User login
Increase in late-stage cancer diagnoses after pandemic
at Moores Cancer Center in La Jolla, Calif., according to a research letter in JAMA Network Open.
“The number of patients presenting at late, incurable stages is increasing,” say the authors, led by Jade Zifei Zhou, MD, PhD, a hematology/oncology fellow at the center, which is affiliated with the University of California, San Diego.
As the pandemic unfolded and much of routine medicine was put on hold, the postponement or delay in mammograms, colonoscopies, and other screenings led many cancer experts to warn of trouble ahead. In June 2020, for instance, the National Cancer Institute predicted tens of thousands of excess cancer deaths through 2030 because of missed screenings and delays in care.
The message now, Dr. Zhou and colleagues say, is that “patients who have delayed preventative care during the pandemic should be encouraged to resume treatment as soon as possible.”
The team compared the number of people presenting to their cancer center with stage I and IV disease, either for a new diagnosis or a second opinion, during 2019 and with the number during 2020, the first year of the pandemic. The review included over 500 patients, almost 90% of whom were women aged 58 years on average.
While 63.9% of patients with breast cancer presented with stage I disease in 2019, 51.3% did so in 2020. Conversely, while just 1.9% presented with stage IV breast cancer in 2019, the number went up to 6.2% in 2020.
The numbers were even worse from January through March 2021, with only 41.9% of women presenting with stage I and 8% presenting with stage IV breast cancer.
It was the same story for colon cancer, but because of smaller numbers, the findings were not statistically significant.
After the start of the pandemic, the number of patients presenting with stage I colon cancer fell from 17.8% (eight patients) to 14.6% (six patients), while stage IV presentations climbed from 6.7% (three) to 19.5% (eight).
Across all cancer types, stage I presentations fell from 31.9% in 2019 to 29% in 2020, while stage IV presentations rose from 26% to 26.4%.
One of the study limitations is that the patients who came in for a second opinion could have been newly diagnosed but might also have been referred for refractory disease, the authors comment.
No funding for this study was reported. Senior author Kathryn Ann Gold, MD, reported personal fees from AstraZeneca, Takeda, Rakuten, and Regeneron as well as grants from Pfizer and Pharmacyclics.
A version of this article first appeared on Medscape.com.
at Moores Cancer Center in La Jolla, Calif., according to a research letter in JAMA Network Open.
“The number of patients presenting at late, incurable stages is increasing,” say the authors, led by Jade Zifei Zhou, MD, PhD, a hematology/oncology fellow at the center, which is affiliated with the University of California, San Diego.
As the pandemic unfolded and much of routine medicine was put on hold, the postponement or delay in mammograms, colonoscopies, and other screenings led many cancer experts to warn of trouble ahead. In June 2020, for instance, the National Cancer Institute predicted tens of thousands of excess cancer deaths through 2030 because of missed screenings and delays in care.
The message now, Dr. Zhou and colleagues say, is that “patients who have delayed preventative care during the pandemic should be encouraged to resume treatment as soon as possible.”
The team compared the number of people presenting to their cancer center with stage I and IV disease, either for a new diagnosis or a second opinion, during 2019 and with the number during 2020, the first year of the pandemic. The review included over 500 patients, almost 90% of whom were women aged 58 years on average.
While 63.9% of patients with breast cancer presented with stage I disease in 2019, 51.3% did so in 2020. Conversely, while just 1.9% presented with stage IV breast cancer in 2019, the number went up to 6.2% in 2020.
The numbers were even worse from January through March 2021, with only 41.9% of women presenting with stage I and 8% presenting with stage IV breast cancer.
It was the same story for colon cancer, but because of smaller numbers, the findings were not statistically significant.
After the start of the pandemic, the number of patients presenting with stage I colon cancer fell from 17.8% (eight patients) to 14.6% (six patients), while stage IV presentations climbed from 6.7% (three) to 19.5% (eight).
Across all cancer types, stage I presentations fell from 31.9% in 2019 to 29% in 2020, while stage IV presentations rose from 26% to 26.4%.
One of the study limitations is that the patients who came in for a second opinion could have been newly diagnosed but might also have been referred for refractory disease, the authors comment.
No funding for this study was reported. Senior author Kathryn Ann Gold, MD, reported personal fees from AstraZeneca, Takeda, Rakuten, and Regeneron as well as grants from Pfizer and Pharmacyclics.
A version of this article first appeared on Medscape.com.
at Moores Cancer Center in La Jolla, Calif., according to a research letter in JAMA Network Open.
“The number of patients presenting at late, incurable stages is increasing,” say the authors, led by Jade Zifei Zhou, MD, PhD, a hematology/oncology fellow at the center, which is affiliated with the University of California, San Diego.
As the pandemic unfolded and much of routine medicine was put on hold, the postponement or delay in mammograms, colonoscopies, and other screenings led many cancer experts to warn of trouble ahead. In June 2020, for instance, the National Cancer Institute predicted tens of thousands of excess cancer deaths through 2030 because of missed screenings and delays in care.
The message now, Dr. Zhou and colleagues say, is that “patients who have delayed preventative care during the pandemic should be encouraged to resume treatment as soon as possible.”
The team compared the number of people presenting to their cancer center with stage I and IV disease, either for a new diagnosis or a second opinion, during 2019 and with the number during 2020, the first year of the pandemic. The review included over 500 patients, almost 90% of whom were women aged 58 years on average.
While 63.9% of patients with breast cancer presented with stage I disease in 2019, 51.3% did so in 2020. Conversely, while just 1.9% presented with stage IV breast cancer in 2019, the number went up to 6.2% in 2020.
The numbers were even worse from January through March 2021, with only 41.9% of women presenting with stage I and 8% presenting with stage IV breast cancer.
It was the same story for colon cancer, but because of smaller numbers, the findings were not statistically significant.
After the start of the pandemic, the number of patients presenting with stage I colon cancer fell from 17.8% (eight patients) to 14.6% (six patients), while stage IV presentations climbed from 6.7% (three) to 19.5% (eight).
Across all cancer types, stage I presentations fell from 31.9% in 2019 to 29% in 2020, while stage IV presentations rose from 26% to 26.4%.
One of the study limitations is that the patients who came in for a second opinion could have been newly diagnosed but might also have been referred for refractory disease, the authors comment.
No funding for this study was reported. Senior author Kathryn Ann Gold, MD, reported personal fees from AstraZeneca, Takeda, Rakuten, and Regeneron as well as grants from Pfizer and Pharmacyclics.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Late-onset recurrence in breast cancer: Implications for women’s health clinicians in survivorship care
Improved treatments for breast cancer (BC) and effective screening programs have resulted in a BC mortality rate reduction of 41% since 1989.1 Because BC is the leading cause of cancer in women, these mortality improvements have resulted in more than 3 million BC survivors in the United States.2,3 With longer-term survival, there is increasing interest in late-onset recurrences.4,5 A recent study has provided an improved understanding of the risk of lateonset recurrence in women with 10 years of disease-free survival, an important finding for women’s health providers because oncologists do not typically follow survivors after 10 years of disease-free survival.4
Recent study looks at incidence of late-onset recurrence
Pederson and colleagues evaluated all patients diagnosed with BC in Denmark from 1987 through 2004.4 Those patients without evidence of recurrence at 10 years were then followed utilizing population-based linked registries to identify patients who subsequently developed a local, regional, or distant late-onset recurrence. The authors evaluated the frequency of late recurrence and identified associations with demographic and tumor characteristics.
What they found
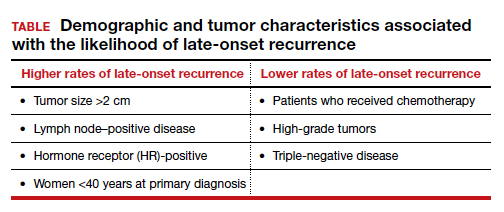
A total of 36,920 patients were diagnosed with BC in Denmark between 1987-2004, of whom 20,315 (55%) were identified as disease free for at least 10 years. Late-onset recurrence occurred in 2,595 (12.8%) with the strongest associations of recurrence seen in patients who had a tumor size >2 cm and lymph node‒positive (involving 4 or more nodes) disease (24.6%), compared with 12.7% in patients with tumors <2 cm and node-negative disease. Several other factors were associated with a higher risk of late-onset recurrence and are included in the TABLE. Half of the recurrences occurred between 10 and 15 years after the primary diagnosis.
Prior research
These findings are consistent with another recent study showing that BC patients have a 1% to 2%/year risk of recurrence after 10 disease-free years.5 Strengths of this study include:
- population-based, including all women with BC
- long-term follow-up for up to 32 years
- universal health care in Denmark, which results in robust and linked databases and very few missing data points.
There were two notable weaknesses to consider:
- Treatment regimens changed considerably during the time frame of the study (1997-2018), particularly the duration of tamoxifen use in patients with HR-positive disease. In this study nearly all patients received 5 years or less of tamoxifen. Since the mid-2010s, 10 years of hormonal adjuvant therapy has become routine in HR-positive BC, which reduces recurrences, including late-onset recurrence.6 The effect of 10 years of tamoxifen would very likely have resulted in less late-onset recurrence in the HR-positive population in this study.
- There is a lack of racial diversity in the Danish population, and the study findings may not translate to Black patients who have a higher frequency of triple-negative BC with a different risk of late-onset recurrence.7
Practice takeaways
Cancer surveillance. There are 3+ million BC survivors in the United States, and a 55%+ likelihood that they will be disease free for 10 years. This is clearly an important population to the women’s health care provider. This study, and previous research, suggests that among 10-year-disease-free survivors, 1% to 2% will recur annually, with higher rates amongst HR-positive, lymph-node positive women under age 40, and in the first 5 years following the 10-year post–initial diagnosis mark, so ongoing surveillance is imperative. Annual clinical breast examinations along with annual (not biennial) mammography should be performed.8 Digital breast tomosynthesis has improved specificity and sensitivity for BC detection and is the preferred modality when it is available.
Management of menopausal symptoms. These findings also have implications for menopausal hormone therapy for patients with symptoms. Because HR-positive patients have an increased risk of late-onset recurrence, nonhormonal therapies should be considered as first-line therapy for patients with menopausal symptoms. If hormone therapy is being considered, providers and patients should use shared decision making to balance the potential benefits (reduction in symptoms, possible cardiovascular benefits, and reduction in bone loss) with the risks (increased risk of recurrence and venous thromboembolism), even if patients are remote from the original diagnosis (ie, 10-year disease-free survival).
Topical estrogen therapies would be preferred for patients with significant urogenital atrophic symptoms who fail nonhormonal therapies due to substantially less systemic absorption and the lack of need to add a progestin.9,10 If oral therapy is being considered, I carefully counsel these women about the likely increased risk of recurrence and, if possible, include their breast oncologist in the discussion. ●
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654.
- de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22:561- 570. doi: 10.1158/1055-9965.EPI-12-1356.
- Carreira H, Williams R, Funston G, et al. Associations between breast cancer survivorship and adverse mental health outcomes: a matched population-based cohort study in the United Kingdom. PLOS Med. 2021;18:e1003504. doi: 10.1371/journal.pmed.1003504.
- Pedersen RN, Esen BÖ, Mellemkjær L, et al. The incidence of breast cancer recurrence 10-32 years after primary diagnosis. J Natl Cancer Inst. November 8, 2021. doi: 10.1093/jnci/djab202.
- Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836- 1846. doi: 10.1056/NEJMoa1701830.
- Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805-816. doi: 10.1016/S0140-6736(12)61963-1.
- Scott LC, Mobley LR, Kuo TM, et al. Update on triple‐negative breast cancer disparities for the United States: a population‐based study from the United States Cancer Statistics database, 2010 through 2014. Cancer. 2019;125:3412-3417. doi: 10.1002/cncr.32207.
- NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. 2021; Version 2.2022.
- Crandall CJ, Diamant A, Santoro N. Safety of vaginal estrogens: a systematic review. Menopause. 2020;27:339-360. doi: 10.1097 /GME.0000000000001468.
- Treatment of urogenital symptoms in individuals with a history of estrogen-dependent breast cancer: clinical consensus. Obstet Gynecol. 2021;138:950-960. doi: 10.1097/AOG .0000000000004601.
Improved treatments for breast cancer (BC) and effective screening programs have resulted in a BC mortality rate reduction of 41% since 1989.1 Because BC is the leading cause of cancer in women, these mortality improvements have resulted in more than 3 million BC survivors in the United States.2,3 With longer-term survival, there is increasing interest in late-onset recurrences.4,5 A recent study has provided an improved understanding of the risk of lateonset recurrence in women with 10 years of disease-free survival, an important finding for women’s health providers because oncologists do not typically follow survivors after 10 years of disease-free survival.4
Recent study looks at incidence of late-onset recurrence
Pederson and colleagues evaluated all patients diagnosed with BC in Denmark from 1987 through 2004.4 Those patients without evidence of recurrence at 10 years were then followed utilizing population-based linked registries to identify patients who subsequently developed a local, regional, or distant late-onset recurrence. The authors evaluated the frequency of late recurrence and identified associations with demographic and tumor characteristics.
What they found
A total of 36,920 patients were diagnosed with BC in Denmark between 1987-2004, of whom 20,315 (55%) were identified as disease free for at least 10 years. Late-onset recurrence occurred in 2,595 (12.8%) with the strongest associations of recurrence seen in patients who had a tumor size >2 cm and lymph node‒positive (involving 4 or more nodes) disease (24.6%), compared with 12.7% in patients with tumors <2 cm and node-negative disease. Several other factors were associated with a higher risk of late-onset recurrence and are included in the TABLE. Half of the recurrences occurred between 10 and 15 years after the primary diagnosis.

Prior research
These findings are consistent with another recent study showing that BC patients have a 1% to 2%/year risk of recurrence after 10 disease-free years.5 Strengths of this study include:
- population-based, including all women with BC
- long-term follow-up for up to 32 years
- universal health care in Denmark, which results in robust and linked databases and very few missing data points.
There were two notable weaknesses to consider:
- Treatment regimens changed considerably during the time frame of the study (1997-2018), particularly the duration of tamoxifen use in patients with HR-positive disease. In this study nearly all patients received 5 years or less of tamoxifen. Since the mid-2010s, 10 years of hormonal adjuvant therapy has become routine in HR-positive BC, which reduces recurrences, including late-onset recurrence.6 The effect of 10 years of tamoxifen would very likely have resulted in less late-onset recurrence in the HR-positive population in this study.
