User login
ACC, AHA release first cardiovascular disease primary prevention guideline
that takes into account each person’s social determinants of health. The guideline substantially dialed down prior recommendations on aspirin for primary prevention by calling for no use in people older than 70 years and infrequent use in those 40-70 years old.

The American College of Cardiology and the American Heart Association released their 2019 guideline on the primary prevention of cardiovascular disease on March 17, during the annual meeting of the American College of Cardiology (J Amer Coll Cardiol. 2019 March 17;doi: 10.1016/j.jacc.2019.03.010.). The guideline is a “one-stop shop” that pulls together existing recommendations from the two organizations and combines it with some new recommendations that address issues such as aspirin prophylaxis, and the social setting of each person, said Donna K. Arnett, Ph.D., professor of epidemiology at the University of Kentucky, dean of the university’s College of Public Health, and co-chair of the guideline writing panel.
“We made the social determinants of health front and center. With many people, clinicians don’t ask whether they have access to healthy foods or a way to get to the pharmacy. Asking about these issues is step one,” toward helping people address their social situation, Dr. Arnett said while introducing the new guideline in a press briefing. The guideline recommends that clinicians assess the social determinants for each person treated for cardiovascular disease prevention using a screening tool developed by the U.S. Centers for Medicare & Medicaid Services and made available by the National Academy of Medicine (NAM Perspectives. 2017; doi:10.31478/201705b).
“No other guideline has highlighted the social determinants of health,” noted Erin D. Michos, MD, associate director of preventive cardiology at Johns Hopkins Medicine in Baltimore, and a member of the guideline-writing panel. Other overarching themes of the guideline are its emphasis on the need for a team of clinicians to deliver all the disparate and time-consuming facets of care needed for comprehensive primary prevention of cardiovascular disease, and its call for a healthy lifestyle throughout life as foundations for prevention, Dr. Michos said in an interview.
With 48 recommendations, the guideline also deals with prevention issues such as a healthy diet and body mass, appropriate control of diabetes, smoking cessation, and control of blood pressure and cholesterol (see chart). The writing committee took the cholesterol and blood pressure recommendations directly from recent guidelines from the ACC and AHA in 2017 (blood pressure:J Amer Coll Cardiol. 2018 May;71[19]:e177-e248) and 2018 (cholesterol:Circulation. 2018 Nov 10;doi: 10.1161/CIR.0000000000000625).
The other major, new recommendations in the guideline deal with aspirin use for primary prevention, which recently underwent a shake up with publication of results from several studies that showed less cardiovascular benefit and more potential bleeding harm from routine aspirin prophylaxis than previously appreciated. Among the most notable of these reports, which led to a class III recommendation – do not use – for aspirin in people more than 70 years old came from the ASPREE (Aspirin in Reducing Events in the Elderly) study (New Engl J Med. 2018 Oct 18;379[16]:1519-28). For those 40-70 years old, the recommendation is class IIb, worded as “might be considered for select adults.”

“Generally no, occasionally yes,” is aspirin appropriate for people in this age group, notably those at high risk for cardiovascular disease and also at low risk for bleeding, explained Amit Khera, MD, a guideline-panel member, and professor of medicine and director of preventive cardiology at the University of Texas Southwestern Medical Center in Dallas.
As a guideline for primary prevention, a prime target audience is primary care physicians, who would need to be instrumental in applying the guideline. But the guideline recommendations released by the ACC and AHA for blood pressure management in 2017 were not accepted by U.S. groups that represent primary care physicians, the American College of Physicians, and the American Academy of Family Physicians.
John J. Warner, MD, an interventional cardiologist, executive vice president for health system affairs at UT Southwestern, and president of the AHA when the blood pressure guideline came out said that the ACC and AHA “learned some lessons” from the blood pressure experience. The societies responded this time around by “trying to view the document through as many lenses as possible” during the peer review process, Dr. Warner said during the press conference.
“I don’t think the new guideline will be seen as anything except positive,” commented Martha Gulati, MD, professor of medicine and chief of cardiology at the University of Arizona in Phoenix. Collecting all the cardiovascular disease recommendations for primary prevention in one document “helps clinicians access the information easily and helps patients see the big picture,” said Dr. Gulati, who was not involved in the guideline’s writing or review.
She especially applauded the recommendations to assess each person’s social determinants of health, the team-care approach, and the recommendations dealing with diet and other aspects of a healthy lifestyle. “This was a perfect time” to bring together the existing blood pressure and cholesterol guidelines, the new guidance on aspirin use, and the other recommendation in a single document, she said in an interview.
Dr. Arnett, Dr. Michos, Dr. Khera, Dr. Warner, and Dr. Gulati had no disclosures.
mzoler@mdedge.com
On Twitter @mitchelzoler
SOURCE: Arnett DK et al. J Amer Coll Cardiol. 2019 March 17;doi: 10.1016/j.jacc.2019.03.010.
that takes into account each person’s social determinants of health. The guideline substantially dialed down prior recommendations on aspirin for primary prevention by calling for no use in people older than 70 years and infrequent use in those 40-70 years old.

The American College of Cardiology and the American Heart Association released their 2019 guideline on the primary prevention of cardiovascular disease on March 17, during the annual meeting of the American College of Cardiology (J Amer Coll Cardiol. 2019 March 17;doi: 10.1016/j.jacc.2019.03.010.). The guideline is a “one-stop shop” that pulls together existing recommendations from the two organizations and combines it with some new recommendations that address issues such as aspirin prophylaxis, and the social setting of each person, said Donna K. Arnett, Ph.D., professor of epidemiology at the University of Kentucky, dean of the university’s College of Public Health, and co-chair of the guideline writing panel.
“We made the social determinants of health front and center. With many people, clinicians don’t ask whether they have access to healthy foods or a way to get to the pharmacy. Asking about these issues is step one,” toward helping people address their social situation, Dr. Arnett said while introducing the new guideline in a press briefing. The guideline recommends that clinicians assess the social determinants for each person treated for cardiovascular disease prevention using a screening tool developed by the U.S. Centers for Medicare & Medicaid Services and made available by the National Academy of Medicine (NAM Perspectives. 2017; doi:10.31478/201705b).
“No other guideline has highlighted the social determinants of health,” noted Erin D. Michos, MD, associate director of preventive cardiology at Johns Hopkins Medicine in Baltimore, and a member of the guideline-writing panel. Other overarching themes of the guideline are its emphasis on the need for a team of clinicians to deliver all the disparate and time-consuming facets of care needed for comprehensive primary prevention of cardiovascular disease, and its call for a healthy lifestyle throughout life as foundations for prevention, Dr. Michos said in an interview.
With 48 recommendations, the guideline also deals with prevention issues such as a healthy diet and body mass, appropriate control of diabetes, smoking cessation, and control of blood pressure and cholesterol (see chart). The writing committee took the cholesterol and blood pressure recommendations directly from recent guidelines from the ACC and AHA in 2017 (blood pressure:J Amer Coll Cardiol. 2018 May;71[19]:e177-e248) and 2018 (cholesterol:Circulation. 2018 Nov 10;doi: 10.1161/CIR.0000000000000625).
The other major, new recommendations in the guideline deal with aspirin use for primary prevention, which recently underwent a shake up with publication of results from several studies that showed less cardiovascular benefit and more potential bleeding harm from routine aspirin prophylaxis than previously appreciated. Among the most notable of these reports, which led to a class III recommendation – do not use – for aspirin in people more than 70 years old came from the ASPREE (Aspirin in Reducing Events in the Elderly) study (New Engl J Med. 2018 Oct 18;379[16]:1519-28). For those 40-70 years old, the recommendation is class IIb, worded as “might be considered for select adults.”

