User login
Lifeline calls spike after Robin Williams’ suicide
Suicides and calls to the National Suicide Prevention Lifeline spiked after the suicide of actor Robin Williams, based on data from calls and website visits before and after his death.
Suicides in the United States tend to follow temporal patterns, with spikes in the spring and early summer, but “some events, including celebrity deaths, serve as ‘shocks’ that disrupt seasonal time trends and may prompt imitation,” wrote Rajeev Ramchand, PhD, of the National Institute of Mental Health, Bethesda, Md., and colleagues in a study published online in the journal Psychiatric Services in Advance (2019 Apr 30. doi: 10.1176/appi.ps.201900007).
The National Suicide Prevention Lifeline (NSPL) experienced a 300% increase in call volume the day after Mr. Williams’ death, however, only 57% of these calls were answered, the researchers said.
The researchers compared daily suicide data, NSPL call volume, and visits to two suicide prevention websites before and after Mr. Williams’ death on August 11, 2014.
Before August 11 in 2012, 2013, and 2014, the average number of daily suicides ranged from 113 to 117; after August 11, 2014, this average spiked to 142, an increase not seen in 2012 or 2013, according to data from the National Center for Health Statistics’ Compressed Mortality File. The NSPL received 12,972 calls on August 12, 2014, following Mr. Williams’ death, compared with a daily average of 4,116 to 6,302 calls during the week before his death. In addition, the Suicide Prevention Resource Center (SPRC), a website that provides technical assistance, training, and suicide prevention material; and Suicide Awareness Voices of Education (SAVE), a website with resources for individuals affected by suicide, as well educational information to raise public awareness, saw significant increases in visits on the day after Mr. Williams’ suicide.
The study findings were limited by several factors including the lack of information on whether calls to NSPL were information seekers or individuals in crisis, the researchers noted. However, the results suggest the need for surge capacity to prepare for increased demand in the wake of a celebrity suicide, they said.
The researchers had no financial conflicts to disclose.
SOURCE: Ramchand R et al. Psychiatric Services in Advance. 2019. doi: 10.1176/appi.ps.201900007 .
Suicides and calls to the National Suicide Prevention Lifeline spiked after the suicide of actor Robin Williams, based on data from calls and website visits before and after his death.
Suicides in the United States tend to follow temporal patterns, with spikes in the spring and early summer, but “some events, including celebrity deaths, serve as ‘shocks’ that disrupt seasonal time trends and may prompt imitation,” wrote Rajeev Ramchand, PhD, of the National Institute of Mental Health, Bethesda, Md., and colleagues in a study published online in the journal Psychiatric Services in Advance (2019 Apr 30. doi: 10.1176/appi.ps.201900007).
The National Suicide Prevention Lifeline (NSPL) experienced a 300% increase in call volume the day after Mr. Williams’ death, however, only 57% of these calls were answered, the researchers said.
The researchers compared daily suicide data, NSPL call volume, and visits to two suicide prevention websites before and after Mr. Williams’ death on August 11, 2014.
Before August 11 in 2012, 2013, and 2014, the average number of daily suicides ranged from 113 to 117; after August 11, 2014, this average spiked to 142, an increase not seen in 2012 or 2013, according to data from the National Center for Health Statistics’ Compressed Mortality File. The NSPL received 12,972 calls on August 12, 2014, following Mr. Williams’ death, compared with a daily average of 4,116 to 6,302 calls during the week before his death. In addition, the Suicide Prevention Resource Center (SPRC), a website that provides technical assistance, training, and suicide prevention material; and Suicide Awareness Voices of Education (SAVE), a website with resources for individuals affected by suicide, as well educational information to raise public awareness, saw significant increases in visits on the day after Mr. Williams’ suicide.
The study findings were limited by several factors including the lack of information on whether calls to NSPL were information seekers or individuals in crisis, the researchers noted. However, the results suggest the need for surge capacity to prepare for increased demand in the wake of a celebrity suicide, they said.
The researchers had no financial conflicts to disclose.
SOURCE: Ramchand R et al. Psychiatric Services in Advance. 2019. doi: 10.1176/appi.ps.201900007 .
Suicides and calls to the National Suicide Prevention Lifeline spiked after the suicide of actor Robin Williams, based on data from calls and website visits before and after his death.
Suicides in the United States tend to follow temporal patterns, with spikes in the spring and early summer, but “some events, including celebrity deaths, serve as ‘shocks’ that disrupt seasonal time trends and may prompt imitation,” wrote Rajeev Ramchand, PhD, of the National Institute of Mental Health, Bethesda, Md., and colleagues in a study published online in the journal Psychiatric Services in Advance (2019 Apr 30. doi: 10.1176/appi.ps.201900007).
The National Suicide Prevention Lifeline (NSPL) experienced a 300% increase in call volume the day after Mr. Williams’ death, however, only 57% of these calls were answered, the researchers said.
The researchers compared daily suicide data, NSPL call volume, and visits to two suicide prevention websites before and after Mr. Williams’ death on August 11, 2014.
Before August 11 in 2012, 2013, and 2014, the average number of daily suicides ranged from 113 to 117; after August 11, 2014, this average spiked to 142, an increase not seen in 2012 or 2013, according to data from the National Center for Health Statistics’ Compressed Mortality File. The NSPL received 12,972 calls on August 12, 2014, following Mr. Williams’ death, compared with a daily average of 4,116 to 6,302 calls during the week before his death. In addition, the Suicide Prevention Resource Center (SPRC), a website that provides technical assistance, training, and suicide prevention material; and Suicide Awareness Voices of Education (SAVE), a website with resources for individuals affected by suicide, as well educational information to raise public awareness, saw significant increases in visits on the day after Mr. Williams’ suicide.
The study findings were limited by several factors including the lack of information on whether calls to NSPL were information seekers or individuals in crisis, the researchers noted. However, the results suggest the need for surge capacity to prepare for increased demand in the wake of a celebrity suicide, they said.
The researchers had no financial conflicts to disclose.
SOURCE: Ramchand R et al. Psychiatric Services in Advance. 2019. doi: 10.1176/appi.ps.201900007 .
FROM PS IN ADVANCE
Cluster headache is associated with increased suicidality
Short- and long-term cluster headache disease burden, as well as depressive symptoms, contributes to suicidality, according to research published online Cephalalgia. Development of treatments that reduce the headache-related burden and prevent future bouts could reduce suicidality, said the researchers.
Although cluster headache has been called the “suicide headache,” few studies have examined suicidality in patients with cluster headache. Research by Rozen et al. found that the rate of suicidal attempt among patients was similar to that among the general population. The results have not been replicated, however, and the investigators did not examine whether suicidality varied according to the phases of the disorder.
A prospective, multicenter study
Mi Ji Lee, MD, PhD, clinical assistant professor of neurology at Samsung Medical Center in Seoul, South Korea, and colleagues conducted a prospective study to investigate the suicidality associated with cluster headache and the factors associated with increased suicidality in that disorder. The researchers enrolled 193 consecutive patients with cluster headache between September 2016 and August 2018 at 15 hospitals. They examined the patients and used the Patient Health Questionnaire–9 (PHQ-9) and the General Anxiety Disorder–7 item scale (GAD-7) screening tools. During the ictal and interictal phases, the researchers asked the patients whether they had had passive suicidal ideation, active suicidal ideation, suicidal planning, or suicidal attempt. Dr. Ji Lee and colleagues performed univariable and multivariable logistic regression analyses to evaluate the factors associated with high ictal suicidality, which was defined as two or more positive responses during the ictal phase. Participants were followed up during the between-bout phase.
The researchers excluded 18 patients from analysis because they were between bouts at enrollment. The mean age of the remaining 175 patients was 38.4 years. Mean age at onset was 29.9 years. About 85% of the patients were male. The diagnosis was definite cluster headache for 87.4% of the sample and probable cluster headache for 12.6%. In addition, 88% of the population had episodic cluster headache.
Suicidal ideation increased during the ictal phase
During the ictal phase, 64.2% of participants reported passive suicidal ideation, and 35.8% reported active suicidal ideation. Furthermore, 5.8% of patients had a suicidal plan, and 2.3% attempted suicide. In the interictal phase, 4.0% of patients reported passive suicidal ideation, and 3.5% reported active suicidal ideation. Interictal suicidal planning was reported by 2.9% of participants, and 1.2% of participants attempted suicide interictally. The results were similar between patients with definite and probable cluster headache.
The ictal phase increased the odds of passive suicidal ideation (odds ratio [OR], 42.46), active suicidal ideation (OR, 15.55), suicidal planning (OR, 2.06), and suicidal attempt (OR, 2.02), compared with the interictal phase. The differences in suicidal planning and suicidal attempt between the ictal and interictal phases, however, were not statistically significant.
Longer disease duration, higher attack intensity, higher Headache Impact Test–6 (HIT-6) score, GAD-7 score, and PHQ-9 score were associated with high ictal suicidality. Disease duration, HIT-6, and PHQ-9 remained significantly associated with high ictal suicidality in the multivariate analysis. Younger age at onset, longer disease duration, total number of lifetime bouts, and higher GAD-7 and PHQ-9 scores were significantly associated with interictal suicidality in the univariable analysis. The total number of lifetime bouts and the PHQ-9 scores remained significant in the multivariable analysis.
In all, 54 patients were followed up between bouts. None reported passive suicidal ideation, 1.9% reported active suicidal ideation, 1.9% reported suicidal planning, and none reported suicidal attempt. Compared with the between-bouts period, the ictal phase was associated with significantly higher odds of active suicidal ideation (OR, 37.32) and nonsignificantly increased suicidal planning (OR, 3.20).
Patients need a disease-modifying treatment
Taken together, the study results underscore the importance of proper management of cluster headache to reduce its burden, said the authors. “Given that greater headache-related impact was independently associated with ictal suicidality, an intensive treatment to reduce the headache-related impact might be beneficial to prevent suicide in cluster headache patients,” they said. In addition to reducing headache-related impact and headache intensity, “a disease-modifying treatment to prevent further bouts is warranted to decrease suicidality in cluster headache patients.”
Although patients with cluster headache had increased suicidality in the ictal and interictal phases, they had lower suicidality between bouts, compared with the general population. This result suggests that patients remain mentally healthy when the bouts are over, and that “a strategy to shorten the length of bout is warranted,” said Dr. Ji Lee and colleagues. Furthermore, the fact that suicidality did not differ significantly between patients with definite cluster headache and those with probable cluster headache “prompts clinicians for an increased identification and intensive treatment strategy for probable cluster headache.”
The current study is the first prospective investigation of suicidality in the various phases of cluster headache, according to the investigators. It nevertheless has several limitations. The prevalence of chronic cluster headache was low in the study population, and not all patients presented for follow-up during the period between bouts. In addition, the data were obtained from recall, and consequently may be less accurate than those gained from prospective recording. Finally, Dr. Ji Lee and colleagues did not gather information on personality disorders, insomnia, substance abuse, or addiction, even though these factors can influence suicidality in patients with chronic pain.
The investigators reported no conflicts of interest related to their research. The study was supported by a grant from the Korean Neurological Association.
SOURCE: Ji Lee M et al. Cephalalgia. 2019 Apr 24. doi: 10.1177/0333102419845660.
Short- and long-term cluster headache disease burden, as well as depressive symptoms, contributes to suicidality, according to research published online Cephalalgia. Development of treatments that reduce the headache-related burden and prevent future bouts could reduce suicidality, said the researchers.
Although cluster headache has been called the “suicide headache,” few studies have examined suicidality in patients with cluster headache. Research by Rozen et al. found that the rate of suicidal attempt among patients was similar to that among the general population. The results have not been replicated, however, and the investigators did not examine whether suicidality varied according to the phases of the disorder.
A prospective, multicenter study
Mi Ji Lee, MD, PhD, clinical assistant professor of neurology at Samsung Medical Center in Seoul, South Korea, and colleagues conducted a prospective study to investigate the suicidality associated with cluster headache and the factors associated with increased suicidality in that disorder. The researchers enrolled 193 consecutive patients with cluster headache between September 2016 and August 2018 at 15 hospitals. They examined the patients and used the Patient Health Questionnaire–9 (PHQ-9) and the General Anxiety Disorder–7 item scale (GAD-7) screening tools. During the ictal and interictal phases, the researchers asked the patients whether they had had passive suicidal ideation, active suicidal ideation, suicidal planning, or suicidal attempt. Dr. Ji Lee and colleagues performed univariable and multivariable logistic regression analyses to evaluate the factors associated with high ictal suicidality, which was defined as two or more positive responses during the ictal phase. Participants were followed up during the between-bout phase.
The researchers excluded 18 patients from analysis because they were between bouts at enrollment. The mean age of the remaining 175 patients was 38.4 years. Mean age at onset was 29.9 years. About 85% of the patients were male. The diagnosis was definite cluster headache for 87.4% of the sample and probable cluster headache for 12.6%. In addition, 88% of the population had episodic cluster headache.
Suicidal ideation increased during the ictal phase
During the ictal phase, 64.2% of participants reported passive suicidal ideation, and 35.8% reported active suicidal ideation. Furthermore, 5.8% of patients had a suicidal plan, and 2.3% attempted suicide. In the interictal phase, 4.0% of patients reported passive suicidal ideation, and 3.5% reported active suicidal ideation. Interictal suicidal planning was reported by 2.9% of participants, and 1.2% of participants attempted suicide interictally. The results were similar between patients with definite and probable cluster headache.
The ictal phase increased the odds of passive suicidal ideation (odds ratio [OR], 42.46), active suicidal ideation (OR, 15.55), suicidal planning (OR, 2.06), and suicidal attempt (OR, 2.02), compared with the interictal phase. The differences in suicidal planning and suicidal attempt between the ictal and interictal phases, however, were not statistically significant.
Longer disease duration, higher attack intensity, higher Headache Impact Test–6 (HIT-6) score, GAD-7 score, and PHQ-9 score were associated with high ictal suicidality. Disease duration, HIT-6, and PHQ-9 remained significantly associated with high ictal suicidality in the multivariate analysis. Younger age at onset, longer disease duration, total number of lifetime bouts, and higher GAD-7 and PHQ-9 scores were significantly associated with interictal suicidality in the univariable analysis. The total number of lifetime bouts and the PHQ-9 scores remained significant in the multivariable analysis.
In all, 54 patients were followed up between bouts. None reported passive suicidal ideation, 1.9% reported active suicidal ideation, 1.9% reported suicidal planning, and none reported suicidal attempt. Compared with the between-bouts period, the ictal phase was associated with significantly higher odds of active suicidal ideation (OR, 37.32) and nonsignificantly increased suicidal planning (OR, 3.20).
Patients need a disease-modifying treatment
Taken together, the study results underscore the importance of proper management of cluster headache to reduce its burden, said the authors. “Given that greater headache-related impact was independently associated with ictal suicidality, an intensive treatment to reduce the headache-related impact might be beneficial to prevent suicide in cluster headache patients,” they said. In addition to reducing headache-related impact and headache intensity, “a disease-modifying treatment to prevent further bouts is warranted to decrease suicidality in cluster headache patients.”
Although patients with cluster headache had increased suicidality in the ictal and interictal phases, they had lower suicidality between bouts, compared with the general population. This result suggests that patients remain mentally healthy when the bouts are over, and that “a strategy to shorten the length of bout is warranted,” said Dr. Ji Lee and colleagues. Furthermore, the fact that suicidality did not differ significantly between patients with definite cluster headache and those with probable cluster headache “prompts clinicians for an increased identification and intensive treatment strategy for probable cluster headache.”
The current study is the first prospective investigation of suicidality in the various phases of cluster headache, according to the investigators. It nevertheless has several limitations. The prevalence of chronic cluster headache was low in the study population, and not all patients presented for follow-up during the period between bouts. In addition, the data were obtained from recall, and consequently may be less accurate than those gained from prospective recording. Finally, Dr. Ji Lee and colleagues did not gather information on personality disorders, insomnia, substance abuse, or addiction, even though these factors can influence suicidality in patients with chronic pain.
The investigators reported no conflicts of interest related to their research. The study was supported by a grant from the Korean Neurological Association.
SOURCE: Ji Lee M et al. Cephalalgia. 2019 Apr 24. doi: 10.1177/0333102419845660.
Short- and long-term cluster headache disease burden, as well as depressive symptoms, contributes to suicidality, according to research published online Cephalalgia. Development of treatments that reduce the headache-related burden and prevent future bouts could reduce suicidality, said the researchers.
Although cluster headache has been called the “suicide headache,” few studies have examined suicidality in patients with cluster headache. Research by Rozen et al. found that the rate of suicidal attempt among patients was similar to that among the general population. The results have not been replicated, however, and the investigators did not examine whether suicidality varied according to the phases of the disorder.
A prospective, multicenter study
Mi Ji Lee, MD, PhD, clinical assistant professor of neurology at Samsung Medical Center in Seoul, South Korea, and colleagues conducted a prospective study to investigate the suicidality associated with cluster headache and the factors associated with increased suicidality in that disorder. The researchers enrolled 193 consecutive patients with cluster headache between September 2016 and August 2018 at 15 hospitals. They examined the patients and used the Patient Health Questionnaire–9 (PHQ-9) and the General Anxiety Disorder–7 item scale (GAD-7) screening tools. During the ictal and interictal phases, the researchers asked the patients whether they had had passive suicidal ideation, active suicidal ideation, suicidal planning, or suicidal attempt. Dr. Ji Lee and colleagues performed univariable and multivariable logistic regression analyses to evaluate the factors associated with high ictal suicidality, which was defined as two or more positive responses during the ictal phase. Participants were followed up during the between-bout phase.
The researchers excluded 18 patients from analysis because they were between bouts at enrollment. The mean age of the remaining 175 patients was 38.4 years. Mean age at onset was 29.9 years. About 85% of the patients were male. The diagnosis was definite cluster headache for 87.4% of the sample and probable cluster headache for 12.6%. In addition, 88% of the population had episodic cluster headache.
Suicidal ideation increased during the ictal phase
During the ictal phase, 64.2% of participants reported passive suicidal ideation, and 35.8% reported active suicidal ideation. Furthermore, 5.8% of patients had a suicidal plan, and 2.3% attempted suicide. In the interictal phase, 4.0% of patients reported passive suicidal ideation, and 3.5% reported active suicidal ideation. Interictal suicidal planning was reported by 2.9% of participants, and 1.2% of participants attempted suicide interictally. The results were similar between patients with definite and probable cluster headache.
The ictal phase increased the odds of passive suicidal ideation (odds ratio [OR], 42.46), active suicidal ideation (OR, 15.55), suicidal planning (OR, 2.06), and suicidal attempt (OR, 2.02), compared with the interictal phase. The differences in suicidal planning and suicidal attempt between the ictal and interictal phases, however, were not statistically significant.
Longer disease duration, higher attack intensity, higher Headache Impact Test–6 (HIT-6) score, GAD-7 score, and PHQ-9 score were associated with high ictal suicidality. Disease duration, HIT-6, and PHQ-9 remained significantly associated with high ictal suicidality in the multivariate analysis. Younger age at onset, longer disease duration, total number of lifetime bouts, and higher GAD-7 and PHQ-9 scores were significantly associated with interictal suicidality in the univariable analysis. The total number of lifetime bouts and the PHQ-9 scores remained significant in the multivariable analysis.
In all, 54 patients were followed up between bouts. None reported passive suicidal ideation, 1.9% reported active suicidal ideation, 1.9% reported suicidal planning, and none reported suicidal attempt. Compared with the between-bouts period, the ictal phase was associated with significantly higher odds of active suicidal ideation (OR, 37.32) and nonsignificantly increased suicidal planning (OR, 3.20).
Patients need a disease-modifying treatment
Taken together, the study results underscore the importance of proper management of cluster headache to reduce its burden, said the authors. “Given that greater headache-related impact was independently associated with ictal suicidality, an intensive treatment to reduce the headache-related impact might be beneficial to prevent suicide in cluster headache patients,” they said. In addition to reducing headache-related impact and headache intensity, “a disease-modifying treatment to prevent further bouts is warranted to decrease suicidality in cluster headache patients.”
Although patients with cluster headache had increased suicidality in the ictal and interictal phases, they had lower suicidality between bouts, compared with the general population. This result suggests that patients remain mentally healthy when the bouts are over, and that “a strategy to shorten the length of bout is warranted,” said Dr. Ji Lee and colleagues. Furthermore, the fact that suicidality did not differ significantly between patients with definite cluster headache and those with probable cluster headache “prompts clinicians for an increased identification and intensive treatment strategy for probable cluster headache.”
The current study is the first prospective investigation of suicidality in the various phases of cluster headache, according to the investigators. It nevertheless has several limitations. The prevalence of chronic cluster headache was low in the study population, and not all patients presented for follow-up during the period between bouts. In addition, the data were obtained from recall, and consequently may be less accurate than those gained from prospective recording. Finally, Dr. Ji Lee and colleagues did not gather information on personality disorders, insomnia, substance abuse, or addiction, even though these factors can influence suicidality in patients with chronic pain.
The investigators reported no conflicts of interest related to their research. The study was supported by a grant from the Korean Neurological Association.
SOURCE: Ji Lee M et al. Cephalalgia. 2019 Apr 24. doi: 10.1177/0333102419845660.
FROM CEPHALAGIA
Key clinical point: Cluster headache is associated with increased suicidality during attacks and within the active period.
Major finding: Cluster headache attacks increased the risk of active suicidal ideation (odds ratio, 15.55).
Study details: A prospective, multicenter study of 175 patients with cluster headache.
Disclosures: The study was supported by a grant from the Korean Neurological Association.
Source: Ji Lee M et al. Cephalalgia. 2019 Apr 24. doi: 10.1177/0333102419845660.
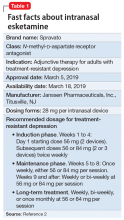
Intranasal esketamine
Treatment-resistant depression (TRD) is a common clinical struggle that practicing clinicians address on a daily basis. Major depressive disorder affects nearly 1 in 5 Americans at some point in their life and, by definition, impairs social and occupational functioning. Historic treatments have focused on the monoamine theories of depression—modulating the monoamines serotonin, norepinephrine, and/or dopamine. Limitations of currently available antidepressants include delayed onset of effect and low remission rates. To further complicate the matter, numerous studies have shown that with each subsequent antidepressant trial, patients have a decreasing likelihood of responding to subsequent antidepressant treatment options. For example, in the classic STAR*D trial, by the time a patient had not responded to the first 2 antidepressant options, the chance that they would respond to a third or fourth antidepressant had decreased to approximately 15% per antidepressant treatment course.1
To address the need for new treatments for patients with TRD, on March 5, 2019 the FDA-approved intranasal
How it works
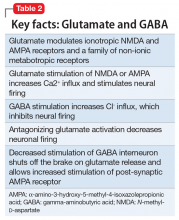
Modern research has looked beyond the monoamine system to explore the neuro-modulatory effects of glutamate and gamma-aminobutyric acid (GABA).3 The yin and yang of glutamate and GABA revolves around neural excitation vs neural inhibition at a local synaptic level. The primary effects of the glutamate and GABA systems (Table 2) can be broken down into several key areas of understanding.
