User login
Tension Pneumothorax After Ultrasound-Guided Interscalene Block and Shoulder Arthroscopy
Interscalene brachial plexus anesthesia is commonly used for arthroscopic and open procedures of the shoulder. This regional anesthetic targets the trunks of the brachial plexus and anesthetizes the area about the shoulder and proximal arm. Its use may obviate the need for concomitant general anesthesia, potentially reducing the use of postoperative intravenous and oral pain medication. Furthermore, patients often bypass the acute postoperative anesthesia care unit and proceed directly to the ambulatory unit, permitting earlier hospital discharge. Previous reports in the literature have demonstrated higher rates of neurologic, cardiac, and pulmonary complications from this procedure; in particular, the incidence of pneumothorax was reported as high as 3%.1 Techniques to localize the nerves, such as electrical nerve stimulation and, more recently, ultrasound guidance, have reduced these complication rates.2,3 Successful administration of the block has been shown to result in satisfactory postoperative pain relief.2 However, ultrasound-guided interscalene nerve blocks remain operator-dependent and complications may still occur.
We report a case of tension pneumothorax after arthroscopic rotator cuff repair and subacromial decompression with an ultrasound-guided interscalene block. Immediate recognition and treatment of this complication resulted in a good clinical outcome. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman presented with 3 months of right shoulder pain after a fall. Examination was pertinent for weakness in forward elevation and positive rotator cuff impingement signs. She remained symptomatic despite a course of nonsurgical management that included cortisone injections and physical therapy. Magnetic resonance imaging of the shoulder showed a full-thickness supraspinatus tear with minimal fatty atrophy. After a discussion of her treatment options, she elected to undergo an arthroscopic rotator cuff repair with subacromial decompression. An evaluation by her internist revealed no pertinent medical history apart from obesity (body mass index, 36). Specifically, there was no reported history of chronic obstructive pulmonary disease or asthma. She denied any prior cigarette smoking.
The patient was evaluated by the regional anesthesia team and was classified as a class 2 airway. An interscalene brachial plexus block was performed using a 2-inch, 22-gauge needle inserted into the interscalene groove. Using an out-of-plane technique under direct ultrasound guidance, 30 mL of 0.52% ropivacaine was injected. The block was considered successful, and no complications, such as resistance, paresthesias, pain, or blood on aspiration, were noted during injection. The patient had no complaints of chest pain or shortness of breath immediately afterward, and all vital signs were stable throughout the procedure.
The patient was brought to the operating room and placed in the beach-chair position. Induction for general anesthesia was started 15 minutes after the regional anesthetic, with 2 intubation attempts necessary because of poor airway visualization. After placement of the endotracheal tube, breath sounds were noted to be equal bilaterally. The arthroscopic procedure consisted of double-row rotator cuff repair, subacromial decompression, and débridement of the glenohumeral joint for synovitis, using standard arthroscopic portals. There were no difficulties with trocar placement, and bleeding was minimal throughout the case. The total surgical time was 150 minutes and a pump pressure of 30 mm Hg was maintained during the arthroscopy.
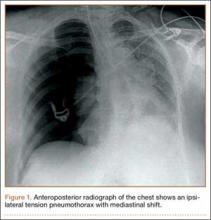
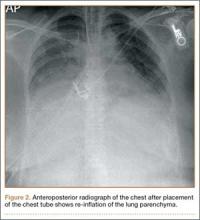
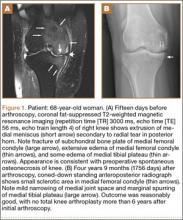
Within the first 60 minutes of the start of the arthroscopic procedure, the patient was noted to be intermittently hypotensive with mean arterial pressure (MAP) ranging from the 30s to 130s mm Hg and pulse in the 70 to 80 beats/min range. FiO2 in the 85% to 95% range was maintained throughout the procedure. During that time, 50 μg phenylephrine was administered on 4 separate occasions to maintain her blood pressure. The labile blood pressure was attributed by the anesthesiologist to the beach-chair position. During an attempted extubation upon conclusion of the surgery, the patient became hypotensive with MAP that ranged from the 40s to 60s mm Hg and tachycardic to 90 beats/min. The oxygen saturation was in the low 90s and tidal volume was poor. Absent lung sounds were noted on the right chest. An urgent portable chest radiograph showed a large right-sided tension pneumothorax with mediastinal shift (Figure 1). After an immediate general surgery consultation, a chest tube was placed in the operating room. The patient’s vital signs improved and a repeat chest radiograph revealed successful re-expansion of the lung (Figure 2). She was transferred to the acute postoperative anesthesia care unit and extubated in the intensive care unit later that day.
The patient’s chest tube was removed 2 days later and she was discharged home on hospital day 5 with a completely resolved pneumothorax. She was seen 1 week later in the office for a postoperative visit and reported feeling well without chest pain or shortness of breath.
Discussion
Interscalene brachial plexus anesthesia was first described by Winnie4 in 1970. This block targets the trunks of the brachial plexus, which are enclosed in a fascial sheath between the anterior and middle scalene muscles. In this region lie several structures at risk: the phrenic nerve superficially and inferiorly; the carotid sheath located superficially and medially; the subclavian artery parallel to the trunks; and the cupula of the lung that lies deep and inferior to the anterior scalene muscle. Recognized complications of the block include vocal hoarseness, Horner syndrome, and hemidiaphragmatic paresis caused by the temporary blockade of the ipsilateral recurrent laryngeal nerve, stellate ganglion, and phrenic nerve, in that order.5 Use of the interscalene block has been associated with minimal risk for pneumothorax, because the needle entry point is superior and directed away from the lung pleura.6 This is in contrast to the more inferiorly placed supraclavicular block, located in closer proximity to the lung cupula.5
Two different approaches are commonly used during ultrasound-guided nerve blocks. The in-plane approach generates a long-axis view of the needle by advancing the needle parallel with the long axis of the ultrasound probe. While this allows direct visualization of the needle tip, it requires deeper needle insertion from lateral to medial, causing puncture of the middle scalene muscle that may increase patient discomfort and risk nerve injury within the muscle.7 The out-of-plane approach used on our patient involves needle insertion parallel to the brachial plexus, but along the short axis of the ultrasound probe. Although this permits the operator to assess the periphery of the nerve, it may lead to poor needle-tip visualization during the procedure. As a result, operators often use a combination of tissue disturbance and “hydrolocation,” in which fluid is injected to indicate the needle-tip location.8,9
Tension pneumothorax represents the accumulation of air in the pleural space that leads to impaired pulmonary and cardiac function. It is often caused by disruption or puncture of the parietal or visceral pleura, creating a connection between the alveoli and pleural cavity. The gradual buildup of air in the pleural cavity results in increased intrapleural pressure, which compresses and ultimately collapses the ipsilateral lung. Venous compression restricts blood return to the heart and reduces cardiac output. Clinical manifestations include dyspnea, hypoxemia, tachycardia, and hypotension.10 Multiple techniques were developed to better localize the brachial plexus while reducing injury to nearby structures, including the lung. These include eliciting needle paresthesias, electrical nerve stimulation, and ultrasound guidance. While nerve stimulation was once the gold standard for brachial plexus localization, ultrasound guidance has gained in popularity because of its noninvasive nature and dynamic capability to identify nerves and surrounding structures.11 Perlas and colleagues12 determined the sensitivity of needle paresthesias and nerve stimulation to be 38% and 75%, respectively, in cases in which plexus localization had been confirmed by ultrasound.
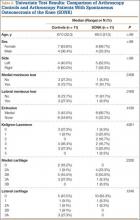
Several studies have reported on the efficacy of interscalene nerve block with either nerve stimulation or ultrasound guidance in the setting of shoulder surgery.2,3 Bishop and colleagues3 reviewed 547 patients who underwent interscalene regional anesthesia with nerve stimulation for both arthroscopic and open-shoulder procedures. They reported a 97% success rate and 12 (2.3%) minor complications, including sensory neuropathy and complex regional pain syndrome. There were no cases of pneumothorax, cardiac events, or other major complications.3 In a prospective study of 1319 patients, Singh and colleagues2 reported a 99.6% success rate using ultrasound-guided interscalene blocks for their shoulder surgeries. A total of 38 adverse events (2.88%) were identified: 14 transient neurologic events, including ear numbness, digital numbness, and brachial plexitis; 1 case of intraoperative bradycardia, and 2 cancellations after the block for chest pain and flank pain, which yielded negative cardiac workups. Other complications included postoperative emergency room visits and hospital admissions for reasons unrelated to the block.2 Interscalene regional anesthesia, therefore, provides effective anesthesia for shoulder surgery with low complication rates.
Pneumothorax after ultrasound-guided interscalene block has rarely been reported.13,14 In a review of 144 ultrasound-guided indwelling interscalene catheter placements, a 98% successful block rate with a single complication of small pneumothorax after total shoulder arthroplasty was reported.13 Mandim and colleagues14 reported a case of pneumothorax in a smoker who underwent an ultrasound-guided brachial plexus block prior to open reduction and internal fixation of an ulnar fracture. While the patient was asymptomatic and vital signs remained stable during the procedure, the patient complained postoperatively of chest pain with hypoxia, tachycardia, and hypotension. A chest radiograph confirmed an ipsilateral pneumothorax, and the patient was treated successfully with chest-tube placement. The authors attributed this complication to a higher pleural dome resulting from a hyperinflated lung caused by chronic smoking. Our patient reported no history of smoking and her preoperative chest radiograph had no evidence of lung disease.
In contrast, several cases of pneumothorax after shoulder surgery have been reported in the absence of nerve block. Oldman and Peng1 reported a 41-year-old nonsmoker who underwent arthroscopic labral repair and subacromial decompression. The preoperative nerve block was cancelled, and the patient received general endotracheal anesthesia alone. Fifty minutes after the case, the patient developed chest pain and hypoxia. A chest radiograph showed a small pneumothorax that was managed conservatively. The pneumothorax was attributed to spontaneous rupture of a preexisting lung bulla, suggesting that blocks are not always the cause of this complication. Furthermore, Dietzel and Ciullo15 reported 4 cases of spontaneous pneumothorax within 24 hours of uncomplicated arthroscopic shoulder procedures under general anesthesia in the lateral decubitus position. The patient ages ranged from 22 to 38 years, and medical histories were all significant for preexisting lung disease, remote history of pneumonia, and heavy smoking. Three of the patients experienced symptoms at home the day after surgery. The authors concluded that these cases were likely caused by rupture of blebs or bullae from underlying lung disease; these ruptured blebs or bullae are difficult to detect and usually located in the upper lung. The pressure gradient from the positive pressure of anesthesia and the ipsilateral upper lung is thought to be highest in the lateral decubitus position, increasing their chance of rupture.15
Finally, Lee and colleagues16 described 3 patients aged 40 to 45 years who underwent uncomplicated subacromial decompression in the beach-chair position under general anesthesia. Significant shoulder, neck, and axillary swelling were noted after surgery, and a chest radiograph showed tension pneumothorax, subcutaneous emphysema, and pneumomediastinum. The authors speculated that pressure in the subacromial space may become negative relative to atmospheric pressure when the shaver and suction are running, drawing in air through other portals. When the suction is discontinued, fluid infusion may push air into the surrounding tissue, leading to subcutaneous emphysema, which may spread to the mediastinum.16
Conclusion
Ultrasound-guided interscalene nerve blocks have successfully provided anesthesia for shoulder surgeries with low complication rates. Although the incidence of pneumothorax has decreased significantly with ultrasound guidance, the success of this procedure is highly operator-dependent. We present the case of an otherwise healthy patient without known pulmonary disease who developed a tension pneumothorax after the administration of ultrasound-guided regional and general anesthesia for arthroscopic shoulder surgery. Orthopedic surgeons and anesthesiologists must remain vigilant for pneumothorax during the perioperative period after shoulder surgery performed under interscalene regional aesthesia, particularly in the setting of hypotension, hypoxia, and/or tachycardia. Risk factors, such as history of smoking and preexisting lung disease, may predispose patients to the development of pneumothorax. Timely recognition and placement of a chest tube result in satisfactory clinical outcomes.
1. Oldman M, Peng Pi P. Pneumothorax after shoulder arthroscopy: don’t blame it on regional anesthesia. Reg Anesth Pain Med. 2004;29(4):382-383.
2. Singh A, Kelly C, O’Brien T, Wilson J, Warner JJ. Ultrasound-guided interscalene block anesthesia for shoulder arthroscopy: a prospective study of 1319 patients. J Bone Joint Surg Am. 2012;94(22):2040-2046.
3. Bishop JY, Sprague M, Gelber J, et al. Interscalene regional anesthesia for shoulder surgery. J Bone Joint Surg Am. 2005;87(5):974-979.
4. Winnie AP. Interscalene brachial plexus block. Anesth Analg. 1970;49(3):455-466.
5. Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat. 2014;27(2):210-221.
6. Brown AR, Weiss R, Greenberg C, Flatow EL, Bigliani LU. Interscalene block for shoulder arthroscopy: comparison with general anesthesia. Arthroscopy. 1993;9(3):295-300.
7. Marhofer P, Harrop-Griffiths W, Willschke H, Kirchmair L. Fifteen years of ultrasound guidance in regional anaesthesia: Part 2 - recent developments in block techniques. Br J Anaesth. 2010;104(6):673-683.
8. Sites BD, Spence BC, Gallagher J, et al. Regional anesthesia meets ultrasound: a specialty in transition. Acta Anaesthesiol Scand. 2008;52(4):456-466.
9. Ilfeld BM, Fredrickson MJ, Mariano ER. Ultrasound-guided perineural catheter insertion: three approaches but few illuminating data. Reg Anesth Pain Med. 2010;35(2):123-126.
10. Choi WI. Pneumothorax. Tuberc Respir Dis (Seoul). 2014;76(3):99-104.
11. Klaastad O, Sauter AR, Dodgson MS. Brachial plexus block with or without ultrasound guidance. Curr Opin Anaesthesiol. 2009;22(5):655-660.
12. Perlas A, Niazi A, McCartney C, Chan V, Xu D, Abbas S. The sensitivity of motor response to nerve stimulation and paresthesia for nerve localization as evaluated by ultrasound. Reg Anesth Pain Med. 2006;31(5):445-450.
13. Bryan NA, Swenson JD, Greis PE, Burks RT. Indwelling interscalene catheter use in an outpatient setting for shoulder surgery: technique, efficacy, and complications. J Shoulder Elbow Surg. 2007;16(4):388-395.
14. Mandim BL, Alves RR, Almeida R, Pontes JP, Arantes LJ, Morais FP. Pneumothorax post brachial plexus block guided by ultrasound: a case report. Rev Bras Anestesiol. 2012;62(5):741-747.
15. Dietzel DP, Ciullo JV. Spontaneous pneumothorax after shoulder arthroscopy: a report of four cases. Arthroscopy. 1996;12(1):99-102.
16. Lee HC, Dewan N, Crosby L. Subcutaneous emphysema, pneumomediastinum, and potentially life-threatening tension pneumothorax. Pulmonary complications from arthroscopic shoulder decompression. Chest. 1992;101(5):1265-1267.
Interscalene brachial plexus anesthesia is commonly used for arthroscopic and open procedures of the shoulder. This regional anesthetic targets the trunks of the brachial plexus and anesthetizes the area about the shoulder and proximal arm. Its use may obviate the need for concomitant general anesthesia, potentially reducing the use of postoperative intravenous and oral pain medication. Furthermore, patients often bypass the acute postoperative anesthesia care unit and proceed directly to the ambulatory unit, permitting earlier hospital discharge. Previous reports in the literature have demonstrated higher rates of neurologic, cardiac, and pulmonary complications from this procedure; in particular, the incidence of pneumothorax was reported as high as 3%.1 Techniques to localize the nerves, such as electrical nerve stimulation and, more recently, ultrasound guidance, have reduced these complication rates.2,3 Successful administration of the block has been shown to result in satisfactory postoperative pain relief.2 However, ultrasound-guided interscalene nerve blocks remain operator-dependent and complications may still occur.
We report a case of tension pneumothorax after arthroscopic rotator cuff repair and subacromial decompression with an ultrasound-guided interscalene block. Immediate recognition and treatment of this complication resulted in a good clinical outcome. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman presented with 3 months of right shoulder pain after a fall. Examination was pertinent for weakness in forward elevation and positive rotator cuff impingement signs. She remained symptomatic despite a course of nonsurgical management that included cortisone injections and physical therapy. Magnetic resonance imaging of the shoulder showed a full-thickness supraspinatus tear with minimal fatty atrophy. After a discussion of her treatment options, she elected to undergo an arthroscopic rotator cuff repair with subacromial decompression. An evaluation by her internist revealed no pertinent medical history apart from obesity (body mass index, 36). Specifically, there was no reported history of chronic obstructive pulmonary disease or asthma. She denied any prior cigarette smoking.
The patient was evaluated by the regional anesthesia team and was classified as a class 2 airway. An interscalene brachial plexus block was performed using a 2-inch, 22-gauge needle inserted into the interscalene groove. Using an out-of-plane technique under direct ultrasound guidance, 30 mL of 0.52% ropivacaine was injected. The block was considered successful, and no complications, such as resistance, paresthesias, pain, or blood on aspiration, were noted during injection. The patient had no complaints of chest pain or shortness of breath immediately afterward, and all vital signs were stable throughout the procedure.
The patient was brought to the operating room and placed in the beach-chair position. Induction for general anesthesia was started 15 minutes after the regional anesthetic, with 2 intubation attempts necessary because of poor airway visualization. After placement of the endotracheal tube, breath sounds were noted to be equal bilaterally. The arthroscopic procedure consisted of double-row rotator cuff repair, subacromial decompression, and débridement of the glenohumeral joint for synovitis, using standard arthroscopic portals. There were no difficulties with trocar placement, and bleeding was minimal throughout the case. The total surgical time was 150 minutes and a pump pressure of 30 mm Hg was maintained during the arthroscopy.
Within the first 60 minutes of the start of the arthroscopic procedure, the patient was noted to be intermittently hypotensive with mean arterial pressure (MAP) ranging from the 30s to 130s mm Hg and pulse in the 70 to 80 beats/min range. FiO2 in the 85% to 95% range was maintained throughout the procedure. During that time, 50 μg phenylephrine was administered on 4 separate occasions to maintain her blood pressure. The labile blood pressure was attributed by the anesthesiologist to the beach-chair position. During an attempted extubation upon conclusion of the surgery, the patient became hypotensive with MAP that ranged from the 40s to 60s mm Hg and tachycardic to 90 beats/min. The oxygen saturation was in the low 90s and tidal volume was poor. Absent lung sounds were noted on the right chest. An urgent portable chest radiograph showed a large right-sided tension pneumothorax with mediastinal shift (Figure 1). After an immediate general surgery consultation, a chest tube was placed in the operating room. The patient’s vital signs improved and a repeat chest radiograph revealed successful re-expansion of the lung (Figure 2). She was transferred to the acute postoperative anesthesia care unit and extubated in the intensive care unit later that day.
The patient’s chest tube was removed 2 days later and she was discharged home on hospital day 5 with a completely resolved pneumothorax. She was seen 1 week later in the office for a postoperative visit and reported feeling well without chest pain or shortness of breath.
Discussion
Interscalene brachial plexus anesthesia was first described by Winnie4 in 1970. This block targets the trunks of the brachial plexus, which are enclosed in a fascial sheath between the anterior and middle scalene muscles. In this region lie several structures at risk: the phrenic nerve superficially and inferiorly; the carotid sheath located superficially and medially; the subclavian artery parallel to the trunks; and the cupula of the lung that lies deep and inferior to the anterior scalene muscle. Recognized complications of the block include vocal hoarseness, Horner syndrome, and hemidiaphragmatic paresis caused by the temporary blockade of the ipsilateral recurrent laryngeal nerve, stellate ganglion, and phrenic nerve, in that order.5 Use of the interscalene block has been associated with minimal risk for pneumothorax, because the needle entry point is superior and directed away from the lung pleura.6 This is in contrast to the more inferiorly placed supraclavicular block, located in closer proximity to the lung cupula.5
Two different approaches are commonly used during ultrasound-guided nerve blocks. The in-plane approach generates a long-axis view of the needle by advancing the needle parallel with the long axis of the ultrasound probe. While this allows direct visualization of the needle tip, it requires deeper needle insertion from lateral to medial, causing puncture of the middle scalene muscle that may increase patient discomfort and risk nerve injury within the muscle.7 The out-of-plane approach used on our patient involves needle insertion parallel to the brachial plexus, but along the short axis of the ultrasound probe. Although this permits the operator to assess the periphery of the nerve, it may lead to poor needle-tip visualization during the procedure. As a result, operators often use a combination of tissue disturbance and “hydrolocation,” in which fluid is injected to indicate the needle-tip location.8,9
Tension pneumothorax represents the accumulation of air in the pleural space that leads to impaired pulmonary and cardiac function. It is often caused by disruption or puncture of the parietal or visceral pleura, creating a connection between the alveoli and pleural cavity. The gradual buildup of air in the pleural cavity results in increased intrapleural pressure, which compresses and ultimately collapses the ipsilateral lung. Venous compression restricts blood return to the heart and reduces cardiac output. Clinical manifestations include dyspnea, hypoxemia, tachycardia, and hypotension.10 Multiple techniques were developed to better localize the brachial plexus while reducing injury to nearby structures, including the lung. These include eliciting needle paresthesias, electrical nerve stimulation, and ultrasound guidance. While nerve stimulation was once the gold standard for brachial plexus localization, ultrasound guidance has gained in popularity because of its noninvasive nature and dynamic capability to identify nerves and surrounding structures.11 Perlas and colleagues12 determined the sensitivity of needle paresthesias and nerve stimulation to be 38% and 75%, respectively, in cases in which plexus localization had been confirmed by ultrasound.
Several studies have reported on the efficacy of interscalene nerve block with either nerve stimulation or ultrasound guidance in the setting of shoulder surgery.2,3 Bishop and colleagues3 reviewed 547 patients who underwent interscalene regional anesthesia with nerve stimulation for both arthroscopic and open-shoulder procedures. They reported a 97% success rate and 12 (2.3%) minor complications, including sensory neuropathy and complex regional pain syndrome. There were no cases of pneumothorax, cardiac events, or other major complications.3 In a prospective study of 1319 patients, Singh and colleagues2 reported a 99.6% success rate using ultrasound-guided interscalene blocks for their shoulder surgeries. A total of 38 adverse events (2.88%) were identified: 14 transient neurologic events, including ear numbness, digital numbness, and brachial plexitis; 1 case of intraoperative bradycardia, and 2 cancellations after the block for chest pain and flank pain, which yielded negative cardiac workups. Other complications included postoperative emergency room visits and hospital admissions for reasons unrelated to the block.2 Interscalene regional anesthesia, therefore, provides effective anesthesia for shoulder surgery with low complication rates.
Pneumothorax after ultrasound-guided interscalene block has rarely been reported.13,14 In a review of 144 ultrasound-guided indwelling interscalene catheter placements, a 98% successful block rate with a single complication of small pneumothorax after total shoulder arthroplasty was reported.13 Mandim and colleagues14 reported a case of pneumothorax in a smoker who underwent an ultrasound-guided brachial plexus block prior to open reduction and internal fixation of an ulnar fracture. While the patient was asymptomatic and vital signs remained stable during the procedure, the patient complained postoperatively of chest pain with hypoxia, tachycardia, and hypotension. A chest radiograph confirmed an ipsilateral pneumothorax, and the patient was treated successfully with chest-tube placement. The authors attributed this complication to a higher pleural dome resulting from a hyperinflated lung caused by chronic smoking. Our patient reported no history of smoking and her preoperative chest radiograph had no evidence of lung disease.
In contrast, several cases of pneumothorax after shoulder surgery have been reported in the absence of nerve block. Oldman and Peng1 reported a 41-year-old nonsmoker who underwent arthroscopic labral repair and subacromial decompression. The preoperative nerve block was cancelled, and the patient received general endotracheal anesthesia alone. Fifty minutes after the case, the patient developed chest pain and hypoxia. A chest radiograph showed a small pneumothorax that was managed conservatively. The pneumothorax was attributed to spontaneous rupture of a preexisting lung bulla, suggesting that blocks are not always the cause of this complication. Furthermore, Dietzel and Ciullo15 reported 4 cases of spontaneous pneumothorax within 24 hours of uncomplicated arthroscopic shoulder procedures under general anesthesia in the lateral decubitus position. The patient ages ranged from 22 to 38 years, and medical histories were all significant for preexisting lung disease, remote history of pneumonia, and heavy smoking. Three of the patients experienced symptoms at home the day after surgery. The authors concluded that these cases were likely caused by rupture of blebs or bullae from underlying lung disease; these ruptured blebs or bullae are difficult to detect and usually located in the upper lung. The pressure gradient from the positive pressure of anesthesia and the ipsilateral upper lung is thought to be highest in the lateral decubitus position, increasing their chance of rupture.15
Finally, Lee and colleagues16 described 3 patients aged 40 to 45 years who underwent uncomplicated subacromial decompression in the beach-chair position under general anesthesia. Significant shoulder, neck, and axillary swelling were noted after surgery, and a chest radiograph showed tension pneumothorax, subcutaneous emphysema, and pneumomediastinum. The authors speculated that pressure in the subacromial space may become negative relative to atmospheric pressure when the shaver and suction are running, drawing in air through other portals. When the suction is discontinued, fluid infusion may push air into the surrounding tissue, leading to subcutaneous emphysema, which may spread to the mediastinum.16
Conclusion
Ultrasound-guided interscalene nerve blocks have successfully provided anesthesia for shoulder surgeries with low complication rates. Although the incidence of pneumothorax has decreased significantly with ultrasound guidance, the success of this procedure is highly operator-dependent. We present the case of an otherwise healthy patient without known pulmonary disease who developed a tension pneumothorax after the administration of ultrasound-guided regional and general anesthesia for arthroscopic shoulder surgery. Orthopedic surgeons and anesthesiologists must remain vigilant for pneumothorax during the perioperative period after shoulder surgery performed under interscalene regional aesthesia, particularly in the setting of hypotension, hypoxia, and/or tachycardia. Risk factors, such as history of smoking and preexisting lung disease, may predispose patients to the development of pneumothorax. Timely recognition and placement of a chest tube result in satisfactory clinical outcomes.
Interscalene brachial plexus anesthesia is commonly used for arthroscopic and open procedures of the shoulder. This regional anesthetic targets the trunks of the brachial plexus and anesthetizes the area about the shoulder and proximal arm. Its use may obviate the need for concomitant general anesthesia, potentially reducing the use of postoperative intravenous and oral pain medication. Furthermore, patients often bypass the acute postoperative anesthesia care unit and proceed directly to the ambulatory unit, permitting earlier hospital discharge. Previous reports in the literature have demonstrated higher rates of neurologic, cardiac, and pulmonary complications from this procedure; in particular, the incidence of pneumothorax was reported as high as 3%.1 Techniques to localize the nerves, such as electrical nerve stimulation and, more recently, ultrasound guidance, have reduced these complication rates.2,3 Successful administration of the block has been shown to result in satisfactory postoperative pain relief.2 However, ultrasound-guided interscalene nerve blocks remain operator-dependent and complications may still occur.
We report a case of tension pneumothorax after arthroscopic rotator cuff repair and subacromial decompression with an ultrasound-guided interscalene block. Immediate recognition and treatment of this complication resulted in a good clinical outcome. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman presented with 3 months of right shoulder pain after a fall. Examination was pertinent for weakness in forward elevation and positive rotator cuff impingement signs. She remained symptomatic despite a course of nonsurgical management that included cortisone injections and physical therapy. Magnetic resonance imaging of the shoulder showed a full-thickness supraspinatus tear with minimal fatty atrophy. After a discussion of her treatment options, she elected to undergo an arthroscopic rotator cuff repair with subacromial decompression. An evaluation by her internist revealed no pertinent medical history apart from obesity (body mass index, 36). Specifically, there was no reported history of chronic obstructive pulmonary disease or asthma. She denied any prior cigarette smoking.
The patient was evaluated by the regional anesthesia team and was classified as a class 2 airway. An interscalene brachial plexus block was performed using a 2-inch, 22-gauge needle inserted into the interscalene groove. Using an out-of-plane technique under direct ultrasound guidance, 30 mL of 0.52% ropivacaine was injected. The block was considered successful, and no complications, such as resistance, paresthesias, pain, or blood on aspiration, were noted during injection. The patient had no complaints of chest pain or shortness of breath immediately afterward, and all vital signs were stable throughout the procedure.
The patient was brought to the operating room and placed in the beach-chair position. Induction for general anesthesia was started 15 minutes after the regional anesthetic, with 2 intubation attempts necessary because of poor airway visualization. After placement of the endotracheal tube, breath sounds were noted to be equal bilaterally. The arthroscopic procedure consisted of double-row rotator cuff repair, subacromial decompression, and débridement of the glenohumeral joint for synovitis, using standard arthroscopic portals. There were no difficulties with trocar placement, and bleeding was minimal throughout the case. The total surgical time was 150 minutes and a pump pressure of 30 mm Hg was maintained during the arthroscopy.
Within the first 60 minutes of the start of the arthroscopic procedure, the patient was noted to be intermittently hypotensive with mean arterial pressure (MAP) ranging from the 30s to 130s mm Hg and pulse in the 70 to 80 beats/min range. FiO2 in the 85% to 95% range was maintained throughout the procedure. During that time, 50 μg phenylephrine was administered on 4 separate occasions to maintain her blood pressure. The labile blood pressure was attributed by the anesthesiologist to the beach-chair position. During an attempted extubation upon conclusion of the surgery, the patient became hypotensive with MAP that ranged from the 40s to 60s mm Hg and tachycardic to 90 beats/min. The oxygen saturation was in the low 90s and tidal volume was poor. Absent lung sounds were noted on the right chest. An urgent portable chest radiograph showed a large right-sided tension pneumothorax with mediastinal shift (Figure 1). After an immediate general surgery consultation, a chest tube was placed in the operating room. The patient’s vital signs improved and a repeat chest radiograph revealed successful re-expansion of the lung (Figure 2). She was transferred to the acute postoperative anesthesia care unit and extubated in the intensive care unit later that day.
The patient’s chest tube was removed 2 days later and she was discharged home on hospital day 5 with a completely resolved pneumothorax. She was seen 1 week later in the office for a postoperative visit and reported feeling well without chest pain or shortness of breath.
Discussion
Interscalene brachial plexus anesthesia was first described by Winnie4 in 1970. This block targets the trunks of the brachial plexus, which are enclosed in a fascial sheath between the anterior and middle scalene muscles. In this region lie several structures at risk: the phrenic nerve superficially and inferiorly; the carotid sheath located superficially and medially; the subclavian artery parallel to the trunks; and the cupula of the lung that lies deep and inferior to the anterior scalene muscle. Recognized complications of the block include vocal hoarseness, Horner syndrome, and hemidiaphragmatic paresis caused by the temporary blockade of the ipsilateral recurrent laryngeal nerve, stellate ganglion, and phrenic nerve, in that order.5 Use of the interscalene block has been associated with minimal risk for pneumothorax, because the needle entry point is superior and directed away from the lung pleura.6 This is in contrast to the more inferiorly placed supraclavicular block, located in closer proximity to the lung cupula.5
Two different approaches are commonly used during ultrasound-guided nerve blocks. The in-plane approach generates a long-axis view of the needle by advancing the needle parallel with the long axis of the ultrasound probe. While this allows direct visualization of the needle tip, it requires deeper needle insertion from lateral to medial, causing puncture of the middle scalene muscle that may increase patient discomfort and risk nerve injury within the muscle.7 The out-of-plane approach used on our patient involves needle insertion parallel to the brachial plexus, but along the short axis of the ultrasound probe. Although this permits the operator to assess the periphery of the nerve, it may lead to poor needle-tip visualization during the procedure. As a result, operators often use a combination of tissue disturbance and “hydrolocation,” in which fluid is injected to indicate the needle-tip location.8,9
Tension pneumothorax represents the accumulation of air in the pleural space that leads to impaired pulmonary and cardiac function. It is often caused by disruption or puncture of the parietal or visceral pleura, creating a connection between the alveoli and pleural cavity. The gradual buildup of air in the pleural cavity results in increased intrapleural pressure, which compresses and ultimately collapses the ipsilateral lung. Venous compression restricts blood return to the heart and reduces cardiac output. Clinical manifestations include dyspnea, hypoxemia, tachycardia, and hypotension.10 Multiple techniques were developed to better localize the brachial plexus while reducing injury to nearby structures, including the lung. These include eliciting needle paresthesias, electrical nerve stimulation, and ultrasound guidance. While nerve stimulation was once the gold standard for brachial plexus localization, ultrasound guidance has gained in popularity because of its noninvasive nature and dynamic capability to identify nerves and surrounding structures.11 Perlas and colleagues12 determined the sensitivity of needle paresthesias and nerve stimulation to be 38% and 75%, respectively, in cases in which plexus localization had been confirmed by ultrasound.
Several studies have reported on the efficacy of interscalene nerve block with either nerve stimulation or ultrasound guidance in the setting of shoulder surgery.2,3 Bishop and colleagues3 reviewed 547 patients who underwent interscalene regional anesthesia with nerve stimulation for both arthroscopic and open-shoulder procedures. They reported a 97% success rate and 12 (2.3%) minor complications, including sensory neuropathy and complex regional pain syndrome. There were no cases of pneumothorax, cardiac events, or other major complications.3 In a prospective study of 1319 patients, Singh and colleagues2 reported a 99.6% success rate using ultrasound-guided interscalene blocks for their shoulder surgeries. A total of 38 adverse events (2.88%) were identified: 14 transient neurologic events, including ear numbness, digital numbness, and brachial plexitis; 1 case of intraoperative bradycardia, and 2 cancellations after the block for chest pain and flank pain, which yielded negative cardiac workups. Other complications included postoperative emergency room visits and hospital admissions for reasons unrelated to the block.2 Interscalene regional anesthesia, therefore, provides effective anesthesia for shoulder surgery with low complication rates.
Pneumothorax after ultrasound-guided interscalene block has rarely been reported.13,14 In a review of 144 ultrasound-guided indwelling interscalene catheter placements, a 98% successful block rate with a single complication of small pneumothorax after total shoulder arthroplasty was reported.13 Mandim and colleagues14 reported a case of pneumothorax in a smoker who underwent an ultrasound-guided brachial plexus block prior to open reduction and internal fixation of an ulnar fracture. While the patient was asymptomatic and vital signs remained stable during the procedure, the patient complained postoperatively of chest pain with hypoxia, tachycardia, and hypotension. A chest radiograph confirmed an ipsilateral pneumothorax, and the patient was treated successfully with chest-tube placement. The authors attributed this complication to a higher pleural dome resulting from a hyperinflated lung caused by chronic smoking. Our patient reported no history of smoking and her preoperative chest radiograph had no evidence of lung disease.
In contrast, several cases of pneumothorax after shoulder surgery have been reported in the absence of nerve block. Oldman and Peng1 reported a 41-year-old nonsmoker who underwent arthroscopic labral repair and subacromial decompression. The preoperative nerve block was cancelled, and the patient received general endotracheal anesthesia alone. Fifty minutes after the case, the patient developed chest pain and hypoxia. A chest radiograph showed a small pneumothorax that was managed conservatively. The pneumothorax was attributed to spontaneous rupture of a preexisting lung bulla, suggesting that blocks are not always the cause of this complication. Furthermore, Dietzel and Ciullo15 reported 4 cases of spontaneous pneumothorax within 24 hours of uncomplicated arthroscopic shoulder procedures under general anesthesia in the lateral decubitus position. The patient ages ranged from 22 to 38 years, and medical histories were all significant for preexisting lung disease, remote history of pneumonia, and heavy smoking. Three of the patients experienced symptoms at home the day after surgery. The authors concluded that these cases were likely caused by rupture of blebs or bullae from underlying lung disease; these ruptured blebs or bullae are difficult to detect and usually located in the upper lung. The pressure gradient from the positive pressure of anesthesia and the ipsilateral upper lung is thought to be highest in the lateral decubitus position, increasing their chance of rupture.15
Finally, Lee and colleagues16 described 3 patients aged 40 to 45 years who underwent uncomplicated subacromial decompression in the beach-chair position under general anesthesia. Significant shoulder, neck, and axillary swelling were noted after surgery, and a chest radiograph showed tension pneumothorax, subcutaneous emphysema, and pneumomediastinum. The authors speculated that pressure in the subacromial space may become negative relative to atmospheric pressure when the shaver and suction are running, drawing in air through other portals. When the suction is discontinued, fluid infusion may push air into the surrounding tissue, leading to subcutaneous emphysema, which may spread to the mediastinum.16
Conclusion
Ultrasound-guided interscalene nerve blocks have successfully provided anesthesia for shoulder surgeries with low complication rates. Although the incidence of pneumothorax has decreased significantly with ultrasound guidance, the success of this procedure is highly operator-dependent. We present the case of an otherwise healthy patient without known pulmonary disease who developed a tension pneumothorax after the administration of ultrasound-guided regional and general anesthesia for arthroscopic shoulder surgery. Orthopedic surgeons and anesthesiologists must remain vigilant for pneumothorax during the perioperative period after shoulder surgery performed under interscalene regional aesthesia, particularly in the setting of hypotension, hypoxia, and/or tachycardia. Risk factors, such as history of smoking and preexisting lung disease, may predispose patients to the development of pneumothorax. Timely recognition and placement of a chest tube result in satisfactory clinical outcomes.
1. Oldman M, Peng Pi P. Pneumothorax after shoulder arthroscopy: don’t blame it on regional anesthesia. Reg Anesth Pain Med. 2004;29(4):382-383.
2. Singh A, Kelly C, O’Brien T, Wilson J, Warner JJ. Ultrasound-guided interscalene block anesthesia for shoulder arthroscopy: a prospective study of 1319 patients. J Bone Joint Surg Am. 2012;94(22):2040-2046.
3. Bishop JY, Sprague M, Gelber J, et al. Interscalene regional anesthesia for shoulder surgery. J Bone Joint Surg Am. 2005;87(5):974-979.
4. Winnie AP. Interscalene brachial plexus block. Anesth Analg. 1970;49(3):455-466.
5. Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat. 2014;27(2):210-221.
6. Brown AR, Weiss R, Greenberg C, Flatow EL, Bigliani LU. Interscalene block for shoulder arthroscopy: comparison with general anesthesia. Arthroscopy. 1993;9(3):295-300.
7. Marhofer P, Harrop-Griffiths W, Willschke H, Kirchmair L. Fifteen years of ultrasound guidance in regional anaesthesia: Part 2 - recent developments in block techniques. Br J Anaesth. 2010;104(6):673-683.
8. Sites BD, Spence BC, Gallagher J, et al. Regional anesthesia meets ultrasound: a specialty in transition. Acta Anaesthesiol Scand. 2008;52(4):456-466.
9. Ilfeld BM, Fredrickson MJ, Mariano ER. Ultrasound-guided perineural catheter insertion: three approaches but few illuminating data. Reg Anesth Pain Med. 2010;35(2):123-126.
10. Choi WI. Pneumothorax. Tuberc Respir Dis (Seoul). 2014;76(3):99-104.
11. Klaastad O, Sauter AR, Dodgson MS. Brachial plexus block with or without ultrasound guidance. Curr Opin Anaesthesiol. 2009;22(5):655-660.
12. Perlas A, Niazi A, McCartney C, Chan V, Xu D, Abbas S. The sensitivity of motor response to nerve stimulation and paresthesia for nerve localization as evaluated by ultrasound. Reg Anesth Pain Med. 2006;31(5):445-450.
13. Bryan NA, Swenson JD, Greis PE, Burks RT. Indwelling interscalene catheter use in an outpatient setting for shoulder surgery: technique, efficacy, and complications. J Shoulder Elbow Surg. 2007;16(4):388-395.
14. Mandim BL, Alves RR, Almeida R, Pontes JP, Arantes LJ, Morais FP. Pneumothorax post brachial plexus block guided by ultrasound: a case report. Rev Bras Anestesiol. 2012;62(5):741-747.
15. Dietzel DP, Ciullo JV. Spontaneous pneumothorax after shoulder arthroscopy: a report of four cases. Arthroscopy. 1996;12(1):99-102.
16. Lee HC, Dewan N, Crosby L. Subcutaneous emphysema, pneumomediastinum, and potentially life-threatening tension pneumothorax. Pulmonary complications from arthroscopic shoulder decompression. Chest. 1992;101(5):1265-1267.
1. Oldman M, Peng Pi P. Pneumothorax after shoulder arthroscopy: don’t blame it on regional anesthesia. Reg Anesth Pain Med. 2004;29(4):382-383.
2. Singh A, Kelly C, O’Brien T, Wilson J, Warner JJ. Ultrasound-guided interscalene block anesthesia for shoulder arthroscopy: a prospective study of 1319 patients. J Bone Joint Surg Am. 2012;94(22):2040-2046.
3. Bishop JY, Sprague M, Gelber J, et al. Interscalene regional anesthesia for shoulder surgery. J Bone Joint Surg Am. 2005;87(5):974-979.
4. Winnie AP. Interscalene brachial plexus block. Anesth Analg. 1970;49(3):455-466.
5. Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat. 2014;27(2):210-221.
6. Brown AR, Weiss R, Greenberg C, Flatow EL, Bigliani LU. Interscalene block for shoulder arthroscopy: comparison with general anesthesia. Arthroscopy. 1993;9(3):295-300.
7. Marhofer P, Harrop-Griffiths W, Willschke H, Kirchmair L. Fifteen years of ultrasound guidance in regional anaesthesia: Part 2 - recent developments in block techniques. Br J Anaesth. 2010;104(6):673-683.
8. Sites BD, Spence BC, Gallagher J, et al. Regional anesthesia meets ultrasound: a specialty in transition. Acta Anaesthesiol Scand. 2008;52(4):456-466.
9. Ilfeld BM, Fredrickson MJ, Mariano ER. Ultrasound-guided perineural catheter insertion: three approaches but few illuminating data. Reg Anesth Pain Med. 2010;35(2):123-126.
10. Choi WI. Pneumothorax. Tuberc Respir Dis (Seoul). 2014;76(3):99-104.
11. Klaastad O, Sauter AR, Dodgson MS. Brachial plexus block with or without ultrasound guidance. Curr Opin Anaesthesiol. 2009;22(5):655-660.
12. Perlas A, Niazi A, McCartney C, Chan V, Xu D, Abbas S. The sensitivity of motor response to nerve stimulation and paresthesia for nerve localization as evaluated by ultrasound. Reg Anesth Pain Med. 2006;31(5):445-450.
13. Bryan NA, Swenson JD, Greis PE, Burks RT. Indwelling interscalene catheter use in an outpatient setting for shoulder surgery: technique, efficacy, and complications. J Shoulder Elbow Surg. 2007;16(4):388-395.
14. Mandim BL, Alves RR, Almeida R, Pontes JP, Arantes LJ, Morais FP. Pneumothorax post brachial plexus block guided by ultrasound: a case report. Rev Bras Anestesiol. 2012;62(5):741-747.
15. Dietzel DP, Ciullo JV. Spontaneous pneumothorax after shoulder arthroscopy: a report of four cases. Arthroscopy. 1996;12(1):99-102.
16. Lee HC, Dewan N, Crosby L. Subcutaneous emphysema, pneumomediastinum, and potentially life-threatening tension pneumothorax. Pulmonary complications from arthroscopic shoulder decompression. Chest. 1992;101(5):1265-1267.
Osteoid Osteoma of the Talar Neck With Subacute Presentation
Osteoid osteoma of the talar neck is an unusual clinical condition that is often overlooked on initial assessment of patients with ankle pain. Here, we present a case report of an adolescent male with talar neck osteoid osteoma who reported persistent pain after an injury. We discuss the differential diagnosis of persistent anterior ankle pain and assess the treatment options for osteoid osteoma of the talar neck. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
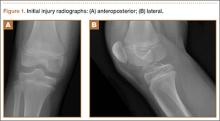
A 13-year-old boy presented to our clinic 3 months after a right ankle sprain. He had visited the emergency department at the time of injury; radiographs of the ankle were reported negative for fractures, dislocations, or bone pathologies. He was treated conservatively with elastic support, icing, rest, elevation, and weight-bearing as tolerated. Upon presentation to our office, his pain involved the entire ankle joint. He had not put weight on it since the injury. On examination, he had a significant limp, anteromedial swelling, and tenderness over the ankle joint anteromedially. His neurologic and vascular examinations were normal.
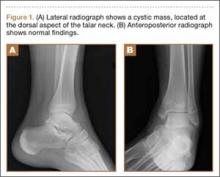
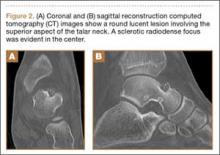
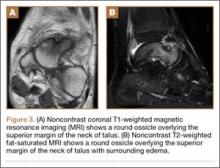
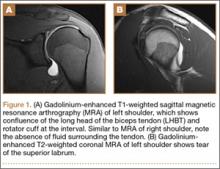
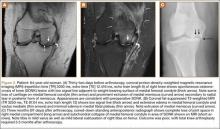
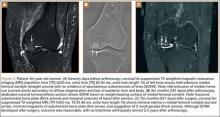
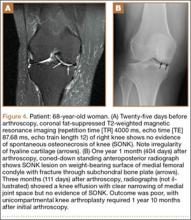
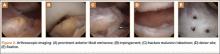
His plain radiographs showed a cystic mass, located at the dorsal aspect of the talar neck (Figures 1A, 1B). Computed tomography (CT) showed a round lucent lesion involving the superior aspect of the talar neck, measuring 9 mm by 6 mm. A sclerotic radiodense focus was evident in the center (Figures 2A, 2B). Noncontrast multiplanar, multisequence magnetic resonance imaging (MRI) showed abnormal edema throughout the talus and a 9-mm rounded ossicle overlying the superior margin of the neck of the talus (Figures 3A, 3B).
Differential Diagnosis
The differential diagnosis for anterior ankle pain includes ankle sprain, monoarticular arthritis, anterior ankle impingement, and talar neck fractures. Other related findings include the presence of a talar ridge and a talar beak.
Ankle sprains are very common injuries. The mainstay treatment consists of ice, resting, elevation, and elastic or semirigid support, and patients usually recover over the course of a few weeks. These sprains are typically injuries of the lateral or medial ligaments of the ankle. Extension of a ligament tear across the anterior capsule can explain persistent anterior ankle pain. The presence of a bony lesion on plain radiographs, however, makes the diagnosis of an ankle sprain, with or without extension into the anterior capsule, less likely.
Monoarticular arthritis, which may present in the ankle and has a wide differential diagnosis, usually involves the whole joint.
Anterior ankle impingement typically occurs in athletes who participate in sports that involve kicking. It can be a bony or soft-tissue impingent. Clinically, patients present with pain and loss of motion, specifically dorsiflexion.
