User login
Class I recall of GE Healthcare TruSignal SpO2 sensors
The Food and Drug Administration has identified this as a class I recall, the most serious type. The company has not received any reports of patient injury or deaths as a result of these issues.*
The recall includes the TruSignal Adult Pediatric Sensor, TruSignal AllFit Sensor, TruSignal Sensitive Skin Sensor, TruSignal Wrap Sensor, TruSignal Ear Sensor, TruSignal Integrated Ear Sensor with GE Connector, TruSignal Integrated Ear Sensor With Datex Connector, TruSignal Integrated Ear Sensor With Datex Connector, and TruSignal Integrated Ear Sensor With Ohmeda Connector.
The sensors were distributed in the United States from Jan. 1, 2021, to March 4, 2023.
According to the recall notice, the malfunctioning sensors “may reduce the amount of energy sent to the heart during defibrillation without any notification to the care provider, which could prevent delivery of lifesaving therapy in a critical situation.
“This issue is most hazardous to hospitalized patients who may need defibrillation for cardiac arrest. Affected sensors may also unintentionally expose patients to electrical currents from other sources or may provide inaccurate measurements of SpO2, which can impact treatment decisions,” the notice warns.
In an urgent device correction letter sent to health care professionals in May, GE HealthCare recommends that health care professionals do the following:
- Use an alternate method for SpO2 monitoring, including TruSignal sensors not impacted or an alternate SpO2 device.
- If alternate methods are not available, use affected TruSignal SpO2 sensors as long as they have not been saturated with fluids.
- If defibrillation is necessary when affected TruSignal SpO2 sensors are being used, remove the affected TruSignal SpO2 sensor, defibrillate per hospital protocol, and reattach the affected TruSignal SpO2 sensor after defibrillation is no longer needed.
- For Adult/Pediatric SpO2 sensors, confirm that material does not cover the emitter or detector before using.
- Discard the sensor and use another sensor if any additional material is present.
- Make sure all potential users are made aware of this safety notification and the recommended actions, and retain this notice.
Customers are also asked to complete and return the acknowledgment form attached to the notice to Recall.39004@ge.com.
For questions or concerns about this recall, contact GE HealthCare Service at 1-800-437-1171 or a local service representative.
Health care professionals can report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
*Correction, 8/3/23: An earlier version of this article mischaracterized the reports received by the company.
The Food and Drug Administration has identified this as a class I recall, the most serious type. The company has not received any reports of patient injury or deaths as a result of these issues.*
The recall includes the TruSignal Adult Pediatric Sensor, TruSignal AllFit Sensor, TruSignal Sensitive Skin Sensor, TruSignal Wrap Sensor, TruSignal Ear Sensor, TruSignal Integrated Ear Sensor with GE Connector, TruSignal Integrated Ear Sensor With Datex Connector, TruSignal Integrated Ear Sensor With Datex Connector, and TruSignal Integrated Ear Sensor With Ohmeda Connector.
The sensors were distributed in the United States from Jan. 1, 2021, to March 4, 2023.
According to the recall notice, the malfunctioning sensors “may reduce the amount of energy sent to the heart during defibrillation without any notification to the care provider, which could prevent delivery of lifesaving therapy in a critical situation.
“This issue is most hazardous to hospitalized patients who may need defibrillation for cardiac arrest. Affected sensors may also unintentionally expose patients to electrical currents from other sources or may provide inaccurate measurements of SpO2, which can impact treatment decisions,” the notice warns.
In an urgent device correction letter sent to health care professionals in May, GE HealthCare recommends that health care professionals do the following:
- Use an alternate method for SpO2 monitoring, including TruSignal sensors not impacted or an alternate SpO2 device.
- If alternate methods are not available, use affected TruSignal SpO2 sensors as long as they have not been saturated with fluids.
- If defibrillation is necessary when affected TruSignal SpO2 sensors are being used, remove the affected TruSignal SpO2 sensor, defibrillate per hospital protocol, and reattach the affected TruSignal SpO2 sensor after defibrillation is no longer needed.
- For Adult/Pediatric SpO2 sensors, confirm that material does not cover the emitter or detector before using.
- Discard the sensor and use another sensor if any additional material is present.
- Make sure all potential users are made aware of this safety notification and the recommended actions, and retain this notice.
Customers are also asked to complete and return the acknowledgment form attached to the notice to Recall.39004@ge.com.
For questions or concerns about this recall, contact GE HealthCare Service at 1-800-437-1171 or a local service representative.
Health care professionals can report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
*Correction, 8/3/23: An earlier version of this article mischaracterized the reports received by the company.
The Food and Drug Administration has identified this as a class I recall, the most serious type. The company has not received any reports of patient injury or deaths as a result of these issues.*
The recall includes the TruSignal Adult Pediatric Sensor, TruSignal AllFit Sensor, TruSignal Sensitive Skin Sensor, TruSignal Wrap Sensor, TruSignal Ear Sensor, TruSignal Integrated Ear Sensor with GE Connector, TruSignal Integrated Ear Sensor With Datex Connector, TruSignal Integrated Ear Sensor With Datex Connector, and TruSignal Integrated Ear Sensor With Ohmeda Connector.
The sensors were distributed in the United States from Jan. 1, 2021, to March 4, 2023.
According to the recall notice, the malfunctioning sensors “may reduce the amount of energy sent to the heart during defibrillation without any notification to the care provider, which could prevent delivery of lifesaving therapy in a critical situation.
“This issue is most hazardous to hospitalized patients who may need defibrillation for cardiac arrest. Affected sensors may also unintentionally expose patients to electrical currents from other sources or may provide inaccurate measurements of SpO2, which can impact treatment decisions,” the notice warns.
In an urgent device correction letter sent to health care professionals in May, GE HealthCare recommends that health care professionals do the following:
- Use an alternate method for SpO2 monitoring, including TruSignal sensors not impacted or an alternate SpO2 device.
- If alternate methods are not available, use affected TruSignal SpO2 sensors as long as they have not been saturated with fluids.
- If defibrillation is necessary when affected TruSignal SpO2 sensors are being used, remove the affected TruSignal SpO2 sensor, defibrillate per hospital protocol, and reattach the affected TruSignal SpO2 sensor after defibrillation is no longer needed.
- For Adult/Pediatric SpO2 sensors, confirm that material does not cover the emitter or detector before using.
- Discard the sensor and use another sensor if any additional material is present.
- Make sure all potential users are made aware of this safety notification and the recommended actions, and retain this notice.
Customers are also asked to complete and return the acknowledgment form attached to the notice to Recall.39004@ge.com.
For questions or concerns about this recall, contact GE HealthCare Service at 1-800-437-1171 or a local service representative.
Health care professionals can report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
*Correction, 8/3/23: An earlier version of this article mischaracterized the reports received by the company.
Mortality post perioperative CPR climbs with patient frailty
And the frailer that patients were going into surgery, according to their scores on an established frailty index, the greater their adjusted mortality risk at 30 days and the likelier they were to be discharged to a location other than their home.
The findings are based on more than 3,000 patients in an American College of Surgeons (ACS) quality improvement registry who underwent CPR at noncardiac surgery, about one-fourth of whom scored a least 40 on the revised Risk Analysis Index (RAI). The frailty index accounts for the patient’s comorbidities, cognition, functional and nutritional status, and other factors as predictors of postoperative mortality risk.
Such CPR for perioperative cardiac arrest “should not be considered futile just because a patient is frail, but neither should cardiac arrest be considered as ‘reversible’ in this population, as previously thought,” lead author Matthew B. Allen, MD, of Brigham and Women’s Hospital, Boston, said in an interview.
“We know that patients who are frail have higher risk of complications and mortality after surgery, and recent studies have demonstrated that frailty is associated with very poor outcomes following CPR in nonsurgical settings,” said Dr. Allen, an attending physician in the department of anesthesiology, perioperative, and pain medicine at his center.
Although cardiac arrest is typically regarded as being “more reversible” in the setting of surgery and anesthesia than elsewhere in the hospital, he observed, there’s very little data on whether that is indeed the case for frail patients.
The current analysis provides “a heretofore absent base of evidence to guide decision-making regarding CPR in patients with frailty who undergo surgery,” states the report, published in JAMA Network Open.
The 3,058 patients in the analysis, from the ACS National Surgical Quality Improvement database, received CPR for cardiac arrest during or soon after noncardiac surgery. Their mean age was 71 and 44% were women.
Their RAI scores ranged from 14 to 71 and averaged 37.7; one-fourth of the patients had scores of 40 or higher, the study’s threshold for identifying patients as “frail.”
Overall in the cohort, more cardiac arrests occurred during surgeries that entailed low-to-moderate physiologic stress (an Operative Stress Score of 1 to 3) than in the setting of emergency surgery: 67.9% vs. 39.1%, respectively.
During emergency surgeries, a greater proportion of frail than nonfrail patients experienced cardiac arrest, 42% and 38%, respectively. The same relationship was observed during low-to-moderate stress surgeries: 76.6% of frail patients and 64.8% of nonfrail patients. General anesthesia was used in about 93% of procedures for both frail and nonfrail patients, the report states.
The primary endpoint, 30-day mortality, was 58.6% overall, 67.4% in frail patients, and 55.6% for nonfrail patients. Frailty and mortality were positively associated, with an adjusted odds ratio (AOR) of 1.35 (95% confidence interval [CI], 1.11-1.65, P = .003) in multivariate analysis.
Of the cohort’s 1,164 patients who had been admitted from home and survived to discharge, 38.6% were discharged to a destination other than home; the corresponding rates for frail and nonfrail patients were 59.3% and 33.9%, respectively. Frailty and nonhome discharge were positively correlated with an AOR of 1.85 (95% CI, 1.31-2.62, P < .001).
“There is no such thing as a low-risk procedure in patients who are frail,” Dr. Allen said in an interview. “Frail patients should be medically optimized prior to undergoing surgery and anesthesia, and plans should be tailored to patients’ vulnerabilities to reduce the risk of complications and facilitate rapid recognition and treatment when they occur.”
Moreover, he said, management of clinical decompensation in the perioperative period should be a part of the shared decision-making process “to establish a plan aligned with the patients’ priorities whenever possible.”
The current study quantifies risk associated with frailty in the surgical setting, and “this quantification can help providers, patients, and insurers better grasp the growing frailty problem,” Balachundhar Subramaniam, MD, MPH, of Harvard Medical School, Boston, said in an interview.
Universal screening for frailty is “a must in all surgical patients” to help identify those who are high-risk and reduce their chances for perioperative adverse events, said Dr. Subramaniam, who was not involved in the study.
“Prehabilitation with education, nutrition, physical fitness, and psychological support offer the best chance of significantly reducing poor outcomes” in frail patients, he said, along with “continuous education” in the care of frail patients.
University of Colorado surgeon Joseph Cleveland, MD, not part of the current study, said that it “provides a framework for counseling patients” regarding their do-not-resuscitate status.
“We can counsel patients with frailty with this information,” he said, “that if their heart should stop or go into in irregular rhythm, their chances of surviving are not greater than 50% and they have a more than 50% chance of not being discharged home.”
Dr. Allen reported receiving a clinical translational starter grant from Brigham and Women’s Hospital Department of Anesthesiology; disclosures for the other authors are in the original article. Dr. Subramaniam disclosed research funding from Masimo and Merck and serving as an education consultant for Masimo. Dr. Cleveland reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
And the frailer that patients were going into surgery, according to their scores on an established frailty index, the greater their adjusted mortality risk at 30 days and the likelier they were to be discharged to a location other than their home.
The findings are based on more than 3,000 patients in an American College of Surgeons (ACS) quality improvement registry who underwent CPR at noncardiac surgery, about one-fourth of whom scored a least 40 on the revised Risk Analysis Index (RAI). The frailty index accounts for the patient’s comorbidities, cognition, functional and nutritional status, and other factors as predictors of postoperative mortality risk.
Such CPR for perioperative cardiac arrest “should not be considered futile just because a patient is frail, but neither should cardiac arrest be considered as ‘reversible’ in this population, as previously thought,” lead author Matthew B. Allen, MD, of Brigham and Women’s Hospital, Boston, said in an interview.
“We know that patients who are frail have higher risk of complications and mortality after surgery, and recent studies have demonstrated that frailty is associated with very poor outcomes following CPR in nonsurgical settings,” said Dr. Allen, an attending physician in the department of anesthesiology, perioperative, and pain medicine at his center.
Although cardiac arrest is typically regarded as being “more reversible” in the setting of surgery and anesthesia than elsewhere in the hospital, he observed, there’s very little data on whether that is indeed the case for frail patients.
The current analysis provides “a heretofore absent base of evidence to guide decision-making regarding CPR in patients with frailty who undergo surgery,” states the report, published in JAMA Network Open.
The 3,058 patients in the analysis, from the ACS National Surgical Quality Improvement database, received CPR for cardiac arrest during or soon after noncardiac surgery. Their mean age was 71 and 44% were women.
Their RAI scores ranged from 14 to 71 and averaged 37.7; one-fourth of the patients had scores of 40 or higher, the study’s threshold for identifying patients as “frail.”
Overall in the cohort, more cardiac arrests occurred during surgeries that entailed low-to-moderate physiologic stress (an Operative Stress Score of 1 to 3) than in the setting of emergency surgery: 67.9% vs. 39.1%, respectively.
During emergency surgeries, a greater proportion of frail than nonfrail patients experienced cardiac arrest, 42% and 38%, respectively. The same relationship was observed during low-to-moderate stress surgeries: 76.6% of frail patients and 64.8% of nonfrail patients. General anesthesia was used in about 93% of procedures for both frail and nonfrail patients, the report states.
The primary endpoint, 30-day mortality, was 58.6% overall, 67.4% in frail patients, and 55.6% for nonfrail patients. Frailty and mortality were positively associated, with an adjusted odds ratio (AOR) of 1.35 (95% confidence interval [CI], 1.11-1.65, P = .003) in multivariate analysis.
Of the cohort’s 1,164 patients who had been admitted from home and survived to discharge, 38.6% were discharged to a destination other than home; the corresponding rates for frail and nonfrail patients were 59.3% and 33.9%, respectively. Frailty and nonhome discharge were positively correlated with an AOR of 1.85 (95% CI, 1.31-2.62, P < .001).
“There is no such thing as a low-risk procedure in patients who are frail,” Dr. Allen said in an interview. “Frail patients should be medically optimized prior to undergoing surgery and anesthesia, and plans should be tailored to patients’ vulnerabilities to reduce the risk of complications and facilitate rapid recognition and treatment when they occur.”
Moreover, he said, management of clinical decompensation in the perioperative period should be a part of the shared decision-making process “to establish a plan aligned with the patients’ priorities whenever possible.”
The current study quantifies risk associated with frailty in the surgical setting, and “this quantification can help providers, patients, and insurers better grasp the growing frailty problem,” Balachundhar Subramaniam, MD, MPH, of Harvard Medical School, Boston, said in an interview.
Universal screening for frailty is “a must in all surgical patients” to help identify those who are high-risk and reduce their chances for perioperative adverse events, said Dr. Subramaniam, who was not involved in the study.
“Prehabilitation with education, nutrition, physical fitness, and psychological support offer the best chance of significantly reducing poor outcomes” in frail patients, he said, along with “continuous education” in the care of frail patients.
University of Colorado surgeon Joseph Cleveland, MD, not part of the current study, said that it “provides a framework for counseling patients” regarding their do-not-resuscitate status.