Glutamate modulates ionotropic N-methyl-
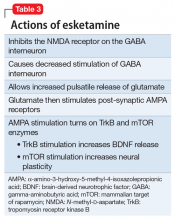
Esketamine, the S-enantiomer of ketamine, has a higher affinity for the NMDA receptor than the R-enantiomer and has been developed as an intranasal adjunctive treatment for TRD. Esketamine blocks NMDA receptors on GABA interneurons. This allows for increased pulsatile release of glutamate into the synapse. Intrasynaptic glutamate then stimulates postsynaptic AMPA receptors. Glutamate stimulation of postsynaptic AMPA receptors results in an intracellular cascade that activates the enzymes tropomyosin receptor kinase B (TrkB) and mammalian target of rapamycin (mTOR). TrkB stimulation results in increased production and release of BDNF. mTor stimulation increases neuronal membrane protein formation with subsequent increased neural plasticity. Taken together, preclinical models show that esketamine’s inhibition of the NMDA receptor on the GABA interneuron results in a cascade of increased BDNF release and synaptogenesis with increased neuroplasticity (Table 3).
Clinical implications
Treatment-resistant depression affects nearly one-third of patients currently receiving standard antidepressant treatment. Major depressive disorder is currently the second leading cause of disability for working adults within the United States and one of the largest causes of disability worldwide. The esketamine nasal spray could be beneficial for patients who have experienced TRD with standard monoamine antidepressants.
Supporting evidence
Clinical trials examining intranasal esketamine include both short- and long-term studies of patients with TRD.
Continue to: Esketamine was evaluated...
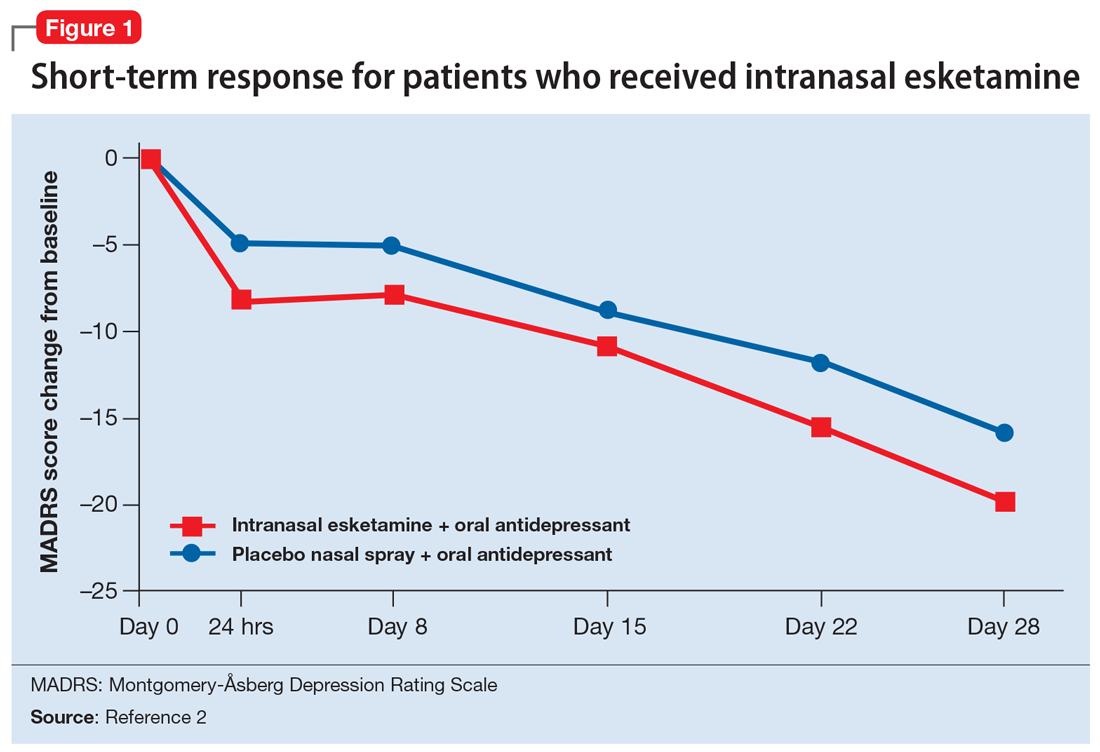
Esketamine was evaluated in a randomized, placebo-controlled, double-blind, multicenter, short-term (4-week) phase III study in adult patients age 18 to 65 with TRD (they had not responded to at least 2 different antidepressants of adequate dose and duration).4 After discontinuing prior antidepressant treatments, all patients were started on a newly initiated antidepressant and were also randomized to concomitant intranasal esketamine or intranasal placebo as follows:
- 114 patients were randomized to the intranasal esketamine plus newly initiated oral antidepressant arm
- 109 patients were randomized to the placebo nasal spray plus newly initiated oral antidepressant arm
- The mean baseline Montgomery-Åsberg Depression Rating Scale (MADRS) score for each group was 37 (ie, moderately to severely depressed).
Newly started antidepressants included esc
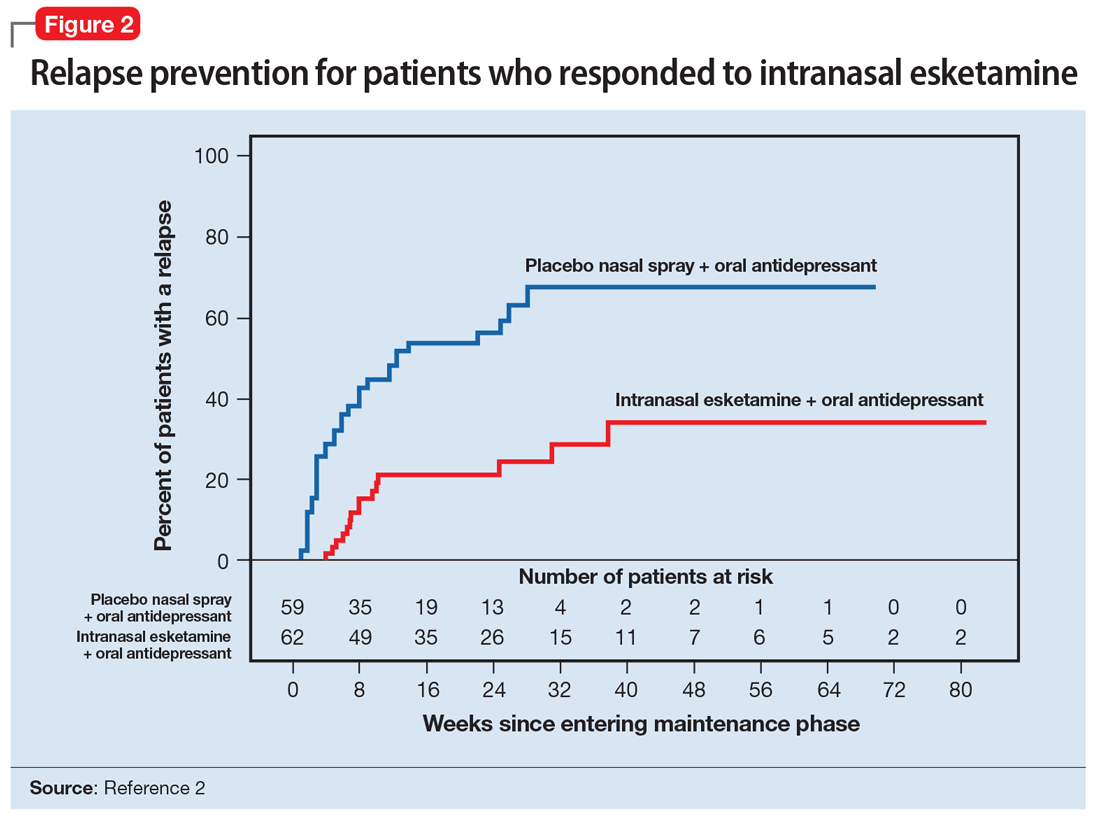
A long-term, double-blind multicenter maintenance-of-effect trial examined adults age 18 to 65 with TRD.5-6 Patients in this study were responders in 1 of 2 short-term studies or in an open-label direct enrollment study. Stable remission was defined as a MADRS total score <12 for at least 3 of the last 4 weeks of the study, and stable response was defined as a MADRS reduction of >50% but not in remission. After 16 weeks of intranasal esketamine plus an oral antidepressant, stable remitters and stable responders were then randomized separately to continue intranasal esketamine or switch to placebo nasal spray, with both groups continuing on their concomitant oral antidepressant. The primary study endpoint was time to relapse. Relapse was defined as a MADRS total score >22 for more than 2 consecutive weeks, hospitalization for worsening of depression, or any other clinically relevant event. The median age was 48, 66% were female, 90% were White and 4% were black. Patients in stable response or stable remission experienced a significantly longer time to relapse compared with patients who continued their oral antidepressant but were switched to placebo intranasal spray. In this remission response study, patients could receive intranasal treatment weekly or bi-weekly based on symptom severity (Figure 22).
Impact on driving. Two studies examined the impact of esketamine on driving performance. One examined adults with major depressive disorder and the other examined healthy participants. The effects of a single 84-mg dose of esketamine nasal spray on a patient’s ability to drive was assessed in 23 healthy adults. In this study, mirt
A second study evaluated the effects of repeated esketamine administration on driving performance in 25 adults with major depressive disorder. In this study, an ethanol-containing beverage was used as an active control. After administration of a single 84-mg dose of intranasal esketamine, driving performance was the same as a placebo at 18 hours. In the multiple dose phase, standard driving performance was similar for esketamine nasal spray and placebo at 6 hours postdose on Days 11, 18, and 25.
Continue to: Pharmacologic profile
Pharmacologic profile
Adverse events. The most common adverse events in patients treated with esketamine nasal spray were dissociation (41%), dizziness (29%), nausea (28%), sedation (23%), and vertigo (23%).2 The majority of these effects were short-term and resolved during the 2-hour observation period.
In addition to spontaneously reported events, sedation and dissociation were further monitored with specific scales. Sedation was measured with the Modified Observer’s Alertness and Sedation Scale. Using this scale, 50% of patients receiving 56 mg and 61% of patients receiving 84 mg of esketamine met criteria for sedation.
Similarly, dissociation/perceptional changes were measured with spontaneously reported events and also with the Clinician Administered Dissociative State Scale. On this scale, 61% of patients receiving the 56-mg dose, and 69% of patients receiving the 84-mg dose met criteria for dissociation/perceptional changes after dose administration.
Increases in blod pressure. Esketamine intranasal spray was associated with a 7 to 9 mm Hg increase in systolic blood pressure and a 4 to 6 mm Hg increase in diastolic blood pressure, both of which peaked 40 minutes post-dose.
Nausea and vomiting. Intranasal esketamine was associated with a 27% rate of nausea at 56 mg, and 32% at 84 mg, with a 6% rate of vomiting at 56 mg and 12% at 84 mg.
Continue to: Pharmacokinetics
Pharmacokinetics
Esketamine exposure increases from 28 to 84 mg in a fairly dose-proportional range. No accumulation of esketamine was observed in the plasma following twice-weekly administration. Bioavailability is approximately 48% following nasal administration. The Tmax for esketamine plasma concentration is 20 to 40 minutes after the last nasal spray. Protein binding of esketamine is approximately 43% to 45%. The brain-to-plasma ratio of noresketamine is 4 to 6 times lower than that of esketamine. The half-life of esketamine ranged from 7 to 12 hours. The mean half-life of nore
Potential drug interactions
Central nervous system depressants. Concomitant use of esketamine and other CNS depressants (ie, benzodiazepines, opioids, alcohol) may increase sedation. Patients receiving esketamine with concomitant use of other CNS depressants should be closely monitored for sedation.
Psychostimulants. Concomitant use of esketamine and psychostimulants (ie, amphetamines, methylphenidates, moda
Monoamine oxidase inhibitors. Concomitant use of esketamine with monoamine oxidase inhibitors may increase blood pressure. Closely monitor blood pressure with concomitant use of esketamine and monoamine oxidase inhibitors.
Use in special populations. Because of concerns of increased sedation, intranasal esketamine should be administered cautiously in patients receiving other CNS depressants, such as benzodiazepines. In patients with psychosis or a prior history of psychosis, esketamine should be used with increased caution and the risk/benefit ratio should be carefully considered.
Continue to: Because of potential teratogenicity...
Because of potential teratogenicity, esketamine is not recommended in women who are pregnant, may become pregnant, or who are currently nursing.
Intranasal esketamine was examined in a phase III trial of 194 patients age ≥65. At the end of 4 weeks, there was no statistically significant difference in groups on the MADRS, the primary efficacy endpoint. There were no overall differences in the safety profile in patients >65 years compared with younger patients; however, the mean esketamine Cmax and area under the curve were higher in older patients compared with younger adults. The mean esketamine half-life was longer in patients with moderate hepatic impairment.
Abuse liability
Esketamine is a CIII controlled substance and concerns about abuse, misuse, and diversion have been taken into account within the REMS drug safety program.2 Patients with a prior history of substance abuse or misuse should be considered with regard to the risk/benefit ratio.
The REMS drug safety program
Due to the nature of its usually transient adverse effects, including sedation, dissociation, hypertension, and nausea, intranasal esketamine will be administered through a REMS drug safety program at certified REMS treatment centers. Certified REMS treatment centers will receive training on how to safely and effectively counsel and monitor patients. Prior to treatment, patients will receive blood pressure monitoring and anticipated adverse effects will be discussed. Patients will be instructed to not eat solid food for 2 hours pre-dose and to not drink anything for 30 minutes prior.
A treatment session consists of nasal administration and a minimum 2-hour post-administration observation period. Blood pressure must be assessed prior to administration and if elevated, (ie, systolic blood pressure >140 mm Hg, diastolic >90 mm Hg), clinicians should consider the risk of short-term increases in blood pressure that may occur. Do not administer if increases in blood pressure or intracranial pressure pose a serious risk.
Continue to: After each intranasal...
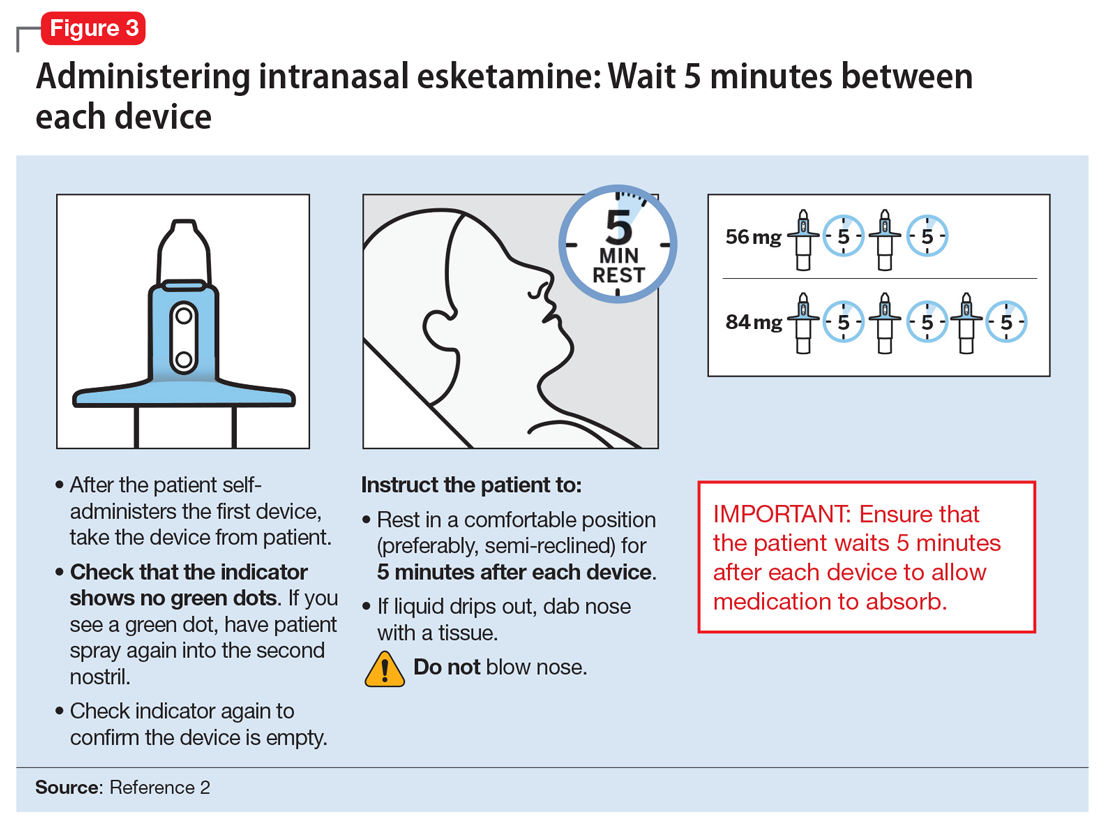
After each intranasal administration the patient will be observed for 5 minutes before the second nasal inhaler is utilized and for another 5 minutes when the patient is receiving 84 mg (ie, each inhaler equals 28 mg). After administering, blood pressure should be reassessed at approximately 40 minutes, which corresponds to the Cmax of intranasal esketamine, and periodically thereafter as warranted.
The patient will then be monitored in a quiet environment for a minimum of 2 hours to make sure that dissociative phenomenon, sedation, and hypertensive reactions have normalized prior to discharge from a certified REMS treatment center.
Dosing and administration
Each intranasal device is primed for 2 infusions (1 in each nostril) for a total dose of 28 mg of esketamine. Combinations of devices can be used to adjust the dose as appropriate for individual patients. The recommended starting dose is 56 mg (ie, 2 devices, with a 5-minute gap between devices). The dose can be increased to 84 mg (ie, 3 intranasal devices spaced at 5-minute intervals) by the second dose based on clinical judgment.
The patient will be instructed to recline the head to a 45° angle, clear his or her nostrils prior to the first treatment, and then self-administer a dose to each nostril while holding the reciprocal nostril closed and inhaling. This process is then repeated every 5 minutes for each subsequent device, with a maximum total dose of 3 devices, or 84 mg (Figure 32). The patient will then be monitored for blood pressure, heart rate, and signs of psychologic or physiologic changes for the next 2 hours. Patients may not drive a car or operate any type of motor equipment until the following day after receiving a normal night’s sleep. Patients will be released from the REMS treatment center after 2 hours if both psychological and physical adverse effects have normalized.
Missed treatment sessions. If a patient misses a treatment session and there is worsening of depressive symptoms, consider returning the patient to the previous dosing schedule (ie, every 2 weeks to once weekly, or weekly to twice weekly).
Continue to: Contraindications for...
Contraindications for intranasal esketamine include:
- aneurysmal vascular disease, including thoracic and abdominal aortic, intracranial, and peripheral arterial vessels, or arterial venous malformations
- history of intracerebral hemorrhage
- hypersensitivity to esketamine, ketamine, or any of the excipients.
Clinical considerations
Intranasal esketamine represents a unique delivery system for the first glutamatergic treatment approved for patients with TRD.
Why Rx? Treatment-resistant depression is found in nearly 1 out of 3 patients with currently available monoaminergic antidepressant treatment options. Patients with TRD are at increased risk of physical and psychological impairment, subsequent worsening of their condition, and social and occupational disability.
Bottom Line
Intranasal esketamine is the first glutamatergic treatment option FDA-approved for patients with treatment-resistant depression who have not responded to standard antidepressant treatment options. In short-term trials, intranasal esketamine significantly improved depressive symptoms as quickly as 24 hours after treatment, with significant improvement maintained through 4 weeks of ongoing administration. In addition, intranasal esketamine was shown to significantly decrease time to relapse for patients who had achieved stable remission or stable response.
Related Resource
- Sullivan MG. FDA approves intranasal esketamine for refractory major depressive disorder. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/195712/depression/fda-approves-intranasal-esketamine-refractory-major-depressive. Published March 5, 2019.
Drug Brand Names
Armodafinil • Nuvigil
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Mirtazapine • Remeron
Modafinil • Provigil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Rush AG, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR D Report. Am J Psychiatry. 2006;163(11):1905-1917.
2. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting anti-depression. Nat Med. 2016;22(3):238-249.
4. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
5. Daly EJ, Trivedi M, Janik A, et al. A randomized withdrawal, double-blind, multicenter study of esketamine nasal spray plus an oral antidepressant for relapse prevention in treatment-resistant depression. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.
6. Wajs E, Aluisio L, Morrison R, et al. Long-term safety of esketamine nasal spray plus oral antidepressants in patients with treatment-resistant depression: phase III open-label safety and efficacy study. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.
Treatment-resistant depression (TRD) is a common clinical struggle that practicing clinicians address on a daily basis. Major depressive disorder affects nearly 1 in 5 Americans at some point in their life and, by definition, impairs social and occupational functioning. Historic treatments have focused on the monoamine theories of depression—modulating the monoamines serotonin, norepinephrine, and/or dopamine. Limitations of currently available antidepressants include delayed onset of effect and low remission rates. To further complicate the matter, numerous studies have shown that with each subsequent antidepressant trial, patients have a decreasing likelihood of responding to subsequent antidepressant treatment options. For example, in the classic STAR*D trial, by the time a patient had not responded to the first 2 antidepressant options, the chance that they would respond to a third or fourth antidepressant had decreased to approximately 15% per antidepressant treatment course.1
To address the need for new treatments for patients with TRD, on March 5, 2019 the FDA-approved intranasal
How it works
Modern research has looked beyond the monoamine system to explore the neuro-modulatory effects of glutamate and gamma-aminobutyric acid (GABA).3 The yin and yang of glutamate and GABA revolves around neural excitation vs neural inhibition at a local synaptic level. The primary effects of the glutamate and GABA systems (Table 2) can be broken down into several key areas of understanding.
Glutamate modulates ionotropic N-methyl-
Esketamine, the S-enantiomer of ketamine, has a higher affinity for the NMDA receptor than the R-enantiomer and has been developed as an intranasal adjunctive treatment for TRD. Esketamine blocks NMDA receptors on GABA interneurons. This allows for increased pulsatile release of glutamate into the synapse. Intrasynaptic glutamate then stimulates postsynaptic AMPA receptors. Glutamate stimulation of postsynaptic AMPA receptors results in an intracellular cascade that activates the enzymes tropomyosin receptor kinase B (TrkB) and mammalian target of rapamycin (mTOR). TrkB stimulation results in increased production and release of BDNF. mTor stimulation increases neuronal membrane protein formation with subsequent increased neural plasticity. Taken together, preclinical models show that esketamine’s inhibition of the NMDA receptor on the GABA interneuron results in a cascade of increased BDNF release and synaptogenesis with increased neuroplasticity (Table 3).