“Generally no, occasionally yes,” is aspirin appropriate for people in this age group, notably those at high risk for cardiovascular disease and also at low risk for bleeding, explained Amit Khera, MD, a guideline-panel member, and professor of medicine and director of preventive cardiology at the University of Texas Southwestern Medical Center in Dallas.
As a guideline for primary prevention, a prime target audience is primary care physicians, who would need to be instrumental in applying the guideline. But the guideline recommendations released by the ACC and AHA for blood pressure management in 2017 were not accepted by U.S. groups that represent primary care physicians, the American College of Physicians, and the American Academy of Family Physicians.
John J. Warner, MD, an interventional cardiologist, executive vice president for health system affairs at UT Southwestern, and president of the AHA when the blood pressure guideline came out said that the ACC and AHA “learned some lessons” from the blood pressure experience. The societies responded this time around by “trying to view the document through as many lenses as possible” during the peer review process, Dr. Warner said during the press conference.
“I don’t think the new guideline will be seen as anything except positive,” commented Martha Gulati, MD, professor of medicine and chief of cardiology at the University of Arizona in Phoenix. Collecting all the cardiovascular disease recommendations for primary prevention in one document “helps clinicians access the information easily and helps patients see the big picture,” said Dr. Gulati, who was not involved in the guideline’s writing or review.
She especially applauded the recommendations to assess each person’s social determinants of health, the team-care approach, and the recommendations dealing with diet and other aspects of a healthy lifestyle. “This was a perfect time” to bring together the existing blood pressure and cholesterol guidelines, the new guidance on aspirin use, and the other recommendation in a single document, she said in an interview.
Dr. Arnett, Dr. Michos, Dr. Khera, Dr. Warner, and Dr. Gulati had no disclosures.
mzoler@mdedge.com
On Twitter @mitchelzoler
SOURCE: Arnett DK et al. J Amer Coll Cardiol. 2019 March 17;doi: 10.1016/j.jacc.2019.03.010.
that takes into account each person’s social determinants of health. The guideline substantially dialed down prior recommendations on aspirin for primary prevention by calling for no use in people older than 70 years and infrequent use in those 40-70 years old.

The American College of Cardiology and the American Heart Association released their 2019 guideline on the primary prevention of cardiovascular disease on March 17, during the annual meeting of the American College of Cardiology (J Amer Coll Cardiol. 2019 March 17;doi: 10.1016/j.jacc.2019.03.010.). The guideline is a “one-stop shop” that pulls together existing recommendations from the two organizations and combines it with some new recommendations that address issues such as aspirin prophylaxis, and the social setting of each person, said Donna K. Arnett, Ph.D., professor of epidemiology at the University of Kentucky, dean of the university’s College of Public Health, and co-chair of the guideline writing panel.
“We made the social determinants of health front and center. With many people, clinicians don’t ask whether they have access to healthy foods or a way to get to the pharmacy. Asking about these issues is step one,” toward helping people address their social situation, Dr. Arnett said while introducing the new guideline in a press briefing. The guideline recommends that clinicians assess the social determinants for each person treated for cardiovascular disease prevention using a screening tool developed by the U.S. Centers for Medicare & Medicaid Services and made available by the National Academy of Medicine (NAM Perspectives. 2017; doi:10.31478/201705b).
“No other guideline has highlighted the social determinants of health,” noted Erin D. Michos, MD, associate director of preventive cardiology at Johns Hopkins Medicine in Baltimore, and a member of the guideline-writing panel. Other overarching themes of the guideline are its emphasis on the need for a team of clinicians to deliver all the disparate and time-consuming facets of care needed for comprehensive primary prevention of cardiovascular disease, and its call for a healthy lifestyle throughout life as foundations for prevention, Dr. Michos said in an interview.
With 48 recommendations, the guideline also deals with prevention issues such as a healthy diet and body mass, appropriate control of diabetes, smoking cessation, and control of blood pressure and cholesterol (see chart). The writing committee took the cholesterol and blood pressure recommendations directly from recent guidelines from the ACC and AHA in 2017 (blood pressure:J Amer Coll Cardiol. 2018 May;71[19]:e177-e248) and 2018 (cholesterol:Circulation. 2018 Nov 10;doi: 10.1161/CIR.0000000000000625).
The other major, new recommendations in the guideline deal with aspirin use for primary prevention, which recently underwent a shake up with publication of results from several studies that showed less cardiovascular benefit and more potential bleeding harm from routine aspirin prophylaxis than previously appreciated. Among the most notable of these reports, which led to a class III recommendation – do not use – for aspirin in people more than 70 years old came from the ASPREE (Aspirin in Reducing Events in the Elderly) study (New Engl J Med. 2018 Oct 18;379[16]:1519-28). For those 40-70 years old, the recommendation is class IIb, worded as “might be considered for select adults.”

“Generally no, occasionally yes,” is aspirin appropriate for people in this age group, notably those at high risk for cardiovascular disease and also at low risk for bleeding, explained Amit Khera, MD, a guideline-panel member, and professor of medicine and director of preventive cardiology at the University of Texas Southwestern Medical Center in Dallas.
As a guideline for primary prevention, a prime target audience is primary care physicians, who would need to be instrumental in applying the guideline. But the guideline recommendations released by the ACC and AHA for blood pressure management in 2017 were not accepted by U.S. groups that represent primary care physicians, the American College of Physicians, and the American Academy of Family Physicians.
John J. Warner, MD, an interventional cardiologist, executive vice president for health system affairs at UT Southwestern, and president of the AHA when the blood pressure guideline came out said that the ACC and AHA “learned some lessons” from the blood pressure experience. The societies responded this time around by “trying to view the document through as many lenses as possible” during the peer review process, Dr. Warner said during the press conference.
“I don’t think the new guideline will be seen as anything except positive,” commented Martha Gulati, MD, professor of medicine and chief of cardiology at the University of Arizona in Phoenix. Collecting all the cardiovascular disease recommendations for primary prevention in one document “helps clinicians access the information easily and helps patients see the big picture,” said Dr. Gulati, who was not involved in the guideline’s writing or review.
She especially applauded the recommendations to assess each person’s social determinants of health, the team-care approach, and the recommendations dealing with diet and other aspects of a healthy lifestyle. “This was a perfect time” to bring together the existing blood pressure and cholesterol guidelines, the new guidance on aspirin use, and the other recommendation in a single document, she said in an interview.
Dr. Arnett, Dr. Michos, Dr. Khera, Dr. Warner, and Dr. Gulati had no disclosures.
mzoler@mdedge.com
On Twitter @mitchelzoler
SOURCE: Arnett DK et al. J Amer Coll Cardiol. 2019 March 17;doi: 10.1016/j.jacc.2019.03.010.
REPORTING FROM ACC 2019
Hypoglycemia in the elderly: Watch for atypical symptoms
We read with interest the review article by Keber and Fiebert, “Diabetes in the elderly: Matching meds to needs” (J Fam Pract. 2018;67:408-410,412-415). The authors have provided a timely overview of antidiabetes medications for elderly people with type 2 diabetes mellitus (T2DM) and their relative risks for hypoglycemia.
We’d like to add to this important conversation.
Aging, per se, modifies the glycemic thresholds for autonomic symptoms and cognitive impairment; in older nondiabetic men (mean + SD: age 65 ± 3 years), autonomic symptoms and cognitive dysfunction commence at identical glycemic thresholds (3 ± 0.2 mmol/L [54 ± 4 mg/dL]). By contrast, in younger men (age 23 ± 2 years), a significant gap is observed between the glycemic threshold for symptom generation (3.6 mmol/L [65 mg/dL]) and the onset of cognitive dysfunction (2.6 mmol/L [47 mg/dL]).1,2 The simultaneous occurrence of symptoms and cognitive impairment in older people may adversely affect their ability to recognize and treat hypoglycemia promptly.