Talar neck fractures are usually the result of high-energy trauma. Stress fractures of the neck of the talus are uncommon and are associated with a recent sudden increase in physical activity, such as running, dancing, or military training. Radiographs, CT scans, and MRI help define the fracture line.
The talar ridge is the site of capsular and ligamentous attachment on the superior aspect of the talar neck and may become hypertrophic in athletes. A hypertrophic talar ridge is asymptomatic and is not considered a pathologic finding on radiographs.
The talar beak, a flaring of the anterosuperior aspect of the talar head, is an indirect sign of tarsal coalition. When symptomatic, patients complain of subtalar symptoms, typically pain and limitation of motion. It usually does not present acutely.
Treatment
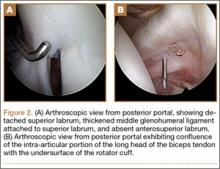
We offered the patient surgical excision, and his guardian consented to left ankle arthroscopy. We performed synovectomy using a combination of 3.5-mm shaver and radiofrequency probe. We identified the mass: round, soft, and located at the superior-medial aspect of the talar neck. We removed it in piecemeal fashion using manual arthroscopic instruments, and cauterized its base using the radiofrequency probe. We allowed the patient weight-bearing as tolerated starting the day after surgery.
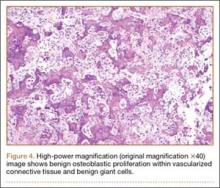
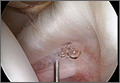
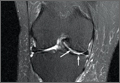
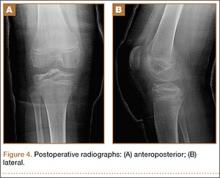
We submitted the specimen for pathologic evaluation (Figure 4). It consisted of multiple pieces of tan/brown tissue. Histologic examination showed benign osteoblastic proliferation composed of anastomosing bony trabeculae with variable mineralization, lined by plump osteoblasts, within vascularized connective tissue; benign giant cells were present, consistent with a nidus of an osteoid osteoma.
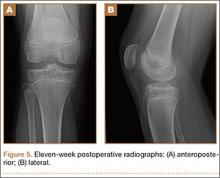
On the first postoperative visit, the patient was pain-free and bearing weight with crutches. He was gradually weaned from his crutches and returned to full weight-bearing over the next 4 weeks. At 12-month follow-up, he was symptom-free with good range of motion and full return to previous level of activity.
Discussion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in a few case series2-7 and is associated with a typical nidus that can be identified on CT scans. It does not present acutely, however. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. However, this presentation is not universal and is frequently missed.2
Juxta-articular osteoid osteomas in the ankle and foot can be difficult to diagnose. The most common site is the talus.3 The majority of patients link their pain to a remote ankle injury. The time delay to diagnosis is on average 2.5 years, but it can be as long as 10 years.4-6 A CT scan is the best method to identify the nidus; MRI can be misleading if it shows only marrow edema but not a nidus.4,5,7 In our patient, an injury was documented, and the patient denied prior symptoms. We cannot explain how an injury would trigger the formation of an osteoid osteoma or cause a previously asymptomatic osteoid osteoma to become symptomatic.
Medical treatment with nonsteroidal anti-inflammatory drugs has been used but is reported to take 2 to 4 years for resolution of symptoms; many patients may consider the treatment time frame too long when other alternatives are available.8 These include open resection, arthroscopic resection, and image-guided ablation. Open surgical techniques include en bloc resection and curettage. Bone grafting or internal fixation may be performed as needed. Arthroscopic excision of juxta-articular osteoid osteomas offers the advantages of good visualization and avoidance of soft-tissue dissection, and allows for complete excision of the lesion as well as synovectomy.6,9,10 Arthroscopic excision also allows for quicker rehabilitation. Image-guided ablation, such as radionuclide-guided excision, CT-guided thermal ablation, and laser photocoagulation, may be even less invasive but do not allow for direct visualization, complete resection, and biopsy.11
Conclusion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in multiple case series and is associated with a typical nidus that can be identified on CT scans. Usually, it does not present acutely. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. This presentation is not universal, however, and is frequently missed, especially when the pain is associated with a prior injury.2 Arthroscopic exploration of the ankle with resection of subperiosteal osteoid osteoma and the associated synovitis using thermal ablation of the base with radiofrequency offers lasting cure with minimal morbidity.
1. Edeiken J, DePalma AF, Hodes PJ. Osteoid osteoma. Clin Orthop Relat Res. 1966;49:201-206.
2. El Rayes MA, El Kordy S. Osteoid osteoma of the talus. Foot. 2003;13(3):166–168.
3. Capanna R, Van Horn JR, Ayala A, Picci P, Bettelli G. Osteoid osteoma and osteoblastoma of the talus. A report of 40 cases. Skeletal Radiol. 1986;15(5):360-364.
4. Chuang SY, Wang SJ, Au MK, Huang GS. Osteoid osteoma in talar neck: a report of two cases. Foot Ankle Int. 1998;19(1):44-47.
5. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
6. Yercan HS, Okcu G, Őzalp T, Ősiç U. Arthroscopic removal of the osteoid osteoma on the neck of the talus. Knee Surg Sports Traumatol Arthrosc. 2004;12(3):246-249.
7. Mazlout O, Saudan M, Ladeb MF, Garcia JF, Bianchi S. Osteoid osteoma of the talar neck: a diagnostic challenge. Eur J Radiol Extra. 2004;49(2):67-70.
8. Kneisl JS, Simon MA. Medical management compared with operative treatment for osteoid-osteoma. J Bone Joint Surg Am. 1992;74(2):179-185.
9. Bojanić I, Orlić D, Ivković A. Arthroscopic removal of a juxtaarticular osteoid osteoma of the talar neck. J Foot Ankle Surg. 2003;42(6):359-362.
10. Tüzüner S, Aydin AT. Arthroscopic removal of an osteoid osteoma at talar neck. Arthroscopy. 1998;14(4):405-409.
11. Amendola A, Vellet D, Willits K. Osteoid osteoma of the neck of the talus: percutaneous, computed tomography-guided technique for complete excision. Foot Ankle Int. 1994;15(8):429-432.
Osteoid osteoma of the talar neck is an unusual clinical condition that is often overlooked on initial assessment of patients with ankle pain. Here, we present a case report of an adolescent male with talar neck osteoid osteoma who reported persistent pain after an injury. We discuss the differential diagnosis of persistent anterior ankle pain and assess the treatment options for osteoid osteoma of the talar neck. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 13-year-old boy presented to our clinic 3 months after a right ankle sprain. He had visited the emergency department at the time of injury; radiographs of the ankle were reported negative for fractures, dislocations, or bone pathologies. He was treated conservatively with elastic support, icing, rest, elevation, and weight-bearing as tolerated. Upon presentation to our office, his pain involved the entire ankle joint. He had not put weight on it since the injury. On examination, he had a significant limp, anteromedial swelling, and tenderness over the ankle joint anteromedially. His neurologic and vascular examinations were normal.
His plain radiographs showed a cystic mass, located at the dorsal aspect of the talar neck (Figures 1A, 1B). Computed tomography (CT) showed a round lucent lesion involving the superior aspect of the talar neck, measuring 9 mm by 6 mm. A sclerotic radiodense focus was evident in the center (Figures 2A, 2B). Noncontrast multiplanar, multisequence magnetic resonance imaging (MRI) showed abnormal edema throughout the talus and a 9-mm rounded ossicle overlying the superior margin of the neck of the talus (Figures 3A, 3B).
Differential Diagnosis
The differential diagnosis for anterior ankle pain includes ankle sprain, monoarticular arthritis, anterior ankle impingement, and talar neck fractures. Other related findings include the presence of a talar ridge and a talar beak.
Ankle sprains are very common injuries. The mainstay treatment consists of ice, resting, elevation, and elastic or semirigid support, and patients usually recover over the course of a few weeks. These sprains are typically injuries of the lateral or medial ligaments of the ankle. Extension of a ligament tear across the anterior capsule can explain persistent anterior ankle pain. The presence of a bony lesion on plain radiographs, however, makes the diagnosis of an ankle sprain, with or without extension into the anterior capsule, less likely.
Monoarticular arthritis, which may present in the ankle and has a wide differential diagnosis, usually involves the whole joint.
Anterior ankle impingement typically occurs in athletes who participate in sports that involve kicking. It can be a bony or soft-tissue impingent. Clinically, patients present with pain and loss of motion, specifically dorsiflexion.
Talar neck fractures are usually the result of high-energy trauma. Stress fractures of the neck of the talus are uncommon and are associated with a recent sudden increase in physical activity, such as running, dancing, or military training. Radiographs, CT scans, and MRI help define the fracture line.
The talar ridge is the site of capsular and ligamentous attachment on the superior aspect of the talar neck and may become hypertrophic in athletes. A hypertrophic talar ridge is asymptomatic and is not considered a pathologic finding on radiographs.
The talar beak, a flaring of the anterosuperior aspect of the talar head, is an indirect sign of tarsal coalition. When symptomatic, patients complain of subtalar symptoms, typically pain and limitation of motion. It usually does not present acutely.
Treatment
We offered the patient surgical excision, and his guardian consented to left ankle arthroscopy. We performed synovectomy using a combination of 3.5-mm shaver and radiofrequency probe. We identified the mass: round, soft, and located at the superior-medial aspect of the talar neck. We removed it in piecemeal fashion using manual arthroscopic instruments, and cauterized its base using the radiofrequency probe. We allowed the patient weight-bearing as tolerated starting the day after surgery.
We submitted the specimen for pathologic evaluation (Figure 4). It consisted of multiple pieces of tan/brown tissue. Histologic examination showed benign osteoblastic proliferation composed of anastomosing bony trabeculae with variable mineralization, lined by plump osteoblasts, within vascularized connective tissue; benign giant cells were present, consistent with a nidus of an osteoid osteoma.
On the first postoperative visit, the patient was pain-free and bearing weight with crutches. He was gradually weaned from his crutches and returned to full weight-bearing over the next 4 weeks. At 12-month follow-up, he was symptom-free with good range of motion and full return to previous level of activity.
Discussion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in a few case series2-7 and is associated with a typical nidus that can be identified on CT scans. It does not present acutely, however. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. However, this presentation is not universal and is frequently missed.2
Juxta-articular osteoid osteomas in the ankle and foot can be difficult to diagnose. The most common site is the talus.3 The majority of patients link their pain to a remote ankle injury. The time delay to diagnosis is on average 2.5 years, but it can be as long as 10 years.4-6 A CT scan is the best method to identify the nidus; MRI can be misleading if it shows only marrow edema but not a nidus.4,5,7 In our patient, an injury was documented, and the patient denied prior symptoms. We cannot explain how an injury would trigger the formation of an osteoid osteoma or cause a previously asymptomatic osteoid osteoma to become symptomatic.
Medical treatment with nonsteroidal anti-inflammatory drugs has been used but is reported to take 2 to 4 years for resolution of symptoms; many patients may consider the treatment time frame too long when other alternatives are available.8 These include open resection, arthroscopic resection, and image-guided ablation. Open surgical techniques include en bloc resection and curettage. Bone grafting or internal fixation may be performed as needed. Arthroscopic excision of juxta-articular osteoid osteomas offers the advantages of good visualization and avoidance of soft-tissue dissection, and allows for complete excision of the lesion as well as synovectomy.6,9,10 Arthroscopic excision also allows for quicker rehabilitation. Image-guided ablation, such as radionuclide-guided excision, CT-guided thermal ablation, and laser photocoagulation, may be even less invasive but do not allow for direct visualization, complete resection, and biopsy.11
Conclusion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in multiple case series and is associated with a typical nidus that can be identified on CT scans. Usually, it does not present acutely. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. This presentation is not universal, however, and is frequently missed, especially when the pain is associated with a prior injury.2 Arthroscopic exploration of the ankle with resection of subperiosteal osteoid osteoma and the associated synovitis using thermal ablation of the base with radiofrequency offers lasting cure with minimal morbidity.
Osteoid osteoma of the talar neck is an unusual clinical condition that is often overlooked on initial assessment of patients with ankle pain. Here, we present a case report of an adolescent male with talar neck osteoid osteoma who reported persistent pain after an injury. We discuss the differential diagnosis of persistent anterior ankle pain and assess the treatment options for osteoid osteoma of the talar neck. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 13-year-old boy presented to our clinic 3 months after a right ankle sprain. He had visited the emergency department at the time of injury; radiographs of the ankle were reported negative for fractures, dislocations, or bone pathologies. He was treated conservatively with elastic support, icing, rest, elevation, and weight-bearing as tolerated. Upon presentation to our office, his pain involved the entire ankle joint. He had not put weight on it since the injury. On examination, he had a significant limp, anteromedial swelling, and tenderness over the ankle joint anteromedially. His neurologic and vascular examinations were normal.
His plain radiographs showed a cystic mass, located at the dorsal aspect of the talar neck (Figures 1A, 1B). Computed tomography (CT) showed a round lucent lesion involving the superior aspect of the talar neck, measuring 9 mm by 6 mm. A sclerotic radiodense focus was evident in the center (Figures 2A, 2B). Noncontrast multiplanar, multisequence magnetic resonance imaging (MRI) showed abnormal edema throughout the talus and a 9-mm rounded ossicle overlying the superior margin of the neck of the talus (Figures 3A, 3B).
Differential Diagnosis
The differential diagnosis for anterior ankle pain includes ankle sprain, monoarticular arthritis, anterior ankle impingement, and talar neck fractures. Other related findings include the presence of a talar ridge and a talar beak.
Ankle sprains are very common injuries. The mainstay treatment consists of ice, resting, elevation, and elastic or semirigid support, and patients usually recover over the course of a few weeks. These sprains are typically injuries of the lateral or medial ligaments of the ankle. Extension of a ligament tear across the anterior capsule can explain persistent anterior ankle pain. The presence of a bony lesion on plain radiographs, however, makes the diagnosis of an ankle sprain, with or without extension into the anterior capsule, less likely.
Monoarticular arthritis, which may present in the ankle and has a wide differential diagnosis, usually involves the whole joint.
Anterior ankle impingement typically occurs in athletes who participate in sports that involve kicking. It can be a bony or soft-tissue impingent. Clinically, patients present with pain and loss of motion, specifically dorsiflexion.
Talar neck fractures are usually the result of high-energy trauma. Stress fractures of the neck of the talus are uncommon and are associated with a recent sudden increase in physical activity, such as running, dancing, or military training. Radiographs, CT scans, and MRI help define the fracture line.
The talar ridge is the site of capsular and ligamentous attachment on the superior aspect of the talar neck and may become hypertrophic in athletes. A hypertrophic talar ridge is asymptomatic and is not considered a pathologic finding on radiographs.
The talar beak, a flaring of the anterosuperior aspect of the talar head, is an indirect sign of tarsal coalition. When symptomatic, patients complain of subtalar symptoms, typically pain and limitation of motion. It usually does not present acutely.
Treatment
We offered the patient surgical excision, and his guardian consented to left ankle arthroscopy. We performed synovectomy using a combination of 3.5-mm shaver and radiofrequency probe. We identified the mass: round, soft, and located at the superior-medial aspect of the talar neck. We removed it in piecemeal fashion using manual arthroscopic instruments, and cauterized its base using the radiofrequency probe. We allowed the patient weight-bearing as tolerated starting the day after surgery.
We submitted the specimen for pathologic evaluation (Figure 4). It consisted of multiple pieces of tan/brown tissue. Histologic examination showed benign osteoblastic proliferation composed of anastomosing bony trabeculae with variable mineralization, lined by plump osteoblasts, within vascularized connective tissue; benign giant cells were present, consistent with a nidus of an osteoid osteoma.
On the first postoperative visit, the patient was pain-free and bearing weight with crutches. He was gradually weaned from his crutches and returned to full weight-bearing over the next 4 weeks. At 12-month follow-up, he was symptom-free with good range of motion and full return to previous level of activity.
Discussion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in a few case series2-7 and is associated with a typical nidus that can be identified on CT scans. It does not present acutely, however. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. However, this presentation is not universal and is frequently missed.2
Juxta-articular osteoid osteomas in the ankle and foot can be difficult to diagnose. The most common site is the talus.3 The majority of patients link their pain to a remote ankle injury. The time delay to diagnosis is on average 2.5 years, but it can be as long as 10 years.4-6 A CT scan is the best method to identify the nidus; MRI can be misleading if it shows only marrow edema but not a nidus.4,5,7 In our patient, an injury was documented, and the patient denied prior symptoms. We cannot explain how an injury would trigger the formation of an osteoid osteoma or cause a previously asymptomatic osteoid osteoma to become symptomatic.
Medical treatment with nonsteroidal anti-inflammatory drugs has been used but is reported to take 2 to 4 years for resolution of symptoms; many patients may consider the treatment time frame too long when other alternatives are available.8 These include open resection, arthroscopic resection, and image-guided ablation. Open surgical techniques include en bloc resection and curettage. Bone grafting or internal fixation may be performed as needed. Arthroscopic excision of juxta-articular osteoid osteomas offers the advantages of good visualization and avoidance of soft-tissue dissection, and allows for complete excision of the lesion as well as synovectomy.6,9,10 Arthroscopic excision also allows for quicker rehabilitation. Image-guided ablation, such as radionuclide-guided excision, CT-guided thermal ablation, and laser photocoagulation, may be even less invasive but do not allow for direct visualization, complete resection, and biopsy.11
Conclusion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in multiple case series and is associated with a typical nidus that can be identified on CT scans. Usually, it does not present acutely. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. This presentation is not universal, however, and is frequently missed, especially when the pain is associated with a prior injury.2 Arthroscopic exploration of the ankle with resection of subperiosteal osteoid osteoma and the associated synovitis using thermal ablation of the base with radiofrequency offers lasting cure with minimal morbidity.
1. Edeiken J, DePalma AF, Hodes PJ. Osteoid osteoma. Clin Orthop Relat Res. 1966;49:201-206.
2. El Rayes MA, El Kordy S. Osteoid osteoma of the talus. Foot. 2003;13(3):166–168.
3. Capanna R, Van Horn JR, Ayala A, Picci P, Bettelli G. Osteoid osteoma and osteoblastoma of the talus. A report of 40 cases. Skeletal Radiol. 1986;15(5):360-364.
4. Chuang SY, Wang SJ, Au MK, Huang GS. Osteoid osteoma in talar neck: a report of two cases. Foot Ankle Int. 1998;19(1):44-47.
5. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
6. Yercan HS, Okcu G, Őzalp T, Ősiç U. Arthroscopic removal of the osteoid osteoma on the neck of the talus. Knee Surg Sports Traumatol Arthrosc. 2004;12(3):246-249.
7. Mazlout O, Saudan M, Ladeb MF, Garcia JF, Bianchi S. Osteoid osteoma of the talar neck: a diagnostic challenge. Eur J Radiol Extra. 2004;49(2):67-70.
8. Kneisl JS, Simon MA. Medical management compared with operative treatment for osteoid-osteoma. J Bone Joint Surg Am. 1992;74(2):179-185.
9. Bojanić I, Orlić D, Ivković A. Arthroscopic removal of a juxtaarticular osteoid osteoma of the talar neck. J Foot Ankle Surg. 2003;42(6):359-362.
10. Tüzüner S, Aydin AT. Arthroscopic removal of an osteoid osteoma at talar neck. Arthroscopy. 1998;14(4):405-409.
11. Amendola A, Vellet D, Willits K. Osteoid osteoma of the neck of the talus: percutaneous, computed tomography-guided technique for complete excision. Foot Ankle Int. 1994;15(8):429-432.
1. Edeiken J, DePalma AF, Hodes PJ. Osteoid osteoma. Clin Orthop Relat Res. 1966;49:201-206.
2. El Rayes MA, El Kordy S. Osteoid osteoma of the talus. Foot. 2003;13(3):166–168.
3. Capanna R, Van Horn JR, Ayala A, Picci P, Bettelli G. Osteoid osteoma and osteoblastoma of the talus. A report of 40 cases. Skeletal Radiol. 1986;15(5):360-364.
4. Chuang SY, Wang SJ, Au MK, Huang GS. Osteoid osteoma in talar neck: a report of two cases. Foot Ankle Int. 1998;19(1):44-47.
5. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
6. Yercan HS, Okcu G, Őzalp T, Ősiç U. Arthroscopic removal of the osteoid osteoma on the neck of the talus. Knee Surg Sports Traumatol Arthrosc. 2004;12(3):246-249.
7. Mazlout O, Saudan M, Ladeb MF, Garcia JF, Bianchi S. Osteoid osteoma of the talar neck: a diagnostic challenge. Eur J Radiol Extra. 2004;49(2):67-70.
8. Kneisl JS, Simon MA. Medical management compared with operative treatment for osteoid-osteoma. J Bone Joint Surg Am. 1992;74(2):179-185.
9. Bojanić I, Orlić D, Ivković A. Arthroscopic removal of a juxtaarticular osteoid osteoma of the talar neck. J Foot Ankle Surg. 2003;42(6):359-362.
10. Tüzüner S, Aydin AT. Arthroscopic removal of an osteoid osteoma at talar neck. Arthroscopy. 1998;14(4):405-409.
11. Amendola A, Vellet D, Willits K. Osteoid osteoma of the neck of the talus: percutaneous, computed tomography-guided technique for complete excision. Foot Ankle Int. 1994;15(8):429-432.
Factors Affecting Perceptions of Open, Mini-Open, and Arthroscopic Rotator Cuff Repair Techniques Among Medical Professionals
Rotator cuff tears are a common condition affecting the shoulder joint. Initial open repair techniques were associated with several complications, including severe early postoperative pain, deltoid detachment and/or weakness, risk for infection, and arthrofibrosis.1-3 In addition, open procedures cannot address other possible diagnoses, such as labral tears and loose bodies. These disadvantages promoted the development of an arthroscopically assisted mini-open technique.4 Superior long-term results, with more than 90% of patients achieving good to excellent results,5-13 established the mini-open rotator cuff repair (RCR) as the gold standard.3,6,10,12,14-16
Recently, as instrumentation for arthroscopy has improved, enthusiasm for all-arthroscopic techniques (hereafter referred to as arthroscopic repair) has grown. The appeal of arthroscopic repair includes potentially less initial pain, ability to treat intra-articular lesions concurrently, smaller skin incisions with better cosmesis, less soft-tissue dissection, and low risk for deltoid detachment.3,17 The potential advantages of arthroscopic repair can lead to perceptions of quicker healing and shorter recovery, which are not supported by the literature. However, arthroscopic repair is technically more challenging, time-consuming, and expensive than open or mini-open repairs,18,19 and though some investigators have reported a trend toward fewer complications,3 the long-term outcome of arthroscopic RCRs has not been shown to be better than that of other techniques.
Given that no differences have been shown between the emerging arthroscopic repair technique and mini-open repair with respect to range of motion or clinical scores in the short term,3 it is unclear what perceptions influence choice of technique for one’s own personal RCR.
We conducted a study to determine which RCR technique medical professionals (orthopedic attendings and residents, anesthesiologists, internal medicine attendings, main operating room nurses, and physical therapists) preferred for their own surgery and to analyze perceptions shaping those opinions. Orthopedic surgeons have the best concept of rotator cuff surgery, but anesthesiologists and nurses have a “front row seat” and opinions on types of rotator cuff surgery. Physical therapists, who treat patients with rotator cuff tears, also have a working knowledge of rotator cuff surgery. Finally, internists represent a rotator cuff injury referral service and may have patients who have undergone rotator cuff surgery. We hypothesized that most medical professionals, irrespective of specialty or career length, would prefer arthroscopic RCR because of its perceived superior outcome and fast recovery.
Materials and Methods
This cross-sectional, descriptive, survey-based study was approved by our institutional review board (IRB) and offered via 3 emails between April 2011 and June 2011 to attendings (orthopedists, internists, anesthesiologists), residents, and allied health professionals (AHPs; operating room nurses, physical therapists) involved in orthopedic care at our institution. Each email contained a hyperlink to the online survey (Appendix), which took about 10 minutes to complete and explored respondent demographics, exposure to the different techniques, and opinions regarding different aspects of RCR surgery and recovery.
There were 84 respondents. The sexes were equally represented, and age ranged from 25 to 78 years (Table 1). Of the respondents, 41 (49%) were attendings, 20 (24%) were residents, and 23 (27%) were AHPs. Of the attendings, 13 (32%) were orthopedic surgeons, 26 (63%) were primary care physicians, and 2 (5%) did not specify their specialty. Four orthopedic surgeons had fellowship training in sports medicine or shoulder and elbow surgery. The attendings were overall more experienced in their profession than the other groups were, with 68% reporting more than 5 years of experience.
Descriptive statistics, including means and standard errors, were calculated. Fisher exact test was used to compare preferences of RCR type according to type of training and years of experience. Significance was set at P ≤ .05.
Results
Overall Responses (Table 2)
Of the 84 respondents, almost half (46%) preferred deferring their choice of RCR to their surgeon. Most of the other respondents preferred the arthroscopic technique (26%) or the mini-open repair (23%). There was no association between technique preference and medical professional type. Most respondents (63%) had never assisted in or performed rotator cuff surgery.
Seventy-four percent of all respondents indicated they thought arthroscopic, mini-open, and open RCRs are safe, and about half thought these procedures are fast. About half expressed no opinion about the cost-effectiveness of arthroscopic, mini-open, or open RCRs (54%, 52%, and 48%, respectively), and slightly more than half expressed no opinion about whether arthroscopic, mini-open, or open RCR provide the best outcome (58%, 60%, and 62%, respectively). Significantly (P < .05) more respondents thought arthroscopic and mini-open repairs, rather than open repairs, promote quick healing (64% and 45%, respectively, vs 15%), good cosmetic results (81% and 51%, respectively, vs 10%), and patient satisfaction (50% and 48%, respectively, vs 30%). However, a significant (P < .05) number also thought arthroscopic and mini-open repairs are harder to learn/more challenging to perform than open repairs (52% and 38%, respectively, vs 17%).
Of all factors considered, safety of arthroscopic repair garnered the highest consensus: 82%. Respondents were least opinionated about the outcome of the open repair technique, with more than 62% expressing no opinion about the outcome. The responses to the questions on the learning curves for the 3 techniques varied the most.
Responses by Group (Table 2)
Attendings. Of the 41 attendings, 24 (59%) responded they would defer to their surgeon’s technique preference for RCR. Of the other 17 who expressed a preference, most indicated arthroscopic or mini-open repair (17% each). There was a difference (P < .05) between years of experience and RCR preference: of the 13 attendings with less than 5 years of experience, arthroscopic repair was preferred by 31%; in contrast, of the 28 attendings with more than 5 years of experience, only 11% preferred arthroscopic repair.
Of the 11 attendings who performed rotator cuff surgery, 55% used the open technique, but most (8) preferred to have their own rotator cuff fixed arthroscopically or according to their surgeon’s preference. Only 1 surgeon preferred open repair for his own rotator cuff. Of the 4 surgeons who performed arthroscopic RCRs, 3 had less than 5 years of experience. Conversely, all 7 surgeons who performed mini-open or open repairs had more than 5 years of experience.
Of the 30 attendings who did not perform rotator cuff surgery, most (20) responded they would defer to their surgeon’s technique preference for RCR.
The attendings’ opinions on factors affecting rotator cuff surgery were similar to those of the other respondents with respect to safety, cost-effectiveness, recovery, cosmesis, patient satisfaction, outcome, and technical difficulty. Unlike the others, however, attendings considered all 3 repair techniques fast.
Residents. Of the 20 residents, 7 preferred arthroscopic, 5 preferred mini-open, and 1 preferred open repair; the other 7 responded they would defer to their surgeon’s preference. Residents’ opinions on each factor were more polarized and consistent across categories than those of the other groups. Residents overwhelmingly thought all 3 techniques (arthroscopic, mini-open, open) are safe (19, 19, and 18, respectively) and cost-effective (12, 14, and 14, respectively). Although most residents considered the open and mini-open repair techniques fast (19 and 15, respectively), only 8 considered arthroscopic RCR fast, and 4 considered it slow. Residents’ opinions about the technique that produces the best outcome were mixed. As with the other respondents, residents thought arthroscopic RCRs heal fast and produce great cosmetic results, but are challenging to perform and have a steep learning curve. Unlike the other respondents, most residents (12) considered open RCR easy to learn (P = .006), with a learning curve of fewer than 20 procedures.
AHPs. No AHP expressed a preference for open RCR. This group was evenly divided among 3 choices: deferring to their surgeon’s preference, arthroscopic repair, and mini-open repair. The 23 AHPs thought arthroscopic, mini-open, and open repairs are safe (17, 15, and 12, respectively), but most indicated they were “equivocal” about which techniques are cost-effective, challenging to perform, and produce the best outcomes. A significantly (P = .014) larger number of AHPs (7) considered open rotator cuff surgery slow compared with arthroscopic (0) and mini-open (2) repair techniques. As with the overall cohort, AHPs reported arthroscopic and mini-open repairs promote quick healing and good cosmetic results, but are challenging to perform.
Discussion
As our population ages and continues to remain active, the demand for RCR has accelerated. National data show that 272,148 ambulatory RCRs and 20,433 inpatient RCRs were performed in 2006—an overall 141% increase in RCR since 1996.20 In 1996, 41 per 100,000 population underwent RCR.20 By 2006, this number ballooned to 98 per 100,000 population.20 There are 3 predominant techniques for repairing the rotator cuff: open, mini-open, and arthroscopic. As RCR use increases, we should consider the factors that medical professionals consider important when choosing a method for their own RCR.
Of the 84 medical professionals in our cohort, 39 (46%) indicated they would defer to their surgeon’s technique preference for RCR. Of the other 45, about equal numbers preferred arthroscopic and mini-open RCRs; only 2 preferred open RCRs. This finding suggests that the individual opinions of surgeons who perform RCRs have a substantial influence on a large proportion of medical professionals’ ultimate choice of RCR method. Interestingly, of the attendings who performed open RCR, only 1 expressed a preference for the open technique for his own RCR. This finding might suggest a shift in opinion and an emerging perception among surgeons performing RCR about the value of this technique.
Several factors may account for these evolving beliefs. We hypothesized that a biased favorable view of arthroscopic repair outcome might influence opinions. However, our results did not support the hypothesis. Medical professionals in our cohort were equivocal about the best RCR technique. No consensus was evident among attendings, residents, or AHPs. This lack of clinical agreement about rotator cuff surgery has been observed elsewhere—for example, among members of the American Academy of Orthopaedic Surgeons (AAOS)21 and the European Society of Sports Traumatology, Knee Surgery, and Arthroscopy.22 Despite theoretical advantages of arthroscopic repair, there has been no documented significant difference in patient outcomes when compared with other techniques.23 To our knowledge, there have been only a few clinical studies comparing the different RCR techniques. A meta-analysis of 5 clinical studies comparing arthroscopic and mini-open RCR techniques showed no difference in clinical outcomes or complication rates.8 The 2012 AAOS clinical practice guidelines for RCR reflect these observations.24 That consortium of leading shoulder surgeons could not recommend a modality of surgical rotator cuff tear repair given the lack of conclusive evidence.24
At our institution, arthroscopic, mini-open, and open RCRs were performed by 36%, 9%, and 55% of our surgeons, respectively. A survey of AAOS surgeons showed that, of those who perform RCRs, 14.5%, 46.2%, and 36.6% used arthroscopic, mini-open, and open techniques, respectively.21 The greater use of open repairs at our institution might reflect the seniority of our faculty. Dunn and colleagues21 found that surgeons who preferred open RCR had been in practice longer than those who preferred the arthroscopic or mini-open technique. Of our 4 faculty who performed arthroscopic repairs, 3 were less than 5 years from completing their training. In contrast, all faculty who performed mini-open or open repairs were more than 5 years from completing their training. Furthermore, mean age of the surgeons who performed arthroscopic repair was 39.8 years (range, 32-51 years), and these surgeons were significantly younger than those who performed mini-open or open repair (mean age, 56.3 years; range, 41-78 years). Younger surgeon age has been associated with higher rates of arthroscopic repair.25
Attendings unaccustomed to arthroscopy may find it more challenging than the younger generation of surgeons, who are exposed to it early in training. Dunn and colleagues21 noted that the likelihood of performing an arthroscopic repair was influenced by the surgeon’s experience level. Fellowship-trained shoulder and sports medicine surgeons are also more likely to perform arthroscopic repairs than those with training limited to orthopedic residency.25 Arthroscopic RCR demands a high level of technical skill that many acquire in fellowship training.26 Mauro and colleagues26 found that surgeons trained in a sports medicine fellowship performed 82.6% of subacromial decompression and/or RCR procedures arthroscopically, compared with 54.5% to 70.1% for surgeons trained in other fellowships. In our cohort, with the exception of 1 surgeon, all fellowship-trained shoulder and sports medicine surgeons performed arthroscopic RCRs.
Although no conclusive evidence in the literature supports arthroscopic over the other repair types, the demand for arthroscopic RCR has rapidly increased relative to that for the others. Between 1996 and 2006, use of arthroscopic RCR increased 600%, from 8 to 58 per 100,000 population.20 In that same period, use of open RCR increased by only 34%.20 Similarly, Mauro and colleagues26 found that the proportion of subacromial decompression and RCRs performed arthroscopically rose from 58.3% in 2004 to 83.7% in 2009. Using the 2006 New York State Ambulatory Surgery Database, Churchill and Ghorai27 found that 74.5% of RCRs with acromioplasty were performed arthroscopically.
Respondent-indicated factors that may have contributed to the more favorable opinion of arthroscopic and mini-open repair include quick healing, good cosmetic results, and better perceived patient satisfaction. The literature supports these perceptions. Baker and Liu14 found shorter hospital stays and quicker return to activity with arthroscopic repair compared with open repair. Vitale and colleagues25 also noted that, compared with open or mini-open repair techniques, arthroscopic repair resulted in shorter hospitalization and quicker overall recovery.
If these selected health care professionals with some inside information on rotator cuff surgery have biases that affect their selection of rotator cuff procedures, we should acknowledge that nonmedical personnel, in particular our patients, also have biases. The knowledge base of patients may be further influenced by friends or family members who have had rotator cuff surgery, by lay publications, and by the Internet. Satisfaction with any surgical procedure depends not only on the success of the surgery and the rehabilitation but also on patient and provider expectations. Such expectations are influenced, in part, by biases.
Our medical professionals had similar opinions on safety, recovery, cosmesis, and overall outcome of the RCR techniques, but different opinions on procedure durations and associated training requirements. All residents except one indicated open repair was a quick procedure. In contrast, a significant number of AHPs thought open repair was time-consuming. The attendings considered all the methods fast. The residents’ opinions were the most consistent with the true operating times reported. According to the literature, total operating time for mini-open repair ranges from 10 to 16 minutes faster than that for arthroscopic repair.18,20,27 Ultimately, procedure duration did not affect the respondents’ technique preference for RCR.
There was substantial disagreement about the number of procedures needed to become proficient in the different repair techniques. Overall, however, there was consensus that arthroscopic and mini-open repairs had longer learning curves than open repair. Given the lack of agreement among orthopedic department chairmen and sports medicine fellowship directors regarding the minimum exposure needed (during residency) to become proficient in diagnostic shoulder arthroscopy,28 this finding is not surprising. Guttmann and colleagues29 attempted to quantify the learning curve for arthroscopic RCR by tracking operating time as a surrogate measure. They found that RCR operative time decreased rapidly during the initial block of 10 cases to the second block of 10 cases, but thereafter improvement continued at a much lower rate.29 None of our respondents thought the learning curve for arthroscopic RCR was under 10 cases, but no group, not even the attendings who performed RCRs, could agree on the minimum number of cases needed for proficiency. The longer learning curve for arthroscopic RCR did not discourage the respondents who preferred arthroscopic or mini-open RCR.
Cost was not an influential factor in opinions about which RCR method is optimal. Medical professionals were ambivalent about the cost-effectiveness of the different procedures, with most expressing no opinion on cost. Multiple investigators have shown that arthroscopic RCR costs as much as $1144 more than mini-open RCR,18,27 which has many of the advantages of arthroscopic repair but not the costly implants and instruments. As our medical community becomes more cost-conscious, concern about this factor may increase among medical professionals.
Our study had several limitations. Its results must be interpreted carefully, given they represent the viewpoints of a nonrandomized sample of motivated respondents at one institution. A selection bias excluded surgeons who were uncomfortable with RCR and unwilling to report any shortcomings. The conclusions cannot be generalized to other medical professionals or to other institutions. Furthermore, to develop a simple, straightforward survey focused on a specific type of rotator cuff tear, and to avoid confusion, we assumed that the treatment preference for the described tear was generalizable to all encountered tears. However, some surgeons have reported different repair techniques for different types and sizes of rotator cuff tears.25
Conclusion
Most of our surveyed medical professionals were willing to defer to their surgeon’s decision about which technique would be appropriate for their own personal RCR. There is a trend nationally, and at our institution, for increased use of arthroscopic RCR. Although medical professionals readily acknowledge it is unclear which repair method provides the best ultimate outcome, many perceive fast recovery and good cosmetic results with arthroscopic and mini-open repairs. When medical professionals are counseling patients, we need to recognize these personal biases because many patients defer to their surgeon’s counsel. For some medical professionals, cosmesis can be an important factor, but cost, procedure duration, potential technical challenges of arthroscopic repair, and other considerations may make other techniques more desirable for others.
1. Bennett WF. Arthroscopic repair of massive rotator cuff tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(4):380-390.
2. Bennett WF. Arthroscopic repair of full-thickness supraspinatus tears (small-to-medium): a prospective study with 2- to 4-year follow-up. Arthroscopy. 2003;19(3):249-256.
3. Nho SJ, Shindle MK, Sherman SL, Freedman KB, Lyman S, MacGillivray JD. Systematic review of arthroscopic rotator cuff repair and mini-open rotator cuff repair. J Bone Joint Surg Am. 2007;89(suppl 3):127-136.
4. Duralde XA, Greene RT. Mini-open rotator cuff repair via an anterosuperior approach. J Shoulder Elbow Surg. 2008;17(5):715-721.
5. Blevins FT, Warren RF, Cavo C, et al. Arthroscopic assisted rotator cuff repair: results using a mini-open deltoid splitting approach. Arthroscopy. 1996;12(1):50-59.
6. Levy HJ, Uribe JW, Delaney LG. Arthroscopic assisted rotator cuff repair: preliminary results. Arthroscopy. 1990;6(1):55-60.
7. Liu SH. Arthroscopically-assisted rotator-cuff repair. J Bone Joint Surg Br. 1994;76(4):592-595.
8. Morse K, Davis AD, Afra R, Kaye EK, Schepsis A, Voloshin I. Arthroscopic versus mini-open rotator cuff repair: a comprehensive review and meta-analysis. Am J Sports Med. 2008;36(9):1824-1828.
9. Park JY, Levine WN, Marra G, Pollock RG, Flatow EL, Bigliani LU. Portal-extension approach for the repair of small and medium rotator cuff tears. Am J Sports Med. 2000;28(3):312-316.
10. Paulos LE, Kody MH. Arthroscopically enhanced “miniapproach” to rotator cuff repair. Am J Sports Med. 1994;22(1):19-25.
11. Posada A, Uribe JW, Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE. Mini-deltoid splitting rotator cuff repair: do results deteriorate with time? Arthroscopy. 2000;16(2):137-141.
12. Shinners TJ, Noordsij PG, Orwin JF. Arthroscopically assisted mini-open rotator cuff repair. Arthroscopy. 2002;18(1):21-26.
13. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
14. Baker CL, Liu SH. Comparison of open and arthroscopically assisted rotator cuff repairs. Am J Sports Med. 1995;23(1):99-104.
15. Liu SH, Baker CL. Arthroscopically assisted rotator cuff repair: correlation of functional results with integrity of the cuff. Arthroscopy. 1994;10(1):54-60.
16. Pollock RG, Flatow EL. The rotator cuff, part II. Full-thickness tears. Mini-open repair. Orthop Clin North Am. 1997;28(2):169-177.
17. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
18. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
19. Kose KC, Tezen E, Cebesoy O, et al. Mini-open versus all-arthroscopic rotator cuff repair: comparison of the operative costs and the clinical outcomes. Adv Ther. 2008;25(3):249-259.
20. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
21. Dunn WR, Schackman BR, Walsh C, et al. Variation in orthopaedic surgeons’ perceptions about the indications for rotator cuff surgery. J Bone Joint Surg Am. 2005;87(9):1978-1984.
22. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):803-815.
23. Aleem AW, Brophy RH. Outcomes of rotator cuff surgery: what does the evidence tell us? Clin Sports Med. 2012;31(4):665-674.
24. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
25. Vitale MA, Kleweno CP, Jacir AM, Levine WN, Bigliani LU, Ahmad CS. Training resources in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2007;89(6):1393-1398.
26. Mauro CS, Jordan SS, Irrgang JJ, Harner CD. Practice patterns for subacromial decompression and rotator cuff repair: an analysis of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2012;94(16):1492-1499.
27. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
28. O’Neill PJ, Cosgarea AJ, Freedman JA, Queale WS, McFarland EG. Arthroscopic proficiency: a survey of orthopaedic sports medicine fellowship directors and orthopaedic surgery department chairs. Arthroscopy. 2002;18(7):795-800.
29. Guttmann D, Graham RD, MacLennan MJ, Lubowitz JH. Arthroscopic rotator cuff repair: the learning curve. Arthroscopy. 2005;21(4):394-400.
Rotator cuff tears are a common condition affecting the shoulder joint. Initial open repair techniques were associated with several complications, including severe early postoperative pain, deltoid detachment and/or weakness, risk for infection, and arthrofibrosis.1-3 In addition, open procedures cannot address other possible diagnoses, such as labral tears and loose bodies. These disadvantages promoted the development of an arthroscopically assisted mini-open technique.4 Superior long-term results, with more than 90% of patients achieving good to excellent results,5-13 established the mini-open rotator cuff repair (RCR) as the gold standard.3,6,10,12,14-16
Recently, as instrumentation for arthroscopy has improved, enthusiasm for all-arthroscopic techniques (hereafter referred to as arthroscopic repair) has grown. The appeal of arthroscopic repair includes potentially less initial pain, ability to treat intra-articular lesions concurrently, smaller skin incisions with better cosmesis, less soft-tissue dissection, and low risk for deltoid detachment.3,17 The potential advantages of arthroscopic repair can lead to perceptions of quicker healing and shorter recovery, which are not supported by the literature. However, arthroscopic repair is technically more challenging, time-consuming, and expensive than open or mini-open repairs,18,19 and though some investigators have reported a trend toward fewer complications,3 the long-term outcome of arthroscopic RCRs has not been shown to be better than that of other techniques.
Given that no differences have been shown between the emerging arthroscopic repair technique and mini-open repair with respect to range of motion or clinical scores in the short term,3 it is unclear what perceptions influence choice of technique for one’s own personal RCR.
We conducted a study to determine which RCR technique medical professionals (orthopedic attendings and residents, anesthesiologists, internal medicine attendings, main operating room nurses, and physical therapists) preferred for their own surgery and to analyze perceptions shaping those opinions. Orthopedic surgeons have the best concept of rotator cuff surgery, but anesthesiologists and nurses have a “front row seat” and opinions on types of rotator cuff surgery. Physical therapists, who treat patients with rotator cuff tears, also have a working knowledge of rotator cuff surgery. Finally, internists represent a rotator cuff injury referral service and may have patients who have undergone rotator cuff surgery. We hypothesized that most medical professionals, irrespective of specialty or career length, would prefer arthroscopic RCR because of its perceived superior outcome and fast recovery.
Materials and Methods
This cross-sectional, descriptive, survey-based study was approved by our institutional review board (IRB) and offered via 3 emails between April 2011 and June 2011 to attendings (orthopedists, internists, anesthesiologists), residents, and allied health professionals (AHPs; operating room nurses, physical therapists) involved in orthopedic care at our institution. Each email contained a hyperlink to the online survey (Appendix), which took about 10 minutes to complete and explored respondent demographics, exposure to the different techniques, and opinions regarding different aspects of RCR surgery and recovery.
There were 84 respondents. The sexes were equally represented, and age ranged from 25 to 78 years (Table 1). Of the respondents, 41 (49%) were attendings, 20 (24%) were residents, and 23 (27%) were AHPs. Of the attendings, 13 (32%) were orthopedic surgeons, 26 (63%) were primary care physicians, and 2 (5%) did not specify their specialty. Four orthopedic surgeons had fellowship training in sports medicine or shoulder and elbow surgery. The attendings were overall more experienced in their profession than the other groups were, with 68% reporting more than 5 years of experience.
Descriptive statistics, including means and standard errors, were calculated. Fisher exact test was used to compare preferences of RCR type according to type of training and years of experience. Significance was set at P ≤ .05.
Results
Overall Responses (Table 2)
Of the 84 respondents, almost half (46%) preferred deferring their choice of RCR to their surgeon. Most of the other respondents preferred the arthroscopic technique (26%) or the mini-open repair (23%). There was no association between technique preference and medical professional type. Most respondents (63%) had never assisted in or performed rotator cuff surgery.
Seventy-four percent of all respondents indicated they thought arthroscopic, mini-open, and open RCRs are safe, and about half thought these procedures are fast. About half expressed no opinion about the cost-effectiveness of arthroscopic, mini-open, or open RCRs (54%, 52%, and 48%, respectively), and slightly more than half expressed no opinion about whether arthroscopic, mini-open, or open RCR provide the best outcome (58%, 60%, and 62%, respectively). Significantly (P < .05) more respondents thought arthroscopic and mini-open repairs, rather than open repairs, promote quick healing (64% and 45%, respectively, vs 15%), good cosmetic results (81% and 51%, respectively, vs 10%), and patient satisfaction (50% and 48%, respectively, vs 30%). However, a significant (P < .05) number also thought arthroscopic and mini-open repairs are harder to learn/more challenging to perform than open repairs (52% and 38%, respectively, vs 17%).
Of all factors considered, safety of arthroscopic repair garnered the highest consensus: 82%. Respondents were least opinionated about the outcome of the open repair technique, with more than 62% expressing no opinion about the outcome. The responses to the questions on the learning curves for the 3 techniques varied the most.
Responses by Group (Table 2)
Attendings. Of the 41 attendings, 24 (59%) responded they would defer to their surgeon’s technique preference for RCR. Of the other 17 who expressed a preference, most indicated arthroscopic or mini-open repair (17% each). There was a difference (P < .05) between years of experience and RCR preference: of the 13 attendings with less than 5 years of experience, arthroscopic repair was preferred by 31%; in contrast, of the 28 attendings with more than 5 years of experience, only 11% preferred arthroscopic repair.