Clinical implications
Treatment-resistant depression affects nearly one-third of patients currently receiving standard antidepressant treatment. Major depressive disorder is currently the second leading cause of disability for working adults within the United States and one of the largest causes of disability worldwide. The esketamine nasal spray could be beneficial for patients who have experienced TRD with standard monoamine antidepressants.
Supporting evidence
Clinical trials examining intranasal esketamine include both short- and long-term studies of patients with TRD.
Continue to: Esketamine was evaluated...
Esketamine was evaluated in a randomized, placebo-controlled, double-blind, multicenter, short-term (4-week) phase III study in adult patients age 18 to 65 with TRD (they had not responded to at least 2 different antidepressants of adequate dose and duration).4 After discontinuing prior antidepressant treatments, all patients were started on a newly initiated antidepressant and were also randomized to concomitant intranasal esketamine or intranasal placebo as follows:
- 114 patients were randomized to the intranasal esketamine plus newly initiated oral antidepressant arm
- 109 patients were randomized to the placebo nasal spray plus newly initiated oral antidepressant arm
- The mean baseline Montgomery-Åsberg Depression Rating Scale (MADRS) score for each group was 37 (ie, moderately to severely depressed).
Newly started antidepressants included esc
A long-term, double-blind multicenter maintenance-of-effect trial examined adults age 18 to 65 with TRD.5-6 Patients in this study were responders in 1 of 2 short-term studies or in an open-label direct enrollment study. Stable remission was defined as a MADRS total score <12 for at least 3 of the last 4 weeks of the study, and stable response was defined as a MADRS reduction of >50% but not in remission. After 16 weeks of intranasal esketamine plus an oral antidepressant, stable remitters and stable responders were then randomized separately to continue intranasal esketamine or switch to placebo nasal spray, with both groups continuing on their concomitant oral antidepressant. The primary study endpoint was time to relapse. Relapse was defined as a MADRS total score >22 for more than 2 consecutive weeks, hospitalization for worsening of depression, or any other clinically relevant event. The median age was 48, 66% were female, 90% were White and 4% were black. Patients in stable response or stable remission experienced a significantly longer time to relapse compared with patients who continued their oral antidepressant but were switched to placebo intranasal spray. In this remission response study, patients could receive intranasal treatment weekly or bi-weekly based on symptom severity (Figure 22).
Impact on driving. Two studies examined the impact of esketamine on driving performance. One examined adults with major depressive disorder and the other examined healthy participants. The effects of a single 84-mg dose of esketamine nasal spray on a patient’s ability to drive was assessed in 23 healthy adults. In this study, mirt
A second study evaluated the effects of repeated esketamine administration on driving performance in 25 adults with major depressive disorder. In this study, an ethanol-containing beverage was used as an active control. After administration of a single 84-mg dose of intranasal esketamine, driving performance was the same as a placebo at 18 hours. In the multiple dose phase, standard driving performance was similar for esketamine nasal spray and placebo at 6 hours postdose on Days 11, 18, and 25.
Continue to: Pharmacologic profile
Pharmacologic profile
Adverse events. The most common adverse events in patients treated with esketamine nasal spray were dissociation (41%), dizziness (29%), nausea (28%), sedation (23%), and vertigo (23%).2 The majority of these effects were short-term and resolved during the 2-hour observation period.
In addition to spontaneously reported events, sedation and dissociation were further monitored with specific scales. Sedation was measured with the Modified Observer’s Alertness and Sedation Scale. Using this scale, 50% of patients receiving 56 mg and 61% of patients receiving 84 mg of esketamine met criteria for sedation.
Similarly, dissociation/perceptional changes were measured with spontaneously reported events and also with the Clinician Administered Dissociative State Scale. On this scale, 61% of patients receiving the 56-mg dose, and 69% of patients receiving the 84-mg dose met criteria for dissociation/perceptional changes after dose administration.
Increases in blod pressure. Esketamine intranasal spray was associated with a 7 to 9 mm Hg increase in systolic blood pressure and a 4 to 6 mm Hg increase in diastolic blood pressure, both of which peaked 40 minutes post-dose.
Nausea and vomiting. Intranasal esketamine was associated with a 27% rate of nausea at 56 mg, and 32% at 84 mg, with a 6% rate of vomiting at 56 mg and 12% at 84 mg.
Continue to: Pharmacokinetics
Pharmacokinetics
Esketamine exposure increases from 28 to 84 mg in a fairly dose-proportional range. No accumulation of esketamine was observed in the plasma following twice-weekly administration. Bioavailability is approximately 48% following nasal administration. The Tmax for esketamine plasma concentration is 20 to 40 minutes after the last nasal spray. Protein binding of esketamine is approximately 43% to 45%. The brain-to-plasma ratio of noresketamine is 4 to 6 times lower than that of esketamine. The half-life of esketamine ranged from 7 to 12 hours. The mean half-life of nore
Potential drug interactions
Central nervous system depressants. Concomitant use of esketamine and other CNS depressants (ie, benzodiazepines, opioids, alcohol) may increase sedation. Patients receiving esketamine with concomitant use of other CNS depressants should be closely monitored for sedation.
Psychostimulants. Concomitant use of esketamine and psychostimulants (ie, amphetamines, methylphenidates, moda
Monoamine oxidase inhibitors. Concomitant use of esketamine with monoamine oxidase inhibitors may increase blood pressure. Closely monitor blood pressure with concomitant use of esketamine and monoamine oxidase inhibitors.
Use in special populations. Because of concerns of increased sedation, intranasal esketamine should be administered cautiously in patients receiving other CNS depressants, such as benzodiazepines. In patients with psychosis or a prior history of psychosis, esketamine should be used with increased caution and the risk/benefit ratio should be carefully considered.
Continue to: Because of potential teratogenicity...
Because of potential teratogenicity, esketamine is not recommended in women who are pregnant, may become pregnant, or who are currently nursing.
Intranasal esketamine was examined in a phase III trial of 194 patients age ≥65. At the end of 4 weeks, there was no statistically significant difference in groups on the MADRS, the primary efficacy endpoint. There were no overall differences in the safety profile in patients >65 years compared with younger patients; however, the mean esketamine Cmax and area under the curve were higher in older patients compared with younger adults. The mean esketamine half-life was longer in patients with moderate hepatic impairment.
Abuse liability
Esketamine is a CIII controlled substance and concerns about abuse, misuse, and diversion have been taken into account within the REMS drug safety program.2 Patients with a prior history of substance abuse or misuse should be considered with regard to the risk/benefit ratio.
The REMS drug safety program
Due to the nature of its usually transient adverse effects, including sedation, dissociation, hypertension, and nausea, intranasal esketamine will be administered through a REMS drug safety program at certified REMS treatment centers. Certified REMS treatment centers will receive training on how to safely and effectively counsel and monitor patients. Prior to treatment, patients will receive blood pressure monitoring and anticipated adverse effects will be discussed. Patients will be instructed to not eat solid food for 2 hours pre-dose and to not drink anything for 30 minutes prior.
A treatment session consists of nasal administration and a minimum 2-hour post-administration observation period. Blood pressure must be assessed prior to administration and if elevated, (ie, systolic blood pressure >140 mm Hg, diastolic >90 mm Hg), clinicians should consider the risk of short-term increases in blood pressure that may occur. Do not administer if increases in blood pressure or intracranial pressure pose a serious risk.
Continue to: After each intranasal...
After each intranasal administration the patient will be observed for 5 minutes before the second nasal inhaler is utilized and for another 5 minutes when the patient is receiving 84 mg (ie, each inhaler equals 28 mg). After administering, blood pressure should be reassessed at approximately 40 minutes, which corresponds to the Cmax of intranasal esketamine, and periodically thereafter as warranted.
The patient will then be monitored in a quiet environment for a minimum of 2 hours to make sure that dissociative phenomenon, sedation, and hypertensive reactions have normalized prior to discharge from a certified REMS treatment center.
Dosing and administration
Each intranasal device is primed for 2 infusions (1 in each nostril) for a total dose of 28 mg of esketamine. Combinations of devices can be used to adjust the dose as appropriate for individual patients. The recommended starting dose is 56 mg (ie, 2 devices, with a 5-minute gap between devices). The dose can be increased to 84 mg (ie, 3 intranasal devices spaced at 5-minute intervals) by the second dose based on clinical judgment.
The patient will be instructed to recline the head to a 45° angle, clear his or her nostrils prior to the first treatment, and then self-administer a dose to each nostril while holding the reciprocal nostril closed and inhaling. This process is then repeated every 5 minutes for each subsequent device, with a maximum total dose of 3 devices, or 84 mg (Figure 32). The patient will then be monitored for blood pressure, heart rate, and signs of psychologic or physiologic changes for the next 2 hours. Patients may not drive a car or operate any type of motor equipment until the following day after receiving a normal night’s sleep. Patients will be released from the REMS treatment center after 2 hours if both psychological and physical adverse effects have normalized.
Missed treatment sessions. If a patient misses a treatment session and there is worsening of depressive symptoms, consider returning the patient to the previous dosing schedule (ie, every 2 weeks to once weekly, or weekly to twice weekly).
Continue to: Contraindications for...
Contraindications for intranasal esketamine include:
- aneurysmal vascular disease, including thoracic and abdominal aortic, intracranial, and peripheral arterial vessels, or arterial venous malformations
- history of intracerebral hemorrhage
- hypersensitivity to esketamine, ketamine, or any of the excipients.
Clinical considerations
Intranasal esketamine represents a unique delivery system for the first glutamatergic treatment approved for patients with TRD.
Why Rx? Treatment-resistant depression is found in nearly 1 out of 3 patients with currently available monoaminergic antidepressant treatment options. Patients with TRD are at increased risk of physical and psychological impairment, subsequent worsening of their condition, and social and occupational disability.
Bottom Line
Intranasal esketamine is the first glutamatergic treatment option FDA-approved for patients with treatment-resistant depression who have not responded to standard antidepressant treatment options. In short-term trials, intranasal esketamine significantly improved depressive symptoms as quickly as 24 hours after treatment, with significant improvement maintained through 4 weeks of ongoing administration. In addition, intranasal esketamine was shown to significantly decrease time to relapse for patients who had achieved stable remission or stable response.
Related Resource
- Sullivan MG. FDA approves intranasal esketamine for refractory major depressive disorder. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/195712/depression/fda-approves-intranasal-esketamine-refractory-major-depressive. Published March 5, 2019.
Drug Brand Names
Armodafinil • Nuvigil
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Mirtazapine • Remeron
Modafinil • Provigil
Sertraline • Zoloft
Venlafaxine • Effexor
Treatment-resistant depression (TRD) is a common clinical struggle that practicing clinicians address on a daily basis. Major depressive disorder affects nearly 1 in 5 Americans at some point in their life and, by definition, impairs social and occupational functioning. Historic treatments have focused on the monoamine theories of depression—modulating the monoamines serotonin, norepinephrine, and/or dopamine. Limitations of currently available antidepressants include delayed onset of effect and low remission rates. To further complicate the matter, numerous studies have shown that with each subsequent antidepressant trial, patients have a decreasing likelihood of responding to subsequent antidepressant treatment options. For example, in the classic STAR*D trial, by the time a patient had not responded to the first 2 antidepressant options, the chance that they would respond to a third or fourth antidepressant had decreased to approximately 15% per antidepressant treatment course.1
To address the need for new treatments for patients with TRD, on March 5, 2019 the FDA-approved intranasal
How it works
Modern research has looked beyond the monoamine system to explore the neuro-modulatory effects of glutamate and gamma-aminobutyric acid (GABA).3 The yin and yang of glutamate and GABA revolves around neural excitation vs neural inhibition at a local synaptic level. The primary effects of the glutamate and GABA systems (Table 2) can be broken down into several key areas of understanding.
Glutamate modulates ionotropic N-methyl-
Esketamine, the S-enantiomer of ketamine, has a higher affinity for the NMDA receptor than the R-enantiomer and has been developed as an intranasal adjunctive treatment for TRD. Esketamine blocks NMDA receptors on GABA interneurons. This allows for increased pulsatile release of glutamate into the synapse. Intrasynaptic glutamate then stimulates postsynaptic AMPA receptors. Glutamate stimulation of postsynaptic AMPA receptors results in an intracellular cascade that activates the enzymes tropomyosin receptor kinase B (TrkB) and mammalian target of rapamycin (mTOR). TrkB stimulation results in increased production and release of BDNF. mTor stimulation increases neuronal membrane protein formation with subsequent increased neural plasticity. Taken together, preclinical models show that esketamine’s inhibition of the NMDA receptor on the GABA interneuron results in a cascade of increased BDNF release and synaptogenesis with increased neuroplasticity (Table 3).
Clinical implications
Treatment-resistant depression affects nearly one-third of patients currently receiving standard antidepressant treatment. Major depressive disorder is currently the second leading cause of disability for working adults within the United States and one of the largest causes of disability worldwide. The esketamine nasal spray could be beneficial for patients who have experienced TRD with standard monoamine antidepressants.
Supporting evidence
Clinical trials examining intranasal esketamine include both short- and long-term studies of patients with TRD.
Continue to: Esketamine was evaluated...
Esketamine was evaluated in a randomized, placebo-controlled, double-blind, multicenter, short-term (4-week) phase III study in adult patients age 18 to 65 with TRD (they had not responded to at least 2 different antidepressants of adequate dose and duration).4 After discontinuing prior antidepressant treatments, all patients were started on a newly initiated antidepressant and were also randomized to concomitant intranasal esketamine or intranasal placebo as follows:
- 114 patients were randomized to the intranasal esketamine plus newly initiated oral antidepressant arm
- 109 patients were randomized to the placebo nasal spray plus newly initiated oral antidepressant arm
- The mean baseline Montgomery-Åsberg Depression Rating Scale (MADRS) score for each group was 37 (ie, moderately to severely depressed).
Newly started antidepressants included esc
A long-term, double-blind multicenter maintenance-of-effect trial examined adults age 18 to 65 with TRD.5-6 Patients in this study were responders in 1 of 2 short-term studies or in an open-label direct enrollment study. Stable remission was defined as a MADRS total score <12 for at least 3 of the last 4 weeks of the study, and stable response was defined as a MADRS reduction of >50% but not in remission. After 16 weeks of intranasal esketamine plus an oral antidepressant, stable remitters and stable responders were then randomized separately to continue intranasal esketamine or switch to placebo nasal spray, with both groups continuing on their concomitant oral antidepressant. The primary study endpoint was time to relapse. Relapse was defined as a MADRS total score >22 for more than 2 consecutive weeks, hospitalization for worsening of depression, or any other clinically relevant event. The median age was 48, 66% were female, 90% were White and 4% were black. Patients in stable response or stable remission experienced a significantly longer time to relapse compared with patients who continued their oral antidepressant but were switched to placebo intranasal spray. In this remission response study, patients could receive intranasal treatment weekly or bi-weekly based on symptom severity (Figure 22).
Impact on driving. Two studies examined the impact of esketamine on driving performance. One examined adults with major depressive disorder and the other examined healthy participants. The effects of a single 84-mg dose of esketamine nasal spray on a patient’s ability to drive was assessed in 23 healthy adults. In this study, mirt
A second study evaluated the effects of repeated esketamine administration on driving performance in 25 adults with major depressive disorder. In this study, an ethanol-containing beverage was used as an active control. After administration of a single 84-mg dose of intranasal esketamine, driving performance was the same as a placebo at 18 hours. In the multiple dose phase, standard driving performance was similar for esketamine nasal spray and placebo at 6 hours postdose on Days 11, 18, and 25.
Continue to: Pharmacologic profile
Pharmacologic profile
Adverse events. The most common adverse events in patients treated with esketamine nasal spray were dissociation (41%), dizziness (29%), nausea (28%), sedation (23%), and vertigo (23%).2 The majority of these effects were short-term and resolved during the 2-hour observation period.
In addition to spontaneously reported events, sedation and dissociation were further monitored with specific scales. Sedation was measured with the Modified Observer’s Alertness and Sedation Scale. Using this scale, 50% of patients receiving 56 mg and 61% of patients receiving 84 mg of esketamine met criteria for sedation.
Similarly, dissociation/perceptional changes were measured with spontaneously reported events and also with the Clinician Administered Dissociative State Scale. On this scale, 61% of patients receiving the 56-mg dose, and 69% of patients receiving the 84-mg dose met criteria for dissociation/perceptional changes after dose administration.
Increases in blod pressure. Esketamine intranasal spray was associated with a 7 to 9 mm Hg increase in systolic blood pressure and a 4 to 6 mm Hg increase in diastolic blood pressure, both of which peaked 40 minutes post-dose.
Nausea and vomiting. Intranasal esketamine was associated with a 27% rate of nausea at 56 mg, and 32% at 84 mg, with a 6% rate of vomiting at 56 mg and 12% at 84 mg.
Continue to: Pharmacokinetics
Pharmacokinetics
Esketamine exposure increases from 28 to 84 mg in a fairly dose-proportional range. No accumulation of esketamine was observed in the plasma following twice-weekly administration. Bioavailability is approximately 48% following nasal administration. The Tmax for esketamine plasma concentration is 20 to 40 minutes after the last nasal spray. Protein binding of esketamine is approximately 43% to 45%. The brain-to-plasma ratio of noresketamine is 4 to 6 times lower than that of esketamine. The half-life of esketamine ranged from 7 to 12 hours. The mean half-life of nore
Potential drug interactions
Central nervous system depressants. Concomitant use of esketamine and other CNS depressants (ie, benzodiazepines, opioids, alcohol) may increase sedation. Patients receiving esketamine with concomitant use of other CNS depressants should be closely monitored for sedation.
Psychostimulants. Concomitant use of esketamine and psychostimulants (ie, amphetamines, methylphenidates, moda
Monoamine oxidase inhibitors. Concomitant use of esketamine with monoamine oxidase inhibitors may increase blood pressure. Closely monitor blood pressure with concomitant use of esketamine and monoamine oxidase inhibitors.
Use in special populations. Because of concerns of increased sedation, intranasal esketamine should be administered cautiously in patients receiving other CNS depressants, such as benzodiazepines. In patients with psychosis or a prior history of psychosis, esketamine should be used with increased caution and the risk/benefit ratio should be carefully considered.
Continue to: Because of potential teratogenicity...
Because of potential teratogenicity, esketamine is not recommended in women who are pregnant, may become pregnant, or who are currently nursing.
Intranasal esketamine was examined in a phase III trial of 194 patients age ≥65. At the end of 4 weeks, there was no statistically significant difference in groups on the MADRS, the primary efficacy endpoint. There were no overall differences in the safety profile in patients >65 years compared with younger patients; however, the mean esketamine Cmax and area under the curve were higher in older patients compared with younger adults. The mean esketamine half-life was longer in patients with moderate hepatic impairment.
Abuse liability
Esketamine is a CIII controlled substance and concerns about abuse, misuse, and diversion have been taken into account within the REMS drug safety program.2 Patients with a prior history of substance abuse or misuse should be considered with regard to the risk/benefit ratio.
The REMS drug safety program
Due to the nature of its usually transient adverse effects, including sedation, dissociation, hypertension, and nausea, intranasal esketamine will be administered through a REMS drug safety program at certified REMS treatment centers. Certified REMS treatment centers will receive training on how to safely and effectively counsel and monitor patients. Prior to treatment, patients will receive blood pressure monitoring and anticipated adverse effects will be discussed. Patients will be instructed to not eat solid food for 2 hours pre-dose and to not drink anything for 30 minutes prior.
A treatment session consists of nasal administration and a minimum 2-hour post-administration observation period. Blood pressure must be assessed prior to administration and if elevated, (ie, systolic blood pressure >140 mm Hg, diastolic >90 mm Hg), clinicians should consider the risk of short-term increases in blood pressure that may occur. Do not administer if increases in blood pressure or intracranial pressure pose a serious risk.
Continue to: After each intranasal...
After each intranasal administration the patient will be observed for 5 minutes before the second nasal inhaler is utilized and for another 5 minutes when the patient is receiving 84 mg (ie, each inhaler equals 28 mg). After administering, blood pressure should be reassessed at approximately 40 minutes, which corresponds to the Cmax of intranasal esketamine, and periodically thereafter as warranted.
The patient will then be monitored in a quiet environment for a minimum of 2 hours to make sure that dissociative phenomenon, sedation, and hypertensive reactions have normalized prior to discharge from a certified REMS treatment center.
Dosing and administration
Each intranasal device is primed for 2 infusions (1 in each nostril) for a total dose of 28 mg of esketamine. Combinations of devices can be used to adjust the dose as appropriate for individual patients. The recommended starting dose is 56 mg (ie, 2 devices, with a 5-minute gap between devices). The dose can be increased to 84 mg (ie, 3 intranasal devices spaced at 5-minute intervals) by the second dose based on clinical judgment.
The patient will be instructed to recline the head to a 45° angle, clear his or her nostrils prior to the first treatment, and then self-administer a dose to each nostril while holding the reciprocal nostril closed and inhaling. This process is then repeated every 5 minutes for each subsequent device, with a maximum total dose of 3 devices, or 84 mg (Figure 32). The patient will then be monitored for blood pressure, heart rate, and signs of psychologic or physiologic changes for the next 2 hours. Patients may not drive a car or operate any type of motor equipment until the following day after receiving a normal night’s sleep. Patients will be released from the REMS treatment center after 2 hours if both psychological and physical adverse effects have normalized.
Missed treatment sessions. If a patient misses a treatment session and there is worsening of depressive symptoms, consider returning the patient to the previous dosing schedule (ie, every 2 weeks to once weekly, or weekly to twice weekly).
Continue to: Contraindications for...
Contraindications for intranasal esketamine include:
- aneurysmal vascular disease, including thoracic and abdominal aortic, intracranial, and peripheral arterial vessels, or arterial venous malformations
- history of intracerebral hemorrhage
- hypersensitivity to esketamine, ketamine, or any of the excipients.
Clinical considerations
Intranasal esketamine represents a unique delivery system for the first glutamatergic treatment approved for patients with TRD.
Why Rx? Treatment-resistant depression is found in nearly 1 out of 3 patients with currently available monoaminergic antidepressant treatment options. Patients with TRD are at increased risk of physical and psychological impairment, subsequent worsening of their condition, and social and occupational disability.
Bottom Line
Intranasal esketamine is the first glutamatergic treatment option FDA-approved for patients with treatment-resistant depression who have not responded to standard antidepressant treatment options. In short-term trials, intranasal esketamine significantly improved depressive symptoms as quickly as 24 hours after treatment, with significant improvement maintained through 4 weeks of ongoing administration. In addition, intranasal esketamine was shown to significantly decrease time to relapse for patients who had achieved stable remission or stable response.
