User login
PRESERVED-HF: Dapagliflozin improves physical limitations in patients with HFpEF
The SGLT2 inhibitor dapagliflozin scored a clear win in a randomized, controlled trial with more than 300 U.S. patients with heart failure with preserved ejection fraction (HFpEF), showing a significant and clinically meaningful benefit for the primary endpoint, a KCCQ measure of symptoms and physical limitations, after 12 weeks of treatment.
These results in the PRESERVED-HF study follow closely on the heals of the initial report from the EMPEROR-Preserved trial that showed a benefit from a different sodium-glucose cotransporter 2 (SGLT2) inhibitor, empagliflozin (Jardiance) in nearly 6,000 randomized patients for the primary endpoint of preventing cardiovascular death or hospitalizations for heart failure.
In PRESERVED-HF, patients with HFpEF who received a standard, once-daily dose of dapagliflozin (Farxiga) had an average 5.8-point improvement in their condition as measured by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CS), the study’s primary endpoint.
This is “the first study to demonstrate that an SGLT2 inhibitor dapagliflozin significantly improves symptoms, physical limitations, and 6-minute walking distance in patients with HFpEF,” Mikhail N. Kosiborod, MD, reported at the annual scientific meeting of the Heart Failure Society of America. The secondary endpoint of 6-minute walking distance “has been very difficult to improve in many previous studies of other treatments” tested in patients with HFpEF, noted Dr. Kosiborod, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute.
The results are “highly complementary” to the findings from large outcome trials, such as the findings from EMPEROR-Preserved, he said, and collectively the recent findings from these studies of SGLT2 inhibitors in patients with HFpEF identify drugs in this class as a “new treatment option” for patients with a disorder that until now had no treatment with unequivocally proven efficacy and safety.
‘Impressive and unprecedented’ findings
The findings are “really impressive and unprecedented,” said Milton Packer, MD, a cardiologist at Baylor University Medical Center in Dallas who was not involved in the study. “This is the largest KCCQ benefit ever seen in either patients with HFpEF or in patients with heart failure with reduced ejection fraction,” said Dr. Packer, one of the investigators who led the EMPEROR-Preserved trial.
PRESERVED-HF randomized 324 patients diagnosed with heart failure and with a left ventricular ejection fraction of 45% or higher at any of 26 U.S. centers, with 304 patients completing the planned final analysis after 12 weeks on treatment. Patients could be in New York Heart Association (NYHA) functional class II-IV, they had to have a baseline N-terminal pro-brain natriuretic peptide (NT-proBNP) level of at least 225 pg/mL (or higher if they also had atrial fibrillation), and they required at least one of three markers of established heart failure: recent hospitalization for heart failure or an urgent outpatient visit that required treatment with an IV diuretic, elevated filling pressure measured by left or right catheterization, or structural heart disease detected by echocardiography.
The average age of the enrolled patients was 70 years, and they had been diagnosed with heart failure for about 3 years; 57% were women, 30% were African American, and their median body mass index was 35 kg/m2. Roughly 42% had NYHA class III or IV disease, 56% had type 2 diabetes, their median estimated glomerular filtration rate was about 55 mL/min per 1.73m2, their median KCCQ-CS score at baseline was about 62, and their average 6-minute walk distance was 244 m.
These and other features of the enrolled population define a distinctly U.S. patient population, stressed Dr. Kosiborod, professor of medicine at the University of Missouri–Kansas City.
“The patients we enrolled are the patients we see in U.S. clinical practice,” he said in an interview. Importantly, the patient profile of a median BMI of 35 kg/m2, a median KCCQ-CS score of 62 – “quite low,” noted Dr. Kosiborod – and having more than 40% of patients in NYHA functional class III defines a study population with a substantially greater burden of obesity, symptoms, and functional impairment compared with those enrolled in prior trials involving patients with HFpEF such as EMPEROR-Preserved.
Results complement findings from larger trials
PRESERVED-HF was an investigator-initiated study designed to inform clinical practice, not as a pivotal trial like EMPEROR-Preserved, which aims to gather evidence to support a new indication for regulatory approval. (On Sept. 9, 2021, the Food and Drug Administration granted empagliflozin “breakthrough therapy” status for treating HFpEF based on the EMPEROR-Preserved results, which will fast-track the agency’s decision on this indication.)
Dr. Kosiborod noted that he and his associates designed PRESERVED-HF with adequate patient numbers to power a statistically valid assessment of effect on KCCQ-CS score. While the new findings will not by themselves lead to a new indication for dapagliflozin to treat patients with HFpEF, they will potentially complement the pending results of another trial, DELIVER, by showing efficacy and safety in a uniquely U.S. patient population. DELIVER is a pivotal, global trial of dapagliflozin in more than 6,000 patients with HFpEF that’s on track to report findings in 2022.
Dr. Kosiborod also stressed that dapagliflozin has U.S.-approved indications for treating patients with type 2 diabetes, and for patients with chronic kidney disease, and that a majority of patients enrolled in PRESERVED-HF had one or both of these conditions. That makes the new findings especially compelling for patients with either type 2 diabetes or chronic kidney disease and HFpEF who are not already receiving an SGLT2 inhibitor.
Other findings that he reported showed a range of benefits consistent with the primary endpoint, including the KCCQ overall summary score, which also showed a significant 4.5-point average increase over placebo after 12 weeks. Analysis by the percentage of patients achieving at least a 5-point improvement in the KCCQ clinical summary score (the threshold for a clinically meaningful improvement) showed that about 45% of patients treated with dapagliflozin reached this mark compared with roughly 35% of patients in the placebo arm, indicating a number needed to treat of nine to have one additional patient achieve this threshold after 12 weeks. Average improvement in 6-minute walk distance was about 20 m with dapagliflozin compared with placebo.
No heterogeneity of effect by baseline ejection fraction.
Subgroup analyses showed no heterogeneity of response across 12 different ways of subdividing the study population, including age, sex, race, diabetes status, and BMI. The median left ventricular ejection fraction among enrolled patients was 60%, and the findings showed identical KCCQ improvements among patients with ejection fractions less than the median and those with an ejection fraction above the median.
This last finding was especially relevant because the EMPEROR-Preserved results showed a possible signal of heterogeneity by ejection fraction and an attenuated effect among patients with HFpEF and an ejection fraction above the 60%-65% range, although the certainty of this finding is currently controversial.
The impact of empagliflozin on KCCQ clinical summary score in EMPEROR-Preserved showed an average incremental improvement of 1.32 points compared with placebo, a significant difference, but more modest than the increment from dapagliflozin treatment seen in PRESERVED-HF. Dr. Kosiborod hypothesized that this difference might be mostly because of the different patient populations enrolled in the two studies.
Dr. Kosiborod noted that a report on the PRESERVED-HF results will soon appear in Nature Medicine.
PRESERVED-HF was funded by AstraZeneca, which markets dapagliflozin (Farxiga), but the trials’ design and conduct were independent of this funding source. Dr. Kosiborod has been a consultant to AstraZeneca and numerous other companies, and he has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Packer has had financial relationships with AstraZeneca and numerous other companies.
The SGLT2 inhibitor dapagliflozin scored a clear win in a randomized, controlled trial with more than 300 U.S. patients with heart failure with preserved ejection fraction (HFpEF), showing a significant and clinically meaningful benefit for the primary endpoint, a KCCQ measure of symptoms and physical limitations, after 12 weeks of treatment.
These results in the PRESERVED-HF study follow closely on the heals of the initial report from the EMPEROR-Preserved trial that showed a benefit from a different sodium-glucose cotransporter 2 (SGLT2) inhibitor, empagliflozin (Jardiance) in nearly 6,000 randomized patients for the primary endpoint of preventing cardiovascular death or hospitalizations for heart failure.
In PRESERVED-HF, patients with HFpEF who received a standard, once-daily dose of dapagliflozin (Farxiga) had an average 5.8-point improvement in their condition as measured by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CS), the study’s primary endpoint.
This is “the first study to demonstrate that an SGLT2 inhibitor dapagliflozin significantly improves symptoms, physical limitations, and 6-minute walking distance in patients with HFpEF,” Mikhail N. Kosiborod, MD, reported at the annual scientific meeting of the Heart Failure Society of America. The secondary endpoint of 6-minute walking distance “has been very difficult to improve in many previous studies of other treatments” tested in patients with HFpEF, noted Dr. Kosiborod, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute.
The results are “highly complementary” to the findings from large outcome trials, such as the findings from EMPEROR-Preserved, he said, and collectively the recent findings from these studies of SGLT2 inhibitors in patients with HFpEF identify drugs in this class as a “new treatment option” for patients with a disorder that until now had no treatment with unequivocally proven efficacy and safety.
‘Impressive and unprecedented’ findings
The findings are “really impressive and unprecedented,” said Milton Packer, MD, a cardiologist at Baylor University Medical Center in Dallas who was not involved in the study. “This is the largest KCCQ benefit ever seen in either patients with HFpEF or in patients with heart failure with reduced ejection fraction,” said Dr. Packer, one of the investigators who led the EMPEROR-Preserved trial.
PRESERVED-HF randomized 324 patients diagnosed with heart failure and with a left ventricular ejection fraction of 45% or higher at any of 26 U.S. centers, with 304 patients completing the planned final analysis after 12 weeks on treatment. Patients could be in New York Heart Association (NYHA) functional class II-IV, they had to have a baseline N-terminal pro-brain natriuretic peptide (NT-proBNP) level of at least 225 pg/mL (or higher if they also had atrial fibrillation), and they required at least one of three markers of established heart failure: recent hospitalization for heart failure or an urgent outpatient visit that required treatment with an IV diuretic, elevated filling pressure measured by left or right catheterization, or structural heart disease detected by echocardiography.
The average age of the enrolled patients was 70 years, and they had been diagnosed with heart failure for about 3 years; 57% were women, 30% were African American, and their median body mass index was 35 kg/m2. Roughly 42% had NYHA class III or IV disease, 56% had type 2 diabetes, their median estimated glomerular filtration rate was about 55 mL/min per 1.73m2, their median KCCQ-CS score at baseline was about 62, and their average 6-minute walk distance was 244 m.
These and other features of the enrolled population define a distinctly U.S. patient population, stressed Dr. Kosiborod, professor of medicine at the University of Missouri–Kansas City.
“The patients we enrolled are the patients we see in U.S. clinical practice,” he said in an interview. Importantly, the patient profile of a median BMI of 35 kg/m2, a median KCCQ-CS score of 62 – “quite low,” noted Dr. Kosiborod – and having more than 40% of patients in NYHA functional class III defines a study population with a substantially greater burden of obesity, symptoms, and functional impairment compared with those enrolled in prior trials involving patients with HFpEF such as EMPEROR-Preserved.
Results complement findings from larger trials
PRESERVED-HF was an investigator-initiated study designed to inform clinical practice, not as a pivotal trial like EMPEROR-Preserved, which aims to gather evidence to support a new indication for regulatory approval. (On Sept. 9, 2021, the Food and Drug Administration granted empagliflozin “breakthrough therapy” status for treating HFpEF based on the EMPEROR-Preserved results, which will fast-track the agency’s decision on this indication.)
Dr. Kosiborod noted that he and his associates designed PRESERVED-HF with adequate patient numbers to power a statistically valid assessment of effect on KCCQ-CS score. While the new findings will not by themselves lead to a new indication for dapagliflozin to treat patients with HFpEF, they will potentially complement the pending results of another trial, DELIVER, by showing efficacy and safety in a uniquely U.S. patient population. DELIVER is a pivotal, global trial of dapagliflozin in more than 6,000 patients with HFpEF that’s on track to report findings in 2022.
Dr. Kosiborod also stressed that dapagliflozin has U.S.-approved indications for treating patients with type 2 diabetes, and for patients with chronic kidney disease, and that a majority of patients enrolled in PRESERVED-HF had one or both of these conditions. That makes the new findings especially compelling for patients with either type 2 diabetes or chronic kidney disease and HFpEF who are not already receiving an SGLT2 inhibitor.
Other findings that he reported showed a range of benefits consistent with the primary endpoint, including the KCCQ overall summary score, which also showed a significant 4.5-point average increase over placebo after 12 weeks. Analysis by the percentage of patients achieving at least a 5-point improvement in the KCCQ clinical summary score (the threshold for a clinically meaningful improvement) showed that about 45% of patients treated with dapagliflozin reached this mark compared with roughly 35% of patients in the placebo arm, indicating a number needed to treat of nine to have one additional patient achieve this threshold after 12 weeks. Average improvement in 6-minute walk distance was about 20 m with dapagliflozin compared with placebo.
No heterogeneity of effect by baseline ejection fraction.