Of the 11 attendings who performed rotator cuff surgery, 55% used the open technique, but most (8) preferred to have their own rotator cuff fixed arthroscopically or according to their surgeon’s preference. Only 1 surgeon preferred open repair for his own rotator cuff. Of the 4 surgeons who performed arthroscopic RCRs, 3 had less than 5 years of experience. Conversely, all 7 surgeons who performed mini-open or open repairs had more than 5 years of experience.
Of the 30 attendings who did not perform rotator cuff surgery, most (20) responded they would defer to their surgeon’s technique preference for RCR.
The attendings’ opinions on factors affecting rotator cuff surgery were similar to those of the other respondents with respect to safety, cost-effectiveness, recovery, cosmesis, patient satisfaction, outcome, and technical difficulty. Unlike the others, however, attendings considered all 3 repair techniques fast.
Residents. Of the 20 residents, 7 preferred arthroscopic, 5 preferred mini-open, and 1 preferred open repair; the other 7 responded they would defer to their surgeon’s preference. Residents’ opinions on each factor were more polarized and consistent across categories than those of the other groups. Residents overwhelmingly thought all 3 techniques (arthroscopic, mini-open, open) are safe (19, 19, and 18, respectively) and cost-effective (12, 14, and 14, respectively). Although most residents considered the open and mini-open repair techniques fast (19 and 15, respectively), only 8 considered arthroscopic RCR fast, and 4 considered it slow. Residents’ opinions about the technique that produces the best outcome were mixed. As with the other respondents, residents thought arthroscopic RCRs heal fast and produce great cosmetic results, but are challenging to perform and have a steep learning curve. Unlike the other respondents, most residents (12) considered open RCR easy to learn (P = .006), with a learning curve of fewer than 20 procedures.
AHPs. No AHP expressed a preference for open RCR. This group was evenly divided among 3 choices: deferring to their surgeon’s preference, arthroscopic repair, and mini-open repair. The 23 AHPs thought arthroscopic, mini-open, and open repairs are safe (17, 15, and 12, respectively), but most indicated they were “equivocal” about which techniques are cost-effective, challenging to perform, and produce the best outcomes. A significantly (P = .014) larger number of AHPs (7) considered open rotator cuff surgery slow compared with arthroscopic (0) and mini-open (2) repair techniques. As with the overall cohort, AHPs reported arthroscopic and mini-open repairs promote quick healing and good cosmetic results, but are challenging to perform.
Discussion
As our population ages and continues to remain active, the demand for RCR has accelerated. National data show that 272,148 ambulatory RCRs and 20,433 inpatient RCRs were performed in 2006—an overall 141% increase in RCR since 1996.20 In 1996, 41 per 100,000 population underwent RCR.20 By 2006, this number ballooned to 98 per 100,000 population.20 There are 3 predominant techniques for repairing the rotator cuff: open, mini-open, and arthroscopic. As RCR use increases, we should consider the factors that medical professionals consider important when choosing a method for their own RCR.
Of the 84 medical professionals in our cohort, 39 (46%) indicated they would defer to their surgeon’s technique preference for RCR. Of the other 45, about equal numbers preferred arthroscopic and mini-open RCRs; only 2 preferred open RCRs. This finding suggests that the individual opinions of surgeons who perform RCRs have a substantial influence on a large proportion of medical professionals’ ultimate choice of RCR method. Interestingly, of the attendings who performed open RCR, only 1 expressed a preference for the open technique for his own RCR. This finding might suggest a shift in opinion and an emerging perception among surgeons performing RCR about the value of this technique.
Several factors may account for these evolving beliefs. We hypothesized that a biased favorable view of arthroscopic repair outcome might influence opinions. However, our results did not support the hypothesis. Medical professionals in our cohort were equivocal about the best RCR technique. No consensus was evident among attendings, residents, or AHPs. This lack of clinical agreement about rotator cuff surgery has been observed elsewhere—for example, among members of the American Academy of Orthopaedic Surgeons (AAOS)21 and the European Society of Sports Traumatology, Knee Surgery, and Arthroscopy.22 Despite theoretical advantages of arthroscopic repair, there has been no documented significant difference in patient outcomes when compared with other techniques.23 To our knowledge, there have been only a few clinical studies comparing the different RCR techniques. A meta-analysis of 5 clinical studies comparing arthroscopic and mini-open RCR techniques showed no difference in clinical outcomes or complication rates.8 The 2012 AAOS clinical practice guidelines for RCR reflect these observations.24 That consortium of leading shoulder surgeons could not recommend a modality of surgical rotator cuff tear repair given the lack of conclusive evidence.24
At our institution, arthroscopic, mini-open, and open RCRs were performed by 36%, 9%, and 55% of our surgeons, respectively. A survey of AAOS surgeons showed that, of those who perform RCRs, 14.5%, 46.2%, and 36.6% used arthroscopic, mini-open, and open techniques, respectively.21 The greater use of open repairs at our institution might reflect the seniority of our faculty. Dunn and colleagues21 found that surgeons who preferred open RCR had been in practice longer than those who preferred the arthroscopic or mini-open technique. Of our 4 faculty who performed arthroscopic repairs, 3 were less than 5 years from completing their training. In contrast, all faculty who performed mini-open or open repairs were more than 5 years from completing their training. Furthermore, mean age of the surgeons who performed arthroscopic repair was 39.8 years (range, 32-51 years), and these surgeons were significantly younger than those who performed mini-open or open repair (mean age, 56.3 years; range, 41-78 years). Younger surgeon age has been associated with higher rates of arthroscopic repair.25
Attendings unaccustomed to arthroscopy may find it more challenging than the younger generation of surgeons, who are exposed to it early in training. Dunn and colleagues21 noted that the likelihood of performing an arthroscopic repair was influenced by the surgeon’s experience level. Fellowship-trained shoulder and sports medicine surgeons are also more likely to perform arthroscopic repairs than those with training limited to orthopedic residency.25 Arthroscopic RCR demands a high level of technical skill that many acquire in fellowship training.26 Mauro and colleagues26 found that surgeons trained in a sports medicine fellowship performed 82.6% of subacromial decompression and/or RCR procedures arthroscopically, compared with 54.5% to 70.1% for surgeons trained in other fellowships. In our cohort, with the exception of 1 surgeon, all fellowship-trained shoulder and sports medicine surgeons performed arthroscopic RCRs.
Although no conclusive evidence in the literature supports arthroscopic over the other repair types, the demand for arthroscopic RCR has rapidly increased relative to that for the others. Between 1996 and 2006, use of arthroscopic RCR increased 600%, from 8 to 58 per 100,000 population.20 In that same period, use of open RCR increased by only 34%.20 Similarly, Mauro and colleagues26 found that the proportion of subacromial decompression and RCRs performed arthroscopically rose from 58.3% in 2004 to 83.7% in 2009. Using the 2006 New York State Ambulatory Surgery Database, Churchill and Ghorai27 found that 74.5% of RCRs with acromioplasty were performed arthroscopically.
Respondent-indicated factors that may have contributed to the more favorable opinion of arthroscopic and mini-open repair include quick healing, good cosmetic results, and better perceived patient satisfaction. The literature supports these perceptions. Baker and Liu14 found shorter hospital stays and quicker return to activity with arthroscopic repair compared with open repair. Vitale and colleagues25 also noted that, compared with open or mini-open repair techniques, arthroscopic repair resulted in shorter hospitalization and quicker overall recovery.
If these selected health care professionals with some inside information on rotator cuff surgery have biases that affect their selection of rotator cuff procedures, we should acknowledge that nonmedical personnel, in particular our patients, also have biases. The knowledge base of patients may be further influenced by friends or family members who have had rotator cuff surgery, by lay publications, and by the Internet. Satisfaction with any surgical procedure depends not only on the success of the surgery and the rehabilitation but also on patient and provider expectations. Such expectations are influenced, in part, by biases.
Our medical professionals had similar opinions on safety, recovery, cosmesis, and overall outcome of the RCR techniques, but different opinions on procedure durations and associated training requirements. All residents except one indicated open repair was a quick procedure. In contrast, a significant number of AHPs thought open repair was time-consuming. The attendings considered all the methods fast. The residents’ opinions were the most consistent with the true operating times reported. According to the literature, total operating time for mini-open repair ranges from 10 to 16 minutes faster than that for arthroscopic repair.18,20,27 Ultimately, procedure duration did not affect the respondents’ technique preference for RCR.
There was substantial disagreement about the number of procedures needed to become proficient in the different repair techniques. Overall, however, there was consensus that arthroscopic and mini-open repairs had longer learning curves than open repair. Given the lack of agreement among orthopedic department chairmen and sports medicine fellowship directors regarding the minimum exposure needed (during residency) to become proficient in diagnostic shoulder arthroscopy,28 this finding is not surprising. Guttmann and colleagues29 attempted to quantify the learning curve for arthroscopic RCR by tracking operating time as a surrogate measure. They found that RCR operative time decreased rapidly during the initial block of 10 cases to the second block of 10 cases, but thereafter improvement continued at a much lower rate.29 None of our respondents thought the learning curve for arthroscopic RCR was under 10 cases, but no group, not even the attendings who performed RCRs, could agree on the minimum number of cases needed for proficiency. The longer learning curve for arthroscopic RCR did not discourage the respondents who preferred arthroscopic or mini-open RCR.
Cost was not an influential factor in opinions about which RCR method is optimal. Medical professionals were ambivalent about the cost-effectiveness of the different procedures, with most expressing no opinion on cost. Multiple investigators have shown that arthroscopic RCR costs as much as $1144 more than mini-open RCR,18,27 which has many of the advantages of arthroscopic repair but not the costly implants and instruments. As our medical community becomes more cost-conscious, concern about this factor may increase among medical professionals.
Our study had several limitations. Its results must be interpreted carefully, given they represent the viewpoints of a nonrandomized sample of motivated respondents at one institution. A selection bias excluded surgeons who were uncomfortable with RCR and unwilling to report any shortcomings. The conclusions cannot be generalized to other medical professionals or to other institutions. Furthermore, to develop a simple, straightforward survey focused on a specific type of rotator cuff tear, and to avoid confusion, we assumed that the treatment preference for the described tear was generalizable to all encountered tears. However, some surgeons have reported different repair techniques for different types and sizes of rotator cuff tears.25
Conclusion
Most of our surveyed medical professionals were willing to defer to their surgeon’s decision about which technique would be appropriate for their own personal RCR. There is a trend nationally, and at our institution, for increased use of arthroscopic RCR. Although medical professionals readily acknowledge it is unclear which repair method provides the best ultimate outcome, many perceive fast recovery and good cosmetic results with arthroscopic and mini-open repairs. When medical professionals are counseling patients, we need to recognize these personal biases because many patients defer to their surgeon’s counsel. For some medical professionals, cosmesis can be an important factor, but cost, procedure duration, potential technical challenges of arthroscopic repair, and other considerations may make other techniques more desirable for others.
Rotator cuff tears are a common condition affecting the shoulder joint. Initial open repair techniques were associated with several complications, including severe early postoperative pain, deltoid detachment and/or weakness, risk for infection, and arthrofibrosis.1-3 In addition, open procedures cannot address other possible diagnoses, such as labral tears and loose bodies. These disadvantages promoted the development of an arthroscopically assisted mini-open technique.4 Superior long-term results, with more than 90% of patients achieving good to excellent results,5-13 established the mini-open rotator cuff repair (RCR) as the gold standard.3,6,10,12,14-16
Recently, as instrumentation for arthroscopy has improved, enthusiasm for all-arthroscopic techniques (hereafter referred to as arthroscopic repair) has grown. The appeal of arthroscopic repair includes potentially less initial pain, ability to treat intra-articular lesions concurrently, smaller skin incisions with better cosmesis, less soft-tissue dissection, and low risk for deltoid detachment.3,17 The potential advantages of arthroscopic repair can lead to perceptions of quicker healing and shorter recovery, which are not supported by the literature. However, arthroscopic repair is technically more challenging, time-consuming, and expensive than open or mini-open repairs,18,19 and though some investigators have reported a trend toward fewer complications,3 the long-term outcome of arthroscopic RCRs has not been shown to be better than that of other techniques.
Given that no differences have been shown between the emerging arthroscopic repair technique and mini-open repair with respect to range of motion or clinical scores in the short term,3 it is unclear what perceptions influence choice of technique for one’s own personal RCR.
We conducted a study to determine which RCR technique medical professionals (orthopedic attendings and residents, anesthesiologists, internal medicine attendings, main operating room nurses, and physical therapists) preferred for their own surgery and to analyze perceptions shaping those opinions. Orthopedic surgeons have the best concept of rotator cuff surgery, but anesthesiologists and nurses have a “front row seat” and opinions on types of rotator cuff surgery. Physical therapists, who treat patients with rotator cuff tears, also have a working knowledge of rotator cuff surgery. Finally, internists represent a rotator cuff injury referral service and may have patients who have undergone rotator cuff surgery. We hypothesized that most medical professionals, irrespective of specialty or career length, would prefer arthroscopic RCR because of its perceived superior outcome and fast recovery.
Materials and Methods
This cross-sectional, descriptive, survey-based study was approved by our institutional review board (IRB) and offered via 3 emails between April 2011 and June 2011 to attendings (orthopedists, internists, anesthesiologists), residents, and allied health professionals (AHPs; operating room nurses, physical therapists) involved in orthopedic care at our institution. Each email contained a hyperlink to the online survey (Appendix), which took about 10 minutes to complete and explored respondent demographics, exposure to the different techniques, and opinions regarding different aspects of RCR surgery and recovery.
There were 84 respondents. The sexes were equally represented, and age ranged from 25 to 78 years (Table 1). Of the respondents, 41 (49%) were attendings, 20 (24%) were residents, and 23 (27%) were AHPs. Of the attendings, 13 (32%) were orthopedic surgeons, 26 (63%) were primary care physicians, and 2 (5%) did not specify their specialty. Four orthopedic surgeons had fellowship training in sports medicine or shoulder and elbow surgery. The attendings were overall more experienced in their profession than the other groups were, with 68% reporting more than 5 years of experience.
Descriptive statistics, including means and standard errors, were calculated. Fisher exact test was used to compare preferences of RCR type according to type of training and years of experience. Significance was set at P ≤ .05.
Results
Overall Responses (Table 2)
Of the 84 respondents, almost half (46%) preferred deferring their choice of RCR to their surgeon. Most of the other respondents preferred the arthroscopic technique (26%) or the mini-open repair (23%). There was no association between technique preference and medical professional type. Most respondents (63%) had never assisted in or performed rotator cuff surgery.
Seventy-four percent of all respondents indicated they thought arthroscopic, mini-open, and open RCRs are safe, and about half thought these procedures are fast. About half expressed no opinion about the cost-effectiveness of arthroscopic, mini-open, or open RCRs (54%, 52%, and 48%, respectively), and slightly more than half expressed no opinion about whether arthroscopic, mini-open, or open RCR provide the best outcome (58%, 60%, and 62%, respectively). Significantly (P < .05) more respondents thought arthroscopic and mini-open repairs, rather than open repairs, promote quick healing (64% and 45%, respectively, vs 15%), good cosmetic results (81% and 51%, respectively, vs 10%), and patient satisfaction (50% and 48%, respectively, vs 30%). However, a significant (P < .05) number also thought arthroscopic and mini-open repairs are harder to learn/more challenging to perform than open repairs (52% and 38%, respectively, vs 17%).
Of all factors considered, safety of arthroscopic repair garnered the highest consensus: 82%. Respondents were least opinionated about the outcome of the open repair technique, with more than 62% expressing no opinion about the outcome. The responses to the questions on the learning curves for the 3 techniques varied the most.
Responses by Group (Table 2)
Attendings. Of the 41 attendings, 24 (59%) responded they would defer to their surgeon’s technique preference for RCR. Of the other 17 who expressed a preference, most indicated arthroscopic or mini-open repair (17% each). There was a difference (P < .05) between years of experience and RCR preference: of the 13 attendings with less than 5 years of experience, arthroscopic repair was preferred by 31%; in contrast, of the 28 attendings with more than 5 years of experience, only 11% preferred arthroscopic repair.
Of the 11 attendings who performed rotator cuff surgery, 55% used the open technique, but most (8) preferred to have their own rotator cuff fixed arthroscopically or according to their surgeon’s preference. Only 1 surgeon preferred open repair for his own rotator cuff. Of the 4 surgeons who performed arthroscopic RCRs, 3 had less than 5 years of experience. Conversely, all 7 surgeons who performed mini-open or open repairs had more than 5 years of experience.
Of the 30 attendings who did not perform rotator cuff surgery, most (20) responded they would defer to their surgeon’s technique preference for RCR.
The attendings’ opinions on factors affecting rotator cuff surgery were similar to those of the other respondents with respect to safety, cost-effectiveness, recovery, cosmesis, patient satisfaction, outcome, and technical difficulty. Unlike the others, however, attendings considered all 3 repair techniques fast.
Residents. Of the 20 residents, 7 preferred arthroscopic, 5 preferred mini-open, and 1 preferred open repair; the other 7 responded they would defer to their surgeon’s preference. Residents’ opinions on each factor were more polarized and consistent across categories than those of the other groups. Residents overwhelmingly thought all 3 techniques (arthroscopic, mini-open, open) are safe (19, 19, and 18, respectively) and cost-effective (12, 14, and 14, respectively). Although most residents considered the open and mini-open repair techniques fast (19 and 15, respectively), only 8 considered arthroscopic RCR fast, and 4 considered it slow. Residents’ opinions about the technique that produces the best outcome were mixed. As with the other respondents, residents thought arthroscopic RCRs heal fast and produce great cosmetic results, but are challenging to perform and have a steep learning curve. Unlike the other respondents, most residents (12) considered open RCR easy to learn (P = .006), with a learning curve of fewer than 20 procedures.
AHPs. No AHP expressed a preference for open RCR. This group was evenly divided among 3 choices: deferring to their surgeon’s preference, arthroscopic repair, and mini-open repair. The 23 AHPs thought arthroscopic, mini-open, and open repairs are safe (17, 15, and 12, respectively), but most indicated they were “equivocal” about which techniques are cost-effective, challenging to perform, and produce the best outcomes. A significantly (P = .014) larger number of AHPs (7) considered open rotator cuff surgery slow compared with arthroscopic (0) and mini-open (2) repair techniques. As with the overall cohort, AHPs reported arthroscopic and mini-open repairs promote quick healing and good cosmetic results, but are challenging to perform.
Discussion
As our population ages and continues to remain active, the demand for RCR has accelerated. National data show that 272,148 ambulatory RCRs and 20,433 inpatient RCRs were performed in 2006—an overall 141% increase in RCR since 1996.20 In 1996, 41 per 100,000 population underwent RCR.20 By 2006, this number ballooned to 98 per 100,000 population.20 There are 3 predominant techniques for repairing the rotator cuff: open, mini-open, and arthroscopic. As RCR use increases, we should consider the factors that medical professionals consider important when choosing a method for their own RCR.
Of the 84 medical professionals in our cohort, 39 (46%) indicated they would defer to their surgeon’s technique preference for RCR. Of the other 45, about equal numbers preferred arthroscopic and mini-open RCRs; only 2 preferred open RCRs. This finding suggests that the individual opinions of surgeons who perform RCRs have a substantial influence on a large proportion of medical professionals’ ultimate choice of RCR method. Interestingly, of the attendings who performed open RCR, only 1 expressed a preference for the open technique for his own RCR. This finding might suggest a shift in opinion and an emerging perception among surgeons performing RCR about the value of this technique.
Several factors may account for these evolving beliefs. We hypothesized that a biased favorable view of arthroscopic repair outcome might influence opinions. However, our results did not support the hypothesis. Medical professionals in our cohort were equivocal about the best RCR technique. No consensus was evident among attendings, residents, or AHPs. This lack of clinical agreement about rotator cuff surgery has been observed elsewhere—for example, among members of the American Academy of Orthopaedic Surgeons (AAOS)21 and the European Society of Sports Traumatology, Knee Surgery, and Arthroscopy.22 Despite theoretical advantages of arthroscopic repair, there has been no documented significant difference in patient outcomes when compared with other techniques.23 To our knowledge, there have been only a few clinical studies comparing the different RCR techniques. A meta-analysis of 5 clinical studies comparing arthroscopic and mini-open RCR techniques showed no difference in clinical outcomes or complication rates.8 The 2012 AAOS clinical practice guidelines for RCR reflect these observations.24 That consortium of leading shoulder surgeons could not recommend a modality of surgical rotator cuff tear repair given the lack of conclusive evidence.24
At our institution, arthroscopic, mini-open, and open RCRs were performed by 36%, 9%, and 55% of our surgeons, respectively. A survey of AAOS surgeons showed that, of those who perform RCRs, 14.5%, 46.2%, and 36.6% used arthroscopic, mini-open, and open techniques, respectively.21 The greater use of open repairs at our institution might reflect the seniority of our faculty. Dunn and colleagues21 found that surgeons who preferred open RCR had been in practice longer than those who preferred the arthroscopic or mini-open technique. Of our 4 faculty who performed arthroscopic repairs, 3 were less than 5 years from completing their training. In contrast, all faculty who performed mini-open or open repairs were more than 5 years from completing their training. Furthermore, mean age of the surgeons who performed arthroscopic repair was 39.8 years (range, 32-51 years), and these surgeons were significantly younger than those who performed mini-open or open repair (mean age, 56.3 years; range, 41-78 years). Younger surgeon age has been associated with higher rates of arthroscopic repair.25
Attendings unaccustomed to arthroscopy may find it more challenging than the younger generation of surgeons, who are exposed to it early in training. Dunn and colleagues21 noted that the likelihood of performing an arthroscopic repair was influenced by the surgeon’s experience level. Fellowship-trained shoulder and sports medicine surgeons are also more likely to perform arthroscopic repairs than those with training limited to orthopedic residency.25 Arthroscopic RCR demands a high level of technical skill that many acquire in fellowship training.26 Mauro and colleagues26 found that surgeons trained in a sports medicine fellowship performed 82.6% of subacromial decompression and/or RCR procedures arthroscopically, compared with 54.5% to 70.1% for surgeons trained in other fellowships. In our cohort, with the exception of 1 surgeon, all fellowship-trained shoulder and sports medicine surgeons performed arthroscopic RCRs.
Although no conclusive evidence in the literature supports arthroscopic over the other repair types, the demand for arthroscopic RCR has rapidly increased relative to that for the others. Between 1996 and 2006, use of arthroscopic RCR increased 600%, from 8 to 58 per 100,000 population.20 In that same period, use of open RCR increased by only 34%.20 Similarly, Mauro and colleagues26 found that the proportion of subacromial decompression and RCRs performed arthroscopically rose from 58.3% in 2004 to 83.7% in 2009. Using the 2006 New York State Ambulatory Surgery Database, Churchill and Ghorai27 found that 74.5% of RCRs with acromioplasty were performed arthroscopically.
Respondent-indicated factors that may have contributed to the more favorable opinion of arthroscopic and mini-open repair include quick healing, good cosmetic results, and better perceived patient satisfaction. The literature supports these perceptions. Baker and Liu14 found shorter hospital stays and quicker return to activity with arthroscopic repair compared with open repair. Vitale and colleagues25 also noted that, compared with open or mini-open repair techniques, arthroscopic repair resulted in shorter hospitalization and quicker overall recovery.
If these selected health care professionals with some inside information on rotator cuff surgery have biases that affect their selection of rotator cuff procedures, we should acknowledge that nonmedical personnel, in particular our patients, also have biases. The knowledge base of patients may be further influenced by friends or family members who have had rotator cuff surgery, by lay publications, and by the Internet. Satisfaction with any surgical procedure depends not only on the success of the surgery and the rehabilitation but also on patient and provider expectations. Such expectations are influenced, in part, by biases.
Our medical professionals had similar opinions on safety, recovery, cosmesis, and overall outcome of the RCR techniques, but different opinions on procedure durations and associated training requirements. All residents except one indicated open repair was a quick procedure. In contrast, a significant number of AHPs thought open repair was time-consuming. The attendings considered all the methods fast. The residents’ opinions were the most consistent with the true operating times reported. According to the literature, total operating time for mini-open repair ranges from 10 to 16 minutes faster than that for arthroscopic repair.18,20,27 Ultimately, procedure duration did not affect the respondents’ technique preference for RCR.
There was substantial disagreement about the number of procedures needed to become proficient in the different repair techniques. Overall, however, there was consensus that arthroscopic and mini-open repairs had longer learning curves than open repair. Given the lack of agreement among orthopedic department chairmen and sports medicine fellowship directors regarding the minimum exposure needed (during residency) to become proficient in diagnostic shoulder arthroscopy,28 this finding is not surprising. Guttmann and colleagues29 attempted to quantify the learning curve for arthroscopic RCR by tracking operating time as a surrogate measure. They found that RCR operative time decreased rapidly during the initial block of 10 cases to the second block of 10 cases, but thereafter improvement continued at a much lower rate.29 None of our respondents thought the learning curve for arthroscopic RCR was under 10 cases, but no group, not even the attendings who performed RCRs, could agree on the minimum number of cases needed for proficiency. The longer learning curve for arthroscopic RCR did not discourage the respondents who preferred arthroscopic or mini-open RCR.
Cost was not an influential factor in opinions about which RCR method is optimal. Medical professionals were ambivalent about the cost-effectiveness of the different procedures, with most expressing no opinion on cost. Multiple investigators have shown that arthroscopic RCR costs as much as $1144 more than mini-open RCR,18,27 which has many of the advantages of arthroscopic repair but not the costly implants and instruments. As our medical community becomes more cost-conscious, concern about this factor may increase among medical professionals.
Our study had several limitations. Its results must be interpreted carefully, given they represent the viewpoints of a nonrandomized sample of motivated respondents at one institution. A selection bias excluded surgeons who were uncomfortable with RCR and unwilling to report any shortcomings. The conclusions cannot be generalized to other medical professionals or to other institutions. Furthermore, to develop a simple, straightforward survey focused on a specific type of rotator cuff tear, and to avoid confusion, we assumed that the treatment preference for the described tear was generalizable to all encountered tears. However, some surgeons have reported different repair techniques for different types and sizes of rotator cuff tears.25
Conclusion
Most of our surveyed medical professionals were willing to defer to their surgeon’s decision about which technique would be appropriate for their own personal RCR. There is a trend nationally, and at our institution, for increased use of arthroscopic RCR. Although medical professionals readily acknowledge it is unclear which repair method provides the best ultimate outcome, many perceive fast recovery and good cosmetic results with arthroscopic and mini-open repairs. When medical professionals are counseling patients, we need to recognize these personal biases because many patients defer to their surgeon’s counsel. For some medical professionals, cosmesis can be an important factor, but cost, procedure duration, potential technical challenges of arthroscopic repair, and other considerations may make other techniques more desirable for others.
1. Bennett WF. Arthroscopic repair of massive rotator cuff tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(4):380-390.
2. Bennett WF. Arthroscopic repair of full-thickness supraspinatus tears (small-to-medium): a prospective study with 2- to 4-year follow-up. Arthroscopy. 2003;19(3):249-256.
3. Nho SJ, Shindle MK, Sherman SL, Freedman KB, Lyman S, MacGillivray JD. Systematic review of arthroscopic rotator cuff repair and mini-open rotator cuff repair. J Bone Joint Surg Am. 2007;89(suppl 3):127-136.
4. Duralde XA, Greene RT. Mini-open rotator cuff repair via an anterosuperior approach. J Shoulder Elbow Surg. 2008;17(5):715-721.
5. Blevins FT, Warren RF, Cavo C, et al. Arthroscopic assisted rotator cuff repair: results using a mini-open deltoid splitting approach. Arthroscopy. 1996;12(1):50-59.
6. Levy HJ, Uribe JW, Delaney LG. Arthroscopic assisted rotator cuff repair: preliminary results. Arthroscopy. 1990;6(1):55-60.
7. Liu SH. Arthroscopically-assisted rotator-cuff repair. J Bone Joint Surg Br. 1994;76(4):592-595.
8. Morse K, Davis AD, Afra R, Kaye EK, Schepsis A, Voloshin I. Arthroscopic versus mini-open rotator cuff repair: a comprehensive review and meta-analysis. Am J Sports Med. 2008;36(9):1824-1828.
9. Park JY, Levine WN, Marra G, Pollock RG, Flatow EL, Bigliani LU. Portal-extension approach for the repair of small and medium rotator cuff tears. Am J Sports Med. 2000;28(3):312-316.
10. Paulos LE, Kody MH. Arthroscopically enhanced “miniapproach” to rotator cuff repair. Am J Sports Med. 1994;22(1):19-25.
11. Posada A, Uribe JW, Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE. Mini-deltoid splitting rotator cuff repair: do results deteriorate with time? Arthroscopy. 2000;16(2):137-141.
12. Shinners TJ, Noordsij PG, Orwin JF. Arthroscopically assisted mini-open rotator cuff repair. Arthroscopy. 2002;18(1):21-26.
13. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
14. Baker CL, Liu SH. Comparison of open and arthroscopically assisted rotator cuff repairs. Am J Sports Med. 1995;23(1):99-104.
15. Liu SH, Baker CL. Arthroscopically assisted rotator cuff repair: correlation of functional results with integrity of the cuff. Arthroscopy. 1994;10(1):54-60.
16. Pollock RG, Flatow EL. The rotator cuff, part II. Full-thickness tears. Mini-open repair. Orthop Clin North Am. 1997;28(2):169-177.
17. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
18. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
19. Kose KC, Tezen E, Cebesoy O, et al. Mini-open versus all-arthroscopic rotator cuff repair: comparison of the operative costs and the clinical outcomes. Adv Ther. 2008;25(3):249-259.
20. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
21. Dunn WR, Schackman BR, Walsh C, et al. Variation in orthopaedic surgeons’ perceptions about the indications for rotator cuff surgery. J Bone Joint Surg Am. 2005;87(9):1978-1984.
22. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):803-815.
23. Aleem AW, Brophy RH. Outcomes of rotator cuff surgery: what does the evidence tell us? Clin Sports Med. 2012;31(4):665-674.
24. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
25. Vitale MA, Kleweno CP, Jacir AM, Levine WN, Bigliani LU, Ahmad CS. Training resources in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2007;89(6):1393-1398.
26. Mauro CS, Jordan SS, Irrgang JJ, Harner CD. Practice patterns for subacromial decompression and rotator cuff repair: an analysis of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2012;94(16):1492-1499.
27. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
28. O’Neill PJ, Cosgarea AJ, Freedman JA, Queale WS, McFarland EG. Arthroscopic proficiency: a survey of orthopaedic sports medicine fellowship directors and orthopaedic surgery department chairs. Arthroscopy. 2002;18(7):795-800.
29. Guttmann D, Graham RD, MacLennan MJ, Lubowitz JH. Arthroscopic rotator cuff repair: the learning curve. Arthroscopy. 2005;21(4):394-400.
1. Bennett WF. Arthroscopic repair of massive rotator cuff tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(4):380-390.
2. Bennett WF. Arthroscopic repair of full-thickness supraspinatus tears (small-to-medium): a prospective study with 2- to 4-year follow-up. Arthroscopy. 2003;19(3):249-256.
3. Nho SJ, Shindle MK, Sherman SL, Freedman KB, Lyman S, MacGillivray JD. Systematic review of arthroscopic rotator cuff repair and mini-open rotator cuff repair. J Bone Joint Surg Am. 2007;89(suppl 3):127-136.
4. Duralde XA, Greene RT. Mini-open rotator cuff repair via an anterosuperior approach. J Shoulder Elbow Surg. 2008;17(5):715-721.
5. Blevins FT, Warren RF, Cavo C, et al. Arthroscopic assisted rotator cuff repair: results using a mini-open deltoid splitting approach. Arthroscopy. 1996;12(1):50-59.
6. Levy HJ, Uribe JW, Delaney LG. Arthroscopic assisted rotator cuff repair: preliminary results. Arthroscopy. 1990;6(1):55-60.
7. Liu SH. Arthroscopically-assisted rotator-cuff repair. J Bone Joint Surg Br. 1994;76(4):592-595.
8. Morse K, Davis AD, Afra R, Kaye EK, Schepsis A, Voloshin I. Arthroscopic versus mini-open rotator cuff repair: a comprehensive review and meta-analysis. Am J Sports Med. 2008;36(9):1824-1828.
9. Park JY, Levine WN, Marra G, Pollock RG, Flatow EL, Bigliani LU. Portal-extension approach for the repair of small and medium rotator cuff tears. Am J Sports Med. 2000;28(3):312-316.
10. Paulos LE, Kody MH. Arthroscopically enhanced “miniapproach” to rotator cuff repair. Am J Sports Med. 1994;22(1):19-25.
11. Posada A, Uribe JW, Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE. Mini-deltoid splitting rotator cuff repair: do results deteriorate with time? Arthroscopy. 2000;16(2):137-141.
12. Shinners TJ, Noordsij PG, Orwin JF. Arthroscopically assisted mini-open rotator cuff repair. Arthroscopy. 2002;18(1):21-26.
13. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
14. Baker CL, Liu SH. Comparison of open and arthroscopically assisted rotator cuff repairs. Am J Sports Med. 1995;23(1):99-104.
15. Liu SH, Baker CL. Arthroscopically assisted rotator cuff repair: correlation of functional results with integrity of the cuff. Arthroscopy. 1994;10(1):54-60.
16. Pollock RG, Flatow EL. The rotator cuff, part II. Full-thickness tears. Mini-open repair. Orthop Clin North Am. 1997;28(2):169-177.
17. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
18. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
19. Kose KC, Tezen E, Cebesoy O, et al. Mini-open versus all-arthroscopic rotator cuff repair: comparison of the operative costs and the clinical outcomes. Adv Ther. 2008;25(3):249-259.
20. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
21. Dunn WR, Schackman BR, Walsh C, et al. Variation in orthopaedic surgeons’ perceptions about the indications for rotator cuff surgery. J Bone Joint Surg Am. 2005;87(9):1978-1984.
22. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):803-815.
23. Aleem AW, Brophy RH. Outcomes of rotator cuff surgery: what does the evidence tell us? Clin Sports Med. 2012;31(4):665-674.
24. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
25. Vitale MA, Kleweno CP, Jacir AM, Levine WN, Bigliani LU, Ahmad CS. Training resources in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2007;89(6):1393-1398.
26. Mauro CS, Jordan SS, Irrgang JJ, Harner CD. Practice patterns for subacromial decompression and rotator cuff repair: an analysis of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2012;94(16):1492-1499.
27. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
28. O’Neill PJ, Cosgarea AJ, Freedman JA, Queale WS, McFarland EG. Arthroscopic proficiency: a survey of orthopaedic sports medicine fellowship directors and orthopaedic surgery department chairs. Arthroscopy. 2002;18(7):795-800.
29. Guttmann D, Graham RD, MacLennan MJ, Lubowitz JH. Arthroscopic rotator cuff repair: the learning curve. Arthroscopy. 2005;21(4):394-400.
Bilateral Superior Labrum Anterior to Posterior (SLAP) Tears With Abnormal Anatomy of Biceps Tendon
The biceps brachii derives its name from the 2 heads of the muscle. The short head originates from the coracoid apex, with the coracobrachialis muscle. The long head of the biceps tendon (LHBT) starts within the capsule of the shoulder joint, running from the supraglenoid tubercle or labrum.1 The tendon typically runs free along its intra-articular course, but it is also extrasynovial and ensheathed by a continuation of the synovial lining of the articular capsule that extends to the inferior-most extent of the bicipital groove.2 Congenital anomalies of the LHBT are uncommon, although several atypical forms have been described. A literature search for anomalous LHBT identified several variations in anatomic descriptions, including Y-shaped variant, complete absence of tendon, extra-articular attachment, and a variety of intracapsular attachments. In all, 8 case reports of aberrant intracapsular attachment of LHBT3-12 were identified. These cases presented with a variety of clinical manifestations and pathologic changes. Often, these anatomic variations are considered innocuous, yet some present with pathologic findings.
We present the clinical, magnetic resonance imaging (MRI), and arthroscopic findings of a relatively young athletic patient who was experiencing symptoms of bilateral superior labrum anterior to posterior (SLAP) tears that were unresponsive to conservative management. A unique anatomic variant of the LHBT that involved confluence of the LHBT with the undersurface of the anterosuperior capsule at the rotator interval, as well as a Buford complex anteriorly, was identified and treated. We believe that the tethering of the biceps tendon to the capsule combined with the Buford complex created increased stress on the superior labrum and biceps anchor variant, leading to the development of bilateral symptomatic type II SLAP tears. Knowledge of this variant, though perhaps rare, may be relevant for diagnostic recognition of young athletic patients who present with recalcitrant shoulder symptoms. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
A 15-year-old healthy and active athletic boy presented with pain in the right shoulder without history of trauma. He was active in both swimming and baseball. He complained of pain that was present with activities, such as lifting weights, swimming, and throwing. His treatment prior to the office visit consisted of nonsteroidal anti-inflammatory medication, rest, and a therapy program initiated by his high school athletic trainer.
Physical examination demonstrated tenderness to palpation over the posterior capsule and biceps. Motion was full, cuff strength was normal, and SLAP signs (O’Brien, Speed, and Jobe relocation) were positive. A radiograph showed no sign of fracture or dislocation, and no evidence of bony abnormality.
The patient was sent for an MRI arthrogram, which showed a SLAP tear extending from 1 o’clock anteriorly to 10 o’clock posteriorly without intra-articular displacement. No rotator cuff tear was noted. The biceps tendon was noted to be unremarkable and located within the bicipital groove, although retrospective review of the MRI showed that the intra-articular biceps tendon was somewhat confluent with the adjacent tissues.
The patient underwent right shoulder arthroscopy. The shoulder was stable to ligamentous examination under anesthesia. Arthroscopic evaluation revealed that there was a type II SLAP tear extending from the 11-o’clock to the 2-o’clock positions. The superior glenohumeral ligament was identified as it arose from the upper pole of the glenoid labrum and then ran parallel and inferior to the tendon of the biceps towards the lesser tubercle. Surprisingly, there was a very unusual attachment of the intracapsular LHBT to the undersurface of the rotator interval, which restricted biceps excursion in relation to the rotator cuff. Additionally, there was a thick cord-like middle glenohumeral ligament anteriorly that lacked the normal glenoid attachments, thus representing a Buford complex. Interestingly, the labral tear could not only be displaced with a probe, but placing the shoulder through a range of motion also led to increased displacement of the labrum from the glenoid, likely because the biceps tendon was tethered to the undersurface of the capsule.
At the time of arthroscopy, the LHBT was released from its attachment to the capsule at the rotator interval with a radiofrequency wand and shaver. A labral repair was performed using three 2.9-mm bioabsorbable suture anchors, placing 2 posterior and 1 anterior to the biceps tendon. The integrity of the labral repair was observed while placing the shoulder through range of motion.
Postoperatively, the patient was kept in a sling for 5 weeks. Home exercises were initiated at 2 weeks, and outpatient physical therapy was implemented at 4 weeks. The patient resumed swimming, throwing, and other activities—with minimal discomfort—at 6 months postoperatively.
Three years after his initial visit, the patient returned to the office with a similar complaint of pain and limitation of function in his left shoulder after returning to full athletic competition. Once again, there was no history of injury, and history, physical examination, and MRI arthrogram (Figures 1A, 1B) evaluation proved to be very similar to this young athlete’s right shoulder work-up.
The patient once again underwent shoulder arthroscopy and treatment. Although this was now the left shoulder, the findings were essentially identical to the right shoulder. Once again, the labrum was detached from the 11-o’clock to 2-o’clock positions, and a Buford complex was present anteriorly (Figure 2A). The labral tear was easily displaceable from the glenoid with a probe, and placing the shoulder through a range of motion led to increased displacement of the labrum from the glenoid. There was also confluence of the intra-articular LHBT with the undersurface of the capsule within the rotator interval (Figure 2B). A radiofrequency wand, shaver, and elevator were used to define the biceps tendon and separate it from the undersurface of the capsule. The SLAP repair was performed using three 2.9-mm absorbable suture anchors with 2 posterior and 1 anterior to the biceps tendon insertion. The labral repair was observed while placing the shoulder through range of motion and the shoulder was seen to be free of any undue tension on the labrum.
Postoperatively, the patient’s sling and rehabilitation protocol was identical to that of the right shoulder. The patient progressed well, was released to full activity at 6 months, and has not returned with any further complaints of left or right shoulder pain. Approximately 3 years after treatment the patient was contacted via phone and asked about symptoms, pain, and activity. He denies current symptoms of clicking or instability and has no pain that he can identify as being related to previous pathology or treatment. Since the surgery, he has ceased competitive sports and weight lifting, which he attributes to deconditioning associated with postsurgical immobilization and lack of motivation.
Discussion
Of the 8 case reports in the literature that identified variable intra-articular biceps insertional anatomy, only 2 reports represented confluence of the biceps within the rotator interval.7 Interestingly, of the cases identified, the single case that presented a patient with similar pathology of a type II SLAP lesion had an almost identical anatomical variant presentation consisting of both the anomalous insertion of the LHBT into the undersurface of the rotator interval and a Buford variant of the anterosuperior glenohumeral ligament complex. To our knowledge, our bilateral case of an altered intra-articular biceps insertion and a concomitant SLAP tear supports the theory that this pattern of anomalous insertion may very well have altered the biomechanics of the tendon, resulting in acquired pathology to the superior labrum.
The literature reviewed showed the prevalence of anatomic variations of the LHBT ranged from 1.9% to 7.4%.13,14 These variations are generally considered benign; however, in some cases—as in the cases of the young athletes presented by Wahl and MacGillivray7 and in this report—anatomic variation may play an important role in pathogenesis of different injury patterns. The primary function of the LHBT is the stabilization of the glenohumeral joint during abduction and external rotation.15 When the insertion diverges from normal (eg, when the tendon is tethered to the undersurface of the rotator cuff), the biomechanical stresses on the tendon likely change. As a result of the anomalous position of the LHBT origin, there may be a change in the shoulder joint’s biomechanics, with increased strain on the glenohumeral ligament and its attachment onto the glenoid.16
This case report differs from publications on variable superior glenohumeral ligament attachments because a discrete superior glenohumeral ligament structure was isolated from the biceps tendon. Although a larger case series or patient cohort, as well as more involved biomechanical analysis, would certainly be necessary to prove our hypothesis, we believe that this case suggests certain anatomic LHBT and labral variations can contribute to the develop of SLAP tears in younger individuals.
1. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
2. Burkhead WZ Jr. The biceps tendon. In: Rockwood CA Jr, Matsen FA III, eds. The Shoulder. Vol. 2. Philadelphia: WB Saunders; 1990:791-836.
3. Parikh SN, Bonnaig N, Zbojniewicz A. Intracapsular origin of the long head biceps tendon with glenoid avulsion of the glenohumeral ligaments. Orthopedics. 2011;34(11):781-784.
4. Gaskin CM, Golish SR, Blount KJ, Diduch DR. Anomalies of the long head of the biceps brachii tendon: clinical significance, MR arthrographic findings, and arthroscopic correlation in two patients. Skeletal Radiol. 2007;36(8):785-789.
5. Yeh L, Pedowitz R, Kwak S, et al. Intracapsular origin of the long head of the biceps tendon. Skeletal Radiol. 1999;28(3):178-181.
6. Richards DP, Schwartz M. Anomalous intraarticular origin of the long head of the biceps brachii. Clin J Sport Med. 2003;13(2):122-124.
7. Wahl CJ, MacGillivray JD. Three congenital variations in the long head of the biceps tendon: a review of the pathoanatomic considerations and case reports. J Shoulder Elbow Surg. 2007;16(6):e25-e30.I
8. Egea JM, Melguizo C, Prados J, Aránega A. Capsular origin of the long head of the biceps tendon: a clinical case. Rom J Morphol Embryol. 2010;51(2):375-377.
9. Hyman JL, Warren RF. Extra-articular origin of biceps brachii. Arthroscopy. 2001;17(7): E29.
10. Enad JG. Bifurcate origin of the long head of the biceps tendon. Arthroscopy. 2004;20(10):1081-1083.
11. Mariani PP, Bellelli A, Botticella C. Arthroscopic absence of the long head of the biceps tendon. Arthroscopy. 1997;13(4):499-501.
12. Koplas MC, Winalski CS, Ulmer WH Jr, Recht M. Bilateral congenital absence of the long head of the biceps tendon. Skeletal Radiol. 2009;38(7):715-719.
13. Kanatli U, Ozturk BY, Eisen E, Bolukbasi S. Intra-articular variations of the long head of the biceps tendon. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1576-1581.
14. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
15. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
16. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
The biceps brachii derives its name from the 2 heads of the muscle. The short head originates from the coracoid apex, with the coracobrachialis muscle. The long head of the biceps tendon (LHBT) starts within the capsule of the shoulder joint, running from the supraglenoid tubercle or labrum.1 The tendon typically runs free along its intra-articular course, but it is also extrasynovial and ensheathed by a continuation of the synovial lining of the articular capsule that extends to the inferior-most extent of the bicipital groove.2 Congenital anomalies of the LHBT are uncommon, although several atypical forms have been described. A literature search for anomalous LHBT identified several variations in anatomic descriptions, including Y-shaped variant, complete absence of tendon, extra-articular attachment, and a variety of intracapsular attachments. In all, 8 case reports of aberrant intracapsular attachment of LHBT3-12 were identified. These cases presented with a variety of clinical manifestations and pathologic changes. Often, these anatomic variations are considered innocuous, yet some present with pathologic findings.
We present the clinical, magnetic resonance imaging (MRI), and arthroscopic findings of a relatively young athletic patient who was experiencing symptoms of bilateral superior labrum anterior to posterior (SLAP) tears that were unresponsive to conservative management. A unique anatomic variant of the LHBT that involved confluence of the LHBT with the undersurface of the anterosuperior capsule at the rotator interval, as well as a Buford complex anteriorly, was identified and treated. We believe that the tethering of the biceps tendon to the capsule combined with the Buford complex created increased stress on the superior labrum and biceps anchor variant, leading to the development of bilateral symptomatic type II SLAP tears. Knowledge of this variant, though perhaps rare, may be relevant for diagnostic recognition of young athletic patients who present with recalcitrant shoulder symptoms. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
A 15-year-old healthy and active athletic boy presented with pain in the right shoulder without history of trauma. He was active in both swimming and baseball. He complained of pain that was present with activities, such as lifting weights, swimming, and throwing. His treatment prior to the office visit consisted of nonsteroidal anti-inflammatory medication, rest, and a therapy program initiated by his high school athletic trainer.
Physical examination demonstrated tenderness to palpation over the posterior capsule and biceps. Motion was full, cuff strength was normal, and SLAP signs (O’Brien, Speed, and Jobe relocation) were positive. A radiograph showed no sign of fracture or dislocation, and no evidence of bony abnormality.