- There is a lack of racial diversity in the Danish population, and the study findings may not translate to Black patients who have a higher frequency of triple-negative BC with a different risk of late-onset recurrence.7
Practice takeaways
Cancer surveillance. There are 3+ million BC survivors in the United States, and a 55%+ likelihood that they will be disease free for 10 years. This is clearly an important population to the women’s health care provider. This study, and previous research, suggests that among 10-year-disease-free survivors, 1% to 2% will recur annually, with higher rates amongst HR-positive, lymph-node positive women under age 40, and in the first 5 years following the 10-year post–initial diagnosis mark, so ongoing surveillance is imperative. Annual clinical breast examinations along with annual (not biennial) mammography should be performed.8 Digital breast tomosynthesis has improved specificity and sensitivity for BC detection and is the preferred modality when it is available.
Management of menopausal symptoms. These findings also have implications for menopausal hormone therapy for patients with symptoms. Because HR-positive patients have an increased risk of late-onset recurrence, nonhormonal therapies should be considered as first-line therapy for patients with menopausal symptoms. If hormone therapy is being considered, providers and patients should use shared decision making to balance the potential benefits (reduction in symptoms, possible cardiovascular benefits, and reduction in bone loss) with the risks (increased risk of recurrence and venous thromboembolism), even if patients are remote from the original diagnosis (ie, 10-year disease-free survival).
Topical estrogen therapies would be preferred for patients with significant urogenital atrophic symptoms who fail nonhormonal therapies due to substantially less systemic absorption and the lack of need to add a progestin.9,10 If oral therapy is being considered, I carefully counsel these women about the likely increased risk of recurrence and, if possible, include their breast oncologist in the discussion. ●
Improved treatments for breast cancer (BC) and effective screening programs have resulted in a BC mortality rate reduction of 41% since 1989.1 Because BC is the leading cause of cancer in women, these mortality improvements have resulted in more than 3 million BC survivors in the United States.2,3 With longer-term survival, there is increasing interest in late-onset recurrences.4,5 A recent study has provided an improved understanding of the risk of lateonset recurrence in women with 10 years of disease-free survival, an important finding for women’s health providers because oncologists do not typically follow survivors after 10 years of disease-free survival.4
Recent study looks at incidence of late-onset recurrence
Pederson and colleagues evaluated all patients diagnosed with BC in Denmark from 1987 through 2004.4 Those patients without evidence of recurrence at 10 years were then followed utilizing population-based linked registries to identify patients who subsequently developed a local, regional, or distant late-onset recurrence. The authors evaluated the frequency of late recurrence and identified associations with demographic and tumor characteristics.
What they found
A total of 36,920 patients were diagnosed with BC in Denmark between 1987-2004, of whom 20,315 (55%) were identified as disease free for at least 10 years. Late-onset recurrence occurred in 2,595 (12.8%) with the strongest associations of recurrence seen in patients who had a tumor size >2 cm and lymph node‒positive (involving 4 or more nodes) disease (24.6%), compared with 12.7% in patients with tumors <2 cm and node-negative disease. Several other factors were associated with a higher risk of late-onset recurrence and are included in the TABLE. Half of the recurrences occurred between 10 and 15 years after the primary diagnosis.

Prior research
These findings are consistent with another recent study showing that BC patients have a 1% to 2%/year risk of recurrence after 10 disease-free years.5 Strengths of this study include:
- population-based, including all women with BC
- long-term follow-up for up to 32 years
- universal health care in Denmark, which results in robust and linked databases and very few missing data points.
There were two notable weaknesses to consider:
- Treatment regimens changed considerably during the time frame of the study (1997-2018), particularly the duration of tamoxifen use in patients with HR-positive disease. In this study nearly all patients received 5 years or less of tamoxifen. Since the mid-2010s, 10 years of hormonal adjuvant therapy has become routine in HR-positive BC, which reduces recurrences, including late-onset recurrence.6 The effect of 10 years of tamoxifen would very likely have resulted in less late-onset recurrence in the HR-positive population in this study.
- There is a lack of racial diversity in the Danish population, and the study findings may not translate to Black patients who have a higher frequency of triple-negative BC with a different risk of late-onset recurrence.7
Practice takeaways
Cancer surveillance. There are 3+ million BC survivors in the United States, and a 55%+ likelihood that they will be disease free for 10 years. This is clearly an important population to the women’s health care provider. This study, and previous research, suggests that among 10-year-disease-free survivors, 1% to 2% will recur annually, with higher rates amongst HR-positive, lymph-node positive women under age 40, and in the first 5 years following the 10-year post–initial diagnosis mark, so ongoing surveillance is imperative. Annual clinical breast examinations along with annual (not biennial) mammography should be performed.8 Digital breast tomosynthesis has improved specificity and sensitivity for BC detection and is the preferred modality when it is available.
Management of menopausal symptoms. These findings also have implications for menopausal hormone therapy for patients with symptoms. Because HR-positive patients have an increased risk of late-onset recurrence, nonhormonal therapies should be considered as first-line therapy for patients with menopausal symptoms. If hormone therapy is being considered, providers and patients should use shared decision making to balance the potential benefits (reduction in symptoms, possible cardiovascular benefits, and reduction in bone loss) with the risks (increased risk of recurrence and venous thromboembolism), even if patients are remote from the original diagnosis (ie, 10-year disease-free survival).
Topical estrogen therapies would be preferred for patients with significant urogenital atrophic symptoms who fail nonhormonal therapies due to substantially less systemic absorption and the lack of need to add a progestin.9,10 If oral therapy is being considered, I carefully counsel these women about the likely increased risk of recurrence and, if possible, include their breast oncologist in the discussion. ●
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654.
- de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22:561- 570. doi: 10.1158/1055-9965.EPI-12-1356.
- Carreira H, Williams R, Funston G, et al. Associations between breast cancer survivorship and adverse mental health outcomes: a matched population-based cohort study in the United Kingdom. PLOS Med. 2021;18:e1003504. doi: 10.1371/journal.pmed.1003504.
- Pedersen RN, Esen BÖ, Mellemkjær L, et al. The incidence of breast cancer recurrence 10-32 years after primary diagnosis. J Natl Cancer Inst. November 8, 2021. doi: 10.1093/jnci/djab202.
- Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836- 1846. doi: 10.1056/NEJMoa1701830.
- Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805-816. doi: 10.1016/S0140-6736(12)61963-1.
- Scott LC, Mobley LR, Kuo TM, et al. Update on triple‐negative breast cancer disparities for the United States: a population‐based study from the United States Cancer Statistics database, 2010 through 2014. Cancer. 2019;125:3412-3417. doi: 10.1002/cncr.32207.
- NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. 2021; Version 2.2022.
- Crandall CJ, Diamant A, Santoro N. Safety of vaginal estrogens: a systematic review. Menopause. 2020;27:339-360. doi: 10.1097 /GME.0000000000001468.
- Treatment of urogenital symptoms in individuals with a history of estrogen-dependent breast cancer: clinical consensus. Obstet Gynecol. 2021;138:950-960. doi: 10.1097/AOG .0000000000004601.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654.
- de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22:561- 570. doi: 10.1158/1055-9965.EPI-12-1356.
- Carreira H, Williams R, Funston G, et al. Associations between breast cancer survivorship and adverse mental health outcomes: a matched population-based cohort study in the United Kingdom. PLOS Med. 2021;18:e1003504. doi: 10.1371/journal.pmed.1003504.
- Pedersen RN, Esen BÖ, Mellemkjær L, et al. The incidence of breast cancer recurrence 10-32 years after primary diagnosis. J Natl Cancer Inst. November 8, 2021. doi: 10.1093/jnci/djab202.
- Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836- 1846. doi: 10.1056/NEJMoa1701830.
- Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805-816. doi: 10.1016/S0140-6736(12)61963-1.
- Scott LC, Mobley LR, Kuo TM, et al. Update on triple‐negative breast cancer disparities for the United States: a population‐based study from the United States Cancer Statistics database, 2010 through 2014. Cancer. 2019;125:3412-3417. doi: 10.1002/cncr.32207.
- NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. 2021; Version 2.2022.
- Crandall CJ, Diamant A, Santoro N. Safety of vaginal estrogens: a systematic review. Menopause. 2020;27:339-360. doi: 10.1097 /GME.0000000000001468.
- Treatment of urogenital symptoms in individuals with a history of estrogen-dependent breast cancer: clinical consensus. Obstet Gynecol. 2021;138:950-960. doi: 10.1097/AOG .0000000000004601.
Breast cancer now leading cause of cancer death in Black women
Breast cancer has replaced lung cancer as the leading cause of cancer-related death among Black women, but lung cancer remains the leading cause of cancer death in Black men, according to a new report from the American Cancer Society (ACS).
Lung cancer remains the second most commonly diagnosed cancer in both Black women and Black men.
These are among the key findings of the report, Cancer Statistics for African American/Black People 2022 – a triannual compilation of U.S. data on cancer incidence, mortality, survival, screening, and risk factors for Black people – and it marks a major shift as of 2019.
“African American/Black people have a disproportionately high cancer burden compared to other population groups. According to the report, the risk of cancer death for Black individuals remains 19% higher for men and 12% higher for women compared to White individuals,” the ACS says in a statement.
“The gap for breast cancer is more alarming,” it adds. “Black women are 41% more likely to die from breast cancer than White women despite a lower risk of being diagnosed with the disease.”
The new report, published online on Feb. 10 in CA: A Cancer Journal for Clinicians, also notes the following:
An estimated 224,080 new cancer cases and 73,680 cancer deaths will occur among Black people in 2022.
Over the past 5 data years, Black women had an 8% lower overall cancer incidence than White women but 12% higher mortality; Black men have 6% higher cancer incidence than White men but 19% higher cancer mortality.
Prostate cancer mortality among Black men decreased by 1.3% per year from 2015 to 2019 despite a 5% increase in the diagnosis of distant-stage prostate cancer annually since 2012, but the decline was slower than the 5% per year decline from 2010 to 2014.
The overall cancer mortality gap between Black and White people is narrowing. This is due to a steeper drop in prostate, lung, and other smoking-related cancers among Black people.
Colorectal cancer incidence and mortality rates are 21% and 44% higher, respectively, in Black men in comparison with White men and 18% and 31% higher, respectively, in Black women in comparison with White women.
The reasons for the disparities are complex but “largely stem from less access to high-quality care and optimal treatment as a repercussion of long-standing institutional racism,” the report concludes.
“We must address structural racism as a public health issue to close the gaps and advance health equity,” Tawana Thomas-Johnson, senior vice president and chief diversity officer at the ACS, said in the press release.
A version of this article first appeared on Medscape.com.
Breast cancer has replaced lung cancer as the leading cause of cancer-related death among Black women, but lung cancer remains the leading cause of cancer death in Black men, according to a new report from the American Cancer Society (ACS).
Lung cancer remains the second most commonly diagnosed cancer in both Black women and Black men.
These are among the key findings of the report, Cancer Statistics for African American/Black People 2022 – a triannual compilation of U.S. data on cancer incidence, mortality, survival, screening, and risk factors for Black people – and it marks a major shift as of 2019.
“African American/Black people have a disproportionately high cancer burden compared to other population groups. According to the report, the risk of cancer death for Black individuals remains 19% higher for men and 12% higher for women compared to White individuals,” the ACS says in a statement.
“The gap for breast cancer is more alarming,” it adds. “Black women are 41% more likely to die from breast cancer than White women despite a lower risk of being diagnosed with the disease.”
The new report, published online on Feb. 10 in CA: A Cancer Journal for Clinicians, also notes the following:
An estimated 224,080 new cancer cases and 73,680 cancer deaths will occur among Black people in 2022.
Over the past 5 data years, Black women had an 8% lower overall cancer incidence than White women but 12% higher mortality; Black men have 6% higher cancer incidence than White men but 19% higher cancer mortality.
Prostate cancer mortality among Black men decreased by 1.3% per year from 2015 to 2019 despite a 5% increase in the diagnosis of distant-stage prostate cancer annually since 2012, but the decline was slower than the 5% per year decline from 2010 to 2014.
The overall cancer mortality gap between Black and White people is narrowing. This is due to a steeper drop in prostate, lung, and other smoking-related cancers among Black people.
Colorectal cancer incidence and mortality rates are 21% and 44% higher, respectively, in Black men in comparison with White men and 18% and 31% higher, respectively, in Black women in comparison with White women.
The reasons for the disparities are complex but “largely stem from less access to high-quality care and optimal treatment as a repercussion of long-standing institutional racism,” the report concludes.
“We must address structural racism as a public health issue to close the gaps and advance health equity,” Tawana Thomas-Johnson, senior vice president and chief diversity officer at the ACS, said in the press release.
A version of this article first appeared on Medscape.com.
Breast cancer has replaced lung cancer as the leading cause of cancer-related death among Black women, but lung cancer remains the leading cause of cancer death in Black men, according to a new report from the American Cancer Society (ACS).
Lung cancer remains the second most commonly diagnosed cancer in both Black women and Black men.
These are among the key findings of the report, Cancer Statistics for African American/Black People 2022 – a triannual compilation of U.S. data on cancer incidence, mortality, survival, screening, and risk factors for Black people – and it marks a major shift as of 2019.
“African American/Black people have a disproportionately high cancer burden compared to other population groups. According to the report, the risk of cancer death for Black individuals remains 19% higher for men and 12% higher for women compared to White individuals,” the ACS says in a statement.
“The gap for breast cancer is more alarming,” it adds. “Black women are 41% more likely to die from breast cancer than White women despite a lower risk of being diagnosed with the disease.”
The new report, published online on Feb. 10 in CA: A Cancer Journal for Clinicians, also notes the following:
An estimated 224,080 new cancer cases and 73,680 cancer deaths will occur among Black people in 2022.
Over the past 5 data years, Black women had an 8% lower overall cancer incidence than White women but 12% higher mortality; Black men have 6% higher cancer incidence than White men but 19% higher cancer mortality.
Prostate cancer mortality among Black men decreased by 1.3% per year from 2015 to 2019 despite a 5% increase in the diagnosis of distant-stage prostate cancer annually since 2012, but the decline was slower than the 5% per year decline from 2010 to 2014.
The overall cancer mortality gap between Black and White people is narrowing. This is due to a steeper drop in prostate, lung, and other smoking-related cancers among Black people.
Colorectal cancer incidence and mortality rates are 21% and 44% higher, respectively, in Black men in comparison with White men and 18% and 31% higher, respectively, in Black women in comparison with White women.
The reasons for the disparities are complex but “largely stem from less access to high-quality care and optimal treatment as a repercussion of long-standing institutional racism,” the report concludes.
“We must address structural racism as a public health issue to close the gaps and advance health equity,” Tawana Thomas-Johnson, senior vice president and chief diversity officer at the ACS, said in the press release.
A version of this article first appeared on Medscape.com.
Cost not a factor in radiotherapy type for breast cancer patients
A study comparing the cost of hypofractionated radiotherapy for early-stage breast cancer with the more expensive multidose conventional form, finds that physicians are increasingly opting for hypofractionated radiotherapy despite lower reimbursements rates for the procedure.
