User login
Folic acid tied to a reduction in suicide attempts
new research suggests.
After adjusting for multiple factors, results from a large pharmaco-epidemiological study showed taking folic acid was associated with a 44% reduction in suicide events.
“These results are really putting folic acid squarely on the map as a potential for large-scale, population-level prevention,” lead author Robert D. Gibbons, PhD, professor of biostatistics, Center for Health Statistics, University of Chicago, said in an interview.
“Folic acid is safe, inexpensive, and generally available, and if future randomized controlled trials show this association is beyond a shadow of a doubt causal, we have a new tool in the arsenal,” Dr. Gibbons said.
Having such a tool would be extremely important given that suicide is such a significant public health crisis worldwide, he added.
The findings were published online in JAMA Psychiatry.
Previous research ‘fairly thin’
Folate, the naturally occurring form of B9, is essential for neurogenesis, nucleotide synthesis, and methylation of homocysteine. Past research has suggested that taking folate can prevent neural tube and heart defects in the fetus during pregnancy – and may prevent strokes and reduce age-related hearing loss in adults.
In psychiatry, the role of folate has been recognized for more than a decade. It may enhance the effects of antidepressants; and folate deficiency can predict poorer response to SSRIs.
This has led to recommendations for folate augmentation in patients with low or normal levels at the start of depression treatment.
Although previous research has shown a link between folic acid and suicidality, the findings have been “fairly thin,” with studies being “generally small, and many are case series,” Dr. Gibbons said.
The current study follows an earlier analysis that used a novel statistical methodology for generating drug safety signals that was developed by Dr. Gibbons and colleagues. That study compared rates of suicide attempts before and after initiation of 922 drugs with at least 3,000 prescriptions.
Its results showed 10 drugs were associated with increased risk after exposure, with the strongest associations for alprazolam, butalbital, hydrocodone, and combination codeine/promethazine. In addition, 44 drugs were associated with decreased risk, many of which were antidepressants and antipsychotics.
“One of the most interesting findings in terms of the decreased risk was for folic acid,” said Dr. Gibbons.
He and his colleagues initially thought this was because of women taking folic acid during pregnancy. But when restricting the analysis to men, they found the same effect.
Their next step was to carry out the current large-scale pharmaco-epidemiological study.
Prescriptions for pain
The researchers used a health claims database that included 164 million enrollees. The study cohort was comprised of 866,586 adults with private health insurance (81.3% women; 10.4% aged 60 years and older) who filled a folic acid prescription between 2012 and 2017.
More than half of the folic acid prescriptions were associated with pain disorders. About 48% were for a single agent at a dosage of 1 mg/d, which is the upper tolerable limit for adults – including in pregnancy and lactation.
Other single-agent daily dosages ranging from 0.4 mg to 5 mg accounted for 0.11% of prescriptions. The remainder were multivitamins.
The participants were followed for 24 months. The within-person analysis compared suicide attempts or self-harm events resulting in an outpatient visit or inpatient admission during periods of folic acid treatment versus during periods without treatment.
During the study period, the overall suicidal event rate was 133 per 100,000 population, which is one-fourth the national rate reported by the National Institutes of Health of 600 per 100,000.
After adjusting for age, sex, diagnoses related to suicidal behavior and folic acid deficiency, history of folate-reducing medications, and history of suicidal events, the estimated hazard ratio for suicide events when taking folic acid was 0.56 (95% confidence interal, 0.48-0.65) – which indicates a 44% reduction in suicide events.
“This is a very large decrease and is extremely significant and exciting,” Dr. Gibbons said.
He noted the decrease in suicidal events may have been even greater considering the study captured only prescription folic acid, and participants may also have also taken over-the-counter products.
“The 44% reduction in suicide attempts may actually be an underestimate,” said Dr. Gibbons.
Age and sex did not moderate the association between folic acid and suicide attempts, and a similar association was found in women of childbearing age.
Provocative results?
The investigators also assessed a negative control group of 236,610 individuals using cyanocobalamin during the study period. Cyanocobalamin is a form of vitamin B12 that is essential for metabolism, blood cell synthesis, and the nervous system. It does not contain folic acid and is commonly used to treat anemia.
Results showed no association between cyanocobalamin and suicidal events in the adjusted analysis (HR, 1.01; 95% CI, 0.80-1.27) or unadjusted analysis (HR, 1.02; 95% CI, 0.80-1.28).
Dr. Gibbons noted this result boosts the argument that the association between folic acid and reduced suicidal attempts “isn’t just about health-seeking behavior like taking vitamin supplements.”
Another sensitivity analysis showed every additional month of treatment was associated with a 5% reduction in the suicidal event rate.
“This means the longer you take folic acid, the greater the benefit, which is what you would expect to see if there was a real association between a treatment and an outcome,” said Dr. Gibbons.
The new results “are so provocative that they really mandate the need for a well-controlled randomized controlled trial of folic acid and suicide events,” possibly in a high-risk population such as veterans, he noted.
Such a study could use longitudinal assessments of suicidal events, such as the validated Computerized Adaptive Test Suicide Scale, he added. This continuous scale of suicidality ranges from subclinical, signifying helplessness, hopelessness, and loss of pleasure, to suicide attempts and completion.
As for study limitations, the investigators noted that this study was observational, so there could be selection effects. And using claims data likely underrepresented the number of suicidal events because of incomplete reporting. As the researchers pointed out, the rate of suicidal events in this study was much lower than the national rate.
Other limitations cited were that the association between folic acid and suicidal events may be explained by healthy user bias; and although the investigators conducted a sensitivity analysis in women of childbearing age, they did not have data on women actively planning for a pregnancy.
‘Impressive, encouraging’
In a comment, Shirley Yen, PhD, associate professor of psychology, Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, described the new findings as “quite impressive” and “extremely encouraging.”
However, she noted “it’s too premature” to suggest widespread use of folic acid in patients with depressive symptoms.
Dr. Yen, who has researched suicide risks previously, was not involved with the current study.
She did agree with the investigators that the results call for “more robustly controlled studies. These could include double-blind, randomized, controlled trials that could “more formally assess” all folic acid usage as opposed to prescriptions only, Dr. Yen said.
The study was funded by the NIH, the Agency for Healthcare Research and Quality, and the Center of Excellence for Suicide Prevention. Dr. Gibbons reported serving as an expert witness in cases for the Department of Justice; receiving expert witness fees from Merck, GlaxoSmithKline, Pfizer, and Wyeth; and having founded Adaptive Testing Technologies, which distributes the Computerized Adaptive Test Suicide Scale. Dr. Yen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
After adjusting for multiple factors, results from a large pharmaco-epidemiological study showed taking folic acid was associated with a 44% reduction in suicide events.
“These results are really putting folic acid squarely on the map as a potential for large-scale, population-level prevention,” lead author Robert D. Gibbons, PhD, professor of biostatistics, Center for Health Statistics, University of Chicago, said in an interview.
“Folic acid is safe, inexpensive, and generally available, and if future randomized controlled trials show this association is beyond a shadow of a doubt causal, we have a new tool in the arsenal,” Dr. Gibbons said.
Having such a tool would be extremely important given that suicide is such a significant public health crisis worldwide, he added.
The findings were published online in JAMA Psychiatry.
Previous research ‘fairly thin’
Folate, the naturally occurring form of B9, is essential for neurogenesis, nucleotide synthesis, and methylation of homocysteine. Past research has suggested that taking folate can prevent neural tube and heart defects in the fetus during pregnancy – and may prevent strokes and reduce age-related hearing loss in adults.
In psychiatry, the role of folate has been recognized for more than a decade. It may enhance the effects of antidepressants; and folate deficiency can predict poorer response to SSRIs.
This has led to recommendations for folate augmentation in patients with low or normal levels at the start of depression treatment.
Although previous research has shown a link between folic acid and suicidality, the findings have been “fairly thin,” with studies being “generally small, and many are case series,” Dr. Gibbons said.
The current study follows an earlier analysis that used a novel statistical methodology for generating drug safety signals that was developed by Dr. Gibbons and colleagues. That study compared rates of suicide attempts before and after initiation of 922 drugs with at least 3,000 prescriptions.
Its results showed 10 drugs were associated with increased risk after exposure, with the strongest associations for alprazolam, butalbital, hydrocodone, and combination codeine/promethazine. In addition, 44 drugs were associated with decreased risk, many of which were antidepressants and antipsychotics.
“One of the most interesting findings in terms of the decreased risk was for folic acid,” said Dr. Gibbons.
He and his colleagues initially thought this was because of women taking folic acid during pregnancy. But when restricting the analysis to men, they found the same effect.
Their next step was to carry out the current large-scale pharmaco-epidemiological study.
Prescriptions for pain
The researchers used a health claims database that included 164 million enrollees. The study cohort was comprised of 866,586 adults with private health insurance (81.3% women; 10.4% aged 60 years and older) who filled a folic acid prescription between 2012 and 2017.
More than half of the folic acid prescriptions were associated with pain disorders. About 48% were for a single agent at a dosage of 1 mg/d, which is the upper tolerable limit for adults – including in pregnancy and lactation.
Other single-agent daily dosages ranging from 0.4 mg to 5 mg accounted for 0.11% of prescriptions. The remainder were multivitamins.
The participants were followed for 24 months. The within-person analysis compared suicide attempts or self-harm events resulting in an outpatient visit or inpatient admission during periods of folic acid treatment versus during periods without treatment.
During the study period, the overall suicidal event rate was 133 per 100,000 population, which is one-fourth the national rate reported by the National Institutes of Health of 600 per 100,000.
After adjusting for age, sex, diagnoses related to suicidal behavior and folic acid deficiency, history of folate-reducing medications, and history of suicidal events, the estimated hazard ratio for suicide events when taking folic acid was 0.56 (95% confidence interal, 0.48-0.65) – which indicates a 44% reduction in suicide events.
“This is a very large decrease and is extremely significant and exciting,” Dr. Gibbons said.
He noted the decrease in suicidal events may have been even greater considering the study captured only prescription folic acid, and participants may also have also taken over-the-counter products.
“The 44% reduction in suicide attempts may actually be an underestimate,” said Dr. Gibbons.
Age and sex did not moderate the association between folic acid and suicide attempts, and a similar association was found in women of childbearing age.
Provocative results?
The investigators also assessed a negative control group of 236,610 individuals using cyanocobalamin during the study period. Cyanocobalamin is a form of vitamin B12 that is essential for metabolism, blood cell synthesis, and the nervous system. It does not contain folic acid and is commonly used to treat anemia.
Results showed no association between cyanocobalamin and suicidal events in the adjusted analysis (HR, 1.01; 95% CI, 0.80-1.27) or unadjusted analysis (HR, 1.02; 95% CI, 0.80-1.28).
Dr. Gibbons noted this result boosts the argument that the association between folic acid and reduced suicidal attempts “isn’t just about health-seeking behavior like taking vitamin supplements.”
Another sensitivity analysis showed every additional month of treatment was associated with a 5% reduction in the suicidal event rate.
“This means the longer you take folic acid, the greater the benefit, which is what you would expect to see if there was a real association between a treatment and an outcome,” said Dr. Gibbons.
The new results “are so provocative that they really mandate the need for a well-controlled randomized controlled trial of folic acid and suicide events,” possibly in a high-risk population such as veterans, he noted.
Such a study could use longitudinal assessments of suicidal events, such as the validated Computerized Adaptive Test Suicide Scale, he added. This continuous scale of suicidality ranges from subclinical, signifying helplessness, hopelessness, and loss of pleasure, to suicide attempts and completion.
As for study limitations, the investigators noted that this study was observational, so there could be selection effects. And using claims data likely underrepresented the number of suicidal events because of incomplete reporting. As the researchers pointed out, the rate of suicidal events in this study was much lower than the national rate.
Other limitations cited were that the association between folic acid and suicidal events may be explained by healthy user bias; and although the investigators conducted a sensitivity analysis in women of childbearing age, they did not have data on women actively planning for a pregnancy.
‘Impressive, encouraging’
In a comment, Shirley Yen, PhD, associate professor of psychology, Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, described the new findings as “quite impressive” and “extremely encouraging.”
However, she noted “it’s too premature” to suggest widespread use of folic acid in patients with depressive symptoms.
Dr. Yen, who has researched suicide risks previously, was not involved with the current study.
She did agree with the investigators that the results call for “more robustly controlled studies. These could include double-blind, randomized, controlled trials that could “more formally assess” all folic acid usage as opposed to prescriptions only, Dr. Yen said.
The study was funded by the NIH, the Agency for Healthcare Research and Quality, and the Center of Excellence for Suicide Prevention. Dr. Gibbons reported serving as an expert witness in cases for the Department of Justice; receiving expert witness fees from Merck, GlaxoSmithKline, Pfizer, and Wyeth; and having founded Adaptive Testing Technologies, which distributes the Computerized Adaptive Test Suicide Scale. Dr. Yen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
After adjusting for multiple factors, results from a large pharmaco-epidemiological study showed taking folic acid was associated with a 44% reduction in suicide events.
“These results are really putting folic acid squarely on the map as a potential for large-scale, population-level prevention,” lead author Robert D. Gibbons, PhD, professor of biostatistics, Center for Health Statistics, University of Chicago, said in an interview.
“Folic acid is safe, inexpensive, and generally available, and if future randomized controlled trials show this association is beyond a shadow of a doubt causal, we have a new tool in the arsenal,” Dr. Gibbons said.
Having such a tool would be extremely important given that suicide is such a significant public health crisis worldwide, he added.
The findings were published online in JAMA Psychiatry.
Previous research ‘fairly thin’
Folate, the naturally occurring form of B9, is essential for neurogenesis, nucleotide synthesis, and methylation of homocysteine. Past research has suggested that taking folate can prevent neural tube and heart defects in the fetus during pregnancy – and may prevent strokes and reduce age-related hearing loss in adults.
In psychiatry, the role of folate has been recognized for more than a decade. It may enhance the effects of antidepressants; and folate deficiency can predict poorer response to SSRIs.
This has led to recommendations for folate augmentation in patients with low or normal levels at the start of depression treatment.
Although previous research has shown a link between folic acid and suicidality, the findings have been “fairly thin,” with studies being “generally small, and many are case series,” Dr. Gibbons said.
The current study follows an earlier analysis that used a novel statistical methodology for generating drug safety signals that was developed by Dr. Gibbons and colleagues. That study compared rates of suicide attempts before and after initiation of 922 drugs with at least 3,000 prescriptions.
Its results showed 10 drugs were associated with increased risk after exposure, with the strongest associations for alprazolam, butalbital, hydrocodone, and combination codeine/promethazine. In addition, 44 drugs were associated with decreased risk, many of which were antidepressants and antipsychotics.
“One of the most interesting findings in terms of the decreased risk was for folic acid,” said Dr. Gibbons.
He and his colleagues initially thought this was because of women taking folic acid during pregnancy. But when restricting the analysis to men, they found the same effect.
Their next step was to carry out the current large-scale pharmaco-epidemiological study.
Prescriptions for pain
The researchers used a health claims database that included 164 million enrollees. The study cohort was comprised of 866,586 adults with private health insurance (81.3% women; 10.4% aged 60 years and older) who filled a folic acid prescription between 2012 and 2017.
More than half of the folic acid prescriptions were associated with pain disorders. About 48% were for a single agent at a dosage of 1 mg/d, which is the upper tolerable limit for adults – including in pregnancy and lactation.
Other single-agent daily dosages ranging from 0.4 mg to 5 mg accounted for 0.11% of prescriptions. The remainder were multivitamins.
The participants were followed for 24 months. The within-person analysis compared suicide attempts or self-harm events resulting in an outpatient visit or inpatient admission during periods of folic acid treatment versus during periods without treatment.
During the study period, the overall suicidal event rate was 133 per 100,000 population, which is one-fourth the national rate reported by the National Institutes of Health of 600 per 100,000.
After adjusting for age, sex, diagnoses related to suicidal behavior and folic acid deficiency, history of folate-reducing medications, and history of suicidal events, the estimated hazard ratio for suicide events when taking folic acid was 0.56 (95% confidence interal, 0.48-0.65) – which indicates a 44% reduction in suicide events.
“This is a very large decrease and is extremely significant and exciting,” Dr. Gibbons said.
He noted the decrease in suicidal events may have been even greater considering the study captured only prescription folic acid, and participants may also have also taken over-the-counter products.
“The 44% reduction in suicide attempts may actually be an underestimate,” said Dr. Gibbons.
Age and sex did not moderate the association between folic acid and suicide attempts, and a similar association was found in women of childbearing age.
Provocative results?
The investigators also assessed a negative control group of 236,610 individuals using cyanocobalamin during the study period. Cyanocobalamin is a form of vitamin B12 that is essential for metabolism, blood cell synthesis, and the nervous system. It does not contain folic acid and is commonly used to treat anemia.
Results showed no association between cyanocobalamin and suicidal events in the adjusted analysis (HR, 1.01; 95% CI, 0.80-1.27) or unadjusted analysis (HR, 1.02; 95% CI, 0.80-1.28).
Dr. Gibbons noted this result boosts the argument that the association between folic acid and reduced suicidal attempts “isn’t just about health-seeking behavior like taking vitamin supplements.”
Another sensitivity analysis showed every additional month of treatment was associated with a 5% reduction in the suicidal event rate.
“This means the longer you take folic acid, the greater the benefit, which is what you would expect to see if there was a real association between a treatment and an outcome,” said Dr. Gibbons.
The new results “are so provocative that they really mandate the need for a well-controlled randomized controlled trial of folic acid and suicide events,” possibly in a high-risk population such as veterans, he noted.
Such a study could use longitudinal assessments of suicidal events, such as the validated Computerized Adaptive Test Suicide Scale, he added. This continuous scale of suicidality ranges from subclinical, signifying helplessness, hopelessness, and loss of pleasure, to suicide attempts and completion.
As for study limitations, the investigators noted that this study was observational, so there could be selection effects. And using claims data likely underrepresented the number of suicidal events because of incomplete reporting. As the researchers pointed out, the rate of suicidal events in this study was much lower than the national rate.
Other limitations cited were that the association between folic acid and suicidal events may be explained by healthy user bias; and although the investigators conducted a sensitivity analysis in women of childbearing age, they did not have data on women actively planning for a pregnancy.
‘Impressive, encouraging’
In a comment, Shirley Yen, PhD, associate professor of psychology, Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, described the new findings as “quite impressive” and “extremely encouraging.”
However, she noted “it’s too premature” to suggest widespread use of folic acid in patients with depressive symptoms.
Dr. Yen, who has researched suicide risks previously, was not involved with the current study.
She did agree with the investigators that the results call for “more robustly controlled studies. These could include double-blind, randomized, controlled trials that could “more formally assess” all folic acid usage as opposed to prescriptions only, Dr. Yen said.
The study was funded by the NIH, the Agency for Healthcare Research and Quality, and the Center of Excellence for Suicide Prevention. Dr. Gibbons reported serving as an expert witness in cases for the Department of Justice; receiving expert witness fees from Merck, GlaxoSmithKline, Pfizer, and Wyeth; and having founded Adaptive Testing Technologies, which distributes the Computerized Adaptive Test Suicide Scale. Dr. Yen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Using SNRIs to prevent migraines in patients with depression
Ms. D, age 45, has major depressive disorder (MDD), generalized anxiety disorder (GAD), migraines, and hypertension. At a follow-up visit, she says she has been under a lot of stress at work in the past several months and feels her antidepressant is not working well for her depression or anxiety. Ms. D notes that lately she has had more frequent migraines, occurring approximately 4 times per month during the past 3 months. She describes a severe throbbing frontal pain that occurs primarily on the left side of her head, but sometimes on the right side. Ms. D says she experiences nausea, vomiting, and photophobia during these migraine episodes. The migraines last up to 12 hours, but often resolve with sumatriptan 50 mg as needed.