Related Resource
- Sullivan MG. FDA approves intranasal esketamine for refractory major depressive disorder. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/195712/depression/fda-approves-intranasal-esketamine-refractory-major-depressive. Published March 5, 2019.
Drug Brand Names
Armodafinil • Nuvigil
Duloxetine • Cymbalta
Escitalopram • Lexapro
Esketamine • Spravato
Mirtazapine • Remeron
Modafinil • Provigil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Rush AG, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR D Report. Am J Psychiatry. 2006;163(11):1905-1917.
2. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting anti-depression. Nat Med. 2016;22(3):238-249.
4. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
5. Daly EJ, Trivedi M, Janik A, et al. A randomized withdrawal, double-blind, multicenter study of esketamine nasal spray plus an oral antidepressant for relapse prevention in treatment-resistant depression. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.
6. Wajs E, Aluisio L, Morrison R, et al. Long-term safety of esketamine nasal spray plus oral antidepressants in patients with treatment-resistant depression: phase III open-label safety and efficacy study. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.
1. Rush AG, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR D Report. Am J Psychiatry. 2006;163(11):1905-1917.
2. Spravato [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting anti-depression. Nat Med. 2016;22(3):238-249.
4. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
5. Daly EJ, Trivedi M, Janik A, et al. A randomized withdrawal, double-blind, multicenter study of esketamine nasal spray plus an oral antidepressant for relapse prevention in treatment-resistant depression. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.
6. Wajs E, Aluisio L, Morrison R, et al. Long-term safety of esketamine nasal spray plus oral antidepressants in patients with treatment-resistant depression: phase III open-label safety and efficacy study. Poster presented at the 2018 American Society of Clinical Psychopharmacology Annual Meeting; May 2018; Miami, Florida.
Depression treatment rates rose with expanded insurance coverage
Multiple national policies designed to expand insurance coverage for mental health services in the United States likely contributed to modest increases in treatment for depression, according to an analysis of three national medical expenditure surveys.
for their depression,” wrote Jason M. Hockenberry, PhD, of Emory University in Atlanta and his associates. The study was published in JAMA Psychiatry.
To examine trends in depression treatment and spending, especially after the passage of the Mental Health Parity and Addiction Equity Act in 2008 and the Affordable Care Act in 2010, the authors analyzed responses to the 1998, 2007, and 2015 Medical Expenditure Panel Surveys (MEPSs). The final analysis included 86,216 individuals who were a mean (SD) age of 37.2 years.
From 1998 to 2015, rates of outpatient treatment for depression increased from 2.36 (95% confidence interval, 2.12-2.61) per 100 to 3.47 (95% CI, 3.16-3.79) per 100. The treated prevalence among white survey respondents was more than double that of black respondents in 2015, at 4.00 (95% CI, 3.58-4.43) per 100, compared with 1.91 (95% CI, 1.55-2.28) per 100. Though psychotherapy use declined from 1998 to 2007 and then increased slightly in 2015, the proportion of patients treated using pharmacotherapy stayed relatively constant at 81.9% (95% CI, 77.9%-85.9%) in 1998 and 80.8% (95% CI, 77.9%-83.7%) in 2015.
Total spending on outpatient depression treatment increased from $12,430,000 in 1998 to $15,554,000 in 2007, and $17,404,000 in 2015. The percentage of spending that came from self-pay decreased from 32% in 1998 to 20% in 2015. At the same time, the percentage of spending covered by Medicaid increased, from 19% in 1998 to 36% in 2015.
Dr. Hockenberry and his coauthors acknowledged the limitations of their study, including the pitfalls of relying on national surveys over long periods of time. Specifically, the MEPSs depended in part on inexact measures, such as memory of health care visits; the 2015 survey also had a response rate of only 47.7%. That said, they reinforced their findings by citing how additional surveys that assess major depression – including the 2016 National Survey on Drug Use and Health – “have found similar proportions of treated depression to what we find in the 2015 MEPS.”
The study was supported in part by the Commonwealth Fund, and Dr. Hockenberry also reported receiving grants from the Commonwealth Fund. No other conflicts of interest were reported.
SOURCE: Hockenberry JM et al. JAMA Psychiatry. 2019 Apr 24. doi: 10.1001/jamapsychiatry.2019.0633.
Multiple national policies designed to expand insurance coverage for mental health services in the United States likely contributed to modest increases in treatment for depression, according to an analysis of three national medical expenditure surveys.
for their depression,” wrote Jason M. Hockenberry, PhD, of Emory University in Atlanta and his associates. The study was published in JAMA Psychiatry.
To examine trends in depression treatment and spending, especially after the passage of the Mental Health Parity and Addiction Equity Act in 2008 and the Affordable Care Act in 2010, the authors analyzed responses to the 1998, 2007, and 2015 Medical Expenditure Panel Surveys (MEPSs). The final analysis included 86,216 individuals who were a mean (SD) age of 37.2 years.
From 1998 to 2015, rates of outpatient treatment for depression increased from 2.36 (95% confidence interval, 2.12-2.61) per 100 to 3.47 (95% CI, 3.16-3.79) per 100. The treated prevalence among white survey respondents was more than double that of black respondents in 2015, at 4.00 (95% CI, 3.58-4.43) per 100, compared with 1.91 (95% CI, 1.55-2.28) per 100. Though psychotherapy use declined from 1998 to 2007 and then increased slightly in 2015, the proportion of patients treated using pharmacotherapy stayed relatively constant at 81.9% (95% CI, 77.9%-85.9%) in 1998 and 80.8% (95% CI, 77.9%-83.7%) in 2015.
Total spending on outpatient depression treatment increased from $12,430,000 in 1998 to $15,554,000 in 2007, and $17,404,000 in 2015. The percentage of spending that came from self-pay decreased from 32% in 1998 to 20% in 2015. At the same time, the percentage of spending covered by Medicaid increased, from 19% in 1998 to 36% in 2015.
Dr. Hockenberry and his coauthors acknowledged the limitations of their study, including the pitfalls of relying on national surveys over long periods of time. Specifically, the MEPSs depended in part on inexact measures, such as memory of health care visits; the 2015 survey also had a response rate of only 47.7%. That said, they reinforced their findings by citing how additional surveys that assess major depression – including the 2016 National Survey on Drug Use and Health – “have found similar proportions of treated depression to what we find in the 2015 MEPS.”
The study was supported in part by the Commonwealth Fund, and Dr. Hockenberry also reported receiving grants from the Commonwealth Fund. No other conflicts of interest were reported.
SOURCE: Hockenberry JM et al. JAMA Psychiatry. 2019 Apr 24. doi: 10.1001/jamapsychiatry.2019.0633.
Multiple national policies designed to expand insurance coverage for mental health services in the United States likely contributed to modest increases in treatment for depression, according to an analysis of three national medical expenditure surveys.
for their depression,” wrote Jason M. Hockenberry, PhD, of Emory University in Atlanta and his associates. The study was published in JAMA Psychiatry.
To examine trends in depression treatment and spending, especially after the passage of the Mental Health Parity and Addiction Equity Act in 2008 and the Affordable Care Act in 2010, the authors analyzed responses to the 1998, 2007, and 2015 Medical Expenditure Panel Surveys (MEPSs). The final analysis included 86,216 individuals who were a mean (SD) age of 37.2 years.
From 1998 to 2015, rates of outpatient treatment for depression increased from 2.36 (95% confidence interval, 2.12-2.61) per 100 to 3.47 (95% CI, 3.16-3.79) per 100. The treated prevalence among white survey respondents was more than double that of black respondents in 2015, at 4.00 (95% CI, 3.58-4.43) per 100, compared with 1.91 (95% CI, 1.55-2.28) per 100. Though psychotherapy use declined from 1998 to 2007 and then increased slightly in 2015, the proportion of patients treated using pharmacotherapy stayed relatively constant at 81.9% (95% CI, 77.9%-85.9%) in 1998 and 80.8% (95% CI, 77.9%-83.7%) in 2015.
Total spending on outpatient depression treatment increased from $12,430,000 in 1998 to $15,554,000 in 2007, and $17,404,000 in 2015. The percentage of spending that came from self-pay decreased from 32% in 1998 to 20% in 2015. At the same time, the percentage of spending covered by Medicaid increased, from 19% in 1998 to 36% in 2015.
Dr. Hockenberry and his coauthors acknowledged the limitations of their study, including the pitfalls of relying on national surveys over long periods of time. Specifically, the MEPSs depended in part on inexact measures, such as memory of health care visits; the 2015 survey also had a response rate of only 47.7%. That said, they reinforced their findings by citing how additional surveys that assess major depression – including the 2016 National Survey on Drug Use and Health – “have found similar proportions of treated depression to what we find in the 2015 MEPS.”
The study was supported in part by the Commonwealth Fund, and Dr. Hockenberry also reported receiving grants from the Commonwealth Fund. No other conflicts of interest were reported.
SOURCE: Hockenberry JM et al. JAMA Psychiatry. 2019 Apr 24. doi: 10.1001/jamapsychiatry.2019.0633.
FROM JAMA PSYCHIATRY
Key clinical point: Treatment for – and spending on – depression both saw modest increases from 1998 to 2015.
Major finding: Rates of outpatient treatment for depression increased from 2.36 (95% confidence interval, 2.12-2.61) per 100 in 1998 to 3.47 (95% CI, 3.16-3.79) per 100 in 2015.
Study details: An analysis of 86,216 individuals from the 1998, 2007, and 2015 Medical Expenditure Panel Surveys.
Disclosures: The study was supported in part by the Commonwealth Fund, and the lead author also reported receiving grants from the Commonwealth Fund. No other conflicts of interest were reported.
Source: Hockenberry JM et al. JAMA Psychiatry. 2019 Apr 24. doi: 10.1001/jamapsychiatry.2019.0633.
Losing a patient to suicide: ‘Never Worry Alone’
Losing a patient to suicide is one of the most difficult and painful experiences a psychiatrist will face. In addition to concern for the patient and his or her family, psychiatrists may experience thoughts of responsibility and what they could have done to prevent the suicide. Although often trained in helping patients address grief, psychiatrists may not be as comfortable processing their own grief after the loss of a patient to suicide.
On April 24, MDedge Psychiatry hosted a conversation on Twitter to help psychiatrists examine some of their own feelings about losing patients in this way. Two psychiatrists – Dinah Miller, MD, and Eric Plakun, MD – responded to questions on this topic.
Dr. Miller is the author of numerous books and articles, including “Committed: The Battle Over Involuntary Care” (Baltimore: Johns Hopkins University Press, 2016), which she wrote with Dr. Annette Hanson, and a piece in the New England Journal of Medicine about her own experience with the death of a patient to suicide. She has a private practice in Baltimore and is affiliated with Johns Hopkins University there. Dr. Plakun is the medical director and CEO of the Austen Riggs Center based in Stockbridge, Mass., a “Top 10” U.S. News and World Report “Best Hospital” in psychiatry. He also serves on the board of trustees of the American Psychiatric Association representing New England and Eastern Canada, and was the founding leader of the APA Psychotherapy Caucus. Dr. Plakun is a board-certified psychiatrist, psychoanalyst, former member of the Harvard Medical School clinical faculty, and author of more than 50 publications.
Some of the conversation focused on the impact of patient suicide on young doctors. “I tell the residents: Never Worry Alone,” Dr. Miller wrote. “When in doubt, get supervision, curbside consult, formal paid supervision, or send the patient for a second opinion. Have friends.”
Dr. Miller also wrote that these kinds of losses are difficult for experienced doctors, but “just awful when you’re just starting out. A young psychiatrist I knew lost two patients early on in her career. Hang in there – sending support.”
Dr. Plakun wrote. “Questioning whether one wants to do such work is not unusual.”
The following is an edited version of the discussion.
Question: Have you ever lost a patient to suicide?
Dr. Plakun: This is an important subject and worth bringing to wider discussion. Thirty-eight percent of clinicians experience a significant reaction to this kind of loss.
Dr. Miller: I spent decades worrying that a patient might die by suicide. The reality was more troubling to me than I imagined. It left me more hesitant, less trusting of both the patients and myself. Not always, just at moments. The work we do is hard.
Dr. Plakun: Not one I was working with, but several after we terminated. And it has happened to patients I admitted to Austen Riggs during 35 years as director of admissions.
Dr. Miller: You know, I was struck by the fact that we have no formal way to approach this ... the APA info is for residents. This was part of why I wrote the NEJM piece, to open the conversation.
Question: How do you think the loss of your patient changed your approach to psychiatry?
Dr. Miller: One of the things I’ve heard is that recovering from this tremendous loss helps doctors/therapists to know that others have had a rough time, and that this isn’t weird or odd, and that they are not alone.
Question: How did the loss change you?
Dr. Miller: I did not feel a sense of “blame.” I imagine that would have made it much more difficult. An internist wrote to me discussing how he’d dismissed a patient’s chest pain and the patient died – there are so many layers of complexity here. So many different stories.
Dr. Plakun: I think the impact on us is great because in suicide the deceased is both victim and perpetrator of murder, while we have tried to empathize with both sides.
Dr. Miller: I imagine everyone feels some distress, some emotional response. It’s hard to imagine that a doctor or therapist would hear a patient under their care died of any cause and would feel nothing.
Closing observations
Dr. Plakun: If you lose a patient to suicide, seek consultation and support from a trusted colleague. Remember that isolation will be part of the problem, not the solution. Remember to call your insurance carrier for consultation regarding risk-management issues.
Dr. Miller: I think this topic is difficult for docs/therapists to listen to. It’s hard to sit with a friend/colleague’s pain.
Dr. Plakun: Meeting with family is an issue. The primary purpose of such meetings is to meet their needs – not your own. Help them deal with a traumatic loss causing powerful and complicated feelings. If it helps you, that’s a bonus rather than the goal of the meeting.
References
Miller D. When a patient dies by suicide –The physician’s silent sorrow. (N Engl J Med. 2019 Jan 24;380:311-3).
Plakun EM. Psychotherapy with suicidal patients, Part 1: Expert consensus recommendations. (J Psychiatr Prac. 2018 Nov;249[6]:420-3).
Plakun EM and Jane G. Tillman. Responding to clinicians after loss of a patient to suicide. Directions in Psychiatry. 2005 Oct.
Connect with Dr. Miller on Twitter at @shrinkrapdinah and with Dr. Plakun at @EricPlakunMD. And look for #MDedgeChats to find the complete conversation on Twitter. Dr. Miller and Dr. Plakun will be joining Lorenzo Norris, MD, and Jane Tillman, PhD, for a discussion of this topic from noon to 1 p.m. on Monday, May 20, at the American Psychiatric Association annual meeting in San Francisco for a live recording of the MDedge Psychcast. Join them at booth 1518!
Also, as in past years, Dr. Plakun will join Dr. Tillman to offer a workshop at the APA meeting called “Responding to the Impact of Suicide on Clinicians.” The workshop (session ID: 1054) will be held on Sunday, May 19, 10 a.m. – 11:30 a.m., in Room 153, upper mezzanine, Moscone South.
Losing a patient to suicide is one of the most difficult and painful experiences a psychiatrist will face. In addition to concern for the patient and his or her family, psychiatrists may experience thoughts of responsibility and what they could have done to prevent the suicide. Although often trained in helping patients address grief, psychiatrists may not be as comfortable processing their own grief after the loss of a patient to suicide.
On April 24, MDedge Psychiatry hosted a conversation on Twitter to help psychiatrists examine some of their own feelings about losing patients in this way. Two psychiatrists – Dinah Miller, MD, and Eric Plakun, MD – responded to questions on this topic.
Dr. Miller is the author of numerous books and articles, including “Committed: The Battle Over Involuntary Care” (Baltimore: Johns Hopkins University Press, 2016), which she wrote with Dr. Annette Hanson, and a piece in the New England Journal of Medicine about her own experience with the death of a patient to suicide. She has a private practice in Baltimore and is affiliated with Johns Hopkins University there. Dr. Plakun is the medical director and CEO of the Austen Riggs Center based in Stockbridge, Mass., a “Top 10” U.S. News and World Report “Best Hospital” in psychiatry. He also serves on the board of trustees of the American Psychiatric Association representing New England and Eastern Canada, and was the founding leader of the APA Psychotherapy Caucus. Dr. Plakun is a board-certified psychiatrist, psychoanalyst, former member of the Harvard Medical School clinical faculty, and author of more than 50 publications.
Some of the conversation focused on the impact of patient suicide on young doctors. “I tell the residents: Never Worry Alone,” Dr. Miller wrote. “When in doubt, get supervision, curbside consult, formal paid supervision, or send the patient for a second opinion. Have friends.”
Dr. Miller also wrote that these kinds of losses are difficult for experienced doctors, but “just awful when you’re just starting out. A young psychiatrist I knew lost two patients early on in her career. Hang in there – sending support.”
Dr. Plakun wrote. “Questioning whether one wants to do such work is not unusual.”
The following is an edited version of the discussion.
Question: Have you ever lost a patient to suicide?
Dr. Plakun: This is an important subject and worth bringing to wider discussion. Thirty-eight percent of clinicians experience a significant reaction to this kind of loss.
Dr. Miller: I spent decades worrying that a patient might die by suicide. The reality was more troubling to me than I imagined. It left me more hesitant, less trusting of both the patients and myself. Not always, just at moments. The work we do is hard.
Dr. Plakun: Not one I was working with, but several after we terminated. And it has happened to patients I admitted to Austen Riggs during 35 years as director of admissions.
Dr. Miller: You know, I was struck by the fact that we have no formal way to approach this ... the APA info is for residents. This was part of why I wrote the NEJM piece, to open the conversation.
Question: How do you think the loss of your patient changed your approach to psychiatry?
Dr. Miller: One of the things I’ve heard is that recovering from this tremendous loss helps doctors/therapists to know that others have had a rough time, and that this isn’t weird or odd, and that they are not alone.
Question: How did the loss change you?
Dr. Miller: I did not feel a sense of “blame.” I imagine that would have made it much more difficult. An internist wrote to me discussing how he’d dismissed a patient’s chest pain and the patient died – there are so many layers of complexity here. So many different stories.
Dr. Plakun: I think the impact on us is great because in suicide the deceased is both victim and perpetrator of murder, while we have tried to empathize with both sides.
Dr. Miller: I imagine everyone feels some distress, some emotional response. It’s hard to imagine that a doctor or therapist would hear a patient under their care died of any cause and would feel nothing.
Closing observations
Dr. Plakun: If you lose a patient to suicide, seek consultation and support from a trusted colleague. Remember that isolation will be part of the problem, not the solution. Remember to call your insurance carrier for consultation regarding risk-management issues.
Dr. Miller: I think this topic is difficult for docs/therapists to listen to. It’s hard to sit with a friend/colleague’s pain.
Dr. Plakun: Meeting with family is an issue. The primary purpose of such meetings is to meet their needs – not your own. Help them deal with a traumatic loss causing powerful and complicated feelings. If it helps you, that’s a bonus rather than the goal of the meeting.
References
Miller D. When a patient dies by suicide –The physician’s silent sorrow. (N Engl J Med. 2019 Jan 24;380:311-3).
Plakun EM. Psychotherapy with suicidal patients, Part 1: Expert consensus recommendations. (J Psychiatr Prac. 2018 Nov;249[6]:420-3).
Plakun EM and Jane G. Tillman. Responding to clinicians after loss of a patient to suicide. Directions in Psychiatry. 2005 Oct.
Connect with Dr. Miller on Twitter at @shrinkrapdinah and with Dr. Plakun at @EricPlakunMD. And look for #MDedgeChats to find the complete conversation on Twitter. Dr. Miller and Dr. Plakun will be joining Lorenzo Norris, MD, and Jane Tillman, PhD, for a discussion of this topic from noon to 1 p.m. on Monday, May 20, at the American Psychiatric Association annual meeting in San Francisco for a live recording of the MDedge Psychcast. Join them at booth 1518!
Also, as in past years, Dr. Plakun will join Dr. Tillman to offer a workshop at the APA meeting called “Responding to the Impact of Suicide on Clinicians.” The workshop (session ID: 1054) will be held on Sunday, May 19, 10 a.m. – 11:30 a.m., in Room 153, upper mezzanine, Moscone South.
Losing a patient to suicide is one of the most difficult and painful experiences a psychiatrist will face. In addition to concern for the patient and his or her family, psychiatrists may experience thoughts of responsibility and what they could have done to prevent the suicide. Although often trained in helping patients address grief, psychiatrists may not be as comfortable processing their own grief after the loss of a patient to suicide.
On April 24, MDedge Psychiatry hosted a conversation on Twitter to help psychiatrists examine some of their own feelings about losing patients in this way. Two psychiatrists – Dinah Miller, MD, and Eric Plakun, MD – responded to questions on this topic.
Dr. Miller is the author of numerous books and articles, including “Committed: The Battle Over Involuntary Care” (Baltimore: Johns Hopkins University Press, 2016), which she wrote with Dr. Annette Hanson, and a piece in the New England Journal of Medicine about her own experience with the death of a patient to suicide. She has a private practice in Baltimore and is affiliated with Johns Hopkins University there. Dr. Plakun is the medical director and CEO of the Austen Riggs Center based in Stockbridge, Mass., a “Top 10” U.S. News and World Report “Best Hospital” in psychiatry. He also serves on the board of trustees of the American Psychiatric Association representing New England and Eastern Canada, and was the founding leader of the APA Psychotherapy Caucus. Dr. Plakun is a board-certified psychiatrist, psychoanalyst, former member of the Harvard Medical School clinical faculty, and author of more than 50 publications.
Some of the conversation focused on the impact of patient suicide on young doctors. “I tell the residents: Never Worry Alone,” Dr. Miller wrote. “When in doubt, get supervision, curbside consult, formal paid supervision, or send the patient for a second opinion. Have friends.”
Dr. Miller also wrote that these kinds of losses are difficult for experienced doctors, but “just awful when you’re just starting out. A young psychiatrist I knew lost two patients early on in her career. Hang in there – sending support.”
Dr. Plakun wrote. “Questioning whether one wants to do such work is not unusual.”
The following is an edited version of the discussion.
Question: Have you ever lost a patient to suicide?
Dr. Plakun: This is an important subject and worth bringing to wider discussion. Thirty-eight percent of clinicians experience a significant reaction to this kind of loss.
Dr. Miller: I spent decades worrying that a patient might die by suicide. The reality was more troubling to me than I imagined. It left me more hesitant, less trusting of both the patients and myself. Not always, just at moments. The work we do is hard.
Dr. Plakun: Not one I was working with, but several after we terminated. And it has happened to patients I admitted to Austen Riggs during 35 years as director of admissions.
Dr. Miller: You know, I was struck by the fact that we have no formal way to approach this ... the APA info is for residents. This was part of why I wrote the NEJM piece, to open the conversation.
