User login
A New Valve—and a Change of Heart?
ANSWER
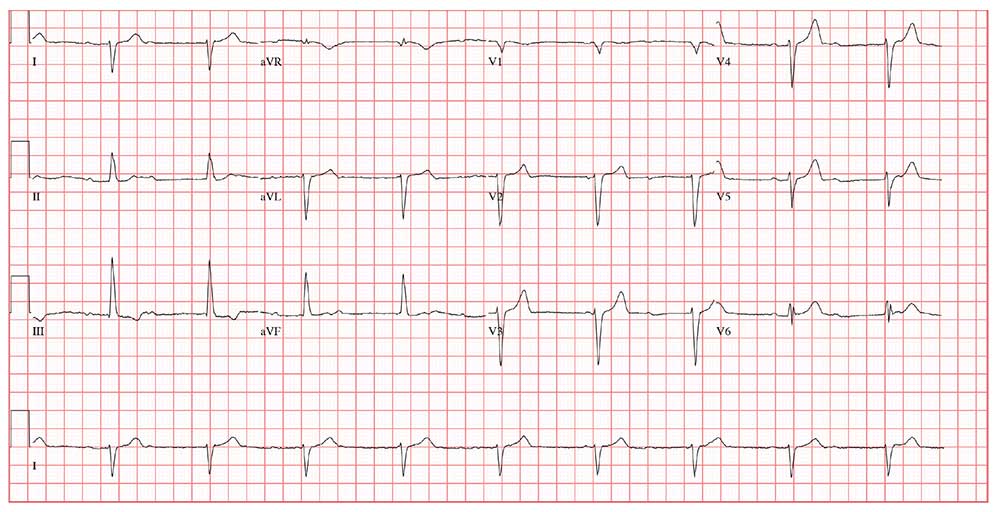
This ECG shows sinus rhythm with complete heart block and a junctional rhythm with a right-axis deviation. Additionally, ventricular depolarization in the precordial leads is suggestive of an anterior myocardial infarction.
Sinus rhythm is evidenced by the regular, steady progression of P waves with a P-P interval of about 90 beats/min. Complete atrioventricular dissociation indicates complete heart block.
A normal QRS duration of 106 ms at a rate of 56 beats/min supports the diagnosis of a junctional escape rhythm. Right-axis deviation is evidenced by an R axis of 120°.
Finally, poor R-wave progression with deep S waves in leads V1 through V5 is suggestive of an anterior myocardial infarction. However, in this case, there is no evidence of ischemia or history of infarction—so these are thought to be early postoperative findings.
ANSWER
This ECG shows sinus rhythm with complete heart block and a junctional rhythm with a right-axis deviation. Additionally, ventricular depolarization in the precordial leads is suggestive of an anterior myocardial infarction.
Sinus rhythm is evidenced by the regular, steady progression of P waves with a P-P interval of about 90 beats/min. Complete atrioventricular dissociation indicates complete heart block.
A normal QRS duration of 106 ms at a rate of 56 beats/min supports the diagnosis of a junctional escape rhythm. Right-axis deviation is evidenced by an R axis of 120°.
Finally, poor R-wave progression with deep S waves in leads V1 through V5 is suggestive of an anterior myocardial infarction. However, in this case, there is no evidence of ischemia or history of infarction—so these are thought to be early postoperative findings.
ANSWER
This ECG shows sinus rhythm with complete heart block and a junctional rhythm with a right-axis deviation. Additionally, ventricular depolarization in the precordial leads is suggestive of an anterior myocardial infarction.
Sinus rhythm is evidenced by the regular, steady progression of P waves with a P-P interval of about 90 beats/min. Complete atrioventricular dissociation indicates complete heart block.
A normal QRS duration of 106 ms at a rate of 56 beats/min supports the diagnosis of a junctional escape rhythm. Right-axis deviation is evidenced by an R axis of 120°.
Finally, poor R-wave progression with deep S waves in leads V1 through V5 is suggestive of an anterior myocardial infarction. However, in this case, there is no evidence of ischemia or history of infarction—so these are thought to be early postoperative findings.
Three days ago, a 64-year-old man underwent a tricuspid valve replacement for severe tricuspid regurgitation of unknown etiology. The surgical procedure included implantation of a 29-mm porcine valve and 2 epicardial right ventricular epicardial pacing leads.
The patient’s preoperative echocardiogram had shown severe tricuspid regurgitation with anterior leaflet prolapse and severe right atrial and ventricular enlargement. Preoperatively, the peak velocity of the tricuspid valve was 3.4 m/s, and the right ventricular systolic pressure was measured at 55 mm Hg. There was no mitral valvular disease or evidence of ischemia, and the overall left ventricular function was preserved, with a normal ejection fraction.
Preoperative history included 3-year progression of shortness of breath, dyspnea on exertion, and bilateral lower extremity edema. Over the past 2 months, he has had signs of hepatic congestion, including elevated serum transaminase, alkaline phosphatase, and direct bilirubin levels. A physical exam had revealed an enlarged liver that was tender to deep palpation. Social and family histories are noncontributory to the case as presented.
This morning, the patient is in no distress, sitting comfortably in a chair, and is alert and cooperative. Vital signs include a blood pressure of 118/64 mm Hg; pulse, 60 beats/min; respiratory rate, 16 breaths/min; and temperature, 96.4°F.
The surgical incision is clean, dry, and well approximated, and the patient is back to his preoperative weight. Pulmonary exam reveals clear breath sounds, with the exception of the left base, which demonstrates crackles that change with coughing. There are no wheezes. The cardiac exam reveals a regular rhythm at a rate of 60 beats/min, with a soft grade II/VI systolic murmur at the left lower sternal border. A late systolic friction rub is also evident. The abdomen is soft and nontender, with good bowel sounds and no organomegaly. Peripheral pulses are strong bilaterally, and there is trace pitting edema in both lower extremities. Neurologic exam is normal.
This morning’s ECG reveals a ventricular rate of 56 beats/min; PR interval, unmeasurable; QRS duration, 106 ms; QT/QTc interval, 400/386 ms; P axis, 36°; R axis, 120°; and T axis, 7°. What is your interpretation of this ECG?
Getting high heightens stroke, arrhythmia risks
Stoners, beware: , and people with cannabis use disorder are at a 50% greater risk of being hospitalized for arrhythmias, according to new research presented at the American Heart Association Scientific Sessions 2019.
An analysis of pooled data on nearly 44,000 participants in a cross-sectional survey showed that, among the 13.6% who reported using marijuana within the last 30 days, the adjusted odds ratio for young-onset stroke (aged 18-44 years), compared with non-users, was 2.75, reported Tarang Parekh, MBBS, a health policy researcher of George Mason University in Fairfax, Va., and colleagues.
In a separate study, a retrospective analysis of national inpatient data showed that people diagnosed with cannabis use disorder – a pathological pattern of impaired control, social impairment, risky behavior or physiological adaptation similar in nature to alcoholism – had a 47%-52% increased likelihood of hospitalization for an arrhythmia, reported Rikinkumar S. Patel, MD, a psychiatry resident at Griffin Memorial Hospital in Norman, Okla.
“As these [cannabis] products become increasingly used across the country, getting clearer, scientifically rigorous data is going to be important as we try to understand the overall health effects of cannabis,” said AHA President Robert Harrington, MD, of Stanford (Calif.) University in a statement.
Currently, use of both medical and recreational marijuana is fully legal in 11 U.S. states and the District of Columbia. Medical marijuana is legal with recreational use decriminalized (or penalties reduced) in 28 other states, and totally illegal in 11 other states, according to employee screening firm DISA Global Solutions.
Stroke study
In an oral presentation with simultaneous publication in the AHA journal Stroke, Dr. Parekh and colleagues presented an analysis of pooled data from the Behavioral Risk Factor Surveillance System (BRFSS), a nationally representative cross-sectional survey collected by the Centers for Disease Control and Prevention in 2016 and 2017.
They looked at baseline sociodemographic data and created multivariable logistic regression models with state fixed effects to determine whether marijuana use within the last 30 days was associated with young-onset stroke.
They identified 43,860 participants representing a weighted sample of 35.5 million Americans. Of the sample, 63.3% were male, and 13.6 % of all participants reported using marijuana in the last 30 days.
They found in an unadjusted model that marijuana users had an odds ratio for stroke, compared with nonusers, of 1.59 (P less than.1), and in a model adjusted for demographic factors (gender, race, ethnicity, and education) the OR increased to 1.76 (P less than .05).
When they threw risk behavior into the model (physical activity, body mass index, heavy drinking, and cigarette smoking), they saw that the OR for stroke shot up to 2.75 (P less than .01).
“Physicians should ask patients if they use cannabis and counsel them about its potential stroke risk as part of regular doctor visits,” Dr. Parekh said in a statement.
Arrhythmias study
Based on recent studies suggesting that cannabis use may trigger cardiovascular events, Dr. Patel and colleagues studied whether cannabis use disorder may be related to arrhythmias, approaching the question through hospital records.
“The effects of using cannabis are seen within 15 minutes and last for around 3 hours. At lower doses, it is linked to a rapid heartbeat. At higher doses, it is linked to a too-slow heartbeat,” he said in a statement.
Dr. Patel and colleagues conducted a retrospective analysis of the Nationwide Inpatient Sample from 2010-2014, a period during which medical marijuana became legal in several states and recreational marijuana became legal in Colorado and Washington. The sample is a database maintained by the Healthcare Cost and Utilization Project of the U.S. Office of Disease Prevention and Health Promotion.
They identified 570,557 patients aged 15-54 years with a primary diagnosis of arrhythmia, and compared them with a sample of 67,662,082 patients hospitalized with no arrhythmia diagnosed during the same period.
They found a 2.6% incidence of cannabis use disorder among patients hospitalized for arrhythmias. Patients with cannabis use disorder tended to be younger (15- to 24-years-old; OR, 4.23), male (OR, 1.70) and African American (OR, 2.70).
In regression analysis adjusted for demographics and comorbidities, cannabis use disorder was associated with higher odds of arrhythmia hospitalization in young patients, at 1.28 times among 15- to 24-year-olds (95% confidence interval, 1.229-1.346) and 1.52 times for 25- to 34-year-olds (95% CI, 1.469-1.578).
“As medical and recreational cannabis is legalized in many states, it is important to know the difference between therapeutic cannabis dosing for medical purposes and the consequences of cannabis abuse. We urgently need additional research to understand these issues,” Dr. Patel said.
“It’s not proving that there’s a direct link, but it’s raising a suggestion in an observational analysis that [this] indeed might be the case. What that means for clinicians is that, if you’re seeing a patient who is presenting with a symptomatic arrhythmia, adding cannabis usage to your list of questions as you begin to try to understand possible precipitating factors for this arrhythmia seems to be a reasonable thing to do,” Dr. Harrington commented.
Stoners, beware: , and people with cannabis use disorder are at a 50% greater risk of being hospitalized for arrhythmias, according to new research presented at the American Heart Association Scientific Sessions 2019.
An analysis of pooled data on nearly 44,000 participants in a cross-sectional survey showed that, among the 13.6% who reported using marijuana within the last 30 days, the adjusted odds ratio for young-onset stroke (aged 18-44 years), compared with non-users, was 2.75, reported Tarang Parekh, MBBS, a health policy researcher of George Mason University in Fairfax, Va., and colleagues.
In a separate study, a retrospective analysis of national inpatient data showed that people diagnosed with cannabis use disorder – a pathological pattern of impaired control, social impairment, risky behavior or physiological adaptation similar in nature to alcoholism – had a 47%-52% increased likelihood of hospitalization for an arrhythmia, reported Rikinkumar S. Patel, MD, a psychiatry resident at Griffin Memorial Hospital in Norman, Okla.
“As these [cannabis] products become increasingly used across the country, getting clearer, scientifically rigorous data is going to be important as we try to understand the overall health effects of cannabis,” said AHA President Robert Harrington, MD, of Stanford (Calif.) University in a statement.
Currently, use of both medical and recreational marijuana is fully legal in 11 U.S. states and the District of Columbia. Medical marijuana is legal with recreational use decriminalized (or penalties reduced) in 28 other states, and totally illegal in 11 other states, according to employee screening firm DISA Global Solutions.
Stroke study
In an oral presentation with simultaneous publication in the AHA journal Stroke, Dr. Parekh and colleagues presented an analysis of pooled data from the Behavioral Risk Factor Surveillance System (BRFSS), a nationally representative cross-sectional survey collected by the Centers for Disease Control and Prevention in 2016 and 2017.
They looked at baseline sociodemographic data and created multivariable logistic regression models with state fixed effects to determine whether marijuana use within the last 30 days was associated with young-onset stroke.
They identified 43,860 participants representing a weighted sample of 35.5 million Americans. Of the sample, 63.3% were male, and 13.6 % of all participants reported using marijuana in the last 30 days.
They found in an unadjusted model that marijuana users had an odds ratio for stroke, compared with nonusers, of 1.59 (P less than.1), and in a model adjusted for demographic factors (gender, race, ethnicity, and education) the OR increased to 1.76 (P less than .05).
When they threw risk behavior into the model (physical activity, body mass index, heavy drinking, and cigarette smoking), they saw that the OR for stroke shot up to 2.75 (P less than .01).
“Physicians should ask patients if they use cannabis and counsel them about its potential stroke risk as part of regular doctor visits,” Dr. Parekh said in a statement.
Arrhythmias study
Based on recent studies suggesting that cannabis use may trigger cardiovascular events, Dr. Patel and colleagues studied whether cannabis use disorder may be related to arrhythmias, approaching the question through hospital records.
“The effects of using cannabis are seen within 15 minutes and last for around 3 hours. At lower doses, it is linked to a rapid heartbeat. At higher doses, it is linked to a too-slow heartbeat,” he said in a statement.
Dr. Patel and colleagues conducted a retrospective analysis of the Nationwide Inpatient Sample from 2010-2014, a period during which medical marijuana became legal in several states and recreational marijuana became legal in Colorado and Washington. The sample is a database maintained by the Healthcare Cost and Utilization Project of the U.S. Office of Disease Prevention and Health Promotion.
They identified 570,557 patients aged 15-54 years with a primary diagnosis of arrhythmia, and compared them with a sample of 67,662,082 patients hospitalized with no arrhythmia diagnosed during the same period.
They found a 2.6% incidence of cannabis use disorder among patients hospitalized for arrhythmias. Patients with cannabis use disorder tended to be younger (15- to 24-years-old; OR, 4.23), male (OR, 1.70) and African American (OR, 2.70).
In regression analysis adjusted for demographics and comorbidities, cannabis use disorder was associated with higher odds of arrhythmia hospitalization in young patients, at 1.28 times among 15- to 24-year-olds (95% confidence interval, 1.229-1.346) and 1.52 times for 25- to 34-year-olds (95% CI, 1.469-1.578).
“As medical and recreational cannabis is legalized in many states, it is important to know the difference between therapeutic cannabis dosing for medical purposes and the consequences of cannabis abuse. We urgently need additional research to understand these issues,” Dr. Patel said.
“It’s not proving that there’s a direct link, but it’s raising a suggestion in an observational analysis that [this] indeed might be the case. What that means for clinicians is that, if you’re seeing a patient who is presenting with a symptomatic arrhythmia, adding cannabis usage to your list of questions as you begin to try to understand possible precipitating factors for this arrhythmia seems to be a reasonable thing to do,” Dr. Harrington commented.
Stoners, beware: , and people with cannabis use disorder are at a 50% greater risk of being hospitalized for arrhythmias, according to new research presented at the American Heart Association Scientific Sessions 2019.
An analysis of pooled data on nearly 44,000 participants in a cross-sectional survey showed that, among the 13.6% who reported using marijuana within the last 30 days, the adjusted odds ratio for young-onset stroke (aged 18-44 years), compared with non-users, was 2.75, reported Tarang Parekh, MBBS, a health policy researcher of George Mason University in Fairfax, Va., and colleagues.
In a separate study, a retrospective analysis of national inpatient data showed that people diagnosed with cannabis use disorder – a pathological pattern of impaired control, social impairment, risky behavior or physiological adaptation similar in nature to alcoholism – had a 47%-52% increased likelihood of hospitalization for an arrhythmia, reported Rikinkumar S. Patel, MD, a psychiatry resident at Griffin Memorial Hospital in Norman, Okla.
“As these [cannabis] products become increasingly used across the country, getting clearer, scientifically rigorous data is going to be important as we try to understand the overall health effects of cannabis,” said AHA President Robert Harrington, MD, of Stanford (Calif.) University in a statement.
Currently, use of both medical and recreational marijuana is fully legal in 11 U.S. states and the District of Columbia. Medical marijuana is legal with recreational use decriminalized (or penalties reduced) in 28 other states, and totally illegal in 11 other states, according to employee screening firm DISA Global Solutions.
Stroke study
In an oral presentation with simultaneous publication in the AHA journal Stroke, Dr. Parekh and colleagues presented an analysis of pooled data from the Behavioral Risk Factor Surveillance System (BRFSS), a nationally representative cross-sectional survey collected by the Centers for Disease Control and Prevention in 2016 and 2017.
They looked at baseline sociodemographic data and created multivariable logistic regression models with state fixed effects to determine whether marijuana use within the last 30 days was associated with young-onset stroke.
They identified 43,860 participants representing a weighted sample of 35.5 million Americans. Of the sample, 63.3% were male, and 13.6 % of all participants reported using marijuana in the last 30 days.
They found in an unadjusted model that marijuana users had an odds ratio for stroke, compared with nonusers, of 1.59 (P less than.1), and in a model adjusted for demographic factors (gender, race, ethnicity, and education) the OR increased to 1.76 (P less than .05).
When they threw risk behavior into the model (physical activity, body mass index, heavy drinking, and cigarette smoking), they saw that the OR for stroke shot up to 2.75 (P less than .01).
“Physicians should ask patients if they use cannabis and counsel them about its potential stroke risk as part of regular doctor visits,” Dr. Parekh said in a statement.
Arrhythmias study
Based on recent studies suggesting that cannabis use may trigger cardiovascular events, Dr. Patel and colleagues studied whether cannabis use disorder may be related to arrhythmias, approaching the question through hospital records.
“The effects of using cannabis are seen within 15 minutes and last for around 3 hours. At lower doses, it is linked to a rapid heartbeat. At higher doses, it is linked to a too-slow heartbeat,” he said in a statement.
Dr. Patel and colleagues conducted a retrospective analysis of the Nationwide Inpatient Sample from 2010-2014, a period during which medical marijuana became legal in several states and recreational marijuana became legal in Colorado and Washington. The sample is a database maintained by the Healthcare Cost and Utilization Project of the U.S. Office of Disease Prevention and Health Promotion.
They identified 570,557 patients aged 15-54 years with a primary diagnosis of arrhythmia, and compared them with a sample of 67,662,082 patients hospitalized with no arrhythmia diagnosed during the same period.
They found a 2.6% incidence of cannabis use disorder among patients hospitalized for arrhythmias. Patients with cannabis use disorder tended to be younger (15- to 24-years-old; OR, 4.23), male (OR, 1.70) and African American (OR, 2.70).
In regression analysis adjusted for demographics and comorbidities, cannabis use disorder was associated with higher odds of arrhythmia hospitalization in young patients, at 1.28 times among 15- to 24-year-olds (95% confidence interval, 1.229-1.346) and 1.52 times for 25- to 34-year-olds (95% CI, 1.469-1.578).
“As medical and recreational cannabis is legalized in many states, it is important to know the difference between therapeutic cannabis dosing for medical purposes and the consequences of cannabis abuse. We urgently need additional research to understand these issues,” Dr. Patel said.
“It’s not proving that there’s a direct link, but it’s raising a suggestion in an observational analysis that [this] indeed might be the case. What that means for clinicians is that, if you’re seeing a patient who is presenting with a symptomatic arrhythmia, adding cannabis usage to your list of questions as you begin to try to understand possible precipitating factors for this arrhythmia seems to be a reasonable thing to do,” Dr. Harrington commented.
REPORTING FROM AHA 2019
Aspirin for primary prevention reduces risk of CV events, increases bleeding
Background: Aspirin is beneficial in secondary prevention of stroke and MI. There is no consensus on its role in primary prevention of the same.
Study design: Systematic review and meta-analysis.
Setting: PubMed and Embase search on Cochrane from the earliest publication available through Nov. 1, 2018.
Synopsis: This meta-analysis included randomized, controlled trials that compared aspirin use versus no aspirin use in more than 1,000 participants without known cardiovascular (CV) disease. The primary CV outcome was a composite of CV mortality, nonfatal MI, and nonfatal stroke. The primary bleeding outcome was major bleeding (defined by individual studies). Thirteen studies enrolling 164,225 participants and including 1,050,511 participant-years were included. Compared with no aspirin use, aspirin use showed a reduction in composite CV outcomes (hazard ratio, 0.89; 95% confidence interval, 0.84-0.95; number needed to treat, 265) and an increased risk of major bleeding (HR, 1.43; 95% CI, 1.30-1.56; number needed to harm, 210). Limitations of the study include variations in data quality, outcome definitions, and aspirin doses among trials. The study authors advocate for including the lower risk of CV events and increased risk of major bleeding as part of discussions with patients about the use of aspirin for primary prevention.
Bottom line: Aspirin for primary prevention lowers risk of CV events and increases risk of major bleeding. Health care providers should include this as part of informed decision-making discussions with patients about the use of aspirin for primary prevention.
Citation: Zheng S et al. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: A systematic review and meta-analysis. JAMA. 2019 Jan 22;321(3):277-87.
Dr. Radhakrishnan is a hospitalist at Beth Israel Deaconess Medical Center.
Background: Aspirin is beneficial in secondary prevention of stroke and MI. There is no consensus on its role in primary prevention of the same.
Study design: Systematic review and meta-analysis.
