User login
Veterans’ Well-Being Tools Aim to Improve Quality of Life
Could assessing the well-being of older patients create better treatment plans?
Researchers with the US Department of Veterans Affairs posit that doing so just might improve patient quality of life.
In an article in Medical Care, Dawne Vogt, PhD, and her colleagues described two surveys of well-being developed for use in clinical settings.
“Well-Being Signs” (WBS), a 1-minute screening, asks patients about how satisfied they are with the most important parts of their daily life, which could include time with family. It also asks how regularly involved they are in the activities and their level of functioning.
“Well-Being Brief” (WBB) is self-administered and asks more in-depth questions about finances, health, social relationships, and vocation. Clinicians can use the tool to make referrals to appropriate services like counseling or resources like senior centers.
“They’re not things that we’ve historically paid a lot of attention to, at least in the healthcare setting,” said Vogt, a research psychologist in the Women’s Health Sciences Division of the VA Boston Healthcare System in Massachusetts. “A growing body of research shows that they have really big implications for health.”
The two approaches stem from an increased awareness of the relationship between social determinants of health and outcomes. Both screenings can be implemented more effectively in a clinical setting than other measures because of their brevity and ease of use, she said.
Vogt shared that anecdotally, she finds patients are pleasantly surprised by the questionnaires “because they’re being seen in a way that they don’t always feel like they’re seen.”
Vogt said that the two well-being measurements are more nuanced than standard screenings for depression.
“A measure of depression tells you something much more narrow than a measure of well-being tells you,” she said, adding that identifying problem areas early can help prevent developing mental health disorders. For example, Vogt said that veterans with higher well-being are less likely to develop posttraumatic stress disorder when exposed to trauma.
The WBS has been validated, while the WBB questionnaire awaits final testing.
James Michail, MD, a family and geriatric physician with Providence Health & Services in Los Angeles, California, said he views the well-being screeners as launching points into discussing whether a treatment is enhancing or inhibiting a patient’s life.
“We have screenings for everything else but not for wellness, and the goal of care isn’t necessarily always treatment,” Michail said. “It’s taking the whole person into consideration. There’s a person behind the disease.”
Kendra Segura, MD, an obstetrician-gynecologist in Los Angeles, said she is open to using a well-being screener. Usually, building repertoire with a patient takes time, and sometimes only then can it allow for a more candid assessment of well-being.
“Over the course of several visits, that is when patients open up,” she said. “It’s when that starts to happen where they start to tell you about their well-being. It’s not an easy thing to establish.”
The authors of the article reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Could assessing the well-being of older patients create better treatment plans?
Researchers with the US Department of Veterans Affairs posit that doing so just might improve patient quality of life.
In an article in Medical Care, Dawne Vogt, PhD, and her colleagues described two surveys of well-being developed for use in clinical settings.
“Well-Being Signs” (WBS), a 1-minute screening, asks patients about how satisfied they are with the most important parts of their daily life, which could include time with family. It also asks how regularly involved they are in the activities and their level of functioning.
“Well-Being Brief” (WBB) is self-administered and asks more in-depth questions about finances, health, social relationships, and vocation. Clinicians can use the tool to make referrals to appropriate services like counseling or resources like senior centers.
“They’re not things that we’ve historically paid a lot of attention to, at least in the healthcare setting,” said Vogt, a research psychologist in the Women’s Health Sciences Division of the VA Boston Healthcare System in Massachusetts. “A growing body of research shows that they have really big implications for health.”
The two approaches stem from an increased awareness of the relationship between social determinants of health and outcomes. Both screenings can be implemented more effectively in a clinical setting than other measures because of their brevity and ease of use, she said.
Vogt shared that anecdotally, she finds patients are pleasantly surprised by the questionnaires “because they’re being seen in a way that they don’t always feel like they’re seen.”
Vogt said that the two well-being measurements are more nuanced than standard screenings for depression.
“A measure of depression tells you something much more narrow than a measure of well-being tells you,” she said, adding that identifying problem areas early can help prevent developing mental health disorders. For example, Vogt said that veterans with higher well-being are less likely to develop posttraumatic stress disorder when exposed to trauma.
The WBS has been validated, while the WBB questionnaire awaits final testing.
James Michail, MD, a family and geriatric physician with Providence Health & Services in Los Angeles, California, said he views the well-being screeners as launching points into discussing whether a treatment is enhancing or inhibiting a patient’s life.
“We have screenings for everything else but not for wellness, and the goal of care isn’t necessarily always treatment,” Michail said. “It’s taking the whole person into consideration. There’s a person behind the disease.”
Kendra Segura, MD, an obstetrician-gynecologist in Los Angeles, said she is open to using a well-being screener. Usually, building repertoire with a patient takes time, and sometimes only then can it allow for a more candid assessment of well-being.
“Over the course of several visits, that is when patients open up,” she said. “It’s when that starts to happen where they start to tell you about their well-being. It’s not an easy thing to establish.”
The authors of the article reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Could assessing the well-being of older patients create better treatment plans?
Researchers with the US Department of Veterans Affairs posit that doing so just might improve patient quality of life.
In an article in Medical Care, Dawne Vogt, PhD, and her colleagues described two surveys of well-being developed for use in clinical settings.
“Well-Being Signs” (WBS), a 1-minute screening, asks patients about how satisfied they are with the most important parts of their daily life, which could include time with family. It also asks how regularly involved they are in the activities and their level of functioning.
“Well-Being Brief” (WBB) is self-administered and asks more in-depth questions about finances, health, social relationships, and vocation. Clinicians can use the tool to make referrals to appropriate services like counseling or resources like senior centers.
“They’re not things that we’ve historically paid a lot of attention to, at least in the healthcare setting,” said Vogt, a research psychologist in the Women’s Health Sciences Division of the VA Boston Healthcare System in Massachusetts. “A growing body of research shows that they have really big implications for health.”
The two approaches stem from an increased awareness of the relationship between social determinants of health and outcomes. Both screenings can be implemented more effectively in a clinical setting than other measures because of their brevity and ease of use, she said.
Vogt shared that anecdotally, she finds patients are pleasantly surprised by the questionnaires “because they’re being seen in a way that they don’t always feel like they’re seen.”
Vogt said that the two well-being measurements are more nuanced than standard screenings for depression.
“A measure of depression tells you something much more narrow than a measure of well-being tells you,” she said, adding that identifying problem areas early can help prevent developing mental health disorders. For example, Vogt said that veterans with higher well-being are less likely to develop posttraumatic stress disorder when exposed to trauma.
The WBS has been validated, while the WBB questionnaire awaits final testing.
James Michail, MD, a family and geriatric physician with Providence Health & Services in Los Angeles, California, said he views the well-being screeners as launching points into discussing whether a treatment is enhancing or inhibiting a patient’s life.
“We have screenings for everything else but not for wellness, and the goal of care isn’t necessarily always treatment,” Michail said. “It’s taking the whole person into consideration. There’s a person behind the disease.”
Kendra Segura, MD, an obstetrician-gynecologist in Los Angeles, said she is open to using a well-being screener. Usually, building repertoire with a patient takes time, and sometimes only then can it allow for a more candid assessment of well-being.
“Over the course of several visits, that is when patients open up,” she said. “It’s when that starts to happen where they start to tell you about their well-being. It’s not an easy thing to establish.”
The authors of the article reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MEDICAL CARE
Two Brain Stim Methods Better Than One for Depression?
TOPLINE:
a new study showed.
METHODOLOGY:
- Researchers conducted a double-blind, sham-controlled randomized clinical trial from 2021 to 2023 at three hospitals in China with 240 participants with MDD (mean age, 32.5 years; 58% women).
- Participants received active tDCS + active rTMS, sham tDCS + active rTMS, active tDCS + sham rTMS, or sham tDCS + sham rTMS with treatments administered five times per week for 2 weeks.
- tDCS was administered in 20-minute sessions using a 2-mA direct current stimulator, whereas rTMS involved 1600 pulses of 10-Hz stimulation targeting the left dorsolateral prefrontal cortex. Sham treatments used a pseudostimulation coil and only emitted sound.
- The primary outcome was change in the 24-item Hamilton Depression Rating Scale (HDRS-24) total score from baseline to week 2.
- Secondary outcomes included HDRS-24 total score change at week 4, remission rate (HDRS-24 total score ≤ 9), response rate (≥ 50% reduction in HDRS-24 total score), and adverse events.
TAKEAWAY:
- The active tDCS + active rTMS group demonstrated the greatest reduction in mean HDRS-24 score (18.33 ± 5.39) at week 2 compared with sham tDCS + active rTMS, active tDCS + sham rTMS, and sham tDCS + sham rTMS (P < .001).
- Response rates at week 2 were notably higher in the active tDCS + active rTMS group (85%) than in the active tDCS + sham rTMS (30%) and sham tDCS + sham rTMS groups (32%).
- The remission rate at week 4 reached 83% in the active tDCS + active rTMS group, which was significantly higher than the remission rates with the other interventions (P < .001).
- The treatments were well tolerated, with no serious adverse events, seizures, or manic symptoms reported across all intervention groups.
IN PRACTICE:
This trial “was the first to evaluate the safety, feasibility, and efficacy of combining tDCS and rTMS in treating depression. Future studies should focus on investigating the mechanism of this synergistic effect and improving the stimulation parameters to optimize the therapeutic effect,” the investigators wrote.
SOURCE:
This study was led by Dongsheng Zhou, MD, Ningbo Kangning Hospital, Ningbo, China. It was published online in JAMA Network Open.
LIMITATIONS:
The brief treatment duration involving 10 sessions may have been insufficient for tDCS and rTMS to demonstrate their full antidepressant potential. The inability to regulate participants’ antidepressant medications throughout the study period presented another limitation. Additionally, the lack of stratified randomization and adjustment for center effects may have introduced variability in the results.
DISCLOSURES:
This study received support from multiple grants, including from the Natural Science Foundation of Zhejiang Province, Basic Public Welfare Research Project of Zhejiang Province, Ningbo Medical and Health Brand Discipline, Ningbo Clinical Medical Research Centre for Mental Health, Ningbo Top Medical and Health Research Program, and the Zhejiang Medical and Health Science and Technology Plan Project. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
a new study showed.
METHODOLOGY:
- Researchers conducted a double-blind, sham-controlled randomized clinical trial from 2021 to 2023 at three hospitals in China with 240 participants with MDD (mean age, 32.5 years; 58% women).
- Participants received active tDCS + active rTMS, sham tDCS + active rTMS, active tDCS + sham rTMS, or sham tDCS + sham rTMS with treatments administered five times per week for 2 weeks.
- tDCS was administered in 20-minute sessions using a 2-mA direct current stimulator, whereas rTMS involved 1600 pulses of 10-Hz stimulation targeting the left dorsolateral prefrontal cortex. Sham treatments used a pseudostimulation coil and only emitted sound.
- The primary outcome was change in the 24-item Hamilton Depression Rating Scale (HDRS-24) total score from baseline to week 2.
- Secondary outcomes included HDRS-24 total score change at week 4, remission rate (HDRS-24 total score ≤ 9), response rate (≥ 50% reduction in HDRS-24 total score), and adverse events.
TAKEAWAY:
- The active tDCS + active rTMS group demonstrated the greatest reduction in mean HDRS-24 score (18.33 ± 5.39) at week 2 compared with sham tDCS + active rTMS, active tDCS + sham rTMS, and sham tDCS + sham rTMS (P < .001).
- Response rates at week 2 were notably higher in the active tDCS + active rTMS group (85%) than in the active tDCS + sham rTMS (30%) and sham tDCS + sham rTMS groups (32%).
- The remission rate at week 4 reached 83% in the active tDCS + active rTMS group, which was significantly higher than the remission rates with the other interventions (P < .001).
- The treatments were well tolerated, with no serious adverse events, seizures, or manic symptoms reported across all intervention groups.
IN PRACTICE:
This trial “was the first to evaluate the safety, feasibility, and efficacy of combining tDCS and rTMS in treating depression. Future studies should focus on investigating the mechanism of this synergistic effect and improving the stimulation parameters to optimize the therapeutic effect,” the investigators wrote.
SOURCE:
This study was led by Dongsheng Zhou, MD, Ningbo Kangning Hospital, Ningbo, China. It was published online in JAMA Network Open.
LIMITATIONS:
The brief treatment duration involving 10 sessions may have been insufficient for tDCS and rTMS to demonstrate their full antidepressant potential. The inability to regulate participants’ antidepressant medications throughout the study period presented another limitation. Additionally, the lack of stratified randomization and adjustment for center effects may have introduced variability in the results.
DISCLOSURES:
This study received support from multiple grants, including from the Natural Science Foundation of Zhejiang Province, Basic Public Welfare Research Project of Zhejiang Province, Ningbo Medical and Health Brand Discipline, Ningbo Clinical Medical Research Centre for Mental Health, Ningbo Top Medical and Health Research Program, and the Zhejiang Medical and Health Science and Technology Plan Project. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
a new study showed.
METHODOLOGY:
- Researchers conducted a double-blind, sham-controlled randomized clinical trial from 2021 to 2023 at three hospitals in China with 240 participants with MDD (mean age, 32.5 years; 58% women).
- Participants received active tDCS + active rTMS, sham tDCS + active rTMS, active tDCS + sham rTMS, or sham tDCS + sham rTMS with treatments administered five times per week for 2 weeks.
- tDCS was administered in 20-minute sessions using a 2-mA direct current stimulator, whereas rTMS involved 1600 pulses of 10-Hz stimulation targeting the left dorsolateral prefrontal cortex. Sham treatments used a pseudostimulation coil and only emitted sound.
- The primary outcome was change in the 24-item Hamilton Depression Rating Scale (HDRS-24) total score from baseline to week 2.
- Secondary outcomes included HDRS-24 total score change at week 4, remission rate (HDRS-24 total score ≤ 9), response rate (≥ 50% reduction in HDRS-24 total score), and adverse events.
TAKEAWAY:
- The active tDCS + active rTMS group demonstrated the greatest reduction in mean HDRS-24 score (18.33 ± 5.39) at week 2 compared with sham tDCS + active rTMS, active tDCS + sham rTMS, and sham tDCS + sham rTMS (P < .001).
- Response rates at week 2 were notably higher in the active tDCS + active rTMS group (85%) than in the active tDCS + sham rTMS (30%) and sham tDCS + sham rTMS groups (32%).
- The remission rate at week 4 reached 83% in the active tDCS + active rTMS group, which was significantly higher than the remission rates with the other interventions (P < .001).
- The treatments were well tolerated, with no serious adverse events, seizures, or manic symptoms reported across all intervention groups.
IN PRACTICE:
This trial “was the first to evaluate the safety, feasibility, and efficacy of combining tDCS and rTMS in treating depression. Future studies should focus on investigating the mechanism of this synergistic effect and improving the stimulation parameters to optimize the therapeutic effect,” the investigators wrote.
SOURCE:
This study was led by Dongsheng Zhou, MD, Ningbo Kangning Hospital, Ningbo, China. It was published online in JAMA Network Open.
LIMITATIONS:
The brief treatment duration involving 10 sessions may have been insufficient for tDCS and rTMS to demonstrate their full antidepressant potential. The inability to regulate participants’ antidepressant medications throughout the study period presented another limitation. Additionally, the lack of stratified randomization and adjustment for center effects may have introduced variability in the results.
DISCLOSURES:
This study received support from multiple grants, including from the Natural Science Foundation of Zhejiang Province, Basic Public Welfare Research Project of Zhejiang Province, Ningbo Medical and Health Brand Discipline, Ningbo Clinical Medical Research Centre for Mental Health, Ningbo Top Medical and Health Research Program, and the Zhejiang Medical and Health Science and Technology Plan Project. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The Emotional Cost of Nursing School: Depression
Nursing is a competitive field. In 2022, nursing schools rejected more than 78,000 qualified applications, and the students whose applications were accepted faced demanding schedules and rigorous academics and clinical rotations. Is this a recipe for depression?
In 2024, 38% of nursing students experienced depression — a 9.3% increase over 2019, according to research from higher education research group Degreechoices. Catherine A. Stubin, PhD, RN, assistant professor of nursing at Rutgers University–Camden in New Jersey, calls it “a mental health crisis in nursing.”
“Nursing is a very rigorous, difficult, psychologically and physically demanding profession,” she said. “If students don’t have the tools and resources to adequately deal with these stressors in nursing school, it’s going to carry over to their professional practice.”
A growing recognition of the toll that nursing programs may have on students’ mental health has led schools to launch initiatives to better support the next generation of nurses.
Diagnosing the Problem
Higher than average rates of depression among nursing students are not new. Nursing students often work long shifts with limited breaks. The academic rigors and clinical demands of caring for patients with acute and chronic conditions while instructors evaluate and watch for mistakes can cause high levels of stress, Stubin told this news organization. “Eventually, something has to give, and it’s usually their mental health.”
Clinical practicums often start when nursing students are still freshmen, and asking 18-year-old students to provide patient care in often-chaotic clinical environments is “overwhelming,” according to Stubin. The COVID-19 pandemic further exacerbated the issue.
During lockdown, more than half the nursing students reported moderate to severe symptoms of anxiety and depression, which was attributed to the transition to online learning, fear of infection, burnout, and the psychological distress of lockdown.
“The pandemic exacerbated existing mental health problems in undergraduate nursing students,” said Stubin. “In the wake of it ... a lot of [registered nurses] have mental health issues and are leaving the profession.”
