User login
Comorbidities and Nonalcoholic Fatty Liver Disease: The Chicken, the Egg, or Both?
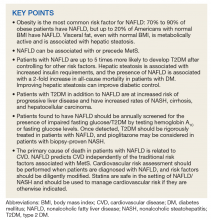
Nonalcoholic fatty liver disease (NALFD) is now the most common chronic liver disease in the developed world and affects about 25% to 30% of adults in the US and 30% of veterans who receive care in the VHA system (Figure 1).
Related:
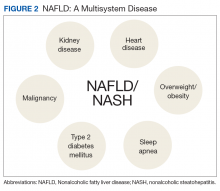
NAFLD is significantly associated with the presence of MetS, so much so that it has been considered the hepatic manifestation of MetS. NAFLD also is strongly associated with type 2 diabetes mellitus (T2DM), CVD, chronic kidney disease (CKD), and obstructive sleep apnea (OSA) (Figure 2).
Obesity/Visceral Adiposity
Obesity (body mass index [BMI] > 30) prevalence in the US has almost doubled over the past 30 years and continues to climb.1 Obesity affects 41% of veterans in the Veterans Health Administration and is the most common risk factor for NAFLD.2 NAFLD is 4 times more prevalent in obese patients, thus, it is not surprising that 80% to 90% of patients evaluated in bariatric centers have NAFLD, reported in 2 large series.3,4 Increased BMI and waist circumference predict the presence of NASH and advanced fibrosis.5
While obesity is a hallmark for NAFLD, particularly in the US, it is important to note that up to 20% of Americans with normal BMI have NAFLD, based on findings of steatosis on ultrasound.6 These patients with lean NAFLD are often underdiagnosed. In addition to the patient’s BMI, it is important to recognize that in NAFLD, the distribution and type of fat deposition is more important than just BMI. Visceral fat refers to fat accumulation within the abdominal cavity and is key to the pathogenesis of NAFLD. Visceral fat, compared with subcutaneous fat, is metabolically active and can deliver an overabundance of free fatty acids to the liver as well as secrete proinflammatory mediators in the setting of insulin resistance. Visceral fat stores can predict increased hepatic fat content, inflammation, and fibrosis.5 Thus, it is important to recognize that those patients with relatively more visceral fat are more prone to NAFLD. The best clinical indicator of visceral adiposity is abdominal obesity, indicated by waist circumference > 40 inches in men and > 35 inches in women.
Metabolic Syndrome
Hepatic fat deposition can be associated with or precede MetS. MetS is defined as having at least 3 of the following characteristics: abdominal obesity, elevated triglycerides (TGs) (≥ 150 mg/dL), reduced high-density lipoprotein cholesterol (< 40 mg/dL in men or < 50 mg/dL in women), elevated blood pressure (BP) (systolic BP ≥ 130 mm Hg or diastolic BP ≥ 85 mm Hg), or elevated fasting glucose (≥ 110 mg/dL). Population studies have found that 50% of patients with MetS have NAFLD, and liver fat content is strongly correlated with the number of MetS features present in an individual.5,7 In addition to this association, NAFLD also promotes the development of MetS. Increased energy intake relative to energy expenditure will facilitate ectopic fat accumulation in the liver, which then increases hepatic gluconeogenesis and drives the pathogenesis of insulin resistance.8 Therefore, the presence of NAFLD is both a marker and a promotor of insulin resistance and its complications.
Related:
Type 2 Diabetes Mellitus
At 70% to 75%, the prevalence of NAFLD in patients with T2DM is more than twice as high as that in the general US adult population. Conversely, about 23% of patients with NAFLD also have T2DM.9
Influence of NAFLD on T2DM
Patients with ultrasound-based evidence of NAFLD are 2 to 5 times more likely to develop T2DM after adjusting for lifestyle and metabolic risk factors in multiple epidemiologic studies.10,11 The severity of hepaticfat content measured by ultrasound also is associated with an increasing risk of T2DM incidence over the next 5 years (normal,7%; mild, 9.8%; moderate-severe, 17.8%; P < .001).12 In another study, 58% of patientswith biopsy-proven NAFLD developed T2DM after a mean follow-up of 13.7 years.13 Those who were found to have NASH had a 3-fold higher risk of developing T2DM than did those with simple steatosis. This finding was confirmed in another study where T2DM incidence was 2 times higher in patients predicted to have advanced fibrosis compared with those who did not.14
Because liver steatosis interferes with insulin-induced glycogen production and suppression of gluconeogenesis, hepatic fat content predicts the insulin dose required for adequate glucose control in patients with diabetes mellitus (DM) and NAFLD.15 Higher levels of insulin are required in patients with DM and NAFLD compared with those without NAFLD.5
Additionally, a 10-year cohort study found that resolution of ultrasound-based NAFLD in patients without baseline T2DM, was associated with a reduced T2DM incidence (multivariate odds ratio [OR] 0.27, 95% CI, 0.12-0.61) after controlling for factors such as age, BMI, and impaired fasting glucose.11,17
Given this close relationship between T2DM and NAFLD, both the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of Liver Diseases (EASL) guidelines recommend that patients found to have NAFLD should be screened for the presence of impaired fasting glucose/T2DM by testing hemoglobin A1c or fasting glucose levels.18,19 Recognizing the role that NAFLD can play in patients with DM also is important, as improving hepatic steatosis may also improve DM.
Influence of DM on NAFLD
Patients with T2DM and NAFLD are at increased risk of progressive liver disease and have increased rates of NASH, cirrhosis, and HCC. In a paired-biopsy study, the development of T2DM was the strongest predictor of progression of NASH and hepatic fibrosis.20 This fibrosis progression can easily go undetected, as NASH can be present even with normal aminotransferases. This increased risk of fibrosis progression in the setting of comorbid T2DM is clinically important, as it is the severity of fibrosis that predicts all-cause and liver-related mortality in patients with NAFLD/NASH.21,22 In fact, the prevalence of biopsy-proven NASH in overweight/obese patients with DM with normal liver aminotransferases (defined as aspartate aminotransferase and alanine aminotransferase < 40 U/L) was found to be 58%.23 Because chronic liver disease, including NAFLD, is underrecognized in the “healthy population” used to establish normal aminotransferase levels, more recent AASLD and ACG guidelines now define normal aminotransferase levels as < 35 U/L for males and < 25 U/L for females.24 These stricter cutoffs are based on populations with normal BMI and negative testing for chronic liver diseases.24 The lower cutoffs may improve recognition of progressive liver disease in NAFLD and NASH patients.
Medications used in the treatment of T2DM, such as metformin, pioglitazone, and liraglutide, have been studied in patients with biopsy-proven NASH. The initial data showing histologic improvement in NAFLD patients taking metformin was more likely related to the associated weight loss in the treatment group. In a study by Loomba and colleagues the improvement in the NAFLD activity score was only seen in patients who lost ≥ 5% of their total body weight.25 Pioglitazone is a PPAR-γ agonist that helps regulate glucose and lipid metabolism as well as inflammation. Pioglitazone helps adipose tissue, hepatocytes, and muscle cells restore insulin sensitivity. A recent trial in 100 patients with prediabetes or T2DM as well as NASH showed that 36 weeks of pioglitazone treatment was associated with significant improvements in steatosis, inflammation, and most important, in stage of fibrosis compared with that of placebo.26
Related:
Glucagon-like peptide-1 (GLP-1) receptor agonists, such as liraglutide, have effects on lipid and glucose metabolism as well. They can lower glucose levels by increasing insulin secretion, reducing glucagon concentration, suppressing appetite (resulting in weight loss), and increasing sensitivity to insulin in hepatocytes and adipocytes. Liraglutide has been studied in patients with NASH both with and without DM, and results of the largest study to date show that it is associated with significant improvement in hepatic inflammation compared with that of placebo.27 Additional phase 3 clinical trials are currently underway.
Current AASLD guidelines do not recommend routine screening for NAFLD, even among high-risk patients, such as patients with DM.18 This is due to the widespread prevalence of NAFLD, the unclear utility of diagnostic tests, and limited efficacy of available treatment. Lifestyle modification to achieve weight loss remains the backbone of management, and rates of successful adherence are low.28 Contrary to this, EASL guidelines state that NAFLD screening with ultrasound even in patients with normal liver enzymes should be performed in high-risk patients with T2DM.19
Once detected, T2DM should be diligently treated in patients with NAFLD, and pioglitazone may be considered in patients with biopsy-proven NASH per AASLD guidelines.18 Pioglitazone has been studied in patients with biopsy-proven NASH both with and without DM and has been associated with significant resolution of NASH, as well as improvement in histologic changes of NASH and improvement in fibrosis.29,30 Because of potential medication AEs, including a mean weight gain of 2.5 kg to 4.7 kg in trials of 12- to 36-months’ duration, as well as potential bone loss in women, discussions about the risks and benefits of treatment should occur prior to treatment initiation.18 Additionally, pioglitazone is not safe in the setting of left ventricular heart failure. Future studies may point to the utility of other DM medications, such as GLP-receptor agonists.
Cardiovascular Disease
Given the association between features of MetS and NAFLD, it is not surprising that the primary cause of death in patients with NAFLD is related to CVD.21,22,31 However, it is increasingly recognized that NAFLD predicts CVD independently of the traditional risk factors associated with MetS. The increase in cardiovascular risk in the setting of NAFLD can be partly explained by the increased hepatic de novo lipogenesis that is associated with increased production of highly atherogenic small dense low-density lipoproteins (sd-LDL) independent of BMI and presence of insulin resistance.32 Additionally, increased intracellular free fatty acids can activate proinflammatory cytokine production by hepatocytes in addition to the increase in systemic inflammatory mediators and oxidative stress associated with NASH.
A recent meta-analysis of 27 studies confirmed the association between NAFLD and many subclinical features of CVD, including increases in coronary-artery calcium score, carotid artery intimal media thickness, and arterial wall stiffness, as well as impaired flow-mediated vasodilation after controlling for classic CVD risk factors.33 The risk of subclinical carotid and coronary atherosclerosis progression was higher in NAFLD patients with evidence of advanced fibrosis using noninvasive measures. Additionally, NAFLD was associated with increased severity of coronary artery disease in > 600 patients undergoing cardiac angiograms.34 Conversely, the regression of NAFLD on ultrasound was associated with a decreased risk of carotid atherosclerosis progression.35
Multiple epidemiologic studies have found an increased incidence of clinically overt CVD in patients with NAFLD after controlling for confounders. The largest updated meta-analysis, which included more than 34,000 patients with 2,600 CVD outcomes over a median of 6.9 years found that the presence of NAFLD (based on imaging or biopsy) was associated with an odds ratio (OR) of 1.64 (95% CI, 1.26-2.13) for fatal and nonfatal incident CVD.36 In the same meta-analysis, patients with NASH, with or without fibrosis, were at an even higher risk, with an OR of 2.58 (95% CI, 1.78-3.75).
Initial studies of statin medications for the treatment of NASH using surrogate endpoints like improvement in aminotransferases or imaging, suggested a potential liver-related benefit. However, there was no histologic improvement in the single study comparing 12 months of simvastatin therapy with placebo in patients with NASH.37 Although it is unclear whether statin use will directly improve NAFLD, there is no evidence to suggest that statin use should be avoided in patients with elevated CVD risk.38 Treatment with atorvastatin has been shown to be associated with a greater reduction in cardiovascular events in patients with NAFLD compared with that of patients without NAFLD.39
The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies. The clinical algorithms currently used to help risk stratify patients and determine appropriate preventative strategies, the Framingham risk equation or the systemic coronary risk evaluation, do not incorporate NAFLD as a potential risk factor for CVD. Additional studies are needed to determine whether adding NAFLD to the assessment will improve the predictive accuracy of future CVD events. Nevertheless, European clinical guidelines recommend performing a CVD risk assessment for patients with NAFLD.19
Chronic Kidney Disease
The prevalence of CKD, defined as estimated glomerular filtration rate (GFR) < 60 mL/min/1.72 m2, abnormal albuminuria, or proteinuria, is significantly increased in patients with NAFLD. Several epidemiologic studies have shown the prevalence of CKD in NAFLD patients ranges from 20% to 55% compared with 5% to 30% among patients without NAFLD.40 Overall, patients with NAFLD have a 2-fold increased risk of prevalent (OR 2.12; 95% CI, 1.69-2.66) or incident (hazard ratio 1.79; 95% CI, 1.65-1.95) CKD, even after adjusting for T2DM, visceral fat, and insulin resistance.40 There is an additional 2-fold increase in CKD risk in patients with NASH and advanced fibrosis compared with those with NASH and mild/no fibrosis. Additionally, advancing NASH fibrosis stage is independently associated with worsening stage of CKD.41
Data regarding the exact mechanism of kidney pathology in the setting of NAFLD is lacking. The accelerated atherogenesis in NAFLD likely contributes to renal damage. Another potential mechanism to explain the association between NASH and CKD involves the increased activation of the angiotensin-aldosterone system (RAAS) seen in NASH, which leads to increased hepatic fibrogenesis as well as kidney damage.42
Similar to the previously listed comorbidities, there is evidence that improvement in NAFLD can lead to improvements in renal disease. A prospective study of NASH patients undergoing 52 weeks of lifestyle modification found that the patients who had improvements in histologic NASH endpoints also had improvement in renal function.43
There are currently no specific recommendations on screening for CKD in professionalguidelines, but many experts propose monitoring for CKD yearly with serum creatinine and urinalysis and referring to nephrology if needed. Given the association between NASH and activation of the RAAS pathway that is associated with worsening hepatic fibrosis, RAAS-inhibitors should be a first-line agent in the treatment of hypertension in patients with NAFLD.
Obstructive Sleep Apnea
OSA is characterized by repeated pharyngeal collapse during sleep, which leads to chronic intermittent hypoxia and is associated with increased metabolic and cardiovascular morbidity and mortality. The cycle of intermittent hypoxia and reoxygenation in OSA results in inflammation and oxidative stress. Multiple studies have supported a link between NAFLD and OSA.
Hepatic fat content on ultrasound was increased in patients with OSA independent of BMI. There also has been evidence of a positive association between the severity of chronic intermittent hypoxia and increased hepatic fibrosis based on liver elastography.44 A meta-analysis using histologic NAFLD diagnosis showed that the presence of OSA was associated with a higher risk of fibrosis compared with that of patients with NAFLD without OSA (OR 2.6; 95% CI, 1.3-5.2).45
Based on animal models, hypoxia can drive fat accumulation and inflammation in the liver via multiple different pathways. Hypoxia can increase fasting glucose and systemic TG levels and induce hepatic lipogenesis by altering gene expression.45 Hypoxia also can increase oxidative stress and reduce β-oxidation, which leads to the production of lipotoxic lipids. These hypoxia-induced changes are typically more pronounced in subjects with obesity compared with that in subjects without obesity. Despite multiple adverse metabolic effects of OSA-induced hypoxia in the setting of NAFLD, preliminary, short-term studies have failed to find an association with OSA treatment with continuous positive airway pressure and improvement in NAFLD.45 Perhaps larger, long-term prospective trials will clarify this question.
Malignancy
Extrahepatic malignancy (colon, esophagus, stomach, pancreas, kidney, and breast) is the second most common cause of death in patients with NAFLD.21,22 The primary association between NAFLD and malignancy is found in the colon. Most large population-based studies have been performed in East Asia and have found that NAFLD is associated with a 1.5 to 1.7-fold increased risk for colonic adenomas and a 1.9 to 3.1-fold increased risk of colorectal cancer.46-49 Using magnetic resonance spectroscopy and liver biopsy to diagnose NAFLD and NASH, respectively, Wong and colleagues found that NASH, but not simple steatosis, is associated with a higher risk of advanced colorectalneoplasia (OR 5.34; 95% CI, 1.9-14.8), after adjusting for age, gender, BMI, family history, smoking, and T2DM.50
Data showing a definitive causative role of NAFLD in the development of colorectal cancer are lacking, but the presence of increased insulin levels has many potential effects on carcinogenesis in general, including stimulation of cell proliferation and apoptosis. Currently, there are no recommended changes to the standard colorectal cancer screening recommendations specifically for patients with NAFLD.
Conclusion
NAFLD is a multisystem disease that is associated with increased liver-related and all-cause mortality. Data on the close association between NAFLD and several extrahepatic complications, including MetS, T2DM, CVD, CKD, and malignancy are well established. There also is growing evidence of a bidirectional relationship between some of these diagnoses, whereas NAFLD is not only a consequence, but also a cause of MetS, T2DM, and CKD independent of other typical risk factors.
Given the multiple comorbidities associated with NAFLD and its potential to influence the severity of these diagnoses, management of these complex patients requires diligence and a multidisciplinary approach. In order to engage in early recognition and intervention to prevent potential morbidity and mortality, regular screening and surveillance for the development of NAFLD in patients with metabolic risk factors can be considered, and careful screening for metabolic complications in patients with established NAFLD is important.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2014.
2. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
3. Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45(4):600-606.
4. Subichin M, Clanton J, Makuszewski M, Bohon A, Zografakis JG, Dan A. Liver disease in the morbidly obese: a review of 1000 consecutive patients undergoing weight loss surgery. Surg Obes Relat Dis. 2015;11(1):137-141.
5. Non-alcoholic Fatty Liver Disease Study Group, Lonardo A, Bellentani S, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: focus on high-risk groups. Dig Liver Dis. 2015;47(12):997-1006.
6. Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15(4):474-485.
7. Kotronen A, Westerbacka J, Bergholm R, Pietiläinen KH, Yki-Järvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92(9):3490-3497.
8. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131-1141.
9. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
10. Armstrong MJ, Adams LA, Canbay A, et al. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174-1197.
11. Kashanian S, Fuchs M. Non-alcoholic fatty liver disease in patients with diabetes mellitus: a clinician’s perspective. Int J Dig Dis. 2015;1:1.
12. Park SK, Seo MH, Shin HC, Ryoo JH. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology. 2013;57(4):1378-1383.
13. Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
14. Chang Y, Jung HS, Yun KE, Cho J, Cho YK, Ryu S. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am J Gastroenterol. 2013;108(12):1861-1868.
15. Ryysy L, Hakkinen AM, Goto T, et al. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes. 2000;49(5):749-758.
16. Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105(7):1567-1573.
17. Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H. Independent association between improvement in nonalcoholic fatty liver disease and reduced risk of incidence of type 2 diabetes. Diabetes Care. 2015;38(9):1673-1679.
18. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
19. European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. EASL-EASD-EASO clinical practice guidelines for the management of nonalcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
20. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148-1155.
21. Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547-1554.
22. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic feature, is associated with long-term outcomes in patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389-397.
23. Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal aminotransferases. J Clin Endocrinol. Metab. 2015;100(6):2231-2238.
24. Kwo PY, Cohen SM, and Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18-35.
25. Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29(2):172-182.
26. Cusi K, Orsak B, Lomonaco R, et al. Extended treatment with pioglitazone improves liver histology in patients with pre-diabetes or type 2 diabetes mellitus and NASH. Hepatology. 2013;58(supp 1):248a.
27. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690.
28. Patel YA, Gifford EJ, Glass LM, et al. Risk factors for biopsy-proven advanced non-alcoholic fatty liver disease in the Veterans Health Administration. Aliment Pharmacol Ther. 2018;47(2):268-278.
29. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305-315.
30. Sanyal AJ, Chalasani N, Kowdley KV, et al; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675-1685.
31. Ekstedt M, Frazen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
32. Vanni E, Marengo A, Mezzabotta L, Bugianesi E. Systemic complications of nonalcoholic fatty liver disease: when the liver is not an innocent bystander. Semin Liver Dis. 2015;35(3): 236-249.
33. Oni ET, Agatston AS, Blaha MJ, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver: should we care? Atherosclerosis. 2013;230(2):358-367.
34. Wong VW, Wong GL, Yip GW, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60(12):1721-1727.
35. Sinn DH, Cho SJ, Gu S. Persistent nonalcoholic fatty liver disease increased risk for carotid atherosclerosis. Gastroenterology. 2016;151(3):481-488.
36. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589-600.
37. Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized, placebo-controlled trial. J Clin Gastroenterol. 2009;43(10):900-904.
38. Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46(5):1453-1463.
39. Athyros VG, Tziomalos K, Gossios TD, et al; GREACE Study Collaborative Group. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary artery disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study: a post-hoc analysis. Lancet. 2010;376(9756):1916-1922.
40. Musso G, Gambino R, Tabibian JH, et al. Association with non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11(7):e1001680.
41. Targher G, Bertolini L, Rodella S, Lippi G, Zoppini G, Chonchol M. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol. 2010;5(12):2166-2171.
42. Vilar-Gomez E, Galzadilla-Bertot L, Friedman SL, et al. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2017;45(2):332-344
43. Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9(2):283-291.
44. Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23(11):1815-1825.
45. Aron-Wisnewsky J, Clement K, Pépin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65(8):1124-1135.
46. Ding W, Fan J, Qin J. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(1):322-333.
47. Shen H, Lipka S, Kumar A, Mustacchia P. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. J Gastrointest Oncol. 2014:5(6):440-446.
48. Lee YI, Lim YS, Park HS. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: a retrospective cohort study. J Gastroenterol Hepatol. 2012;27(1):91-95.
49. Lin XF, Shi KQ, You J, et al. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: a large study. Mol Biol Rep. 2014;41(5):2989-2997.
50. Wong VW, Wong GL, Tsang SW, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut. 2011;60(6):829-836.
51. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123-133.
Nonalcoholic fatty liver disease (NALFD) is now the most common chronic liver disease in the developed world and affects about 25% to 30% of adults in the US and 30% of veterans who receive care in the VHA system (Figure 1).
Related:
NAFLD is significantly associated with the presence of MetS, so much so that it has been considered the hepatic manifestation of MetS. NAFLD also is strongly associated with type 2 diabetes mellitus (T2DM), CVD, chronic kidney disease (CKD), and obstructive sleep apnea (OSA) (Figure 2).
