User login
Time-restricted eating ‘promising, but more data are needed’
Time-restricted eating – that is, reducing the number of hours a person is allowed to eat during the day – may produce a modest 1%-4% weight loss, even without cutting calories, early studies in humans suggest. But more research is needed to provide definitive evidence.
This type of intermittent fasting also appears to improve blood glucose, blood pressure, and oxidative stress, said Courtney M. Peterson, PhD, a researcher at the University of Alabama at Birmingham, summarizing what is known about the potential weight-loss strategy at the annual scientific sessions of the American Diabetes Association.
The best results were seen with early time-restricted eating (that is, ending the nighttime fasting early in the day) and allowing a person to eat 8-10 hours each day (for example, 8 a.m. to 4 p.m. or 8 a.m. to 6 p.m.), with fasting and only water allowed the remaining hours, she reported.
However, the 3 dozen or so studies in humans to date are mainly small, pilot, or single-arm studies lasting up to 3 months, and there are only three main randomized, controlled trials with 25 or more participants in each group.
Large trials with around 260 participants are needed, Dr. Peterson said, “before drawing definitive conclusions” about the weight-loss and cardiometabolic benefits of time-restricted eating.
Invited to comment, session chair Lisa S. Chow, MD, an associate professor of medicine in the endocrine and diabetes division at the University of Minnesota, Minneapolis, similarly said: “I think time-restricted eating is promising because of its simple message and noted weight-loss benefit, yet more data are needed.”
“Many uncertainties remain,” she added, “including the potential concern that time-restricted eating may be associated with lean [muscle] mass loss and identifying the populations most likely to benefit from time-restricted eating,” she said.
36 small studies, a review, a meta-analysis, 3 RCTs
There have been about three dozen small studies of time-restricted eating in humans, which examined 4- to 11-hour eating windows, Dr. Peterson explained.
A systematic review of 23 trials of time-restricted eating reported that, on average, participants lost 3% of their initial weight. And a meta-analysis of 19 trials in 475 participants found a –0.9 kg mean difference effect for weight loss.
However, those two analyses did not compare time-restricted eating with a control treatment, she stressed.
The largest randomized, controlled trial is a 12-week study in 271 adults with nonalcoholic fatty liver disease in China, Dr. Peterson said.
The researchers compared three groups:
- Alternate-day modified fasting: healthy meal provided.
- Time-restricted eating: 8-hour window, healthy meal provided.
- Control: 20% calorie reduction, no meal provided.
At 4 and 12 weeks, adults in the two treatment groups lost more weight than those in the control group, but “this was not a fair comparison” because of the lack of a provided meal in the control group, Dr. Peterson pointed out.
The next largest randomized, controlled study is the 12-week TREAT trial, published online in JAMA Internal Medicine in October 2020.
The researchers, from the University of California, San Francisco, randomized 116 adults into two groups:
- 8-hour time-restricted eating from noon to 8 p.m..
- Control: three meals/day.
Time-restricted eating did not lead to greater weight loss, compared with three structured meals a day, which was not surprising, Dr. Chow said, as “participants just reported whether they were engaged in time-restricted eating in a yes/no answer.”
Moreover, “there was no objective measure of their eating window. From our study, we showed that the extent of eating window restriction matters, not just time-restricted eating participation.”
Also, in TREAT, the eating window was noon to 8 p.m. (considered late for time-restricted eating), and the trial also allowed noncaloric beverages outside the window, whereas most studies only allow water and medications.
Lastly, TREAT showed that time-restricted eating reduced weight, compared with baseline, but the weight loss was not significant, compared with the control group, and there was a wide spread of effects (that is, some lost a lot of weight, others didn’t lose much weight).
“That being said, the JAMA Internal Medicine paper is the largest paper to date of time-restricted eating randomized versus control, so its findings need to be acknowledged and recognized,” Dr. Chow said.
Peterson reported that her group recently completed a 14-week intervention in 90 adults with obesity divided into two groups:
- Control: Continuous energy restriction, self-selected ≥ 12-hour window.
- Early time-restricted eating: 8-hour window from 7 a.m. to 3 p.m.
The findings will provide further insight into the benefits of time-restricted eating.
How might time-restricted eating lead to weight loss?
Dr. Peterson concluded by presenting data suggesting how time-restricted eating may induce weight loss.
In a 4-day crossover study in 11 overweight adults, time-restricted eating did not affect energy expenditure, but it lessened swings in subjective hunger, improved appetite hormones including ghrelin, and increased fat oxidation.
Most trials have reported that time-restricted eating improves one or more cardiometabolic endpoints, she noted.
Early time-restricted eating was associated with improved insulin sensitivity and secretion, blood pressure, and oxidative stress, but not better lipid levels.
In contrast, compared with eating 3 meals/day (control), late time-restricted eating (eating 1 meal/day from 5 p.m. to 9 p.m.) was associated with worsened cardiometabolic health (glucose, insulin, blood pressure, and lipid levels) in an 8-week crossover study in 15 participants.
Dr. Peterson and Dr. Chow reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Time-restricted eating – that is, reducing the number of hours a person is allowed to eat during the day – may produce a modest 1%-4% weight loss, even without cutting calories, early studies in humans suggest. But more research is needed to provide definitive evidence.
This type of intermittent fasting also appears to improve blood glucose, blood pressure, and oxidative stress, said Courtney M. Peterson, PhD, a researcher at the University of Alabama at Birmingham, summarizing what is known about the potential weight-loss strategy at the annual scientific sessions of the American Diabetes Association.
The best results were seen with early time-restricted eating (that is, ending the nighttime fasting early in the day) and allowing a person to eat 8-10 hours each day (for example, 8 a.m. to 4 p.m. or 8 a.m. to 6 p.m.), with fasting and only water allowed the remaining hours, she reported.
However, the 3 dozen or so studies in humans to date are mainly small, pilot, or single-arm studies lasting up to 3 months, and there are only three main randomized, controlled trials with 25 or more participants in each group.
Large trials with around 260 participants are needed, Dr. Peterson said, “before drawing definitive conclusions” about the weight-loss and cardiometabolic benefits of time-restricted eating.
Invited to comment, session chair Lisa S. Chow, MD, an associate professor of medicine in the endocrine and diabetes division at the University of Minnesota, Minneapolis, similarly said: “I think time-restricted eating is promising because of its simple message and noted weight-loss benefit, yet more data are needed.”
“Many uncertainties remain,” she added, “including the potential concern that time-restricted eating may be associated with lean [muscle] mass loss and identifying the populations most likely to benefit from time-restricted eating,” she said.
36 small studies, a review, a meta-analysis, 3 RCTs
There have been about three dozen small studies of time-restricted eating in humans, which examined 4- to 11-hour eating windows, Dr. Peterson explained.
A systematic review of 23 trials of time-restricted eating reported that, on average, participants lost 3% of their initial weight. And a meta-analysis of 19 trials in 475 participants found a –0.9 kg mean difference effect for weight loss.
However, those two analyses did not compare time-restricted eating with a control treatment, she stressed.
The largest randomized, controlled trial is a 12-week study in 271 adults with nonalcoholic fatty liver disease in China, Dr. Peterson said.
The researchers compared three groups:
- Alternate-day modified fasting: healthy meal provided.
- Time-restricted eating: 8-hour window, healthy meal provided.
- Control: 20% calorie reduction, no meal provided.
At 4 and 12 weeks, adults in the two treatment groups lost more weight than those in the control group, but “this was not a fair comparison” because of the lack of a provided meal in the control group, Dr. Peterson pointed out.
The next largest randomized, controlled study is the 12-week TREAT trial, published online in JAMA Internal Medicine in October 2020.
The researchers, from the University of California, San Francisco, randomized 116 adults into two groups:
- 8-hour time-restricted eating from noon to 8 p.m..
- Control: three meals/day.
Time-restricted eating did not lead to greater weight loss, compared with three structured meals a day, which was not surprising, Dr. Chow said, as “participants just reported whether they were engaged in time-restricted eating in a yes/no answer.”
Moreover, “there was no objective measure of their eating window. From our study, we showed that the extent of eating window restriction matters, not just time-restricted eating participation.”
Also, in TREAT, the eating window was noon to 8 p.m. (considered late for time-restricted eating), and the trial also allowed noncaloric beverages outside the window, whereas most studies only allow water and medications.
Lastly, TREAT showed that time-restricted eating reduced weight, compared with baseline, but the weight loss was not significant, compared with the control group, and there was a wide spread of effects (that is, some lost a lot of weight, others didn’t lose much weight).
“That being said, the JAMA Internal Medicine paper is the largest paper to date of time-restricted eating randomized versus control, so its findings need to be acknowledged and recognized,” Dr. Chow said.
Peterson reported that her group recently completed a 14-week intervention in 90 adults with obesity divided into two groups:
- Control: Continuous energy restriction, self-selected ≥ 12-hour window.
- Early time-restricted eating: 8-hour window from 7 a.m. to 3 p.m.
The findings will provide further insight into the benefits of time-restricted eating.
How might time-restricted eating lead to weight loss?
Dr. Peterson concluded by presenting data suggesting how time-restricted eating may induce weight loss.
In a 4-day crossover study in 11 overweight adults, time-restricted eating did not affect energy expenditure, but it lessened swings in subjective hunger, improved appetite hormones including ghrelin, and increased fat oxidation.
Most trials have reported that time-restricted eating improves one or more cardiometabolic endpoints, she noted.
Early time-restricted eating was associated with improved insulin sensitivity and secretion, blood pressure, and oxidative stress, but not better lipid levels.
In contrast, compared with eating 3 meals/day (control), late time-restricted eating (eating 1 meal/day from 5 p.m. to 9 p.m.) was associated with worsened cardiometabolic health (glucose, insulin, blood pressure, and lipid levels) in an 8-week crossover study in 15 participants.
Dr. Peterson and Dr. Chow reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Time-restricted eating – that is, reducing the number of hours a person is allowed to eat during the day – may produce a modest 1%-4% weight loss, even without cutting calories, early studies in humans suggest. But more research is needed to provide definitive evidence.
This type of intermittent fasting also appears to improve blood glucose, blood pressure, and oxidative stress, said Courtney M. Peterson, PhD, a researcher at the University of Alabama at Birmingham, summarizing what is known about the potential weight-loss strategy at the annual scientific sessions of the American Diabetes Association.
The best results were seen with early time-restricted eating (that is, ending the nighttime fasting early in the day) and allowing a person to eat 8-10 hours each day (for example, 8 a.m. to 4 p.m. or 8 a.m. to 6 p.m.), with fasting and only water allowed the remaining hours, she reported.
However, the 3 dozen or so studies in humans to date are mainly small, pilot, or single-arm studies lasting up to 3 months, and there are only three main randomized, controlled trials with 25 or more participants in each group.
Large trials with around 260 participants are needed, Dr. Peterson said, “before drawing definitive conclusions” about the weight-loss and cardiometabolic benefits of time-restricted eating.
Invited to comment, session chair Lisa S. Chow, MD, an associate professor of medicine in the endocrine and diabetes division at the University of Minnesota, Minneapolis, similarly said: “I think time-restricted eating is promising because of its simple message and noted weight-loss benefit, yet more data are needed.”
“Many uncertainties remain,” she added, “including the potential concern that time-restricted eating may be associated with lean [muscle] mass loss and identifying the populations most likely to benefit from time-restricted eating,” she said.
36 small studies, a review, a meta-analysis, 3 RCTs
There have been about three dozen small studies of time-restricted eating in humans, which examined 4- to 11-hour eating windows, Dr. Peterson explained.
A systematic review of 23 trials of time-restricted eating reported that, on average, participants lost 3% of their initial weight. And a meta-analysis of 19 trials in 475 participants found a –0.9 kg mean difference effect for weight loss.
However, those two analyses did not compare time-restricted eating with a control treatment, she stressed.
The largest randomized, controlled trial is a 12-week study in 271 adults with nonalcoholic fatty liver disease in China, Dr. Peterson said.
The researchers compared three groups:
- Alternate-day modified fasting: healthy meal provided.
- Time-restricted eating: 8-hour window, healthy meal provided.
- Control: 20% calorie reduction, no meal provided.
At 4 and 12 weeks, adults in the two treatment groups lost more weight than those in the control group, but “this was not a fair comparison” because of the lack of a provided meal in the control group, Dr. Peterson pointed out.
The next largest randomized, controlled study is the 12-week TREAT trial, published online in JAMA Internal Medicine in October 2020.
The researchers, from the University of California, San Francisco, randomized 116 adults into two groups:
- 8-hour time-restricted eating from noon to 8 p.m..
- Control: three meals/day.
Time-restricted eating did not lead to greater weight loss, compared with three structured meals a day, which was not surprising, Dr. Chow said, as “participants just reported whether they were engaged in time-restricted eating in a yes/no answer.”
Moreover, “there was no objective measure of their eating window. From our study, we showed that the extent of eating window restriction matters, not just time-restricted eating participation.”
Also, in TREAT, the eating window was noon to 8 p.m. (considered late for time-restricted eating), and the trial also allowed noncaloric beverages outside the window, whereas most studies only allow water and medications.
Lastly, TREAT showed that time-restricted eating reduced weight, compared with baseline, but the weight loss was not significant, compared with the control group, and there was a wide spread of effects (that is, some lost a lot of weight, others didn’t lose much weight).
“That being said, the JAMA Internal Medicine paper is the largest paper to date of time-restricted eating randomized versus control, so its findings need to be acknowledged and recognized,” Dr. Chow said.
Peterson reported that her group recently completed a 14-week intervention in 90 adults with obesity divided into two groups:
- Control: Continuous energy restriction, self-selected ≥ 12-hour window.
- Early time-restricted eating: 8-hour window from 7 a.m. to 3 p.m.
The findings will provide further insight into the benefits of time-restricted eating.
How might time-restricted eating lead to weight loss?
Dr. Peterson concluded by presenting data suggesting how time-restricted eating may induce weight loss.
In a 4-day crossover study in 11 overweight adults, time-restricted eating did not affect energy expenditure, but it lessened swings in subjective hunger, improved appetite hormones including ghrelin, and increased fat oxidation.
Most trials have reported that time-restricted eating improves one or more cardiometabolic endpoints, she noted.
Early time-restricted eating was associated with improved insulin sensitivity and secretion, blood pressure, and oxidative stress, but not better lipid levels.
In contrast, compared with eating 3 meals/day (control), late time-restricted eating (eating 1 meal/day from 5 p.m. to 9 p.m.) was associated with worsened cardiometabolic health (glucose, insulin, blood pressure, and lipid levels) in an 8-week crossover study in 15 participants.
Dr. Peterson and Dr. Chow reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Bariatric surgery leads to better cardiovascular function in pregnancy
Pregnant women with a history of bariatric surgery have better cardiovascular adaptation to pregnancy compared with women who have similar early-pregnancy body mass index (BMI) but no history of weight loss surgery, new data suggest.
“Pregnant women who have had bariatric surgery demonstrate better cardiovascular adaptation through lower blood pressure, heart rate, and cardiac output, more favorable diastolic indices, and better systolic function,” reported Deesha Patel, MBBS MRCOG, specialist registrar, Chelsea and Westminster Hospital, London.
“Because the groups were matched for early pregnancy BMI, it’s unlikely that the results are due to weight loss alone but indicate that the metabolic alterations as a result of the surgery, via the enterocardiac axis, play an important role,” Dr. Patel continued.
The findings were presented at the Royal College of Obstetricians and Gynecologists 2021 Virtual World Congress.
Although obesity is known for its inflammatory and toxic effects on the cardiovascular system, it is not clear to what extent the various treatment options for obesity modify these risks in the long term, said Hutan Ashrafian, MD, clinical lecturer in surgery, Imperial College London.
“It is even less clear how anti-obesity interventions affect the cardiovascular system in pregnancy,” Dr. Ashrafian told this news organization.