The patient was sent for an MRI arthrogram, which showed a SLAP tear extending from 1 o’clock anteriorly to 10 o’clock posteriorly without intra-articular displacement. No rotator cuff tear was noted. The biceps tendon was noted to be unremarkable and located within the bicipital groove, although retrospective review of the MRI showed that the intra-articular biceps tendon was somewhat confluent with the adjacent tissues.
The patient underwent right shoulder arthroscopy. The shoulder was stable to ligamentous examination under anesthesia. Arthroscopic evaluation revealed that there was a type II SLAP tear extending from the 11-o’clock to the 2-o’clock positions. The superior glenohumeral ligament was identified as it arose from the upper pole of the glenoid labrum and then ran parallel and inferior to the tendon of the biceps towards the lesser tubercle. Surprisingly, there was a very unusual attachment of the intracapsular LHBT to the undersurface of the rotator interval, which restricted biceps excursion in relation to the rotator cuff. Additionally, there was a thick cord-like middle glenohumeral ligament anteriorly that lacked the normal glenoid attachments, thus representing a Buford complex. Interestingly, the labral tear could not only be displaced with a probe, but placing the shoulder through a range of motion also led to increased displacement of the labrum from the glenoid, likely because the biceps tendon was tethered to the undersurface of the capsule.
At the time of arthroscopy, the LHBT was released from its attachment to the capsule at the rotator interval with a radiofrequency wand and shaver. A labral repair was performed using three 2.9-mm bioabsorbable suture anchors, placing 2 posterior and 1 anterior to the biceps tendon. The integrity of the labral repair was observed while placing the shoulder through range of motion.
Postoperatively, the patient was kept in a sling for 5 weeks. Home exercises were initiated at 2 weeks, and outpatient physical therapy was implemented at 4 weeks. The patient resumed swimming, throwing, and other activities—with minimal discomfort—at 6 months postoperatively.
Three years after his initial visit, the patient returned to the office with a similar complaint of pain and limitation of function in his left shoulder after returning to full athletic competition. Once again, there was no history of injury, and history, physical examination, and MRI arthrogram (Figures 1A, 1B) evaluation proved to be very similar to this young athlete’s right shoulder work-up.
The patient once again underwent shoulder arthroscopy and treatment. Although this was now the left shoulder, the findings were essentially identical to the right shoulder. Once again, the labrum was detached from the 11-o’clock to 2-o’clock positions, and a Buford complex was present anteriorly (Figure 2A). The labral tear was easily displaceable from the glenoid with a probe, and placing the shoulder through a range of motion led to increased displacement of the labrum from the glenoid. There was also confluence of the intra-articular LHBT with the undersurface of the capsule within the rotator interval (Figure 2B). A radiofrequency wand, shaver, and elevator were used to define the biceps tendon and separate it from the undersurface of the capsule. The SLAP repair was performed using three 2.9-mm absorbable suture anchors with 2 posterior and 1 anterior to the biceps tendon insertion. The labral repair was observed while placing the shoulder through range of motion and the shoulder was seen to be free of any undue tension on the labrum.
Postoperatively, the patient’s sling and rehabilitation protocol was identical to that of the right shoulder. The patient progressed well, was released to full activity at 6 months, and has not returned with any further complaints of left or right shoulder pain. Approximately 3 years after treatment the patient was contacted via phone and asked about symptoms, pain, and activity. He denies current symptoms of clicking or instability and has no pain that he can identify as being related to previous pathology or treatment. Since the surgery, he has ceased competitive sports and weight lifting, which he attributes to deconditioning associated with postsurgical immobilization and lack of motivation.
Discussion
Of the 8 case reports in the literature that identified variable intra-articular biceps insertional anatomy, only 2 reports represented confluence of the biceps within the rotator interval.7 Interestingly, of the cases identified, the single case that presented a patient with similar pathology of a type II SLAP lesion had an almost identical anatomical variant presentation consisting of both the anomalous insertion of the LHBT into the undersurface of the rotator interval and a Buford variant of the anterosuperior glenohumeral ligament complex. To our knowledge, our bilateral case of an altered intra-articular biceps insertion and a concomitant SLAP tear supports the theory that this pattern of anomalous insertion may very well have altered the biomechanics of the tendon, resulting in acquired pathology to the superior labrum.
The literature reviewed showed the prevalence of anatomic variations of the LHBT ranged from 1.9% to 7.4%.13,14 These variations are generally considered benign; however, in some cases—as in the cases of the young athletes presented by Wahl and MacGillivray7 and in this report—anatomic variation may play an important role in pathogenesis of different injury patterns. The primary function of the LHBT is the stabilization of the glenohumeral joint during abduction and external rotation.15 When the insertion diverges from normal (eg, when the tendon is tethered to the undersurface of the rotator cuff), the biomechanical stresses on the tendon likely change. As a result of the anomalous position of the LHBT origin, there may be a change in the shoulder joint’s biomechanics, with increased strain on the glenohumeral ligament and its attachment onto the glenoid.16
This case report differs from publications on variable superior glenohumeral ligament attachments because a discrete superior glenohumeral ligament structure was isolated from the biceps tendon. Although a larger case series or patient cohort, as well as more involved biomechanical analysis, would certainly be necessary to prove our hypothesis, we believe that this case suggests certain anatomic LHBT and labral variations can contribute to the develop of SLAP tears in younger individuals.
The biceps brachii derives its name from the 2 heads of the muscle. The short head originates from the coracoid apex, with the coracobrachialis muscle. The long head of the biceps tendon (LHBT) starts within the capsule of the shoulder joint, running from the supraglenoid tubercle or labrum.1 The tendon typically runs free along its intra-articular course, but it is also extrasynovial and ensheathed by a continuation of the synovial lining of the articular capsule that extends to the inferior-most extent of the bicipital groove.2 Congenital anomalies of the LHBT are uncommon, although several atypical forms have been described. A literature search for anomalous LHBT identified several variations in anatomic descriptions, including Y-shaped variant, complete absence of tendon, extra-articular attachment, and a variety of intracapsular attachments. In all, 8 case reports of aberrant intracapsular attachment of LHBT3-12 were identified. These cases presented with a variety of clinical manifestations and pathologic changes. Often, these anatomic variations are considered innocuous, yet some present with pathologic findings.
We present the clinical, magnetic resonance imaging (MRI), and arthroscopic findings of a relatively young athletic patient who was experiencing symptoms of bilateral superior labrum anterior to posterior (SLAP) tears that were unresponsive to conservative management. A unique anatomic variant of the LHBT that involved confluence of the LHBT with the undersurface of the anterosuperior capsule at the rotator interval, as well as a Buford complex anteriorly, was identified and treated. We believe that the tethering of the biceps tendon to the capsule combined with the Buford complex created increased stress on the superior labrum and biceps anchor variant, leading to the development of bilateral symptomatic type II SLAP tears. Knowledge of this variant, though perhaps rare, may be relevant for diagnostic recognition of young athletic patients who present with recalcitrant shoulder symptoms. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
A 15-year-old healthy and active athletic boy presented with pain in the right shoulder without history of trauma. He was active in both swimming and baseball. He complained of pain that was present with activities, such as lifting weights, swimming, and throwing. His treatment prior to the office visit consisted of nonsteroidal anti-inflammatory medication, rest, and a therapy program initiated by his high school athletic trainer.
Physical examination demonstrated tenderness to palpation over the posterior capsule and biceps. Motion was full, cuff strength was normal, and SLAP signs (O’Brien, Speed, and Jobe relocation) were positive. A radiograph showed no sign of fracture or dislocation, and no evidence of bony abnormality.
The patient was sent for an MRI arthrogram, which showed a SLAP tear extending from 1 o’clock anteriorly to 10 o’clock posteriorly without intra-articular displacement. No rotator cuff tear was noted. The biceps tendon was noted to be unremarkable and located within the bicipital groove, although retrospective review of the MRI showed that the intra-articular biceps tendon was somewhat confluent with the adjacent tissues.
The patient underwent right shoulder arthroscopy. The shoulder was stable to ligamentous examination under anesthesia. Arthroscopic evaluation revealed that there was a type II SLAP tear extending from the 11-o’clock to the 2-o’clock positions. The superior glenohumeral ligament was identified as it arose from the upper pole of the glenoid labrum and then ran parallel and inferior to the tendon of the biceps towards the lesser tubercle. Surprisingly, there was a very unusual attachment of the intracapsular LHBT to the undersurface of the rotator interval, which restricted biceps excursion in relation to the rotator cuff. Additionally, there was a thick cord-like middle glenohumeral ligament anteriorly that lacked the normal glenoid attachments, thus representing a Buford complex. Interestingly, the labral tear could not only be displaced with a probe, but placing the shoulder through a range of motion also led to increased displacement of the labrum from the glenoid, likely because the biceps tendon was tethered to the undersurface of the capsule.
At the time of arthroscopy, the LHBT was released from its attachment to the capsule at the rotator interval with a radiofrequency wand and shaver. A labral repair was performed using three 2.9-mm bioabsorbable suture anchors, placing 2 posterior and 1 anterior to the biceps tendon. The integrity of the labral repair was observed while placing the shoulder through range of motion.
Postoperatively, the patient was kept in a sling for 5 weeks. Home exercises were initiated at 2 weeks, and outpatient physical therapy was implemented at 4 weeks. The patient resumed swimming, throwing, and other activities—with minimal discomfort—at 6 months postoperatively.
Three years after his initial visit, the patient returned to the office with a similar complaint of pain and limitation of function in his left shoulder after returning to full athletic competition. Once again, there was no history of injury, and history, physical examination, and MRI arthrogram (Figures 1A, 1B) evaluation proved to be very similar to this young athlete’s right shoulder work-up.
The patient once again underwent shoulder arthroscopy and treatment. Although this was now the left shoulder, the findings were essentially identical to the right shoulder. Once again, the labrum was detached from the 11-o’clock to 2-o’clock positions, and a Buford complex was present anteriorly (Figure 2A). The labral tear was easily displaceable from the glenoid with a probe, and placing the shoulder through a range of motion led to increased displacement of the labrum from the glenoid. There was also confluence of the intra-articular LHBT with the undersurface of the capsule within the rotator interval (Figure 2B). A radiofrequency wand, shaver, and elevator were used to define the biceps tendon and separate it from the undersurface of the capsule. The SLAP repair was performed using three 2.9-mm absorbable suture anchors with 2 posterior and 1 anterior to the biceps tendon insertion. The labral repair was observed while placing the shoulder through range of motion and the shoulder was seen to be free of any undue tension on the labrum.
Postoperatively, the patient’s sling and rehabilitation protocol was identical to that of the right shoulder. The patient progressed well, was released to full activity at 6 months, and has not returned with any further complaints of left or right shoulder pain. Approximately 3 years after treatment the patient was contacted via phone and asked about symptoms, pain, and activity. He denies current symptoms of clicking or instability and has no pain that he can identify as being related to previous pathology or treatment. Since the surgery, he has ceased competitive sports and weight lifting, which he attributes to deconditioning associated with postsurgical immobilization and lack of motivation.
Discussion
Of the 8 case reports in the literature that identified variable intra-articular biceps insertional anatomy, only 2 reports represented confluence of the biceps within the rotator interval.7 Interestingly, of the cases identified, the single case that presented a patient with similar pathology of a type II SLAP lesion had an almost identical anatomical variant presentation consisting of both the anomalous insertion of the LHBT into the undersurface of the rotator interval and a Buford variant of the anterosuperior glenohumeral ligament complex. To our knowledge, our bilateral case of an altered intra-articular biceps insertion and a concomitant SLAP tear supports the theory that this pattern of anomalous insertion may very well have altered the biomechanics of the tendon, resulting in acquired pathology to the superior labrum.
The literature reviewed showed the prevalence of anatomic variations of the LHBT ranged from 1.9% to 7.4%.13,14 These variations are generally considered benign; however, in some cases—as in the cases of the young athletes presented by Wahl and MacGillivray7 and in this report—anatomic variation may play an important role in pathogenesis of different injury patterns. The primary function of the LHBT is the stabilization of the glenohumeral joint during abduction and external rotation.15 When the insertion diverges from normal (eg, when the tendon is tethered to the undersurface of the rotator cuff), the biomechanical stresses on the tendon likely change. As a result of the anomalous position of the LHBT origin, there may be a change in the shoulder joint’s biomechanics, with increased strain on the glenohumeral ligament and its attachment onto the glenoid.16
This case report differs from publications on variable superior glenohumeral ligament attachments because a discrete superior glenohumeral ligament structure was isolated from the biceps tendon. Although a larger case series or patient cohort, as well as more involved biomechanical analysis, would certainly be necessary to prove our hypothesis, we believe that this case suggests certain anatomic LHBT and labral variations can contribute to the develop of SLAP tears in younger individuals.
1. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
2. Burkhead WZ Jr. The biceps tendon. In: Rockwood CA Jr, Matsen FA III, eds. The Shoulder. Vol. 2. Philadelphia: WB Saunders; 1990:791-836.
3. Parikh SN, Bonnaig N, Zbojniewicz A. Intracapsular origin of the long head biceps tendon with glenoid avulsion of the glenohumeral ligaments. Orthopedics. 2011;34(11):781-784.
4. Gaskin CM, Golish SR, Blount KJ, Diduch DR. Anomalies of the long head of the biceps brachii tendon: clinical significance, MR arthrographic findings, and arthroscopic correlation in two patients. Skeletal Radiol. 2007;36(8):785-789.
5. Yeh L, Pedowitz R, Kwak S, et al. Intracapsular origin of the long head of the biceps tendon. Skeletal Radiol. 1999;28(3):178-181.
6. Richards DP, Schwartz M. Anomalous intraarticular origin of the long head of the biceps brachii. Clin J Sport Med. 2003;13(2):122-124.
7. Wahl CJ, MacGillivray JD. Three congenital variations in the long head of the biceps tendon: a review of the pathoanatomic considerations and case reports. J Shoulder Elbow Surg. 2007;16(6):e25-e30.I
8. Egea JM, Melguizo C, Prados J, Aránega A. Capsular origin of the long head of the biceps tendon: a clinical case. Rom J Morphol Embryol. 2010;51(2):375-377.
9. Hyman JL, Warren RF. Extra-articular origin of biceps brachii. Arthroscopy. 2001;17(7): E29.
10. Enad JG. Bifurcate origin of the long head of the biceps tendon. Arthroscopy. 2004;20(10):1081-1083.
11. Mariani PP, Bellelli A, Botticella C. Arthroscopic absence of the long head of the biceps tendon. Arthroscopy. 1997;13(4):499-501.
12. Koplas MC, Winalski CS, Ulmer WH Jr, Recht M. Bilateral congenital absence of the long head of the biceps tendon. Skeletal Radiol. 2009;38(7):715-719.
13. Kanatli U, Ozturk BY, Eisen E, Bolukbasi S. Intra-articular variations of the long head of the biceps tendon. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1576-1581.
14. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
15. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
16. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
1. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
2. Burkhead WZ Jr. The biceps tendon. In: Rockwood CA Jr, Matsen FA III, eds. The Shoulder. Vol. 2. Philadelphia: WB Saunders; 1990:791-836.
3. Parikh SN, Bonnaig N, Zbojniewicz A. Intracapsular origin of the long head biceps tendon with glenoid avulsion of the glenohumeral ligaments. Orthopedics. 2011;34(11):781-784.
4. Gaskin CM, Golish SR, Blount KJ, Diduch DR. Anomalies of the long head of the biceps brachii tendon: clinical significance, MR arthrographic findings, and arthroscopic correlation in two patients. Skeletal Radiol. 2007;36(8):785-789.
5. Yeh L, Pedowitz R, Kwak S, et al. Intracapsular origin of the long head of the biceps tendon. Skeletal Radiol. 1999;28(3):178-181.
6. Richards DP, Schwartz M. Anomalous intraarticular origin of the long head of the biceps brachii. Clin J Sport Med. 2003;13(2):122-124.
7. Wahl CJ, MacGillivray JD. Three congenital variations in the long head of the biceps tendon: a review of the pathoanatomic considerations and case reports. J Shoulder Elbow Surg. 2007;16(6):e25-e30.I
8. Egea JM, Melguizo C, Prados J, Aránega A. Capsular origin of the long head of the biceps tendon: a clinical case. Rom J Morphol Embryol. 2010;51(2):375-377.
9. Hyman JL, Warren RF. Extra-articular origin of biceps brachii. Arthroscopy. 2001;17(7): E29.
10. Enad JG. Bifurcate origin of the long head of the biceps tendon. Arthroscopy. 2004;20(10):1081-1083.
11. Mariani PP, Bellelli A, Botticella C. Arthroscopic absence of the long head of the biceps tendon. Arthroscopy. 1997;13(4):499-501.
12. Koplas MC, Winalski CS, Ulmer WH Jr, Recht M. Bilateral congenital absence of the long head of the biceps tendon. Skeletal Radiol. 2009;38(7):715-719.
13. Kanatli U, Ozturk BY, Eisen E, Bolukbasi S. Intra-articular variations of the long head of the biceps tendon. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1576-1581.
14. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
15. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
16. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
Commentary to "The Burden of Craft in Arthroscopic Rotator Cuff Repair: Where We Have Been and Where We Are Going"
“The Burden of Craft in Arthroscopic Rotator Cuff Repair” is a summary of the annual Neer Lecture that was delivered by Stephen S. Burkhart, MD, at the 2014 annual meeting of American Shoulder and Elbow Surgeons. It is a fascinating personal story of the 35-year evolution of arthroscopic rotator cuff surgery presented by one of the most respected arthroscopic innovators of our times. I especially enjoyed his apt citations of classic leaders—Churchill and Gandhi—but 3 points I believe deserve special comment.
First, Steve describes the challenges he faced bringing new products to market in the 1980s. How do we resolve the inherent conflict between innovation that introduces new technology and the “tried and true” standards of established practice? Do the hard work that Steve has done over the years: pose a hypothesis, design a study to answer the question, publish results in peer-reviewed journals, and embrace the techniques that demonstrate better outcomes for patients.
My second point relates to surgeon–device industry relationships, a subject of great interest to The American Journal of Orthopedics dating back to 2006.1-3 Dr. Burkhart learned early on that he could not fashion new arthroscopic instruments in his garage. Nor could a company develop useful instruments without a knowledgeable surgeon’s input. Hence, a partnership between the innovator-surgeon and the device industry is essential to bring new and effective “tools” to market. Dr. Burkhart’s partnership with Arthrex has benefited many thousands of patients.
The agreements announced in 2007 between the US Department of Justice and 5 orthopedic device manufacturers (interestingly, current presidential candidate and Governor of New Jersey Chris Christie was the lead US Attorney on the case!) dramatically altered the surgeon–industry interaction and established strict guidelines that governed these relationships.4 These were needed reforms. However, the changes did not preclude an entrepreneurial surgeon with great ideas and a device manufacturer from profiting from excellent products that advanced patient care, provided, quoting from my editorial of 2006, “that these partnerships comply with legal and ethical standards” and are transparent as well as fully disclosed.1
Finally, Steve’s last point focuses on the “burden of craft,” a topic dear to all orthopedic surgeons and our professional societies. All of us are committed to improving our surgical skills and, as a profession, we are consistently engaged in learning from our talented colleagues, who are only too willing to share their expertise. The burden of craft requires eager students and dedicated teachers, all committed to the same goal—better outcomes for our patients. We are indeed fortunate that, as orthopedic surgeons, we fundamentally support a culture of continued learning.
I thank Steve for his eloquent paper on this important principle.
1. McCann PD. Are surgeons accepting bribes? Am J Orthop. 2006;35(3):114.
2. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part I. Am J Orthop. 2006;35(3):117-120.
3. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part II. Am J Orthop. 2006;35(4):166-171.
4 Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed July 14, 2015.
“The Burden of Craft in Arthroscopic Rotator Cuff Repair” is a summary of the annual Neer Lecture that was delivered by Stephen S. Burkhart, MD, at the 2014 annual meeting of American Shoulder and Elbow Surgeons. It is a fascinating personal story of the 35-year evolution of arthroscopic rotator cuff surgery presented by one of the most respected arthroscopic innovators of our times. I especially enjoyed his apt citations of classic leaders—Churchill and Gandhi—but 3 points I believe deserve special comment.
First, Steve describes the challenges he faced bringing new products to market in the 1980s. How do we resolve the inherent conflict between innovation that introduces new technology and the “tried and true” standards of established practice? Do the hard work that Steve has done over the years: pose a hypothesis, design a study to answer the question, publish results in peer-reviewed journals, and embrace the techniques that demonstrate better outcomes for patients.
My second point relates to surgeon–device industry relationships, a subject of great interest to The American Journal of Orthopedics dating back to 2006.1-3 Dr. Burkhart learned early on that he could not fashion new arthroscopic instruments in his garage. Nor could a company develop useful instruments without a knowledgeable surgeon’s input. Hence, a partnership between the innovator-surgeon and the device industry is essential to bring new and effective “tools” to market. Dr. Burkhart’s partnership with Arthrex has benefited many thousands of patients.
The agreements announced in 2007 between the US Department of Justice and 5 orthopedic device manufacturers (interestingly, current presidential candidate and Governor of New Jersey Chris Christie was the lead US Attorney on the case!) dramatically altered the surgeon–industry interaction and established strict guidelines that governed these relationships.4 These were needed reforms. However, the changes did not preclude an entrepreneurial surgeon with great ideas and a device manufacturer from profiting from excellent products that advanced patient care, provided, quoting from my editorial of 2006, “that these partnerships comply with legal and ethical standards” and are transparent as well as fully disclosed.1
Finally, Steve’s last point focuses on the “burden of craft,” a topic dear to all orthopedic surgeons and our professional societies. All of us are committed to improving our surgical skills and, as a profession, we are consistently engaged in learning from our talented colleagues, who are only too willing to share their expertise. The burden of craft requires eager students and dedicated teachers, all committed to the same goal—better outcomes for our patients. We are indeed fortunate that, as orthopedic surgeons, we fundamentally support a culture of continued learning.
I thank Steve for his eloquent paper on this important principle.
“The Burden of Craft in Arthroscopic Rotator Cuff Repair” is a summary of the annual Neer Lecture that was delivered by Stephen S. Burkhart, MD, at the 2014 annual meeting of American Shoulder and Elbow Surgeons. It is a fascinating personal story of the 35-year evolution of arthroscopic rotator cuff surgery presented by one of the most respected arthroscopic innovators of our times. I especially enjoyed his apt citations of classic leaders—Churchill and Gandhi—but 3 points I believe deserve special comment.
First, Steve describes the challenges he faced bringing new products to market in the 1980s. How do we resolve the inherent conflict between innovation that introduces new technology and the “tried and true” standards of established practice? Do the hard work that Steve has done over the years: pose a hypothesis, design a study to answer the question, publish results in peer-reviewed journals, and embrace the techniques that demonstrate better outcomes for patients.
My second point relates to surgeon–device industry relationships, a subject of great interest to The American Journal of Orthopedics dating back to 2006.1-3 Dr. Burkhart learned early on that he could not fashion new arthroscopic instruments in his garage. Nor could a company develop useful instruments without a knowledgeable surgeon’s input. Hence, a partnership between the innovator-surgeon and the device industry is essential to bring new and effective “tools” to market. Dr. Burkhart’s partnership with Arthrex has benefited many thousands of patients.
The agreements announced in 2007 between the US Department of Justice and 5 orthopedic device manufacturers (interestingly, current presidential candidate and Governor of New Jersey Chris Christie was the lead US Attorney on the case!) dramatically altered the surgeon–industry interaction and established strict guidelines that governed these relationships.4 These were needed reforms. However, the changes did not preclude an entrepreneurial surgeon with great ideas and a device manufacturer from profiting from excellent products that advanced patient care, provided, quoting from my editorial of 2006, “that these partnerships comply with legal and ethical standards” and are transparent as well as fully disclosed.1
Finally, Steve’s last point focuses on the “burden of craft,” a topic dear to all orthopedic surgeons and our professional societies. All of us are committed to improving our surgical skills and, as a profession, we are consistently engaged in learning from our talented colleagues, who are only too willing to share their expertise. The burden of craft requires eager students and dedicated teachers, all committed to the same goal—better outcomes for our patients. We are indeed fortunate that, as orthopedic surgeons, we fundamentally support a culture of continued learning.
I thank Steve for his eloquent paper on this important principle.
1. McCann PD. Are surgeons accepting bribes? Am J Orthop. 2006;35(3):114.
2. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part I. Am J Orthop. 2006;35(3):117-120.
3. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part II. Am J Orthop. 2006;35(4):166-171.
4 Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed July 14, 2015.
1. McCann PD. Are surgeons accepting bribes? Am J Orthop. 2006;35(3):114.
2. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part I. Am J Orthop. 2006;35(3):117-120.
3. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part II. Am J Orthop. 2006;35(4):166-171.
4 Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed July 14, 2015.
The Burden of Craft in Arthroscopic Rotator Cuff Repair: Where We Have Been and Where We Are Going
I am very honored that Dr. Rob Bell, past president of the American Shoulder and Elbow Surgeons, invited me to give last year’s Neer Lecture. Dr. Bell asked me to specifically address my role in the development of arthroscopic rotator cuff repair and to recount the significant resistance that the early arthroscopic shoulder surgeons faced from the shoulder establishment as we struggled to achieve mainstream acceptance for this new technology. Tasked with such a personal topic, I find myself in a position analogous to that of Winston Churchill at the end of World War II. When a journalist asked him to speculate on how historians would portray his role in the war, he replied without hesitation, “History will be kind to me because I intend to write it.”
So let’s start at the beginning. And for me it makes the most sense to travel back to the year I started my practice: 1981. The world then was very different from today’s world. On January 20, 1981, Ronald Reagan was inaugurated President of the United States. The same day, 52 US hostages in Iran were released after having been held captive for 442 days. In March 1981, Reagan survived an assassination attempt; 3 months earlier, John Lennon had not been so lucky. Lennon’s hit song “Starting Over” garnered the highest musical awards posthumously.
The world of shoulder surgery was also very different in 1981. The arthroscope was the “instrument of the devil,” according to Dr. Rockwood. And shoulder surgery was ruled by the Charlies—Dr. Charles Neer, Dr. Charlie Rockwood, and any other Charlie who felt compelled to marginalize shoulder arthroscopy.
My personal world in the early 1980s was daunting as well. I had just completed my residency at the Mayo Clinic and my sports medicine fellowship in Eugene, Oregon. I had a young son, a new daughter, and a new job with the San Antonio Orthopaedic Group. I had a new house with a 21% mortgage loan and a “new” used car with a 23% car loan.
I was simultaneously energized and intimidated by my new job, where I was doing general orthopedics with a “special interest” in shoulder surgery and sports medicine. I was initially very proud and humbled by the fact that my senior partners had entrusted me with the care of the most difficult shoulder cases within the practice. But that pride got cut down to its appropriate size the day after I had thanked one of my partners, Dr. Lamar Collie, for his confidence in my potential as a shoulder surgeon. Dr. Collie replied matter-of-factly, “Sure … but you need to understand that we always make the new guy the shoulder expert because shoulders never do worth a damn.”
For shoulder arthroscopy, the early 1980s were exciting. Most of us who were scoping shoulders had already been doing knee arthroscopy and were trying to adapt knee instruments to the shoulder. This worked for some simple excisional cases. For example, I recall excising the bucket-handle portion of a type III SLAP (superior labral tear from anterior to posterior) lesion in 1983. In general, however, shoulder problems were different from knee problems and usually involved repair rather than excision of damaged tissues. Therefore, the technology used in knee arthroscopy was often not directly transferable to the shoulder. Furthermore, treatment of the rotator cuff necessitated development of arthroscopic techniques in a virtual space, the subacromial space, and this was an entirely new arthroscopic concept.
Development of Arthroscopic Rotator Cuff Repair
A major mind-expanding turning point for me occurred in 1984 when I attended one of Dr. Jim Esch’s early San Diego shoulder courses. During that course, Dr. Harvard Ellman of Los Angeles demonstrated to me on a cadaver shoulder how he created a virtual subacromial working space that allowed enough visualization for an arthroscopic acromioplasty. At that moment, I knew that arthroscopic rotator cuff repair was just around the corner. Up until then, I had not been able to envision complex extra-articular reconstructive surgery, as all previous arthroscopic surgery had been intra-articular. But now, having realized a virtual working space could always be created, I knew it would be relatively straightforward to develop the portals to approach the cuff as well as the implants and the instruments to repair it. But I also knew that progression to all-arthroscopic repair techniques would have to be stepwise and that the final repair constructs would need to be at least as strong as those of open repair in order to be acceptable. With an undergraduate degree in mechanical engineering, I had a reasonably clear idea of the concepts I wanted to apply to the instrumentation and techniques, though I could never have envisioned how circuitous the route to the end result would be.
First Steps
I sketched out my ideas for arthroscopic suture passers and knot-tying instruments and presented them to a couple of the major arthroscopy companies in the United States, but the companies were not interested. They did not believe arthroscopy would have any meaningful applications in the shoulder. So, I enlisted the services of a local San Antonio aircraft machinist to fabricate instruments for me. By 1987, I was doing arthroscopic side-to-side margin convergence1 cuff repairs for U-shape tears on a regular basis. And I was doing these at the most hostile point in the universe for arthroscopic shoulder surgery: San Antonio, Texas.
Only a few surgeons were doing arthroscopic shoulder surgery in the 1980s and early 1990s, and without exception these surgeons became the leader-pioneers in the new discipline. In general, these were young surgeons who were in private practice and removed from academia and professional organizations, and thus relatively sheltered from the actions of the shoulder rule-makers of the day. They accepted their status as pariahs as they developed their techniques out of the view of mainstream orthopedics. These leaders included Jim Esch, Steve Snyder, Dick Caspari, Lanny Johnson, Gene Wolf, Gary Gartsman, Rob Bell, and Howard Sweeney. We shared our techniques and our ideas with one another, encouraged one another, and generally became good friends.
Thomas Kuhn, in his classic book The Structure of Scientific Revolutions,2 observed that paradigm shifts within a given field were usually achieved by practitioners who were either very young (naïve) or outside the established hierarchy in the field. The surgeons who contributed most to the shift of shoulder surgery from open to arthroscopic techniques were generally young men who were in private practice and had little to lose by inciting the disdain of the shoulder establishment. Predictably, resistance from the mainstream open shoulder surgeons increased as arthroscopic techniques became more successful and more threatening to the primacy of the open shoulder surgeons. The disdain yielded to disruption and finally to transformation as the paradigm shift occurred. The conflict between the open shoulder surgeons and the arthroscopic shoulder surgeons passed through all the phases that Mahatma Gandhi had described many years before. “First they ignore you; then they laugh at you; then they fight you; then you win.”
Building a Ship in a Bottle
At the start of the 1990s, I recognized that my progress in arthroscopic rotator cuff repair would be extremely slow unless I could find an industry partner who shared my vision for full-scale conversion to arthroscopic means of repair and would be willing to help make it a reality. In 1991, I happened to meet Reinhold Schmieding, the owner of Arthrex, a small arthroscopic device company in Naples, Florida. Reinhold invited me to visit him to discuss the feasibility of developing arthroscopic repair systems for the shoulder. At the time, the world headquarters of Arthrex was a 20×30-ft storage room in an office service center, and there were 2 employees. One employee, Don Grafton, was a talented engineer without medical experience. By the end of my first day there, Reinhold and Don and I had agreed that developing arthroscopic repair systems for shoulder instability and rotator cuff repair would become a top priority for Arthrex.
My initial bias toward arthroscopic cuff repair was that a transosseous bone tunnel technique not only would be possible but would be superior to suture anchor fixation. In fact, my first 2 patents with Arthrex were for instrumentation for an arthroscopic transosseous repair technique. I tested my hypothesis with 2 successive biomechanical studies. The first examined cyclic loading of bone tunnel repairs, and the second examined cyclic loading of anchor-based repairs.3,4 Evaluating the data from these 2 studies, I was surprised to find that anchor-based repairs were significantly stronger than bone tunnel repairs. In addition, anchors shifted the weak link from the bone–suture interface to the tendon–suture interface; in essence, anchors optimized bone fixation by shifting the weak link in the construct to the tendon. I was then completely convinced of the superiority of suture anchors over bone tunnels, and that conviction has become even stronger over the years. After these 2 cyclic loading studies, I shifted my focus, and that of Arthrex, toward arthroscopic suture anchor repair of the rotator cuff.
Reconciling Technique and Instrumentation With Anatomy and Biomechanics
Having recognized the importance of the rotator cable attachments both anatomically5 and biomechanically,6,7 I thought it important to reinforce them as a routine part of performing rotator cuff repairs. Our anatomical and biomechanical studies had had great translational implications in the development of our techniques and instrumentation.
As mentioned earlier, Don Grafton was the chief (and for a long time only) engineer at Arthrex. As he had no medical experience, I invited him to come to San Antonio to observe surgery. During Don’s many visits, I showed him pathology in the operating room and pointed out what I could do with the instruments I had and what I could not do. Then in the evening we went to my house and brainstormed how to perform the “missing” surgical manipulations, how to improve manipulations that were suboptimal, and how to optimize final surgical constructs.
Passing suture through tendon was an early challenge. One must remember that, in the early 1990s, it was not possible for machinists to fabricate complex shapes. Therefore, straight tubular retrograde suture passers were the logical first option. We initially developed spring-loaded retrograde hook retrievers (Figure 1) and then curved suture hooks with shuttling wires (Lasso). To me, the most unappealing feature of retrograde suture passage was the oblique angle of approach through the tendon, which caused a length–tension mismatch between the upper fibers and the lower fibers of the muscle–tendon unit. We recognized we could eliminate the mismatch if we passed the suture antegrade, such that it would pass perpendicular to the tendon fibers. These insights and efforts culminated in development of the Viper suture passer and then the FastPass Scorpion suture passer, which has a spring-loaded trapdoor on the upper jaw for ergonomic self-retrieving of the suture once it is passed through the tendon.
To develop a knot pusher that optimized knot tying (yielding the highest knot security and the tightest loop security), we used prototype instruments to tie and test literally thousands of knots in the laboratory. We were thus able to verify that the Surgeon’s Sixth Finger Knot Pusher (Arthrex) reproducibly tied optimized knots8,9 and also optimized knot fixation and bone fixation. However, our suture was not yet optimized and was prone to breakage, and our suture–tendon interface was not yet optimized. Clearly, improvement was needed in 2 more areas.
Don came up with the idea for a virtually unbreakable suture and developed that idea into FiberWire.10 Shortly thereafter, I contributed the idea and design for FiberTape, which dramatically enhanced suture pullout strength and footprint compression.
Anchor designs improved rapidly and dramatically. We made the second-generation BioCorkscrew fully threaded, which virtually eliminated anchor failure, even in soft bone.
Optimization of the suture–tendon interface took a giant step forward when Park and colleagues11,12 introduced linked double-row rotator cuff repair. Much as with a Chinese finger trap, the harder you pull, the stronger it becomes, with yield load approaching ultimate load.
At this point, it seemed we had optimized virtually every segment of the rotator cuff repair construct. Each component was just about as good as it could be. Or was it?
The Accidental Quest for Knotless Fixation
In November 1998, I made my first trip to China as a guest speaker at the Congress of the Hong Kong Orthopaedic Association. My first view of the magnificent Hong Kong skyline across Victoria Harbour was truly breathtaking. As I admired the gleaming glass towers and the concrete canyons of the city, I had no idea that the very next day these modern skyscrapers would reveal an ancient secret that would change my approach to arthroscopic rotator cuff repair.
The day after my arrival, Dr. James Lam took me to lunch. As we approached the restaurant, he pointed across the street to a tall building that was being renovated and had scaffolding supporting workers alongside the first 9 stories of the exterior wall. Dr. Lam said that, after lunch, he would take me to the construction site for a closer look at the scaffolding.
After lunch, we walked to the base of the scaffolding. Dr. Lam told me it was constructed entirely of bamboo poles held together with lashings but no knots (Figure 2). Lashings were secured by turning them back on themselves and wrapping them in an entirely knotless manner.13 I found it incredible that this knotless fixation was so secure that it could support the weight of workers many stories above the ground. I resolved to determine how this fixation method worked and see if the same mechanism might help us achieve reliable knotless fixation in surgery.
When I returned home, I broke out my college engineering books and reacquainted myself with the concept of cable friction. As has happened so often in the past, however, it took a practical lesson from the ranch to truly illustrate for me how cable friction works.
Every cowboy knows that a spirited horse cannot be restrained with only one lead rope. However, a cowboy can wrap a lead rope around a “snubbing post” and thereby gain complete control over the animal, despite the horse’s superior size and strength. The cable friction between the rope and the post creates such a large restraining force that the cowboy can easily hold the animal without the help of a knotted rope (Figure 3). In similar fashion in the Hong Kong scaffolding, fixation strength results from the significant amount of cable friction produced when the lashings wrap around one another and around the bamboo poles.
The cable friction concept was pivotal in the development of knotless fixation in arthroscopic rotator cuff repair. In lateral row fixation, the eyelet of the PushLock and SwiveLock suture anchors (Arthrex) produces significant cable friction at the eyelet–suture interface, in addition to frictional force wedging the suture between anchor and bone.
As with so many other devices in shoulder arthroscopy, the SwiveLock suture anchor developed in stages. In the first stage, a chainlike suture with consecutive intersecting links was used (FiberChain). The idea for an adjustable fixation construct came to me because I thought that a forked eyelet on a SwiveLock would provide a firm fixation point when inserted into the appropriate suture link, yet would be totally adjustable simply by choosing a tighter or looser link (Figure 4). Although the system worked very well, it was technically challenging. The process was greatly simplified after Don Grafton and I developed FiberTape and recognized that the power of cable friction was dramatically increased by the larger contact area between the eyelet and the braided FiberTape. The SpeedBridge construct (Arthrex), which enhanced cable friction fixation by means of passing FiberTape through the anchor eyelets, also provided a larger compressive interface at the repair site by using FiberTape rather than conventional suture. These incremental improvements led to what I would characterize as today’s gold standard for arthroscopic rotator cuff repair: a largely knotless linked double-row construct using FiberTape, with cinch-loop sutures at the anterior and posterior margins of the tear to reinforce the cable attachments and simultaneously reduce the dog-ears that typically occur in those locations, and a double-pulley medial mattress if tendon quality is poor (Figure 5).
The Burden of Craft
With all the recent enthusiasm for level I studies, I think we need to examine whether they will accelerate technological advancement in rotator cuff repair. The answer, in my opinion, is a resounding no. This answer is based on a major disconnect I have detected in how we evaluate these studies in rotator cuff disease and repair.
An irony related to technological advancement in surgery is that the more technically advanced the surgery becomes, the more skill is required. This fact is completely at odds with the public’s perception that technological advances make procedures easier. In arthroscopic rotator cuff repair, the surgeon must look, feel, and be aware to a greater degree than in open surgery.
Edward Tenner, in his book Why Things Bite Back, described the burden of the practitioner of any advanced technology as the burden of craft.14 The burden of craft is the inherent demand on all craftspeople, but particularly surgeons, to “up our game” if we are to be successful in our craft. For arthroscopic rotator cuff repair, the burden of craft requires patience, attention to detail, and the ability to work in a virtual space. Not everyone has these skills. But anyone who wants to practice in this discipline has an obligation to learn the skills required, and then to teach them to others and assess how well they are being applied.
The problem with relying on level I studies to assess the efficacy of a surgical procedure is that they are inherently biased by the surgeons involved. As results depend on surgeons’ skills, and surgeons’ skill levels are not equal, level I studies cannot prove what is possible, cannot demonstrate the limits of a technique, and cannot demonstrate the equivalence of techniques.
Amazingly enough, there are still rotator cuff repair “deniers” who confidently assert from the podium that a large percentage of massive cuff tears cannot be repaired and that, even if they can be repaired, they do not have the biological potential to heal. Given the disparity in surgeons’ skills and results, however, one must ask whether poor results are a consequence of a biological deficit in the patient, or of a skill deficit in the surgeon.
What I know is that we have techniques for predictable arthroscopic repair and healing of the vast majority of rotator cuff tears, even massive tears,15-17 and patients do very well clinically. Yet, among many orthopedic surgeons, there is a trend to go straight to reverse total shoulder arthroplasty (rTSA) for massive tears—despite the evidence against it. As reported in the literature, rTSA results are not as good as arthroscopic cuff repair results, and the complication rate for rTSA is much higher.
Why has this trend toward rTSA for massive tears gained so much momentum? The only reason I can surmise is that, for the average surgeon, rTSA is easier and quicker than arthroscopic repair for massive tears. But the reason for choosing a specific type of surgery for a given problem should not be that it is easiest for the surgeon; it should be that it is best for the patient.
The surgeon should start by asking what procedure he or she would want if the roles were reversed—if the surgeon were the patient with the massive rotator cuff tear. If a surgeon does not have the skill set for the best procedure for a particular patient, he or she is obligated to send that patient to a surgeon who does have the skills. In addition, given that infection is the most feared complication in most shoulder surgeries, the surgeon should ask which infection rate would be personally acceptable. Arthroscopic rotator cuff repair has a reported infection rate of 1.6 per 1000, or .0016,18 whereas rTSA has an infection rate about 25 times higher, or .04.19 Further, the surgeon must consider the relative severity of the consequences of infection. By any measure, an infected arthroscopy is a straightforward treatable complication, but an infected shoulder replacement is a human tragedy. Patients vastly prefer the minimally invasive arthroscopic approach, and through online searches can easily identify who can offer an arthroscopic solution.
To reproducibly achieve successful arthroscopic repair of massive rotator cuff tears, the surgeon must know advanced techniques, including subscapularis repair techniques,20,21 interval slides,22,23 and self-reinforcing constructs.24,25
“It’s a poor carpenter who blames his tools.” This 18th-century English proverb is as true today as it was 300 years ago. The tools for arthroscopic cuff repair exist, and they are excellent. The burden of craft is the surgeon’s burden and obligation. As surgeons, we must accept that obligation and the responsibility of that burden.
As mentioned earlier, Dr. Rob Bell’s charge to me when he invited me to give the Neer Lecture was to sum up my involvement in the development of arthroscopic shoulder surgery. The short version is that I have been doing shoulder arthroscopy for 31 years; have received 28 US patents related to shoulder instruments and implants and have 12 US patents pending; have published 167 peer-reviewed articles, a couple dozen book chapters, and 2 textbooks on shoulder arthroscopy; have trained 25 fellows; and have hosted approximately 3000 visiting surgeons in my operating room. My greatest professional dream was to see the standard of care for rotator cuff repair and shoulder instability transition from open to arthroscopic techniques, and I have been fortunate enough to have observed that paradigm shift during my career.
What do I envision over the next 31 years? As we all know, history runs in both directions, and some things simply have not happened yet. In terms of rotator cuff treatment, I think over the next few years the guiding principle of treatment will be joint preservation. All rotator cuff tears, even massive tears, will be repaired arthroscopically. Patients and insurers will demand arthroscopic repair, and surgeons without the skill set will migrate to other subspecialties. As for the role of arthroplasty in the treatment of rotator cuff tears, rTSA will be indicated only for pseudoparalysis after failed cuff repair in low-demand elderly patients.
In rotator cuff treatment, I envision a standard of care that is almost entirely arthroscopic. This standard will demand that surgeons who treat rotator cuff tears be proficient in arthroscopic repair of the full range of tears. Acquiring the skills for arthroscopic repair may not be easy, but then “there’s the easy way, and there’s the Cowboy Way.” As my dad used to tell me when I complained about working too hard, “No man ever drowned in his own sweat.” We shoulder surgeons must accept the burden of craft that accompanies the new standard of arthroscopic cuff repair, and we must offer our patients the same level of care we would choose for ourselves.
Happy trails!
1. Burkhart SS, Athanasiou KA, Wirth MA. Margin convergence: a method of reducing strain in massive rotator cuff tears. Arthroscopy. 1996:12(3):335-338.
2. Kuhn TS. The Structure of Scientific Revolutions. Chicago, IL: University of Chicago Press; 1962.
3. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176.
4. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athansiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
5. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
6. Burkhart SS, Nottage WM, Ogilvie-Harris DJ, Kohn HS, Pachelli A. Partial repair of irreparable rotator cuff tears. Arthroscopy. 1994;10(4):363-370.
7. Halder AM, O’Driscoll SW, Heers G, et al. Biomechanical comparison of effects of supraspinatus tendon detachments, tendon defects, and muscle retractions. J Bone Joint Surg Am. 2002;84(5):780-785.
8. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
9. Lo IK, Ochoa E Jr, Burkhart SS. A comparison of knot security and loop security in arthroscopic knots tied with newer high-strength suture materials. Arthroscopy. 2010;26(9 suppl):S120-S126.
10. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
11. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a “transosseous-equivalent” rotator cuff repair technique compared to a double-row technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
12. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
13. Burkhart SS, Athanasiou KA. The twist-lock concept of tissue transport and suture fixation without knots: observations along the Hong Kong skyline. Arthroscopy. 2003;19(6):613-625.
14. Tenner E. Why Things Bite Back. New York, NY: Random House; 1996.
15. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of arthroscopic massive rotator cuff repair: the importance of double-row fixation. Arthroscopy. 2012;28(7):909-915.
16. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
17. Lädermann A, Denard PJ, Burkhart SS. Revision arthroscopic rotator cuff repair: systematic review and authors’ preferred surgical technique. Arthroscopy. 2012;28(8):1160-1169.
18. Randelli P, Castagna A, Cabitza F, Cabitza P, Arrigoni P, Denti M. Infectious and thromboembolic complications of arthroscopic shoulder surgery. J Shoulder Elbow Surg. 2010;19(1):97-101.
19. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
20. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
21. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
22. Lo IK, Burkhart SS. Arthroscopic repair of massive, contracted, immobile rotator cuff tears using single and double interval slides: technique and preliminary results. Arthroscopy. 2004;20(1):22-33.
23. Lo IK, Burkhart SS. The interval slide in continuity: a method of mobilizing the anterosuperior rotator cuff without disrupting the tear margins. Arthroscopy. 2004;20(4):435-441.
24. Denard PJ, Burkhart SS. Techniques for managing poor quality tissue and bone during arthroscopic rotator cuff repair. Arthroscopy. 2011;27(10):1409-1421.
25. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
I am very honored that Dr. Rob Bell, past president of the American Shoulder and Elbow Surgeons, invited me to give last year’s Neer Lecture. Dr. Bell asked me to specifically address my role in the development of arthroscopic rotator cuff repair and to recount the significant resistance that the early arthroscopic shoulder surgeons faced from the shoulder establishment as we struggled to achieve mainstream acceptance for this new technology. Tasked with such a personal topic, I find myself in a position analogous to that of Winston Churchill at the end of World War II. When a journalist asked him to speculate on how historians would portray his role in the war, he replied without hesitation, “History will be kind to me because I intend to write it.”