Question: How do you think the loss of your patient changed your approach to psychiatry?
Dr. Miller: One of the things I’ve heard is that recovering from this tremendous loss helps doctors/therapists to know that others have had a rough time, and that this isn’t weird or odd, and that they are not alone.
Question: How did the loss change you?
Dr. Miller: I did not feel a sense of “blame.” I imagine that would have made it much more difficult. An internist wrote to me discussing how he’d dismissed a patient’s chest pain and the patient died – there are so many layers of complexity here. So many different stories.
Dr. Plakun: I think the impact on us is great because in suicide the deceased is both victim and perpetrator of murder, while we have tried to empathize with both sides.
Dr. Miller: I imagine everyone feels some distress, some emotional response. It’s hard to imagine that a doctor or therapist would hear a patient under their care died of any cause and would feel nothing.
Closing observations
Dr. Plakun: If you lose a patient to suicide, seek consultation and support from a trusted colleague. Remember that isolation will be part of the problem, not the solution. Remember to call your insurance carrier for consultation regarding risk-management issues.
Dr. Miller: I think this topic is difficult for docs/therapists to listen to. It’s hard to sit with a friend/colleague’s pain.
Dr. Plakun: Meeting with family is an issue. The primary purpose of such meetings is to meet their needs – not your own. Help them deal with a traumatic loss causing powerful and complicated feelings. If it helps you, that’s a bonus rather than the goal of the meeting.
References
Miller D. When a patient dies by suicide –The physician’s silent sorrow. (N Engl J Med. 2019 Jan 24;380:311-3).
Plakun EM. Psychotherapy with suicidal patients, Part 1: Expert consensus recommendations. (J Psychiatr Prac. 2018 Nov;249[6]:420-3).
Plakun EM and Jane G. Tillman. Responding to clinicians after loss of a patient to suicide. Directions in Psychiatry. 2005 Oct.
Connect with Dr. Miller on Twitter at @shrinkrapdinah and with Dr. Plakun at @EricPlakunMD. And look for #MDedgeChats to find the complete conversation on Twitter. Dr. Miller and Dr. Plakun will be joining Lorenzo Norris, MD, and Jane Tillman, PhD, for a discussion of this topic from noon to 1 p.m. on Monday, May 20, at the American Psychiatric Association annual meeting in San Francisco for a live recording of the MDedge Psychcast. Join them at booth 1518!
Also, as in past years, Dr. Plakun will join Dr. Tillman to offer a workshop at the APA meeting called “Responding to the Impact of Suicide on Clinicians.” The workshop (session ID: 1054) will be held on Sunday, May 19, 10 a.m. – 11:30 a.m., in Room 153, upper mezzanine, Moscone South.
Anxiety, not depression, commonly afflicts euthyroid patients with thyroid disease
LOS ANGELES – results from a cross-sectional study have shown.
“Thyroid disease is often associated with impaired quality of life and psychological well-being,” lead study author Anette Merke, MD, MS, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “In daily practice, anxiety and depression complaints are common in patients with thyroid dysfunctions. While depressive symptoms are often associated with hypothyroidism, anxiety is claimed to be mainly linked to hyperthyroidism. Data on euthyroid patients with thyroid disease are controversial. Some studies point out that autoimmunity itself contributes to psychosomatic malfunctions. Overall, the mechanisms underlying the interaction between thyroid dysfunction and neuropsychiatric processes are still unknown.”
Dr. Merke, of the Thyroid Center Bergstrasse in Bensheim, Germany, and her husband/coauthor Jüergen Merke, MD, PhD, used the self-administered German version of the validated Hospital Anxiety and Depression Scale (HADS-D) to perform a cross-sectional study of 215 euthyroid adults with thyroid disease between January and February of 2019. Of the 14 items on the measure, half relate to anxiety and the other half to depression. Each item on the HADS-D is scored from 0-3, and a score of 10 or higher is considered a positive case of anxiety or depression. Patients completed the HADS-D within 3 months of routine lab testing, and the researchers collected the patients’ demographic data after they had assessed the individual scores.
Of the 215 study participants, most (89%) were women, the mean age was 47 years, and the mean anxiety and depression scores were 6.68 and 4.68, respectively (P = .0001). There was no significant difference in severity with respect to anxiety or depression. Of the 70 patients (33%) with antibody-positive Hashimoto’s thyroiditis, the mean anxiety and depression scores were 7.26 and 4.17.
In patients with HADS-D scores of 10 or greater, 50 (23%) had prominent anxiety scores (mean, 12.4), whereas 22 (10%) had prominent depression scores (mean, 13.18). Among the subset of Hashimoto’s thyroiditis patients, 18 (26%) had a mean anxiety score of 12.6 and 8 (11%) had a mean depression score of 13.18. Overall, significantly more cases were found in those who met criteria for anxiety, compared with those who met criteria for depression (P = .0001), with no significant difference in severity of either condition.
Depressive symptoms are usually more closely associated with thyroid disease, and there are more studies that have examined that relationship, so “we were surprised to find no significant difference in depressive symptoms between our study cohort and the German general population,” Dr. Merke said. “We were also surprised that anxiety had a significantly higher incidence in the cohort.”
The findings suggest that clinicians should focus on signs of anxiety symptoms when dealing with euthyroid patients with thyroid disease who report psychosomatic impairments, she continued, especially when patients complain of not being able to relax and release somatic tension.
“According to HADS, fears and worries are an expression of anxiety, and not of depression,” Dr. Merke said. “With this in mind, doctors [should] actively ask [about] the above-mentioned symptoms and advise patients to learn relaxation techniques to improve their quality of life. Interdisciplinary collaboration is needed between clinicians and psychotherapeutic professionals for the sake of the patients and to evaluate a cause-and-effect relationship and potential risk factors for development of psychosomatic cofactors in thyroid and other chronic somatic diseases. We should all be aware that misinterpretation or even denial of psychosomatic complaints may lead to complications and even a higher mortality of somatic diseases, as shown for chronic heart failure. This could also be true for thyroid disease.”
Dr Merke acknowledged certain limitations of the study, including the fact that neither the duration of thyroid disease nor the use of specific thyroid medications was assessed. In addition, “the definition of euthyroidism in our study is somewhat broad, especially compared with the recommendations of the [American Association of Clinical Endocrinologists],” she said. “Division of the respective thyroid hormone status into quartiles may be helpful to indicate the critical thyroid hormone serum concentration, which may be associated with clinically relevant anxiety symptoms in euthyroid patients.”
Dr. Merke reported having no financial disclosures or conflict of interest.
LOS ANGELES – results from a cross-sectional study have shown.
“Thyroid disease is often associated with impaired quality of life and psychological well-being,” lead study author Anette Merke, MD, MS, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “In daily practice, anxiety and depression complaints are common in patients with thyroid dysfunctions. While depressive symptoms are often associated with hypothyroidism, anxiety is claimed to be mainly linked to hyperthyroidism. Data on euthyroid patients with thyroid disease are controversial. Some studies point out that autoimmunity itself contributes to psychosomatic malfunctions. Overall, the mechanisms underlying the interaction between thyroid dysfunction and neuropsychiatric processes are still unknown.”
Dr. Merke, of the Thyroid Center Bergstrasse in Bensheim, Germany, and her husband/coauthor Jüergen Merke, MD, PhD, used the self-administered German version of the validated Hospital Anxiety and Depression Scale (HADS-D) to perform a cross-sectional study of 215 euthyroid adults with thyroid disease between January and February of 2019. Of the 14 items on the measure, half relate to anxiety and the other half to depression. Each item on the HADS-D is scored from 0-3, and a score of 10 or higher is considered a positive case of anxiety or depression. Patients completed the HADS-D within 3 months of routine lab testing, and the researchers collected the patients’ demographic data after they had assessed the individual scores.
Of the 215 study participants, most (89%) were women, the mean age was 47 years, and the mean anxiety and depression scores were 6.68 and 4.68, respectively (P = .0001). There was no significant difference in severity with respect to anxiety or depression. Of the 70 patients (33%) with antibody-positive Hashimoto’s thyroiditis, the mean anxiety and depression scores were 7.26 and 4.17.
In patients with HADS-D scores of 10 or greater, 50 (23%) had prominent anxiety scores (mean, 12.4), whereas 22 (10%) had prominent depression scores (mean, 13.18). Among the subset of Hashimoto’s thyroiditis patients, 18 (26%) had a mean anxiety score of 12.6 and 8 (11%) had a mean depression score of 13.18. Overall, significantly more cases were found in those who met criteria for anxiety, compared with those who met criteria for depression (P = .0001), with no significant difference in severity of either condition.
Depressive symptoms are usually more closely associated with thyroid disease, and there are more studies that have examined that relationship, so “we were surprised to find no significant difference in depressive symptoms between our study cohort and the German general population,” Dr. Merke said. “We were also surprised that anxiety had a significantly higher incidence in the cohort.”
The findings suggest that clinicians should focus on signs of anxiety symptoms when dealing with euthyroid patients with thyroid disease who report psychosomatic impairments, she continued, especially when patients complain of not being able to relax and release somatic tension.
“According to HADS, fears and worries are an expression of anxiety, and not of depression,” Dr. Merke said. “With this in mind, doctors [should] actively ask [about] the above-mentioned symptoms and advise patients to learn relaxation techniques to improve their quality of life. Interdisciplinary collaboration is needed between clinicians and psychotherapeutic professionals for the sake of the patients and to evaluate a cause-and-effect relationship and potential risk factors for development of psychosomatic cofactors in thyroid and other chronic somatic diseases. We should all be aware that misinterpretation or even denial of psychosomatic complaints may lead to complications and even a higher mortality of somatic diseases, as shown for chronic heart failure. This could also be true for thyroid disease.”
Dr Merke acknowledged certain limitations of the study, including the fact that neither the duration of thyroid disease nor the use of specific thyroid medications was assessed. In addition, “the definition of euthyroidism in our study is somewhat broad, especially compared with the recommendations of the [American Association of Clinical Endocrinologists],” she said. “Division of the respective thyroid hormone status into quartiles may be helpful to indicate the critical thyroid hormone serum concentration, which may be associated with clinically relevant anxiety symptoms in euthyroid patients.”
Dr. Merke reported having no financial disclosures or conflict of interest.
LOS ANGELES – results from a cross-sectional study have shown.
“Thyroid disease is often associated with impaired quality of life and psychological well-being,” lead study author Anette Merke, MD, MS, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “In daily practice, anxiety and depression complaints are common in patients with thyroid dysfunctions. While depressive symptoms are often associated with hypothyroidism, anxiety is claimed to be mainly linked to hyperthyroidism. Data on euthyroid patients with thyroid disease are controversial. Some studies point out that autoimmunity itself contributes to psychosomatic malfunctions. Overall, the mechanisms underlying the interaction between thyroid dysfunction and neuropsychiatric processes are still unknown.”
Dr. Merke, of the Thyroid Center Bergstrasse in Bensheim, Germany, and her husband/coauthor Jüergen Merke, MD, PhD, used the self-administered German version of the validated Hospital Anxiety and Depression Scale (HADS-D) to perform a cross-sectional study of 215 euthyroid adults with thyroid disease between January and February of 2019. Of the 14 items on the measure, half relate to anxiety and the other half to depression. Each item on the HADS-D is scored from 0-3, and a score of 10 or higher is considered a positive case of anxiety or depression. Patients completed the HADS-D within 3 months of routine lab testing, and the researchers collected the patients’ demographic data after they had assessed the individual scores.
Of the 215 study participants, most (89%) were women, the mean age was 47 years, and the mean anxiety and depression scores were 6.68 and 4.68, respectively (P = .0001). There was no significant difference in severity with respect to anxiety or depression. Of the 70 patients (33%) with antibody-positive Hashimoto’s thyroiditis, the mean anxiety and depression scores were 7.26 and 4.17.
In patients with HADS-D scores of 10 or greater, 50 (23%) had prominent anxiety scores (mean, 12.4), whereas 22 (10%) had prominent depression scores (mean, 13.18). Among the subset of Hashimoto’s thyroiditis patients, 18 (26%) had a mean anxiety score of 12.6 and 8 (11%) had a mean depression score of 13.18. Overall, significantly more cases were found in those who met criteria for anxiety, compared with those who met criteria for depression (P = .0001), with no significant difference in severity of either condition.
Depressive symptoms are usually more closely associated with thyroid disease, and there are more studies that have examined that relationship, so “we were surprised to find no significant difference in depressive symptoms between our study cohort and the German general population,” Dr. Merke said. “We were also surprised that anxiety had a significantly higher incidence in the cohort.”
The findings suggest that clinicians should focus on signs of anxiety symptoms when dealing with euthyroid patients with thyroid disease who report psychosomatic impairments, she continued, especially when patients complain of not being able to relax and release somatic tension.
“According to HADS, fears and worries are an expression of anxiety, and not of depression,” Dr. Merke said. “With this in mind, doctors [should] actively ask [about] the above-mentioned symptoms and advise patients to learn relaxation techniques to improve their quality of life. Interdisciplinary collaboration is needed between clinicians and psychotherapeutic professionals for the sake of the patients and to evaluate a cause-and-effect relationship and potential risk factors for development of psychosomatic cofactors in thyroid and other chronic somatic diseases. We should all be aware that misinterpretation or even denial of psychosomatic complaints may lead to complications and even a higher mortality of somatic diseases, as shown for chronic heart failure. This could also be true for thyroid disease.”
Dr Merke acknowledged certain limitations of the study, including the fact that neither the duration of thyroid disease nor the use of specific thyroid medications was assessed. In addition, “the definition of euthyroidism in our study is somewhat broad, especially compared with the recommendations of the [American Association of Clinical Endocrinologists],” she said. “Division of the respective thyroid hormone status into quartiles may be helpful to indicate the critical thyroid hormone serum concentration, which may be associated with clinically relevant anxiety symptoms in euthyroid patients.”
Dr. Merke reported having no financial disclosures or conflict of interest.
REPORTING FROM AACE 2019
Key clinical point: Anxiety in patients with thyroid disease might have a pathological pathway different from depressive disorders.
Major finding: In patients with HADS-D scores of 10 or greater, 50 (23%) had prominent anxiety scores (mean, 12.4) and 22 (10%) had prominent depression scores (mean, 13.18).
Study details: A cross-sectional study of 215 euthyroid patients with thyroid disease.
Disclosures: Dr. Merke reported having no financial disclosures or conflicts of interest.
Suicide barriers on the Golden Gate Bridge: Will they save lives?
Ultimately, we need to find better treatments for depression and anxiety
San Francisco entrances people. Photographers capture more images of the Golden Gate Bridge than any other bridge in the world.1 And only the Nanjing Yangtze River Bridge in China surpasses the Golden Gate as a destination for dying by suicide.2 At least 1,700 people reportedly have plunged from the bridge to their deaths since its opening in 1937.3
Despite concerted efforts by bridge security, the local mental health community, and a volunteer organization – Bridgewatch Angels – suicides continue at the pace of about 1 every 2 weeks. After more than 60 years of discussion, transportation officials allocated funding and have started building a suicide prevention barrier system on the Golden Gate.
Extrapolating from the success of barriers built on other bridges that were “suicide magnets,” we should be able to assure people that suicide deaths from the Golden Gate will dramatically decrease, and perhaps cease completely.4 Certainly, some in the mental health community think this barrier will save lives. They support this claim by citing research showing that removing highly accessible and lethal means of suicide reduces overall suicide rates, and that suicidal individuals, when thwarted, do not seek alternate modes of death.
I support building the Golden Gate suicide barrier, partly because symbolically, it should deliver a powerful message that we value all human life. But will the barrier save lives? I don’t think it will. As the American Psychiatric Association prepares to gather for its annual meeting in San Francisco, I would like to share my reasoning.
What the evidence shows
The most robust evidence that restricting availability of highly lethal and accessible means of suicide reduces overall suicide deaths comes from studies looking at self-poisoning in Asian countries and Great Britain. In many parts of Asia, ingestion of pesticides constitutes a significant proportion of suicide deaths, and several studies have found that, in localities where sales of highly lethal pesticides were restricted, overall suicide deaths decreased.5,6 Conversely, suicide rates increased when more lethal varieties of pesticides became more available. In Great Britain, overall suicide rates decreased when natural gas replaced coal gas for home heating and cooking.7 For decades preceding this change, more Britons had killed themselves by inhaling coal gas than by any other method.
Strong correlations exist between regional levels of gun ownership and suicide rates by shooting,8 but several potentially confounding sociopolitical factors explain some portion of this connection. Stronger evidence of gun availability affecting suicide rates has been demonstrated by decreases in suicide rates after restrictions in gun access in Switzerland,9 Israel,10 and other areas. These studies show correlations – not causality. However, the number of studies, links between increases and decreases in suicide rates with changes in access to guns, absence of changes in suicide rates during the same time periods among ostensibly similar control populations, and lack of other compelling explanations support the argument that restricting access to highly lethal and accessible means of suicide prevents suicide deaths overall.
The installation of suicide barriers on bridges that have been the sites of multiple suicides robustly reduces or even eliminates suicide deaths from those bridges,11 but the effect on overall suicide rates remains less clear. Various studies have found subsequent increases or no changes12-14 in suicide deaths from other bridges or tall buildings in the vicinity after the installation of suicide barriers on a “suicide magnet.” Many of the studies failed to find any impact on overall suicide rates in the regions investigated. Deaths from jumping off tall structures constitute a tiny proportion of total suicide deaths, making it difficult to detect any changes in overall suicide rates. In the United States, suicides by jumping/falling constituted 1%-2% of total suicides over the last several decades.15
If we know that restricting highly lethal and accessible methods of killing reduces suicide deaths, why would I question the value of the Golden Gate suicide barrier in preventing overall suicide deaths?
Unique aspects of the bridge
The World High Dive Federation recommends keeping dives to less than 20 meters (65.5 feet), with a few exceptions.16 The rail of the Golden Gate Bridge stands 67 meters (220 feet) above the water, and assuming minimal wind resistance, a falling person traverses that distance in about 3.7 seconds and lands with an impact of 130 km/hour (81 miles per hour).17 Only about 1%-2% of those jumping from the Golden Gate survive that fall.18
A 99% likelihood of death sounds pretty lethal; however, death by jumping from the Golden Gate inherently takes place in a public space, with the opportunity for interventions by other people. A more realistic calculation of the lethality would start the instant that someone initiates a sequence of behaviors leading to the intended death. By that criteria, measuring the lethality of the Golden Gate would begin when an individual enters a vehicle or sets off on foot with the plan of going over the railing.
Unless our surveillance-oriented society makes substantially greater advances (which I oppose), we will remain unable to assess suicide lethality by starting at the moment of inception. However, we do have data showing what happens once someone with suicidal intentions walks onto the bridge.
Between 2000 and 2018, observers noted 2,546 people on the Golden Gate who appeared to be considering a suicide attempt, the San Francisco Chronicle has reported. Five hundred sixty-four confirmed suicides occurred. In an additional 71 cases, suicide is presumed but bodies were not recovered. In the 1,911 remaining instances, mental health interventions were made, with individuals taken to local hospitals and psychiatric wards, and released when no longer overtly suicidal. Interventions successfully diverted 75% (1,911/2,546) of those intending to end their own lives, which suggests that the current lethality of the Golden Gate as a means of suicide is only 25%. Even in the bridge’s first half-century, without constant camera monitoring, and a cadre of volunteers and professionals scanning for those attempting suicide, the lethality rate approached about 50%.19
We face even more difficulties measuring accessibility than in determining lethality. The Golden Gate appears to be accessible to almost anyone – drivers have to pay a toll only when traveling from the north, and then only after they have traversed the span. Pedestrians retain unfettered admittance to the east sidewalk (facing San Francisco city and bay) throughout daylight hours. But any determination of accessibility must include how quickly and easily one can make use of an opportunity.
Both entrances to the Golden Gate are embedded in the Golden Gate National Recreational Area, part of the National Park system. The south entrance to the bridge arises from The Presidio, a former military installation that housed about 4,000 people.20 Even fewer people live in the parklands at the north end of the bridge. The Presidio extends far enough so that the closest San Francisco neighborhoods outside of the park are a full 2.2 km (1.36 miles) from the bridge railing. A brisk walk would still require a minimum of about 20 minutes to get to the bridge; it is difficult to arrive at the bridge without a trek.
Researchers define impulsivity, like accessibility, inconsistently – and often imprecisely. Impulsivity, which clearly exists on a spectrum, connotes overvaluing of immediate feelings and thoughts at the expense of longer term goals and aspirations. Some suicide research appears to define impulsivity as the antithesis of planned behavior;21,22 others define it pragmatically as behaviors executed within 5 minutes of a decision,23 and still others contend that “suicidal behavior is rarely if ever impulsive.”24 Furthermore, when we assess impulsivity, we must acknowledge a fundamental difference between “impulsive” shootings and poisonings that are accomplished at home and within seconds or minutes, from “impulsive” Golden Gate Bridge suicide attempts, which require substantial travel and time commitments, and inherently involve the potential for others to intervene.
Those arguing that the bridge suicide barrier will save lives often bring up two additional sets of numbers to back up their assertions. They provide evidence that most of those people who were stopped in their attempts at suicide at the Golden Gate do not go on to commit suicide elsewhere, and that many of those who survived their attempts express regret at having tried to kill themselves. Specifically, 94% of those who were prevented from jumping from the Golden Gate had not committed suicide after a median follow-up of 26 years, according to a follow-up study published a few years ago. On the other hand, those who have made a serious suicide attempt have a substantially increased risk, relative to the general population, of dying from a later attempt,25,26 and the strongest predictor for death by suicide is having made a previous, serious suicide attempt.27
While all of these studies provide important and interesting information regarding suicide, none directly address the question of whether individuals will substitute attempts by other methods if the Golden Gate Bridge were no longer available. Many discussions blur the distinction between how individuals behave after a thwarted Golden Gate suicide attempt and how other people might act if we secured the bridge from any potential future suicide attempts. I hope that the following analogy makes this distinction clearer without trivializing: Imagine that we know that everyone who was interrupted while eating their dinner in a particular restaurant never went back and ate out anywhere, ever again. We could not conclude from this that another individual, who learned that the intended restaurant was indefinitely closed, would never dine out again. Once effective suicide barriers exist on the Golden Gate, this will likely become widely known, thereby greatly reducing the likelihood that any individuals will consider the possibility of jumping from the bridge. But it seems very unlikely that this would vanquish all suicidal impulses from the northern California population.
Lessons from patients
Two former patients of mine ended their lives by suicide from the Golden Gate. P, a solitary and lonely man in his 50s, was referred to me by his neighbor, Q, one of my long-term patients. P had a history of repeated assessments for lifelong depression, with minimal follow-up. I made a treatment plan with P that we hoped would address both his depression and his reluctance to engage with mental health professionals. He did not return for his follow-up appointment and ignored all my attempts to contact him.
P continued to have intermittent contact with Q. A decade after I had evaluated him, P was finally hospitalized for depression. Since P had no local family or friends, he asked Q to pick him up from the hospital at the time of his discharge. P asked Q to drive him to the Golden Gate Bridge, ostensibly to relish his release by partaking of the panoramic view of San Francisco from the bridge. They parked in the lot at the north end of the bridge, where Q stayed with the car at the vista point. The last that anyone saw of P was when Q noticed him walking on the bridge; nobody saw him go over and his body was not recovered.
In contrast to my brief connection with P, I worked with S over the course of 8 years to deal with her very severe attention-deficit/hyperactivity disorder and associated depression, which destroyed jobs and friendships, and estranged her from her family. She moved to Hawaii in hopes of “starting over with less baggage,” but I received a few phone calls over the next few years detailing suicide attempts, including driving her car off a bridge. Floundering in life, she returned to San Francisco and was hospitalized with suicidal ideation. The inpatient team sedated her heavily, ignored her past treatments and diagnoses, and discharged her after several days. Within a day of discharge, S’s sister called to say that S’s body had been recovered from the water below the bridge.
I don’t think that suicide was inevitable for either P or S, but I also lack any indication that either would be alive today had we installed suicide barriers on the Golden Gate years ago. Unless we eliminate access to guns, cars, trains, poisons, ropes, tall buildings and cliffs, people contemplating suicide will have numerous options at their disposal. We are likely to save lives by continuing to find ways to restrict access to means of death that can be used within seconds and have a high degree of lethality, and we should persist with such efforts. Buying a $5 trigger lock for every gun in California, and spending tens of millions on a public service campaign would cost less and may well save more lives than the Golden Gate suicide barrier. Unfortunately, we still possess very limited knowledge regarding which suicide prevention measures have an “impact on actual deaths or behavior.”28
To increase our efficacy in reducing suicide, we need to find better treatments for depression and anxiety. We also need to identify better ways of targeting those most at risk for suicide,29 improve our delivery of such treatments, and mitigate the social factors that contribute to such misery and unhappiness.
As a psychiatrist who has lost not only patients but also family members to suicide, I appreciate the hole in the soul these deaths create. I understand the drive to find ways to prevent additional deaths and save future survivors from such grief. But we must design psychiatric interventions that do the maximum good. To be imprecise in the lessons we learn from those who have killed themselves doubles down on the disservice to those lives already lost.
Dr. Kruse is a psychiatrist who practices in San Francisco. Several key details about the patients were changed to protect confidentiality.
References
1. Frommer’s Comprehensive Travel Guide, California. New York: Prentice Hall Travel, 1993.
2. “Chen Si, the ‘Angel of Nanjing,’ has saved more than 330 people from suicide,” by Matt Young, News.com.au. May 14, 2017.
3. “Finding Kyle,” by Lizzie Johnson, San Francisco Chronicle. Feb 8, 2019.
4. Beautrais A. Suicide by jumping. A review of research and prevention strategies. Crisis. 2007 Jan;28 Suppl 1:58-63. Crisis: The J of Crisis Interven Suicide Preven. 2007 Jan. (28)[Suppl1]:58-63.
5. Gunnell D et al. The global distribution of fatal pesticide self-poisoning: Systematic review. BMC Public Health. 2007 Dec. 21;7:357.
6. Vijayakumar L and Satheesh-Babu R. Does ‘no pesticide’ reduce suicides? Int J Soc Psychiatry. 2009 Jul 17;55:401-6.
7. Kreitman N. The coal gas story. United Kingdom suicide rates, 1960-71. Br J Prev Soc Med. 1976 Jun;30(2)86-93.
8. Ajdacic-Gross V et al. Changing times: A longitudinal analysis of international firearm suicide data. Am J Public Health. 2006 Oct;96(10):1752-5.
9. Reisch T et al. Change in suicide rates in Switzerland before and after firearm restriction resulting from the 2003 “Army XXI” reform. Am J Psychiatry. 2013 Sep170(9):977-84.
10. Lubin G et al. Decrease in suicide rates after a change of policy reducing access to firearms in adolescents: A naturalistic epidemiological study. Suicide Life Threat Behav. 2010 Oct;40(5):421-4.
11. Sinyor M and Levitt A. Effect of a barrier at Bloor Street Viaduct on suicide rates in Toronto: Natural experiment BMJ. 2010;341. doi: 1136/bmjc2884.
12. O’Carroll P and Silverman M. Community suicide prevention: The effectiveness of bridge barriers. Suicide Life Threat Behav. 1994 Spring;24(1):89-91; discussion 91-9.
13. Pelletier A. Preventing suicide by jumping: The effect of a bridge safety fence. Inj Prev. 2007 Feb;13(1):57-9.
14. Bennewith O et al. Effect of barriers on the Clifton suspension bridge, England, on local patterns of suicide: Implications for prevention. Br J Psychiatry. 2007 Mar;190:266-7.
15. Harvard T.H. Chan School of Public Health. 2004. “How do people most commonly complete suicide?”
16. “How cliff diving works,” by Heather Kolich, HowStuffWorks.com. Oct 5, 2009.
17. “Bridge design and construction statistics.” Goldengate.org
18. “How did teen survive fall from Golden Gate Bridge?” by Remy Molina, Live Science. Apr 19, 2011.
19. Seiden R. Where are they now? A follow-up study of suicide attempters from the Golden Gate Bridge. Suicide Life Threat Behav. 1978 Winter;8(4):203-16.
20. Presidio demographics. Point2homes.com.
21. Baca-García E et al. A prospective study of the paradoxical relationship between impulsivity and lethality of suicide attempts. J Clin Psychiatry. 2001 Jul;62(7):560-4.
22. Lim M et al. Differences between impulsive and non-impulsive suicide attempts among individuals treated in emergency rooms of South Korea. Psychiatry Investig. 2016 Jul;13(4):389-96.
23. Simon O et al. Characteristics of impulsive suicide attempts and attempters. Suicide Life Threat Behav. 2001;32(1 Suppl):49-59.
24. Anestis M et al. Reconsidering the link between impulsivity and suicidal behavior. Pers Soc Psychol Rev. 2014 Nov;18(4):366-86.
25. Ostamo A et al. Excess mortality of suicide attempters. Psychiatry Psychiatr Epidemiol. 2001 Jan;36(1):29-35.
26. Leon A et al. Statistical issues in the identification of risk factors for suicidal behavior: The application of survival analysis. Psychiatry Res. 1990 Jan;31(1):99-108.
27. Bostwick J et al. Suicide attempt as a risk factor for completed suicide: Even more lethal than we knew. Am J Psychiatry. 2016 Nov 1;173(11):1094-100.
28. Stone D and Crosby A. Suicide prevention. Am J Lifestyle Med. 2014;8(6):404-20.
29. Belsher B et al. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Mar 13. doi: 10.1001/jamapsychiatry.2019.0174.
Ultimately, we need to find better treatments for depression and anxiety
Ultimately, we need to find better treatments for depression and anxiety
San Francisco entrances people. Photographers capture more images of the Golden Gate Bridge than any other bridge in the world.1 And only the Nanjing Yangtze River Bridge in China surpasses the Golden Gate as a destination for dying by suicide.2 At least 1,700 people reportedly have plunged from the bridge to their deaths since its opening in 1937.3
Despite concerted efforts by bridge security, the local mental health community, and a volunteer organization – Bridgewatch Angels – suicides continue at the pace of about 1 every 2 weeks. After more than 60 years of discussion, transportation officials allocated funding and have started building a suicide prevention barrier system on the Golden Gate.
Extrapolating from the success of barriers built on other bridges that were “suicide magnets,” we should be able to assure people that suicide deaths from the Golden Gate will dramatically decrease, and perhaps cease completely.4 Certainly, some in the mental health community think this barrier will save lives. They support this claim by citing research showing that removing highly accessible and lethal means of suicide reduces overall suicide rates, and that suicidal individuals, when thwarted, do not seek alternate modes of death.
I support building the Golden Gate suicide barrier, partly because symbolically, it should deliver a powerful message that we value all human life. But will the barrier save lives? I don’t think it will. As the American Psychiatric Association prepares to gather for its annual meeting in San Francisco, I would like to share my reasoning.
What the evidence shows
The most robust evidence that restricting availability of highly lethal and accessible means of suicide reduces overall suicide deaths comes from studies looking at self-poisoning in Asian countries and Great Britain. In many parts of Asia, ingestion of pesticides constitutes a significant proportion of suicide deaths, and several studies have found that, in localities where sales of highly lethal pesticides were restricted, overall suicide deaths decreased.5,6 Conversely, suicide rates increased when more lethal varieties of pesticides became more available. In Great Britain, overall suicide rates decreased when natural gas replaced coal gas for home heating and cooking.7 For decades preceding this change, more Britons had killed themselves by inhaling coal gas than by any other method.
Strong correlations exist between regional levels of gun ownership and suicide rates by shooting,8 but several potentially confounding sociopolitical factors explain some portion of this connection. Stronger evidence of gun availability affecting suicide rates has been demonstrated by decreases in suicide rates after restrictions in gun access in Switzerland,9 Israel,10 and other areas. These studies show correlations – not causality. However, the number of studies, links between increases and decreases in suicide rates with changes in access to guns, absence of changes in suicide rates during the same time periods among ostensibly similar control populations, and lack of other compelling explanations support the argument that restricting access to highly lethal and accessible means of suicide prevents suicide deaths overall.
The installation of suicide barriers on bridges that have been the sites of multiple suicides robustly reduces or even eliminates suicide deaths from those bridges,11 but the effect on overall suicide rates remains less clear. Various studies have found subsequent increases or no changes12-14 in suicide deaths from other bridges or tall buildings in the vicinity after the installation of suicide barriers on a “suicide magnet.” Many of the studies failed to find any impact on overall suicide rates in the regions investigated. Deaths from jumping off tall structures constitute a tiny proportion of total suicide deaths, making it difficult to detect any changes in overall suicide rates. In the United States, suicides by jumping/falling constituted 1%-2% of total suicides over the last several decades.15
If we know that restricting highly lethal and accessible methods of killing reduces suicide deaths, why would I question the value of the Golden Gate suicide barrier in preventing overall suicide deaths?
Unique aspects of the bridge
The World High Dive Federation recommends keeping dives to less than 20 meters (65.5 feet), with a few exceptions.16 The rail of the Golden Gate Bridge stands 67 meters (220 feet) above the water, and assuming minimal wind resistance, a falling person traverses that distance in about 3.7 seconds and lands with an impact of 130 km/hour (81 miles per hour).17 Only about 1%-2% of those jumping from the Golden Gate survive that fall.18
A 99% likelihood of death sounds pretty lethal; however, death by jumping from the Golden Gate inherently takes place in a public space, with the opportunity for interventions by other people. A more realistic calculation of the lethality would start the instant that someone initiates a sequence of behaviors leading to the intended death. By that criteria, measuring the lethality of the Golden Gate would begin when an individual enters a vehicle or sets off on foot with the plan of going over the railing.
Unless our surveillance-oriented society makes substantially greater advances (which I oppose), we will remain unable to assess suicide lethality by starting at the moment of inception. However, we do have data showing what happens once someone with suicidal intentions walks onto the bridge.
Between 2000 and 2018, observers noted 2,546 people on the Golden Gate who appeared to be considering a suicide attempt, the San Francisco Chronicle has reported. Five hundred sixty-four confirmed suicides occurred. In an additional 71 cases, suicide is presumed but bodies were not recovered. In the 1,911 remaining instances, mental health interventions were made, with individuals taken to local hospitals and psychiatric wards, and released when no longer overtly suicidal. Interventions successfully diverted 75% (1,911/2,546) of those intending to end their own lives, which suggests that the current lethality of the Golden Gate as a means of suicide is only 25%. Even in the bridge’s first half-century, without constant camera monitoring, and a cadre of volunteers and professionals scanning for those attempting suicide, the lethality rate approached about 50%.19
We face even more difficulties measuring accessibility than in determining lethality. The Golden Gate appears to be accessible to almost anyone – drivers have to pay a toll only when traveling from the north, and then only after they have traversed the span. Pedestrians retain unfettered admittance to the east sidewalk (facing San Francisco city and bay) throughout daylight hours. But any determination of accessibility must include how quickly and easily one can make use of an opportunity.
Both entrances to the Golden Gate are embedded in the Golden Gate National Recreational Area, part of the National Park system. The south entrance to the bridge arises from The Presidio, a former military installation that housed about 4,000 people.20 Even fewer people live in the parklands at the north end of the bridge. The Presidio extends far enough so that the closest San Francisco neighborhoods outside of the park are a full 2.2 km (1.36 miles) from the bridge railing. A brisk walk would still require a minimum of about 20 minutes to get to the bridge; it is difficult to arrive at the bridge without a trek.
Researchers define impulsivity, like accessibility, inconsistently – and often imprecisely. Impulsivity, which clearly exists on a spectrum, connotes overvaluing of immediate feelings and thoughts at the expense of longer term goals and aspirations. Some suicide research appears to define impulsivity as the antithesis of planned behavior;21,22 others define it pragmatically as behaviors executed within 5 minutes of a decision,23 and still others contend that “suicidal behavior is rarely if ever impulsive.”24 Furthermore, when we assess impulsivity, we must acknowledge a fundamental difference between “impulsive” shootings and poisonings that are accomplished at home and within seconds or minutes, from “impulsive” Golden Gate Bridge suicide attempts, which require substantial travel and time commitments, and inherently involve the potential for others to intervene.
Those arguing that the bridge suicide barrier will save lives often bring up two additional sets of numbers to back up their assertions. They provide evidence that most of those people who were stopped in their attempts at suicide at the Golden Gate do not go on to commit suicide elsewhere, and that many of those who survived their attempts express regret at having tried to kill themselves. Specifically, 94% of those who were prevented from jumping from the Golden Gate had not committed suicide after a median follow-up of 26 years, according to a follow-up study published a few years ago. On the other hand, those who have made a serious suicide attempt have a substantially increased risk, relative to the general population, of dying from a later attempt,25,26 and the strongest predictor for death by suicide is having made a previous, serious suicide attempt.27
While all of these studies provide important and interesting information regarding suicide, none directly address the question of whether individuals will substitute attempts by other methods if the Golden Gate Bridge were no longer available. Many discussions blur the distinction between how individuals behave after a thwarted Golden Gate suicide attempt and how other people might act if we secured the bridge from any potential future suicide attempts. I hope that the following analogy makes this distinction clearer without trivializing: Imagine that we know that everyone who was interrupted while eating their dinner in a particular restaurant never went back and ate out anywhere, ever again. We could not conclude from this that another individual, who learned that the intended restaurant was indefinitely closed, would never dine out again. Once effective suicide barriers exist on the Golden Gate, this will likely become widely known, thereby greatly reducing the likelihood that any individuals will consider the possibility of jumping from the bridge. But it seems very unlikely that this would vanquish all suicidal impulses from the northern California population.
Lessons from patients
Two former patients of mine ended their lives by suicide from the Golden Gate. P, a solitary and lonely man in his 50s, was referred to me by his neighbor, Q, one of my long-term patients. P had a history of repeated assessments for lifelong depression, with minimal follow-up. I made a treatment plan with P that we hoped would address both his depression and his reluctance to engage with mental health professionals. He did not return for his follow-up appointment and ignored all my attempts to contact him.
P continued to have intermittent contact with Q. A decade after I had evaluated him, P was finally hospitalized for depression. Since P had no local family or friends, he asked Q to pick him up from the hospital at the time of his discharge. P asked Q to drive him to the Golden Gate Bridge, ostensibly to relish his release by partaking of the panoramic view of San Francisco from the bridge. They parked in the lot at the north end of the bridge, where Q stayed with the car at the vista point. The last that anyone saw of P was when Q noticed him walking on the bridge; nobody saw him go over and his body was not recovered.
In contrast to my brief connection with P, I worked with S over the course of 8 years to deal with her very severe attention-deficit/hyperactivity disorder and associated depression, which destroyed jobs and friendships, and estranged her from her family. She moved to Hawaii in hopes of “starting over with less baggage,” but I received a few phone calls over the next few years detailing suicide attempts, including driving her car off a bridge. Floundering in life, she returned to San Francisco and was hospitalized with suicidal ideation. The inpatient team sedated her heavily, ignored her past treatments and diagnoses, and discharged her after several days. Within a day of discharge, S’s sister called to say that S’s body had been recovered from the water below the bridge.
I don’t think that suicide was inevitable for either P or S, but I also lack any indication that either would be alive today had we installed suicide barriers on the Golden Gate years ago. Unless we eliminate access to guns, cars, trains, poisons, ropes, tall buildings and cliffs, people contemplating suicide will have numerous options at their disposal. We are likely to save lives by continuing to find ways to restrict access to means of death that can be used within seconds and have a high degree of lethality, and we should persist with such efforts. Buying a $5 trigger lock for every gun in California, and spending tens of millions on a public service campaign would cost less and may well save more lives than the Golden Gate suicide barrier. Unfortunately, we still possess very limited knowledge regarding which suicide prevention measures have an “impact on actual deaths or behavior.”28
To increase our efficacy in reducing suicide, we need to find better treatments for depression and anxiety. We also need to identify better ways of targeting those most at risk for suicide,29 improve our delivery of such treatments, and mitigate the social factors that contribute to such misery and unhappiness.
As a psychiatrist who has lost not only patients but also family members to suicide, I appreciate the hole in the soul these deaths create. I understand the drive to find ways to prevent additional deaths and save future survivors from such grief. But we must design psychiatric interventions that do the maximum good. To be imprecise in the lessons we learn from those who have killed themselves doubles down on the disservice to those lives already lost.
Dr. Kruse is a psychiatrist who practices in San Francisco. Several key details about the patients were changed to protect confidentiality.
References
1. Frommer’s Comprehensive Travel Guide, California. New York: Prentice Hall Travel, 1993.
2. “Chen Si, the ‘Angel of Nanjing,’ has saved more than 330 people from suicide,” by Matt Young, News.com.au. May 14, 2017.
3. “Finding Kyle,” by Lizzie Johnson, San Francisco Chronicle. Feb 8, 2019.
4. Beautrais A. Suicide by jumping. A review of research and prevention strategies. Crisis. 2007 Jan;28 Suppl 1:58-63. Crisis: The J of Crisis Interven Suicide Preven. 2007 Jan. (28)[Suppl1]:58-63.
5. Gunnell D et al. The global distribution of fatal pesticide self-poisoning: Systematic review. BMC Public Health. 2007 Dec. 21;7:357.
6. Vijayakumar L and Satheesh-Babu R. Does ‘no pesticide’ reduce suicides? Int J Soc Psychiatry. 2009 Jul 17;55:401-6.
7. Kreitman N. The coal gas story. United Kingdom suicide rates, 1960-71. Br J Prev Soc Med. 1976 Jun;30(2)86-93.
8. Ajdacic-Gross V et al. Changing times: A longitudinal analysis of international firearm suicide data. Am J Public Health. 2006 Oct;96(10):1752-5.
9. Reisch T et al. Change in suicide rates in Switzerland before and after firearm restriction resulting from the 2003 “Army XXI” reform. Am J Psychiatry. 2013 Sep170(9):977-84.
10. Lubin G et al. Decrease in suicide rates after a change of policy reducing access to firearms in adolescents: A naturalistic epidemiological study. Suicide Life Threat Behav. 2010 Oct;40(5):421-4.
11. Sinyor M and Levitt A. Effect of a barrier at Bloor Street Viaduct on suicide rates in Toronto: Natural experiment BMJ. 2010;341. doi: 1136/bmjc2884.
12. O’Carroll P and Silverman M. Community suicide prevention: The effectiveness of bridge barriers. Suicide Life Threat Behav. 1994 Spring;24(1):89-91; discussion 91-9.
13. Pelletier A. Preventing suicide by jumping: The effect of a bridge safety fence. Inj Prev. 2007 Feb;13(1):57-9.
14. Bennewith O et al. Effect of barriers on the Clifton suspension bridge, England, on local patterns of suicide: Implications for prevention. Br J Psychiatry. 2007 Mar;190:266-7.
15. Harvard T.H. Chan School of Public Health. 2004. “How do people most commonly complete suicide?”
16. “How cliff diving works,” by Heather Kolich, HowStuffWorks.com. Oct 5, 2009.
17. “Bridge design and construction statistics.” Goldengate.org
18. “How did teen survive fall from Golden Gate Bridge?” by Remy Molina, Live Science. Apr 19, 2011.
19. Seiden R. Where are they now? A follow-up study of suicide attempters from the Golden Gate Bridge. Suicide Life Threat Behav. 1978 Winter;8(4):203-16.
20. Presidio demographics. Point2homes.com.
21. Baca-García E et al. A prospective study of the paradoxical relationship between impulsivity and lethality of suicide attempts. J Clin Psychiatry. 2001 Jul;62(7):560-4.
22. Lim M et al. Differences between impulsive and non-impulsive suicide attempts among individuals treated in emergency rooms of South Korea. Psychiatry Investig. 2016 Jul;13(4):389-96.
23. Simon O et al. Characteristics of impulsive suicide attempts and attempters. Suicide Life Threat Behav. 2001;32(1 Suppl):49-59.
24. Anestis M et al. Reconsidering the link between impulsivity and suicidal behavior. Pers Soc Psychol Rev. 2014 Nov;18(4):366-86.
25. Ostamo A et al. Excess mortality of suicide attempters. Psychiatry Psychiatr Epidemiol. 2001 Jan;36(1):29-35.
26. Leon A et al. Statistical issues in the identification of risk factors for suicidal behavior: The application of survival analysis. Psychiatry Res. 1990 Jan;31(1):99-108.
27. Bostwick J et al. Suicide attempt as a risk factor for completed suicide: Even more lethal than we knew. Am J Psychiatry. 2016 Nov 1;173(11):1094-100.
28. Stone D and Crosby A. Suicide prevention. Am J Lifestyle Med. 2014;8(6):404-20.
29. Belsher B et al. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Mar 13. doi: 10.1001/jamapsychiatry.2019.0174.
San Francisco entrances people. Photographers capture more images of the Golden Gate Bridge than any other bridge in the world.1 And only the Nanjing Yangtze River Bridge in China surpasses the Golden Gate as a destination for dying by suicide.2 At least 1,700 people reportedly have plunged from the bridge to their deaths since its opening in 1937.3
Despite concerted efforts by bridge security, the local mental health community, and a volunteer organization – Bridgewatch Angels – suicides continue at the pace of about 1 every 2 weeks. After more than 60 years of discussion, transportation officials allocated funding and have started building a suicide prevention barrier system on the Golden Gate.