“This very novel study in pregnant mothers having undergone the most successful and consistent intervention for severe obesity – bariatric or metabolic surgery – gives new clues as to the extent that bariatric procedures can alter cardiovascular risk in pregnant mothers,” continued Dr. Ashrafian, who was not involved in the study.
The results show how bariatric surgery has favorable effects on cardiac adaptation in pregnancy and in turn “might offer protection from pregnancy-related cardiovascular pathology such as preeclampsia,” explained Dr. Ashrafian. “This adds to the known effects of cardiovascular protection of bariatric surgery through the enterocardiac axis, which may explain a wider range of effects that can be translated within pregnancy and possibly following pregnancy in the postpartum era and beyond.”
A history of bariatric surgery versus no surgery
The prospective, longitudinal study compared 41 women who had a history of bariatric surgery with 41 women who had not undergone surgery. Patients’ characteristics were closely matched for age, BMI (34.5 kg/m2 and 34.3 kg/m2 in the surgery and bariatric surgery groups, respectively) and race. Hypertensive disorders in the post-surgery group were significantly less common compared with the no-surgery group (0% vs. 9.8%).
During the study, participants underwent cardiovascular assessment at 12-14 weeks, 20-24 weeks, and 30-32 weeks of gestation. The assessment included measurement of blood pressure and heart rate, transthoracic echocardiography, and 2D speckle tracking, performed offline to assess global longitudinal and circumferential strain.
Blood pressure readings across the three trimesters were consistently lower in the women who had undergone bariatric surgery compared with those in the no-surgery group, and all differences were statistically significant. Likewise, heart rate and cardiac output across the three trimesters were lower in the post-surgery cohort. However, there was no difference in stroke volume between the two groups.
As for diastolic function, there were more favorable indices in the post-surgery group with a higher E/A ratio, a marker of left ventricle filling (P < .001), and lower left atrial volume (P < .05), Dr. Patel reported.
With respect to systolic function, there was no difference in ejection fraction, but there was lower global longitudinal strain (P < .01) and global circumferential strain in the post-bariatric group (P = .02), suggesting better systolic function.
“Strain is a measure of differences in motion and velocity between regions of the myocardium through the cardiac cycle and can detect subclinical changes when ejection fraction is normal,” she added.
“This is a fascinating piece of work. The author should be congratulated on gathering so many [pregnant] women who had had bariatric surgery. The work gives a unique glimpse into metabolic syndrome,” said Philip Toozs-Hobson, MD, who moderated the session.
“We are increasingly recognizing the impact [of bariatric surgery] on metabolic syndrome, and the fact that this study demonstrates that there is more to it than just weight is important,” continued Dr. Toosz-Hobson, who is a consultant gynecologist at Birmingham Women’s Hospital NHS Foundation Trust, United Kingdom.
Cardiovascular benefits of bariatric surgery
Bariatric surgery has been associated with loss of excess body weight of up to 55% and with approximately 40% reduction in all-cause mortality in the general population. The procedure also reduces the risk for heart disease, diabetes, and cancer.
The cardiovascular benefits of bariatric surgery include reduced hypertension, remodeling of the heart with a reduction in left ventricular mass, and an improvement in diastolic and systolic function.
“Traditionally, the cardiac changes were thought to be due to weight loss and blood pressure reduction, but it is now conceivable that the metabolic components contribute to the reverse modeling via changes to the enterocardiac axis involving changes to gut hormones,” said Dr. Patel. These hormones include secretin, glucagon, and vasoactive intestinal peptide, which are known to have inotropic effects, as well as adiponectin and leptin, which are known to have cardiac effects, she added.
“Pregnancy following bariatric surgery is associated with a reduced risk of hypertensive disorders, as well as a reduced risk of gestational diabetes, large-for-gestational-age neonates, and a small increased risk of small-for-gestational-age neonates,” said Dr. Patel.
Dr. Patel and Dr. Toosz-Hobson have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women with a history of bariatric surgery have better cardiovascular adaptation to pregnancy compared with women who have similar early-pregnancy body mass index (BMI) but no history of weight loss surgery, new data suggest.
“Pregnant women who have had bariatric surgery demonstrate better cardiovascular adaptation through lower blood pressure, heart rate, and cardiac output, more favorable diastolic indices, and better systolic function,” reported Deesha Patel, MBBS MRCOG, specialist registrar, Chelsea and Westminster Hospital, London.
“Because the groups were matched for early pregnancy BMI, it’s unlikely that the results are due to weight loss alone but indicate that the metabolic alterations as a result of the surgery, via the enterocardiac axis, play an important role,” Dr. Patel continued.
The findings were presented at the Royal College of Obstetricians and Gynecologists 2021 Virtual World Congress.
Although obesity is known for its inflammatory and toxic effects on the cardiovascular system, it is not clear to what extent the various treatment options for obesity modify these risks in the long term, said Hutan Ashrafian, MD, clinical lecturer in surgery, Imperial College London.
“It is even less clear how anti-obesity interventions affect the cardiovascular system in pregnancy,” Dr. Ashrafian told this news organization.
“This very novel study in pregnant mothers having undergone the most successful and consistent intervention for severe obesity – bariatric or metabolic surgery – gives new clues as to the extent that bariatric procedures can alter cardiovascular risk in pregnant mothers,” continued Dr. Ashrafian, who was not involved in the study.
The results show how bariatric surgery has favorable effects on cardiac adaptation in pregnancy and in turn “might offer protection from pregnancy-related cardiovascular pathology such as preeclampsia,” explained Dr. Ashrafian. “This adds to the known effects of cardiovascular protection of bariatric surgery through the enterocardiac axis, which may explain a wider range of effects that can be translated within pregnancy and possibly following pregnancy in the postpartum era and beyond.”
A history of bariatric surgery versus no surgery
The prospective, longitudinal study compared 41 women who had a history of bariatric surgery with 41 women who had not undergone surgery. Patients’ characteristics were closely matched for age, BMI (34.5 kg/m2 and 34.3 kg/m2 in the surgery and bariatric surgery groups, respectively) and race. Hypertensive disorders in the post-surgery group were significantly less common compared with the no-surgery group (0% vs. 9.8%).
During the study, participants underwent cardiovascular assessment at 12-14 weeks, 20-24 weeks, and 30-32 weeks of gestation. The assessment included measurement of blood pressure and heart rate, transthoracic echocardiography, and 2D speckle tracking, performed offline to assess global longitudinal and circumferential strain.
Blood pressure readings across the three trimesters were consistently lower in the women who had undergone bariatric surgery compared with those in the no-surgery group, and all differences were statistically significant. Likewise, heart rate and cardiac output across the three trimesters were lower in the post-surgery cohort. However, there was no difference in stroke volume between the two groups.
As for diastolic function, there were more favorable indices in the post-surgery group with a higher E/A ratio, a marker of left ventricle filling (P < .001), and lower left atrial volume (P < .05), Dr. Patel reported.
With respect to systolic function, there was no difference in ejection fraction, but there was lower global longitudinal strain (P < .01) and global circumferential strain in the post-bariatric group (P = .02), suggesting better systolic function.
“Strain is a measure of differences in motion and velocity between regions of the myocardium through the cardiac cycle and can detect subclinical changes when ejection fraction is normal,” she added.
“This is a fascinating piece of work. The author should be congratulated on gathering so many [pregnant] women who had had bariatric surgery. The work gives a unique glimpse into metabolic syndrome,” said Philip Toozs-Hobson, MD, who moderated the session.
“We are increasingly recognizing the impact [of bariatric surgery] on metabolic syndrome, and the fact that this study demonstrates that there is more to it than just weight is important,” continued Dr. Toosz-Hobson, who is a consultant gynecologist at Birmingham Women’s Hospital NHS Foundation Trust, United Kingdom.
Cardiovascular benefits of bariatric surgery
Bariatric surgery has been associated with loss of excess body weight of up to 55% and with approximately 40% reduction in all-cause mortality in the general population. The procedure also reduces the risk for heart disease, diabetes, and cancer.
The cardiovascular benefits of bariatric surgery include reduced hypertension, remodeling of the heart with a reduction in left ventricular mass, and an improvement in diastolic and systolic function.
“Traditionally, the cardiac changes were thought to be due to weight loss and blood pressure reduction, but it is now conceivable that the metabolic components contribute to the reverse modeling via changes to the enterocardiac axis involving changes to gut hormones,” said Dr. Patel. These hormones include secretin, glucagon, and vasoactive intestinal peptide, which are known to have inotropic effects, as well as adiponectin and leptin, which are known to have cardiac effects, she added.
“Pregnancy following bariatric surgery is associated with a reduced risk of hypertensive disorders, as well as a reduced risk of gestational diabetes, large-for-gestational-age neonates, and a small increased risk of small-for-gestational-age neonates,” said Dr. Patel.
Dr. Patel and Dr. Toosz-Hobson have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women with a history of bariatric surgery have better cardiovascular adaptation to pregnancy compared with women who have similar early-pregnancy body mass index (BMI) but no history of weight loss surgery, new data suggest.
“Pregnant women who have had bariatric surgery demonstrate better cardiovascular adaptation through lower blood pressure, heart rate, and cardiac output, more favorable diastolic indices, and better systolic function,” reported Deesha Patel, MBBS MRCOG, specialist registrar, Chelsea and Westminster Hospital, London.
“Because the groups were matched for early pregnancy BMI, it’s unlikely that the results are due to weight loss alone but indicate that the metabolic alterations as a result of the surgery, via the enterocardiac axis, play an important role,” Dr. Patel continued.
The findings were presented at the Royal College of Obstetricians and Gynecologists 2021 Virtual World Congress.
Although obesity is known for its inflammatory and toxic effects on the cardiovascular system, it is not clear to what extent the various treatment options for obesity modify these risks in the long term, said Hutan Ashrafian, MD, clinical lecturer in surgery, Imperial College London.
“It is even less clear how anti-obesity interventions affect the cardiovascular system in pregnancy,” Dr. Ashrafian told this news organization.
“This very novel study in pregnant mothers having undergone the most successful and consistent intervention for severe obesity – bariatric or metabolic surgery – gives new clues as to the extent that bariatric procedures can alter cardiovascular risk in pregnant mothers,” continued Dr. Ashrafian, who was not involved in the study.
The results show how bariatric surgery has favorable effects on cardiac adaptation in pregnancy and in turn “might offer protection from pregnancy-related cardiovascular pathology such as preeclampsia,” explained Dr. Ashrafian. “This adds to the known effects of cardiovascular protection of bariatric surgery through the enterocardiac axis, which may explain a wider range of effects that can be translated within pregnancy and possibly following pregnancy in the postpartum era and beyond.”
A history of bariatric surgery versus no surgery
The prospective, longitudinal study compared 41 women who had a history of bariatric surgery with 41 women who had not undergone surgery. Patients’ characteristics were closely matched for age, BMI (34.5 kg/m2 and 34.3 kg/m2 in the surgery and bariatric surgery groups, respectively) and race. Hypertensive disorders in the post-surgery group were significantly less common compared with the no-surgery group (0% vs. 9.8%).
During the study, participants underwent cardiovascular assessment at 12-14 weeks, 20-24 weeks, and 30-32 weeks of gestation. The assessment included measurement of blood pressure and heart rate, transthoracic echocardiography, and 2D speckle tracking, performed offline to assess global longitudinal and circumferential strain.
Blood pressure readings across the three trimesters were consistently lower in the women who had undergone bariatric surgery compared with those in the no-surgery group, and all differences were statistically significant. Likewise, heart rate and cardiac output across the three trimesters were lower in the post-surgery cohort. However, there was no difference in stroke volume between the two groups.
As for diastolic function, there were more favorable indices in the post-surgery group with a higher E/A ratio, a marker of left ventricle filling (P < .001), and lower left atrial volume (P < .05), Dr. Patel reported.
With respect to systolic function, there was no difference in ejection fraction, but there was lower global longitudinal strain (P < .01) and global circumferential strain in the post-bariatric group (P = .02), suggesting better systolic function.
“Strain is a measure of differences in motion and velocity between regions of the myocardium through the cardiac cycle and can detect subclinical changes when ejection fraction is normal,” she added.
“This is a fascinating piece of work. The author should be congratulated on gathering so many [pregnant] women who had had bariatric surgery. The work gives a unique glimpse into metabolic syndrome,” said Philip Toozs-Hobson, MD, who moderated the session.
“We are increasingly recognizing the impact [of bariatric surgery] on metabolic syndrome, and the fact that this study demonstrates that there is more to it than just weight is important,” continued Dr. Toosz-Hobson, who is a consultant gynecologist at Birmingham Women’s Hospital NHS Foundation Trust, United Kingdom.
Cardiovascular benefits of bariatric surgery
Bariatric surgery has been associated with loss of excess body weight of up to 55% and with approximately 40% reduction in all-cause mortality in the general population. The procedure also reduces the risk for heart disease, diabetes, and cancer.
The cardiovascular benefits of bariatric surgery include reduced hypertension, remodeling of the heart with a reduction in left ventricular mass, and an improvement in diastolic and systolic function.
“Traditionally, the cardiac changes were thought to be due to weight loss and blood pressure reduction, but it is now conceivable that the metabolic components contribute to the reverse modeling via changes to the enterocardiac axis involving changes to gut hormones,” said Dr. Patel. These hormones include secretin, glucagon, and vasoactive intestinal peptide, which are known to have inotropic effects, as well as adiponectin and leptin, which are known to have cardiac effects, she added.
“Pregnancy following bariatric surgery is associated with a reduced risk of hypertensive disorders, as well as a reduced risk of gestational diabetes, large-for-gestational-age neonates, and a small increased risk of small-for-gestational-age neonates,” said Dr. Patel.
Dr. Patel and Dr. Toosz-Hobson have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Stopping statins linked to death, CV events in elderly
Deprescribing may help in reducing inappropriate medication use and adverse events, but for cardiovascular care in the elderly, eliminating statins among patients taking other medications may have negative effects that far outweigh the benefits, a new study suggests.
In a large cohort study, researchers found that the withdrawal of statins from an elderly population receiving polypharmacy was associated with an increase in the risk for hospital admission for heart failure and any cardiovascular outcome, as well as death from any cause.
Statins are “lifesaving” drugs, and “according to the findings of our study, the discontinuation of this therapy has significant effects,” lead study author Federico Rea, PhD, research fellow, Laboratory of Healthcare Research and Pharmacoepidemiology, the department of statistics and quantitative methods, the University of Milano-Bicocca, said in an interview.
The article was published online June 14, 2021, in JAMA Network Open.
Negative clinical consequences, including adverse drug reactions leading to hospitalizations, are causing more physicians to consider deprescribing as a way to reduce problems associated with polypharmacy, the researchers noted.
Statins are “the most widely prescribed medication in the Western world, being a pivotal component in the primary and secondary prevention of cardiovascular (CV) diseases,” they wrote, but because randomized trials usually exclude patients with serious clinical conditions, the precise role statins play for frail patients, such as those with polypharmacy, “is still unclear.”
The population-based cohort study examined 29,047 Italian residents aged 65 years and older who were receiving uninterrupted treatment with statins as well as blood pressure–lowering, antidiabetic, and antiplatelet agents over 16 months. The follow-up period was more than 3 years.
The cohort members were followed to identify those for whom statins were discontinued. Those who continued taking other therapies during the first 6 months after stopping statins were propensity score matched in a 1:1 ratio with patients who did not discontinue taking statins or other drugs. The patient pairs were then followed for fatal and nonfatal outcomes to estimate the risk associated with statin discontinuation.
Of the overall cohort exposed to polypharmacy, 5819 (20.0%) discontinued statins while continuing to take their other medications. Of those, 4,010 were matched with a comparator.