Helping Nurses Heal
A significant shift in the willingness to talk about mental health and seek treatment could help. In 2011, just one third of students participated in the treatment for a mental health disorder. The latest data show that 61% of students experiencing symptoms of depression or anxiety take medication or seek therapy or counseling.
Incoming health sciences students at Ohio State University (OSU), Columbus, are screened for depression, anxiety, and suicidal ideation and directed to campus health services as needed. Bernadette Mazurek Melnyk, PhD, APRN-CNP, OSU’s chief wellness officer and former dean in the College of Nursing, believes it’s an essential step in supporting students, adding, “If you don’t screen, you don’t know the students are suffering, and we’re able to get help to the students who need it quickly.”
Prioritizing Solutions
Counseling services available through campus health centers are just one part of a multipronged approach that nursing schools have taken to improve the health and well-being of students. Nursing programs have also introduced initiatives to lower stress, prevent burnout, and relieve emotional trauma.
“In nursing education, we have to lay the groundwork for the self-care, wellness, and resilience practices that can, hopefully, be carried over into their professional practices,” Stubin said.
At Rutgers University–Camden, the wellness center provides counseling services, and the Student Nursing Association offers a pet therapy program. Stubin also incorporates self-care, resilience-building strategies, and wellness programming into the curriculum.
During the pandemic, the University of Colorado College of Nursing, Aurora, created a class called Stress Impact and Care for COVID-19 to provide content, exercises, and support groups for nursing students. The class was so popular that it was adapted and integrated into the curriculum.
The University of Vermont, Burlington, introduced the Benson-Henry Institute Stress Management and Resiliency Training program in 2021. The 8-week program was designed to teach nursing students coping strategies to reduce stress.
Offering stress management programs to first-year nursing students has been linked to improved problem-solving skills and fewer emotional and social behavioral symptoms. However, for programs to be effective, Melnyk believes that they need to be integrated into the curriculum, not offered as electives.
“We know mindfulness works, we know cognitive behavior skills-building works, and these types of evidence-based programs with such efficacy behind them should not be optional,” she said. “Students are overwhelmed just with their coursework, so if these programs exist for extra credit, students won’t take them.”
Creating a Culture of Wellness
Teaching nursing students how to manage stress and providing the resources to combat depression and anxiety is just the first step in building a healthy, resilient nursing workforce.
Prioritizing wellness in nursing isn’t just essential for addressing the nationwide nursing shortage. Burnout in the medical field costs the United States healthcare system $4.6 billion per year, and preventable medical errors are the third leading cause of death in the United States.
“There is a nice movement across the United States to reduce these mental health issues because they’re so costly,” Melnyk said.
There are also national efforts to address the issue. The National Academy of Medicine introduced the Action Collaborative on Clinician Well-Being and Resilience, which has grown to include more than 200 organizations committed to reversing burnout and improving mental health in the clinical workforce. The American Nurses Foundation created The Nurse Well-Being: Building Peer and Leadership Support Program to provide resources and peer support to help nurses manage stress.
Health systems and hospitals also need to prioritize clinical well-being to reduce stress and burnout — and these efforts must be ongoing.
“These resources have to be extended into the working world ... and not just once a year for Nurses Week in May, but on a regular continued basis,” said Stubin. “Healthcare corporations and hospitals have to continue these resources and this help; it has to be a priority.”
Until the culture changes, Stubin fears that nursing students will continue facing barriers to completing their programs and maintaining nursing careers. Currently, 43% of college students considered leaving their program for mental health reasons, and 21.7% of nurses reported suicidal ideation.
“There’s a nursing shortage, and the acuity of patient care is increasing, so the stressors in the clinical area aren’t going to decrease,” Stubin said. “We as nursing faculty must teach our students how to manage these stressors to build a resilient, mentally and physically healthy workforce.”
A version of this article first appeared on Medscape.com.
Nursing is a competitive field. In 2022, nursing schools rejected more than 78,000 qualified applications, and the students whose applications were accepted faced demanding schedules and rigorous academics and clinical rotations. Is this a recipe for depression?
In 2024, 38% of nursing students experienced depression — a 9.3% increase over 2019, according to research from higher education research group Degreechoices. Catherine A. Stubin, PhD, RN, assistant professor of nursing at Rutgers University–Camden in New Jersey, calls it “a mental health crisis in nursing.”
“Nursing is a very rigorous, difficult, psychologically and physically demanding profession,” she said. “If students don’t have the tools and resources to adequately deal with these stressors in nursing school, it’s going to carry over to their professional practice.”
A growing recognition of the toll that nursing programs may have on students’ mental health has led schools to launch initiatives to better support the next generation of nurses.
Diagnosing the Problem
Higher than average rates of depression among nursing students are not new. Nursing students often work long shifts with limited breaks. The academic rigors and clinical demands of caring for patients with acute and chronic conditions while instructors evaluate and watch for mistakes can cause high levels of stress, Stubin told this news organization. “Eventually, something has to give, and it’s usually their mental health.”
Clinical practicums often start when nursing students are still freshmen, and asking 18-year-old students to provide patient care in often-chaotic clinical environments is “overwhelming,” according to Stubin. The COVID-19 pandemic further exacerbated the issue.
During lockdown, more than half the nursing students reported moderate to severe symptoms of anxiety and depression, which was attributed to the transition to online learning, fear of infection, burnout, and the psychological distress of lockdown.
“The pandemic exacerbated existing mental health problems in undergraduate nursing students,” said Stubin. “In the wake of it ... a lot of [registered nurses] have mental health issues and are leaving the profession.”
Helping Nurses Heal
A significant shift in the willingness to talk about mental health and seek treatment could help. In 2011, just one third of students participated in the treatment for a mental health disorder. The latest data show that 61% of students experiencing symptoms of depression or anxiety take medication or seek therapy or counseling.
Incoming health sciences students at Ohio State University (OSU), Columbus, are screened for depression, anxiety, and suicidal ideation and directed to campus health services as needed. Bernadette Mazurek Melnyk, PhD, APRN-CNP, OSU’s chief wellness officer and former dean in the College of Nursing, believes it’s an essential step in supporting students, adding, “If you don’t screen, you don’t know the students are suffering, and we’re able to get help to the students who need it quickly.”
Prioritizing Solutions
Counseling services available through campus health centers are just one part of a multipronged approach that nursing schools have taken to improve the health and well-being of students. Nursing programs have also introduced initiatives to lower stress, prevent burnout, and relieve emotional trauma.
“In nursing education, we have to lay the groundwork for the self-care, wellness, and resilience practices that can, hopefully, be carried over into their professional practices,” Stubin said.
At Rutgers University–Camden, the wellness center provides counseling services, and the Student Nursing Association offers a pet therapy program. Stubin also incorporates self-care, resilience-building strategies, and wellness programming into the curriculum.
During the pandemic, the University of Colorado College of Nursing, Aurora, created a class called Stress Impact and Care for COVID-19 to provide content, exercises, and support groups for nursing students. The class was so popular that it was adapted and integrated into the curriculum.
The University of Vermont, Burlington, introduced the Benson-Henry Institute Stress Management and Resiliency Training program in 2021. The 8-week program was designed to teach nursing students coping strategies to reduce stress.
Offering stress management programs to first-year nursing students has been linked to improved problem-solving skills and fewer emotional and social behavioral symptoms. However, for programs to be effective, Melnyk believes that they need to be integrated into the curriculum, not offered as electives.
“We know mindfulness works, we know cognitive behavior skills-building works, and these types of evidence-based programs with such efficacy behind them should not be optional,” she said. “Students are overwhelmed just with their coursework, so if these programs exist for extra credit, students won’t take them.”
Creating a Culture of Wellness
Teaching nursing students how to manage stress and providing the resources to combat depression and anxiety is just the first step in building a healthy, resilient nursing workforce.
Prioritizing wellness in nursing isn’t just essential for addressing the nationwide nursing shortage. Burnout in the medical field costs the United States healthcare system $4.6 billion per year, and preventable medical errors are the third leading cause of death in the United States.
“There is a nice movement across the United States to reduce these mental health issues because they’re so costly,” Melnyk said.
There are also national efforts to address the issue. The National Academy of Medicine introduced the Action Collaborative on Clinician Well-Being and Resilience, which has grown to include more than 200 organizations committed to reversing burnout and improving mental health in the clinical workforce. The American Nurses Foundation created The Nurse Well-Being: Building Peer and Leadership Support Program to provide resources and peer support to help nurses manage stress.
Health systems and hospitals also need to prioritize clinical well-being to reduce stress and burnout — and these efforts must be ongoing.
“These resources have to be extended into the working world ... and not just once a year for Nurses Week in May, but on a regular continued basis,” said Stubin. “Healthcare corporations and hospitals have to continue these resources and this help; it has to be a priority.”
Until the culture changes, Stubin fears that nursing students will continue facing barriers to completing their programs and maintaining nursing careers. Currently, 43% of college students considered leaving their program for mental health reasons, and 21.7% of nurses reported suicidal ideation.
“There’s a nursing shortage, and the acuity of patient care is increasing, so the stressors in the clinical area aren’t going to decrease,” Stubin said. “We as nursing faculty must teach our students how to manage these stressors to build a resilient, mentally and physically healthy workforce.”
A version of this article first appeared on Medscape.com.
Nursing is a competitive field. In 2022, nursing schools rejected more than 78,000 qualified applications, and the students whose applications were accepted faced demanding schedules and rigorous academics and clinical rotations. Is this a recipe for depression?
In 2024, 38% of nursing students experienced depression — a 9.3% increase over 2019, according to research from higher education research group Degreechoices. Catherine A. Stubin, PhD, RN, assistant professor of nursing at Rutgers University–Camden in New Jersey, calls it “a mental health crisis in nursing.”
“Nursing is a very rigorous, difficult, psychologically and physically demanding profession,” she said. “If students don’t have the tools and resources to adequately deal with these stressors in nursing school, it’s going to carry over to their professional practice.”
A growing recognition of the toll that nursing programs may have on students’ mental health has led schools to launch initiatives to better support the next generation of nurses.
Diagnosing the Problem
Higher than average rates of depression among nursing students are not new. Nursing students often work long shifts with limited breaks. The academic rigors and clinical demands of caring for patients with acute and chronic conditions while instructors evaluate and watch for mistakes can cause high levels of stress, Stubin told this news organization. “Eventually, something has to give, and it’s usually their mental health.”
Clinical practicums often start when nursing students are still freshmen, and asking 18-year-old students to provide patient care in often-chaotic clinical environments is “overwhelming,” according to Stubin. The COVID-19 pandemic further exacerbated the issue.
During lockdown, more than half the nursing students reported moderate to severe symptoms of anxiety and depression, which was attributed to the transition to online learning, fear of infection, burnout, and the psychological distress of lockdown.
“The pandemic exacerbated existing mental health problems in undergraduate nursing students,” said Stubin. “In the wake of it ... a lot of [registered nurses] have mental health issues and are leaving the profession.”
Helping Nurses Heal
A significant shift in the willingness to talk about mental health and seek treatment could help. In 2011, just one third of students participated in the treatment for a mental health disorder. The latest data show that 61% of students experiencing symptoms of depression or anxiety take medication or seek therapy or counseling.
Incoming health sciences students at Ohio State University (OSU), Columbus, are screened for depression, anxiety, and suicidal ideation and directed to campus health services as needed. Bernadette Mazurek Melnyk, PhD, APRN-CNP, OSU’s chief wellness officer and former dean in the College of Nursing, believes it’s an essential step in supporting students, adding, “If you don’t screen, you don’t know the students are suffering, and we’re able to get help to the students who need it quickly.”
Prioritizing Solutions
Counseling services available through campus health centers are just one part of a multipronged approach that nursing schools have taken to improve the health and well-being of students. Nursing programs have also introduced initiatives to lower stress, prevent burnout, and relieve emotional trauma.
“In nursing education, we have to lay the groundwork for the self-care, wellness, and resilience practices that can, hopefully, be carried over into their professional practices,” Stubin said.
At Rutgers University–Camden, the wellness center provides counseling services, and the Student Nursing Association offers a pet therapy program. Stubin also incorporates self-care, resilience-building strategies, and wellness programming into the curriculum.
During the pandemic, the University of Colorado College of Nursing, Aurora, created a class called Stress Impact and Care for COVID-19 to provide content, exercises, and support groups for nursing students. The class was so popular that it was adapted and integrated into the curriculum.
The University of Vermont, Burlington, introduced the Benson-Henry Institute Stress Management and Resiliency Training program in 2021. The 8-week program was designed to teach nursing students coping strategies to reduce stress.
Offering stress management programs to first-year nursing students has been linked to improved problem-solving skills and fewer emotional and social behavioral symptoms. However, for programs to be effective, Melnyk believes that they need to be integrated into the curriculum, not offered as electives.
“We know mindfulness works, we know cognitive behavior skills-building works, and these types of evidence-based programs with such efficacy behind them should not be optional,” she said. “Students are overwhelmed just with their coursework, so if these programs exist for extra credit, students won’t take them.”
Creating a Culture of Wellness
Teaching nursing students how to manage stress and providing the resources to combat depression and anxiety is just the first step in building a healthy, resilient nursing workforce.
Prioritizing wellness in nursing isn’t just essential for addressing the nationwide nursing shortage. Burnout in the medical field costs the United States healthcare system $4.6 billion per year, and preventable medical errors are the third leading cause of death in the United States.
“There is a nice movement across the United States to reduce these mental health issues because they’re so costly,” Melnyk said.
There are also national efforts to address the issue. The National Academy of Medicine introduced the Action Collaborative on Clinician Well-Being and Resilience, which has grown to include more than 200 organizations committed to reversing burnout and improving mental health in the clinical workforce. The American Nurses Foundation created The Nurse Well-Being: Building Peer and Leadership Support Program to provide resources and peer support to help nurses manage stress.
Health systems and hospitals also need to prioritize clinical well-being to reduce stress and burnout — and these efforts must be ongoing.
“These resources have to be extended into the working world ... and not just once a year for Nurses Week in May, but on a regular continued basis,” said Stubin. “Healthcare corporations and hospitals have to continue these resources and this help; it has to be a priority.”
Until the culture changes, Stubin fears that nursing students will continue facing barriers to completing their programs and maintaining nursing careers. Currently, 43% of college students considered leaving their program for mental health reasons, and 21.7% of nurses reported suicidal ideation.
“There’s a nursing shortage, and the acuity of patient care is increasing, so the stressors in the clinical area aren’t going to decrease,” Stubin said. “We as nursing faculty must teach our students how to manage these stressors to build a resilient, mentally and physically healthy workforce.”
A version of this article first appeared on Medscape.com.
‘Round Face’: A Viral Term’s Real Diagnostic Implications
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
Additional symptoms of PCOS and its impact are found in the figure below.
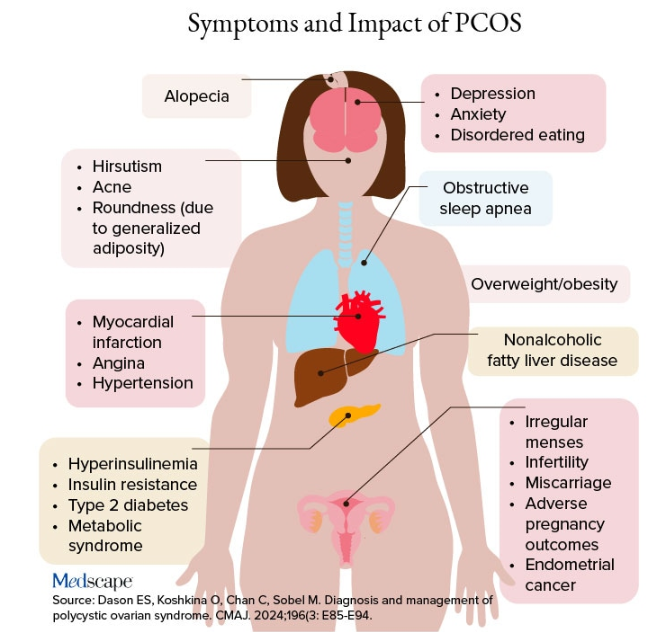
PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
Additional symptoms of PCOS and its impact are found in the figure below.

PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
Additional symptoms of PCOS and its impact are found in the figure below.

PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
Postpartum Exercise Reduces Depression and Anxiety Symptoms
TOPLINE:
Postpartum exercise reduces the severity of depressive and anxiety symptoms. Initiating exercise within 12 weeks post partum is linked to greater reductions in depressive symptoms.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis including 35 studies with a total of 4072 participants.
- The review included randomized controlled trials and nonrandomized interventions examining the impact of postpartum exercise on depression and anxiety.
- Participants were postpartum individuals within the first year after childbirth, with interventions including various types of exercise.
- Data sources included online databases with data up to January 2024, reference lists, and hand searches.
- The Grading of Recommendations, Assessment, Development, and Evaluation framework was used to assess the certainty of evidence.
TAKEAWAY:
- Postpartum exercise-only interventions resulted in a moderate reduction in the severity of depressive symptoms (standardized mean difference [SMD], –0.52; 95% CI, –0.80 to –0.24).
- Exercise-only interventions were associated with a small reduction in the severity of anxiety symptoms (SMD, –0.25; 95% CI, –0.43 to –0.08).
- Initiating exercise within 12 weeks post partum was associated with a greater reduction in depressive symptoms, compared with starting later.