Obesity/Visceral Adiposity
Obesity (body mass index [BMI] > 30) prevalence in the US has almost doubled over the past 30 years and continues to climb.1 Obesity affects 41% of veterans in the Veterans Health Administration and is the most common risk factor for NAFLD.2 NAFLD is 4 times more prevalent in obese patients, thus, it is not surprising that 80% to 90% of patients evaluated in bariatric centers have NAFLD, reported in 2 large series.3,4 Increased BMI and waist circumference predict the presence of NASH and advanced fibrosis.5
While obesity is a hallmark for NAFLD, particularly in the US, it is important to note that up to 20% of Americans with normal BMI have NAFLD, based on findings of steatosis on ultrasound.6 These patients with lean NAFLD are often underdiagnosed. In addition to the patient’s BMI, it is important to recognize that in NAFLD, the distribution and type of fat deposition is more important than just BMI. Visceral fat refers to fat accumulation within the abdominal cavity and is key to the pathogenesis of NAFLD. Visceral fat, compared with subcutaneous fat, is metabolically active and can deliver an overabundance of free fatty acids to the liver as well as secrete proinflammatory mediators in the setting of insulin resistance. Visceral fat stores can predict increased hepatic fat content, inflammation, and fibrosis.5 Thus, it is important to recognize that those patients with relatively more visceral fat are more prone to NAFLD. The best clinical indicator of visceral adiposity is abdominal obesity, indicated by waist circumference > 40 inches in men and > 35 inches in women.
Metabolic Syndrome
Hepatic fat deposition can be associated with or precede MetS. MetS is defined as having at least 3 of the following characteristics: abdominal obesity, elevated triglycerides (TGs) (≥ 150 mg/dL), reduced high-density lipoprotein cholesterol (< 40 mg/dL in men or < 50 mg/dL in women), elevated blood pressure (BP) (systolic BP ≥ 130 mm Hg or diastolic BP ≥ 85 mm Hg), or elevated fasting glucose (≥ 110 mg/dL). Population studies have found that 50% of patients with MetS have NAFLD, and liver fat content is strongly correlated with the number of MetS features present in an individual.5,7 In addition to this association, NAFLD also promotes the development of MetS. Increased energy intake relative to energy expenditure will facilitate ectopic fat accumulation in the liver, which then increases hepatic gluconeogenesis and drives the pathogenesis of insulin resistance.8 Therefore, the presence of NAFLD is both a marker and a promotor of insulin resistance and its complications.
Related:
Type 2 Diabetes Mellitus
At 70% to 75%, the prevalence of NAFLD in patients with T2DM is more than twice as high as that in the general US adult population. Conversely, about 23% of patients with NAFLD also have T2DM.9
Influence of NAFLD on T2DM
Patients with ultrasound-based evidence of NAFLD are 2 to 5 times more likely to develop T2DM after adjusting for lifestyle and metabolic risk factors in multiple epidemiologic studies.10,11 The severity of hepaticfat content measured by ultrasound also is associated with an increasing risk of T2DM incidence over the next 5 years (normal,7%; mild, 9.8%; moderate-severe, 17.8%; P < .001).12 In another study, 58% of patientswith biopsy-proven NAFLD developed T2DM after a mean follow-up of 13.7 years.13 Those who were found to have NASH had a 3-fold higher risk of developing T2DM than did those with simple steatosis. This finding was confirmed in another study where T2DM incidence was 2 times higher in patients predicted to have advanced fibrosis compared with those who did not.14
Because liver steatosis interferes with insulin-induced glycogen production and suppression of gluconeogenesis, hepatic fat content predicts the insulin dose required for adequate glucose control in patients with diabetes mellitus (DM) and NAFLD.15 Higher levels of insulin are required in patients with DM and NAFLD compared with those without NAFLD.5
Additionally, a 10-year cohort study found that resolution of ultrasound-based NAFLD in patients without baseline T2DM, was associated with a reduced T2DM incidence (multivariate odds ratio [OR] 0.27, 95% CI, 0.12-0.61) after controlling for factors such as age, BMI, and impaired fasting glucose.11,17
Given this close relationship between T2DM and NAFLD, both the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of Liver Diseases (EASL) guidelines recommend that patients found to have NAFLD should be screened for the presence of impaired fasting glucose/T2DM by testing hemoglobin A1c or fasting glucose levels.18,19 Recognizing the role that NAFLD can play in patients with DM also is important, as improving hepatic steatosis may also improve DM.
Influence of DM on NAFLD
Patients with T2DM and NAFLD are at increased risk of progressive liver disease and have increased rates of NASH, cirrhosis, and HCC. In a paired-biopsy study, the development of T2DM was the strongest predictor of progression of NASH and hepatic fibrosis.20 This fibrosis progression can easily go undetected, as NASH can be present even with normal aminotransferases. This increased risk of fibrosis progression in the setting of comorbid T2DM is clinically important, as it is the severity of fibrosis that predicts all-cause and liver-related mortality in patients with NAFLD/NASH.21,22 In fact, the prevalence of biopsy-proven NASH in overweight/obese patients with DM with normal liver aminotransferases (defined as aspartate aminotransferase and alanine aminotransferase < 40 U/L) was found to be 58%.23 Because chronic liver disease, including NAFLD, is underrecognized in the “healthy population” used to establish normal aminotransferase levels, more recent AASLD and ACG guidelines now define normal aminotransferase levels as < 35 U/L for males and < 25 U/L for females.24 These stricter cutoffs are based on populations with normal BMI and negative testing for chronic liver diseases.24 The lower cutoffs may improve recognition of progressive liver disease in NAFLD and NASH patients.
Medications used in the treatment of T2DM, such as metformin, pioglitazone, and liraglutide, have been studied in patients with biopsy-proven NASH. The initial data showing histologic improvement in NAFLD patients taking metformin was more likely related to the associated weight loss in the treatment group. In a study by Loomba and colleagues the improvement in the NAFLD activity score was only seen in patients who lost ≥ 5% of their total body weight.25 Pioglitazone is a PPAR-γ agonist that helps regulate glucose and lipid metabolism as well as inflammation. Pioglitazone helps adipose tissue, hepatocytes, and muscle cells restore insulin sensitivity. A recent trial in 100 patients with prediabetes or T2DM as well as NASH showed that 36 weeks of pioglitazone treatment was associated with significant improvements in steatosis, inflammation, and most important, in stage of fibrosis compared with that of placebo.26
Related:
Glucagon-like peptide-1 (GLP-1) receptor agonists, such as liraglutide, have effects on lipid and glucose metabolism as well. They can lower glucose levels by increasing insulin secretion, reducing glucagon concentration, suppressing appetite (resulting in weight loss), and increasing sensitivity to insulin in hepatocytes and adipocytes. Liraglutide has been studied in patients with NASH both with and without DM, and results of the largest study to date show that it is associated with significant improvement in hepatic inflammation compared with that of placebo.27 Additional phase 3 clinical trials are currently underway.
Current AASLD guidelines do not recommend routine screening for NAFLD, even among high-risk patients, such as patients with DM.18 This is due to the widespread prevalence of NAFLD, the unclear utility of diagnostic tests, and limited efficacy of available treatment. Lifestyle modification to achieve weight loss remains the backbone of management, and rates of successful adherence are low.28 Contrary to this, EASL guidelines state that NAFLD screening with ultrasound even in patients with normal liver enzymes should be performed in high-risk patients with T2DM.19
Once detected, T2DM should be diligently treated in patients with NAFLD, and pioglitazone may be considered in patients with biopsy-proven NASH per AASLD guidelines.18 Pioglitazone has been studied in patients with biopsy-proven NASH both with and without DM and has been associated with significant resolution of NASH, as well as improvement in histologic changes of NASH and improvement in fibrosis.29,30 Because of potential medication AEs, including a mean weight gain of 2.5 kg to 4.7 kg in trials of 12- to 36-months’ duration, as well as potential bone loss in women, discussions about the risks and benefits of treatment should occur prior to treatment initiation.18 Additionally, pioglitazone is not safe in the setting of left ventricular heart failure. Future studies may point to the utility of other DM medications, such as GLP-receptor agonists.
Cardiovascular Disease
Given the association between features of MetS and NAFLD, it is not surprising that the primary cause of death in patients with NAFLD is related to CVD.21,22,31 However, it is increasingly recognized that NAFLD predicts CVD independently of the traditional risk factors associated with MetS. The increase in cardiovascular risk in the setting of NAFLD can be partly explained by the increased hepatic de novo lipogenesis that is associated with increased production of highly atherogenic small dense low-density lipoproteins (sd-LDL) independent of BMI and presence of insulin resistance.32 Additionally, increased intracellular free fatty acids can activate proinflammatory cytokine production by hepatocytes in addition to the increase in systemic inflammatory mediators and oxidative stress associated with NASH.
A recent meta-analysis of 27 studies confirmed the association between NAFLD and many subclinical features of CVD, including increases in coronary-artery calcium score, carotid artery intimal media thickness, and arterial wall stiffness, as well as impaired flow-mediated vasodilation after controlling for classic CVD risk factors.33 The risk of subclinical carotid and coronary atherosclerosis progression was higher in NAFLD patients with evidence of advanced fibrosis using noninvasive measures. Additionally, NAFLD was associated with increased severity of coronary artery disease in > 600 patients undergoing cardiac angiograms.34 Conversely, the regression of NAFLD on ultrasound was associated with a decreased risk of carotid atherosclerosis progression.35
Multiple epidemiologic studies have found an increased incidence of clinically overt CVD in patients with NAFLD after controlling for confounders. The largest updated meta-analysis, which included more than 34,000 patients with 2,600 CVD outcomes over a median of 6.9 years found that the presence of NAFLD (based on imaging or biopsy) was associated with an odds ratio (OR) of 1.64 (95% CI, 1.26-2.13) for fatal and nonfatal incident CVD.36 In the same meta-analysis, patients with NASH, with or without fibrosis, were at an even higher risk, with an OR of 2.58 (95% CI, 1.78-3.75).
Initial studies of statin medications for the treatment of NASH using surrogate endpoints like improvement in aminotransferases or imaging, suggested a potential liver-related benefit. However, there was no histologic improvement in the single study comparing 12 months of simvastatin therapy with placebo in patients with NASH.37 Although it is unclear whether statin use will directly improve NAFLD, there is no evidence to suggest that statin use should be avoided in patients with elevated CVD risk.38 Treatment with atorvastatin has been shown to be associated with a greater reduction in cardiovascular events in patients with NAFLD compared with that of patients without NAFLD.39
The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies. The clinical algorithms currently used to help risk stratify patients and determine appropriate preventative strategies, the Framingham risk equation or the systemic coronary risk evaluation, do not incorporate NAFLD as a potential risk factor for CVD. Additional studies are needed to determine whether adding NAFLD to the assessment will improve the predictive accuracy of future CVD events. Nevertheless, European clinical guidelines recommend performing a CVD risk assessment for patients with NAFLD.19
Chronic Kidney Disease
The prevalence of CKD, defined as estimated glomerular filtration rate (GFR) < 60 mL/min/1.72 m2, abnormal albuminuria, or proteinuria, is significantly increased in patients with NAFLD. Several epidemiologic studies have shown the prevalence of CKD in NAFLD patients ranges from 20% to 55% compared with 5% to 30% among patients without NAFLD.40 Overall, patients with NAFLD have a 2-fold increased risk of prevalent (OR 2.12; 95% CI, 1.69-2.66) or incident (hazard ratio 1.79; 95% CI, 1.65-1.95) CKD, even after adjusting for T2DM, visceral fat, and insulin resistance.40 There is an additional 2-fold increase in CKD risk in patients with NASH and advanced fibrosis compared with those with NASH and mild/no fibrosis. Additionally, advancing NASH fibrosis stage is independently associated with worsening stage of CKD.41
Data regarding the exact mechanism of kidney pathology in the setting of NAFLD is lacking. The accelerated atherogenesis in NAFLD likely contributes to renal damage. Another potential mechanism to explain the association between NASH and CKD involves the increased activation of the angiotensin-aldosterone system (RAAS) seen in NASH, which leads to increased hepatic fibrogenesis as well as kidney damage.42
Similar to the previously listed comorbidities, there is evidence that improvement in NAFLD can lead to improvements in renal disease. A prospective study of NASH patients undergoing 52 weeks of lifestyle modification found that the patients who had improvements in histologic NASH endpoints also had improvement in renal function.43
There are currently no specific recommendations on screening for CKD in professionalguidelines, but many experts propose monitoring for CKD yearly with serum creatinine and urinalysis and referring to nephrology if needed. Given the association between NASH and activation of the RAAS pathway that is associated with worsening hepatic fibrosis, RAAS-inhibitors should be a first-line agent in the treatment of hypertension in patients with NAFLD.
Obstructive Sleep Apnea
OSA is characterized by repeated pharyngeal collapse during sleep, which leads to chronic intermittent hypoxia and is associated with increased metabolic and cardiovascular morbidity and mortality. The cycle of intermittent hypoxia and reoxygenation in OSA results in inflammation and oxidative stress. Multiple studies have supported a link between NAFLD and OSA.
Hepatic fat content on ultrasound was increased in patients with OSA independent of BMI. There also has been evidence of a positive association between the severity of chronic intermittent hypoxia and increased hepatic fibrosis based on liver elastography.44 A meta-analysis using histologic NAFLD diagnosis showed that the presence of OSA was associated with a higher risk of fibrosis compared with that of patients with NAFLD without OSA (OR 2.6; 95% CI, 1.3-5.2).45
Based on animal models, hypoxia can drive fat accumulation and inflammation in the liver via multiple different pathways. Hypoxia can increase fasting glucose and systemic TG levels and induce hepatic lipogenesis by altering gene expression.45 Hypoxia also can increase oxidative stress and reduce β-oxidation, which leads to the production of lipotoxic lipids. These hypoxia-induced changes are typically more pronounced in subjects with obesity compared with that in subjects without obesity. Despite multiple adverse metabolic effects of OSA-induced hypoxia in the setting of NAFLD, preliminary, short-term studies have failed to find an association with OSA treatment with continuous positive airway pressure and improvement in NAFLD.45 Perhaps larger, long-term prospective trials will clarify this question.
Malignancy
Extrahepatic malignancy (colon, esophagus, stomach, pancreas, kidney, and breast) is the second most common cause of death in patients with NAFLD.21,22 The primary association between NAFLD and malignancy is found in the colon. Most large population-based studies have been performed in East Asia and have found that NAFLD is associated with a 1.5 to 1.7-fold increased risk for colonic adenomas and a 1.9 to 3.1-fold increased risk of colorectal cancer.46-49 Using magnetic resonance spectroscopy and liver biopsy to diagnose NAFLD and NASH, respectively, Wong and colleagues found that NASH, but not simple steatosis, is associated with a higher risk of advanced colorectalneoplasia (OR 5.34; 95% CI, 1.9-14.8), after adjusting for age, gender, BMI, family history, smoking, and T2DM.50
Data showing a definitive causative role of NAFLD in the development of colorectal cancer are lacking, but the presence of increased insulin levels has many potential effects on carcinogenesis in general, including stimulation of cell proliferation and apoptosis. Currently, there are no recommended changes to the standard colorectal cancer screening recommendations specifically for patients with NAFLD.
Conclusion
NAFLD is a multisystem disease that is associated with increased liver-related and all-cause mortality. Data on the close association between NAFLD and several extrahepatic complications, including MetS, T2DM, CVD, CKD, and malignancy are well established. There also is growing evidence of a bidirectional relationship between some of these diagnoses, whereas NAFLD is not only a consequence, but also a cause of MetS, T2DM, and CKD independent of other typical risk factors.
Given the multiple comorbidities associated with NAFLD and its potential to influence the severity of these diagnoses, management of these complex patients requires diligence and a multidisciplinary approach. In order to engage in early recognition and intervention to prevent potential morbidity and mortality, regular screening and surveillance for the development of NAFLD in patients with metabolic risk factors can be considered, and careful screening for metabolic complications in patients with established NAFLD is important.
Nonalcoholic fatty liver disease (NALFD) is now the most common chronic liver disease in the developed world and affects about 25% to 30% of adults in the US and 30% of veterans who receive care in the VHA system (Figure 1).
Related:
NAFLD is significantly associated with the presence of MetS, so much so that it has been considered the hepatic manifestation of MetS. NAFLD also is strongly associated with type 2 diabetes mellitus (T2DM), CVD, chronic kidney disease (CKD), and obstructive sleep apnea (OSA) (Figure 2).
Obesity/Visceral Adiposity
Obesity (body mass index [BMI] > 30) prevalence in the US has almost doubled over the past 30 years and continues to climb.1 Obesity affects 41% of veterans in the Veterans Health Administration and is the most common risk factor for NAFLD.2 NAFLD is 4 times more prevalent in obese patients, thus, it is not surprising that 80% to 90% of patients evaluated in bariatric centers have NAFLD, reported in 2 large series.3,4 Increased BMI and waist circumference predict the presence of NASH and advanced fibrosis.5
While obesity is a hallmark for NAFLD, particularly in the US, it is important to note that up to 20% of Americans with normal BMI have NAFLD, based on findings of steatosis on ultrasound.6 These patients with lean NAFLD are often underdiagnosed. In addition to the patient’s BMI, it is important to recognize that in NAFLD, the distribution and type of fat deposition is more important than just BMI. Visceral fat refers to fat accumulation within the abdominal cavity and is key to the pathogenesis of NAFLD. Visceral fat, compared with subcutaneous fat, is metabolically active and can deliver an overabundance of free fatty acids to the liver as well as secrete proinflammatory mediators in the setting of insulin resistance. Visceral fat stores can predict increased hepatic fat content, inflammation, and fibrosis.5 Thus, it is important to recognize that those patients with relatively more visceral fat are more prone to NAFLD. The best clinical indicator of visceral adiposity is abdominal obesity, indicated by waist circumference > 40 inches in men and > 35 inches in women.
Metabolic Syndrome
Hepatic fat deposition can be associated with or precede MetS. MetS is defined as having at least 3 of the following characteristics: abdominal obesity, elevated triglycerides (TGs) (≥ 150 mg/dL), reduced high-density lipoprotein cholesterol (< 40 mg/dL in men or < 50 mg/dL in women), elevated blood pressure (BP) (systolic BP ≥ 130 mm Hg or diastolic BP ≥ 85 mm Hg), or elevated fasting glucose (≥ 110 mg/dL). Population studies have found that 50% of patients with MetS have NAFLD, and liver fat content is strongly correlated with the number of MetS features present in an individual.5,7 In addition to this association, NAFLD also promotes the development of MetS. Increased energy intake relative to energy expenditure will facilitate ectopic fat accumulation in the liver, which then increases hepatic gluconeogenesis and drives the pathogenesis of insulin resistance.8 Therefore, the presence of NAFLD is both a marker and a promotor of insulin resistance and its complications.
Related:
Type 2 Diabetes Mellitus
At 70% to 75%, the prevalence of NAFLD in patients with T2DM is more than twice as high as that in the general US adult population. Conversely, about 23% of patients with NAFLD also have T2DM.9
Influence of NAFLD on T2DM
Patients with ultrasound-based evidence of NAFLD are 2 to 5 times more likely to develop T2DM after adjusting for lifestyle and metabolic risk factors in multiple epidemiologic studies.10,11 The severity of hepaticfat content measured by ultrasound also is associated with an increasing risk of T2DM incidence over the next 5 years (normal,7%; mild, 9.8%; moderate-severe, 17.8%; P < .001).12 In another study, 58% of patientswith biopsy-proven NAFLD developed T2DM after a mean follow-up of 13.7 years.13 Those who were found to have NASH had a 3-fold higher risk of developing T2DM than did those with simple steatosis. This finding was confirmed in another study where T2DM incidence was 2 times higher in patients predicted to have advanced fibrosis compared with those who did not.14
Because liver steatosis interferes with insulin-induced glycogen production and suppression of gluconeogenesis, hepatic fat content predicts the insulin dose required for adequate glucose control in patients with diabetes mellitus (DM) and NAFLD.15 Higher levels of insulin are required in patients with DM and NAFLD compared with those without NAFLD.5
Additionally, a 10-year cohort study found that resolution of ultrasound-based NAFLD in patients without baseline T2DM, was associated with a reduced T2DM incidence (multivariate odds ratio [OR] 0.27, 95% CI, 0.12-0.61) after controlling for factors such as age, BMI, and impaired fasting glucose.11,17
Given this close relationship between T2DM and NAFLD, both the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of Liver Diseases (EASL) guidelines recommend that patients found to have NAFLD should be screened for the presence of impaired fasting glucose/T2DM by testing hemoglobin A1c or fasting glucose levels.18,19 Recognizing the role that NAFLD can play in patients with DM also is important, as improving hepatic steatosis may also improve DM.
Influence of DM on NAFLD
Patients with T2DM and NAFLD are at increased risk of progressive liver disease and have increased rates of NASH, cirrhosis, and HCC. In a paired-biopsy study, the development of T2DM was the strongest predictor of progression of NASH and hepatic fibrosis.20 This fibrosis progression can easily go undetected, as NASH can be present even with normal aminotransferases. This increased risk of fibrosis progression in the setting of comorbid T2DM is clinically important, as it is the severity of fibrosis that predicts all-cause and liver-related mortality in patients with NAFLD/NASH.21,22 In fact, the prevalence of biopsy-proven NASH in overweight/obese patients with DM with normal liver aminotransferases (defined as aspartate aminotransferase and alanine aminotransferase < 40 U/L) was found to be 58%.23 Because chronic liver disease, including NAFLD, is underrecognized in the “healthy population” used to establish normal aminotransferase levels, more recent AASLD and ACG guidelines now define normal aminotransferase levels as < 35 U/L for males and < 25 U/L for females.24 These stricter cutoffs are based on populations with normal BMI and negative testing for chronic liver diseases.24 The lower cutoffs may improve recognition of progressive liver disease in NAFLD and NASH patients.
Medications used in the treatment of T2DM, such as metformin, pioglitazone, and liraglutide, have been studied in patients with biopsy-proven NASH. The initial data showing histologic improvement in NAFLD patients taking metformin was more likely related to the associated weight loss in the treatment group. In a study by Loomba and colleagues the improvement in the NAFLD activity score was only seen in patients who lost ≥ 5% of their total body weight.25 Pioglitazone is a PPAR-γ agonist that helps regulate glucose and lipid metabolism as well as inflammation. Pioglitazone helps adipose tissue, hepatocytes, and muscle cells restore insulin sensitivity. A recent trial in 100 patients with prediabetes or T2DM as well as NASH showed that 36 weeks of pioglitazone treatment was associated with significant improvements in steatosis, inflammation, and most important, in stage of fibrosis compared with that of placebo.26
Related:
Glucagon-like peptide-1 (GLP-1) receptor agonists, such as liraglutide, have effects on lipid and glucose metabolism as well. They can lower glucose levels by increasing insulin secretion, reducing glucagon concentration, suppressing appetite (resulting in weight loss), and increasing sensitivity to insulin in hepatocytes and adipocytes. Liraglutide has been studied in patients with NASH both with and without DM, and results of the largest study to date show that it is associated with significant improvement in hepatic inflammation compared with that of placebo.27 Additional phase 3 clinical trials are currently underway.
Current AASLD guidelines do not recommend routine screening for NAFLD, even among high-risk patients, such as patients with DM.18 This is due to the widespread prevalence of NAFLD, the unclear utility of diagnostic tests, and limited efficacy of available treatment. Lifestyle modification to achieve weight loss remains the backbone of management, and rates of successful adherence are low.28 Contrary to this, EASL guidelines state that NAFLD screening with ultrasound even in patients with normal liver enzymes should be performed in high-risk patients with T2DM.19
Once detected, T2DM should be diligently treated in patients with NAFLD, and pioglitazone may be considered in patients with biopsy-proven NASH per AASLD guidelines.18 Pioglitazone has been studied in patients with biopsy-proven NASH both with and without DM and has been associated with significant resolution of NASH, as well as improvement in histologic changes of NASH and improvement in fibrosis.29,30 Because of potential medication AEs, including a mean weight gain of 2.5 kg to 4.7 kg in trials of 12- to 36-months’ duration, as well as potential bone loss in women, discussions about the risks and benefits of treatment should occur prior to treatment initiation.18 Additionally, pioglitazone is not safe in the setting of left ventricular heart failure. Future studies may point to the utility of other DM medications, such as GLP-receptor agonists.
Cardiovascular Disease
Given the association between features of MetS and NAFLD, it is not surprising that the primary cause of death in patients with NAFLD is related to CVD.21,22,31 However, it is increasingly recognized that NAFLD predicts CVD independently of the traditional risk factors associated with MetS. The increase in cardiovascular risk in the setting of NAFLD can be partly explained by the increased hepatic de novo lipogenesis that is associated with increased production of highly atherogenic small dense low-density lipoproteins (sd-LDL) independent of BMI and presence of insulin resistance.32 Additionally, increased intracellular free fatty acids can activate proinflammatory cytokine production by hepatocytes in addition to the increase in systemic inflammatory mediators and oxidative stress associated with NASH.
A recent meta-analysis of 27 studies confirmed the association between NAFLD and many subclinical features of CVD, including increases in coronary-artery calcium score, carotid artery intimal media thickness, and arterial wall stiffness, as well as impaired flow-mediated vasodilation after controlling for classic CVD risk factors.33 The risk of subclinical carotid and coronary atherosclerosis progression was higher in NAFLD patients with evidence of advanced fibrosis using noninvasive measures. Additionally, NAFLD was associated with increased severity of coronary artery disease in > 600 patients undergoing cardiac angiograms.34 Conversely, the regression of NAFLD on ultrasound was associated with a decreased risk of carotid atherosclerosis progression.35
Multiple epidemiologic studies have found an increased incidence of clinically overt CVD in patients with NAFLD after controlling for confounders. The largest updated meta-analysis, which included more than 34,000 patients with 2,600 CVD outcomes over a median of 6.9 years found that the presence of NAFLD (based on imaging or biopsy) was associated with an odds ratio (OR) of 1.64 (95% CI, 1.26-2.13) for fatal and nonfatal incident CVD.36 In the same meta-analysis, patients with NASH, with or without fibrosis, were at an even higher risk, with an OR of 2.58 (95% CI, 1.78-3.75).
Initial studies of statin medications for the treatment of NASH using surrogate endpoints like improvement in aminotransferases or imaging, suggested a potential liver-related benefit. However, there was no histologic improvement in the single study comparing 12 months of simvastatin therapy with placebo in patients with NASH.37 Although it is unclear whether statin use will directly improve NAFLD, there is no evidence to suggest that statin use should be avoided in patients with elevated CVD risk.38 Treatment with atorvastatin has been shown to be associated with a greater reduction in cardiovascular events in patients with NAFLD compared with that of patients without NAFLD.39
The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies. The clinical algorithms currently used to help risk stratify patients and determine appropriate preventative strategies, the Framingham risk equation or the systemic coronary risk evaluation, do not incorporate NAFLD as a potential risk factor for CVD. Additional studies are needed to determine whether adding NAFLD to the assessment will improve the predictive accuracy of future CVD events. Nevertheless, European clinical guidelines recommend performing a CVD risk assessment for patients with NAFLD.19
Chronic Kidney Disease
The prevalence of CKD, defined as estimated glomerular filtration rate (GFR) < 60 mL/min/1.72 m2, abnormal albuminuria, or proteinuria, is significantly increased in patients with NAFLD. Several epidemiologic studies have shown the prevalence of CKD in NAFLD patients ranges from 20% to 55% compared with 5% to 30% among patients without NAFLD.40 Overall, patients with NAFLD have a 2-fold increased risk of prevalent (OR 2.12; 95% CI, 1.69-2.66) or incident (hazard ratio 1.79; 95% CI, 1.65-1.95) CKD, even after adjusting for T2DM, visceral fat, and insulin resistance.40 There is an additional 2-fold increase in CKD risk in patients with NASH and advanced fibrosis compared with those with NASH and mild/no fibrosis. Additionally, advancing NASH fibrosis stage is independently associated with worsening stage of CKD.41
Data regarding the exact mechanism of kidney pathology in the setting of NAFLD is lacking. The accelerated atherogenesis in NAFLD likely contributes to renal damage. Another potential mechanism to explain the association between NASH and CKD involves the increased activation of the angiotensin-aldosterone system (RAAS) seen in NASH, which leads to increased hepatic fibrogenesis as well as kidney damage.42
Similar to the previously listed comorbidities, there is evidence that improvement in NAFLD can lead to improvements in renal disease. A prospective study of NASH patients undergoing 52 weeks of lifestyle modification found that the patients who had improvements in histologic NASH endpoints also had improvement in renal function.43
There are currently no specific recommendations on screening for CKD in professionalguidelines, but many experts propose monitoring for CKD yearly with serum creatinine and urinalysis and referring to nephrology if needed. Given the association between NASH and activation of the RAAS pathway that is associated with worsening hepatic fibrosis, RAAS-inhibitors should be a first-line agent in the treatment of hypertension in patients with NAFLD.
Obstructive Sleep Apnea
OSA is characterized by repeated pharyngeal collapse during sleep, which leads to chronic intermittent hypoxia and is associated with increased metabolic and cardiovascular morbidity and mortality. The cycle of intermittent hypoxia and reoxygenation in OSA results in inflammation and oxidative stress. Multiple studies have supported a link between NAFLD and OSA.
Hepatic fat content on ultrasound was increased in patients with OSA independent of BMI. There also has been evidence of a positive association between the severity of chronic intermittent hypoxia and increased hepatic fibrosis based on liver elastography.44 A meta-analysis using histologic NAFLD diagnosis showed that the presence of OSA was associated with a higher risk of fibrosis compared with that of patients with NAFLD without OSA (OR 2.6; 95% CI, 1.3-5.2).45
Based on animal models, hypoxia can drive fat accumulation and inflammation in the liver via multiple different pathways. Hypoxia can increase fasting glucose and systemic TG levels and induce hepatic lipogenesis by altering gene expression.45 Hypoxia also can increase oxidative stress and reduce β-oxidation, which leads to the production of lipotoxic lipids. These hypoxia-induced changes are typically more pronounced in subjects with obesity compared with that in subjects without obesity. Despite multiple adverse metabolic effects of OSA-induced hypoxia in the setting of NAFLD, preliminary, short-term studies have failed to find an association with OSA treatment with continuous positive airway pressure and improvement in NAFLD.45 Perhaps larger, long-term prospective trials will clarify this question.
Malignancy
Extrahepatic malignancy (colon, esophagus, stomach, pancreas, kidney, and breast) is the second most common cause of death in patients with NAFLD.21,22 The primary association between NAFLD and malignancy is found in the colon. Most large population-based studies have been performed in East Asia and have found that NAFLD is associated with a 1.5 to 1.7-fold increased risk for colonic adenomas and a 1.9 to 3.1-fold increased risk of colorectal cancer.46-49 Using magnetic resonance spectroscopy and liver biopsy to diagnose NAFLD and NASH, respectively, Wong and colleagues found that NASH, but not simple steatosis, is associated with a higher risk of advanced colorectalneoplasia (OR 5.34; 95% CI, 1.9-14.8), after adjusting for age, gender, BMI, family history, smoking, and T2DM.50
Data showing a definitive causative role of NAFLD in the development of colorectal cancer are lacking, but the presence of increased insulin levels has many potential effects on carcinogenesis in general, including stimulation of cell proliferation and apoptosis. Currently, there are no recommended changes to the standard colorectal cancer screening recommendations specifically for patients with NAFLD.
Conclusion
NAFLD is a multisystem disease that is associated with increased liver-related and all-cause mortality. Data on the close association between NAFLD and several extrahepatic complications, including MetS, T2DM, CVD, CKD, and malignancy are well established. There also is growing evidence of a bidirectional relationship between some of these diagnoses, whereas NAFLD is not only a consequence, but also a cause of MetS, T2DM, and CKD independent of other typical risk factors.
Given the multiple comorbidities associated with NAFLD and its potential to influence the severity of these diagnoses, management of these complex patients requires diligence and a multidisciplinary approach. In order to engage in early recognition and intervention to prevent potential morbidity and mortality, regular screening and surveillance for the development of NAFLD in patients with metabolic risk factors can be considered, and careful screening for metabolic complications in patients with established NAFLD is important.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2014.
2. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
3. Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45(4):600-606.
4. Subichin M, Clanton J, Makuszewski M, Bohon A, Zografakis JG, Dan A. Liver disease in the morbidly obese: a review of 1000 consecutive patients undergoing weight loss surgery. Surg Obes Relat Dis. 2015;11(1):137-141.
5. Non-alcoholic Fatty Liver Disease Study Group, Lonardo A, Bellentani S, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: focus on high-risk groups. Dig Liver Dis. 2015;47(12):997-1006.
6. Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15(4):474-485.
7. Kotronen A, Westerbacka J, Bergholm R, Pietiläinen KH, Yki-Järvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92(9):3490-3497.
8. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131-1141.
9. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
10. Armstrong MJ, Adams LA, Canbay A, et al. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174-1197.
11. Kashanian S, Fuchs M. Non-alcoholic fatty liver disease in patients with diabetes mellitus: a clinician’s perspective. Int J Dig Dis. 2015;1:1.
12. Park SK, Seo MH, Shin HC, Ryoo JH. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology. 2013;57(4):1378-1383.
13. Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
14. Chang Y, Jung HS, Yun KE, Cho J, Cho YK, Ryu S. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am J Gastroenterol. 2013;108(12):1861-1868.
15. Ryysy L, Hakkinen AM, Goto T, et al. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes. 2000;49(5):749-758.
16. Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105(7):1567-1573.
17. Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H. Independent association between improvement in nonalcoholic fatty liver disease and reduced risk of incidence of type 2 diabetes. Diabetes Care. 2015;38(9):1673-1679.
18. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
19. European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. EASL-EASD-EASO clinical practice guidelines for the management of nonalcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
20. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148-1155.
21. Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547-1554.
22. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic feature, is associated with long-term outcomes in patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389-397.
23. Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal aminotransferases. J Clin Endocrinol. Metab. 2015;100(6):2231-2238.
24. Kwo PY, Cohen SM, and Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18-35.
25. Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29(2):172-182.
26. Cusi K, Orsak B, Lomonaco R, et al. Extended treatment with pioglitazone improves liver histology in patients with pre-diabetes or type 2 diabetes mellitus and NASH. Hepatology. 2013;58(supp 1):248a.
27. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690.
28. Patel YA, Gifford EJ, Glass LM, et al. Risk factors for biopsy-proven advanced non-alcoholic fatty liver disease in the Veterans Health Administration. Aliment Pharmacol Ther. 2018;47(2):268-278.
29. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305-315.
30. Sanyal AJ, Chalasani N, Kowdley KV, et al; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675-1685.
31. Ekstedt M, Frazen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
32. Vanni E, Marengo A, Mezzabotta L, Bugianesi E. Systemic complications of nonalcoholic fatty liver disease: when the liver is not an innocent bystander. Semin Liver Dis. 2015;35(3): 236-249.
33. Oni ET, Agatston AS, Blaha MJ, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver: should we care? Atherosclerosis. 2013;230(2):358-367.
34. Wong VW, Wong GL, Yip GW, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60(12):1721-1727.
35. Sinn DH, Cho SJ, Gu S. Persistent nonalcoholic fatty liver disease increased risk for carotid atherosclerosis. Gastroenterology. 2016;151(3):481-488.
36. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589-600.
37. Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized, placebo-controlled trial. J Clin Gastroenterol. 2009;43(10):900-904.
38. Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46(5):1453-1463.
39. Athyros VG, Tziomalos K, Gossios TD, et al; GREACE Study Collaborative Group. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary artery disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study: a post-hoc analysis. Lancet. 2010;376(9756):1916-1922.
40. Musso G, Gambino R, Tabibian JH, et al. Association with non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11(7):e1001680.
41. Targher G, Bertolini L, Rodella S, Lippi G, Zoppini G, Chonchol M. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol. 2010;5(12):2166-2171.
42. Vilar-Gomez E, Galzadilla-Bertot L, Friedman SL, et al. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2017;45(2):332-344
43. Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9(2):283-291.
44. Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23(11):1815-1825.
45. Aron-Wisnewsky J, Clement K, Pépin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65(8):1124-1135.
46. Ding W, Fan J, Qin J. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(1):322-333.
47. Shen H, Lipka S, Kumar A, Mustacchia P. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. J Gastrointest Oncol. 2014:5(6):440-446.
48. Lee YI, Lim YS, Park HS. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: a retrospective cohort study. J Gastroenterol Hepatol. 2012;27(1):91-95.
49. Lin XF, Shi KQ, You J, et al. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: a large study. Mol Biol Rep. 2014;41(5):2989-2997.
50. Wong VW, Wong GL, Tsang SW, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut. 2011;60(6):829-836.
51. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123-133.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2014.
2. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
3. Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45(4):600-606.
4. Subichin M, Clanton J, Makuszewski M, Bohon A, Zografakis JG, Dan A. Liver disease in the morbidly obese: a review of 1000 consecutive patients undergoing weight loss surgery. Surg Obes Relat Dis. 2015;11(1):137-141.
5. Non-alcoholic Fatty Liver Disease Study Group, Lonardo A, Bellentani S, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: focus on high-risk groups. Dig Liver Dis. 2015;47(12):997-1006.
6. Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15(4):474-485.
7. Kotronen A, Westerbacka J, Bergholm R, Pietiläinen KH, Yki-Järvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92(9):3490-3497.
8. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131-1141.
9. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
10. Armstrong MJ, Adams LA, Canbay A, et al. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174-1197.
11. Kashanian S, Fuchs M. Non-alcoholic fatty liver disease in patients with diabetes mellitus: a clinician’s perspective. Int J Dig Dis. 2015;1:1.
12. Park SK, Seo MH, Shin HC, Ryoo JH. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology. 2013;57(4):1378-1383.
13. Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
14. Chang Y, Jung HS, Yun KE, Cho J, Cho YK, Ryu S. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am J Gastroenterol. 2013;108(12):1861-1868.
15. Ryysy L, Hakkinen AM, Goto T, et al. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes. 2000;49(5):749-758.
16. Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105(7):1567-1573.
17. Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H. Independent association between improvement in nonalcoholic fatty liver disease and reduced risk of incidence of type 2 diabetes. Diabetes Care. 2015;38(9):1673-1679.
18. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
19. European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. EASL-EASD-EASO clinical practice guidelines for the management of nonalcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
20. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148-1155.
21. Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547-1554.
22. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic feature, is associated with long-term outcomes in patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389-397.
23. Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal aminotransferases. J Clin Endocrinol. Metab. 2015;100(6):2231-2238.
24. Kwo PY, Cohen SM, and Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18-35.
25. Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29(2):172-182.
26. Cusi K, Orsak B, Lomonaco R, et al. Extended treatment with pioglitazone improves liver histology in patients with pre-diabetes or type 2 diabetes mellitus and NASH. Hepatology. 2013;58(supp 1):248a.
27. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690.
28. Patel YA, Gifford EJ, Glass LM, et al. Risk factors for biopsy-proven advanced non-alcoholic fatty liver disease in the Veterans Health Administration. Aliment Pharmacol Ther. 2018;47(2):268-278.
29. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305-315.
30. Sanyal AJ, Chalasani N, Kowdley KV, et al; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675-1685.
31. Ekstedt M, Frazen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
32. Vanni E, Marengo A, Mezzabotta L, Bugianesi E. Systemic complications of nonalcoholic fatty liver disease: when the liver is not an innocent bystander. Semin Liver Dis. 2015;35(3): 236-249.
33. Oni ET, Agatston AS, Blaha MJ, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver: should we care? Atherosclerosis. 2013;230(2):358-367.
34. Wong VW, Wong GL, Yip GW, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60(12):1721-1727.
35. Sinn DH, Cho SJ, Gu S. Persistent nonalcoholic fatty liver disease increased risk for carotid atherosclerosis. Gastroenterology. 2016;151(3):481-488.
36. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589-600.
37. Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized, placebo-controlled trial. J Clin Gastroenterol. 2009;43(10):900-904.
38. Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46(5):1453-1463.
39. Athyros VG, Tziomalos K, Gossios TD, et al; GREACE Study Collaborative Group. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary artery disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study: a post-hoc analysis. Lancet. 2010;376(9756):1916-1922.
40. Musso G, Gambino R, Tabibian JH, et al. Association with non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11(7):e1001680.
41. Targher G, Bertolini L, Rodella S, Lippi G, Zoppini G, Chonchol M. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol. 2010;5(12):2166-2171.
42. Vilar-Gomez E, Galzadilla-Bertot L, Friedman SL, et al. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2017;45(2):332-344
43. Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9(2):283-291.
44. Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23(11):1815-1825.
45. Aron-Wisnewsky J, Clement K, Pépin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65(8):1124-1135.
46. Ding W, Fan J, Qin J. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(1):322-333.
47. Shen H, Lipka S, Kumar A, Mustacchia P. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. J Gastrointest Oncol. 2014:5(6):440-446.
48. Lee YI, Lim YS, Park HS. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: a retrospective cohort study. J Gastroenterol Hepatol. 2012;27(1):91-95.
49. Lin XF, Shi KQ, You J, et al. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: a large study. Mol Biol Rep. 2014;41(5):2989-2997.
50. Wong VW, Wong GL, Tsang SW, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut. 2011;60(6):829-836.
51. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123-133.
Physiological versus pathological cardiac remodeling in athletes
SNOWMASS, COLO. – according to Matthew W. Martinez, MD, medical director of the Sports Cardiology and Hypertrophic Cardiomyopathy Center at the Lehigh Valley Health Network in Allentown, Pa.
“The MRI may be the single test that best helps you sort out when you’re not quite sure. If you think about a single study that’s going to help you identify cardiac arrest etiologies – hypertrophic cardiomyopathy, myocarditis, anomalous coronaries, left-sided disease, right-sided disease like arrhythmogenic right ventricular cardiomyopathy, valvular heart disease, aortic disease – MRI is a very powerful tool because it will help you evaluate all of those groups more than 90% of the time,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Dr. Martinez, who serves as lead cardiologist for U.S. Major League Soccer and is also heavily involved with the National Football League, spends a lot of time with elite professional or Olympic athletes who fall into what he calls “the gray zone,” with a left ventricular wall thickness of 12-15 mm as measured on echocardiography. While that would clearly be considered abnormal in a nonathlete or a recreational sports enthusiast, his experience as well as that of other sports cardiologists working with professional soccer, football, and basketball players, bicyclists, and high-level track and field competitors has been that wall thickness in the 12- to 15-mm range in elite athletes can represent physiological adaptation to their enormous cardiovascular workloads. For example, more than 10% of National Football League players have a maximum left ventricular wall thickness of 13 mm or more, as do more than 10% of National Basketball Association players.
But what if that echocardiographic measurement of wall thickness is off by a millimeter or two, as is often par for the course?
“It’s well described that MRI gives a better look at wall thickness than echocardiography, especially where there’s areas of hypertrophy next to normal wall. In that gray zone, where we have to know if it’s really 10-12 or 14-16 mm, the MRI better identifies the actual thickness,” he said.
In addition, cardiac MRI readily provides accurate, reproducible measurements of left and right ventricular chamber size. But the most important way in which cardiac MRI helps in evaluating the significance of cardiac remodeling in athletes is via the gadolinium study. Late gadolinium enhancement is a concerning finding. It indicates the presence of myocardial fibrosis and scar, which at least in the general population is a prognostic sign for worse outcome.
“If you detect fibrosis, the search for pathology has to start,” the cardiologist emphasized.
He noted that the most comprehensive review to date of myocardial fibrosis in endurance athletes identified the intraventricular septum and the junction of the right ventricle and septum as the most common sites of involvement. The investigators concluded that, while there is a lack of compelling data on the clinical impact and prognosis of myocardial fibrosis in athletes, potential mechanisms include exercise-induced repetitive microinjury, pulmonary artery pressure overload, genetic predisposition, and silent myocarditis (Mayo Clin Proc. 2016 Nov;91[11]:1617-31).
That being said about the value of cardiac MRI as a tiebreaker, Dr. Martinez asserted that “there’s no specific test that’s going to get you out of jail. ... I would submit to you that you have to load the boat. Be comprehensive and try to build a case for one side or the other. And I would encourage you to ask for help; we do it all the time.”
Dilated chambers outside the normal range are common in competitive athletes. Don’t accept the echocardiographic hard numeric cutoffs that have been established as “normal” in the general population. For example, 36% of National Basketball Association players have a left ventricular end diastolic dimension (LVEDD) greater than 60 mm.
“I’ve seen LVEDDs up to 70 mm in cyclists. And all but a handful have a normal left ventricular ejection fraction greater than 50%,” he noted.
Dilated chambers in elite athletes are reassuring, provided stroke volume is preserved or, as is more often the case, enhanced.
“One of the hallmarks of being an athlete is the ability to suck in blood and increase stroke volume as a result. A typical stroke volume in an athlete is well above normal, with 85-90 cc or more being common. On tissue Doppler assessment, you shouldn’t have a normal inflow pattern or normal relaxation. A septal E prime velocity of 11-14 cm/sec is what I typically expect in an athlete. A lower E prime velocity suggests early pathologic change. If you find an E prime velocity of less than 9 cm/sec on tissue Doppler, or an elevated filling pressure like 15 mm Hg, that correlates with a greater than 90% sensitivity for pathology, such as hypertrophic cardiomyopathy. The average E prime velocity in Major League Soccer players is about 13 cm/sec, so that’s an important number to keep in your head,” according to the cardiologist.
Cardiac remodeling in elite athletes tends towards one of two forms, depending upon their sport. Endurance athletes, such as marathon runners, are repetitively volume challenged, so expect a tendency towards aortic regurgitation. For pressure-challenged athletes, such as power weightlifters, the tendency is toward aortic stenosis.
“But also expect a blend. It’s rarely just one or the other. Understanding that can help you discern the gray zone athlete,” he said.
Dr. Martinez reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – according to Matthew W. Martinez, MD, medical director of the Sports Cardiology and Hypertrophic Cardiomyopathy Center at the Lehigh Valley Health Network in Allentown, Pa.
“The MRI may be the single test that best helps you sort out when you’re not quite sure. If you think about a single study that’s going to help you identify cardiac arrest etiologies – hypertrophic cardiomyopathy, myocarditis, anomalous coronaries, left-sided disease, right-sided disease like arrhythmogenic right ventricular cardiomyopathy, valvular heart disease, aortic disease – MRI is a very powerful tool because it will help you evaluate all of those groups more than 90% of the time,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Dr. Martinez, who serves as lead cardiologist for U.S. Major League Soccer and is also heavily involved with the National Football League, spends a lot of time with elite professional or Olympic athletes who fall into what he calls “the gray zone,” with a left ventricular wall thickness of 12-15 mm as measured on echocardiography. While that would clearly be considered abnormal in a nonathlete or a recreational sports enthusiast, his experience as well as that of other sports cardiologists working with professional soccer, football, and basketball players, bicyclists, and high-level track and field competitors has been that wall thickness in the 12- to 15-mm range in elite athletes can represent physiological adaptation to their enormous cardiovascular workloads. For example, more than 10% of National Football League players have a maximum left ventricular wall thickness of 13 mm or more, as do more than 10% of National Basketball Association players.
But what if that echocardiographic measurement of wall thickness is off by a millimeter or two, as is often par for the course?
“It’s well described that MRI gives a better look at wall thickness than echocardiography, especially where there’s areas of hypertrophy next to normal wall. In that gray zone, where we have to know if it’s really 10-12 or 14-16 mm, the MRI better identifies the actual thickness,” he said.
In addition, cardiac MRI readily provides accurate, reproducible measurements of left and right ventricular chamber size. But the most important way in which cardiac MRI helps in evaluating the significance of cardiac remodeling in athletes is via the gadolinium study. Late gadolinium enhancement is a concerning finding. It indicates the presence of myocardial fibrosis and scar, which at least in the general population is a prognostic sign for worse outcome.
“If you detect fibrosis, the search for pathology has to start,” the cardiologist emphasized.
He noted that the most comprehensive review to date of myocardial fibrosis in endurance athletes identified the intraventricular septum and the junction of the right ventricle and septum as the most common sites of involvement. The investigators concluded that, while there is a lack of compelling data on the clinical impact and prognosis of myocardial fibrosis in athletes, potential mechanisms include exercise-induced repetitive microinjury, pulmonary artery pressure overload, genetic predisposition, and silent myocarditis (Mayo Clin Proc. 2016 Nov;91[11]:1617-31).
That being said about the value of cardiac MRI as a tiebreaker, Dr. Martinez asserted that “there’s no specific test that’s going to get you out of jail. ... I would submit to you that you have to load the boat. Be comprehensive and try to build a case for one side or the other. And I would encourage you to ask for help; we do it all the time.”
Dilated chambers outside the normal range are common in competitive athletes. Don’t accept the echocardiographic hard numeric cutoffs that have been established as “normal” in the general population. For example, 36% of National Basketball Association players have a left ventricular end diastolic dimension (LVEDD) greater than 60 mm.
“I’ve seen LVEDDs up to 70 mm in cyclists. And all but a handful have a normal left ventricular ejection fraction greater than 50%,” he noted.
Dilated chambers in elite athletes are reassuring, provided stroke volume is preserved or, as is more often the case, enhanced.
“One of the hallmarks of being an athlete is the ability to suck in blood and increase stroke volume as a result. A typical stroke volume in an athlete is well above normal, with 85-90 cc or more being common. On tissue Doppler assessment, you shouldn’t have a normal inflow pattern or normal relaxation. A septal E prime velocity of 11-14 cm/sec is what I typically expect in an athlete. A lower E prime velocity suggests early pathologic change. If you find an E prime velocity of less than 9 cm/sec on tissue Doppler, or an elevated filling pressure like 15 mm Hg, that correlates with a greater than 90% sensitivity for pathology, such as hypertrophic cardiomyopathy. The average E prime velocity in Major League Soccer players is about 13 cm/sec, so that’s an important number to keep in your head,” according to the cardiologist.
Cardiac remodeling in elite athletes tends towards one of two forms, depending upon their sport. Endurance athletes, such as marathon runners, are repetitively volume challenged, so expect a tendency towards aortic regurgitation. For pressure-challenged athletes, such as power weightlifters, the tendency is toward aortic stenosis.
“But also expect a blend. It’s rarely just one or the other. Understanding that can help you discern the gray zone athlete,” he said.
Dr. Martinez reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – according to Matthew W. Martinez, MD, medical director of the Sports Cardiology and Hypertrophic Cardiomyopathy Center at the Lehigh Valley Health Network in Allentown, Pa.
“The MRI may be the single test that best helps you sort out when you’re not quite sure. If you think about a single study that’s going to help you identify cardiac arrest etiologies – hypertrophic cardiomyopathy, myocarditis, anomalous coronaries, left-sided disease, right-sided disease like arrhythmogenic right ventricular cardiomyopathy, valvular heart disease, aortic disease – MRI is a very powerful tool because it will help you evaluate all of those groups more than 90% of the time,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Dr. Martinez, who serves as lead cardiologist for U.S. Major League Soccer and is also heavily involved with the National Football League, spends a lot of time with elite professional or Olympic athletes who fall into what he calls “the gray zone,” with a left ventricular wall thickness of 12-15 mm as measured on echocardiography. While that would clearly be considered abnormal in a nonathlete or a recreational sports enthusiast, his experience as well as that of other sports cardiologists working with professional soccer, football, and basketball players, bicyclists, and high-level track and field competitors has been that wall thickness in the 12- to 15-mm range in elite athletes can represent physiological adaptation to their enormous cardiovascular workloads. For example, more than 10% of National Football League players have a maximum left ventricular wall thickness of 13 mm or more, as do more than 10% of National Basketball Association players.
But what if that echocardiographic measurement of wall thickness is off by a millimeter or two, as is often par for the course?
“It’s well described that MRI gives a better look at wall thickness than echocardiography, especially where there’s areas of hypertrophy next to normal wall. In that gray zone, where we have to know if it’s really 10-12 or 14-16 mm, the MRI better identifies the actual thickness,” he said.
In addition, cardiac MRI readily provides accurate, reproducible measurements of left and right ventricular chamber size. But the most important way in which cardiac MRI helps in evaluating the significance of cardiac remodeling in athletes is via the gadolinium study. Late gadolinium enhancement is a concerning finding. It indicates the presence of myocardial fibrosis and scar, which at least in the general population is a prognostic sign for worse outcome.
“If you detect fibrosis, the search for pathology has to start,” the cardiologist emphasized.
He noted that the most comprehensive review to date of myocardial fibrosis in endurance athletes identified the intraventricular septum and the junction of the right ventricle and septum as the most common sites of involvement. The investigators concluded that, while there is a lack of compelling data on the clinical impact and prognosis of myocardial fibrosis in athletes, potential mechanisms include exercise-induced repetitive microinjury, pulmonary artery pressure overload, genetic predisposition, and silent myocarditis (Mayo Clin Proc. 2016 Nov;91[11]:1617-31).
That being said about the value of cardiac MRI as a tiebreaker, Dr. Martinez asserted that “there’s no specific test that’s going to get you out of jail. ... I would submit to you that you have to load the boat. Be comprehensive and try to build a case for one side or the other. And I would encourage you to ask for help; we do it all the time.”
Dilated chambers outside the normal range are common in competitive athletes. Don’t accept the echocardiographic hard numeric cutoffs that have been established as “normal” in the general population. For example, 36% of National Basketball Association players have a left ventricular end diastolic dimension (LVEDD) greater than 60 mm.
“I’ve seen LVEDDs up to 70 mm in cyclists. And all but a handful have a normal left ventricular ejection fraction greater than 50%,” he noted.
Dilated chambers in elite athletes are reassuring, provided stroke volume is preserved or, as is more often the case, enhanced.
“One of the hallmarks of being an athlete is the ability to suck in blood and increase stroke volume as a result. A typical stroke volume in an athlete is well above normal, with 85-90 cc or more being common. On tissue Doppler assessment, you shouldn’t have a normal inflow pattern or normal relaxation. A septal E prime velocity of 11-14 cm/sec is what I typically expect in an athlete. A lower E prime velocity suggests early pathologic change. If you find an E prime velocity of less than 9 cm/sec on tissue Doppler, or an elevated filling pressure like 15 mm Hg, that correlates with a greater than 90% sensitivity for pathology, such as hypertrophic cardiomyopathy. The average E prime velocity in Major League Soccer players is about 13 cm/sec, so that’s an important number to keep in your head,” according to the cardiologist.
Cardiac remodeling in elite athletes tends towards one of two forms, depending upon their sport. Endurance athletes, such as marathon runners, are repetitively volume challenged, so expect a tendency towards aortic regurgitation. For pressure-challenged athletes, such as power weightlifters, the tendency is toward aortic stenosis.
“But also expect a blend. It’s rarely just one or the other. Understanding that can help you discern the gray zone athlete,” he said.
Dr. Martinez reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM ACC SNOWMASS 2019
Evaluation of Interventions by Clinical Pharmacy Specialists in Cardiology at a VA Ambulatory Cardiology Clinic
Integration of CPSs into an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for other cardiology health care providers.
Health care providers face many challenges in utilizing cardiovascular therapies, such as anticipated shortages in physicians, patients with more complicated conditions, shifting medication regimens, management needs, and increased accountability for quality and performance measures.1 To meet the potential increase in service demand, cardiology practices are embracing cardiovascular team-based care.1 Advanced practice providers, such as advanced practice registered nurses (APRNs), physician assistants (PAs), and clinical pharmacy specialists (CPSs), have education, training, and experience to extend the team’s capability to meet these complex management needs.1
The role of CPSs within a cardiovascular care team includes providing a variety of patient-specific services, such as collaborating with other cardiology providers, to optimize evidence-based pharmacotherapy, preventing medication-related adverse events/errors, improving patient understanding of their medication regimen, and ultimately, improving patient outcomes.2 Health care systems, such as Kaiser Permanente of Colorado, have demonstrated improved clinical outcomes for patients with coronary artery disease (CAD) by implementing a multidisciplinary collaborative cardiac care service, including a clinical pharmacy cardiac risk service, in which CPSs assisted with management of cholesterol-lowering, hypertension, diabetes mellitus (DM), and smoking-cessation therapies, which resulted in a 76% to 89% reduction in all-cause mortality associated with CAD in multiple evaluations.3,4
Pharmacists providing medication therapy management (MTM) services in Minnesota had higher goal attainment for patients with hypertension and hyperlipidemia than did pharmacists who did not provide MTM services.5 MTM services provided by pharmacists led to an improvement in clinical outcomes for patients as well as a reduction in overall health care expenditures compared with that of a control group of patients who did not receive MTM services.5 Furthermore, CPS integration in the heart failure (HF) setting has led to improvements in utilization and optimization of guideline-directed medical therapies, an area in which recent data have suggested deficiencies exist.6-8 A full review of the outcomes associated with CPS involvement in cardiovascular care is beyond the scope of this article; but the recent review by Dunn and colleagues provides more detail.2
With the increasing number of patients with cardiovascular disease,expanding integration of CPSs in the cardiovascular team providing MTM services may reduce the burden of other providers (MD, PA, APRN, etc), thereby increasing access for not only new patients, but also diagnostic and interventional work, while potentially improving clinical and economic outcomes.2 The value of integrating CPSs as members of the cardiovascular care team is recognized in a variety of inpatient and ambulatory practice settings.2-6 However, data are limited on the number and types of interventions made per encounter as direct patient care providers. Expanded granularity regarding the effect of CPSs as active members of the cardiovascular team is an essential component to evaluate the potential benefit of CPS integration into direct patient care.
Methods
The West Palm Beach (WPB) Veteran Affairs Medical Center (VAMC) outpatient cardiology clinic consists of 6 full-time employee (FTE) cardiologists, 4 PAs or APRNs, 10 other cardiology health care staff members (registered/license practical nurses and technicians), and 2 cardiology CPSs providing direct patient care
The cardiology pharmacotherapy clinic is open 20.5 hours per week with 41 appointment slots (30 minutes each), of which 7 appointments are delivered via clinic video telehealth and 34 appointments are traditional face-to-face visits.9 The remaining CPS time is assigned to other clinical care and administrative areas to fit facility need, including oversight of the CPS-run 24-hour ambulatory blood pressure clinic, postgraduate year 2 cardiology pharmacy practice residency program directorship, and other administrative activities for the facility.10
The cardiology CPSs practice under an advanced scope of practice in which they independently manage medications (initiate, modify, discontinue), order diagnostic testing (laboratory, monitoring, imaging, etc) needed for medication management, and create monitoring and treatment plans for patients referred to the cardiology pharmacotherapy clinic by other cardiology providers. The diseases managed within the clinic vary based on patient-specific needs, but may include HF, dyslipidemia, hypertension, anticoagulation, CAD, arrhythmias, cardiovascular risk factor assessment and reduction, and medication reconciliation and teaching. Patients are referred for CPS management directly from facility cardiologist and cardiology clinic PAs and APRNs. Workload and interventions carried out are captured in the Pharmacists Achieve Results with Medications Demonstration (PhARMD) tool and patient care encounter tracking.9
Data Collection
Using local data from workload tracking, the number of CPS encounters was determined from July 6, 2015, to October 1, 2015. Data were collected on the types and volume of interventions made by CPSs in the cardiology pharmacotherapy clinic using the PhARMD tool (Figure).
The PhARMD tool was initially developed and implemented for CPSs in primary care pharmacotherapy clinics and was used to evaluate the types and volume of CPS interventions made in this setting.11 Since this initial evaluation, the tool has been updated, standardized nationally by the Department of Veterans Affairs (VA) Pharmacy Benefits Management Clinical Pharmacy Practice Office, and integrated across numerous VAMCs and associated outpatient clinics. The tool remains embedded within the VA electronic health record (EHR) and allows the capture of specific CPS interventions of several types (ie, both pharmacologic and nonpharmacologic interventions, including adjust dose or frequency; change or discontinue medication; initiate medication; monitor medication; counsel on adherence, contraindications, drug interactions, and drugs not indicated; reconcile medication; and prevent or manage adverse drug events [ADEs]) specific to certain diseases, such as anemia, anticoagulation, HF, type 2 DM (T2DM), hypertension, dyslipidemia, and tobacco cessation.
Given that the interventions captured by the PhARMD tool are based on self-report of the CPS performing the intervention, a quality assurance (QA) measure was taken to audit a random sample of interventions to validate the accuracy of reported data. A Pharmacy Benefits Management PhARMD Project QA report provided the 20% random sample of encounters for each cardiology CPS to be reviewed. This percentage was determined by VAMC Clinical Pharmacy Program Office (CPPO) directives on implementation of the PhARMD tool. During the QA period, the provided sample was reviewed to determine whether the intervention(s) recorded with the PhARMD tool matched the actions documented in the EHR. The QA review was done through a manual chart review by an author not involved in recording the original interventions. Both WPB VAMC cardiology CPSs passed the QA review (> 80% concurrence with tool logged and chart documented interventions as required by VA CPPO directive), with a 90.9% concurrence between the EHR and PhARMD tool documentation.
Statistical Analyses
Data on intervention type and encounter number were evaluated with descriptive statistics. The information was characterized and diagrammed with Excel (Microsoft, Redmond, WA) charts and graphs.
Cost-avoidance calculations were done using previously described methods and are included for exploratory analysis.11,12 Briefly, published estimates of cost avoidance associated with various interventions from the outpatient setting within a VAMC setting were applied as appropriate to the various interventions captured with the PhARMD tool.11,12 These estimates from Lee and colleagues were derived from detailed chart review of interventions made and the potential harm prevented.12 Costs or cost avoidances associated with interventions were calculated from pooled examination of 600 interventions in a VAMC with drug costs before and after the intervention, costs associated with harms prevented by the intervention, as well as the VAMC hourly pharmacist wages associated with making an intervention and processing and filling original vs recommended therapies.
The costs presented represent a “best-case” scenario in which all interventions made are expected to prevent patient harms. The costs related to avoided outcomes, facility overhead, and auxiliary staff cannot be included but highlight the many considerations that must be considered when examining potential cost-avoidance calculations. The estimates and methods at hand were chosen because, to our knowledge, no other consensus model exists that would be more appropriate for use in the situation and health care system at hand. Cost-avoidance estimates were calculated by extrapolating the 88-day study period values to a yearly estimate. All cost estimates were adjusted for inflation using the consumer price index calculator as per convention in previous analyses using the cost-avoidance estimates at hand.11-13
Results
From July 6, 2015, through October 1, 2015, 301 patient encounters occurred, and 529 interventions were documented with the PhARMD tool. The mean number of interventions per encounter was 1.8. Interventions were 65.2% pharmacologic and 34.8% nonpharmacologic. Of pharmacologic interventions, 27.1% were for HF, 12.7% for hypertension, 8.8% for dyslipidemia, 2.8% for anticoagulation, 1.4% for tobacco cessation, 1.1% for T2DM, 0.3% for anemia, and 45.8% for other conditions (Table 1).
The main types of pharmacologic interventions across all diseases were related to adjustments in medication dose or frequency (42.3%) and change or discontinuation of medications (20.0%).
Discussion
Evaluation of the interventions and encounters at the WPB VAMC ambulatory cardiology pharmacotherapy clinic suggests that CPSs are able to contribute to direct patient care independently of interventions performed by other cardiology providers. Specifically, 1.8 interventions per encounter were made by CPSs in this study. In a prior evaluation of CPS interventions recorded with the PhARMD tool in a VAMC primary care setting, 2.3 interventions per encounter were recorded.11 In comparing the present volume of interventions with the volume recorded in the study by Hough and colleagues, the difference in practice setting may account for differences seen.11
The primary care medication management setting would capture a broader array of clinical interventions than would the ambulatory cardiology clinic of the present study, so it is reasonable that more interventions would be captured per encounter in the primary care clinic. The difference in practice settings affecting the character of collected interventions can be seen because most interventions in this study at an ambulatory cardiology clinic were related to HF, whereas in Hough and colleagues 39.2% of the disease-specific interventions were related to DM, and only 2.9% were related to HF.11 The differences inherent in the intervention populations can also be seen by comparing the percentage of interventions related to hypertension and dyslipidemia: 30% and 28% in the study by Hough and colleagues compared with 13% and 9%, respectively, in the present study.11
Comparison of the present evaluation and Hough and colleagues is also hindered by the PhARMD tool used. The PhARMD tool used in the initial evaluation has been modified on a national level to improve the granularity of intervention data collected.
Our cost-avoidance estimate of $433,324.06 per year seems lower than that estimated in the previous evaluation when all applicable interventions were included.11 However, this study had several differences compared with those of previous VAMC studies looking at clinical interventions performed by CPSs. The main differences are the volume and setting in which interventions were being made. For example, in comparison with Hough and colleagues, the studies include different practice settings (primary care vs cardiology specialty clinic) and number of FTEs involved in the study (4.65 vs 1). If the cost avoidance is distributed evenly per FTE in the previous study, the following calculation is observed: $649,551.99 per FTE, which is closer to this study’s estimation. Given that primary care is a broader setting than is ambulatory cardiology, it is not surprising that more types of interventions and the overall volume/absolute number of interventions would be higher. Thus, the lower estimated cost avoidance in our study may be attributed to the lower volume of intervention opportunities availed to the cardiology CPS. Another difference is that detailed types of interventions related to hypertension, DM, dyslipidemia, and HF were not included in Hough and colleagues, whereas our study included all applicable interventions regardless of relation to diseases, which may account for a degree of the variation in intervention breakdown between the 2 studies.11 However, as noted previously, some interventions for these particular diseases may not fully capture the rationale for pharmacotherapy interventions, such as drug dose changes or discontinuations, which may misrepresent the potential cost avoidance associated with them in reality.
Limitations
Of general importance, the PhARMD tool may underestimate the number of interventions made such that multiple interventions for a medical condition may have been completed but only captured as 1 intervention, which may represent a limitation of the tool when multiple interventions are made for the same disease (eg, titration of both β-blocker and angiotensin-converting enzyme inhibitor doses at a single appointment in a patient with HF with reduced left ventricular ejection fraction). Improved clarity about interventions made would require laborious chart review, which was not feasible. The evaluation at hand included a preliminary QA review, adding confidence that overdocumentation was not being done and the values represented at worst an underestimation of actual CPS intervention impact. Because this study was an initial evaluation of interventions made by CPSs in an ambulatory cardiology pharmacotherapy setting, whether these same outcomes would exist in other patient cohorts is unclear. However, these data do provide a foundational understanding of what may be expected from CPS integration into a cardiovascular care team.
These findings may be limited in generalizability to other health care systems and situations in which CPSs are afforded the regulatory opportunity to practice independently within an established scope of practice or collaborative practice agreements. The Veterans Health Administration system has been a leader in integrating CPSs into direct patient care roles and serves as a potential model for application by other groups. This evaluation’s data support continued efforts to create such independent practice environments as they allow for qualified CPSs to practice to their full clinical potential and have the fullest possible effect on cardiovascular outcomes.
Previous studies looking at cost savings in MTM programs have established a substantial return in economic investment with patients being managed by pharmacists.5,14 Given that the interventions made in this study were not tied to attainment of clinical outcomes, a limitation to our study, the cost-avoidance estimates should be interpreted cautiously. However, we know of no such tool that is available to allow accurate capture of clinical event reduction in a single center with consistent CPS involvement in care. A clear opportunity exists regarding design of a model that measures clinical, economic, and humanistic outcomes related to the interventions performed by cardiology CPSs, but developing and deploying such a model may be challenging because guideline-directed medical therapies vary significantly based on many patient-specific issues, and identifying optimal or truly optimized medical therapy is at times a subjective task, especially in a single center. Using the types and volumes of interventions made by CPSs as a surrogate for these higher-level outcomes is still of value in order to understand the effect and role of CPSs in cardiovascular care. At present, the cost-avoidance estimates presented in this evaluation are based on the most appropriate system-specific data at hand, with the realization that actual cost avoidance in practice may vary widely and should be the topic of future research.
Conclusion
As cardiovascular team-based care continues to expand with the support of large organizations, such as the American College of Cardiology Foundation, Heart Failure Society of America, and American College of Clinical Pharmacy Cardiology Practice and Research Network, the need for understanding the effect of CPSs on patient care measures and health care costs becomes more pronounced.2,15 The results of this study demonstrate how integration of CPSs in an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for cardiology physicians and providers, allowing more availability for diagnostic testing and care.
Interventions made by CPSs functioning as independent providers
1. Brush JE Jr, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65(19):2118-2136.
2. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66(19):2129-2139.
3. Sandoff BG, Kuca S, Rasmussen J, Merenich JA. Collaborative cardiac care service: a multidisciplinary approach to caring for patients with coronary artery disease. Perm J. 2008;12(3):4-11.
4. Merenich JA, Olson KL, Delate T, Rasmussen J, Helling DK, Ward DG; Clinical Pharmacy Cardiac Risk Service Study Group. Mortality reduction benefits of a comprehensive cardiac care program for patients with occlusive coronary disease. Pharmacotherapy. 2007;27(10):1370-1378.
5. Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience.
6. Martinez AS, Saef J, Paszcuzuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70(12):1070-1076.
7. Roth GA, Poole JE, Zaha R, Zhou W, Skinner J, Morden NE. Use of guideline-directed medications for heart failure before cardioverter-defibrillator implantation. J Am Coll Cardiol. 2016;67(9):1062-1069.
8. Noschese LA, Bergman CL, Brar CK, Kansal MM. The pharmacist’s role in medication optimization for patients with chronic heart failure. Fed Pract. 2017;34(suppl 10):S10-S15.
9. Coakley C, Hough A, Dwyer D, Parra D. Clinical video telehealth in a cardiology pharmacotherapy clinic. Am J Health Syst Pharm. 2013;70(22):1974-1975.
10. Khazan E, Anastasia E, Hough A, Parra D. Pharmacist-managed ambulatory blood pressure monitoring service. Am J Health Syst Pharm. 2017;74(4):190-195.
11. Hough A, Vartan CM, Groppi JA, Reyes S, Beckey NP. Evaluation of clinical pharmacy interventions in a Veterans Affairs medical center primary care clinic. Am J Health Syst Pharm. 2013;70(13):1168-1172.
12. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070-2077.
13. US Department of Labor. CPI inflation calculator. www.bls.gov/data/inflation_calculator.htm. Accessed January 18, 2019.
14. Perez A, Doloresco F, Hoffman JM, et al. Economic evaluations of clinical pharmacy services: 2001-2005. Pharmacotherapy. 2008;29(1):128.
15. Milfred-LaForest SK, Chow SL, DiDomenico RJ, et al. Clinical pharmacy services in heart failure: an opinion paper from the Heart Failure Society of America and American College of Clinical Pharmacy Cardiology Practice and Research Network. Pharmacotherapy. 2013;33(5):529-548.
Integration of CPSs into an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for other cardiology health care providers.
Integration of CPSs into an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for other cardiology health care providers.
Health care providers face many challenges in utilizing cardiovascular therapies, such as anticipated shortages in physicians, patients with more complicated conditions, shifting medication regimens, management needs, and increased accountability for quality and performance measures.1 To meet the potential increase in service demand, cardiology practices are embracing cardiovascular team-based care.1 Advanced practice providers, such as advanced practice registered nurses (APRNs), physician assistants (PAs), and clinical pharmacy specialists (CPSs), have education, training, and experience to extend the team’s capability to meet these complex management needs.1
The role of CPSs within a cardiovascular care team includes providing a variety of patient-specific services, such as collaborating with other cardiology providers, to optimize evidence-based pharmacotherapy, preventing medication-related adverse events/errors, improving patient understanding of their medication regimen, and ultimately, improving patient outcomes.2 Health care systems, such as Kaiser Permanente of Colorado, have demonstrated improved clinical outcomes for patients with coronary artery disease (CAD) by implementing a multidisciplinary collaborative cardiac care service, including a clinical pharmacy cardiac risk service, in which CPSs assisted with management of cholesterol-lowering, hypertension, diabetes mellitus (DM), and smoking-cessation therapies, which resulted in a 76% to 89% reduction in all-cause mortality associated with CAD in multiple evaluations.3,4
Pharmacists providing medication therapy management (MTM) services in Minnesota had higher goal attainment for patients with hypertension and hyperlipidemia than did pharmacists who did not provide MTM services.5 MTM services provided by pharmacists led to an improvement in clinical outcomes for patients as well as a reduction in overall health care expenditures compared with that of a control group of patients who did not receive MTM services.5 Furthermore, CPS integration in the heart failure (HF) setting has led to improvements in utilization and optimization of guideline-directed medical therapies, an area in which recent data have suggested deficiencies exist.6-8 A full review of the outcomes associated with CPS involvement in cardiovascular care is beyond the scope of this article; but the recent review by Dunn and colleagues provides more detail.2
With the increasing number of patients with cardiovascular disease,expanding integration of CPSs in the cardiovascular team providing MTM services may reduce the burden of other providers (MD, PA, APRN, etc), thereby increasing access for not only new patients, but also diagnostic and interventional work, while potentially improving clinical and economic outcomes.2 The value of integrating CPSs as members of the cardiovascular care team is recognized in a variety of inpatient and ambulatory practice settings.2-6 However, data are limited on the number and types of interventions made per encounter as direct patient care providers. Expanded granularity regarding the effect of CPSs as active members of the cardiovascular team is an essential component to evaluate the potential benefit of CPS integration into direct patient care.
Methods
The West Palm Beach (WPB) Veteran Affairs Medical Center (VAMC) outpatient cardiology clinic consists of 6 full-time employee (FTE) cardiologists, 4 PAs or APRNs, 10 other cardiology health care staff members (registered/license practical nurses and technicians), and 2 cardiology CPSs providing direct patient care
The cardiology pharmacotherapy clinic is open 20.5 hours per week with 41 appointment slots (30 minutes each), of which 7 appointments are delivered via clinic video telehealth and 34 appointments are traditional face-to-face visits.9 The remaining CPS time is assigned to other clinical care and administrative areas to fit facility need, including oversight of the CPS-run 24-hour ambulatory blood pressure clinic, postgraduate year 2 cardiology pharmacy practice residency program directorship, and other administrative activities for the facility.10
The cardiology CPSs practice under an advanced scope of practice in which they independently manage medications (initiate, modify, discontinue), order diagnostic testing (laboratory, monitoring, imaging, etc) needed for medication management, and create monitoring and treatment plans for patients referred to the cardiology pharmacotherapy clinic by other cardiology providers. The diseases managed within the clinic vary based on patient-specific needs, but may include HF, dyslipidemia, hypertension, anticoagulation, CAD, arrhythmias, cardiovascular risk factor assessment and reduction, and medication reconciliation and teaching. Patients are referred for CPS management directly from facility cardiologist and cardiology clinic PAs and APRNs. Workload and interventions carried out are captured in the Pharmacists Achieve Results with Medications Demonstration (PhARMD) tool and patient care encounter tracking.9
Data Collection
Using local data from workload tracking, the number of CPS encounters was determined from July 6, 2015, to October 1, 2015. Data were collected on the types and volume of interventions made by CPSs in the cardiology pharmacotherapy clinic using the PhARMD tool (Figure).
The PhARMD tool was initially developed and implemented for CPSs in primary care pharmacotherapy clinics and was used to evaluate the types and volume of CPS interventions made in this setting.11 Since this initial evaluation, the tool has been updated, standardized nationally by the Department of Veterans Affairs (VA) Pharmacy Benefits Management Clinical Pharmacy Practice Office, and integrated across numerous VAMCs and associated outpatient clinics. The tool remains embedded within the VA electronic health record (EHR) and allows the capture of specific CPS interventions of several types (ie, both pharmacologic and nonpharmacologic interventions, including adjust dose or frequency; change or discontinue medication; initiate medication; monitor medication; counsel on adherence, contraindications, drug interactions, and drugs not indicated; reconcile medication; and prevent or manage adverse drug events [ADEs]) specific to certain diseases, such as anemia, anticoagulation, HF, type 2 DM (T2DM), hypertension, dyslipidemia, and tobacco cessation.
Given that the interventions captured by the PhARMD tool are based on self-report of the CPS performing the intervention, a quality assurance (QA) measure was taken to audit a random sample of interventions to validate the accuracy of reported data. A Pharmacy Benefits Management PhARMD Project QA report provided the 20% random sample of encounters for each cardiology CPS to be reviewed. This percentage was determined by VAMC Clinical Pharmacy Program Office (CPPO) directives on implementation of the PhARMD tool. During the QA period, the provided sample was reviewed to determine whether the intervention(s) recorded with the PhARMD tool matched the actions documented in the EHR. The QA review was done through a manual chart review by an author not involved in recording the original interventions. Both WPB VAMC cardiology CPSs passed the QA review (> 80% concurrence with tool logged and chart documented interventions as required by VA CPPO directive), with a 90.9% concurrence between the EHR and PhARMD tool documentation.
Statistical Analyses
Data on intervention type and encounter number were evaluated with descriptive statistics. The information was characterized and diagrammed with Excel (Microsoft, Redmond, WA) charts and graphs.
Cost-avoidance calculations were done using previously described methods and are included for exploratory analysis.11,12 Briefly, published estimates of cost avoidance associated with various interventions from the outpatient setting within a VAMC setting were applied as appropriate to the various interventions captured with the PhARMD tool.11,12 These estimates from Lee and colleagues were derived from detailed chart review of interventions made and the potential harm prevented.12 Costs or cost avoidances associated with interventions were calculated from pooled examination of 600 interventions in a VAMC with drug costs before and after the intervention, costs associated with harms prevented by the intervention, as well as the VAMC hourly pharmacist wages associated with making an intervention and processing and filling original vs recommended therapies.
The costs presented represent a “best-case” scenario in which all interventions made are expected to prevent patient harms. The costs related to avoided outcomes, facility overhead, and auxiliary staff cannot be included but highlight the many considerations that must be considered when examining potential cost-avoidance calculations. The estimates and methods at hand were chosen because, to our knowledge, no other consensus model exists that would be more appropriate for use in the situation and health care system at hand. Cost-avoidance estimates were calculated by extrapolating the 88-day study period values to a yearly estimate. All cost estimates were adjusted for inflation using the consumer price index calculator as per convention in previous analyses using the cost-avoidance estimates at hand.11-13
Results
From July 6, 2015, through October 1, 2015, 301 patient encounters occurred, and 529 interventions were documented with the PhARMD tool. The mean number of interventions per encounter was 1.8. Interventions were 65.2% pharmacologic and 34.8% nonpharmacologic. Of pharmacologic interventions, 27.1% were for HF, 12.7% for hypertension, 8.8% for dyslipidemia, 2.8% for anticoagulation, 1.4% for tobacco cessation, 1.1% for T2DM, 0.3% for anemia, and 45.8% for other conditions (Table 1).
The main types of pharmacologic interventions across all diseases were related to adjustments in medication dose or frequency (42.3%) and change or discontinuation of medications (20.0%).
Discussion
Evaluation of the interventions and encounters at the WPB VAMC ambulatory cardiology pharmacotherapy clinic suggests that CPSs are able to contribute to direct patient care independently of interventions performed by other cardiology providers. Specifically, 1.8 interventions per encounter were made by CPSs in this study. In a prior evaluation of CPS interventions recorded with the PhARMD tool in a VAMC primary care setting, 2.3 interventions per encounter were recorded.11 In comparing the present volume of interventions with the volume recorded in the study by Hough and colleagues, the difference in practice setting may account for differences seen.11
The primary care medication management setting would capture a broader array of clinical interventions than would the ambulatory cardiology clinic of the present study, so it is reasonable that more interventions would be captured per encounter in the primary care clinic. The difference in practice settings affecting the character of collected interventions can be seen because most interventions in this study at an ambulatory cardiology clinic were related to HF, whereas in Hough and colleagues 39.2% of the disease-specific interventions were related to DM, and only 2.9% were related to HF.11 The differences inherent in the intervention populations can also be seen by comparing the percentage of interventions related to hypertension and dyslipidemia: 30% and 28% in the study by Hough and colleagues compared with 13% and 9%, respectively, in the present study.11
Comparison of the present evaluation and Hough and colleagues is also hindered by the PhARMD tool used. The PhARMD tool used in the initial evaluation has been modified on a national level to improve the granularity of intervention data collected.
Our cost-avoidance estimate of $433,324.06 per year seems lower than that estimated in the previous evaluation when all applicable interventions were included.11 However, this study had several differences compared with those of previous VAMC studies looking at clinical interventions performed by CPSs. The main differences are the volume and setting in which interventions were being made. For example, in comparison with Hough and colleagues, the studies include different practice settings (primary care vs cardiology specialty clinic) and number of FTEs involved in the study (4.65 vs 1). If the cost avoidance is distributed evenly per FTE in the previous study, the following calculation is observed: $649,551.99 per FTE, which is closer to this study’s estimation. Given that primary care is a broader setting than is ambulatory cardiology, it is not surprising that more types of interventions and the overall volume/absolute number of interventions would be higher. Thus, the lower estimated cost avoidance in our study may be attributed to the lower volume of intervention opportunities availed to the cardiology CPS. Another difference is that detailed types of interventions related to hypertension, DM, dyslipidemia, and HF were not included in Hough and colleagues, whereas our study included all applicable interventions regardless of relation to diseases, which may account for a degree of the variation in intervention breakdown between the 2 studies.11 However, as noted previously, some interventions for these particular diseases may not fully capture the rationale for pharmacotherapy interventions, such as drug dose changes or discontinuations, which may misrepresent the potential cost avoidance associated with them in reality.
Limitations
Of general importance, the PhARMD tool may underestimate the number of interventions made such that multiple interventions for a medical condition may have been completed but only captured as 1 intervention, which may represent a limitation of the tool when multiple interventions are made for the same disease (eg, titration of both β-blocker and angiotensin-converting enzyme inhibitor doses at a single appointment in a patient with HF with reduced left ventricular ejection fraction). Improved clarity about interventions made would require laborious chart review, which was not feasible. The evaluation at hand included a preliminary QA review, adding confidence that overdocumentation was not being done and the values represented at worst an underestimation of actual CPS intervention impact. Because this study was an initial evaluation of interventions made by CPSs in an ambulatory cardiology pharmacotherapy setting, whether these same outcomes would exist in other patient cohorts is unclear. However, these data do provide a foundational understanding of what may be expected from CPS integration into a cardiovascular care team.
These findings may be limited in generalizability to other health care systems and situations in which CPSs are afforded the regulatory opportunity to practice independently within an established scope of practice or collaborative practice agreements. The Veterans Health Administration system has been a leader in integrating CPSs into direct patient care roles and serves as a potential model for application by other groups. This evaluation’s data support continued efforts to create such independent practice environments as they allow for qualified CPSs to practice to their full clinical potential and have the fullest possible effect on cardiovascular outcomes.
Previous studies looking at cost savings in MTM programs have established a substantial return in economic investment with patients being managed by pharmacists.5,14 Given that the interventions made in this study were not tied to attainment of clinical outcomes, a limitation to our study, the cost-avoidance estimates should be interpreted cautiously. However, we know of no such tool that is available to allow accurate capture of clinical event reduction in a single center with consistent CPS involvement in care. A clear opportunity exists regarding design of a model that measures clinical, economic, and humanistic outcomes related to the interventions performed by cardiology CPSs, but developing and deploying such a model may be challenging because guideline-directed medical therapies vary significantly based on many patient-specific issues, and identifying optimal or truly optimized medical therapy is at times a subjective task, especially in a single center. Using the types and volumes of interventions made by CPSs as a surrogate for these higher-level outcomes is still of value in order to understand the effect and role of CPSs in cardiovascular care. At present, the cost-avoidance estimates presented in this evaluation are based on the most appropriate system-specific data at hand, with the realization that actual cost avoidance in practice may vary widely and should be the topic of future research.
Conclusion
As cardiovascular team-based care continues to expand with the support of large organizations, such as the American College of Cardiology Foundation, Heart Failure Society of America, and American College of Clinical Pharmacy Cardiology Practice and Research Network, the need for understanding the effect of CPSs on patient care measures and health care costs becomes more pronounced.2,15 The results of this study demonstrate how integration of CPSs in an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for cardiology physicians and providers, allowing more availability for diagnostic testing and care.
Interventions made by CPSs functioning as independent providers
Health care providers face many challenges in utilizing cardiovascular therapies, such as anticipated shortages in physicians, patients with more complicated conditions, shifting medication regimens, management needs, and increased accountability for quality and performance measures.1 To meet the potential increase in service demand, cardiology practices are embracing cardiovascular team-based care.1 Advanced practice providers, such as advanced practice registered nurses (APRNs), physician assistants (PAs), and clinical pharmacy specialists (CPSs), have education, training, and experience to extend the team’s capability to meet these complex management needs.1
The role of CPSs within a cardiovascular care team includes providing a variety of patient-specific services, such as collaborating with other cardiology providers, to optimize evidence-based pharmacotherapy, preventing medication-related adverse events/errors, improving patient understanding of their medication regimen, and ultimately, improving patient outcomes.2 Health care systems, such as Kaiser Permanente of Colorado, have demonstrated improved clinical outcomes for patients with coronary artery disease (CAD) by implementing a multidisciplinary collaborative cardiac care service, including a clinical pharmacy cardiac risk service, in which CPSs assisted with management of cholesterol-lowering, hypertension, diabetes mellitus (DM), and smoking-cessation therapies, which resulted in a 76% to 89% reduction in all-cause mortality associated with CAD in multiple evaluations.3,4
Pharmacists providing medication therapy management (MTM) services in Minnesota had higher goal attainment for patients with hypertension and hyperlipidemia than did pharmacists who did not provide MTM services.5 MTM services provided by pharmacists led to an improvement in clinical outcomes for patients as well as a reduction in overall health care expenditures compared with that of a control group of patients who did not receive MTM services.5 Furthermore, CPS integration in the heart failure (HF) setting has led to improvements in utilization and optimization of guideline-directed medical therapies, an area in which recent data have suggested deficiencies exist.6-8 A full review of the outcomes associated with CPS involvement in cardiovascular care is beyond the scope of this article; but the recent review by Dunn and colleagues provides more detail.2
With the increasing number of patients with cardiovascular disease,expanding integration of CPSs in the cardiovascular team providing MTM services may reduce the burden of other providers (MD, PA, APRN, etc), thereby increasing access for not only new patients, but also diagnostic and interventional work, while potentially improving clinical and economic outcomes.2 The value of integrating CPSs as members of the cardiovascular care team is recognized in a variety of inpatient and ambulatory practice settings.2-6 However, data are limited on the number and types of interventions made per encounter as direct patient care providers. Expanded granularity regarding the effect of CPSs as active members of the cardiovascular team is an essential component to evaluate the potential benefit of CPS integration into direct patient care.
Methods
The West Palm Beach (WPB) Veteran Affairs Medical Center (VAMC) outpatient cardiology clinic consists of 6 full-time employee (FTE) cardiologists, 4 PAs or APRNs, 10 other cardiology health care staff members (registered/license practical nurses and technicians), and 2 cardiology CPSs providing direct patient care
The cardiology pharmacotherapy clinic is open 20.5 hours per week with 41 appointment slots (30 minutes each), of which 7 appointments are delivered via clinic video telehealth and 34 appointments are traditional face-to-face visits.9 The remaining CPS time is assigned to other clinical care and administrative areas to fit facility need, including oversight of the CPS-run 24-hour ambulatory blood pressure clinic, postgraduate year 2 cardiology pharmacy practice residency program directorship, and other administrative activities for the facility.10
The cardiology CPSs practice under an advanced scope of practice in which they independently manage medications (initiate, modify, discontinue), order diagnostic testing (laboratory, monitoring, imaging, etc) needed for medication management, and create monitoring and treatment plans for patients referred to the cardiology pharmacotherapy clinic by other cardiology providers. The diseases managed within the clinic vary based on patient-specific needs, but may include HF, dyslipidemia, hypertension, anticoagulation, CAD, arrhythmias, cardiovascular risk factor assessment and reduction, and medication reconciliation and teaching. Patients are referred for CPS management directly from facility cardiologist and cardiology clinic PAs and APRNs. Workload and interventions carried out are captured in the Pharmacists Achieve Results with Medications Demonstration (PhARMD) tool and patient care encounter tracking.9
Data Collection
Using local data from workload tracking, the number of CPS encounters was determined from July 6, 2015, to October 1, 2015. Data were collected on the types and volume of interventions made by CPSs in the cardiology pharmacotherapy clinic using the PhARMD tool (Figure).
The PhARMD tool was initially developed and implemented for CPSs in primary care pharmacotherapy clinics and was used to evaluate the types and volume of CPS interventions made in this setting.11 Since this initial evaluation, the tool has been updated, standardized nationally by the Department of Veterans Affairs (VA) Pharmacy Benefits Management Clinical Pharmacy Practice Office, and integrated across numerous VAMCs and associated outpatient clinics. The tool remains embedded within the VA electronic health record (EHR) and allows the capture of specific CPS interventions of several types (ie, both pharmacologic and nonpharmacologic interventions, including adjust dose or frequency; change or discontinue medication; initiate medication; monitor medication; counsel on adherence, contraindications, drug interactions, and drugs not indicated; reconcile medication; and prevent or manage adverse drug events [ADEs]) specific to certain diseases, such as anemia, anticoagulation, HF, type 2 DM (T2DM), hypertension, dyslipidemia, and tobacco cessation.
Given that the interventions captured by the PhARMD tool are based on self-report of the CPS performing the intervention, a quality assurance (QA) measure was taken to audit a random sample of interventions to validate the accuracy of reported data. A Pharmacy Benefits Management PhARMD Project QA report provided the 20% random sample of encounters for each cardiology CPS to be reviewed. This percentage was determined by VAMC Clinical Pharmacy Program Office (CPPO) directives on implementation of the PhARMD tool. During the QA period, the provided sample was reviewed to determine whether the intervention(s) recorded with the PhARMD tool matched the actions documented in the EHR. The QA review was done through a manual chart review by an author not involved in recording the original interventions. Both WPB VAMC cardiology CPSs passed the QA review (> 80% concurrence with tool logged and chart documented interventions as required by VA CPPO directive), with a 90.9% concurrence between the EHR and PhARMD tool documentation.
Statistical Analyses
Data on intervention type and encounter number were evaluated with descriptive statistics. The information was characterized and diagrammed with Excel (Microsoft, Redmond, WA) charts and graphs.
Cost-avoidance calculations were done using previously described methods and are included for exploratory analysis.11,12 Briefly, published estimates of cost avoidance associated with various interventions from the outpatient setting within a VAMC setting were applied as appropriate to the various interventions captured with the PhARMD tool.11,12 These estimates from Lee and colleagues were derived from detailed chart review of interventions made and the potential harm prevented.12 Costs or cost avoidances associated with interventions were calculated from pooled examination of 600 interventions in a VAMC with drug costs before and after the intervention, costs associated with harms prevented by the intervention, as well as the VAMC hourly pharmacist wages associated with making an intervention and processing and filling original vs recommended therapies.
The costs presented represent a “best-case” scenario in which all interventions made are expected to prevent patient harms. The costs related to avoided outcomes, facility overhead, and auxiliary staff cannot be included but highlight the many considerations that must be considered when examining potential cost-avoidance calculations. The estimates and methods at hand were chosen because, to our knowledge, no other consensus model exists that would be more appropriate for use in the situation and health care system at hand. Cost-avoidance estimates were calculated by extrapolating the 88-day study period values to a yearly estimate. All cost estimates were adjusted for inflation using the consumer price index calculator as per convention in previous analyses using the cost-avoidance estimates at hand.11-13
Results
From July 6, 2015, through October 1, 2015, 301 patient encounters occurred, and 529 interventions were documented with the PhARMD tool. The mean number of interventions per encounter was 1.8. Interventions were 65.2% pharmacologic and 34.8% nonpharmacologic. Of pharmacologic interventions, 27.1% were for HF, 12.7% for hypertension, 8.8% for dyslipidemia, 2.8% for anticoagulation, 1.4% for tobacco cessation, 1.1% for T2DM, 0.3% for anemia, and 45.8% for other conditions (Table 1).
The main types of pharmacologic interventions across all diseases were related to adjustments in medication dose or frequency (42.3%) and change or discontinuation of medications (20.0%).
Discussion
Evaluation of the interventions and encounters at the WPB VAMC ambulatory cardiology pharmacotherapy clinic suggests that CPSs are able to contribute to direct patient care independently of interventions performed by other cardiology providers. Specifically, 1.8 interventions per encounter were made by CPSs in this study. In a prior evaluation of CPS interventions recorded with the PhARMD tool in a VAMC primary care setting, 2.3 interventions per encounter were recorded.11 In comparing the present volume of interventions with the volume recorded in the study by Hough and colleagues, the difference in practice setting may account for differences seen.11
The primary care medication management setting would capture a broader array of clinical interventions than would the ambulatory cardiology clinic of the present study, so it is reasonable that more interventions would be captured per encounter in the primary care clinic. The difference in practice settings affecting the character of collected interventions can be seen because most interventions in this study at an ambulatory cardiology clinic were related to HF, whereas in Hough and colleagues 39.2% of the disease-specific interventions were related to DM, and only 2.9% were related to HF.11 The differences inherent in the intervention populations can also be seen by comparing the percentage of interventions related to hypertension and dyslipidemia: 30% and 28% in the study by Hough and colleagues compared with 13% and 9%, respectively, in the present study.11
Comparison of the present evaluation and Hough and colleagues is also hindered by the PhARMD tool used. The PhARMD tool used in the initial evaluation has been modified on a national level to improve the granularity of intervention data collected.
Our cost-avoidance estimate of $433,324.06 per year seems lower than that estimated in the previous evaluation when all applicable interventions were included.11 However, this study had several differences compared with those of previous VAMC studies looking at clinical interventions performed by CPSs. The main differences are the volume and setting in which interventions were being made. For example, in comparison with Hough and colleagues, the studies include different practice settings (primary care vs cardiology specialty clinic) and number of FTEs involved in the study (4.65 vs 1). If the cost avoidance is distributed evenly per FTE in the previous study, the following calculation is observed: $649,551.99 per FTE, which is closer to this study’s estimation. Given that primary care is a broader setting than is ambulatory cardiology, it is not surprising that more types of interventions and the overall volume/absolute number of interventions would be higher. Thus, the lower estimated cost avoidance in our study may be attributed to the lower volume of intervention opportunities availed to the cardiology CPS. Another difference is that detailed types of interventions related to hypertension, DM, dyslipidemia, and HF were not included in Hough and colleagues, whereas our study included all applicable interventions regardless of relation to diseases, which may account for a degree of the variation in intervention breakdown between the 2 studies.11 However, as noted previously, some interventions for these particular diseases may not fully capture the rationale for pharmacotherapy interventions, such as drug dose changes or discontinuations, which may misrepresent the potential cost avoidance associated with them in reality.
Limitations
Of general importance, the PhARMD tool may underestimate the number of interventions made such that multiple interventions for a medical condition may have been completed but only captured as 1 intervention, which may represent a limitation of the tool when multiple interventions are made for the same disease (eg, titration of both β-blocker and angiotensin-converting enzyme inhibitor doses at a single appointment in a patient with HF with reduced left ventricular ejection fraction). Improved clarity about interventions made would require laborious chart review, which was not feasible. The evaluation at hand included a preliminary QA review, adding confidence that overdocumentation was not being done and the values represented at worst an underestimation of actual CPS intervention impact. Because this study was an initial evaluation of interventions made by CPSs in an ambulatory cardiology pharmacotherapy setting, whether these same outcomes would exist in other patient cohorts is unclear. However, these data do provide a foundational understanding of what may be expected from CPS integration into a cardiovascular care team.
These findings may be limited in generalizability to other health care systems and situations in which CPSs are afforded the regulatory opportunity to practice independently within an established scope of practice or collaborative practice agreements. The Veterans Health Administration system has been a leader in integrating CPSs into direct patient care roles and serves as a potential model for application by other groups. This evaluation’s data support continued efforts to create such independent practice environments as they allow for qualified CPSs to practice to their full clinical potential and have the fullest possible effect on cardiovascular outcomes.
Previous studies looking at cost savings in MTM programs have established a substantial return in economic investment with patients being managed by pharmacists.5,14 Given that the interventions made in this study were not tied to attainment of clinical outcomes, a limitation to our study, the cost-avoidance estimates should be interpreted cautiously. However, we know of no such tool that is available to allow accurate capture of clinical event reduction in a single center with consistent CPS involvement in care. A clear opportunity exists regarding design of a model that measures clinical, economic, and humanistic outcomes related to the interventions performed by cardiology CPSs, but developing and deploying such a model may be challenging because guideline-directed medical therapies vary significantly based on many patient-specific issues, and identifying optimal or truly optimized medical therapy is at times a subjective task, especially in a single center. Using the types and volumes of interventions made by CPSs as a surrogate for these higher-level outcomes is still of value in order to understand the effect and role of CPSs in cardiovascular care. At present, the cost-avoidance estimates presented in this evaluation are based on the most appropriate system-specific data at hand, with the realization that actual cost avoidance in practice may vary widely and should be the topic of future research.
Conclusion
As cardiovascular team-based care continues to expand with the support of large organizations, such as the American College of Cardiology Foundation, Heart Failure Society of America, and American College of Clinical Pharmacy Cardiology Practice and Research Network, the need for understanding the effect of CPSs on patient care measures and health care costs becomes more pronounced.2,15 The results of this study demonstrate how integration of CPSs in an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for cardiology physicians and providers, allowing more availability for diagnostic testing and care.
Interventions made by CPSs functioning as independent providers
1. Brush JE Jr, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65(19):2118-2136.
2. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66(19):2129-2139.
3. Sandoff BG, Kuca S, Rasmussen J, Merenich JA. Collaborative cardiac care service: a multidisciplinary approach to caring for patients with coronary artery disease. Perm J. 2008;12(3):4-11.
4. Merenich JA, Olson KL, Delate T, Rasmussen J, Helling DK, Ward DG; Clinical Pharmacy Cardiac Risk Service Study Group. Mortality reduction benefits of a comprehensive cardiac care program for patients with occlusive coronary disease. Pharmacotherapy. 2007;27(10):1370-1378.
5. Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience.
6. Martinez AS, Saef J, Paszcuzuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70(12):1070-1076.
7. Roth GA, Poole JE, Zaha R, Zhou W, Skinner J, Morden NE. Use of guideline-directed medications for heart failure before cardioverter-defibrillator implantation. J Am Coll Cardiol. 2016;67(9):1062-1069.
8. Noschese LA, Bergman CL, Brar CK, Kansal MM. The pharmacist’s role in medication optimization for patients with chronic heart failure. Fed Pract. 2017;34(suppl 10):S10-S15.
9. Coakley C, Hough A, Dwyer D, Parra D. Clinical video telehealth in a cardiology pharmacotherapy clinic. Am J Health Syst Pharm. 2013;70(22):1974-1975.
10. Khazan E, Anastasia E, Hough A, Parra D. Pharmacist-managed ambulatory blood pressure monitoring service. Am J Health Syst Pharm. 2017;74(4):190-195.
11. Hough A, Vartan CM, Groppi JA, Reyes S, Beckey NP. Evaluation of clinical pharmacy interventions in a Veterans Affairs medical center primary care clinic. Am J Health Syst Pharm. 2013;70(13):1168-1172.
12. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070-2077.
13. US Department of Labor. CPI inflation calculator. www.bls.gov/data/inflation_calculator.htm. Accessed January 18, 2019.
14. Perez A, Doloresco F, Hoffman JM, et al. Economic evaluations of clinical pharmacy services: 2001-2005. Pharmacotherapy. 2008;29(1):128.
15. Milfred-LaForest SK, Chow SL, DiDomenico RJ, et al. Clinical pharmacy services in heart failure: an opinion paper from the Heart Failure Society of America and American College of Clinical Pharmacy Cardiology Practice and Research Network. Pharmacotherapy. 2013;33(5):529-548.
1. Brush JE Jr, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65(19):2118-2136.
2. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66(19):2129-2139.
3. Sandoff BG, Kuca S, Rasmussen J, Merenich JA. Collaborative cardiac care service: a multidisciplinary approach to caring for patients with coronary artery disease. Perm J. 2008;12(3):4-11.
4. Merenich JA, Olson KL, Delate T, Rasmussen J, Helling DK, Ward DG; Clinical Pharmacy Cardiac Risk Service Study Group. Mortality reduction benefits of a comprehensive cardiac care program for patients with occlusive coronary disease. Pharmacotherapy. 2007;27(10):1370-1378.
5. Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience.
6. Martinez AS, Saef J, Paszcuzuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70(12):1070-1076.
7. Roth GA, Poole JE, Zaha R, Zhou W, Skinner J, Morden NE. Use of guideline-directed medications for heart failure before cardioverter-defibrillator implantation. J Am Coll Cardiol. 2016;67(9):1062-1069.
8. Noschese LA, Bergman CL, Brar CK, Kansal MM. The pharmacist’s role in medication optimization for patients with chronic heart failure. Fed Pract. 2017;34(suppl 10):S10-S15.
9. Coakley C, Hough A, Dwyer D, Parra D. Clinical video telehealth in a cardiology pharmacotherapy clinic. Am J Health Syst Pharm. 2013;70(22):1974-1975.
10. Khazan E, Anastasia E, Hough A, Parra D. Pharmacist-managed ambulatory blood pressure monitoring service. Am J Health Syst Pharm. 2017;74(4):190-195.
11. Hough A, Vartan CM, Groppi JA, Reyes S, Beckey NP. Evaluation of clinical pharmacy interventions in a Veterans Affairs medical center primary care clinic. Am J Health Syst Pharm. 2013;70(13):1168-1172.
12. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070-2077.
13. US Department of Labor. CPI inflation calculator. www.bls.gov/data/inflation_calculator.htm. Accessed January 18, 2019.
14. Perez A, Doloresco F, Hoffman JM, et al. Economic evaluations of clinical pharmacy services: 2001-2005. Pharmacotherapy. 2008;29(1):128.
15. Milfred-LaForest SK, Chow SL, DiDomenico RJ, et al. Clinical pharmacy services in heart failure: an opinion paper from the Heart Failure Society of America and American College of Clinical Pharmacy Cardiology Practice and Research Network. Pharmacotherapy. 2013;33(5):529-548.
She Won’t Quit—But Will Her Heart?
ANSWER
The correct interpretation is sinus rhythm with a first-degree atrioventricular (AV) block and possible left atrial enlargement. Criteria for sinus rhythm include a P wave for every QRS complex and a QRS complex for every P wave with a consistent PR interval.
Criteria for a first-degree AV block include a consistent PR interval > 200 ms in all leads. Criteria for left atrial enlargement include a P-wave duration > 120 ms in lead II or a downward deflection of the P wave in lead V1 > 40 ms in length with > 1-mm negative deflection.
The P wave in leads II and V1, along with a murmur, is consistent with mitral regurgitation and a history of palpitations and paroxysmal atrial fibrillation. Left atrial enlargement should be considered. An echocardiogram was ordered and confirmed the diagnosis of left atrial enlargement in this patient.
ANSWER
The correct interpretation is sinus rhythm with a first-degree atrioventricular (AV) block and possible left atrial enlargement. Criteria for sinus rhythm include a P wave for every QRS complex and a QRS complex for every P wave with a consistent PR interval.
Criteria for a first-degree AV block include a consistent PR interval > 200 ms in all leads. Criteria for left atrial enlargement include a P-wave duration > 120 ms in lead II or a downward deflection of the P wave in lead V1 > 40 ms in length with > 1-mm negative deflection.
The P wave in leads II and V1, along with a murmur, is consistent with mitral regurgitation and a history of palpitations and paroxysmal atrial fibrillation. Left atrial enlargement should be considered. An echocardiogram was ordered and confirmed the diagnosis of left atrial enlargement in this patient.
ANSWER
The correct interpretation is sinus rhythm with a first-degree atrioventricular (AV) block and possible left atrial enlargement. Criteria for sinus rhythm include a P wave for every QRS complex and a QRS complex for every P wave with a consistent PR interval.
Criteria for a first-degree AV block include a consistent PR interval > 200 ms in all leads. Criteria for left atrial enlargement include a P-wave duration > 120 ms in lead II or a downward deflection of the P wave in lead V1 > 40 ms in length with > 1-mm negative deflection.
The P wave in leads II and V1, along with a murmur, is consistent with mitral regurgitation and a history of palpitations and paroxysmal atrial fibrillation. Left atrial enlargement should be considered. An echocardiogram was ordered and confirmed the diagnosis of left atrial enlargement in this patient.
A 58-year-old woman presents for preoperative workup for surgical repair of a distal left tibial fracture sustained while snowshoeing. She had been descending a side slope when she lost her footing. Her left snowshoe became entangled in a large granite rock, which defined the lateral wall of the path she was traversing. She has no prior orthopedic injuries.
Cardiac history is remarkable for hypertension, palpitations, and two episodes of paroxysmal atrial fibrillation. A Holter monitor, worn to help determine the etiology of her palpitations, captured the atrial fibrillation episodes, each of which was cardioverted within 48 hours of onset without complication. Following the second cardioversion, about 6 months ago, a novel oral anticoagulation agent was recommended, but she refused to take it because she felt such medication would interfere with her active lifestyle.
The patient is otherwise quite healthy and has been personally active in her preventive health maintenance.
Her current medications include lisinopril (5 mg/d) and aspirin (81 mg/d). She also takes a multivitamin daily. In the past 24 hours, she has taken two doses of acetaminophen/oxycodone (325/5 mg) for left ankle pain. She has no drug allergies.
The patient, an immigration attorney for a prominent law firm, is divorced and has no children. She works as a Zumba instructor on the weekends and has run 3 marathons within the past 2 years. She has never smoked or used recreational drugs, but she does partake in one or two glasses of wine with friends on weekends.
Family history is remarkable for hypertension in both parents and two of her three siblings. All are alive and otherwise healthy.
Review of systems reveals no current problems. She states she went through menopause about 10 years ago and was recommended to start estrogen therapy but refused this treatment.
Vital signs include a blood pressure of 118/88 mm Hg; pulse, 90 beats/min; temperature, 98.4°F; and O2 saturation, 98% on room air. Her weight is 129 lb, and her height, 64 in.
Physical exam reveals a healthy, athletic woman in no distress. She wears an orthopedic boot on her left foot, but it isn’t removed to examine the affected ankle. She wears contact lenses and has a posterior lingual brace on her lower teeth.
The HEENT exam is normal. The neck is supple without masses. There is no thyromegaly, carotid bruits, or jugular venous distention. The lungs are clear in all fields. The breasts are symmetrical without palpable nodules.
Cardiac exam is remarkable for a regular rate and rhythm at 90 beats/min. There is a soft end-systolic murmur consistent with mild mitral regurgitation. S1 and S2 are of normal intensity, and there are no extra heart sounds.
The abdomen is nontender with no organomegaly; the patient proudly shows her core strength and “a hint of a six-pack.” The genitourinary exam is deferred. Peripheral pulses are strong and equal bilaterally. The neurologic exam is grossly intact.
A preoperative chest x-ray is performed; results are pending.
The ECG shows a ventricular rate of 89 beats/min; PR interval, 232 ms; QRS duration, 82 ms; QT/QTc interval, 364/442 ms; P axis, 86°; R axis, 23°
ADT harms likely limited to men with CV comorbidities
WASHINGTON – The cardiovascular effects of androgen deprivation therapy (ADT) for men with advanced prostate cancer are less severe than once feared, but there is evidence to suggest that men with preexisting heart failure or a history of myocardial infarction could be at excess risk for death from cardiovascular causes when they receive ADT, according to a leading prostate cancer expert.
“I think there are concerns about potential cardiovascular harm of ADT, and I think this has reduced ADT use, despite the fact that we know for most men it improves overall survival,” said Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“In fact, when we looked recently at men with high-risk prostate cancer, this is a group where overall survival is improved by 50% if they get ADT – so it cuts the risk of death in half – but it turns out that nearly a quarter of those patients are not receiving ADT. I think that the concern about cardiovascular harm and the confusion as to where that data stands is a lot of what’s driving that right now,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Randomized trial data
Dr. Nguyen noted that the evidence suggesting that ADT can increase the risk of death from cardiovascular causes came largely from three major studies:
- A 2006 study of 73,196 Medicare enrollees aged 66 or older, which found that ADT with a gonadotropin-releasing hormone (GnRH) agonist was possibly associated with increased risk of incident diabetes and cardiovascular disease (J Clin Oncol. 2006 Sep 20;24[27]:4448-56.).
- A 2007 analysis of data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CAPSURE) database on 3,262 men treated with radical prostatectomy and 1,630 men treated with radiation or cryotherapy for localized prostate cancer, which found that among those 65 and older the 5-year cumulative incidence of cardiovascular death was 5.5% for patients who received ADT, vs. 2% for those who did not (J Natl Cancer Inst. 2007 Oct 17;99[20]:1516-24).
- A 2007 study of 1,372 men in three randomized trials of radiation therapy with or without androgen suppression therapy up to 8 months in duration, which found that men 65 and older who received 6 months of androgen suppression had significantly shorter times to fatal MIs than did men who did not receive the therapy (J Clin Oncol. 2007;25[17]:2420-5).
These studies, combined with observational data, led to a 2010 consensus statement from the American Heart Association, American Cancer Society, and American Urological Association, with endorsement from the American Society for Radiation Oncology, which stated that “there may be a relation between ADT and cardiovascular events and death.”
Also in 2010, the Food and Drug Administration required new labeling on GnRH agonists warning of “increased risk of diabetes and certain cardiovascular diseases (heart attack, sudden cardiac death, stroke).”
Not unanimous
Two other large randomized studies (J Clin Oncol. 2008 Feb 1;26[4]:585-91 and J Clin Oncol. 2009 Jan 1;27[1]:92-9) and two retrospective studies (J Clin Oncol. 2009 Jul 20;27[21]:3452-8 and J Clin Oncol. 2011 Sep 10;29[26]3510-16) found no excess risk of cardiovascular disease from ADT, Dr. Nguyen said, prompting him and his colleagues to see whether they could get a better estimate of the actual risk.
They did so through a 2011 meta-analysis (JAMA. 2011;306[21]:2359-66) of data on 4,141 patients from eight randomized trials. They found that among patients with unfavorable-risk prostate cancer, ADT was not associated with an increased risk of cardiovascular death, but was associated with lower risks for both prostate-specific and all-cause mortality.
Subpopulations may still be at risk
Dr. Nguyen said that the principal finding of the meta-analysis, while reassuring, “doesn’t let ADT off the hook for metabolic events, diabetes which we know happens, and the possibility of nonfatal cardiac events.”
He noted that while ADT was not associated with cardiovascular disease in clinical trials, observational studies showed significantly increased risk for fatal or non-fatal MI.
One possible explanation for the difference is that observational studies included nonfatal MI, while randomized trials looked only at cardiovascular deaths. It’s also possible that ADT causes harm primarily in men with preexisting comorbidities, who are often excluded from or underrepresented in clinical trials.
Evidence from a 2009 study (JAMA. 2009 Aug 26;302[8]:866-73) showed that among men with clinical stage T1 to T3 noninvasive, nonmetastatic prostate cancer, neoadjuvant hormonal therapy with both a luteinizing hormone-releasing hormone (LHRH) agonist and a nonsteroidal antiandrogen was associated with increased risk for all-cause mortality for those with a history of coronary artery disease–induced heart failure, but not for men with either no comorbidities or only a single comorbidity such as hypertension, hypercholesterolemia, or diabetes.
Clinical considerations
The decision to treat men with prostate cancer with ADT is therefore a balancing act, Dr. Nguyen said.
“As the risk of prostate cancer death goes up, the benefit of ADT goes up. However, as the comorbidity level goes up, the potential cardiovascular harm of ADT goes up,” he said.
For patients at the extreme ends of each continuum, such as a patient with high-risk prostate cancer and no cardiovascular comorbidities or a patient with low-risk cancer but multiple CV risk factors, the decision to give or withhold ADT is relatively simple, he said.
But for patients in between, such as a man with intermediate-risk cancer and one risk factor or a man with high risk disease with multiple comorbidities, the decision is far more complex.
“This where I think the dialogue with the cardiologist really needs to come into this decision,” he said.
Evidence to support the decision comes from retrospective studies suggesting that even men with high-risk prostate cancer have poorer overall survival with ADT if they have a history of heart failure or MI.
For patients with low-risk cancer and diabetes, ADT is associated with worse overall survival, but ADT does not cause additional harm to men with intermediate- to high-risk prostate cancer who have concomitant diabetes, Dr. Nguyen said.
“My view is that ADT has not been shown to increase cardiovascular death in randomized trials, so I think that for the vast majority of patients it probably does not increase cardiovascular deaths. But I think there could very well be a vulnerable 5% of patients who might have an excess risk of cardiovascular death, and I think we have to be careful, but we still have to balance it out against their risks for prostate cancer death,” he said.
Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
WASHINGTON – The cardiovascular effects of androgen deprivation therapy (ADT) for men with advanced prostate cancer are less severe than once feared, but there is evidence to suggest that men with preexisting heart failure or a history of myocardial infarction could be at excess risk for death from cardiovascular causes when they receive ADT, according to a leading prostate cancer expert.
“I think there are concerns about potential cardiovascular harm of ADT, and I think this has reduced ADT use, despite the fact that we know for most men it improves overall survival,” said Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“In fact, when we looked recently at men with high-risk prostate cancer, this is a group where overall survival is improved by 50% if they get ADT – so it cuts the risk of death in half – but it turns out that nearly a quarter of those patients are not receiving ADT. I think that the concern about cardiovascular harm and the confusion as to where that data stands is a lot of what’s driving that right now,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Randomized trial data
Dr. Nguyen noted that the evidence suggesting that ADT can increase the risk of death from cardiovascular causes came largely from three major studies:
- A 2006 study of 73,196 Medicare enrollees aged 66 or older, which found that ADT with a gonadotropin-releasing hormone (GnRH) agonist was possibly associated with increased risk of incident diabetes and cardiovascular disease (J Clin Oncol. 2006 Sep 20;24[27]:4448-56.).
- A 2007 analysis of data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CAPSURE) database on 3,262 men treated with radical prostatectomy and 1,630 men treated with radiation or cryotherapy for localized prostate cancer, which found that among those 65 and older the 5-year cumulative incidence of cardiovascular death was 5.5% for patients who received ADT, vs. 2% for those who did not (J Natl Cancer Inst. 2007 Oct 17;99[20]:1516-24).
- A 2007 study of 1,372 men in three randomized trials of radiation therapy with or without androgen suppression therapy up to 8 months in duration, which found that men 65 and older who received 6 months of androgen suppression had significantly shorter times to fatal MIs than did men who did not receive the therapy (J Clin Oncol. 2007;25[17]:2420-5).
These studies, combined with observational data, led to a 2010 consensus statement from the American Heart Association, American Cancer Society, and American Urological Association, with endorsement from the American Society for Radiation Oncology, which stated that “there may be a relation between ADT and cardiovascular events and death.”
Also in 2010, the Food and Drug Administration required new labeling on GnRH agonists warning of “increased risk of diabetes and certain cardiovascular diseases (heart attack, sudden cardiac death, stroke).”
Not unanimous
Two other large randomized studies (J Clin Oncol. 2008 Feb 1;26[4]:585-91 and J Clin Oncol. 2009 Jan 1;27[1]:92-9) and two retrospective studies (J Clin Oncol. 2009 Jul 20;27[21]:3452-8 and J Clin Oncol. 2011 Sep 10;29[26]3510-16) found no excess risk of cardiovascular disease from ADT, Dr. Nguyen said, prompting him and his colleagues to see whether they could get a better estimate of the actual risk.
They did so through a 2011 meta-analysis (JAMA. 2011;306[21]:2359-66) of data on 4,141 patients from eight randomized trials. They found that among patients with unfavorable-risk prostate cancer, ADT was not associated with an increased risk of cardiovascular death, but was associated with lower risks for both prostate-specific and all-cause mortality.
Subpopulations may still be at risk
Dr. Nguyen said that the principal finding of the meta-analysis, while reassuring, “doesn’t let ADT off the hook for metabolic events, diabetes which we know happens, and the possibility of nonfatal cardiac events.”
He noted that while ADT was not associated with cardiovascular disease in clinical trials, observational studies showed significantly increased risk for fatal or non-fatal MI.
One possible explanation for the difference is that observational studies included nonfatal MI, while randomized trials looked only at cardiovascular deaths. It’s also possible that ADT causes harm primarily in men with preexisting comorbidities, who are often excluded from or underrepresented in clinical trials.
Evidence from a 2009 study (JAMA. 2009 Aug 26;302[8]:866-73) showed that among men with clinical stage T1 to T3 noninvasive, nonmetastatic prostate cancer, neoadjuvant hormonal therapy with both a luteinizing hormone-releasing hormone (LHRH) agonist and a nonsteroidal antiandrogen was associated with increased risk for all-cause mortality for those with a history of coronary artery disease–induced heart failure, but not for men with either no comorbidities or only a single comorbidity such as hypertension, hypercholesterolemia, or diabetes.
Clinical considerations
The decision to treat men with prostate cancer with ADT is therefore a balancing act, Dr. Nguyen said.
“As the risk of prostate cancer death goes up, the benefit of ADT goes up. However, as the comorbidity level goes up, the potential cardiovascular harm of ADT goes up,” he said.
For patients at the extreme ends of each continuum, such as a patient with high-risk prostate cancer and no cardiovascular comorbidities or a patient with low-risk cancer but multiple CV risk factors, the decision to give or withhold ADT is relatively simple, he said.
But for patients in between, such as a man with intermediate-risk cancer and one risk factor or a man with high risk disease with multiple comorbidities, the decision is far more complex.
“This where I think the dialogue with the cardiologist really needs to come into this decision,” he said.
Evidence to support the decision comes from retrospective studies suggesting that even men with high-risk prostate cancer have poorer overall survival with ADT if they have a history of heart failure or MI.
For patients with low-risk cancer and diabetes, ADT is associated with worse overall survival, but ADT does not cause additional harm to men with intermediate- to high-risk prostate cancer who have concomitant diabetes, Dr. Nguyen said.
“My view is that ADT has not been shown to increase cardiovascular death in randomized trials, so I think that for the vast majority of patients it probably does not increase cardiovascular deaths. But I think there could very well be a vulnerable 5% of patients who might have an excess risk of cardiovascular death, and I think we have to be careful, but we still have to balance it out against their risks for prostate cancer death,” he said.
Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
WASHINGTON – The cardiovascular effects of androgen deprivation therapy (ADT) for men with advanced prostate cancer are less severe than once feared, but there is evidence to suggest that men with preexisting heart failure or a history of myocardial infarction could be at excess risk for death from cardiovascular causes when they receive ADT, according to a leading prostate cancer expert.
“I think there are concerns about potential cardiovascular harm of ADT, and I think this has reduced ADT use, despite the fact that we know for most men it improves overall survival,” said Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“In fact, when we looked recently at men with high-risk prostate cancer, this is a group where overall survival is improved by 50% if they get ADT – so it cuts the risk of death in half – but it turns out that nearly a quarter of those patients are not receiving ADT. I think that the concern about cardiovascular harm and the confusion as to where that data stands is a lot of what’s driving that right now,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Randomized trial data
Dr. Nguyen noted that the evidence suggesting that ADT can increase the risk of death from cardiovascular causes came largely from three major studies:
- A 2006 study of 73,196 Medicare enrollees aged 66 or older, which found that ADT with a gonadotropin-releasing hormone (GnRH) agonist was possibly associated with increased risk of incident diabetes and cardiovascular disease (J Clin Oncol. 2006 Sep 20;24[27]:4448-56.).
- A 2007 analysis of data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CAPSURE) database on 3,262 men treated with radical prostatectomy and 1,630 men treated with radiation or cryotherapy for localized prostate cancer, which found that among those 65 and older the 5-year cumulative incidence of cardiovascular death was 5.5% for patients who received ADT, vs. 2% for those who did not (J Natl Cancer Inst. 2007 Oct 17;99[20]:1516-24).
- A 2007 study of 1,372 men in three randomized trials of radiation therapy with or without androgen suppression therapy up to 8 months in duration, which found that men 65 and older who received 6 months of androgen suppression had significantly shorter times to fatal MIs than did men who did not receive the therapy (J Clin Oncol. 2007;25[17]:2420-5).
These studies, combined with observational data, led to a 2010 consensus statement from the American Heart Association, American Cancer Society, and American Urological Association, with endorsement from the American Society for Radiation Oncology, which stated that “there may be a relation between ADT and cardiovascular events and death.”
Also in 2010, the Food and Drug Administration required new labeling on GnRH agonists warning of “increased risk of diabetes and certain cardiovascular diseases (heart attack, sudden cardiac death, stroke).”
Not unanimous
Two other large randomized studies (J Clin Oncol. 2008 Feb 1;26[4]:585-91 and J Clin Oncol. 2009 Jan 1;27[1]:92-9) and two retrospective studies (J Clin Oncol. 2009 Jul 20;27[21]:3452-8 and J Clin Oncol. 2011 Sep 10;29[26]3510-16) found no excess risk of cardiovascular disease from ADT, Dr. Nguyen said, prompting him and his colleagues to see whether they could get a better estimate of the actual risk.
They did so through a 2011 meta-analysis (JAMA. 2011;306[21]:2359-66) of data on 4,141 patients from eight randomized trials. They found that among patients with unfavorable-risk prostate cancer, ADT was not associated with an increased risk of cardiovascular death, but was associated with lower risks for both prostate-specific and all-cause mortality.
Subpopulations may still be at risk
Dr. Nguyen said that the principal finding of the meta-analysis, while reassuring, “doesn’t let ADT off the hook for metabolic events, diabetes which we know happens, and the possibility of nonfatal cardiac events.”
He noted that while ADT was not associated with cardiovascular disease in clinical trials, observational studies showed significantly increased risk for fatal or non-fatal MI.
One possible explanation for the difference is that observational studies included nonfatal MI, while randomized trials looked only at cardiovascular deaths. It’s also possible that ADT causes harm primarily in men with preexisting comorbidities, who are often excluded from or underrepresented in clinical trials.
Evidence from a 2009 study (JAMA. 2009 Aug 26;302[8]:866-73) showed that among men with clinical stage T1 to T3 noninvasive, nonmetastatic prostate cancer, neoadjuvant hormonal therapy with both a luteinizing hormone-releasing hormone (LHRH) agonist and a nonsteroidal antiandrogen was associated with increased risk for all-cause mortality for those with a history of coronary artery disease–induced heart failure, but not for men with either no comorbidities or only a single comorbidity such as hypertension, hypercholesterolemia, or diabetes.
Clinical considerations
The decision to treat men with prostate cancer with ADT is therefore a balancing act, Dr. Nguyen said.
“As the risk of prostate cancer death goes up, the benefit of ADT goes up. However, as the comorbidity level goes up, the potential cardiovascular harm of ADT goes up,” he said.
For patients at the extreme ends of each continuum, such as a patient with high-risk prostate cancer and no cardiovascular comorbidities or a patient with low-risk cancer but multiple CV risk factors, the decision to give or withhold ADT is relatively simple, he said.
But for patients in between, such as a man with intermediate-risk cancer and one risk factor or a man with high risk disease with multiple comorbidities, the decision is far more complex.
“This where I think the dialogue with the cardiologist really needs to come into this decision,” he said.
Evidence to support the decision comes from retrospective studies suggesting that even men with high-risk prostate cancer have poorer overall survival with ADT if they have a history of heart failure or MI.
For patients with low-risk cancer and diabetes, ADT is associated with worse overall survival, but ADT does not cause additional harm to men with intermediate- to high-risk prostate cancer who have concomitant diabetes, Dr. Nguyen said.
“My view is that ADT has not been shown to increase cardiovascular death in randomized trials, so I think that for the vast majority of patients it probably does not increase cardiovascular deaths. But I think there could very well be a vulnerable 5% of patients who might have an excess risk of cardiovascular death, and I think we have to be careful, but we still have to balance it out against their risks for prostate cancer death,” he said.
Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
REPORTING FROM ACC CARDIO-ONCOLOGY
Key clinical point: Risk of cardiovascular death should be weighed against proven ADT benefits.
Major finding: ADT-related cardiovascular events appear limited to men with comorbid cardiovascular disease.
Study details: Review of clinical data on the cardiovascular consequences of ADT.
Disclosures: Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
African American Smokers May Have Higher Risk of PAD
Even though peripheral artery disease (PAD) is almost 3 times more prevalent among African Americans compared with that of whites, it is understudied, say researchers from University of Mississippi. They say earlier studies did not include significant numbers of African Americans, limiting the ability to single out the effects of smoking in African Americans as distinct from, for example, diabetes mellitus, hypertension, and obesity.
This National Institute of Health (NIH)-funded study, however, provides some new information about what raises the risks of PAD in African Americans. The researchers studied participants in the Jackson Heart Study, the largest single-site cohort study investigating cardiovascular disease in African Americans.
They divided 5,258 participants into 3 groups: smokers, past smokers, never smokers. After taking other risk factors into account, they found people who smoked > 1 pack a day had a significantly higher risk than did those smoking < 19 cigarettes a day. A longer history of smoking also raised the risk of PAD.
Their findings point to the benefits of stopping smoking, the researchers say: Although never smokers had the lowest risk, past smokers also had lower odds.
The researchers caution, though, that despite strong associations between smoking and PAD, their findings do not establish a causal link.
Even though peripheral artery disease (PAD) is almost 3 times more prevalent among African Americans compared with that of whites, it is understudied, say researchers from University of Mississippi. They say earlier studies did not include significant numbers of African Americans, limiting the ability to single out the effects of smoking in African Americans as distinct from, for example, diabetes mellitus, hypertension, and obesity.
This National Institute of Health (NIH)-funded study, however, provides some new information about what raises the risks of PAD in African Americans. The researchers studied participants in the Jackson Heart Study, the largest single-site cohort study investigating cardiovascular disease in African Americans.
They divided 5,258 participants into 3 groups: smokers, past smokers, never smokers. After taking other risk factors into account, they found people who smoked > 1 pack a day had a significantly higher risk than did those smoking < 19 cigarettes a day. A longer history of smoking also raised the risk of PAD.
Their findings point to the benefits of stopping smoking, the researchers say: Although never smokers had the lowest risk, past smokers also had lower odds.
The researchers caution, though, that despite strong associations between smoking and PAD, their findings do not establish a causal link.
Even though peripheral artery disease (PAD) is almost 3 times more prevalent among African Americans compared with that of whites, it is understudied, say researchers from University of Mississippi. They say earlier studies did not include significant numbers of African Americans, limiting the ability to single out the effects of smoking in African Americans as distinct from, for example, diabetes mellitus, hypertension, and obesity.
This National Institute of Health (NIH)-funded study, however, provides some new information about what raises the risks of PAD in African Americans. The researchers studied participants in the Jackson Heart Study, the largest single-site cohort study investigating cardiovascular disease in African Americans.
They divided 5,258 participants into 3 groups: smokers, past smokers, never smokers. After taking other risk factors into account, they found people who smoked > 1 pack a day had a significantly higher risk than did those smoking < 19 cigarettes a day. A longer history of smoking also raised the risk of PAD.
Their findings point to the benefits of stopping smoking, the researchers say: Although never smokers had the lowest risk, past smokers also had lower odds.
The researchers caution, though, that despite strong associations between smoking and PAD, their findings do not establish a causal link.
SGLT2 inhibitors morph into HF drugs
SNOWMASS, COLO. – The oral sodium-glucose cotransporter-2 (SGLT2) inhibitors are the focus of a slew of ongoing phase 3 clinical trials in patients with symptomatic heart failure but no diabetes.
“We have a wide array of exciting opportunities to modify cardiovascular risk with agents that were initially developed for the therapy of diabetes. I think we’re increasingly moving to an age where these agents are actually cardiovascular drugs that happen to lower blood glucose, rather than the other way around, which is how they were initially conceived,” Akshay S. Desai, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
These are each multi-thousand-patient trials, variously due to be completed in 2019-2021. Of note, several of them are restricted to nondiabetic patients with heart failure with preserved ejection fraction (HFpEF), a common, serious, understudied, extremely high-cost disease sorely in need of effective pharmacotherapies, added Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
All of these placebo-controlled trials have as their composite primary endpoint cardiovascular death and heart failure hospitalization.
EMPEROR-Preserved has randomized 4,126 patients with HFpEF to empagliflozin (Jardiance) or placebo, while EMPEROR-Reduced involves 2,850 patients with heart failure with reduced ejection fraction (HFrEF). Both are due to be completed in 2020.
In addition, the DELIVER trial is focused on 4,700 HFpEF patients randomized to dapagliflozin (Farxiga) or placebo, while Dapa-HF employs the SGLT2 inhibitor in a study of 4,500 patients with HFrEF. Dapa-HF will be completed by late 2019. DELIVER wraps up in mid-2021.
Again, remarkably, none of the participants in these trials has diabetes. All have symptomatic heart failure with elevated N-terminal pro b-type natriuretic peptide levels. The impetus for this ongoing round of studies was the impressive reduction in the risk of hospitalization for heart failure seen in the pivotal trials that earned the SGLT2 inhibitors empagliflozin, canagliflozin (Invokana), and dapagliflozin marketing approval for treatment of type 2 diabetes from the Food and Drug Administration.
Dr. Desai called attention to a new systematic review and meta-analysis of cardiovascular outcomes in randomized, placebo-controlled trials of SGLT2 inhibitors in more than 34,000 patients with type 2 diabetes. The conclusion: These drugs impressively reduced the risk of heart failure hospitalization by 32% in patients with a baseline history of heart failure and similarly by 29% in those with no such history. Also notable was the 45% reduction in the risk of progression of renal disease regardless of whether patients had atherosclerotic cardiovascular disease (Lancet. 2019 Jan 5;393[10166]:31-9).
Only one of the ongoing round of phase 3 trials of SGLT2 inhibitors in heart failure is being conducted in patients with comorbid type 2 diabetes: the 4,000-subject SOLOIST-WHF trial. This study features the investigational dual inhibitor of SGLT1 and 2, sotagliflozin, with a primary outcome of cardiovascular death or heart failure hospitalization. Results are expected in early 2021.
What the latest guidelines say
The 2018 American Diabetes Association/European Association for the Study of Diabetes joint consensus statement on management of hyperglycemia in type 2 diabetes reflects an appreciation of the cardiovascular benefits of the SGLT2 inhibitors as well as the injectable glucagon-like peptide-1 receptor (GLP-1) agonists, which have shown significant reductions in major adverse cardiovascular events in pivotal trials including LEADER, HARMONY, and REWIND, albeit without the impressive reduction in heart failure hospitalizations documented with the SGLT2 inhibitors.