Hypofractionated radiotherapy is administered in fewer fractions requiring fewer hospital visits, which, in turn, should lead to less expensive procedures. According to previously reported randomized controlled trials of patients with early breast cancer, both procedures are equally efficacious. In 2011, the American Society of Radiation Oncology published guidelines recommending hypofractionated whole-breast irradiation for patients who have not undergone chemotherapy and who are at least 50 years old with a small primary tumor (T1-2).
In the new study, Loren Saulsberry, PhD, of the department of public health at the University of Chicago, and colleagues Chuanhong Liao and Dezheng Huo, hypothesized that a fee-for-service incentive structure in which doctors are paid by volume and quantity of services, would drive up use of conventional therapy among patients with commercial insurance. And, they hypothesized that, when presented with a smaller cost difference between the two procedures, physicians would recommend hypofractionated radiotherapy over the conventional form, but neither theory was proven true.
This was a retrospective study of private employer–sponsored health insurance claims processed between 2008 and 2017 for women with early-stage breast cancer who were treated with lumpectomy and whole-breast irradiation.
The study included 15,869 women who received hypofractionated radiotherapy and 59,328 who received the conventional form. Women who underwent hypofractionated radiotherapy received 15-24 fractions over 21-31 days. Those who received conventional radiotherapy received 25-40 fractions over 39-120 days. The primary outcomes and measures were the use of hypofractionated or conventional radiotherapy, costs incurred by insurers and out-of-pocket patient expenses.
Dr. Saulsberry and colleagues found the use of hypofractionated radiotherapy increased during this period. They found no association between the likelihood of receiving hypofractionated radiotherapy and insurance plan characteristics. At $23,286, conventional radiotherapy was $6,253 more expensive than hypofractionated radiotherapy which averaged $17,763.
After out-of-pocket expenses were paid (average of $502 for conventional and $363 for hypofractionated radiotherapy), insurers paid an average of $6,375 more for conventional therapy after adjustments.
“Hypofractionated radiotherapy represents significant savings to both the health care system and to individual patients. It may soon become the dominant form of radiation treatment in the U.S. if current trends continue,” Dr. Saulsberry said in an interview after she presented the study (Abstract P3-19-07) at the San Antonio Breast Cancer Symposium.
According to the National Cancer Institute, the cost of cancer care grew from $190.2 billion in 2015 to $208.9 billion in 2020.
Dr. Saulsberry declared no conflicts of interest.
A study comparing the cost of hypofractionated radiotherapy for early-stage breast cancer with the more expensive multidose conventional form, finds that physicians are increasingly opting for hypofractionated radiotherapy despite lower reimbursements rates for the procedure.
Hypofractionated radiotherapy is administered in fewer fractions requiring fewer hospital visits, which, in turn, should lead to less expensive procedures. According to previously reported randomized controlled trials of patients with early breast cancer, both procedures are equally efficacious. In 2011, the American Society of Radiation Oncology published guidelines recommending hypofractionated whole-breast irradiation for patients who have not undergone chemotherapy and who are at least 50 years old with a small primary tumor (T1-2).
In the new study, Loren Saulsberry, PhD, of the department of public health at the University of Chicago, and colleagues Chuanhong Liao and Dezheng Huo, hypothesized that a fee-for-service incentive structure in which doctors are paid by volume and quantity of services, would drive up use of conventional therapy among patients with commercial insurance. And, they hypothesized that, when presented with a smaller cost difference between the two procedures, physicians would recommend hypofractionated radiotherapy over the conventional form, but neither theory was proven true.
This was a retrospective study of private employer–sponsored health insurance claims processed between 2008 and 2017 for women with early-stage breast cancer who were treated with lumpectomy and whole-breast irradiation.
The study included 15,869 women who received hypofractionated radiotherapy and 59,328 who received the conventional form. Women who underwent hypofractionated radiotherapy received 15-24 fractions over 21-31 days. Those who received conventional radiotherapy received 25-40 fractions over 39-120 days. The primary outcomes and measures were the use of hypofractionated or conventional radiotherapy, costs incurred by insurers and out-of-pocket patient expenses.
Dr. Saulsberry and colleagues found the use of hypofractionated radiotherapy increased during this period. They found no association between the likelihood of receiving hypofractionated radiotherapy and insurance plan characteristics. At $23,286, conventional radiotherapy was $6,253 more expensive than hypofractionated radiotherapy which averaged $17,763.
After out-of-pocket expenses were paid (average of $502 for conventional and $363 for hypofractionated radiotherapy), insurers paid an average of $6,375 more for conventional therapy after adjustments.
“Hypofractionated radiotherapy represents significant savings to both the health care system and to individual patients. It may soon become the dominant form of radiation treatment in the U.S. if current trends continue,” Dr. Saulsberry said in an interview after she presented the study (Abstract P3-19-07) at the San Antonio Breast Cancer Symposium.
According to the National Cancer Institute, the cost of cancer care grew from $190.2 billion in 2015 to $208.9 billion in 2020.
Dr. Saulsberry declared no conflicts of interest.
A study comparing the cost of hypofractionated radiotherapy for early-stage breast cancer with the more expensive multidose conventional form, finds that physicians are increasingly opting for hypofractionated radiotherapy despite lower reimbursements rates for the procedure.
Hypofractionated radiotherapy is administered in fewer fractions requiring fewer hospital visits, which, in turn, should lead to less expensive procedures. According to previously reported randomized controlled trials of patients with early breast cancer, both procedures are equally efficacious. In 2011, the American Society of Radiation Oncology published guidelines recommending hypofractionated whole-breast irradiation for patients who have not undergone chemotherapy and who are at least 50 years old with a small primary tumor (T1-2).
In the new study, Loren Saulsberry, PhD, of the department of public health at the University of Chicago, and colleagues Chuanhong Liao and Dezheng Huo, hypothesized that a fee-for-service incentive structure in which doctors are paid by volume and quantity of services, would drive up use of conventional therapy among patients with commercial insurance. And, they hypothesized that, when presented with a smaller cost difference between the two procedures, physicians would recommend hypofractionated radiotherapy over the conventional form, but neither theory was proven true.
This was a retrospective study of private employer–sponsored health insurance claims processed between 2008 and 2017 for women with early-stage breast cancer who were treated with lumpectomy and whole-breast irradiation.
The study included 15,869 women who received hypofractionated radiotherapy and 59,328 who received the conventional form. Women who underwent hypofractionated radiotherapy received 15-24 fractions over 21-31 days. Those who received conventional radiotherapy received 25-40 fractions over 39-120 days. The primary outcomes and measures were the use of hypofractionated or conventional radiotherapy, costs incurred by insurers and out-of-pocket patient expenses.
Dr. Saulsberry and colleagues found the use of hypofractionated radiotherapy increased during this period. They found no association between the likelihood of receiving hypofractionated radiotherapy and insurance plan characteristics. At $23,286, conventional radiotherapy was $6,253 more expensive than hypofractionated radiotherapy which averaged $17,763.
After out-of-pocket expenses were paid (average of $502 for conventional and $363 for hypofractionated radiotherapy), insurers paid an average of $6,375 more for conventional therapy after adjustments.
“Hypofractionated radiotherapy represents significant savings to both the health care system and to individual patients. It may soon become the dominant form of radiation treatment in the U.S. if current trends continue,” Dr. Saulsberry said in an interview after she presented the study (Abstract P3-19-07) at the San Antonio Breast Cancer Symposium.
According to the National Cancer Institute, the cost of cancer care grew from $190.2 billion in 2015 to $208.9 billion in 2020.
Dr. Saulsberry declared no conflicts of interest.
FROM SABCS 2021
Some U.S. women not getting ET for curable breast cancer
A standard treatment for early breast cancer is endocrine therapy (ET), with drugs such a tamoxifen and aromatase inhibitors.
But the study found that ET was not being used in about half of the eligible patients.
For example, only 13,115 of 26,255 eligible patients (48.8%) initiated ET within 1 year of diagnosis, and only 13,944 (52.1%) continued with ET.
“This is remarkable, considering that ET confers an impressive one-third reduction in the risk of death from breast cancer in the first 15 years after diagnosis,” comment authors Michael J. Hassett, MD, of the Dana-Farber Cancer Institute, Boston, and colleagues.
The findings were published online on Jan. 27 in JAMA Oncology.
This study provides an “important and disturbing” glimpse of the hidden barriers patients face when seeking quality, guideline-concordant care, says Kathy Miller, MD, the Ballve Lantero professor of oncology at Indiana University School of Medicine and associate director of clinical research at the IU Simon Comprehensive Cancer Center, Indianapolis, who was approached for comment.
Geographical variations
In their study, Dr. Hasset and colleagues set out determine the extent to which geospatial variations in early breast cancer care are attributable to health service area versus patient factors. They analyzed Surveillance, Epidemiology, and End Results (SEER) Medicare data for 31,571 patients with newly diagnosed with stage I-II nonmetastatic breast cancer between 2007 and 2013 who were followed for at least 3 years.
The patients had a median age of 71 years, and 61.4% had stage I disease at diagnosis.
Geospatial density maps (heat maps) in the paper highlight regional performance patterns. For initiation of ET within 1 year of diagnosis, the regions that appeared the worst (with less than 50% of patients getting this treatment) were parts of California, Utah, New Mexico, Louisiana, Georgia, Kentucky, Washington, and an isolated patch in Michigan.
In addition to the striking finding that nearly half of all women who are eligible for ET did not receive that therapy, the investigators found that 81.6% of 21,190 eligible patients received radiation therapy and 72.8% of 9,903 eligible patients received chemotherapy.
This also varied across the graphical regions, with the heat maps showing that the areas that were delivering radiation and chemotherapy to 70% to 80% of women were similar to the areas that were not initiating ET in about half of these women.
The authors found that the geographical region and health service area (HSA) explained more observed variation (24% to 48%) than patient factors (1% to 4%).
“While patient characteristics, such as race and ethnicity, were significantly associated with variation in breast cancer care, they explained a relatively small proportion of the total observed geospatial variance,” the authors comment.
“In fact, most of the total observed variance was owing to randomness or unexplained factors,” they add. The largest share of variation – 35% to 45% – was unexplained.
“The ET metrics demonstrated the largest total observed variance, the lowest absolute performance (only 49% of patients had an ET prescription within 1 year of diagnosis), and the strongest association with region/HSA,” they conclude.
Though limited by factors inherent in a retrospective review of SEER-Medicare data, the “unexplained nature of most geospatial variation in initial breast cancer care is not likely to change,” they comment.
Future quality improvement efforts should focus on reducing this unwarranted geospatial variation, particularly through the use of ET in eligible patients and with strategies that work across health care delivery systems, they suggest.
Approached for comment on the new findings, Dr. Miller posits that “many factors may be at play.”
“Unfortunately, the SEER database doesn’t allow us to sort out the impact of poverty/cost of care, distance to medical care, availability of specialty and subspecialty care, and payer/provider networks that may limit choices and options for second opinions,” Dr. Miller told this news organization.
She said that patients should be encouraged to consult reliable patient-focused information, such as that provided by the American Society of Clinical Oncology through its disease-specific sites, and to seek a second opinion from a university center. In many cases, major centers have become more accessible through virtual visits made available in the wake of the COVID-19 pandemic, she noted.
This study was supported by Dana-Farber Cancer Institute and the American Cancer Society. The authors and Dr. Miller have disclosed no relevant financial relationships. Dr. Miller is a regular contributor to Medscape with her Miller on Oncology column.
A version of this article first appeared on Medscape.com.
A standard treatment for early breast cancer is endocrine therapy (ET), with drugs such a tamoxifen and aromatase inhibitors.
But the study found that ET was not being used in about half of the eligible patients.
For example, only 13,115 of 26,255 eligible patients (48.8%) initiated ET within 1 year of diagnosis, and only 13,944 (52.1%) continued with ET.
“This is remarkable, considering that ET confers an impressive one-third reduction in the risk of death from breast cancer in the first 15 years after diagnosis,” comment authors Michael J. Hassett, MD, of the Dana-Farber Cancer Institute, Boston, and colleagues.
The findings were published online on Jan. 27 in JAMA Oncology.
This study provides an “important and disturbing” glimpse of the hidden barriers patients face when seeking quality, guideline-concordant care, says Kathy Miller, MD, the Ballve Lantero professor of oncology at Indiana University School of Medicine and associate director of clinical research at the IU Simon Comprehensive Cancer Center, Indianapolis, who was approached for comment.
Geographical variations
In their study, Dr. Hasset and colleagues set out determine the extent to which geospatial variations in early breast cancer care are attributable to health service area versus patient factors. They analyzed Surveillance, Epidemiology, and End Results (SEER) Medicare data for 31,571 patients with newly diagnosed with stage I-II nonmetastatic breast cancer between 2007 and 2013 who were followed for at least 3 years.
The patients had a median age of 71 years, and 61.4% had stage I disease at diagnosis.
Geospatial density maps (heat maps) in the paper highlight regional performance patterns. For initiation of ET within 1 year of diagnosis, the regions that appeared the worst (with less than 50% of patients getting this treatment) were parts of California, Utah, New Mexico, Louisiana, Georgia, Kentucky, Washington, and an isolated patch in Michigan.
In addition to the striking finding that nearly half of all women who are eligible for ET did not receive that therapy, the investigators found that 81.6% of 21,190 eligible patients received radiation therapy and 72.8% of 9,903 eligible patients received chemotherapy.
This also varied across the graphical regions, with the heat maps showing that the areas that were delivering radiation and chemotherapy to 70% to 80% of women were similar to the areas that were not initiating ET in about half of these women.
The authors found that the geographical region and health service area (HSA) explained more observed variation (24% to 48%) than patient factors (1% to 4%).
“While patient characteristics, such as race and ethnicity, were significantly associated with variation in breast cancer care, they explained a relatively small proportion of the total observed geospatial variance,” the authors comment.
“In fact, most of the total observed variance was owing to randomness or unexplained factors,” they add. The largest share of variation – 35% to 45% – was unexplained.
“The ET metrics demonstrated the largest total observed variance, the lowest absolute performance (only 49% of patients had an ET prescription within 1 year of diagnosis), and the strongest association with region/HSA,” they conclude.
Though limited by factors inherent in a retrospective review of SEER-Medicare data, the “unexplained nature of most geospatial variation in initial breast cancer care is not likely to change,” they comment.
Future quality improvement efforts should focus on reducing this unwarranted geospatial variation, particularly through the use of ET in eligible patients and with strategies that work across health care delivery systems, they suggest.
Approached for comment on the new findings, Dr. Miller posits that “many factors may be at play.”
