User login
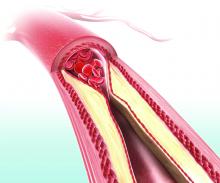
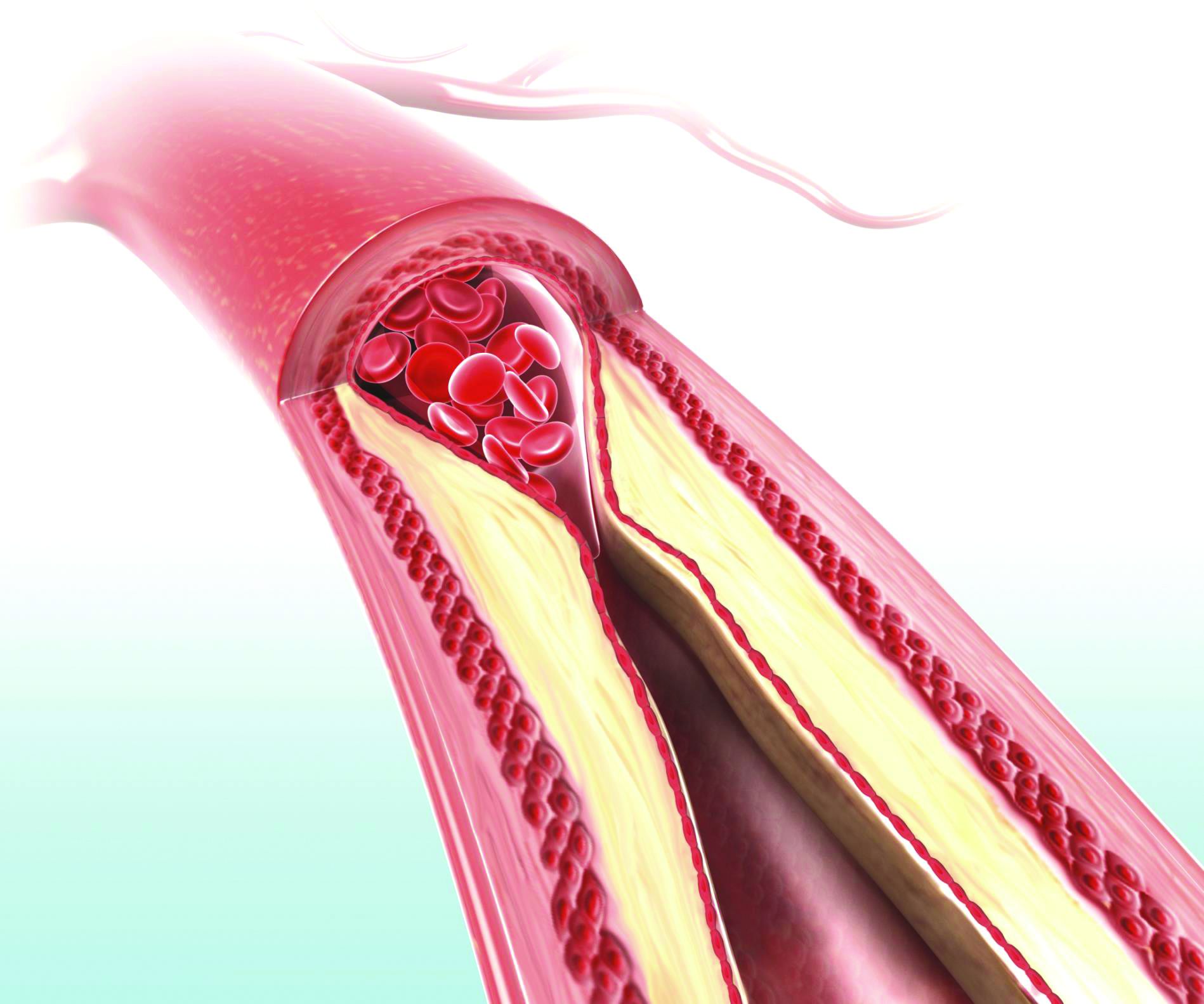
Therapy ups breast cancer survivors’ cardiac risks
WASHINGTON – Oncologists and cardiologists need to work hand-in-hand when managing the care of women with breast cancer whose treatment plan includes cardiotoxic therapies and breast irradiation, reported specialists.
Depending on the cancer subtype, women with breast cancer may receive chemotherapy with a cardiotoxic anthracycline such as doxorubicin or epirubicin, or a HER2-targeted agent such as trastuzumab (Herceptin), pertuzumab (Perjeta), or ado-trastuzumab emtansine (Kadcyla).
“The cardiotoxicity related to breast cancer has been a well publicized issue, and chances are your patients know about it and are concerned as well,” Jennifer E. Liu, MD, director of cardiovascular laboratories at Memorial Sloan Kettering Cancer Center in New York, said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Anthracyclines, trastuzumab, and HF
In large adjuvant therapy trials of anthracyclines and trastuzumab in women with breast cancer, doxorubicin alone was associated with an asymptomatic decline in left ventricular ejection fraction (LVEF) of 4% to 11%, and a less than 1% incidence of heart failure (HF), Dr. Liu noted.
When patients received an anthracycline followed by trastuzumab, the incidence of asymptomatic LVEF decline ranged from 4% to 19%, the incidence of clinical HF was 2% to 4%, and the rate of trastuzumab interruption for cardiac adverse events ranged from 5% to 18%.
In comparison, in trials with trastuzumab in combination therapy that did not contain an anthracycline, the risk of cardiovascular complications was lower, with asymptomatic decline in LVEF ranging from 3.2% to 9.4%, and class III/IV HF occurring in just 0.5% of patients. In trials combining trastuzumab and pertuzumab, there were no increases in cardiac toxicity over trastuzumab alone.
Although with longer follow-up, the approximately 4% rate of HF in patients treated with anthracycline-based chemotherapy, paclitaxel, and trastuzumab in the NSABP B-31 trial has not changed significantly; retrospective claims-based studies reflecting daily practice have shown significantly higher rates of HF and or cardiomyopathy, Dr. Liu said.
She cited a 2012 study showing that among 45,537 women with a mean age of 76 years who were treated for breast cancer, the 3-year incidence rates of HF and/or cardiomyopathy were 32% for patients treated with trastuzumab alone, and 41.9% for those treated with an anthracycline followed by trastuzumab. Other, smaller studies also showed lower but significantly elevated risks for the drugs.
The discrepancy between clinical trial and “real world” results may be chalked up to the fact that claims-based data rely on diagnostic codes that may not accurately reflect the actual cardiac diagnosis, and by the fact that clinical trials have strict entry criteria that exclude patients with cardiovascular disease, she said.
Radiation risks
Radiation therapy is associated with a more than 7% increase in major coronary events per Gy of mean heart dose, Dr. Liu noted, citing a 2013 study (N Engl J Med. 2013;368:987-98).
Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston, said that risk factors for radiation-induced heart disease include anterior or left chest irradiation, cumulative doses above 30 Gy, patient age younger than 50 years, doses of more than 2 Gy per fraction, presence and extent of tumor in or near the heart, lack of radiation shielding, concomitant chemotherapy (especially with anthracyclines), and preexisting cardiovascular disease or risk factors.
For patients with breast cancer, the risk of developing radiation-induced heart disease has diminished considerably with the adoption of heart-sparing techniques over the last several decades, including 3-D conformal techniques, intensity-modulated radiation therapy, proton beam therapy, novel patient positioning techniques that allow radiation only to the cancer-involved breast, and deep inspiration breath holds in which the radiation beam is gated to turn on only when the patient is holding a deep breath, Dr. Nguyen noted.
Treatment options for LVEF decline
The package insert for trastuzumab recommends withholding the drug for a minimum of 4 weeks if the patient has a 16% or greater decline in LVEF from baseline, or a 10% or greater decline from baseline to below the lower limit of normal. The insert recommends LVEF monitoring every 3 or 4 weeks, and says that trastuzumab can be resumed if LVEF improves to above the lower limit of normal with an absolute decrease from baseline of not more than 15%. The insert also states, however, that “the safety of continuation or resumption of trastuzumab in patients with trastuzumab induced LV dysfunction has never been studied, “ Dr. Liu noted.
She cited an American Society of Clinical Oncology guideline on the prevention and monitoring of cardiac dysfunction in survivors of adult cancers, which states in part that the decision to continue or discontinue cancer therapy in patients with evidence of cardiac dysfunction “made by the oncologist, should be informed by close collaboration with a cardiologist, fully evaluating the clinical circumstances and considering the risks and benefits of continuation of therapy responsible for the cardiac dysfunction.”
“I want to emphasize the importance of accepting and managing cardiovascular risk in patients priors to and during potentially cardiotoxic therapy. To optimize cardiologic and oncologic outcomes, we need to avoid or minimize treatment interruptions of life-saving therapy, and mitigate cardiac events with aggressive cardiovascular risk-factor modification,” Dr. Liu said.
She called for development of better risk stratification tools to tailor cardiac surveillance during therapy, based on both patient-specific and treatment-specific risk factors.
Dr. Liu reported nothing to disclose. Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics. Cota, Dendreon, Ferring Pharmaceuticals. GenomeDx, Janssen, and Nanobiotix.
WASHINGTON – Oncologists and cardiologists need to work hand-in-hand when managing the care of women with breast cancer whose treatment plan includes cardiotoxic therapies and breast irradiation, reported specialists.
Depending on the cancer subtype, women with breast cancer may receive chemotherapy with a cardiotoxic anthracycline such as doxorubicin or epirubicin, or a HER2-targeted agent such as trastuzumab (Herceptin), pertuzumab (Perjeta), or ado-trastuzumab emtansine (Kadcyla).
“The cardiotoxicity related to breast cancer has been a well publicized issue, and chances are your patients know about it and are concerned as well,” Jennifer E. Liu, MD, director of cardiovascular laboratories at Memorial Sloan Kettering Cancer Center in New York, said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Anthracyclines, trastuzumab, and HF
In large adjuvant therapy trials of anthracyclines and trastuzumab in women with breast cancer, doxorubicin alone was associated with an asymptomatic decline in left ventricular ejection fraction (LVEF) of 4% to 11%, and a less than 1% incidence of heart failure (HF), Dr. Liu noted.
When patients received an anthracycline followed by trastuzumab, the incidence of asymptomatic LVEF decline ranged from 4% to 19%, the incidence of clinical HF was 2% to 4%, and the rate of trastuzumab interruption for cardiac adverse events ranged from 5% to 18%.
In comparison, in trials with trastuzumab in combination therapy that did not contain an anthracycline, the risk of cardiovascular complications was lower, with asymptomatic decline in LVEF ranging from 3.2% to 9.4%, and class III/IV HF occurring in just 0.5% of patients. In trials combining trastuzumab and pertuzumab, there were no increases in cardiac toxicity over trastuzumab alone.
Although with longer follow-up, the approximately 4% rate of HF in patients treated with anthracycline-based chemotherapy, paclitaxel, and trastuzumab in the NSABP B-31 trial has not changed significantly; retrospective claims-based studies reflecting daily practice have shown significantly higher rates of HF and or cardiomyopathy, Dr. Liu said.
She cited a 2012 study showing that among 45,537 women with a mean age of 76 years who were treated for breast cancer, the 3-year incidence rates of HF and/or cardiomyopathy were 32% for patients treated with trastuzumab alone, and 41.9% for those treated with an anthracycline followed by trastuzumab. Other, smaller studies also showed lower but significantly elevated risks for the drugs.
The discrepancy between clinical trial and “real world” results may be chalked up to the fact that claims-based data rely on diagnostic codes that may not accurately reflect the actual cardiac diagnosis, and by the fact that clinical trials have strict entry criteria that exclude patients with cardiovascular disease, she said.
Radiation risks
Radiation therapy is associated with a more than 7% increase in major coronary events per Gy of mean heart dose, Dr. Liu noted, citing a 2013 study (N Engl J Med. 2013;368:987-98).
Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston, said that risk factors for radiation-induced heart disease include anterior or left chest irradiation, cumulative doses above 30 Gy, patient age younger than 50 years, doses of more than 2 Gy per fraction, presence and extent of tumor in or near the heart, lack of radiation shielding, concomitant chemotherapy (especially with anthracyclines), and preexisting cardiovascular disease or risk factors.
For patients with breast cancer, the risk of developing radiation-induced heart disease has diminished considerably with the adoption of heart-sparing techniques over the last several decades, including 3-D conformal techniques, intensity-modulated radiation therapy, proton beam therapy, novel patient positioning techniques that allow radiation only to the cancer-involved breast, and deep inspiration breath holds in which the radiation beam is gated to turn on only when the patient is holding a deep breath, Dr. Nguyen noted.
Treatment options for LVEF decline
The package insert for trastuzumab recommends withholding the drug for a minimum of 4 weeks if the patient has a 16% or greater decline in LVEF from baseline, or a 10% or greater decline from baseline to below the lower limit of normal. The insert recommends LVEF monitoring every 3 or 4 weeks, and says that trastuzumab can be resumed if LVEF improves to above the lower limit of normal with an absolute decrease from baseline of not more than 15%. The insert also states, however, that “the safety of continuation or resumption of trastuzumab in patients with trastuzumab induced LV dysfunction has never been studied, “ Dr. Liu noted.
