insurance coverage, and difficulty identifying reliable dentists in the patient’s geographic area. Clinicians also identified problems coordinating smoking cessation referrals for patients.
In addition, they identified the hospitalization and/or home care phases as areas for potential improvement. During hospitalization, patients often expressed surprise upon learning that they had a feeding tube and/or tracheostomy despite having received pre-operative education. This misunderstanding by the patient was likely related to the clinicians’ assumptions about the best timing for patient education and the amount of time needed for education before the surgical procedure. The surgical team provided patient education based on individual needs, and it has not been standardized because they felt that patients’ education needs vary from person to person. In contrast, patient education prior radiation therapy is standardized, and all patients received a comprehensive package of information that is re-enforced by direct patient education by the clinicians.
Another gap in care identified by the inpatient team was a prolonged intensive care unit (ICU) stay for the HNC patients. These patients remained in the ICU for the entirety of their stay. Not only was this causing overuse of resources, but patients also felt unprepared for an independent discharge home given the high level of care received in the ICU.
A range of suggestions were made to solve these problems. The most prevalent suggestion was to use a nurse navigator to coordinate referrals, schedule appointments, facilitate interdisciplinary communication, and to address social, financial, and transport needs for HNC patients. Several other suggestions referred to standardizing treatment procedures and pre-operative patient education.
Patient survey
Forty-three patients were identified for the patient satisfaction survey. Each patient was contacted at least three times over the course of 3 weeks. Of the 43 patients, 20 had an invalid phone number, 10 were not available for participation, and 1 declined to participate. A total of 12 patients completed the survey.
Although the sample size was small, the patients surveyed were very satisfied with their care. Of the 12 patients, 5 patients rated all of the services relevant to their treatment as a 5 (Great). Areas of particular concern for the patients included the waiting time to see a physician in the ENT clinic, the explanation/collection of charges, and the accessibility of support groups. Services rated 3 (Satisfactory) included waiting time to schedule appointments; the amount of information and patient education provided by about radiation, nutrition, physical therapy (PT), occupational therapy (OT), and SLP; and overall satisfaction with care.
Surgical patients. The Danish Head and Neck Society guidelines state that the interval between the initial visit diagnosis and surgery should be within 30 days.12A comparison of the average intervals between important treatment points for the surgical sample patients with the benchmark timing recommended in the literature are shown in Table 2. The mean time from initial visit to surgery was 28 days in the cancer center sample; 67% of patients (n = 14) had surgery within 30 days, and 33% of patients (n = 7) had surgery beyond 30 days. The interval re-assessment showed improvements in this area: the mean time from initial visit to surgery went from 28 to 18 days, and 100% of patients
n = 10) had surgery within 30 days.
Huang and colleagues have indicated that postoperative radiation therapy should ideally occur within 42 days of surgery;13 however, in the present study, 79% (n = 11) of the sample surgical patients undergoing radiation began their therapy on average more than 63 days after surgery. The interval re-assessment found the same results with 80% of patients starting radiation over 42 days after surgery although the average time lag decreased from 68 days to 53 days.
Nonsurgical patients. Huang and colleagues have indicated that for patients undergoing radiation as their primary form of treatment, an interval of 40 days between biopsy and the start of radiation is ideal.13 The average intervals between important time points of treatment for patients who did not require surgery in their treatment are shown in Table 2. The cancer center met the benchmark at baseline with an average of 38 days (n = 11 patients). The re-assessment showed improvement in this area with 100% of cases (n = 10) meeting the benchmark with an average of 32 days. Likewise, the benchmark waiting time from RT consultation to RT start of less than 30 days11 was met by the cancer center for the nonsurgical group (n = 11).
Access to supportive services
Nutrition care. Studies have shown that standard nutritional care for HNC patients should start before treatment.18,19 In the present study, the waiting time from surgery to outpatient nutrition assessment improved from 61 days to 50 days (Table 2). For patients in the surgical group, the time interval between the initial ENT visit to the outpatient nutrition assessment decreased from 85 days at baseline to 66 days at reassessment, and 82 days to 35 days, respectively, for the nonsurgical group. The time interval from surgery to nutrition assessment has not reached the recommended pretreatment benchmark, but data showed a trend of improvement from 61 days at baseline to 50 days at reassessment for patients in the group.
Patients were typically referred to outpatient nutrition at the start of radiation therapy. In the initial assessment, all patients (n = 33) had access to nutrition services, but 21% (n = 7) never spoke to the nutritionist. The re-assessment found all but one (n = 7) of the patients had been seen by a nutritionist at some point during the treatment period. The benchmark of preradiation nutrition assessment was met by 2 postsurgical patients, with the remainder of the patients being seen within 3 days of the initiation of radiation.
Speech-language pathology management. The literature recommends that patients receive SLP management before the surgery.14-17 In this gap analysis, a difference in access to SLP services was identified between inpatient and outpatient settings. On average, patients within the sample were referred to outpatient SLP over a month after their surgery. In contrast, inpatient surgical patients had access to rapid consultations with SLP (eg, 1 day after surgery for total laryngectomy, and 4 days after surgery for oropharyngeal and oral surgery patients; T Hogan, unpublished data, June 2014). Overall, the benchmark was not met, as patients were not seen by the SPL prior to treatment.
New baseline data was collected about SLP services and showed that 70% of patients had contact with the outpatient SLP at some point during their treatment. Of those, only 29% of patients saw SLP before surgery, meeting the benchmark. The baseline waiting time was an average of 15 days before surgery and 43 days after surgery. Overall, the trend is moving toward the benchmark of care.
Similarly, studies determined that the gold standard of care for nonsurgical patients is that SLPs begin pretreatment management of HNC.16Patients in the baseline sample were typically referred to outpatient SLP about a month after biopsy (presumably diagnosis), but before the start of chemo-radiation. There were no data available for the number of patients who were actually seen by the outpatient SLP before the start of chemo-radiation.
The new baseline data found that 100% of nonsurgical patients were referred to SLP, but only25% (n = 2) were seen before they started chemo-radiation therapy (an average 5 days before) and 75% (n = 6) were seen after starting chemo-radiation therapy (an average 23 days after).
SWOT analysis
The SWOT analysis included strengths, weaknesses, opportunities and threats of the care provided to HNC patients at the cancer center. The gap analysis based on the results of the clinician surveys, process mapping, and patient satisfaction survey is summarized in Table 3. Three main gaps were identified: waiting time to treatment, education, and coordination of and transitions in care.
Quality improvement actions
Interventions by the outpatient MTD team included changing the process of scheduling dental appointments, creating a new approach to outpatient nutrition by proactively meeting patients in the ENT clinic, and conducting PT and SLP assessments to patients in the chemotherapy unit while receiving their treatment. A nurse navigator position for this patient population was approved and an expedited referral system was initiated. At the same time, the inpatient team implemented a specialized HNC unit in the medical-surgical floor, developed the protocols for the management of postsurgery HNC patients, educated nursing staff, and standardized patient education to facilitate transition to the next level of care (Table 3).
Discussion
The gap analysis of services provided to HNC patients at the cancer center identified three gaps in care: delay in treatment and supportive services, nonstandardized patient education, and lack of care coordination.
All patients should have access to a timely treatment initiation. In this analysis, surgical patients encountered a delay between surgery and the start of radiation therapy, about 3 weeks beyond the recommended in the literature.12 Clinicians mentioned delays in ensuring preradiation dental consultations as a significant issue affecting the patient treatment process. Re-assessment data reported that despite interventions for early dental referrals, 80% of patients still started radiation over 6 weeks after surgery; however, the average time lag decreased from 68 days to 53 days.
RT delays in HNC patients not only affect patients’ emotional state but may also impact clinical outcomes. Treatment delays have the potential to harm patients by: allowing tumor growth that impact on the curative outcomes of RT; postponing the benefits of palliative RT on symptom relief; and causing psychological distress.25 In addition, delay in starting treatment has shown to increase the risk for local recurrence,13,26 and decrease survival.27
Higher demand for advanced RT modalities has been linked to treatment delays. Waiting times from initial RT evaluation to start RT have increased over time, from <14 days in 1989 to 31 days in 1997.11 This is explained by the complexity of the pretreatment evaluations and the increasing demand of radiation services, especially in high volume institutions.25,27A fast-track program to reduce waiting time in the treatment of HNC patients reported to be effective.22 This program includes a patient coordinator, a hotline for referral procedures, prebooked slots for ENT and RT clinics, faster pathology and imaging reports, and the establishment of an MTD team.
The clinician survey identified patient needs classified in three categories: pre-operative education, hospitalization process, and access to support services. Regarding pre-operative education, clinicians acknowledged that although patients were educated about their surgical options and possible outcomes prior to hospitalization, they often could not fully understand this information at the time of the instruction. The high need for education particularly in the pretreatment phase was documented in a needs assessment survey for HNC patients conducted at the cancer center D DeMille, RD, unpublished data, August 2013).