Subgroup analyses showed no heterogeneity of response across 12 different ways of subdividing the study population, including age, sex, race, diabetes status, and BMI. The median left ventricular ejection fraction among enrolled patients was 60%, and the findings showed identical KCCQ improvements among patients with ejection fractions less than the median and those with an ejection fraction above the median.
This last finding was especially relevant because the EMPEROR-Preserved results showed a possible signal of heterogeneity by ejection fraction and an attenuated effect among patients with HFpEF and an ejection fraction above the 60%-65% range, although the certainty of this finding is currently controversial.
The impact of empagliflozin on KCCQ clinical summary score in EMPEROR-Preserved showed an average incremental improvement of 1.32 points compared with placebo, a significant difference, but more modest than the increment from dapagliflozin treatment seen in PRESERVED-HF. Dr. Kosiborod hypothesized that this difference might be mostly because of the different patient populations enrolled in the two studies.
Dr. Kosiborod noted that a report on the PRESERVED-HF results will soon appear in Nature Medicine.
PRESERVED-HF was funded by AstraZeneca, which markets dapagliflozin (Farxiga), but the trials’ design and conduct were independent of this funding source. Dr. Kosiborod has been a consultant to AstraZeneca and numerous other companies, and he has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Packer has had financial relationships with AstraZeneca and numerous other companies.
The SGLT2 inhibitor dapagliflozin scored a clear win in a randomized, controlled trial with more than 300 U.S. patients with heart failure with preserved ejection fraction (HFpEF), showing a significant and clinically meaningful benefit for the primary endpoint, a KCCQ measure of symptoms and physical limitations, after 12 weeks of treatment.
These results in the PRESERVED-HF study follow closely on the heals of the initial report from the EMPEROR-Preserved trial that showed a benefit from a different sodium-glucose cotransporter 2 (SGLT2) inhibitor, empagliflozin (Jardiance) in nearly 6,000 randomized patients for the primary endpoint of preventing cardiovascular death or hospitalizations for heart failure.
In PRESERVED-HF, patients with HFpEF who received a standard, once-daily dose of dapagliflozin (Farxiga) had an average 5.8-point improvement in their condition as measured by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CS), the study’s primary endpoint.
This is “the first study to demonstrate that an SGLT2 inhibitor dapagliflozin significantly improves symptoms, physical limitations, and 6-minute walking distance in patients with HFpEF,” Mikhail N. Kosiborod, MD, reported at the annual scientific meeting of the Heart Failure Society of America. The secondary endpoint of 6-minute walking distance “has been very difficult to improve in many previous studies of other treatments” tested in patients with HFpEF, noted Dr. Kosiborod, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute.
The results are “highly complementary” to the findings from large outcome trials, such as the findings from EMPEROR-Preserved, he said, and collectively the recent findings from these studies of SGLT2 inhibitors in patients with HFpEF identify drugs in this class as a “new treatment option” for patients with a disorder that until now had no treatment with unequivocally proven efficacy and safety.
‘Impressive and unprecedented’ findings
The findings are “really impressive and unprecedented,” said Milton Packer, MD, a cardiologist at Baylor University Medical Center in Dallas who was not involved in the study. “This is the largest KCCQ benefit ever seen in either patients with HFpEF or in patients with heart failure with reduced ejection fraction,” said Dr. Packer, one of the investigators who led the EMPEROR-Preserved trial.
PRESERVED-HF randomized 324 patients diagnosed with heart failure and with a left ventricular ejection fraction of 45% or higher at any of 26 U.S. centers, with 304 patients completing the planned final analysis after 12 weeks on treatment. Patients could be in New York Heart Association (NYHA) functional class II-IV, they had to have a baseline N-terminal pro-brain natriuretic peptide (NT-proBNP) level of at least 225 pg/mL (or higher if they also had atrial fibrillation), and they required at least one of three markers of established heart failure: recent hospitalization for heart failure or an urgent outpatient visit that required treatment with an IV diuretic, elevated filling pressure measured by left or right catheterization, or structural heart disease detected by echocardiography.
The average age of the enrolled patients was 70 years, and they had been diagnosed with heart failure for about 3 years; 57% were women, 30% were African American, and their median body mass index was 35 kg/m2. Roughly 42% had NYHA class III or IV disease, 56% had type 2 diabetes, their median estimated glomerular filtration rate was about 55 mL/min per 1.73m2, their median KCCQ-CS score at baseline was about 62, and their average 6-minute walk distance was 244 m.
These and other features of the enrolled population define a distinctly U.S. patient population, stressed Dr. Kosiborod, professor of medicine at the University of Missouri–Kansas City.
“The patients we enrolled are the patients we see in U.S. clinical practice,” he said in an interview. Importantly, the patient profile of a median BMI of 35 kg/m2, a median KCCQ-CS score of 62 – “quite low,” noted Dr. Kosiborod – and having more than 40% of patients in NYHA functional class III defines a study population with a substantially greater burden of obesity, symptoms, and functional impairment compared with those enrolled in prior trials involving patients with HFpEF such as EMPEROR-Preserved.
Results complement findings from larger trials
PRESERVED-HF was an investigator-initiated study designed to inform clinical practice, not as a pivotal trial like EMPEROR-Preserved, which aims to gather evidence to support a new indication for regulatory approval. (On Sept. 9, 2021, the Food and Drug Administration granted empagliflozin “breakthrough therapy” status for treating HFpEF based on the EMPEROR-Preserved results, which will fast-track the agency’s decision on this indication.)
Dr. Kosiborod noted that he and his associates designed PRESERVED-HF with adequate patient numbers to power a statistically valid assessment of effect on KCCQ-CS score. While the new findings will not by themselves lead to a new indication for dapagliflozin to treat patients with HFpEF, they will potentially complement the pending results of another trial, DELIVER, by showing efficacy and safety in a uniquely U.S. patient population. DELIVER is a pivotal, global trial of dapagliflozin in more than 6,000 patients with HFpEF that’s on track to report findings in 2022.
Dr. Kosiborod also stressed that dapagliflozin has U.S.-approved indications for treating patients with type 2 diabetes, and for patients with chronic kidney disease, and that a majority of patients enrolled in PRESERVED-HF had one or both of these conditions. That makes the new findings especially compelling for patients with either type 2 diabetes or chronic kidney disease and HFpEF who are not already receiving an SGLT2 inhibitor.
Other findings that he reported showed a range of benefits consistent with the primary endpoint, including the KCCQ overall summary score, which also showed a significant 4.5-point average increase over placebo after 12 weeks. Analysis by the percentage of patients achieving at least a 5-point improvement in the KCCQ clinical summary score (the threshold for a clinically meaningful improvement) showed that about 45% of patients treated with dapagliflozin reached this mark compared with roughly 35% of patients in the placebo arm, indicating a number needed to treat of nine to have one additional patient achieve this threshold after 12 weeks. Average improvement in 6-minute walk distance was about 20 m with dapagliflozin compared with placebo.
No heterogeneity of effect by baseline ejection fraction.
Subgroup analyses showed no heterogeneity of response across 12 different ways of subdividing the study population, including age, sex, race, diabetes status, and BMI. The median left ventricular ejection fraction among enrolled patients was 60%, and the findings showed identical KCCQ improvements among patients with ejection fractions less than the median and those with an ejection fraction above the median.
This last finding was especially relevant because the EMPEROR-Preserved results showed a possible signal of heterogeneity by ejection fraction and an attenuated effect among patients with HFpEF and an ejection fraction above the 60%-65% range, although the certainty of this finding is currently controversial.
The impact of empagliflozin on KCCQ clinical summary score in EMPEROR-Preserved showed an average incremental improvement of 1.32 points compared with placebo, a significant difference, but more modest than the increment from dapagliflozin treatment seen in PRESERVED-HF. Dr. Kosiborod hypothesized that this difference might be mostly because of the different patient populations enrolled in the two studies.
Dr. Kosiborod noted that a report on the PRESERVED-HF results will soon appear in Nature Medicine.
PRESERVED-HF was funded by AstraZeneca, which markets dapagliflozin (Farxiga), but the trials’ design and conduct were independent of this funding source. Dr. Kosiborod has been a consultant to AstraZeneca and numerous other companies, and he has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Packer has had financial relationships with AstraZeneca and numerous other companies.
FROM HFSA 2021
Cavernous gender gap in Medicare payments to cardiologists
Women cardiologists receive dramatically smaller payments from the U.S. Centers for Medicare & Medicaid Services (CMS) than their male counterparts, new research suggests.
An analysis of 2016 claims data revealed male cardiologists received on average 45% more reimbursement than women in the inpatient setting, with the median payment 39% higher ($62,897 vs. $45,288).
In the outpatient setting, men received on average 62% more annual CMS payments, with the median payment 75% higher ($91,053 vs. $51,975; P < .001 for both).
The difference remained significant after the exclusion of the top and bottom 2.5% of earning physicians and cardiology subspecialties, like electrophysiology and interventional cardiology, with high procedural volumes and greater gender imbalances.
“This is one study among others which demonstrates a wage gap between men and women in medicine in cardiology,” lead author Inbar Raber, MD, Beth Israel Deaconess Medical Center, Boston, said in an interview. “I hope by increasing awareness [and] understanding of possible etiologies, it will enable some sustainable solutions, and those include access to additional support staff and equitable models surrounding parental leave and childcare support.”
The study, published online September 8 in JAMA Cardiology, comes on the heels of a recent cross-sectional analysis that put cardiology at the bottom of 13 internal medicine subspecialties with just 21% female faculty representation and one of only three specialties in which women’s median salaries did not reach 90% of men’s.
The new findings build on a 2017 report that showed Medicare payments to women physicians in 2013 were 55% of those to male physicians across all specialties.
“It can be disheartening, especially as an early career woman cardiologist, seeing these differences, but I think the responsibility on all of us is to take these observations and really try to understand more deeply why they exist,” Nosheen Reza, MD, from the University of Pennsylvania, Philadelphia, and coauthor of the cross-sectional analysis, told this news organization.
Several factors could be contributing to the disparity, but “it’s not gender discrimination from Medicare,” Dr. Raber said. “The gap in reimbursement is really driven by the types and the volume of charges submitted.”
Indeed, a direct comparison of the three most common inpatient and outpatient billing codes showed no difference in payments between the sexes.
Men, however, submitted 24% more median inpatient charges to CMS than women (1,190 vs. 959), and 94% more outpatient charges (1,685 vs. 870).
Men also submitted slightly more unique billing codes (median inpatient, 10 vs. 9; median outpatient, 11 vs. 8).
Notably, women made up just 13% of the 17,524 cardiologists who received CMS payments in the inpatient setting in 2016 and 13% of the 16,929 cardiologists who did so in the outpatient setting.
Louisiana had the dubious distinction of having the largest gender gap in mean CMS payments, with male cardiologists earning $145,323 (235%) more than women, whereas women cardiologists in Vermont out-earned men by $31,483 (38%).
Overall, male cardiologists had more years in practice than women cardiologists and cared for slightly older Medicare beneficiaries.
Differences in CMS payments persisted, however, after adjustment for years since graduation, physician subspecialty, number of charges, number of unique billing codes, and patient complexity. The resulting β coefficient was -0.06, which translates into women receiving an average of 94% of the CMS payments received by men.
“The first takeaway, if you were really crass and focused on the bottom line, might be: ‘Hey, let me get a few more male cardiologists because they’re going to bring more into the organization.’ But we shouldn’t do that because, unless you link these data with quality outcomes, they’re an interesting observation and hypothesis-generating,” said Sharonne Hayes, MD, coauthor of the 2017 report and professor of cardiovascular medicine at Mayo Clinic in Rochester, Minn., where she has served as director of diversity and inclusion for a decade.
She noted that there are multiple examples that the style of medicine women practice, on average, may be more effective, may be more outcomes based, and may save lives, as suggested by a recent analysis of hospitalized Medicare beneficiaries.
“The gap was not much different, like within 1% or so, but when you take that over the literally millions of Medicare patients cared for each year by hospitalists, that’s a substantial number of people,” Dr. Hayes said. “So, I think we need to take a step back, and we have to include these observations on studies like this and better understand the compensation gaps.”
She pointed out that the present study lacks data on full-time-equivalent status but that female physicians are more likely to work part-time, thus reducing the volume of claims.
Women might also care for different patient populations. “I practice in a women’s heart clinic and take care of [spontaneous coronary artery dissection] SCAD patients where the average age of SCAD is 42. So, the vast majority of patients I see on a day-to-day basis aren’t going to be Medicare age,” observed Dr. Hayes.
The differences in charges might also reflect the increased obligations in nonreimbursed work that women can have, Dr. Raber said. These can be things like mentoring, teaching roles, and serving on committees, which is a hypothesis supported by a 2021 study that showed women physicians spend more time on these “citizenship tasks” than men.
Finally, there could be organizational barriers that affect women’s clinical volumes, including less access to support from health care personnel. Added support is especially important, though, amid a 100-year pandemic, the women agreed.
