User login
‘Baby TAM’ effective, tolerable for breast cancer prevention
SAN ANTONIO — The drug can reduce incidence of breast cancer in high-risk individuals, but side effects that mimic menopause have led to low rates of uptake. Lower-dose tamoxifen aims to reduce those side effects, but there remains some uncertainty about the minimum dose required to maintain efficacy.
The TAM-01 study, first published in 2019, demonstrated that a 5-mg dose of tamoxifen led to a reduction in recurrence of invasive breast cancer or ductal carcinoma in situ (DCIS). At the San Antonio Breast Cancer Symposium, two studies were presented that provided insight into dose efficacy and likelihood of medication adherence in women taking baby TAM.
“We all know that women who are at increased risk for breast cancer may benefit from the use of tamoxifen to help lower their risk, although historical uptake to tamoxifen in the prevention setting has been quite low,” said Lauren Cornell, MD, during a presentation. Her team investigated the impact of patient counseling on how well they understood their risk, as well as their likelihood of adherence to the medication.
The study included 41 women, and 31 completed follow-up at 1 year. “We saw that 90% of our patients reported good or complete understanding of their breast cancer risk after the consultation, emphasizing the benefit of that consult, and 73% reported that the availability of baby tamoxifen helped in their decision to consider a preventative medication,” said Dr. Cornell during her presentation. After 1 year of follow-up, 74% said that they had initiated baby tamoxifen, and 78% of those who started taking the drug were still taking it at 1 year.
Participants who continued to take baby TAM at 1 year had a higher estimated breast cancer risk (IBIS 10-year risk, 12.7% vs 7.6%; P = .027) than those who discontinued. “We saw that uptake to baby TAM after informed discussion in patients who qualify is high, especially in those patients with high risk and intraepithelial lesions or DCIS, and adherence and tolerability at 1 year follow up is improved, compared to what we would expect with traditional dosing of tamoxifen. It’s important to note that the NCCN guidelines and the ASCO clinical practice update now include low-dose tamoxifen as an option for select women, and future randomized control trials on de-escalation of tamoxifen and high-risk patients based on their risk model assessment still need to be done. Future study should also focus on markers to identify candidates best suited for low versus standard dose of tamoxifen,” said Dr. Cornell, who is an assistant professor of medicine at Mayo Clinic Florida in Jacksonville.
At another SABCS session, Per Hall, MD, PhD, discussed findings from the previously published KARISMA-2 study, which examined efficacy of various doses of tamoxifen. A total of 1440 participants, 240 in each arm, received tamoxifen doses of 20 mg, 10 mg, 5 mg, 2.5 mg, 1 mg, or placebo. During his talk, Dr. Hall pointed out that measuring outcomes would take a very large number of participants to identify small differences in breast cancer rates. Therefore, the researchers examined breast density changes as a proxy. As a noninferiority outcome, the researchers used the proportion of women in each arm who achieved the median decrease in breast density seen at 20 mg of tamoxifen, which is 10.1%.
The women underwent mammograms at baseline and again at 6 months to determine change in breast density. Among all women in the study, the proportion of patients who had a similar breast density reduction as the 20-mg dose were very similar in the 10 mg (50.0%; P = .002), 5 mg (49.3%; P < .001), and 2.5 mg (52.5%; P < .001) groups. The 1 mg group had a proportion of 39.5% (P = .138), while the placebo group had 38.9% (P = .161). However, the results were driven by premenopausal women, where the values were 63.3%, 70.7%, 74.4%, and 69.7% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, respectively, and 32.9% at 1 mg and 29.7% on placebo. In postmenopausal women, the values were 41.9%, 36.7%, 33.3%, and 41.9% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, with values of 44.2% in the 1-mg group and 43.8% in the placebo group.
The median density change was 18.5% in premenopausal women and 4.0% in postmenopausal women.
“We didn’t see anything in the postmenopausal women. The decrease for those on 20 milligrams and those on placebo were exactly the same. Why this is, we still don’t know because we do know that tamoxifen in the adjuvant setting could be used for postmenopausal women. It could be that 6 months is too short of a time [to see a benefit]. We don’t know,” said Dr. Hall, who is a medical epidemiologist and biostatistician at Karolinska Institutet, Stockholm, Sweden.
Severe vasomotor side effects like hot flashes, cold flashes, and night sweats were reduced by about 50% in the lower tamoxifen doses, compared with 20 mg.
Dr. Hall also pointed out that tamoxifen is a prodrug. The CYP2D6 enzyme produces a range of metabolites, with endoxifen having the strongest affinity to the estrogen receptor and being present at the highest plasma concentration. He showed a table of endoxifen plasma levels at various tamoxifen doses in women of various metabolizer status, ranging from poor to ultrafast. Among intermediate, normal, and ultrarapid metabolizers, 5- and 10-mg doses produced plasma endoxifen levels ranging from 2.4 to 6.2 ng/mL, which represents a good therapeutic window. “For intermediate and normal metabolizers, it could be that 5 mg [of tamoxifen] is enough, but I want to underline that we didn’t use breast cancer incidence or recurrence in this study, we used density change, so we should be careful when we use these results,” said Dr. Hall. His group is now conducting the KARISMA Endoxifen trial, which will test endoxifen directly at doses of 1 and 2 mg.
Dr. Cornell has no relevant financial disclosures. Dr. Hall is a member of the scientific advisory board for Atossa Therapeutics.
SAN ANTONIO — The drug can reduce incidence of breast cancer in high-risk individuals, but side effects that mimic menopause have led to low rates of uptake. Lower-dose tamoxifen aims to reduce those side effects, but there remains some uncertainty about the minimum dose required to maintain efficacy.
The TAM-01 study, first published in 2019, demonstrated that a 5-mg dose of tamoxifen led to a reduction in recurrence of invasive breast cancer or ductal carcinoma in situ (DCIS). At the San Antonio Breast Cancer Symposium, two studies were presented that provided insight into dose efficacy and likelihood of medication adherence in women taking baby TAM.
“We all know that women who are at increased risk for breast cancer may benefit from the use of tamoxifen to help lower their risk, although historical uptake to tamoxifen in the prevention setting has been quite low,” said Lauren Cornell, MD, during a presentation. Her team investigated the impact of patient counseling on how well they understood their risk, as well as their likelihood of adherence to the medication.
The study included 41 women, and 31 completed follow-up at 1 year. “We saw that 90% of our patients reported good or complete understanding of their breast cancer risk after the consultation, emphasizing the benefit of that consult, and 73% reported that the availability of baby tamoxifen helped in their decision to consider a preventative medication,” said Dr. Cornell during her presentation. After 1 year of follow-up, 74% said that they had initiated baby tamoxifen, and 78% of those who started taking the drug were still taking it at 1 year.
Participants who continued to take baby TAM at 1 year had a higher estimated breast cancer risk (IBIS 10-year risk, 12.7% vs 7.6%; P = .027) than those who discontinued. “We saw that uptake to baby TAM after informed discussion in patients who qualify is high, especially in those patients with high risk and intraepithelial lesions or DCIS, and adherence and tolerability at 1 year follow up is improved, compared to what we would expect with traditional dosing of tamoxifen. It’s important to note that the NCCN guidelines and the ASCO clinical practice update now include low-dose tamoxifen as an option for select women, and future randomized control trials on de-escalation of tamoxifen and high-risk patients based on their risk model assessment still need to be done. Future study should also focus on markers to identify candidates best suited for low versus standard dose of tamoxifen,” said Dr. Cornell, who is an assistant professor of medicine at Mayo Clinic Florida in Jacksonville.
At another SABCS session, Per Hall, MD, PhD, discussed findings from the previously published KARISMA-2 study, which examined efficacy of various doses of tamoxifen. A total of 1440 participants, 240 in each arm, received tamoxifen doses of 20 mg, 10 mg, 5 mg, 2.5 mg, 1 mg, or placebo. During his talk, Dr. Hall pointed out that measuring outcomes would take a very large number of participants to identify small differences in breast cancer rates. Therefore, the researchers examined breast density changes as a proxy. As a noninferiority outcome, the researchers used the proportion of women in each arm who achieved the median decrease in breast density seen at 20 mg of tamoxifen, which is 10.1%.
The women underwent mammograms at baseline and again at 6 months to determine change in breast density. Among all women in the study, the proportion of patients who had a similar breast density reduction as the 20-mg dose were very similar in the 10 mg (50.0%; P = .002), 5 mg (49.3%; P < .001), and 2.5 mg (52.5%; P < .001) groups. The 1 mg group had a proportion of 39.5% (P = .138), while the placebo group had 38.9% (P = .161). However, the results were driven by premenopausal women, where the values were 63.3%, 70.7%, 74.4%, and 69.7% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, respectively, and 32.9% at 1 mg and 29.7% on placebo. In postmenopausal women, the values were 41.9%, 36.7%, 33.3%, and 41.9% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, with values of 44.2% in the 1-mg group and 43.8% in the placebo group.
The median density change was 18.5% in premenopausal women and 4.0% in postmenopausal women.
“We didn’t see anything in the postmenopausal women. The decrease for those on 20 milligrams and those on placebo were exactly the same. Why this is, we still don’t know because we do know that tamoxifen in the adjuvant setting could be used for postmenopausal women. It could be that 6 months is too short of a time [to see a benefit]. We don’t know,” said Dr. Hall, who is a medical epidemiologist and biostatistician at Karolinska Institutet, Stockholm, Sweden.
Severe vasomotor side effects like hot flashes, cold flashes, and night sweats were reduced by about 50% in the lower tamoxifen doses, compared with 20 mg.
Dr. Hall also pointed out that tamoxifen is a prodrug. The CYP2D6 enzyme produces a range of metabolites, with endoxifen having the strongest affinity to the estrogen receptor and being present at the highest plasma concentration. He showed a table of endoxifen plasma levels at various tamoxifen doses in women of various metabolizer status, ranging from poor to ultrafast. Among intermediate, normal, and ultrarapid metabolizers, 5- and 10-mg doses produced plasma endoxifen levels ranging from 2.4 to 6.2 ng/mL, which represents a good therapeutic window. “For intermediate and normal metabolizers, it could be that 5 mg [of tamoxifen] is enough, but I want to underline that we didn’t use breast cancer incidence or recurrence in this study, we used density change, so we should be careful when we use these results,” said Dr. Hall. His group is now conducting the KARISMA Endoxifen trial, which will test endoxifen directly at doses of 1 and 2 mg.
Dr. Cornell has no relevant financial disclosures. Dr. Hall is a member of the scientific advisory board for Atossa Therapeutics.
SAN ANTONIO — The drug can reduce incidence of breast cancer in high-risk individuals, but side effects that mimic menopause have led to low rates of uptake. Lower-dose tamoxifen aims to reduce those side effects, but there remains some uncertainty about the minimum dose required to maintain efficacy.
The TAM-01 study, first published in 2019, demonstrated that a 5-mg dose of tamoxifen led to a reduction in recurrence of invasive breast cancer or ductal carcinoma in situ (DCIS). At the San Antonio Breast Cancer Symposium, two studies were presented that provided insight into dose efficacy and likelihood of medication adherence in women taking baby TAM.
“We all know that women who are at increased risk for breast cancer may benefit from the use of tamoxifen to help lower their risk, although historical uptake to tamoxifen in the prevention setting has been quite low,” said Lauren Cornell, MD, during a presentation. Her team investigated the impact of patient counseling on how well they understood their risk, as well as their likelihood of adherence to the medication.
The study included 41 women, and 31 completed follow-up at 1 year. “We saw that 90% of our patients reported good or complete understanding of their breast cancer risk after the consultation, emphasizing the benefit of that consult, and 73% reported that the availability of baby tamoxifen helped in their decision to consider a preventative medication,” said Dr. Cornell during her presentation. After 1 year of follow-up, 74% said that they had initiated baby tamoxifen, and 78% of those who started taking the drug were still taking it at 1 year.
Participants who continued to take baby TAM at 1 year had a higher estimated breast cancer risk (IBIS 10-year risk, 12.7% vs 7.6%; P = .027) than those who discontinued. “We saw that uptake to baby TAM after informed discussion in patients who qualify is high, especially in those patients with high risk and intraepithelial lesions or DCIS, and adherence and tolerability at 1 year follow up is improved, compared to what we would expect with traditional dosing of tamoxifen. It’s important to note that the NCCN guidelines and the ASCO clinical practice update now include low-dose tamoxifen as an option for select women, and future randomized control trials on de-escalation of tamoxifen and high-risk patients based on their risk model assessment still need to be done. Future study should also focus on markers to identify candidates best suited for low versus standard dose of tamoxifen,” said Dr. Cornell, who is an assistant professor of medicine at Mayo Clinic Florida in Jacksonville.
At another SABCS session, Per Hall, MD, PhD, discussed findings from the previously published KARISMA-2 study, which examined efficacy of various doses of tamoxifen. A total of 1440 participants, 240 in each arm, received tamoxifen doses of 20 mg, 10 mg, 5 mg, 2.5 mg, 1 mg, or placebo. During his talk, Dr. Hall pointed out that measuring outcomes would take a very large number of participants to identify small differences in breast cancer rates. Therefore, the researchers examined breast density changes as a proxy. As a noninferiority outcome, the researchers used the proportion of women in each arm who achieved the median decrease in breast density seen at 20 mg of tamoxifen, which is 10.1%.
The women underwent mammograms at baseline and again at 6 months to determine change in breast density. Among all women in the study, the proportion of patients who had a similar breast density reduction as the 20-mg dose were very similar in the 10 mg (50.0%; P = .002), 5 mg (49.3%; P < .001), and 2.5 mg (52.5%; P < .001) groups. The 1 mg group had a proportion of 39.5% (P = .138), while the placebo group had 38.9% (P = .161). However, the results were driven by premenopausal women, where the values were 63.3%, 70.7%, 74.4%, and 69.7% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, respectively, and 32.9% at 1 mg and 29.7% on placebo. In postmenopausal women, the values were 41.9%, 36.7%, 33.3%, and 41.9% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, with values of 44.2% in the 1-mg group and 43.8% in the placebo group.
The median density change was 18.5% in premenopausal women and 4.0% in postmenopausal women.
“We didn’t see anything in the postmenopausal women. The decrease for those on 20 milligrams and those on placebo were exactly the same. Why this is, we still don’t know because we do know that tamoxifen in the adjuvant setting could be used for postmenopausal women. It could be that 6 months is too short of a time [to see a benefit]. We don’t know,” said Dr. Hall, who is a medical epidemiologist and biostatistician at Karolinska Institutet, Stockholm, Sweden.
Severe vasomotor side effects like hot flashes, cold flashes, and night sweats were reduced by about 50% in the lower tamoxifen doses, compared with 20 mg.
Dr. Hall also pointed out that tamoxifen is a prodrug. The CYP2D6 enzyme produces a range of metabolites, with endoxifen having the strongest affinity to the estrogen receptor and being present at the highest plasma concentration. He showed a table of endoxifen plasma levels at various tamoxifen doses in women of various metabolizer status, ranging from poor to ultrafast. Among intermediate, normal, and ultrarapid metabolizers, 5- and 10-mg doses produced plasma endoxifen levels ranging from 2.4 to 6.2 ng/mL, which represents a good therapeutic window. “For intermediate and normal metabolizers, it could be that 5 mg [of tamoxifen] is enough, but I want to underline that we didn’t use breast cancer incidence or recurrence in this study, we used density change, so we should be careful when we use these results,” said Dr. Hall. His group is now conducting the KARISMA Endoxifen trial, which will test endoxifen directly at doses of 1 and 2 mg.
Dr. Cornell has no relevant financial disclosures. Dr. Hall is a member of the scientific advisory board for Atossa Therapeutics.
FROM SABCS 2023
Living in a Food Swamp Tied to High Breast Cancer Mortality
SAN ANTONIO — a novel ecological study has found.
“Food deserts and food swamps are both bad, but it’s worse in food swamps,” Malcolm Bevel, PhD, MSPH, with Augusta University in Georgia, said in an interview.
He presented his research at the San Antonio Breast Cancer Symposium.
Breast cancer is the fourth leading cause of cancer death in the United States and is one of 13 obesity-related cancers. Healthy food consumption is a protective factor shown to decrease obesity risk and postmenopausal breast cancer mortality.
However, residing in food deserts or food swamps reduces access to healthy foods and has been severely understudied regarding postmenopausal breast cancer mortality, Dr. Bevel explained.
To investigate, Dr. Bevel and colleagues did a cross-sectional, ecological analysis where they merged 2010 to 2020 postmenopausal breast cancer mortality data from the Centers for Disease Control and Prevention (CDC) with aggregated 2012 to 2020 data from the US Department of Agriculture Food Environment Atlas.
A food swamp score was calculated as the ratio of fast-food and convenience stores to grocery stores and farmer’s markets.
A food desert score was calculated as the proportion of residents living more than 1 mile (urban) or 10 miles (rural) from a grocery store and household income ≤ 200% of the federal poverty threshold.
The researchers categorized food deserts and food swamps as low, moderate, or high, with higher scores denoting counties with fewer resources for healthy food.
Counties with high postmenopausal breast cancer mortality rates had a higher percentage of non-Hispanic Black population (5.8% vs. 2.1%), poverty rates (17.2% vs 14.2%), and adult obesity (32.5% vs 32%) and diabetes rates (11.8% vs 10.5%), compared with counties with low postmenopausal breast cancer mortality rates, Dr. Bevel reported.
The age-adjusted odds of counties having high postmenopausal breast cancer mortality was 53% higher in counties with high food desert scores (adjusted odds ratio [aOR] 1.53; 95% CI, 1.26 - 1.88), and over twofold higher in those with high food swamp scores (aOR, 2.09; 95% CI: 1.69 - 2.58).
In fully adjusted models, the likelihood of counties having moderate postmenopausal breast cancer mortality rates was 32% higher in those with moderate food swamp scores (aOR, 1.32; 95% CI, 1.03 - 1.70).
Growing Epidemic Requires System Change
These findings are in line with another study by Dr. Bevel and his colleagues published earlier this year in JAMA Oncology.
In that study, communities with easy access to fast food were 77% more likely to have high levels of obesity-related cancer mortality, as reported by this news organization.
There is a “growing epidemic” of food deserts and food swamps in the US, which could be due to systemic issues such as gentrification/redlining and lack of investment with chain grocery stores that provide healthy food options, said Dr. Bevel.
Local policymakers and community stakeholders could implement culturally tailored, sustainable interventions for obesity and obesity-related cancer prevention, including postmenopausal breast cancer. These could include creating more walkable neighborhoods and community vegetable gardens, he suggested.
“This is an important study demonstrating how the environment impacts outcomes in postmenopausal women diagnosed with breast cancer,” said Lia Scott, PhD, MPH, discussant for the study.
“Most of the literature is primarily focused on food deserts to characterize the food environment. However, these authors looked at both food deserts and food swamps. And even after adjusting for various factors and age, counties with high food swamp scores at greater odds of having higher postmenopausal breast cancer mortality rates,” said Dr. Scott, who is from Georgia State University School of Public Health in Atlanta.
“There is a clear need for systems change. With ecological studies like this one, we could potentially drive policy by providing actionable data,” she added.
The study had no specific funding. Dr. Bevel and Dr. Scott report no relevant financial relationships.
A version of this article appeared on Medscape.com.
SAN ANTONIO — a novel ecological study has found.
“Food deserts and food swamps are both bad, but it’s worse in food swamps,” Malcolm Bevel, PhD, MSPH, with Augusta University in Georgia, said in an interview.
He presented his research at the San Antonio Breast Cancer Symposium.
Breast cancer is the fourth leading cause of cancer death in the United States and is one of 13 obesity-related cancers. Healthy food consumption is a protective factor shown to decrease obesity risk and postmenopausal breast cancer mortality.
However, residing in food deserts or food swamps reduces access to healthy foods and has been severely understudied regarding postmenopausal breast cancer mortality, Dr. Bevel explained.
To investigate, Dr. Bevel and colleagues did a cross-sectional, ecological analysis where they merged 2010 to 2020 postmenopausal breast cancer mortality data from the Centers for Disease Control and Prevention (CDC) with aggregated 2012 to 2020 data from the US Department of Agriculture Food Environment Atlas.
A food swamp score was calculated as the ratio of fast-food and convenience stores to grocery stores and farmer’s markets.
A food desert score was calculated as the proportion of residents living more than 1 mile (urban) or 10 miles (rural) from a grocery store and household income ≤ 200% of the federal poverty threshold.
The researchers categorized food deserts and food swamps as low, moderate, or high, with higher scores denoting counties with fewer resources for healthy food.
Counties with high postmenopausal breast cancer mortality rates had a higher percentage of non-Hispanic Black population (5.8% vs. 2.1%), poverty rates (17.2% vs 14.2%), and adult obesity (32.5% vs 32%) and diabetes rates (11.8% vs 10.5%), compared with counties with low postmenopausal breast cancer mortality rates, Dr. Bevel reported.
