User login
Geographic Clusters Show Uneven Cancer Screening in the US
Geographic Clusters Show Uneven Cancer Screening in the US
TOPLINE:
An analysis of 3142 US counties revealed that county-level screening for breast, cervical, and colorectal cancer increased overall between 1997 and 2019; however, despite the reduced geographic variation, persistently high-screening clusters remained in the Northeast, whereas persistently low-screening clusters remained in the Southwest.
METHODOLOGY:
- Cancer screening reduces mortality. Despite guideline recommendation, the uptake of breast, cervical, and colorectal cancer screening in the US falls short of national goals and varies across sociodemographic groups. To date, only a few studies have examined geographic and temporal patterns of screening.
- To address this gap, researchers conducted a cross-sectional study using an ecological panel design to analyze county-level screening prevalence across 3142 US mainland counties from 1997 to 2019, deriving prevalence estimates from Behavioral Risk Factor Surveillance System (BRFSS) and National Health Interview Survey (NHIS) data over 3- to 5-year periods.
- Spatial autocorrelation analyses, including Global Moran I and the bivariate local indicator of spatial autocorrelation, were performed to assess geographic clusters of cancer screening within each period. Four types of local geographic clusters of county-level cancer screening were identified: counties with persistently high screening rates, counties with persistently low screening rates, counties in which screening rates decreased from high to low, and counties in which screening rates increased from low to high.
- Screening prevalence was compared across multiple time windows for different modalities (mammography, a Papanicolaou test, colonoscopy, colorectal cancer test, endoscopy, and a fecal occult blood test [FOBT]). Overall, 3101 counties were analyzed for mammography and the Papanicolaou test, 3107 counties for colonoscopy, 3100 counties for colorectal cancer test, 3089 counties for endoscopy, and 3090 counties for the FOBT.
TAKEAWAY:
- Overall screening prevalence increased from 1997 to 2019, and global spatial autocorrelation declined over time. For instance, the distribution of mammography screening became 83% more uniform in more recent years (Moran I, 0.57 in 1997-1999 vs 0.10 in 2017-2019). Similarly, Papanicolaou test screening became more uniform in more recent years (Moran I, 0.44 vs. 0.07). These changes indicate reduced geographic heterogeneity.
- Colonoscopy and endoscopy use increased, surpassing a 50% prevalence in many counties for 2010; however, FOBT use declined. Spatial clustering also attenuated, with a 23.4% declined in Moran I for colonoscopy from 2011-2016 to 2017-2019, a 12.3% decline in the colorectal cancer test from 2004-2007 to 2008-2010, and a 14.0% decline for endoscopy from 2004-2007 to 2008-2010.
- Persistently high-/high-screening clusters were concentrated in the Northeast for mammography and colorectal cancer screening and in the East for Papanicolaou test screening, whereas persistently low-/low-screening clusters were concentrated in the Southwest for the same modalities.
- Clusters of low- and high-screening counties were more disadvantaged -- with lower socioeconomic status and a higher proportion of non-White residents -- than other cluster types, suggesting some improvement in screening uptake in more disadvantaged areas. Counties with persistently low screening exhibited greater socioeconomic disadvantages -- lower media household income, higher poverty, lower home values, and lower educational attainment -- than those with persistently high screening.
IN PRACTICE:
"This cross-sectional study found that despite secular increases that reduced geographic variation in screening, local clusters of high and low screening persisted in the Northeast and Southwest US, respectively. Future studies could incorporate health care access characteristics to explain why areas of low screening did not catch up to optimize cancer screening practice," the authors wrote.
SOURCE:
The study, led by Pranoti Pradhan, PhD, Harvard T.H. Chan School of Public Health, Boston, was published online in JAMA Network Open.
LIMITATIONS:
The county-level estimates were modeled using BRFSS, NHIS, and US Census data, which might be susceptible to sampling biases despite corrections for nonresponse and noncoverage. Researchers lacked data on specific health systems characteristics that may have directly driven changes in prevalence and were restricted to using screening time intervals available from the Small Area Estimates for Cancer-Relates Measures from the National Cancer Institute, rather than those according to US Preventive Services Task Force guidelines. Additionally, the spatial cluster method was sensitive to county size and arrangement, which may have influenced local cluster detection.
DISCLOSURES:
This research was supported by the T32 Cancer Prevention and Control Funding Fellowship and T32 Cancer Epidemiology Fellowship at the Harvard T.H. Chan School of Public Health. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
An analysis of 3142 US counties revealed that county-level screening for breast, cervical, and colorectal cancer increased overall between 1997 and 2019; however, despite the reduced geographic variation, persistently high-screening clusters remained in the Northeast, whereas persistently low-screening clusters remained in the Southwest.
METHODOLOGY:
- Cancer screening reduces mortality. Despite guideline recommendation, the uptake of breast, cervical, and colorectal cancer screening in the US falls short of national goals and varies across sociodemographic groups. To date, only a few studies have examined geographic and temporal patterns of screening.
- To address this gap, researchers conducted a cross-sectional study using an ecological panel design to analyze county-level screening prevalence across 3142 US mainland counties from 1997 to 2019, deriving prevalence estimates from Behavioral Risk Factor Surveillance System (BRFSS) and National Health Interview Survey (NHIS) data over 3- to 5-year periods.
- Spatial autocorrelation analyses, including Global Moran I and the bivariate local indicator of spatial autocorrelation, were performed to assess geographic clusters of cancer screening within each period. Four types of local geographic clusters of county-level cancer screening were identified: counties with persistently high screening rates, counties with persistently low screening rates, counties in which screening rates decreased from high to low, and counties in which screening rates increased from low to high.
- Screening prevalence was compared across multiple time windows for different modalities (mammography, a Papanicolaou test, colonoscopy, colorectal cancer test, endoscopy, and a fecal occult blood test [FOBT]). Overall, 3101 counties were analyzed for mammography and the Papanicolaou test, 3107 counties for colonoscopy, 3100 counties for colorectal cancer test, 3089 counties for endoscopy, and 3090 counties for the FOBT.
TAKEAWAY:
- Overall screening prevalence increased from 1997 to 2019, and global spatial autocorrelation declined over time. For instance, the distribution of mammography screening became 83% more uniform in more recent years (Moran I, 0.57 in 1997-1999 vs 0.10 in 2017-2019). Similarly, Papanicolaou test screening became more uniform in more recent years (Moran I, 0.44 vs. 0.07). These changes indicate reduced geographic heterogeneity.
- Colonoscopy and endoscopy use increased, surpassing a 50% prevalence in many counties for 2010; however, FOBT use declined. Spatial clustering also attenuated, with a 23.4% declined in Moran I for colonoscopy from 2011-2016 to 2017-2019, a 12.3% decline in the colorectal cancer test from 2004-2007 to 2008-2010, and a 14.0% decline for endoscopy from 2004-2007 to 2008-2010.
- Persistently high-/high-screening clusters were concentrated in the Northeast for mammography and colorectal cancer screening and in the East for Papanicolaou test screening, whereas persistently low-/low-screening clusters were concentrated in the Southwest for the same modalities.
- Clusters of low- and high-screening counties were more disadvantaged -- with lower socioeconomic status and a higher proportion of non-White residents -- than other cluster types, suggesting some improvement in screening uptake in more disadvantaged areas. Counties with persistently low screening exhibited greater socioeconomic disadvantages -- lower media household income, higher poverty, lower home values, and lower educational attainment -- than those with persistently high screening.
IN PRACTICE:
"This cross-sectional study found that despite secular increases that reduced geographic variation in screening, local clusters of high and low screening persisted in the Northeast and Southwest US, respectively. Future studies could incorporate health care access characteristics to explain why areas of low screening did not catch up to optimize cancer screening practice," the authors wrote.
SOURCE:
The study, led by Pranoti Pradhan, PhD, Harvard T.H. Chan School of Public Health, Boston, was published online in JAMA Network Open.
LIMITATIONS:
The county-level estimates were modeled using BRFSS, NHIS, and US Census data, which might be susceptible to sampling biases despite corrections for nonresponse and noncoverage. Researchers lacked data on specific health systems characteristics that may have directly driven changes in prevalence and were restricted to using screening time intervals available from the Small Area Estimates for Cancer-Relates Measures from the National Cancer Institute, rather than those according to US Preventive Services Task Force guidelines. Additionally, the spatial cluster method was sensitive to county size and arrangement, which may have influenced local cluster detection.
DISCLOSURES:
This research was supported by the T32 Cancer Prevention and Control Funding Fellowship and T32 Cancer Epidemiology Fellowship at the Harvard T.H. Chan School of Public Health. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
An analysis of 3142 US counties revealed that county-level screening for breast, cervical, and colorectal cancer increased overall between 1997 and 2019; however, despite the reduced geographic variation, persistently high-screening clusters remained in the Northeast, whereas persistently low-screening clusters remained in the Southwest.
METHODOLOGY:
- Cancer screening reduces mortality. Despite guideline recommendation, the uptake of breast, cervical, and colorectal cancer screening in the US falls short of national goals and varies across sociodemographic groups. To date, only a few studies have examined geographic and temporal patterns of screening.
- To address this gap, researchers conducted a cross-sectional study using an ecological panel design to analyze county-level screening prevalence across 3142 US mainland counties from 1997 to 2019, deriving prevalence estimates from Behavioral Risk Factor Surveillance System (BRFSS) and National Health Interview Survey (NHIS) data over 3- to 5-year periods.
- Spatial autocorrelation analyses, including Global Moran I and the bivariate local indicator of spatial autocorrelation, were performed to assess geographic clusters of cancer screening within each period. Four types of local geographic clusters of county-level cancer screening were identified: counties with persistently high screening rates, counties with persistently low screening rates, counties in which screening rates decreased from high to low, and counties in which screening rates increased from low to high.
- Screening prevalence was compared across multiple time windows for different modalities (mammography, a Papanicolaou test, colonoscopy, colorectal cancer test, endoscopy, and a fecal occult blood test [FOBT]). Overall, 3101 counties were analyzed for mammography and the Papanicolaou test, 3107 counties for colonoscopy, 3100 counties for colorectal cancer test, 3089 counties for endoscopy, and 3090 counties for the FOBT.
TAKEAWAY:
- Overall screening prevalence increased from 1997 to 2019, and global spatial autocorrelation declined over time. For instance, the distribution of mammography screening became 83% more uniform in more recent years (Moran I, 0.57 in 1997-1999 vs 0.10 in 2017-2019). Similarly, Papanicolaou test screening became more uniform in more recent years (Moran I, 0.44 vs. 0.07). These changes indicate reduced geographic heterogeneity.
- Colonoscopy and endoscopy use increased, surpassing a 50% prevalence in many counties for 2010; however, FOBT use declined. Spatial clustering also attenuated, with a 23.4% declined in Moran I for colonoscopy from 2011-2016 to 2017-2019, a 12.3% decline in the colorectal cancer test from 2004-2007 to 2008-2010, and a 14.0% decline for endoscopy from 2004-2007 to 2008-2010.
- Persistently high-/high-screening clusters were concentrated in the Northeast for mammography and colorectal cancer screening and in the East for Papanicolaou test screening, whereas persistently low-/low-screening clusters were concentrated in the Southwest for the same modalities.
- Clusters of low- and high-screening counties were more disadvantaged -- with lower socioeconomic status and a higher proportion of non-White residents -- than other cluster types, suggesting some improvement in screening uptake in more disadvantaged areas. Counties with persistently low screening exhibited greater socioeconomic disadvantages -- lower media household income, higher poverty, lower home values, and lower educational attainment -- than those with persistently high screening.
IN PRACTICE:
"This cross-sectional study found that despite secular increases that reduced geographic variation in screening, local clusters of high and low screening persisted in the Northeast and Southwest US, respectively. Future studies could incorporate health care access characteristics to explain why areas of low screening did not catch up to optimize cancer screening practice," the authors wrote.
SOURCE:
The study, led by Pranoti Pradhan, PhD, Harvard T.H. Chan School of Public Health, Boston, was published online in JAMA Network Open.
LIMITATIONS:
The county-level estimates were modeled using BRFSS, NHIS, and US Census data, which might be susceptible to sampling biases despite corrections for nonresponse and noncoverage. Researchers lacked data on specific health systems characteristics that may have directly driven changes in prevalence and were restricted to using screening time intervals available from the Small Area Estimates for Cancer-Relates Measures from the National Cancer Institute, rather than those according to US Preventive Services Task Force guidelines. Additionally, the spatial cluster method was sensitive to county size and arrangement, which may have influenced local cluster detection.
