User login
Task Force: Start biennial mammograms at age 50
The U.S. Preventive Services Task Force is continuing to recommend routine mammograms every 2 years for women starting at age 50, according to newly released draft screening recommendations for breast cancer.
The draft recommendations, issued on April 20, largely reaffirm the group’s advice from 2009. Recent research findings have not altered the USPSTF’s key recommendation: routine mammograms every 2 years for women 50-74 years old. The USPSTF gave this recommendation a grade B, meaning that the evidence is strong enough for clinicians to adopt this practice. For women 75 years or older, the evidence remains inadequate to recommend for or against routine mammography.
For women 40-49 years old, the task force continued to state that the decision to start screening should be an individual one. The USPSTF gave mammography screening in this age group a grade C, concluding that the benefit of screening mammography outweighs the harms, but only by a small amount.
“Women who place a higher value on the potential benefit than the potential harms may choose to begin biennial screening between the ages of 40 and 49 years,” the task force members wrote.
In contrast, the American College of Obstetricians and Gynecologists, the American Cancer Society, the American College of Radiology, and the Society for Breast Imaging all recommend annual mammography starting at age 40.
The USPSTF is not advising against screening at age 40, but the task force members wrote that younger women with average breast cancer risk “must weigh a very important but infrequent benefit (small reduction in breast cancer deaths) against a group of meaningful and much more common harms (overdiagnosis and overtreatment; unnecessary and sometimes invasive follow-up testing; psychological harms associated with false-positive tests; and false reassurance from false-negative tests).”
The USPSTF’s recommendations apply to asymptomatic women without preexisting breast cancer, a previously diagnosed high-risk breast lesion, underlying genetic mutations, or a family history that puts them at high risk.
New for 2015, the group also weighed in on tomosynthesis (3-D mammography) for primary screening, and additional screening – ultrasound, MRI, tomosynthesis, or other methods – in women with dense breasts but otherwise negative mammogram results.
“In both cases, while there is some information about the accuracy of these modalities, there is no information on the effects of their use on health outcomes, such as breast cancer incidence, mortality, or overdiagnosis rates,” the USPSTF wrote.
The task force did not recommend for or against their use, stating only that more research is needed.
“From the limited data available, tomosynthesis appears to reduce recall rates for false-positive tests, compared with 2-D digital mammography alone. Available data also suggest that tomosynthesis increases the cancer detection rate, compared with 2-D digital mammography alone. However, current study designs do not answer the question of whether all of the additional cancers detected would have become clinically significant or whether there is an incremental clinical benefit to detecting these cancers earlier,” the task force members wrote.
Also, as currently practiced in most settings, tomosynthesis exposes women to about twice the amount of radiation of 2-D digital mammography. Tomosynthesis may also increase the rate of breast biopsy in women with abnormal findings, compared with 2-D digital mammography, according to the draft recommendations.
Meanwhile, “many important questions remain about the potential role of breast density in individualizing screening approaches; the current evidence is insufficient to recommend a specific screening strategy for women with increased breast density,” the group said.
The draft recommendations are available at www.screeningforbreastcancer.org. The group will accept public comments on the recommendations through May 18.
The U.S. Preventive Services Task Force is continuing to recommend routine mammograms every 2 years for women starting at age 50, according to newly released draft screening recommendations for breast cancer.
The draft recommendations, issued on April 20, largely reaffirm the group’s advice from 2009. Recent research findings have not altered the USPSTF’s key recommendation: routine mammograms every 2 years for women 50-74 years old. The USPSTF gave this recommendation a grade B, meaning that the evidence is strong enough for clinicians to adopt this practice. For women 75 years or older, the evidence remains inadequate to recommend for or against routine mammography.
For women 40-49 years old, the task force continued to state that the decision to start screening should be an individual one. The USPSTF gave mammography screening in this age group a grade C, concluding that the benefit of screening mammography outweighs the harms, but only by a small amount.
“Women who place a higher value on the potential benefit than the potential harms may choose to begin biennial screening between the ages of 40 and 49 years,” the task force members wrote.
In contrast, the American College of Obstetricians and Gynecologists, the American Cancer Society, the American College of Radiology, and the Society for Breast Imaging all recommend annual mammography starting at age 40.
The USPSTF is not advising against screening at age 40, but the task force members wrote that younger women with average breast cancer risk “must weigh a very important but infrequent benefit (small reduction in breast cancer deaths) against a group of meaningful and much more common harms (overdiagnosis and overtreatment; unnecessary and sometimes invasive follow-up testing; psychological harms associated with false-positive tests; and false reassurance from false-negative tests).”
The USPSTF’s recommendations apply to asymptomatic women without preexisting breast cancer, a previously diagnosed high-risk breast lesion, underlying genetic mutations, or a family history that puts them at high risk.
New for 2015, the group also weighed in on tomosynthesis (3-D mammography) for primary screening, and additional screening – ultrasound, MRI, tomosynthesis, or other methods – in women with dense breasts but otherwise negative mammogram results.
“In both cases, while there is some information about the accuracy of these modalities, there is no information on the effects of their use on health outcomes, such as breast cancer incidence, mortality, or overdiagnosis rates,” the USPSTF wrote.
The task force did not recommend for or against their use, stating only that more research is needed.
“From the limited data available, tomosynthesis appears to reduce recall rates for false-positive tests, compared with 2-D digital mammography alone. Available data also suggest that tomosynthesis increases the cancer detection rate, compared with 2-D digital mammography alone. However, current study designs do not answer the question of whether all of the additional cancers detected would have become clinically significant or whether there is an incremental clinical benefit to detecting these cancers earlier,” the task force members wrote.
Also, as currently practiced in most settings, tomosynthesis exposes women to about twice the amount of radiation of 2-D digital mammography. Tomosynthesis may also increase the rate of breast biopsy in women with abnormal findings, compared with 2-D digital mammography, according to the draft recommendations.
Meanwhile, “many important questions remain about the potential role of breast density in individualizing screening approaches; the current evidence is insufficient to recommend a specific screening strategy for women with increased breast density,” the group said.
The draft recommendations are available at www.screeningforbreastcancer.org. The group will accept public comments on the recommendations through May 18.
The U.S. Preventive Services Task Force is continuing to recommend routine mammograms every 2 years for women starting at age 50, according to newly released draft screening recommendations for breast cancer.
The draft recommendations, issued on April 20, largely reaffirm the group’s advice from 2009. Recent research findings have not altered the USPSTF’s key recommendation: routine mammograms every 2 years for women 50-74 years old. The USPSTF gave this recommendation a grade B, meaning that the evidence is strong enough for clinicians to adopt this practice. For women 75 years or older, the evidence remains inadequate to recommend for or against routine mammography.
For women 40-49 years old, the task force continued to state that the decision to start screening should be an individual one. The USPSTF gave mammography screening in this age group a grade C, concluding that the benefit of screening mammography outweighs the harms, but only by a small amount.
“Women who place a higher value on the potential benefit than the potential harms may choose to begin biennial screening between the ages of 40 and 49 years,” the task force members wrote.
In contrast, the American College of Obstetricians and Gynecologists, the American Cancer Society, the American College of Radiology, and the Society for Breast Imaging all recommend annual mammography starting at age 40.
The USPSTF is not advising against screening at age 40, but the task force members wrote that younger women with average breast cancer risk “must weigh a very important but infrequent benefit (small reduction in breast cancer deaths) against a group of meaningful and much more common harms (overdiagnosis and overtreatment; unnecessary and sometimes invasive follow-up testing; psychological harms associated with false-positive tests; and false reassurance from false-negative tests).”
The USPSTF’s recommendations apply to asymptomatic women without preexisting breast cancer, a previously diagnosed high-risk breast lesion, underlying genetic mutations, or a family history that puts them at high risk.
New for 2015, the group also weighed in on tomosynthesis (3-D mammography) for primary screening, and additional screening – ultrasound, MRI, tomosynthesis, or other methods – in women with dense breasts but otherwise negative mammogram results.
“In both cases, while there is some information about the accuracy of these modalities, there is no information on the effects of their use on health outcomes, such as breast cancer incidence, mortality, or overdiagnosis rates,” the USPSTF wrote.
The task force did not recommend for or against their use, stating only that more research is needed.
“From the limited data available, tomosynthesis appears to reduce recall rates for false-positive tests, compared with 2-D digital mammography alone. Available data also suggest that tomosynthesis increases the cancer detection rate, compared with 2-D digital mammography alone. However, current study designs do not answer the question of whether all of the additional cancers detected would have become clinically significant or whether there is an incremental clinical benefit to detecting these cancers earlier,” the task force members wrote.
Also, as currently practiced in most settings, tomosynthesis exposes women to about twice the amount of radiation of 2-D digital mammography. Tomosynthesis may also increase the rate of breast biopsy in women with abnormal findings, compared with 2-D digital mammography, according to the draft recommendations.
Meanwhile, “many important questions remain about the potential role of breast density in individualizing screening approaches; the current evidence is insufficient to recommend a specific screening strategy for women with increased breast density,” the group said.
The draft recommendations are available at www.screeningforbreastcancer.org. The group will accept public comments on the recommendations through May 18.
FROM THE U.S. PREVENTIVE SERVICES TASK FORCE
Class of 2015: New drugs projected to earn billions and billions
Of all drugs to be released in 2015, the melanoma drug Opdivo (nivolumab) is expected to have the brightest future, according to a report from Thomson Reuters.
With sales forecast to reach nearly $5.7 billion by 2019, Opdivo is at the head of a large 2015 “blockbuster” drug class. Opdivo is followed by a pair of drugs for the cardiovascular system: Praluent (alirocumab) for hypercholesterolemia with projected sales of $4.4 billion and LCZ-696 (sacubitril and valsartan) for chronic heart failure with projected 2019 sales of $3.7 billion, Thomson Reuters said.
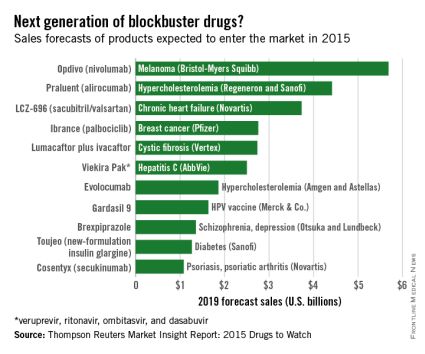
With estimated sales of $2.8 billion, the breast cancer drug Ibrance (palbociclib) is the second oncologic drug making the blockbuster list, with the first noncancer or non-CV drug – lumacaftor plus ivacaftor for cystic fibrosis – rounding out the Top 5 with projected sales of $2.7 billion by 2019.