“Within the first year of the pandemic, we saw women leaving the workforce in droves across all sectors, including medicine, including academic medicine. And, as the pandemic goes on without any signs of abatement, those threats continue to exist and continue to be amplified,” Dr. Reza said.
The groundswell of support surrounding the importance of diversity, equity, and inclusion initiatives across the board has helped bring attention to the issue, she said. Some institutions, including the National Institutes of Health, are making efforts to extend relief to women with young families, caregivers, or those in academic medicine who, for example, need extensions on grants or bridge funding.
“There’s certainly a lot left to do, but I do think within the last year, there’s been an acceleration of literature that has come out, not only pointing out the disparities, but pointing out that perhaps women physicians do have better outcomes and are better liked by their patients and that losing women in the workforce would be a huge detriment to the field overall,” Dr. Reza said.
Dr. Raber, Dr. Reza, and Dr. Hayes reports no relevant financial relationships. Coauthor conflict of interest disclosures are listed in the paper.
A version of this article first appeared on Medscape.com.
Women cardiologists receive dramatically smaller payments from the U.S. Centers for Medicare & Medicaid Services (CMS) than their male counterparts, new research suggests.
An analysis of 2016 claims data revealed male cardiologists received on average 45% more reimbursement than women in the inpatient setting, with the median payment 39% higher ($62,897 vs. $45,288).
In the outpatient setting, men received on average 62% more annual CMS payments, with the median payment 75% higher ($91,053 vs. $51,975; P < .001 for both).
The difference remained significant after the exclusion of the top and bottom 2.5% of earning physicians and cardiology subspecialties, like electrophysiology and interventional cardiology, with high procedural volumes and greater gender imbalances.
“This is one study among others which demonstrates a wage gap between men and women in medicine in cardiology,” lead author Inbar Raber, MD, Beth Israel Deaconess Medical Center, Boston, said in an interview. “I hope by increasing awareness [and] understanding of possible etiologies, it will enable some sustainable solutions, and those include access to additional support staff and equitable models surrounding parental leave and childcare support.”
The study, published online September 8 in JAMA Cardiology, comes on the heels of a recent cross-sectional analysis that put cardiology at the bottom of 13 internal medicine subspecialties with just 21% female faculty representation and one of only three specialties in which women’s median salaries did not reach 90% of men’s.
The new findings build on a 2017 report that showed Medicare payments to women physicians in 2013 were 55% of those to male physicians across all specialties.
“It can be disheartening, especially as an early career woman cardiologist, seeing these differences, but I think the responsibility on all of us is to take these observations and really try to understand more deeply why they exist,” Nosheen Reza, MD, from the University of Pennsylvania, Philadelphia, and coauthor of the cross-sectional analysis, told this news organization.
Several factors could be contributing to the disparity, but “it’s not gender discrimination from Medicare,” Dr. Raber said. “The gap in reimbursement is really driven by the types and the volume of charges submitted.”
Indeed, a direct comparison of the three most common inpatient and outpatient billing codes showed no difference in payments between the sexes.
Men, however, submitted 24% more median inpatient charges to CMS than women (1,190 vs. 959), and 94% more outpatient charges (1,685 vs. 870).
Men also submitted slightly more unique billing codes (median inpatient, 10 vs. 9; median outpatient, 11 vs. 8).
Notably, women made up just 13% of the 17,524 cardiologists who received CMS payments in the inpatient setting in 2016 and 13% of the 16,929 cardiologists who did so in the outpatient setting.
Louisiana had the dubious distinction of having the largest gender gap in mean CMS payments, with male cardiologists earning $145,323 (235%) more than women, whereas women cardiologists in Vermont out-earned men by $31,483 (38%).
Overall, male cardiologists had more years in practice than women cardiologists and cared for slightly older Medicare beneficiaries.
Differences in CMS payments persisted, however, after adjustment for years since graduation, physician subspecialty, number of charges, number of unique billing codes, and patient complexity. The resulting β coefficient was -0.06, which translates into women receiving an average of 94% of the CMS payments received by men.
“The first takeaway, if you were really crass and focused on the bottom line, might be: ‘Hey, let me get a few more male cardiologists because they’re going to bring more into the organization.’ But we shouldn’t do that because, unless you link these data with quality outcomes, they’re an interesting observation and hypothesis-generating,” said Sharonne Hayes, MD, coauthor of the 2017 report and professor of cardiovascular medicine at Mayo Clinic in Rochester, Minn., where she has served as director of diversity and inclusion for a decade.
She noted that there are multiple examples that the style of medicine women practice, on average, may be more effective, may be more outcomes based, and may save lives, as suggested by a recent analysis of hospitalized Medicare beneficiaries.
“The gap was not much different, like within 1% or so, but when you take that over the literally millions of Medicare patients cared for each year by hospitalists, that’s a substantial number of people,” Dr. Hayes said. “So, I think we need to take a step back, and we have to include these observations on studies like this and better understand the compensation gaps.”
She pointed out that the present study lacks data on full-time-equivalent status but that female physicians are more likely to work part-time, thus reducing the volume of claims.
Women might also care for different patient populations. “I practice in a women’s heart clinic and take care of [spontaneous coronary artery dissection] SCAD patients where the average age of SCAD is 42. So, the vast majority of patients I see on a day-to-day basis aren’t going to be Medicare age,” observed Dr. Hayes.
The differences in charges might also reflect the increased obligations in nonreimbursed work that women can have, Dr. Raber said. These can be things like mentoring, teaching roles, and serving on committees, which is a hypothesis supported by a 2021 study that showed women physicians spend more time on these “citizenship tasks” than men.
Finally, there could be organizational barriers that affect women’s clinical volumes, including less access to support from health care personnel. Added support is especially important, though, amid a 100-year pandemic, the women agreed.
“Within the first year of the pandemic, we saw women leaving the workforce in droves across all sectors, including medicine, including academic medicine. And, as the pandemic goes on without any signs of abatement, those threats continue to exist and continue to be amplified,” Dr. Reza said.
The groundswell of support surrounding the importance of diversity, equity, and inclusion initiatives across the board has helped bring attention to the issue, she said. Some institutions, including the National Institutes of Health, are making efforts to extend relief to women with young families, caregivers, or those in academic medicine who, for example, need extensions on grants or bridge funding.
“There’s certainly a lot left to do, but I do think within the last year, there’s been an acceleration of literature that has come out, not only pointing out the disparities, but pointing out that perhaps women physicians do have better outcomes and are better liked by their patients and that losing women in the workforce would be a huge detriment to the field overall,” Dr. Reza said.
Dr. Raber, Dr. Reza, and Dr. Hayes reports no relevant financial relationships. Coauthor conflict of interest disclosures are listed in the paper.
A version of this article first appeared on Medscape.com.
Women cardiologists receive dramatically smaller payments from the U.S. Centers for Medicare & Medicaid Services (CMS) than their male counterparts, new research suggests.
An analysis of 2016 claims data revealed male cardiologists received on average 45% more reimbursement than women in the inpatient setting, with the median payment 39% higher ($62,897 vs. $45,288).
In the outpatient setting, men received on average 62% more annual CMS payments, with the median payment 75% higher ($91,053 vs. $51,975; P < .001 for both).
The difference remained significant after the exclusion of the top and bottom 2.5% of earning physicians and cardiology subspecialties, like electrophysiology and interventional cardiology, with high procedural volumes and greater gender imbalances.
“This is one study among others which demonstrates a wage gap between men and women in medicine in cardiology,” lead author Inbar Raber, MD, Beth Israel Deaconess Medical Center, Boston, said in an interview. “I hope by increasing awareness [and] understanding of possible etiologies, it will enable some sustainable solutions, and those include access to additional support staff and equitable models surrounding parental leave and childcare support.”
The study, published online September 8 in JAMA Cardiology, comes on the heels of a recent cross-sectional analysis that put cardiology at the bottom of 13 internal medicine subspecialties with just 21% female faculty representation and one of only three specialties in which women’s median salaries did not reach 90% of men’s.
The new findings build on a 2017 report that showed Medicare payments to women physicians in 2013 were 55% of those to male physicians across all specialties.
“It can be disheartening, especially as an early career woman cardiologist, seeing these differences, but I think the responsibility on all of us is to take these observations and really try to understand more deeply why they exist,” Nosheen Reza, MD, from the University of Pennsylvania, Philadelphia, and coauthor of the cross-sectional analysis, told this news organization.
Several factors could be contributing to the disparity, but “it’s not gender discrimination from Medicare,” Dr. Raber said. “The gap in reimbursement is really driven by the types and the volume of charges submitted.”
Indeed, a direct comparison of the three most common inpatient and outpatient billing codes showed no difference in payments between the sexes.
Men, however, submitted 24% more median inpatient charges to CMS than women (1,190 vs. 959), and 94% more outpatient charges (1,685 vs. 870).
Men also submitted slightly more unique billing codes (median inpatient, 10 vs. 9; median outpatient, 11 vs. 8).
Notably, women made up just 13% of the 17,524 cardiologists who received CMS payments in the inpatient setting in 2016 and 13% of the 16,929 cardiologists who did so in the outpatient setting.
Louisiana had the dubious distinction of having the largest gender gap in mean CMS payments, with male cardiologists earning $145,323 (235%) more than women, whereas women cardiologists in Vermont out-earned men by $31,483 (38%).
Overall, male cardiologists had more years in practice than women cardiologists and cared for slightly older Medicare beneficiaries.
Differences in CMS payments persisted, however, after adjustment for years since graduation, physician subspecialty, number of charges, number of unique billing codes, and patient complexity. The resulting β coefficient was -0.06, which translates into women receiving an average of 94% of the CMS payments received by men.
“The first takeaway, if you were really crass and focused on the bottom line, might be: ‘Hey, let me get a few more male cardiologists because they’re going to bring more into the organization.’ But we shouldn’t do that because, unless you link these data with quality outcomes, they’re an interesting observation and hypothesis-generating,” said Sharonne Hayes, MD, coauthor of the 2017 report and professor of cardiovascular medicine at Mayo Clinic in Rochester, Minn., where she has served as director of diversity and inclusion for a decade.
She noted that there are multiple examples that the style of medicine women practice, on average, may be more effective, may be more outcomes based, and may save lives, as suggested by a recent analysis of hospitalized Medicare beneficiaries.
“The gap was not much different, like within 1% or so, but when you take that over the literally millions of Medicare patients cared for each year by hospitalists, that’s a substantial number of people,” Dr. Hayes said. “So, I think we need to take a step back, and we have to include these observations on studies like this and better understand the compensation gaps.”
She pointed out that the present study lacks data on full-time-equivalent status but that female physicians are more likely to work part-time, thus reducing the volume of claims.
Women might also care for different patient populations. “I practice in a women’s heart clinic and take care of [spontaneous coronary artery dissection] SCAD patients where the average age of SCAD is 42. So, the vast majority of patients I see on a day-to-day basis aren’t going to be Medicare age,” observed Dr. Hayes.
The differences in charges might also reflect the increased obligations in nonreimbursed work that women can have, Dr. Raber said. These can be things like mentoring, teaching roles, and serving on committees, which is a hypothesis supported by a 2021 study that showed women physicians spend more time on these “citizenship tasks” than men.
Finally, there could be organizational barriers that affect women’s clinical volumes, including less access to support from health care personnel. Added support is especially important, though, amid a 100-year pandemic, the women agreed.
“Within the first year of the pandemic, we saw women leaving the workforce in droves across all sectors, including medicine, including academic medicine. And, as the pandemic goes on without any signs of abatement, those threats continue to exist and continue to be amplified,” Dr. Reza said.
The groundswell of support surrounding the importance of diversity, equity, and inclusion initiatives across the board has helped bring attention to the issue, she said. Some institutions, including the National Institutes of Health, are making efforts to extend relief to women with young families, caregivers, or those in academic medicine who, for example, need extensions on grants or bridge funding.
“There’s certainly a lot left to do, but I do think within the last year, there’s been an acceleration of literature that has come out, not only pointing out the disparities, but pointing out that perhaps women physicians do have better outcomes and are better liked by their patients and that losing women in the workforce would be a huge detriment to the field overall,” Dr. Reza said.
Dr. Raber, Dr. Reza, and Dr. Hayes reports no relevant financial relationships. Coauthor conflict of interest disclosures are listed in the paper.
A version of this article first appeared on Medscape.com.
Are ESC’s new heart failure guidelines already outdated?