“Unfortunately, the SEER database doesn’t allow us to sort out the impact of poverty/cost of care, distance to medical care, availability of specialty and subspecialty care, and payer/provider networks that may limit choices and options for second opinions,” Dr. Miller told this news organization.
She said that patients should be encouraged to consult reliable patient-focused information, such as that provided by the American Society of Clinical Oncology through its disease-specific sites, and to seek a second opinion from a university center. In many cases, major centers have become more accessible through virtual visits made available in the wake of the COVID-19 pandemic, she noted.
This study was supported by Dana-Farber Cancer Institute and the American Cancer Society. The authors and Dr. Miller have disclosed no relevant financial relationships. Dr. Miller is a regular contributor to Medscape with her Miller on Oncology column.
A version of this article first appeared on Medscape.com.
A standard treatment for early breast cancer is endocrine therapy (ET), with drugs such a tamoxifen and aromatase inhibitors.
But the study found that ET was not being used in about half of the eligible patients.
For example, only 13,115 of 26,255 eligible patients (48.8%) initiated ET within 1 year of diagnosis, and only 13,944 (52.1%) continued with ET.
“This is remarkable, considering that ET confers an impressive one-third reduction in the risk of death from breast cancer in the first 15 years after diagnosis,” comment authors Michael J. Hassett, MD, of the Dana-Farber Cancer Institute, Boston, and colleagues.
The findings were published online on Jan. 27 in JAMA Oncology.
This study provides an “important and disturbing” glimpse of the hidden barriers patients face when seeking quality, guideline-concordant care, says Kathy Miller, MD, the Ballve Lantero professor of oncology at Indiana University School of Medicine and associate director of clinical research at the IU Simon Comprehensive Cancer Center, Indianapolis, who was approached for comment.
Geographical variations
In their study, Dr. Hasset and colleagues set out determine the extent to which geospatial variations in early breast cancer care are attributable to health service area versus patient factors. They analyzed Surveillance, Epidemiology, and End Results (SEER) Medicare data for 31,571 patients with newly diagnosed with stage I-II nonmetastatic breast cancer between 2007 and 2013 who were followed for at least 3 years.
The patients had a median age of 71 years, and 61.4% had stage I disease at diagnosis.
Geospatial density maps (heat maps) in the paper highlight regional performance patterns. For initiation of ET within 1 year of diagnosis, the regions that appeared the worst (with less than 50% of patients getting this treatment) were parts of California, Utah, New Mexico, Louisiana, Georgia, Kentucky, Washington, and an isolated patch in Michigan.
In addition to the striking finding that nearly half of all women who are eligible for ET did not receive that therapy, the investigators found that 81.6% of 21,190 eligible patients received radiation therapy and 72.8% of 9,903 eligible patients received chemotherapy.
This also varied across the graphical regions, with the heat maps showing that the areas that were delivering radiation and chemotherapy to 70% to 80% of women were similar to the areas that were not initiating ET in about half of these women.
The authors found that the geographical region and health service area (HSA) explained more observed variation (24% to 48%) than patient factors (1% to 4%).
“While patient characteristics, such as race and ethnicity, were significantly associated with variation in breast cancer care, they explained a relatively small proportion of the total observed geospatial variance,” the authors comment.
“In fact, most of the total observed variance was owing to randomness or unexplained factors,” they add. The largest share of variation – 35% to 45% – was unexplained.
“The ET metrics demonstrated the largest total observed variance, the lowest absolute performance (only 49% of patients had an ET prescription within 1 year of diagnosis), and the strongest association with region/HSA,” they conclude.
Though limited by factors inherent in a retrospective review of SEER-Medicare data, the “unexplained nature of most geospatial variation in initial breast cancer care is not likely to change,” they comment.
Future quality improvement efforts should focus on reducing this unwarranted geospatial variation, particularly through the use of ET in eligible patients and with strategies that work across health care delivery systems, they suggest.
Approached for comment on the new findings, Dr. Miller posits that “many factors may be at play.”
“Unfortunately, the SEER database doesn’t allow us to sort out the impact of poverty/cost of care, distance to medical care, availability of specialty and subspecialty care, and payer/provider networks that may limit choices and options for second opinions,” Dr. Miller told this news organization.
She said that patients should be encouraged to consult reliable patient-focused information, such as that provided by the American Society of Clinical Oncology through its disease-specific sites, and to seek a second opinion from a university center. In many cases, major centers have become more accessible through virtual visits made available in the wake of the COVID-19 pandemic, she noted.
This study was supported by Dana-Farber Cancer Institute and the American Cancer Society. The authors and Dr. Miller have disclosed no relevant financial relationships. Dr. Miller is a regular contributor to Medscape with her Miller on Oncology column.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
Clinical Edge Journal Scan Commentary: Breast Cancer February 2022
Breast cancer diagnosis and treatment in young women can present unique challenges based on their life stage, including potential impact on fertility and future pregnancy. The role of GnRH analogues for ovarian protection during chemotherapy has been shown in both the POEMS-SWOG S0230 and PROMISE-GIM6 studies. Zong and colleagues conducted a phase 3 trial in China among premenopausal women with stage I-III breast cancer receiving cyclophosphamide-containing chemotherapy, with randomization to GnRHa + chemotherapy vs chemotherapy alone. Among 301 patients eligible for primary endpoint analysis, the premature ovarian insufficiency rate at 12 months was 10.3% for the GnRHa group vs 44.5% for the control group (odds ratio 0.23; P < 0.001). The rate of ovarian function recovery was also 46.4% higher in the GnRHa group. Furthermore, although survival outcomes were similar between groups, in patients <35 years of age, the tumor-free survival was higher in the GnRHa group vs control (93% vs 62%, P = 0.004) (Zong et al). These data reinforce the role of GnRHa as a means to reduce POI risk and support ovarian function recovery in young women undergoing chemotherapy for breast cancer. Measures of fertility and timing of pregnancy after breast cancer diagnosis continue to be areas of active research.
The treatment landscape for early-stage HER2-positive breast cancer continues to rapidly evolve with efforts to enhance efficacy and minimize toxicity for patients. The phase 3 KAITLIN study included 1846 patients with early-stage HER2-positive breast cancer (node-positive or node-negative, hormone receptor-negative and ≥T2 primary tumor) with randomization after surgery to adjuvant AC followed by taxane + trastuzumab + pertuzumab (AC-THP) or AC followed by T-DM1 + pertuzumab (AC-KP). In both the overall and node-positive populations, there was no significant difference in IDFS between the arms (stratified HR 0.98 and 0.97, respectively). In the overall population, the 3-year IDFS was 93.1% for AC-KP and 94.2% for AC-THP. Treatment completion rates were lower for AC-KP vs AC-THP (65.0% vs 88.4%), with T-DM1 discontinuation driven mostly by lab abnormalities (elevated liver function tests and thrombocytopenia) (Krop et al). Many patients diagnosed with early HER2-positive breast cancer (specifically those with tumors >2cm or node-positive) are treated with neoadjuvant chemotherapy + HER2-targeted therapy with subsequent tailoring of adjuvant treatment pending response, including use of T-DM1 if residual disease present. Future escalation and de-escalation strategies are being explored to further optimize outcomes and decrease side effects.
The addition of CDK 4/6 inhibitors to endocrine therapy has led to improved survival outcomes for patients diagnosed with advanced HR-positive-HER2-negative breast cancer. Lu and colleagues presented exploratory updated OS results among 672 patients with extended follow-up (median 53.5 months) from MONALEESA-7, which was a phase 3 randomized trial of ribociclib + endocrine therapy vs endocrine therapy alone among peri/pre-menopausal patients with HR-positive/HER2-negative advanced breast cancer. Median OS was 58.7 months vs 48.0 months for the ribociclib and placebo arms, respectively (HR 0.76), and a more pronounced benefit was seen in patients <40 years of age (median OS 51.3 months vs 40.5 months for ribociclib vs placebo arm; HR 0.65) (Lu et al). Furthermore, there was a significant delay in time to chemotherapy with ribociclib vs placebo (50.9 months vs 36.8 months; HR 0.69) which can certainly impact quality of life. A prior pooled analysis of the various MONALEESA trials demonstrated consistent PFS benefit with ribociclib across all intrinsic breast cancer subtypes, with the exception of basal-like and a more pronounced favorable impact in HER2-enriched. Future research to elucidate differences among CDK 4/6 inhibitors, influence of breast cancer subtype on their effect and how this can be translated to routine clinical practice are warranted.
Breast cancer diagnosis and treatment in young women can present unique challenges based on their life stage, including potential impact on fertility and future pregnancy. The role of GnRH analogues for ovarian protection during chemotherapy has been shown in both the POEMS-SWOG S0230 and PROMISE-GIM6 studies. Zong and colleagues conducted a phase 3 trial in China among premenopausal women with stage I-III breast cancer receiving cyclophosphamide-containing chemotherapy, with randomization to GnRHa + chemotherapy vs chemotherapy alone. Among 301 patients eligible for primary endpoint analysis, the premature ovarian insufficiency rate at 12 months was 10.3% for the GnRHa group vs 44.5% for the control group (odds ratio 0.23; P < 0.001). The rate of ovarian function recovery was also 46.4% higher in the GnRHa group. Furthermore, although survival outcomes were similar between groups, in patients <35 years of age, the tumor-free survival was higher in the GnRHa group vs control (93% vs 62%, P = 0.004) (Zong et al). These data reinforce the role of GnRHa as a means to reduce POI risk and support ovarian function recovery in young women undergoing chemotherapy for breast cancer. Measures of fertility and timing of pregnancy after breast cancer diagnosis continue to be areas of active research.
The treatment landscape for early-stage HER2-positive breast cancer continues to rapidly evolve with efforts to enhance efficacy and minimize toxicity for patients. The phase 3 KAITLIN study included 1846 patients with early-stage HER2-positive breast cancer (node-positive or node-negative, hormone receptor-negative and ≥T2 primary tumor) with randomization after surgery to adjuvant AC followed by taxane + trastuzumab + pertuzumab (AC-THP) or AC followed by T-DM1 + pertuzumab (AC-KP). In both the overall and node-positive populations, there was no significant difference in IDFS between the arms (stratified HR 0.98 and 0.97, respectively). In the overall population, the 3-year IDFS was 93.1% for AC-KP and 94.2% for AC-THP. Treatment completion rates were lower for AC-KP vs AC-THP (65.0% vs 88.4%), with T-DM1 discontinuation driven mostly by lab abnormalities (elevated liver function tests and thrombocytopenia) (Krop et al). Many patients diagnosed with early HER2-positive breast cancer (specifically those with tumors >2cm or node-positive) are treated with neoadjuvant chemotherapy + HER2-targeted therapy with subsequent tailoring of adjuvant treatment pending response, including use of T-DM1 if residual disease present. Future escalation and de-escalation strategies are being explored to further optimize outcomes and decrease side effects.
The addition of CDK 4/6 inhibitors to endocrine therapy has led to improved survival outcomes for patients diagnosed with advanced HR-positive-HER2-negative breast cancer. Lu and colleagues presented exploratory updated OS results among 672 patients with extended follow-up (median 53.5 months) from MONALEESA-7, which was a phase 3 randomized trial of ribociclib + endocrine therapy vs endocrine therapy alone among peri/pre-menopausal patients with HR-positive/HER2-negative advanced breast cancer. Median OS was 58.7 months vs 48.0 months for the ribociclib and placebo arms, respectively (HR 0.76), and a more pronounced benefit was seen in patients <40 years of age (median OS 51.3 months vs 40.5 months for ribociclib vs placebo arm; HR 0.65) (Lu et al). Furthermore, there was a significant delay in time to chemotherapy with ribociclib vs placebo (50.9 months vs 36.8 months; HR 0.69) which can certainly impact quality of life. A prior pooled analysis of the various MONALEESA trials demonstrated consistent PFS benefit with ribociclib across all intrinsic breast cancer subtypes, with the exception of basal-like and a more pronounced favorable impact in HER2-enriched. Future research to elucidate differences among CDK 4/6 inhibitors, influence of breast cancer subtype on their effect and how this can be translated to routine clinical practice are warranted.
Breast cancer diagnosis and treatment in young women can present unique challenges based on their life stage, including potential impact on fertility and future pregnancy. The role of GnRH analogues for ovarian protection during chemotherapy has been shown in both the POEMS-SWOG S0230 and PROMISE-GIM6 studies. Zong and colleagues conducted a phase 3 trial in China among premenopausal women with stage I-III breast cancer receiving cyclophosphamide-containing chemotherapy, with randomization to GnRHa + chemotherapy vs chemotherapy alone. Among 301 patients eligible for primary endpoint analysis, the premature ovarian insufficiency rate at 12 months was 10.3% for the GnRHa group vs 44.5% for the control group (odds ratio 0.23; P < 0.001). The rate of ovarian function recovery was also 46.4% higher in the GnRHa group. Furthermore, although survival outcomes were similar between groups, in patients <35 years of age, the tumor-free survival was higher in the GnRHa group vs control (93% vs 62%, P = 0.004) (Zong et al). These data reinforce the role of GnRHa as a means to reduce POI risk and support ovarian function recovery in young women undergoing chemotherapy for breast cancer. Measures of fertility and timing of pregnancy after breast cancer diagnosis continue to be areas of active research.
The treatment landscape for early-stage HER2-positive breast cancer continues to rapidly evolve with efforts to enhance efficacy and minimize toxicity for patients. The phase 3 KAITLIN study included 1846 patients with early-stage HER2-positive breast cancer (node-positive or node-negative, hormone receptor-negative and ≥T2 primary tumor) with randomization after surgery to adjuvant AC followed by taxane + trastuzumab + pertuzumab (AC-THP) or AC followed by T-DM1 + pertuzumab (AC-KP). In both the overall and node-positive populations, there was no significant difference in IDFS between the arms (stratified HR 0.98 and 0.97, respectively). In the overall population, the 3-year IDFS was 93.1% for AC-KP and 94.2% for AC-THP. Treatment completion rates were lower for AC-KP vs AC-THP (65.0% vs 88.4%), with T-DM1 discontinuation driven mostly by lab abnormalities (elevated liver function tests and thrombocytopenia) (Krop et al). Many patients diagnosed with early HER2-positive breast cancer (specifically those with tumors >2cm or node-positive) are treated with neoadjuvant chemotherapy + HER2-targeted therapy with subsequent tailoring of adjuvant treatment pending response, including use of T-DM1 if residual disease present. Future escalation and de-escalation strategies are being explored to further optimize outcomes and decrease side effects.