Studies have looked at the effectiveness of education in cancer patients. The use of teaching interventions (written information, audiotapes, videotapes, and computer programs) has proven to be valuable for educating patients prior to experiencing cancer treatments.20Further, a systematic review of preparatory education for cancer patients undergoing surgery reported that face-to-face discussions appear to be effective at improving patient outcomes with regards to increasing knowledge and decreasing anxiety.21 However, it was stated that the timing of the delivery of education is critical to be efficient. For example, an education session provided one day prior the day of surgery is not useful as it may place additional stress on a patient who is already highly anxious and decreases the likelihood for the information to be managed. It is recommended to deliver education early enough prior surgery to allow time for the patient to process the information. Also, a study reported that presurgical education on potential side effects; the assessment of patients’ needs by an SLP, physical therapist, nutritionist, and social worker; and pre-operative nutritional support decrease postoperative complications.4
The education committee was created in response to the gap on patient education. The inpatient team took the lead and provided intense education on the care of HNC patients to the nursing staff and to HNC patients and their families about postoperative care at home. Education was also extended to rehabilitation facilities caring for this cancer population at discharge from the hospital.
Clinicians identified a gap during the hospitalization process. The gap included prolonged stay of patients in the ICU postsurgery, inefficient interclinician communication, lack of standardization of postsurgical care, and difficulty communicating with external home care teams. A major intervention was implemented that included the creation of a HNC specialized unit that offered a structured setting for standardized care and communication between patients and clinicians. Dedicated units for the management of HNC patients highly enhance the quality of care provided because it enables the MTD team to work properly by clearly defining roles and responsibilities, delineating evidence-based clinical interventions, and promoting expert care for this patient population.23In addition, several key steps have been recommended to reduce the fragmentation of care for hospital patients, including developing a referral/transition tracking system, organizing and training staff members to coordinate transition/referrals, and identifying and creating agreements with key care providers.28
Early patient access to supportive services was a concern to most clinicians. HNC providers were not consulting the CARE clinic about patients’ nutritional, physical and SLP needs until the patient was having serious problems. Patient tracking found that the minority of patients met the standard of having a presurgical speech referral. Most patients had access to outpatient nutrition services during radiation therapy but the majority of patients in the sample did not attend CARE clinic. The literature strongly supports early management of HNC patients by the SPL and nutrition counselor. Van der Molen and colleagues demonstrated that a pretreatment SPL rehabilitation program is feasible and offers reasonable patient compliance despite of the burden caused by ongoing chemo-radiation therapy for HNC patients.16Similarly, early nutrition counseling for HNC patients undergoing RT has reported to decrease unintended weight loss and malnutrition compared with late nutrition intervention.19
Although there are clear gaps in care for HNC patients from the clinicians’ perspective, the patients surveyed indicated a clear satisfaction with their care at the cancer center. Almost all patients were satisfied with their relationships with clinicians in the team. Some patients mentioned complaints of insufficient pre-operative education and waiting time, but there were not significant complaints about coordination, which clinicians had identified as a major issue. This is likely explained by the small sample size and the patients’ inability to see the background interclinician communication.
A crucial suggestion to address all of these gaps in care was the implementation of a nurse navigator. With the support of hospital and cancer center administration, a nurse navigator was hired to address the needs of HNC patients throughout their disease trajectory. The team agreed that the nurse navigator should make contact with HNC patients during their initial appointment at the surgical ENT office. This initial contact allows the nurse navigator to provide support and connection to resources. Thereafter, early contact with this patient population allows the nurse navigator to follow the patient through the continuum of care from biopsy and diagnosis to survivorship. The nurse navigator facilitates communication between clinicians, patients and their families; and provides emotional support to patients while helping to manage their financial and transportation needs.29
Limitations
This is a quality improvement project with a small sample size of HNC cases. Data from this gap analysis are not statistically significant; yet, are clinically relevant in the management of the HNC population at the cancer center. Likewise, the patient sample size was small, making definitive generalizations about patient experience difficult; however, the data are helpful in highlighting possible problems for patients.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2016;67:7-30.
2. National Cancer Institute. Head and neck cancers. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet. Reviewed February 1, 2013. Accessed January 26, 2017
3. Weiderholt PA, Connor NP, Hartig GK, Harari PM. Bridging gaps in multidisciplinary head and neck cancer care: nursing coordination and case management. Int J Radiat Oncol Biol Phys. 2007;69(2 suppl):S88-S91.
4. Dingman C, Hegedus PD, Likes C, McDowell P, McCarthy E, Zwilling C. A coordinated, multidisciplinary approach to caring for the patient with head and neck cancer. J Support Oncol. 2008;6(3):125-131.
5. Liao C, Kang CJ, Lee LY, et al. Association between multidisciplinary team care approach and survival rates in patients with oral cavity squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1544-1553.
6. Starmer H, Sanguineti G, Marur S, Gourin CG. Multidisciplinary head and neck cancer clinic and adherence with speech pathology. Laryngoscope. 2011;121(10):2131-2135.
7. Prades J, Remue E, van Hoof E, Borras JM. Is it worth reorganizing cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organization of MDTs and their impact on patient outcomes. Health Pol. 2015;119(4):464-474.
8. Institute of Medicine. Delivering high-quality cancer care: charting a new course for a system in crisis. 2013. www.nationalacademies.org/hmd/Reports/2013/Delivering-High-Quality-Cancer-Care-Charting-a-New-Course-for-a-System-in-Crisis.aspx. Published September 10, 2013. Accessed May 29, 2016.
9. Granda-Cameron C, DeMille D, Lynch MP, et al. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs. 2010;14(1):72-80.
10. Davis-Ajami ML, Costa L, Kulik S. Gap analysis: synergies and opportunities for effective nursing leadership. Nurs Econ. 2014;32(1):17-25.
11. Fortin A, Bairati I, Albert M, et al. Effect of treatment delay on outcome of patients with early-stage head-and-neck carcinoma receiving radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52(4):929-936.
12. Van Harten MC, Ridder M, Hamming-Vrieze O, et al. The association of treatment delay and prognosis in head and neck squamous cell carcinoma (HNSCC) in a Dutch comprehensive cancer center. Oral Oncol. 2014;50:282-290.
13. Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J ClinOncol. 2003;21(3):555-563.
14. Lazarus CL. Management of swallowing disorders in head and neck cancer patients: optimal patterns of care. Sem Speech Lang. 2000;21(4):293-310.
15. Mayer KR. Learning to speak after laryngectomy. http://speech-language-pathology-audiology.advanceweb.com/Features/Articles/Learning-to-Speak-After-Laryngectomy.aspx. Posted October 27, 2014. Accessed January 17, 2017.
16. van der Molen L, van Rossum MA, Burkhead LM, et al. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemo-radiotherapy: feasibility, compliance, and short-term effects. Dysphagia. 2011;26:155-170.
17. Starmer HM, Gourin CG. Is speech language pathologist evaluation necessary in the nonoperative treatment of head and neck cancer? Laryngoscope. 2013;123(7):1571-1572.
18. [Article in French] Meuric J, Garabige V, Blanc-Vincent MP, et al. Good clinical practice in nutritional management of head and neck cancer patients. Bull Cancer. 1999;86(10):843-854.
19. van den Berg MG, Rasmussen-Conrad EL, Wei KH, et al. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br J Nutr. 2010;104:872-877.
20. Waller A, Forshaw K, Bryant J, Mair S. Interventions for preparing patients for chemotherapy and radiotherapy: a systematic review. Supp Care Ca. 2014;22(8):2297-2308.
21. Waller A, Forshaw K, Bryant J, et al. Preparatory education for cancer patients undergoing surgery: a systematic review of volume and quality of research output over time. Patient Educ Couns. 2015;98:1540-1549.
22. Toustrup K, Lambersten K, Birke-Sorensen H, et al. Reduction in waiting time for diagnosis and treatment of head and neck cancer – a fast track study. Acta Oncol. 2011;50:636-641.
23. Bergamini C, Locati L, Bossi P et al. Does a multidisciplinary team approach in a tertiary referral centre impact on the initial management of head and neck cancer? Oral Oncol. 2016;54:54-57.
24. AHRQ. Pediatric toolkit for using the AHRQ quality indicators. http://www.ahrq.gov/professionals/systems/hospital/qitoolkit/pediatrictoolkit.html . Reviewed July 2016. Accessed January 26, 2017.
25. Mackillop WJ. Killing time: the consequences of delays in radiotherapy. Radiother Oncol. 2007;84:1-4.
26.Chen Z, King, W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87:3-16.
27. Sharma S, Bekelman J, Lin A et al. Clinical impact of prolonged diagnosis to treatment interval (DTI) among patients with ororpharyngeal squamous cell carcinoma. Oral Oncol. 2016;56:17-24.
28. Improving chronic illness care. Reducing care fragmentation. Care coordination. http://www.improvingchroniccare.org/index.php?p=Care_Coordination&s=326. Published 2010. Accessed May 28, 2016.
29. Fillion L, de Serres M, Cook S, et al. Professional patient navigation in head and neck cancer. Sem Oncol Nurs. 2009;25(3):212-221.