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
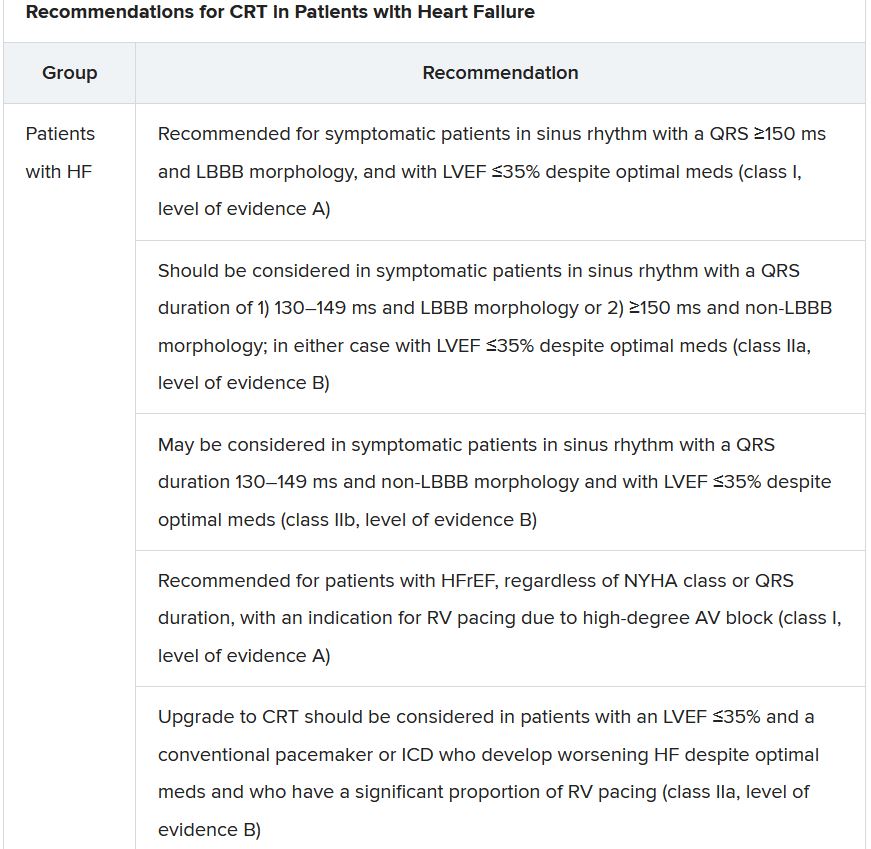
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
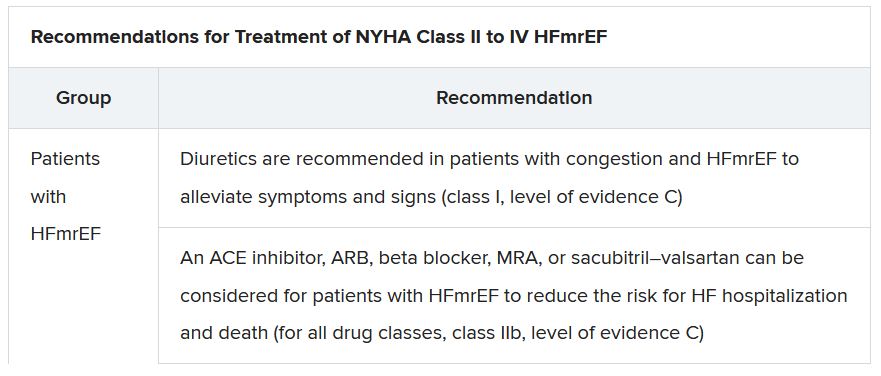
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
Study gives bleeding risk estimates for VTE patients on anticoagulants
The meta-analysis of data from 27 studies with 17,202 patients was published in the Annals of Internal Medicine. According to two of the paper’s coauthors, Faizan Khan, MSc, and Marc A. Rodger, MD, it “provides best available estimates of long-term bleeding risk with different anticoagulants in patients with unprovoked VTE,” including subgroups at increased risk.
Patients at increased risk for major bleeding include those who are older; those using antiplatelet therapy; and patients with kidney disease, a history of bleeding, or anemia, noted the coauthors, who work for the Ottawa Hospital Research Institute.
The researchers focused on randomized controlled trials (RCTs) and prospective cohort studies that reported major bleeding among patients with a first unprovoked or weakly provoked VTE who received oral anticoagulation for at least 6 months beyond an initial anticoagulant treatment course of at least 3 months.
The investigators analyzed data from 14 RCTs and 13 cohort studies. In all, 9,982 patients received a vitamin K antagonist (VKA), and 7,220 received a direct oral anticoagulant (DOAC).
The incidence of major bleeding per 100 person-years was 1.7 events with VKAs, compared with 1.1 events with DOACs. The researchers estimated that the 5-year cumulative incidence of major bleeding with VKAs was 6.3%. The available data for DOACs were insufficient to estimate the incidence of major bleeding beyond 1 year.
“This information can help clinicians counsel patients and inform shared decision-making about extended therapy,” the researchers said.
Risks of serious bleeding ‘not trivial’
Margaret Fang, MD, with the University of California, San Francisco, agreed that the study can help clinicians and patients weigh the risks of extended anticoagulation for common types of VTE.
The study also “highlights that the risks of serious bleeding are not trivial” and points out gaps in the literature regarding the long-term use of DOACs for extended VTE therapy, Dr. Fang said.
Better ways to predict which patients will develop bleeding on anticoagulants are needed, Dr. Fang added. “It will also be important to establish which of the various therapies for preventing recurrent VTE – full dose versus lowered dose, or even aspirin – has the best balance of safety and efficacy,” she said.
‘Standardized approach’ for identifying high-risk patients lacking
Clinical practice guidelines recommend indefinite anticoagulation for an unprovoked VTE, except when patients are at high risk of bleeding, the authors noted. But clinicians lack a “standardized approach to identify patients at high risk of bleeding,” Mr. Khan and Dr. Rodger said. “Evidence from randomized trials on net long-term benefit of extended therapy is limited, and current guideline recommendations are largely based on expert consensus opinion. Major bleeding events are two to three times more likely to be fatal than recurrent VTE events, so extended therapy is not always associated with a net mortality benefit, particularly in patients at low risk of recurrent VTE or high risk of bleeding.”
The analysis indicates that there is “a clinically meaningful difference in long-term risk for anticoagulant-related major bleeding among patients with a first unprovoked VTE stratified according to presence or absence of the following risk factors: age older than 65 years, creatinine clearance less than 50 mL/min, history of bleeding, concomitant use of antiplatelet therapy, and hemoglobin level less than 100 g/L,” the authors said.
For example, the researchers found that the incidence of major bleeding was higher among those older than 65 years, compared with younger patients (incidence rate ratio, 1.84 with VKAs and 2.92 with DOACs), and among those with creatinine clearance less than 50 mL/min (IRR, 2.83 with VKAs and 3.71 with DOACs).
The case-fatality rate of major bleeding was 8.3% with VKAs and 9.7% with DOACs.
The study received funding from the Canadian Institutes of Health Research. Some of the coauthors are employees of or have financial ties to pharmaceutical companies. Mr. Khan, Dr. Rodger, and Dr. Fang had no relevant disclosures.
The meta-analysis of data from 27 studies with 17,202 patients was published in the Annals of Internal Medicine. According to two of the paper’s coauthors, Faizan Khan, MSc, and Marc A. Rodger, MD, it “provides best available estimates of long-term bleeding risk with different anticoagulants in patients with unprovoked VTE,” including subgroups at increased risk.
Patients at increased risk for major bleeding include those who are older; those using antiplatelet therapy; and patients with kidney disease, a history of bleeding, or anemia, noted the coauthors, who work for the Ottawa Hospital Research Institute.
The researchers focused on randomized controlled trials (RCTs) and prospective cohort studies that reported major bleeding among patients with a first unprovoked or weakly provoked VTE who received oral anticoagulation for at least 6 months beyond an initial anticoagulant treatment course of at least 3 months.
The investigators analyzed data from 14 RCTs and 13 cohort studies. In all, 9,982 patients received a vitamin K antagonist (VKA), and 7,220 received a direct oral anticoagulant (DOAC).
The incidence of major bleeding per 100 person-years was 1.7 events with VKAs, compared with 1.1 events with DOACs. The researchers estimated that the 5-year cumulative incidence of major bleeding with VKAs was 6.3%. The available data for DOACs were insufficient to estimate the incidence of major bleeding beyond 1 year.
“This information can help clinicians counsel patients and inform shared decision-making about extended therapy,” the researchers said.
Risks of serious bleeding ‘not trivial’
Margaret Fang, MD, with the University of California, San Francisco, agreed that the study can help clinicians and patients weigh the risks of extended anticoagulation for common types of VTE.
The study also “highlights that the risks of serious bleeding are not trivial” and points out gaps in the literature regarding the long-term use of DOACs for extended VTE therapy, Dr. Fang said.
Better ways to predict which patients will develop bleeding on anticoagulants are needed, Dr. Fang added. “It will also be important to establish which of the various therapies for preventing recurrent VTE – full dose versus lowered dose, or even aspirin – has the best balance of safety and efficacy,” she said.
‘Standardized approach’ for identifying high-risk patients lacking
Clinical practice guidelines recommend indefinite anticoagulation for an unprovoked VTE, except when patients are at high risk of bleeding, the authors noted. But clinicians lack a “standardized approach to identify patients at high risk of bleeding,” Mr. Khan and Dr. Rodger said. “Evidence from randomized trials on net long-term benefit of extended therapy is limited, and current guideline recommendations are largely based on expert consensus opinion. Major bleeding events are two to three times more likely to be fatal than recurrent VTE events, so extended therapy is not always associated with a net mortality benefit, particularly in patients at low risk of recurrent VTE or high risk of bleeding.”
The analysis indicates that there is “a clinically meaningful difference in long-term risk for anticoagulant-related major bleeding among patients with a first unprovoked VTE stratified according to presence or absence of the following risk factors: age older than 65 years, creatinine clearance less than 50 mL/min, history of bleeding, concomitant use of antiplatelet therapy, and hemoglobin level less than 100 g/L,” the authors said.
For example, the researchers found that the incidence of major bleeding was higher among those older than 65 years, compared with younger patients (incidence rate ratio, 1.84 with VKAs and 2.92 with DOACs), and among those with creatinine clearance less than 50 mL/min (IRR, 2.83 with VKAs and 3.71 with DOACs).
The case-fatality rate of major bleeding was 8.3% with VKAs and 9.7% with DOACs.
The study received funding from the Canadian Institutes of Health Research. Some of the coauthors are employees of or have financial ties to pharmaceutical companies. Mr. Khan, Dr. Rodger, and Dr. Fang had no relevant disclosures.
The meta-analysis of data from 27 studies with 17,202 patients was published in the Annals of Internal Medicine. According to two of the paper’s coauthors, Faizan Khan, MSc, and Marc A. Rodger, MD, it “provides best available estimates of long-term bleeding risk with different anticoagulants in patients with unprovoked VTE,” including subgroups at increased risk.
Patients at increased risk for major bleeding include those who are older; those using antiplatelet therapy; and patients with kidney disease, a history of bleeding, or anemia, noted the coauthors, who work for the Ottawa Hospital Research Institute.
The researchers focused on randomized controlled trials (RCTs) and prospective cohort studies that reported major bleeding among patients with a first unprovoked or weakly provoked VTE who received oral anticoagulation for at least 6 months beyond an initial anticoagulant treatment course of at least 3 months.
The investigators analyzed data from 14 RCTs and 13 cohort studies. In all, 9,982 patients received a vitamin K antagonist (VKA), and 7,220 received a direct oral anticoagulant (DOAC).
The incidence of major bleeding per 100 person-years was 1.7 events with VKAs, compared with 1.1 events with DOACs. The researchers estimated that the 5-year cumulative incidence of major bleeding with VKAs was 6.3%. The available data for DOACs were insufficient to estimate the incidence of major bleeding beyond 1 year.
“This information can help clinicians counsel patients and inform shared decision-making about extended therapy,” the researchers said.
Risks of serious bleeding ‘not trivial’
Margaret Fang, MD, with the University of California, San Francisco, agreed that the study can help clinicians and patients weigh the risks of extended anticoagulation for common types of VTE.
The study also “highlights that the risks of serious bleeding are not trivial” and points out gaps in the literature regarding the long-term use of DOACs for extended VTE therapy, Dr. Fang said.
Better ways to predict which patients will develop bleeding on anticoagulants are needed, Dr. Fang added. “It will also be important to establish which of the various therapies for preventing recurrent VTE – full dose versus lowered dose, or even aspirin – has the best balance of safety and efficacy,” she said.
‘Standardized approach’ for identifying high-risk patients lacking
Clinical practice guidelines recommend indefinite anticoagulation for an unprovoked VTE, except when patients are at high risk of bleeding, the authors noted. But clinicians lack a “standardized approach to identify patients at high risk of bleeding,” Mr. Khan and Dr. Rodger said. “Evidence from randomized trials on net long-term benefit of extended therapy is limited, and current guideline recommendations are largely based on expert consensus opinion. Major bleeding events are two to three times more likely to be fatal than recurrent VTE events, so extended therapy is not always associated with a net mortality benefit, particularly in patients at low risk of recurrent VTE or high risk of bleeding.”