The age-adjusted odds of counties having high postmenopausal breast cancer mortality was 53% higher in counties with high food desert scores (adjusted odds ratio [aOR] 1.53; 95% CI, 1.26 - 1.88), and over twofold higher in those with high food swamp scores (aOR, 2.09; 95% CI: 1.69 - 2.58).
In fully adjusted models, the likelihood of counties having moderate postmenopausal breast cancer mortality rates was 32% higher in those with moderate food swamp scores (aOR, 1.32; 95% CI, 1.03 - 1.70).
Growing Epidemic Requires System Change
These findings are in line with another study by Dr. Bevel and his colleagues published earlier this year in JAMA Oncology.
In that study, communities with easy access to fast food were 77% more likely to have high levels of obesity-related cancer mortality, as reported by this news organization.
There is a “growing epidemic” of food deserts and food swamps in the US, which could be due to systemic issues such as gentrification/redlining and lack of investment with chain grocery stores that provide healthy food options, said Dr. Bevel.
Local policymakers and community stakeholders could implement culturally tailored, sustainable interventions for obesity and obesity-related cancer prevention, including postmenopausal breast cancer. These could include creating more walkable neighborhoods and community vegetable gardens, he suggested.
“This is an important study demonstrating how the environment impacts outcomes in postmenopausal women diagnosed with breast cancer,” said Lia Scott, PhD, MPH, discussant for the study.
“Most of the literature is primarily focused on food deserts to characterize the food environment. However, these authors looked at both food deserts and food swamps. And even after adjusting for various factors and age, counties with high food swamp scores at greater odds of having higher postmenopausal breast cancer mortality rates,” said Dr. Scott, who is from Georgia State University School of Public Health in Atlanta.
“There is a clear need for systems change. With ecological studies like this one, we could potentially drive policy by providing actionable data,” she added.
The study had no specific funding. Dr. Bevel and Dr. Scott report no relevant financial relationships.
A version of this article appeared on Medscape.com.
SAN ANTONIO — a novel ecological study has found.
“Food deserts and food swamps are both bad, but it’s worse in food swamps,” Malcolm Bevel, PhD, MSPH, with Augusta University in Georgia, said in an interview.
He presented his research at the San Antonio Breast Cancer Symposium.
Breast cancer is the fourth leading cause of cancer death in the United States and is one of 13 obesity-related cancers. Healthy food consumption is a protective factor shown to decrease obesity risk and postmenopausal breast cancer mortality.
However, residing in food deserts or food swamps reduces access to healthy foods and has been severely understudied regarding postmenopausal breast cancer mortality, Dr. Bevel explained.
To investigate, Dr. Bevel and colleagues did a cross-sectional, ecological analysis where they merged 2010 to 2020 postmenopausal breast cancer mortality data from the Centers for Disease Control and Prevention (CDC) with aggregated 2012 to 2020 data from the US Department of Agriculture Food Environment Atlas.
A food swamp score was calculated as the ratio of fast-food and convenience stores to grocery stores and farmer’s markets.
A food desert score was calculated as the proportion of residents living more than 1 mile (urban) or 10 miles (rural) from a grocery store and household income ≤ 200% of the federal poverty threshold.
The researchers categorized food deserts and food swamps as low, moderate, or high, with higher scores denoting counties with fewer resources for healthy food.
Counties with high postmenopausal breast cancer mortality rates had a higher percentage of non-Hispanic Black population (5.8% vs. 2.1%), poverty rates (17.2% vs 14.2%), and adult obesity (32.5% vs 32%) and diabetes rates (11.8% vs 10.5%), compared with counties with low postmenopausal breast cancer mortality rates, Dr. Bevel reported.
The age-adjusted odds of counties having high postmenopausal breast cancer mortality was 53% higher in counties with high food desert scores (adjusted odds ratio [aOR] 1.53; 95% CI, 1.26 - 1.88), and over twofold higher in those with high food swamp scores (aOR, 2.09; 95% CI: 1.69 - 2.58).
In fully adjusted models, the likelihood of counties having moderate postmenopausal breast cancer mortality rates was 32% higher in those with moderate food swamp scores (aOR, 1.32; 95% CI, 1.03 - 1.70).
Growing Epidemic Requires System Change
These findings are in line with another study by Dr. Bevel and his colleagues published earlier this year in JAMA Oncology.
In that study, communities with easy access to fast food were 77% more likely to have high levels of obesity-related cancer mortality, as reported by this news organization.
There is a “growing epidemic” of food deserts and food swamps in the US, which could be due to systemic issues such as gentrification/redlining and lack of investment with chain grocery stores that provide healthy food options, said Dr. Bevel.
Local policymakers and community stakeholders could implement culturally tailored, sustainable interventions for obesity and obesity-related cancer prevention, including postmenopausal breast cancer. These could include creating more walkable neighborhoods and community vegetable gardens, he suggested.
“This is an important study demonstrating how the environment impacts outcomes in postmenopausal women diagnosed with breast cancer,” said Lia Scott, PhD, MPH, discussant for the study.
“Most of the literature is primarily focused on food deserts to characterize the food environment. However, these authors looked at both food deserts and food swamps. And even after adjusting for various factors and age, counties with high food swamp scores at greater odds of having higher postmenopausal breast cancer mortality rates,” said Dr. Scott, who is from Georgia State University School of Public Health in Atlanta.
“There is a clear need for systems change. With ecological studies like this one, we could potentially drive policy by providing actionable data,” she added.
The study had no specific funding. Dr. Bevel and Dr. Scott report no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM SABCS 2023
Oncotype Score Helps Avoid Unnecessary Radiation in DCIS
SAN ANTONIO — There’s a long-standing concern among oncologists that many women with ductal carcinoma in situ (DCIS), a potential precursor to invasive breast cancer, receive more treatment than they need. The potential for overtreatment largely revolves around the extent of surgery and the use of radiation.
Using the Oncotype DX Breast DCIS Score test, a laboratory test that estimates DCIS recurrence risk, may help identify patients with low-risk DCIS who can safely avoid adjuvant radiation after surgery, according to new research (abstract GS03-01) presented at the San Antonio Breast Cancer Symposium.
Low-risk patients who skipped adjuvant radiotherapy after breast-conserving surgery demonstrated similar 5-year recurrence rates compared with high-risk patients who received adjuvant radiotherapy.
This is the first prospective study to evaluate radiation decisions among patients with DCIS.
Lead author Seema A. Khan, MD, who presented the research, called the findings “reassuring.”
However, “we need larger and better trials” as well as longer follow-up to confirm this less-is-more approach, said Dr. Khan, a breast cancer surgeon and researcher at Northwestern University, Chicago.
Virginia Kaklamani, MD, who moderated the presentation, noted that it is good to finally have prospective data on this topic. And although they are not definitive, “I personally think these results should be used” for counseling, said Dr. Kaklamani, leader of the breast cancer program at UT Health San Antonio.
To reduce the risk for DCIS recurrence or progression to invasive breast cancer, most patients with DCIS undergo breast-conserving surgery followed by adjuvant radiotherapy, Dr. Khan explained. Instead of breast-conserving surgery, about one in four patients opt for mastectomy.
Earlier results from this trial revealed that MRI helped identify patients who can safely receive breast-conserving surgery instead of mastectomy.
The current results assessed whether the Oncotype DX score can guide radiation treatment decisions.
The study included 171 patients with DCIS who had wide local excisions after MRI confirmed that they could forgo more extensive surgery.
Surgical specimens were then sent for testing to determine the DCIS score using the 12-gene Oncotype DX test.
Women who scored < 39 points on the 100-point Oncotype DX scale were considered to be at low risk for recurrence and were advised to skip radiation. Women who scored > 39 were advised to undergo radiation. Overall, 93% of the patients followed the radiation recommendations: 75 of 82 patients (91.4%) deemed as low risk skipped adjuvant radiotherapy and 84 of 89 patients (94.4%) deemed as high risk had radiotherapy.
At a median follow-up of 5 years, 5.1% (4 of 82) of low-risk patients experienced a recurrence vs. 4.5% (4 of 89) of higher-risk patients.
Recurrence rates among patients who followed the radiation recommendations mirrored these overall findings: 5.5% of 75 patients with low-risk DCIS who skipped radiotherapy experienced disease recurrence vs. 4.8% of 84 patients with high-risk DCIS who received radiotherapy.
Age did not appear to affect the outcomes. Among the 33 women younger than 50 years, two experienced a recurrence (4%), both invasive. One occurred in the low-risk group and the other in the higher-risk group. Among the 138 older women, six had recurrences, three in each group, and one recurrence in each was invasive.
In short, “women who skipped radiation based on this score did not experience an excess risk of” ipsilateral recurrence over 5 years, said Dr. Khan.
Overall, the study offers “strong evidence” that the DCIS score might help “prevent excessive treatment for some patients,” she concluded, adding that 10-year outcomes will be reported.
The work was funded by the National Cancer Institute. Dr. Khan has no conflicts of interest. Dr. Kaklamani has extensive industry ties, including being a speaker for Pfizer, Genentech, Novartis, and AstraZeneca.
A version in the article appeared on Medscape.com.
SAN ANTONIO — There’s a long-standing concern among oncologists that many women with ductal carcinoma in situ (DCIS), a potential precursor to invasive breast cancer, receive more treatment than they need. The potential for overtreatment largely revolves around the extent of surgery and the use of radiation.
Using the Oncotype DX Breast DCIS Score test, a laboratory test that estimates DCIS recurrence risk, may help identify patients with low-risk DCIS who can safely avoid adjuvant radiation after surgery, according to new research (abstract GS03-01) presented at the San Antonio Breast Cancer Symposium.
Low-risk patients who skipped adjuvant radiotherapy after breast-conserving surgery demonstrated similar 5-year recurrence rates compared with high-risk patients who received adjuvant radiotherapy.
This is the first prospective study to evaluate radiation decisions among patients with DCIS.
Lead author Seema A. Khan, MD, who presented the research, called the findings “reassuring.”
However, “we need larger and better trials” as well as longer follow-up to confirm this less-is-more approach, said Dr. Khan, a breast cancer surgeon and researcher at Northwestern University, Chicago.
Virginia Kaklamani, MD, who moderated the presentation, noted that it is good to finally have prospective data on this topic. And although they are not definitive, “I personally think these results should be used” for counseling, said Dr. Kaklamani, leader of the breast cancer program at UT Health San Antonio.
To reduce the risk for DCIS recurrence or progression to invasive breast cancer, most patients with DCIS undergo breast-conserving surgery followed by adjuvant radiotherapy, Dr. Khan explained. Instead of breast-conserving surgery, about one in four patients opt for mastectomy.
Earlier results from this trial revealed that MRI helped identify patients who can safely receive breast-conserving surgery instead of mastectomy.
The current results assessed whether the Oncotype DX score can guide radiation treatment decisions.
The study included 171 patients with DCIS who had wide local excisions after MRI confirmed that they could forgo more extensive surgery.
Surgical specimens were then sent for testing to determine the DCIS score using the 12-gene Oncotype DX test.
Women who scored < 39 points on the 100-point Oncotype DX scale were considered to be at low risk for recurrence and were advised to skip radiation. Women who scored > 39 were advised to undergo radiation. Overall, 93% of the patients followed the radiation recommendations: 75 of 82 patients (91.4%) deemed as low risk skipped adjuvant radiotherapy and 84 of 89 patients (94.4%) deemed as high risk had radiotherapy.
At a median follow-up of 5 years, 5.1% (4 of 82) of low-risk patients experienced a recurrence vs. 4.5% (4 of 89) of higher-risk patients.
Recurrence rates among patients who followed the radiation recommendations mirrored these overall findings: 5.5% of 75 patients with low-risk DCIS who skipped radiotherapy experienced disease recurrence vs. 4.8% of 84 patients with high-risk DCIS who received radiotherapy.
Age did not appear to affect the outcomes. Among the 33 women younger than 50 years, two experienced a recurrence (4%), both invasive. One occurred in the low-risk group and the other in the higher-risk group. Among the 138 older women, six had recurrences, three in each group, and one recurrence in each was invasive.
In short, “women who skipped radiation based on this score did not experience an excess risk of” ipsilateral recurrence over 5 years, said Dr. Khan.
Overall, the study offers “strong evidence” that the DCIS score might help “prevent excessive treatment for some patients,” she concluded, adding that 10-year outcomes will be reported.
The work was funded by the National Cancer Institute. Dr. Khan has no conflicts of interest. Dr. Kaklamani has extensive industry ties, including being a speaker for Pfizer, Genentech, Novartis, and AstraZeneca.
A version in the article appeared on Medscape.com.
SAN ANTONIO — There’s a long-standing concern among oncologists that many women with ductal carcinoma in situ (DCIS), a potential precursor to invasive breast cancer, receive more treatment than they need. The potential for overtreatment largely revolves around the extent of surgery and the use of radiation.
Using the Oncotype DX Breast DCIS Score test, a laboratory test that estimates DCIS recurrence risk, may help identify patients with low-risk DCIS who can safely avoid adjuvant radiation after surgery, according to new research (abstract GS03-01) presented at the San Antonio Breast Cancer Symposium.
Low-risk patients who skipped adjuvant radiotherapy after breast-conserving surgery demonstrated similar 5-year recurrence rates compared with high-risk patients who received adjuvant radiotherapy.
This is the first prospective study to evaluate radiation decisions among patients with DCIS.
Lead author Seema A. Khan, MD, who presented the research, called the findings “reassuring.”
However, “we need larger and better trials” as well as longer follow-up to confirm this less-is-more approach, said Dr. Khan, a breast cancer surgeon and researcher at Northwestern University, Chicago.
Virginia Kaklamani, MD, who moderated the presentation, noted that it is good to finally have prospective data on this topic. And although they are not definitive, “I personally think these results should be used” for counseling, said Dr. Kaklamani, leader of the breast cancer program at UT Health San Antonio.
To reduce the risk for DCIS recurrence or progression to invasive breast cancer, most patients with DCIS undergo breast-conserving surgery followed by adjuvant radiotherapy, Dr. Khan explained. Instead of breast-conserving surgery, about one in four patients opt for mastectomy.
Earlier results from this trial revealed that MRI helped identify patients who can safely receive breast-conserving surgery instead of mastectomy.
The current results assessed whether the Oncotype DX score can guide radiation treatment decisions.
The study included 171 patients with DCIS who had wide local excisions after MRI confirmed that they could forgo more extensive surgery.
Surgical specimens were then sent for testing to determine the DCIS score using the 12-gene Oncotype DX test.
Women who scored < 39 points on the 100-point Oncotype DX scale were considered to be at low risk for recurrence and were advised to skip radiation. Women who scored > 39 were advised to undergo radiation. Overall, 93% of the patients followed the radiation recommendations: 75 of 82 patients (91.4%) deemed as low risk skipped adjuvant radiotherapy and 84 of 89 patients (94.4%) deemed as high risk had radiotherapy.
At a median follow-up of 5 years, 5.1% (4 of 82) of low-risk patients experienced a recurrence vs. 4.5% (4 of 89) of higher-risk patients.
Recurrence rates among patients who followed the radiation recommendations mirrored these overall findings: 5.5% of 75 patients with low-risk DCIS who skipped radiotherapy experienced disease recurrence vs. 4.8% of 84 patients with high-risk DCIS who received radiotherapy.
Age did not appear to affect the outcomes. Among the 33 women younger than 50 years, two experienced a recurrence (4%), both invasive. One occurred in the low-risk group and the other in the higher-risk group. Among the 138 older women, six had recurrences, three in each group, and one recurrence in each was invasive.
In short, “women who skipped radiation based on this score did not experience an excess risk of” ipsilateral recurrence over 5 years, said Dr. Khan.
Overall, the study offers “strong evidence” that the DCIS score might help “prevent excessive treatment for some patients,” she concluded, adding that 10-year outcomes will be reported.
The work was funded by the National Cancer Institute. Dr. Khan has no conflicts of interest. Dr. Kaklamani has extensive industry ties, including being a speaker for Pfizer, Genentech, Novartis, and AstraZeneca.
A version in the article appeared on Medscape.com.
FROM SABCS 2023
Patient counseling for breast cancer screening: Taking changes to USPSTF recommendations into account
Breast cancer represents the most commonly diagnosed cancer in the nation.1 However, unlike other cancers, most breast cancers are identified at stage I and have a 90% survival rate 5-year prognosis.2 These outcomes are attributable to various factors, one of the most significant being screening mammography—a largely accessible, highly sensitive and specific screening tool.3 Data demonstrate that malignant tumors detected on screening mammography have more favorable profiles in tumor size and nodal status compared with symptomatic breast cancers,4 which make it critical for early diagnosis. Most importantly, the research overwhelmingly demonstrates that screening mammography decreases breast cancer–related mortality.5-7
The USPSTF big change: Mammography starting at age 40 for all recommended
Despite the general accessibility and mortality benefits of screening mammography (in light of the high lifetime 12% prevalence of breast cancer in the United States8), recommendations still conflict across medical societies regarding optimal timing and frequency.9-12 Previously, the US Preventive Services Task Force (USPSTF) recommended that screening mammography should occur at age 50 biennially and that screening between ages 40 and 49 should be an individualized decision.13,14 In the draft recommendation statement issued on May 9, 2023, however, the USPSTF now recommends screening every other year starting at age 40 to decrease the risk of dying from breast cancer.15
This change represents a critically important shift. The new guidance:
- acknowledges the increasing incidence of early-onset breast cancer
- reinforces a national consciousness toward screening mammography in decreasing mortality,17 even among a younger age group for whom the perception of risk may be lower.
The USPSTF statement represents a significant change in how patients should be counseled. Practitioners now have more direct guidance that is concordant with what other national medical organizations offer or recommend, including the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR), and the National Comprehensive Cancer Network (NCCN).
However, while the USPSTF statement can and should encourage health care practitioners to initiate mammography earlier than prior recommendations, ongoing discussion regarding the optimal screening interval is warranted. The USPSTF recommendations state that mammography should be performed biennially. While the age at initiation represents a step in the right direction, this recommended screening interval should be reevaluated.
Annual vs biennial screening?
The debate between annual and biennial screening mammography is not new. While many randomized trials on screening mammography have evaluated such factors as breast cancer mortality by age or rate of false positives,18 fewer trials have evaluated the optimal screening interval.
One randomized trial from the United Kingdom evaluated 99,389 people aged 50 to 62 from 1989 to 1996 who underwent annual screening (study arm) versus 3 years later (control).19 Findings demonstrated a significantly smaller tumor size in the study arm (P=.05) as well as an increased total cancer detection rate. However, the authors concluded that shortening the screening interval (from 3 years) would not yield a statistically significant decrease in mortality.19
In a randomized trial from Finland, researchers screened those aged older than 50 at biennial intervals and those aged younger than 50 at either annual or triennial intervals.20 Results demonstrated that, among those aged 40 to 49, the frequency of stage I cancers was not significantly different from screen-detected cancers, interval cancers, or cancers detected outside of screening (50%, 42%, and 44%, respectively; P=.73). Furthermore, there was a greater likelihood of interval cancers among those aged 40 to 49 at 1-year (27%) and 3-year (39%) screening intervals compared with those aged older than 50 screened biennially (18%; P=.08 and P=.0009, respectively).20
These randomized trials, however, have been scrutinized because of factors such as discrepancies in screening intervals by country as well as substantial improvements made in screening mammography since the time these trials were conducted.5 Due to the dearth of more contemporary randomized controlled trials accounting for more up-to-date training and technology, most of the more recent data has been largely observational, retrospective, or used modeling.21 The TABLE outlines some of the major studies on this topic.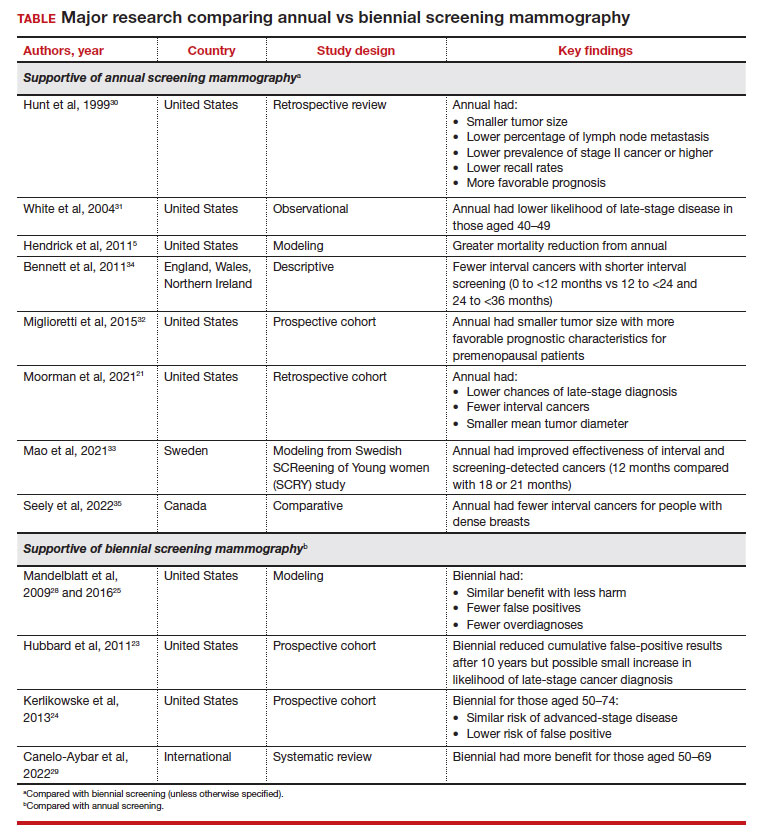
False-positive results, biopsy rates. The arguments against more frequent screening include the possibility of false positives that require callbacks and biopsies, which may be more frequent among those who undergo annual mammography.22 A systematic review from the Breast Cancer Surveillance Consortium demonstrated a 61.3% annual (confidence interval [CI], 59.4%–63.1%) versus 41.6% biennial (CI, 40.6%–42.5%) false-positive rate, resulting in a 7% (CI, 6.1%–7.8%) versus 4.8% (CI, 4.4–5.2%) rate of biopsy, respectively.23 This false-positive rate, however, also may be increased in younger patients aged 40 to 49 and in those with dense breasts.22,24 These callbacks and biopsies could induce significant patient stress, pain, and anxiety, as well as carry financial implications related to subsequent diagnostic imaging.
