User login
Model reveals genes associated with prognosis in ER+, HER2– breast cancer
ORLANDO – , according to new research.
Yara Abdou, MD, of Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., and colleagues presented this work in a poster at the ASCO-SITC Clinical Immuno-Oncology Symposium.
The model used 50 cycles of machine learning to cluster 98 patients from The Cancer Genome Atlas Program into high- and low-risk groups based on mRNA expression of 26 gene groups.
The gene groups consisted of 191 genes enriched in cellular and noncellular elements of the tumor microenvironment. Mutational burden and clinical outcomes data for the patients also were considered, Dr. Abdou explained in an interview.
Kaplan-Meier curves were created for each group by K-means clustering, survival differences between the two groups were assessed, and correlations among the various gene groups were analyzed.
Five identified genes were associated with poor prognosis: LOXL2, PHEX, ACTA2, MEGF9, and TNFSF4. Fifteen genes were associated with good prognosis: CD8A, CD8B, FCRL3, GZMK, CD3E, CCL5, TP53, ICAM3, CD247, IFNG, IFNGR1, ICAM4, SHH, HLA-DOB, and CXCR3.
The Kaplan-Meier curves showed a significant difference in survival between the two groups (hazard ratio, 2.878; P = .05), confirming the validity of the risk score modeling, Dr. Abdou said.
Immune profiling showed that expression of genes associated with desmoplastic reaction, neutrophils, and immunosuppressive cytokines were higher in the high-risk group, whereas expression of genes related to immune system activation were higher in the low-risk group (P less than .05).
Stroma in the tumor microenvironment is known to affect prognosis and response to therapy in patients with breast cancer, but few mathematical models exist to determine prognosis based on mRNA expressivity in the tumor microenvironment, Dr. Abdou said, explaining the rationale for the study.
The findings suggest that when genomic profile information is available for a given patient in the clinic, this machine learning–assisted risk scoring approach could have prognostic value, she said, noting that the model also will be assessed in patients with other types of breast cancer.
Dr. Abdou reported having no disclosures.
SOURCE: Abdou Y et al. ASCO-SITC. Poster A3.
ORLANDO – , according to new research.
Yara Abdou, MD, of Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., and colleagues presented this work in a poster at the ASCO-SITC Clinical Immuno-Oncology Symposium.
The model used 50 cycles of machine learning to cluster 98 patients from The Cancer Genome Atlas Program into high- and low-risk groups based on mRNA expression of 26 gene groups.
The gene groups consisted of 191 genes enriched in cellular and noncellular elements of the tumor microenvironment. Mutational burden and clinical outcomes data for the patients also were considered, Dr. Abdou explained in an interview.
Kaplan-Meier curves were created for each group by K-means clustering, survival differences between the two groups were assessed, and correlations among the various gene groups were analyzed.
Five identified genes were associated with poor prognosis: LOXL2, PHEX, ACTA2, MEGF9, and TNFSF4. Fifteen genes were associated with good prognosis: CD8A, CD8B, FCRL3, GZMK, CD3E, CCL5, TP53, ICAM3, CD247, IFNG, IFNGR1, ICAM4, SHH, HLA-DOB, and CXCR3.
The Kaplan-Meier curves showed a significant difference in survival between the two groups (hazard ratio, 2.878; P = .05), confirming the validity of the risk score modeling, Dr. Abdou said.
Immune profiling showed that expression of genes associated with desmoplastic reaction, neutrophils, and immunosuppressive cytokines were higher in the high-risk group, whereas expression of genes related to immune system activation were higher in the low-risk group (P less than .05).
Stroma in the tumor microenvironment is known to affect prognosis and response to therapy in patients with breast cancer, but few mathematical models exist to determine prognosis based on mRNA expressivity in the tumor microenvironment, Dr. Abdou said, explaining the rationale for the study.
The findings suggest that when genomic profile information is available for a given patient in the clinic, this machine learning–assisted risk scoring approach could have prognostic value, she said, noting that the model also will be assessed in patients with other types of breast cancer.
Dr. Abdou reported having no disclosures.
SOURCE: Abdou Y et al. ASCO-SITC. Poster A3.
ORLANDO – , according to new research.
Yara Abdou, MD, of Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., and colleagues presented this work in a poster at the ASCO-SITC Clinical Immuno-Oncology Symposium.
The model used 50 cycles of machine learning to cluster 98 patients from The Cancer Genome Atlas Program into high- and low-risk groups based on mRNA expression of 26 gene groups.
The gene groups consisted of 191 genes enriched in cellular and noncellular elements of the tumor microenvironment. Mutational burden and clinical outcomes data for the patients also were considered, Dr. Abdou explained in an interview.
Kaplan-Meier curves were created for each group by K-means clustering, survival differences between the two groups were assessed, and correlations among the various gene groups were analyzed.
Five identified genes were associated with poor prognosis: LOXL2, PHEX, ACTA2, MEGF9, and TNFSF4. Fifteen genes were associated with good prognosis: CD8A, CD8B, FCRL3, GZMK, CD3E, CCL5, TP53, ICAM3, CD247, IFNG, IFNGR1, ICAM4, SHH, HLA-DOB, and CXCR3.
The Kaplan-Meier curves showed a significant difference in survival between the two groups (hazard ratio, 2.878; P = .05), confirming the validity of the risk score modeling, Dr. Abdou said.
Immune profiling showed that expression of genes associated with desmoplastic reaction, neutrophils, and immunosuppressive cytokines were higher in the high-risk group, whereas expression of genes related to immune system activation were higher in the low-risk group (P less than .05).
Stroma in the tumor microenvironment is known to affect prognosis and response to therapy in patients with breast cancer, but few mathematical models exist to determine prognosis based on mRNA expressivity in the tumor microenvironment, Dr. Abdou said, explaining the rationale for the study.
The findings suggest that when genomic profile information is available for a given patient in the clinic, this machine learning–assisted risk scoring approach could have prognostic value, she said, noting that the model also will be assessed in patients with other types of breast cancer.
Dr. Abdou reported having no disclosures.
SOURCE: Abdou Y et al. ASCO-SITC. Poster A3.
REPORTING FROM THE CLINICAL IMMUNO-ONCOLOGY SYMPOSIUM
FDA: Cell phones still look safe
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
Breast cancer treatments veer from guidelines
Women with breast cancer may be receiving treatments that are discordant with guideline recommendations for genetic subtypes of disease, based on a retrospective analysis of more than 20,000 patients.
Radiotherapy and chemotherapy practices were particularly out of alignment with guidelines, reported lead author Allison W. Kurian, MD, of Stanford (Calif.) University, and colleagues.
“Integrating genetic testing into breast cancer care has been complex and challenging,” the investigators wrote in JAMA Oncology. “There is wide variability in which clinicians order testing and disclose results, in the clinical significance of results, and in how clinicians interpret results to patients.”
According to the investigators, while germline testing is on the rise, little is known about how these test results are translating to clinical care.
To learn more, the investigators evaluated data from 20,568 women with stage 0-III breast cancer who entered the Surveillance, Epidemiology, and End Results registries of Georgia and California between 2014 and 2016.
Three treatment types were evaluated: surgery (bilateral vs. unilateral mastectomy), radiotherapy after lumpectomy, and chemotherapy. Treatment selection was compared with test results for breast cancer–associated genes, such as BRCA1/2, TP53, PTEN, and others. Associations were then compared with guideline recommendations.
Data analysis suggested that many clinicians were correctly using genetic test results to guide surgical decisions. For example, almost two-thirds (61.7%) of women with a BRCA mutation underwent bilateral mastectomy, compared with one-quarter (24.3%) who were BRCA negative (odds ratio, 5.52). For other pathogenic variants, the rate of bilateral mastectomy was still elevated, albeit to a lesser degree (OR, 2.41).
Generally, these practices align with recommendations, the investigators wrote, noting that research supports bilateral mastectomy with BRCA1/2, TP53, and PTEN variants, while data are lacking for other genetic subtypes.
Radiotherapy and chemotherapy practices were more discordant with guidelines. For example, women with a BRCA mutation were 78% less likely to receive radiotherapy after lumpectomy (OR, 0.22) and 76% more likely to receive chemotherapy for early-stage, hormone-positive disease (OR, 1.76). According to investigators, these findings suggest possible trends in undertreatment and overtreatment, respectively.
“We believe more research is needed to confirm our results and to evaluate long-term outcomes of pathogenic variant carriers to understand treatment decision making and consequences,” the investigators concluded.
The study was funded by the National Institutes of Health and the California Department of Public Health. The investigators reported relationships with Myriad Genetics, Genomic Health, Roche, and other companies.
SOURCE: Kurian AW et al. JAMA Oncol. 2020 Feb 6. doi: 10.1001/jamaoncol.2019.6400.
Women with breast cancer may be receiving treatments that are discordant with guideline recommendations for genetic subtypes of disease, based on a retrospective analysis of more than 20,000 patients.
Radiotherapy and chemotherapy practices were particularly out of alignment with guidelines, reported lead author Allison W. Kurian, MD, of Stanford (Calif.) University, and colleagues.
“Integrating genetic testing into breast cancer care has been complex and challenging,” the investigators wrote in JAMA Oncology. “There is wide variability in which clinicians order testing and disclose results, in the clinical significance of results, and in how clinicians interpret results to patients.”
According to the investigators, while germline testing is on the rise, little is known about how these test results are translating to clinical care.
To learn more, the investigators evaluated data from 20,568 women with stage 0-III breast cancer who entered the Surveillance, Epidemiology, and End Results registries of Georgia and California between 2014 and 2016.
Three treatment types were evaluated: surgery (bilateral vs. unilateral mastectomy), radiotherapy after lumpectomy, and chemotherapy. Treatment selection was compared with test results for breast cancer–associated genes, such as BRCA1/2, TP53, PTEN, and others. Associations were then compared with guideline recommendations.
Data analysis suggested that many clinicians were correctly using genetic test results to guide surgical decisions. For example, almost two-thirds (61.7%) of women with a BRCA mutation underwent bilateral mastectomy, compared with one-quarter (24.3%) who were BRCA negative (odds ratio, 5.52). For other pathogenic variants, the rate of bilateral mastectomy was still elevated, albeit to a lesser degree (OR, 2.41).
Generally, these practices align with recommendations, the investigators wrote, noting that research supports bilateral mastectomy with BRCA1/2, TP53, and PTEN variants, while data are lacking for other genetic subtypes.
Radiotherapy and chemotherapy practices were more discordant with guidelines. For example, women with a BRCA mutation were 78% less likely to receive radiotherapy after lumpectomy (OR, 0.22) and 76% more likely to receive chemotherapy for early-stage, hormone-positive disease (OR, 1.76). According to investigators, these findings suggest possible trends in undertreatment and overtreatment, respectively.
“We believe more research is needed to confirm our results and to evaluate long-term outcomes of pathogenic variant carriers to understand treatment decision making and consequences,” the investigators concluded.
The study was funded by the National Institutes of Health and the California Department of Public Health. The investigators reported relationships with Myriad Genetics, Genomic Health, Roche, and other companies.
SOURCE: Kurian AW et al. JAMA Oncol. 2020 Feb 6. doi: 10.1001/jamaoncol.2019.6400.
Women with breast cancer may be receiving treatments that are discordant with guideline recommendations for genetic subtypes of disease, based on a retrospective analysis of more than 20,000 patients.
Radiotherapy and chemotherapy practices were particularly out of alignment with guidelines, reported lead author Allison W. Kurian, MD, of Stanford (Calif.) University, and colleagues.
“Integrating genetic testing into breast cancer care has been complex and challenging,” the investigators wrote in JAMA Oncology. “There is wide variability in which clinicians order testing and disclose results, in the clinical significance of results, and in how clinicians interpret results to patients.”
According to the investigators, while germline testing is on the rise, little is known about how these test results are translating to clinical care.
To learn more, the investigators evaluated data from 20,568 women with stage 0-III breast cancer who entered the Surveillance, Epidemiology, and End Results registries of Georgia and California between 2014 and 2016.
Three treatment types were evaluated: surgery (bilateral vs. unilateral mastectomy), radiotherapy after lumpectomy, and chemotherapy. Treatment selection was compared with test results for breast cancer–associated genes, such as BRCA1/2, TP53, PTEN, and others. Associations were then compared with guideline recommendations.
Data analysis suggested that many clinicians were correctly using genetic test results to guide surgical decisions. For example, almost two-thirds (61.7%) of women with a BRCA mutation underwent bilateral mastectomy, compared with one-quarter (24.3%) who were BRCA negative (odds ratio, 5.52). For other pathogenic variants, the rate of bilateral mastectomy was still elevated, albeit to a lesser degree (OR, 2.41).
Generally, these practices align with recommendations, the investigators wrote, noting that research supports bilateral mastectomy with BRCA1/2, TP53, and PTEN variants, while data are lacking for other genetic subtypes.
Radiotherapy and chemotherapy practices were more discordant with guidelines. For example, women with a BRCA mutation were 78% less likely to receive radiotherapy after lumpectomy (OR, 0.22) and 76% more likely to receive chemotherapy for early-stage, hormone-positive disease (OR, 1.76). According to investigators, these findings suggest possible trends in undertreatment and overtreatment, respectively.
“We believe more research is needed to confirm our results and to evaluate long-term outcomes of pathogenic variant carriers to understand treatment decision making and consequences,” the investigators concluded.
The study was funded by the National Institutes of Health and the California Department of Public Health. The investigators reported relationships with Myriad Genetics, Genomic Health, Roche, and other companies.
SOURCE: Kurian AW et al. JAMA Oncol. 2020 Feb 6. doi: 10.1001/jamaoncol.2019.6400.
FROM JAMA ONCOLOGY
Abbreviated MRI equals standard protocol for high-risk breast cancer screens
CHICAGO – An abbreviated magnetic resonance imaging (MRI) protocol for screening individuals at high risk for breast cancer performed as well as a standard protocol, in about half the time and with greater patient satisfaction.
The abbreviated protocol also resulted in fewer false positive findings, with 5% fewer patients receiving biopsies for benign lesions than with a standard protocol (8.4% versus 13.7%, P less than .001).
Findings from the prospective 10-month trial conducted in the province of Ontario were presented by Jean Seely, MD, professor of radiology at the University of Ottawa, at the annual meeting of the Radiological Society of North America.
“The abbreviated protocol was shown to be as effective as the standard protocol in high-risk screening breast MRI, supporting previous studies,” said Dr. Seely. The shorter protocol took 16.3 minutes on average, compared with 27 minutes for the standard MRI protocol. This difference resulted in a 50% increase in institutional capacity, or a jump from two to three patients screened per hour.
In the province of Ontario, said Dr. Seely, women assessed at being at a 25% or greater lifetime risk of breast cancer receive MRIs as part of the Ontario Breast Screening Program (OBSP), which calculates risk by using the International Breast Cancer Intervention Study model.
For high-risk patients, the OBSP model provides annual mammography and an MRI for women between the ages of 30 and 60 years. Not only is the half-hour duration of the standard protocol resource-intensive, especially in regions with limited scanner availability, but patients may either be reluctant to undergo a half-hour scan, or not tolerate a lengthy scan very well.
Dr. Seely cited previous work (J Clin Oncol. 2014 Aug 1;32[22]:2304-10) showing that an abbreviated MRI protocol has similar accuracy as the full standard protocol. With the foundation of evidence from this study, Dr. Seely and her collaborators compared outcomes for high-risk patients who were screened with an abbreviated versus a standard protocol.
The abbreviated protocol, approved by the American College of Radiology, omits a final round of image acquisition sequences at the 9-minute mark after gadolinium administration, instead performing acquisition at 1, 2, 3, and 4 minutes after contrast delivery. Total time required for this protocol is just over 13 minutes, said Dr. Seely, and additional diagnostic MRIs were not required.
The trial was constructed so that the abbreviated protocol was used for the entire OBSP cohort for 10 months in 2018. Results were compared with those from the 12 previous months, when OBSP patients’ MRIs were performed using the standard protocol.