“We can counsel patients with frailty with this information,” he said, “that if their heart should stop or go into in irregular rhythm, their chances of surviving are not greater than 50% and they have a more than 50% chance of not being discharged home.”
Dr. Allen reported receiving a clinical translational starter grant from Brigham and Women’s Hospital Department of Anesthesiology; disclosures for the other authors are in the original article. Dr. Subramaniam disclosed research funding from Masimo and Merck and serving as an education consultant for Masimo. Dr. Cleveland reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
And the frailer that patients were going into surgery, according to their scores on an established frailty index, the greater their adjusted mortality risk at 30 days and the likelier they were to be discharged to a location other than their home.
The findings are based on more than 3,000 patients in an American College of Surgeons (ACS) quality improvement registry who underwent CPR at noncardiac surgery, about one-fourth of whom scored a least 40 on the revised Risk Analysis Index (RAI). The frailty index accounts for the patient’s comorbidities, cognition, functional and nutritional status, and other factors as predictors of postoperative mortality risk.
Such CPR for perioperative cardiac arrest “should not be considered futile just because a patient is frail, but neither should cardiac arrest be considered as ‘reversible’ in this population, as previously thought,” lead author Matthew B. Allen, MD, of Brigham and Women’s Hospital, Boston, said in an interview.
“We know that patients who are frail have higher risk of complications and mortality after surgery, and recent studies have demonstrated that frailty is associated with very poor outcomes following CPR in nonsurgical settings,” said Dr. Allen, an attending physician in the department of anesthesiology, perioperative, and pain medicine at his center.
Although cardiac arrest is typically regarded as being “more reversible” in the setting of surgery and anesthesia than elsewhere in the hospital, he observed, there’s very little data on whether that is indeed the case for frail patients.
The current analysis provides “a heretofore absent base of evidence to guide decision-making regarding CPR in patients with frailty who undergo surgery,” states the report, published in JAMA Network Open.
The 3,058 patients in the analysis, from the ACS National Surgical Quality Improvement database, received CPR for cardiac arrest during or soon after noncardiac surgery. Their mean age was 71 and 44% were women.
Their RAI scores ranged from 14 to 71 and averaged 37.7; one-fourth of the patients had scores of 40 or higher, the study’s threshold for identifying patients as “frail.”
Overall in the cohort, more cardiac arrests occurred during surgeries that entailed low-to-moderate physiologic stress (an Operative Stress Score of 1 to 3) than in the setting of emergency surgery: 67.9% vs. 39.1%, respectively.
During emergency surgeries, a greater proportion of frail than nonfrail patients experienced cardiac arrest, 42% and 38%, respectively. The same relationship was observed during low-to-moderate stress surgeries: 76.6% of frail patients and 64.8% of nonfrail patients. General anesthesia was used in about 93% of procedures for both frail and nonfrail patients, the report states.
The primary endpoint, 30-day mortality, was 58.6% overall, 67.4% in frail patients, and 55.6% for nonfrail patients. Frailty and mortality were positively associated, with an adjusted odds ratio (AOR) of 1.35 (95% confidence interval [CI], 1.11-1.65, P = .003) in multivariate analysis.
Of the cohort’s 1,164 patients who had been admitted from home and survived to discharge, 38.6% were discharged to a destination other than home; the corresponding rates for frail and nonfrail patients were 59.3% and 33.9%, respectively. Frailty and nonhome discharge were positively correlated with an AOR of 1.85 (95% CI, 1.31-2.62, P < .001).
“There is no such thing as a low-risk procedure in patients who are frail,” Dr. Allen said in an interview. “Frail patients should be medically optimized prior to undergoing surgery and anesthesia, and plans should be tailored to patients’ vulnerabilities to reduce the risk of complications and facilitate rapid recognition and treatment when they occur.”
Moreover, he said, management of clinical decompensation in the perioperative period should be a part of the shared decision-making process “to establish a plan aligned with the patients’ priorities whenever possible.”
The current study quantifies risk associated with frailty in the surgical setting, and “this quantification can help providers, patients, and insurers better grasp the growing frailty problem,” Balachundhar Subramaniam, MD, MPH, of Harvard Medical School, Boston, said in an interview.
Universal screening for frailty is “a must in all surgical patients” to help identify those who are high-risk and reduce their chances for perioperative adverse events, said Dr. Subramaniam, who was not involved in the study.
“Prehabilitation with education, nutrition, physical fitness, and psychological support offer the best chance of significantly reducing poor outcomes” in frail patients, he said, along with “continuous education” in the care of frail patients.
University of Colorado surgeon Joseph Cleveland, MD, not part of the current study, said that it “provides a framework for counseling patients” regarding their do-not-resuscitate status.
“We can counsel patients with frailty with this information,” he said, “that if their heart should stop or go into in irregular rhythm, their chances of surviving are not greater than 50% and they have a more than 50% chance of not being discharged home.”
Dr. Allen reported receiving a clinical translational starter grant from Brigham and Women’s Hospital Department of Anesthesiology; disclosures for the other authors are in the original article. Dr. Subramaniam disclosed research funding from Masimo and Merck and serving as an education consultant for Masimo. Dr. Cleveland reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
MD rushes in after lightning strikes four people at White House
It was one of those dog days of August where the humidity is palpable and the pressure is so hot and thick you can almost feel the ions in the air. At the time (2022), I was a White House fellow and senior adviser in the West Wing Office of Public Engagement and in the Office of the Vice President.
I was leaving the White House around 7:00 p.m. through the front gate on Lafayette Square. I had a dinner reservation with a friend, so I was in a rush. It was super overcast. Lo and behold, three steps after I closed the gate behind me, it started pouring. Rain came down so hard I had to take shelter.
There’s a stone building in front of the White House with archways, so I took cover underneath one of them, hoping that in a couple of minutes the rain would pass. Behind the archways are these thick, black, iron gates.
Just as I was about to make a run for it, I heard: BOOM!
It was like a bomb had gone off. In one moment, I saw the lightning bolt, heard the thunder, and felt the heat. It was all one rush of sensation. I couldn’t remember having been that scared in a long time.
I thought, “I definitely have to get out of here. In a couple of minutes there might be another strike, and I’m sitting next to iron gates!” I saw a little bit of a window in the downpour, so I started booking it. I knew there was a sheltered Secret Service area around the corner where they park their cars. A much safer place to be.
I was sprinting on the sidewalk and spotted a bunch of Secret Service agents on their bikes riding in the opposite direction, back toward the park. I knew they wouldn’t be out on bikes in this mess without a reason. As they reached me, one agent said, “Clear the sidewalk! We’re coming through with a bunch of equipment.”
I yelled, “What’s going on?”
“Four people were just struck by lightning,” he said as he zoomed past.
I thought: “Sh*t. I have to go back.”
It was like two different parts of my brain were active at the exact same time. My subcortical brain at the level of the amygdala was like: “You just ran from there, idiot. Why are you running back?” And another part of my brain was like: “This is who you are.”
The lightning had struck one of the largest trees in the park. Four bodies splayed out in one direction from the tree. They’d been taking shelter underneath it when they were hit and were blown off to one side. By the time I got there, two Secret Service agents were on the scene doing CPR. Some bystanders had started to run over.
I did a quick round of pulse checks to see everyone’s status, and all four were apneic and pulseless. I told the two Secret Service agents to keep doing compressions on the first person. Two bystanders also began compressions on another person, an older man.
More Secret Service agents arrived, and I said, “We need to do compressions on this other person right now.” One of the agents took a moment to question who I could be and why I was there. I said, “I’m a doctor. I know I’m not dressed like one, but I’m a physician.”
I told some agents to go find an AED, because these people needed to be shocked.
After they left, I was effectively trying to triage which of these four people would get the AED first. Initially, I spent more of my time on the young man, and we began to get some response from him. I then spent some time with the young woman.
It turned out there were AEDs in the pouches on the Secret Service bikes, but they were very small, dinky AEDs. We tried to apply the pads, but it was downpouring so much that the adhesive wouldn’t stick. I told one of the agents we needed a towel.
Through all this I was concerned we were going to be struck again. I mean, the metal statue of Lafayette was right there! They say lighting doesn’t strike in the same place twice, but who knows if that’s really true?
The towel arrived, and we were able to get the chests of the younger people dry enough for the AED pads. We applied two shocks first to the woman, then the young man. We got his pulse back quickly. The woman’s came back as well, but it felt much weaker.
EMS arrived shortly thereafter. We got all four patients on the transport, and they were transferred to the hospital.
The whole experience had taken 14 minutes.
At the time, I felt confident that the young man was going to survive. We’re taught that lightning bolt strikes are survivable if you can shock someone quickly. He also got pretty good CPR. But the next day I was watching the news and learned that he had passed away. So, of course I was thinking the worst about the others as well.
But a week and a half later, I learned that the young woman had been discharged from the ICU. She was the only one who made it. Her name is Amber, and we got connected through a reporter. About 2 weeks later, I invited her to the White House. I took her to the Oval Office. I met her mom and dad and husband, and we had dinner. We’ve been in touch ever since.
I remember the first time we talked on the phone, Amber said something along the lines of, “This sucks. Obviously, I was not planning for any of this to happen. But I also think there’s something good that could come from this.”
I was so surprised and happy to hear her say that. I had something similar happen to me when I was a teenager – caught in the wrong place at the wrong time. I tried to intervene in a gang fight in my neighborhood. I thought a kid was going to get killed, so I jumped in, imagining I could save the day. I didn’t. They broke a bunch of my bones and I was in the hospital for a bit.
I remember thinking then that my life was over. But after some time, I found a new perspective, which was: Maybe that life is over. But maybe this could be the beginning of a new one. And maybe those things that I’ve been afraid of doing, the dreams that I have, maybe now I’m actually free to go after them.
I told Amber, if there are things that you have been waiting to do, this could be the time. She wants to be an international human rights activist, and she is kicking butt in a graduate school program to begin on that pathway. It’s been really cool to watch her chase this dream with way more vigor than she had before.
I think we bonded because we’ve gone through – obviously not the same thing, but a similar moment of being confronted with your own mortality. Realizing that life can just shatter. And so, while we’re here, we might as well go for it with all the force of a person who knows this could all disappear in an instant.
It was an extremely humbling moment. It reaffirmed that my life is not about me. I have to use the time that I’ve got on behalf of other people as much as I can. What is my life about if not being useful?
Dr. Martin is an emergency medicine physician and faculty member at the MGH Center for Social Justice and Health Equity at Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
It was one of those dog days of August where the humidity is palpable and the pressure is so hot and thick you can almost feel the ions in the air. At the time (2022), I was a White House fellow and senior adviser in the West Wing Office of Public Engagement and in the Office of the Vice President.
I was leaving the White House around 7:00 p.m. through the front gate on Lafayette Square. I had a dinner reservation with a friend, so I was in a rush. It was super overcast. Lo and behold, three steps after I closed the gate behind me, it started pouring. Rain came down so hard I had to take shelter.
There’s a stone building in front of the White House with archways, so I took cover underneath one of them, hoping that in a couple of minutes the rain would pass. Behind the archways are these thick, black, iron gates.
Just as I was about to make a run for it, I heard: BOOM!
It was like a bomb had gone off. In one moment, I saw the lightning bolt, heard the thunder, and felt the heat. It was all one rush of sensation. I couldn’t remember having been that scared in a long time.
I thought, “I definitely have to get out of here. In a couple of minutes there might be another strike, and I’m sitting next to iron gates!” I saw a little bit of a window in the downpour, so I started booking it. I knew there was a sheltered Secret Service area around the corner where they park their cars. A much safer place to be.
I was sprinting on the sidewalk and spotted a bunch of Secret Service agents on their bikes riding in the opposite direction, back toward the park. I knew they wouldn’t be out on bikes in this mess without a reason. As they reached me, one agent said, “Clear the sidewalk! We’re coming through with a bunch of equipment.”
I yelled, “What’s going on?”
“Four people were just struck by lightning,” he said as he zoomed past.
I thought: “Sh*t. I have to go back.”
It was like two different parts of my brain were active at the exact same time. My subcortical brain at the level of the amygdala was like: “You just ran from there, idiot. Why are you running back?” And another part of my brain was like: “This is who you are.”
The lightning had struck one of the largest trees in the park. Four bodies splayed out in one direction from the tree. They’d been taking shelter underneath it when they were hit and were blown off to one side. By the time I got there, two Secret Service agents were on the scene doing CPR. Some bystanders had started to run over.
I did a quick round of pulse checks to see everyone’s status, and all four were apneic and pulseless. I told the two Secret Service agents to keep doing compressions on the first person. Two bystanders also began compressions on another person, an older man.
More Secret Service agents arrived, and I said, “We need to do compressions on this other person right now.” One of the agents took a moment to question who I could be and why I was there. I said, “I’m a doctor. I know I’m not dressed like one, but I’m a physician.”
I told some agents to go find an AED, because these people needed to be shocked.
After they left, I was effectively trying to triage which of these four people would get the AED first. Initially, I spent more of my time on the young man, and we began to get some response from him. I then spent some time with the young woman.
It turned out there were AEDs in the pouches on the Secret Service bikes, but they were very small, dinky AEDs. We tried to apply the pads, but it was downpouring so much that the adhesive wouldn’t stick. I told one of the agents we needed a towel.
Through all this I was concerned we were going to be struck again. I mean, the metal statue of Lafayette was right there! They say lighting doesn’t strike in the same place twice, but who knows if that’s really true?
The towel arrived, and we were able to get the chests of the younger people dry enough for the AED pads. We applied two shocks first to the woman, then the young man. We got his pulse back quickly. The woman’s came back as well, but it felt much weaker.
EMS arrived shortly thereafter. We got all four patients on the transport, and they were transferred to the hospital.
The whole experience had taken 14 minutes.
At the time, I felt confident that the young man was going to survive. We’re taught that lightning bolt strikes are survivable if you can shock someone quickly. He also got pretty good CPR. But the next day I was watching the news and learned that he had passed away. So, of course I was thinking the worst about the others as well.
But a week and a half later, I learned that the young woman had been discharged from the ICU. She was the only one who made it. Her name is Amber, and we got connected through a reporter. About 2 weeks later, I invited her to the White House. I took her to the Oval Office. I met her mom and dad and husband, and we had dinner. We’ve been in touch ever since.
I remember the first time we talked on the phone, Amber said something along the lines of, “This sucks. Obviously, I was not planning for any of this to happen. But I also think there’s something good that could come from this.”
I was so surprised and happy to hear her say that. I had something similar happen to me when I was a teenager – caught in the wrong place at the wrong time. I tried to intervene in a gang fight in my neighborhood. I thought a kid was going to get killed, so I jumped in, imagining I could save the day. I didn’t. They broke a bunch of my bones and I was in the hospital for a bit.
I remember thinking then that my life was over. But after some time, I found a new perspective, which was: Maybe that life is over. But maybe this could be the beginning of a new one. And maybe those things that I’ve been afraid of doing, the dreams that I have, maybe now I’m actually free to go after them.
I told Amber, if there are things that you have been waiting to do, this could be the time. She wants to be an international human rights activist, and she is kicking butt in a graduate school program to begin on that pathway. It’s been really cool to watch her chase this dream with way more vigor than she had before.