Overdiagnosis. There is also the risk of overdiagnosis, in which an indolent breast cancer that otherwise would not grow or progress to become symptomatic is identified. This could lead to overtreatment. While the exact incidence of overdiagnosis is unclear (due to recommendations for universal treatment of ductal carcinoma in situ), some data suggest that overdiagnosis could be decreased with biennial screening.25
While discomfort could also be a barrier, it may not necessarily be prohibitive for some to continue with future screening mammograms.22 Further, increased radiation with annual mammography is a concern. However, modeling studies have shown that the mortality benefit for annual mammography starting at age 40 outweighs (by 60-fold) the mortality risk from a radiation-induced breast cancer.26
Benefit from biennial screening
Some research suggests overall benefit from biennial screening. One study that used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer microsimulation was adapted to measure the incidence, mortality, and life-years gained for Canadian patients.27 This model demonstrated that mortality reduction was linked to greater lifetime screens for breast cancer, but this applied primarily to patients aged 50 and older. Overall, a larger impact was observed by initiating screening at age 40 than by decreasing screening intervals.27
Using modeling, Mandelblatt and colleagues demonstrated that biennial screening could capture most of the benefit of annual screening with less harm.28 In another study in 2016, Mandelblatt and colleagues used updated and revised versions of these simulation models and maintained that biennial screening upheld 79.8% to 81.3% of the benefits of annual screening mammography but with fewer overdiagnoses and false-positive results.25 The authors concluded that while biennial screening is equally effective for average-risk populations, there should be an evaluation of benefits and harms based on the clinical scenario (suggesting that annual screening for those at age 40 who carried elevated risk was similar to biennial screening for average-risk patients starting at age 50).25
Another study that served to inform the European Commission Initiative on Breast Cancer recommendations evaluated randomized controlled trials and observational and modeling studies that assessed breast screening intervals.29 The authors concluded that each screening interval has risks and benefits, with data suggesting more benefit with biennial screening for people aged 50 to 69 years and more possible harm with annual screening in younger people (aged 45–49).29
Continue to: Benefit from annual screening...
Benefit from annual screening
However, these data conflict with other studies that demonstrate the benefit of annual compared with biennial screening mammography. One large retrospective review of prospectively collected data evaluated outcome differences based on mammography frequency.30 For those undergoing annual versus biennial screening, the median tumor size was 11 mm (versus 15 mm), the percentage of lymph node metastasis was 14% (versus 24%), and cancer stage II or higher was 17% (versus 29%). The study overall demonstrated that annual screening resulted in lower recall rates (P<.0001) and detection of smaller tumors that carried a more favorable prognosis (P<.04).30
Another observational study from 2004 that assessed data from 7 different mammography registries nationwide noted that, among those aged 40 to 49, patients who underwent biennial screening had an increased likelihood of late-stage disease compared with those with annual screening (28% vs 21%, respectively; odds ratio [OR], 1.35; 95% CI, 1.01–1.81), although this discrepancy was not observed in people aged 50 or older.31
A study that critiqued the previous 2012 version of the USPSTF guidelines used CISNET modeling, which demonstrated a 39.6% mortality reduction with annual screening for those aged 40 to 84 versus 23.2% for biennial screening for those aged 50 to 74.5
More recent data also reflect these findings. A retrospective cohort study that evaluated patients aged 40 to 84 diagnosed with breast cancer found that those who previously underwent annual versus biennial screening mammography had lower incidences of late-stage diagnoses (24.0% vs 43.8%, respectively; P=.02), fewer interval cancers (10.5% vs 37.5%; P<.001), and smaller mean (SD) tumor diameter (1.4 [1.2] cm vs 1.8 [1.6] cm; P=.04).21 Postmenopausal patients in this cohort also demonstrated similar findings when comparing mammogram frequency. Although not significant, biennial (or greater) frequency of screening mammography also resulted in an increased likelihood of axillary lymph node dissection and chemotherapy.
Similarly, authors of another large prospective cohort study concluded that breast cancers diagnosed in premenopausal patients were more likely to be larger with less favorable prognostic characteristics (tumor size >15 mm, relative risk [RR], 1.21 [95% CI, 1.07–1.37]; P=.002); any less favorable prognostic characteristics (RR, 1.11 [95% CI, 1.00–1.22]; P=.047), and higher stage (stage IIB or higher, RR, 1.28 [95% CI, 1.01–1.63]; P=.04) for those who underwent biennial screening compared with breast cancers diagnosed by annual screening.32 However, this trend was not observed in postmenopausal patients not taking hormone therapy.32
Some international studies also show more favorable outcomes with annual screening mammography. A Swedish study evaluated mammography screening intervals of 21 months compared with 18 or 12 months in patients aged 40 to 49.33 Data showed an improved effectiveness of 1.6% to 9.8% for interval cancers and 2.9% to 17.4% for both interval and screening-detected cancers by reducing the screening frequency to 12 months, with authors suggesting a further reduction in breast cancer–related mortality rates for this age group.33
Results from another descriptive study from Europe also showed increasing interval breast cancer rates with increasing screening intervals.34 After a negative screen, the interval cancer rates and regional ranges for 0 to less than 12 months, 12 to less than 24 months, and 24 to less than 36 months per 1,000 screened were 0.55 (0.43–0.76), 1.13 (0.92–1.47), and 1.22 (0.93–1.57), respectively.34
Finally, a study conducted in Canada evaluated interval breast cancers among people with dense breasts screened between 2008 and 2010.35 Those with screening programs with policies that offered annual screening reported fewer interval cancers (interval cancer rate, 0.89 per 1,000; 95% CI, 0.67–1.11) compared with those who had policies that used biennial screening (interval cancer rate, 1.45 per 1,000 [annualized]; 95% CI, 1.19–1.72), which was 63% higher (P=.002). For those for whom radiologists recommended screening, interval cancer was lower for annual (0.93 per 1,000; 95% CI, 0.71–1.16) versus biennial screening (1.70 per 1,000 [annualized]; 95% CI, 0.70–2.71) (P=.061).35
Continue to: Black patients have a worse breast cancer prognosis...
Black patients have a worse breast cancer prognosis
Additional consideration should be given to populations with worse survival outcomes at baseline for whom screening mammography could play a significant role. In particular, Black people have similar rates of breast cancer compared with White people (127.8 cases per 100,000 vs 133.7 cases per 100,000, respectively) but have a 40% increased breast cancer–related mortality.8 The USPSTF recognizes this disparity and mentions it in their recommendations, encouraging health care clinicians to engage in shared decision making with Black patients and asserting that more research is needed on screening mammography in Black communities.15
While the age modification to the new guidelines better addresses the disparities that impact the Black community (such as increased likelihood of early-onset breast cancer36 and increased rate of breast cancer diagnosis at first mammogram37), the next obvious question is: Can groups with higher breast cancer mortality such as Black communities afford to undergo mammography every 2 years (as opposed to every year)?
Although some data specifically have evaluated the age of initiation and frequency of screening mammography among Black patients,38,39 little data have specifically assessed outcomes for annual versus biennial screening among Black people. Despite these research gaps, risk factors among the Black community should be considered. There is an increased risk of triple-negative breast cancer that can contribute to higher mortality among Black communities.40 Black people also tend to be diagnosed with more aggressive subtypes overall,41,42 are more likely to have dense breasts,43,44 have a higher likelihood of advanced stages at the time of diagnosis compared with White people,8,45 and have a greater chance of diagnosis of a second primary or contralateral breast cancer46-48—all risk factors that support the importance of regular and early-screening mammography.
How I counsel my patients
As Director of the Cancer Genetics and Breast Health Clinic, I am a gynecologist who primarily evaluates patients at increased risk for breast cancer (and other cancers). As an initial step, I strongly encourage all patients (especially Black patients and those of Ashkenazi Jewish ancestry as per the American College of Radiology recommendations9) to undergo risk assessment at age 25 to determine if they may be at increased risk for breast cancer. This first step may include genetic testing if the patient meets NCCN testing criteria based on personal or family history. If results are positive for a germline pathogenic variant, the timing and nature of breast screening would be based on NCCN recommendations for that particular variant (with possible modification of age of initiation based on family history). If testing is negative, lifetime risk assessment would then be performed using risk calculators—such as Tyrer-Cuzick—to determine if the patient meets criteria for intensive surveillance with supplemental breast magnetic resonance imaging. If the patient is subsequently determined to be at average risk after these assessments, I recommend they undergo screening mammography annually starting at age 40. However, it must be recognized that risk may change over time. A patient’s risk can continue to be assessed over a lifetime—with changing family history, personal risk factors, and new discoveries in genetics.
Summary
Ultimately, it is reassuring that the USPSTF guidelines have been updated to be concordant with other national medical society recommendations. They reflect the increasing nationwide trends that clearly demonstrate the high overall prevalence of breast cancer as well as the increasing incidence of early-onset breast cancer.
The updated guidelines, however, do not reflect the entirety of breast cancer trends in this country. With breast cancer being the most commonly diagnosed cancer in the United States, it is imperative to consider the data that demonstrate improved prognostics with annual compared with biennial mammography. Furthermore, the guidelines only begin to explore the disparities that Black patients face regarding breast cancer–related mortality. The risks of younger age at diagnosis, greater likelihood of aggressive subtypes, increased risk of second primary and contralateral breast cancer, and later stage at diagnosis must be seriously evaluated when counseling this patient population.
While the USPSTF recommendations for age at initiation reflect national statistics, recommendations by the ACR and NCCN more appropriately recognize that the benefits of annual screening outweigh the potential risks. Annual screening frequency should be adopted when counseling patients, particularly for the Black community. ●
- Cancer stat facts: Common cancer sites. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed November 7, 2023. https://seer .cancer.gov/statfacts/html/common.html#:~:text=An%20 estimated%20297%2C790%20women%20and,overall%20 with%20288%2C300%20expected%20cases
- Survival rates for breast cancer. American Cancer Society. March 1, 2023. Accessed November 16, 2023. https://www .cancer.org/cancer/breast-cancer/understanding-a-breast -cancer-diagnosis/breast-cancer-survival-rates.html
- Ambinder EB, Lee E, Nguyen DL, et al. Interval breast cancers versus screen detected breast cancers: a retrospective cohort study. Acad Radiol. 2023;30(suppl 2):S154-S160.
- Allgood PC, Duffy SW, Kearins O, et al. Explaining the difference in prognosis between screen-detected and symptomatic breast cancers. Br J Cancer. 2011;104:1680-1685.
- Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
- Oeffinger KC, Fontham ETH, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019;125:1482-1488.
- Breast cancer facts & figures 2022-2024. American Cancer Society. 2022. Accessed September 7, 2023. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/breast-cancer-facts-and-figures/2022-2024 -breast-cancer-fact-figures-acs.pdf
- New ACR breast cancer screening guidelines call for earlier and more-intensive screening for high-risk women. American College of Radiology. May 3, 2023. Accessed October 8, 2023. https://www.acr.org/Media-Center/ACR -News-Releases/2023/New-ACR-Breast-Cancer-Screening -Guidelines-call-for-earlier-screening-for-high-risk-women
- American Cancer Society recommendations for the early detection of breast cancer. American Cancer Society. January 14, 2022. Accessed October 30, 2023. https://www.cancer .org/cancer/types/breast-cancer/screening-tests-and-early -detection/american-cancer-society-recommendations-for -the-early-detection-of-breast-cancer.html
- Breast cancer screening and diagnosis. National Comprehensive Cancer Network. Published Version 1.2023. June 19, 2023. Accessed September 21, 2023. https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No 179. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Final recommendation statement. Breast cancer: screening. US Preventive Services Task Force. January 11, 2016. Accessed September 1, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/recommendation breast-cancer-screening
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Breast cancer: screening. US Preventive Services Task Force. May 9, 2023. Accessed October 7, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/document/draft -evidence-review/breast-cancer-screening-adults
- Breast cancer in young women. Centers for Disease Control and Prevention. June 21, 2023. Accessed October 30, 2023. https://www.cdc.gov/cancer/breast/young_women/index .htm
- Arleo EK, Hendrick RE, Helvie MA, et al. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123:3673-3680.
- Nelson HD, Tyne K, Naik A, et al; US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727737, W237-W242.
- Breast Screening Frequency Trial Group. The frequency of breast cancer screening: results from the UKCCCR randomised trial. United Kingdom Co-ordinating Committee on Cancer Research. Eur J Cancer. 2002;38:1458-1464.
- Klemi PJ, Toikkanen S, Räsänen O, et al. Mammography screening interval and the frequency of interval cancers in a population-based screening. Br J Cancer. 1997;75:762-766.
- Moorman SEH, Pujara AC, Sakala MD, et al. Annual screening mammography associated with lower stage breast cancer compared with biennial screening. AJR Am J Roentgenol. 2021;217:40-47.
- Nelson HD, Pappas M, Cantor A, et al. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:256-267.
- Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173:807-816.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies. Ann Intern Med. 2016;164:215-225.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiationinduced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med. 2016;164:205-214.
- Yaffe MJ, Mittmann N, Lee P, et al. Clinical outcomes of modelling mammography screening strategies. Health Rep. 2015;26:9-15.
- Mandelblatt JS, Cronin KA, Bailey S, et al; Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151: 738-747.
- Canelo-Aybar C, Posso M, Montero N, et al. Benefits and harms of annual, biennial, or triennial breast cancer mammography screening for women at average risk of breast cancer: a systematic review for the European Commission Initiative on Breast Cancer (ECIBC). Br J Cancer. 2022;126:673-688.
- Hunt KA, Rosen EL, Sickles EA. Outcome analysis for women undergoing annual versus biennial screening mammography: a review of 24,211 examinations. AJR Am J Roentgenol. 1999;173:285-289.
- White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96:1832-1839.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol. 2015;1:1069-1077.
- Mao Z, Nyström L, Jonsson H. Breast cancer screening with mammography in women aged 40-49 years: impact of length of screening interval on effectiveness of the program. J Med Screen. 2021;28:200-206.
- Bennett RL, Sellars SJ, Moss SM. Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland. Br J Cancer. 2011;104:571-577.
- Seely JM, Peddle SE, Yang H, et al. Breast density and risk of interval cancers: the effect of annual versus biennial screening mammography policies in Canada. Can Assoc Radiol J. 2022;73:90-100.
- Liu Q, Yao S, Zhao H, et al. Early-onset triple-negative breast cancer in multiracial/ethnic populations: distinct trends of prevalence of truncation mutations. Cancer Med. 2019;8:1845-1853.
- Wilkerson AD, Obi M, Ortega C, et al. Young Black women may be more likely to have first mammogram cancers: a new perspective in breast cancer disparities. Ann Surg Oncol. 2023;30:2856-2869.
- Chen T, Kharazmi E, Fallah M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open. 2023;6:e238893.
- Chapman CH, Schechter CB, Cadham CJ, et al. Identifying equitable screening mammography strategies for Black women in the United States using simulation modeling. Ann Intern Med. 2021;174:1637-1646.
- Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8-16.
- Stringer-Reasor EM, Elkhanany A, Khoury K, et al. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46.
- Newman LA. Parsing the etiology of breast cancer disparities. J Clin Oncol. 2016;34:1013-1014.
- Moore JX, Han Y, Appleton C, et al. Determinants of mammographic breast density by race among a large screening population. JNCI Cancer Spectr. 2020;4:pkaa010.
- McCarthy AM, Keller BM, Pantalone LM, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst. 2016;108:djw104.
- Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24:1666-1672.
- Terman E, Sheade J, Zhao F, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in Black and White women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200:75-83.
- Watt GP, John EM, Bandera EV, et al. Race, ethnicity and risk of second primary contralateral breast cancer in the United States. Int J Cancer. 2021;148:2748-2758.
- Giannakeas V, Lim DW, Narod SA. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer. 2021;125:601-610.
Breast cancer represents the most commonly diagnosed cancer in the nation.1 However, unlike other cancers, most breast cancers are identified at stage I and have a 90% survival rate 5-year prognosis.2 These outcomes are attributable to various factors, one of the most significant being screening mammography—a largely accessible, highly sensitive and specific screening tool.3 Data demonstrate that malignant tumors detected on screening mammography have more favorable profiles in tumor size and nodal status compared with symptomatic breast cancers,4 which make it critical for early diagnosis. Most importantly, the research overwhelmingly demonstrates that screening mammography decreases breast cancer–related mortality.5-7
The USPSTF big change: Mammography starting at age 40 for all recommended
Despite the general accessibility and mortality benefits of screening mammography (in light of the high lifetime 12% prevalence of breast cancer in the United States8), recommendations still conflict across medical societies regarding optimal timing and frequency.9-12 Previously, the US Preventive Services Task Force (USPSTF) recommended that screening mammography should occur at age 50 biennially and that screening between ages 40 and 49 should be an individualized decision.13,14 In the draft recommendation statement issued on May 9, 2023, however, the USPSTF now recommends screening every other year starting at age 40 to decrease the risk of dying from breast cancer.15
This change represents a critically important shift. The new guidance:
- acknowledges the increasing incidence of early-onset breast cancer
- reinforces a national consciousness toward screening mammography in decreasing mortality,17 even among a younger age group for whom the perception of risk may be lower.
The USPSTF statement represents a significant change in how patients should be counseled. Practitioners now have more direct guidance that is concordant with what other national medical organizations offer or recommend, including the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR), and the National Comprehensive Cancer Network (NCCN).
However, while the USPSTF statement can and should encourage health care practitioners to initiate mammography earlier than prior recommendations, ongoing discussion regarding the optimal screening interval is warranted. The USPSTF recommendations state that mammography should be performed biennially. While the age at initiation represents a step in the right direction, this recommended screening interval should be reevaluated.
Annual vs biennial screening?
The debate between annual and biennial screening mammography is not new. While many randomized trials on screening mammography have evaluated such factors as breast cancer mortality by age or rate of false positives,18 fewer trials have evaluated the optimal screening interval.
One randomized trial from the United Kingdom evaluated 99,389 people aged 50 to 62 from 1989 to 1996 who underwent annual screening (study arm) versus 3 years later (control).19 Findings demonstrated a significantly smaller tumor size in the study arm (P=.05) as well as an increased total cancer detection rate. However, the authors concluded that shortening the screening interval (from 3 years) would not yield a statistically significant decrease in mortality.19
In a randomized trial from Finland, researchers screened those aged older than 50 at biennial intervals and those aged younger than 50 at either annual or triennial intervals.20 Results demonstrated that, among those aged 40 to 49, the frequency of stage I cancers was not significantly different from screen-detected cancers, interval cancers, or cancers detected outside of screening (50%, 42%, and 44%, respectively; P=.73). Furthermore, there was a greater likelihood of interval cancers among those aged 40 to 49 at 1-year (27%) and 3-year (39%) screening intervals compared with those aged older than 50 screened biennially (18%; P=.08 and P=.0009, respectively).20
These randomized trials, however, have been scrutinized because of factors such as discrepancies in screening intervals by country as well as substantial improvements made in screening mammography since the time these trials were conducted.5 Due to the dearth of more contemporary randomized controlled trials accounting for more up-to-date training and technology, most of the more recent data has been largely observational, retrospective, or used modeling.21 The TABLE outlines some of the major studies on this topic.
False-positive results, biopsy rates. The arguments against more frequent screening include the possibility of false positives that require callbacks and biopsies, which may be more frequent among those who undergo annual mammography.22 A systematic review from the Breast Cancer Surveillance Consortium demonstrated a 61.3% annual (confidence interval [CI], 59.4%–63.1%) versus 41.6% biennial (CI, 40.6%–42.5%) false-positive rate, resulting in a 7% (CI, 6.1%–7.8%) versus 4.8% (CI, 4.4–5.2%) rate of biopsy, respectively.23 This false-positive rate, however, also may be increased in younger patients aged 40 to 49 and in those with dense breasts.22,24 These callbacks and biopsies could induce significant patient stress, pain, and anxiety, as well as carry financial implications related to subsequent diagnostic imaging.