DISCLOSURES:
This research was supported by the T32 Cancer Prevention and Control Funding Fellowship and T32 Cancer Epidemiology Fellowship at the Harvard T.H. Chan School of Public Health. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Geographic Clusters Show Uneven Cancer Screening in the US
Geographic Clusters Show Uneven Cancer Screening in the US
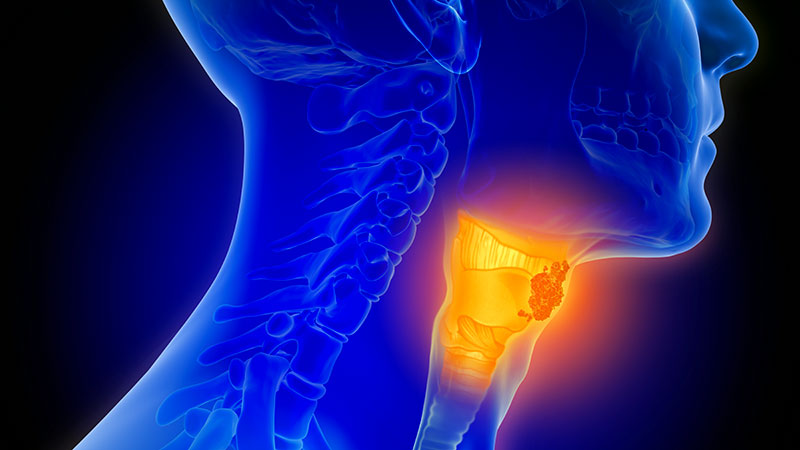
Team-Based Care is Crucial for Head-and-Neck Cancer Cases
Team-Based Care is Crucial for Head-and-Neck Cancer Cases
PHOENIX – A 70-year-old Vietnam veteran with oropharyngeal cancer presented challenges beyond his disease.
He couldn’t afford transportation for daily radiation treatments and had lost > 10% of his body weight due to pain and eating difficulties, recalled radiation oncologist Vinita Takiar, MD, PhD, in a presentation at the annual meeting of the Association of VA Hematology/Oncology.
To make matters more difficult, his wife held medical power of attorney despite his apparent competence to make decisions, said Takiar, who formerly worked with the US Department of Veterans Affairs (VA) Cincinnati Healthcare System and is now chair of radiation oncology at Penn State University.
All these factors would likely have derailed his treatment if not for a coordinated team intervention, Takiar said. Fortunately, the clinic launched a multifaceted effort involving representatives from the social work, dentistry, ethics, nutrition, and chaplaincy departments.
When surgery became impossible because the patient couldn’t lie on the operating table for adequate tumor exposure, she said, the existing team framework enabled a seamless and rapid transition to radiation with concurrent chemotherapy.
The patient completed treatment with an excellent response, offering a lesson in the importance of multidisciplinary care in head-and-neck cancers, she said.
In fact, when it comes to these forms of cancer, coordinated care “is probably more impactful than any treatment that we’re going to come up with,” she said. “The data show that when we do multidisciplinary care and we do it well, it actually improves the patient experience and outcomes.”
As Takiar noted, teamwork matters in many ways. It leads to better logistics and can address disparities, reduce financial burden and stigma, and even increase clinical trial involvement.
She pointed to studies linking teamwork to better outcomes, support for patients, and overall survival.
Takiar highlighted different parts of teams headed by radiation oncologists who act as “a node to improve multimodal care delivery.”
Speech and swallowing specialists, for example, are helpful in head-and-neck cancer because “there’s an impact on speech, swallowing, and appearance. Our patients don’t want to go out to dinner with friends because they can’t do it.”
Dentists and prosthodontists are key team members too: “I have dentists who have my cell phone number. They just call me: ‘Can I do this extraction? Was this in your radiation field? What was the dose?’”
Other team members include ear, nose, and throat specialists, palliative and supportive care specialists, medical oncologists, nurses, pathologists, transportation workers, and service connection specialists. She noted that previous military experience can affect radiation therapy. For example, the physical restraints required during treatment present particular challenges for veterans who’ve had wartime trauma. These patients may require therapy adjustments.
What’s next on the horizon? Takiar highlighted precision oncology and molecular profiling, artificial intelligence in care decisions and in radiation planning, telemedicine and virtual tumor boards, and expanded survivorship programs.
As for now, she urged colleagues to not be afraid to chat with radiation oncologists. “Please talk to us. We prioritize open communication and shared decision-making with the entire team,” she said. “If you see something and think your radiation oncologist should know about it, you think it was caused by the radiation, you should reach out to us.”
Takiar reported no disclosures.
PHOENIX – A 70-year-old Vietnam veteran with oropharyngeal cancer presented challenges beyond his disease.
He couldn’t afford transportation for daily radiation treatments and had lost > 10% of his body weight due to pain and eating difficulties, recalled radiation oncologist Vinita Takiar, MD, PhD, in a presentation at the annual meeting of the Association of VA Hematology/Oncology.
To make matters more difficult, his wife held medical power of attorney despite his apparent competence to make decisions, said Takiar, who formerly worked with the US Department of Veterans Affairs (VA) Cincinnati Healthcare System and is now chair of radiation oncology at Penn State University.
All these factors would likely have derailed his treatment if not for a coordinated team intervention, Takiar said. Fortunately, the clinic launched a multifaceted effort involving representatives from the social work, dentistry, ethics, nutrition, and chaplaincy departments.
When surgery became impossible because the patient couldn’t lie on the operating table for adequate tumor exposure, she said, the existing team framework enabled a seamless and rapid transition to radiation with concurrent chemotherapy.
The patient completed treatment with an excellent response, offering a lesson in the importance of multidisciplinary care in head-and-neck cancers, she said.
In fact, when it comes to these forms of cancer, coordinated care “is probably more impactful than any treatment that we’re going to come up with,” she said. “The data show that when we do multidisciplinary care and we do it well, it actually improves the patient experience and outcomes.”
As Takiar noted, teamwork matters in many ways. It leads to better logistics and can address disparities, reduce financial burden and stigma, and even increase clinical trial involvement.
She pointed to studies linking teamwork to better outcomes, support for patients, and overall survival.
Takiar highlighted different parts of teams headed by radiation oncologists who act as “a node to improve multimodal care delivery.”
Speech and swallowing specialists, for example, are helpful in head-and-neck cancer because “there’s an impact on speech, swallowing, and appearance. Our patients don’t want to go out to dinner with friends because they can’t do it.”
Dentists and prosthodontists are key team members too: “I have dentists who have my cell phone number. They just call me: ‘Can I do this extraction? Was this in your radiation field? What was the dose?’”
Other team members include ear, nose, and throat specialists, palliative and supportive care specialists, medical oncologists, nurses, pathologists, transportation workers, and service connection specialists. She noted that previous military experience can affect radiation therapy. For example, the physical restraints required during treatment present particular challenges for veterans who’ve had wartime trauma. These patients may require therapy adjustments.
What’s next on the horizon? Takiar highlighted precision oncology and molecular profiling, artificial intelligence in care decisions and in radiation planning, telemedicine and virtual tumor boards, and expanded survivorship programs.
As for now, she urged colleagues to not be afraid to chat with radiation oncologists. “Please talk to us. We prioritize open communication and shared decision-making with the entire team,” she said. “If you see something and think your radiation oncologist should know about it, you think it was caused by the radiation, you should reach out to us.”
Takiar reported no disclosures.
PHOENIX – A 70-year-old Vietnam veteran with oropharyngeal cancer presented challenges beyond his disease.
He couldn’t afford transportation for daily radiation treatments and had lost > 10% of his body weight due to pain and eating difficulties, recalled radiation oncologist Vinita Takiar, MD, PhD, in a presentation at the annual meeting of the Association of VA Hematology/Oncology.
To make matters more difficult, his wife held medical power of attorney despite his apparent competence to make decisions, said Takiar, who formerly worked with the US Department of Veterans Affairs (VA) Cincinnati Healthcare System and is now chair of radiation oncology at Penn State University.
All these factors would likely have derailed his treatment if not for a coordinated team intervention, Takiar said. Fortunately, the clinic launched a multifaceted effort involving representatives from the social work, dentistry, ethics, nutrition, and chaplaincy departments.
When surgery became impossible because the patient couldn’t lie on the operating table for adequate tumor exposure, she said, the existing team framework enabled a seamless and rapid transition to radiation with concurrent chemotherapy.
The patient completed treatment with an excellent response, offering a lesson in the importance of multidisciplinary care in head-and-neck cancers, she said.
In fact, when it comes to these forms of cancer, coordinated care “is probably more impactful than any treatment that we’re going to come up with,” she said. “The data show that when we do multidisciplinary care and we do it well, it actually improves the patient experience and outcomes.”
As Takiar noted, teamwork matters in many ways. It leads to better logistics and can address disparities, reduce financial burden and stigma, and even increase clinical trial involvement.
She pointed to studies linking teamwork to better outcomes, support for patients, and overall survival.
Takiar highlighted different parts of teams headed by radiation oncologists who act as “a node to improve multimodal care delivery.”
Speech and swallowing specialists, for example, are helpful in head-and-neck cancer because “there’s an impact on speech, swallowing, and appearance. Our patients don’t want to go out to dinner with friends because they can’t do it.”
Dentists and prosthodontists are key team members too: “I have dentists who have my cell phone number. They just call me: ‘Can I do this extraction? Was this in your radiation field? What was the dose?’”
Other team members include ear, nose, and throat specialists, palliative and supportive care specialists, medical oncologists, nurses, pathologists, transportation workers, and service connection specialists. She noted that previous military experience can affect radiation therapy. For example, the physical restraints required during treatment present particular challenges for veterans who’ve had wartime trauma. These patients may require therapy adjustments.
What’s next on the horizon? Takiar highlighted precision oncology and molecular profiling, artificial intelligence in care decisions and in radiation planning, telemedicine and virtual tumor boards, and expanded survivorship programs.
As for now, she urged colleagues to not be afraid to chat with radiation oncologists. “Please talk to us. We prioritize open communication and shared decision-making with the entire team,” she said. “If you see something and think your radiation oncologist should know about it, you think it was caused by the radiation, you should reach out to us.”
Takiar reported no disclosures.
Team-Based Care is Crucial for Head-and-Neck Cancer Cases
Team-Based Care is Crucial for Head-and-Neck Cancer Cases
Rising Cancer Rates Among Young People Spur New Fertility Preservation Options
Rising Cancer Rates Among Young People Spur New Fertility Preservation Options
ATLANTA —Jacqueline Lee, MD, a reproductive endocrinologist at Emory School of Medicine, frequently treats patients with cancer. Recently, she treated 4 women in their 30s with histories of colon cancer, acute lymphoblastic leukemia, lymphoma, and breast cancer. A young man in his 20s sought her care, to discuss his case of lymphoma.
All these patients sought guidance from Lee because they want to protect their ability to have children. At the annual meeting of the Association of VA Hematology/Oncology, Lee explained that plenty of patients are finding themselves in similar straits due in part to recent trends.
Cancer rates in the US have been rising among people aged 15 to 39 years, who now account for 4.2% of all cancer cases. An estimated 84,100 people in this age group are expected to be diagnosed with cancer this year. Meanwhile, women are having children later in life-birth rates are up among those aged 25 to 49 years-making it more likely that they have histories of cancer.
Although it's difficult to predict how cancer will affect fertility, Lee emphasized that many chemotherapy medications, including cisplatin and carboplatin, are cytotoxic. "It's hard to always predict what someone's arc of care is going to be," she said, "so I really have a low threshold for recommending fertility preservation in patients who have a strong desire to have future childbearing."
For women with cancer, egg preservation isn't the only strategy. Clinicians can also try to protect ovarian tissue from pelvic radiation through surgical reposition of the ovaries, Lee noted. In addition goserelin, a hormone-suppressing therapy, may protect the ovaries from chemotherapy, though its effectiveness in boosting pregnancy rates is still unclear.
"When I mentioned this option, it's usually for patients who can't preserve fertility via egg or embryo preservation, or we don't have the luxury of that kind of time," Lee said. "I say that if helps at all, it might help you resume menses after treatment. But infertility is still very common."
For some patients, freezing eggs is an easy decision. "They don't have a reproductive partner they're ready to make embryos with, so we proceed with egg preservation. It's no longer considered experimental and comes with lower upfront costs since the costs of actually making embryos are deferred until the future."
In addition, she said, freezing eggs also avoids the touchy topic of disposing of embryos. Lee cautions patients that retrieving eggs is a 2-week process that requires any initiation of cancer care to be delayed. However, the retrieval process can be adjusted in patients with special needs due to the type of cancer they have.
For prepubertal girls with cancer, ovarian tissue can be removed and frozen as a fertility preservation option. However, this is not considered standard of care. "We don't do it," she said. "We refer out if needed. Hopefully we'll develop a program in the future."