The analysis indicates that there is “a clinically meaningful difference in long-term risk for anticoagulant-related major bleeding among patients with a first unprovoked VTE stratified according to presence or absence of the following risk factors: age older than 65 years, creatinine clearance less than 50 mL/min, history of bleeding, concomitant use of antiplatelet therapy, and hemoglobin level less than 100 g/L,” the authors said.
For example, the researchers found that the incidence of major bleeding was higher among those older than 65 years, compared with younger patients (incidence rate ratio, 1.84 with VKAs and 2.92 with DOACs), and among those with creatinine clearance less than 50 mL/min (IRR, 2.83 with VKAs and 3.71 with DOACs).
The case-fatality rate of major bleeding was 8.3% with VKAs and 9.7% with DOACs.
The study received funding from the Canadian Institutes of Health Research. Some of the coauthors are employees of or have financial ties to pharmaceutical companies. Mr. Khan, Dr. Rodger, and Dr. Fang had no relevant disclosures.
FROM ANNALS OF INTERNAL MEDICINE
Feds slap UPMC, lead cardiothoracic surgeon with fraud lawsuit
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
Case: Patient with statin-associated muscle symptoms
A 66-year-old woman is discharged from the hospital after an MI. Her discharge medications include atorvastatin 40 mg, lisinopril 20 mg, acetylsalicylic acid 81 mg, and clopidogrel 75 mg. At this patient’s follow-up appointment, she mentions that she has muscle pain and stiffness in both legs and her back. Her labs include thyroid-stimulating hormone of 2.0 and vitamin D of 40. She stops the atorvastatin for 2 weeks with resolution of her symptoms.
Which treatment recommendation would you make for this patient?
A. Restart atorvastatin
B. Start rosuvastatin twice a week
C. Start ezetimibe
D. Start a PCSK9 inhibitor
We often see high-risk cardiovascular disease patients who are concerned about muscle side effects brought on by statins. I think we all can agree that this patient needs aggressive medical therapy for prevention of secondary cardiovascular events. I would restart her atorvastatin.
Neilsen and Nordestgaard found that early statin discontinuation rates increased from 6% in 1995 to 18% in 2010.1
Early statin discontinuation correlated with negative statin-related news stories, their paper states. This suggests either an increased awareness of side effects or a possible nocebo effect.
Statin rechallenge results
Joy and colleagues reported the results on eight patients who had developed myalgias within 3 weeks of starting a statin. These patients, who received placebo or statin, completed an N-of-1 trial with three double-blind, crossover comparisons separated by 3-week washout periods.
Patients were evaluated pain on a visual analog scale (VAS). For each N-of-1 trial there was no statistically significant difference in pain or myalgia score between those who took statin and placebo. Five of the eight patients chose to continue on statins at the end of the trial.
Herrett and colleagues performed a more extensive series of N-of-1 trials involving 200 patients who had stopped or were considering stopping statins because of muscle symptoms.3 Participants either received 2 months of atorvastatin 20 mg or placebo for 2-month blocks six times. They rated their muscle symptoms on a VAS at the end of each block. There was no difference in muscle symptom scores between the statin and placebo periods.
Wood and colleagues took it a step further, when they studied an N-of-1 trial that included statin, placebo, and no treatment.4 Each participant received four bottles of atorvastatin 20 mg, four bottles of placebo, and four empty bottles. Each month they used treatment from the bottles based on random sequence and reported daily symptom scores. The mean symptom intensity was 8.0 during no-tablet months, 15.4 during placebo months (P < .001, compared with no-tablet months), and 16.3 during statin months (P < .001, compared with no-tablet months; P = .39, compared with placebo).
Taylor and colleagues studied 120 patients who had prior statin-associated muscle complaints.5 Each patient received either simvastatin 20 mg or placebo for 4 weeks, and then were switched for an additional 4 weeks. A total of 43 patients (36%) had pain on simvastatin but not placebo, 21 (17%) had no pain with either treatment, 21 (17%) reported pain with both treatments, and 35 (29%) had pain with placebo but not simvastatin. These studies support the concept of nocebo effect in patients who have muscle symptoms on statins.
So what should be done? Brennan and Roy did a retrospective study of 118 patients referred to a lipid clinic as being statin intolerant to two or more statins.6 Most of the patients were able to tolerate a statin: 71% tolerated same statin rechallenge, 53% tolerated statin switch, and 57% tolerated a nonstatin therapy.
In the Prosisa study, only 27% of patients who reported statin-associated muscle symptoms had reappearance of muscle symptoms after rechallenge with a statin.7
Research implications
Rechallenge with the same statin seems to be a reasonable first step, followed by switching to a different statin. I also share the concept of nocebo effect with my patients, and tell them I believe they have an excellent chance of tolerating the statin.
Pearl: The majority of patients with muscle symptoms while taking a statin likely have a nocebo effect, and are likely to tolerate rechallenge with the same statin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Nielsen SF and Nordestgaard BG. Eur Heart J. 2016;37:908-16.
2. Joy TR et al. Ann Intern Med. 2014;160:301-10.
3. Herrett E et al. BMJ. 2021 Feb 24;372:n135.
4. Wood FA et al. N Engl J Med 2020;383:2182-4.
5. Taylor BA et al. Atherosclerosis. 2017;256:100-4.
6. Brennen ET and Roy TR. Can J Card. 2017;33(5):666-73.
7. Bonaiti Fet al. Atherosclerosis. 2020;315:E13-4.
A 66-year-old woman is discharged from the hospital after an MI. Her discharge medications include atorvastatin 40 mg, lisinopril 20 mg, acetylsalicylic acid 81 mg, and clopidogrel 75 mg. At this patient’s follow-up appointment, she mentions that she has muscle pain and stiffness in both legs and her back. Her labs include thyroid-stimulating hormone of 2.0 and vitamin D of 40. She stops the atorvastatin for 2 weeks with resolution of her symptoms.
Which treatment recommendation would you make for this patient?
A. Restart atorvastatin
B. Start rosuvastatin twice a week
C. Start ezetimibe
D. Start a PCSK9 inhibitor
We often see high-risk cardiovascular disease patients who are concerned about muscle side effects brought on by statins. I think we all can agree that this patient needs aggressive medical therapy for prevention of secondary cardiovascular events. I would restart her atorvastatin.
Neilsen and Nordestgaard found that early statin discontinuation rates increased from 6% in 1995 to 18% in 2010.1
Early statin discontinuation correlated with negative statin-related news stories, their paper states. This suggests either an increased awareness of side effects or a possible nocebo effect.
Statin rechallenge results
Joy and colleagues reported the results on eight patients who had developed myalgias within 3 weeks of starting a statin. These patients, who received placebo or statin, completed an N-of-1 trial with three double-blind, crossover comparisons separated by 3-week washout periods.
Patients were evaluated pain on a visual analog scale (VAS). For each N-of-1 trial there was no statistically significant difference in pain or myalgia score between those who took statin and placebo. Five of the eight patients chose to continue on statins at the end of the trial.
Herrett and colleagues performed a more extensive series of N-of-1 trials involving 200 patients who had stopped or were considering stopping statins because of muscle symptoms.3 Participants either received 2 months of atorvastatin 20 mg or placebo for 2-month blocks six times. They rated their muscle symptoms on a VAS at the end of each block. There was no difference in muscle symptom scores between the statin and placebo periods.
Wood and colleagues took it a step further, when they studied an N-of-1 trial that included statin, placebo, and no treatment.4 Each participant received four bottles of atorvastatin 20 mg, four bottles of placebo, and four empty bottles. Each month they used treatment from the bottles based on random sequence and reported daily symptom scores. The mean symptom intensity was 8.0 during no-tablet months, 15.4 during placebo months (P < .001, compared with no-tablet months), and 16.3 during statin months (P < .001, compared with no-tablet months; P = .39, compared with placebo).
Taylor and colleagues studied 120 patients who had prior statin-associated muscle complaints.5 Each patient received either simvastatin 20 mg or placebo for 4 weeks, and then were switched for an additional 4 weeks. A total of 43 patients (36%) had pain on simvastatin but not placebo, 21 (17%) had no pain with either treatment, 21 (17%) reported pain with both treatments, and 35 (29%) had pain with placebo but not simvastatin. These studies support the concept of nocebo effect in patients who have muscle symptoms on statins.
So what should be done? Brennan and Roy did a retrospective study of 118 patients referred to a lipid clinic as being statin intolerant to two or more statins.6 Most of the patients were able to tolerate a statin: 71% tolerated same statin rechallenge, 53% tolerated statin switch, and 57% tolerated a nonstatin therapy.
In the Prosisa study, only 27% of patients who reported statin-associated muscle symptoms had reappearance of muscle symptoms after rechallenge with a statin.7
Research implications
Rechallenge with the same statin seems to be a reasonable first step, followed by switching to a different statin. I also share the concept of nocebo effect with my patients, and tell them I believe they have an excellent chance of tolerating the statin.
Pearl: The majority of patients with muscle symptoms while taking a statin likely have a nocebo effect, and are likely to tolerate rechallenge with the same statin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Nielsen SF and Nordestgaard BG. Eur Heart J. 2016;37:908-16.
2. Joy TR et al. Ann Intern Med. 2014;160:301-10.
3. Herrett E et al. BMJ. 2021 Feb 24;372:n135.
4. Wood FA et al. N Engl J Med 2020;383:2182-4.
5. Taylor BA et al. Atherosclerosis. 2017;256:100-4.
6. Brennen ET and Roy TR. Can J Card. 2017;33(5):666-73.
7. Bonaiti Fet al. Atherosclerosis. 2020;315:E13-4.
A 66-year-old woman is discharged from the hospital after an MI. Her discharge medications include atorvastatin 40 mg, lisinopril 20 mg, acetylsalicylic acid 81 mg, and clopidogrel 75 mg. At this patient’s follow-up appointment, she mentions that she has muscle pain and stiffness in both legs and her back. Her labs include thyroid-stimulating hormone of 2.0 and vitamin D of 40. She stops the atorvastatin for 2 weeks with resolution of her symptoms.
Which treatment recommendation would you make for this patient?
A. Restart atorvastatin
B. Start rosuvastatin twice a week
C. Start ezetimibe
D. Start a PCSK9 inhibitor
We often see high-risk cardiovascular disease patients who are concerned about muscle side effects brought on by statins. I think we all can agree that this patient needs aggressive medical therapy for prevention of secondary cardiovascular events. I would restart her atorvastatin.
Neilsen and Nordestgaard found that early statin discontinuation rates increased from 6% in 1995 to 18% in 2010.1
Early statin discontinuation correlated with negative statin-related news stories, their paper states. This suggests either an increased awareness of side effects or a possible nocebo effect.
Statin rechallenge results
Joy and colleagues reported the results on eight patients who had developed myalgias within 3 weeks of starting a statin. These patients, who received placebo or statin, completed an N-of-1 trial with three double-blind, crossover comparisons separated by 3-week washout periods.
Patients were evaluated pain on a visual analog scale (VAS). For each N-of-1 trial there was no statistically significant difference in pain or myalgia score between those who took statin and placebo. Five of the eight patients chose to continue on statins at the end of the trial.
Herrett and colleagues performed a more extensive series of N-of-1 trials involving 200 patients who had stopped or were considering stopping statins because of muscle symptoms.3 Participants either received 2 months of atorvastatin 20 mg or placebo for 2-month blocks six times. They rated their muscle symptoms on a VAS at the end of each block. There was no difference in muscle symptom scores between the statin and placebo periods.
Wood and colleagues took it a step further, when they studied an N-of-1 trial that included statin, placebo, and no treatment.4 Each participant received four bottles of atorvastatin 20 mg, four bottles of placebo, and four empty bottles. Each month they used treatment from the bottles based on random sequence and reported daily symptom scores. The mean symptom intensity was 8.0 during no-tablet months, 15.4 during placebo months (P < .001, compared with no-tablet months), and 16.3 during statin months (P < .001, compared with no-tablet months; P = .39, compared with placebo).
Taylor and colleagues studied 120 patients who had prior statin-associated muscle complaints.5 Each patient received either simvastatin 20 mg or placebo for 4 weeks, and then were switched for an additional 4 weeks. A total of 43 patients (36%) had pain on simvastatin but not placebo, 21 (17%) had no pain with either treatment, 21 (17%) reported pain with both treatments, and 35 (29%) had pain with placebo but not simvastatin. These studies support the concept of nocebo effect in patients who have muscle symptoms on statins.