Overdiagnosis. There is also the risk of overdiagnosis, in which an indolent breast cancer that otherwise would not grow or progress to become symptomatic is identified. This could lead to overtreatment. While the exact incidence of overdiagnosis is unclear (due to recommendations for universal treatment of ductal carcinoma in situ), some data suggest that overdiagnosis could be decreased with biennial screening.25
While discomfort could also be a barrier, it may not necessarily be prohibitive for some to continue with future screening mammograms.22 Further, increased radiation with annual mammography is a concern. However, modeling studies have shown that the mortality benefit for annual mammography starting at age 40 outweighs (by 60-fold) the mortality risk from a radiation-induced breast cancer.26
Benefit from biennial screening
Some research suggests overall benefit from biennial screening. One study that used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer microsimulation was adapted to measure the incidence, mortality, and life-years gained for Canadian patients.27 This model demonstrated that mortality reduction was linked to greater lifetime screens for breast cancer, but this applied primarily to patients aged 50 and older. Overall, a larger impact was observed by initiating screening at age 40 than by decreasing screening intervals.27
Using modeling, Mandelblatt and colleagues demonstrated that biennial screening could capture most of the benefit of annual screening with less harm.28 In another study in 2016, Mandelblatt and colleagues used updated and revised versions of these simulation models and maintained that biennial screening upheld 79.8% to 81.3% of the benefits of annual screening mammography but with fewer overdiagnoses and false-positive results.25 The authors concluded that while biennial screening is equally effective for average-risk populations, there should be an evaluation of benefits and harms based on the clinical scenario (suggesting that annual screening for those at age 40 who carried elevated risk was similar to biennial screening for average-risk patients starting at age 50).25
Another study that served to inform the European Commission Initiative on Breast Cancer recommendations evaluated randomized controlled trials and observational and modeling studies that assessed breast screening intervals.29 The authors concluded that each screening interval has risks and benefits, with data suggesting more benefit with biennial screening for people aged 50 to 69 years and more possible harm with annual screening in younger people (aged 45–49).29
Continue to: Benefit from annual screening...
Benefit from annual screening
However, these data conflict with other studies that demonstrate the benefit of annual compared with biennial screening mammography. One large retrospective review of prospectively collected data evaluated outcome differences based on mammography frequency.30 For those undergoing annual versus biennial screening, the median tumor size was 11 mm (versus 15 mm), the percentage of lymph node metastasis was 14% (versus 24%), and cancer stage II or higher was 17% (versus 29%). The study overall demonstrated that annual screening resulted in lower recall rates (P<.0001) and detection of smaller tumors that carried a more favorable prognosis (P<.04).30
Another observational study from 2004 that assessed data from 7 different mammography registries nationwide noted that, among those aged 40 to 49, patients who underwent biennial screening had an increased likelihood of late-stage disease compared with those with annual screening (28% vs 21%, respectively; odds ratio [OR], 1.35; 95% CI, 1.01–1.81), although this discrepancy was not observed in people aged 50 or older.31
A study that critiqued the previous 2012 version of the USPSTF guidelines used CISNET modeling, which demonstrated a 39.6% mortality reduction with annual screening for those aged 40 to 84 versus 23.2% for biennial screening for those aged 50 to 74.5
More recent data also reflect these findings. A retrospective cohort study that evaluated patients aged 40 to 84 diagnosed with breast cancer found that those who previously underwent annual versus biennial screening mammography had lower incidences of late-stage diagnoses (24.0% vs 43.8%, respectively; P=.02), fewer interval cancers (10.5% vs 37.5%; P<.001), and smaller mean (SD) tumor diameter (1.4 [1.2] cm vs 1.8 [1.6] cm; P=.04).21 Postmenopausal patients in this cohort also demonstrated similar findings when comparing mammogram frequency. Although not significant, biennial (or greater) frequency of screening mammography also resulted in an increased likelihood of axillary lymph node dissection and chemotherapy.
Similarly, authors of another large prospective cohort study concluded that breast cancers diagnosed in premenopausal patients were more likely to be larger with less favorable prognostic characteristics (tumor size >15 mm, relative risk [RR], 1.21 [95% CI, 1.07–1.37]; P=.002); any less favorable prognostic characteristics (RR, 1.11 [95% CI, 1.00–1.22]; P=.047), and higher stage (stage IIB or higher, RR, 1.28 [95% CI, 1.01–1.63]; P=.04) for those who underwent biennial screening compared with breast cancers diagnosed by annual screening.32 However, this trend was not observed in postmenopausal patients not taking hormone therapy.32
Some international studies also show more favorable outcomes with annual screening mammography. A Swedish study evaluated mammography screening intervals of 21 months compared with 18 or 12 months in patients aged 40 to 49.33 Data showed an improved effectiveness of 1.6% to 9.8% for interval cancers and 2.9% to 17.4% for both interval and screening-detected cancers by reducing the screening frequency to 12 months, with authors suggesting a further reduction in breast cancer–related mortality rates for this age group.33
Results from another descriptive study from Europe also showed increasing interval breast cancer rates with increasing screening intervals.34 After a negative screen, the interval cancer rates and regional ranges for 0 to less than 12 months, 12 to less than 24 months, and 24 to less than 36 months per 1,000 screened were 0.55 (0.43–0.76), 1.13 (0.92–1.47), and 1.22 (0.93–1.57), respectively.34
Finally, a study conducted in Canada evaluated interval breast cancers among people with dense breasts screened between 2008 and 2010.35 Those with screening programs with policies that offered annual screening reported fewer interval cancers (interval cancer rate, 0.89 per 1,000; 95% CI, 0.67–1.11) compared with those who had policies that used biennial screening (interval cancer rate, 1.45 per 1,000 [annualized]; 95% CI, 1.19–1.72), which was 63% higher (P=.002). For those for whom radiologists recommended screening, interval cancer was lower for annual (0.93 per 1,000; 95% CI, 0.71–1.16) versus biennial screening (1.70 per 1,000 [annualized]; 95% CI, 0.70–2.71) (P=.061).35
Continue to: Black patients have a worse breast cancer prognosis...
Black patients have a worse breast cancer prognosis
Additional consideration should be given to populations with worse survival outcomes at baseline for whom screening mammography could play a significant role. In particular, Black people have similar rates of breast cancer compared with White people (127.8 cases per 100,000 vs 133.7 cases per 100,000, respectively) but have a 40% increased breast cancer–related mortality.8 The USPSTF recognizes this disparity and mentions it in their recommendations, encouraging health care clinicians to engage in shared decision making with Black patients and asserting that more research is needed on screening mammography in Black communities.15
While the age modification to the new guidelines better addresses the disparities that impact the Black community (such as increased likelihood of early-onset breast cancer36 and increased rate of breast cancer diagnosis at first mammogram37), the next obvious question is: Can groups with higher breast cancer mortality such as Black communities afford to undergo mammography every 2 years (as opposed to every year)?
Although some data specifically have evaluated the age of initiation and frequency of screening mammography among Black patients,38,39 little data have specifically assessed outcomes for annual versus biennial screening among Black people. Despite these research gaps, risk factors among the Black community should be considered. There is an increased risk of triple-negative breast cancer that can contribute to higher mortality among Black communities.40 Black people also tend to be diagnosed with more aggressive subtypes overall,41,42 are more likely to have dense breasts,43,44 have a higher likelihood of advanced stages at the time of diagnosis compared with White people,8,45 and have a greater chance of diagnosis of a second primary or contralateral breast cancer46-48—all risk factors that support the importance of regular and early-screening mammography.
How I counsel my patients
As Director of the Cancer Genetics and Breast Health Clinic, I am a gynecologist who primarily evaluates patients at increased risk for breast cancer (and other cancers). As an initial step, I strongly encourage all patients (especially Black patients and those of Ashkenazi Jewish ancestry as per the American College of Radiology recommendations9) to undergo risk assessment at age 25 to determine if they may be at increased risk for breast cancer. This first step may include genetic testing if the patient meets NCCN testing criteria based on personal or family history. If results are positive for a germline pathogenic variant, the timing and nature of breast screening would be based on NCCN recommendations for that particular variant (with possible modification of age of initiation based on family history). If testing is negative, lifetime risk assessment would then be performed using risk calculators—such as Tyrer-Cuzick—to determine if the patient meets criteria for intensive surveillance with supplemental breast magnetic resonance imaging. If the patient is subsequently determined to be at average risk after these assessments, I recommend they undergo screening mammography annually starting at age 40. However, it must be recognized that risk may change over time. A patient’s risk can continue to be assessed over a lifetime—with changing family history, personal risk factors, and new discoveries in genetics.
Summary
Ultimately, it is reassuring that the USPSTF guidelines have been updated to be concordant with other national medical society recommendations. They reflect the increasing nationwide trends that clearly demonstrate the high overall prevalence of breast cancer as well as the increasing incidence of early-onset breast cancer.
The updated guidelines, however, do not reflect the entirety of breast cancer trends in this country. With breast cancer being the most commonly diagnosed cancer in the United States, it is imperative to consider the data that demonstrate improved prognostics with annual compared with biennial mammography. Furthermore, the guidelines only begin to explore the disparities that Black patients face regarding breast cancer–related mortality. The risks of younger age at diagnosis, greater likelihood of aggressive subtypes, increased risk of second primary and contralateral breast cancer, and later stage at diagnosis must be seriously evaluated when counseling this patient population.
While the USPSTF recommendations for age at initiation reflect national statistics, recommendations by the ACR and NCCN more appropriately recognize that the benefits of annual screening outweigh the potential risks. Annual screening frequency should be adopted when counseling patients, particularly for the Black community. ●
Breast cancer represents the most commonly diagnosed cancer in the nation.1 However, unlike other cancers, most breast cancers are identified at stage I and have a 90% survival rate 5-year prognosis.2 These outcomes are attributable to various factors, one of the most significant being screening mammography—a largely accessible, highly sensitive and specific screening tool.3 Data demonstrate that malignant tumors detected on screening mammography have more favorable profiles in tumor size and nodal status compared with symptomatic breast cancers,4 which make it critical for early diagnosis. Most importantly, the research overwhelmingly demonstrates that screening mammography decreases breast cancer–related mortality.5-7
The USPSTF big change: Mammography starting at age 40 for all recommended
Despite the general accessibility and mortality benefits of screening mammography (in light of the high lifetime 12% prevalence of breast cancer in the United States8), recommendations still conflict across medical societies regarding optimal timing and frequency.9-12 Previously, the US Preventive Services Task Force (USPSTF) recommended that screening mammography should occur at age 50 biennially and that screening between ages 40 and 49 should be an individualized decision.13,14 In the draft recommendation statement issued on May 9, 2023, however, the USPSTF now recommends screening every other year starting at age 40 to decrease the risk of dying from breast cancer.15
This change represents a critically important shift. The new guidance:
- acknowledges the increasing incidence of early-onset breast cancer
- reinforces a national consciousness toward screening mammography in decreasing mortality,17 even among a younger age group for whom the perception of risk may be lower.
The USPSTF statement represents a significant change in how patients should be counseled. Practitioners now have more direct guidance that is concordant with what other national medical organizations offer or recommend, including the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR), and the National Comprehensive Cancer Network (NCCN).
However, while the USPSTF statement can and should encourage health care practitioners to initiate mammography earlier than prior recommendations, ongoing discussion regarding the optimal screening interval is warranted. The USPSTF recommendations state that mammography should be performed biennially. While the age at initiation represents a step in the right direction, this recommended screening interval should be reevaluated.
Annual vs biennial screening?
The debate between annual and biennial screening mammography is not new. While many randomized trials on screening mammography have evaluated such factors as breast cancer mortality by age or rate of false positives,18 fewer trials have evaluated the optimal screening interval.
One randomized trial from the United Kingdom evaluated 99,389 people aged 50 to 62 from 1989 to 1996 who underwent annual screening (study arm) versus 3 years later (control).19 Findings demonstrated a significantly smaller tumor size in the study arm (P=.05) as well as an increased total cancer detection rate. However, the authors concluded that shortening the screening interval (from 3 years) would not yield a statistically significant decrease in mortality.19
In a randomized trial from Finland, researchers screened those aged older than 50 at biennial intervals and those aged younger than 50 at either annual or triennial intervals.20 Results demonstrated that, among those aged 40 to 49, the frequency of stage I cancers was not significantly different from screen-detected cancers, interval cancers, or cancers detected outside of screening (50%, 42%, and 44%, respectively; P=.73). Furthermore, there was a greater likelihood of interval cancers among those aged 40 to 49 at 1-year (27%) and 3-year (39%) screening intervals compared with those aged older than 50 screened biennially (18%; P=.08 and P=.0009, respectively).20
These randomized trials, however, have been scrutinized because of factors such as discrepancies in screening intervals by country as well as substantial improvements made in screening mammography since the time these trials were conducted.5 Due to the dearth of more contemporary randomized controlled trials accounting for more up-to-date training and technology, most of the more recent data has been largely observational, retrospective, or used modeling.21 The TABLE outlines some of the major studies on this topic.
False-positive results, biopsy rates. The arguments against more frequent screening include the possibility of false positives that require callbacks and biopsies, which may be more frequent among those who undergo annual mammography.22 A systematic review from the Breast Cancer Surveillance Consortium demonstrated a 61.3% annual (confidence interval [CI], 59.4%–63.1%) versus 41.6% biennial (CI, 40.6%–42.5%) false-positive rate, resulting in a 7% (CI, 6.1%–7.8%) versus 4.8% (CI, 4.4–5.2%) rate of biopsy, respectively.23 This false-positive rate, however, also may be increased in younger patients aged 40 to 49 and in those with dense breasts.22,24 These callbacks and biopsies could induce significant patient stress, pain, and anxiety, as well as carry financial implications related to subsequent diagnostic imaging.
Overdiagnosis. There is also the risk of overdiagnosis, in which an indolent breast cancer that otherwise would not grow or progress to become symptomatic is identified. This could lead to overtreatment. While the exact incidence of overdiagnosis is unclear (due to recommendations for universal treatment of ductal carcinoma in situ), some data suggest that overdiagnosis could be decreased with biennial screening.25
While discomfort could also be a barrier, it may not necessarily be prohibitive for some to continue with future screening mammograms.22 Further, increased radiation with annual mammography is a concern. However, modeling studies have shown that the mortality benefit for annual mammography starting at age 40 outweighs (by 60-fold) the mortality risk from a radiation-induced breast cancer.26
Benefit from biennial screening
Some research suggests overall benefit from biennial screening. One study that used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer microsimulation was adapted to measure the incidence, mortality, and life-years gained for Canadian patients.27 This model demonstrated that mortality reduction was linked to greater lifetime screens for breast cancer, but this applied primarily to patients aged 50 and older. Overall, a larger impact was observed by initiating screening at age 40 than by decreasing screening intervals.27
Using modeling, Mandelblatt and colleagues demonstrated that biennial screening could capture most of the benefit of annual screening with less harm.28 In another study in 2016, Mandelblatt and colleagues used updated and revised versions of these simulation models and maintained that biennial screening upheld 79.8% to 81.3% of the benefits of annual screening mammography but with fewer overdiagnoses and false-positive results.25 The authors concluded that while biennial screening is equally effective for average-risk populations, there should be an evaluation of benefits and harms based on the clinical scenario (suggesting that annual screening for those at age 40 who carried elevated risk was similar to biennial screening for average-risk patients starting at age 50).25
Another study that served to inform the European Commission Initiative on Breast Cancer recommendations evaluated randomized controlled trials and observational and modeling studies that assessed breast screening intervals.29 The authors concluded that each screening interval has risks and benefits, with data suggesting more benefit with biennial screening for people aged 50 to 69 years and more possible harm with annual screening in younger people (aged 45–49).29
Continue to: Benefit from annual screening...
Benefit from annual screening
However, these data conflict with other studies that demonstrate the benefit of annual compared with biennial screening mammography. One large retrospective review of prospectively collected data evaluated outcome differences based on mammography frequency.30 For those undergoing annual versus biennial screening, the median tumor size was 11 mm (versus 15 mm), the percentage of lymph node metastasis was 14% (versus 24%), and cancer stage II or higher was 17% (versus 29%). The study overall demonstrated that annual screening resulted in lower recall rates (P<.0001) and detection of smaller tumors that carried a more favorable prognosis (P<.04).30
Another observational study from 2004 that assessed data from 7 different mammography registries nationwide noted that, among those aged 40 to 49, patients who underwent biennial screening had an increased likelihood of late-stage disease compared with those with annual screening (28% vs 21%, respectively; odds ratio [OR], 1.35; 95% CI, 1.01–1.81), although this discrepancy was not observed in people aged 50 or older.31
A study that critiqued the previous 2012 version of the USPSTF guidelines used CISNET modeling, which demonstrated a 39.6% mortality reduction with annual screening for those aged 40 to 84 versus 23.2% for biennial screening for those aged 50 to 74.5
More recent data also reflect these findings. A retrospective cohort study that evaluated patients aged 40 to 84 diagnosed with breast cancer found that those who previously underwent annual versus biennial screening mammography had lower incidences of late-stage diagnoses (24.0% vs 43.8%, respectively; P=.02), fewer interval cancers (10.5% vs 37.5%; P<.001), and smaller mean (SD) tumor diameter (1.4 [1.2] cm vs 1.8 [1.6] cm; P=.04).21 Postmenopausal patients in this cohort also demonstrated similar findings when comparing mammogram frequency. Although not significant, biennial (or greater) frequency of screening mammography also resulted in an increased likelihood of axillary lymph node dissection and chemotherapy.
Similarly, authors of another large prospective cohort study concluded that breast cancers diagnosed in premenopausal patients were more likely to be larger with less favorable prognostic characteristics (tumor size >15 mm, relative risk [RR], 1.21 [95% CI, 1.07–1.37]; P=.002); any less favorable prognostic characteristics (RR, 1.11 [95% CI, 1.00–1.22]; P=.047), and higher stage (stage IIB or higher, RR, 1.28 [95% CI, 1.01–1.63]; P=.04) for those who underwent biennial screening compared with breast cancers diagnosed by annual screening.32 However, this trend was not observed in postmenopausal patients not taking hormone therapy.32
Some international studies also show more favorable outcomes with annual screening mammography. A Swedish study evaluated mammography screening intervals of 21 months compared with 18 or 12 months in patients aged 40 to 49.33 Data showed an improved effectiveness of 1.6% to 9.8% for interval cancers and 2.9% to 17.4% for both interval and screening-detected cancers by reducing the screening frequency to 12 months, with authors suggesting a further reduction in breast cancer–related mortality rates for this age group.33
Results from another descriptive study from Europe also showed increasing interval breast cancer rates with increasing screening intervals.34 After a negative screen, the interval cancer rates and regional ranges for 0 to less than 12 months, 12 to less than 24 months, and 24 to less than 36 months per 1,000 screened were 0.55 (0.43–0.76), 1.13 (0.92–1.47), and 1.22 (0.93–1.57), respectively.34
Finally, a study conducted in Canada evaluated interval breast cancers among people with dense breasts screened between 2008 and 2010.35 Those with screening programs with policies that offered annual screening reported fewer interval cancers (interval cancer rate, 0.89 per 1,000; 95% CI, 0.67–1.11) compared with those who had policies that used biennial screening (interval cancer rate, 1.45 per 1,000 [annualized]; 95% CI, 1.19–1.72), which was 63% higher (P=.002). For those for whom radiologists recommended screening, interval cancer was lower for annual (0.93 per 1,000; 95% CI, 0.71–1.16) versus biennial screening (1.70 per 1,000 [annualized]; 95% CI, 0.70–2.71) (P=.061).35
Continue to: Black patients have a worse breast cancer prognosis...
Black patients have a worse breast cancer prognosis
Additional consideration should be given to populations with worse survival outcomes at baseline for whom screening mammography could play a significant role. In particular, Black people have similar rates of breast cancer compared with White people (127.8 cases per 100,000 vs 133.7 cases per 100,000, respectively) but have a 40% increased breast cancer–related mortality.8 The USPSTF recognizes this disparity and mentions it in their recommendations, encouraging health care clinicians to engage in shared decision making with Black patients and asserting that more research is needed on screening mammography in Black communities.15
While the age modification to the new guidelines better addresses the disparities that impact the Black community (such as increased likelihood of early-onset breast cancer36 and increased rate of breast cancer diagnosis at first mammogram37), the next obvious question is: Can groups with higher breast cancer mortality such as Black communities afford to undergo mammography every 2 years (as opposed to every year)?
Although some data specifically have evaluated the age of initiation and frequency of screening mammography among Black patients,38,39 little data have specifically assessed outcomes for annual versus biennial screening among Black people. Despite these research gaps, risk factors among the Black community should be considered. There is an increased risk of triple-negative breast cancer that can contribute to higher mortality among Black communities.40 Black people also tend to be diagnosed with more aggressive subtypes overall,41,42 are more likely to have dense breasts,43,44 have a higher likelihood of advanced stages at the time of diagnosis compared with White people,8,45 and have a greater chance of diagnosis of a second primary or contralateral breast cancer46-48—all risk factors that support the importance of regular and early-screening mammography.
How I counsel my patients
As Director of the Cancer Genetics and Breast Health Clinic, I am a gynecologist who primarily evaluates patients at increased risk for breast cancer (and other cancers). As an initial step, I strongly encourage all patients (especially Black patients and those of Ashkenazi Jewish ancestry as per the American College of Radiology recommendations9) to undergo risk assessment at age 25 to determine if they may be at increased risk for breast cancer. This first step may include genetic testing if the patient meets NCCN testing criteria based on personal or family history. If results are positive for a germline pathogenic variant, the timing and nature of breast screening would be based on NCCN recommendations for that particular variant (with possible modification of age of initiation based on family history). If testing is negative, lifetime risk assessment would then be performed using risk calculators—such as Tyrer-Cuzick—to determine if the patient meets criteria for intensive surveillance with supplemental breast magnetic resonance imaging. If the patient is subsequently determined to be at average risk after these assessments, I recommend they undergo screening mammography annually starting at age 40. However, it must be recognized that risk may change over time. A patient’s risk can continue to be assessed over a lifetime—with changing family history, personal risk factors, and new discoveries in genetics.
Summary
Ultimately, it is reassuring that the USPSTF guidelines have been updated to be concordant with other national medical society recommendations. They reflect the increasing nationwide trends that clearly demonstrate the high overall prevalence of breast cancer as well as the increasing incidence of early-onset breast cancer.
The updated guidelines, however, do not reflect the entirety of breast cancer trends in this country. With breast cancer being the most commonly diagnosed cancer in the United States, it is imperative to consider the data that demonstrate improved prognostics with annual compared with biennial mammography. Furthermore, the guidelines only begin to explore the disparities that Black patients face regarding breast cancer–related mortality. The risks of younger age at diagnosis, greater likelihood of aggressive subtypes, increased risk of second primary and contralateral breast cancer, and later stage at diagnosis must be seriously evaluated when counseling this patient population.
While the USPSTF recommendations for age at initiation reflect national statistics, recommendations by the ACR and NCCN more appropriately recognize that the benefits of annual screening outweigh the potential risks. Annual screening frequency should be adopted when counseling patients, particularly for the Black community. ●
- Cancer stat facts: Common cancer sites. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed November 7, 2023. https://seer .cancer.gov/statfacts/html/common.html#:~:text=An%20 estimated%20297%2C790%20women%20and,overall%20 with%20288%2C300%20expected%20cases
- Survival rates for breast cancer. American Cancer Society. March 1, 2023. Accessed November 16, 2023. https://www .cancer.org/cancer/breast-cancer/understanding-a-breast -cancer-diagnosis/breast-cancer-survival-rates.html
- Ambinder EB, Lee E, Nguyen DL, et al. Interval breast cancers versus screen detected breast cancers: a retrospective cohort study. Acad Radiol. 2023;30(suppl 2):S154-S160.
- Allgood PC, Duffy SW, Kearins O, et al. Explaining the difference in prognosis between screen-detected and symptomatic breast cancers. Br J Cancer. 2011;104:1680-1685.
- Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
- Oeffinger KC, Fontham ETH, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019;125:1482-1488.
- Breast cancer facts & figures 2022-2024. American Cancer Society. 2022. Accessed September 7, 2023. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/breast-cancer-facts-and-figures/2022-2024 -breast-cancer-fact-figures-acs.pdf
- New ACR breast cancer screening guidelines call for earlier and more-intensive screening for high-risk women. American College of Radiology. May 3, 2023. Accessed October 8, 2023. https://www.acr.org/Media-Center/ACR -News-Releases/2023/New-ACR-Breast-Cancer-Screening -Guidelines-call-for-earlier-screening-for-high-risk-women
- American Cancer Society recommendations for the early detection of breast cancer. American Cancer Society. January 14, 2022. Accessed October 30, 2023. https://www.cancer .org/cancer/types/breast-cancer/screening-tests-and-early -detection/american-cancer-society-recommendations-for -the-early-detection-of-breast-cancer.html
- Breast cancer screening and diagnosis. National Comprehensive Cancer Network. Published Version 1.2023. June 19, 2023. Accessed September 21, 2023. https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No 179. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Final recommendation statement. Breast cancer: screening. US Preventive Services Task Force. January 11, 2016. Accessed September 1, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/recommendation breast-cancer-screening
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Breast cancer: screening. US Preventive Services Task Force. May 9, 2023. Accessed October 7, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/document/draft -evidence-review/breast-cancer-screening-adults
- Breast cancer in young women. Centers for Disease Control and Prevention. June 21, 2023. Accessed October 30, 2023. https://www.cdc.gov/cancer/breast/young_women/index .htm
- Arleo EK, Hendrick RE, Helvie MA, et al. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123:3673-3680.
- Nelson HD, Tyne K, Naik A, et al; US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727737, W237-W242.
- Breast Screening Frequency Trial Group. The frequency of breast cancer screening: results from the UKCCCR randomised trial. United Kingdom Co-ordinating Committee on Cancer Research. Eur J Cancer. 2002;38:1458-1464.
- Klemi PJ, Toikkanen S, Räsänen O, et al. Mammography screening interval and the frequency of interval cancers in a population-based screening. Br J Cancer. 1997;75:762-766.
- Moorman SEH, Pujara AC, Sakala MD, et al. Annual screening mammography associated with lower stage breast cancer compared with biennial screening. AJR Am J Roentgenol. 2021;217:40-47.
- Nelson HD, Pappas M, Cantor A, et al. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:256-267.
- Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173:807-816.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies. Ann Intern Med. 2016;164:215-225.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiationinduced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med. 2016;164:205-214.
- Yaffe MJ, Mittmann N, Lee P, et al. Clinical outcomes of modelling mammography screening strategies. Health Rep. 2015;26:9-15.
- Mandelblatt JS, Cronin KA, Bailey S, et al; Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151: 738-747.
- Canelo-Aybar C, Posso M, Montero N, et al. Benefits and harms of annual, biennial, or triennial breast cancer mammography screening for women at average risk of breast cancer: a systematic review for the European Commission Initiative on Breast Cancer (ECIBC). Br J Cancer. 2022;126:673-688.
- Hunt KA, Rosen EL, Sickles EA. Outcome analysis for women undergoing annual versus biennial screening mammography: a review of 24,211 examinations. AJR Am J Roentgenol. 1999;173:285-289.
- White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96:1832-1839.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol. 2015;1:1069-1077.
- Mao Z, Nyström L, Jonsson H. Breast cancer screening with mammography in women aged 40-49 years: impact of length of screening interval on effectiveness of the program. J Med Screen. 2021;28:200-206.
- Bennett RL, Sellars SJ, Moss SM. Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland. Br J Cancer. 2011;104:571-577.
- Seely JM, Peddle SE, Yang H, et al. Breast density and risk of interval cancers: the effect of annual versus biennial screening mammography policies in Canada. Can Assoc Radiol J. 2022;73:90-100.
- Liu Q, Yao S, Zhao H, et al. Early-onset triple-negative breast cancer in multiracial/ethnic populations: distinct trends of prevalence of truncation mutations. Cancer Med. 2019;8:1845-1853.
- Wilkerson AD, Obi M, Ortega C, et al. Young Black women may be more likely to have first mammogram cancers: a new perspective in breast cancer disparities. Ann Surg Oncol. 2023;30:2856-2869.
- Chen T, Kharazmi E, Fallah M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open. 2023;6:e238893.
- Chapman CH, Schechter CB, Cadham CJ, et al. Identifying equitable screening mammography strategies for Black women in the United States using simulation modeling. Ann Intern Med. 2021;174:1637-1646.
- Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8-16.
- Stringer-Reasor EM, Elkhanany A, Khoury K, et al. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46.
- Newman LA. Parsing the etiology of breast cancer disparities. J Clin Oncol. 2016;34:1013-1014.
- Moore JX, Han Y, Appleton C, et al. Determinants of mammographic breast density by race among a large screening population. JNCI Cancer Spectr. 2020;4:pkaa010.
- McCarthy AM, Keller BM, Pantalone LM, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst. 2016;108:djw104.
- Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24:1666-1672.
- Terman E, Sheade J, Zhao F, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in Black and White women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200:75-83.
- Watt GP, John EM, Bandera EV, et al. Race, ethnicity and risk of second primary contralateral breast cancer in the United States. Int J Cancer. 2021;148:2748-2758.
- Giannakeas V, Lim DW, Narod SA. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer. 2021;125:601-610.
- Cancer stat facts: Common cancer sites. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed November 7, 2023. https://seer .cancer.gov/statfacts/html/common.html#:~:text=An%20 estimated%20297%2C790%20women%20and,overall%20 with%20288%2C300%20expected%20cases
- Survival rates for breast cancer. American Cancer Society. March 1, 2023. Accessed November 16, 2023. https://www .cancer.org/cancer/breast-cancer/understanding-a-breast -cancer-diagnosis/breast-cancer-survival-rates.html
- Ambinder EB, Lee E, Nguyen DL, et al. Interval breast cancers versus screen detected breast cancers: a retrospective cohort study. Acad Radiol. 2023;30(suppl 2):S154-S160.
- Allgood PC, Duffy SW, Kearins O, et al. Explaining the difference in prognosis between screen-detected and symptomatic breast cancers. Br J Cancer. 2011;104:1680-1685.
- Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
- Oeffinger KC, Fontham ETH, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019;125:1482-1488.
- Breast cancer facts & figures 2022-2024. American Cancer Society. 2022. Accessed September 7, 2023. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/breast-cancer-facts-and-figures/2022-2024 -breast-cancer-fact-figures-acs.pdf
- New ACR breast cancer screening guidelines call for earlier and more-intensive screening for high-risk women. American College of Radiology. May 3, 2023. Accessed October 8, 2023. https://www.acr.org/Media-Center/ACR -News-Releases/2023/New-ACR-Breast-Cancer-Screening -Guidelines-call-for-earlier-screening-for-high-risk-women
- American Cancer Society recommendations for the early detection of breast cancer. American Cancer Society. January 14, 2022. Accessed October 30, 2023. https://www.cancer .org/cancer/types/breast-cancer/screening-tests-and-early -detection/american-cancer-society-recommendations-for -the-early-detection-of-breast-cancer.html
- Breast cancer screening and diagnosis. National Comprehensive Cancer Network. Published Version 1.2023. June 19, 2023. Accessed September 21, 2023. https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No 179. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Final recommendation statement. Breast cancer: screening. US Preventive Services Task Force. January 11, 2016. Accessed September 1, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/recommendation breast-cancer-screening
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Breast cancer: screening. US Preventive Services Task Force. May 9, 2023. Accessed October 7, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/document/draft -evidence-review/breast-cancer-screening-adults
- Breast cancer in young women. Centers for Disease Control and Prevention. June 21, 2023. Accessed October 30, 2023. https://www.cdc.gov/cancer/breast/young_women/index .htm
- Arleo EK, Hendrick RE, Helvie MA, et al. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123:3673-3680.
- Nelson HD, Tyne K, Naik A, et al; US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727737, W237-W242.
- Breast Screening Frequency Trial Group. The frequency of breast cancer screening: results from the UKCCCR randomised trial. United Kingdom Co-ordinating Committee on Cancer Research. Eur J Cancer. 2002;38:1458-1464.
- Klemi PJ, Toikkanen S, Räsänen O, et al. Mammography screening interval and the frequency of interval cancers in a population-based screening. Br J Cancer. 1997;75:762-766.
- Moorman SEH, Pujara AC, Sakala MD, et al. Annual screening mammography associated with lower stage breast cancer compared with biennial screening. AJR Am J Roentgenol. 2021;217:40-47.
- Nelson HD, Pappas M, Cantor A, et al. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:256-267.
- Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173:807-816.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies. Ann Intern Med. 2016;164:215-225.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiationinduced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med. 2016;164:205-214.
- Yaffe MJ, Mittmann N, Lee P, et al. Clinical outcomes of modelling mammography screening strategies. Health Rep. 2015;26:9-15.
- Mandelblatt JS, Cronin KA, Bailey S, et al; Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151: 738-747.
- Canelo-Aybar C, Posso M, Montero N, et al. Benefits and harms of annual, biennial, or triennial breast cancer mammography screening for women at average risk of breast cancer: a systematic review for the European Commission Initiative on Breast Cancer (ECIBC). Br J Cancer. 2022;126:673-688.
- Hunt KA, Rosen EL, Sickles EA. Outcome analysis for women undergoing annual versus biennial screening mammography: a review of 24,211 examinations. AJR Am J Roentgenol. 1999;173:285-289.
- White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96:1832-1839.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol. 2015;1:1069-1077.
- Mao Z, Nyström L, Jonsson H. Breast cancer screening with mammography in women aged 40-49 years: impact of length of screening interval on effectiveness of the program. J Med Screen. 2021;28:200-206.
- Bennett RL, Sellars SJ, Moss SM. Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland. Br J Cancer. 2011;104:571-577.
- Seely JM, Peddle SE, Yang H, et al. Breast density and risk of interval cancers: the effect of annual versus biennial screening mammography policies in Canada. Can Assoc Radiol J. 2022;73:90-100.
- Liu Q, Yao S, Zhao H, et al. Early-onset triple-negative breast cancer in multiracial/ethnic populations: distinct trends of prevalence of truncation mutations. Cancer Med. 2019;8:1845-1853.
- Wilkerson AD, Obi M, Ortega C, et al. Young Black women may be more likely to have first mammogram cancers: a new perspective in breast cancer disparities. Ann Surg Oncol. 2023;30:2856-2869.
- Chen T, Kharazmi E, Fallah M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open. 2023;6:e238893.
- Chapman CH, Schechter CB, Cadham CJ, et al. Identifying equitable screening mammography strategies for Black women in the United States using simulation modeling. Ann Intern Med. 2021;174:1637-1646.
- Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8-16.
- Stringer-Reasor EM, Elkhanany A, Khoury K, et al. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46.
- Newman LA. Parsing the etiology of breast cancer disparities. J Clin Oncol. 2016;34:1013-1014.
- Moore JX, Han Y, Appleton C, et al. Determinants of mammographic breast density by race among a large screening population. JNCI Cancer Spectr. 2020;4:pkaa010.
- McCarthy AM, Keller BM, Pantalone LM, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst. 2016;108:djw104.
- Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24:1666-1672.
- Terman E, Sheade J, Zhao F, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in Black and White women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200:75-83.
- Watt GP, John EM, Bandera EV, et al. Race, ethnicity and risk of second primary contralateral breast cancer in the United States. Int J Cancer. 2021;148:2748-2758.
- Giannakeas V, Lim DW, Narod SA. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer. 2021;125:601-610.
Answering the unknowns of taxanes for breast cancer during pregnancy
San Antonio – The findings shed light on a relatively unstudied topic. “Our cohort with 103 patients represents the most extensive study to date, and our main goal was to have homogeneous reporting of adverse events,” Ana Ferrigno Guajardo, MD, said in an interview. She presented the results at the San Antonio Breast Cancer Symposium.
“Breast cancer during pregnancy is a very challenging clinical situation as the expected antineoplastic effects of treatment must be carefully balanced against potential detrimental consequences on the developing fetus,” said Dr. Guajardo. She is a resident physician at Yale University School of Medicine.
Anthracycline-based chemotherapy agents are generally used during pregnancy because there is more safety data available for them, but some studies have shown that taxanes may have better efficacy in some clinical situations. “Cohort studies that have been done in the past [show] that taxane use is mostly deferred to the postpartum period, and we are not really sure of the impact that can have on survival in patients postponing treatment,” said Dr. Guajardo.
There are potential safety concerns with taxanes because neonates lack the cytochrome enzymes to metabolize the drugs, which creates a theoretical risk of adverse effects due to prolonged activity. On the other hand, pregnant women metabolize taxanes faster, and there are placental barriers that can inhibit high molecular weight molecules like taxanes from reaching the fetus, according to Dr. Guajardo.
In addition to pregnancy outcomes, the researchers followed 28 infants, and found that 87% were found to be completely healthy, “so we were relatively reassured. But of course we think that there’s a need for prospective studies that validate our findings regarding the safety taxanes,” said Dr. Guajardo.
Although there is no direct comparison group, the findings correlate well with studies of the general population and other chemotherapy agents. “We have large cohorts with mostly anthracycline-based chemotherapy agents during pregnancy that we can compare our results to, and overall, we were reassured that the prevalence of complications that we found in our cohort was very similar or even lower to those reported in the literature with patients treated with anthracycline-based therapy,” said Dr. Guajardo.
Compared with the general population, the team found higher rates of preterm births, neonatal ICU admissions, and premature membrane rupture, and infants that are small for gestational age. However, with the exception of the latter, all of these risks have been seen in pregnant women treated with other types of chemotherapy. “Perhaps it would be interesting to see if the incidence of small for gestational age neonates might be a bit higher in this population when compared to anthracycline-based therapy agents, but that does require a study that has a comparator group,” said Dr. Guajardo.
The researchers recruited 103 women with an average age of 34 years from 10 centers in 6 countries: United States, France, Spain, Mexico, Italy, and Costa Rica. The great majority were also treated with anthracyclines during gestation, and nearly all (97%) were treated with paclitaxel. The live birth rate was 98%, and 43.4% were preterm, 24% were small for gestational age, 16% were admitted to the neonate ICU, and 12.5% had hyperbilirubinemia.
Obstetric complications included intrauterine growth restriction (9%), preterm premature rupture of membranes (5%), gestational diabetes mellitus (5%), hypertensive disorders (4%), and pregnancy loss (2%).
After the presentation, Virginia Borges, MD, professor of medical oncology at University of Colorado Anschutz Medical Center, served as a discussant.
“Highlights of this study [include] that it is an international cohort from over six countries with over 100 cases of women included specifically focusing on the use of paclitaxel. They demonstrated safe outcomes for the pregnancies and the mothers,” Dr. Borges said during her presentation.
She went on to highlight several key points that physicians should consider when treating pregnancy-related breast cancer. “We want to achieve prepartum treatment wherever feasible to tackle that cancer before delivery of the child to prevent a pregnancy-related breast cancer from potentially turning into a postpartum breast cancer,” she said.
“If the tumor is ER+/HER2-, we now see we can safely give anthracyclines and taxanes from 12 to about 35 weeks of gestation. We don’t want to get too close to the delivery with chemotherapy. If a patient is HER2+, I prefer to give the anthracycline portion while the person is pregnant and then after delivery incorporate the taxane with the HER2 targeted therapies as there’s some older data showing concurrent therapy looks a bit better than sequential. In triple negative breast cancer, again I prefer to give the anthracycline and delay the taxane and carboplatin to overlap with immunotherapy so we are getting the necessary synergy there as well,” Dr. Borges added.
Dr. Guajardo has no relevant financial disclosures. Dr. Borges has consulted for SeaGen, Gilead, and AstraZeneca, and has received research funding from AstraZeneca, Gilead, Olema, and SeaGen.
San Antonio – The findings shed light on a relatively unstudied topic. “Our cohort with 103 patients represents the most extensive study to date, and our main goal was to have homogeneous reporting of adverse events,” Ana Ferrigno Guajardo, MD, said in an interview. She presented the results at the San Antonio Breast Cancer Symposium.
“Breast cancer during pregnancy is a very challenging clinical situation as the expected antineoplastic effects of treatment must be carefully balanced against potential detrimental consequences on the developing fetus,” said Dr. Guajardo. She is a resident physician at Yale University School of Medicine.
Anthracycline-based chemotherapy agents are generally used during pregnancy because there is more safety data available for them, but some studies have shown that taxanes may have better efficacy in some clinical situations. “Cohort studies that have been done in the past [show] that taxane use is mostly deferred to the postpartum period, and we are not really sure of the impact that can have on survival in patients postponing treatment,” said Dr. Guajardo.
There are potential safety concerns with taxanes because neonates lack the cytochrome enzymes to metabolize the drugs, which creates a theoretical risk of adverse effects due to prolonged activity. On the other hand, pregnant women metabolize taxanes faster, and there are placental barriers that can inhibit high molecular weight molecules like taxanes from reaching the fetus, according to Dr. Guajardo.
In addition to pregnancy outcomes, the researchers followed 28 infants, and found that 87% were found to be completely healthy, “so we were relatively reassured. But of course we think that there’s a need for prospective studies that validate our findings regarding the safety taxanes,” said Dr. Guajardo.
Although there is no direct comparison group, the findings correlate well with studies of the general population and other chemotherapy agents. “We have large cohorts with mostly anthracycline-based chemotherapy agents during pregnancy that we can compare our results to, and overall, we were reassured that the prevalence of complications that we found in our cohort was very similar or even lower to those reported in the literature with patients treated with anthracycline-based therapy,” said Dr. Guajardo.