So let’s start at the beginning. And for me it makes the most sense to travel back to the year I started my practice: 1981. The world then was very different from today’s world. On January 20, 1981, Ronald Reagan was inaugurated President of the United States. The same day, 52 US hostages in Iran were released after having been held captive for 442 days. In March 1981, Reagan survived an assassination attempt; 3 months earlier, John Lennon had not been so lucky. Lennon’s hit song “Starting Over” garnered the highest musical awards posthumously.
The world of shoulder surgery was also very different in 1981. The arthroscope was the “instrument of the devil,” according to Dr. Rockwood. And shoulder surgery was ruled by the Charlies—Dr. Charles Neer, Dr. Charlie Rockwood, and any other Charlie who felt compelled to marginalize shoulder arthroscopy.
My personal world in the early 1980s was daunting as well. I had just completed my residency at the Mayo Clinic and my sports medicine fellowship in Eugene, Oregon. I had a young son, a new daughter, and a new job with the San Antonio Orthopaedic Group. I had a new house with a 21% mortgage loan and a “new” used car with a 23% car loan.
I was simultaneously energized and intimidated by my new job, where I was doing general orthopedics with a “special interest” in shoulder surgery and sports medicine. I was initially very proud and humbled by the fact that my senior partners had entrusted me with the care of the most difficult shoulder cases within the practice. But that pride got cut down to its appropriate size the day after I had thanked one of my partners, Dr. Lamar Collie, for his confidence in my potential as a shoulder surgeon. Dr. Collie replied matter-of-factly, “Sure … but you need to understand that we always make the new guy the shoulder expert because shoulders never do worth a damn.”
For shoulder arthroscopy, the early 1980s were exciting. Most of us who were scoping shoulders had already been doing knee arthroscopy and were trying to adapt knee instruments to the shoulder. This worked for some simple excisional cases. For example, I recall excising the bucket-handle portion of a type III SLAP (superior labral tear from anterior to posterior) lesion in 1983. In general, however, shoulder problems were different from knee problems and usually involved repair rather than excision of damaged tissues. Therefore, the technology used in knee arthroscopy was often not directly transferable to the shoulder. Furthermore, treatment of the rotator cuff necessitated development of arthroscopic techniques in a virtual space, the subacromial space, and this was an entirely new arthroscopic concept.
Development of Arthroscopic Rotator Cuff Repair
A major mind-expanding turning point for me occurred in 1984 when I attended one of Dr. Jim Esch’s early San Diego shoulder courses. During that course, Dr. Harvard Ellman of Los Angeles demonstrated to me on a cadaver shoulder how he created a virtual subacromial working space that allowed enough visualization for an arthroscopic acromioplasty. At that moment, I knew that arthroscopic rotator cuff repair was just around the corner. Up until then, I had not been able to envision complex extra-articular reconstructive surgery, as all previous arthroscopic surgery had been intra-articular. But now, having realized a virtual working space could always be created, I knew it would be relatively straightforward to develop the portals to approach the cuff as well as the implants and the instruments to repair it. But I also knew that progression to all-arthroscopic repair techniques would have to be stepwise and that the final repair constructs would need to be at least as strong as those of open repair in order to be acceptable. With an undergraduate degree in mechanical engineering, I had a reasonably clear idea of the concepts I wanted to apply to the instrumentation and techniques, though I could never have envisioned how circuitous the route to the end result would be.
First Steps
I sketched out my ideas for arthroscopic suture passers and knot-tying instruments and presented them to a couple of the major arthroscopy companies in the United States, but the companies were not interested. They did not believe arthroscopy would have any meaningful applications in the shoulder. So, I enlisted the services of a local San Antonio aircraft machinist to fabricate instruments for me. By 1987, I was doing arthroscopic side-to-side margin convergence1 cuff repairs for U-shape tears on a regular basis. And I was doing these at the most hostile point in the universe for arthroscopic shoulder surgery: San Antonio, Texas.
Only a few surgeons were doing arthroscopic shoulder surgery in the 1980s and early 1990s, and without exception these surgeons became the leader-pioneers in the new discipline. In general, these were young surgeons who were in private practice and removed from academia and professional organizations, and thus relatively sheltered from the actions of the shoulder rule-makers of the day. They accepted their status as pariahs as they developed their techniques out of the view of mainstream orthopedics. These leaders included Jim Esch, Steve Snyder, Dick Caspari, Lanny Johnson, Gene Wolf, Gary Gartsman, Rob Bell, and Howard Sweeney. We shared our techniques and our ideas with one another, encouraged one another, and generally became good friends.
Thomas Kuhn, in his classic book The Structure of Scientific Revolutions,2 observed that paradigm shifts within a given field were usually achieved by practitioners who were either very young (naïve) or outside the established hierarchy in the field. The surgeons who contributed most to the shift of shoulder surgery from open to arthroscopic techniques were generally young men who were in private practice and had little to lose by inciting the disdain of the shoulder establishment. Predictably, resistance from the mainstream open shoulder surgeons increased as arthroscopic techniques became more successful and more threatening to the primacy of the open shoulder surgeons. The disdain yielded to disruption and finally to transformation as the paradigm shift occurred. The conflict between the open shoulder surgeons and the arthroscopic shoulder surgeons passed through all the phases that Mahatma Gandhi had described many years before. “First they ignore you; then they laugh at you; then they fight you; then you win.”
Building a Ship in a Bottle
At the start of the 1990s, I recognized that my progress in arthroscopic rotator cuff repair would be extremely slow unless I could find an industry partner who shared my vision for full-scale conversion to arthroscopic means of repair and would be willing to help make it a reality. In 1991, I happened to meet Reinhold Schmieding, the owner of Arthrex, a small arthroscopic device company in Naples, Florida. Reinhold invited me to visit him to discuss the feasibility of developing arthroscopic repair systems for the shoulder. At the time, the world headquarters of Arthrex was a 20×30-ft storage room in an office service center, and there were 2 employees. One employee, Don Grafton, was a talented engineer without medical experience. By the end of my first day there, Reinhold and Don and I had agreed that developing arthroscopic repair systems for shoulder instability and rotator cuff repair would become a top priority for Arthrex.
My initial bias toward arthroscopic cuff repair was that a transosseous bone tunnel technique not only would be possible but would be superior to suture anchor fixation. In fact, my first 2 patents with Arthrex were for instrumentation for an arthroscopic transosseous repair technique. I tested my hypothesis with 2 successive biomechanical studies. The first examined cyclic loading of bone tunnel repairs, and the second examined cyclic loading of anchor-based repairs.3,4 Evaluating the data from these 2 studies, I was surprised to find that anchor-based repairs were significantly stronger than bone tunnel repairs. In addition, anchors shifted the weak link from the bone–suture interface to the tendon–suture interface; in essence, anchors optimized bone fixation by shifting the weak link in the construct to the tendon. I was then completely convinced of the superiority of suture anchors over bone tunnels, and that conviction has become even stronger over the years. After these 2 cyclic loading studies, I shifted my focus, and that of Arthrex, toward arthroscopic suture anchor repair of the rotator cuff.
Reconciling Technique and Instrumentation With Anatomy and Biomechanics
Having recognized the importance of the rotator cable attachments both anatomically5 and biomechanically,6,7 I thought it important to reinforce them as a routine part of performing rotator cuff repairs. Our anatomical and biomechanical studies had had great translational implications in the development of our techniques and instrumentation.
As mentioned earlier, Don Grafton was the chief (and for a long time only) engineer at Arthrex. As he had no medical experience, I invited him to come to San Antonio to observe surgery. During Don’s many visits, I showed him pathology in the operating room and pointed out what I could do with the instruments I had and what I could not do. Then in the evening we went to my house and brainstormed how to perform the “missing” surgical manipulations, how to improve manipulations that were suboptimal, and how to optimize final surgical constructs.
Passing suture through tendon was an early challenge. One must remember that, in the early 1990s, it was not possible for machinists to fabricate complex shapes. Therefore, straight tubular retrograde suture passers were the logical first option. We initially developed spring-loaded retrograde hook retrievers (Figure 1) and then curved suture hooks with shuttling wires (Lasso). To me, the most unappealing feature of retrograde suture passage was the oblique angle of approach through the tendon, which caused a length–tension mismatch between the upper fibers and the lower fibers of the muscle–tendon unit. We recognized we could eliminate the mismatch if we passed the suture antegrade, such that it would pass perpendicular to the tendon fibers. These insights and efforts culminated in development of the Viper suture passer and then the FastPass Scorpion suture passer, which has a spring-loaded trapdoor on the upper jaw for ergonomic self-retrieving of the suture once it is passed through the tendon.
To develop a knot pusher that optimized knot tying (yielding the highest knot security and the tightest loop security), we used prototype instruments to tie and test literally thousands of knots in the laboratory. We were thus able to verify that the Surgeon’s Sixth Finger Knot Pusher (Arthrex) reproducibly tied optimized knots8,9 and also optimized knot fixation and bone fixation. However, our suture was not yet optimized and was prone to breakage, and our suture–tendon interface was not yet optimized. Clearly, improvement was needed in 2 more areas.
Don came up with the idea for a virtually unbreakable suture and developed that idea into FiberWire.10 Shortly thereafter, I contributed the idea and design for FiberTape, which dramatically enhanced suture pullout strength and footprint compression.
Anchor designs improved rapidly and dramatically. We made the second-generation BioCorkscrew fully threaded, which virtually eliminated anchor failure, even in soft bone.
Optimization of the suture–tendon interface took a giant step forward when Park and colleagues11,12 introduced linked double-row rotator cuff repair. Much as with a Chinese finger trap, the harder you pull, the stronger it becomes, with yield load approaching ultimate load.
At this point, it seemed we had optimized virtually every segment of the rotator cuff repair construct. Each component was just about as good as it could be. Or was it?
The Accidental Quest for Knotless Fixation
In November 1998, I made my first trip to China as a guest speaker at the Congress of the Hong Kong Orthopaedic Association. My first view of the magnificent Hong Kong skyline across Victoria Harbour was truly breathtaking. As I admired the gleaming glass towers and the concrete canyons of the city, I had no idea that the very next day these modern skyscrapers would reveal an ancient secret that would change my approach to arthroscopic rotator cuff repair.
The day after my arrival, Dr. James Lam took me to lunch. As we approached the restaurant, he pointed across the street to a tall building that was being renovated and had scaffolding supporting workers alongside the first 9 stories of the exterior wall. Dr. Lam said that, after lunch, he would take me to the construction site for a closer look at the scaffolding.
After lunch, we walked to the base of the scaffolding. Dr. Lam told me it was constructed entirely of bamboo poles held together with lashings but no knots (Figure 2). Lashings were secured by turning them back on themselves and wrapping them in an entirely knotless manner.13 I found it incredible that this knotless fixation was so secure that it could support the weight of workers many stories above the ground. I resolved to determine how this fixation method worked and see if the same mechanism might help us achieve reliable knotless fixation in surgery.
When I returned home, I broke out my college engineering books and reacquainted myself with the concept of cable friction. As has happened so often in the past, however, it took a practical lesson from the ranch to truly illustrate for me how cable friction works.
Every cowboy knows that a spirited horse cannot be restrained with only one lead rope. However, a cowboy can wrap a lead rope around a “snubbing post” and thereby gain complete control over the animal, despite the horse’s superior size and strength. The cable friction between the rope and the post creates such a large restraining force that the cowboy can easily hold the animal without the help of a knotted rope (Figure 3). In similar fashion in the Hong Kong scaffolding, fixation strength results from the significant amount of cable friction produced when the lashings wrap around one another and around the bamboo poles.
The cable friction concept was pivotal in the development of knotless fixation in arthroscopic rotator cuff repair. In lateral row fixation, the eyelet of the PushLock and SwiveLock suture anchors (Arthrex) produces significant cable friction at the eyelet–suture interface, in addition to frictional force wedging the suture between anchor and bone.
As with so many other devices in shoulder arthroscopy, the SwiveLock suture anchor developed in stages. In the first stage, a chainlike suture with consecutive intersecting links was used (FiberChain). The idea for an adjustable fixation construct came to me because I thought that a forked eyelet on a SwiveLock would provide a firm fixation point when inserted into the appropriate suture link, yet would be totally adjustable simply by choosing a tighter or looser link (Figure 4). Although the system worked very well, it was technically challenging. The process was greatly simplified after Don Grafton and I developed FiberTape and recognized that the power of cable friction was dramatically increased by the larger contact area between the eyelet and the braided FiberTape. The SpeedBridge construct (Arthrex), which enhanced cable friction fixation by means of passing FiberTape through the anchor eyelets, also provided a larger compressive interface at the repair site by using FiberTape rather than conventional suture. These incremental improvements led to what I would characterize as today’s gold standard for arthroscopic rotator cuff repair: a largely knotless linked double-row construct using FiberTape, with cinch-loop sutures at the anterior and posterior margins of the tear to reinforce the cable attachments and simultaneously reduce the dog-ears that typically occur in those locations, and a double-pulley medial mattress if tendon quality is poor (Figure 5).
The Burden of Craft
With all the recent enthusiasm for level I studies, I think we need to examine whether they will accelerate technological advancement in rotator cuff repair. The answer, in my opinion, is a resounding no. This answer is based on a major disconnect I have detected in how we evaluate these studies in rotator cuff disease and repair.
An irony related to technological advancement in surgery is that the more technically advanced the surgery becomes, the more skill is required. This fact is completely at odds with the public’s perception that technological advances make procedures easier. In arthroscopic rotator cuff repair, the surgeon must look, feel, and be aware to a greater degree than in open surgery.
Edward Tenner, in his book Why Things Bite Back, described the burden of the practitioner of any advanced technology as the burden of craft.14 The burden of craft is the inherent demand on all craftspeople, but particularly surgeons, to “up our game” if we are to be successful in our craft. For arthroscopic rotator cuff repair, the burden of craft requires patience, attention to detail, and the ability to work in a virtual space. Not everyone has these skills. But anyone who wants to practice in this discipline has an obligation to learn the skills required, and then to teach them to others and assess how well they are being applied.
The problem with relying on level I studies to assess the efficacy of a surgical procedure is that they are inherently biased by the surgeons involved. As results depend on surgeons’ skills, and surgeons’ skill levels are not equal, level I studies cannot prove what is possible, cannot demonstrate the limits of a technique, and cannot demonstrate the equivalence of techniques.
Amazingly enough, there are still rotator cuff repair “deniers” who confidently assert from the podium that a large percentage of massive cuff tears cannot be repaired and that, even if they can be repaired, they do not have the biological potential to heal. Given the disparity in surgeons’ skills and results, however, one must ask whether poor results are a consequence of a biological deficit in the patient, or of a skill deficit in the surgeon.
What I know is that we have techniques for predictable arthroscopic repair and healing of the vast majority of rotator cuff tears, even massive tears,15-17 and patients do very well clinically. Yet, among many orthopedic surgeons, there is a trend to go straight to reverse total shoulder arthroplasty (rTSA) for massive tears—despite the evidence against it. As reported in the literature, rTSA results are not as good as arthroscopic cuff repair results, and the complication rate for rTSA is much higher.
Why has this trend toward rTSA for massive tears gained so much momentum? The only reason I can surmise is that, for the average surgeon, rTSA is easier and quicker than arthroscopic repair for massive tears. But the reason for choosing a specific type of surgery for a given problem should not be that it is easiest for the surgeon; it should be that it is best for the patient.
The surgeon should start by asking what procedure he or she would want if the roles were reversed—if the surgeon were the patient with the massive rotator cuff tear. If a surgeon does not have the skill set for the best procedure for a particular patient, he or she is obligated to send that patient to a surgeon who does have the skills. In addition, given that infection is the most feared complication in most shoulder surgeries, the surgeon should ask which infection rate would be personally acceptable. Arthroscopic rotator cuff repair has a reported infection rate of 1.6 per 1000, or .0016,18 whereas rTSA has an infection rate about 25 times higher, or .04.19 Further, the surgeon must consider the relative severity of the consequences of infection. By any measure, an infected arthroscopy is a straightforward treatable complication, but an infected shoulder replacement is a human tragedy. Patients vastly prefer the minimally invasive arthroscopic approach, and through online searches can easily identify who can offer an arthroscopic solution.
To reproducibly achieve successful arthroscopic repair of massive rotator cuff tears, the surgeon must know advanced techniques, including subscapularis repair techniques,20,21 interval slides,22,23 and self-reinforcing constructs.24,25
“It’s a poor carpenter who blames his tools.” This 18th-century English proverb is as true today as it was 300 years ago. The tools for arthroscopic cuff repair exist, and they are excellent. The burden of craft is the surgeon’s burden and obligation. As surgeons, we must accept that obligation and the responsibility of that burden.
As mentioned earlier, Dr. Rob Bell’s charge to me when he invited me to give the Neer Lecture was to sum up my involvement in the development of arthroscopic shoulder surgery. The short version is that I have been doing shoulder arthroscopy for 31 years; have received 28 US patents related to shoulder instruments and implants and have 12 US patents pending; have published 167 peer-reviewed articles, a couple dozen book chapters, and 2 textbooks on shoulder arthroscopy; have trained 25 fellows; and have hosted approximately 3000 visiting surgeons in my operating room. My greatest professional dream was to see the standard of care for rotator cuff repair and shoulder instability transition from open to arthroscopic techniques, and I have been fortunate enough to have observed that paradigm shift during my career.
What do I envision over the next 31 years? As we all know, history runs in both directions, and some things simply have not happened yet. In terms of rotator cuff treatment, I think over the next few years the guiding principle of treatment will be joint preservation. All rotator cuff tears, even massive tears, will be repaired arthroscopically. Patients and insurers will demand arthroscopic repair, and surgeons without the skill set will migrate to other subspecialties. As for the role of arthroplasty in the treatment of rotator cuff tears, rTSA will be indicated only for pseudoparalysis after failed cuff repair in low-demand elderly patients.
In rotator cuff treatment, I envision a standard of care that is almost entirely arthroscopic. This standard will demand that surgeons who treat rotator cuff tears be proficient in arthroscopic repair of the full range of tears. Acquiring the skills for arthroscopic repair may not be easy, but then “there’s the easy way, and there’s the Cowboy Way.” As my dad used to tell me when I complained about working too hard, “No man ever drowned in his own sweat.” We shoulder surgeons must accept the burden of craft that accompanies the new standard of arthroscopic cuff repair, and we must offer our patients the same level of care we would choose for ourselves.
Happy trails!
I am very honored that Dr. Rob Bell, past president of the American Shoulder and Elbow Surgeons, invited me to give last year’s Neer Lecture. Dr. Bell asked me to specifically address my role in the development of arthroscopic rotator cuff repair and to recount the significant resistance that the early arthroscopic shoulder surgeons faced from the shoulder establishment as we struggled to achieve mainstream acceptance for this new technology. Tasked with such a personal topic, I find myself in a position analogous to that of Winston Churchill at the end of World War II. When a journalist asked him to speculate on how historians would portray his role in the war, he replied without hesitation, “History will be kind to me because I intend to write it.”
So let’s start at the beginning. And for me it makes the most sense to travel back to the year I started my practice: 1981. The world then was very different from today’s world. On January 20, 1981, Ronald Reagan was inaugurated President of the United States. The same day, 52 US hostages in Iran were released after having been held captive for 442 days. In March 1981, Reagan survived an assassination attempt; 3 months earlier, John Lennon had not been so lucky. Lennon’s hit song “Starting Over” garnered the highest musical awards posthumously.
The world of shoulder surgery was also very different in 1981. The arthroscope was the “instrument of the devil,” according to Dr. Rockwood. And shoulder surgery was ruled by the Charlies—Dr. Charles Neer, Dr. Charlie Rockwood, and any other Charlie who felt compelled to marginalize shoulder arthroscopy.
My personal world in the early 1980s was daunting as well. I had just completed my residency at the Mayo Clinic and my sports medicine fellowship in Eugene, Oregon. I had a young son, a new daughter, and a new job with the San Antonio Orthopaedic Group. I had a new house with a 21% mortgage loan and a “new” used car with a 23% car loan.
I was simultaneously energized and intimidated by my new job, where I was doing general orthopedics with a “special interest” in shoulder surgery and sports medicine. I was initially very proud and humbled by the fact that my senior partners had entrusted me with the care of the most difficult shoulder cases within the practice. But that pride got cut down to its appropriate size the day after I had thanked one of my partners, Dr. Lamar Collie, for his confidence in my potential as a shoulder surgeon. Dr. Collie replied matter-of-factly, “Sure … but you need to understand that we always make the new guy the shoulder expert because shoulders never do worth a damn.”
For shoulder arthroscopy, the early 1980s were exciting. Most of us who were scoping shoulders had already been doing knee arthroscopy and were trying to adapt knee instruments to the shoulder. This worked for some simple excisional cases. For example, I recall excising the bucket-handle portion of a type III SLAP (superior labral tear from anterior to posterior) lesion in 1983. In general, however, shoulder problems were different from knee problems and usually involved repair rather than excision of damaged tissues. Therefore, the technology used in knee arthroscopy was often not directly transferable to the shoulder. Furthermore, treatment of the rotator cuff necessitated development of arthroscopic techniques in a virtual space, the subacromial space, and this was an entirely new arthroscopic concept.
Development of Arthroscopic Rotator Cuff Repair
A major mind-expanding turning point for me occurred in 1984 when I attended one of Dr. Jim Esch’s early San Diego shoulder courses. During that course, Dr. Harvard Ellman of Los Angeles demonstrated to me on a cadaver shoulder how he created a virtual subacromial working space that allowed enough visualization for an arthroscopic acromioplasty. At that moment, I knew that arthroscopic rotator cuff repair was just around the corner. Up until then, I had not been able to envision complex extra-articular reconstructive surgery, as all previous arthroscopic surgery had been intra-articular. But now, having realized a virtual working space could always be created, I knew it would be relatively straightforward to develop the portals to approach the cuff as well as the implants and the instruments to repair it. But I also knew that progression to all-arthroscopic repair techniques would have to be stepwise and that the final repair constructs would need to be at least as strong as those of open repair in order to be acceptable. With an undergraduate degree in mechanical engineering, I had a reasonably clear idea of the concepts I wanted to apply to the instrumentation and techniques, though I could never have envisioned how circuitous the route to the end result would be.
First Steps
I sketched out my ideas for arthroscopic suture passers and knot-tying instruments and presented them to a couple of the major arthroscopy companies in the United States, but the companies were not interested. They did not believe arthroscopy would have any meaningful applications in the shoulder. So, I enlisted the services of a local San Antonio aircraft machinist to fabricate instruments for me. By 1987, I was doing arthroscopic side-to-side margin convergence1 cuff repairs for U-shape tears on a regular basis. And I was doing these at the most hostile point in the universe for arthroscopic shoulder surgery: San Antonio, Texas.
Only a few surgeons were doing arthroscopic shoulder surgery in the 1980s and early 1990s, and without exception these surgeons became the leader-pioneers in the new discipline. In general, these were young surgeons who were in private practice and removed from academia and professional organizations, and thus relatively sheltered from the actions of the shoulder rule-makers of the day. They accepted their status as pariahs as they developed their techniques out of the view of mainstream orthopedics. These leaders included Jim Esch, Steve Snyder, Dick Caspari, Lanny Johnson, Gene Wolf, Gary Gartsman, Rob Bell, and Howard Sweeney. We shared our techniques and our ideas with one another, encouraged one another, and generally became good friends.
Thomas Kuhn, in his classic book The Structure of Scientific Revolutions,2 observed that paradigm shifts within a given field were usually achieved by practitioners who were either very young (naïve) or outside the established hierarchy in the field. The surgeons who contributed most to the shift of shoulder surgery from open to arthroscopic techniques were generally young men who were in private practice and had little to lose by inciting the disdain of the shoulder establishment. Predictably, resistance from the mainstream open shoulder surgeons increased as arthroscopic techniques became more successful and more threatening to the primacy of the open shoulder surgeons. The disdain yielded to disruption and finally to transformation as the paradigm shift occurred. The conflict between the open shoulder surgeons and the arthroscopic shoulder surgeons passed through all the phases that Mahatma Gandhi had described many years before. “First they ignore you; then they laugh at you; then they fight you; then you win.”
Building a Ship in a Bottle
At the start of the 1990s, I recognized that my progress in arthroscopic rotator cuff repair would be extremely slow unless I could find an industry partner who shared my vision for full-scale conversion to arthroscopic means of repair and would be willing to help make it a reality. In 1991, I happened to meet Reinhold Schmieding, the owner of Arthrex, a small arthroscopic device company in Naples, Florida. Reinhold invited me to visit him to discuss the feasibility of developing arthroscopic repair systems for the shoulder. At the time, the world headquarters of Arthrex was a 20×30-ft storage room in an office service center, and there were 2 employees. One employee, Don Grafton, was a talented engineer without medical experience. By the end of my first day there, Reinhold and Don and I had agreed that developing arthroscopic repair systems for shoulder instability and rotator cuff repair would become a top priority for Arthrex.
My initial bias toward arthroscopic cuff repair was that a transosseous bone tunnel technique not only would be possible but would be superior to suture anchor fixation. In fact, my first 2 patents with Arthrex were for instrumentation for an arthroscopic transosseous repair technique. I tested my hypothesis with 2 successive biomechanical studies. The first examined cyclic loading of bone tunnel repairs, and the second examined cyclic loading of anchor-based repairs.3,4 Evaluating the data from these 2 studies, I was surprised to find that anchor-based repairs were significantly stronger than bone tunnel repairs. In addition, anchors shifted the weak link from the bone–suture interface to the tendon–suture interface; in essence, anchors optimized bone fixation by shifting the weak link in the construct to the tendon. I was then completely convinced of the superiority of suture anchors over bone tunnels, and that conviction has become even stronger over the years. After these 2 cyclic loading studies, I shifted my focus, and that of Arthrex, toward arthroscopic suture anchor repair of the rotator cuff.
Reconciling Technique and Instrumentation With Anatomy and Biomechanics
Having recognized the importance of the rotator cable attachments both anatomically5 and biomechanically,6,7 I thought it important to reinforce them as a routine part of performing rotator cuff repairs. Our anatomical and biomechanical studies had had great translational implications in the development of our techniques and instrumentation.
As mentioned earlier, Don Grafton was the chief (and for a long time only) engineer at Arthrex. As he had no medical experience, I invited him to come to San Antonio to observe surgery. During Don’s many visits, I showed him pathology in the operating room and pointed out what I could do with the instruments I had and what I could not do. Then in the evening we went to my house and brainstormed how to perform the “missing” surgical manipulations, how to improve manipulations that were suboptimal, and how to optimize final surgical constructs.
Passing suture through tendon was an early challenge. One must remember that, in the early 1990s, it was not possible for machinists to fabricate complex shapes. Therefore, straight tubular retrograde suture passers were the logical first option. We initially developed spring-loaded retrograde hook retrievers (Figure 1) and then curved suture hooks with shuttling wires (Lasso). To me, the most unappealing feature of retrograde suture passage was the oblique angle of approach through the tendon, which caused a length–tension mismatch between the upper fibers and the lower fibers of the muscle–tendon unit. We recognized we could eliminate the mismatch if we passed the suture antegrade, such that it would pass perpendicular to the tendon fibers. These insights and efforts culminated in development of the Viper suture passer and then the FastPass Scorpion suture passer, which has a spring-loaded trapdoor on the upper jaw for ergonomic self-retrieving of the suture once it is passed through the tendon.
To develop a knot pusher that optimized knot tying (yielding the highest knot security and the tightest loop security), we used prototype instruments to tie and test literally thousands of knots in the laboratory. We were thus able to verify that the Surgeon’s Sixth Finger Knot Pusher (Arthrex) reproducibly tied optimized knots8,9 and also optimized knot fixation and bone fixation. However, our suture was not yet optimized and was prone to breakage, and our suture–tendon interface was not yet optimized. Clearly, improvement was needed in 2 more areas.
Don came up with the idea for a virtually unbreakable suture and developed that idea into FiberWire.10 Shortly thereafter, I contributed the idea and design for FiberTape, which dramatically enhanced suture pullout strength and footprint compression.
Anchor designs improved rapidly and dramatically. We made the second-generation BioCorkscrew fully threaded, which virtually eliminated anchor failure, even in soft bone.
Optimization of the suture–tendon interface took a giant step forward when Park and colleagues11,12 introduced linked double-row rotator cuff repair. Much as with a Chinese finger trap, the harder you pull, the stronger it becomes, with yield load approaching ultimate load.
At this point, it seemed we had optimized virtually every segment of the rotator cuff repair construct. Each component was just about as good as it could be. Or was it?
The Accidental Quest for Knotless Fixation
In November 1998, I made my first trip to China as a guest speaker at the Congress of the Hong Kong Orthopaedic Association. My first view of the magnificent Hong Kong skyline across Victoria Harbour was truly breathtaking. As I admired the gleaming glass towers and the concrete canyons of the city, I had no idea that the very next day these modern skyscrapers would reveal an ancient secret that would change my approach to arthroscopic rotator cuff repair.
The day after my arrival, Dr. James Lam took me to lunch. As we approached the restaurant, he pointed across the street to a tall building that was being renovated and had scaffolding supporting workers alongside the first 9 stories of the exterior wall. Dr. Lam said that, after lunch, he would take me to the construction site for a closer look at the scaffolding.
After lunch, we walked to the base of the scaffolding. Dr. Lam told me it was constructed entirely of bamboo poles held together with lashings but no knots (Figure 2). Lashings were secured by turning them back on themselves and wrapping them in an entirely knotless manner.13 I found it incredible that this knotless fixation was so secure that it could support the weight of workers many stories above the ground. I resolved to determine how this fixation method worked and see if the same mechanism might help us achieve reliable knotless fixation in surgery.
When I returned home, I broke out my college engineering books and reacquainted myself with the concept of cable friction. As has happened so often in the past, however, it took a practical lesson from the ranch to truly illustrate for me how cable friction works.
Every cowboy knows that a spirited horse cannot be restrained with only one lead rope. However, a cowboy can wrap a lead rope around a “snubbing post” and thereby gain complete control over the animal, despite the horse’s superior size and strength. The cable friction between the rope and the post creates such a large restraining force that the cowboy can easily hold the animal without the help of a knotted rope (Figure 3). In similar fashion in the Hong Kong scaffolding, fixation strength results from the significant amount of cable friction produced when the lashings wrap around one another and around the bamboo poles.
The cable friction concept was pivotal in the development of knotless fixation in arthroscopic rotator cuff repair. In lateral row fixation, the eyelet of the PushLock and SwiveLock suture anchors (Arthrex) produces significant cable friction at the eyelet–suture interface, in addition to frictional force wedging the suture between anchor and bone.
As with so many other devices in shoulder arthroscopy, the SwiveLock suture anchor developed in stages. In the first stage, a chainlike suture with consecutive intersecting links was used (FiberChain). The idea for an adjustable fixation construct came to me because I thought that a forked eyelet on a SwiveLock would provide a firm fixation point when inserted into the appropriate suture link, yet would be totally adjustable simply by choosing a tighter or looser link (Figure 4). Although the system worked very well, it was technically challenging. The process was greatly simplified after Don Grafton and I developed FiberTape and recognized that the power of cable friction was dramatically increased by the larger contact area between the eyelet and the braided FiberTape. The SpeedBridge construct (Arthrex), which enhanced cable friction fixation by means of passing FiberTape through the anchor eyelets, also provided a larger compressive interface at the repair site by using FiberTape rather than conventional suture. These incremental improvements led to what I would characterize as today’s gold standard for arthroscopic rotator cuff repair: a largely knotless linked double-row construct using FiberTape, with cinch-loop sutures at the anterior and posterior margins of the tear to reinforce the cable attachments and simultaneously reduce the dog-ears that typically occur in those locations, and a double-pulley medial mattress if tendon quality is poor (Figure 5).
The Burden of Craft
With all the recent enthusiasm for level I studies, I think we need to examine whether they will accelerate technological advancement in rotator cuff repair. The answer, in my opinion, is a resounding no. This answer is based on a major disconnect I have detected in how we evaluate these studies in rotator cuff disease and repair.
An irony related to technological advancement in surgery is that the more technically advanced the surgery becomes, the more skill is required. This fact is completely at odds with the public’s perception that technological advances make procedures easier. In arthroscopic rotator cuff repair, the surgeon must look, feel, and be aware to a greater degree than in open surgery.
Edward Tenner, in his book Why Things Bite Back, described the burden of the practitioner of any advanced technology as the burden of craft.14 The burden of craft is the inherent demand on all craftspeople, but particularly surgeons, to “up our game” if we are to be successful in our craft. For arthroscopic rotator cuff repair, the burden of craft requires patience, attention to detail, and the ability to work in a virtual space. Not everyone has these skills. But anyone who wants to practice in this discipline has an obligation to learn the skills required, and then to teach them to others and assess how well they are being applied.
The problem with relying on level I studies to assess the efficacy of a surgical procedure is that they are inherently biased by the surgeons involved. As results depend on surgeons’ skills, and surgeons’ skill levels are not equal, level I studies cannot prove what is possible, cannot demonstrate the limits of a technique, and cannot demonstrate the equivalence of techniques.
Amazingly enough, there are still rotator cuff repair “deniers” who confidently assert from the podium that a large percentage of massive cuff tears cannot be repaired and that, even if they can be repaired, they do not have the biological potential to heal. Given the disparity in surgeons’ skills and results, however, one must ask whether poor results are a consequence of a biological deficit in the patient, or of a skill deficit in the surgeon.
What I know is that we have techniques for predictable arthroscopic repair and healing of the vast majority of rotator cuff tears, even massive tears,15-17 and patients do very well clinically. Yet, among many orthopedic surgeons, there is a trend to go straight to reverse total shoulder arthroplasty (rTSA) for massive tears—despite the evidence against it. As reported in the literature, rTSA results are not as good as arthroscopic cuff repair results, and the complication rate for rTSA is much higher.
Why has this trend toward rTSA for massive tears gained so much momentum? The only reason I can surmise is that, for the average surgeon, rTSA is easier and quicker than arthroscopic repair for massive tears. But the reason for choosing a specific type of surgery for a given problem should not be that it is easiest for the surgeon; it should be that it is best for the patient.
The surgeon should start by asking what procedure he or she would want if the roles were reversed—if the surgeon were the patient with the massive rotator cuff tear. If a surgeon does not have the skill set for the best procedure for a particular patient, he or she is obligated to send that patient to a surgeon who does have the skills. In addition, given that infection is the most feared complication in most shoulder surgeries, the surgeon should ask which infection rate would be personally acceptable. Arthroscopic rotator cuff repair has a reported infection rate of 1.6 per 1000, or .0016,18 whereas rTSA has an infection rate about 25 times higher, or .04.19 Further, the surgeon must consider the relative severity of the consequences of infection. By any measure, an infected arthroscopy is a straightforward treatable complication, but an infected shoulder replacement is a human tragedy. Patients vastly prefer the minimally invasive arthroscopic approach, and through online searches can easily identify who can offer an arthroscopic solution.
To reproducibly achieve successful arthroscopic repair of massive rotator cuff tears, the surgeon must know advanced techniques, including subscapularis repair techniques,20,21 interval slides,22,23 and self-reinforcing constructs.24,25
“It’s a poor carpenter who blames his tools.” This 18th-century English proverb is as true today as it was 300 years ago. The tools for arthroscopic cuff repair exist, and they are excellent. The burden of craft is the surgeon’s burden and obligation. As surgeons, we must accept that obligation and the responsibility of that burden.
As mentioned earlier, Dr. Rob Bell’s charge to me when he invited me to give the Neer Lecture was to sum up my involvement in the development of arthroscopic shoulder surgery. The short version is that I have been doing shoulder arthroscopy for 31 years; have received 28 US patents related to shoulder instruments and implants and have 12 US patents pending; have published 167 peer-reviewed articles, a couple dozen book chapters, and 2 textbooks on shoulder arthroscopy; have trained 25 fellows; and have hosted approximately 3000 visiting surgeons in my operating room. My greatest professional dream was to see the standard of care for rotator cuff repair and shoulder instability transition from open to arthroscopic techniques, and I have been fortunate enough to have observed that paradigm shift during my career.
What do I envision over the next 31 years? As we all know, history runs in both directions, and some things simply have not happened yet. In terms of rotator cuff treatment, I think over the next few years the guiding principle of treatment will be joint preservation. All rotator cuff tears, even massive tears, will be repaired arthroscopically. Patients and insurers will demand arthroscopic repair, and surgeons without the skill set will migrate to other subspecialties. As for the role of arthroplasty in the treatment of rotator cuff tears, rTSA will be indicated only for pseudoparalysis after failed cuff repair in low-demand elderly patients.
In rotator cuff treatment, I envision a standard of care that is almost entirely arthroscopic. This standard will demand that surgeons who treat rotator cuff tears be proficient in arthroscopic repair of the full range of tears. Acquiring the skills for arthroscopic repair may not be easy, but then “there’s the easy way, and there’s the Cowboy Way.” As my dad used to tell me when I complained about working too hard, “No man ever drowned in his own sweat.” We shoulder surgeons must accept the burden of craft that accompanies the new standard of arthroscopic cuff repair, and we must offer our patients the same level of care we would choose for ourselves.
Happy trails!
1. Burkhart SS, Athanasiou KA, Wirth MA. Margin convergence: a method of reducing strain in massive rotator cuff tears. Arthroscopy. 1996:12(3):335-338.
2. Kuhn TS. The Structure of Scientific Revolutions. Chicago, IL: University of Chicago Press; 1962.
3. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176.
4. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athansiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
5. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
6. Burkhart SS, Nottage WM, Ogilvie-Harris DJ, Kohn HS, Pachelli A. Partial repair of irreparable rotator cuff tears. Arthroscopy. 1994;10(4):363-370.
7. Halder AM, O’Driscoll SW, Heers G, et al. Biomechanical comparison of effects of supraspinatus tendon detachments, tendon defects, and muscle retractions. J Bone Joint Surg Am. 2002;84(5):780-785.
8. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
9. Lo IK, Ochoa E Jr, Burkhart SS. A comparison of knot security and loop security in arthroscopic knots tied with newer high-strength suture materials. Arthroscopy. 2010;26(9 suppl):S120-S126.
10. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
11. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a “transosseous-equivalent” rotator cuff repair technique compared to a double-row technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
12. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
13. Burkhart SS, Athanasiou KA. The twist-lock concept of tissue transport and suture fixation without knots: observations along the Hong Kong skyline. Arthroscopy. 2003;19(6):613-625.
14. Tenner E. Why Things Bite Back. New York, NY: Random House; 1996.
15. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of arthroscopic massive rotator cuff repair: the importance of double-row fixation. Arthroscopy. 2012;28(7):909-915.
16. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
17. Lädermann A, Denard PJ, Burkhart SS. Revision arthroscopic rotator cuff repair: systematic review and authors’ preferred surgical technique. Arthroscopy. 2012;28(8):1160-1169.
18. Randelli P, Castagna A, Cabitza F, Cabitza P, Arrigoni P, Denti M. Infectious and thromboembolic complications of arthroscopic shoulder surgery. J Shoulder Elbow Surg. 2010;19(1):97-101.
19. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
20. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
21. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
22. Lo IK, Burkhart SS. Arthroscopic repair of massive, contracted, immobile rotator cuff tears using single and double interval slides: technique and preliminary results. Arthroscopy. 2004;20(1):22-33.
23. Lo IK, Burkhart SS. The interval slide in continuity: a method of mobilizing the anterosuperior rotator cuff without disrupting the tear margins. Arthroscopy. 2004;20(4):435-441.
24. Denard PJ, Burkhart SS. Techniques for managing poor quality tissue and bone during arthroscopic rotator cuff repair. Arthroscopy. 2011;27(10):1409-1421.
25. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
1. Burkhart SS, Athanasiou KA, Wirth MA. Margin convergence: a method of reducing strain in massive rotator cuff tears. Arthroscopy. 1996:12(3):335-338.
2. Kuhn TS. The Structure of Scientific Revolutions. Chicago, IL: University of Chicago Press; 1962.
3. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176.
4. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athansiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
5. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
6. Burkhart SS, Nottage WM, Ogilvie-Harris DJ, Kohn HS, Pachelli A. Partial repair of irreparable rotator cuff tears. Arthroscopy. 1994;10(4):363-370.
7. Halder AM, O’Driscoll SW, Heers G, et al. Biomechanical comparison of effects of supraspinatus tendon detachments, tendon defects, and muscle retractions. J Bone Joint Surg Am. 2002;84(5):780-785.
8. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
9. Lo IK, Ochoa E Jr, Burkhart SS. A comparison of knot security and loop security in arthroscopic knots tied with newer high-strength suture materials. Arthroscopy. 2010;26(9 suppl):S120-S126.
10. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
11. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a “transosseous-equivalent” rotator cuff repair technique compared to a double-row technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
12. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
13. Burkhart SS, Athanasiou KA. The twist-lock concept of tissue transport and suture fixation without knots: observations along the Hong Kong skyline. Arthroscopy. 2003;19(6):613-625.
14. Tenner E. Why Things Bite Back. New York, NY: Random House; 1996.
15. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of arthroscopic massive rotator cuff repair: the importance of double-row fixation. Arthroscopy. 2012;28(7):909-915.
16. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
17. Lädermann A, Denard PJ, Burkhart SS. Revision arthroscopic rotator cuff repair: systematic review and authors’ preferred surgical technique. Arthroscopy. 2012;28(8):1160-1169.
18. Randelli P, Castagna A, Cabitza F, Cabitza P, Arrigoni P, Denti M. Infectious and thromboembolic complications of arthroscopic shoulder surgery. J Shoulder Elbow Surg. 2010;19(1):97-101.
19. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
20. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
21. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
22. Lo IK, Burkhart SS. Arthroscopic repair of massive, contracted, immobile rotator cuff tears using single and double interval slides: technique and preliminary results. Arthroscopy. 2004;20(1):22-33.
23. Lo IK, Burkhart SS. The interval slide in continuity: a method of mobilizing the anterosuperior rotator cuff without disrupting the tear margins. Arthroscopy. 2004;20(4):435-441.
24. Denard PJ, Burkhart SS. Techniques for managing poor quality tissue and bone during arthroscopic rotator cuff repair. Arthroscopy. 2011;27(10):1409-1421.
25. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
Isolating Suture Slippage During Cadaveric Testing of Knotless Anchors
Knotless suture anchor fixation techniques continue to evolve as efficient, low-profile options for arthroscopic rotator cuff repair (RCR).1,2 Excellent outcomes have been reported for constructs that use knotless fixation laterally, typically in suture bridge-type configurations.2-4 Early comparative biomechanical and clinical studies have also demonstrated equivalent results for all-knotless versus conventional constructs for arthroscopic RCR.5-10 Given the increased use and availability of multiple implant designs, it is important to supplement our clinical knowledge of these devices with laboratory studies delineating the biomechanical properties of the anchors that are used to help guide appropriate clinical use of the implants in specific patient populations.
Several biomechanical studies have shown suture slippage to be the weak but crucial link in the design of knotless anchors and the most likely mode of in vivo failure.11,12 Other studies have demonstrated frequent anchor dislodgement from bone, but these analyses involved use of elderly cadaveric specimens and relatively high-force testing protocols.12,13 Because suture-retention force may have exceeded anchor resistance to pullout (imparted by weak cadaveric bone in such biomechanical settings), the focus on suture-retention properties was limited.11 It is thought that, in clinical practice, the majority of patients who undergo RCR tend not to generate the high forces (relative to resistance to bone pullout) used to cause the anchor pullouts observed in biomechanical studies, particularly in the early postoperative setting.11-15 Cadaveric testing, however, often involves use of specimens with diminished bone mineral density (BMD), relative to age, because of the illness and other factors leading to death and donation.
Using a novel testing apparatus, we isolated, analyzed, and compared suture slippage in 2 anchor designs, one with entirely press-fit suture clamping and the other reliant on an intrinsic suture-locking mechanism.
Materials and Methods
Six human cadaveric proximal humeri specimens were used for this biomechanical study. Mean (SD) age was 53.3 (5.7) years (range, 46-59 years). Middle-aged specimens were used in order to best represent the quality of bone typically encountered in RCR surgery. To approximate tissue in clinical use, we used fresh-frozen cadaver tissue. Specimens were maintained at –20°C until about 24 hours before use and then were thawed to room temperature for testing. Specimens were included only if they had a completely intact humeral head and no prior surgery or hardware placement. Before instrumentation, dual-energy x-ray absorptiometry with a QDR-1000 scanner (Hologic) was used to determine BMD of all proximal humeri.
Two knotless suture anchors were compared: PushLock (4.5×18.5 mm; Arthrex) and ReelX STT (5.5×19.4 mm; Stryker). These anchors have multiple surgical indications (including RCR), allow patient-specific tissue tensioning, and use polyetheretherketone eyelets. The clamping force for PushLock depends entirely on the interference fit achieved for the suture between the outside of the anchor and the surrounding trabecular/cortical bone after device insertion, whereas the suture in ReelX is secured within the anchor shaft entirely by an internal ratchet-locking mechanism.
For anchor insertion, shoulders were dissected down to the greater tuberosity of the proximal humerus, and all implants were inserted (by a fellowship-trained surgeon in accordance with manufacturer guidelines) at a 25° insertion angle with manufacturer-supplied instruments. One anchor of each type (Figure 1) was inserted into the center of the rotator cuff footprint on the greater tuberosity of each specimen. Anterior and posterior positions were randomized, and an anchor from the other group was inserted into the matching location on the contralateral matched-pair specimen. In all instances, distance between the anterior and posterior anchors was 2 cm, and anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity (Figure 2). Two strands of size 2 ultrahigh-molecular-weight–polyethylene Force Fiber (Stryker) were loaded into all anchors.
A custom urethane fixture was secured over the center of each anchor to allow testing to focus on suture slippage by minimizing anchor migration (Figure 3). The small aperture of this device allowed suture tails to pass freely through the center of the fixture but prevented disengagement and proximal migration of the suture anchor from the underlying bone through contact of the urethane fixture with the anchor perimeter. Any system deformation observed during testing was restricted to the suture and/or the anchor’s suture-locking mechanism. Testing fixtures also oriented the suture anchor coaxial with the axis of tension, creating a worst-case loading scenario (Figure 3).