- Postpartum exercise was associated with a 45% reduction in the odds of developing depression (odds ratio, 0.55; 95% CI, 0.32-0.95).
IN PRACTICE:
“Further investigation should aim to investigate the effects of postpartum exercise in individuals who experienced perinatal complications and in those who had limitations to exercise during pregnancy. Additionally, more investigation is required to address the possible lasting effects of postpartum exercise on maternal mental health as there were very limited studies reporting on this outcome,” the authors of the study wrote.
SOURCE:
This study was led by Margie H. Davenport, University of Alberta in Edmonton, Canada. It was published online in British Journal of Sports Medicine.
LIMITATIONS:
This study’s limitations included high heterogeneity among included studies, small sample sizes in some studies, and the combination of exercise with other interventions in some cases. These factors may have affected the generalizability and precision of the findings.
DISCLOSURES:
This study was funded by the Christenson Professorship in Active Healthy Living. Davenport is funded by a Christenson Professorship in Active Healthy Living. One coauthor is funded by the Université du Québec à Trois-Rivières research chair in physical activity and maternal and neonatal health. No relevant conflicts of interest were disclosed by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Postpartum exercise reduces the severity of depressive and anxiety symptoms. Initiating exercise within 12 weeks post partum is linked to greater reductions in depressive symptoms.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis including 35 studies with a total of 4072 participants.
- The review included randomized controlled trials and nonrandomized interventions examining the impact of postpartum exercise on depression and anxiety.
- Participants were postpartum individuals within the first year after childbirth, with interventions including various types of exercise.
- Data sources included online databases with data up to January 2024, reference lists, and hand searches.
- The Grading of Recommendations, Assessment, Development, and Evaluation framework was used to assess the certainty of evidence.
TAKEAWAY:
- Postpartum exercise-only interventions resulted in a moderate reduction in the severity of depressive symptoms (standardized mean difference [SMD], –0.52; 95% CI, –0.80 to –0.24).
- Exercise-only interventions were associated with a small reduction in the severity of anxiety symptoms (SMD, –0.25; 95% CI, –0.43 to –0.08).
- Initiating exercise within 12 weeks post partum was associated with a greater reduction in depressive symptoms, compared with starting later.
- Postpartum exercise was associated with a 45% reduction in the odds of developing depression (odds ratio, 0.55; 95% CI, 0.32-0.95).
IN PRACTICE:
“Further investigation should aim to investigate the effects of postpartum exercise in individuals who experienced perinatal complications and in those who had limitations to exercise during pregnancy. Additionally, more investigation is required to address the possible lasting effects of postpartum exercise on maternal mental health as there were very limited studies reporting on this outcome,” the authors of the study wrote.
SOURCE:
This study was led by Margie H. Davenport, University of Alberta in Edmonton, Canada. It was published online in British Journal of Sports Medicine.
LIMITATIONS:
This study’s limitations included high heterogeneity among included studies, small sample sizes in some studies, and the combination of exercise with other interventions in some cases. These factors may have affected the generalizability and precision of the findings.
DISCLOSURES:
This study was funded by the Christenson Professorship in Active Healthy Living. Davenport is funded by a Christenson Professorship in Active Healthy Living. One coauthor is funded by the Université du Québec à Trois-Rivières research chair in physical activity and maternal and neonatal health. No relevant conflicts of interest were disclosed by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Postpartum exercise reduces the severity of depressive and anxiety symptoms. Initiating exercise within 12 weeks post partum is linked to greater reductions in depressive symptoms.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis including 35 studies with a total of 4072 participants.
- The review included randomized controlled trials and nonrandomized interventions examining the impact of postpartum exercise on depression and anxiety.
- Participants were postpartum individuals within the first year after childbirth, with interventions including various types of exercise.
- Data sources included online databases with data up to January 2024, reference lists, and hand searches.
- The Grading of Recommendations, Assessment, Development, and Evaluation framework was used to assess the certainty of evidence.
TAKEAWAY:
- Postpartum exercise-only interventions resulted in a moderate reduction in the severity of depressive symptoms (standardized mean difference [SMD], –0.52; 95% CI, –0.80 to –0.24).
- Exercise-only interventions were associated with a small reduction in the severity of anxiety symptoms (SMD, –0.25; 95% CI, –0.43 to –0.08).
- Initiating exercise within 12 weeks post partum was associated with a greater reduction in depressive symptoms, compared with starting later.
- Postpartum exercise was associated with a 45% reduction in the odds of developing depression (odds ratio, 0.55; 95% CI, 0.32-0.95).
IN PRACTICE:
“Further investigation should aim to investigate the effects of postpartum exercise in individuals who experienced perinatal complications and in those who had limitations to exercise during pregnancy. Additionally, more investigation is required to address the possible lasting effects of postpartum exercise on maternal mental health as there were very limited studies reporting on this outcome,” the authors of the study wrote.
SOURCE:
This study was led by Margie H. Davenport, University of Alberta in Edmonton, Canada. It was published online in British Journal of Sports Medicine.
LIMITATIONS:
This study’s limitations included high heterogeneity among included studies, small sample sizes in some studies, and the combination of exercise with other interventions in some cases. These factors may have affected the generalizability and precision of the findings.
DISCLOSURES:
This study was funded by the Christenson Professorship in Active Healthy Living. Davenport is funded by a Christenson Professorship in Active Healthy Living. One coauthor is funded by the Université du Québec à Trois-Rivières research chair in physical activity and maternal and neonatal health. No relevant conflicts of interest were disclosed by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Postpartum Depression Common After Cesarean Delivery
TOPLINE:
About one in six women experience symptoms of postpartum depression (PPD) 2 months after cesarean delivery, with certain obstetric factors such as emergency cesarean delivery before labor, cesarean delivery after labor induction, lack of social support in the operating room, and severe postoperative pain influencing the risk.
METHODOLOGY:
- Researchers conducted a prospective ancillary cohort study of the Tranexamic Acid for Preventing Postpartum Hemorrhage after Cesarean Delivery (TRAAP2) trial to examine the prevalence of PPD 2 months after cesarean delivery and associated risk factors.
- A total of 2793 women (median age, 33.5 years) were included who had a cesarean delivery at 34 or more weeks of gestation; they completed the Edinburgh Postnatal Depression Scale (EPDS), a self-administered questionnaire, at 2 months after delivery.
- Information about the cesarean delivery, postpartum blood loss, immediate postpartum period, psychiatric history, and memories of delivery and postoperative pain were prospectively collected.
- Medical records were used to obtain details about characteristics of patients; 5.0% had a psychiatric history (2.4% composed of depression).
- The main endpoint was a positive screening for symptoms consistent with this depression — defined as a PPD diagnosis — 2 months after caesarian delivery, with an EPDS score of 13 or higher.
TAKEAWAY:
- The prevalence of a provisional PPD diagnosis at 2 months after cesarean delivery was 16.4% (95% CI, 14.9-18.0) with an EPDS score of 13 or higher and was 23.1% (95% CI, 21.4-24.9%) with a cutoff value of 11 or higher.
- Women who had an emergency cesarean delivery before labor had a higher risk for PPD than those who had a normal cesarean delivery before labor started (adjusted odds ratio [aOR], 1.70; 95% CI, 1.15-2.50); women who had started labor after induction but then had a cesarean delivery also had a higher risk for PPD than those who had a cesarean delivery before going into labor (aOR, 1.36; 95% CI, 1.03-1.84).
- Severe pain during the postpartum stay (aOR, 1.73; 95% CI, 1.32-2.26) and bad memories of delivery (aOR, 1.67; 95% CI, 1.14-2.45) were also risk factors for PPD.
- However, women who had social support in the operating room showed a 27% lower risk for PPD (P = .02).
IN PRACTICE:
“Identifying subgroups of women at risk for PPD based on aspects of their obstetric experience could help to screen for women who might benefit from early screening and interventions,” the authors wrote.
SOURCE:
This study was led by Alizée Froeliger, MD, MPH, of the Department of Obstetrics and Gynecology at Bordeaux University Hospital in France, and was published online in American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study population was derived from a randomized controlled trial, which may have underestimated the prevalence of PPD. The use of a self-administered questionnaire for PPD screening may not have provided a definitive diagnosis. Moreover, this study did not assess the prevalence of depressive symptoms during pregnancy.
DISCLOSURES:
The TRAAP2 trial was supported by a grant from the French Ministry of Health under its Clinical Research Hospital Program. One author reported carrying out consultancy work and lecturing for Ferring Laboratories, GlaxoSmithKline, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
About one in six women experience symptoms of postpartum depression (PPD) 2 months after cesarean delivery, with certain obstetric factors such as emergency cesarean delivery before labor, cesarean delivery after labor induction, lack of social support in the operating room, and severe postoperative pain influencing the risk.
METHODOLOGY:
- Researchers conducted a prospective ancillary cohort study of the Tranexamic Acid for Preventing Postpartum Hemorrhage after Cesarean Delivery (TRAAP2) trial to examine the prevalence of PPD 2 months after cesarean delivery and associated risk factors.
- A total of 2793 women (median age, 33.5 years) were included who had a cesarean delivery at 34 or more weeks of gestation; they completed the Edinburgh Postnatal Depression Scale (EPDS), a self-administered questionnaire, at 2 months after delivery.
- Information about the cesarean delivery, postpartum blood loss, immediate postpartum period, psychiatric history, and memories of delivery and postoperative pain were prospectively collected.
- Medical records were used to obtain details about characteristics of patients; 5.0% had a psychiatric history (2.4% composed of depression).
- The main endpoint was a positive screening for symptoms consistent with this depression — defined as a PPD diagnosis — 2 months after caesarian delivery, with an EPDS score of 13 or higher.
TAKEAWAY:
- The prevalence of a provisional PPD diagnosis at 2 months after cesarean delivery was 16.4% (95% CI, 14.9-18.0) with an EPDS score of 13 or higher and was 23.1% (95% CI, 21.4-24.9%) with a cutoff value of 11 or higher.
- Women who had an emergency cesarean delivery before labor had a higher risk for PPD than those who had a normal cesarean delivery before labor started (adjusted odds ratio [aOR], 1.70; 95% CI, 1.15-2.50); women who had started labor after induction but then had a cesarean delivery also had a higher risk for PPD than those who had a cesarean delivery before going into labor (aOR, 1.36; 95% CI, 1.03-1.84).
- Severe pain during the postpartum stay (aOR, 1.73; 95% CI, 1.32-2.26) and bad memories of delivery (aOR, 1.67; 95% CI, 1.14-2.45) were also risk factors for PPD.
- However, women who had social support in the operating room showed a 27% lower risk for PPD (P = .02).
IN PRACTICE:
“Identifying subgroups of women at risk for PPD based on aspects of their obstetric experience could help to screen for women who might benefit from early screening and interventions,” the authors wrote.
SOURCE:
This study was led by Alizée Froeliger, MD, MPH, of the Department of Obstetrics and Gynecology at Bordeaux University Hospital in France, and was published online in American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study population was derived from a randomized controlled trial, which may have underestimated the prevalence of PPD. The use of a self-administered questionnaire for PPD screening may not have provided a definitive diagnosis. Moreover, this study did not assess the prevalence of depressive symptoms during pregnancy.
DISCLOSURES:
The TRAAP2 trial was supported by a grant from the French Ministry of Health under its Clinical Research Hospital Program. One author reported carrying out consultancy work and lecturing for Ferring Laboratories, GlaxoSmithKline, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
About one in six women experience symptoms of postpartum depression (PPD) 2 months after cesarean delivery, with certain obstetric factors such as emergency cesarean delivery before labor, cesarean delivery after labor induction, lack of social support in the operating room, and severe postoperative pain influencing the risk.
METHODOLOGY:
- Researchers conducted a prospective ancillary cohort study of the Tranexamic Acid for Preventing Postpartum Hemorrhage after Cesarean Delivery (TRAAP2) trial to examine the prevalence of PPD 2 months after cesarean delivery and associated risk factors.
- A total of 2793 women (median age, 33.5 years) were included who had a cesarean delivery at 34 or more weeks of gestation; they completed the Edinburgh Postnatal Depression Scale (EPDS), a self-administered questionnaire, at 2 months after delivery.
- Information about the cesarean delivery, postpartum blood loss, immediate postpartum period, psychiatric history, and memories of delivery and postoperative pain were prospectively collected.
- Medical records were used to obtain details about characteristics of patients; 5.0% had a psychiatric history (2.4% composed of depression).
- The main endpoint was a positive screening for symptoms consistent with this depression — defined as a PPD diagnosis — 2 months after caesarian delivery, with an EPDS score of 13 or higher.
TAKEAWAY:
- The prevalence of a provisional PPD diagnosis at 2 months after cesarean delivery was 16.4% (95% CI, 14.9-18.0) with an EPDS score of 13 or higher and was 23.1% (95% CI, 21.4-24.9%) with a cutoff value of 11 or higher.
- Women who had an emergency cesarean delivery before labor had a higher risk for PPD than those who had a normal cesarean delivery before labor started (adjusted odds ratio [aOR], 1.70; 95% CI, 1.15-2.50); women who had started labor after induction but then had a cesarean delivery also had a higher risk for PPD than those who had a cesarean delivery before going into labor (aOR, 1.36; 95% CI, 1.03-1.84).
- Severe pain during the postpartum stay (aOR, 1.73; 95% CI, 1.32-2.26) and bad memories of delivery (aOR, 1.67; 95% CI, 1.14-2.45) were also risk factors for PPD.
- However, women who had social support in the operating room showed a 27% lower risk for PPD (P = .02).
IN PRACTICE:
“Identifying subgroups of women at risk for PPD based on aspects of their obstetric experience could help to screen for women who might benefit from early screening and interventions,” the authors wrote.
SOURCE:
This study was led by Alizée Froeliger, MD, MPH, of the Department of Obstetrics and Gynecology at Bordeaux University Hospital in France, and was published online in American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study population was derived from a randomized controlled trial, which may have underestimated the prevalence of PPD. The use of a self-administered questionnaire for PPD screening may not have provided a definitive diagnosis. Moreover, this study did not assess the prevalence of depressive symptoms during pregnancy.
DISCLOSURES:
The TRAAP2 trial was supported by a grant from the French Ministry of Health under its Clinical Research Hospital Program. One author reported carrying out consultancy work and lecturing for Ferring Laboratories, GlaxoSmithKline, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
A History of Concussion Linked to Maternal Mental Illness
A history of concussion can have serious long-term mental health implications for women, even years after giving birth, according to a new study.
Researchers looked at all people who delivered babies in Ontario, Canada, and found that those with a predelivery history of concussion were 25% more likely to have a serious mental illness up to 14 years after giving birth than those with no history of concussion.
The findings indicate the need for early identification and screening of women with a history of concussion, as well as ongoing, long-term supports to prevent adverse psychiatric outcomes, wrote the authors.
“I played a lot of sports growing up, and I definitely would not have thought about how a concussion could affect childbearing or parenting,” author Samantha Krueger, RM, MSc, told this news organization. She completed the research as part of her studies at the University of Toronto, Ontario.
The data were published on November 4 in The Journal of Clinical Psychiatry.
Implications for Prevention
“Birthing people, and women in general, are an often-overlooked population in the scientific literature on traumatic brain injury, including concussion. There is a potential interplay between concussion history and the challenges of being a new parent (such as labor and birth, lack of sleep, and increased noise) that make this an important population to study,” said Krueger.
The researchers conducted a population-based cohort study of all women who gave birth in Ontario between 2007 and 2017. Follow-up continued until 2021. The primary outcome was severe maternal mental illness, which was defined as a psychiatric emergency department visit, psychiatric hospital admission, or self-harm or suicide in the 14 years after delivery.
The researchers identified 18,064 women with a predelivery history of concussion and 736,689 women without a history of concussion during the study period. Women with a predelivery history of concussion were more likely than those without such a history to live in a rural area and have a history of assault or mental illness.
Overall, 11.3% (n = 2033) of the women with a predelivery history of concussion developed severe maternal mental illness (14.7 per 1000 person-years), compared with 6.8% (n = 49,928) of the women without a predelivery history of concussion (7.9 per 1000 person-years).
The adjusted hazard ratio (aHR) was 1.25. The association was strongest in women who had a predelivery history of concussion but no history of mental illness (aHR, 1.33).
“We hope to increase awareness of the seriousness of having a concussion, even when it is considered a mild head injury,” Krueger said. “The results have important implications for concussion prevention measures for young people and for the provision of postpartum supports (such as mental health and other social supports like sleep relief) to mitigate the risk of serious mental illness outcomes in birthing people with a history of concussion.”
Healthcare providers, including maternity care providers, should be asking about concussion history and providing mental health screening and supports to clients and their families to detect mental illness before a serious outcome occurs, Krueger added.
“Maternity care providers can help birthing people and their families set up supports for after the baby is born and teach families about mental health symptoms to look out for. It’s also important that providers be certain that their care is trauma informed to avoid triggering a trauma response when providing care,” she said.
Area of Concern
“This research is novel and highlights an area of major concern,” Simon Sherry, PhD, professor of psychology and neuroscience at Dalhousie University in Halifax, Nova Scotia, Canada, told this news organization. Sherry did not participate in the study.
“Postpartum depression occurs in approximately 10%-25% of mothers, but it is likely that many more cases go undiagnosed. It is attributed to hormonal changes, genetic predisposition, and environmental factors, and while previous depression or mental illness is frequently considered a risk factor, traumatic brain injuries or concussions usually are not,” Sherry said.
