User login
Yoga, CBT provide long-term improvement in insomnia, worry
new research suggests.
The study is the first to compare the long-term effects from the two interventions; and the results offer clinicians and patients two effective choices for reducing worry and anxiety, researchers noted.
“Anxiety can be a really big problem for older adults,” lead investigator Suzanne Danhauer, PhD, professor of social sciences and health policy at Wake Forest University, Winston-Salem, N.C., said in an interview.
“So to find something they can do that lasts ... and has some enduring impact on their quality of life and their mental health, and they’re both nonpharmacologic treatments, I think for a lot of older people that’s really attractive,” Dr. Danhauer said.
The findings are published in the September issue of the American Journal of Geriatric Psychiatry.
Long-term benefits
The two-stage randomized preference trial included 500 community-dwelling individuals over age 60 who scored 26 or above on the Penn State Worry Questionnaire–Abbreviated (PSWQ-A), indicating heightened anxiety and worry.
Half the group took part in a randomized, controlled trial comparing CBT (n = 125) with yoga (n = 125). The other half participated in a preference trial where they were allowed to choose between CBT (n = 120) and yoga (n = 130).
Participants completed 20 yoga sessions over 10 weeks or 10 weekly CBT calls between May 2017 and November 2018.
Measures used included the PSWQ-A, the Insomnia Severity Index (ISI), the Patient Reported Outcomes Measurement Information System (PROMIS) Short Form v1.0 – Anxiety 8a, and the PROMIS-29 to assess depression, fatigue, physical function, social participation, and pain.
In 2020, the researchers published results at 11 weeks showing improvements from baseline in all areas. The scores for anxiety and worry were similar between the CBT and yoga groups, but CBT yielded significantly higher improvement in insomnia.
At 37 weeks, about 6 months after the interventions had ended, the investigators found even greater improvements from baseline in all areas measured – except physical function.
However, at that point, there were no significant differences between the two interventions in either the randomized controlled trial or the preference trial. There were also no differences in the results between the two trial designs.
“There were some little differences, but by and large we found both interventions to be efficacious,” Dr. Danhauer said. “This gives clinicians [the] choice to be able to say, ‘you can try either one of these and they’re probably going to help.’ ”
Beyond statistically significant
The researchers also found the improvements were not just statistically significant, but were also clinically meaningful for worry, anxiety, and insomnia.
Meaningful changes were defined as a decrease of at least 5.5 points on the PSWQ-A for worry, a decrease of at least 3 points on the PROMIS Anxiety scale for anxiety, and a decrease of at least 6 points in the ISI for insomnia.
At long-term follow-up, the majority of participants in both the CBT and yoga arms of the randomized, controlled trial demonstrated meaningful change in worry (85.7% and 77.6%, respectively), anxiety (82.1% and 80.8%), and insomnia (52.8% and 44.3%).
The majority of participants also reported meaningful improvements in generalized anxiety symptoms, depressive symptoms, and fatigue, but not for physical function, pain interference, or pain intensity.
“That’s the part to me that’s particularly notable. The improvements weren’t just statistically significant, they were clinically meaningful as well,” Dr. Danhauer said.
“When it comes right down to people’s lives, they want differences they can feel and see and not just what a P value looks like,” she added.
Real-world impact
In an accompanying editorial, Carmen Andreescu, MD, associate professor of psychiatry at the University of Pittsburgh, agreed that the results have “real-world impact.”
“Clinicians can direct their patients toward interventions that may be beneficial, consolidate the results over time and avoid fueling the well-trained worry cognitive loop with concerns related to potential side effects,” Dr. Andreescu wrote.
She adds that interventions such as these “may increase accessibility and provide relief for the immediate suffering of our patients.”
The study was funded by the Patient-Centered Outcomes Research Institute Program. Dr. Danhauer and Dr. Andreescu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
The study is the first to compare the long-term effects from the two interventions; and the results offer clinicians and patients two effective choices for reducing worry and anxiety, researchers noted.
“Anxiety can be a really big problem for older adults,” lead investigator Suzanne Danhauer, PhD, professor of social sciences and health policy at Wake Forest University, Winston-Salem, N.C., said in an interview.
“So to find something they can do that lasts ... and has some enduring impact on their quality of life and their mental health, and they’re both nonpharmacologic treatments, I think for a lot of older people that’s really attractive,” Dr. Danhauer said.
The findings are published in the September issue of the American Journal of Geriatric Psychiatry.
Long-term benefits
The two-stage randomized preference trial included 500 community-dwelling individuals over age 60 who scored 26 or above on the Penn State Worry Questionnaire–Abbreviated (PSWQ-A), indicating heightened anxiety and worry.
Half the group took part in a randomized, controlled trial comparing CBT (n = 125) with yoga (n = 125). The other half participated in a preference trial where they were allowed to choose between CBT (n = 120) and yoga (n = 130).
Participants completed 20 yoga sessions over 10 weeks or 10 weekly CBT calls between May 2017 and November 2018.
Measures used included the PSWQ-A, the Insomnia Severity Index (ISI), the Patient Reported Outcomes Measurement Information System (PROMIS) Short Form v1.0 – Anxiety 8a, and the PROMIS-29 to assess depression, fatigue, physical function, social participation, and pain.
In 2020, the researchers published results at 11 weeks showing improvements from baseline in all areas. The scores for anxiety and worry were similar between the CBT and yoga groups, but CBT yielded significantly higher improvement in insomnia.
At 37 weeks, about 6 months after the interventions had ended, the investigators found even greater improvements from baseline in all areas measured – except physical function.
However, at that point, there were no significant differences between the two interventions in either the randomized controlled trial or the preference trial. There were also no differences in the results between the two trial designs.
“There were some little differences, but by and large we found both interventions to be efficacious,” Dr. Danhauer said. “This gives clinicians [the] choice to be able to say, ‘you can try either one of these and they’re probably going to help.’ ”
Beyond statistically significant
The researchers also found the improvements were not just statistically significant, but were also clinically meaningful for worry, anxiety, and insomnia.
Meaningful changes were defined as a decrease of at least 5.5 points on the PSWQ-A for worry, a decrease of at least 3 points on the PROMIS Anxiety scale for anxiety, and a decrease of at least 6 points in the ISI for insomnia.
At long-term follow-up, the majority of participants in both the CBT and yoga arms of the randomized, controlled trial demonstrated meaningful change in worry (85.7% and 77.6%, respectively), anxiety (82.1% and 80.8%), and insomnia (52.8% and 44.3%).
The majority of participants also reported meaningful improvements in generalized anxiety symptoms, depressive symptoms, and fatigue, but not for physical function, pain interference, or pain intensity.
“That’s the part to me that’s particularly notable. The improvements weren’t just statistically significant, they were clinically meaningful as well,” Dr. Danhauer said.
“When it comes right down to people’s lives, they want differences they can feel and see and not just what a P value looks like,” she added.
Real-world impact
In an accompanying editorial, Carmen Andreescu, MD, associate professor of psychiatry at the University of Pittsburgh, agreed that the results have “real-world impact.”
“Clinicians can direct their patients toward interventions that may be beneficial, consolidate the results over time and avoid fueling the well-trained worry cognitive loop with concerns related to potential side effects,” Dr. Andreescu wrote.
She adds that interventions such as these “may increase accessibility and provide relief for the immediate suffering of our patients.”
The study was funded by the Patient-Centered Outcomes Research Institute Program. Dr. Danhauer and Dr. Andreescu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
The study is the first to compare the long-term effects from the two interventions; and the results offer clinicians and patients two effective choices for reducing worry and anxiety, researchers noted.
“Anxiety can be a really big problem for older adults,” lead investigator Suzanne Danhauer, PhD, professor of social sciences and health policy at Wake Forest University, Winston-Salem, N.C., said in an interview.
“So to find something they can do that lasts ... and has some enduring impact on their quality of life and their mental health, and they’re both nonpharmacologic treatments, I think for a lot of older people that’s really attractive,” Dr. Danhauer said.
The findings are published in the September issue of the American Journal of Geriatric Psychiatry.
Long-term benefits
The two-stage randomized preference trial included 500 community-dwelling individuals over age 60 who scored 26 or above on the Penn State Worry Questionnaire–Abbreviated (PSWQ-A), indicating heightened anxiety and worry.
Half the group took part in a randomized, controlled trial comparing CBT (n = 125) with yoga (n = 125). The other half participated in a preference trial where they were allowed to choose between CBT (n = 120) and yoga (n = 130).
Participants completed 20 yoga sessions over 10 weeks or 10 weekly CBT calls between May 2017 and November 2018.
Measures used included the PSWQ-A, the Insomnia Severity Index (ISI), the Patient Reported Outcomes Measurement Information System (PROMIS) Short Form v1.0 – Anxiety 8a, and the PROMIS-29 to assess depression, fatigue, physical function, social participation, and pain.
In 2020, the researchers published results at 11 weeks showing improvements from baseline in all areas. The scores for anxiety and worry were similar between the CBT and yoga groups, but CBT yielded significantly higher improvement in insomnia.
At 37 weeks, about 6 months after the interventions had ended, the investigators found even greater improvements from baseline in all areas measured – except physical function.
However, at that point, there were no significant differences between the two interventions in either the randomized controlled trial or the preference trial. There were also no differences in the results between the two trial designs.
“There were some little differences, but by and large we found both interventions to be efficacious,” Dr. Danhauer said. “This gives clinicians [the] choice to be able to say, ‘you can try either one of these and they’re probably going to help.’ ”
Beyond statistically significant
The researchers also found the improvements were not just statistically significant, but were also clinically meaningful for worry, anxiety, and insomnia.
Meaningful changes were defined as a decrease of at least 5.5 points on the PSWQ-A for worry, a decrease of at least 3 points on the PROMIS Anxiety scale for anxiety, and a decrease of at least 6 points in the ISI for insomnia.
At long-term follow-up, the majority of participants in both the CBT and yoga arms of the randomized, controlled trial demonstrated meaningful change in worry (85.7% and 77.6%, respectively), anxiety (82.1% and 80.8%), and insomnia (52.8% and 44.3%).
The majority of participants also reported meaningful improvements in generalized anxiety symptoms, depressive symptoms, and fatigue, but not for physical function, pain interference, or pain intensity.
“That’s the part to me that’s particularly notable. The improvements weren’t just statistically significant, they were clinically meaningful as well,” Dr. Danhauer said.
“When it comes right down to people’s lives, they want differences they can feel and see and not just what a P value looks like,” she added.
Real-world impact
In an accompanying editorial, Carmen Andreescu, MD, associate professor of psychiatry at the University of Pittsburgh, agreed that the results have “real-world impact.”
“Clinicians can direct their patients toward interventions that may be beneficial, consolidate the results over time and avoid fueling the well-trained worry cognitive loop with concerns related to potential side effects,” Dr. Andreescu wrote.
She adds that interventions such as these “may increase accessibility and provide relief for the immediate suffering of our patients.”
The study was funded by the Patient-Centered Outcomes Research Institute Program. Dr. Danhauer and Dr. Andreescu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AMERICAN JOURNAL OF GERIATRIC PSYCHIATRY
Neuropsychiatric symptoms after stroke
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.
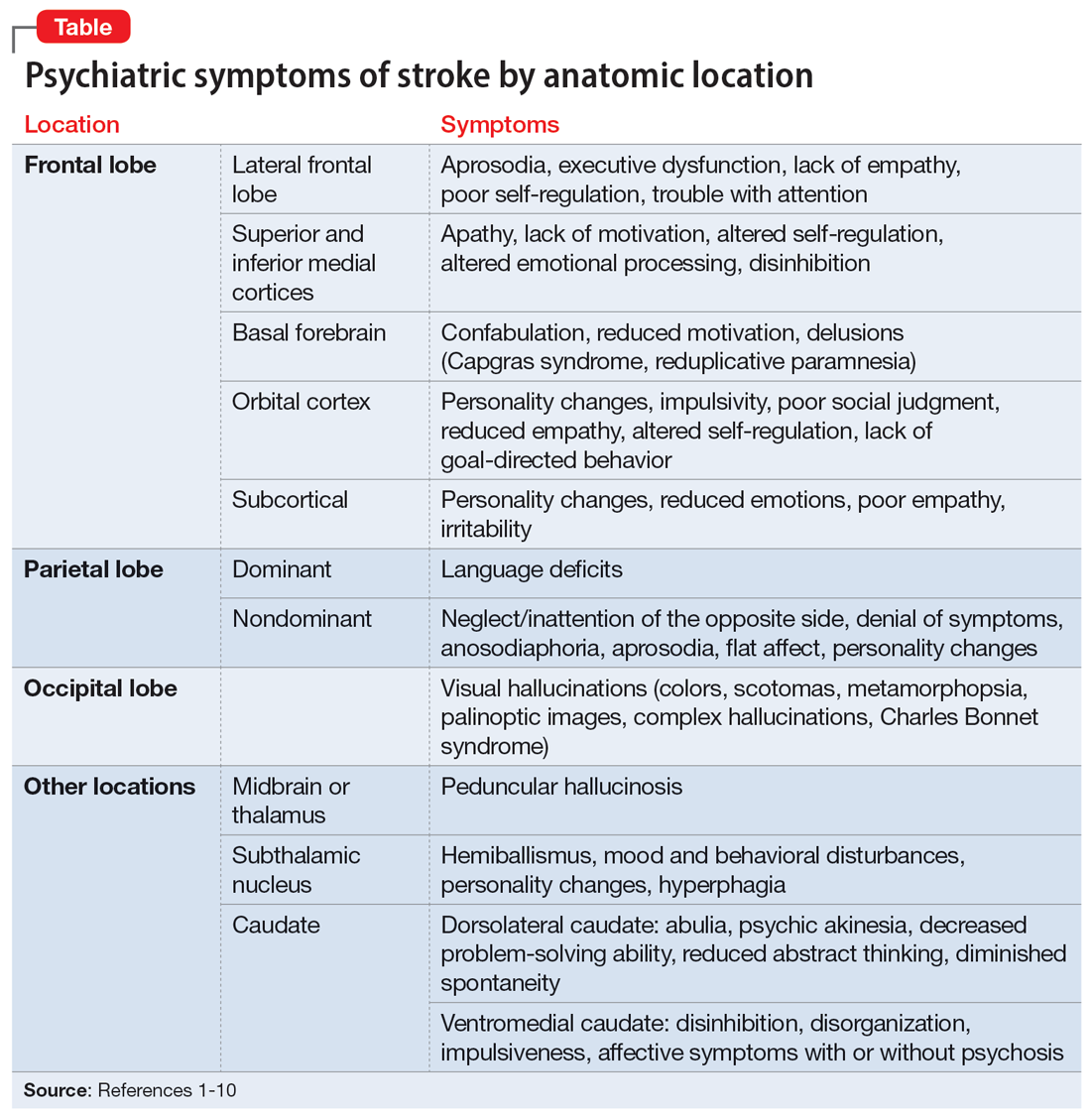
Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.

Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.

Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.
Proposal for a new diagnosis: Acute anxiety disorder
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Mr. F, age 42, says he has always been a very anxious person and has chronically found his worrying to negatively affect his life. He says that over the last month his anxiety has been “off the charts” and he is worrying “24/7” due to taking on new responsibilities at his job and his son being diagnosed with lupus. He says his constant worrying is significantly impairing his ability to focus at his job, and he is considering taking a mental health leave from work. His wife reports that she is extremely frustrated because Mr. F has been isolating himself from family and friends; he admits this is true and attributes it to being preoccupied by his worries.
Mr. F endorses chronic insomnia, muscle tension, and irritability associated with anxiety; these have all substantially worsened over the last month. He admits that recently he has occasionally thought it would be easier if he weren’t alive. Mr. F denies having problems with his energy or motivation levels and insists that he generally feels very anxious, but not depressed. He says he drinks 1 alcoholic drink per week and denies any other substance use. Mr. F is overweight and has slightly elevated cholesterol but denies any other health conditions. He takes melatonin to help him sleep but does not take any prescribed medications.
Although this vignette provides limited details, on the surface it appears that Mr. F is experiencing an exacerbation of chronic generalized anxiety disorder (GAD). However, in this article, I propose establishing a new diagnosis: “acute anxiety disorder,” which would encapsulate severe exacerbations of a pre-existing anxiety disorder. Among the patients I have encountered for whom this diagnosis would fit, most have pre-existing GAD or panic disorder.
A look at the differential diagnosis
It is important to determine whether Mr. F is using any substances or has a medical condition that could be contributing to his anxiety. Other psychiatric diagnoses that could be considered include:
Adjustment disorder. This diagnosis would make sense if Mr. F didn’t have an apparent chronic history of symptoms that meet criteria for GAD.
Major depressive disorder with anxious distress. Many patients experiencing a major depressive episode meet the criteria for the specifier “with anxious distress,” even those who do not have a comorbid anxiety disorder.1 However, it is not evident from this vignette that Mr. F is experiencing a major depressive episode.
Continue to: Panic disorder and GAD...
Panic disorder and GAD. It is possible for a patient with GAD to develop panic disorder, which, at times, occurs after experiencing significant life stressors. Panic disorder requires the presence of recurrent panic attacks. Mr. F describes experiencing chronic, intense symptoms of anxiety rather than the discreet episodes of acute symptoms that characterize panic attacks.
Acute stress disorder. This diagnosis involves psychological symptoms that occur in response to exposure to actual or threatened death, serious injury, or sexual violation. Mr. F was not exposed to any of these stressors.
Why this new diagnosis would be helpful
A new diagnosis, acute anxiety disorder, would indicate that a patient is currently experiencing an acute exacerbation of a chronic anxiety disorder that is leading to a significant decrease in their baseline functioning. My proposed criteria for acute anxiety disorder appear in the Table. Here are some reasons this diagnosis would be helpful:

Signifier of severity. Anxiety disorders such as GAD are generally not considered severe conditions and not considered to fall under the rubric of SPMI (severe and persistent mental illness).2 Posttraumatic stress disorder is the anxiety disorder–like condition most often found in the SPMI category. A diagnosis of acute anxiety disorder would indicate a patient is experiencing an episode of anxiety that is distinct from their chronic anxiety condition due to its severe impact on functional capabilities. Acute anxiety disorder would certainly not qualify as a “SPMI diagnosis” that would facilitate someone being considered eligible for supplemental security income, but it might be a legitimate justification for someone to receive short-term disability.
Treatment approach. The pharmacologic treatment of anxiety disorders usually involves a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI). However, these medications can sometimes briefly increase anxiety when they are started. Individuals with acute anxiety are the most vulnerable to the possibility of experiencing increased anxiety when starting an SSRI or SNRI and may benefit from a slower titration of these medications. In light of this and the length of time required for SSRIs or SNRIs to exert a positive effect (typically a few weeks), patients with acute anxiety are best served by treatment with a medication with an immediate onset of action, such as a benzodiazepine or a sleep medication (eg, zolpidem). Benzodiazepines and hypnotics such as zolpidem are best prescribed for as-needed use because they carry a risk of dependence. One might consider prescribing mirtazapine or pregabalin (both of which are used off-label to treat anxiety) because these medications also have a relatively rapid onset of action and can treat both anxiety and insomnia (particularly mirtazapine).
Research considerations. It would be helpful to study which treatments are most effective for the subset of patients who experience acute anxiety disorder as I define it. Perhaps psychotherapy treatment protocols could be adapted or created. Treatment with esketamine or IV ketamine might be further studied as a treatment for acute anxiety because some evidence suggests ketamine is efficacious for this indication.3
1. Otsubo T, Hokama C, Sano N, et al. How significant is the assessment of the DSM-5 ‘anxious distress’ specifier in patients with major depressive disorder without comorbid anxiety disorders in the continuation/maintenance phase? Int J Psychiatry Clin Pract. 2021;25(4):385-392. doi:10.1080/13651501.2021.1907415
2. Butler H, O’Brien AJ. Access to specialist palliative care services by people with severe and persistent mental illness: a retrospective cohort study. Int J Ment Health Nurs. 2018;27(2):737-746. doi:10.1111/inm.12360
3. Glue P, Neehoff SM, Medlicott NJ, et al. Safety and efficacy of maintenance ketamine treatment in patients with treatment-refractory generalised anxiety and social anxiety disorders. J Psychopharmacol. 2018;32(6):663-667. doi:10.1177/0269881118762073
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Mr. F, age 42, says he has always been a very anxious person and has chronically found his worrying to negatively affect his life. He says that over the last month his anxiety has been “off the charts” and he is worrying “24/7” due to taking on new responsibilities at his job and his son being diagnosed with lupus. He says his constant worrying is significantly impairing his ability to focus at his job, and he is considering taking a mental health leave from work. His wife reports that she is extremely frustrated because Mr. F has been isolating himself from family and friends; he admits this is true and attributes it to being preoccupied by his worries.
Mr. F endorses chronic insomnia, muscle tension, and irritability associated with anxiety; these have all substantially worsened over the last month. He admits that recently he has occasionally thought it would be easier if he weren’t alive. Mr. F denies having problems with his energy or motivation levels and insists that he generally feels very anxious, but not depressed. He says he drinks 1 alcoholic drink per week and denies any other substance use. Mr. F is overweight and has slightly elevated cholesterol but denies any other health conditions. He takes melatonin to help him sleep but does not take any prescribed medications.
Although this vignette provides limited details, on the surface it appears that Mr. F is experiencing an exacerbation of chronic generalized anxiety disorder (GAD). However, in this article, I propose establishing a new diagnosis: “acute anxiety disorder,” which would encapsulate severe exacerbations of a pre-existing anxiety disorder. Among the patients I have encountered for whom this diagnosis would fit, most have pre-existing GAD or panic disorder.
A look at the differential diagnosis
It is important to determine whether Mr. F is using any substances or has a medical condition that could be contributing to his anxiety. Other psychiatric diagnoses that could be considered include:
Adjustment disorder. This diagnosis would make sense if Mr. F didn’t have an apparent chronic history of symptoms that meet criteria for GAD.
Major depressive disorder with anxious distress. Many patients experiencing a major depressive episode meet the criteria for the specifier “with anxious distress,” even those who do not have a comorbid anxiety disorder.1 However, it is not evident from this vignette that Mr. F is experiencing a major depressive episode.
Continue to: Panic disorder and GAD...
Panic disorder and GAD. It is possible for a patient with GAD to develop panic disorder, which, at times, occurs after experiencing significant life stressors. Panic disorder requires the presence of recurrent panic attacks. Mr. F describes experiencing chronic, intense symptoms of anxiety rather than the discreet episodes of acute symptoms that characterize panic attacks.
Acute stress disorder. This diagnosis involves psychological symptoms that occur in response to exposure to actual or threatened death, serious injury, or sexual violation. Mr. F was not exposed to any of these stressors.
Why this new diagnosis would be helpful
A new diagnosis, acute anxiety disorder, would indicate that a patient is currently experiencing an acute exacerbation of a chronic anxiety disorder that is leading to a significant decrease in their baseline functioning. My proposed criteria for acute anxiety disorder appear in the Table. Here are some reasons this diagnosis would be helpful:

Signifier of severity. Anxiety disorders such as GAD are generally not considered severe conditions and not considered to fall under the rubric of SPMI (severe and persistent mental illness).2 Posttraumatic stress disorder is the anxiety disorder–like condition most often found in the SPMI category. A diagnosis of acute anxiety disorder would indicate a patient is experiencing an episode of anxiety that is distinct from their chronic anxiety condition due to its severe impact on functional capabilities. Acute anxiety disorder would certainly not qualify as a “SPMI diagnosis” that would facilitate someone being considered eligible for supplemental security income, but it might be a legitimate justification for someone to receive short-term disability.
Treatment approach. The pharmacologic treatment of anxiety disorders usually involves a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI). However, these medications can sometimes briefly increase anxiety when they are started. Individuals with acute anxiety are the most vulnerable to the possibility of experiencing increased anxiety when starting an SSRI or SNRI and may benefit from a slower titration of these medications. In light of this and the length of time required for SSRIs or SNRIs to exert a positive effect (typically a few weeks), patients with acute anxiety are best served by treatment with a medication with an immediate onset of action, such as a benzodiazepine or a sleep medication (eg, zolpidem). Benzodiazepines and hypnotics such as zolpidem are best prescribed for as-needed use because they carry a risk of dependence. One might consider prescribing mirtazapine or pregabalin (both of which are used off-label to treat anxiety) because these medications also have a relatively rapid onset of action and can treat both anxiety and insomnia (particularly mirtazapine).
Research considerations. It would be helpful to study which treatments are most effective for the subset of patients who experience acute anxiety disorder as I define it. Perhaps psychotherapy treatment protocols could be adapted or created. Treatment with esketamine or IV ketamine might be further studied as a treatment for acute anxiety because some evidence suggests ketamine is efficacious for this indication.3
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Mr. F, age 42, says he has always been a very anxious person and has chronically found his worrying to negatively affect his life. He says that over the last month his anxiety has been “off the charts” and he is worrying “24/7” due to taking on new responsibilities at his job and his son being diagnosed with lupus. He says his constant worrying is significantly impairing his ability to focus at his job, and he is considering taking a mental health leave from work. His wife reports that she is extremely frustrated because Mr. F has been isolating himself from family and friends; he admits this is true and attributes it to being preoccupied by his worries.
Mr. F endorses chronic insomnia, muscle tension, and irritability associated with anxiety; these have all substantially worsened over the last month. He admits that recently he has occasionally thought it would be easier if he weren’t alive. Mr. F denies having problems with his energy or motivation levels and insists that he generally feels very anxious, but not depressed. He says he drinks 1 alcoholic drink per week and denies any other substance use. Mr. F is overweight and has slightly elevated cholesterol but denies any other health conditions. He takes melatonin to help him sleep but does not take any prescribed medications.
Although this vignette provides limited details, on the surface it appears that Mr. F is experiencing an exacerbation of chronic generalized anxiety disorder (GAD). However, in this article, I propose establishing a new diagnosis: “acute anxiety disorder,” which would encapsulate severe exacerbations of a pre-existing anxiety disorder. Among the patients I have encountered for whom this diagnosis would fit, most have pre-existing GAD or panic disorder.
A look at the differential diagnosis
It is important to determine whether Mr. F is using any substances or has a medical condition that could be contributing to his anxiety. Other psychiatric diagnoses that could be considered include:
Adjustment disorder. This diagnosis would make sense if Mr. F didn’t have an apparent chronic history of symptoms that meet criteria for GAD.
Major depressive disorder with anxious distress. Many patients experiencing a major depressive episode meet the criteria for the specifier “with anxious distress,” even those who do not have a comorbid anxiety disorder.1 However, it is not evident from this vignette that Mr. F is experiencing a major depressive episode.
Continue to: Panic disorder and GAD...
Panic disorder and GAD. It is possible for a patient with GAD to develop panic disorder, which, at times, occurs after experiencing significant life stressors. Panic disorder requires the presence of recurrent panic attacks. Mr. F describes experiencing chronic, intense symptoms of anxiety rather than the discreet episodes of acute symptoms that characterize panic attacks.
Acute stress disorder. This diagnosis involves psychological symptoms that occur in response to exposure to actual or threatened death, serious injury, or sexual violation. Mr. F was not exposed to any of these stressors.
Why this new diagnosis would be helpful
A new diagnosis, acute anxiety disorder, would indicate that a patient is currently experiencing an acute exacerbation of a chronic anxiety disorder that is leading to a significant decrease in their baseline functioning. My proposed criteria for acute anxiety disorder appear in the Table. Here are some reasons this diagnosis would be helpful:

Signifier of severity. Anxiety disorders such as GAD are generally not considered severe conditions and not considered to fall under the rubric of SPMI (severe and persistent mental illness).2 Posttraumatic stress disorder is the anxiety disorder–like condition most often found in the SPMI category. A diagnosis of acute anxiety disorder would indicate a patient is experiencing an episode of anxiety that is distinct from their chronic anxiety condition due to its severe impact on functional capabilities. Acute anxiety disorder would certainly not qualify as a “SPMI diagnosis” that would facilitate someone being considered eligible for supplemental security income, but it might be a legitimate justification for someone to receive short-term disability.
Treatment approach. The pharmacologic treatment of anxiety disorders usually involves a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI). However, these medications can sometimes briefly increase anxiety when they are started. Individuals with acute anxiety are the most vulnerable to the possibility of experiencing increased anxiety when starting an SSRI or SNRI and may benefit from a slower titration of these medications. In light of this and the length of time required for SSRIs or SNRIs to exert a positive effect (typically a few weeks), patients with acute anxiety are best served by treatment with a medication with an immediate onset of action, such as a benzodiazepine or a sleep medication (eg, zolpidem). Benzodiazepines and hypnotics such as zolpidem are best prescribed for as-needed use because they carry a risk of dependence. One might consider prescribing mirtazapine or pregabalin (both of which are used off-label to treat anxiety) because these medications also have a relatively rapid onset of action and can treat both anxiety and insomnia (particularly mirtazapine).
Research considerations. It would be helpful to study which treatments are most effective for the subset of patients who experience acute anxiety disorder as I define it. Perhaps psychotherapy treatment protocols could be adapted or created. Treatment with esketamine or IV ketamine might be further studied as a treatment for acute anxiety because some evidence suggests ketamine is efficacious for this indication.3
1. Otsubo T, Hokama C, Sano N, et al. How significant is the assessment of the DSM-5 ‘anxious distress’ specifier in patients with major depressive disorder without comorbid anxiety disorders in the continuation/maintenance phase? Int J Psychiatry Clin Pract. 2021;25(4):385-392. doi:10.1080/13651501.2021.1907415
2. Butler H, O’Brien AJ. Access to specialist palliative care services by people with severe and persistent mental illness: a retrospective cohort study. Int J Ment Health Nurs. 2018;27(2):737-746. doi:10.1111/inm.12360
3. Glue P, Neehoff SM, Medlicott NJ, et al. Safety and efficacy of maintenance ketamine treatment in patients with treatment-refractory generalised anxiety and social anxiety disorders. J Psychopharmacol. 2018;32(6):663-667. doi:10.1177/0269881118762073
1. Otsubo T, Hokama C, Sano N, et al. How significant is the assessment of the DSM-5 ‘anxious distress’ specifier in patients with major depressive disorder without comorbid anxiety disorders in the continuation/maintenance phase? Int J Psychiatry Clin Pract. 2021;25(4):385-392. doi:10.1080/13651501.2021.1907415
2. Butler H, O’Brien AJ. Access to specialist palliative care services by people with severe and persistent mental illness: a retrospective cohort study. Int J Ment Health Nurs. 2018;27(2):737-746. doi:10.1111/inm.12360
3. Glue P, Neehoff SM, Medlicott NJ, et al. Safety and efficacy of maintenance ketamine treatment in patients with treatment-refractory generalised anxiety and social anxiety disorders. J Psychopharmacol. 2018;32(6):663-667. doi:10.1177/0269881118762073
Inhaled, systemic steroids linked to changes in brain structure
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN
‘Doomscrolling’ may be a significant driver of poor mental health
The past 2 years have been filled with worrisome global events, from the pandemic to the war in Ukraine, large-scale protests, mass shootings, and devastating wildfires. The 24-hour media coverage of these events can take a toll on “news addicts” who have an excessive urge to constantly check the news, researchers note.
Results from an online survey of more than 1,000 adults showed that nearly 17% showed signs of “severely problematic” news consumption.
These “doomscrollers” or “doomsurfers” scored high on all five problematic news consumption dimensions: being absorbed in news content, being consumed by thoughts about the news, attempting to alleviate feelings of threat by consuming more news, losing control over news consumption, and having news consumption interfere in daily life.
“We anticipated that a sizable portion of our sample would show signs of problematic news consumption. However, we were surprised to find that 17% of study participants suffer from the most severe level of problematic news consumption,” lead author Bryan McLaughlin, PhD, Texas Tech University, Lubbock, told this news organization. “This is certainly concerning and suggests the problem may be more widespread than we expected,” he said.
In addition, 74% of those with severe levels of problematic news consumption reported experiencing mental problems, and 61% reported physical problems.
“It’s important for health care providers to be aware that problematic news consumption may be a significant driver of mental and physical ill-being, especially because a lot of people might be unaware of the negative impact the news is having on their health,” Dr. McLaughlin said.
The findings were published online in Health Communication.
Emotionally invested
The researchers assessed data from an online survey of 1,100 adults (mean age, 40.5 years; 51% women) in the United States who were recruited in August 2021.
Among those surveyed, 27.3% reported “moderately problematic” news consumption, 27.5% reported minimally problematic news consumption, and 28.7% reported no problematic news consumption.
Perhaps not surprisingly, respondents with higher levels of problematic news consumption were significantly more likely to experience mental and physical ill-being than those with lower levels, even after accounting for demographics, personality traits, and overall news use, the researchers note.
Nearly three-quarters (74%) of those with severe levels of problematic news consumption reported experiencing mental ill-being “quite a bit” or “very much” – whereas frequent symptoms were only reported by 8% of all other study participants.
In addition, 61% of adults with severe problematic news consumption reported experiencing physical ill-being “quite a bit” or “very much,” compared with only 6.1% for all other study participants.
Dr. McLaughlin noted that one way to combat this problem is to help individuals develop a healthier relationship with the news – and mindfulness training may be one way to accomplish that.
“We have some preliminary evidence that individuals with high levels of mindfulness are much less susceptible to developing higher levels of problematic news consumption,” he said.
“Given this, mindfulness-based training could potentially help problematic news consumers follow the news without becoming so emotionally invested in it. We hope to examine the effectiveness of a mindfulness intervention in our future research,” he added.
Increased distress
Commenting on the study, Steven R. Thorp, PhD, ABPP, a professor at California School of Professional Psychology, Alliant International University, San Diego, said that he and his colleagues have noticed an increase in clients reporting distress about news consumption.
The survey by Dr. McLaughlin and colleagues “appears to be representative and has sufficient statistical power to address the issues,” said Dr. Thorp, who was not involved with the research.
“However, as the researchers note, it is a cross-sectional and correlational survey. So it’s possible that, as implied, people who ‘doomscroll’ are more likely to have physical and mental health problems that interfere with their functioning,” he added.
It is also possible that individuals with physical and mental health problems are more likely to be isolated and have restricted activities, thus leading to greater news consumption, Dr. Thorp noted. Alternatively, there could be an independent link between health and news consumption.
Most news is “sensational and not representative,” Dr. Thorp pointed out.
For example, “we are far more likely to hear about deaths from terrorist attacks or plane crashes than from heart attacks, though deaths from heart attacks are far more common,” he said.
“News also tends to be negative, rather than uplifting, and most news is not directly relevant to a person’s day-to-day functioning. Thus, for most people, the consumption of news may have more downsides than upsides,” Dr. Thorp added.
Still, many people want to stay informed about national and international events. So rather than following a “cold turkey” or abstinence model of stopping all news consumption, individuals could consider a “harm reduction” model of reducing time spent consuming news, Dr. Thorp noted.
Another thing to consider is the news source. “Some outlets and social media sites are designed to instill outrage, fear, or anger and to increase polarization, while others have been shown to provide balanced and less sensational coverage,” Dr. Thorp said.
“I also think it’s a good idea for providers to regularly ask about news consumption, along with learning about other daily activities that may enhance or diminish mental and physical health,” he added.
The research had no specific funding. Dr. McLaughlin and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The past 2 years have been filled with worrisome global events, from the pandemic to the war in Ukraine, large-scale protests, mass shootings, and devastating wildfires. The 24-hour media coverage of these events can take a toll on “news addicts” who have an excessive urge to constantly check the news, researchers note.
Results from an online survey of more than 1,000 adults showed that nearly 17% showed signs of “severely problematic” news consumption.
These “doomscrollers” or “doomsurfers” scored high on all five problematic news consumption dimensions: being absorbed in news content, being consumed by thoughts about the news, attempting to alleviate feelings of threat by consuming more news, losing control over news consumption, and having news consumption interfere in daily life.
“We anticipated that a sizable portion of our sample would show signs of problematic news consumption. However, we were surprised to find that 17% of study participants suffer from the most severe level of problematic news consumption,” lead author Bryan McLaughlin, PhD, Texas Tech University, Lubbock, told this news organization. “This is certainly concerning and suggests the problem may be more widespread than we expected,” he said.
In addition, 74% of those with severe levels of problematic news consumption reported experiencing mental problems, and 61% reported physical problems.
“It’s important for health care providers to be aware that problematic news consumption may be a significant driver of mental and physical ill-being, especially because a lot of people might be unaware of the negative impact the news is having on their health,” Dr. McLaughlin said.
The findings were published online in Health Communication.
Emotionally invested
The researchers assessed data from an online survey of 1,100 adults (mean age, 40.5 years; 51% women) in the United States who were recruited in August 2021.
Among those surveyed, 27.3% reported “moderately problematic” news consumption, 27.5% reported minimally problematic news consumption, and 28.7% reported no problematic news consumption.
Perhaps not surprisingly, respondents with higher levels of problematic news consumption were significantly more likely to experience mental and physical ill-being than those with lower levels, even after accounting for demographics, personality traits, and overall news use, the researchers note.
Nearly three-quarters (74%) of those with severe levels of problematic news consumption reported experiencing mental ill-being “quite a bit” or “very much” – whereas frequent symptoms were only reported by 8% of all other study participants.
In addition, 61% of adults with severe problematic news consumption reported experiencing physical ill-being “quite a bit” or “very much,” compared with only 6.1% for all other study participants.
Dr. McLaughlin noted that one way to combat this problem is to help individuals develop a healthier relationship with the news – and mindfulness training may be one way to accomplish that.
“We have some preliminary evidence that individuals with high levels of mindfulness are much less susceptible to developing higher levels of problematic news consumption,” he said.
“Given this, mindfulness-based training could potentially help problematic news consumers follow the news without becoming so emotionally invested in it. We hope to examine the effectiveness of a mindfulness intervention in our future research,” he added.
Increased distress
Commenting on the study, Steven R. Thorp, PhD, ABPP, a professor at California School of Professional Psychology, Alliant International University, San Diego, said that he and his colleagues have noticed an increase in clients reporting distress about news consumption.
The survey by Dr. McLaughlin and colleagues “appears to be representative and has sufficient statistical power to address the issues,” said Dr. Thorp, who was not involved with the research.
“However, as the researchers note, it is a cross-sectional and correlational survey. So it’s possible that, as implied, people who ‘doomscroll’ are more likely to have physical and mental health problems that interfere with their functioning,” he added.
It is also possible that individuals with physical and mental health problems are more likely to be isolated and have restricted activities, thus leading to greater news consumption, Dr. Thorp noted. Alternatively, there could be an independent link between health and news consumption.
Most news is “sensational and not representative,” Dr. Thorp pointed out.
For example, “we are far more likely to hear about deaths from terrorist attacks or plane crashes than from heart attacks, though deaths from heart attacks are far more common,” he said.
“News also tends to be negative, rather than uplifting, and most news is not directly relevant to a person’s day-to-day functioning. Thus, for most people, the consumption of news may have more downsides than upsides,” Dr. Thorp added.
Still, many people want to stay informed about national and international events. So rather than following a “cold turkey” or abstinence model of stopping all news consumption, individuals could consider a “harm reduction” model of reducing time spent consuming news, Dr. Thorp noted.
Another thing to consider is the news source. “Some outlets and social media sites are designed to instill outrage, fear, or anger and to increase polarization, while others have been shown to provide balanced and less sensational coverage,” Dr. Thorp said.
“I also think it’s a good idea for providers to regularly ask about news consumption, along with learning about other daily activities that may enhance or diminish mental and physical health,” he added.
The research had no specific funding. Dr. McLaughlin and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The past 2 years have been filled with worrisome global events, from the pandemic to the war in Ukraine, large-scale protests, mass shootings, and devastating wildfires. The 24-hour media coverage of these events can take a toll on “news addicts” who have an excessive urge to constantly check the news, researchers note.
Results from an online survey of more than 1,000 adults showed that nearly 17% showed signs of “severely problematic” news consumption.
These “doomscrollers” or “doomsurfers” scored high on all five problematic news consumption dimensions: being absorbed in news content, being consumed by thoughts about the news, attempting to alleviate feelings of threat by consuming more news, losing control over news consumption, and having news consumption interfere in daily life.
“We anticipated that a sizable portion of our sample would show signs of problematic news consumption. However, we were surprised to find that 17% of study participants suffer from the most severe level of problematic news consumption,” lead author Bryan McLaughlin, PhD, Texas Tech University, Lubbock, told this news organization. “This is certainly concerning and suggests the problem may be more widespread than we expected,” he said.
In addition, 74% of those with severe levels of problematic news consumption reported experiencing mental problems, and 61% reported physical problems.
“It’s important for health care providers to be aware that problematic news consumption may be a significant driver of mental and physical ill-being, especially because a lot of people might be unaware of the negative impact the news is having on their health,” Dr. McLaughlin said.
The findings were published online in Health Communication.
Emotionally invested
The researchers assessed data from an online survey of 1,100 adults (mean age, 40.5 years; 51% women) in the United States who were recruited in August 2021.
Among those surveyed, 27.3% reported “moderately problematic” news consumption, 27.5% reported minimally problematic news consumption, and 28.7% reported no problematic news consumption.
Perhaps not surprisingly, respondents with higher levels of problematic news consumption were significantly more likely to experience mental and physical ill-being than those with lower levels, even after accounting for demographics, personality traits, and overall news use, the researchers note.
Nearly three-quarters (74%) of those with severe levels of problematic news consumption reported experiencing mental ill-being “quite a bit” or “very much” – whereas frequent symptoms were only reported by 8% of all other study participants.
In addition, 61% of adults with severe problematic news consumption reported experiencing physical ill-being “quite a bit” or “very much,” compared with only 6.1% for all other study participants.
Dr. McLaughlin noted that one way to combat this problem is to help individuals develop a healthier relationship with the news – and mindfulness training may be one way to accomplish that.
“We have some preliminary evidence that individuals with high levels of mindfulness are much less susceptible to developing higher levels of problematic news consumption,” he said.
“Given this, mindfulness-based training could potentially help problematic news consumers follow the news without becoming so emotionally invested in it. We hope to examine the effectiveness of a mindfulness intervention in our future research,” he added.
Increased distress
Commenting on the study, Steven R. Thorp, PhD, ABPP, a professor at California School of Professional Psychology, Alliant International University, San Diego, said that he and his colleagues have noticed an increase in clients reporting distress about news consumption.
The survey by Dr. McLaughlin and colleagues “appears to be representative and has sufficient statistical power to address the issues,” said Dr. Thorp, who was not involved with the research.
“However, as the researchers note, it is a cross-sectional and correlational survey. So it’s possible that, as implied, people who ‘doomscroll’ are more likely to have physical and mental health problems that interfere with their functioning,” he added.
It is also possible that individuals with physical and mental health problems are more likely to be isolated and have restricted activities, thus leading to greater news consumption, Dr. Thorp noted. Alternatively, there could be an independent link between health and news consumption.
Most news is “sensational and not representative,” Dr. Thorp pointed out.
For example, “we are far more likely to hear about deaths from terrorist attacks or plane crashes than from heart attacks, though deaths from heart attacks are far more common,” he said.
“News also tends to be negative, rather than uplifting, and most news is not directly relevant to a person’s day-to-day functioning. Thus, for most people, the consumption of news may have more downsides than upsides,” Dr. Thorp added.
Still, many people want to stay informed about national and international events. So rather than following a “cold turkey” or abstinence model of stopping all news consumption, individuals could consider a “harm reduction” model of reducing time spent consuming news, Dr. Thorp noted.
Another thing to consider is the news source. “Some outlets and social media sites are designed to instill outrage, fear, or anger and to increase polarization, while others have been shown to provide balanced and less sensational coverage,” Dr. Thorp said.
“I also think it’s a good idea for providers to regularly ask about news consumption, along with learning about other daily activities that may enhance or diminish mental and physical health,” he added.
The research had no specific funding. Dr. McLaughlin and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HEALTH COMMUNICATION
TikTok’s impact on adolescent mental health
For younger generations, TikTok is a go-to site for those who like short and catchy video clips. As a social media platform that allows concise video sharing, TikTok has over 1 billion monthly global users. Because of its platform size, a plethora of resources, and influence on media discourse, TikTok is the place for content creators to share visual media. Its cursory, condensed content delivery with videos capped at 1-minute focuses on high-yield information and rapid identification of fundamental points that are both engaging and entertaining.
Currently, on TikTok, 40 billion views are associated with the hashtag #mentalhealth. Content creators and regular users are employing this platform to share their own experiences, opinions, and strategies to overcome their struggles. While it is understandable for creators to share their personal stories that may be abusive, traumatic, or violent, they may not be prepared for their video to “go viral.”
Like any other social media platform, hateful speech such as racism, sexism, or xenophobia can accumulate on TikTok, which may cause more self-harm than self-help. Oversharing about personal strategies may lead to misconceived advice for TikTok viewers, while watching these TikTok videos can have negative mental health effects, even though there are no malicious intentions behind the creators who post these videos.
Hence, public health should pay more attention to the potential health-related implications this platform can create, as the quality of the information and the qualifications of the creators are mostly unrevealed. The concerns include undisclosed conflicts of interest, unchecked spread of misinformation, difficulty identifying source credibility, and excessive false information that viewers must filter through.1,2
Individual TikTok users may follow accounts and interpret these content creators as therapists and the content they see as therapy. They may also believe that a close relationship with the content creator exists when it does not. Specifically, these relationships may be defined as parasocial relationships, which are one-sided relationships where one person (the TikTok viewer) extends emotional energy, interest, and time, and the other party (the content creator) is completely unaware of the other’s existence.3 Additionally, Americans who are uninsured/underinsured may turn to this diluted version of therapy to compensate for the one-on-one or group therapy they need.
While TikTok may seem like a dangerous platform to browse through or post on, its growing influence cannot be underestimated. With 41% of TikTok users between the ages of 16 and 24, this is an ideal platform to disseminate public health information pertaining to this age group (for example, safe sex practices, substance abuse, and mental health issues).4 Because younger generations have incorporated social media into their daily lives, the medical community can harness TikTok’s potential to disseminate accurate information to potential patients for targeted medical education.
For example, Jake Goodman, MD, MBA, and Melissa Shepard, MD, each have more than a million TikTok followers and are notable psychiatrists who post a variety of content ranging from recognizing signs of depression to reducing stigma around mental health. Similarly, Justin Puder, PhD, is a licensed psychologist who advocates for ways to overcome mental health issues. By creating diverse content with appealing strategies, spreading accurate medical knowledge, and answering common medical questions for the public, these ‘mental health influencers’ educate potential patients to create patient-centered interactions.
While there are many pros and cons to social media platforms, it is undeniable that these platforms – such as TikTok – are here to stay. It is crucial for members of the medical community to recognize the outlets that younger generations use to express themselves and to exploit these media channels therapeutically.
Ms. Wong is a fourth-year medical student at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y. Dr. Chua is a psychiatrist with the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia, and assistant professor of clinical psychiatry at the University of Pennsylvania, also in Philadelphia.
References
1. Gottlieb M and Dyer S. Information and Disinformation: Social Media in the COVID-19 Crisis. Acad Emerg Med. 2020 Jul;27(7):640-1. doi: 10.1111/acem.14036.
2. De Veirman M et al. Front Psychol. 2019;10:2685. doi: 10.3389/fpsyg.2019.02685.
3. Bennett N-K et al. “Parasocial Relationships: The Nature of Celebrity Fascinations.” National Register of Health Service Psychologists. https://www.findapsychologist.org/parasocial-relationships-the-nature-of-celebrity-fascinations/.
4. Eghtesadi M and Florea A. Can J Public Health. 2020 Jun;111(3):389-91. doi: 10.17269/s41997-020-00343-0.
For younger generations, TikTok is a go-to site for those who like short and catchy video clips. As a social media platform that allows concise video sharing, TikTok has over 1 billion monthly global users. Because of its platform size, a plethora of resources, and influence on media discourse, TikTok is the place for content creators to share visual media. Its cursory, condensed content delivery with videos capped at 1-minute focuses on high-yield information and rapid identification of fundamental points that are both engaging and entertaining.
Currently, on TikTok, 40 billion views are associated with the hashtag #mentalhealth. Content creators and regular users are employing this platform to share their own experiences, opinions, and strategies to overcome their struggles. While it is understandable for creators to share their personal stories that may be abusive, traumatic, or violent, they may not be prepared for their video to “go viral.”
Like any other social media platform, hateful speech such as racism, sexism, or xenophobia can accumulate on TikTok, which may cause more self-harm than self-help. Oversharing about personal strategies may lead to misconceived advice for TikTok viewers, while watching these TikTok videos can have negative mental health effects, even though there are no malicious intentions behind the creators who post these videos.
Hence, public health should pay more attention to the potential health-related implications this platform can create, as the quality of the information and the qualifications of the creators are mostly unrevealed. The concerns include undisclosed conflicts of interest, unchecked spread of misinformation, difficulty identifying source credibility, and excessive false information that viewers must filter through.1,2
Individual TikTok users may follow accounts and interpret these content creators as therapists and the content they see as therapy. They may also believe that a close relationship with the content creator exists when it does not. Specifically, these relationships may be defined as parasocial relationships, which are one-sided relationships where one person (the TikTok viewer) extends emotional energy, interest, and time, and the other party (the content creator) is completely unaware of the other’s existence.3 Additionally, Americans who are uninsured/underinsured may turn to this diluted version of therapy to compensate for the one-on-one or group therapy they need.
While TikTok may seem like a dangerous platform to browse through or post on, its growing influence cannot be underestimated. With 41% of TikTok users between the ages of 16 and 24, this is an ideal platform to disseminate public health information pertaining to this age group (for example, safe sex practices, substance abuse, and mental health issues).4 Because younger generations have incorporated social media into their daily lives, the medical community can harness TikTok’s potential to disseminate accurate information to potential patients for targeted medical education.
For example, Jake Goodman, MD, MBA, and Melissa Shepard, MD, each have more than a million TikTok followers and are notable psychiatrists who post a variety of content ranging from recognizing signs of depression to reducing stigma around mental health. Similarly, Justin Puder, PhD, is a licensed psychologist who advocates for ways to overcome mental health issues. By creating diverse content with appealing strategies, spreading accurate medical knowledge, and answering common medical questions for the public, these ‘mental health influencers’ educate potential patients to create patient-centered interactions.
While there are many pros and cons to social media platforms, it is undeniable that these platforms – such as TikTok – are here to stay. It is crucial for members of the medical community to recognize the outlets that younger generations use to express themselves and to exploit these media channels therapeutically.
Ms. Wong is a fourth-year medical student at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y. Dr. Chua is a psychiatrist with the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia, and assistant professor of clinical psychiatry at the University of Pennsylvania, also in Philadelphia.
References
1. Gottlieb M and Dyer S. Information and Disinformation: Social Media in the COVID-19 Crisis. Acad Emerg Med. 2020 Jul;27(7):640-1. doi: 10.1111/acem.14036.
2. De Veirman M et al. Front Psychol. 2019;10:2685. doi: 10.3389/fpsyg.2019.02685.
3. Bennett N-K et al. “Parasocial Relationships: The Nature of Celebrity Fascinations.” National Register of Health Service Psychologists. https://www.findapsychologist.org/parasocial-relationships-the-nature-of-celebrity-fascinations/.
4. Eghtesadi M and Florea A. Can J Public Health. 2020 Jun;111(3):389-91. doi: 10.17269/s41997-020-00343-0.
For younger generations, TikTok is a go-to site for those who like short and catchy video clips. As a social media platform that allows concise video sharing, TikTok has over 1 billion monthly global users. Because of its platform size, a plethora of resources, and influence on media discourse, TikTok is the place for content creators to share visual media. Its cursory, condensed content delivery with videos capped at 1-minute focuses on high-yield information and rapid identification of fundamental points that are both engaging and entertaining.
Currently, on TikTok, 40 billion views are associated with the hashtag #mentalhealth. Content creators and regular users are employing this platform to share their own experiences, opinions, and strategies to overcome their struggles. While it is understandable for creators to share their personal stories that may be abusive, traumatic, or violent, they may not be prepared for their video to “go viral.”
Like any other social media platform, hateful speech such as racism, sexism, or xenophobia can accumulate on TikTok, which may cause more self-harm than self-help. Oversharing about personal strategies may lead to misconceived advice for TikTok viewers, while watching these TikTok videos can have negative mental health effects, even though there are no malicious intentions behind the creators who post these videos.
Hence, public health should pay more attention to the potential health-related implications this platform can create, as the quality of the information and the qualifications of the creators are mostly unrevealed. The concerns include undisclosed conflicts of interest, unchecked spread of misinformation, difficulty identifying source credibility, and excessive false information that viewers must filter through.1,2
Individual TikTok users may follow accounts and interpret these content creators as therapists and the content they see as therapy. They may also believe that a close relationship with the content creator exists when it does not. Specifically, these relationships may be defined as parasocial relationships, which are one-sided relationships where one person (the TikTok viewer) extends emotional energy, interest, and time, and the other party (the content creator) is completely unaware of the other’s existence.3 Additionally, Americans who are uninsured/underinsured may turn to this diluted version of therapy to compensate for the one-on-one or group therapy they need.
While TikTok may seem like a dangerous platform to browse through or post on, its growing influence cannot be underestimated. With 41% of TikTok users between the ages of 16 and 24, this is an ideal platform to disseminate public health information pertaining to this age group (for example, safe sex practices, substance abuse, and mental health issues).4 Because younger generations have incorporated social media into their daily lives, the medical community can harness TikTok’s potential to disseminate accurate information to potential patients for targeted medical education.
For example, Jake Goodman, MD, MBA, and Melissa Shepard, MD, each have more than a million TikTok followers and are notable psychiatrists who post a variety of content ranging from recognizing signs of depression to reducing stigma around mental health. Similarly, Justin Puder, PhD, is a licensed psychologist who advocates for ways to overcome mental health issues. By creating diverse content with appealing strategies, spreading accurate medical knowledge, and answering common medical questions for the public, these ‘mental health influencers’ educate potential patients to create patient-centered interactions.
While there are many pros and cons to social media platforms, it is undeniable that these platforms – such as TikTok – are here to stay. It is crucial for members of the medical community to recognize the outlets that younger generations use to express themselves and to exploit these media channels therapeutically.
Ms. Wong is a fourth-year medical student at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y. Dr. Chua is a psychiatrist with the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia, and assistant professor of clinical psychiatry at the University of Pennsylvania, also in Philadelphia.
References
1. Gottlieb M and Dyer S. Information and Disinformation: Social Media in the COVID-19 Crisis. Acad Emerg Med. 2020 Jul;27(7):640-1. doi: 10.1111/acem.14036.
2. De Veirman M et al. Front Psychol. 2019;10:2685. doi: 10.3389/fpsyg.2019.02685.
3. Bennett N-K et al. “Parasocial Relationships: The Nature of Celebrity Fascinations.” National Register of Health Service Psychologists. https://www.findapsychologist.org/parasocial-relationships-the-nature-of-celebrity-fascinations/.
4. Eghtesadi M and Florea A. Can J Public Health. 2020 Jun;111(3):389-91. doi: 10.17269/s41997-020-00343-0.
Brief Psychiatric Rating Scale succeeds as transdiagnostic measure
“Current DSM and ICD diagnoses do not depict psychopathology accurately, therefore their validity in research and utility in clinical practice is questioned,” wrote Andreas B. Hofmann, PhD, of the University of Zürich and colleagues.
The BPRS was developed to assess changes in psychopathology across a range of severe psychiatric disorders, but its potential to assess symptoms in nonpsychotic disorders has not been explored, the researchers said.
In a study published in Psychiatry Research, the investigators analyzed data from 600 adult psychiatric inpatients divided equally into six diagnostic categories: alcohol use disorder, major depressive disorder, anxiety disorders, bipolar disorder, schizophrenia, and personality disorders. The mean age of the patients was 41.5 years and 45.5% were women. The demographic characteristics were similar across most groups, although patients with a personality disorder were significantly more likely than other patients to be younger and female.
Patients were assessed using the BPRS based on their main diagnosis. The mini-ICF-APP, another validated measure for assessing psychiatric disorders, served as a comparator, and both were compared to the Clinical Global Impression Scale (CGI).
Overall, the BPRS and mini-ICF-APP showed moderate correlation and good agreement, the researchers said. The Pearson correlation coefficient for the BPRS and mini-ICF-APP scales was 0.53 and the concordance correlation coefficient was 0.52. The mean sum scores for the BPRS, the mini-ICF-APP, and the CGI were 45.4 (standard deviation, 14.4), 19.93 (SD, 8.21), and 5.55 (SD, 0.84), respectively, which indicated “markedly ill” to “severely ill” patients, the researchers said.
The researchers were able to detect three clusters of symptoms corresponding to externalizing, internalizing, and thought disturbance domains using the BPRS, and four clusters using the mini-ICF-APP.
The symptoms using BPRS and the functionality domains using the mini-ICF-APP “showed a close interplay,” the researchers noted.
“The symptoms and functional domains we found to be central within the network structure are among the first targets of any psychiatric or psychotherapeutic intervention, namely the building of a common language and understanding as well as the establishment of confidence in relationships and a trustworthy therapeutic alliance,” they wrote in their discussion.
The study findings were limited by several factors including the collection of data from routine practice rather than clinical trials, the focus on only the main diagnosis without comorbidities, and the inclusion only of patients requiring hospitalization, the researchers noted.
However, the results were strengthened by the large sample size, and demonstrate the validity of the BPRS as a measurement tool across a range of psychiatric diagnoses, they said.
“Since the BPRS is a widely known and readily available psychometric scale, our results support its use as a transdiagnostic measurement instrument of psychopathology,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
“Current DSM and ICD diagnoses do not depict psychopathology accurately, therefore their validity in research and utility in clinical practice is questioned,” wrote Andreas B. Hofmann, PhD, of the University of Zürich and colleagues.
The BPRS was developed to assess changes in psychopathology across a range of severe psychiatric disorders, but its potential to assess symptoms in nonpsychotic disorders has not been explored, the researchers said.
In a study published in Psychiatry Research, the investigators analyzed data from 600 adult psychiatric inpatients divided equally into six diagnostic categories: alcohol use disorder, major depressive disorder, anxiety disorders, bipolar disorder, schizophrenia, and personality disorders. The mean age of the patients was 41.5 years and 45.5% were women. The demographic characteristics were similar across most groups, although patients with a personality disorder were significantly more likely than other patients to be younger and female.
Patients were assessed using the BPRS based on their main diagnosis. The mini-ICF-APP, another validated measure for assessing psychiatric disorders, served as a comparator, and both were compared to the Clinical Global Impression Scale (CGI).
Overall, the BPRS and mini-ICF-APP showed moderate correlation and good agreement, the researchers said. The Pearson correlation coefficient for the BPRS and mini-ICF-APP scales was 0.53 and the concordance correlation coefficient was 0.52. The mean sum scores for the BPRS, the mini-ICF-APP, and the CGI were 45.4 (standard deviation, 14.4), 19.93 (SD, 8.21), and 5.55 (SD, 0.84), respectively, which indicated “markedly ill” to “severely ill” patients, the researchers said.
The researchers were able to detect three clusters of symptoms corresponding to externalizing, internalizing, and thought disturbance domains using the BPRS, and four clusters using the mini-ICF-APP.
The symptoms using BPRS and the functionality domains using the mini-ICF-APP “showed a close interplay,” the researchers noted.
“The symptoms and functional domains we found to be central within the network structure are among the first targets of any psychiatric or psychotherapeutic intervention, namely the building of a common language and understanding as well as the establishment of confidence in relationships and a trustworthy therapeutic alliance,” they wrote in their discussion.
The study findings were limited by several factors including the collection of data from routine practice rather than clinical trials, the focus on only the main diagnosis without comorbidities, and the inclusion only of patients requiring hospitalization, the researchers noted.
However, the results were strengthened by the large sample size, and demonstrate the validity of the BPRS as a measurement tool across a range of psychiatric diagnoses, they said.
“Since the BPRS is a widely known and readily available psychometric scale, our results support its use as a transdiagnostic measurement instrument of psychopathology,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
“Current DSM and ICD diagnoses do not depict psychopathology accurately, therefore their validity in research and utility in clinical practice is questioned,” wrote Andreas B. Hofmann, PhD, of the University of Zürich and colleagues.
The BPRS was developed to assess changes in psychopathology across a range of severe psychiatric disorders, but its potential to assess symptoms in nonpsychotic disorders has not been explored, the researchers said.
In a study published in Psychiatry Research, the investigators analyzed data from 600 adult psychiatric inpatients divided equally into six diagnostic categories: alcohol use disorder, major depressive disorder, anxiety disorders, bipolar disorder, schizophrenia, and personality disorders. The mean age of the patients was 41.5 years and 45.5% were women. The demographic characteristics were similar across most groups, although patients with a personality disorder were significantly more likely than other patients to be younger and female.
Patients were assessed using the BPRS based on their main diagnosis. The mini-ICF-APP, another validated measure for assessing psychiatric disorders, served as a comparator, and both were compared to the Clinical Global Impression Scale (CGI).
Overall, the BPRS and mini-ICF-APP showed moderate correlation and good agreement, the researchers said. The Pearson correlation coefficient for the BPRS and mini-ICF-APP scales was 0.53 and the concordance correlation coefficient was 0.52. The mean sum scores for the BPRS, the mini-ICF-APP, and the CGI were 45.4 (standard deviation, 14.4), 19.93 (SD, 8.21), and 5.55 (SD, 0.84), respectively, which indicated “markedly ill” to “severely ill” patients, the researchers said.
The researchers were able to detect three clusters of symptoms corresponding to externalizing, internalizing, and thought disturbance domains using the BPRS, and four clusters using the mini-ICF-APP.
The symptoms using BPRS and the functionality domains using the mini-ICF-APP “showed a close interplay,” the researchers noted.
“The symptoms and functional domains we found to be central within the network structure are among the first targets of any psychiatric or psychotherapeutic intervention, namely the building of a common language and understanding as well as the establishment of confidence in relationships and a trustworthy therapeutic alliance,” they wrote in their discussion.
The study findings were limited by several factors including the collection of data from routine practice rather than clinical trials, the focus on only the main diagnosis without comorbidities, and the inclusion only of patients requiring hospitalization, the researchers noted.
However, the results were strengthened by the large sample size, and demonstrate the validity of the BPRS as a measurement tool across a range of psychiatric diagnoses, they said.
“Since the BPRS is a widely known and readily available psychometric scale, our results support its use as a transdiagnostic measurement instrument of psychopathology,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM PSYCHIATRY RESEARCH
New panic disorder model flags risk for recurrence, persistence
Investigators based in France and the United States analyzed data for almost 800 patients with DSM-IV–diagnosed PD.
Results showed that having a “general psychopathology factor,” defined as the shared effects of all comorbid conditions, or PD liability, significantly and independently predicted 3-year recurrence or persistence of PD symptoms.
Having a lower physical health-related quality of life (QOL), a greater number of stressful life events, and not seeking treatment at baseline were also significant and independent predictors.
“This integrative model could help clinicians to identify individuals at high risk of recurrence or persistence of panic disorder and provide content for future research,” Valentin Scheer, MD, MPH, a resident in psychiatry at AP-HP, Assistance Publique, Hôpitaux de Paris, and colleagues wrote.
The findings were published online in the Journal of Clinical Psychiatry.
Integration needed
PD is a disabling disorder with a “chronic course” – and a recurrence rate ranging from 25% to 50%, the investigators noted.
“Because of the heterogeneous course of PD, there is a need to develop a comprehensive predictive model of recurrence or persistence,” they wrote. This could “help practitioners adapt therapeutic strategies and develop prevention strategies in high-risk individuals.”
Most previous studies that have investigated risk factors for PD recurrence and persistence have relied on clinical samples, often with limited sample sizes.
Moreover, each risk factor, when considered individually, accounts for only a “small proportion” of the variance in risk, the researchers noted. The co-occurrence of these risk factors “suggests the need to combine them into a broad multivariable model.”
However, currently proposed integrative models do not identify independent predictors or mitigate the influence of confounding variables. To fill this gap, the investigators conducted a study using structural equation modeling “to take into account multiple correlations across predictors.”
They drew on data from 775 participants (mean age, 40 years) in the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). For the current analysis, they examined two waves of NESARC (2001-2002 and 2004-2005) to “build a comprehensive model” of the 3-year recurrence or persistence of PD.
The researchers used a “latent variable approach” that simultaneously examined the effect of the following five groups of potential predictors of recurrence or persistence: PD severity, severity of comorbidity, family history of psychiatric disorders, sociodemographic characteristics, and treatment-seeking behavior.
They also distinguished between risk factors responsible for recurrence and those responsible for persistence.
Psychiatric diagnoses were determined on the basis of the Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV. Participants also completed Version 2 of the Short Form 12-Item Health Survey, which assesses both mental and physical QOL over the previous 4 weeks.
Early treatment needed
Among participants with a 12-month diagnosis of PD at wave 1, 13% had persistent PD and 27.6% had recurrent PD during the 3-year period. The mean duration of illness was 9.5 years.
A greater number of lifetime panic attacks, the presence of any Axis I or II comorbid disorder, and any Axis I disorder, especially social anxiety disorder, were significantly associated with 3-year risk for recurrence and for persistence.
Sweating, choking, paresthesias, the comorbid disorders of mania/hypomania and general anxiety disorder, nicotine dependence, lower mental and physical QOL scores, and exposure to a greater number of stressful life events in the previous year were all significantly associated with 3-year risk for recurrence.
Only variables shown with a P value were statistically significant, “with the a priori fixed at .05,” the researchers noted.
A combination of psychopathology factors, such as the shared effect of all comorbid psychiatric conditions, PD liability, lower physical health-related QOL, more life stressors during the past year, and not seeking treatment at baseline “significantly and independently” predicted recurrence or persistence of symptoms between the two waves (all Ps < .05), the investigators reported.
One study limitation cited was that several psychiatric disorders known to be associated with PD recurrence or persistence, such as borderline personality disorder, were not examined. Additionally, the study used a 3-year follow-up period – and the results might have differed for other follow-up time frames, the researchers noted.
Nevertheless, the findings constitute a “comprehensive model” to predict recurrence and persistence of PD, they wrote. Moreover, early treatment-seeking behavior “should be promoted, as it may reduce the risk of recurrence.”
Not much new?
Commenting on the study, Peter Roy-Byrne, MD, professor of psychiatry, University of Washington, Seattle, noted, “there is not much that is new here.”
Dr. Roy-Byrne, who was not involved with the study, said that a “general theme for years has been that more severe illness, whether you measure it by greater number of other Axis I disorders or symptom severity or a general psychopathology factor, usually predicts worse outcome – here codified as persistence and recurrence.”
Greater stress and reluctance to seek treatment may also predict worse outcomes, he noted.
In addition, the study “did not examine another very important factor: the degree of social connection/social support that someone has,” Dr. Roy-Byrne said. However, “perhaps some of this was contained in specific life events.”
A version of this article first appeared on Medscape.com.
Investigators based in France and the United States analyzed data for almost 800 patients with DSM-IV–diagnosed PD.
Results showed that having a “general psychopathology factor,” defined as the shared effects of all comorbid conditions, or PD liability, significantly and independently predicted 3-year recurrence or persistence of PD symptoms.
Having a lower physical health-related quality of life (QOL), a greater number of stressful life events, and not seeking treatment at baseline were also significant and independent predictors.
“This integrative model could help clinicians to identify individuals at high risk of recurrence or persistence of panic disorder and provide content for future research,” Valentin Scheer, MD, MPH, a resident in psychiatry at AP-HP, Assistance Publique, Hôpitaux de Paris, and colleagues wrote.
The findings were published online in the Journal of Clinical Psychiatry.
Integration needed
PD is a disabling disorder with a “chronic course” – and a recurrence rate ranging from 25% to 50%, the investigators noted.
“Because of the heterogeneous course of PD, there is a need to develop a comprehensive predictive model of recurrence or persistence,” they wrote. This could “help practitioners adapt therapeutic strategies and develop prevention strategies in high-risk individuals.”
Most previous studies that have investigated risk factors for PD recurrence and persistence have relied on clinical samples, often with limited sample sizes.
Moreover, each risk factor, when considered individually, accounts for only a “small proportion” of the variance in risk, the researchers noted. The co-occurrence of these risk factors “suggests the need to combine them into a broad multivariable model.”
However, currently proposed integrative models do not identify independent predictors or mitigate the influence of confounding variables. To fill this gap, the investigators conducted a study using structural equation modeling “to take into account multiple correlations across predictors.”
They drew on data from 775 participants (mean age, 40 years) in the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). For the current analysis, they examined two waves of NESARC (2001-2002 and 2004-2005) to “build a comprehensive model” of the 3-year recurrence or persistence of PD.
The researchers used a “latent variable approach” that simultaneously examined the effect of the following five groups of potential predictors of recurrence or persistence: PD severity, severity of comorbidity, family history of psychiatric disorders, sociodemographic characteristics, and treatment-seeking behavior.
They also distinguished between risk factors responsible for recurrence and those responsible for persistence.
Psychiatric diagnoses were determined on the basis of the Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV. Participants also completed Version 2 of the Short Form 12-Item Health Survey, which assesses both mental and physical QOL over the previous 4 weeks.
Early treatment needed
Among participants with a 12-month diagnosis of PD at wave 1, 13% had persistent PD and 27.6% had recurrent PD during the 3-year period. The mean duration of illness was 9.5 years.
A greater number of lifetime panic attacks, the presence of any Axis I or II comorbid disorder, and any Axis I disorder, especially social anxiety disorder, were significantly associated with 3-year risk for recurrence and for persistence.
Sweating, choking, paresthesias, the comorbid disorders of mania/hypomania and general anxiety disorder, nicotine dependence, lower mental and physical QOL scores, and exposure to a greater number of stressful life events in the previous year were all significantly associated with 3-year risk for recurrence.
Only variables shown with a P value were statistically significant, “with the a priori fixed at .05,” the researchers noted.
A combination of psychopathology factors, such as the shared effect of all comorbid psychiatric conditions, PD liability, lower physical health-related QOL, more life stressors during the past year, and not seeking treatment at baseline “significantly and independently” predicted recurrence or persistence of symptoms between the two waves (all Ps < .05), the investigators reported.
One study limitation cited was that several psychiatric disorders known to be associated with PD recurrence or persistence, such as borderline personality disorder, were not examined. Additionally, the study used a 3-year follow-up period – and the results might have differed for other follow-up time frames, the researchers noted.
Nevertheless, the findings constitute a “comprehensive model” to predict recurrence and persistence of PD, they wrote. Moreover, early treatment-seeking behavior “should be promoted, as it may reduce the risk of recurrence.”
Not much new?
Commenting on the study, Peter Roy-Byrne, MD, professor of psychiatry, University of Washington, Seattle, noted, “there is not much that is new here.”
Dr. Roy-Byrne, who was not involved with the study, said that a “general theme for years has been that more severe illness, whether you measure it by greater number of other Axis I disorders or symptom severity or a general psychopathology factor, usually predicts worse outcome – here codified as persistence and recurrence.”
Greater stress and reluctance to seek treatment may also predict worse outcomes, he noted.
In addition, the study “did not examine another very important factor: the degree of social connection/social support that someone has,” Dr. Roy-Byrne said. However, “perhaps some of this was contained in specific life events.”
A version of this article first appeared on Medscape.com.
Investigators based in France and the United States analyzed data for almost 800 patients with DSM-IV–diagnosed PD.
Results showed that having a “general psychopathology factor,” defined as the shared effects of all comorbid conditions, or PD liability, significantly and independently predicted 3-year recurrence or persistence of PD symptoms.
Having a lower physical health-related quality of life (QOL), a greater number of stressful life events, and not seeking treatment at baseline were also significant and independent predictors.
“This integrative model could help clinicians to identify individuals at high risk of recurrence or persistence of panic disorder and provide content for future research,” Valentin Scheer, MD, MPH, a resident in psychiatry at AP-HP, Assistance Publique, Hôpitaux de Paris, and colleagues wrote.
The findings were published online in the Journal of Clinical Psychiatry.
Integration needed
PD is a disabling disorder with a “chronic course” – and a recurrence rate ranging from 25% to 50%, the investigators noted.
“Because of the heterogeneous course of PD, there is a need to develop a comprehensive predictive model of recurrence or persistence,” they wrote. This could “help practitioners adapt therapeutic strategies and develop prevention strategies in high-risk individuals.”
Most previous studies that have investigated risk factors for PD recurrence and persistence have relied on clinical samples, often with limited sample sizes.
Moreover, each risk factor, when considered individually, accounts for only a “small proportion” of the variance in risk, the researchers noted. The co-occurrence of these risk factors “suggests the need to combine them into a broad multivariable model.”
However, currently proposed integrative models do not identify independent predictors or mitigate the influence of confounding variables. To fill this gap, the investigators conducted a study using structural equation modeling “to take into account multiple correlations across predictors.”
They drew on data from 775 participants (mean age, 40 years) in the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). For the current analysis, they examined two waves of NESARC (2001-2002 and 2004-2005) to “build a comprehensive model” of the 3-year recurrence or persistence of PD.
The researchers used a “latent variable approach” that simultaneously examined the effect of the following five groups of potential predictors of recurrence or persistence: PD severity, severity of comorbidity, family history of psychiatric disorders, sociodemographic characteristics, and treatment-seeking behavior.
They also distinguished between risk factors responsible for recurrence and those responsible for persistence.
Psychiatric diagnoses were determined on the basis of the Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV. Participants also completed Version 2 of the Short Form 12-Item Health Survey, which assesses both mental and physical QOL over the previous 4 weeks.
Early treatment needed
Among participants with a 12-month diagnosis of PD at wave 1, 13% had persistent PD and 27.6% had recurrent PD during the 3-year period. The mean duration of illness was 9.5 years.
A greater number of lifetime panic attacks, the presence of any Axis I or II comorbid disorder, and any Axis I disorder, especially social anxiety disorder, were significantly associated with 3-year risk for recurrence and for persistence.
Sweating, choking, paresthesias, the comorbid disorders of mania/hypomania and general anxiety disorder, nicotine dependence, lower mental and physical QOL scores, and exposure to a greater number of stressful life events in the previous year were all significantly associated with 3-year risk for recurrence.
Only variables shown with a P value were statistically significant, “with the a priori fixed at .05,” the researchers noted.
A combination of psychopathology factors, such as the shared effect of all comorbid psychiatric conditions, PD liability, lower physical health-related QOL, more life stressors during the past year, and not seeking treatment at baseline “significantly and independently” predicted recurrence or persistence of symptoms between the two waves (all Ps < .05), the investigators reported.
One study limitation cited was that several psychiatric disorders known to be associated with PD recurrence or persistence, such as borderline personality disorder, were not examined. Additionally, the study used a 3-year follow-up period – and the results might have differed for other follow-up time frames, the researchers noted.
Nevertheless, the findings constitute a “comprehensive model” to predict recurrence and persistence of PD, they wrote. Moreover, early treatment-seeking behavior “should be promoted, as it may reduce the risk of recurrence.”
Not much new?
Commenting on the study, Peter Roy-Byrne, MD, professor of psychiatry, University of Washington, Seattle, noted, “there is not much that is new here.”
Dr. Roy-Byrne, who was not involved with the study, said that a “general theme for years has been that more severe illness, whether you measure it by greater number of other Axis I disorders or symptom severity or a general psychopathology factor, usually predicts worse outcome – here codified as persistence and recurrence.”
Greater stress and reluctance to seek treatment may also predict worse outcomes, he noted.
In addition, the study “did not examine another very important factor: the degree of social connection/social support that someone has,” Dr. Roy-Byrne said. However, “perhaps some of this was contained in specific life events.”
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Active shooter drills may be harming children, but doctors offer help
The drills can range from staging lockdowns and sheltering in place to quasi dramas with mock shooters roaming the halls. Although the goals of these training exercises are important, equally important are the potential negative effects of drills on students’ mental health, according to doctors with expertise in pediatrics and mental health.
“Dramatic simulation of an active shooter event at school would be expected to provoke the same stress response as the real thing,” said Peter L. Loper Jr., MD, a pediatrician and psychiatrist, in an interview. “While ensuring their physical safety is very important, we must be intentional about making sure that we are not doing so at the expense of their psychosocial or emotional safety.”
“Children may not be able to differentiate a dramatic drill from a real event,” emphasized Dr. Loper, of the neuropsychiatry and behavioral science departments at the University of South Carolina, Columbia. “The parts of the brain responsible for our flight-fight-or-freeze response would interpret both simulated and real events identically and produce the same neurohormonal stress-response.”
Indeed, a study published in the journal Humanities & Social Sciences Communications suggested children experienced mental health problems related to participating in active shooter drills. In the large study, a team of statisticians from the Georgia Institute of Technology found that students reported a 42% increase in stress and anxiety and a 38.7% increase in depression during the 90 days following active shooter drills, compared with the 90 days before the drills.
The authors of this study, including Mai ElSherief, PhD, drew these conclusions after analyzing 54 million social media posts before and after drills in 114 schools across 33 states. The researchers analyzed the language of the social media posts by teachers, parents, and students and found increased use of the words hope, love, home, school, kids, community, support, and help after the drills. The researchers considered posting with these terms in the aftermath of the drills to be indicative of having high anxiety.
They included examples of how high stress, anxiety, and depression manifested in specific posts from parents in their report. The following is an example of a poster expressing high anxiety and stress: “are we really gonna normalize school shooter drills?! holy sh* there has to be a real way to avoid these tragedies. sh*t like this cannot be normalized. teachers injured after being shot with plastic pellets ‘execution style’ in active shooter drill.”
The authors also shared this post to serve as an example of a person who seems depressed: “and now we are revisiting the trauma on our kids, forcing them to act out school drills monthly. i don’t get why gen x parents buy into this concept wholeheartedly. things need to change.”
The published material did not include posts from students, but the researchers’ analysis of the content of posts overall showed increased concerns for health and increased concerns about death during the period after drills, compared with before drills.
The authors also conducted focus groups in communities in which drills occurred, and many teachers and parents reported anecdotal evidence of children who were nervous long after the drills were over, with some showing extreme reactions such as panic over a standard fire alarm at school. Overall, the results show that school shooter drills can negatively affect school communities over prolonged periods of time, they concluded.
According to a statement from the American Academy of Pediatrics, “there is a need to be cautious about the potential psychological risks and other unintended consequences of directly involving children in live exercises and drills.”
“These risks and consequences are especially a concern when children are deceived and led to believe there is an actual attack and not a drill,” wrote David Schonfeld, MD, the lead author of the statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, and colleagues.
Managing the fallout
Physicians can help students experiencing mental health problems from these drills, according to doctors interviewed for this piece.
It’s important for providers to know that stress will show up differently in children than in adults, said Chelsea Younghans, MD, a psychiatrist and military officer in Bethesda, Md., in an interview.
“They may see children with headaches, stomach aches, or nonspecific complaints. They may also see children who have not had difficulty with sleep present with nightmares or bed wetting,” she added.
For teens and preteens, validated tools such as the Child PTSD Symptom Scale (CPSS-5) and Child and Adolescent Trauma Screen (CATS) to assess PTSD in youth, may help serve as a starting point for a conversation between providers and their older child population, she noted.
Children who exhibit avoidance or withdrawal behaviors including consistent school refusal, an increase in reassurance-seeking behaviors, or somatic symptoms like vague abdominal pain or headaches that prevent school attendance after participating in a drill, may need more robust mental health services, Dr. Loper noted.
Dr. Schonfeld, who is also director of the National Center for School Crisis and Bereavement at Children’s Hospital Los Angeles, called for health care providers to be available to help children process traumatic reactions to these exercises.
Agreeing with Dr. Schonfeld, Dr. Younghans said: “It is vital to debrief with students and staff after drills, making sure that students have a safe space and ample time to speak with trusted staff. As children will undoubtedly have questions and concerns, creating open lines of communication will help alleviate any traumatic effect these drills may have.”
Communicating with various stakeholders
Experts also gave recommendations for how clinicians communicate with leaders in their area’s school districts and other members of their communities about these training exercises.
“For primary care providers, it is important to establish meaningful relationships within your community and patient population as much as possible,” Dr. Younghans said. “Having a good relationship with the local schools and being part of the conversation can help increase school and community awareness on the impact these drills can have on students and staff,” she added.
For those pediatricians or other health care providers who serve as consultants to schools, Dr. Schonfeld advised they ask about policies related to exercises and drills, such as what are the limits to what children might be exposed to in a drill, and what requirements there might be at the local and state level in terms of frequency and what the drills will and will not involve.
He also noted that clinicians should encourage school leaders to consider the fact that kids may have personal histories of trauma that are completely unknown to the school when they design these exercises.
School staff and health care providers should explain the nature and reasons for drills, invite family members to express concerns, and make accommodations if necessary for some children to participate in drills in a more limited way, noted Dr. Schonfeld, who is also clinical professor of pediatrics at the University of Southern California, Los Angeles.
“I think health care providers should work with legislators, so that if they require a drill, it must be done in a way that is physically and emotionally safe,” he added.
Executing better drills for students’ mental health
Experts also advised on ways to execute these drills that will be least damaging to students.
The AAP statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, for example, advocates eliminating high-intensity drills, prohibiting deception in drills, and providing accommodations based on children’s vulnerabilities.
Dr. Schonfeld also emphasized, in an interview, that training for an attack need not be extremely realistic to be effective.
“When you are preparing for a crisis, the drills and exercises are for children to practice and develop mastery over something they don’t know how to do fully yet,” said Dr. Schonfeld.
Citing a suggestion from a 2020 report conducted by Everytown for Gun Safety on keeping schools safe from gun violence, Dr. Younghans said, “Schools should be in clear communication with communities and families regarding when drills will be happening,” and advised ensuring that the explanation of drills is developmentally appropriate to the age of the children participating.
The report also recommends conducting drills that do not simulate an actual incident, combining drills with trauma-informed approaches to address students’ well-being during and for a sustained period after the drills, and tracking data on the efficacy and effects of drills.
Dr. Loper suggested ways that clinicians and parents can help navigate the tricky territory of school safety drills.
In his view, they should not be random or unexpected, and anticipatory guidance should be given regarding any visual or auditory stimuli, such as flashing lights or sirens, alarms, or announcements.
“A preventive approach should be utilized to ensure that any child who is experiencing extreme drill-distress be excused from any future disaster drills to prevent retraumatization,” Dr. Loper said.
Physicians interviewed for this piece also provided tips on how to talk about these events with children in a way that is beneficial to their mental health.
“What we want to do is [have a] calm discussion [with kids] about what we are doing and why we are doing it” and guide them through the movements, Dr. Schonfeld said.
When teaching children how to respond to an emergency, some elements of uncertainty need to be discussed. Children need to anticipate “what you might do if you are not in the classroom if something occurs, such as being in the bathroom, or out at recess,” he continued.
Dr. Younghans recommended that parents and staff schedule time to prepare children for the drill and practice in advance, and that behavioral health providers, counselors, and/or primary care providers should be involved in the planning and execution of the drill.
The Georgia Tech study was supported through a grant from Everytown for Gun Safety.
The study authors and experts interviewed for this piece had no financial conflicts to disclose.
The drills can range from staging lockdowns and sheltering in place to quasi dramas with mock shooters roaming the halls. Although the goals of these training exercises are important, equally important are the potential negative effects of drills on students’ mental health, according to doctors with expertise in pediatrics and mental health.
“Dramatic simulation of an active shooter event at school would be expected to provoke the same stress response as the real thing,” said Peter L. Loper Jr., MD, a pediatrician and psychiatrist, in an interview. “While ensuring their physical safety is very important, we must be intentional about making sure that we are not doing so at the expense of their psychosocial or emotional safety.”
“Children may not be able to differentiate a dramatic drill from a real event,” emphasized Dr. Loper, of the neuropsychiatry and behavioral science departments at the University of South Carolina, Columbia. “The parts of the brain responsible for our flight-fight-or-freeze response would interpret both simulated and real events identically and produce the same neurohormonal stress-response.”
Indeed, a study published in the journal Humanities & Social Sciences Communications suggested children experienced mental health problems related to participating in active shooter drills. In the large study, a team of statisticians from the Georgia Institute of Technology found that students reported a 42% increase in stress and anxiety and a 38.7% increase in depression during the 90 days following active shooter drills, compared with the 90 days before the drills.
The authors of this study, including Mai ElSherief, PhD, drew these conclusions after analyzing 54 million social media posts before and after drills in 114 schools across 33 states. The researchers analyzed the language of the social media posts by teachers, parents, and students and found increased use of the words hope, love, home, school, kids, community, support, and help after the drills. The researchers considered posting with these terms in the aftermath of the drills to be indicative of having high anxiety.
They included examples of how high stress, anxiety, and depression manifested in specific posts from parents in their report. The following is an example of a poster expressing high anxiety and stress: “are we really gonna normalize school shooter drills?! holy sh* there has to be a real way to avoid these tragedies. sh*t like this cannot be normalized. teachers injured after being shot with plastic pellets ‘execution style’ in active shooter drill.”
The authors also shared this post to serve as an example of a person who seems depressed: “and now we are revisiting the trauma on our kids, forcing them to act out school drills monthly. i don’t get why gen x parents buy into this concept wholeheartedly. things need to change.”
The published material did not include posts from students, but the researchers’ analysis of the content of posts overall showed increased concerns for health and increased concerns about death during the period after drills, compared with before drills.
The authors also conducted focus groups in communities in which drills occurred, and many teachers and parents reported anecdotal evidence of children who were nervous long after the drills were over, with some showing extreme reactions such as panic over a standard fire alarm at school. Overall, the results show that school shooter drills can negatively affect school communities over prolonged periods of time, they concluded.
According to a statement from the American Academy of Pediatrics, “there is a need to be cautious about the potential psychological risks and other unintended consequences of directly involving children in live exercises and drills.”
“These risks and consequences are especially a concern when children are deceived and led to believe there is an actual attack and not a drill,” wrote David Schonfeld, MD, the lead author of the statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, and colleagues.
Managing the fallout
Physicians can help students experiencing mental health problems from these drills, according to doctors interviewed for this piece.
It’s important for providers to know that stress will show up differently in children than in adults, said Chelsea Younghans, MD, a psychiatrist and military officer in Bethesda, Md., in an interview.
“They may see children with headaches, stomach aches, or nonspecific complaints. They may also see children who have not had difficulty with sleep present with nightmares or bed wetting,” she added.
For teens and preteens, validated tools such as the Child PTSD Symptom Scale (CPSS-5) and Child and Adolescent Trauma Screen (CATS) to assess PTSD in youth, may help serve as a starting point for a conversation between providers and their older child population, she noted.
Children who exhibit avoidance or withdrawal behaviors including consistent school refusal, an increase in reassurance-seeking behaviors, or somatic symptoms like vague abdominal pain or headaches that prevent school attendance after participating in a drill, may need more robust mental health services, Dr. Loper noted.
Dr. Schonfeld, who is also director of the National Center for School Crisis and Bereavement at Children’s Hospital Los Angeles, called for health care providers to be available to help children process traumatic reactions to these exercises.
Agreeing with Dr. Schonfeld, Dr. Younghans said: “It is vital to debrief with students and staff after drills, making sure that students have a safe space and ample time to speak with trusted staff. As children will undoubtedly have questions and concerns, creating open lines of communication will help alleviate any traumatic effect these drills may have.”
Communicating with various stakeholders
Experts also gave recommendations for how clinicians communicate with leaders in their area’s school districts and other members of their communities about these training exercises.
“For primary care providers, it is important to establish meaningful relationships within your community and patient population as much as possible,” Dr. Younghans said. “Having a good relationship with the local schools and being part of the conversation can help increase school and community awareness on the impact these drills can have on students and staff,” she added.
For those pediatricians or other health care providers who serve as consultants to schools, Dr. Schonfeld advised they ask about policies related to exercises and drills, such as what are the limits to what children might be exposed to in a drill, and what requirements there might be at the local and state level in terms of frequency and what the drills will and will not involve.
He also noted that clinicians should encourage school leaders to consider the fact that kids may have personal histories of trauma that are completely unknown to the school when they design these exercises.
School staff and health care providers should explain the nature and reasons for drills, invite family members to express concerns, and make accommodations if necessary for some children to participate in drills in a more limited way, noted Dr. Schonfeld, who is also clinical professor of pediatrics at the University of Southern California, Los Angeles.
“I think health care providers should work with legislators, so that if they require a drill, it must be done in a way that is physically and emotionally safe,” he added.
Executing better drills for students’ mental health
Experts also advised on ways to execute these drills that will be least damaging to students.
The AAP statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, for example, advocates eliminating high-intensity drills, prohibiting deception in drills, and providing accommodations based on children’s vulnerabilities.
Dr. Schonfeld also emphasized, in an interview, that training for an attack need not be extremely realistic to be effective.
“When you are preparing for a crisis, the drills and exercises are for children to practice and develop mastery over something they don’t know how to do fully yet,” said Dr. Schonfeld.
Citing a suggestion from a 2020 report conducted by Everytown for Gun Safety on keeping schools safe from gun violence, Dr. Younghans said, “Schools should be in clear communication with communities and families regarding when drills will be happening,” and advised ensuring that the explanation of drills is developmentally appropriate to the age of the children participating.
The report also recommends conducting drills that do not simulate an actual incident, combining drills with trauma-informed approaches to address students’ well-being during and for a sustained period after the drills, and tracking data on the efficacy and effects of drills.
Dr. Loper suggested ways that clinicians and parents can help navigate the tricky territory of school safety drills.
In his view, they should not be random or unexpected, and anticipatory guidance should be given regarding any visual or auditory stimuli, such as flashing lights or sirens, alarms, or announcements.
“A preventive approach should be utilized to ensure that any child who is experiencing extreme drill-distress be excused from any future disaster drills to prevent retraumatization,” Dr. Loper said.
Physicians interviewed for this piece also provided tips on how to talk about these events with children in a way that is beneficial to their mental health.
“What we want to do is [have a] calm discussion [with kids] about what we are doing and why we are doing it” and guide them through the movements, Dr. Schonfeld said.
When teaching children how to respond to an emergency, some elements of uncertainty need to be discussed. Children need to anticipate “what you might do if you are not in the classroom if something occurs, such as being in the bathroom, or out at recess,” he continued.
Dr. Younghans recommended that parents and staff schedule time to prepare children for the drill and practice in advance, and that behavioral health providers, counselors, and/or primary care providers should be involved in the planning and execution of the drill.
The Georgia Tech study was supported through a grant from Everytown for Gun Safety.
The study authors and experts interviewed for this piece had no financial conflicts to disclose.
The drills can range from staging lockdowns and sheltering in place to quasi dramas with mock shooters roaming the halls. Although the goals of these training exercises are important, equally important are the potential negative effects of drills on students’ mental health, according to doctors with expertise in pediatrics and mental health.
“Dramatic simulation of an active shooter event at school would be expected to provoke the same stress response as the real thing,” said Peter L. Loper Jr., MD, a pediatrician and psychiatrist, in an interview. “While ensuring their physical safety is very important, we must be intentional about making sure that we are not doing so at the expense of their psychosocial or emotional safety.”
“Children may not be able to differentiate a dramatic drill from a real event,” emphasized Dr. Loper, of the neuropsychiatry and behavioral science departments at the University of South Carolina, Columbia. “The parts of the brain responsible for our flight-fight-or-freeze response would interpret both simulated and real events identically and produce the same neurohormonal stress-response.”
Indeed, a study published in the journal Humanities & Social Sciences Communications suggested children experienced mental health problems related to participating in active shooter drills. In the large study, a team of statisticians from the Georgia Institute of Technology found that students reported a 42% increase in stress and anxiety and a 38.7% increase in depression during the 90 days following active shooter drills, compared with the 90 days before the drills.
The authors of this study, including Mai ElSherief, PhD, drew these conclusions after analyzing 54 million social media posts before and after drills in 114 schools across 33 states. The researchers analyzed the language of the social media posts by teachers, parents, and students and found increased use of the words hope, love, home, school, kids, community, support, and help after the drills. The researchers considered posting with these terms in the aftermath of the drills to be indicative of having high anxiety.
They included examples of how high stress, anxiety, and depression manifested in specific posts from parents in their report. The following is an example of a poster expressing high anxiety and stress: “are we really gonna normalize school shooter drills?! holy sh* there has to be a real way to avoid these tragedies. sh*t like this cannot be normalized. teachers injured after being shot with plastic pellets ‘execution style’ in active shooter drill.”
The authors also shared this post to serve as an example of a person who seems depressed: “and now we are revisiting the trauma on our kids, forcing them to act out school drills monthly. i don’t get why gen x parents buy into this concept wholeheartedly. things need to change.”
The published material did not include posts from students, but the researchers’ analysis of the content of posts overall showed increased concerns for health and increased concerns about death during the period after drills, compared with before drills.
The authors also conducted focus groups in communities in which drills occurred, and many teachers and parents reported anecdotal evidence of children who were nervous long after the drills were over, with some showing extreme reactions such as panic over a standard fire alarm at school. Overall, the results show that school shooter drills can negatively affect school communities over prolonged periods of time, they concluded.
According to a statement from the American Academy of Pediatrics, “there is a need to be cautious about the potential psychological risks and other unintended consequences of directly involving children in live exercises and drills.”
“These risks and consequences are especially a concern when children are deceived and led to believe there is an actual attack and not a drill,” wrote David Schonfeld, MD, the lead author of the statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, and colleagues.
Managing the fallout
Physicians can help students experiencing mental health problems from these drills, according to doctors interviewed for this piece.
It’s important for providers to know that stress will show up differently in children than in adults, said Chelsea Younghans, MD, a psychiatrist and military officer in Bethesda, Md., in an interview.
“They may see children with headaches, stomach aches, or nonspecific complaints. They may also see children who have not had difficulty with sleep present with nightmares or bed wetting,” she added.
For teens and preteens, validated tools such as the Child PTSD Symptom Scale (CPSS-5) and Child and Adolescent Trauma Screen (CATS) to assess PTSD in youth, may help serve as a starting point for a conversation between providers and their older child population, she noted.
Children who exhibit avoidance or withdrawal behaviors including consistent school refusal, an increase in reassurance-seeking behaviors, or somatic symptoms like vague abdominal pain or headaches that prevent school attendance after participating in a drill, may need more robust mental health services, Dr. Loper noted.
Dr. Schonfeld, who is also director of the National Center for School Crisis and Bereavement at Children’s Hospital Los Angeles, called for health care providers to be available to help children process traumatic reactions to these exercises.
Agreeing with Dr. Schonfeld, Dr. Younghans said: “It is vital to debrief with students and staff after drills, making sure that students have a safe space and ample time to speak with trusted staff. As children will undoubtedly have questions and concerns, creating open lines of communication will help alleviate any traumatic effect these drills may have.”
Communicating with various stakeholders
Experts also gave recommendations for how clinicians communicate with leaders in their area’s school districts and other members of their communities about these training exercises.
“For primary care providers, it is important to establish meaningful relationships within your community and patient population as much as possible,” Dr. Younghans said. “Having a good relationship with the local schools and being part of the conversation can help increase school and community awareness on the impact these drills can have on students and staff,” she added.
For those pediatricians or other health care providers who serve as consultants to schools, Dr. Schonfeld advised they ask about policies related to exercises and drills, such as what are the limits to what children might be exposed to in a drill, and what requirements there might be at the local and state level in terms of frequency and what the drills will and will not involve.
He also noted that clinicians should encourage school leaders to consider the fact that kids may have personal histories of trauma that are completely unknown to the school when they design these exercises.
School staff and health care providers should explain the nature and reasons for drills, invite family members to express concerns, and make accommodations if necessary for some children to participate in drills in a more limited way, noted Dr. Schonfeld, who is also clinical professor of pediatrics at the University of Southern California, Los Angeles.
“I think health care providers should work with legislators, so that if they require a drill, it must be done in a way that is physically and emotionally safe,” he added.
Executing better drills for students’ mental health
Experts also advised on ways to execute these drills that will be least damaging to students.
The AAP statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, for example, advocates eliminating high-intensity drills, prohibiting deception in drills, and providing accommodations based on children’s vulnerabilities.
Dr. Schonfeld also emphasized, in an interview, that training for an attack need not be extremely realistic to be effective.
“When you are preparing for a crisis, the drills and exercises are for children to practice and develop mastery over something they don’t know how to do fully yet,” said Dr. Schonfeld.
Citing a suggestion from a 2020 report conducted by Everytown for Gun Safety on keeping schools safe from gun violence, Dr. Younghans said, “Schools should be in clear communication with communities and families regarding when drills will be happening,” and advised ensuring that the explanation of drills is developmentally appropriate to the age of the children participating.
The report also recommends conducting drills that do not simulate an actual incident, combining drills with trauma-informed approaches to address students’ well-being during and for a sustained period after the drills, and tracking data on the efficacy and effects of drills.
Dr. Loper suggested ways that clinicians and parents can help navigate the tricky territory of school safety drills.
In his view, they should not be random or unexpected, and anticipatory guidance should be given regarding any visual or auditory stimuli, such as flashing lights or sirens, alarms, or announcements.
“A preventive approach should be utilized to ensure that any child who is experiencing extreme drill-distress be excused from any future disaster drills to prevent retraumatization,” Dr. Loper said.
Physicians interviewed for this piece also provided tips on how to talk about these events with children in a way that is beneficial to their mental health.
“What we want to do is [have a] calm discussion [with kids] about what we are doing and why we are doing it” and guide them through the movements, Dr. Schonfeld said.
When teaching children how to respond to an emergency, some elements of uncertainty need to be discussed. Children need to anticipate “what you might do if you are not in the classroom if something occurs, such as being in the bathroom, or out at recess,” he continued.
Dr. Younghans recommended that parents and staff schedule time to prepare children for the drill and practice in advance, and that behavioral health providers, counselors, and/or primary care providers should be involved in the planning and execution of the drill.
The Georgia Tech study was supported through a grant from Everytown for Gun Safety.
The study authors and experts interviewed for this piece had no financial conflicts to disclose.
Reassessing benzodiazepines: What role should this medication class play in psychiatry?
Many psychiatrists have had the grim experience of a newly referred patient explaining that her (and it is most often “her”) primary care doctor has been prescribing lorazepam 8 mg per day or alprazolam 6 mg per day and is sending her to you for help with ongoing anxiety. For conscientious psychiatrists, this means the beginning of a long tapering process along with a great deal of reassuring of a patient who is terrified of feeling overwhelmed with anxiety. The same problem occurs with patients taking large doses of sedatives who are still unable to sleep.
Mark Olfson and coauthors quantified benzodiazepine use in the United States in 2008 using a large prescription database, and found that 5.2% of adults between 18 and 80 years old were taking these drugs.1 The percentage increased with age, to 8.7% of those 65-80 years, in whom 31% received long-term prescriptions from a psychiatrist. Benzodiazepine use was twice as prevalent in women, compared with men. This occurs despite peer-reviewed publications and articles in the popular press regarding the risks of long-term benzodiazepine use in the elderly. Fang-Yu Lin and coauthors documented a 2.23-fold higher risk of hip fracture in zolpidem users that increased with age; elderly users had a 21-fold higher incidence of fracture, compared with younger users, and were twice as likely to sustain a fracture than elderly nonusers.2
Rashona Thomas and Edid Ramos-Rivas reviewed the risks of benzodiazepines in older patients with insomnia and document the increase in serious adverse events such as falls, fractures, and cognitive and behavioral changes.3 Many patients have ongoing prescriptions that make discontinuation difficult, given the potential for withdrawal agitation, seizures, insomnia, nightmares and even psychosis.
Greta Bushnell and coauthors pointed to the problem of simultaneous prescribing of a new antidepressant with a benzodiazepine by 10% of doctors initiating antidepressants.4 Over 12% of this group of patients continued benzodiazepines long term, even though there was no difference in the response to antidepressant treatment at 6 months. Those with long-term benzodiazepine use were also more likely to have recent prescriptions for opiates.
A Finnish research team found that 34% of middle-aged and 55% of elderly people developed long-term use of benzodiazepines after an initial prescription.5 Those who became long-term users were more often older male receivers of social benefits, with psychiatric comorbidities and substance abuse histories.
Kevin Xu and coauthors reviewed a National Health and Nutrition Examination Survey dataset from 1999 to 2015 with follow-up on over 5,000 individuals in that period.6 They found doubling of all-cause mortality in users of benzodiazepines with or without accompanying use of opiates, a statistically significant increase.
Perhaps most alarming is the increased risk for Alzheimer’s dementia diagnosis in users of benzodiazepines. Two separate studies (Billoti de Gage and colleagues and Ettcheto and colleagues7,8) provided reviews of evidence for the relationship between use of benzodiazepines and development of dementia, and repeated warnings about close monitoring of patients and the need for alternative treatments for anxiety and insomnia in the elderly.
Be alert to underlying issues
Overburdened primary practitioners faced with complaints about sleep and anxiety understandably turn to medication rather than taking time to discuss the reasons for these problems or to describe nonmedication approaches to relief of symptoms. Even insured patients may have very limited options for “covered” psychiatric consultation, as many competent psychiatrists have moved to a cash-only system. It is easier to renew prescriptions than to counsel patients or refer them, and many primary care practitioners have limited experience with diagnosing causes of anxiety and insomnia, much less alternative medication approaches.
Psychiatrists should be aware of the frequency of underlying mood disorders that include sleep and anxiety as prominent symptoms; in fact, these symptoms are often what motivates patients to pursue treatment. It is critical to obtain not only a personal history of symptoms beginning in childhood up to the present, but also a family history of mood and anxiety problems. Mood dysregulation disorders are highly hereditary and a family history of mania or psychosis should raise concern about the cause of symptoms in one’s patient. A strong personal and/or family history of alcohol abuse and dependence may cover underlying undiagnosed mood dysregulation. Primary care physicians may not recognize mood dysregulation unless a patient is clearly manic or psychotic.
There is a cohort of patients who do well on antidepressant medication, but anorgasmia, fatigue, and emotional blunting are common side effects that affect compliance. When patients have unexpected responses to SSRI medications such as euphoria, agitation, anxiety, insomnia, and more prominent mood swings, primary care physicians may add a benzodiazepine, expecting the problem to abate with time. Unfortunately, this often leads to ongoing use of benzodiazepines, since attempts to stop them causes withdrawal effects that are indistinguishable from the original anxiety symptoms.
Most psychiatrists are aware that some patients need mood stabilization rather than mood elevation to maintain an adequate baseline mood. Lithium, anticonvulsants, and second-generation antipsychotics may be effective without adding antidepressant medication. Managing dosing and side effects requires time for follow-up visits with patients after initiating treatment but leads to more stability and better outcomes.
Benzodiazepines are appropriate and helpful in situations that cause transient anxiety and with patients who have done poorly with other options. Intermittent use is key to avoiding tolerance and inevitable dose increases. Some individuals can take low daily doses that are harmless, though these likely only prevent withdrawal rather than preventing anxiety. The placebo effect of taking a pill is powerful. And some patients take more doses than they admit to. Most practitioners have heard stories about the alprazolam that was accidentally spilled into the sink or the prescription bottle of diazepam that was lost or the lorazepam supply that was stolen by the babysitter.
These concepts are illustrated in case examples below.
Case one
Ms. A, a 55-year-old married female business administrator, admitted to using zolpidem at 40 mg per night for the past several months. She began with the typical dose of 10 mg at bedtime prescribed by her internist, but after several weeks, needed an additional 10 mg at 2 a.m. to stay asleep. As weeks passed, she found that she needed an additional 20 mg when she awoke at 2 a.m. Within months, she needed 20 mg to initiate sleep and 20 mg to maintain sleep. She obtained extra zolpidem from her gynecologist and came for consultation when refill requests were refused.
Ms. A had a family history of high anxiety in her mother and depressed mood in multiple paternal relatives, including her father. She had trouble sleeping beginning in adolescence, significant premenstrual dysphoria, and postpartum depression that led to a prescription for sertraline. Instead of feeling better, Ms. A remembers being agitated and unable to sleep, so she stopped it. Ms. A was now perimenopausal, and insomnia was worse. She had gradually increased wine consumption to a bottle of wine each night after work to “settle down.” This allowed her to fall asleep, but she inevitably awoke within 4 hours. Her internist noted an elevation in ALT and asked Ms. A about alcohol consumption. She was alarmed and cut back to one glass of wine per night but again couldn’t sleep. Her internist started zolpidem at that point.
The psychiatrist explained the concepts of tolerance and addiction and a plan to slowly taper off zolpidem while using quetiapine for sleep. She decreased to 20 mg of zolpidem at bedtime with quetiapine 50 mg and was able to stay asleep. After 3 weeks, Ms. A took zolpidem 10 mg at bedtime with quetiapine 75 mg and again, was able to fall asleep and stay asleep. After another 3 weeks, she increased quetiapine to 100 mg and stopped zolpidem without difficulty. This dose of quetiapine has continued to work well without significant side effects.
Case two
Ms. B, a 70-year-old married housewife, was referred for help with longstanding anxiety when her primary care doctor recognized that lorazepam, initially helpful at 1 mg twice daily, had required titration to 2 mg three times daily. Ms. B was preoccupied with having lorazepam on hand and never missed a dose. She had little interest in activities beyond her home, rarely socialized, and had fallen twice. She napped for 2 hours each afternoon, and sometimes had trouble staying asleep through the night.
Ms. B was reluctant to talk about her childhood history of hostility and undermining by her mother, who clearly preferred her older brother and was competitive with Ms. B. Her father traveled for work during the week and had little time for her. Ms. B had always seen herself as stupid and unlovable, which interfered with making friends. She attended college for 1 year but dropped out to marry her husband. He was also anxious and had difficulty socializing, but they found reassurance in each other. Their only child, a son in his 40s, was estranged from them, having married a woman who disliked Ms. B. Ms. B felt hopeless about developing a relationship with her grandchildren who were rarely allowed to visit. Despite her initial shame in talking about these painful problems, Ms. B realized that she felt better and scheduled monthly visits to check in.
Ms. B understood the risks of using lorazepam and wanted to stop it but was terrified of becoming anxious again. We set up a very slow tapering schedule that lowered her total dose by 0.5 mg every 2 weeks. At the same time, she began escitalopram which was effective at 20 mg. Ms. B noted that she no longer felt anxious upon awakening but was still afraid to miss a dose of lorazepam. As she felt more confident and alert, Ms. B joined a painting class at a local community center and was gratified to find that she was good at working with watercolors. She invited her neighbors to come for dinner and was surprised at how friendly and open they were. Once she had tapered to 1 mg twice daily, Ms. B began walking for exercise as she now had enough energy that it felt good to move around. After 6 months, she was completely off lorazepam, and very grateful to have discovered her capacity to improve her pleasure in life.
Case three
Ms. C, a 48-year-old attorney was referred for help with anxiety and distress in the face of separation from her husband who had admitted to an affair after she heard him talking to his girlfriend from their basement. She was unsure whether she wanted to save the marriage or end it and was horrified at the thought of dating. She had never felt especially anxious or depressed and had a supportive circle of close friends. She was uncharacteristically unable to concentrate long enough to consider her options because of anxiety.
A dose of clonazepam 0.5 mg allowed her to stay alert but calm enough to reflect on her feelings. She used it intermittently over several months and maintained regular individual psychotherapy sessions that allowed her to review the situation thoroughly. On her psychiatrist’s recommendation, she contacted a colleague to represent her if she decided to initiate divorce proceedings. She attempted to engage her husband in marital therapy, and his reluctance made it clear to her that she could no longer trust him. Ms. C offered him the option of a dissolution if he was willing to cooperate, or to sue for divorce if not. Once Ms. C regained her confidence and recognized that she would survive this emotionally fraught situation, she no longer needed clonazepam.
Summary
The risks, which include cognitive slowing, falls and fractures, and withdrawal phenomena when abruptly stopped, make this class dangerous for all patients but particularly the elderly. Benzodiazepines are nonetheless useful medications for patients able to use them intermittently, whether on an alternating basis with other medications (for example, quetiapine alternating with clonazepam for chronic insomnia) or because symptoms of anxiety are intermittent. Psychiatrists treating tolerant patients should be familiar with the approach of tapering slowly while introducing more appropriate medications at adequate doses to manage symptoms.
Dr. Kaplan is training and supervising psychoanalyst at the Cincinnati Psychoanalytic Institute and volunteer professor of clinical psychiatry at the University of Cincinnati. The author reported no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Olfson M et al. JAMA Psychiatry. 2015 Feb;72(2):136-42. doi: 10.1001/jamapsychiatry.2014.1763.
2. Lin FY et al. Sleep. 2014 Apr 1;37(4):673-9. doi: 10.5665/sleep.3566.
3. Thomas R and Ramos-Rivas E. Psychiatr Ann. 2018;48(6):266-70. doi: 10.3928/00485713-20180513-01.
4. Bushnell GA et al. JAMA Psychiatry. 2017 Jul 1;74(7):747-55. doi: 10.1001/jamapsychiatry.2017.1273.
5. Taipale H et al. JAMA Netw Open. 2020;3(10):e2019029. doi: 10.1001/jamanetworkopen.2020.19029.
6. Xu KY et al. JAMA Netw Open. 2020;3(12):e2028557. doi: 10.1001/jamanetworkopen.2020.28557.
7. Billioti de Gage S et al. BMJ. 2014;349:g5205. doi: 10.1136/bmj.g5205.
8. Ettcheto M et al. Front Aging Neurosci. 2020 Jan 8;11:344. doi: 10.3389/fnagi.2019.00344.
Many psychiatrists have had the grim experience of a newly referred patient explaining that her (and it is most often “her”) primary care doctor has been prescribing lorazepam 8 mg per day or alprazolam 6 mg per day and is sending her to you for help with ongoing anxiety. For conscientious psychiatrists, this means the beginning of a long tapering process along with a great deal of reassuring of a patient who is terrified of feeling overwhelmed with anxiety. The same problem occurs with patients taking large doses of sedatives who are still unable to sleep.
Mark Olfson and coauthors quantified benzodiazepine use in the United States in 2008 using a large prescription database, and found that 5.2% of adults between 18 and 80 years old were taking these drugs.1 The percentage increased with age, to 8.7% of those 65-80 years, in whom 31% received long-term prescriptions from a psychiatrist. Benzodiazepine use was twice as prevalent in women, compared with men. This occurs despite peer-reviewed publications and articles in the popular press regarding the risks of long-term benzodiazepine use in the elderly. Fang-Yu Lin and coauthors documented a 2.23-fold higher risk of hip fracture in zolpidem users that increased with age; elderly users had a 21-fold higher incidence of fracture, compared with younger users, and were twice as likely to sustain a fracture than elderly nonusers.2
Rashona Thomas and Edid Ramos-Rivas reviewed the risks of benzodiazepines in older patients with insomnia and document the increase in serious adverse events such as falls, fractures, and cognitive and behavioral changes.3 Many patients have ongoing prescriptions that make discontinuation difficult, given the potential for withdrawal agitation, seizures, insomnia, nightmares and even psychosis.
Greta Bushnell and coauthors pointed to the problem of simultaneous prescribing of a new antidepressant with a benzodiazepine by 10% of doctors initiating antidepressants.4 Over 12% of this group of patients continued benzodiazepines long term, even though there was no difference in the response to antidepressant treatment at 6 months. Those with long-term benzodiazepine use were also more likely to have recent prescriptions for opiates.
A Finnish research team found that 34% of middle-aged and 55% of elderly people developed long-term use of benzodiazepines after an initial prescription.5 Those who became long-term users were more often older male receivers of social benefits, with psychiatric comorbidities and substance abuse histories.
Kevin Xu and coauthors reviewed a National Health and Nutrition Examination Survey dataset from 1999 to 2015 with follow-up on over 5,000 individuals in that period.6 They found doubling of all-cause mortality in users of benzodiazepines with or without accompanying use of opiates, a statistically significant increase.
Perhaps most alarming is the increased risk for Alzheimer’s dementia diagnosis in users of benzodiazepines. Two separate studies (Billoti de Gage and colleagues and Ettcheto and colleagues7,8) provided reviews of evidence for the relationship between use of benzodiazepines and development of dementia, and repeated warnings about close monitoring of patients and the need for alternative treatments for anxiety and insomnia in the elderly.
Be alert to underlying issues
Overburdened primary practitioners faced with complaints about sleep and anxiety understandably turn to medication rather than taking time to discuss the reasons for these problems or to describe nonmedication approaches to relief of symptoms. Even insured patients may have very limited options for “covered” psychiatric consultation, as many competent psychiatrists have moved to a cash-only system. It is easier to renew prescriptions than to counsel patients or refer them, and many primary care practitioners have limited experience with diagnosing causes of anxiety and insomnia, much less alternative medication approaches.
Psychiatrists should be aware of the frequency of underlying mood disorders that include sleep and anxiety as prominent symptoms; in fact, these symptoms are often what motivates patients to pursue treatment. It is critical to obtain not only a personal history of symptoms beginning in childhood up to the present, but also a family history of mood and anxiety problems. Mood dysregulation disorders are highly hereditary and a family history of mania or psychosis should raise concern about the cause of symptoms in one’s patient. A strong personal and/or family history of alcohol abuse and dependence may cover underlying undiagnosed mood dysregulation. Primary care physicians may not recognize mood dysregulation unless a patient is clearly manic or psychotic.
There is a cohort of patients who do well on antidepressant medication, but anorgasmia, fatigue, and emotional blunting are common side effects that affect compliance. When patients have unexpected responses to SSRI medications such as euphoria, agitation, anxiety, insomnia, and more prominent mood swings, primary care physicians may add a benzodiazepine, expecting the problem to abate with time. Unfortunately, this often leads to ongoing use of benzodiazepines, since attempts to stop them causes withdrawal effects that are indistinguishable from the original anxiety symptoms.
Most psychiatrists are aware that some patients need mood stabilization rather than mood elevation to maintain an adequate baseline mood. Lithium, anticonvulsants, and second-generation antipsychotics may be effective without adding antidepressant medication. Managing dosing and side effects requires time for follow-up visits with patients after initiating treatment but leads to more stability and better outcomes.
Benzodiazepines are appropriate and helpful in situations that cause transient anxiety and with patients who have done poorly with other options. Intermittent use is key to avoiding tolerance and inevitable dose increases. Some individuals can take low daily doses that are harmless, though these likely only prevent withdrawal rather than preventing anxiety. The placebo effect of taking a pill is powerful. And some patients take more doses than they admit to. Most practitioners have heard stories about the alprazolam that was accidentally spilled into the sink or the prescription bottle of diazepam that was lost or the lorazepam supply that was stolen by the babysitter.
These concepts are illustrated in case examples below.
Case one
Ms. A, a 55-year-old married female business administrator, admitted to using zolpidem at 40 mg per night for the past several months. She began with the typical dose of 10 mg at bedtime prescribed by her internist, but after several weeks, needed an additional 10 mg at 2 a.m. to stay asleep. As weeks passed, she found that she needed an additional 20 mg when she awoke at 2 a.m. Within months, she needed 20 mg to initiate sleep and 20 mg to maintain sleep. She obtained extra zolpidem from her gynecologist and came for consultation when refill requests were refused.
Ms. A had a family history of high anxiety in her mother and depressed mood in multiple paternal relatives, including her father. She had trouble sleeping beginning in adolescence, significant premenstrual dysphoria, and postpartum depression that led to a prescription for sertraline. Instead of feeling better, Ms. A remembers being agitated and unable to sleep, so she stopped it. Ms. A was now perimenopausal, and insomnia was worse. She had gradually increased wine consumption to a bottle of wine each night after work to “settle down.” This allowed her to fall asleep, but she inevitably awoke within 4 hours. Her internist noted an elevation in ALT and asked Ms. A about alcohol consumption. She was alarmed and cut back to one glass of wine per night but again couldn’t sleep. Her internist started zolpidem at that point.
The psychiatrist explained the concepts of tolerance and addiction and a plan to slowly taper off zolpidem while using quetiapine for sleep. She decreased to 20 mg of zolpidem at bedtime with quetiapine 50 mg and was able to stay asleep. After 3 weeks, Ms. A took zolpidem 10 mg at bedtime with quetiapine 75 mg and again, was able to fall asleep and stay asleep. After another 3 weeks, she increased quetiapine to 100 mg and stopped zolpidem without difficulty. This dose of quetiapine has continued to work well without significant side effects.
Case two
Ms. B, a 70-year-old married housewife, was referred for help with longstanding anxiety when her primary care doctor recognized that lorazepam, initially helpful at 1 mg twice daily, had required titration to 2 mg three times daily. Ms. B was preoccupied with having lorazepam on hand and never missed a dose. She had little interest in activities beyond her home, rarely socialized, and had fallen twice. She napped for 2 hours each afternoon, and sometimes had trouble staying asleep through the night.
Ms. B was reluctant to talk about her childhood history of hostility and undermining by her mother, who clearly preferred her older brother and was competitive with Ms. B. Her father traveled for work during the week and had little time for her. Ms. B had always seen herself as stupid and unlovable, which interfered with making friends. She attended college for 1 year but dropped out to marry her husband. He was also anxious and had difficulty socializing, but they found reassurance in each other. Their only child, a son in his 40s, was estranged from them, having married a woman who disliked Ms. B. Ms. B felt hopeless about developing a relationship with her grandchildren who were rarely allowed to visit. Despite her initial shame in talking about these painful problems, Ms. B realized that she felt better and scheduled monthly visits to check in.
Ms. B understood the risks of using lorazepam and wanted to stop it but was terrified of becoming anxious again. We set up a very slow tapering schedule that lowered her total dose by 0.5 mg every 2 weeks. At the same time, she began escitalopram which was effective at 20 mg. Ms. B noted that she no longer felt anxious upon awakening but was still afraid to miss a dose of lorazepam. As she felt more confident and alert, Ms. B joined a painting class at a local community center and was gratified to find that she was good at working with watercolors. She invited her neighbors to come for dinner and was surprised at how friendly and open they were. Once she had tapered to 1 mg twice daily, Ms. B began walking for exercise as she now had enough energy that it felt good to move around. After 6 months, she was completely off lorazepam, and very grateful to have discovered her capacity to improve her pleasure in life.
Case three
Ms. C, a 48-year-old attorney was referred for help with anxiety and distress in the face of separation from her husband who had admitted to an affair after she heard him talking to his girlfriend from their basement. She was unsure whether she wanted to save the marriage or end it and was horrified at the thought of dating. She had never felt especially anxious or depressed and had a supportive circle of close friends. She was uncharacteristically unable to concentrate long enough to consider her options because of anxiety.
A dose of clonazepam 0.5 mg allowed her to stay alert but calm enough to reflect on her feelings. She used it intermittently over several months and maintained regular individual psychotherapy sessions that allowed her to review the situation thoroughly. On her psychiatrist’s recommendation, she contacted a colleague to represent her if she decided to initiate divorce proceedings. She attempted to engage her husband in marital therapy, and his reluctance made it clear to her that she could no longer trust him. Ms. C offered him the option of a dissolution if he was willing to cooperate, or to sue for divorce if not. Once Ms. C regained her confidence and recognized that she would survive this emotionally fraught situation, she no longer needed clonazepam.
Summary
The risks, which include cognitive slowing, falls and fractures, and withdrawal phenomena when abruptly stopped, make this class dangerous for all patients but particularly the elderly. Benzodiazepines are nonetheless useful medications for patients able to use them intermittently, whether on an alternating basis with other medications (for example, quetiapine alternating with clonazepam for chronic insomnia) or because symptoms of anxiety are intermittent. Psychiatrists treating tolerant patients should be familiar with the approach of tapering slowly while introducing more appropriate medications at adequate doses to manage symptoms.
Dr. Kaplan is training and supervising psychoanalyst at the Cincinnati Psychoanalytic Institute and volunteer professor of clinical psychiatry at the University of Cincinnati. The author reported no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Olfson M et al. JAMA Psychiatry. 2015 Feb;72(2):136-42. doi: 10.1001/jamapsychiatry.2014.1763.
2. Lin FY et al. Sleep. 2014 Apr 1;37(4):673-9. doi: 10.5665/sleep.3566.
3. Thomas R and Ramos-Rivas E. Psychiatr Ann. 2018;48(6):266-70. doi: 10.3928/00485713-20180513-01.
4. Bushnell GA et al. JAMA Psychiatry. 2017 Jul 1;74(7):747-55. doi: 10.1001/jamapsychiatry.2017.1273.
5. Taipale H et al. JAMA Netw Open. 2020;3(10):e2019029. doi: 10.1001/jamanetworkopen.2020.19029.
6. Xu KY et al. JAMA Netw Open. 2020;3(12):e2028557. doi: 10.1001/jamanetworkopen.2020.28557.
7. Billioti de Gage S et al. BMJ. 2014;349:g5205. doi: 10.1136/bmj.g5205.
8. Ettcheto M et al. Front Aging Neurosci. 2020 Jan 8;11:344. doi: 10.3389/fnagi.2019.00344.
Many psychiatrists have had the grim experience of a newly referred patient explaining that her (and it is most often “her”) primary care doctor has been prescribing lorazepam 8 mg per day or alprazolam 6 mg per day and is sending her to you for help with ongoing anxiety. For conscientious psychiatrists, this means the beginning of a long tapering process along with a great deal of reassuring of a patient who is terrified of feeling overwhelmed with anxiety. The same problem occurs with patients taking large doses of sedatives who are still unable to sleep.
Mark Olfson and coauthors quantified benzodiazepine use in the United States in 2008 using a large prescription database, and found that 5.2% of adults between 18 and 80 years old were taking these drugs.1 The percentage increased with age, to 8.7% of those 65-80 years, in whom 31% received long-term prescriptions from a psychiatrist. Benzodiazepine use was twice as prevalent in women, compared with men. This occurs despite peer-reviewed publications and articles in the popular press regarding the risks of long-term benzodiazepine use in the elderly. Fang-Yu Lin and coauthors documented a 2.23-fold higher risk of hip fracture in zolpidem users that increased with age; elderly users had a 21-fold higher incidence of fracture, compared with younger users, and were twice as likely to sustain a fracture than elderly nonusers.2
Rashona Thomas and Edid Ramos-Rivas reviewed the risks of benzodiazepines in older patients with insomnia and document the increase in serious adverse events such as falls, fractures, and cognitive and behavioral changes.3 Many patients have ongoing prescriptions that make discontinuation difficult, given the potential for withdrawal agitation, seizures, insomnia, nightmares and even psychosis.
Greta Bushnell and coauthors pointed to the problem of simultaneous prescribing of a new antidepressant with a benzodiazepine by 10% of doctors initiating antidepressants.4 Over 12% of this group of patients continued benzodiazepines long term, even though there was no difference in the response to antidepressant treatment at 6 months. Those with long-term benzodiazepine use were also more likely to have recent prescriptions for opiates.
A Finnish research team found that 34% of middle-aged and 55% of elderly people developed long-term use of benzodiazepines after an initial prescription.5 Those who became long-term users were more often older male receivers of social benefits, with psychiatric comorbidities and substance abuse histories.
Kevin Xu and coauthors reviewed a National Health and Nutrition Examination Survey dataset from 1999 to 2015 with follow-up on over 5,000 individuals in that period.6 They found doubling of all-cause mortality in users of benzodiazepines with or without accompanying use of opiates, a statistically significant increase.
Perhaps most alarming is the increased risk for Alzheimer’s dementia diagnosis in users of benzodiazepines. Two separate studies (Billoti de Gage and colleagues and Ettcheto and colleagues7,8) provided reviews of evidence for the relationship between use of benzodiazepines and development of dementia, and repeated warnings about close monitoring of patients and the need for alternative treatments for anxiety and insomnia in the elderly.
Be alert to underlying issues
Overburdened primary practitioners faced with complaints about sleep and anxiety understandably turn to medication rather than taking time to discuss the reasons for these problems or to describe nonmedication approaches to relief of symptoms. Even insured patients may have very limited options for “covered” psychiatric consultation, as many competent psychiatrists have moved to a cash-only system. It is easier to renew prescriptions than to counsel patients or refer them, and many primary care practitioners have limited experience with diagnosing causes of anxiety and insomnia, much less alternative medication approaches.
Psychiatrists should be aware of the frequency of underlying mood disorders that include sleep and anxiety as prominent symptoms; in fact, these symptoms are often what motivates patients to pursue treatment. It is critical to obtain not only a personal history of symptoms beginning in childhood up to the present, but also a family history of mood and anxiety problems. Mood dysregulation disorders are highly hereditary and a family history of mania or psychosis should raise concern about the cause of symptoms in one’s patient. A strong personal and/or family history of alcohol abuse and dependence may cover underlying undiagnosed mood dysregulation. Primary care physicians may not recognize mood dysregulation unless a patient is clearly manic or psychotic.
There is a cohort of patients who do well on antidepressant medication, but anorgasmia, fatigue, and emotional blunting are common side effects that affect compliance. When patients have unexpected responses to SSRI medications such as euphoria, agitation, anxiety, insomnia, and more prominent mood swings, primary care physicians may add a benzodiazepine, expecting the problem to abate with time. Unfortunately, this often leads to ongoing use of benzodiazepines, since attempts to stop them causes withdrawal effects that are indistinguishable from the original anxiety symptoms.
Most psychiatrists are aware that some patients need mood stabilization rather than mood elevation to maintain an adequate baseline mood. Lithium, anticonvulsants, and second-generation antipsychotics may be effective without adding antidepressant medication. Managing dosing and side effects requires time for follow-up visits with patients after initiating treatment but leads to more stability and better outcomes.
Benzodiazepines are appropriate and helpful in situations that cause transient anxiety and with patients who have done poorly with other options. Intermittent use is key to avoiding tolerance and inevitable dose increases. Some individuals can take low daily doses that are harmless, though these likely only prevent withdrawal rather than preventing anxiety. The placebo effect of taking a pill is powerful. And some patients take more doses than they admit to. Most practitioners have heard stories about the alprazolam that was accidentally spilled into the sink or the prescription bottle of diazepam that was lost or the lorazepam supply that was stolen by the babysitter.
These concepts are illustrated in case examples below.
Case one
Ms. A, a 55-year-old married female business administrator, admitted to using zolpidem at 40 mg per night for the past several months. She began with the typical dose of 10 mg at bedtime prescribed by her internist, but after several weeks, needed an additional 10 mg at 2 a.m. to stay asleep. As weeks passed, she found that she needed an additional 20 mg when she awoke at 2 a.m. Within months, she needed 20 mg to initiate sleep and 20 mg to maintain sleep. She obtained extra zolpidem from her gynecologist and came for consultation when refill requests were refused.
Ms. A had a family history of high anxiety in her mother and depressed mood in multiple paternal relatives, including her father. She had trouble sleeping beginning in adolescence, significant premenstrual dysphoria, and postpartum depression that led to a prescription for sertraline. Instead of feeling better, Ms. A remembers being agitated and unable to sleep, so she stopped it. Ms. A was now perimenopausal, and insomnia was worse. She had gradually increased wine consumption to a bottle of wine each night after work to “settle down.” This allowed her to fall asleep, but she inevitably awoke within 4 hours. Her internist noted an elevation in ALT and asked Ms. A about alcohol consumption. She was alarmed and cut back to one glass of wine per night but again couldn’t sleep. Her internist started zolpidem at that point.
The psychiatrist explained the concepts of tolerance and addiction and a plan to slowly taper off zolpidem while using quetiapine for sleep. She decreased to 20 mg of zolpidem at bedtime with quetiapine 50 mg and was able to stay asleep. After 3 weeks, Ms. A took zolpidem 10 mg at bedtime with quetiapine 75 mg and again, was able to fall asleep and stay asleep. After another 3 weeks, she increased quetiapine to 100 mg and stopped zolpidem without difficulty. This dose of quetiapine has continued to work well without significant side effects.
Case two
Ms. B, a 70-year-old married housewife, was referred for help with longstanding anxiety when her primary care doctor recognized that lorazepam, initially helpful at 1 mg twice daily, had required titration to 2 mg three times daily. Ms. B was preoccupied with having lorazepam on hand and never missed a dose. She had little interest in activities beyond her home, rarely socialized, and had fallen twice. She napped for 2 hours each afternoon, and sometimes had trouble staying asleep through the night.
Ms. B was reluctant to talk about her childhood history of hostility and undermining by her mother, who clearly preferred her older brother and was competitive with Ms. B. Her father traveled for work during the week and had little time for her. Ms. B had always seen herself as stupid and unlovable, which interfered with making friends. She attended college for 1 year but dropped out to marry her husband. He was also anxious and had difficulty socializing, but they found reassurance in each other. Their only child, a son in his 40s, was estranged from them, having married a woman who disliked Ms. B. Ms. B felt hopeless about developing a relationship with her grandchildren who were rarely allowed to visit. Despite her initial shame in talking about these painful problems, Ms. B realized that she felt better and scheduled monthly visits to check in.
Ms. B understood the risks of using lorazepam and wanted to stop it but was terrified of becoming anxious again. We set up a very slow tapering schedule that lowered her total dose by 0.5 mg every 2 weeks. At the same time, she began escitalopram which was effective at 20 mg. Ms. B noted that she no longer felt anxious upon awakening but was still afraid to miss a dose of lorazepam. As she felt more confident and alert, Ms. B joined a painting class at a local community center and was gratified to find that she was good at working with watercolors. She invited her neighbors to come for dinner and was surprised at how friendly and open they were. Once she had tapered to 1 mg twice daily, Ms. B began walking for exercise as she now had enough energy that it felt good to move around. After 6 months, she was completely off lorazepam, and very grateful to have discovered her capacity to improve her pleasure in life.
Case three
Ms. C, a 48-year-old attorney was referred for help with anxiety and distress in the face of separation from her husband who had admitted to an affair after she heard him talking to his girlfriend from their basement. She was unsure whether she wanted to save the marriage or end it and was horrified at the thought of dating. She had never felt especially anxious or depressed and had a supportive circle of close friends. She was uncharacteristically unable to concentrate long enough to consider her options because of anxiety.
A dose of clonazepam 0.5 mg allowed her to stay alert but calm enough to reflect on her feelings. She used it intermittently over several months and maintained regular individual psychotherapy sessions that allowed her to review the situation thoroughly. On her psychiatrist’s recommendation, she contacted a colleague to represent her if she decided to initiate divorce proceedings. She attempted to engage her husband in marital therapy, and his reluctance made it clear to her that she could no longer trust him. Ms. C offered him the option of a dissolution if he was willing to cooperate, or to sue for divorce if not. Once Ms. C regained her confidence and recognized that she would survive this emotionally fraught situation, she no longer needed clonazepam.
Summary
The risks, which include cognitive slowing, falls and fractures, and withdrawal phenomena when abruptly stopped, make this class dangerous for all patients but particularly the elderly. Benzodiazepines are nonetheless useful medications for patients able to use them intermittently, whether on an alternating basis with other medications (for example, quetiapine alternating with clonazepam for chronic insomnia) or because symptoms of anxiety are intermittent. Psychiatrists treating tolerant patients should be familiar with the approach of tapering slowly while introducing more appropriate medications at adequate doses to manage symptoms.
Dr. Kaplan is training and supervising psychoanalyst at the Cincinnati Psychoanalytic Institute and volunteer professor of clinical psychiatry at the University of Cincinnati. The author reported no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Olfson M et al. JAMA Psychiatry. 2015 Feb;72(2):136-42. doi: 10.1001/jamapsychiatry.2014.1763.
2. Lin FY et al. Sleep. 2014 Apr 1;37(4):673-9. doi: 10.5665/sleep.3566.
3. Thomas R and Ramos-Rivas E. Psychiatr Ann. 2018;48(6):266-70. doi: 10.3928/00485713-20180513-01.
4. Bushnell GA et al. JAMA Psychiatry. 2017 Jul 1;74(7):747-55. doi: 10.1001/jamapsychiatry.2017.1273.
5. Taipale H et al. JAMA Netw Open. 2020;3(10):e2019029. doi: 10.1001/jamanetworkopen.2020.19029.
6. Xu KY et al. JAMA Netw Open. 2020;3(12):e2028557. doi: 10.1001/jamanetworkopen.2020.28557.
7. Billioti de Gage S et al. BMJ. 2014;349:g5205. doi: 10.1136/bmj.g5205.
8. Ettcheto M et al. Front Aging Neurosci. 2020 Jan 8;11:344. doi: 10.3389/fnagi.2019.00344.