Ms. D takes fluoxetine 60 mg/d for depression and anxiety, lisinopril 20 mg/d for hypertension, as well as a women’s multivitamin and vitamin D3 daily. She has not tried other antidepressants and misses doses of her medications about once every other week. Her blood pressure is 125/80 mm Hg; heart rate is 80 beats per minute; and temperature is 37° C. Ms. D’s treatment team is considering switching her to a medication that can act as preventative therapy for migraines while also treating her depression and anxiety.
Migraine is a chronic, disabling neurovascular disorder that affects approximately 15% of the United States population.1 It is the second-leading disabling condition worldwide and may negatively affect social, family, personal, academic, and occupational domains.2 Migraine is often characterized by throbbing pain, is frequently unilateral, and may last 24 to 72 hours.3 It may occur with or without aura and can be associated with nausea, vomiting, or sensitivity to light.3 Episodic migraines occur <15 days a month, while chronic migraines occur ≥15 days a month.4
Many psychiatric, neurologic, vascular, and cardiac comorbidities are more prevalent in individuals who experience migraine headaches compared to the general population. Common psychiatric comorbidities found in patients with migraines are depression, bipolar disorder, GAD, panic disorder, and posttraumatic stress disorder5; MDD is the most common.4 A person who experiences migraine headaches is 2 to 4 times more likely to develop MDD than one who does not experience migraine headaches.4
First-line treatments for preventing migraine including divalproex, topiramate, metoprolol, propranolol, and timolol.6 However, for some patients with migraines and comorbid depression or anxiety, an antidepressant may be an option. This article briefly reviews the evidence for using antidepressants that have been studied for their ability to decrease migraine frequency.
Antidepressants that can prevent migraine
Tricyclic antidepressants (TCAs) are second- or third-line options for migraine prevention.6 While TCAs have proven to be effective for preventing migraines, many patients are unable to tolerate their adverse effects (ie, anticholinergic effects, sedation).7 TCAs may be more appealing for younger patients, who may be less bothered by anticholinergic burden, or those who have difficulty sleeping.
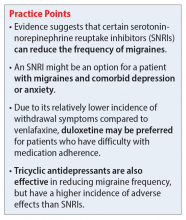
Serotonin-norepinephrine reuptake inhibitors (SNRIs). There has been growing interest in understanding the potential utility of SNRIs as a preventative treatment for migraines. Research has found that SNRIs are as effective as TCAs for preventing migraines and also more tolerable in terms of adverse effects.7 SNRIs such as venlafaxine and duloxetine are currently prescribed off-label to prevent migraines despite a lack of FDA approval for this indication.8
Continue to: Understanding the safety and efficacy...
Understanding the safety and efficacy of SNRIs as preventative treatment for episodic migraines is useful, particularly for patients with comorbid depression. The Table8-17 details clinical information related to SNRI use.
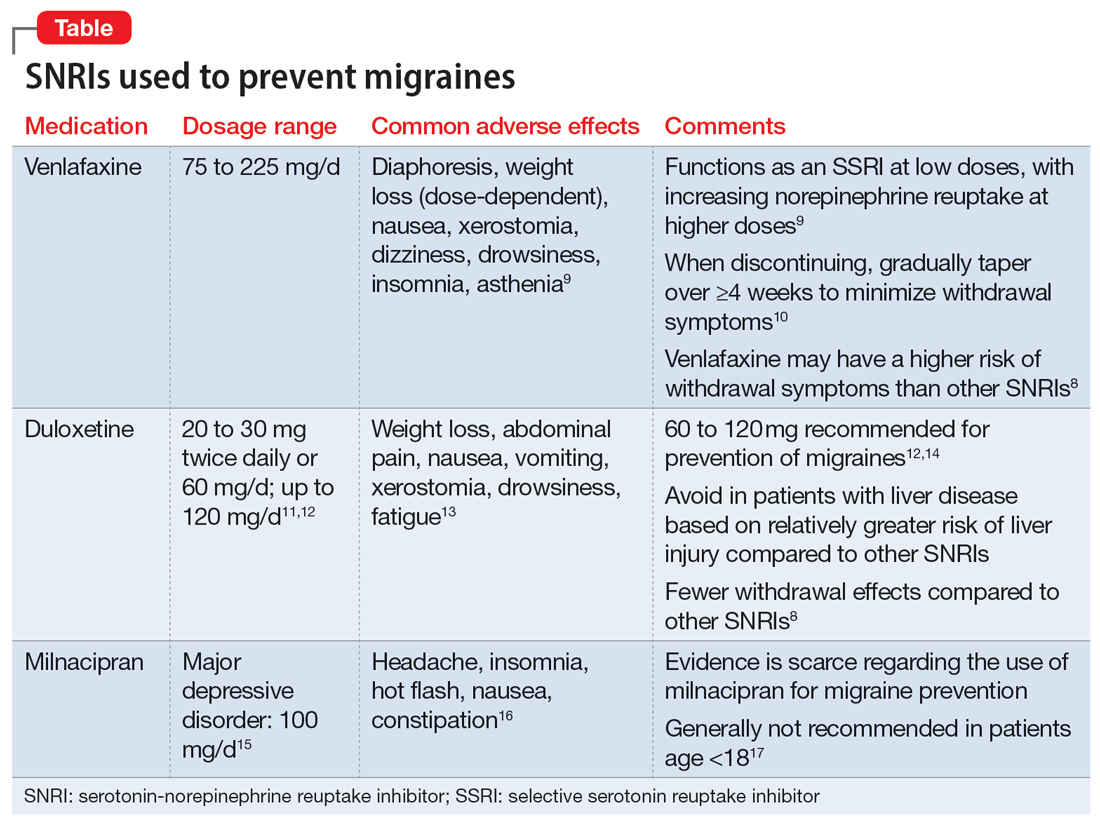
Duloxetine has demonstrated efficacy in preventing migraines in patients with comorbid depression.8 In a 2019 study, Kisler et al14 found that duloxetine 60 mg/d for 7 weeks was more effective for migraine prophylaxis than placebo as measured by the percentage of self-estimated migraine improvement by each patient compared to pretreatment levels (duloxetine: 52.3% ± 30.4%; placebo: 26.0% ± 27.3%; P = .001).
Venlafaxine has also demonstrated efficacy for preventing migraines in patients with comorbid depression.8 One study demonstrated a significant decrease in headaches per month with the use of venlafaxine 150 mg/d compared to placebo.18 Adelman et al19 found a reduction in migraine headaches per month (16.1 to 11.1, P < .0001) in patients who took venlafaxine for an average of 6 months with a mean dose of 150 mg/d. In a study of patients who did not have a mood disorder, Tarlaci20 found that venlafaxine reduced migraine headache independent of its antidepressant action.
Though milnacipran has not been studied as extensively as other SNRIs, evidence suggests it reduces the incidence of headaches and migraines, especially among episodic migraine patients. Although it has an equipotent effect on both serotonin and norepinephrine (NE) reuptake, milnacipran has a greater NE effect compared to other SNRIs approved for treating mood disorders. A prospective, single-arm study by Engel et al21 found a significant (P < .005) reduction from baseline in all headache and migraine days per month with the use of milnacipran 100 mg/d over the course of 3 months. The number of headache days per month was reduced by 4.2 compared to baseline. This same study reported improved functionality and reduced use of acute and symptomatic medications overall due to the decrease in headaches and migraines.21
In addition to demonstrating that certain SNRIs can effectively prevent migraine, some evidence suggests certain patients may benefit from the opportunity to decrease pill burden by using a single medication to treat both depression and migraine.22 Duloxetine may be preferred for patients who struggle with adherence (such as Ms. D) due to its relatively lower incidence of withdrawal symptoms compared to venlafaxine.8
CASE CONTINUED
Ms. D’s psychiatrist concludes she would be an appropriate candidate for treatment with an SNRI due to her history of MDD and chronic migraines. Because Ms. D expresses some difficulty remembering to take her medications, the psychiatrist recommends duloxetine because it is less likely to produce withdrawal symptoms compared to venlafaxine. To decrease pill burden, fluoxetine 60 mg is stopped with no taper due to its long half-life, and duloxetine is started at 30 mg/d, with a planned increase to 60 mg/d after 1 to 2 weeks as tolerated to target both mood and migraine prophylaxis. Duloxetine will not interact with Ms. D’s current medication regimen, including lisinopril, women’s multivitamin, or vitamin D3. The psychiatrist discusses the importance of medication adherence to improve her conditions effectively and safely. Ms. D’s heart rate and blood pressure will continue to be monitored.
Related Resources
- Leo RJ, Khalid K. Antidepressants for chronic pain. Current Psychiatry. 2019;18(2):8-16,21-22.
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
Drug Brand Names
Divalproex • Depakote
Duloxetine • Cymbalta
Fluoxetine • Prozac
Lisinopril • Zestril, Prinivil
Milnacipran • Savella
Sumatriptan • Imitrex
Topiramate • Topamax
Venlafaxine • Effexor
1. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496-505. doi:10.1111/head.13281
2. GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954-976. doi:10.1016/S1474-4422(18)30322-3
3. Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346(4):257-270. doi:10.1056/NEJMra010917
4. Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. 2017;29(5):504-515. doi:10.1080/09540261.2017.1326882
5. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649. doi:10.1016/j.ncl.2019.06.001
6. Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17-24.
7. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine (Baltimore). 2017;96(22):e6989. doi:10.1097/MD.0000000000006989
8. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
9. Venlafaxine. Lexicomp. 2021. http://online.lexi.com/
10. Ogle NR, Akkerman SR. Guidance for the discontinuation or switching of antidepressant therapies in adults. J Pharm Pract. 2013;26(4):389-396. doi:10.1177/0897190012467210
11. Duloxetine [package insert]. Indianapolis, IN: Eli Lilly and Company; 2004.
12. Young WB, Bradley KC, Anjum MW, et al. Duloxetine prophylaxis for episodic migraine in persons without depression: a prospective study. Headache. 2013;53(9):1430-1437.
13. Duloxetine. Lexicomp. 2021. http://online.lexi.com/
14. Kisler LB, Weissman-Fogel I, Coghill RC, et al. Individualization of migraine prevention: a randomized controlled trial of psychophysical-based prediction of duloxetine efficacy. Clin J Pain. 2019;35(9):753-765.
15. Mansuy L. Antidepressant therapy with milnacipran and venlafaxine. Neuropsychiatr Dis Treat. 2010;6 (Suppl I):17-22.
16. Milnacipran. Lexicomp. 2021. http://online.lexi.com/
17. Milnacipran. MedlinePlus. Updated January 22, 2022. Accessed August 19, 2022. https://medlineplus.gov/druginfo/meds/a609016.html
18. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152. doi:10.1111/j.1526-4610.2005.05029.x
19. Adelman LC, Adelman JU, Von Seggern R, et al. Venlafaxine extended release (XR) for the prophylaxis of migraine and tension-type headache: a retrospective study in a clinical setting. Headache. 2000;40(7):572-580. doi:10.1046/j.1526-4610.2000.00089.x
20. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258. doi:10.1097/WNF.0b013e3181a8c84f
21. Engel ER, Kudrow D, Rapoport AM. A prospective, open-label study of milnacipran in the prevention of headache in patients with episodic or chronic migraine. Neurol Sci. 2014;35(3):429-435. doi:10.1007/s10072-013-1536-0
22. Baumgartner A, Drame K, Geutjens S, et al. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. 2020;12(2):190.
Ms. D, age 45, has major depressive disorder (MDD), generalized anxiety disorder (GAD), migraines, and hypertension. At a follow-up visit, she says she has been under a lot of stress at work in the past several months and feels her antidepressant is not working well for her depression or anxiety. Ms. D notes that lately she has had more frequent migraines, occurring approximately 4 times per month during the past 3 months. She describes a severe throbbing frontal pain that occurs primarily on the left side of her head, but sometimes on the right side. Ms. D says she experiences nausea, vomiting, and photophobia during these migraine episodes. The migraines last up to 12 hours, but often resolve with sumatriptan 50 mg as needed.
Ms. D takes fluoxetine 60 mg/d for depression and anxiety, lisinopril 20 mg/d for hypertension, as well as a women’s multivitamin and vitamin D3 daily. She has not tried other antidepressants and misses doses of her medications about once every other week. Her blood pressure is 125/80 mm Hg; heart rate is 80 beats per minute; and temperature is 37° C. Ms. D’s treatment team is considering switching her to a medication that can act as preventative therapy for migraines while also treating her depression and anxiety.
Migraine is a chronic, disabling neurovascular disorder that affects approximately 15% of the United States population.1 It is the second-leading disabling condition worldwide and may negatively affect social, family, personal, academic, and occupational domains.2 Migraine is often characterized by throbbing pain, is frequently unilateral, and may last 24 to 72 hours.3 It may occur with or without aura and can be associated with nausea, vomiting, or sensitivity to light.3 Episodic migraines occur <15 days a month, while chronic migraines occur ≥15 days a month.4
Many psychiatric, neurologic, vascular, and cardiac comorbidities are more prevalent in individuals who experience migraine headaches compared to the general population. Common psychiatric comorbidities found in patients with migraines are depression, bipolar disorder, GAD, panic disorder, and posttraumatic stress disorder5; MDD is the most common.4 A person who experiences migraine headaches is 2 to 4 times more likely to develop MDD than one who does not experience migraine headaches.4
First-line treatments for preventing migraine including divalproex, topiramate, metoprolol, propranolol, and timolol.6 However, for some patients with migraines and comorbid depression or anxiety, an antidepressant may be an option. This article briefly reviews the evidence for using antidepressants that have been studied for their ability to decrease migraine frequency.
Antidepressants that can prevent migraine
Tricyclic antidepressants (TCAs) are second- or third-line options for migraine prevention.6 While TCAs have proven to be effective for preventing migraines, many patients are unable to tolerate their adverse effects (ie, anticholinergic effects, sedation).7 TCAs may be more appealing for younger patients, who may be less bothered by anticholinergic burden, or those who have difficulty sleeping.
Serotonin-norepinephrine reuptake inhibitors (SNRIs). There has been growing interest in understanding the potential utility of SNRIs as a preventative treatment for migraines. Research has found that SNRIs are as effective as TCAs for preventing migraines and also more tolerable in terms of adverse effects.7 SNRIs such as venlafaxine and duloxetine are currently prescribed off-label to prevent migraines despite a lack of FDA approval for this indication.8
Continue to: Understanding the safety and efficacy...
Understanding the safety and efficacy of SNRIs as preventative treatment for episodic migraines is useful, particularly for patients with comorbid depression. The Table8-17 details clinical information related to SNRI use.

Duloxetine has demonstrated efficacy in preventing migraines in patients with comorbid depression.8 In a 2019 study, Kisler et al14 found that duloxetine 60 mg/d for 7 weeks was more effective for migraine prophylaxis than placebo as measured by the percentage of self-estimated migraine improvement by each patient compared to pretreatment levels (duloxetine: 52.3% ± 30.4%; placebo: 26.0% ± 27.3%; P = .001).
Venlafaxine has also demonstrated efficacy for preventing migraines in patients with comorbid depression.8 One study demonstrated a significant decrease in headaches per month with the use of venlafaxine 150 mg/d compared to placebo.18 Adelman et al19 found a reduction in migraine headaches per month (16.1 to 11.1, P < .0001) in patients who took venlafaxine for an average of 6 months with a mean dose of 150 mg/d. In a study of patients who did not have a mood disorder, Tarlaci20 found that venlafaxine reduced migraine headache independent of its antidepressant action.
Though milnacipran has not been studied as extensively as other SNRIs, evidence suggests it reduces the incidence of headaches and migraines, especially among episodic migraine patients. Although it has an equipotent effect on both serotonin and norepinephrine (NE) reuptake, milnacipran has a greater NE effect compared to other SNRIs approved for treating mood disorders. A prospective, single-arm study by Engel et al21 found a significant (P < .005) reduction from baseline in all headache and migraine days per month with the use of milnacipran 100 mg/d over the course of 3 months. The number of headache days per month was reduced by 4.2 compared to baseline. This same study reported improved functionality and reduced use of acute and symptomatic medications overall due to the decrease in headaches and migraines.21
In addition to demonstrating that certain SNRIs can effectively prevent migraine, some evidence suggests certain patients may benefit from the opportunity to decrease pill burden by using a single medication to treat both depression and migraine.22 Duloxetine may be preferred for patients who struggle with adherence (such as Ms. D) due to its relatively lower incidence of withdrawal symptoms compared to venlafaxine.8
CASE CONTINUED
Ms. D’s psychiatrist concludes she would be an appropriate candidate for treatment with an SNRI due to her history of MDD and chronic migraines. Because Ms. D expresses some difficulty remembering to take her medications, the psychiatrist recommends duloxetine because it is less likely to produce withdrawal symptoms compared to venlafaxine. To decrease pill burden, fluoxetine 60 mg is stopped with no taper due to its long half-life, and duloxetine is started at 30 mg/d, with a planned increase to 60 mg/d after 1 to 2 weeks as tolerated to target both mood and migraine prophylaxis. Duloxetine will not interact with Ms. D’s current medication regimen, including lisinopril, women’s multivitamin, or vitamin D3. The psychiatrist discusses the importance of medication adherence to improve her conditions effectively and safely. Ms. D’s heart rate and blood pressure will continue to be monitored.
Related Resources
- Leo RJ, Khalid K. Antidepressants for chronic pain. Current Psychiatry. 2019;18(2):8-16,21-22.
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
Drug Brand Names
Divalproex • Depakote
Duloxetine • Cymbalta
Fluoxetine • Prozac
Lisinopril • Zestril, Prinivil
Milnacipran • Savella
Sumatriptan • Imitrex
Topiramate • Topamax
Venlafaxine • Effexor
Ms. D, age 45, has major depressive disorder (MDD), generalized anxiety disorder (GAD), migraines, and hypertension. At a follow-up visit, she says she has been under a lot of stress at work in the past several months and feels her antidepressant is not working well for her depression or anxiety. Ms. D notes that lately she has had more frequent migraines, occurring approximately 4 times per month during the past 3 months. She describes a severe throbbing frontal pain that occurs primarily on the left side of her head, but sometimes on the right side. Ms. D says she experiences nausea, vomiting, and photophobia during these migraine episodes. The migraines last up to 12 hours, but often resolve with sumatriptan 50 mg as needed.
Ms. D takes fluoxetine 60 mg/d for depression and anxiety, lisinopril 20 mg/d for hypertension, as well as a women’s multivitamin and vitamin D3 daily. She has not tried other antidepressants and misses doses of her medications about once every other week. Her blood pressure is 125/80 mm Hg; heart rate is 80 beats per minute; and temperature is 37° C. Ms. D’s treatment team is considering switching her to a medication that can act as preventative therapy for migraines while also treating her depression and anxiety.