Next comes Viekira Pak (ombitasvir, paritaprevir, and ritonavir tablets, copackaged with dasabuvir tablets), a hepatitis C virus drug with estimated 2019 sales of $2.5 billion, followed by the hypercholesterolemia/hyperlipidemia drug evolocumab, with projected sales of $1.9 billion. This $2.5 billion disparity between evolocumab and Praluent may be explained by Praluent’s arrival on the market a month sooner, and also because Praluent had a reduced rate of cardiac death, heart attack, and stroke in a phase III trial, a point likely to be relevant to most patients, according to the report.
Overall, 11 drugs are expected to reach $1 billion in sales by 2019, many more than the three blockbusters predicted from the 2014 stock of drugs. However, the two highest-selling new drugs from 2014, Sovaldi (sofosbuvir) and Harvoni (sofosbuvir plus ledipasvir) – both HCV drugs – are each predicted to reach sales of more than $10 billion by 2017, far exceeding anything from 2015, the report said.
The Thomson Reuters Market Insight Report used data collected from 2013 through early February 2015.
Of all drugs to be released in 2015, the melanoma drug Opdivo (nivolumab) is expected to have the brightest future, according to a report from Thomson Reuters.
With sales forecast to reach nearly $5.7 billion by 2019, Opdivo is at the head of a large 2015 “blockbuster” drug class. Opdivo is followed by a pair of drugs for the cardiovascular system: Praluent (alirocumab) for hypercholesterolemia with projected sales of $4.4 billion and LCZ-696 (sacubitril and valsartan) for chronic heart failure with projected 2019 sales of $3.7 billion, Thomson Reuters said.

With estimated sales of $2.8 billion, the breast cancer drug Ibrance (palbociclib) is the second oncologic drug making the blockbuster list, with the first noncancer or non-CV drug – lumacaftor plus ivacaftor for cystic fibrosis – rounding out the Top 5 with projected sales of $2.7 billion by 2019.
Next comes Viekira Pak (ombitasvir, paritaprevir, and ritonavir tablets, copackaged with dasabuvir tablets), a hepatitis C virus drug with estimated 2019 sales of $2.5 billion, followed by the hypercholesterolemia/hyperlipidemia drug evolocumab, with projected sales of $1.9 billion. This $2.5 billion disparity between evolocumab and Praluent may be explained by Praluent’s arrival on the market a month sooner, and also because Praluent had a reduced rate of cardiac death, heart attack, and stroke in a phase III trial, a point likely to be relevant to most patients, according to the report.
Overall, 11 drugs are expected to reach $1 billion in sales by 2019, many more than the three blockbusters predicted from the 2014 stock of drugs. However, the two highest-selling new drugs from 2014, Sovaldi (sofosbuvir) and Harvoni (sofosbuvir plus ledipasvir) – both HCV drugs – are each predicted to reach sales of more than $10 billion by 2017, far exceeding anything from 2015, the report said.
The Thomson Reuters Market Insight Report used data collected from 2013 through early February 2015.
Of all drugs to be released in 2015, the melanoma drug Opdivo (nivolumab) is expected to have the brightest future, according to a report from Thomson Reuters.
With sales forecast to reach nearly $5.7 billion by 2019, Opdivo is at the head of a large 2015 “blockbuster” drug class. Opdivo is followed by a pair of drugs for the cardiovascular system: Praluent (alirocumab) for hypercholesterolemia with projected sales of $4.4 billion and LCZ-696 (sacubitril and valsartan) for chronic heart failure with projected 2019 sales of $3.7 billion, Thomson Reuters said.

With estimated sales of $2.8 billion, the breast cancer drug Ibrance (palbociclib) is the second oncologic drug making the blockbuster list, with the first noncancer or non-CV drug – lumacaftor plus ivacaftor for cystic fibrosis – rounding out the Top 5 with projected sales of $2.7 billion by 2019.
Next comes Viekira Pak (ombitasvir, paritaprevir, and ritonavir tablets, copackaged with dasabuvir tablets), a hepatitis C virus drug with estimated 2019 sales of $2.5 billion, followed by the hypercholesterolemia/hyperlipidemia drug evolocumab, with projected sales of $1.9 billion. This $2.5 billion disparity between evolocumab and Praluent may be explained by Praluent’s arrival on the market a month sooner, and also because Praluent had a reduced rate of cardiac death, heart attack, and stroke in a phase III trial, a point likely to be relevant to most patients, according to the report.
Overall, 11 drugs are expected to reach $1 billion in sales by 2019, many more than the three blockbusters predicted from the 2014 stock of drugs. However, the two highest-selling new drugs from 2014, Sovaldi (sofosbuvir) and Harvoni (sofosbuvir plus ledipasvir) – both HCV drugs – are each predicted to reach sales of more than $10 billion by 2017, far exceeding anything from 2015, the report said.
The Thomson Reuters Market Insight Report used data collected from 2013 through early February 2015.
Sleep disorders in patients with cancer
Sleep disturbances are common among patients with cancer for many reasons. Sleep problems can be present at any stage during treatment for cancer and in some patients, sleep disturbance may be the presenting symptoms that lead to the diagnosis of some types of cancer. Poor sleep impairs quality of life In people with cancer, but most do not specifically complain of sleep problems unless they are explicitly asked. Insomnia and fatigue are most common sleep disorders in this cohort, although primary sleep disorders, including obstructive sleep apnea and restless legs syndrome, which are common in the general population, have not been carefully studied in the oncology setting despite significant their impairment of quality of life.
Click on the PDF icon at the top of this introduction to read the full article.
disorder
Sleep disturbances are common among patients with cancer for many reasons. Sleep problems can be present at any stage during treatment for cancer and in some patients, sleep disturbance may be the presenting symptoms that lead to the diagnosis of some types of cancer. Poor sleep impairs quality of life In people with cancer, but most do not specifically complain of sleep problems unless they are explicitly asked. Insomnia and fatigue are most common sleep disorders in this cohort, although primary sleep disorders, including obstructive sleep apnea and restless legs syndrome, which are common in the general population, have not been carefully studied in the oncology setting despite significant their impairment of quality of life.
Click on the PDF icon at the top of this introduction to read the full article.
Sleep disturbances are common among patients with cancer for many reasons. Sleep problems can be present at any stage during treatment for cancer and in some patients, sleep disturbance may be the presenting symptoms that lead to the diagnosis of some types of cancer. Poor sleep impairs quality of life In people with cancer, but most do not specifically complain of sleep problems unless they are explicitly asked. Insomnia and fatigue are most common sleep disorders in this cohort, although primary sleep disorders, including obstructive sleep apnea and restless legs syndrome, which are common in the general population, have not been carefully studied in the oncology setting despite significant their impairment of quality of life.
Click on the PDF icon at the top of this introduction to read the full article.
disorder
disorder
Neoadjuvant therapy facilitates breast conservation
HOUSTON – For some women with breast cancer, neoadjuvant therapy can increase the likelihood of breast-conserving treatment and may limit the extent of axillary dissection, a breast cancer researcher says.
“Neoadjuvant chemotherapy has long been used in the management of inflammatory breast cancer, in patients with locally advanced, or inoperable disease, and it’s increasingly being used in patients who have operable breast cancer,” said Dr. Elizabeth A. Mittendorf of the University of Texas MD Anderson Cancer Center, Houston.
A meta-analysis published in 2007 suggested that neoadjuvant therapy in patients with operable breast cancer reduced the mastectomy rate by 17%, a figure that Dr. Mittendorf said likely underestimates the benefit, because many of the trials included in the analysis did not require patients to be considered for breast conservation at presentation.
The meta-analysis also showed that local recurrence rates did not differ from those seen with mastectomy when patients treated with neoadjuvant therapy were downstaged to breast-conserving therapy, and that there were no differences in local recurrence rates for neoadjuvant vs. adjuvant chemotherapy stratified by type of surgery, Dr. Mittendorf said at the annual Society of Surgical Oncology Cancer Symposium.
Key clinical trials, including the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and B-27 trials, showed that neoadjuvant chemotherapy did not have an effect on either disease-free or overall survival compared with adjuvant chemotherapy, Dr. Mittendorf noted.
Response to neoadjuvant chemotherapy is also a good predictor of prognosis, she said, pointing to a pooled analysis of 12 studies published in 2014 in The Lancet. The authors of the analysis reported that patients with a pathologic complete response (pCR; no invasive disease in either the breast or axilla) after neoadjuvant chemotherapy had significantly improved survival, with the greatest prognostic values seen in patients with aggressive tumor subtypes.
Factors to consider when selecting neoadjuvant chemotherapy include:
• Tumor size.
• Lymph node status.
• Estrogen, progesterone, and/or HER2 status.
• Treatment sensitivity (as measured by Ki-67 or other markers).
• Pathologic complete response rates.
Chemo for HR-positive?
“With respect to hormone receptor–positive breast cancer, I think the most important question for these patients is do they even need chemotherapy?” Dr. Mittendorf said.
Hormone receptor–positive (HR-positive) breast cancers have been shown to be less responsive to neoadjuvant chemotherapy, and pCR is less prognostic of outcome in this tumor subtype. Older patients with HR-positive cancers who are borderline candidates for breast-conserving therapy might benefit from neoadjuvant therapy with an aromatase inhibitor, she noted.
HER2-positive disease
For patients with HER2-positive breast cancers, it may be possible to tailor neoadjuvant therapy, so that patients who achieve a pCR with neoadjuvant trastuzumab (Herceptin) might be spared an additional 6 months of adjuvant therapy. Dr. Mittendorf’s group published a recent study
Combination anti-HER2 therapies (trastuzumab and pertuzumab [Perjeta] as used in the NeoSphere Trial may help to improve pCR rates and outcomes in patients with HER2-positive tumors, Dr. Mittendorf said.
Triple negative disease
Among patients with triple-negative breast cancer (tumors lacking hormonal receptors and HER2), those who have residual cancer after neoadjuvant chemotherapy have a poor prognosis. At MD Anderson, patients with localized triple-negative breast cancer who are scheduled to receive neoadjuvant chemotherapy first have a biopsy with molecular profiling, and are immediately started on an anthracycline-based regimen.