I think we bonded because we’ve gone through – obviously not the same thing, but a similar moment of being confronted with your own mortality. Realizing that life can just shatter. And so, while we’re here, we might as well go for it with all the force of a person who knows this could all disappear in an instant.
It was an extremely humbling moment. It reaffirmed that my life is not about me. I have to use the time that I’ve got on behalf of other people as much as I can. What is my life about if not being useful?
Dr. Martin is an emergency medicine physician and faculty member at the MGH Center for Social Justice and Health Equity at Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
It was one of those dog days of August where the humidity is palpable and the pressure is so hot and thick you can almost feel the ions in the air. At the time (2022), I was a White House fellow and senior adviser in the West Wing Office of Public Engagement and in the Office of the Vice President.
I was leaving the White House around 7:00 p.m. through the front gate on Lafayette Square. I had a dinner reservation with a friend, so I was in a rush. It was super overcast. Lo and behold, three steps after I closed the gate behind me, it started pouring. Rain came down so hard I had to take shelter.
There’s a stone building in front of the White House with archways, so I took cover underneath one of them, hoping that in a couple of minutes the rain would pass. Behind the archways are these thick, black, iron gates.
Just as I was about to make a run for it, I heard: BOOM!
It was like a bomb had gone off. In one moment, I saw the lightning bolt, heard the thunder, and felt the heat. It was all one rush of sensation. I couldn’t remember having been that scared in a long time.
I thought, “I definitely have to get out of here. In a couple of minutes there might be another strike, and I’m sitting next to iron gates!” I saw a little bit of a window in the downpour, so I started booking it. I knew there was a sheltered Secret Service area around the corner where they park their cars. A much safer place to be.
I was sprinting on the sidewalk and spotted a bunch of Secret Service agents on their bikes riding in the opposite direction, back toward the park. I knew they wouldn’t be out on bikes in this mess without a reason. As they reached me, one agent said, “Clear the sidewalk! We’re coming through with a bunch of equipment.”
I yelled, “What’s going on?”
“Four people were just struck by lightning,” he said as he zoomed past.
I thought: “Sh*t. I have to go back.”
It was like two different parts of my brain were active at the exact same time. My subcortical brain at the level of the amygdala was like: “You just ran from there, idiot. Why are you running back?” And another part of my brain was like: “This is who you are.”
The lightning had struck one of the largest trees in the park. Four bodies splayed out in one direction from the tree. They’d been taking shelter underneath it when they were hit and were blown off to one side. By the time I got there, two Secret Service agents were on the scene doing CPR. Some bystanders had started to run over.
I did a quick round of pulse checks to see everyone’s status, and all four were apneic and pulseless. I told the two Secret Service agents to keep doing compressions on the first person. Two bystanders also began compressions on another person, an older man.
More Secret Service agents arrived, and I said, “We need to do compressions on this other person right now.” One of the agents took a moment to question who I could be and why I was there. I said, “I’m a doctor. I know I’m not dressed like one, but I’m a physician.”
I told some agents to go find an AED, because these people needed to be shocked.
After they left, I was effectively trying to triage which of these four people would get the AED first. Initially, I spent more of my time on the young man, and we began to get some response from him. I then spent some time with the young woman.
It turned out there were AEDs in the pouches on the Secret Service bikes, but they were very small, dinky AEDs. We tried to apply the pads, but it was downpouring so much that the adhesive wouldn’t stick. I told one of the agents we needed a towel.
Through all this I was concerned we were going to be struck again. I mean, the metal statue of Lafayette was right there! They say lighting doesn’t strike in the same place twice, but who knows if that’s really true?
The towel arrived, and we were able to get the chests of the younger people dry enough for the AED pads. We applied two shocks first to the woman, then the young man. We got his pulse back quickly. The woman’s came back as well, but it felt much weaker.
EMS arrived shortly thereafter. We got all four patients on the transport, and they were transferred to the hospital.
The whole experience had taken 14 minutes.
At the time, I felt confident that the young man was going to survive. We’re taught that lightning bolt strikes are survivable if you can shock someone quickly. He also got pretty good CPR. But the next day I was watching the news and learned that he had passed away. So, of course I was thinking the worst about the others as well.
But a week and a half later, I learned that the young woman had been discharged from the ICU. She was the only one who made it. Her name is Amber, and we got connected through a reporter. About 2 weeks later, I invited her to the White House. I took her to the Oval Office. I met her mom and dad and husband, and we had dinner. We’ve been in touch ever since.
I remember the first time we talked on the phone, Amber said something along the lines of, “This sucks. Obviously, I was not planning for any of this to happen. But I also think there’s something good that could come from this.”
I was so surprised and happy to hear her say that. I had something similar happen to me when I was a teenager – caught in the wrong place at the wrong time. I tried to intervene in a gang fight in my neighborhood. I thought a kid was going to get killed, so I jumped in, imagining I could save the day. I didn’t. They broke a bunch of my bones and I was in the hospital for a bit.
I remember thinking then that my life was over. But after some time, I found a new perspective, which was: Maybe that life is over. But maybe this could be the beginning of a new one. And maybe those things that I’ve been afraid of doing, the dreams that I have, maybe now I’m actually free to go after them.
I told Amber, if there are things that you have been waiting to do, this could be the time. She wants to be an international human rights activist, and she is kicking butt in a graduate school program to begin on that pathway. It’s been really cool to watch her chase this dream with way more vigor than she had before.
I think we bonded because we’ve gone through – obviously not the same thing, but a similar moment of being confronted with your own mortality. Realizing that life can just shatter. And so, while we’re here, we might as well go for it with all the force of a person who knows this could all disappear in an instant.
It was an extremely humbling moment. It reaffirmed that my life is not about me. I have to use the time that I’ve got on behalf of other people as much as I can. What is my life about if not being useful?
Dr. Martin is an emergency medicine physician and faculty member at the MGH Center for Social Justice and Health Equity at Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
AHA statement addresses equity in cardio-oncology care
A new scientific statement from the American Heart Association focuses on equity in cardio-oncology care and research.
A “growing body of evidence” suggests that women and people from underrepresented patient groups experience disproportionately higher cardiovascular effects from new and emerging anticancer therapies, the writing group, led by Daniel Addison, MD, with the Ohio State University, Columbus, pointed out.
For example, women appear to be at higher risk of immune checkpoint inhibitor–related toxicities, whereas Black patients with cancer face up to a threefold higher risk of cardiotoxicity with anticancer therapies.
With reduced screening and delayed preventive measures, Hispanic patients have more complex heart disease, cancer is diagnosed at later stages, and they receive more cardiotoxic regimens because of a lack of eligibility for novel treatments. Ultimately, this contributes to a higher incidence of treatment complications, cardiac dysfunction, and adverse patient outcomes for this patient group, they write.
Although no studies have specifically addressed cardio-oncology disparities in the LGBTQIA+ population, such disparities can be inferred from known cardiovascular disease and oncology disparities, the writing group noted.
These disparities are supported by “disparately high” risk of death after a cancer diagnosis among women and individuals from underrepresented groups, even after accounting for socioeconomic and behavioral patterns, they pointed out.
The scientific statement was published online in Circulation.
Evidence gaps and the path forward
“Despite advances in strategies to limit the risks of cardiovascular events among cancer survivors, relatively limited guidance is available to address the rapidly growing problem of disparate cardiotoxic risks among women and underrepresented patient populations,” the writing group said.
Decentralized and sporadic evaluations have led to a lack of consensus on the definitions, investigations, and potential optimal strategies to address disparate cardiotoxicity with contemporary cancer immunotherapy, as well as biologic and cytotoxic therapies, they noted.
They said caution is needed when interpreting clinical trial data about cardiotoxicity and in generalizing the results because people from diverse racial and ethnic groups have not been well represented in many trials.
The writing group outlined key evidence gaps and future research directions for addressing cardio-oncology disparities, as well as strategies to improve equity in cardio-oncology care and research.
These include the following:
- Identifying specific predictive factors of long-term cardiotoxic risk with targeted and immune-based cancer therapies in women and underrepresented populations.
- Investigating biological mechanisms that may underlie differences in cardiotoxicities between different patient groups.
- Developing personalized cardioprotection strategies that integrate biological, genetic, and social determinant markers.
- Intentionally diversifying clinical trials and identifying optimal strategies to improve representation in cancer clinical trials.
- Determining the role of technology, such as artificial intelligence, in improving cardiotoxicity disparities.
“Conscientiously leveraging technology and designing trials with outcomes related to these issues in practice (considering feasibility and cost) will critically accelerate the field of cardio-oncology in the 21st century. With tangible goals, we can improve health inequities in cardio-oncology,” the writing group said.
The research had no commercial funding. No conflicts of interest were reported.
A version of this article originally appeared on Medscape.com.
A new scientific statement from the American Heart Association focuses on equity in cardio-oncology care and research.
A “growing body of evidence” suggests that women and people from underrepresented patient groups experience disproportionately higher cardiovascular effects from new and emerging anticancer therapies, the writing group, led by Daniel Addison, MD, with the Ohio State University, Columbus, pointed out.
For example, women appear to be at higher risk of immune checkpoint inhibitor–related toxicities, whereas Black patients with cancer face up to a threefold higher risk of cardiotoxicity with anticancer therapies.
With reduced screening and delayed preventive measures, Hispanic patients have more complex heart disease, cancer is diagnosed at later stages, and they receive more cardiotoxic regimens because of a lack of eligibility for novel treatments. Ultimately, this contributes to a higher incidence of treatment complications, cardiac dysfunction, and adverse patient outcomes for this patient group, they write.
Although no studies have specifically addressed cardio-oncology disparities in the LGBTQIA+ population, such disparities can be inferred from known cardiovascular disease and oncology disparities, the writing group noted.
These disparities are supported by “disparately high” risk of death after a cancer diagnosis among women and individuals from underrepresented groups, even after accounting for socioeconomic and behavioral patterns, they pointed out.
The scientific statement was published online in Circulation.
Evidence gaps and the path forward
“Despite advances in strategies to limit the risks of cardiovascular events among cancer survivors, relatively limited guidance is available to address the rapidly growing problem of disparate cardiotoxic risks among women and underrepresented patient populations,” the writing group said.
Decentralized and sporadic evaluations have led to a lack of consensus on the definitions, investigations, and potential optimal strategies to address disparate cardiotoxicity with contemporary cancer immunotherapy, as well as biologic and cytotoxic therapies, they noted.
They said caution is needed when interpreting clinical trial data about cardiotoxicity and in generalizing the results because people from diverse racial and ethnic groups have not been well represented in many trials.
The writing group outlined key evidence gaps and future research directions for addressing cardio-oncology disparities, as well as strategies to improve equity in cardio-oncology care and research.
These include the following:
- Identifying specific predictive factors of long-term cardiotoxic risk with targeted and immune-based cancer therapies in women and underrepresented populations.
- Investigating biological mechanisms that may underlie differences in cardiotoxicities between different patient groups.
- Developing personalized cardioprotection strategies that integrate biological, genetic, and social determinant markers.
- Intentionally diversifying clinical trials and identifying optimal strategies to improve representation in cancer clinical trials.
- Determining the role of technology, such as artificial intelligence, in improving cardiotoxicity disparities.
“Conscientiously leveraging technology and designing trials with outcomes related to these issues in practice (considering feasibility and cost) will critically accelerate the field of cardio-oncology in the 21st century. With tangible goals, we can improve health inequities in cardio-oncology,” the writing group said.
The research had no commercial funding. No conflicts of interest were reported.
A version of this article originally appeared on Medscape.com.
A new scientific statement from the American Heart Association focuses on equity in cardio-oncology care and research.
A “growing body of evidence” suggests that women and people from underrepresented patient groups experience disproportionately higher cardiovascular effects from new and emerging anticancer therapies, the writing group, led by Daniel Addison, MD, with the Ohio State University, Columbus, pointed out.
For example, women appear to be at higher risk of immune checkpoint inhibitor–related toxicities, whereas Black patients with cancer face up to a threefold higher risk of cardiotoxicity with anticancer therapies.
With reduced screening and delayed preventive measures, Hispanic patients have more complex heart disease, cancer is diagnosed at later stages, and they receive more cardiotoxic regimens because of a lack of eligibility for novel treatments. Ultimately, this contributes to a higher incidence of treatment complications, cardiac dysfunction, and adverse patient outcomes for this patient group, they write.
Although no studies have specifically addressed cardio-oncology disparities in the LGBTQIA+ population, such disparities can be inferred from known cardiovascular disease and oncology disparities, the writing group noted.
These disparities are supported by “disparately high” risk of death after a cancer diagnosis among women and individuals from underrepresented groups, even after accounting for socioeconomic and behavioral patterns, they pointed out.
The scientific statement was published online in Circulation.
Evidence gaps and the path forward
“Despite advances in strategies to limit the risks of cardiovascular events among cancer survivors, relatively limited guidance is available to address the rapidly growing problem of disparate cardiotoxic risks among women and underrepresented patient populations,” the writing group said.
Decentralized and sporadic evaluations have led to a lack of consensus on the definitions, investigations, and potential optimal strategies to address disparate cardiotoxicity with contemporary cancer immunotherapy, as well as biologic and cytotoxic therapies, they noted.
They said caution is needed when interpreting clinical trial data about cardiotoxicity and in generalizing the results because people from diverse racial and ethnic groups have not been well represented in many trials.
The writing group outlined key evidence gaps and future research directions for addressing cardio-oncology disparities, as well as strategies to improve equity in cardio-oncology care and research.
These include the following:
- Identifying specific predictive factors of long-term cardiotoxic risk with targeted and immune-based cancer therapies in women and underrepresented populations.
- Investigating biological mechanisms that may underlie differences in cardiotoxicities between different patient groups.
- Developing personalized cardioprotection strategies that integrate biological, genetic, and social determinant markers.
- Intentionally diversifying clinical trials and identifying optimal strategies to improve representation in cancer clinical trials.
- Determining the role of technology, such as artificial intelligence, in improving cardiotoxicity disparities.
“Conscientiously leveraging technology and designing trials with outcomes related to these issues in practice (considering feasibility and cost) will critically accelerate the field of cardio-oncology in the 21st century. With tangible goals, we can improve health inequities in cardio-oncology,” the writing group said.
The research had no commercial funding. No conflicts of interest were reported.
A version of this article originally appeared on Medscape.com.
FROM CIRCULATION
Upping CO2 does not benefit OHCA patients: TAME
The Targeted Therapeutic Mild Hypercapnia After Resuscitated Cardiac Arrest (TAME) study showed that the intervention failed to improve neurologic or functional outcomes or quality of life at 6 months. However, the researchers also found that slightly elevated CO2 levels were not associated with worse outcomes.
“I think these results show that our hypothesis – that raising CO2 levels as applied in this trial may be beneficial for these patients – was not effective, even though previous work suggested that it would be,” co–lead investigator Alistair Nichol, MD, said in an interview.
“This was a rigorous trial; the intervention was well delivered, and the results are pretty clear. Unfortunately, we have proved a null hypothesis – that this approach doesn’t seem to work,” Dr. Nichol, who is professor of critical care medicine at University College Dublin, said.
“However, we did find that hypercapnia was safe. This is an important finding, as sometimes in very sick patients such as those who develop pneumonia, we have to drive the ventilator less hard to minimize injury to the lungs, and this can lead to higher CO2 levels,” he added. “Our results show that this practice should not be harmful, which is reassuring.”