In addition, hypoglycemia in older T2DM patients often presents with atypical neurologic symptoms, including incoordination and ataxia, slurring of speech, and visual disturbances, which either are not identified as hypoglycemia or are misdiagnosed as other medical disorders (eg, transient ischemic attack).3 Knowledge about hypoglycemia symptoms is poor, in both elderly people with diabetes and their relatives and caregivers, which compromises the ability to identify hypoglycemia and provide effective treatment.4 Education about the possible presentations of hypoglycemia and its effective treatment is essential for older patients and their relatives.
Jan Brož, MD
Prague, Czech Republic
1. Meneilly GS, Elahi D. Physiological importance of first-phase insulin release in elderly patients with diabetes. Diabetes Care. 1998;21:1326-1329.
2. Matyka K, Evans M, Lomas J, et al. Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Diabetes Care. 1997;20:135-141.
3. Jaap AJ, Jones GC, McCrimmon RJ, et al. Perceived symptoms of hypoglycaemia in elderly type 2 diabetic patients treated with insulin. Diabet Med. 1998;15:398-401.
4. Thomson FJ, Masson EA, Leeming JT, et al. Lack of knowledge of symptoms of hypoglycaemia by elderly diabetic patients. Age Ageing. 1991;20:404-406.
We read with interest the review article by Keber and Fiebert, “Diabetes in the elderly: Matching meds to needs” (J Fam Pract. 2018;67:408-410,412-415). The authors have provided a timely overview of antidiabetes medications for elderly people with type 2 diabetes mellitus (T2DM) and their relative risks for hypoglycemia.
We’d like to add to this important conversation.
Aging, per se, modifies the glycemic thresholds for autonomic symptoms and cognitive impairment; in older nondiabetic men (mean + SD: age 65 ± 3 years), autonomic symptoms and cognitive dysfunction commence at identical glycemic thresholds (3 ± 0.2 mmol/L [54 ± 4 mg/dL]). By contrast, in younger men (age 23 ± 2 years), a significant gap is observed between the glycemic threshold for symptom generation (3.6 mmol/L [65 mg/dL]) and the onset of cognitive dysfunction (2.6 mmol/L [47 mg/dL]).1,2 The simultaneous occurrence of symptoms and cognitive impairment in older people may adversely affect their ability to recognize and treat hypoglycemia promptly.
In addition, hypoglycemia in older T2DM patients often presents with atypical neurologic symptoms, including incoordination and ataxia, slurring of speech, and visual disturbances, which either are not identified as hypoglycemia or are misdiagnosed as other medical disorders (eg, transient ischemic attack).3 Knowledge about hypoglycemia symptoms is poor, in both elderly people with diabetes and their relatives and caregivers, which compromises the ability to identify hypoglycemia and provide effective treatment.4 Education about the possible presentations of hypoglycemia and its effective treatment is essential for older patients and their relatives.
Jan Brož, MD
Prague, Czech Republic
We read with interest the review article by Keber and Fiebert, “Diabetes in the elderly: Matching meds to needs” (J Fam Pract. 2018;67:408-410,412-415). The authors have provided a timely overview of antidiabetes medications for elderly people with type 2 diabetes mellitus (T2DM) and their relative risks for hypoglycemia.
We’d like to add to this important conversation.
Aging, per se, modifies the glycemic thresholds for autonomic symptoms and cognitive impairment; in older nondiabetic men (mean + SD: age 65 ± 3 years), autonomic symptoms and cognitive dysfunction commence at identical glycemic thresholds (3 ± 0.2 mmol/L [54 ± 4 mg/dL]). By contrast, in younger men (age 23 ± 2 years), a significant gap is observed between the glycemic threshold for symptom generation (3.6 mmol/L [65 mg/dL]) and the onset of cognitive dysfunction (2.6 mmol/L [47 mg/dL]).1,2 The simultaneous occurrence of symptoms and cognitive impairment in older people may adversely affect their ability to recognize and treat hypoglycemia promptly.
In addition, hypoglycemia in older T2DM patients often presents with atypical neurologic symptoms, including incoordination and ataxia, slurring of speech, and visual disturbances, which either are not identified as hypoglycemia or are misdiagnosed as other medical disorders (eg, transient ischemic attack).3 Knowledge about hypoglycemia symptoms is poor, in both elderly people with diabetes and their relatives and caregivers, which compromises the ability to identify hypoglycemia and provide effective treatment.4 Education about the possible presentations of hypoglycemia and its effective treatment is essential for older patients and their relatives.
Jan Brož, MD
Prague, Czech Republic
1. Meneilly GS, Elahi D. Physiological importance of first-phase insulin release in elderly patients with diabetes. Diabetes Care. 1998;21:1326-1329.
2. Matyka K, Evans M, Lomas J, et al. Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Diabetes Care. 1997;20:135-141.
3. Jaap AJ, Jones GC, McCrimmon RJ, et al. Perceived symptoms of hypoglycaemia in elderly type 2 diabetic patients treated with insulin. Diabet Med. 1998;15:398-401.
4. Thomson FJ, Masson EA, Leeming JT, et al. Lack of knowledge of symptoms of hypoglycaemia by elderly diabetic patients. Age Ageing. 1991;20:404-406.
1. Meneilly GS, Elahi D. Physiological importance of first-phase insulin release in elderly patients with diabetes. Diabetes Care. 1998;21:1326-1329.
2. Matyka K, Evans M, Lomas J, et al. Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Diabetes Care. 1997;20:135-141.
3. Jaap AJ, Jones GC, McCrimmon RJ, et al. Perceived symptoms of hypoglycaemia in elderly type 2 diabetic patients treated with insulin. Diabet Med. 1998;15:398-401.
4. Thomson FJ, Masson EA, Leeming JT, et al. Lack of knowledge of symptoms of hypoglycaemia by elderly diabetic patients. Age Ageing. 1991;20:404-406.
Newer antihyperglycemic drugs have distinctive CV, kidney benefits
The two newer classes of antihyperglycemic drugs that lower cardiovascular risk have different effects on specific cardiovascular and kidney disease outcomes in patients with type 2 diabetes, results of a meta-analysis suggest. Sodium-glucose contransporter-2 (SGLT2) inhibitors significantly reduced hospitalization from heart failure, whereas glucagon-like peptide-1 receptor agonists (GLP-1 RAs) did not, according to the reported results.
The GLP-1–RA class reduced risk of kidney disease progression, largely driven by a reduction in macroalbuminuria, according to the authors, whereas only the SGLT2 inhibitors reduced adverse kidney disease outcomes in a composite excluding that biomarker.
“The prevention of heart failure and progression of kidney disease by SGLT2 [inhibitors] should be considered in the decision-making process when treating patients with type 2 diabetes,” study senior author Marc S. Sabatine, MD, MPH, of Brigham and Women’s Hospital, Boston, and his coauthors wrote in a report on the study appearing in Circulation.
Both GLP-1 RAs and SGLT2 inhibitors significantly reduced major adverse cardiovascular events (MACE) and, as shown in other recent findings, their benefits were confined to patients with established atherosclerotic cardiovascular disease, Dr. Sabatine and his colleagues wrote.
The systematic review and meta-analysis of eight cardiovascular outcomes trials included 77,242 patients, of whom about 56% participated in GLP-1–RA studies and 44% in SGLT2-inhibitor trials. Just under three-quarters of the patients had established atherosclerotic cardiovascular disease, while the remainder had multiple risk factors for it.
Relative risk of hospitalization for heart failure was reduced by 31% with SGLT2 inhibitors, but it was not significantly reduced by GLP-1 RAs, the authors noted.
Risk of kidney disease progression was reduced by 38% with SGLT2 inhibitors and by 18% with GLP-1 RAs when the researchers used a broad composite endpoint including macroalbuminuria, estimated glomerular filtration rate (eGFR), end-stage kidney disease, and death due to renal causes.