A total of 881 patients received standard-protocol MRIs; about three quarters (651) of those patients had previous MRI screening, while the remaining 230 patients had a baseline screen via the standard protocol.
Of the 658 patients in the abbreviated protocol group, 135, or about 20%, received the briefer scans as a baseline screen; the remast of the patients in this arm had received earlier MRI screening.
In addition to tracking scanning times, Dr. Seely and her collaborators also compared cancer detection rates, Breast Imaging Reporting and Data System (BI-RADS) assessment categories, positive predictive values, and the abnormal interpretation rate – that is, how many scans fell into BI-RADS categories 0, 4, and 5.
No significant difference was found between the rates of BI-RADs 0, 3, or 5 studies between the groups. Significantly fewer abbreviated scans fell into the BI-RADS 4 category, however (9.3% vs. 14.9%; P less than .001).
Similarly, the abnormal interpretation rate was 12.5% for the abbreviated protocol, compared with 17.5% for the standard protocol (P less than .007), with a correspondingly lower biopsy rate of 8.4% for the abbreviated protocol, compared with 13.7% for the standard protocol (P less than .001). The overall cancer detection rate did not differ between groups.
She and her colleagues will continue to track outcomes for those receiving abbreviated screening within the province of Ontario to track performance over time.
Dr. Seely reported that she had no relevant conflicts of interest. She reported no funding source beyond the province of Ontario.
koakes@mdedge.com
SOURCE: Seely J et al. RSNA 2019, Session RC-215-04.
CHICAGO – An abbreviated magnetic resonance imaging (MRI) protocol for screening individuals at high risk for breast cancer performed as well as a standard protocol, in about half the time and with greater patient satisfaction.
The abbreviated protocol also resulted in fewer false positive findings, with 5% fewer patients receiving biopsies for benign lesions than with a standard protocol (8.4% versus 13.7%, P less than .001).
Findings from the prospective 10-month trial conducted in the province of Ontario were presented by Jean Seely, MD, professor of radiology at the University of Ottawa, at the annual meeting of the Radiological Society of North America.
“The abbreviated protocol was shown to be as effective as the standard protocol in high-risk screening breast MRI, supporting previous studies,” said Dr. Seely. The shorter protocol took 16.3 minutes on average, compared with 27 minutes for the standard MRI protocol. This difference resulted in a 50% increase in institutional capacity, or a jump from two to three patients screened per hour.
In the province of Ontario, said Dr. Seely, women assessed at being at a 25% or greater lifetime risk of breast cancer receive MRIs as part of the Ontario Breast Screening Program (OBSP), which calculates risk by using the International Breast Cancer Intervention Study model.
For high-risk patients, the OBSP model provides annual mammography and an MRI for women between the ages of 30 and 60 years. Not only is the half-hour duration of the standard protocol resource-intensive, especially in regions with limited scanner availability, but patients may either be reluctant to undergo a half-hour scan, or not tolerate a lengthy scan very well.
Dr. Seely cited previous work (J Clin Oncol. 2014 Aug 1;32[22]:2304-10) showing that an abbreviated MRI protocol has similar accuracy as the full standard protocol. With the foundation of evidence from this study, Dr. Seely and her collaborators compared outcomes for high-risk patients who were screened with an abbreviated versus a standard protocol.
The abbreviated protocol, approved by the American College of Radiology, omits a final round of image acquisition sequences at the 9-minute mark after gadolinium administration, instead performing acquisition at 1, 2, 3, and 4 minutes after contrast delivery. Total time required for this protocol is just over 13 minutes, said Dr. Seely, and additional diagnostic MRIs were not required.
The trial was constructed so that the abbreviated protocol was used for the entire OBSP cohort for 10 months in 2018. Results were compared with those from the 12 previous months, when OBSP patients’ MRIs were performed using the standard protocol.
A total of 881 patients received standard-protocol MRIs; about three quarters (651) of those patients had previous MRI screening, while the remaining 230 patients had a baseline screen via the standard protocol.
Of the 658 patients in the abbreviated protocol group, 135, or about 20%, received the briefer scans as a baseline screen; the remast of the patients in this arm had received earlier MRI screening.
In addition to tracking scanning times, Dr. Seely and her collaborators also compared cancer detection rates, Breast Imaging Reporting and Data System (BI-RADS) assessment categories, positive predictive values, and the abnormal interpretation rate – that is, how many scans fell into BI-RADS categories 0, 4, and 5.
No significant difference was found between the rates of BI-RADs 0, 3, or 5 studies between the groups. Significantly fewer abbreviated scans fell into the BI-RADS 4 category, however (9.3% vs. 14.9%; P less than .001).
Similarly, the abnormal interpretation rate was 12.5% for the abbreviated protocol, compared with 17.5% for the standard protocol (P less than .007), with a correspondingly lower biopsy rate of 8.4% for the abbreviated protocol, compared with 13.7% for the standard protocol (P less than .001). The overall cancer detection rate did not differ between groups.
She and her colleagues will continue to track outcomes for those receiving abbreviated screening within the province of Ontario to track performance over time.
Dr. Seely reported that she had no relevant conflicts of interest. She reported no funding source beyond the province of Ontario.
koakes@mdedge.com
SOURCE: Seely J et al. RSNA 2019, Session RC-215-04.
CHICAGO – An abbreviated magnetic resonance imaging (MRI) protocol for screening individuals at high risk for breast cancer performed as well as a standard protocol, in about half the time and with greater patient satisfaction.
The abbreviated protocol also resulted in fewer false positive findings, with 5% fewer patients receiving biopsies for benign lesions than with a standard protocol (8.4% versus 13.7%, P less than .001).
Findings from the prospective 10-month trial conducted in the province of Ontario were presented by Jean Seely, MD, professor of radiology at the University of Ottawa, at the annual meeting of the Radiological Society of North America.
“The abbreviated protocol was shown to be as effective as the standard protocol in high-risk screening breast MRI, supporting previous studies,” said Dr. Seely. The shorter protocol took 16.3 minutes on average, compared with 27 minutes for the standard MRI protocol. This difference resulted in a 50% increase in institutional capacity, or a jump from two to three patients screened per hour.
In the province of Ontario, said Dr. Seely, women assessed at being at a 25% or greater lifetime risk of breast cancer receive MRIs as part of the Ontario Breast Screening Program (OBSP), which calculates risk by using the International Breast Cancer Intervention Study model.
For high-risk patients, the OBSP model provides annual mammography and an MRI for women between the ages of 30 and 60 years. Not only is the half-hour duration of the standard protocol resource-intensive, especially in regions with limited scanner availability, but patients may either be reluctant to undergo a half-hour scan, or not tolerate a lengthy scan very well.
Dr. Seely cited previous work (J Clin Oncol. 2014 Aug 1;32[22]:2304-10) showing that an abbreviated MRI protocol has similar accuracy as the full standard protocol. With the foundation of evidence from this study, Dr. Seely and her collaborators compared outcomes for high-risk patients who were screened with an abbreviated versus a standard protocol.
The abbreviated protocol, approved by the American College of Radiology, omits a final round of image acquisition sequences at the 9-minute mark after gadolinium administration, instead performing acquisition at 1, 2, 3, and 4 minutes after contrast delivery. Total time required for this protocol is just over 13 minutes, said Dr. Seely, and additional diagnostic MRIs were not required.
The trial was constructed so that the abbreviated protocol was used for the entire OBSP cohort for 10 months in 2018. Results were compared with those from the 12 previous months, when OBSP patients’ MRIs were performed using the standard protocol.
A total of 881 patients received standard-protocol MRIs; about three quarters (651) of those patients had previous MRI screening, while the remaining 230 patients had a baseline screen via the standard protocol.
Of the 658 patients in the abbreviated protocol group, 135, or about 20%, received the briefer scans as a baseline screen; the remast of the patients in this arm had received earlier MRI screening.
In addition to tracking scanning times, Dr. Seely and her collaborators also compared cancer detection rates, Breast Imaging Reporting and Data System (BI-RADS) assessment categories, positive predictive values, and the abnormal interpretation rate – that is, how many scans fell into BI-RADS categories 0, 4, and 5.
No significant difference was found between the rates of BI-RADs 0, 3, or 5 studies between the groups. Significantly fewer abbreviated scans fell into the BI-RADS 4 category, however (9.3% vs. 14.9%; P less than .001).
Similarly, the abnormal interpretation rate was 12.5% for the abbreviated protocol, compared with 17.5% for the standard protocol (P less than .007), with a correspondingly lower biopsy rate of 8.4% for the abbreviated protocol, compared with 13.7% for the standard protocol (P less than .001). The overall cancer detection rate did not differ between groups.
She and her colleagues will continue to track outcomes for those receiving abbreviated screening within the province of Ontario to track performance over time.
Dr. Seely reported that she had no relevant conflicts of interest. She reported no funding source beyond the province of Ontario.
koakes@mdedge.com
SOURCE: Seely J et al. RSNA 2019, Session RC-215-04.
REPORTING FROM RSNA 2019
Data emerging to support personalized nutrition in oncology
SAN DIEGO – When Dawn Lemanne, MD, MPH, meets with cancer patients and their families, the question invariably comes up: “What should I eat?”
“The answer always is, ‘It depends,’” Dr. Lemanne, an oncologist who founded Oregon Integrative Oncology in Ashland, said at Natural Supplements: An Evidence-Based Update, presented by Scripps Center for Integrative Medicine. “The answers are not the same for each of these patients.”
According to Dr. Lemanne, targeted nutrition is evolving as a key component of cancer care. One of the goals of this approach is to decrease mTOR signaling. Normally, mTOR signaling promotes cell proliferation and metabolism; aberrant mTOR signaling can contribute to cancer initiation and progression.
“When mTOR speaks it says, ‘grow,’” said Dr. Lemanne, who is also an assistant professor of clinical medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona in Tucson. This message is meant to be heard by normal tissues, to stimulate normal tissue proliferation, such as in growing children or when a wound needs to be healed.
“However, cancer cells can hear and respond to mTOR’s message,” she said. “Normal cells may listen to mTOR’s ‘grow’ message or not, depending on the task they perform. Once we reach adulthood, we all likely have some precancerous or cancerous cells around, but they’re usually dormant. That’s why once you’re an adult, however, you don’t want too much mTOR signaling, because that might stimulate growth of things you definitely don’t want to grow.”
Having excessive levels of the growth hormone insulin-like growth factor-1 (IGF-1) also appears to play a role in cancer risk. Researchers studying members of a South American clan with Laron dwarfism – an inherited IGF-1 deficiency – found that besides being very short, affected members of this family rarely develop cancer (Cells. 2019;8[6]:596). “They also don’t get diabetes,” Dr. Lemanne said. “What we see in those with Laron dwarfism is that mTOR signaling is missing.”
She went on to note that studying type 2 diabetes gives physicians “a clue as to what dietary measures we might offer our patients in terms of decreasing their risk of dying from cancer or getting cancer.” The most common types of cancer are indeed more common in patients with type 2 diabetes. In addition, once someone with type 2 diabetes is diagnosed with cancer, their prognosis is poorer, compared with a cancer patient without diabetes.
“Metformin is often prescribed to patients with type 2 diabetes because it helps keep blood sugar low,” she said. “What’s fascinating is that diabetics on metformin develop cancer less frequently than diabetics not taking this drug. And also interesting, those diabetics who do develop cancer seem to do better if they’re on metformin before and after diagnosis.”
On the other hand, exogenous insulin therapy given to people with type 2 diabetes doubles the risk of cancer. Consistent with this is the two-decades-old finding that an elevated fasting insulin level also is associated with a poor breast cancer prognosis (J Clin Oncol. 2002 Jan 1;20[1]:42-51). “It’s really important to understand that, in a person destined to become a type 2 diabetic, the level of fasting insulin rises long before fasting glucose becomes abnormally high,” Dr. Lemanne explained. “A normal fasting glucose doesn’t let you off the hook in terms of checking your patient for insulin resistance.
“We will miss diagnosing many patients with dangerous insulin resistance and prediabetes if we don’t check the fasting glucose and the fasting insulin levels together. If the fasting insulin level is high, it’s important to limit carbohydrate intake enough to bring it down permanently, even when the fasting glucose is normal, or the patient is likely at increased risk for developing cancer.”
Two large, prospective randomized trials have examined breast cancer and diet: the Women’s Intervention Study (WINS) and the Women’s Health Eating and Living Study (WHEL). Patients in both trials had early stage breast cancer and were put on low-fat diets. In the end, there was a weak to negligible connection between breast cancer survival and dietary fat restriction. “That kind of shook up the oncology world,” Dr. Lemanne said, “because before these two studies, everyone ‘knew’ that dietary fat was related to breast cancer risk. These studies showed that wasn’t the case at all.”
According to Dr. Lemanne, unexpectedly, moderate carbohydrate restriction has been associated with lower risk of breast cancer recurrence in patients with postmenopausal hormone-receptor expressing breast cancer. Researchers at the University of California, San Diego, conducted a subanalysis of 265 postmenopausal patients with estrogen receptor positive breast cancer from the WHEL cohort (Cancer Epidemiol Biomarkers Prev. 2014 23[7]:1273-9). The recurrence risk was halved in those who cut their carbohydrate intake after diagnosis. The amount of decrease was modest, only 27 grams per day – the equivalent of one banana. “That is on par with a lot of our drugs, and maybe a little bit better,” she said. The effect was strongest if the breast tumor expressed IGF-1 receptor. Dr. Lemanne pointed out that decreasing dietary carbohydrate load was not the only treatment. These patients also had appropriate conventional cancer treatments, including surgery, radiation, and chemotherapy. “If we cut just some of the daily carb load in these patients, they might have a better cancer prognosis,” she said.
Overweight or obese patients with colon cancer also may benefit from moderate carbohydrate restriction. The CALGB 89803 study assessed 1,011 subjects with stage III colon cancer. It found that the subjects in the highest quintile of daily glycemic load and total carbohydrate intake had an increased risk of cancer recurrence and mortality (hazard ratio, 2.26; J Nat Cancer Inst. 2012;104[22]:1702-11). “This is pretty strong evidence that glycemic load and total carbohydrate intake play a role in colon cancer recurrence, but there’s a caveat here,” she said. “The effect was seen only in patients who were overweight or obese.” There was no association between carbohydrate intake and colon cancer recurrence in the absence of overweight or obesity.
Based on existing evidence, she said,
“That’s pretty modest; that’s 400 calories of carbohydrates per day,” Dr. Lemanne said. “I tell patients that they can have fruit, starchy vegetables, and even very small amounts of healthy whole grains, although I’m not a fan of grains due to the heavy carbohydrate load. All those things are OK. We’re not talking about jelly beans and white sugar.
“I also have them measure their fasting glucose each day, because different people have different blood glucose responses to the same food.” The goals she aims for with many of her patients are a fasting morning glucose between 79 and 83 mg/dL consistently, an HbA1c of 5.4 or less, and a BMI of 24.9 kg/m2 or less. “This set of goals, however, has to be individualized,” she said.
The ketogenic diet is another form of carb restriction, “but it’s much more drastic,” Dr. Lemanne said. “Most people require a carbohydrate load below 30 grams a day to enter a state of ketosis. But ketosis lowers the blood sugar and dampens the mTOR signaling.”
Evidence is emerging to support the use of a ketogenic diet as an adjunct to radiation therapy and as part of a complete course of treatment for glioblastoma multiforme and cancer cachexia. As an adjunct to radiation, a ketogenic diet decreases insulin and IGF-1 signaling. “This causes normal cells to enter dormancy, decreasing oxidative damage in normal cells,” Dr. Lemanne said. “There is also suppression of tumor angiogenesis, and thus poor DNA repair of radiation damage in tumor cells (Cancer Metastasis Rev. 2014;33[1]:217-29). Being in ketosis widens the therapeutic window. There are many animal studies which show that the ketogenic diet is helpful in cancer, mainly when combined with other anticancer treatments, such as radiation. Unfortunately, the evidence in humans is very anecdotal.”