The TAME study was presented at the Critical Care Reviews 2023 Meeting (CCR23) held in Belfast, Northern Ireland.
It was simultaneously published online in the New England Journal of Medicine.
The researchers explain that after the return of spontaneous circulation, brain hypoperfusion may contribute to cerebral hypoxia, exacerbate brain damage, and lead to poor neurologic outcomes. The partial pressure of arterial carbon dioxide (PaCO2) is the major physiologic regulator of cerebrovascular tone, and increasing CO2 levels increases cerebral blood flow.
Two previous observational studies showed that exposure to hypercapnia was associated with an increase in the likelihood of being discharged home and better neurologic outcomes at 12 months, compared with hypocapnia or normocapnia.
In addition, a physiologic study showed that deliberate increases in PaCO2 induced higher cerebral oxygen saturations, compared with normocapnia. A phase 2 randomized trial showed that hypercapnia significantly attenuated the release of neuron-specific enolase, a biomarker of brain injury, and also suggested better 6-month neurologic recovery with hypercapnia compared with normocapnia.
The current TAME trial was conducted to try to confirm these results in a larger, more definitive study.
For the trial, 1,700 adults with coma who had been resuscitated after out-of-hospital cardiac arrest were randomly assigned to receive either 24 hours of mild hypercapnia (target PaCO2, 50-55 mm Hg) or normocapnia (target PaCO2, 35-45 mm Hg).
The primary outcome – a favorable neurologic outcome, defined as a score of 5 or higher on the Glasgow Outcome Scale–Extended at 6 months – occurred in 43.5% in the mild hypercapnia group and in 44.6% in the normocapnia group (relative risk, 0.98; P = .76).
By 6 months, 48.2% of those in the mild hypercapnia group and 45.9% in the normocapnia group had died (relative risk with mild hypercapnia, 1.05; 95% confidence interval, 0.94-1.16). In the mild hypercapnia group, 53.4% had a poor functional outcome, defined as a Modified Rankin Scale score of 4-6, compared with 51.3% in the normocapnia group.
Health-related quality of life, as assessed by the EQ Visual Analogue Scale component of the EuroQol-5D-5L, was similar in the two groups.
In terms of safety, results showed that mild hypercapnia did not increase the incidence of prespecified adverse events.
The authors note that there is concern that mild hypercapnia may worsen cerebral edema and elevate intracranial pressure; however, elevated intracranial pressure is uncommon in the first 72 hours after the return of spontaneous circulation.
In the TAME trial, there was one case of cerebral edema in the hypercapnia group. “This is a very low rate and would be expected in a group this size, so this does not indicate a safety concern,” Dr. Nichol commented.
The researchers are planning further analyses of biological samples to look for possible prognostic markers.
“These out-of-hospital cardiac arrest patients are a very diverse group, and it may be possible that some patients could have benefited from hypercapnia while others may have been harmed,” Dr. Nichol noted.
“Raising CO2 levels does improve overall delivery of oxygen to the brain, but this might not have occurred in the right areas. It may be possible that some patients benefited, and analysis of biological samples will help us look more closely at this.”
He added that other ongoing trials are investigating hypercapnia in patients with traumatic brain injury.
“These patients are managed differently and often have probes in their brain to measure the response to CO2, so more of a precision medicine approach is possible,” he explained.
He also noted that the TAME study, which was conducted in conjunction with the TTM-2 study investigating hypothermia in out-of-hospital cardiac arrest patients, has established a network of ICU teams around the world, providing an infrastructure for further trials to be performed in this patient population in the future.
The TAME trial was funded by the National Health and Medical Research Council of Australia, the Health Research Board of Ireland, and the Health Research Council of New Zealand.
A version of this article originally appeared on Medscape.com.
The Targeted Therapeutic Mild Hypercapnia After Resuscitated Cardiac Arrest (TAME) study showed that the intervention failed to improve neurologic or functional outcomes or quality of life at 6 months. However, the researchers also found that slightly elevated CO2 levels were not associated with worse outcomes.
“I think these results show that our hypothesis – that raising CO2 levels as applied in this trial may be beneficial for these patients – was not effective, even though previous work suggested that it would be,” co–lead investigator Alistair Nichol, MD, said in an interview.
“This was a rigorous trial; the intervention was well delivered, and the results are pretty clear. Unfortunately, we have proved a null hypothesis – that this approach doesn’t seem to work,” Dr. Nichol, who is professor of critical care medicine at University College Dublin, said.
“However, we did find that hypercapnia was safe. This is an important finding, as sometimes in very sick patients such as those who develop pneumonia, we have to drive the ventilator less hard to minimize injury to the lungs, and this can lead to higher CO2 levels,” he added. “Our results show that this practice should not be harmful, which is reassuring.”
The TAME study was presented at the Critical Care Reviews 2023 Meeting (CCR23) held in Belfast, Northern Ireland.
It was simultaneously published online in the New England Journal of Medicine.
The researchers explain that after the return of spontaneous circulation, brain hypoperfusion may contribute to cerebral hypoxia, exacerbate brain damage, and lead to poor neurologic outcomes. The partial pressure of arterial carbon dioxide (PaCO2) is the major physiologic regulator of cerebrovascular tone, and increasing CO2 levels increases cerebral blood flow.
Two previous observational studies showed that exposure to hypercapnia was associated with an increase in the likelihood of being discharged home and better neurologic outcomes at 12 months, compared with hypocapnia or normocapnia.
In addition, a physiologic study showed that deliberate increases in PaCO2 induced higher cerebral oxygen saturations, compared with normocapnia. A phase 2 randomized trial showed that hypercapnia significantly attenuated the release of neuron-specific enolase, a biomarker of brain injury, and also suggested better 6-month neurologic recovery with hypercapnia compared with normocapnia.
The current TAME trial was conducted to try to confirm these results in a larger, more definitive study.
For the trial, 1,700 adults with coma who had been resuscitated after out-of-hospital cardiac arrest were randomly assigned to receive either 24 hours of mild hypercapnia (target PaCO2, 50-55 mm Hg) or normocapnia (target PaCO2, 35-45 mm Hg).
The primary outcome – a favorable neurologic outcome, defined as a score of 5 or higher on the Glasgow Outcome Scale–Extended at 6 months – occurred in 43.5% in the mild hypercapnia group and in 44.6% in the normocapnia group (relative risk, 0.98; P = .76).
By 6 months, 48.2% of those in the mild hypercapnia group and 45.9% in the normocapnia group had died (relative risk with mild hypercapnia, 1.05; 95% confidence interval, 0.94-1.16). In the mild hypercapnia group, 53.4% had a poor functional outcome, defined as a Modified Rankin Scale score of 4-6, compared with 51.3% in the normocapnia group.
Health-related quality of life, as assessed by the EQ Visual Analogue Scale component of the EuroQol-5D-5L, was similar in the two groups.
In terms of safety, results showed that mild hypercapnia did not increase the incidence of prespecified adverse events.
The authors note that there is concern that mild hypercapnia may worsen cerebral edema and elevate intracranial pressure; however, elevated intracranial pressure is uncommon in the first 72 hours after the return of spontaneous circulation.
In the TAME trial, there was one case of cerebral edema in the hypercapnia group. “This is a very low rate and would be expected in a group this size, so this does not indicate a safety concern,” Dr. Nichol commented.
The researchers are planning further analyses of biological samples to look for possible prognostic markers.
“These out-of-hospital cardiac arrest patients are a very diverse group, and it may be possible that some patients could have benefited from hypercapnia while others may have been harmed,” Dr. Nichol noted.
“Raising CO2 levels does improve overall delivery of oxygen to the brain, but this might not have occurred in the right areas. It may be possible that some patients benefited, and analysis of biological samples will help us look more closely at this.”
He added that other ongoing trials are investigating hypercapnia in patients with traumatic brain injury.
“These patients are managed differently and often have probes in their brain to measure the response to CO2, so more of a precision medicine approach is possible,” he explained.
He also noted that the TAME study, which was conducted in conjunction with the TTM-2 study investigating hypothermia in out-of-hospital cardiac arrest patients, has established a network of ICU teams around the world, providing an infrastructure for further trials to be performed in this patient population in the future.
The TAME trial was funded by the National Health and Medical Research Council of Australia, the Health Research Board of Ireland, and the Health Research Council of New Zealand.
A version of this article originally appeared on Medscape.com.
The Targeted Therapeutic Mild Hypercapnia After Resuscitated Cardiac Arrest (TAME) study showed that the intervention failed to improve neurologic or functional outcomes or quality of life at 6 months. However, the researchers also found that slightly elevated CO2 levels were not associated with worse outcomes.
“I think these results show that our hypothesis – that raising CO2 levels as applied in this trial may be beneficial for these patients – was not effective, even though previous work suggested that it would be,” co–lead investigator Alistair Nichol, MD, said in an interview.
“This was a rigorous trial; the intervention was well delivered, and the results are pretty clear. Unfortunately, we have proved a null hypothesis – that this approach doesn’t seem to work,” Dr. Nichol, who is professor of critical care medicine at University College Dublin, said.
“However, we did find that hypercapnia was safe. This is an important finding, as sometimes in very sick patients such as those who develop pneumonia, we have to drive the ventilator less hard to minimize injury to the lungs, and this can lead to higher CO2 levels,” he added. “Our results show that this practice should not be harmful, which is reassuring.”
The TAME study was presented at the Critical Care Reviews 2023 Meeting (CCR23) held in Belfast, Northern Ireland.
It was simultaneously published online in the New England Journal of Medicine.
The researchers explain that after the return of spontaneous circulation, brain hypoperfusion may contribute to cerebral hypoxia, exacerbate brain damage, and lead to poor neurologic outcomes. The partial pressure of arterial carbon dioxide (PaCO2) is the major physiologic regulator of cerebrovascular tone, and increasing CO2 levels increases cerebral blood flow.
Two previous observational studies showed that exposure to hypercapnia was associated with an increase in the likelihood of being discharged home and better neurologic outcomes at 12 months, compared with hypocapnia or normocapnia.
In addition, a physiologic study showed that deliberate increases in PaCO2 induced higher cerebral oxygen saturations, compared with normocapnia. A phase 2 randomized trial showed that hypercapnia significantly attenuated the release of neuron-specific enolase, a biomarker of brain injury, and also suggested better 6-month neurologic recovery with hypercapnia compared with normocapnia.
The current TAME trial was conducted to try to confirm these results in a larger, more definitive study.
For the trial, 1,700 adults with coma who had been resuscitated after out-of-hospital cardiac arrest were randomly assigned to receive either 24 hours of mild hypercapnia (target PaCO2, 50-55 mm Hg) or normocapnia (target PaCO2, 35-45 mm Hg).
The primary outcome – a favorable neurologic outcome, defined as a score of 5 or higher on the Glasgow Outcome Scale–Extended at 6 months – occurred in 43.5% in the mild hypercapnia group and in 44.6% in the normocapnia group (relative risk, 0.98; P = .76).
By 6 months, 48.2% of those in the mild hypercapnia group and 45.9% in the normocapnia group had died (relative risk with mild hypercapnia, 1.05; 95% confidence interval, 0.94-1.16). In the mild hypercapnia group, 53.4% had a poor functional outcome, defined as a Modified Rankin Scale score of 4-6, compared with 51.3% in the normocapnia group.
Health-related quality of life, as assessed by the EQ Visual Analogue Scale component of the EuroQol-5D-5L, was similar in the two groups.
In terms of safety, results showed that mild hypercapnia did not increase the incidence of prespecified adverse events.
The authors note that there is concern that mild hypercapnia may worsen cerebral edema and elevate intracranial pressure; however, elevated intracranial pressure is uncommon in the first 72 hours after the return of spontaneous circulation.
In the TAME trial, there was one case of cerebral edema in the hypercapnia group. “This is a very low rate and would be expected in a group this size, so this does not indicate a safety concern,” Dr. Nichol commented.
The researchers are planning further analyses of biological samples to look for possible prognostic markers.
“These out-of-hospital cardiac arrest patients are a very diverse group, and it may be possible that some patients could have benefited from hypercapnia while others may have been harmed,” Dr. Nichol noted.
“Raising CO2 levels does improve overall delivery of oxygen to the brain, but this might not have occurred in the right areas. It may be possible that some patients benefited, and analysis of biological samples will help us look more closely at this.”
He added that other ongoing trials are investigating hypercapnia in patients with traumatic brain injury.
“These patients are managed differently and often have probes in their brain to measure the response to CO2, so more of a precision medicine approach is possible,” he explained.
He also noted that the TAME study, which was conducted in conjunction with the TTM-2 study investigating hypothermia in out-of-hospital cardiac arrest patients, has established a network of ICU teams around the world, providing an infrastructure for further trials to be performed in this patient population in the future.
The TAME trial was funded by the National Health and Medical Research Council of Australia, the Health Research Board of Ireland, and the Health Research Council of New Zealand.
A version of this article originally appeared on Medscape.com.
FROM CCR23
Guide explains nonsurgical management of major hemorrhage
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
PTSD, anxiety linked to out-of-hospital cardiac arrest
Investigators compared more than 35,000 OHCA case patients with a similar number of matched control persons and found an almost 1.5 times higher hazard of long-term stress conditions among OHCA case patients, compared with control persons, with a similar hazard for anxiety. Posttraumatic stress disorder was associated with an almost twofold higher risk of OHCA.
The findings applied equally to men and women and were independent of the presence of cardiovascular disease (CVD).
“This study raises awareness of the higher risks of OHCA and early risk monitoring to prevent OHCA in patients with stress-related disorders and anxiety,” write Talip Eroglu, of the department of cardiology, Copenhagen University Hospital, and colleagues.
The study was published online in BMJ Open Heart.
Stress disorders and anxiety overrepresented
OHCA “predominantly arises from lethal cardiac arrhythmias ... that occur most frequently in the setting of coronary heart disease,” the authors write. However, increasing evidence suggests that rates of OHCA may also be increased in association with noncardiac diseases.
Individuals with stress-related disorders and anxiety are “overrepresented” among victims of cardiac arrest as well as those with multiple CVDs. But previous studies of OHCA have been limited by small numbers of cardiac arrests. In addition, those studies involved only data from selected populations or used in-hospital diagnosis to identify cardiac arrest, thereby potentially omitting OHCA patients who died prior to hospital admission.
The researchers therefore turned to data from Danish health registries that include a large, unselected cohort of patients with OHCA to investigate whether long-term stress conditions (that is, PTSD and adjustment disorder) or anxiety disorder were associated with OHCA.
They stratified the cohort according to sex, age, and CVD to identify which risk factor confers the highest risk of OHCA in patients with long-term stress conditions or anxiety, and they conducted sensitivity analyses of potential confounders, such as depression.
The design was a nested-case control model in which records at an individual patient level across registries were cross-linked to data from other national registries and were compared to matched control persons from the general population (35,195 OHCAs and 351,950 matched control persons; median IQR age, 72 [62-81] years; 66.82% men).
The prevalence of comorbidities and use of cardiovascular drugs were higher among OHCA case patients than among non-OHCA control persons.
Keep aware of stress and anxiety as risk factors
Among OHCA and non-OHCA participants, long-term stress conditions were diagnosed in 0.92% and 0.45%, respectively. Anxiety was diagnosed in 0.85% of OHCA case patients and in 0.37% of non-OHCA control persons.