Patients who have a response to the chemotherapy proceed to receive a taxane, while nonresponders will be triaged onto phase II studies based on the subtype of triple-negative breast cancer. Patients who are positive for BRCA mutations will be started on a carboplatin/paclitaxel regimen, while those with mesenchymal tumor subtypes will be started on a phosphoinositide 3-kinase (PI3K) inhibitor, and those with basal-like tumors will be started on an immunotherapy protocol.Better understanding of the biology of different tumor subtypes may also help to reduce the extent of axillary surgery, by helping clinicians to identify those patients who are likely to have a nodal pCR, Dr. Mittendorf said.
HOUSTON – For some women with breast cancer, neoadjuvant therapy can increase the likelihood of breast-conserving treatment and may limit the extent of axillary dissection, a breast cancer researcher says.
“Neoadjuvant chemotherapy has long been used in the management of inflammatory breast cancer, in patients with locally advanced, or inoperable disease, and it’s increasingly being used in patients who have operable breast cancer,” said Dr. Elizabeth A. Mittendorf of the University of Texas MD Anderson Cancer Center, Houston.
A meta-analysis published in 2007 suggested that neoadjuvant therapy in patients with operable breast cancer reduced the mastectomy rate by 17%, a figure that Dr. Mittendorf said likely underestimates the benefit, because many of the trials included in the analysis did not require patients to be considered for breast conservation at presentation.
The meta-analysis also showed that local recurrence rates did not differ from those seen with mastectomy when patients treated with neoadjuvant therapy were downstaged to breast-conserving therapy, and that there were no differences in local recurrence rates for neoadjuvant vs. adjuvant chemotherapy stratified by type of surgery, Dr. Mittendorf said at the annual Society of Surgical Oncology Cancer Symposium.
Key clinical trials, including the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and B-27 trials, showed that neoadjuvant chemotherapy did not have an effect on either disease-free or overall survival compared with adjuvant chemotherapy, Dr. Mittendorf noted.
Response to neoadjuvant chemotherapy is also a good predictor of prognosis, she said, pointing to a pooled analysis of 12 studies published in 2014 in The Lancet. The authors of the analysis reported that patients with a pathologic complete response (pCR; no invasive disease in either the breast or axilla) after neoadjuvant chemotherapy had significantly improved survival, with the greatest prognostic values seen in patients with aggressive tumor subtypes.
Factors to consider when selecting neoadjuvant chemotherapy include:
• Tumor size.
• Lymph node status.
• Estrogen, progesterone, and/or HER2 status.
• Treatment sensitivity (as measured by Ki-67 or other markers).
• Pathologic complete response rates.
Chemo for HR-positive?
“With respect to hormone receptor–positive breast cancer, I think the most important question for these patients is do they even need chemotherapy?” Dr. Mittendorf said.
Hormone receptor–positive (HR-positive) breast cancers have been shown to be less responsive to neoadjuvant chemotherapy, and pCR is less prognostic of outcome in this tumor subtype. Older patients with HR-positive cancers who are borderline candidates for breast-conserving therapy might benefit from neoadjuvant therapy with an aromatase inhibitor, she noted.
HER2-positive disease
For patients with HER2-positive breast cancers, it may be possible to tailor neoadjuvant therapy, so that patients who achieve a pCR with neoadjuvant trastuzumab (Herceptin) might be spared an additional 6 months of adjuvant therapy. Dr. Mittendorf’s group published a recent study
Combination anti-HER2 therapies (trastuzumab and pertuzumab [Perjeta] as used in the NeoSphere Trial may help to improve pCR rates and outcomes in patients with HER2-positive tumors, Dr. Mittendorf said.
Triple negative disease
Among patients with triple-negative breast cancer (tumors lacking hormonal receptors and HER2), those who have residual cancer after neoadjuvant chemotherapy have a poor prognosis. At MD Anderson, patients with localized triple-negative breast cancer who are scheduled to receive neoadjuvant chemotherapy first have a biopsy with molecular profiling, and are immediately started on an anthracycline-based regimen.
Patients who have a response to the chemotherapy proceed to receive a taxane, while nonresponders will be triaged onto phase II studies based on the subtype of triple-negative breast cancer. Patients who are positive for BRCA mutations will be started on a carboplatin/paclitaxel regimen, while those with mesenchymal tumor subtypes will be started on a phosphoinositide 3-kinase (PI3K) inhibitor, and those with basal-like tumors will be started on an immunotherapy protocol.Better understanding of the biology of different tumor subtypes may also help to reduce the extent of axillary surgery, by helping clinicians to identify those patients who are likely to have a nodal pCR, Dr. Mittendorf said.
HOUSTON – For some women with breast cancer, neoadjuvant therapy can increase the likelihood of breast-conserving treatment and may limit the extent of axillary dissection, a breast cancer researcher says.
“Neoadjuvant chemotherapy has long been used in the management of inflammatory breast cancer, in patients with locally advanced, or inoperable disease, and it’s increasingly being used in patients who have operable breast cancer,” said Dr. Elizabeth A. Mittendorf of the University of Texas MD Anderson Cancer Center, Houston.
A meta-analysis published in 2007 suggested that neoadjuvant therapy in patients with operable breast cancer reduced the mastectomy rate by 17%, a figure that Dr. Mittendorf said likely underestimates the benefit, because many of the trials included in the analysis did not require patients to be considered for breast conservation at presentation.
The meta-analysis also showed that local recurrence rates did not differ from those seen with mastectomy when patients treated with neoadjuvant therapy were downstaged to breast-conserving therapy, and that there were no differences in local recurrence rates for neoadjuvant vs. adjuvant chemotherapy stratified by type of surgery, Dr. Mittendorf said at the annual Society of Surgical Oncology Cancer Symposium.
Key clinical trials, including the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and B-27 trials, showed that neoadjuvant chemotherapy did not have an effect on either disease-free or overall survival compared with adjuvant chemotherapy, Dr. Mittendorf noted.
Response to neoadjuvant chemotherapy is also a good predictor of prognosis, she said, pointing to a pooled analysis of 12 studies published in 2014 in The Lancet. The authors of the analysis reported that patients with a pathologic complete response (pCR; no invasive disease in either the breast or axilla) after neoadjuvant chemotherapy had significantly improved survival, with the greatest prognostic values seen in patients with aggressive tumor subtypes.
Factors to consider when selecting neoadjuvant chemotherapy include:
• Tumor size.
• Lymph node status.
• Estrogen, progesterone, and/or HER2 status.
• Treatment sensitivity (as measured by Ki-67 or other markers).
• Pathologic complete response rates.
Chemo for HR-positive?
“With respect to hormone receptor–positive breast cancer, I think the most important question for these patients is do they even need chemotherapy?” Dr. Mittendorf said.
Hormone receptor–positive (HR-positive) breast cancers have been shown to be less responsive to neoadjuvant chemotherapy, and pCR is less prognostic of outcome in this tumor subtype. Older patients with HR-positive cancers who are borderline candidates for breast-conserving therapy might benefit from neoadjuvant therapy with an aromatase inhibitor, she noted.
HER2-positive disease
For patients with HER2-positive breast cancers, it may be possible to tailor neoadjuvant therapy, so that patients who achieve a pCR with neoadjuvant trastuzumab (Herceptin) might be spared an additional 6 months of adjuvant therapy. Dr. Mittendorf’s group published a recent study
Combination anti-HER2 therapies (trastuzumab and pertuzumab [Perjeta] as used in the NeoSphere Trial may help to improve pCR rates and outcomes in patients with HER2-positive tumors, Dr. Mittendorf said.
Triple negative disease
Among patients with triple-negative breast cancer (tumors lacking hormonal receptors and HER2), those who have residual cancer after neoadjuvant chemotherapy have a poor prognosis. At MD Anderson, patients with localized triple-negative breast cancer who are scheduled to receive neoadjuvant chemotherapy first have a biopsy with molecular profiling, and are immediately started on an anthracycline-based regimen.
Patients who have a response to the chemotherapy proceed to receive a taxane, while nonresponders will be triaged onto phase II studies based on the subtype of triple-negative breast cancer. Patients who are positive for BRCA mutations will be started on a carboplatin/paclitaxel regimen, while those with mesenchymal tumor subtypes will be started on a phosphoinositide 3-kinase (PI3K) inhibitor, and those with basal-like tumors will be started on an immunotherapy protocol.Better understanding of the biology of different tumor subtypes may also help to reduce the extent of axillary surgery, by helping clinicians to identify those patients who are likely to have a nodal pCR, Dr. Mittendorf said.
Type, location of BRCA mutations influence risk
Among women who carry BRCA1 or BRCA2 mutations, the type and exact location of the mutation influences the risk it confers for breast and ovarian cancer, according to a report published online April 7 in JAMA.
Investigators examined differences in cancer risks by analyzing data in the Consortium of Investigators of Modifiers of BRCA (CIMBA), a collection of clinical and genetic information for carriers of disease-associated BRCA mutations in 33 countries.
For this study, data were assessed for 19,581 women with BRCA1 mutations and 11,900 women with BRCA2 mutations for whom there was sufficient information to estimate hazard ratios. The types of mutations included nonsense, frame shift, in-frame, missense, splicing, rearrangement, premature termination codons, and nonsense-mediated decay mutations, said Timothy R. Rebbeck, Ph.D., of Abramson Cancer Center and the Center for Clinical Epidemiology and Biostatistics at the University of Pennsylvania, Philadelphia, and his associates.
Among the study participants with BRCA1 mutations, 9,052 women (46%) developed breast cancer, 2,317 (12%) developed ovarian cancer, and 1,041 (5%) developed both breast and ovarian cancer. Among the women with BRCA2 mutations, 6,180 (52%) developed breast cancer, 682 (6%) developed ovarian cancer, and 272 (2%) developed both breast and ovarian cancer. A woman’s risk for cancer differed significantly according to the type of BRCA1 or BRCA2 mutation she carried and according to the location of the mutation on the nucleotide, the investigators said (JAMA 2015 April 7 [doi:10.1001/jama.2014.5985]).
Further research is needed to determine the absolute risks of cancer associated with each mutation and “to better understand what level of risk difference will change decision-making and standards of care, such as preventive surgery” for BRCA1 and BRCA2 carriers, Dr. Rebbeck and his associates added.