One study found that if you feed mice with cancer ketogenic chow versus standard chow, they have a modestly improved survival (a mean of 43 days vs. 33 days; PLoS ONE. 2012;7[5]:e36197). However, when radiation was added to the keto diet, there was a dramatic improvement in survival (P less than 0.001). In fact, 75% survived to 250 days. “That’s pretty spectacular,” Dr. Lemanne said.
A ketogenic diet is standard therapy for several nonmalignant conditions, including glucose transporter 1 deficiency syndrome, pyruvate dehydrogenase deficiency syndrome, and refractory infantile epilepsy. The three major ketone bodies involved in human nutrition are acetoacetate, beta hydroxybutyrate, and acetone. Dr. Lemanne said beta hydroxybutyrate decreases inflammation and inhibits hexadecynoic acids (which induces apoptosis in cancer cells). Beta hydroxybutyrate also increases sirtuins, innate immunity, and seizure threshold; modulates circadian rhythm; and decreases insulin levels, she said.
In one case report from the scientific literature, a 38-year-old male with glioblastoma multiforme was placed on a hypocaloric ketogenic diet (Front Nutr. 2018 Mar 29;5:20). The patient had surgery, radiation, chemotherapy, and hyperbaric oxygen, and was given high doses of green tea extract in an attempt to antagonize glutamine metabolism. Two years after the beginning of his treatment, he was alive and had maintained a good level of tumor regression.
“We’ll see how he does,” said Dr. Lemanne, who was not involved in the report. “In my experience, I have a patient right now with a diagnosis of glioblastoma multiforme. She’s getting a keto diet in combo with intensive chemo, radiation, and surgery. She’s also had some hyperbaric oxygen and IV ozone therapy and is taking repurposed drugs. She has exceeded her expected survival, but she continues to have disease and symptoms. We are by no means out of the woods with this patient. But the keto diet has been quite feasible for her, because she has a lot of family and outside support.”
A ketogenic diet also may benefit patients with cancer cachexia, which is a loss of lean tissue. “Cancer cachexia is not completely understood,” Dr. Lemanne said. “What we know is that it is caused by inflammation created by the tumor itself, and this, in turn results in severe insulin resistance. Therefore, giving more calories as carbohydrate makes the cancer cachexia situation worse. Animal models of cancer cachexia have shown that the ketogenic diet normalizes metabolism and prevents lean tissue loss. Human studies are underway; we’ll see how they turn out.”
She closed her presentation by noting that in copious amounts of animal studies, fasting has been linked to improvements in chemotherapy efficacy and decreased side effects. In one study carried out at the University of Southern California in Los Angeles, volunteers fasted up to 140 hours before chemotherapy and an additional 156 hours afterward (Aging. 2009;1[12]:988-1007). The researchers found that the fasting was well-tolerated.
“The patients had some mild light-headedness, but there were no adverse effects on tumor volume or serum tumor markers,” Dr. Lemanne said. A more recent study of patients on cisplatin found that acaloric fasting led to decreased DNA damage in white blood cells, decreased IFG-1, and better white blood cell counts (BMC Cancer. 2016 Jun 10;16:360). “The benefits are immediate, and the optimal fasting time appears to be 48 hours,” Dr. Lemanne said.
One of her patients is a 64-year-old man on adjuvant cisplatin-based chemotherapy for cholangiocarcinoma. He fasts 24 hours before and 24 hours after each infusion, and has experienced no emesis or nausea. “His immune suppression and anemia are much milder than we expected, and he has not required any treatment for chemotherapy-related side effects,” Dr. Lemanne said. “That’s a big monetary value.”
Fasting 13 hours overnight has been associated with fewer breast cancer-related problems in patients already diagnosed with the disease. Chronic caloric restriction, just cutting calories by 25%-40% daily, has been shown to delay all diseases of aging, including cancer, and is associated with increased longevity in many species. “Chronic caloric restriction is difficult, however, because it results in chronic hunger and weight loss,” she said. “Occasional fasting is superior to chronic caloric restriction because it maintains normal weight, preserves lean muscle mass, enhances tumor sensitivity to chemotherapy and radiotherapy, and diminishes the side effects of chemotherapy.”
Dr. Lemanne reported having no financial disclosures.
dbrunk@mdedge.com
SAN DIEGO – When Dawn Lemanne, MD, MPH, meets with cancer patients and their families, the question invariably comes up: “What should I eat?”
“The answer always is, ‘It depends,’” Dr. Lemanne, an oncologist who founded Oregon Integrative Oncology in Ashland, said at Natural Supplements: An Evidence-Based Update, presented by Scripps Center for Integrative Medicine. “The answers are not the same for each of these patients.”
According to Dr. Lemanne, targeted nutrition is evolving as a key component of cancer care. One of the goals of this approach is to decrease mTOR signaling. Normally, mTOR signaling promotes cell proliferation and metabolism; aberrant mTOR signaling can contribute to cancer initiation and progression.
“When mTOR speaks it says, ‘grow,’” said Dr. Lemanne, who is also an assistant professor of clinical medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona in Tucson. This message is meant to be heard by normal tissues, to stimulate normal tissue proliferation, such as in growing children or when a wound needs to be healed.
“However, cancer cells can hear and respond to mTOR’s message,” she said. “Normal cells may listen to mTOR’s ‘grow’ message or not, depending on the task they perform. Once we reach adulthood, we all likely have some precancerous or cancerous cells around, but they’re usually dormant. That’s why once you’re an adult, however, you don’t want too much mTOR signaling, because that might stimulate growth of things you definitely don’t want to grow.”
Having excessive levels of the growth hormone insulin-like growth factor-1 (IGF-1) also appears to play a role in cancer risk. Researchers studying members of a South American clan with Laron dwarfism – an inherited IGF-1 deficiency – found that besides being very short, affected members of this family rarely develop cancer (Cells. 2019;8[6]:596). “They also don’t get diabetes,” Dr. Lemanne said. “What we see in those with Laron dwarfism is that mTOR signaling is missing.”
She went on to note that studying type 2 diabetes gives physicians “a clue as to what dietary measures we might offer our patients in terms of decreasing their risk of dying from cancer or getting cancer.” The most common types of cancer are indeed more common in patients with type 2 diabetes. In addition, once someone with type 2 diabetes is diagnosed with cancer, their prognosis is poorer, compared with a cancer patient without diabetes.
“Metformin is often prescribed to patients with type 2 diabetes because it helps keep blood sugar low,” she said. “What’s fascinating is that diabetics on metformin develop cancer less frequently than diabetics not taking this drug. And also interesting, those diabetics who do develop cancer seem to do better if they’re on metformin before and after diagnosis.”
On the other hand, exogenous insulin therapy given to people with type 2 diabetes doubles the risk of cancer. Consistent with this is the two-decades-old finding that an elevated fasting insulin level also is associated with a poor breast cancer prognosis (J Clin Oncol. 2002 Jan 1;20[1]:42-51). “It’s really important to understand that, in a person destined to become a type 2 diabetic, the level of fasting insulin rises long before fasting glucose becomes abnormally high,” Dr. Lemanne explained. “A normal fasting glucose doesn’t let you off the hook in terms of checking your patient for insulin resistance.
“We will miss diagnosing many patients with dangerous insulin resistance and prediabetes if we don’t check the fasting glucose and the fasting insulin levels together. If the fasting insulin level is high, it’s important to limit carbohydrate intake enough to bring it down permanently, even when the fasting glucose is normal, or the patient is likely at increased risk for developing cancer.”
Two large, prospective randomized trials have examined breast cancer and diet: the Women’s Intervention Study (WINS) and the Women’s Health Eating and Living Study (WHEL). Patients in both trials had early stage breast cancer and were put on low-fat diets. In the end, there was a weak to negligible connection between breast cancer survival and dietary fat restriction. “That kind of shook up the oncology world,” Dr. Lemanne said, “because before these two studies, everyone ‘knew’ that dietary fat was related to breast cancer risk. These studies showed that wasn’t the case at all.”
According to Dr. Lemanne, unexpectedly, moderate carbohydrate restriction has been associated with lower risk of breast cancer recurrence in patients with postmenopausal hormone-receptor expressing breast cancer. Researchers at the University of California, San Diego, conducted a subanalysis of 265 postmenopausal patients with estrogen receptor positive breast cancer from the WHEL cohort (Cancer Epidemiol Biomarkers Prev. 2014 23[7]:1273-9). The recurrence risk was halved in those who cut their carbohydrate intake after diagnosis. The amount of decrease was modest, only 27 grams per day – the equivalent of one banana. “That is on par with a lot of our drugs, and maybe a little bit better,” she said. The effect was strongest if the breast tumor expressed IGF-1 receptor. Dr. Lemanne pointed out that decreasing dietary carbohydrate load was not the only treatment. These patients also had appropriate conventional cancer treatments, including surgery, radiation, and chemotherapy. “If we cut just some of the daily carb load in these patients, they might have a better cancer prognosis,” she said.
Overweight or obese patients with colon cancer also may benefit from moderate carbohydrate restriction. The CALGB 89803 study assessed 1,011 subjects with stage III colon cancer. It found that the subjects in the highest quintile of daily glycemic load and total carbohydrate intake had an increased risk of cancer recurrence and mortality (hazard ratio, 2.26; J Nat Cancer Inst. 2012;104[22]:1702-11). “This is pretty strong evidence that glycemic load and total carbohydrate intake play a role in colon cancer recurrence, but there’s a caveat here,” she said. “The effect was seen only in patients who were overweight or obese.” There was no association between carbohydrate intake and colon cancer recurrence in the absence of overweight or obesity.
Based on existing evidence, she said,
“That’s pretty modest; that’s 400 calories of carbohydrates per day,” Dr. Lemanne said. “I tell patients that they can have fruit, starchy vegetables, and even very small amounts of healthy whole grains, although I’m not a fan of grains due to the heavy carbohydrate load. All those things are OK. We’re not talking about jelly beans and white sugar.
“I also have them measure their fasting glucose each day, because different people have different blood glucose responses to the same food.” The goals she aims for with many of her patients are a fasting morning glucose between 79 and 83 mg/dL consistently, an HbA1c of 5.4 or less, and a BMI of 24.9 kg/m2 or less. “This set of goals, however, has to be individualized,” she said.
The ketogenic diet is another form of carb restriction, “but it’s much more drastic,” Dr. Lemanne said. “Most people require a carbohydrate load below 30 grams a day to enter a state of ketosis. But ketosis lowers the blood sugar and dampens the mTOR signaling.”
Evidence is emerging to support the use of a ketogenic diet as an adjunct to radiation therapy and as part of a complete course of treatment for glioblastoma multiforme and cancer cachexia. As an adjunct to radiation, a ketogenic diet decreases insulin and IGF-1 signaling. “This causes normal cells to enter dormancy, decreasing oxidative damage in normal cells,” Dr. Lemanne said. “There is also suppression of tumor angiogenesis, and thus poor DNA repair of radiation damage in tumor cells (Cancer Metastasis Rev. 2014;33[1]:217-29). Being in ketosis widens the therapeutic window. There are many animal studies which show that the ketogenic diet is helpful in cancer, mainly when combined with other anticancer treatments, such as radiation. Unfortunately, the evidence in humans is very anecdotal.”
One study found that if you feed mice with cancer ketogenic chow versus standard chow, they have a modestly improved survival (a mean of 43 days vs. 33 days; PLoS ONE. 2012;7[5]:e36197). However, when radiation was added to the keto diet, there was a dramatic improvement in survival (P less than 0.001). In fact, 75% survived to 250 days. “That’s pretty spectacular,” Dr. Lemanne said.
A ketogenic diet is standard therapy for several nonmalignant conditions, including glucose transporter 1 deficiency syndrome, pyruvate dehydrogenase deficiency syndrome, and refractory infantile epilepsy. The three major ketone bodies involved in human nutrition are acetoacetate, beta hydroxybutyrate, and acetone. Dr. Lemanne said beta hydroxybutyrate decreases inflammation and inhibits hexadecynoic acids (which induces apoptosis in cancer cells). Beta hydroxybutyrate also increases sirtuins, innate immunity, and seizure threshold; modulates circadian rhythm; and decreases insulin levels, she said.
In one case report from the scientific literature, a 38-year-old male with glioblastoma multiforme was placed on a hypocaloric ketogenic diet (Front Nutr. 2018 Mar 29;5:20). The patient had surgery, radiation, chemotherapy, and hyperbaric oxygen, and was given high doses of green tea extract in an attempt to antagonize glutamine metabolism. Two years after the beginning of his treatment, he was alive and had maintained a good level of tumor regression.
“We’ll see how he does,” said Dr. Lemanne, who was not involved in the report. “In my experience, I have a patient right now with a diagnosis of glioblastoma multiforme. She’s getting a keto diet in combo with intensive chemo, radiation, and surgery. She’s also had some hyperbaric oxygen and IV ozone therapy and is taking repurposed drugs. She has exceeded her expected survival, but she continues to have disease and symptoms. We are by no means out of the woods with this patient. But the keto diet has been quite feasible for her, because she has a lot of family and outside support.”
A ketogenic diet also may benefit patients with cancer cachexia, which is a loss of lean tissue. “Cancer cachexia is not completely understood,” Dr. Lemanne said. “What we know is that it is caused by inflammation created by the tumor itself, and this, in turn results in severe insulin resistance. Therefore, giving more calories as carbohydrate makes the cancer cachexia situation worse. Animal models of cancer cachexia have shown that the ketogenic diet normalizes metabolism and prevents lean tissue loss. Human studies are underway; we’ll see how they turn out.”
She closed her presentation by noting that in copious amounts of animal studies, fasting has been linked to improvements in chemotherapy efficacy and decreased side effects. In one study carried out at the University of Southern California in Los Angeles, volunteers fasted up to 140 hours before chemotherapy and an additional 156 hours afterward (Aging. 2009;1[12]:988-1007). The researchers found that the fasting was well-tolerated.
“The patients had some mild light-headedness, but there were no adverse effects on tumor volume or serum tumor markers,” Dr. Lemanne said. A more recent study of patients on cisplatin found that acaloric fasting led to decreased DNA damage in white blood cells, decreased IFG-1, and better white blood cell counts (BMC Cancer. 2016 Jun 10;16:360). “The benefits are immediate, and the optimal fasting time appears to be 48 hours,” Dr. Lemanne said.
One of her patients is a 64-year-old man on adjuvant cisplatin-based chemotherapy for cholangiocarcinoma. He fasts 24 hours before and 24 hours after each infusion, and has experienced no emesis or nausea. “His immune suppression and anemia are much milder than we expected, and he has not required any treatment for chemotherapy-related side effects,” Dr. Lemanne said. “That’s a big monetary value.”
Fasting 13 hours overnight has been associated with fewer breast cancer-related problems in patients already diagnosed with the disease. Chronic caloric restriction, just cutting calories by 25%-40% daily, has been shown to delay all diseases of aging, including cancer, and is associated with increased longevity in many species. “Chronic caloric restriction is difficult, however, because it results in chronic hunger and weight loss,” she said. “Occasional fasting is superior to chronic caloric restriction because it maintains normal weight, preserves lean muscle mass, enhances tumor sensitivity to chemotherapy and radiotherapy, and diminishes the side effects of chemotherapy.”
Dr. Lemanne reported having no financial disclosures.
dbrunk@mdedge.com
SAN DIEGO – When Dawn Lemanne, MD, MPH, meets with cancer patients and their families, the question invariably comes up: “What should I eat?”
“The answer always is, ‘It depends,’” Dr. Lemanne, an oncologist who founded Oregon Integrative Oncology in Ashland, said at Natural Supplements: An Evidence-Based Update, presented by Scripps Center for Integrative Medicine. “The answers are not the same for each of these patients.”
According to Dr. Lemanne, targeted nutrition is evolving as a key component of cancer care. One of the goals of this approach is to decrease mTOR signaling. Normally, mTOR signaling promotes cell proliferation and metabolism; aberrant mTOR signaling can contribute to cancer initiation and progression.
