User login
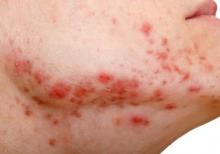
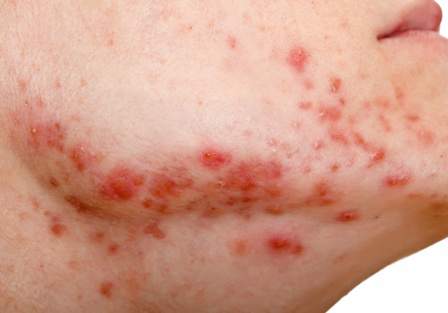
Wrinkle filler also works for acne scars
ORLANDO – A non-resorbable wrinkle filler proved highly effective and durable for the treatment of atrophic acne scars in a randomized, controlled, multicenter study.
At 1 month after treatment with polymethylmethacrylate-collagen, or PMMA-collagen (Artefill, Suneva Medical, Inc.), nearly 70% of 97 subjects showed at least a 2-point improvement on the validated 4-point Acne Scar Rating Scale, compared with about 40% of 50 control subjects injected with saline. At 6 months, the response rate remained above 60% in the PMMA-collagen group, but dropped closer to 30% among those in the control group, Dr. James M. Spencer of Mount Sinai School of Medicine, New York reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
The control group subjects were then crossed over to the treatment group, and, at 12 months, the response rates were about 70% and nearly 60% in the treatment and control groups, respectively, he said.
Similarly, both Physician and Subject Global Aesthetic Improvement Scale (PGAIS/SGAIS) scores diverged during a 6-month evaluator-blinded phase of the study, then converged after crossover by the control group subjects. For example, the percentage of treatment and control group subjects with improvement at 1 month and 6 months according to the 5-point PGAIS was about 90% vs. less than 65%, and about 80% vs. about 30%, respectively. More than 90% in both groups showed improvement at 12 months, after control group crossover.
Additionally, subject satisfaction at 1 and 6 months in the treatment and control groups based on assessment of scar correction using Patient Satisfaction Scale scores was above 80% vs. about 60%, and about 80% vs. about 50%, respectively. Satisfaction in both groups was between 80% and 90% at 12 months, after control group crossover.
Study subjects, who had a mean age of 44 years, were enrolled from 10 U.S. centers and were treated during one injection session. An additional touch-up injection was allowed as needed. A total of 1,292 scars were treated in the 97 treatment group subjects, and 424 were treated in the 50 control group subjects. Participants were evaluated by blinded assessors at 2 weeks and 1, 3, and 6 months, after which control group subjects were treated with PMMA-collagen. Assessments were made in open-label fashion at 9 and 12 months. Most subjects (61%) were women, and 20% had Fitzpatrick skin types 5 or 6.
During the blinded portion of the study, six treatment-related adverse events were reported among treatment group subjects, and two were reported among control group subjects. None of the subjects experienced granulomas, changes in pigmentation, or hypertrophic scarring.
This study is the first randomized, blinded study of PMMA-collagen for treating acne scars, Dr. Spencer noted, adding that the findings demonstrate the efficacy and safety of PMMA-collagen for this purpose.
“The improvement is durable, lasting for 12 months,” he wrote, noting that the filler is easily administered and requires minimal training in those who are familiar with dermal fillers.
“PMMA-collagen works very well on deep, severe acne scars, and should also work very well on shallow scars,” he said.
The product may enable practitioners to effectively treat acne scarring without a large capital equipment expenditure and without the risks associated with resurfacing procedures,” he said.
This study was sponsored by Suneva Medical, Inc.
ORLANDO – A non-resorbable wrinkle filler proved highly effective and durable for the treatment of atrophic acne scars in a randomized, controlled, multicenter study.
At 1 month after treatment with polymethylmethacrylate-collagen, or PMMA-collagen (Artefill, Suneva Medical, Inc.), nearly 70% of 97 subjects showed at least a 2-point improvement on the validated 4-point Acne Scar Rating Scale, compared with about 40% of 50 control subjects injected with saline. At 6 months, the response rate remained above 60% in the PMMA-collagen group, but dropped closer to 30% among those in the control group, Dr. James M. Spencer of Mount Sinai School of Medicine, New York reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
The control group subjects were then crossed over to the treatment group, and, at 12 months, the response rates were about 70% and nearly 60% in the treatment and control groups, respectively, he said.
Similarly, both Physician and Subject Global Aesthetic Improvement Scale (PGAIS/SGAIS) scores diverged during a 6-month evaluator-blinded phase of the study, then converged after crossover by the control group subjects. For example, the percentage of treatment and control group subjects with improvement at 1 month and 6 months according to the 5-point PGAIS was about 90% vs. less than 65%, and about 80% vs. about 30%, respectively. More than 90% in both groups showed improvement at 12 months, after control group crossover.
Additionally, subject satisfaction at 1 and 6 months in the treatment and control groups based on assessment of scar correction using Patient Satisfaction Scale scores was above 80% vs. about 60%, and about 80% vs. about 50%, respectively. Satisfaction in both groups was between 80% and 90% at 12 months, after control group crossover.
Study subjects, who had a mean age of 44 years, were enrolled from 10 U.S. centers and were treated during one injection session. An additional touch-up injection was allowed as needed. A total of 1,292 scars were treated in the 97 treatment group subjects, and 424 were treated in the 50 control group subjects. Participants were evaluated by blinded assessors at 2 weeks and 1, 3, and 6 months, after which control group subjects were treated with PMMA-collagen. Assessments were made in open-label fashion at 9 and 12 months. Most subjects (61%) were women, and 20% had Fitzpatrick skin types 5 or 6.
During the blinded portion of the study, six treatment-related adverse events were reported among treatment group subjects, and two were reported among control group subjects. None of the subjects experienced granulomas, changes in pigmentation, or hypertrophic scarring.
This study is the first randomized, blinded study of PMMA-collagen for treating acne scars, Dr. Spencer noted, adding that the findings demonstrate the efficacy and safety of PMMA-collagen for this purpose.
“The improvement is durable, lasting for 12 months,” he wrote, noting that the filler is easily administered and requires minimal training in those who are familiar with dermal fillers.
“PMMA-collagen works very well on deep, severe acne scars, and should also work very well on shallow scars,” he said.
The product may enable practitioners to effectively treat acne scarring without a large capital equipment expenditure and without the risks associated with resurfacing procedures,” he said.
This study was sponsored by Suneva Medical, Inc.
ORLANDO – A non-resorbable wrinkle filler proved highly effective and durable for the treatment of atrophic acne scars in a randomized, controlled, multicenter study.
At 1 month after treatment with polymethylmethacrylate-collagen, or PMMA-collagen (Artefill, Suneva Medical, Inc.), nearly 70% of 97 subjects showed at least a 2-point improvement on the validated 4-point Acne Scar Rating Scale, compared with about 40% of 50 control subjects injected with saline. At 6 months, the response rate remained above 60% in the PMMA-collagen group, but dropped closer to 30% among those in the control group, Dr. James M. Spencer of Mount Sinai School of Medicine, New York reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
The control group subjects were then crossed over to the treatment group, and, at 12 months, the response rates were about 70% and nearly 60% in the treatment and control groups, respectively, he said.
Similarly, both Physician and Subject Global Aesthetic Improvement Scale (PGAIS/SGAIS) scores diverged during a 6-month evaluator-blinded phase of the study, then converged after crossover by the control group subjects. For example, the percentage of treatment and control group subjects with improvement at 1 month and 6 months according to the 5-point PGAIS was about 90% vs. less than 65%, and about 80% vs. about 30%, respectively. More than 90% in both groups showed improvement at 12 months, after control group crossover.
Additionally, subject satisfaction at 1 and 6 months in the treatment and control groups based on assessment of scar correction using Patient Satisfaction Scale scores was above 80% vs. about 60%, and about 80% vs. about 50%, respectively. Satisfaction in both groups was between 80% and 90% at 12 months, after control group crossover.
Study subjects, who had a mean age of 44 years, were enrolled from 10 U.S. centers and were treated during one injection session. An additional touch-up injection was allowed as needed. A total of 1,292 scars were treated in the 97 treatment group subjects, and 424 were treated in the 50 control group subjects. Participants were evaluated by blinded assessors at 2 weeks and 1, 3, and 6 months, after which control group subjects were treated with PMMA-collagen. Assessments were made in open-label fashion at 9 and 12 months. Most subjects (61%) were women, and 20% had Fitzpatrick skin types 5 or 6.
During the blinded portion of the study, six treatment-related adverse events were reported among treatment group subjects, and two were reported among control group subjects. None of the subjects experienced granulomas, changes in pigmentation, or hypertrophic scarring.
This study is the first randomized, blinded study of PMMA-collagen for treating acne scars, Dr. Spencer noted, adding that the findings demonstrate the efficacy and safety of PMMA-collagen for this purpose.
“The improvement is durable, lasting for 12 months,” he wrote, noting that the filler is easily administered and requires minimal training in those who are familiar with dermal fillers.
“PMMA-collagen works very well on deep, severe acne scars, and should also work very well on shallow scars,” he said.
The product may enable practitioners to effectively treat acne scarring without a large capital equipment expenditure and without the risks associated with resurfacing procedures,” he said.
This study was sponsored by Suneva Medical, Inc.
AT THE ODAC CONFERENCE
Key clinical point: PMMA-collagen is safe, effective, and practical for treating atrophic acne scars.
Major finding: Nearly 70% of treated subjects vs. 40% of controls showed at least a 2-point improvement on the Acne Scar Rating Scale
Data source: A randomized, controlled, multicenter study of 147 subjects.
Disclosures: This study was sponsored by Suneva Medical, Inc.
Cosmetic Corner: Dermatologists Weigh in on Eyelash Enhancers
To improve patient care and outcomes, leading dermatologists offered their recommendations on the eyelash enhancers. Consideration must be given to:
- Latisse
Allergan, Inc.
“It’s the only eyelash-enhancing product that is FDA approved and has been proven to increase eyelash size.”—Gary Goldenberg, MD, New York, New York
Recommended by Elizabeth K. Hale, MD, New York, New York
“It’s an excellent treatment for stimulating the length and diameter of eyelashes (as well as eyebrows). Most patients respond well to it and side effects are minimal and rare.”—Mark G. Rubin, MD, Beverly Hills, California
Recommended by Joel Schlessinger, MD, Omaha, Nebraska
“My choice is definitely Latisse, but inform patients that they should not stop abruptly as this can cause acute eyelash loss.”—Antonella Tosti, MD, Miami, Florida
- RapidLash
Rocasuba, Inc
“This active ingredient is proving to have good clinical results at a good price point for consumers.”—Anthony M. Rossi, MD, New York, New York
- RevitaLash Advanced
Athena Cosmetics
“A great alternative to Latisse or one that patients can transition to once they are satisfied with the growth that Latisse has brought them or if they are allergic/sensitive to Latisse.”—Joel Schlessinger, MD, Omaha, Nebraska
Cutis invites readers to send us their recommendations. Skin care products for babies, hair straighteners, aftershaves, and cleansing pads will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the eyelash enhancers. Consideration must be given to:
- Latisse
Allergan, Inc.
“It’s the only eyelash-enhancing product that is FDA approved and has been proven to increase eyelash size.”—Gary Goldenberg, MD, New York, New York
Recommended by Elizabeth K. Hale, MD, New York, New York
“It’s an excellent treatment for stimulating the length and diameter of eyelashes (as well as eyebrows). Most patients respond well to it and side effects are minimal and rare.”—Mark G. Rubin, MD, Beverly Hills, California
Recommended by Joel Schlessinger, MD, Omaha, Nebraska
“My choice is definitely Latisse, but inform patients that they should not stop abruptly as this can cause acute eyelash loss.”—Antonella Tosti, MD, Miami, Florida
- RapidLash
Rocasuba, Inc
“This active ingredient is proving to have good clinical results at a good price point for consumers.”—Anthony M. Rossi, MD, New York, New York
- RevitaLash Advanced
Athena Cosmetics
“A great alternative to Latisse or one that patients can transition to once they are satisfied with the growth that Latisse has brought them or if they are allergic/sensitive to Latisse.”—Joel Schlessinger, MD, Omaha, Nebraska
Cutis invites readers to send us their recommendations. Skin care products for babies, hair straighteners, aftershaves, and cleansing pads will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the eyelash enhancers. Consideration must be given to:
- Latisse
Allergan, Inc.
“It’s the only eyelash-enhancing product that is FDA approved and has been proven to increase eyelash size.”—Gary Goldenberg, MD, New York, New York
Recommended by Elizabeth K. Hale, MD, New York, New York
“It’s an excellent treatment for stimulating the length and diameter of eyelashes (as well as eyebrows). Most patients respond well to it and side effects are minimal and rare.”—Mark G. Rubin, MD, Beverly Hills, California
Recommended by Joel Schlessinger, MD, Omaha, Nebraska
“My choice is definitely Latisse, but inform patients that they should not stop abruptly as this can cause acute eyelash loss.”—Antonella Tosti, MD, Miami, Florida
- RapidLash
Rocasuba, Inc
“This active ingredient is proving to have good clinical results at a good price point for consumers.”—Anthony M. Rossi, MD, New York, New York
- RevitaLash Advanced
Athena Cosmetics
“A great alternative to Latisse or one that patients can transition to once they are satisfied with the growth that Latisse has brought them or if they are allergic/sensitive to Latisse.”—Joel Schlessinger, MD, Omaha, Nebraska
Cutis invites readers to send us their recommendations. Skin care products for babies, hair straighteners, aftershaves, and cleansing pads will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Sunscreens Causing Cancer? The Facts
Skin cancer is the most common form of cancer in the United States and continues to rise in incidence and mortality each year.1 It is common knowledge that UV light plays a major role in the development of skin cancer.2,3 Studies have long demonstrated that using sunscreen on a daily basis can help prevent the development of skin cancer, premature aging, and exacerbation of photodermatoses.4-7 Although there are several photoprotective measures available, sunscreen remains the most popular and widely used among patients.8 Sunscreens that are on the market today contain either organic or inorganic UV filters or a combination of both based on their chemical composition and photoprotection mechanisms.9 Concerns about these ingredients causing cancer have created confusion among consumers. I will attempt to clarify these concerns by critically analyzing available evidence-based data on sunscreen use so that as dermatology residents we will be more knowledgeable about sunscreen safety topics and will be able to provide accurate and up-to-date information to our patients.
Organic UV Filters
Organic UV filters are classified as aromatic compounds that provide photoprotection by absorbing UV light.10 Aside from the photoallergic potential of organic UV filters, controversy has arisen in response to studies reporting their possible hormone disruptive effects.11-18 Although there are several US Food and Drug Administration (FDA)–approved organic UV filters in use today, one of the most commonly manufactured and controversial agents is oxybenzone.10 Claims regarding the estrogenic and antiandrogenic effects of oxybenzone have been investigated with results refuting the claims or concluding that more sensitive studies are needed to determine if these organic ingredients pose such risks.10,19,20 One study demonstrated that nearly 300 years of daily sunscreen application would be needed to reach similar exposure levels of oxybenzone used and described in prior animal studies.21 Additionally, most of the studied adverse effects of UV filters have been evaluated based on oral exposure rather than actual dermal application.11 Although these compounds are absorbed systemically, studies have reported that the amounts are insignificant and noncumulative in the body.10,22-24 Furthermore, the binding affinity of oxybenzone for estrogen receptors has been shown to be much weaker and near insignificant compared to estrogen and estradiol.24,25 Although numerous important studies examining systemic absorption have not shown a clinically significant disruption of hormonal homeostasis or acute toxicity in humans by organic UV filters, further studies are needed.