Migraine is a chronic, disabling neurovascular disorder that affects approximately 15% of the United States population.1 It is the second-leading disabling condition worldwide and may negatively affect social, family, personal, academic, and occupational domains.2 Migraine is often characterized by throbbing pain, is frequently unilateral, and may last 24 to 72 hours.3 It may occur with or without aura and can be associated with nausea, vomiting, or sensitivity to light.3 Episodic migraines occur <15 days a month, while chronic migraines occur ≥15 days a month.4
Many psychiatric, neurologic, vascular, and cardiac comorbidities are more prevalent in individuals who experience migraine headaches compared to the general population. Common psychiatric comorbidities found in patients with migraines are depression, bipolar disorder, GAD, panic disorder, and posttraumatic stress disorder5; MDD is the most common.4 A person who experiences migraine headaches is 2 to 4 times more likely to develop MDD than one who does not experience migraine headaches.4
First-line treatments for preventing migraine including divalproex, topiramate, metoprolol, propranolol, and timolol.6 However, for some patients with migraines and comorbid depression or anxiety, an antidepressant may be an option. This article briefly reviews the evidence for using antidepressants that have been studied for their ability to decrease migraine frequency.
Antidepressants that can prevent migraine
Tricyclic antidepressants (TCAs) are second- or third-line options for migraine prevention.6 While TCAs have proven to be effective for preventing migraines, many patients are unable to tolerate their adverse effects (ie, anticholinergic effects, sedation).7 TCAs may be more appealing for younger patients, who may be less bothered by anticholinergic burden, or those who have difficulty sleeping.
Serotonin-norepinephrine reuptake inhibitors (SNRIs). There has been growing interest in understanding the potential utility of SNRIs as a preventative treatment for migraines. Research has found that SNRIs are as effective as TCAs for preventing migraines and also more tolerable in terms of adverse effects.7 SNRIs such as venlafaxine and duloxetine are currently prescribed off-label to prevent migraines despite a lack of FDA approval for this indication.8
Continue to: Understanding the safety and efficacy...
Understanding the safety and efficacy of SNRIs as preventative treatment for episodic migraines is useful, particularly for patients with comorbid depression. The Table8-17 details clinical information related to SNRI use.

Duloxetine has demonstrated efficacy in preventing migraines in patients with comorbid depression.8 In a 2019 study, Kisler et al14 found that duloxetine 60 mg/d for 7 weeks was more effective for migraine prophylaxis than placebo as measured by the percentage of self-estimated migraine improvement by each patient compared to pretreatment levels (duloxetine: 52.3% ± 30.4%; placebo: 26.0% ± 27.3%; P = .001).
Venlafaxine has also demonstrated efficacy for preventing migraines in patients with comorbid depression.8 One study demonstrated a significant decrease in headaches per month with the use of venlafaxine 150 mg/d compared to placebo.18 Adelman et al19 found a reduction in migraine headaches per month (16.1 to 11.1, P < .0001) in patients who took venlafaxine for an average of 6 months with a mean dose of 150 mg/d. In a study of patients who did not have a mood disorder, Tarlaci20 found that venlafaxine reduced migraine headache independent of its antidepressant action.
Though milnacipran has not been studied as extensively as other SNRIs, evidence suggests it reduces the incidence of headaches and migraines, especially among episodic migraine patients. Although it has an equipotent effect on both serotonin and norepinephrine (NE) reuptake, milnacipran has a greater NE effect compared to other SNRIs approved for treating mood disorders. A prospective, single-arm study by Engel et al21 found a significant (P < .005) reduction from baseline in all headache and migraine days per month with the use of milnacipran 100 mg/d over the course of 3 months. The number of headache days per month was reduced by 4.2 compared to baseline. This same study reported improved functionality and reduced use of acute and symptomatic medications overall due to the decrease in headaches and migraines.21
In addition to demonstrating that certain SNRIs can effectively prevent migraine, some evidence suggests certain patients may benefit from the opportunity to decrease pill burden by using a single medication to treat both depression and migraine.22 Duloxetine may be preferred for patients who struggle with adherence (such as Ms. D) due to its relatively lower incidence of withdrawal symptoms compared to venlafaxine.8
CASE CONTINUED
Ms. D’s psychiatrist concludes she would be an appropriate candidate for treatment with an SNRI due to her history of MDD and chronic migraines. Because Ms. D expresses some difficulty remembering to take her medications, the psychiatrist recommends duloxetine because it is less likely to produce withdrawal symptoms compared to venlafaxine. To decrease pill burden, fluoxetine 60 mg is stopped with no taper due to its long half-life, and duloxetine is started at 30 mg/d, with a planned increase to 60 mg/d after 1 to 2 weeks as tolerated to target both mood and migraine prophylaxis. Duloxetine will not interact with Ms. D’s current medication regimen, including lisinopril, women’s multivitamin, or vitamin D3. The psychiatrist discusses the importance of medication adherence to improve her conditions effectively and safely. Ms. D’s heart rate and blood pressure will continue to be monitored.
Related Resources
- Leo RJ, Khalid K. Antidepressants for chronic pain. Current Psychiatry. 2019;18(2):8-16,21-22.
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
Drug Brand Names
Divalproex • Depakote
Duloxetine • Cymbalta
Fluoxetine • Prozac
Lisinopril • Zestril, Prinivil
Milnacipran • Savella
Sumatriptan • Imitrex
Topiramate • Topamax
Venlafaxine • Effexor
1. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496-505. doi:10.1111/head.13281
2. GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954-976. doi:10.1016/S1474-4422(18)30322-3
3. Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346(4):257-270. doi:10.1056/NEJMra010917
4. Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. 2017;29(5):504-515. doi:10.1080/09540261.2017.1326882
5. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649. doi:10.1016/j.ncl.2019.06.001
6. Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17-24.
7. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine (Baltimore). 2017;96(22):e6989. doi:10.1097/MD.0000000000006989
8. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
9. Venlafaxine. Lexicomp. 2021. http://online.lexi.com/
10. Ogle NR, Akkerman SR. Guidance for the discontinuation or switching of antidepressant therapies in adults. J Pharm Pract. 2013;26(4):389-396. doi:10.1177/0897190012467210
11. Duloxetine [package insert]. Indianapolis, IN: Eli Lilly and Company; 2004.
12. Young WB, Bradley KC, Anjum MW, et al. Duloxetine prophylaxis for episodic migraine in persons without depression: a prospective study. Headache. 2013;53(9):1430-1437.
13. Duloxetine. Lexicomp. 2021. http://online.lexi.com/
14. Kisler LB, Weissman-Fogel I, Coghill RC, et al. Individualization of migraine prevention: a randomized controlled trial of psychophysical-based prediction of duloxetine efficacy. Clin J Pain. 2019;35(9):753-765.
15. Mansuy L. Antidepressant therapy with milnacipran and venlafaxine. Neuropsychiatr Dis Treat. 2010;6 (Suppl I):17-22.
16. Milnacipran. Lexicomp. 2021. http://online.lexi.com/
17. Milnacipran. MedlinePlus. Updated January 22, 2022. Accessed August 19, 2022. https://medlineplus.gov/druginfo/meds/a609016.html
18. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152. doi:10.1111/j.1526-4610.2005.05029.x
19. Adelman LC, Adelman JU, Von Seggern R, et al. Venlafaxine extended release (XR) for the prophylaxis of migraine and tension-type headache: a retrospective study in a clinical setting. Headache. 2000;40(7):572-580. doi:10.1046/j.1526-4610.2000.00089.x
20. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258. doi:10.1097/WNF.0b013e3181a8c84f
21. Engel ER, Kudrow D, Rapoport AM. A prospective, open-label study of milnacipran in the prevention of headache in patients with episodic or chronic migraine. Neurol Sci. 2014;35(3):429-435. doi:10.1007/s10072-013-1536-0
22. Baumgartner A, Drame K, Geutjens S, et al. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. 2020;12(2):190.
1. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496-505. doi:10.1111/head.13281
2. GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954-976. doi:10.1016/S1474-4422(18)30322-3
3. Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346(4):257-270. doi:10.1056/NEJMra010917
4. Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. 2017;29(5):504-515. doi:10.1080/09540261.2017.1326882
5. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649. doi:10.1016/j.ncl.2019.06.001
6. Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17-24.
7. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine (Baltimore). 2017;96(22):e6989. doi:10.1097/MD.0000000000006989
8. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
9. Venlafaxine. Lexicomp. 2021. http://online.lexi.com/
10. Ogle NR, Akkerman SR. Guidance for the discontinuation or switching of antidepressant therapies in adults. J Pharm Pract. 2013;26(4):389-396. doi:10.1177/0897190012467210
11. Duloxetine [package insert]. Indianapolis, IN: Eli Lilly and Company; 2004.
12. Young WB, Bradley KC, Anjum MW, et al. Duloxetine prophylaxis for episodic migraine in persons without depression: a prospective study. Headache. 2013;53(9):1430-1437.
13. Duloxetine. Lexicomp. 2021. http://online.lexi.com/
14. Kisler LB, Weissman-Fogel I, Coghill RC, et al. Individualization of migraine prevention: a randomized controlled trial of psychophysical-based prediction of duloxetine efficacy. Clin J Pain. 2019;35(9):753-765.
15. Mansuy L. Antidepressant therapy with milnacipran and venlafaxine. Neuropsychiatr Dis Treat. 2010;6 (Suppl I):17-22.
16. Milnacipran. Lexicomp. 2021. http://online.lexi.com/
17. Milnacipran. MedlinePlus. Updated January 22, 2022. Accessed August 19, 2022. https://medlineplus.gov/druginfo/meds/a609016.html
18. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152. doi:10.1111/j.1526-4610.2005.05029.x
19. Adelman LC, Adelman JU, Von Seggern R, et al. Venlafaxine extended release (XR) for the prophylaxis of migraine and tension-type headache: a retrospective study in a clinical setting. Headache. 2000;40(7):572-580. doi:10.1046/j.1526-4610.2000.00089.x
20. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258. doi:10.1097/WNF.0b013e3181a8c84f
21. Engel ER, Kudrow D, Rapoport AM. A prospective, open-label study of milnacipran in the prevention of headache in patients with episodic or chronic migraine. Neurol Sci. 2014;35(3):429-435. doi:10.1007/s10072-013-1536-0
22. Baumgartner A, Drame K, Geutjens S, et al. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. 2020;12(2):190.
Timing of food intake a novel strategy for treating mood disorders?
new research suggests.
Investigators at Brigham and Women’s Hospital, Boston, created a simulated nightwork schedule for 19 individuals in a laboratory setting. Participants then engaged in two different meal timing models – daytime-only meals (DMI), and meals taken during both daytime and nighttime (DNMC).
Depression- and anxiety-like mood levels increased by 26% and 16%, respectively, among the daytime and nighttime eaters, but there was no such increase in daytime-only eaters.
“Our findings provide evidence for the timing of food intake as a novel strategy to potentially minimize mood vulnerability in individuals experiencing circadian misalignment, such as people engaged in shift work, experiencing jet lag, or suffering from circadian rhythm disorders,” co–corresponding author Frank A.J.L. Scheer, PhD, director of the medical chronobiology program, Brigham and Women’s Hospital, Boston, said in a news release.
The study was published online in the Proceedings of the National Academy of Sciences.
Misaligned circadian clock
“Shift workers often experience a misalignment between their central circadian clock in the brain and daily behaviors, such as sleep/wake and fasting/eating cycles,” senior author Sarah Chellappa, MD, PhD, currently the Alexander Von Humboldt Experienced Fellow in the department of nuclear medicine, University of Cologne (Germany). Dr. Chellappa was a postdoctoral fellow at Brigham and Women’s Hospital when the study was conducted.
“They also have a 25%-40% higher risk of depression and anxiety,” she continued. “Since meal timing is important for physical health and diet is important for mood, we sought to find out whether meal timing can benefit mental health as well.”
Given that impaired glycemic control is a “risk factor for mood disruption,” the researchers tested the prediction that daytime eating “would prevent mood vulnerability, despite simulated night work.”
To investigate the question, they conducted a parallel-design, randomized clinical trial that included a 14-day circadian laboratory protocol with 19 healthy adults (12 men, 7 women; mean age, 26.5 ± 4.1 years) who underwent a forced desynchrony (FD) in dim light for 4 “days,” each of which consisted of 28 hours. Each 28-hour “day” resulted in an additional 4-hour misalignment between the central circadian clock and external behavioral/environmental cycles.
By the fourth day, the participants were misaligned by 12 hours, compared to baseline (that is, the first day). They were then randomly assigned to two groups.
The DNMC group – the control group – had a “typical 28-hour FD protocol,” with behavioral and environmental cycles (sleep/wake, rest/activity, supine/upright posture, dark during scheduled sleep/dim light during wakefulness) scheduled on a 28-hour cycle. Thus, they took their meals during both “daytime” and “nighttime,” which is the typical way that night workers eat.
The DMI group underwent a modified 28-hour FD protocol, with all cycles scheduled on a 28-hour basis, except for the fasting/eating cycle, which was scheduled on a 24-hour basis, resulting in meals consumed only during the “daytime.”
Depression- and anxiety-like mood (which “correspond to an amalgam of mood states typically observed in depression and anxiety) were assessed every hour during the 4 FD days, using computerized visual analogue scales.
Nutritional psychiatry
Participants in the DNMC group experienced an increase from baseline in depression- and anxiety-like mood levels of 26.2% (95% confidence interval, 21-31.5; P = .001; P value using false discovery rate, .01; effect-size r, 0.78) and 16.1% (95% CI, 8.5-23.6; P = .005; PFDR, .001; effect-size r, 0.47), respectively.
By contrast, a similar increase did not take place in the DMI group for either depression- or anxiety-like mood levels (95% CI, –5.7% to 7.4%, P not significant and 95% CI, –3.1% to 9.9%, P not significant, respectively).
The researchers tested “whether increase mood vulnerability during simulated night work was associated with the degree of internal circadian misalignment” — defined as “change in the phase difference between the acrophase of circadian glucose rhythms and the bathyphase of circadian body temperature rhythms.”
They found that a larger degree of internal circadian misalignment was “robustly associated” with more depression-like (r, 0.77; P = .001) and anxiety-like (r, 0.67; P = .002) mood levels during simulated night work.
The findings imply that meal timing had “moderate to large effects in depression-like and anxiety-like mood levels during night work, and that such effects were associated with the degree of internal circadian misalignment,” the authors wrote.
The laboratory protocol of both groups was identical except for the timing of meals. The authors noted that the “relevance of diet on sleep, circadian rhythms, and mental health is receiving growing awareness with the emergence of a new field, nutritional psychiatry.”
People who experience depression “often report poor-quality diets with high carbohydrate intake,” and there is evidence that adherence to the Mediterranean diet is associated “with lower odds of depression, anxiety, and psychological distress.”
They cautioned that although these emerging studies suggest an association between dietary factors and mental health, “experimental studies in individuals with depression and/or anxiety/anxiety-related disorders are required to determine causality and direction of effects.”
They described meal timing as “an emerging aspect of nutrition, with increasing research interest because of its influence on physical health.” However, they noted, “the causal role of the timing of food intake on mental health remains to be tested.”
Novel findings
Commenting for this article, Kathleen Merikangas, PhD, distinguished investigator and chief, genetic epidemiology research branch, intramural research program, National Institute of Mental Health, Bethesda, Md., described the research as important with novel findings.
The research “employs the elegant, carefully controlled laboratory procedures that have unraveled the influence of light and other environmental cues on sleep and circadian rhythms over the past 2 decades,” said Dr. Merikangas, who was not involved with the study.
“One of the most significant contributions of this work is its demonstration of the importance of investigating circadian rhythms of multiple systems rather than solely focusing on sleep, eating, or emotional states that have often been studied in isolation,” she pointed out.
“Growing evidence from basic research highlights the interdependence of multiple human systems that should be built into interventions that tend to focus on one or two domains.”
She recommended that this work be replicated “in more diverse samples ... in both controlled and naturalistic settings...to test both the generalizability and mechanism of these intriguing findings.”
The study was funded by the National Institutes of Health. Individual investigators were funded by the Alexander Von Humboldt Foundation and the American Diabetes Association. Dr. Chellappa disclosed no relevant financial relationships. Dr. Merikangas disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Investigators at Brigham and Women’s Hospital, Boston, created a simulated nightwork schedule for 19 individuals in a laboratory setting. Participants then engaged in two different meal timing models – daytime-only meals (DMI), and meals taken during both daytime and nighttime (DNMC).
Depression- and anxiety-like mood levels increased by 26% and 16%, respectively, among the daytime and nighttime eaters, but there was no such increase in daytime-only eaters.
“Our findings provide evidence for the timing of food intake as a novel strategy to potentially minimize mood vulnerability in individuals experiencing circadian misalignment, such as people engaged in shift work, experiencing jet lag, or suffering from circadian rhythm disorders,” co–corresponding author Frank A.J.L. Scheer, PhD, director of the medical chronobiology program, Brigham and Women’s Hospital, Boston, said in a news release.
The study was published online in the Proceedings of the National Academy of Sciences.
Misaligned circadian clock
“Shift workers often experience a misalignment between their central circadian clock in the brain and daily behaviors, such as sleep/wake and fasting/eating cycles,” senior author Sarah Chellappa, MD, PhD, currently the Alexander Von Humboldt Experienced Fellow in the department of nuclear medicine, University of Cologne (Germany). Dr. Chellappa was a postdoctoral fellow at Brigham and Women’s Hospital when the study was conducted.
“They also have a 25%-40% higher risk of depression and anxiety,” she continued. “Since meal timing is important for physical health and diet is important for mood, we sought to find out whether meal timing can benefit mental health as well.”
Given that impaired glycemic control is a “risk factor for mood disruption,” the researchers tested the prediction that daytime eating “would prevent mood vulnerability, despite simulated night work.”
To investigate the question, they conducted a parallel-design, randomized clinical trial that included a 14-day circadian laboratory protocol with 19 healthy adults (12 men, 7 women; mean age, 26.5 ± 4.1 years) who underwent a forced desynchrony (FD) in dim light for 4 “days,” each of which consisted of 28 hours. Each 28-hour “day” resulted in an additional 4-hour misalignment between the central circadian clock and external behavioral/environmental cycles.
By the fourth day, the participants were misaligned by 12 hours, compared to baseline (that is, the first day). They were then randomly assigned to two groups.
The DNMC group – the control group – had a “typical 28-hour FD protocol,” with behavioral and environmental cycles (sleep/wake, rest/activity, supine/upright posture, dark during scheduled sleep/dim light during wakefulness) scheduled on a 28-hour cycle. Thus, they took their meals during both “daytime” and “nighttime,” which is the typical way that night workers eat.
The DMI group underwent a modified 28-hour FD protocol, with all cycles scheduled on a 28-hour basis, except for the fasting/eating cycle, which was scheduled on a 24-hour basis, resulting in meals consumed only during the “daytime.”
Depression- and anxiety-like mood (which “correspond to an amalgam of mood states typically observed in depression and anxiety) were assessed every hour during the 4 FD days, using computerized visual analogue scales.
Nutritional psychiatry
Participants in the DNMC group experienced an increase from baseline in depression- and anxiety-like mood levels of 26.2% (95% confidence interval, 21-31.5; P = .001; P value using false discovery rate, .01; effect-size r, 0.78) and 16.1% (95% CI, 8.5-23.6; P = .005; PFDR, .001; effect-size r, 0.47), respectively.
By contrast, a similar increase did not take place in the DMI group for either depression- or anxiety-like mood levels (95% CI, –5.7% to 7.4%, P not significant and 95% CI, –3.1% to 9.9%, P not significant, respectively).
The researchers tested “whether increase mood vulnerability during simulated night work was associated with the degree of internal circadian misalignment” — defined as “change in the phase difference between the acrophase of circadian glucose rhythms and the bathyphase of circadian body temperature rhythms.”