As for the 5 patients that Lee mentioned, with details changed to protect their privacy, their outcomes were as follows:
- The woman with colon cancer, who had undergone a hemicolectomy, chose to defer fertility preservation.
- The woman with acute lymphoblastic leukemia, who was taking depo-Lupron, had undetectable anti-Müllerian hormone (AMH) levels. Lee discussed the possibility of IVF with a donor egg.
- The woman with breast cancer, who was newly diagnosed, deferred fertility preservation.
- The man with lymphoma (Hodgkin's), who was awaiting chemotherapy, had his sperm frozen.
- The woman with lymphoma (new diagnosis) had 27 eggs frozen.
Lee had no disclosures to report.
ATLANTA —Jacqueline Lee, MD, a reproductive endocrinologist at Emory School of Medicine, frequently treats patients with cancer. Recently, she treated 4 women in their 30s with histories of colon cancer, acute lymphoblastic leukemia, lymphoma, and breast cancer. A young man in his 20s sought her care, to discuss his case of lymphoma.
All these patients sought guidance from Lee because they want to protect their ability to have children. At the annual meeting of the Association of VA Hematology/Oncology, Lee explained that plenty of patients are finding themselves in similar straits due in part to recent trends.
Cancer rates in the US have been rising among people aged 15 to 39 years, who now account for 4.2% of all cancer cases. An estimated 84,100 people in this age group are expected to be diagnosed with cancer this year. Meanwhile, women are having children later in life-birth rates are up among those aged 25 to 49 years-making it more likely that they have histories of cancer.
Although it's difficult to predict how cancer will affect fertility, Lee emphasized that many chemotherapy medications, including cisplatin and carboplatin, are cytotoxic. "It's hard to always predict what someone's arc of care is going to be," she said, "so I really have a low threshold for recommending fertility preservation in patients who have a strong desire to have future childbearing."
For women with cancer, egg preservation isn't the only strategy. Clinicians can also try to protect ovarian tissue from pelvic radiation through surgical reposition of the ovaries, Lee noted. In addition goserelin, a hormone-suppressing therapy, may protect the ovaries from chemotherapy, though its effectiveness in boosting pregnancy rates is still unclear.
"When I mentioned this option, it's usually for patients who can't preserve fertility via egg or embryo preservation, or we don't have the luxury of that kind of time," Lee said. "I say that if helps at all, it might help you resume menses after treatment. But infertility is still very common."
For some patients, freezing eggs is an easy decision. "They don't have a reproductive partner they're ready to make embryos with, so we proceed with egg preservation. It's no longer considered experimental and comes with lower upfront costs since the costs of actually making embryos are deferred until the future."
In addition, she said, freezing eggs also avoids the touchy topic of disposing of embryos. Lee cautions patients that retrieving eggs is a 2-week process that requires any initiation of cancer care to be delayed. However, the retrieval process can be adjusted in patients with special needs due to the type of cancer they have.
For prepubertal girls with cancer, ovarian tissue can be removed and frozen as a fertility preservation option. However, this is not considered standard of care. "We don't do it," she said. "We refer out if needed. Hopefully we'll develop a program in the future."
As for the 5 patients that Lee mentioned, with details changed to protect their privacy, their outcomes were as follows:
- The woman with colon cancer, who had undergone a hemicolectomy, chose to defer fertility preservation.
- The woman with acute lymphoblastic leukemia, who was taking depo-Lupron, had undetectable anti-Müllerian hormone (AMH) levels. Lee discussed the possibility of IVF with a donor egg.
- The woman with breast cancer, who was newly diagnosed, deferred fertility preservation.
- The man with lymphoma (Hodgkin's), who was awaiting chemotherapy, had his sperm frozen.
- The woman with lymphoma (new diagnosis) had 27 eggs frozen.
Lee had no disclosures to report.
ATLANTA —Jacqueline Lee, MD, a reproductive endocrinologist at Emory School of Medicine, frequently treats patients with cancer. Recently, she treated 4 women in their 30s with histories of colon cancer, acute lymphoblastic leukemia, lymphoma, and breast cancer. A young man in his 20s sought her care, to discuss his case of lymphoma.
All these patients sought guidance from Lee because they want to protect their ability to have children. At the annual meeting of the Association of VA Hematology/Oncology, Lee explained that plenty of patients are finding themselves in similar straits due in part to recent trends.
Cancer rates in the US have been rising among people aged 15 to 39 years, who now account for 4.2% of all cancer cases. An estimated 84,100 people in this age group are expected to be diagnosed with cancer this year. Meanwhile, women are having children later in life-birth rates are up among those aged 25 to 49 years-making it more likely that they have histories of cancer.
Although it's difficult to predict how cancer will affect fertility, Lee emphasized that many chemotherapy medications, including cisplatin and carboplatin, are cytotoxic. "It's hard to always predict what someone's arc of care is going to be," she said, "so I really have a low threshold for recommending fertility preservation in patients who have a strong desire to have future childbearing."
For women with cancer, egg preservation isn't the only strategy. Clinicians can also try to protect ovarian tissue from pelvic radiation through surgical reposition of the ovaries, Lee noted. In addition goserelin, a hormone-suppressing therapy, may protect the ovaries from chemotherapy, though its effectiveness in boosting pregnancy rates is still unclear.
"When I mentioned this option, it's usually for patients who can't preserve fertility via egg or embryo preservation, or we don't have the luxury of that kind of time," Lee said. "I say that if helps at all, it might help you resume menses after treatment. But infertility is still very common."
For some patients, freezing eggs is an easy decision. "They don't have a reproductive partner they're ready to make embryos with, so we proceed with egg preservation. It's no longer considered experimental and comes with lower upfront costs since the costs of actually making embryos are deferred until the future."
In addition, she said, freezing eggs also avoids the touchy topic of disposing of embryos. Lee cautions patients that retrieving eggs is a 2-week process that requires any initiation of cancer care to be delayed. However, the retrieval process can be adjusted in patients with special needs due to the type of cancer they have.
For prepubertal girls with cancer, ovarian tissue can be removed and frozen as a fertility preservation option. However, this is not considered standard of care. "We don't do it," she said. "We refer out if needed. Hopefully we'll develop a program in the future."
As for the 5 patients that Lee mentioned, with details changed to protect their privacy, their outcomes were as follows:
- The woman with colon cancer, who had undergone a hemicolectomy, chose to defer fertility preservation.
- The woman with acute lymphoblastic leukemia, who was taking depo-Lupron, had undetectable anti-Müllerian hormone (AMH) levels. Lee discussed the possibility of IVF with a donor egg.
- The woman with breast cancer, who was newly diagnosed, deferred fertility preservation.
- The man with lymphoma (Hodgkin's), who was awaiting chemotherapy, had his sperm frozen.
- The woman with lymphoma (new diagnosis) had 27 eggs frozen.
Lee had no disclosures to report.
Rising Cancer Rates Among Young People Spur New Fertility Preservation Options
Rising Cancer Rates Among Young People Spur New Fertility Preservation Options
VA Cancer Clinical Trials as a Strategy for Increasing Accrual of Racial and Ethnic Underrepresented Groups
Background
Cancer clinical trials (CCTs) are central to improving cancer care. However, generalizability of findings from CCTs is difficult due to the lack of diversity in most United States CCTs. Clinical trial accrual of underrepresented groups, is low throughout the United States and is approximately 4-5% in most CCTs. Reasons for low accrual in this population are multifactorial. Despite numerous factors related to accruing racial and ethnic underrepresented groups, many institutions have sought to address these barriers. We conducted a scoping review to identify evidence-based approaches to increase participation in cancer treatment clinical trials.
Methods
We reviewed the Salisbury VA Medical Center Oncology clinical trial database from October 2019 to June 2024. The participants in these clinical trials required consent. These clinical trials included treatment interventional as well as non-treatment interventional. Fifteen studies were included and over 260 Veterans participated.
Results
Key themes emerged that included a focus on patient education, cultural competency, and building capacity in the clinics to care for the Veteran population at three separate sites in the Salisbury VA system. The Black Veteran accrual rate of 29% was achieved. This accrual rate is representative of our VA catchment population of 33% for Black Veterans, and is five times the national average.
Conclusions
The research team’s success in enrolling Black Veterans in clinical trials is attributed to several factors. The demographic composition of Veterans served by the Salisbury, Charlotte, and Kernersville VA provided a diverse population that included a 33% Black group. The type of clinical trials focused on patients who were most impacted by the disease. The VA did afford less barriers to access to health care.
Background
Cancer clinical trials (CCTs) are central to improving cancer care. However, generalizability of findings from CCTs is difficult due to the lack of diversity in most United States CCTs. Clinical trial accrual of underrepresented groups, is low throughout the United States and is approximately 4-5% in most CCTs. Reasons for low accrual in this population are multifactorial. Despite numerous factors related to accruing racial and ethnic underrepresented groups, many institutions have sought to address these barriers. We conducted a scoping review to identify evidence-based approaches to increase participation in cancer treatment clinical trials.
Methods
We reviewed the Salisbury VA Medical Center Oncology clinical trial database from October 2019 to June 2024. The participants in these clinical trials required consent. These clinical trials included treatment interventional as well as non-treatment interventional. Fifteen studies were included and over 260 Veterans participated.
Results
Key themes emerged that included a focus on patient education, cultural competency, and building capacity in the clinics to care for the Veteran population at three separate sites in the Salisbury VA system. The Black Veteran accrual rate of 29% was achieved. This accrual rate is representative of our VA catchment population of 33% for Black Veterans, and is five times the national average.
Conclusions
The research team’s success in enrolling Black Veterans in clinical trials is attributed to several factors. The demographic composition of Veterans served by the Salisbury, Charlotte, and Kernersville VA provided a diverse population that included a 33% Black group. The type of clinical trials focused on patients who were most impacted by the disease. The VA did afford less barriers to access to health care.
Background
Cancer clinical trials (CCTs) are central to improving cancer care. However, generalizability of findings from CCTs is difficult due to the lack of diversity in most United States CCTs. Clinical trial accrual of underrepresented groups, is low throughout the United States and is approximately 4-5% in most CCTs. Reasons for low accrual in this population are multifactorial. Despite numerous factors related to accruing racial and ethnic underrepresented groups, many institutions have sought to address these barriers. We conducted a scoping review to identify evidence-based approaches to increase participation in cancer treatment clinical trials.
Methods
We reviewed the Salisbury VA Medical Center Oncology clinical trial database from October 2019 to June 2024. The participants in these clinical trials required consent. These clinical trials included treatment interventional as well as non-treatment interventional. Fifteen studies were included and over 260 Veterans participated.
Results
Key themes emerged that included a focus on patient education, cultural competency, and building capacity in the clinics to care for the Veteran population at three separate sites in the Salisbury VA system. The Black Veteran accrual rate of 29% was achieved. This accrual rate is representative of our VA catchment population of 33% for Black Veterans, and is five times the national average.
Conclusions
The research team’s success in enrolling Black Veterans in clinical trials is attributed to several factors. The demographic composition of Veterans served by the Salisbury, Charlotte, and Kernersville VA provided a diverse population that included a 33% Black group. The type of clinical trials focused on patients who were most impacted by the disease. The VA did afford less barriers to access to health care.

Cannabis May Ease Symptoms in Advanced Pancreatic Cancer
Cannabis May Ease Symptoms in Advanced Pancreatic Cancer
TOPLINE:
A randomized trial of 32 patients with advanced pancreatic cancer found that early access to medical cannabis patients' symptom burden, with minimal side effects.
METHODOLOGY:
- Patients with pancreatic cancer commonly experience moderate-to-severe pain, nausea, insomnia, and other symptoms that significantly affect their quality of life. Current management approaches are insufficient. Preliminary evidence suggests that medical cannabis has efficacy against multiple cancer-related symptoms, but high-quality data remain limited due to regulatory barriers.
- Researchers conducted a pilot randomized, waitlist-controlled trial involving 32 patients (median age, 71 years) with newly diagnosed locally advanced or metastatic pancreatic adenocarcinoma and at least one burdensome symptom.
- Patients were randomly assigned in a 1:1 ratio to early (0-8 weeks) or delayed (9-16 weeks) cannabis intervention through the Minnesota Medical Cannabis Program, which provided cannabis products and education in how to use them.
- Primary outcomes focused on feasibility, while secondary outcomes examined acceptability, changes in symptom burden, and quality of life in exploratory efficacy analyses.
TAKEAWAY:
- At baseline, patients reported a substantial moderate-to-severe symptom burden — most commonly insomnia (85%), pain (77%), and appetite loss (69%); 10 patients (31%) were using opioids.
- The study met all of its feasibility metrics, with 74% of the patients meeting enrollment eligibility and 81% complying with their random assignment. Patients in the arm with early cannabis access typically picked up their products 3 days after starting chemotherapy. Most used tablets or other oral cannabis formulations.