Setting: PubMed and Embase search on Cochrane from the earliest publication available through Nov. 1, 2018.
Synopsis: This meta-analysis included randomized, controlled trials that compared aspirin use versus no aspirin use in more than 1,000 participants without known cardiovascular (CV) disease. The primary CV outcome was a composite of CV mortality, nonfatal MI, and nonfatal stroke. The primary bleeding outcome was major bleeding (defined by individual studies). Thirteen studies enrolling 164,225 participants and including 1,050,511 participant-years were included. Compared with no aspirin use, aspirin use showed a reduction in composite CV outcomes (hazard ratio, 0.89; 95% confidence interval, 0.84-0.95; number needed to treat, 265) and an increased risk of major bleeding (HR, 1.43; 95% CI, 1.30-1.56; number needed to harm, 210). Limitations of the study include variations in data quality, outcome definitions, and aspirin doses among trials. The study authors advocate for including the lower risk of CV events and increased risk of major bleeding as part of discussions with patients about the use of aspirin for primary prevention.
Bottom line: Aspirin for primary prevention lowers risk of CV events and increases risk of major bleeding. Health care providers should include this as part of informed decision-making discussions with patients about the use of aspirin for primary prevention.
Citation: Zheng S et al. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: A systematic review and meta-analysis. JAMA. 2019 Jan 22;321(3):277-87.
Dr. Radhakrishnan is a hospitalist at Beth Israel Deaconess Medical Center.
Background: Aspirin is beneficial in secondary prevention of stroke and MI. There is no consensus on its role in primary prevention of the same.
Study design: Systematic review and meta-analysis.
Setting: PubMed and Embase search on Cochrane from the earliest publication available through Nov. 1, 2018.
Synopsis: This meta-analysis included randomized, controlled trials that compared aspirin use versus no aspirin use in more than 1,000 participants without known cardiovascular (CV) disease. The primary CV outcome was a composite of CV mortality, nonfatal MI, and nonfatal stroke. The primary bleeding outcome was major bleeding (defined by individual studies). Thirteen studies enrolling 164,225 participants and including 1,050,511 participant-years were included. Compared with no aspirin use, aspirin use showed a reduction in composite CV outcomes (hazard ratio, 0.89; 95% confidence interval, 0.84-0.95; number needed to treat, 265) and an increased risk of major bleeding (HR, 1.43; 95% CI, 1.30-1.56; number needed to harm, 210). Limitations of the study include variations in data quality, outcome definitions, and aspirin doses among trials. The study authors advocate for including the lower risk of CV events and increased risk of major bleeding as part of discussions with patients about the use of aspirin for primary prevention.
Bottom line: Aspirin for primary prevention lowers risk of CV events and increases risk of major bleeding. Health care providers should include this as part of informed decision-making discussions with patients about the use of aspirin for primary prevention.
Citation: Zheng S et al. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: A systematic review and meta-analysis. JAMA. 2019 Jan 22;321(3):277-87.
Dr. Radhakrishnan is a hospitalist at Beth Israel Deaconess Medical Center.
Heart Failure in Older Adults: A Geriatrician Call for Action (FULL)
As the population ages, heart failure is becoming a major public health challenge; clinicians need further evidence-based treatments to bridge the existing gap between guidelines and real-world clinical practice.
In 2050, persons aged ≥ 85 years, also known as the oldest old, are projected to reach 18 million, accounting for 4.5% of the US population, up from 2.5% in 2030.1 These patients are the fastest growing segment of the US population.
Advances in treating cardiovascular (CV) disease over the past 2 decades have led to an increased incidence of heart failure (HF) and hospitalizations among older patients.2 Total costs of care for persons with HF have exceeded $30 billion annually and are expected to rise to more than $70 billion by 2030 due to growth of the aging population.3,4 Moreover, the Framingham Study reported mortality increases with advancing age (HR 1.27 and 1.61 per decade in men and women, respectively).5
The prevalence of HF is also high and increasing over time. The National Health and Nutrition Examination Survey reported that about 5.7 million Americans have HF.6 The prevalence of HF is expected to reach 8 million by 2030.6 The higher numbers of HF among patients with advanced age is associated with age-related changes in CV structure and function, including reduced responsiveness to β-adrenergic stimulation, impaired left ventricular diastolic filling, and increased vascular stiffness. In addition, age-related changes in other systems might contribute to a HF diagnosis or worsening of the condition.7
Older adults experience physiologic changes in pharmacokinetics and pharmacodynamics, including decreased volume of distribution and creatinine clearance, which lead to significant changes in drug concentration and effectiveness.8
Geriatric patients aged > 65 years who have comorbidities and those who reside in long-term care settings are underrepresented in clinical trials, leading clinicians to make treatment decisions based on data from younger, community-dwelling individuals. Researchers have questioned whether to include elderly patients and those with comorbidities in clinical trials, given that their diminished response may produce less conclusive results with smaller treatment effects. Exclusion criteria based on comorbid conditions or functional status disqualify many older adults from clinical trials.
This article reviews evidence from major randomized controlled trials over the past 2 decades and explores their applicability to support HF treatment guidelines in patients with advanced age (Table).
Pharmacotherapy for Heart Failure
Angiotensin-Converting Enzyme Inhibitors
Several randomized clinical trials have found that angiotensin-converting enzyme (ACE) inhibitors improve symptoms in patients with HF. The CooperativeNorth Scandinavian Enalapril Survival Study (CONSENSUS), demonstrated that enalapril improves survival in patients with New York Heart Association (NYHA) class IV HF with reduced ejection fraction (HFrEF) when added to standard therapy.9 However, the duration of beneficial effect of reduced mortality could not be assessed because the benefit of enalapril in NYHA class I to III HF was not evaluated, and follow-up data are limited. The average age of patients in the study was 71 years, and individuals with significant comorbidities were excluded.
ACE inhibitors also were found to reduce mortality even in asymptomatic patients with HFrEF in the Studies of Left Ventricular Dysfunction trial (SOLVD).10 Enalapril was found to reduce 4-year mortality by 16% and decrease HF hospitalizations when added to conventional therapy consisting primarily of digitalis, diuretics, and nitrates in patients with HFrEF. In this trial, patients aged ≥ 80 years were excluded as well as those with serum creatinine > 2 mg/dL or other conditions that could shorten survival or otherwise impede participation in a long-term trial.
PARADIGM-HF trial patients with HFrEF were randomized to enalapril or the angiotensin receptor-neprilysin inhibitor LCZ696. After a median of 27 months of follow-up, treatment with the angiotensin receptor-neprilysin inhibitor demonstrated greater reduction in CV mortality and HF hospitalizations than enalapril did and was associated with reduced all-cause mortality.11 The trial was stopped early because of evidence of overwhelming benefit with LCZ696. This study of mainly white men included no patients aged ≥ 75 years.
Angiotensin Receptor Blockers
Although less studied than ACE inhibitors, angiotensin receptor blockers (ARBs) share similar benefits. Among patients with symptomatic HFrEF taking an ACE inhibitor, the addition of candesartan reduced the risk of CV death and HF hospitalization as demonstrated in the Candesartan in Heart Failure Assessment of Reduction Mortality and Morbidity (CHARM-added and CHARM-alternative trials).12,13 The CHARM-added trial targeted patients with left ventricular ejection fraction (LVEF) ≤ 40% and NYHA class II to IV HF symptoms who were taking an ACE inhibitor. Adding candesartan reduced CV mortality by 37.9% and HF hospitalization by 42.3% compared with that of placebo.
The CHARM-alternative study found that use of candesartan in symptomatic HFrEF patients who do not tolerate ACE inhibitors,resulted in a 20% reduction in CV mortality as well as a 40% reduction in hospitalization for HF. Among patients with HF with preserved ejection fraction (HFpEF) and NYHA class II to IV symptoms, adding candesartan modestly reduced the rate of HF-related hospitalizations and had no effect on CV mortality in the CHARM-preserved study.14 The CHARM trials examined mostly white men, but 26% of patients were aged > 75 years. However, there was no subgroup analysis for patients aged > 75 years. The study excluded patients with serum creatinine > 2 mg/dL.
Other ARB trials included the following:
- The I-PRESERVE trial, which found that irbesartan did not improve outcomes of patients with HF with preserved ejection fraction (HFpEF).15 The study of mostly white patients did not include patients aged ≥ 80 years.
- A randomized trial of valsartan in HF improved symptoms and mortality in NYHA II to IV HF but showed no benefit when added to ACE inhibitors.16 The trial had no patients aged ≥ 75 years and excluded those with several common comorbidities.
- A randomized, double-blind trial studied the effects of high-dose vs low-dose losartan on clinical outcomes in 3,846 patients with HF and demonstrated that high-dose losartan (150 mg/d) reduces all-cause mortality and hospitalization for HF more effectively than does low-dose losartan (50 mg/d).17 The study, however, had several exclusion criteria, and no patients were aged ≥ 75 years.
Mineralocorticoid Receptor Antagonists
Major studies of aldosterone antagonists demonstrated extra benefit when added to ACE inhibitors/ARBs in patients with HFrEF and NYHA class II HF.18,19
In the RALES study, spironolactone was found to reduce all-cause mortality by 30% and symptoms in NYHA III HF without a significant increase in the risk of serious hyperkalemia or renal failure.18 Most patients were white men aged < 80 years. This study demonstrated the importance of closely following serum potassium levels after initiating aldosterone antagonists in patients with subclinical renal disease because extensive structural damage within the kidney occurs before serum creatinine increases. Patients with advanced renal failure or those who cannot have close monitoring of serum potassium levels have an unfavorable risk–benefit ratio with aldosterone antagonists. Patients with cancer and liver failure were excluded from this trial.
In the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure study, (EMPHASIS-HF Study) eplerenone was found to reduce all-cause mortality and hospitalization for HFrEF.19 Similar to RALES, patients were mostly white males aged < 80 years, and patients with clinically significant, coexisting conditions were excluded.
The 2014 Treatment of Preserved Cardiac FunctionHeart Failure with an Aldosterone Antagonist Trial (TOPCAT) randomized 3,445 patients with well-controlled blood pressure to spironolactone or placebo.20 Inclusion criteria were LVEF ≥ 45%, findings of HF, and either a HF hospitalization or elevated B-type natriuretic peptide level. There was no difference in the primary composite outcome of CV mortality, aborted cardiac arrest, or HF hospitalization over the 3.3-year follow-up period. The study found that among patients with HFpEF, spironolactone does not reduce the composite endpoint of CV mortality, aborted cardiac arrest, or HF hospitalizations compared with that of placebo.20 In the trial, 29% of patients were aged > 75 years, and most were white men. There was no subgroup analysis for older patients.20 In all 3 trials, patients with kidney injury (serum creatinine of ≥ 2.5 or estimated glomerular filtration rate of ≤ 30 mL/min) were excluded because of the risk of hyperkalemia.
An observational study after the RALES trial demonstrated a nearly 4-fold increase in admissions for hyperkalemia with a 6-fold increase in associated mortality in patients taking spirolactone.21 Therefore, it is important to closely follow serum potassium levels after initiating aldosterone antagonists in older patients with subclinical renal disease. Patients with advanced renal failure or those without close monitoring of serum potassium levels have an unfavorable risk–benefit ratio with aldosterone antagonists.
Antithrombotic Therapy
The large multicenter, double-blind randomized trial WARCEF found no added benefit with warfarin vs aspirin for patients with HFrEF in sinus rhythm.22 There was no reduced time to first stroke or death, and the reduced ischemic stroke risk was offset by an increase in major hemorrhage. It is not clear whether subgroup analysis for the etiology of patients’ HF was performed in WARCEF.
The Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial (N = 1,587) found that treatment with warfarin resulted in significantly fewer strokes in patients with ischemic cardiomyopathy.23 Randomization was not stratified by age group in both trials, and baseline characteristics included mostly white men, and no patients were older than aged > 75 years.
The risk of bleeding with prophylactic aspirin use for CV disease is dose dependent and increases with higher aspirin doses.24 The use of aspirin, 325 mg/d, in the WARCEF study might have contributed to the increased risk of hemorrhage.
Recently published results of COMMANDER HF found that the addition of rivaroxaban at a dose of 2.5 mg twice daily to standard care, including clinically selected antiplatelet therapies was not associated with a significantly lower rate of the composite primary outcome composite outcome of death, myocardial infarction (MI), or stroke among 5,022 patients with a recent episode of worsening heart failure compared with that of placebo.25
Several medical conditions are known to increase bleeding risk, including hypertension, cerebrovascular disease, ischemic stroke, serious heart disease, diabetes mellitus, renal insufficiency, alcoholism, liver disease, and falls.26 Many of these conditions are common among very old patients and should be considered when estimating risk–benefit ratio of oral anticoagulation therapy.
β-blockers
In several large studies, β-blockers have been shown to be effective in reducing mortality in patients with HFrEF. In the Cardiac Insufficiency Bisoprolol Study II, bisoprolol improved all-cause mortality and all-cause hospitalizations, and reduced sudden death in patients with NYHA III or IV HF.27 In the Carvedilol or Metoprolol European Trial (COMET), carvedilol was superior to metoprolol in reducing all-cause mortality for patients with NYHA II or IV HF.28 Both trials included mostly white men; patients with several comorbidities were excluded, and no patients were aged > 80 years.
COMET compared carvedilol with metoprolol tartrate, the short-acting form of metoprolol that has not shown a survival benefit for patients with HF. However, the Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure trial demonstrated survival benefits with metoprolol CR/XL and included patients aged > 80 years.29
In the SENIORS study, patients treated with nebivolol had a 4.2% absolute risk reduction in a composite of mortality or hospital admission at a mean follow-up of 21 months.30 It is reasonable to use nebivolol for managing HF in older patients. Careful monitoring of heart rate is necessary when prescribing β-blockers for older patients.
Cardiac Glycosides
Digoxin with diuretics was the first-line treatment for HF for many decades and the mainstay of HF therapy until the first large HF trials were performed in the 1980s. One trial initiated by the Digoxin Investigation Group (DIG) studied patients with HFrEF who were already receiving treatment for HF (including 94% taking ACE inhibitors and 82% on diuretics) and randomized them to either digoxin or placebo.31 The study found no significant difference in mortality between the groups at the 3-year follow-up; however, the digoxin group had significantly fewer hospitalizations compared with that of the placebo group.
A post-hoc analysis of patients by age found no difference in mortality between patients aged 70 to 79 years and those ≥ 80 years, with a persistent benefit in fewer hospitalizations. Digoxin continues to be recommended as a reasonable medication for treating symptomatic HFrEF. However, caution is advised in older patients, especially women, who are at higher risk of digoxin toxicity.
No current evidence exists that digoxin adds any benefits for patients with HFpEF of any age and therefore, it should not be used.
Diuretics
Diuretic therapy is important for managing shortness of breath and congestion related to fluid volume overload in patients with HF. Although diuretics have not been shown to reduce mortality in patients with HF, they are the mainstay treatment for patients with HFpEF.32 In a post-hoc analysis of the DIG study, diuretic use was associated with increased risk of mortality and hospitalizations in patients aged > 65 years.33 Hyponatremia is one of the most serious adverse effects (AEs) with these agents and occurs in about one-fifth of elderly patients taking diuretics.
In severe cases hyponatremia can cause a range of problems, including weakness, confusion, postural giddiness, postural hypotension, falls, transient hemiparesis, and seizures. In older patients with diminished renal reserve, diuretics are more likely to precipitate prerenal uremia than it does in younger patients. Prerequisites for diuretic use are an accurate diagnosis, careful monitoring of blood pressure and serum electrolytes, and regular review of their efficacy, AEs, and the need for continued treatment.
Statins
The Controlled Rosuvastatin Multinational Trial in Heart Failure demonstrated that low-dose rosuvastatin (10 mg/d) does not improve survival among patients with moderate-to-severe ischemic cardiomyopathy but could reduce the rate of CV hospitalizations.34 Patients in this study had a mean age of 73 years, and 41% of them were aged ≥ 75 years. However, the study used a low-dose rosuvastatin, and patients with several common comorbidities were excluded. Evidence exists that treatment with other statins may improve outcomes in patients with HF. There is also evidence that among elderly patients with HF, low serum total cholesterol is independently associated with a worse prognosis.35
Comorbidities
Anemia
In patients with iron-deficiency anemia (ferritin 15-100 ng/mL or 100-299 ng/mL with transferrin saturation < 20%) and symptomatic HFrEF (LVEF ≤ 40% with NYHA II to IV HF), oral iron replacement had no effect on exercise capacity as measured using change in peak oxygen uptake.36 However, IV iron replacement might be a reasonable option to improve functional status and quality of life (QOL) for patients with HF.37 In these studies, participants were aged < 75 years, and there is no evidence that treating other types of anemia improves outcomes in patients with HF.
Hypertension
The Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated that controlling blood pressure to a goal systolic pressure of < 120 mm Hg is associated with significant reduction in the mortality among patients with increased CV risk (aged > 75 years, vascular disease, kidney injury, or a Framingham Risk Score >15%).38 The SPRINT study included patients aged > 75 (25%); however, the study excluded older adults living in nursing homes and those with diabetes mellitus, symptomatic HF, dementia, or stroke. The subgroup analysis did not stratify patients based on age nor provided sufficient evidence regarding treatment targets for this vulnerable population. Therefore, clinicians cannot draw any conclusions about managing hypertension among patients with HF from this study.
Sleep Apnea
Sleep apnea is common among patients with HF. A study of adults with chronic HF treated with evidence-based therapies found that 61% of participants had central or obstructive sleep apnea.39 In elderly patients, sleep apnea is further complicated by insomnia and disturbance of sleep cycle that often occur with the aging process.
It is crucial to differentiate central sleep apnea from obstructive sleep apnea, because the treatment approaches differ. Central sleep apnea is associated with poor prognosis in patients with HF.40 Adaptive servo ventilation for central sleep apnea uses a noninvasive ventilator to delivering servo controlled inspiratory pressure support on top of expiratory positive airway pressure. Adaptive servo ventilation for central sleep apnea is associated with higher all-cause mortality and CV mortality.41 Continuous positive airway pressure for obstructive sleep apnea improves sleep quality, reduces the apnea-hypopnea index, and improves nocturnal oxygenation.42
Depression
Clinically significant depression occurs in 21% of patients with HF, and the relationship between depression and poor HF outcomes is consistent and strong across several endpoints. However, in a randomized, 12-week study, the selective serotonin reuptake inhibitor sertraline did not improve depression symptoms or clinical status among patients with HF.43 Depression symptoms might overlap with fatigue and low energy expenditure experienced by oldest old patients with HF who do not have depression.
Furthermore, studies describing depression treatments among patients with HF are too small and heterogeneous to permit definitive conclusions about intervention effectiveness. These results identify areas requiring further development, raise questions regarding the association between depression and clinical outcomes in patients with HF, and provide information on depression prevalence that may help researchers design studies with appropriate depression measures and adequately powered sample sizes.
Frailty
Although frailty is prevalent in the elderly and is independently associated with poor outcomes, there is no standardized definition for frailty. The Fried Frailty Index is a widely used scale that incorporates criteria including weakness, slowness, exhaustion, and low physical activity in the diagnosis of frailty.44 However these symptoms are common among patients with advanced HF with and without depression or frailty.
Frailty should be defined collaboratively by the clinician and the patient and should include multidimensional aspects of health, function, and well-being. The treatment goal for patients with HF with frailty is to establish patient-centered goals based on preferences of care.45
Discussion
Although several novel approaches to improve outcomes of patients with HF have been developed, it continues to be the leading cause of cardiovascular death among older patients and the leading cause of hospital admissions.46 About 50% of newly diagnosed patients with HF die within 5 years.47 Current guidelines for managing HF are based on clinical trials that either include few or completely exclude patients aged > 80 years, minorities, and patients with comorbidities clinicians encounter daily in clinical practice.
Furthermore, most clinical trials are designed with mortality as the primary endpoint, which might be as important to our patients with advanced age as their ability to function with a reasonable QOL and less dependence on caregivers.
Decision making in managing HF in our oldest patients should start with an open discussion of the disease and its prognosis, goals of care, and available treatment options. The discussion should also cover all dimensions of suffering, including physical, spiritual, and psychosocial domains. Interviews of patients dying of HF and their caregivers conducted in the United Kingdom identified several communication and transition of care challenges specific to treating this population.48 The study revealed in most cases, patients did not recall receiving any written information about the severity of their disease and often did not understand the association among symptoms, such as shortness of breath, edema, and HF. Patients and caregivers did not feel involved in the decision-making process regarding their illness.
The concurrent presence of comorbidity, frailty, and cognitive impairment in our aging population with HF might add to the burden of the primary condition. Care often is perceived as fragmented. Polypharmacy negatively impacts HF management by increasing risk of drug nonadherence, drug interactions, and AEs in an already vulnerable population. There is a need for more effective interpersonal and easy to understand communication and resources.
In many situations, support services might be best facilitated by a dedicated palliative medicine team with significant experience in managing patients with HF.Although palliative medicine should always be considered for patients with HF with advanced age,consultations often are not obtained unless the patient decides to forgo medical treatment or until the last month of life.49
Although not all end-of-life symptoms can realistically be palliated, earlier involvement of multidisciplinary palliative medicine specialists may improve symptom control, functional status, and QOL. The team may help patients and caregivers cope with uncertainty, and make informed decisions that are person centered based on value system and beliefs.51
Conclusion
Randomized control trials as well as thoughtful observational studies of HF in patients with advanced age and comorbidities, although challenging, are needed to create the evidence base for treatment interventions and assessing their impact on mortality, morbidity, and QOL in this rapidly growing segment of our population.
Given the lack of evidence for HF treatment in patients with advanced age, the clinician should weigh the knowledge of the effect of aging on the CV system, and the lived experience of patients with HF, with the evidence that exists for making the best decision to relieve bothersome symptoms and improve outcomes of care as determined by patients and their caregivers.