“Mothers are already an at-risk population for mental illness, as illustrated by the high rates of postpartum depression, and so are people with a history of concussion or traumatic brain injury. What sets this study apart is that it shows the heightened risk for women with the combination of those two distinct risk factors. Identifying these risk factors is essential to providing preventive care. If care providers know a patient is at increased risk when starting a pregnancy, then they will likely catch warning signs earlier,” he said.
“Additionally, as the article suggests, maternal mental health often is not studied beyond the first postpartum year,” Sherry said.
“Mental health struggles during the first postpartum year have largely been normalized as part of the transition into parenthood, but mental health issues among parents later in life are less accepted. After birth, so much emphasis is moved from the parent to the child. Parents rightly prioritize their children, but our job as care providers is to ensure we are also prioritizing them. The prolonged period of this study helps illustrate how important the practice of prioritizing mothers’ mental health is,” he added.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care. The Canadian Institutes of Health Research also supported the study. Krueger is supported by a Canadian Institutes of Health Research Canada Graduate Scholarship Masters Award. Sherry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A history of concussion can have serious long-term mental health implications for women, even years after giving birth, according to a new study.
Researchers looked at all people who delivered babies in Ontario, Canada, and found that those with a predelivery history of concussion were 25% more likely to have a serious mental illness up to 14 years after giving birth than those with no history of concussion.
The findings indicate the need for early identification and screening of women with a history of concussion, as well as ongoing, long-term supports to prevent adverse psychiatric outcomes, wrote the authors.
“I played a lot of sports growing up, and I definitely would not have thought about how a concussion could affect childbearing or parenting,” author Samantha Krueger, RM, MSc, told this news organization. She completed the research as part of her studies at the University of Toronto, Ontario.
The data were published on November 4 in The Journal of Clinical Psychiatry.
Implications for Prevention
“Birthing people, and women in general, are an often-overlooked population in the scientific literature on traumatic brain injury, including concussion. There is a potential interplay between concussion history and the challenges of being a new parent (such as labor and birth, lack of sleep, and increased noise) that make this an important population to study,” said Krueger.
The researchers conducted a population-based cohort study of all women who gave birth in Ontario between 2007 and 2017. Follow-up continued until 2021. The primary outcome was severe maternal mental illness, which was defined as a psychiatric emergency department visit, psychiatric hospital admission, or self-harm or suicide in the 14 years after delivery.
The researchers identified 18,064 women with a predelivery history of concussion and 736,689 women without a history of concussion during the study period. Women with a predelivery history of concussion were more likely than those without such a history to live in a rural area and have a history of assault or mental illness.
Overall, 11.3% (n = 2033) of the women with a predelivery history of concussion developed severe maternal mental illness (14.7 per 1000 person-years), compared with 6.8% (n = 49,928) of the women without a predelivery history of concussion (7.9 per 1000 person-years).
The adjusted hazard ratio (aHR) was 1.25. The association was strongest in women who had a predelivery history of concussion but no history of mental illness (aHR, 1.33).
“We hope to increase awareness of the seriousness of having a concussion, even when it is considered a mild head injury,” Krueger said. “The results have important implications for concussion prevention measures for young people and for the provision of postpartum supports (such as mental health and other social supports like sleep relief) to mitigate the risk of serious mental illness outcomes in birthing people with a history of concussion.”
Healthcare providers, including maternity care providers, should be asking about concussion history and providing mental health screening and supports to clients and their families to detect mental illness before a serious outcome occurs, Krueger added.
“Maternity care providers can help birthing people and their families set up supports for after the baby is born and teach families about mental health symptoms to look out for. It’s also important that providers be certain that their care is trauma informed to avoid triggering a trauma response when providing care,” she said.
Area of Concern
“This research is novel and highlights an area of major concern,” Simon Sherry, PhD, professor of psychology and neuroscience at Dalhousie University in Halifax, Nova Scotia, Canada, told this news organization. Sherry did not participate in the study.
“Postpartum depression occurs in approximately 10%-25% of mothers, but it is likely that many more cases go undiagnosed. It is attributed to hormonal changes, genetic predisposition, and environmental factors, and while previous depression or mental illness is frequently considered a risk factor, traumatic brain injuries or concussions usually are not,” Sherry said.
“Mothers are already an at-risk population for mental illness, as illustrated by the high rates of postpartum depression, and so are people with a history of concussion or traumatic brain injury. What sets this study apart is that it shows the heightened risk for women with the combination of those two distinct risk factors. Identifying these risk factors is essential to providing preventive care. If care providers know a patient is at increased risk when starting a pregnancy, then they will likely catch warning signs earlier,” he said.
“Additionally, as the article suggests, maternal mental health often is not studied beyond the first postpartum year,” Sherry said.
“Mental health struggles during the first postpartum year have largely been normalized as part of the transition into parenthood, but mental health issues among parents later in life are less accepted. After birth, so much emphasis is moved from the parent to the child. Parents rightly prioritize their children, but our job as care providers is to ensure we are also prioritizing them. The prolonged period of this study helps illustrate how important the practice of prioritizing mothers’ mental health is,” he added.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care. The Canadian Institutes of Health Research also supported the study. Krueger is supported by a Canadian Institutes of Health Research Canada Graduate Scholarship Masters Award. Sherry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A history of concussion can have serious long-term mental health implications for women, even years after giving birth, according to a new study.
Researchers looked at all people who delivered babies in Ontario, Canada, and found that those with a predelivery history of concussion were 25% more likely to have a serious mental illness up to 14 years after giving birth than those with no history of concussion.
The findings indicate the need for early identification and screening of women with a history of concussion, as well as ongoing, long-term supports to prevent adverse psychiatric outcomes, wrote the authors.
“I played a lot of sports growing up, and I definitely would not have thought about how a concussion could affect childbearing or parenting,” author Samantha Krueger, RM, MSc, told this news organization. She completed the research as part of her studies at the University of Toronto, Ontario.
The data were published on November 4 in The Journal of Clinical Psychiatry.
Implications for Prevention
“Birthing people, and women in general, are an often-overlooked population in the scientific literature on traumatic brain injury, including concussion. There is a potential interplay between concussion history and the challenges of being a new parent (such as labor and birth, lack of sleep, and increased noise) that make this an important population to study,” said Krueger.
The researchers conducted a population-based cohort study of all women who gave birth in Ontario between 2007 and 2017. Follow-up continued until 2021. The primary outcome was severe maternal mental illness, which was defined as a psychiatric emergency department visit, psychiatric hospital admission, or self-harm or suicide in the 14 years after delivery.
The researchers identified 18,064 women with a predelivery history of concussion and 736,689 women without a history of concussion during the study period. Women with a predelivery history of concussion were more likely than those without such a history to live in a rural area and have a history of assault or mental illness.
Overall, 11.3% (n = 2033) of the women with a predelivery history of concussion developed severe maternal mental illness (14.7 per 1000 person-years), compared with 6.8% (n = 49,928) of the women without a predelivery history of concussion (7.9 per 1000 person-years).
The adjusted hazard ratio (aHR) was 1.25. The association was strongest in women who had a predelivery history of concussion but no history of mental illness (aHR, 1.33).
“We hope to increase awareness of the seriousness of having a concussion, even when it is considered a mild head injury,” Krueger said. “The results have important implications for concussion prevention measures for young people and for the provision of postpartum supports (such as mental health and other social supports like sleep relief) to mitigate the risk of serious mental illness outcomes in birthing people with a history of concussion.”
Healthcare providers, including maternity care providers, should be asking about concussion history and providing mental health screening and supports to clients and their families to detect mental illness before a serious outcome occurs, Krueger added.
“Maternity care providers can help birthing people and their families set up supports for after the baby is born and teach families about mental health symptoms to look out for. It’s also important that providers be certain that their care is trauma informed to avoid triggering a trauma response when providing care,” she said.
Area of Concern
“This research is novel and highlights an area of major concern,” Simon Sherry, PhD, professor of psychology and neuroscience at Dalhousie University in Halifax, Nova Scotia, Canada, told this news organization. Sherry did not participate in the study.
“Postpartum depression occurs in approximately 10%-25% of mothers, but it is likely that many more cases go undiagnosed. It is attributed to hormonal changes, genetic predisposition, and environmental factors, and while previous depression or mental illness is frequently considered a risk factor, traumatic brain injuries or concussions usually are not,” Sherry said.
“Mothers are already an at-risk population for mental illness, as illustrated by the high rates of postpartum depression, and so are people with a history of concussion or traumatic brain injury. What sets this study apart is that it shows the heightened risk for women with the combination of those two distinct risk factors. Identifying these risk factors is essential to providing preventive care. If care providers know a patient is at increased risk when starting a pregnancy, then they will likely catch warning signs earlier,” he said.
“Additionally, as the article suggests, maternal mental health often is not studied beyond the first postpartum year,” Sherry said.
“Mental health struggles during the first postpartum year have largely been normalized as part of the transition into parenthood, but mental health issues among parents later in life are less accepted. After birth, so much emphasis is moved from the parent to the child. Parents rightly prioritize their children, but our job as care providers is to ensure we are also prioritizing them. The prolonged period of this study helps illustrate how important the practice of prioritizing mothers’ mental health is,” he added.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care. The Canadian Institutes of Health Research also supported the study. Krueger is supported by a Canadian Institutes of Health Research Canada Graduate Scholarship Masters Award. Sherry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Silent Epidemic: Loneliness a Serious Threat to Both Brain and Body
In a world that is more connected than ever, a silent epidemic is taking its toll. Overall, one in three US adults report chronic loneliness — a condition so detrimental that it rivals smoking and obesity with respect to its negative effect on health and well-being. From anxiety and depression to life-threatening conditions like cardiovascular disease, stroke, and Alzheimer’s and Parkinson’s diseases, loneliness is more than an emotion — it’s a serious threat to both the brain and body.
In 2023, a US Surgeon General advisory raised the alarm about the national problem of loneliness and isolation, describing it as an epidemic.
“Given the significant health consequences of loneliness and isolation, we must prioritize building social connection in the same way we have prioritized other critical public health issues such as tobacco, obesity, and substance use disorders. Together, we can build a country that’s healthier, more resilient, less lonely, and more connected,” the report concluded.
But how, exactly, does chronic loneliness affect the physiology and function of the brain? What does the latest research reveal about the link between loneliness and neurologic and psychiatric illness, and what can clinicians do to address the issue?
This news organization spoke to multiple experts in the field to explore these issues.
A Major Risk Factor
Anna Finley, PhD, assistant professor of psychology at North Dakota State University, Fargo, explained that loneliness and social isolation are different entities. Social isolation is an objective measure of the number of people someone interacts with on a regular basis, whereas loneliness is a subjective feeling that occurs when close connections are lacking.
“These two things are not actually as related as you think they would be. People can feel lonely in a crowd or feel well connected with only a few friendships. It’s more about the quality of the connection and the quality of your perception of it. So someone could be in some very supportive relationships but still feel that there’s something missing,” she said in an interview.
So what do we know about how loneliness affects health? Evidence supporting the hypothesis that loneliness is an emerging risk factor for many diseases is steadily building.
Recently, the American Heart Association published a statement summarizing the evidence for a direct association between social isolation and loneliness and coronary heart disease and stroke mortality.
In addition, many studies have shown that individuals experiencing social isolation or loneliness have an increased risk for anxiety and depression, dementia, infectious disease, hospitalization, and all-cause death, even after adjusting for age and many other traditional risk factors.
One study revealed that eliminating loneliness has the potential to prevent nearly 20% of cases of depression in adults aged 50 years or older.
Indu Subramanian, MD, professor of neurology at the University of California, Los Angeles, and colleagues conducted a study involving patients with Parkinson’s disease, which showed that the negative impact of loneliness on disease severity was as significant as the positive effects of 30 minutes of daily exercise.
“The importance of loneliness is under-recognized and undervalued, and it poses a major risk for health outcomes and quality of life,” said Subramanian.
Subramanian noted that loneliness is stigmatizing, causing people to feel unlikable and blame themselves, which prevents them from opening up to doctors or loved ones about their struggle. At the same time, healthcare providers may not think to ask about loneliness or know about potential interventions. She emphasized that much more work is needed to address this issue.
Early Mortality Risk
Julianne Holt-Lunstad, PhD, professor of psychology and neuroscience at Brigham Young University in Provo, Utah, is the author of two large meta-analyses that suggest loneliness, social isolation, or living alone are independent risk factors for early mortality, increasing this risk by about a third — the equivalent to the risk of smoking 15 cigarettes per day.
“We have quite robust evidence across a number of health outcomes implicating the harmful effects of loneliness and social isolation. While these are observational studies and show mainly associations, we do have evidence from longitudinal studies that show lacking social connection, whether that be loneliness or social isolation, predicts subsequent worse outcomes, and most of these studies have adjusted for alternative kinds of explanations, like age, initial health status, lifestyle factors,” Holt-Lunstad said.
There is some evidence to suggest that isolation is more predictive of physical health outcomes, whereas loneliness is more predictive of mental health outcomes. That said, both isolation and loneliness have significant effects on mental and physical health outcomes, she noted.
There is also the question of whether loneliness is causing poor health or whether people who are in poor health feel lonely because poor health can lead to social isolation.
Finley said there’s probably a bit of both going on, but longitudinal studies, where loneliness is measured at a fixed timepoint then health outcomes are reported a few years later, suggest that loneliness is contributing to these adverse outcomes.
She added that there is also some evidence in animal models to suggest that loneliness is a causal risk factor for adverse health outcomes. “But you can’t ask a mouse or rat how lonely they’re feeling. All you can do is house them individually — removing them from social connection. This isn’t necessarily the same thing as loneliness in humans.”
Finley is studying mechanisms in the brain that may be involved in mediating the adverse health consequences of loneliness.
“What I’ve been seeing in the data so far is that it tends to be the self-report of how lonely folks are feeling that has the associations with differences in the brain, as opposed to the number of social connections people have. It does seem to be the more subjective, emotional perception of loneliness that is important.”
In a review of potential mechanisms involved, she concluded that it is dysregulated emotions and altered perceptions of social interactions that has profound impacts on the brain, suggesting that people who are lonely may have a tendency to interpret social cues in a negative way, preventing them from forming productive positive relationships.
Lack of Trust
One researcher who has studied this phenomenon is Dirk Scheele, PhD, professor of social neuroscience at Ruhr University Bochum in Germany.
“We were interested to find out why people remained lonely,” he said in an interview. “Loneliness is an unpleasant experience, and there are so many opportunities for social contacts nowadays, it’s not really clear at first sight why people are chronically lonely.”
To examine this question, Scheele and his team conducted a study in which functional MRI was used to examine the brain in otherwise healthy individuals with high or low loneliness scores while they played a trust game.
They also simulated a positive social interaction between participants and researchers, in which they talked about plans for a fictitious lottery win, and about their hobbies and interests, during which mood was measured with questionnaires, and saliva samples were collected to measure hormone levels.
Results showed that the high-lonely individuals had reduced activation in the insula cortex during the trust decisions. “This area of the brain is involved in the processing of bodily signals, such as ‘gut feelings.’ So reduced activity here could be interpreted as fewer gut feelings on who can be trusted,” Scheele explained.
The high-lonely individuals also had reduced responsiveness to the positive social interaction with a lower release of oxytocin and a smaller elevation in mood compared with the control individuals.
Scheele pointed out that there is some evidence that oxytocin might increase trust, and there is reduced release of endogenous oxytocin in high loneliness.
“Our results are consistent with the idea that loneliness is associated with negative biases about other people. So if we expect negative things from other people — for instance, that they cannot be trusted — then that would hamper further social interactions and could lead to loneliness,” he added.
A Role for Oxytocin?
In another study, the same researchers tested short-term (five weekly sessions) group psychotherapy to reduce loneliness using established techniques to target these negative biases. They also investigated whether the effects of this group psychotherapy could be augmented by administering intranasal oxytocin (vs placebo) before the group psychotherapy sessions.
Results showed that the group psychotherapy intervention reduced trait loneliness (loneliness experienced over a prolonged period). The oxytocin did not show a significant effect on trait loneliness, but there was a suggestion that it may enhance the reduction in state loneliness (how someone is feeling at a specific time) brought about by the psychotherapy sessions.
“We found that bonding within the groups was experienced as more positive in the oxytocin treated groups. It is possible that a longer intervention would be helpful for longer-term results,” Scheele concluded. “It’s not going to be a quick fix for loneliness, but there may be a role for oxytocin as an adjunct to psychotherapy.”
A Basic Human Need
Another loneliness researcher, Livia Tomova, PhD, assistant professor of psychology at Cardiff University in Wales, has used social isolation to induce loneliness in young people and found that this intervention was linked to brain patterns similar to those associated with hunger.
“We know that the drive to eat food is a very basic human need. We know quite well how it is represented in the brain,” she explained.
The researchers tested how the brains of the participants responded to seeing pictures of social interactions after they underwent a prolonged period of social isolation. In a subsequent session, the same people were asked to undergo food fasting and then underwent brain scans when looking at pictures of food. Results showed that the neural patterns were similar in the two situations with increased activity in the substantia nigra area within the midbrain.
“This area of the brain processes rewards and motivation. It consists primarily of dopamine neurons and increased activity corresponds to a feeling of craving something. So this area of the brain that controls essential homeostatic needs is activated when people feel lonely, suggesting that our need for social contact with others is potentially a very basic need similar to eating,” Tomova said.