Extrapolating from the success of barriers built on other bridges that were “suicide magnets,” we should be able to assure people that suicide deaths from the Golden Gate will dramatically decrease, and perhaps cease completely.4 Certainly, some in the mental health community think this barrier will save lives. They support this claim by citing research showing that removing highly accessible and lethal means of suicide reduces overall suicide rates, and that suicidal individuals, when thwarted, do not seek alternate modes of death.
I support building the Golden Gate suicide barrier, partly because symbolically, it should deliver a powerful message that we value all human life. But will the barrier save lives? I don’t think it will. As the American Psychiatric Association prepares to gather for its annual meeting in San Francisco, I would like to share my reasoning.
What the evidence shows
The most robust evidence that restricting availability of highly lethal and accessible means of suicide reduces overall suicide deaths comes from studies looking at self-poisoning in Asian countries and Great Britain. In many parts of Asia, ingestion of pesticides constitutes a significant proportion of suicide deaths, and several studies have found that, in localities where sales of highly lethal pesticides were restricted, overall suicide deaths decreased.5,6 Conversely, suicide rates increased when more lethal varieties of pesticides became more available. In Great Britain, overall suicide rates decreased when natural gas replaced coal gas for home heating and cooking.7 For decades preceding this change, more Britons had killed themselves by inhaling coal gas than by any other method.
Strong correlations exist between regional levels of gun ownership and suicide rates by shooting,8 but several potentially confounding sociopolitical factors explain some portion of this connection. Stronger evidence of gun availability affecting suicide rates has been demonstrated by decreases in suicide rates after restrictions in gun access in Switzerland,9 Israel,10 and other areas. These studies show correlations – not causality. However, the number of studies, links between increases and decreases in suicide rates with changes in access to guns, absence of changes in suicide rates during the same time periods among ostensibly similar control populations, and lack of other compelling explanations support the argument that restricting access to highly lethal and accessible means of suicide prevents suicide deaths overall.
The installation of suicide barriers on bridges that have been the sites of multiple suicides robustly reduces or even eliminates suicide deaths from those bridges,11 but the effect on overall suicide rates remains less clear. Various studies have found subsequent increases or no changes12-14 in suicide deaths from other bridges or tall buildings in the vicinity after the installation of suicide barriers on a “suicide magnet.” Many of the studies failed to find any impact on overall suicide rates in the regions investigated. Deaths from jumping off tall structures constitute a tiny proportion of total suicide deaths, making it difficult to detect any changes in overall suicide rates. In the United States, suicides by jumping/falling constituted 1%-2% of total suicides over the last several decades.15
If we know that restricting highly lethal and accessible methods of killing reduces suicide deaths, why would I question the value of the Golden Gate suicide barrier in preventing overall suicide deaths?
Unique aspects of the bridge
The World High Dive Federation recommends keeping dives to less than 20 meters (65.5 feet), with a few exceptions.16 The rail of the Golden Gate Bridge stands 67 meters (220 feet) above the water, and assuming minimal wind resistance, a falling person traverses that distance in about 3.7 seconds and lands with an impact of 130 km/hour (81 miles per hour).17 Only about 1%-2% of those jumping from the Golden Gate survive that fall.18
A 99% likelihood of death sounds pretty lethal; however, death by jumping from the Golden Gate inherently takes place in a public space, with the opportunity for interventions by other people. A more realistic calculation of the lethality would start the instant that someone initiates a sequence of behaviors leading to the intended death. By that criteria, measuring the lethality of the Golden Gate would begin when an individual enters a vehicle or sets off on foot with the plan of going over the railing.
Unless our surveillance-oriented society makes substantially greater advances (which I oppose), we will remain unable to assess suicide lethality by starting at the moment of inception. However, we do have data showing what happens once someone with suicidal intentions walks onto the bridge.
Between 2000 and 2018, observers noted 2,546 people on the Golden Gate who appeared to be considering a suicide attempt, the San Francisco Chronicle has reported. Five hundred sixty-four confirmed suicides occurred. In an additional 71 cases, suicide is presumed but bodies were not recovered. In the 1,911 remaining instances, mental health interventions were made, with individuals taken to local hospitals and psychiatric wards, and released when no longer overtly suicidal. Interventions successfully diverted 75% (1,911/2,546) of those intending to end their own lives, which suggests that the current lethality of the Golden Gate as a means of suicide is only 25%. Even in the bridge’s first half-century, without constant camera monitoring, and a cadre of volunteers and professionals scanning for those attempting suicide, the lethality rate approached about 50%.19
We face even more difficulties measuring accessibility than in determining lethality. The Golden Gate appears to be accessible to almost anyone – drivers have to pay a toll only when traveling from the north, and then only after they have traversed the span. Pedestrians retain unfettered admittance to the east sidewalk (facing San Francisco city and bay) throughout daylight hours. But any determination of accessibility must include how quickly and easily one can make use of an opportunity.
Both entrances to the Golden Gate are embedded in the Golden Gate National Recreational Area, part of the National Park system. The south entrance to the bridge arises from The Presidio, a former military installation that housed about 4,000 people.20 Even fewer people live in the parklands at the north end of the bridge. The Presidio extends far enough so that the closest San Francisco neighborhoods outside of the park are a full 2.2 km (1.36 miles) from the bridge railing. A brisk walk would still require a minimum of about 20 minutes to get to the bridge; it is difficult to arrive at the bridge without a trek.
Researchers define impulsivity, like accessibility, inconsistently – and often imprecisely. Impulsivity, which clearly exists on a spectrum, connotes overvaluing of immediate feelings and thoughts at the expense of longer term goals and aspirations. Some suicide research appears to define impulsivity as the antithesis of planned behavior;21,22 others define it pragmatically as behaviors executed within 5 minutes of a decision,23 and still others contend that “suicidal behavior is rarely if ever impulsive.”24 Furthermore, when we assess impulsivity, we must acknowledge a fundamental difference between “impulsive” shootings and poisonings that are accomplished at home and within seconds or minutes, from “impulsive” Golden Gate Bridge suicide attempts, which require substantial travel and time commitments, and inherently involve the potential for others to intervene.
Those arguing that the bridge suicide barrier will save lives often bring up two additional sets of numbers to back up their assertions. They provide evidence that most of those people who were stopped in their attempts at suicide at the Golden Gate do not go on to commit suicide elsewhere, and that many of those who survived their attempts express regret at having tried to kill themselves. Specifically, 94% of those who were prevented from jumping from the Golden Gate had not committed suicide after a median follow-up of 26 years, according to a follow-up study published a few years ago. On the other hand, those who have made a serious suicide attempt have a substantially increased risk, relative to the general population, of dying from a later attempt,25,26 and the strongest predictor for death by suicide is having made a previous, serious suicide attempt.27
While all of these studies provide important and interesting information regarding suicide, none directly address the question of whether individuals will substitute attempts by other methods if the Golden Gate Bridge were no longer available. Many discussions blur the distinction between how individuals behave after a thwarted Golden Gate suicide attempt and how other people might act if we secured the bridge from any potential future suicide attempts. I hope that the following analogy makes this distinction clearer without trivializing: Imagine that we know that everyone who was interrupted while eating their dinner in a particular restaurant never went back and ate out anywhere, ever again. We could not conclude from this that another individual, who learned that the intended restaurant was indefinitely closed, would never dine out again. Once effective suicide barriers exist on the Golden Gate, this will likely become widely known, thereby greatly reducing the likelihood that any individuals will consider the possibility of jumping from the bridge. But it seems very unlikely that this would vanquish all suicidal impulses from the northern California population.
Lessons from patients
Two former patients of mine ended their lives by suicide from the Golden Gate. P, a solitary and lonely man in his 50s, was referred to me by his neighbor, Q, one of my long-term patients. P had a history of repeated assessments for lifelong depression, with minimal follow-up. I made a treatment plan with P that we hoped would address both his depression and his reluctance to engage with mental health professionals. He did not return for his follow-up appointment and ignored all my attempts to contact him.
P continued to have intermittent contact with Q. A decade after I had evaluated him, P was finally hospitalized for depression. Since P had no local family or friends, he asked Q to pick him up from the hospital at the time of his discharge. P asked Q to drive him to the Golden Gate Bridge, ostensibly to relish his release by partaking of the panoramic view of San Francisco from the bridge. They parked in the lot at the north end of the bridge, where Q stayed with the car at the vista point. The last that anyone saw of P was when Q noticed him walking on the bridge; nobody saw him go over and his body was not recovered.
In contrast to my brief connection with P, I worked with S over the course of 8 years to deal with her very severe attention-deficit/hyperactivity disorder and associated depression, which destroyed jobs and friendships, and estranged her from her family. She moved to Hawaii in hopes of “starting over with less baggage,” but I received a few phone calls over the next few years detailing suicide attempts, including driving her car off a bridge. Floundering in life, she returned to San Francisco and was hospitalized with suicidal ideation. The inpatient team sedated her heavily, ignored her past treatments and diagnoses, and discharged her after several days. Within a day of discharge, S’s sister called to say that S’s body had been recovered from the water below the bridge.
I don’t think that suicide was inevitable for either P or S, but I also lack any indication that either would be alive today had we installed suicide barriers on the Golden Gate years ago. Unless we eliminate access to guns, cars, trains, poisons, ropes, tall buildings and cliffs, people contemplating suicide will have numerous options at their disposal. We are likely to save lives by continuing to find ways to restrict access to means of death that can be used within seconds and have a high degree of lethality, and we should persist with such efforts. Buying a $5 trigger lock for every gun in California, and spending tens of millions on a public service campaign would cost less and may well save more lives than the Golden Gate suicide barrier. Unfortunately, we still possess very limited knowledge regarding which suicide prevention measures have an “impact on actual deaths or behavior.”28
To increase our efficacy in reducing suicide, we need to find better treatments for depression and anxiety. We also need to identify better ways of targeting those most at risk for suicide,29 improve our delivery of such treatments, and mitigate the social factors that contribute to such misery and unhappiness.
As a psychiatrist who has lost not only patients but also family members to suicide, I appreciate the hole in the soul these deaths create. I understand the drive to find ways to prevent additional deaths and save future survivors from such grief. But we must design psychiatric interventions that do the maximum good. To be imprecise in the lessons we learn from those who have killed themselves doubles down on the disservice to those lives already lost.
Dr. Kruse is a psychiatrist who practices in San Francisco. Several key details about the patients were changed to protect confidentiality.
References
1. Frommer’s Comprehensive Travel Guide, California. New York: Prentice Hall Travel, 1993.
2. “Chen Si, the ‘Angel of Nanjing,’ has saved more than 330 people from suicide,” by Matt Young, News.com.au. May 14, 2017.
3. “Finding Kyle,” by Lizzie Johnson, San Francisco Chronicle. Feb 8, 2019.
4. Beautrais A. Suicide by jumping. A review of research and prevention strategies. Crisis. 2007 Jan;28 Suppl 1:58-63. Crisis: The J of Crisis Interven Suicide Preven. 2007 Jan. (28)[Suppl1]:58-63.
5. Gunnell D et al. The global distribution of fatal pesticide self-poisoning: Systematic review. BMC Public Health. 2007 Dec. 21;7:357.
6. Vijayakumar L and Satheesh-Babu R. Does ‘no pesticide’ reduce suicides? Int J Soc Psychiatry. 2009 Jul 17;55:401-6.
7. Kreitman N. The coal gas story. United Kingdom suicide rates, 1960-71. Br J Prev Soc Med. 1976 Jun;30(2)86-93.
8. Ajdacic-Gross V et al. Changing times: A longitudinal analysis of international firearm suicide data. Am J Public Health. 2006 Oct;96(10):1752-5.
9. Reisch T et al. Change in suicide rates in Switzerland before and after firearm restriction resulting from the 2003 “Army XXI” reform. Am J Psychiatry. 2013 Sep170(9):977-84.
10. Lubin G et al. Decrease in suicide rates after a change of policy reducing access to firearms in adolescents: A naturalistic epidemiological study. Suicide Life Threat Behav. 2010 Oct;40(5):421-4.
11. Sinyor M and Levitt A. Effect of a barrier at Bloor Street Viaduct on suicide rates in Toronto: Natural experiment BMJ. 2010;341. doi: 1136/bmjc2884.
12. O’Carroll P and Silverman M. Community suicide prevention: The effectiveness of bridge barriers. Suicide Life Threat Behav. 1994 Spring;24(1):89-91; discussion 91-9.
13. Pelletier A. Preventing suicide by jumping: The effect of a bridge safety fence. Inj Prev. 2007 Feb;13(1):57-9.
14. Bennewith O et al. Effect of barriers on the Clifton suspension bridge, England, on local patterns of suicide: Implications for prevention. Br J Psychiatry. 2007 Mar;190:266-7.
15. Harvard T.H. Chan School of Public Health. 2004. “How do people most commonly complete suicide?”
16. “How cliff diving works,” by Heather Kolich, HowStuffWorks.com. Oct 5, 2009.
17. “Bridge design and construction statistics.” Goldengate.org
18. “How did teen survive fall from Golden Gate Bridge?” by Remy Molina, Live Science. Apr 19, 2011.
19. Seiden R. Where are they now? A follow-up study of suicide attempters from the Golden Gate Bridge. Suicide Life Threat Behav. 1978 Winter;8(4):203-16.
20. Presidio demographics. Point2homes.com.
21. Baca-García E et al. A prospective study of the paradoxical relationship between impulsivity and lethality of suicide attempts. J Clin Psychiatry. 2001 Jul;62(7):560-4.
22. Lim M et al. Differences between impulsive and non-impulsive suicide attempts among individuals treated in emergency rooms of South Korea. Psychiatry Investig. 2016 Jul;13(4):389-96.
23. Simon O et al. Characteristics of impulsive suicide attempts and attempters. Suicide Life Threat Behav. 2001;32(1 Suppl):49-59.
24. Anestis M et al. Reconsidering the link between impulsivity and suicidal behavior. Pers Soc Psychol Rev. 2014 Nov;18(4):366-86.
25. Ostamo A et al. Excess mortality of suicide attempters. Psychiatry Psychiatr Epidemiol. 2001 Jan;36(1):29-35.
26. Leon A et al. Statistical issues in the identification of risk factors for suicidal behavior: The application of survival analysis. Psychiatry Res. 1990 Jan;31(1):99-108.
27. Bostwick J et al. Suicide attempt as a risk factor for completed suicide: Even more lethal than we knew. Am J Psychiatry. 2016 Nov 1;173(11):1094-100.
28. Stone D and Crosby A. Suicide prevention. Am J Lifestyle Med. 2014;8(6):404-20.
29. Belsher B et al. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Mar 13. doi: 10.1001/jamapsychiatry.2019.0174.
Losing a patient to suicide

Join us Wednesday, April 24, 2019, at 6:00 p.m. Eastern/5:00 p.m. Central as we open a lively Twitter discussion in psychiatry on the topic of losing a patient to suicide. Our special guests include physicians with expertise on the topic of patient suicide, Dinah Miller, MD, (@shrinkrapdinah) and Eric Plakun, MD, (@EricPlakunMD). We hope you join us April 24 at 6 p.m. ET. #MDedgeChats.
Suicides in the United States are on the rise; according to the Centers for Disease Control and Prevention, suicide was the cause of death of almost 45,000 people in 2016. Overall, it was the 10th leading cause of death, and the second leading cause of death among people aged 10-34 years. Twice as many people completed suicide as were victims of homicides.
Losing a patient to suicide is one of the most difficult and painful experiences a psychiatrist will face. In addition to concern for the patient and his or her family, psychiatrists may experience thoughts of responsibility and what they could have done differently to prevent the suicide. Although often trained in helping patients address grief, psychiatrists may not be as comfortable processing their own grief after the loss of a patient to suicide.
Topics of conversation
Q1: Have you ever lost a patient to suicide?
Q2: How do you think the loss of your patient changed your approach to psychiatry?
Q3: How did the loss change you?
Q4: If you did not discuss the suicide with your colleagues, what held you back?
Q5: How can you support medical professionals who lose patients to suicide?
Resources
Preventing suicide: What should clinicians do differently?
Individualized intervention key to reducing suicide attempts
Helping survivors in the aftermath of suicide loss
The blinding lies of depression
Suicide symposium: A multidisciplinary approach to risk assessment and the emotional aftermath of patient suicide
About Dr. Miller
Dr. Miller is the author of numerous books and articles, including “Committed: The Battle Over Involuntary Care” (Baltimore: Johns Hopkins University Press, 2016), which she wrote with Dr. Annette Hanson (@clinkshrink), and “When a Patient Dies by Suicide – The Physician’s Silent Sorrow” in the New England Journal of Medicine (@NEJM) (2019;380:311-13). She has a private practice in Baltimore and is affiliated with Johns Hopkins University (@HopkinsMedicine).
About Dr. Plakun
Dr. Plakun is the medical director and CEO of the Austen Riggs Center (@Austen Riggs), a “top 10” U.S. News and World Report “Best Hospital” in psychiatry based in Stockbridge, Mass. He also serves on the board of trustees of the American Psychiatric Association (@APA Psychiatric) representing New England and Eastern Canada, and was the founding leader of the APA Psychotherapy Caucus. Dr. Plakun is a board-certified psychiatrist, psychoanalyst, former member of the Harvard Medical School clinical faculty, and author of more than 50 publications.
Are you new to Twitter chats? We have included simple steps below to help you join and participate in the conversation.

Join us Wednesday, April 24, 2019, at 6:00 p.m. Eastern/5:00 p.m. Central as we open a lively Twitter discussion in psychiatry on the topic of losing a patient to suicide. Our special guests include physicians with expertise on the topic of patient suicide, Dinah Miller, MD, (@shrinkrapdinah) and Eric Plakun, MD, (@EricPlakunMD). We hope you join us April 24 at 6 p.m. ET. #MDedgeChats.
Suicides in the United States are on the rise; according to the Centers for Disease Control and Prevention, suicide was the cause of death of almost 45,000 people in 2016. Overall, it was the 10th leading cause of death, and the second leading cause of death among people aged 10-34 years. Twice as many people completed suicide as were victims of homicides.
Losing a patient to suicide is one of the most difficult and painful experiences a psychiatrist will face. In addition to concern for the patient and his or her family, psychiatrists may experience thoughts of responsibility and what they could have done differently to prevent the suicide. Although often trained in helping patients address grief, psychiatrists may not be as comfortable processing their own grief after the loss of a patient to suicide.
Topics of conversation
Q1: Have you ever lost a patient to suicide?
Q2: How do you think the loss of your patient changed your approach to psychiatry?
Q3: How did the loss change you?
Q4: If you did not discuss the suicide with your colleagues, what held you back?
Q5: How can you support medical professionals who lose patients to suicide?
Resources
Preventing suicide: What should clinicians do differently?
Individualized intervention key to reducing suicide attempts
Helping survivors in the aftermath of suicide loss
The blinding lies of depression
Suicide symposium: A multidisciplinary approach to risk assessment and the emotional aftermath of patient suicide
About Dr. Miller
Dr. Miller is the author of numerous books and articles, including “Committed: The Battle Over Involuntary Care” (Baltimore: Johns Hopkins University Press, 2016), which she wrote with Dr. Annette Hanson (@clinkshrink), and “When a Patient Dies by Suicide – The Physician’s Silent Sorrow” in the New England Journal of Medicine (@NEJM) (2019;380:311-13). She has a private practice in Baltimore and is affiliated with Johns Hopkins University (@HopkinsMedicine).
About Dr. Plakun
Dr. Plakun is the medical director and CEO of the Austen Riggs Center (@Austen Riggs), a “top 10” U.S. News and World Report “Best Hospital” in psychiatry based in Stockbridge, Mass. He also serves on the board of trustees of the American Psychiatric Association (@APA Psychiatric) representing New England and Eastern Canada, and was the founding leader of the APA Psychotherapy Caucus. Dr. Plakun is a board-certified psychiatrist, psychoanalyst, former member of the Harvard Medical School clinical faculty, and author of more than 50 publications.
Are you new to Twitter chats? We have included simple steps below to help you join and participate in the conversation.

Join us Wednesday, April 24, 2019, at 6:00 p.m. Eastern/5:00 p.m. Central as we open a lively Twitter discussion in psychiatry on the topic of losing a patient to suicide. Our special guests include physicians with expertise on the topic of patient suicide, Dinah Miller, MD, (@shrinkrapdinah) and Eric Plakun, MD, (@EricPlakunMD). We hope you join us April 24 at 6 p.m. ET. #MDedgeChats.
Suicides in the United States are on the rise; according to the Centers for Disease Control and Prevention, suicide was the cause of death of almost 45,000 people in 2016. Overall, it was the 10th leading cause of death, and the second leading cause of death among people aged 10-34 years. Twice as many people completed suicide as were victims of homicides.
Losing a patient to suicide is one of the most difficult and painful experiences a psychiatrist will face. In addition to concern for the patient and his or her family, psychiatrists may experience thoughts of responsibility and what they could have done differently to prevent the suicide. Although often trained in helping patients address grief, psychiatrists may not be as comfortable processing their own grief after the loss of a patient to suicide.
Topics of conversation
Q1: Have you ever lost a patient to suicide?
Q2: How do you think the loss of your patient changed your approach to psychiatry?
Q3: How did the loss change you?
Q4: If you did not discuss the suicide with your colleagues, what held you back?
Q5: How can you support medical professionals who lose patients to suicide?
Resources
Preventing suicide: What should clinicians do differently?
Individualized intervention key to reducing suicide attempts
Helping survivors in the aftermath of suicide loss
The blinding lies of depression
Suicide symposium: A multidisciplinary approach to risk assessment and the emotional aftermath of patient suicide
About Dr. Miller
Dr. Miller is the author of numerous books and articles, including “Committed: The Battle Over Involuntary Care” (Baltimore: Johns Hopkins University Press, 2016), which she wrote with Dr. Annette Hanson (@clinkshrink), and “When a Patient Dies by Suicide – The Physician’s Silent Sorrow” in the New England Journal of Medicine (@NEJM) (2019;380:311-13). She has a private practice in Baltimore and is affiliated with Johns Hopkins University (@HopkinsMedicine).
About Dr. Plakun
Dr. Plakun is the medical director and CEO of the Austen Riggs Center (@Austen Riggs), a “top 10” U.S. News and World Report “Best Hospital” in psychiatry based in Stockbridge, Mass. He also serves on the board of trustees of the American Psychiatric Association (@APA Psychiatric) representing New England and Eastern Canada, and was the founding leader of the APA Psychotherapy Caucus. Dr. Plakun is a board-certified psychiatrist, psychoanalyst, former member of the Harvard Medical School clinical faculty, and author of more than 50 publications.
Are you new to Twitter chats? We have included simple steps below to help you join and participate in the conversation.
Most CBT delivery methods effective for depression
Most modes of delivery for cognitive-behavioral therapy appear to be effective interventions for the acute symptoms of depression, with the exception of unguided self-help therapy, a study has found.