In the United States, there will be an estimated 49,670 new cases of head and neck cancer for 2017.1 Head and neck cancer (HNC) is a term used to describe a range of tumors that originate in the area of the body spanning from the lower neck to the upper nasal cavity.2 Specifically, they are malignancies arising in the mouth, larynx, nasal cavity, sinuses, tongue, lips, and numerous glands such as the thyroid and salivary.2 To clarify, HNC, despite the encompassing name, does not include growths of the bones, teeth, skin, brain parenchyma, and eye; therefore, such tumors will not be addressed in this article.
Patients with HNC often experience fragmented and uncoordinated care that leads to delays in cancer treatment, severe distress in patients and families, and dissatisfaction with care. Literature reports that these patients face numerous stressors including aggressive cancer treatments, severe symptoms, body image concerns, loss of speech, difficulty swallowing, nutritional issues, and respiratory problems that affect their quality of life and ability to function on a day-to-day basis.3,4In addition, patients with HNC and their families are challenged to navigate the health care system and to overcome the difficulties of accessing services within the context of financial constraints. A multidisciplinary team (MDT) approach is the standard of care for HNC patients, as demonstrated in studies reporting better 5-year survival outcomes, increased completion of adjuvant therapy, and higher compliance with speech-language pathologist (SLP) recommendations.5, 6 Furthermore, a recent systematic review of cancer teams concluded that the MDT approach leads to improved clinical outcomes and enhanced communication between the patient and the team.7
The Institute of Medicine (IOM) stated in its 2013 report on cancer care that a high-quality care delivery system requires continuing measurement of cancer care and strategies to carry out performance improvement.8 Following the IOM premise, the cancer center at an academic medical center in Philadelphia made efforts to improve patient access to multidisciplinary services, first, by creating a multidisciplinary Cancer Appetite and Rehabilitation (CARE)clinic to address the symptoms and nutritional needs of HNC patients,9 and second, by using a gap analysis to conduct an assessment of the cancer care services provided to this cancer population. The need to conduct this assessment was generated by the desire to improve access to multidisciplinary care, with the goal of meeting standard benchmarks for completion of treatment while increasing the use of ancillary services. This article describes the process of conducting a gap analysis of cancer services for HNC patients, and includes discussion of the findings, recommendations for improving care, a description of the quality improvement interventions, and a report of the outcomes based on an interval re-assessment 18 months later.
Methods
Methods included a gap analysis, implementation of quality improvement recommendations, and re-assessment of indicators (Figure). A gap analysis “identifies differences between desired and actual practice conditions, including service delivery and quality patient outcomes as measured against evidence-based benchmarks while incorporating key stakeholder concerns and expectations.”10 The gap analysis of cancer care services offered to HNC patients was achieved through the step-by-step process described hereinafter. The implementation of quality improvement recommendations was accomplished by establishing two task force committees focused respectively on education and transitions in care coordination. Re-assessment of indicators related to timeliness of delivery of cancer treatments and collection of additional baseline data regarding supportive services.
Gap analysis
Identification of the scope of the problem. Members of the HNC multidisciplinary team raised concerns about unintended breaches in care for HNC patients that resulted not only in delay of the patients’ cancer treatments, but also in unnecessary distress for the patients and their families. As a result, the HNC team decided to conduct a gap analysis to identify the barriers in care for HNC patients, and by doing this, to determine possible solutions.
Identification of best practice care indicators. The indicators of best practice care (benchmarks) for HNC patients were identified after exhaustive review of the literature11-22(Table 1). For this gap analysis, the indicators focused on waiting time to treatment (surgery, chemotherapy, radiation therapy) and to supportive care interventions (nutrition, speech and language pathology) as follows:
- 9.2Futura StdInitial ear-nose-throat (ENT) visit to surgery: <30 days
- Biopsy to start radiation therapy (RT) for nonsurgical patients: 40 days
- Surgery to RT start: 42 days
- Surgery to nutrition consultation (outpatient), start RT to nutrition: Pretreatment
- Surgery to outpatient SLP, initial ENT visit to SLP referral, surgery to SLP referral, RT start to outpatient SLP start: Pretreatment
Measure gaps against benchmarks. To Gap analysis of measure gaps against benchmarks, the authors used the Agency for Health Care Research and Quality tool that provides a systematic method to compare current practice with best practices and determine the barriers to best practice and the feasibility of implementing best practices by the institution23 (Table 1). For this project, a process map of waiting time to treatment and supportive care interventions was created, so that real-world conditions could be measured against benchmarks.
Process map. The authors identified 67 newly diagnosed HNC patients during January-July 2014 from the surgery, radiation therapy, and nutrition departments, but only 33 patients were able to be tracked from their initial visit at the cancer center until the completion of their treatment through the electronic medical record (EMR) system. Their information was compiled in a spreadsheet based on the EMR information. Data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
To map the typical patient process, the patients were split into two groups: surgical (n = 22) and nonsurgical (n = 11). Surgical patients underwent surgery as their primary treatment and received adjuvant radiation therapy or concurrent chemotherapy. Nonsurgical patients did not require surgery other than biopsy as a part of their treatment. Most of the nonsurgical patients received chemotherapy, and 1 patient received palliative radiation therapy.
SWOT analysis. The SWOT analysis is used to chart institution performance in relation to benchmarks while describing stakeholders’ perceptions.24The stakeholder perspective for this project focused on the views of the health care providers from all disciplines regarding the quality of care provided to the HNC population. In addition, a patient survey was conducted to assess their perception of the care they received.
Clinician survey. We surveyed 25 clinicians, including physicians, advanced practice providers, nurses, and allied health professionals, from the surgical (n = 3), hospitalization (n = 6), radiation (n = 3), chemotherapy (n = 3) and supportive services teams (n = 10). The survey was conducted face to face and included 7 open-ended questions designed to gain insight about problems encountered with coordination of care, suggestions to improve coordination of care, factors in treatment delays, suggestions to decrease treatment delays, factors in excellent patient outcomes, rate overall patient care, and suggestions for improvement of service. Initial survey responses were filtered by recurring themes in each question among the different patient service teams.
Patient satisfaction survey. The sample of patients was obtained from the surgery, radiation therapy, and nutrition departments during January-July 2014. Sixty-seven initial patients were identified but only 43 were eligible for interview because they had a listed phone number. A six-question nonvalidated survey was developed by the authors to measure patient satisfaction with the scheduling process, waiting time, information provided about treatment and their medical status, emotional support, the coordination of care, and the payment process. Satisfaction was rated on a scale from 1 to 5 (1 = Poor, 2 = Fair, 3 = Satisfactory, 5 = Great).
Analysis and final report. See Results section.
Quality improvement implementation. The transitions and the education committees were created to address the gaps identified during the analysis. The transitions committee developed strategies to improve the coordination of care of HNC patients throughout their cancer treatment and the education committee elaborated new ways to enhance patient education while meeting treatment timeline standards. The implementation of the interventions was developed by the inpatient and outpatient MTD teams caring for the HNC population.
Re-assessment of indicators. During January-December 2015, a total of 58 patients diagnosed with HNC were identified. Of those, 40 patients with recurrent disease were eliminated, leaving 18 patients (10 surgical, 8 nonsurgical). Similar to the initial assessment for the gap analysis, data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
Results
Most of the patients were men (70%), white (70%), and 60% were within the 50-69 years age range at the time of diagnosis.
Clinician survey
The clinicians were surveyed and their responses analyzed by two people, the project leader and the project assistant. The most commonly identified weaknesses in care that the clinicians identified were delayed access to dental referrals, insufficient preoperative patient education, and inefficient discharge planning and/or home care coordination. Dental referrals were identified as a major cause of delay in starting radiation therapy because of scheduling issues, a lack of patient motivation, limited insurance coverage, and difficulty identifying reliable dentists in the patient’s geographic area. Clinicians also identified problems coordinating smoking cessation referrals for patients.
In addition, they identified the hospitalization and/or home care phases as areas for potential improvement. During hospitalization, patients often expressed surprise upon learning that they had a feeding tube and/or tracheostomy despite having received pre-operative education. This misunderstanding by the patient was likely related to the clinicians’ assumptions about the best timing for patient education and the amount of time needed for education before the surgical procedure. The surgical team provided patient education based on individual needs, and it has not been standardized because they felt that patients’ education needs vary from person to person. In contrast, patient education prior radiation therapy is standardized, and all patients received a comprehensive package of information that is re-enforced by direct patient education by the clinicians.
Another gap in care identified by the inpatient team was a prolonged intensive care unit (ICU) stay for the HNC patients. These patients remained in the ICU for the entirety of their stay. Not only was this causing overuse of resources, but patients also felt unprepared for an independent discharge home given the high level of care received in the ICU.
A range of suggestions were made to solve these problems. The most prevalent suggestion was to use a nurse navigator to coordinate referrals, schedule appointments, facilitate interdisciplinary communication, and to address social, financial, and transport needs for HNC patients. Several other suggestions referred to standardizing treatment procedures and pre-operative patient education.
Patient survey
Forty-three patients were identified for the patient satisfaction survey. Each patient was contacted at least three times over the course of 3 weeks. Of the 43 patients, 20 had an invalid phone number, 10 were not available for participation, and 1 declined to participate. A total of 12 patients completed the survey.