This study was funded primarily by Cancer Research UK and also was supported by the U.S. National Institutes of Health, the Basser Research Center at the University of Pennsylvania, the Breast Cancer Research Foundation, and the Rooney Family Foundation. Dr. Rebbeck reported having no financial disclosures; his associates reported numerous ties to industry sources.
Among women who carry BRCA1 or BRCA2 mutations, the type and exact location of the mutation influences the risk it confers for breast and ovarian cancer, according to a report published online April 7 in JAMA.
Investigators examined differences in cancer risks by analyzing data in the Consortium of Investigators of Modifiers of BRCA (CIMBA), a collection of clinical and genetic information for carriers of disease-associated BRCA mutations in 33 countries.
For this study, data were assessed for 19,581 women with BRCA1 mutations and 11,900 women with BRCA2 mutations for whom there was sufficient information to estimate hazard ratios. The types of mutations included nonsense, frame shift, in-frame, missense, splicing, rearrangement, premature termination codons, and nonsense-mediated decay mutations, said Timothy R. Rebbeck, Ph.D., of Abramson Cancer Center and the Center for Clinical Epidemiology and Biostatistics at the University of Pennsylvania, Philadelphia, and his associates.
Among the study participants with BRCA1 mutations, 9,052 women (46%) developed breast cancer, 2,317 (12%) developed ovarian cancer, and 1,041 (5%) developed both breast and ovarian cancer. Among the women with BRCA2 mutations, 6,180 (52%) developed breast cancer, 682 (6%) developed ovarian cancer, and 272 (2%) developed both breast and ovarian cancer. A woman’s risk for cancer differed significantly according to the type of BRCA1 or BRCA2 mutation she carried and according to the location of the mutation on the nucleotide, the investigators said (JAMA 2015 April 7 [doi:10.1001/jama.2014.5985]).
Further research is needed to determine the absolute risks of cancer associated with each mutation and “to better understand what level of risk difference will change decision-making and standards of care, such as preventive surgery” for BRCA1 and BRCA2 carriers, Dr. Rebbeck and his associates added.
This study was funded primarily by Cancer Research UK and also was supported by the U.S. National Institutes of Health, the Basser Research Center at the University of Pennsylvania, the Breast Cancer Research Foundation, and the Rooney Family Foundation. Dr. Rebbeck reported having no financial disclosures; his associates reported numerous ties to industry sources.
Among women who carry BRCA1 or BRCA2 mutations, the type and exact location of the mutation influences the risk it confers for breast and ovarian cancer, according to a report published online April 7 in JAMA.
Investigators examined differences in cancer risks by analyzing data in the Consortium of Investigators of Modifiers of BRCA (CIMBA), a collection of clinical and genetic information for carriers of disease-associated BRCA mutations in 33 countries.
For this study, data were assessed for 19,581 women with BRCA1 mutations and 11,900 women with BRCA2 mutations for whom there was sufficient information to estimate hazard ratios. The types of mutations included nonsense, frame shift, in-frame, missense, splicing, rearrangement, premature termination codons, and nonsense-mediated decay mutations, said Timothy R. Rebbeck, Ph.D., of Abramson Cancer Center and the Center for Clinical Epidemiology and Biostatistics at the University of Pennsylvania, Philadelphia, and his associates.
Among the study participants with BRCA1 mutations, 9,052 women (46%) developed breast cancer, 2,317 (12%) developed ovarian cancer, and 1,041 (5%) developed both breast and ovarian cancer. Among the women with BRCA2 mutations, 6,180 (52%) developed breast cancer, 682 (6%) developed ovarian cancer, and 272 (2%) developed both breast and ovarian cancer. A woman’s risk for cancer differed significantly according to the type of BRCA1 or BRCA2 mutation she carried and according to the location of the mutation on the nucleotide, the investigators said (JAMA 2015 April 7 [doi:10.1001/jama.2014.5985]).
Further research is needed to determine the absolute risks of cancer associated with each mutation and “to better understand what level of risk difference will change decision-making and standards of care, such as preventive surgery” for BRCA1 and BRCA2 carriers, Dr. Rebbeck and his associates added.
This study was funded primarily by Cancer Research UK and also was supported by the U.S. National Institutes of Health, the Basser Research Center at the University of Pennsylvania, the Breast Cancer Research Foundation, and the Rooney Family Foundation. Dr. Rebbeck reported having no financial disclosures; his associates reported numerous ties to industry sources.
FROM JAMA
Key clinical point: The type and exact location of BRCA1 and BRCA2 mutations affect the risk they confer for breast and ovarian cancer.
Major finding: Among the study participants with BRCA1 mutations, 46% developed breast cancer, 12% developed ovarian cancer, and 5% developed both breast and ovarian cancer; among those with BRCA2 mutations, 52% developed breast cancer, 6% developed ovarian cancer, and 2% developed both breast and ovarian cancer.
Data source: An observational analysis of cancer risks among 31,481 women in an international database of carriers of any deleterious BRCA mutations.
Disclosures: This study was funded primarily by Cancer Research UK and also was supported by the U.S. National Institutes of Health, the Basser Research Center at the University of Pennsylvania, the Breast Cancer Research Foundation, and the Rooney Family Foundation. Dr. Rebbeck reported having no financial disclosures; his associates reported numerous ties to industry sources.
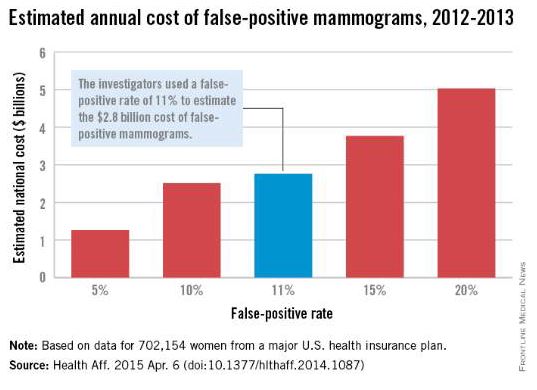
False-positive mammograms cost $2.8 billion a year
False-positive mammograms in women aged 40-59 years cost an estimated $2.8 billion per year in the United States in 2012-2013, according to a report published April 6 in Health Affairs.
The cost of screen-detected invasive breast cancer overdiagnoses was $1.2 billion a year for that time period and age group. Invasive breast cancers represent about $1 billion of that, with the rest coming from overdiagnoses of ductal carcinoma in situ (DCIS), Mei-Sing Ong, Ph.D., and Dr. Kenneth D. Mandl, both of Boston Children’s Hospital, estimated.
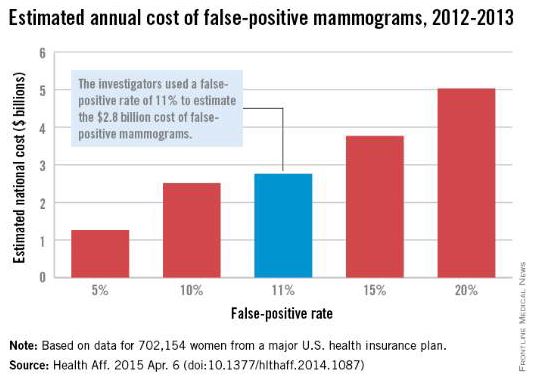
Using expenditure data for 702,154 women from a major U.S. health care insurance plan, the investigators calculated that the average cost of a false-positive mammogram – the mammography plus all related costs over the next 12 months – was $852. The average 12-month costs for invasive breast cancer and DCIS were $51,837 and $12,369, they reported (Health Aff. 2015 Apr. 6 [doi:10.1377/hlthaff.2014.1087]).
Dr. Ong and Dr. Mandl used a false-positive rate of 11% for the mammogram estimate, although they also calculated costs based on a range of rates from 5% to 20%. Based on recent studies in the New England Journal of Medicine and the BMJ, they used an overdiagnosis rate of 22% for the breast cancer estimate; the overdiagnosis rate of 86% for DCIS came from a 2004 study.
“The disutility of overdiagnosis,” together with the high diagnostic and treatment costs of mammography, “may tilt the balance to the point where screening [based on age] appears relatively cost ineffective,” Dr. Ong and Dr. Mandl wrote. It may be time to “shape a more individualized approach to determining who should receive screening, focusing on women who are most likely to benefit.”
Dr. Ong is supported by a fellowship from the National Health and Medical Research Council in Australia, which played no role in the study. Dr. Mandl did not report any conflicts.
False-positive mammograms in women aged 40-59 years cost an estimated $2.8 billion per year in the United States in 2012-2013, according to a report published April 6 in Health Affairs.
The cost of screen-detected invasive breast cancer overdiagnoses was $1.2 billion a year for that time period and age group. Invasive breast cancers represent about $1 billion of that, with the rest coming from overdiagnoses of ductal carcinoma in situ (DCIS), Mei-Sing Ong, Ph.D., and Dr. Kenneth D. Mandl, both of Boston Children’s Hospital, estimated.

Using expenditure data for 702,154 women from a major U.S. health care insurance plan, the investigators calculated that the average cost of a false-positive mammogram – the mammography plus all related costs over the next 12 months – was $852. The average 12-month costs for invasive breast cancer and DCIS were $51,837 and $12,369, they reported (Health Aff. 2015 Apr. 6 [doi:10.1377/hlthaff.2014.1087]).
Dr. Ong and Dr. Mandl used a false-positive rate of 11% for the mammogram estimate, although they also calculated costs based on a range of rates from 5% to 20%. Based on recent studies in the New England Journal of Medicine and the BMJ, they used an overdiagnosis rate of 22% for the breast cancer estimate; the overdiagnosis rate of 86% for DCIS came from a 2004 study.
“The disutility of overdiagnosis,” together with the high diagnostic and treatment costs of mammography, “may tilt the balance to the point where screening [based on age] appears relatively cost ineffective,” Dr. Ong and Dr. Mandl wrote. It may be time to “shape a more individualized approach to determining who should receive screening, focusing on women who are most likely to benefit.”
Dr. Ong is supported by a fellowship from the National Health and Medical Research Council in Australia, which played no role in the study. Dr. Mandl did not report any conflicts.
False-positive mammograms in women aged 40-59 years cost an estimated $2.8 billion per year in the United States in 2012-2013, according to a report published April 6 in Health Affairs.
The cost of screen-detected invasive breast cancer overdiagnoses was $1.2 billion a year for that time period and age group. Invasive breast cancers represent about $1 billion of that, with the rest coming from overdiagnoses of ductal carcinoma in situ (DCIS), Mei-Sing Ong, Ph.D., and Dr. Kenneth D. Mandl, both of Boston Children’s Hospital, estimated.