Inorganic UV Filters
Used as the main active ingredients in sunscreen for decades, titanium dioxide (TiO2) and zinc oxide (ZnO) compounds generally are more photostable and less photoallergic than their organic counterparts.10 In recent years, the safety of these long-used photoprotectors has been questioned because of the development of nanoparticle (<100 nm) formulas that are less opaque on application. Although this formula provides a thin, transparent, and cosmetically appealing medium, there is concern that the metal oxides penetrate the skin and cause local and systemic toxicities.26-28 Several recent scientific studies have shown no percutaneous permeation of these particles in normal adult human skin and reported no causal damage to mammalian cells.10,29-31 Although skin penetration of TiO2 and ZnO has been described as insignificant, focus has shifted to health risks associated with inhaling TiO2 through the use of spray or powder products following statements made by the International Agency for Research on Cancer in 2006.32 Several studies investigating increased health risks, specifically lung cancer, in factory workers who were subjected to TiO2 and ZnO inhalation concluded that exposure was unlikely to pose substantial health risks or subchronic toxicity.33,34 Despite a relatively strong safety profile, a major concern of using these metal oxides as UV filters has been potential free radical formation.35-39 For this reason, the Scientific Committee on Emerging and Newly Identified Health Risks extensively researched and delivered opinions on the use of TiO2 and ZnO in cosmetics, concluding that topical application of either compound does not result in toxicity or other adverse effects.30,40-42 Additionally, an effort has been made by manufacturers to encapsulate nanoparticles with magnesium and other materials to quench the reactive oxygen species along with the human body’s own antioxidant defense system.10 In summary, it appears that the current weight of scientific evidence suggests that percutaneous absorption and toxicity by UV filters in humans may be overestimated and that the use of nanoparticles in sunscreens poses no or negligible potential risks to human health.43,44
Concerns Beyond Organic and Inorganic UV Filters
Beyond these concerns with organic and inorganic UV filters, there are several other claims regarding sunscreen safety that have stirred up controversy, including the side-effect profile of retinyl palmitate, vitamin D deficiency, phototoxicity, environmental effects, futility of sun protection factor levels greater than 50, and increased health risks in children. Although some studies report mixed results, the majority of scientific investigations have addressed and refuted several of these claims, again confirming the relative safety of sunscreen use. It is beyond the scope of this article to further discuss these topics specifically. However, it is worth mentioning that consumer studies report that the actual use of sunscreens is 0.5 mg/cm2 or less compared to the ideal application of 2 mg/cm2, thereby confounding many of the claims made about sunscreen use, such as vitamin D deficiency.45 Sunscreens often contain a combination of several UV filters. To date, only a few existing studies have shown that mixtures of the photoprotective agents discussed might interact and exhibit toxic activity when combined, even when there is no observed adverse toxic effect when used individually in products.46-48
The current FDA ruling on sunscreen labeling does not require manufacturers to state if inorganic UV filters have been formulated into nanoparticles; however, manufacturers are now required to include a statement on all sunscreen labels warning consumers to avoid using sunscreen on damaged or broken skin49 in an effort to prevent the active ingredients from getting under the skin, potentially causing inflammation and/or health risks, because available data do not provide conclusive evidence on increased penetration of open skin.50 Additional information regarding the 2011 FDA sunscreen ruling can be found in a prior Cutis Resident Corner column.51
Final Thoughts
As health care providers, we should take advantage of opportunities to educate our patients about other sun safety practices, such as avoiding excessive sun exposure during peak hours (10 am to 2 pm), seeking shade, and wearing photoprotective clothing (eg, wide-brimmed hats, sunglasses).
The research is quite clear: Using broadband sunscreens that absorb and/or block UV radiation results in reduced damage to the skin’s DNA, a fact that should be considered when taking into account the risks and benefits of sunscreen use.2,3 Although sunscreen use is highly recommended in addition to the other sun protection methods, it is ultimately the patient’s choice. If a patient is still concerned about the active ingredients of UV filters, even given the high probability of safety, there are products available on the market that do not include organic filters or nanoparticles. Given the established benefits of UV protection, the use of sunscreens remain one of the most important photoprotective methods, and with increased usage by the public, continuous monitoring of the overall safety and benefit profile of future products is prudent.
1. Skin cancer statistics. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/cancer/skin/statistics/index.htm. Updated September 2, 2014. Accessed December 30, 2014.
2. World Health Organization, International Agency for Research on Cancer. Solar and ultraviolet radiation. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 55. Lyon, France: International Agency for Research on Cancer; 1992.
3. Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29:257-263.
4. Darlington S, Williams G, Neale R, et al. A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Arch Dermatol. 2003;139:451-455.
5. Van der Pols JC, Williams GM, Pandeya N, et al. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev. 2006;15:2546-2548.
6. Hughes MC, Williams GM, Baker P, et al. Sunscreen and prevention of skin aging: a randomized trial. Ann Intern Med. 2013;158:781-790.
7. Bissonnette R, Nigen S, Bolduc C. Influence of the quantity of sunscreen applied on the ability to protect against ultraviolet-induced polymorphous light eruption. Photodermatol Photoimmunol Photomed. 2012;28:240-243.
8. Cancer trends progress report 2011/2012 update: sun protection. National Cancer Institute Web site. http://progressreport.cancer.gov/doc_detail.asp?pid¡1&did¡2009&chid¡91&coid¡911. Accessed December 30, 2014.
9. Sunscreen Drug Products for Over-the-counter Human Use, 21 CFR §352.10. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=352.10. Updated September 1, 2014. Accessed December 30, 2014.
10. Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photoimmunol Photomed. 2011;27:58-67.
11. Krause M, Klit A, Blomberg Jensen M, et al. Sunscreens: are they beneficial for health? an overview of endocrine disrupting properties of UV-filters. Int J Androl. 2012;35:424-436.
12. Schlumpf M, Cotton B, Conscience M, et al. In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect. 2001;109:239-244.
13. Schlumpf M, Schmid P, Durrer S, et al. Endocrine activity and developmental toxicity of cosmetic UV filters–an update. Toxicol. 2004;205:113-122.
14. Schlumpf M, Kypke K, Vökt C, et al. Endocrine active UV filters: developmental toxicity and exposure through breast milk. Chimia. 2008;62:345-351.
15. Nakagawa Y, Suzuki T. Metabolism of 2-hydroxy-4-methoxybenzophenone in isolated rat hepatocytes and xenoestrogenic effects of its metabolites on MCF-7 human breast cancer cells. Chem Biol Interact. 2002;139:115-128.
16. Ma R, Cotton B, Lichtensteiger W, et al. UV filters with antagonistic action at androgen receptors in the MDA-kb2 cell transcriptional-activation assay. Toxicol Sci. 2003;74:43-50.
17. Heneweer M, Muusse M, van den Berg M, et al. Additive estrogenic effects of mixtures of frequently used UV filters on pS2-gene transcription in MCF-7 cells. Toxicol Appl Pharmacol. 2005;208:170-177.
18. Knobler E, Almeida L, Ruzkowski AM, et al. Photoallergy to benzophenone. Arch Dermatol. 1989;125:801-804.
19. Draelos ZD. Are sunscreens safe? J Cosmet Dermatol. 2010;9:1-2.
20. Gilbert E, Pirot F, Bertholle V. Commonly used UV filter toxicity on biological functions: review of last decade studies. Int J of Cosmet Sci. 2013;35:208-219.
21. Wang SQ, Burnett ME, Lim HW. Safety of oxybenzone: putting numbers into perspective. Arch Dermatol. 2011;147:865-866.
22. Mancebo SE, Hu JY, Wang SQ. Sunscreens: a review of health benefits, regulations, and controversies. Dermatol Clin. 2014;32:427-438.
23. Jansen R, Osterwalder U, Wang SQ, et al. Photoprotection: part II. sunscreen: development, efficacy, and controversies. J Am Acad Dermatol. 2013;69:867.e1-867.e14.
24. Janjua NR, Mogensen B, Andersson AM, et al. Systemic absorption of the sunscreens benzo- phenone-3, octyl-methoxycinnamate, and 3-(4-methyl-benzy-lidene) camphor after whole-body topical application and reproductive hormone levels in humans. J Invest Dermatol. 2004;123:57-61.
25. Kadry AM, Chukwuemeka SO, Mohamed S, et al. Pharmacokinetics of benzophenone-3 after oral exposure in male rats. J Appl Toxicol. 1995;15:97-102.
26. Gulson B, McCall M, Korsch M, et al. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol Sci. 2010;118:140-149.
27. Gulson B, Wong H, Korsch M, et al. Comparison of dermal absorption of zinc from different sunscreen formulations and differing UV exposure based on stable isotope tracing. Sci Total Environ. 2012:420:313-318.
28. Benech-Kieffer F, Meuling WJ, Leclerc C, et al. Percutaneous absorption of Mexoryl SX in human volunteers: comparison with in vitro data. Skin Pharmacol Appl Skin Physiol. 2003;16:343-355.
29. Nash JF. Human safety and efficacy of ultraviolet filters and sunscreen products. Dermatol Clin. 2006;24:35-51.
30. Nohynek GJ, Lademann J, Ribaud C, et al. Grey goo on the skin? nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol. 2007;37:251-277.
31. Sadrieh N, Wokovich AM, Gopee NV, et al. Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles. Toxicol Sci. 2010;115:156-166.
32. International Agency for Research on Cancer. Carbon black, titanium dioxide, and talc. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 93. Lyon, France: International Agency for Research on Cancer; 2006.
33. Liao CM, Chiang YH, Chio CP. Model-based assessment for human inhalation exposure risk to airborne nano/fine titanium dioxide particles. Sci Total Environ. 2008:15;407:165-177.
34. Adamcakova-Dodd A, Stebounova LV, Kim JS, et al. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part Fibre Toxicol. 2014;11:15.
35. Wamer WG, Yin JJ, Wei RR. Oxidative damage to nucleic acids photosensitized by titanium dioxide. Free Radic Biol Med. 1997;23:851-858.
36. Nakagawa Y, Wakuri S, Sakamoto K, et al. The photogenotoxicity of titanium dioxide particles. Mutat Res. 1997;394:125-132.
37. Dunford R, Salinaro A, Cai L, et al. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997;418:87-90, 99.
38. Hidaka H, Kobayashi H, Koike T, et al. DNA damage photoinduced by cosmetic pigments and sunscreen agents under solar exposure and artificial UV illumination. J Oleo Sci. 2006;55:249-261.
39. Dufour EK, Kumaravel T, Nohynek GJ, et al. Clastogenicity, photo-clastogenicity or pseudo-photo-clastogenicity: genotoxic effects of zinc oxide in the dark, in pre-irradiated or simultaneously irradiated Chinese hamster ovary cells [published online ahead of print June 21, 2006]. Mutat Res. 2006;607:215-224.
40. Opinion of the Scientific Committee on Cosmetic Products and Non-Food Products intended for Consumers concerning titanium dioxide. http://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out135_en.pdf. Published October 24, 2000. Accessed December 30, 2014.
41. The Scientific Committee on Cosmetic Products and Non-Food Products intended for Consumers opinion concerning zinc oxide. http://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out222_en.pdf. Published June 24-25, 2003. Accessed December 30, 2014.
42. Hackenberg S, Friehs G, Kessler M, et al. Nanosized titanium dioxide particles do not induce DNA damage in human peripheral blood lymphocytes. Environ Mol Mutagen. 2010;52:264-268.
43. Bach-Thomsen M, Wulf HC. Sunbather’s application of sunscreen is probably inadequate to obtain the sun protection factor assigned to the preparation. Photodermatol Photoimmunol Photomed. 1993:9;242-244.
44. Nohynek GJ, Antignac E, Re T, et al. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol Appl Pharmacol. 2010:1;243:239-259.
45. Diffey BL. Sunscreens: use and misuse. In: Giacomoni PU, ed. Sun Protection in Man. Vol 3. Amsterdam, the Netherlands: Elsevier Science BV; 2001:521-534.
46. Heneweer M, Muusse M, Van den BM, et al. Additive estrogenic effects of mixtures of frequently used UV-filters on pS2-gene transcription in MCF-7 cells. Toxicol Appl Pharmacol. 2005;208:170-177.
47. Kunz PY, Galicia HF, Fent K. Comparison of in vitro and in vivo estrogenic activity of UV-filters in fish. Toxicol Sci. 2006;90:349-361.
48. Kortenkamp A, Faust M, Scholze M, et al. Low-level exposure to multiple chemicals: reason for human health concerns? Environ Health Perspect. 2007;115(suppl 1):106-114.
49. Labeling and effectiveness testing: sunscreen drug products for over-the-counter human use—small entity compliance guide. US Food and Drug Administration Web site. http://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/guidances/ucm330694.htm. Published December 2012. Updated May 13, 2014. Accessed December 30, 2014.
50. Schafer-Korting M, Korting HC, Ponce-Poschl E. Liposomal tretinoin for uncomplicated acne vulgaris. Clin Investig. 1994;72:1086-1091.
51. Bronfenbrener R. Simplifying sun safety: a guide to the new FDA sunscreen monograph. Cutis. 2014;93:e17-e19.
Skin cancer is the most common form of cancer in the United States and continues to rise in incidence and mortality each year.1 It is common knowledge that UV light plays a major role in the development of skin cancer.2,3 Studies have long demonstrated that using sunscreen on a daily basis can help prevent the development of skin cancer, premature aging, and exacerbation of photodermatoses.4-7 Although there are several photoprotective measures available, sunscreen remains the most popular and widely used among patients.8 Sunscreens that are on the market today contain either organic or inorganic UV filters or a combination of both based on their chemical composition and photoprotection mechanisms.9 Concerns about these ingredients causing cancer have created confusion among consumers. I will attempt to clarify these concerns by critically analyzing available evidence-based data on sunscreen use so that as dermatology residents we will be more knowledgeable about sunscreen safety topics and will be able to provide accurate and up-to-date information to our patients.
Organic UV Filters
Organic UV filters are classified as aromatic compounds that provide photoprotection by absorbing UV light.10 Aside from the photoallergic potential of organic UV filters, controversy has arisen in response to studies reporting their possible hormone disruptive effects.11-18 Although there are several US Food and Drug Administration (FDA)–approved organic UV filters in use today, one of the most commonly manufactured and controversial agents is oxybenzone.10 Claims regarding the estrogenic and antiandrogenic effects of oxybenzone have been investigated with results refuting the claims or concluding that more sensitive studies are needed to determine if these organic ingredients pose such risks.10,19,20 One study demonstrated that nearly 300 years of daily sunscreen application would be needed to reach similar exposure levels of oxybenzone used and described in prior animal studies.21 Additionally, most of the studied adverse effects of UV filters have been evaluated based on oral exposure rather than actual dermal application.11 Although these compounds are absorbed systemically, studies have reported that the amounts are insignificant and noncumulative in the body.10,22-24 Furthermore, the binding affinity of oxybenzone for estrogen receptors has been shown to be much weaker and near insignificant compared to estrogen and estradiol.24,25 Although numerous important studies examining systemic absorption have not shown a clinically significant disruption of hormonal homeostasis or acute toxicity in humans by organic UV filters, further studies are needed.