“When mTOR speaks it says, ‘grow,’” said Dr. Lemanne, who is also an assistant professor of clinical medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona in Tucson. This message is meant to be heard by normal tissues, to stimulate normal tissue proliferation, such as in growing children or when a wound needs to be healed.
“However, cancer cells can hear and respond to mTOR’s message,” she said. “Normal cells may listen to mTOR’s ‘grow’ message or not, depending on the task they perform. Once we reach adulthood, we all likely have some precancerous or cancerous cells around, but they’re usually dormant. That’s why once you’re an adult, however, you don’t want too much mTOR signaling, because that might stimulate growth of things you definitely don’t want to grow.”
Having excessive levels of the growth hormone insulin-like growth factor-1 (IGF-1) also appears to play a role in cancer risk. Researchers studying members of a South American clan with Laron dwarfism – an inherited IGF-1 deficiency – found that besides being very short, affected members of this family rarely develop cancer (Cells. 2019;8[6]:596). “They also don’t get diabetes,” Dr. Lemanne said. “What we see in those with Laron dwarfism is that mTOR signaling is missing.”
She went on to note that studying type 2 diabetes gives physicians “a clue as to what dietary measures we might offer our patients in terms of decreasing their risk of dying from cancer or getting cancer.” The most common types of cancer are indeed more common in patients with type 2 diabetes. In addition, once someone with type 2 diabetes is diagnosed with cancer, their prognosis is poorer, compared with a cancer patient without diabetes.
“Metformin is often prescribed to patients with type 2 diabetes because it helps keep blood sugar low,” she said. “What’s fascinating is that diabetics on metformin develop cancer less frequently than diabetics not taking this drug. And also interesting, those diabetics who do develop cancer seem to do better if they’re on metformin before and after diagnosis.”
On the other hand, exogenous insulin therapy given to people with type 2 diabetes doubles the risk of cancer. Consistent with this is the two-decades-old finding that an elevated fasting insulin level also is associated with a poor breast cancer prognosis (J Clin Oncol. 2002 Jan 1;20[1]:42-51). “It’s really important to understand that, in a person destined to become a type 2 diabetic, the level of fasting insulin rises long before fasting glucose becomes abnormally high,” Dr. Lemanne explained. “A normal fasting glucose doesn’t let you off the hook in terms of checking your patient for insulin resistance.
“We will miss diagnosing many patients with dangerous insulin resistance and prediabetes if we don’t check the fasting glucose and the fasting insulin levels together. If the fasting insulin level is high, it’s important to limit carbohydrate intake enough to bring it down permanently, even when the fasting glucose is normal, or the patient is likely at increased risk for developing cancer.”
Two large, prospective randomized trials have examined breast cancer and diet: the Women’s Intervention Study (WINS) and the Women’s Health Eating and Living Study (WHEL). Patients in both trials had early stage breast cancer and were put on low-fat diets. In the end, there was a weak to negligible connection between breast cancer survival and dietary fat restriction. “That kind of shook up the oncology world,” Dr. Lemanne said, “because before these two studies, everyone ‘knew’ that dietary fat was related to breast cancer risk. These studies showed that wasn’t the case at all.”
According to Dr. Lemanne, unexpectedly, moderate carbohydrate restriction has been associated with lower risk of breast cancer recurrence in patients with postmenopausal hormone-receptor expressing breast cancer. Researchers at the University of California, San Diego, conducted a subanalysis of 265 postmenopausal patients with estrogen receptor positive breast cancer from the WHEL cohort (Cancer Epidemiol Biomarkers Prev. 2014 23[7]:1273-9). The recurrence risk was halved in those who cut their carbohydrate intake after diagnosis. The amount of decrease was modest, only 27 grams per day – the equivalent of one banana. “That is on par with a lot of our drugs, and maybe a little bit better,” she said. The effect was strongest if the breast tumor expressed IGF-1 receptor. Dr. Lemanne pointed out that decreasing dietary carbohydrate load was not the only treatment. These patients also had appropriate conventional cancer treatments, including surgery, radiation, and chemotherapy. “If we cut just some of the daily carb load in these patients, they might have a better cancer prognosis,” she said.
Overweight or obese patients with colon cancer also may benefit from moderate carbohydrate restriction. The CALGB 89803 study assessed 1,011 subjects with stage III colon cancer. It found that the subjects in the highest quintile of daily glycemic load and total carbohydrate intake had an increased risk of cancer recurrence and mortality (hazard ratio, 2.26; J Nat Cancer Inst. 2012;104[22]:1702-11). “This is pretty strong evidence that glycemic load and total carbohydrate intake play a role in colon cancer recurrence, but there’s a caveat here,” she said. “The effect was seen only in patients who were overweight or obese.” There was no association between carbohydrate intake and colon cancer recurrence in the absence of overweight or obesity.
Based on existing evidence, she said,
“That’s pretty modest; that’s 400 calories of carbohydrates per day,” Dr. Lemanne said. “I tell patients that they can have fruit, starchy vegetables, and even very small amounts of healthy whole grains, although I’m not a fan of grains due to the heavy carbohydrate load. All those things are OK. We’re not talking about jelly beans and white sugar.
“I also have them measure their fasting glucose each day, because different people have different blood glucose responses to the same food.” The goals she aims for with many of her patients are a fasting morning glucose between 79 and 83 mg/dL consistently, an HbA1c of 5.4 or less, and a BMI of 24.9 kg/m2 or less. “This set of goals, however, has to be individualized,” she said.
The ketogenic diet is another form of carb restriction, “but it’s much more drastic,” Dr. Lemanne said. “Most people require a carbohydrate load below 30 grams a day to enter a state of ketosis. But ketosis lowers the blood sugar and dampens the mTOR signaling.”
Evidence is emerging to support the use of a ketogenic diet as an adjunct to radiation therapy and as part of a complete course of treatment for glioblastoma multiforme and cancer cachexia. As an adjunct to radiation, a ketogenic diet decreases insulin and IGF-1 signaling. “This causes normal cells to enter dormancy, decreasing oxidative damage in normal cells,” Dr. Lemanne said. “There is also suppression of tumor angiogenesis, and thus poor DNA repair of radiation damage in tumor cells (Cancer Metastasis Rev. 2014;33[1]:217-29). Being in ketosis widens the therapeutic window. There are many animal studies which show that the ketogenic diet is helpful in cancer, mainly when combined with other anticancer treatments, such as radiation. Unfortunately, the evidence in humans is very anecdotal.”
One study found that if you feed mice with cancer ketogenic chow versus standard chow, they have a modestly improved survival (a mean of 43 days vs. 33 days; PLoS ONE. 2012;7[5]:e36197). However, when radiation was added to the keto diet, there was a dramatic improvement in survival (P less than 0.001). In fact, 75% survived to 250 days. “That’s pretty spectacular,” Dr. Lemanne said.
A ketogenic diet is standard therapy for several nonmalignant conditions, including glucose transporter 1 deficiency syndrome, pyruvate dehydrogenase deficiency syndrome, and refractory infantile epilepsy. The three major ketone bodies involved in human nutrition are acetoacetate, beta hydroxybutyrate, and acetone. Dr. Lemanne said beta hydroxybutyrate decreases inflammation and inhibits hexadecynoic acids (which induces apoptosis in cancer cells). Beta hydroxybutyrate also increases sirtuins, innate immunity, and seizure threshold; modulates circadian rhythm; and decreases insulin levels, she said.
In one case report from the scientific literature, a 38-year-old male with glioblastoma multiforme was placed on a hypocaloric ketogenic diet (Front Nutr. 2018 Mar 29;5:20). The patient had surgery, radiation, chemotherapy, and hyperbaric oxygen, and was given high doses of green tea extract in an attempt to antagonize glutamine metabolism. Two years after the beginning of his treatment, he was alive and had maintained a good level of tumor regression.
“We’ll see how he does,” said Dr. Lemanne, who was not involved in the report. “In my experience, I have a patient right now with a diagnosis of glioblastoma multiforme. She’s getting a keto diet in combo with intensive chemo, radiation, and surgery. She’s also had some hyperbaric oxygen and IV ozone therapy and is taking repurposed drugs. She has exceeded her expected survival, but she continues to have disease and symptoms. We are by no means out of the woods with this patient. But the keto diet has been quite feasible for her, because she has a lot of family and outside support.”
A ketogenic diet also may benefit patients with cancer cachexia, which is a loss of lean tissue. “Cancer cachexia is not completely understood,” Dr. Lemanne said. “What we know is that it is caused by inflammation created by the tumor itself, and this, in turn results in severe insulin resistance. Therefore, giving more calories as carbohydrate makes the cancer cachexia situation worse. Animal models of cancer cachexia have shown that the ketogenic diet normalizes metabolism and prevents lean tissue loss. Human studies are underway; we’ll see how they turn out.”
She closed her presentation by noting that in copious amounts of animal studies, fasting has been linked to improvements in chemotherapy efficacy and decreased side effects. In one study carried out at the University of Southern California in Los Angeles, volunteers fasted up to 140 hours before chemotherapy and an additional 156 hours afterward (Aging. 2009;1[12]:988-1007). The researchers found that the fasting was well-tolerated.
“The patients had some mild light-headedness, but there were no adverse effects on tumor volume or serum tumor markers,” Dr. Lemanne said. A more recent study of patients on cisplatin found that acaloric fasting led to decreased DNA damage in white blood cells, decreased IFG-1, and better white blood cell counts (BMC Cancer. 2016 Jun 10;16:360). “The benefits are immediate, and the optimal fasting time appears to be 48 hours,” Dr. Lemanne said.
One of her patients is a 64-year-old man on adjuvant cisplatin-based chemotherapy for cholangiocarcinoma. He fasts 24 hours before and 24 hours after each infusion, and has experienced no emesis or nausea. “His immune suppression and anemia are much milder than we expected, and he has not required any treatment for chemotherapy-related side effects,” Dr. Lemanne said. “That’s a big monetary value.”
Fasting 13 hours overnight has been associated with fewer breast cancer-related problems in patients already diagnosed with the disease. Chronic caloric restriction, just cutting calories by 25%-40% daily, has been shown to delay all diseases of aging, including cancer, and is associated with increased longevity in many species. “Chronic caloric restriction is difficult, however, because it results in chronic hunger and weight loss,” she said. “Occasional fasting is superior to chronic caloric restriction because it maintains normal weight, preserves lean muscle mass, enhances tumor sensitivity to chemotherapy and radiotherapy, and diminishes the side effects of chemotherapy.”
Dr. Lemanne reported having no financial disclosures.
dbrunk@mdedge.com
REPORTING FROM A NATURAL SUPPLEMENTS UPDATE
Breast cancer chemoprophylaxis in high-risk women: How persistent is the impact of an aromatase inhibitor after 5 years of use?
Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
EXPERT COMMENTARY
A manufacturer-sponsored trial initiated in 2003, IBIS-II (International Breast Cancer Intervention Study II) included 3,864 menopausal women (mean age at baseline, 59.4 years) at elevated risk for breast cancer. The women were randomly assigned to 5-year treatment with either placebo (N = 1,944) or the aromatase inhibitor anastrozole 1 mg daily (N = 1,920).1
Reporting on the long-term follow-up results of the trial, Cuzick and colleagues found that anastrozole use substantially reduced the incidence of all breast cancer, including invasive breast cancer and ductal carcinoma in situ. Key adverse events associated with anastrozole were fractures, arthralgias, and menopausal symptoms (vasomotor symptoms and vaginal dryness).
To determine whether anastrozole had any persistent impact, the investigators continued to follow participants for all breast cancers and other outcomes.2
Details of the study
This randomized controlled trial that included 3,864 postmenopausal women had a median overall follow-up of 131 months; the primary outcome was all breast cancer. Random assignment to anastrozole use (1,920 women) was associated with a 49% reduction in all breast cancer (85 cases vs 165 cases in the placebo group [N = 1,944]; HR, 0.51; 95% CI, 0.39–0.66; P<.0001).
In the first 5 years, risk reduction was 61% with anastrozole (P<.0001 for overall and the first 5 years of follow-up). Subsequently, the magnitude of the risk reduction attenuated to 37% (P = .014). With 12 years of follow-up, the estimated risk of being diagnosed with breast cancer was 8.8% and 5.3% in the placebo and anastrozole groups, respectively. The number needed to treat for 5 years to prevent 1 breast cancer was 29.
With anastrozole, prevention of estrogen–receptor positive tumors was substantially more robust at 54% (HR, 0.46; 95% CI, 0.33–0.65; P<.0001) than for estrogen–receptor negative tumors at 27% (HR, 0.77; 95% CI, 0.41–1.44; P = .41).
Over the course of the long-term study, the incidence of fractures and cardiovascular events was similar in the placebo and anastrozole groups. Arthralgias and menopausal symptoms were not assessed after the trial’s initial 5 years. Overall, the number of deaths (all cause as well as breast cancer related) were similar in the placebo and anastrozole groups.
Continue to: Study strengths and limitations...
Study strengths and limitations
The authors noted that this updated analysis of the IBIS-II trial data offers further support for the use of anastrozole in breast cancer prevention for high-risk postmenopausal women. The extended posttreatment follow-up showed a significant continuing reduction in breast cancer, and there was no evidence of new late adverse effects. A limitation of the analysis, however, is that very few deaths from breast cancer occurred during the study timeframe. Thus, additional follow-up would be needed to assess anastrozole’s effect on breast cancer mortality.
The breast cancer chemoprophylactic efficacy of anastrozole compares favorably with that of tamoxifen. Furthermore, in women with an intact uterus, the increased risks of gynecologic problems, including endometrial cancer, associated with tamoxifen do not occur with aromatase inhibitors. However, the lack of any obvious mortality benefit means the ultimate value of estrogen deprivation breast cancer chemoprophylaxis continues to be uncertain, especially given other risks, including bone loss. In view of these new data, it will be important for high-risk women considering aromatase inhibitor prophylaxis to understand that these medications have not been associated with a mortality benefit.
ANDREW M. KAUNITZ, MD, NCMP
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041-1048.
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
EXPERT COMMENTARY
A manufacturer-sponsored trial initiated in 2003, IBIS-II (International Breast Cancer Intervention Study II) included 3,864 menopausal women (mean age at baseline, 59.4 years) at elevated risk for breast cancer. The women were randomly assigned to 5-year treatment with either placebo (N = 1,944) or the aromatase inhibitor anastrozole 1 mg daily (N = 1,920).1
Reporting on the long-term follow-up results of the trial, Cuzick and colleagues found that anastrozole use substantially reduced the incidence of all breast cancer, including invasive breast cancer and ductal carcinoma in situ. Key adverse events associated with anastrozole were fractures, arthralgias, and menopausal symptoms (vasomotor symptoms and vaginal dryness).
To determine whether anastrozole had any persistent impact, the investigators continued to follow participants for all breast cancers and other outcomes.2
Details of the study
This randomized controlled trial that included 3,864 postmenopausal women had a median overall follow-up of 131 months; the primary outcome was all breast cancer. Random assignment to anastrozole use (1,920 women) was associated with a 49% reduction in all breast cancer (85 cases vs 165 cases in the placebo group [N = 1,944]; HR, 0.51; 95% CI, 0.39–0.66; P<.0001).
In the first 5 years, risk reduction was 61% with anastrozole (P<.0001 for overall and the first 5 years of follow-up). Subsequently, the magnitude of the risk reduction attenuated to 37% (P = .014). With 12 years of follow-up, the estimated risk of being diagnosed with breast cancer was 8.8% and 5.3% in the placebo and anastrozole groups, respectively. The number needed to treat for 5 years to prevent 1 breast cancer was 29.
With anastrozole, prevention of estrogen–receptor positive tumors was substantially more robust at 54% (HR, 0.46; 95% CI, 0.33–0.65; P<.0001) than for estrogen–receptor negative tumors at 27% (HR, 0.77; 95% CI, 0.41–1.44; P = .41).