So what should be done? Brennan and Roy did a retrospective study of 118 patients referred to a lipid clinic as being statin intolerant to two or more statins.6 Most of the patients were able to tolerate a statin: 71% tolerated same statin rechallenge, 53% tolerated statin switch, and 57% tolerated a nonstatin therapy.
In the Prosisa study, only 27% of patients who reported statin-associated muscle symptoms had reappearance of muscle symptoms after rechallenge with a statin.7
Research implications
Rechallenge with the same statin seems to be a reasonable first step, followed by switching to a different statin. I also share the concept of nocebo effect with my patients, and tell them I believe they have an excellent chance of tolerating the statin.
Pearl: The majority of patients with muscle symptoms while taking a statin likely have a nocebo effect, and are likely to tolerate rechallenge with the same statin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Nielsen SF and Nordestgaard BG. Eur Heart J. 2016;37:908-16.
2. Joy TR et al. Ann Intern Med. 2014;160:301-10.
3. Herrett E et al. BMJ. 2021 Feb 24;372:n135.
4. Wood FA et al. N Engl J Med 2020;383:2182-4.
5. Taylor BA et al. Atherosclerosis. 2017;256:100-4.
6. Brennen ET and Roy TR. Can J Card. 2017;33(5):666-73.
7. Bonaiti Fet al. Atherosclerosis. 2020;315:E13-4.
‘Quadpill’ bests monotherapy for initial BP lowering: QUARTET
A “quadpill” containing quarter doses of four blood pressure (BP)–lowering medications was more effective than monotherapy for initial treatment of hypertension, with similar tolerability, in the 1-year, phase 3 QUARTET randomized, active-control trial.
Clara Chow, MD, PhD, academic director of the Westmead Applied Research Centre, University of Sydney, presented the findings in a late-breaking trial session at the annual congress of the European Society of Cardiology. The study was simultaneously published in The Lancet.
The primary outcome, mean unattended office BP at 12 weeks, dropped from 142/86 mm Hg to 120/71 mm Hg in patients who received the daily quadpill – a capsule containing irbesartan, amlodipine, indapamide, and bisoprolol – and fell from 140/83 mm Hg to 127/79 mm Hg in patients who received a daily full dose of irbesartan.
This 6.9 mm Hg greater drop in systolic BP at 12 months is clinically meaningful, Dr. Chow told this news organization. “If maintained, it would be expected to confer about a 15%-20% reduction” in heart disease, stroke, and heart failure.
In the SPRINT study, she noted, the final systolic BP was 120 mm Hg in the intervention group and 134 mm Hg in the control group, and the difference was associated with a 27% reduction in the composite cardiovascular (CV) outcome.
The results of QUARTET suggest that, “even in those with stage 1 hypertension, we can safely reduce BP to a significant degree by this simple approach, compared to usual care,” Salim Yusuf, MD, DPhil, a long-time advocate of a polypill approach, said in an email.
Importantly, Dr. Chow pointed out, at 12 months, 81% of patients treated with the quadpill versus 62% of patients treated with monotherapy had BP control (<140/90 mm Hg). Patients who received monotherapy did not “catch up,” even though a higher percentage received stepped-up therapy.
The quadpill dosing strategy aligns with the latest 2018 ESC/European Society of Hypertension guidelines, which recommend starting antihypertensive treatment with more than one drug, session cochair Thomas Kahan, MD, PhD, Karolinska Institute, Danderyd Hospital, department of clinical sciences, Stockholm, commented.
“How many drugs should be in the initial step?” he asked. “Is four better than three or two, or should we have even more drugs at low doses?”
The trial was not designed to answer these questions, Dr. Chow replied. “We were really comparing [the quadpill] against what the majority of people around the planet are still doing, which is starting on one drug and slowly but surely stepping it up,” she said.
The quadpill was actually a capsule, she clarified, that contained four generic BP medications available in half doses in Australia. The half doses were cut in half and the medications were encapsulated. The control drug was prepared in an identical-looking capsule.
It is important to note that “the time to BP control was shorter in patients who received the quadpill versus monotherapy,” session cochair Felix Mahfoud, MD, internal medicine and cardiology, Saarland University Hospital, Hamburg, Germany, pointed out, because “in clinical practice we aim to get patients to BP control as quickly as possible.”
“What is new here is the use of four drugs, each given at quarter doses,” Dr. Yusuf, director of the Population Health Research Institute, McMaster University, Hamilton, Ont., said. Although a few questions remain, “this study emphasizes the importance and potential benefits and simplicity of using combination BP-lowering drugs at low doses.”
For guidelines to be changed, he observed, the findings would have to be replicated in independent studies, and the quadpill would likely have to be shown to be superior to the dual pill.
“It took about 20 years [to change guidelines] after the first evidence that combinations of two pills were preferable to single-drug combinations,” he noted.
“I hope that in most people with elevated BP, at least a two-drug combination plus a statin plus aspirin will be prescribed,” Dr. Yusuf said. “This can reduce the risk of CVD events by 50% or more – a big impact both for the individual and for populations. The quad pill may have a role in this approach.”
Four-in-one pill
Worldwide, hypertension control is poor, Dr. Chow said, because of the need for multiple medications, treatment inertia, and concerns about adverse events.
The researchers hypothesized that initial antihypertensive treatment with a four-in-one pill with quarter doses of each medication would minimize side effects, maximize BP lowering, and overcome these treatment barriers and concerns. A pilot study of this strategy published by the group in 2017 showed promise.
QUARTET randomized 591 adults with hypertension, seen at clinics in four states in Australia from June 2017 through August 2020.
Patients were either receiving no antihypertensive medication and had an unattended standard office BP of 140/90 to 179/109 mm Hg or daytime ambulatory BP greater than 135/95 mm Hg, or they were on BP-lowering monotherapy and had a BP of 130/85 to 179/109 mm Hg or daytime ambulatory BP greater than 125/80 mm Hg. Patients who were taking antihypertensive therapy entered a washout period before the trial.
The researchers randomized 291 participants to receive 150 mg irbesartan daily (usual care or control group).
The other 300 participants received a daily quadpill containing 37.5 mg irbesartan, 1.25 mg amlodipine, 0.625 mg indapamide, and 2.5 mg bisoprolol. The first three drugs are the most commonly prescribed angiotensin II receptor blocker, calcium channel blocker, and thiazide or thiazidelike diuretic in Australia, and the last drug, a beta-blocker, has a long duration of action, the study protocol explains.
Patients in both groups had similar baseline characteristics. They were a mean age of 58 years, 40% were women, and 82% were White. They also had a mean body mass index of 31 kg/m2. About 8% were current smokers, and about 54% were not taking a BP-lowering drug.
Participants had clinic visits at baseline, 6 weeks, and 12 weeks, and if they continued the study, at 26 weeks and 52 weeks.
If a patient’s blood pressure was higher than 140/90 mm Hg, clinicians could add another medication, starting with amlodipine 5 mg.
At 12 weeks, 15% of patients in the intervention group and 40% in the control group had stepped up treatment.
Despite greater up-titration in the usual care group, BP control remained higher in the quadpill group, Dr. Chow pointed out. That is, patients in the quadpill group were more likely than patients in the usual care group to have a BP less than 140/90 mm Hg (76% vs. 58% respectively; P < .0001).
Patients in the quadpill group also had lower daytime and nighttime ambulatory systolic BP.
At 12 months, among the 417 patients who continued treatment, patients in the quadpill group had a 7.7 mm Hg greater drop in systolic BP, compared with patients in the control group, (P < .001).
There were no significant differences in adverse events, which were most commonly dizziness (31% and 25%) or muscle cramps, gastrointestinal complaints, headache, or musculoskeletal complaints.
At 12 weeks, there were seven serious adverse events in the intervention group versus three in the control group. There were 12 treatment withdrawals in the intervention group versus seven in the control group (P = .27).
Remaining questions, upcoming phase 3 U.S. study
“While the [QUARTET] results are impressive, we are left with a number of questions,” Dr. Yusuf said.
Would the results be the same with a three-drug combo or even a two-drug combo at half doses? In the HOPE 3 trial, a two-drug combo at half doses provided similar results to the current study, over a much longer mean follow-up of 5.6 years, he noted.
Also, is the quadpill associated with higher rates of diabetes or higher creatinine levels in the long term? “Given that we do not have any data on long-term clinical outcomes from a four-drug combination,” Dr. Yusuf said, “caution should be utilized.”
Would the reduced risk of CVD be greater with a combination of low doses of two BP-lowering drugs plus a statin plus aspirin? That may be superior, he said, “based on recent information published on the polypill indicating a 50% relative risk reduction in CVD events.”
The related phase 2 QUARTET US trial should shed further light on a quadpill strategy. Patients with hypertension are being randomized to a daily quadpill containing 2 mg candesartan, 1.25 mg amlodipine besylate, 0.625 mg indapamide, and 2.5 mg bisoprolol, or to usual care, 8 mg candesartan daily.
Investigators plan to enroll 87 participants in the Chicago area, with estimated study completion by March 31, 2023.
The study was supported by an Australian National Health and Medical Research Council grant. The George Institute for Global Health has submitted patent applications for low–fixed-dose combination products to treat CV or cardiometabolic disease. Dr. Chow and coauthor Kris Rogers, PhD, senior biostatistician at The George Institute for Global Health, Newtown, Australia, are listed as inventors, but they do not have direct financial interests in these patent applications.
A version of this article first appeared on Medscape.com.
A “quadpill” containing quarter doses of four blood pressure (BP)–lowering medications was more effective than monotherapy for initial treatment of hypertension, with similar tolerability, in the 1-year, phase 3 QUARTET randomized, active-control trial.
Clara Chow, MD, PhD, academic director of the Westmead Applied Research Centre, University of Sydney, presented the findings in a late-breaking trial session at the annual congress of the European Society of Cardiology. The study was simultaneously published in The Lancet.
The primary outcome, mean unattended office BP at 12 weeks, dropped from 142/86 mm Hg to 120/71 mm Hg in patients who received the daily quadpill – a capsule containing irbesartan, amlodipine, indapamide, and bisoprolol – and fell from 140/83 mm Hg to 127/79 mm Hg in patients who received a daily full dose of irbesartan.
This 6.9 mm Hg greater drop in systolic BP at 12 months is clinically meaningful, Dr. Chow told this news organization. “If maintained, it would be expected to confer about a 15%-20% reduction” in heart disease, stroke, and heart failure.
In the SPRINT study, she noted, the final systolic BP was 120 mm Hg in the intervention group and 134 mm Hg in the control group, and the difference was associated with a 27% reduction in the composite cardiovascular (CV) outcome.
The results of QUARTET suggest that, “even in those with stage 1 hypertension, we can safely reduce BP to a significant degree by this simple approach, compared to usual care,” Salim Yusuf, MD, DPhil, a long-time advocate of a polypill approach, said in an email.
Importantly, Dr. Chow pointed out, at 12 months, 81% of patients treated with the quadpill versus 62% of patients treated with monotherapy had BP control (<140/90 mm Hg). Patients who received monotherapy did not “catch up,” even though a higher percentage received stepped-up therapy.
The quadpill dosing strategy aligns with the latest 2018 ESC/European Society of Hypertension guidelines, which recommend starting antihypertensive treatment with more than one drug, session cochair Thomas Kahan, MD, PhD, Karolinska Institute, Danderyd Hospital, department of clinical sciences, Stockholm, commented.
“How many drugs should be in the initial step?” he asked. “Is four better than three or two, or should we have even more drugs at low doses?”
The trial was not designed to answer these questions, Dr. Chow replied. “We were really comparing [the quadpill] against what the majority of people around the planet are still doing, which is starting on one drug and slowly but surely stepping it up,” she said.
The quadpill was actually a capsule, she clarified, that contained four generic BP medications available in half doses in Australia. The half doses were cut in half and the medications were encapsulated. The control drug was prepared in an identical-looking capsule.
It is important to note that “the time to BP control was shorter in patients who received the quadpill versus monotherapy,” session cochair Felix Mahfoud, MD, internal medicine and cardiology, Saarland University Hospital, Hamburg, Germany, pointed out, because “in clinical practice we aim to get patients to BP control as quickly as possible.”
“What is new here is the use of four drugs, each given at quarter doses,” Dr. Yusuf, director of the Population Health Research Institute, McMaster University, Hamilton, Ont., said. Although a few questions remain, “this study emphasizes the importance and potential benefits and simplicity of using combination BP-lowering drugs at low doses.”
For guidelines to be changed, he observed, the findings would have to be replicated in independent studies, and the quadpill would likely have to be shown to be superior to the dual pill.
“It took about 20 years [to change guidelines] after the first evidence that combinations of two pills were preferable to single-drug combinations,” he noted.
“I hope that in most people with elevated BP, at least a two-drug combination plus a statin plus aspirin will be prescribed,” Dr. Yusuf said. “This can reduce the risk of CVD events by 50% or more – a big impact both for the individual and for populations. The quad pill may have a role in this approach.”