By contrast, SGLT2 inhibitors reduced by 45% the relative risk of a narrower kidney outcome that excluded macroalbuminuria, whereas GLP-1 RAs had only a nonsignificant effect on the risk of doubling serum creatinine. That suggests the relative risk reduction of the kidney composite with GLP-1 RAs was driven mainly by a reduction in macroalbuminuria, the authors wrote.
Although albuminuria is an established biomarker for kidney and cardiovascular disease, it is a surrogate marker and can even be absent in patients with reduced eGFR, they said.
“Reduction in eGFR has emerged as a more meaningful endpoint of greater importance and is used in ongoing diabetes trials for kidney outcomes,” the authors said in a discussion of their results.
Relative risk of the composite MACE endpoint, including myocardial infarction, stroke, and cardiovascular death, was reduced by 12% for GLP-1 RAs and by 11% for SGLT2 [inhibitors], according to results of the analysis. However, the benefit was confined to patients with established cardiovascular disease, who had a 14% reduction of risk, compared with no treatment effect in patients who had multiple risk factors only.
Looking at individual MACE components, investigators found that both drug classes significantly reduced relative risk of myocardial infarction and of cardiovascular death, whereas only GLP-1 RAs significantly reduced relative risk of stroke.
Study authors provided disclosures related to AstraZeneca, Amgen, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen Research and Development, and Medimmune, among others.
SOURCE: Zelniker TA et al. Circulation. 2019 Feb 21. doi: 10.1161/CIRCULATIONAHA.118.038868.
The two newer classes of antihyperglycemic drugs that lower cardiovascular risk have different effects on specific cardiovascular and kidney disease outcomes in patients with type 2 diabetes, results of a meta-analysis suggest. Sodium-glucose contransporter-2 (SGLT2) inhibitors significantly reduced hospitalization from heart failure, whereas glucagon-like peptide-1 receptor agonists (GLP-1 RAs) did not, according to the reported results.
The GLP-1–RA class reduced risk of kidney disease progression, largely driven by a reduction in macroalbuminuria, according to the authors, whereas only the SGLT2 inhibitors reduced adverse kidney disease outcomes in a composite excluding that biomarker.
“The prevention of heart failure and progression of kidney disease by SGLT2 [inhibitors] should be considered in the decision-making process when treating patients with type 2 diabetes,” study senior author Marc S. Sabatine, MD, MPH, of Brigham and Women’s Hospital, Boston, and his coauthors wrote in a report on the study appearing in Circulation.
Both GLP-1 RAs and SGLT2 inhibitors significantly reduced major adverse cardiovascular events (MACE) and, as shown in other recent findings, their benefits were confined to patients with established atherosclerotic cardiovascular disease, Dr. Sabatine and his colleagues wrote.
The systematic review and meta-analysis of eight cardiovascular outcomes trials included 77,242 patients, of whom about 56% participated in GLP-1–RA studies and 44% in SGLT2-inhibitor trials. Just under three-quarters of the patients had established atherosclerotic cardiovascular disease, while the remainder had multiple risk factors for it.
Relative risk of hospitalization for heart failure was reduced by 31% with SGLT2 inhibitors, but it was not significantly reduced by GLP-1 RAs, the authors noted.
Risk of kidney disease progression was reduced by 38% with SGLT2 inhibitors and by 18% with GLP-1 RAs when the researchers used a broad composite endpoint including macroalbuminuria, estimated glomerular filtration rate (eGFR), end-stage kidney disease, and death due to renal causes.
By contrast, SGLT2 inhibitors reduced by 45% the relative risk of a narrower kidney outcome that excluded macroalbuminuria, whereas GLP-1 RAs had only a nonsignificant effect on the risk of doubling serum creatinine. That suggests the relative risk reduction of the kidney composite with GLP-1 RAs was driven mainly by a reduction in macroalbuminuria, the authors wrote.
Although albuminuria is an established biomarker for kidney and cardiovascular disease, it is a surrogate marker and can even be absent in patients with reduced eGFR, they said.
“Reduction in eGFR has emerged as a more meaningful endpoint of greater importance and is used in ongoing diabetes trials for kidney outcomes,” the authors said in a discussion of their results.
Relative risk of the composite MACE endpoint, including myocardial infarction, stroke, and cardiovascular death, was reduced by 12% for GLP-1 RAs and by 11% for SGLT2 [inhibitors], according to results of the analysis. However, the benefit was confined to patients with established cardiovascular disease, who had a 14% reduction of risk, compared with no treatment effect in patients who had multiple risk factors only.
Looking at individual MACE components, investigators found that both drug classes significantly reduced relative risk of myocardial infarction and of cardiovascular death, whereas only GLP-1 RAs significantly reduced relative risk of stroke.
Study authors provided disclosures related to AstraZeneca, Amgen, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen Research and Development, and Medimmune, among others.
SOURCE: Zelniker TA et al. Circulation. 2019 Feb 21. doi: 10.1161/CIRCULATIONAHA.118.038868.
The two newer classes of antihyperglycemic drugs that lower cardiovascular risk have different effects on specific cardiovascular and kidney disease outcomes in patients with type 2 diabetes, results of a meta-analysis suggest. Sodium-glucose contransporter-2 (SGLT2) inhibitors significantly reduced hospitalization from heart failure, whereas glucagon-like peptide-1 receptor agonists (GLP-1 RAs) did not, according to the reported results.
The GLP-1–RA class reduced risk of kidney disease progression, largely driven by a reduction in macroalbuminuria, according to the authors, whereas only the SGLT2 inhibitors reduced adverse kidney disease outcomes in a composite excluding that biomarker.
“The prevention of heart failure and progression of kidney disease by SGLT2 [inhibitors] should be considered in the decision-making process when treating patients with type 2 diabetes,” study senior author Marc S. Sabatine, MD, MPH, of Brigham and Women’s Hospital, Boston, and his coauthors wrote in a report on the study appearing in Circulation.
Both GLP-1 RAs and SGLT2 inhibitors significantly reduced major adverse cardiovascular events (MACE) and, as shown in other recent findings, their benefits were confined to patients with established atherosclerotic cardiovascular disease, Dr. Sabatine and his colleagues wrote.
The systematic review and meta-analysis of eight cardiovascular outcomes trials included 77,242 patients, of whom about 56% participated in GLP-1–RA studies and 44% in SGLT2-inhibitor trials. Just under three-quarters of the patients had established atherosclerotic cardiovascular disease, while the remainder had multiple risk factors for it.
Relative risk of hospitalization for heart failure was reduced by 31% with SGLT2 inhibitors, but it was not significantly reduced by GLP-1 RAs, the authors noted.
Risk of kidney disease progression was reduced by 38% with SGLT2 inhibitors and by 18% with GLP-1 RAs when the researchers used a broad composite endpoint including macroalbuminuria, estimated glomerular filtration rate (eGFR), end-stage kidney disease, and death due to renal causes.
By contrast, SGLT2 inhibitors reduced by 45% the relative risk of a narrower kidney outcome that excluded macroalbuminuria, whereas GLP-1 RAs had only a nonsignificant effect on the risk of doubling serum creatinine. That suggests the relative risk reduction of the kidney composite with GLP-1 RAs was driven mainly by a reduction in macroalbuminuria, the authors wrote.
Although albuminuria is an established biomarker for kidney and cardiovascular disease, it is a surrogate marker and can even be absent in patients with reduced eGFR, they said.
“Reduction in eGFR has emerged as a more meaningful endpoint of greater importance and is used in ongoing diabetes trials for kidney outcomes,” the authors said in a discussion of their results.