Lower Gray Matter Volumes in Key Brain Areas
And another group from Germany has found that higher loneliness scores are negatively associated with specific brain regions responsible for memory, emotion regulation, and social processing.
Sandra Düzel, PhD, and colleagues from the Max Planck Institute for Human Development and the Charité – Universitätsmedizin Berlin, both in Berlin, Germany, reported a study in which individuals who reported higher loneliness had smaller gray matter volumes in brain regions such as the left amygdala, anterior hippocampus, and cerebellum, regions which are crucial for both emotional regulation and higher-order cognitive processes, such as self-reflection and executive function.
Düzel believes that possible mechanisms behind the link between loneliness and brain volume differences could include stress-related damage, with prolonged loneliness associated with elevated levels of stress hormones, which can damage the hippocampus over time, and reduced cognitive and social stimulation, which may contribute to brain volume reductions in regions critical for memory and emotional processing.
“Loneliness is often characterized by reduced social and environmental diversity, leading to less engagement with novel experiences and potentially lower hippocampal-striatal connectivity.
Since novelty-seeking and environmental diversity are associated with positive emotional states, individuals experiencing loneliness might benefit from increased exposure to new environments which could stimulate the brain’s reward circuits, fostering positive affect and potentially mitigating the emotional burden of loneliness,” she said.
Is Social Prescribing the Answer?
So are there enough data now to act and attempt to develop interventions to reduce loneliness? Most of these researchers believe so.
“I think we have enough information to act on this now. There are a number of national academies consensus reports, which suggest that, while certainly there are still gaps in our evidence and more to be learned, there is sufficient evidence that a concerning portion of the population seems to lack connection, and that the consequences are serious enough that we need to do something about it,” said Holt-Lunstad.
Some countries have introduced social prescribing where doctors can prescribe a group activity or a regular visit or telephone conversation with a supportive person.
Subramanian pointed out that it’s easier to implement in countries with national health services and may be more difficult to embrace in the US healthcare system.
“We are not so encouraged from a financial perspective to think about preventive care in the US. We don’t have an easy way to recognize in any tangible way the downstream of such activities in terms of preventing future problems. That is something we need to work on,” she said.
Finley cautioned that to work well, social prescribing will require an understanding of each person’s individual situation.
“Some people may only receive benefit of interacting with others if they are also getting some sort of support to address the social and emotional concerns that are tagging along with loneliness. I’m not sure that just telling people to go join their local gardening club or whatever will be the correct answer for everyone.”
She pointed out that many people will have issues in their life that are making it hard for them to be social. These could be mobility or financial challenges, care responsibilities, or concerns about illnesses or life events. “We need to figure out what would have the most bang for the person’s buck, so to speak, as an intervention. That could mean connecting them to a group relevant to their individual situation.”
Opportunity to Connect Not Enough?
Tomova believes that training people in social skills may be a better option. “It appears that some people who are chronically lonely seem to struggle to make relationships with others. So just encouraging them to interact with others more will not necessarily help. We need to better understand the pathways involved and who are the people who become ill. We can then develop and target better interventions and teach people coping strategies for that situation.”
Scheele agreed. “While just giving people the opportunity to connect may work for some, others who are experiencing really chronic loneliness may not benefit very much from this unless their negative belief systems are addressed.” He suggested some sort of psychotherapy may be helpful in this situation.
But at least all seem to agree that healthcare providers need to be more aware of loneliness as a health risk factor, try to identify people at risk, and to think about how best to support them.
Holt-Lunstad noted that one of the recommendations in the US Surgeon General’s advisory was to increase the education, training, and resources on loneliness for healthcare providers.
“If we want this to be addressed, we need to give healthcare providers the time, resources, and training in order to do that, otherwise, we are adding one more thing to an already overburdened system. They need to understand how important it is, and how it might help them take care of the patient.”
“Our hope is that we can start to reverse some of the trends that we are seeing, both in terms of the prevalence rates of loneliness, but also that we could start seeing improvements in health and other kinds of outcomes,” she concluded.
Progress is being made in increasing awareness about the dangers of chronic loneliness. It’s now recognized as a serious health risk, but there are actionable steps that can help. Loneliness doesn’t have to be a permanent condition for anyone, said Scheele.
Holt-Lunstad served as an adviser for Foundation for Social Connection, Global Initiative on Loneliness and Connection, and Nextdoor Neighborhood Vitality Board and received research grants/income from Templeton Foundation, Eventbrite, Foundation for Social Connection, and Triple-S Foundation. Subramanian served as a speaker bureau for Acorda Pharma. The other researchers reported no disclosures.
A version of this article first appeared on Medscape.com.
In a world that is more connected than ever, a silent epidemic is taking its toll. Overall, one in three US adults report chronic loneliness — a condition so detrimental that it rivals smoking and obesity with respect to its negative effect on health and well-being. From anxiety and depression to life-threatening conditions like cardiovascular disease, stroke, and Alzheimer’s and Parkinson’s diseases, loneliness is more than an emotion — it’s a serious threat to both the brain and body.
In 2023, a US Surgeon General advisory raised the alarm about the national problem of loneliness and isolation, describing it as an epidemic.
“Given the significant health consequences of loneliness and isolation, we must prioritize building social connection in the same way we have prioritized other critical public health issues such as tobacco, obesity, and substance use disorders. Together, we can build a country that’s healthier, more resilient, less lonely, and more connected,” the report concluded.
But how, exactly, does chronic loneliness affect the physiology and function of the brain? What does the latest research reveal about the link between loneliness and neurologic and psychiatric illness, and what can clinicians do to address the issue?
This news organization spoke to multiple experts in the field to explore these issues.
A Major Risk Factor
Anna Finley, PhD, assistant professor of psychology at North Dakota State University, Fargo, explained that loneliness and social isolation are different entities. Social isolation is an objective measure of the number of people someone interacts with on a regular basis, whereas loneliness is a subjective feeling that occurs when close connections are lacking.
“These two things are not actually as related as you think they would be. People can feel lonely in a crowd or feel well connected with only a few friendships. It’s more about the quality of the connection and the quality of your perception of it. So someone could be in some very supportive relationships but still feel that there’s something missing,” she said in an interview.
So what do we know about how loneliness affects health? Evidence supporting the hypothesis that loneliness is an emerging risk factor for many diseases is steadily building.
Recently, the American Heart Association published a statement summarizing the evidence for a direct association between social isolation and loneliness and coronary heart disease and stroke mortality.
In addition, many studies have shown that individuals experiencing social isolation or loneliness have an increased risk for anxiety and depression, dementia, infectious disease, hospitalization, and all-cause death, even after adjusting for age and many other traditional risk factors.
One study revealed that eliminating loneliness has the potential to prevent nearly 20% of cases of depression in adults aged 50 years or older.
Indu Subramanian, MD, professor of neurology at the University of California, Los Angeles, and colleagues conducted a study involving patients with Parkinson’s disease, which showed that the negative impact of loneliness on disease severity was as significant as the positive effects of 30 minutes of daily exercise.
“The importance of loneliness is under-recognized and undervalued, and it poses a major risk for health outcomes and quality of life,” said Subramanian.
Subramanian noted that loneliness is stigmatizing, causing people to feel unlikable and blame themselves, which prevents them from opening up to doctors or loved ones about their struggle. At the same time, healthcare providers may not think to ask about loneliness or know about potential interventions. She emphasized that much more work is needed to address this issue.
Early Mortality Risk
Julianne Holt-Lunstad, PhD, professor of psychology and neuroscience at Brigham Young University in Provo, Utah, is the author of two large meta-analyses that suggest loneliness, social isolation, or living alone are independent risk factors for early mortality, increasing this risk by about a third — the equivalent to the risk of smoking 15 cigarettes per day.
“We have quite robust evidence across a number of health outcomes implicating the harmful effects of loneliness and social isolation. While these are observational studies and show mainly associations, we do have evidence from longitudinal studies that show lacking social connection, whether that be loneliness or social isolation, predicts subsequent worse outcomes, and most of these studies have adjusted for alternative kinds of explanations, like age, initial health status, lifestyle factors,” Holt-Lunstad said.
There is some evidence to suggest that isolation is more predictive of physical health outcomes, whereas loneliness is more predictive of mental health outcomes. That said, both isolation and loneliness have significant effects on mental and physical health outcomes, she noted.
There is also the question of whether loneliness is causing poor health or whether people who are in poor health feel lonely because poor health can lead to social isolation.
Finley said there’s probably a bit of both going on, but longitudinal studies, where loneliness is measured at a fixed timepoint then health outcomes are reported a few years later, suggest that loneliness is contributing to these adverse outcomes.
She added that there is also some evidence in animal models to suggest that loneliness is a causal risk factor for adverse health outcomes. “But you can’t ask a mouse or rat how lonely they’re feeling. All you can do is house them individually — removing them from social connection. This isn’t necessarily the same thing as loneliness in humans.”
Finley is studying mechanisms in the brain that may be involved in mediating the adverse health consequences of loneliness.
“What I’ve been seeing in the data so far is that it tends to be the self-report of how lonely folks are feeling that has the associations with differences in the brain, as opposed to the number of social connections people have. It does seem to be the more subjective, emotional perception of loneliness that is important.”
In a review of potential mechanisms involved, she concluded that it is dysregulated emotions and altered perceptions of social interactions that has profound impacts on the brain, suggesting that people who are lonely may have a tendency to interpret social cues in a negative way, preventing them from forming productive positive relationships.
Lack of Trust
One researcher who has studied this phenomenon is Dirk Scheele, PhD, professor of social neuroscience at Ruhr University Bochum in Germany.
“We were interested to find out why people remained lonely,” he said in an interview. “Loneliness is an unpleasant experience, and there are so many opportunities for social contacts nowadays, it’s not really clear at first sight why people are chronically lonely.”
To examine this question, Scheele and his team conducted a study in which functional MRI was used to examine the brain in otherwise healthy individuals with high or low loneliness scores while they played a trust game.
They also simulated a positive social interaction between participants and researchers, in which they talked about plans for a fictitious lottery win, and about their hobbies and interests, during which mood was measured with questionnaires, and saliva samples were collected to measure hormone levels.
Results showed that the high-lonely individuals had reduced activation in the insula cortex during the trust decisions. “This area of the brain is involved in the processing of bodily signals, such as ‘gut feelings.’ So reduced activity here could be interpreted as fewer gut feelings on who can be trusted,” Scheele explained.
The high-lonely individuals also had reduced responsiveness to the positive social interaction with a lower release of oxytocin and a smaller elevation in mood compared with the control individuals.
Scheele pointed out that there is some evidence that oxytocin might increase trust, and there is reduced release of endogenous oxytocin in high loneliness.
“Our results are consistent with the idea that loneliness is associated with negative biases about other people. So if we expect negative things from other people — for instance, that they cannot be trusted — then that would hamper further social interactions and could lead to loneliness,” he added.
A Role for Oxytocin?
In another study, the same researchers tested short-term (five weekly sessions) group psychotherapy to reduce loneliness using established techniques to target these negative biases. They also investigated whether the effects of this group psychotherapy could be augmented by administering intranasal oxytocin (vs placebo) before the group psychotherapy sessions.
Results showed that the group psychotherapy intervention reduced trait loneliness (loneliness experienced over a prolonged period). The oxytocin did not show a significant effect on trait loneliness, but there was a suggestion that it may enhance the reduction in state loneliness (how someone is feeling at a specific time) brought about by the psychotherapy sessions.
“We found that bonding within the groups was experienced as more positive in the oxytocin treated groups. It is possible that a longer intervention would be helpful for longer-term results,” Scheele concluded. “It’s not going to be a quick fix for loneliness, but there may be a role for oxytocin as an adjunct to psychotherapy.”
A Basic Human Need
Another loneliness researcher, Livia Tomova, PhD, assistant professor of psychology at Cardiff University in Wales, has used social isolation to induce loneliness in young people and found that this intervention was linked to brain patterns similar to those associated with hunger.
“We know that the drive to eat food is a very basic human need. We know quite well how it is represented in the brain,” she explained.
The researchers tested how the brains of the participants responded to seeing pictures of social interactions after they underwent a prolonged period of social isolation. In a subsequent session, the same people were asked to undergo food fasting and then underwent brain scans when looking at pictures of food. Results showed that the neural patterns were similar in the two situations with increased activity in the substantia nigra area within the midbrain.
“This area of the brain processes rewards and motivation. It consists primarily of dopamine neurons and increased activity corresponds to a feeling of craving something. So this area of the brain that controls essential homeostatic needs is activated when people feel lonely, suggesting that our need for social contact with others is potentially a very basic need similar to eating,” Tomova said.
Lower Gray Matter Volumes in Key Brain Areas
And another group from Germany has found that higher loneliness scores are negatively associated with specific brain regions responsible for memory, emotion regulation, and social processing.
Sandra Düzel, PhD, and colleagues from the Max Planck Institute for Human Development and the Charité – Universitätsmedizin Berlin, both in Berlin, Germany, reported a study in which individuals who reported higher loneliness had smaller gray matter volumes in brain regions such as the left amygdala, anterior hippocampus, and cerebellum, regions which are crucial for both emotional regulation and higher-order cognitive processes, such as self-reflection and executive function.
Düzel believes that possible mechanisms behind the link between loneliness and brain volume differences could include stress-related damage, with prolonged loneliness associated with elevated levels of stress hormones, which can damage the hippocampus over time, and reduced cognitive and social stimulation, which may contribute to brain volume reductions in regions critical for memory and emotional processing.
“Loneliness is often characterized by reduced social and environmental diversity, leading to less engagement with novel experiences and potentially lower hippocampal-striatal connectivity.
Since novelty-seeking and environmental diversity are associated with positive emotional states, individuals experiencing loneliness might benefit from increased exposure to new environments which could stimulate the brain’s reward circuits, fostering positive affect and potentially mitigating the emotional burden of loneliness,” she said.
Is Social Prescribing the Answer?
So are there enough data now to act and attempt to develop interventions to reduce loneliness? Most of these researchers believe so.
“I think we have enough information to act on this now. There are a number of national academies consensus reports, which suggest that, while certainly there are still gaps in our evidence and more to be learned, there is sufficient evidence that a concerning portion of the population seems to lack connection, and that the consequences are serious enough that we need to do something about it,” said Holt-Lunstad.
Some countries have introduced social prescribing where doctors can prescribe a group activity or a regular visit or telephone conversation with a supportive person.
Subramanian pointed out that it’s easier to implement in countries with national health services and may be more difficult to embrace in the US healthcare system.
“We are not so encouraged from a financial perspective to think about preventive care in the US. We don’t have an easy way to recognize in any tangible way the downstream of such activities in terms of preventing future problems. That is something we need to work on,” she said.
Finley cautioned that to work well, social prescribing will require an understanding of each person’s individual situation.
“Some people may only receive benefit of interacting with others if they are also getting some sort of support to address the social and emotional concerns that are tagging along with loneliness. I’m not sure that just telling people to go join their local gardening club or whatever will be the correct answer for everyone.”
She pointed out that many people will have issues in their life that are making it hard for them to be social. These could be mobility or financial challenges, care responsibilities, or concerns about illnesses or life events. “We need to figure out what would have the most bang for the person’s buck, so to speak, as an intervention. That could mean connecting them to a group relevant to their individual situation.”
Opportunity to Connect Not Enough?
Tomova believes that training people in social skills may be a better option. “It appears that some people who are chronically lonely seem to struggle to make relationships with others. So just encouraging them to interact with others more will not necessarily help. We need to better understand the pathways involved and who are the people who become ill. We can then develop and target better interventions and teach people coping strategies for that situation.”
Scheele agreed. “While just giving people the opportunity to connect may work for some, others who are experiencing really chronic loneliness may not benefit very much from this unless their negative belief systems are addressed.” He suggested some sort of psychotherapy may be helpful in this situation.
But at least all seem to agree that healthcare providers need to be more aware of loneliness as a health risk factor, try to identify people at risk, and to think about how best to support them.
Holt-Lunstad noted that one of the recommendations in the US Surgeon General’s advisory was to increase the education, training, and resources on loneliness for healthcare providers.
“If we want this to be addressed, we need to give healthcare providers the time, resources, and training in order to do that, otherwise, we are adding one more thing to an already overburdened system. They need to understand how important it is, and how it might help them take care of the patient.”
“Our hope is that we can start to reverse some of the trends that we are seeing, both in terms of the prevalence rates of loneliness, but also that we could start seeing improvements in health and other kinds of outcomes,” she concluded.
Progress is being made in increasing awareness about the dangers of chronic loneliness. It’s now recognized as a serious health risk, but there are actionable steps that can help. Loneliness doesn’t have to be a permanent condition for anyone, said Scheele.
Holt-Lunstad served as an adviser for Foundation for Social Connection, Global Initiative on Loneliness and Connection, and Nextdoor Neighborhood Vitality Board and received research grants/income from Templeton Foundation, Eventbrite, Foundation for Social Connection, and Triple-S Foundation. Subramanian served as a speaker bureau for Acorda Pharma. The other researchers reported no disclosures.
A version of this article first appeared on Medscape.com.
In a world that is more connected than ever, a silent epidemic is taking its toll. Overall, one in three US adults report chronic loneliness — a condition so detrimental that it rivals smoking and obesity with respect to its negative effect on health and well-being. From anxiety and depression to life-threatening conditions like cardiovascular disease, stroke, and Alzheimer’s and Parkinson’s diseases, loneliness is more than an emotion — it’s a serious threat to both the brain and body.