Four-in-one pill
Worldwide, hypertension control is poor, Dr. Chow said, because of the need for multiple medications, treatment inertia, and concerns about adverse events.
The researchers hypothesized that initial antihypertensive treatment with a four-in-one pill with quarter doses of each medication would minimize side effects, maximize BP lowering, and overcome these treatment barriers and concerns. A pilot study of this strategy published by the group in 2017 showed promise.
QUARTET randomized 591 adults with hypertension, seen at clinics in four states in Australia from June 2017 through August 2020.
Patients were either receiving no antihypertensive medication and had an unattended standard office BP of 140/90 to 179/109 mm Hg or daytime ambulatory BP greater than 135/95 mm Hg, or they were on BP-lowering monotherapy and had a BP of 130/85 to 179/109 mm Hg or daytime ambulatory BP greater than 125/80 mm Hg. Patients who were taking antihypertensive therapy entered a washout period before the trial.
The researchers randomized 291 participants to receive 150 mg irbesartan daily (usual care or control group).
The other 300 participants received a daily quadpill containing 37.5 mg irbesartan, 1.25 mg amlodipine, 0.625 mg indapamide, and 2.5 mg bisoprolol. The first three drugs are the most commonly prescribed angiotensin II receptor blocker, calcium channel blocker, and thiazide or thiazidelike diuretic in Australia, and the last drug, a beta-blocker, has a long duration of action, the study protocol explains.
Patients in both groups had similar baseline characteristics. They were a mean age of 58 years, 40% were women, and 82% were White. They also had a mean body mass index of 31 kg/m2. About 8% were current smokers, and about 54% were not taking a BP-lowering drug.
Participants had clinic visits at baseline, 6 weeks, and 12 weeks, and if they continued the study, at 26 weeks and 52 weeks.
If a patient’s blood pressure was higher than 140/90 mm Hg, clinicians could add another medication, starting with amlodipine 5 mg.
At 12 weeks, 15% of patients in the intervention group and 40% in the control group had stepped up treatment.
Despite greater up-titration in the usual care group, BP control remained higher in the quadpill group, Dr. Chow pointed out. That is, patients in the quadpill group were more likely than patients in the usual care group to have a BP less than 140/90 mm Hg (76% vs. 58% respectively; P < .0001).
Patients in the quadpill group also had lower daytime and nighttime ambulatory systolic BP.
At 12 months, among the 417 patients who continued treatment, patients in the quadpill group had a 7.7 mm Hg greater drop in systolic BP, compared with patients in the control group, (P < .001).
There were no significant differences in adverse events, which were most commonly dizziness (31% and 25%) or muscle cramps, gastrointestinal complaints, headache, or musculoskeletal complaints.
At 12 weeks, there were seven serious adverse events in the intervention group versus three in the control group. There were 12 treatment withdrawals in the intervention group versus seven in the control group (P = .27).
Remaining questions, upcoming phase 3 U.S. study
“While the [QUARTET] results are impressive, we are left with a number of questions,” Dr. Yusuf said.
Would the results be the same with a three-drug combo or even a two-drug combo at half doses? In the HOPE 3 trial, a two-drug combo at half doses provided similar results to the current study, over a much longer mean follow-up of 5.6 years, he noted.
Also, is the quadpill associated with higher rates of diabetes or higher creatinine levels in the long term? “Given that we do not have any data on long-term clinical outcomes from a four-drug combination,” Dr. Yusuf said, “caution should be utilized.”
Would the reduced risk of CVD be greater with a combination of low doses of two BP-lowering drugs plus a statin plus aspirin? That may be superior, he said, “based on recent information published on the polypill indicating a 50% relative risk reduction in CVD events.”
The related phase 2 QUARTET US trial should shed further light on a quadpill strategy. Patients with hypertension are being randomized to a daily quadpill containing 2 mg candesartan, 1.25 mg amlodipine besylate, 0.625 mg indapamide, and 2.5 mg bisoprolol, or to usual care, 8 mg candesartan daily.
Investigators plan to enroll 87 participants in the Chicago area, with estimated study completion by March 31, 2023.
The study was supported by an Australian National Health and Medical Research Council grant. The George Institute for Global Health has submitted patent applications for low–fixed-dose combination products to treat CV or cardiometabolic disease. Dr. Chow and coauthor Kris Rogers, PhD, senior biostatistician at The George Institute for Global Health, Newtown, Australia, are listed as inventors, but they do not have direct financial interests in these patent applications.
A version of this article first appeared on Medscape.com.
A “quadpill” containing quarter doses of four blood pressure (BP)–lowering medications was more effective than monotherapy for initial treatment of hypertension, with similar tolerability, in the 1-year, phase 3 QUARTET randomized, active-control trial.
Clara Chow, MD, PhD, academic director of the Westmead Applied Research Centre, University of Sydney, presented the findings in a late-breaking trial session at the annual congress of the European Society of Cardiology. The study was simultaneously published in The Lancet.
The primary outcome, mean unattended office BP at 12 weeks, dropped from 142/86 mm Hg to 120/71 mm Hg in patients who received the daily quadpill – a capsule containing irbesartan, amlodipine, indapamide, and bisoprolol – and fell from 140/83 mm Hg to 127/79 mm Hg in patients who received a daily full dose of irbesartan.
This 6.9 mm Hg greater drop in systolic BP at 12 months is clinically meaningful, Dr. Chow told this news organization. “If maintained, it would be expected to confer about a 15%-20% reduction” in heart disease, stroke, and heart failure.
In the SPRINT study, she noted, the final systolic BP was 120 mm Hg in the intervention group and 134 mm Hg in the control group, and the difference was associated with a 27% reduction in the composite cardiovascular (CV) outcome.
The results of QUARTET suggest that, “even in those with stage 1 hypertension, we can safely reduce BP to a significant degree by this simple approach, compared to usual care,” Salim Yusuf, MD, DPhil, a long-time advocate of a polypill approach, said in an email.
Importantly, Dr. Chow pointed out, at 12 months, 81% of patients treated with the quadpill versus 62% of patients treated with monotherapy had BP control (<140/90 mm Hg). Patients who received monotherapy did not “catch up,” even though a higher percentage received stepped-up therapy.
The quadpill dosing strategy aligns with the latest 2018 ESC/European Society of Hypertension guidelines, which recommend starting antihypertensive treatment with more than one drug, session cochair Thomas Kahan, MD, PhD, Karolinska Institute, Danderyd Hospital, department of clinical sciences, Stockholm, commented.
“How many drugs should be in the initial step?” he asked. “Is four better than three or two, or should we have even more drugs at low doses?”
The trial was not designed to answer these questions, Dr. Chow replied. “We were really comparing [the quadpill] against what the majority of people around the planet are still doing, which is starting on one drug and slowly but surely stepping it up,” she said.
The quadpill was actually a capsule, she clarified, that contained four generic BP medications available in half doses in Australia. The half doses were cut in half and the medications were encapsulated. The control drug was prepared in an identical-looking capsule.
It is important to note that “the time to BP control was shorter in patients who received the quadpill versus monotherapy,” session cochair Felix Mahfoud, MD, internal medicine and cardiology, Saarland University Hospital, Hamburg, Germany, pointed out, because “in clinical practice we aim to get patients to BP control as quickly as possible.”
“What is new here is the use of four drugs, each given at quarter doses,” Dr. Yusuf, director of the Population Health Research Institute, McMaster University, Hamilton, Ont., said. Although a few questions remain, “this study emphasizes the importance and potential benefits and simplicity of using combination BP-lowering drugs at low doses.”
For guidelines to be changed, he observed, the findings would have to be replicated in independent studies, and the quadpill would likely have to be shown to be superior to the dual pill.
“It took about 20 years [to change guidelines] after the first evidence that combinations of two pills were preferable to single-drug combinations,” he noted.
“I hope that in most people with elevated BP, at least a two-drug combination plus a statin plus aspirin will be prescribed,” Dr. Yusuf said. “This can reduce the risk of CVD events by 50% or more – a big impact both for the individual and for populations. The quad pill may have a role in this approach.”
Four-in-one pill
Worldwide, hypertension control is poor, Dr. Chow said, because of the need for multiple medications, treatment inertia, and concerns about adverse events.
The researchers hypothesized that initial antihypertensive treatment with a four-in-one pill with quarter doses of each medication would minimize side effects, maximize BP lowering, and overcome these treatment barriers and concerns. A pilot study of this strategy published by the group in 2017 showed promise.
QUARTET randomized 591 adults with hypertension, seen at clinics in four states in Australia from June 2017 through August 2020.
Patients were either receiving no antihypertensive medication and had an unattended standard office BP of 140/90 to 179/109 mm Hg or daytime ambulatory BP greater than 135/95 mm Hg, or they were on BP-lowering monotherapy and had a BP of 130/85 to 179/109 mm Hg or daytime ambulatory BP greater than 125/80 mm Hg. Patients who were taking antihypertensive therapy entered a washout period before the trial.
The researchers randomized 291 participants to receive 150 mg irbesartan daily (usual care or control group).
The other 300 participants received a daily quadpill containing 37.5 mg irbesartan, 1.25 mg amlodipine, 0.625 mg indapamide, and 2.5 mg bisoprolol. The first three drugs are the most commonly prescribed angiotensin II receptor blocker, calcium channel blocker, and thiazide or thiazidelike diuretic in Australia, and the last drug, a beta-blocker, has a long duration of action, the study protocol explains.
Patients in both groups had similar baseline characteristics. They were a mean age of 58 years, 40% were women, and 82% were White. They also had a mean body mass index of 31 kg/m2. About 8% were current smokers, and about 54% were not taking a BP-lowering drug.
Participants had clinic visits at baseline, 6 weeks, and 12 weeks, and if they continued the study, at 26 weeks and 52 weeks.
If a patient’s blood pressure was higher than 140/90 mm Hg, clinicians could add another medication, starting with amlodipine 5 mg.
At 12 weeks, 15% of patients in the intervention group and 40% in the control group had stepped up treatment.
Despite greater up-titration in the usual care group, BP control remained higher in the quadpill group, Dr. Chow pointed out. That is, patients in the quadpill group were more likely than patients in the usual care group to have a BP less than 140/90 mm Hg (76% vs. 58% respectively; P < .0001).
Patients in the quadpill group also had lower daytime and nighttime ambulatory systolic BP.
At 12 months, among the 417 patients who continued treatment, patients in the quadpill group had a 7.7 mm Hg greater drop in systolic BP, compared with patients in the control group, (P < .001).
There were no significant differences in adverse events, which were most commonly dizziness (31% and 25%) or muscle cramps, gastrointestinal complaints, headache, or musculoskeletal complaints.
At 12 weeks, there were seven serious adverse events in the intervention group versus three in the control group. There were 12 treatment withdrawals in the intervention group versus seven in the control group (P = .27).
Remaining questions, upcoming phase 3 U.S. study
“While the [QUARTET] results are impressive, we are left with a number of questions,” Dr. Yusuf said.
Would the results be the same with a three-drug combo or even a two-drug combo at half doses? In the HOPE 3 trial, a two-drug combo at half doses provided similar results to the current study, over a much longer mean follow-up of 5.6 years, he noted.
Also, is the quadpill associated with higher rates of diabetes or higher creatinine levels in the long term? “Given that we do not have any data on long-term clinical outcomes from a four-drug combination,” Dr. Yusuf said, “caution should be utilized.”
Would the reduced risk of CVD be greater with a combination of low doses of two BP-lowering drugs plus a statin plus aspirin? That may be superior, he said, “based on recent information published on the polypill indicating a 50% relative risk reduction in CVD events.”
The related phase 2 QUARTET US trial should shed further light on a quadpill strategy. Patients with hypertension are being randomized to a daily quadpill containing 2 mg candesartan, 1.25 mg amlodipine besylate, 0.625 mg indapamide, and 2.5 mg bisoprolol, or to usual care, 8 mg candesartan daily.
Investigators plan to enroll 87 participants in the Chicago area, with estimated study completion by March 31, 2023.
The study was supported by an Australian National Health and Medical Research Council grant. The George Institute for Global Health has submitted patent applications for low–fixed-dose combination products to treat CV or cardiometabolic disease. Dr. Chow and coauthor Kris Rogers, PhD, senior biostatistician at The George Institute for Global Health, Newtown, Australia, are listed as inventors, but they do not have direct financial interests in these patent applications.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2021
Growing proportion of cardiac arrests in U.S. considered opioid related
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
FROM ESC 2021
STOP-DAPT 2 ACS: 1 month of DAPT proves inadequate for patients with recent ACS
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
New European guidelines on CVD prevention
The new guidelines were published online Aug. 30 in the European Heart Journal to coincide with presentation at the European Stroke Congress (ESOC) 2021.