Compared with the maintaining group, those who discontinued statins had the following outcomes: an increased risk for hospital admissions for heart failure (hazard ratio, 1.24; 95% confidence interval, 1.07-1.43), any cardiovascular outcomes (HR, 1.14; 95% CI, 1.03-1.26), death from any cause (HR, 1.15; 95% CI, 1.02-1.30), and emergency admissions for any cause (HR, 1.12; 95% CI, 1.01-1.19)
The increased risk occurred in patients with mild or severe profiles, regardless of gender and whether statins were prescribed as primary or secondary CV prevention.
“We expected that the discontinuation of statins could reduce the risk of access to the emergency department for neurological causes, considered a proxy for the onset of episodes of delirium, [but] this was not observed, suggesting that statin therapy has essential benefits on the reduction of fatal/nonfatal cardiovascular events with no harm effect,” said Dr. Rea, “at least considering major adverse events like hospital and emergency department admissions.”
Findings no surprise
Neil Stone, MD, Bonow Professor of Medicine (Cardiology) and Preventive Medicine at Northwestern University, Chicago, said the study results aren’t surprising.
“Older patients have a higher absolute risk of dying, and withdrawing proven therapy shown to reduce risk of coronary/stroke events in randomized, controlled trials would be expected to result in more cardiovascular events,” Dr. Stone said.
Although polypharmacy is a concern for the elderly and is a factor in decreased adherence, he said better solutions are needed than withdrawing proven, effective therapy. “In that sense, this study indirectly supports more research in the use of polypills to address cardiovascular risk factors,” he said. Giving a single pill that combines medications of proven value in reducing blood pressure and cholesterol might be preferable to reducing the total number of medications.
Given the complexity of polypharmacy, the study investigators say more attention is needed from all health care professionals who care for elderly patients.
“We hope that future studies can shed light on the best way to balance the undeniable benefit of [statins] and the harms, especially among the elderly exposed to polypharmacy,” said Rea.
Further research is also needed into why statins are discontinued in the first place, added Dr. Stone. “We know that statins often are stopped due to symptoms that on further scrutiny may not be related to statin use.”
The study was funded by grants from Fondo d’Ateneo per la Ricerca and Modelling Effectiveness, Cost-effectiveness, and Promoting Health Care Value in the Real World: the Motive Project from the Italian Ministry of the Education, University, and Research. One coauthor served on the advisory board of Roche and has received grants from Bristol Myers Squibb, GlaxoSmithKline, and Novartis outside the submitted work. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Deprescribing may help in reducing inappropriate medication use and adverse events, but for cardiovascular care in the elderly, eliminating statins among patients taking other medications may have negative effects that far outweigh the benefits, a new study suggests.
In a large cohort study, researchers found that the withdrawal of statins from an elderly population receiving polypharmacy was associated with an increase in the risk for hospital admission for heart failure and any cardiovascular outcome, as well as death from any cause.
Statins are “lifesaving” drugs, and “according to the findings of our study, the discontinuation of this therapy has significant effects,” lead study author Federico Rea, PhD, research fellow, Laboratory of Healthcare Research and Pharmacoepidemiology, the department of statistics and quantitative methods, the University of Milano-Bicocca, said in an interview.
The article was published online June 14, 2021, in JAMA Network Open.
Negative clinical consequences, including adverse drug reactions leading to hospitalizations, are causing more physicians to consider deprescribing as a way to reduce problems associated with polypharmacy, the researchers noted.
Statins are “the most widely prescribed medication in the Western world, being a pivotal component in the primary and secondary prevention of cardiovascular (CV) diseases,” they wrote, but because randomized trials usually exclude patients with serious clinical conditions, the precise role statins play for frail patients, such as those with polypharmacy, “is still unclear.”
The population-based cohort study examined 29,047 Italian residents aged 65 years and older who were receiving uninterrupted treatment with statins as well as blood pressure–lowering, antidiabetic, and antiplatelet agents over 16 months. The follow-up period was more than 3 years.
The cohort members were followed to identify those for whom statins were discontinued. Those who continued taking other therapies during the first 6 months after stopping statins were propensity score matched in a 1:1 ratio with patients who did not discontinue taking statins or other drugs. The patient pairs were then followed for fatal and nonfatal outcomes to estimate the risk associated with statin discontinuation.
Of the overall cohort exposed to polypharmacy, 5819 (20.0%) discontinued statins while continuing to take their other medications. Of those, 4,010 were matched with a comparator.
Compared with the maintaining group, those who discontinued statins had the following outcomes: an increased risk for hospital admissions for heart failure (hazard ratio, 1.24; 95% confidence interval, 1.07-1.43), any cardiovascular outcomes (HR, 1.14; 95% CI, 1.03-1.26), death from any cause (HR, 1.15; 95% CI, 1.02-1.30), and emergency admissions for any cause (HR, 1.12; 95% CI, 1.01-1.19)
The increased risk occurred in patients with mild or severe profiles, regardless of gender and whether statins were prescribed as primary or secondary CV prevention.
“We expected that the discontinuation of statins could reduce the risk of access to the emergency department for neurological causes, considered a proxy for the onset of episodes of delirium, [but] this was not observed, suggesting that statin therapy has essential benefits on the reduction of fatal/nonfatal cardiovascular events with no harm effect,” said Dr. Rea, “at least considering major adverse events like hospital and emergency department admissions.”
Findings no surprise
Neil Stone, MD, Bonow Professor of Medicine (Cardiology) and Preventive Medicine at Northwestern University, Chicago, said the study results aren’t surprising.
“Older patients have a higher absolute risk of dying, and withdrawing proven therapy shown to reduce risk of coronary/stroke events in randomized, controlled trials would be expected to result in more cardiovascular events,” Dr. Stone said.
Although polypharmacy is a concern for the elderly and is a factor in decreased adherence, he said better solutions are needed than withdrawing proven, effective therapy. “In that sense, this study indirectly supports more research in the use of polypills to address cardiovascular risk factors,” he said. Giving a single pill that combines medications of proven value in reducing blood pressure and cholesterol might be preferable to reducing the total number of medications.
Given the complexity of polypharmacy, the study investigators say more attention is needed from all health care professionals who care for elderly patients.
“We hope that future studies can shed light on the best way to balance the undeniable benefit of [statins] and the harms, especially among the elderly exposed to polypharmacy,” said Rea.
Further research is also needed into why statins are discontinued in the first place, added Dr. Stone. “We know that statins often are stopped due to symptoms that on further scrutiny may not be related to statin use.”
The study was funded by grants from Fondo d’Ateneo per la Ricerca and Modelling Effectiveness, Cost-effectiveness, and Promoting Health Care Value in the Real World: the Motive Project from the Italian Ministry of the Education, University, and Research. One coauthor served on the advisory board of Roche and has received grants from Bristol Myers Squibb, GlaxoSmithKline, and Novartis outside the submitted work. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Deprescribing may help in reducing inappropriate medication use and adverse events, but for cardiovascular care in the elderly, eliminating statins among patients taking other medications may have negative effects that far outweigh the benefits, a new study suggests.
In a large cohort study, researchers found that the withdrawal of statins from an elderly population receiving polypharmacy was associated with an increase in the risk for hospital admission for heart failure and any cardiovascular outcome, as well as death from any cause.
Statins are “lifesaving” drugs, and “according to the findings of our study, the discontinuation of this therapy has significant effects,” lead study author Federico Rea, PhD, research fellow, Laboratory of Healthcare Research and Pharmacoepidemiology, the department of statistics and quantitative methods, the University of Milano-Bicocca, said in an interview.
The article was published online June 14, 2021, in JAMA Network Open.
Negative clinical consequences, including adverse drug reactions leading to hospitalizations, are causing more physicians to consider deprescribing as a way to reduce problems associated with polypharmacy, the researchers noted.
Statins are “the most widely prescribed medication in the Western world, being a pivotal component in the primary and secondary prevention of cardiovascular (CV) diseases,” they wrote, but because randomized trials usually exclude patients with serious clinical conditions, the precise role statins play for frail patients, such as those with polypharmacy, “is still unclear.”
The population-based cohort study examined 29,047 Italian residents aged 65 years and older who were receiving uninterrupted treatment with statins as well as blood pressure–lowering, antidiabetic, and antiplatelet agents over 16 months. The follow-up period was more than 3 years.
The cohort members were followed to identify those for whom statins were discontinued. Those who continued taking other therapies during the first 6 months after stopping statins were propensity score matched in a 1:1 ratio with patients who did not discontinue taking statins or other drugs. The patient pairs were then followed for fatal and nonfatal outcomes to estimate the risk associated with statin discontinuation.
Of the overall cohort exposed to polypharmacy, 5819 (20.0%) discontinued statins while continuing to take their other medications. Of those, 4,010 were matched with a comparator.
Compared with the maintaining group, those who discontinued statins had the following outcomes: an increased risk for hospital admissions for heart failure (hazard ratio, 1.24; 95% confidence interval, 1.07-1.43), any cardiovascular outcomes (HR, 1.14; 95% CI, 1.03-1.26), death from any cause (HR, 1.15; 95% CI, 1.02-1.30), and emergency admissions for any cause (HR, 1.12; 95% CI, 1.01-1.19)
The increased risk occurred in patients with mild or severe profiles, regardless of gender and whether statins were prescribed as primary or secondary CV prevention.
“We expected that the discontinuation of statins could reduce the risk of access to the emergency department for neurological causes, considered a proxy for the onset of episodes of delirium, [but] this was not observed, suggesting that statin therapy has essential benefits on the reduction of fatal/nonfatal cardiovascular events with no harm effect,” said Dr. Rea, “at least considering major adverse events like hospital and emergency department admissions.”
Findings no surprise
Neil Stone, MD, Bonow Professor of Medicine (Cardiology) and Preventive Medicine at Northwestern University, Chicago, said the study results aren’t surprising.
“Older patients have a higher absolute risk of dying, and withdrawing proven therapy shown to reduce risk of coronary/stroke events in randomized, controlled trials would be expected to result in more cardiovascular events,” Dr. Stone said.
Although polypharmacy is a concern for the elderly and is a factor in decreased adherence, he said better solutions are needed than withdrawing proven, effective therapy. “In that sense, this study indirectly supports more research in the use of polypills to address cardiovascular risk factors,” he said. Giving a single pill that combines medications of proven value in reducing blood pressure and cholesterol might be preferable to reducing the total number of medications.
Given the complexity of polypharmacy, the study investigators say more attention is needed from all health care professionals who care for elderly patients.
“We hope that future studies can shed light on the best way to balance the undeniable benefit of [statins] and the harms, especially among the elderly exposed to polypharmacy,” said Rea.
Further research is also needed into why statins are discontinued in the first place, added Dr. Stone. “We know that statins often are stopped due to symptoms that on further scrutiny may not be related to statin use.”
The study was funded by grants from Fondo d’Ateneo per la Ricerca and Modelling Effectiveness, Cost-effectiveness, and Promoting Health Care Value in the Real World: the Motive Project from the Italian Ministry of the Education, University, and Research. One coauthor served on the advisory board of Roche and has received grants from Bristol Myers Squibb, GlaxoSmithKline, and Novartis outside the submitted work. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ADA scientific sessions address the old and the new
Long-awaited twincretin data, a study to inform prescribing in type 2 diabetes, COVID-19 and diabetes, and new guidance for treating type 1 diabetes in adults will be among the hot topics at the annual scientific sessions of the American Diabetes Association.
The meeting, to be held virtually for a second year, will take place June 25-29. As usual, the sessions will cover a wide range of basic, translational, and clinical material pertaining to type 1 and type 2 diabetes, complications, related subjects such as obesity and cardiovascular disease, and health care delivery.
New to this year’s agenda is COVID-19 and the many ways it has affected people with diabetes and health care delivery. And, more than in the past, the meeting will focus on ethnic and racial disparities in the delivery of care to people with diabetes.
And of course, there will be a tribute to another special aspect of 2021: the 100th anniversary of the discovery of insulin.
“I think there will undoubtedly be several things that will come out of this meeting that will change practice, and it will be important for clinicians to be aware of those, whether that’s groundbreaking trials or interpretation of data that will help us understand the interrelation between diabetes and COVID-19, which is still with us,” ADA chief scientific and medical officer Robert A. Gabbay, MD, PhD, said in an interview.
And ADA president of medicine and science Ruth S. Weinstock, MD, PhD, said in an interview: “I think there are many exciting sessions at this year’s meeting. ...I hope that it will help [clinicians] take better care of their patients with diabetes.
”
Will the twincretin tirzepatide live up to the hype?
Between December 2020 and May 2021, Eli Lilly issued a series of four press releases touting positive top-line results from a series of phase 3 studies on its novel agent tirzepatide, dubbed a twincretin for its dual actions as an agonist of the glucose-dependent insulinotropic polypeptide (GIP) and glucagonlike peptide-1 receptors.
Detailed results from those four trials, SURPASS-1, -2, -3, and -5, will be presented in a symposium on Tuesday, June 29. Results from SURPASS-4 will be presented at the annual meeting of the European Association for the Study of Diabetes in September 2021.
According to the company, the drug met its phase 3 primary efficacy endpoints for both hemoglobin A1c reduction and weight loss.
“At least the buzz on it has been good, but now we want to see the real data,” Dr. Gabbay said, noting that “the early data on weight loss in particular were quite good. So then the question would be: Do you go to a GLP-1 [agonist] or a dual agonist? There will be studies to tease that out.”
Regarding tirzepatide, Dr. Weinstock said: “Hopefully, more people with type 2 diabetes could achieve their glycemic goals, and those who would benefit from weight loss could have better weight loss. I haven’t seen the data, but if the addition of GIP can further improve glucose lowering as well as weight loss that would be great.”
How far will GRADE go in answering the second-drug question?
On Monday, June 28, results will be presented from the long-awaited Glycemia Reduction Approaches in Diabetes – A Comparative Effectiveness (GRADE) study.
Launched in 2013, the trial is funded by the National Institutes of Health and several pharmaceutical company partners. Over 5,000 patients diagnosed with type 2 diabetes within the prior 10 years and already taking metformin were randomized to one of four commonly used second-line glucose-lowering agents: glimepiride, sitagliptin, liraglutide, and basal insulin glargine. The aim was to determine which combination produced the best glycemic control with the fewest side effects.
Dr. Weinstock said: “Clinicians now have increasing numbers of medications to choose from when treating hyperglycemia in type 2 diabetes, and a common dilemma is which one to select. The results of GRADE should be informative for people taking care of type 2 diabetes in different populations.”
However, she also pointed out that GRADE does not include a group with a sodium-glucose transporter 2 inhibitor, as the trial was designed prior to the availability of the drug class. Now, SGLT2 inhibitors are widely used and recommended for cardiovascular and kidney benefit as well as glucose lowering.
“I believe the future is really precision medicine where we individualize treatment. So, for someone with heart failure you might choose an SGLT2 inhibitor, but there are plenty of other subpopulations. They are going to be looking at different subpopulations. I think we’re all very interested in seeing what the results are, but it’s not the end of the story. We will still have to individualize therapy and keep in mind their kidney, heart, heart failure status, and other factors,” she said.
Dr. Gabbay pointed out that GRADE is important because it’s one of the few comparative effectiveness trials conducted in diabetes. “I think it will be very rich [data] that will impact practice in a variety of ways. On the one hand, it doesn’t do everything we’d want it to do, but on the other hand, if you think of the number of comparative effectiveness trials in diabetes, there are not a lot ... I think it will be big.”
COVID-19 and diabetes: A lot to discuss
In contrast to the ADA scientific sessions in 2020, which took place too soon after the start of the COVID-19 pandemic to include much material about it, this year’s meeting will address many different aspects of the novel coronavirus.
Sessions will cover minimizing risk in people with diabetes during the pandemic, the latest data on whether COVID-19 triggers diabetes, and if so, by what mechanism, mental health issues related to COVID-19, as well as the management of foot care, pregnancy, and the pediatric population during the pandemic.