She cited an American Society of Clinical Oncology guideline on the prevention and monitoring of cardiac dysfunction in survivors of adult cancers, which states in part that the decision to continue or discontinue cancer therapy in patients with evidence of cardiac dysfunction “made by the oncologist, should be informed by close collaboration with a cardiologist, fully evaluating the clinical circumstances and considering the risks and benefits of continuation of therapy responsible for the cardiac dysfunction.”
“I want to emphasize the importance of accepting and managing cardiovascular risk in patients priors to and during potentially cardiotoxic therapy. To optimize cardiologic and oncologic outcomes, we need to avoid or minimize treatment interruptions of life-saving therapy, and mitigate cardiac events with aggressive cardiovascular risk-factor modification,” Dr. Liu said.
She called for development of better risk stratification tools to tailor cardiac surveillance during therapy, based on both patient-specific and treatment-specific risk factors.
Dr. Liu reported nothing to disclose. Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics. Cota, Dendreon, Ferring Pharmaceuticals. GenomeDx, Janssen, and Nanobiotix.
WASHINGTON – Oncologists and cardiologists need to work hand-in-hand when managing the care of women with breast cancer whose treatment plan includes cardiotoxic therapies and breast irradiation, reported specialists.
Depending on the cancer subtype, women with breast cancer may receive chemotherapy with a cardiotoxic anthracycline such as doxorubicin or epirubicin, or a HER2-targeted agent such as trastuzumab (Herceptin), pertuzumab (Perjeta), or ado-trastuzumab emtansine (Kadcyla).
“The cardiotoxicity related to breast cancer has been a well publicized issue, and chances are your patients know about it and are concerned as well,” Jennifer E. Liu, MD, director of cardiovascular laboratories at Memorial Sloan Kettering Cancer Center in New York, said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Anthracyclines, trastuzumab, and HF
In large adjuvant therapy trials of anthracyclines and trastuzumab in women with breast cancer, doxorubicin alone was associated with an asymptomatic decline in left ventricular ejection fraction (LVEF) of 4% to 11%, and a less than 1% incidence of heart failure (HF), Dr. Liu noted.
When patients received an anthracycline followed by trastuzumab, the incidence of asymptomatic LVEF decline ranged from 4% to 19%, the incidence of clinical HF was 2% to 4%, and the rate of trastuzumab interruption for cardiac adverse events ranged from 5% to 18%.
In comparison, in trials with trastuzumab in combination therapy that did not contain an anthracycline, the risk of cardiovascular complications was lower, with asymptomatic decline in LVEF ranging from 3.2% to 9.4%, and class III/IV HF occurring in just 0.5% of patients. In trials combining trastuzumab and pertuzumab, there were no increases in cardiac toxicity over trastuzumab alone.
Although with longer follow-up, the approximately 4% rate of HF in patients treated with anthracycline-based chemotherapy, paclitaxel, and trastuzumab in the NSABP B-31 trial has not changed significantly; retrospective claims-based studies reflecting daily practice have shown significantly higher rates of HF and or cardiomyopathy, Dr. Liu said.
She cited a 2012 study showing that among 45,537 women with a mean age of 76 years who were treated for breast cancer, the 3-year incidence rates of HF and/or cardiomyopathy were 32% for patients treated with trastuzumab alone, and 41.9% for those treated with an anthracycline followed by trastuzumab. Other, smaller studies also showed lower but significantly elevated risks for the drugs.
The discrepancy between clinical trial and “real world” results may be chalked up to the fact that claims-based data rely on diagnostic codes that may not accurately reflect the actual cardiac diagnosis, and by the fact that clinical trials have strict entry criteria that exclude patients with cardiovascular disease, she said.
Radiation risks
Radiation therapy is associated with a more than 7% increase in major coronary events per Gy of mean heart dose, Dr. Liu noted, citing a 2013 study (N Engl J Med. 2013;368:987-98).
Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston, said that risk factors for radiation-induced heart disease include anterior or left chest irradiation, cumulative doses above 30 Gy, patient age younger than 50 years, doses of more than 2 Gy per fraction, presence and extent of tumor in or near the heart, lack of radiation shielding, concomitant chemotherapy (especially with anthracyclines), and preexisting cardiovascular disease or risk factors.
For patients with breast cancer, the risk of developing radiation-induced heart disease has diminished considerably with the adoption of heart-sparing techniques over the last several decades, including 3-D conformal techniques, intensity-modulated radiation therapy, proton beam therapy, novel patient positioning techniques that allow radiation only to the cancer-involved breast, and deep inspiration breath holds in which the radiation beam is gated to turn on only when the patient is holding a deep breath, Dr. Nguyen noted.
Treatment options for LVEF decline
The package insert for trastuzumab recommends withholding the drug for a minimum of 4 weeks if the patient has a 16% or greater decline in LVEF from baseline, or a 10% or greater decline from baseline to below the lower limit of normal. The insert recommends LVEF monitoring every 3 or 4 weeks, and says that trastuzumab can be resumed if LVEF improves to above the lower limit of normal with an absolute decrease from baseline of not more than 15%. The insert also states, however, that “the safety of continuation or resumption of trastuzumab in patients with trastuzumab induced LV dysfunction has never been studied, “ Dr. Liu noted.
She cited an American Society of Clinical Oncology guideline on the prevention and monitoring of cardiac dysfunction in survivors of adult cancers, which states in part that the decision to continue or discontinue cancer therapy in patients with evidence of cardiac dysfunction “made by the oncologist, should be informed by close collaboration with a cardiologist, fully evaluating the clinical circumstances and considering the risks and benefits of continuation of therapy responsible for the cardiac dysfunction.”
“I want to emphasize the importance of accepting and managing cardiovascular risk in patients priors to and during potentially cardiotoxic therapy. To optimize cardiologic and oncologic outcomes, we need to avoid or minimize treatment interruptions of life-saving therapy, and mitigate cardiac events with aggressive cardiovascular risk-factor modification,” Dr. Liu said.
She called for development of better risk stratification tools to tailor cardiac surveillance during therapy, based on both patient-specific and treatment-specific risk factors.
Dr. Liu reported nothing to disclose. Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics. Cota, Dendreon, Ferring Pharmaceuticals. GenomeDx, Janssen, and Nanobiotix.
REPORTING FROM ACC CARDIO-ONCOLOGY
Key clinical point: Oncologists should work with cardiologists to mitigate heart disease risk.
Major finding: Anthracyclines followed by trastuzumab significantly increase risk of HF.
Study details: Review of risk for heart disease in breast cancer survivors.
Disclosures: Dr. Liu reported nothing to disclose. Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
Moment of truth approaches for low-risk TAVR
SNOWMASS, COLO. – There are now more transcatheter aortic valve replacements performed each year than surgical ones in the United States, a disparity that may grow vastly larger.
That’s if the results of the two pivotal randomized trials comparing transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) in low-surgical-risk patients scheduled for presentation at the annual scientific session of the American College of Cardiology in March turn out to show TAVR outcomes are equivalent or superior to SAVR.
And that just might be the scenario, provided the eye-popping results already reported from another, much smaller study – the Low Risk TAVR study, a 200-patient, prospective, nonrandomized, observational study – are at all reflective of what’s to come when the pivotal PARTNER 3 and EVOLUT R trials are released at the ACC meeting in New Orleans, Michael J. Mack, MD, said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“The TAVR train has left the station on the way to low risk, and I don’t really see it coming back,” said Dr. Mack, medical director for cardiothoracic surgery at Baylor Scott & White Health in Dallas.
He wasn’t part of the Low Risk TAVR study, in which 200 low-surgical-risk patients with symptomatic severe aortic stenosis underwent TAVR with contemporary devices at 11 centers and were matched to 719 historical control SAVR patients at the same centers. But he called the study results “pretty spectacular”: zero 30-day all cause mortality in the TAVR group versus 1.7% with SAVR, no in-hospital strokes with TAVR versus a 0.6% rate with SAVR, and similar permanent pacemaker implantation rates of 5.0% with TAVR and 4.5% with SAVR.
Also, the TAVR group had a mere 3.0% rate of new-onset atrial fibrillation, a 2-day hospital length of stay, and a 0.5% incidence of greater-than-mild paravalvular leak at 30 days (J Am Coll Cardiol. 2018 Oct 30;72[18]:2095-105).
The two major trials due to report 1-year outcomes at the ACC meeting in March are similarly designed. The PARTNER 3 trial includes 1,000 low-surgical-risk patients with a mean age of 73 years and a predicted 30-day surgical mortality risk of 1.9%. Seventy-one percent of them were New York Heart Association (NYHA) Class II at enrollment. Participants were randomized to TAVR with the Edwards Lifesciences Sapien 3 valve or to SAVR, with the primary outcome being a composite of all-cause mortality, stroke, and rehospitalization 1 year post procedure. The EVOLUT R trial is similar, except the TAVR valve is the Medtronic CoreValve.
Both trials will continue to follow patients annually for 10 years in order to address the still-open issue of TAVR and SAVR valve durability. Also, the Food and Drug Administration has mandated that 4D CT imaging substudies be conducted in 800 of the combined 2,000 participants in the two trials in order to provide new insight into the issue of subclinical valve leaflet thrombosis, which was detected in 14% of participants in the Low Risk TAVR study 30 days post procedure.
“The clinical impact and need for anticoagulant therapy are currently unknown. However, clot anywhere else in the body doesn’t do good things, so it’s hard to imagine it’s helping here. Pretending it doesn’t exist isn’t going to make the problem go away,” Dr. Mack said.
The 4D CT imaging substudy results are expected to be presented later this year at the Transcatheter Cardiovascular Therapeutics conference in San Francisco.
In 2017, 51,064 TAVR procedures for symptomatic severe aortic stenosis were done in the United States, compared with 41,490 SAVRs. The past several years have seen a decreasing proportion of TAVRs being done in high-surgical-risk patients and a growing proportion in intermediate-risk patients.
Even if PARTNER 3 and EVOLUT R prove to be resoundingly positive for TAVR in low-risk patients, however, SAVR is not going to vanish, according to Dr. Mack. He cited four factors working against universal adoption of TAVR: the uncertainty surrounding valve durability, which will take years to resolve; the issue of TAVR valve leaflet thrombosis and the for-now theoretic possibility that all TAVR patients might need to receive postprocedure oral anticoagulation; the high rate of new permanent pacemaker implantation associated with TAVR, which Dr. Mack called the procedure’s Achilles heel; and the total absence of high-quality data on TAVR in patients with bicuspid aortic stenosis.
Even though TAVR for diseased bicuspid valves is not off-label therapy – the FDA’s indication for TAVR is for native valve aortic stenosis – patients with bicuspid valves weren’t included in any of the randomized trials, he explained.
Younger patients are likely to stick with SAVR for the foreseeable future, regardless of the outcomes of PARTNER 3 and EVOLUT R, according to the surgeon, because of the unresolved issue of valve durability, as well as TAVR’s greater associated need for a permanent pacemaker, both significant considerations in individuals with a life expectancy of another 20-30 years.
There are now roughly 600 TAVR centers and 1,150 SAVR centers nationally. One of the hot topics in the field stems from the fact that half of these TAVR centers do only one TAVR per week or less. That’s concerning in light of a recent New York State study showing a clear association between operator volume and outcomes.
“The more you do, the better your outcomes are, similar to many other procedures in medicine,” Dr. Mack commented.
On the other hand, it’s unlikely that patients who present to one of the roughly 550 SAVR-only centers are truly getting informed consent as to their options, he added.
TAVR timeline for 2019
March
PARTNER 3 and EVOLUT R primary outcomes to be presented at the American College of Cardiology annual scientific session.
Centers for Medicare & Medicaid Services to issue proposal for a revised National Coverage Determination for TAVR reimbursement.
June
Following a public comment period, CMS will release final revised criteria for TAVR reimbursement.
September
Results of the PARTNER 3 and EVOLUT R 4D CT imaging substudies will probably be presented late in the month at the annual Transcatheter Cardiovascular Therapeutics conference in San Francisco.
Late 2019
If PARTNER 3 and EVOLUT R trials are positive, FDA approval of the TAVR valves in low-surgical-risk patients is expected.
Dr. Mack is coprincipal investigator of PARTNER 3, which was sponsored by Edwards Lifesciences, and of Abbott Vascular’s COAPT trial. He’s also on the executive committee of the INTREPID trial, sponsored by Medtronic.
SNOWMASS, COLO. – There are now more transcatheter aortic valve replacements performed each year than surgical ones in the United States, a disparity that may grow vastly larger.
That’s if the results of the two pivotal randomized trials comparing transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) in low-surgical-risk patients scheduled for presentation at the annual scientific session of the American College of Cardiology in March turn out to show TAVR outcomes are equivalent or superior to SAVR.
And that just might be the scenario, provided the eye-popping results already reported from another, much smaller study – the Low Risk TAVR study, a 200-patient, prospective, nonrandomized, observational study – are at all reflective of what’s to come when the pivotal PARTNER 3 and EVOLUT R trials are released at the ACC meeting in New Orleans, Michael J. Mack, MD, said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“The TAVR train has left the station on the way to low risk, and I don’t really see it coming back,” said Dr. Mack, medical director for cardiothoracic surgery at Baylor Scott & White Health in Dallas.
He wasn’t part of the Low Risk TAVR study, in which 200 low-surgical-risk patients with symptomatic severe aortic stenosis underwent TAVR with contemporary devices at 11 centers and were matched to 719 historical control SAVR patients at the same centers. But he called the study results “pretty spectacular”: zero 30-day all cause mortality in the TAVR group versus 1.7% with SAVR, no in-hospital strokes with TAVR versus a 0.6% rate with SAVR, and similar permanent pacemaker implantation rates of 5.0% with TAVR and 4.5% with SAVR.
Also, the TAVR group had a mere 3.0% rate of new-onset atrial fibrillation, a 2-day hospital length of stay, and a 0.5% incidence of greater-than-mild paravalvular leak at 30 days (J Am Coll Cardiol. 2018 Oct 30;72[18]:2095-105).
The two major trials due to report 1-year outcomes at the ACC meeting in March are similarly designed. The PARTNER 3 trial includes 1,000 low-surgical-risk patients with a mean age of 73 years and a predicted 30-day surgical mortality risk of 1.9%. Seventy-one percent of them were New York Heart Association (NYHA) Class II at enrollment. Participants were randomized to TAVR with the Edwards Lifesciences Sapien 3 valve or to SAVR, with the primary outcome being a composite of all-cause mortality, stroke, and rehospitalization 1 year post procedure. The EVOLUT R trial is similar, except the TAVR valve is the Medtronic CoreValve.
Both trials will continue to follow patients annually for 10 years in order to address the still-open issue of TAVR and SAVR valve durability. Also, the Food and Drug Administration has mandated that 4D CT imaging substudies be conducted in 800 of the combined 2,000 participants in the two trials in order to provide new insight into the issue of subclinical valve leaflet thrombosis, which was detected in 14% of participants in the Low Risk TAVR study 30 days post procedure.
“The clinical impact and need for anticoagulant therapy are currently unknown. However, clot anywhere else in the body doesn’t do good things, so it’s hard to imagine it’s helping here. Pretending it doesn’t exist isn’t going to make the problem go away,” Dr. Mack said.
The 4D CT imaging substudy results are expected to be presented later this year at the Transcatheter Cardiovascular Therapeutics conference in San Francisco.
In 2017, 51,064 TAVR procedures for symptomatic severe aortic stenosis were done in the United States, compared with 41,490 SAVRs. The past several years have seen a decreasing proportion of TAVRs being done in high-surgical-risk patients and a growing proportion in intermediate-risk patients.
Even if PARTNER 3 and EVOLUT R prove to be resoundingly positive for TAVR in low-risk patients, however, SAVR is not going to vanish, according to Dr. Mack. He cited four factors working against universal adoption of TAVR: the uncertainty surrounding valve durability, which will take years to resolve; the issue of TAVR valve leaflet thrombosis and the for-now theoretic possibility that all TAVR patients might need to receive postprocedure oral anticoagulation; the high rate of new permanent pacemaker implantation associated with TAVR, which Dr. Mack called the procedure’s Achilles heel; and the total absence of high-quality data on TAVR in patients with bicuspid aortic stenosis.
Even though TAVR for diseased bicuspid valves is not off-label therapy – the FDA’s indication for TAVR is for native valve aortic stenosis – patients with bicuspid valves weren’t included in any of the randomized trials, he explained.
Younger patients are likely to stick with SAVR for the foreseeable future, regardless of the outcomes of PARTNER 3 and EVOLUT R, according to the surgeon, because of the unresolved issue of valve durability, as well as TAVR’s greater associated need for a permanent pacemaker, both significant considerations in individuals with a life expectancy of another 20-30 years.
There are now roughly 600 TAVR centers and 1,150 SAVR centers nationally. One of the hot topics in the field stems from the fact that half of these TAVR centers do only one TAVR per week or less. That’s concerning in light of a recent New York State study showing a clear association between operator volume and outcomes.
“The more you do, the better your outcomes are, similar to many other procedures in medicine,” Dr. Mack commented.
On the other hand, it’s unlikely that patients who present to one of the roughly 550 SAVR-only centers are truly getting informed consent as to their options, he added.
TAVR timeline for 2019
March
PARTNER 3 and EVOLUT R primary outcomes to be presented at the American College of Cardiology annual scientific session.
Centers for Medicare & Medicaid Services to issue proposal for a revised National Coverage Determination for TAVR reimbursement.
June
Following a public comment period, CMS will release final revised criteria for TAVR reimbursement.
September
Results of the PARTNER 3 and EVOLUT R 4D CT imaging substudies will probably be presented late in the month at the annual Transcatheter Cardiovascular Therapeutics conference in San Francisco.
Late 2019
If PARTNER 3 and EVOLUT R trials are positive, FDA approval of the TAVR valves in low-surgical-risk patients is expected.
Dr. Mack is coprincipal investigator of PARTNER 3, which was sponsored by Edwards Lifesciences, and of Abbott Vascular’s COAPT trial. He’s also on the executive committee of the INTREPID trial, sponsored by Medtronic.
SNOWMASS, COLO. – There are now more transcatheter aortic valve replacements performed each year than surgical ones in the United States, a disparity that may grow vastly larger.
That’s if the results of the two pivotal randomized trials comparing transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) in low-surgical-risk patients scheduled for presentation at the annual scientific session of the American College of Cardiology in March turn out to show TAVR outcomes are equivalent or superior to SAVR.
And that just might be the scenario, provided the eye-popping results already reported from another, much smaller study – the Low Risk TAVR study, a 200-patient, prospective, nonrandomized, observational study – are at all reflective of what’s to come when the pivotal PARTNER 3 and EVOLUT R trials are released at the ACC meeting in New Orleans, Michael J. Mack, MD, said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“The TAVR train has left the station on the way to low risk, and I don’t really see it coming back,” said Dr. Mack, medical director for cardiothoracic surgery at Baylor Scott & White Health in Dallas.
He wasn’t part of the Low Risk TAVR study, in which 200 low-surgical-risk patients with symptomatic severe aortic stenosis underwent TAVR with contemporary devices at 11 centers and were matched to 719 historical control SAVR patients at the same centers. But he called the study results “pretty spectacular”: zero 30-day all cause mortality in the TAVR group versus 1.7% with SAVR, no in-hospital strokes with TAVR versus a 0.6% rate with SAVR, and similar permanent pacemaker implantation rates of 5.0% with TAVR and 4.5% with SAVR.
Also, the TAVR group had a mere 3.0% rate of new-onset atrial fibrillation, a 2-day hospital length of stay, and a 0.5% incidence of greater-than-mild paravalvular leak at 30 days (J Am Coll Cardiol. 2018 Oct 30;72[18]:2095-105).
The two major trials due to report 1-year outcomes at the ACC meeting in March are similarly designed. The PARTNER 3 trial includes 1,000 low-surgical-risk patients with a mean age of 73 years and a predicted 30-day surgical mortality risk of 1.9%. Seventy-one percent of them were New York Heart Association (NYHA) Class II at enrollment. Participants were randomized to TAVR with the Edwards Lifesciences Sapien 3 valve or to SAVR, with the primary outcome being a composite of all-cause mortality, stroke, and rehospitalization 1 year post procedure. The EVOLUT R trial is similar, except the TAVR valve is the Medtronic CoreValve.
Both trials will continue to follow patients annually for 10 years in order to address the still-open issue of TAVR and SAVR valve durability. Also, the Food and Drug Administration has mandated that 4D CT imaging substudies be conducted in 800 of the combined 2,000 participants in the two trials in order to provide new insight into the issue of subclinical valve leaflet thrombosis, which was detected in 14% of participants in the Low Risk TAVR study 30 days post procedure.
“The clinical impact and need for anticoagulant therapy are currently unknown. However, clot anywhere else in the body doesn’t do good things, so it’s hard to imagine it’s helping here. Pretending it doesn’t exist isn’t going to make the problem go away,” Dr. Mack said.
The 4D CT imaging substudy results are expected to be presented later this year at the Transcatheter Cardiovascular Therapeutics conference in San Francisco.
In 2017, 51,064 TAVR procedures for symptomatic severe aortic stenosis were done in the United States, compared with 41,490 SAVRs. The past several years have seen a decreasing proportion of TAVRs being done in high-surgical-risk patients and a growing proportion in intermediate-risk patients.
Even if PARTNER 3 and EVOLUT R prove to be resoundingly positive for TAVR in low-risk patients, however, SAVR is not going to vanish, according to Dr. Mack. He cited four factors working against universal adoption of TAVR: the uncertainty surrounding valve durability, which will take years to resolve; the issue of TAVR valve leaflet thrombosis and the for-now theoretic possibility that all TAVR patients might need to receive postprocedure oral anticoagulation; the high rate of new permanent pacemaker implantation associated with TAVR, which Dr. Mack called the procedure’s Achilles heel; and the total absence of high-quality data on TAVR in patients with bicuspid aortic stenosis.
Even though TAVR for diseased bicuspid valves is not off-label therapy – the FDA’s indication for TAVR is for native valve aortic stenosis – patients with bicuspid valves weren’t included in any of the randomized trials, he explained.
Younger patients are likely to stick with SAVR for the foreseeable future, regardless of the outcomes of PARTNER 3 and EVOLUT R, according to the surgeon, because of the unresolved issue of valve durability, as well as TAVR’s greater associated need for a permanent pacemaker, both significant considerations in individuals with a life expectancy of another 20-30 years.
There are now roughly 600 TAVR centers and 1,150 SAVR centers nationally. One of the hot topics in the field stems from the fact that half of these TAVR centers do only one TAVR per week or less. That’s concerning in light of a recent New York State study showing a clear association between operator volume and outcomes.
“The more you do, the better your outcomes are, similar to many other procedures in medicine,” Dr. Mack commented.
On the other hand, it’s unlikely that patients who present to one of the roughly 550 SAVR-only centers are truly getting informed consent as to their options, he added.
TAVR timeline for 2019
March
PARTNER 3 and EVOLUT R primary outcomes to be presented at the American College of Cardiology annual scientific session.
Centers for Medicare & Medicaid Services to issue proposal for a revised National Coverage Determination for TAVR reimbursement.
June
Following a public comment period, CMS will release final revised criteria for TAVR reimbursement.
September
Results of the PARTNER 3 and EVOLUT R 4D CT imaging substudies will probably be presented late in the month at the annual Transcatheter Cardiovascular Therapeutics conference in San Francisco.
Late 2019
If PARTNER 3 and EVOLUT R trials are positive, FDA approval of the TAVR valves in low-surgical-risk patients is expected.
Dr. Mack is coprincipal investigator of PARTNER 3, which was sponsored by Edwards Lifesciences, and of Abbott Vascular’s COAPT trial. He’s also on the executive committee of the INTREPID trial, sponsored by Medtronic.
EXPERT ANALYSIS FROM ACC SNOWMASS 2019
Biomarkers predict VTE risk with menopausal oral hormone therapy
CHICAGO – An elevated baseline D-dimer level is helpful to women and their physicians in clarifying decision making about oral hormone therapy for troublesome menopausal symptoms, Mary Cushman, MD, said at the American Heart Association scientific sessions.
She was lead investigator in a nested case-control study embedded in the landmark Women’s Health Initiative (WHI), which showed that participants who had a baseline D-dimer greater than 0.54 mg/L – putting them in the top 25% – and were randomized to oral menopausal hormone therapy had a 5-year incidence of venous thromboembolism (VTE) of 6%. That’s 500% higher than in women with a lower D-dimer randomized to placebo.
“The number needed to test for D-dimer in advance of prescribing in order to prevent one VTE over 5 years of hormone therapy was only 33. So this is potentially something in the toolbox you can use in counseling women about oral hormone therapy,” said Dr. Cushman, professor of medicine and pathology and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
The biomarker study included 1,082 WHI participants aged 50-79 years randomized to oral conjugated equine estrogen with or without medroxyprogesterone acetate or to placebo, 215 of whom experienced VTE during a mean 4.1 years of follow-up. Levels of a variety of biomarkers obtained at baseline were assessed in terms of their associated risk of future VTE. The biomarkers included C-reactive protein and procoagulant, anticoagulant, and fibrinolytic factors.
In a logistic regression analysis adjusted for age, race, body mass index, and hysterectomy, the strongest association with VTE was a high D-dimer. That 500% increased risk of VTE with hormone therapy in women with a D-dimer greater than 0.54 mg/L was comparable in magnitude with the risk Dr. Cushman and her coinvestigators previously reported for the combination of factor V Leiden and hormone therapy.
Dr. Cushman and her associates also took a first step towards developing a multibiomarker risk score. They found that WHI participants randomized to hormone therapy who had abnormal baseline values for any three or more of eight biomarkers had a 1,450% greater risk of future VTE than women with zero or one abnormal biomarker who were assigned to placebo. The eight-biomarker panel described in the recently published study comprised D-dimer, factor V Leiden, protein C, total protein S, free protein S, antithrombin, plasmin-antiplasmin complex, and fragment 1.2. However, the investigators indicated the risk score needs further study before it’s ready for adoption in clinical practice (Res Pract Thromb Haemost. 2018 Apr 17;2[2]:310-9).
Dr. Cushman noted that, although the main findings of the WHI have largely resulted in abandonment of menopausal hormone therapy for disease prevention, many women still want to take oral hormone therapy for relief of bothersome menopausal symptoms. She tries to steer them instead to safer nonoral formulations. Transdermal estrogen replacement has no associated risk of VTE and doesn’t activate anticoagulation. Neither does vaginal estradiol.
In offering what she called “the 30,000-foot view of the impact of venous thrombosis on women’s health,” Dr. Cushman noted that VTE is the third-most common vascular disease in the United States, with up to 900,000 cases per year. The lifetime risk in women after age 45 is 8.4%. Half of VTEs are provoked and therefore potentially preventable, with common triggers being surgery, cancer, pregnancy, trauma, and immobilization, especially during travel.
In addition, a retrospective study conducted in the Worcester, Mass., area showed that 1-month mortality after VTE remained static in the 5%-10% range during 1999-2009.
“This is a fatal disease, even though we treat it as an outpatient quite a lot,” Dr. Cushman observed.
Common nonfatal complications of VTE include major bleeding in 5%-10% of cases, a recurrence rate of 5%-10% annually, a 20%-40% of the burdensome and not infrequently disabling condition known as postthrombotic syndrome, and a 3%-4% incidence of chronic thromboembolic pulmonary hypertension. Yet despite the seriousness of VTE, awareness about VTE is poor among both patients and physicians, and appropriate prophylaxis is underutilized, she said.
The key to improved primary prevention of VTE, Dr. Cushman continued, is greater attention to modifiable behavioral risk factors, along with more use of prophylactic medication when needed.
The traditional cardiovascular risk factors, like hypertension, smoking, and hyperlipidemia, aren’t relevant to VTE risk. But obesity and sedentary lifestyle have come to be recognized as important modifiable risk factors. In one study of more than 30,000 Americans, the risk of VTE was shown to be reduced by 40% in individuals who exercised at least four times per week, compared with the physically inactive.
And in an analysis led by Dr. Cushman of nearly 21,000 participants over age 45 years with 12.6 years of follow-up in the Longitudinal Investigation of Thromboembolism Etiology (LITE), the investigators found that greater levels of all body size measures – not just body mass index, but calf circumference, waist-hip ratio, hip circumference, and others – were associated with increased VTE risk. These associations weren’t affected by levels of circulating biomarkers for inflammation or hypercoagulability, suggesting that it’s obesity per se, with its associated adverse impact on blood flow caused by physical factors, that explains the mechanism underlying obesity as a risk factor for VTE (Thromb Res. 2016 Aug;144:127-32).
At the meeting’s opening ceremonies, AHA President Ivor Benjamin, MD, of the Medical College of Wisconsin, Milwaukee, presented Dr. Cushman with the AHA Population Research Prize. She was honored for her “critically acclaimed research utilizing biomarker assessments in population studies to elucidate pathways of disease etiology for the three most common vascular diseases – coronary heart disease, stroke, and venous thromboembolism – as well as their risk factors,” said Dr. Benjamin.
Dr. Cushman reported having no financial conflicts regarding her D-dimer study, which was funded by the National Institutes of Health.
CHICAGO – An elevated baseline D-dimer level is helpful to women and their physicians in clarifying decision making about oral hormone therapy for troublesome menopausal symptoms, Mary Cushman, MD, said at the American Heart Association scientific sessions.
She was lead investigator in a nested case-control study embedded in the landmark Women’s Health Initiative (WHI), which showed that participants who had a baseline D-dimer greater than 0.54 mg/L – putting them in the top 25% – and were randomized to oral menopausal hormone therapy had a 5-year incidence of venous thromboembolism (VTE) of 6%. That’s 500% higher than in women with a lower D-dimer randomized to placebo.
“The number needed to test for D-dimer in advance of prescribing in order to prevent one VTE over 5 years of hormone therapy was only 33. So this is potentially something in the toolbox you can use in counseling women about oral hormone therapy,” said Dr. Cushman, professor of medicine and pathology and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
The biomarker study included 1,082 WHI participants aged 50-79 years randomized to oral conjugated equine estrogen with or without medroxyprogesterone acetate or to placebo, 215 of whom experienced VTE during a mean 4.1 years of follow-up. Levels of a variety of biomarkers obtained at baseline were assessed in terms of their associated risk of future VTE. The biomarkers included C-reactive protein and procoagulant, anticoagulant, and fibrinolytic factors.
In a logistic regression analysis adjusted for age, race, body mass index, and hysterectomy, the strongest association with VTE was a high D-dimer. That 500% increased risk of VTE with hormone therapy in women with a D-dimer greater than 0.54 mg/L was comparable in magnitude with the risk Dr. Cushman and her coinvestigators previously reported for the combination of factor V Leiden and hormone therapy.
Dr. Cushman and her associates also took a first step towards developing a multibiomarker risk score. They found that WHI participants randomized to hormone therapy who had abnormal baseline values for any three or more of eight biomarkers had a 1,450% greater risk of future VTE than women with zero or one abnormal biomarker who were assigned to placebo. The eight-biomarker panel described in the recently published study comprised D-dimer, factor V Leiden, protein C, total protein S, free protein S, antithrombin, plasmin-antiplasmin complex, and fragment 1.2. However, the investigators indicated the risk score needs further study before it’s ready for adoption in clinical practice (Res Pract Thromb Haemost. 2018 Apr 17;2[2]:310-9).
Dr. Cushman noted that, although the main findings of the WHI have largely resulted in abandonment of menopausal hormone therapy for disease prevention, many women still want to take oral hormone therapy for relief of bothersome menopausal symptoms. She tries to steer them instead to safer nonoral formulations. Transdermal estrogen replacement has no associated risk of VTE and doesn’t activate anticoagulation. Neither does vaginal estradiol.
In offering what she called “the 30,000-foot view of the impact of venous thrombosis on women’s health,” Dr. Cushman noted that VTE is the third-most common vascular disease in the United States, with up to 900,000 cases per year. The lifetime risk in women after age 45 is 8.4%. Half of VTEs are provoked and therefore potentially preventable, with common triggers being surgery, cancer, pregnancy, trauma, and immobilization, especially during travel.
In addition, a retrospective study conducted in the Worcester, Mass., area showed that 1-month mortality after VTE remained static in the 5%-10% range during 1999-2009.
“This is a fatal disease, even though we treat it as an outpatient quite a lot,” Dr. Cushman observed.
Common nonfatal complications of VTE include major bleeding in 5%-10% of cases, a recurrence rate of 5%-10% annually, a 20%-40% of the burdensome and not infrequently disabling condition known as postthrombotic syndrome, and a 3%-4% incidence of chronic thromboembolic pulmonary hypertension. Yet despite the seriousness of VTE, awareness about VTE is poor among both patients and physicians, and appropriate prophylaxis is underutilized, she said.
The key to improved primary prevention of VTE, Dr. Cushman continued, is greater attention to modifiable behavioral risk factors, along with more use of prophylactic medication when needed.
The traditional cardiovascular risk factors, like hypertension, smoking, and hyperlipidemia, aren’t relevant to VTE risk. But obesity and sedentary lifestyle have come to be recognized as important modifiable risk factors. In one study of more than 30,000 Americans, the risk of VTE was shown to be reduced by 40% in individuals who exercised at least four times per week, compared with the physically inactive.
And in an analysis led by Dr. Cushman of nearly 21,000 participants over age 45 years with 12.6 years of follow-up in the Longitudinal Investigation of Thromboembolism Etiology (LITE), the investigators found that greater levels of all body size measures – not just body mass index, but calf circumference, waist-hip ratio, hip circumference, and others – were associated with increased VTE risk. These associations weren’t affected by levels of circulating biomarkers for inflammation or hypercoagulability, suggesting that it’s obesity per se, with its associated adverse impact on blood flow caused by physical factors, that explains the mechanism underlying obesity as a risk factor for VTE (Thromb Res. 2016 Aug;144:127-32).
At the meeting’s opening ceremonies, AHA President Ivor Benjamin, MD, of the Medical College of Wisconsin, Milwaukee, presented Dr. Cushman with the AHA Population Research Prize. She was honored for her “critically acclaimed research utilizing biomarker assessments in population studies to elucidate pathways of disease etiology for the three most common vascular diseases – coronary heart disease, stroke, and venous thromboembolism – as well as their risk factors,” said Dr. Benjamin.
Dr. Cushman reported having no financial conflicts regarding her D-dimer study, which was funded by the National Institutes of Health.
CHICAGO – An elevated baseline D-dimer level is helpful to women and their physicians in clarifying decision making about oral hormone therapy for troublesome menopausal symptoms, Mary Cushman, MD, said at the American Heart Association scientific sessions.
She was lead investigator in a nested case-control study embedded in the landmark Women’s Health Initiative (WHI), which showed that participants who had a baseline D-dimer greater than 0.54 mg/L – putting them in the top 25% – and were randomized to oral menopausal hormone therapy had a 5-year incidence of venous thromboembolism (VTE) of 6%. That’s 500% higher than in women with a lower D-dimer randomized to placebo.
“The number needed to test for D-dimer in advance of prescribing in order to prevent one VTE over 5 years of hormone therapy was only 33. So this is potentially something in the toolbox you can use in counseling women about oral hormone therapy,” said Dr. Cushman, professor of medicine and pathology and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
The biomarker study included 1,082 WHI participants aged 50-79 years randomized to oral conjugated equine estrogen with or without medroxyprogesterone acetate or to placebo, 215 of whom experienced VTE during a mean 4.1 years of follow-up. Levels of a variety of biomarkers obtained at baseline were assessed in terms of their associated risk of future VTE. The biomarkers included C-reactive protein and procoagulant, anticoagulant, and fibrinolytic factors.
In a logistic regression analysis adjusted for age, race, body mass index, and hysterectomy, the strongest association with VTE was a high D-dimer. That 500% increased risk of VTE with hormone therapy in women with a D-dimer greater than 0.54 mg/L was comparable in magnitude with the risk Dr. Cushman and her coinvestigators previously reported for the combination of factor V Leiden and hormone therapy.
Dr. Cushman and her associates also took a first step towards developing a multibiomarker risk score. They found that WHI participants randomized to hormone therapy who had abnormal baseline values for any three or more of eight biomarkers had a 1,450% greater risk of future VTE than women with zero or one abnormal biomarker who were assigned to placebo. The eight-biomarker panel described in the recently published study comprised D-dimer, factor V Leiden, protein C, total protein S, free protein S, antithrombin, plasmin-antiplasmin complex, and fragment 1.2. However, the investigators indicated the risk score needs further study before it’s ready for adoption in clinical practice (Res Pract Thromb Haemost. 2018 Apr 17;2[2]:310-9).
Dr. Cushman noted that, although the main findings of the WHI have largely resulted in abandonment of menopausal hormone therapy for disease prevention, many women still want to take oral hormone therapy for relief of bothersome menopausal symptoms. She tries to steer them instead to safer nonoral formulations. Transdermal estrogen replacement has no associated risk of VTE and doesn’t activate anticoagulation. Neither does vaginal estradiol.
In offering what she called “the 30,000-foot view of the impact of venous thrombosis on women’s health,” Dr. Cushman noted that VTE is the third-most common vascular disease in the United States, with up to 900,000 cases per year. The lifetime risk in women after age 45 is 8.4%. Half of VTEs are provoked and therefore potentially preventable, with common triggers being surgery, cancer, pregnancy, trauma, and immobilization, especially during travel.
In addition, a retrospective study conducted in the Worcester, Mass., area showed that 1-month mortality after VTE remained static in the 5%-10% range during 1999-2009.
“This is a fatal disease, even though we treat it as an outpatient quite a lot,” Dr. Cushman observed.
Common nonfatal complications of VTE include major bleeding in 5%-10% of cases, a recurrence rate of 5%-10% annually, a 20%-40% of the burdensome and not infrequently disabling condition known as postthrombotic syndrome, and a 3%-4% incidence of chronic thromboembolic pulmonary hypertension. Yet despite the seriousness of VTE, awareness about VTE is poor among both patients and physicians, and appropriate prophylaxis is underutilized, she said.
The key to improved primary prevention of VTE, Dr. Cushman continued, is greater attention to modifiable behavioral risk factors, along with more use of prophylactic medication when needed.
The traditional cardiovascular risk factors, like hypertension, smoking, and hyperlipidemia, aren’t relevant to VTE risk. But obesity and sedentary lifestyle have come to be recognized as important modifiable risk factors. In one study of more than 30,000 Americans, the risk of VTE was shown to be reduced by 40% in individuals who exercised at least four times per week, compared with the physically inactive.
And in an analysis led by Dr. Cushman of nearly 21,000 participants over age 45 years with 12.6 years of follow-up in the Longitudinal Investigation of Thromboembolism Etiology (LITE), the investigators found that greater levels of all body size measures – not just body mass index, but calf circumference, waist-hip ratio, hip circumference, and others – were associated with increased VTE risk. These associations weren’t affected by levels of circulating biomarkers for inflammation or hypercoagulability, suggesting that it’s obesity per se, with its associated adverse impact on blood flow caused by physical factors, that explains the mechanism underlying obesity as a risk factor for VTE (Thromb Res. 2016 Aug;144:127-32).
At the meeting’s opening ceremonies, AHA President Ivor Benjamin, MD, of the Medical College of Wisconsin, Milwaukee, presented Dr. Cushman with the AHA Population Research Prize. She was honored for her “critically acclaimed research utilizing biomarker assessments in population studies to elucidate pathways of disease etiology for the three most common vascular diseases – coronary heart disease, stroke, and venous thromboembolism – as well as their risk factors,” said Dr. Benjamin.
Dr. Cushman reported having no financial conflicts regarding her D-dimer study, which was funded by the National Institutes of Health.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Women in the top 25% for D-dimer level before going on menopausal hormone therapy had a 6% incidence of venous thromboembolism over 5 years.
Study details: This was a nested case-control study focused on identifying biomarkers for venous thromboembolism risk which included 1,082 participants in the Women’s Health Initiative randomized to menopausal hormone therapy or placebo.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was funded by the National Institutes of Health.
Arterial stiffness index may be potent tool for PAD risk stratification
An increased peripheral arterial stiffness index was associated with an increased rate of major adverse cardiac events (MACEs), independent of age, coronary artery disease, and Rutherford category, according to the results of a study of patients with peripheral arterial disease (PAD) presenting to the vascular surgery outpatient clinic at the San Francisco Veterans Affairs Medical Center.
Seventy-two patients with PAD were recruited during 2011-2016 and each had baseline radial artery applanation tonometry performed at a baseline visit. Their central radial artery augmentation index (AIX), normalized to 75 beats/min, and the peripheral AIX were calculated using pulse wave analysis. Subsequent MACEs were identified by chart review in the Journal of Surgical Research.
The cohort was predominately male (96%) and white (69%), with an average age of 69.4 years.
During a median follow-up period of 34 months, 14 patients experienced a MACE (19%). These events included eight myocardial infarctions or coronary revascularizations, four deaths from a cardiac cause, one stroke, and one transient ischemic attack. Unadjusted Cox proportional hazards models identified a significant association between the peripheral AIX and rate of MACE (hazard ratio, 1.54; 95% confidence interval, 1.06-2.22; P less than .02).
the researchers concluded.
The study was funded by public institutions including the National Institutes of Heath, the University of California, San Francisco, and a Society for Vascular Surgery Seed Grant and Career Development Award. The authors reported that they had no disclosures.
SOURCE: Ramirez JL et al. J Surg Res 2019;235:250-7.
An increased peripheral arterial stiffness index was associated with an increased rate of major adverse cardiac events (MACEs), independent of age, coronary artery disease, and Rutherford category, according to the results of a study of patients with peripheral arterial disease (PAD) presenting to the vascular surgery outpatient clinic at the San Francisco Veterans Affairs Medical Center.
Seventy-two patients with PAD were recruited during 2011-2016 and each had baseline radial artery applanation tonometry performed at a baseline visit. Their central radial artery augmentation index (AIX), normalized to 75 beats/min, and the peripheral AIX were calculated using pulse wave analysis. Subsequent MACEs were identified by chart review in the Journal of Surgical Research.
The cohort was predominately male (96%) and white (69%), with an average age of 69.4 years.
During a median follow-up period of 34 months, 14 patients experienced a MACE (19%). These events included eight myocardial infarctions or coronary revascularizations, four deaths from a cardiac cause, one stroke, and one transient ischemic attack. Unadjusted Cox proportional hazards models identified a significant association between the peripheral AIX and rate of MACE (hazard ratio, 1.54; 95% confidence interval, 1.06-2.22; P less than .02).
the researchers concluded.
The study was funded by public institutions including the National Institutes of Heath, the University of California, San Francisco, and a Society for Vascular Surgery Seed Grant and Career Development Award. The authors reported that they had no disclosures.
SOURCE: Ramirez JL et al. J Surg Res 2019;235:250-7.
An increased peripheral arterial stiffness index was associated with an increased rate of major adverse cardiac events (MACEs), independent of age, coronary artery disease, and Rutherford category, according to the results of a study of patients with peripheral arterial disease (PAD) presenting to the vascular surgery outpatient clinic at the San Francisco Veterans Affairs Medical Center.
Seventy-two patients with PAD were recruited during 2011-2016 and each had baseline radial artery applanation tonometry performed at a baseline visit. Their central radial artery augmentation index (AIX), normalized to 75 beats/min, and the peripheral AIX were calculated using pulse wave analysis. Subsequent MACEs were identified by chart review in the Journal of Surgical Research.
The cohort was predominately male (96%) and white (69%), with an average age of 69.4 years.
During a median follow-up period of 34 months, 14 patients experienced a MACE (19%). These events included eight myocardial infarctions or coronary revascularizations, four deaths from a cardiac cause, one stroke, and one transient ischemic attack. Unadjusted Cox proportional hazards models identified a significant association between the peripheral AIX and rate of MACE (hazard ratio, 1.54; 95% confidence interval, 1.06-2.22; P less than .02).
the researchers concluded.
The study was funded by public institutions including the National Institutes of Heath, the University of California, San Francisco, and a Society for Vascular Surgery Seed Grant and Career Development Award. The authors reported that they had no disclosures.
SOURCE: Ramirez JL et al. J Surg Res 2019;235:250-7.
FROM THE JOURNAL OF SURGICAL RESEARCH
Key clinical point: An increased peripheral arterial stiffness index was associated with an increased rate of MACEs.
Major finding: There was significant association between peripheral arterial stiffness and the rate of MACE (P less than .02).
Study details: Chart review study of 72 patients with PAD over time.
Disclosures: The study was funded by various public institutions. The authors reported that they had no disclosures.
Source: Ramirez JL et al. J Surg Res 2019 Mar;235:250-7.
Life’s Simple 7 tied to lowered PAD risk in long-term study
Adherence to the American Heart Association’s Life’s Simple 7 is associated with lower incidence of peripheral artery disease and less decline in ankle brachial index, according to the results of a retrospective analysis of patients in the Multi-Ethnic Study of Atherosclerosis (MESA) trial.
“These results support the use of LS7 to prevent PAD and decline in ABI in multiple racial/ethnic groups,” according to Jonathan T. Unkart, MD, of the department of medicine and public health, University of California, San Diego, and his colleagues.
MESA recruited 6,814 men and women aged 45-84 years who were free of clinical cardiovascular disease. The cohort comprised 53% women and had the following racial/ethnic composition: 38% non-Hispanic white; 28% African American; 23% Hispanic, and 11% Asian. MESA consisted of six exams, with the baseline exam occurring from 2000 to 2002, including assessment of all LS7 components and PAD assessment using ABI calculated on both left and right sides. The final exam was performed from 2010 to 2012.
As background, the metrics for Life’s Simple 7 consist of total cholesterol, blood pressure, blood glucose, smoking status, body mass index, physical activity, and adherence to a healthy diet score. Each element can be scored 2 points for optimum, 1 for average, and 0 for inadequate. The investigators assessed overall LS7 scores on a continuous 0-14 scale, as well as the overall categorical indications of inadequate, average, and optimum. Cox proportional hazard models were used to assess the association of individual LS7 components by overall LS7 with incident PAD, according to the researchers.
Interactions of race/ethnicity by LS7 score were assessed on a multiplicative scale for both incident PAD and decline in ABI outcomes, adjusted for age, sex, education, and income (Am J Prev Med. 2019;56:262-70).
A total of 5,529 participants had complete LS7 information and met inclusion criteria to assess incident PAD. Over a median follow-up of 9.2 years, 251 (4.5%) participants developed incident PAD and 419 (9.8%) participants had a decline of at least 0.15 in ABI. In addition, each point higher on the continuous LS7 scale was associated with 0.94-fold lower odds of decline in ABI (odds ratio, 0.94; 95% confidence interval, 0.87-0.97; P =.003).
Each point higher on the continuous LS7 scale was associated with a 17% lower rate of incident PAD (HR, 0.83; 95% CI, 0.78-0.88; P less than .001), according to the researchers.
The study showed that there was a significant prospective association between LS7 score and incident PAD in African Americans, Hispanics, and non-Hispanic whites. Although the association was not statistically significant for the Chinese Americans in MESA, this was likely because of the low number of incident PAD cases (only 18) in this group, according to the authors.
Analysis by individual LS7 components showed that more optimal levels of smoking, physical activity, glucose, and blood pressure were significantly associated with lower rates of incident PAD. Similarly, after adjustment for age, sex, race/ethnicity, income, education, and baseline ABI, more optimal levels of smoking, and glucose were significantly associated with lower odds of decline. These results for the decline in ABI did not appear to differ across race/ethnicity, according to Dr. Unkart and his colleagues.
In contrast, BMI, diet, and cholesterol were not associated with incident PAD or decline in ABI.
“Higher scores on the AHA LS7 were associated with lower incident PAD and less decline in ABI. Preventive measures targeting LS7 components could assist with reducing PAD-related morbidity and mortality,” the researchers concluded.
The work was supported by the National, Heart, Lung, and Blood Institute. Dr. Unkart and his colleagues reported that they had no disclosures.
SOURCE: Unkart JT et al. Am J Prev Med 2019;56:262-270.
Adherence to the American Heart Association’s Life’s Simple 7 is associated with lower incidence of peripheral artery disease and less decline in ankle brachial index, according to the results of a retrospective analysis of patients in the Multi-Ethnic Study of Atherosclerosis (MESA) trial.
“These results support the use of LS7 to prevent PAD and decline in ABI in multiple racial/ethnic groups,” according to Jonathan T. Unkart, MD, of the department of medicine and public health, University of California, San Diego, and his colleagues.
MESA recruited 6,814 men and women aged 45-84 years who were free of clinical cardiovascular disease. The cohort comprised 53% women and had the following racial/ethnic composition: 38% non-Hispanic white; 28% African American; 23% Hispanic, and 11% Asian. MESA consisted of six exams, with the baseline exam occurring from 2000 to 2002, including assessment of all LS7 components and PAD assessment using ABI calculated on both left and right sides. The final exam was performed from 2010 to 2012.
As background, the metrics for Life’s Simple 7 consist of total cholesterol, blood pressure, blood glucose, smoking status, body mass index, physical activity, and adherence to a healthy diet score. Each element can be scored 2 points for optimum, 1 for average, and 0 for inadequate. The investigators assessed overall LS7 scores on a continuous 0-14 scale, as well as the overall categorical indications of inadequate, average, and optimum. Cox proportional hazard models were used to assess the association of individual LS7 components by overall LS7 with incident PAD, according to the researchers.
Interactions of race/ethnicity by LS7 score were assessed on a multiplicative scale for both incident PAD and decline in ABI outcomes, adjusted for age, sex, education, and income (Am J Prev Med. 2019;56:262-70).
A total of 5,529 participants had complete LS7 information and met inclusion criteria to assess incident PAD. Over a median follow-up of 9.2 years, 251 (4.5%) participants developed incident PAD and 419 (9.8%) participants had a decline of at least 0.15 in ABI. In addition, each point higher on the continuous LS7 scale was associated with 0.94-fold lower odds of decline in ABI (odds ratio, 0.94; 95% confidence interval, 0.87-0.97; P =.003).
Each point higher on the continuous LS7 scale was associated with a 17% lower rate of incident PAD (HR, 0.83; 95% CI, 0.78-0.88; P less than .001), according to the researchers.
The study showed that there was a significant prospective association between LS7 score and incident PAD in African Americans, Hispanics, and non-Hispanic whites. Although the association was not statistically significant for the Chinese Americans in MESA, this was likely because of the low number of incident PAD cases (only 18) in this group, according to the authors.
Analysis by individual LS7 components showed that more optimal levels of smoking, physical activity, glucose, and blood pressure were significantly associated with lower rates of incident PAD. Similarly, after adjustment for age, sex, race/ethnicity, income, education, and baseline ABI, more optimal levels of smoking, and glucose were significantly associated with lower odds of decline. These results for the decline in ABI did not appear to differ across race/ethnicity, according to Dr. Unkart and his colleagues.
In contrast, BMI, diet, and cholesterol were not associated with incident PAD or decline in ABI.
“Higher scores on the AHA LS7 were associated with lower incident PAD and less decline in ABI. Preventive measures targeting LS7 components could assist with reducing PAD-related morbidity and mortality,” the researchers concluded.
The work was supported by the National, Heart, Lung, and Blood Institute. Dr. Unkart and his colleagues reported that they had no disclosures.
SOURCE: Unkart JT et al. Am J Prev Med 2019;56:262-270.
Adherence to the American Heart Association’s Life’s Simple 7 is associated with lower incidence of peripheral artery disease and less decline in ankle brachial index, according to the results of a retrospective analysis of patients in the Multi-Ethnic Study of Atherosclerosis (MESA) trial.
“These results support the use of LS7 to prevent PAD and decline in ABI in multiple racial/ethnic groups,” according to Jonathan T. Unkart, MD, of the department of medicine and public health, University of California, San Diego, and his colleagues.
MESA recruited 6,814 men and women aged 45-84 years who were free of clinical cardiovascular disease. The cohort comprised 53% women and had the following racial/ethnic composition: 38% non-Hispanic white; 28% African American; 23% Hispanic, and 11% Asian. MESA consisted of six exams, with the baseline exam occurring from 2000 to 2002, including assessment of all LS7 components and PAD assessment using ABI calculated on both left and right sides. The final exam was performed from 2010 to 2012.
As background, the metrics for Life’s Simple 7 consist of total cholesterol, blood pressure, blood glucose, smoking status, body mass index, physical activity, and adherence to a healthy diet score. Each element can be scored 2 points for optimum, 1 for average, and 0 for inadequate. The investigators assessed overall LS7 scores on a continuous 0-14 scale, as well as the overall categorical indications of inadequate, average, and optimum. Cox proportional hazard models were used to assess the association of individual LS7 components by overall LS7 with incident PAD, according to the researchers.
Interactions of race/ethnicity by LS7 score were assessed on a multiplicative scale for both incident PAD and decline in ABI outcomes, adjusted for age, sex, education, and income (Am J Prev Med. 2019;56:262-70).
A total of 5,529 participants had complete LS7 information and met inclusion criteria to assess incident PAD. Over a median follow-up of 9.2 years, 251 (4.5%) participants developed incident PAD and 419 (9.8%) participants had a decline of at least 0.15 in ABI. In addition, each point higher on the continuous LS7 scale was associated with 0.94-fold lower odds of decline in ABI (odds ratio, 0.94; 95% confidence interval, 0.87-0.97; P =.003).
Each point higher on the continuous LS7 scale was associated with a 17% lower rate of incident PAD (HR, 0.83; 95% CI, 0.78-0.88; P less than .001), according to the researchers.
The study showed that there was a significant prospective association between LS7 score and incident PAD in African Americans, Hispanics, and non-Hispanic whites. Although the association was not statistically significant for the Chinese Americans in MESA, this was likely because of the low number of incident PAD cases (only 18) in this group, according to the authors.
Analysis by individual LS7 components showed that more optimal levels of smoking, physical activity, glucose, and blood pressure were significantly associated with lower rates of incident PAD. Similarly, after adjustment for age, sex, race/ethnicity, income, education, and baseline ABI, more optimal levels of smoking, and glucose were significantly associated with lower odds of decline. These results for the decline in ABI did not appear to differ across race/ethnicity, according to Dr. Unkart and his colleagues.
In contrast, BMI, diet, and cholesterol were not associated with incident PAD or decline in ABI.
“Higher scores on the AHA LS7 were associated with lower incident PAD and less decline in ABI. Preventive measures targeting LS7 components could assist with reducing PAD-related morbidity and mortality,” the researchers concluded.
The work was supported by the National, Heart, Lung, and Blood Institute. Dr. Unkart and his colleagues reported that they had no disclosures.
SOURCE: Unkart JT et al. Am J Prev Med 2019;56:262-270.
FROM THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Key clinical point: Life’s Simple 7 appears to be a valid tool for modifying PAD risk.
Major finding: Each point higher for the overall Life’s Simple 7 score was associated with a 17% lower rate of incident PAD (HR, 0.83; P less than .001).
Study details: Retrospective analysis of 5,529 individuals from the Multi-Ethnic Study of Atherosclerosis who were followed more than 10 years.
Disclosures: The work was supported by the National, Heart, Lung, and Blood Institute. The authors reported that they had no disclosures..
Source: Unkart JT et al. Am J Prev Med. 2019;56:262-70.
Statins cut vascular events in elderly patients
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
FROM THE LANCET
Key clinical point: but patients older than 75 years with occlusive vascular disease have a smaller reduction in major coronary events.
Major finding: Major vascular coronary events were reduced by 24% (risk ratio, 0.76; 95% confidence interval, 0.73-0.79) with a decrease in the reduction of coronary events among patients older than 75 years. Study details: A meta-analysis of 28 trials with 186,854 individuals undergoing statin therapy from the Cholesterol Treatment Trialists’ Collaboration.
Disclosures: This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
Source: Fulcher J et al. Lancet. 2019;393:407-15.
Immunotherapy’s cardiac effects require early monitoring, management
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
REPORTING FROM ACC CARDIO-ONCOLOGY
Key clinical point: Monitor for cardiac symptoms and treat or interrupt immunotherapy as needed.
Major finding: Immune checkpoint inhibitors and CAR T-cell therapies are associated with distinct cardiovascular adverse events.
Study details: Review of strategies for managing the cardiovascular consequences of cancer immunotherapies.
Disclosures: Dr. Cornell reported having nothing to disclose.
When is it safe to resume anticoagulation in my patient with hemorrhagic stroke?
Balancing risk is critical to decision making
Department of Medicine, Massachusetts General Hospital, Boston
Case
A 75 year-old woman with a history of hypertension, diabetes mellitus, heart failure and nonvalvular atrial fibrillation (CHA2DS2-VASc score, 8) on anticoagulation is admitted with weakness and dysarthria. Exam is notable for hypertension and right-sided hemiparesis. CT of the head shows an intraparenchymal hemorrhage in the left putamen. Her anticoagulation is reversed and blood pressure well controlled. She is discharged 12 days later.
Brief overview of the issue
Intracranial hemorrhage (ICH) is the second most common cause of stroke and is associated with high morbidity and mortality.1 It is estimated that 10%-15% of spontaneous ICH cases occur in patients on therapeutic anticoagulation for atrial fibrillation.2 As our population ages and more people develop atrial fibrillation, anticoagulation for primary or secondary prevention of embolic stroke also will likely increase, placing more people at risk for ICH. Even stringently controlled therapeutic international normalized ratios (INRs) between 2 and 3 may double the risk of ICH.3
Patients with ICH require close monitoring and treatment, including blood pressure control, reversal of anticoagulation, reduction of intracranial pressure and, at times, neurosurgery.4 Although anticoagulation is discontinued and reversed at the onset of ICH, no clear consensus exists as to when it is safe to resume it. Although anticoagulation decreases the risk of stroke/thromboembolism, it may also increase the amount of bleeding associated with the initial ICH or lead to its recurrence.
Factors that may contribute to rebleeding include uncontrolled hypertension, advanced age, time to resumption of anticoagulation, and lobar location of ICH (i.e., in cerebral cortex and/or underlying white matter).5 Traditionally, lobar ICH has high incidence of cerebral amyloid angiopathy and has been associated with higher bleeding rates than has deep ICH (i.e., involving the thalami, basal ganglia, cerebellum, or brainstem) where cerebral amyloid angiopathy is rare and ICH is usually from hypertensive vessel disease. However, in patients with active thromboembolic disease, high-risk atrial fibrillation, and mechanical valves, withholding anticoagulation could place them at high risk of stroke.
Two questions should be addressed in the case presented: Is it safe to restart therapeutic anticoagulation; and if so, what is the optimal time interval between ICH and reinitiation of anticoagulation?
Overview of the data
There is limited guidance from major professional societies regarding the reinitiation of anticoagulation and the optimal timing of safely resuming anticoagulation in patients with prior ICH.
Current European Stroke Organization guidelines provide no specific recommendations for anticoagulation resumption after ICH.7 The American Heart Association/American Stroke Association guideline has a class IIA (weak) recommendation to avoid anticoagulation in spontaneous lobar ICH and a class IIB (very weak) recommendation to consider resuming anticoagulation in nonlobar ICH on a case-by-case basis.4
Two recent meta-analyses have examined outcomes of resuming anticoagulation after ICH. In a meta-analysis of 5,300 patients with nonlobar ICH involving eight retrospective studies, Murthy et al. evaluated the risk of thromboembolic events (described as a composite outcome of MI and stroke) and the risk of recurrent ICH.8 They reported that resumption of therapeutic anticoagulation was associated with a decrease in the rate of thromboembolic events (6.7% vs. 17.6%; risk ratio, 0.35; 95% confidence interval, 0.25-0.45) with no significant change in the rate of repeat ICH (8.7% vs. 7.8%).
A second meta-analysis of three retrospective trials conducted by Biffi et al. examined anticoagulation resumption in 1,012 patients with ICH solely in the setting of thromboprophylaxis for nonvalvular atrial fibrillation.9 Reinitiation of anticoagulation after ICH was associated with decreased mortality (hazard ratio, 0.27; 95% CI, 0.19-0.40; P less than .0001), improved functional outcome (HR, 4.15; 95% CI, 2.92-5.90; P less than .0001), and reduction in all-cause stroke recurrence (HR 0.47; 95% CI, 0.36-0.64; P less than .0001). There was no significant difference in the rate of recurrent ICH when anticoagulation was resumed. Despite the notion that patients with cerebral amyloid angiopathy are at high risk of rebleeding, this positive association still held irrespective of lobar vs. nonlobar location of ICH.
Collectively, these studies suggest that resumption of anticoagulation may be effective in decreasing the rates of thromboembolism, as well as provide a functional and mortality benefit without increasing the risk of rebleeding, irrespective of the location of the bleed.
Less is known about the optimal timing of resumption of therapeutic anticoagulation, with data ranging from 72 hours to 30 weeks.10 The American Heart Association/American Stroke Association has a class IIB (very weak) recommendation to avoid anticoagulation for at least 4 weeks in patients without mechanical heart valves.4 The median time to resumption of therapeutic anticoagulation in aforementioned meta-analyses ranged from 10 to 44 days.8,9
A recent observational study of 2,619 ICH survivors explored the relationship between the timing of reinitiation of anticoagulation and the incidence of thrombotic events (defined as ischemic stroke or death because of MI or systemic arterial thromboembolism) and hemorrhagic events (defined as recurrent ICH or bleeding event leading to death) occurring at least 28 days after initial ICH in patients with atrial fibrillation.11
A decrease in thrombotic events was demonstrated if anticoagulation was started 4-16 weeks after ICH. However, when anticoagulation was started more than 16 weeks after ICH, no benefit was seen. Additionally, there was no significant difference in hemorrhagic events between men and women who resumed anticoagulation. In patients with high venous thromboembolism risk based on CHA2DS2-VASc score, resumption of anticoagulation was associated with a decreased predicted incidence of vascular death and nonfatal stroke, with the greatest benefit observed when anticoagulation was started at 7-8 weeks after ICH.
Unfortunately, published literature to date on anticoagulation after ICH is based entirely on retrospective studies – not randomized, controlled studies – making it more likely that anticoagulation would have been resumed in healthier patients, not those left debilitated by the ICH.
Furthermore, information on the location and size of the hemorrhages – which may serve as another confounding factor – often has not been reported. This is important since patients with smaller hemorrhages in less precarious areas also may be more likely to have resumption of anticoagulation. Another limitation of the current literature is that warfarin is the most common anticoagulant studied, with few studies involving the increasingly prescribed newer direct oral anticoagulants. It is also important to stress that a causal relationship between use of anticoagulants and certain outcomes or adverse effects following ICH may be more difficult to invoke in the absence of randomized controlled study designs.
Application of the data to our patient
Resumption of anticoagulation in our patient with ICH requires balancing the risk of hemorrhage expansion and recurrent ICH with the risk of thromboembolic disease.
Our patient is at higher risk of bleeding because of her advanced age, but adequate control of her blood pressure and nonlobar location of her ICH in the basal ganglia also may decrease her risk of recurrent ICH. Her high CHA2DS2-VASc score places her at high risk of thromboembolic event and stroke, making it more likely for reinitiation of anticoagulation to confer a mortality benefit.
Based on AHA guidelines,4 we should wait at least 4 weeks, or possibly wait until weeks 7-8 after ICH when the greatest benefit may be expected based on prediction models.11
Bottom line
It would likely be safe to resume anticoagulation 4-8 weeks after ICH in our patient.
Dr. Gibson, Dr. Restrepo, Dr. Sasidhara, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. An SJ et al. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke. 2017 Jan;19:3-10.
2. Horstmann S et al. Intracerebral hemorrhage during anticoagulation with vitamin K antagonists: a consecutive observational study. J Neurol. 2013 Aug;260:2046-51.
3. Rosand J et al. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004 Apr 26;164:880-4.
4. Hemphill JC et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015 Jul;46:2032-60.
5. Aguillar MI et al. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1:148-59.
6. Hill MD et al. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123-7.
7. Steiner T et al. European Stroke Organization (ESO) guidelines for the management of spontaneous cerebral hemorrhage. Int J Stroke. 2014;9:840-55.
8. Murthy SB et al. Restarting anticoagulation therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke. 2017 Jun;48:1594-600.
9. Biffi A et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017 Nov;82:755-65.
10. Witt DM. What to do after the bleed: Resuming anticoagulation after major bleeding. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;206:620-4.
11. Pennlert J et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017 Feb;48:314-20.
Key Points
- Robust scientific data on when to resume anticoagulation after ICH does not exist.
- Retrospective studies have shown that anticoagulation resumption after 4-8 weeks decreases the risk of thromboembolic events, decreases mortality, and improves functional status following ICH with no significant change in the risk of its recurrence.
- Prospective, randomized controlled trials are needed to explore risks/benefits of anticoagulation resumption and better define its optimal timing in relation to ICH.
Quiz
Which of the following is false regarding ICH?
A. Lobar ICHs are usually associated with cerebral amyloid angiopathy which are prone to bleeding.
B. Randomized, controlled studies have helped guide the decision as to when to resume anticoagulation in patients with ICH.
C. Current guidelines suggest deferring therapeutic anticoagulation for at least 4 weeks following ICH.
D. Resumption of anticoagulation after 4-8 weeks does not lead to increased risk of rebleeding in patients with prior ICH.
The false answer is B: Current recommendations regarding resumption of anticoagulation in patients with ICH are based solely on retrospective observational studies; there are no randomized, control trials to date.
A is true: In contrast to hypertensive vessel disease associated with deep ICH, lobar hemorrhages are usually associated with cerebral amyloid angiopathy, which are more prone to bleeding.
C is true: The AHA/ASA has a class IIB recommendation to avoid anticoagulation for at least 4 weeks after ICH in patients without mechanical heart valves.
D is true: Several studies have shown that resumption of anticoagulation 4-8 weeks after ICH does not increase the risk of rebleeding.
Balancing risk is critical to decision making
Balancing risk is critical to decision making
Department of Medicine, Massachusetts General Hospital, Boston
Case
A 75 year-old woman with a history of hypertension, diabetes mellitus, heart failure and nonvalvular atrial fibrillation (CHA2DS2-VASc score, 8) on anticoagulation is admitted with weakness and dysarthria. Exam is notable for hypertension and right-sided hemiparesis. CT of the head shows an intraparenchymal hemorrhage in the left putamen. Her anticoagulation is reversed and blood pressure well controlled. She is discharged 12 days later.
Brief overview of the issue
Intracranial hemorrhage (ICH) is the second most common cause of stroke and is associated with high morbidity and mortality.1 It is estimated that 10%-15% of spontaneous ICH cases occur in patients on therapeutic anticoagulation for atrial fibrillation.2 As our population ages and more people develop atrial fibrillation, anticoagulation for primary or secondary prevention of embolic stroke also will likely increase, placing more people at risk for ICH. Even stringently controlled therapeutic international normalized ratios (INRs) between 2 and 3 may double the risk of ICH.3
Patients with ICH require close monitoring and treatment, including blood pressure control, reversal of anticoagulation, reduction of intracranial pressure and, at times, neurosurgery.4 Although anticoagulation is discontinued and reversed at the onset of ICH, no clear consensus exists as to when it is safe to resume it. Although anticoagulation decreases the risk of stroke/thromboembolism, it may also increase the amount of bleeding associated with the initial ICH or lead to its recurrence.
Factors that may contribute to rebleeding include uncontrolled hypertension, advanced age, time to resumption of anticoagulation, and lobar location of ICH (i.e., in cerebral cortex and/or underlying white matter).5 Traditionally, lobar ICH has high incidence of cerebral amyloid angiopathy and has been associated with higher bleeding rates than has deep ICH (i.e., involving the thalami, basal ganglia, cerebellum, or brainstem) where cerebral amyloid angiopathy is rare and ICH is usually from hypertensive vessel disease. However, in patients with active thromboembolic disease, high-risk atrial fibrillation, and mechanical valves, withholding anticoagulation could place them at high risk of stroke.
Two questions should be addressed in the case presented: Is it safe to restart therapeutic anticoagulation; and if so, what is the optimal time interval between ICH and reinitiation of anticoagulation?
Overview of the data
There is limited guidance from major professional societies regarding the reinitiation of anticoagulation and the optimal timing of safely resuming anticoagulation in patients with prior ICH.
Current European Stroke Organization guidelines provide no specific recommendations for anticoagulation resumption after ICH.7 The American Heart Association/American Stroke Association guideline has a class IIA (weak) recommendation to avoid anticoagulation in spontaneous lobar ICH and a class IIB (very weak) recommendation to consider resuming anticoagulation in nonlobar ICH on a case-by-case basis.4
Two recent meta-analyses have examined outcomes of resuming anticoagulation after ICH. In a meta-analysis of 5,300 patients with nonlobar ICH involving eight retrospective studies, Murthy et al. evaluated the risk of thromboembolic events (described as a composite outcome of MI and stroke) and the risk of recurrent ICH.8 They reported that resumption of therapeutic anticoagulation was associated with a decrease in the rate of thromboembolic events (6.7% vs. 17.6%; risk ratio, 0.35; 95% confidence interval, 0.25-0.45) with no significant change in the rate of repeat ICH (8.7% vs. 7.8%).
A second meta-analysis of three retrospective trials conducted by Biffi et al. examined anticoagulation resumption in 1,012 patients with ICH solely in the setting of thromboprophylaxis for nonvalvular atrial fibrillation.9 Reinitiation of anticoagulation after ICH was associated with decreased mortality (hazard ratio, 0.27; 95% CI, 0.19-0.40; P less than .0001), improved functional outcome (HR, 4.15; 95% CI, 2.92-5.90; P less than .0001), and reduction in all-cause stroke recurrence (HR 0.47; 95% CI, 0.36-0.64; P less than .0001). There was no significant difference in the rate of recurrent ICH when anticoagulation was resumed. Despite the notion that patients with cerebral amyloid angiopathy are at high risk of rebleeding, this positive association still held irrespective of lobar vs. nonlobar location of ICH.
Collectively, these studies suggest that resumption of anticoagulation may be effective in decreasing the rates of thromboembolism, as well as provide a functional and mortality benefit without increasing the risk of rebleeding, irrespective of the location of the bleed.
Less is known about the optimal timing of resumption of therapeutic anticoagulation, with data ranging from 72 hours to 30 weeks.10 The American Heart Association/American Stroke Association has a class IIB (very weak) recommendation to avoid anticoagulation for at least 4 weeks in patients without mechanical heart valves.4 The median time to resumption of therapeutic anticoagulation in aforementioned meta-analyses ranged from 10 to 44 days.8,9
A recent observational study of 2,619 ICH survivors explored the relationship between the timing of reinitiation of anticoagulation and the incidence of thrombotic events (defined as ischemic stroke or death because of MI or systemic arterial thromboembolism) and hemorrhagic events (defined as recurrent ICH or bleeding event leading to death) occurring at least 28 days after initial ICH in patients with atrial fibrillation.11
A decrease in thrombotic events was demonstrated if anticoagulation was started 4-16 weeks after ICH. However, when anticoagulation was started more than 16 weeks after ICH, no benefit was seen. Additionally, there was no significant difference in hemorrhagic events between men and women who resumed anticoagulation. In patients with high venous thromboembolism risk based on CHA2DS2-VASc score, resumption of anticoagulation was associated with a decreased predicted incidence of vascular death and nonfatal stroke, with the greatest benefit observed when anticoagulation was started at 7-8 weeks after ICH.
Unfortunately, published literature to date on anticoagulation after ICH is based entirely on retrospective studies – not randomized, controlled studies – making it more likely that anticoagulation would have been resumed in healthier patients, not those left debilitated by the ICH.
Furthermore, information on the location and size of the hemorrhages – which may serve as another confounding factor – often has not been reported. This is important since patients with smaller hemorrhages in less precarious areas also may be more likely to have resumption of anticoagulation. Another limitation of the current literature is that warfarin is the most common anticoagulant studied, with few studies involving the increasingly prescribed newer direct oral anticoagulants. It is also important to stress that a causal relationship between use of anticoagulants and certain outcomes or adverse effects following ICH may be more difficult to invoke in the absence of randomized controlled study designs.
Application of the data to our patient
Resumption of anticoagulation in our patient with ICH requires balancing the risk of hemorrhage expansion and recurrent ICH with the risk of thromboembolic disease.
Our patient is at higher risk of bleeding because of her advanced age, but adequate control of her blood pressure and nonlobar location of her ICH in the basal ganglia also may decrease her risk of recurrent ICH. Her high CHA2DS2-VASc score places her at high risk of thromboembolic event and stroke, making it more likely for reinitiation of anticoagulation to confer a mortality benefit.
Based on AHA guidelines,4 we should wait at least 4 weeks, or possibly wait until weeks 7-8 after ICH when the greatest benefit may be expected based on prediction models.11
Bottom line
It would likely be safe to resume anticoagulation 4-8 weeks after ICH in our patient.
Dr. Gibson, Dr. Restrepo, Dr. Sasidhara, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. An SJ et al. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke. 2017 Jan;19:3-10.
2. Horstmann S et al. Intracerebral hemorrhage during anticoagulation with vitamin K antagonists: a consecutive observational study. J Neurol. 2013 Aug;260:2046-51.
3. Rosand J et al. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004 Apr 26;164:880-4.
4. Hemphill JC et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015 Jul;46:2032-60.
5. Aguillar MI et al. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1:148-59.
6. Hill MD et al. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123-7.
7. Steiner T et al. European Stroke Organization (ESO) guidelines for the management of spontaneous cerebral hemorrhage. Int J Stroke. 2014;9:840-55.
8. Murthy SB et al. Restarting anticoagulation therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke. 2017 Jun;48:1594-600.
9. Biffi A et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017 Nov;82:755-65.
10. Witt DM. What to do after the bleed: Resuming anticoagulation after major bleeding. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;206:620-4.
11. Pennlert J et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017 Feb;48:314-20.
Key Points
- Robust scientific data on when to resume anticoagulation after ICH does not exist.
- Retrospective studies have shown that anticoagulation resumption after 4-8 weeks decreases the risk of thromboembolic events, decreases mortality, and improves functional status following ICH with no significant change in the risk of its recurrence.
- Prospective, randomized controlled trials are needed to explore risks/benefits of anticoagulation resumption and better define its optimal timing in relation to ICH.
Quiz
Which of the following is false regarding ICH?
A. Lobar ICHs are usually associated with cerebral amyloid angiopathy which are prone to bleeding.
B. Randomized, controlled studies have helped guide the decision as to when to resume anticoagulation in patients with ICH.
C. Current guidelines suggest deferring therapeutic anticoagulation for at least 4 weeks following ICH.
D. Resumption of anticoagulation after 4-8 weeks does not lead to increased risk of rebleeding in patients with prior ICH.
The false answer is B: Current recommendations regarding resumption of anticoagulation in patients with ICH are based solely on retrospective observational studies; there are no randomized, control trials to date.
A is true: In contrast to hypertensive vessel disease associated with deep ICH, lobar hemorrhages are usually associated with cerebral amyloid angiopathy, which are more prone to bleeding.
C is true: The AHA/ASA has a class IIB recommendation to avoid anticoagulation for at least 4 weeks after ICH in patients without mechanical heart valves.
D is true: Several studies have shown that resumption of anticoagulation 4-8 weeks after ICH does not increase the risk of rebleeding.
Department of Medicine, Massachusetts General Hospital, Boston
Case
A 75 year-old woman with a history of hypertension, diabetes mellitus, heart failure and nonvalvular atrial fibrillation (CHA2DS2-VASc score, 8) on anticoagulation is admitted with weakness and dysarthria. Exam is notable for hypertension and right-sided hemiparesis. CT of the head shows an intraparenchymal hemorrhage in the left putamen. Her anticoagulation is reversed and blood pressure well controlled. She is discharged 12 days later.
Brief overview of the issue
Intracranial hemorrhage (ICH) is the second most common cause of stroke and is associated with high morbidity and mortality.1 It is estimated that 10%-15% of spontaneous ICH cases occur in patients on therapeutic anticoagulation for atrial fibrillation.2 As our population ages and more people develop atrial fibrillation, anticoagulation for primary or secondary prevention of embolic stroke also will likely increase, placing more people at risk for ICH. Even stringently controlled therapeutic international normalized ratios (INRs) between 2 and 3 may double the risk of ICH.3
Patients with ICH require close monitoring and treatment, including blood pressure control, reversal of anticoagulation, reduction of intracranial pressure and, at times, neurosurgery.4 Although anticoagulation is discontinued and reversed at the onset of ICH, no clear consensus exists as to when it is safe to resume it. Although anticoagulation decreases the risk of stroke/thromboembolism, it may also increase the amount of bleeding associated with the initial ICH or lead to its recurrence.
Factors that may contribute to rebleeding include uncontrolled hypertension, advanced age, time to resumption of anticoagulation, and lobar location of ICH (i.e., in cerebral cortex and/or underlying white matter).5 Traditionally, lobar ICH has high incidence of cerebral amyloid angiopathy and has been associated with higher bleeding rates than has deep ICH (i.e., involving the thalami, basal ganglia, cerebellum, or brainstem) where cerebral amyloid angiopathy is rare and ICH is usually from hypertensive vessel disease. However, in patients with active thromboembolic disease, high-risk atrial fibrillation, and mechanical valves, withholding anticoagulation could place them at high risk of stroke.
Two questions should be addressed in the case presented: Is it safe to restart therapeutic anticoagulation; and if so, what is the optimal time interval between ICH and reinitiation of anticoagulation?
Overview of the data
There is limited guidance from major professional societies regarding the reinitiation of anticoagulation and the optimal timing of safely resuming anticoagulation in patients with prior ICH.
Current European Stroke Organization guidelines provide no specific recommendations for anticoagulation resumption after ICH.7 The American Heart Association/American Stroke Association guideline has a class IIA (weak) recommendation to avoid anticoagulation in spontaneous lobar ICH and a class IIB (very weak) recommendation to consider resuming anticoagulation in nonlobar ICH on a case-by-case basis.4
Two recent meta-analyses have examined outcomes of resuming anticoagulation after ICH. In a meta-analysis of 5,300 patients with nonlobar ICH involving eight retrospective studies, Murthy et al. evaluated the risk of thromboembolic events (described as a composite outcome of MI and stroke) and the risk of recurrent ICH.8 They reported that resumption of therapeutic anticoagulation was associated with a decrease in the rate of thromboembolic events (6.7% vs. 17.6%; risk ratio, 0.35; 95% confidence interval, 0.25-0.45) with no significant change in the rate of repeat ICH (8.7% vs. 7.8%).
A second meta-analysis of three retrospective trials conducted by Biffi et al. examined anticoagulation resumption in 1,012 patients with ICH solely in the setting of thromboprophylaxis for nonvalvular atrial fibrillation.9 Reinitiation of anticoagulation after ICH was associated with decreased mortality (hazard ratio, 0.27; 95% CI, 0.19-0.40; P less than .0001), improved functional outcome (HR, 4.15; 95% CI, 2.92-5.90; P less than .0001), and reduction in all-cause stroke recurrence (HR 0.47; 95% CI, 0.36-0.64; P less than .0001). There was no significant difference in the rate of recurrent ICH when anticoagulation was resumed. Despite the notion that patients with cerebral amyloid angiopathy are at high risk of rebleeding, this positive association still held irrespective of lobar vs. nonlobar location of ICH.
Collectively, these studies suggest that resumption of anticoagulation may be effective in decreasing the rates of thromboembolism, as well as provide a functional and mortality benefit without increasing the risk of rebleeding, irrespective of the location of the bleed.