They were developed by an ESOC task force in collaboration with 12 medical societies and with special contribution of the European Association of Preventive Cardiology.
“A chief goal of the task force was to create a single CVD prevention guideline for everyone – for primary care, for hospital care, for guiding clinical practice – so one guideline for all,” said cochair of the guideline committee Frank Visseren, MD, PhD, University Medical Center Utrecht, Netherlands. “We also wanted to make a more personalized CVD prevention guideline, instead of a one-size-fits-all. In clinical practice, people are very, very different, and we really want to have a more individualized prevention guideline,” said Dr. Visseren, as well as provide “more room for shared decision-making.”
Prevention at the individual and population levels
The new guidelines also give more attention to CVD prevention in older persons. “Many of our patients are over 70 years old and we really want to have more detail, more guidance on older persons,” said Dr. Visseren.
The guideline is divided into two sections. One section covers CVD prevention at the individual level in apparently healthy people, in patients with established CVD, and in those with diabetes, familial hypercholesterolemia, or chronic kidney disease.
The other section covers CVD prevention at the population level, including public health policy, interventions, and the environment, including putting in place measures to reduce air pollution, use of fossil fuels, and limiting carbon dioxide emissions.
Targets for blood lipids, blood pressure, and glycemic control in diabetes remain in line with recent ESC guidelines on dyslipidemias, hypertension, or diabetes.
However, the guidelines introduce a new stepwise treatment-intensification approach to achieve these targets, with consideration of CVD risk, treatment benefit of risk factors, risk modifiers, comorbidities, and patient preferences.
The 2021 CVD prevention guidelines also embrace the recently published Systemic Coronary Risk Estimation 2 (SCORE2) and Systemic Coronary Risk Estimation 2-Older Persons (SCORE2-OP) algorithms. “The algorithms we are using are a bit old and we want to have more updated risk prediction, because that’s the starting point of CVD prevention,” Dr. Visseren said.
The guidelines also introduce age-specific risk thresholds for risk factor treatments in apparently healthy people and provide estimation of lifetime CVD risk and treatment benefit. This will allow clinicians to have “an informed discussion with patients on lifetime risk and potential treatment benefits,” Dr. Visseren said.
For the first time, the guidelines recommend smoking cessation regardless of whether it leads to weight gain, as weight gain does not lessen the benefits of cessation.
Regarding exercise, adults of all ages should aim for at least 150-300 minutes a week of moderate, or 75-150 minutes a week of vigorous, aerobic physical activity. The guidelines recommend reducing sedentary time and engaging in at least light activity throughout the day.
Regarding nutrition, the guidelines advise adopting a Mediterranean or similar diet; restricting alcohol intake to a maximum of 100 g per week (a standard drink is 8-14 g); eating fish, preferably fatty fish, at least once a week; and restricting consumption of meat, particularly processed meat.
Also for the first time, the guidelines state that bariatric surgery should be considered for obese individuals at elevated risk of CVD when a healthy diet and exercise fail to lead to weight loss that is maintained.
They note that individuals with mental disorders need additional attention and support to improve adherence to lifestyle changes and drug treatment.
They advise consideration of referring patients with heart disease and significant stress and anxiety to psychotherapeutic stress management to reduce stress symptoms and improve CV outcomes.
Potential cost issues that could be considered when implementing the guidelines are also reviewed.
Dr. Visseren acknowledged and thanked the task force members for continuing their work on the guidelines over the 2 “challenging” years.
Setting the bar lower?
Discussant for the guideline presentation, Diederick Grobbee, MD, University Medical Center Utrecht, who was not involved in drafting the guidelines, said he does have one conflict of interest, which is a “passion for prevention.” From that perspective, he said the guideline panel “should be applauded; the once-every-5-year issuing of the prevention guidelines is a major event.”
Dr. Grobbee noted that the working group “really tried to follow their ambitions and goals, in a way making the guidelines simpler, or perhaps setting the bar not initially as high as we used to do, which may, in fact, sometimes scare off physicians and patients alike.”
“We’ve had prevention guidelines for quite some time now, yet looking at what is accomplished in practice is sobering,” said Dr. Grobbee. Introducing a stepwise approach is “really appealing,” he added.
A version of this article first appeared on Medscape.com.
The new guidelines were published online Aug. 30 in the European Heart Journal to coincide with presentation at the European Stroke Congress (ESOC) 2021.
They were developed by an ESOC task force in collaboration with 12 medical societies and with special contribution of the European Association of Preventive Cardiology.
“A chief goal of the task force was to create a single CVD prevention guideline for everyone – for primary care, for hospital care, for guiding clinical practice – so one guideline for all,” said cochair of the guideline committee Frank Visseren, MD, PhD, University Medical Center Utrecht, Netherlands. “We also wanted to make a more personalized CVD prevention guideline, instead of a one-size-fits-all. In clinical practice, people are very, very different, and we really want to have a more individualized prevention guideline,” said Dr. Visseren, as well as provide “more room for shared decision-making.”
Prevention at the individual and population levels
The new guidelines also give more attention to CVD prevention in older persons. “Many of our patients are over 70 years old and we really want to have more detail, more guidance on older persons,” said Dr. Visseren.
The guideline is divided into two sections. One section covers CVD prevention at the individual level in apparently healthy people, in patients with established CVD, and in those with diabetes, familial hypercholesterolemia, or chronic kidney disease.
The other section covers CVD prevention at the population level, including public health policy, interventions, and the environment, including putting in place measures to reduce air pollution, use of fossil fuels, and limiting carbon dioxide emissions.
Targets for blood lipids, blood pressure, and glycemic control in diabetes remain in line with recent ESC guidelines on dyslipidemias, hypertension, or diabetes.
However, the guidelines introduce a new stepwise treatment-intensification approach to achieve these targets, with consideration of CVD risk, treatment benefit of risk factors, risk modifiers, comorbidities, and patient preferences.
The 2021 CVD prevention guidelines also embrace the recently published Systemic Coronary Risk Estimation 2 (SCORE2) and Systemic Coronary Risk Estimation 2-Older Persons (SCORE2-OP) algorithms. “The algorithms we are using are a bit old and we want to have more updated risk prediction, because that’s the starting point of CVD prevention,” Dr. Visseren said.
The guidelines also introduce age-specific risk thresholds for risk factor treatments in apparently healthy people and provide estimation of lifetime CVD risk and treatment benefit. This will allow clinicians to have “an informed discussion with patients on lifetime risk and potential treatment benefits,” Dr. Visseren said.
For the first time, the guidelines recommend smoking cessation regardless of whether it leads to weight gain, as weight gain does not lessen the benefits of cessation.
Regarding exercise, adults of all ages should aim for at least 150-300 minutes a week of moderate, or 75-150 minutes a week of vigorous, aerobic physical activity. The guidelines recommend reducing sedentary time and engaging in at least light activity throughout the day.
Regarding nutrition, the guidelines advise adopting a Mediterranean or similar diet; restricting alcohol intake to a maximum of 100 g per week (a standard drink is 8-14 g); eating fish, preferably fatty fish, at least once a week; and restricting consumption of meat, particularly processed meat.
Also for the first time, the guidelines state that bariatric surgery should be considered for obese individuals at elevated risk of CVD when a healthy diet and exercise fail to lead to weight loss that is maintained.
They note that individuals with mental disorders need additional attention and support to improve adherence to lifestyle changes and drug treatment.
They advise consideration of referring patients with heart disease and significant stress and anxiety to psychotherapeutic stress management to reduce stress symptoms and improve CV outcomes.
Potential cost issues that could be considered when implementing the guidelines are also reviewed.
Dr. Visseren acknowledged and thanked the task force members for continuing their work on the guidelines over the 2 “challenging” years.
Setting the bar lower?
Discussant for the guideline presentation, Diederick Grobbee, MD, University Medical Center Utrecht, who was not involved in drafting the guidelines, said he does have one conflict of interest, which is a “passion for prevention.” From that perspective, he said the guideline panel “should be applauded; the once-every-5-year issuing of the prevention guidelines is a major event.”
Dr. Grobbee noted that the working group “really tried to follow their ambitions and goals, in a way making the guidelines simpler, or perhaps setting the bar not initially as high as we used to do, which may, in fact, sometimes scare off physicians and patients alike.”
“We’ve had prevention guidelines for quite some time now, yet looking at what is accomplished in practice is sobering,” said Dr. Grobbee. Introducing a stepwise approach is “really appealing,” he added.
A version of this article first appeared on Medscape.com.
The new guidelines were published online Aug. 30 in the European Heart Journal to coincide with presentation at the European Stroke Congress (ESOC) 2021.
They were developed by an ESOC task force in collaboration with 12 medical societies and with special contribution of the European Association of Preventive Cardiology.
“A chief goal of the task force was to create a single CVD prevention guideline for everyone – for primary care, for hospital care, for guiding clinical practice – so one guideline for all,” said cochair of the guideline committee Frank Visseren, MD, PhD, University Medical Center Utrecht, Netherlands. “We also wanted to make a more personalized CVD prevention guideline, instead of a one-size-fits-all. In clinical practice, people are very, very different, and we really want to have a more individualized prevention guideline,” said Dr. Visseren, as well as provide “more room for shared decision-making.”
Prevention at the individual and population levels
The new guidelines also give more attention to CVD prevention in older persons. “Many of our patients are over 70 years old and we really want to have more detail, more guidance on older persons,” said Dr. Visseren.
The guideline is divided into two sections. One section covers CVD prevention at the individual level in apparently healthy people, in patients with established CVD, and in those with diabetes, familial hypercholesterolemia, or chronic kidney disease.
The other section covers CVD prevention at the population level, including public health policy, interventions, and the environment, including putting in place measures to reduce air pollution, use of fossil fuels, and limiting carbon dioxide emissions.
Targets for blood lipids, blood pressure, and glycemic control in diabetes remain in line with recent ESC guidelines on dyslipidemias, hypertension, or diabetes.
However, the guidelines introduce a new stepwise treatment-intensification approach to achieve these targets, with consideration of CVD risk, treatment benefit of risk factors, risk modifiers, comorbidities, and patient preferences.
The 2021 CVD prevention guidelines also embrace the recently published Systemic Coronary Risk Estimation 2 (SCORE2) and Systemic Coronary Risk Estimation 2-Older Persons (SCORE2-OP) algorithms. “The algorithms we are using are a bit old and we want to have more updated risk prediction, because that’s the starting point of CVD prevention,” Dr. Visseren said.
The guidelines also introduce age-specific risk thresholds for risk factor treatments in apparently healthy people and provide estimation of lifetime CVD risk and treatment benefit. This will allow clinicians to have “an informed discussion with patients on lifetime risk and potential treatment benefits,” Dr. Visseren said.
For the first time, the guidelines recommend smoking cessation regardless of whether it leads to weight gain, as weight gain does not lessen the benefits of cessation.
Regarding exercise, adults of all ages should aim for at least 150-300 minutes a week of moderate, or 75-150 minutes a week of vigorous, aerobic physical activity. The guidelines recommend reducing sedentary time and engaging in at least light activity throughout the day.
Regarding nutrition, the guidelines advise adopting a Mediterranean or similar diet; restricting alcohol intake to a maximum of 100 g per week (a standard drink is 8-14 g); eating fish, preferably fatty fish, at least once a week; and restricting consumption of meat, particularly processed meat.
Also for the first time, the guidelines state that bariatric surgery should be considered for obese individuals at elevated risk of CVD when a healthy diet and exercise fail to lead to weight loss that is maintained.
They note that individuals with mental disorders need additional attention and support to improve adherence to lifestyle changes and drug treatment.
They advise consideration of referring patients with heart disease and significant stress and anxiety to psychotherapeutic stress management to reduce stress symptoms and improve CV outcomes.
Potential cost issues that could be considered when implementing the guidelines are also reviewed.
Dr. Visseren acknowledged and thanked the task force members for continuing their work on the guidelines over the 2 “challenging” years.
Setting the bar lower?
Discussant for the guideline presentation, Diederick Grobbee, MD, University Medical Center Utrecht, who was not involved in drafting the guidelines, said he does have one conflict of interest, which is a “passion for prevention.” From that perspective, he said the guideline panel “should be applauded; the once-every-5-year issuing of the prevention guidelines is a major event.”
Dr. Grobbee noted that the working group “really tried to follow their ambitions and goals, in a way making the guidelines simpler, or perhaps setting the bar not initially as high as we used to do, which may, in fact, sometimes scare off physicians and patients alike.”
“We’ve had prevention guidelines for quite some time now, yet looking at what is accomplished in practice is sobering,” said Dr. Grobbee. Introducing a stepwise approach is “really appealing,” he added.
A version of this article first appeared on Medscape.com.
FROM ESC 2021