PushLock implants were inserted with 5 pounds of tension, as indicated, using a manufacturer-supplied suture tensioner, and ReelX devices were inserted and locked with 2 full rotations, as specified by the manufacturer. After one end of each suture was cut, as would be done in vivo, the 2 other suture ends, which would have been part of the RCR in vivo, were tied together to form an 8-cm circumference loop that was brought through the urethane fixture. Humeri were then mounted in a materials testing system (MTS 810; MTS Systems) servohydraulic load frame, and the suture loop was passed around a cross-bar on the actuator of the testing device. A mechanical testing protocol consisting of modest repetitive forces was carefully chosen to simulate expected activity during rehabilitation after RCR.15 In this protocol, a 60-second preload of 10 N was followed by tensile loading between 10 N and 90 N at a frequency of 0.5 Hz for 500 cycles.15 Cycle duration at 3 mm and 5 mm of suture slippage (threshold for clinical failure) was recorded.12,16,17 In addition, suture slippage was measured after 1, 10, 50, 100, 200, 300, 400, and 500 cycles. The first 5 test cycles were not counted in the analysis to control for initial knot slippage. Finally, after completion of dynamic testing, samples were loaded at a displacement rate of 0.5 mm/s for tension-to-failure testing in the custom fixtures. Maximum failure load, stiffness, and failure mode were recorded. Ultimate failure was defined as suture breakage or gross suture slippage.
Paired Student t test was used to determine significant differences in suture slippage distance between the 2 groups at various cycle durations. In addition, Kaplan-Meier survival test was used to determine statistical differences in sample survival during the dynamic loading test.
Results
Mean (SD) BMD of the cadaveric shoulder specimens was 0.55 (0.13) g/cm2 (range, 0.29-0.68 g/cm2). The testing fixtures isolated suture slippage from anchor–bone disengagement. All 6 PushLock implants demonstrated slippage of more than 3 mm, and 5 of the 6 demonstrated slippage of more than 5 mm. All 6 ReelX devices exhibited slippage of less than 3 mm. In addition, PushLock demonstrated more suture slippage at cycles 1, 10, and 100 (P < .05) and more maximum slippage after 500 cycles (mean, 11.2 mm; SD, 4.7 mm) compared with ReelX (mean, 1.9 mm; SD, 0.5 mm) (P = .004). Figure 4 shows mean suture slippage at each cycle.
Kaplan-Meier analysis revealed significantly (λ2 = 8.170; P = .0043) decreased survival after dynamic testing for PushLock versus ReelX (Figure 5). Survival was defined as suture slippage of less than 5 mm after completion of dynamic testing. Only 1 of the 6 PushLock anchors completed dynamic testing; the other 5 failed via complete suture slippage from the anchor before testing could be completed. All 6 ReelX devices survived dynamic testing.
Therefore, 1 PushLock implant and all 6 ReelX devices were available for subsequent load-to-failure testing. Failure in this setting was defined as suture slippage of more than 10 mm or suture breakage. The PushLock implant failed at a maximum force of 171.8 N with a stiffness of 74.4 N/mm and eventually exhibited gross suture slippage. All 6 ReelX devices failed at a mean (SD) maximum of 273.5 (20.2) N, with a mean (SD) stiffness of 74.1 (17) N/mm. Mechanism of failure for all ReelX devices was suture breakage during the tensile load-to-failure test.
Discussion
We evaluated a new technique designed to isolate suture slippage in knotless anchors used for RCR. The impetus for developing this new method was to provide a means for better analyzing the ability of a knotless anchor to resist suture slippage in the cadaveric biomechanical testing setting. Suture slippage is an important mode of failure during such analyses.11,12 Significant slippage occurred in a range of implants before half the anchor–bone pullout strength was reached in a study using young bovine femoral heads.11 In another study, using young, high-BMD cadaveric humeral heads, initial slippage and maximum failure loads were equivalent among numerous devices using various suture-retention mechanisms, and suture slippage was the most common failure mode.12 Nevertheless, other biomechanical studies have demonstrated frequent failure caused by anchor pullout in elderly human cadaveric specimens with diminished BMD, often with high-force testing protocols.12,13 In the more modest-force, in vivo rehabilitative environment, suture slippage rather than anchor dislodgement may be the main failure mode.11-15
We compared the PushLock implant and its entirely press-fit suture clamping design with the ReelX device, which relies on an intrinsic suture-locking mechanism. Middle-aged (mean, 53.3 years; SD, 5.7 years) cadaveric humeri were tested under physiologically relevant biomechanical conditions to begin to help identify how relatively osteopenic bone may affect suture-retention properties for a given implant. The results showed that the study methodology prevented implant failure via anchor–bone pullout. To our knowledge, this was the first study to exclusively analyze suture slippage in knotless anchors. The findings indicated that implants that rely heavily on a tight interference fit of the suture between the anchor and the surrounding bone may exhibit early slippage and failure after RCR in middle-aged patients with relative osteopenia.11,12 However, this study also demonstrated that devices with intrinsic clamping mechanisms that do not depend on the quality of surrounding bone may better resist suture slippage. It is not clear that all knotless anchors with intrinsic locking mechanisms function equivalently. For instance, Pietschmann and colleagues12 found that 2 of 10 implants with a different internal clamping device were unable to resist failure via suture slippage, even in healthy bone. Similarly, in a study comparing ReelX devices with implants having a different internal suture-retention mechanism, ReelX failed at higher ultimate loads, and typically via anchor dislodgement, versus suture slippage in the other implants.18
It is important to note that, in the present study, the loads at which sutures broke in the intrinsic clamping anchors approached the maximum contractile force of the supraspinatus muscle (302 N).19,20 In addition, these loads were above the resistance of the rotator cuff tendon to cut out with modern suture material.21
This study’s limitations include use of an in vitro human cadaveric model that precluded analysis of the effects of postoperative healing. Biomechanical testing was also performed in a single row-type suture configuration with the rotator cuff tendon removed. Fixtures used during testing oriented the load coaxially with the axis of tension, creating a worst-case loading scenario. Although this form of testing may limit its clinical applicability, its purpose was to critically isolate how well a knotless anchor could resist suture slippage. The methods we used were also limited because the stability of the bone–anchor interface was not assessed. For patients with osteopenia, anchor pullout rather than suture slippage could be the most limiting factor for knotless anchor construct failure, and therefore further testing of both failure modes is needed. Future biomechanical studies should compare various knotless anchors’ suture-slippage characteristics in other constructs in physiologic testing orientations, including double-row and suture-bridge configurations, as well as with intact rotator cuff tendons. In addition, use of labral tape as a substitute for polyblend suture has been suggested to limit suture slippage, and this technique theoretically could have changed the results of this study.22
Conclusion
An implant with an internal ratcheting mechanism for suture retention demonstrated significantly less suture slippage in an axial tension evaluation protocol than a device reliant on interference fit of the suture between the anchor and surrounding bone. In the clinical setting, this may allow for less gap formation during the healing phase following RCR with a knotless anchor. There was also increased maximum load to failure, demonstrating an increased load until catastrophic failure using a device with a ratcheting internal locking mechanism.
1. Thal R. A knotless suture anchor. Design, function, and biomechanical testing. Am J Sports Med. 2001;29(5):646-649.
2. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
3. Kim KC, Shin HD, Cha SM, Lee WY. Comparison of repair integrity and functional outcomes for 3 arthroscopic suture bridge rotator cuff repair techniques. Am J Sports Med. 2013;41(2):271-277.
4. Choi CH, Kim SK, Cho MR, et al. Functional outcomes and structural integrity after double-pulley suture bridge rotator cuff repair using serial ultrasonographic examination. J Shoulder Elbow Surg. 2012;21(12):1753-1763.
5. Brown BS, Cooper AD, McIff TE, Key VH, Toby EB. Initial fixation and cyclic loading stability of knotless suture anchors for rotator cuff repair. J Shoulder Elbow Surg. 2008;17(2):313-318.
6. Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD. A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy. 2009;25(3):274-281.
7. Hepp P, Osterhoff G, Engel T, Marquass B, Klink T, Josten C. Biomechanical evaluation of knotless anatomical double-layer double-row rotator cuff repair: a comparative ex vivo study. Am J Sports Med. 2009;37(7):1363-1369.
8. Maguire M, Goldberg J, Bokor D, et al. Biomechanical evaluation of four different transosseous-equivalent/suture bridge rotator cuff repairs. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1582-1587.
9. Millar NL, Wu X, Tantau R, Silverstone E, Murrell GA. Open versus two forms of arthroscopic rotator cuff repair. Clin Orthop Relat Res. 2009;467(4):966-978.
10. Rhee YG, Cho NS, Parke CS. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med. 2012;40(11):2440-2447.
11. Wieser K, Farshad M, Vlachopoulos L, Ruffieux K, Gerber C, Meyer DC. Suture slippage in knotless suture anchors as a potential failure mechanism in rotator cuff repair. Arthroscopy. 2012;28(11):1622-1627.
12. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
13. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
14. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
15. Bynum CK, Lee S, Mahar A, Tasto J, Pedowitz R. Failure mode of suture anchors as a function of insertion depth. Am J Sports Med. 2005;33(7):1030-1034.
16. Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76(3):371-380.
17. Schneeberger AG, von Roll A, Kalberer F, Jacob HA, Gerber C. Mechanical strength of arthroscopic rotator cuff repair techniques: an in vitro study. J Bone Joint Surg Am. 2002;84(12):2152-2160.
18. Efird C, Traub S, Baldini T, et al. Knotless single-row rotator cuff repair: a comparative biomechanical study of 2 knotless suture anchors. Orthopedics. 2013;36(8):e1033-e1037.
19. Wright PB, Budoff JE, Yeh ML, Kelm ZS, Luo ZP. Strength of damaged suture: an in vitro study. Arthroscopy. 2006;22(12):1270-1275.
20. Burkhart SS. A stepwise approach to arthroscopic rotator cuff repair based on biomechanical principles. Arthroscopy. 2000;16(1):82-90.
21. Bisson LJ, Manohar LM. A biomechanical comparison of the pullout strength of No. 2 FiberWire suture and 2-mm FiberWire tape in bovine rotator cuff tendons. Arthroscopy. 2010;26(11):1463-1468.
22. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
Knotless suture anchor fixation techniques continue to evolve as efficient, low-profile options for arthroscopic rotator cuff repair (RCR).1,2 Excellent outcomes have been reported for constructs that use knotless fixation laterally, typically in suture bridge-type configurations.2-4 Early comparative biomechanical and clinical studies have also demonstrated equivalent results for all-knotless versus conventional constructs for arthroscopic RCR.5-10 Given the increased use and availability of multiple implant designs, it is important to supplement our clinical knowledge of these devices with laboratory studies delineating the biomechanical properties of the anchors that are used to help guide appropriate clinical use of the implants in specific patient populations.
Several biomechanical studies have shown suture slippage to be the weak but crucial link in the design of knotless anchors and the most likely mode of in vivo failure.11,12 Other studies have demonstrated frequent anchor dislodgement from bone, but these analyses involved use of elderly cadaveric specimens and relatively high-force testing protocols.12,13 Because suture-retention force may have exceeded anchor resistance to pullout (imparted by weak cadaveric bone in such biomechanical settings), the focus on suture-retention properties was limited.11 It is thought that, in clinical practice, the majority of patients who undergo RCR tend not to generate the high forces (relative to resistance to bone pullout) used to cause the anchor pullouts observed in biomechanical studies, particularly in the early postoperative setting.11-15 Cadaveric testing, however, often involves use of specimens with diminished bone mineral density (BMD), relative to age, because of the illness and other factors leading to death and donation.
Using a novel testing apparatus, we isolated, analyzed, and compared suture slippage in 2 anchor designs, one with entirely press-fit suture clamping and the other reliant on an intrinsic suture-locking mechanism.
Materials and Methods
Six human cadaveric proximal humeri specimens were used for this biomechanical study. Mean (SD) age was 53.3 (5.7) years (range, 46-59 years). Middle-aged specimens were used in order to best represent the quality of bone typically encountered in RCR surgery. To approximate tissue in clinical use, we used fresh-frozen cadaver tissue. Specimens were maintained at –20°C until about 24 hours before use and then were thawed to room temperature for testing. Specimens were included only if they had a completely intact humeral head and no prior surgery or hardware placement. Before instrumentation, dual-energy x-ray absorptiometry with a QDR-1000 scanner (Hologic) was used to determine BMD of all proximal humeri.
Two knotless suture anchors were compared: PushLock (4.5×18.5 mm; Arthrex) and ReelX STT (5.5×19.4 mm; Stryker). These anchors have multiple surgical indications (including RCR), allow patient-specific tissue tensioning, and use polyetheretherketone eyelets. The clamping force for PushLock depends entirely on the interference fit achieved for the suture between the outside of the anchor and the surrounding trabecular/cortical bone after device insertion, whereas the suture in ReelX is secured within the anchor shaft entirely by an internal ratchet-locking mechanism.
For anchor insertion, shoulders were dissected down to the greater tuberosity of the proximal humerus, and all implants were inserted (by a fellowship-trained surgeon in accordance with manufacturer guidelines) at a 25° insertion angle with manufacturer-supplied instruments. One anchor of each type (Figure 1) was inserted into the center of the rotator cuff footprint on the greater tuberosity of each specimen. Anterior and posterior positions were randomized, and an anchor from the other group was inserted into the matching location on the contralateral matched-pair specimen. In all instances, distance between the anterior and posterior anchors was 2 cm, and anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity (Figure 2). Two strands of size 2 ultrahigh-molecular-weight–polyethylene Force Fiber (Stryker) were loaded into all anchors.
A custom urethane fixture was secured over the center of each anchor to allow testing to focus on suture slippage by minimizing anchor migration (Figure 3). The small aperture of this device allowed suture tails to pass freely through the center of the fixture but prevented disengagement and proximal migration of the suture anchor from the underlying bone through contact of the urethane fixture with the anchor perimeter. Any system deformation observed during testing was restricted to the suture and/or the anchor’s suture-locking mechanism. Testing fixtures also oriented the suture anchor coaxial with the axis of tension, creating a worst-case loading scenario (Figure 3).
PushLock implants were inserted with 5 pounds of tension, as indicated, using a manufacturer-supplied suture tensioner, and ReelX devices were inserted and locked with 2 full rotations, as specified by the manufacturer. After one end of each suture was cut, as would be done in vivo, the 2 other suture ends, which would have been part of the RCR in vivo, were tied together to form an 8-cm circumference loop that was brought through the urethane fixture. Humeri were then mounted in a materials testing system (MTS 810; MTS Systems) servohydraulic load frame, and the suture loop was passed around a cross-bar on the actuator of the testing device. A mechanical testing protocol consisting of modest repetitive forces was carefully chosen to simulate expected activity during rehabilitation after RCR.15 In this protocol, a 60-second preload of 10 N was followed by tensile loading between 10 N and 90 N at a frequency of 0.5 Hz for 500 cycles.15 Cycle duration at 3 mm and 5 mm of suture slippage (threshold for clinical failure) was recorded.12,16,17 In addition, suture slippage was measured after 1, 10, 50, 100, 200, 300, 400, and 500 cycles. The first 5 test cycles were not counted in the analysis to control for initial knot slippage. Finally, after completion of dynamic testing, samples were loaded at a displacement rate of 0.5 mm/s for tension-to-failure testing in the custom fixtures. Maximum failure load, stiffness, and failure mode were recorded. Ultimate failure was defined as suture breakage or gross suture slippage.
Paired Student t test was used to determine significant differences in suture slippage distance between the 2 groups at various cycle durations. In addition, Kaplan-Meier survival test was used to determine statistical differences in sample survival during the dynamic loading test.
Results
Mean (SD) BMD of the cadaveric shoulder specimens was 0.55 (0.13) g/cm2 (range, 0.29-0.68 g/cm2). The testing fixtures isolated suture slippage from anchor–bone disengagement. All 6 PushLock implants demonstrated slippage of more than 3 mm, and 5 of the 6 demonstrated slippage of more than 5 mm. All 6 ReelX devices exhibited slippage of less than 3 mm. In addition, PushLock demonstrated more suture slippage at cycles 1, 10, and 100 (P < .05) and more maximum slippage after 500 cycles (mean, 11.2 mm; SD, 4.7 mm) compared with ReelX (mean, 1.9 mm; SD, 0.5 mm) (P = .004). Figure 4 shows mean suture slippage at each cycle.
Kaplan-Meier analysis revealed significantly (λ2 = 8.170; P = .0043) decreased survival after dynamic testing for PushLock versus ReelX (Figure 5). Survival was defined as suture slippage of less than 5 mm after completion of dynamic testing. Only 1 of the 6 PushLock anchors completed dynamic testing; the other 5 failed via complete suture slippage from the anchor before testing could be completed. All 6 ReelX devices survived dynamic testing.
Therefore, 1 PushLock implant and all 6 ReelX devices were available for subsequent load-to-failure testing. Failure in this setting was defined as suture slippage of more than 10 mm or suture breakage. The PushLock implant failed at a maximum force of 171.8 N with a stiffness of 74.4 N/mm and eventually exhibited gross suture slippage. All 6 ReelX devices failed at a mean (SD) maximum of 273.5 (20.2) N, with a mean (SD) stiffness of 74.1 (17) N/mm. Mechanism of failure for all ReelX devices was suture breakage during the tensile load-to-failure test.
Discussion
We evaluated a new technique designed to isolate suture slippage in knotless anchors used for RCR. The impetus for developing this new method was to provide a means for better analyzing the ability of a knotless anchor to resist suture slippage in the cadaveric biomechanical testing setting. Suture slippage is an important mode of failure during such analyses.11,12 Significant slippage occurred in a range of implants before half the anchor–bone pullout strength was reached in a study using young bovine femoral heads.11 In another study, using young, high-BMD cadaveric humeral heads, initial slippage and maximum failure loads were equivalent among numerous devices using various suture-retention mechanisms, and suture slippage was the most common failure mode.12 Nevertheless, other biomechanical studies have demonstrated frequent failure caused by anchor pullout in elderly human cadaveric specimens with diminished BMD, often with high-force testing protocols.12,13 In the more modest-force, in vivo rehabilitative environment, suture slippage rather than anchor dislodgement may be the main failure mode.11-15
We compared the PushLock implant and its entirely press-fit suture clamping design with the ReelX device, which relies on an intrinsic suture-locking mechanism. Middle-aged (mean, 53.3 years; SD, 5.7 years) cadaveric humeri were tested under physiologically relevant biomechanical conditions to begin to help identify how relatively osteopenic bone may affect suture-retention properties for a given implant. The results showed that the study methodology prevented implant failure via anchor–bone pullout. To our knowledge, this was the first study to exclusively analyze suture slippage in knotless anchors. The findings indicated that implants that rely heavily on a tight interference fit of the suture between the anchor and the surrounding bone may exhibit early slippage and failure after RCR in middle-aged patients with relative osteopenia.11,12 However, this study also demonstrated that devices with intrinsic clamping mechanisms that do not depend on the quality of surrounding bone may better resist suture slippage. It is not clear that all knotless anchors with intrinsic locking mechanisms function equivalently. For instance, Pietschmann and colleagues12 found that 2 of 10 implants with a different internal clamping device were unable to resist failure via suture slippage, even in healthy bone. Similarly, in a study comparing ReelX devices with implants having a different internal suture-retention mechanism, ReelX failed at higher ultimate loads, and typically via anchor dislodgement, versus suture slippage in the other implants.18
It is important to note that, in the present study, the loads at which sutures broke in the intrinsic clamping anchors approached the maximum contractile force of the supraspinatus muscle (302 N).19,20 In addition, these loads were above the resistance of the rotator cuff tendon to cut out with modern suture material.21
This study’s limitations include use of an in vitro human cadaveric model that precluded analysis of the effects of postoperative healing. Biomechanical testing was also performed in a single row-type suture configuration with the rotator cuff tendon removed. Fixtures used during testing oriented the load coaxially with the axis of tension, creating a worst-case loading scenario. Although this form of testing may limit its clinical applicability, its purpose was to critically isolate how well a knotless anchor could resist suture slippage. The methods we used were also limited because the stability of the bone–anchor interface was not assessed. For patients with osteopenia, anchor pullout rather than suture slippage could be the most limiting factor for knotless anchor construct failure, and therefore further testing of both failure modes is needed. Future biomechanical studies should compare various knotless anchors’ suture-slippage characteristics in other constructs in physiologic testing orientations, including double-row and suture-bridge configurations, as well as with intact rotator cuff tendons. In addition, use of labral tape as a substitute for polyblend suture has been suggested to limit suture slippage, and this technique theoretically could have changed the results of this study.22
Conclusion
An implant with an internal ratcheting mechanism for suture retention demonstrated significantly less suture slippage in an axial tension evaluation protocol than a device reliant on interference fit of the suture between the anchor and surrounding bone. In the clinical setting, this may allow for less gap formation during the healing phase following RCR with a knotless anchor. There was also increased maximum load to failure, demonstrating an increased load until catastrophic failure using a device with a ratcheting internal locking mechanism.
Knotless suture anchor fixation techniques continue to evolve as efficient, low-profile options for arthroscopic rotator cuff repair (RCR).1,2 Excellent outcomes have been reported for constructs that use knotless fixation laterally, typically in suture bridge-type configurations.2-4 Early comparative biomechanical and clinical studies have also demonstrated equivalent results for all-knotless versus conventional constructs for arthroscopic RCR.5-10 Given the increased use and availability of multiple implant designs, it is important to supplement our clinical knowledge of these devices with laboratory studies delineating the biomechanical properties of the anchors that are used to help guide appropriate clinical use of the implants in specific patient populations.
Several biomechanical studies have shown suture slippage to be the weak but crucial link in the design of knotless anchors and the most likely mode of in vivo failure.11,12 Other studies have demonstrated frequent anchor dislodgement from bone, but these analyses involved use of elderly cadaveric specimens and relatively high-force testing protocols.12,13 Because suture-retention force may have exceeded anchor resistance to pullout (imparted by weak cadaveric bone in such biomechanical settings), the focus on suture-retention properties was limited.11 It is thought that, in clinical practice, the majority of patients who undergo RCR tend not to generate the high forces (relative to resistance to bone pullout) used to cause the anchor pullouts observed in biomechanical studies, particularly in the early postoperative setting.11-15 Cadaveric testing, however, often involves use of specimens with diminished bone mineral density (BMD), relative to age, because of the illness and other factors leading to death and donation.
Using a novel testing apparatus, we isolated, analyzed, and compared suture slippage in 2 anchor designs, one with entirely press-fit suture clamping and the other reliant on an intrinsic suture-locking mechanism.
Materials and Methods
Six human cadaveric proximal humeri specimens were used for this biomechanical study. Mean (SD) age was 53.3 (5.7) years (range, 46-59 years). Middle-aged specimens were used in order to best represent the quality of bone typically encountered in RCR surgery. To approximate tissue in clinical use, we used fresh-frozen cadaver tissue. Specimens were maintained at –20°C until about 24 hours before use and then were thawed to room temperature for testing. Specimens were included only if they had a completely intact humeral head and no prior surgery or hardware placement. Before instrumentation, dual-energy x-ray absorptiometry with a QDR-1000 scanner (Hologic) was used to determine BMD of all proximal humeri.
Two knotless suture anchors were compared: PushLock (4.5×18.5 mm; Arthrex) and ReelX STT (5.5×19.4 mm; Stryker). These anchors have multiple surgical indications (including RCR), allow patient-specific tissue tensioning, and use polyetheretherketone eyelets. The clamping force for PushLock depends entirely on the interference fit achieved for the suture between the outside of the anchor and the surrounding trabecular/cortical bone after device insertion, whereas the suture in ReelX is secured within the anchor shaft entirely by an internal ratchet-locking mechanism.
For anchor insertion, shoulders were dissected down to the greater tuberosity of the proximal humerus, and all implants were inserted (by a fellowship-trained surgeon in accordance with manufacturer guidelines) at a 25° insertion angle with manufacturer-supplied instruments. One anchor of each type (Figure 1) was inserted into the center of the rotator cuff footprint on the greater tuberosity of each specimen. Anterior and posterior positions were randomized, and an anchor from the other group was inserted into the matching location on the contralateral matched-pair specimen. In all instances, distance between the anterior and posterior anchors was 2 cm, and anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity (Figure 2). Two strands of size 2 ultrahigh-molecular-weight–polyethylene Force Fiber (Stryker) were loaded into all anchors.
A custom urethane fixture was secured over the center of each anchor to allow testing to focus on suture slippage by minimizing anchor migration (Figure 3). The small aperture of this device allowed suture tails to pass freely through the center of the fixture but prevented disengagement and proximal migration of the suture anchor from the underlying bone through contact of the urethane fixture with the anchor perimeter. Any system deformation observed during testing was restricted to the suture and/or the anchor’s suture-locking mechanism. Testing fixtures also oriented the suture anchor coaxial with the axis of tension, creating a worst-case loading scenario (Figure 3).
PushLock implants were inserted with 5 pounds of tension, as indicated, using a manufacturer-supplied suture tensioner, and ReelX devices were inserted and locked with 2 full rotations, as specified by the manufacturer. After one end of each suture was cut, as would be done in vivo, the 2 other suture ends, which would have been part of the RCR in vivo, were tied together to form an 8-cm circumference loop that was brought through the urethane fixture. Humeri were then mounted in a materials testing system (MTS 810; MTS Systems) servohydraulic load frame, and the suture loop was passed around a cross-bar on the actuator of the testing device. A mechanical testing protocol consisting of modest repetitive forces was carefully chosen to simulate expected activity during rehabilitation after RCR.15 In this protocol, a 60-second preload of 10 N was followed by tensile loading between 10 N and 90 N at a frequency of 0.5 Hz for 500 cycles.15 Cycle duration at 3 mm and 5 mm of suture slippage (threshold for clinical failure) was recorded.12,16,17 In addition, suture slippage was measured after 1, 10, 50, 100, 200, 300, 400, and 500 cycles. The first 5 test cycles were not counted in the analysis to control for initial knot slippage. Finally, after completion of dynamic testing, samples were loaded at a displacement rate of 0.5 mm/s for tension-to-failure testing in the custom fixtures. Maximum failure load, stiffness, and failure mode were recorded. Ultimate failure was defined as suture breakage or gross suture slippage.
Paired Student t test was used to determine significant differences in suture slippage distance between the 2 groups at various cycle durations. In addition, Kaplan-Meier survival test was used to determine statistical differences in sample survival during the dynamic loading test.
Results
Mean (SD) BMD of the cadaveric shoulder specimens was 0.55 (0.13) g/cm2 (range, 0.29-0.68 g/cm2). The testing fixtures isolated suture slippage from anchor–bone disengagement. All 6 PushLock implants demonstrated slippage of more than 3 mm, and 5 of the 6 demonstrated slippage of more than 5 mm. All 6 ReelX devices exhibited slippage of less than 3 mm. In addition, PushLock demonstrated more suture slippage at cycles 1, 10, and 100 (P < .05) and more maximum slippage after 500 cycles (mean, 11.2 mm; SD, 4.7 mm) compared with ReelX (mean, 1.9 mm; SD, 0.5 mm) (P = .004). Figure 4 shows mean suture slippage at each cycle.
Kaplan-Meier analysis revealed significantly (λ2 = 8.170; P = .0043) decreased survival after dynamic testing for PushLock versus ReelX (Figure 5). Survival was defined as suture slippage of less than 5 mm after completion of dynamic testing. Only 1 of the 6 PushLock anchors completed dynamic testing; the other 5 failed via complete suture slippage from the anchor before testing could be completed. All 6 ReelX devices survived dynamic testing.
Therefore, 1 PushLock implant and all 6 ReelX devices were available for subsequent load-to-failure testing. Failure in this setting was defined as suture slippage of more than 10 mm or suture breakage. The PushLock implant failed at a maximum force of 171.8 N with a stiffness of 74.4 N/mm and eventually exhibited gross suture slippage. All 6 ReelX devices failed at a mean (SD) maximum of 273.5 (20.2) N, with a mean (SD) stiffness of 74.1 (17) N/mm. Mechanism of failure for all ReelX devices was suture breakage during the tensile load-to-failure test.
Discussion
We evaluated a new technique designed to isolate suture slippage in knotless anchors used for RCR. The impetus for developing this new method was to provide a means for better analyzing the ability of a knotless anchor to resist suture slippage in the cadaveric biomechanical testing setting. Suture slippage is an important mode of failure during such analyses.11,12 Significant slippage occurred in a range of implants before half the anchor–bone pullout strength was reached in a study using young bovine femoral heads.11 In another study, using young, high-BMD cadaveric humeral heads, initial slippage and maximum failure loads were equivalent among numerous devices using various suture-retention mechanisms, and suture slippage was the most common failure mode.12 Nevertheless, other biomechanical studies have demonstrated frequent failure caused by anchor pullout in elderly human cadaveric specimens with diminished BMD, often with high-force testing protocols.12,13 In the more modest-force, in vivo rehabilitative environment, suture slippage rather than anchor dislodgement may be the main failure mode.11-15
We compared the PushLock implant and its entirely press-fit suture clamping design with the ReelX device, which relies on an intrinsic suture-locking mechanism. Middle-aged (mean, 53.3 years; SD, 5.7 years) cadaveric humeri were tested under physiologically relevant biomechanical conditions to begin to help identify how relatively osteopenic bone may affect suture-retention properties for a given implant. The results showed that the study methodology prevented implant failure via anchor–bone pullout. To our knowledge, this was the first study to exclusively analyze suture slippage in knotless anchors. The findings indicated that implants that rely heavily on a tight interference fit of the suture between the anchor and the surrounding bone may exhibit early slippage and failure after RCR in middle-aged patients with relative osteopenia.11,12 However, this study also demonstrated that devices with intrinsic clamping mechanisms that do not depend on the quality of surrounding bone may better resist suture slippage. It is not clear that all knotless anchors with intrinsic locking mechanisms function equivalently. For instance, Pietschmann and colleagues12 found that 2 of 10 implants with a different internal clamping device were unable to resist failure via suture slippage, even in healthy bone. Similarly, in a study comparing ReelX devices with implants having a different internal suture-retention mechanism, ReelX failed at higher ultimate loads, and typically via anchor dislodgement, versus suture slippage in the other implants.18
It is important to note that, in the present study, the loads at which sutures broke in the intrinsic clamping anchors approached the maximum contractile force of the supraspinatus muscle (302 N).19,20 In addition, these loads were above the resistance of the rotator cuff tendon to cut out with modern suture material.21
This study’s limitations include use of an in vitro human cadaveric model that precluded analysis of the effects of postoperative healing. Biomechanical testing was also performed in a single row-type suture configuration with the rotator cuff tendon removed. Fixtures used during testing oriented the load coaxially with the axis of tension, creating a worst-case loading scenario. Although this form of testing may limit its clinical applicability, its purpose was to critically isolate how well a knotless anchor could resist suture slippage. The methods we used were also limited because the stability of the bone–anchor interface was not assessed. For patients with osteopenia, anchor pullout rather than suture slippage could be the most limiting factor for knotless anchor construct failure, and therefore further testing of both failure modes is needed. Future biomechanical studies should compare various knotless anchors’ suture-slippage characteristics in other constructs in physiologic testing orientations, including double-row and suture-bridge configurations, as well as with intact rotator cuff tendons. In addition, use of labral tape as a substitute for polyblend suture has been suggested to limit suture slippage, and this technique theoretically could have changed the results of this study.22
Conclusion
An implant with an internal ratcheting mechanism for suture retention demonstrated significantly less suture slippage in an axial tension evaluation protocol than a device reliant on interference fit of the suture between the anchor and surrounding bone. In the clinical setting, this may allow for less gap formation during the healing phase following RCR with a knotless anchor. There was also increased maximum load to failure, demonstrating an increased load until catastrophic failure using a device with a ratcheting internal locking mechanism.
1. Thal R. A knotless suture anchor. Design, function, and biomechanical testing. Am J Sports Med. 2001;29(5):646-649.
2. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
3. Kim KC, Shin HD, Cha SM, Lee WY. Comparison of repair integrity and functional outcomes for 3 arthroscopic suture bridge rotator cuff repair techniques. Am J Sports Med. 2013;41(2):271-277.
4. Choi CH, Kim SK, Cho MR, et al. Functional outcomes and structural integrity after double-pulley suture bridge rotator cuff repair using serial ultrasonographic examination. J Shoulder Elbow Surg. 2012;21(12):1753-1763.
5. Brown BS, Cooper AD, McIff TE, Key VH, Toby EB. Initial fixation and cyclic loading stability of knotless suture anchors for rotator cuff repair. J Shoulder Elbow Surg. 2008;17(2):313-318.
6. Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD. A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy. 2009;25(3):274-281.
7. Hepp P, Osterhoff G, Engel T, Marquass B, Klink T, Josten C. Biomechanical evaluation of knotless anatomical double-layer double-row rotator cuff repair: a comparative ex vivo study. Am J Sports Med. 2009;37(7):1363-1369.
8. Maguire M, Goldberg J, Bokor D, et al. Biomechanical evaluation of four different transosseous-equivalent/suture bridge rotator cuff repairs. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1582-1587.
9. Millar NL, Wu X, Tantau R, Silverstone E, Murrell GA. Open versus two forms of arthroscopic rotator cuff repair. Clin Orthop Relat Res. 2009;467(4):966-978.
10. Rhee YG, Cho NS, Parke CS. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med. 2012;40(11):2440-2447.
11. Wieser K, Farshad M, Vlachopoulos L, Ruffieux K, Gerber C, Meyer DC. Suture slippage in knotless suture anchors as a potential failure mechanism in rotator cuff repair. Arthroscopy. 2012;28(11):1622-1627.
12. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
13. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
14. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
15. Bynum CK, Lee S, Mahar A, Tasto J, Pedowitz R. Failure mode of suture anchors as a function of insertion depth. Am J Sports Med. 2005;33(7):1030-1034.
16. Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76(3):371-380.
17. Schneeberger AG, von Roll A, Kalberer F, Jacob HA, Gerber C. Mechanical strength of arthroscopic rotator cuff repair techniques: an in vitro study. J Bone Joint Surg Am. 2002;84(12):2152-2160.
18. Efird C, Traub S, Baldini T, et al. Knotless single-row rotator cuff repair: a comparative biomechanical study of 2 knotless suture anchors. Orthopedics. 2013;36(8):e1033-e1037.
19. Wright PB, Budoff JE, Yeh ML, Kelm ZS, Luo ZP. Strength of damaged suture: an in vitro study. Arthroscopy. 2006;22(12):1270-1275.
20. Burkhart SS. A stepwise approach to arthroscopic rotator cuff repair based on biomechanical principles. Arthroscopy. 2000;16(1):82-90.
21. Bisson LJ, Manohar LM. A biomechanical comparison of the pullout strength of No. 2 FiberWire suture and 2-mm FiberWire tape in bovine rotator cuff tendons. Arthroscopy. 2010;26(11):1463-1468.
22. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
1. Thal R. A knotless suture anchor. Design, function, and biomechanical testing. Am J Sports Med. 2001;29(5):646-649.
2. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
3. Kim KC, Shin HD, Cha SM, Lee WY. Comparison of repair integrity and functional outcomes for 3 arthroscopic suture bridge rotator cuff repair techniques. Am J Sports Med. 2013;41(2):271-277.
4. Choi CH, Kim SK, Cho MR, et al. Functional outcomes and structural integrity after double-pulley suture bridge rotator cuff repair using serial ultrasonographic examination. J Shoulder Elbow Surg. 2012;21(12):1753-1763.
5. Brown BS, Cooper AD, McIff TE, Key VH, Toby EB. Initial fixation and cyclic loading stability of knotless suture anchors for rotator cuff repair. J Shoulder Elbow Surg. 2008;17(2):313-318.
6. Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD. A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy. 2009;25(3):274-281.
7. Hepp P, Osterhoff G, Engel T, Marquass B, Klink T, Josten C. Biomechanical evaluation of knotless anatomical double-layer double-row rotator cuff repair: a comparative ex vivo study. Am J Sports Med. 2009;37(7):1363-1369.
8. Maguire M, Goldberg J, Bokor D, et al. Biomechanical evaluation of four different transosseous-equivalent/suture bridge rotator cuff repairs. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1582-1587.
9. Millar NL, Wu X, Tantau R, Silverstone E, Murrell GA. Open versus two forms of arthroscopic rotator cuff repair. Clin Orthop Relat Res. 2009;467(4):966-978.
10. Rhee YG, Cho NS, Parke CS. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med. 2012;40(11):2440-2447.
11. Wieser K, Farshad M, Vlachopoulos L, Ruffieux K, Gerber C, Meyer DC. Suture slippage in knotless suture anchors as a potential failure mechanism in rotator cuff repair. Arthroscopy. 2012;28(11):1622-1627.
12. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
13. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
14. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
15. Bynum CK, Lee S, Mahar A, Tasto J, Pedowitz R. Failure mode of suture anchors as a function of insertion depth. Am J Sports Med. 2005;33(7):1030-1034.
16. Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76(3):371-380.
17. Schneeberger AG, von Roll A, Kalberer F, Jacob HA, Gerber C. Mechanical strength of arthroscopic rotator cuff repair techniques: an in vitro study. J Bone Joint Surg Am. 2002;84(12):2152-2160.
18. Efird C, Traub S, Baldini T, et al. Knotless single-row rotator cuff repair: a comparative biomechanical study of 2 knotless suture anchors. Orthopedics. 2013;36(8):e1033-e1037.
19. Wright PB, Budoff JE, Yeh ML, Kelm ZS, Luo ZP. Strength of damaged suture: an in vitro study. Arthroscopy. 2006;22(12):1270-1275.
20. Burkhart SS. A stepwise approach to arthroscopic rotator cuff repair based on biomechanical principles. Arthroscopy. 2000;16(1):82-90.
21. Bisson LJ, Manohar LM. A biomechanical comparison of the pullout strength of No. 2 FiberWire suture and 2-mm FiberWire tape in bovine rotator cuff tendons. Arthroscopy. 2010;26(11):1463-1468.
22. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
The Effect of Arthroscopic Rotator Interval Closure on Glenohumeral Volume
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
Spontaneous Osteonecrosis of Knee After Arthroscopy Is Not Necessarily Related to the Procedure
The term spontaneous osteonecrosis of the knee was first used by Ahlbäck1 in 1968. This term, and the acronym SONK (sometimes SPONK2), has subsequently been used by other authors to refer to an apparent osteonecrosis of the knee, most commonly occurring within the medial femoral condyle. SONK typically occurs in older women who usually do not have the typical osteonecrosis risk factors, such as steroid use, sickle-cell anemia, and excessive alcohol intake. Furthermore, the radiologic appearance of SONK differs from the typical avascular necrosis findings seen with radiography and magnetic resonance imaging (MRI). In particular, on MRI, the abnormality of SONK does not have the typical serpiginous margin of bone infarction, or the double-line sign indicating both sclerosis and granulation tissue.3 SONK is normally seen as a line of signal intensity on T1- and T2-weighted sequences; this line is adjacent to or parallels the subchondral bone with an adjacent area of extensive edema.
There is dispute over the cause of SONK. Yamamoto and Bullough4 proposed the lesion is in part a subchondral insufficiency fracture and staged it into 4 parts. Histologic findings suggest at least some SONK lesions are subchondral insufficiency fractures.5 Brahme and colleagues6 were the first to describe SONK occurring after arthroscopy, and others have documented this finding. The condition has also been referred to as osteonecrosis in the postoperative knee.7-13 An association of postoperative SONK with cartilage loss and meniscal tear has been proposed.7-13
We reviewed the clinical, radiologic, and MRI findings in 11 patients with evidence of postarthroscopy SONK to try to identify any risk factors that might predispose them to poor outcomes. Our study population consisted of 11 patients (12 knees) with SONK; 6 of the knees had the lesion before knee arthroscopy, and the other 6 developed the lesion after arthroscopy. We also considered MRI findings in a group of 11 age- and sex-matched patients who underwent knee arthroscopy and did not have or develop SONK. We reviewed the preoperative MRI findings of both groups for meniscal tear, meniscal extrusion, and cartilage loss. We had 2 hypotheses. First, patients with preoperative MRI findings of SONK would have articular cartilage changes, posterior root degeneration, and meniscal extrusion similar to those of patients who developed SONK after arthroscopy. Second, an age- and sex-matched group of patients who underwent arthroscopy and did not develop SONK would be similar in articular cartilage changes, posterior root degeneration or tear, and meniscal extrusion.
Materials and Methods
With institutional review board approval and waived informed consent, we reviewed all imaging studies, particularly the radiographs and MRI studies, of 11 patients (12 knees) who either had SONK before arthroscopy or developed it after arthroscopy. In all these cases, arthroscopy was performed to alleviate mechanical symptoms associated with meniscal tear.
On subsequent review by a musculoskeletal radiologist, 6 patients with SONK had an identifiable lesion before surgery. All patients’ symptoms had not improved with an earlier trial of conservative management. All preoperative and postoperative radiologic and MRI findings were reviewed. The patient group was assembled by writing to all the orthopedic surgeons who performed arthroscopy at our institution and asking for SONK cases seen in their practices. All but 2 cases were performed by a surgeon who treated a predominantly older, less active population. Clinical notes were reviewed for outcomes, and the musculoskeletal radiologist reviewed all radiologic studies. The 4 men and 7 women in the SONK group (1 woman had bilateral knee lesions) ranged in age from 43 to 74 years (mean, 63.8 years), and the 4 men and 7 women in the control group were age-matched to 43 to 75 years (mean, 63.6 years). The controls were chosen from a pool of patients who underwent knee arthroscopy at our institution.
MRI was performed using General Electric 1-T, 1.5-T, or 3-T magnets (GE Healthcare, Milwaukee, Wisconsin) or using Philips 1.5-T or open 0.7-T magnets (Philips Healthcare, Andover, Massachusetts). Imaging included sagittal and coronal proton density–weighted sequences and coronal and axial fat-suppressed T2-weighted sequences. SONK was diagnosed when a low signal line adjacent to the subchondral bone plate on the femoral or tibial condyles was present with an adjacent area of bone marrow edema in the respective condyle or when there was depression of the subchondral bone plate with adjacent edema. The MRI studies were reviewed for lesion location, and medial meniscus and lateral meniscus were reviewed for tear. Type of meniscal tear (horizontal cleavage, radial, complex degenerative) was documented, as was meniscal extrusion. The meniscus was regarded as extruded if the body extended more than 3 mm from the joint margin. Cartilage in the medial and lateral compartment was reviewed according to a modified Noyes scale listing 0 as normal, 1 as internal changes only, 2A as 1% to 49% cartilage loss, 2B as 50% to 90% loss of articular cartilage, 3A as 100% articular cartilage loss with subchondral bone plate intact, and 3B as 100% articular cartilage loss with ulcerated subchondral bone plate.14 Osteoarthritic severity was similarly classified using the Kellgren-Lawrence scale,15 where grade 0 is normal; grade 1 is unlikely to have narrowing of the joint space but potentially has osteophytic lipping; grade 2 has both definite narrowing of the joint space and osteophytes; grade 3 has narrowing of the joint space and multiple osteophytes, some sclerosis, and possible deformity of bone contour; and grade 4 has marked narrowing of the joint space, large osteophytes, severe sclerosis, and definite deformity of bone contour. Follow-up clinical notes and radiologic studies were reviewed in the assessment of patient outcomes.
All statistical analyses were performed with SAS 9.2 software (SAS Institute, Cary, North Carolina). Age data were evaluated with the Shapiro-Wilk test and graphical displays and were found to violate normality assumptions, so they are presented as medians and ranges; other variables are presented as count and column percentages. The Wilcoxon rank sum test was used to compare the 2 groups’ age distributions. Fisher exact tests were used to compare proportions between the 2 groups for the other variables. Statistical significance was set at P < .05.
Results
Table 1 lists the demographics and imaging characteristics of the 11 patients—6 had SONK before arthroscopy and 6 developed it after arthroscopy. Comparison of the 11 patients with SONK and the 11 controls is summarized with P values in Table 2. Representative cases that either presented before surgery or developed after surgery are shown in Figures 1 to 4. There were 6 prearthroscopy lesions and 6 postarthroscopy lesions—all 12 in the medial femoral condyle. Eleven of the 12 knees had a medial meniscal tear, and 1 knee had both medial and lateral meniscal tears. In 8 of the 12 knees, the lateral meniscus was normal; in 2 knees, it had mild degeneration; and, in 1 knee, it had a complex tear. Assessment of hyaline cartilage revealed medial cartilage loss ranging from 2A to 3B (median, 2B) in the patients with SONK, and lateral cartilage loss ranging from 0 to 2A (median, 0). At surgery, all knees had a partial medial meniscectomy, and 6 had a partial lateral meniscectomy. Ten of the 12 knees had chondroplasty, 9 patellar and 5 of the medial femoral condyle. Only 4 of the 11 patients with follow-up of more than 1 year went on to joint replacement. Six of the 12 had follow-up of more than 2 years. Of the 6 patients without an identifiable SONK lesion on MRI before arthroscopy, 4 had mild to moderate knee pain 0.5, 2.4, 3.5, and 4 years after surgery. For the other 2 patients, knee replacement was performed 1.5 and 1.8 years after surgery. Of the 6 patients with prearthroscopy SONK, 4 had mild to moderate knee pain 1.5, 3.7, 6.5, and 6.8 years after surgery; the other 2 had knee replacement 0.5 and 1.8 years after surgery. Articular cartilage degeneration and meniscal extrusion were similar (Table 1). In the control group, there was only 1 knee replacement, at 3 years, and the other 11 were functioning 2.6 to 5 years later. The longer follow-up resulted from selection of appropriate controls from the same year. Of the 6 SONK lesions found on preoperative MRI, 3 were read by the interpreting radiologist before surgery as possible SONK lesions, 2 were read as insufficiency fractures, and 1 was read as a possible insufficiency fracture.
Discussion
SONK is well described as a complication of arthroscopic knee surgery. However, this condition more commonly appears spontaneously in a population that has not had surgery. It has become clear that the term SONK may be misleading.16 In a recent series of postoperative subchondral fractures reported by MacDessi and colleagues,5 the average age of patients included in their study was 64 years. Pathologic analysis revealed subchondral fracture with callus formation in all cases. Only 2 knees had evidence of osteonecrosis, which appeared to be secondary to the fracture. Based on these findings, the authors concluded that “further investigation into the etiology of this condition is warranted.” A prominent association with medial meniscal tear has been noted, with the medial femoral condyle predominantly affected. As already mentioned, SONK differs from classical avascular necrosis on several points, including lack of the typical avascular osteonecrosis risk factors and absence of the serpiginous margin and double-line sign seen with typical bone infarction. In addition, the SONK lesions seen on radiographs and MRIs of the knee typically are in the medial femoral condyle and are very different from the typical area of infarction seen in patients with known risk factors for secondary osteonecrosis.
The cause of SONK is not known. Of more importance from a medicolegal standpoint is that these lesions are not necessarily related to arthroscopy.17 Interestingly, Pape and colleagues17 noted that some of the lesions they studied may have been present before surgery, which is what we found in 6 (50%) of the SONK knees in our study. Our data thus support the proposition that some SONK lesions are present before arthroscopy, and some cases of so-called postarthroscopy SONK may in fact have been progressing before surgery.
Our data also reinforce the importance of radiologist–orthopedic surgeon communication regarding the presence of SONK. We emphasize the importance of communicating the MRI findings clearly, whether the lesion is called SONK, SPONK, or insufficiency fracture. The orthopedic surgeons in our series may have been unaware of the presence of these lesions before arthroscopic meniscectomy, given the wide variety of terms being used in radiologic reports.