These conditions were associated with a higher rate of OHCA after adjustment for common OHCA risk factors.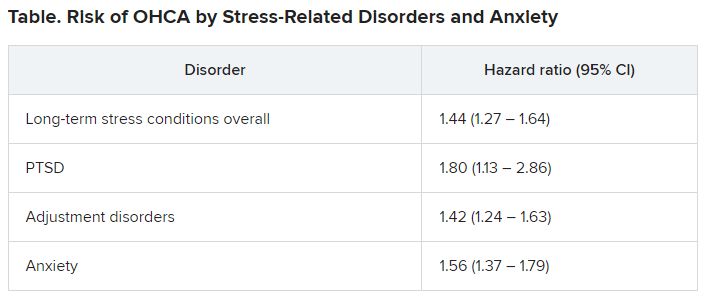
There were no significant differences in results when the researchers adjusted for the use of anxiolytics and antidepressants.
When they examined the prevalence of concomitant medication use or comorbidities, they found that depression was more frequent among patients with long-term stress and anxiety, compared with individuals with neither of those diagnoses. Additionally, patients with long-term stress and anxiety more often used anxiolytics, antidepressants, and QT-prolonging drugs.
Stratification of the analyses according to sex revealed that the OHCA rate was increased in both women and men with long-term stress and anxiety. There were no significant differences between the sexes. There were also no significant differences between the association among different age groups, nor between patients with and those without CVD, ischemic heart disease, or heart failure.
Previous research has shown associations of stress-related disorders or anxiety with cardiovascular outcomes, including myocardial infarction, heart failure, and cerebrovascular disease. These disorders might be “biological mediators in the causal pathway of OHCA” and contribute to the increased OHCA rate associated with stress-related disorders and anxiety, the authors suggest.
Nevertheless, they note, stress-related disorders and anxiety remained significantly associated with OHCA after controlling for these variables, “suggesting that it is unlikely that traditional risk factors of OHCA alone explain this relationship.”
They suggest several potential mechanisms. One is that the relationship is likely mediated by the activity of the sympathetic autonomic nervous system, which “leads to an increase in heart rate, release of neurotransmitters into the circulation, and local release of neurotransmitters in the heart.”
Each of these factors “may potentially influence cardiac electrophysiology and facilitate ventricular arrhythmias and OHCA.”
In addition to a biological mechanism, behavioral and psychosocial factors may also contribute to OHCA risk, since stress-related disorders and anxiety “often lead to unhealthy lifestyle, such as smoking and lower physical activity, which in turn may increase the risk of OHCA.” Given the absence of data on these features in the registries the investigators used, they were unable to account for them.
However, “it is unlikely that knowledge of these factors would have altered our conclusions considering that we have adjusted for all the relevant cardiovascular comorbidities.”
Similarly, other psychiatric disorders, such as depression, can contribute to OHCA risk, but they adjusted for depression in their multivariable analyses.
“Awareness of the higher risks of OHCA in patients with stress-related disorders and anxiety is important when treating these patients,” they conclude.
Detrimental to the heart, not just the psyche
Glenn Levine, MD, master clinician and professor of medicine, Baylor College of Medicine, Houston, called it an “important study in that it is a large, nationwide cohort study and thus provides important information to complement much smaller, focused studies.”
Like those other studies, “it finds that negative psychological health, specifically, long-term stress (as well as anxiety), is associated with a significantly increased risk of out-of-hospital cardiac arrest,” continued Dr. Levine, who is the chief of the cardiology section at Michael E. DeBakey VA Medical Center, Houston, and was not involved with the study.
Dr. Levine thinks the study “does a good job, as best one can for such a study, in trying to control for other factors, and zeroing in specifically on stress (and anxiety), trying to assess their independent contributions to the risk of developing cardiac arrest.”
The take-home message for clinicians and patients “is that negative psychological stress factors, such as stress and anxiety, are not only detrimental to one’s psychological health but likely increase one’s risk for adverse cardiac events, such as cardiac arrest,” he stated.
No specific funding for the study was disclosed. Mr. Eroglu has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Levine reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared more than 35,000 OHCA case patients with a similar number of matched control persons and found an almost 1.5 times higher hazard of long-term stress conditions among OHCA case patients, compared with control persons, with a similar hazard for anxiety. Posttraumatic stress disorder was associated with an almost twofold higher risk of OHCA.
The findings applied equally to men and women and were independent of the presence of cardiovascular disease (CVD).
“This study raises awareness of the higher risks of OHCA and early risk monitoring to prevent OHCA in patients with stress-related disorders and anxiety,” write Talip Eroglu, of the department of cardiology, Copenhagen University Hospital, and colleagues.
The study was published online in BMJ Open Heart.
Stress disorders and anxiety overrepresented
OHCA “predominantly arises from lethal cardiac arrhythmias ... that occur most frequently in the setting of coronary heart disease,” the authors write. However, increasing evidence suggests that rates of OHCA may also be increased in association with noncardiac diseases.
Individuals with stress-related disorders and anxiety are “overrepresented” among victims of cardiac arrest as well as those with multiple CVDs. But previous studies of OHCA have been limited by small numbers of cardiac arrests. In addition, those studies involved only data from selected populations or used in-hospital diagnosis to identify cardiac arrest, thereby potentially omitting OHCA patients who died prior to hospital admission.
The researchers therefore turned to data from Danish health registries that include a large, unselected cohort of patients with OHCA to investigate whether long-term stress conditions (that is, PTSD and adjustment disorder) or anxiety disorder were associated with OHCA.
They stratified the cohort according to sex, age, and CVD to identify which risk factor confers the highest risk of OHCA in patients with long-term stress conditions or anxiety, and they conducted sensitivity analyses of potential confounders, such as depression.
The design was a nested-case control model in which records at an individual patient level across registries were cross-linked to data from other national registries and were compared to matched control persons from the general population (35,195 OHCAs and 351,950 matched control persons; median IQR age, 72 [62-81] years; 66.82% men).
The prevalence of comorbidities and use of cardiovascular drugs were higher among OHCA case patients than among non-OHCA control persons.
Keep aware of stress and anxiety as risk factors
Among OHCA and non-OHCA participants, long-term stress conditions were diagnosed in 0.92% and 0.45%, respectively. Anxiety was diagnosed in 0.85% of OHCA case patients and in 0.37% of non-OHCA control persons.
These conditions were associated with a higher rate of OHCA after adjustment for common OHCA risk factors.
There were no significant differences in results when the researchers adjusted for the use of anxiolytics and antidepressants.
When they examined the prevalence of concomitant medication use or comorbidities, they found that depression was more frequent among patients with long-term stress and anxiety, compared with individuals with neither of those diagnoses. Additionally, patients with long-term stress and anxiety more often used anxiolytics, antidepressants, and QT-prolonging drugs.
Stratification of the analyses according to sex revealed that the OHCA rate was increased in both women and men with long-term stress and anxiety. There were no significant differences between the sexes. There were also no significant differences between the association among different age groups, nor between patients with and those without CVD, ischemic heart disease, or heart failure.
Previous research has shown associations of stress-related disorders or anxiety with cardiovascular outcomes, including myocardial infarction, heart failure, and cerebrovascular disease. These disorders might be “biological mediators in the causal pathway of OHCA” and contribute to the increased OHCA rate associated with stress-related disorders and anxiety, the authors suggest.
Nevertheless, they note, stress-related disorders and anxiety remained significantly associated with OHCA after controlling for these variables, “suggesting that it is unlikely that traditional risk factors of OHCA alone explain this relationship.”
They suggest several potential mechanisms. One is that the relationship is likely mediated by the activity of the sympathetic autonomic nervous system, which “leads to an increase in heart rate, release of neurotransmitters into the circulation, and local release of neurotransmitters in the heart.”
Each of these factors “may potentially influence cardiac electrophysiology and facilitate ventricular arrhythmias and OHCA.”
In addition to a biological mechanism, behavioral and psychosocial factors may also contribute to OHCA risk, since stress-related disorders and anxiety “often lead to unhealthy lifestyle, such as smoking and lower physical activity, which in turn may increase the risk of OHCA.” Given the absence of data on these features in the registries the investigators used, they were unable to account for them.
However, “it is unlikely that knowledge of these factors would have altered our conclusions considering that we have adjusted for all the relevant cardiovascular comorbidities.”
Similarly, other psychiatric disorders, such as depression, can contribute to OHCA risk, but they adjusted for depression in their multivariable analyses.
“Awareness of the higher risks of OHCA in patients with stress-related disorders and anxiety is important when treating these patients,” they conclude.
Detrimental to the heart, not just the psyche
Glenn Levine, MD, master clinician and professor of medicine, Baylor College of Medicine, Houston, called it an “important study in that it is a large, nationwide cohort study and thus provides important information to complement much smaller, focused studies.”
Like those other studies, “it finds that negative psychological health, specifically, long-term stress (as well as anxiety), is associated with a significantly increased risk of out-of-hospital cardiac arrest,” continued Dr. Levine, who is the chief of the cardiology section at Michael E. DeBakey VA Medical Center, Houston, and was not involved with the study.
Dr. Levine thinks the study “does a good job, as best one can for such a study, in trying to control for other factors, and zeroing in specifically on stress (and anxiety), trying to assess their independent contributions to the risk of developing cardiac arrest.”
The take-home message for clinicians and patients “is that negative psychological stress factors, such as stress and anxiety, are not only detrimental to one’s psychological health but likely increase one’s risk for adverse cardiac events, such as cardiac arrest,” he stated.
No specific funding for the study was disclosed. Mr. Eroglu has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Levine reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared more than 35,000 OHCA case patients with a similar number of matched control persons and found an almost 1.5 times higher hazard of long-term stress conditions among OHCA case patients, compared with control persons, with a similar hazard for anxiety. Posttraumatic stress disorder was associated with an almost twofold higher risk of OHCA.
The findings applied equally to men and women and were independent of the presence of cardiovascular disease (CVD).
“This study raises awareness of the higher risks of OHCA and early risk monitoring to prevent OHCA in patients with stress-related disorders and anxiety,” write Talip Eroglu, of the department of cardiology, Copenhagen University Hospital, and colleagues.
The study was published online in BMJ Open Heart.
Stress disorders and anxiety overrepresented
OHCA “predominantly arises from lethal cardiac arrhythmias ... that occur most frequently in the setting of coronary heart disease,” the authors write. However, increasing evidence suggests that rates of OHCA may also be increased in association with noncardiac diseases.
Individuals with stress-related disorders and anxiety are “overrepresented” among victims of cardiac arrest as well as those with multiple CVDs. But previous studies of OHCA have been limited by small numbers of cardiac arrests. In addition, those studies involved only data from selected populations or used in-hospital diagnosis to identify cardiac arrest, thereby potentially omitting OHCA patients who died prior to hospital admission.
The researchers therefore turned to data from Danish health registries that include a large, unselected cohort of patients with OHCA to investigate whether long-term stress conditions (that is, PTSD and adjustment disorder) or anxiety disorder were associated with OHCA.
They stratified the cohort according to sex, age, and CVD to identify which risk factor confers the highest risk of OHCA in patients with long-term stress conditions or anxiety, and they conducted sensitivity analyses of potential confounders, such as depression.
The design was a nested-case control model in which records at an individual patient level across registries were cross-linked to data from other national registries and were compared to matched control persons from the general population (35,195 OHCAs and 351,950 matched control persons; median IQR age, 72 [62-81] years; 66.82% men).
The prevalence of comorbidities and use of cardiovascular drugs were higher among OHCA case patients than among non-OHCA control persons.
Keep aware of stress and anxiety as risk factors
Among OHCA and non-OHCA participants, long-term stress conditions were diagnosed in 0.92% and 0.45%, respectively. Anxiety was diagnosed in 0.85% of OHCA case patients and in 0.37% of non-OHCA control persons.
These conditions were associated with a higher rate of OHCA after adjustment for common OHCA risk factors.
There were no significant differences in results when the researchers adjusted for the use of anxiolytics and antidepressants.
When they examined the prevalence of concomitant medication use or comorbidities, they found that depression was more frequent among patients with long-term stress and anxiety, compared with individuals with neither of those diagnoses. Additionally, patients with long-term stress and anxiety more often used anxiolytics, antidepressants, and QT-prolonging drugs.
Stratification of the analyses according to sex revealed that the OHCA rate was increased in both women and men with long-term stress and anxiety. There were no significant differences between the sexes. There were also no significant differences between the association among different age groups, nor between patients with and those without CVD, ischemic heart disease, or heart failure.
Previous research has shown associations of stress-related disorders or anxiety with cardiovascular outcomes, including myocardial infarction, heart failure, and cerebrovascular disease. These disorders might be “biological mediators in the causal pathway of OHCA” and contribute to the increased OHCA rate associated with stress-related disorders and anxiety, the authors suggest.
Nevertheless, they note, stress-related disorders and anxiety remained significantly associated with OHCA after controlling for these variables, “suggesting that it is unlikely that traditional risk factors of OHCA alone explain this relationship.”
They suggest several potential mechanisms. One is that the relationship is likely mediated by the activity of the sympathetic autonomic nervous system, which “leads to an increase in heart rate, release of neurotransmitters into the circulation, and local release of neurotransmitters in the heart.”
Each of these factors “may potentially influence cardiac electrophysiology and facilitate ventricular arrhythmias and OHCA.”
In addition to a biological mechanism, behavioral and psychosocial factors may also contribute to OHCA risk, since stress-related disorders and anxiety “often lead to unhealthy lifestyle, such as smoking and lower physical activity, which in turn may increase the risk of OHCA.” Given the absence of data on these features in the registries the investigators used, they were unable to account for them.
However, “it is unlikely that knowledge of these factors would have altered our conclusions considering that we have adjusted for all the relevant cardiovascular comorbidities.”
Similarly, other psychiatric disorders, such as depression, can contribute to OHCA risk, but they adjusted for depression in their multivariable analyses.
“Awareness of the higher risks of OHCA in patients with stress-related disorders and anxiety is important when treating these patients,” they conclude.
Detrimental to the heart, not just the psyche
Glenn Levine, MD, master clinician and professor of medicine, Baylor College of Medicine, Houston, called it an “important study in that it is a large, nationwide cohort study and thus provides important information to complement much smaller, focused studies.”
Like those other studies, “it finds that negative psychological health, specifically, long-term stress (as well as anxiety), is associated with a significantly increased risk of out-of-hospital cardiac arrest,” continued Dr. Levine, who is the chief of the cardiology section at Michael E. DeBakey VA Medical Center, Houston, and was not involved with the study.
Dr. Levine thinks the study “does a good job, as best one can for such a study, in trying to control for other factors, and zeroing in specifically on stress (and anxiety), trying to assess their independent contributions to the risk of developing cardiac arrest.”
The take-home message for clinicians and patients “is that negative psychological stress factors, such as stress and anxiety, are not only detrimental to one’s psychological health but likely increase one’s risk for adverse cardiac events, such as cardiac arrest,” he stated.
No specific funding for the study was disclosed. Mr. Eroglu has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Levine reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN HEART
ECMO signals benefit for cardiogenic shock after MI in halted trial
Data support new randomized trial
At the time that it was halted, a multicenter randomized trial was associating venoarterial extracorporeal membrane oxygenation (VA-ECMO) with an intriguing signal of benefit for patients in cardiogenic shock undergoing percutaneous intervention (PCI) for acute myocardial infarction.