Inorganic UV Filters
Used as the main active ingredients in sunscreen for decades, titanium dioxide (TiO2) and zinc oxide (ZnO) compounds generally are more photostable and less photoallergic than their organic counterparts.10 In recent years, the safety of these long-used photoprotectors has been questioned because of the development of nanoparticle (<100 nm) formulas that are less opaque on application. Although this formula provides a thin, transparent, and cosmetically appealing medium, there is concern that the metal oxides penetrate the skin and cause local and systemic toxicities.26-28 Several recent scientific studies have shown no percutaneous permeation of these particles in normal adult human skin and reported no causal damage to mammalian cells.10,29-31 Although skin penetration of TiO2 and ZnO has been described as insignificant, focus has shifted to health risks associated with inhaling TiO2 through the use of spray or powder products following statements made by the International Agency for Research on Cancer in 2006.32 Several studies investigating increased health risks, specifically lung cancer, in factory workers who were subjected to TiO2 and ZnO inhalation concluded that exposure was unlikely to pose substantial health risks or subchronic toxicity.33,34 Despite a relatively strong safety profile, a major concern of using these metal oxides as UV filters has been potential free radical formation.35-39 For this reason, the Scientific Committee on Emerging and Newly Identified Health Risks extensively researched and delivered opinions on the use of TiO2 and ZnO in cosmetics, concluding that topical application of either compound does not result in toxicity or other adverse effects.30,40-42 Additionally, an effort has been made by manufacturers to encapsulate nanoparticles with magnesium and other materials to quench the reactive oxygen species along with the human body’s own antioxidant defense system.10 In summary, it appears that the current weight of scientific evidence suggests that percutaneous absorption and toxicity by UV filters in humans may be overestimated and that the use of nanoparticles in sunscreens poses no or negligible potential risks to human health.43,44
Concerns Beyond Organic and Inorganic UV Filters
Beyond these concerns with organic and inorganic UV filters, there are several other claims regarding sunscreen safety that have stirred up controversy, including the side-effect profile of retinyl palmitate, vitamin D deficiency, phototoxicity, environmental effects, futility of sun protection factor levels greater than 50, and increased health risks in children. Although some studies report mixed results, the majority of scientific investigations have addressed and refuted several of these claims, again confirming the relative safety of sunscreen use. It is beyond the scope of this article to further discuss these topics specifically. However, it is worth mentioning that consumer studies report that the actual use of sunscreens is 0.5 mg/cm2 or less compared to the ideal application of 2 mg/cm2, thereby confounding many of the claims made about sunscreen use, such as vitamin D deficiency.45 Sunscreens often contain a combination of several UV filters. To date, only a few existing studies have shown that mixtures of the photoprotective agents discussed might interact and exhibit toxic activity when combined, even when there is no observed adverse toxic effect when used individually in products.46-48
The current FDA ruling on sunscreen labeling does not require manufacturers to state if inorganic UV filters have been formulated into nanoparticles; however, manufacturers are now required to include a statement on all sunscreen labels warning consumers to avoid using sunscreen on damaged or broken skin49 in an effort to prevent the active ingredients from getting under the skin, potentially causing inflammation and/or health risks, because available data do not provide conclusive evidence on increased penetration of open skin.50 Additional information regarding the 2011 FDA sunscreen ruling can be found in a prior Cutis Resident Corner column.51
Final Thoughts
As health care providers, we should take advantage of opportunities to educate our patients about other sun safety practices, such as avoiding excessive sun exposure during peak hours (10 am to 2 pm), seeking shade, and wearing photoprotective clothing (eg, wide-brimmed hats, sunglasses).
The research is quite clear: Using broadband sunscreens that absorb and/or block UV radiation results in reduced damage to the skin’s DNA, a fact that should be considered when taking into account the risks and benefits of sunscreen use.2,3 Although sunscreen use is highly recommended in addition to the other sun protection methods, it is ultimately the patient’s choice. If a patient is still concerned about the active ingredients of UV filters, even given the high probability of safety, there are products available on the market that do not include organic filters or nanoparticles. Given the established benefits of UV protection, the use of sunscreens remain one of the most important photoprotective methods, and with increased usage by the public, continuous monitoring of the overall safety and benefit profile of future products is prudent.
Skin cancer is the most common form of cancer in the United States and continues to rise in incidence and mortality each year.1 It is common knowledge that UV light plays a major role in the development of skin cancer.2,3 Studies have long demonstrated that using sunscreen on a daily basis can help prevent the development of skin cancer, premature aging, and exacerbation of photodermatoses.4-7 Although there are several photoprotective measures available, sunscreen remains the most popular and widely used among patients.8 Sunscreens that are on the market today contain either organic or inorganic UV filters or a combination of both based on their chemical composition and photoprotection mechanisms.9 Concerns about these ingredients causing cancer have created confusion among consumers. I will attempt to clarify these concerns by critically analyzing available evidence-based data on sunscreen use so that as dermatology residents we will be more knowledgeable about sunscreen safety topics and will be able to provide accurate and up-to-date information to our patients.
Organic UV Filters
Organic UV filters are classified as aromatic compounds that provide photoprotection by absorbing UV light.10 Aside from the photoallergic potential of organic UV filters, controversy has arisen in response to studies reporting their possible hormone disruptive effects.11-18 Although there are several US Food and Drug Administration (FDA)–approved organic UV filters in use today, one of the most commonly manufactured and controversial agents is oxybenzone.10 Claims regarding the estrogenic and antiandrogenic effects of oxybenzone have been investigated with results refuting the claims or concluding that more sensitive studies are needed to determine if these organic ingredients pose such risks.10,19,20 One study demonstrated that nearly 300 years of daily sunscreen application would be needed to reach similar exposure levels of oxybenzone used and described in prior animal studies.21 Additionally, most of the studied adverse effects of UV filters have been evaluated based on oral exposure rather than actual dermal application.11 Although these compounds are absorbed systemically, studies have reported that the amounts are insignificant and noncumulative in the body.10,22-24 Furthermore, the binding affinity of oxybenzone for estrogen receptors has been shown to be much weaker and near insignificant compared to estrogen and estradiol.24,25 Although numerous important studies examining systemic absorption have not shown a clinically significant disruption of hormonal homeostasis or acute toxicity in humans by organic UV filters, further studies are needed.
Inorganic UV Filters
Used as the main active ingredients in sunscreen for decades, titanium dioxide (TiO2) and zinc oxide (ZnO) compounds generally are more photostable and less photoallergic than their organic counterparts.10 In recent years, the safety of these long-used photoprotectors has been questioned because of the development of nanoparticle (<100 nm) formulas that are less opaque on application. Although this formula provides a thin, transparent, and cosmetically appealing medium, there is concern that the metal oxides penetrate the skin and cause local and systemic toxicities.26-28 Several recent scientific studies have shown no percutaneous permeation of these particles in normal adult human skin and reported no causal damage to mammalian cells.10,29-31 Although skin penetration of TiO2 and ZnO has been described as insignificant, focus has shifted to health risks associated with inhaling TiO2 through the use of spray or powder products following statements made by the International Agency for Research on Cancer in 2006.32 Several studies investigating increased health risks, specifically lung cancer, in factory workers who were subjected to TiO2 and ZnO inhalation concluded that exposure was unlikely to pose substantial health risks or subchronic toxicity.33,34 Despite a relatively strong safety profile, a major concern of using these metal oxides as UV filters has been potential free radical formation.35-39 For this reason, the Scientific Committee on Emerging and Newly Identified Health Risks extensively researched and delivered opinions on the use of TiO2 and ZnO in cosmetics, concluding that topical application of either compound does not result in toxicity or other adverse effects.30,40-42 Additionally, an effort has been made by manufacturers to encapsulate nanoparticles with magnesium and other materials to quench the reactive oxygen species along with the human body’s own antioxidant defense system.10 In summary, it appears that the current weight of scientific evidence suggests that percutaneous absorption and toxicity by UV filters in humans may be overestimated and that the use of nanoparticles in sunscreens poses no or negligible potential risks to human health.43,44
Concerns Beyond Organic and Inorganic UV Filters
Beyond these concerns with organic and inorganic UV filters, there are several other claims regarding sunscreen safety that have stirred up controversy, including the side-effect profile of retinyl palmitate, vitamin D deficiency, phototoxicity, environmental effects, futility of sun protection factor levels greater than 50, and increased health risks in children. Although some studies report mixed results, the majority of scientific investigations have addressed and refuted several of these claims, again confirming the relative safety of sunscreen use. It is beyond the scope of this article to further discuss these topics specifically. However, it is worth mentioning that consumer studies report that the actual use of sunscreens is 0.5 mg/cm2 or less compared to the ideal application of 2 mg/cm2, thereby confounding many of the claims made about sunscreen use, such as vitamin D deficiency.45 Sunscreens often contain a combination of several UV filters. To date, only a few existing studies have shown that mixtures of the photoprotective agents discussed might interact and exhibit toxic activity when combined, even when there is no observed adverse toxic effect when used individually in products.46-48
The current FDA ruling on sunscreen labeling does not require manufacturers to state if inorganic UV filters have been formulated into nanoparticles; however, manufacturers are now required to include a statement on all sunscreen labels warning consumers to avoid using sunscreen on damaged or broken skin49 in an effort to prevent the active ingredients from getting under the skin, potentially causing inflammation and/or health risks, because available data do not provide conclusive evidence on increased penetration of open skin.50 Additional information regarding the 2011 FDA sunscreen ruling can be found in a prior Cutis Resident Corner column.51
Final Thoughts
As health care providers, we should take advantage of opportunities to educate our patients about other sun safety practices, such as avoiding excessive sun exposure during peak hours (10 am to 2 pm), seeking shade, and wearing photoprotective clothing (eg, wide-brimmed hats, sunglasses).
The research is quite clear: Using broadband sunscreens that absorb and/or block UV radiation results in reduced damage to the skin’s DNA, a fact that should be considered when taking into account the risks and benefits of sunscreen use.2,3 Although sunscreen use is highly recommended in addition to the other sun protection methods, it is ultimately the patient’s choice. If a patient is still concerned about the active ingredients of UV filters, even given the high probability of safety, there are products available on the market that do not include organic filters or nanoparticles. Given the established benefits of UV protection, the use of sunscreens remain one of the most important photoprotective methods, and with increased usage by the public, continuous monitoring of the overall safety and benefit profile of future products is prudent.
1. Skin cancer statistics. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/cancer/skin/statistics/index.htm. Updated September 2, 2014. Accessed December 30, 2014.
2. World Health Organization, International Agency for Research on Cancer. Solar and ultraviolet radiation. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 55. Lyon, France: International Agency for Research on Cancer; 1992.
3. Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29:257-263.
4. Darlington S, Williams G, Neale R, et al. A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Arch Dermatol. 2003;139:451-455.
5. Van der Pols JC, Williams GM, Pandeya N, et al. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev. 2006;15:2546-2548.
6. Hughes MC, Williams GM, Baker P, et al. Sunscreen and prevention of skin aging: a randomized trial. Ann Intern Med. 2013;158:781-790.
7. Bissonnette R, Nigen S, Bolduc C. Influence of the quantity of sunscreen applied on the ability to protect against ultraviolet-induced polymorphous light eruption. Photodermatol Photoimmunol Photomed. 2012;28:240-243.
8. Cancer trends progress report 2011/2012 update: sun protection. National Cancer Institute Web site. http://progressreport.cancer.gov/doc_detail.asp?pid¡1&did¡2009&chid¡91&coid¡911. Accessed December 30, 2014.
9. Sunscreen Drug Products for Over-the-counter Human Use, 21 CFR §352.10. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=352.10. Updated September 1, 2014. Accessed December 30, 2014.
10. Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photoimmunol Photomed. 2011;27:58-67.
11. Krause M, Klit A, Blomberg Jensen M, et al. Sunscreens: are they beneficial for health? an overview of endocrine disrupting properties of UV-filters. Int J Androl. 2012;35:424-436.
12. Schlumpf M, Cotton B, Conscience M, et al. In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect. 2001;109:239-244.
13. Schlumpf M, Schmid P, Durrer S, et al. Endocrine activity and developmental toxicity of cosmetic UV filters–an update. Toxicol. 2004;205:113-122.
14. Schlumpf M, Kypke K, Vökt C, et al. Endocrine active UV filters: developmental toxicity and exposure through breast milk. Chimia. 2008;62:345-351.
15. Nakagawa Y, Suzuki T. Metabolism of 2-hydroxy-4-methoxybenzophenone in isolated rat hepatocytes and xenoestrogenic effects of its metabolites on MCF-7 human breast cancer cells. Chem Biol Interact. 2002;139:115-128.
16. Ma R, Cotton B, Lichtensteiger W, et al. UV filters with antagonistic action at androgen receptors in the MDA-kb2 cell transcriptional-activation assay. Toxicol Sci. 2003;74:43-50.
17. Heneweer M, Muusse M, van den Berg M, et al. Additive estrogenic effects of mixtures of frequently used UV filters on pS2-gene transcription in MCF-7 cells. Toxicol Appl Pharmacol. 2005;208:170-177.
18. Knobler E, Almeida L, Ruzkowski AM, et al. Photoallergy to benzophenone. Arch Dermatol. 1989;125:801-804.
19. Draelos ZD. Are sunscreens safe? J Cosmet Dermatol. 2010;9:1-2.
20. Gilbert E, Pirot F, Bertholle V. Commonly used UV filter toxicity on biological functions: review of last decade studies. Int J of Cosmet Sci. 2013;35:208-219.
21. Wang SQ, Burnett ME, Lim HW. Safety of oxybenzone: putting numbers into perspective. Arch Dermatol. 2011;147:865-866.
22. Mancebo SE, Hu JY, Wang SQ. Sunscreens: a review of health benefits, regulations, and controversies. Dermatol Clin. 2014;32:427-438.
23. Jansen R, Osterwalder U, Wang SQ, et al. Photoprotection: part II. sunscreen: development, efficacy, and controversies. J Am Acad Dermatol. 2013;69:867.e1-867.e14.
24. Janjua NR, Mogensen B, Andersson AM, et al. Systemic absorption of the sunscreens benzo- phenone-3, octyl-methoxycinnamate, and 3-(4-methyl-benzy-lidene) camphor after whole-body topical application and reproductive hormone levels in humans. J Invest Dermatol. 2004;123:57-61.
25. Kadry AM, Chukwuemeka SO, Mohamed S, et al. Pharmacokinetics of benzophenone-3 after oral exposure in male rats. J Appl Toxicol. 1995;15:97-102.
26. Gulson B, McCall M, Korsch M, et al. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol Sci. 2010;118:140-149.
27. Gulson B, Wong H, Korsch M, et al. Comparison of dermal absorption of zinc from different sunscreen formulations and differing UV exposure based on stable isotope tracing. Sci Total Environ. 2012:420:313-318.
28. Benech-Kieffer F, Meuling WJ, Leclerc C, et al. Percutaneous absorption of Mexoryl SX in human volunteers: comparison with in vitro data. Skin Pharmacol Appl Skin Physiol. 2003;16:343-355.
29. Nash JF. Human safety and efficacy of ultraviolet filters and sunscreen products. Dermatol Clin. 2006;24:35-51.
30. Nohynek GJ, Lademann J, Ribaud C, et al. Grey goo on the skin? nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol. 2007;37:251-277.
31. Sadrieh N, Wokovich AM, Gopee NV, et al. Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles. Toxicol Sci. 2010;115:156-166.
32. International Agency for Research on Cancer. Carbon black, titanium dioxide, and talc. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 93. Lyon, France: International Agency for Research on Cancer; 2006.
33. Liao CM, Chiang YH, Chio CP. Model-based assessment for human inhalation exposure risk to airborne nano/fine titanium dioxide particles. Sci Total Environ. 2008:15;407:165-177.