On Sunday, June 27, a symposium will be devoted to results of the DARE-19 trial, which explored the effects of the SGLT2 inhibitor dapagliflozin in more than 1200 patients hospitalized with COVID-19. The overall results, presented in May 2021 at the scientific sessions of the American College of Cardiology, showed a nonsignificant trend for benefit in time to organ failure or death compared with placebo. At ADA, separate efficacy and safety results for patients with and without diabetes will be presented.
According to Dr. Weinstock, “We know that in nonhospitalized patients with type 2 diabetes the SGLT2 inhibitors can help preserve kidney function and reduce heart failure. But we also know there can be diabetic ketoacidosis and genital infections and other side effects, so it’s been unclear up till now in type 2 diabetes whether they are safe and effective in people hospitalized in respiratory failure with COVID-19. And, given that people with type 2 diabetes and COVID-19 are more likely to require mechanical ventilation and are at greater risk of mortality, we’re anxious to see what these results are.”
Dr. Gabbay commented that, when the DARE study was initiated in April 2020, there were concerns about whether it was safe. And even now, “we’re still not sure about whether SGLT2 inhibitors should be stopped in hospitalized patients. The recommendations say to stop. I think this will be interesting.”
Also to be addressed in several meeting sessions are related issues the pandemic has brought forth, such as the use of telehealth for routine diabetes management, inpatient use of continuous glucose monitoring, and, of course, health care disparities.
“A lot of important issues related to COVID-19 of great interest will be discussed in a variety of sessions,” Dr. Weinstock said.
Type 1 diabetes in adults: It’s not just a pediatric disease
On Monday, June 28, a draft of the first-ever ADA/EASD consensus report on the management of type 1 diabetes in adults will be presented, with the final version slated for the annual meeting of the EASD in September 2021.
A previous ADA position statement had addressed management of type 1 diabetes across all age groups, but this will be the first to focus on adults. This is important, given that type 1 diabetes was formerly called juvenile diabetes and is still often perceived as a childhood disease. Adults who develop it are commonly misdiagnosed as having type 2 diabetes, Dr. Gabbay noted.
“A big-time issue is recognition of type 1 in adults. We often see patients come in who were misdiagnosed, on metformin, and not given insulin. Often they go for a while and get sicker and sicker.” Or, he said, sometimes they’re prescribed insulin but not the intensive regimens that are required for adequate glycemic control in type 1 diabetes. “They can be suboptimally treated and it can take years to get the right therapy. ... It’s unfortunate that they have to experience that.”
Dr. Weinstock, one of the authors of the statement, said it will cover a range of issues, including care schedules, therapies, psychosocial issues, and social determinants of health. “We tried to be comprehensive in this in terms of glycemic management. It doesn’t include a discussion of complications or their management. It really focuses on diagnosis and glycemic management.”
Dealing with disparities: ADA has taken several steps
A priority of the ADA is addressing disparities in the delivery of health care to people with diabetes, both Dr. Weinstock and Dr. Gabbay stressed. Quite a few sessions at the meeting will touch on various aspects, including sessions on Friday afternoon on “Health Care as a Social Justice Issue in the Diagnosis and Management of Diabetes,” and separate sessions on “Challenges and Successes With Health Inequities and Health Disparities in Diabetes” in adult and pediatric populations.
“For us at ADA, addressing health disparities is extremely important and we have a number of new programs this year to address this very important issue,” Dr. Weinstock said.
In August 2020, the ADA issued a Health Equity Bill of Rights, which includes access to insulin and other medications, affordable health care, and freedom from stigma and discrimination. The Association has also requested applications from researchers studying disparities in diabetes care.
Celebrating 100 years of lifesaving medication
Of course, the ADA will be celebrating the 100th anniversary of the discovery of insulin. A session on Saturday afternoon, entitled, “Insulin at Its 100th Birthday,” will cover the history of the landmark discovery, as well as insulin biosynthesis and mechanisms of action, and “the future of insulin as a therapy.”
Dr. Weinstock noted: “The discovery of insulin was an incredible achievement that, of course, saved the lives of many millions of children and adults. Before insulin became available, children and adults only survived for days or at most a few years after diagnosis. We will commemorate this anniversary.”
The virtual platform: Like last year, only better
Dr. Gabbay said in an interview that the virtual setup will be similar to last year’s in that talks will be prerecorded to ensure there are no technical glitches, but for many, presenters will be available afterward for live question and answers.
This year, though, the chat functionality will be enhanced to allow for discussion during the presentation, separate from the scientific question and answers. And, he noted, the virtual exhibit hall will be “bigger and better.”
Despite these improvements, Dr. Gabbay said, the plan is to go back to an in-person meeting in 2022 in New Orleans.
Dr. Weinstock’s institution receives research grants from Medtronic, Insulet, Lilly, Novo Nordisk, and Boehringer Ingelheim. Dr. Gabbay reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-awaited twincretin data, a study to inform prescribing in type 2 diabetes, COVID-19 and diabetes, and new guidance for treating type 1 diabetes in adults will be among the hot topics at the annual scientific sessions of the American Diabetes Association.
The meeting, to be held virtually for a second year, will take place June 25-29. As usual, the sessions will cover a wide range of basic, translational, and clinical material pertaining to type 1 and type 2 diabetes, complications, related subjects such as obesity and cardiovascular disease, and health care delivery.
New to this year’s agenda is COVID-19 and the many ways it has affected people with diabetes and health care delivery. And, more than in the past, the meeting will focus on ethnic and racial disparities in the delivery of care to people with diabetes.
And of course, there will be a tribute to another special aspect of 2021: the 100th anniversary of the discovery of insulin.
“I think there will undoubtedly be several things that will come out of this meeting that will change practice, and it will be important for clinicians to be aware of those, whether that’s groundbreaking trials or interpretation of data that will help us understand the interrelation between diabetes and COVID-19, which is still with us,” ADA chief scientific and medical officer Robert A. Gabbay, MD, PhD, said in an interview.
And ADA president of medicine and science Ruth S. Weinstock, MD, PhD, said in an interview: “I think there are many exciting sessions at this year’s meeting. ...I hope that it will help [clinicians] take better care of their patients with diabetes.
”
Will the twincretin tirzepatide live up to the hype?
Between December 2020 and May 2021, Eli Lilly issued a series of four press releases touting positive top-line results from a series of phase 3 studies on its novel agent tirzepatide, dubbed a twincretin for its dual actions as an agonist of the glucose-dependent insulinotropic polypeptide (GIP) and glucagonlike peptide-1 receptors.
Detailed results from those four trials, SURPASS-1, -2, -3, and -5, will be presented in a symposium on Tuesday, June 29. Results from SURPASS-4 will be presented at the annual meeting of the European Association for the Study of Diabetes in September 2021.
According to the company, the drug met its phase 3 primary efficacy endpoints for both hemoglobin A1c reduction and weight loss.
“At least the buzz on it has been good, but now we want to see the real data,” Dr. Gabbay said, noting that “the early data on weight loss in particular were quite good. So then the question would be: Do you go to a GLP-1 [agonist] or a dual agonist? There will be studies to tease that out.”
Regarding tirzepatide, Dr. Weinstock said: “Hopefully, more people with type 2 diabetes could achieve their glycemic goals, and those who would benefit from weight loss could have better weight loss. I haven’t seen the data, but if the addition of GIP can further improve glucose lowering as well as weight loss that would be great.”
How far will GRADE go in answering the second-drug question?
On Monday, June 28, results will be presented from the long-awaited Glycemia Reduction Approaches in Diabetes – A Comparative Effectiveness (GRADE) study.
Launched in 2013, the trial is funded by the National Institutes of Health and several pharmaceutical company partners. Over 5,000 patients diagnosed with type 2 diabetes within the prior 10 years and already taking metformin were randomized to one of four commonly used second-line glucose-lowering agents: glimepiride, sitagliptin, liraglutide, and basal insulin glargine. The aim was to determine which combination produced the best glycemic control with the fewest side effects.
Dr. Weinstock said: “Clinicians now have increasing numbers of medications to choose from when treating hyperglycemia in type 2 diabetes, and a common dilemma is which one to select. The results of GRADE should be informative for people taking care of type 2 diabetes in different populations.”
However, she also pointed out that GRADE does not include a group with a sodium-glucose transporter 2 inhibitor, as the trial was designed prior to the availability of the drug class. Now, SGLT2 inhibitors are widely used and recommended for cardiovascular and kidney benefit as well as glucose lowering.
“I believe the future is really precision medicine where we individualize treatment. So, for someone with heart failure you might choose an SGLT2 inhibitor, but there are plenty of other subpopulations. They are going to be looking at different subpopulations. I think we’re all very interested in seeing what the results are, but it’s not the end of the story. We will still have to individualize therapy and keep in mind their kidney, heart, heart failure status, and other factors,” she said.
Dr. Gabbay pointed out that GRADE is important because it’s one of the few comparative effectiveness trials conducted in diabetes. “I think it will be very rich [data] that will impact practice in a variety of ways. On the one hand, it doesn’t do everything we’d want it to do, but on the other hand, if you think of the number of comparative effectiveness trials in diabetes, there are not a lot ... I think it will be big.”
COVID-19 and diabetes: A lot to discuss
In contrast to the ADA scientific sessions in 2020, which took place too soon after the start of the COVID-19 pandemic to include much material about it, this year’s meeting will address many different aspects of the novel coronavirus.
Sessions will cover minimizing risk in people with diabetes during the pandemic, the latest data on whether COVID-19 triggers diabetes, and if so, by what mechanism, mental health issues related to COVID-19, as well as the management of foot care, pregnancy, and the pediatric population during the pandemic.
On Sunday, June 27, a symposium will be devoted to results of the DARE-19 trial, which explored the effects of the SGLT2 inhibitor dapagliflozin in more than 1200 patients hospitalized with COVID-19. The overall results, presented in May 2021 at the scientific sessions of the American College of Cardiology, showed a nonsignificant trend for benefit in time to organ failure or death compared with placebo. At ADA, separate efficacy and safety results for patients with and without diabetes will be presented.
According to Dr. Weinstock, “We know that in nonhospitalized patients with type 2 diabetes the SGLT2 inhibitors can help preserve kidney function and reduce heart failure. But we also know there can be diabetic ketoacidosis and genital infections and other side effects, so it’s been unclear up till now in type 2 diabetes whether they are safe and effective in people hospitalized in respiratory failure with COVID-19. And, given that people with type 2 diabetes and COVID-19 are more likely to require mechanical ventilation and are at greater risk of mortality, we’re anxious to see what these results are.”
Dr. Gabbay commented that, when the DARE study was initiated in April 2020, there were concerns about whether it was safe. And even now, “we’re still not sure about whether SGLT2 inhibitors should be stopped in hospitalized patients. The recommendations say to stop. I think this will be interesting.”
Also to be addressed in several meeting sessions are related issues the pandemic has brought forth, such as the use of telehealth for routine diabetes management, inpatient use of continuous glucose monitoring, and, of course, health care disparities.
“A lot of important issues related to COVID-19 of great interest will be discussed in a variety of sessions,” Dr. Weinstock said.
Type 1 diabetes in adults: It’s not just a pediatric disease
On Monday, June 28, a draft of the first-ever ADA/EASD consensus report on the management of type 1 diabetes in adults will be presented, with the final version slated for the annual meeting of the EASD in September 2021.
A previous ADA position statement had addressed management of type 1 diabetes across all age groups, but this will be the first to focus on adults. This is important, given that type 1 diabetes was formerly called juvenile diabetes and is still often perceived as a childhood disease. Adults who develop it are commonly misdiagnosed as having type 2 diabetes, Dr. Gabbay noted.
“A big-time issue is recognition of type 1 in adults. We often see patients come in who were misdiagnosed, on metformin, and not given insulin. Often they go for a while and get sicker and sicker.” Or, he said, sometimes they’re prescribed insulin but not the intensive regimens that are required for adequate glycemic control in type 1 diabetes. “They can be suboptimally treated and it can take years to get the right therapy. ... It’s unfortunate that they have to experience that.”
Dr. Weinstock, one of the authors of the statement, said it will cover a range of issues, including care schedules, therapies, psychosocial issues, and social determinants of health. “We tried to be comprehensive in this in terms of glycemic management. It doesn’t include a discussion of complications or their management. It really focuses on diagnosis and glycemic management.”
Dealing with disparities: ADA has taken several steps
A priority of the ADA is addressing disparities in the delivery of health care to people with diabetes, both Dr. Weinstock and Dr. Gabbay stressed. Quite a few sessions at the meeting will touch on various aspects, including sessions on Friday afternoon on “Health Care as a Social Justice Issue in the Diagnosis and Management of Diabetes,” and separate sessions on “Challenges and Successes With Health Inequities and Health Disparities in Diabetes” in adult and pediatric populations.
“For us at ADA, addressing health disparities is extremely important and we have a number of new programs this year to address this very important issue,” Dr. Weinstock said.
In August 2020, the ADA issued a Health Equity Bill of Rights, which includes access to insulin and other medications, affordable health care, and freedom from stigma and discrimination. The Association has also requested applications from researchers studying disparities in diabetes care.
Celebrating 100 years of lifesaving medication
Of course, the ADA will be celebrating the 100th anniversary of the discovery of insulin. A session on Saturday afternoon, entitled, “Insulin at Its 100th Birthday,” will cover the history of the landmark discovery, as well as insulin biosynthesis and mechanisms of action, and “the future of insulin as a therapy.”
Dr. Weinstock noted: “The discovery of insulin was an incredible achievement that, of course, saved the lives of many millions of children and adults. Before insulin became available, children and adults only survived for days or at most a few years after diagnosis. We will commemorate this anniversary.”
The virtual platform: Like last year, only better
Dr. Gabbay said in an interview that the virtual setup will be similar to last year’s in that talks will be prerecorded to ensure there are no technical glitches, but for many, presenters will be available afterward for live question and answers.
This year, though, the chat functionality will be enhanced to allow for discussion during the presentation, separate from the scientific question and answers. And, he noted, the virtual exhibit hall will be “bigger and better.”
Despite these improvements, Dr. Gabbay said, the plan is to go back to an in-person meeting in 2022 in New Orleans.
Dr. Weinstock’s institution receives research grants from Medtronic, Insulet, Lilly, Novo Nordisk, and Boehringer Ingelheim. Dr. Gabbay reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-awaited twincretin data, a study to inform prescribing in type 2 diabetes, COVID-19 and diabetes, and new guidance for treating type 1 diabetes in adults will be among the hot topics at the annual scientific sessions of the American Diabetes Association.
The meeting, to be held virtually for a second year, will take place June 25-29. As usual, the sessions will cover a wide range of basic, translational, and clinical material pertaining to type 1 and type 2 diabetes, complications, related subjects such as obesity and cardiovascular disease, and health care delivery.
New to this year’s agenda is COVID-19 and the many ways it has affected people with diabetes and health care delivery. And, more than in the past, the meeting will focus on ethnic and racial disparities in the delivery of care to people with diabetes.
And of course, there will be a tribute to another special aspect of 2021: the 100th anniversary of the discovery of insulin.
“I think there will undoubtedly be several things that will come out of this meeting that will change practice, and it will be important for clinicians to be aware of those, whether that’s groundbreaking trials or interpretation of data that will help us understand the interrelation between diabetes and COVID-19, which is still with us,” ADA chief scientific and medical officer Robert A. Gabbay, MD, PhD, said in an interview.
And ADA president of medicine and science Ruth S. Weinstock, MD, PhD, said in an interview: “I think there are many exciting sessions at this year’s meeting. ...I hope that it will help [clinicians] take better care of their patients with diabetes.
”
Will the twincretin tirzepatide live up to the hype?
Between December 2020 and May 2021, Eli Lilly issued a series of four press releases touting positive top-line results from a series of phase 3 studies on its novel agent tirzepatide, dubbed a twincretin for its dual actions as an agonist of the glucose-dependent insulinotropic polypeptide (GIP) and glucagonlike peptide-1 receptors.
Detailed results from those four trials, SURPASS-1, -2, -3, and -5, will be presented in a symposium on Tuesday, June 29. Results from SURPASS-4 will be presented at the annual meeting of the European Association for the Study of Diabetes in September 2021.
According to the company, the drug met its phase 3 primary efficacy endpoints for both hemoglobin A1c reduction and weight loss.
“At least the buzz on it has been good, but now we want to see the real data,” Dr. Gabbay said, noting that “the early data on weight loss in particular were quite good. So then the question would be: Do you go to a GLP-1 [agonist] or a dual agonist? There will be studies to tease that out.”
Regarding tirzepatide, Dr. Weinstock said: “Hopefully, more people with type 2 diabetes could achieve their glycemic goals, and those who would benefit from weight loss could have better weight loss. I haven’t seen the data, but if the addition of GIP can further improve glucose lowering as well as weight loss that would be great.”
How far will GRADE go in answering the second-drug question?
On Monday, June 28, results will be presented from the long-awaited Glycemia Reduction Approaches in Diabetes – A Comparative Effectiveness (GRADE) study.
Launched in 2013, the trial is funded by the National Institutes of Health and several pharmaceutical company partners. Over 5,000 patients diagnosed with type 2 diabetes within the prior 10 years and already taking metformin were randomized to one of four commonly used second-line glucose-lowering agents: glimepiride, sitagliptin, liraglutide, and basal insulin glargine. The aim was to determine which combination produced the best glycemic control with the fewest side effects.
Dr. Weinstock said: “Clinicians now have increasing numbers of medications to choose from when treating hyperglycemia in type 2 diabetes, and a common dilemma is which one to select. The results of GRADE should be informative for people taking care of type 2 diabetes in different populations.”
However, she also pointed out that GRADE does not include a group with a sodium-glucose transporter 2 inhibitor, as the trial was designed prior to the availability of the drug class. Now, SGLT2 inhibitors are widely used and recommended for cardiovascular and kidney benefit as well as glucose lowering.
“I believe the future is really precision medicine where we individualize treatment. So, for someone with heart failure you might choose an SGLT2 inhibitor, but there are plenty of other subpopulations. They are going to be looking at different subpopulations. I think we’re all very interested in seeing what the results are, but it’s not the end of the story. We will still have to individualize therapy and keep in mind their kidney, heart, heart failure status, and other factors,” she said.
Dr. Gabbay pointed out that GRADE is important because it’s one of the few comparative effectiveness trials conducted in diabetes. “I think it will be very rich [data] that will impact practice in a variety of ways. On the one hand, it doesn’t do everything we’d want it to do, but on the other hand, if you think of the number of comparative effectiveness trials in diabetes, there are not a lot ... I think it will be big.”
COVID-19 and diabetes: A lot to discuss
In contrast to the ADA scientific sessions in 2020, which took place too soon after the start of the COVID-19 pandemic to include much material about it, this year’s meeting will address many different aspects of the novel coronavirus.
Sessions will cover minimizing risk in people with diabetes during the pandemic, the latest data on whether COVID-19 triggers diabetes, and if so, by what mechanism, mental health issues related to COVID-19, as well as the management of foot care, pregnancy, and the pediatric population during the pandemic.
On Sunday, June 27, a symposium will be devoted to results of the DARE-19 trial, which explored the effects of the SGLT2 inhibitor dapagliflozin in more than 1200 patients hospitalized with COVID-19. The overall results, presented in May 2021 at the scientific sessions of the American College of Cardiology, showed a nonsignificant trend for benefit in time to organ failure or death compared with placebo. At ADA, separate efficacy and safety results for patients with and without diabetes will be presented.
According to Dr. Weinstock, “We know that in nonhospitalized patients with type 2 diabetes the SGLT2 inhibitors can help preserve kidney function and reduce heart failure. But we also know there can be diabetic ketoacidosis and genital infections and other side effects, so it’s been unclear up till now in type 2 diabetes whether they are safe and effective in people hospitalized in respiratory failure with COVID-19. And, given that people with type 2 diabetes and COVID-19 are more likely to require mechanical ventilation and are at greater risk of mortality, we’re anxious to see what these results are.”
Dr. Gabbay commented that, when the DARE study was initiated in April 2020, there were concerns about whether it was safe. And even now, “we’re still not sure about whether SGLT2 inhibitors should be stopped in hospitalized patients. The recommendations say to stop. I think this will be interesting.”
Also to be addressed in several meeting sessions are related issues the pandemic has brought forth, such as the use of telehealth for routine diabetes management, inpatient use of continuous glucose monitoring, and, of course, health care disparities.
“A lot of important issues related to COVID-19 of great interest will be discussed in a variety of sessions,” Dr. Weinstock said.
Type 1 diabetes in adults: It’s not just a pediatric disease
On Monday, June 28, a draft of the first-ever ADA/EASD consensus report on the management of type 1 diabetes in adults will be presented, with the final version slated for the annual meeting of the EASD in September 2021.
A previous ADA position statement had addressed management of type 1 diabetes across all age groups, but this will be the first to focus on adults. This is important, given that type 1 diabetes was formerly called juvenile diabetes and is still often perceived as a childhood disease. Adults who develop it are commonly misdiagnosed as having type 2 diabetes, Dr. Gabbay noted.
“A big-time issue is recognition of type 1 in adults. We often see patients come in who were misdiagnosed, on metformin, and not given insulin. Often they go for a while and get sicker and sicker.” Or, he said, sometimes they’re prescribed insulin but not the intensive regimens that are required for adequate glycemic control in type 1 diabetes. “They can be suboptimally treated and it can take years to get the right therapy. ... It’s unfortunate that they have to experience that.”
Dr. Weinstock, one of the authors of the statement, said it will cover a range of issues, including care schedules, therapies, psychosocial issues, and social determinants of health. “We tried to be comprehensive in this in terms of glycemic management. It doesn’t include a discussion of complications or their management. It really focuses on diagnosis and glycemic management.”
Dealing with disparities: ADA has taken several steps
A priority of the ADA is addressing disparities in the delivery of health care to people with diabetes, both Dr. Weinstock and Dr. Gabbay stressed. Quite a few sessions at the meeting will touch on various aspects, including sessions on Friday afternoon on “Health Care as a Social Justice Issue in the Diagnosis and Management of Diabetes,” and separate sessions on “Challenges and Successes With Health Inequities and Health Disparities in Diabetes” in adult and pediatric populations.
“For us at ADA, addressing health disparities is extremely important and we have a number of new programs this year to address this very important issue,” Dr. Weinstock said.
In August 2020, the ADA issued a Health Equity Bill of Rights, which includes access to insulin and other medications, affordable health care, and freedom from stigma and discrimination. The Association has also requested applications from researchers studying disparities in diabetes care.
Celebrating 100 years of lifesaving medication
Of course, the ADA will be celebrating the 100th anniversary of the discovery of insulin. A session on Saturday afternoon, entitled, “Insulin at Its 100th Birthday,” will cover the history of the landmark discovery, as well as insulin biosynthesis and mechanisms of action, and “the future of insulin as a therapy.”
Dr. Weinstock noted: “The discovery of insulin was an incredible achievement that, of course, saved the lives of many millions of children and adults. Before insulin became available, children and adults only survived for days or at most a few years after diagnosis. We will commemorate this anniversary.”
The virtual platform: Like last year, only better
Dr. Gabbay said in an interview that the virtual setup will be similar to last year’s in that talks will be prerecorded to ensure there are no technical glitches, but for many, presenters will be available afterward for live question and answers.
This year, though, the chat functionality will be enhanced to allow for discussion during the presentation, separate from the scientific question and answers. And, he noted, the virtual exhibit hall will be “bigger and better.”
Despite these improvements, Dr. Gabbay said, the plan is to go back to an in-person meeting in 2022 in New Orleans.
Dr. Weinstock’s institution receives research grants from Medtronic, Insulet, Lilly, Novo Nordisk, and Boehringer Ingelheim. Dr. Gabbay reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
No overall statin effect seen on dementia, cognition in ASPREE analysis
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Sotagliflozin use in T2D patients linked with posthospitalization benefits in analysis
The outcome measure –days alive and out of the hospital – may be a meaningful, patient-centered way of capturing disease burden, the researchers wrote in their paper, published in Annals of Internal Medicine.
“The question was: Can we keep patients alive and out of the hospital for any reason, accounting for the duration of each hospitalization?” author Michael Szarek, PhD, a visiting professor in the division of cardiology at the University of Colorado at Denver, Aurora, said in an interview.
“For every 100 days of follow-up, patients in the sotagliflozin group were alive and out of the hospital 3% more days in relative terms or 2.9 days in absolute terms than those in the placebo group (91.8 vs. 88.9 days),” the researchers reported in their analysis of data from the SOLOIST-WHF trial.
“If you translate that to over the course of a year, that’s more than 10 days,” said Dr. Szarek, who is also a faculty member of CPC Clinical Research, an academic research organization affiliated with the University of Colorado.
Most patients in both groups survived to the end of the study without hospitalization, according to the paper.
Sotagliflozin, a sodium-glucose cotransporter 1 and SGLT2 inhibitor, is not approved in the United States. In 2019, the Food and Drug Administration rejected sotagliflozin as an adjunct to insulin for the treatment of type 1 diabetes after members of an advisory committee expressed concerns about an increased risk for diabetic ketoacidosis with the drug.
Methods and results
To examine whether sotagliflozin increased days alive and out of the hospital in the SOLOIST-WHF trial, Dr. Szarek and colleagues analyzed data from this randomized, double-blind, placebo-controlled study. The trial’s primary results were published in the New England Journal of Medicine in January 2021. Researchers conducted SOLOIST-WHF at more than 300 sites in 32 countries. The trial included 1,222 patients with type 2 diabetes and reduced or preserved ejection fraction who were recently hospitalized for worsening heart failure.
In the new analysis the researchers looked at hospitalizations for any reason and the duration of hospital admissions after randomization. They analyzed days alive and out of the hospital using prespecified models.
Similar proportions of patients who received sotagliflozin and placebo were hospitalized at least once (38.5% vs. 41.4%) during a median follow-up of 9 months. Fewer patients who received sotagliflozin were hospitalized more than once (16.3% vs. 22.1%). In all, 64 patients in the sotagliflozin group and 76 patients in the placebo group died.
The reason for each hospitalization was unspecified, except for cases of heart failure, the authors noted. About 62% of hospitalizations during the trial were for reasons other than heart failure.
Outside expert cites similarities to initial trial
The results for days alive and out of the hospital are “not particularly surprising given the previous publication” of the trial’s primary results, but the new analysis provides a “different view of outcomes that might be clinically meaningful for patients,” commented Frank Brosius, MD, a professor of medicine at the University of Arizona, Tucson.
The SOLOIST-WHF trial indicated that doctors may be able to effectively treat patients with relatively new heart failure with sotagliflozin as long as patients are relatively stable, said Dr. Brosius, who coauthored an editorial in the New England Journal of Medicine that accompanied the initial results from the SOLOIST-WHF trial. It appears that previously reported benefits with regard to heart failure outcomes “showed up in these other indicators” in the secondary analysis.
Still, the effect sizes in the new analysis were relatively small and “probably more studies will be necessary” to examine these end points, he added.
SOLOIST-WHF was funded by Sanofi at initiation and by Lexicon Pharmaceuticals at completion. Dr. Szarek disclosed grants from Lexicon and grants and personal fees from Sanofi, as well as personal fees from other companies. His coauthors included employees of Lexicon and other researchers with financial ties to Lexicon and other pharmaceutical companies. Dr. Brosius disclosed personal fees from the American Diabetes Association and is a member of the Diabetic Kidney Disease Collaborative task force for the American Society of Nephrology that is broadly advocating the use of SGLT2 inhibitors by patients with diabetic kidney diseases. He also has participated in an advisory group for treatment of diabetic kidney disease for Gilead.
The outcome measure –days alive and out of the hospital – may be a meaningful, patient-centered way of capturing disease burden, the researchers wrote in their paper, published in Annals of Internal Medicine.
“The question was: Can we keep patients alive and out of the hospital for any reason, accounting for the duration of each hospitalization?” author Michael Szarek, PhD, a visiting professor in the division of cardiology at the University of Colorado at Denver, Aurora, said in an interview.
“For every 100 days of follow-up, patients in the sotagliflozin group were alive and out of the hospital 3% more days in relative terms or 2.9 days in absolute terms than those in the placebo group (91.8 vs. 88.9 days),” the researchers reported in their analysis of data from the SOLOIST-WHF trial.
“If you translate that to over the course of a year, that’s more than 10 days,” said Dr. Szarek, who is also a faculty member of CPC Clinical Research, an academic research organization affiliated with the University of Colorado.
Most patients in both groups survived to the end of the study without hospitalization, according to the paper.
Sotagliflozin, a sodium-glucose cotransporter 1 and SGLT2 inhibitor, is not approved in the United States. In 2019, the Food and Drug Administration rejected sotagliflozin as an adjunct to insulin for the treatment of type 1 diabetes after members of an advisory committee expressed concerns about an increased risk for diabetic ketoacidosis with the drug.
Methods and results
To examine whether sotagliflozin increased days alive and out of the hospital in the SOLOIST-WHF trial, Dr. Szarek and colleagues analyzed data from this randomized, double-blind, placebo-controlled study. The trial’s primary results were published in the New England Journal of Medicine in January 2021. Researchers conducted SOLOIST-WHF at more than 300 sites in 32 countries. The trial included 1,222 patients with type 2 diabetes and reduced or preserved ejection fraction who were recently hospitalized for worsening heart failure.
In the new analysis the researchers looked at hospitalizations for any reason and the duration of hospital admissions after randomization. They analyzed days alive and out of the hospital using prespecified models.
Similar proportions of patients who received sotagliflozin and placebo were hospitalized at least once (38.5% vs. 41.4%) during a median follow-up of 9 months. Fewer patients who received sotagliflozin were hospitalized more than once (16.3% vs. 22.1%). In all, 64 patients in the sotagliflozin group and 76 patients in the placebo group died.
The reason for each hospitalization was unspecified, except for cases of heart failure, the authors noted. About 62% of hospitalizations during the trial were for reasons other than heart failure.
Outside expert cites similarities to initial trial
The results for days alive and out of the hospital are “not particularly surprising given the previous publication” of the trial’s primary results, but the new analysis provides a “different view of outcomes that might be clinically meaningful for patients,” commented Frank Brosius, MD, a professor of medicine at the University of Arizona, Tucson.
The SOLOIST-WHF trial indicated that doctors may be able to effectively treat patients with relatively new heart failure with sotagliflozin as long as patients are relatively stable, said Dr. Brosius, who coauthored an editorial in the New England Journal of Medicine that accompanied the initial results from the SOLOIST-WHF trial. It appears that previously reported benefits with regard to heart failure outcomes “showed up in these other indicators” in the secondary analysis.
Still, the effect sizes in the new analysis were relatively small and “probably more studies will be necessary” to examine these end points, he added.
SOLOIST-WHF was funded by Sanofi at initiation and by Lexicon Pharmaceuticals at completion. Dr. Szarek disclosed grants from Lexicon and grants and personal fees from Sanofi, as well as personal fees from other companies. His coauthors included employees of Lexicon and other researchers with financial ties to Lexicon and other pharmaceutical companies. Dr. Brosius disclosed personal fees from the American Diabetes Association and is a member of the Diabetic Kidney Disease Collaborative task force for the American Society of Nephrology that is broadly advocating the use of SGLT2 inhibitors by patients with diabetic kidney diseases. He also has participated in an advisory group for treatment of diabetic kidney disease for Gilead.
The outcome measure –days alive and out of the hospital – may be a meaningful, patient-centered way of capturing disease burden, the researchers wrote in their paper, published in Annals of Internal Medicine.
“The question was: Can we keep patients alive and out of the hospital for any reason, accounting for the duration of each hospitalization?” author Michael Szarek, PhD, a visiting professor in the division of cardiology at the University of Colorado at Denver, Aurora, said in an interview.
“For every 100 days of follow-up, patients in the sotagliflozin group were alive and out of the hospital 3% more days in relative terms or 2.9 days in absolute terms than those in the placebo group (91.8 vs. 88.9 days),” the researchers reported in their analysis of data from the SOLOIST-WHF trial.
“If you translate that to over the course of a year, that’s more than 10 days,” said Dr. Szarek, who is also a faculty member of CPC Clinical Research, an academic research organization affiliated with the University of Colorado.
Most patients in both groups survived to the end of the study without hospitalization, according to the paper.
Sotagliflozin, a sodium-glucose cotransporter 1 and SGLT2 inhibitor, is not approved in the United States. In 2019, the Food and Drug Administration rejected sotagliflozin as an adjunct to insulin for the treatment of type 1 diabetes after members of an advisory committee expressed concerns about an increased risk for diabetic ketoacidosis with the drug.
Methods and results
To examine whether sotagliflozin increased days alive and out of the hospital in the SOLOIST-WHF trial, Dr. Szarek and colleagues analyzed data from this randomized, double-blind, placebo-controlled study. The trial’s primary results were published in the New England Journal of Medicine in January 2021. Researchers conducted SOLOIST-WHF at more than 300 sites in 32 countries. The trial included 1,222 patients with type 2 diabetes and reduced or preserved ejection fraction who were recently hospitalized for worsening heart failure.
In the new analysis the researchers looked at hospitalizations for any reason and the duration of hospital admissions after randomization. They analyzed days alive and out of the hospital using prespecified models.
Similar proportions of patients who received sotagliflozin and placebo were hospitalized at least once (38.5% vs. 41.4%) during a median follow-up of 9 months. Fewer patients who received sotagliflozin were hospitalized more than once (16.3% vs. 22.1%). In all, 64 patients in the sotagliflozin group and 76 patients in the placebo group died.
The reason for each hospitalization was unspecified, except for cases of heart failure, the authors noted. About 62% of hospitalizations during the trial were for reasons other than heart failure.
Outside expert cites similarities to initial trial
The results for days alive and out of the hospital are “not particularly surprising given the previous publication” of the trial’s primary results, but the new analysis provides a “different view of outcomes that might be clinically meaningful for patients,” commented Frank Brosius, MD, a professor of medicine at the University of Arizona, Tucson.
The SOLOIST-WHF trial indicated that doctors may be able to effectively treat patients with relatively new heart failure with sotagliflozin as long as patients are relatively stable, said Dr. Brosius, who coauthored an editorial in the New England Journal of Medicine that accompanied the initial results from the SOLOIST-WHF trial. It appears that previously reported benefits with regard to heart failure outcomes “showed up in these other indicators” in the secondary analysis.
Still, the effect sizes in the new analysis were relatively small and “probably more studies will be necessary” to examine these end points, he added.
SOLOIST-WHF was funded by Sanofi at initiation and by Lexicon Pharmaceuticals at completion. Dr. Szarek disclosed grants from Lexicon and grants and personal fees from Sanofi, as well as personal fees from other companies. His coauthors included employees of Lexicon and other researchers with financial ties to Lexicon and other pharmaceutical companies. Dr. Brosius disclosed personal fees from the American Diabetes Association and is a member of the Diabetic Kidney Disease Collaborative task force for the American Society of Nephrology that is broadly advocating the use of SGLT2 inhibitors by patients with diabetic kidney diseases. He also has participated in an advisory group for treatment of diabetic kidney disease for Gilead.
FROM ANNALS OF INTERNAL MEDICINE
Medicare rule changes allow for broader CGM use
Beginning July 18, 2021, the Centers for Medicare & Medicaid Services will no longer require that beneficiaries test their blood sugar four times a day in order to qualify for CGM. In addition, the term “multiple daily injections” of insulin has been changed to multiple daily “administrations” in order to allow coverage for people who use inhaled insulin.
The changes are among those lobbied for by several organizations, including the American Diabetes Association and the Association of Diabetes Care and Education Specialists, which represents the professionals formerly known as “diabetes educators.”
The ADA tweeted on July 11 that “the removal of this criterion has been an effort long-led by the ADA, on which we have been actively engaged with CMS. People with diabetes on Medicare will now be able to more easily access this critical piece of technology, leading to better diabetes management and better health outcomes. A big win for the diabetes community!”
“After years of advocacy from the diabetes community and ADCES, Medicare has taken an important step to make [CGM] more accessible for Medicare beneficiaries with diabetes,” Kate Thomas, ADCES chief advocacy and external affairs officer, wrote in a blog post. “This updated [Local Coverage Determination] was a direct result of coordinated advocacy efforts among patient and provider groups, as well as industry partners, coalitions and other entities.”
It’s tough to test four times a day with only three strips
In a Jan. 29, 2021, letter to the Medicare Administrative Contractors, who oversee the policies for durable medical equipment, ADCES explained why the organization strongly supported removal of the four-daily fingerstick requirement, noting that “There is no evidence to suggest that requiring four or more fingerstick tests per day significantly impacts the outcomes of CGM therapy.”
Moreover, they pointed out that the requirement was particularly burdensome, considering the fact that Medicare only covers three test strips per day for insulin-using beneficiaries. “Removing this coverage requirement would allow for increased access to CGM systems and improved health outcomes for beneficiaries with diabetes by improving glycemic control. This also represents a step toward addressing the disparities that exist around diabetes technology under the Medicare program.”
As for the terminology change from “injection” to “administration,” ADCES said that, in addition to allowing CGM coverage for individuals who use rapid-acting inhaled insulin, “we also hope that updating this terminology will help to expedite coverage as future innovations in insulin delivery methods come to market.”
More changes needed, ADCES says
In that January 2021 letter, ADCES recommended several other changes, including covering CGM for anyone diagnosed with type 1 diabetes at any age and without having to meet other requirements except for twice-yearly clinician visits, and for anyone with type 2 diabetes who uses any type of insulin or who has had documented hypoglycemia regardless of therapy.
They also recommended that CGM coverage be considered for patients with chronic kidney disease, and that the required 6-month clinician visits be allowed to take place via telehealth. “ADCES believes that allowing the initiation of CGM therapy through a virtual visit will reduce barriers associated with travel and difficulty accessing a trained provider that are experienced by Medicare beneficiaries.”
In addition, ADCES requested that CMS eliminate the requirement that beneficiaries use insulin three times a day to qualify for CGM, noting that this creates a barrier for patients who can’t afford insulin at all but are at risk for hypoglycemia because they take sulfonylureas or other insulin secretagogues, or for those who use cheaper synthetic human insulins that are only taken twice a day, such as NPH.
“The existing CGM coverage criteria creates an unbalanced and disparate system that excludes from coverage beneficiaries who could greatly benefit from a CGM system, but do not qualify due to issues with insulin affordability,” ADCES wrote in the January letter.
Ms. Thomas wrote in the June 14th blog: “Our work is not done. We know there are more changes that must be made.”
Beginning July 18, 2021, the Centers for Medicare & Medicaid Services will no longer require that beneficiaries test their blood sugar four times a day in order to qualify for CGM. In addition, the term “multiple daily injections” of insulin has been changed to multiple daily “administrations” in order to allow coverage for people who use inhaled insulin.
The changes are among those lobbied for by several organizations, including the American Diabetes Association and the Association of Diabetes Care and Education Specialists, which represents the professionals formerly known as “diabetes educators.”
The ADA tweeted on July 11 that “the removal of this criterion has been an effort long-led by the ADA, on which we have been actively engaged with CMS. People with diabetes on Medicare will now be able to more easily access this critical piece of technology, leading to better diabetes management and better health outcomes. A big win for the diabetes community!”
“After years of advocacy from the diabetes community and ADCES, Medicare has taken an important step to make [CGM] more accessible for Medicare beneficiaries with diabetes,” Kate Thomas, ADCES chief advocacy and external affairs officer, wrote in a blog post. “This updated [Local Coverage Determination] was a direct result of coordinated advocacy efforts among patient and provider groups, as well as industry partners, coalitions and other entities.”
It’s tough to test four times a day with only three strips
In a Jan. 29, 2021, letter to the Medicare Administrative Contractors, who oversee the policies for durable medical equipment, ADCES explained why the organization strongly supported removal of the four-daily fingerstick requirement, noting that “There is no evidence to suggest that requiring four or more fingerstick tests per day significantly impacts the outcomes of CGM therapy.”
Moreover, they pointed out that the requirement was particularly burdensome, considering the fact that Medicare only covers three test strips per day for insulin-using beneficiaries. “Removing this coverage requirement would allow for increased access to CGM systems and improved health outcomes for beneficiaries with diabetes by improving glycemic control. This also represents a step toward addressing the disparities that exist around diabetes technology under the Medicare program.”
As for the terminology change from “injection” to “administration,” ADCES said that, in addition to allowing CGM coverage for individuals who use rapid-acting inhaled insulin, “we also hope that updating this terminology will help to expedite coverage as future innovations in insulin delivery methods come to market.”
More changes needed, ADCES says
In that January 2021 letter, ADCES recommended several other changes, including covering CGM for anyone diagnosed with type 1 diabetes at any age and without having to meet other requirements except for twice-yearly clinician visits, and for anyone with type 2 diabetes who uses any type of insulin or who has had documented hypoglycemia regardless of therapy.
They also recommended that CGM coverage be considered for patients with chronic kidney disease, and that the required 6-month clinician visits be allowed to take place via telehealth. “ADCES believes that allowing the initiation of CGM therapy through a virtual visit will reduce barriers associated with travel and difficulty accessing a trained provider that are experienced by Medicare beneficiaries.”
In addition, ADCES requested that CMS eliminate the requirement that beneficiaries use insulin three times a day to qualify for CGM, noting that this creates a barrier for patients who can’t afford insulin at all but are at risk for hypoglycemia because they take sulfonylureas or other insulin secretagogues, or for those who use cheaper synthetic human insulins that are only taken twice a day, such as NPH.
“The existing CGM coverage criteria creates an unbalanced and disparate system that excludes from coverage beneficiaries who could greatly benefit from a CGM system, but do not qualify due to issues with insulin affordability,” ADCES wrote in the January letter.
Ms. Thomas wrote in the June 14th blog: “Our work is not done. We know there are more changes that must be made.”
Beginning July 18, 2021, the Centers for Medicare & Medicaid Services will no longer require that beneficiaries test their blood sugar four times a day in order to qualify for CGM. In addition, the term “multiple daily injections” of insulin has been changed to multiple daily “administrations” in order to allow coverage for people who use inhaled insulin.
The changes are among those lobbied for by several organizations, including the American Diabetes Association and the Association of Diabetes Care and Education Specialists, which represents the professionals formerly known as “diabetes educators.”
The ADA tweeted on July 11 that “the removal of this criterion has been an effort long-led by the ADA, on which we have been actively engaged with CMS. People with diabetes on Medicare will now be able to more easily access this critical piece of technology, leading to better diabetes management and better health outcomes. A big win for the diabetes community!”
“After years of advocacy from the diabetes community and ADCES, Medicare has taken an important step to make [CGM] more accessible for Medicare beneficiaries with diabetes,” Kate Thomas, ADCES chief advocacy and external affairs officer, wrote in a blog post. “This updated [Local Coverage Determination] was a direct result of coordinated advocacy efforts among patient and provider groups, as well as industry partners, coalitions and other entities.”
It’s tough to test four times a day with only three strips
In a Jan. 29, 2021, letter to the Medicare Administrative Contractors, who oversee the policies for durable medical equipment, ADCES explained why the organization strongly supported removal of the four-daily fingerstick requirement, noting that “There is no evidence to suggest that requiring four or more fingerstick tests per day significantly impacts the outcomes of CGM therapy.”
Moreover, they pointed out that the requirement was particularly burdensome, considering the fact that Medicare only covers three test strips per day for insulin-using beneficiaries. “Removing this coverage requirement would allow for increased access to CGM systems and improved health outcomes for beneficiaries with diabetes by improving glycemic control. This also represents a step toward addressing the disparities that exist around diabetes technology under the Medicare program.”
As for the terminology change from “injection” to “administration,” ADCES said that, in addition to allowing CGM coverage for individuals who use rapid-acting inhaled insulin, “we also hope that updating this terminology will help to expedite coverage as future innovations in insulin delivery methods come to market.”
More changes needed, ADCES says
In that January 2021 letter, ADCES recommended several other changes, including covering CGM for anyone diagnosed with type 1 diabetes at any age and without having to meet other requirements except for twice-yearly clinician visits, and for anyone with type 2 diabetes who uses any type of insulin or who has had documented hypoglycemia regardless of therapy.
They also recommended that CGM coverage be considered for patients with chronic kidney disease, and that the required 6-month clinician visits be allowed to take place via telehealth. “ADCES believes that allowing the initiation of CGM therapy through a virtual visit will reduce barriers associated with travel and difficulty accessing a trained provider that are experienced by Medicare beneficiaries.”
In addition, ADCES requested that CMS eliminate the requirement that beneficiaries use insulin three times a day to qualify for CGM, noting that this creates a barrier for patients who can’t afford insulin at all but are at risk for hypoglycemia because they take sulfonylureas or other insulin secretagogues, or for those who use cheaper synthetic human insulins that are only taken twice a day, such as NPH.
“The existing CGM coverage criteria creates an unbalanced and disparate system that excludes from coverage beneficiaries who could greatly benefit from a CGM system, but do not qualify due to issues with insulin affordability,” ADCES wrote in the January letter.
Ms. Thomas wrote in the June 14th blog: “Our work is not done. We know there are more changes that must be made.”
Bariatric surgery cuts insulin needs in type 1 diabetes with severe obesity
While bariatric surgery does nothing to directly improve the disease of patients with type 1 diabetes, it can work indirectly by moderating severe obesity and improving insulin sensitivity to cut the total insulin needs of patients with type 1 diabetes and obesity, based on a single-center, retrospective chart review of 38 U.S. patients.
Two years following their bariatric surgery, these 38 patients with confirmed type 1 diabetes and an average body mass index of 43 kg/m2 before surgery saw their average daily insulin requirement nearly halved, dropping from 118 units/day to 60 units/day, a significant decrease, Brian J. Dessify, DO, said in a presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Another measure of this effect showed that the percentage of patients who required more than one drug for treating their hyperglycemia fell from 66% before surgery to 52% 2 years after surgery, a change that was not statistically significant, said Dr. Dessify, a bariatric surgeon at Geisinger Medical Center in Danville, Pa.
Appropriate for patients with ‘double diabetes’
These results “provide good evidence for [using] bariatric surgery” in people with both obesity and type 1 diabetes,” he concluded. This includes people with what Dr. Dessify called “double diabetes,” meaning that they do not make endogenous insulin, and are also resistant to the effects of exogenous insulin and hence have features of both type 2 and type 1 diabetes.
“This is a really important study,” commented Ali Aminian, MD, director of the Bariatric and Metabolic Institute of the Cleveland Clinic. “For patients with type 1 diabetes, the primary goal of bariatric surgery is weight loss and improvement of obesity-related comorbidities. Patients with type 2 diabetes can be a candidate for bariatric surgery regardless of their weight,” Dr. Aminian said as designated discussant for the report.
