User login
Colombia reports first Zika deaths, all in medically compromised patients
AMSTERDAM – Five people with confirmed Zika virus infections have died in Colombia, and all had medical comorbidities, including leukemia, diabetes, sickle cell anemia, and hypertension.
All of the deaths occurred last October in northern and central Colombia, Dr. Alfonso Rodriguez-Morales said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Four of the cases were simultaneously published April 7 in the Lancet Infectious Diseases (2016 Apr 7. doi: 10.1016/S1473-3099[16]30006-8). The fifth case occurred in northern Colombia, and was reported in Emerging Infectious Diseases (2016 May. doi: 10.3201/eid2205.151934).
Reports of confirmed Zika-related deaths are rare. Brazil, the only other country to disclose them, has now reported three, said Dr. Rodriguez-Morales of the Universidad Tecnológica de Pereira, Colombia.
“Before the current outbreak in Latin America, Zika virus was not linked to deaths,” he noted. But the eight confirmed Zika-related deaths in South America “call attention to the need for evidence-based guidelines for clinical management of Zika, as well as the possible occurrence of atypical and severe cases, including possibly congenitally related microcephaly.”
Because they all occurred in medically compromised patients, Dr. Rodriguez-Morales also urged clinicians to cast a wary eye on such patients who present with arbovirus-type symptoms, including fever and rash.
From September 2015 to March 2016, Colombia had 58,838 reported cases of Zika. Of those, only 2,361 were lab confirmed. The rest were either diagnosed clinically or were suspected cases, Dr. Rodriguez-Morales said. Although Colombia has a much smaller population than Brazil (49 million vs. 210 million), its Zika case rate is much higher, 120 cases per 100,000 people vs. 34 cases per 100,000 people.
The group of four deaths occurred in central Colombia, and included a 2-year-old girl, a 30-year-old woman, a 61-year-old man, and a 72-year-old woman. All presented with 2-6 days of fever. All were initially suspected to have dengue fever or chikungunya. None tested positive for dengue, but the man was coinfected with chikungunya.
All patients presented with anemia. All but the older man also had severe thrombocytopenia.
The toddler presented with hepatomegaly, mucosal hemorrhage, progressive respiratory collapse, progressive thrombocytopenia, and intravascular coagulation. She died 5 days after symptom onset and was found to have had unrecognized lymphoblastic leukemia.
The 30-year-old woman presented with a severe rash on both arms. She also exhibited coagulation dysfunction, including severe thrombocytopenia and leukopenia that progressed to intracerebral and subarachnoid hemorrhage. She died 12 days after symptom onset. She was determined to have had unrecognized acute myeloid leukemia.
The elderly man had a history of medically controlled hypertension. He experienced mucosal hemorrhage and respiratory distress. He died 7 days after symptom onset. On autopsy, his liver showed necrotic areas, and his spleen indicated a systemic inflammatory response.
The elderly woman had a history of insulin-controlled type 2 diabetes. Her symptoms included gastrointestinal distress, thrombocytopenia, and acute respiratory failure. She died 48 hours after symptom onset; her brain showed edema and ischemic lesions.
The 15-year-old girl in northern Colombia had a 5-year history of sickle cell disease, which, Dr. Rodriguez-Morales pointed out, is a risk factor for arbovirus diseases. However, the patient had never been hospitalized for a vasoocclusive crisis. She presented with a high fever; joint, muscle, and abdominal pain; and jaundice. She was assumed to have dengue virus. Within another day, she had progressed into respiratory failure and was on a ventilator. She died less than 2 days later.
Her autopsy showed hepatic necrosis and severe decrease of splenic lymphoid tissue with splenic sequestration. Systemic inflammation probably triggered a fatal vasoocclusive crisis and splenic sequestration.
Dr. Rodriguez-Morales had no financial disclosures.
AMSTERDAM – Five people with confirmed Zika virus infections have died in Colombia, and all had medical comorbidities, including leukemia, diabetes, sickle cell anemia, and hypertension.
All of the deaths occurred last October in northern and central Colombia, Dr. Alfonso Rodriguez-Morales said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Four of the cases were simultaneously published April 7 in the Lancet Infectious Diseases (2016 Apr 7. doi: 10.1016/S1473-3099[16]30006-8). The fifth case occurred in northern Colombia, and was reported in Emerging Infectious Diseases (2016 May. doi: 10.3201/eid2205.151934).
Reports of confirmed Zika-related deaths are rare. Brazil, the only other country to disclose them, has now reported three, said Dr. Rodriguez-Morales of the Universidad Tecnológica de Pereira, Colombia.
“Before the current outbreak in Latin America, Zika virus was not linked to deaths,” he noted. But the eight confirmed Zika-related deaths in South America “call attention to the need for evidence-based guidelines for clinical management of Zika, as well as the possible occurrence of atypical and severe cases, including possibly congenitally related microcephaly.”
Because they all occurred in medically compromised patients, Dr. Rodriguez-Morales also urged clinicians to cast a wary eye on such patients who present with arbovirus-type symptoms, including fever and rash.
From September 2015 to March 2016, Colombia had 58,838 reported cases of Zika. Of those, only 2,361 were lab confirmed. The rest were either diagnosed clinically or were suspected cases, Dr. Rodriguez-Morales said. Although Colombia has a much smaller population than Brazil (49 million vs. 210 million), its Zika case rate is much higher, 120 cases per 100,000 people vs. 34 cases per 100,000 people.
The group of four deaths occurred in central Colombia, and included a 2-year-old girl, a 30-year-old woman, a 61-year-old man, and a 72-year-old woman. All presented with 2-6 days of fever. All were initially suspected to have dengue fever or chikungunya. None tested positive for dengue, but the man was coinfected with chikungunya.
All patients presented with anemia. All but the older man also had severe thrombocytopenia.
The toddler presented with hepatomegaly, mucosal hemorrhage, progressive respiratory collapse, progressive thrombocytopenia, and intravascular coagulation. She died 5 days after symptom onset and was found to have had unrecognized lymphoblastic leukemia.
The 30-year-old woman presented with a severe rash on both arms. She also exhibited coagulation dysfunction, including severe thrombocytopenia and leukopenia that progressed to intracerebral and subarachnoid hemorrhage. She died 12 days after symptom onset. She was determined to have had unrecognized acute myeloid leukemia.
The elderly man had a history of medically controlled hypertension. He experienced mucosal hemorrhage and respiratory distress. He died 7 days after symptom onset. On autopsy, his liver showed necrotic areas, and his spleen indicated a systemic inflammatory response.
The elderly woman had a history of insulin-controlled type 2 diabetes. Her symptoms included gastrointestinal distress, thrombocytopenia, and acute respiratory failure. She died 48 hours after symptom onset; her brain showed edema and ischemic lesions.
The 15-year-old girl in northern Colombia had a 5-year history of sickle cell disease, which, Dr. Rodriguez-Morales pointed out, is a risk factor for arbovirus diseases. However, the patient had never been hospitalized for a vasoocclusive crisis. She presented with a high fever; joint, muscle, and abdominal pain; and jaundice. She was assumed to have dengue virus. Within another day, she had progressed into respiratory failure and was on a ventilator. She died less than 2 days later.
Her autopsy showed hepatic necrosis and severe decrease of splenic lymphoid tissue with splenic sequestration. Systemic inflammation probably triggered a fatal vasoocclusive crisis and splenic sequestration.
Dr. Rodriguez-Morales had no financial disclosures.
AMSTERDAM – Five people with confirmed Zika virus infections have died in Colombia, and all had medical comorbidities, including leukemia, diabetes, sickle cell anemia, and hypertension.
All of the deaths occurred last October in northern and central Colombia, Dr. Alfonso Rodriguez-Morales said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Four of the cases were simultaneously published April 7 in the Lancet Infectious Diseases (2016 Apr 7. doi: 10.1016/S1473-3099[16]30006-8). The fifth case occurred in northern Colombia, and was reported in Emerging Infectious Diseases (2016 May. doi: 10.3201/eid2205.151934).
Reports of confirmed Zika-related deaths are rare. Brazil, the only other country to disclose them, has now reported three, said Dr. Rodriguez-Morales of the Universidad Tecnológica de Pereira, Colombia.
“Before the current outbreak in Latin America, Zika virus was not linked to deaths,” he noted. But the eight confirmed Zika-related deaths in South America “call attention to the need for evidence-based guidelines for clinical management of Zika, as well as the possible occurrence of atypical and severe cases, including possibly congenitally related microcephaly.”
Because they all occurred in medically compromised patients, Dr. Rodriguez-Morales also urged clinicians to cast a wary eye on such patients who present with arbovirus-type symptoms, including fever and rash.
From September 2015 to March 2016, Colombia had 58,838 reported cases of Zika. Of those, only 2,361 were lab confirmed. The rest were either diagnosed clinically or were suspected cases, Dr. Rodriguez-Morales said. Although Colombia has a much smaller population than Brazil (49 million vs. 210 million), its Zika case rate is much higher, 120 cases per 100,000 people vs. 34 cases per 100,000 people.
The group of four deaths occurred in central Colombia, and included a 2-year-old girl, a 30-year-old woman, a 61-year-old man, and a 72-year-old woman. All presented with 2-6 days of fever. All were initially suspected to have dengue fever or chikungunya. None tested positive for dengue, but the man was coinfected with chikungunya.
All patients presented with anemia. All but the older man also had severe thrombocytopenia.
The toddler presented with hepatomegaly, mucosal hemorrhage, progressive respiratory collapse, progressive thrombocytopenia, and intravascular coagulation. She died 5 days after symptom onset and was found to have had unrecognized lymphoblastic leukemia.
The 30-year-old woman presented with a severe rash on both arms. She also exhibited coagulation dysfunction, including severe thrombocytopenia and leukopenia that progressed to intracerebral and subarachnoid hemorrhage. She died 12 days after symptom onset. She was determined to have had unrecognized acute myeloid leukemia.
The elderly man had a history of medically controlled hypertension. He experienced mucosal hemorrhage and respiratory distress. He died 7 days after symptom onset. On autopsy, his liver showed necrotic areas, and his spleen indicated a systemic inflammatory response.
The elderly woman had a history of insulin-controlled type 2 diabetes. Her symptoms included gastrointestinal distress, thrombocytopenia, and acute respiratory failure. She died 48 hours after symptom onset; her brain showed edema and ischemic lesions.
The 15-year-old girl in northern Colombia had a 5-year history of sickle cell disease, which, Dr. Rodriguez-Morales pointed out, is a risk factor for arbovirus diseases. However, the patient had never been hospitalized for a vasoocclusive crisis. She presented with a high fever; joint, muscle, and abdominal pain; and jaundice. She was assumed to have dengue virus. Within another day, she had progressed into respiratory failure and was on a ventilator. She died less than 2 days later.
Her autopsy showed hepatic necrosis and severe decrease of splenic lymphoid tissue with splenic sequestration. Systemic inflammation probably triggered a fatal vasoocclusive crisis and splenic sequestration.
Dr. Rodriguez-Morales had no financial disclosures.
AT ECCMID 2016
Slowing the progression of sickle cell disease
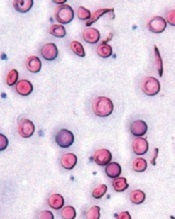
Image courtesy of the
University of Michigan
Activating the antioxidant regulator Nrf2 may slow the progression of sickle cell disease (SCD), according to preclinical research published in JCI Insight.
Investigators found the severity of hemolytic anemia, vascular inflammation, and lung injury increased with age in mice with SCD.
However, activating Nrf2 in young animals had a prophylactic effect, reducing the severity of these adverse effects and improving survival.
To uncover these findings, Solomon Ofori-Acquah, PhD, of the University of Pittsburgh in Pennsylvania, and his colleagues conducted a 10-month longitudinal observational study of mice with SCD.
The team found that, in mice with homozygous SCD (SS), there was a link between intravascular hemolysis, vascular inflammation, lung injury, and early death.
Mice as young as 2 months showed exacerbation of intravascular hemolysis. And additional investigation linked worsening intravascular hemolysis and oxidative stress to the release of VE-cadherin and progressive lung damage in aging SS mice.
The investigators knew that Nrf2 regulates the expression of genes that protect against the effects of intravascular hemolysis. So they decided to see if activating Nrf2 in young mice with SCD would slow the disease progression that occurs with age.
The team took SS mice that were about a month old and randomized them to receive 3H-1, 2-dithiole-3-thione (D3T) or a DMSO vehicle for 3 months or longer.
Treatment with D3T stabilized the concentration of hemoglobin, increased white blood cell counts, increased reticulocyte counts (though not significantly), kept HO-1 levels stable, increased levels of NQO1 and ferritin, and impeded the progression of endothelial dysfunction.
The investigators also looked at the role of Nrf2 in nonhematopoietic tissues and were surprised to find that Nrf2 deficiency in nonhematopoietic tissues exacerbated anemia and caused premature pulmonary edema in mice with SCD.
The team said this suggests a dominant protective role for nonhematopoietic Nrf2 against tissue damage in both erythroid and nonerythroid tissues in SCD.
And, when taken together, the results of this research indicate that activating Nrf2 can impede the onset of the severe adult phenotype of SCD in mice. ![]()

Image courtesy of the
University of Michigan
Activating the antioxidant regulator Nrf2 may slow the progression of sickle cell disease (SCD), according to preclinical research published in JCI Insight.
Investigators found the severity of hemolytic anemia, vascular inflammation, and lung injury increased with age in mice with SCD.
However, activating Nrf2 in young animals had a prophylactic effect, reducing the severity of these adverse effects and improving survival.
To uncover these findings, Solomon Ofori-Acquah, PhD, of the University of Pittsburgh in Pennsylvania, and his colleagues conducted a 10-month longitudinal observational study of mice with SCD.
The team found that, in mice with homozygous SCD (SS), there was a link between intravascular hemolysis, vascular inflammation, lung injury, and early death.
Mice as young as 2 months showed exacerbation of intravascular hemolysis. And additional investigation linked worsening intravascular hemolysis and oxidative stress to the release of VE-cadherin and progressive lung damage in aging SS mice.
The investigators knew that Nrf2 regulates the expression of genes that protect against the effects of intravascular hemolysis. So they decided to see if activating Nrf2 in young mice with SCD would slow the disease progression that occurs with age.
The team took SS mice that were about a month old and randomized them to receive 3H-1, 2-dithiole-3-thione (D3T) or a DMSO vehicle for 3 months or longer.
Treatment with D3T stabilized the concentration of hemoglobin, increased white blood cell counts, increased reticulocyte counts (though not significantly), kept HO-1 levels stable, increased levels of NQO1 and ferritin, and impeded the progression of endothelial dysfunction.
The investigators also looked at the role of Nrf2 in nonhematopoietic tissues and were surprised to find that Nrf2 deficiency in nonhematopoietic tissues exacerbated anemia and caused premature pulmonary edema in mice with SCD.
The team said this suggests a dominant protective role for nonhematopoietic Nrf2 against tissue damage in both erythroid and nonerythroid tissues in SCD.
And, when taken together, the results of this research indicate that activating Nrf2 can impede the onset of the severe adult phenotype of SCD in mice. ![]()

Image courtesy of the
University of Michigan
Activating the antioxidant regulator Nrf2 may slow the progression of sickle cell disease (SCD), according to preclinical research published in JCI Insight.
Investigators found the severity of hemolytic anemia, vascular inflammation, and lung injury increased with age in mice with SCD.
However, activating Nrf2 in young animals had a prophylactic effect, reducing the severity of these adverse effects and improving survival.
To uncover these findings, Solomon Ofori-Acquah, PhD, of the University of Pittsburgh in Pennsylvania, and his colleagues conducted a 10-month longitudinal observational study of mice with SCD.
The team found that, in mice with homozygous SCD (SS), there was a link between intravascular hemolysis, vascular inflammation, lung injury, and early death.
Mice as young as 2 months showed exacerbation of intravascular hemolysis. And additional investigation linked worsening intravascular hemolysis and oxidative stress to the release of VE-cadherin and progressive lung damage in aging SS mice.
The investigators knew that Nrf2 regulates the expression of genes that protect against the effects of intravascular hemolysis. So they decided to see if activating Nrf2 in young mice with SCD would slow the disease progression that occurs with age.
The team took SS mice that were about a month old and randomized them to receive 3H-1, 2-dithiole-3-thione (D3T) or a DMSO vehicle for 3 months or longer.
Treatment with D3T stabilized the concentration of hemoglobin, increased white blood cell counts, increased reticulocyte counts (though not significantly), kept HO-1 levels stable, increased levels of NQO1 and ferritin, and impeded the progression of endothelial dysfunction.
The investigators also looked at the role of Nrf2 in nonhematopoietic tissues and were surprised to find that Nrf2 deficiency in nonhematopoietic tissues exacerbated anemia and caused premature pulmonary edema in mice with SCD.
The team said this suggests a dominant protective role for nonhematopoietic Nrf2 against tissue damage in both erythroid and nonerythroid tissues in SCD.
And, when taken together, the results of this research indicate that activating Nrf2 can impede the onset of the severe adult phenotype of SCD in mice. ![]()
EC approves drug for pediatric ITP

Photo by Logan Tuttle
The European Commission (EC) has approved eltrombopag (Revolade), a once-daily oral thrombopoietin receptor agonist, to treat pediatric patients (age 1 and older) with chronic immune thrombocytopenia (ITP) that is refractory to other therapies.
This approval includes the use of tablets and a new oral suspension formulation of eltrombopag, which is designed for younger children who may not be able to swallow tablets.
The approval applies to all 28 member states of the European Union plus Iceland, Norway, and Liechtenstein.
Eltrombopag was previously approved by the EC for use in adults with refractory chronic ITP. The drug is also approved in the EC to treat adults with severe aplastic anemia and adults with chronic hepatitis C virus infection who have thrombocytopenia.
Eltrombopag is made by Novartis. For more details on the drug, see the full Summary of Product Characteristics, available on the European Medicines Agency’s website.
The EC’s latest approval of eltrombopag was based on data from 2 double-blind, placebo-controlled trials—the phase 2 PETIT trial and the phase 3 PETIT2 trial.
PETIT trials: Efficacy
The PETIT trial included 67 ITP patients stratified by age cohort (12-17 years, 6-11 years, and 1-5 years). They were randomized (2:1) to receive eltrombopag or placebo for 7 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects achieving platelet counts of 50 x 109/L or higher at least once between days 8 and 43 of the randomized period of the study.
Significantly more patients in the eltrombopag arm met this endpoint—62.2%—compared to 31.8% in the placebo arm (P=0.011).
The PETIT2 trial enrolled 92 patients with chronic ITP who were randomized (2:1) to receive eltrombopag or placebo for 13 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects who achieved platelet counts of 50 x 109/L or higher for at least 6 out of 8 weeks, between weeks 5 and 12 of the randomized period.
Significantly more patients in the eltrombopag arm met this endpoint—41.3%—compared to 3.4% of patients in the placebo arm (P<0.001).
PETIT trials: Safety
For both trials, there were 107 eltrombopag-treated patients evaluable for safety.
The most common adverse events that occurred more frequently in the eltrombopag arms than the placebo arms were upper respiratory tract infection, nasopharyngitis, cough, diarrhea, pyrexia, rhinitis, abdominal pain, oropharyngeal pain, toothache, increased ALT/AST, rash, and rhinorrhea.
Serious adverse events were reported in 8% of patients during the randomized part of both trials, although no serious adverse event occurred in more than 1 patient.
An ALT elevation of at least 3 times the upper limit of normal occurred in 5% of eltrombopag-treated patients. Of those patients, 2% had ALT increases of at least 5 times the upper limit of normal.
There were no deaths or thromboembolic events during either study. ![]()

Photo by Logan Tuttle
The European Commission (EC) has approved eltrombopag (Revolade), a once-daily oral thrombopoietin receptor agonist, to treat pediatric patients (age 1 and older) with chronic immune thrombocytopenia (ITP) that is refractory to other therapies.
This approval includes the use of tablets and a new oral suspension formulation of eltrombopag, which is designed for younger children who may not be able to swallow tablets.
The approval applies to all 28 member states of the European Union plus Iceland, Norway, and Liechtenstein.
Eltrombopag was previously approved by the EC for use in adults with refractory chronic ITP. The drug is also approved in the EC to treat adults with severe aplastic anemia and adults with chronic hepatitis C virus infection who have thrombocytopenia.
Eltrombopag is made by Novartis. For more details on the drug, see the full Summary of Product Characteristics, available on the European Medicines Agency’s website.
The EC’s latest approval of eltrombopag was based on data from 2 double-blind, placebo-controlled trials—the phase 2 PETIT trial and the phase 3 PETIT2 trial.
PETIT trials: Efficacy
The PETIT trial included 67 ITP patients stratified by age cohort (12-17 years, 6-11 years, and 1-5 years). They were randomized (2:1) to receive eltrombopag or placebo for 7 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects achieving platelet counts of 50 x 109/L or higher at least once between days 8 and 43 of the randomized period of the study.
Significantly more patients in the eltrombopag arm met this endpoint—62.2%—compared to 31.8% in the placebo arm (P=0.011).
The PETIT2 trial enrolled 92 patients with chronic ITP who were randomized (2:1) to receive eltrombopag or placebo for 13 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects who achieved platelet counts of 50 x 109/L or higher for at least 6 out of 8 weeks, between weeks 5 and 12 of the randomized period.
Significantly more patients in the eltrombopag arm met this endpoint—41.3%—compared to 3.4% of patients in the placebo arm (P<0.001).
PETIT trials: Safety
For both trials, there were 107 eltrombopag-treated patients evaluable for safety.
The most common adverse events that occurred more frequently in the eltrombopag arms than the placebo arms were upper respiratory tract infection, nasopharyngitis, cough, diarrhea, pyrexia, rhinitis, abdominal pain, oropharyngeal pain, toothache, increased ALT/AST, rash, and rhinorrhea.
Serious adverse events were reported in 8% of patients during the randomized part of both trials, although no serious adverse event occurred in more than 1 patient.
An ALT elevation of at least 3 times the upper limit of normal occurred in 5% of eltrombopag-treated patients. Of those patients, 2% had ALT increases of at least 5 times the upper limit of normal.
There were no deaths or thromboembolic events during either study. ![]()

Photo by Logan Tuttle
The European Commission (EC) has approved eltrombopag (Revolade), a once-daily oral thrombopoietin receptor agonist, to treat pediatric patients (age 1 and older) with chronic immune thrombocytopenia (ITP) that is refractory to other therapies.
This approval includes the use of tablets and a new oral suspension formulation of eltrombopag, which is designed for younger children who may not be able to swallow tablets.
The approval applies to all 28 member states of the European Union plus Iceland, Norway, and Liechtenstein.
Eltrombopag was previously approved by the EC for use in adults with refractory chronic ITP. The drug is also approved in the EC to treat adults with severe aplastic anemia and adults with chronic hepatitis C virus infection who have thrombocytopenia.
Eltrombopag is made by Novartis. For more details on the drug, see the full Summary of Product Characteristics, available on the European Medicines Agency’s website.
The EC’s latest approval of eltrombopag was based on data from 2 double-blind, placebo-controlled trials—the phase 2 PETIT trial and the phase 3 PETIT2 trial.
PETIT trials: Efficacy
The PETIT trial included 67 ITP patients stratified by age cohort (12-17 years, 6-11 years, and 1-5 years). They were randomized (2:1) to receive eltrombopag or placebo for 7 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects achieving platelet counts of 50 x 109/L or higher at least once between days 8 and 43 of the randomized period of the study.
Significantly more patients in the eltrombopag arm met this endpoint—62.2%—compared to 31.8% in the placebo arm (P=0.011).
The PETIT2 trial enrolled 92 patients with chronic ITP who were randomized (2:1) to receive eltrombopag or placebo for 13 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects who achieved platelet counts of 50 x 109/L or higher for at least 6 out of 8 weeks, between weeks 5 and 12 of the randomized period.
Significantly more patients in the eltrombopag arm met this endpoint—41.3%—compared to 3.4% of patients in the placebo arm (P<0.001).
PETIT trials: Safety
For both trials, there were 107 eltrombopag-treated patients evaluable for safety.
The most common adverse events that occurred more frequently in the eltrombopag arms than the placebo arms were upper respiratory tract infection, nasopharyngitis, cough, diarrhea, pyrexia, rhinitis, abdominal pain, oropharyngeal pain, toothache, increased ALT/AST, rash, and rhinorrhea.
Serious adverse events were reported in 8% of patients during the randomized part of both trials, although no serious adverse event occurred in more than 1 patient.
An ALT elevation of at least 3 times the upper limit of normal occurred in 5% of eltrombopag-treated patients. Of those patients, 2% had ALT increases of at least 5 times the upper limit of normal.
There were no deaths or thromboembolic events during either study. ![]()
Drug bests placebo in iron deficiency anemia trial

Top-line results from a phase 3 trial suggest the oral, iron-based drug ferric citrate is more effective than placebo for treating iron deficiency anemia in adults with stage 3-5, non-dialysis-dependent chronic kidney disease.
Fifty-two percent of patients who received ferric citrate achieved at least a 1 g/dL increase in hemoglobin over a 16-week period, compared to 19% of patients who received placebo.
Researchers said the safety profile of ferric citrate in this trial was consistent with that in previous studies.
Keryx Biopharmaceuticals, Inc., the company developing ferric citrate, recently announced these results.
Patients and treatment
In this phase 3 study, researchers compared treatment with ferric citrate to placebo in 234 patients who previously had not adequately responded to or tolerated current oral iron therapies. The patients were not allowed to receive any iron (intravenous or oral) or erythropoiesis-stimulating agents during this study.
The patients were randomized 1:1 to receive ferric citrate (n=117) or placebo (n=115). Two patients in the placebo arm discontinued the study and were not included in the efficacy analysis. One discontinued after randomization prior to receiving placebo, and the other discontinued after taking a dose of placebo but before having laboratory values drawn.
The study had a 16-week, randomized, double-blind, placebo-controlled efficacy period, followed by an 8-week, open-label safety extension period. During the extension period, all patients remaining in the study, including the placebo arm, received ferric citrate.
During the efficacy period, ferric citrate was administered at a starting dose of 3 tablets per day, with food, and could be titrated every 4 weeks by an additional 3 tablets, for up to 12 tablets per day. The mean dose of ferric citrate was 5 tablets per day.
Baseline laboratory values were similar between the treatment arms. The mean hemoglobin was 10.4 g/dL in both arms.
The mean transferrin saturation was 20.2% in the ferric citrate arm and 19.6% in the placebo arm. The mean ferritin was 85.9 ng/mL and 81.7 ng/mL, respectively. And the mean serum phosphate was 4.2 mg/dL and 4.1 mg/dL, respectively.
Efficacy results
The study achieved its primary endpoint, with 52.1% (61/117) of patients who received ferric citrate achieving a 1g/dL or greater rise in hemoglobin at any time point during the 16-week efficacy period, compared to 19.1% (22/115) of patients in the placebo arm (P<0.001).
The researchers also observed significant differences in all pre-specified secondary efficacy endpoints.
The mean change in hemoglobin was 0.75 g/dL in the ferric citrate arm and -0.08 g/dL in the placebo arm (P<0.001). The mean change in transferrin saturation was 17.8% and -0.6%, respectively (P<0.001).
The mean change in ferritin was 162.5 ng/mL and -7.7 ng/mL, respectively (P<0.001). And the mean change in serum phosphate was -0.43 mg/dL and -0.22 mg/dL, respectively (P=0.02).
The proportion of patients with a durable response during the efficacy period was 48.7% in the ferric citrate arm and 14.8% in the placebo arm (P<0.001).
A durable response was defined as a mean change in hemoglobin from baseline of at least 0.75 g/dL over any 4-week time period during the efficacy period, provided that an increase of at least 1.0 g/dL had occurred during that 4-week period.
Safety results
During the efficacy period, the majority of adverse events (AEs) were mild to moderate. The most common AEs—in the ferric citrate and placebo arms, respectively—were diarrhea (20.5% vs 16.4%), constipation (18.8% vs 12.9%), discolored feces (14.5% vs 0%), and nausea (11.1% vs 2.6%).
Hypophosphatemia was reported in 4 patients—1 in the ferric citrate arm and 3 in the placebo arm.
Twenty-six percent (31/117) of ferric citrate-treated patients and 30% (35/116) of patients receiving placebo discontinued treatment during the efficacy period. Twelve patients treated with ferric citrate discontinued due to an AE, as did 10 patients who received placebo.
During the efficacy period, the rate of serious AEs was balanced between the ferric citrate and placebo arms, at 12% and 10%, respectively. None of the serious AEs were deemed drug-related.
Over the course of the study, there were 2 deaths reported. Both occurred in patients receiving ferric citrate, but neither were considered drug-related. ![]()

Top-line results from a phase 3 trial suggest the oral, iron-based drug ferric citrate is more effective than placebo for treating iron deficiency anemia in adults with stage 3-5, non-dialysis-dependent chronic kidney disease.
Fifty-two percent of patients who received ferric citrate achieved at least a 1 g/dL increase in hemoglobin over a 16-week period, compared to 19% of patients who received placebo.
Researchers said the safety profile of ferric citrate in this trial was consistent with that in previous studies.
Keryx Biopharmaceuticals, Inc., the company developing ferric citrate, recently announced these results.
Patients and treatment
In this phase 3 study, researchers compared treatment with ferric citrate to placebo in 234 patients who previously had not adequately responded to or tolerated current oral iron therapies. The patients were not allowed to receive any iron (intravenous or oral) or erythropoiesis-stimulating agents during this study.
The patients were randomized 1:1 to receive ferric citrate (n=117) or placebo (n=115). Two patients in the placebo arm discontinued the study and were not included in the efficacy analysis. One discontinued after randomization prior to receiving placebo, and the other discontinued after taking a dose of placebo but before having laboratory values drawn.
The study had a 16-week, randomized, double-blind, placebo-controlled efficacy period, followed by an 8-week, open-label safety extension period. During the extension period, all patients remaining in the study, including the placebo arm, received ferric citrate.
During the efficacy period, ferric citrate was administered at a starting dose of 3 tablets per day, with food, and could be titrated every 4 weeks by an additional 3 tablets, for up to 12 tablets per day. The mean dose of ferric citrate was 5 tablets per day.
Baseline laboratory values were similar between the treatment arms. The mean hemoglobin was 10.4 g/dL in both arms.
The mean transferrin saturation was 20.2% in the ferric citrate arm and 19.6% in the placebo arm. The mean ferritin was 85.9 ng/mL and 81.7 ng/mL, respectively. And the mean serum phosphate was 4.2 mg/dL and 4.1 mg/dL, respectively.
Efficacy results
The study achieved its primary endpoint, with 52.1% (61/117) of patients who received ferric citrate achieving a 1g/dL or greater rise in hemoglobin at any time point during the 16-week efficacy period, compared to 19.1% (22/115) of patients in the placebo arm (P<0.001).
The researchers also observed significant differences in all pre-specified secondary efficacy endpoints.
The mean change in hemoglobin was 0.75 g/dL in the ferric citrate arm and -0.08 g/dL in the placebo arm (P<0.001). The mean change in transferrin saturation was 17.8% and -0.6%, respectively (P<0.001).
The mean change in ferritin was 162.5 ng/mL and -7.7 ng/mL, respectively (P<0.001). And the mean change in serum phosphate was -0.43 mg/dL and -0.22 mg/dL, respectively (P=0.02).
The proportion of patients with a durable response during the efficacy period was 48.7% in the ferric citrate arm and 14.8% in the placebo arm (P<0.001).
A durable response was defined as a mean change in hemoglobin from baseline of at least 0.75 g/dL over any 4-week time period during the efficacy period, provided that an increase of at least 1.0 g/dL had occurred during that 4-week period.
Safety results
During the efficacy period, the majority of adverse events (AEs) were mild to moderate. The most common AEs—in the ferric citrate and placebo arms, respectively—were diarrhea (20.5% vs 16.4%), constipation (18.8% vs 12.9%), discolored feces (14.5% vs 0%), and nausea (11.1% vs 2.6%).
Hypophosphatemia was reported in 4 patients—1 in the ferric citrate arm and 3 in the placebo arm.
Twenty-six percent (31/117) of ferric citrate-treated patients and 30% (35/116) of patients receiving placebo discontinued treatment during the efficacy period. Twelve patients treated with ferric citrate discontinued due to an AE, as did 10 patients who received placebo.
During the efficacy period, the rate of serious AEs was balanced between the ferric citrate and placebo arms, at 12% and 10%, respectively. None of the serious AEs were deemed drug-related.
Over the course of the study, there were 2 deaths reported. Both occurred in patients receiving ferric citrate, but neither were considered drug-related. ![]()

Top-line results from a phase 3 trial suggest the oral, iron-based drug ferric citrate is more effective than placebo for treating iron deficiency anemia in adults with stage 3-5, non-dialysis-dependent chronic kidney disease.
Fifty-two percent of patients who received ferric citrate achieved at least a 1 g/dL increase in hemoglobin over a 16-week period, compared to 19% of patients who received placebo.
Researchers said the safety profile of ferric citrate in this trial was consistent with that in previous studies.
Keryx Biopharmaceuticals, Inc., the company developing ferric citrate, recently announced these results.
Patients and treatment
In this phase 3 study, researchers compared treatment with ferric citrate to placebo in 234 patients who previously had not adequately responded to or tolerated current oral iron therapies. The patients were not allowed to receive any iron (intravenous or oral) or erythropoiesis-stimulating agents during this study.
The patients were randomized 1:1 to receive ferric citrate (n=117) or placebo (n=115). Two patients in the placebo arm discontinued the study and were not included in the efficacy analysis. One discontinued after randomization prior to receiving placebo, and the other discontinued after taking a dose of placebo but before having laboratory values drawn.
The study had a 16-week, randomized, double-blind, placebo-controlled efficacy period, followed by an 8-week, open-label safety extension period. During the extension period, all patients remaining in the study, including the placebo arm, received ferric citrate.
During the efficacy period, ferric citrate was administered at a starting dose of 3 tablets per day, with food, and could be titrated every 4 weeks by an additional 3 tablets, for up to 12 tablets per day. The mean dose of ferric citrate was 5 tablets per day.
Baseline laboratory values were similar between the treatment arms. The mean hemoglobin was 10.4 g/dL in both arms.
The mean transferrin saturation was 20.2% in the ferric citrate arm and 19.6% in the placebo arm. The mean ferritin was 85.9 ng/mL and 81.7 ng/mL, respectively. And the mean serum phosphate was 4.2 mg/dL and 4.1 mg/dL, respectively.
Efficacy results
The study achieved its primary endpoint, with 52.1% (61/117) of patients who received ferric citrate achieving a 1g/dL or greater rise in hemoglobin at any time point during the 16-week efficacy period, compared to 19.1% (22/115) of patients in the placebo arm (P<0.001).
The researchers also observed significant differences in all pre-specified secondary efficacy endpoints.
The mean change in hemoglobin was 0.75 g/dL in the ferric citrate arm and -0.08 g/dL in the placebo arm (P<0.001). The mean change in transferrin saturation was 17.8% and -0.6%, respectively (P<0.001).
The mean change in ferritin was 162.5 ng/mL and -7.7 ng/mL, respectively (P<0.001). And the mean change in serum phosphate was -0.43 mg/dL and -0.22 mg/dL, respectively (P=0.02).
The proportion of patients with a durable response during the efficacy period was 48.7% in the ferric citrate arm and 14.8% in the placebo arm (P<0.001).
A durable response was defined as a mean change in hemoglobin from baseline of at least 0.75 g/dL over any 4-week time period during the efficacy period, provided that an increase of at least 1.0 g/dL had occurred during that 4-week period.
Safety results
During the efficacy period, the majority of adverse events (AEs) were mild to moderate. The most common AEs—in the ferric citrate and placebo arms, respectively—were diarrhea (20.5% vs 16.4%), constipation (18.8% vs 12.9%), discolored feces (14.5% vs 0%), and nausea (11.1% vs 2.6%).
Hypophosphatemia was reported in 4 patients—1 in the ferric citrate arm and 3 in the placebo arm.
Twenty-six percent (31/117) of ferric citrate-treated patients and 30% (35/116) of patients receiving placebo discontinued treatment during the efficacy period. Twelve patients treated with ferric citrate discontinued due to an AE, as did 10 patients who received placebo.
During the efficacy period, the rate of serious AEs was balanced between the ferric citrate and placebo arms, at 12% and 10%, respectively. None of the serious AEs were deemed drug-related.
Over the course of the study, there were 2 deaths reported. Both occurred in patients receiving ferric citrate, but neither were considered drug-related. ![]()
Drug exhibits preclinical activity against MDS

Researchers have found the fusion protein APG101 can rescue erythropoiesis in bone marrow samples from patients with lower-risk myelodysplastic syndromes (MDS).
Previous research suggested that CD95—a receptor that can induce apoptosis when triggered by the CD95 ligand—is overexpressed in two-thirds of patients with lower-risk MDS, and overexpression of CD95 is predictive of a lower response to erythropoiesis-stimulating agents (ESAs).
APG101, which consists of the extracellular domain of the CD95 receptor and the Fc domain of an IgG antibody, is designed to block the CD95 ligand.
The new study showed that APG101 can inhibit apoptosis in erythrocyte precursor cells and improve their overall proliferation rate. The drug increased the number of burst-forming unit-erythroid (BFU-E) progenitors in samples from MDS patients with low BFU-E numbers at baseline.
“APG101 added to cellular assays efficiently rescued the growth of erythroid progenitors in MDS patients harboring a profound defect of erythropoiesis, independent of the expression level of CD95 or CD95 ligand,” said Michaela Fontenay, MD, PhD, of the Institut Cochin in Paris, France.
Dr Fontenay and her colleagues described these results in Oncotarget. The research was funded by Apogenix, the company developing APG101.
By comparing bone marrow samples from MDS patients and healthy control subjects, the researchers found that CD95, but not CD95 ligand, was overexpressed in patients with lower-risk MDS.
Further analysis revealed that a patient’s CD95 expression level at diagnosis could predict response to an ESA. Specifically, CD95 overexpression was predictive of a lower response rate to ESAs in patients with low- or intermediate-1-risk MDS.
Next, the researchers tested bone marrow erythroid progenitors from 3 control subjects and 5 patients with MDS and found that CD95 expression increased during MDS erythroid progenitor amplification but remained lower in control cultures.
On day 5 of culture, the mean number of BFU-Es was significantly lower in MDS patient samples than in controls. And treatment with APG101 prompted a dose-dependent increase in BFU-E growth in MDS samples but not in controls.
When the researchers added APG101 (at 10 μg/mL) to the cultures over 7 days, they observed an improvement in the proliferation of erythroblasts but no significant effect on the kinetics of differentiation.
They also found that APG101 reduced apoptosis in immature precursors by 30% but had no effect on apoptosis in mature precursors.
Baseline BFU-E number affects response
The researchers then assessed the effects of APG101 in samples from 5 control subjects and 20 MDS patients with varying responses to ESAs and varying baseline levels of BFU-Es.
Fifteen of the MDS patients had a significantly lower number of baseline BFU-Es than controls (P=0.005), but 5 MDS patients had a mean number of BFU-Es that was comparable to controls (P=0.429). There was no significant difference in WHO classification or CD95 expression between the 2 groups of MDS patients (P=0.612).
However, 11 of the 15 patients with low erythropoiesis had received an ESA, and all of them were resistant to this treatment. In all, 15 of the MDS patients had received an ESA, and 14 were resistant to it (6 primary and 8 secondary).
The researchers found that APG101 induced a dose-dependent increase of BFU-Es in samples from the 15 MDS patients with low erythropoiesis but not in samples from the 5 patients with normal erythropoiesis or in the control samples (P<0.001).
The team said that a low BFU-E number at baseline was significantly associated with in vitro response to APG101 among the 20 MDS patients (P=0.031) and the 14 ESA-resistant patients (P=0.027).
Further investigation confirmed that a low clonogenic progenitor number at baseline, but not the level of CD95 or CD95 ligand expression, was predictive of the response to APG101.
“This study provides a rationale for further clinical investigation of this potential new therapeutic option in patients with severely impaired erythropoiesis who are resistant to erythropoiesis-stimulating agents,” Dr Fontenay said.
Apogenix has conducted a phase 1 trial of APG101 in transfusion-dependent patients with low- to intermediate-1-risk MDS. The company expects the results of this trial will be available in the coming months. ![]()

Researchers have found the fusion protein APG101 can rescue erythropoiesis in bone marrow samples from patients with lower-risk myelodysplastic syndromes (MDS).
Previous research suggested that CD95—a receptor that can induce apoptosis when triggered by the CD95 ligand—is overexpressed in two-thirds of patients with lower-risk MDS, and overexpression of CD95 is predictive of a lower response to erythropoiesis-stimulating agents (ESAs).
APG101, which consists of the extracellular domain of the CD95 receptor and the Fc domain of an IgG antibody, is designed to block the CD95 ligand.
The new study showed that APG101 can inhibit apoptosis in erythrocyte precursor cells and improve their overall proliferation rate. The drug increased the number of burst-forming unit-erythroid (BFU-E) progenitors in samples from MDS patients with low BFU-E numbers at baseline.
“APG101 added to cellular assays efficiently rescued the growth of erythroid progenitors in MDS patients harboring a profound defect of erythropoiesis, independent of the expression level of CD95 or CD95 ligand,” said Michaela Fontenay, MD, PhD, of the Institut Cochin in Paris, France.
Dr Fontenay and her colleagues described these results in Oncotarget. The research was funded by Apogenix, the company developing APG101.
By comparing bone marrow samples from MDS patients and healthy control subjects, the researchers found that CD95, but not CD95 ligand, was overexpressed in patients with lower-risk MDS.
Further analysis revealed that a patient’s CD95 expression level at diagnosis could predict response to an ESA. Specifically, CD95 overexpression was predictive of a lower response rate to ESAs in patients with low- or intermediate-1-risk MDS.
Next, the researchers tested bone marrow erythroid progenitors from 3 control subjects and 5 patients with MDS and found that CD95 expression increased during MDS erythroid progenitor amplification but remained lower in control cultures.
On day 5 of culture, the mean number of BFU-Es was significantly lower in MDS patient samples than in controls. And treatment with APG101 prompted a dose-dependent increase in BFU-E growth in MDS samples but not in controls.
When the researchers added APG101 (at 10 μg/mL) to the cultures over 7 days, they observed an improvement in the proliferation of erythroblasts but no significant effect on the kinetics of differentiation.
They also found that APG101 reduced apoptosis in immature precursors by 30% but had no effect on apoptosis in mature precursors.
Baseline BFU-E number affects response
The researchers then assessed the effects of APG101 in samples from 5 control subjects and 20 MDS patients with varying responses to ESAs and varying baseline levels of BFU-Es.
Fifteen of the MDS patients had a significantly lower number of baseline BFU-Es than controls (P=0.005), but 5 MDS patients had a mean number of BFU-Es that was comparable to controls (P=0.429). There was no significant difference in WHO classification or CD95 expression between the 2 groups of MDS patients (P=0.612).
However, 11 of the 15 patients with low erythropoiesis had received an ESA, and all of them were resistant to this treatment. In all, 15 of the MDS patients had received an ESA, and 14 were resistant to it (6 primary and 8 secondary).
The researchers found that APG101 induced a dose-dependent increase of BFU-Es in samples from the 15 MDS patients with low erythropoiesis but not in samples from the 5 patients with normal erythropoiesis or in the control samples (P<0.001).
The team said that a low BFU-E number at baseline was significantly associated with in vitro response to APG101 among the 20 MDS patients (P=0.031) and the 14 ESA-resistant patients (P=0.027).
Further investigation confirmed that a low clonogenic progenitor number at baseline, but not the level of CD95 or CD95 ligand expression, was predictive of the response to APG101.
“This study provides a rationale for further clinical investigation of this potential new therapeutic option in patients with severely impaired erythropoiesis who are resistant to erythropoiesis-stimulating agents,” Dr Fontenay said.
Apogenix has conducted a phase 1 trial of APG101 in transfusion-dependent patients with low- to intermediate-1-risk MDS. The company expects the results of this trial will be available in the coming months. ![]()

Researchers have found the fusion protein APG101 can rescue erythropoiesis in bone marrow samples from patients with lower-risk myelodysplastic syndromes (MDS).
Previous research suggested that CD95—a receptor that can induce apoptosis when triggered by the CD95 ligand—is overexpressed in two-thirds of patients with lower-risk MDS, and overexpression of CD95 is predictive of a lower response to erythropoiesis-stimulating agents (ESAs).
APG101, which consists of the extracellular domain of the CD95 receptor and the Fc domain of an IgG antibody, is designed to block the CD95 ligand.
The new study showed that APG101 can inhibit apoptosis in erythrocyte precursor cells and improve their overall proliferation rate. The drug increased the number of burst-forming unit-erythroid (BFU-E) progenitors in samples from MDS patients with low BFU-E numbers at baseline.
“APG101 added to cellular assays efficiently rescued the growth of erythroid progenitors in MDS patients harboring a profound defect of erythropoiesis, independent of the expression level of CD95 or CD95 ligand,” said Michaela Fontenay, MD, PhD, of the Institut Cochin in Paris, France.
Dr Fontenay and her colleagues described these results in Oncotarget. The research was funded by Apogenix, the company developing APG101.
By comparing bone marrow samples from MDS patients and healthy control subjects, the researchers found that CD95, but not CD95 ligand, was overexpressed in patients with lower-risk MDS.
Further analysis revealed that a patient’s CD95 expression level at diagnosis could predict response to an ESA. Specifically, CD95 overexpression was predictive of a lower response rate to ESAs in patients with low- or intermediate-1-risk MDS.
Next, the researchers tested bone marrow erythroid progenitors from 3 control subjects and 5 patients with MDS and found that CD95 expression increased during MDS erythroid progenitor amplification but remained lower in control cultures.
On day 5 of culture, the mean number of BFU-Es was significantly lower in MDS patient samples than in controls. And treatment with APG101 prompted a dose-dependent increase in BFU-E growth in MDS samples but not in controls.
When the researchers added APG101 (at 10 μg/mL) to the cultures over 7 days, they observed an improvement in the proliferation of erythroblasts but no significant effect on the kinetics of differentiation.
They also found that APG101 reduced apoptosis in immature precursors by 30% but had no effect on apoptosis in mature precursors.
Baseline BFU-E number affects response
The researchers then assessed the effects of APG101 in samples from 5 control subjects and 20 MDS patients with varying responses to ESAs and varying baseline levels of BFU-Es.
Fifteen of the MDS patients had a significantly lower number of baseline BFU-Es than controls (P=0.005), but 5 MDS patients had a mean number of BFU-Es that was comparable to controls (P=0.429). There was no significant difference in WHO classification or CD95 expression between the 2 groups of MDS patients (P=0.612).
However, 11 of the 15 patients with low erythropoiesis had received an ESA, and all of them were resistant to this treatment. In all, 15 of the MDS patients had received an ESA, and 14 were resistant to it (6 primary and 8 secondary).
The researchers found that APG101 induced a dose-dependent increase of BFU-Es in samples from the 15 MDS patients with low erythropoiesis but not in samples from the 5 patients with normal erythropoiesis or in the control samples (P<0.001).
The team said that a low BFU-E number at baseline was significantly associated with in vitro response to APG101 among the 20 MDS patients (P=0.031) and the 14 ESA-resistant patients (P=0.027).
Further investigation confirmed that a low clonogenic progenitor number at baseline, but not the level of CD95 or CD95 ligand expression, was predictive of the response to APG101.
“This study provides a rationale for further clinical investigation of this potential new therapeutic option in patients with severely impaired erythropoiesis who are resistant to erythropoiesis-stimulating agents,” Dr Fontenay said.
Apogenix has conducted a phase 1 trial of APG101 in transfusion-dependent patients with low- to intermediate-1-risk MDS. The company expects the results of this trial will be available in the coming months. ![]()
mAb gets breakthrough designation for HLH

Photo by Linda Bartlett
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to NI-0501 for the treatment of patients with primary hemophagocytic lymphohistiocytosis (HLH) who have refractory disease or recurrent/progressive disease during conventional therapy.
NI-0501 is a fully human monoclonal antibody (mAb) targeting interferon-gamma (IFNγ) that is being developed by Novimmune.
The biological activity of IFNγ, which is considered to have a pivotal pathogenic role in HLH, is neutralized by NI-0501.
“The FDA’s designation of NI-0501 as a breakthrough therapy recognizes the potential of NI-0501 to address an important unmet medical need in a disease with still high mortality, and for which there are no approved treatments,” said Novimmune Chairman and Chief Executive Officer Eduard Holdener.
The breakthrough therapy designation is intended to expedite the development and review of new therapies for serious or life threatening conditions, which have shown encouraging early clinical results demonstrating substantial improvement on a clinically significant endpoint over available therapies.
The FDA granted breakthrough designation to NI-0501 on the basis of data from a phase 2 study in children with primary HLH. Preliminary results from this study were presented at the 2015 ASH Annual Meeting.
The trial included 16 patients—8 males and 8 females. Their median age was 1.2 years (range, 0.2-13).
Two patients were receiving NI-0501 as first-line treatment, and the rest were receiving the mAb as second-line treatment. Patients had previously received dexamethasone (n=13), methylprednisone (n=2), etoposide (n=13), ATG (n=4), cyclosporine A (n=6), and “other” therapy (n=4).
NI-0501 was given at a starting dose of 1 mg/kg every 3 days, with possible dose increases guided by pharmacokinetic data and/or clinical response in each patient. The mAb was administered with dexamethasone at a dose of 5 mg/m2 to 10 mg/m2, but dexamethasone could be tapered during the treatment course.
The treatment duration ranged from 4 weeks to 8 weeks, and the follow-up period was 4 weeks.
Efficacy
One patient was excluded from the analysis due to a lymphoma diagnosis after enrollment. Two patients were still receiving treatment as of the ASH presentation, and 13 have completed treatment.
Among the patients who completed therapy, 4 had an insufficient response. Two of these patients died, and 2 proceeded to allogeneic hematopoietic stem cell transplant (HSCT) after receiving additional agents to control their disease.
Nine patients achieved a favorable response to NI-0501. Seven of these patients proceeded to HSCT, and 2 were awaiting HSCT at the time of the ASH presentation, with their disease well-controlled.
Post-transplant follow-up is still early for most patients, but 2 patients have follow-up greater than 1 year. One child died of graft-vs-host disease around day 45, but the remaining patients who went on to HSCT were still alive as of the ASH presentation.
Safety
No off-target effects of NI-0501 were observed, and none of the patients withdrew from the study for safety reasons.
There were 14 serious adverse events reported in 8 patients, but only 1 of these events was considered treatment-related.
The patient had necrotizing fasciitis following P aeruginosa skin infection, which resolved. This event was considered treatment-related by an investigator but not by the data monitoring committee or the sponsor.
Three patients had died as of the ASH presentation, but none of the deaths were related to NI-0501. Two patients died of HLH/multi-organ failure, and 1 died of graft-vs-host disease. ![]()

Photo by Linda Bartlett
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to NI-0501 for the treatment of patients with primary hemophagocytic lymphohistiocytosis (HLH) who have refractory disease or recurrent/progressive disease during conventional therapy.
NI-0501 is a fully human monoclonal antibody (mAb) targeting interferon-gamma (IFNγ) that is being developed by Novimmune.
The biological activity of IFNγ, which is considered to have a pivotal pathogenic role in HLH, is neutralized by NI-0501.
“The FDA’s designation of NI-0501 as a breakthrough therapy recognizes the potential of NI-0501 to address an important unmet medical need in a disease with still high mortality, and for which there are no approved treatments,” said Novimmune Chairman and Chief Executive Officer Eduard Holdener.
The breakthrough therapy designation is intended to expedite the development and review of new therapies for serious or life threatening conditions, which have shown encouraging early clinical results demonstrating substantial improvement on a clinically significant endpoint over available therapies.
The FDA granted breakthrough designation to NI-0501 on the basis of data from a phase 2 study in children with primary HLH. Preliminary results from this study were presented at the 2015 ASH Annual Meeting.
The trial included 16 patients—8 males and 8 females. Their median age was 1.2 years (range, 0.2-13).
Two patients were receiving NI-0501 as first-line treatment, and the rest were receiving the mAb as second-line treatment. Patients had previously received dexamethasone (n=13), methylprednisone (n=2), etoposide (n=13), ATG (n=4), cyclosporine A (n=6), and “other” therapy (n=4).
NI-0501 was given at a starting dose of 1 mg/kg every 3 days, with possible dose increases guided by pharmacokinetic data and/or clinical response in each patient. The mAb was administered with dexamethasone at a dose of 5 mg/m2 to 10 mg/m2, but dexamethasone could be tapered during the treatment course.
The treatment duration ranged from 4 weeks to 8 weeks, and the follow-up period was 4 weeks.
Efficacy
One patient was excluded from the analysis due to a lymphoma diagnosis after enrollment. Two patients were still receiving treatment as of the ASH presentation, and 13 have completed treatment.
Among the patients who completed therapy, 4 had an insufficient response. Two of these patients died, and 2 proceeded to allogeneic hematopoietic stem cell transplant (HSCT) after receiving additional agents to control their disease.
Nine patients achieved a favorable response to NI-0501. Seven of these patients proceeded to HSCT, and 2 were awaiting HSCT at the time of the ASH presentation, with their disease well-controlled.
Post-transplant follow-up is still early for most patients, but 2 patients have follow-up greater than 1 year. One child died of graft-vs-host disease around day 45, but the remaining patients who went on to HSCT were still alive as of the ASH presentation.
Safety
No off-target effects of NI-0501 were observed, and none of the patients withdrew from the study for safety reasons.
There were 14 serious adverse events reported in 8 patients, but only 1 of these events was considered treatment-related.
The patient had necrotizing fasciitis following P aeruginosa skin infection, which resolved. This event was considered treatment-related by an investigator but not by the data monitoring committee or the sponsor.
Three patients had died as of the ASH presentation, but none of the deaths were related to NI-0501. Two patients died of HLH/multi-organ failure, and 1 died of graft-vs-host disease. ![]()

Photo by Linda Bartlett
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to NI-0501 for the treatment of patients with primary hemophagocytic lymphohistiocytosis (HLH) who have refractory disease or recurrent/progressive disease during conventional therapy.
NI-0501 is a fully human monoclonal antibody (mAb) targeting interferon-gamma (IFNγ) that is being developed by Novimmune.
The biological activity of IFNγ, which is considered to have a pivotal pathogenic role in HLH, is neutralized by NI-0501.
“The FDA’s designation of NI-0501 as a breakthrough therapy recognizes the potential of NI-0501 to address an important unmet medical need in a disease with still high mortality, and for which there are no approved treatments,” said Novimmune Chairman and Chief Executive Officer Eduard Holdener.
The breakthrough therapy designation is intended to expedite the development and review of new therapies for serious or life threatening conditions, which have shown encouraging early clinical results demonstrating substantial improvement on a clinically significant endpoint over available therapies.
The FDA granted breakthrough designation to NI-0501 on the basis of data from a phase 2 study in children with primary HLH. Preliminary results from this study were presented at the 2015 ASH Annual Meeting.
The trial included 16 patients—8 males and 8 females. Their median age was 1.2 years (range, 0.2-13).
Two patients were receiving NI-0501 as first-line treatment, and the rest were receiving the mAb as second-line treatment. Patients had previously received dexamethasone (n=13), methylprednisone (n=2), etoposide (n=13), ATG (n=4), cyclosporine A (n=6), and “other” therapy (n=4).
NI-0501 was given at a starting dose of 1 mg/kg every 3 days, with possible dose increases guided by pharmacokinetic data and/or clinical response in each patient. The mAb was administered with dexamethasone at a dose of 5 mg/m2 to 10 mg/m2, but dexamethasone could be tapered during the treatment course.
The treatment duration ranged from 4 weeks to 8 weeks, and the follow-up period was 4 weeks.
Efficacy
One patient was excluded from the analysis due to a lymphoma diagnosis after enrollment. Two patients were still receiving treatment as of the ASH presentation, and 13 have completed treatment.
Among the patients who completed therapy, 4 had an insufficient response. Two of these patients died, and 2 proceeded to allogeneic hematopoietic stem cell transplant (HSCT) after receiving additional agents to control their disease.
Nine patients achieved a favorable response to NI-0501. Seven of these patients proceeded to HSCT, and 2 were awaiting HSCT at the time of the ASH presentation, with their disease well-controlled.
Post-transplant follow-up is still early for most patients, but 2 patients have follow-up greater than 1 year. One child died of graft-vs-host disease around day 45, but the remaining patients who went on to HSCT were still alive as of the ASH presentation.
Safety
No off-target effects of NI-0501 were observed, and none of the patients withdrew from the study for safety reasons.
There were 14 serious adverse events reported in 8 patients, but only 1 of these events was considered treatment-related.
The patient had necrotizing fasciitis following P aeruginosa skin infection, which resolved. This event was considered treatment-related by an investigator but not by the data monitoring committee or the sponsor.
Three patients had died as of the ASH presentation, but none of the deaths were related to NI-0501. Two patients died of HLH/multi-organ failure, and 1 died of graft-vs-host disease. ![]()
Drug may best BSC in some high-risk MDS patients

Results of a phase 3 trial suggest the small-molecule inhibitor rigosertib may improve overall survival (OS) in some patients with higher-risk myelodysplastic syndromes (HR-MDS).
Overall, researchers found no significant difference in OS between patients who received rigosertib and those who received best supportive care (BSC).
However, the data indicate that rigosertib can confer a survival benefit in certain subgroups of HR-MDS patients.
The results of this trial, known as ONTIME, were published in The Lancet Oncology. The trial was sponsored by Onconova Therapeutics, Inc., the company developing rigosertib.
The trial enrolled 299 HR-MDS patients. They had refractory anemia with excess blasts (RAEB)-1, RAEB-2, RAEB-t, or chronic myelomonocytic leukemia based on local site assessment. They had all failed treatment with a hypomethylating agent (HMA) in the past 2 years.
The patients were randomized (2:1) to receive rigosertib at 1800 mg per 24 hours via 72-hour continuous intravenous (IV) infusion, administered every other week (n=199), or BSC with or without low-dose cytarabine (n=100).
At a median follow-up of 19.5 months, there was no significant difference in OS between the treatment arms. The median OS was 8.2 months in the rigosertib arm and 5.9 months in the BSC arm. The hazard ratio (HR) was 0.87 (P=0.33).
However, the researchers said that subgroup analyses suggested rigosertib may provide a survival benefit over BSC in some HR-MDS patients. This includes:
- Patients younger than 75 years of age (HR=0.55, P=0.0010)
- Patients who received HMA therapy for 9 months or fewer (HR=0.54, P=0.0016)
- Patients with primary, rather than secondary, HMA failure (HR=0.72, P=0.060)
- Patients who were classified as “very high risk” according to the Revised International Prognostic Scoring System (HR=0.61, P=0.015)
- Patients with monosomy 7 (HR=0.26, P=0.0041)
- Patients with trisomy 8 (HR=0.28, P=0.0083).
The most common grade 3 or higher adverse events—in the rigosertib and BSC arms, respectively—were anemia (18% vs 8%), thrombocytopenia (19% vs 7%), neutropenia (17% vs 8%), febrile neutropenia (12% vs 11%), and pneumonia (12% vs 11%).
Twenty-two percent of patients in the rigosertib arm and 33% in the BSC arm died due to adverse events. Three deaths were attributed to rigosertib.
“Rigosertib was well-tolerated in patients with a high unmet medical need who have no approved therapeutic options,” said study author Guillermo Garcia-Manero, MD, of The University of Texas MD Anderson Cancer Center in Houston.
“We are impressed by the trend to notable efficacy in well-defined, well-balanced subgroups of HR-MDS patients with very poor prognosis. Based on these findings, we have designed the new phase 3 INSPIRE study with IV rigosertib, which is currently enrolling patients.”
INSPIRE is a randomized, controlled study designed to assess the efficacy and safety of IV rigosertib in HR-MDS patients under 80 years of age who had progressed on, failed to respond to, or relapsed after previous treatment with an HMA within the first 9 months of HMA treatment initiation.
The trial is expected to enroll approximately 225 patients, who will be randomized at a 2:1 ratio into 2 treatment arms: IV rigosertib plus BSC versus physician’s choice plus BSC. The primary endpoint is OS. Full details on the trial can be found on clinicaltrials.gov (NCT02562443). ![]()

Results of a phase 3 trial suggest the small-molecule inhibitor rigosertib may improve overall survival (OS) in some patients with higher-risk myelodysplastic syndromes (HR-MDS).
Overall, researchers found no significant difference in OS between patients who received rigosertib and those who received best supportive care (BSC).
However, the data indicate that rigosertib can confer a survival benefit in certain subgroups of HR-MDS patients.
The results of this trial, known as ONTIME, were published in The Lancet Oncology. The trial was sponsored by Onconova Therapeutics, Inc., the company developing rigosertib.
The trial enrolled 299 HR-MDS patients. They had refractory anemia with excess blasts (RAEB)-1, RAEB-2, RAEB-t, or chronic myelomonocytic leukemia based on local site assessment. They had all failed treatment with a hypomethylating agent (HMA) in the past 2 years.
The patients were randomized (2:1) to receive rigosertib at 1800 mg per 24 hours via 72-hour continuous intravenous (IV) infusion, administered every other week (n=199), or BSC with or without low-dose cytarabine (n=100).
At a median follow-up of 19.5 months, there was no significant difference in OS between the treatment arms. The median OS was 8.2 months in the rigosertib arm and 5.9 months in the BSC arm. The hazard ratio (HR) was 0.87 (P=0.33).
However, the researchers said that subgroup analyses suggested rigosertib may provide a survival benefit over BSC in some HR-MDS patients. This includes:
- Patients younger than 75 years of age (HR=0.55, P=0.0010)
- Patients who received HMA therapy for 9 months or fewer (HR=0.54, P=0.0016)
- Patients with primary, rather than secondary, HMA failure (HR=0.72, P=0.060)
- Patients who were classified as “very high risk” according to the Revised International Prognostic Scoring System (HR=0.61, P=0.015)
- Patients with monosomy 7 (HR=0.26, P=0.0041)
- Patients with trisomy 8 (HR=0.28, P=0.0083).
The most common grade 3 or higher adverse events—in the rigosertib and BSC arms, respectively—were anemia (18% vs 8%), thrombocytopenia (19% vs 7%), neutropenia (17% vs 8%), febrile neutropenia (12% vs 11%), and pneumonia (12% vs 11%).
Twenty-two percent of patients in the rigosertib arm and 33% in the BSC arm died due to adverse events. Three deaths were attributed to rigosertib.
“Rigosertib was well-tolerated in patients with a high unmet medical need who have no approved therapeutic options,” said study author Guillermo Garcia-Manero, MD, of The University of Texas MD Anderson Cancer Center in Houston.
“We are impressed by the trend to notable efficacy in well-defined, well-balanced subgroups of HR-MDS patients with very poor prognosis. Based on these findings, we have designed the new phase 3 INSPIRE study with IV rigosertib, which is currently enrolling patients.”
INSPIRE is a randomized, controlled study designed to assess the efficacy and safety of IV rigosertib in HR-MDS patients under 80 years of age who had progressed on, failed to respond to, or relapsed after previous treatment with an HMA within the first 9 months of HMA treatment initiation.
The trial is expected to enroll approximately 225 patients, who will be randomized at a 2:1 ratio into 2 treatment arms: IV rigosertib plus BSC versus physician’s choice plus BSC. The primary endpoint is OS. Full details on the trial can be found on clinicaltrials.gov (NCT02562443). ![]()

Results of a phase 3 trial suggest the small-molecule inhibitor rigosertib may improve overall survival (OS) in some patients with higher-risk myelodysplastic syndromes (HR-MDS).
Overall, researchers found no significant difference in OS between patients who received rigosertib and those who received best supportive care (BSC).
However, the data indicate that rigosertib can confer a survival benefit in certain subgroups of HR-MDS patients.
The results of this trial, known as ONTIME, were published in The Lancet Oncology. The trial was sponsored by Onconova Therapeutics, Inc., the company developing rigosertib.
The trial enrolled 299 HR-MDS patients. They had refractory anemia with excess blasts (RAEB)-1, RAEB-2, RAEB-t, or chronic myelomonocytic leukemia based on local site assessment. They had all failed treatment with a hypomethylating agent (HMA) in the past 2 years.
The patients were randomized (2:1) to receive rigosertib at 1800 mg per 24 hours via 72-hour continuous intravenous (IV) infusion, administered every other week (n=199), or BSC with or without low-dose cytarabine (n=100).
At a median follow-up of 19.5 months, there was no significant difference in OS between the treatment arms. The median OS was 8.2 months in the rigosertib arm and 5.9 months in the BSC arm. The hazard ratio (HR) was 0.87 (P=0.33).
However, the researchers said that subgroup analyses suggested rigosertib may provide a survival benefit over BSC in some HR-MDS patients. This includes:
- Patients younger than 75 years of age (HR=0.55, P=0.0010)
- Patients who received HMA therapy for 9 months or fewer (HR=0.54, P=0.0016)
- Patients with primary, rather than secondary, HMA failure (HR=0.72, P=0.060)
- Patients who were classified as “very high risk” according to the Revised International Prognostic Scoring System (HR=0.61, P=0.015)
- Patients with monosomy 7 (HR=0.26, P=0.0041)
- Patients with trisomy 8 (HR=0.28, P=0.0083).
The most common grade 3 or higher adverse events—in the rigosertib and BSC arms, respectively—were anemia (18% vs 8%), thrombocytopenia (19% vs 7%), neutropenia (17% vs 8%), febrile neutropenia (12% vs 11%), and pneumonia (12% vs 11%).
Twenty-two percent of patients in the rigosertib arm and 33% in the BSC arm died due to adverse events. Three deaths were attributed to rigosertib.
“Rigosertib was well-tolerated in patients with a high unmet medical need who have no approved therapeutic options,” said study author Guillermo Garcia-Manero, MD, of The University of Texas MD Anderson Cancer Center in Houston.
“We are impressed by the trend to notable efficacy in well-defined, well-balanced subgroups of HR-MDS patients with very poor prognosis. Based on these findings, we have designed the new phase 3 INSPIRE study with IV rigosertib, which is currently enrolling patients.”
INSPIRE is a randomized, controlled study designed to assess the efficacy and safety of IV rigosertib in HR-MDS patients under 80 years of age who had progressed on, failed to respond to, or relapsed after previous treatment with an HMA within the first 9 months of HMA treatment initiation.
The trial is expected to enroll approximately 225 patients, who will be randomized at a 2:1 ratio into 2 treatment arms: IV rigosertib plus BSC versus physician’s choice plus BSC. The primary endpoint is OS. Full details on the trial can be found on clinicaltrials.gov (NCT02562443). ![]()
Germline mutations linked to hematologic malignancies

A new study suggests mutations in the gene DDX41 occur in families where hematologic malignancies are common.
Previous research showed that both germline and acquired DDX41 mutations occur in families with multiple cases of late-onset myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).
The new study, published in Blood, has linked germline mutations in DDX41 to chronic myeloid leukemia and lymphomas as well.
“This is the first gene identified in families with lymphoma and represents a major breakthrough for the field,” said study author Hamish Scott, PhD, of the University of Adelaide in South Australia.
“Researchers are recognizing now that genetic predisposition to blood cancer is more common than previously thought, and our study shows the importance of taking a thorough family history at diagnosis.”
To conduct this study, Dr Scott and his colleagues screened 2 cohorts of families with a range of hematologic disorders (malignant and non-malignant). One cohort included 240 individuals from 93 families in Australia. The other included 246 individuals from 198 families in the US.
In all, 9 of the families (3%) had germline DDX41 mutations.
Three families carried the recurrent p.D140Gfs*2 mutation, which was linked to AML.
One family carried a germline mutation—p.R525H, c.1574G.A—that was previously described only as a somatic mutation at the time of progression to MDS or AML. In the current study, the mutation was again linked to MDS and AML.
Five families carried novel DDX41 mutations.
One of these mutations was a germline substitution—c.435-2_435-1delAGinsCA—that was linked to MDS in 1 family.
Two families had a missense start-loss substitution—c.3G.A, p.M1I—that was linked to MDS, AML, chronic myeloid leukemia, and non-Hodgkin lymphoma.
One family had a DDX41 missense variant—c.490C.T, p.R164W. This was linked to Hodgkin and non-Hodgkin lymphoma (including 3 cases of follicular lymphoma). There was a possible link to multiple myeloma as well, but the diagnosis could not be confirmed.
And 1 family had a missense mutation in the helicase domain—p.G530D—that was linked to AML.
“DDX41 is a new type of cancer predisposition gene, and we are still investigating its function,” Dr Scott noted.
“But it appears to have dual roles in regulating the correct expression of genes in the cell and also enabling the immune system to respond to threats such as bacteria and viruses, as well as the development of cancer cells. Immunotherapy is a promising approach for cancer treatment, and our research to understand the function of DDX41 will help design better therapies.” ![]()

A new study suggests mutations in the gene DDX41 occur in families where hematologic malignancies are common.
Previous research showed that both germline and acquired DDX41 mutations occur in families with multiple cases of late-onset myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).
The new study, published in Blood, has linked germline mutations in DDX41 to chronic myeloid leukemia and lymphomas as well.
“This is the first gene identified in families with lymphoma and represents a major breakthrough for the field,” said study author Hamish Scott, PhD, of the University of Adelaide in South Australia.
“Researchers are recognizing now that genetic predisposition to blood cancer is more common than previously thought, and our study shows the importance of taking a thorough family history at diagnosis.”
To conduct this study, Dr Scott and his colleagues screened 2 cohorts of families with a range of hematologic disorders (malignant and non-malignant). One cohort included 240 individuals from 93 families in Australia. The other included 246 individuals from 198 families in the US.
In all, 9 of the families (3%) had germline DDX41 mutations.
Three families carried the recurrent p.D140Gfs*2 mutation, which was linked to AML.
One family carried a germline mutation—p.R525H, c.1574G.A—that was previously described only as a somatic mutation at the time of progression to MDS or AML. In the current study, the mutation was again linked to MDS and AML.
Five families carried novel DDX41 mutations.
One of these mutations was a germline substitution—c.435-2_435-1delAGinsCA—that was linked to MDS in 1 family.
Two families had a missense start-loss substitution—c.3G.A, p.M1I—that was linked to MDS, AML, chronic myeloid leukemia, and non-Hodgkin lymphoma.
One family had a DDX41 missense variant—c.490C.T, p.R164W. This was linked to Hodgkin and non-Hodgkin lymphoma (including 3 cases of follicular lymphoma). There was a possible link to multiple myeloma as well, but the diagnosis could not be confirmed.
And 1 family had a missense mutation in the helicase domain—p.G530D—that was linked to AML.
“DDX41 is a new type of cancer predisposition gene, and we are still investigating its function,” Dr Scott noted.
“But it appears to have dual roles in regulating the correct expression of genes in the cell and also enabling the immune system to respond to threats such as bacteria and viruses, as well as the development of cancer cells. Immunotherapy is a promising approach for cancer treatment, and our research to understand the function of DDX41 will help design better therapies.” ![]()

A new study suggests mutations in the gene DDX41 occur in families where hematologic malignancies are common.
Previous research showed that both germline and acquired DDX41 mutations occur in families with multiple cases of late-onset myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).
The new study, published in Blood, has linked germline mutations in DDX41 to chronic myeloid leukemia and lymphomas as well.
“This is the first gene identified in families with lymphoma and represents a major breakthrough for the field,” said study author Hamish Scott, PhD, of the University of Adelaide in South Australia.
“Researchers are recognizing now that genetic predisposition to blood cancer is more common than previously thought, and our study shows the importance of taking a thorough family history at diagnosis.”
To conduct this study, Dr Scott and his colleagues screened 2 cohorts of families with a range of hematologic disorders (malignant and non-malignant). One cohort included 240 individuals from 93 families in Australia. The other included 246 individuals from 198 families in the US.
In all, 9 of the families (3%) had germline DDX41 mutations.
Three families carried the recurrent p.D140Gfs*2 mutation, which was linked to AML.
One family carried a germline mutation—p.R525H, c.1574G.A—that was previously described only as a somatic mutation at the time of progression to MDS or AML. In the current study, the mutation was again linked to MDS and AML.
Five families carried novel DDX41 mutations.
One of these mutations was a germline substitution—c.435-2_435-1delAGinsCA—that was linked to MDS in 1 family.
Two families had a missense start-loss substitution—c.3G.A, p.M1I—that was linked to MDS, AML, chronic myeloid leukemia, and non-Hodgkin lymphoma.
One family had a DDX41 missense variant—c.490C.T, p.R164W. This was linked to Hodgkin and non-Hodgkin lymphoma (including 3 cases of follicular lymphoma). There was a possible link to multiple myeloma as well, but the diagnosis could not be confirmed.
And 1 family had a missense mutation in the helicase domain—p.G530D—that was linked to AML.
“DDX41 is a new type of cancer predisposition gene, and we are still investigating its function,” Dr Scott noted.
“But it appears to have dual roles in regulating the correct expression of genes in the cell and also enabling the immune system to respond to threats such as bacteria and viruses, as well as the development of cancer cells. Immunotherapy is a promising approach for cancer treatment, and our research to understand the function of DDX41 will help design better therapies.”
System could aid assessment of sickle cell disease

and a normal one
Image by Betty Pace
A microfluidic system can measure the deformability and adhesion of red blood cells (RBCs) in samples from patients with sickle cell disease (SCD), according to research published in Technology.
The researchers noted that RBC deformability has been associated with vaso-occlusion in SCD, but we have limited knowledge on deformation characteristics of RBCs adhered to endothelium-associated proteins in microphysiological fluid flow conditions.
In the past, various approaches have been used to measure RBC deformability, including optical tweezers, micropipette aspiration, and atomic force microscopy. These methods have enabled sensitive and controlled measurement of RBC mechanical properties, but they are typically performed in open environments without fluid flow.
“Microfluidic techniques allow incorporation of physiological flow conditions, as well as biologically relevant adhesion surfaces in a closed setting, which better mimic the natural physiological environment of the RBCs in blood flow,” said study author Umut Gurkan, PhD, of the Case Western Reserve University in Cleveland, Ohio.
For their system, Dr Gurkan and his colleagues integrated a microfluidic approach with a cell-dimensioning algorithm.
They introduced a new parameter to assess the deformability of RBCs. It is known as the dynamic deformability index (DDI), which they defined as the time-dependent change of the cell’s aspect ratio in response to fluid flow shear stress.
The researchers assessed the deformability and adhesion of RBCs containing healthy hemoglobin A (HbA) and homozygous sickle hemoglobin (HbS). And they found the DDI of HbS-containing RBCs was significantly lower than the DDI of HbA-containing RBCs.
The team also found they could divide HbS-containing RBCs into 2 groups—deformable and non-deformable RBCs.
“We report, for the first time, on the subpopulations of RBCs in terms of dynamic deformation characteristics in SCD: deformable and non-deformable RBCs,” said Yunus Alapan, a PhD candidate at Case Western Reserve University.
“Furthermore, we analyzed adhesion of non-deformable RBCs, in comparison to deformable RBCs, quantitatively at physiological and above physiological flow shear stresses in blood samples obtained from SCD patients.”
“We observed significantly greater numbers of adhered non-deformable sickle RBCs than deformable sickle RBCs at flow shear stresses well above the physiological range, suggesting an interplay between dynamic deformability and increased adhesion of RBCs in vaso-occlusive events.”
Now, the researchers are working to further characterize deformability and adhesion of RBCs in a greater number of SCD patients to analyze their associations with clinical phenotypes and complications.
The team said their system may provide important biophysical insights into disease pathophysiology when widely applied in SCD.
They also believe the microfluidic platform has the potential to be used as an in vitro assay for monitoring disease activity at baseline, during clinical flux after treatment, during painful episodes, and in association with long-term complications.

and a normal one
Image by Betty Pace
A microfluidic system can measure the deformability and adhesion of red blood cells (RBCs) in samples from patients with sickle cell disease (SCD), according to research published in Technology.
The researchers noted that RBC deformability has been associated with vaso-occlusion in SCD, but we have limited knowledge on deformation characteristics of RBCs adhered to endothelium-associated proteins in microphysiological fluid flow conditions.
In the past, various approaches have been used to measure RBC deformability, including optical tweezers, micropipette aspiration, and atomic force microscopy. These methods have enabled sensitive and controlled measurement of RBC mechanical properties, but they are typically performed in open environments without fluid flow.
“Microfluidic techniques allow incorporation of physiological flow conditions, as well as biologically relevant adhesion surfaces in a closed setting, which better mimic the natural physiological environment of the RBCs in blood flow,” said study author Umut Gurkan, PhD, of the Case Western Reserve University in Cleveland, Ohio.
For their system, Dr Gurkan and his colleagues integrated a microfluidic approach with a cell-dimensioning algorithm.
They introduced a new parameter to assess the deformability of RBCs. It is known as the dynamic deformability index (DDI), which they defined as the time-dependent change of the cell’s aspect ratio in response to fluid flow shear stress.
The researchers assessed the deformability and adhesion of RBCs containing healthy hemoglobin A (HbA) and homozygous sickle hemoglobin (HbS). And they found the DDI of HbS-containing RBCs was significantly lower than the DDI of HbA-containing RBCs.
The team also found they could divide HbS-containing RBCs into 2 groups—deformable and non-deformable RBCs.
“We report, for the first time, on the subpopulations of RBCs in terms of dynamic deformation characteristics in SCD: deformable and non-deformable RBCs,” said Yunus Alapan, a PhD candidate at Case Western Reserve University.
“Furthermore, we analyzed adhesion of non-deformable RBCs, in comparison to deformable RBCs, quantitatively at physiological and above physiological flow shear stresses in blood samples obtained from SCD patients.”
“We observed significantly greater numbers of adhered non-deformable sickle RBCs than deformable sickle RBCs at flow shear stresses well above the physiological range, suggesting an interplay between dynamic deformability and increased adhesion of RBCs in vaso-occlusive events.”
Now, the researchers are working to further characterize deformability and adhesion of RBCs in a greater number of SCD patients to analyze their associations with clinical phenotypes and complications.
The team said their system may provide important biophysical insights into disease pathophysiology when widely applied in SCD.
They also believe the microfluidic platform has the potential to be used as an in vitro assay for monitoring disease activity at baseline, during clinical flux after treatment, during painful episodes, and in association with long-term complications.

and a normal one
Image by Betty Pace
A microfluidic system can measure the deformability and adhesion of red blood cells (RBCs) in samples from patients with sickle cell disease (SCD), according to research published in Technology.
The researchers noted that RBC deformability has been associated with vaso-occlusion in SCD, but we have limited knowledge on deformation characteristics of RBCs adhered to endothelium-associated proteins in microphysiological fluid flow conditions.
In the past, various approaches have been used to measure RBC deformability, including optical tweezers, micropipette aspiration, and atomic force microscopy. These methods have enabled sensitive and controlled measurement of RBC mechanical properties, but they are typically performed in open environments without fluid flow.
“Microfluidic techniques allow incorporation of physiological flow conditions, as well as biologically relevant adhesion surfaces in a closed setting, which better mimic the natural physiological environment of the RBCs in blood flow,” said study author Umut Gurkan, PhD, of the Case Western Reserve University in Cleveland, Ohio.
For their system, Dr Gurkan and his colleagues integrated a microfluidic approach with a cell-dimensioning algorithm.
They introduced a new parameter to assess the deformability of RBCs. It is known as the dynamic deformability index (DDI), which they defined as the time-dependent change of the cell’s aspect ratio in response to fluid flow shear stress.
The researchers assessed the deformability and adhesion of RBCs containing healthy hemoglobin A (HbA) and homozygous sickle hemoglobin (HbS). And they found the DDI of HbS-containing RBCs was significantly lower than the DDI of HbA-containing RBCs.
The team also found they could divide HbS-containing RBCs into 2 groups—deformable and non-deformable RBCs.
“We report, for the first time, on the subpopulations of RBCs in terms of dynamic deformation characteristics in SCD: deformable and non-deformable RBCs,” said Yunus Alapan, a PhD candidate at Case Western Reserve University.
“Furthermore, we analyzed adhesion of non-deformable RBCs, in comparison to deformable RBCs, quantitatively at physiological and above physiological flow shear stresses in blood samples obtained from SCD patients.”
“We observed significantly greater numbers of adhered non-deformable sickle RBCs than deformable sickle RBCs at flow shear stresses well above the physiological range, suggesting an interplay between dynamic deformability and increased adhesion of RBCs in vaso-occlusive events.”
Now, the researchers are working to further characterize deformability and adhesion of RBCs in a greater number of SCD patients to analyze their associations with clinical phenotypes and complications.
The team said their system may provide important biophysical insights into disease pathophysiology when widely applied in SCD.
They also believe the microfluidic platform has the potential to be used as an in vitro assay for monitoring disease activity at baseline, during clinical flux after treatment, during painful episodes, and in association with long-term complications.
NICE recommends device for managing SCD

Image courtesy of Terumo BCT
The National Institute for Health and Care Excellence (NICE) has issued a guidance recommending a new device for managing sickle cell disease (SCD).
The device is the Spectra Optia Apheresis System for automated red blood cell (RBC) exchange in patients with SCD who need regular blood transfusions.
The Spectra Optia Apheresis System automatically replaces sickled RBCs with healthy RBCs.
The system is made up of 3 components: an apheresis machine, embedded software, and a single-use, disposable blood tubing set.
NICE said the Spectra Optia system is faster than manual RBC exchange, and patients need RBC exchange less often with this device. In addition, the system could provide considerable savings to the National Health Service (NHS) in England.
“The device could save the NHS in England an estimated £13 million each year—around £18,000 per patient—with the size of the saving depending on the patient’s condition and the equipment already owned by the NHS,” said Carole Longson, director of the NICE Centre for Health Technology Evaluation.
“We also recommend that specialists collaborate to collect and publish data on some outcomes of treatment with Spectra Optia to provide further clinical evidence. It would be particularly helpful to have long-term data on how automated and manual exchange affects the amount of iron in the body and the need to treat this complication.”
Treatment with the Spectra Optia system is intended to be iron-neutral, meaning that patients who are already iron-overloaded can have their condition managed effectively.
The Spectra Optia Apheresis System is manufactured by Terumo BCT.
Costs and savings
The list prices (excluding tax) for the components of the Spectra Optia Apheresis System are as follows:
- Spectra Optia device: £45,350
- RBC exchange software: £6700
- Spectra Optia exchange set: £1007 per 6
- Astotube with injection port: £218 per 50
- ACD-A anticoagulant (750 ml): £57 per 12
- Service charge: £4572 per year.
Bulk order discounts are available.
Based on current evidence and expert advice on the anticipated benefits of the technology when used in patients with iron overload, cost modelling shows that, in most cases, using Spectra Optia is cost-saving compared with manual RBC exchange or top-up transfusion.
The savings depend on the iron overload status of the patient and are more likely to be achieved if devices already owned by the NHS can be used to treat SCD.
The estimated cost saving for adopting Spectra Optia is £18,100 per patient per year, which has the potential to save the NHS £12.9 million each year.

Image courtesy of Terumo BCT
The National Institute for Health and Care Excellence (NICE) has issued a guidance recommending a new device for managing sickle cell disease (SCD).
The device is the Spectra Optia Apheresis System for automated red blood cell (RBC) exchange in patients with SCD who need regular blood transfusions.
The Spectra Optia Apheresis System automatically replaces sickled RBCs with healthy RBCs.
The system is made up of 3 components: an apheresis machine, embedded software, and a single-use, disposable blood tubing set.
NICE said the Spectra Optia system is faster than manual RBC exchange, and patients need RBC exchange less often with this device. In addition, the system could provide considerable savings to the National Health Service (NHS) in England.
“The device could save the NHS in England an estimated £13 million each year—around £18,000 per patient—with the size of the saving depending on the patient’s condition and the equipment already owned by the NHS,” said Carole Longson, director of the NICE Centre for Health Technology Evaluation.
“We also recommend that specialists collaborate to collect and publish data on some outcomes of treatment with Spectra Optia to provide further clinical evidence. It would be particularly helpful to have long-term data on how automated and manual exchange affects the amount of iron in the body and the need to treat this complication.”
Treatment with the Spectra Optia system is intended to be iron-neutral, meaning that patients who are already iron-overloaded can have their condition managed effectively.
The Spectra Optia Apheresis System is manufactured by Terumo BCT.
Costs and savings
The list prices (excluding tax) for the components of the Spectra Optia Apheresis System are as follows:
- Spectra Optia device: £45,350
- RBC exchange software: £6700
- Spectra Optia exchange set: £1007 per 6
- Astotube with injection port: £218 per 50
- ACD-A anticoagulant (750 ml): £57 per 12
- Service charge: £4572 per year.
Bulk order discounts are available.
Based on current evidence and expert advice on the anticipated benefits of the technology when used in patients with iron overload, cost modelling shows that, in most cases, using Spectra Optia is cost-saving compared with manual RBC exchange or top-up transfusion.
The savings depend on the iron overload status of the patient and are more likely to be achieved if devices already owned by the NHS can be used to treat SCD.
The estimated cost saving for adopting Spectra Optia is £18,100 per patient per year, which has the potential to save the NHS £12.9 million each year.

Image courtesy of Terumo BCT
The National Institute for Health and Care Excellence (NICE) has issued a guidance recommending a new device for managing sickle cell disease (SCD).
The device is the Spectra Optia Apheresis System for automated red blood cell (RBC) exchange in patients with SCD who need regular blood transfusions.
The Spectra Optia Apheresis System automatically replaces sickled RBCs with healthy RBCs.
The system is made up of 3 components: an apheresis machine, embedded software, and a single-use, disposable blood tubing set.
NICE said the Spectra Optia system is faster than manual RBC exchange, and patients need RBC exchange less often with this device. In addition, the system could provide considerable savings to the National Health Service (NHS) in England.
“The device could save the NHS in England an estimated £13 million each year—around £18,000 per patient—with the size of the saving depending on the patient’s condition and the equipment already owned by the NHS,” said Carole Longson, director of the NICE Centre for Health Technology Evaluation.
“We also recommend that specialists collaborate to collect and publish data on some outcomes of treatment with Spectra Optia to provide further clinical evidence. It would be particularly helpful to have long-term data on how automated and manual exchange affects the amount of iron in the body and the need to treat this complication.”
Treatment with the Spectra Optia system is intended to be iron-neutral, meaning that patients who are already iron-overloaded can have their condition managed effectively.
The Spectra Optia Apheresis System is manufactured by Terumo BCT.
Costs and savings
The list prices (excluding tax) for the components of the Spectra Optia Apheresis System are as follows:
- Spectra Optia device: £45,350
- RBC exchange software: £6700
- Spectra Optia exchange set: £1007 per 6
- Astotube with injection port: £218 per 50
- ACD-A anticoagulant (750 ml): £57 per 12
- Service charge: £4572 per year.
Bulk order discounts are available.
Based on current evidence and expert advice on the anticipated benefits of the technology when used in patients with iron overload, cost modelling shows that, in most cases, using Spectra Optia is cost-saving compared with manual RBC exchange or top-up transfusion.
The savings depend on the iron overload status of the patient and are more likely to be achieved if devices already owned by the NHS can be used to treat SCD.
The estimated cost saving for adopting Spectra Optia is £18,100 per patient per year, which has the potential to save the NHS £12.9 million each year.