34. Adamcakova-Dodd A, Stebounova LV, Kim JS, et al. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part Fibre Toxicol. 2014;11:15.
35. Wamer WG, Yin JJ, Wei RR. Oxidative damage to nucleic acids photosensitized by titanium dioxide. Free Radic Biol Med. 1997;23:851-858.
36. Nakagawa Y, Wakuri S, Sakamoto K, et al. The photogenotoxicity of titanium dioxide particles. Mutat Res. 1997;394:125-132.
37. Dunford R, Salinaro A, Cai L, et al. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997;418:87-90, 99.
38. Hidaka H, Kobayashi H, Koike T, et al. DNA damage photoinduced by cosmetic pigments and sunscreen agents under solar exposure and artificial UV illumination. J Oleo Sci. 2006;55:249-261.
39. Dufour EK, Kumaravel T, Nohynek GJ, et al. Clastogenicity, photo-clastogenicity or pseudo-photo-clastogenicity: genotoxic effects of zinc oxide in the dark, in pre-irradiated or simultaneously irradiated Chinese hamster ovary cells [published online ahead of print June 21, 2006]. Mutat Res. 2006;607:215-224.
40. Opinion of the Scientific Committee on Cosmetic Products and Non-Food Products intended for Consumers concerning titanium dioxide. http://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out135_en.pdf. Published October 24, 2000. Accessed December 30, 2014.
41. The Scientific Committee on Cosmetic Products and Non-Food Products intended for Consumers opinion concerning zinc oxide. http://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out222_en.pdf. Published June 24-25, 2003. Accessed December 30, 2014.
42. Hackenberg S, Friehs G, Kessler M, et al. Nanosized titanium dioxide particles do not induce DNA damage in human peripheral blood lymphocytes. Environ Mol Mutagen. 2010;52:264-268.
43. Bach-Thomsen M, Wulf HC. Sunbather’s application of sunscreen is probably inadequate to obtain the sun protection factor assigned to the preparation. Photodermatol Photoimmunol Photomed. 1993:9;242-244.
44. Nohynek GJ, Antignac E, Re T, et al. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol Appl Pharmacol. 2010:1;243:239-259.
45. Diffey BL. Sunscreens: use and misuse. In: Giacomoni PU, ed. Sun Protection in Man. Vol 3. Amsterdam, the Netherlands: Elsevier Science BV; 2001:521-534.
46. Heneweer M, Muusse M, Van den BM, et al. Additive estrogenic effects of mixtures of frequently used UV-filters on pS2-gene transcription in MCF-7 cells. Toxicol Appl Pharmacol. 2005;208:170-177.
47. Kunz PY, Galicia HF, Fent K. Comparison of in vitro and in vivo estrogenic activity of UV-filters in fish. Toxicol Sci. 2006;90:349-361.
48. Kortenkamp A, Faust M, Scholze M, et al. Low-level exposure to multiple chemicals: reason for human health concerns? Environ Health Perspect. 2007;115(suppl 1):106-114.
49. Labeling and effectiveness testing: sunscreen drug products for over-the-counter human use—small entity compliance guide. US Food and Drug Administration Web site. http://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/guidances/ucm330694.htm. Published December 2012. Updated May 13, 2014. Accessed December 30, 2014.
50. Schafer-Korting M, Korting HC, Ponce-Poschl E. Liposomal tretinoin for uncomplicated acne vulgaris. Clin Investig. 1994;72:1086-1091.
51. Bronfenbrener R. Simplifying sun safety: a guide to the new FDA sunscreen monograph. Cutis. 2014;93:e17-e19.
1. Skin cancer statistics. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/cancer/skin/statistics/index.htm. Updated September 2, 2014. Accessed December 30, 2014.
2. World Health Organization, International Agency for Research on Cancer. Solar and ultraviolet radiation. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 55. Lyon, France: International Agency for Research on Cancer; 1992.
3. Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29:257-263.
4. Darlington S, Williams G, Neale R, et al. A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Arch Dermatol. 2003;139:451-455.
5. Van der Pols JC, Williams GM, Pandeya N, et al. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev. 2006;15:2546-2548.
6. Hughes MC, Williams GM, Baker P, et al. Sunscreen and prevention of skin aging: a randomized trial. Ann Intern Med. 2013;158:781-790.
7. Bissonnette R, Nigen S, Bolduc C. Influence of the quantity of sunscreen applied on the ability to protect against ultraviolet-induced polymorphous light eruption. Photodermatol Photoimmunol Photomed. 2012;28:240-243.
8. Cancer trends progress report 2011/2012 update: sun protection. National Cancer Institute Web site. http://progressreport.cancer.gov/doc_detail.asp?pid¡1&did¡2009&chid¡91&coid¡911. Accessed December 30, 2014.
9. Sunscreen Drug Products for Over-the-counter Human Use, 21 CFR §352.10. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=352.10. Updated September 1, 2014. Accessed December 30, 2014.
10. Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photoimmunol Photomed. 2011;27:58-67.
11. Krause M, Klit A, Blomberg Jensen M, et al. Sunscreens: are they beneficial for health? an overview of endocrine disrupting properties of UV-filters. Int J Androl. 2012;35:424-436.
12. Schlumpf M, Cotton B, Conscience M, et al. In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect. 2001;109:239-244.
13. Schlumpf M, Schmid P, Durrer S, et al. Endocrine activity and developmental toxicity of cosmetic UV filters–an update. Toxicol. 2004;205:113-122.
14. Schlumpf M, Kypke K, Vökt C, et al. Endocrine active UV filters: developmental toxicity and exposure through breast milk. Chimia. 2008;62:345-351.
15. Nakagawa Y, Suzuki T. Metabolism of 2-hydroxy-4-methoxybenzophenone in isolated rat hepatocytes and xenoestrogenic effects of its metabolites on MCF-7 human breast cancer cells. Chem Biol Interact. 2002;139:115-128.
16. Ma R, Cotton B, Lichtensteiger W, et al. UV filters with antagonistic action at androgen receptors in the MDA-kb2 cell transcriptional-activation assay. Toxicol Sci. 2003;74:43-50.
17. Heneweer M, Muusse M, van den Berg M, et al. Additive estrogenic effects of mixtures of frequently used UV filters on pS2-gene transcription in MCF-7 cells. Toxicol Appl Pharmacol. 2005;208:170-177.
18. Knobler E, Almeida L, Ruzkowski AM, et al. Photoallergy to benzophenone. Arch Dermatol. 1989;125:801-804.
19. Draelos ZD. Are sunscreens safe? J Cosmet Dermatol. 2010;9:1-2.
20. Gilbert E, Pirot F, Bertholle V. Commonly used UV filter toxicity on biological functions: review of last decade studies. Int J of Cosmet Sci. 2013;35:208-219.
21. Wang SQ, Burnett ME, Lim HW. Safety of oxybenzone: putting numbers into perspective. Arch Dermatol. 2011;147:865-866.
22. Mancebo SE, Hu JY, Wang SQ. Sunscreens: a review of health benefits, regulations, and controversies. Dermatol Clin. 2014;32:427-438.
23. Jansen R, Osterwalder U, Wang SQ, et al. Photoprotection: part II. sunscreen: development, efficacy, and controversies. J Am Acad Dermatol. 2013;69:867.e1-867.e14.
24. Janjua NR, Mogensen B, Andersson AM, et al. Systemic absorption of the sunscreens benzo- phenone-3, octyl-methoxycinnamate, and 3-(4-methyl-benzy-lidene) camphor after whole-body topical application and reproductive hormone levels in humans. J Invest Dermatol. 2004;123:57-61.
25. Kadry AM, Chukwuemeka SO, Mohamed S, et al. Pharmacokinetics of benzophenone-3 after oral exposure in male rats. J Appl Toxicol. 1995;15:97-102.
26. Gulson B, McCall M, Korsch M, et al. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol Sci. 2010;118:140-149.
27. Gulson B, Wong H, Korsch M, et al. Comparison of dermal absorption of zinc from different sunscreen formulations and differing UV exposure based on stable isotope tracing. Sci Total Environ. 2012:420:313-318.
28. Benech-Kieffer F, Meuling WJ, Leclerc C, et al. Percutaneous absorption of Mexoryl SX in human volunteers: comparison with in vitro data. Skin Pharmacol Appl Skin Physiol. 2003;16:343-355.
29. Nash JF. Human safety and efficacy of ultraviolet filters and sunscreen products. Dermatol Clin. 2006;24:35-51.
30. Nohynek GJ, Lademann J, Ribaud C, et al. Grey goo on the skin? nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol. 2007;37:251-277.
31. Sadrieh N, Wokovich AM, Gopee NV, et al. Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles. Toxicol Sci. 2010;115:156-166.
32. International Agency for Research on Cancer. Carbon black, titanium dioxide, and talc. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 93. Lyon, France: International Agency for Research on Cancer; 2006.
33. Liao CM, Chiang YH, Chio CP. Model-based assessment for human inhalation exposure risk to airborne nano/fine titanium dioxide particles. Sci Total Environ. 2008:15;407:165-177.
34. Adamcakova-Dodd A, Stebounova LV, Kim JS, et al. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part Fibre Toxicol. 2014;11:15.
35. Wamer WG, Yin JJ, Wei RR. Oxidative damage to nucleic acids photosensitized by titanium dioxide. Free Radic Biol Med. 1997;23:851-858.
36. Nakagawa Y, Wakuri S, Sakamoto K, et al. The photogenotoxicity of titanium dioxide particles. Mutat Res. 1997;394:125-132.
37. Dunford R, Salinaro A, Cai L, et al. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997;418:87-90, 99.
38. Hidaka H, Kobayashi H, Koike T, et al. DNA damage photoinduced by cosmetic pigments and sunscreen agents under solar exposure and artificial UV illumination. J Oleo Sci. 2006;55:249-261.
39. Dufour EK, Kumaravel T, Nohynek GJ, et al. Clastogenicity, photo-clastogenicity or pseudo-photo-clastogenicity: genotoxic effects of zinc oxide in the dark, in pre-irradiated or simultaneously irradiated Chinese hamster ovary cells [published online ahead of print June 21, 2006]. Mutat Res. 2006;607:215-224.
40. Opinion of the Scientific Committee on Cosmetic Products and Non-Food Products intended for Consumers concerning titanium dioxide. http://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out135_en.pdf. Published October 24, 2000. Accessed December 30, 2014.
41. The Scientific Committee on Cosmetic Products and Non-Food Products intended for Consumers opinion concerning zinc oxide. http://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out222_en.pdf. Published June 24-25, 2003. Accessed December 30, 2014.
42. Hackenberg S, Friehs G, Kessler M, et al. Nanosized titanium dioxide particles do not induce DNA damage in human peripheral blood lymphocytes. Environ Mol Mutagen. 2010;52:264-268.
43. Bach-Thomsen M, Wulf HC. Sunbather’s application of sunscreen is probably inadequate to obtain the sun protection factor assigned to the preparation. Photodermatol Photoimmunol Photomed. 1993:9;242-244.
44. Nohynek GJ, Antignac E, Re T, et al. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol Appl Pharmacol. 2010:1;243:239-259.
45. Diffey BL. Sunscreens: use and misuse. In: Giacomoni PU, ed. Sun Protection in Man. Vol 3. Amsterdam, the Netherlands: Elsevier Science BV; 2001:521-534.
46. Heneweer M, Muusse M, Van den BM, et al. Additive estrogenic effects of mixtures of frequently used UV-filters on pS2-gene transcription in MCF-7 cells. Toxicol Appl Pharmacol. 2005;208:170-177.
47. Kunz PY, Galicia HF, Fent K. Comparison of in vitro and in vivo estrogenic activity of UV-filters in fish. Toxicol Sci. 2006;90:349-361.
48. Kortenkamp A, Faust M, Scholze M, et al. Low-level exposure to multiple chemicals: reason for human health concerns? Environ Health Perspect. 2007;115(suppl 1):106-114.
49. Labeling and effectiveness testing: sunscreen drug products for over-the-counter human use—small entity compliance guide. US Food and Drug Administration Web site. http://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/guidances/ucm330694.htm. Published December 2012. Updated May 13, 2014. Accessed December 30, 2014.
50. Schafer-Korting M, Korting HC, Ponce-Poschl E. Liposomal tretinoin for uncomplicated acne vulgaris. Clin Investig. 1994;72:1086-1091.
51. Bronfenbrener R. Simplifying sun safety: a guide to the new FDA sunscreen monograph. Cutis. 2014;93:e17-e19.
Mango
Mangifera indica (mango) is a member of the Anacardiaceae family with a tradition of use as a medicinal plant. Mango extracts have been characterized as exhibiting antioxidant, anti-inflammatory, analgesic, and immunomodulatory activities (Photodermatol. Photoimmunol. Photomed. 2013;29:84-9; Drug Chem. Toxicol. 2009;32:53-8). Mango is grown in more than 100 countries, primarily in Asia, in tropical as well as subtropical regions (Molecules 2014;19:17107-29). Mango stem bark and leaves have been used in traditional medicine to treat anemia, cutaneous infections, diabetes, diarrhea, scabies, syphilis, and malignant tumors (Pharmacol. Res. 2007;55:351-8). Polyphenols and carotenoids are among the phytonutrients identified as responsible for the biologic activity of mango (Photodermatol. Photoimmunol. Photomed. 2013;29:84-9).
Various biologic activities and traditional uses
Ojewole investigated the anti-inflammatory, analgesic, and antidiabetic activity of M. indica stem bark aqueous extract in rats and mice in 2005. In mice, mango extract dose-dependently delivered significant analgesic effects against thermally and chemically-generated pain. The investigators attributed the observed salutary effects of the plant to its constituent polyphenolics, flavonoids, triterpenoids, and mangiferin. They also noted that their findings support the folkloric uses of the plant for treating arthritic and other inflammatory conditions, as well as type 2 diabetes (Methods Find. Exp. Clin. Pharmacol. 2005;27:547-54).
Another important constituent of mango (also found in olive, strawberry, fig, and various medicinal herbs) is the triterpene lupeol, which has been characterized as exhibiting potent antioxidant, antimutagenic, anti-inflammatory, and antiarthritic activity (Oncogene 2004;23:5203-14). A 2014 study by Sahu et al. also showed that M. indica leaves display some antityrosinase activity, though not as strongly as other medicinal plants, such as Emblica officinalis (Pak. J. Biol. Sci. 2014;17:146-50).
Anticancer, antioxidant, and antiphotoaging activity
In 2004, Saleem et al. demonstrated that topically applied lupeol exhibited anti–tumor-promoting effects in a CD-1 mouse skin tumorigenesis model. Pretreatment with the mango constituent time- and dose-dependently inhibited multiple 12-O-tetradecanoyl-phorbol-13-acetate (TPA)-mediated increases in edema, hyperplasia, epidermal ornithine decarboxylase (ODC) activity, as well as protein expression of ODC, cyclooxygenase 2 (COX-2) and nitric oxide synthase. Pretreated animals also experienced significantly lower tumor incidence and tumor body burden as well as a significant delay in tumor latency period. The researchers concluded that lupeol exerts anti–skin tumor promoting effects on CD-1 mice (Oncogene 2004;23:5203-14).
Three years later, Núñez-Sellés et al. reported that a mango stem bark extract (Vimang) developed in Cuba exhibited antioxidant, analgesic, anti-inflammatory, and immunomodulating activity in basic, preclinical, and clinical studies (Pharmacol. Res. 2007;55:351-8).