Using expenditure data for 702,154 women from a major U.S. health care insurance plan, the investigators calculated that the average cost of a false-positive mammogram – the mammography plus all related costs over the next 12 months – was $852. The average 12-month costs for invasive breast cancer and DCIS were $51,837 and $12,369, they reported (Health Aff. 2015 Apr. 6 [doi:10.1377/hlthaff.2014.1087]).
Dr. Ong and Dr. Mandl used a false-positive rate of 11% for the mammogram estimate, although they also calculated costs based on a range of rates from 5% to 20%. Based on recent studies in the New England Journal of Medicine and the BMJ, they used an overdiagnosis rate of 22% for the breast cancer estimate; the overdiagnosis rate of 86% for DCIS came from a 2004 study.
“The disutility of overdiagnosis,” together with the high diagnostic and treatment costs of mammography, “may tilt the balance to the point where screening [based on age] appears relatively cost ineffective,” Dr. Ong and Dr. Mandl wrote. It may be time to “shape a more individualized approach to determining who should receive screening, focusing on women who are most likely to benefit.”
Dr. Ong is supported by a fellowship from the National Health and Medical Research Council in Australia, which played no role in the study. Dr. Mandl did not report any conflicts.
FROM HEALTH AFFAIRS
Does a family history of both breast and prostate cancer (vs breast only) put a woman at greater risk for future breast cancer?
The most common invasive cancers diagnosed in US women and men are breast and prostate cancers, respectively. This analysis from the Women’s Health Initiative observational study involved 78,171 women aged 50 to 79 years at enrollment. Invasive breast cancer was diagnosed in 3,506 women (4.5%) during a median of 132 months of follow-up. Having a first-degree relative with breast or prostate cancer was associated with an elevated adjusted hazard ratio of breast cancer of 1.42 and 1.14, respectively. Women who had a history of both cancers among first-degree relatives had an adjusted HR of 1.78. Although the difference did not achieve statistical significance, there was a suggestion that the elevated risk for breast cancer associated with relatives with prostate and breast cancer was higher in African-American women compared with white women. The risk for breast cancer was not elevated in women who had first-degree relatives with cancers other than breast or prostate.
The authors point out that another study also reported that a family history that includes both cancers is associated with a greater elevation in the risk for breast cancer than family history of prostate cancer alone. Although BRCA 1 and 2 mutations are associated with an elevated risk of not only breast but also prostate cancer, the authors indicate that such mutations account for only a small proportion of the observed aggregation of breast and prostate cancer in first-degree relatives of women with breast cancer in their analysis.
What this evidence means for practice
The associations observed by these authors underscore that, when taking family histories, women’s health clinicians should pay attention not only to breast but also to prostate cancer, and counsel patients regarding risk and screening practices accordingly.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The most common invasive cancers diagnosed in US women and men are breast and prostate cancers, respectively. This analysis from the Women’s Health Initiative observational study involved 78,171 women aged 50 to 79 years at enrollment. Invasive breast cancer was diagnosed in 3,506 women (4.5%) during a median of 132 months of follow-up. Having a first-degree relative with breast or prostate cancer was associated with an elevated adjusted hazard ratio of breast cancer of 1.42 and 1.14, respectively. Women who had a history of both cancers among first-degree relatives had an adjusted HR of 1.78. Although the difference did not achieve statistical significance, there was a suggestion that the elevated risk for breast cancer associated with relatives with prostate and breast cancer was higher in African-American women compared with white women. The risk for breast cancer was not elevated in women who had first-degree relatives with cancers other than breast or prostate.
The authors point out that another study also reported that a family history that includes both cancers is associated with a greater elevation in the risk for breast cancer than family history of prostate cancer alone. Although BRCA 1 and 2 mutations are associated with an elevated risk of not only breast but also prostate cancer, the authors indicate that such mutations account for only a small proportion of the observed aggregation of breast and prostate cancer in first-degree relatives of women with breast cancer in their analysis.
What this evidence means for practice
The associations observed by these authors underscore that, when taking family histories, women’s health clinicians should pay attention not only to breast but also to prostate cancer, and counsel patients regarding risk and screening practices accordingly.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The most common invasive cancers diagnosed in US women and men are breast and prostate cancers, respectively. This analysis from the Women’s Health Initiative observational study involved 78,171 women aged 50 to 79 years at enrollment. Invasive breast cancer was diagnosed in 3,506 women (4.5%) during a median of 132 months of follow-up. Having a first-degree relative with breast or prostate cancer was associated with an elevated adjusted hazard ratio of breast cancer of 1.42 and 1.14, respectively. Women who had a history of both cancers among first-degree relatives had an adjusted HR of 1.78. Although the difference did not achieve statistical significance, there was a suggestion that the elevated risk for breast cancer associated with relatives with prostate and breast cancer was higher in African-American women compared with white women. The risk for breast cancer was not elevated in women who had first-degree relatives with cancers other than breast or prostate.
The authors point out that another study also reported that a family history that includes both cancers is associated with a greater elevation in the risk for breast cancer than family history of prostate cancer alone. Although BRCA 1 and 2 mutations are associated with an elevated risk of not only breast but also prostate cancer, the authors indicate that such mutations account for only a small proportion of the observed aggregation of breast and prostate cancer in first-degree relatives of women with breast cancer in their analysis.
What this evidence means for practice
The associations observed by these authors underscore that, when taking family histories, women’s health clinicians should pay attention not only to breast but also to prostate cancer, and counsel patients regarding risk and screening practices accordingly.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Breast cancer survivors should try glycerin-containing products
“UPDATE ON SEXUAL DYSFUNCTION”
BARBARA S. LEVY, MD (SEPTEMBER 2014)
Breast cancer survivors should try glycerin-containing products
Dr. Levy’s well-written article on dyspareunia said everything I would tell a patient, until I had breast cancer and started estrogen antagonist therapy. Not only does the vagina lose elasticity but there is a similar sensation to spilling a strong acid on your skin in chemistry lab!
The silicone products Dr. Levy suggests may not be enough. Online support groups suggest a glycerin-containing product called “Probe Personal Lubricant.” If women find it too slippery to handle, they can mix it with an unscented petroleum gel product, such as “Albolene” [Albolene Moisturizing Cleanser] or “Aquaphor” [Aquaphor Healing Ointment]. I cannot tell you how many marriages this has saved for my patients and our local breast cancer survivors.
Joan Eggert, MD, MPH
St. George, Utah
Dr. Levy responds
I thank Dr. Eggert for sharing her personal experience and offering readers excellent practical advice. There is no substitute for listening to our patients and modifying recommendations based on their input and feedback. This is an important part of continuous quality improvement and experiential learning. I truly appreciate the suggestion from someone far more expert than I.
I do want to express a concern about using a petroleum jelly or mineral oil–based product as a lubricant with condoms. Albolene and Aquaphor dissolve latex and increase the chance of rupture. I do not recommend their use when a woman is using a condom for birth control or prevention of sexually transmitted disease.
Clarification requested
In the February issue of OBG Management, you quoted me as saying that the recent FDA analysis of power morcellation was inadequate. Actually, what I said was that the “FDA did an inadequate and irresponsible analysis and it has been a disservice to women.” I didn’t mince words when I spoke and I am appalled by the FDA’s lack of rigor in this important matter.
William H. Parker, MD
Santa Monica, California
The editors respond
We thank Dr. Parker for expressing his concern to us. Although the full title of Dr. Parker’s Web exclusive audio was included online, it was truncated in print due to space and may have not conveyed his full meaning to print readers. Dr. Parker’s voice, and how it is portrayed within the journal’s pages and online, is very important to us.
ANSWERING YOUR CODING QUESTIONS
A reader recently requested assistance for a specific coding challenge. We’ve asked our reimbursement specialist, Melanie Witt, RN, CPC, COBGC, MA, to provide her insight.
What billing code for patients with inconclusive viability?
Dr. Barbieri’s editorial on suspected nonviable pregnancy (“Stop using the hCG discriminatory zone of 1,500 to 2,000 mIU/mL to guide intervention during early pregnancy,” January 2015) and other recent articles help guide our trainees to not “pull the trigger,” so to speak, so quickly on early pregnancies with uncertain viability. It confirms our teaching to be patient and let the pregnancy develop, or not, especially when patients are stable.
I find billing for these encounters to be difficult, however. What do you recommend as the billing code for patients with inconclusive viability—V23.87? Is there anything other than a V-code?
Rana Snipe Berry, MD
Indianapolis, Indiana
Ms. Witt responds
Currently there is only one ICD-9-CM code that describes uncertain fetal viability: V23.87 (Pregnancy with inconclusive fetal viability). This code represents the supervision of a high-risk pregnancy for this reason, and it helps to explain additional testing that may be required. Unlike other “V” codes that many payers ignore, the V codes for pregnancy care, whether for routine supervision, high-risk supervision, or antenatal screening, are accepted by payers as reasons for care.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
“UPDATE ON SEXUAL DYSFUNCTION”
BARBARA S. LEVY, MD (SEPTEMBER 2014)
Breast cancer survivors should try glycerin-containing products
Dr. Levy’s well-written article on dyspareunia said everything I would tell a patient, until I had breast cancer and started estrogen antagonist therapy. Not only does the vagina lose elasticity but there is a similar sensation to spilling a strong acid on your skin in chemistry lab!
The silicone products Dr. Levy suggests may not be enough. Online support groups suggest a glycerin-containing product called “Probe Personal Lubricant.” If women find it too slippery to handle, they can mix it with an unscented petroleum gel product, such as “Albolene” [Albolene Moisturizing Cleanser] or “Aquaphor” [Aquaphor Healing Ointment]. I cannot tell you how many marriages this has saved for my patients and our local breast cancer survivors.
Joan Eggert, MD, MPH
St. George, Utah
Dr. Levy responds
I thank Dr. Eggert for sharing her personal experience and offering readers excellent practical advice. There is no substitute for listening to our patients and modifying recommendations based on their input and feedback. This is an important part of continuous quality improvement and experiential learning. I truly appreciate the suggestion from someone far more expert than I.
I do want to express a concern about using a petroleum jelly or mineral oil–based product as a lubricant with condoms. Albolene and Aquaphor dissolve latex and increase the chance of rupture. I do not recommend their use when a woman is using a condom for birth control or prevention of sexually transmitted disease.