They found that a larger degree of internal circadian misalignment was “robustly associated” with more depression-like (r, 0.77; P = .001) and anxiety-like (r, 0.67; P = .002) mood levels during simulated night work.
The findings imply that meal timing had “moderate to large effects in depression-like and anxiety-like mood levels during night work, and that such effects were associated with the degree of internal circadian misalignment,” the authors wrote.
The laboratory protocol of both groups was identical except for the timing of meals. The authors noted that the “relevance of diet on sleep, circadian rhythms, and mental health is receiving growing awareness with the emergence of a new field, nutritional psychiatry.”
People who experience depression “often report poor-quality diets with high carbohydrate intake,” and there is evidence that adherence to the Mediterranean diet is associated “with lower odds of depression, anxiety, and psychological distress.”
They cautioned that although these emerging studies suggest an association between dietary factors and mental health, “experimental studies in individuals with depression and/or anxiety/anxiety-related disorders are required to determine causality and direction of effects.”
They described meal timing as “an emerging aspect of nutrition, with increasing research interest because of its influence on physical health.” However, they noted, “the causal role of the timing of food intake on mental health remains to be tested.”
Novel findings
Commenting for this article, Kathleen Merikangas, PhD, distinguished investigator and chief, genetic epidemiology research branch, intramural research program, National Institute of Mental Health, Bethesda, Md., described the research as important with novel findings.
The research “employs the elegant, carefully controlled laboratory procedures that have unraveled the influence of light and other environmental cues on sleep and circadian rhythms over the past 2 decades,” said Dr. Merikangas, who was not involved with the study.
“One of the most significant contributions of this work is its demonstration of the importance of investigating circadian rhythms of multiple systems rather than solely focusing on sleep, eating, or emotional states that have often been studied in isolation,” she pointed out.
“Growing evidence from basic research highlights the interdependence of multiple human systems that should be built into interventions that tend to focus on one or two domains.”
She recommended that this work be replicated “in more diverse samples ... in both controlled and naturalistic settings...to test both the generalizability and mechanism of these intriguing findings.”
The study was funded by the National Institutes of Health. Individual investigators were funded by the Alexander Von Humboldt Foundation and the American Diabetes Association. Dr. Chellappa disclosed no relevant financial relationships. Dr. Merikangas disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Investigators at Brigham and Women’s Hospital, Boston, created a simulated nightwork schedule for 19 individuals in a laboratory setting. Participants then engaged in two different meal timing models – daytime-only meals (DMI), and meals taken during both daytime and nighttime (DNMC).
Depression- and anxiety-like mood levels increased by 26% and 16%, respectively, among the daytime and nighttime eaters, but there was no such increase in daytime-only eaters.
“Our findings provide evidence for the timing of food intake as a novel strategy to potentially minimize mood vulnerability in individuals experiencing circadian misalignment, such as people engaged in shift work, experiencing jet lag, or suffering from circadian rhythm disorders,” co–corresponding author Frank A.J.L. Scheer, PhD, director of the medical chronobiology program, Brigham and Women’s Hospital, Boston, said in a news release.
The study was published online in the Proceedings of the National Academy of Sciences.
Misaligned circadian clock
“Shift workers often experience a misalignment between their central circadian clock in the brain and daily behaviors, such as sleep/wake and fasting/eating cycles,” senior author Sarah Chellappa, MD, PhD, currently the Alexander Von Humboldt Experienced Fellow in the department of nuclear medicine, University of Cologne (Germany). Dr. Chellappa was a postdoctoral fellow at Brigham and Women’s Hospital when the study was conducted.
“They also have a 25%-40% higher risk of depression and anxiety,” she continued. “Since meal timing is important for physical health and diet is important for mood, we sought to find out whether meal timing can benefit mental health as well.”
Given that impaired glycemic control is a “risk factor for mood disruption,” the researchers tested the prediction that daytime eating “would prevent mood vulnerability, despite simulated night work.”
To investigate the question, they conducted a parallel-design, randomized clinical trial that included a 14-day circadian laboratory protocol with 19 healthy adults (12 men, 7 women; mean age, 26.5 ± 4.1 years) who underwent a forced desynchrony (FD) in dim light for 4 “days,” each of which consisted of 28 hours. Each 28-hour “day” resulted in an additional 4-hour misalignment between the central circadian clock and external behavioral/environmental cycles.
By the fourth day, the participants were misaligned by 12 hours, compared to baseline (that is, the first day). They were then randomly assigned to two groups.
The DNMC group – the control group – had a “typical 28-hour FD protocol,” with behavioral and environmental cycles (sleep/wake, rest/activity, supine/upright posture, dark during scheduled sleep/dim light during wakefulness) scheduled on a 28-hour cycle. Thus, they took their meals during both “daytime” and “nighttime,” which is the typical way that night workers eat.
The DMI group underwent a modified 28-hour FD protocol, with all cycles scheduled on a 28-hour basis, except for the fasting/eating cycle, which was scheduled on a 24-hour basis, resulting in meals consumed only during the “daytime.”
Depression- and anxiety-like mood (which “correspond to an amalgam of mood states typically observed in depression and anxiety) were assessed every hour during the 4 FD days, using computerized visual analogue scales.
Nutritional psychiatry
Participants in the DNMC group experienced an increase from baseline in depression- and anxiety-like mood levels of 26.2% (95% confidence interval, 21-31.5; P = .001; P value using false discovery rate, .01; effect-size r, 0.78) and 16.1% (95% CI, 8.5-23.6; P = .005; PFDR, .001; effect-size r, 0.47), respectively.
By contrast, a similar increase did not take place in the DMI group for either depression- or anxiety-like mood levels (95% CI, –5.7% to 7.4%, P not significant and 95% CI, –3.1% to 9.9%, P not significant, respectively).
The researchers tested “whether increase mood vulnerability during simulated night work was associated with the degree of internal circadian misalignment” — defined as “change in the phase difference between the acrophase of circadian glucose rhythms and the bathyphase of circadian body temperature rhythms.”
They found that a larger degree of internal circadian misalignment was “robustly associated” with more depression-like (r, 0.77; P = .001) and anxiety-like (r, 0.67; P = .002) mood levels during simulated night work.
The findings imply that meal timing had “moderate to large effects in depression-like and anxiety-like mood levels during night work, and that such effects were associated with the degree of internal circadian misalignment,” the authors wrote.
The laboratory protocol of both groups was identical except for the timing of meals. The authors noted that the “relevance of diet on sleep, circadian rhythms, and mental health is receiving growing awareness with the emergence of a new field, nutritional psychiatry.”
People who experience depression “often report poor-quality diets with high carbohydrate intake,” and there is evidence that adherence to the Mediterranean diet is associated “with lower odds of depression, anxiety, and psychological distress.”
They cautioned that although these emerging studies suggest an association between dietary factors and mental health, “experimental studies in individuals with depression and/or anxiety/anxiety-related disorders are required to determine causality and direction of effects.”
They described meal timing as “an emerging aspect of nutrition, with increasing research interest because of its influence on physical health.” However, they noted, “the causal role of the timing of food intake on mental health remains to be tested.”
Novel findings
Commenting for this article, Kathleen Merikangas, PhD, distinguished investigator and chief, genetic epidemiology research branch, intramural research program, National Institute of Mental Health, Bethesda, Md., described the research as important with novel findings.
The research “employs the elegant, carefully controlled laboratory procedures that have unraveled the influence of light and other environmental cues on sleep and circadian rhythms over the past 2 decades,” said Dr. Merikangas, who was not involved with the study.
“One of the most significant contributions of this work is its demonstration of the importance of investigating circadian rhythms of multiple systems rather than solely focusing on sleep, eating, or emotional states that have often been studied in isolation,” she pointed out.
“Growing evidence from basic research highlights the interdependence of multiple human systems that should be built into interventions that tend to focus on one or two domains.”
She recommended that this work be replicated “in more diverse samples ... in both controlled and naturalistic settings...to test both the generalizability and mechanism of these intriguing findings.”
The study was funded by the National Institutes of Health. Individual investigators were funded by the Alexander Von Humboldt Foundation and the American Diabetes Association. Dr. Chellappa disclosed no relevant financial relationships. Dr. Merikangas disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCE
Urgent need for research into psychedelic therapy for older adults
new research shows.
“Geriatric psychiatrists and others caring for older adults are interested in how much is known about psychedelic use in older adults,” study investigator C. Bree Johnston, MD, MPH, University of Arizona, Tucson, told this news organization.
“A major concern is how safe psychedelic-assisted therapy is for patients with heart disease, hypertension, neurological disorders, and multimorbidity,” Dr. Johnston said.
The study is published online in the American Journal of Geriatric Psychiatry.
‘Groundswell’ of research
The past few years have brought a “groundswell” of interest and promising research into the potential therapeutic benefit of psychedelic-assisted therapy for a variety of conditions affecting adults, the researchers noted.
They include psilocybin-assisted therapy for the distress associated with a terminal diagnosis, depression, and addiction, and MDMA-assisted therapy for PTSD.
However, in most studies, psychedelic therapy has been tested in relatively young healthy adults, raising the question of how generalizable the study results are for the patients that most geropsychiatrists will be treating, the investigators noted.
They reviewed “the most important” research studies on psilocybin- and MDMA-assisted therapies published over the past 2 decades that are likely to be relevant for geriatric psychiatrists and other professionals caring for older adults.
The researchers point out that psychedelics and related compounds have shown efficacy for the treatment of a number of conditions that are common among older adults, including mood disorders, distress associated with a serious medical illness, PTSD, substance use problems, and prolonged grief.
Psychedelics also have properties that may provide for cognitive impairment and dementia and promote personal growth among healthy older adults.
Research has shown that psychedelics can be safely administered to healthy adults in controlled conditions.
However, both psilocybin and MDMA can increase blood pressure and heart rate, which could be a concern if used in older adults with cardiovascular disease, the investigators noted.
“Healthy older adults are likely to face similar risks when undergoing psychedelic-assisted therapy as healthy younger adults,” said Dr. Johnston.
“In carefully selected adults, those risks appear to be minor when psychedelics are administered in controlled conditions under the guidance of a skilled therapist,” she added.
Given the potential of psychedelic compounds to benefit older adults, the authors call for more research to establish the safety and efficacy among older adults, particularly those with multiple comorbidities.
Pressing knowledge gaps
The exclusion of older adults from clinical trials of novel treatments is “one of contemporary psychiatry’s more pressing problems – one that extends beyond psychedelics,” Ipsit V. Vahia, MD, associate chief of the division of geriatric psychiatry, McLean Hospital, Belmont, Mass., who wasn’t involved in the review, told this news organization.
“Currently, there is little evidence that clinicians can lean on while considering the use of psychedelics in older adults,” Dr. Vahia said.
This paper highlights “the most pressing gaps in the evidence that bear addressing in order to develop more substantial best practices around the use of these drugs,” he added.
For example, little is known about appropriate dosing, pharmacokinetics, and pharmacodynamics of psychedelics in older adults, Dr. Vahia said.
“Their risks, particularly cardiovascular risks, are barely studied, and almost nothing is known about how these drugs may impact those in their 80s or older, or those with serious medical comorbidities who use multiple medications,” Dr. Vahia said. “The majority of the existing literature has excluded older adults, and the extremely limited evidence that does exist has been collected in relatively healthy, and relatively young (aged below 75) persons.”
Dr. Vahia noted that, before psychedelics as a class can be considered viable treatment options for a broader group of older adults, “more research is needed, particularly to establish safety.”
This research had no specific funding. Dr. Johnston and Dr. Vahia have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
new research shows.
“Geriatric psychiatrists and others caring for older adults are interested in how much is known about psychedelic use in older adults,” study investigator C. Bree Johnston, MD, MPH, University of Arizona, Tucson, told this news organization.
“A major concern is how safe psychedelic-assisted therapy is for patients with heart disease, hypertension, neurological disorders, and multimorbidity,” Dr. Johnston said.
The study is published online in the American Journal of Geriatric Psychiatry.
‘Groundswell’ of research
The past few years have brought a “groundswell” of interest and promising research into the potential therapeutic benefit of psychedelic-assisted therapy for a variety of conditions affecting adults, the researchers noted.
They include psilocybin-assisted therapy for the distress associated with a terminal diagnosis, depression, and addiction, and MDMA-assisted therapy for PTSD.
However, in most studies, psychedelic therapy has been tested in relatively young healthy adults, raising the question of how generalizable the study results are for the patients that most geropsychiatrists will be treating, the investigators noted.
They reviewed “the most important” research studies on psilocybin- and MDMA-assisted therapies published over the past 2 decades that are likely to be relevant for geriatric psychiatrists and other professionals caring for older adults.
The researchers point out that psychedelics and related compounds have shown efficacy for the treatment of a number of conditions that are common among older adults, including mood disorders, distress associated with a serious medical illness, PTSD, substance use problems, and prolonged grief.
Psychedelics also have properties that may provide for cognitive impairment and dementia and promote personal growth among healthy older adults.
Research has shown that psychedelics can be safely administered to healthy adults in controlled conditions.
However, both psilocybin and MDMA can increase blood pressure and heart rate, which could be a concern if used in older adults with cardiovascular disease, the investigators noted.
“Healthy older adults are likely to face similar risks when undergoing psychedelic-assisted therapy as healthy younger adults,” said Dr. Johnston.
“In carefully selected adults, those risks appear to be minor when psychedelics are administered in controlled conditions under the guidance of a skilled therapist,” she added.
Given the potential of psychedelic compounds to benefit older adults, the authors call for more research to establish the safety and efficacy among older adults, particularly those with multiple comorbidities.
Pressing knowledge gaps
The exclusion of older adults from clinical trials of novel treatments is “one of contemporary psychiatry’s more pressing problems – one that extends beyond psychedelics,” Ipsit V. Vahia, MD, associate chief of the division of geriatric psychiatry, McLean Hospital, Belmont, Mass., who wasn’t involved in the review, told this news organization.
“Currently, there is little evidence that clinicians can lean on while considering the use of psychedelics in older adults,” Dr. Vahia said.
This paper highlights “the most pressing gaps in the evidence that bear addressing in order to develop more substantial best practices around the use of these drugs,” he added.
For example, little is known about appropriate dosing, pharmacokinetics, and pharmacodynamics of psychedelics in older adults, Dr. Vahia said.
“Their risks, particularly cardiovascular risks, are barely studied, and almost nothing is known about how these drugs may impact those in their 80s or older, or those with serious medical comorbidities who use multiple medications,” Dr. Vahia said. “The majority of the existing literature has excluded older adults, and the extremely limited evidence that does exist has been collected in relatively healthy, and relatively young (aged below 75) persons.”
Dr. Vahia noted that, before psychedelics as a class can be considered viable treatment options for a broader group of older adults, “more research is needed, particularly to establish safety.”
This research had no specific funding. Dr. Johnston and Dr. Vahia have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
new research shows.
“Geriatric psychiatrists and others caring for older adults are interested in how much is known about psychedelic use in older adults,” study investigator C. Bree Johnston, MD, MPH, University of Arizona, Tucson, told this news organization.
“A major concern is how safe psychedelic-assisted therapy is for patients with heart disease, hypertension, neurological disorders, and multimorbidity,” Dr. Johnston said.
The study is published online in the American Journal of Geriatric Psychiatry.
‘Groundswell’ of research
The past few years have brought a “groundswell” of interest and promising research into the potential therapeutic benefit of psychedelic-assisted therapy for a variety of conditions affecting adults, the researchers noted.
They include psilocybin-assisted therapy for the distress associated with a terminal diagnosis, depression, and addiction, and MDMA-assisted therapy for PTSD.
However, in most studies, psychedelic therapy has been tested in relatively young healthy adults, raising the question of how generalizable the study results are for the patients that most geropsychiatrists will be treating, the investigators noted.
They reviewed “the most important” research studies on psilocybin- and MDMA-assisted therapies published over the past 2 decades that are likely to be relevant for geriatric psychiatrists and other professionals caring for older adults.
The researchers point out that psychedelics and related compounds have shown efficacy for the treatment of a number of conditions that are common among older adults, including mood disorders, distress associated with a serious medical illness, PTSD, substance use problems, and prolonged grief.
Psychedelics also have properties that may provide for cognitive impairment and dementia and promote personal growth among healthy older adults.
Research has shown that psychedelics can be safely administered to healthy adults in controlled conditions.
However, both psilocybin and MDMA can increase blood pressure and heart rate, which could be a concern if used in older adults with cardiovascular disease, the investigators noted.
“Healthy older adults are likely to face similar risks when undergoing psychedelic-assisted therapy as healthy younger adults,” said Dr. Johnston.
“In carefully selected adults, those risks appear to be minor when psychedelics are administered in controlled conditions under the guidance of a skilled therapist,” she added.
Given the potential of psychedelic compounds to benefit older adults, the authors call for more research to establish the safety and efficacy among older adults, particularly those with multiple comorbidities.
Pressing knowledge gaps
The exclusion of older adults from clinical trials of novel treatments is “one of contemporary psychiatry’s more pressing problems – one that extends beyond psychedelics,” Ipsit V. Vahia, MD, associate chief of the division of geriatric psychiatry, McLean Hospital, Belmont, Mass., who wasn’t involved in the review, told this news organization.
“Currently, there is little evidence that clinicians can lean on while considering the use of psychedelics in older adults,” Dr. Vahia said.
This paper highlights “the most pressing gaps in the evidence that bear addressing in order to develop more substantial best practices around the use of these drugs,” he added.
For example, little is known about appropriate dosing, pharmacokinetics, and pharmacodynamics of psychedelics in older adults, Dr. Vahia said.
“Their risks, particularly cardiovascular risks, are barely studied, and almost nothing is known about how these drugs may impact those in their 80s or older, or those with serious medical comorbidities who use multiple medications,” Dr. Vahia said. “The majority of the existing literature has excluded older adults, and the extremely limited evidence that does exist has been collected in relatively healthy, and relatively young (aged below 75) persons.”
Dr. Vahia noted that, before psychedelics as a class can be considered viable treatment options for a broader group of older adults, “more research is needed, particularly to establish safety.”
This research had no specific funding. Dr. Johnston and Dr. Vahia have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GERIATRIC PSYCHIATRY
CRP levels could predict SSRI success
C-reactive protein (CRP) has been shown to predict antidepressant treatment outcomes in depressed patients, but previous studies have been small and under restricted conditions, and data from large, real-world studies are lacking, wrote Yuqian Pan of First Affiliated Hospital of Zhengzhou University, Henan, China, and colleagues.
In a study published in the Journal of Affective Disorders , the researchers identified depressed patients aged 12-60 years who had tested CRP levels. The participants were followed through outpatient visits or telephone interviews to collect information on medication use and assess efficacy based on the Clinical Global Impressions–Improvement scale.
CRP was separated into the low CRP group of 709 patients (CRP < 1 mg/L) and a high CRP group of 209 patients (CRP ≥ 1 mg/L). The primary outcome was efficacy defined as effective and ineffective for high and low CRP levels in patients using different medications: Selected serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors, (SNRIs), melatonin receptor agonists (MTs), and norepinephrinergic and specific serotonergic antidepressants (NaSSAs).
The researchers compared efficacy in different groups according to CRP levels.
Overall, patients with low CRP showed significantly greater efficacy with SSRIs than did those with high CRP (hazard ratio [HR], 1.257, P = .047). SNRIs were more effective than SSRIs for treating patients with high CRP levels (HR, 1.652, P = .037).
A possible reason for the difference in efficacy is the correlation between CRP and body mass index; previous studies have shown that SSRIs may be less effective in obese individuals, the researchers said.