The natural history of spontaneous osteonecrosis of the medial tibial plateau has also been studied.18 There were 3 outcome patterns—acute extensive collapse of the medial tibial plateau, rapid progression to varying degrees of osteoarthritis, and complete resolution. It has been shown that resolution of SONK can occur in the early stages of the disease, within several months, but often the changes progress to bone destruction and articular cartilage collapse.19
In our series of patients, there was a female predominance, and mean age was 64 years. We investigated cartilage loss, meniscal tear, and meniscal extrusion to see if we could predict outcomes in patients who had the lesion before arthroscopy and if we could predict who might be at risk for developing the lesion after arthroscopy. Type of surgical procedure was also reviewed. For the sake of simplicity, we divided the follow-up patients into 2 groups: those managed with conservative treatment, which we deemed a reasonable outcome, and those who subsequently required knee joint replacement, which we deemed a poor outcome. As seen from our representative cases, both groups had patients with cartilage loss, meniscal tear, and meniscal extrusion to varying degrees. There were no risk factors pointing to a reasonable or poor outcome. In the group of patients with prearthroscopy lesions, we found the same problem. We were unable to identify a risk factor that might suggest a poor rather than a reasonable outcome. We must also emphasize that, in our review of patient charts, we could find no other causes for osteonecrosis. In particular, arthroscopic causes of acute chondral loss (eg, thermal wash, laser, bupivacaine pain pumps, epinephrine in irrigant) were not identified.
This study consisted of a series of cases managed at our institution over the past 8 years. Our data and this study had several limitations:
We may have been unable to identify other SONK cases that belonged in the group from our institution. In addition, we had only 11 patients for comparison with patients without SONK. Likewise, there were only 6 knees each in the prearthroscopy and postarthroscopy SONK groups. We also used images obtained from 1-T, 1.5-T, and 3-T closed MRI devices and one 0.7-T open device. These were, however, at the same institution.
Timing of our imaging was not uniform. In particular, in 3 of the patients who developed SONK after arthroscopy, preoperative MRI studies were performed quite some time before surgery. However, in these patients, more recent preoperative radiographs did not show any evidence of lesions. It can also be seen that postarthroscopy follow-up of patients varied. It is possible that, on longer follow-up, some of the cases we classified as having a reasonable outcome may have gone on to require total knee arthroplasty. One could argue that, in the patient who developed SONK within 1 year after surgery (Figure 4), the lesion was not related to the surgery. However, this patient’s radiographs 3 months after surgery did not show the SONK lesion but clearly showed prominent medial joint space narrowing—a new finding.
Only 1 musculoskeletal radiologist evaluated the radiographs, MRIs, and tomosynthesis (similar to computed tomography) studies for this investigation.
This lesion is not common, thus giving us a small group to analyze.
Despite our data limitations and the retrospective nature of this study, we compiled a reasonably representative sample of surgical SONK patients that matches other samples reported in the literature. Unfortunately, we could not identify any risk factors pointing to the likelihood of developing SONK or any risk factors pointing to either a reasonable or a poor prognosis in these patients. The etiology of the lesion remains an enigma. Our finding 6 cases of prearthroscopy lesions that did not necessarily result in a poor outcome, combined with our inability to identify any risk factors for SONK, points to the lack of a causal relationship with arthroscopy.
1. Ahlbäck S. Osteoarthritis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
2. Juréus J, Lindstrand A, Geijer M, Robertsson O, Tägil M. The natural course of spontaneous osteonecrosis of the knee (SPONK): a 1- to 27-year follow-up of 40 patients. Acta Orthop. 2013;84(4):410-414.
3. Zurlo JV. The double-line sign. Radiology. 1999;212(2):541-542.
4. Yamamoto T, Bullough PG. Spontaneous osteonecrosis of the knee: the result of subchondral insufficiency fracture. J Bone Joint Surg Am. 2000;82(6):858-866.
5. MacDessi SJ, Brophy RH, Bullough PG, Windsor RE, Sculco TP. Subchondral fracture following arthroscopic knee surgery. A series of eight cases. J Bone Joint Surg Am. 2008;90(5):1007-1012.
6. Brahme SK, Fox JM, Ferkel RD, Friedman MJ, Flannigan BD, Resnick DL. Osteonecrosis of the knee after arthroscopic surgery: diagnosis with MR imaging. Radiology. 1991;178(3):851-853.
7. Faletti C, Robba T, de Petro P. Postmeniscectomy osteonecrosis. Arthroscopy. 2002;18(1):91-94.
8. Johnson TC, Evans JA, Gilley JA, DeLee JC. Osteonecrosis of the knee after arthroscopic surgery for meniscal tears and chondral lesions. Arthroscopy. 2000;16(3):254-261.
9. al-Kaar M, Garcia J, Fritschy D, Bonvin JC. Aseptic osteonecrosis of the femoral condyle after meniscectomy by the arthroscopic approach. J Radiol. 1997;78(4):283-288.
10. DeFalco RA, Ricci AR, Balduini FC. Osteonecrosis of the knee after arthroscopic meniscectomy and chondroplasty: a case report and literature review. Am J Sports Med. 2003;31(6):1013-1016.
11. Kusayama T. Idiopathic osteonecrosis of the femoral condyle after meniscectomy. Tokai J Exp Clin Med. 2003;28(4):145-150.
12. Prues-Latour V, Bonvin JC, Fritschy D. Nine cases of osteonecrosis in elderly patients following arthroscopic meniscectomy. Knee Surg Sports Traumatol Arthrosc. 1998;6(3):142-147.
13. Santori N, Condello V, Adriani E, Mariani PP. Osteonecrosis after arthroscopic medial meniscectomy. Arthroscopy. 1995;11(2):220-224.
14. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
15. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
16. Kidwai AS, Hemphill SD, Griffiths HJ. Radiologic case study. Spontaneous osteonecrosis of the knee reclassified as insufficiency fracture. Orthopedics. 2005;28(3):236, 333-236.
17. Pape D, Lorbach O, Anagnostakos K, Kohn D. Osteonecrosis in the postarthroscopic knee. Orthopade. 2008;37(11):1099-1107.
18. Satku K, Kumar VP, Chacha PB. Stress fractures around the knee in elderly patients. A cause of acute pain in the knee. J Bone Joint Surg Am. 1990;72(6):918-922.
19. Soucacos PN, Xenakis TH, Beris AE, Soucacos PK, Georgoulis A. Idiopathic osteonecrosis of the medial femoral condyle. Classification and treatment. Clin Orthop. 1997;(341):82-89.
The term spontaneous osteonecrosis of the knee was first used by Ahlbäck1 in 1968. This term, and the acronym SONK (sometimes SPONK2), has subsequently been used by other authors to refer to an apparent osteonecrosis of the knee, most commonly occurring within the medial femoral condyle. SONK typically occurs in older women who usually do not have the typical osteonecrosis risk factors, such as steroid use, sickle-cell anemia, and excessive alcohol intake. Furthermore, the radiologic appearance of SONK differs from the typical avascular necrosis findings seen with radiography and magnetic resonance imaging (MRI). In particular, on MRI, the abnormality of SONK does not have the typical serpiginous margin of bone infarction, or the double-line sign indicating both sclerosis and granulation tissue.3 SONK is normally seen as a line of signal intensity on T1- and T2-weighted sequences; this line is adjacent to or parallels the subchondral bone with an adjacent area of extensive edema.
There is dispute over the cause of SONK. Yamamoto and Bullough4 proposed the lesion is in part a subchondral insufficiency fracture and staged it into 4 parts. Histologic findings suggest at least some SONK lesions are subchondral insufficiency fractures.5 Brahme and colleagues6 were the first to describe SONK occurring after arthroscopy, and others have documented this finding. The condition has also been referred to as osteonecrosis in the postoperative knee.7-13 An association of postoperative SONK with cartilage loss and meniscal tear has been proposed.7-13
We reviewed the clinical, radiologic, and MRI findings in 11 patients with evidence of postarthroscopy SONK to try to identify any risk factors that might predispose them to poor outcomes. Our study population consisted of 11 patients (12 knees) with SONK; 6 of the knees had the lesion before knee arthroscopy, and the other 6 developed the lesion after arthroscopy. We also considered MRI findings in a group of 11 age- and sex-matched patients who underwent knee arthroscopy and did not have or develop SONK. We reviewed the preoperative MRI findings of both groups for meniscal tear, meniscal extrusion, and cartilage loss. We had 2 hypotheses. First, patients with preoperative MRI findings of SONK would have articular cartilage changes, posterior root degeneration, and meniscal extrusion similar to those of patients who developed SONK after arthroscopy. Second, an age- and sex-matched group of patients who underwent arthroscopy and did not develop SONK would be similar in articular cartilage changes, posterior root degeneration or tear, and meniscal extrusion.
Materials and Methods
With institutional review board approval and waived informed consent, we reviewed all imaging studies, particularly the radiographs and MRI studies, of 11 patients (12 knees) who either had SONK before arthroscopy or developed it after arthroscopy. In all these cases, arthroscopy was performed to alleviate mechanical symptoms associated with meniscal tear.
On subsequent review by a musculoskeletal radiologist, 6 patients with SONK had an identifiable lesion before surgery. All patients’ symptoms had not improved with an earlier trial of conservative management. All preoperative and postoperative radiologic and MRI findings were reviewed. The patient group was assembled by writing to all the orthopedic surgeons who performed arthroscopy at our institution and asking for SONK cases seen in their practices. All but 2 cases were performed by a surgeon who treated a predominantly older, less active population. Clinical notes were reviewed for outcomes, and the musculoskeletal radiologist reviewed all radiologic studies. The 4 men and 7 women in the SONK group (1 woman had bilateral knee lesions) ranged in age from 43 to 74 years (mean, 63.8 years), and the 4 men and 7 women in the control group were age-matched to 43 to 75 years (mean, 63.6 years). The controls were chosen from a pool of patients who underwent knee arthroscopy at our institution.
MRI was performed using General Electric 1-T, 1.5-T, or 3-T magnets (GE Healthcare, Milwaukee, Wisconsin) or using Philips 1.5-T or open 0.7-T magnets (Philips Healthcare, Andover, Massachusetts). Imaging included sagittal and coronal proton density–weighted sequences and coronal and axial fat-suppressed T2-weighted sequences. SONK was diagnosed when a low signal line adjacent to the subchondral bone plate on the femoral or tibial condyles was present with an adjacent area of bone marrow edema in the respective condyle or when there was depression of the subchondral bone plate with adjacent edema. The MRI studies were reviewed for lesion location, and medial meniscus and lateral meniscus were reviewed for tear. Type of meniscal tear (horizontal cleavage, radial, complex degenerative) was documented, as was meniscal extrusion. The meniscus was regarded as extruded if the body extended more than 3 mm from the joint margin. Cartilage in the medial and lateral compartment was reviewed according to a modified Noyes scale listing 0 as normal, 1 as internal changes only, 2A as 1% to 49% cartilage loss, 2B as 50% to 90% loss of articular cartilage, 3A as 100% articular cartilage loss with subchondral bone plate intact, and 3B as 100% articular cartilage loss with ulcerated subchondral bone plate.14 Osteoarthritic severity was similarly classified using the Kellgren-Lawrence scale,15 where grade 0 is normal; grade 1 is unlikely to have narrowing of the joint space but potentially has osteophytic lipping; grade 2 has both definite narrowing of the joint space and osteophytes; grade 3 has narrowing of the joint space and multiple osteophytes, some sclerosis, and possible deformity of bone contour; and grade 4 has marked narrowing of the joint space, large osteophytes, severe sclerosis, and definite deformity of bone contour. Follow-up clinical notes and radiologic studies were reviewed in the assessment of patient outcomes.
All statistical analyses were performed with SAS 9.2 software (SAS Institute, Cary, North Carolina). Age data were evaluated with the Shapiro-Wilk test and graphical displays and were found to violate normality assumptions, so they are presented as medians and ranges; other variables are presented as count and column percentages. The Wilcoxon rank sum test was used to compare the 2 groups’ age distributions. Fisher exact tests were used to compare proportions between the 2 groups for the other variables. Statistical significance was set at P < .05.
Results
Table 1 lists the demographics and imaging characteristics of the 11 patients—6 had SONK before arthroscopy and 6 developed it after arthroscopy. Comparison of the 11 patients with SONK and the 11 controls is summarized with P values in Table 2. Representative cases that either presented before surgery or developed after surgery are shown in Figures 1 to 4. There were 6 prearthroscopy lesions and 6 postarthroscopy lesions—all 12 in the medial femoral condyle. Eleven of the 12 knees had a medial meniscal tear, and 1 knee had both medial and lateral meniscal tears. In 8 of the 12 knees, the lateral meniscus was normal; in 2 knees, it had mild degeneration; and, in 1 knee, it had a complex tear. Assessment of hyaline cartilage revealed medial cartilage loss ranging from 2A to 3B (median, 2B) in the patients with SONK, and lateral cartilage loss ranging from 0 to 2A (median, 0). At surgery, all knees had a partial medial meniscectomy, and 6 had a partial lateral meniscectomy. Ten of the 12 knees had chondroplasty, 9 patellar and 5 of the medial femoral condyle. Only 4 of the 11 patients with follow-up of more than 1 year went on to joint replacement. Six of the 12 had follow-up of more than 2 years. Of the 6 patients without an identifiable SONK lesion on MRI before arthroscopy, 4 had mild to moderate knee pain 0.5, 2.4, 3.5, and 4 years after surgery. For the other 2 patients, knee replacement was performed 1.5 and 1.8 years after surgery. Of the 6 patients with prearthroscopy SONK, 4 had mild to moderate knee pain 1.5, 3.7, 6.5, and 6.8 years after surgery; the other 2 had knee replacement 0.5 and 1.8 years after surgery. Articular cartilage degeneration and meniscal extrusion were similar (Table 1). In the control group, there was only 1 knee replacement, at 3 years, and the other 11 were functioning 2.6 to 5 years later. The longer follow-up resulted from selection of appropriate controls from the same year. Of the 6 SONK lesions found on preoperative MRI, 3 were read by the interpreting radiologist before surgery as possible SONK lesions, 2 were read as insufficiency fractures, and 1 was read as a possible insufficiency fracture.
Discussion
SONK is well described as a complication of arthroscopic knee surgery. However, this condition more commonly appears spontaneously in a population that has not had surgery. It has become clear that the term SONK may be misleading.16 In a recent series of postoperative subchondral fractures reported by MacDessi and colleagues,5 the average age of patients included in their study was 64 years. Pathologic analysis revealed subchondral fracture with callus formation in all cases. Only 2 knees had evidence of osteonecrosis, which appeared to be secondary to the fracture. Based on these findings, the authors concluded that “further investigation into the etiology of this condition is warranted.” A prominent association with medial meniscal tear has been noted, with the medial femoral condyle predominantly affected. As already mentioned, SONK differs from classical avascular necrosis on several points, including lack of the typical avascular osteonecrosis risk factors and absence of the serpiginous margin and double-line sign seen with typical bone infarction. In addition, the SONK lesions seen on radiographs and MRIs of the knee typically are in the medial femoral condyle and are very different from the typical area of infarction seen in patients with known risk factors for secondary osteonecrosis.
The cause of SONK is not known. Of more importance from a medicolegal standpoint is that these lesions are not necessarily related to arthroscopy.17 Interestingly, Pape and colleagues17 noted that some of the lesions they studied may have been present before surgery, which is what we found in 6 (50%) of the SONK knees in our study. Our data thus support the proposition that some SONK lesions are present before arthroscopy, and some cases of so-called postarthroscopy SONK may in fact have been progressing before surgery.
Our data also reinforce the importance of radiologist–orthopedic surgeon communication regarding the presence of SONK. We emphasize the importance of communicating the MRI findings clearly, whether the lesion is called SONK, SPONK, or insufficiency fracture. The orthopedic surgeons in our series may have been unaware of the presence of these lesions before arthroscopic meniscectomy, given the wide variety of terms being used in radiologic reports.
The natural history of spontaneous osteonecrosis of the medial tibial plateau has also been studied.18 There were 3 outcome patterns—acute extensive collapse of the medial tibial plateau, rapid progression to varying degrees of osteoarthritis, and complete resolution. It has been shown that resolution of SONK can occur in the early stages of the disease, within several months, but often the changes progress to bone destruction and articular cartilage collapse.19
In our series of patients, there was a female predominance, and mean age was 64 years. We investigated cartilage loss, meniscal tear, and meniscal extrusion to see if we could predict outcomes in patients who had the lesion before arthroscopy and if we could predict who might be at risk for developing the lesion after arthroscopy. Type of surgical procedure was also reviewed. For the sake of simplicity, we divided the follow-up patients into 2 groups: those managed with conservative treatment, which we deemed a reasonable outcome, and those who subsequently required knee joint replacement, which we deemed a poor outcome. As seen from our representative cases, both groups had patients with cartilage loss, meniscal tear, and meniscal extrusion to varying degrees. There were no risk factors pointing to a reasonable or poor outcome. In the group of patients with prearthroscopy lesions, we found the same problem. We were unable to identify a risk factor that might suggest a poor rather than a reasonable outcome. We must also emphasize that, in our review of patient charts, we could find no other causes for osteonecrosis. In particular, arthroscopic causes of acute chondral loss (eg, thermal wash, laser, bupivacaine pain pumps, epinephrine in irrigant) were not identified.
This study consisted of a series of cases managed at our institution over the past 8 years. Our data and this study had several limitations:
We may have been unable to identify other SONK cases that belonged in the group from our institution. In addition, we had only 11 patients for comparison with patients without SONK. Likewise, there were only 6 knees each in the prearthroscopy and postarthroscopy SONK groups. We also used images obtained from 1-T, 1.5-T, and 3-T closed MRI devices and one 0.7-T open device. These were, however, at the same institution.
Timing of our imaging was not uniform. In particular, in 3 of the patients who developed SONK after arthroscopy, preoperative MRI studies were performed quite some time before surgery. However, in these patients, more recent preoperative radiographs did not show any evidence of lesions. It can also be seen that postarthroscopy follow-up of patients varied. It is possible that, on longer follow-up, some of the cases we classified as having a reasonable outcome may have gone on to require total knee arthroplasty. One could argue that, in the patient who developed SONK within 1 year after surgery (Figure 4), the lesion was not related to the surgery. However, this patient’s radiographs 3 months after surgery did not show the SONK lesion but clearly showed prominent medial joint space narrowing—a new finding.
Only 1 musculoskeletal radiologist evaluated the radiographs, MRIs, and tomosynthesis (similar to computed tomography) studies for this investigation.
This lesion is not common, thus giving us a small group to analyze.
Despite our data limitations and the retrospective nature of this study, we compiled a reasonably representative sample of surgical SONK patients that matches other samples reported in the literature. Unfortunately, we could not identify any risk factors pointing to the likelihood of developing SONK or any risk factors pointing to either a reasonable or a poor prognosis in these patients. The etiology of the lesion remains an enigma. Our finding 6 cases of prearthroscopy lesions that did not necessarily result in a poor outcome, combined with our inability to identify any risk factors for SONK, points to the lack of a causal relationship with arthroscopy.
The term spontaneous osteonecrosis of the knee was first used by Ahlbäck1 in 1968. This term, and the acronym SONK (sometimes SPONK2), has subsequently been used by other authors to refer to an apparent osteonecrosis of the knee, most commonly occurring within the medial femoral condyle. SONK typically occurs in older women who usually do not have the typical osteonecrosis risk factors, such as steroid use, sickle-cell anemia, and excessive alcohol intake. Furthermore, the radiologic appearance of SONK differs from the typical avascular necrosis findings seen with radiography and magnetic resonance imaging (MRI). In particular, on MRI, the abnormality of SONK does not have the typical serpiginous margin of bone infarction, or the double-line sign indicating both sclerosis and granulation tissue.3 SONK is normally seen as a line of signal intensity on T1- and T2-weighted sequences; this line is adjacent to or parallels the subchondral bone with an adjacent area of extensive edema.
There is dispute over the cause of SONK. Yamamoto and Bullough4 proposed the lesion is in part a subchondral insufficiency fracture and staged it into 4 parts. Histologic findings suggest at least some SONK lesions are subchondral insufficiency fractures.5 Brahme and colleagues6 were the first to describe SONK occurring after arthroscopy, and others have documented this finding. The condition has also been referred to as osteonecrosis in the postoperative knee.7-13 An association of postoperative SONK with cartilage loss and meniscal tear has been proposed.7-13
We reviewed the clinical, radiologic, and MRI findings in 11 patients with evidence of postarthroscopy SONK to try to identify any risk factors that might predispose them to poor outcomes. Our study population consisted of 11 patients (12 knees) with SONK; 6 of the knees had the lesion before knee arthroscopy, and the other 6 developed the lesion after arthroscopy. We also considered MRI findings in a group of 11 age- and sex-matched patients who underwent knee arthroscopy and did not have or develop SONK. We reviewed the preoperative MRI findings of both groups for meniscal tear, meniscal extrusion, and cartilage loss. We had 2 hypotheses. First, patients with preoperative MRI findings of SONK would have articular cartilage changes, posterior root degeneration, and meniscal extrusion similar to those of patients who developed SONK after arthroscopy. Second, an age- and sex-matched group of patients who underwent arthroscopy and did not develop SONK would be similar in articular cartilage changes, posterior root degeneration or tear, and meniscal extrusion.
Materials and Methods
With institutional review board approval and waived informed consent, we reviewed all imaging studies, particularly the radiographs and MRI studies, of 11 patients (12 knees) who either had SONK before arthroscopy or developed it after arthroscopy. In all these cases, arthroscopy was performed to alleviate mechanical symptoms associated with meniscal tear.
On subsequent review by a musculoskeletal radiologist, 6 patients with SONK had an identifiable lesion before surgery. All patients’ symptoms had not improved with an earlier trial of conservative management. All preoperative and postoperative radiologic and MRI findings were reviewed. The patient group was assembled by writing to all the orthopedic surgeons who performed arthroscopy at our institution and asking for SONK cases seen in their practices. All but 2 cases were performed by a surgeon who treated a predominantly older, less active population. Clinical notes were reviewed for outcomes, and the musculoskeletal radiologist reviewed all radiologic studies. The 4 men and 7 women in the SONK group (1 woman had bilateral knee lesions) ranged in age from 43 to 74 years (mean, 63.8 years), and the 4 men and 7 women in the control group were age-matched to 43 to 75 years (mean, 63.6 years). The controls were chosen from a pool of patients who underwent knee arthroscopy at our institution.
MRI was performed using General Electric 1-T, 1.5-T, or 3-T magnets (GE Healthcare, Milwaukee, Wisconsin) or using Philips 1.5-T or open 0.7-T magnets (Philips Healthcare, Andover, Massachusetts). Imaging included sagittal and coronal proton density–weighted sequences and coronal and axial fat-suppressed T2-weighted sequences. SONK was diagnosed when a low signal line adjacent to the subchondral bone plate on the femoral or tibial condyles was present with an adjacent area of bone marrow edema in the respective condyle or when there was depression of the subchondral bone plate with adjacent edema. The MRI studies were reviewed for lesion location, and medial meniscus and lateral meniscus were reviewed for tear. Type of meniscal tear (horizontal cleavage, radial, complex degenerative) was documented, as was meniscal extrusion. The meniscus was regarded as extruded if the body extended more than 3 mm from the joint margin. Cartilage in the medial and lateral compartment was reviewed according to a modified Noyes scale listing 0 as normal, 1 as internal changes only, 2A as 1% to 49% cartilage loss, 2B as 50% to 90% loss of articular cartilage, 3A as 100% articular cartilage loss with subchondral bone plate intact, and 3B as 100% articular cartilage loss with ulcerated subchondral bone plate.14 Osteoarthritic severity was similarly classified using the Kellgren-Lawrence scale,15 where grade 0 is normal; grade 1 is unlikely to have narrowing of the joint space but potentially has osteophytic lipping; grade 2 has both definite narrowing of the joint space and osteophytes; grade 3 has narrowing of the joint space and multiple osteophytes, some sclerosis, and possible deformity of bone contour; and grade 4 has marked narrowing of the joint space, large osteophytes, severe sclerosis, and definite deformity of bone contour. Follow-up clinical notes and radiologic studies were reviewed in the assessment of patient outcomes.
All statistical analyses were performed with SAS 9.2 software (SAS Institute, Cary, North Carolina). Age data were evaluated with the Shapiro-Wilk test and graphical displays and were found to violate normality assumptions, so they are presented as medians and ranges; other variables are presented as count and column percentages. The Wilcoxon rank sum test was used to compare the 2 groups’ age distributions. Fisher exact tests were used to compare proportions between the 2 groups for the other variables. Statistical significance was set at P < .05.
Results
Table 1 lists the demographics and imaging characteristics of the 11 patients—6 had SONK before arthroscopy and 6 developed it after arthroscopy. Comparison of the 11 patients with SONK and the 11 controls is summarized with P values in Table 2. Representative cases that either presented before surgery or developed after surgery are shown in Figures 1 to 4. There were 6 prearthroscopy lesions and 6 postarthroscopy lesions—all 12 in the medial femoral condyle. Eleven of the 12 knees had a medial meniscal tear, and 1 knee had both medial and lateral meniscal tears. In 8 of the 12 knees, the lateral meniscus was normal; in 2 knees, it had mild degeneration; and, in 1 knee, it had a complex tear. Assessment of hyaline cartilage revealed medial cartilage loss ranging from 2A to 3B (median, 2B) in the patients with SONK, and lateral cartilage loss ranging from 0 to 2A (median, 0). At surgery, all knees had a partial medial meniscectomy, and 6 had a partial lateral meniscectomy. Ten of the 12 knees had chondroplasty, 9 patellar and 5 of the medial femoral condyle. Only 4 of the 11 patients with follow-up of more than 1 year went on to joint replacement. Six of the 12 had follow-up of more than 2 years. Of the 6 patients without an identifiable SONK lesion on MRI before arthroscopy, 4 had mild to moderate knee pain 0.5, 2.4, 3.5, and 4 years after surgery. For the other 2 patients, knee replacement was performed 1.5 and 1.8 years after surgery. Of the 6 patients with prearthroscopy SONK, 4 had mild to moderate knee pain 1.5, 3.7, 6.5, and 6.8 years after surgery; the other 2 had knee replacement 0.5 and 1.8 years after surgery. Articular cartilage degeneration and meniscal extrusion were similar (Table 1). In the control group, there was only 1 knee replacement, at 3 years, and the other 11 were functioning 2.6 to 5 years later. The longer follow-up resulted from selection of appropriate controls from the same year. Of the 6 SONK lesions found on preoperative MRI, 3 were read by the interpreting radiologist before surgery as possible SONK lesions, 2 were read as insufficiency fractures, and 1 was read as a possible insufficiency fracture.
Discussion
SONK is well described as a complication of arthroscopic knee surgery. However, this condition more commonly appears spontaneously in a population that has not had surgery. It has become clear that the term SONK may be misleading.16 In a recent series of postoperative subchondral fractures reported by MacDessi and colleagues,5 the average age of patients included in their study was 64 years. Pathologic analysis revealed subchondral fracture with callus formation in all cases. Only 2 knees had evidence of osteonecrosis, which appeared to be secondary to the fracture. Based on these findings, the authors concluded that “further investigation into the etiology of this condition is warranted.” A prominent association with medial meniscal tear has been noted, with the medial femoral condyle predominantly affected. As already mentioned, SONK differs from classical avascular necrosis on several points, including lack of the typical avascular osteonecrosis risk factors and absence of the serpiginous margin and double-line sign seen with typical bone infarction. In addition, the SONK lesions seen on radiographs and MRIs of the knee typically are in the medial femoral condyle and are very different from the typical area of infarction seen in patients with known risk factors for secondary osteonecrosis.
The cause of SONK is not known. Of more importance from a medicolegal standpoint is that these lesions are not necessarily related to arthroscopy.17 Interestingly, Pape and colleagues17 noted that some of the lesions they studied may have been present before surgery, which is what we found in 6 (50%) of the SONK knees in our study. Our data thus support the proposition that some SONK lesions are present before arthroscopy, and some cases of so-called postarthroscopy SONK may in fact have been progressing before surgery.
Our data also reinforce the importance of radiologist–orthopedic surgeon communication regarding the presence of SONK. We emphasize the importance of communicating the MRI findings clearly, whether the lesion is called SONK, SPONK, or insufficiency fracture. The orthopedic surgeons in our series may have been unaware of the presence of these lesions before arthroscopic meniscectomy, given the wide variety of terms being used in radiologic reports.
The natural history of spontaneous osteonecrosis of the medial tibial plateau has also been studied.18 There were 3 outcome patterns—acute extensive collapse of the medial tibial plateau, rapid progression to varying degrees of osteoarthritis, and complete resolution. It has been shown that resolution of SONK can occur in the early stages of the disease, within several months, but often the changes progress to bone destruction and articular cartilage collapse.19
In our series of patients, there was a female predominance, and mean age was 64 years. We investigated cartilage loss, meniscal tear, and meniscal extrusion to see if we could predict outcomes in patients who had the lesion before arthroscopy and if we could predict who might be at risk for developing the lesion after arthroscopy. Type of surgical procedure was also reviewed. For the sake of simplicity, we divided the follow-up patients into 2 groups: those managed with conservative treatment, which we deemed a reasonable outcome, and those who subsequently required knee joint replacement, which we deemed a poor outcome. As seen from our representative cases, both groups had patients with cartilage loss, meniscal tear, and meniscal extrusion to varying degrees. There were no risk factors pointing to a reasonable or poor outcome. In the group of patients with prearthroscopy lesions, we found the same problem. We were unable to identify a risk factor that might suggest a poor rather than a reasonable outcome. We must also emphasize that, in our review of patient charts, we could find no other causes for osteonecrosis. In particular, arthroscopic causes of acute chondral loss (eg, thermal wash, laser, bupivacaine pain pumps, epinephrine in irrigant) were not identified.
This study consisted of a series of cases managed at our institution over the past 8 years. Our data and this study had several limitations:
We may have been unable to identify other SONK cases that belonged in the group from our institution. In addition, we had only 11 patients for comparison with patients without SONK. Likewise, there were only 6 knees each in the prearthroscopy and postarthroscopy SONK groups. We also used images obtained from 1-T, 1.5-T, and 3-T closed MRI devices and one 0.7-T open device. These were, however, at the same institution.
Timing of our imaging was not uniform. In particular, in 3 of the patients who developed SONK after arthroscopy, preoperative MRI studies were performed quite some time before surgery. However, in these patients, more recent preoperative radiographs did not show any evidence of lesions. It can also be seen that postarthroscopy follow-up of patients varied. It is possible that, on longer follow-up, some of the cases we classified as having a reasonable outcome may have gone on to require total knee arthroplasty. One could argue that, in the patient who developed SONK within 1 year after surgery (Figure 4), the lesion was not related to the surgery. However, this patient’s radiographs 3 months after surgery did not show the SONK lesion but clearly showed prominent medial joint space narrowing—a new finding.
Only 1 musculoskeletal radiologist evaluated the radiographs, MRIs, and tomosynthesis (similar to computed tomography) studies for this investigation.
This lesion is not common, thus giving us a small group to analyze.
Despite our data limitations and the retrospective nature of this study, we compiled a reasonably representative sample of surgical SONK patients that matches other samples reported in the literature. Unfortunately, we could not identify any risk factors pointing to the likelihood of developing SONK or any risk factors pointing to either a reasonable or a poor prognosis in these patients. The etiology of the lesion remains an enigma. Our finding 6 cases of prearthroscopy lesions that did not necessarily result in a poor outcome, combined with our inability to identify any risk factors for SONK, points to the lack of a causal relationship with arthroscopy.
1. Ahlbäck S. Osteoarthritis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
2. Juréus J, Lindstrand A, Geijer M, Robertsson O, Tägil M. The natural course of spontaneous osteonecrosis of the knee (SPONK): a 1- to 27-year follow-up of 40 patients. Acta Orthop. 2013;84(4):410-414.
3. Zurlo JV. The double-line sign. Radiology. 1999;212(2):541-542.
4. Yamamoto T, Bullough PG. Spontaneous osteonecrosis of the knee: the result of subchondral insufficiency fracture. J Bone Joint Surg Am. 2000;82(6):858-866.
5. MacDessi SJ, Brophy RH, Bullough PG, Windsor RE, Sculco TP. Subchondral fracture following arthroscopic knee surgery. A series of eight cases. J Bone Joint Surg Am. 2008;90(5):1007-1012.
6. Brahme SK, Fox JM, Ferkel RD, Friedman MJ, Flannigan BD, Resnick DL. Osteonecrosis of the knee after arthroscopic surgery: diagnosis with MR imaging. Radiology. 1991;178(3):851-853.
7. Faletti C, Robba T, de Petro P. Postmeniscectomy osteonecrosis. Arthroscopy. 2002;18(1):91-94.
8. Johnson TC, Evans JA, Gilley JA, DeLee JC. Osteonecrosis of the knee after arthroscopic surgery for meniscal tears and chondral lesions. Arthroscopy. 2000;16(3):254-261.
9. al-Kaar M, Garcia J, Fritschy D, Bonvin JC. Aseptic osteonecrosis of the femoral condyle after meniscectomy by the arthroscopic approach. J Radiol. 1997;78(4):283-288.
10. DeFalco RA, Ricci AR, Balduini FC. Osteonecrosis of the knee after arthroscopic meniscectomy and chondroplasty: a case report and literature review. Am J Sports Med. 2003;31(6):1013-1016.
11. Kusayama T. Idiopathic osteonecrosis of the femoral condyle after meniscectomy. Tokai J Exp Clin Med. 2003;28(4):145-150.
12. Prues-Latour V, Bonvin JC, Fritschy D. Nine cases of osteonecrosis in elderly patients following arthroscopic meniscectomy. Knee Surg Sports Traumatol Arthrosc. 1998;6(3):142-147.
13. Santori N, Condello V, Adriani E, Mariani PP. Osteonecrosis after arthroscopic medial meniscectomy. Arthroscopy. 1995;11(2):220-224.
14. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
15. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
16. Kidwai AS, Hemphill SD, Griffiths HJ. Radiologic case study. Spontaneous osteonecrosis of the knee reclassified as insufficiency fracture. Orthopedics. 2005;28(3):236, 333-236.
17. Pape D, Lorbach O, Anagnostakos K, Kohn D. Osteonecrosis in the postarthroscopic knee. Orthopade. 2008;37(11):1099-1107.
18. Satku K, Kumar VP, Chacha PB. Stress fractures around the knee in elderly patients. A cause of acute pain in the knee. J Bone Joint Surg Am. 1990;72(6):918-922.
19. Soucacos PN, Xenakis TH, Beris AE, Soucacos PK, Georgoulis A. Idiopathic osteonecrosis of the medial femoral condyle. Classification and treatment. Clin Orthop. 1997;(341):82-89.
1. Ahlbäck S. Osteoarthritis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
2. Juréus J, Lindstrand A, Geijer M, Robertsson O, Tägil M. The natural course of spontaneous osteonecrosis of the knee (SPONK): a 1- to 27-year follow-up of 40 patients. Acta Orthop. 2013;84(4):410-414.
3. Zurlo JV. The double-line sign. Radiology. 1999;212(2):541-542.
4. Yamamoto T, Bullough PG. Spontaneous osteonecrosis of the knee: the result of subchondral insufficiency fracture. J Bone Joint Surg Am. 2000;82(6):858-866.
5. MacDessi SJ, Brophy RH, Bullough PG, Windsor RE, Sculco TP. Subchondral fracture following arthroscopic knee surgery. A series of eight cases. J Bone Joint Surg Am. 2008;90(5):1007-1012.
6. Brahme SK, Fox JM, Ferkel RD, Friedman MJ, Flannigan BD, Resnick DL. Osteonecrosis of the knee after arthroscopic surgery: diagnosis with MR imaging. Radiology. 1991;178(3):851-853.
7. Faletti C, Robba T, de Petro P. Postmeniscectomy osteonecrosis. Arthroscopy. 2002;18(1):91-94.
8. Johnson TC, Evans JA, Gilley JA, DeLee JC. Osteonecrosis of the knee after arthroscopic surgery for meniscal tears and chondral lesions. Arthroscopy. 2000;16(3):254-261.
9. al-Kaar M, Garcia J, Fritschy D, Bonvin JC. Aseptic osteonecrosis of the femoral condyle after meniscectomy by the arthroscopic approach. J Radiol. 1997;78(4):283-288.
10. DeFalco RA, Ricci AR, Balduini FC. Osteonecrosis of the knee after arthroscopic meniscectomy and chondroplasty: a case report and literature review. Am J Sports Med. 2003;31(6):1013-1016.
11. Kusayama T. Idiopathic osteonecrosis of the femoral condyle after meniscectomy. Tokai J Exp Clin Med. 2003;28(4):145-150.
12. Prues-Latour V, Bonvin JC, Fritschy D. Nine cases of osteonecrosis in elderly patients following arthroscopic meniscectomy. Knee Surg Sports Traumatol Arthrosc. 1998;6(3):142-147.
13. Santori N, Condello V, Adriani E, Mariani PP. Osteonecrosis after arthroscopic medial meniscectomy. Arthroscopy. 1995;11(2):220-224.
14. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
15. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
16. Kidwai AS, Hemphill SD, Griffiths HJ. Radiologic case study. Spontaneous osteonecrosis of the knee reclassified as insufficiency fracture. Orthopedics. 2005;28(3):236, 333-236.
17. Pape D, Lorbach O, Anagnostakos K, Kohn D. Osteonecrosis in the postarthroscopic knee. Orthopade. 2008;37(11):1099-1107.
18. Satku K, Kumar VP, Chacha PB. Stress fractures around the knee in elderly patients. A cause of acute pain in the knee. J Bone Joint Surg Am. 1990;72(6):918-922.
19. Soucacos PN, Xenakis TH, Beris AE, Soucacos PK, Georgoulis A. Idiopathic osteonecrosis of the medial femoral condyle. Classification and treatment. Clin Orthop. 1997;(341):82-89.
Arthroscopic Treatment of Tibial Spine Malunion With Resorbable Screws
Anterior tibial spine fractures are rare, occurring with an incidence of 3 per 100,000 per year.1,2 Historically, this fracture has occurred more frequently in children,3-5 and was considered a condition of skeletal immaturity and the pediatric equivalent of an anterior cruciate ligament (ACL) rupture.6 However, recent literature indicates that this fracture is more common in the adult population than previously thought.7 The tibial spine is an attachment point for the ACL and an avulsion may produce ACL laxity,8 predisposing to further symptomatic laxity and premature osteoarthritis. Nearly 40% of these fractures are associated with concomitant injuries to surrounding structures.9
Meyers and McKeever10,11 originally classified these fractures into 3 groups on the basis of displacement. Type I fractures present with no significant displacement of the anterior margin, type II involve displacement and are hinged, while type III have complete displacement.10,11 More recently, a type IV fracture has been added, involving comminution of the displaced fragment. Nondisplaced fractures are commonly treated with immobilization in varying degrees of extension; this allows the femoral condyles to compress and to reduce the fracture while arthroscopic or open reduction is the preferred method for displaced fractures of the tibial spine.2,4,8,10
We report the case of an 11-year-old boy with a tibial spine fracture that failed conservative management. He developed a subsequent malunion with impingement anteriorly of the tibial spine on the notch, and residual instability of the ACL. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old Caucasian boy was referred to our office for evaluation of right knee injury. He sustained the injury approximately 3 months earlier, and it was determined that he had a tibial spine fracture. Conservative management with immobilization in extension and activity modification was undertaken; however, he was referred for further evaluation because of healing in a malreduced position and residual ACL laxity. Physical examination showed a grade 2A Lachman test (contralateral limb with negative Lachman examination), negative McMurray test, and pain with forced hyperextension; range-of-motion examination showed lack of the terminal 5º of extension. Magnetic resonance and computed tomography imaging from an outside facility showed a skeletally immature individual with a large tibial spine fracture that had healed in a malunited position with the fragment extended on a posterior hinge, creating a large prominence anteriorly (Figures 1A, 1B). Magnetic resonance imaging showed that the ACL fibers were likely to remain intact but would lack appropriate tension secondary to the displacement of the tibial insertion.
Because of healing in a displaced position, lack of terminal extension, ACL laxity, and subjective complaints of pain, we discussed surgery with the patient and his parents (Figures 2A, 2B). Four months after the initial injury, the patient underwent surgery for a right tibial spine malunion arthroscopic takedown and repair, as well as an intraoperative evaluation of the ACL. Standard arthroscopy was performed, using anterolateral and anteromedial arthroscopic portals, and an accessory medial peripatellar portal. During surgery, a large prominence was noted in the region of the anterior tibial spine (Figure 3A). The ACL fibers maintained a slack position secondary to the elevation of the tibial insertion point, and intraoperative Lachman examination showed anterior translation of the tibia on the femur as the slack was removed from the ACL. During surgery, impingement of the anterior tibial spine along the femoral notch was shown to be significant by taking the knee into near-full extension (Figure 3B). A cam-like effect was noted at the time of impingement with the posterior soft tissues relaxing to accommodate slight further extension.
Based on these findings, we chose to take down the malunited fracture and repair it (Figure 3C). PDS suture (Ethicon, Somerville, New Jersey) was temporarily placed along the intermeniscal ligament and anterior horns of the medial and lateral menisci, using a system of spinal needles to facilitate suture passage. Surgical clamps were hung from the suture to provide traction on the sutures throughout the case, allowing the intermeniscal ligament and menisci to recede anteriorly to improve working space and aid in preventing iatrogenic injury. These sutures were removed at the conclusion of the case. Using a combination of curettes, elevator, and small shaver, we were able to meticulously remove interposed malunited callus to allow for mobilization of the displaced fragment. After removal of the excess bone formation, a typical donor site was created, allowing the displaced spine fragment to be hinged into appropriate alignment (Figure 3D). We were able to maintain a posterior cortical hinge to facilitate this process.
Then, we placed Kirschner wires (K-wires) across the fracture in an antegrade fashion, anterior to the trochlea and notch, using an accessory medial peripatellar starting point percutaneously, under direct visualization to avoid iatrogenic chondral injury. The tibial spine fragment was temporarily maintained in a reduced position with an arthroscopic probe and pinned in place with two 0.062-in K-wires. The fracture was stabilized with 8 resorbable 1.6-mm poly-L-lactic/polyglycolic acid (PLLA/PGA) nails, in varying lengths from 18 mm to 22 mm. Excellent fixation was obtained, and range of motion was tested from 0º to 80º, without movement of the fracture site (Figure 3E). Fluoroscopy with multi-axial views verified adequate fixation and reduction. Further, we examined and noted a taut ACL after fixation. The patient was placed in a long leg cast for 3 weeks at 30º, based upon intraoperative determination of the position of least tension on the fracture fragment.
At 3-week follow-up, the patient was progressing well and transitioned from a long leg cast to a hinged knee brace, to allow for early range of motion. Radiographs showed appropriate alignment of the tibial spine fracture with no significant loss of fixation (Figures 4A, 4B). Physical therapy was initiated between 0º and 30º, and flexion was progressively increased over the course of the first 3 weeks. Active and active-assist, closed-chain activities were maintained. Seven weeks postoperatively, the patient displayed continued clinical progression. Radiographs showed interval healing with slight lucency over the anterolateral aspect of the fracture fragment, likely related to the early resorptive process of healing. Physical examination showed movement between 0º and 120º, stable Lachman test, and stable anterior drawer. Crutches were discontinued and hinged knee brace was converted to an ACL brace. By the 11th week, motion had increased to 140º, and radiographs continued to show acceptable alignment and healing (Figures 5A, 5B). The patient was released to return to play as tolerated; however, an ACL brace was recommended during his initial return to provide additional support.
Discussion
In this report, we present an approach for arthroscopic reduction of a malunited tibial spine fracture using resorbable PLLA/PGA nails. The number of polyglycolic nails employed is individualized per case, dependent on the surface area and the quality of the bone within the fractured fragment. Preoperative templating allows for measurements from the fractured fragment to the level of the proximal tibial physis. Based on these measurements, nails are chosen to maximize fixation length and avoid the physis. Despite studies that have examined the effect of transphyseal K-wire pinning or drilling on subsequent growth, there is no consensus about optimal technique. Experiments in animal models indicate that drill injuries destroying less than 8% to 9% of the physis do not impact total bone growth.12,13 Further, temporary crossing of the physeal plate for internal fixation of dislocated joint injuries has not been shown to result in bone bridging or growth disturbance.14,15
Each nail is 1.6 mm in diameter, leaving a small footprint. The nails are used judiciously to provide effective stabilization of the fragment and to maintain a cost-conscious approach. An accessory superomedial peripatellar portal allows an appropriate angle for nail placement. This portal allows access to all regions of the fractured fragment, while an anteromedial and anterolateral portal are used as working and camera portals, respectively. Nails are placed to provide an axis perpendicular to the fracture line to allow appropriate compression. By virtue of the shape of the typical fragment in a tibial spine fracture, the nails vary in insertion angle.
The occurrence of anterior tibial spine fractures is rare, and while several techniques have been described to repair this fracture, there remains a great deal of uncertainty regarding the best course of treatment. A review of the literature finds arthroscopic and open approaches, as well as techniques employing K-wire fixation, metal screw fixation, staple fixation, absorbable fixation, and fixation with sutures passed through the tibial tunnel.16-18
Avulsion fractures of the tibial eminence were treated with open fixation until McLennan8 first reported the benefits of reduction with an arthroscope. Open reduction and internal fixation provide the benefit of direct visualization,9 while arthroscopic reduction offers decreased morbidity and an accelerated recovery of knee functions,8 despite the fact that a higher rate of range-of-motion deficits were seen in patients treated arthroscopically.19 We feel that with proper early rehabilitation to achieve range of motion, the risk of this can be minimal.