Although the sample size was small, the patients surveyed were very satisfied with their care. Of the 12 patients, 5 patients rated all of the services relevant to their treatment as a 5 (Great). Areas of particular concern for the patients included the waiting time to see a physician in the ENT clinic, the explanation/collection of charges, and the accessibility of support groups. Services rated 3 (Satisfactory) included waiting time to schedule appointments; the amount of information and patient education provided by about radiation, nutrition, physical therapy (PT), occupational therapy (OT), and SLP; and overall satisfaction with care.
Surgical patients. The Danish Head and Neck Society guidelines state that the interval between the initial visit diagnosis and surgery should be within 30 days.12A comparison of the average intervals between important treatment points for the surgical sample patients with the benchmark timing recommended in the literature are shown in Table 2. The mean time from initial visit to surgery was 28 days in the cancer center sample; 67% of patients (n = 14) had surgery within 30 days, and 33% of patients (n = 7) had surgery beyond 30 days. The interval re-assessment showed improvements in this area: the mean time from initial visit to surgery went from 28 to 18 days, and 100% of patients
n = 10) had surgery within 30 days.
Huang and colleagues have indicated that postoperative radiation therapy should ideally occur within 42 days of surgery;13 however, in the present study, 79% (n = 11) of the sample surgical patients undergoing radiation began their therapy on average more than 63 days after surgery. The interval re-assessment found the same results with 80% of patients starting radiation over 42 days after surgery although the average time lag decreased from 68 days to 53 days.
Nonsurgical patients. Huang and colleagues have indicated that for patients undergoing radiation as their primary form of treatment, an interval of 40 days between biopsy and the start of radiation is ideal.13 The average intervals between important time points of treatment for patients who did not require surgery in their treatment are shown in Table 2. The cancer center met the benchmark at baseline with an average of 38 days (n = 11 patients). The re-assessment showed improvement in this area with 100% of cases (n = 10) meeting the benchmark with an average of 32 days. Likewise, the benchmark waiting time from RT consultation to RT start of less than 30 days11 was met by the cancer center for the nonsurgical group (n = 11).
Access to supportive services
Nutrition care. Studies have shown that standard nutritional care for HNC patients should start before treatment.18,19 In the present study, the waiting time from surgery to outpatient nutrition assessment improved from 61 days to 50 days (Table 2). For patients in the surgical group, the time interval between the initial ENT visit to the outpatient nutrition assessment decreased from 85 days at baseline to 66 days at reassessment, and 82 days to 35 days, respectively, for the nonsurgical group. The time interval from surgery to nutrition assessment has not reached the recommended pretreatment benchmark, but data showed a trend of improvement from 61 days at baseline to 50 days at reassessment for patients in the group.
Patients were typically referred to outpatient nutrition at the start of radiation therapy. In the initial assessment, all patients (n = 33) had access to nutrition services, but 21% (n = 7) never spoke to the nutritionist. The re-assessment found all but one (n = 7) of the patients had been seen by a nutritionist at some point during the treatment period. The benchmark of preradiation nutrition assessment was met by 2 postsurgical patients, with the remainder of the patients being seen within 3 days of the initiation of radiation.
Speech-language pathology management. The literature recommends that patients receive SLP management before the surgery.14-17 In this gap analysis, a difference in access to SLP services was identified between inpatient and outpatient settings. On average, patients within the sample were referred to outpatient SLP over a month after their surgery. In contrast, inpatient surgical patients had access to rapid consultations with SLP (eg, 1 day after surgery for total laryngectomy, and 4 days after surgery for oropharyngeal and oral surgery patients; T Hogan, unpublished data, June 2014). Overall, the benchmark was not met, as patients were not seen by the SPL prior to treatment.
New baseline data was collected about SLP services and showed that 70% of patients had contact with the outpatient SLP at some point during their treatment. Of those, only 29% of patients saw SLP before surgery, meeting the benchmark. The baseline waiting time was an average of 15 days before surgery and 43 days after surgery. Overall, the trend is moving toward the benchmark of care.
Similarly, studies determined that the gold standard of care for nonsurgical patients is that SLPs begin pretreatment management of HNC.16Patients in the baseline sample were typically referred to outpatient SLP about a month after biopsy (presumably diagnosis), but before the start of chemo-radiation. There were no data available for the number of patients who were actually seen by the outpatient SLP before the start of chemo-radiation.
The new baseline data found that 100% of nonsurgical patients were referred to SLP, but only25% (n = 2) were seen before they started chemo-radiation therapy (an average 5 days before) and 75% (n = 6) were seen after starting chemo-radiation therapy (an average 23 days after).
SWOT analysis
The SWOT analysis included strengths, weaknesses, opportunities and threats of the care provided to HNC patients at the cancer center. The gap analysis based on the results of the clinician surveys, process mapping, and patient satisfaction survey is summarized in Table 3. Three main gaps were identified: waiting time to treatment, education, and coordination of and transitions in care.
Quality improvement actions
Interventions by the outpatient MTD team included changing the process of scheduling dental appointments, creating a new approach to outpatient nutrition by proactively meeting patients in the ENT clinic, and conducting PT and SLP assessments to patients in the chemotherapy unit while receiving their treatment. A nurse navigator position for this patient population was approved and an expedited referral system was initiated. At the same time, the inpatient team implemented a specialized HNC unit in the medical-surgical floor, developed the protocols for the management of postsurgery HNC patients, educated nursing staff, and standardized patient education to facilitate transition to the next level of care (Table 3).
Discussion
The gap analysis of services provided to HNC patients at the cancer center identified three gaps in care: delay in treatment and supportive services, nonstandardized patient education, and lack of care coordination.
All patients should have access to a timely treatment initiation. In this analysis, surgical patients encountered a delay between surgery and the start of radiation therapy, about 3 weeks beyond the recommended in the literature.12 Clinicians mentioned delays in ensuring preradiation dental consultations as a significant issue affecting the patient treatment process. Re-assessment data reported that despite interventions for early dental referrals, 80% of patients still started radiation over 6 weeks after surgery; however, the average time lag decreased from 68 days to 53 days.
RT delays in HNC patients not only affect patients’ emotional state but may also impact clinical outcomes. Treatment delays have the potential to harm patients by: allowing tumor growth that impact on the curative outcomes of RT; postponing the benefits of palliative RT on symptom relief; and causing psychological distress.25 In addition, delay in starting treatment has shown to increase the risk for local recurrence,13,26 and decrease survival.27
Higher demand for advanced RT modalities has been linked to treatment delays. Waiting times from initial RT evaluation to start RT have increased over time, from <14 days in 1989 to 31 days in 1997.11 This is explained by the complexity of the pretreatment evaluations and the increasing demand of radiation services, especially in high volume institutions.25,27A fast-track program to reduce waiting time in the treatment of HNC patients reported to be effective.22 This program includes a patient coordinator, a hotline for referral procedures, prebooked slots for ENT and RT clinics, faster pathology and imaging reports, and the establishment of an MTD team.
The clinician survey identified patient needs classified in three categories: pre-operative education, hospitalization process, and access to support services. Regarding pre-operative education, clinicians acknowledged that although patients were educated about their surgical options and possible outcomes prior to hospitalization, they often could not fully understand this information at the time of the instruction. The high need for education particularly in the pretreatment phase was documented in a needs assessment survey for HNC patients conducted at the cancer center D DeMille, RD, unpublished data, August 2013).
Studies have looked at the effectiveness of education in cancer patients. The use of teaching interventions (written information, audiotapes, videotapes, and computer programs) has proven to be valuable for educating patients prior to experiencing cancer treatments.20Further, a systematic review of preparatory education for cancer patients undergoing surgery reported that face-to-face discussions appear to be effective at improving patient outcomes with regards to increasing knowledge and decreasing anxiety.21 However, it was stated that the timing of the delivery of education is critical to be efficient. For example, an education session provided one day prior the day of surgery is not useful as it may place additional stress on a patient who is already highly anxious and decreases the likelihood for the information to be managed. It is recommended to deliver education early enough prior surgery to allow time for the patient to process the information. Also, a study reported that presurgical education on potential side effects; the assessment of patients’ needs by an SLP, physical therapist, nutritionist, and social worker; and pre-operative nutritional support decrease postoperative complications.4
The education committee was created in response to the gap on patient education. The inpatient team took the lead and provided intense education on the care of HNC patients to the nursing staff and to HNC patients and their families about postoperative care at home. Education was also extended to rehabilitation facilities caring for this cancer population at discharge from the hospital.