A 2009 toxicological analysis of Vimang, which has been formulated into tablets, creams, capsules, syrup, vaginal oval, and suppositories for various applications, revealed via irritant tests conducted on rabbits that the topical formulation was not irritating to the skin, generally, with minimal irritancy noted after vaginal application. No adverse effects were reported (Drug Chem. Toxicol. 2009;32:53-8).
In 2012, Li et al. discovered norathyriol (1,3,6,7-tetrahydroxy-9H-xanthen-9-one), a plant-derived chemopreventive metabolite of mangiferin, found in mango, Hypericum elegans, and Tripterospermum lanceolatum. They found that norathyriol significantly inhibited solar UV-induced skin carcinogenesis in mouse models. In vitro investigations revealed that the compound suppressed cell growth in mouse skin epidermal JB6 P+ cells at the level of G2-M phase arrest. The investigators concluded that this newly identified substance appears to act as a safe chemopreventive agent against UV-induced skin cancer (Cancer Res. 2012;72:260-70).
A year later, Song et al. assessed the protective effects of orally administered mango extract against UVB-induced cutaneous aging in HR-1 hairless male mice. The animals were divided into control, UVB-treated vehicle, and UVB-treated mango extract groups. The researchers found that mango extract significantly suppressed the increase in epidermal thickness and hypertrophy indicative of UVB treatment, with mean length of wrinkles significantly lower in the mango group compared with the UVB-treated vehicle group. Treatment with mango extract also led to a significant increase in collagen bundles in animals treated with UVB. The authors concluded that mango extract displayed antiphotoaging properties in hairless mice exposed to UVB (Photodermatol. Photoimmunol. Photomed. 2013;29:84-9).
Further, a 2014 in vitro study revealed that extracts of Helicanthus elastica growing on M. indica exhibited antioxidant activity. H. elastica is a hemiparasite that often grows on mango trees in India and is known to be a rich source of phenolic substances (J. Tradit. Complement. Med. 2014;4:285-8).
Topical delivery
Mandawgade and Patravale developed a mango butter skin care formulation in 2008 that was used to test skin repair in rat excision and incision wound models. A healing response was noted in both animal models. The formulation also was found to be effective in achieving complete repair of worn and cracked skin on the feet of all human volunteers in the study. The investigators concluded that the mango butter preparation delivers superlative emolliency and warrants consideration as an excipient agent in cosmeceutical products (Indian J. Pharm. Sci. 2008;70:539-42).
It is worth noting that cases of “mango dermatitis” (allergic contact dermatitis to the sap or skin of M. indica), manifesting in urticaria and eczematous rashes, have been reported (Australas. J. Dermatol. 1996;37:59-60; Int. J. Dermatol. 2004;43:195-6).
In 2014, Leanpolchareanchai et al. developed a microemulsion system containing Thai mango seed kernel extract that displayed strong skin enhancement results in ex vivo skin permeation studies (penetrating skin layers up to 60-fold higher than controls) and physicochemical stability over 6 months (Drug Chem. Toxicol. 2009;32:53-8). Thai mango seed kernel extract had previously been shown to exhibit anti–methicillin-resistant Staphylococcus aureus and antityrosinase characteristics, as well as strong free radical scavenging, antioxidant, anti-inflammatory, and hepatoprotective activities.(Molecules 2014;19:17107-29).
Conclusion
Evidence on the cutaneous applications of mango is emerging, but does not have a significant track record. That said, this fruit has long been used in traditional medicine for a range of indications, including skin disorders. Much more research is necessary, however, to ascertain how beneficial this fruit and its extracts may be. At the very least, there are few reports of adverse events associated with topical application.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Mangifera indica (mango) is a member of the Anacardiaceae family with a tradition of use as a medicinal plant. Mango extracts have been characterized as exhibiting antioxidant, anti-inflammatory, analgesic, and immunomodulatory activities (Photodermatol. Photoimmunol. Photomed. 2013;29:84-9; Drug Chem. Toxicol. 2009;32:53-8). Mango is grown in more than 100 countries, primarily in Asia, in tropical as well as subtropical regions (Molecules 2014;19:17107-29). Mango stem bark and leaves have been used in traditional medicine to treat anemia, cutaneous infections, diabetes, diarrhea, scabies, syphilis, and malignant tumors (Pharmacol. Res. 2007;55:351-8). Polyphenols and carotenoids are among the phytonutrients identified as responsible for the biologic activity of mango (Photodermatol. Photoimmunol. Photomed. 2013;29:84-9).
Various biologic activities and traditional uses
Ojewole investigated the anti-inflammatory, analgesic, and antidiabetic activity of M. indica stem bark aqueous extract in rats and mice in 2005. In mice, mango extract dose-dependently delivered significant analgesic effects against thermally and chemically-generated pain. The investigators attributed the observed salutary effects of the plant to its constituent polyphenolics, flavonoids, triterpenoids, and mangiferin. They also noted that their findings support the folkloric uses of the plant for treating arthritic and other inflammatory conditions, as well as type 2 diabetes (Methods Find. Exp. Clin. Pharmacol. 2005;27:547-54).
Another important constituent of mango (also found in olive, strawberry, fig, and various medicinal herbs) is the triterpene lupeol, which has been characterized as exhibiting potent antioxidant, antimutagenic, anti-inflammatory, and antiarthritic activity (Oncogene 2004;23:5203-14). A 2014 study by Sahu et al. also showed that M. indica leaves display some antityrosinase activity, though not as strongly as other medicinal plants, such as Emblica officinalis (Pak. J. Biol. Sci. 2014;17:146-50).
Anticancer, antioxidant, and antiphotoaging activity
In 2004, Saleem et al. demonstrated that topically applied lupeol exhibited anti–tumor-promoting effects in a CD-1 mouse skin tumorigenesis model. Pretreatment with the mango constituent time- and dose-dependently inhibited multiple 12-O-tetradecanoyl-phorbol-13-acetate (TPA)-mediated increases in edema, hyperplasia, epidermal ornithine decarboxylase (ODC) activity, as well as protein expression of ODC, cyclooxygenase 2 (COX-2) and nitric oxide synthase. Pretreated animals also experienced significantly lower tumor incidence and tumor body burden as well as a significant delay in tumor latency period. The researchers concluded that lupeol exerts anti–skin tumor promoting effects on CD-1 mice (Oncogene 2004;23:5203-14).
Three years later, Núñez-Sellés et al. reported that a mango stem bark extract (Vimang) developed in Cuba exhibited antioxidant, analgesic, anti-inflammatory, and immunomodulating activity in basic, preclinical, and clinical studies (Pharmacol. Res. 2007;55:351-8).
A 2009 toxicological analysis of Vimang, which has been formulated into tablets, creams, capsules, syrup, vaginal oval, and suppositories for various applications, revealed via irritant tests conducted on rabbits that the topical formulation was not irritating to the skin, generally, with minimal irritancy noted after vaginal application. No adverse effects were reported (Drug Chem. Toxicol. 2009;32:53-8).
In 2012, Li et al. discovered norathyriol (1,3,6,7-tetrahydroxy-9H-xanthen-9-one), a plant-derived chemopreventive metabolite of mangiferin, found in mango, Hypericum elegans, and Tripterospermum lanceolatum. They found that norathyriol significantly inhibited solar UV-induced skin carcinogenesis in mouse models. In vitro investigations revealed that the compound suppressed cell growth in mouse skin epidermal JB6 P+ cells at the level of G2-M phase arrest. The investigators concluded that this newly identified substance appears to act as a safe chemopreventive agent against UV-induced skin cancer (Cancer Res. 2012;72:260-70).
A year later, Song et al. assessed the protective effects of orally administered mango extract against UVB-induced cutaneous aging in HR-1 hairless male mice. The animals were divided into control, UVB-treated vehicle, and UVB-treated mango extract groups. The researchers found that mango extract significantly suppressed the increase in epidermal thickness and hypertrophy indicative of UVB treatment, with mean length of wrinkles significantly lower in the mango group compared with the UVB-treated vehicle group. Treatment with mango extract also led to a significant increase in collagen bundles in animals treated with UVB. The authors concluded that mango extract displayed antiphotoaging properties in hairless mice exposed to UVB (Photodermatol. Photoimmunol. Photomed. 2013;29:84-9).
Further, a 2014 in vitro study revealed that extracts of Helicanthus elastica growing on M. indica exhibited antioxidant activity. H. elastica is a hemiparasite that often grows on mango trees in India and is known to be a rich source of phenolic substances (J. Tradit. Complement. Med. 2014;4:285-8).
Topical delivery
Mandawgade and Patravale developed a mango butter skin care formulation in 2008 that was used to test skin repair in rat excision and incision wound models. A healing response was noted in both animal models. The formulation also was found to be effective in achieving complete repair of worn and cracked skin on the feet of all human volunteers in the study. The investigators concluded that the mango butter preparation delivers superlative emolliency and warrants consideration as an excipient agent in cosmeceutical products (Indian J. Pharm. Sci. 2008;70:539-42).
It is worth noting that cases of “mango dermatitis” (allergic contact dermatitis to the sap or skin of M. indica), manifesting in urticaria and eczematous rashes, have been reported (Australas. J. Dermatol. 1996;37:59-60; Int. J. Dermatol. 2004;43:195-6).
In 2014, Leanpolchareanchai et al. developed a microemulsion system containing Thai mango seed kernel extract that displayed strong skin enhancement results in ex vivo skin permeation studies (penetrating skin layers up to 60-fold higher than controls) and physicochemical stability over 6 months (Drug Chem. Toxicol. 2009;32:53-8). Thai mango seed kernel extract had previously been shown to exhibit anti–methicillin-resistant Staphylococcus aureus and antityrosinase characteristics, as well as strong free radical scavenging, antioxidant, anti-inflammatory, and hepatoprotective activities.(Molecules 2014;19:17107-29).
Conclusion
Evidence on the cutaneous applications of mango is emerging, but does not have a significant track record. That said, this fruit has long been used in traditional medicine for a range of indications, including skin disorders. Much more research is necessary, however, to ascertain how beneficial this fruit and its extracts may be. At the very least, there are few reports of adverse events associated with topical application.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Mangifera indica (mango) is a member of the Anacardiaceae family with a tradition of use as a medicinal plant. Mango extracts have been characterized as exhibiting antioxidant, anti-inflammatory, analgesic, and immunomodulatory activities (Photodermatol. Photoimmunol. Photomed. 2013;29:84-9; Drug Chem. Toxicol. 2009;32:53-8). Mango is grown in more than 100 countries, primarily in Asia, in tropical as well as subtropical regions (Molecules 2014;19:17107-29). Mango stem bark and leaves have been used in traditional medicine to treat anemia, cutaneous infections, diabetes, diarrhea, scabies, syphilis, and malignant tumors (Pharmacol. Res. 2007;55:351-8). Polyphenols and carotenoids are among the phytonutrients identified as responsible for the biologic activity of mango (Photodermatol. Photoimmunol. Photomed. 2013;29:84-9).
Various biologic activities and traditional uses
Ojewole investigated the anti-inflammatory, analgesic, and antidiabetic activity of M. indica stem bark aqueous extract in rats and mice in 2005. In mice, mango extract dose-dependently delivered significant analgesic effects against thermally and chemically-generated pain. The investigators attributed the observed salutary effects of the plant to its constituent polyphenolics, flavonoids, triterpenoids, and mangiferin. They also noted that their findings support the folkloric uses of the plant for treating arthritic and other inflammatory conditions, as well as type 2 diabetes (Methods Find. Exp. Clin. Pharmacol. 2005;27:547-54).
Another important constituent of mango (also found in olive, strawberry, fig, and various medicinal herbs) is the triterpene lupeol, which has been characterized as exhibiting potent antioxidant, antimutagenic, anti-inflammatory, and antiarthritic activity (Oncogene 2004;23:5203-14). A 2014 study by Sahu et al. also showed that M. indica leaves display some antityrosinase activity, though not as strongly as other medicinal plants, such as Emblica officinalis (Pak. J. Biol. Sci. 2014;17:146-50).
Anticancer, antioxidant, and antiphotoaging activity
In 2004, Saleem et al. demonstrated that topically applied lupeol exhibited anti–tumor-promoting effects in a CD-1 mouse skin tumorigenesis model. Pretreatment with the mango constituent time- and dose-dependently inhibited multiple 12-O-tetradecanoyl-phorbol-13-acetate (TPA)-mediated increases in edema, hyperplasia, epidermal ornithine decarboxylase (ODC) activity, as well as protein expression of ODC, cyclooxygenase 2 (COX-2) and nitric oxide synthase. Pretreated animals also experienced significantly lower tumor incidence and tumor body burden as well as a significant delay in tumor latency period. The researchers concluded that lupeol exerts anti–skin tumor promoting effects on CD-1 mice (Oncogene 2004;23:5203-14).
Three years later, Núñez-Sellés et al. reported that a mango stem bark extract (Vimang) developed in Cuba exhibited antioxidant, analgesic, anti-inflammatory, and immunomodulating activity in basic, preclinical, and clinical studies (Pharmacol. Res. 2007;55:351-8).
A 2009 toxicological analysis of Vimang, which has been formulated into tablets, creams, capsules, syrup, vaginal oval, and suppositories for various applications, revealed via irritant tests conducted on rabbits that the topical formulation was not irritating to the skin, generally, with minimal irritancy noted after vaginal application. No adverse effects were reported (Drug Chem. Toxicol. 2009;32:53-8).
In 2012, Li et al. discovered norathyriol (1,3,6,7-tetrahydroxy-9H-xanthen-9-one), a plant-derived chemopreventive metabolite of mangiferin, found in mango, Hypericum elegans, and Tripterospermum lanceolatum. They found that norathyriol significantly inhibited solar UV-induced skin carcinogenesis in mouse models. In vitro investigations revealed that the compound suppressed cell growth in mouse skin epidermal JB6 P+ cells at the level of G2-M phase arrest. The investigators concluded that this newly identified substance appears to act as a safe chemopreventive agent against UV-induced skin cancer (Cancer Res. 2012;72:260-70).
A year later, Song et al. assessed the protective effects of orally administered mango extract against UVB-induced cutaneous aging in HR-1 hairless male mice. The animals were divided into control, UVB-treated vehicle, and UVB-treated mango extract groups. The researchers found that mango extract significantly suppressed the increase in epidermal thickness and hypertrophy indicative of UVB treatment, with mean length of wrinkles significantly lower in the mango group compared with the UVB-treated vehicle group. Treatment with mango extract also led to a significant increase in collagen bundles in animals treated with UVB. The authors concluded that mango extract displayed antiphotoaging properties in hairless mice exposed to UVB (Photodermatol. Photoimmunol. Photomed. 2013;29:84-9).
Further, a 2014 in vitro study revealed that extracts of Helicanthus elastica growing on M. indica exhibited antioxidant activity. H. elastica is a hemiparasite that often grows on mango trees in India and is known to be a rich source of phenolic substances (J. Tradit. Complement. Med. 2014;4:285-8).
Topical delivery
Mandawgade and Patravale developed a mango butter skin care formulation in 2008 that was used to test skin repair in rat excision and incision wound models. A healing response was noted in both animal models. The formulation also was found to be effective in achieving complete repair of worn and cracked skin on the feet of all human volunteers in the study. The investigators concluded that the mango butter preparation delivers superlative emolliency and warrants consideration as an excipient agent in cosmeceutical products (Indian J. Pharm. Sci. 2008;70:539-42).
It is worth noting that cases of “mango dermatitis” (allergic contact dermatitis to the sap or skin of M. indica), manifesting in urticaria and eczematous rashes, have been reported (Australas. J. Dermatol. 1996;37:59-60; Int. J. Dermatol. 2004;43:195-6).