In 2023, a US Surgeon General advisory raised the alarm about the national problem of loneliness and isolation, describing it as an epidemic.
“Given the significant health consequences of loneliness and isolation, we must prioritize building social connection in the same way we have prioritized other critical public health issues such as tobacco, obesity, and substance use disorders. Together, we can build a country that’s healthier, more resilient, less lonely, and more connected,” the report concluded.
But how, exactly, does chronic loneliness affect the physiology and function of the brain? What does the latest research reveal about the link between loneliness and neurologic and psychiatric illness, and what can clinicians do to address the issue?
This news organization spoke to multiple experts in the field to explore these issues.
A Major Risk Factor
Anna Finley, PhD, assistant professor of psychology at North Dakota State University, Fargo, explained that loneliness and social isolation are different entities. Social isolation is an objective measure of the number of people someone interacts with on a regular basis, whereas loneliness is a subjective feeling that occurs when close connections are lacking.
“These two things are not actually as related as you think they would be. People can feel lonely in a crowd or feel well connected with only a few friendships. It’s more about the quality of the connection and the quality of your perception of it. So someone could be in some very supportive relationships but still feel that there’s something missing,” she said in an interview.
So what do we know about how loneliness affects health? Evidence supporting the hypothesis that loneliness is an emerging risk factor for many diseases is steadily building.
Recently, the American Heart Association published a statement summarizing the evidence for a direct association between social isolation and loneliness and coronary heart disease and stroke mortality.
In addition, many studies have shown that individuals experiencing social isolation or loneliness have an increased risk for anxiety and depression, dementia, infectious disease, hospitalization, and all-cause death, even after adjusting for age and many other traditional risk factors.
One study revealed that eliminating loneliness has the potential to prevent nearly 20% of cases of depression in adults aged 50 years or older.
Indu Subramanian, MD, professor of neurology at the University of California, Los Angeles, and colleagues conducted a study involving patients with Parkinson’s disease, which showed that the negative impact of loneliness on disease severity was as significant as the positive effects of 30 minutes of daily exercise.
“The importance of loneliness is under-recognized and undervalued, and it poses a major risk for health outcomes and quality of life,” said Subramanian.
Subramanian noted that loneliness is stigmatizing, causing people to feel unlikable and blame themselves, which prevents them from opening up to doctors or loved ones about their struggle. At the same time, healthcare providers may not think to ask about loneliness or know about potential interventions. She emphasized that much more work is needed to address this issue.
Early Mortality Risk
Julianne Holt-Lunstad, PhD, professor of psychology and neuroscience at Brigham Young University in Provo, Utah, is the author of two large meta-analyses that suggest loneliness, social isolation, or living alone are independent risk factors for early mortality, increasing this risk by about a third — the equivalent to the risk of smoking 15 cigarettes per day.
“We have quite robust evidence across a number of health outcomes implicating the harmful effects of loneliness and social isolation. While these are observational studies and show mainly associations, we do have evidence from longitudinal studies that show lacking social connection, whether that be loneliness or social isolation, predicts subsequent worse outcomes, and most of these studies have adjusted for alternative kinds of explanations, like age, initial health status, lifestyle factors,” Holt-Lunstad said.
There is some evidence to suggest that isolation is more predictive of physical health outcomes, whereas loneliness is more predictive of mental health outcomes. That said, both isolation and loneliness have significant effects on mental and physical health outcomes, she noted.
There is also the question of whether loneliness is causing poor health or whether people who are in poor health feel lonely because poor health can lead to social isolation.
Finley said there’s probably a bit of both going on, but longitudinal studies, where loneliness is measured at a fixed timepoint then health outcomes are reported a few years later, suggest that loneliness is contributing to these adverse outcomes.
She added that there is also some evidence in animal models to suggest that loneliness is a causal risk factor for adverse health outcomes. “But you can’t ask a mouse or rat how lonely they’re feeling. All you can do is house them individually — removing them from social connection. This isn’t necessarily the same thing as loneliness in humans.”
Finley is studying mechanisms in the brain that may be involved in mediating the adverse health consequences of loneliness.
“What I’ve been seeing in the data so far is that it tends to be the self-report of how lonely folks are feeling that has the associations with differences in the brain, as opposed to the number of social connections people have. It does seem to be the more subjective, emotional perception of loneliness that is important.”
In a review of potential mechanisms involved, she concluded that it is dysregulated emotions and altered perceptions of social interactions that has profound impacts on the brain, suggesting that people who are lonely may have a tendency to interpret social cues in a negative way, preventing them from forming productive positive relationships.
Lack of Trust
One researcher who has studied this phenomenon is Dirk Scheele, PhD, professor of social neuroscience at Ruhr University Bochum in Germany.
“We were interested to find out why people remained lonely,” he said in an interview. “Loneliness is an unpleasant experience, and there are so many opportunities for social contacts nowadays, it’s not really clear at first sight why people are chronically lonely.”
To examine this question, Scheele and his team conducted a study in which functional MRI was used to examine the brain in otherwise healthy individuals with high or low loneliness scores while they played a trust game.
They also simulated a positive social interaction between participants and researchers, in which they talked about plans for a fictitious lottery win, and about their hobbies and interests, during which mood was measured with questionnaires, and saliva samples were collected to measure hormone levels.
Results showed that the high-lonely individuals had reduced activation in the insula cortex during the trust decisions. “This area of the brain is involved in the processing of bodily signals, such as ‘gut feelings.’ So reduced activity here could be interpreted as fewer gut feelings on who can be trusted,” Scheele explained.
The high-lonely individuals also had reduced responsiveness to the positive social interaction with a lower release of oxytocin and a smaller elevation in mood compared with the control individuals.
Scheele pointed out that there is some evidence that oxytocin might increase trust, and there is reduced release of endogenous oxytocin in high loneliness.
“Our results are consistent with the idea that loneliness is associated with negative biases about other people. So if we expect negative things from other people — for instance, that they cannot be trusted — then that would hamper further social interactions and could lead to loneliness,” he added.
A Role for Oxytocin?
In another study, the same researchers tested short-term (five weekly sessions) group psychotherapy to reduce loneliness using established techniques to target these negative biases. They also investigated whether the effects of this group psychotherapy could be augmented by administering intranasal oxytocin (vs placebo) before the group psychotherapy sessions.
Results showed that the group psychotherapy intervention reduced trait loneliness (loneliness experienced over a prolonged period). The oxytocin did not show a significant effect on trait loneliness, but there was a suggestion that it may enhance the reduction in state loneliness (how someone is feeling at a specific time) brought about by the psychotherapy sessions.
“We found that bonding within the groups was experienced as more positive in the oxytocin treated groups. It is possible that a longer intervention would be helpful for longer-term results,” Scheele concluded. “It’s not going to be a quick fix for loneliness, but there may be a role for oxytocin as an adjunct to psychotherapy.”
A Basic Human Need
Another loneliness researcher, Livia Tomova, PhD, assistant professor of psychology at Cardiff University in Wales, has used social isolation to induce loneliness in young people and found that this intervention was linked to brain patterns similar to those associated with hunger.
“We know that the drive to eat food is a very basic human need. We know quite well how it is represented in the brain,” she explained.
The researchers tested how the brains of the participants responded to seeing pictures of social interactions after they underwent a prolonged period of social isolation. In a subsequent session, the same people were asked to undergo food fasting and then underwent brain scans when looking at pictures of food. Results showed that the neural patterns were similar in the two situations with increased activity in the substantia nigra area within the midbrain.
“This area of the brain processes rewards and motivation. It consists primarily of dopamine neurons and increased activity corresponds to a feeling of craving something. So this area of the brain that controls essential homeostatic needs is activated when people feel lonely, suggesting that our need for social contact with others is potentially a very basic need similar to eating,” Tomova said.
Lower Gray Matter Volumes in Key Brain Areas
And another group from Germany has found that higher loneliness scores are negatively associated with specific brain regions responsible for memory, emotion regulation, and social processing.
Sandra Düzel, PhD, and colleagues from the Max Planck Institute for Human Development and the Charité – Universitätsmedizin Berlin, both in Berlin, Germany, reported a study in which individuals who reported higher loneliness had smaller gray matter volumes in brain regions such as the left amygdala, anterior hippocampus, and cerebellum, regions which are crucial for both emotional regulation and higher-order cognitive processes, such as self-reflection and executive function.
Düzel believes that possible mechanisms behind the link between loneliness and brain volume differences could include stress-related damage, with prolonged loneliness associated with elevated levels of stress hormones, which can damage the hippocampus over time, and reduced cognitive and social stimulation, which may contribute to brain volume reductions in regions critical for memory and emotional processing.
“Loneliness is often characterized by reduced social and environmental diversity, leading to less engagement with novel experiences and potentially lower hippocampal-striatal connectivity.
Since novelty-seeking and environmental diversity are associated with positive emotional states, individuals experiencing loneliness might benefit from increased exposure to new environments which could stimulate the brain’s reward circuits, fostering positive affect and potentially mitigating the emotional burden of loneliness,” she said.
Is Social Prescribing the Answer?
So are there enough data now to act and attempt to develop interventions to reduce loneliness? Most of these researchers believe so.
“I think we have enough information to act on this now. There are a number of national academies consensus reports, which suggest that, while certainly there are still gaps in our evidence and more to be learned, there is sufficient evidence that a concerning portion of the population seems to lack connection, and that the consequences are serious enough that we need to do something about it,” said Holt-Lunstad.
Some countries have introduced social prescribing where doctors can prescribe a group activity or a regular visit or telephone conversation with a supportive person.
Subramanian pointed out that it’s easier to implement in countries with national health services and may be more difficult to embrace in the US healthcare system.
“We are not so encouraged from a financial perspective to think about preventive care in the US. We don’t have an easy way to recognize in any tangible way the downstream of such activities in terms of preventing future problems. That is something we need to work on,” she said.
Finley cautioned that to work well, social prescribing will require an understanding of each person’s individual situation.
“Some people may only receive benefit of interacting with others if they are also getting some sort of support to address the social and emotional concerns that are tagging along with loneliness. I’m not sure that just telling people to go join their local gardening club or whatever will be the correct answer for everyone.”
She pointed out that many people will have issues in their life that are making it hard for them to be social. These could be mobility or financial challenges, care responsibilities, or concerns about illnesses or life events. “We need to figure out what would have the most bang for the person’s buck, so to speak, as an intervention. That could mean connecting them to a group relevant to their individual situation.”
Opportunity to Connect Not Enough?
Tomova believes that training people in social skills may be a better option. “It appears that some people who are chronically lonely seem to struggle to make relationships with others. So just encouraging them to interact with others more will not necessarily help. We need to better understand the pathways involved and who are the people who become ill. We can then develop and target better interventions and teach people coping strategies for that situation.”
Scheele agreed. “While just giving people the opportunity to connect may work for some, others who are experiencing really chronic loneliness may not benefit very much from this unless their negative belief systems are addressed.” He suggested some sort of psychotherapy may be helpful in this situation.
But at least all seem to agree that healthcare providers need to be more aware of loneliness as a health risk factor, try to identify people at risk, and to think about how best to support them.
Holt-Lunstad noted that one of the recommendations in the US Surgeon General’s advisory was to increase the education, training, and resources on loneliness for healthcare providers.
“If we want this to be addressed, we need to give healthcare providers the time, resources, and training in order to do that, otherwise, we are adding one more thing to an already overburdened system. They need to understand how important it is, and how it might help them take care of the patient.”
“Our hope is that we can start to reverse some of the trends that we are seeing, both in terms of the prevalence rates of loneliness, but also that we could start seeing improvements in health and other kinds of outcomes,” she concluded.
Progress is being made in increasing awareness about the dangers of chronic loneliness. It’s now recognized as a serious health risk, but there are actionable steps that can help. Loneliness doesn’t have to be a permanent condition for anyone, said Scheele.
Holt-Lunstad served as an adviser for Foundation for Social Connection, Global Initiative on Loneliness and Connection, and Nextdoor Neighborhood Vitality Board and received research grants/income from Templeton Foundation, Eventbrite, Foundation for Social Connection, and Triple-S Foundation. Subramanian served as a speaker bureau for Acorda Pharma. The other researchers reported no disclosures.
A version of this article first appeared on Medscape.com.
Novel Intervention Slows Cognitive Decline in At-Risk Adults
new research suggests.
The cognitive remediation intervention included a series of progressively difficult computer-based and facilitator-monitored mental exercises designed to sharpen cognitive function.
Researchers found that using cognitive remediation with tDCS slowed decline in executive function and verbal memory more than other cognitive functions. The effect was stronger among people with rMDD versus those with MCI and in those at low genetic risk for Alzheimer’s disease.
“We have developed a novel intervention, combining two interventions that if used separately have a weak effect but together have substantial and clinically meaningful effect of slowing the progression of cognitive decline,” said study author Benoit H. Mulsant, MD, chair of the Department of Psychiatry, University of Toronto, Ontario, Canada, and senior scientist at the Center for Addiction and Mental Health, also in Toronto.
The findings were published online in JAMA Psychiatry.
High-Risk Group
Research shows that older adults with MDD or MCI are at high risk for cognitive decline and dementia. Evidence also suggests that depression in early or mid-life significantly increases the risk for dementia in late life, even if the depression has been in remission for decades.
A potential mechanism underlying this increased risk for dementia could be impaired cortical plasticity, or the ability of the brain to compensate for damage.
The PACt-MD trial included 375 older adults with rMDD, MCI, or both (mean age, 72 years; 62% women) at five academic hospitals in Toronto.
Participants received either cognitive remediation plus tDCS or sham intervention 5 days per week for 8 weeks (acute phase), followed by 5-day “boosters” every 6 months.
tDCS was administered by trained personnel and involved active stimulation for 30 minutes at the beginning of each cognitive remediation group session. The intervention targets the prefrontal cortex, a critical region for cognitive compensation in normal cognitive aging.
The sham group received a weakened version of cognitive remediation, with exercises that did not get progressively more difficult. For the sham stimulation, the current flowed at full intensity for only 54 seconds before and after 30-second ramp-up and ramp-down phases, to create a blinding effect, the authors noted.
A geriatric psychiatrist followed all participants throughout the study, conducting assessments at baseline, month 2, and yearly for 3-7 years (mean follow-up, 48.3 months).
Participants’ depressive symptoms were evaluated at baseline and at all follow-ups and underwent neuropsychological testing to assess six cognitive domains: processing speed, working memory, executive functioning, verbal memory, visual memory, and language.
To get a norm for the cognitive tests, researchers recruited a comparator group of 75 subjects similar in age, gender, and years of education, with no neuropsychiatric disorder or cognitive impairment. They completed the same assessments but not the intervention.
Study participants and assessors were blinded to treatment assignment.
Slower Cognitive Decline
Participants in the intervention group had a significantly slower decline in cognitive function, compared with those in the sham group (adjusted z score difference [active – sham] at month 60, 0.21; P = .006). This is equivalent to slowing cognitive decline by about 4 years, researchers reported. The intervention also showed a positive effect on executive function and verbal memory.
“If I can push dementia from 85 to 89 years and you die at 86, in practice, I have prevented you from ever developing dementia,” Mulsant said.
The efficacy of cognitive remediation plus tDCS in rMDD could be tied to enhanced neuroplasticity, said Mulsant.
The treatment worked well in people with a history of depression, regardless of MCI status, but was not as effective for people with just MCI, researchers noted. The intervention also did not work as well among people at genetic risk for Alzheimer’s disease.
“We don’t believe we have discovered an intervention to prevent dementia in people who are at high risk for Alzheimer disease, but we have discovered an intervention that could prevent dementia in people who have an history of depression,” said Mulsant.
These results suggest the pathways to dementia among people with MCI and rMDD are different, he added.
Because previous research showed either treatment alone demonstrated little efficacy, researchers said the new results indicate that there may be a synergistic effect of combining the two.
The ideal amount of treatment and optimal age for initiation still need to be determined, said Mulsant. The study did not include a comparator group without rMDD or MCI, so the observed cognitive benefits might be specific to people with these high-risk conditions. Another study limitation is lack of diversity in terms of ethnicity, race, and education.
Promising, Important Findings
Commenting on the research, Badr Ratnakaran, MD, assistant professor and division director of geriatric psychiatry at Carilion Clinic–Virginia Tech Carilion School of Medicine, Roanoke, said the results are promising and important because there are so few treatment options for the increasing number of older patients with depression and dementia.
The side-effect profile of the combined treatment is better than that of many pharmacologic treatments, Ratnakaran noted. As more research like this comes out, Ratnakaran predicts that cognitive remediation and tCDS will become more readily available.
“This is telling us that the field of psychiatry, and also dementia, is progressing beyond your usual pharmacotherapy treatments,” said Ratnakaran, who also is chair of the American Psychiatric Association’s Council on Geriatric Psychiatry.
The study received support from the Canada Brain Research Fund of Brain Canada, Health Canada, the Chagnon Family, and the Centre for Addiction and Mental Health Discovery Fund. Mulsant reported holding and receiving support from the Labatt Family Chair in Biology of Depression in Late-Life Adults at the University of Toronto; being a member of the Center for Addiction and Mental Health Board of Trustees; research support from Brain Canada, Canadian Institutes of Health Research, Center for Addiction and Mental Health Foundation, Patient-Centered Outcomes Research Institute, and National Institutes of Health; and nonfinancial support from Capital Solution Design and HappyNeuron. Ratnakaran reported no relevant conflicts.