Over the course of the long-term study, the incidence of fractures and cardiovascular events was similar in the placebo and anastrozole groups. Arthralgias and menopausal symptoms were not assessed after the trial’s initial 5 years. Overall, the number of deaths (all cause as well as breast cancer related) were similar in the placebo and anastrozole groups.
Continue to: Study strengths and limitations...
Study strengths and limitations
The authors noted that this updated analysis of the IBIS-II trial data offers further support for the use of anastrozole in breast cancer prevention for high-risk postmenopausal women. The extended posttreatment follow-up showed a significant continuing reduction in breast cancer, and there was no evidence of new late adverse effects. A limitation of the analysis, however, is that very few deaths from breast cancer occurred during the study timeframe. Thus, additional follow-up would be needed to assess anastrozole’s effect on breast cancer mortality.
The breast cancer chemoprophylactic efficacy of anastrozole compares favorably with that of tamoxifen. Furthermore, in women with an intact uterus, the increased risks of gynecologic problems, including endometrial cancer, associated with tamoxifen do not occur with aromatase inhibitors. However, the lack of any obvious mortality benefit means the ultimate value of estrogen deprivation breast cancer chemoprophylaxis continues to be uncertain, especially given other risks, including bone loss. In view of these new data, it will be important for high-risk women considering aromatase inhibitor prophylaxis to understand that these medications have not been associated with a mortality benefit.
ANDREW M. KAUNITZ, MD, NCMP
Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
EXPERT COMMENTARY
A manufacturer-sponsored trial initiated in 2003, IBIS-II (International Breast Cancer Intervention Study II) included 3,864 menopausal women (mean age at baseline, 59.4 years) at elevated risk for breast cancer. The women were randomly assigned to 5-year treatment with either placebo (N = 1,944) or the aromatase inhibitor anastrozole 1 mg daily (N = 1,920).1
Reporting on the long-term follow-up results of the trial, Cuzick and colleagues found that anastrozole use substantially reduced the incidence of all breast cancer, including invasive breast cancer and ductal carcinoma in situ. Key adverse events associated with anastrozole were fractures, arthralgias, and menopausal symptoms (vasomotor symptoms and vaginal dryness).
To determine whether anastrozole had any persistent impact, the investigators continued to follow participants for all breast cancers and other outcomes.2
Details of the study
This randomized controlled trial that included 3,864 postmenopausal women had a median overall follow-up of 131 months; the primary outcome was all breast cancer. Random assignment to anastrozole use (1,920 women) was associated with a 49% reduction in all breast cancer (85 cases vs 165 cases in the placebo group [N = 1,944]; HR, 0.51; 95% CI, 0.39–0.66; P<.0001).
In the first 5 years, risk reduction was 61% with anastrozole (P<.0001 for overall and the first 5 years of follow-up). Subsequently, the magnitude of the risk reduction attenuated to 37% (P = .014). With 12 years of follow-up, the estimated risk of being diagnosed with breast cancer was 8.8% and 5.3% in the placebo and anastrozole groups, respectively. The number needed to treat for 5 years to prevent 1 breast cancer was 29.
With anastrozole, prevention of estrogen–receptor positive tumors was substantially more robust at 54% (HR, 0.46; 95% CI, 0.33–0.65; P<.0001) than for estrogen–receptor negative tumors at 27% (HR, 0.77; 95% CI, 0.41–1.44; P = .41).
Over the course of the long-term study, the incidence of fractures and cardiovascular events was similar in the placebo and anastrozole groups. Arthralgias and menopausal symptoms were not assessed after the trial’s initial 5 years. Overall, the number of deaths (all cause as well as breast cancer related) were similar in the placebo and anastrozole groups.
Continue to: Study strengths and limitations...
Study strengths and limitations
The authors noted that this updated analysis of the IBIS-II trial data offers further support for the use of anastrozole in breast cancer prevention for high-risk postmenopausal women. The extended posttreatment follow-up showed a significant continuing reduction in breast cancer, and there was no evidence of new late adverse effects. A limitation of the analysis, however, is that very few deaths from breast cancer occurred during the study timeframe. Thus, additional follow-up would be needed to assess anastrozole’s effect on breast cancer mortality.
The breast cancer chemoprophylactic efficacy of anastrozole compares favorably with that of tamoxifen. Furthermore, in women with an intact uterus, the increased risks of gynecologic problems, including endometrial cancer, associated with tamoxifen do not occur with aromatase inhibitors. However, the lack of any obvious mortality benefit means the ultimate value of estrogen deprivation breast cancer chemoprophylaxis continues to be uncertain, especially given other risks, including bone loss. In view of these new data, it will be important for high-risk women considering aromatase inhibitor prophylaxis to understand that these medications have not been associated with a mortality benefit.
ANDREW M. KAUNITZ, MD, NCMP
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041-1048.
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041-1048.
- Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395;117-122.
New tools could help predict complication risks in lung and breast cancer
In this edition of “How I Will Treat My Next Patient,” I highlight the potential role of new models for predicting risks of common, clinically important situations in general oncology practice: severe neutropenia in lung cancer patients and locoregional recurrence of breast cancer.
Predicting neutropenia
Accurate, lung cancer–specific prediction models would be useful to estimate risk of chemotherapy-induced neutropenia (CIN), especially febrile neutropenia (FN), since that particular toxicity is linked to infection, dose delays and dose reductions that can compromise treatment efficacy, and poor health-related quality of life. Lung cancer patients are often older adults, with advanced disease and comorbid conditions, so they are a particularly vulnerable population for CIN.
Xiaowen Cao of Duke University, Durham, N.C., and coinvestigators published a model for predicting risk of severe CIN in advanced lung cancer patients, based on 10 pretreatment variables (Lung Cancer. 2020 Jan 5. doi: 10.1016/j.lungcan.2020.01.004). They developed their model to overcome limitations of the previously published work of Gary H. Lyman, MD, and colleagues that is not specific to lung cancer and incorporated relative dose intensity as a predictor (Cancer. 2011;117:1917-27). Relative dose intensity is not determined until after a treatment course is completed.
The new prediction model was based on a lung cancer data set encompassing 11,352 patients from 67 phase 2-3 cooperative group studies conducted between 1991 and 2010. In this data set, the Lyman model had an area under the curve of 0.8772 in patients with small cell lung cancer, but an area under the curve of just 0.6787 in non–small cell lung cancer.
The derivation model was derived from about two-thirds of the patients, randomly selected. The validation set was conducted using the remaining third. The variables included were readily clinically available: age, gender, weight, body mass index, insurance status, disease stage, number of metastatic sites, chemotherapy agents used, number of chemotherapy agents, planned growth factor use, duration of planned therapy, pleural effusion, presence of symptoms, and performance status. Their model had an area under the curve of 0.8348 in the training set and 0.8234 in the testing set.
How these results influence practice
The risk of an initial episode of FN is highest during a patient’s initial cycle of chemotherapy, when most patients are receiving full-dose treatment, often without prophylactic measures. Guidelines from the National Comprehensive Cancer Network suggest the use of prophylactic growth factors in patients with more than a 20% risk of FN, and considering using prophylaxis in patients with 10%-20% risk of FN. Underestimating those risks and failure to take adequate precautions may be particularly important for patients with lung cancer who are generally older adults, with comorbid conditions.
The comprehensive risk model for neutropenic complications that was developed by Dr. Lyman and colleagues was based on a large, prospective cohort including nearly 3,800 patients. The model had a 90% sensitivity and 96% predictive value, but was not lung cancer specific and, in this latest study, did not perform as well in the 85% of lung cancer patients with non–small cell lung cancer. The Lyman data, however, was obtained in cancer patients treated with investigator-choice chemotherapy in community practices. It remains the National Comprehensive Cancer Network standard for evaluating FN risk in patients embarking on chemotherapy for advanced malignancies. That should remain the case, pending the additional validation testing of the new lung cancer–specific model at independent institutions, treating heterogeneous patients in real-world settings.
Locoregional recurrence
A retrospective cohort analysis of SWOG 8814, a phase 3 study of tamoxifen alone versus chemotherapy plus by tamoxifen in postmenopausal, node-positive, hormone receptor–positive breast cancer patients suggests that the 21-gene assay recurrence score (RS) can aid decisions about radiotherapy (RT).
Wendy A. Woodward, MD, PhD, and colleagues, analyzed patients who underwent mastectomy or breast-conserving surgery as their local therapy (JAMA Oncol. 2020 Jan 9. doi: 10.1001/jamaoncol.2019.5559). They found that patients with an intermediate or high RS – according to the 21-gene assay OncotypeDX – had more locoregional recurrences (LRR; breast, chest wall, axilla, internal mammary, supraclavicular or infraclavicular nodes).
There were 367 patients in SWOG 8814 who received tamoxifen alone or cyclophosphamide, doxorubicin, and fluorouracil followed by tamoxifen. LRR was observed in 5.8% of patients with a low RS (less than 18) and in 13.8% of patients with an intermediate or high RS (more than 18). The estimated 10-year cumulative LRR incidence rates were 9.7% and 16.5%, respectively (P = .02).
In the subset of patients with one to three positive nodes who had mastectomy without radiotherapy, the LRR was 1.5% for those with low RS and 11.1% for those with intermediate or high RS (P = .051). No difference by RS was found in the 10-year rates of LRR among patients with four or more involved nodes who received a mastectomy without RT (25.9% vs. 27.0%; P = .27).
In multivariate analysis, incorporating RS, type of surgery, and number of involved nodes, intermediate or high RS was a significant predictor of LRR, with a hazard ratio of 2.36 (P = .04). The investigators suggested that RS, when available, should be one of the factors considered in selecting patients for postmastectomy RT.
How these results influence practice
Selecting the node-positive, hormone receptor–positive, breast cancer patients who should receive postmastectomy RT is difficult and controversial. This is particularly true for those postmenopausal patients with fewer than four involved nodes, no lymphatic or vascular invasion, and no extracapsular spread of disease into the axillary fat. Limited information exists on the ability of genomic assays to identify LRR risk.
Eleftherios P. Mamounas, MD, and colleagues examined the results of NSABP B-28, a trial of chemotherapy plus tamoxifen (J Natl Cancer Inst. 2017;109[4]. doi:10.1093/jnci/djw259). Postmastectomy RT was not permitted. They found high RS correlated with greater LRR and low RS with decreased LRR among patients with one to three positive nodes. At first blush, the prospectively treated cohort of SWOG 8814 represents a uniformly treated cohort with long-term follow-up (median, 8.5 years) and extends in an independent analysis the findings of NSABP B-28.
However, as Dr. Woodward and colleagues point out, the current study has limitations. The use of RT was extracted retrospectively and may be underreported. More modern chemotherapy and RT may lower LRR from the risks observed in SWOG 8814. Finally, the modest numbers of LRR events precluded secondary analysis of RS as a continuous variable. This is important because the risk group cutoffs suggested by the authors are not aligned with those in the recently published TailorRx study or the ongoing RxPonder trial.
The TailorRT (Regional Radiotherapy in Biomarker Low Risk Node Positive Breast Cancer) study examines the safety of omitting RT among patients with low RS and one to three positive nodes. Until the TailorRT results are reported, the controversy regarding the role of postmastectomy RT in this group will continue for patients with low nodal tumor burden and less aggressive tumor features, including low RS.
An observed LRR risk of 11.1% in SWOG 8814 among patients with N1 disease and an RS above 18 suggest that genomic risk could be one of the factors that may justify postmastectomy RT in postmenopausal patients with node-positive, hormone receptor–positive breast cancer until additional data emerge from the contemporary trials.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight the potential role of new models for predicting risks of common, clinically important situations in general oncology practice: severe neutropenia in lung cancer patients and locoregional recurrence of breast cancer.
Predicting neutropenia
Accurate, lung cancer–specific prediction models would be useful to estimate risk of chemotherapy-induced neutropenia (CIN), especially febrile neutropenia (FN), since that particular toxicity is linked to infection, dose delays and dose reductions that can compromise treatment efficacy, and poor health-related quality of life. Lung cancer patients are often older adults, with advanced disease and comorbid conditions, so they are a particularly vulnerable population for CIN.
Xiaowen Cao of Duke University, Durham, N.C., and coinvestigators published a model for predicting risk of severe CIN in advanced lung cancer patients, based on 10 pretreatment variables (Lung Cancer. 2020 Jan 5. doi: 10.1016/j.lungcan.2020.01.004). They developed their model to overcome limitations of the previously published work of Gary H. Lyman, MD, and colleagues that is not specific to lung cancer and incorporated relative dose intensity as a predictor (Cancer. 2011;117:1917-27). Relative dose intensity is not determined until after a treatment course is completed.
The new prediction model was based on a lung cancer data set encompassing 11,352 patients from 67 phase 2-3 cooperative group studies conducted between 1991 and 2010. In this data set, the Lyman model had an area under the curve of 0.8772 in patients with small cell lung cancer, but an area under the curve of just 0.6787 in non–small cell lung cancer.
The derivation model was derived from about two-thirds of the patients, randomly selected. The validation set was conducted using the remaining third. The variables included were readily clinically available: age, gender, weight, body mass index, insurance status, disease stage, number of metastatic sites, chemotherapy agents used, number of chemotherapy agents, planned growth factor use, duration of planned therapy, pleural effusion, presence of symptoms, and performance status. Their model had an area under the curve of 0.8348 in the training set and 0.8234 in the testing set.
How these results influence practice
The risk of an initial episode of FN is highest during a patient’s initial cycle of chemotherapy, when most patients are receiving full-dose treatment, often without prophylactic measures. Guidelines from the National Comprehensive Cancer Network suggest the use of prophylactic growth factors in patients with more than a 20% risk of FN, and considering using prophylaxis in patients with 10%-20% risk of FN. Underestimating those risks and failure to take adequate precautions may be particularly important for patients with lung cancer who are generally older adults, with comorbid conditions.
The comprehensive risk model for neutropenic complications that was developed by Dr. Lyman and colleagues was based on a large, prospective cohort including nearly 3,800 patients. The model had a 90% sensitivity and 96% predictive value, but was not lung cancer specific and, in this latest study, did not perform as well in the 85% of lung cancer patients with non–small cell lung cancer. The Lyman data, however, was obtained in cancer patients treated with investigator-choice chemotherapy in community practices. It remains the National Comprehensive Cancer Network standard for evaluating FN risk in patients embarking on chemotherapy for advanced malignancies. That should remain the case, pending the additional validation testing of the new lung cancer–specific model at independent institutions, treating heterogeneous patients in real-world settings.
Locoregional recurrence
A retrospective cohort analysis of SWOG 8814, a phase 3 study of tamoxifen alone versus chemotherapy plus by tamoxifen in postmenopausal, node-positive, hormone receptor–positive breast cancer patients suggests that the 21-gene assay recurrence score (RS) can aid decisions about radiotherapy (RT).
Wendy A. Woodward, MD, PhD, and colleagues, analyzed patients who underwent mastectomy or breast-conserving surgery as their local therapy (JAMA Oncol. 2020 Jan 9. doi: 10.1001/jamaoncol.2019.5559). They found that patients with an intermediate or high RS – according to the 21-gene assay OncotypeDX – had more locoregional recurrences (LRR; breast, chest wall, axilla, internal mammary, supraclavicular or infraclavicular nodes).
There were 367 patients in SWOG 8814 who received tamoxifen alone or cyclophosphamide, doxorubicin, and fluorouracil followed by tamoxifen. LRR was observed in 5.8% of patients with a low RS (less than 18) and in 13.8% of patients with an intermediate or high RS (more than 18). The estimated 10-year cumulative LRR incidence rates were 9.7% and 16.5%, respectively (P = .02).
In the subset of patients with one to three positive nodes who had mastectomy without radiotherapy, the LRR was 1.5% for those with low RS and 11.1% for those with intermediate or high RS (P = .051). No difference by RS was found in the 10-year rates of LRR among patients with four or more involved nodes who received a mastectomy without RT (25.9% vs. 27.0%; P = .27).
In multivariate analysis, incorporating RS, type of surgery, and number of involved nodes, intermediate or high RS was a significant predictor of LRR, with a hazard ratio of 2.36 (P = .04). The investigators suggested that RS, when available, should be one of the factors considered in selecting patients for postmastectomy RT.