Often the most important intervention we can offer our patients, especially those nearing the end of life, is dedicating our time to truly and actively listen with empathy, understating, and respect for their autonomy and for their decision making. And in doing so we accept our own limitations with humility.
Acknowledgments
Dr. Kheirbek received funds from the Veterans Affairs Capitol Health Care Network to establish the Center for Health and Aging at the Washington DC VA Medical Center.
1. Ortman JM, Velkoff AV, Hogan H. An aging nation: the older population in the United States. https://www.census.gov/prod/2014pubs/p25-1140.pdf. Published May 2014. Accessed September 30, 2018.
2. Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;52(6):428-434.
3. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619.
4. National Heart, Lung, and Blood Institute, National Institutes of Health. Incidence and Prevalence: 2006 Chart Book on Cardiovascular and Lung Diseases. Bethesda, MD: National Institutes of Health; 2006.
5. Curtis LH, Whellan DJ, Hammill BG, et al. Incidence and prevalence of heart failure in elderly persons, 1994-2003. Arch Intern Med. 2008;168(4):418-424.
6. Writing Group, Mozaffarian D, Benjamin EJ, et al; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e360.
7. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107(1):139-146.
8. Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6-14.
9. CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316(23):1429-1435.
10. SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB Jr, Cohn JN. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327(10):685-691.
11. McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004.
12. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362(9386):767-771.
13. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362(9386):772-776.
14. Yusuf S, Pfeffer MA, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362(9386):777-781.
15. Massie BM, Carson PE, McMurray JJ, et al; I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456-2467.
16. Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345(23):1667-1675.
17. Konstam MA, Neaton JD, Dickstein K, et al; HEAAL Investigators. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374(9704):1840-1848.
18. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709-717.
19. Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11-21.
20. Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383-1392.
21. Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351(6):543-551.
22. Homma S, Thompson JL, Pullicino PM, et al; WARCEF Investigators. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366(20):1859-1869.
23. Massie BM, Collins JF, Ammon SE, et al; WATCH Trial Investigators. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation. 2009;119(12):1616-1624.
24. Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA. 2007;297(18):2018-2024.
25. Zannad F, Anker, SD, Byra WM, et al; COMMANDER HF Investigators. Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N Engl J Med. 2018;379(14):1332-1342.
26. Schulman S, Beyth RJ, Kearon C, Levine MN. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):257S-298S.
27. CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9-13.
28. Poole-Wilson PA, Swedberg K, Cleland JG, et al; Carvedilol Or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomized controlled trial. Lancet. 2003;362(9377):7-13.
29. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353(9169):2001-2007.
30. Flather MD, Shibata MC, Coats AJ, et al; SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26(3):215-225.
31. Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336(8):525-533.
32. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239.
33 Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351(6):543-551.
34. Kjekshus J, Apetrei E, Barrios V, et al; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357(22):2248-2261.
35. Rauchhaus M, Clark AL, Doehner W, et al. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol. 2003;42(11):1933-1940.
36. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137-e161.
37. Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36(11):657-668.
38. SPRINT Research Group, Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116.
39. MacDonald M, Fang J, Pittman SD, White DP, Malhotra A. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med. 2008;4(1):38-42.
40. Bradley TD, Floras JS. Sleep Apnea and heart failure: part II: Central sleep apnea. Circulation. 2003;107(13):1822-1826.
41. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-1105.
42. McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931.
43. O’Connor CM, Jiang W, Kuchibhatla M, et al; SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692-699.
44. Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-156.
45. Pilotto A, Addante F, Franceschi M, et al. Multidimensional Prognostic Index based on a comprehensive geriatric assessment predicts short-term mortality in older patients with heart failure. Circ Heart Fail. 2010;3(1):14-20.
46. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
47. Goldberg, RJ, Ciampa, J, Lessard D,, et al. Long-term survival after heart failure: a contemporary population-based perspective. Arch Intern Med. 2007;167(5):490-496.
48. Murray SA, Boyd K, Kendall M, Worth A, Benton TF, Clausen H. Dying of lung cancer or cardiac failure: prospective qualitative interview study of patients and their carers in the community. BMJ. 2002;325(7370):929.
49. Gibbs JS, McCoy AS, Gibbs LM, Rogers AE, Addington-Hall JM. Living with and dying from heart failure: the role of palliative care. Heart. 2002;88(suppl 2):ii36-39.
50. Quill TE, Dresser R, Brock DW. The rule of double effect—a critique of its role in end-of-life decision making. N Engl J Med. 1997;337(24):1768-1771.
51. Nieminen MS, Dickstein K, Fonseca C, et al. The patient perspective: quality of life in advanced heart failure with frequent hospitalizations. Int J Cardiol. 2015;191:256-264.
As the population ages, heart failure is becoming a major public health challenge; clinicians need further evidence-based treatments to bridge the existing gap between guidelines and real-world clinical practice.
As the population ages, heart failure is becoming a major public health challenge; clinicians need further evidence-based treatments to bridge the existing gap between guidelines and real-world clinical practice.
In 2050, persons aged ≥ 85 years, also known as the oldest old, are projected to reach 18 million, accounting for 4.5% of the US population, up from 2.5% in 2030.1 These patients are the fastest growing segment of the US population.
Advances in treating cardiovascular (CV) disease over the past 2 decades have led to an increased incidence of heart failure (HF) and hospitalizations among older patients.2 Total costs of care for persons with HF have exceeded $30 billion annually and are expected to rise to more than $70 billion by 2030 due to growth of the aging population.3,4 Moreover, the Framingham Study reported mortality increases with advancing age (HR 1.27 and 1.61 per decade in men and women, respectively).5
The prevalence of HF is also high and increasing over time. The National Health and Nutrition Examination Survey reported that about 5.7 million Americans have HF.6 The prevalence of HF is expected to reach 8 million by 2030.6 The higher numbers of HF among patients with advanced age is associated with age-related changes in CV structure and function, including reduced responsiveness to β-adrenergic stimulation, impaired left ventricular diastolic filling, and increased vascular stiffness. In addition, age-related changes in other systems might contribute to a HF diagnosis or worsening of the condition.7
Older adults experience physiologic changes in pharmacokinetics and pharmacodynamics, including decreased volume of distribution and creatinine clearance, which lead to significant changes in drug concentration and effectiveness.8
Geriatric patients aged > 65 years who have comorbidities and those who reside in long-term care settings are underrepresented in clinical trials, leading clinicians to make treatment decisions based on data from younger, community-dwelling individuals. Researchers have questioned whether to include elderly patients and those with comorbidities in clinical trials, given that their diminished response may produce less conclusive results with smaller treatment effects. Exclusion criteria based on comorbid conditions or functional status disqualify many older adults from clinical trials.
This article reviews evidence from major randomized controlled trials over the past 2 decades and explores their applicability to support HF treatment guidelines in patients with advanced age (Table).
Pharmacotherapy for Heart Failure
Angiotensin-Converting Enzyme Inhibitors
Several randomized clinical trials have found that angiotensin-converting enzyme (ACE) inhibitors improve symptoms in patients with HF. The CooperativeNorth Scandinavian Enalapril Survival Study (CONSENSUS), demonstrated that enalapril improves survival in patients with New York Heart Association (NYHA) class IV HF with reduced ejection fraction (HFrEF) when added to standard therapy.9 However, the duration of beneficial effect of reduced mortality could not be assessed because the benefit of enalapril in NYHA class I to III HF was not evaluated, and follow-up data are limited. The average age of patients in the study was 71 years, and individuals with significant comorbidities were excluded.
ACE inhibitors also were found to reduce mortality even in asymptomatic patients with HFrEF in the Studies of Left Ventricular Dysfunction trial (SOLVD).10 Enalapril was found to reduce 4-year mortality by 16% and decrease HF hospitalizations when added to conventional therapy consisting primarily of digitalis, diuretics, and nitrates in patients with HFrEF. In this trial, patients aged ≥ 80 years were excluded as well as those with serum creatinine > 2 mg/dL or other conditions that could shorten survival or otherwise impede participation in a long-term trial.
PARADIGM-HF trial patients with HFrEF were randomized to enalapril or the angiotensin receptor-neprilysin inhibitor LCZ696. After a median of 27 months of follow-up, treatment with the angiotensin receptor-neprilysin inhibitor demonstrated greater reduction in CV mortality and HF hospitalizations than enalapril did and was associated with reduced all-cause mortality.11 The trial was stopped early because of evidence of overwhelming benefit with LCZ696. This study of mainly white men included no patients aged ≥ 75 years.
Angiotensin Receptor Blockers
Although less studied than ACE inhibitors, angiotensin receptor blockers (ARBs) share similar benefits. Among patients with symptomatic HFrEF taking an ACE inhibitor, the addition of candesartan reduced the risk of CV death and HF hospitalization as demonstrated in the Candesartan in Heart Failure Assessment of Reduction Mortality and Morbidity (CHARM-added and CHARM-alternative trials).12,13 The CHARM-added trial targeted patients with left ventricular ejection fraction (LVEF) ≤ 40% and NYHA class II to IV HF symptoms who were taking an ACE inhibitor. Adding candesartan reduced CV mortality by 37.9% and HF hospitalization by 42.3% compared with that of placebo.
The CHARM-alternative study found that use of candesartan in symptomatic HFrEF patients who do not tolerate ACE inhibitors,resulted in a 20% reduction in CV mortality as well as a 40% reduction in hospitalization for HF. Among patients with HF with preserved ejection fraction (HFpEF) and NYHA class II to IV symptoms, adding candesartan modestly reduced the rate of HF-related hospitalizations and had no effect on CV mortality in the CHARM-preserved study.14 The CHARM trials examined mostly white men, but 26% of patients were aged > 75 years. However, there was no subgroup analysis for patients aged > 75 years. The study excluded patients with serum creatinine > 2 mg/dL.
Other ARB trials included the following:
- The I-PRESERVE trial, which found that irbesartan did not improve outcomes of patients with HF with preserved ejection fraction (HFpEF).15 The study of mostly white patients did not include patients aged ≥ 80 years.
- A randomized trial of valsartan in HF improved symptoms and mortality in NYHA II to IV HF but showed no benefit when added to ACE inhibitors.16 The trial had no patients aged ≥ 75 years and excluded those with several common comorbidities.
- A randomized, double-blind trial studied the effects of high-dose vs low-dose losartan on clinical outcomes in 3,846 patients with HF and demonstrated that high-dose losartan (150 mg/d) reduces all-cause mortality and hospitalization for HF more effectively than does low-dose losartan (50 mg/d).17 The study, however, had several exclusion criteria, and no patients were aged ≥ 75 years.
Mineralocorticoid Receptor Antagonists
Major studies of aldosterone antagonists demonstrated extra benefit when added to ACE inhibitors/ARBs in patients with HFrEF and NYHA class II HF.18,19
In the RALES study, spironolactone was found to reduce all-cause mortality by 30% and symptoms in NYHA III HF without a significant increase in the risk of serious hyperkalemia or renal failure.18 Most patients were white men aged < 80 years. This study demonstrated the importance of closely following serum potassium levels after initiating aldosterone antagonists in patients with subclinical renal disease because extensive structural damage within the kidney occurs before serum creatinine increases. Patients with advanced renal failure or those who cannot have close monitoring of serum potassium levels have an unfavorable risk–benefit ratio with aldosterone antagonists. Patients with cancer and liver failure were excluded from this trial.
In the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure study, (EMPHASIS-HF Study) eplerenone was found to reduce all-cause mortality and hospitalization for HFrEF.19 Similar to RALES, patients were mostly white males aged < 80 years, and patients with clinically significant, coexisting conditions were excluded.
The 2014 Treatment of Preserved Cardiac FunctionHeart Failure with an Aldosterone Antagonist Trial (TOPCAT) randomized 3,445 patients with well-controlled blood pressure to spironolactone or placebo.20 Inclusion criteria were LVEF ≥ 45%, findings of HF, and either a HF hospitalization or elevated B-type natriuretic peptide level. There was no difference in the primary composite outcome of CV mortality, aborted cardiac arrest, or HF hospitalization over the 3.3-year follow-up period. The study found that among patients with HFpEF, spironolactone does not reduce the composite endpoint of CV mortality, aborted cardiac arrest, or HF hospitalizations compared with that of placebo.20 In the trial, 29% of patients were aged > 75 years, and most were white men. There was no subgroup analysis for older patients.20 In all 3 trials, patients with kidney injury (serum creatinine of ≥ 2.5 or estimated glomerular filtration rate of ≤ 30 mL/min) were excluded because of the risk of hyperkalemia.
An observational study after the RALES trial demonstrated a nearly 4-fold increase in admissions for hyperkalemia with a 6-fold increase in associated mortality in patients taking spirolactone.21 Therefore, it is important to closely follow serum potassium levels after initiating aldosterone antagonists in older patients with subclinical renal disease. Patients with advanced renal failure or those without close monitoring of serum potassium levels have an unfavorable risk–benefit ratio with aldosterone antagonists.
Antithrombotic Therapy
The large multicenter, double-blind randomized trial WARCEF found no added benefit with warfarin vs aspirin for patients with HFrEF in sinus rhythm.22 There was no reduced time to first stroke or death, and the reduced ischemic stroke risk was offset by an increase in major hemorrhage. It is not clear whether subgroup analysis for the etiology of patients’ HF was performed in WARCEF.
The Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial (N = 1,587) found that treatment with warfarin resulted in significantly fewer strokes in patients with ischemic cardiomyopathy.23 Randomization was not stratified by age group in both trials, and baseline characteristics included mostly white men, and no patients were older than aged > 75 years.
The risk of bleeding with prophylactic aspirin use for CV disease is dose dependent and increases with higher aspirin doses.24 The use of aspirin, 325 mg/d, in the WARCEF study might have contributed to the increased risk of hemorrhage.
Recently published results of COMMANDER HF found that the addition of rivaroxaban at a dose of 2.5 mg twice daily to standard care, including clinically selected antiplatelet therapies was not associated with a significantly lower rate of the composite primary outcome composite outcome of death, myocardial infarction (MI), or stroke among 5,022 patients with a recent episode of worsening heart failure compared with that of placebo.25
Several medical conditions are known to increase bleeding risk, including hypertension, cerebrovascular disease, ischemic stroke, serious heart disease, diabetes mellitus, renal insufficiency, alcoholism, liver disease, and falls.26 Many of these conditions are common among very old patients and should be considered when estimating risk–benefit ratio of oral anticoagulation therapy.
β-blockers
In several large studies, β-blockers have been shown to be effective in reducing mortality in patients with HFrEF. In the Cardiac Insufficiency Bisoprolol Study II, bisoprolol improved all-cause mortality and all-cause hospitalizations, and reduced sudden death in patients with NYHA III or IV HF.27 In the Carvedilol or Metoprolol European Trial (COMET), carvedilol was superior to metoprolol in reducing all-cause mortality for patients with NYHA II or IV HF.28 Both trials included mostly white men; patients with several comorbidities were excluded, and no patients were aged > 80 years.
COMET compared carvedilol with metoprolol tartrate, the short-acting form of metoprolol that has not shown a survival benefit for patients with HF. However, the Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure trial demonstrated survival benefits with metoprolol CR/XL and included patients aged > 80 years.29
In the SENIORS study, patients treated with nebivolol had a 4.2% absolute risk reduction in a composite of mortality or hospital admission at a mean follow-up of 21 months.30 It is reasonable to use nebivolol for managing HF in older patients. Careful monitoring of heart rate is necessary when prescribing β-blockers for older patients.
Cardiac Glycosides
Digoxin with diuretics was the first-line treatment for HF for many decades and the mainstay of HF therapy until the first large HF trials were performed in the 1980s. One trial initiated by the Digoxin Investigation Group (DIG) studied patients with HFrEF who were already receiving treatment for HF (including 94% taking ACE inhibitors and 82% on diuretics) and randomized them to either digoxin or placebo.31 The study found no significant difference in mortality between the groups at the 3-year follow-up; however, the digoxin group had significantly fewer hospitalizations compared with that of the placebo group.
A post-hoc analysis of patients by age found no difference in mortality between patients aged 70 to 79 years and those ≥ 80 years, with a persistent benefit in fewer hospitalizations. Digoxin continues to be recommended as a reasonable medication for treating symptomatic HFrEF. However, caution is advised in older patients, especially women, who are at higher risk of digoxin toxicity.
No current evidence exists that digoxin adds any benefits for patients with HFpEF of any age and therefore, it should not be used.
Diuretics
Diuretic therapy is important for managing shortness of breath and congestion related to fluid volume overload in patients with HF. Although diuretics have not been shown to reduce mortality in patients with HF, they are the mainstay treatment for patients with HFpEF.32 In a post-hoc analysis of the DIG study, diuretic use was associated with increased risk of mortality and hospitalizations in patients aged > 65 years.33 Hyponatremia is one of the most serious adverse effects (AEs) with these agents and occurs in about one-fifth of elderly patients taking diuretics.
In severe cases hyponatremia can cause a range of problems, including weakness, confusion, postural giddiness, postural hypotension, falls, transient hemiparesis, and seizures. In older patients with diminished renal reserve, diuretics are more likely to precipitate prerenal uremia than it does in younger patients. Prerequisites for diuretic use are an accurate diagnosis, careful monitoring of blood pressure and serum electrolytes, and regular review of their efficacy, AEs, and the need for continued treatment.
Statins
The Controlled Rosuvastatin Multinational Trial in Heart Failure demonstrated that low-dose rosuvastatin (10 mg/d) does not improve survival among patients with moderate-to-severe ischemic cardiomyopathy but could reduce the rate of CV hospitalizations.34 Patients in this study had a mean age of 73 years, and 41% of them were aged ≥ 75 years. However, the study used a low-dose rosuvastatin, and patients with several common comorbidities were excluded. Evidence exists that treatment with other statins may improve outcomes in patients with HF. There is also evidence that among elderly patients with HF, low serum total cholesterol is independently associated with a worse prognosis.35
Comorbidities
Anemia
In patients with iron-deficiency anemia (ferritin 15-100 ng/mL or 100-299 ng/mL with transferrin saturation < 20%) and symptomatic HFrEF (LVEF ≤ 40% with NYHA II to IV HF), oral iron replacement had no effect on exercise capacity as measured using change in peak oxygen uptake.36 However, IV iron replacement might be a reasonable option to improve functional status and quality of life (QOL) for patients with HF.37 In these studies, participants were aged < 75 years, and there is no evidence that treating other types of anemia improves outcomes in patients with HF.
Hypertension
The Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated that controlling blood pressure to a goal systolic pressure of < 120 mm Hg is associated with significant reduction in the mortality among patients with increased CV risk (aged > 75 years, vascular disease, kidney injury, or a Framingham Risk Score >15%).38 The SPRINT study included patients aged > 75 (25%); however, the study excluded older adults living in nursing homes and those with diabetes mellitus, symptomatic HF, dementia, or stroke. The subgroup analysis did not stratify patients based on age nor provided sufficient evidence regarding treatment targets for this vulnerable population. Therefore, clinicians cannot draw any conclusions about managing hypertension among patients with HF from this study.
Sleep Apnea
Sleep apnea is common among patients with HF. A study of adults with chronic HF treated with evidence-based therapies found that 61% of participants had central or obstructive sleep apnea.39 In elderly patients, sleep apnea is further complicated by insomnia and disturbance of sleep cycle that often occur with the aging process.
It is crucial to differentiate central sleep apnea from obstructive sleep apnea, because the treatment approaches differ. Central sleep apnea is associated with poor prognosis in patients with HF.40 Adaptive servo ventilation for central sleep apnea uses a noninvasive ventilator to delivering servo controlled inspiratory pressure support on top of expiratory positive airway pressure. Adaptive servo ventilation for central sleep apnea is associated with higher all-cause mortality and CV mortality.41 Continuous positive airway pressure for obstructive sleep apnea improves sleep quality, reduces the apnea-hypopnea index, and improves nocturnal oxygenation.42
Depression
Clinically significant depression occurs in 21% of patients with HF, and the relationship between depression and poor HF outcomes is consistent and strong across several endpoints. However, in a randomized, 12-week study, the selective serotonin reuptake inhibitor sertraline did not improve depression symptoms or clinical status among patients with HF.43 Depression symptoms might overlap with fatigue and low energy expenditure experienced by oldest old patients with HF who do not have depression.
Furthermore, studies describing depression treatments among patients with HF are too small and heterogeneous to permit definitive conclusions about intervention effectiveness. These results identify areas requiring further development, raise questions regarding the association between depression and clinical outcomes in patients with HF, and provide information on depression prevalence that may help researchers design studies with appropriate depression measures and adequately powered sample sizes.
Frailty
Although frailty is prevalent in the elderly and is independently associated with poor outcomes, there is no standardized definition for frailty. The Fried Frailty Index is a widely used scale that incorporates criteria including weakness, slowness, exhaustion, and low physical activity in the diagnosis of frailty.44 However these symptoms are common among patients with advanced HF with and without depression or frailty.
Frailty should be defined collaboratively by the clinician and the patient and should include multidimensional aspects of health, function, and well-being. The treatment goal for patients with HF with frailty is to establish patient-centered goals based on preferences of care.45
Discussion
Although several novel approaches to improve outcomes of patients with HF have been developed, it continues to be the leading cause of cardiovascular death among older patients and the leading cause of hospital admissions.46 About 50% of newly diagnosed patients with HF die within 5 years.47 Current guidelines for managing HF are based on clinical trials that either include few or completely exclude patients aged > 80 years, minorities, and patients with comorbidities clinicians encounter daily in clinical practice.
Furthermore, most clinical trials are designed with mortality as the primary endpoint, which might be as important to our patients with advanced age as their ability to function with a reasonable QOL and less dependence on caregivers.