Relative risk of the composite MACE endpoint, including myocardial infarction, stroke, and cardiovascular death, was reduced by 12% for GLP-1 RAs and by 11% for SGLT2 [inhibitors], according to results of the analysis. However, the benefit was confined to patients with established cardiovascular disease, who had a 14% reduction of risk, compared with no treatment effect in patients who had multiple risk factors only.
Looking at individual MACE components, investigators found that both drug classes significantly reduced relative risk of myocardial infarction and of cardiovascular death, whereas only GLP-1 RAs significantly reduced relative risk of stroke.
Study authors provided disclosures related to AstraZeneca, Amgen, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen Research and Development, and Medimmune, among others.
SOURCE: Zelniker TA et al. Circulation. 2019 Feb 21. doi: 10.1161/CIRCULATIONAHA.118.038868.
FROM CIRCULATION
Semaglutide plus SGLT2 inhibitors improves glycemic control in type 2 diabetes
Adding once-weekly treatment with the glucagon-like peptide-1 receptor agonist (GLP-1 RA) semaglutide helped most patients with type 2 diabetes already being treated with sodium-glucose cotransporter-2 (SGLT2) inhibitors meet their glycemic targets and lose weight, researchers reported.
“Combining the distinct modes of action of these two drug classes has beneficial effects on glucose and weight outcomes,” wrote Bernard Zinman, MD, of Mount Sinai Hospital, Toronto, and his coauthors. Their report is in Lancet Diabetes and Endocrinology.
In the international, double-blind, phase 3 SUSTAIN 9 trial, 302 patients with type 2 diabetes whose hemoglobin A1c (HbA1c) levels were 7.0% to 10.0% (53 to 86 mmol/mol) despite at least 90 days of SGLT2-inhibitor therapy (alone or with metformin or sulfonylurea) were randomly assigned to add either once-weekly semaglutide (1-mg injection) or placebo to their regimen.
A total of 294 patients completed the trial. After 30 weeks, those who had received adjunctive semaglutide had significantly greater reductions in their HbA1c levels (estimated treatment difference, –1.42%; P less than .0001). They also lost about 3.81 kg more bodyweight than did patients in the placebo group and they had significantly greater reductions in mean body mass index, waist circumference, fasting and self-measured blood glucose, systolic blood pressure, pulse rate, total cholesterol, and low-density lipoprotein and triglyceride levels.
The most commonly reported adverse effects of semaglutide were nausea, diarrhea, vomiting, and constipation, which were usually mild in severity. Severe or blood-glucose–confirmed hypoglycemia occurred in 2.7% patients on semaglutide and none on placebo. However, most patients who received semaglutide achieved an HbA1c of 7.0% (53 mmol/mol) or less without weight gain or severe or blood-glucose–confirmed hypoglycemia (P less than .0001 vs. placebo).
Novo Nordisk funded the study. Three of the authors were employees of Novo Nordisk at the time of the study, and the remaining authors reported that they and/or their institutions had received funding from the company.
SOURCE: Zinman B et al. Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587(19)30066-X.
Adding once-weekly treatment with the glucagon-like peptide-1 receptor agonist (GLP-1 RA) semaglutide helped most patients with type 2 diabetes already being treated with sodium-glucose cotransporter-2 (SGLT2) inhibitors meet their glycemic targets and lose weight, researchers reported.
“Combining the distinct modes of action of these two drug classes has beneficial effects on glucose and weight outcomes,” wrote Bernard Zinman, MD, of Mount Sinai Hospital, Toronto, and his coauthors. Their report is in Lancet Diabetes and Endocrinology.
In the international, double-blind, phase 3 SUSTAIN 9 trial, 302 patients with type 2 diabetes whose hemoglobin A1c (HbA1c) levels were 7.0% to 10.0% (53 to 86 mmol/mol) despite at least 90 days of SGLT2-inhibitor therapy (alone or with metformin or sulfonylurea) were randomly assigned to add either once-weekly semaglutide (1-mg injection) or placebo to their regimen.
A total of 294 patients completed the trial. After 30 weeks, those who had received adjunctive semaglutide had significantly greater reductions in their HbA1c levels (estimated treatment difference, –1.42%; P less than .0001). They also lost about 3.81 kg more bodyweight than did patients in the placebo group and they had significantly greater reductions in mean body mass index, waist circumference, fasting and self-measured blood glucose, systolic blood pressure, pulse rate, total cholesterol, and low-density lipoprotein and triglyceride levels.
The most commonly reported adverse effects of semaglutide were nausea, diarrhea, vomiting, and constipation, which were usually mild in severity. Severe or blood-glucose–confirmed hypoglycemia occurred in 2.7% patients on semaglutide and none on placebo. However, most patients who received semaglutide achieved an HbA1c of 7.0% (53 mmol/mol) or less without weight gain or severe or blood-glucose–confirmed hypoglycemia (P less than .0001 vs. placebo).
Novo Nordisk funded the study. Three of the authors were employees of Novo Nordisk at the time of the study, and the remaining authors reported that they and/or their institutions had received funding from the company.
SOURCE: Zinman B et al. Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587(19)30066-X.
Adding once-weekly treatment with the glucagon-like peptide-1 receptor agonist (GLP-1 RA) semaglutide helped most patients with type 2 diabetes already being treated with sodium-glucose cotransporter-2 (SGLT2) inhibitors meet their glycemic targets and lose weight, researchers reported.
“Combining the distinct modes of action of these two drug classes has beneficial effects on glucose and weight outcomes,” wrote Bernard Zinman, MD, of Mount Sinai Hospital, Toronto, and his coauthors. Their report is in Lancet Diabetes and Endocrinology.
In the international, double-blind, phase 3 SUSTAIN 9 trial, 302 patients with type 2 diabetes whose hemoglobin A1c (HbA1c) levels were 7.0% to 10.0% (53 to 86 mmol/mol) despite at least 90 days of SGLT2-inhibitor therapy (alone or with metformin or sulfonylurea) were randomly assigned to add either once-weekly semaglutide (1-mg injection) or placebo to their regimen.
A total of 294 patients completed the trial. After 30 weeks, those who had received adjunctive semaglutide had significantly greater reductions in their HbA1c levels (estimated treatment difference, –1.42%; P less than .0001). They also lost about 3.81 kg more bodyweight than did patients in the placebo group and they had significantly greater reductions in mean body mass index, waist circumference, fasting and self-measured blood glucose, systolic blood pressure, pulse rate, total cholesterol, and low-density lipoprotein and triglyceride levels.
The most commonly reported adverse effects of semaglutide were nausea, diarrhea, vomiting, and constipation, which were usually mild in severity. Severe or blood-glucose–confirmed hypoglycemia occurred in 2.7% patients on semaglutide and none on placebo. However, most patients who received semaglutide achieved an HbA1c of 7.0% (53 mmol/mol) or less without weight gain or severe or blood-glucose–confirmed hypoglycemia (P less than .0001 vs. placebo).
Novo Nordisk funded the study. Three of the authors were employees of Novo Nordisk at the time of the study, and the remaining authors reported that they and/or their institutions had received funding from the company.
SOURCE: Zinman B et al. Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587(19)30066-X.
FROM LANCET DIABETES AND ENDOCRINOLOGY
Key clinical point: Adding once-weekly treatment with the glucagon-like peptide-1 receptor agonist (GLP-1 RA) semaglutide helped most patients with type 2 diabetes on sodium-glucose contransporter-2 (SGLT2) inhibitors meet glycemic targets and lose weight.
Major finding: After 30 weeks, the estimated treatment difference for hemoglobin A1c (HbA1c) favored semaglutide over placebo (–1.42; P less than .0001). Patients also lost about 3.81 kg more bodyweight with adjunctive semaglutide, compared with placebo (P less than .0001)
Study details: International, double-blind, phase 3 trial of 302 patients with type 2 diabetes with HbA1c levels of 7.0% to 10.0% despite having received at least 90 days of SGLT2-inhibitor therapy (SUSTAIN 9).