The consensus statement emphasizes that aggressive lifestyle modification advice is step No. 1, with the first-line medication being metformin titrated to a target of 1,000 mg twice daily. For patients with clinical heart failure or chronic kidney disease and atherosclerotic cardiovascular heart disease, the next drug recommended is an SGLT2 inhibitor with proven cardiovascular benefit. A GLP-1 agonist is recommended as the first injectable medication, ahead of insulin.
Who will take the lead in this new treatment strategy?
Dr. Desai presented data showing that overall utilization of SGLT2 inhibitors and GLP-1 agonists is going up, but not as steeply as it should.
“Cardiologists need to take a more active role,” he declared.
“It’s increasingly clear that, if we’re interested in modifying cardiovascular outcomes, we need to take ownership of this problem, much as we’ve done for lipids and hypertension, because modulating cardiovascular risk is our job,” Dr. Desai asserted. “These drugs may have modest influence on glycemic control, but the primary goal with these agents is to influence cardiovascular outcomes – and if we leave that job to our colleagues, then it often is just a can that gets kicked down the road.”
As a practical matter in prescribing SGLT2 inhibitors and GLP-1 agonists, he emphasized the value of partnering with a primary care physician, endocrinologist, and/or pharmacist by creating pathways for accelerated referral for pharmacologic teaching and, in the case of GLP-1 agonists, injection-related instruction. Pharmacists are often particularly helpful in obtaining prior authorization and financial approval for these medications, and they are familiar with drug discounts and vouchers.
“A great way to jump start collaboration is to provide the patient with a prescription before leaving your office. I think often what we do is just suggest it to the patient, and then a year later they come back and nothing has changed,” the cardiologist said.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
SNOWMASS, COLO. – The oral sodium-glucose cotransporter-2 (SGLT2) inhibitors are the focus of a slew of ongoing phase 3 clinical trials in patients with symptomatic heart failure but no diabetes.
“We have a wide array of exciting opportunities to modify cardiovascular risk with agents that were initially developed for the therapy of diabetes. I think we’re increasingly moving to an age where these agents are actually cardiovascular drugs that happen to lower blood glucose, rather than the other way around, which is how they were initially conceived,” Akshay S. Desai, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
These are each multi-thousand-patient trials, variously due to be completed in 2019-2021. Of note, several of them are restricted to nondiabetic patients with heart failure with preserved ejection fraction (HFpEF), a common, serious, understudied, extremely high-cost disease sorely in need of effective pharmacotherapies, added Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
All of these placebo-controlled trials have as their composite primary endpoint cardiovascular death and heart failure hospitalization.
EMPEROR-Preserved has randomized 4,126 patients with HFpEF to empagliflozin (Jardiance) or placebo, while EMPEROR-Reduced involves 2,850 patients with heart failure with reduced ejection fraction (HFrEF). Both are due to be completed in 2020.
In addition, the DELIVER trial is focused on 4,700 HFpEF patients randomized to dapagliflozin (Farxiga) or placebo, while Dapa-HF employs the SGLT2 inhibitor in a study of 4,500 patients with HFrEF. Dapa-HF will be completed by late 2019. DELIVER wraps up in mid-2021.
Again, remarkably, none of the participants in these trials has diabetes. All have symptomatic heart failure with elevated N-terminal pro b-type natriuretic peptide levels. The impetus for this ongoing round of studies was the impressive reduction in the risk of hospitalization for heart failure seen in the pivotal trials that earned the SGLT2 inhibitors empagliflozin, canagliflozin (Invokana), and dapagliflozin marketing approval for treatment of type 2 diabetes from the Food and Drug Administration.
Dr. Desai called attention to a new systematic review and meta-analysis of cardiovascular outcomes in randomized, placebo-controlled trials of SGLT2 inhibitors in more than 34,000 patients with type 2 diabetes. The conclusion: These drugs impressively reduced the risk of heart failure hospitalization by 32% in patients with a baseline history of heart failure and similarly by 29% in those with no such history. Also notable was the 45% reduction in the risk of progression of renal disease regardless of whether patients had atherosclerotic cardiovascular disease (Lancet. 2019 Jan 5;393[10166]:31-9).
Only one of the ongoing round of phase 3 trials of SGLT2 inhibitors in heart failure is being conducted in patients with comorbid type 2 diabetes: the 4,000-subject SOLOIST-WHF trial. This study features the investigational dual inhibitor of SGLT1 and 2, sotagliflozin, with a primary outcome of cardiovascular death or heart failure hospitalization. Results are expected in early 2021.
What the latest guidelines say
The 2018 American Diabetes Association/European Association for the Study of Diabetes joint consensus statement on management of hyperglycemia in type 2 diabetes reflects an appreciation of the cardiovascular benefits of the SGLT2 inhibitors as well as the injectable glucagon-like peptide-1 receptor (GLP-1) agonists, which have shown significant reductions in major adverse cardiovascular events in pivotal trials including LEADER, HARMONY, and REWIND, albeit without the impressive reduction in heart failure hospitalizations documented with the SGLT2 inhibitors.
The consensus statement emphasizes that aggressive lifestyle modification advice is step No. 1, with the first-line medication being metformin titrated to a target of 1,000 mg twice daily. For patients with clinical heart failure or chronic kidney disease and atherosclerotic cardiovascular heart disease, the next drug recommended is an SGLT2 inhibitor with proven cardiovascular benefit. A GLP-1 agonist is recommended as the first injectable medication, ahead of insulin.
Who will take the lead in this new treatment strategy?
Dr. Desai presented data showing that overall utilization of SGLT2 inhibitors and GLP-1 agonists is going up, but not as steeply as it should.
“Cardiologists need to take a more active role,” he declared.
“It’s increasingly clear that, if we’re interested in modifying cardiovascular outcomes, we need to take ownership of this problem, much as we’ve done for lipids and hypertension, because modulating cardiovascular risk is our job,” Dr. Desai asserted. “These drugs may have modest influence on glycemic control, but the primary goal with these agents is to influence cardiovascular outcomes – and if we leave that job to our colleagues, then it often is just a can that gets kicked down the road.”
As a practical matter in prescribing SGLT2 inhibitors and GLP-1 agonists, he emphasized the value of partnering with a primary care physician, endocrinologist, and/or pharmacist by creating pathways for accelerated referral for pharmacologic teaching and, in the case of GLP-1 agonists, injection-related instruction. Pharmacists are often particularly helpful in obtaining prior authorization and financial approval for these medications, and they are familiar with drug discounts and vouchers.
“A great way to jump start collaboration is to provide the patient with a prescription before leaving your office. I think often what we do is just suggest it to the patient, and then a year later they come back and nothing has changed,” the cardiologist said.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
SNOWMASS, COLO. – The oral sodium-glucose cotransporter-2 (SGLT2) inhibitors are the focus of a slew of ongoing phase 3 clinical trials in patients with symptomatic heart failure but no diabetes.
“We have a wide array of exciting opportunities to modify cardiovascular risk with agents that were initially developed for the therapy of diabetes. I think we’re increasingly moving to an age where these agents are actually cardiovascular drugs that happen to lower blood glucose, rather than the other way around, which is how they were initially conceived,” Akshay S. Desai, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
These are each multi-thousand-patient trials, variously due to be completed in 2019-2021. Of note, several of them are restricted to nondiabetic patients with heart failure with preserved ejection fraction (HFpEF), a common, serious, understudied, extremely high-cost disease sorely in need of effective pharmacotherapies, added Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
All of these placebo-controlled trials have as their composite primary endpoint cardiovascular death and heart failure hospitalization.
EMPEROR-Preserved has randomized 4,126 patients with HFpEF to empagliflozin (Jardiance) or placebo, while EMPEROR-Reduced involves 2,850 patients with heart failure with reduced ejection fraction (HFrEF). Both are due to be completed in 2020.
In addition, the DELIVER trial is focused on 4,700 HFpEF patients randomized to dapagliflozin (Farxiga) or placebo, while Dapa-HF employs the SGLT2 inhibitor in a study of 4,500 patients with HFrEF. Dapa-HF will be completed by late 2019. DELIVER wraps up in mid-2021.
Again, remarkably, none of the participants in these trials has diabetes. All have symptomatic heart failure with elevated N-terminal pro b-type natriuretic peptide levels. The impetus for this ongoing round of studies was the impressive reduction in the risk of hospitalization for heart failure seen in the pivotal trials that earned the SGLT2 inhibitors empagliflozin, canagliflozin (Invokana), and dapagliflozin marketing approval for treatment of type 2 diabetes from the Food and Drug Administration.
Dr. Desai called attention to a new systematic review and meta-analysis of cardiovascular outcomes in randomized, placebo-controlled trials of SGLT2 inhibitors in more than 34,000 patients with type 2 diabetes. The conclusion: These drugs impressively reduced the risk of heart failure hospitalization by 32% in patients with a baseline history of heart failure and similarly by 29% in those with no such history. Also notable was the 45% reduction in the risk of progression of renal disease regardless of whether patients had atherosclerotic cardiovascular disease (Lancet. 2019 Jan 5;393[10166]:31-9).
Only one of the ongoing round of phase 3 trials of SGLT2 inhibitors in heart failure is being conducted in patients with comorbid type 2 diabetes: the 4,000-subject SOLOIST-WHF trial. This study features the investigational dual inhibitor of SGLT1 and 2, sotagliflozin, with a primary outcome of cardiovascular death or heart failure hospitalization. Results are expected in early 2021.
What the latest guidelines say
The 2018 American Diabetes Association/European Association for the Study of Diabetes joint consensus statement on management of hyperglycemia in type 2 diabetes reflects an appreciation of the cardiovascular benefits of the SGLT2 inhibitors as well as the injectable glucagon-like peptide-1 receptor (GLP-1) agonists, which have shown significant reductions in major adverse cardiovascular events in pivotal trials including LEADER, HARMONY, and REWIND, albeit without the impressive reduction in heart failure hospitalizations documented with the SGLT2 inhibitors.
The consensus statement emphasizes that aggressive lifestyle modification advice is step No. 1, with the first-line medication being metformin titrated to a target of 1,000 mg twice daily. For patients with clinical heart failure or chronic kidney disease and atherosclerotic cardiovascular heart disease, the next drug recommended is an SGLT2 inhibitor with proven cardiovascular benefit. A GLP-1 agonist is recommended as the first injectable medication, ahead of insulin.
Who will take the lead in this new treatment strategy?
Dr. Desai presented data showing that overall utilization of SGLT2 inhibitors and GLP-1 agonists is going up, but not as steeply as it should.
“Cardiologists need to take a more active role,” he declared.
“It’s increasingly clear that, if we’re interested in modifying cardiovascular outcomes, we need to take ownership of this problem, much as we’ve done for lipids and hypertension, because modulating cardiovascular risk is our job,” Dr. Desai asserted. “These drugs may have modest influence on glycemic control, but the primary goal with these agents is to influence cardiovascular outcomes – and if we leave that job to our colleagues, then it often is just a can that gets kicked down the road.”
As a practical matter in prescribing SGLT2 inhibitors and GLP-1 agonists, he emphasized the value of partnering with a primary care physician, endocrinologist, and/or pharmacist by creating pathways for accelerated referral for pharmacologic teaching and, in the case of GLP-1 agonists, injection-related instruction. Pharmacists are often particularly helpful in obtaining prior authorization and financial approval for these medications, and they are familiar with drug discounts and vouchers.
“A great way to jump start collaboration is to provide the patient with a prescription before leaving your office. I think often what we do is just suggest it to the patient, and then a year later they come back and nothing has changed,” the cardiologist said.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
EXPERT ANALYSIS FROM ACC SNOWMASS 2019
‘Simple’ way to cut PAD risk, misguided ED visits for atrial fib, and more
This week in MDedge Cardiocast: Elevated CAC in highly active men doesn’t raise risk of death, Life’s Simple 7 scores can be used to modify PAD risk, medical guidance often leads atrial fibrillation patients to needlessly seek emergency department care, and thinking of pregnancy as a stress test can help predict women’s future cardiovascular risk.
Amazon AlexaApple Podcasts
Google Podcasts
TuneIn
This week in MDedge Cardiocast: Elevated CAC in highly active men doesn’t raise risk of death, Life’s Simple 7 scores can be used to modify PAD risk, medical guidance often leads atrial fibrillation patients to needlessly seek emergency department care, and thinking of pregnancy as a stress test can help predict women’s future cardiovascular risk.
Amazon AlexaApple Podcasts
Google Podcasts
TuneIn
This week in MDedge Cardiocast: Elevated CAC in highly active men doesn’t raise risk of death, Life’s Simple 7 scores can be used to modify PAD risk, medical guidance often leads atrial fibrillation patients to needlessly seek emergency department care, and thinking of pregnancy as a stress test can help predict women’s future cardiovascular risk.
Amazon AlexaApple Podcasts
Google Podcasts
TuneIn
Cilostazol plus aspirin or clopidogrel reduces the risk of recurrent stroke
HONOLULU – The combination also entails a similar risk of major bleeding, compared with aspirin and clopidogrel alone, according to results from the Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com).
Dual-antiplatelet therapy with aspirin and clopidogrel reduced the rate of recurrent stroke in previous studies. The benefit of this drug combination is relatively short-lived, however, and long-term concomitant use of aspirin and clopidogrel entails a risk of major bleeding. Other data have indicated that cilostazol, which is approved by the Food and Drug Administration to alleviate intermittent claudication in patients with peripheral vascular disease, prevents stroke recurrence without increasing the incidence of serious bleeding, compared with aspirin, said Kazunori Toyoda, MD, PhD, who presented the results of the CSPS.com trial at the International Stroke Conference sponsored by the American Heart Association.
Dr. Toyoda of the National Cerebral and Cardiovascular Center in Osaka, Japan, and his colleagues randomized 1,879 high-risk patients at 8-180 days after the onset of noncardioembolic ischemic stroke identified on MRI to receive 81 or 100 mg aspirin or 50 or 75 mg clopidogrel alone, or a combination of cilostazol 100 mg twice daily with aspirin or clopidogrel. They conducted their open-label, parallel-group trial at 292 sites in Japan from December 2013 through March 2017.
To be considered at high risk, participants had to meet one or more of the following criteria: 50% or greater stenosis of a major intracranial artery, 50% or greater stenosis of an extracranial artery, and two or more vascular risk factors. The trial’s primary efficacy outcome was the first recurrence of ischemic stroke. Safety outcomes included severe or life-threatening bleeding.
The investigators ended the trial early because of a delay in recruiting patients. They enrolled 1,884 and randomized 1,879 of an anticipated 4,000 patients. At randomization, 41% in the dual-therapy group received aspirin and 59% clopidogrel, and in the monotherapy group, 40% received aspirin and 60% clopidogrel. Baseline characteristics were similar between the treatment groups. The population’s mean age was 70. Approximately 30% of patients were women.
During a median follow-up period of 17 months, ischemic stroke recurred in 29 of 932 patients receiving dual therapy including cilostazol for an annual rate of 2.2% and in 64 of 947 patients receiving monotherapy for an annual rate of 4.5% (hazard ratio, 0.49; 95% confidence interval, 0.31-0.76; P = .001). Severe or life-threatening bleeding occurred in 8 patients (0.6% per year) receiving dual therapy and 13 patients (0.9% per year) receiving monotherapy (HR, 0.66; 95% CI, 0.27-1.60; P = .354).
The study was funded by Otsuka Pharmaceutical, which manufactures cilostazol. Dr. Toyoda reported receiving support from Bayer Yakuhin, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
SOURCE: Toyoda K et al. ISC 2019, Abstract LB3.
HONOLULU – The combination also entails a similar risk of major bleeding, compared with aspirin and clopidogrel alone, according to results from the Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com).
Dual-antiplatelet therapy with aspirin and clopidogrel reduced the rate of recurrent stroke in previous studies. The benefit of this drug combination is relatively short-lived, however, and long-term concomitant use of aspirin and clopidogrel entails a risk of major bleeding. Other data have indicated that cilostazol, which is approved by the Food and Drug Administration to alleviate intermittent claudication in patients with peripheral vascular disease, prevents stroke recurrence without increasing the incidence of serious bleeding, compared with aspirin, said Kazunori Toyoda, MD, PhD, who presented the results of the CSPS.com trial at the International Stroke Conference sponsored by the American Heart Association.
Dr. Toyoda of the National Cerebral and Cardiovascular Center in Osaka, Japan, and his colleagues randomized 1,879 high-risk patients at 8-180 days after the onset of noncardioembolic ischemic stroke identified on MRI to receive 81 or 100 mg aspirin or 50 or 75 mg clopidogrel alone, or a combination of cilostazol 100 mg twice daily with aspirin or clopidogrel. They conducted their open-label, parallel-group trial at 292 sites in Japan from December 2013 through March 2017.
To be considered at high risk, participants had to meet one or more of the following criteria: 50% or greater stenosis of a major intracranial artery, 50% or greater stenosis of an extracranial artery, and two or more vascular risk factors. The trial’s primary efficacy outcome was the first recurrence of ischemic stroke. Safety outcomes included severe or life-threatening bleeding.
The investigators ended the trial early because of a delay in recruiting patients. They enrolled 1,884 and randomized 1,879 of an anticipated 4,000 patients. At randomization, 41% in the dual-therapy group received aspirin and 59% clopidogrel, and in the monotherapy group, 40% received aspirin and 60% clopidogrel. Baseline characteristics were similar between the treatment groups. The population’s mean age was 70. Approximately 30% of patients were women.
During a median follow-up period of 17 months, ischemic stroke recurred in 29 of 932 patients receiving dual therapy including cilostazol for an annual rate of 2.2% and in 64 of 947 patients receiving monotherapy for an annual rate of 4.5% (hazard ratio, 0.49; 95% confidence interval, 0.31-0.76; P = .001). Severe or life-threatening bleeding occurred in 8 patients (0.6% per year) receiving dual therapy and 13 patients (0.9% per year) receiving monotherapy (HR, 0.66; 95% CI, 0.27-1.60; P = .354).
The study was funded by Otsuka Pharmaceutical, which manufactures cilostazol. Dr. Toyoda reported receiving support from Bayer Yakuhin, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
SOURCE: Toyoda K et al. ISC 2019, Abstract LB3.
HONOLULU – The combination also entails a similar risk of major bleeding, compared with aspirin and clopidogrel alone, according to results from the Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com).
Dual-antiplatelet therapy with aspirin and clopidogrel reduced the rate of recurrent stroke in previous studies. The benefit of this drug combination is relatively short-lived, however, and long-term concomitant use of aspirin and clopidogrel entails a risk of major bleeding. Other data have indicated that cilostazol, which is approved by the Food and Drug Administration to alleviate intermittent claudication in patients with peripheral vascular disease, prevents stroke recurrence without increasing the incidence of serious bleeding, compared with aspirin, said Kazunori Toyoda, MD, PhD, who presented the results of the CSPS.com trial at the International Stroke Conference sponsored by the American Heart Association.
Dr. Toyoda of the National Cerebral and Cardiovascular Center in Osaka, Japan, and his colleagues randomized 1,879 high-risk patients at 8-180 days after the onset of noncardioembolic ischemic stroke identified on MRI to receive 81 or 100 mg aspirin or 50 or 75 mg clopidogrel alone, or a combination of cilostazol 100 mg twice daily with aspirin or clopidogrel. They conducted their open-label, parallel-group trial at 292 sites in Japan from December 2013 through March 2017.
To be considered at high risk, participants had to meet one or more of the following criteria: 50% or greater stenosis of a major intracranial artery, 50% or greater stenosis of an extracranial artery, and two or more vascular risk factors. The trial’s primary efficacy outcome was the first recurrence of ischemic stroke. Safety outcomes included severe or life-threatening bleeding.
The investigators ended the trial early because of a delay in recruiting patients. They enrolled 1,884 and randomized 1,879 of an anticipated 4,000 patients. At randomization, 41% in the dual-therapy group received aspirin and 59% clopidogrel, and in the monotherapy group, 40% received aspirin and 60% clopidogrel. Baseline characteristics were similar between the treatment groups. The population’s mean age was 70. Approximately 30% of patients were women.
During a median follow-up period of 17 months, ischemic stroke recurred in 29 of 932 patients receiving dual therapy including cilostazol for an annual rate of 2.2% and in 64 of 947 patients receiving monotherapy for an annual rate of 4.5% (hazard ratio, 0.49; 95% confidence interval, 0.31-0.76; P = .001). Severe or life-threatening bleeding occurred in 8 patients (0.6% per year) receiving dual therapy and 13 patients (0.9% per year) receiving monotherapy (HR, 0.66; 95% CI, 0.27-1.60; P = .354).
The study was funded by Otsuka Pharmaceutical, which manufactures cilostazol. Dr. Toyoda reported receiving support from Bayer Yakuhin, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
SOURCE: Toyoda K et al. ISC 2019, Abstract LB3.
REPORTING FROM ISC
Key clinical point: Treating patients at high risk of recurrent stroke with cilostazol and aspirin or clopidogrel reduced the risk of recurrent stroke more than either aspirin or clopidogrel alone and was just as safe.
Major finding: Dual therapy with cilostazol and aspirin or clopidogrel reduced the risk of recurrent stroke by approximately half, compared with aspirin or clopidogrel alone.
Study details: A multicenter, randomized, open-label, parallel-group trial including 1,879 patients at high risk of recurrent stroke.
Disclosures: Otsuka Pharmaceutical funded the study. The presenter reported receiving support from Bayer Yakuhin, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
Source: Toyoda K et al. ISC 2019, Abstract LB3.
Intensive insulin added no benefit for hyperglycemia after ischemic stroke
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
REPORTING FROM ISC 2019
Key clinical point: Aggressive insulin management of hyperglycemia following an ischemic stroke gave no clinical benefit, compared with a standard approach.
Major finding: After 90 days, favorable outcomes occurred in 21% of patients on aggressive insulin treatment and 22% on standard treatment.
Study details: SHINE, a multicenter, randomized trial with 1,151 acute ischemic stroke patients.
Disclosures: SHINE received no commercial funding. Dr. Johnston had no disclosures.
Source: Johnston KC et al. ISC 2019, Abstract LB1.