Clarification requested
In the February issue of OBG Management, you quoted me as saying that the recent FDA analysis of power morcellation was inadequate. Actually, what I said was that the “FDA did an inadequate and irresponsible analysis and it has been a disservice to women.” I didn’t mince words when I spoke and I am appalled by the FDA’s lack of rigor in this important matter.
William H. Parker, MD
Santa Monica, California
The editors respond
We thank Dr. Parker for expressing his concern to us. Although the full title of Dr. Parker’s Web exclusive audio was included online, it was truncated in print due to space and may have not conveyed his full meaning to print readers. Dr. Parker’s voice, and how it is portrayed within the journal’s pages and online, is very important to us.
ANSWERING YOUR CODING QUESTIONS
A reader recently requested assistance for a specific coding challenge. We’ve asked our reimbursement specialist, Melanie Witt, RN, CPC, COBGC, MA, to provide her insight.
What billing code for patients with inconclusive viability?
Dr. Barbieri’s editorial on suspected nonviable pregnancy (“Stop using the hCG discriminatory zone of 1,500 to 2,000 mIU/mL to guide intervention during early pregnancy,” January 2015) and other recent articles help guide our trainees to not “pull the trigger,” so to speak, so quickly on early pregnancies with uncertain viability. It confirms our teaching to be patient and let the pregnancy develop, or not, especially when patients are stable.
I find billing for these encounters to be difficult, however. What do you recommend as the billing code for patients with inconclusive viability—V23.87? Is there anything other than a V-code?
Rana Snipe Berry, MD
Indianapolis, Indiana
Ms. Witt responds
Currently there is only one ICD-9-CM code that describes uncertain fetal viability: V23.87 (Pregnancy with inconclusive fetal viability). This code represents the supervision of a high-risk pregnancy for this reason, and it helps to explain additional testing that may be required. Unlike other “V” codes that many payers ignore, the V codes for pregnancy care, whether for routine supervision, high-risk supervision, or antenatal screening, are accepted by payers as reasons for care.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
“UPDATE ON SEXUAL DYSFUNCTION”
BARBARA S. LEVY, MD (SEPTEMBER 2014)
Breast cancer survivors should try glycerin-containing products
Dr. Levy’s well-written article on dyspareunia said everything I would tell a patient, until I had breast cancer and started estrogen antagonist therapy. Not only does the vagina lose elasticity but there is a similar sensation to spilling a strong acid on your skin in chemistry lab!
The silicone products Dr. Levy suggests may not be enough. Online support groups suggest a glycerin-containing product called “Probe Personal Lubricant.” If women find it too slippery to handle, they can mix it with an unscented petroleum gel product, such as “Albolene” [Albolene Moisturizing Cleanser] or “Aquaphor” [Aquaphor Healing Ointment]. I cannot tell you how many marriages this has saved for my patients and our local breast cancer survivors.
Joan Eggert, MD, MPH
St. George, Utah
Dr. Levy responds
I thank Dr. Eggert for sharing her personal experience and offering readers excellent practical advice. There is no substitute for listening to our patients and modifying recommendations based on their input and feedback. This is an important part of continuous quality improvement and experiential learning. I truly appreciate the suggestion from someone far more expert than I.
I do want to express a concern about using a petroleum jelly or mineral oil–based product as a lubricant with condoms. Albolene and Aquaphor dissolve latex and increase the chance of rupture. I do not recommend their use when a woman is using a condom for birth control or prevention of sexually transmitted disease.
Clarification requested
In the February issue of OBG Management, you quoted me as saying that the recent FDA analysis of power morcellation was inadequate. Actually, what I said was that the “FDA did an inadequate and irresponsible analysis and it has been a disservice to women.” I didn’t mince words when I spoke and I am appalled by the FDA’s lack of rigor in this important matter.
William H. Parker, MD
Santa Monica, California
The editors respond
We thank Dr. Parker for expressing his concern to us. Although the full title of Dr. Parker’s Web exclusive audio was included online, it was truncated in print due to space and may have not conveyed his full meaning to print readers. Dr. Parker’s voice, and how it is portrayed within the journal’s pages and online, is very important to us.
ANSWERING YOUR CODING QUESTIONS
A reader recently requested assistance for a specific coding challenge. We’ve asked our reimbursement specialist, Melanie Witt, RN, CPC, COBGC, MA, to provide her insight.
What billing code for patients with inconclusive viability?
Dr. Barbieri’s editorial on suspected nonviable pregnancy (“Stop using the hCG discriminatory zone of 1,500 to 2,000 mIU/mL to guide intervention during early pregnancy,” January 2015) and other recent articles help guide our trainees to not “pull the trigger,” so to speak, so quickly on early pregnancies with uncertain viability. It confirms our teaching to be patient and let the pregnancy develop, or not, especially when patients are stable.
I find billing for these encounters to be difficult, however. What do you recommend as the billing code for patients with inconclusive viability—V23.87? Is there anything other than a V-code?
Rana Snipe Berry, MD
Indianapolis, Indiana
Ms. Witt responds
Currently there is only one ICD-9-CM code that describes uncertain fetal viability: V23.87 (Pregnancy with inconclusive fetal viability). This code represents the supervision of a high-risk pregnancy for this reason, and it helps to explain additional testing that may be required. Unlike other “V” codes that many payers ignore, the V codes for pregnancy care, whether for routine supervision, high-risk supervision, or antenatal screening, are accepted by payers as reasons for care.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Physician-patient communication, terminology play important role in CPM decisions
BIRMINGHAM, ALA. – The perception that one’s physician had recommended contralateral prophylactic mastectomy was a particularly important factor in the decision to undergo the procedure among BRCA1/2 mutation noncarriers with newly diagnosed breast cancer in a prospective study.
Of 90 BRCA noncarriers with newly diagnosed breast cancer, a “sizable minority” (24.4%) chose to undergo contralateral prophylactic mastectomy (CPM) after learning their mutation status, Jada G. Hamilton, Ph.D., reported at the annual meeting of the American Society of Preventive Oncology.
By comparison, 88% of eight BRCA1/2 carriers who participated in the study chose to undergo CPM, and neither of two women with a BRCA1/2 variant of undetermined significance chose to undergo CPM.
On multivariate analysis, the perception that one’s physician had recommended CPM was most strongly associated with the decision to undergo the procedure (odds ratio, 11.1), said Dr. Hamilton of Memorial Sloan-Kettering Cancer Center, New York.
Other factors associated with the decision were a perception of greater risk for contralateral breast cancer (OR, 6.46) and a perception of greater pros of CPM (OR, 1.37), she said, noting that those who indicated they would feel good about having CPM and those who indicated they might feel regret if they didn’t have CPM were most likely to elect CPM.
Age, Ashkenazi Jewish ethnicity, breast cancer–related distress, perceived cons of CPM (such as disfigurement and concerns regarding a negative impact on one’s sex life), and decisional conflict regarding CPM were not significantly associated with the decision.
Presurgical genetic testing provides valuable information to women with newly diagnosed breast cancer as they begin to make decisions about treatment, Dr. Hamilton said. Although BRCA1/2 mutation noncarriers have a low (3%-10%) risk, compared with carriers (27%-37%), studies suggest that about 18% nevertheless choose to undergo CPM.
The psychosocial factors that may affect the decision are not well understood, Dr. Hamilton said.
For the current analysis, participants who were part of a larger prospective study on presurgical BRCA1/2 testing completed a questionnaire, and the frequency and psychosocial correlates of the decision to undergo a CPM were assessed. The participants were adult women with a median age of 43 years (range of 29-59 years).
The findings raise interesting questions for future work, Dr. Hamilton said.
“I think it’s really critical for future studies to dig in to what’s happening in terms of physician-patient communication around CPM,” she said, noting that it will be important to explore how such communication interacts with a woman’s past experiences, emotions, and beliefs to shape her cancer prevention decisions.
Further, the women who undergo CPM should be followed to assess their long-term outcomes with respect to factors such as quality of life, satisfaction, and decisional regret, she concluded.
The decision to undergo CPM and the effects of physician-patient communication on that decision were also addressed in another study presented at the meeting.
In that study – a mixed methods pilot study looking mainly at factors affecting informed decision making in women who had ductal carcinoma in situ (DCIS) or who were considered to be at increased risk of invasive breast cancer risk because of a diagnosis of lobular carcinoma in situ (LCIS), BRCA positivity, or 20% or greater calculated lifetime risk – anxiety about cancer recurrence was the top reason for pursuing CPM.
Despite a lack of survival benefit, an increasing number of women with DCIS are undergoing CPM, but little is known about the decision-making process, said Jessica Valente of Emory University, Atlanta.
She and her colleagues sought to identify factors impacting risk comprehension and decision making. Of 68 women with DCIS or who were at high risk for development of invasive breast cancer, 33 considered CPM and 11 underwent the procedure.
The choice to undergo CPM was significantly associated with plastic surgery consultation, increased 10-year breast cancer risk, genetic counseling, genetic testing, and higher income, she said.
The study also highlighted a lack of health literacy and understanding of related terminology.
Most participants (nearly 84%) stated that DCIS qualified as breast cancer, but only about 40% correctly defined DCIS, Ms. Valente said.
When asked what they would recommend as a treatment strategy for a friend with DCIS, 35% thought surgery would be best. A similar percentage would recommend surgery for LCIS.
“When we looked at ductal hyperplasia, fewer people thought that that qualified as cancer, and they were more likely to recommend observation,” she said.
Further, when asked to interpret the phrase “indolent lesion of epithelial origin,” which is a phrase that has been promoted as a replacement for “DCIS” in light of concerns that women are increasingly electing CPM for DCIS because of fear of the word “carcinoma” despite a 99% survival rate, only 28% of patients believed it referred to cancer.
Observation was one of the highly recommended interventions for “indolent lesion of epithelial origin,” followed by biopsy, she said, noting that only 13% recommended surgery when this phrase was used.
“Interestingly, 7.4% said an oral or topical medication [should be used for “indolent lesion of epithelial origin]” – a finding likely explained by the fact that some women interpreted the word “lesion” to mean a wound or sore on the skin, she said.
Additionally, few women were able to define contralateral prophylactic mastectomy.
Overall, despite the study population being very well educated and from a higher socioeconomic status, they had low scores for understanding terminology (8.21 out of 20), Ms. Valente noted.