How these results influence practice
Selecting the node-positive, hormone receptor–positive, breast cancer patients who should receive postmastectomy RT is difficult and controversial. This is particularly true for those postmenopausal patients with fewer than four involved nodes, no lymphatic or vascular invasion, and no extracapsular spread of disease into the axillary fat. Limited information exists on the ability of genomic assays to identify LRR risk.
Eleftherios P. Mamounas, MD, and colleagues examined the results of NSABP B-28, a trial of chemotherapy plus tamoxifen (J Natl Cancer Inst. 2017;109[4]. doi:10.1093/jnci/djw259). Postmastectomy RT was not permitted. They found high RS correlated with greater LRR and low RS with decreased LRR among patients with one to three positive nodes. At first blush, the prospectively treated cohort of SWOG 8814 represents a uniformly treated cohort with long-term follow-up (median, 8.5 years) and extends in an independent analysis the findings of NSABP B-28.
However, as Dr. Woodward and colleagues point out, the current study has limitations. The use of RT was extracted retrospectively and may be underreported. More modern chemotherapy and RT may lower LRR from the risks observed in SWOG 8814. Finally, the modest numbers of LRR events precluded secondary analysis of RS as a continuous variable. This is important because the risk group cutoffs suggested by the authors are not aligned with those in the recently published TailorRx study or the ongoing RxPonder trial.
The TailorRT (Regional Radiotherapy in Biomarker Low Risk Node Positive Breast Cancer) study examines the safety of omitting RT among patients with low RS and one to three positive nodes. Until the TailorRT results are reported, the controversy regarding the role of postmastectomy RT in this group will continue for patients with low nodal tumor burden and less aggressive tumor features, including low RS.
An observed LRR risk of 11.1% in SWOG 8814 among patients with N1 disease and an RS above 18 suggest that genomic risk could be one of the factors that may justify postmastectomy RT in postmenopausal patients with node-positive, hormone receptor–positive breast cancer until additional data emerge from the contemporary trials.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight the potential role of new models for predicting risks of common, clinically important situations in general oncology practice: severe neutropenia in lung cancer patients and locoregional recurrence of breast cancer.
Predicting neutropenia
Accurate, lung cancer–specific prediction models would be useful to estimate risk of chemotherapy-induced neutropenia (CIN), especially febrile neutropenia (FN), since that particular toxicity is linked to infection, dose delays and dose reductions that can compromise treatment efficacy, and poor health-related quality of life. Lung cancer patients are often older adults, with advanced disease and comorbid conditions, so they are a particularly vulnerable population for CIN.
Xiaowen Cao of Duke University, Durham, N.C., and coinvestigators published a model for predicting risk of severe CIN in advanced lung cancer patients, based on 10 pretreatment variables (Lung Cancer. 2020 Jan 5. doi: 10.1016/j.lungcan.2020.01.004). They developed their model to overcome limitations of the previously published work of Gary H. Lyman, MD, and colleagues that is not specific to lung cancer and incorporated relative dose intensity as a predictor (Cancer. 2011;117:1917-27). Relative dose intensity is not determined until after a treatment course is completed.
The new prediction model was based on a lung cancer data set encompassing 11,352 patients from 67 phase 2-3 cooperative group studies conducted between 1991 and 2010. In this data set, the Lyman model had an area under the curve of 0.8772 in patients with small cell lung cancer, but an area under the curve of just 0.6787 in non–small cell lung cancer.
The derivation model was derived from about two-thirds of the patients, randomly selected. The validation set was conducted using the remaining third. The variables included were readily clinically available: age, gender, weight, body mass index, insurance status, disease stage, number of metastatic sites, chemotherapy agents used, number of chemotherapy agents, planned growth factor use, duration of planned therapy, pleural effusion, presence of symptoms, and performance status. Their model had an area under the curve of 0.8348 in the training set and 0.8234 in the testing set.
How these results influence practice
The risk of an initial episode of FN is highest during a patient’s initial cycle of chemotherapy, when most patients are receiving full-dose treatment, often without prophylactic measures. Guidelines from the National Comprehensive Cancer Network suggest the use of prophylactic growth factors in patients with more than a 20% risk of FN, and considering using prophylaxis in patients with 10%-20% risk of FN. Underestimating those risks and failure to take adequate precautions may be particularly important for patients with lung cancer who are generally older adults, with comorbid conditions.
The comprehensive risk model for neutropenic complications that was developed by Dr. Lyman and colleagues was based on a large, prospective cohort including nearly 3,800 patients. The model had a 90% sensitivity and 96% predictive value, but was not lung cancer specific and, in this latest study, did not perform as well in the 85% of lung cancer patients with non–small cell lung cancer. The Lyman data, however, was obtained in cancer patients treated with investigator-choice chemotherapy in community practices. It remains the National Comprehensive Cancer Network standard for evaluating FN risk in patients embarking on chemotherapy for advanced malignancies. That should remain the case, pending the additional validation testing of the new lung cancer–specific model at independent institutions, treating heterogeneous patients in real-world settings.
Locoregional recurrence
A retrospective cohort analysis of SWOG 8814, a phase 3 study of tamoxifen alone versus chemotherapy plus by tamoxifen in postmenopausal, node-positive, hormone receptor–positive breast cancer patients suggests that the 21-gene assay recurrence score (RS) can aid decisions about radiotherapy (RT).
Wendy A. Woodward, MD, PhD, and colleagues, analyzed patients who underwent mastectomy or breast-conserving surgery as their local therapy (JAMA Oncol. 2020 Jan 9. doi: 10.1001/jamaoncol.2019.5559). They found that patients with an intermediate or high RS – according to the 21-gene assay OncotypeDX – had more locoregional recurrences (LRR; breast, chest wall, axilla, internal mammary, supraclavicular or infraclavicular nodes).
There were 367 patients in SWOG 8814 who received tamoxifen alone or cyclophosphamide, doxorubicin, and fluorouracil followed by tamoxifen. LRR was observed in 5.8% of patients with a low RS (less than 18) and in 13.8% of patients with an intermediate or high RS (more than 18). The estimated 10-year cumulative LRR incidence rates were 9.7% and 16.5%, respectively (P = .02).
In the subset of patients with one to three positive nodes who had mastectomy without radiotherapy, the LRR was 1.5% for those with low RS and 11.1% for those with intermediate or high RS (P = .051). No difference by RS was found in the 10-year rates of LRR among patients with four or more involved nodes who received a mastectomy without RT (25.9% vs. 27.0%; P = .27).
In multivariate analysis, incorporating RS, type of surgery, and number of involved nodes, intermediate or high RS was a significant predictor of LRR, with a hazard ratio of 2.36 (P = .04). The investigators suggested that RS, when available, should be one of the factors considered in selecting patients for postmastectomy RT.
How these results influence practice
Selecting the node-positive, hormone receptor–positive, breast cancer patients who should receive postmastectomy RT is difficult and controversial. This is particularly true for those postmenopausal patients with fewer than four involved nodes, no lymphatic or vascular invasion, and no extracapsular spread of disease into the axillary fat. Limited information exists on the ability of genomic assays to identify LRR risk.
Eleftherios P. Mamounas, MD, and colleagues examined the results of NSABP B-28, a trial of chemotherapy plus tamoxifen (J Natl Cancer Inst. 2017;109[4]. doi:10.1093/jnci/djw259). Postmastectomy RT was not permitted. They found high RS correlated with greater LRR and low RS with decreased LRR among patients with one to three positive nodes. At first blush, the prospectively treated cohort of SWOG 8814 represents a uniformly treated cohort with long-term follow-up (median, 8.5 years) and extends in an independent analysis the findings of NSABP B-28.
However, as Dr. Woodward and colleagues point out, the current study has limitations. The use of RT was extracted retrospectively and may be underreported. More modern chemotherapy and RT may lower LRR from the risks observed in SWOG 8814. Finally, the modest numbers of LRR events precluded secondary analysis of RS as a continuous variable. This is important because the risk group cutoffs suggested by the authors are not aligned with those in the recently published TailorRx study or the ongoing RxPonder trial.
The TailorRT (Regional Radiotherapy in Biomarker Low Risk Node Positive Breast Cancer) study examines the safety of omitting RT among patients with low RS and one to three positive nodes. Until the TailorRT results are reported, the controversy regarding the role of postmastectomy RT in this group will continue for patients with low nodal tumor burden and less aggressive tumor features, including low RS.
An observed LRR risk of 11.1% in SWOG 8814 among patients with N1 disease and an RS above 18 suggest that genomic risk could be one of the factors that may justify postmastectomy RT in postmenopausal patients with node-positive, hormone receptor–positive breast cancer until additional data emerge from the contemporary trials.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
Should supplemental MRI be used in otherwise average-risk women with extremely dense breasts?
While the frequency of dense breasts decreases with age, approximately 10% of women in the United States have extremely dense breasts (Breast Imaging, Reporting, and Data System [BI-RADS] category D), and another 40% have heterogeneously dense breasts (BI-RADS category C).1 Women with dense breasts have both an increased risk for developing breast cancer and reduced mammographic sensitivity for breast cancer detection compared with women who have nondense breasts.2
These 2 observations have led the majority of states to pass legislation requiring that women with dense breasts be informed of their breast density, and most require that providers discuss these results with their patients. Thoughtful clinicians who review the available literature, however, will find sparse evidence on which to counsel patients as to next steps.
Now, a recent trial adds to our knowledge about supplemental magnetic resonance imaging (MRI) breast screening in women with extremely dense breasts.
DENSE trial offers high-quality data
Bakker and colleagues studied women aged 50 to 74 who were participating in a Netherlands population-based biennial mammography screening program.3 They enrolled average-risk women with extremely dense breasts who had a negative screening digital mammogram into the Dense Tissue and Early Breast Neoplasm Screening (DENSE) multicenter trial. The women were randomly assigned to receive either continued biennial digital mammography or supplemental breast MRI.
The primary outcome was the between-group difference in the development of interval breast cancers—that is, breast cancers detected by women or their providers between rounds of screening mammography. Interval breast cancers were chosen as the primary outcome for 2 reasons:
- interval cancers appear to be more aggressive tumors than those cancers detected by screening mammography
- interval cancers can be identified over a shorter time interval, making them easier to study than outcomes such as breast cancer mortality, which typically require more than a decade to identify.
The DENSE trial’s secondary outcomes included recall rates from MRI, cancer detection rates on MRI, positive predictive value of MRIs requiring biopsy, and breast cancer characteristics (size, stage) diagnosed in the different groups.
Between-group difference in incidence of interval cancers
A total of 40,373 women with extremely dense breasts were screened; 8,061 of these were randomly assigned to receive breast MRI and 32,312 to continued mammography only (1:4 cluster randomization) across 12 mammography centers in the Netherlands. Among the women assigned to the MRI group, 59% actually underwent MRI (4,783 of the 8,061).
The interval cancer rate in the mammography-only group was 5.0 per 1,000 screenings (95% confidence interval [CI], 4.3–5.8), while the interval cancer rate in the MRI-assigned group was 2.5 per 1,000 screenings (95% CI, 1.6–3.8) (TABLE 1).3
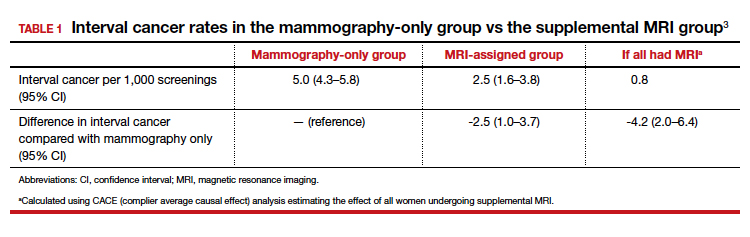
Key secondary outcomes
Of the women who underwent supplemental MRI, 9.49% were recalled for additional imaging, follow-up, or biopsy. Of the 4,783 women who had an MRI, 300 (6.3%) underwent a breast biopsy, and 79 breast cancers (1.65%) were detected. Sixty-four of these cancers were invasive, and 15 were ductal carcinoma in situ (DCIS). Among women who underwent a biopsy for an MRI-detected abnormality, the positive predictive value was 26.3%.
Tumor characteristics. For women who developed breast cancer during the study, both tumor size at diagnosis and tumor stage (early vs late) were described. TABLE 2 shows these results in the women who had their breast cancer detected on MRI, those in the MRI-assigned group who developed interval cancer, and those in the mammography-only group who had interval cancers.3 Overall, tumor size was smaller in the interval group who underwent MRI compared with those who underwent mammography only.
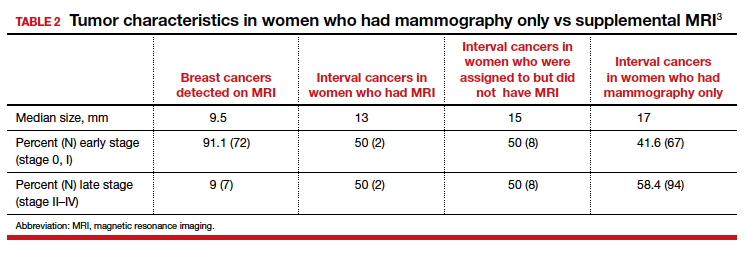
Continue to: Study contributes valuable data, but we need more on long-term outcomes...
Study contributes valuable data, but we need more on long-term outcomes
The trial by Bakker and colleagues employed a solid study design as women were randomly assigned to supplemental MRI screening or ongoing biennial mammography, and nearly all cancers were identified in the short-term of follow-up. In addition, very few women were lost to follow-up, and secondary outcomes, including false-positive rates, were collected to help providers and patients better understand some of the potential downsides of supplemental screening.
The substantial reduction in interval cancers (50% in the intent-to-screen analysis and 84% in the women who actually underwent supplemental MRI) was highly statistically significant (P<.001). While there were substantially fewer interval cancers in the MRI-assigned group, the interval cancers that did occur were of similar stage as those in the women assigned to the mammography-only group (TABLE 2).
Data demonstrate that interval cancers appear to be more aggressive than screen-detected cancers.4 While reducing interval cancers should be a good thing overall, it remains unproven that using supplemental MRI in all women with dense breasts would reduce breast cancer specific mortality, all-cause mortality, or the risk of more invasive treatments (for example, the need for chemotherapy or requirement for mastectomy).
On the other hand, using routine supplemental breast MRI in women with extremely dense breasts would result in very substantial use of resources, including cost, radiologist time, provider time, and machine time. In the United States, approximately 49 million women are aged 50 to 74.5 Breast MRI charges commonly range from $1,000 to $4,000. If the 4.9 million women with extremely dense breasts underwent supplemental MRI this year, the approximate cost would be somewhere between $4.9 and $19.5 billion for imaging alone. This does not include callbacks, biopsies, or provider time for ordering, interpreting, and arranging for follow-up.
While the reduction in interval cancers seen in this study is promising, more assurance of improvement in important outcomes—such as reduced mortality or reduced need for more invasive breast cancer treatments—should precede any routine change in practice.
Unanswered questions
This study did not address a number of other important questions, including:
Should MRI be done with every round of breast cancer screening given the possibility of prevalence bias? Prevalence bias can be defined as more cancers detected in the first round of MRI screening with possible reduced benefit in future rounds of screening. The study authors indicated that they will continue to analyze the study results to see what occurs in the next round of screening.
Is there a similar impact on decreased interval cancers in women undergoing annual mammography or in women screened between ages 40 and 49? This study was conducted in women aged 50 to 74 undergoing mammography every 2 years. In the United States, annual mammography in women aged 40 to 49 is frequently recommended.
What effect does supplemental MRI screening have in women with heterogeneously dense breasts, which represents 40% of the population? The US Food and Drug Administration recommends that all women with dense breasts be counseled regarding options for management.6
Do these results translate to the more racially and ethnically diverse populations of the United States? In the Netherlands, where this study was conducted, 85% to 90% of women are either Dutch or of western European origin. Women of different racial and ancestral backgrounds have biologically different breast cancers and cancer risk (for example, higher rates of triple-negative breast cancers in African American women; 10-fold higher rates of BRCA pathogenic variants in Ashkenazi Jewish women).