Decision making in managing HF in our oldest patients should start with an open discussion of the disease and its prognosis, goals of care, and available treatment options. The discussion should also cover all dimensions of suffering, including physical, spiritual, and psychosocial domains. Interviews of patients dying of HF and their caregivers conducted in the United Kingdom identified several communication and transition of care challenges specific to treating this population.48 The study revealed in most cases, patients did not recall receiving any written information about the severity of their disease and often did not understand the association among symptoms, such as shortness of breath, edema, and HF. Patients and caregivers did not feel involved in the decision-making process regarding their illness.
The concurrent presence of comorbidity, frailty, and cognitive impairment in our aging population with HF might add to the burden of the primary condition. Care often is perceived as fragmented. Polypharmacy negatively impacts HF management by increasing risk of drug nonadherence, drug interactions, and AEs in an already vulnerable population. There is a need for more effective interpersonal and easy to understand communication and resources.
In many situations, support services might be best facilitated by a dedicated palliative medicine team with significant experience in managing patients with HF.Although palliative medicine should always be considered for patients with HF with advanced age,consultations often are not obtained unless the patient decides to forgo medical treatment or until the last month of life.49
Although not all end-of-life symptoms can realistically be palliated, earlier involvement of multidisciplinary palliative medicine specialists may improve symptom control, functional status, and QOL. The team may help patients and caregivers cope with uncertainty, and make informed decisions that are person centered based on value system and beliefs.51
Conclusion
Randomized control trials as well as thoughtful observational studies of HF in patients with advanced age and comorbidities, although challenging, are needed to create the evidence base for treatment interventions and assessing their impact on mortality, morbidity, and QOL in this rapidly growing segment of our population.
Given the lack of evidence for HF treatment in patients with advanced age, the clinician should weigh the knowledge of the effect of aging on the CV system, and the lived experience of patients with HF, with the evidence that exists for making the best decision to relieve bothersome symptoms and improve outcomes of care as determined by patients and their caregivers.
Often the most important intervention we can offer our patients, especially those nearing the end of life, is dedicating our time to truly and actively listen with empathy, understating, and respect for their autonomy and for their decision making. And in doing so we accept our own limitations with humility.
Acknowledgments
Dr. Kheirbek received funds from the Veterans Affairs Capitol Health Care Network to establish the Center for Health and Aging at the Washington DC VA Medical Center.
In 2050, persons aged ≥ 85 years, also known as the oldest old, are projected to reach 18 million, accounting for 4.5% of the US population, up from 2.5% in 2030.1 These patients are the fastest growing segment of the US population.
Advances in treating cardiovascular (CV) disease over the past 2 decades have led to an increased incidence of heart failure (HF) and hospitalizations among older patients.2 Total costs of care for persons with HF have exceeded $30 billion annually and are expected to rise to more than $70 billion by 2030 due to growth of the aging population.3,4 Moreover, the Framingham Study reported mortality increases with advancing age (HR 1.27 and 1.61 per decade in men and women, respectively).5
The prevalence of HF is also high and increasing over time. The National Health and Nutrition Examination Survey reported that about 5.7 million Americans have HF.6 The prevalence of HF is expected to reach 8 million by 2030.6 The higher numbers of HF among patients with advanced age is associated with age-related changes in CV structure and function, including reduced responsiveness to β-adrenergic stimulation, impaired left ventricular diastolic filling, and increased vascular stiffness. In addition, age-related changes in other systems might contribute to a HF diagnosis or worsening of the condition.7
Older adults experience physiologic changes in pharmacokinetics and pharmacodynamics, including decreased volume of distribution and creatinine clearance, which lead to significant changes in drug concentration and effectiveness.8
Geriatric patients aged > 65 years who have comorbidities and those who reside in long-term care settings are underrepresented in clinical trials, leading clinicians to make treatment decisions based on data from younger, community-dwelling individuals. Researchers have questioned whether to include elderly patients and those with comorbidities in clinical trials, given that their diminished response may produce less conclusive results with smaller treatment effects. Exclusion criteria based on comorbid conditions or functional status disqualify many older adults from clinical trials.
This article reviews evidence from major randomized controlled trials over the past 2 decades and explores their applicability to support HF treatment guidelines in patients with advanced age (Table).
Pharmacotherapy for Heart Failure
Angiotensin-Converting Enzyme Inhibitors
Several randomized clinical trials have found that angiotensin-converting enzyme (ACE) inhibitors improve symptoms in patients with HF. The CooperativeNorth Scandinavian Enalapril Survival Study (CONSENSUS), demonstrated that enalapril improves survival in patients with New York Heart Association (NYHA) class IV HF with reduced ejection fraction (HFrEF) when added to standard therapy.9 However, the duration of beneficial effect of reduced mortality could not be assessed because the benefit of enalapril in NYHA class I to III HF was not evaluated, and follow-up data are limited. The average age of patients in the study was 71 years, and individuals with significant comorbidities were excluded.
ACE inhibitors also were found to reduce mortality even in asymptomatic patients with HFrEF in the Studies of Left Ventricular Dysfunction trial (SOLVD).10 Enalapril was found to reduce 4-year mortality by 16% and decrease HF hospitalizations when added to conventional therapy consisting primarily of digitalis, diuretics, and nitrates in patients with HFrEF. In this trial, patients aged ≥ 80 years were excluded as well as those with serum creatinine > 2 mg/dL or other conditions that could shorten survival or otherwise impede participation in a long-term trial.
PARADIGM-HF trial patients with HFrEF were randomized to enalapril or the angiotensin receptor-neprilysin inhibitor LCZ696. After a median of 27 months of follow-up, treatment with the angiotensin receptor-neprilysin inhibitor demonstrated greater reduction in CV mortality and HF hospitalizations than enalapril did and was associated with reduced all-cause mortality.11 The trial was stopped early because of evidence of overwhelming benefit with LCZ696. This study of mainly white men included no patients aged ≥ 75 years.
Angiotensin Receptor Blockers
Although less studied than ACE inhibitors, angiotensin receptor blockers (ARBs) share similar benefits. Among patients with symptomatic HFrEF taking an ACE inhibitor, the addition of candesartan reduced the risk of CV death and HF hospitalization as demonstrated in the Candesartan in Heart Failure Assessment of Reduction Mortality and Morbidity (CHARM-added and CHARM-alternative trials).12,13 The CHARM-added trial targeted patients with left ventricular ejection fraction (LVEF) ≤ 40% and NYHA class II to IV HF symptoms who were taking an ACE inhibitor. Adding candesartan reduced CV mortality by 37.9% and HF hospitalization by 42.3% compared with that of placebo.
The CHARM-alternative study found that use of candesartan in symptomatic HFrEF patients who do not tolerate ACE inhibitors,resulted in a 20% reduction in CV mortality as well as a 40% reduction in hospitalization for HF. Among patients with HF with preserved ejection fraction (HFpEF) and NYHA class II to IV symptoms, adding candesartan modestly reduced the rate of HF-related hospitalizations and had no effect on CV mortality in the CHARM-preserved study.14 The CHARM trials examined mostly white men, but 26% of patients were aged > 75 years. However, there was no subgroup analysis for patients aged > 75 years. The study excluded patients with serum creatinine > 2 mg/dL.
Other ARB trials included the following:
- The I-PRESERVE trial, which found that irbesartan did not improve outcomes of patients with HF with preserved ejection fraction (HFpEF).15 The study of mostly white patients did not include patients aged ≥ 80 years.
- A randomized trial of valsartan in HF improved symptoms and mortality in NYHA II to IV HF but showed no benefit when added to ACE inhibitors.16 The trial had no patients aged ≥ 75 years and excluded those with several common comorbidities.
- A randomized, double-blind trial studied the effects of high-dose vs low-dose losartan on clinical outcomes in 3,846 patients with HF and demonstrated that high-dose losartan (150 mg/d) reduces all-cause mortality and hospitalization for HF more effectively than does low-dose losartan (50 mg/d).17 The study, however, had several exclusion criteria, and no patients were aged ≥ 75 years.
Mineralocorticoid Receptor Antagonists
Major studies of aldosterone antagonists demonstrated extra benefit when added to ACE inhibitors/ARBs in patients with HFrEF and NYHA class II HF.18,19
In the RALES study, spironolactone was found to reduce all-cause mortality by 30% and symptoms in NYHA III HF without a significant increase in the risk of serious hyperkalemia or renal failure.18 Most patients were white men aged < 80 years. This study demonstrated the importance of closely following serum potassium levels after initiating aldosterone antagonists in patients with subclinical renal disease because extensive structural damage within the kidney occurs before serum creatinine increases. Patients with advanced renal failure or those who cannot have close monitoring of serum potassium levels have an unfavorable risk–benefit ratio with aldosterone antagonists. Patients with cancer and liver failure were excluded from this trial.
In the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure study, (EMPHASIS-HF Study) eplerenone was found to reduce all-cause mortality and hospitalization for HFrEF.19 Similar to RALES, patients were mostly white males aged < 80 years, and patients with clinically significant, coexisting conditions were excluded.
The 2014 Treatment of Preserved Cardiac FunctionHeart Failure with an Aldosterone Antagonist Trial (TOPCAT) randomized 3,445 patients with well-controlled blood pressure to spironolactone or placebo.20 Inclusion criteria were LVEF ≥ 45%, findings of HF, and either a HF hospitalization or elevated B-type natriuretic peptide level. There was no difference in the primary composite outcome of CV mortality, aborted cardiac arrest, or HF hospitalization over the 3.3-year follow-up period. The study found that among patients with HFpEF, spironolactone does not reduce the composite endpoint of CV mortality, aborted cardiac arrest, or HF hospitalizations compared with that of placebo.20 In the trial, 29% of patients were aged > 75 years, and most were white men. There was no subgroup analysis for older patients.20 In all 3 trials, patients with kidney injury (serum creatinine of ≥ 2.5 or estimated glomerular filtration rate of ≤ 30 mL/min) were excluded because of the risk of hyperkalemia.
An observational study after the RALES trial demonstrated a nearly 4-fold increase in admissions for hyperkalemia with a 6-fold increase in associated mortality in patients taking spirolactone.21 Therefore, it is important to closely follow serum potassium levels after initiating aldosterone antagonists in older patients with subclinical renal disease. Patients with advanced renal failure or those without close monitoring of serum potassium levels have an unfavorable risk–benefit ratio with aldosterone antagonists.
Antithrombotic Therapy
The large multicenter, double-blind randomized trial WARCEF found no added benefit with warfarin vs aspirin for patients with HFrEF in sinus rhythm.22 There was no reduced time to first stroke or death, and the reduced ischemic stroke risk was offset by an increase in major hemorrhage. It is not clear whether subgroup analysis for the etiology of patients’ HF was performed in WARCEF.
The Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial (N = 1,587) found that treatment with warfarin resulted in significantly fewer strokes in patients with ischemic cardiomyopathy.23 Randomization was not stratified by age group in both trials, and baseline characteristics included mostly white men, and no patients were older than aged > 75 years.
The risk of bleeding with prophylactic aspirin use for CV disease is dose dependent and increases with higher aspirin doses.24 The use of aspirin, 325 mg/d, in the WARCEF study might have contributed to the increased risk of hemorrhage.
Recently published results of COMMANDER HF found that the addition of rivaroxaban at a dose of 2.5 mg twice daily to standard care, including clinically selected antiplatelet therapies was not associated with a significantly lower rate of the composite primary outcome composite outcome of death, myocardial infarction (MI), or stroke among 5,022 patients with a recent episode of worsening heart failure compared with that of placebo.25
Several medical conditions are known to increase bleeding risk, including hypertension, cerebrovascular disease, ischemic stroke, serious heart disease, diabetes mellitus, renal insufficiency, alcoholism, liver disease, and falls.26 Many of these conditions are common among very old patients and should be considered when estimating risk–benefit ratio of oral anticoagulation therapy.
β-blockers
In several large studies, β-blockers have been shown to be effective in reducing mortality in patients with HFrEF. In the Cardiac Insufficiency Bisoprolol Study II, bisoprolol improved all-cause mortality and all-cause hospitalizations, and reduced sudden death in patients with NYHA III or IV HF.27 In the Carvedilol or Metoprolol European Trial (COMET), carvedilol was superior to metoprolol in reducing all-cause mortality for patients with NYHA II or IV HF.28 Both trials included mostly white men; patients with several comorbidities were excluded, and no patients were aged > 80 years.
COMET compared carvedilol with metoprolol tartrate, the short-acting form of metoprolol that has not shown a survival benefit for patients with HF. However, the Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure trial demonstrated survival benefits with metoprolol CR/XL and included patients aged > 80 years.29
In the SENIORS study, patients treated with nebivolol had a 4.2% absolute risk reduction in a composite of mortality or hospital admission at a mean follow-up of 21 months.30 It is reasonable to use nebivolol for managing HF in older patients. Careful monitoring of heart rate is necessary when prescribing β-blockers for older patients.
Cardiac Glycosides
Digoxin with diuretics was the first-line treatment for HF for many decades and the mainstay of HF therapy until the first large HF trials were performed in the 1980s. One trial initiated by the Digoxin Investigation Group (DIG) studied patients with HFrEF who were already receiving treatment for HF (including 94% taking ACE inhibitors and 82% on diuretics) and randomized them to either digoxin or placebo.31 The study found no significant difference in mortality between the groups at the 3-year follow-up; however, the digoxin group had significantly fewer hospitalizations compared with that of the placebo group.
A post-hoc analysis of patients by age found no difference in mortality between patients aged 70 to 79 years and those ≥ 80 years, with a persistent benefit in fewer hospitalizations. Digoxin continues to be recommended as a reasonable medication for treating symptomatic HFrEF. However, caution is advised in older patients, especially women, who are at higher risk of digoxin toxicity.
No current evidence exists that digoxin adds any benefits for patients with HFpEF of any age and therefore, it should not be used.
Diuretics
Diuretic therapy is important for managing shortness of breath and congestion related to fluid volume overload in patients with HF. Although diuretics have not been shown to reduce mortality in patients with HF, they are the mainstay treatment for patients with HFpEF.32 In a post-hoc analysis of the DIG study, diuretic use was associated with increased risk of mortality and hospitalizations in patients aged > 65 years.33 Hyponatremia is one of the most serious adverse effects (AEs) with these agents and occurs in about one-fifth of elderly patients taking diuretics.
In severe cases hyponatremia can cause a range of problems, including weakness, confusion, postural giddiness, postural hypotension, falls, transient hemiparesis, and seizures. In older patients with diminished renal reserve, diuretics are more likely to precipitate prerenal uremia than it does in younger patients. Prerequisites for diuretic use are an accurate diagnosis, careful monitoring of blood pressure and serum electrolytes, and regular review of their efficacy, AEs, and the need for continued treatment.
Statins
The Controlled Rosuvastatin Multinational Trial in Heart Failure demonstrated that low-dose rosuvastatin (10 mg/d) does not improve survival among patients with moderate-to-severe ischemic cardiomyopathy but could reduce the rate of CV hospitalizations.34 Patients in this study had a mean age of 73 years, and 41% of them were aged ≥ 75 years. However, the study used a low-dose rosuvastatin, and patients with several common comorbidities were excluded. Evidence exists that treatment with other statins may improve outcomes in patients with HF. There is also evidence that among elderly patients with HF, low serum total cholesterol is independently associated with a worse prognosis.35
Comorbidities
Anemia
In patients with iron-deficiency anemia (ferritin 15-100 ng/mL or 100-299 ng/mL with transferrin saturation < 20%) and symptomatic HFrEF (LVEF ≤ 40% with NYHA II to IV HF), oral iron replacement had no effect on exercise capacity as measured using change in peak oxygen uptake.36 However, IV iron replacement might be a reasonable option to improve functional status and quality of life (QOL) for patients with HF.37 In these studies, participants were aged < 75 years, and there is no evidence that treating other types of anemia improves outcomes in patients with HF.
Hypertension
The Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated that controlling blood pressure to a goal systolic pressure of < 120 mm Hg is associated with significant reduction in the mortality among patients with increased CV risk (aged > 75 years, vascular disease, kidney injury, or a Framingham Risk Score >15%).38 The SPRINT study included patients aged > 75 (25%); however, the study excluded older adults living in nursing homes and those with diabetes mellitus, symptomatic HF, dementia, or stroke. The subgroup analysis did not stratify patients based on age nor provided sufficient evidence regarding treatment targets for this vulnerable population. Therefore, clinicians cannot draw any conclusions about managing hypertension among patients with HF from this study.
Sleep Apnea
Sleep apnea is common among patients with HF. A study of adults with chronic HF treated with evidence-based therapies found that 61% of participants had central or obstructive sleep apnea.39 In elderly patients, sleep apnea is further complicated by insomnia and disturbance of sleep cycle that often occur with the aging process.
It is crucial to differentiate central sleep apnea from obstructive sleep apnea, because the treatment approaches differ. Central sleep apnea is associated with poor prognosis in patients with HF.40 Adaptive servo ventilation for central sleep apnea uses a noninvasive ventilator to delivering servo controlled inspiratory pressure support on top of expiratory positive airway pressure. Adaptive servo ventilation for central sleep apnea is associated with higher all-cause mortality and CV mortality.41 Continuous positive airway pressure for obstructive sleep apnea improves sleep quality, reduces the apnea-hypopnea index, and improves nocturnal oxygenation.42
Depression
Clinically significant depression occurs in 21% of patients with HF, and the relationship between depression and poor HF outcomes is consistent and strong across several endpoints. However, in a randomized, 12-week study, the selective serotonin reuptake inhibitor sertraline did not improve depression symptoms or clinical status among patients with HF.43 Depression symptoms might overlap with fatigue and low energy expenditure experienced by oldest old patients with HF who do not have depression.
Furthermore, studies describing depression treatments among patients with HF are too small and heterogeneous to permit definitive conclusions about intervention effectiveness. These results identify areas requiring further development, raise questions regarding the association between depression and clinical outcomes in patients with HF, and provide information on depression prevalence that may help researchers design studies with appropriate depression measures and adequately powered sample sizes.
Frailty
Although frailty is prevalent in the elderly and is independently associated with poor outcomes, there is no standardized definition for frailty. The Fried Frailty Index is a widely used scale that incorporates criteria including weakness, slowness, exhaustion, and low physical activity in the diagnosis of frailty.44 However these symptoms are common among patients with advanced HF with and without depression or frailty.
Frailty should be defined collaboratively by the clinician and the patient and should include multidimensional aspects of health, function, and well-being. The treatment goal for patients with HF with frailty is to establish patient-centered goals based on preferences of care.45
Discussion
Although several novel approaches to improve outcomes of patients with HF have been developed, it continues to be the leading cause of cardiovascular death among older patients and the leading cause of hospital admissions.46 About 50% of newly diagnosed patients with HF die within 5 years.47 Current guidelines for managing HF are based on clinical trials that either include few or completely exclude patients aged > 80 years, minorities, and patients with comorbidities clinicians encounter daily in clinical practice.
Furthermore, most clinical trials are designed with mortality as the primary endpoint, which might be as important to our patients with advanced age as their ability to function with a reasonable QOL and less dependence on caregivers.
Decision making in managing HF in our oldest patients should start with an open discussion of the disease and its prognosis, goals of care, and available treatment options. The discussion should also cover all dimensions of suffering, including physical, spiritual, and psychosocial domains. Interviews of patients dying of HF and their caregivers conducted in the United Kingdom identified several communication and transition of care challenges specific to treating this population.48 The study revealed in most cases, patients did not recall receiving any written information about the severity of their disease and often did not understand the association among symptoms, such as shortness of breath, edema, and HF. Patients and caregivers did not feel involved in the decision-making process regarding their illness.
The concurrent presence of comorbidity, frailty, and cognitive impairment in our aging population with HF might add to the burden of the primary condition. Care often is perceived as fragmented. Polypharmacy negatively impacts HF management by increasing risk of drug nonadherence, drug interactions, and AEs in an already vulnerable population. There is a need for more effective interpersonal and easy to understand communication and resources.
In many situations, support services might be best facilitated by a dedicated palliative medicine team with significant experience in managing patients with HF.Although palliative medicine should always be considered for patients with HF with advanced age,consultations often are not obtained unless the patient decides to forgo medical treatment or until the last month of life.49
Although not all end-of-life symptoms can realistically be palliated, earlier involvement of multidisciplinary palliative medicine specialists may improve symptom control, functional status, and QOL. The team may help patients and caregivers cope with uncertainty, and make informed decisions that are person centered based on value system and beliefs.51
Conclusion
Randomized control trials as well as thoughtful observational studies of HF in patients with advanced age and comorbidities, although challenging, are needed to create the evidence base for treatment interventions and assessing their impact on mortality, morbidity, and QOL in this rapidly growing segment of our population.
Given the lack of evidence for HF treatment in patients with advanced age, the clinician should weigh the knowledge of the effect of aging on the CV system, and the lived experience of patients with HF, with the evidence that exists for making the best decision to relieve bothersome symptoms and improve outcomes of care as determined by patients and their caregivers.
Often the most important intervention we can offer our patients, especially those nearing the end of life, is dedicating our time to truly and actively listen with empathy, understating, and respect for their autonomy and for their decision making. And in doing so we accept our own limitations with humility.
Acknowledgments
Dr. Kheirbek received funds from the Veterans Affairs Capitol Health Care Network to establish the Center for Health and Aging at the Washington DC VA Medical Center.
1. Ortman JM, Velkoff AV, Hogan H. An aging nation: the older population in the United States. https://www.census.gov/prod/2014pubs/p25-1140.pdf. Published May 2014. Accessed September 30, 2018.
2. Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;52(6):428-434.
3. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619.