“Another possible explanation is that at high levels of inflammation, neurons, microglia, and macrophages respond to inflammatory challenges at the cellular level by activating metabolic pathways,” they said.
No significant changes in CRP levels were observed before and after starting medication use, which supports the stability of CRP as a biomarker under normal circumstances.
No difference in efficacy appeared between SSRIs and SNRIs in patients with low CRP, “which may indicate that SNRIs have stronger anti-inflammatory effects than SSRIs,” a finding consistent with previous studies, they said.
The study findings were limited by several factors including the small number of patients taking MT and NaSSA, the irregular time intervals for before and after SSRI treatment in 90 patients, the lack of classification by antidepressant type, and the potential for recall bias, the researchers noted.
However, the results suggest that CRP could predict the efficacy of SSRIs in depressed patients in a real-world setting, which may inform treatment decisions, they said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
C-reactive protein (CRP) has been shown to predict antidepressant treatment outcomes in depressed patients, but previous studies have been small and under restricted conditions, and data from large, real-world studies are lacking, wrote Yuqian Pan of First Affiliated Hospital of Zhengzhou University, Henan, China, and colleagues.
In a study published in the Journal of Affective Disorders , the researchers identified depressed patients aged 12-60 years who had tested CRP levels. The participants were followed through outpatient visits or telephone interviews to collect information on medication use and assess efficacy based on the Clinical Global Impressions–Improvement scale.
CRP was separated into the low CRP group of 709 patients (CRP < 1 mg/L) and a high CRP group of 209 patients (CRP ≥ 1 mg/L). The primary outcome was efficacy defined as effective and ineffective for high and low CRP levels in patients using different medications: Selected serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors, (SNRIs), melatonin receptor agonists (MTs), and norepinephrinergic and specific serotonergic antidepressants (NaSSAs).
The researchers compared efficacy in different groups according to CRP levels.
Overall, patients with low CRP showed significantly greater efficacy with SSRIs than did those with high CRP (hazard ratio [HR], 1.257, P = .047). SNRIs were more effective than SSRIs for treating patients with high CRP levels (HR, 1.652, P = .037).
A possible reason for the difference in efficacy is the correlation between CRP and body mass index; previous studies have shown that SSRIs may be less effective in obese individuals, the researchers said.
“Another possible explanation is that at high levels of inflammation, neurons, microglia, and macrophages respond to inflammatory challenges at the cellular level by activating metabolic pathways,” they said.
No significant changes in CRP levels were observed before and after starting medication use, which supports the stability of CRP as a biomarker under normal circumstances.
No difference in efficacy appeared between SSRIs and SNRIs in patients with low CRP, “which may indicate that SNRIs have stronger anti-inflammatory effects than SSRIs,” a finding consistent with previous studies, they said.
The study findings were limited by several factors including the small number of patients taking MT and NaSSA, the irregular time intervals for before and after SSRI treatment in 90 patients, the lack of classification by antidepressant type, and the potential for recall bias, the researchers noted.
However, the results suggest that CRP could predict the efficacy of SSRIs in depressed patients in a real-world setting, which may inform treatment decisions, they said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
C-reactive protein (CRP) has been shown to predict antidepressant treatment outcomes in depressed patients, but previous studies have been small and under restricted conditions, and data from large, real-world studies are lacking, wrote Yuqian Pan of First Affiliated Hospital of Zhengzhou University, Henan, China, and colleagues.
In a study published in the Journal of Affective Disorders , the researchers identified depressed patients aged 12-60 years who had tested CRP levels. The participants were followed through outpatient visits or telephone interviews to collect information on medication use and assess efficacy based on the Clinical Global Impressions–Improvement scale.
CRP was separated into the low CRP group of 709 patients (CRP < 1 mg/L) and a high CRP group of 209 patients (CRP ≥ 1 mg/L). The primary outcome was efficacy defined as effective and ineffective for high and low CRP levels in patients using different medications: Selected serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors, (SNRIs), melatonin receptor agonists (MTs), and norepinephrinergic and specific serotonergic antidepressants (NaSSAs).
The researchers compared efficacy in different groups according to CRP levels.
Overall, patients with low CRP showed significantly greater efficacy with SSRIs than did those with high CRP (hazard ratio [HR], 1.257, P = .047). SNRIs were more effective than SSRIs for treating patients with high CRP levels (HR, 1.652, P = .037).
A possible reason for the difference in efficacy is the correlation between CRP and body mass index; previous studies have shown that SSRIs may be less effective in obese individuals, the researchers said.
“Another possible explanation is that at high levels of inflammation, neurons, microglia, and macrophages respond to inflammatory challenges at the cellular level by activating metabolic pathways,” they said.
No significant changes in CRP levels were observed before and after starting medication use, which supports the stability of CRP as a biomarker under normal circumstances.
No difference in efficacy appeared between SSRIs and SNRIs in patients with low CRP, “which may indicate that SNRIs have stronger anti-inflammatory effects than SSRIs,” a finding consistent with previous studies, they said.
The study findings were limited by several factors including the small number of patients taking MT and NaSSA, the irregular time intervals for before and after SSRI treatment in 90 patients, the lack of classification by antidepressant type, and the potential for recall bias, the researchers noted.
However, the results suggest that CRP could predict the efficacy of SSRIs in depressed patients in a real-world setting, which may inform treatment decisions, they said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
A ‘setback’ for anti-inflammatory treatment in depression
In the MinoTRD trial, 6 weeks of minocycline 200 mg daily added to stable antidepressant therapy did not show a statistically significant advantage over placebo on the overall course of depressive symptoms.
“The failure of minocycline treatment to reduce depressive symptoms in a naturalistic sample of patients with TRD is a setback for anti-inflammatory treatment strategies in this clinical population,” Julian Hellmann-Regen, MD, department of psychiatry and neurosciences, Charité – Universitätsmedizin Berlin, and colleagues wrote.
The findings were published online in JAMA Network Open.
No additional benefit
The “inflammatory hypothesis” of depression maintains that depression arises from increased immune activation and neurotrophic mechanisms.
This view is supported by observations that depression is accompanied by increased levels of proinflammatory cytokines. In addition, low-grade inflammatory processes may interfere with response to typical antidepressant medications.
Minocycline has been put forth as a novel antidepressant, and a few small trials have hinted at a benefit.
In the MinoTRD trial, 168 patients (mean age, 46 years; 53% men) with TRD were randomly allocated minocycline 200 mg/day or matching placebo in addition to usual antidepressant treatment for 6 weeks.
Results showed minocycline was well tolerated but was not superior to placebo in reducing depressive symptoms, the researchers report.
Overall, the mean Montgomery-Åsberg Depression Rating Scale (MADRS) score at baseline was 26.5. There was no significant between-group difference in mean change in MADRS score from baseline to 6 weeks, the primary outcome.
After 6 weeks of treatment, the mean reduction in MADRS score was 8.46 points in the minocycline group versus 8.01 points in the placebo group, and the difference of 1.46 points was not significant (P = .25).
Six weeks of minocycline treatment did not alter the course of depression severity, compared with placebo, the investigators noted.
Minocycline treatment also showed no statistically significant effect on secondary outcomes of response, remission, and various other clinical rating scales.
Caveats and cautionary notes
The researchers noted that one explanation for the null result of the MinoTRD trial could be that the 6-week treatment duration was not long enough to reveal detectable differences. They point to a small study that showed a strong effect of minocycline, compared with placebo by and after 8 weeks of treatment.
However, a closer look at that study suggests the overall effect in the minocycline group was caused by an almost-complete lack of improvement in the placebo group, “which is very unusual,” the investigators wrote.
In the MinoTRD trial, the magnitude of improvement for placebo was as expected from similar trials in TRD, they pointed out.
They also noted that, unlike some other trials, the MinoTRD trial purposely recruited a naturalistic population of TRD, assuming (but not confirming) elevated baseline inflammation as a potential underlying cause in at least a subgroup of the patients.
Post hoc stratification by baseline CRP levels in MinoTRD participants did not yield any results supporting the hypothesis of minocycline treatment possibly being more effective in participants with higher-grade baseline inflammation, the researchers reported.
“Our results from this large randomized controlled trial of a pleiotropic anti-inflammatory drug in this difficult-to-treat patient population are of great clinical importance, robustly demonstrating that minocycline add-on treatment does not outperform placebo, not even in those participants with elevated levels of CRP prior to treatment initiation,” they wrote.
The trial was funded by a grant from the German Federal Ministry of Education and Research within the Consortium Optimizing Treatment of Depression, as part of the Research Network for Psychiatric Disorders. Dr. Hellmann-Regen reported no relevant financial relationship.
A version of this article first appeared on Medscape.com.
In the MinoTRD trial, 6 weeks of minocycline 200 mg daily added to stable antidepressant therapy did not show a statistically significant advantage over placebo on the overall course of depressive symptoms.
“The failure of minocycline treatment to reduce depressive symptoms in a naturalistic sample of patients with TRD is a setback for anti-inflammatory treatment strategies in this clinical population,” Julian Hellmann-Regen, MD, department of psychiatry and neurosciences, Charité – Universitätsmedizin Berlin, and colleagues wrote.
The findings were published online in JAMA Network Open.
No additional benefit
The “inflammatory hypothesis” of depression maintains that depression arises from increased immune activation and neurotrophic mechanisms.
This view is supported by observations that depression is accompanied by increased levels of proinflammatory cytokines. In addition, low-grade inflammatory processes may interfere with response to typical antidepressant medications.
Minocycline has been put forth as a novel antidepressant, and a few small trials have hinted at a benefit.
In the MinoTRD trial, 168 patients (mean age, 46 years; 53% men) with TRD were randomly allocated minocycline 200 mg/day or matching placebo in addition to usual antidepressant treatment for 6 weeks.
Results showed minocycline was well tolerated but was not superior to placebo in reducing depressive symptoms, the researchers report.
Overall, the mean Montgomery-Åsberg Depression Rating Scale (MADRS) score at baseline was 26.5. There was no significant between-group difference in mean change in MADRS score from baseline to 6 weeks, the primary outcome.
After 6 weeks of treatment, the mean reduction in MADRS score was 8.46 points in the minocycline group versus 8.01 points in the placebo group, and the difference of 1.46 points was not significant (P = .25).
Six weeks of minocycline treatment did not alter the course of depression severity, compared with placebo, the investigators noted.
Minocycline treatment also showed no statistically significant effect on secondary outcomes of response, remission, and various other clinical rating scales.
Caveats and cautionary notes
The researchers noted that one explanation for the null result of the MinoTRD trial could be that the 6-week treatment duration was not long enough to reveal detectable differences. They point to a small study that showed a strong effect of minocycline, compared with placebo by and after 8 weeks of treatment.
However, a closer look at that study suggests the overall effect in the minocycline group was caused by an almost-complete lack of improvement in the placebo group, “which is very unusual,” the investigators wrote.
In the MinoTRD trial, the magnitude of improvement for placebo was as expected from similar trials in TRD, they pointed out.
They also noted that, unlike some other trials, the MinoTRD trial purposely recruited a naturalistic population of TRD, assuming (but not confirming) elevated baseline inflammation as a potential underlying cause in at least a subgroup of the patients.
Post hoc stratification by baseline CRP levels in MinoTRD participants did not yield any results supporting the hypothesis of minocycline treatment possibly being more effective in participants with higher-grade baseline inflammation, the researchers reported.
“Our results from this large randomized controlled trial of a pleiotropic anti-inflammatory drug in this difficult-to-treat patient population are of great clinical importance, robustly demonstrating that minocycline add-on treatment does not outperform placebo, not even in those participants with elevated levels of CRP prior to treatment initiation,” they wrote.
The trial was funded by a grant from the German Federal Ministry of Education and Research within the Consortium Optimizing Treatment of Depression, as part of the Research Network for Psychiatric Disorders. Dr. Hellmann-Regen reported no relevant financial relationship.
A version of this article first appeared on Medscape.com.
In the MinoTRD trial, 6 weeks of minocycline 200 mg daily added to stable antidepressant therapy did not show a statistically significant advantage over placebo on the overall course of depressive symptoms.
“The failure of minocycline treatment to reduce depressive symptoms in a naturalistic sample of patients with TRD is a setback for anti-inflammatory treatment strategies in this clinical population,” Julian Hellmann-Regen, MD, department of psychiatry and neurosciences, Charité – Universitätsmedizin Berlin, and colleagues wrote.
The findings were published online in JAMA Network Open.
No additional benefit
The “inflammatory hypothesis” of depression maintains that depression arises from increased immune activation and neurotrophic mechanisms.
This view is supported by observations that depression is accompanied by increased levels of proinflammatory cytokines. In addition, low-grade inflammatory processes may interfere with response to typical antidepressant medications.
Minocycline has been put forth as a novel antidepressant, and a few small trials have hinted at a benefit.
In the MinoTRD trial, 168 patients (mean age, 46 years; 53% men) with TRD were randomly allocated minocycline 200 mg/day or matching placebo in addition to usual antidepressant treatment for 6 weeks.
Results showed minocycline was well tolerated but was not superior to placebo in reducing depressive symptoms, the researchers report.
Overall, the mean Montgomery-Åsberg Depression Rating Scale (MADRS) score at baseline was 26.5. There was no significant between-group difference in mean change in MADRS score from baseline to 6 weeks, the primary outcome.
After 6 weeks of treatment, the mean reduction in MADRS score was 8.46 points in the minocycline group versus 8.01 points in the placebo group, and the difference of 1.46 points was not significant (P = .25).
Six weeks of minocycline treatment did not alter the course of depression severity, compared with placebo, the investigators noted.
Minocycline treatment also showed no statistically significant effect on secondary outcomes of response, remission, and various other clinical rating scales.
Caveats and cautionary notes
The researchers noted that one explanation for the null result of the MinoTRD trial could be that the 6-week treatment duration was not long enough to reveal detectable differences. They point to a small study that showed a strong effect of minocycline, compared with placebo by and after 8 weeks of treatment.
However, a closer look at that study suggests the overall effect in the minocycline group was caused by an almost-complete lack of improvement in the placebo group, “which is very unusual,” the investigators wrote.
In the MinoTRD trial, the magnitude of improvement for placebo was as expected from similar trials in TRD, they pointed out.
They also noted that, unlike some other trials, the MinoTRD trial purposely recruited a naturalistic population of TRD, assuming (but not confirming) elevated baseline inflammation as a potential underlying cause in at least a subgroup of the patients.
Post hoc stratification by baseline CRP levels in MinoTRD participants did not yield any results supporting the hypothesis of minocycline treatment possibly being more effective in participants with higher-grade baseline inflammation, the researchers reported.
“Our results from this large randomized controlled trial of a pleiotropic anti-inflammatory drug in this difficult-to-treat patient population are of great clinical importance, robustly demonstrating that minocycline add-on treatment does not outperform placebo, not even in those participants with elevated levels of CRP prior to treatment initiation,” they wrote.
The trial was funded by a grant from the German Federal Ministry of Education and Research within the Consortium Optimizing Treatment of Depression, as part of the Research Network for Psychiatric Disorders. Dr. Hellmann-Regen reported no relevant financial relationship.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Eighty percent of U.S. maternal deaths are preventable: Study
More than 80% of U.S. maternal deaths across a 2-year period were due to preventable causes, according to a new CDC report.
Black mothers made up about a third of deaths, and more than 90% of deaths among Indigenous mothers were preventable.
“It’s significant. It’s staggering. It’s heartbreaking,” Allison Bryant, MD, a high-risk pregnancy specialist and senior medical director for health equity at Massachusetts General Hospital, told USA Today.
“It just means that we have so much work to do,” she said.
In the report, CDC researchers looked at pregnancy-related deaths between 2017 to 2019 based on numbers from maternal mortality review committees, which are multidisciplinary groups in 36 states that investigate the circumstances around maternal deaths.
Of the 1,018 deaths during the 2-year period, 839 occurred up to a year after delivery. About 22% of deaths happened during pregnancy, and 25% happened on the day of delivery or within a week after delivery. But 53% occurred more than 7 days after delivery.
Mental health conditions, such as overdoses and deaths by suicide, were the top underlying cause, followed by hemorrhage, or extreme bleeding. About a quarter of deaths were due to mental health conditions, followed by 14% due to hemorrhage and 13% due to heart problems. The rest were related to infection, embolism, cardiomyopathy, and high blood pressure-related disorders.
The analysis included a section on maternal deaths for American Indian and Alaska Native mothers, who are more than twice as likely as White mothers to die but are often undercounted in health data due to misclassification. More than 90% of their deaths were preventable between 2017 to 2019, with most due to mental health conditions and hemorrhage.
“It’s incredibly distressful,” Brian Thompson, MD, of the Oneida Nation and assistant professor of obstetrics and gynecology at Upstate Medical University, New York, told USA Today.
Dr. Thompson is working with the National Indian Health Board to create the first national tribal review committee for maternal deaths.
“It really needs to be looked at and examined why that is the case if essentially all of them are preventable,” he said.
Black mothers were also three times as likely as White mothers to die and more likely to die from heart problems. Hispanic mothers, who made up 14% of deaths, were more likely to die from mental health conditions.
Some of the deaths, such as hemorrhage, should be highly preventable. Existing toolkits for clinicians provide evidence-based guidelines to prevent and treat excessive bleeding.
“No pregnant person should be passing away from a hemorrhage,” Andrea Jackson, MD, division chief of obstetrics and gynecology at the University of California, San Francisco, told USA Today.
“We have the tools in the United States, and we know how to deal with it,” she said. “That was really disheartening to see.”
What’s more, the new CDC report highlights the need for more mental health resources during pregnancy and the postpartum period – up to a year or more after delivery – including improvements in access to care, diagnosis, and treatment.
“These are things that need to happen systemically,” LeThenia Baker, MD, an obstetrician and gynecologist at Wellstar Health, Georgia, told USA Today.
“It can’t just be a few practices here or there who are adopting best practices,” she said. “It has to be a systemic change.”
A version of this article first appeared on WebMD.com.
More than 80% of U.S. maternal deaths across a 2-year period were due to preventable causes, according to a new CDC report.
Black mothers made up about a third of deaths, and more than 90% of deaths among Indigenous mothers were preventable.
“It’s significant. It’s staggering. It’s heartbreaking,” Allison Bryant, MD, a high-risk pregnancy specialist and senior medical director for health equity at Massachusetts General Hospital, told USA Today.
“It just means that we have so much work to do,” she said.
In the report, CDC researchers looked at pregnancy-related deaths between 2017 to 2019 based on numbers from maternal mortality review committees, which are multidisciplinary groups in 36 states that investigate the circumstances around maternal deaths.
Of the 1,018 deaths during the 2-year period, 839 occurred up to a year after delivery. About 22% of deaths happened during pregnancy, and 25% happened on the day of delivery or within a week after delivery. But 53% occurred more than 7 days after delivery.
Mental health conditions, such as overdoses and deaths by suicide, were the top underlying cause, followed by hemorrhage, or extreme bleeding. About a quarter of deaths were due to mental health conditions, followed by 14% due to hemorrhage and 13% due to heart problems. The rest were related to infection, embolism, cardiomyopathy, and high blood pressure-related disorders.
The analysis included a section on maternal deaths for American Indian and Alaska Native mothers, who are more than twice as likely as White mothers to die but are often undercounted in health data due to misclassification. More than 90% of their deaths were preventable between 2017 to 2019, with most due to mental health conditions and hemorrhage.