Stopped early because of the pandemic, the EURO SHOCK trial has data on only 35 patients, but all-cause mortality at 30 days was nearly 30% lower in the VA-ECMO arm than in the standard-therapy arm, reported Manel Sabate, MD, PhD, chief of the interventional cardiology unit, Clinic University Hospital, Barcelona.
When patients were followed out to 12 months, the numerical survival advantage appeared to persist.
Yet, because of the early trial termination, “there really are no definite conclusions to be drawn from these results,” acknowledged Dr. Sabate, who noted that less than 10% of the planned enrollment had been reached. In addition, the survival benefit in the VA-ECMO arm was achieved at the cost of a higher rate of complications.
Despite the small numbers, results from the halted trial were presented as a late-breaker at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions. They were also simultaneously published in EuroIntervention.
The interest is based on an important unmet need, said Dr. Sabate. Cardiogenic shock occurs in about 10% of acute MI patients. Of those that continue on to revascularization, the 30-day mortality can approach 50%.
Meanwhile, the potential of mechanical circulatory support to maintain perfusion during cardiogenic shock makes it one of the most attractive, if unproven, approaches for improving outcomes.
Major multicenter trial terminated
The EURO SHOCK trial had a planned enrollment of 428 patients when it was initiated; 15 centers in six European countries participated. Recruitment and the trial were brought to a halt by the COVID-19 pandemic.
When trial recruitment was stopped, 18 patients had been assigned to standard supportive care and 17 patients to VA-ECMO. The primary endpoint of the trial was all-cause mortality at 30 days. Mortality at 12 months along with bleeding complications, cerebrovascular events, and readmission for heart failure, were among secondary endpoints.
At 30 days, the mortality rate was 61.1% among patients randomized to standard care, versus 43.8% among patients randomized to VA ECMO (hazard ratio, 0.56; 95% confidence interval, 0.21-01.45; P = 0.22).
At 12 months, the numerical advantage of VA-ECMO persisted (81.5% vs. 51.8%) with a similar nonsignificant signal for potential benefit despite the small sample size (HR, 0.52; 95% CI, 0.21-1.26; P = 0.14).
There were also numerically lower rates of cardiovascular death, ischemic stroke, recurrent MI, and acute kidney injury among patients in the VA-ECMO group relative to those in the standard-care group, Dr. Sabate reported.
However, VA-ECMO was associated with more vascular complications (21.4% vs. 0%) and bleeding events (35.7% vs. 5.6%).
Furthermore, although quality of life data were limited, Dr. Sabate noted that about half of patients in the VA-ECMO group reported problems with mobility, self-care, or usual activities on the basis of the EQ-5D-3L questionnaire at 30 days. None of the patients in the standard-care group reported any such difficulties.
When standard care was compared with VA-ECMO, rates of readmission for heart failure over 12 months (8.0% vs. 6.9%) were not different.
To be enrolled in this study, patients being treated for MI had to be in cardiogenic shock for at least 30 minutes following primary PCI. The median time from onset of cardiogenic shock to VA-ECMO in the active treatment arm was 4.8 hours.
Patient enrollment was challenging
Even independent of the COVID-19 pandemic, enrolling patients proved to be difficult. The 35 patients enrolled represented about 10% of the 333 patients screened at the participating centers. Unwitnessed out-of-hospital cardiac arrest, cardiogenic shock from a cause other than MI, and recovery from cardiogenic shock after the PCI was performed were among reasons for the high rate of exclusions.
The difficulty of identifying and engaging appropriate candidates for VA-ECMO, along with a substantial crossover rate, should be among lessons for investigators planning the next trial, said Dr. Sabate, who pointed out that 5 of the 17 patients assigned to VA-ECMO were never treated due to complications or patient refusal.
Dr. Sabate said.
Davide Capodanno, MD, PhD, a professor of cardiology and interventional cardiologist at the University of Catania (Italy), agreed.
“It was a good decision to publish these results,” he said. Noting that there were challenges in conducting the trial unrelated to COVID-19, Dr. Capodanno acknowledged the promise of mechanical ventilatory support for a relatively common and life-threatening complication.
“This study must be read for the lessons it will provide for future trials,” he said.
Dr. Sabate reported he has no potential conflicts of interest. Dr. Capodanno reported financial relationships with Amgen, Daiichi Sankyo, and Sanofi.
Data support new randomized trial
Data support new randomized trial
At the time that it was halted, a multicenter randomized trial was associating venoarterial extracorporeal membrane oxygenation (VA-ECMO) with an intriguing signal of benefit for patients in cardiogenic shock undergoing percutaneous intervention (PCI) for acute myocardial infarction.
Stopped early because of the pandemic, the EURO SHOCK trial has data on only 35 patients, but all-cause mortality at 30 days was nearly 30% lower in the VA-ECMO arm than in the standard-therapy arm, reported Manel Sabate, MD, PhD, chief of the interventional cardiology unit, Clinic University Hospital, Barcelona.
When patients were followed out to 12 months, the numerical survival advantage appeared to persist.
Yet, because of the early trial termination, “there really are no definite conclusions to be drawn from these results,” acknowledged Dr. Sabate, who noted that less than 10% of the planned enrollment had been reached. In addition, the survival benefit in the VA-ECMO arm was achieved at the cost of a higher rate of complications.
Despite the small numbers, results from the halted trial were presented as a late-breaker at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions. They were also simultaneously published in EuroIntervention.
The interest is based on an important unmet need, said Dr. Sabate. Cardiogenic shock occurs in about 10% of acute MI patients. Of those that continue on to revascularization, the 30-day mortality can approach 50%.
Meanwhile, the potential of mechanical circulatory support to maintain perfusion during cardiogenic shock makes it one of the most attractive, if unproven, approaches for improving outcomes.
Major multicenter trial terminated
The EURO SHOCK trial had a planned enrollment of 428 patients when it was initiated; 15 centers in six European countries participated. Recruitment and the trial were brought to a halt by the COVID-19 pandemic.
When trial recruitment was stopped, 18 patients had been assigned to standard supportive care and 17 patients to VA-ECMO. The primary endpoint of the trial was all-cause mortality at 30 days. Mortality at 12 months along with bleeding complications, cerebrovascular events, and readmission for heart failure, were among secondary endpoints.
At 30 days, the mortality rate was 61.1% among patients randomized to standard care, versus 43.8% among patients randomized to VA ECMO (hazard ratio, 0.56; 95% confidence interval, 0.21-01.45; P = 0.22).
At 12 months, the numerical advantage of VA-ECMO persisted (81.5% vs. 51.8%) with a similar nonsignificant signal for potential benefit despite the small sample size (HR, 0.52; 95% CI, 0.21-1.26; P = 0.14).
There were also numerically lower rates of cardiovascular death, ischemic stroke, recurrent MI, and acute kidney injury among patients in the VA-ECMO group relative to those in the standard-care group, Dr. Sabate reported.
However, VA-ECMO was associated with more vascular complications (21.4% vs. 0%) and bleeding events (35.7% vs. 5.6%).
Furthermore, although quality of life data were limited, Dr. Sabate noted that about half of patients in the VA-ECMO group reported problems with mobility, self-care, or usual activities on the basis of the EQ-5D-3L questionnaire at 30 days. None of the patients in the standard-care group reported any such difficulties.
When standard care was compared with VA-ECMO, rates of readmission for heart failure over 12 months (8.0% vs. 6.9%) were not different.
To be enrolled in this study, patients being treated for MI had to be in cardiogenic shock for at least 30 minutes following primary PCI. The median time from onset of cardiogenic shock to VA-ECMO in the active treatment arm was 4.8 hours.
Patient enrollment was challenging
Even independent of the COVID-19 pandemic, enrolling patients proved to be difficult. The 35 patients enrolled represented about 10% of the 333 patients screened at the participating centers. Unwitnessed out-of-hospital cardiac arrest, cardiogenic shock from a cause other than MI, and recovery from cardiogenic shock after the PCI was performed were among reasons for the high rate of exclusions.
The difficulty of identifying and engaging appropriate candidates for VA-ECMO, along with a substantial crossover rate, should be among lessons for investigators planning the next trial, said Dr. Sabate, who pointed out that 5 of the 17 patients assigned to VA-ECMO were never treated due to complications or patient refusal.
Dr. Sabate said.
Davide Capodanno, MD, PhD, a professor of cardiology and interventional cardiologist at the University of Catania (Italy), agreed.
“It was a good decision to publish these results,” he said. Noting that there were challenges in conducting the trial unrelated to COVID-19, Dr. Capodanno acknowledged the promise of mechanical ventilatory support for a relatively common and life-threatening complication.
“This study must be read for the lessons it will provide for future trials,” he said.
Dr. Sabate reported he has no potential conflicts of interest. Dr. Capodanno reported financial relationships with Amgen, Daiichi Sankyo, and Sanofi.
At the time that it was halted, a multicenter randomized trial was associating venoarterial extracorporeal membrane oxygenation (VA-ECMO) with an intriguing signal of benefit for patients in cardiogenic shock undergoing percutaneous intervention (PCI) for acute myocardial infarction.
Stopped early because of the pandemic, the EURO SHOCK trial has data on only 35 patients, but all-cause mortality at 30 days was nearly 30% lower in the VA-ECMO arm than in the standard-therapy arm, reported Manel Sabate, MD, PhD, chief of the interventional cardiology unit, Clinic University Hospital, Barcelona.
When patients were followed out to 12 months, the numerical survival advantage appeared to persist.
Yet, because of the early trial termination, “there really are no definite conclusions to be drawn from these results,” acknowledged Dr. Sabate, who noted that less than 10% of the planned enrollment had been reached. In addition, the survival benefit in the VA-ECMO arm was achieved at the cost of a higher rate of complications.
Despite the small numbers, results from the halted trial were presented as a late-breaker at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions. They were also simultaneously published in EuroIntervention.
The interest is based on an important unmet need, said Dr. Sabate. Cardiogenic shock occurs in about 10% of acute MI patients. Of those that continue on to revascularization, the 30-day mortality can approach 50%.
Meanwhile, the potential of mechanical circulatory support to maintain perfusion during cardiogenic shock makes it one of the most attractive, if unproven, approaches for improving outcomes.
Major multicenter trial terminated
The EURO SHOCK trial had a planned enrollment of 428 patients when it was initiated; 15 centers in six European countries participated. Recruitment and the trial were brought to a halt by the COVID-19 pandemic.
When trial recruitment was stopped, 18 patients had been assigned to standard supportive care and 17 patients to VA-ECMO. The primary endpoint of the trial was all-cause mortality at 30 days. Mortality at 12 months along with bleeding complications, cerebrovascular events, and readmission for heart failure, were among secondary endpoints.
At 30 days, the mortality rate was 61.1% among patients randomized to standard care, versus 43.8% among patients randomized to VA ECMO (hazard ratio, 0.56; 95% confidence interval, 0.21-01.45; P = 0.22).
At 12 months, the numerical advantage of VA-ECMO persisted (81.5% vs. 51.8%) with a similar nonsignificant signal for potential benefit despite the small sample size (HR, 0.52; 95% CI, 0.21-1.26; P = 0.14).
There were also numerically lower rates of cardiovascular death, ischemic stroke, recurrent MI, and acute kidney injury among patients in the VA-ECMO group relative to those in the standard-care group, Dr. Sabate reported.
However, VA-ECMO was associated with more vascular complications (21.4% vs. 0%) and bleeding events (35.7% vs. 5.6%).
Furthermore, although quality of life data were limited, Dr. Sabate noted that about half of patients in the VA-ECMO group reported problems with mobility, self-care, or usual activities on the basis of the EQ-5D-3L questionnaire at 30 days. None of the patients in the standard-care group reported any such difficulties.
When standard care was compared with VA-ECMO, rates of readmission for heart failure over 12 months (8.0% vs. 6.9%) were not different.
To be enrolled in this study, patients being treated for MI had to be in cardiogenic shock for at least 30 minutes following primary PCI. The median time from onset of cardiogenic shock to VA-ECMO in the active treatment arm was 4.8 hours.
Patient enrollment was challenging
Even independent of the COVID-19 pandemic, enrolling patients proved to be difficult. The 35 patients enrolled represented about 10% of the 333 patients screened at the participating centers. Unwitnessed out-of-hospital cardiac arrest, cardiogenic shock from a cause other than MI, and recovery from cardiogenic shock after the PCI was performed were among reasons for the high rate of exclusions.
The difficulty of identifying and engaging appropriate candidates for VA-ECMO, along with a substantial crossover rate, should be among lessons for investigators planning the next trial, said Dr. Sabate, who pointed out that 5 of the 17 patients assigned to VA-ECMO were never treated due to complications or patient refusal.
Dr. Sabate said.
Davide Capodanno, MD, PhD, a professor of cardiology and interventional cardiologist at the University of Catania (Italy), agreed.
“It was a good decision to publish these results,” he said. Noting that there were challenges in conducting the trial unrelated to COVID-19, Dr. Capodanno acknowledged the promise of mechanical ventilatory support for a relatively common and life-threatening complication.
“This study must be read for the lessons it will provide for future trials,” he said.
Dr. Sabate reported he has no potential conflicts of interest. Dr. Capodanno reported financial relationships with Amgen, Daiichi Sankyo, and Sanofi.
FROM EUROPCR 2023
Real-world data validate ESC risk model in NSTE-ACS
ESC model appropriately identifies risk
A real-world study of more than 12,000 cases over 7 years has validated the predictive ability of the proposed guidelines for stratifying thrombotic risks at 1 year for patients with non–ST-elevated acute coronary syndrome (NSTE-ACS) undergoing percutaneous coronary intervention (PCI).
In research presented at the Society for Cardiovascular Angiography & Interventions annual scientific sessions, George Dangas, MD, PhD, current SCAI president and professor of cardiology and vascular surgery at the Icahn School of Medicine at Mount Sinai, New York, reported that the European Society of Cardiology risk stratification criteria appropriately predicted risk in 12,538 patients treated from 2012 to 2019.
Despite these proposed guidelines put forward by the ESC in 2020, no consensus exists on criteria for ischemic or thrombotic risk in NSTE-ACS patients, Dr. Dangas noted.
The new study shows that the 1-year major adverse cardiovascular events (MACE) risk was four times greater in patients classified as medium risk (hazard ratio, 4.31; 95% confidence interval, 2.47-7.52) and six times greater in high-risk patients (HR, 6.16; 95% CI, 3.52-10.8), compared with the low-risk group, mostly because of higher rates of all-cause death and myocardial infarction, Dr. Dangas said in an interview.
“Indeed, we found some good correlation between the three risk categories and gradation of risk that validates essentially, but with the statistical testing that we need, that this classification is meaningful if not perfect,” Dr. Dangas said. “In the future we may perform calibrations to enhance its performance.”
The study used data on consecutive patients from the Angioplasty and Stent Procedures Database of Mount Sinai, grouping them into low, medium, and high thrombotic risk based on the proposed ESC guidelines for the management of NSTE-ACS.
The guidelines included a subset of criteria to identify patients with increased thrombotic risk who may benefit from extended treatment with a second antithrombotic agent.
This study aimed to evaluate the value of the criteria to identify patients at higher risk of ischemic events. “That’s why we went to our database to see how this might work,” Dr. Dangas said.
The researchers also found that high-risk patients had about a 40% greater risk of major bleeding (HR, 1.39; 95% CI, 1.06-1.84). Bleeding risks were similar between the low- and moderate-risk groups.