4. National Heart, Lung, and Blood Institute, National Institutes of Health. Incidence and Prevalence: 2006 Chart Book on Cardiovascular and Lung Diseases. Bethesda, MD: National Institutes of Health; 2006.
5. Curtis LH, Whellan DJ, Hammill BG, et al. Incidence and prevalence of heart failure in elderly persons, 1994-2003. Arch Intern Med. 2008;168(4):418-424.
6. Writing Group, Mozaffarian D, Benjamin EJ, et al; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e360.
7. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107(1):139-146.
8. Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6-14.
9. CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316(23):1429-1435.
10. SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB Jr, Cohn JN. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327(10):685-691.
11. McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004.
12. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362(9386):767-771.
13. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362(9386):772-776.
14. Yusuf S, Pfeffer MA, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362(9386):777-781.
15. Massie BM, Carson PE, McMurray JJ, et al; I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456-2467.
16. Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345(23):1667-1675.
17. Konstam MA, Neaton JD, Dickstein K, et al; HEAAL Investigators. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374(9704):1840-1848.
18. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709-717.
19. Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11-21.
20. Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383-1392.
21. Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351(6):543-551.
22. Homma S, Thompson JL, Pullicino PM, et al; WARCEF Investigators. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366(20):1859-1869.
23. Massie BM, Collins JF, Ammon SE, et al; WATCH Trial Investigators. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation. 2009;119(12):1616-1624.
24. Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA. 2007;297(18):2018-2024.
25. Zannad F, Anker, SD, Byra WM, et al; COMMANDER HF Investigators. Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N Engl J Med. 2018;379(14):1332-1342.
26. Schulman S, Beyth RJ, Kearon C, Levine MN. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):257S-298S.
27. CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9-13.
28. Poole-Wilson PA, Swedberg K, Cleland JG, et al; Carvedilol Or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomized controlled trial. Lancet. 2003;362(9377):7-13.
29. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353(9169):2001-2007.
30. Flather MD, Shibata MC, Coats AJ, et al; SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26(3):215-225.
31. Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336(8):525-533.
32. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239.
33 Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351(6):543-551.
34. Kjekshus J, Apetrei E, Barrios V, et al; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357(22):2248-2261.
35. Rauchhaus M, Clark AL, Doehner W, et al. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol. 2003;42(11):1933-1940.
36. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137-e161.
37. Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36(11):657-668.
38. SPRINT Research Group, Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116.
39. MacDonald M, Fang J, Pittman SD, White DP, Malhotra A. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med. 2008;4(1):38-42.
40. Bradley TD, Floras JS. Sleep Apnea and heart failure: part II: Central sleep apnea. Circulation. 2003;107(13):1822-1826.
41. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-1105.
42. McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931.
43. O’Connor CM, Jiang W, Kuchibhatla M, et al; SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692-699.
44. Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-156.
45. Pilotto A, Addante F, Franceschi M, et al. Multidimensional Prognostic Index based on a comprehensive geriatric assessment predicts short-term mortality in older patients with heart failure. Circ Heart Fail. 2010;3(1):14-20.
46. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
47. Goldberg, RJ, Ciampa, J, Lessard D,, et al. Long-term survival after heart failure: a contemporary population-based perspective. Arch Intern Med. 2007;167(5):490-496.
48. Murray SA, Boyd K, Kendall M, Worth A, Benton TF, Clausen H. Dying of lung cancer or cardiac failure: prospective qualitative interview study of patients and their carers in the community. BMJ. 2002;325(7370):929.
49. Gibbs JS, McCoy AS, Gibbs LM, Rogers AE, Addington-Hall JM. Living with and dying from heart failure: the role of palliative care. Heart. 2002;88(suppl 2):ii36-39.
50. Quill TE, Dresser R, Brock DW. The rule of double effect—a critique of its role in end-of-life decision making. N Engl J Med. 1997;337(24):1768-1771.
51. Nieminen MS, Dickstein K, Fonseca C, et al. The patient perspective: quality of life in advanced heart failure with frequent hospitalizations. Int J Cardiol. 2015;191:256-264.
1. Ortman JM, Velkoff AV, Hogan H. An aging nation: the older population in the United States. https://www.census.gov/prod/2014pubs/p25-1140.pdf. Published May 2014. Accessed September 30, 2018.
2. Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;52(6):428-434.
3. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619.
4. National Heart, Lung, and Blood Institute, National Institutes of Health. Incidence and Prevalence: 2006 Chart Book on Cardiovascular and Lung Diseases. Bethesda, MD: National Institutes of Health; 2006.
5. Curtis LH, Whellan DJ, Hammill BG, et al. Incidence and prevalence of heart failure in elderly persons, 1994-2003. Arch Intern Med. 2008;168(4):418-424.
6. Writing Group, Mozaffarian D, Benjamin EJ, et al; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e360.
7. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107(1):139-146.
8. Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6-14.
9. CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316(23):1429-1435.
10. SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB Jr, Cohn JN. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327(10):685-691.
11. McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004.
12. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362(9386):767-771.
13. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362(9386):772-776.
14. Yusuf S, Pfeffer MA, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362(9386):777-781.
15. Massie BM, Carson PE, McMurray JJ, et al; I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456-2467.
16. Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345(23):1667-1675.
17. Konstam MA, Neaton JD, Dickstein K, et al; HEAAL Investigators. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374(9704):1840-1848.
18. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709-717.
19. Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11-21.
20. Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383-1392.
21. Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351(6):543-551.
22. Homma S, Thompson JL, Pullicino PM, et al; WARCEF Investigators. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366(20):1859-1869.
23. Massie BM, Collins JF, Ammon SE, et al; WATCH Trial Investigators. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation. 2009;119(12):1616-1624.
24. Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA. 2007;297(18):2018-2024.
25. Zannad F, Anker, SD, Byra WM, et al; COMMANDER HF Investigators. Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N Engl J Med. 2018;379(14):1332-1342.
26. Schulman S, Beyth RJ, Kearon C, Levine MN. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):257S-298S.
27. CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9-13.
28. Poole-Wilson PA, Swedberg K, Cleland JG, et al; Carvedilol Or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomized controlled trial. Lancet. 2003;362(9377):7-13.
29. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353(9169):2001-2007.
30. Flather MD, Shibata MC, Coats AJ, et al; SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26(3):215-225.
31. Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336(8):525-533.
32. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239.
33 Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351(6):543-551.
34. Kjekshus J, Apetrei E, Barrios V, et al; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357(22):2248-2261.
35. Rauchhaus M, Clark AL, Doehner W, et al. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol. 2003;42(11):1933-1940.
36. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137-e161.
37. Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36(11):657-668.
38. SPRINT Research Group, Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116.
39. MacDonald M, Fang J, Pittman SD, White DP, Malhotra A. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med. 2008;4(1):38-42.
40. Bradley TD, Floras JS. Sleep Apnea and heart failure: part II: Central sleep apnea. Circulation. 2003;107(13):1822-1826.
41. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-1105.
42. McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931.
43. O’Connor CM, Jiang W, Kuchibhatla M, et al; SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692-699.
44. Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-156.
45. Pilotto A, Addante F, Franceschi M, et al. Multidimensional Prognostic Index based on a comprehensive geriatric assessment predicts short-term mortality in older patients with heart failure. Circ Heart Fail. 2010;3(1):14-20.
46. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
47. Goldberg, RJ, Ciampa, J, Lessard D,, et al. Long-term survival after heart failure: a contemporary population-based perspective. Arch Intern Med. 2007;167(5):490-496.
48. Murray SA, Boyd K, Kendall M, Worth A, Benton TF, Clausen H. Dying of lung cancer or cardiac failure: prospective qualitative interview study of patients and their carers in the community. BMJ. 2002;325(7370):929.
49. Gibbs JS, McCoy AS, Gibbs LM, Rogers AE, Addington-Hall JM. Living with and dying from heart failure: the role of palliative care. Heart. 2002;88(suppl 2):ii36-39.
50. Quill TE, Dresser R, Brock DW. The rule of double effect—a critique of its role in end-of-life decision making. N Engl J Med. 1997;337(24):1768-1771.
51. Nieminen MS, Dickstein K, Fonseca C, et al. The patient perspective: quality of life in advanced heart failure with frequent hospitalizations. Int J Cardiol. 2015;191:256-264.
U.S. deaths from preventable causes occur more often in rural areas
compared with the most urban counties during 2010-2017, according to study published in CDC’s Morbidity and Mortality Weekly Report.
These leading causes of death comprised heart disease, cancer, unintentional injury, chronic lower respiratory disease, and stroke and accounted for approximately 1.7 million deaths or 61% of all deaths in 2017.
The study presents estimates, percentages, and annual percent changes for potentially excess deaths by urban-rural classification from heart disease, cancer, unintentional injury, chronic lower respiratory disease, and stroke. Urban-rural categories were identified using the National Center for Health Statistics 2013 urban-rural classification scheme for counties.
The report’s main findings include the following statistics:
- In 2010, 28.7% of deaths from cancer in the most rural counties were potentially preventable, compared with 17.9% in the most urban counties. By 2017, 21.7% of cancer deaths in the most rural counties were potentially preventable, compared with 3.2% in the most urban counties.
- In 2010, 45.1% of deaths from heart disease in the most rural counties were potentially preventable, compared with 33.5% in the most urban counties. By 2017, 44.9% of deaths from heart disease in the most rural counties were potentially preventable, compared with 28.0% in the most urban counties.
- In 2010, 60.9% of deaths from unintentional injury in the most rural counties were potentially preventable, compared with 25.4% in the most urban counties. By 2017, 64.1% of deaths from unintentional injury in the most rural counties were potentially preventable, compared with 47.8% in the most urban counties.
- In 2010, 54.3% of deaths from chronic lower respiratory disease (such as COPD) in the most rural counties were potentially preventable, compared with 23.4% in the most urban counties. By 2017, 57.1% of deaths from chronic lower respiratory disease in the most rural counties were potentially preventable, compared with 13% in the most urban counties.
- In 2010, 41.6% of deaths from stroke in the most rural counties were potentially preventable, compared with 31.7% in most urban areas. By 2017, 37.8% of deaths from stroke in the most rural counties were potentially preventable, compared with 27.4% most urban counties.
“This report demonstrates the value of analyzing potentially excess deaths according to the six 2013 [National Center for Health Statistics] urban-rural county classifications. Reporting trends in potentially excess deaths over an 8-year period highlights differences over time, independent of traditional underlying structural, environmental, and genetic factors,” wrote Macarena C. Garcia, DrPH, and coauthors.
“Because of increasing percentages of potentially excess deaths in recent years for certain causes of death and certain demographic groups, these data can be used, with traditional rate comparisons, by public health practitioners who are involved in planning interventions. Comparing the findings in this report with data from tools such as the CDC Interactive Atlas of Heart Disease and Stroke might help identify the social determinants, health care infrastructures, and public policies that could increase or decrease numbers of deaths in specific nonmetropolitan areas,” they added.
The study authors did not disclose any potential conflicts of interest.
SOURCE: Garcia MC et al. MMWR Morb Mortal Wkly Rep. 2019 Nov 8: 68(10);1-11.
compared with the most urban counties during 2010-2017, according to study published in CDC’s Morbidity and Mortality Weekly Report.
These leading causes of death comprised heart disease, cancer, unintentional injury, chronic lower respiratory disease, and stroke and accounted for approximately 1.7 million deaths or 61% of all deaths in 2017.
The study presents estimates, percentages, and annual percent changes for potentially excess deaths by urban-rural classification from heart disease, cancer, unintentional injury, chronic lower respiratory disease, and stroke. Urban-rural categories were identified using the National Center for Health Statistics 2013 urban-rural classification scheme for counties.
The report’s main findings include the following statistics:
- In 2010, 28.7% of deaths from cancer in the most rural counties were potentially preventable, compared with 17.9% in the most urban counties. By 2017, 21.7% of cancer deaths in the most rural counties were potentially preventable, compared with 3.2% in the most urban counties.
- In 2010, 45.1% of deaths from heart disease in the most rural counties were potentially preventable, compared with 33.5% in the most urban counties. By 2017, 44.9% of deaths from heart disease in the most rural counties were potentially preventable, compared with 28.0% in the most urban counties.
- In 2010, 60.9% of deaths from unintentional injury in the most rural counties were potentially preventable, compared with 25.4% in the most urban counties. By 2017, 64.1% of deaths from unintentional injury in the most rural counties were potentially preventable, compared with 47.8% in the most urban counties.
- In 2010, 54.3% of deaths from chronic lower respiratory disease (such as COPD) in the most rural counties were potentially preventable, compared with 23.4% in the most urban counties. By 2017, 57.1% of deaths from chronic lower respiratory disease in the most rural counties were potentially preventable, compared with 13% in the most urban counties.
- In 2010, 41.6% of deaths from stroke in the most rural counties were potentially preventable, compared with 31.7% in most urban areas. By 2017, 37.8% of deaths from stroke in the most rural counties were potentially preventable, compared with 27.4% most urban counties.
“This report demonstrates the value of analyzing potentially excess deaths according to the six 2013 [National Center for Health Statistics] urban-rural county classifications. Reporting trends in potentially excess deaths over an 8-year period highlights differences over time, independent of traditional underlying structural, environmental, and genetic factors,” wrote Macarena C. Garcia, DrPH, and coauthors.
“Because of increasing percentages of potentially excess deaths in recent years for certain causes of death and certain demographic groups, these data can be used, with traditional rate comparisons, by public health practitioners who are involved in planning interventions. Comparing the findings in this report with data from tools such as the CDC Interactive Atlas of Heart Disease and Stroke might help identify the social determinants, health care infrastructures, and public policies that could increase or decrease numbers of deaths in specific nonmetropolitan areas,” they added.
The study authors did not disclose any potential conflicts of interest.
SOURCE: Garcia MC et al. MMWR Morb Mortal Wkly Rep. 2019 Nov 8: 68(10);1-11.
compared with the most urban counties during 2010-2017, according to study published in CDC’s Morbidity and Mortality Weekly Report.
These leading causes of death comprised heart disease, cancer, unintentional injury, chronic lower respiratory disease, and stroke and accounted for approximately 1.7 million deaths or 61% of all deaths in 2017.
The study presents estimates, percentages, and annual percent changes for potentially excess deaths by urban-rural classification from heart disease, cancer, unintentional injury, chronic lower respiratory disease, and stroke. Urban-rural categories were identified using the National Center for Health Statistics 2013 urban-rural classification scheme for counties.
The report’s main findings include the following statistics:
- In 2010, 28.7% of deaths from cancer in the most rural counties were potentially preventable, compared with 17.9% in the most urban counties. By 2017, 21.7% of cancer deaths in the most rural counties were potentially preventable, compared with 3.2% in the most urban counties.
- In 2010, 45.1% of deaths from heart disease in the most rural counties were potentially preventable, compared with 33.5% in the most urban counties. By 2017, 44.9% of deaths from heart disease in the most rural counties were potentially preventable, compared with 28.0% in the most urban counties.
- In 2010, 60.9% of deaths from unintentional injury in the most rural counties were potentially preventable, compared with 25.4% in the most urban counties. By 2017, 64.1% of deaths from unintentional injury in the most rural counties were potentially preventable, compared with 47.8% in the most urban counties.
- In 2010, 54.3% of deaths from chronic lower respiratory disease (such as COPD) in the most rural counties were potentially preventable, compared with 23.4% in the most urban counties. By 2017, 57.1% of deaths from chronic lower respiratory disease in the most rural counties were potentially preventable, compared with 13% in the most urban counties.
- In 2010, 41.6% of deaths from stroke in the most rural counties were potentially preventable, compared with 31.7% in most urban areas. By 2017, 37.8% of deaths from stroke in the most rural counties were potentially preventable, compared with 27.4% most urban counties.
“This report demonstrates the value of analyzing potentially excess deaths according to the six 2013 [National Center for Health Statistics] urban-rural county classifications. Reporting trends in potentially excess deaths over an 8-year period highlights differences over time, independent of traditional underlying structural, environmental, and genetic factors,” wrote Macarena C. Garcia, DrPH, and coauthors.
“Because of increasing percentages of potentially excess deaths in recent years for certain causes of death and certain demographic groups, these data can be used, with traditional rate comparisons, by public health practitioners who are involved in planning interventions. Comparing the findings in this report with data from tools such as the CDC Interactive Atlas of Heart Disease and Stroke might help identify the social determinants, health care infrastructures, and public policies that could increase or decrease numbers of deaths in specific nonmetropolitan areas,” they added.
The study authors did not disclose any potential conflicts of interest.
SOURCE: Garcia MC et al. MMWR Morb Mortal Wkly Rep. 2019 Nov 8: 68(10);1-11.
Spanish risk score predicts 30-day mortality in acute HF in ED patients
Background: The MEESSI-AHF (Multiple Estimation of Risk based on the Emergency Department Spanish Score In patients with Acute Heart Failure) score is a risk-stratification tool that includes systolic blood pressure, age, NT-proBNP, potassium, cardiac troponin T, New York Heart Association class 4 disease, respiratory rate, low-output symptoms, oxygen saturation, episode associated with acute coronary syndrome, signs of left ventricular hypertrophy on EKG, creatinine, and Barthel Index Score. Prior research has shown that it accurately risk-stratified ED patients with AHF in Spain. It has not been studied in other populations.
Study design: Prospective multicenter cohort study.
Setting: Adult ED patients with acute dyspnea in four hospitals in Switzerland.
Synopsis: The study included 1,247 nonhemodialysis patients who presented to the ED with acute dyspnea, were found to have all the necessary variables to calculate the MEESSI-AHF score, and were adjudicated to have acute heart failure. The authors calculated a modified MEESSI-AHF score, excluding the Barthel Index for all patients. The authors found that a six-group modified MEESSI-AHF risk-stratification model could predict 30-day mortality with excellent discrimination (C-Statistic, 0.80). Limitations of the study include the exclusion of all hemodynamically unstable patients and those on hemodialysis.
Bottom line: The MEESSI-AHF score effectively predicts 30-day mortality in AHF in Swiss and Spanish ED patients.
Citation: Wussler D et al. External validation of the MEESSI acute heart failure risk score: A cohort study. Ann Intern Med. 2019;170:248-56.
Dr. Radhakrishnan is a hospitalist at Beth Israel Deaconess Medical Center.
Background: The MEESSI-AHF (Multiple Estimation of Risk based on the Emergency Department Spanish Score In patients with Acute Heart Failure) score is a risk-stratification tool that includes systolic blood pressure, age, NT-proBNP, potassium, cardiac troponin T, New York Heart Association class 4 disease, respiratory rate, low-output symptoms, oxygen saturation, episode associated with acute coronary syndrome, signs of left ventricular hypertrophy on EKG, creatinine, and Barthel Index Score. Prior research has shown that it accurately risk-stratified ED patients with AHF in Spain. It has not been studied in other populations.
Study design: Prospective multicenter cohort study.
Setting: Adult ED patients with acute dyspnea in four hospitals in Switzerland.
Synopsis: The study included 1,247 nonhemodialysis patients who presented to the ED with acute dyspnea, were found to have all the necessary variables to calculate the MEESSI-AHF score, and were adjudicated to have acute heart failure. The authors calculated a modified MEESSI-AHF score, excluding the Barthel Index for all patients. The authors found that a six-group modified MEESSI-AHF risk-stratification model could predict 30-day mortality with excellent discrimination (C-Statistic, 0.80). Limitations of the study include the exclusion of all hemodynamically unstable patients and those on hemodialysis.
Bottom line: The MEESSI-AHF score effectively predicts 30-day mortality in AHF in Swiss and Spanish ED patients.
Citation: Wussler D et al. External validation of the MEESSI acute heart failure risk score: A cohort study. Ann Intern Med. 2019;170:248-56.
Dr. Radhakrishnan is a hospitalist at Beth Israel Deaconess Medical Center.
Background: The MEESSI-AHF (Multiple Estimation of Risk based on the Emergency Department Spanish Score In patients with Acute Heart Failure) score is a risk-stratification tool that includes systolic blood pressure, age, NT-proBNP, potassium, cardiac troponin T, New York Heart Association class 4 disease, respiratory rate, low-output symptoms, oxygen saturation, episode associated with acute coronary syndrome, signs of left ventricular hypertrophy on EKG, creatinine, and Barthel Index Score. Prior research has shown that it accurately risk-stratified ED patients with AHF in Spain. It has not been studied in other populations.
Study design: Prospective multicenter cohort study.
Setting: Adult ED patients with acute dyspnea in four hospitals in Switzerland.
Synopsis: The study included 1,247 nonhemodialysis patients who presented to the ED with acute dyspnea, were found to have all the necessary variables to calculate the MEESSI-AHF score, and were adjudicated to have acute heart failure. The authors calculated a modified MEESSI-AHF score, excluding the Barthel Index for all patients. The authors found that a six-group modified MEESSI-AHF risk-stratification model could predict 30-day mortality with excellent discrimination (C-Statistic, 0.80). Limitations of the study include the exclusion of all hemodynamically unstable patients and those on hemodialysis.
Bottom line: The MEESSI-AHF score effectively predicts 30-day mortality in AHF in Swiss and Spanish ED patients.
Citation: Wussler D et al. External validation of the MEESSI acute heart failure risk score: A cohort study. Ann Intern Med. 2019;170:248-56.
Dr. Radhakrishnan is a hospitalist at Beth Israel Deaconess Medical Center.
Best timing for measuring orthostatic vital signs?
ILLUSTRATIVE CASE
A 54-year-old woman with a history of hypertension presents with a chief complaint of dizziness. You require an assessment of orthostatic vital signs to proceed. In your busy clinical practice, when should assessment take place to be most useful?