In 2014, Leanpolchareanchai et al. developed a microemulsion system containing Thai mango seed kernel extract that displayed strong skin enhancement results in ex vivo skin permeation studies (penetrating skin layers up to 60-fold higher than controls) and physicochemical stability over 6 months (Drug Chem. Toxicol. 2009;32:53-8). Thai mango seed kernel extract had previously been shown to exhibit anti–methicillin-resistant Staphylococcus aureus and antityrosinase characteristics, as well as strong free radical scavenging, antioxidant, anti-inflammatory, and hepatoprotective activities.(Molecules 2014;19:17107-29).
Conclusion
Evidence on the cutaneous applications of mango is emerging, but does not have a significant track record. That said, this fruit has long been used in traditional medicine for a range of indications, including skin disorders. Much more research is necessary, however, to ascertain how beneficial this fruit and its extracts may be. At the very least, there are few reports of adverse events associated with topical application.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Microneedling
Microneedling, or skin needling, is an aesthetic technique used for decades prior to resurfacing lasers, but it has recently experienced a surge in popularity, particularly for ethnic skin. In 1995, subcision or dermal needling was identified as an effective treatment for scars. Since then, the technique initially referred to as collagen induction therapy has become a staple in the treatment of acne scars, surgical scars, photo aging, and stretch marks.
The skin needling technique involves using fine sterile needles 0.1mm-2.5 mm in length that repeatedly pierce the stratum corneum, producing microscopic “holes” in the dermis. These microscopic wounds lead to the release of growth factors stimulating the formation of new collagen, elastin, and neovascularization in the dermis. There are many brands and manufacturers of microneedling tools on the market, including dermarollers, Dermapen, Dermastamp, Cosmopen, and multiple other in-office and at-home devices. At-home devices usually have shorter needles and provide significantly less penetration and injury, and therefore may be less effective.
Prior to the procedure, patients are often anesthetized with topical anesthesia without vasoconstrictors for 1 hour. The area is cleaned with sterile gauze and alcohol or Hibiclens, and a microneedling device is used to either roll or prick the skin in multiple alternating passes. The depth of penetration, number of passes, and degree of overlap is highly dependent on the underlying condition, the area being treated, the brand of device used, and the length and frequency of the needle insertion. Petechiae and pinpoint bleeding occur during the treatment. Treatments are usually done 4-6 weeks apart. Post procedure, the patient often experiences mild erythema, bruising, and some mild edema.
This technique has been particularly beneficial to patients with skin of color who are not candidates for factional lasers because of the risks of hyperpigmentation and scarring. There is low risk of hyper- or hypopigmentation with microneedling, and multiple treatments can be performed in patients with types III-VI skin and those with a history of melasma.
Contraindications and precautions when considering microneedling include: history of keloid or hypertrophic scarring,recent skin rashes, history of herpes simplex infections if the perioral area is being treated, and the presence of raised moles, warts, or any raised lesions on the targeted area. Absolute contraindications include: scleroderma, collagen vascular diseases clotting problems, active bacterial or fungal infection, and immunosuppression.
Microneedling is a safe, effective, in-office procedure with a range of uses. Many new indications are currently being explored. In my practice, we have used microneedling for atrophic scars, repigmentation of depigmented scars and vitiligo, stimulation of hair regrowth in noninflammatory alopecias, and treatment of burn scars. Patients are generally very happy with the quick treatment time, minimal downtime, and overall long-term results.
References
1. Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol. Surg. 1995;21:6543-9.
2. Camirand A, Doucet J. Needle dermabrasion. Aesthetic Plast. Surg. 1997;21:48-51.
3. Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac. Surg. Clin. North Am. 2006;17:51-63.
4. Aust MC, Fernandes D, Kolokythas P, Kaplan HM, Vogt PM. Percutaneous collagen induction therapy: An alternative treatment for scars, wrinkles and skin laxity. Plast. Reconstr. Surg. 2008;21:1421-9.
5. Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin. Dermatol. 2008;26:192-9.
6. Aust MC, Reimers K, Repenning C, Stahl F, Jahn S, Guggenheim M et al. Percutaneous collagen induction: Minimally invasive skin rejuvenation without risk of hyperpigmentation – fact or fiction? Plast. Reconstr. Surg. 2008;122:1553-63.
7. Fabbrocini G, De Vita V, Pastore F, et al. Collagen induction therapy for the treatment of upper lip wrinkles. J. Dermatolog. Treat. 2012;23:144-52. 8. Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J. Cutan. Aesthet. Surg. 2009;2:26-30.
9. Doddaballapur S. Microneedling with dermaroller. J. Cutan. Aesthet. Surg 2009;2: 110-11.
10. Dogra S, Yadav S. Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J. Cosmet. Dermatol. 2014;13:180-7.
Dr. Talakoub and Dr. Wesley are cocontributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
Microneedling, or skin needling, is an aesthetic technique used for decades prior to resurfacing lasers, but it has recently experienced a surge in popularity, particularly for ethnic skin. In 1995, subcision or dermal needling was identified as an effective treatment for scars. Since then, the technique initially referred to as collagen induction therapy has become a staple in the treatment of acne scars, surgical scars, photo aging, and stretch marks.
The skin needling technique involves using fine sterile needles 0.1mm-2.5 mm in length that repeatedly pierce the stratum corneum, producing microscopic “holes” in the dermis. These microscopic wounds lead to the release of growth factors stimulating the formation of new collagen, elastin, and neovascularization in the dermis. There are many brands and manufacturers of microneedling tools on the market, including dermarollers, Dermapen, Dermastamp, Cosmopen, and multiple other in-office and at-home devices. At-home devices usually have shorter needles and provide significantly less penetration and injury, and therefore may be less effective.
Prior to the procedure, patients are often anesthetized with topical anesthesia without vasoconstrictors for 1 hour. The area is cleaned with sterile gauze and alcohol or Hibiclens, and a microneedling device is used to either roll or prick the skin in multiple alternating passes. The depth of penetration, number of passes, and degree of overlap is highly dependent on the underlying condition, the area being treated, the brand of device used, and the length and frequency of the needle insertion. Petechiae and pinpoint bleeding occur during the treatment. Treatments are usually done 4-6 weeks apart. Post procedure, the patient often experiences mild erythema, bruising, and some mild edema.
This technique has been particularly beneficial to patients with skin of color who are not candidates for factional lasers because of the risks of hyperpigmentation and scarring. There is low risk of hyper- or hypopigmentation with microneedling, and multiple treatments can be performed in patients with types III-VI skin and those with a history of melasma.
Contraindications and precautions when considering microneedling include: history of keloid or hypertrophic scarring,recent skin rashes, history of herpes simplex infections if the perioral area is being treated, and the presence of raised moles, warts, or any raised lesions on the targeted area. Absolute contraindications include: scleroderma, collagen vascular diseases clotting problems, active bacterial or fungal infection, and immunosuppression.
Microneedling is a safe, effective, in-office procedure with a range of uses. Many new indications are currently being explored. In my practice, we have used microneedling for atrophic scars, repigmentation of depigmented scars and vitiligo, stimulation of hair regrowth in noninflammatory alopecias, and treatment of burn scars. Patients are generally very happy with the quick treatment time, minimal downtime, and overall long-term results.
References
1. Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol. Surg. 1995;21:6543-9.
2. Camirand A, Doucet J. Needle dermabrasion. Aesthetic Plast. Surg. 1997;21:48-51.
3. Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac. Surg. Clin. North Am. 2006;17:51-63.
4. Aust MC, Fernandes D, Kolokythas P, Kaplan HM, Vogt PM. Percutaneous collagen induction therapy: An alternative treatment for scars, wrinkles and skin laxity. Plast. Reconstr. Surg. 2008;21:1421-9.
5. Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin. Dermatol. 2008;26:192-9.
6. Aust MC, Reimers K, Repenning C, Stahl F, Jahn S, Guggenheim M et al. Percutaneous collagen induction: Minimally invasive skin rejuvenation without risk of hyperpigmentation – fact or fiction? Plast. Reconstr. Surg. 2008;122:1553-63.
7. Fabbrocini G, De Vita V, Pastore F, et al. Collagen induction therapy for the treatment of upper lip wrinkles. J. Dermatolog. Treat. 2012;23:144-52. 8. Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J. Cutan. Aesthet. Surg. 2009;2:26-30.
9. Doddaballapur S. Microneedling with dermaroller. J. Cutan. Aesthet. Surg 2009;2: 110-11.
10. Dogra S, Yadav S. Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J. Cosmet. Dermatol. 2014;13:180-7.
Dr. Talakoub and Dr. Wesley are cocontributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
Microneedling, or skin needling, is an aesthetic technique used for decades prior to resurfacing lasers, but it has recently experienced a surge in popularity, particularly for ethnic skin. In 1995, subcision or dermal needling was identified as an effective treatment for scars. Since then, the technique initially referred to as collagen induction therapy has become a staple in the treatment of acne scars, surgical scars, photo aging, and stretch marks.
The skin needling technique involves using fine sterile needles 0.1mm-2.5 mm in length that repeatedly pierce the stratum corneum, producing microscopic “holes” in the dermis. These microscopic wounds lead to the release of growth factors stimulating the formation of new collagen, elastin, and neovascularization in the dermis. There are many brands and manufacturers of microneedling tools on the market, including dermarollers, Dermapen, Dermastamp, Cosmopen, and multiple other in-office and at-home devices. At-home devices usually have shorter needles and provide significantly less penetration and injury, and therefore may be less effective.
Prior to the procedure, patients are often anesthetized with topical anesthesia without vasoconstrictors for 1 hour. The area is cleaned with sterile gauze and alcohol or Hibiclens, and a microneedling device is used to either roll or prick the skin in multiple alternating passes. The depth of penetration, number of passes, and degree of overlap is highly dependent on the underlying condition, the area being treated, the brand of device used, and the length and frequency of the needle insertion. Petechiae and pinpoint bleeding occur during the treatment. Treatments are usually done 4-6 weeks apart. Post procedure, the patient often experiences mild erythema, bruising, and some mild edema.
This technique has been particularly beneficial to patients with skin of color who are not candidates for factional lasers because of the risks of hyperpigmentation and scarring. There is low risk of hyper- or hypopigmentation with microneedling, and multiple treatments can be performed in patients with types III-VI skin and those with a history of melasma.
Contraindications and precautions when considering microneedling include: history of keloid or hypertrophic scarring,recent skin rashes, history of herpes simplex infections if the perioral area is being treated, and the presence of raised moles, warts, or any raised lesions on the targeted area. Absolute contraindications include: scleroderma, collagen vascular diseases clotting problems, active bacterial or fungal infection, and immunosuppression.
Microneedling is a safe, effective, in-office procedure with a range of uses. Many new indications are currently being explored. In my practice, we have used microneedling for atrophic scars, repigmentation of depigmented scars and vitiligo, stimulation of hair regrowth in noninflammatory alopecias, and treatment of burn scars. Patients are generally very happy with the quick treatment time, minimal downtime, and overall long-term results.
References
1. Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol. Surg. 1995;21:6543-9.
2. Camirand A, Doucet J. Needle dermabrasion. Aesthetic Plast. Surg. 1997;21:48-51.
3. Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac. Surg. Clin. North Am. 2006;17:51-63.
4. Aust MC, Fernandes D, Kolokythas P, Kaplan HM, Vogt PM. Percutaneous collagen induction therapy: An alternative treatment for scars, wrinkles and skin laxity. Plast. Reconstr. Surg. 2008;21:1421-9.
5. Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin. Dermatol. 2008;26:192-9.
6. Aust MC, Reimers K, Repenning C, Stahl F, Jahn S, Guggenheim M et al. Percutaneous collagen induction: Minimally invasive skin rejuvenation without risk of hyperpigmentation – fact or fiction? Plast. Reconstr. Surg. 2008;122:1553-63.
7. Fabbrocini G, De Vita V, Pastore F, et al. Collagen induction therapy for the treatment of upper lip wrinkles. J. Dermatolog. Treat. 2012;23:144-52. 8. Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J. Cutan. Aesthet. Surg. 2009;2:26-30.
9. Doddaballapur S. Microneedling with dermaroller. J. Cutan. Aesthet. Surg 2009;2: 110-11.
10. Dogra S, Yadav S. Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J. Cosmet. Dermatol. 2014;13:180-7.
Dr. Talakoub and Dr. Wesley are cocontributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
RNA sequencing characterized high-risk squamous cell carcinomas
SAN DIEGO – Cutaneous squamous cell carcinomas from organ transplant recipients had a more aggressive molecular profile than did tumor samples from immunocompetent patients, according to an RNA sequencing study presented at the annual meeting of the American Society for Dermatologic Surgery.
Specimens from organ transplant recipients showed greater induction of biologic pathways related to cancer signaling, fibrosis, and extracellular matrix remodeling, said Dr. Cameron Chesnut, a dermatologist in private practice in Spokane, Wash., who carried out the research while he was a dermatologic surgery resident at the University of California, Los Angeles.
Furthermore, the TP53 tumor suppressor gene was inhibited at least five times more in samples from organ transplant recipients, compared with those from immunocompetent patients, Dr. Chesnut said in an interview.
Squamous cell carcinoma (SCC) is the most common cancer to occur after organ transplantation, Dr. Chesnut and his associates noted. The malignancy is 65-250 times more common, is more than 4 times more likely to metastasize, and has a mortality rate of 5% compared with a rate of less than 1% in immunocompetent patients, based on data published online in the journal F1000 Prime Reports, they said.
To characterize these high-risk SCCs and compare them with lower-risk SCCs, the researchers performed RNA sequencing of three normal skin samples and SCC specimens from 15 patients – 7 organ transplant recipients and 8 otherwise healthy individuals. The researchers used an Illumina GAIIx RNA Seq instrument to generate RNA sequencing libraries of the specimens. They also used the web-based Ingenuity Pathway Analysis technique to identify the major biological pathways regulated within the tumors.
In all, 690 highly expressed genes were induced at least fivefold in SCCs from organ transplant recipients compared with those from otherwise healthy patients. These genes encoded pathways related to fibrosis, extracellular remodeling, the cell cycle, and tumor signaling, the investigators said. The COX-2 pathway for prostaglandin synthesis also was induced fivefold or more in the high-risk SCCs compared with those from immunocompetent patients, Dr. Chesnut added.
The researchers also identified 1,290 highly expressed genes that were inhibited at least fivefold in SCCs from organ transplant recipients compared with specimens from immunocompetent patients. The most strongly inhibited pathways were related to sterol biosynthesis and epithelial differentiation, followed by nucleotide excision repair, interleukin-6 and IL-17, and apoptosis, they said.
Based on these findings, novel therapeutics might someday be able to target specific biologic pathways that are highly induced in SCCs from organ transplant recipients, Dr. Chesnut said. “It’s hard to say what the most likely candidates are,” but based on the study findings, “regulating inflammation may be a target,” he added. Dr. Chesnut and his associates reported no external funding sources or conflicts of interest.
SAN DIEGO – Cutaneous squamous cell carcinomas from organ transplant recipients had a more aggressive molecular profile than did tumor samples from immunocompetent patients, according to an RNA sequencing study presented at the annual meeting of the American Society for Dermatologic Surgery.
Specimens from organ transplant recipients showed greater induction of biologic pathways related to cancer signaling, fibrosis, and extracellular matrix remodeling, said Dr. Cameron Chesnut, a dermatologist in private practice in Spokane, Wash., who carried out the research while he was a dermatologic surgery resident at the University of California, Los Angeles.