“The goal of bariatric surgery in patients with type 1 diabetes is to promote sensitivity to the exogenous insulin they receive,” agreed Julie Kim, MD, a bariatric surgeon at Mount Auburn Hospital in Waltham, Mass., and a second discussant for the report. Patients with double diabetes “are probably a subclass of patients [with type 1 diabetes] who might benefit even more from bariatric surgery.”
Using gastric sleeves to avoid diabetic ketoacidosis
Dr. Aminian also noted that “at the Cleveland Clinic we consider a sleeve gastrectomy the procedure of choice” for patients with type 1 diabetes or type 2 diabetes with insulin insufficiency “unless the patient has an absolute contraindication” because of the increased risk for diabetic ketoacidosis in these patients “undergoing any surgery, including bariatric surgery.” Patients with insulin insufficiency “require intensive diabetes and insulin management preoperatively to reduce their risk for developing diabetic ketoacidosis,” and using a sleeve rather than bypass generally results in “more reliable absorption of carbohydrates and nutrients” while also reducing the risk for hypoglycemia, Dr. Aminian said.
In the series reported by Dr. Dessify, 33 patients underwent gastric bypass and 5 had sleeve gastrectomy. The decision to use bypass usually stemmed from its “marginal” improvement in weight loss, compared with a sleeve procedure, and an overall preference at Geisinger for bypass procedures. Dr. Dessify added that he had not yet run a comprehensive assessment of diabetic ketoacidosis complications among patients in his reported series.
Those 38 patients underwent their bariatric procedure during 2002-2019, constituting fewer than 1% of the 4,549 total bariatric surgeries done at Geisinger during that period. The 38 patients with type 1 diabetes averaged 41 years of age, 33 (87%) were women, and 37 (97%) were White. Dr. Dessify and associates undertook this review “to help provide supporting evidence for using bariatric surgery in people with obesity and type 1 diabetes,” he noted.
Dr. Dessify, Dr. Aminian, and Dr. Kim had no disclosures.
While bariatric surgery does nothing to directly improve the disease of patients with type 1 diabetes, it can work indirectly by moderating severe obesity and improving insulin sensitivity to cut the total insulin needs of patients with type 1 diabetes and obesity, based on a single-center, retrospective chart review of 38 U.S. patients.
Two years following their bariatric surgery, these 38 patients with confirmed type 1 diabetes and an average body mass index of 43 kg/m2 before surgery saw their average daily insulin requirement nearly halved, dropping from 118 units/day to 60 units/day, a significant decrease, Brian J. Dessify, DO, said in a presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Another measure of this effect showed that the percentage of patients who required more than one drug for treating their hyperglycemia fell from 66% before surgery to 52% 2 years after surgery, a change that was not statistically significant, said Dr. Dessify, a bariatric surgeon at Geisinger Medical Center in Danville, Pa.
Appropriate for patients with ‘double diabetes’
These results “provide good evidence for [using] bariatric surgery” in people with both obesity and type 1 diabetes,” he concluded. This includes people with what Dr. Dessify called “double diabetes,” meaning that they do not make endogenous insulin, and are also resistant to the effects of exogenous insulin and hence have features of both type 2 and type 1 diabetes.
“This is a really important study,” commented Ali Aminian, MD, director of the Bariatric and Metabolic Institute of the Cleveland Clinic. “For patients with type 1 diabetes, the primary goal of bariatric surgery is weight loss and improvement of obesity-related comorbidities. Patients with type 2 diabetes can be a candidate for bariatric surgery regardless of their weight,” Dr. Aminian said as designated discussant for the report.
“The goal of bariatric surgery in patients with type 1 diabetes is to promote sensitivity to the exogenous insulin they receive,” agreed Julie Kim, MD, a bariatric surgeon at Mount Auburn Hospital in Waltham, Mass., and a second discussant for the report. Patients with double diabetes “are probably a subclass of patients [with type 1 diabetes] who might benefit even more from bariatric surgery.”
Using gastric sleeves to avoid diabetic ketoacidosis
Dr. Aminian also noted that “at the Cleveland Clinic we consider a sleeve gastrectomy the procedure of choice” for patients with type 1 diabetes or type 2 diabetes with insulin insufficiency “unless the patient has an absolute contraindication” because of the increased risk for diabetic ketoacidosis in these patients “undergoing any surgery, including bariatric surgery.” Patients with insulin insufficiency “require intensive diabetes and insulin management preoperatively to reduce their risk for developing diabetic ketoacidosis,” and using a sleeve rather than bypass generally results in “more reliable absorption of carbohydrates and nutrients” while also reducing the risk for hypoglycemia, Dr. Aminian said.
In the series reported by Dr. Dessify, 33 patients underwent gastric bypass and 5 had sleeve gastrectomy. The decision to use bypass usually stemmed from its “marginal” improvement in weight loss, compared with a sleeve procedure, and an overall preference at Geisinger for bypass procedures. Dr. Dessify added that he had not yet run a comprehensive assessment of diabetic ketoacidosis complications among patients in his reported series.
Those 38 patients underwent their bariatric procedure during 2002-2019, constituting fewer than 1% of the 4,549 total bariatric surgeries done at Geisinger during that period. The 38 patients with type 1 diabetes averaged 41 years of age, 33 (87%) were women, and 37 (97%) were White. Dr. Dessify and associates undertook this review “to help provide supporting evidence for using bariatric surgery in people with obesity and type 1 diabetes,” he noted.
Dr. Dessify, Dr. Aminian, and Dr. Kim had no disclosures.
While bariatric surgery does nothing to directly improve the disease of patients with type 1 diabetes, it can work indirectly by moderating severe obesity and improving insulin sensitivity to cut the total insulin needs of patients with type 1 diabetes and obesity, based on a single-center, retrospective chart review of 38 U.S. patients.
Two years following their bariatric surgery, these 38 patients with confirmed type 1 diabetes and an average body mass index of 43 kg/m2 before surgery saw their average daily insulin requirement nearly halved, dropping from 118 units/day to 60 units/day, a significant decrease, Brian J. Dessify, DO, said in a presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Another measure of this effect showed that the percentage of patients who required more than one drug for treating their hyperglycemia fell from 66% before surgery to 52% 2 years after surgery, a change that was not statistically significant, said Dr. Dessify, a bariatric surgeon at Geisinger Medical Center in Danville, Pa.
Appropriate for patients with ‘double diabetes’
These results “provide good evidence for [using] bariatric surgery” in people with both obesity and type 1 diabetes,” he concluded. This includes people with what Dr. Dessify called “double diabetes,” meaning that they do not make endogenous insulin, and are also resistant to the effects of exogenous insulin and hence have features of both type 2 and type 1 diabetes.
“This is a really important study,” commented Ali Aminian, MD, director of the Bariatric and Metabolic Institute of the Cleveland Clinic. “For patients with type 1 diabetes, the primary goal of bariatric surgery is weight loss and improvement of obesity-related comorbidities. Patients with type 2 diabetes can be a candidate for bariatric surgery regardless of their weight,” Dr. Aminian said as designated discussant for the report.
“The goal of bariatric surgery in patients with type 1 diabetes is to promote sensitivity to the exogenous insulin they receive,” agreed Julie Kim, MD, a bariatric surgeon at Mount Auburn Hospital in Waltham, Mass., and a second discussant for the report. Patients with double diabetes “are probably a subclass of patients [with type 1 diabetes] who might benefit even more from bariatric surgery.”
Using gastric sleeves to avoid diabetic ketoacidosis
Dr. Aminian also noted that “at the Cleveland Clinic we consider a sleeve gastrectomy the procedure of choice” for patients with type 1 diabetes or type 2 diabetes with insulin insufficiency “unless the patient has an absolute contraindication” because of the increased risk for diabetic ketoacidosis in these patients “undergoing any surgery, including bariatric surgery.” Patients with insulin insufficiency “require intensive diabetes and insulin management preoperatively to reduce their risk for developing diabetic ketoacidosis,” and using a sleeve rather than bypass generally results in “more reliable absorption of carbohydrates and nutrients” while also reducing the risk for hypoglycemia, Dr. Aminian said.
In the series reported by Dr. Dessify, 33 patients underwent gastric bypass and 5 had sleeve gastrectomy. The decision to use bypass usually stemmed from its “marginal” improvement in weight loss, compared with a sleeve procedure, and an overall preference at Geisinger for bypass procedures. Dr. Dessify added that he had not yet run a comprehensive assessment of diabetic ketoacidosis complications among patients in his reported series.
Those 38 patients underwent their bariatric procedure during 2002-2019, constituting fewer than 1% of the 4,549 total bariatric surgeries done at Geisinger during that period. The 38 patients with type 1 diabetes averaged 41 years of age, 33 (87%) were women, and 37 (97%) were White. Dr. Dessify and associates undertook this review “to help provide supporting evidence for using bariatric surgery in people with obesity and type 1 diabetes,” he noted.
Dr. Dessify, Dr. Aminian, and Dr. Kim had no disclosures.
FROM ASMBS 2021
Getting hypertension under control in the youngest of patients
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7
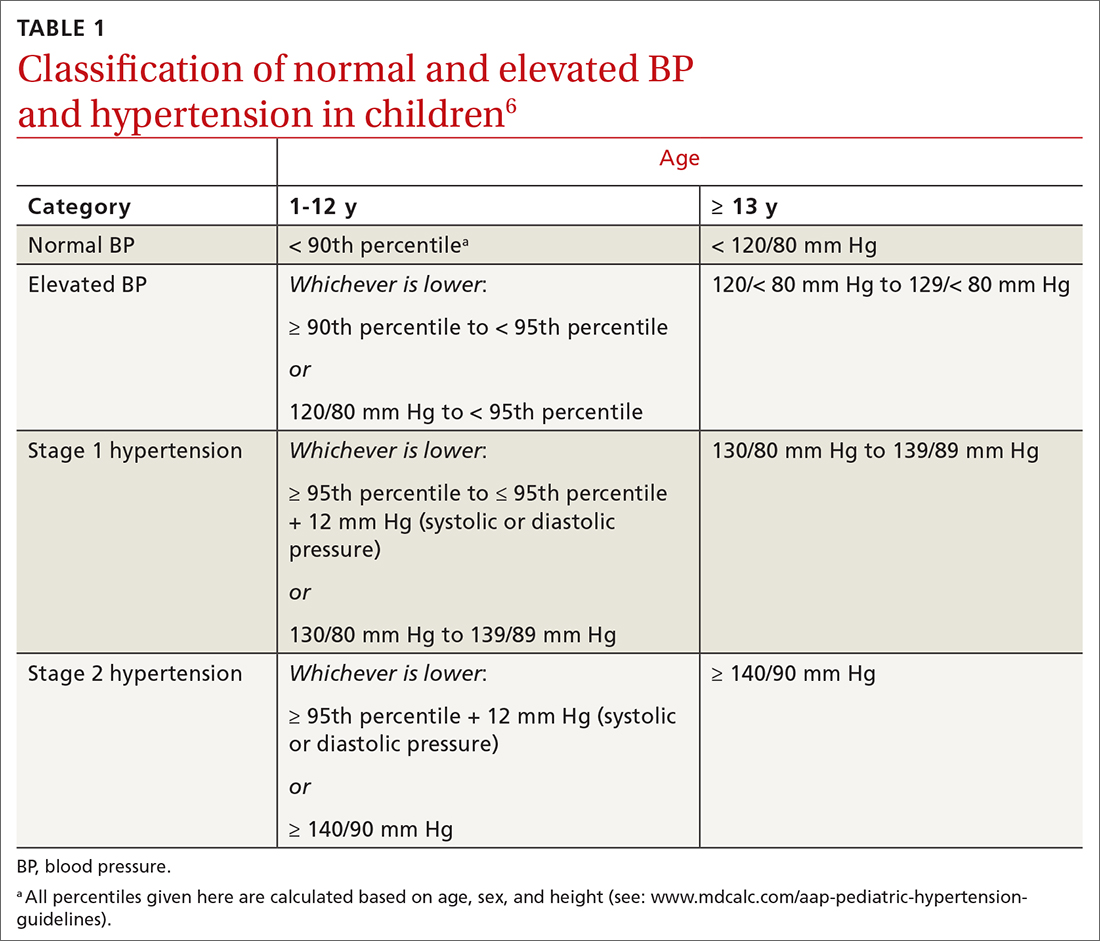
As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16
Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.
ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.
TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.
Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.
Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; dustinksmith@yahoo.com
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7

As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16
Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.
ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.
TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.
Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.
Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; dustinksmith@yahoo.com
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7

As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16
Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.
ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.
TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.
Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.
Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; dustinksmith@yahoo.com
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
PRACTICE RECOMMENDATIONS
› Measure the blood pressure (BP) of all children 3 years and older annually; those who have a specific comorbid condition (eg, obesity, diabetes, renal disease, or an aortic-arch abnormality) or who are taking medication known to elevate BP should have their BP checked at every health care visit. C
› Encourage lifestyle modification as the initial treatment for elevated BP or hypertension in children. A
› Utilize pharmacotherapy for (1) children with stage 1 hypertension who have failed to meet BP goals after 3 to 6 months of lifestyle modification and (2) children with stage 2 hypertension who do not have a modifiable risk factor, such as obesity. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Healthy with obesity? The latest study casts doubt
compared with people without obesity and or adverse metabolic profiles, new research suggests.
The latest data on this controversial subject come from an analysis of nearly 400,000 people in the U.K. Biobank. Although the data also showed that metabolically healthy obesity poses less risk than “metabolically unhealthy” obesity, the risk of progression from healthy to unhealthy within 3-5 years was high.
“People with metabolically healthy obesity are not ‘healthy’ as they are at higher risk of atherosclerotic cardiovascular disease [ASCVD], heart failure, and respiratory diseases, compared with nonobese people with a normal metabolic profile. As such, weight management could be beneficial to all people with obesity irrespective of metabolic profile,” Ziyi Zhou and colleagues wrote in their report, published June 10, 2021, in Diabetologia.
Moreover, they advised avoiding the term metabolically healthy obesity entirely in clinical medicine “as it is misleading, and different strategies for risk stratification should be explored.”
In interviews, two experts provided somewhat different takes on the study and the overall subject.
‘Lifestyle should be explored with every single patient regardless of their weight’
Yoni Freedhoff, MD, medical director of the Bariatric Medical Institute, Ottawa, said “clinicians and patients need to be aware that obesity increases a person’s risk of various medical problems, and in turn this might lead to more frequent screening. This increased screening might be analogous to that of a person with a strong familial history of cancer who of course we would never describe as being ‘unhealthy’ as a consequence of their increased risk.”
In addition to screening, “lifestyle should be explored with every single patient regardless of their weight, and if a person’s weight is not affecting their health or their quality of life, a clinician need only let the patient know that, were they to want to discuss weight management options in the future, that they’d be there for them,” said Dr. Freedhoff.
‘Metabolically healthy obesity’ has had many definitions
Matthias Schulze, DrPH, head of the molecular epidemiology at the German Institute of Human Nutrition, Potsdam, and professor at the University of Potsdam, pointed out that the way metabolically healthy obesity is defined and the outcomes assessed make a difference.
In the current study, the term is defined as having a body mass index of at least 30 kg/m2 and at least four of six metabolically healthy criteria: blood pressure, C-reactive protein, triacylglycerols, LDL cholesterol, HDL cholesterol, and hemoglobin A1c.
In May 2021, Dr. Schulze and associates reported in JAMA Network Open on a different definition that they found to identify individuals who do not have an increased risk of cardiovascular disease death and total mortality. Interestingly, they also used the U.K. Biobank as their validation cohort.
“We derived a new definition of metabolic health ... that is different from those used in [the current] article. Importantly, we included a measure of body fat distribution, waist-to-hip ratio. On the other side, we investigated only mortality outcomes and we can therefore not exclude the possibility that other outcomes may still be related. [For example], a higher diabetes risk may still be present among those we have defined as having metabolically healthy obesity.”
Dr. Schulze also said that several previous studies and meta-analyses have suggested that “previous common definitions of metabolically healthy obesity do not identify a subgroup without risk, or being at risk comparable to normal-weight metabolically healthy. Thus, this study confirms this conclusion. [But] this doesn’t rule out that there are better ways of defining subgroups.”
Clinically, he said “given that we investigated only mortality, we cannot conclude that our ‘metabolically healthy obesity’ group doesn’t require intervention.”
Higher rates of diabetes, ASCVD, heart failure, death
The current population-based study included 381,363 U.K. Biobank participants who were followed up for a median 11.2 years. Overall, about 55% did not have obesity or metabolic abnormalities, 9% had metabolically healthy obesity, 20% were metabolically unhealthy but did not have obesity, and 16% had metabolically unhealthy obesity as defined by the investigators.
The investigators adjusted the data for several potential confounders, including age, sex, ethnicity, education, socioeconomic status, smoking status, physical activity, and dietary factors.
Compared with individuals without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher rates of incident diabetes (hazard ratio, 4.32), ASCVD (HR, 1.18), myocardial infarction (HR, 1.23), stroke (HR, 1.10), heart failure (HR, 1.76), respiratory diseases (HR, 1.28), and chronic obstructive pulmonary disease (HR, 1.19).
In general, rates of cardiovascular and respiratory outcomes were highest in metabolically unhealthy obesity, followed by those without obesity but with metabolic abnormalities and those with metabolically healthy obesity. However, for incident and fatal heart failure and incident respiratory diseases, those with metabolically healthy obesity had higher rates than did those without obesity but with metabolic abnormalities.
Compared with those without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher all-cause mortality rates (HR, 1.22). And, compared with those without obesity (regardless of metabolic status) at baseline, those with metabolically healthy obesity were significantly more likely to have diabetes (HR, 2.06), heart failure (HR, 1.6), and respiratory diseases (HR, 1.2), but not ASCVD. The association was also significant for all-cause and heart failure mortality (HR, 1.12 and 1.44, respectively), but not for other causes of death.
Progression from metabolically healthy to unhealthy is common
Among 8,512 participants for whom longitudinal data were available for a median of 4.4 years, half of those with metabolically healthy obesity remained in that category, 20% no longer had obesity, and more than a quarter transitioned to metabolically unhealthy obesity. Compared with those without obesity or metabolic abnormalities throughout, those who transitioned from metabolically healthy to metabolically unhealthy had significantly higher rates of incident ASCVD (HR, 2.46) and all-cause mortality (HR, 3.07).
But those who remained in the metabolically healthy obesity category throughout did not have significantly increased risks for the adverse outcomes measured.
Ms. Zhou and colleagues noted that the data demonstrate heterogeneity among people with obesity, which offers the potential to stratify risk based on prognosis. For example, “people with [metabolically unhealthy obesity] were at a higher risk of mortality and morbidity than everyone else, and thus they should be prioritized for intervention.”
However, they add, “Obesity is associated with a wide range of diseases, and using a single label or categorical risk algorithm is unlikely to be effective compared with prediction algorithms based on disease-specific and continuous risk markers.”
Ms. Zhou has no disclosures. One coauthor has relationships with numerous pharmaceutical companies; the rest have none. Dr. Freedhoff has served as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health. He is a speaker or a member of a speakers bureau for Obesity Canada and Novo Nordisk, received research grant from Novo Nordisk, and received income of at least $250 from WebMD, CTV, and Random House. Dr/ Schulze has received grants from German Federal Ministry of Education and Research.
compared with people without obesity and or adverse metabolic profiles, new research suggests.
The latest data on this controversial subject come from an analysis of nearly 400,000 people in the U.K. Biobank. Although the data also showed that metabolically healthy obesity poses less risk than “metabolically unhealthy” obesity, the risk of progression from healthy to unhealthy within 3-5 years was high.
“People with metabolically healthy obesity are not ‘healthy’ as they are at higher risk of atherosclerotic cardiovascular disease [ASCVD], heart failure, and respiratory diseases, compared with nonobese people with a normal metabolic profile. As such, weight management could be beneficial to all people with obesity irrespective of metabolic profile,” Ziyi Zhou and colleagues wrote in their report, published June 10, 2021, in Diabetologia.
Moreover, they advised avoiding the term metabolically healthy obesity entirely in clinical medicine “as it is misleading, and different strategies for risk stratification should be explored.”
In interviews, two experts provided somewhat different takes on the study and the overall subject.
‘Lifestyle should be explored with every single patient regardless of their weight’
Yoni Freedhoff, MD, medical director of the Bariatric Medical Institute, Ottawa, said “clinicians and patients need to be aware that obesity increases a person’s risk of various medical problems, and in turn this might lead to more frequent screening. This increased screening might be analogous to that of a person with a strong familial history of cancer who of course we would never describe as being ‘unhealthy’ as a consequence of their increased risk.”
In addition to screening, “lifestyle should be explored with every single patient regardless of their weight, and if a person’s weight is not affecting their health or their quality of life, a clinician need only let the patient know that, were they to want to discuss weight management options in the future, that they’d be there for them,” said Dr. Freedhoff.
‘Metabolically healthy obesity’ has had many definitions
Matthias Schulze, DrPH, head of the molecular epidemiology at the German Institute of Human Nutrition, Potsdam, and professor at the University of Potsdam, pointed out that the way metabolically healthy obesity is defined and the outcomes assessed make a difference.
In the current study, the term is defined as having a body mass index of at least 30 kg/m2 and at least four of six metabolically healthy criteria: blood pressure, C-reactive protein, triacylglycerols, LDL cholesterol, HDL cholesterol, and hemoglobin A1c.
In May 2021, Dr. Schulze and associates reported in JAMA Network Open on a different definition that they found to identify individuals who do not have an increased risk of cardiovascular disease death and total mortality. Interestingly, they also used the U.K. Biobank as their validation cohort.
“We derived a new definition of metabolic health ... that is different from those used in [the current] article. Importantly, we included a measure of body fat distribution, waist-to-hip ratio. On the other side, we investigated only mortality outcomes and we can therefore not exclude the possibility that other outcomes may still be related. [For example], a higher diabetes risk may still be present among those we have defined as having metabolically healthy obesity.”
Dr. Schulze also said that several previous studies and meta-analyses have suggested that “previous common definitions of metabolically healthy obesity do not identify a subgroup without risk, or being at risk comparable to normal-weight metabolically healthy. Thus, this study confirms this conclusion. [But] this doesn’t rule out that there are better ways of defining subgroups.”
Clinically, he said “given that we investigated only mortality, we cannot conclude that our ‘metabolically healthy obesity’ group doesn’t require intervention.”
Higher rates of diabetes, ASCVD, heart failure, death
The current population-based study included 381,363 U.K. Biobank participants who were followed up for a median 11.2 years. Overall, about 55% did not have obesity or metabolic abnormalities, 9% had metabolically healthy obesity, 20% were metabolically unhealthy but did not have obesity, and 16% had metabolically unhealthy obesity as defined by the investigators.
The investigators adjusted the data for several potential confounders, including age, sex, ethnicity, education, socioeconomic status, smoking status, physical activity, and dietary factors.
Compared with individuals without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher rates of incident diabetes (hazard ratio, 4.32), ASCVD (HR, 1.18), myocardial infarction (HR, 1.23), stroke (HR, 1.10), heart failure (HR, 1.76), respiratory diseases (HR, 1.28), and chronic obstructive pulmonary disease (HR, 1.19).
In general, rates of cardiovascular and respiratory outcomes were highest in metabolically unhealthy obesity, followed by those without obesity but with metabolic abnormalities and those with metabolically healthy obesity. However, for incident and fatal heart failure and incident respiratory diseases, those with metabolically healthy obesity had higher rates than did those without obesity but with metabolic abnormalities.
Compared with those without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher all-cause mortality rates (HR, 1.22). And, compared with those without obesity (regardless of metabolic status) at baseline, those with metabolically healthy obesity were significantly more likely to have diabetes (HR, 2.06), heart failure (HR, 1.6), and respiratory diseases (HR, 1.2), but not ASCVD. The association was also significant for all-cause and heart failure mortality (HR, 1.12 and 1.44, respectively), but not for other causes of death.
Progression from metabolically healthy to unhealthy is common
Among 8,512 participants for whom longitudinal data were available for a median of 4.4 years, half of those with metabolically healthy obesity remained in that category, 20% no longer had obesity, and more than a quarter transitioned to metabolically unhealthy obesity. Compared with those without obesity or metabolic abnormalities throughout, those who transitioned from metabolically healthy to metabolically unhealthy had significantly higher rates of incident ASCVD (HR, 2.46) and all-cause mortality (HR, 3.07).
But those who remained in the metabolically healthy obesity category throughout did not have significantly increased risks for the adverse outcomes measured.
Ms. Zhou and colleagues noted that the data demonstrate heterogeneity among people with obesity, which offers the potential to stratify risk based on prognosis. For example, “people with [metabolically unhealthy obesity] were at a higher risk of mortality and morbidity than everyone else, and thus they should be prioritized for intervention.”
However, they add, “Obesity is associated with a wide range of diseases, and using a single label or categorical risk algorithm is unlikely to be effective compared with prediction algorithms based on disease-specific and continuous risk markers.”
Ms. Zhou has no disclosures. One coauthor has relationships with numerous pharmaceutical companies; the rest have none. Dr. Freedhoff has served as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health. He is a speaker or a member of a speakers bureau for Obesity Canada and Novo Nordisk, received research grant from Novo Nordisk, and received income of at least $250 from WebMD, CTV, and Random House. Dr/ Schulze has received grants from German Federal Ministry of Education and Research.
compared with people without obesity and or adverse metabolic profiles, new research suggests.
The latest data on this controversial subject come from an analysis of nearly 400,000 people in the U.K. Biobank. Although the data also showed that metabolically healthy obesity poses less risk than “metabolically unhealthy” obesity, the risk of progression from healthy to unhealthy within 3-5 years was high.
“People with metabolically healthy obesity are not ‘healthy’ as they are at higher risk of atherosclerotic cardiovascular disease [ASCVD], heart failure, and respiratory diseases, compared with nonobese people with a normal metabolic profile. As such, weight management could be beneficial to all people with obesity irrespective of metabolic profile,” Ziyi Zhou and colleagues wrote in their report, published June 10, 2021, in Diabetologia.
Moreover, they advised avoiding the term metabolically healthy obesity entirely in clinical medicine “as it is misleading, and different strategies for risk stratification should be explored.”
In interviews, two experts provided somewhat different takes on the study and the overall subject.
‘Lifestyle should be explored with every single patient regardless of their weight’
Yoni Freedhoff, MD, medical director of the Bariatric Medical Institute, Ottawa, said “clinicians and patients need to be aware that obesity increases a person’s risk of various medical problems, and in turn this might lead to more frequent screening. This increased screening might be analogous to that of a person with a strong familial history of cancer who of course we would never describe as being ‘unhealthy’ as a consequence of their increased risk.”
In addition to screening, “lifestyle should be explored with every single patient regardless of their weight, and if a person’s weight is not affecting their health or their quality of life, a clinician need only let the patient know that, were they to want to discuss weight management options in the future, that they’d be there for them,” said Dr. Freedhoff.
‘Metabolically healthy obesity’ has had many definitions
Matthias Schulze, DrPH, head of the molecular epidemiology at the German Institute of Human Nutrition, Potsdam, and professor at the University of Potsdam, pointed out that the way metabolically healthy obesity is defined and the outcomes assessed make a difference.
In the current study, the term is defined as having a body mass index of at least 30 kg/m2 and at least four of six metabolically healthy criteria: blood pressure, C-reactive protein, triacylglycerols, LDL cholesterol, HDL cholesterol, and hemoglobin A1c.
In May 2021, Dr. Schulze and associates reported in JAMA Network Open on a different definition that they found to identify individuals who do not have an increased risk of cardiovascular disease death and total mortality. Interestingly, they also used the U.K. Biobank as their validation cohort.
“We derived a new definition of metabolic health ... that is different from those used in [the current] article. Importantly, we included a measure of body fat distribution, waist-to-hip ratio. On the other side, we investigated only mortality outcomes and we can therefore not exclude the possibility that other outcomes may still be related. [For example], a higher diabetes risk may still be present among those we have defined as having metabolically healthy obesity.”
Dr. Schulze also said that several previous studies and meta-analyses have suggested that “previous common definitions of metabolically healthy obesity do not identify a subgroup without risk, or being at risk comparable to normal-weight metabolically healthy. Thus, this study confirms this conclusion. [But] this doesn’t rule out that there are better ways of defining subgroups.”
Clinically, he said “given that we investigated only mortality, we cannot conclude that our ‘metabolically healthy obesity’ group doesn’t require intervention.”
Higher rates of diabetes, ASCVD, heart failure, death
The current population-based study included 381,363 U.K. Biobank participants who were followed up for a median 11.2 years. Overall, about 55% did not have obesity or metabolic abnormalities, 9% had metabolically healthy obesity, 20% were metabolically unhealthy but did not have obesity, and 16% had metabolically unhealthy obesity as defined by the investigators.
The investigators adjusted the data for several potential confounders, including age, sex, ethnicity, education, socioeconomic status, smoking status, physical activity, and dietary factors.
Compared with individuals without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher rates of incident diabetes (hazard ratio, 4.32), ASCVD (HR, 1.18), myocardial infarction (HR, 1.23), stroke (HR, 1.10), heart failure (HR, 1.76), respiratory diseases (HR, 1.28), and chronic obstructive pulmonary disease (HR, 1.19).
In general, rates of cardiovascular and respiratory outcomes were highest in metabolically unhealthy obesity, followed by those without obesity but with metabolic abnormalities and those with metabolically healthy obesity. However, for incident and fatal heart failure and incident respiratory diseases, those with metabolically healthy obesity had higher rates than did those without obesity but with metabolic abnormalities.
Compared with those without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher all-cause mortality rates (HR, 1.22). And, compared with those without obesity (regardless of metabolic status) at baseline, those with metabolically healthy obesity were significantly more likely to have diabetes (HR, 2.06), heart failure (HR, 1.6), and respiratory diseases (HR, 1.2), but not ASCVD. The association was also significant for all-cause and heart failure mortality (HR, 1.12 and 1.44, respectively), but not for other causes of death.
Progression from metabolically healthy to unhealthy is common
Among 8,512 participants for whom longitudinal data were available for a median of 4.4 years, half of those with metabolically healthy obesity remained in that category, 20% no longer had obesity, and more than a quarter transitioned to metabolically unhealthy obesity. Compared with those without obesity or metabolic abnormalities throughout, those who transitioned from metabolically healthy to metabolically unhealthy had significantly higher rates of incident ASCVD (HR, 2.46) and all-cause mortality (HR, 3.07).
But those who remained in the metabolically healthy obesity category throughout did not have significantly increased risks for the adverse outcomes measured.
Ms. Zhou and colleagues noted that the data demonstrate heterogeneity among people with obesity, which offers the potential to stratify risk based on prognosis. For example, “people with [metabolically unhealthy obesity] were at a higher risk of mortality and morbidity than everyone else, and thus they should be prioritized for intervention.”
However, they add, “Obesity is associated with a wide range of diseases, and using a single label or categorical risk algorithm is unlikely to be effective compared with prediction algorithms based on disease-specific and continuous risk markers.”
Ms. Zhou has no disclosures. One coauthor has relationships with numerous pharmaceutical companies; the rest have none. Dr. Freedhoff has served as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health. He is a speaker or a member of a speakers bureau for Obesity Canada and Novo Nordisk, received research grant from Novo Nordisk, and received income of at least $250 from WebMD, CTV, and Random House. Dr/ Schulze has received grants from German Federal Ministry of Education and Research.
FROM DIABETOLOGIA