“It’s incredibly distressful,” Brian Thompson, MD, of the Oneida Nation and assistant professor of obstetrics and gynecology at Upstate Medical University, New York, told USA Today.
Dr. Thompson is working with the National Indian Health Board to create the first national tribal review committee for maternal deaths.
“It really needs to be looked at and examined why that is the case if essentially all of them are preventable,” he said.
Black mothers were also three times as likely as White mothers to die and more likely to die from heart problems. Hispanic mothers, who made up 14% of deaths, were more likely to die from mental health conditions.
Some of the deaths, such as hemorrhage, should be highly preventable. Existing toolkits for clinicians provide evidence-based guidelines to prevent and treat excessive bleeding.
“No pregnant person should be passing away from a hemorrhage,” Andrea Jackson, MD, division chief of obstetrics and gynecology at the University of California, San Francisco, told USA Today.
“We have the tools in the United States, and we know how to deal with it,” she said. “That was really disheartening to see.”
What’s more, the new CDC report highlights the need for more mental health resources during pregnancy and the postpartum period – up to a year or more after delivery – including improvements in access to care, diagnosis, and treatment.
“These are things that need to happen systemically,” LeThenia Baker, MD, an obstetrician and gynecologist at Wellstar Health, Georgia, told USA Today.
“It can’t just be a few practices here or there who are adopting best practices,” she said. “It has to be a systemic change.”
A version of this article first appeared on WebMD.com.
More than 80% of U.S. maternal deaths across a 2-year period were due to preventable causes, according to a new CDC report.
Black mothers made up about a third of deaths, and more than 90% of deaths among Indigenous mothers were preventable.
“It’s significant. It’s staggering. It’s heartbreaking,” Allison Bryant, MD, a high-risk pregnancy specialist and senior medical director for health equity at Massachusetts General Hospital, told USA Today.
“It just means that we have so much work to do,” she said.
In the report, CDC researchers looked at pregnancy-related deaths between 2017 to 2019 based on numbers from maternal mortality review committees, which are multidisciplinary groups in 36 states that investigate the circumstances around maternal deaths.
Of the 1,018 deaths during the 2-year period, 839 occurred up to a year after delivery. About 22% of deaths happened during pregnancy, and 25% happened on the day of delivery or within a week after delivery. But 53% occurred more than 7 days after delivery.
Mental health conditions, such as overdoses and deaths by suicide, were the top underlying cause, followed by hemorrhage, or extreme bleeding. About a quarter of deaths were due to mental health conditions, followed by 14% due to hemorrhage and 13% due to heart problems. The rest were related to infection, embolism, cardiomyopathy, and high blood pressure-related disorders.
The analysis included a section on maternal deaths for American Indian and Alaska Native mothers, who are more than twice as likely as White mothers to die but are often undercounted in health data due to misclassification. More than 90% of their deaths were preventable between 2017 to 2019, with most due to mental health conditions and hemorrhage.
“It’s incredibly distressful,” Brian Thompson, MD, of the Oneida Nation and assistant professor of obstetrics and gynecology at Upstate Medical University, New York, told USA Today.
Dr. Thompson is working with the National Indian Health Board to create the first national tribal review committee for maternal deaths.
“It really needs to be looked at and examined why that is the case if essentially all of them are preventable,” he said.
Black mothers were also three times as likely as White mothers to die and more likely to die from heart problems. Hispanic mothers, who made up 14% of deaths, were more likely to die from mental health conditions.
Some of the deaths, such as hemorrhage, should be highly preventable. Existing toolkits for clinicians provide evidence-based guidelines to prevent and treat excessive bleeding.
“No pregnant person should be passing away from a hemorrhage,” Andrea Jackson, MD, division chief of obstetrics and gynecology at the University of California, San Francisco, told USA Today.
“We have the tools in the United States, and we know how to deal with it,” she said. “That was really disheartening to see.”
What’s more, the new CDC report highlights the need for more mental health resources during pregnancy and the postpartum period – up to a year or more after delivery – including improvements in access to care, diagnosis, and treatment.
“These are things that need to happen systemically,” LeThenia Baker, MD, an obstetrician and gynecologist at Wellstar Health, Georgia, told USA Today.
“It can’t just be a few practices here or there who are adopting best practices,” she said. “It has to be a systemic change.”
A version of this article first appeared on WebMD.com.
Ketamine linked to reduced suicidal thoughts, depression, anxiety
, new research suggests.
Results from a retrospective chart review analysis, which included more than 400 participants with TRD, illustrate that ketamine is a safe and rapid treatment in a real-world patient population, lead author Patrick A. Oliver, MD, founder and medical director, MindPeace Clinics, Richmond, Va., told this news organization.
The effect was perhaps most notable for reducing suicidal ideation, he said.
“In 2 weeks, we can take somebody from being suicidal to nonsuicidal. It’s a total game changer,” Dr. Oliver added.
Every year in the United States, about 12 million individuals think about suicide, 3.2 million make a plan to kill themselves, and more than 46,000 succeed, the investigators note.
The findings were published online in the Journal of Clinical Psychiatry.
Molecule mixture
Primarily used as an anesthetic in hospitals, ketamine is also taken illegally as a recreational drug. Users may aim for an intense high or feeling of dissociation, or an out-of-body–type experience.
Ketamine is a mixture of two mirror-image molecules. An intranasal version of one of these molecules (esketamine) is approved by the U.S. Food and Drug Administration for TRD. Both esketamine and ketamine are believed to increase neurotrophic signaling that affects synaptic function.
The study included 424 patients (mean age, 41.7 years) with major depressive disorder or another mood disorder and who received at least one ketamine infusion at a specialty clinic. Most participants had failed prior medication trials.
Patients in the study were typically started on 0.5 mg/kg of ketamine, with the dose titrated to achieve symptoms of partial dissociation. The median dose administered after titration was 0.93 mg/kg over 40 minutes.
The main treatment course of at least six infusions within 21 days was completed by 70% of the patients.
At each clinic visit, all participants completed the Patient Health Questionnaire-9 (PHQ-9) and the Generalized Anxiety Disorder-7 (GAD-7).
The primary outcome was PHQ-9 total scores, for which researchers looked at seven time periods: 1 week, 2-3 weeks, 4-6 weeks, 7-12 weeks, 13-24 weeks, 25-51 weeks, and 52+ weeks.
‘Blows it out of the water’
Results showed PHQ-9 total scores declined by 50% throughout the course of treatment, with much of the improvement gained within 4-6 weeks. There was a significant difference between week 1 and all later time periods (all P values < .001) and between weeks 2 and 3 and all later periods (all P values < .001).
Other measures included treatment response, defined as at least a 50% improvement on the PHQ-9, and depression remission, defined as a PHQ-9 score of less than 5. After three infusions, 14% of the patients responded and 7% were in remission. After 10 infusions, 72% responded and 38% were in remission.
These results compare favorably to other depression treatments, said Dr. Oliver. “Truthfully, with the exception of ECT [electroconvulsive therapy], this blows it all out of the water,” he added.
Dr. Oliver noted that the success rate for repetitive transcranial magnetic stimulation is 40%-60% depending on the modality; and for selective serotonin reuptake inhibitors, the success rate “is somewhere between the mid-20s and low-30s percent range.”
Another outcome measure was the self-harm/suicidal ideation item of the PHQ-9 questionnaire, which asks about “thoughts that you would be better off dead, or of hurting yourself in some way.” About 22% of the study participants no longer reported suicidal ideation after 3 infusions, 50% by 6 infusions, and 75% by 10 infusions.
By 15 infusions, 85% no longer reported these thoughts. “Nothing else has shown that, ever,” said Dr. Oliver.
Symptoms of generalized anxiety were also substantially improved. There was about a 30% reduction in the GAD-7 score during treatment and, again, most of the response occurred by 4-6 weeks.
Study limitations
Sex, age, and other demographic characteristics did not predict response or remission, but suicide planning trended toward higher response rates (P = .083). This suggests that a more depressed subgroup can achieve greater benefit from the treatment than can less symptomatic patients, the investigators note.
A history of psychosis also trended toward better response to treatment (P = .086) but not remission.
The researchers note that study limitations include that it was retrospective, lacked a control group, and did not require patients to be hospitalized – so the study sample may have been less severely ill than in other studies.
In addition, most patients paid out of pocket for the treatment at $495 per infusion, and they self-reported their symptoms.
As well, the researchers did not assess adverse events, although nurses made follow-up calls to patients. Dr. Oliver noted the most common side effects of ketamine are nausea, vomiting, and anxiety.
Previous research has suggested that ketamine therapy is not linked to long-term side effects, such as sexual dysfunction, weight gain, lethargy, or cognitive issues, said Dr. Oliver.
The investigators point out another study limitation was lack of detailed demographic information, such as race, income, and education, which might affect its generalizability.
Concerns and questions
Pouya Movahed Rad, MD, PhD, senior consultant and researcher in psychiatry, Lund (Sweden) University, noted several concerns, including that the clinics treating the study participants with ketamine profited from it.
He also speculated about who can afford the treatment because only a few patients in the study were reimbursed through insurance.
Dr. Movahed Rad was not involved with the current research but was principal investigator for a recent study that compared intravenous ketamine to ECT.
He questioned whether the patient population in the new study really was “real world.” Well-designed randomized controlled trials have been carried out in a “naturalistic setting, [which] get closer to real-life patients,” he said.
He also noted that the median dose after clinician titration (0.93 mg/kg over 40 minutes) “may be considered very high.”
With regard to doses being titrated to achieve symptoms of partial dissociation, “there is no obvious evidence to my knowledge that patients need to develop dissociative symptoms in order to have antidepressant effect,” said Dr. Movahed Rad.
Finally, he noted that the finding that 28% of the participants were using illegal drugs “is worrying” and wondered what drugs they were taking; he also questioned why 81% of the study population needed to take antidepressants.
The study did not receive outside funding. Dr. Oliver is the founder of MindPeace Clinics, which specialize in ketamine therapeutics. Dr. Movahed Rad has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a retrospective chart review analysis, which included more than 400 participants with TRD, illustrate that ketamine is a safe and rapid treatment in a real-world patient population, lead author Patrick A. Oliver, MD, founder and medical director, MindPeace Clinics, Richmond, Va., told this news organization.
The effect was perhaps most notable for reducing suicidal ideation, he said.
“In 2 weeks, we can take somebody from being suicidal to nonsuicidal. It’s a total game changer,” Dr. Oliver added.
Every year in the United States, about 12 million individuals think about suicide, 3.2 million make a plan to kill themselves, and more than 46,000 succeed, the investigators note.
The findings were published online in the Journal of Clinical Psychiatry.
Molecule mixture
Primarily used as an anesthetic in hospitals, ketamine is also taken illegally as a recreational drug. Users may aim for an intense high or feeling of dissociation, or an out-of-body–type experience.
Ketamine is a mixture of two mirror-image molecules. An intranasal version of one of these molecules (esketamine) is approved by the U.S. Food and Drug Administration for TRD. Both esketamine and ketamine are believed to increase neurotrophic signaling that affects synaptic function.
The study included 424 patients (mean age, 41.7 years) with major depressive disorder or another mood disorder and who received at least one ketamine infusion at a specialty clinic. Most participants had failed prior medication trials.
Patients in the study were typically started on 0.5 mg/kg of ketamine, with the dose titrated to achieve symptoms of partial dissociation. The median dose administered after titration was 0.93 mg/kg over 40 minutes.
The main treatment course of at least six infusions within 21 days was completed by 70% of the patients.
At each clinic visit, all participants completed the Patient Health Questionnaire-9 (PHQ-9) and the Generalized Anxiety Disorder-7 (GAD-7).
The primary outcome was PHQ-9 total scores, for which researchers looked at seven time periods: 1 week, 2-3 weeks, 4-6 weeks, 7-12 weeks, 13-24 weeks, 25-51 weeks, and 52+ weeks.
‘Blows it out of the water’
Results showed PHQ-9 total scores declined by 50% throughout the course of treatment, with much of the improvement gained within 4-6 weeks. There was a significant difference between week 1 and all later time periods (all P values < .001) and between weeks 2 and 3 and all later periods (all P values < .001).
Other measures included treatment response, defined as at least a 50% improvement on the PHQ-9, and depression remission, defined as a PHQ-9 score of less than 5. After three infusions, 14% of the patients responded and 7% were in remission. After 10 infusions, 72% responded and 38% were in remission.
These results compare favorably to other depression treatments, said Dr. Oliver. “Truthfully, with the exception of ECT [electroconvulsive therapy], this blows it all out of the water,” he added.
Dr. Oliver noted that the success rate for repetitive transcranial magnetic stimulation is 40%-60% depending on the modality; and for selective serotonin reuptake inhibitors, the success rate “is somewhere between the mid-20s and low-30s percent range.”
Another outcome measure was the self-harm/suicidal ideation item of the PHQ-9 questionnaire, which asks about “thoughts that you would be better off dead, or of hurting yourself in some way.” About 22% of the study participants no longer reported suicidal ideation after 3 infusions, 50% by 6 infusions, and 75% by 10 infusions.
By 15 infusions, 85% no longer reported these thoughts. “Nothing else has shown that, ever,” said Dr. Oliver.
Symptoms of generalized anxiety were also substantially improved. There was about a 30% reduction in the GAD-7 score during treatment and, again, most of the response occurred by 4-6 weeks.
Study limitations
Sex, age, and other demographic characteristics did not predict response or remission, but suicide planning trended toward higher response rates (P = .083). This suggests that a more depressed subgroup can achieve greater benefit from the treatment than can less symptomatic patients, the investigators note.
A history of psychosis also trended toward better response to treatment (P = .086) but not remission.
The researchers note that study limitations include that it was retrospective, lacked a control group, and did not require patients to be hospitalized – so the study sample may have been less severely ill than in other studies.
In addition, most patients paid out of pocket for the treatment at $495 per infusion, and they self-reported their symptoms.
As well, the researchers did not assess adverse events, although nurses made follow-up calls to patients. Dr. Oliver noted the most common side effects of ketamine are nausea, vomiting, and anxiety.
Previous research has suggested that ketamine therapy is not linked to long-term side effects, such as sexual dysfunction, weight gain, lethargy, or cognitive issues, said Dr. Oliver.
The investigators point out another study limitation was lack of detailed demographic information, such as race, income, and education, which might affect its generalizability.
Concerns and questions
Pouya Movahed Rad, MD, PhD, senior consultant and researcher in psychiatry, Lund (Sweden) University, noted several concerns, including that the clinics treating the study participants with ketamine profited from it.
He also speculated about who can afford the treatment because only a few patients in the study were reimbursed through insurance.
Dr. Movahed Rad was not involved with the current research but was principal investigator for a recent study that compared intravenous ketamine to ECT.
He questioned whether the patient population in the new study really was “real world.” Well-designed randomized controlled trials have been carried out in a “naturalistic setting, [which] get closer to real-life patients,” he said.
He also noted that the median dose after clinician titration (0.93 mg/kg over 40 minutes) “may be considered very high.”
With regard to doses being titrated to achieve symptoms of partial dissociation, “there is no obvious evidence to my knowledge that patients need to develop dissociative symptoms in order to have antidepressant effect,” said Dr. Movahed Rad.
Finally, he noted that the finding that 28% of the participants were using illegal drugs “is worrying” and wondered what drugs they were taking; he also questioned why 81% of the study population needed to take antidepressants.
The study did not receive outside funding. Dr. Oliver is the founder of MindPeace Clinics, which specialize in ketamine therapeutics. Dr. Movahed Rad has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a retrospective chart review analysis, which included more than 400 participants with TRD, illustrate that ketamine is a safe and rapid treatment in a real-world patient population, lead author Patrick A. Oliver, MD, founder and medical director, MindPeace Clinics, Richmond, Va., told this news organization.
The effect was perhaps most notable for reducing suicidal ideation, he said.
“In 2 weeks, we can take somebody from being suicidal to nonsuicidal. It’s a total game changer,” Dr. Oliver added.
Every year in the United States, about 12 million individuals think about suicide, 3.2 million make a plan to kill themselves, and more than 46,000 succeed, the investigators note.
The findings were published online in the Journal of Clinical Psychiatry.
Molecule mixture
Primarily used as an anesthetic in hospitals, ketamine is also taken illegally as a recreational drug. Users may aim for an intense high or feeling of dissociation, or an out-of-body–type experience.
Ketamine is a mixture of two mirror-image molecules. An intranasal version of one of these molecules (esketamine) is approved by the U.S. Food and Drug Administration for TRD. Both esketamine and ketamine are believed to increase neurotrophic signaling that affects synaptic function.
The study included 424 patients (mean age, 41.7 years) with major depressive disorder or another mood disorder and who received at least one ketamine infusion at a specialty clinic. Most participants had failed prior medication trials.
Patients in the study were typically started on 0.5 mg/kg of ketamine, with the dose titrated to achieve symptoms of partial dissociation. The median dose administered after titration was 0.93 mg/kg over 40 minutes.
The main treatment course of at least six infusions within 21 days was completed by 70% of the patients.
At each clinic visit, all participants completed the Patient Health Questionnaire-9 (PHQ-9) and the Generalized Anxiety Disorder-7 (GAD-7).
The primary outcome was PHQ-9 total scores, for which researchers looked at seven time periods: 1 week, 2-3 weeks, 4-6 weeks, 7-12 weeks, 13-24 weeks, 25-51 weeks, and 52+ weeks.
‘Blows it out of the water’
Results showed PHQ-9 total scores declined by 50% throughout the course of treatment, with much of the improvement gained within 4-6 weeks. There was a significant difference between week 1 and all later time periods (all P values < .001) and between weeks 2 and 3 and all later periods (all P values < .001).
Other measures included treatment response, defined as at least a 50% improvement on the PHQ-9, and depression remission, defined as a PHQ-9 score of less than 5. After three infusions, 14% of the patients responded and 7% were in remission. After 10 infusions, 72% responded and 38% were in remission.
These results compare favorably to other depression treatments, said Dr. Oliver. “Truthfully, with the exception of ECT [electroconvulsive therapy], this blows it all out of the water,” he added.
Dr. Oliver noted that the success rate for repetitive transcranial magnetic stimulation is 40%-60% depending on the modality; and for selective serotonin reuptake inhibitors, the success rate “is somewhere between the mid-20s and low-30s percent range.”
Another outcome measure was the self-harm/suicidal ideation item of the PHQ-9 questionnaire, which asks about “thoughts that you would be better off dead, or of hurting yourself in some way.” About 22% of the study participants no longer reported suicidal ideation after 3 infusions, 50% by 6 infusions, and 75% by 10 infusions.
By 15 infusions, 85% no longer reported these thoughts. “Nothing else has shown that, ever,” said Dr. Oliver.
Symptoms of generalized anxiety were also substantially improved. There was about a 30% reduction in the GAD-7 score during treatment and, again, most of the response occurred by 4-6 weeks.
Study limitations
Sex, age, and other demographic characteristics did not predict response or remission, but suicide planning trended toward higher response rates (P = .083). This suggests that a more depressed subgroup can achieve greater benefit from the treatment than can less symptomatic patients, the investigators note.
A history of psychosis also trended toward better response to treatment (P = .086) but not remission.
The researchers note that study limitations include that it was retrospective, lacked a control group, and did not require patients to be hospitalized – so the study sample may have been less severely ill than in other studies.
In addition, most patients paid out of pocket for the treatment at $495 per infusion, and they self-reported their symptoms.
As well, the researchers did not assess adverse events, although nurses made follow-up calls to patients. Dr. Oliver noted the most common side effects of ketamine are nausea, vomiting, and anxiety.
Previous research has suggested that ketamine therapy is not linked to long-term side effects, such as sexual dysfunction, weight gain, lethargy, or cognitive issues, said Dr. Oliver.
The investigators point out another study limitation was lack of detailed demographic information, such as race, income, and education, which might affect its generalizability.
Concerns and questions
Pouya Movahed Rad, MD, PhD, senior consultant and researcher in psychiatry, Lund (Sweden) University, noted several concerns, including that the clinics treating the study participants with ketamine profited from it.
He also speculated about who can afford the treatment because only a few patients in the study were reimbursed through insurance.
Dr. Movahed Rad was not involved with the current research but was principal investigator for a recent study that compared intravenous ketamine to ECT.
He questioned whether the patient population in the new study really was “real world.” Well-designed randomized controlled trials have been carried out in a “naturalistic setting, [which] get closer to real-life patients,” he said.
He also noted that the median dose after clinician titration (0.93 mg/kg over 40 minutes) “may be considered very high.”
With regard to doses being titrated to achieve symptoms of partial dissociation, “there is no obvious evidence to my knowledge that patients need to develop dissociative symptoms in order to have antidepressant effect,” said Dr. Movahed Rad.
Finally, he noted that the finding that 28% of the participants were using illegal drugs “is worrying” and wondered what drugs they were taking; he also questioned why 81% of the study population needed to take antidepressants.
The study did not receive outside funding. Dr. Oliver is the founder of MindPeace Clinics, which specialize in ketamine therapeutics. Dr. Movahed Rad has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF CLINICAL PSYCHIATRY
Me, my spouse, and COVID
Managing family conflict and cohesion
I watched you in the garage, with your wipes and your mask, your gloves and bottles of sprays and potions. I admired your fealty to CNN’s Dr. Sanjay Gupta as he demonstrated the proper technique for disinfecting groceries. I watched sterile protocol being broken and quietly closed the garage door.
I listened to your descriptions of the agility of the virus with each exhalation of breath, and how far the virus could travel with a tailwind and in cold dry air. I listen as closely and with the same intention as I listen to my yoga teacher’s explication of the benefits of attention to the breath.
Relatives and friends came prepared to be entertained outdoors. Even masked, you eschewed the world. Your version of science clashes with my laissez-faire attitude. We blow up as a couple. Then we settle down and learn how to cope with the stress, as a team, together.
The COVID factor
In the first few months of any stressor, family and couple functioning must reorganize to manage well.
During lockdown, social scientists accessed an eager public ready to participate in their studies. With nowhere to go, many people, especially women, completed online COVID surveys. Community-based tools such as the Centers for Disease Control and Prevention’s Social Vulnerability Index identified populations of high social vulnerability (as caused by external stresses on human health, such as unemployment, overcrowding, presence of an individual with caregiving needs, and low educational attainment). It is assumed that such populations will experience more stress and have more difficulty coping and adjusting.
In a study by a team at the University of Miami, social vulnerability was associated with more disrupted family functioning, except when households with children (n = 2,666) were compared to households without children (n = 1,456).1 What allowed these families with children to enjoy better functioning?
Looking more closely at the Miami study, what can we find? It is a large survey study (n = 4,122), disseminated through professional networks and social media via purchased Facebook and Instagram ads. Data were logged in REDCap, and participants had the option of taking the survey in English or Spanish. Most participants were female (93.5%), 55.7% responded in English, and 44.3% in Spanish. There were few differences between the women who had and did not have children, in terms of their age, employment status, and education level. The number of children in the household did not affect the results.
This study used a new tool called the COVID-19 Household Environment Scale. This tool has 25 items measuring individual and household characteristics, and associated COVID-19 stressors. This tool also includes two family functioning measures: conflict and cohesion, asking the respondent to reflect on the change in “conflict” or “togetherness,” as it relates to household experiences and activities, compared with the period before social distancing.
The surprising finding was that even though households with children reported more conflict than before the start of the pandemic, they also reported more cohesion. This syncs with my experience. My niece and nephew found that having their teenage children at home brought them closer as a family, cut down on some of the extracurricular activities they did not support, and generally “slowed the world down.”
However, in a study in Germany, survey respondents (n = 1,042) noted that having children up to 17 years old was associated with decreases in satisfaction with family life, although this was not related to changes in family demands. The study assessed changes over 6 months and underscores the fact that perceptions of family demands and family well-being are independent of each other.2
These findings also resonate with prior research that measured burden and reward in couples. High burden is not associated with low reward; these two constructs are independent of each other.3
What about couples?
It is no surprise that poor relationships begat poor coping. In an online Belgian survey of 1,491 cohabiting couples during the shutdown, both men and women felt significantly more stress than before, because they felt restricted in their relationship.4
However, only women reported significantly more stress during the lockdown than before, because of relationship conflicts, such as feeling neglected by their partner. These feelings had predated lockdown.
In another lockdown online survey of 782 U.S. adults (89.8% White, 84.5% female), cohabitating intimate partners reported that there were higher thoughts of separation if the participants were younger, or if there was higher verbal aggression, higher relationship invalidation, and lower relationship satisfaction. Higher relationship satisfaction was reported when there was lower money stress, higher sexual fulfillment, lower relationship invalidation, and higher perceived fairness of relationship power. High relationship satisfaction was also reported where there were no children in the home.5
It should be noted that none of these relationship variables was measured in the Miami study discussed above, and this study did not measure perceived conflict or perceived cohesion, so we know less about these aspects of the family unit.
What about teens?
The COVID-19 lockdown had a positive effect on the dynamics in some families, according to a naturalistic study of adolescents (n = 155) who completed surveys at two time periods (initial and 8 weeks).6
These adolescents reported a reduction in perceived psychological control by their mothers, and no change in autonomy support. The changes did not vary according to gender or the mother’s employment situation. The decrease in psychological control was greater with higher initial levels of satisfaction with the mother, and lower levels of the teens disobeying their parents.
What about hospital settings?
The worst of the COVID experience was in the hospital. The pain was displayed on the faces of the staff as they labored to figure out how to care for the dying patients who had no contact with their families. Hospitals, out of fear of contamination and viral dissemination, excluded visitors. In those early days of uncertainty, the stress among staff, patients, and family members was high.
In response to family members feeling disconnected from the health care team and the psychological and moral distress of the staff, Nadine J. Kaslow and colleagues revised policies and procedures at Emory University, Atlanta, facilities to reprioritize patient- and family-centered care.7
The guiding principles focus on providing safe yet compassionate and ethical care, balancing community health and the mitigation of viral transmission, while appreciating family members as essential partners in care; fostering communication between patients and their families; and promoting interactions and decision-making among health care providers, patients, and families.
COVID continues to intrude in many of our lives. Many people are mourning family members and friends who died after contracting the disease. Many people choose to ignore their risk and live their lives as before. Many people, like my spouse and me, continue to debate the merits of venturing into public spaces. Personally, COVID has given me time to read many more books than I could ever have imagined and allowed my spouse to explore the delicate nuances of cooking.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at alisonheru@gmail.com.
References
1. Chavez JV et al. Assessing the impact of COVID-19 social distancing and social vulnerability on family functioning in an international sample of households with and without children. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 233-48. doi: 10.1037/cfp0000166.
2. Rudolph CW, Zacher H. Family demands and satisfaction with family life during the COVID-19 pandemic. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 249-59. doi: 10.1037/cfp0000170.
3. Heru AM et al. Family functioning in the caregivers of patients with dementia. Int J Geriatr Psychiatry. 2004 Jun;19(6):533-7. doi: 10.1002/gps.1119.
4. Schokkenbroek JM et al. Partners in lockdown: Relationship stress in men and women during the COVID-19 pandemic. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 149-57. doi: 10.1037/cfp0000172.
5. Eubanks Fleming CJ, Franzese AT. Should I stay or should I go? Evaluating intimate relationship outcomes during the 2020 pandemic shutdown. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 158-67. doi: 10.1037/cfp0000169.
6. Bacikova-Sleskova M,et al. Did perceived parenting in adolescence change as a result of the COVID-19 lockdown? A natural experiment. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 271-80. doi: 10.1037/cfp0000167.
7. Kaslow NJ et al. A roadmap for patient- and family-centered care during the pandemic. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 223-32. doi: 10.1037/cfp0000176.
Managing family conflict and cohesion
Managing family conflict and cohesion
I watched you in the garage, with your wipes and your mask, your gloves and bottles of sprays and potions. I admired your fealty to CNN’s Dr. Sanjay Gupta as he demonstrated the proper technique for disinfecting groceries. I watched sterile protocol being broken and quietly closed the garage door.
I listened to your descriptions of the agility of the virus with each exhalation of breath, and how far the virus could travel with a tailwind and in cold dry air. I listen as closely and with the same intention as I listen to my yoga teacher’s explication of the benefits of attention to the breath.
Relatives and friends came prepared to be entertained outdoors. Even masked, you eschewed the world. Your version of science clashes with my laissez-faire attitude. We blow up as a couple. Then we settle down and learn how to cope with the stress, as a team, together.
The COVID factor
In the first few months of any stressor, family and couple functioning must reorganize to manage well.
During lockdown, social scientists accessed an eager public ready to participate in their studies. With nowhere to go, many people, especially women, completed online COVID surveys. Community-based tools such as the Centers for Disease Control and Prevention’s Social Vulnerability Index identified populations of high social vulnerability (as caused by external stresses on human health, such as unemployment, overcrowding, presence of an individual with caregiving needs, and low educational attainment). It is assumed that such populations will experience more stress and have more difficulty coping and adjusting.
In a study by a team at the University of Miami, social vulnerability was associated with more disrupted family functioning, except when households with children (n = 2,666) were compared to households without children (n = 1,456).1 What allowed these families with children to enjoy better functioning?
Looking more closely at the Miami study, what can we find? It is a large survey study (n = 4,122), disseminated through professional networks and social media via purchased Facebook and Instagram ads. Data were logged in REDCap, and participants had the option of taking the survey in English or Spanish. Most participants were female (93.5%), 55.7% responded in English, and 44.3% in Spanish. There were few differences between the women who had and did not have children, in terms of their age, employment status, and education level. The number of children in the household did not affect the results.
This study used a new tool called the COVID-19 Household Environment Scale. This tool has 25 items measuring individual and household characteristics, and associated COVID-19 stressors. This tool also includes two family functioning measures: conflict and cohesion, asking the respondent to reflect on the change in “conflict” or “togetherness,” as it relates to household experiences and activities, compared with the period before social distancing.
The surprising finding was that even though households with children reported more conflict than before the start of the pandemic, they also reported more cohesion. This syncs with my experience. My niece and nephew found that having their teenage children at home brought them closer as a family, cut down on some of the extracurricular activities they did not support, and generally “slowed the world down.”
However, in a study in Germany, survey respondents (n = 1,042) noted that having children up to 17 years old was associated with decreases in satisfaction with family life, although this was not related to changes in family demands. The study assessed changes over 6 months and underscores the fact that perceptions of family demands and family well-being are independent of each other.2
These findings also resonate with prior research that measured burden and reward in couples. High burden is not associated with low reward; these two constructs are independent of each other.3
What about couples?
It is no surprise that poor relationships begat poor coping. In an online Belgian survey of 1,491 cohabiting couples during the shutdown, both men and women felt significantly more stress than before, because they felt restricted in their relationship.4
However, only women reported significantly more stress during the lockdown than before, because of relationship conflicts, such as feeling neglected by their partner. These feelings had predated lockdown.
In another lockdown online survey of 782 U.S. adults (89.8% White, 84.5% female), cohabitating intimate partners reported that there were higher thoughts of separation if the participants were younger, or if there was higher verbal aggression, higher relationship invalidation, and lower relationship satisfaction. Higher relationship satisfaction was reported when there was lower money stress, higher sexual fulfillment, lower relationship invalidation, and higher perceived fairness of relationship power. High relationship satisfaction was also reported where there were no children in the home.5
It should be noted that none of these relationship variables was measured in the Miami study discussed above, and this study did not measure perceived conflict or perceived cohesion, so we know less about these aspects of the family unit.
What about teens?
The COVID-19 lockdown had a positive effect on the dynamics in some families, according to a naturalistic study of adolescents (n = 155) who completed surveys at two time periods (initial and 8 weeks).6
These adolescents reported a reduction in perceived psychological control by their mothers, and no change in autonomy support. The changes did not vary according to gender or the mother’s employment situation. The decrease in psychological control was greater with higher initial levels of satisfaction with the mother, and lower levels of the teens disobeying their parents.
What about hospital settings?
The worst of the COVID experience was in the hospital. The pain was displayed on the faces of the staff as they labored to figure out how to care for the dying patients who had no contact with their families. Hospitals, out of fear of contamination and viral dissemination, excluded visitors. In those early days of uncertainty, the stress among staff, patients, and family members was high.
In response to family members feeling disconnected from the health care team and the psychological and moral distress of the staff, Nadine J. Kaslow and colleagues revised policies and procedures at Emory University, Atlanta, facilities to reprioritize patient- and family-centered care.7
The guiding principles focus on providing safe yet compassionate and ethical care, balancing community health and the mitigation of viral transmission, while appreciating family members as essential partners in care; fostering communication between patients and their families; and promoting interactions and decision-making among health care providers, patients, and families.
COVID continues to intrude in many of our lives. Many people are mourning family members and friends who died after contracting the disease. Many people choose to ignore their risk and live their lives as before. Many people, like my spouse and me, continue to debate the merits of venturing into public spaces. Personally, COVID has given me time to read many more books than I could ever have imagined and allowed my spouse to explore the delicate nuances of cooking.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at alisonheru@gmail.com.
References
1. Chavez JV et al. Assessing the impact of COVID-19 social distancing and social vulnerability on family functioning in an international sample of households with and without children. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 233-48. doi: 10.1037/cfp0000166.
2. Rudolph CW, Zacher H. Family demands and satisfaction with family life during the COVID-19 pandemic. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 249-59. doi: 10.1037/cfp0000170.
3. Heru AM et al. Family functioning in the caregivers of patients with dementia. Int J Geriatr Psychiatry. 2004 Jun;19(6):533-7. doi: 10.1002/gps.1119.
4. Schokkenbroek JM et al. Partners in lockdown: Relationship stress in men and women during the COVID-19 pandemic. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 149-57. doi: 10.1037/cfp0000172.
5. Eubanks Fleming CJ, Franzese AT. Should I stay or should I go? Evaluating intimate relationship outcomes during the 2020 pandemic shutdown. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 158-67. doi: 10.1037/cfp0000169.
6. Bacikova-Sleskova M,et al. Did perceived parenting in adolescence change as a result of the COVID-19 lockdown? A natural experiment. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 271-80. doi: 10.1037/cfp0000167.
7. Kaslow NJ et al. A roadmap for patient- and family-centered care during the pandemic. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 223-32. doi: 10.1037/cfp0000176.
I watched you in the garage, with your wipes and your mask, your gloves and bottles of sprays and potions. I admired your fealty to CNN’s Dr. Sanjay Gupta as he demonstrated the proper technique for disinfecting groceries. I watched sterile protocol being broken and quietly closed the garage door.
I listened to your descriptions of the agility of the virus with each exhalation of breath, and how far the virus could travel with a tailwind and in cold dry air. I listen as closely and with the same intention as I listen to my yoga teacher’s explication of the benefits of attention to the breath.
Relatives and friends came prepared to be entertained outdoors. Even masked, you eschewed the world. Your version of science clashes with my laissez-faire attitude. We blow up as a couple. Then we settle down and learn how to cope with the stress, as a team, together.
The COVID factor
In the first few months of any stressor, family and couple functioning must reorganize to manage well.
During lockdown, social scientists accessed an eager public ready to participate in their studies. With nowhere to go, many people, especially women, completed online COVID surveys. Community-based tools such as the Centers for Disease Control and Prevention’s Social Vulnerability Index identified populations of high social vulnerability (as caused by external stresses on human health, such as unemployment, overcrowding, presence of an individual with caregiving needs, and low educational attainment). It is assumed that such populations will experience more stress and have more difficulty coping and adjusting.
In a study by a team at the University of Miami, social vulnerability was associated with more disrupted family functioning, except when households with children (n = 2,666) were compared to households without children (n = 1,456).1 What allowed these families with children to enjoy better functioning?
Looking more closely at the Miami study, what can we find? It is a large survey study (n = 4,122), disseminated through professional networks and social media via purchased Facebook and Instagram ads. Data were logged in REDCap, and participants had the option of taking the survey in English or Spanish. Most participants were female (93.5%), 55.7% responded in English, and 44.3% in Spanish. There were few differences between the women who had and did not have children, in terms of their age, employment status, and education level. The number of children in the household did not affect the results.
This study used a new tool called the COVID-19 Household Environment Scale. This tool has 25 items measuring individual and household characteristics, and associated COVID-19 stressors. This tool also includes two family functioning measures: conflict and cohesion, asking the respondent to reflect on the change in “conflict” or “togetherness,” as it relates to household experiences and activities, compared with the period before social distancing.
The surprising finding was that even though households with children reported more conflict than before the start of the pandemic, they also reported more cohesion. This syncs with my experience. My niece and nephew found that having their teenage children at home brought them closer as a family, cut down on some of the extracurricular activities they did not support, and generally “slowed the world down.”
However, in a study in Germany, survey respondents (n = 1,042) noted that having children up to 17 years old was associated with decreases in satisfaction with family life, although this was not related to changes in family demands. The study assessed changes over 6 months and underscores the fact that perceptions of family demands and family well-being are independent of each other.2
These findings also resonate with prior research that measured burden and reward in couples. High burden is not associated with low reward; these two constructs are independent of each other.3
What about couples?
It is no surprise that poor relationships begat poor coping. In an online Belgian survey of 1,491 cohabiting couples during the shutdown, both men and women felt significantly more stress than before, because they felt restricted in their relationship.4
However, only women reported significantly more stress during the lockdown than before, because of relationship conflicts, such as feeling neglected by their partner. These feelings had predated lockdown.
In another lockdown online survey of 782 U.S. adults (89.8% White, 84.5% female), cohabitating intimate partners reported that there were higher thoughts of separation if the participants were younger, or if there was higher verbal aggression, higher relationship invalidation, and lower relationship satisfaction. Higher relationship satisfaction was reported when there was lower money stress, higher sexual fulfillment, lower relationship invalidation, and higher perceived fairness of relationship power. High relationship satisfaction was also reported where there were no children in the home.5
It should be noted that none of these relationship variables was measured in the Miami study discussed above, and this study did not measure perceived conflict or perceived cohesion, so we know less about these aspects of the family unit.
What about teens?
The COVID-19 lockdown had a positive effect on the dynamics in some families, according to a naturalistic study of adolescents (n = 155) who completed surveys at two time periods (initial and 8 weeks).6
These adolescents reported a reduction in perceived psychological control by their mothers, and no change in autonomy support. The changes did not vary according to gender or the mother’s employment situation. The decrease in psychological control was greater with higher initial levels of satisfaction with the mother, and lower levels of the teens disobeying their parents.
What about hospital settings?
The worst of the COVID experience was in the hospital. The pain was displayed on the faces of the staff as they labored to figure out how to care for the dying patients who had no contact with their families. Hospitals, out of fear of contamination and viral dissemination, excluded visitors. In those early days of uncertainty, the stress among staff, patients, and family members was high.