- At 8 weeks, patients in the early-access arm experienced numerically higher rates of improvement in pain (44% vs 20%; P = .35), appetite (56% vs 30%; P = .37), and insomnia (67% vs 30%; P = .18), as well as a reduction in opioid use. Their rates of potential cannabis side effects, including dry mouth, dizziness, and concentration problems, were lower compared with the waitlist group — possibly, the authors noted, due to their education to “start low, go slow.”
- Patients made a median of two trips to a cannabis dispensary during the study period, and most said that using cannabis was “easy” and “practical.”
IN PRACTICE:
“Early access to medical cannabis was associated with improvement in certain symptoms, such as insomnia, with minimal harms,” the authors wrote, adding that the research design offers a model collaboration between investigators and state cannabis programs.
“The encouraging preliminary efficacy and safety of cannabis in managing symptoms supports further exploration," they concluded.
SOURCE:
The study was led by Dylan Zylla, MD, MS, of HealthPartners Institute, Cancer Research Center, Minneapolis, Minnesota. It was presented on January 9 at the ASCO Gastrointestinal Cancers Symposium 2026 and simultaneously published in JCO Oncology Practice.
LIMITATIONS:
The trial was small and the 8-week primary study period precluded conclusions about longer-term benefits and safety. Generalizability may be limited as the trial was conducted in a single state with a predominantly urban and White patient population. Additionally, heterogeneity in state cannabis programs and laws may limit national applicability.
DISCLOSURES:
The study was supported by philanthropic support to the HealthPartners Cancer Research Center. Cannabis products were provided by Vireo Health (GreenGoods, Minnesota). Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A randomized trial of 32 patients with advanced pancreatic cancer found that early access to medical cannabis patients' symptom burden, with minimal side effects.
METHODOLOGY:
- Patients with pancreatic cancer commonly experience moderate-to-severe pain, nausea, insomnia, and other symptoms that significantly affect their quality of life. Current management approaches are insufficient. Preliminary evidence suggests that medical cannabis has efficacy against multiple cancer-related symptoms, but high-quality data remain limited due to regulatory barriers.
- Researchers conducted a pilot randomized, waitlist-controlled trial involving 32 patients (median age, 71 years) with newly diagnosed locally advanced or metastatic pancreatic adenocarcinoma and at least one burdensome symptom.
- Patients were randomly assigned in a 1:1 ratio to early (0-8 weeks) or delayed (9-16 weeks) cannabis intervention through the Minnesota Medical Cannabis Program, which provided cannabis products and education in how to use them.
- Primary outcomes focused on feasibility, while secondary outcomes examined acceptability, changes in symptom burden, and quality of life in exploratory efficacy analyses.
TAKEAWAY:
- At baseline, patients reported a substantial moderate-to-severe symptom burden — most commonly insomnia (85%), pain (77%), and appetite loss (69%); 10 patients (31%) were using opioids.
- The study met all of its feasibility metrics, with 74% of the patients meeting enrollment eligibility and 81% complying with their random assignment. Patients in the arm with early cannabis access typically picked up their products 3 days after starting chemotherapy. Most used tablets or other oral cannabis formulations.
- At 8 weeks, patients in the early-access arm experienced numerically higher rates of improvement in pain (44% vs 20%; P = .35), appetite (56% vs 30%; P = .37), and insomnia (67% vs 30%; P = .18), as well as a reduction in opioid use. Their rates of potential cannabis side effects, including dry mouth, dizziness, and concentration problems, were lower compared with the waitlist group — possibly, the authors noted, due to their education to “start low, go slow.”
- Patients made a median of two trips to a cannabis dispensary during the study period, and most said that using cannabis was “easy” and “practical.”
IN PRACTICE:
“Early access to medical cannabis was associated with improvement in certain symptoms, such as insomnia, with minimal harms,” the authors wrote, adding that the research design offers a model collaboration between investigators and state cannabis programs.
“The encouraging preliminary efficacy and safety of cannabis in managing symptoms supports further exploration," they concluded.
SOURCE:
The study was led by Dylan Zylla, MD, MS, of HealthPartners Institute, Cancer Research Center, Minneapolis, Minnesota. It was presented on January 9 at the ASCO Gastrointestinal Cancers Symposium 2026 and simultaneously published in JCO Oncology Practice.
LIMITATIONS:
The trial was small and the 8-week primary study period precluded conclusions about longer-term benefits and safety. Generalizability may be limited as the trial was conducted in a single state with a predominantly urban and White patient population. Additionally, heterogeneity in state cannabis programs and laws may limit national applicability.
DISCLOSURES:
The study was supported by philanthropic support to the HealthPartners Cancer Research Center. Cannabis products were provided by Vireo Health (GreenGoods, Minnesota). Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A randomized trial of 32 patients with advanced pancreatic cancer found that early access to medical cannabis patients' symptom burden, with minimal side effects.
METHODOLOGY:
- Patients with pancreatic cancer commonly experience moderate-to-severe pain, nausea, insomnia, and other symptoms that significantly affect their quality of life. Current management approaches are insufficient. Preliminary evidence suggests that medical cannabis has efficacy against multiple cancer-related symptoms, but high-quality data remain limited due to regulatory barriers.
- Researchers conducted a pilot randomized, waitlist-controlled trial involving 32 patients (median age, 71 years) with newly diagnosed locally advanced or metastatic pancreatic adenocarcinoma and at least one burdensome symptom.
- Patients were randomly assigned in a 1:1 ratio to early (0-8 weeks) or delayed (9-16 weeks) cannabis intervention through the Minnesota Medical Cannabis Program, which provided cannabis products and education in how to use them.
- Primary outcomes focused on feasibility, while secondary outcomes examined acceptability, changes in symptom burden, and quality of life in exploratory efficacy analyses.
TAKEAWAY:
- At baseline, patients reported a substantial moderate-to-severe symptom burden — most commonly insomnia (85%), pain (77%), and appetite loss (69%); 10 patients (31%) were using opioids.
- The study met all of its feasibility metrics, with 74% of the patients meeting enrollment eligibility and 81% complying with their random assignment. Patients in the arm with early cannabis access typically picked up their products 3 days after starting chemotherapy. Most used tablets or other oral cannabis formulations.
- At 8 weeks, patients in the early-access arm experienced numerically higher rates of improvement in pain (44% vs 20%; P = .35), appetite (56% vs 30%; P = .37), and insomnia (67% vs 30%; P = .18), as well as a reduction in opioid use. Their rates of potential cannabis side effects, including dry mouth, dizziness, and concentration problems, were lower compared with the waitlist group — possibly, the authors noted, due to their education to “start low, go slow.”
- Patients made a median of two trips to a cannabis dispensary during the study period, and most said that using cannabis was “easy” and “practical.”
IN PRACTICE:
“Early access to medical cannabis was associated with improvement in certain symptoms, such as insomnia, with minimal harms,” the authors wrote, adding that the research design offers a model collaboration between investigators and state cannabis programs.
“The encouraging preliminary efficacy and safety of cannabis in managing symptoms supports further exploration," they concluded.
SOURCE:
The study was led by Dylan Zylla, MD, MS, of HealthPartners Institute, Cancer Research Center, Minneapolis, Minnesota. It was presented on January 9 at the ASCO Gastrointestinal Cancers Symposium 2026 and simultaneously published in JCO Oncology Practice.
LIMITATIONS:
The trial was small and the 8-week primary study period precluded conclusions about longer-term benefits and safety. Generalizability may be limited as the trial was conducted in a single state with a predominantly urban and White patient population. Additionally, heterogeneity in state cannabis programs and laws may limit national applicability.
DISCLOSURES:
The study was supported by philanthropic support to the HealthPartners Cancer Research Center. Cannabis products were provided by Vireo Health (GreenGoods, Minnesota). Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Cannabis May Ease Symptoms in Advanced Pancreatic Cancer
Cannabis May Ease Symptoms in Advanced Pancreatic Cancer
Home Screening Cost-Effective for Anal Cancer
Home Screening Cost-Effective for Anal Cancer
TOPLINE:
A recent analysis suggested that home-based screening for anal cancer is a cost-effective way to increase screening compared to clinic-based screening. The study found that a home-based approach led to higher participation rates (89.2% vs 74.2% for a clinic-based approach) among sexual and gender minority individuals and was cost-effective, costing $25.19 per additional individual screened when accounting for both direct and indirect costs and $132.36 per additional individual screened when only accounting for direct medical costs.
METHODOLOGY:
- Anal cancer screening is recommended for high-risk populations, such as sexual and gender minority individuals. However, it's unclear how cost-effective home-based self-sampling is compared to clinic-based screening.
- Researchers conducted an economic evaluation using data from a randomized clinical trial that included 240 sexual and gender minority individuals in Milwaukee from January 2020 to August 2022.
- Participants, aged ≥ 25 years, were randomized to either home-based self-sampling or clinic-based screening.
- Researchers evaluated direct home-based screening costs from the trial, and sourced clinic-based costs from the Medicare reimbursement schedule. Travel and time costs were determined from participant self-reports.
- The primary outcome was the incremental cost-effectiveness ratio (ICER), which was the additional cost needed to increase screening participation by one person. The researchers calculated ICERs from both a healthcare payer and societal perspective. The healthcare perspective included only direct medical costs and the societal perspective accounted for direct medical costs as well as indirect time and travel costs.
TAKEAWAY:
- Home-based screening led to higher participation rates than clinic-based screening—89.2% vs 74.2%—with 107 participants completing home-based screening compared with 89 participants doing clinic-based screening.
- The cost per participant was $64.18 for home-based screening and $60.40 for clinic-based screening from the societal perspective, and $61.91 for home-based screening and $42.06 for clinic-based screening from the healthcare payer perspective.
- With home-based screening, the ICER per additional screened participant was $25.19 from a societal perspective and $132.36 from a healthcare payer perspective.
- From the societal perspective, the probability that home-based screening was cost-effective compared with clinic-based screening was nearly 50% at a willingness-to-pay threshold of $25 and 99.99% at a threshold of $100. From the healthcare perspective, the probability was 3.8% at a threshold of $100 and 90.9% at a threshold of $200.
IN PRACTICE:
"These findings suggest that home-based screening promises to be a cost-effective option to enhance anal cancer screening participation," the study authors concluded.
SOURCE:
The study, led by Haluk Damgacioglu, PhD, Department of Public Health Sciences, Medical University of South Carolina in Charleston, South Carolina, was published online in JAMA Network Open.
LIMITATIONS:
The study was conducted in an urban setting where proximity to clinics may reduce structural barriers, potentially limiting generalizability to rural areas where longer travel distances and limited clinician availability could affect participation rates. The analysis did not include downstream steps such as follow-up clinic visits, confirmatory testing, treatment of precancerous lesions, or cancer prevention outcomes. While home-based screening participants were required to visit clinics for digital anal rectal examination to exclude prevalent anal cancer, these follow-up visit costs were not included in the cost-effectiveness analysis.
DISCLOSURES:
Elizabeth Chiao, PhD, reported receiving grants from the National Institutes of Health during the study. Jennifer S. Smith, PhD, MPH, disclosed receiving personal fees from Hologic, Inc., and materials for research purposes from Rovers Medical Devices. Ashish A. Deshmukh, PhD, MPH, reported receiving personal fees from Value Analytics Lab. Alan G. Nyitray, PhD, reported receiving grants from the National Cancer Institute and test kits from Copan Diagnostics.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A recent analysis suggested that home-based screening for anal cancer is a cost-effective way to increase screening compared to clinic-based screening. The study found that a home-based approach led to higher participation rates (89.2% vs 74.2% for a clinic-based approach) among sexual and gender minority individuals and was cost-effective, costing $25.19 per additional individual screened when accounting for both direct and indirect costs and $132.36 per additional individual screened when only accounting for direct medical costs.
METHODOLOGY:
- Anal cancer screening is recommended for high-risk populations, such as sexual and gender minority individuals. However, it's unclear how cost-effective home-based self-sampling is compared to clinic-based screening.
- Researchers conducted an economic evaluation using data from a randomized clinical trial that included 240 sexual and gender minority individuals in Milwaukee from January 2020 to August 2022.
- Participants, aged ≥ 25 years, were randomized to either home-based self-sampling or clinic-based screening.
- Researchers evaluated direct home-based screening costs from the trial, and sourced clinic-based costs from the Medicare reimbursement schedule. Travel and time costs were determined from participant self-reports.
- The primary outcome was the incremental cost-effectiveness ratio (ICER), which was the additional cost needed to increase screening participation by one person. The researchers calculated ICERs from both a healthcare payer and societal perspective. The healthcare perspective included only direct medical costs and the societal perspective accounted for direct medical costs as well as indirect time and travel costs.
TAKEAWAY:
- Home-based screening led to higher participation rates than clinic-based screening—89.2% vs 74.2%—with 107 participants completing home-based screening compared with 89 participants doing clinic-based screening.
- The cost per participant was $64.18 for home-based screening and $60.40 for clinic-based screening from the societal perspective, and $61.91 for home-based screening and $42.06 for clinic-based screening from the healthcare payer perspective.
- With home-based screening, the ICER per additional screened participant was $25.19 from a societal perspective and $132.36 from a healthcare payer perspective.
- From the societal perspective, the probability that home-based screening was cost-effective compared with clinic-based screening was nearly 50% at a willingness-to-pay threshold of $25 and 99.99% at a threshold of $100. From the healthcare perspective, the probability was 3.8% at a threshold of $100 and 90.9% at a threshold of $200.
IN PRACTICE:
"These findings suggest that home-based screening promises to be a cost-effective option to enhance anal cancer screening participation," the study authors concluded.
SOURCE:
The study, led by Haluk Damgacioglu, PhD, Department of Public Health Sciences, Medical University of South Carolina in Charleston, South Carolina, was published online in JAMA Network Open.
LIMITATIONS:
The study was conducted in an urban setting where proximity to clinics may reduce structural barriers, potentially limiting generalizability to rural areas where longer travel distances and limited clinician availability could affect participation rates. The analysis did not include downstream steps such as follow-up clinic visits, confirmatory testing, treatment of precancerous lesions, or cancer prevention outcomes. While home-based screening participants were required to visit clinics for digital anal rectal examination to exclude prevalent anal cancer, these follow-up visit costs were not included in the cost-effectiveness analysis.
DISCLOSURES:
Elizabeth Chiao, PhD, reported receiving grants from the National Institutes of Health during the study. Jennifer S. Smith, PhD, MPH, disclosed receiving personal fees from Hologic, Inc., and materials for research purposes from Rovers Medical Devices. Ashish A. Deshmukh, PhD, MPH, reported receiving personal fees from Value Analytics Lab. Alan G. Nyitray, PhD, reported receiving grants from the National Cancer Institute and test kits from Copan Diagnostics.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A recent analysis suggested that home-based screening for anal cancer is a cost-effective way to increase screening compared to clinic-based screening. The study found that a home-based approach led to higher participation rates (89.2% vs 74.2% for a clinic-based approach) among sexual and gender minority individuals and was cost-effective, costing $25.19 per additional individual screened when accounting for both direct and indirect costs and $132.36 per additional individual screened when only accounting for direct medical costs.
METHODOLOGY:
- Anal cancer screening is recommended for high-risk populations, such as sexual and gender minority individuals. However, it's unclear how cost-effective home-based self-sampling is compared to clinic-based screening.
- Researchers conducted an economic evaluation using data from a randomized clinical trial that included 240 sexual and gender minority individuals in Milwaukee from January 2020 to August 2022.
- Participants, aged ≥ 25 years, were randomized to either home-based self-sampling or clinic-based screening.
- Researchers evaluated direct home-based screening costs from the trial, and sourced clinic-based costs from the Medicare reimbursement schedule. Travel and time costs were determined from participant self-reports.
- The primary outcome was the incremental cost-effectiveness ratio (ICER), which was the additional cost needed to increase screening participation by one person. The researchers calculated ICERs from both a healthcare payer and societal perspective. The healthcare perspective included only direct medical costs and the societal perspective accounted for direct medical costs as well as indirect time and travel costs.
TAKEAWAY:
- Home-based screening led to higher participation rates than clinic-based screening—89.2% vs 74.2%—with 107 participants completing home-based screening compared with 89 participants doing clinic-based screening.
- The cost per participant was $64.18 for home-based screening and $60.40 for clinic-based screening from the societal perspective, and $61.91 for home-based screening and $42.06 for clinic-based screening from the healthcare payer perspective.
- With home-based screening, the ICER per additional screened participant was $25.19 from a societal perspective and $132.36 from a healthcare payer perspective.
- From the societal perspective, the probability that home-based screening was cost-effective compared with clinic-based screening was nearly 50% at a willingness-to-pay threshold of $25 and 99.99% at a threshold of $100. From the healthcare perspective, the probability was 3.8% at a threshold of $100 and 90.9% at a threshold of $200.
IN PRACTICE:
"These findings suggest that home-based screening promises to be a cost-effective option to enhance anal cancer screening participation," the study authors concluded.
SOURCE:
The study, led by Haluk Damgacioglu, PhD, Department of Public Health Sciences, Medical University of South Carolina in Charleston, South Carolina, was published online in JAMA Network Open.
LIMITATIONS:
The study was conducted in an urban setting where proximity to clinics may reduce structural barriers, potentially limiting generalizability to rural areas where longer travel distances and limited clinician availability could affect participation rates. The analysis did not include downstream steps such as follow-up clinic visits, confirmatory testing, treatment of precancerous lesions, or cancer prevention outcomes. While home-based screening participants were required to visit clinics for digital anal rectal examination to exclude prevalent anal cancer, these follow-up visit costs were not included in the cost-effectiveness analysis.
DISCLOSURES:
Elizabeth Chiao, PhD, reported receiving grants from the National Institutes of Health during the study. Jennifer S. Smith, PhD, MPH, disclosed receiving personal fees from Hologic, Inc., and materials for research purposes from Rovers Medical Devices. Ashish A. Deshmukh, PhD, MPH, reported receiving personal fees from Value Analytics Lab. Alan G. Nyitray, PhD, reported receiving grants from the National Cancer Institute and test kits from Copan Diagnostics.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Home Screening Cost-Effective for Anal Cancer
Home Screening Cost-Effective for Anal Cancer
Simple Steps: Walking May Ease Colorectal Cancer Fatigue
Simple Steps: Walking May Ease Colorectal Cancer Fatigue
Regular physical activity—especially walking—may improve fatigue and boost quality of life for people with nonmetastatic colorectal cancer during the first 2 years after diagnosis, according to research presented at ASCO Gastrointestinal Cancers Symposium 2026.
The study, which tracked over 1700 patients with colorectal cancer, found that those with nonmetastatic disease who walked for exercise 6-12 months after their diagnosis showed significant improvement in their fatigue scores over time. Their quality-of-life ratings rose in tandem.
The findings suggest that simple, sustained movement may play a meaningful role in long-term survivorship care, lead investigator Louisa Liu, MD, of Cedars-Sinai Medical Center in Los Angeles, said during a press briefing.
“Fatigue is one of the most common and debilitating symptoms our patients experience, often long after treatment ends,” Liu noted.
The new data, she said, show that an accessible form of exercise, especially when maintained over time, “can make a real difference in how patients feel and function during recovery.”
Joel Saltzman, MD, an ASCO expert in gastrointestinal cancers based at Taussig Cancer Center, Cleveland Clinic, Cleveland, agreed.
This is a “super-important study for all of us in the cancer community,” Saltzman told the briefing, especially in light of the CHALLENGE trial.
That study demonstrated that a structured exercise program can actually improve overall survival for patients with early-stage colon cancer who completed surgery and adjuvant chemotherapy.
“When you couple that with how patients feel, it really begs the question: Are we as a society doing enough cancer rehabilitation?” Saltzman said. “Everyone’s familiar with cardiac rehab, but oncologic rehabilitation is really something that really should be thought about in the future.”
Among long-term colorectal cancer survivors, nearly 40% continue to experience moderate-to-severe fatigue years after treatment — a challenge that affects functional recovery, daily activity, and quality of life.
“Yet,” Liu said, “our toolbox of effective interventions remains limited.”
Growing evidence supports physical activity as a nonpharmacologic approach for managing cancer-related fatigue. The mechanisms, Liu noted, may be multiple and include reductions in systemic inflammation, preserved muscle mass, better sleep quality and improvements in psychological stress.
In fact, current clinical guidelines recommend physical activity as part of survivorship care, but some key questions remain unanswered, Liu said.
“We still don’t fully understand when during recovery activity is most beneficial, what types of activity are best for different patients, or how these effects play out in real-world longitudinal settings, especially in colorectal cancer survivors,” she explained.
To address some of those gaps, Liu and colleagues analyzed data from 1718 patients with colorectal cancer (mean age, 67 years; 48% women) enrolled in the International ColoCare prospective cohort study. Nearly 1 in 5 had metastatic disease at diagnosis.
Physical activity was assessed at baseline and at 6, 12, and 24 months after diagnosis using a validated questionnaire. Participants’ total number of metabolic equivalent of task (MET) minutes per week — a measurement of energy spent during physical activity — were calculated for walking, moderate activities, and vigorous activities.
Total physical activity was categorized as low (fewer than 600 MET min/wk), moderate (600-3000 MET min/wk), or high (over 3000 MET min/wk).
Cancer-related fatigue and quality of life were measured using the European Organization for Research and Treatment of Cancer QLQ-C30 scale.
Overall, patients who were more physically active reported less fatigue and better quality of life as they moved further into recovery. And walking, Liu said, showed the “clearest and most consistent” association with these improved outcomes.
Among patients with nonmetastatic disease, those who reported regular walking 6-12 months after diagnosis showed significantly lower fatigue and higher quality-of-life scores over 2 years. Fatigue scores in this group improved steadily with time, from 32.5 at diagnosis to 29 at 12 months post-diagnosis and 26.8 at 24 months post-diagnosis.
Patients with metastatic disease also showed reductions in fatigue scores — from 40.7 at diagnosis to 37.1 at 12 months and 36.4 at 24 months — although those differences did not reach statistical significance.
Liu pointed out that patients with metastatic disease, not surprisingly, reported greater fatigue and poorer quality of life across all time points vs those with early-stage disease.
So, she said, “we don’t yet have strong evidence that physical activity changes the fatigue trajectory in the long run for metastatic patients. But this is an area where more targeted research is really needed.”
Looking at patterns of physical activity, the researchers found that activity levels at the time of diagnosis did not reliably predict long-term fatigue and quality-of-life outcomes. Instead, a patient’s activity level maintained between diagnosis and 1 year follow-up was a predictor of better outcomes.
“Short-term increases in physical activity didn’t seem to make a meaningful difference,” Liu said. “This suggests that when it comes to managing cancer-related fatigue, the key is to build steady, lasting habits that patients can stick with throughout their recovery.”
The study had no commercial funding. Liu and Saltzman had no disclosures.
A version of this article first appeared on Medscape.com.
Regular physical activity—especially walking—may improve fatigue and boost quality of life for people with nonmetastatic colorectal cancer during the first 2 years after diagnosis, according to research presented at ASCO Gastrointestinal Cancers Symposium 2026.
The study, which tracked over 1700 patients with colorectal cancer, found that those with nonmetastatic disease who walked for exercise 6-12 months after their diagnosis showed significant improvement in their fatigue scores over time. Their quality-of-life ratings rose in tandem.
The findings suggest that simple, sustained movement may play a meaningful role in long-term survivorship care, lead investigator Louisa Liu, MD, of Cedars-Sinai Medical Center in Los Angeles, said during a press briefing.
“Fatigue is one of the most common and debilitating symptoms our patients experience, often long after treatment ends,” Liu noted.
The new data, she said, show that an accessible form of exercise, especially when maintained over time, “can make a real difference in how patients feel and function during recovery.”
Joel Saltzman, MD, an ASCO expert in gastrointestinal cancers based at Taussig Cancer Center, Cleveland Clinic, Cleveland, agreed.
This is a “super-important study for all of us in the cancer community,” Saltzman told the briefing, especially in light of the CHALLENGE trial.
That study demonstrated that a structured exercise program can actually improve overall survival for patients with early-stage colon cancer who completed surgery and adjuvant chemotherapy.
“When you couple that with how patients feel, it really begs the question: Are we as a society doing enough cancer rehabilitation?” Saltzman said. “Everyone’s familiar with cardiac rehab, but oncologic rehabilitation is really something that really should be thought about in the future.”
Among long-term colorectal cancer survivors, nearly 40% continue to experience moderate-to-severe fatigue years after treatment — a challenge that affects functional recovery, daily activity, and quality of life.
“Yet,” Liu said, “our toolbox of effective interventions remains limited.”
Growing evidence supports physical activity as a nonpharmacologic approach for managing cancer-related fatigue. The mechanisms, Liu noted, may be multiple and include reductions in systemic inflammation, preserved muscle mass, better sleep quality and improvements in psychological stress.
In fact, current clinical guidelines recommend physical activity as part of survivorship care, but some key questions remain unanswered, Liu said.
“We still don’t fully understand when during recovery activity is most beneficial, what types of activity are best for different patients, or how these effects play out in real-world longitudinal settings, especially in colorectal cancer survivors,” she explained.
To address some of those gaps, Liu and colleagues analyzed data from 1718 patients with colorectal cancer (mean age, 67 years; 48% women) enrolled in the International ColoCare prospective cohort study. Nearly 1 in 5 had metastatic disease at diagnosis.
Physical activity was assessed at baseline and at 6, 12, and 24 months after diagnosis using a validated questionnaire. Participants’ total number of metabolic equivalent of task (MET) minutes per week — a measurement of energy spent during physical activity — were calculated for walking, moderate activities, and vigorous activities.
Total physical activity was categorized as low (fewer than 600 MET min/wk), moderate (600-3000 MET min/wk), or high (over 3000 MET min/wk).
Cancer-related fatigue and quality of life were measured using the European Organization for Research and Treatment of Cancer QLQ-C30 scale.
Overall, patients who were more physically active reported less fatigue and better quality of life as they moved further into recovery. And walking, Liu said, showed the “clearest and most consistent” association with these improved outcomes.
Among patients with nonmetastatic disease, those who reported regular walking 6-12 months after diagnosis showed significantly lower fatigue and higher quality-of-life scores over 2 years. Fatigue scores in this group improved steadily with time, from 32.5 at diagnosis to 29 at 12 months post-diagnosis and 26.8 at 24 months post-diagnosis.
Patients with metastatic disease also showed reductions in fatigue scores — from 40.7 at diagnosis to 37.1 at 12 months and 36.4 at 24 months — although those differences did not reach statistical significance.
Liu pointed out that patients with metastatic disease, not surprisingly, reported greater fatigue and poorer quality of life across all time points vs those with early-stage disease.
So, she said, “we don’t yet have strong evidence that physical activity changes the fatigue trajectory in the long run for metastatic patients. But this is an area where more targeted research is really needed.”
Looking at patterns of physical activity, the researchers found that activity levels at the time of diagnosis did not reliably predict long-term fatigue and quality-of-life outcomes. Instead, a patient’s activity level maintained between diagnosis and 1 year follow-up was a predictor of better outcomes.
“Short-term increases in physical activity didn’t seem to make a meaningful difference,” Liu said. “This suggests that when it comes to managing cancer-related fatigue, the key is to build steady, lasting habits that patients can stick with throughout their recovery.”
The study had no commercial funding. Liu and Saltzman had no disclosures.
A version of this article first appeared on Medscape.com.
Regular physical activity—especially walking—may improve fatigue and boost quality of life for people with nonmetastatic colorectal cancer during the first 2 years after diagnosis, according to research presented at ASCO Gastrointestinal Cancers Symposium 2026.
The study, which tracked over 1700 patients with colorectal cancer, found that those with nonmetastatic disease who walked for exercise 6-12 months after their diagnosis showed significant improvement in their fatigue scores over time. Their quality-of-life ratings rose in tandem.
The findings suggest that simple, sustained movement may play a meaningful role in long-term survivorship care, lead investigator Louisa Liu, MD, of Cedars-Sinai Medical Center in Los Angeles, said during a press briefing.
“Fatigue is one of the most common and debilitating symptoms our patients experience, often long after treatment ends,” Liu noted.
The new data, she said, show that an accessible form of exercise, especially when maintained over time, “can make a real difference in how patients feel and function during recovery.”
Joel Saltzman, MD, an ASCO expert in gastrointestinal cancers based at Taussig Cancer Center, Cleveland Clinic, Cleveland, agreed.
This is a “super-important study for all of us in the cancer community,” Saltzman told the briefing, especially in light of the CHALLENGE trial.
That study demonstrated that a structured exercise program can actually improve overall survival for patients with early-stage colon cancer who completed surgery and adjuvant chemotherapy.
“When you couple that with how patients feel, it really begs the question: Are we as a society doing enough cancer rehabilitation?” Saltzman said. “Everyone’s familiar with cardiac rehab, but oncologic rehabilitation is really something that really should be thought about in the future.”
Among long-term colorectal cancer survivors, nearly 40% continue to experience moderate-to-severe fatigue years after treatment — a challenge that affects functional recovery, daily activity, and quality of life.
“Yet,” Liu said, “our toolbox of effective interventions remains limited.”
Growing evidence supports physical activity as a nonpharmacologic approach for managing cancer-related fatigue. The mechanisms, Liu noted, may be multiple and include reductions in systemic inflammation, preserved muscle mass, better sleep quality and improvements in psychological stress.
In fact, current clinical guidelines recommend physical activity as part of survivorship care, but some key questions remain unanswered, Liu said.
“We still don’t fully understand when during recovery activity is most beneficial, what types of activity are best for different patients, or how these effects play out in real-world longitudinal settings, especially in colorectal cancer survivors,” she explained.
To address some of those gaps, Liu and colleagues analyzed data from 1718 patients with colorectal cancer (mean age, 67 years; 48% women) enrolled in the International ColoCare prospective cohort study. Nearly 1 in 5 had metastatic disease at diagnosis.
Physical activity was assessed at baseline and at 6, 12, and 24 months after diagnosis using a validated questionnaire. Participants’ total number of metabolic equivalent of task (MET) minutes per week — a measurement of energy spent during physical activity — were calculated for walking, moderate activities, and vigorous activities.
Total physical activity was categorized as low (fewer than 600 MET min/wk), moderate (600-3000 MET min/wk), or high (over 3000 MET min/wk).
Cancer-related fatigue and quality of life were measured using the European Organization for Research and Treatment of Cancer QLQ-C30 scale.
Overall, patients who were more physically active reported less fatigue and better quality of life as they moved further into recovery. And walking, Liu said, showed the “clearest and most consistent” association with these improved outcomes.
Among patients with nonmetastatic disease, those who reported regular walking 6-12 months after diagnosis showed significantly lower fatigue and higher quality-of-life scores over 2 years. Fatigue scores in this group improved steadily with time, from 32.5 at diagnosis to 29 at 12 months post-diagnosis and 26.8 at 24 months post-diagnosis.
Patients with metastatic disease also showed reductions in fatigue scores — from 40.7 at diagnosis to 37.1 at 12 months and 36.4 at 24 months — although those differences did not reach statistical significance.
Liu pointed out that patients with metastatic disease, not surprisingly, reported greater fatigue and poorer quality of life across all time points vs those with early-stage disease.
So, she said, “we don’t yet have strong evidence that physical activity changes the fatigue trajectory in the long run for metastatic patients. But this is an area where more targeted research is really needed.”
Looking at patterns of physical activity, the researchers found that activity levels at the time of diagnosis did not reliably predict long-term fatigue and quality-of-life outcomes. Instead, a patient’s activity level maintained between diagnosis and 1 year follow-up was a predictor of better outcomes.
“Short-term increases in physical activity didn’t seem to make a meaningful difference,” Liu said. “This suggests that when it comes to managing cancer-related fatigue, the key is to build steady, lasting habits that patients can stick with throughout their recovery.”
The study had no commercial funding. Liu and Saltzman had no disclosures.
A version of this article first appeared on Medscape.com.
Simple Steps: Walking May Ease Colorectal Cancer Fatigue
Simple Steps: Walking May Ease Colorectal Cancer Fatigue
Rural Cancer Survivors Are More Likely to Have Chronic Pain
TOPLINE:
Rural cancer survivors experience significantly higher rates of chronic pain at 43.0% than those among urban survivors at 33.5%. Even after controlling for demographics and health conditions, rural residents showed 21% higher odds of experiencing chronic pain.
METHODOLOGY:
- Chronic pain prevalence among cancer survivors is twice that of the general US population and is associated with numerous negative outcomes. Rural residence is frequently linked to debilitating long-term survivorship effects, and current data lack information on whether chronic pain disparity exists specifically for rural cancer survivors.
- Researchers pooled data from the 2019–2021 and 2023 National Health Interview Survey, a cross–sectional survey conducted by the National Center for Health Statistics.
- Analysis included 5542 adult cancer survivors diagnosed within the previous 5 years, with 51.6% female participants and 48.4% male participants.
- Chronic pain was defined as pain experienced on most or all days over the past 3 months, following National Center for Health Statistics conventions.
- Rural residence classification was based on noncore or nonmetropolitan counties using the modified National Center for Health Statistics Urban–Rural Classification Scheme for Counties.
TAKEAWAY:
- Rural cancer survivors showed significantly higher odds of experiencing chronic pain compared with urban survivors (odds ratio [OR], 1.21; 95% CI, 1.01-1.45).
- Rural survivors were more likely to be non–Hispanic White, have less than a 4-year college degree, have an income below 200% of the federal poverty level, and have slightly more chronic health conditions.
- Having an income below 100% of the federal poverty level was associated with doubled odds of chronic pain (OR, 2.07; 95% CI, 1.54-2.77) compared with having an income at least four times the federal poverty level.
- Each additional health condition increased the odds of experiencing chronic pain by 32% (OR, 1.32; 95% CI, 1.26-1.39).
IN PRACTICE:
“Policymakers and health systems should work to close this gap by increasing the availability of pain management resources for rural cancer survivors. Approaches could include innovative payment models for integrative medicine in rural areas or supporting rural clinician access to pain specialists,” the authors of the study wrote.
SOURCE:
This study was led by Hyojin Choi, PhD, Department of Family Medicine, The Robert Larner MD College of Medicine, University of Vermont in Burlington, Vermont. It was published online in JAMA Network Open.
LIMITATIONS:
The authors note that the cross–sectional design of the study and limited information on individual respondents’ use of multimodal pain treatment options constrain the interpretation of findings.
DISCLOSURES:
The authors did not report any relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Rural cancer survivors experience significantly higher rates of chronic pain at 43.0% than those among urban survivors at 33.5%. Even after controlling for demographics and health conditions, rural residents showed 21% higher odds of experiencing chronic pain.
METHODOLOGY:
- Chronic pain prevalence among cancer survivors is twice that of the general US population and is associated with numerous negative outcomes. Rural residence is frequently linked to debilitating long-term survivorship effects, and current data lack information on whether chronic pain disparity exists specifically for rural cancer survivors.
- Researchers pooled data from the 2019–2021 and 2023 National Health Interview Survey, a cross–sectional survey conducted by the National Center for Health Statistics.
- Analysis included 5542 adult cancer survivors diagnosed within the previous 5 years, with 51.6% female participants and 48.4% male participants.
- Chronic pain was defined as pain experienced on most or all days over the past 3 months, following National Center for Health Statistics conventions.
- Rural residence classification was based on noncore or nonmetropolitan counties using the modified National Center for Health Statistics Urban–Rural Classification Scheme for Counties.
TAKEAWAY:
- Rural cancer survivors showed significantly higher odds of experiencing chronic pain compared with urban survivors (odds ratio [OR], 1.21; 95% CI, 1.01-1.45).
- Rural survivors were more likely to be non–Hispanic White, have less than a 4-year college degree, have an income below 200% of the federal poverty level, and have slightly more chronic health conditions.
- Having an income below 100% of the federal poverty level was associated with doubled odds of chronic pain (OR, 2.07; 95% CI, 1.54-2.77) compared with having an income at least four times the federal poverty level.
- Each additional health condition increased the odds of experiencing chronic pain by 32% (OR, 1.32; 95% CI, 1.26-1.39).
IN PRACTICE:
“Policymakers and health systems should work to close this gap by increasing the availability of pain management resources for rural cancer survivors. Approaches could include innovative payment models for integrative medicine in rural areas or supporting rural clinician access to pain specialists,” the authors of the study wrote.
SOURCE:
This study was led by Hyojin Choi, PhD, Department of Family Medicine, The Robert Larner MD College of Medicine, University of Vermont in Burlington, Vermont. It was published online in JAMA Network Open.
LIMITATIONS:
The authors note that the cross–sectional design of the study and limited information on individual respondents’ use of multimodal pain treatment options constrain the interpretation of findings.
DISCLOSURES:
The authors did not report any relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Rural cancer survivors experience significantly higher rates of chronic pain at 43.0% than those among urban survivors at 33.5%. Even after controlling for demographics and health conditions, rural residents showed 21% higher odds of experiencing chronic pain.
METHODOLOGY:
- Chronic pain prevalence among cancer survivors is twice that of the general US population and is associated with numerous negative outcomes. Rural residence is frequently linked to debilitating long-term survivorship effects, and current data lack information on whether chronic pain disparity exists specifically for rural cancer survivors.
- Researchers pooled data from the 2019–2021 and 2023 National Health Interview Survey, a cross–sectional survey conducted by the National Center for Health Statistics.
- Analysis included 5542 adult cancer survivors diagnosed within the previous 5 years, with 51.6% female participants and 48.4% male participants.
- Chronic pain was defined as pain experienced on most or all days over the past 3 months, following National Center for Health Statistics conventions.