A version of this article appeared on Medscape.com.
new research suggests.
The cognitive remediation intervention included a series of progressively difficult computer-based and facilitator-monitored mental exercises designed to sharpen cognitive function.
Researchers found that using cognitive remediation with tDCS slowed decline in executive function and verbal memory more than other cognitive functions. The effect was stronger among people with rMDD versus those with MCI and in those at low genetic risk for Alzheimer’s disease.
“We have developed a novel intervention, combining two interventions that if used separately have a weak effect but together have substantial and clinically meaningful effect of slowing the progression of cognitive decline,” said study author Benoit H. Mulsant, MD, chair of the Department of Psychiatry, University of Toronto, Ontario, Canada, and senior scientist at the Center for Addiction and Mental Health, also in Toronto.
The findings were published online in JAMA Psychiatry.
High-Risk Group
Research shows that older adults with MDD or MCI are at high risk for cognitive decline and dementia. Evidence also suggests that depression in early or mid-life significantly increases the risk for dementia in late life, even if the depression has been in remission for decades.
A potential mechanism underlying this increased risk for dementia could be impaired cortical plasticity, or the ability of the brain to compensate for damage.
The PACt-MD trial included 375 older adults with rMDD, MCI, or both (mean age, 72 years; 62% women) at five academic hospitals in Toronto.
Participants received either cognitive remediation plus tDCS or sham intervention 5 days per week for 8 weeks (acute phase), followed by 5-day “boosters” every 6 months.
tDCS was administered by trained personnel and involved active stimulation for 30 minutes at the beginning of each cognitive remediation group session. The intervention targets the prefrontal cortex, a critical region for cognitive compensation in normal cognitive aging.
The sham group received a weakened version of cognitive remediation, with exercises that did not get progressively more difficult. For the sham stimulation, the current flowed at full intensity for only 54 seconds before and after 30-second ramp-up and ramp-down phases, to create a blinding effect, the authors noted.
A geriatric psychiatrist followed all participants throughout the study, conducting assessments at baseline, month 2, and yearly for 3-7 years (mean follow-up, 48.3 months).
Participants’ depressive symptoms were evaluated at baseline and at all follow-ups and underwent neuropsychological testing to assess six cognitive domains: processing speed, working memory, executive functioning, verbal memory, visual memory, and language.
To get a norm for the cognitive tests, researchers recruited a comparator group of 75 subjects similar in age, gender, and years of education, with no neuropsychiatric disorder or cognitive impairment. They completed the same assessments but not the intervention.
Study participants and assessors were blinded to treatment assignment.
Slower Cognitive Decline
Participants in the intervention group had a significantly slower decline in cognitive function, compared with those in the sham group (adjusted z score difference [active – sham] at month 60, 0.21; P = .006). This is equivalent to slowing cognitive decline by about 4 years, researchers reported. The intervention also showed a positive effect on executive function and verbal memory.
“If I can push dementia from 85 to 89 years and you die at 86, in practice, I have prevented you from ever developing dementia,” Mulsant said.
The efficacy of cognitive remediation plus tDCS in rMDD could be tied to enhanced neuroplasticity, said Mulsant.
The treatment worked well in people with a history of depression, regardless of MCI status, but was not as effective for people with just MCI, researchers noted. The intervention also did not work as well among people at genetic risk for Alzheimer’s disease.
“We don’t believe we have discovered an intervention to prevent dementia in people who are at high risk for Alzheimer disease, but we have discovered an intervention that could prevent dementia in people who have an history of depression,” said Mulsant.
These results suggest the pathways to dementia among people with MCI and rMDD are different, he added.
Because previous research showed either treatment alone demonstrated little efficacy, researchers said the new results indicate that there may be a synergistic effect of combining the two.
The ideal amount of treatment and optimal age for initiation still need to be determined, said Mulsant. The study did not include a comparator group without rMDD or MCI, so the observed cognitive benefits might be specific to people with these high-risk conditions. Another study limitation is lack of diversity in terms of ethnicity, race, and education.
Promising, Important Findings
Commenting on the research, Badr Ratnakaran, MD, assistant professor and division director of geriatric psychiatry at Carilion Clinic–Virginia Tech Carilion School of Medicine, Roanoke, said the results are promising and important because there are so few treatment options for the increasing number of older patients with depression and dementia.
The side-effect profile of the combined treatment is better than that of many pharmacologic treatments, Ratnakaran noted. As more research like this comes out, Ratnakaran predicts that cognitive remediation and tCDS will become more readily available.
“This is telling us that the field of psychiatry, and also dementia, is progressing beyond your usual pharmacotherapy treatments,” said Ratnakaran, who also is chair of the American Psychiatric Association’s Council on Geriatric Psychiatry.
The study received support from the Canada Brain Research Fund of Brain Canada, Health Canada, the Chagnon Family, and the Centre for Addiction and Mental Health Discovery Fund. Mulsant reported holding and receiving support from the Labatt Family Chair in Biology of Depression in Late-Life Adults at the University of Toronto; being a member of the Center for Addiction and Mental Health Board of Trustees; research support from Brain Canada, Canadian Institutes of Health Research, Center for Addiction and Mental Health Foundation, Patient-Centered Outcomes Research Institute, and National Institutes of Health; and nonfinancial support from Capital Solution Design and HappyNeuron. Ratnakaran reported no relevant conflicts.
A version of this article appeared on Medscape.com.
new research suggests.
The cognitive remediation intervention included a series of progressively difficult computer-based and facilitator-monitored mental exercises designed to sharpen cognitive function.
Researchers found that using cognitive remediation with tDCS slowed decline in executive function and verbal memory more than other cognitive functions. The effect was stronger among people with rMDD versus those with MCI and in those at low genetic risk for Alzheimer’s disease.
“We have developed a novel intervention, combining two interventions that if used separately have a weak effect but together have substantial and clinically meaningful effect of slowing the progression of cognitive decline,” said study author Benoit H. Mulsant, MD, chair of the Department of Psychiatry, University of Toronto, Ontario, Canada, and senior scientist at the Center for Addiction and Mental Health, also in Toronto.
The findings were published online in JAMA Psychiatry.
High-Risk Group
Research shows that older adults with MDD or MCI are at high risk for cognitive decline and dementia. Evidence also suggests that depression in early or mid-life significantly increases the risk for dementia in late life, even if the depression has been in remission for decades.
A potential mechanism underlying this increased risk for dementia could be impaired cortical plasticity, or the ability of the brain to compensate for damage.
The PACt-MD trial included 375 older adults with rMDD, MCI, or both (mean age, 72 years; 62% women) at five academic hospitals in Toronto.
Participants received either cognitive remediation plus tDCS or sham intervention 5 days per week for 8 weeks (acute phase), followed by 5-day “boosters” every 6 months.
tDCS was administered by trained personnel and involved active stimulation for 30 minutes at the beginning of each cognitive remediation group session. The intervention targets the prefrontal cortex, a critical region for cognitive compensation in normal cognitive aging.
The sham group received a weakened version of cognitive remediation, with exercises that did not get progressively more difficult. For the sham stimulation, the current flowed at full intensity for only 54 seconds before and after 30-second ramp-up and ramp-down phases, to create a blinding effect, the authors noted.
A geriatric psychiatrist followed all participants throughout the study, conducting assessments at baseline, month 2, and yearly for 3-7 years (mean follow-up, 48.3 months).
Participants’ depressive symptoms were evaluated at baseline and at all follow-ups and underwent neuropsychological testing to assess six cognitive domains: processing speed, working memory, executive functioning, verbal memory, visual memory, and language.
To get a norm for the cognitive tests, researchers recruited a comparator group of 75 subjects similar in age, gender, and years of education, with no neuropsychiatric disorder or cognitive impairment. They completed the same assessments but not the intervention.
Study participants and assessors were blinded to treatment assignment.
Slower Cognitive Decline
Participants in the intervention group had a significantly slower decline in cognitive function, compared with those in the sham group (adjusted z score difference [active – sham] at month 60, 0.21; P = .006). This is equivalent to slowing cognitive decline by about 4 years, researchers reported. The intervention also showed a positive effect on executive function and verbal memory.
“If I can push dementia from 85 to 89 years and you die at 86, in practice, I have prevented you from ever developing dementia,” Mulsant said.
The efficacy of cognitive remediation plus tDCS in rMDD could be tied to enhanced neuroplasticity, said Mulsant.
The treatment worked well in people with a history of depression, regardless of MCI status, but was not as effective for people with just MCI, researchers noted. The intervention also did not work as well among people at genetic risk for Alzheimer’s disease.
“We don’t believe we have discovered an intervention to prevent dementia in people who are at high risk for Alzheimer disease, but we have discovered an intervention that could prevent dementia in people who have an history of depression,” said Mulsant.
These results suggest the pathways to dementia among people with MCI and rMDD are different, he added.
Because previous research showed either treatment alone demonstrated little efficacy, researchers said the new results indicate that there may be a synergistic effect of combining the two.
The ideal amount of treatment and optimal age for initiation still need to be determined, said Mulsant. The study did not include a comparator group without rMDD or MCI, so the observed cognitive benefits might be specific to people with these high-risk conditions. Another study limitation is lack of diversity in terms of ethnicity, race, and education.
Promising, Important Findings
Commenting on the research, Badr Ratnakaran, MD, assistant professor and division director of geriatric psychiatry at Carilion Clinic–Virginia Tech Carilion School of Medicine, Roanoke, said the results are promising and important because there are so few treatment options for the increasing number of older patients with depression and dementia.
The side-effect profile of the combined treatment is better than that of many pharmacologic treatments, Ratnakaran noted. As more research like this comes out, Ratnakaran predicts that cognitive remediation and tCDS will become more readily available.
“This is telling us that the field of psychiatry, and also dementia, is progressing beyond your usual pharmacotherapy treatments,” said Ratnakaran, who also is chair of the American Psychiatric Association’s Council on Geriatric Psychiatry.
The study received support from the Canada Brain Research Fund of Brain Canada, Health Canada, the Chagnon Family, and the Centre for Addiction and Mental Health Discovery Fund. Mulsant reported holding and receiving support from the Labatt Family Chair in Biology of Depression in Late-Life Adults at the University of Toronto; being a member of the Center for Addiction and Mental Health Board of Trustees; research support from Brain Canada, Canadian Institutes of Health Research, Center for Addiction and Mental Health Foundation, Patient-Centered Outcomes Research Institute, and National Institutes of Health; and nonfinancial support from Capital Solution Design and HappyNeuron. Ratnakaran reported no relevant conflicts.
A version of this article appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Will Psychedelics Break the Major Depression Logjam?
With tens of millions of Euros in the offing, researchers across the European Union have eagerly taken up the gauntlet to find novel interventions for difficult-to-treat mental health and pain conditions. Their target is psychedelics, including classic compounds like psilocybin and atypical ones like ketamine and MDMA. Some of these still carry a stigma as party drugs and spiritual gateways to holotropic experiences.
Twelve groups make up the European College of Neuropsychopharmacology’s psychedelic research network. Along with several affiliates, the groups span nine countries (Denmark, Sweden, Finland, Switzerland, Germany, the Netherlands, the Czech Republic, Greece, and the United Kingdom) and are focused on the use of psychedelic compounds as potential treatments for psychiatric conditions like treatment-resistant depression, addiction, attention-deficit/hyperactivity disorder, anorexia, and obsessive-compulsive disorder.
One of the largest endeavors is PsyPal, a 4-year, randomized controlled trial investigating the potential of psilocybin for treating psychological distress in palliative care patients with life-limiting conditions. PsyPal is the first multisite clinical trial funded by the European Union to explore psychedelic-assisted therapy.
At PsyPal’s helm is Robert Schoevers, MD, PhD, professor of psychiatry and Department Head at University Medical Center Groningen (UMCG), Groningen, the Netherlands, a major hub for psychedelic research.
Schoevers is a bit of a pioneer who said he entered psychedelic research somewhat reluctantly. A decade ago, a colleague showed him a few papers on ketamine and depression. Because UMCG has a large population of patients with treatment-resistant depression, he agreed to do a pilot study. Since then, he has put together an interdisciplinary team of 25 researchers, published numerous papers, and currently has seven studies, including PsyPal, in various stages of progress.
Schoevers is also building a large national consortium that aims to investigate and, if shown effective, implement novel psychiatry treatments much more rapidly and efficiently than current drug development and approval processes, which can take 12 years or longer. He has just secured millions in government funding to start this process.
Next year, Schoevers and his team will decide to test either MDMA for posttraumatic stress disorder (PTSD) or psilocybin for depression in a large clinical trial, with the aim of getting a treatment as near to formal registration as possible. This will involve working with the European Medicines Agency and its Dutch counterpart, talking to experts who are familiar with the US Food and Drug Administration’s rejection of Lykos Therapeutics’ MDMA treatment for PTSD, and working directly with patients through the National Patient Alliance. The team is also talking to insurers and pharma companies.
The ultimate goal is to “see if we can build a platform in the Netherlands that would have a European perspective, serve as a point of entry for researchers with good ideas, and [attract] public funding as well as companies who have interesting compounds we think would be worthwhile to study,” Schoevers said.
His multiple endeavors emphasize a transdisciplinary and transdiagnostic approach he has been honing for decades. He and his colleagues are investigating the clinical, psychological, and neurobiological parallels between different treatment- resistant conditions and seeking to understand how contextual factors might influence patients, experiences, and outcomes.
Connecting the Dots
Jens H. van Dalfsen, PhD, a postdoctoral researcher in biological psychiatry, is the principal investigator of another UMCG group looking into the neurobiological mechanisms of major depressive disorder and treatment-resistant depression. His team’s strategy entails an elaborate coordination between the preclinical and clinical research settings.
For example, Sarah Massetti, a PhD candidate in biological psychiatry, is using blood samples collected in clinical trials to investigate the molecular mechanisms underpinning the neuroplasticity and immune-modulating effects produced by psychedelic compounds.
Another line of research spearheaded by Rutger Boesjes, a PhD candidate in biological psychiatry, is exploring the interactions between drugs like ketamine and the circadian system and how they might relate to antidepressant responses in animal models. It could be that the timing of administration of these drugs is relevant, he explained.
The Patient Factor
How psychedelics work and in whom is a big question for the UMCG team and across the research landscape.
“When researchers and the general public talk about psychedelics, they frequently refer to how they promote synaptic plasticity and new connections in the brain,” said van Dalfsen. “But traditional compounds also do that. So the ultimate question that we’ve been exploring is whether findings reflect an actual pharmacological effect or if expectancy also plays a role. In other words: How can we explain why psychedelics might or might not be effective in treatment-resistant patients?” he explained.
This is where the connection to the clinical experience becomes paramount and Joost Breeksema, PhD, comes in.
Breeksema divides his time between UMCG research and his role as executive director and co-founder of the Open Foundation, a nonprofit dedicated to advancing scientific psychedelic research. The work he’s doing outside the university is helping to frame the investigations of the wider group.
So far he has conducted two qualitative studies.
One was an off-label study in which patients with treatment-resistant depression were administered esketamine.
The other was a randomized clinical trial in which participants were blinded to a single 10- or 25-mg dose of psilocybin versus a 1-mg psilocybin microdose placebo that is too small to invoke any effects.
A key insight was the degree to which participants were unprepared for the intensity of their experiences, especially with regards to ketamine. Breeksema said the sessions might not have been so intense or negative for some participants had they been informed beforehand to expect the drugs could provoke “quite overwhelming experiences” and had they been accompanied by an experienced guide providing reassurance and support.
The format for the psilocybin trial met part of this criteria. Participants received a micro (placebo), medium, or high dose in a single session accompanied by two trained therapists. They then engaged in two sessions afterward to process their experiences. A single psilocybin experience appeared to be not enough or too much depending on the dose they were assigned and if they had prior experience with the compound.
Trial participants also felt they needed more help making sense of the experience. “This is a common and important theme,” said Breeksema. “Think about it. If you’ve been depressed for 10, 15 years and … you uncover something and break through something that’s been stuck, you need to process it.”
Jeanine Kamphuis, PhD, a psychiatrist and senior researcher at UMCG and one of the trial study co-authors, explained that they want to find a way to identify who will be too overwhelmed by these experiences if the dose should be adjusted or if some time needs to pass between dosing sessions. They also want to spend more time preparing patients for these sessions.
She emphasized that the studies have provided a reality check. “These are not wonder pills or wonder experiences. And in these types of patients, they’re not intended for a personal growth experience,” she said. “You have a patient who is sitting in front of you who seeks therapy and relief from very severe mood complaints, and the suffering is high,” she said, adding that expectancy bias further complicates patient participation and, likely, outcomes.
The Challenges
For all the potential and opportunity that psychedelics may hold for treatment, UMCG’s work has underscored some challenges.
The field of psychedelic research is characterized by methodological issues, explained van Dalfsen, such as blinding, expectancy, and overestimation of treatment effects. When looking at efficacy, “Is it the compound or the expectancy and promise? This is why it’s important to study how the drugs differ from each other in their biological effects and why they are or are not effective,” he said.
The team has also experienced issues with trial recruitment.
Martijn Godschalk, MD, a PhD candidate in psychiatry, has been addressing this problem while working on RESET-TRD, a phase 3, randomized controlled trial comparing an oral esketamine drink with electroconvulsive therapy in patients with treatment-resistant depression.
He’s been coordinating with local university hospitals, general hospitals, and municipal healthcare clinics to meet inclusion criteria and ensure the trial has enough power to demonstrate effectiveness. In turn, these sites are able to participate in a trial they wouldn’t normally be involved in due to lack of resources.