In the study, published in JAMA Psychiatry, : individual, group, telephone, guided, and unguided self-help.
In general, CBT delivered individually, in a group, by guided self-help, or by telephone were all significantly more effective at improving the severity of depression than unguided, self-administered CBT, and significantly more effective than the controls of waiting list or usual care.
However, even unguided self-help CBT was more effective than the waiting list, although not more effective than care as usual.
“This study suggests that group, telephone, and guided self-help treatments are effective interventions that may be considered as alternatives to individual CBT,” wrote Pim Cuijpers, PhD, who is affiliated with the Amsterdam Public Health Research Institute at the Vrije Universiteit Amsterdam, and his coauthors. “Applying effective and acceptable CBT in a range of different formats will make CBT easier to implement, disseminate, and deliver across different settings and diverse patient populations.”
In terms of acceptability, individual-, group-, and telephone-delivered CBT were all equally acceptable. The analysis showed that guided self-help had lower acceptability than individual or group therapy, care as usual, and the waiting list, while unguided self-help therapy was less acceptable than being on a waiting list.
The authors said it was not clear why guided self-help CBT showed lower acceptability, compared with the other CBT formats.
“Maybe the absence of direct contact with a professional makes it easier to stop the treatment because there is less personal relationship pressure to continue with the treatment or the study,” they wrote.
The analysis also explored the long-term effectiveness of different delivery methods, although the authors cautioned that this was based on small numbers of comparisons. They found significantly greater long-term effectiveness associated with individual, group, guided self-help, and telephone CBT, compared with usual care, but telephone CBT was less effective than individual CBT.
Two authors reported receiving personal fees from private industry outside of the submitted work, and one reported receiving grants and support from the National Institute for Health Research.
SOURCE: Cuijpers P et al. JAMA Psychiatry. 2019 Apr 17. doi: 10.1001/jamapsychiatry.2019.0268.
Most modes of delivery for cognitive-behavioral therapy appear to be effective interventions for the acute symptoms of depression, with the exception of unguided self-help therapy, a study has found.
In the study, published in JAMA Psychiatry, : individual, group, telephone, guided, and unguided self-help.
In general, CBT delivered individually, in a group, by guided self-help, or by telephone were all significantly more effective at improving the severity of depression than unguided, self-administered CBT, and significantly more effective than the controls of waiting list or usual care.
However, even unguided self-help CBT was more effective than the waiting list, although not more effective than care as usual.
“This study suggests that group, telephone, and guided self-help treatments are effective interventions that may be considered as alternatives to individual CBT,” wrote Pim Cuijpers, PhD, who is affiliated with the Amsterdam Public Health Research Institute at the Vrije Universiteit Amsterdam, and his coauthors. “Applying effective and acceptable CBT in a range of different formats will make CBT easier to implement, disseminate, and deliver across different settings and diverse patient populations.”
In terms of acceptability, individual-, group-, and telephone-delivered CBT were all equally acceptable. The analysis showed that guided self-help had lower acceptability than individual or group therapy, care as usual, and the waiting list, while unguided self-help therapy was less acceptable than being on a waiting list.
The authors said it was not clear why guided self-help CBT showed lower acceptability, compared with the other CBT formats.
“Maybe the absence of direct contact with a professional makes it easier to stop the treatment because there is less personal relationship pressure to continue with the treatment or the study,” they wrote.
The analysis also explored the long-term effectiveness of different delivery methods, although the authors cautioned that this was based on small numbers of comparisons. They found significantly greater long-term effectiveness associated with individual, group, guided self-help, and telephone CBT, compared with usual care, but telephone CBT was less effective than individual CBT.
Two authors reported receiving personal fees from private industry outside of the submitted work, and one reported receiving grants and support from the National Institute for Health Research.
SOURCE: Cuijpers P et al. JAMA Psychiatry. 2019 Apr 17. doi: 10.1001/jamapsychiatry.2019.0268.
Most modes of delivery for cognitive-behavioral therapy appear to be effective interventions for the acute symptoms of depression, with the exception of unguided self-help therapy, a study has found.
In the study, published in JAMA Psychiatry, : individual, group, telephone, guided, and unguided self-help.
In general, CBT delivered individually, in a group, by guided self-help, or by telephone were all significantly more effective at improving the severity of depression than unguided, self-administered CBT, and significantly more effective than the controls of waiting list or usual care.
However, even unguided self-help CBT was more effective than the waiting list, although not more effective than care as usual.
“This study suggests that group, telephone, and guided self-help treatments are effective interventions that may be considered as alternatives to individual CBT,” wrote Pim Cuijpers, PhD, who is affiliated with the Amsterdam Public Health Research Institute at the Vrije Universiteit Amsterdam, and his coauthors. “Applying effective and acceptable CBT in a range of different formats will make CBT easier to implement, disseminate, and deliver across different settings and diverse patient populations.”
In terms of acceptability, individual-, group-, and telephone-delivered CBT were all equally acceptable. The analysis showed that guided self-help had lower acceptability than individual or group therapy, care as usual, and the waiting list, while unguided self-help therapy was less acceptable than being on a waiting list.
The authors said it was not clear why guided self-help CBT showed lower acceptability, compared with the other CBT formats.
“Maybe the absence of direct contact with a professional makes it easier to stop the treatment because there is less personal relationship pressure to continue with the treatment or the study,” they wrote.
The analysis also explored the long-term effectiveness of different delivery methods, although the authors cautioned that this was based on small numbers of comparisons. They found significantly greater long-term effectiveness associated with individual, group, guided self-help, and telephone CBT, compared with usual care, but telephone CBT was less effective than individual CBT.
Two authors reported receiving personal fees from private industry outside of the submitted work, and one reported receiving grants and support from the National Institute for Health Research.
SOURCE: Cuijpers P et al. JAMA Psychiatry. 2019 Apr 17. doi: 10.1001/jamapsychiatry.2019.0268.
FROM JAMA PSYCHIATRY
Should we defend the unrestrained availability of patented psychotropics?
Since the biological revolution in psychiatry, with the introduction of chlorpromazine in the 1950s,1 psychiatrists have been introduced to the economic questions inherent in the tension between funding psychotropic medications for the treatment of mental illness versus funding psychosocial interventions. Of course, our natural inclination is to advocate for all available treatments for our patients, but the economic realities of medical care – especially government-subsidized or regulated medical care – force us to weigh the relative advantages of these treatments and to promote our patients’ interests with a wise allocation of limited resources.
It has become common practice for the American Psychiatric Association to advocate for additional funds for both research into mental illness as well as treatment. The promotion of mental health parity and the demonization of prior authorizations are examples of our natural priorities in the debates over funding for medical care. A bias has played out in the national conversation about medical care in general regarding the right to said care, but economists understand that medical care is a limited resource and, as such, treating it as a “right,” per se, does not make sense: One has to make hard decisions about its allocation or simply leave it to the free market to make said decisions.
Recently, the government has proposed to eliminate certain psychotropic medications from their protected status within Medicare Part D. Those medications include all drugs labeled as antidepressants, antipsychotics, and anticonvulsants. As expected, the APA’s medical director has written a formal statement opposing the proposal. His statement includes warnings about suicides and overwhelmed emergency departments. He compared the mental health situation in the United States to a crisis. He described the availability of expensive and new psychotropics to be “lifesaving.”2
The goals of the APA and its leaders are honorable. We are inspired by the dedication that some psychiatrists have to advocate for us all as well as for our patients. However, we are concerned that unfounded claims are being made. We are even more troubled when those claims promote the interests of a fallible pharmaceutical industry, an industry that has opened up our field to extensive critical scrutiny over the past few years. We wonder whether a brief examination of the scientific evidence warrants the statements made by the APA.
After reviewing clinical textbooks and search engines, we were not able to find reliable and convincing evidence that newer psychotropics reduce emergency department stays or that lengths of stay in the hospital correlate with the use of newer agents. We have actually not even heard of that claim made before in any serious forum. Reviews of predictors of length of stay in psychiatric hospitals have typically included demographic factors, diagnostic factors, logistical factors such as time of day, and social factors, such as insurance status and homelessness.3,4 We found no review mentioning the use of patented drugs as a predictor of shorter stays.
At a larger level, The Food and Drug Administration approves psychiatric medications based on superiority to placebo and not superiority to existing – and usually much cheaper – medications. Our subscription to Epocrates informs us that a 1-month supply of once-a-day brand-name Abilify, Invega, or Latuda is more than $1,000.5 Alternatively, a 1-month supply of generic olanzapine, risperidone, or quetiapine is available for $4 at Walmart.6 As famously described in the CATIE trial7 of patients with schizophrenia, newer antipsychotics are not particularly better than older ones. In addition, a more recent meta-analysis8 did not find significant differences among antipsychotics’ efficacy.
A similar analysis can be made of antidepressants without addressing debates surrounding the effectiveness of antidepressants as a class and the value of psychological interventions over chemical ones. Reviews of the literature do not suggest that newer antidepressants are more effective than older ones. A recent meta-analysis of antidepressant efficacy did not find significant differences among antidepressants and, when looking at trends, amitriptyline, a much older antidepressant, was most effective.9
The most surprising part of the APA medical director’s statement was the claim of reduced suicidality. While lithium and clozapine have some evidence for reducing the risk of suicide, the evidence that antidepressants reduce suicide is equivocal. Quite the contrary, some evidence exists that antidepressants may increase the risk of suicide,10 and we are not aware of evidence suggesting that any newer agents can reduce suicide at any higher rate. One psychiatrist has even made a career out of testifying that antidepressants increase impulsivity and suicide.11
We are not politicians, and we trust the APA to have good intentions with a desire to help patients suffering from mental illness. We understand the need to advocate for any measure that provides additional resources for the treatment of mental illness. We have no doubt that a publicly funded and appropriately regulated mental health system is a wise goal from both an ethical as well as a societal perspective. The APA has an imperative to advocate for our patients with the goal to improve our society.
However, we are concerned when our field makes unfounded claims. Advocating that insurance companies and the government provide most psychotropics without prior authorization and without discrimination does not appear to be based on scientific evidence and has serious economic implications that are not being weighed in a transparent manner. Whatever funding levels the APA recommends for the treatment of mental illness, said treatments will remain a limited resource, and then it becomes a question not just of ethics but of economics. What combination of resources produce the most benefit for the most people in question? Would the increased cost of a newer psychotropic be better spent on a system with more elaborate psychosocial interventions? In making this argument, does one risk repeating the historical blunder made when, in the 1960s, long-term psychiatric hospitals were closed with the intention of replacing their costs with outpatient treatments that then never materialized?
A review of the literature does not support the claim that newer psychotropic agents are more effective from either a clinical or an economic perspective. Cost-saving measures are ethical and possibly beneficial if they permit a more justifiable allocation of resources.
Dr. Lehman is an associate professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He is also the course director for the UCSD third-year medical student psychiatry clerkship. Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. Among his writings is Chapter 7 in the new book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019).
References
1. Ann Med Psychol (Paris). 1952 Jun;110(2 1):112-7.
2. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2019.3b26.
3. Am J Emerg Med. 2016 Feb;34(2):133-9.
4. Eur Psychiatry. 2018 Feb;48:6-12.
5. https://online.epocrates.com/drugs. Retrieved March 3, 2019.
6. https://www.walmart.com/cp/$4-prescriptions/1078664. Retrieved March 27, 2019.
7. N Engl J Med. 2005 Sep 22;353(12):1209-23.
8. Am J Psychiatry. 2017. 174(10):927-42.
9. Lancet. 2018 Apr 7. 391(10128):P1357-66.
10. BMJ. 2009.339;b2880.
11. https://breggin.com/.
Since the biological revolution in psychiatry, with the introduction of chlorpromazine in the 1950s,1 psychiatrists have been introduced to the economic questions inherent in the tension between funding psychotropic medications for the treatment of mental illness versus funding psychosocial interventions. Of course, our natural inclination is to advocate for all available treatments for our patients, but the economic realities of medical care – especially government-subsidized or regulated medical care – force us to weigh the relative advantages of these treatments and to promote our patients’ interests with a wise allocation of limited resources.
It has become common practice for the American Psychiatric Association to advocate for additional funds for both research into mental illness as well as treatment. The promotion of mental health parity and the demonization of prior authorizations are examples of our natural priorities in the debates over funding for medical care. A bias has played out in the national conversation about medical care in general regarding the right to said care, but economists understand that medical care is a limited resource and, as such, treating it as a “right,” per se, does not make sense: One has to make hard decisions about its allocation or simply leave it to the free market to make said decisions.
Recently, the government has proposed to eliminate certain psychotropic medications from their protected status within Medicare Part D. Those medications include all drugs labeled as antidepressants, antipsychotics, and anticonvulsants. As expected, the APA’s medical director has written a formal statement opposing the proposal. His statement includes warnings about suicides and overwhelmed emergency departments. He compared the mental health situation in the United States to a crisis. He described the availability of expensive and new psychotropics to be “lifesaving.”2
The goals of the APA and its leaders are honorable. We are inspired by the dedication that some psychiatrists have to advocate for us all as well as for our patients. However, we are concerned that unfounded claims are being made. We are even more troubled when those claims promote the interests of a fallible pharmaceutical industry, an industry that has opened up our field to extensive critical scrutiny over the past few years. We wonder whether a brief examination of the scientific evidence warrants the statements made by the APA.
After reviewing clinical textbooks and search engines, we were not able to find reliable and convincing evidence that newer psychotropics reduce emergency department stays or that lengths of stay in the hospital correlate with the use of newer agents. We have actually not even heard of that claim made before in any serious forum. Reviews of predictors of length of stay in psychiatric hospitals have typically included demographic factors, diagnostic factors, logistical factors such as time of day, and social factors, such as insurance status and homelessness.3,4 We found no review mentioning the use of patented drugs as a predictor of shorter stays.
At a larger level, The Food and Drug Administration approves psychiatric medications based on superiority to placebo and not superiority to existing – and usually much cheaper – medications. Our subscription to Epocrates informs us that a 1-month supply of once-a-day brand-name Abilify, Invega, or Latuda is more than $1,000.5 Alternatively, a 1-month supply of generic olanzapine, risperidone, or quetiapine is available for $4 at Walmart.6 As famously described in the CATIE trial7 of patients with schizophrenia, newer antipsychotics are not particularly better than older ones. In addition, a more recent meta-analysis8 did not find significant differences among antipsychotics’ efficacy.
A similar analysis can be made of antidepressants without addressing debates surrounding the effectiveness of antidepressants as a class and the value of psychological interventions over chemical ones. Reviews of the literature do not suggest that newer antidepressants are more effective than older ones. A recent meta-analysis of antidepressant efficacy did not find significant differences among antidepressants and, when looking at trends, amitriptyline, a much older antidepressant, was most effective.9
The most surprising part of the APA medical director’s statement was the claim of reduced suicidality. While lithium and clozapine have some evidence for reducing the risk of suicide, the evidence that antidepressants reduce suicide is equivocal. Quite the contrary, some evidence exists that antidepressants may increase the risk of suicide,10 and we are not aware of evidence suggesting that any newer agents can reduce suicide at any higher rate. One psychiatrist has even made a career out of testifying that antidepressants increase impulsivity and suicide.11
We are not politicians, and we trust the APA to have good intentions with a desire to help patients suffering from mental illness. We understand the need to advocate for any measure that provides additional resources for the treatment of mental illness. We have no doubt that a publicly funded and appropriately regulated mental health system is a wise goal from both an ethical as well as a societal perspective. The APA has an imperative to advocate for our patients with the goal to improve our society.
However, we are concerned when our field makes unfounded claims. Advocating that insurance companies and the government provide most psychotropics without prior authorization and without discrimination does not appear to be based on scientific evidence and has serious economic implications that are not being weighed in a transparent manner. Whatever funding levels the APA recommends for the treatment of mental illness, said treatments will remain a limited resource, and then it becomes a question not just of ethics but of economics. What combination of resources produce the most benefit for the most people in question? Would the increased cost of a newer psychotropic be better spent on a system with more elaborate psychosocial interventions? In making this argument, does one risk repeating the historical blunder made when, in the 1960s, long-term psychiatric hospitals were closed with the intention of replacing their costs with outpatient treatments that then never materialized?
A review of the literature does not support the claim that newer psychotropic agents are more effective from either a clinical or an economic perspective. Cost-saving measures are ethical and possibly beneficial if they permit a more justifiable allocation of resources.
Dr. Lehman is an associate professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He is also the course director for the UCSD third-year medical student psychiatry clerkship. Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. Among his writings is Chapter 7 in the new book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019).
References
1. Ann Med Psychol (Paris). 1952 Jun;110(2 1):112-7.
2. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2019.3b26.
3. Am J Emerg Med. 2016 Feb;34(2):133-9.
4. Eur Psychiatry. 2018 Feb;48:6-12.
5. https://online.epocrates.com/drugs. Retrieved March 3, 2019.
6. https://www.walmart.com/cp/$4-prescriptions/1078664. Retrieved March 27, 2019.
7. N Engl J Med. 2005 Sep 22;353(12):1209-23.
8. Am J Psychiatry. 2017. 174(10):927-42.
9. Lancet. 2018 Apr 7. 391(10128):P1357-66.
10. BMJ. 2009.339;b2880.
11. https://breggin.com/.
Since the biological revolution in psychiatry, with the introduction of chlorpromazine in the 1950s,1 psychiatrists have been introduced to the economic questions inherent in the tension between funding psychotropic medications for the treatment of mental illness versus funding psychosocial interventions. Of course, our natural inclination is to advocate for all available treatments for our patients, but the economic realities of medical care – especially government-subsidized or regulated medical care – force us to weigh the relative advantages of these treatments and to promote our patients’ interests with a wise allocation of limited resources.
It has become common practice for the American Psychiatric Association to advocate for additional funds for both research into mental illness as well as treatment. The promotion of mental health parity and the demonization of prior authorizations are examples of our natural priorities in the debates over funding for medical care. A bias has played out in the national conversation about medical care in general regarding the right to said care, but economists understand that medical care is a limited resource and, as such, treating it as a “right,” per se, does not make sense: One has to make hard decisions about its allocation or simply leave it to the free market to make said decisions.
Recently, the government has proposed to eliminate certain psychotropic medications from their protected status within Medicare Part D. Those medications include all drugs labeled as antidepressants, antipsychotics, and anticonvulsants. As expected, the APA’s medical director has written a formal statement opposing the proposal. His statement includes warnings about suicides and overwhelmed emergency departments. He compared the mental health situation in the United States to a crisis. He described the availability of expensive and new psychotropics to be “lifesaving.”2
The goals of the APA and its leaders are honorable. We are inspired by the dedication that some psychiatrists have to advocate for us all as well as for our patients. However, we are concerned that unfounded claims are being made. We are even more troubled when those claims promote the interests of a fallible pharmaceutical industry, an industry that has opened up our field to extensive critical scrutiny over the past few years. We wonder whether a brief examination of the scientific evidence warrants the statements made by the APA.
After reviewing clinical textbooks and search engines, we were not able to find reliable and convincing evidence that newer psychotropics reduce emergency department stays or that lengths of stay in the hospital correlate with the use of newer agents. We have actually not even heard of that claim made before in any serious forum. Reviews of predictors of length of stay in psychiatric hospitals have typically included demographic factors, diagnostic factors, logistical factors such as time of day, and social factors, such as insurance status and homelessness.3,4 We found no review mentioning the use of patented drugs as a predictor of shorter stays.
At a larger level, The Food and Drug Administration approves psychiatric medications based on superiority to placebo and not superiority to existing – and usually much cheaper – medications. Our subscription to Epocrates informs us that a 1-month supply of once-a-day brand-name Abilify, Invega, or Latuda is more than $1,000.5 Alternatively, a 1-month supply of generic olanzapine, risperidone, or quetiapine is available for $4 at Walmart.6 As famously described in the CATIE trial7 of patients with schizophrenia, newer antipsychotics are not particularly better than older ones. In addition, a more recent meta-analysis8 did not find significant differences among antipsychotics’ efficacy.
A similar analysis can be made of antidepressants without addressing debates surrounding the effectiveness of antidepressants as a class and the value of psychological interventions over chemical ones. Reviews of the literature do not suggest that newer antidepressants are more effective than older ones. A recent meta-analysis of antidepressant efficacy did not find significant differences among antidepressants and, when looking at trends, amitriptyline, a much older antidepressant, was most effective.9
The most surprising part of the APA medical director’s statement was the claim of reduced suicidality. While lithium and clozapine have some evidence for reducing the risk of suicide, the evidence that antidepressants reduce suicide is equivocal. Quite the contrary, some evidence exists that antidepressants may increase the risk of suicide,10 and we are not aware of evidence suggesting that any newer agents can reduce suicide at any higher rate. One psychiatrist has even made a career out of testifying that antidepressants increase impulsivity and suicide.11
We are not politicians, and we trust the APA to have good intentions with a desire to help patients suffering from mental illness. We understand the need to advocate for any measure that provides additional resources for the treatment of mental illness. We have no doubt that a publicly funded and appropriately regulated mental health system is a wise goal from both an ethical as well as a societal perspective. The APA has an imperative to advocate for our patients with the goal to improve our society.
However, we are concerned when our field makes unfounded claims. Advocating that insurance companies and the government provide most psychotropics without prior authorization and without discrimination does not appear to be based on scientific evidence and has serious economic implications that are not being weighed in a transparent manner. Whatever funding levels the APA recommends for the treatment of mental illness, said treatments will remain a limited resource, and then it becomes a question not just of ethics but of economics. What combination of resources produce the most benefit for the most people in question? Would the increased cost of a newer psychotropic be better spent on a system with more elaborate psychosocial interventions? In making this argument, does one risk repeating the historical blunder made when, in the 1960s, long-term psychiatric hospitals were closed with the intention of replacing their costs with outpatient treatments that then never materialized?
A review of the literature does not support the claim that newer psychotropic agents are more effective from either a clinical or an economic perspective. Cost-saving measures are ethical and possibly beneficial if they permit a more justifiable allocation of resources.
Dr. Lehman is an associate professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He is also the course director for the UCSD third-year medical student psychiatry clerkship. Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. Among his writings is Chapter 7 in the new book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019).
References
1. Ann Med Psychol (Paris). 1952 Jun;110(2 1):112-7.
2. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2019.3b26.
3. Am J Emerg Med. 2016 Feb;34(2):133-9.
4. Eur Psychiatry. 2018 Feb;48:6-12.
5. https://online.epocrates.com/drugs. Retrieved March 3, 2019.
6. https://www.walmart.com/cp/$4-prescriptions/1078664. Retrieved March 27, 2019.
7. N Engl J Med. 2005 Sep 22;353(12):1209-23.
8. Am J Psychiatry. 2017. 174(10):927-42.
9. Lancet. 2018 Apr 7. 391(10128):P1357-66.
10. BMJ. 2009.339;b2880.
11. https://breggin.com/.