Compared with the general population, the team found higher rates of preterm births, neonatal ICU admissions, and premature membrane rupture, and infants that are small for gestational age. However, with the exception of the latter, all of these risks have been seen in pregnant women treated with other types of chemotherapy. “Perhaps it would be interesting to see if the incidence of small for gestational age neonates might be a bit higher in this population when compared to anthracycline-based therapy agents, but that does require a study that has a comparator group,” said Dr. Guajardo.
The researchers recruited 103 women with an average age of 34 years from 10 centers in 6 countries: United States, France, Spain, Mexico, Italy, and Costa Rica. The great majority were also treated with anthracyclines during gestation, and nearly all (97%) were treated with paclitaxel. The live birth rate was 98%, and 43.4% were preterm, 24% were small for gestational age, 16% were admitted to the neonate ICU, and 12.5% had hyperbilirubinemia.
Obstetric complications included intrauterine growth restriction (9%), preterm premature rupture of membranes (5%), gestational diabetes mellitus (5%), hypertensive disorders (4%), and pregnancy loss (2%).
After the presentation, Virginia Borges, MD, professor of medical oncology at University of Colorado Anschutz Medical Center, served as a discussant.
“Highlights of this study [include] that it is an international cohort from over six countries with over 100 cases of women included specifically focusing on the use of paclitaxel. They demonstrated safe outcomes for the pregnancies and the mothers,” Dr. Borges said during her presentation.
She went on to highlight several key points that physicians should consider when treating pregnancy-related breast cancer. “We want to achieve prepartum treatment wherever feasible to tackle that cancer before delivery of the child to prevent a pregnancy-related breast cancer from potentially turning into a postpartum breast cancer,” she said.
“If the tumor is ER+/HER2-, we now see we can safely give anthracyclines and taxanes from 12 to about 35 weeks of gestation. We don’t want to get too close to the delivery with chemotherapy. If a patient is HER2+, I prefer to give the anthracycline portion while the person is pregnant and then after delivery incorporate the taxane with the HER2 targeted therapies as there’s some older data showing concurrent therapy looks a bit better than sequential. In triple negative breast cancer, again I prefer to give the anthracycline and delay the taxane and carboplatin to overlap with immunotherapy so we are getting the necessary synergy there as well,” Dr. Borges added.
Dr. Guajardo has no relevant financial disclosures. Dr. Borges has consulted for SeaGen, Gilead, and AstraZeneca, and has received research funding from AstraZeneca, Gilead, Olema, and SeaGen.
San Antonio – The findings shed light on a relatively unstudied topic. “Our cohort with 103 patients represents the most extensive study to date, and our main goal was to have homogeneous reporting of adverse events,” Ana Ferrigno Guajardo, MD, said in an interview. She presented the results at the San Antonio Breast Cancer Symposium.
“Breast cancer during pregnancy is a very challenging clinical situation as the expected antineoplastic effects of treatment must be carefully balanced against potential detrimental consequences on the developing fetus,” said Dr. Guajardo. She is a resident physician at Yale University School of Medicine.
Anthracycline-based chemotherapy agents are generally used during pregnancy because there is more safety data available for them, but some studies have shown that taxanes may have better efficacy in some clinical situations. “Cohort studies that have been done in the past [show] that taxane use is mostly deferred to the postpartum period, and we are not really sure of the impact that can have on survival in patients postponing treatment,” said Dr. Guajardo.
There are potential safety concerns with taxanes because neonates lack the cytochrome enzymes to metabolize the drugs, which creates a theoretical risk of adverse effects due to prolonged activity. On the other hand, pregnant women metabolize taxanes faster, and there are placental barriers that can inhibit high molecular weight molecules like taxanes from reaching the fetus, according to Dr. Guajardo.
In addition to pregnancy outcomes, the researchers followed 28 infants, and found that 87% were found to be completely healthy, “so we were relatively reassured. But of course we think that there’s a need for prospective studies that validate our findings regarding the safety taxanes,” said Dr. Guajardo.
Although there is no direct comparison group, the findings correlate well with studies of the general population and other chemotherapy agents. “We have large cohorts with mostly anthracycline-based chemotherapy agents during pregnancy that we can compare our results to, and overall, we were reassured that the prevalence of complications that we found in our cohort was very similar or even lower to those reported in the literature with patients treated with anthracycline-based therapy,” said Dr. Guajardo.
Compared with the general population, the team found higher rates of preterm births, neonatal ICU admissions, and premature membrane rupture, and infants that are small for gestational age. However, with the exception of the latter, all of these risks have been seen in pregnant women treated with other types of chemotherapy. “Perhaps it would be interesting to see if the incidence of small for gestational age neonates might be a bit higher in this population when compared to anthracycline-based therapy agents, but that does require a study that has a comparator group,” said Dr. Guajardo.
The researchers recruited 103 women with an average age of 34 years from 10 centers in 6 countries: United States, France, Spain, Mexico, Italy, and Costa Rica. The great majority were also treated with anthracyclines during gestation, and nearly all (97%) were treated with paclitaxel. The live birth rate was 98%, and 43.4% were preterm, 24% were small for gestational age, 16% were admitted to the neonate ICU, and 12.5% had hyperbilirubinemia.
Obstetric complications included intrauterine growth restriction (9%), preterm premature rupture of membranes (5%), gestational diabetes mellitus (5%), hypertensive disorders (4%), and pregnancy loss (2%).
After the presentation, Virginia Borges, MD, professor of medical oncology at University of Colorado Anschutz Medical Center, served as a discussant.
“Highlights of this study [include] that it is an international cohort from over six countries with over 100 cases of women included specifically focusing on the use of paclitaxel. They demonstrated safe outcomes for the pregnancies and the mothers,” Dr. Borges said during her presentation.
She went on to highlight several key points that physicians should consider when treating pregnancy-related breast cancer. “We want to achieve prepartum treatment wherever feasible to tackle that cancer before delivery of the child to prevent a pregnancy-related breast cancer from potentially turning into a postpartum breast cancer,” she said.
“If the tumor is ER+/HER2-, we now see we can safely give anthracyclines and taxanes from 12 to about 35 weeks of gestation. We don’t want to get too close to the delivery with chemotherapy. If a patient is HER2+, I prefer to give the anthracycline portion while the person is pregnant and then after delivery incorporate the taxane with the HER2 targeted therapies as there’s some older data showing concurrent therapy looks a bit better than sequential. In triple negative breast cancer, again I prefer to give the anthracycline and delay the taxane and carboplatin to overlap with immunotherapy so we are getting the necessary synergy there as well,” Dr. Borges added.
Dr. Guajardo has no relevant financial disclosures. Dr. Borges has consulted for SeaGen, Gilead, and AstraZeneca, and has received research funding from AstraZeneca, Gilead, Olema, and SeaGen.
FROM SABCS 2023
Women over 50 may safely de-escalate mammogram frequency following surgery
SAN ANTONIO – , according to results from a new randomized trial.
In the UK study, researchers found similar recurrence rates and overall survival between patients who continued to undergo annual screening versus women who underwent screening every 2 years after breast-conserving surgery or every 3 years after a mastectomy.
Current US guidelines recommend annual screening with no stopping point, while UK guidelines recommend annual screening for 5 years, followed by every 3 years after that.
The study was commissioned by the UK government after a previously commissioned systematic review showed lack of evidence for the existing frequency and duration of mammograms in this patient population, and a survey showed wide-ranging clinical practices. In response, the UK government funded the new study to evaluate whether it would be safe to reduce screening, according to Janet Dunn, PhD, who presented the study at the San Antonio Breast Cancer Symposium.
“Ladies are going back for years and years and years [for mammograms], and it’s inappropriate, it’s not necessary,” Dr. Dunn, professor of clinical trials and head of cancer trials at University of Warwick in the UK, said in an interview.
The lower frequency schedule requires that the patient be cancer free at 3 years following curative treatment. “We know for anybody going through breast cancer there are a couple of peaks of recurrence. One peak is 2 to 3 years [after curative treatment] for high-risk patients. The other peak is when they start hormone therapy, so at 5 to 6 years you get the peak. But this particular set of patients are at low to moderate risk, so they’re not the high-risk patients going into treatment trials,” said Dr. Dunn.
When asked if the findings should change practice, Dr. Dunn suggested that they could. “We would say that this is providing clinical evidence and probably changing guidance for the management of these patients: Instead of giving annual mammograms for years and years and years, after three years post curative surgery, and having gone through treatment with a baseline mammogram that’s clear, in the UK we can manage these patients back within the screening program, certainly for the mastectomy patients,” said Dr. Dunn.
She emphasized the potential mental health impact. “Women who are going through mammograms waiting for the results are much more anxious if they’ve had breast cancer before than those who were who were just going through normal screening. So [deescalation] is reducing anxiety. It’s also reducing the cost. It’s just reducing the burden on the whole health care system,” said Dr. Dunn.
During the Q&A session after the talk, Bruce Mann, MBBS, PhD, asked if there were differences in the pathology of the cancers that occurred in the less frequent group than those that arose in the annual group. If more frequent screening leads to earlier diagnosis of a tumor, “you would expect that those who have less [frequent screening] may be diagnosed with more advanced recurrences or new cancers, and that would eventually lead to a difference in outcome,” Dr. Mann said in an interview. He is director of breast cancer services at the Royal Melbourne Hospital in Australia.
He acknowledged the need to reconsider screening frequency, and complimented the researchers. “This is a nice pragmatic study. It’s interesting. It’s provocative. I think it’s a bit too early [to change practice]. I think we do need to see more information,” he said.
The researchers randomized 5235 women aged 50 and older to annual or less frequent mammography. The disease type was invasive in 87% of women, while 13% had ductal carcinoma in situ.
Over a median follow-up of 8.7 years, 7% experienced a recurrence. At 5 years, breast cancer–specific survival was 98.1% and 98.3% in the annual and less frequent groups, respectively (hazard ratio [HR], 0.92; 95% CI, 0.64-1.32). There was also no difference in recurrence-free survival or overall survival.
Analyses showed noninferiority of less frequent mammograms at a 3% margin (P < .0001) and a 1% margin (P = .003).
The researchers found that 83% of women were compliant with the mammogram schedule in the annual group, versus 69% in the less frequent arm. The COVID-19 pandemic was responsible for 35% of missed mammograms overall.
Quality of life measures showed that levels of distress over mammograms was similar across time and between the two groups, with 24% of women reporting medium or high levels of distress.
Dr. Dunn and Dr. Mann have no relevant financial disclosures.
SAN ANTONIO – , according to results from a new randomized trial.
In the UK study, researchers found similar recurrence rates and overall survival between patients who continued to undergo annual screening versus women who underwent screening every 2 years after breast-conserving surgery or every 3 years after a mastectomy.
Current US guidelines recommend annual screening with no stopping point, while UK guidelines recommend annual screening for 5 years, followed by every 3 years after that.
The study was commissioned by the UK government after a previously commissioned systematic review showed lack of evidence for the existing frequency and duration of mammograms in this patient population, and a survey showed wide-ranging clinical practices. In response, the UK government funded the new study to evaluate whether it would be safe to reduce screening, according to Janet Dunn, PhD, who presented the study at the San Antonio Breast Cancer Symposium.
“Ladies are going back for years and years and years [for mammograms], and it’s inappropriate, it’s not necessary,” Dr. Dunn, professor of clinical trials and head of cancer trials at University of Warwick in the UK, said in an interview.
The lower frequency schedule requires that the patient be cancer free at 3 years following curative treatment. “We know for anybody going through breast cancer there are a couple of peaks of recurrence. One peak is 2 to 3 years [after curative treatment] for high-risk patients. The other peak is when they start hormone therapy, so at 5 to 6 years you get the peak. But this particular set of patients are at low to moderate risk, so they’re not the high-risk patients going into treatment trials,” said Dr. Dunn.
When asked if the findings should change practice, Dr. Dunn suggested that they could. “We would say that this is providing clinical evidence and probably changing guidance for the management of these patients: Instead of giving annual mammograms for years and years and years, after three years post curative surgery, and having gone through treatment with a baseline mammogram that’s clear, in the UK we can manage these patients back within the screening program, certainly for the mastectomy patients,” said Dr. Dunn.
She emphasized the potential mental health impact. “Women who are going through mammograms waiting for the results are much more anxious if they’ve had breast cancer before than those who were who were just going through normal screening. So [deescalation] is reducing anxiety. It’s also reducing the cost. It’s just reducing the burden on the whole health care system,” said Dr. Dunn.
During the Q&A session after the talk, Bruce Mann, MBBS, PhD, asked if there were differences in the pathology of the cancers that occurred in the less frequent group than those that arose in the annual group. If more frequent screening leads to earlier diagnosis of a tumor, “you would expect that those who have less [frequent screening] may be diagnosed with more advanced recurrences or new cancers, and that would eventually lead to a difference in outcome,” Dr. Mann said in an interview. He is director of breast cancer services at the Royal Melbourne Hospital in Australia.
He acknowledged the need to reconsider screening frequency, and complimented the researchers. “This is a nice pragmatic study. It’s interesting. It’s provocative. I think it’s a bit too early [to change practice]. I think we do need to see more information,” he said.
The researchers randomized 5235 women aged 50 and older to annual or less frequent mammography. The disease type was invasive in 87% of women, while 13% had ductal carcinoma in situ.
Over a median follow-up of 8.7 years, 7% experienced a recurrence. At 5 years, breast cancer–specific survival was 98.1% and 98.3% in the annual and less frequent groups, respectively (hazard ratio [HR], 0.92; 95% CI, 0.64-1.32). There was also no difference in recurrence-free survival or overall survival.
Analyses showed noninferiority of less frequent mammograms at a 3% margin (P < .0001) and a 1% margin (P = .003).
The researchers found that 83% of women were compliant with the mammogram schedule in the annual group, versus 69% in the less frequent arm. The COVID-19 pandemic was responsible for 35% of missed mammograms overall.
Quality of life measures showed that levels of distress over mammograms was similar across time and between the two groups, with 24% of women reporting medium or high levels of distress.
Dr. Dunn and Dr. Mann have no relevant financial disclosures.
SAN ANTONIO – , according to results from a new randomized trial.
In the UK study, researchers found similar recurrence rates and overall survival between patients who continued to undergo annual screening versus women who underwent screening every 2 years after breast-conserving surgery or every 3 years after a mastectomy.
Current US guidelines recommend annual screening with no stopping point, while UK guidelines recommend annual screening for 5 years, followed by every 3 years after that.
The study was commissioned by the UK government after a previously commissioned systematic review showed lack of evidence for the existing frequency and duration of mammograms in this patient population, and a survey showed wide-ranging clinical practices. In response, the UK government funded the new study to evaluate whether it would be safe to reduce screening, according to Janet Dunn, PhD, who presented the study at the San Antonio Breast Cancer Symposium.
“Ladies are going back for years and years and years [for mammograms], and it’s inappropriate, it’s not necessary,” Dr. Dunn, professor of clinical trials and head of cancer trials at University of Warwick in the UK, said in an interview.
The lower frequency schedule requires that the patient be cancer free at 3 years following curative treatment. “We know for anybody going through breast cancer there are a couple of peaks of recurrence. One peak is 2 to 3 years [after curative treatment] for high-risk patients. The other peak is when they start hormone therapy, so at 5 to 6 years you get the peak. But this particular set of patients are at low to moderate risk, so they’re not the high-risk patients going into treatment trials,” said Dr. Dunn.
When asked if the findings should change practice, Dr. Dunn suggested that they could. “We would say that this is providing clinical evidence and probably changing guidance for the management of these patients: Instead of giving annual mammograms for years and years and years, after three years post curative surgery, and having gone through treatment with a baseline mammogram that’s clear, in the UK we can manage these patients back within the screening program, certainly for the mastectomy patients,” said Dr. Dunn.
She emphasized the potential mental health impact. “Women who are going through mammograms waiting for the results are much more anxious if they’ve had breast cancer before than those who were who were just going through normal screening. So [deescalation] is reducing anxiety. It’s also reducing the cost. It’s just reducing the burden on the whole health care system,” said Dr. Dunn.
During the Q&A session after the talk, Bruce Mann, MBBS, PhD, asked if there were differences in the pathology of the cancers that occurred in the less frequent group than those that arose in the annual group. If more frequent screening leads to earlier diagnosis of a tumor, “you would expect that those who have less [frequent screening] may be diagnosed with more advanced recurrences or new cancers, and that would eventually lead to a difference in outcome,” Dr. Mann said in an interview. He is director of breast cancer services at the Royal Melbourne Hospital in Australia.
He acknowledged the need to reconsider screening frequency, and complimented the researchers. “This is a nice pragmatic study. It’s interesting. It’s provocative. I think it’s a bit too early [to change practice]. I think we do need to see more information,” he said.
The researchers randomized 5235 women aged 50 and older to annual or less frequent mammography. The disease type was invasive in 87% of women, while 13% had ductal carcinoma in situ.
Over a median follow-up of 8.7 years, 7% experienced a recurrence. At 5 years, breast cancer–specific survival was 98.1% and 98.3% in the annual and less frequent groups, respectively (hazard ratio [HR], 0.92; 95% CI, 0.64-1.32). There was also no difference in recurrence-free survival or overall survival.
Analyses showed noninferiority of less frequent mammograms at a 3% margin (P < .0001) and a 1% margin (P = .003).
The researchers found that 83% of women were compliant with the mammogram schedule in the annual group, versus 69% in the less frequent arm. The COVID-19 pandemic was responsible for 35% of missed mammograms overall.
Quality of life measures showed that levels of distress over mammograms was similar across time and between the two groups, with 24% of women reporting medium or high levels of distress.
Dr. Dunn and Dr. Mann have no relevant financial disclosures.
FROM SABCS 2023
Trop-2 drug conjugate may trump chemo in HR+, HER2- breast cancer
SAN ANTONIO — In endocrine-resistant, HR+/HER2- metastatic breast cancer, the antibody-drug conjugate (ADC) datopotamab deruxtecan (dato-DXd, Daiichi Sankyo/AstraZeneca) has greater efficacy and a better safety profile than investigator-chosen chemotherapy, according to the latest results from the TROPION-Breast01 clinical trial.
If approved, the ADC would join sacituzumab govitecan (Trodelvy, Gilead) as agents that target trophoblast cell-surface antigen-2 (Trop-2), which is universally expressed in breast cancer, according to Aditya Bardia, MD, who presented the new results at the San Antonio Breast Cancer Symposium.
“ If this drug gets approved, we need more work in terms of biomarkers of response and resistance to understand how to select these agents and how to sequence these different agents,” Dr. Bardia said in an interview. He is associate professor of medicine at Harvard Medical School and Massachusetts General Hospital Cancer Center.
Dato-DXd has a proprietary linker technology that makes it more stable in plasma and more selective for tumor cells, where overexpressed enzymes cleave it and lead to release of the drug. This reduces off-target toxicity, said Dr. Bardia.
The primary results from TROPION-Breast01, presented at ESMO 2023, showed statistically significant improvement in progression-free survival compared to investigator’s choice of chemotherapy (hazard ratio, [HR], 0.63; P < .0001) and a higher overall response rate (36.4% versus 22.9%).
At SABCS, Dr. Bardia presented additional PFS sub-analyses and safety data.
TROPION-Breast01 included 732 patients who had failed or were ineligible for endocrine therapy, and who had received 1-2 lines of chemotherapy in the metastatic or inoperable setting. They were randomized to dato-DXd or chemotherapy.
The median PFS as determined by blinded independent central review was longer in the dato-DXd group (6.9 versus 4.5 months; P < .0001). Time to first subsequent therapy was also longer (median 8.2 versus 5.0 months; HR, 0.53; 95% CI, 0.45-0.64).
PFS benefit was similar regardless of duration of previous CDK4/6 inhibitor treatment. There was no significant difference in median PFS among patients with brain metastases at baseline (HR, 0.73; 95% CI, 0.39-1.42).
Grade 3 or higher treatment-related adverse events were less common in the dato-DXd group (21% versus 45%), as were the incidences of dose interruption (12% versus 25%), treatment-related neutropenia (11% versus 42%), grade 3 or higher treatment-related neutropenia (1% versus 31%), neutropenia-related dose interruption (0% versus 17%), and neutropenia-related dose reduction (0.3% versus 13%). G-CSF usage was lower in the dato-DXd group during treatment (3% versus 22%) and after treatment (0.3% versus 8%).
Stomatitis was more common in the dato-DXd group (50% versus 13%), including grade 3 (6% versus 3%). Dose reduction due to stomatitis was also more common (12% versus 1%), and discontinuation occurred in just 1 patient (0.3%) in the dato-DXd group.
The median time to confirmed deterioration, as measured by the Global Health Status/Quality of Life scale, was longer in the dato-DXd group (9.0 versus 4.8 months; HR, 0.76; 95% CI, 0.58-0.98).
During the Q & A period after the talk, Marc E. Lippman, MD, professor of oncology and director of the breast cancer program at Georgetown University’s Lombardi Comprehensive Cancer Center, questioned the assumption that Trop-2 is universally expressed in breast cancer, and asked if there were any data on outcomes associated with its expression. “That’s a very good question,” said Dr. Bardia. He said that the team is working on the problem, including identifying the best tool to measure Trop-2 expression, but also addressing whether expression changes over time. Finally, the team hopes to determine if treatment response might relate to levels of expression.
Trop-2 expression was studied in the ASCENT trial that examined sacituzumab govitecan in metastatic triple-negative breast cancer, and there was no apparent link. “In general, we don’t see a very strong correlation between Trop-2 expression and outcomes. In the ASCENT trial, even in patients who had low expression of Trop-2, the outcomes with Trop-2 antibody drug conjugates [were] superior to standard chemotherapy,” replied Dr. Bardia.
Ron Bose, MD, PhD, also asked if there would be broader biomarker analyses of responders versus nonresponders to dato-dxd. “I think it’s very important to know, what are the biomarkers that predict efficacy for dato-dxd. The median progression free survival improvement was only about two months, maybe a little bit more, so knowing which patients are going to get the most benefit will be very important,” Dr. Bose said in an interview. Dr. Bose is associate professor of oncology at the Washington University School of Medicine in St. Louis.
Overall, he was impressed by the results. “The median progression free survival benefit is moderate, but the safety I think is really particularly strong, and when I’m thinking about this for my patients, the fact that there is a progression free survival benefit, plus a safety benefit [compared to chemotherapy] makes it very appealing,” he said.
Dr. Bose has consulted for Genentech. Dr. Bardia has been on advisory boards for Pfizer, Novartis, Genentech, Merck, Radius Health/Menarini, Immunomedics/Gilead, Sanofi, Daiichi Pharma/AstraZeneca, Phillips, Eli Lilly, Mersana, and Foundation Medicine. He has received research grants from Genentech, Novartis, Pfizer, Merck, Sanofi, Radius Health/Menarini, Immunomedics/Gilead, Daiichi Pharma/AstraZeneca, Natera, and Eli Lilly.
SAN ANTONIO — In endocrine-resistant, HR+/HER2- metastatic breast cancer, the antibody-drug conjugate (ADC) datopotamab deruxtecan (dato-DXd, Daiichi Sankyo/AstraZeneca) has greater efficacy and a better safety profile than investigator-chosen chemotherapy, according to the latest results from the TROPION-Breast01 clinical trial.
If approved, the ADC would join sacituzumab govitecan (Trodelvy, Gilead) as agents that target trophoblast cell-surface antigen-2 (Trop-2), which is universally expressed in breast cancer, according to Aditya Bardia, MD, who presented the new results at the San Antonio Breast Cancer Symposium.
“ If this drug gets approved, we need more work in terms of biomarkers of response and resistance to understand how to select these agents and how to sequence these different agents,” Dr. Bardia said in an interview. He is associate professor of medicine at Harvard Medical School and Massachusetts General Hospital Cancer Center.
Dato-DXd has a proprietary linker technology that makes it more stable in plasma and more selective for tumor cells, where overexpressed enzymes cleave it and lead to release of the drug. This reduces off-target toxicity, said Dr. Bardia.
The primary results from TROPION-Breast01, presented at ESMO 2023, showed statistically significant improvement in progression-free survival compared to investigator’s choice of chemotherapy (hazard ratio, [HR], 0.63; P < .0001) and a higher overall response rate (36.4% versus 22.9%).
At SABCS, Dr. Bardia presented additional PFS sub-analyses and safety data.
TROPION-Breast01 included 732 patients who had failed or were ineligible for endocrine therapy, and who had received 1-2 lines of chemotherapy in the metastatic or inoperable setting. They were randomized to dato-DXd or chemotherapy.
The median PFS as determined by blinded independent central review was longer in the dato-DXd group (6.9 versus 4.5 months; P < .0001). Time to first subsequent therapy was also longer (median 8.2 versus 5.0 months; HR, 0.53; 95% CI, 0.45-0.64).
PFS benefit was similar regardless of duration of previous CDK4/6 inhibitor treatment. There was no significant difference in median PFS among patients with brain metastases at baseline (HR, 0.73; 95% CI, 0.39-1.42).
Grade 3 or higher treatment-related adverse events were less common in the dato-DXd group (21% versus 45%), as were the incidences of dose interruption (12% versus 25%), treatment-related neutropenia (11% versus 42%), grade 3 or higher treatment-related neutropenia (1% versus 31%), neutropenia-related dose interruption (0% versus 17%), and neutropenia-related dose reduction (0.3% versus 13%). G-CSF usage was lower in the dato-DXd group during treatment (3% versus 22%) and after treatment (0.3% versus 8%).
Stomatitis was more common in the dato-DXd group (50% versus 13%), including grade 3 (6% versus 3%). Dose reduction due to stomatitis was also more common (12% versus 1%), and discontinuation occurred in just 1 patient (0.3%) in the dato-DXd group.
The median time to confirmed deterioration, as measured by the Global Health Status/Quality of Life scale, was longer in the dato-DXd group (9.0 versus 4.8 months; HR, 0.76; 95% CI, 0.58-0.98).
During the Q & A period after the talk, Marc E. Lippman, MD, professor of oncology and director of the breast cancer program at Georgetown University’s Lombardi Comprehensive Cancer Center, questioned the assumption that Trop-2 is universally expressed in breast cancer, and asked if there were any data on outcomes associated with its expression. “That’s a very good question,” said Dr. Bardia. He said that the team is working on the problem, including identifying the best tool to measure Trop-2 expression, but also addressing whether expression changes over time. Finally, the team hopes to determine if treatment response might relate to levels of expression.
Trop-2 expression was studied in the ASCENT trial that examined sacituzumab govitecan in metastatic triple-negative breast cancer, and there was no apparent link. “In general, we don’t see a very strong correlation between Trop-2 expression and outcomes. In the ASCENT trial, even in patients who had low expression of Trop-2, the outcomes with Trop-2 antibody drug conjugates [were] superior to standard chemotherapy,” replied Dr. Bardia.
Ron Bose, MD, PhD, also asked if there would be broader biomarker analyses of responders versus nonresponders to dato-dxd. “I think it’s very important to know, what are the biomarkers that predict efficacy for dato-dxd. The median progression free survival improvement was only about two months, maybe a little bit more, so knowing which patients are going to get the most benefit will be very important,” Dr. Bose said in an interview. Dr. Bose is associate professor of oncology at the Washington University School of Medicine in St. Louis.
Overall, he was impressed by the results. “The median progression free survival benefit is moderate, but the safety I think is really particularly strong, and when I’m thinking about this for my patients, the fact that there is a progression free survival benefit, plus a safety benefit [compared to chemotherapy] makes it very appealing,” he said.
Dr. Bose has consulted for Genentech. Dr. Bardia has been on advisory boards for Pfizer, Novartis, Genentech, Merck, Radius Health/Menarini, Immunomedics/Gilead, Sanofi, Daiichi Pharma/AstraZeneca, Phillips, Eli Lilly, Mersana, and Foundation Medicine. He has received research grants from Genentech, Novartis, Pfizer, Merck, Sanofi, Radius Health/Menarini, Immunomedics/Gilead, Daiichi Pharma/AstraZeneca, Natera, and Eli Lilly.
SAN ANTONIO — In endocrine-resistant, HR+/HER2- metastatic breast cancer, the antibody-drug conjugate (ADC) datopotamab deruxtecan (dato-DXd, Daiichi Sankyo/AstraZeneca) has greater efficacy and a better safety profile than investigator-chosen chemotherapy, according to the latest results from the TROPION-Breast01 clinical trial.
If approved, the ADC would join sacituzumab govitecan (Trodelvy, Gilead) as agents that target trophoblast cell-surface antigen-2 (Trop-2), which is universally expressed in breast cancer, according to Aditya Bardia, MD, who presented the new results at the San Antonio Breast Cancer Symposium.
“ If this drug gets approved, we need more work in terms of biomarkers of response and resistance to understand how to select these agents and how to sequence these different agents,” Dr. Bardia said in an interview. He is associate professor of medicine at Harvard Medical School and Massachusetts General Hospital Cancer Center.
Dato-DXd has a proprietary linker technology that makes it more stable in plasma and more selective for tumor cells, where overexpressed enzymes cleave it and lead to release of the drug. This reduces off-target toxicity, said Dr. Bardia.
The primary results from TROPION-Breast01, presented at ESMO 2023, showed statistically significant improvement in progression-free survival compared to investigator’s choice of chemotherapy (hazard ratio, [HR], 0.63; P < .0001) and a higher overall response rate (36.4% versus 22.9%).
At SABCS, Dr. Bardia presented additional PFS sub-analyses and safety data.
TROPION-Breast01 included 732 patients who had failed or were ineligible for endocrine therapy, and who had received 1-2 lines of chemotherapy in the metastatic or inoperable setting. They were randomized to dato-DXd or chemotherapy.
The median PFS as determined by blinded independent central review was longer in the dato-DXd group (6.9 versus 4.5 months; P < .0001). Time to first subsequent therapy was also longer (median 8.2 versus 5.0 months; HR, 0.53; 95% CI, 0.45-0.64).
PFS benefit was similar regardless of duration of previous CDK4/6 inhibitor treatment. There was no significant difference in median PFS among patients with brain metastases at baseline (HR, 0.73; 95% CI, 0.39-1.42).
Grade 3 or higher treatment-related adverse events were less common in the dato-DXd group (21% versus 45%), as were the incidences of dose interruption (12% versus 25%), treatment-related neutropenia (11% versus 42%), grade 3 or higher treatment-related neutropenia (1% versus 31%), neutropenia-related dose interruption (0% versus 17%), and neutropenia-related dose reduction (0.3% versus 13%). G-CSF usage was lower in the dato-DXd group during treatment (3% versus 22%) and after treatment (0.3% versus 8%).
Stomatitis was more common in the dato-DXd group (50% versus 13%), including grade 3 (6% versus 3%). Dose reduction due to stomatitis was also more common (12% versus 1%), and discontinuation occurred in just 1 patient (0.3%) in the dato-DXd group.
The median time to confirmed deterioration, as measured by the Global Health Status/Quality of Life scale, was longer in the dato-DXd group (9.0 versus 4.8 months; HR, 0.76; 95% CI, 0.58-0.98).
During the Q & A period after the talk, Marc E. Lippman, MD, professor of oncology and director of the breast cancer program at Georgetown University’s Lombardi Comprehensive Cancer Center, questioned the assumption that Trop-2 is universally expressed in breast cancer, and asked if there were any data on outcomes associated with its expression. “That’s a very good question,” said Dr. Bardia. He said that the team is working on the problem, including identifying the best tool to measure Trop-2 expression, but also addressing whether expression changes over time. Finally, the team hopes to determine if treatment response might relate to levels of expression.
Trop-2 expression was studied in the ASCENT trial that examined sacituzumab govitecan in metastatic triple-negative breast cancer, and there was no apparent link. “In general, we don’t see a very strong correlation between Trop-2 expression and outcomes. In the ASCENT trial, even in patients who had low expression of Trop-2, the outcomes with Trop-2 antibody drug conjugates [were] superior to standard chemotherapy,” replied Dr. Bardia.
Ron Bose, MD, PhD, also asked if there would be broader biomarker analyses of responders versus nonresponders to dato-dxd. “I think it’s very important to know, what are the biomarkers that predict efficacy for dato-dxd. The median progression free survival improvement was only about two months, maybe a little bit more, so knowing which patients are going to get the most benefit will be very important,” Dr. Bose said in an interview. Dr. Bose is associate professor of oncology at the Washington University School of Medicine in St. Louis.
Overall, he was impressed by the results. “The median progression free survival benefit is moderate, but the safety I think is really particularly strong, and when I’m thinking about this for my patients, the fact that there is a progression free survival benefit, plus a safety benefit [compared to chemotherapy] makes it very appealing,” he said.
Dr. Bose has consulted for Genentech. Dr. Bardia has been on advisory boards for Pfizer, Novartis, Genentech, Merck, Radius Health/Menarini, Immunomedics/Gilead, Sanofi, Daiichi Pharma/AstraZeneca, Phillips, Eli Lilly, Mersana, and Foundation Medicine. He has received research grants from Genentech, Novartis, Pfizer, Merck, Sanofi, Radius Health/Menarini, Immunomedics/Gilead, Daiichi Pharma/AstraZeneca, Natera, and Eli Lilly.
AT SABCS 2023
Can younger postmenopausal women with low-risk BC skip radiation?
SAN ANTONIO — Women 65-70 years old are often offered the option of skipping radiation after lumpectomy for hormone receptor–positive early-stage breast cancer and moving straight to endocrine therapy.
The recurrence rate with and without radiation is well known so women can be counseled accurately about their options.
Omitting radiation for older postmenopausal women is “very reasonable to offer so long as they are willing to accept the risk,” said Reshma Jagsi, MD, chief of radiation oncology at Emory University, Atlanta.
The option, however, isn’t generally offered to postmenopausal women younger than 65 years old because their risk from skipping adjuvant radiation isn’t known, but that’s about to change.
Several teams are investigating the issue, including one led by Dr. Jagsi, who presented her and her colleagues’ latest results at the San Antonio Breast Cancer Symposium.
In the single-arm IDEA [Individualized Decisions for Endocrine therapy Alone] study, 200 women 50-69 years old with pT1N0 unifocal hormone receptor–positive, HER2-negative invasive breast cancer agreed to the approach when it was offered to them following lumpectomy with sentinel lymph node biopsy. The mean tumor size was 10 mm with margins of at least 2 mm.
The women were at low risk for recurrence, with recurrence risk scores no higher than 18 points on the Oncotype DX 21-gene assay; the mean score was 11 points.
Radiation would have been the usual next step after lumpectomy, but instead the patients went directly to endocrine therapy for 5 years, with adherence above 80%.
At 5 years, the results are “promising,” Dr. Jagsi said at the meeting. Overall and breast cancer–specific survival were both 100%, and the recurrence rate was just 1%, with two recurrences before the 5-year point. The women were a mean of 62 years old.
A similar single-arm trial, LUMINA, recently reported comparable results.
Dr. Jagsi called the findings of the studies “reassuring,” but cautioned that it will be a while before younger postmenopausal women can be offered radiation-free treatment like their older peers.
Even though the results suggest “that this might well be a really good idea,” longer follow-up and randomized data are needed “before we change the standard of care,” she said.
Of concern, for instance, is that there were six additional recurrences in the IDEA study past the 5-year mark, for a total of three recurrences among the 60 women 50-59 years old (5%) and five among the 140 women 60-69 years old (3.6%). Five of the recurrent cases were adherent to endocrine therapy.
Also, so few women in IDEA have passed the 5-year mark that “we can’t [conclude] anything” about long-term relapse risks, Dr. Jagsi said. Besides that, skipping radiation for such women at this point is “not reasonable,” Dr. Jagsi added.
Carlos Arteaga, MD, director of the UT Southwestern Simmons Cancer Center, Dallas, agreed.
“I think we have to wait. We have randomized studies that will test this in a formal way. Be that as it may, this provides the basis for a conversation physicians can have with patients because this could be an option” at some point, said Dr. Arteaga, who moderated Dr. Jagsi’s presentation.
“This is a big step in trying not to do too much for patients who don’t need it,” Virginia Kaklamani, MD, leader of the breast cancer program at UT Health San Antonio, said in an interview.
IDEA was published in the Journal of Clinical Oncology to coincide with Dr. Jagsi’s presentation.
The study was funded by the Susan G. Komen Breast Cancer Foundation and the University of Michigan Rogel Cancer Center. Dr. Jagsi has stock in Equity Quotient and research support form Genentech. Disclosure information for Arteaga was not available. Dr. Kaklamani has extensive industry ties, including being a speaker for Pfizer, Genentech, Novartis, and AstraZeneca.
SAN ANTONIO — Women 65-70 years old are often offered the option of skipping radiation after lumpectomy for hormone receptor–positive early-stage breast cancer and moving straight to endocrine therapy.
The recurrence rate with and without radiation is well known so women can be counseled accurately about their options.
Omitting radiation for older postmenopausal women is “very reasonable to offer so long as they are willing to accept the risk,” said Reshma Jagsi, MD, chief of radiation oncology at Emory University, Atlanta.
The option, however, isn’t generally offered to postmenopausal women younger than 65 years old because their risk from skipping adjuvant radiation isn’t known, but that’s about to change.
Several teams are investigating the issue, including one led by Dr. Jagsi, who presented her and her colleagues’ latest results at the San Antonio Breast Cancer Symposium.
In the single-arm IDEA [Individualized Decisions for Endocrine therapy Alone] study, 200 women 50-69 years old with pT1N0 unifocal hormone receptor–positive, HER2-negative invasive breast cancer agreed to the approach when it was offered to them following lumpectomy with sentinel lymph node biopsy. The mean tumor size was 10 mm with margins of at least 2 mm.
The women were at low risk for recurrence, with recurrence risk scores no higher than 18 points on the Oncotype DX 21-gene assay; the mean score was 11 points.
Radiation would have been the usual next step after lumpectomy, but instead the patients went directly to endocrine therapy for 5 years, with adherence above 80%.
At 5 years, the results are “promising,” Dr. Jagsi said at the meeting. Overall and breast cancer–specific survival were both 100%, and the recurrence rate was just 1%, with two recurrences before the 5-year point. The women were a mean of 62 years old.
A similar single-arm trial, LUMINA, recently reported comparable results.
Dr. Jagsi called the findings of the studies “reassuring,” but cautioned that it will be a while before younger postmenopausal women can be offered radiation-free treatment like their older peers.
Even though the results suggest “that this might well be a really good idea,” longer follow-up and randomized data are needed “before we change the standard of care,” she said.
Of concern, for instance, is that there were six additional recurrences in the IDEA study past the 5-year mark, for a total of three recurrences among the 60 women 50-59 years old (5%) and five among the 140 women 60-69 years old (3.6%). Five of the recurrent cases were adherent to endocrine therapy.
Also, so few women in IDEA have passed the 5-year mark that “we can’t [conclude] anything” about long-term relapse risks, Dr. Jagsi said. Besides that, skipping radiation for such women at this point is “not reasonable,” Dr. Jagsi added.
Carlos Arteaga, MD, director of the UT Southwestern Simmons Cancer Center, Dallas, agreed.
“I think we have to wait. We have randomized studies that will test this in a formal way. Be that as it may, this provides the basis for a conversation physicians can have with patients because this could be an option” at some point, said Dr. Arteaga, who moderated Dr. Jagsi’s presentation.
“This is a big step in trying not to do too much for patients who don’t need it,” Virginia Kaklamani, MD, leader of the breast cancer program at UT Health San Antonio, said in an interview.
IDEA was published in the Journal of Clinical Oncology to coincide with Dr. Jagsi’s presentation.
The study was funded by the Susan G. Komen Breast Cancer Foundation and the University of Michigan Rogel Cancer Center. Dr. Jagsi has stock in Equity Quotient and research support form Genentech. Disclosure information for Arteaga was not available. Dr. Kaklamani has extensive industry ties, including being a speaker for Pfizer, Genentech, Novartis, and AstraZeneca.
SAN ANTONIO — Women 65-70 years old are often offered the option of skipping radiation after lumpectomy for hormone receptor–positive early-stage breast cancer and moving straight to endocrine therapy.
The recurrence rate with and without radiation is well known so women can be counseled accurately about their options.
Omitting radiation for older postmenopausal women is “very reasonable to offer so long as they are willing to accept the risk,” said Reshma Jagsi, MD, chief of radiation oncology at Emory University, Atlanta.
The option, however, isn’t generally offered to postmenopausal women younger than 65 years old because their risk from skipping adjuvant radiation isn’t known, but that’s about to change.
Several teams are investigating the issue, including one led by Dr. Jagsi, who presented her and her colleagues’ latest results at the San Antonio Breast Cancer Symposium.
In the single-arm IDEA [Individualized Decisions for Endocrine therapy Alone] study, 200 women 50-69 years old with pT1N0 unifocal hormone receptor–positive, HER2-negative invasive breast cancer agreed to the approach when it was offered to them following lumpectomy with sentinel lymph node biopsy. The mean tumor size was 10 mm with margins of at least 2 mm.
The women were at low risk for recurrence, with recurrence risk scores no higher than 18 points on the Oncotype DX 21-gene assay; the mean score was 11 points.
Radiation would have been the usual next step after lumpectomy, but instead the patients went directly to endocrine therapy for 5 years, with adherence above 80%.
At 5 years, the results are “promising,” Dr. Jagsi said at the meeting. Overall and breast cancer–specific survival were both 100%, and the recurrence rate was just 1%, with two recurrences before the 5-year point. The women were a mean of 62 years old.
A similar single-arm trial, LUMINA, recently reported comparable results.
Dr. Jagsi called the findings of the studies “reassuring,” but cautioned that it will be a while before younger postmenopausal women can be offered radiation-free treatment like their older peers.