Clinicians identified a gap during the hospitalization process. The gap included prolonged stay of patients in the ICU postsurgery, inefficient interclinician communication, lack of standardization of postsurgical care, and difficulty communicating with external home care teams. A major intervention was implemented that included the creation of a HNC specialized unit that offered a structured setting for standardized care and communication between patients and clinicians. Dedicated units for the management of HNC patients highly enhance the quality of care provided because it enables the MTD team to work properly by clearly defining roles and responsibilities, delineating evidence-based clinical interventions, and promoting expert care for this patient population.23In addition, several key steps have been recommended to reduce the fragmentation of care for hospital patients, including developing a referral/transition tracking system, organizing and training staff members to coordinate transition/referrals, and identifying and creating agreements with key care providers.28
Early patient access to supportive services was a concern to most clinicians. HNC providers were not consulting the CARE clinic about patients’ nutritional, physical and SLP needs until the patient was having serious problems. Patient tracking found that the minority of patients met the standard of having a presurgical speech referral. Most patients had access to outpatient nutrition services during radiation therapy but the majority of patients in the sample did not attend CARE clinic. The literature strongly supports early management of HNC patients by the SPL and nutrition counselor. Van der Molen and colleagues demonstrated that a pretreatment SPL rehabilitation program is feasible and offers reasonable patient compliance despite of the burden caused by ongoing chemo-radiation therapy for HNC patients.16Similarly, early nutrition counseling for HNC patients undergoing RT has reported to decrease unintended weight loss and malnutrition compared with late nutrition intervention.19
Although there are clear gaps in care for HNC patients from the clinicians’ perspective, the patients surveyed indicated a clear satisfaction with their care at the cancer center. Almost all patients were satisfied with their relationships with clinicians in the team. Some patients mentioned complaints of insufficient pre-operative education and waiting time, but there were not significant complaints about coordination, which clinicians had identified as a major issue. This is likely explained by the small sample size and the patients’ inability to see the background interclinician communication.
A crucial suggestion to address all of these gaps in care was the implementation of a nurse navigator. With the support of hospital and cancer center administration, a nurse navigator was hired to address the needs of HNC patients throughout their disease trajectory. The team agreed that the nurse navigator should make contact with HNC patients during their initial appointment at the surgical ENT office. This initial contact allows the nurse navigator to provide support and connection to resources. Thereafter, early contact with this patient population allows the nurse navigator to follow the patient through the continuum of care from biopsy and diagnosis to survivorship. The nurse navigator facilitates communication between clinicians, patients and their families; and provides emotional support to patients while helping to manage their financial and transportation needs.29
Limitations
This is a quality improvement project with a small sample size of HNC cases. Data from this gap analysis are not statistically significant; yet, are clinically relevant in the management of the HNC population at the cancer center. Likewise, the patient sample size was small, making definitive generalizations about patient experience difficult; however, the data are helpful in highlighting possible problems for patients.
In the United States, there will be an estimated 49,670 new cases of head and neck cancer for 2017.1 Head and neck cancer (HNC) is a term used to describe a range of tumors that originate in the area of the body spanning from the lower neck to the upper nasal cavity.2 Specifically, they are malignancies arising in the mouth, larynx, nasal cavity, sinuses, tongue, lips, and numerous glands such as the thyroid and salivary.2 To clarify, HNC, despite the encompassing name, does not include growths of the bones, teeth, skin, brain parenchyma, and eye; therefore, such tumors will not be addressed in this article.
Patients with HNC often experience fragmented and uncoordinated care that leads to delays in cancer treatment, severe distress in patients and families, and dissatisfaction with care. Literature reports that these patients face numerous stressors including aggressive cancer treatments, severe symptoms, body image concerns, loss of speech, difficulty swallowing, nutritional issues, and respiratory problems that affect their quality of life and ability to function on a day-to-day basis.3,4In addition, patients with HNC and their families are challenged to navigate the health care system and to overcome the difficulties of accessing services within the context of financial constraints. A multidisciplinary team (MDT) approach is the standard of care for HNC patients, as demonstrated in studies reporting better 5-year survival outcomes, increased completion of adjuvant therapy, and higher compliance with speech-language pathologist (SLP) recommendations.5, 6 Furthermore, a recent systematic review of cancer teams concluded that the MDT approach leads to improved clinical outcomes and enhanced communication between the patient and the team.7
The Institute of Medicine (IOM) stated in its 2013 report on cancer care that a high-quality care delivery system requires continuing measurement of cancer care and strategies to carry out performance improvement.8 Following the IOM premise, the cancer center at an academic medical center in Philadelphia made efforts to improve patient access to multidisciplinary services, first, by creating a multidisciplinary Cancer Appetite and Rehabilitation (CARE)clinic to address the symptoms and nutritional needs of HNC patients,9 and second, by using a gap analysis to conduct an assessment of the cancer care services provided to this cancer population. The need to conduct this assessment was generated by the desire to improve access to multidisciplinary care, with the goal of meeting standard benchmarks for completion of treatment while increasing the use of ancillary services. This article describes the process of conducting a gap analysis of cancer services for HNC patients, and includes discussion of the findings, recommendations for improving care, a description of the quality improvement interventions, and a report of the outcomes based on an interval re-assessment 18 months later.
Methods
Methods included a gap analysis, implementation of quality improvement recommendations, and re-assessment of indicators (Figure). A gap analysis “identifies differences between desired and actual practice conditions, including service delivery and quality patient outcomes as measured against evidence-based benchmarks while incorporating key stakeholder concerns and expectations.”10 The gap analysis of cancer care services offered to HNC patients was achieved through the step-by-step process described hereinafter. The implementation of quality improvement recommendations was accomplished by establishing two task force committees focused respectively on education and transitions in care coordination. Re-assessment of indicators related to timeliness of delivery of cancer treatments and collection of additional baseline data regarding supportive services.
Gap analysis
Identification of the scope of the problem. Members of the HNC multidisciplinary team raised concerns about unintended breaches in care for HNC patients that resulted not only in delay of the patients’ cancer treatments, but also in unnecessary distress for the patients and their families. As a result, the HNC team decided to conduct a gap analysis to identify the barriers in care for HNC patients, and by doing this, to determine possible solutions.
Identification of best practice care indicators. The indicators of best practice care (benchmarks) for HNC patients were identified after exhaustive review of the literature11-22(Table 1). For this gap analysis, the indicators focused on waiting time to treatment (surgery, chemotherapy, radiation therapy) and to supportive care interventions (nutrition, speech and language pathology) as follows:
- 9.2Futura StdInitial ear-nose-throat (ENT) visit to surgery: <30 days
- Biopsy to start radiation therapy (RT) for nonsurgical patients: 40 days
- Surgery to RT start: 42 days
- Surgery to nutrition consultation (outpatient), start RT to nutrition: Pretreatment
- Surgery to outpatient SLP, initial ENT visit to SLP referral, surgery to SLP referral, RT start to outpatient SLP start: Pretreatment
Measure gaps against benchmarks. To Gap analysis of measure gaps against benchmarks, the authors used the Agency for Health Care Research and Quality tool that provides a systematic method to compare current practice with best practices and determine the barriers to best practice and the feasibility of implementing best practices by the institution23 (Table 1). For this project, a process map of waiting time to treatment and supportive care interventions was created, so that real-world conditions could be measured against benchmarks.
Process map. The authors identified 67 newly diagnosed HNC patients during January-July 2014 from the surgery, radiation therapy, and nutrition departments, but only 33 patients were able to be tracked from their initial visit at the cancer center until the completion of their treatment through the electronic medical record (EMR) system. Their information was compiled in a spreadsheet based on the EMR information. Data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
To map the typical patient process, the patients were split into two groups: surgical (n = 22) and nonsurgical (n = 11). Surgical patients underwent surgery as their primary treatment and received adjuvant radiation therapy or concurrent chemotherapy. Nonsurgical patients did not require surgery other than biopsy as a part of their treatment. Most of the nonsurgical patients received chemotherapy, and 1 patient received palliative radiation therapy.
SWOT analysis. The SWOT analysis is used to chart institution performance in relation to benchmarks while describing stakeholders’ perceptions.24The stakeholder perspective for this project focused on the views of the health care providers from all disciplines regarding the quality of care provided to the HNC population. In addition, a patient survey was conducted to assess their perception of the care they received.
Clinician survey. We surveyed 25 clinicians, including physicians, advanced practice providers, nurses, and allied health professionals, from the surgical (n = 3), hospitalization (n = 6), radiation (n = 3), chemotherapy (n = 3) and supportive services teams (n = 10). The survey was conducted face to face and included 7 open-ended questions designed to gain insight about problems encountered with coordination of care, suggestions to improve coordination of care, factors in treatment delays, suggestions to decrease treatment delays, factors in excellent patient outcomes, rate overall patient care, and suggestions for improvement of service. Initial survey responses were filtered by recurring themes in each question among the different patient service teams.
Patient satisfaction survey. The sample of patients was obtained from the surgery, radiation therapy, and nutrition departments during January-July 2014. Sixty-seven initial patients were identified but only 43 were eligible for interview because they had a listed phone number. A six-question nonvalidated survey was developed by the authors to measure patient satisfaction with the scheduling process, waiting time, information provided about treatment and their medical status, emotional support, the coordination of care, and the payment process. Satisfaction was rated on a scale from 1 to 5 (1 = Poor, 2 = Fair, 3 = Satisfactory, 5 = Great).
Analysis and final report. See Results section.
Quality improvement implementation. The transitions and the education committees were created to address the gaps identified during the analysis. The transitions committee developed strategies to improve the coordination of care of HNC patients throughout their cancer treatment and the education committee elaborated new ways to enhance patient education while meeting treatment timeline standards. The implementation of the interventions was developed by the inpatient and outpatient MTD teams caring for the HNC population.