The findings demonstrate that decision making in the context of DCIS remains complex and underscore the importance of recognizing patients’ knowledge of risk communication and terminology for supporting shared and informed surgical decision making, she said.
The findings also demonstrate that while fewer women felt that surgery was appropriate when the term “indolent lesion of epithelial origin” was used instead of “DCIS,” the proposed new terminology doesn’t necessarily provide the desired clarity, she noted.
“They still came up with such a broad range of interpretations that we really might be introducing a new set of conflicts and confusion when we think about changing to that terminology when we talk to patients,” she concluded.
Dr. Hamilton and Ms. Valente each reported having no disclosures.
BIRMINGHAM, ALA. – The perception that one’s physician had recommended contralateral prophylactic mastectomy was a particularly important factor in the decision to undergo the procedure among BRCA1/2 mutation noncarriers with newly diagnosed breast cancer in a prospective study.
Of 90 BRCA noncarriers with newly diagnosed breast cancer, a “sizable minority” (24.4%) chose to undergo contralateral prophylactic mastectomy (CPM) after learning their mutation status, Jada G. Hamilton, Ph.D., reported at the annual meeting of the American Society of Preventive Oncology.
By comparison, 88% of eight BRCA1/2 carriers who participated in the study chose to undergo CPM, and neither of two women with a BRCA1/2 variant of undetermined significance chose to undergo CPM.
On multivariate analysis, the perception that one’s physician had recommended CPM was most strongly associated with the decision to undergo the procedure (odds ratio, 11.1), said Dr. Hamilton of Memorial Sloan-Kettering Cancer Center, New York.
Other factors associated with the decision were a perception of greater risk for contralateral breast cancer (OR, 6.46) and a perception of greater pros of CPM (OR, 1.37), she said, noting that those who indicated they would feel good about having CPM and those who indicated they might feel regret if they didn’t have CPM were most likely to elect CPM.
Age, Ashkenazi Jewish ethnicity, breast cancer–related distress, perceived cons of CPM (such as disfigurement and concerns regarding a negative impact on one’s sex life), and decisional conflict regarding CPM were not significantly associated with the decision.
Presurgical genetic testing provides valuable information to women with newly diagnosed breast cancer as they begin to make decisions about treatment, Dr. Hamilton said. Although BRCA1/2 mutation noncarriers have a low (3%-10%) risk, compared with carriers (27%-37%), studies suggest that about 18% nevertheless choose to undergo CPM.
The psychosocial factors that may affect the decision are not well understood, Dr. Hamilton said.
For the current analysis, participants who were part of a larger prospective study on presurgical BRCA1/2 testing completed a questionnaire, and the frequency and psychosocial correlates of the decision to undergo a CPM were assessed. The participants were adult women with a median age of 43 years (range of 29-59 years).
The findings raise interesting questions for future work, Dr. Hamilton said.
“I think it’s really critical for future studies to dig in to what’s happening in terms of physician-patient communication around CPM,” she said, noting that it will be important to explore how such communication interacts with a woman’s past experiences, emotions, and beliefs to shape her cancer prevention decisions.
Further, the women who undergo CPM should be followed to assess their long-term outcomes with respect to factors such as quality of life, satisfaction, and decisional regret, she concluded.
The decision to undergo CPM and the effects of physician-patient communication on that decision were also addressed in another study presented at the meeting.
In that study – a mixed methods pilot study looking mainly at factors affecting informed decision making in women who had ductal carcinoma in situ (DCIS) or who were considered to be at increased risk of invasive breast cancer risk because of a diagnosis of lobular carcinoma in situ (LCIS), BRCA positivity, or 20% or greater calculated lifetime risk – anxiety about cancer recurrence was the top reason for pursuing CPM.
Despite a lack of survival benefit, an increasing number of women with DCIS are undergoing CPM, but little is known about the decision-making process, said Jessica Valente of Emory University, Atlanta.
She and her colleagues sought to identify factors impacting risk comprehension and decision making. Of 68 women with DCIS or who were at high risk for development of invasive breast cancer, 33 considered CPM and 11 underwent the procedure.
The choice to undergo CPM was significantly associated with plastic surgery consultation, increased 10-year breast cancer risk, genetic counseling, genetic testing, and higher income, she said.
The study also highlighted a lack of health literacy and understanding of related terminology.
Most participants (nearly 84%) stated that DCIS qualified as breast cancer, but only about 40% correctly defined DCIS, Ms. Valente said.
When asked what they would recommend as a treatment strategy for a friend with DCIS, 35% thought surgery would be best. A similar percentage would recommend surgery for LCIS.
“When we looked at ductal hyperplasia, fewer people thought that that qualified as cancer, and they were more likely to recommend observation,” she said.
Further, when asked to interpret the phrase “indolent lesion of epithelial origin,” which is a phrase that has been promoted as a replacement for “DCIS” in light of concerns that women are increasingly electing CPM for DCIS because of fear of the word “carcinoma” despite a 99% survival rate, only 28% of patients believed it referred to cancer.
Observation was one of the highly recommended interventions for “indolent lesion of epithelial origin,” followed by biopsy, she said, noting that only 13% recommended surgery when this phrase was used.
“Interestingly, 7.4% said an oral or topical medication [should be used for “indolent lesion of epithelial origin]” – a finding likely explained by the fact that some women interpreted the word “lesion” to mean a wound or sore on the skin, she said.
Additionally, few women were able to define contralateral prophylactic mastectomy.
Overall, despite the study population being very well educated and from a higher socioeconomic status, they had low scores for understanding terminology (8.21 out of 20), Ms. Valente noted.
The findings demonstrate that decision making in the context of DCIS remains complex and underscore the importance of recognizing patients’ knowledge of risk communication and terminology for supporting shared and informed surgical decision making, she said.
The findings also demonstrate that while fewer women felt that surgery was appropriate when the term “indolent lesion of epithelial origin” was used instead of “DCIS,” the proposed new terminology doesn’t necessarily provide the desired clarity, she noted.
“They still came up with such a broad range of interpretations that we really might be introducing a new set of conflicts and confusion when we think about changing to that terminology when we talk to patients,” she concluded.
Dr. Hamilton and Ms. Valente each reported having no disclosures.
BIRMINGHAM, ALA. – The perception that one’s physician had recommended contralateral prophylactic mastectomy was a particularly important factor in the decision to undergo the procedure among BRCA1/2 mutation noncarriers with newly diagnosed breast cancer in a prospective study.
Of 90 BRCA noncarriers with newly diagnosed breast cancer, a “sizable minority” (24.4%) chose to undergo contralateral prophylactic mastectomy (CPM) after learning their mutation status, Jada G. Hamilton, Ph.D., reported at the annual meeting of the American Society of Preventive Oncology.
By comparison, 88% of eight BRCA1/2 carriers who participated in the study chose to undergo CPM, and neither of two women with a BRCA1/2 variant of undetermined significance chose to undergo CPM.
On multivariate analysis, the perception that one’s physician had recommended CPM was most strongly associated with the decision to undergo the procedure (odds ratio, 11.1), said Dr. Hamilton of Memorial Sloan-Kettering Cancer Center, New York.
Other factors associated with the decision were a perception of greater risk for contralateral breast cancer (OR, 6.46) and a perception of greater pros of CPM (OR, 1.37), she said, noting that those who indicated they would feel good about having CPM and those who indicated they might feel regret if they didn’t have CPM were most likely to elect CPM.
Age, Ashkenazi Jewish ethnicity, breast cancer–related distress, perceived cons of CPM (such as disfigurement and concerns regarding a negative impact on one’s sex life), and decisional conflict regarding CPM were not significantly associated with the decision.
Presurgical genetic testing provides valuable information to women with newly diagnosed breast cancer as they begin to make decisions about treatment, Dr. Hamilton said. Although BRCA1/2 mutation noncarriers have a low (3%-10%) risk, compared with carriers (27%-37%), studies suggest that about 18% nevertheless choose to undergo CPM.
The psychosocial factors that may affect the decision are not well understood, Dr. Hamilton said.
For the current analysis, participants who were part of a larger prospective study on presurgical BRCA1/2 testing completed a questionnaire, and the frequency and psychosocial correlates of the decision to undergo a CPM were assessed. The participants were adult women with a median age of 43 years (range of 29-59 years).
The findings raise interesting questions for future work, Dr. Hamilton said.
“I think it’s really critical for future studies to dig in to what’s happening in terms of physician-patient communication around CPM,” she said, noting that it will be important to explore how such communication interacts with a woman’s past experiences, emotions, and beliefs to shape her cancer prevention decisions.
Further, the women who undergo CPM should be followed to assess their long-term outcomes with respect to factors such as quality of life, satisfaction, and decisional regret, she concluded.
The decision to undergo CPM and the effects of physician-patient communication on that decision were also addressed in another study presented at the meeting.
In that study – a mixed methods pilot study looking mainly at factors affecting informed decision making in women who had ductal carcinoma in situ (DCIS) or who were considered to be at increased risk of invasive breast cancer risk because of a diagnosis of lobular carcinoma in situ (LCIS), BRCA positivity, or 20% or greater calculated lifetime risk – anxiety about cancer recurrence was the top reason for pursuing CPM.
Despite a lack of survival benefit, an increasing number of women with DCIS are undergoing CPM, but little is known about the decision-making process, said Jessica Valente of Emory University, Atlanta.
She and her colleagues sought to identify factors impacting risk comprehension and decision making. Of 68 women with DCIS or who were at high risk for development of invasive breast cancer, 33 considered CPM and 11 underwent the procedure.
The choice to undergo CPM was significantly associated with plastic surgery consultation, increased 10-year breast cancer risk, genetic counseling, genetic testing, and higher income, she said.
The study also highlighted a lack of health literacy and understanding of related terminology.
Most participants (nearly 84%) stated that DCIS qualified as breast cancer, but only about 40% correctly defined DCIS, Ms. Valente said.
When asked what they would recommend as a treatment strategy for a friend with DCIS, 35% thought surgery would be best. A similar percentage would recommend surgery for LCIS.
“When we looked at ductal hyperplasia, fewer people thought that that qualified as cancer, and they were more likely to recommend observation,” she said.
Further, when asked to interpret the phrase “indolent lesion of epithelial origin,” which is a phrase that has been promoted as a replacement for “DCIS” in light of concerns that women are increasingly electing CPM for DCIS because of fear of the word “carcinoma” despite a 99% survival rate, only 28% of patients believed it referred to cancer.