Orthostatic hypotension (OH) is defined as a postural reduction in systolic blood pressure (BP) of ≥ 20 mm Hg or diastolic BP of ≥ 10 mm Hg, measured within 3 minutes of rising from supine to standing. This definition is based on consensus guidelines from the American Academy of Neurology and the American Autonomic Society2 and has been upheld by European guidelines.3
The prevalence of OH is approximately 6% in the general population, with estimates ranging from 10% to 55% in older adults.4 Etiology is often multifactorial; causes may be neurogenic (mediated by autonomic failure as in Parkinson’s disease, multiple system atrophy, or diabetic neuropathy), non-neurogenic (related to medications or hypovolemia), or idiopathic.
It’s important to identify OH because of its associated increase in morbidities, such as an increased risk of falls (hazard ratio [HR] = 1.5),5 coronary heart disease (HR = 1.3), stroke (HR = 1.2), and all-cause mortality (HR = 1.4).6 Treatments include physical maneuvers (getting up slowly, leg crossing, and muscle clenching), increased salt and water intake, compression stockings, the addition of medications (such as fludrocortisone or midodrine), and the avoidance of other medications (such as benzodiazepines and diuretics).
The guideline-recommended 3-minute delay in assessment can be impractical in a busy clinical setting. Using data from the Atherosclerosis Risk in Communities (ARIC) study, investigators correlated the timing of measurements of postural change in BP with long-term adverse outcomes.1
STUDY SUMMARY
Early vs late OH assessment in middle-aged adults
The ARIC study is a longitudinal, prospective, cohort study of almost 16,000 adults followed since 1987. Juraschek et al1 assessed the optimal time to identify OH and its association with the adverse clinical outcomes of fall, fracture, syncope, motor vehicle crash, and mortality. The researchers sought to discover whether BP measurements determined immediately after standing predict adverse events as well as BP measurements taken closer to 3 minutes.
Study participants were between the ages of 45 and 64 years (mean 54 years), and 26% were black and 54% were female. They lived in 4 different US communities. The researchers excluded patients with missing OH assessments or other relevant cohort or historical data, leaving a cohort of 11,429 subjects.
Continue to: As part of their...
As part of their enrollment into the ARIC study, subjects had their BP measurements taken 2 to 5 times in the lying position (90% of participants had ≥ 4 measurements) and after standing (91% participants had ≥ 4 measurements) using a programmable automatic BP cuff. All 5 standing BP measurements (taken at a mean of 28, 53, 76, 100, and 116 seconds after standing) were measured for 7385 out of 11,429 (64.6%) participants. Subjects were asked if he or she “usually gets dizzy on standing up.”
Researchers determined the association between OH and postural change in systolic BP or postural change in diastolic BP with history of dizziness after standing. They also determined the incidence of falls, fracture, syncope, motor vehicle crash, and mortality via a review of hospitalizations and billing for Medicaid and Medicare services. Subjects were followed for a median of 23 years.
Results
Of the entire cohort, 1138 (10%) reported dizziness on standing. Only OH identified at the first BP measurement (mean 28 secs) was associated with a history of dizziness upon standing (odds ratio [OR] = 1.49; 95% confidence interval [CI], 1.18-1.89). Also, it was associated with the highest incidence of fracture, syncope, and death (18.9, 17, and 31.4 per 1000 person-years, respectively).
After adjusting for age, sex, and multiple other cardiovascular risk factors, the risk of falls was significantly associated with OH at BP measurements 1 to 4, but was most strongly associated with BP measurement 2 (taken at a mean of 53 secs after standing) (HR = 1.29; 95% CI, 1.12-1.49), which translates to 13.2 falls per 1000 patient-years. Fracture was associated with OH at measurements 1 (HR = 1.16; 95% CI, 1.01-1.34) and 2 (HR = 1.14; 95% CI, 1.01-1.29). Motor vehicle crashes were associated only with BP measurement 2 (HR = 1.43; 95% CI, 1.04-1.96). Finally, risk of syncope and risk of death were statistically associated with the presence of OH at all 5 BP measurements.
WHAT’S NEW
Earlier OH assessments are more informative than late ones
This study found OH identified within 1 minute of standing to be more clinically meaningful than OH identified after 1 minute. Also, the findings reinforce the relationship between OH and adverse events, including injury and overall mortality. Evaluation for OH performed only at 3 minutes may miss symptomatic OH.
Continue to: CAVEATS
CAVEATS
Could a healthy population skew the results?
The population in this study was relatively healthy, with a lower prevalence of diabetes and coronary artery disease than the general population. While there is no reason to expect detection of OH to differ in a population with more comorbidities, the possibility exists.
If OH is not identified in < 1 minute of standing, standard OH evaluation within 3 minutes after standing should be performed, as OH identified at any time point after standing is associated with adverse events and increased mortality.
This study did not address the effects of medical intervention for OH on injury or mortality. Also, whether OH is the direct cause of the adverse outcomes or a marker for other disease is unknown.
CHALLENGES TO IMPLEMENTATION
A change to protocols and guidelines
Although none were noted, any change in practice requires updating clinical protocols and guidelines, which can take time.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Internal Med. 2017;177:1316-1323.
2. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470.
3. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930-936.
4. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
5. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults: the Cardiovascular Health Study. Hypertension. 1992;19(6 Pt 1):508-519.
6. Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100:406-413.
ILLUSTRATIVE CASE
A 54-year-old woman with a history of hypertension presents with a chief complaint of dizziness. You require an assessment of orthostatic vital signs to proceed. In your busy clinical practice, when should assessment take place to be most useful?
Orthostatic hypotension (OH) is defined as a postural reduction in systolic blood pressure (BP) of ≥ 20 mm Hg or diastolic BP of ≥ 10 mm Hg, measured within 3 minutes of rising from supine to standing. This definition is based on consensus guidelines from the American Academy of Neurology and the American Autonomic Society2 and has been upheld by European guidelines.3
The prevalence of OH is approximately 6% in the general population, with estimates ranging from 10% to 55% in older adults.4 Etiology is often multifactorial; causes may be neurogenic (mediated by autonomic failure as in Parkinson’s disease, multiple system atrophy, or diabetic neuropathy), non-neurogenic (related to medications or hypovolemia), or idiopathic.
It’s important to identify OH because of its associated increase in morbidities, such as an increased risk of falls (hazard ratio [HR] = 1.5),5 coronary heart disease (HR = 1.3), stroke (HR = 1.2), and all-cause mortality (HR = 1.4).6 Treatments include physical maneuvers (getting up slowly, leg crossing, and muscle clenching), increased salt and water intake, compression stockings, the addition of medications (such as fludrocortisone or midodrine), and the avoidance of other medications (such as benzodiazepines and diuretics).
The guideline-recommended 3-minute delay in assessment can be impractical in a busy clinical setting. Using data from the Atherosclerosis Risk in Communities (ARIC) study, investigators correlated the timing of measurements of postural change in BP with long-term adverse outcomes.1
STUDY SUMMARY
Early vs late OH assessment in middle-aged adults
The ARIC study is a longitudinal, prospective, cohort study of almost 16,000 adults followed since 1987. Juraschek et al1 assessed the optimal time to identify OH and its association with the adverse clinical outcomes of fall, fracture, syncope, motor vehicle crash, and mortality. The researchers sought to discover whether BP measurements determined immediately after standing predict adverse events as well as BP measurements taken closer to 3 minutes.
Study participants were between the ages of 45 and 64 years (mean 54 years), and 26% were black and 54% were female. They lived in 4 different US communities. The researchers excluded patients with missing OH assessments or other relevant cohort or historical data, leaving a cohort of 11,429 subjects.
Continue to: As part of their...
As part of their enrollment into the ARIC study, subjects had their BP measurements taken 2 to 5 times in the lying position (90% of participants had ≥ 4 measurements) and after standing (91% participants had ≥ 4 measurements) using a programmable automatic BP cuff. All 5 standing BP measurements (taken at a mean of 28, 53, 76, 100, and 116 seconds after standing) were measured for 7385 out of 11,429 (64.6%) participants. Subjects were asked if he or she “usually gets dizzy on standing up.”
Researchers determined the association between OH and postural change in systolic BP or postural change in diastolic BP with history of dizziness after standing. They also determined the incidence of falls, fracture, syncope, motor vehicle crash, and mortality via a review of hospitalizations and billing for Medicaid and Medicare services. Subjects were followed for a median of 23 years.
Results
Of the entire cohort, 1138 (10%) reported dizziness on standing. Only OH identified at the first BP measurement (mean 28 secs) was associated with a history of dizziness upon standing (odds ratio [OR] = 1.49; 95% confidence interval [CI], 1.18-1.89). Also, it was associated with the highest incidence of fracture, syncope, and death (18.9, 17, and 31.4 per 1000 person-years, respectively).
After adjusting for age, sex, and multiple other cardiovascular risk factors, the risk of falls was significantly associated with OH at BP measurements 1 to 4, but was most strongly associated with BP measurement 2 (taken at a mean of 53 secs after standing) (HR = 1.29; 95% CI, 1.12-1.49), which translates to 13.2 falls per 1000 patient-years. Fracture was associated with OH at measurements 1 (HR = 1.16; 95% CI, 1.01-1.34) and 2 (HR = 1.14; 95% CI, 1.01-1.29). Motor vehicle crashes were associated only with BP measurement 2 (HR = 1.43; 95% CI, 1.04-1.96). Finally, risk of syncope and risk of death were statistically associated with the presence of OH at all 5 BP measurements.
WHAT’S NEW
Earlier OH assessments are more informative than late ones
This study found OH identified within 1 minute of standing to be more clinically meaningful than OH identified after 1 minute. Also, the findings reinforce the relationship between OH and adverse events, including injury and overall mortality. Evaluation for OH performed only at 3 minutes may miss symptomatic OH.
Continue to: CAVEATS
CAVEATS
Could a healthy population skew the results?
The population in this study was relatively healthy, with a lower prevalence of diabetes and coronary artery disease than the general population. While there is no reason to expect detection of OH to differ in a population with more comorbidities, the possibility exists.
If OH is not identified in < 1 minute of standing, standard OH evaluation within 3 minutes after standing should be performed, as OH identified at any time point after standing is associated with adverse events and increased mortality.
This study did not address the effects of medical intervention for OH on injury or mortality. Also, whether OH is the direct cause of the adverse outcomes or a marker for other disease is unknown.
CHALLENGES TO IMPLEMENTATION
A change to protocols and guidelines
Although none were noted, any change in practice requires updating clinical protocols and guidelines, which can take time.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 54-year-old woman with a history of hypertension presents with a chief complaint of dizziness. You require an assessment of orthostatic vital signs to proceed. In your busy clinical practice, when should assessment take place to be most useful?
Orthostatic hypotension (OH) is defined as a postural reduction in systolic blood pressure (BP) of ≥ 20 mm Hg or diastolic BP of ≥ 10 mm Hg, measured within 3 minutes of rising from supine to standing. This definition is based on consensus guidelines from the American Academy of Neurology and the American Autonomic Society2 and has been upheld by European guidelines.3
The prevalence of OH is approximately 6% in the general population, with estimates ranging from 10% to 55% in older adults.4 Etiology is often multifactorial; causes may be neurogenic (mediated by autonomic failure as in Parkinson’s disease, multiple system atrophy, or diabetic neuropathy), non-neurogenic (related to medications or hypovolemia), or idiopathic.
It’s important to identify OH because of its associated increase in morbidities, such as an increased risk of falls (hazard ratio [HR] = 1.5),5 coronary heart disease (HR = 1.3), stroke (HR = 1.2), and all-cause mortality (HR = 1.4).6 Treatments include physical maneuvers (getting up slowly, leg crossing, and muscle clenching), increased salt and water intake, compression stockings, the addition of medications (such as fludrocortisone or midodrine), and the avoidance of other medications (such as benzodiazepines and diuretics).
The guideline-recommended 3-minute delay in assessment can be impractical in a busy clinical setting. Using data from the Atherosclerosis Risk in Communities (ARIC) study, investigators correlated the timing of measurements of postural change in BP with long-term adverse outcomes.1
STUDY SUMMARY
Early vs late OH assessment in middle-aged adults
The ARIC study is a longitudinal, prospective, cohort study of almost 16,000 adults followed since 1987. Juraschek et al1 assessed the optimal time to identify OH and its association with the adverse clinical outcomes of fall, fracture, syncope, motor vehicle crash, and mortality. The researchers sought to discover whether BP measurements determined immediately after standing predict adverse events as well as BP measurements taken closer to 3 minutes.
Study participants were between the ages of 45 and 64 years (mean 54 years), and 26% were black and 54% were female. They lived in 4 different US communities. The researchers excluded patients with missing OH assessments or other relevant cohort or historical data, leaving a cohort of 11,429 subjects.
Continue to: As part of their...
As part of their enrollment into the ARIC study, subjects had their BP measurements taken 2 to 5 times in the lying position (90% of participants had ≥ 4 measurements) and after standing (91% participants had ≥ 4 measurements) using a programmable automatic BP cuff. All 5 standing BP measurements (taken at a mean of 28, 53, 76, 100, and 116 seconds after standing) were measured for 7385 out of 11,429 (64.6%) participants. Subjects were asked if he or she “usually gets dizzy on standing up.”
Researchers determined the association between OH and postural change in systolic BP or postural change in diastolic BP with history of dizziness after standing. They also determined the incidence of falls, fracture, syncope, motor vehicle crash, and mortality via a review of hospitalizations and billing for Medicaid and Medicare services. Subjects were followed for a median of 23 years.
Results
Of the entire cohort, 1138 (10%) reported dizziness on standing. Only OH identified at the first BP measurement (mean 28 secs) was associated with a history of dizziness upon standing (odds ratio [OR] = 1.49; 95% confidence interval [CI], 1.18-1.89). Also, it was associated with the highest incidence of fracture, syncope, and death (18.9, 17, and 31.4 per 1000 person-years, respectively).
After adjusting for age, sex, and multiple other cardiovascular risk factors, the risk of falls was significantly associated with OH at BP measurements 1 to 4, but was most strongly associated with BP measurement 2 (taken at a mean of 53 secs after standing) (HR = 1.29; 95% CI, 1.12-1.49), which translates to 13.2 falls per 1000 patient-years. Fracture was associated with OH at measurements 1 (HR = 1.16; 95% CI, 1.01-1.34) and 2 (HR = 1.14; 95% CI, 1.01-1.29). Motor vehicle crashes were associated only with BP measurement 2 (HR = 1.43; 95% CI, 1.04-1.96). Finally, risk of syncope and risk of death were statistically associated with the presence of OH at all 5 BP measurements.
WHAT’S NEW
Earlier OH assessments are more informative than late ones
This study found OH identified within 1 minute of standing to be more clinically meaningful than OH identified after 1 minute. Also, the findings reinforce the relationship between OH and adverse events, including injury and overall mortality. Evaluation for OH performed only at 3 minutes may miss symptomatic OH.
Continue to: CAVEATS
CAVEATS
Could a healthy population skew the results?
The population in this study was relatively healthy, with a lower prevalence of diabetes and coronary artery disease than the general population. While there is no reason to expect detection of OH to differ in a population with more comorbidities, the possibility exists.
If OH is not identified in < 1 minute of standing, standard OH evaluation within 3 minutes after standing should be performed, as OH identified at any time point after standing is associated with adverse events and increased mortality.
This study did not address the effects of medical intervention for OH on injury or mortality. Also, whether OH is the direct cause of the adverse outcomes or a marker for other disease is unknown.
CHALLENGES TO IMPLEMENTATION
A change to protocols and guidelines
Although none were noted, any change in practice requires updating clinical protocols and guidelines, which can take time.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Internal Med. 2017;177:1316-1323.
2. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470.
3. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930-936.
4. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
5. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults: the Cardiovascular Health Study. Hypertension. 1992;19(6 Pt 1):508-519.
6. Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100:406-413.
1. Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Internal Med. 2017;177:1316-1323.
2. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470.
3. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930-936.
4. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
5. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults: the Cardiovascular Health Study. Hypertension. 1992;19(6 Pt 1):508-519.
6. Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100:406-413.
PRACTICE CHANGER
Measure orthostatic vital signs within 1 minute of standing to most accurately correlate dizziness with long-term adverse outcomes. 1
STRENGTH OF RECOMMENDATION
B: Based on a single, high-quality, prospective cohort study with patient-oriented outcomes and good follow-up.
Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Intern Med. 2017;177:1316-1323.
Insomnia symptoms increase likelihood of stroke and heart disease
, according to a large cohort study of adults in China. A greater number of insomnia symptoms is associated with increased risk, and this relationship is more evident in younger adults and in adults without hypertension at baseline, researchers reported Nov. 6 in Neurology.
“These results suggest that, if we can target people who are having trouble sleeping with behavioral therapies, it’s possible that we could reduce the number of cases of stroke, heart attack, and other diseases later down the line,” study author Liming Li, MD, professor of epidemiology at Peking University, Beijing, said in a news release.
To clarify the relationships between individual insomnia symptoms, cardiocerebral vascular diseases, and potential effect modifiers, Dr. Li and colleagues analyzed data from the China Kadoorie Biobank Study. For this study, more than 500,000 adults in China aged 30-79 years completed a baseline survey during 2004-2008. The present analysis included data from 487,200 participants who did not have a history of stroke, coronary heart disease, or cancer at baseline.
For the baseline survey, participants answered questions about whether specific insomnia symptoms occurred at least 3 days per week during the past month. The symptoms included difficulty initiating or maintaining sleep (that is, sleep onset latency of 30 minutes or more after going to bed or waking up in the middle of the night); waking too early and being unable to fall back asleep; and trouble functioning during the day because of bad sleep.
The researchers assessed the incidence of cardiocerebral vascular diseases through 2016 by examining disease registries, national health insurance claims databases, and local records. Investigators identified participants with any cardiocerebral vascular disease and assessed the incidence of ischemic heart disease, acute myocardial infarction, hemorrhagic stroke, and ischemic stroke. The researchers followed each participant until the diagnosis of a cardiocerebral vascular disease outcome, death from any cause, loss to follow-up, or Dec. 31, 2016. The researchers used Cox proportional hazard models to estimate hazard ratios for the association between each insomnia symptom and cardiocerebral vascular disease outcomes. They adjusted the models for established and potential confounding factors, including age, income, smoking status, diet, and physical activity.
More than 16% had any insomnia symptom
Of the 487,200 participants, 11.3% had difficulty initiating or maintaining sleep, 10.4% had early morning awakening, and 2.2% had daytime dysfunction attributed to poor sleep. Compared with participants without insomnia symptoms, participants with insomnia symptoms tended to be older and were more likely to be female, not married, and from a rural area. In addition, those with insomnia symptoms were more likely have depression or anxiety symptoms, lower education level, lower household income, and lower body mass index. They also were more likely to have a history of diabetes mellitus. During a median follow-up of 9.6 years, 130,032 cases of cardiocerebral vascular disease occurred, including 40,348 cases of ischemic heart disease and 45,316 cases of stroke.
After adjustment for potential confounders, each insomnia symptom was associated with greater risk of cardiocerebral vascular disease. For difficulty initiating or maintaining sleep, the hazard ratio was 1.09. For early-morning awakening, the HR was 1.07. For daytime dysfunction, the HR was 1.13. Each insomnia symptom was associated with increased risk of ischemic heart disease and ischemic stroke, whereas only difficulty initiating or maintaining sleep was associated with increased risk of acute MI.
In all, 16.4% of participants reported any insomnia symptom; 10% had one symptom, 5.2% had two symptoms, and 1.2% had three symptoms. “Compared with those without any insomnia symptoms, participants with one, two, or three symptoms had a 7%, 10%, or 18% higher risk of total [cardiocerebral vascular disease] incidence, respectively,” the authors wrote. “Our study is the first large-scale cohort study that identified positive dose-response relationships between the number of insomnia symptoms and risks of [cardiocerebral vascular diseases, ischemic heart disease] and stroke incidence.”
Opportunity for intervention
Compared with clinical diagnostic criteria for insomnia, “individual insomnia symptoms are better defined and more feasible to assess with questionnaires in large-scale population studies and clinical practice,” Dr. Li and colleagues wrote. “Moreover, it is reasonable that insomnia symptoms are more modifiable and precisely targetable through behavioral therapies before developing into clinically significant insomnia disorder. Therefore, future clinical trials or community-based intervention studies should be conducted to test whether lifestyle or sleep hygiene interventions for insomnia symptoms can reduce subsequent [cardiocerebral vascular disease] risks.”
The results suggest that efforts aimed at early detection and intervention should include a focus on younger adults and people who do not have high blood pressure, Dr. Li said.
The self-reported insomnia symptoms used in this study have not been fully validated, the investigators noted. The researchers also lacked information about potential confounders, such as shift work and obstructive sleep apnea, that are risk factors for coronary heart disease or stroke and may interfere with insomnia symptoms. In addition, the study did not capture changes in insomnia symptoms over time.
This study was supported by the National Key Research and Development Program of China, the Chinese Ministry of Science and Technology, and the National Natural Science Foundation of China. The China Kadoorie Biobank surveys were supported by grants from the Kadoorie Charitable Foundation and the U.K. Wellcome Trust. The authors had no relevant disclosures.
SOURCE: Zheng B et al. Neurology. 2019 Nov 6. doi: 10.1212/WNL.0000000000008581.
, according to a large cohort study of adults in China. A greater number of insomnia symptoms is associated with increased risk, and this relationship is more evident in younger adults and in adults without hypertension at baseline, researchers reported Nov. 6 in Neurology.
“These results suggest that, if we can target people who are having trouble sleeping with behavioral therapies, it’s possible that we could reduce the number of cases of stroke, heart attack, and other diseases later down the line,” study author Liming Li, MD, professor of epidemiology at Peking University, Beijing, said in a news release.
To clarify the relationships between individual insomnia symptoms, cardiocerebral vascular diseases, and potential effect modifiers, Dr. Li and colleagues analyzed data from the China Kadoorie Biobank Study. For this study, more than 500,000 adults in China aged 30-79 years completed a baseline survey during 2004-2008. The present analysis included data from 487,200 participants who did not have a history of stroke, coronary heart disease, or cancer at baseline.
For the baseline survey, participants answered questions about whether specific insomnia symptoms occurred at least 3 days per week during the past month. The symptoms included difficulty initiating or maintaining sleep (that is, sleep onset latency of 30 minutes or more after going to bed or waking up in the middle of the night); waking too early and being unable to fall back asleep; and trouble functioning during the day because of bad sleep.
The researchers assessed the incidence of cardiocerebral vascular diseases through 2016 by examining disease registries, national health insurance claims databases, and local records. Investigators identified participants with any cardiocerebral vascular disease and assessed the incidence of ischemic heart disease, acute myocardial infarction, hemorrhagic stroke, and ischemic stroke. The researchers followed each participant until the diagnosis of a cardiocerebral vascular disease outcome, death from any cause, loss to follow-up, or Dec. 31, 2016. The researchers used Cox proportional hazard models to estimate hazard ratios for the association between each insomnia symptom and cardiocerebral vascular disease outcomes. They adjusted the models for established and potential confounding factors, including age, income, smoking status, diet, and physical activity.
More than 16% had any insomnia symptom
Of the 487,200 participants, 11.3% had difficulty initiating or maintaining sleep, 10.4% had early morning awakening, and 2.2% had daytime dysfunction attributed to poor sleep. Compared with participants without insomnia symptoms, participants with insomnia symptoms tended to be older and were more likely to be female, not married, and from a rural area. In addition, those with insomnia symptoms were more likely have depression or anxiety symptoms, lower education level, lower household income, and lower body mass index. They also were more likely to have a history of diabetes mellitus. During a median follow-up of 9.6 years, 130,032 cases of cardiocerebral vascular disease occurred, including 40,348 cases of ischemic heart disease and 45,316 cases of stroke.
After adjustment for potential confounders, each insomnia symptom was associated with greater risk of cardiocerebral vascular disease. For difficulty initiating or maintaining sleep, the hazard ratio was 1.09. For early-morning awakening, the HR was 1.07. For daytime dysfunction, the HR was 1.13. Each insomnia symptom was associated with increased risk of ischemic heart disease and ischemic stroke, whereas only difficulty initiating or maintaining sleep was associated with increased risk of acute MI.
In all, 16.4% of participants reported any insomnia symptom; 10% had one symptom, 5.2% had two symptoms, and 1.2% had three symptoms. “Compared with those without any insomnia symptoms, participants with one, two, or three symptoms had a 7%, 10%, or 18% higher risk of total [cardiocerebral vascular disease] incidence, respectively,” the authors wrote. “Our study is the first large-scale cohort study that identified positive dose-response relationships between the number of insomnia symptoms and risks of [cardiocerebral vascular diseases, ischemic heart disease] and stroke incidence.”
Opportunity for intervention
Compared with clinical diagnostic criteria for insomnia, “individual insomnia symptoms are better defined and more feasible to assess with questionnaires in large-scale population studies and clinical practice,” Dr. Li and colleagues wrote. “Moreover, it is reasonable that insomnia symptoms are more modifiable and precisely targetable through behavioral therapies before developing into clinically significant insomnia disorder. Therefore, future clinical trials or community-based intervention studies should be conducted to test whether lifestyle or sleep hygiene interventions for insomnia symptoms can reduce subsequent [cardiocerebral vascular disease] risks.”
The results suggest that efforts aimed at early detection and intervention should include a focus on younger adults and people who do not have high blood pressure, Dr. Li said.
The self-reported insomnia symptoms used in this study have not been fully validated, the investigators noted. The researchers also lacked information about potential confounders, such as shift work and obstructive sleep apnea, that are risk factors for coronary heart disease or stroke and may interfere with insomnia symptoms. In addition, the study did not capture changes in insomnia symptoms over time.
This study was supported by the National Key Research and Development Program of China, the Chinese Ministry of Science and Technology, and the National Natural Science Foundation of China. The China Kadoorie Biobank surveys were supported by grants from the Kadoorie Charitable Foundation and the U.K. Wellcome Trust. The authors had no relevant disclosures.
SOURCE: Zheng B et al. Neurology. 2019 Nov 6. doi: 10.1212/WNL.0000000000008581.
, according to a large cohort study of adults in China. A greater number of insomnia symptoms is associated with increased risk, and this relationship is more evident in younger adults and in adults without hypertension at baseline, researchers reported Nov. 6 in Neurology.
“These results suggest that, if we can target people who are having trouble sleeping with behavioral therapies, it’s possible that we could reduce the number of cases of stroke, heart attack, and other diseases later down the line,” study author Liming Li, MD, professor of epidemiology at Peking University, Beijing, said in a news release.
To clarify the relationships between individual insomnia symptoms, cardiocerebral vascular diseases, and potential effect modifiers, Dr. Li and colleagues analyzed data from the China Kadoorie Biobank Study. For this study, more than 500,000 adults in China aged 30-79 years completed a baseline survey during 2004-2008. The present analysis included data from 487,200 participants who did not have a history of stroke, coronary heart disease, or cancer at baseline.
For the baseline survey, participants answered questions about whether specific insomnia symptoms occurred at least 3 days per week during the past month. The symptoms included difficulty initiating or maintaining sleep (that is, sleep onset latency of 30 minutes or more after going to bed or waking up in the middle of the night); waking too early and being unable to fall back asleep; and trouble functioning during the day because of bad sleep.
The researchers assessed the incidence of cardiocerebral vascular diseases through 2016 by examining disease registries, national health insurance claims databases, and local records. Investigators identified participants with any cardiocerebral vascular disease and assessed the incidence of ischemic heart disease, acute myocardial infarction, hemorrhagic stroke, and ischemic stroke. The researchers followed each participant until the diagnosis of a cardiocerebral vascular disease outcome, death from any cause, loss to follow-up, or Dec. 31, 2016. The researchers used Cox proportional hazard models to estimate hazard ratios for the association between each insomnia symptom and cardiocerebral vascular disease outcomes. They adjusted the models for established and potential confounding factors, including age, income, smoking status, diet, and physical activity.
More than 16% had any insomnia symptom
Of the 487,200 participants, 11.3% had difficulty initiating or maintaining sleep, 10.4% had early morning awakening, and 2.2% had daytime dysfunction attributed to poor sleep. Compared with participants without insomnia symptoms, participants with insomnia symptoms tended to be older and were more likely to be female, not married, and from a rural area. In addition, those with insomnia symptoms were more likely have depression or anxiety symptoms, lower education level, lower household income, and lower body mass index. They also were more likely to have a history of diabetes mellitus. During a median follow-up of 9.6 years, 130,032 cases of cardiocerebral vascular disease occurred, including 40,348 cases of ischemic heart disease and 45,316 cases of stroke.
After adjustment for potential confounders, each insomnia symptom was associated with greater risk of cardiocerebral vascular disease. For difficulty initiating or maintaining sleep, the hazard ratio was 1.09. For early-morning awakening, the HR was 1.07. For daytime dysfunction, the HR was 1.13. Each insomnia symptom was associated with increased risk of ischemic heart disease and ischemic stroke, whereas only difficulty initiating or maintaining sleep was associated with increased risk of acute MI.
In all, 16.4% of participants reported any insomnia symptom; 10% had one symptom, 5.2% had two symptoms, and 1.2% had three symptoms. “Compared with those without any insomnia symptoms, participants with one, two, or three symptoms had a 7%, 10%, or 18% higher risk of total [cardiocerebral vascular disease] incidence, respectively,” the authors wrote. “Our study is the first large-scale cohort study that identified positive dose-response relationships between the number of insomnia symptoms and risks of [cardiocerebral vascular diseases, ischemic heart disease] and stroke incidence.”
Opportunity for intervention
Compared with clinical diagnostic criteria for insomnia, “individual insomnia symptoms are better defined and more feasible to assess with questionnaires in large-scale population studies and clinical practice,” Dr. Li and colleagues wrote. “Moreover, it is reasonable that insomnia symptoms are more modifiable and precisely targetable through behavioral therapies before developing into clinically significant insomnia disorder. Therefore, future clinical trials or community-based intervention studies should be conducted to test whether lifestyle or sleep hygiene interventions for insomnia symptoms can reduce subsequent [cardiocerebral vascular disease] risks.”
The results suggest that efforts aimed at early detection and intervention should include a focus on younger adults and people who do not have high blood pressure, Dr. Li said.
The self-reported insomnia symptoms used in this study have not been fully validated, the investigators noted. The researchers also lacked information about potential confounders, such as shift work and obstructive sleep apnea, that are risk factors for coronary heart disease or stroke and may interfere with insomnia symptoms. In addition, the study did not capture changes in insomnia symptoms over time.
This study was supported by the National Key Research and Development Program of China, the Chinese Ministry of Science and Technology, and the National Natural Science Foundation of China. The China Kadoorie Biobank surveys were supported by grants from the Kadoorie Charitable Foundation and the U.K. Wellcome Trust. The authors had no relevant disclosures.
SOURCE: Zheng B et al. Neurology. 2019 Nov 6. doi: 10.1212/WNL.0000000000008581.
FROM NEUROLOGY
Key clinical point: The presence of insomnia symptoms increases the likelihood of cardiovascular or cerebrovascular disease during approximately 10 years of follow-up.
Major finding: After adjustment for potential confounders, each insomnia symptom was associated with greater risk of cardiocerebral vascular disease. For difficulty initiating or maintaining sleep, the hazard ratio was 1.09. For early-morning awakening, the HR was 1.07. For daytime dysfunction, the HR was 1.13.
Study details: An analysis of data from 487,200 adults in China aged 30-79 years who completed a baseline survey during 2004-2008 and were followed through 2016.
Disclosures: This study was supported by the National Key Research and Development Program of China, the Chinese Ministry of Science and Technology, and the National Natural Science Foundation of China. The China Kadoorie Biobank surveys were supported by grants from the Kadoorie Charitable Foundation and the U.K. Wellcome Trust. The authors had no relevant disclosures.
Source: Zheng B et al. Neurology. 2019 Nov 6. doi: 10.1212/WNL.0000000000008581.
Cardiometabolic risk burden is high in under-50s with type 2 diabetes
BARCELONA – People diagnosed with type 2 diabetes when they are 18-39 years old have significantly higher cardiometabolic risk burden, compared with older people, according to the results of a large study from the United Kingdom presented at the annual meeting of the European Association for the Study of Diabetes.
Patients in that younger age group were found to have higher glycated hemoglobin (HbA1c) levels, along with higher levels of low-density lipoprotein cholesterol and higher body weight.
“We wanted to evaluate the population-level trend in the incidence of young-onset type 2 diabetes in the United Kingdom, compared with later-onset diabetes,” said senior study author Sanjoy Paul, PhD, the director of the Melbourne EpiCentre at the University of Melbourne at a press briefing during the meeting.
Other aims of the study were to compare temporal trends in the incidence of atherosclerotic cardiovascular disease in younger and older patients with type 2 diabetes, and to see how being “high risk” at diagnosis affected patients’ risk of ASCVD and subsequent risk of death.
High-risk status was defined as having at least two of the risk factors for ASCVD – smoking, high systolic blood pressure, high low-density lipoprotein cholesterol, or chronic kidney disease.
The investigators searched a nationally representative sample of primary care electronic medical records from The Health Improvement Network (THIN) database to find incident cases of type 2 diabetes that occurred between 2000 and 2017, with a total of 370,854 cases identified.
At diagnosis of type 2 diabetes, 8% of the sample (n = 29,678) was aged 18-39 years; 15% (n = 56,798), 40-49 years; 25% (n = 93,698), 50-59 years; 29% (n = 107,261), 60-69 years; and 23% (n = 83,419), 70-79 years. Follow-up was just more than 6 years.
Baseline HbA1c in the respective age groups was 8.6%, 8.4%, 8.1%, 7.8%, and 7.6%, with more than 55% of patients in the two youngest age groups having an HbA1c of 7.5% or higher, compared with 34%-47% in the three oldest age groups.
The percentage of patients with a high LDL cholesterol value (2.6 mmol/L or higher in those without ASCVD, and 1.8 or higher in those with ASCVD) was 71%, 75%, 74%, 69%, and 65%, from the youngest to oldest age groups. A respective 71%, 70%, 66%, 57%, and 44% of the patients had a body mass index of 35 kg/m2 or higher.
Few younger patients had ASCVD at diagnosis (2% of the 18-39 age group; 6% of the 40-49 group), with higher rates in the older age groups (13% of the 50-59 group; 23% of the 60-69 group; and 33% of the 70-79 group).
The percentage of patients considered to be at high risk of ASCVD at diagnosis was 23%, 37%, 45%, 50%, and 53%, respectively, across the five age groups.
Although high systolic blood pressure (SBP; 130 mmHg in those with ASCVD, 140 mmHg in those without) was more common in the older age groups (52% at 50-59 years; 60% at 60-69 years, and 64% at 70-79 years,) a substantial proportion of the younger patients also had a high SBP (27% at 18-39 years and 41% at 40-49 years).
Digsu Koye, PhD, also of the Melbourne EpiCentre, presented the main findings of the study during the meeting, noting that the proportion of people diagnosed when they were younger than 50 years remained stable between 2000 and 2017, with a marginal increase in those diagnosed when they were aged 50-59 years, and a decline in those diagnosed when they were older than 70 years.
In the youngest and oldest age groups, equal numbers of men and women were diagnosed with type 2 diabetes, and more women than men were diagnosed in the 60-69 age group, Dr. Koye said. However, for the 40-49 and 50-59 age groups, there were more men than women diagnosed with type 2 diabetes.
Patients were followed for an average of just more than 6 years. “The rate of atherosclerotic cardiovascular disease was declining in all age categories during 2000-2006, but after that, we saw a stable and consistent pattern for all age categories after 2007,” Dr. Koye observed.
In regard to all-cause mortality, there was a 30% decline in the oldest age group (70-79 years), and a 20% decline in the 60-69 age group, but there was no significant decline in the younger age groups, he added.
The investigators determined the average time to event (ASCVD or all-cause mortality) by high-risk status at type 2 diabetes diagnosis for each age group. These analyses showed that there was little difference between the high- and low-risk groups for the average time to ASCVD or all-cause mortality in the youngest age group, with wider differences in the older patients of 1-2 years for ASCVD and 0.5-2 years for all-cause mortality.
Dr. Koye noted that people with young-onset type 2 diabetes had a risk of ASCVD or all-cause mortality that was similar to that of older people, irrespective of whether or not they were considered to be at high or low risk of events. “So we need a more focused treatment strategy for the youngest age group, irrespective of the cardiometabolic risk level at diagnosis,” he said.
Dr. Paul and Dr. Koye reported having no conflicts of interest.
SOURCE: Koye D et al. EASD 2019, Abstract 82.
BARCELONA – People diagnosed with type 2 diabetes when they are 18-39 years old have significantly higher cardiometabolic risk burden, compared with older people, according to the results of a large study from the United Kingdom presented at the annual meeting of the European Association for the Study of Diabetes.
Patients in that younger age group were found to have higher glycated hemoglobin (HbA1c) levels, along with higher levels of low-density lipoprotein cholesterol and higher body weight.
“We wanted to evaluate the population-level trend in the incidence of young-onset type 2 diabetes in the United Kingdom, compared with later-onset diabetes,” said senior study author Sanjoy Paul, PhD, the director of the Melbourne EpiCentre at the University of Melbourne at a press briefing during the meeting.
Other aims of the study were to compare temporal trends in the incidence of atherosclerotic cardiovascular disease in younger and older patients with type 2 diabetes, and to see how being “high risk” at diagnosis affected patients’ risk of ASCVD and subsequent risk of death.
High-risk status was defined as having at least two of the risk factors for ASCVD – smoking, high systolic blood pressure, high low-density lipoprotein cholesterol, or chronic kidney disease.
The investigators searched a nationally representative sample of primary care electronic medical records from The Health Improvement Network (THIN) database to find incident cases of type 2 diabetes that occurred between 2000 and 2017, with a total of 370,854 cases identified.
At diagnosis of type 2 diabetes, 8% of the sample (n = 29,678) was aged 18-39 years; 15% (n = 56,798), 40-49 years; 25% (n = 93,698), 50-59 years; 29% (n = 107,261), 60-69 years; and 23% (n = 83,419), 70-79 years. Follow-up was just more than 6 years.
Baseline HbA1c in the respective age groups was 8.6%, 8.4%, 8.1%, 7.8%, and 7.6%, with more than 55% of patients in the two youngest age groups having an HbA1c of 7.5% or higher, compared with 34%-47% in the three oldest age groups.
The percentage of patients with a high LDL cholesterol value (2.6 mmol/L or higher in those without ASCVD, and 1.8 or higher in those with ASCVD) was 71%, 75%, 74%, 69%, and 65%, from the youngest to oldest age groups. A respective 71%, 70%, 66%, 57%, and 44% of the patients had a body mass index of 35 kg/m2 or higher.
Few younger patients had ASCVD at diagnosis (2% of the 18-39 age group; 6% of the 40-49 group), with higher rates in the older age groups (13% of the 50-59 group; 23% of the 60-69 group; and 33% of the 70-79 group).
The percentage of patients considered to be at high risk of ASCVD at diagnosis was 23%, 37%, 45%, 50%, and 53%, respectively, across the five age groups.
Although high systolic blood pressure (SBP; 130 mmHg in those with ASCVD, 140 mmHg in those without) was more common in the older age groups (52% at 50-59 years; 60% at 60-69 years, and 64% at 70-79 years,) a substantial proportion of the younger patients also had a high SBP (27% at 18-39 years and 41% at 40-49 years).
Digsu Koye, PhD, also of the Melbourne EpiCentre, presented the main findings of the study during the meeting, noting that the proportion of people diagnosed when they were younger than 50 years remained stable between 2000 and 2017, with a marginal increase in those diagnosed when they were aged 50-59 years, and a decline in those diagnosed when they were older than 70 years.
In the youngest and oldest age groups, equal numbers of men and women were diagnosed with type 2 diabetes, and more women than men were diagnosed in the 60-69 age group, Dr. Koye said. However, for the 40-49 and 50-59 age groups, there were more men than women diagnosed with type 2 diabetes.
Patients were followed for an average of just more than 6 years. “The rate of atherosclerotic cardiovascular disease was declining in all age categories during 2000-2006, but after that, we saw a stable and consistent pattern for all age categories after 2007,” Dr. Koye observed.
In regard to all-cause mortality, there was a 30% decline in the oldest age group (70-79 years), and a 20% decline in the 60-69 age group, but there was no significant decline in the younger age groups, he added.
The investigators determined the average time to event (ASCVD or all-cause mortality) by high-risk status at type 2 diabetes diagnosis for each age group. These analyses showed that there was little difference between the high- and low-risk groups for the average time to ASCVD or all-cause mortality in the youngest age group, with wider differences in the older patients of 1-2 years for ASCVD and 0.5-2 years for all-cause mortality.
Dr. Koye noted that people with young-onset type 2 diabetes had a risk of ASCVD or all-cause mortality that was similar to that of older people, irrespective of whether or not they were considered to be at high or low risk of events. “So we need a more focused treatment strategy for the youngest age group, irrespective of the cardiometabolic risk level at diagnosis,” he said.
Dr. Paul and Dr. Koye reported having no conflicts of interest.
SOURCE: Koye D et al. EASD 2019, Abstract 82.
BARCELONA – People diagnosed with type 2 diabetes when they are 18-39 years old have significantly higher cardiometabolic risk burden, compared with older people, according to the results of a large study from the United Kingdom presented at the annual meeting of the European Association for the Study of Diabetes.
Patients in that younger age group were found to have higher glycated hemoglobin (HbA1c) levels, along with higher levels of low-density lipoprotein cholesterol and higher body weight.
“We wanted to evaluate the population-level trend in the incidence of young-onset type 2 diabetes in the United Kingdom, compared with later-onset diabetes,” said senior study author Sanjoy Paul, PhD, the director of the Melbourne EpiCentre at the University of Melbourne at a press briefing during the meeting.
Other aims of the study were to compare temporal trends in the incidence of atherosclerotic cardiovascular disease in younger and older patients with type 2 diabetes, and to see how being “high risk” at diagnosis affected patients’ risk of ASCVD and subsequent risk of death.
High-risk status was defined as having at least two of the risk factors for ASCVD – smoking, high systolic blood pressure, high low-density lipoprotein cholesterol, or chronic kidney disease.
The investigators searched a nationally representative sample of primary care electronic medical records from The Health Improvement Network (THIN) database to find incident cases of type 2 diabetes that occurred between 2000 and 2017, with a total of 370,854 cases identified.
At diagnosis of type 2 diabetes, 8% of the sample (n = 29,678) was aged 18-39 years; 15% (n = 56,798), 40-49 years; 25% (n = 93,698), 50-59 years; 29% (n = 107,261), 60-69 years; and 23% (n = 83,419), 70-79 years. Follow-up was just more than 6 years.
Baseline HbA1c in the respective age groups was 8.6%, 8.4%, 8.1%, 7.8%, and 7.6%, with more than 55% of patients in the two youngest age groups having an HbA1c of 7.5% or higher, compared with 34%-47% in the three oldest age groups.
The percentage of patients with a high LDL cholesterol value (2.6 mmol/L or higher in those without ASCVD, and 1.8 or higher in those with ASCVD) was 71%, 75%, 74%, 69%, and 65%, from the youngest to oldest age groups. A respective 71%, 70%, 66%, 57%, and 44% of the patients had a body mass index of 35 kg/m2 or higher.
Few younger patients had ASCVD at diagnosis (2% of the 18-39 age group; 6% of the 40-49 group), with higher rates in the older age groups (13% of the 50-59 group; 23% of the 60-69 group; and 33% of the 70-79 group).