Re-assessment of indicators. During January-December 2015, a total of 58 patients diagnosed with HNC were identified. Of those, 40 patients with recurrent disease were eliminated, leaving 18 patients (10 surgical, 8 nonsurgical). Similar to the initial assessment for the gap analysis, data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
Results
Most of the patients were men (70%), white (70%), and 60% were within the 50-69 years age range at the time of diagnosis.
Clinician survey
The clinicians were surveyed and their responses analyzed by two people, the project leader and the project assistant. The most commonly identified weaknesses in care that the clinicians identified were delayed access to dental referrals, insufficient preoperative patient education, and inefficient discharge planning and/or home care coordination. Dental referrals were identified as a major cause of delay in starting radiation therapy because of scheduling issues, a lack of patient motivation, limited insurance coverage, and difficulty identifying reliable dentists in the patient’s geographic area. Clinicians also identified problems coordinating smoking cessation referrals for patients.
In addition, they identified the hospitalization and/or home care phases as areas for potential improvement. During hospitalization, patients often expressed surprise upon learning that they had a feeding tube and/or tracheostomy despite having received pre-operative education. This misunderstanding by the patient was likely related to the clinicians’ assumptions about the best timing for patient education and the amount of time needed for education before the surgical procedure. The surgical team provided patient education based on individual needs, and it has not been standardized because they felt that patients’ education needs vary from person to person. In contrast, patient education prior radiation therapy is standardized, and all patients received a comprehensive package of information that is re-enforced by direct patient education by the clinicians.
Another gap in care identified by the inpatient team was a prolonged intensive care unit (ICU) stay for the HNC patients. These patients remained in the ICU for the entirety of their stay. Not only was this causing overuse of resources, but patients also felt unprepared for an independent discharge home given the high level of care received in the ICU.
A range of suggestions were made to solve these problems. The most prevalent suggestion was to use a nurse navigator to coordinate referrals, schedule appointments, facilitate interdisciplinary communication, and to address social, financial, and transport needs for HNC patients. Several other suggestions referred to standardizing treatment procedures and pre-operative patient education.
Patient survey
Forty-three patients were identified for the patient satisfaction survey. Each patient was contacted at least three times over the course of 3 weeks. Of the 43 patients, 20 had an invalid phone number, 10 were not available for participation, and 1 declined to participate. A total of 12 patients completed the survey.
Although the sample size was small, the patients surveyed were very satisfied with their care. Of the 12 patients, 5 patients rated all of the services relevant to their treatment as a 5 (Great). Areas of particular concern for the patients included the waiting time to see a physician in the ENT clinic, the explanation/collection of charges, and the accessibility of support groups. Services rated 3 (Satisfactory) included waiting time to schedule appointments; the amount of information and patient education provided by about radiation, nutrition, physical therapy (PT), occupational therapy (OT), and SLP; and overall satisfaction with care.
Surgical patients. The Danish Head and Neck Society guidelines state that the interval between the initial visit diagnosis and surgery should be within 30 days.12A comparison of the average intervals between important treatment points for the surgical sample patients with the benchmark timing recommended in the literature are shown in Table 2. The mean time from initial visit to surgery was 28 days in the cancer center sample; 67% of patients (n = 14) had surgery within 30 days, and 33% of patients (n = 7) had surgery beyond 30 days. The interval re-assessment showed improvements in this area: the mean time from initial visit to surgery went from 28 to 18 days, and 100% of patients
n = 10) had surgery within 30 days.
Huang and colleagues have indicated that postoperative radiation therapy should ideally occur within 42 days of surgery;13 however, in the present study, 79% (n = 11) of the sample surgical patients undergoing radiation began their therapy on average more than 63 days after surgery. The interval re-assessment found the same results with 80% of patients starting radiation over 42 days after surgery although the average time lag decreased from 68 days to 53 days.
Nonsurgical patients. Huang and colleagues have indicated that for patients undergoing radiation as their primary form of treatment, an interval of 40 days between biopsy and the start of radiation is ideal.13 The average intervals between important time points of treatment for patients who did not require surgery in their treatment are shown in Table 2. The cancer center met the benchmark at baseline with an average of 38 days (n = 11 patients). The re-assessment showed improvement in this area with 100% of cases (n = 10) meeting the benchmark with an average of 32 days. Likewise, the benchmark waiting time from RT consultation to RT start of less than 30 days11 was met by the cancer center for the nonsurgical group (n = 11).
Access to supportive services
Nutrition care. Studies have shown that standard nutritional care for HNC patients should start before treatment.18,19 In the present study, the waiting time from surgery to outpatient nutrition assessment improved from 61 days to 50 days (Table 2). For patients in the surgical group, the time interval between the initial ENT visit to the outpatient nutrition assessment decreased from 85 days at baseline to 66 days at reassessment, and 82 days to 35 days, respectively, for the nonsurgical group. The time interval from surgery to nutrition assessment has not reached the recommended pretreatment benchmark, but data showed a trend of improvement from 61 days at baseline to 50 days at reassessment for patients in the group.
Patients were typically referred to outpatient nutrition at the start of radiation therapy. In the initial assessment, all patients (n = 33) had access to nutrition services, but 21% (n = 7) never spoke to the nutritionist. The re-assessment found all but one (n = 7) of the patients had been seen by a nutritionist at some point during the treatment period. The benchmark of preradiation nutrition assessment was met by 2 postsurgical patients, with the remainder of the patients being seen within 3 days of the initiation of radiation.
Speech-language pathology management. The literature recommends that patients receive SLP management before the surgery.14-17 In this gap analysis, a difference in access to SLP services was identified between inpatient and outpatient settings. On average, patients within the sample were referred to outpatient SLP over a month after their surgery. In contrast, inpatient surgical patients had access to rapid consultations with SLP (eg, 1 day after surgery for total laryngectomy, and 4 days after surgery for oropharyngeal and oral surgery patients; T Hogan, unpublished data, June 2014). Overall, the benchmark was not met, as patients were not seen by the SPL prior to treatment.
New baseline data was collected about SLP services and showed that 70% of patients had contact with the outpatient SLP at some point during their treatment. Of those, only 29% of patients saw SLP before surgery, meeting the benchmark. The baseline waiting time was an average of 15 days before surgery and 43 days after surgery. Overall, the trend is moving toward the benchmark of care.
Similarly, studies determined that the gold standard of care for nonsurgical patients is that SLPs begin pretreatment management of HNC.16Patients in the baseline sample were typically referred to outpatient SLP about a month after biopsy (presumably diagnosis), but before the start of chemo-radiation. There were no data available for the number of patients who were actually seen by the outpatient SLP before the start of chemo-radiation.
The new baseline data found that 100% of nonsurgical patients were referred to SLP, but only25% (n = 2) were seen before they started chemo-radiation therapy (an average 5 days before) and 75% (n = 6) were seen after starting chemo-radiation therapy (an average 23 days after).
SWOT analysis
The SWOT analysis included strengths, weaknesses, opportunities and threats of the care provided to HNC patients at the cancer center. The gap analysis based on the results of the clinician surveys, process mapping, and patient satisfaction survey is summarized in Table 3. Three main gaps were identified: waiting time to treatment, education, and coordination of and transitions in care.
Quality improvement actions
Interventions by the outpatient MTD team included changing the process of scheduling dental appointments, creating a new approach to outpatient nutrition by proactively meeting patients in the ENT clinic, and conducting PT and SLP assessments to patients in the chemotherapy unit while receiving their treatment. A nurse navigator position for this patient population was approved and an expedited referral system was initiated. At the same time, the inpatient team implemented a specialized HNC unit in the medical-surgical floor, developed the protocols for the management of postsurgery HNC patients, educated nursing staff, and standardized patient education to facilitate transition to the next level of care (Table 3).
Discussion
The gap analysis of services provided to HNC patients at the cancer center identified three gaps in care: delay in treatment and supportive services, nonstandardized patient education, and lack of care coordination.
All patients should have access to a timely treatment initiation. In this analysis, surgical patients encountered a delay between surgery and the start of radiation therapy, about 3 weeks beyond the recommended in the literature.12 Clinicians mentioned delays in ensuring preradiation dental consultations as a significant issue affecting the patient treatment process. Re-assessment data reported that despite interventions for early dental referrals, 80% of patients still started radiation over 6 weeks after surgery; however, the average time lag decreased from 68 days to 53 days.
RT delays in HNC patients not only affect patients’ emotional state but may also impact clinical outcomes. Treatment delays have the potential to harm patients by: allowing tumor growth that impact on the curative outcomes of RT; postponing the benefits of palliative RT on symptom relief; and causing psychological distress.25 In addition, delay in starting treatment has shown to increase the risk for local recurrence,13,26 and decrease survival.27
Higher demand for advanced RT modalities has been linked to treatment delays. Waiting times from initial RT evaluation to start RT have increased over time, from <14 days in 1989 to 31 days in 1997.11 This is explained by the complexity of the pretreatment evaluations and the increasing demand of radiation services, especially in high volume institutions.25,27A fast-track program to reduce waiting time in the treatment of HNC patients reported to be effective.22 This program includes a patient coordinator, a hotline for referral procedures, prebooked slots for ENT and RT clinics, faster pathology and imaging reports, and the establishment of an MTD team.