- Rural residence classification was based on noncore or nonmetropolitan counties using the modified National Center for Health Statistics Urban–Rural Classification Scheme for Counties.
TAKEAWAY:
- Rural cancer survivors showed significantly higher odds of experiencing chronic pain compared with urban survivors (odds ratio [OR], 1.21; 95% CI, 1.01-1.45).
- Rural survivors were more likely to be non–Hispanic White, have less than a 4-year college degree, have an income below 200% of the federal poverty level, and have slightly more chronic health conditions.
- Having an income below 100% of the federal poverty level was associated with doubled odds of chronic pain (OR, 2.07; 95% CI, 1.54-2.77) compared with having an income at least four times the federal poverty level.
- Each additional health condition increased the odds of experiencing chronic pain by 32% (OR, 1.32; 95% CI, 1.26-1.39).
IN PRACTICE:
“Policymakers and health systems should work to close this gap by increasing the availability of pain management resources for rural cancer survivors. Approaches could include innovative payment models for integrative medicine in rural areas or supporting rural clinician access to pain specialists,” the authors of the study wrote.
SOURCE:
This study was led by Hyojin Choi, PhD, Department of Family Medicine, The Robert Larner MD College of Medicine, University of Vermont in Burlington, Vermont. It was published online in JAMA Network Open.
LIMITATIONS:
The authors note that the cross–sectional design of the study and limited information on individual respondents’ use of multimodal pain treatment options constrain the interpretation of findings.
DISCLOSURES:
The authors did not report any relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
NICE Endorses Chemo-Free First-Line Options for EGFR NSCLC
NICE Endorses Chemo-Free First-Line Options for EGFR NSCLC
The National Institute for Health and Care Excellence (NICE) has recommended amivantamab (Rybrevant) plus lazertinib (Lazcluze) as a first-line option for adults with previously untreated advanced non–small-cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R substitution mutations.
In final draft guidance, NICE said the combination therapy should be funded by the NHS in England for eligible patients when it is the most appropriate option. Around 1115 people are expected to benefit.
Lung cancer is the third most common cancer and the leading cause of cancer mortality in the UK. It accounted for 10% of all new cancer diagnoses and 20% of cancer deaths in 2020. Approximately 31,000 people received NSCLC diagnoses in England in 2021, comprising 91% of all lung cancer cases. EGFR mutation-positive NSCLC is more common in women, non-smokers, and individuals from Asian ethnic backgrounds.
Welcoming the decision, Virginia Harrison, research trustee, EGFR+ UK, said, “This is a meaningful advance for patients and their families facing this diagnosis. [It] provides something the EGFR community urgently needs: more choice in first-line treatment.”
How Practice May Shift
The recommendation adds an alternative to existing standards, including osimertinib monotherapy or osimertinib plus pemetrexed/platinum-based chemotherapy. Clinical specialists noted that no single standard care exists for this patient group.
Younger patients and those willing to accept greater side effects may choose between amivantamab plus lazertinib or osimertinib plus chemotherapy. Patients older than 80 years might prefer osimertinib monotherapy due to adverse event considerations.
Mechanism of Action and Clinical Evidence
Amivantamab is a bispecific antibody that simultaneously binds EGFR and mesenchymal-epithelial transition receptors, blocking downstream signaling pathways that drive tumor growth and promoting immune-mediated cancer cell killing. Lazertinib is an oral third-generation EGFR TKI that selectively inhibits mutant EGFR signaling. Together, the agents provides complementary suppression of EGFR-driven tumour growth and resistance mechanisms.
The NICE recommendation is supported by results from the phase 3 MARIPOSA trial, which met its primary endpoint of progression-free survival (PFS). Treatment with amivantamab plus lazertinib significantly prolonged median PFS to 23.7 months compared with 16.6 months with osimertinib. The combination also demonstrated a significant improvement in overall survival, reducing the risk for death by 25% vs osimertinib. Median OS was not reached in the combination arm and was 36.7 months with osimertinib.
The most common adverse reactions with the combination included rash, nail toxicity, hypoalbuminaemia, hepatotoxicity, and stomatitis.
A Medicines and Healthcare products Regulatory Agency-approved subcutaneous formulation of amivantamab, authorized after the committee’s initial meeting, may further improve tolerability and convenience. Administration-related reactions occurred in 63% of patients with the intravenous formulation vs 14% with the subcutaneous formulation. Clinicians expect subcutaneous dosing to replace intravenous use in practice.
Dosing, Access, and Implementation
Amivantamab is administered every 2 weeks, either intravenously or subcutaneously. Lazertinib is taken as a daily oral tablet.
Rybrevant costs £1079 for a 350-mg per 7-mL vial. Lazcluze is priced at £4128.50 for 56 x 80-mg tablets and £6192.75 for 28 x 240-mg tablets. Confidential NHS discounts are available through simple patient access schemes.
Integrated care boards, NHS England, and local authorities must implement the guidance within 90 days of publication. For drugs receiving positive draft recommendations for routine commissioning, interim funding becomes accessible from the Cancer Drugs Fund budget starting from the point of marketing authorisation or publication of draft guidance.
The National Institute for Health and Care Excellence (NICE) has recommended amivantamab (Rybrevant) plus lazertinib (Lazcluze) as a first-line option for adults with previously untreated advanced non–small-cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R substitution mutations.
In final draft guidance, NICE said the combination therapy should be funded by the NHS in England for eligible patients when it is the most appropriate option. Around 1115 people are expected to benefit.
Lung cancer is the third most common cancer and the leading cause of cancer mortality in the UK. It accounted for 10% of all new cancer diagnoses and 20% of cancer deaths in 2020. Approximately 31,000 people received NSCLC diagnoses in England in 2021, comprising 91% of all lung cancer cases. EGFR mutation-positive NSCLC is more common in women, non-smokers, and individuals from Asian ethnic backgrounds.
Welcoming the decision, Virginia Harrison, research trustee, EGFR+ UK, said, “This is a meaningful advance for patients and their families facing this diagnosis. [It] provides something the EGFR community urgently needs: more choice in first-line treatment.”
How Practice May Shift
The recommendation adds an alternative to existing standards, including osimertinib monotherapy or osimertinib plus pemetrexed/platinum-based chemotherapy. Clinical specialists noted that no single standard care exists for this patient group.
Younger patients and those willing to accept greater side effects may choose between amivantamab plus lazertinib or osimertinib plus chemotherapy. Patients older than 80 years might prefer osimertinib monotherapy due to adverse event considerations.
Mechanism of Action and Clinical Evidence
Amivantamab is a bispecific antibody that simultaneously binds EGFR and mesenchymal-epithelial transition receptors, blocking downstream signaling pathways that drive tumor growth and promoting immune-mediated cancer cell killing. Lazertinib is an oral third-generation EGFR TKI that selectively inhibits mutant EGFR signaling. Together, the agents provides complementary suppression of EGFR-driven tumour growth and resistance mechanisms.
The NICE recommendation is supported by results from the phase 3 MARIPOSA trial, which met its primary endpoint of progression-free survival (PFS). Treatment with amivantamab plus lazertinib significantly prolonged median PFS to 23.7 months compared with 16.6 months with osimertinib. The combination also demonstrated a significant improvement in overall survival, reducing the risk for death by 25% vs osimertinib. Median OS was not reached in the combination arm and was 36.7 months with osimertinib.
The most common adverse reactions with the combination included rash, nail toxicity, hypoalbuminaemia, hepatotoxicity, and stomatitis.
A Medicines and Healthcare products Regulatory Agency-approved subcutaneous formulation of amivantamab, authorized after the committee’s initial meeting, may further improve tolerability and convenience. Administration-related reactions occurred in 63% of patients with the intravenous formulation vs 14% with the subcutaneous formulation. Clinicians expect subcutaneous dosing to replace intravenous use in practice.
Dosing, Access, and Implementation
Amivantamab is administered every 2 weeks, either intravenously or subcutaneously. Lazertinib is taken as a daily oral tablet.
Rybrevant costs £1079 for a 350-mg per 7-mL vial. Lazcluze is priced at £4128.50 for 56 x 80-mg tablets and £6192.75 for 28 x 240-mg tablets. Confidential NHS discounts are available through simple patient access schemes.
Integrated care boards, NHS England, and local authorities must implement the guidance within 90 days of publication. For drugs receiving positive draft recommendations for routine commissioning, interim funding becomes accessible from the Cancer Drugs Fund budget starting from the point of marketing authorisation or publication of draft guidance.
The National Institute for Health and Care Excellence (NICE) has recommended amivantamab (Rybrevant) plus lazertinib (Lazcluze) as a first-line option for adults with previously untreated advanced non–small-cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R substitution mutations.
In final draft guidance, NICE said the combination therapy should be funded by the NHS in England for eligible patients when it is the most appropriate option. Around 1115 people are expected to benefit.
Lung cancer is the third most common cancer and the leading cause of cancer mortality in the UK. It accounted for 10% of all new cancer diagnoses and 20% of cancer deaths in 2020. Approximately 31,000 people received NSCLC diagnoses in England in 2021, comprising 91% of all lung cancer cases. EGFR mutation-positive NSCLC is more common in women, non-smokers, and individuals from Asian ethnic backgrounds.
Welcoming the decision, Virginia Harrison, research trustee, EGFR+ UK, said, “This is a meaningful advance for patients and their families facing this diagnosis. [It] provides something the EGFR community urgently needs: more choice in first-line treatment.”
How Practice May Shift
The recommendation adds an alternative to existing standards, including osimertinib monotherapy or osimertinib plus pemetrexed/platinum-based chemotherapy. Clinical specialists noted that no single standard care exists for this patient group.
Younger patients and those willing to accept greater side effects may choose between amivantamab plus lazertinib or osimertinib plus chemotherapy. Patients older than 80 years might prefer osimertinib monotherapy due to adverse event considerations.
Mechanism of Action and Clinical Evidence
Amivantamab is a bispecific antibody that simultaneously binds EGFR and mesenchymal-epithelial transition receptors, blocking downstream signaling pathways that drive tumor growth and promoting immune-mediated cancer cell killing. Lazertinib is an oral third-generation EGFR TKI that selectively inhibits mutant EGFR signaling. Together, the agents provides complementary suppression of EGFR-driven tumour growth and resistance mechanisms.
The NICE recommendation is supported by results from the phase 3 MARIPOSA trial, which met its primary endpoint of progression-free survival (PFS). Treatment with amivantamab plus lazertinib significantly prolonged median PFS to 23.7 months compared with 16.6 months with osimertinib. The combination also demonstrated a significant improvement in overall survival, reducing the risk for death by 25% vs osimertinib. Median OS was not reached in the combination arm and was 36.7 months with osimertinib.
The most common adverse reactions with the combination included rash, nail toxicity, hypoalbuminaemia, hepatotoxicity, and stomatitis.
A Medicines and Healthcare products Regulatory Agency-approved subcutaneous formulation of amivantamab, authorized after the committee’s initial meeting, may further improve tolerability and convenience. Administration-related reactions occurred in 63% of patients with the intravenous formulation vs 14% with the subcutaneous formulation. Clinicians expect subcutaneous dosing to replace intravenous use in practice.
Dosing, Access, and Implementation
Amivantamab is administered every 2 weeks, either intravenously or subcutaneously. Lazertinib is taken as a daily oral tablet.
Rybrevant costs £1079 for a 350-mg per 7-mL vial. Lazcluze is priced at £4128.50 for 56 x 80-mg tablets and £6192.75 for 28 x 240-mg tablets. Confidential NHS discounts are available through simple patient access schemes.
Integrated care boards, NHS England, and local authorities must implement the guidance within 90 days of publication. For drugs receiving positive draft recommendations for routine commissioning, interim funding becomes accessible from the Cancer Drugs Fund budget starting from the point of marketing authorisation or publication of draft guidance.
NICE Endorses Chemo-Free First-Line Options for EGFR NSCLC
NICE Endorses Chemo-Free First-Line Options for EGFR NSCLC
Turning the Cancer Research Problem Into an Opportunity
Turning the Cancer Research Problem Into an Opportunity
The War on Cancer, declared by President Richard Nixon some 50 years ago, has been canceled during the second Trump administration in 2025 — so saith The New York Times Sunday magazine cover story on September 14, 2025. This war seems now to be best described as "The War on Cancer Research."
To our horror and disbelief, we've witnessed the slow but persistent drift of much of the United States citizenry away from science and the sudden and severe movement of the US government to crush much medical research. But it is not as if we were not warned.
In August 2024, on these pages and without political bias, I urged Medscape readers to pay attention to Project 2025. A great deal of what we as a population are now experiencing was laid out as a carefully constructed plan.