The percentage of patients considered to be at high risk of ASCVD at diagnosis was 23%, 37%, 45%, 50%, and 53%, respectively, across the five age groups.
Although high systolic blood pressure (SBP; 130 mmHg in those with ASCVD, 140 mmHg in those without) was more common in the older age groups (52% at 50-59 years; 60% at 60-69 years, and 64% at 70-79 years,) a substantial proportion of the younger patients also had a high SBP (27% at 18-39 years and 41% at 40-49 years).
Digsu Koye, PhD, also of the Melbourne EpiCentre, presented the main findings of the study during the meeting, noting that the proportion of people diagnosed when they were younger than 50 years remained stable between 2000 and 2017, with a marginal increase in those diagnosed when they were aged 50-59 years, and a decline in those diagnosed when they were older than 70 years.
In the youngest and oldest age groups, equal numbers of men and women were diagnosed with type 2 diabetes, and more women than men were diagnosed in the 60-69 age group, Dr. Koye said. However, for the 40-49 and 50-59 age groups, there were more men than women diagnosed with type 2 diabetes.
Patients were followed for an average of just more than 6 years. “The rate of atherosclerotic cardiovascular disease was declining in all age categories during 2000-2006, but after that, we saw a stable and consistent pattern for all age categories after 2007,” Dr. Koye observed.
In regard to all-cause mortality, there was a 30% decline in the oldest age group (70-79 years), and a 20% decline in the 60-69 age group, but there was no significant decline in the younger age groups, he added.
The investigators determined the average time to event (ASCVD or all-cause mortality) by high-risk status at type 2 diabetes diagnosis for each age group. These analyses showed that there was little difference between the high- and low-risk groups for the average time to ASCVD or all-cause mortality in the youngest age group, with wider differences in the older patients of 1-2 years for ASCVD and 0.5-2 years for all-cause mortality.
Dr. Koye noted that people with young-onset type 2 diabetes had a risk of ASCVD or all-cause mortality that was similar to that of older people, irrespective of whether or not they were considered to be at high or low risk of events. “So we need a more focused treatment strategy for the youngest age group, irrespective of the cardiometabolic risk level at diagnosis,” he said.
Dr. Paul and Dr. Koye reported having no conflicts of interest.
SOURCE: Koye D et al. EASD 2019, Abstract 82.
REPORTING FROM EASD 2019
Adverse childhood experiences increase the risk of poor long-term health
and societal outcomes, according to a new report by the Centers for Disease Control and Prevention.
“Our analysis suggests that preventing or reducing these adverse childhood experiences [ACEs] could potentially reduce the annual number of coronary heart disease cases by up to 13%,” said Ann Schuchat, MD, the CDC’s principal deputy director. “If we apply this analysis to other national disease estimates, preventing ACEs could prevent 1.9 million cases of heart disease, 2.5 million cases of overweight or obesity, 21 million cases of depression, and 1.5 million high-school incompletions.”
The analysis, conducted by Melissa T. Merrick, PhD, and colleagues at the National Center for Injury Prevention and Control at the CDC, Atlanta, is based on data acquired from more than 144,000 adults in 27 states.
It’s the first time the CDC has waded into this territory, Dr. Schuchat said during a press briefing. But a hard look into the data is long overdue. ACEs have been linked to at least 5 of the top 10 leading causes of death in the United States: heart disease, cancer, respiratory disease, diabetes, and suicide.
“It’s been proven that exposure to abuse, violence, and familial substance abuse and mental health problems can lead to health and social problems during the entire lifespan. Multiple exposures can produce toxic stress and chronic activation of the stress response system,” Dr. Schuchat continued. “Our report found that more than half of adults have experienced at least one type of ACE, and one in six adults has been exposed to four or more. The effects add up – the more types of ACE encountered, the higher the risk for negative outcomes that limit their entire lives.”
Dr. Merrick, a behavioral scientist with the CDC, and her team reviewed data collected from the Behavioral Risk Factor Surveillance System (BRFSS), a telephone survey of noninstitutionalized adults administered every year within each state. During the 2015-2017 data collection years, 27 states included questions about ACEs. The experiences included childhood exposure to three types of abuse (physical, emotional, and sexual) and five types of household challenges (household member substance misuse, incarceration, mental illness, parental divorce, or witnessing intimate partner violence) before age 18 years.
In all, 61% of respondents reported experiencing at least one of the events; 16% reported experiencing four or more. Women, Native Americans, Native Alaskans, and blacks were more likely to have these experiences than were men and whites.
A multivariate regression analysis found that adults with the highest level of ACE exposure had significantly elevated risks of several chronic health issues and social challenges, compared with nonexposed subjects. These included increased risk of overweight or obesity (adjusted odds ratio, 1.2), chronic obstructive pulmonary disease (aOR, 2.8), depression (aOR 5.3), smoking (aOR 3.1), heavy drinking (aOR 1.8), and underemployment (aOR 1.7), compared with adults reporting no ACEs.
Reducing ACE exposures could in turn reduce many of these challenges, especially among people with the highest number of exposures. Among this group, preventing all ACE exposure could cut overweight and obesity by up to 1.7%, chronic obstructive pulmonary disease by up to 27%, depression by up to 44%, smoking by up to 33%, and heavy drinking by 24%. Preventing ACE exposure also could reduce lack of health insurance by 4% and unemployment by 15%, the researchers said.
The good news, Dr. Merrick and associates said, is that ACE exposure can be at least partially offset by positive interactions with adults and in social and community settings.
“Prevention of adverse childhood experiences is possible with state and community efforts to build resilient families and communities, provide parental support to develop positive parenting and coping skills, and increase access to, and use of, comprehensive health services,” they said.
The CDC recommends a comprehensive approach to preventing ACEs and mitigating their impact. The data-driven suggestions include:
- Promoting family economic health, including tax credits and family-focused work policy.
- Endorsing programs to mitigate violence and adversity, including public education programs that support parents.
- Promoting early childhood development with high-quality child care and preschool programs.
- Recommending stress reduction skills for parents and young people, and programs that teach safe dating and healthy relationship skills.
- Supporting youth development by connecting youth to adult mentors and after-school programs.
- Encouraging clinicians to identify and address ACE exposure with screening, referral, and support.
“This is important for reducing the consequences of adverse childhood experiences and for helping to protect the next generation of children from exposure to violence and other adverse experiences, such as witnessing substance misuse in their household,” Dr. Merrick and associates said.
The researchers had no relevant financial disclosures.
SOURCE: Merrick M et al. MMWR. 2019 Nov 5. doi: 10.15585/mmwr.mm6844e1.
Adverse childhood experiences (ACEs) trigger pathophysiologic responses that exert real physical and psychological harm. Thus, clinicians can and should address them as part of good medical care, Christopher M. Jones, PharmD, Melissa T. Merrick, PhD, and Debra E. Houry, MD, MPH, said in a JAMA commentary.
“A large and growing body of research indicates that the underlying mechanism by which ACEs are associated with health outcomes is through the development of toxic stress, a chronic activation of the stress response system. Toxic stress results in dysregulation of the limbic-hypothalamic-pituitary-adrenal axis, elevating levels of catecholamines (“fight or flight” response), cortisol, and proinflammatory cytokines, leading to cascading effects on the nervous, endocrine, and immune systems. These changes can affect attention and other executive functioning, impulsive behavior, brain reward systems, decision-making, and response to stress throughout the life span,” they said.
While societies and communities at large must work together to reduce ACE exposure, clinicians also have a role. Research indicates that many don’t routinely ask questions about these issues, in a large part because they lack training in how and when to screen.
“Incorporating components of primary ACEs prevention into everyday clinical practice may be achievable through talking with parents and caregivers about creating safe, stable, nurturing environments and protective relationships, and reinforcing positive parenting techniques and coping skills at routine clinical visits,” the editorialists said. “In addition, clinicians can refer parents to parenting skills classes or refer higher-risk parents to home visitation programs such as Healthy Families America and Nurse-Family Partnership. Home visitation programs have demonstrated significant reductions in rates of child abuse and neglect and have improved substance use, violence, and parenting outcomes.”
Clinicians also may have a role to play in mitigating the harms of ACEs, by incorporating trauma-informed care and services into their daily practice.
“Important elements of trauma-informed care include understanding how trauma affects health, routinely screening for ACEs and trauma, using culturally responsive assessments, promoting resilience and protective factors, addressing trauma-related somatic and mental health issues, and ensuring appropriate linkage to services and supports for identified issues,” the editorialists concluded.
Dr. Jones is associate director in the Office of Strategy and Innovation in the CDC Injury Center. Dr. Merrick is president and CEO of Prevent Childhood Abuse America, Chicago. Dr. Houry is director of the National Center for Injury Prevention and Control at the CDC, Atlanta. They discussed the MMWR analysis in a commentary (JAMA. 2019 Nov 5. doi: 10.1001/jama.2019.18499). They had no relevant financial disclosures.
Adverse childhood experiences (ACEs) trigger pathophysiologic responses that exert real physical and psychological harm. Thus, clinicians can and should address them as part of good medical care, Christopher M. Jones, PharmD, Melissa T. Merrick, PhD, and Debra E. Houry, MD, MPH, said in a JAMA commentary.
“A large and growing body of research indicates that the underlying mechanism by which ACEs are associated with health outcomes is through the development of toxic stress, a chronic activation of the stress response system. Toxic stress results in dysregulation of the limbic-hypothalamic-pituitary-adrenal axis, elevating levels of catecholamines (“fight or flight” response), cortisol, and proinflammatory cytokines, leading to cascading effects on the nervous, endocrine, and immune systems. These changes can affect attention and other executive functioning, impulsive behavior, brain reward systems, decision-making, and response to stress throughout the life span,” they said.
While societies and communities at large must work together to reduce ACE exposure, clinicians also have a role. Research indicates that many don’t routinely ask questions about these issues, in a large part because they lack training in how and when to screen.
“Incorporating components of primary ACEs prevention into everyday clinical practice may be achievable through talking with parents and caregivers about creating safe, stable, nurturing environments and protective relationships, and reinforcing positive parenting techniques and coping skills at routine clinical visits,” the editorialists said. “In addition, clinicians can refer parents to parenting skills classes or refer higher-risk parents to home visitation programs such as Healthy Families America and Nurse-Family Partnership. Home visitation programs have demonstrated significant reductions in rates of child abuse and neglect and have improved substance use, violence, and parenting outcomes.”
Clinicians also may have a role to play in mitigating the harms of ACEs, by incorporating trauma-informed care and services into their daily practice.
“Important elements of trauma-informed care include understanding how trauma affects health, routinely screening for ACEs and trauma, using culturally responsive assessments, promoting resilience and protective factors, addressing trauma-related somatic and mental health issues, and ensuring appropriate linkage to services and supports for identified issues,” the editorialists concluded.
Dr. Jones is associate director in the Office of Strategy and Innovation in the CDC Injury Center. Dr. Merrick is president and CEO of Prevent Childhood Abuse America, Chicago. Dr. Houry is director of the National Center for Injury Prevention and Control at the CDC, Atlanta. They discussed the MMWR analysis in a commentary (JAMA. 2019 Nov 5. doi: 10.1001/jama.2019.18499). They had no relevant financial disclosures.
Adverse childhood experiences (ACEs) trigger pathophysiologic responses that exert real physical and psychological harm. Thus, clinicians can and should address them as part of good medical care, Christopher M. Jones, PharmD, Melissa T. Merrick, PhD, and Debra E. Houry, MD, MPH, said in a JAMA commentary.
“A large and growing body of research indicates that the underlying mechanism by which ACEs are associated with health outcomes is through the development of toxic stress, a chronic activation of the stress response system. Toxic stress results in dysregulation of the limbic-hypothalamic-pituitary-adrenal axis, elevating levels of catecholamines (“fight or flight” response), cortisol, and proinflammatory cytokines, leading to cascading effects on the nervous, endocrine, and immune systems. These changes can affect attention and other executive functioning, impulsive behavior, brain reward systems, decision-making, and response to stress throughout the life span,” they said.
While societies and communities at large must work together to reduce ACE exposure, clinicians also have a role. Research indicates that many don’t routinely ask questions about these issues, in a large part because they lack training in how and when to screen.
“Incorporating components of primary ACEs prevention into everyday clinical practice may be achievable through talking with parents and caregivers about creating safe, stable, nurturing environments and protective relationships, and reinforcing positive parenting techniques and coping skills at routine clinical visits,” the editorialists said. “In addition, clinicians can refer parents to parenting skills classes or refer higher-risk parents to home visitation programs such as Healthy Families America and Nurse-Family Partnership. Home visitation programs have demonstrated significant reductions in rates of child abuse and neglect and have improved substance use, violence, and parenting outcomes.”
Clinicians also may have a role to play in mitigating the harms of ACEs, by incorporating trauma-informed care and services into their daily practice.
“Important elements of trauma-informed care include understanding how trauma affects health, routinely screening for ACEs and trauma, using culturally responsive assessments, promoting resilience and protective factors, addressing trauma-related somatic and mental health issues, and ensuring appropriate linkage to services and supports for identified issues,” the editorialists concluded.
Dr. Jones is associate director in the Office of Strategy and Innovation in the CDC Injury Center. Dr. Merrick is president and CEO of Prevent Childhood Abuse America, Chicago. Dr. Houry is director of the National Center for Injury Prevention and Control at the CDC, Atlanta. They discussed the MMWR analysis in a commentary (JAMA. 2019 Nov 5. doi: 10.1001/jama.2019.18499). They had no relevant financial disclosures.
and societal outcomes, according to a new report by the Centers for Disease Control and Prevention.
“Our analysis suggests that preventing or reducing these adverse childhood experiences [ACEs] could potentially reduce the annual number of coronary heart disease cases by up to 13%,” said Ann Schuchat, MD, the CDC’s principal deputy director. “If we apply this analysis to other national disease estimates, preventing ACEs could prevent 1.9 million cases of heart disease, 2.5 million cases of overweight or obesity, 21 million cases of depression, and 1.5 million high-school incompletions.”
The analysis, conducted by Melissa T. Merrick, PhD, and colleagues at the National Center for Injury Prevention and Control at the CDC, Atlanta, is based on data acquired from more than 144,000 adults in 27 states.
It’s the first time the CDC has waded into this territory, Dr. Schuchat said during a press briefing. But a hard look into the data is long overdue. ACEs have been linked to at least 5 of the top 10 leading causes of death in the United States: heart disease, cancer, respiratory disease, diabetes, and suicide.
“It’s been proven that exposure to abuse, violence, and familial substance abuse and mental health problems can lead to health and social problems during the entire lifespan. Multiple exposures can produce toxic stress and chronic activation of the stress response system,” Dr. Schuchat continued. “Our report found that more than half of adults have experienced at least one type of ACE, and one in six adults has been exposed to four or more. The effects add up – the more types of ACE encountered, the higher the risk for negative outcomes that limit their entire lives.”
Dr. Merrick, a behavioral scientist with the CDC, and her team reviewed data collected from the Behavioral Risk Factor Surveillance System (BRFSS), a telephone survey of noninstitutionalized adults administered every year within each state. During the 2015-2017 data collection years, 27 states included questions about ACEs. The experiences included childhood exposure to three types of abuse (physical, emotional, and sexual) and five types of household challenges (household member substance misuse, incarceration, mental illness, parental divorce, or witnessing intimate partner violence) before age 18 years.
In all, 61% of respondents reported experiencing at least one of the events; 16% reported experiencing four or more. Women, Native Americans, Native Alaskans, and blacks were more likely to have these experiences than were men and whites.
A multivariate regression analysis found that adults with the highest level of ACE exposure had significantly elevated risks of several chronic health issues and social challenges, compared with nonexposed subjects. These included increased risk of overweight or obesity (adjusted odds ratio, 1.2), chronic obstructive pulmonary disease (aOR, 2.8), depression (aOR 5.3), smoking (aOR 3.1), heavy drinking (aOR 1.8), and underemployment (aOR 1.7), compared with adults reporting no ACEs.
Reducing ACE exposures could in turn reduce many of these challenges, especially among people with the highest number of exposures. Among this group, preventing all ACE exposure could cut overweight and obesity by up to 1.7%, chronic obstructive pulmonary disease by up to 27%, depression by up to 44%, smoking by up to 33%, and heavy drinking by 24%. Preventing ACE exposure also could reduce lack of health insurance by 4% and unemployment by 15%, the researchers said.
The good news, Dr. Merrick and associates said, is that ACE exposure can be at least partially offset by positive interactions with adults and in social and community settings.
“Prevention of adverse childhood experiences is possible with state and community efforts to build resilient families and communities, provide parental support to develop positive parenting and coping skills, and increase access to, and use of, comprehensive health services,” they said.
The CDC recommends a comprehensive approach to preventing ACEs and mitigating their impact. The data-driven suggestions include:
- Promoting family economic health, including tax credits and family-focused work policy.
- Endorsing programs to mitigate violence and adversity, including public education programs that support parents.
- Promoting early childhood development with high-quality child care and preschool programs.
- Recommending stress reduction skills for parents and young people, and programs that teach safe dating and healthy relationship skills.
- Supporting youth development by connecting youth to adult mentors and after-school programs.
- Encouraging clinicians to identify and address ACE exposure with screening, referral, and support.
“This is important for reducing the consequences of adverse childhood experiences and for helping to protect the next generation of children from exposure to violence and other adverse experiences, such as witnessing substance misuse in their household,” Dr. Merrick and associates said.
The researchers had no relevant financial disclosures.
SOURCE: Merrick M et al. MMWR. 2019 Nov 5. doi: 10.15585/mmwr.mm6844e1.
and societal outcomes, according to a new report by the Centers for Disease Control and Prevention.
“Our analysis suggests that preventing or reducing these adverse childhood experiences [ACEs] could potentially reduce the annual number of coronary heart disease cases by up to 13%,” said Ann Schuchat, MD, the CDC’s principal deputy director. “If we apply this analysis to other national disease estimates, preventing ACEs could prevent 1.9 million cases of heart disease, 2.5 million cases of overweight or obesity, 21 million cases of depression, and 1.5 million high-school incompletions.”
The analysis, conducted by Melissa T. Merrick, PhD, and colleagues at the National Center for Injury Prevention and Control at the CDC, Atlanta, is based on data acquired from more than 144,000 adults in 27 states.
It’s the first time the CDC has waded into this territory, Dr. Schuchat said during a press briefing. But a hard look into the data is long overdue. ACEs have been linked to at least 5 of the top 10 leading causes of death in the United States: heart disease, cancer, respiratory disease, diabetes, and suicide.
“It’s been proven that exposure to abuse, violence, and familial substance abuse and mental health problems can lead to health and social problems during the entire lifespan. Multiple exposures can produce toxic stress and chronic activation of the stress response system,” Dr. Schuchat continued. “Our report found that more than half of adults have experienced at least one type of ACE, and one in six adults has been exposed to four or more. The effects add up – the more types of ACE encountered, the higher the risk for negative outcomes that limit their entire lives.”
Dr. Merrick, a behavioral scientist with the CDC, and her team reviewed data collected from the Behavioral Risk Factor Surveillance System (BRFSS), a telephone survey of noninstitutionalized adults administered every year within each state. During the 2015-2017 data collection years, 27 states included questions about ACEs. The experiences included childhood exposure to three types of abuse (physical, emotional, and sexual) and five types of household challenges (household member substance misuse, incarceration, mental illness, parental divorce, or witnessing intimate partner violence) before age 18 years.
In all, 61% of respondents reported experiencing at least one of the events; 16% reported experiencing four or more. Women, Native Americans, Native Alaskans, and blacks were more likely to have these experiences than were men and whites.
A multivariate regression analysis found that adults with the highest level of ACE exposure had significantly elevated risks of several chronic health issues and social challenges, compared with nonexposed subjects. These included increased risk of overweight or obesity (adjusted odds ratio, 1.2), chronic obstructive pulmonary disease (aOR, 2.8), depression (aOR 5.3), smoking (aOR 3.1), heavy drinking (aOR 1.8), and underemployment (aOR 1.7), compared with adults reporting no ACEs.
Reducing ACE exposures could in turn reduce many of these challenges, especially among people with the highest number of exposures. Among this group, preventing all ACE exposure could cut overweight and obesity by up to 1.7%, chronic obstructive pulmonary disease by up to 27%, depression by up to 44%, smoking by up to 33%, and heavy drinking by 24%. Preventing ACE exposure also could reduce lack of health insurance by 4% and unemployment by 15%, the researchers said.
The good news, Dr. Merrick and associates said, is that ACE exposure can be at least partially offset by positive interactions with adults and in social and community settings.
“Prevention of adverse childhood experiences is possible with state and community efforts to build resilient families and communities, provide parental support to develop positive parenting and coping skills, and increase access to, and use of, comprehensive health services,” they said.
The CDC recommends a comprehensive approach to preventing ACEs and mitigating their impact. The data-driven suggestions include:
- Promoting family economic health, including tax credits and family-focused work policy.
- Endorsing programs to mitigate violence and adversity, including public education programs that support parents.
- Promoting early childhood development with high-quality child care and preschool programs.
- Recommending stress reduction skills for parents and young people, and programs that teach safe dating and healthy relationship skills.
- Supporting youth development by connecting youth to adult mentors and after-school programs.
- Encouraging clinicians to identify and address ACE exposure with screening, referral, and support.
“This is important for reducing the consequences of adverse childhood experiences and for helping to protect the next generation of children from exposure to violence and other adverse experiences, such as witnessing substance misuse in their household,” Dr. Merrick and associates said.
The researchers had no relevant financial disclosures.
SOURCE: Merrick M et al. MMWR. 2019 Nov 5. doi: 10.15585/mmwr.mm6844e1.
FROM MMWR