But Godschalk said he was concerned that many patients have gotten wind of the hype surrounding psychedelic treatments within psychiatry — a factor that has contributed to recruitment challenges. “There are a lot of patients who are interested in the non-registered drug and don’t necessarily have an interest in the other [control] arm,” he said.
Despite the challenges, the classic psychedelics such as MDMA and psilocybin “seem to catalyze a psychological process that may be harder to get with regular psychotherapies,” said Schoevers.
He remains cautious, noting there are still unanswered questions, such as who are the best candidates for these drugs and whether they might cause harm in certain patients while benefiting others. “I do think that this is the first time in 20 or 30 years that there is a group of potential treatments that would really make a difference.”
Schoevers received grants and other funding from The Netherlands Organisation for Health Research & Development, Horizon 2020, Horizon 2023, the National Institute of Mental Health (USA), UMCG, Stichting tot Steun VCVGZ, Nationaal programma Groningen, Healthcare Innovation Funds, Janssen Pharmaceuticals, Novartis, Compass Pathways, Clexio Biosciences, and GH research.
A version of this article appeared on Medscape.com.
With tens of millions of Euros in the offing, researchers across the European Union have eagerly taken up the gauntlet to find novel interventions for difficult-to-treat mental health and pain conditions. Their target is psychedelics, including classic compounds like psilocybin and atypical ones like ketamine and MDMA. Some of these still carry a stigma as party drugs and spiritual gateways to holotropic experiences.
Twelve groups make up the European College of Neuropsychopharmacology’s psychedelic research network. Along with several affiliates, the groups span nine countries (Denmark, Sweden, Finland, Switzerland, Germany, the Netherlands, the Czech Republic, Greece, and the United Kingdom) and are focused on the use of psychedelic compounds as potential treatments for psychiatric conditions like treatment-resistant depression, addiction, attention-deficit/hyperactivity disorder, anorexia, and obsessive-compulsive disorder.
One of the largest endeavors is PsyPal, a 4-year, randomized controlled trial investigating the potential of psilocybin for treating psychological distress in palliative care patients with life-limiting conditions. PsyPal is the first multisite clinical trial funded by the European Union to explore psychedelic-assisted therapy.
At PsyPal’s helm is Robert Schoevers, MD, PhD, professor of psychiatry and Department Head at University Medical Center Groningen (UMCG), Groningen, the Netherlands, a major hub for psychedelic research.
Schoevers is a bit of a pioneer who said he entered psychedelic research somewhat reluctantly. A decade ago, a colleague showed him a few papers on ketamine and depression. Because UMCG has a large population of patients with treatment-resistant depression, he agreed to do a pilot study. Since then, he has put together an interdisciplinary team of 25 researchers, published numerous papers, and currently has seven studies, including PsyPal, in various stages of progress.
Schoevers is also building a large national consortium that aims to investigate and, if shown effective, implement novel psychiatry treatments much more rapidly and efficiently than current drug development and approval processes, which can take 12 years or longer. He has just secured millions in government funding to start this process.
Next year, Schoevers and his team will decide to test either MDMA for posttraumatic stress disorder (PTSD) or psilocybin for depression in a large clinical trial, with the aim of getting a treatment as near to formal registration as possible. This will involve working with the European Medicines Agency and its Dutch counterpart, talking to experts who are familiar with the US Food and Drug Administration’s rejection of Lykos Therapeutics’ MDMA treatment for PTSD, and working directly with patients through the National Patient Alliance. The team is also talking to insurers and pharma companies.
The ultimate goal is to “see if we can build a platform in the Netherlands that would have a European perspective, serve as a point of entry for researchers with good ideas, and [attract] public funding as well as companies who have interesting compounds we think would be worthwhile to study,” Schoevers said.
His multiple endeavors emphasize a transdisciplinary and transdiagnostic approach he has been honing for decades. He and his colleagues are investigating the clinical, psychological, and neurobiological parallels between different treatment- resistant conditions and seeking to understand how contextual factors might influence patients, experiences, and outcomes.
Connecting the Dots
Jens H. van Dalfsen, PhD, a postdoctoral researcher in biological psychiatry, is the principal investigator of another UMCG group looking into the neurobiological mechanisms of major depressive disorder and treatment-resistant depression. His team’s strategy entails an elaborate coordination between the preclinical and clinical research settings.
For example, Sarah Massetti, a PhD candidate in biological psychiatry, is using blood samples collected in clinical trials to investigate the molecular mechanisms underpinning the neuroplasticity and immune-modulating effects produced by psychedelic compounds.
Another line of research spearheaded by Rutger Boesjes, a PhD candidate in biological psychiatry, is exploring the interactions between drugs like ketamine and the circadian system and how they might relate to antidepressant responses in animal models. It could be that the timing of administration of these drugs is relevant, he explained.
The Patient Factor
How psychedelics work and in whom is a big question for the UMCG team and across the research landscape.
“When researchers and the general public talk about psychedelics, they frequently refer to how they promote synaptic plasticity and new connections in the brain,” said van Dalfsen. “But traditional compounds also do that. So the ultimate question that we’ve been exploring is whether findings reflect an actual pharmacological effect or if expectancy also plays a role. In other words: How can we explain why psychedelics might or might not be effective in treatment-resistant patients?” he explained.
This is where the connection to the clinical experience becomes paramount and Joost Breeksema, PhD, comes in.
Breeksema divides his time between UMCG research and his role as executive director and co-founder of the Open Foundation, a nonprofit dedicated to advancing scientific psychedelic research. The work he’s doing outside the university is helping to frame the investigations of the wider group.
So far he has conducted two qualitative studies.
One was an off-label study in which patients with treatment-resistant depression were administered esketamine.
The other was a randomized clinical trial in which participants were blinded to a single 10- or 25-mg dose of psilocybin versus a 1-mg psilocybin microdose placebo that is too small to invoke any effects.
A key insight was the degree to which participants were unprepared for the intensity of their experiences, especially with regards to ketamine. Breeksema said the sessions might not have been so intense or negative for some participants had they been informed beforehand to expect the drugs could provoke “quite overwhelming experiences” and had they been accompanied by an experienced guide providing reassurance and support.
The format for the psilocybin trial met part of this criteria. Participants received a micro (placebo), medium, or high dose in a single session accompanied by two trained therapists. They then engaged in two sessions afterward to process their experiences. A single psilocybin experience appeared to be not enough or too much depending on the dose they were assigned and if they had prior experience with the compound.
Trial participants also felt they needed more help making sense of the experience. “This is a common and important theme,” said Breeksema. “Think about it. If you’ve been depressed for 10, 15 years and … you uncover something and break through something that’s been stuck, you need to process it.”
Jeanine Kamphuis, PhD, a psychiatrist and senior researcher at UMCG and one of the trial study co-authors, explained that they want to find a way to identify who will be too overwhelmed by these experiences if the dose should be adjusted or if some time needs to pass between dosing sessions. They also want to spend more time preparing patients for these sessions.
She emphasized that the studies have provided a reality check. “These are not wonder pills or wonder experiences. And in these types of patients, they’re not intended for a personal growth experience,” she said. “You have a patient who is sitting in front of you who seeks therapy and relief from very severe mood complaints, and the suffering is high,” she said, adding that expectancy bias further complicates patient participation and, likely, outcomes.
The Challenges
For all the potential and opportunity that psychedelics may hold for treatment, UMCG’s work has underscored some challenges.
The field of psychedelic research is characterized by methodological issues, explained van Dalfsen, such as blinding, expectancy, and overestimation of treatment effects. When looking at efficacy, “Is it the compound or the expectancy and promise? This is why it’s important to study how the drugs differ from each other in their biological effects and why they are or are not effective,” he said.
The team has also experienced issues with trial recruitment.
Martijn Godschalk, MD, a PhD candidate in psychiatry, has been addressing this problem while working on RESET-TRD, a phase 3, randomized controlled trial comparing an oral esketamine drink with electroconvulsive therapy in patients with treatment-resistant depression.
He’s been coordinating with local university hospitals, general hospitals, and municipal healthcare clinics to meet inclusion criteria and ensure the trial has enough power to demonstrate effectiveness. In turn, these sites are able to participate in a trial they wouldn’t normally be involved in due to lack of resources.
But Godschalk said he was concerned that many patients have gotten wind of the hype surrounding psychedelic treatments within psychiatry — a factor that has contributed to recruitment challenges. “There are a lot of patients who are interested in the non-registered drug and don’t necessarily have an interest in the other [control] arm,” he said.
Despite the challenges, the classic psychedelics such as MDMA and psilocybin “seem to catalyze a psychological process that may be harder to get with regular psychotherapies,” said Schoevers.
He remains cautious, noting there are still unanswered questions, such as who are the best candidates for these drugs and whether they might cause harm in certain patients while benefiting others. “I do think that this is the first time in 20 or 30 years that there is a group of potential treatments that would really make a difference.”
Schoevers received grants and other funding from The Netherlands Organisation for Health Research & Development, Horizon 2020, Horizon 2023, the National Institute of Mental Health (USA), UMCG, Stichting tot Steun VCVGZ, Nationaal programma Groningen, Healthcare Innovation Funds, Janssen Pharmaceuticals, Novartis, Compass Pathways, Clexio Biosciences, and GH research.
A version of this article appeared on Medscape.com.
With tens of millions of Euros in the offing, researchers across the European Union have eagerly taken up the gauntlet to find novel interventions for difficult-to-treat mental health and pain conditions. Their target is psychedelics, including classic compounds like psilocybin and atypical ones like ketamine and MDMA. Some of these still carry a stigma as party drugs and spiritual gateways to holotropic experiences.
Twelve groups make up the European College of Neuropsychopharmacology’s psychedelic research network. Along with several affiliates, the groups span nine countries (Denmark, Sweden, Finland, Switzerland, Germany, the Netherlands, the Czech Republic, Greece, and the United Kingdom) and are focused on the use of psychedelic compounds as potential treatments for psychiatric conditions like treatment-resistant depression, addiction, attention-deficit/hyperactivity disorder, anorexia, and obsessive-compulsive disorder.
One of the largest endeavors is PsyPal, a 4-year, randomized controlled trial investigating the potential of psilocybin for treating psychological distress in palliative care patients with life-limiting conditions. PsyPal is the first multisite clinical trial funded by the European Union to explore psychedelic-assisted therapy.
At PsyPal’s helm is Robert Schoevers, MD, PhD, professor of psychiatry and Department Head at University Medical Center Groningen (UMCG), Groningen, the Netherlands, a major hub for psychedelic research.
Schoevers is a bit of a pioneer who said he entered psychedelic research somewhat reluctantly. A decade ago, a colleague showed him a few papers on ketamine and depression. Because UMCG has a large population of patients with treatment-resistant depression, he agreed to do a pilot study. Since then, he has put together an interdisciplinary team of 25 researchers, published numerous papers, and currently has seven studies, including PsyPal, in various stages of progress.
Schoevers is also building a large national consortium that aims to investigate and, if shown effective, implement novel psychiatry treatments much more rapidly and efficiently than current drug development and approval processes, which can take 12 years or longer. He has just secured millions in government funding to start this process.
Next year, Schoevers and his team will decide to test either MDMA for posttraumatic stress disorder (PTSD) or psilocybin for depression in a large clinical trial, with the aim of getting a treatment as near to formal registration as possible. This will involve working with the European Medicines Agency and its Dutch counterpart, talking to experts who are familiar with the US Food and Drug Administration’s rejection of Lykos Therapeutics’ MDMA treatment for PTSD, and working directly with patients through the National Patient Alliance. The team is also talking to insurers and pharma companies.
The ultimate goal is to “see if we can build a platform in the Netherlands that would have a European perspective, serve as a point of entry for researchers with good ideas, and [attract] public funding as well as companies who have interesting compounds we think would be worthwhile to study,” Schoevers said.
His multiple endeavors emphasize a transdisciplinary and transdiagnostic approach he has been honing for decades. He and his colleagues are investigating the clinical, psychological, and neurobiological parallels between different treatment- resistant conditions and seeking to understand how contextual factors might influence patients, experiences, and outcomes.
Connecting the Dots
Jens H. van Dalfsen, PhD, a postdoctoral researcher in biological psychiatry, is the principal investigator of another UMCG group looking into the neurobiological mechanisms of major depressive disorder and treatment-resistant depression. His team’s strategy entails an elaborate coordination between the preclinical and clinical research settings.
For example, Sarah Massetti, a PhD candidate in biological psychiatry, is using blood samples collected in clinical trials to investigate the molecular mechanisms underpinning the neuroplasticity and immune-modulating effects produced by psychedelic compounds.
Another line of research spearheaded by Rutger Boesjes, a PhD candidate in biological psychiatry, is exploring the interactions between drugs like ketamine and the circadian system and how they might relate to antidepressant responses in animal models. It could be that the timing of administration of these drugs is relevant, he explained.
The Patient Factor
How psychedelics work and in whom is a big question for the UMCG team and across the research landscape.
“When researchers and the general public talk about psychedelics, they frequently refer to how they promote synaptic plasticity and new connections in the brain,” said van Dalfsen. “But traditional compounds also do that. So the ultimate question that we’ve been exploring is whether findings reflect an actual pharmacological effect or if expectancy also plays a role. In other words: How can we explain why psychedelics might or might not be effective in treatment-resistant patients?” he explained.
This is where the connection to the clinical experience becomes paramount and Joost Breeksema, PhD, comes in.
Breeksema divides his time between UMCG research and his role as executive director and co-founder of the Open Foundation, a nonprofit dedicated to advancing scientific psychedelic research. The work he’s doing outside the university is helping to frame the investigations of the wider group.
So far he has conducted two qualitative studies.
One was an off-label study in which patients with treatment-resistant depression were administered esketamine.
The other was a randomized clinical trial in which participants were blinded to a single 10- or 25-mg dose of psilocybin versus a 1-mg psilocybin microdose placebo that is too small to invoke any effects.
A key insight was the degree to which participants were unprepared for the intensity of their experiences, especially with regards to ketamine. Breeksema said the sessions might not have been so intense or negative for some participants had they been informed beforehand to expect the drugs could provoke “quite overwhelming experiences” and had they been accompanied by an experienced guide providing reassurance and support.
The format for the psilocybin trial met part of this criteria. Participants received a micro (placebo), medium, or high dose in a single session accompanied by two trained therapists. They then engaged in two sessions afterward to process their experiences. A single psilocybin experience appeared to be not enough or too much depending on the dose they were assigned and if they had prior experience with the compound.
Trial participants also felt they needed more help making sense of the experience. “This is a common and important theme,” said Breeksema. “Think about it. If you’ve been depressed for 10, 15 years and … you uncover something and break through something that’s been stuck, you need to process it.”
Jeanine Kamphuis, PhD, a psychiatrist and senior researcher at UMCG and one of the trial study co-authors, explained that they want to find a way to identify who will be too overwhelmed by these experiences if the dose should be adjusted or if some time needs to pass between dosing sessions. They also want to spend more time preparing patients for these sessions.
She emphasized that the studies have provided a reality check. “These are not wonder pills or wonder experiences. And in these types of patients, they’re not intended for a personal growth experience,” she said. “You have a patient who is sitting in front of you who seeks therapy and relief from very severe mood complaints, and the suffering is high,” she said, adding that expectancy bias further complicates patient participation and, likely, outcomes.
The Challenges
For all the potential and opportunity that psychedelics may hold for treatment, UMCG’s work has underscored some challenges.
The field of psychedelic research is characterized by methodological issues, explained van Dalfsen, such as blinding, expectancy, and overestimation of treatment effects. When looking at efficacy, “Is it the compound or the expectancy and promise? This is why it’s important to study how the drugs differ from each other in their biological effects and why they are or are not effective,” he said.
The team has also experienced issues with trial recruitment.
Martijn Godschalk, MD, a PhD candidate in psychiatry, has been addressing this problem while working on RESET-TRD, a phase 3, randomized controlled trial comparing an oral esketamine drink with electroconvulsive therapy in patients with treatment-resistant depression.
He’s been coordinating with local university hospitals, general hospitals, and municipal healthcare clinics to meet inclusion criteria and ensure the trial has enough power to demonstrate effectiveness. In turn, these sites are able to participate in a trial they wouldn’t normally be involved in due to lack of resources.
But Godschalk said he was concerned that many patients have gotten wind of the hype surrounding psychedelic treatments within psychiatry — a factor that has contributed to recruitment challenges. “There are a lot of patients who are interested in the non-registered drug and don’t necessarily have an interest in the other [control] arm,” he said.
Despite the challenges, the classic psychedelics such as MDMA and psilocybin “seem to catalyze a psychological process that may be harder to get with regular psychotherapies,” said Schoevers.
He remains cautious, noting there are still unanswered questions, such as who are the best candidates for these drugs and whether they might cause harm in certain patients while benefiting others. “I do think that this is the first time in 20 or 30 years that there is a group of potential treatments that would really make a difference.”
Schoevers received grants and other funding from The Netherlands Organisation for Health Research & Development, Horizon 2020, Horizon 2023, the National Institute of Mental Health (USA), UMCG, Stichting tot Steun VCVGZ, Nationaal programma Groningen, Healthcare Innovation Funds, Janssen Pharmaceuticals, Novartis, Compass Pathways, Clexio Biosciences, and GH research.
A version of this article appeared on Medscape.com.