Furthermore, the TP53 tumor suppressor gene was inhibited at least five times more in samples from organ transplant recipients, compared with those from immunocompetent patients, Dr. Chesnut said in an interview.
Squamous cell carcinoma (SCC) is the most common cancer to occur after organ transplantation, Dr. Chesnut and his associates noted. The malignancy is 65-250 times more common, is more than 4 times more likely to metastasize, and has a mortality rate of 5% compared with a rate of less than 1% in immunocompetent patients, based on data published online in the journal F1000 Prime Reports, they said.
To characterize these high-risk SCCs and compare them with lower-risk SCCs, the researchers performed RNA sequencing of three normal skin samples and SCC specimens from 15 patients – 7 organ transplant recipients and 8 otherwise healthy individuals. The researchers used an Illumina GAIIx RNA Seq instrument to generate RNA sequencing libraries of the specimens. They also used the web-based Ingenuity Pathway Analysis technique to identify the major biological pathways regulated within the tumors.
In all, 690 highly expressed genes were induced at least fivefold in SCCs from organ transplant recipients compared with those from otherwise healthy patients. These genes encoded pathways related to fibrosis, extracellular remodeling, the cell cycle, and tumor signaling, the investigators said. The COX-2 pathway for prostaglandin synthesis also was induced fivefold or more in the high-risk SCCs compared with those from immunocompetent patients, Dr. Chesnut added.
The researchers also identified 1,290 highly expressed genes that were inhibited at least fivefold in SCCs from organ transplant recipients compared with specimens from immunocompetent patients. The most strongly inhibited pathways were related to sterol biosynthesis and epithelial differentiation, followed by nucleotide excision repair, interleukin-6 and IL-17, and apoptosis, they said.
Based on these findings, novel therapeutics might someday be able to target specific biologic pathways that are highly induced in SCCs from organ transplant recipients, Dr. Chesnut said. “It’s hard to say what the most likely candidates are,” but based on the study findings, “regulating inflammation may be a target,” he added. Dr. Chesnut and his associates reported no external funding sources or conflicts of interest.
SAN DIEGO – Cutaneous squamous cell carcinomas from organ transplant recipients had a more aggressive molecular profile than did tumor samples from immunocompetent patients, according to an RNA sequencing study presented at the annual meeting of the American Society for Dermatologic Surgery.
Specimens from organ transplant recipients showed greater induction of biologic pathways related to cancer signaling, fibrosis, and extracellular matrix remodeling, said Dr. Cameron Chesnut, a dermatologist in private practice in Spokane, Wash., who carried out the research while he was a dermatologic surgery resident at the University of California, Los Angeles.
Furthermore, the TP53 tumor suppressor gene was inhibited at least five times more in samples from organ transplant recipients, compared with those from immunocompetent patients, Dr. Chesnut said in an interview.
Squamous cell carcinoma (SCC) is the most common cancer to occur after organ transplantation, Dr. Chesnut and his associates noted. The malignancy is 65-250 times more common, is more than 4 times more likely to metastasize, and has a mortality rate of 5% compared with a rate of less than 1% in immunocompetent patients, based on data published online in the journal F1000 Prime Reports, they said.
To characterize these high-risk SCCs and compare them with lower-risk SCCs, the researchers performed RNA sequencing of three normal skin samples and SCC specimens from 15 patients – 7 organ transplant recipients and 8 otherwise healthy individuals. The researchers used an Illumina GAIIx RNA Seq instrument to generate RNA sequencing libraries of the specimens. They also used the web-based Ingenuity Pathway Analysis technique to identify the major biological pathways regulated within the tumors.
In all, 690 highly expressed genes were induced at least fivefold in SCCs from organ transplant recipients compared with those from otherwise healthy patients. These genes encoded pathways related to fibrosis, extracellular remodeling, the cell cycle, and tumor signaling, the investigators said. The COX-2 pathway for prostaglandin synthesis also was induced fivefold or more in the high-risk SCCs compared with those from immunocompetent patients, Dr. Chesnut added.
The researchers also identified 1,290 highly expressed genes that were inhibited at least fivefold in SCCs from organ transplant recipients compared with specimens from immunocompetent patients. The most strongly inhibited pathways were related to sterol biosynthesis and epithelial differentiation, followed by nucleotide excision repair, interleukin-6 and IL-17, and apoptosis, they said.
Based on these findings, novel therapeutics might someday be able to target specific biologic pathways that are highly induced in SCCs from organ transplant recipients, Dr. Chesnut said. “It’s hard to say what the most likely candidates are,” but based on the study findings, “regulating inflammation may be a target,” he added. Dr. Chesnut and his associates reported no external funding sources or conflicts of interest.
Key clinical point: Squamous cell carcinomas from organ transplant recipients showed a more aggressive molecular profile than did those from immunocompetent individuals.
Major finding: The high-risk tumors showed greater induction of biologic pathways related to cancer signaling, fibrosis, and extracellular matrix remodeling, and inhibition of the tp53 tumor suppressor gene.
Data source: RNA sequencing of 15 squamous cell carcinomas, including seven from organ transplant recipients.
Disclosures: The investigators reported no external funding sources or conflicts of interest.
Lesions With a Distinct Fingerprint Presentation
The Diagnosis: Phytophotodermatitis
Phytophotodermatitis (PPD) is a nonimmunologic cutaneous phototoxic inflammatory reaction resulting from the activation of photosensitizing botanical agents such as furanocoumarins in contact with the skin by exposure to UVA light.1,2 Furanocoumarins, including psoralens and angelicins, become photoexcited and covalently bind to pyrimidine bases on DNA strands, resulting in acute damage to epidermal, dermal, and endothelial cells.1,3
Vegetation most commonly implicated in this plant solar dermatitis are celery, fennel, parsnip, parsley, and hogweed (Apiaceae [formerly known as the Umbelliferae family]), as well as oranges, lemons, limes, and grapefruits (Rutaceae or citrus family).1,3 Psoralens found in the Persian lime have been noted to cause phototoxic eruptions in the United States, with the rind containing higher concentrations than the pulp.4
Clinical features of PPD include erythema, edema, and vesicle or bullae formation 12 to 36 hours after psoralen and UV light exposure. Burning and pain may be present, but pruritus is not a common characteristic of the eruptions, distinguishing PPD from allergic phytodermatitis.
Hyperpigmentation appears on resolution of the lesions and slowly fades over months to years.1,3,5 Mild exposure may lead to hyperpigmentation without a vesicular or erythematous eruption.1 Phytophotodermatitis follows a benign course and often spontaneously resolves; however, prolonged hyperpigmentation may cause concern for these patients.
Phytophotodermatitis is common among patients preparing drinks and foods with citrus juices or after gardening. Our patient had prepared limeade 3 weeks prior to presentation. The distribution of cutaneous exposure to furanocoumarins influences clinical presentation and may range from blotches and streaks to distinct fingerprint smudges and handprints, as seen in our patient. The distinct full handprint on the right arm was striking. The bullous lesions and resulting hyperpigmentation may mimic burns and healing bruises. In children, PPD often is mistaken for child abuse.1,6,7 In adults, it often is misdiagnosed as poison oak dermatitis, erythema multiforme, and thrombocytopenic purpura.1,3 It is important to recognize PPD to avoid delay in or misdiagnosis and to better counsel patients on how to avoid recurrent episodes of PPD.
1. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Maryland Heights, MO: Mosby; 2008.
2. Pomeranz MK, Karen JK. Phytophotodermatitis and limes. N Engl J Med. 2007;357:e1.
3. Sassiville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
4. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
5. Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
6. Mill J, Wallis B, Cuttle L, et al. Phytophotodermatitis: case reports of children presenting with blistering after preparing lime juice. Burns. 2008;34:731-733.
7. Carlsen K, Weismann K. Phytophotodermatitis in 19 children admitted to hospital and their differential diagnoses: child abuse and herpes simplex virus infection. J Am Acad Dermatol. 2007;57(suppl):S88-S91.
The Diagnosis: Phytophotodermatitis
Phytophotodermatitis (PPD) is a nonimmunologic cutaneous phototoxic inflammatory reaction resulting from the activation of photosensitizing botanical agents such as furanocoumarins in contact with the skin by exposure to UVA light.1,2 Furanocoumarins, including psoralens and angelicins, become photoexcited and covalently bind to pyrimidine bases on DNA strands, resulting in acute damage to epidermal, dermal, and endothelial cells.1,3
Vegetation most commonly implicated in this plant solar dermatitis are celery, fennel, parsnip, parsley, and hogweed (Apiaceae [formerly known as the Umbelliferae family]), as well as oranges, lemons, limes, and grapefruits (Rutaceae or citrus family).1,3 Psoralens found in the Persian lime have been noted to cause phototoxic eruptions in the United States, with the rind containing higher concentrations than the pulp.4
Clinical features of PPD include erythema, edema, and vesicle or bullae formation 12 to 36 hours after psoralen and UV light exposure. Burning and pain may be present, but pruritus is not a common characteristic of the eruptions, distinguishing PPD from allergic phytodermatitis.
Hyperpigmentation appears on resolution of the lesions and slowly fades over months to years.1,3,5 Mild exposure may lead to hyperpigmentation without a vesicular or erythematous eruption.1 Phytophotodermatitis follows a benign course and often spontaneously resolves; however, prolonged hyperpigmentation may cause concern for these patients.
Phytophotodermatitis is common among patients preparing drinks and foods with citrus juices or after gardening. Our patient had prepared limeade 3 weeks prior to presentation. The distribution of cutaneous exposure to furanocoumarins influences clinical presentation and may range from blotches and streaks to distinct fingerprint smudges and handprints, as seen in our patient. The distinct full handprint on the right arm was striking. The bullous lesions and resulting hyperpigmentation may mimic burns and healing bruises. In children, PPD often is mistaken for child abuse.1,6,7 In adults, it often is misdiagnosed as poison oak dermatitis, erythema multiforme, and thrombocytopenic purpura.1,3 It is important to recognize PPD to avoid delay in or misdiagnosis and to better counsel patients on how to avoid recurrent episodes of PPD.
The Diagnosis: Phytophotodermatitis
Phytophotodermatitis (PPD) is a nonimmunologic cutaneous phototoxic inflammatory reaction resulting from the activation of photosensitizing botanical agents such as furanocoumarins in contact with the skin by exposure to UVA light.1,2 Furanocoumarins, including psoralens and angelicins, become photoexcited and covalently bind to pyrimidine bases on DNA strands, resulting in acute damage to epidermal, dermal, and endothelial cells.1,3
Vegetation most commonly implicated in this plant solar dermatitis are celery, fennel, parsnip, parsley, and hogweed (Apiaceae [formerly known as the Umbelliferae family]), as well as oranges, lemons, limes, and grapefruits (Rutaceae or citrus family).1,3 Psoralens found in the Persian lime have been noted to cause phototoxic eruptions in the United States, with the rind containing higher concentrations than the pulp.4
Clinical features of PPD include erythema, edema, and vesicle or bullae formation 12 to 36 hours after psoralen and UV light exposure. Burning and pain may be present, but pruritus is not a common characteristic of the eruptions, distinguishing PPD from allergic phytodermatitis.
Hyperpigmentation appears on resolution of the lesions and slowly fades over months to years.1,3,5 Mild exposure may lead to hyperpigmentation without a vesicular or erythematous eruption.1 Phytophotodermatitis follows a benign course and often spontaneously resolves; however, prolonged hyperpigmentation may cause concern for these patients.
Phytophotodermatitis is common among patients preparing drinks and foods with citrus juices or after gardening. Our patient had prepared limeade 3 weeks prior to presentation. The distribution of cutaneous exposure to furanocoumarins influences clinical presentation and may range from blotches and streaks to distinct fingerprint smudges and handprints, as seen in our patient. The distinct full handprint on the right arm was striking. The bullous lesions and resulting hyperpigmentation may mimic burns and healing bruises. In children, PPD often is mistaken for child abuse.1,6,7 In adults, it often is misdiagnosed as poison oak dermatitis, erythema multiforme, and thrombocytopenic purpura.1,3 It is important to recognize PPD to avoid delay in or misdiagnosis and to better counsel patients on how to avoid recurrent episodes of PPD.
1. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Maryland Heights, MO: Mosby; 2008.
2. Pomeranz MK, Karen JK. Phytophotodermatitis and limes. N Engl J Med. 2007;357:e1.
3. Sassiville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
4. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
5. Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
6. Mill J, Wallis B, Cuttle L, et al. Phytophotodermatitis: case reports of children presenting with blistering after preparing lime juice. Burns. 2008;34:731-733.
7. Carlsen K, Weismann K. Phytophotodermatitis in 19 children admitted to hospital and their differential diagnoses: child abuse and herpes simplex virus infection. J Am Acad Dermatol. 2007;57(suppl):S88-S91.
1. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Maryland Heights, MO: Mosby; 2008.
2. Pomeranz MK, Karen JK. Phytophotodermatitis and limes. N Engl J Med. 2007;357:e1.
3. Sassiville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
4. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
5. Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
6. Mill J, Wallis B, Cuttle L, et al. Phytophotodermatitis: case reports of children presenting with blistering after preparing lime juice. Burns. 2008;34:731-733.
7. Carlsen K, Weismann K. Phytophotodermatitis in 19 children admitted to hospital and their differential diagnoses: child abuse and herpes simplex virus infection. J Am Acad Dermatol. 2007;57(suppl):S88-S91.

A 17-year-old adolescent girl presented with scattered brown macules over the dorsal aspect of the hands bilaterally and a brown patch in the shape of a hand on the right upper arm of 3 weeks’ duration.
Burn injury risk doubles in HOT patients who smoke
AUSTIN, TEX. – Smokers offered home oxygen therapy were found to be at twice the risk for burn injuries, based on data from a retrospective study.
Even so, almost all the home oxygen therapy (HOT) burn victims were discharged with a prescription for oxygen, including the 15% of patients who had incurred similar injuries at least once, and in some cases, three times.
“I have a problem with this,” said Dr. Mary Baker, a critical care fellow at Indiana University and a medical ethics fellow at the university’s Richard M. Fairbanks Burn Center at Wishard-Eskenazi Health, both in Indianapolis. Dr. Baker presented the findings at the annual meeting of the American College of Chest Physicians.
“Should we be prescribing oxygen to patients who smoke? Maybe the bigger question is [whether] it is ever ethically defensible to take oxygen away once someone has sustained a combustion injury from smoking while using HOT,” she said.
Dr. Baker and her colleagues conducted a chart review of patients admitted to a single site for home oxygen–related burns between 2008 and 2013. They found that 55 of all such burn unit admissions were smokers, representing 4% of the center’s annual admissions rate and twice that of the national burn rate for smokers in general. Nearly all the patients, a balance of men and women with a median age of 61 years, were using HOT for chronic obstructive pulmonary disease.
“The location of the burns, probably not surprisingly, was the face. Probably the most common was the nasal cannula,” Dr. Baker said.
Although nearly three-quarters of the 55-member cohort had less than a 5% total body surface–area burn, Dr. Baker said that in a patient population with baseline respiratory compromise and respiratory failure, this was an alarming rate of morbidity, particularly since half of the injured were intubated, and bronchonscopic exam revealed a third of these patients also had inhalation injuries.
“And here’s the kicker,” said Dr. Baker. “Eight deaths over 5 years. This is huge. So when these [individuals] get burned, it’s often really bad. Several of them had house fires, and we were able to find in the chart where other people [in the home] were burned and admitted to the hospital.”
Still, after a median 5-day stay, almost all the patients who survived were discharged with prescriptions for HOT, including the so-called “repeat offenders.” Because nearly half of all surviving smoking-related HOT patients were discharged to a higher level of care, this cohort tended to have higher health care utilization rates as well, Dr. Baker noted.
A surprise finding was that more than a quarter of the cohort had either current or concomitant problems with substance abuse. “We were not expecting that, and it has not been previously reported,” Dr. Baker said.
The data demonstrate a need for the screening of HOT patients as to whether they smoke and whether they have substance use issues, she said. If either condition applies, then faster follow-up and, potentially, counseling could be offered, including better education about the risks of oxygen therapy. “Currently, we have no formalized way to educate patients on the dangers of those tanks in the home,” said Dr. Baker.
The data raise questions about the risk-benefit ratio of prescribing any breathing aid to COPD patients who are also smokers.
“I don’t know how much sense it makes to keep throwing these inhalers, which cost hundreds of dollars a month, at people who continue to smoke,” Dr. Baker said in an interview. “We take all comers, and we think oxygen therapy helps, and prolongs life, but when you factor in smoking, we don’t really know what the risks and benefits are.”
A large study population would be needed to determine the risks and benefits, she added.
On Twitter @whitneymcknight
AUSTIN, TEX. – Smokers offered home oxygen therapy were found to be at twice the risk for burn injuries, based on data from a retrospective study.
Even so, almost all the home oxygen therapy (HOT) burn victims were discharged with a prescription for oxygen, including the 15% of patients who had incurred similar injuries at least once, and in some cases, three times.
“I have a problem with this,” said Dr. Mary Baker, a critical care fellow at Indiana University and a medical ethics fellow at the university’s Richard M. Fairbanks Burn Center at Wishard-Eskenazi Health, both in Indianapolis. Dr. Baker presented the findings at the annual meeting of the American College of Chest Physicians.
“Should we be prescribing oxygen to patients who smoke? Maybe the bigger question is [whether] it is ever ethically defensible to take oxygen away once someone has sustained a combustion injury from smoking while using HOT,” she said.
Dr. Baker and her colleagues conducted a chart review of patients admitted to a single site for home oxygen–related burns between 2008 and 2013. They found that 55 of all such burn unit admissions were smokers, representing 4% of the center’s annual admissions rate and twice that of the national burn rate for smokers in general. Nearly all the patients, a balance of men and women with a median age of 61 years, were using HOT for chronic obstructive pulmonary disease.
“The location of the burns, probably not surprisingly, was the face. Probably the most common was the nasal cannula,” Dr. Baker said.
Although nearly three-quarters of the 55-member cohort had less than a 5% total body surface–area burn, Dr. Baker said that in a patient population with baseline respiratory compromise and respiratory failure, this was an alarming rate of morbidity, particularly since half of the injured were intubated, and bronchonscopic exam revealed a third of these patients also had inhalation injuries.
“And here’s the kicker,” said Dr. Baker. “Eight deaths over 5 years. This is huge. So when these [individuals] get burned, it’s often really bad. Several of them had house fires, and we were able to find in the chart where other people [in the home] were burned and admitted to the hospital.”
Still, after a median 5-day stay, almost all the patients who survived were discharged with prescriptions for HOT, including the so-called “repeat offenders.” Because nearly half of all surviving smoking-related HOT patients were discharged to a higher level of care, this cohort tended to have higher health care utilization rates as well, Dr. Baker noted.
A surprise finding was that more than a quarter of the cohort had either current or concomitant problems with substance abuse. “We were not expecting that, and it has not been previously reported,” Dr. Baker said.
The data demonstrate a need for the screening of HOT patients as to whether they smoke and whether they have substance use issues, she said. If either condition applies, then faster follow-up and, potentially, counseling could be offered, including better education about the risks of oxygen therapy. “Currently, we have no formalized way to educate patients on the dangers of those tanks in the home,” said Dr. Baker.
The data raise questions about the risk-benefit ratio of prescribing any breathing aid to COPD patients who are also smokers.
“I don’t know how much sense it makes to keep throwing these inhalers, which cost hundreds of dollars a month, at people who continue to smoke,” Dr. Baker said in an interview. “We take all comers, and we think oxygen therapy helps, and prolongs life, but when you factor in smoking, we don’t really know what the risks and benefits are.”
A large study population would be needed to determine the risks and benefits, she added.
On Twitter @whitneymcknight
AUSTIN, TEX. – Smokers offered home oxygen therapy were found to be at twice the risk for burn injuries, based on data from a retrospective study.
Even so, almost all the home oxygen therapy (HOT) burn victims were discharged with a prescription for oxygen, including the 15% of patients who had incurred similar injuries at least once, and in some cases, three times.
“I have a problem with this,” said Dr. Mary Baker, a critical care fellow at Indiana University and a medical ethics fellow at the university’s Richard M. Fairbanks Burn Center at Wishard-Eskenazi Health, both in Indianapolis. Dr. Baker presented the findings at the annual meeting of the American College of Chest Physicians.
“Should we be prescribing oxygen to patients who smoke? Maybe the bigger question is [whether] it is ever ethically defensible to take oxygen away once someone has sustained a combustion injury from smoking while using HOT,” she said.
Dr. Baker and her colleagues conducted a chart review of patients admitted to a single site for home oxygen–related burns between 2008 and 2013. They found that 55 of all such burn unit admissions were smokers, representing 4% of the center’s annual admissions rate and twice that of the national burn rate for smokers in general. Nearly all the patients, a balance of men and women with a median age of 61 years, were using HOT for chronic obstructive pulmonary disease.
“The location of the burns, probably not surprisingly, was the face. Probably the most common was the nasal cannula,” Dr. Baker said.
Although nearly three-quarters of the 55-member cohort had less than a 5% total body surface–area burn, Dr. Baker said that in a patient population with baseline respiratory compromise and respiratory failure, this was an alarming rate of morbidity, particularly since half of the injured were intubated, and bronchonscopic exam revealed a third of these patients also had inhalation injuries.
“And here’s the kicker,” said Dr. Baker. “Eight deaths over 5 years. This is huge. So when these [individuals] get burned, it’s often really bad. Several of them had house fires, and we were able to find in the chart where other people [in the home] were burned and admitted to the hospital.”
Still, after a median 5-day stay, almost all the patients who survived were discharged with prescriptions for HOT, including the so-called “repeat offenders.” Because nearly half of all surviving smoking-related HOT patients were discharged to a higher level of care, this cohort tended to have higher health care utilization rates as well, Dr. Baker noted.
A surprise finding was that more than a quarter of the cohort had either current or concomitant problems with substance abuse. “We were not expecting that, and it has not been previously reported,” Dr. Baker said.
The data demonstrate a need for the screening of HOT patients as to whether they smoke and whether they have substance use issues, she said. If either condition applies, then faster follow-up and, potentially, counseling could be offered, including better education about the risks of oxygen therapy. “Currently, we have no formalized way to educate patients on the dangers of those tanks in the home,” said Dr. Baker.
The data raise questions about the risk-benefit ratio of prescribing any breathing aid to COPD patients who are also smokers.
“I don’t know how much sense it makes to keep throwing these inhalers, which cost hundreds of dollars a month, at people who continue to smoke,” Dr. Baker said in an interview. “We take all comers, and we think oxygen therapy helps, and prolongs life, but when you factor in smoking, we don’t really know what the risks and benefits are.”
A large study population would be needed to determine the risks and benefits, she added.
On Twitter @whitneymcknight
AT CHEST 2014
Key clinical point: Counsel patients on the elevated risk of mortality and morbidity when HOT and smoking are combined.
Major finding: The burn injury rate for smokers with COPD using HOT was 4%, compared with 2% in smokers not using HOT.
Data source: Retrospective analysis of single site burn injury admissions beween 2008 and 2013.
Disclosures: Dr. Baker reported that she had no relevant disclosures.
Indoor tanning rates down for high school students in 2013
Indoor tanning by high school girls decreased from 2009 to 2013, according to a recent study from the Centers for Disease Control and Prevention.
The overall indoor tanning rate for all high school girls dropped to about 20% in 2013, down from just over 25% in 2009. There was a significant drop in indoor tanning for non-Hispanic white girls and a slight decrease for Hispanic girls. The rate of indoor tanning for non-Hispanic black girls remained steady.
Decreases in indoor tanning “may be partly attributable to increased awareness of its harms,” with new or strengthened laws in 40 states having an impact as well, according to Gery P. Guy Jr., Ph.D., of the Division of Cancer Prevention and Control at the CDC in Atlanta.
Non-Hispanic white girls were by far the most likely to indoor tan in 2013, with nearly 31% tanning at least once in the previous year and almost 17% tanning at least 10 times in the same period. No other measured ethnic group had such high rate of usage, with only 2.5% of non-Hispanic blacks, about 8% of Hispanics, and just under 10% of non-Hispanic others engaging in indoor tanning at least once, the investigators reported (JAMA Dermatol. 2014 Dec. 23 [doi:10.1001/jamadermatol.2014.4677]).
Indoor tanning by high school boys was much lower than for girls, with about 5% of all boys tanning at least once in 2013. White boys had the highest rate of measured ethnicities, but this was only at about 6%, the researchers said.
The study is based on data collected for the 2009, 2011, and 2013 Youth Risk Behavior Surveys.
Indoor tanning by high school girls decreased from 2009 to 2013, according to a recent study from the Centers for Disease Control and Prevention.
The overall indoor tanning rate for all high school girls dropped to about 20% in 2013, down from just over 25% in 2009. There was a significant drop in indoor tanning for non-Hispanic white girls and a slight decrease for Hispanic girls. The rate of indoor tanning for non-Hispanic black girls remained steady.
Decreases in indoor tanning “may be partly attributable to increased awareness of its harms,” with new or strengthened laws in 40 states having an impact as well, according to Gery P. Guy Jr., Ph.D., of the Division of Cancer Prevention and Control at the CDC in Atlanta.
Non-Hispanic white girls were by far the most likely to indoor tan in 2013, with nearly 31% tanning at least once in the previous year and almost 17% tanning at least 10 times in the same period. No other measured ethnic group had such high rate of usage, with only 2.5% of non-Hispanic blacks, about 8% of Hispanics, and just under 10% of non-Hispanic others engaging in indoor tanning at least once, the investigators reported (JAMA Dermatol. 2014 Dec. 23 [doi:10.1001/jamadermatol.2014.4677]).
Indoor tanning by high school boys was much lower than for girls, with about 5% of all boys tanning at least once in 2013. White boys had the highest rate of measured ethnicities, but this was only at about 6%, the researchers said.
The study is based on data collected for the 2009, 2011, and 2013 Youth Risk Behavior Surveys.
Indoor tanning by high school girls decreased from 2009 to 2013, according to a recent study from the Centers for Disease Control and Prevention.
The overall indoor tanning rate for all high school girls dropped to about 20% in 2013, down from just over 25% in 2009. There was a significant drop in indoor tanning for non-Hispanic white girls and a slight decrease for Hispanic girls. The rate of indoor tanning for non-Hispanic black girls remained steady.
Decreases in indoor tanning “may be partly attributable to increased awareness of its harms,” with new or strengthened laws in 40 states having an impact as well, according to Gery P. Guy Jr., Ph.D., of the Division of Cancer Prevention and Control at the CDC in Atlanta.
Non-Hispanic white girls were by far the most likely to indoor tan in 2013, with nearly 31% tanning at least once in the previous year and almost 17% tanning at least 10 times in the same period. No other measured ethnic group had such high rate of usage, with only 2.5% of non-Hispanic blacks, about 8% of Hispanics, and just under 10% of non-Hispanic others engaging in indoor tanning at least once, the investigators reported (JAMA Dermatol. 2014 Dec. 23 [doi:10.1001/jamadermatol.2014.4677]).
Indoor tanning by high school boys was much lower than for girls, with about 5% of all boys tanning at least once in 2013. White boys had the highest rate of measured ethnicities, but this was only at about 6%, the researchers said.
The study is based on data collected for the 2009, 2011, and 2013 Youth Risk Behavior Surveys.
FROM JAMA DERMATOLOGY
Cosmetic Corner: Dermatologists Weigh in on Mineral Makeup
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top mineral makeup products. Consideration must be given to:
- bareMinerals
Bare Escentuals Beauty, Inc
“I recommend bareMinerals everyday to patients with various facial blemishes, acne, and dermatoses. This brand has products for patients of all skin types and colors.”—Gary Goldenberg, MD, New York, New York
Recommended by Elizabeth K. Hale, MD, New York, New York
“Very light and noncomedogenic and does not cake up.”—Anthony M. Rossi, MD, New York, New York
- Jane Iredale
Iredale Mineral Cosmetics, Ltd
“My patients and staff love this brand as a whole. They find the options are terrific for various skin types and the company support is very strong, which is a huge part of the equation. The brush-on sunscreen is terrific.”—Joel Schlessinger, MD, Omaha, Nebraska
- Météorites Powder
Guerlain
“Adds brightness but does not dry the skin.”—Antonella Tosti, MD, Miami, Florida
- Sheer Cover Studio
Guthy-Renker
“It provides very effective coverage without appearing heavy.”—Whitney Bowe, MD, Brooklyn, New York
Cutis invites readers to send us their recommendations. Skin care products for babies, eyelash enhancers, and cleansing pads will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top mineral makeup products. Consideration must be given to:
- bareMinerals
Bare Escentuals Beauty, Inc
“I recommend bareMinerals everyday to patients with various facial blemishes, acne, and dermatoses. This brand has products for patients of all skin types and colors.”—Gary Goldenberg, MD, New York, New York
Recommended by Elizabeth K. Hale, MD, New York, New York
“Very light and noncomedogenic and does not cake up.”—Anthony M. Rossi, MD, New York, New York
- Jane Iredale
Iredale Mineral Cosmetics, Ltd
“My patients and staff love this brand as a whole. They find the options are terrific for various skin types and the company support is very strong, which is a huge part of the equation. The brush-on sunscreen is terrific.”—Joel Schlessinger, MD, Omaha, Nebraska
- Météorites Powder
Guerlain
“Adds brightness but does not dry the skin.”—Antonella Tosti, MD, Miami, Florida
- Sheer Cover Studio
Guthy-Renker
“It provides very effective coverage without appearing heavy.”—Whitney Bowe, MD, Brooklyn, New York
Cutis invites readers to send us their recommendations. Skin care products for babies, eyelash enhancers, and cleansing pads will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top mineral makeup products. Consideration must be given to:
- bareMinerals
Bare Escentuals Beauty, Inc
“I recommend bareMinerals everyday to patients with various facial blemishes, acne, and dermatoses. This brand has products for patients of all skin types and colors.”—Gary Goldenberg, MD, New York, New York
Recommended by Elizabeth K. Hale, MD, New York, New York
“Very light and noncomedogenic and does not cake up.”—Anthony M. Rossi, MD, New York, New York
- Jane Iredale
Iredale Mineral Cosmetics, Ltd
“My patients and staff love this brand as a whole. They find the options are terrific for various skin types and the company support is very strong, which is a huge part of the equation. The brush-on sunscreen is terrific.”—Joel Schlessinger, MD, Omaha, Nebraska
- Météorites Powder
Guerlain
“Adds brightness but does not dry the skin.”—Antonella Tosti, MD, Miami, Florida
- Sheer Cover Studio
Guthy-Renker
“It provides very effective coverage without appearing heavy.”—Whitney Bowe, MD, Brooklyn, New York
Cutis invites readers to send us their recommendations. Skin care products for babies, eyelash enhancers, and cleansing pads will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.