The addition of CDK 4/6 inhibitors to endocrine therapy has led to improved survival outcomes for patients diagnosed with advanced HR-positive-HER2-negative breast cancer. Lu and colleagues presented exploratory updated OS results among 672 patients with extended follow-up (median 53.5 months) from MONALEESA-7, which was a phase 3 randomized trial of ribociclib + endocrine therapy vs endocrine therapy alone among peri/pre-menopausal patients with HR-positive/HER2-negative advanced breast cancer. Median OS was 58.7 months vs 48.0 months for the ribociclib and placebo arms, respectively (HR 0.76), and a more pronounced benefit was seen in patients <40 years of age (median OS 51.3 months vs 40.5 months for ribociclib vs placebo arm; HR 0.65) (Lu et al). Furthermore, there was a significant delay in time to chemotherapy with ribociclib vs placebo (50.9 months vs 36.8 months; HR 0.69) which can certainly impact quality of life. A prior pooled analysis of the various MONALEESA trials demonstrated consistent PFS benefit with ribociclib across all intrinsic breast cancer subtypes, with the exception of basal-like and a more pronounced favorable impact in HER2-enriched. Future research to elucidate differences among CDK 4/6 inhibitors, influence of breast cancer subtype on their effect and how this can be translated to routine clinical practice are warranted.
New combo therapy for breast implant–associated lymphoma
The immediate treatment is surgical removal of the implant, which is sometimes followed with chemotherapy.
New data show that women who develop breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) who require chemotherapy can achieve excellent results with a combination of chemotherapy (cyclophosphamide, doxorubicin, and prednisone) and the antibody–drug conjugate brentuximab vedotin.
The findings were published in Blood.
The authors, led by Fabien Le Bras, MD, from the Henri Mondor Hospital, Créteil, France, note that despite BIA-ALCL being recently recognized as a provisional entity by the World Health Organization, its pathogenesis has yet to be fully elucidated, and a standard of care has not been established.
Results from the ECHELON 2 trial established brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisone (BV-CHP) as a new standard of care in CD30-positive peripheral T-cell lymphoma.
That trial included 316 patients with ACLC, although none of these cases were associated with breast implants.
The principal investigator on that trial, Steven Horwitz, MD, from Memorial Sloan Kettering Center, New York, told this news organization that although BIA-ALCL is “incredibly rare,” it causes “distress” to patients, as “many of them made a choice for reconstruction ... that they thought was safe.”
He said that the latest data from France is “interesting” and that the application of the ECHELON-2 findings to BIA-ALCL is “very logical.”
“For the people who need systemic therapy,” it appears from the current results that BV-CHP “is a very good option,” he said.
The “difficulty” in interpreting the data, however, is that “perhaps 80% of people with BIA-ALCL don’t need any systemic therapy” and are “cured with surgery alone.”
Dr. Horwitz said that while patients with infiltrative disease have a “higher risk of recurrence ... many of those are still cured with surgery alone.”
The main outstanding question he has is how many of the patients who received BV-CHP “might have been okay with observation.”
Details of the new data from France
For their study, Dr. Le Bras and colleagues analyzed data from the Lymphoma Study Association registry between 2009 and 2021 and identified 85 patients with BIA-ALCL, including 73 in France and 12 in Belgium.
Most of these patients (whose median age was 57 years) had unilateral lymphoma (94.1%), and only a few patients (5.9%) had bilateral disease.
The team notes that 41.2% of these women had received breast implants once, 41.2% received implants twice, and 17.6% received them three times or more.
In 45.9% of cases, the first implant followed mastectomy for breast cancer.
All patients had at least one textured implant. These have been associated with more cases of BIA-ALCL than smooth implants, and in 2019, Allergan recalled all BioCell textured breast implant products from the United States and around the world, due to the risk for BIA-ALCL, as reported, at the time, by this news organization.
For the women in this registry, the median time from the last implant to BIA-ALCL diagnosis was 7 years.
The most common presentation was seroma, which occurred in 75.3% of patients, while 21.2% of had a breast tumor mass with or without seroma.
Stage I-II disease was identified in 76.5% of patients, and 21.2% of cases were stage IV. Infiltrative disease was present in 24.7%.
Implant removal with total capsulectomy was performed in 77.6%; 29.4% of women also received chemotherapy, with 11.8% receiving BV-CHP.
A complete response was achieved in 84% of patients who received chemotherapy, while 8% failed to respond. Among the patients who received BV-CHP, 80% achieved a complete response.
After a median follow-up of 28.6 months, 91.8% patients were alive and progression free. All patients treated with BV-CHP were alive and progression free after a median follow-up of 1 year.
Patients with infiltrative disease had a significantly worse 2-year progression-free survival than those with in situ/mixed disease, at 73.8% versus 96.7%, or a hazard ratio for progression of 5.3 (P = .0039).
They also had worse 2-year overall survival, at 78.7% versus 100%, or a hazard ratio for death of 8.5 (P = .0022).
The authors note that these patients with infiltrative disease had significantly worse survival outcomes and may benefit most from BV-CHP.
No funding for the study was declared. Dr. Le Bras reports relationships with Novartis, Celgene, BMS, Takeda, Kite, and Gilead. Other authors declare numerous relevant financial relationships.
A version of this article first appeared on Medscape.com.
The immediate treatment is surgical removal of the implant, which is sometimes followed with chemotherapy.
New data show that women who develop breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) who require chemotherapy can achieve excellent results with a combination of chemotherapy (cyclophosphamide, doxorubicin, and prednisone) and the antibody–drug conjugate brentuximab vedotin.
The findings were published in Blood.
The authors, led by Fabien Le Bras, MD, from the Henri Mondor Hospital, Créteil, France, note that despite BIA-ALCL being recently recognized as a provisional entity by the World Health Organization, its pathogenesis has yet to be fully elucidated, and a standard of care has not been established.
Results from the ECHELON 2 trial established brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisone (BV-CHP) as a new standard of care in CD30-positive peripheral T-cell lymphoma.
That trial included 316 patients with ACLC, although none of these cases were associated with breast implants.
The principal investigator on that trial, Steven Horwitz, MD, from Memorial Sloan Kettering Center, New York, told this news organization that although BIA-ALCL is “incredibly rare,” it causes “distress” to patients, as “many of them made a choice for reconstruction ... that they thought was safe.”
He said that the latest data from France is “interesting” and that the application of the ECHELON-2 findings to BIA-ALCL is “very logical.”
“For the people who need systemic therapy,” it appears from the current results that BV-CHP “is a very good option,” he said.
The “difficulty” in interpreting the data, however, is that “perhaps 80% of people with BIA-ALCL don’t need any systemic therapy” and are “cured with surgery alone.”
Dr. Horwitz said that while patients with infiltrative disease have a “higher risk of recurrence ... many of those are still cured with surgery alone.”
The main outstanding question he has is how many of the patients who received BV-CHP “might have been okay with observation.”
Details of the new data from France
For their study, Dr. Le Bras and colleagues analyzed data from the Lymphoma Study Association registry between 2009 and 2021 and identified 85 patients with BIA-ALCL, including 73 in France and 12 in Belgium.
Most of these patients (whose median age was 57 years) had unilateral lymphoma (94.1%), and only a few patients (5.9%) had bilateral disease.
The team notes that 41.2% of these women had received breast implants once, 41.2% received implants twice, and 17.6% received them three times or more.
In 45.9% of cases, the first implant followed mastectomy for breast cancer.
All patients had at least one textured implant. These have been associated with more cases of BIA-ALCL than smooth implants, and in 2019, Allergan recalled all BioCell textured breast implant products from the United States and around the world, due to the risk for BIA-ALCL, as reported, at the time, by this news organization.
For the women in this registry, the median time from the last implant to BIA-ALCL diagnosis was 7 years.
The most common presentation was seroma, which occurred in 75.3% of patients, while 21.2% of had a breast tumor mass with or without seroma.
Stage I-II disease was identified in 76.5% of patients, and 21.2% of cases were stage IV. Infiltrative disease was present in 24.7%.
Implant removal with total capsulectomy was performed in 77.6%; 29.4% of women also received chemotherapy, with 11.8% receiving BV-CHP.
A complete response was achieved in 84% of patients who received chemotherapy, while 8% failed to respond. Among the patients who received BV-CHP, 80% achieved a complete response.
After a median follow-up of 28.6 months, 91.8% patients were alive and progression free. All patients treated with BV-CHP were alive and progression free after a median follow-up of 1 year.
Patients with infiltrative disease had a significantly worse 2-year progression-free survival than those with in situ/mixed disease, at 73.8% versus 96.7%, or a hazard ratio for progression of 5.3 (P = .0039).
They also had worse 2-year overall survival, at 78.7% versus 100%, or a hazard ratio for death of 8.5 (P = .0022).
The authors note that these patients with infiltrative disease had significantly worse survival outcomes and may benefit most from BV-CHP.
No funding for the study was declared. Dr. Le Bras reports relationships with Novartis, Celgene, BMS, Takeda, Kite, and Gilead. Other authors declare numerous relevant financial relationships.
A version of this article first appeared on Medscape.com.
The immediate treatment is surgical removal of the implant, which is sometimes followed with chemotherapy.
New data show that women who develop breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) who require chemotherapy can achieve excellent results with a combination of chemotherapy (cyclophosphamide, doxorubicin, and prednisone) and the antibody–drug conjugate brentuximab vedotin.
The findings were published in Blood.
The authors, led by Fabien Le Bras, MD, from the Henri Mondor Hospital, Créteil, France, note that despite BIA-ALCL being recently recognized as a provisional entity by the World Health Organization, its pathogenesis has yet to be fully elucidated, and a standard of care has not been established.
Results from the ECHELON 2 trial established brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisone (BV-CHP) as a new standard of care in CD30-positive peripheral T-cell lymphoma.
That trial included 316 patients with ACLC, although none of these cases were associated with breast implants.
The principal investigator on that trial, Steven Horwitz, MD, from Memorial Sloan Kettering Center, New York, told this news organization that although BIA-ALCL is “incredibly rare,” it causes “distress” to patients, as “many of them made a choice for reconstruction ... that they thought was safe.”
He said that the latest data from France is “interesting” and that the application of the ECHELON-2 findings to BIA-ALCL is “very logical.”
“For the people who need systemic therapy,” it appears from the current results that BV-CHP “is a very good option,” he said.
The “difficulty” in interpreting the data, however, is that “perhaps 80% of people with BIA-ALCL don’t need any systemic therapy” and are “cured with surgery alone.”
Dr. Horwitz said that while patients with infiltrative disease have a “higher risk of recurrence ... many of those are still cured with surgery alone.”
The main outstanding question he has is how many of the patients who received BV-CHP “might have been okay with observation.”
Details of the new data from France
For their study, Dr. Le Bras and colleagues analyzed data from the Lymphoma Study Association registry between 2009 and 2021 and identified 85 patients with BIA-ALCL, including 73 in France and 12 in Belgium.
Most of these patients (whose median age was 57 years) had unilateral lymphoma (94.1%), and only a few patients (5.9%) had bilateral disease.
The team notes that 41.2% of these women had received breast implants once, 41.2% received implants twice, and 17.6% received them three times or more.
In 45.9% of cases, the first implant followed mastectomy for breast cancer.
All patients had at least one textured implant. These have been associated with more cases of BIA-ALCL than smooth implants, and in 2019, Allergan recalled all BioCell textured breast implant products from the United States and around the world, due to the risk for BIA-ALCL, as reported, at the time, by this news organization.
For the women in this registry, the median time from the last implant to BIA-ALCL diagnosis was 7 years.
The most common presentation was seroma, which occurred in 75.3% of patients, while 21.2% of had a breast tumor mass with or without seroma.
Stage I-II disease was identified in 76.5% of patients, and 21.2% of cases were stage IV. Infiltrative disease was present in 24.7%.
Implant removal with total capsulectomy was performed in 77.6%; 29.4% of women also received chemotherapy, with 11.8% receiving BV-CHP.
A complete response was achieved in 84% of patients who received chemotherapy, while 8% failed to respond. Among the patients who received BV-CHP, 80% achieved a complete response.
After a median follow-up of 28.6 months, 91.8% patients were alive and progression free. All patients treated with BV-CHP were alive and progression free after a median follow-up of 1 year.
Patients with infiltrative disease had a significantly worse 2-year progression-free survival than those with in situ/mixed disease, at 73.8% versus 96.7%, or a hazard ratio for progression of 5.3 (P = .0039).
They also had worse 2-year overall survival, at 78.7% versus 100%, or a hazard ratio for death of 8.5 (P = .0022).
The authors note that these patients with infiltrative disease had significantly worse survival outcomes and may benefit most from BV-CHP.
No funding for the study was declared. Dr. Le Bras reports relationships with Novartis, Celgene, BMS, Takeda, Kite, and Gilead. Other authors declare numerous relevant financial relationships.
A version of this article first appeared on Medscape.com.
Medicare NCDs hinder access to cancer biomarker testing for minorities
of data from patients with advanced non–small cell lung cancer (aNSCLC), metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma. The finding was reported in JAMA Network Open.
Biomarker testing has become an essential tool in cancer care over the last decade. In 2011, for example, less than 1% of patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, and advanced melanoma underwent NGS testing, but by 2019, 40% of patients with these cancers received the testing.
“Next-generation sequencing testing has become increasingly important because it enables identification of multiple biomarkers simultaneously and efficiently while minimizing the number of biopsies required,” wrote the authors, led by William B. Wong, PharmD, of Genentech.
It has been unknown whether for Medicare beneficiaries and the overall population, if the NCD affected health equity issues, the authors wrote. While increased use of appropriate targeted therapies facilitated by NGS testing is associated with improved survival rates in patients with advanced or metastatic cancer, variability in health care coverage policies has posed a significant barrier to obtaining NGS testing for cancer patients, specifically through policy coverage limitations. It has remained unclear if the NCD has influenced NGS testing coverage in insurance types (for example, Medicaid) encompassing a larger population of minority racial and ethnic groups often experiencing poorer care and outcomes.
The retrospective cohort analysis compared EHR data from 280 U.S. cancer clinics in the (800 sites of care) pre- versus post-NCD period for patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma (January 2011–March 2020). Nearly 70% of all patients in the study were Medicare recipients who needed NCD approval to cover the cost of testing.
Among 92,687 patients (mean age, 66.6 years; 55.7% women), compared with Medicare beneficiaries, changes in pre- to post-NCD NGS testing trends were similar in commercially insured patients (odds ratio, 1.03; 95% CI, 0.98-1.08; P = .25). Pre- to post-NCD NGS testing trends increased at a slower rate among patients in assistance programs (OR, 0.93; 95% CI, 0.87-0.99; P = .03), compared with Medicare beneficiaries. The rate of increase for patients receiving Medicaid was not significantly different statistically compared with those receiving Medicare (OR, 0.92; 95% CI, 0.84-1.01; P = .07). Also, the NCD was not associated with racial and ethnic groups within Medicare beneficiaries alone or across all insurance types.