Even though the results suggest “that this might well be a really good idea,” longer follow-up and randomized data are needed “before we change the standard of care,” she said.
Of concern, for instance, is that there were six additional recurrences in the IDEA study past the 5-year mark, for a total of three recurrences among the 60 women 50-59 years old (5%) and five among the 140 women 60-69 years old (3.6%). Five of the recurrent cases were adherent to endocrine therapy.
Also, so few women in IDEA have passed the 5-year mark that “we can’t [conclude] anything” about long-term relapse risks, Dr. Jagsi said. Besides that, skipping radiation for such women at this point is “not reasonable,” Dr. Jagsi added.
Carlos Arteaga, MD, director of the UT Southwestern Simmons Cancer Center, Dallas, agreed.
“I think we have to wait. We have randomized studies that will test this in a formal way. Be that as it may, this provides the basis for a conversation physicians can have with patients because this could be an option” at some point, said Dr. Arteaga, who moderated Dr. Jagsi’s presentation.
“This is a big step in trying not to do too much for patients who don’t need it,” Virginia Kaklamani, MD, leader of the breast cancer program at UT Health San Antonio, said in an interview.
IDEA was published in the Journal of Clinical Oncology to coincide with Dr. Jagsi’s presentation.
The study was funded by the Susan G. Komen Breast Cancer Foundation and the University of Michigan Rogel Cancer Center. Dr. Jagsi has stock in Equity Quotient and research support form Genentech. Disclosure information for Arteaga was not available. Dr. Kaklamani has extensive industry ties, including being a speaker for Pfizer, Genentech, Novartis, and AstraZeneca.
FROM SABCS 2023
Sleep problems exact high toll in women with breast cancer
SAN ANTONIO — Poor sleep quality and short sleep duration in particular were associated with poorer mental well-being.
“It’s important to ask patients [with breast cancer] about sleep and provide targeted interventions to improve sleep, when needed, to improve quality of life,” Lin Yang, PhD, with Cancer Care Alberta and University of Calgary, Canada, said in an interview.
The growing population of breast cancer survivors, particularly in developed countries, is burdened by a high prevalence of sleep problems, affecting more than half of the survivors, Dr. Yang said at the San Antonio Breast Cancer Symposium.
The AMBER cohort study delved into how sleep health aspects, including sleep duration, timing, and quality, relate to the physical and mental well-being of women recently diagnosed with breast cancer.
The study recruited 1409 women with newly diagnosed early-stage breast cancer from Edmonton and Calgary, Canada, between 2012 and 2019.
The women completed the Pittsburgh Sleep Quality Index (PSQI) to assess habitual sleep duration and timing as well as sleep latency, efficiency, disturbance, medication, and daytime dysfunction and version two of the Short Form-36 (SF-36) to assess physical and mental well-being.
Multivariable linear regressions were used to estimate the association of sleep characteristics with physical and mental well-being, adjusting for sociodemographic, disease, clinical, and lifestyle behavior factors.
Among the total patient cohort (mean age, 55 years), 41% experienced either short sleep duration (less than 6 h/d) or long sleep duration (more than 9 h/d), and the same percentage also reported regularly going to bed after 11 PM.
Of note, said Dr. Yang, in the multivariable model, short sleep duration was significantly associated with poorer mental well-being (beta-coefficient, -3.6; 95% CI, -4.7 to -2.4) but not poorer physical well-being (beta-coefficient, -1.5; 95% CI, -2.3 to -0.7).
Sleep timing didn’t appear to have a meaningful impact on quality of life.
However, poor sleep quality, measured through various metrics like sleep efficiency, disturbances, medication use, and daytime dysfunction, correlated with reduced physical and mental well-being, Dr. Yang said.
She noted that targeted interventions to improve sleep health may lead to improvements in the quality of life among women with newly diagnosed breast cancer.
“Sleep is something we don’t necessarily think about in patients with breast cancer,” said Don Dizon, MD, with Brown University, Providence, Rhode Island, discussant for the study presentation.
However, this study shows the “clinical significance” of sleep, he said. “Notably 35% of this population is taking a sleeping pill.”
Dr. Yang is an editorial board member of the Journal of Healthy Eating and Active Living. Dr. Dizon receives consulting fees from Astra Zeneca, Glaxo Smith Kline, Kronos Bio, and Pfizer and industry grant support from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
SAN ANTONIO — Poor sleep quality and short sleep duration in particular were associated with poorer mental well-being.
“It’s important to ask patients [with breast cancer] about sleep and provide targeted interventions to improve sleep, when needed, to improve quality of life,” Lin Yang, PhD, with Cancer Care Alberta and University of Calgary, Canada, said in an interview.
The growing population of breast cancer survivors, particularly in developed countries, is burdened by a high prevalence of sleep problems, affecting more than half of the survivors, Dr. Yang said at the San Antonio Breast Cancer Symposium.
The AMBER cohort study delved into how sleep health aspects, including sleep duration, timing, and quality, relate to the physical and mental well-being of women recently diagnosed with breast cancer.
The study recruited 1409 women with newly diagnosed early-stage breast cancer from Edmonton and Calgary, Canada, between 2012 and 2019.
The women completed the Pittsburgh Sleep Quality Index (PSQI) to assess habitual sleep duration and timing as well as sleep latency, efficiency, disturbance, medication, and daytime dysfunction and version two of the Short Form-36 (SF-36) to assess physical and mental well-being.
Multivariable linear regressions were used to estimate the association of sleep characteristics with physical and mental well-being, adjusting for sociodemographic, disease, clinical, and lifestyle behavior factors.
Among the total patient cohort (mean age, 55 years), 41% experienced either short sleep duration (less than 6 h/d) or long sleep duration (more than 9 h/d), and the same percentage also reported regularly going to bed after 11 PM.
Of note, said Dr. Yang, in the multivariable model, short sleep duration was significantly associated with poorer mental well-being (beta-coefficient, -3.6; 95% CI, -4.7 to -2.4) but not poorer physical well-being (beta-coefficient, -1.5; 95% CI, -2.3 to -0.7).
Sleep timing didn’t appear to have a meaningful impact on quality of life.
However, poor sleep quality, measured through various metrics like sleep efficiency, disturbances, medication use, and daytime dysfunction, correlated with reduced physical and mental well-being, Dr. Yang said.
She noted that targeted interventions to improve sleep health may lead to improvements in the quality of life among women with newly diagnosed breast cancer.
“Sleep is something we don’t necessarily think about in patients with breast cancer,” said Don Dizon, MD, with Brown University, Providence, Rhode Island, discussant for the study presentation.
However, this study shows the “clinical significance” of sleep, he said. “Notably 35% of this population is taking a sleeping pill.”
Dr. Yang is an editorial board member of the Journal of Healthy Eating and Active Living. Dr. Dizon receives consulting fees from Astra Zeneca, Glaxo Smith Kline, Kronos Bio, and Pfizer and industry grant support from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
SAN ANTONIO — Poor sleep quality and short sleep duration in particular were associated with poorer mental well-being.
“It’s important to ask patients [with breast cancer] about sleep and provide targeted interventions to improve sleep, when needed, to improve quality of life,” Lin Yang, PhD, with Cancer Care Alberta and University of Calgary, Canada, said in an interview.
The growing population of breast cancer survivors, particularly in developed countries, is burdened by a high prevalence of sleep problems, affecting more than half of the survivors, Dr. Yang said at the San Antonio Breast Cancer Symposium.
The AMBER cohort study delved into how sleep health aspects, including sleep duration, timing, and quality, relate to the physical and mental well-being of women recently diagnosed with breast cancer.
The study recruited 1409 women with newly diagnosed early-stage breast cancer from Edmonton and Calgary, Canada, between 2012 and 2019.
The women completed the Pittsburgh Sleep Quality Index (PSQI) to assess habitual sleep duration and timing as well as sleep latency, efficiency, disturbance, medication, and daytime dysfunction and version two of the Short Form-36 (SF-36) to assess physical and mental well-being.
Multivariable linear regressions were used to estimate the association of sleep characteristics with physical and mental well-being, adjusting for sociodemographic, disease, clinical, and lifestyle behavior factors.
Among the total patient cohort (mean age, 55 years), 41% experienced either short sleep duration (less than 6 h/d) or long sleep duration (more than 9 h/d), and the same percentage also reported regularly going to bed after 11 PM.
Of note, said Dr. Yang, in the multivariable model, short sleep duration was significantly associated with poorer mental well-being (beta-coefficient, -3.6; 95% CI, -4.7 to -2.4) but not poorer physical well-being (beta-coefficient, -1.5; 95% CI, -2.3 to -0.7).
Sleep timing didn’t appear to have a meaningful impact on quality of life.
However, poor sleep quality, measured through various metrics like sleep efficiency, disturbances, medication use, and daytime dysfunction, correlated with reduced physical and mental well-being, Dr. Yang said.
She noted that targeted interventions to improve sleep health may lead to improvements in the quality of life among women with newly diagnosed breast cancer.
“Sleep is something we don’t necessarily think about in patients with breast cancer,” said Don Dizon, MD, with Brown University, Providence, Rhode Island, discussant for the study presentation.
However, this study shows the “clinical significance” of sleep, he said. “Notably 35% of this population is taking a sleeping pill.”
Dr. Yang is an editorial board member of the Journal of Healthy Eating and Active Living. Dr. Dizon receives consulting fees from Astra Zeneca, Glaxo Smith Kline, Kronos Bio, and Pfizer and industry grant support from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
FROM SABCS 2023
When to skip regional nodal radiation in breast cancer
SAN ANTONIO — There’s a long-standing debate in breast oncology about what to do when positive axillary lymph nodes turn negative after neoadjuvant chemotherapy.
Currently, it’s about a 50/50 split among oncologists, according to breast cancer surgeon Eleftherios P. Mamounas, MD, medical director of the Comprehensive Breast Program at the Orlando Health Cancer Institute in Florida.
Until now, Dr. Mamounas’s own institution has opted for regional irradiation just to be on the safe side, but he said that’s going to change in the wake of a multicenter trial he presented at the San Antonio Breast Cancer Symposium.
Dr. Mamounas led a team that randomized 1556 women equally to either regional nodal irradiation or no nodal irradiation following surgery, which was lumpectomy in just over half the subjects and mastectomy in the rest.
Women were a median of 52 years old. Almost 60% had T2 disease and the rest were split about evenly between T1 and T3 disease. Nearly a quarter of the tumors were triple-negative, and over half were HER2 positive.
In the regional nodal irradiation arm, women who had mastectomies had chest wall irradiation in addition to regional nodal irradiation, while women who underwent lumpectomies had whole breast irradiation with regional nodal irradiation. In the no-irradiation group, mastectomies were followed by observation and lumpectomies by whole breast irradiation alone.
All the women had positive axillary lymph nodes (N1) on needle biopsies at baseline that were found to be free of cancer at surgery (ypN0) following neoadjuvant chemotherapy. Neoadjuvant therapy consisted of at least 8 weeks of chemotherapy plus anti-HER2 therapy for HER2-positive patients.
Dr. Mamounas and colleagues observed no meaningful differences in outcomes between the two groups: 92.7% of women in the nodal irradiation arm and 91.8% in the no-irradiation arm were free from invasive recurrences 5 years after surgery.
Patients in both groups demonstrated similar 5-year disease-free survival and overall survival. The 5-year disease-free survival was 88.5% without and 88.3% with regional nodal irradiation and 5-year overall survival was 94% without and 93.6% with regional nodal irradiation.
The team did not observe study-related deaths or unexpected toxicities. Overall, 6.5% of patients without regional nodal irradiation developed grade 3 toxicity vs. 10% of patients with irradiation. Grade 4 toxicity was rare, occurring in 0.1% of patients in the no-irradiation group vs. 0.5% in the irradiation group.
The trial answers “a very important question,” according to Kate Lathrop, MD, a breast medical oncologist at UT Health San Antonio, who moderated the presentation.
The trial results were “highly awaited” because “we didn’t have the data to make these” decisions, Dr. Lathrop said.
Knowing these patients do just as well without regional nodal irradiation is “going to change a lot of opinions,” said Dr. Lathrop, because we can avoid subjecting patients to unnecessary toxicity, including lymphedema with regional nodal irradiation as well as problems with breast reconstruction after mastectomy.
Because recurrences can still occur after 5 years, Dr. Mamounas’s team will continue to follow the women, but he believes “it’s very unlikely that long-term distant disease-free survival will change.”
Based on the study, “we will omit” regional nodal irradiation for women who fit the study criteria, Dr. Mamounas said.
When asked if women should ask their doctors about skipping regional nodal irradiation, Dr. Mamounas said “absolutely.”
“I think it requires a discussion at this point,” he explained. “Based on the data, it’s a reasonable conclusion that radiotherapy can be avoided” in many cases, such as in lower-stage women with one initially positive node.
Dr. Mamounas said he thinks patients will be interested in the approach “because they are really looking to avoid radiotherapy” if they can.
The work was funded by the National Cancer Institute. Dr. Mamounas has been a consultant and speaker for Genentech, Merck, and an adviser for TerSera Therapeutics, Biotheranostics Inc., and Sanofi. He owns stock in Moderna. Dr. Lathrop is a consultant for Pfizer, GE Healthcare, and Biotheranostics, and a speaker for Biotheranostics.
A version of this article first appeared on Medscape.com.
SAN ANTONIO — There’s a long-standing debate in breast oncology about what to do when positive axillary lymph nodes turn negative after neoadjuvant chemotherapy.
Currently, it’s about a 50/50 split among oncologists, according to breast cancer surgeon Eleftherios P. Mamounas, MD, medical director of the Comprehensive Breast Program at the Orlando Health Cancer Institute in Florida.
Until now, Dr. Mamounas’s own institution has opted for regional irradiation just to be on the safe side, but he said that’s going to change in the wake of a multicenter trial he presented at the San Antonio Breast Cancer Symposium.
Dr. Mamounas led a team that randomized 1556 women equally to either regional nodal irradiation or no nodal irradiation following surgery, which was lumpectomy in just over half the subjects and mastectomy in the rest.
Women were a median of 52 years old. Almost 60% had T2 disease and the rest were split about evenly between T1 and T3 disease. Nearly a quarter of the tumors were triple-negative, and over half were HER2 positive.
In the regional nodal irradiation arm, women who had mastectomies had chest wall irradiation in addition to regional nodal irradiation, while women who underwent lumpectomies had whole breast irradiation with regional nodal irradiation. In the no-irradiation group, mastectomies were followed by observation and lumpectomies by whole breast irradiation alone.
All the women had positive axillary lymph nodes (N1) on needle biopsies at baseline that were found to be free of cancer at surgery (ypN0) following neoadjuvant chemotherapy. Neoadjuvant therapy consisted of at least 8 weeks of chemotherapy plus anti-HER2 therapy for HER2-positive patients.
Dr. Mamounas and colleagues observed no meaningful differences in outcomes between the two groups: 92.7% of women in the nodal irradiation arm and 91.8% in the no-irradiation arm were free from invasive recurrences 5 years after surgery.
Patients in both groups demonstrated similar 5-year disease-free survival and overall survival. The 5-year disease-free survival was 88.5% without and 88.3% with regional nodal irradiation and 5-year overall survival was 94% without and 93.6% with regional nodal irradiation.
The team did not observe study-related deaths or unexpected toxicities. Overall, 6.5% of patients without regional nodal irradiation developed grade 3 toxicity vs. 10% of patients with irradiation. Grade 4 toxicity was rare, occurring in 0.1% of patients in the no-irradiation group vs. 0.5% in the irradiation group.
The trial answers “a very important question,” according to Kate Lathrop, MD, a breast medical oncologist at UT Health San Antonio, who moderated the presentation.
The trial results were “highly awaited” because “we didn’t have the data to make these” decisions, Dr. Lathrop said.
Knowing these patients do just as well without regional nodal irradiation is “going to change a lot of opinions,” said Dr. Lathrop, because we can avoid subjecting patients to unnecessary toxicity, including lymphedema with regional nodal irradiation as well as problems with breast reconstruction after mastectomy.
Because recurrences can still occur after 5 years, Dr. Mamounas’s team will continue to follow the women, but he believes “it’s very unlikely that long-term distant disease-free survival will change.”
Based on the study, “we will omit” regional nodal irradiation for women who fit the study criteria, Dr. Mamounas said.
When asked if women should ask their doctors about skipping regional nodal irradiation, Dr. Mamounas said “absolutely.”
“I think it requires a discussion at this point,” he explained. “Based on the data, it’s a reasonable conclusion that radiotherapy can be avoided” in many cases, such as in lower-stage women with one initially positive node.
Dr. Mamounas said he thinks patients will be interested in the approach “because they are really looking to avoid radiotherapy” if they can.
The work was funded by the National Cancer Institute. Dr. Mamounas has been a consultant and speaker for Genentech, Merck, and an adviser for TerSera Therapeutics, Biotheranostics Inc., and Sanofi. He owns stock in Moderna. Dr. Lathrop is a consultant for Pfizer, GE Healthcare, and Biotheranostics, and a speaker for Biotheranostics.
A version of this article first appeared on Medscape.com.
SAN ANTONIO — There’s a long-standing debate in breast oncology about what to do when positive axillary lymph nodes turn negative after neoadjuvant chemotherapy.
Currently, it’s about a 50/50 split among oncologists, according to breast cancer surgeon Eleftherios P. Mamounas, MD, medical director of the Comprehensive Breast Program at the Orlando Health Cancer Institute in Florida.
Until now, Dr. Mamounas’s own institution has opted for regional irradiation just to be on the safe side, but he said that’s going to change in the wake of a multicenter trial he presented at the San Antonio Breast Cancer Symposium.
Dr. Mamounas led a team that randomized 1556 women equally to either regional nodal irradiation or no nodal irradiation following surgery, which was lumpectomy in just over half the subjects and mastectomy in the rest.
Women were a median of 52 years old. Almost 60% had T2 disease and the rest were split about evenly between T1 and T3 disease. Nearly a quarter of the tumors were triple-negative, and over half were HER2 positive.
In the regional nodal irradiation arm, women who had mastectomies had chest wall irradiation in addition to regional nodal irradiation, while women who underwent lumpectomies had whole breast irradiation with regional nodal irradiation. In the no-irradiation group, mastectomies were followed by observation and lumpectomies by whole breast irradiation alone.
All the women had positive axillary lymph nodes (N1) on needle biopsies at baseline that were found to be free of cancer at surgery (ypN0) following neoadjuvant chemotherapy. Neoadjuvant therapy consisted of at least 8 weeks of chemotherapy plus anti-HER2 therapy for HER2-positive patients.
Dr. Mamounas and colleagues observed no meaningful differences in outcomes between the two groups: 92.7% of women in the nodal irradiation arm and 91.8% in the no-irradiation arm were free from invasive recurrences 5 years after surgery.
Patients in both groups demonstrated similar 5-year disease-free survival and overall survival. The 5-year disease-free survival was 88.5% without and 88.3% with regional nodal irradiation and 5-year overall survival was 94% without and 93.6% with regional nodal irradiation.
The team did not observe study-related deaths or unexpected toxicities. Overall, 6.5% of patients without regional nodal irradiation developed grade 3 toxicity vs. 10% of patients with irradiation. Grade 4 toxicity was rare, occurring in 0.1% of patients in the no-irradiation group vs. 0.5% in the irradiation group.
The trial answers “a very important question,” according to Kate Lathrop, MD, a breast medical oncologist at UT Health San Antonio, who moderated the presentation.
The trial results were “highly awaited” because “we didn’t have the data to make these” decisions, Dr. Lathrop said.
Knowing these patients do just as well without regional nodal irradiation is “going to change a lot of opinions,” said Dr. Lathrop, because we can avoid subjecting patients to unnecessary toxicity, including lymphedema with regional nodal irradiation as well as problems with breast reconstruction after mastectomy.
Because recurrences can still occur after 5 years, Dr. Mamounas’s team will continue to follow the women, but he believes “it’s very unlikely that long-term distant disease-free survival will change.”
Based on the study, “we will omit” regional nodal irradiation for women who fit the study criteria, Dr. Mamounas said.
When asked if women should ask their doctors about skipping regional nodal irradiation, Dr. Mamounas said “absolutely.”
“I think it requires a discussion at this point,” he explained. “Based on the data, it’s a reasonable conclusion that radiotherapy can be avoided” in many cases, such as in lower-stage women with one initially positive node.
Dr. Mamounas said he thinks patients will be interested in the approach “because they are really looking to avoid radiotherapy” if they can.
The work was funded by the National Cancer Institute. Dr. Mamounas has been a consultant and speaker for Genentech, Merck, and an adviser for TerSera Therapeutics, Biotheranostics Inc., and Sanofi. He owns stock in Moderna. Dr. Lathrop is a consultant for Pfizer, GE Healthcare, and Biotheranostics, and a speaker for Biotheranostics.
A version of this article first appeared on Medscape.com.
AT SABCS 2023