Disclosures: Novo Nordisk funded the study. Three of the authors were employees of Novo Nordisk at the time of the study, and the remaining authors reported that they and/or their institutions had received funding from the company.
Source: Zinman B et al. Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587(19)30066-X.
How much difference will Eli Lilly’s half-price insulin make?
When Erin Gilmer filled her insulin prescription at a Denver-area Walgreens in January, she paid $8.50. U.S. taxpayers paid another $280.51.
“It eats at me to know that taxpayer money is being wasted,” said Gilmer, who has Medicare and was diagnosed with type 1 diabetes while a sophomore at the University of Colorado in 2002.
The diagnosis meant that for the rest of her life she’d require daily insulin shots to stay alive. But the price of that insulin is skyrocketing.
Between 2009 and 2017 the wholesale price of a single vial of Humalog, the Eli Lilly and Co.–manufactured insulin Gilmer uses, nearly tripled – rising from $92.70 to $274.70, according to data from IBM Watson Health.
Six years ago, Gilmer qualified for Social Security Disability Insurance – and thus, Medicare – because of a range of health issues. At the time, the insulin she needed cost $167.70 per vial, according to IBM Watson Health.
“When it’s taxpayer money paying for medication for someone like me, it makes it a national issue, not just a diabetic issue,” Gilmer said.
Stories about people with type 1 diabetes dying when they couldn’t afford insulin have made headlines. Patient activists like Gilmer have protested high prices outside Lilly’s headquarters in Indianapolis.
Last October in Minnesota, State Attorney General Lori Swanson sued insulin manufacturers, alleging price gouging. Pharmaceutical executives were grilled about high drug prices by the Senate Finance Committee on Feb. 26.
This is the backdrop for Lilly’s announcement March 4 that it is rolling out a half-priced, generic version of Humalog called “insulin lispro.” The list price: $137.35 per vial.
“Patients, doctors and policymakers are demanding lower list prices for medicines and lower patient costs at the pharmacy counter,” Eli Lilly CEO David Ricks wrote in a blog post about the move. “You might be surprised to hear that we agree – it’s time for change in our system and for consumer prices to come down.”
No panacea
When Lilly’s Humalog, the first short-acting insulin, came to market in 1996, the list price was about $21 per vial. The price didn’t reach $275 overnight, but yearly price increases added up.
In February 2009, for example, the wholesale price was $92.70, according to IBM Watson Health. It rose to $99.65 in December 2009, then to $107.60 in September 2010, $115.70 in May 2011, and so on.
“There’s no justification for why prices should keep increasing at an average rate of 10% every year,” said Inmaculada Hernandez of the University of Pittsburgh School of Pharmacy, who was lead author of a January report in Health Affairs attributing the skyrocketing cost of prescription drugs to accumulated yearly price hikes.
“The public perception that we have in general is that drugs are so expensive because we need to pay for research and development, and that’s true,” Hernandez said. “However, usually research and development is paid for in the first years of life of a drug.”
At $137.35 per vial, Lilly’s generic insulin is priced at about the same level as Humalog was in 2012, 16 years after it came to market.
“We want to recognize that this is not a panacea,” said company spokesman Greg Kueterman. “This is an option that we hope can help people in the current system that we work with.”
It’s worth noting that Humalog is a rapid-acting insulin, but that’s only one of the two types of insulin most people with type 1 diabetes use every day. The second kind is long-lasting. Lilly makes one called Basaglar. The most popular long-lasting insulin is Lantus, produced by Sanofi. Neither has a lower-cost alternative.
Still, Lilly’s move on Humalog could put pressure on the other two big makers of insulin to act.
Novo Nordisk called Lilly’s lower-priced generic insulin “an important development,” in an emailed statement.
“Bringing affordable insulin to the market requires ideas from all stakeholders,” Novo Nordisk’s Ken Inchausti said in an email, which also listed steps the company has taken, such as a patient assistance program. The statement didn’t say whether Novo Nordisk is considering offering a lower-priced version of its popular insulin Novolog, a rival of Humalog.
A statement from Sanofi, the third major insulin maker, also didn’t say whether the company would offer lower-priced versions of its insulins.
“Sanofi supports any actions that increase access to insulins for patients living with diabetes at an affordable price,” spokeswoman Ashleigh Koss said in the email, which also touted the company’s patient assistance program.
A different kind of generic
One twist in this story is that Lilly’s new insulin is just a repackaged version of Humalog, minus the brand name. It’s called an “authorized generic.”
“Whoever came up with the term ‘authorized generic’?” Dr. Vincent Rajkumar said, laughing. Rajkumar is a hematologist at the Mayo Clinic in Rochester, Minn.
“It’s the same exact drug” as the brand name, he continued.
Typically, Rajkumar said, authorized generics are introduced by brand-name drugmakers to compete with generic versions of their drugs made by rival companies.
But in the case of Humalog and other insulins, there are no generics made by competitors, as there are for, say, the cholesterol medicine Lipitor or even other diabetes drugs, such as metformin.
So when Lilly’s authorized generic comes to market, the company will have both Humalog insulin and the authorized generic version of that medicine on the market.
Rajkumar said it’s a public relations move.
“There’s outrage over the price of insulin that is being discussed in Congress and elsewhere. And so the company basically says, ‘Hey, we will make the identical product available at half price.’ On the surface that sounds great,” Rajkumar said.
“But you look at the problems and you think, ‘OK, how crazy is this that someone is actually going to be buying the brand-name drug?’ ”
In fact, it’s possible that Lilly could break even or profit off its authorized generic compared to the name-brand Humalog, according to University of Pittsburgh’s Hernandez.
The profit margin would depend on the rebates paid by the company to insurers and pharmacy benefit managers. Rebates are getting a lot of attention these days as one factor that pushes drug prices higher. They’re usually not disclosed and increase as a drug’s price increases, providing an incentive to some companies to raise prices.
“Doing an authorized generic is nothing else than giving insurers two options,” Hernandez said: Pay the full list price for a brand-name drug and receive a higher rebate, or pay the lower price for the authorized generic and receive a presumably smaller rebate.
“What we really need to get insulin prices down is to get generics into the market, and we need more than one,” Hernandez said, adding that previous research has shown that prices begin to go down when two or three generics are competing in the marketplace.
Even so, Lillly’s Kueterman said the authorized generic insulin “is going to help hopefully move the system toward a more sustainable model.”
“I can guarantee you the reason that we’re doing this is to help people,” Kueterman said, noting the company’s Diabetes Solution Center has also helped “10,000 people each month pay significantly less for their insulin” since it opened in August.
For Erin Gilmer, the news about an authorized generic insulin from Lilly has left her mildly encouraged.
“It sounds really good, and it will help some people, which is great,” Gilmer said. “It’s Eli Lilly and pharma starting to understand that grassroots activism has to be taken seriously, and we are at a tipping point.”
This story is part of a partnership that includes NPR and Kaiser Health News. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
When Erin Gilmer filled her insulin prescription at a Denver-area Walgreens in January, she paid $8.50. U.S. taxpayers paid another $280.51.
“It eats at me to know that taxpayer money is being wasted,” said Gilmer, who has Medicare and was diagnosed with type 1 diabetes while a sophomore at the University of Colorado in 2002.
The diagnosis meant that for the rest of her life she’d require daily insulin shots to stay alive. But the price of that insulin is skyrocketing.
Between 2009 and 2017 the wholesale price of a single vial of Humalog, the Eli Lilly and Co.–manufactured insulin Gilmer uses, nearly tripled – rising from $92.70 to $274.70, according to data from IBM Watson Health.
Six years ago, Gilmer qualified for Social Security Disability Insurance – and thus, Medicare – because of a range of health issues. At the time, the insulin she needed cost $167.70 per vial, according to IBM Watson Health.