Compared with non-Hispanic White individuals, increases in average NGS use from the pre-NCD to post-NCD period were 14% lower (OR, 0.86; 95% CI, 0.74-0.99; P = .04) among African American and 23% lower (OR, 0.77; 95% CI, 0.62-0.96; P = .02) among Hispanic/Latino individuals; increases were similar, however, among Asian individuals and other races and ethnicities.
The authors observed that the post-NCD trend of increasing NGS testing seen in Medicare beneficiaries was similarly observed in those with commercial insurance. Testing rate differences, however, widened or were maintained after versus before the NCD in PAP (personal assistance program) and Medicaid beneficiaries relative to Medicare beneficiaries, suggesting that access to NGS testing did not improve equally across insurance types. Since Medicare coverage is determined at the state level, the authors urged research examining individual state coverage policies to further elucidate factors slowing uptake among Medicaid beneficiaries. “Additional efforts beyond coverage policies,” the authors concluded, “are needed to ensure equitable access to the benefits of precision medicine.”
The study was supported by Genentech.
of data from patients with advanced non–small cell lung cancer (aNSCLC), metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma. The finding was reported in JAMA Network Open.
Biomarker testing has become an essential tool in cancer care over the last decade. In 2011, for example, less than 1% of patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, and advanced melanoma underwent NGS testing, but by 2019, 40% of patients with these cancers received the testing.
“Next-generation sequencing testing has become increasingly important because it enables identification of multiple biomarkers simultaneously and efficiently while minimizing the number of biopsies required,” wrote the authors, led by William B. Wong, PharmD, of Genentech.
It has been unknown whether for Medicare beneficiaries and the overall population, if the NCD affected health equity issues, the authors wrote. While increased use of appropriate targeted therapies facilitated by NGS testing is associated with improved survival rates in patients with advanced or metastatic cancer, variability in health care coverage policies has posed a significant barrier to obtaining NGS testing for cancer patients, specifically through policy coverage limitations. It has remained unclear if the NCD has influenced NGS testing coverage in insurance types (for example, Medicaid) encompassing a larger population of minority racial and ethnic groups often experiencing poorer care and outcomes.
The retrospective cohort analysis compared EHR data from 280 U.S. cancer clinics in the (800 sites of care) pre- versus post-NCD period for patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma (January 2011–March 2020). Nearly 70% of all patients in the study were Medicare recipients who needed NCD approval to cover the cost of testing.
Among 92,687 patients (mean age, 66.6 years; 55.7% women), compared with Medicare beneficiaries, changes in pre- to post-NCD NGS testing trends were similar in commercially insured patients (odds ratio, 1.03; 95% CI, 0.98-1.08; P = .25). Pre- to post-NCD NGS testing trends increased at a slower rate among patients in assistance programs (OR, 0.93; 95% CI, 0.87-0.99; P = .03), compared with Medicare beneficiaries. The rate of increase for patients receiving Medicaid was not significantly different statistically compared with those receiving Medicare (OR, 0.92; 95% CI, 0.84-1.01; P = .07). Also, the NCD was not associated with racial and ethnic groups within Medicare beneficiaries alone or across all insurance types.
Compared with non-Hispanic White individuals, increases in average NGS use from the pre-NCD to post-NCD period were 14% lower (OR, 0.86; 95% CI, 0.74-0.99; P = .04) among African American and 23% lower (OR, 0.77; 95% CI, 0.62-0.96; P = .02) among Hispanic/Latino individuals; increases were similar, however, among Asian individuals and other races and ethnicities.
The authors observed that the post-NCD trend of increasing NGS testing seen in Medicare beneficiaries was similarly observed in those with commercial insurance. Testing rate differences, however, widened or were maintained after versus before the NCD in PAP (personal assistance program) and Medicaid beneficiaries relative to Medicare beneficiaries, suggesting that access to NGS testing did not improve equally across insurance types. Since Medicare coverage is determined at the state level, the authors urged research examining individual state coverage policies to further elucidate factors slowing uptake among Medicaid beneficiaries. “Additional efforts beyond coverage policies,” the authors concluded, “are needed to ensure equitable access to the benefits of precision medicine.”
The study was supported by Genentech.
of data from patients with advanced non–small cell lung cancer (aNSCLC), metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma. The finding was reported in JAMA Network Open.
Biomarker testing has become an essential tool in cancer care over the last decade. In 2011, for example, less than 1% of patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, and advanced melanoma underwent NGS testing, but by 2019, 40% of patients with these cancers received the testing.
“Next-generation sequencing testing has become increasingly important because it enables identification of multiple biomarkers simultaneously and efficiently while minimizing the number of biopsies required,” wrote the authors, led by William B. Wong, PharmD, of Genentech.
It has been unknown whether for Medicare beneficiaries and the overall population, if the NCD affected health equity issues, the authors wrote. While increased use of appropriate targeted therapies facilitated by NGS testing is associated with improved survival rates in patients with advanced or metastatic cancer, variability in health care coverage policies has posed a significant barrier to obtaining NGS testing for cancer patients, specifically through policy coverage limitations. It has remained unclear if the NCD has influenced NGS testing coverage in insurance types (for example, Medicaid) encompassing a larger population of minority racial and ethnic groups often experiencing poorer care and outcomes.
The retrospective cohort analysis compared EHR data from 280 U.S. cancer clinics in the (800 sites of care) pre- versus post-NCD period for patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma (January 2011–March 2020). Nearly 70% of all patients in the study were Medicare recipients who needed NCD approval to cover the cost of testing.
Among 92,687 patients (mean age, 66.6 years; 55.7% women), compared with Medicare beneficiaries, changes in pre- to post-NCD NGS testing trends were similar in commercially insured patients (odds ratio, 1.03; 95% CI, 0.98-1.08; P = .25). Pre- to post-NCD NGS testing trends increased at a slower rate among patients in assistance programs (OR, 0.93; 95% CI, 0.87-0.99; P = .03), compared with Medicare beneficiaries. The rate of increase for patients receiving Medicaid was not significantly different statistically compared with those receiving Medicare (OR, 0.92; 95% CI, 0.84-1.01; P = .07). Also, the NCD was not associated with racial and ethnic groups within Medicare beneficiaries alone or across all insurance types.
Compared with non-Hispanic White individuals, increases in average NGS use from the pre-NCD to post-NCD period were 14% lower (OR, 0.86; 95% CI, 0.74-0.99; P = .04) among African American and 23% lower (OR, 0.77; 95% CI, 0.62-0.96; P = .02) among Hispanic/Latino individuals; increases were similar, however, among Asian individuals and other races and ethnicities.
The authors observed that the post-NCD trend of increasing NGS testing seen in Medicare beneficiaries was similarly observed in those with commercial insurance. Testing rate differences, however, widened or were maintained after versus before the NCD in PAP (personal assistance program) and Medicaid beneficiaries relative to Medicare beneficiaries, suggesting that access to NGS testing did not improve equally across insurance types. Since Medicare coverage is determined at the state level, the authors urged research examining individual state coverage policies to further elucidate factors slowing uptake among Medicaid beneficiaries. “Additional efforts beyond coverage policies,” the authors concluded, “are needed to ensure equitable access to the benefits of precision medicine.”
The study was supported by Genentech.
FROM JAMA NETWORK OPEN
Could probiotics reduce ‘chemo brain’ in breast cancer patients?
compared with a control group taking placebo capsules, reports the first study of its kind.
“Our finding[s] provide a simple, inexpensive, and effective prevention strategy for chemotherapy-related side effects, including cognitive impairment,” senior author Jianbin Tong, MD, PhD, of the department of anesthesiology, Third Xiangya Hospital, Central South University, Changsha, Hunan, China, said in an interview.
The research “is the first study showing that probiotics supplementation during chemotherapy can prevent chemotherapy-related brain impairment,” he noted.
The double-blind, randomized study was published in the European Journal of Cancer. It involved 159 patients in China with stage I-III breast cancer who required adjuvant chemotherapy between 2018 and 2019. These patients were randomized to receive a regimen of three capsules twice per day containing either probiotics (n = 80) or placebo (n = 79) during their chemotherapy.
The probiotic capsule (Bifico, Sine Pharmaceuticals) contained Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis (210 mg of each).
The reductions in symptoms seen with the supplementation “exceed our expectations,” Dr. Tong said in an interview.
He speculated that this may have longer-term effects, with the prevention of initial cognitive impairment potentially “changing the neurodegenerative trajectory of patients after chemotherapy.”
“Patients don’t need to take probiotics continuously, but it’s better to take probiotics intermittently,” he said.
Approached for comment, Melanie Sekeres, PhD, Canada Research Chair and assistant professor at the University of Ottawa, said the improvements, such as those seen in delayed recall, are especially of interest.
“This is particularly notable because one of the brain regions that is critically involved in long-term memory processing, the hippocampus, is known to be highly sensitive to chemotherapy-induced neurotoxicity,” she said in an interview.
“The finding that probiotic treatment given alongside chemotherapy is sufficient to, in part, protect against memory disturbances in these patients suggests that there may be some neuroprotection conferred by the probiotic treatment,” she said.
A key question is whether similar results would be seen with other chemotherapy regimens, Dr. Sekeres added. “To better understand the effectiveness of these probiotics in preventing CRCI, they should be tested using other classes of chemotherapies before any broad conclusions can be made.”
Measuring the effect on ‘chemo brain’
“Chemo brain” is commonly reported after chemotherapy, and some 35% of patients report having long-term effects. Key symptoms include deficits in memory, attention, and executive and processing speed skills.
In their study, Dr. Tong and colleagues assessed patients on their cognitive status with a number of validated neuropsychological battery tests 1 day prior to initiating chemotherapy and 21 days after the last cycle of chemotherapy. Tests included the Hopkins Verbal Learning Test–Revised for verbal memory, the Brief Visuospatial Memory Test–Revised for visuospatial memory, and various others.
The team reports that, after adjustment for confounding factors, the total incidence of CRCI was significantly lower in the probiotics group versus the placebo group 21 days post chemotherapy (35% vs. 81%; relative risk, 0.43).
Rates of mild cognitive impairment were also lower in the probiotics group (29% vs 52%; RR, 0.55), as were rates of moderate cognitive impairment (6% vs. 29%; RR, 0.22).
The improvements with probiotics were observed across most other neuropsychological domains, including instantaneous verbal memory and delayed visuospatial memory (for both, P = .003) and visuospatial interference and verbal fluency (for both, P < .001).
The greater improvements in the probiotics group were seen regardless of use of other medications or the type of chemotherapy regimen received, which could have included epirubicin or docetaxel and/or cyclophosphamide.
CRCI was more common in patients who were older and had lower education or a higher body mass index; however, the improvements in the probiotics group were observed regardless of those factors, the authors commented.
In addition to the reduction in cognitive impairment that was seen, the treatment with probiotics was also associated with lower blood glucose (mean, 4.96 vs. 5.30; P = .02) and lower LDL cholesterol (2.61 vs. 2.89; P = .03) versus placebo, while there were no significant differences between the groups prior to chemotherapy.
There were no reports of severe emesis or constipation (grade 3 or higher) in either group; however, the probiotics group did have a significantly lower incidence of both, the authors note.
How does it work?
The potential benefits with probiotics are theorized to result from stabilizing the colonic and bacterial disruptions that are caused by chemotherapy, potentially offsetting the neuroinflammation that is linked to the cancer treatment, the authors speculated.
A subanalysis of 78 stool samples from 20 patients in the study showed no differences in alpha diversity or beta diversity before or after chemotherapy; however, there were significant reductions in the abundance of Streptococcus and Tyzzerella (P = .023 and P = .033, respectively) in the probiotics group after chemotherapy.
Further analysis showed that probiotics supplement modulated the levels of nine plasma metabolites in patients with breast cancer, with the results suggesting that metabolites (including p-mentha-1,8-dien-7-ol) “may be modulators in preventing CRCI by probiotics,” the authors noted.
Benefits reported beyond breast cancer
A subsequent trial conducted by Dr. Tong and colleagues following the CRCI study further showed similar protective benefits with probiotics in the prevention of chemotherapy-related hand-foot syndrome and oral mucositis.
And in a recent study, the research team found evidence of probiotic supplements protecting against cognitive impairment in the elderly following surgery.
The study received support from the National Natural Science Foundation of China, Subproject of the National Key Research and Development Program Project of China, science and technology innovation platform and talent plan of Hunan province and Natural Science Foundation of Hunan Province.
A version of this article first appeared on Medscape.com.
compared with a control group taking placebo capsules, reports the first study of its kind.
“Our finding[s] provide a simple, inexpensive, and effective prevention strategy for chemotherapy-related side effects, including cognitive impairment,” senior author Jianbin Tong, MD, PhD, of the department of anesthesiology, Third Xiangya Hospital, Central South University, Changsha, Hunan, China, said in an interview.
The research “is the first study showing that probiotics supplementation during chemotherapy can prevent chemotherapy-related brain impairment,” he noted.
The double-blind, randomized study was published in the European Journal of Cancer. It involved 159 patients in China with stage I-III breast cancer who required adjuvant chemotherapy between 2018 and 2019. These patients were randomized to receive a regimen of three capsules twice per day containing either probiotics (n = 80) or placebo (n = 79) during their chemotherapy.
The probiotic capsule (Bifico, Sine Pharmaceuticals) contained Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis (210 mg of each).
The reductions in symptoms seen with the supplementation “exceed our expectations,” Dr. Tong said in an interview.
He speculated that this may have longer-term effects, with the prevention of initial cognitive impairment potentially “changing the neurodegenerative trajectory of patients after chemotherapy.”
“Patients don’t need to take probiotics continuously, but it’s better to take probiotics intermittently,” he said.
Approached for comment, Melanie Sekeres, PhD, Canada Research Chair and assistant professor at the University of Ottawa, said the improvements, such as those seen in delayed recall, are especially of interest.
“This is particularly notable because one of the brain regions that is critically involved in long-term memory processing, the hippocampus, is known to be highly sensitive to chemotherapy-induced neurotoxicity,” she said in an interview.
“The finding that probiotic treatment given alongside chemotherapy is sufficient to, in part, protect against memory disturbances in these patients suggests that there may be some neuroprotection conferred by the probiotic treatment,” she said.
A key question is whether similar results would be seen with other chemotherapy regimens, Dr. Sekeres added. “To better understand the effectiveness of these probiotics in preventing CRCI, they should be tested using other classes of chemotherapies before any broad conclusions can be made.”
Measuring the effect on ‘chemo brain’
“Chemo brain” is commonly reported after chemotherapy, and some 35% of patients report having long-term effects. Key symptoms include deficits in memory, attention, and executive and processing speed skills.