The risk categories reflected the rates of all-cause death, myocardial infarction, or stroke: 5.4%, 4.1%, and 1.6% in the high-, moderate-, and low-risks groups, respectively (P < .001).
“This identification of ischemic risks worked very well for all-cause mortality,” Dr. Dangas said. “I feel this is a strength because mortality is a leader of outcomes. And of course, we’ve had some associations with all events like mortality, myocardial infarction, repeat revascularization, which are interesting and valid, but I think a study result that indicates the mortality itself is known to be unidirectional and a very good correlation makes the result more robust.”
Critical role
Risk prediction models such as the proposed ESC guidelines will play a critical role as individualized medicine continues to evolve, Somjot Brar, MD, MPH, director of the regional department of cardiac catheterization at Kaiser Permanente, Los Angeles Medical Center, and associate clinical professor at the University of California, Los Angeles, said in an interview.
“This study highlights again the importance of the value for predictive and precision medicine,” Dr. Brar said. “Everything is moving in this direction where we make decisions that are more appropriate for a given patient as opposed to a population of patients.”
Study strengths are the large sample size in a real-world setting and thorough 1-year follow-up, Dr. Brar said.
A limitation is the three risk categories the guidelines proposed. “These are still pretty big boxes,” he said. “The low-, moderate- and high-risk categorization is still very, very broad and can be very vague.”
The relatively low percentage of low-risk patients – 12% versus 56% and 32% for the moderate- and high-risk groups – in this data set may also skew results, Dr. Brar said.
“As we move toward predictive analytics and medicine, we want to make these boxes smaller and smaller and smaller to be able to better understand which treatments should be administered to which patients to maximize the benefit against the risk,” he said. That would be a focus for future analyses, Dr. Brar said.
Dr. Dangas and Dr. Brar have no relevant financial disclosures.
ESC model appropriately identifies risk
ESC model appropriately identifies risk
A real-world study of more than 12,000 cases over 7 years has validated the predictive ability of the proposed guidelines for stratifying thrombotic risks at 1 year for patients with non–ST-elevated acute coronary syndrome (NSTE-ACS) undergoing percutaneous coronary intervention (PCI).
In research presented at the Society for Cardiovascular Angiography & Interventions annual scientific sessions, George Dangas, MD, PhD, current SCAI president and professor of cardiology and vascular surgery at the Icahn School of Medicine at Mount Sinai, New York, reported that the European Society of Cardiology risk stratification criteria appropriately predicted risk in 12,538 patients treated from 2012 to 2019.
Despite these proposed guidelines put forward by the ESC in 2020, no consensus exists on criteria for ischemic or thrombotic risk in NSTE-ACS patients, Dr. Dangas noted.
The new study shows that the 1-year major adverse cardiovascular events (MACE) risk was four times greater in patients classified as medium risk (hazard ratio, 4.31; 95% confidence interval, 2.47-7.52) and six times greater in high-risk patients (HR, 6.16; 95% CI, 3.52-10.8), compared with the low-risk group, mostly because of higher rates of all-cause death and myocardial infarction, Dr. Dangas said in an interview.
“Indeed, we found some good correlation between the three risk categories and gradation of risk that validates essentially, but with the statistical testing that we need, that this classification is meaningful if not perfect,” Dr. Dangas said. “In the future we may perform calibrations to enhance its performance.”
The study used data on consecutive patients from the Angioplasty and Stent Procedures Database of Mount Sinai, grouping them into low, medium, and high thrombotic risk based on the proposed ESC guidelines for the management of NSTE-ACS.
The guidelines included a subset of criteria to identify patients with increased thrombotic risk who may benefit from extended treatment with a second antithrombotic agent.
This study aimed to evaluate the value of the criteria to identify patients at higher risk of ischemic events. “That’s why we went to our database to see how this might work,” Dr. Dangas said.
The researchers also found that high-risk patients had about a 40% greater risk of major bleeding (HR, 1.39; 95% CI, 1.06-1.84). Bleeding risks were similar between the low- and moderate-risk groups.
The risk categories reflected the rates of all-cause death, myocardial infarction, or stroke: 5.4%, 4.1%, and 1.6% in the high-, moderate-, and low-risks groups, respectively (P < .001).
“This identification of ischemic risks worked very well for all-cause mortality,” Dr. Dangas said. “I feel this is a strength because mortality is a leader of outcomes. And of course, we’ve had some associations with all events like mortality, myocardial infarction, repeat revascularization, which are interesting and valid, but I think a study result that indicates the mortality itself is known to be unidirectional and a very good correlation makes the result more robust.”
Critical role
Risk prediction models such as the proposed ESC guidelines will play a critical role as individualized medicine continues to evolve, Somjot Brar, MD, MPH, director of the regional department of cardiac catheterization at Kaiser Permanente, Los Angeles Medical Center, and associate clinical professor at the University of California, Los Angeles, said in an interview.
“This study highlights again the importance of the value for predictive and precision medicine,” Dr. Brar said. “Everything is moving in this direction where we make decisions that are more appropriate for a given patient as opposed to a population of patients.”
Study strengths are the large sample size in a real-world setting and thorough 1-year follow-up, Dr. Brar said.
A limitation is the three risk categories the guidelines proposed. “These are still pretty big boxes,” he said. “The low-, moderate- and high-risk categorization is still very, very broad and can be very vague.”
The relatively low percentage of low-risk patients – 12% versus 56% and 32% for the moderate- and high-risk groups – in this data set may also skew results, Dr. Brar said.
“As we move toward predictive analytics and medicine, we want to make these boxes smaller and smaller and smaller to be able to better understand which treatments should be administered to which patients to maximize the benefit against the risk,” he said. That would be a focus for future analyses, Dr. Brar said.
Dr. Dangas and Dr. Brar have no relevant financial disclosures.
A real-world study of more than 12,000 cases over 7 years has validated the predictive ability of the proposed guidelines for stratifying thrombotic risks at 1 year for patients with non–ST-elevated acute coronary syndrome (NSTE-ACS) undergoing percutaneous coronary intervention (PCI).
In research presented at the Society for Cardiovascular Angiography & Interventions annual scientific sessions, George Dangas, MD, PhD, current SCAI president and professor of cardiology and vascular surgery at the Icahn School of Medicine at Mount Sinai, New York, reported that the European Society of Cardiology risk stratification criteria appropriately predicted risk in 12,538 patients treated from 2012 to 2019.
Despite these proposed guidelines put forward by the ESC in 2020, no consensus exists on criteria for ischemic or thrombotic risk in NSTE-ACS patients, Dr. Dangas noted.
The new study shows that the 1-year major adverse cardiovascular events (MACE) risk was four times greater in patients classified as medium risk (hazard ratio, 4.31; 95% confidence interval, 2.47-7.52) and six times greater in high-risk patients (HR, 6.16; 95% CI, 3.52-10.8), compared with the low-risk group, mostly because of higher rates of all-cause death and myocardial infarction, Dr. Dangas said in an interview.
“Indeed, we found some good correlation between the three risk categories and gradation of risk that validates essentially, but with the statistical testing that we need, that this classification is meaningful if not perfect,” Dr. Dangas said. “In the future we may perform calibrations to enhance its performance.”
The study used data on consecutive patients from the Angioplasty and Stent Procedures Database of Mount Sinai, grouping them into low, medium, and high thrombotic risk based on the proposed ESC guidelines for the management of NSTE-ACS.
The guidelines included a subset of criteria to identify patients with increased thrombotic risk who may benefit from extended treatment with a second antithrombotic agent.
This study aimed to evaluate the value of the criteria to identify patients at higher risk of ischemic events. “That’s why we went to our database to see how this might work,” Dr. Dangas said.
The researchers also found that high-risk patients had about a 40% greater risk of major bleeding (HR, 1.39; 95% CI, 1.06-1.84). Bleeding risks were similar between the low- and moderate-risk groups.
The risk categories reflected the rates of all-cause death, myocardial infarction, or stroke: 5.4%, 4.1%, and 1.6% in the high-, moderate-, and low-risks groups, respectively (P < .001).
“This identification of ischemic risks worked very well for all-cause mortality,” Dr. Dangas said. “I feel this is a strength because mortality is a leader of outcomes. And of course, we’ve had some associations with all events like mortality, myocardial infarction, repeat revascularization, which are interesting and valid, but I think a study result that indicates the mortality itself is known to be unidirectional and a very good correlation makes the result more robust.”
Critical role
Risk prediction models such as the proposed ESC guidelines will play a critical role as individualized medicine continues to evolve, Somjot Brar, MD, MPH, director of the regional department of cardiac catheterization at Kaiser Permanente, Los Angeles Medical Center, and associate clinical professor at the University of California, Los Angeles, said in an interview.
“This study highlights again the importance of the value for predictive and precision medicine,” Dr. Brar said. “Everything is moving in this direction where we make decisions that are more appropriate for a given patient as opposed to a population of patients.”
Study strengths are the large sample size in a real-world setting and thorough 1-year follow-up, Dr. Brar said.
A limitation is the three risk categories the guidelines proposed. “These are still pretty big boxes,” he said. “The low-, moderate- and high-risk categorization is still very, very broad and can be very vague.”
The relatively low percentage of low-risk patients – 12% versus 56% and 32% for the moderate- and high-risk groups – in this data set may also skew results, Dr. Brar said.
“As we move toward predictive analytics and medicine, we want to make these boxes smaller and smaller and smaller to be able to better understand which treatments should be administered to which patients to maximize the benefit against the risk,” he said. That would be a focus for future analyses, Dr. Brar said.
Dr. Dangas and Dr. Brar have no relevant financial disclosures.
FROM SCAI 2023
Machine-learning model improves MI diagnosis
Use of a machine-learning model that incorporates information from a single troponin test as well as other clinical data was superior to current practice as an aid to the diagnosis of myocardial infarction in the emergency department in a new study.
“Our results suggest that and free up space in the emergency department,” senior author Nicholas L. Mills, MD, University of Edinburgh, Scotland, said in an interview.
“And, perhaps even more importantly, use of this model could also increase the proportion of patients who are correctly identified as at a high probability of having an MI,” he added.
The study was published online in Nature Medicine.
The authors explained that at present, the likelihood of an MI diagnosis for patients presenting to the emergency department with chest pain is based on a fixed troponin threshold in serial tests at specific time points, but there are several problems with this approach.
First, a fixed troponin threshold is generally used for all patients, which does not account for age, sex, or comorbidities that are known to influence cardiac troponin concentrations. Second, the need to perform tests at specific time points for serial testing can be challenging in busy emergency departments.
And third, patients are categorized as being at low, intermediate, or high risk of MI on the basis of troponin thresholds alone, and the test does not take into account other important factors, such as the time of symptom onset or findings on the electrocardiogram.
“Our current practice of using the same threshold to rule in and rule out an MI for everyone, regardless of whether they are an 18-year-old female without a history of heart disease or an 85-year-old male with known heart failure, doesn’t perform well, and there’s a significant risk of misdiagnosis. There is also a high likelihood for inequalities in care, particularly between men and women,” Dr. Mills said.
The current study evaluated whether use of a machine learning model known as CoDE-ACS to guide decision-making could overcome some of these challenges.
The machine learning model assesses the whole spectrum of troponin levels as a continuous variable (rather than use of a single threshold) and turns this measurement into a probability that an individual patient is having an MI after accounting for other factors, including age, sex, comorbidities, and time from symptom onset.
For the current study, the CoDE-ACS model was trained in 10,000 patients with suspected acute coronary syndrome (ACS) who presented to 10 hospitals in Scotland as part of the High-STEACS trial evaluating the implementation of a high-sensitivity cardiac troponin I assay. The results were then validated in another 10,000 patients from six countries around the world.
“Using this model, the patient can have a troponin test on arrival at the emergency department. The other information on age, sex, clinical history, and time since symptom onset is keyed in, and it gives a probability on a scale of 0–100 as to whether the patient is having an MI,” Dr. Mills noted.
“It also has the capacity to incorporate more information over time. So, if there is a second troponin measurement made, then the model automatically refines the probability score,” he added.
The current study showed that use of the CoDE-ACS model identified more patients at presentation as having a low probability of having an MI than fixed cardiac troponin thresholds (61% vs. 27%) with a similar negative predictive value.
It also identified fewer patients as having a high probability of having an MI (10% vs. 16%) with a greater positive predictive value.
Among patients who were identified as having a low probability of MI, the rate of cardiac death was lower than the rate among those with intermediate or high probability at 30 days (0.1% vs. 0.5% and 1.8%) and 1 year (0.3% vs. 2.8% and 4.2%).
“The results show that the machine learning model doubles the proportion of patients who can be discharged with a single test compared to the current practice of using the threshold approach. It really is a game changer in terms of its potential to improve health efficiency,” Dr. Mills said.
In terms of ruling patients in as possibly having an MI, he pointed out that troponin levels are increased in patients with a wide range of other conditions, including heart failure, kidney failure, and atrial fibrillation.
“When using the threshold approach, only one in four patients with an elevated troponin level will actually be having an MI, and that leads to confusion,” he said. “This model takes into consideration these other conditions and so it can correctly identify three out of four patients with a high probability of having an MI. We can therefore be more confident that it is appropriate to refer those patients to cardiology and save a lot of potentially unnecessary investigations and treatments in the others.”
Dr. Mills said the model also seems to work when assessing patients early on.
“Around one-third of patients present within 3 hours of symptom onset, and actually these are a high-risk group because people who have genuine cardiac pain are normally extremely uncomfortable and tend to present quickly. Current guidelines require that we do two tests in all these individuals, but this new model incorporates the time of symptom onset into its estimates of probability and therefore allows us to rule out patients even when they present very early.”
He reported that a second test is required in only one in five patients – those whose first test indicated intermediate probability.
“The second test allows us to refine the probability further, allowing us to rule another half of those patients out. We are then left with a small proportion of patients – about 1 in 10 – who remain of intermediate probability and will require additional clinical judgment.”
Should improve inequities in MI diagnosis
Dr. Mills said the CoDE-ACS model will improve current inequities in MI diagnosis, because of which MI is underrecognized in women and younger people.
“Women have troponin concentrations that are half those of men, and although sex-specific troponin thresholds are recommended in the guidelines, they are not widely used. This automatically leads to underrecognition of heart disease in women. But this new machine learning model performs identically in men and women because it has been trained to recognize the different normal levels in men and women,” he explained.
“It will also help us not to underdiagnose MI in younger people who often have a less classical presentation of MI, and they also generally have very low concentrations of troponin, so any increase in troponin way below the current diagnostic threshold may be very relevant to their risk,” he added.
The researchers are planning a randomized trial of the new model to demonstrate the impact it could have on equality of care and overcrowding in the emergency department. In the trial, patients will be randomly assigned to undergo decision-making on the basis of troponin thresholds (current practice) or to undergo decision-making through the CoDE-ACS model.
“The hope is that this trial will show reductions in unnecessary hospital admissions and length of stay in the emergency department,” Dr. Mills said. Results are expected sometime next year.
“This algorithm is very well trained. It has learned on 20,000 patients, so it has a lot more experience than I have, and I have been a professor of cardiology for 20 years,” Dr. Mills said.
He said he believes these models will get even smarter in the future as more data are added.
“I think the future for good decision-making in emergency care will be informed by clinical decision support from well-trained machine learning algorithms and they will help us guide not just the diagnosis of MI but also heart failure and other important cardiac conditions,” he said.