Observation was one of the highly recommended interventions for “indolent lesion of epithelial origin,” followed by biopsy, she said, noting that only 13% recommended surgery when this phrase was used.
“Interestingly, 7.4% said an oral or topical medication [should be used for “indolent lesion of epithelial origin]” – a finding likely explained by the fact that some women interpreted the word “lesion” to mean a wound or sore on the skin, she said.
Additionally, few women were able to define contralateral prophylactic mastectomy.
Overall, despite the study population being very well educated and from a higher socioeconomic status, they had low scores for understanding terminology (8.21 out of 20), Ms. Valente noted.
The findings demonstrate that decision making in the context of DCIS remains complex and underscore the importance of recognizing patients’ knowledge of risk communication and terminology for supporting shared and informed surgical decision making, she said.
The findings also demonstrate that while fewer women felt that surgery was appropriate when the term “indolent lesion of epithelial origin” was used instead of “DCIS,” the proposed new terminology doesn’t necessarily provide the desired clarity, she noted.
“They still came up with such a broad range of interpretations that we really might be introducing a new set of conflicts and confusion when we think about changing to that terminology when we talk to patients,” she concluded.
Dr. Hamilton and Ms. Valente each reported having no disclosures.
AT THE ASPO ANNUAL MEETING
Key clinical point: Physician-patient communication plays an important role in a woman’s decision to undergo CPM.
Major finding: Of 90 women with invasive breast cancer and without BRCA mutations, 24% chose to undergo CPM; of 68 women with DCIS, 33 considered CPM and 11 underwent the procedure.The choice to undergo CPM was significantly associated with the perception that one’s physician had recommended CPM in the first study, and with a plastic surgery consultation, increased 10-year breast cancer risk, genetic counseling, genetic testing, and higher income in the DCIS study.
Data source: A prospective study of 90 patients with invasive breast cancer and a mixed methods pilot study of 68 patients with DCIS.
Disclosures: Dr. Hamilton and Ms. Valente each reported having no disclosures.
Nurses’ Health Study: No link between depression and breast cancer
BIRMINGHAM, ALA. – Neither depression nor antidepressant use are associated with an increased risk of breast cancer among participants in the Nurses’ Health Study, according to an analysis of data from 67,120 women enrolled in the ongoing prospective cohort study.
Of the women included in the analysis, 2,904 had confirmed breast cancer as of the end of December 2012, including 2,333 with invasive disease. After adjusting for age, body mass index, and menopausal status, no statistically significant associations were seen between invasive or in situ breast cancer and depression or antidepressant use, Katherine W. Reeves, Ph.D. reported at the annual meeting of the American Society of Preventive Oncology.
The point estimates for the odds ratios were all below 1 for in situ disease, indicating a potential protective effect of depression, Dr. Reeves said.
“These were not statistically significant, so I would caution against overinterpreting the results, but it is kind of curious,” she said, noting that the finding may indicate that depressed women are less likely than nondepressed women are to have a mammogram – and thus are less likely to have the opportunity to be diagnosed with in situ disease.
When depression and antidepressant use were included together in the same model, they remained unassociated with breast cancer risk (odds ratio, 0.87), said Dr. Reeves of the University of Massachusetts Amherst.
Study subjects were an average age of 66 years, 8.7% were clinically depressed, and 9.7% used antidepressants. Data on depression and antidepressant use among Nurses’ Health Study participants were collected simultaneously beginning in 2000.
Depression and antidepressant use were self-reported, and depressive symptoms were confirmed using the five-item Mental Health Inventory.
The findings are encouraging; depression and antidepressant use are common and both have been hypothesized to increase breast cancer risk. Some prior studies have found a link between either depression or antidepressant use and breast cancer, and others have not – but most have had important limitations, including retrospective design and inclusion of major depression only, among others, she said.
“To me, though, the most important limitation is that previous studies have not evaluated depression and antidepressant use together,” Dr. Reeves said.
In the current study, which did consider both, no evidence was seen to suggest that depression or antidepressant use affects breast cancer risk.
“It’s typically very unexciting to have to report null results, but in this case, I think this is excellent news and really the best we could have hoped for,” she said, adding that although they are preliminary, the findings should be “very reassuring for the millions of women with depression and/or those using antidepressants.”
“Depression is a very serious medical condition. It deserves to be treated, and it’s nice that these women can take the antidepressants, which so effectively treat this condition without worry that they’re doing something that would adversely affect their breast cancer risk in the future,” she concluded, noting that more sophisticated analyses of the data are planned to consider additional variables, including treatment duration. The analyses will also be repeated in the Nurses’ Health Study II cohort, which is a younger cohort with a higher incidence of premenopausal breast cancer and a greater prevalence of both depression and antidepressant use.
BIRMINGHAM, ALA. – Neither depression nor antidepressant use are associated with an increased risk of breast cancer among participants in the Nurses’ Health Study, according to an analysis of data from 67,120 women enrolled in the ongoing prospective cohort study.
Of the women included in the analysis, 2,904 had confirmed breast cancer as of the end of December 2012, including 2,333 with invasive disease. After adjusting for age, body mass index, and menopausal status, no statistically significant associations were seen between invasive or in situ breast cancer and depression or antidepressant use, Katherine W. Reeves, Ph.D. reported at the annual meeting of the American Society of Preventive Oncology.
The point estimates for the odds ratios were all below 1 for in situ disease, indicating a potential protective effect of depression, Dr. Reeves said.
“These were not statistically significant, so I would caution against overinterpreting the results, but it is kind of curious,” she said, noting that the finding may indicate that depressed women are less likely than nondepressed women are to have a mammogram – and thus are less likely to have the opportunity to be diagnosed with in situ disease.
When depression and antidepressant use were included together in the same model, they remained unassociated with breast cancer risk (odds ratio, 0.87), said Dr. Reeves of the University of Massachusetts Amherst.
Study subjects were an average age of 66 years, 8.7% were clinically depressed, and 9.7% used antidepressants. Data on depression and antidepressant use among Nurses’ Health Study participants were collected simultaneously beginning in 2000.
Depression and antidepressant use were self-reported, and depressive symptoms were confirmed using the five-item Mental Health Inventory.
The findings are encouraging; depression and antidepressant use are common and both have been hypothesized to increase breast cancer risk. Some prior studies have found a link between either depression or antidepressant use and breast cancer, and others have not – but most have had important limitations, including retrospective design and inclusion of major depression only, among others, she said.
“To me, though, the most important limitation is that previous studies have not evaluated depression and antidepressant use together,” Dr. Reeves said.
In the current study, which did consider both, no evidence was seen to suggest that depression or antidepressant use affects breast cancer risk.
“It’s typically very unexciting to have to report null results, but in this case, I think this is excellent news and really the best we could have hoped for,” she said, adding that although they are preliminary, the findings should be “very reassuring for the millions of women with depression and/or those using antidepressants.”
“Depression is a very serious medical condition. It deserves to be treated, and it’s nice that these women can take the antidepressants, which so effectively treat this condition without worry that they’re doing something that would adversely affect their breast cancer risk in the future,” she concluded, noting that more sophisticated analyses of the data are planned to consider additional variables, including treatment duration. The analyses will also be repeated in the Nurses’ Health Study II cohort, which is a younger cohort with a higher incidence of premenopausal breast cancer and a greater prevalence of both depression and antidepressant use.
BIRMINGHAM, ALA. – Neither depression nor antidepressant use are associated with an increased risk of breast cancer among participants in the Nurses’ Health Study, according to an analysis of data from 67,120 women enrolled in the ongoing prospective cohort study.
Of the women included in the analysis, 2,904 had confirmed breast cancer as of the end of December 2012, including 2,333 with invasive disease. After adjusting for age, body mass index, and menopausal status, no statistically significant associations were seen between invasive or in situ breast cancer and depression or antidepressant use, Katherine W. Reeves, Ph.D. reported at the annual meeting of the American Society of Preventive Oncology.
The point estimates for the odds ratios were all below 1 for in situ disease, indicating a potential protective effect of depression, Dr. Reeves said.
“These were not statistically significant, so I would caution against overinterpreting the results, but it is kind of curious,” she said, noting that the finding may indicate that depressed women are less likely than nondepressed women are to have a mammogram – and thus are less likely to have the opportunity to be diagnosed with in situ disease.
When depression and antidepressant use were included together in the same model, they remained unassociated with breast cancer risk (odds ratio, 0.87), said Dr. Reeves of the University of Massachusetts Amherst.
Study subjects were an average age of 66 years, 8.7% were clinically depressed, and 9.7% used antidepressants. Data on depression and antidepressant use among Nurses’ Health Study participants were collected simultaneously beginning in 2000.
Depression and antidepressant use were self-reported, and depressive symptoms were confirmed using the five-item Mental Health Inventory.
The findings are encouraging; depression and antidepressant use are common and both have been hypothesized to increase breast cancer risk. Some prior studies have found a link between either depression or antidepressant use and breast cancer, and others have not – but most have had important limitations, including retrospective design and inclusion of major depression only, among others, she said.
“To me, though, the most important limitation is that previous studies have not evaluated depression and antidepressant use together,” Dr. Reeves said.
In the current study, which did consider both, no evidence was seen to suggest that depression or antidepressant use affects breast cancer risk.
“It’s typically very unexciting to have to report null results, but in this case, I think this is excellent news and really the best we could have hoped for,” she said, adding that although they are preliminary, the findings should be “very reassuring for the millions of women with depression and/or those using antidepressants.”
“Depression is a very serious medical condition. It deserves to be treated, and it’s nice that these women can take the antidepressants, which so effectively treat this condition without worry that they’re doing something that would adversely affect their breast cancer risk in the future,” she concluded, noting that more sophisticated analyses of the data are planned to consider additional variables, including treatment duration. The analyses will also be repeated in the Nurses’ Health Study II cohort, which is a younger cohort with a higher incidence of premenopausal breast cancer and a greater prevalence of both depression and antidepressant use.
AT THE ASPO ANNUAL MEETING
Key clinical point: Depression and antidepressant use do not appear to increase breast cancer risk.
Major finding: No statistically significant associations were seen between either invasive or in situ breast cancer and depression or antidepressant use.
Data source: 67,120 women fromthe Nurses’ Health Study.
Disclosures: The investigator reported no conflicts.