What is surprising is the cruel ruthlessness of the "move fast and break things" approach, taken with little apparent concern about the resultant human tragedies (workforce and patients) and no clear care about the resulting fallout. As we've now learned, destroying something as grand as our cancer research enterprise can be accomplished very quickly. Rebuilding it is certain to be slow and difficult and perhaps can never be accomplished.
In this new anti-science, anti-research, and anti-researcher reality, what can we now do?
First and foremost, we must recognize that the war on cancer is not over. Cancer is not canceled, even if much of the US government's research effort/funding has been. Those of us in medicine and public health often speak in quantification of causes of death of our populations. As such, I'll remind Medscape readers that cancer afflicts some 20 million humans worldwide each year, killing nearly 10 million. Although two-thirds of Americans diagnosed with a potentially lethal malignancy are cured, cancer still kills roughly 600,000 Americans each year. Cancer has been the second most frequent cause of death of Americans for 75 years.
Being inevitable and immutable, death itself is not the enemy. We all die. Disease, disability, pain, and human suffering are the real enemies of us all. Cancer maims, pains, diabetes, and torments some 20 million humans worldwide each year. That is a huge humanitarian problem that should be recognized by individuals of all creeds and backgrounds.
With this depletion of our domestic government basic and applied cancer research program, what can we do?
- Think globally and look to the international scientific research enterprises — relying on them, much as they have relied on us.
- Defend the universal importance of reliable and available literature on medical science.
- Continue to translate and apply the vast amount of available published research in clinical practice and publish the results.
- Urge private industries to expand their research budgets into areas of study that may not produce quickly tangible positive bottom-line results.
- Remind the Secretary of the Department of Health and Human Services (for whom chronic diseases seem paramount) that cancer is the second leading American chronic disease by morbidity.
- Redouble efforts of cancer prevention, especially urging the FDA to ban combustible tobacco and strive more diligently to decrease obesity.
- Appeal to our vast philanthropic universe to increase its funding of nonprofit organizations active in the cancer investigation, diagnosis, and management space.
One such 501c3 organization is California-based Cancer Commons. (Disclosure: I named it in 2010 and serve as its editor in chief).
A commons is a space shared by a community to use for the common interest. As we originally envisioned it, a cancer commons is an open access internet location where individuals and organizations (eg, corporations, universities, government agencies, philanthropies) will voluntarily share their data to work together to defeat the common enemy of humans: cancer.
On September 8, 2025, Cancer Commons was the 15th annual Lundberg Institute Lecturer at the Commonwealth Club of California in San Francisco. At the lecture, Cancer Commons founder (and long-term survivor of metastatic malignant melanoma), Jay Martin "Martin" Tenenbaum, PhD, spoke of the need for a cancer commons and the founder's vision. Emma Shtivelman, PhD, the long-time compassionate chief scientist, described some of the thousands of patients with advanced cancer that she has helped — all free of charge. And newly named CEO Clifford Reid, MBA, PhD, used his entrepreneurial prowess to envision an ambitious future.
Cancer Commons has always focused on patients with cancer who are beyond standards of curative care. As Cancer Commons evolves, it anticipates focusing on patients with cancer who are beyond National Comprehensive Cancer Network Guidelines. The organization intends to greatly expand its 1000 patients per year with "high touch" engagement with PhD clinical scientists to many thousands by including artificial intelligence. It plans to extend its N-of-One approach to create new knowledge — especially regarding the hundreds of drugs that are FDA-approved for use in treating cancer but have not been further assessed for the utility in actually treating patients with cancer.
The war on cancer is not over. It remains a persistent foe that causes immense disability, pain, and human suffering. With government support depleted, the burden now shifts to the private sector and philanthropic organizations, such as Cancer Commons, to serve as the new vital infrastructure in the fight for a cure. Now, we must redouble our efforts to ensure that these research endeavors are supported if the US government will not do its part.
A version of this article first appeared on Medscape.com.
The War on Cancer, declared by President Richard Nixon some 50 years ago, has been canceled during the second Trump administration in 2025 — so saith The New York Times Sunday magazine cover story on September 14, 2025. This war seems now to be best described as "The War on Cancer Research."
To our horror and disbelief, we've witnessed the slow but persistent drift of much of the United States citizenry away from science and the sudden and severe movement of the US government to crush much medical research. But it is not as if we were not warned.
In August 2024, on these pages and without political bias, I urged Medscape readers to pay attention to Project 2025. A great deal of what we as a population are now experiencing was laid out as a carefully constructed plan.
What is surprising is the cruel ruthlessness of the "move fast and break things" approach, taken with little apparent concern about the resultant human tragedies (workforce and patients) and no clear care about the resulting fallout. As we've now learned, destroying something as grand as our cancer research enterprise can be accomplished very quickly. Rebuilding it is certain to be slow and difficult and perhaps can never be accomplished.
In this new anti-science, anti-research, and anti-researcher reality, what can we now do?
First and foremost, we must recognize that the war on cancer is not over. Cancer is not canceled, even if much of the US government's research effort/funding has been. Those of us in medicine and public health often speak in quantification of causes of death of our populations. As such, I'll remind Medscape readers that cancer afflicts some 20 million humans worldwide each year, killing nearly 10 million. Although two-thirds of Americans diagnosed with a potentially lethal malignancy are cured, cancer still kills roughly 600,000 Americans each year. Cancer has been the second most frequent cause of death of Americans for 75 years.
Being inevitable and immutable, death itself is not the enemy. We all die. Disease, disability, pain, and human suffering are the real enemies of us all. Cancer maims, pains, diabetes, and torments some 20 million humans worldwide each year. That is a huge humanitarian problem that should be recognized by individuals of all creeds and backgrounds.
With this depletion of our domestic government basic and applied cancer research program, what can we do?
- Think globally and look to the international scientific research enterprises — relying on them, much as they have relied on us.
- Defend the universal importance of reliable and available literature on medical science.
- Continue to translate and apply the vast amount of available published research in clinical practice and publish the results.
- Urge private industries to expand their research budgets into areas of study that may not produce quickly tangible positive bottom-line results.
- Remind the Secretary of the Department of Health and Human Services (for whom chronic diseases seem paramount) that cancer is the second leading American chronic disease by morbidity.
- Redouble efforts of cancer prevention, especially urging the FDA to ban combustible tobacco and strive more diligently to decrease obesity.
- Appeal to our vast philanthropic universe to increase its funding of nonprofit organizations active in the cancer investigation, diagnosis, and management space.
One such 501c3 organization is California-based Cancer Commons. (Disclosure: I named it in 2010 and serve as its editor in chief).
A commons is a space shared by a community to use for the common interest. As we originally envisioned it, a cancer commons is an open access internet location where individuals and organizations (eg, corporations, universities, government agencies, philanthropies) will voluntarily share their data to work together to defeat the common enemy of humans: cancer.
On September 8, 2025, Cancer Commons was the 15th annual Lundberg Institute Lecturer at the Commonwealth Club of California in San Francisco. At the lecture, Cancer Commons founder (and long-term survivor of metastatic malignant melanoma), Jay Martin "Martin" Tenenbaum, PhD, spoke of the need for a cancer commons and the founder's vision. Emma Shtivelman, PhD, the long-time compassionate chief scientist, described some of the thousands of patients with advanced cancer that she has helped — all free of charge. And newly named CEO Clifford Reid, MBA, PhD, used his entrepreneurial prowess to envision an ambitious future.
Cancer Commons has always focused on patients with cancer who are beyond standards of curative care. As Cancer Commons evolves, it anticipates focusing on patients with cancer who are beyond National Comprehensive Cancer Network Guidelines. The organization intends to greatly expand its 1000 patients per year with "high touch" engagement with PhD clinical scientists to many thousands by including artificial intelligence. It plans to extend its N-of-One approach to create new knowledge — especially regarding the hundreds of drugs that are FDA-approved for use in treating cancer but have not been further assessed for the utility in actually treating patients with cancer.
The war on cancer is not over. It remains a persistent foe that causes immense disability, pain, and human suffering. With government support depleted, the burden now shifts to the private sector and philanthropic organizations, such as Cancer Commons, to serve as the new vital infrastructure in the fight for a cure. Now, we must redouble our efforts to ensure that these research endeavors are supported if the US government will not do its part.
A version of this article first appeared on Medscape.com.
The War on Cancer, declared by President Richard Nixon some 50 years ago, has been canceled during the second Trump administration in 2025 — so saith The New York Times Sunday magazine cover story on September 14, 2025. This war seems now to be best described as "The War on Cancer Research."
To our horror and disbelief, we've witnessed the slow but persistent drift of much of the United States citizenry away from science and the sudden and severe movement of the US government to crush much medical research. But it is not as if we were not warned.
In August 2024, on these pages and without political bias, I urged Medscape readers to pay attention to Project 2025. A great deal of what we as a population are now experiencing was laid out as a carefully constructed plan.
What is surprising is the cruel ruthlessness of the "move fast and break things" approach, taken with little apparent concern about the resultant human tragedies (workforce and patients) and no clear care about the resulting fallout. As we've now learned, destroying something as grand as our cancer research enterprise can be accomplished very quickly. Rebuilding it is certain to be slow and difficult and perhaps can never be accomplished.
In this new anti-science, anti-research, and anti-researcher reality, what can we now do?
First and foremost, we must recognize that the war on cancer is not over. Cancer is not canceled, even if much of the US government's research effort/funding has been. Those of us in medicine and public health often speak in quantification of causes of death of our populations. As such, I'll remind Medscape readers that cancer afflicts some 20 million humans worldwide each year, killing nearly 10 million. Although two-thirds of Americans diagnosed with a potentially lethal malignancy are cured, cancer still kills roughly 600,000 Americans each year. Cancer has been the second most frequent cause of death of Americans for 75 years.
Being inevitable and immutable, death itself is not the enemy. We all die. Disease, disability, pain, and human suffering are the real enemies of us all. Cancer maims, pains, diabetes, and torments some 20 million humans worldwide each year. That is a huge humanitarian problem that should be recognized by individuals of all creeds and backgrounds.
With this depletion of our domestic government basic and applied cancer research program, what can we do?
- Think globally and look to the international scientific research enterprises — relying on them, much as they have relied on us.
- Defend the universal importance of reliable and available literature on medical science.
- Continue to translate and apply the vast amount of available published research in clinical practice and publish the results.
- Urge private industries to expand their research budgets into areas of study that may not produce quickly tangible positive bottom-line results.
- Remind the Secretary of the Department of Health and Human Services (for whom chronic diseases seem paramount) that cancer is the second leading American chronic disease by morbidity.
- Redouble efforts of cancer prevention, especially urging the FDA to ban combustible tobacco and strive more diligently to decrease obesity.
- Appeal to our vast philanthropic universe to increase its funding of nonprofit organizations active in the cancer investigation, diagnosis, and management space.
One such 501c3 organization is California-based Cancer Commons. (Disclosure: I named it in 2010 and serve as its editor in chief).
A commons is a space shared by a community to use for the common interest. As we originally envisioned it, a cancer commons is an open access internet location where individuals and organizations (eg, corporations, universities, government agencies, philanthropies) will voluntarily share their data to work together to defeat the common enemy of humans: cancer.
On September 8, 2025, Cancer Commons was the 15th annual Lundberg Institute Lecturer at the Commonwealth Club of California in San Francisco. At the lecture, Cancer Commons founder (and long-term survivor of metastatic malignant melanoma), Jay Martin "Martin" Tenenbaum, PhD, spoke of the need for a cancer commons and the founder's vision. Emma Shtivelman, PhD, the long-time compassionate chief scientist, described some of the thousands of patients with advanced cancer that she has helped — all free of charge. And newly named CEO Clifford Reid, MBA, PhD, used his entrepreneurial prowess to envision an ambitious future.
Cancer Commons has always focused on patients with cancer who are beyond standards of curative care. As Cancer Commons evolves, it anticipates focusing on patients with cancer who are beyond National Comprehensive Cancer Network Guidelines. The organization intends to greatly expand its 1000 patients per year with "high touch" engagement with PhD clinical scientists to many thousands by including artificial intelligence. It plans to extend its N-of-One approach to create new knowledge — especially regarding the hundreds of drugs that are FDA-approved for use in treating cancer but have not been further assessed for the utility in actually treating patients with cancer.
The war on cancer is not over. It remains a persistent foe that causes immense disability, pain, and human suffering. With government support depleted, the burden now shifts to the private sector and philanthropic organizations, such as Cancer Commons, to serve as the new vital infrastructure in the fight for a cure. Now, we must redouble our efforts to ensure that these research endeavors are supported if the US government will not do its part.
A version of this article first appeared on Medscape.com.
Turning the Cancer Research Problem Into an Opportunity
Turning the Cancer Research Problem Into an Opportunity