‘Elegant and exciting’ data
Commenting on the study, John W. McEvoy, MB, University of Galway, Ireland, said: “These are elegant and exciting data; however, the inputs into the machine learning algorithm include all the necessary information to actually diagnose MI. So why predict MI, when a human diagnosis can just be made directly? The answer to this question may depend on whether we trust machines more than humans.”
Dr. Mills noted that clinical judgment will always be an important part of MI diagnosis.
“Currently, using the troponin threshold approach, experienced clinicians will be able to nuance the results, but invariably, there is disagreement on this, and this can be a major source of tension within clinical care. By providing more individualized information, this will help enormously in the decision-making process,” he said.
“This model is not about replacing clinical decision-making. It’s more about augmenting decision-making and giving clinicians guidance to be able to improve efficiency and reduce inequality,” he added.
The study was funded with support from the National Institute for Health Research and NHSX, the British Heart Foundation, and Wellcome Leap. Dr. Mills has received honoraria or consultancy from Abbott Diagnostics, Roche Diagnostics, Siemens Healthineers, and LumiraDx. He is employed by the University of Edinburgh, which has filed a patent on the Collaboration for the Diagnosis and Evaluation of Acute Coronary Syndrome score.
A version of this article first appeared on Medscape.com.
Use of a machine-learning model that incorporates information from a single troponin test as well as other clinical data was superior to current practice as an aid to the diagnosis of myocardial infarction in the emergency department in a new study.
“Our results suggest that and free up space in the emergency department,” senior author Nicholas L. Mills, MD, University of Edinburgh, Scotland, said in an interview.
“And, perhaps even more importantly, use of this model could also increase the proportion of patients who are correctly identified as at a high probability of having an MI,” he added.
The study was published online in Nature Medicine.
The authors explained that at present, the likelihood of an MI diagnosis for patients presenting to the emergency department with chest pain is based on a fixed troponin threshold in serial tests at specific time points, but there are several problems with this approach.
First, a fixed troponin threshold is generally used for all patients, which does not account for age, sex, or comorbidities that are known to influence cardiac troponin concentrations. Second, the need to perform tests at specific time points for serial testing can be challenging in busy emergency departments.
And third, patients are categorized as being at low, intermediate, or high risk of MI on the basis of troponin thresholds alone, and the test does not take into account other important factors, such as the time of symptom onset or findings on the electrocardiogram.
“Our current practice of using the same threshold to rule in and rule out an MI for everyone, regardless of whether they are an 18-year-old female without a history of heart disease or an 85-year-old male with known heart failure, doesn’t perform well, and there’s a significant risk of misdiagnosis. There is also a high likelihood for inequalities in care, particularly between men and women,” Dr. Mills said.
The current study evaluated whether use of a machine learning model known as CoDE-ACS to guide decision-making could overcome some of these challenges.
The machine learning model assesses the whole spectrum of troponin levels as a continuous variable (rather than use of a single threshold) and turns this measurement into a probability that an individual patient is having an MI after accounting for other factors, including age, sex, comorbidities, and time from symptom onset.
For the current study, the CoDE-ACS model was trained in 10,000 patients with suspected acute coronary syndrome (ACS) who presented to 10 hospitals in Scotland as part of the High-STEACS trial evaluating the implementation of a high-sensitivity cardiac troponin I assay. The results were then validated in another 10,000 patients from six countries around the world.
“Using this model, the patient can have a troponin test on arrival at the emergency department. The other information on age, sex, clinical history, and time since symptom onset is keyed in, and it gives a probability on a scale of 0–100 as to whether the patient is having an MI,” Dr. Mills noted.
“It also has the capacity to incorporate more information over time. So, if there is a second troponin measurement made, then the model automatically refines the probability score,” he added.
The current study showed that use of the CoDE-ACS model identified more patients at presentation as having a low probability of having an MI than fixed cardiac troponin thresholds (61% vs. 27%) with a similar negative predictive value.
It also identified fewer patients as having a high probability of having an MI (10% vs. 16%) with a greater positive predictive value.
Among patients who were identified as having a low probability of MI, the rate of cardiac death was lower than the rate among those with intermediate or high probability at 30 days (0.1% vs. 0.5% and 1.8%) and 1 year (0.3% vs. 2.8% and 4.2%).
“The results show that the machine learning model doubles the proportion of patients who can be discharged with a single test compared to the current practice of using the threshold approach. It really is a game changer in terms of its potential to improve health efficiency,” Dr. Mills said.
In terms of ruling patients in as possibly having an MI, he pointed out that troponin levels are increased in patients with a wide range of other conditions, including heart failure, kidney failure, and atrial fibrillation.
“When using the threshold approach, only one in four patients with an elevated troponin level will actually be having an MI, and that leads to confusion,” he said. “This model takes into consideration these other conditions and so it can correctly identify three out of four patients with a high probability of having an MI. We can therefore be more confident that it is appropriate to refer those patients to cardiology and save a lot of potentially unnecessary investigations and treatments in the others.”
Dr. Mills said the model also seems to work when assessing patients early on.
“Around one-third of patients present within 3 hours of symptom onset, and actually these are a high-risk group because people who have genuine cardiac pain are normally extremely uncomfortable and tend to present quickly. Current guidelines require that we do two tests in all these individuals, but this new model incorporates the time of symptom onset into its estimates of probability and therefore allows us to rule out patients even when they present very early.”
He reported that a second test is required in only one in five patients – those whose first test indicated intermediate probability.
“The second test allows us to refine the probability further, allowing us to rule another half of those patients out. We are then left with a small proportion of patients – about 1 in 10 – who remain of intermediate probability and will require additional clinical judgment.”
Should improve inequities in MI diagnosis
Dr. Mills said the CoDE-ACS model will improve current inequities in MI diagnosis, because of which MI is underrecognized in women and younger people.
“Women have troponin concentrations that are half those of men, and although sex-specific troponin thresholds are recommended in the guidelines, they are not widely used. This automatically leads to underrecognition of heart disease in women. But this new machine learning model performs identically in men and women because it has been trained to recognize the different normal levels in men and women,” he explained.
“It will also help us not to underdiagnose MI in younger people who often have a less classical presentation of MI, and they also generally have very low concentrations of troponin, so any increase in troponin way below the current diagnostic threshold may be very relevant to their risk,” he added.
The researchers are planning a randomized trial of the new model to demonstrate the impact it could have on equality of care and overcrowding in the emergency department. In the trial, patients will be randomly assigned to undergo decision-making on the basis of troponin thresholds (current practice) or to undergo decision-making through the CoDE-ACS model.
“The hope is that this trial will show reductions in unnecessary hospital admissions and length of stay in the emergency department,” Dr. Mills said. Results are expected sometime next year.
“This algorithm is very well trained. It has learned on 20,000 patients, so it has a lot more experience than I have, and I have been a professor of cardiology for 20 years,” Dr. Mills said.
He said he believes these models will get even smarter in the future as more data are added.
“I think the future for good decision-making in emergency care will be informed by clinical decision support from well-trained machine learning algorithms and they will help us guide not just the diagnosis of MI but also heart failure and other important cardiac conditions,” he said.
‘Elegant and exciting’ data
Commenting on the study, John W. McEvoy, MB, University of Galway, Ireland, said: “These are elegant and exciting data; however, the inputs into the machine learning algorithm include all the necessary information to actually diagnose MI. So why predict MI, when a human diagnosis can just be made directly? The answer to this question may depend on whether we trust machines more than humans.”
Dr. Mills noted that clinical judgment will always be an important part of MI diagnosis.
“Currently, using the troponin threshold approach, experienced clinicians will be able to nuance the results, but invariably, there is disagreement on this, and this can be a major source of tension within clinical care. By providing more individualized information, this will help enormously in the decision-making process,” he said.
“This model is not about replacing clinical decision-making. It’s more about augmenting decision-making and giving clinicians guidance to be able to improve efficiency and reduce inequality,” he added.
The study was funded with support from the National Institute for Health Research and NHSX, the British Heart Foundation, and Wellcome Leap. Dr. Mills has received honoraria or consultancy from Abbott Diagnostics, Roche Diagnostics, Siemens Healthineers, and LumiraDx. He is employed by the University of Edinburgh, which has filed a patent on the Collaboration for the Diagnosis and Evaluation of Acute Coronary Syndrome score.
A version of this article first appeared on Medscape.com.
Use of a machine-learning model that incorporates information from a single troponin test as well as other clinical data was superior to current practice as an aid to the diagnosis of myocardial infarction in the emergency department in a new study.
“Our results suggest that and free up space in the emergency department,” senior author Nicholas L. Mills, MD, University of Edinburgh, Scotland, said in an interview.
“And, perhaps even more importantly, use of this model could also increase the proportion of patients who are correctly identified as at a high probability of having an MI,” he added.
The study was published online in Nature Medicine.
The authors explained that at present, the likelihood of an MI diagnosis for patients presenting to the emergency department with chest pain is based on a fixed troponin threshold in serial tests at specific time points, but there are several problems with this approach.
First, a fixed troponin threshold is generally used for all patients, which does not account for age, sex, or comorbidities that are known to influence cardiac troponin concentrations. Second, the need to perform tests at specific time points for serial testing can be challenging in busy emergency departments.
And third, patients are categorized as being at low, intermediate, or high risk of MI on the basis of troponin thresholds alone, and the test does not take into account other important factors, such as the time of symptom onset or findings on the electrocardiogram.
“Our current practice of using the same threshold to rule in and rule out an MI for everyone, regardless of whether they are an 18-year-old female without a history of heart disease or an 85-year-old male with known heart failure, doesn’t perform well, and there’s a significant risk of misdiagnosis. There is also a high likelihood for inequalities in care, particularly between men and women,” Dr. Mills said.
The current study evaluated whether use of a machine learning model known as CoDE-ACS to guide decision-making could overcome some of these challenges.
The machine learning model assesses the whole spectrum of troponin levels as a continuous variable (rather than use of a single threshold) and turns this measurement into a probability that an individual patient is having an MI after accounting for other factors, including age, sex, comorbidities, and time from symptom onset.
For the current study, the CoDE-ACS model was trained in 10,000 patients with suspected acute coronary syndrome (ACS) who presented to 10 hospitals in Scotland as part of the High-STEACS trial evaluating the implementation of a high-sensitivity cardiac troponin I assay. The results were then validated in another 10,000 patients from six countries around the world.
“Using this model, the patient can have a troponin test on arrival at the emergency department. The other information on age, sex, clinical history, and time since symptom onset is keyed in, and it gives a probability on a scale of 0–100 as to whether the patient is having an MI,” Dr. Mills noted.
“It also has the capacity to incorporate more information over time. So, if there is a second troponin measurement made, then the model automatically refines the probability score,” he added.
The current study showed that use of the CoDE-ACS model identified more patients at presentation as having a low probability of having an MI than fixed cardiac troponin thresholds (61% vs. 27%) with a similar negative predictive value.
It also identified fewer patients as having a high probability of having an MI (10% vs. 16%) with a greater positive predictive value.
Among patients who were identified as having a low probability of MI, the rate of cardiac death was lower than the rate among those with intermediate or high probability at 30 days (0.1% vs. 0.5% and 1.8%) and 1 year (0.3% vs. 2.8% and 4.2%).
“The results show that the machine learning model doubles the proportion of patients who can be discharged with a single test compared to the current practice of using the threshold approach. It really is a game changer in terms of its potential to improve health efficiency,” Dr. Mills said.
In terms of ruling patients in as possibly having an MI, he pointed out that troponin levels are increased in patients with a wide range of other conditions, including heart failure, kidney failure, and atrial fibrillation.
“When using the threshold approach, only one in four patients with an elevated troponin level will actually be having an MI, and that leads to confusion,” he said. “This model takes into consideration these other conditions and so it can correctly identify three out of four patients with a high probability of having an MI. We can therefore be more confident that it is appropriate to refer those patients to cardiology and save a lot of potentially unnecessary investigations and treatments in the others.”
Dr. Mills said the model also seems to work when assessing patients early on.
“Around one-third of patients present within 3 hours of symptom onset, and actually these are a high-risk group because people who have genuine cardiac pain are normally extremely uncomfortable and tend to present quickly. Current guidelines require that we do two tests in all these individuals, but this new model incorporates the time of symptom onset into its estimates of probability and therefore allows us to rule out patients even when they present very early.”
He reported that a second test is required in only one in five patients – those whose first test indicated intermediate probability.
“The second test allows us to refine the probability further, allowing us to rule another half of those patients out. We are then left with a small proportion of patients – about 1 in 10 – who remain of intermediate probability and will require additional clinical judgment.”
Should improve inequities in MI diagnosis
Dr. Mills said the CoDE-ACS model will improve current inequities in MI diagnosis, because of which MI is underrecognized in women and younger people.
“Women have troponin concentrations that are half those of men, and although sex-specific troponin thresholds are recommended in the guidelines, they are not widely used. This automatically leads to underrecognition of heart disease in women. But this new machine learning model performs identically in men and women because it has been trained to recognize the different normal levels in men and women,” he explained.
“It will also help us not to underdiagnose MI in younger people who often have a less classical presentation of MI, and they also generally have very low concentrations of troponin, so any increase in troponin way below the current diagnostic threshold may be very relevant to their risk,” he added.
The researchers are planning a randomized trial of the new model to demonstrate the impact it could have on equality of care and overcrowding in the emergency department. In the trial, patients will be randomly assigned to undergo decision-making on the basis of troponin thresholds (current practice) or to undergo decision-making through the CoDE-ACS model.
“The hope is that this trial will show reductions in unnecessary hospital admissions and length of stay in the emergency department,” Dr. Mills said. Results are expected sometime next year.
“This algorithm is very well trained. It has learned on 20,000 patients, so it has a lot more experience than I have, and I have been a professor of cardiology for 20 years,” Dr. Mills said.
He said he believes these models will get even smarter in the future as more data are added.
“I think the future for good decision-making in emergency care will be informed by clinical decision support from well-trained machine learning algorithms and they will help us guide not just the diagnosis of MI but also heart failure and other important cardiac conditions,” he said.
‘Elegant and exciting’ data
Commenting on the study, John W. McEvoy, MB, University of Galway, Ireland, said: “These are elegant and exciting data; however, the inputs into the machine learning algorithm include all the necessary information to actually diagnose MI. So why predict MI, when a human diagnosis can just be made directly? The answer to this question may depend on whether we trust machines more than humans.”
Dr. Mills noted that clinical judgment will always be an important part of MI diagnosis.
“Currently, using the troponin threshold approach, experienced clinicians will be able to nuance the results, but invariably, there is disagreement on this, and this can be a major source of tension within clinical care. By providing more individualized information, this will help enormously in the decision-making process,” he said.
“This model is not about replacing clinical decision-making. It’s more about augmenting decision-making and giving clinicians guidance to be able to improve efficiency and reduce inequality,” he added.
The study was funded with support from the National Institute for Health Research and NHSX, the British Heart Foundation, and Wellcome Leap. Dr. Mills has received honoraria or consultancy from Abbott Diagnostics, Roche Diagnostics, Siemens Healthineers, and LumiraDx. He is employed by the University of Edinburgh, which has filed a patent on the Collaboration for the Diagnosis and Evaluation of Acute Coronary Syndrome score.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE




