User login
FDA requests more data on antiseptics used in health care settings
The Food and Drug Administration is requesting more scientific data on the safety and effectiveness of the active ingredients in antiseptic products used in hospitals, physician’s offices, and other health care settings in a proposed rule issued April 30.
“Today’s proposal seeks to ensure the FDA’s evaluations and determinations for all health care antiseptic active ingredients are consistent, up-to-date and appropriately reflect current scientific knowledge and patterns of use by health care professionals,” Dr. Theresa Michele, director of the division of nonprescription drug products in the FDA’s Center for Drug Evaluation and Research, said in a statement. The proposed rule “should not be taken to mean” that the agency believes these products are not effective or safe, according to the statement.
Alcohol and iodine are among the most common active ingredients in these products, which include hand washes and rubs, surgical hand scrubs and rubs, and preoperative skin preparations used on patients. These products are marketed under an over-the-counter drug monograph and are different than antibacterial soaps, hand sanitizers, and other consumer antiseptic products, which are not included in this proposed rule. To continue to market these products under the monograph, manufacturers must provide the FDA with more data on the safety and effectiveness of the active ingredients, including absorption, hormonal effects, and bacterial resistance, the statement said.
“Emerging science” suggests that, for at least some active ingredients used in these products, “systemic exposure … is higher than previously thought, and existing data raise potential concerns about the effects of repeated daily human exposure to some antiseptic active ingredients,” the statement said. The agency “is particularly interested in gathering additional data on the long-term safety of daily, repeated exposure to these ingredients in the health care setting and on the use of these products by certain populations, including pregnant and breastfeeding health care workers, for which topical absorption of the active ingredients may be important.”
The proposed rule is available at www.federalregister.gov. Public comments can be submitted until Oct. 27.
The Food and Drug Administration is requesting more scientific data on the safety and effectiveness of the active ingredients in antiseptic products used in hospitals, physician’s offices, and other health care settings in a proposed rule issued April 30.
“Today’s proposal seeks to ensure the FDA’s evaluations and determinations for all health care antiseptic active ingredients are consistent, up-to-date and appropriately reflect current scientific knowledge and patterns of use by health care professionals,” Dr. Theresa Michele, director of the division of nonprescription drug products in the FDA’s Center for Drug Evaluation and Research, said in a statement. The proposed rule “should not be taken to mean” that the agency believes these products are not effective or safe, according to the statement.
Alcohol and iodine are among the most common active ingredients in these products, which include hand washes and rubs, surgical hand scrubs and rubs, and preoperative skin preparations used on patients. These products are marketed under an over-the-counter drug monograph and are different than antibacterial soaps, hand sanitizers, and other consumer antiseptic products, which are not included in this proposed rule. To continue to market these products under the monograph, manufacturers must provide the FDA with more data on the safety and effectiveness of the active ingredients, including absorption, hormonal effects, and bacterial resistance, the statement said.
“Emerging science” suggests that, for at least some active ingredients used in these products, “systemic exposure … is higher than previously thought, and existing data raise potential concerns about the effects of repeated daily human exposure to some antiseptic active ingredients,” the statement said. The agency “is particularly interested in gathering additional data on the long-term safety of daily, repeated exposure to these ingredients in the health care setting and on the use of these products by certain populations, including pregnant and breastfeeding health care workers, for which topical absorption of the active ingredients may be important.”
The proposed rule is available at www.federalregister.gov. Public comments can be submitted until Oct. 27.
The Food and Drug Administration is requesting more scientific data on the safety and effectiveness of the active ingredients in antiseptic products used in hospitals, physician’s offices, and other health care settings in a proposed rule issued April 30.
“Today’s proposal seeks to ensure the FDA’s evaluations and determinations for all health care antiseptic active ingredients are consistent, up-to-date and appropriately reflect current scientific knowledge and patterns of use by health care professionals,” Dr. Theresa Michele, director of the division of nonprescription drug products in the FDA’s Center for Drug Evaluation and Research, said in a statement. The proposed rule “should not be taken to mean” that the agency believes these products are not effective or safe, according to the statement.
Alcohol and iodine are among the most common active ingredients in these products, which include hand washes and rubs, surgical hand scrubs and rubs, and preoperative skin preparations used on patients. These products are marketed under an over-the-counter drug monograph and are different than antibacterial soaps, hand sanitizers, and other consumer antiseptic products, which are not included in this proposed rule. To continue to market these products under the monograph, manufacturers must provide the FDA with more data on the safety and effectiveness of the active ingredients, including absorption, hormonal effects, and bacterial resistance, the statement said.
“Emerging science” suggests that, for at least some active ingredients used in these products, “systemic exposure … is higher than previously thought, and existing data raise potential concerns about the effects of repeated daily human exposure to some antiseptic active ingredients,” the statement said. The agency “is particularly interested in gathering additional data on the long-term safety of daily, repeated exposure to these ingredients in the health care setting and on the use of these products by certain populations, including pregnant and breastfeeding health care workers, for which topical absorption of the active ingredients may be important.”
The proposed rule is available at www.federalregister.gov. Public comments can be submitted until Oct. 27.
FDA approves Kybella for chin fat
The Food and Drug Administration on April 29 approved deoxycholic acid (DCA) injection for the treatment of submental fat.
The cytolytic agent, a synthetic version of naturally occurring DCA, physically destroys fat cells when injected properly into submental fat. It is the first drug approved for the treatment of submental fat, and it received unanimous support from an FDA advisory panel in March based on two placebo-controlled phase III trials involving more than 1,000 adults.
The studies demonstrated the safety and effectiveness of treatment with up to 50 injections of 0.2 mL of the 1% DCA solution administered in a single treatment. Up to six treatments can be administered at least 1 month apart.
Serious side effects associated with injection of DCA may include nerve injury in the jaw and trouble swallowing, but the most common side effects are swelling, bruising, pain, numbness, redness, and areas of hardness in the treatment area.
DCA will be marketed as Kybella by Kythera Biopharmaceuticals.
“It is important to remember that Kybella is only approved for the treatment of fat occurring below the chin, and it is not known if Kybella is safe or effective for treatment outside of this area,” Dr. Amy G. Egan, deputy director of the Office of Drug Evaluation III in the FDA Center for Drug Evaluation and Research said in the statement announcing the approval.
Kybella will be provided in single-patient–use vials and should not be diluted or mixed with other compounds. The dispensing pack has a unique hologram on the vial label, and the product should not be used if there is no hologram.
Physicians and patients are encouraged to report adverse reactions from the use of Kybella to the FDA’s MedWatch Adverse Event Reporting program at www.fda.gov/MedWatch or by calling 800-FDA-1088.
The Food and Drug Administration on April 29 approved deoxycholic acid (DCA) injection for the treatment of submental fat.
The cytolytic agent, a synthetic version of naturally occurring DCA, physically destroys fat cells when injected properly into submental fat. It is the first drug approved for the treatment of submental fat, and it received unanimous support from an FDA advisory panel in March based on two placebo-controlled phase III trials involving more than 1,000 adults.
The studies demonstrated the safety and effectiveness of treatment with up to 50 injections of 0.2 mL of the 1% DCA solution administered in a single treatment. Up to six treatments can be administered at least 1 month apart.
Serious side effects associated with injection of DCA may include nerve injury in the jaw and trouble swallowing, but the most common side effects are swelling, bruising, pain, numbness, redness, and areas of hardness in the treatment area.
DCA will be marketed as Kybella by Kythera Biopharmaceuticals.
“It is important to remember that Kybella is only approved for the treatment of fat occurring below the chin, and it is not known if Kybella is safe or effective for treatment outside of this area,” Dr. Amy G. Egan, deputy director of the Office of Drug Evaluation III in the FDA Center for Drug Evaluation and Research said in the statement announcing the approval.
Kybella will be provided in single-patient–use vials and should not be diluted or mixed with other compounds. The dispensing pack has a unique hologram on the vial label, and the product should not be used if there is no hologram.
Physicians and patients are encouraged to report adverse reactions from the use of Kybella to the FDA’s MedWatch Adverse Event Reporting program at www.fda.gov/MedWatch or by calling 800-FDA-1088.
The Food and Drug Administration on April 29 approved deoxycholic acid (DCA) injection for the treatment of submental fat.
The cytolytic agent, a synthetic version of naturally occurring DCA, physically destroys fat cells when injected properly into submental fat. It is the first drug approved for the treatment of submental fat, and it received unanimous support from an FDA advisory panel in March based on two placebo-controlled phase III trials involving more than 1,000 adults.
The studies demonstrated the safety and effectiveness of treatment with up to 50 injections of 0.2 mL of the 1% DCA solution administered in a single treatment. Up to six treatments can be administered at least 1 month apart.
Serious side effects associated with injection of DCA may include nerve injury in the jaw and trouble swallowing, but the most common side effects are swelling, bruising, pain, numbness, redness, and areas of hardness in the treatment area.
DCA will be marketed as Kybella by Kythera Biopharmaceuticals.
“It is important to remember that Kybella is only approved for the treatment of fat occurring below the chin, and it is not known if Kybella is safe or effective for treatment outside of this area,” Dr. Amy G. Egan, deputy director of the Office of Drug Evaluation III in the FDA Center for Drug Evaluation and Research said in the statement announcing the approval.
Kybella will be provided in single-patient–use vials and should not be diluted or mixed with other compounds. The dispensing pack has a unique hologram on the vial label, and the product should not be used if there is no hologram.
Physicians and patients are encouraged to report adverse reactions from the use of Kybella to the FDA’s MedWatch Adverse Event Reporting program at www.fda.gov/MedWatch or by calling 800-FDA-1088.
Imaging guides BCC laser ablation
KISSIMMEE, FLA. – Reflective confocal microscopic imaging successfully guided carbon dioxide laser ablation of basal cell carcinomas, and imaging results fully matched those from Mohs histology, a small study found.
“Our results suggest that reflective confocal microscopy can accurately guide carbon dioxide laser ablation of superficial and early nodular basal cell carcinomas,” said Dr. Brian Hibler of Memorial Sloan Kettering Cancer Center in New York. The technique provides a real-time, noninvasive way to delineate the tumor area before ablation and to check for residual tumor between passes with the laser, he said at the annual meeting of the American Society for Laser Medicine and surgery.
While conventional and Mohs microscopic surgeries remain the gold standard for removing basal carcinomas (BCC), surgery is not an option for some patients because of tumor location, comorbidities, or personal preferences, Dr. Hibler noted. Past studies have reported good clinical and cosmetic outcomes with laser ablation of BCCs, but use of the modality has been limited because there was no way to assess response without excision or biopsies. Reflective confocal microscopy (RCM) uses a low-powered laser system that provides cellular-level imaging and can distinguish BCCs, he said.
Dr. Hibler and his colleagues performed baseline RCM of eight BCCs (three on the trunk, three on the extremities, and two on the head and neck) from seven patients aged 29-83 years. Two patients were men and five were women. The patients then underwent carbon dioxide laser ablation with a wavelength of 10,600 nm, pulse duration of 750 microseconds, and fluence of 7.5 J/cm2, using a square pattern and density of 30%. If RCM revealed residual BCC, the researchers repeated the process up to two more times, for a maximum of three passes. They then removed the entire lesion using Mohs micrographic surgery, performing vertical histologic sectioning of the tissue.
Reflective confocal microscopy generated reliable cellular-level images in real time on the tumor surface and up to 150 mcm deep, Dr. Hibler reported. Tissue from BCCs appears as dense nodular areas with adjacent spaces and red blood cell trafficking, he noted. Microscopy results were consistent with Mohs histology findings in all eight cases, including six in which the tumor was completely removed and two with residual tumor. One of these two cases was the only infiltrative BCC in the series, while the other might have been tissue artifact, Dr. Hibler said.
Patients experienced no adverse effects from the interventions, Dr. Hibler reported. “Future studies are planned are planned without Mohs, so we can use reflective confocal microscopy to longitudinally monitor for recurrence,” he added.
The study won an award at the meeting.
Dr. Hibler reported no funding sources and made no disclosures.
KISSIMMEE, FLA. – Reflective confocal microscopic imaging successfully guided carbon dioxide laser ablation of basal cell carcinomas, and imaging results fully matched those from Mohs histology, a small study found.
“Our results suggest that reflective confocal microscopy can accurately guide carbon dioxide laser ablation of superficial and early nodular basal cell carcinomas,” said Dr. Brian Hibler of Memorial Sloan Kettering Cancer Center in New York. The technique provides a real-time, noninvasive way to delineate the tumor area before ablation and to check for residual tumor between passes with the laser, he said at the annual meeting of the American Society for Laser Medicine and surgery.
While conventional and Mohs microscopic surgeries remain the gold standard for removing basal carcinomas (BCC), surgery is not an option for some patients because of tumor location, comorbidities, or personal preferences, Dr. Hibler noted. Past studies have reported good clinical and cosmetic outcomes with laser ablation of BCCs, but use of the modality has been limited because there was no way to assess response without excision or biopsies. Reflective confocal microscopy (RCM) uses a low-powered laser system that provides cellular-level imaging and can distinguish BCCs, he said.
Dr. Hibler and his colleagues performed baseline RCM of eight BCCs (three on the trunk, three on the extremities, and two on the head and neck) from seven patients aged 29-83 years. Two patients were men and five were women. The patients then underwent carbon dioxide laser ablation with a wavelength of 10,600 nm, pulse duration of 750 microseconds, and fluence of 7.5 J/cm2, using a square pattern and density of 30%. If RCM revealed residual BCC, the researchers repeated the process up to two more times, for a maximum of three passes. They then removed the entire lesion using Mohs micrographic surgery, performing vertical histologic sectioning of the tissue.
Reflective confocal microscopy generated reliable cellular-level images in real time on the tumor surface and up to 150 mcm deep, Dr. Hibler reported. Tissue from BCCs appears as dense nodular areas with adjacent spaces and red blood cell trafficking, he noted. Microscopy results were consistent with Mohs histology findings in all eight cases, including six in which the tumor was completely removed and two with residual tumor. One of these two cases was the only infiltrative BCC in the series, while the other might have been tissue artifact, Dr. Hibler said.
Patients experienced no adverse effects from the interventions, Dr. Hibler reported. “Future studies are planned are planned without Mohs, so we can use reflective confocal microscopy to longitudinally monitor for recurrence,” he added.
The study won an award at the meeting.
Dr. Hibler reported no funding sources and made no disclosures.
KISSIMMEE, FLA. – Reflective confocal microscopic imaging successfully guided carbon dioxide laser ablation of basal cell carcinomas, and imaging results fully matched those from Mohs histology, a small study found.
“Our results suggest that reflective confocal microscopy can accurately guide carbon dioxide laser ablation of superficial and early nodular basal cell carcinomas,” said Dr. Brian Hibler of Memorial Sloan Kettering Cancer Center in New York. The technique provides a real-time, noninvasive way to delineate the tumor area before ablation and to check for residual tumor between passes with the laser, he said at the annual meeting of the American Society for Laser Medicine and surgery.
While conventional and Mohs microscopic surgeries remain the gold standard for removing basal carcinomas (BCC), surgery is not an option for some patients because of tumor location, comorbidities, or personal preferences, Dr. Hibler noted. Past studies have reported good clinical and cosmetic outcomes with laser ablation of BCCs, but use of the modality has been limited because there was no way to assess response without excision or biopsies. Reflective confocal microscopy (RCM) uses a low-powered laser system that provides cellular-level imaging and can distinguish BCCs, he said.
Dr. Hibler and his colleagues performed baseline RCM of eight BCCs (three on the trunk, three on the extremities, and two on the head and neck) from seven patients aged 29-83 years. Two patients were men and five were women. The patients then underwent carbon dioxide laser ablation with a wavelength of 10,600 nm, pulse duration of 750 microseconds, and fluence of 7.5 J/cm2, using a square pattern and density of 30%. If RCM revealed residual BCC, the researchers repeated the process up to two more times, for a maximum of three passes. They then removed the entire lesion using Mohs micrographic surgery, performing vertical histologic sectioning of the tissue.
Reflective confocal microscopy generated reliable cellular-level images in real time on the tumor surface and up to 150 mcm deep, Dr. Hibler reported. Tissue from BCCs appears as dense nodular areas with adjacent spaces and red blood cell trafficking, he noted. Microscopy results were consistent with Mohs histology findings in all eight cases, including six in which the tumor was completely removed and two with residual tumor. One of these two cases was the only infiltrative BCC in the series, while the other might have been tissue artifact, Dr. Hibler said.
Patients experienced no adverse effects from the interventions, Dr. Hibler reported. “Future studies are planned are planned without Mohs, so we can use reflective confocal microscopy to longitudinally monitor for recurrence,” he added.
The study won an award at the meeting.
Dr. Hibler reported no funding sources and made no disclosures.
Key clinical point: Reflective confocal microscopy offers noninvasive, real-time imaging to guide laser ablation of basal cell carcinomas.
Major finding: Results from RCM matched those from Mohs histology in all patients.
Data source: Prospective study of eight BCCs in seven patients.
Disclosures: Dr. Hibler reported no funding sources and made no disclosures.
Radiofrequency improves lower face laxity
KISSIMMEE, FLA. – Three treatments with a noninvasive radiofrequency device significantly improved mild to moderate laxity of the lower face and neck, a prospective study of 30 patients showed.
Downtime after treatment ranged from 2 to 4 days, and adverse effects included mild stippling and purpura at the treatment sites, Dr. Girish Munavalli reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Aging causes progressive loosening of the tissues of the lower face and neck, which manifests as narrowing of the cervicomental angle (between the neck and the lowest point under the chin) and the gnathion angle (between the lowest point under the chin and the most anterior or prominent point on the front of the chin). Historically, patients resorted to invasive surgical lifting procedures to restore these angles, said Dr. Munavalli of Wake Forest University, Charlotte, N.C.
To explore noninvasive alternatives, he and his associates conducted a pilot study of a bipolar fractionated microneedle radiofrequency device that consisted of a handpiece and a 1 cm2 square disposable microneedle tip with 49 proximally insulated 34-G microneedle electrodes. The study comprised 7 men and 23 women aged 37-71 years. One physician treated all patients, and each patient underwent three treatment sessions lasting about 45 minutes and spaced a month apart.
Each session included three passes across the lower face and submental area at a maximum tissue depth of 1-3 mm, Dr. Munavalli said. Thicker skin requires deeper needle penetration, which in turn requires more treatment energy and longer pulse duration, he noted. Therefore, the physician performed the first pass at a depth of about 2.5 mm with an energy setting of 9-11 and a pulse duration of 280-320 msec, the second pass at a depth of 1.5 mm with a setting of 8-9 and a pulse duration of 230-250 msec, and the third pass at a depth of 1 mm with a setting of 6 and a pulse duration of 160 msec.
Six months after the end of treatment, patients underwent computerized measurements of the cervicomental and gnathion angles, and a panel of blinded investigators compared pre- and posttreatment photographs, Dr. Munavalli said. On average, the cervicomental angle increased by 27 degrees (range, 18 to 36 degrees; P < .01), and the gnathion angle increased by 16 degrees (range, 12 to 20 degrees; P < .01). The blinded assessors correctly chose the posttreatment photographs 90% of the time. Taken together, the results suggest that the device is a safe and effective option for improving laxity of the lower face and neck, Dr. Munavalli concluded.
Lutronic Corp is the maker of the Infini device, which the FDA approved for dermatologic use in July 2013. Dr. Munavalli reported no funding sources or financial disclosures.
KISSIMMEE, FLA. – Three treatments with a noninvasive radiofrequency device significantly improved mild to moderate laxity of the lower face and neck, a prospective study of 30 patients showed.
Downtime after treatment ranged from 2 to 4 days, and adverse effects included mild stippling and purpura at the treatment sites, Dr. Girish Munavalli reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Aging causes progressive loosening of the tissues of the lower face and neck, which manifests as narrowing of the cervicomental angle (between the neck and the lowest point under the chin) and the gnathion angle (between the lowest point under the chin and the most anterior or prominent point on the front of the chin). Historically, patients resorted to invasive surgical lifting procedures to restore these angles, said Dr. Munavalli of Wake Forest University, Charlotte, N.C.
To explore noninvasive alternatives, he and his associates conducted a pilot study of a bipolar fractionated microneedle radiofrequency device that consisted of a handpiece and a 1 cm2 square disposable microneedle tip with 49 proximally insulated 34-G microneedle electrodes. The study comprised 7 men and 23 women aged 37-71 years. One physician treated all patients, and each patient underwent three treatment sessions lasting about 45 minutes and spaced a month apart.
Each session included three passes across the lower face and submental area at a maximum tissue depth of 1-3 mm, Dr. Munavalli said. Thicker skin requires deeper needle penetration, which in turn requires more treatment energy and longer pulse duration, he noted. Therefore, the physician performed the first pass at a depth of about 2.5 mm with an energy setting of 9-11 and a pulse duration of 280-320 msec, the second pass at a depth of 1.5 mm with a setting of 8-9 and a pulse duration of 230-250 msec, and the third pass at a depth of 1 mm with a setting of 6 and a pulse duration of 160 msec.
Six months after the end of treatment, patients underwent computerized measurements of the cervicomental and gnathion angles, and a panel of blinded investigators compared pre- and posttreatment photographs, Dr. Munavalli said. On average, the cervicomental angle increased by 27 degrees (range, 18 to 36 degrees; P < .01), and the gnathion angle increased by 16 degrees (range, 12 to 20 degrees; P < .01). The blinded assessors correctly chose the posttreatment photographs 90% of the time. Taken together, the results suggest that the device is a safe and effective option for improving laxity of the lower face and neck, Dr. Munavalli concluded.
Lutronic Corp is the maker of the Infini device, which the FDA approved for dermatologic use in July 2013. Dr. Munavalli reported no funding sources or financial disclosures.
KISSIMMEE, FLA. – Three treatments with a noninvasive radiofrequency device significantly improved mild to moderate laxity of the lower face and neck, a prospective study of 30 patients showed.
Downtime after treatment ranged from 2 to 4 days, and adverse effects included mild stippling and purpura at the treatment sites, Dr. Girish Munavalli reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Aging causes progressive loosening of the tissues of the lower face and neck, which manifests as narrowing of the cervicomental angle (between the neck and the lowest point under the chin) and the gnathion angle (between the lowest point under the chin and the most anterior or prominent point on the front of the chin). Historically, patients resorted to invasive surgical lifting procedures to restore these angles, said Dr. Munavalli of Wake Forest University, Charlotte, N.C.
To explore noninvasive alternatives, he and his associates conducted a pilot study of a bipolar fractionated microneedle radiofrequency device that consisted of a handpiece and a 1 cm2 square disposable microneedle tip with 49 proximally insulated 34-G microneedle electrodes. The study comprised 7 men and 23 women aged 37-71 years. One physician treated all patients, and each patient underwent three treatment sessions lasting about 45 minutes and spaced a month apart.
Each session included three passes across the lower face and submental area at a maximum tissue depth of 1-3 mm, Dr. Munavalli said. Thicker skin requires deeper needle penetration, which in turn requires more treatment energy and longer pulse duration, he noted. Therefore, the physician performed the first pass at a depth of about 2.5 mm with an energy setting of 9-11 and a pulse duration of 280-320 msec, the second pass at a depth of 1.5 mm with a setting of 8-9 and a pulse duration of 230-250 msec, and the third pass at a depth of 1 mm with a setting of 6 and a pulse duration of 160 msec.
Six months after the end of treatment, patients underwent computerized measurements of the cervicomental and gnathion angles, and a panel of blinded investigators compared pre- and posttreatment photographs, Dr. Munavalli said. On average, the cervicomental angle increased by 27 degrees (range, 18 to 36 degrees; P < .01), and the gnathion angle increased by 16 degrees (range, 12 to 20 degrees; P < .01). The blinded assessors correctly chose the posttreatment photographs 90% of the time. Taken together, the results suggest that the device is a safe and effective option for improving laxity of the lower face and neck, Dr. Munavalli concluded.
Lutronic Corp is the maker of the Infini device, which the FDA approved for dermatologic use in July 2013. Dr. Munavalli reported no funding sources or financial disclosures.
Key clinical point: A noninvasive bipolar fractionated microneedle radiofrequency device significantly improved laxity of the lower face and neck.
Major finding: After three treatment sessions, the cervicomental and gnathion angles increased by an average of 27 degrees and 16 degrees, respectively.
Data source: Prospective study of 30 patients with mild to moderate laxity of the lower face and neck.
Disclosures: Dr. Munavalli reported no funding sources or financial disclosures.
Fractional laser resurfacing plus ALA-PDT upped AK clearance
KISSIMMEE, FLA. – Fractional carbon dioxide laser resurfacing followed by 30 minutes of aminolevulinic acid plus blue light photodynamic therapy cleared 94% of actinic keratoses, significantly more than ALA-PDT alone, according to the findings of a randomized, single-blinded, split-face study of 20 patients.
Laser resurfacing was associated with worse short-term erythema, but erythema resolved in about 7 days and was not associated with other adverse events, Dr. Macrene Alexiades-Armenakas said at the annual meeting of the American Society for Laser Medicine and Surgery.
Historically, two sessions of 20% topical ALA and blue light PDT have yielded actinic keratosis cure rates of 78% to 89%, but only with ALA incubation times of 14-18 hours, said Dr. Alexiades-Armenakas, associate clinical professor at Yale University, New Haven, Conn.
“Increasing drug penetration may serve to enhance PDT efficacy and shorten incubation time,” she said.
To test that hypothesis, she compared the safety and efficacy of 15- and 30-minute incubations of ALA and blue light PDT, with or without CO2 laser resurfacing. After cleaning patients’ faces with acetone wipes and applying a topical anesthetic for 1 hour, she randomly selected one half of each patient’s face for pretreatment with fractional CO2 laser, using settings of 15-28 W, 500 mcm dot spacing, and 600-800 microsecond dwell time.
Next, she applied 5-ALA to the entire face, then performed blue light illumination for 1,000 seconds. Half of the 20 patients were randomly assigned ALA incubation times of 15 minutes, while the other half underwent 30-minute incubations. She rechecked patients at 1 week, 4 weeks, and 8 weeks, and took digital photographs at baseline and at each recheck using identical lighting conditions. A blinded evaluator scored each side of each face, defining clearance as complete regression of actinic keratosis.
At 8 weeks, the rate of complete clearance for the 10 patients who underwent 15-minute ALA incubations was 88% for laser resurfacing followed by ALA-PDT, compared with 74% for ALA-PDT alone (P < .05), Dr. Alexiades-Armenakas reported. Clearance rates for the 30-minute incubation group were 94% for laser followed by ALA-PDT and 82% for ALA-PDT alone (P < .05).
Skin treated only with ALA-PDT developed minimal to moderate erythema that resolved within 5-7 days for all patients, but the laser-resurfaced skin developed “moderate to significant” erythema that resolved within 5-7 days with home care, she said.
Taken together, the results indicate that fractional CO2 laser treatment yields safe and effective clearance of actinic keratoses with “ultra-short” incubation times, Dr. Alexiades-Armenakas said.
Deka manufactures the fractional CO2 laser tested in the study, and DUSA Pharmaceuticals manufactures the blue light PDT device and the ALA product. Dr. Alexiades-Armenakas reported receiving clinical research grants from Deka, DUSA Pharmaceuticals, Alma, and Syneron.
KISSIMMEE, FLA. – Fractional carbon dioxide laser resurfacing followed by 30 minutes of aminolevulinic acid plus blue light photodynamic therapy cleared 94% of actinic keratoses, significantly more than ALA-PDT alone, according to the findings of a randomized, single-blinded, split-face study of 20 patients.
Laser resurfacing was associated with worse short-term erythema, but erythema resolved in about 7 days and was not associated with other adverse events, Dr. Macrene Alexiades-Armenakas said at the annual meeting of the American Society for Laser Medicine and Surgery.
Historically, two sessions of 20% topical ALA and blue light PDT have yielded actinic keratosis cure rates of 78% to 89%, but only with ALA incubation times of 14-18 hours, said Dr. Alexiades-Armenakas, associate clinical professor at Yale University, New Haven, Conn.
“Increasing drug penetration may serve to enhance PDT efficacy and shorten incubation time,” she said.
To test that hypothesis, she compared the safety and efficacy of 15- and 30-minute incubations of ALA and blue light PDT, with or without CO2 laser resurfacing. After cleaning patients’ faces with acetone wipes and applying a topical anesthetic for 1 hour, she randomly selected one half of each patient’s face for pretreatment with fractional CO2 laser, using settings of 15-28 W, 500 mcm dot spacing, and 600-800 microsecond dwell time.
Next, she applied 5-ALA to the entire face, then performed blue light illumination for 1,000 seconds. Half of the 20 patients were randomly assigned ALA incubation times of 15 minutes, while the other half underwent 30-minute incubations. She rechecked patients at 1 week, 4 weeks, and 8 weeks, and took digital photographs at baseline and at each recheck using identical lighting conditions. A blinded evaluator scored each side of each face, defining clearance as complete regression of actinic keratosis.
At 8 weeks, the rate of complete clearance for the 10 patients who underwent 15-minute ALA incubations was 88% for laser resurfacing followed by ALA-PDT, compared with 74% for ALA-PDT alone (P < .05), Dr. Alexiades-Armenakas reported. Clearance rates for the 30-minute incubation group were 94% for laser followed by ALA-PDT and 82% for ALA-PDT alone (P < .05).
Skin treated only with ALA-PDT developed minimal to moderate erythema that resolved within 5-7 days for all patients, but the laser-resurfaced skin developed “moderate to significant” erythema that resolved within 5-7 days with home care, she said.
Taken together, the results indicate that fractional CO2 laser treatment yields safe and effective clearance of actinic keratoses with “ultra-short” incubation times, Dr. Alexiades-Armenakas said.
Deka manufactures the fractional CO2 laser tested in the study, and DUSA Pharmaceuticals manufactures the blue light PDT device and the ALA product. Dr. Alexiades-Armenakas reported receiving clinical research grants from Deka, DUSA Pharmaceuticals, Alma, and Syneron.
KISSIMMEE, FLA. – Fractional carbon dioxide laser resurfacing followed by 30 minutes of aminolevulinic acid plus blue light photodynamic therapy cleared 94% of actinic keratoses, significantly more than ALA-PDT alone, according to the findings of a randomized, single-blinded, split-face study of 20 patients.
Laser resurfacing was associated with worse short-term erythema, but erythema resolved in about 7 days and was not associated with other adverse events, Dr. Macrene Alexiades-Armenakas said at the annual meeting of the American Society for Laser Medicine and Surgery.
Historically, two sessions of 20% topical ALA and blue light PDT have yielded actinic keratosis cure rates of 78% to 89%, but only with ALA incubation times of 14-18 hours, said Dr. Alexiades-Armenakas, associate clinical professor at Yale University, New Haven, Conn.
“Increasing drug penetration may serve to enhance PDT efficacy and shorten incubation time,” she said.
To test that hypothesis, she compared the safety and efficacy of 15- and 30-minute incubations of ALA and blue light PDT, with or without CO2 laser resurfacing. After cleaning patients’ faces with acetone wipes and applying a topical anesthetic for 1 hour, she randomly selected one half of each patient’s face for pretreatment with fractional CO2 laser, using settings of 15-28 W, 500 mcm dot spacing, and 600-800 microsecond dwell time.
Next, she applied 5-ALA to the entire face, then performed blue light illumination for 1,000 seconds. Half of the 20 patients were randomly assigned ALA incubation times of 15 minutes, while the other half underwent 30-minute incubations. She rechecked patients at 1 week, 4 weeks, and 8 weeks, and took digital photographs at baseline and at each recheck using identical lighting conditions. A blinded evaluator scored each side of each face, defining clearance as complete regression of actinic keratosis.
At 8 weeks, the rate of complete clearance for the 10 patients who underwent 15-minute ALA incubations was 88% for laser resurfacing followed by ALA-PDT, compared with 74% for ALA-PDT alone (P < .05), Dr. Alexiades-Armenakas reported. Clearance rates for the 30-minute incubation group were 94% for laser followed by ALA-PDT and 82% for ALA-PDT alone (P < .05).
Skin treated only with ALA-PDT developed minimal to moderate erythema that resolved within 5-7 days for all patients, but the laser-resurfaced skin developed “moderate to significant” erythema that resolved within 5-7 days with home care, she said.
Taken together, the results indicate that fractional CO2 laser treatment yields safe and effective clearance of actinic keratoses with “ultra-short” incubation times, Dr. Alexiades-Armenakas said.
Deka manufactures the fractional CO2 laser tested in the study, and DUSA Pharmaceuticals manufactures the blue light PDT device and the ALA product. Dr. Alexiades-Armenakas reported receiving clinical research grants from Deka, DUSA Pharmaceuticals, Alma, and Syneron.
AT LASER 2015
Key clinical point: Performing fractional CO2 laser resurfacing before aminolevulinic acid blue light photodynamic therapy cleared significantly more actinic keratoses than ALA-PDT alone.
Major finding: For the 30-minute incubation group, laser plus ALA-PDT cleared 94% of actinic keratoses, compared with 82% for ALA-PDT alone.
Data source: A randomized, single-blinded, split-face study of 20 patients.
Disclosures: Deka made the fractional CO2 laser tested in the study and DUSA Pharmaceuticals made the blue light PDT device and the ALA product. Dr. Alexiades-Armenakas reported receiving clinical research grants from Deka, DUSA Pharmaceuticals, Alma, and Syneron.
Skin patch hastened Q-switched laser tattoo removal
KISSIMMEE, FLA. – A perfluorodecalin-infused patch lessened reactive whitening and accelerated clearing in 65% of tattoos treated with 755-nm Q-switched lasers in a single-center, split-tattoo study.
“Most subjects showed obvious accelerated clearing on the patch side of the tattoo, and preferred the patch-treated side compared with the control side,” Dr. Brian Biesman said at the annual meeting of the American Society for Laser Medicine and Surgery. “Despite the small number of patients, the study was powered sufficiently to address our qualitative question: Was there an enhanced rate of clearance relative to control when the PFD [perfluorodecalin] patch is used?”
Passing a laser across a tattoo triggers an immediate whitening reaction that blocks light and takes about 20 minutes to resolve, said Dr. Biesman, who is clinical assistant professor at Vanderbilt University and director of the Nashville Centre for Laser and Facial Surgery. Removing tattoos with Q-switched lasers can take months to years because it involves making a single pass each month. A past study showed that topical PFD resolved whitening in seconds, allowing operators to make multiple passes in 5 minutes. but the patch seems to have additional dermoprotective properties that enable patients to tolerate higher fluence as well as multiple passes, Dr. Biesman said.
The study included 17 patients with Fitzpatrick skin types I through III who had previously untreated, dark blue or black ink tattoos measuring less than 100 cm2 in area. Patients with suntans, blood-borne diseases, oral retinoid exposure in the past 12 months, or lidocaine allergies were excluded, he said. All patients underwent monthly treatments with a conventional Q-switched alexandrite laser after pretreatment with topical lidocaine. The control (uncovered) half of each tattoo received a single laser pass, while the half covered by the patch received the maximum tolerable fluence and number of passes in 5 minutes.
The PFD patch was associated with substantially faster clearance, compared with the control, in 11 of 17 patients (65%). Patients tolerated 1.5 to 1.8 times greater fluence and about four passes per session on the patch side. They also developed no serious or unexpected side effects, said Dr. Biesman.
Responses to the patch varied widely, however, ranging from no visible improvement after many sessions to more than 80% greater clearance after two sessions, compared with the control side. Larger studies would be needed to examine predictors of success, he added, noting that the composition of tattoo ink affects response, and that clinicians rarely, if ever, know which inks were used.
It also remains unclear whether faster clearing was the result of multiple passes or higher fluence. The PFD patch reduces scatter in the dermis and epidermis, which could facilitate use of higher fluence, he said.
On April 20, the Food and Drug Administration cleared the PFD patch for use as an accessory to tattoo removal with a 755-nm Q-switched alexandrite laser in Fitzpatrick skin types I through III under the agency’s 510(k) medical device regulatory process, according to Dr. Biesman.
ON Light Sciences manufactures the patch. Dr. Biesman reported receiving grant support and holding ownership interest in ON Light Sciences and also reported financial relationships with a number of other pharmaceutical and device companies.
KISSIMMEE, FLA. – A perfluorodecalin-infused patch lessened reactive whitening and accelerated clearing in 65% of tattoos treated with 755-nm Q-switched lasers in a single-center, split-tattoo study.
“Most subjects showed obvious accelerated clearing on the patch side of the tattoo, and preferred the patch-treated side compared with the control side,” Dr. Brian Biesman said at the annual meeting of the American Society for Laser Medicine and Surgery. “Despite the small number of patients, the study was powered sufficiently to address our qualitative question: Was there an enhanced rate of clearance relative to control when the PFD [perfluorodecalin] patch is used?”
Passing a laser across a tattoo triggers an immediate whitening reaction that blocks light and takes about 20 minutes to resolve, said Dr. Biesman, who is clinical assistant professor at Vanderbilt University and director of the Nashville Centre for Laser and Facial Surgery. Removing tattoos with Q-switched lasers can take months to years because it involves making a single pass each month. A past study showed that topical PFD resolved whitening in seconds, allowing operators to make multiple passes in 5 minutes. but the patch seems to have additional dermoprotective properties that enable patients to tolerate higher fluence as well as multiple passes, Dr. Biesman said.
The study included 17 patients with Fitzpatrick skin types I through III who had previously untreated, dark blue or black ink tattoos measuring less than 100 cm2 in area. Patients with suntans, blood-borne diseases, oral retinoid exposure in the past 12 months, or lidocaine allergies were excluded, he said. All patients underwent monthly treatments with a conventional Q-switched alexandrite laser after pretreatment with topical lidocaine. The control (uncovered) half of each tattoo received a single laser pass, while the half covered by the patch received the maximum tolerable fluence and number of passes in 5 minutes.
The PFD patch was associated with substantially faster clearance, compared with the control, in 11 of 17 patients (65%). Patients tolerated 1.5 to 1.8 times greater fluence and about four passes per session on the patch side. They also developed no serious or unexpected side effects, said Dr. Biesman.
Responses to the patch varied widely, however, ranging from no visible improvement after many sessions to more than 80% greater clearance after two sessions, compared with the control side. Larger studies would be needed to examine predictors of success, he added, noting that the composition of tattoo ink affects response, and that clinicians rarely, if ever, know which inks were used.
It also remains unclear whether faster clearing was the result of multiple passes or higher fluence. The PFD patch reduces scatter in the dermis and epidermis, which could facilitate use of higher fluence, he said.
On April 20, the Food and Drug Administration cleared the PFD patch for use as an accessory to tattoo removal with a 755-nm Q-switched alexandrite laser in Fitzpatrick skin types I through III under the agency’s 510(k) medical device regulatory process, according to Dr. Biesman.
ON Light Sciences manufactures the patch. Dr. Biesman reported receiving grant support and holding ownership interest in ON Light Sciences and also reported financial relationships with a number of other pharmaceutical and device companies.
KISSIMMEE, FLA. – A perfluorodecalin-infused patch lessened reactive whitening and accelerated clearing in 65% of tattoos treated with 755-nm Q-switched lasers in a single-center, split-tattoo study.
“Most subjects showed obvious accelerated clearing on the patch side of the tattoo, and preferred the patch-treated side compared with the control side,” Dr. Brian Biesman said at the annual meeting of the American Society for Laser Medicine and Surgery. “Despite the small number of patients, the study was powered sufficiently to address our qualitative question: Was there an enhanced rate of clearance relative to control when the PFD [perfluorodecalin] patch is used?”
Passing a laser across a tattoo triggers an immediate whitening reaction that blocks light and takes about 20 minutes to resolve, said Dr. Biesman, who is clinical assistant professor at Vanderbilt University and director of the Nashville Centre for Laser and Facial Surgery. Removing tattoos with Q-switched lasers can take months to years because it involves making a single pass each month. A past study showed that topical PFD resolved whitening in seconds, allowing operators to make multiple passes in 5 minutes. but the patch seems to have additional dermoprotective properties that enable patients to tolerate higher fluence as well as multiple passes, Dr. Biesman said.
The study included 17 patients with Fitzpatrick skin types I through III who had previously untreated, dark blue or black ink tattoos measuring less than 100 cm2 in area. Patients with suntans, blood-borne diseases, oral retinoid exposure in the past 12 months, or lidocaine allergies were excluded, he said. All patients underwent monthly treatments with a conventional Q-switched alexandrite laser after pretreatment with topical lidocaine. The control (uncovered) half of each tattoo received a single laser pass, while the half covered by the patch received the maximum tolerable fluence and number of passes in 5 minutes.
The PFD patch was associated with substantially faster clearance, compared with the control, in 11 of 17 patients (65%). Patients tolerated 1.5 to 1.8 times greater fluence and about four passes per session on the patch side. They also developed no serious or unexpected side effects, said Dr. Biesman.
Responses to the patch varied widely, however, ranging from no visible improvement after many sessions to more than 80% greater clearance after two sessions, compared with the control side. Larger studies would be needed to examine predictors of success, he added, noting that the composition of tattoo ink affects response, and that clinicians rarely, if ever, know which inks were used.
It also remains unclear whether faster clearing was the result of multiple passes or higher fluence. The PFD patch reduces scatter in the dermis and epidermis, which could facilitate use of higher fluence, he said.
On April 20, the Food and Drug Administration cleared the PFD patch for use as an accessory to tattoo removal with a 755-nm Q-switched alexandrite laser in Fitzpatrick skin types I through III under the agency’s 510(k) medical device regulatory process, according to Dr. Biesman.
ON Light Sciences manufactures the patch. Dr. Biesman reported receiving grant support and holding ownership interest in ON Light Sciences and also reported financial relationships with a number of other pharmaceutical and device companies.
AT LASER 2015
Key clinical point: A perfluorodecalin-infused skin patch reduced whitening and facilitated clearance of tattoos treated with 755-nm Q-switched lasers.
Major finding: Nearly two-thirds (11 of 17) of tattoos cleared more rapidly with the PFD patch than without.
Data source: Split-tattoo study of 17 patients with black or blue ink tattoos.
Disclosures: ON Light Sciences makes the patch tested in the study. Dr. Biesman disclosed grant support and ownership interest in ON Light Sciences and financial relationships with a number of other pharmaceutical and device companies.
OTC phototherapy devices can enhance outcomes
MIAMI BEACH – Over-the-counter phototherapy devices for anti-aging and acne treatment can improve office-based procedure results and enhance clinical practice, according to Dr. Z. Paul Lorenc.
Light-emitting diode (LED) devices now available for home use, such as the illuMask (La Lumiere) anti-aging and anti-acne masks, are safe and effective and can be used along with office-based treatments to speed healing and boost results, Dr. Lorenc said at the South Beach Symposium.
The illuMask anti-aging mask uses a combination of 630 nm wavelength red light and 855 nm wavelength infrared light, and the two work synergistically to improve signs of aging, such as wrinkles, photoaging, and hyperpigmentation.
Dr. Lorenc, chief medical and scientific officer for La Lumiere, worked on the pivotal trials for the devices, and said that in a study of 30 patients aged 14 -40 years, 15 minutes of daily mask use was associated with 82% and 94% improvement in 17 clinical skin attributes at weeks 4 and 8, respectively. Improvement in 20 sham-treated subjects was 24% at both 4 and 8 weeks.
Subject self-assessment showed significant improvement in 93% and 100% of 15 clinical attributes at weeks 4 and 8, respectively, in the treated subjects, compared with 33% and 40% in the sham-treated subjects, he said.
The illuMask anti-acne mask uses 630 nm red light and 440 nm blue light, which also work synergistically.
In addition, 15 minutes of daily use was associated with a 71% average decrease in the number of noninflammatory lesions and an 83% decrease in the number of inflammatory lesions at 8 weeks. The median decrease in the 5-point Acne Severity Assessment Scale score was 2 points – from 4 (moderate) to 2 (almost clear).
Dr. Lorenc, who practices plastic surgery in New York, said he does not treat acne very often, but he does encounter patients with adult acne who benefit from the acne mask.
He said he uses the anti-aging mask often.
“I’m using it on every single resurfacing patient,” he said, explaining that red light is anti-inflammatory in nature, and increases production of collagen and elastin.
Patients undergoing partial or total resurfacing, those undergoing other procedures such as microneedling, and even those undergoing a face-lift, can benefit from use of the anti-aging mask beginning the day after the procedure, he said, adding that it decreases inflammatory processes, and can provide some added anti-aging effects that may not be addressed by the initial procedures (dyschromia in face-lift patients, for example).
The masks are simple to use, don’t take much time, can be used with existing treatment regimens, are appropriate for all Fitzpatrick skin types, and are associated with a high compliance rate – even among teens, Dr. Lorenc said.
While some physicians have expressed concern that the OTC availability of phototherapy devices could hurt clinical practice, his experience has been quite the opposite.
The boost that the masks give both to the recovery time and efficacy of office-based procedures makes for happier patients who come back – and send their friends, he said.
“I have incorporated [the masks] into my practice, and what it has done is the opposite of what you would think intuitively; it has expanded my practice – both surgical and nonsurgical, and we think that this is a very good adjunct therapy to both acne and anti-aging treatment,” he said.
Dr. Lorenc had no additional disclosures.
MIAMI BEACH – Over-the-counter phototherapy devices for anti-aging and acne treatment can improve office-based procedure results and enhance clinical practice, according to Dr. Z. Paul Lorenc.
Light-emitting diode (LED) devices now available for home use, such as the illuMask (La Lumiere) anti-aging and anti-acne masks, are safe and effective and can be used along with office-based treatments to speed healing and boost results, Dr. Lorenc said at the South Beach Symposium.
The illuMask anti-aging mask uses a combination of 630 nm wavelength red light and 855 nm wavelength infrared light, and the two work synergistically to improve signs of aging, such as wrinkles, photoaging, and hyperpigmentation.
Dr. Lorenc, chief medical and scientific officer for La Lumiere, worked on the pivotal trials for the devices, and said that in a study of 30 patients aged 14 -40 years, 15 minutes of daily mask use was associated with 82% and 94% improvement in 17 clinical skin attributes at weeks 4 and 8, respectively. Improvement in 20 sham-treated subjects was 24% at both 4 and 8 weeks.
Subject self-assessment showed significant improvement in 93% and 100% of 15 clinical attributes at weeks 4 and 8, respectively, in the treated subjects, compared with 33% and 40% in the sham-treated subjects, he said.
The illuMask anti-acne mask uses 630 nm red light and 440 nm blue light, which also work synergistically.
In addition, 15 minutes of daily use was associated with a 71% average decrease in the number of noninflammatory lesions and an 83% decrease in the number of inflammatory lesions at 8 weeks. The median decrease in the 5-point Acne Severity Assessment Scale score was 2 points – from 4 (moderate) to 2 (almost clear).
Dr. Lorenc, who practices plastic surgery in New York, said he does not treat acne very often, but he does encounter patients with adult acne who benefit from the acne mask.
He said he uses the anti-aging mask often.
“I’m using it on every single resurfacing patient,” he said, explaining that red light is anti-inflammatory in nature, and increases production of collagen and elastin.
Patients undergoing partial or total resurfacing, those undergoing other procedures such as microneedling, and even those undergoing a face-lift, can benefit from use of the anti-aging mask beginning the day after the procedure, he said, adding that it decreases inflammatory processes, and can provide some added anti-aging effects that may not be addressed by the initial procedures (dyschromia in face-lift patients, for example).
The masks are simple to use, don’t take much time, can be used with existing treatment regimens, are appropriate for all Fitzpatrick skin types, and are associated with a high compliance rate – even among teens, Dr. Lorenc said.
While some physicians have expressed concern that the OTC availability of phototherapy devices could hurt clinical practice, his experience has been quite the opposite.
The boost that the masks give both to the recovery time and efficacy of office-based procedures makes for happier patients who come back – and send their friends, he said.
“I have incorporated [the masks] into my practice, and what it has done is the opposite of what you would think intuitively; it has expanded my practice – both surgical and nonsurgical, and we think that this is a very good adjunct therapy to both acne and anti-aging treatment,” he said.
Dr. Lorenc had no additional disclosures.
MIAMI BEACH – Over-the-counter phototherapy devices for anti-aging and acne treatment can improve office-based procedure results and enhance clinical practice, according to Dr. Z. Paul Lorenc.
Light-emitting diode (LED) devices now available for home use, such as the illuMask (La Lumiere) anti-aging and anti-acne masks, are safe and effective and can be used along with office-based treatments to speed healing and boost results, Dr. Lorenc said at the South Beach Symposium.
The illuMask anti-aging mask uses a combination of 630 nm wavelength red light and 855 nm wavelength infrared light, and the two work synergistically to improve signs of aging, such as wrinkles, photoaging, and hyperpigmentation.
Dr. Lorenc, chief medical and scientific officer for La Lumiere, worked on the pivotal trials for the devices, and said that in a study of 30 patients aged 14 -40 years, 15 minutes of daily mask use was associated with 82% and 94% improvement in 17 clinical skin attributes at weeks 4 and 8, respectively. Improvement in 20 sham-treated subjects was 24% at both 4 and 8 weeks.
Subject self-assessment showed significant improvement in 93% and 100% of 15 clinical attributes at weeks 4 and 8, respectively, in the treated subjects, compared with 33% and 40% in the sham-treated subjects, he said.
The illuMask anti-acne mask uses 630 nm red light and 440 nm blue light, which also work synergistically.
In addition, 15 minutes of daily use was associated with a 71% average decrease in the number of noninflammatory lesions and an 83% decrease in the number of inflammatory lesions at 8 weeks. The median decrease in the 5-point Acne Severity Assessment Scale score was 2 points – from 4 (moderate) to 2 (almost clear).
Dr. Lorenc, who practices plastic surgery in New York, said he does not treat acne very often, but he does encounter patients with adult acne who benefit from the acne mask.
He said he uses the anti-aging mask often.
“I’m using it on every single resurfacing patient,” he said, explaining that red light is anti-inflammatory in nature, and increases production of collagen and elastin.
Patients undergoing partial or total resurfacing, those undergoing other procedures such as microneedling, and even those undergoing a face-lift, can benefit from use of the anti-aging mask beginning the day after the procedure, he said, adding that it decreases inflammatory processes, and can provide some added anti-aging effects that may not be addressed by the initial procedures (dyschromia in face-lift patients, for example).
The masks are simple to use, don’t take much time, can be used with existing treatment regimens, are appropriate for all Fitzpatrick skin types, and are associated with a high compliance rate – even among teens, Dr. Lorenc said.
While some physicians have expressed concern that the OTC availability of phototherapy devices could hurt clinical practice, his experience has been quite the opposite.
The boost that the masks give both to the recovery time and efficacy of office-based procedures makes for happier patients who come back – and send their friends, he said.
“I have incorporated [the masks] into my practice, and what it has done is the opposite of what you would think intuitively; it has expanded my practice – both surgical and nonsurgical, and we think that this is a very good adjunct therapy to both acne and anti-aging treatment,” he said.
Dr. Lorenc had no additional disclosures.
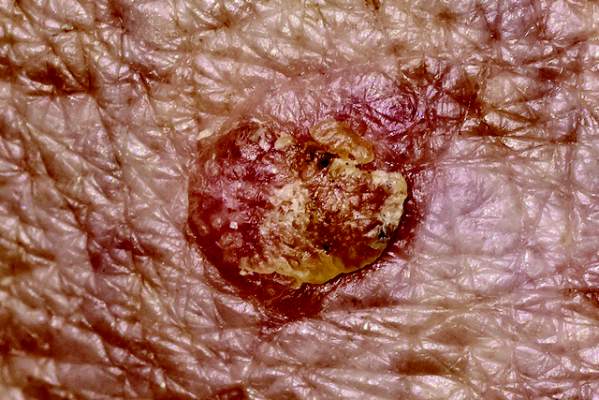
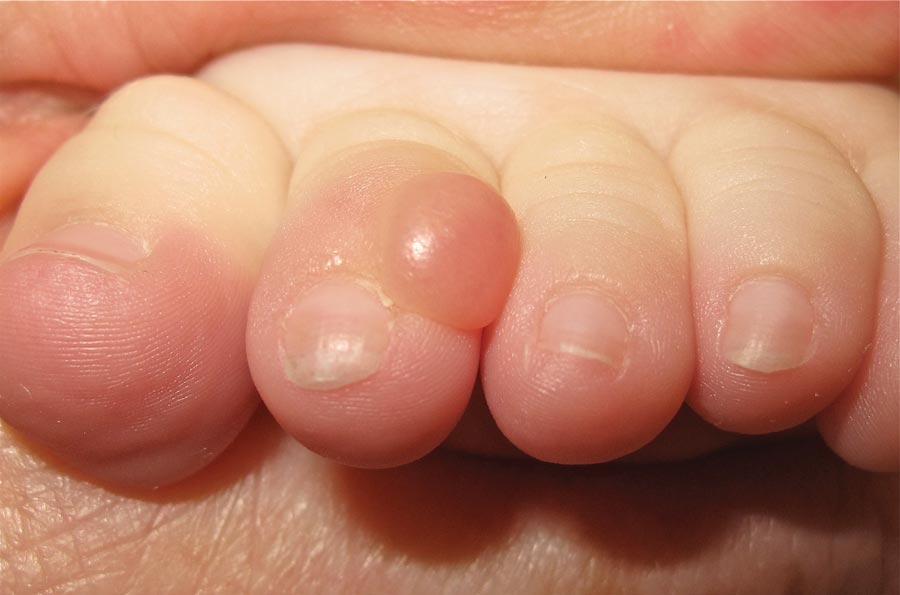
Nodule on the Second Toe in an Infant
The Diagnosis: Infantile Digital Fibromatosis
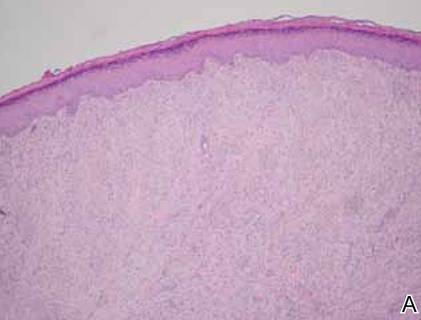
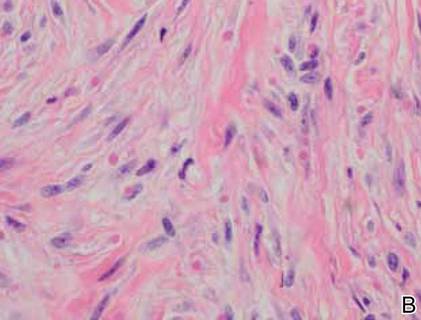
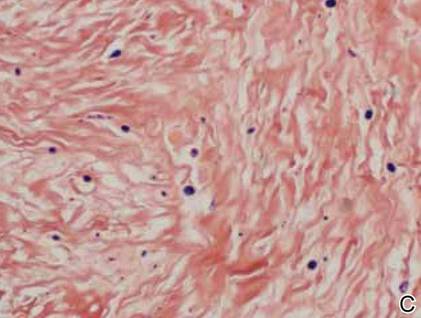
On examination, the patient appeared well developed, well nourished, and had a 1×0.5-cm, flesh-colored, firm, nontender nodule on the dorsolateral aspect of the left second toe. After excision by a pediatric surgeon, the specimen was submitted for histopathologic examination. Dense bands of collagen with spindled myofibroblasts containing characteristic eosinophilic cytoplasmic inclusion bodies staining with phosphotungstic acid hematoxylin confirmed the diagnosis of infantile digital fibromatosis (Figure). Postoperatively the patient did well with normal healing and no complications. After 4 months, a recurrence was noted and the parents were considering reexcision.
|
Infantile digital fibromatosis is a rare, benign, often spontaneously regressing, fibrous tissue tumor of infancy and childhood.1 The prevalence of this tumor is unknown. It can be present at birth or more commonly appears in the first year of life. The lesions present as 1- to 2-cm, firm, flesh-colored nodules that initially grow slowly but have the potential for rapid growth in subsequent months. They occur preferentially on the extensor aspects of the digits, typically sparing the thumb and great toe.1 The clinical differential diagnosis includes keloids or hypertrophic scars, granuloma annulare, sarcoidosis, acral fibrokeratomas, periungual fibromas, supernumerary digits, pachydermodactyly, juvenile aponeurotic fibroma, and terminal osseous dysplasia and pigmentary defects.2
The histology of infantile digital fibromatosis is distinctive. Spindled myofibroblasts that contain round or ovoid, eosinophilic, cytoplasmic inclusion bodies composed of an accumulation of actin and vimentin filaments are characteristic.1 Inclusions are typically juxtanuclear and may indent the adjacent nucleus. The inclusion bodies stain red with Masson trichrome stain and purple with phosphotungstic acid hematoxylin stain.1 The histopathologic differential diagnosis includes scar, angiofibroma, dermatofibroma, neurofibroma, and angiofibromatous verruca vulgaris.
Although many treatments exist for infantile digital fibromatosis, optimal therapy is not standardized. Most lesions spontaneously regress, but func-tional disability with deforming contractures can occur if untreated. Topical therapy with imiquimod cream 5% and diflucortolone valerate cream have been reported to produce no effect on tumor size.3 Intralesional 5-fluorouracil was successful in treating a patient after 5 monthly injections.4 Intralesional triamcinolone 10 mg/cc injections were shown to be a well-tolerated and successful treatment in a case series of 7 patients.5 The most utilized intervention appears to be standard surgery, with a few patients treated with Mohs micrographic surgery.6,7
Treatment with surgical excision often results in recurrence, with studies showing a 50% to 75% recurrence rate.8,9 Our case is not atypical and illustrates this phenomenon. Other reported complications of surgical management include hypertrophic scarring and reduced distal interphalangeal joint mobility.5 Unless infantile digital fibromatosis causes mobility dysfunction or related disabilities, observation with regular follow-up should be considered, as lesions can spontaneously regress.
1. Heymann WR. Infantile digital fibromatosis. J Am Acad Dermatol. 2008;59:122-123.
2. Niamba P, Léauté-Labrèze C, Boralevi F, et al. Further documentation of spontaneous regression of infantile digital fibromatosis. Pediatr Dermatol. 2007;24:280-284.
3. Failla V, Wauters O, Nikkels-Tassoudji N, et al. Congenital infantile digital fibromatosis: a case report and review of the literature. Rare Tumors. 2009;1:e47.
4. Oh CK, Son HS, Kwon YW, et al. Intralesional fluorouracil injection in infantile digital fibromatosis. Arch Dermatol. 2005;141:549-550
5. Holmes WJ, Mishra A, McArthur P. Intra-lesional steroid for the management of symptomatic infantile digital fibromatosis. J Plast Reconstr Aesthet Surg. 2011;64:632-637.
6. Campbell LB, Petrick MG. Mohs micrographic surgery for a problematic infantile digital fibroma. Dermatol Surg. 2007;33:385-387.
7. Albertini JG, Welsch MJ, Conger LA, et al. Infantile digital fibroma treated with Mohs micrographic surgery. Dermatol Surg. 2002;28:959-961.
8. Kang SK, Chang SE, Choi JH, et al. A case of congenital infantile digital fibromatosis. Pediatr Dermatol. 2002;19:462-463.
9. Rimareix F, Bardot J, Andrac L, et al. Infantile digital fibroma—report on eleven cases. Eur J Pediatr Surg. 1997;7:345-348.
The Diagnosis: Infantile Digital Fibromatosis
On examination, the patient appeared well developed, well nourished, and had a 1×0.5-cm, flesh-colored, firm, nontender nodule on the dorsolateral aspect of the left second toe. After excision by a pediatric surgeon, the specimen was submitted for histopathologic examination. Dense bands of collagen with spindled myofibroblasts containing characteristic eosinophilic cytoplasmic inclusion bodies staining with phosphotungstic acid hematoxylin confirmed the diagnosis of infantile digital fibromatosis (Figure). Postoperatively the patient did well with normal healing and no complications. After 4 months, a recurrence was noted and the parents were considering reexcision.
|
Infantile digital fibromatosis is a rare, benign, often spontaneously regressing, fibrous tissue tumor of infancy and childhood.1 The prevalence of this tumor is unknown. It can be present at birth or more commonly appears in the first year of life. The lesions present as 1- to 2-cm, firm, flesh-colored nodules that initially grow slowly but have the potential for rapid growth in subsequent months. They occur preferentially on the extensor aspects of the digits, typically sparing the thumb and great toe.1 The clinical differential diagnosis includes keloids or hypertrophic scars, granuloma annulare, sarcoidosis, acral fibrokeratomas, periungual fibromas, supernumerary digits, pachydermodactyly, juvenile aponeurotic fibroma, and terminal osseous dysplasia and pigmentary defects.2
The histology of infantile digital fibromatosis is distinctive. Spindled myofibroblasts that contain round or ovoid, eosinophilic, cytoplasmic inclusion bodies composed of an accumulation of actin and vimentin filaments are characteristic.1 Inclusions are typically juxtanuclear and may indent the adjacent nucleus. The inclusion bodies stain red with Masson trichrome stain and purple with phosphotungstic acid hematoxylin stain.1 The histopathologic differential diagnosis includes scar, angiofibroma, dermatofibroma, neurofibroma, and angiofibromatous verruca vulgaris.
Although many treatments exist for infantile digital fibromatosis, optimal therapy is not standardized. Most lesions spontaneously regress, but func-tional disability with deforming contractures can occur if untreated. Topical therapy with imiquimod cream 5% and diflucortolone valerate cream have been reported to produce no effect on tumor size.3 Intralesional 5-fluorouracil was successful in treating a patient after 5 monthly injections.4 Intralesional triamcinolone 10 mg/cc injections were shown to be a well-tolerated and successful treatment in a case series of 7 patients.5 The most utilized intervention appears to be standard surgery, with a few patients treated with Mohs micrographic surgery.6,7
Treatment with surgical excision often results in recurrence, with studies showing a 50% to 75% recurrence rate.8,9 Our case is not atypical and illustrates this phenomenon. Other reported complications of surgical management include hypertrophic scarring and reduced distal interphalangeal joint mobility.5 Unless infantile digital fibromatosis causes mobility dysfunction or related disabilities, observation with regular follow-up should be considered, as lesions can spontaneously regress.
The Diagnosis: Infantile Digital Fibromatosis
On examination, the patient appeared well developed, well nourished, and had a 1×0.5-cm, flesh-colored, firm, nontender nodule on the dorsolateral aspect of the left second toe. After excision by a pediatric surgeon, the specimen was submitted for histopathologic examination. Dense bands of collagen with spindled myofibroblasts containing characteristic eosinophilic cytoplasmic inclusion bodies staining with phosphotungstic acid hematoxylin confirmed the diagnosis of infantile digital fibromatosis (Figure). Postoperatively the patient did well with normal healing and no complications. After 4 months, a recurrence was noted and the parents were considering reexcision.
|
Infantile digital fibromatosis is a rare, benign, often spontaneously regressing, fibrous tissue tumor of infancy and childhood.1 The prevalence of this tumor is unknown. It can be present at birth or more commonly appears in the first year of life. The lesions present as 1- to 2-cm, firm, flesh-colored nodules that initially grow slowly but have the potential for rapid growth in subsequent months. They occur preferentially on the extensor aspects of the digits, typically sparing the thumb and great toe.1 The clinical differential diagnosis includes keloids or hypertrophic scars, granuloma annulare, sarcoidosis, acral fibrokeratomas, periungual fibromas, supernumerary digits, pachydermodactyly, juvenile aponeurotic fibroma, and terminal osseous dysplasia and pigmentary defects.2
The histology of infantile digital fibromatosis is distinctive. Spindled myofibroblasts that contain round or ovoid, eosinophilic, cytoplasmic inclusion bodies composed of an accumulation of actin and vimentin filaments are characteristic.1 Inclusions are typically juxtanuclear and may indent the adjacent nucleus. The inclusion bodies stain red with Masson trichrome stain and purple with phosphotungstic acid hematoxylin stain.1 The histopathologic differential diagnosis includes scar, angiofibroma, dermatofibroma, neurofibroma, and angiofibromatous verruca vulgaris.
Although many treatments exist for infantile digital fibromatosis, optimal therapy is not standardized. Most lesions spontaneously regress, but func-tional disability with deforming contractures can occur if untreated. Topical therapy with imiquimod cream 5% and diflucortolone valerate cream have been reported to produce no effect on tumor size.3 Intralesional 5-fluorouracil was successful in treating a patient after 5 monthly injections.4 Intralesional triamcinolone 10 mg/cc injections were shown to be a well-tolerated and successful treatment in a case series of 7 patients.5 The most utilized intervention appears to be standard surgery, with a few patients treated with Mohs micrographic surgery.6,7
Treatment with surgical excision often results in recurrence, with studies showing a 50% to 75% recurrence rate.8,9 Our case is not atypical and illustrates this phenomenon. Other reported complications of surgical management include hypertrophic scarring and reduced distal interphalangeal joint mobility.5 Unless infantile digital fibromatosis causes mobility dysfunction or related disabilities, observation with regular follow-up should be considered, as lesions can spontaneously regress.
1. Heymann WR. Infantile digital fibromatosis. J Am Acad Dermatol. 2008;59:122-123.
2. Niamba P, Léauté-Labrèze C, Boralevi F, et al. Further documentation of spontaneous regression of infantile digital fibromatosis. Pediatr Dermatol. 2007;24:280-284.
3. Failla V, Wauters O, Nikkels-Tassoudji N, et al. Congenital infantile digital fibromatosis: a case report and review of the literature. Rare Tumors. 2009;1:e47.
4. Oh CK, Son HS, Kwon YW, et al. Intralesional fluorouracil injection in infantile digital fibromatosis. Arch Dermatol. 2005;141:549-550
5. Holmes WJ, Mishra A, McArthur P. Intra-lesional steroid for the management of symptomatic infantile digital fibromatosis. J Plast Reconstr Aesthet Surg. 2011;64:632-637.
6. Campbell LB, Petrick MG. Mohs micrographic surgery for a problematic infantile digital fibroma. Dermatol Surg. 2007;33:385-387.
7. Albertini JG, Welsch MJ, Conger LA, et al. Infantile digital fibroma treated with Mohs micrographic surgery. Dermatol Surg. 2002;28:959-961.
8. Kang SK, Chang SE, Choi JH, et al. A case of congenital infantile digital fibromatosis. Pediatr Dermatol. 2002;19:462-463.
9. Rimareix F, Bardot J, Andrac L, et al. Infantile digital fibroma—report on eleven cases. Eur J Pediatr Surg. 1997;7:345-348.
1. Heymann WR. Infantile digital fibromatosis. J Am Acad Dermatol. 2008;59:122-123.
2. Niamba P, Léauté-Labrèze C, Boralevi F, et al. Further documentation of spontaneous regression of infantile digital fibromatosis. Pediatr Dermatol. 2007;24:280-284.
3. Failla V, Wauters O, Nikkels-Tassoudji N, et al. Congenital infantile digital fibromatosis: a case report and review of the literature. Rare Tumors. 2009;1:e47.
4. Oh CK, Son HS, Kwon YW, et al. Intralesional fluorouracil injection in infantile digital fibromatosis. Arch Dermatol. 2005;141:549-550
5. Holmes WJ, Mishra A, McArthur P. Intra-lesional steroid for the management of symptomatic infantile digital fibromatosis. J Plast Reconstr Aesthet Surg. 2011;64:632-637.
6. Campbell LB, Petrick MG. Mohs micrographic surgery for a problematic infantile digital fibroma. Dermatol Surg. 2007;33:385-387.
7. Albertini JG, Welsch MJ, Conger LA, et al. Infantile digital fibroma treated with Mohs micrographic surgery. Dermatol Surg. 2002;28:959-961.
8. Kang SK, Chang SE, Choi JH, et al. A case of congenital infantile digital fibromatosis. Pediatr Dermatol. 2002;19:462-463.
9. Rimareix F, Bardot J, Andrac L, et al. Infantile digital fibroma—report on eleven cases. Eur J Pediatr Surg. 1997;7:345-348.
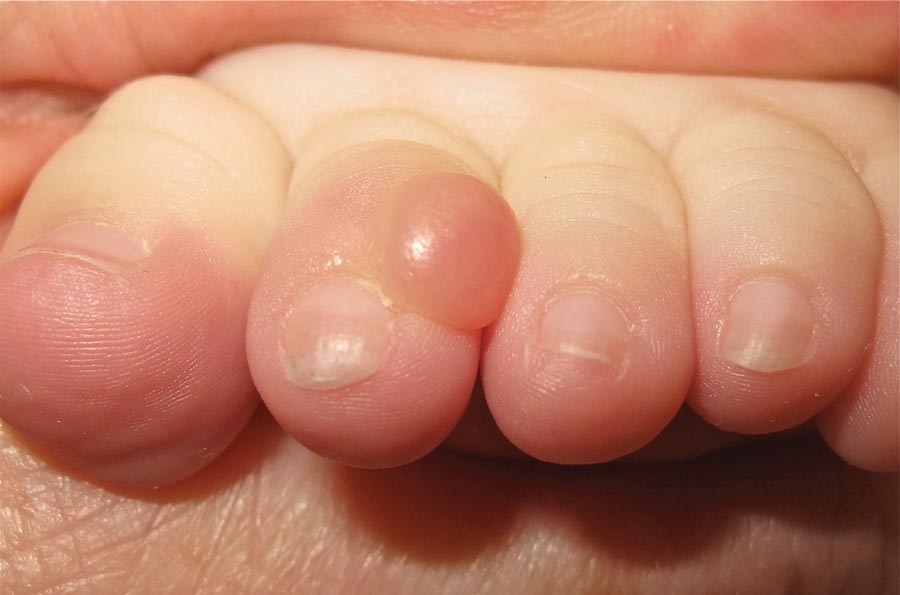
A 6-month-old male infant presented with a 1×0.5-cm, flesh-colored nodule on the dorsolateral aspect of the left second toe. The persistent, slowly enlarging, painless lesion was first noticed at 3 months of age and did not cause functional impairment. There was no preceding trauma and the patient’s medical history was otherwise noncontributory.
FDA moves to alert physicians, public to counterfeit Botox
Counterfeit Botox has been found in the United States and could be in offices and clinics across the country, the Food and Drug Administration announced on April 17.
The counterfeit Botox “may have been sold” to medical clinics and physicians’ offices; the source is an unlicensed supplier that is not authorized to ship or distribute drug products in this country. “The counterfeit products are considered unsafe and should not be used. FDA cannot confirm that the manufacture, quality, storage, and handling of these suspect products follow U.S. standards,” according to the FDA statement, which notes that the agency is not aware of any adverse events resulting from the use of the counterfeit product.
“Currently, there is no indication that Allergan’s FDA-approved version is at risk, and the genuine product should be considered safe and effective for its intended and approved use,” the FDA statement said.
Features that can help identify the counterfeit product include a missing lot number on the vial. And instead of “OnabotulinumtoxinA” on the vial and outer carton (which is printed on the label and outer carton of the FDA-approved Botox manufactured by Allergan), the counterfeit product has “Botulinum Toxin Type A” on the outer carton and vial.
The counterfeit product also does not have anything written after the “LOT: MFG: EXP:” section of the outer carton.
The Allergan website also has information on how to identify counterfeit Botox, which includes a list of authorized distributors. In addition, a list of the state agencies that license wholesale prescription drug distributors is available on the FDA’s website.
The FDA is asking anybody who is aware of any Botox product thought to be counterfeit to contact the agency’s Office of Criminal Investigations (OCI) at 800-551-3989, www.accessdata.fda.gov/scripts/email/oc/oci/contact.cfm, or by e-mail at DrugSupplyChainIntegrity@fda.hhs.gov.
Adverse events thought to be related to counterfeit Botox should be reported to the FDA’s MedWatch program at800-332-1088, or https://www.accessdata.fda.gov/scripts/medwatch/.
Counterfeit Botox has been found in the United States and could be in offices and clinics across the country, the Food and Drug Administration announced on April 17.
The counterfeit Botox “may have been sold” to medical clinics and physicians’ offices; the source is an unlicensed supplier that is not authorized to ship or distribute drug products in this country. “The counterfeit products are considered unsafe and should not be used. FDA cannot confirm that the manufacture, quality, storage, and handling of these suspect products follow U.S. standards,” according to the FDA statement, which notes that the agency is not aware of any adverse events resulting from the use of the counterfeit product.
“Currently, there is no indication that Allergan’s FDA-approved version is at risk, and the genuine product should be considered safe and effective for its intended and approved use,” the FDA statement said.
Features that can help identify the counterfeit product include a missing lot number on the vial. And instead of “OnabotulinumtoxinA” on the vial and outer carton (which is printed on the label and outer carton of the FDA-approved Botox manufactured by Allergan), the counterfeit product has “Botulinum Toxin Type A” on the outer carton and vial.
The counterfeit product also does not have anything written after the “LOT: MFG: EXP:” section of the outer carton.
The Allergan website also has information on how to identify counterfeit Botox, which includes a list of authorized distributors. In addition, a list of the state agencies that license wholesale prescription drug distributors is available on the FDA’s website.
The FDA is asking anybody who is aware of any Botox product thought to be counterfeit to contact the agency’s Office of Criminal Investigations (OCI) at 800-551-3989, www.accessdata.fda.gov/scripts/email/oc/oci/contact.cfm, or by e-mail at DrugSupplyChainIntegrity@fda.hhs.gov.
Adverse events thought to be related to counterfeit Botox should be reported to the FDA’s MedWatch program at800-332-1088, or https://www.accessdata.fda.gov/scripts/medwatch/.
Counterfeit Botox has been found in the United States and could be in offices and clinics across the country, the Food and Drug Administration announced on April 17.
The counterfeit Botox “may have been sold” to medical clinics and physicians’ offices; the source is an unlicensed supplier that is not authorized to ship or distribute drug products in this country. “The counterfeit products are considered unsafe and should not be used. FDA cannot confirm that the manufacture, quality, storage, and handling of these suspect products follow U.S. standards,” according to the FDA statement, which notes that the agency is not aware of any adverse events resulting from the use of the counterfeit product.
“Currently, there is no indication that Allergan’s FDA-approved version is at risk, and the genuine product should be considered safe and effective for its intended and approved use,” the FDA statement said.
Features that can help identify the counterfeit product include a missing lot number on the vial. And instead of “OnabotulinumtoxinA” on the vial and outer carton (which is printed on the label and outer carton of the FDA-approved Botox manufactured by Allergan), the counterfeit product has “Botulinum Toxin Type A” on the outer carton and vial.
The counterfeit product also does not have anything written after the “LOT: MFG: EXP:” section of the outer carton.
The Allergan website also has information on how to identify counterfeit Botox, which includes a list of authorized distributors. In addition, a list of the state agencies that license wholesale prescription drug distributors is available on the FDA’s website.
The FDA is asking anybody who is aware of any Botox product thought to be counterfeit to contact the agency’s Office of Criminal Investigations (OCI) at 800-551-3989, www.accessdata.fda.gov/scripts/email/oc/oci/contact.cfm, or by e-mail at DrugSupplyChainIntegrity@fda.hhs.gov.
Adverse events thought to be related to counterfeit Botox should be reported to the FDA’s MedWatch program at800-332-1088, or https://www.accessdata.fda.gov/scripts/medwatch/.
FROM THE FDA
Plantago major
For centuries, the leaves of Plantago major have been used in most regions of the world in traditional medical treatment of wounds and various diseases, including cutaneous conditions (J. Ethnopharmacol. 2000;71:1-21). P. major, also known as broadleaf plantain or greater plantain, is a member of the Plantaginaceae family, which is now widely dispersed throughout the world, though native to much of Europe as well as northern and central Asia. The Norwegian and Swedish name for the plant, groblad, means “healing leaves” (J. Ethnopharmacol. 2000;71:1-21). It was brought to the Americas by Europeans during the colonial period. Native Americans referred to it as the “white man’s footprint,” which inspired the genus name Plantago from the Latin planta (foot) (J. Ethnopharmacol. 2000;71:1-21).
Among the biologically active constituents of P. major are polysaccharides, lipids, caffeic acid derivatives, flavonoids (apigenin, luteolin, scutellarin, baicalein, nepetin, hispidulin, plantagoside), iridoid glycosides (aucubin, catalpol), terpenoids, and alkaloids (J. Ethnopharmacol. 2000;71:1-21; Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press). In an ethnopharmacologic and folk medicine survey study of 1,225 residents of the Atlantic coast of Colombia completed in 2011, Gómez-Estrada et al. found that P. major was one of the plants traditionally used to treat inflammation; it also was used to treat kidney pain and eye injuries (J. Ethnobiol. Ethnomed. 2011;7:27). P. major also is traditionally used as a mucilage and bulk laxative (Principles and Practice of Phytotherapy: Modern Herbal Medicine, 2013, Churchill Livingstone).
Extracts of the plant have been associated with myriad biologic activities, including wound healing, anti-inflammatory, antimicrobial, analgesic, antioxidant, immunomodulating, and antiulcerogenic action, which Samuelsen suggested may account for the use of the botanical in traditional medicine (J. Ethnopharmacol. 2000;71:1-21; Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press). In fact, the range of biologic properties attributed to P. major also includes astringent, anesthetic, antihelminthic, analeptic, antihistaminic, antirheumatic, antiviral, antitumor, antiulcer, diuretic, hypotensive, and expectorant activity (Exp. Biol. Med. [Maywood] 2012;237:1379-86). Though not the most popular botanical for this indication, P. major is among the plants used in the treatment of cutaneous leishmanial ulcers in Bahia, Brazil, where Leishmania brazilenesis is endemic (Rev. Soc. Bras. Med. Trop. 1996;29:229-32). Other dermatologic uses in traditional medicine include eczema, cuts, hemorrhoids, ulcerations, and wounds (Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press).
Wound healing
The use of P. major for wound healing dates back to the first century, as described by the Greek physician Dioscorides in “De Materia Medica” (J. Ethnopharmacol. 2000;71:1-21).
In 2011, Krasnov et al. developed an experimental model for characterizing proteins and showed that a newly discovered group of tissue-specific biogregulating proteins found previously in animal tissues was also present in P. major and responsible for the wound-healing activity associated with the plant (Prikl Biokhim. Mikrobiol. 2011;47:146-53).
The next year, Thomé et al. investigated and compared the wound-healing effects of P. major and Siparuna guianensis with a commercial product used in Brazil. Mice with cervical dorsal area wounds were treated with the botanical ingredients and the commercial product. Decreases in the wound area occurred earliest in mice treated with P. major, with complete closure (by day 15) seen only in this group. The investigators concluded that their findings support the traditional application of P. major, which shows potential as a viable wound-healing agent (Exp. Biol. Med. [Maywood] 2012;237:1379-86).
Conclusion
The numerous biologic properties of P. major are well established. In addition, use of the plant in traditional medicine for some cutaneous indications warrants consideration for modern therapeutic usage. Much more research is necessary, however, to elucidate the potential incorporation of this botanical into standard topical preparations for any of various skin conditions.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
For centuries, the leaves of Plantago major have been used in most regions of the world in traditional medical treatment of wounds and various diseases, including cutaneous conditions (J. Ethnopharmacol. 2000;71:1-21). P. major, also known as broadleaf plantain or greater plantain, is a member of the Plantaginaceae family, which is now widely dispersed throughout the world, though native to much of Europe as well as northern and central Asia. The Norwegian and Swedish name for the plant, groblad, means “healing leaves” (J. Ethnopharmacol. 2000;71:1-21). It was brought to the Americas by Europeans during the colonial period. Native Americans referred to it as the “white man’s footprint,” which inspired the genus name Plantago from the Latin planta (foot) (J. Ethnopharmacol. 2000;71:1-21).
Among the biologically active constituents of P. major are polysaccharides, lipids, caffeic acid derivatives, flavonoids (apigenin, luteolin, scutellarin, baicalein, nepetin, hispidulin, plantagoside), iridoid glycosides (aucubin, catalpol), terpenoids, and alkaloids (J. Ethnopharmacol. 2000;71:1-21; Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press). In an ethnopharmacologic and folk medicine survey study of 1,225 residents of the Atlantic coast of Colombia completed in 2011, Gómez-Estrada et al. found that P. major was one of the plants traditionally used to treat inflammation; it also was used to treat kidney pain and eye injuries (J. Ethnobiol. Ethnomed. 2011;7:27). P. major also is traditionally used as a mucilage and bulk laxative (Principles and Practice of Phytotherapy: Modern Herbal Medicine, 2013, Churchill Livingstone).
Extracts of the plant have been associated with myriad biologic activities, including wound healing, anti-inflammatory, antimicrobial, analgesic, antioxidant, immunomodulating, and antiulcerogenic action, which Samuelsen suggested may account for the use of the botanical in traditional medicine (J. Ethnopharmacol. 2000;71:1-21; Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press). In fact, the range of biologic properties attributed to P. major also includes astringent, anesthetic, antihelminthic, analeptic, antihistaminic, antirheumatic, antiviral, antitumor, antiulcer, diuretic, hypotensive, and expectorant activity (Exp. Biol. Med. [Maywood] 2012;237:1379-86). Though not the most popular botanical for this indication, P. major is among the plants used in the treatment of cutaneous leishmanial ulcers in Bahia, Brazil, where Leishmania brazilenesis is endemic (Rev. Soc. Bras. Med. Trop. 1996;29:229-32). Other dermatologic uses in traditional medicine include eczema, cuts, hemorrhoids, ulcerations, and wounds (Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press).
Wound healing
The use of P. major for wound healing dates back to the first century, as described by the Greek physician Dioscorides in “De Materia Medica” (J. Ethnopharmacol. 2000;71:1-21).
In 2011, Krasnov et al. developed an experimental model for characterizing proteins and showed that a newly discovered group of tissue-specific biogregulating proteins found previously in animal tissues was also present in P. major and responsible for the wound-healing activity associated with the plant (Prikl Biokhim. Mikrobiol. 2011;47:146-53).
The next year, Thomé et al. investigated and compared the wound-healing effects of P. major and Siparuna guianensis with a commercial product used in Brazil. Mice with cervical dorsal area wounds were treated with the botanical ingredients and the commercial product. Decreases in the wound area occurred earliest in mice treated with P. major, with complete closure (by day 15) seen only in this group. The investigators concluded that their findings support the traditional application of P. major, which shows potential as a viable wound-healing agent (Exp. Biol. Med. [Maywood] 2012;237:1379-86).
Conclusion
The numerous biologic properties of P. major are well established. In addition, use of the plant in traditional medicine for some cutaneous indications warrants consideration for modern therapeutic usage. Much more research is necessary, however, to elucidate the potential incorporation of this botanical into standard topical preparations for any of various skin conditions.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
For centuries, the leaves of Plantago major have been used in most regions of the world in traditional medical treatment of wounds and various diseases, including cutaneous conditions (J. Ethnopharmacol. 2000;71:1-21). P. major, also known as broadleaf plantain or greater plantain, is a member of the Plantaginaceae family, which is now widely dispersed throughout the world, though native to much of Europe as well as northern and central Asia. The Norwegian and Swedish name for the plant, groblad, means “healing leaves” (J. Ethnopharmacol. 2000;71:1-21). It was brought to the Americas by Europeans during the colonial period. Native Americans referred to it as the “white man’s footprint,” which inspired the genus name Plantago from the Latin planta (foot) (J. Ethnopharmacol. 2000;71:1-21).
Among the biologically active constituents of P. major are polysaccharides, lipids, caffeic acid derivatives, flavonoids (apigenin, luteolin, scutellarin, baicalein, nepetin, hispidulin, plantagoside), iridoid glycosides (aucubin, catalpol), terpenoids, and alkaloids (J. Ethnopharmacol. 2000;71:1-21; Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press). In an ethnopharmacologic and folk medicine survey study of 1,225 residents of the Atlantic coast of Colombia completed in 2011, Gómez-Estrada et al. found that P. major was one of the plants traditionally used to treat inflammation; it also was used to treat kidney pain and eye injuries (J. Ethnobiol. Ethnomed. 2011;7:27). P. major also is traditionally used as a mucilage and bulk laxative (Principles and Practice of Phytotherapy: Modern Herbal Medicine, 2013, Churchill Livingstone).
Extracts of the plant have been associated with myriad biologic activities, including wound healing, anti-inflammatory, antimicrobial, analgesic, antioxidant, immunomodulating, and antiulcerogenic action, which Samuelsen suggested may account for the use of the botanical in traditional medicine (J. Ethnopharmacol. 2000;71:1-21; Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press). In fact, the range of biologic properties attributed to P. major also includes astringent, anesthetic, antihelminthic, analeptic, antihistaminic, antirheumatic, antiviral, antitumor, antiulcer, diuretic, hypotensive, and expectorant activity (Exp. Biol. Med. [Maywood] 2012;237:1379-86). Though not the most popular botanical for this indication, P. major is among the plants used in the treatment of cutaneous leishmanial ulcers in Bahia, Brazil, where Leishmania brazilenesis is endemic (Rev. Soc. Bras. Med. Trop. 1996;29:229-32). Other dermatologic uses in traditional medicine include eczema, cuts, hemorrhoids, ulcerations, and wounds (Medical Herbalism: The Science and Practice of Herbal Medicine, 2003, Healing Arts Press).
Wound healing
The use of P. major for wound healing dates back to the first century, as described by the Greek physician Dioscorides in “De Materia Medica” (J. Ethnopharmacol. 2000;71:1-21).
In 2011, Krasnov et al. developed an experimental model for characterizing proteins and showed that a newly discovered group of tissue-specific biogregulating proteins found previously in animal tissues was also present in P. major and responsible for the wound-healing activity associated with the plant (Prikl Biokhim. Mikrobiol. 2011;47:146-53).
The next year, Thomé et al. investigated and compared the wound-healing effects of P. major and Siparuna guianensis with a commercial product used in Brazil. Mice with cervical dorsal area wounds were treated with the botanical ingredients and the commercial product. Decreases in the wound area occurred earliest in mice treated with P. major, with complete closure (by day 15) seen only in this group. The investigators concluded that their findings support the traditional application of P. major, which shows potential as a viable wound-healing agent (Exp. Biol. Med. [Maywood] 2012;237:1379-86).
Conclusion
The numerous biologic properties of P. major are well established. In addition, use of the plant in traditional medicine for some cutaneous indications warrants consideration for modern therapeutic usage. Much more research is necessary, however, to elucidate the potential incorporation of this botanical into standard topical preparations for any of various skin conditions.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.