Various arthroscopic approaches that improve the accuracy of the reduction and decrease surgical invasiveness have been described. Suture and screw fixation are among the most common methods, and both have resulted in positive outcomes.20-24 Suture fixation of the tibial eminence is technically demanding but offers secure fixation without the need for follow-up hardware removal. Screw fixation results in secure fixation; however, numerous hardware-related issues may necessitate removal. Furthermore, in skeletally immature patients, screw fixation may disturb the growth plate if it crosses an open physis.9
Hunter and Willis25 retrospectively reviewed patients with tibial eminence fractures treated with either screw or suture fixation and found a 44% reoperation rate in the screw-fixation group. Removal was often recommended as a result of hardware-related issues. There was a 13% reoperation rate in the suture-fixation group, which resulted largely from stiffness.25 In a recent review, Gans and colleagues19 reviewed 6 publications comparing screw and suture fixation of tibial eminence fractures and found 82.4% of screw patients had laxity on both the anterior drawer and Lachman tests, compared with 18.8% in the suture-fixation group. This study also noted a slightly higher rate of arthrofibrosis in patients treated with suture fixation.19 Biomechanical studies indicate that suture fixation imparts greater strength under cyclic-loading conditions;26 however, there does not appear to be a difference in ultimate force required for fixation failure.27
Ultimately, both suture and screw fixation result in secure methods of fixation; however, there are often greater issues with screw fixation because of the persistent hardware. Metal has been the most popular method for fracture fixation, and while biodegradable materials have been alluring, adverse tissue reactions have slowed implementation. However, these implants have become increasingly sophisticated, thereby reducing disadvantages.28 Previous biodegradable devices were often composed of a single polymer, and many caused adverse reactions by degrading too quickly or provided no real advantages because they degraded too slowly.29 As the number of polymers approved for internal use and surgical applications continues to rise, so too will the benefits of employing this technology. Furthermore, by including multiple polymers in these implants, one is better able to control the degradation rate, limiting the tissue response.
In this study, we employed PLLA/PGA nails. Studies of PGA implants indicate this molecule degrades at a fast rate resulting in adverse tissue reactions. Adverse reactions in studies of PLLA implants are less frequent because of their slower rate of degradation.29,30 Combining these monomers results in appropriate strength and a controlled degradation rate, reducing the likelihood of adverse reactions. Furthermore, numerous studies have reported that inflammatory responses in children are rare and mild in nature.31,32 Absorbable implants have displayed efficacy in numerous orthopedic settings33-36 and are beneficial in procedures that are not suitable for repeated surgeries, such as reconstruction of the ACL.37 There is some concern about the use of absorbable implants in synovial joints. Polyglycolic acid use in synovial joints may cause foreign-body reactions and may increase the risk of intra-articular dissemination of polymeric debris;38 however, use of a multipolymer construct decreases the likelihood of this occurrence.
Polyglycolic nails confer the advantage over nonresorbable screw fixation because further procedure for hardware removal is not required. Although suture fixation has proved to be beneficial over nonresorbable screw fixation, implantation of resorbable nails appears to have several advantages. In Dr. Estes’ experience, placement of resorbable screws through an accessory superomedial portal is far less technically demanding than placement of suture through the fracture fragment. Further, as sutures are passed from the extra-articular to the intra-articular region of the joint, capsular layers of the knee may inadvertently be bound up in the fixation, predisposing to arthrofibrosis.
At the same time, biodegradable devices are often more costly than alternative forms of treatment; however, a true cost-to-benefit analysis requires consideration of other factors. One of the benefits of biodegradable hardware is that there is no need for follow-up hardware removal. Reports have indicated that up to 91% of patients thought that hardware removal was the most negative aspect of metal implants.39 It is estimated that if the removal rate for metallic implants is higher than 19% to 54%, resorbable implants would be more cost-effective.40 The cost of sutures and screws is variable, however; they are invariably less expensive than biodegradable nails. A study of fracture patients determined that biodegradable implants were cheaper on average after considering the cost of implant removal.40 Ultimately, the hardware choice depends on numerous factors, including surgeon’s discretion; however, biodegradable hardware should not be discounted for financial reasons because the difference in cost is likely negligible.
Conclusion
The approach described in this report offers efficient and secure fixation with resorbable hardware without a reduction in range of motion. Resorbable implants may prove beneficial in the treatment of tibial eminence fractures by offering robust fixation without the concerns associated with permanent hardware.
1. Hargrove R, Parsons S, Payne R. Anterior tibial spine fracture – an easy fracture to miss. Accid Emerg Nurs. 2004;12(3):173-175.
2. Aderinto J, Walmsley P, Keating JF. Fractures of the tibial spine: epidemiology and outcome. Knee. 2008;15(3):164-167.
3. Driessen MJ, Winkelman PA. Fractures of the intercondylar eminence of the tibia in childhood. Neth J Surg. 1984;36(3):69-72.
4. Zaricznyj B. Avulsion fracture of the tibial eminence: treatment by open reduction and pinning. J Bone Joint Surg Am. 1977;59(8):1111-1114.
5. Molander ML, Wallin G, Wikstad I. Fracture of the intercondylar eminence of the tibia: a review of 35 patients. J Bone Joint Surg Br. 1981;63(1):89-91.
6. Kieser DC, Gwynne-Jones D, Dreyer S. Displaced tibial intercondylar eminence fractures. J Orthop Surg. 2011;19(3):292-296.
7. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop. 2005;(434):207-212.
8. McLennan JG. The role of arthroscopic surgery in the treatment of fractures of the intercondylar eminence of the tibia. J Bone Joint Surg Br. 1982;64(4):477-480.
9. Lafrance RM, Giordano B, Goldblatt J, Voloshin I, Maloney M. Pediatric tibial eminence fractures: evaluation and management. J Am Acad Orthop Surg. 2010;18(7):395-405.
10. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1959;41(2):209-220.
11. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1970;52(8):1677-1684.
12. Garcés GL, Mugica-Garay I, López-González Coviella N, Guerado E. Growth-plate modifications after drilling. J Pediatr Orthop. 1994;14(2):225-228.
13. Janarv PM, Wikström B, Hirsch G. The influence of transphyseal drilling and tendon grafting on bone growth: an experimental study in the rabbit. J Pediatr Orthop. 1998;18(2):149-154.
14. Boelitz R, Dallek M, Meenen NM, Jungbluth KH. Reaction of the epiphyseal groove to groove-crossing bore-wire osteosynthesis. Results of a histomorphologic small animal study. Unfallchirurgie. 1994;20(3):131-137.
15. Yung PS, Lam CY, Ng BK, Lam TP, Cheng JC. Percutaneous transphyseal intramedullary Kirschner wire pinning: a safe and effective procedure for treatment of displaced diaphyseal forearm fracture in children. J Pediatr Orthop. 2004;24(1):7-12.
16. Bong MR, Romero A, Kubiak E, et al. Suture versus screw fixation of displaced tibial eminence fractures: a biomechanical comparison. Arthroscopy. 2005;21(10):1172-1176.
17. Vega JR, Irribarra LA, Baar AK, Iñiguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
18. Shepley RW. Arthroscopic treatment of type III tibial spine fractures using absorbable fixation. Orthopedics. 2004;27(7):767-769.
19. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2013;42(7):1743-1750.
20. Delcogliano A, Chiossi S, Caporaso A, Menghi A, Rinonapoli G. Tibial intercondylar eminence fractures in adults: arthroscopic treatment. Knee Surg Sports Traumatol Arthrosc. 2003;11(4):255-259.
21. Mulhall KJ, Dowdall J, Grannell M, McCabe JP. Tibial spine fractures: an analysis of outcome in surgically treated type III injuries. Injury. 1999;30(4):289-292.
22. Geissler WB, Matthews DE. Arthroscopic suture fixation of displaced tibial eminence fractures. Orthopedics. 1993;16(3):331-333.
23. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
24. Reynders P, Reynders K, Broos P. Pediatric and adolescent tibial eminence fractures: arthroscopic cannulated screw fixation. J Trauma. 2002;53(1):49-54.
25. Hunter RE, Willis JA. Arthroscopic fixation of avulsion fractures of the tibial eminence: technique and outcome. Arthroscopy. 2004;20(2):113-121.
26. Eggers AK, Becker C, Weimann A, et al. Biomechanical evaluation of different fixation methods for tibial eminence fractures. Am J Sports Med. 2007;35(3):404-410.
27. Mahar AT, Duncan D, Oka R, Lowry A, Gillingham B, Chambers H. Biomechanical comparison of four different fixation techniques for pediatric tibial eminence avulsion fractures. J Pediatr Orthop. 2008;28(2):159-162.
28. Toro C, Robiony M, Zerman N, Politi M. Resorbable plates in maxillary fixation. A 5-year experience. Minerva Stomatol. 2005;54(4):199-206.
29. Andriano KP, Pohjonen T, Törmälä P. Processing and characterization of absorbable polylactide polymers for use in surgical implants. J Appl Biomater.1994;5(2):133-140.
30. Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21(24):2615-2621.
31. Rokkanen PU, Böstman O, Hirvensalo E, et al. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000;21(24):2607-2613.
32. Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93-102.
33. Li ZH, Yu AX, Guo XP, Qi BW, Zhou M, Wang WY. Absorbable implants versus metal implants for the treatment of ankle fractures: A meta-analysis. Exp Ther Med. 2013;5(5):1531-1537.
34. Singh G, Mohammad S, Chak RK, Lepcha N, Singh N, Malkunje LR. Bio-resorbable plates as effective implant in paediatric mandibular fracture. J Maxillofac Oral Surg. 2012;11(4):400-406.
35. Sakamoto Y, Shimizu Y, Nagasao T, Kishi K. Combined use of resorbable poly-L-lactic acid-polyglycolic acid implant and bone cement for treating large orbital floor fractures. J Plast Reconstr Aesthet Surg. 2014;67(3):e88-e90.
36. Benz G, Kallieris D, Seeböck T, McIntosh A, Daum R. Bioresorbable pins and screws in paediatric traumatology. Eur J Pediatr Surg. 1994;4(2):103-107.
37. Gaweda K, Walawski J, Weglowski R, Krzyzanowski W. Comparison of bioabsorbable interference screws and posts for distal fixation in anterior cruciate ligament reconstruction. Int Orthop. 2009;33(1):123-127.
38. Böstman OM. Osteoarthritis of the ankle after foreign-body reaction to absorbable pins and screws: a three- to nine-year follow-up study. J Bone Joint Surg Br. 1998;80(2):333-338.
39. Mittal R, Morley J, Dinopoulos H, Drakoulakis EG, Vermani E, Giannoudis PV. Use of bio-resorbable implants for stabilisation of distal radius fractures: the United Kingdom patients’ perspective. Injury. 2005;36(2):333-338.
40. Böstman OM. Metallic or absorbable fracture fixation devices. A cost minimization analysis. Clin Orthop. 1996;(329):233-239.
Anterior tibial spine fractures are rare, occurring with an incidence of 3 per 100,000 per year.1,2 Historically, this fracture has occurred more frequently in children,3-5 and was considered a condition of skeletal immaturity and the pediatric equivalent of an anterior cruciate ligament (ACL) rupture.6 However, recent literature indicates that this fracture is more common in the adult population than previously thought.7 The tibial spine is an attachment point for the ACL and an avulsion may produce ACL laxity,8 predisposing to further symptomatic laxity and premature osteoarthritis. Nearly 40% of these fractures are associated with concomitant injuries to surrounding structures.9
Meyers and McKeever10,11 originally classified these fractures into 3 groups on the basis of displacement. Type I fractures present with no significant displacement of the anterior margin, type II involve displacement and are hinged, while type III have complete displacement.10,11 More recently, a type IV fracture has been added, involving comminution of the displaced fragment. Nondisplaced fractures are commonly treated with immobilization in varying degrees of extension; this allows the femoral condyles to compress and to reduce the fracture while arthroscopic or open reduction is the preferred method for displaced fractures of the tibial spine.2,4,8,10
We report the case of an 11-year-old boy with a tibial spine fracture that failed conservative management. He developed a subsequent malunion with impingement anteriorly of the tibial spine on the notch, and residual instability of the ACL. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old Caucasian boy was referred to our office for evaluation of right knee injury. He sustained the injury approximately 3 months earlier, and it was determined that he had a tibial spine fracture. Conservative management with immobilization in extension and activity modification was undertaken; however, he was referred for further evaluation because of healing in a malreduced position and residual ACL laxity. Physical examination showed a grade 2A Lachman test (contralateral limb with negative Lachman examination), negative McMurray test, and pain with forced hyperextension; range-of-motion examination showed lack of the terminal 5º of extension. Magnetic resonance and computed tomography imaging from an outside facility showed a skeletally immature individual with a large tibial spine fracture that had healed in a malunited position with the fragment extended on a posterior hinge, creating a large prominence anteriorly (Figures 1A, 1B). Magnetic resonance imaging showed that the ACL fibers were likely to remain intact but would lack appropriate tension secondary to the displacement of the tibial insertion.
Because of healing in a displaced position, lack of terminal extension, ACL laxity, and subjective complaints of pain, we discussed surgery with the patient and his parents (Figures 2A, 2B). Four months after the initial injury, the patient underwent surgery for a right tibial spine malunion arthroscopic takedown and repair, as well as an intraoperative evaluation of the ACL. Standard arthroscopy was performed, using anterolateral and anteromedial arthroscopic portals, and an accessory medial peripatellar portal. During surgery, a large prominence was noted in the region of the anterior tibial spine (Figure 3A). The ACL fibers maintained a slack position secondary to the elevation of the tibial insertion point, and intraoperative Lachman examination showed anterior translation of the tibia on the femur as the slack was removed from the ACL. During surgery, impingement of the anterior tibial spine along the femoral notch was shown to be significant by taking the knee into near-full extension (Figure 3B). A cam-like effect was noted at the time of impingement with the posterior soft tissues relaxing to accommodate slight further extension.
Based on these findings, we chose to take down the malunited fracture and repair it (Figure 3C). PDS suture (Ethicon, Somerville, New Jersey) was temporarily placed along the intermeniscal ligament and anterior horns of the medial and lateral menisci, using a system of spinal needles to facilitate suture passage. Surgical clamps were hung from the suture to provide traction on the sutures throughout the case, allowing the intermeniscal ligament and menisci to recede anteriorly to improve working space and aid in preventing iatrogenic injury. These sutures were removed at the conclusion of the case. Using a combination of curettes, elevator, and small shaver, we were able to meticulously remove interposed malunited callus to allow for mobilization of the displaced fragment. After removal of the excess bone formation, a typical donor site was created, allowing the displaced spine fragment to be hinged into appropriate alignment (Figure 3D). We were able to maintain a posterior cortical hinge to facilitate this process.
Then, we placed Kirschner wires (K-wires) across the fracture in an antegrade fashion, anterior to the trochlea and notch, using an accessory medial peripatellar starting point percutaneously, under direct visualization to avoid iatrogenic chondral injury. The tibial spine fragment was temporarily maintained in a reduced position with an arthroscopic probe and pinned in place with two 0.062-in K-wires. The fracture was stabilized with 8 resorbable 1.6-mm poly-L-lactic/polyglycolic acid (PLLA/PGA) nails, in varying lengths from 18 mm to 22 mm. Excellent fixation was obtained, and range of motion was tested from 0º to 80º, without movement of the fracture site (Figure 3E). Fluoroscopy with multi-axial views verified adequate fixation and reduction. Further, we examined and noted a taut ACL after fixation. The patient was placed in a long leg cast for 3 weeks at 30º, based upon intraoperative determination of the position of least tension on the fracture fragment.
At 3-week follow-up, the patient was progressing well and transitioned from a long leg cast to a hinged knee brace, to allow for early range of motion. Radiographs showed appropriate alignment of the tibial spine fracture with no significant loss of fixation (Figures 4A, 4B). Physical therapy was initiated between 0º and 30º, and flexion was progressively increased over the course of the first 3 weeks. Active and active-assist, closed-chain activities were maintained. Seven weeks postoperatively, the patient displayed continued clinical progression. Radiographs showed interval healing with slight lucency over the anterolateral aspect of the fracture fragment, likely related to the early resorptive process of healing. Physical examination showed movement between 0º and 120º, stable Lachman test, and stable anterior drawer. Crutches were discontinued and hinged knee brace was converted to an ACL brace. By the 11th week, motion had increased to 140º, and radiographs continued to show acceptable alignment and healing (Figures 5A, 5B). The patient was released to return to play as tolerated; however, an ACL brace was recommended during his initial return to provide additional support.
Discussion
In this report, we present an approach for arthroscopic reduction of a malunited tibial spine fracture using resorbable PLLA/PGA nails. The number of polyglycolic nails employed is individualized per case, dependent on the surface area and the quality of the bone within the fractured fragment. Preoperative templating allows for measurements from the fractured fragment to the level of the proximal tibial physis. Based on these measurements, nails are chosen to maximize fixation length and avoid the physis. Despite studies that have examined the effect of transphyseal K-wire pinning or drilling on subsequent growth, there is no consensus about optimal technique. Experiments in animal models indicate that drill injuries destroying less than 8% to 9% of the physis do not impact total bone growth.12,13 Further, temporary crossing of the physeal plate for internal fixation of dislocated joint injuries has not been shown to result in bone bridging or growth disturbance.14,15
Each nail is 1.6 mm in diameter, leaving a small footprint. The nails are used judiciously to provide effective stabilization of the fragment and to maintain a cost-conscious approach. An accessory superomedial peripatellar portal allows an appropriate angle for nail placement. This portal allows access to all regions of the fractured fragment, while an anteromedial and anterolateral portal are used as working and camera portals, respectively. Nails are placed to provide an axis perpendicular to the fracture line to allow appropriate compression. By virtue of the shape of the typical fragment in a tibial spine fracture, the nails vary in insertion angle.
The occurrence of anterior tibial spine fractures is rare, and while several techniques have been described to repair this fracture, there remains a great deal of uncertainty regarding the best course of treatment. A review of the literature finds arthroscopic and open approaches, as well as techniques employing K-wire fixation, metal screw fixation, staple fixation, absorbable fixation, and fixation with sutures passed through the tibial tunnel.16-18
Avulsion fractures of the tibial eminence were treated with open fixation until McLennan8 first reported the benefits of reduction with an arthroscope. Open reduction and internal fixation provide the benefit of direct visualization,9 while arthroscopic reduction offers decreased morbidity and an accelerated recovery of knee functions,8 despite the fact that a higher rate of range-of-motion deficits were seen in patients treated arthroscopically.19 We feel that with proper early rehabilitation to achieve range of motion, the risk of this can be minimal.
Various arthroscopic approaches that improve the accuracy of the reduction and decrease surgical invasiveness have been described. Suture and screw fixation are among the most common methods, and both have resulted in positive outcomes.20-24 Suture fixation of the tibial eminence is technically demanding but offers secure fixation without the need for follow-up hardware removal. Screw fixation results in secure fixation; however, numerous hardware-related issues may necessitate removal. Furthermore, in skeletally immature patients, screw fixation may disturb the growth plate if it crosses an open physis.9
Hunter and Willis25 retrospectively reviewed patients with tibial eminence fractures treated with either screw or suture fixation and found a 44% reoperation rate in the screw-fixation group. Removal was often recommended as a result of hardware-related issues. There was a 13% reoperation rate in the suture-fixation group, which resulted largely from stiffness.25 In a recent review, Gans and colleagues19 reviewed 6 publications comparing screw and suture fixation of tibial eminence fractures and found 82.4% of screw patients had laxity on both the anterior drawer and Lachman tests, compared with 18.8% in the suture-fixation group. This study also noted a slightly higher rate of arthrofibrosis in patients treated with suture fixation.19 Biomechanical studies indicate that suture fixation imparts greater strength under cyclic-loading conditions;26 however, there does not appear to be a difference in ultimate force required for fixation failure.27
Ultimately, both suture and screw fixation result in secure methods of fixation; however, there are often greater issues with screw fixation because of the persistent hardware. Metal has been the most popular method for fracture fixation, and while biodegradable materials have been alluring, adverse tissue reactions have slowed implementation. However, these implants have become increasingly sophisticated, thereby reducing disadvantages.28 Previous biodegradable devices were often composed of a single polymer, and many caused adverse reactions by degrading too quickly or provided no real advantages because they degraded too slowly.29 As the number of polymers approved for internal use and surgical applications continues to rise, so too will the benefits of employing this technology. Furthermore, by including multiple polymers in these implants, one is better able to control the degradation rate, limiting the tissue response.
In this study, we employed PLLA/PGA nails. Studies of PGA implants indicate this molecule degrades at a fast rate resulting in adverse tissue reactions. Adverse reactions in studies of PLLA implants are less frequent because of their slower rate of degradation.29,30 Combining these monomers results in appropriate strength and a controlled degradation rate, reducing the likelihood of adverse reactions. Furthermore, numerous studies have reported that inflammatory responses in children are rare and mild in nature.31,32 Absorbable implants have displayed efficacy in numerous orthopedic settings33-36 and are beneficial in procedures that are not suitable for repeated surgeries, such as reconstruction of the ACL.37 There is some concern about the use of absorbable implants in synovial joints. Polyglycolic acid use in synovial joints may cause foreign-body reactions and may increase the risk of intra-articular dissemination of polymeric debris;38 however, use of a multipolymer construct decreases the likelihood of this occurrence.
Polyglycolic nails confer the advantage over nonresorbable screw fixation because further procedure for hardware removal is not required. Although suture fixation has proved to be beneficial over nonresorbable screw fixation, implantation of resorbable nails appears to have several advantages. In Dr. Estes’ experience, placement of resorbable screws through an accessory superomedial portal is far less technically demanding than placement of suture through the fracture fragment. Further, as sutures are passed from the extra-articular to the intra-articular region of the joint, capsular layers of the knee may inadvertently be bound up in the fixation, predisposing to arthrofibrosis.
At the same time, biodegradable devices are often more costly than alternative forms of treatment; however, a true cost-to-benefit analysis requires consideration of other factors. One of the benefits of biodegradable hardware is that there is no need for follow-up hardware removal. Reports have indicated that up to 91% of patients thought that hardware removal was the most negative aspect of metal implants.39 It is estimated that if the removal rate for metallic implants is higher than 19% to 54%, resorbable implants would be more cost-effective.40 The cost of sutures and screws is variable, however; they are invariably less expensive than biodegradable nails. A study of fracture patients determined that biodegradable implants were cheaper on average after considering the cost of implant removal.40 Ultimately, the hardware choice depends on numerous factors, including surgeon’s discretion; however, biodegradable hardware should not be discounted for financial reasons because the difference in cost is likely negligible.
Conclusion
The approach described in this report offers efficient and secure fixation with resorbable hardware without a reduction in range of motion. Resorbable implants may prove beneficial in the treatment of tibial eminence fractures by offering robust fixation without the concerns associated with permanent hardware.
Anterior tibial spine fractures are rare, occurring with an incidence of 3 per 100,000 per year.1,2 Historically, this fracture has occurred more frequently in children,3-5 and was considered a condition of skeletal immaturity and the pediatric equivalent of an anterior cruciate ligament (ACL) rupture.6 However, recent literature indicates that this fracture is more common in the adult population than previously thought.7 The tibial spine is an attachment point for the ACL and an avulsion may produce ACL laxity,8 predisposing to further symptomatic laxity and premature osteoarthritis. Nearly 40% of these fractures are associated with concomitant injuries to surrounding structures.9
Meyers and McKeever10,11 originally classified these fractures into 3 groups on the basis of displacement. Type I fractures present with no significant displacement of the anterior margin, type II involve displacement and are hinged, while type III have complete displacement.10,11 More recently, a type IV fracture has been added, involving comminution of the displaced fragment. Nondisplaced fractures are commonly treated with immobilization in varying degrees of extension; this allows the femoral condyles to compress and to reduce the fracture while arthroscopic or open reduction is the preferred method for displaced fractures of the tibial spine.2,4,8,10
We report the case of an 11-year-old boy with a tibial spine fracture that failed conservative management. He developed a subsequent malunion with impingement anteriorly of the tibial spine on the notch, and residual instability of the ACL. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old Caucasian boy was referred to our office for evaluation of right knee injury. He sustained the injury approximately 3 months earlier, and it was determined that he had a tibial spine fracture. Conservative management with immobilization in extension and activity modification was undertaken; however, he was referred for further evaluation because of healing in a malreduced position and residual ACL laxity. Physical examination showed a grade 2A Lachman test (contralateral limb with negative Lachman examination), negative McMurray test, and pain with forced hyperextension; range-of-motion examination showed lack of the terminal 5º of extension. Magnetic resonance and computed tomography imaging from an outside facility showed a skeletally immature individual with a large tibial spine fracture that had healed in a malunited position with the fragment extended on a posterior hinge, creating a large prominence anteriorly (Figures 1A, 1B). Magnetic resonance imaging showed that the ACL fibers were likely to remain intact but would lack appropriate tension secondary to the displacement of the tibial insertion.
Because of healing in a displaced position, lack of terminal extension, ACL laxity, and subjective complaints of pain, we discussed surgery with the patient and his parents (Figures 2A, 2B). Four months after the initial injury, the patient underwent surgery for a right tibial spine malunion arthroscopic takedown and repair, as well as an intraoperative evaluation of the ACL. Standard arthroscopy was performed, using anterolateral and anteromedial arthroscopic portals, and an accessory medial peripatellar portal. During surgery, a large prominence was noted in the region of the anterior tibial spine (Figure 3A). The ACL fibers maintained a slack position secondary to the elevation of the tibial insertion point, and intraoperative Lachman examination showed anterior translation of the tibia on the femur as the slack was removed from the ACL. During surgery, impingement of the anterior tibial spine along the femoral notch was shown to be significant by taking the knee into near-full extension (Figure 3B). A cam-like effect was noted at the time of impingement with the posterior soft tissues relaxing to accommodate slight further extension.
Based on these findings, we chose to take down the malunited fracture and repair it (Figure 3C). PDS suture (Ethicon, Somerville, New Jersey) was temporarily placed along the intermeniscal ligament and anterior horns of the medial and lateral menisci, using a system of spinal needles to facilitate suture passage. Surgical clamps were hung from the suture to provide traction on the sutures throughout the case, allowing the intermeniscal ligament and menisci to recede anteriorly to improve working space and aid in preventing iatrogenic injury. These sutures were removed at the conclusion of the case. Using a combination of curettes, elevator, and small shaver, we were able to meticulously remove interposed malunited callus to allow for mobilization of the displaced fragment. After removal of the excess bone formation, a typical donor site was created, allowing the displaced spine fragment to be hinged into appropriate alignment (Figure 3D). We were able to maintain a posterior cortical hinge to facilitate this process.
Then, we placed Kirschner wires (K-wires) across the fracture in an antegrade fashion, anterior to the trochlea and notch, using an accessory medial peripatellar starting point percutaneously, under direct visualization to avoid iatrogenic chondral injury. The tibial spine fragment was temporarily maintained in a reduced position with an arthroscopic probe and pinned in place with two 0.062-in K-wires. The fracture was stabilized with 8 resorbable 1.6-mm poly-L-lactic/polyglycolic acid (PLLA/PGA) nails, in varying lengths from 18 mm to 22 mm. Excellent fixation was obtained, and range of motion was tested from 0º to 80º, without movement of the fracture site (Figure 3E). Fluoroscopy with multi-axial views verified adequate fixation and reduction. Further, we examined and noted a taut ACL after fixation. The patient was placed in a long leg cast for 3 weeks at 30º, based upon intraoperative determination of the position of least tension on the fracture fragment.
At 3-week follow-up, the patient was progressing well and transitioned from a long leg cast to a hinged knee brace, to allow for early range of motion. Radiographs showed appropriate alignment of the tibial spine fracture with no significant loss of fixation (Figures 4A, 4B). Physical therapy was initiated between 0º and 30º, and flexion was progressively increased over the course of the first 3 weeks. Active and active-assist, closed-chain activities were maintained. Seven weeks postoperatively, the patient displayed continued clinical progression. Radiographs showed interval healing with slight lucency over the anterolateral aspect of the fracture fragment, likely related to the early resorptive process of healing. Physical examination showed movement between 0º and 120º, stable Lachman test, and stable anterior drawer. Crutches were discontinued and hinged knee brace was converted to an ACL brace. By the 11th week, motion had increased to 140º, and radiographs continued to show acceptable alignment and healing (Figures 5A, 5B). The patient was released to return to play as tolerated; however, an ACL brace was recommended during his initial return to provide additional support.
Discussion
In this report, we present an approach for arthroscopic reduction of a malunited tibial spine fracture using resorbable PLLA/PGA nails. The number of polyglycolic nails employed is individualized per case, dependent on the surface area and the quality of the bone within the fractured fragment. Preoperative templating allows for measurements from the fractured fragment to the level of the proximal tibial physis. Based on these measurements, nails are chosen to maximize fixation length and avoid the physis. Despite studies that have examined the effect of transphyseal K-wire pinning or drilling on subsequent growth, there is no consensus about optimal technique. Experiments in animal models indicate that drill injuries destroying less than 8% to 9% of the physis do not impact total bone growth.12,13 Further, temporary crossing of the physeal plate for internal fixation of dislocated joint injuries has not been shown to result in bone bridging or growth disturbance.14,15
Each nail is 1.6 mm in diameter, leaving a small footprint. The nails are used judiciously to provide effective stabilization of the fragment and to maintain a cost-conscious approach. An accessory superomedial peripatellar portal allows an appropriate angle for nail placement. This portal allows access to all regions of the fractured fragment, while an anteromedial and anterolateral portal are used as working and camera portals, respectively. Nails are placed to provide an axis perpendicular to the fracture line to allow appropriate compression. By virtue of the shape of the typical fragment in a tibial spine fracture, the nails vary in insertion angle.
The occurrence of anterior tibial spine fractures is rare, and while several techniques have been described to repair this fracture, there remains a great deal of uncertainty regarding the best course of treatment. A review of the literature finds arthroscopic and open approaches, as well as techniques employing K-wire fixation, metal screw fixation, staple fixation, absorbable fixation, and fixation with sutures passed through the tibial tunnel.16-18
Avulsion fractures of the tibial eminence were treated with open fixation until McLennan8 first reported the benefits of reduction with an arthroscope. Open reduction and internal fixation provide the benefit of direct visualization,9 while arthroscopic reduction offers decreased morbidity and an accelerated recovery of knee functions,8 despite the fact that a higher rate of range-of-motion deficits were seen in patients treated arthroscopically.19 We feel that with proper early rehabilitation to achieve range of motion, the risk of this can be minimal.
Various arthroscopic approaches that improve the accuracy of the reduction and decrease surgical invasiveness have been described. Suture and screw fixation are among the most common methods, and both have resulted in positive outcomes.20-24 Suture fixation of the tibial eminence is technically demanding but offers secure fixation without the need for follow-up hardware removal. Screw fixation results in secure fixation; however, numerous hardware-related issues may necessitate removal. Furthermore, in skeletally immature patients, screw fixation may disturb the growth plate if it crosses an open physis.9
Hunter and Willis25 retrospectively reviewed patients with tibial eminence fractures treated with either screw or suture fixation and found a 44% reoperation rate in the screw-fixation group. Removal was often recommended as a result of hardware-related issues. There was a 13% reoperation rate in the suture-fixation group, which resulted largely from stiffness.25 In a recent review, Gans and colleagues19 reviewed 6 publications comparing screw and suture fixation of tibial eminence fractures and found 82.4% of screw patients had laxity on both the anterior drawer and Lachman tests, compared with 18.8% in the suture-fixation group. This study also noted a slightly higher rate of arthrofibrosis in patients treated with suture fixation.19 Biomechanical studies indicate that suture fixation imparts greater strength under cyclic-loading conditions;26 however, there does not appear to be a difference in ultimate force required for fixation failure.27
Ultimately, both suture and screw fixation result in secure methods of fixation; however, there are often greater issues with screw fixation because of the persistent hardware. Metal has been the most popular method for fracture fixation, and while biodegradable materials have been alluring, adverse tissue reactions have slowed implementation. However, these implants have become increasingly sophisticated, thereby reducing disadvantages.28 Previous biodegradable devices were often composed of a single polymer, and many caused adverse reactions by degrading too quickly or provided no real advantages because they degraded too slowly.29 As the number of polymers approved for internal use and surgical applications continues to rise, so too will the benefits of employing this technology. Furthermore, by including multiple polymers in these implants, one is better able to control the degradation rate, limiting the tissue response.
In this study, we employed PLLA/PGA nails. Studies of PGA implants indicate this molecule degrades at a fast rate resulting in adverse tissue reactions. Adverse reactions in studies of PLLA implants are less frequent because of their slower rate of degradation.29,30 Combining these monomers results in appropriate strength and a controlled degradation rate, reducing the likelihood of adverse reactions. Furthermore, numerous studies have reported that inflammatory responses in children are rare and mild in nature.31,32 Absorbable implants have displayed efficacy in numerous orthopedic settings33-36 and are beneficial in procedures that are not suitable for repeated surgeries, such as reconstruction of the ACL.37 There is some concern about the use of absorbable implants in synovial joints. Polyglycolic acid use in synovial joints may cause foreign-body reactions and may increase the risk of intra-articular dissemination of polymeric debris;38 however, use of a multipolymer construct decreases the likelihood of this occurrence.
Polyglycolic nails confer the advantage over nonresorbable screw fixation because further procedure for hardware removal is not required. Although suture fixation has proved to be beneficial over nonresorbable screw fixation, implantation of resorbable nails appears to have several advantages. In Dr. Estes’ experience, placement of resorbable screws through an accessory superomedial portal is far less technically demanding than placement of suture through the fracture fragment. Further, as sutures are passed from the extra-articular to the intra-articular region of the joint, capsular layers of the knee may inadvertently be bound up in the fixation, predisposing to arthrofibrosis.
At the same time, biodegradable devices are often more costly than alternative forms of treatment; however, a true cost-to-benefit analysis requires consideration of other factors. One of the benefits of biodegradable hardware is that there is no need for follow-up hardware removal. Reports have indicated that up to 91% of patients thought that hardware removal was the most negative aspect of metal implants.39 It is estimated that if the removal rate for metallic implants is higher than 19% to 54%, resorbable implants would be more cost-effective.40 The cost of sutures and screws is variable, however; they are invariably less expensive than biodegradable nails. A study of fracture patients determined that biodegradable implants were cheaper on average after considering the cost of implant removal.40 Ultimately, the hardware choice depends on numerous factors, including surgeon’s discretion; however, biodegradable hardware should not be discounted for financial reasons because the difference in cost is likely negligible.
Conclusion
The approach described in this report offers efficient and secure fixation with resorbable hardware without a reduction in range of motion. Resorbable implants may prove beneficial in the treatment of tibial eminence fractures by offering robust fixation without the concerns associated with permanent hardware.
1. Hargrove R, Parsons S, Payne R. Anterior tibial spine fracture – an easy fracture to miss. Accid Emerg Nurs. 2004;12(3):173-175.
2. Aderinto J, Walmsley P, Keating JF. Fractures of the tibial spine: epidemiology and outcome. Knee. 2008;15(3):164-167.
3. Driessen MJ, Winkelman PA. Fractures of the intercondylar eminence of the tibia in childhood. Neth J Surg. 1984;36(3):69-72.
4. Zaricznyj B. Avulsion fracture of the tibial eminence: treatment by open reduction and pinning. J Bone Joint Surg Am. 1977;59(8):1111-1114.
5. Molander ML, Wallin G, Wikstad I. Fracture of the intercondylar eminence of the tibia: a review of 35 patients. J Bone Joint Surg Br. 1981;63(1):89-91.
6. Kieser DC, Gwynne-Jones D, Dreyer S. Displaced tibial intercondylar eminence fractures. J Orthop Surg. 2011;19(3):292-296.
7. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop. 2005;(434):207-212.
8. McLennan JG. The role of arthroscopic surgery in the treatment of fractures of the intercondylar eminence of the tibia. J Bone Joint Surg Br. 1982;64(4):477-480.
9. Lafrance RM, Giordano B, Goldblatt J, Voloshin I, Maloney M. Pediatric tibial eminence fractures: evaluation and management. J Am Acad Orthop Surg. 2010;18(7):395-405.
10. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1959;41(2):209-220.
11. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1970;52(8):1677-1684.
12. Garcés GL, Mugica-Garay I, López-González Coviella N, Guerado E. Growth-plate modifications after drilling. J Pediatr Orthop. 1994;14(2):225-228.
13. Janarv PM, Wikström B, Hirsch G. The influence of transphyseal drilling and tendon grafting on bone growth: an experimental study in the rabbit. J Pediatr Orthop. 1998;18(2):149-154.
14. Boelitz R, Dallek M, Meenen NM, Jungbluth KH. Reaction of the epiphyseal groove to groove-crossing bore-wire osteosynthesis. Results of a histomorphologic small animal study. Unfallchirurgie. 1994;20(3):131-137.
15. Yung PS, Lam CY, Ng BK, Lam TP, Cheng JC. Percutaneous transphyseal intramedullary Kirschner wire pinning: a safe and effective procedure for treatment of displaced diaphyseal forearm fracture in children. J Pediatr Orthop. 2004;24(1):7-12.
16. Bong MR, Romero A, Kubiak E, et al. Suture versus screw fixation of displaced tibial eminence fractures: a biomechanical comparison. Arthroscopy. 2005;21(10):1172-1176.
17. Vega JR, Irribarra LA, Baar AK, Iñiguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
18. Shepley RW. Arthroscopic treatment of type III tibial spine fractures using absorbable fixation. Orthopedics. 2004;27(7):767-769.
19. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2013;42(7):1743-1750.
20. Delcogliano A, Chiossi S, Caporaso A, Menghi A, Rinonapoli G. Tibial intercondylar eminence fractures in adults: arthroscopic treatment. Knee Surg Sports Traumatol Arthrosc. 2003;11(4):255-259.
21. Mulhall KJ, Dowdall J, Grannell M, McCabe JP. Tibial spine fractures: an analysis of outcome in surgically treated type III injuries. Injury. 1999;30(4):289-292.
22. Geissler WB, Matthews DE. Arthroscopic suture fixation of displaced tibial eminence fractures. Orthopedics. 1993;16(3):331-333.
23. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
24. Reynders P, Reynders K, Broos P. Pediatric and adolescent tibial eminence fractures: arthroscopic cannulated screw fixation. J Trauma. 2002;53(1):49-54.
25. Hunter RE, Willis JA. Arthroscopic fixation of avulsion fractures of the tibial eminence: technique and outcome. Arthroscopy. 2004;20(2):113-121.
26. Eggers AK, Becker C, Weimann A, et al. Biomechanical evaluation of different fixation methods for tibial eminence fractures. Am J Sports Med. 2007;35(3):404-410.
27. Mahar AT, Duncan D, Oka R, Lowry A, Gillingham B, Chambers H. Biomechanical comparison of four different fixation techniques for pediatric tibial eminence avulsion fractures. J Pediatr Orthop. 2008;28(2):159-162.
28. Toro C, Robiony M, Zerman N, Politi M. Resorbable plates in maxillary fixation. A 5-year experience. Minerva Stomatol. 2005;54(4):199-206.
29. Andriano KP, Pohjonen T, Törmälä P. Processing and characterization of absorbable polylactide polymers for use in surgical implants. J Appl Biomater.1994;5(2):133-140.
30. Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21(24):2615-2621.
31. Rokkanen PU, Böstman O, Hirvensalo E, et al. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000;21(24):2607-2613.
32. Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93-102.
33. Li ZH, Yu AX, Guo XP, Qi BW, Zhou M, Wang WY. Absorbable implants versus metal implants for the treatment of ankle fractures: A meta-analysis. Exp Ther Med. 2013;5(5):1531-1537.
34. Singh G, Mohammad S, Chak RK, Lepcha N, Singh N, Malkunje LR. Bio-resorbable plates as effective implant in paediatric mandibular fracture. J Maxillofac Oral Surg. 2012;11(4):400-406.
35. Sakamoto Y, Shimizu Y, Nagasao T, Kishi K. Combined use of resorbable poly-L-lactic acid-polyglycolic acid implant and bone cement for treating large orbital floor fractures. J Plast Reconstr Aesthet Surg. 2014;67(3):e88-e90.
36. Benz G, Kallieris D, Seeböck T, McIntosh A, Daum R. Bioresorbable pins and screws in paediatric traumatology. Eur J Pediatr Surg. 1994;4(2):103-107.
37. Gaweda K, Walawski J, Weglowski R, Krzyzanowski W. Comparison of bioabsorbable interference screws and posts for distal fixation in anterior cruciate ligament reconstruction. Int Orthop. 2009;33(1):123-127.
38. Böstman OM. Osteoarthritis of the ankle after foreign-body reaction to absorbable pins and screws: a three- to nine-year follow-up study. J Bone Joint Surg Br. 1998;80(2):333-338.
39. Mittal R, Morley J, Dinopoulos H, Drakoulakis EG, Vermani E, Giannoudis PV. Use of bio-resorbable implants for stabilisation of distal radius fractures: the United Kingdom patients’ perspective. Injury. 2005;36(2):333-338.
40. Böstman OM. Metallic or absorbable fracture fixation devices. A cost minimization analysis. Clin Orthop. 1996;(329):233-239.
1. Hargrove R, Parsons S, Payne R. Anterior tibial spine fracture – an easy fracture to miss. Accid Emerg Nurs. 2004;12(3):173-175.
2. Aderinto J, Walmsley P, Keating JF. Fractures of the tibial spine: epidemiology and outcome. Knee. 2008;15(3):164-167.
3. Driessen MJ, Winkelman PA. Fractures of the intercondylar eminence of the tibia in childhood. Neth J Surg. 1984;36(3):69-72.
4. Zaricznyj B. Avulsion fracture of the tibial eminence: treatment by open reduction and pinning. J Bone Joint Surg Am. 1977;59(8):1111-1114.
5. Molander ML, Wallin G, Wikstad I. Fracture of the intercondylar eminence of the tibia: a review of 35 patients. J Bone Joint Surg Br. 1981;63(1):89-91.
6. Kieser DC, Gwynne-Jones D, Dreyer S. Displaced tibial intercondylar eminence fractures. J Orthop Surg. 2011;19(3):292-296.
7. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop. 2005;(434):207-212.
8. McLennan JG. The role of arthroscopic surgery in the treatment of fractures of the intercondylar eminence of the tibia. J Bone Joint Surg Br. 1982;64(4):477-480.
9. Lafrance RM, Giordano B, Goldblatt J, Voloshin I, Maloney M. Pediatric tibial eminence fractures: evaluation and management. J Am Acad Orthop Surg. 2010;18(7):395-405.
10. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1959;41(2):209-220.
11. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1970;52(8):1677-1684.
12. Garcés GL, Mugica-Garay I, López-González Coviella N, Guerado E. Growth-plate modifications after drilling. J Pediatr Orthop. 1994;14(2):225-228.
13. Janarv PM, Wikström B, Hirsch G. The influence of transphyseal drilling and tendon grafting on bone growth: an experimental study in the rabbit. J Pediatr Orthop. 1998;18(2):149-154.
14. Boelitz R, Dallek M, Meenen NM, Jungbluth KH. Reaction of the epiphyseal groove to groove-crossing bore-wire osteosynthesis. Results of a histomorphologic small animal study. Unfallchirurgie. 1994;20(3):131-137.
15. Yung PS, Lam CY, Ng BK, Lam TP, Cheng JC. Percutaneous transphyseal intramedullary Kirschner wire pinning: a safe and effective procedure for treatment of displaced diaphyseal forearm fracture in children. J Pediatr Orthop. 2004;24(1):7-12.
16. Bong MR, Romero A, Kubiak E, et al. Suture versus screw fixation of displaced tibial eminence fractures: a biomechanical comparison. Arthroscopy. 2005;21(10):1172-1176.
17. Vega JR, Irribarra LA, Baar AK, Iñiguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
18. Shepley RW. Arthroscopic treatment of type III tibial spine fractures using absorbable fixation. Orthopedics. 2004;27(7):767-769.
19. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2013;42(7):1743-1750.
20. Delcogliano A, Chiossi S, Caporaso A, Menghi A, Rinonapoli G. Tibial intercondylar eminence fractures in adults: arthroscopic treatment. Knee Surg Sports Traumatol Arthrosc. 2003;11(4):255-259.
21. Mulhall KJ, Dowdall J, Grannell M, McCabe JP. Tibial spine fractures: an analysis of outcome in surgically treated type III injuries. Injury. 1999;30(4):289-292.
22. Geissler WB, Matthews DE. Arthroscopic suture fixation of displaced tibial eminence fractures. Orthopedics. 1993;16(3):331-333.
23. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
24. Reynders P, Reynders K, Broos P. Pediatric and adolescent tibial eminence fractures: arthroscopic cannulated screw fixation. J Trauma. 2002;53(1):49-54.
25. Hunter RE, Willis JA. Arthroscopic fixation of avulsion fractures of the tibial eminence: technique and outcome. Arthroscopy. 2004;20(2):113-121.
26. Eggers AK, Becker C, Weimann A, et al. Biomechanical evaluation of different fixation methods for tibial eminence fractures. Am J Sports Med. 2007;35(3):404-410.
27. Mahar AT, Duncan D, Oka R, Lowry A, Gillingham B, Chambers H. Biomechanical comparison of four different fixation techniques for pediatric tibial eminence avulsion fractures. J Pediatr Orthop. 2008;28(2):159-162.
28. Toro C, Robiony M, Zerman N, Politi M. Resorbable plates in maxillary fixation. A 5-year experience. Minerva Stomatol. 2005;54(4):199-206.
29. Andriano KP, Pohjonen T, Törmälä P. Processing and characterization of absorbable polylactide polymers for use in surgical implants. J Appl Biomater.1994;5(2):133-140.
30. Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21(24):2615-2621.
31. Rokkanen PU, Böstman O, Hirvensalo E, et al. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000;21(24):2607-2613.
32. Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93-102.
33. Li ZH, Yu AX, Guo XP, Qi BW, Zhou M, Wang WY. Absorbable implants versus metal implants for the treatment of ankle fractures: A meta-analysis. Exp Ther Med. 2013;5(5):1531-1537.
34. Singh G, Mohammad S, Chak RK, Lepcha N, Singh N, Malkunje LR. Bio-resorbable plates as effective implant in paediatric mandibular fracture. J Maxillofac Oral Surg. 2012;11(4):400-406.
35. Sakamoto Y, Shimizu Y, Nagasao T, Kishi K. Combined use of resorbable poly-L-lactic acid-polyglycolic acid implant and bone cement for treating large orbital floor fractures. J Plast Reconstr Aesthet Surg. 2014;67(3):e88-e90.
36. Benz G, Kallieris D, Seeböck T, McIntosh A, Daum R. Bioresorbable pins and screws in paediatric traumatology. Eur J Pediatr Surg. 1994;4(2):103-107.
37. Gaweda K, Walawski J, Weglowski R, Krzyzanowski W. Comparison of bioabsorbable interference screws and posts for distal fixation in anterior cruciate ligament reconstruction. Int Orthop. 2009;33(1):123-127.
38. Böstman OM. Osteoarthritis of the ankle after foreign-body reaction to absorbable pins and screws: a three- to nine-year follow-up study. J Bone Joint Surg Br. 1998;80(2):333-338.
39. Mittal R, Morley J, Dinopoulos H, Drakoulakis EG, Vermani E, Giannoudis PV. Use of bio-resorbable implants for stabilisation of distal radius fractures: the United Kingdom patients’ perspective. Injury. 2005;36(2):333-338.
40. Böstman OM. Metallic or absorbable fracture fixation devices. A cost minimization analysis. Clin Orthop. 1996;(329):233-239.