“When it’s taxpayer money paying for medication for someone like me, it makes it a national issue, not just a diabetic issue,” Gilmer said.
Stories about people with type 1 diabetes dying when they couldn’t afford insulin have made headlines. Patient activists like Gilmer have protested high prices outside Lilly’s headquarters in Indianapolis.
Last October in Minnesota, State Attorney General Lori Swanson sued insulin manufacturers, alleging price gouging. Pharmaceutical executives were grilled about high drug prices by the Senate Finance Committee on Feb. 26.
This is the backdrop for Lilly’s announcement March 4 that it is rolling out a half-priced, generic version of Humalog called “insulin lispro.” The list price: $137.35 per vial.
“Patients, doctors and policymakers are demanding lower list prices for medicines and lower patient costs at the pharmacy counter,” Eli Lilly CEO David Ricks wrote in a blog post about the move. “You might be surprised to hear that we agree – it’s time for change in our system and for consumer prices to come down.”
No panacea
When Lilly’s Humalog, the first short-acting insulin, came to market in 1996, the list price was about $21 per vial. The price didn’t reach $275 overnight, but yearly price increases added up.
In February 2009, for example, the wholesale price was $92.70, according to IBM Watson Health. It rose to $99.65 in December 2009, then to $107.60 in September 2010, $115.70 in May 2011, and so on.
“There’s no justification for why prices should keep increasing at an average rate of 10% every year,” said Inmaculada Hernandez of the University of Pittsburgh School of Pharmacy, who was lead author of a January report in Health Affairs attributing the skyrocketing cost of prescription drugs to accumulated yearly price hikes.
“The public perception that we have in general is that drugs are so expensive because we need to pay for research and development, and that’s true,” Hernandez said. “However, usually research and development is paid for in the first years of life of a drug.”
At $137.35 per vial, Lilly’s generic insulin is priced at about the same level as Humalog was in 2012, 16 years after it came to market.
“We want to recognize that this is not a panacea,” said company spokesman Greg Kueterman. “This is an option that we hope can help people in the current system that we work with.”
It’s worth noting that Humalog is a rapid-acting insulin, but that’s only one of the two types of insulin most people with type 1 diabetes use every day. The second kind is long-lasting. Lilly makes one called Basaglar. The most popular long-lasting insulin is Lantus, produced by Sanofi. Neither has a lower-cost alternative.
Still, Lilly’s move on Humalog could put pressure on the other two big makers of insulin to act.
Novo Nordisk called Lilly’s lower-priced generic insulin “an important development,” in an emailed statement.
“Bringing affordable insulin to the market requires ideas from all stakeholders,” Novo Nordisk’s Ken Inchausti said in an email, which also listed steps the company has taken, such as a patient assistance program. The statement didn’t say whether Novo Nordisk is considering offering a lower-priced version of its popular insulin Novolog, a rival of Humalog.
A statement from Sanofi, the third major insulin maker, also didn’t say whether the company would offer lower-priced versions of its insulins.
“Sanofi supports any actions that increase access to insulins for patients living with diabetes at an affordable price,” spokeswoman Ashleigh Koss said in the email, which also touted the company’s patient assistance program.
A different kind of generic
One twist in this story is that Lilly’s new insulin is just a repackaged version of Humalog, minus the brand name. It’s called an “authorized generic.”
“Whoever came up with the term ‘authorized generic’?” Dr. Vincent Rajkumar said, laughing. Rajkumar is a hematologist at the Mayo Clinic in Rochester, Minn.
“It’s the same exact drug” as the brand name, he continued.
Typically, Rajkumar said, authorized generics are introduced by brand-name drugmakers to compete with generic versions of their drugs made by rival companies.
But in the case of Humalog and other insulins, there are no generics made by competitors, as there are for, say, the cholesterol medicine Lipitor or even other diabetes drugs, such as metformin.
So when Lilly’s authorized generic comes to market, the company will have both Humalog insulin and the authorized generic version of that medicine on the market.
Rajkumar said it’s a public relations move.
“There’s outrage over the price of insulin that is being discussed in Congress and elsewhere. And so the company basically says, ‘Hey, we will make the identical product available at half price.’ On the surface that sounds great,” Rajkumar said.
“But you look at the problems and you think, ‘OK, how crazy is this that someone is actually going to be buying the brand-name drug?’ ”
In fact, it’s possible that Lilly could break even or profit off its authorized generic compared to the name-brand Humalog, according to University of Pittsburgh’s Hernandez.
The profit margin would depend on the rebates paid by the company to insurers and pharmacy benefit managers. Rebates are getting a lot of attention these days as one factor that pushes drug prices higher. They’re usually not disclosed and increase as a drug’s price increases, providing an incentive to some companies to raise prices.
“Doing an authorized generic is nothing else than giving insurers two options,” Hernandez said: Pay the full list price for a brand-name drug and receive a higher rebate, or pay the lower price for the authorized generic and receive a presumably smaller rebate.
“What we really need to get insulin prices down is to get generics into the market, and we need more than one,” Hernandez said, adding that previous research has shown that prices begin to go down when two or three generics are competing in the marketplace.
Even so, Lillly’s Kueterman said the authorized generic insulin “is going to help hopefully move the system toward a more sustainable model.”
“I can guarantee you the reason that we’re doing this is to help people,” Kueterman said, noting the company’s Diabetes Solution Center has also helped “10,000 people each month pay significantly less for their insulin” since it opened in August.
For Erin Gilmer, the news about an authorized generic insulin from Lilly has left her mildly encouraged.
“It sounds really good, and it will help some people, which is great,” Gilmer said. “It’s Eli Lilly and pharma starting to understand that grassroots activism has to be taken seriously, and we are at a tipping point.”
This story is part of a partnership that includes NPR and Kaiser Health News. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
When Erin Gilmer filled her insulin prescription at a Denver-area Walgreens in January, she paid $8.50. U.S. taxpayers paid another $280.51.
“It eats at me to know that taxpayer money is being wasted,” said Gilmer, who has Medicare and was diagnosed with type 1 diabetes while a sophomore at the University of Colorado in 2002.
The diagnosis meant that for the rest of her life she’d require daily insulin shots to stay alive. But the price of that insulin is skyrocketing.
Between 2009 and 2017 the wholesale price of a single vial of Humalog, the Eli Lilly and Co.–manufactured insulin Gilmer uses, nearly tripled – rising from $92.70 to $274.70, according to data from IBM Watson Health.
Six years ago, Gilmer qualified for Social Security Disability Insurance – and thus, Medicare – because of a range of health issues. At the time, the insulin she needed cost $167.70 per vial, according to IBM Watson Health.
“When it’s taxpayer money paying for medication for someone like me, it makes it a national issue, not just a diabetic issue,” Gilmer said.
Stories about people with type 1 diabetes dying when they couldn’t afford insulin have made headlines. Patient activists like Gilmer have protested high prices outside Lilly’s headquarters in Indianapolis.
Last October in Minnesota, State Attorney General Lori Swanson sued insulin manufacturers, alleging price gouging. Pharmaceutical executives were grilled about high drug prices by the Senate Finance Committee on Feb. 26.
This is the backdrop for Lilly’s announcement March 4 that it is rolling out a half-priced, generic version of Humalog called “insulin lispro.” The list price: $137.35 per vial.
“Patients, doctors and policymakers are demanding lower list prices for medicines and lower patient costs at the pharmacy counter,” Eli Lilly CEO David Ricks wrote in a blog post about the move. “You might be surprised to hear that we agree – it’s time for change in our system and for consumer prices to come down.”
No panacea
When Lilly’s Humalog, the first short-acting insulin, came to market in 1996, the list price was about $21 per vial. The price didn’t reach $275 overnight, but yearly price increases added up.