In their study, Dr. Tong and colleagues assessed patients on their cognitive status with a number of validated neuropsychological battery tests 1 day prior to initiating chemotherapy and 21 days after the last cycle of chemotherapy. Tests included the Hopkins Verbal Learning Test–Revised for verbal memory, the Brief Visuospatial Memory Test–Revised for visuospatial memory, and various others.
The team reports that, after adjustment for confounding factors, the total incidence of CRCI was significantly lower in the probiotics group versus the placebo group 21 days post chemotherapy (35% vs. 81%; relative risk, 0.43).
Rates of mild cognitive impairment were also lower in the probiotics group (29% vs 52%; RR, 0.55), as were rates of moderate cognitive impairment (6% vs. 29%; RR, 0.22).
The improvements with probiotics were observed across most other neuropsychological domains, including instantaneous verbal memory and delayed visuospatial memory (for both, P = .003) and visuospatial interference and verbal fluency (for both, P < .001).
The greater improvements in the probiotics group were seen regardless of use of other medications or the type of chemotherapy regimen received, which could have included epirubicin or docetaxel and/or cyclophosphamide.
CRCI was more common in patients who were older and had lower education or a higher body mass index; however, the improvements in the probiotics group were observed regardless of those factors, the authors commented.
In addition to the reduction in cognitive impairment that was seen, the treatment with probiotics was also associated with lower blood glucose (mean, 4.96 vs. 5.30; P = .02) and lower LDL cholesterol (2.61 vs. 2.89; P = .03) versus placebo, while there were no significant differences between the groups prior to chemotherapy.
There were no reports of severe emesis or constipation (grade 3 or higher) in either group; however, the probiotics group did have a significantly lower incidence of both, the authors note.
How does it work?
The potential benefits with probiotics are theorized to result from stabilizing the colonic and bacterial disruptions that are caused by chemotherapy, potentially offsetting the neuroinflammation that is linked to the cancer treatment, the authors speculated.
A subanalysis of 78 stool samples from 20 patients in the study showed no differences in alpha diversity or beta diversity before or after chemotherapy; however, there were significant reductions in the abundance of Streptococcus and Tyzzerella (P = .023 and P = .033, respectively) in the probiotics group after chemotherapy.
Further analysis showed that probiotics supplement modulated the levels of nine plasma metabolites in patients with breast cancer, with the results suggesting that metabolites (including p-mentha-1,8-dien-7-ol) “may be modulators in preventing CRCI by probiotics,” the authors noted.
Benefits reported beyond breast cancer
A subsequent trial conducted by Dr. Tong and colleagues following the CRCI study further showed similar protective benefits with probiotics in the prevention of chemotherapy-related hand-foot syndrome and oral mucositis.
And in a recent study, the research team found evidence of probiotic supplements protecting against cognitive impairment in the elderly following surgery.
The study received support from the National Natural Science Foundation of China, Subproject of the National Key Research and Development Program Project of China, science and technology innovation platform and talent plan of Hunan province and Natural Science Foundation of Hunan Province.
A version of this article first appeared on Medscape.com.
compared with a control group taking placebo capsules, reports the first study of its kind.
“Our finding[s] provide a simple, inexpensive, and effective prevention strategy for chemotherapy-related side effects, including cognitive impairment,” senior author Jianbin Tong, MD, PhD, of the department of anesthesiology, Third Xiangya Hospital, Central South University, Changsha, Hunan, China, said in an interview.
The research “is the first study showing that probiotics supplementation during chemotherapy can prevent chemotherapy-related brain impairment,” he noted.
The double-blind, randomized study was published in the European Journal of Cancer. It involved 159 patients in China with stage I-III breast cancer who required adjuvant chemotherapy between 2018 and 2019. These patients were randomized to receive a regimen of three capsules twice per day containing either probiotics (n = 80) or placebo (n = 79) during their chemotherapy.
The probiotic capsule (Bifico, Sine Pharmaceuticals) contained Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis (210 mg of each).
The reductions in symptoms seen with the supplementation “exceed our expectations,” Dr. Tong said in an interview.
He speculated that this may have longer-term effects, with the prevention of initial cognitive impairment potentially “changing the neurodegenerative trajectory of patients after chemotherapy.”
“Patients don’t need to take probiotics continuously, but it’s better to take probiotics intermittently,” he said.
Approached for comment, Melanie Sekeres, PhD, Canada Research Chair and assistant professor at the University of Ottawa, said the improvements, such as those seen in delayed recall, are especially of interest.
“This is particularly notable because one of the brain regions that is critically involved in long-term memory processing, the hippocampus, is known to be highly sensitive to chemotherapy-induced neurotoxicity,” she said in an interview.
“The finding that probiotic treatment given alongside chemotherapy is sufficient to, in part, protect against memory disturbances in these patients suggests that there may be some neuroprotection conferred by the probiotic treatment,” she said.
A key question is whether similar results would be seen with other chemotherapy regimens, Dr. Sekeres added. “To better understand the effectiveness of these probiotics in preventing CRCI, they should be tested using other classes of chemotherapies before any broad conclusions can be made.”
Measuring the effect on ‘chemo brain’
“Chemo brain” is commonly reported after chemotherapy, and some 35% of patients report having long-term effects. Key symptoms include deficits in memory, attention, and executive and processing speed skills.
In their study, Dr. Tong and colleagues assessed patients on their cognitive status with a number of validated neuropsychological battery tests 1 day prior to initiating chemotherapy and 21 days after the last cycle of chemotherapy. Tests included the Hopkins Verbal Learning Test–Revised for verbal memory, the Brief Visuospatial Memory Test–Revised for visuospatial memory, and various others.
The team reports that, after adjustment for confounding factors, the total incidence of CRCI was significantly lower in the probiotics group versus the placebo group 21 days post chemotherapy (35% vs. 81%; relative risk, 0.43).
Rates of mild cognitive impairment were also lower in the probiotics group (29% vs 52%; RR, 0.55), as were rates of moderate cognitive impairment (6% vs. 29%; RR, 0.22).
The improvements with probiotics were observed across most other neuropsychological domains, including instantaneous verbal memory and delayed visuospatial memory (for both, P = .003) and visuospatial interference and verbal fluency (for both, P < .001).
The greater improvements in the probiotics group were seen regardless of use of other medications or the type of chemotherapy regimen received, which could have included epirubicin or docetaxel and/or cyclophosphamide.
CRCI was more common in patients who were older and had lower education or a higher body mass index; however, the improvements in the probiotics group were observed regardless of those factors, the authors commented.
In addition to the reduction in cognitive impairment that was seen, the treatment with probiotics was also associated with lower blood glucose (mean, 4.96 vs. 5.30; P = .02) and lower LDL cholesterol (2.61 vs. 2.89; P = .03) versus placebo, while there were no significant differences between the groups prior to chemotherapy.
There were no reports of severe emesis or constipation (grade 3 or higher) in either group; however, the probiotics group did have a significantly lower incidence of both, the authors note.
How does it work?
The potential benefits with probiotics are theorized to result from stabilizing the colonic and bacterial disruptions that are caused by chemotherapy, potentially offsetting the neuroinflammation that is linked to the cancer treatment, the authors speculated.
A subanalysis of 78 stool samples from 20 patients in the study showed no differences in alpha diversity or beta diversity before or after chemotherapy; however, there were significant reductions in the abundance of Streptococcus and Tyzzerella (P = .023 and P = .033, respectively) in the probiotics group after chemotherapy.
Further analysis showed that probiotics supplement modulated the levels of nine plasma metabolites in patients with breast cancer, with the results suggesting that metabolites (including p-mentha-1,8-dien-7-ol) “may be modulators in preventing CRCI by probiotics,” the authors noted.
Benefits reported beyond breast cancer
A subsequent trial conducted by Dr. Tong and colleagues following the CRCI study further showed similar protective benefits with probiotics in the prevention of chemotherapy-related hand-foot syndrome and oral mucositis.
And in a recent study, the research team found evidence of probiotic supplements protecting against cognitive impairment in the elderly following surgery.
The study received support from the National Natural Science Foundation of China, Subproject of the National Key Research and Development Program Project of China, science and technology innovation platform and talent plan of Hunan province and Natural Science Foundation of Hunan Province.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN JOURNAL OF CANCER
Radiologist fatigue affects breast imaging interpretation
, based on data from more than 97,000 screening mammograms.
Psychology literature has shown the impact of fatigue on performance in a range of settings, and previous studies have shown that radiologists’ performances are more accurate earlier in their shifts compared to later-shift performance, write Michael H. Bernstein, PhD, and colleagues at Brown University, Providence, R.I., in a study published online Jan. 11 in Radiology.
The effect of time of day on performance may be greater for more detailed imaging modalities that are more “cognitively taxing,” and the effect may be greater in less-experienced radiologists, but the impact of time and experience on overall patient recall and false-positive rates has not been well-studied, the researchers said.
In the retrospective review, the researchers identified 97,671 screening mammograms read by 18 radiologists at one of 12 community sites between Jan. 2018 and Dec. 2019. The researchers analyzed the results by type of image, either standard digital mammography (DM) or the more complex digital breast tomosynthesis (DBT). The researchers separated radiologists into two groups: those with at least 5 post-training years of experience and those with less than 5 post-training years of experience. A total of nine radiologists fell into each category.
Overall, the recall rates were significantly different and higher for DM versus DBT (10.2% vs. 9.0%; P = .006). The false-positive (FP) rate also differed significantly and was higher for DM versus DBT (9.8% vs. 8.6%; P = .004).
The odds of recall increased by 11.5% with each hour of reading time for radiologists with less than 5 post-training years of experience for both DBT (odds ratio, 1.12) and DM (OR, 1.09). For the more experienced radiologists, the odds of recall increased by 1.6% for each hour of reading time for DBT but decreased by 0.1% for DM, with no significant difference.
Similarly, the odds of an FP result increased by 12.1% for DBT and 9% for DM per hour of reading time for radiologists with less experience. For more experienced radiologists, the odds of an FP increased by 1.6% for DBT but decreased by 1.1% for DM per hour of reading time.
Cancer detection (defined as true-positive, or TP) was not higher for DM across time, the researchers note. However, “DBT achieved a higher TP rate than DM regardless of the time of day; this shows that for DBT to maintain a constant and superior TP rate relative to DM, radiologists’ FP rates had to go up as the day went on,” they write. “That is, although DBT achieves a superior TP rate, more junior radiologists appeared to compensate for their fatigue later in the day when using DBT by recalling a broader range of mammograms, more of which were FP findings.”
The researchers caution that their findings were limited by several factors, including the study’s retrospective design and the lack of randomization of the imaging technology, patients, and time of day, which prohibit conclusions regarding causality. Other limitations included the consideration of time of day without the ability to use hours since the start of a clinical shift and the use of a 5-year mark to indicate experience without accounting for work volume.
However, the stronger impact of a time-of-day effect for more junior radiologists agrees with findings from other studies, the researchers add. More empirical research is needed, and the researchers propose a longitudinal study of how time of day affects radiologists as they gain experience, as well as experimental studies to test strategies for mitigating the time-of-day effect observed in the current study.
Scheduled breaks may reduce impact of fatigue
“Digital breast tomosynthesis is increasingly used in clinical practice and takes significantly longer to interpret compared with digital mammography,” said corresponding author Ana P. Lourenco, MD, in an interview. “Radiologists interpret hundreds of images for each screening digital breast tomosynthesis exam, compared with four images for each screening digital mammogram exam; this may certainly contribute to radiologist fatigue.”
“I found it interesting that there was a difference based on years of experience of the radiologist, but I was not surprised that recall rate increased later in the day, as some of us had anecdotally noted this in our clinical practice,” Dr. Lourenco said. In fact, the idea to conduct the study was prompted by a conversation with her statistician colleagues “about how I subjectively felt like my own recall rate increased at the end of the day.”
Ways to counteract the impact of fatigue could include intermittent breaks to refocus attention, said Dr. Lourenco. “Potential barriers would include imaging volumes and attending to patients in the breast imaging center,” she said. “If we can show that decreasing fatigue improves mammography performance metrics, then this may encourage practices to support such interventions.”
However, “more research that includes a larger number of radiologists, wider range of imaging interpretation experience, perhaps even experimental studies comparing metrics for radiologists reading with scheduled breaks versus without such breaks would be of interest,” Dr. Lourenco said.
Fatigue in health care goes beyond radiology
“Due primarily to staffing shortages and increased volume and complexity of patients, burnout and fatigue of all medical personnel, not just physicians, have become hallmarks of modern health care delivery in the United States, and this has been exacerbated by COVID-19 and other societal factors,” said Jeffrey C. Weinreb, MD, professor of radiology and biomedical imaging at Yale University, New Haven, Conn., in an interview.
Previous studies have documented the fact that radiologists are among the specialists most affected by burnout and fatigue, and it has an impact on their performance, Dr. Weinreb said. The current study is important because it tries to pinpoint the key variables that are responsible for fatigue, so resources can be directed to effect change, he said.
Dr. Weinreb said he was not particularly surprised by the study findings. “Diagnostic mammography is a high-volume repetitive enterprise, so it would have been surprising if radiologist experience and time of day had no effect on performance and recall rate,” he said. “As most radiologists will attest based on personal experience, human beings get tired and lose some level of cognition over the course of a long, intense workday,” he added.
“I am a bit surprised that less experienced radiologists were more likely to recommend additional imaging at a higher rate when interpreting DBT but not for DM and only later in the day,” Dr. Weinreb noted. “The authors suggest that this could be due to the increased number of images that are viewed with DBT and the different ways experienced and less experienced radiologists process the information. However, there could be other explanations, such as differences in volumes or differences in ages.”
“Reducing the study volumes per radiologist is one obvious solution to reducing fatigue, but it will not be practical in many practices,” said Dr. Weinreb. “The important work of interpreting diagnostic mammograms needs to continue and grow. Without an increase in radiologist mammographers in the labor pool, this is not going to happen any time soon.”
Instead, “more immediate obvious solutions to radiologist fatigue in clinical practice include more frequent breaks during the workday, which would include walking around and not looking at a computer or cell phone screen, fewer images per study, report templates, streamlined workflow, more variety in daily work, and AI assistance for interpretation and reporting,” said Dr. Weinreb. Using nonradiologists when possible to relieve some of the burden could be considered, “but this is a complex and politically charged issue,” he noted.
Radiology is a well-compensated specialty, but further increasing compensation would help to mitigate burnout, said Dr. Weinreb. However, “perhaps even more important is making certain that the efforts of individual radiologists are appreciated and recognized,” he said.
As for additional research needs, “mammographers are not the only radiologists experiencing fatigue, but the most critical contributing factors for other types of imaging exams and subspecialities may not be identical,” Dr. Weinreb emphasized. “Data for other radiologists, similar to that provided by this study for diagnostic mammography, could be useful.
“An additional area of research could address the issue of individual radiologist circadian rhythms,” said Dr. Weinreb. “Perhaps we could rigorously determine whom amongst us is a ‘morning person’ versus one who performs equally well or better later in the day and use this information for radiologist scheduling,” he said. “Finally, once we know the key factors affecting performance for each type of exam and subspecialty, studies of possible incremental and combined benefits of various interventions would be needed.”
The study received no outside funding. The researchers and Dr. Weinreb have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, based on data from more than 97,000 screening mammograms.
Psychology literature has shown the impact of fatigue on performance in a range of settings, and previous studies have shown that radiologists’ performances are more accurate earlier in their shifts compared to later-shift performance, write Michael H. Bernstein, PhD, and colleagues at Brown University, Providence, R.I., in a study published online Jan. 11 in Radiology.
The effect of time of day on performance may be greater for more detailed imaging modalities that are more “cognitively taxing,” and the effect may be greater in less-experienced radiologists, but the impact of time and experience on overall patient recall and false-positive rates has not been well-studied, the researchers said.
In the retrospective review, the researchers identified 97,671 screening mammograms read by 18 radiologists at one of 12 community sites between Jan. 2018 and Dec. 2019. The researchers analyzed the results by type of image, either standard digital mammography (DM) or the more complex digital breast tomosynthesis (DBT). The researchers separated radiologists into two groups: those with at least 5 post-training years of experience and those with less than 5 post-training years of experience. A total of nine radiologists fell into each category.
Overall, the recall rates were significantly different and higher for DM versus DBT (10.2% vs. 9.0%; P = .006). The false-positive (FP) rate also differed significantly and was higher for DM versus DBT (9.8% vs. 8.6%; P = .004).
The odds of recall increased by 11.5% with each hour of reading time for radiologists with less than 5 post-training years of experience for both DBT (odds ratio, 1.12) and DM (OR, 1.09). For the more experienced radiologists, the odds of recall increased by 1.6% for each hour of reading time for DBT but decreased by 0.1% for DM, with no significant difference.
Similarly, the odds of an FP result increased by 12.1% for DBT and 9% for DM per hour of reading time for radiologists with less experience. For more experienced radiologists, the odds of an FP increased by 1.6% for DBT but decreased by 1.1% for DM per hour of reading time.
Cancer detection (defined as true-positive, or TP) was not higher for DM across time, the researchers note. However, “DBT achieved a higher TP rate than DM regardless of the time of day; this shows that for DBT to maintain a constant and superior TP rate relative to DM, radiologists’ FP rates had to go up as the day went on,” they write. “That is, although DBT achieves a superior TP rate, more junior radiologists appeared to compensate for their fatigue later in the day when using DBT by recalling a broader range of mammograms, more of which were FP findings.”
The researchers caution that their findings were limited by several factors, including the study’s retrospective design and the lack of randomization of the imaging technology, patients, and time of day, which prohibit conclusions regarding causality. Other limitations included the consideration of time of day without the ability to use hours since the start of a clinical shift and the use of a 5-year mark to indicate experience without accounting for work volume.
However, the stronger impact of a time-of-day effect for more junior radiologists agrees with findings from other studies, the researchers add. More empirical research is needed, and the researchers propose a longitudinal study of how time of day affects radiologists as they gain experience, as well as experimental studies to test strategies for mitigating the time-of-day effect observed in the current study.
Scheduled breaks may reduce impact of fatigue
“Digital breast tomosynthesis is increasingly used in clinical practice and takes significantly longer to interpret compared with digital mammography,” said corresponding author Ana P. Lourenco, MD, in an interview. “Radiologists interpret hundreds of images for each screening digital breast tomosynthesis exam, compared with four images for each screening digital mammogram exam; this may certainly contribute to radiologist fatigue.”
“I found it interesting that there was a difference based on years of experience of the radiologist, but I was not surprised that recall rate increased later in the day, as some of us had anecdotally noted this in our clinical practice,” Dr. Lourenco said. In fact, the idea to conduct the study was prompted by a conversation with her statistician colleagues “about how I subjectively felt like my own recall rate increased at the end of the day.”
Ways to counteract the impact of fatigue could include intermittent breaks to refocus attention, said Dr. Lourenco. “Potential barriers would include imaging volumes and attending to patients in the breast imaging center,” she said. “If we can show that decreasing fatigue improves mammography performance metrics, then this may encourage practices to support such interventions.”
However, “more research that includes a larger number of radiologists, wider range of imaging interpretation experience, perhaps even experimental studies comparing metrics for radiologists reading with scheduled breaks versus without such breaks would be of interest,” Dr. Lourenco said.
Fatigue in health care goes beyond radiology
“Due primarily to staffing shortages and increased volume and complexity of patients, burnout and fatigue of all medical personnel, not just physicians, have become hallmarks of modern health care delivery in the United States, and this has been exacerbated by COVID-19 and other societal factors,” said Jeffrey C. Weinreb, MD, professor of radiology and biomedical imaging at Yale University, New Haven, Conn., in an interview.
Previous studies have documented the fact that radiologists are among the specialists most affected by burnout and fatigue, and it has an impact on their performance, Dr. Weinreb said. The current study is important because it tries to pinpoint the key variables that are responsible for fatigue, so resources can be directed to effect change, he said.
Dr. Weinreb said he was not particularly surprised by the study findings. “Diagnostic mammography is a high-volume repetitive enterprise, so it would have been surprising if radiologist experience and time of day had no effect on performance and recall rate,” he said. “As most radiologists will attest based on personal experience, human beings get tired and lose some level of cognition over the course of a long, intense workday,” he added.
“I am a bit surprised that less experienced radiologists were more likely to recommend additional imaging at a higher rate when interpreting DBT but not for DM and only later in the day,” Dr. Weinreb noted. “The authors suggest that this could be due to the increased number of images that are viewed with DBT and the different ways experienced and less experienced radiologists process the information. However, there could be other explanations, such as differences in volumes or differences in ages.”
“Reducing the study volumes per radiologist is one obvious solution to reducing fatigue, but it will not be practical in many practices,” said Dr. Weinreb. “The important work of interpreting diagnostic mammograms needs to continue and grow. Without an increase in radiologist mammographers in the labor pool, this is not going to happen any time soon.”
Instead, “more immediate obvious solutions to radiologist fatigue in clinical practice include more frequent breaks during the workday, which would include walking around and not looking at a computer or cell phone screen, fewer images per study, report templates, streamlined workflow, more variety in daily work, and AI assistance for interpretation and reporting,” said Dr. Weinreb. Using nonradiologists when possible to relieve some of the burden could be considered, “but this is a complex and politically charged issue,” he noted.
Radiology is a well-compensated specialty, but further increasing compensation would help to mitigate burnout, said Dr. Weinreb. However, “perhaps even more important is making certain that the efforts of individual radiologists are appreciated and recognized,” he said.
As for additional research needs, “mammographers are not the only radiologists experiencing fatigue, but the most critical contributing factors for other types of imaging exams and subspecialities may not be identical,” Dr. Weinreb emphasized. “Data for other radiologists, similar to that provided by this study for diagnostic mammography, could be useful.
“An additional area of research could address the issue of individual radiologist circadian rhythms,” said Dr. Weinreb. “Perhaps we could rigorously determine whom amongst us is a ‘morning person’ versus one who performs equally well or better later in the day and use this information for radiologist scheduling,” he said. “Finally, once we know the key factors affecting performance for each type of exam and subspecialty, studies of possible incremental and combined benefits of various interventions would be needed.”
The study received no outside funding. The researchers and Dr. Weinreb have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, based on data from more than 97,000 screening mammograms.
Psychology literature has shown the impact of fatigue on performance in a range of settings, and previous studies have shown that radiologists’ performances are more accurate earlier in their shifts compared to later-shift performance, write Michael H. Bernstein, PhD, and colleagues at Brown University, Providence, R.I., in a study published online Jan. 11 in Radiology.
The effect of time of day on performance may be greater for more detailed imaging modalities that are more “cognitively taxing,” and the effect may be greater in less-experienced radiologists, but the impact of time and experience on overall patient recall and false-positive rates has not been well-studied, the researchers said.
In the retrospective review, the researchers identified 97,671 screening mammograms read by 18 radiologists at one of 12 community sites between Jan. 2018 and Dec. 2019. The researchers analyzed the results by type of image, either standard digital mammography (DM) or the more complex digital breast tomosynthesis (DBT). The researchers separated radiologists into two groups: those with at least 5 post-training years of experience and those with less than 5 post-training years of experience. A total of nine radiologists fell into each category.
Overall, the recall rates were significantly different and higher for DM versus DBT (10.2% vs. 9.0%; P = .006). The false-positive (FP) rate also differed significantly and was higher for DM versus DBT (9.8% vs. 8.6%; P = .004).
The odds of recall increased by 11.5% with each hour of reading time for radiologists with less than 5 post-training years of experience for both DBT (odds ratio, 1.12) and DM (OR, 1.09). For the more experienced radiologists, the odds of recall increased by 1.6% for each hour of reading time for DBT but decreased by 0.1% for DM, with no significant difference.
Similarly, the odds of an FP result increased by 12.1% for DBT and 9% for DM per hour of reading time for radiologists with less experience. For more experienced radiologists, the odds of an FP increased by 1.6% for DBT but decreased by 1.1% for DM per hour of reading time.
Cancer detection (defined as true-positive, or TP) was not higher for DM across time, the researchers note. However, “DBT achieved a higher TP rate than DM regardless of the time of day; this shows that for DBT to maintain a constant and superior TP rate relative to DM, radiologists’ FP rates had to go up as the day went on,” they write. “That is, although DBT achieves a superior TP rate, more junior radiologists appeared to compensate for their fatigue later in the day when using DBT by recalling a broader range of mammograms, more of which were FP findings.”
The researchers caution that their findings were limited by several factors, including the study’s retrospective design and the lack of randomization of the imaging technology, patients, and time of day, which prohibit conclusions regarding causality. Other limitations included the consideration of time of day without the ability to use hours since the start of a clinical shift and the use of a 5-year mark to indicate experience without accounting for work volume.
However, the stronger impact of a time-of-day effect for more junior radiologists agrees with findings from other studies, the researchers add. More empirical research is needed, and the researchers propose a longitudinal study of how time of day affects radiologists as they gain experience, as well as experimental studies to test strategies for mitigating the time-of-day effect observed in the current study.
Scheduled breaks may reduce impact of fatigue
“Digital breast tomosynthesis is increasingly used in clinical practice and takes significantly longer to interpret compared with digital mammography,” said corresponding author Ana P. Lourenco, MD, in an interview. “Radiologists interpret hundreds of images for each screening digital breast tomosynthesis exam, compared with four images for each screening digital mammogram exam; this may certainly contribute to radiologist fatigue.”
“I found it interesting that there was a difference based on years of experience of the radiologist, but I was not surprised that recall rate increased later in the day, as some of us had anecdotally noted this in our clinical practice,” Dr. Lourenco said. In fact, the idea to conduct the study was prompted by a conversation with her statistician colleagues “about how I subjectively felt like my own recall rate increased at the end of the day.”
Ways to counteract the impact of fatigue could include intermittent breaks to refocus attention, said Dr. Lourenco. “Potential barriers would include imaging volumes and attending to patients in the breast imaging center,” she said. “If we can show that decreasing fatigue improves mammography performance metrics, then this may encourage practices to support such interventions.”
However, “more research that includes a larger number of radiologists, wider range of imaging interpretation experience, perhaps even experimental studies comparing metrics for radiologists reading with scheduled breaks versus without such breaks would be of interest,” Dr. Lourenco said.
Fatigue in health care goes beyond radiology
“Due primarily to staffing shortages and increased volume and complexity of patients, burnout and fatigue of all medical personnel, not just physicians, have become hallmarks of modern health care delivery in the United States, and this has been exacerbated by COVID-19 and other societal factors,” said Jeffrey C. Weinreb, MD, professor of radiology and biomedical imaging at Yale University, New Haven, Conn., in an interview.
Previous studies have documented the fact that radiologists are among the specialists most affected by burnout and fatigue, and it has an impact on their performance, Dr. Weinreb said. The current study is important because it tries to pinpoint the key variables that are responsible for fatigue, so resources can be directed to effect change, he said.
Dr. Weinreb said he was not particularly surprised by the study findings. “Diagnostic mammography is a high-volume repetitive enterprise, so it would have been surprising if radiologist experience and time of day had no effect on performance and recall rate,” he said. “As most radiologists will attest based on personal experience, human beings get tired and lose some level of cognition over the course of a long, intense workday,” he added.
“I am a bit surprised that less experienced radiologists were more likely to recommend additional imaging at a higher rate when interpreting DBT but not for DM and only later in the day,” Dr. Weinreb noted. “The authors suggest that this could be due to the increased number of images that are viewed with DBT and the different ways experienced and less experienced radiologists process the information. However, there could be other explanations, such as differences in volumes or differences in ages.”
“Reducing the study volumes per radiologist is one obvious solution to reducing fatigue, but it will not be practical in many practices,” said Dr. Weinreb. “The important work of interpreting diagnostic mammograms needs to continue and grow. Without an increase in radiologist mammographers in the labor pool, this is not going to happen any time soon.”
Instead, “more immediate obvious solutions to radiologist fatigue in clinical practice include more frequent breaks during the workday, which would include walking around and not looking at a computer or cell phone screen, fewer images per study, report templates, streamlined workflow, more variety in daily work, and AI assistance for interpretation and reporting,” said Dr. Weinreb. Using nonradiologists when possible to relieve some of the burden could be considered, “but this is a complex and politically charged issue,” he noted.
Radiology is a well-compensated specialty, but further increasing compensation would help to mitigate burnout, said Dr. Weinreb. However, “perhaps even more important is making certain that the efforts of individual radiologists are appreciated and recognized,” he said.
As for additional research needs, “mammographers are not the only radiologists experiencing fatigue, but the most critical contributing factors for other types of imaging exams and subspecialities may not be identical,” Dr. Weinreb emphasized. “Data for other radiologists, similar to that provided by this study for diagnostic mammography, could be useful.
“An additional area of research could address the issue of individual radiologist circadian rhythms,” said Dr. Weinreb. “Perhaps we could rigorously determine whom amongst us is a ‘morning person’ versus one who performs equally well or better later in the day and use this information for radiologist scheduling,” he said. “Finally, once we know the key factors affecting performance for each type of exam and subspecialty, studies of possible incremental and combined benefits of various interventions would be needed.”
The study received no outside funding. The researchers and Dr. Weinreb have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.