In response to family members feeling disconnected from the health care team and the psychological and moral distress of the staff, Nadine J. Kaslow and colleagues revised policies and procedures at Emory University, Atlanta, facilities to reprioritize patient- and family-centered care.7
The guiding principles focus on providing safe yet compassionate and ethical care, balancing community health and the mitigation of viral transmission, while appreciating family members as essential partners in care; fostering communication between patients and their families; and promoting interactions and decision-making among health care providers, patients, and families.
COVID continues to intrude in many of our lives. Many people are mourning family members and friends who died after contracting the disease. Many people choose to ignore their risk and live their lives as before. Many people, like my spouse and me, continue to debate the merits of venturing into public spaces. Personally, COVID has given me time to read many more books than I could ever have imagined and allowed my spouse to explore the delicate nuances of cooking.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at alisonheru@gmail.com.
References
1. Chavez JV et al. Assessing the impact of COVID-19 social distancing and social vulnerability on family functioning in an international sample of households with and without children. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 233-48. doi: 10.1037/cfp0000166.
2. Rudolph CW, Zacher H. Family demands and satisfaction with family life during the COVID-19 pandemic. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 249-59. doi: 10.1037/cfp0000170.
3. Heru AM et al. Family functioning in the caregivers of patients with dementia. Int J Geriatr Psychiatry. 2004 Jun;19(6):533-7. doi: 10.1002/gps.1119.
4. Schokkenbroek JM et al. Partners in lockdown: Relationship stress in men and women during the COVID-19 pandemic. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 149-57. doi: 10.1037/cfp0000172.
5. Eubanks Fleming CJ, Franzese AT. Should I stay or should I go? Evaluating intimate relationship outcomes during the 2020 pandemic shutdown. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 158-67. doi: 10.1037/cfp0000169.
6. Bacikova-Sleskova M,et al. Did perceived parenting in adolescence change as a result of the COVID-19 lockdown? A natural experiment. Couple Fam Psychol: Res Pract. 2021 Dec;10(4): 271-80. doi: 10.1037/cfp0000167.
7. Kaslow NJ et al. A roadmap for patient- and family-centered care during the pandemic. Couple Fam Psychol: Res Pract. 2021 Sept;10(3): 223-32. doi: 10.1037/cfp0000176.
Detachment predicts worse posttraumatic outcomes
The results highlight the importance of screening for dissociation in patients who have experienced trauma, study investigator Lauren A.M. Lebois, PhD, director of the dissociative disorders and trauma research program at McLean Hospital in Belmont, Mass., told this news organization.
“Clinicians could identify individuals potentially at risk of a chronic, more severe psychiatric course before these people go down that road, and they have the opportunity to connect folks with a phased trauma treatment approach to speed their recovery,” said Dr. Lebois, who is also an assistant professor of psychiatry at Harvard Medical School, Boston.
The study was published in the American Journal of Psychiatry.
Underdiagnosed
Feelings of detachment or derealization are a type of dissociation. Patients with the syndrome report feeling foggy or as if they are in a dream. Dissociative diagnoses are not rare and, in fact, are more prevalent than schizophrenia.
Research supports a powerful relationship between dissociation and traumatic experiences. However, dissociation is among the most stigmatized of psychiatric conditions. Even among clinicians and researchers, beliefs about dissociation are often not based on the scientific literature, said Dr. Lebois.
“For instance, skepticism, misunderstanding, and lack of professional education about dissociation all contribute to striking rates of underdiagnosis and misdiagnoses,” she said.
Dr. Lebois and colleagues used data from the larger Advancing Understanding of Recovery After Trauma (AURORA) study and included 1,464 adults, mean age 35 years, appearing at 22 U.S. emergency departments. Patients experienced a traumatic event such as a motor vehicle crash or physical or sexual assault.
About 2 weeks after the trauma, participants reported symptoms of derealization as measured by a two-item version of the Brief Dissociative Experiences Scale.
Brain imaging data
A subset of 145 patients underwent functional MRI (fMRI), during which they completed an emotion reactivity task (viewing fearful-looking human faces) and a resting-state scan.
In addition to measuring history of childhood maltreatment, researchers assessed posttraumatic stress symptom severity at 2 weeks and again at 3 months using the posttraumatic stress disorder checklist. Also at 3 months, they measured depression and anxiety symptoms, pain, and functional impairment.
About 55% of self-report participants and 50% of MRI participants endorsed some level of persistent derealization at 2 weeks.
After controlling for potential confounders, including sex, age, childhood maltreatment, and current posttraumatic stress symptoms, researchers found persistent derealization was associated with increased ventromedial prefrontal cortex (vmPFC) activity while viewing fearful faces.
The vmPFC helps to regulate emotional and physical reactions. “This region puts the ‘brakes’ on your emotional and physical reactivity – helping you to calm down” after a threatening or stressful experience has passed, said Dr. Lebois.
Researchers also found an association between higher self-reported derealization and decreased resting-state connectivity between the vmPFC and the orbitofrontal cortex and right lobule VIIIa – a region of the cerebellum involved in sensorimotor function.
“This may contribute to perceptual and affective distortions experienced during derealization – for example, feelings that surroundings are fading away, unreal, or strange,” said Dr. Lebois.
More pain, depression, anxiety
Higher levels of self-reported derealization at 2 weeks post trauma predicted higher levels of PTSD, anxiety, and depression as well as more bodily pain and impairment in work, family, and social life at 3 months.
“When we accounted for baseline levels of posttraumatic stress symptoms and trauma history, higher levels of self-reported derealization still predicted higher posttraumatic stress disorder and depression symptoms at 3 months,” said Dr. Lebois.
Additional adjusted analyses showed increased vmPFC activity during the fearful face task predicted 3-month self-reported PTSD symptoms.
Dr. Lebois “highly recommends” clinicians screen for dissociative symptoms, including derealization, in patients with trauma. Self-report screening tools are freely available online.
She noted patients with significant dissociative symptoms often do better with a “phase-oriented” approach to trauma treatment.
“In phase one, they learn emotional regulation skills to help them take more control over when they dissociate. Then they can successfully move on to trauma processing in phase two, which can involve exposure to trauma details.”
Although the field is not yet ready to use brain scans to diagnose dissociative symptoms, the new results “take us one step closer to being able to use objective neuroimaging biomarkers of derealization to augment subjective self-report measures,” said Dr. Lebois.
A limitation of the study was it could not determine a causal relationship, as some derealization may have been present before the traumatic event. The findings may not generalize to other types of dissociation, and the derealization assessment was measured only through a self-report 2 weeks after the trauma.
Another limitation was exclusion of patients with self-inflicted injuries or who were involved in domestic violence. The researchers noted the prevalence of derealization might have been even higher if such individuals were included.
An important investigation
In an accompanying editorial, Lisa M. Shin, PhD, department of psychology, Tufts University, and department of psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, notes having both clinical and neuroimaging variables as well as a large sample size makes the study “an important investigation” into predictors of psychiatric symptoms post-trauma.
Investigating a specific subtype of dissociation – persistent derealization – adds to the “novelty” of the study, she said.
The new findings “are certainly exciting for their potential clinical relevance and contributions to neurocircuitry models of PTSD,” she writes.
Some may argue administering a short, self-report measure of derealization “is far more efficient, cost-effective, and inclusive than conducting a specialized and expensive fMRI scan that is unlikely to be available to everyone,” notes Dr. Shin.
However, she added, a potential benefit of such a scan is identification of specific brain regions as potential targets for intervention. “For example, the results of this and other studies suggest that the vmPFC is a reasonable target for transcranial magnetic stimulation or its variants.”
The new results need to be replicated in a large, independent sample, said Dr. Shin. She added it would be helpful to know if other types of dissociation, and activation in other subregions of the vmPFC, also predict psychiatric outcomes after a trauma.
The study was supported by National Institute of Mental Health grants, the U.S. Army Medical Research and Material Command, One Mind, and the Mayday Fund. Dr. Lebois has received grant support from NIMH, and her spouse receives payments from Vanderbilt University for technology licensed to Acadia Pharmaceuticals. Dr. Shin receives textbook-related royalties from Pearson.
A version of this article first appeared on Medscape.com.
The results highlight the importance of screening for dissociation in patients who have experienced trauma, study investigator Lauren A.M. Lebois, PhD, director of the dissociative disorders and trauma research program at McLean Hospital in Belmont, Mass., told this news organization.
“Clinicians could identify individuals potentially at risk of a chronic, more severe psychiatric course before these people go down that road, and they have the opportunity to connect folks with a phased trauma treatment approach to speed their recovery,” said Dr. Lebois, who is also an assistant professor of psychiatry at Harvard Medical School, Boston.
The study was published in the American Journal of Psychiatry.
Underdiagnosed
Feelings of detachment or derealization are a type of dissociation. Patients with the syndrome report feeling foggy or as if they are in a dream. Dissociative diagnoses are not rare and, in fact, are more prevalent than schizophrenia.
Research supports a powerful relationship between dissociation and traumatic experiences. However, dissociation is among the most stigmatized of psychiatric conditions. Even among clinicians and researchers, beliefs about dissociation are often not based on the scientific literature, said Dr. Lebois.
“For instance, skepticism, misunderstanding, and lack of professional education about dissociation all contribute to striking rates of underdiagnosis and misdiagnoses,” she said.
Dr. Lebois and colleagues used data from the larger Advancing Understanding of Recovery After Trauma (AURORA) study and included 1,464 adults, mean age 35 years, appearing at 22 U.S. emergency departments. Patients experienced a traumatic event such as a motor vehicle crash or physical or sexual assault.
About 2 weeks after the trauma, participants reported symptoms of derealization as measured by a two-item version of the Brief Dissociative Experiences Scale.
Brain imaging data
A subset of 145 patients underwent functional MRI (fMRI), during which they completed an emotion reactivity task (viewing fearful-looking human faces) and a resting-state scan.
In addition to measuring history of childhood maltreatment, researchers assessed posttraumatic stress symptom severity at 2 weeks and again at 3 months using the posttraumatic stress disorder checklist. Also at 3 months, they measured depression and anxiety symptoms, pain, and functional impairment.
About 55% of self-report participants and 50% of MRI participants endorsed some level of persistent derealization at 2 weeks.
After controlling for potential confounders, including sex, age, childhood maltreatment, and current posttraumatic stress symptoms, researchers found persistent derealization was associated with increased ventromedial prefrontal cortex (vmPFC) activity while viewing fearful faces.
The vmPFC helps to regulate emotional and physical reactions. “This region puts the ‘brakes’ on your emotional and physical reactivity – helping you to calm down” after a threatening or stressful experience has passed, said Dr. Lebois.
Researchers also found an association between higher self-reported derealization and decreased resting-state connectivity between the vmPFC and the orbitofrontal cortex and right lobule VIIIa – a region of the cerebellum involved in sensorimotor function.
“This may contribute to perceptual and affective distortions experienced during derealization – for example, feelings that surroundings are fading away, unreal, or strange,” said Dr. Lebois.
More pain, depression, anxiety
Higher levels of self-reported derealization at 2 weeks post trauma predicted higher levels of PTSD, anxiety, and depression as well as more bodily pain and impairment in work, family, and social life at 3 months.
“When we accounted for baseline levels of posttraumatic stress symptoms and trauma history, higher levels of self-reported derealization still predicted higher posttraumatic stress disorder and depression symptoms at 3 months,” said Dr. Lebois.
Additional adjusted analyses showed increased vmPFC activity during the fearful face task predicted 3-month self-reported PTSD symptoms.
Dr. Lebois “highly recommends” clinicians screen for dissociative symptoms, including derealization, in patients with trauma. Self-report screening tools are freely available online.
She noted patients with significant dissociative symptoms often do better with a “phase-oriented” approach to trauma treatment.
“In phase one, they learn emotional regulation skills to help them take more control over when they dissociate. Then they can successfully move on to trauma processing in phase two, which can involve exposure to trauma details.”
Although the field is not yet ready to use brain scans to diagnose dissociative symptoms, the new results “take us one step closer to being able to use objective neuroimaging biomarkers of derealization to augment subjective self-report measures,” said Dr. Lebois.
A limitation of the study was it could not determine a causal relationship, as some derealization may have been present before the traumatic event. The findings may not generalize to other types of dissociation, and the derealization assessment was measured only through a self-report 2 weeks after the trauma.
Another limitation was exclusion of patients with self-inflicted injuries or who were involved in domestic violence. The researchers noted the prevalence of derealization might have been even higher if such individuals were included.
An important investigation
In an accompanying editorial, Lisa M. Shin, PhD, department of psychology, Tufts University, and department of psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, notes having both clinical and neuroimaging variables as well as a large sample size makes the study “an important investigation” into predictors of psychiatric symptoms post-trauma.
Investigating a specific subtype of dissociation – persistent derealization – adds to the “novelty” of the study, she said.
The new findings “are certainly exciting for their potential clinical relevance and contributions to neurocircuitry models of PTSD,” she writes.
Some may argue administering a short, self-report measure of derealization “is far more efficient, cost-effective, and inclusive than conducting a specialized and expensive fMRI scan that is unlikely to be available to everyone,” notes Dr. Shin.
However, she added, a potential benefit of such a scan is identification of specific brain regions as potential targets for intervention. “For example, the results of this and other studies suggest that the vmPFC is a reasonable target for transcranial magnetic stimulation or its variants.”
The new results need to be replicated in a large, independent sample, said Dr. Shin. She added it would be helpful to know if other types of dissociation, and activation in other subregions of the vmPFC, also predict psychiatric outcomes after a trauma.
The study was supported by National Institute of Mental Health grants, the U.S. Army Medical Research and Material Command, One Mind, and the Mayday Fund. Dr. Lebois has received grant support from NIMH, and her spouse receives payments from Vanderbilt University for technology licensed to Acadia Pharmaceuticals. Dr. Shin receives textbook-related royalties from Pearson.
A version of this article first appeared on Medscape.com.
The results highlight the importance of screening for dissociation in patients who have experienced trauma, study investigator Lauren A.M. Lebois, PhD, director of the dissociative disorders and trauma research program at McLean Hospital in Belmont, Mass., told this news organization.
“Clinicians could identify individuals potentially at risk of a chronic, more severe psychiatric course before these people go down that road, and they have the opportunity to connect folks with a phased trauma treatment approach to speed their recovery,” said Dr. Lebois, who is also an assistant professor of psychiatry at Harvard Medical School, Boston.
The study was published in the American Journal of Psychiatry.
Underdiagnosed
Feelings of detachment or derealization are a type of dissociation. Patients with the syndrome report feeling foggy or as if they are in a dream. Dissociative diagnoses are not rare and, in fact, are more prevalent than schizophrenia.
Research supports a powerful relationship between dissociation and traumatic experiences. However, dissociation is among the most stigmatized of psychiatric conditions. Even among clinicians and researchers, beliefs about dissociation are often not based on the scientific literature, said Dr. Lebois.
“For instance, skepticism, misunderstanding, and lack of professional education about dissociation all contribute to striking rates of underdiagnosis and misdiagnoses,” she said.
Dr. Lebois and colleagues used data from the larger Advancing Understanding of Recovery After Trauma (AURORA) study and included 1,464 adults, mean age 35 years, appearing at 22 U.S. emergency departments. Patients experienced a traumatic event such as a motor vehicle crash or physical or sexual assault.
About 2 weeks after the trauma, participants reported symptoms of derealization as measured by a two-item version of the Brief Dissociative Experiences Scale.
Brain imaging data
A subset of 145 patients underwent functional MRI (fMRI), during which they completed an emotion reactivity task (viewing fearful-looking human faces) and a resting-state scan.
In addition to measuring history of childhood maltreatment, researchers assessed posttraumatic stress symptom severity at 2 weeks and again at 3 months using the posttraumatic stress disorder checklist. Also at 3 months, they measured depression and anxiety symptoms, pain, and functional impairment.
About 55% of self-report participants and 50% of MRI participants endorsed some level of persistent derealization at 2 weeks.
After controlling for potential confounders, including sex, age, childhood maltreatment, and current posttraumatic stress symptoms, researchers found persistent derealization was associated with increased ventromedial prefrontal cortex (vmPFC) activity while viewing fearful faces.
The vmPFC helps to regulate emotional and physical reactions. “This region puts the ‘brakes’ on your emotional and physical reactivity – helping you to calm down” after a threatening or stressful experience has passed, said Dr. Lebois.
Researchers also found an association between higher self-reported derealization and decreased resting-state connectivity between the vmPFC and the orbitofrontal cortex and right lobule VIIIa – a region of the cerebellum involved in sensorimotor function.
“This may contribute to perceptual and affective distortions experienced during derealization – for example, feelings that surroundings are fading away, unreal, or strange,” said Dr. Lebois.
More pain, depression, anxiety
Higher levels of self-reported derealization at 2 weeks post trauma predicted higher levels of PTSD, anxiety, and depression as well as more bodily pain and impairment in work, family, and social life at 3 months.
“When we accounted for baseline levels of posttraumatic stress symptoms and trauma history, higher levels of self-reported derealization still predicted higher posttraumatic stress disorder and depression symptoms at 3 months,” said Dr. Lebois.
Additional adjusted analyses showed increased vmPFC activity during the fearful face task predicted 3-month self-reported PTSD symptoms.
Dr. Lebois “highly recommends” clinicians screen for dissociative symptoms, including derealization, in patients with trauma. Self-report screening tools are freely available online.
She noted patients with significant dissociative symptoms often do better with a “phase-oriented” approach to trauma treatment.
“In phase one, they learn emotional regulation skills to help them take more control over when they dissociate. Then they can successfully move on to trauma processing in phase two, which can involve exposure to trauma details.”
Although the field is not yet ready to use brain scans to diagnose dissociative symptoms, the new results “take us one step closer to being able to use objective neuroimaging biomarkers of derealization to augment subjective self-report measures,” said Dr. Lebois.
A limitation of the study was it could not determine a causal relationship, as some derealization may have been present before the traumatic event. The findings may not generalize to other types of dissociation, and the derealization assessment was measured only through a self-report 2 weeks after the trauma.
Another limitation was exclusion of patients with self-inflicted injuries or who were involved in domestic violence. The researchers noted the prevalence of derealization might have been even higher if such individuals were included.
An important investigation
In an accompanying editorial, Lisa M. Shin, PhD, department of psychology, Tufts University, and department of psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, notes having both clinical and neuroimaging variables as well as a large sample size makes the study “an important investigation” into predictors of psychiatric symptoms post-trauma.
Investigating a specific subtype of dissociation – persistent derealization – adds to the “novelty” of the study, she said.
The new findings “are certainly exciting for their potential clinical relevance and contributions to neurocircuitry models of PTSD,” she writes.
Some may argue administering a short, self-report measure of derealization “is far more efficient, cost-effective, and inclusive than conducting a specialized and expensive fMRI scan that is unlikely to be available to everyone,” notes Dr. Shin.
However, she added, a potential benefit of such a scan is identification of specific brain regions as potential targets for intervention. “For example, the results of this and other studies suggest that the vmPFC is a reasonable target for transcranial magnetic stimulation or its variants.”
The new results need to be replicated in a large, independent sample, said Dr. Shin. She added it would be helpful to know if other types of dissociation, and activation in other subregions of the vmPFC, also predict psychiatric outcomes after a trauma.
The study was supported by National Institute of Mental Health grants, the U.S. Army Medical Research and Material Command, One Mind, and the Mayday Fund. Dr. Lebois has received grant support from NIMH, and her spouse receives payments from Vanderbilt University for technology licensed to Acadia Pharmaceuticals. Dr. Shin receives textbook-related royalties from Pearson.
A version of this article first appeared on Medscape.com.
FROM AMERICAN JOURNAL OF PSYCHIATRY