Less is known about the optimal timing of resumption of therapeutic anticoagulation, with data ranging from 72 hours to 30 weeks.10 The American Heart Association/American Stroke Association has a class IIB (very weak) recommendation to avoid anticoagulation for at least 4 weeks in patients without mechanical heart valves.4 The median time to resumption of therapeutic anticoagulation in aforementioned meta-analyses ranged from 10 to 44 days.8,9
A recent observational study of 2,619 ICH survivors explored the relationship between the timing of reinitiation of anticoagulation and the incidence of thrombotic events (defined as ischemic stroke or death because of MI or systemic arterial thromboembolism) and hemorrhagic events (defined as recurrent ICH or bleeding event leading to death) occurring at least 28 days after initial ICH in patients with atrial fibrillation.11
A decrease in thrombotic events was demonstrated if anticoagulation was started 4-16 weeks after ICH. However, when anticoagulation was started more than 16 weeks after ICH, no benefit was seen. Additionally, there was no significant difference in hemorrhagic events between men and women who resumed anticoagulation. In patients with high venous thromboembolism risk based on CHA2DS2-VASc score, resumption of anticoagulation was associated with a decreased predicted incidence of vascular death and nonfatal stroke, with the greatest benefit observed when anticoagulation was started at 7-8 weeks after ICH.
Unfortunately, published literature to date on anticoagulation after ICH is based entirely on retrospective studies – not randomized, controlled studies – making it more likely that anticoagulation would have been resumed in healthier patients, not those left debilitated by the ICH.
Furthermore, information on the location and size of the hemorrhages – which may serve as another confounding factor – often has not been reported. This is important since patients with smaller hemorrhages in less precarious areas also may be more likely to have resumption of anticoagulation. Another limitation of the current literature is that warfarin is the most common anticoagulant studied, with few studies involving the increasingly prescribed newer direct oral anticoagulants. It is also important to stress that a causal relationship between use of anticoagulants and certain outcomes or adverse effects following ICH may be more difficult to invoke in the absence of randomized controlled study designs.
Application of the data to our patient
Resumption of anticoagulation in our patient with ICH requires balancing the risk of hemorrhage expansion and recurrent ICH with the risk of thromboembolic disease.
Our patient is at higher risk of bleeding because of her advanced age, but adequate control of her blood pressure and nonlobar location of her ICH in the basal ganglia also may decrease her risk of recurrent ICH. Her high CHA2DS2-VASc score places her at high risk of thromboembolic event and stroke, making it more likely for reinitiation of anticoagulation to confer a mortality benefit.
Based on AHA guidelines,4 we should wait at least 4 weeks, or possibly wait until weeks 7-8 after ICH when the greatest benefit may be expected based on prediction models.11
Bottom line
It would likely be safe to resume anticoagulation 4-8 weeks after ICH in our patient.
Dr. Gibson, Dr. Restrepo, Dr. Sasidhara, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. An SJ et al. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke. 2017 Jan;19:3-10.
2. Horstmann S et al. Intracerebral hemorrhage during anticoagulation with vitamin K antagonists: a consecutive observational study. J Neurol. 2013 Aug;260:2046-51.
3. Rosand J et al. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004 Apr 26;164:880-4.
4. Hemphill JC et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015 Jul;46:2032-60.
5. Aguillar MI et al. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1:148-59.
6. Hill MD et al. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123-7.
7. Steiner T et al. European Stroke Organization (ESO) guidelines for the management of spontaneous cerebral hemorrhage. Int J Stroke. 2014;9:840-55.
8. Murthy SB et al. Restarting anticoagulation therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke. 2017 Jun;48:1594-600.
9. Biffi A et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017 Nov;82:755-65.
10. Witt DM. What to do after the bleed: Resuming anticoagulation after major bleeding. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;206:620-4.
11. Pennlert J et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017 Feb;48:314-20.
Key Points
- Robust scientific data on when to resume anticoagulation after ICH does not exist.
- Retrospective studies have shown that anticoagulation resumption after 4-8 weeks decreases the risk of thromboembolic events, decreases mortality, and improves functional status following ICH with no significant change in the risk of its recurrence.
- Prospective, randomized controlled trials are needed to explore risks/benefits of anticoagulation resumption and better define its optimal timing in relation to ICH.
Quiz
Which of the following is false regarding ICH?
A. Lobar ICHs are usually associated with cerebral amyloid angiopathy which are prone to bleeding.
B. Randomized, controlled studies have helped guide the decision as to when to resume anticoagulation in patients with ICH.
C. Current guidelines suggest deferring therapeutic anticoagulation for at least 4 weeks following ICH.
D. Resumption of anticoagulation after 4-8 weeks does not lead to increased risk of rebleeding in patients with prior ICH.
The false answer is B: Current recommendations regarding resumption of anticoagulation in patients with ICH are based solely on retrospective observational studies; there are no randomized, control trials to date.
A is true: In contrast to hypertensive vessel disease associated with deep ICH, lobar hemorrhages are usually associated with cerebral amyloid angiopathy, which are more prone to bleeding.
C is true: The AHA/ASA has a class IIB recommendation to avoid anticoagulation for at least 4 weeks after ICH in patients without mechanical heart valves.
D is true: Several studies have shown that resumption of anticoagulation 4-8 weeks after ICH does not increase the risk of rebleeding.
Medical advice prompts unneeded emergency visits by AF patients
BOSTON – Patients with atrial fibrillation who present to emergency departments, despite being asymptomatic, often go based on of their understanding of advice they had previously received from their physicians, according to results from a prospective study of 356 Canadian atrial arrhythmia patients seen in emergency settings.
One way to deal with potentially inappropriate emergency department use is to have concerned patients with atrial fibrillation (AF) record their heart rhythm data with a handheld device or watch, transfer the records to their smartphones, and transmit the information to a remote physician for interpretation and advice, Benedict M. Glover, MD, said at the annual International AF Symposium.
Dr. Glover and his associates are in the process of developing a prototype system of this design to address the need they identified in a recent registry of 356 patients with a primary diagnosis of AF who sought care in the emergency department (ED) of any of seven participating Canadian medical centers, including five academic centers and two community hospitals. The survey results showed that 71% of the patients were symptomatic and 29% were asymptomatic then they first presented to an emergency department.
Case reviews of the 356 patients showed that 152 (43%) came to the EDs for what were classified as inappropriate reasons. The most common cause by far of an inappropriate emergency presentation was prior medical advice the patient had received, cited in 62% of the inappropriate cases, compared with 9% of the appropriate cases, said Dr. Glover, an electrophysiologist at Sunnybrook Health Sciences Centre in Toronto.
The inappropriate ED use by AF patients could be addressed in at least two ways, he said. One solution might be to give patients an alternative destination, so that instead of going to an emergency department they could go to an outpatient AF clinic. A second solution is to give patients a way to have their heart rhythm assessed remotely at the time of their concern. Dr. Glover said that his center had the staff capacity to deal with the potential influx of rhythm data from a pilot-sized program of remote heart-rhythm monitoring, but he conceded that scaling up to deal with the data that could come from the entire panel of AF patients managed by Sunnybrook physicians would be a huge challenge.
“The issue is what do we do with the data after we get it,” Dr. Glover said. “It’s a lot of information.”
Dr. Glover had no disclosures.
BOSTON – Patients with atrial fibrillation who present to emergency departments, despite being asymptomatic, often go based on of their understanding of advice they had previously received from their physicians, according to results from a prospective study of 356 Canadian atrial arrhythmia patients seen in emergency settings.
One way to deal with potentially inappropriate emergency department use is to have concerned patients with atrial fibrillation (AF) record their heart rhythm data with a handheld device or watch, transfer the records to their smartphones, and transmit the information to a remote physician for interpretation and advice, Benedict M. Glover, MD, said at the annual International AF Symposium.
Dr. Glover and his associates are in the process of developing a prototype system of this design to address the need they identified in a recent registry of 356 patients with a primary diagnosis of AF who sought care in the emergency department (ED) of any of seven participating Canadian medical centers, including five academic centers and two community hospitals. The survey results showed that 71% of the patients were symptomatic and 29% were asymptomatic then they first presented to an emergency department.
Case reviews of the 356 patients showed that 152 (43%) came to the EDs for what were classified as inappropriate reasons. The most common cause by far of an inappropriate emergency presentation was prior medical advice the patient had received, cited in 62% of the inappropriate cases, compared with 9% of the appropriate cases, said Dr. Glover, an electrophysiologist at Sunnybrook Health Sciences Centre in Toronto.
The inappropriate ED use by AF patients could be addressed in at least two ways, he said. One solution might be to give patients an alternative destination, so that instead of going to an emergency department they could go to an outpatient AF clinic. A second solution is to give patients a way to have their heart rhythm assessed remotely at the time of their concern. Dr. Glover said that his center had the staff capacity to deal with the potential influx of rhythm data from a pilot-sized program of remote heart-rhythm monitoring, but he conceded that scaling up to deal with the data that could come from the entire panel of AF patients managed by Sunnybrook physicians would be a huge challenge.
“The issue is what do we do with the data after we get it,” Dr. Glover said. “It’s a lot of information.”
Dr. Glover had no disclosures.
BOSTON – Patients with atrial fibrillation who present to emergency departments, despite being asymptomatic, often go based on of their understanding of advice they had previously received from their physicians, according to results from a prospective study of 356 Canadian atrial arrhythmia patients seen in emergency settings.
One way to deal with potentially inappropriate emergency department use is to have concerned patients with atrial fibrillation (AF) record their heart rhythm data with a handheld device or watch, transfer the records to their smartphones, and transmit the information to a remote physician for interpretation and advice, Benedict M. Glover, MD, said at the annual International AF Symposium.
Dr. Glover and his associates are in the process of developing a prototype system of this design to address the need they identified in a recent registry of 356 patients with a primary diagnosis of AF who sought care in the emergency department (ED) of any of seven participating Canadian medical centers, including five academic centers and two community hospitals. The survey results showed that 71% of the patients were symptomatic and 29% were asymptomatic then they first presented to an emergency department.
Case reviews of the 356 patients showed that 152 (43%) came to the EDs for what were classified as inappropriate reasons. The most common cause by far of an inappropriate emergency presentation was prior medical advice the patient had received, cited in 62% of the inappropriate cases, compared with 9% of the appropriate cases, said Dr. Glover, an electrophysiologist at Sunnybrook Health Sciences Centre in Toronto.
The inappropriate ED use by AF patients could be addressed in at least two ways, he said. One solution might be to give patients an alternative destination, so that instead of going to an emergency department they could go to an outpatient AF clinic. A second solution is to give patients a way to have their heart rhythm assessed remotely at the time of their concern. Dr. Glover said that his center had the staff capacity to deal with the potential influx of rhythm data from a pilot-sized program of remote heart-rhythm monitoring, but he conceded that scaling up to deal with the data that could come from the entire panel of AF patients managed by Sunnybrook physicians would be a huge challenge.
“The issue is what do we do with the data after we get it,” Dr. Glover said. “It’s a lot of information.”
Dr. Glover had no disclosures.
REPORTING FROM THE AF SYMPOSIUM 2019
Key clinical point:
Major finding: Among 152 AF patients who made an inappropriate ED visit, 62% cited their prior medical advice.
Study details: Prospective study of 356 AF patients who sought ED care at any of seven Canadian hospitals.
Disclosures: Dr. Glover had no disclosures.
New recall for CoaguChek test strips issued
According to a release, the Food and Drug Administration has identified this recall as Class I, which is the most serious type of recall and indicates that “use of these devices may cause serious injuries or death.”
These strips are used by patients taking warfarin to help determine the patients’ international normalized ratio, which doctors and patients then use to decide whether the dose is appropriate. Roche Diagnostics, the strips’ manufacturer, issued a recall in September 2018; the test strips distributed by Terrific Care and Medex, however, were not labeled or authorized for sale in the United States and were therefore not included in that original recall. According to the release, the strips in this recall, which was initiated Dec. 21, 2018, were purchased by Terrific Care and Medex from an unknown source and then distributed in the United States. On Jan. 28, 2019, Terrific Care sent an Urgent Medical Device Recall Notification Letter to customers.
The full recall is described on the FDA website.
According to a release, the Food and Drug Administration has identified this recall as Class I, which is the most serious type of recall and indicates that “use of these devices may cause serious injuries or death.”
These strips are used by patients taking warfarin to help determine the patients’ international normalized ratio, which doctors and patients then use to decide whether the dose is appropriate. Roche Diagnostics, the strips’ manufacturer, issued a recall in September 2018; the test strips distributed by Terrific Care and Medex, however, were not labeled or authorized for sale in the United States and were therefore not included in that original recall. According to the release, the strips in this recall, which was initiated Dec. 21, 2018, were purchased by Terrific Care and Medex from an unknown source and then distributed in the United States. On Jan. 28, 2019, Terrific Care sent an Urgent Medical Device Recall Notification Letter to customers.
The full recall is described on the FDA website.
According to a release, the Food and Drug Administration has identified this recall as Class I, which is the most serious type of recall and indicates that “use of these devices may cause serious injuries or death.”
These strips are used by patients taking warfarin to help determine the patients’ international normalized ratio, which doctors and patients then use to decide whether the dose is appropriate. Roche Diagnostics, the strips’ manufacturer, issued a recall in September 2018; the test strips distributed by Terrific Care and Medex, however, were not labeled or authorized for sale in the United States and were therefore not included in that original recall. According to the release, the strips in this recall, which was initiated Dec. 21, 2018, were purchased by Terrific Care and Medex from an unknown source and then distributed in the United States. On Jan. 28, 2019, Terrific Care sent an Urgent Medical Device Recall Notification Letter to customers.
The full recall is described on the FDA website.