Continue to: Use validated tools to assess risk comprehensively...
Use validated tools to assess risk comprehensively
Women aged 50 to 74 with extremely dense breasts have reduced interval cancers following a normal biennial mammogram if supplemental MRI is offered, but the long-term benefit of identifying these cancers earlier is unclear. Until more data are available on important long-term outcomes (such as breast cancer mortality and need for more invasive treatments), providers should consider breast density in the context of a more comprehensive assessment of breast cancer risk using a validated breast cancer risk assessment tool.
I prefer the modified version of the International Breast Cancer Intervention Study (IBIS) tool, which is readily available online (https://ibis.ikonopedia.com/).7 This tool incorporates several breast cancer risk factors, including reproductive risk factors, body mass index, BRCA gene status, breast density, and family history. The tool takes 1 to 2 minutes to complete and provides an estimate of a woman’s 10-year risk and lifetime risk of breast cancer.
If the lifetime risk exceeds 20%, I offer the patient supplemental MRI screening, consistent with current recommendations of the National Comprehensive Cancer Network and the American Cancer Society.8,9 I generally recommend starting breast imaging screening 7 to 10 years prior to the youngest breast cancer occurrence in the family, with mammography starting no earlier than age 30 and MRI no earlier than age 25. Other validated tools also can be used.10-13
Incorporating breast density and other important risk factors allows a more comprehensive analysis upon which to counsel women about the value (benefits and harms) of breast imaging.8
- Sprague BL, Gagnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jcni/dju255.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
- Bakker MF, de Lange SV, Pijnappel RM, et al; for the DENSE Trial Study Group. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Drukker CA, Schmidt MK, Rutgers EJT, et al. Mammographic screening detects low-risk tumor biology breast cancers. Breast Cancer Res Treat. 2014;144:103-111.
- Statista website. Resident population of the United States by sex and age as of July 1, 2018. https://www.statista.com/statistics/241488/population-of-the-us-by-sex-and-age. Accessed January 6, 2020.
- US Food and Drug Administration website. Mammography: what you need to know. https://www.fda.gov/consumers/consumer-updates/mammography-what-you-need-know. Accessed January 13, 2020.
- IBIS (International Breast Cancer Intervention Study) website. Online Tyrer-Cuzick Model Breast Cancer Risk Evaluation Tool. ibis.ikonopedia.com. Accessed January 13, 2020.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. Breast cancer screening and diagnosis: NCCN practice guidelines in oncology. JNCCN. 2009;7:1060-1096.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98:1457-1466.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer: implications for risk prediction. Cancer. 1994;73:643-651.
- Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62:145-158.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
While the frequency of dense breasts decreases with age, approximately 10% of women in the United States have extremely dense breasts (Breast Imaging, Reporting, and Data System [BI-RADS] category D), and another 40% have heterogeneously dense breasts (BI-RADS category C).1 Women with dense breasts have both an increased risk for developing breast cancer and reduced mammographic sensitivity for breast cancer detection compared with women who have nondense breasts.2
These 2 observations have led the majority of states to pass legislation requiring that women with dense breasts be informed of their breast density, and most require that providers discuss these results with their patients. Thoughtful clinicians who review the available literature, however, will find sparse evidence on which to counsel patients as to next steps.
Now, a recent trial adds to our knowledge about supplemental magnetic resonance imaging (MRI) breast screening in women with extremely dense breasts.
DENSE trial offers high-quality data
Bakker and colleagues studied women aged 50 to 74 who were participating in a Netherlands population-based biennial mammography screening program.3 They enrolled average-risk women with extremely dense breasts who had a negative screening digital mammogram into the Dense Tissue and Early Breast Neoplasm Screening (DENSE) multicenter trial. The women were randomly assigned to receive either continued biennial digital mammography or supplemental breast MRI.
The primary outcome was the between-group difference in the development of interval breast cancers—that is, breast cancers detected by women or their providers between rounds of screening mammography. Interval breast cancers were chosen as the primary outcome for 2 reasons:
- interval cancers appear to be more aggressive tumors than those cancers detected by screening mammography
- interval cancers can be identified over a shorter time interval, making them easier to study than outcomes such as breast cancer mortality, which typically require more than a decade to identify.
The DENSE trial’s secondary outcomes included recall rates from MRI, cancer detection rates on MRI, positive predictive value of MRIs requiring biopsy, and breast cancer characteristics (size, stage) diagnosed in the different groups.
Between-group difference in incidence of interval cancers
A total of 40,373 women with extremely dense breasts were screened; 8,061 of these were randomly assigned to receive breast MRI and 32,312 to continued mammography only (1:4 cluster randomization) across 12 mammography centers in the Netherlands. Among the women assigned to the MRI group, 59% actually underwent MRI (4,783 of the 8,061).
The interval cancer rate in the mammography-only group was 5.0 per 1,000 screenings (95% confidence interval [CI], 4.3–5.8), while the interval cancer rate in the MRI-assigned group was 2.5 per 1,000 screenings (95% CI, 1.6–3.8) (TABLE 1).3

Key secondary outcomes
Of the women who underwent supplemental MRI, 9.49% were recalled for additional imaging, follow-up, or biopsy. Of the 4,783 women who had an MRI, 300 (6.3%) underwent a breast biopsy, and 79 breast cancers (1.65%) were detected. Sixty-four of these cancers were invasive, and 15 were ductal carcinoma in situ (DCIS). Among women who underwent a biopsy for an MRI-detected abnormality, the positive predictive value was 26.3%.
Tumor characteristics. For women who developed breast cancer during the study, both tumor size at diagnosis and tumor stage (early vs late) were described. TABLE 2 shows these results in the women who had their breast cancer detected on MRI, those in the MRI-assigned group who developed interval cancer, and those in the mammography-only group who had interval cancers.3 Overall, tumor size was smaller in the interval group who underwent MRI compared with those who underwent mammography only.

Continue to: Study contributes valuable data, but we need more on long-term outcomes...
Study contributes valuable data, but we need more on long-term outcomes
The trial by Bakker and colleagues employed a solid study design as women were randomly assigned to supplemental MRI screening or ongoing biennial mammography, and nearly all cancers were identified in the short-term of follow-up. In addition, very few women were lost to follow-up, and secondary outcomes, including false-positive rates, were collected to help providers and patients better understand some of the potential downsides of supplemental screening.
The substantial reduction in interval cancers (50% in the intent-to-screen analysis and 84% in the women who actually underwent supplemental MRI) was highly statistically significant (P<.001). While there were substantially fewer interval cancers in the MRI-assigned group, the interval cancers that did occur were of similar stage as those in the women assigned to the mammography-only group (TABLE 2).
Data demonstrate that interval cancers appear to be more aggressive than screen-detected cancers.4 While reducing interval cancers should be a good thing overall, it remains unproven that using supplemental MRI in all women with dense breasts would reduce breast cancer specific mortality, all-cause mortality, or the risk of more invasive treatments (for example, the need for chemotherapy or requirement for mastectomy).
On the other hand, using routine supplemental breast MRI in women with extremely dense breasts would result in very substantial use of resources, including cost, radiologist time, provider time, and machine time. In the United States, approximately 49 million women are aged 50 to 74.5 Breast MRI charges commonly range from $1,000 to $4,000. If the 4.9 million women with extremely dense breasts underwent supplemental MRI this year, the approximate cost would be somewhere between $4.9 and $19.5 billion for imaging alone. This does not include callbacks, biopsies, or provider time for ordering, interpreting, and arranging for follow-up.
While the reduction in interval cancers seen in this study is promising, more assurance of improvement in important outcomes—such as reduced mortality or reduced need for more invasive breast cancer treatments—should precede any routine change in practice.
Unanswered questions
This study did not address a number of other important questions, including:
Should MRI be done with every round of breast cancer screening given the possibility of prevalence bias? Prevalence bias can be defined as more cancers detected in the first round of MRI screening with possible reduced benefit in future rounds of screening. The study authors indicated that they will continue to analyze the study results to see what occurs in the next round of screening.
Is there a similar impact on decreased interval cancers in women undergoing annual mammography or in women screened between ages 40 and 49? This study was conducted in women aged 50 to 74 undergoing mammography every 2 years. In the United States, annual mammography in women aged 40 to 49 is frequently recommended.
What effect does supplemental MRI screening have in women with heterogeneously dense breasts, which represents 40% of the population? The US Food and Drug Administration recommends that all women with dense breasts be counseled regarding options for management.6
Do these results translate to the more racially and ethnically diverse populations of the United States? In the Netherlands, where this study was conducted, 85% to 90% of women are either Dutch or of western European origin. Women of different racial and ancestral backgrounds have biologically different breast cancers and cancer risk (for example, higher rates of triple-negative breast cancers in African American women; 10-fold higher rates of BRCA pathogenic variants in Ashkenazi Jewish women).
Continue to: Use validated tools to assess risk comprehensively...
Use validated tools to assess risk comprehensively
Women aged 50 to 74 with extremely dense breasts have reduced interval cancers following a normal biennial mammogram if supplemental MRI is offered, but the long-term benefit of identifying these cancers earlier is unclear. Until more data are available on important long-term outcomes (such as breast cancer mortality and need for more invasive treatments), providers should consider breast density in the context of a more comprehensive assessment of breast cancer risk using a validated breast cancer risk assessment tool.
I prefer the modified version of the International Breast Cancer Intervention Study (IBIS) tool, which is readily available online (https://ibis.ikonopedia.com/).7 This tool incorporates several breast cancer risk factors, including reproductive risk factors, body mass index, BRCA gene status, breast density, and family history. The tool takes 1 to 2 minutes to complete and provides an estimate of a woman’s 10-year risk and lifetime risk of breast cancer.
If the lifetime risk exceeds 20%, I offer the patient supplemental MRI screening, consistent with current recommendations of the National Comprehensive Cancer Network and the American Cancer Society.8,9 I generally recommend starting breast imaging screening 7 to 10 years prior to the youngest breast cancer occurrence in the family, with mammography starting no earlier than age 30 and MRI no earlier than age 25. Other validated tools also can be used.10-13
Incorporating breast density and other important risk factors allows a more comprehensive analysis upon which to counsel women about the value (benefits and harms) of breast imaging.8
While the frequency of dense breasts decreases with age, approximately 10% of women in the United States have extremely dense breasts (Breast Imaging, Reporting, and Data System [BI-RADS] category D), and another 40% have heterogeneously dense breasts (BI-RADS category C).1 Women with dense breasts have both an increased risk for developing breast cancer and reduced mammographic sensitivity for breast cancer detection compared with women who have nondense breasts.2
These 2 observations have led the majority of states to pass legislation requiring that women with dense breasts be informed of their breast density, and most require that providers discuss these results with their patients. Thoughtful clinicians who review the available literature, however, will find sparse evidence on which to counsel patients as to next steps.
Now, a recent trial adds to our knowledge about supplemental magnetic resonance imaging (MRI) breast screening in women with extremely dense breasts.
DENSE trial offers high-quality data
Bakker and colleagues studied women aged 50 to 74 who were participating in a Netherlands population-based biennial mammography screening program.3 They enrolled average-risk women with extremely dense breasts who had a negative screening digital mammogram into the Dense Tissue and Early Breast Neoplasm Screening (DENSE) multicenter trial. The women were randomly assigned to receive either continued biennial digital mammography or supplemental breast MRI.
The primary outcome was the between-group difference in the development of interval breast cancers—that is, breast cancers detected by women or their providers between rounds of screening mammography. Interval breast cancers were chosen as the primary outcome for 2 reasons:
- interval cancers appear to be more aggressive tumors than those cancers detected by screening mammography
- interval cancers can be identified over a shorter time interval, making them easier to study than outcomes such as breast cancer mortality, which typically require more than a decade to identify.
The DENSE trial’s secondary outcomes included recall rates from MRI, cancer detection rates on MRI, positive predictive value of MRIs requiring biopsy, and breast cancer characteristics (size, stage) diagnosed in the different groups.
Between-group difference in incidence of interval cancers
A total of 40,373 women with extremely dense breasts were screened; 8,061 of these were randomly assigned to receive breast MRI and 32,312 to continued mammography only (1:4 cluster randomization) across 12 mammography centers in the Netherlands. Among the women assigned to the MRI group, 59% actually underwent MRI (4,783 of the 8,061).
The interval cancer rate in the mammography-only group was 5.0 per 1,000 screenings (95% confidence interval [CI], 4.3–5.8), while the interval cancer rate in the MRI-assigned group was 2.5 per 1,000 screenings (95% CI, 1.6–3.8) (TABLE 1).3

Key secondary outcomes
Of the women who underwent supplemental MRI, 9.49% were recalled for additional imaging, follow-up, or biopsy. Of the 4,783 women who had an MRI, 300 (6.3%) underwent a breast biopsy, and 79 breast cancers (1.65%) were detected. Sixty-four of these cancers were invasive, and 15 were ductal carcinoma in situ (DCIS). Among women who underwent a biopsy for an MRI-detected abnormality, the positive predictive value was 26.3%.
Tumor characteristics. For women who developed breast cancer during the study, both tumor size at diagnosis and tumor stage (early vs late) were described. TABLE 2 shows these results in the women who had their breast cancer detected on MRI, those in the MRI-assigned group who developed interval cancer, and those in the mammography-only group who had interval cancers.3 Overall, tumor size was smaller in the interval group who underwent MRI compared with those who underwent mammography only.

Continue to: Study contributes valuable data, but we need more on long-term outcomes...
Study contributes valuable data, but we need more on long-term outcomes
The trial by Bakker and colleagues employed a solid study design as women were randomly assigned to supplemental MRI screening or ongoing biennial mammography, and nearly all cancers were identified in the short-term of follow-up. In addition, very few women were lost to follow-up, and secondary outcomes, including false-positive rates, were collected to help providers and patients better understand some of the potential downsides of supplemental screening.
The substantial reduction in interval cancers (50% in the intent-to-screen analysis and 84% in the women who actually underwent supplemental MRI) was highly statistically significant (P<.001). While there were substantially fewer interval cancers in the MRI-assigned group, the interval cancers that did occur were of similar stage as those in the women assigned to the mammography-only group (TABLE 2).
Data demonstrate that interval cancers appear to be more aggressive than screen-detected cancers.4 While reducing interval cancers should be a good thing overall, it remains unproven that using supplemental MRI in all women with dense breasts would reduce breast cancer specific mortality, all-cause mortality, or the risk of more invasive treatments (for example, the need for chemotherapy or requirement for mastectomy).
On the other hand, using routine supplemental breast MRI in women with extremely dense breasts would result in very substantial use of resources, including cost, radiologist time, provider time, and machine time. In the United States, approximately 49 million women are aged 50 to 74.5 Breast MRI charges commonly range from $1,000 to $4,000. If the 4.9 million women with extremely dense breasts underwent supplemental MRI this year, the approximate cost would be somewhere between $4.9 and $19.5 billion for imaging alone. This does not include callbacks, biopsies, or provider time for ordering, interpreting, and arranging for follow-up.
While the reduction in interval cancers seen in this study is promising, more assurance of improvement in important outcomes—such as reduced mortality or reduced need for more invasive breast cancer treatments—should precede any routine change in practice.
Unanswered questions
This study did not address a number of other important questions, including:
Should MRI be done with every round of breast cancer screening given the possibility of prevalence bias? Prevalence bias can be defined as more cancers detected in the first round of MRI screening with possible reduced benefit in future rounds of screening. The study authors indicated that they will continue to analyze the study results to see what occurs in the next round of screening.
Is there a similar impact on decreased interval cancers in women undergoing annual mammography or in women screened between ages 40 and 49? This study was conducted in women aged 50 to 74 undergoing mammography every 2 years. In the United States, annual mammography in women aged 40 to 49 is frequently recommended.
What effect does supplemental MRI screening have in women with heterogeneously dense breasts, which represents 40% of the population? The US Food and Drug Administration recommends that all women with dense breasts be counseled regarding options for management.6
Do these results translate to the more racially and ethnically diverse populations of the United States? In the Netherlands, where this study was conducted, 85% to 90% of women are either Dutch or of western European origin. Women of different racial and ancestral backgrounds have biologically different breast cancers and cancer risk (for example, higher rates of triple-negative breast cancers in African American women; 10-fold higher rates of BRCA pathogenic variants in Ashkenazi Jewish women).
Continue to: Use validated tools to assess risk comprehensively...
Use validated tools to assess risk comprehensively
Women aged 50 to 74 with extremely dense breasts have reduced interval cancers following a normal biennial mammogram if supplemental MRI is offered, but the long-term benefit of identifying these cancers earlier is unclear. Until more data are available on important long-term outcomes (such as breast cancer mortality and need for more invasive treatments), providers should consider breast density in the context of a more comprehensive assessment of breast cancer risk using a validated breast cancer risk assessment tool.
I prefer the modified version of the International Breast Cancer Intervention Study (IBIS) tool, which is readily available online (https://ibis.ikonopedia.com/).7 This tool incorporates several breast cancer risk factors, including reproductive risk factors, body mass index, BRCA gene status, breast density, and family history. The tool takes 1 to 2 minutes to complete and provides an estimate of a woman’s 10-year risk and lifetime risk of breast cancer.
If the lifetime risk exceeds 20%, I offer the patient supplemental MRI screening, consistent with current recommendations of the National Comprehensive Cancer Network and the American Cancer Society.8,9 I generally recommend starting breast imaging screening 7 to 10 years prior to the youngest breast cancer occurrence in the family, with mammography starting no earlier than age 30 and MRI no earlier than age 25. Other validated tools also can be used.10-13
Incorporating breast density and other important risk factors allows a more comprehensive analysis upon which to counsel women about the value (benefits and harms) of breast imaging.8
- Sprague BL, Gagnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jcni/dju255.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
- Bakker MF, de Lange SV, Pijnappel RM, et al; for the DENSE Trial Study Group. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Drukker CA, Schmidt MK, Rutgers EJT, et al. Mammographic screening detects low-risk tumor biology breast cancers. Breast Cancer Res Treat. 2014;144:103-111.
- Statista website. Resident population of the United States by sex and age as of July 1, 2018. https://www.statista.com/statistics/241488/population-of-the-us-by-sex-and-age. Accessed January 6, 2020.
- US Food and Drug Administration website. Mammography: what you need to know. https://www.fda.gov/consumers/consumer-updates/mammography-what-you-need-know. Accessed January 13, 2020.
- IBIS (International Breast Cancer Intervention Study) website. Online Tyrer-Cuzick Model Breast Cancer Risk Evaluation Tool. ibis.ikonopedia.com. Accessed January 13, 2020.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. Breast cancer screening and diagnosis: NCCN practice guidelines in oncology. JNCCN. 2009;7:1060-1096.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98:1457-1466.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer: implications for risk prediction. Cancer. 1994;73:643-651.
- Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62:145-158.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
- Sprague BL, Gagnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jcni/dju255.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
- Bakker MF, de Lange SV, Pijnappel RM, et al; for the DENSE Trial Study Group. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Drukker CA, Schmidt MK, Rutgers EJT, et al. Mammographic screening detects low-risk tumor biology breast cancers. Breast Cancer Res Treat. 2014;144:103-111.
- Statista website. Resident population of the United States by sex and age as of July 1, 2018. https://www.statista.com/statistics/241488/population-of-the-us-by-sex-and-age. Accessed January 6, 2020.
- US Food and Drug Administration website. Mammography: what you need to know. https://www.fda.gov/consumers/consumer-updates/mammography-what-you-need-know. Accessed January 13, 2020.
- IBIS (International Breast Cancer Intervention Study) website. Online Tyrer-Cuzick Model Breast Cancer Risk Evaluation Tool. ibis.ikonopedia.com. Accessed January 13, 2020.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. Breast cancer screening and diagnosis: NCCN practice guidelines in oncology. JNCCN. 2009;7:1060-1096.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98:1457-1466.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer: implications for risk prediction. Cancer. 1994;73:643-651.
- Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62:145-158.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
Global project reveals cancer’s genomic playbook
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
FROM NATURE
High-dose chemo offers survival benefit only for highest-risk breast cancer
High-dose chemotherapy in the adjuvant setting offers a long-term survival advantage for women with very-high-risk stage III breast cancer, but does not improve survival odds for women with lower-risk cancers, an analysis of 20 years of follow-up data shows.
Among 885 women younger than 56 years at the time of treatment who had 4 or more involved axilliary lymph nodes, there was no overall survival difference over 2 decades between the total population of women randomized to receive adjuvant high-dose chemotherapy (HDCT) and those assigned to receive conventional-dose chemotherapy (CDCT).
However, women with 10 or more involved axilliary nodes and those with triple-negative breast cancer had an approximately 15% absolute improvement in 20-year overall survival with high-dose chemotherapy, although the difference for triple-negative disease fell just short of statistical significance, reported Tessa G. Steenbruggen, MD, from the Netherlands Cancer Institute in Amsterdam and colleagues.
“Our analysis confirms earlier results that HDCT has no significant overall survival benefit compared with CDCT for unselected patients with stage III [breast cancer]. However, we found a 14.6%improvement in 20-year OS estimates with HDCT in the predefined subgroup of patients with 10 or more involved [axilliary lymph nodes],” they wrote in JAMA Oncology.
And although other studies of chemotherapy regimens containing high doses of alkylating agents have shown increases in risk of late second malignancies and major cardiovascular events, there were no significant increases of either adverse event with HDCT in this study, the authors noted.
They reported 20-year follow-up results for 885 women who were enrolled in a 10-center randomized clinical trial conducted in the Netherlands from August 1, 1993, through July 31, 1999.
The participants were younger than age 56 years with breast cancer involving at least 4 axillary lymph nodes. All patients underwent surgery with complete axillary clearance and were then randomized to receive either conventional chemotherapy, which consisted of five cycles of fluorouracil 500mg/m2, epirubicin 90 mg/m2, and cyclophosphamide 500mg/m2 (FEC), or high-dose chemotherapy, with the first 4 cycles identical to conventional-dose chemotherapy but the fifth cycle consisting of cyclophosphamide 6000 mg/m2, thiotepa 480 mg/m2, and carboplatin 1600 mg/m2, supported with autologous hematopoietic stem cell transplant.
In addition, all patients received radiotherapy according to the local standard and 2 years of adjuvant tamoxifen.
After a median follow-up of 20.4 years, the 20-year overall survival (OS) rates were 45.3% for patients who had received high-dose chemotherapy and 41.5% for those who had received the conventional dose. This translated into a nonsignificant hazard ratio of 0.89.
However, for patients with 10 or more involved axillary nodes, the 20-year OS rates were 44.5% with HDCT and 29.9% with CDCT, translating into an absolute OS advantage for high-dose chemotherapy of 14.6% and an HR of 0.72 (P = .02).
Respective 20-year OS rates for women with triple-negative breast cancer were 52.9% and 37.5%, an absolute difference of 15.4% and a HR of 0.67, which fell just short of statistical significance, possibly because of the small number of patients with triple-negative breast cancer (140).
“In our 20-year follow-up analysis, there was no increase in cumulative risk for a second malignant neoplasm or for incidence of major cardiovascular events after HDCT,” the investigators wrote.
They noted that women randomized to high-dose chemotherapy had more frequent dysrhythmias, hypertension, and hypercholesterolemia, adding that the latter two adverse events may be partly attributable to a higher incidence of menopause induction among women who received HDCT.
The study was sponsored by University Medical Center Groningen and the The Netherlands Cancer Institute. Dr Steenbruggen reported receiving grants from the Dutch Health Insurance Council during the conduct of the study.
SOURCE: Steenbruggen TG et al. JAMA Oncology. 2020 Jan 30. doi: 10.1001/jamaoncol.2019.6276.
High-dose chemotherapy in the adjuvant setting offers a long-term survival advantage for women with very-high-risk stage III breast cancer, but does not improve survival odds for women with lower-risk cancers, an analysis of 20 years of follow-up data shows.
Among 885 women younger than 56 years at the time of treatment who had 4 or more involved axilliary lymph nodes, there was no overall survival difference over 2 decades between the total population of women randomized to receive adjuvant high-dose chemotherapy (HDCT) and those assigned to receive conventional-dose chemotherapy (CDCT).
However, women with 10 or more involved axilliary nodes and those with triple-negative breast cancer had an approximately 15% absolute improvement in 20-year overall survival with high-dose chemotherapy, although the difference for triple-negative disease fell just short of statistical significance, reported Tessa G. Steenbruggen, MD, from the Netherlands Cancer Institute in Amsterdam and colleagues.
“Our analysis confirms earlier results that HDCT has no significant overall survival benefit compared with CDCT for unselected patients with stage III [breast cancer]. However, we found a 14.6%improvement in 20-year OS estimates with HDCT in the predefined subgroup of patients with 10 or more involved [axilliary lymph nodes],” they wrote in JAMA Oncology.
And although other studies of chemotherapy regimens containing high doses of alkylating agents have shown increases in risk of late second malignancies and major cardiovascular events, there were no significant increases of either adverse event with HDCT in this study, the authors noted.
They reported 20-year follow-up results for 885 women who were enrolled in a 10-center randomized clinical trial conducted in the Netherlands from August 1, 1993, through July 31, 1999.
The participants were younger than age 56 years with breast cancer involving at least 4 axillary lymph nodes. All patients underwent surgery with complete axillary clearance and were then randomized to receive either conventional chemotherapy, which consisted of five cycles of fluorouracil 500mg/m2, epirubicin 90 mg/m2, and cyclophosphamide 500mg/m2 (FEC), or high-dose chemotherapy, with the first 4 cycles identical to conventional-dose chemotherapy but the fifth cycle consisting of cyclophosphamide 6000 mg/m2, thiotepa 480 mg/m2, and carboplatin 1600 mg/m2, supported with autologous hematopoietic stem cell transplant.
In addition, all patients received radiotherapy according to the local standard and 2 years of adjuvant tamoxifen.
After a median follow-up of 20.4 years, the 20-year overall survival (OS) rates were 45.3% for patients who had received high-dose chemotherapy and 41.5% for those who had received the conventional dose. This translated into a nonsignificant hazard ratio of 0.89.
However, for patients with 10 or more involved axillary nodes, the 20-year OS rates were 44.5% with HDCT and 29.9% with CDCT, translating into an absolute OS advantage for high-dose chemotherapy of 14.6% and an HR of 0.72 (P = .02).
Respective 20-year OS rates for women with triple-negative breast cancer were 52.9% and 37.5%, an absolute difference of 15.4% and a HR of 0.67, which fell just short of statistical significance, possibly because of the small number of patients with triple-negative breast cancer (140).
“In our 20-year follow-up analysis, there was no increase in cumulative risk for a second malignant neoplasm or for incidence of major cardiovascular events after HDCT,” the investigators wrote.
They noted that women randomized to high-dose chemotherapy had more frequent dysrhythmias, hypertension, and hypercholesterolemia, adding that the latter two adverse events may be partly attributable to a higher incidence of menopause induction among women who received HDCT.
The study was sponsored by University Medical Center Groningen and the The Netherlands Cancer Institute. Dr Steenbruggen reported receiving grants from the Dutch Health Insurance Council during the conduct of the study.
SOURCE: Steenbruggen TG et al. JAMA Oncology. 2020 Jan 30. doi: 10.1001/jamaoncol.2019.6276.
High-dose chemotherapy in the adjuvant setting offers a long-term survival advantage for women with very-high-risk stage III breast cancer, but does not improve survival odds for women with lower-risk cancers, an analysis of 20 years of follow-up data shows.
Among 885 women younger than 56 years at the time of treatment who had 4 or more involved axilliary lymph nodes, there was no overall survival difference over 2 decades between the total population of women randomized to receive adjuvant high-dose chemotherapy (HDCT) and those assigned to receive conventional-dose chemotherapy (CDCT).
However, women with 10 or more involved axilliary nodes and those with triple-negative breast cancer had an approximately 15% absolute improvement in 20-year overall survival with high-dose chemotherapy, although the difference for triple-negative disease fell just short of statistical significance, reported Tessa G. Steenbruggen, MD, from the Netherlands Cancer Institute in Amsterdam and colleagues.
“Our analysis confirms earlier results that HDCT has no significant overall survival benefit compared with CDCT for unselected patients with stage III [breast cancer]. However, we found a 14.6%improvement in 20-year OS estimates with HDCT in the predefined subgroup of patients with 10 or more involved [axilliary lymph nodes],” they wrote in JAMA Oncology.
And although other studies of chemotherapy regimens containing high doses of alkylating agents have shown increases in risk of late second malignancies and major cardiovascular events, there were no significant increases of either adverse event with HDCT in this study, the authors noted.
They reported 20-year follow-up results for 885 women who were enrolled in a 10-center randomized clinical trial conducted in the Netherlands from August 1, 1993, through July 31, 1999.
The participants were younger than age 56 years with breast cancer involving at least 4 axillary lymph nodes. All patients underwent surgery with complete axillary clearance and were then randomized to receive either conventional chemotherapy, which consisted of five cycles of fluorouracil 500mg/m2, epirubicin 90 mg/m2, and cyclophosphamide 500mg/m2 (FEC), or high-dose chemotherapy, with the first 4 cycles identical to conventional-dose chemotherapy but the fifth cycle consisting of cyclophosphamide 6000 mg/m2, thiotepa 480 mg/m2, and carboplatin 1600 mg/m2, supported with autologous hematopoietic stem cell transplant.
In addition, all patients received radiotherapy according to the local standard and 2 years of adjuvant tamoxifen.
After a median follow-up of 20.4 years, the 20-year overall survival (OS) rates were 45.3% for patients who had received high-dose chemotherapy and 41.5% for those who had received the conventional dose. This translated into a nonsignificant hazard ratio of 0.89.
However, for patients with 10 or more involved axillary nodes, the 20-year OS rates were 44.5% with HDCT and 29.9% with CDCT, translating into an absolute OS advantage for high-dose chemotherapy of 14.6% and an HR of 0.72 (P = .02).
Respective 20-year OS rates for women with triple-negative breast cancer were 52.9% and 37.5%, an absolute difference of 15.4% and a HR of 0.67, which fell just short of statistical significance, possibly because of the small number of patients with triple-negative breast cancer (140).
“In our 20-year follow-up analysis, there was no increase in cumulative risk for a second malignant neoplasm or for incidence of major cardiovascular events after HDCT,” the investigators wrote.
They noted that women randomized to high-dose chemotherapy had more frequent dysrhythmias, hypertension, and hypercholesterolemia, adding that the latter two adverse events may be partly attributable to a higher incidence of menopause induction among women who received HDCT.
The study was sponsored by University Medical Center Groningen and the The Netherlands Cancer Institute. Dr Steenbruggen reported receiving grants from the Dutch Health Insurance Council during the conduct of the study.
SOURCE: Steenbruggen TG et al. JAMA Oncology. 2020 Jan 30. doi: 10.1001/jamaoncol.2019.6276.
FROM JAMA ONCOLOGY
Key clinical point: High-dose chemotherapy offers a long-term breast cancer survival advantage only for women with very-high-risk disease.
Major finding: The absolute 20-year overall survival benefit for women with 10 or more involved lymph nodes was 14.6%.
Study details: Long-term, follow-up study of 885 women under age 56 years with stage III breast cancer treated with adjuvant high- or conventional-dose chemotherapy.
Disclosures: The study was sponsored by University Medical Center Groningen and the The Netherlands Cancer Institute. Dr. Steenbruggen reported receiving grants from the Dutch Health Insurance Council during the conduct of the study.
Source: Steenbruggen TG et al. JAMA Oncology. 2020 Jan 30. doi: 10.1001/jamaoncol.2019.6276.