The clinician survey identified patient needs classified in three categories: pre-operative education, hospitalization process, and access to support services. Regarding pre-operative education, clinicians acknowledged that although patients were educated about their surgical options and possible outcomes prior to hospitalization, they often could not fully understand this information at the time of the instruction. The high need for education particularly in the pretreatment phase was documented in a needs assessment survey for HNC patients conducted at the cancer center D DeMille, RD, unpublished data, August 2013).
Studies have looked at the effectiveness of education in cancer patients. The use of teaching interventions (written information, audiotapes, videotapes, and computer programs) has proven to be valuable for educating patients prior to experiencing cancer treatments.20Further, a systematic review of preparatory education for cancer patients undergoing surgery reported that face-to-face discussions appear to be effective at improving patient outcomes with regards to increasing knowledge and decreasing anxiety.21 However, it was stated that the timing of the delivery of education is critical to be efficient. For example, an education session provided one day prior the day of surgery is not useful as it may place additional stress on a patient who is already highly anxious and decreases the likelihood for the information to be managed. It is recommended to deliver education early enough prior surgery to allow time for the patient to process the information. Also, a study reported that presurgical education on potential side effects; the assessment of patients’ needs by an SLP, physical therapist, nutritionist, and social worker; and pre-operative nutritional support decrease postoperative complications.4
The education committee was created in response to the gap on patient education. The inpatient team took the lead and provided intense education on the care of HNC patients to the nursing staff and to HNC patients and their families about postoperative care at home. Education was also extended to rehabilitation facilities caring for this cancer population at discharge from the hospital.
Clinicians identified a gap during the hospitalization process. The gap included prolonged stay of patients in the ICU postsurgery, inefficient interclinician communication, lack of standardization of postsurgical care, and difficulty communicating with external home care teams. A major intervention was implemented that included the creation of a HNC specialized unit that offered a structured setting for standardized care and communication between patients and clinicians. Dedicated units for the management of HNC patients highly enhance the quality of care provided because it enables the MTD team to work properly by clearly defining roles and responsibilities, delineating evidence-based clinical interventions, and promoting expert care for this patient population.23In addition, several key steps have been recommended to reduce the fragmentation of care for hospital patients, including developing a referral/transition tracking system, organizing and training staff members to coordinate transition/referrals, and identifying and creating agreements with key care providers.28
Early patient access to supportive services was a concern to most clinicians. HNC providers were not consulting the CARE clinic about patients’ nutritional, physical and SLP needs until the patient was having serious problems. Patient tracking found that the minority of patients met the standard of having a presurgical speech referral. Most patients had access to outpatient nutrition services during radiation therapy but the majority of patients in the sample did not attend CARE clinic. The literature strongly supports early management of HNC patients by the SPL and nutrition counselor. Van der Molen and colleagues demonstrated that a pretreatment SPL rehabilitation program is feasible and offers reasonable patient compliance despite of the burden caused by ongoing chemo-radiation therapy for HNC patients.16Similarly, early nutrition counseling for HNC patients undergoing RT has reported to decrease unintended weight loss and malnutrition compared with late nutrition intervention.19
Although there are clear gaps in care for HNC patients from the clinicians’ perspective, the patients surveyed indicated a clear satisfaction with their care at the cancer center. Almost all patients were satisfied with their relationships with clinicians in the team. Some patients mentioned complaints of insufficient pre-operative education and waiting time, but there were not significant complaints about coordination, which clinicians had identified as a major issue. This is likely explained by the small sample size and the patients’ inability to see the background interclinician communication.
A crucial suggestion to address all of these gaps in care was the implementation of a nurse navigator. With the support of hospital and cancer center administration, a nurse navigator was hired to address the needs of HNC patients throughout their disease trajectory. The team agreed that the nurse navigator should make contact with HNC patients during their initial appointment at the surgical ENT office. This initial contact allows the nurse navigator to provide support and connection to resources. Thereafter, early contact with this patient population allows the nurse navigator to follow the patient through the continuum of care from biopsy and diagnosis to survivorship. The nurse navigator facilitates communication between clinicians, patients and their families; and provides emotional support to patients while helping to manage their financial and transportation needs.29
Limitations
This is a quality improvement project with a small sample size of HNC cases. Data from this gap analysis are not statistically significant; yet, are clinically relevant in the management of the HNC population at the cancer center. Likewise, the patient sample size was small, making definitive generalizations about patient experience difficult; however, the data are helpful in highlighting possible problems for patients.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2016;67:7-30.
2. National Cancer Institute. Head and neck cancers. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet. Reviewed February 1, 2013. Accessed January 26, 2017
3. Weiderholt PA, Connor NP, Hartig GK, Harari PM. Bridging gaps in multidisciplinary head and neck cancer care: nursing coordination and case management. Int J Radiat Oncol Biol Phys. 2007;69(2 suppl):S88-S91.
4. Dingman C, Hegedus PD, Likes C, McDowell P, McCarthy E, Zwilling C. A coordinated, multidisciplinary approach to caring for the patient with head and neck cancer. J Support Oncol. 2008;6(3):125-131.
5. Liao C, Kang CJ, Lee LY, et al. Association between multidisciplinary team care approach and survival rates in patients with oral cavity squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1544-1553.
6. Starmer H, Sanguineti G, Marur S, Gourin CG. Multidisciplinary head and neck cancer clinic and adherence with speech pathology. Laryngoscope. 2011;121(10):2131-2135.
7. Prades J, Remue E, van Hoof E, Borras JM. Is it worth reorganizing cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organization of MDTs and their impact on patient outcomes. Health Pol. 2015;119(4):464-474.
8. Institute of Medicine. Delivering high-quality cancer care: charting a new course for a system in crisis. 2013. www.nationalacademies.org/hmd/Reports/2013/Delivering-High-Quality-Cancer-Care-Charting-a-New-Course-for-a-System-in-Crisis.aspx. Published September 10, 2013. Accessed May 29, 2016.
9. Granda-Cameron C, DeMille D, Lynch MP, et al. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs. 2010;14(1):72-80.
10. Davis-Ajami ML, Costa L, Kulik S. Gap analysis: synergies and opportunities for effective nursing leadership. Nurs Econ. 2014;32(1):17-25.
11. Fortin A, Bairati I, Albert M, et al. Effect of treatment delay on outcome of patients with early-stage head-and-neck carcinoma receiving radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52(4):929-936.
12. Van Harten MC, Ridder M, Hamming-Vrieze O, et al. The association of treatment delay and prognosis in head and neck squamous cell carcinoma (HNSCC) in a Dutch comprehensive cancer center. Oral Oncol. 2014;50:282-290.
13. Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J ClinOncol. 2003;21(3):555-563.
14. Lazarus CL. Management of swallowing disorders in head and neck cancer patients: optimal patterns of care. Sem Speech Lang. 2000;21(4):293-310.
15. Mayer KR. Learning to speak after laryngectomy. http://speech-language-pathology-audiology.advanceweb.com/Features/Articles/Learning-to-Speak-After-Laryngectomy.aspx. Posted October 27, 2014. Accessed January 17, 2017.
16. van der Molen L, van Rossum MA, Burkhead LM, et al. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemo-radiotherapy: feasibility, compliance, and short-term effects. Dysphagia. 2011;26:155-170.
17. Starmer HM, Gourin CG. Is speech language pathologist evaluation necessary in the nonoperative treatment of head and neck cancer? Laryngoscope. 2013;123(7):1571-1572.
18. [Article in French] Meuric J, Garabige V, Blanc-Vincent MP, et al. Good clinical practice in nutritional management of head and neck cancer patients. Bull Cancer. 1999;86(10):843-854.
19. van den Berg MG, Rasmussen-Conrad EL, Wei KH, et al. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br J Nutr. 2010;104:872-877.
20. Waller A, Forshaw K, Bryant J, Mair S. Interventions for preparing patients for chemotherapy and radiotherapy: a systematic review. Supp Care Ca. 2014;22(8):2297-2308.
21. Waller A, Forshaw K, Bryant J, et al. Preparatory education for cancer patients undergoing surgery: a systematic review of volume and quality of research output over time. Patient Educ Couns. 2015;98:1540-1549.
22. Toustrup K, Lambersten K, Birke-Sorensen H, et al. Reduction in waiting time for diagnosis and treatment of head and neck cancer – a fast track study. Acta Oncol. 2011;50:636-641.
23. Bergamini C, Locati L, Bossi P et al. Does a multidisciplinary team approach in a tertiary referral centre impact on the initial management of head and neck cancer? Oral Oncol. 2016;54:54-57.
24. AHRQ. Pediatric toolkit for using the AHRQ quality indicators. http://www.ahrq.gov/professionals/systems/hospital/qitoolkit/pediatrictoolkit.html . Reviewed July 2016. Accessed January 26, 2017.
25. Mackillop WJ. Killing time: the consequences of delays in radiotherapy. Radiother Oncol. 2007;84:1-4.
26.Chen Z, King, W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87:3-16.
27. Sharma S, Bekelman J, Lin A et al. Clinical impact of prolonged diagnosis to treatment interval (DTI) among patients with ororpharyngeal squamous cell carcinoma. Oral Oncol. 2016;56:17-24.
28. Improving chronic illness care. Reducing care fragmentation. Care coordination. http://www.improvingchroniccare.org/index.php?p=Care_Coordination&s=326. Published 2010. Accessed May 28, 2016.
29. Fillion L, de Serres M, Cook S, et al. Professional patient navigation in head and neck cancer. Sem Oncol Nurs. 2009;25(3):212-221.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2016;67:7-30.
2. National Cancer Institute. Head and neck cancers. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet. Reviewed February 1, 2013. Accessed January 26, 2017
3. Weiderholt PA, Connor NP, Hartig GK, Harari PM. Bridging gaps in multidisciplinary head and neck cancer care: nursing coordination and case management. Int J Radiat Oncol Biol Phys. 2007;69(2 suppl):S88-S91.
4. Dingman C, Hegedus PD, Likes C, McDowell P, McCarthy E, Zwilling C. A coordinated, multidisciplinary approach to caring for the patient with head and neck cancer. J Support Oncol. 2008;6(3):125-131.
5. Liao C, Kang CJ, Lee LY, et al. Association between multidisciplinary team care approach and survival rates in patients with oral cavity squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1544-1553.
6. Starmer H, Sanguineti G, Marur S, Gourin CG. Multidisciplinary head and neck cancer clinic and adherence with speech pathology. Laryngoscope. 2011;121(10):2131-2135.
7. Prades J, Remue E, van Hoof E, Borras JM. Is it worth reorganizing cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organization of MDTs and their impact on patient outcomes. Health Pol. 2015;119(4):464-474.
8. Institute of Medicine. Delivering high-quality cancer care: charting a new course for a system in crisis. 2013. www.nationalacademies.org/hmd/Reports/2013/Delivering-High-Quality-Cancer-Care-Charting-a-New-Course-for-a-System-in-Crisis.aspx. Published September 10, 2013. Accessed May 29, 2016.
9. Granda-Cameron C, DeMille D, Lynch MP, et al. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs. 2010;14(1):72-80.
10. Davis-Ajami ML, Costa L, Kulik S. Gap analysis: synergies and opportunities for effective nursing leadership. Nurs Econ. 2014;32(1):17-25.
11. Fortin A, Bairati I, Albert M, et al. Effect of treatment delay on outcome of patients with early-stage head-and-neck carcinoma receiving radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52(4):929-936.
12. Van Harten MC, Ridder M, Hamming-Vrieze O, et al. The association of treatment delay and prognosis in head and neck squamous cell carcinoma (HNSCC) in a Dutch comprehensive cancer center. Oral Oncol. 2014;50:282-290.
13. Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J ClinOncol. 2003;21(3):555-563.
14. Lazarus CL. Management of swallowing disorders in head and neck cancer patients: optimal patterns of care. Sem Speech Lang. 2000;21(4):293-310.
15. Mayer KR. Learning to speak after laryngectomy. http://speech-language-pathology-audiology.advanceweb.com/Features/Articles/Learning-to-Speak-After-Laryngectomy.aspx. Posted October 27, 2014. Accessed January 17, 2017.
16. van der Molen L, van Rossum MA, Burkhead LM, et al. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemo-radiotherapy: feasibility, compliance, and short-term effects. Dysphagia. 2011;26:155-170.
17. Starmer HM, Gourin CG. Is speech language pathologist evaluation necessary in the nonoperative treatment of head and neck cancer? Laryngoscope. 2013;123(7):1571-1572.
18. [Article in French] Meuric J, Garabige V, Blanc-Vincent MP, et al. Good clinical practice in nutritional management of head and neck cancer patients. Bull Cancer. 1999;86(10):843-854.
19. van den Berg MG, Rasmussen-Conrad EL, Wei KH, et al. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br J Nutr. 2010;104:872-877.
20. Waller A, Forshaw K, Bryant J, Mair S. Interventions for preparing patients for chemotherapy and radiotherapy: a systematic review. Supp Care Ca. 2014;22(8):2297-2308.
21. Waller A, Forshaw K, Bryant J, et al. Preparatory education for cancer patients undergoing surgery: a systematic review of volume and quality of research output over time. Patient Educ Couns. 2015;98:1540-1549.
22. Toustrup K, Lambersten K, Birke-Sorensen H, et al. Reduction in waiting time for diagnosis and treatment of head and neck cancer – a fast track study. Acta Oncol. 2011;50:636-641.
23. Bergamini C, Locati L, Bossi P et al. Does a multidisciplinary team approach in a tertiary referral centre impact on the initial management of head and neck cancer? Oral Oncol. 2016;54:54-57.
24. AHRQ. Pediatric toolkit for using the AHRQ quality indicators. http://www.ahrq.gov/professionals/systems/hospital/qitoolkit/pediatrictoolkit.html . Reviewed July 2016. Accessed January 26, 2017.
25. Mackillop WJ. Killing time: the consequences of delays in radiotherapy. Radiother Oncol. 2007;84:1-4.
26.Chen Z, King, W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87:3-16.
27. Sharma S, Bekelman J, Lin A et al. Clinical impact of prolonged diagnosis to treatment interval (DTI) among patients with ororpharyngeal squamous cell carcinoma. Oral Oncol. 2016;56:17-24.
28. Improving chronic illness care. Reducing care fragmentation. Care coordination. http://www.improvingchroniccare.org/index.php?p=Care_Coordination&s=326. Published 2010. Accessed May 28, 2016.
29. Fillion L, de Serres M, Cook S, et al. Professional patient navigation in head and neck cancer. Sem Oncol Nurs. 2009;25(3):212-221.
Quality of life after surgery for pleural malignant mesothelioma – methodological considerations
Background There is a dearth of literature on patient quality of life (QoL) after treatment for malignant pleural mesothelioma (MPM).
Objectives To review the literature on QoL after surgery for MPM and assess differences in quality of life between patients who have extrapleural pneumonectomy (EPP) and those who have pleurectomy and decortication (P-D).
Methods We retrieved and reviewed original research studies on quality of life after mesothelioma surgery. They had been published from January 1990 through June 2016, and included 15 articles and 12 datasets for a total of 523 patients.
Results QoL data was available for 102 EPP patients and 296 P-D patients. Two studies directly compared QoL outcomes between the 2 techniques. Symptoms, lung function parameters, and physical and social functioning were still compromised 6 months after surgery. However, P-D patients fared better than did EPP patients across QoL measures.
Limitations The amount of available literature is small, and the studies are heterogeneous.
Conclusions QoL is better for a longer period of time in patients who undergo P-D, compared with those who have EPP. Given the need for multimodality therapy for MPM and the aggressive nature of the disease, QoL outcomes should be strongly considered when choosing type of surgery for mesothelioma.
Click on the PDF icon at the top of this introduction to read the full article.
Background There is a dearth of literature on patient quality of life (QoL) after treatment for malignant pleural mesothelioma (MPM).
Objectives To review the literature on QoL after surgery for MPM and assess differences in quality of life between patients who have extrapleural pneumonectomy (EPP) and those who have pleurectomy and decortication (P-D).
Methods We retrieved and reviewed original research studies on quality of life after mesothelioma surgery. They had been published from January 1990 through June 2016, and included 15 articles and 12 datasets for a total of 523 patients.
Results QoL data was available for 102 EPP patients and 296 P-D patients. Two studies directly compared QoL outcomes between the 2 techniques. Symptoms, lung function parameters, and physical and social functioning were still compromised 6 months after surgery. However, P-D patients fared better than did EPP patients across QoL measures.
Limitations The amount of available literature is small, and the studies are heterogeneous.
Conclusions QoL is better for a longer period of time in patients who undergo P-D, compared with those who have EPP. Given the need for multimodality therapy for MPM and the aggressive nature of the disease, QoL outcomes should be strongly considered when choosing type of surgery for mesothelioma.
Click on the PDF icon at the top of this introduction to read the full article.
Background There is a dearth of literature on patient quality of life (QoL) after treatment for malignant pleural mesothelioma (MPM).
Objectives To review the literature on QoL after surgery for MPM and assess differences in quality of life between patients who have extrapleural pneumonectomy (EPP) and those who have pleurectomy and decortication (P-D).
Methods We retrieved and reviewed original research studies on quality of life after mesothelioma surgery. They had been published from January 1990 through June 2016, and included 15 articles and 12 datasets for a total of 523 patients.
Results QoL data was available for 102 EPP patients and 296 P-D patients. Two studies directly compared QoL outcomes between the 2 techniques. Symptoms, lung function parameters, and physical and social functioning were still compromised 6 months after surgery. However, P-D patients fared better than did EPP patients across QoL measures.
Limitations The amount of available literature is small, and the studies are heterogeneous.
Conclusions QoL is better for a longer period of time in patients who undergo P-D, compared with those who have EPP. Given the need for multimodality therapy for MPM and the aggressive nature of the disease, QoL outcomes should be strongly considered when choosing type of surgery for mesothelioma.
Click on the PDF icon at the top of this introduction to read the full article.