In February 2009, for example, the wholesale price was $92.70, according to IBM Watson Health. It rose to $99.65 in December 2009, then to $107.60 in September 2010, $115.70 in May 2011, and so on.
“There’s no justification for why prices should keep increasing at an average rate of 10% every year,” said Inmaculada Hernandez of the University of Pittsburgh School of Pharmacy, who was lead author of a January report in Health Affairs attributing the skyrocketing cost of prescription drugs to accumulated yearly price hikes.
“The public perception that we have in general is that drugs are so expensive because we need to pay for research and development, and that’s true,” Hernandez said. “However, usually research and development is paid for in the first years of life of a drug.”
At $137.35 per vial, Lilly’s generic insulin is priced at about the same level as Humalog was in 2012, 16 years after it came to market.
“We want to recognize that this is not a panacea,” said company spokesman Greg Kueterman. “This is an option that we hope can help people in the current system that we work with.”
It’s worth noting that Humalog is a rapid-acting insulin, but that’s only one of the two types of insulin most people with type 1 diabetes use every day. The second kind is long-lasting. Lilly makes one called Basaglar. The most popular long-lasting insulin is Lantus, produced by Sanofi. Neither has a lower-cost alternative.
Still, Lilly’s move on Humalog could put pressure on the other two big makers of insulin to act.
Novo Nordisk called Lilly’s lower-priced generic insulin “an important development,” in an emailed statement.
“Bringing affordable insulin to the market requires ideas from all stakeholders,” Novo Nordisk’s Ken Inchausti said in an email, which also listed steps the company has taken, such as a patient assistance program. The statement didn’t say whether Novo Nordisk is considering offering a lower-priced version of its popular insulin Novolog, a rival of Humalog.
A statement from Sanofi, the third major insulin maker, also didn’t say whether the company would offer lower-priced versions of its insulins.
“Sanofi supports any actions that increase access to insulins for patients living with diabetes at an affordable price,” spokeswoman Ashleigh Koss said in the email, which also touted the company’s patient assistance program.
A different kind of generic
One twist in this story is that Lilly’s new insulin is just a repackaged version of Humalog, minus the brand name. It’s called an “authorized generic.”
“Whoever came up with the term ‘authorized generic’?” Dr. Vincent Rajkumar said, laughing. Rajkumar is a hematologist at the Mayo Clinic in Rochester, Minn.
“It’s the same exact drug” as the brand name, he continued.
Typically, Rajkumar said, authorized generics are introduced by brand-name drugmakers to compete with generic versions of their drugs made by rival companies.
But in the case of Humalog and other insulins, there are no generics made by competitors, as there are for, say, the cholesterol medicine Lipitor or even other diabetes drugs, such as metformin.
So when Lilly’s authorized generic comes to market, the company will have both Humalog insulin and the authorized generic version of that medicine on the market.
Rajkumar said it’s a public relations move.
“There’s outrage over the price of insulin that is being discussed in Congress and elsewhere. And so the company basically says, ‘Hey, we will make the identical product available at half price.’ On the surface that sounds great,” Rajkumar said.
“But you look at the problems and you think, ‘OK, how crazy is this that someone is actually going to be buying the brand-name drug?’ ”
In fact, it’s possible that Lilly could break even or profit off its authorized generic compared to the name-brand Humalog, according to University of Pittsburgh’s Hernandez.
The profit margin would depend on the rebates paid by the company to insurers and pharmacy benefit managers. Rebates are getting a lot of attention these days as one factor that pushes drug prices higher. They’re usually not disclosed and increase as a drug’s price increases, providing an incentive to some companies to raise prices.
“Doing an authorized generic is nothing else than giving insurers two options,” Hernandez said: Pay the full list price for a brand-name drug and receive a higher rebate, or pay the lower price for the authorized generic and receive a presumably smaller rebate.
“What we really need to get insulin prices down is to get generics into the market, and we need more than one,” Hernandez said, adding that previous research has shown that prices begin to go down when two or three generics are competing in the marketplace.
Even so, Lillly’s Kueterman said the authorized generic insulin “is going to help hopefully move the system toward a more sustainable model.”
“I can guarantee you the reason that we’re doing this is to help people,” Kueterman said, noting the company’s Diabetes Solution Center has also helped “10,000 people each month pay significantly less for their insulin” since it opened in August.
For Erin Gilmer, the news about an authorized generic insulin from Lilly has left her mildly encouraged.
“It sounds really good, and it will help some people, which is great,” Gilmer said. “It’s Eli Lilly and pharma starting to understand that grassroots activism has to be taken seriously, and we are at a tipping point.”
This story is part of a partnership that includes NPR and Kaiser Health News. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Nonadherent Diabetes Patients: An Unexpected Group
“Time-specific” dosing of insulin can be an obstacle to adherence for patients with complicated, busy lives. More than half of patients with type 2 diabetes do not achieve their target HbA1c of 7% after insulin is added to their treatment regimen. Researchers from CAPHRI School for Public Health and Primary Care, and CARIM Institute in The Netherlands, who surveyed 1,483 adults with diabetes suggest that it may be time to rethink both prescribing and patient education, in part because of who fell into the nonadherent group.
The researchers conducted a web-based self-report survey. Of the respondents, 58% used bolus insulin before meals, 24% after meals, and 18% before, during, or after meals. The researchers excluded the “mixed” cohort, including 1,218 in the analysis.
Half the respondents in the postmeal cohort reported experiencing minor hypoglycemic events at least once a week compared with 35% of the premeal cohort. Similarly, more in the postmeal group had had major hypoglycemic events (38% vs 26%). The postmeal respondents also were more likely to have HbA1c ≥ 9% (40% vs 29%). And they were less likely to report always testing their blood glucose before injecting insulin (36% vs 54%).
Perhaps contrary to some expectations, the respondents who injected insulin postmeal were younger, had shorter duration of diabetes, had the highest level of college or university education, were more likely to be employed, and more frequently participated in diabetes education programs (including one-on-one programs).
The researchers say those data suggest that factors other than lack of diabetes education, education, or low socioeconomic status should be considered in explaining the nonadherence. They add that some research has shown that education programs have an “inconsistent relationship with patient adherence.” They suggest that such programs might be improved by placing greater emphasis on the importance of dosing insulin before meals.
Of the nearly 20% of patients who use insulin treatment, > 90% receive bolus insulin. The researchers note that respondents preferred a form of bolus insulin they can administer before, after, or during meals as they see fit. The respondents who injected postmeal were more likely than the premeal respondents to prefer this formulation.
“Time-specific” dosing of insulin can be an obstacle to adherence for patients with complicated, busy lives. More than half of patients with type 2 diabetes do not achieve their target HbA1c of 7% after insulin is added to their treatment regimen. Researchers from CAPHRI School for Public Health and Primary Care, and CARIM Institute in The Netherlands, who surveyed 1,483 adults with diabetes suggest that it may be time to rethink both prescribing and patient education, in part because of who fell into the nonadherent group.
The researchers conducted a web-based self-report survey. Of the respondents, 58% used bolus insulin before meals, 24% after meals, and 18% before, during, or after meals. The researchers excluded the “mixed” cohort, including 1,218 in the analysis.
Half the respondents in the postmeal cohort reported experiencing minor hypoglycemic events at least once a week compared with 35% of the premeal cohort. Similarly, more in the postmeal group had had major hypoglycemic events (38% vs 26%). The postmeal respondents also were more likely to have HbA1c ≥ 9% (40% vs 29%). And they were less likely to report always testing their blood glucose before injecting insulin (36% vs 54%).
Perhaps contrary to some expectations, the respondents who injected insulin postmeal were younger, had shorter duration of diabetes, had the highest level of college or university education, were more likely to be employed, and more frequently participated in diabetes education programs (including one-on-one programs).
The researchers say those data suggest that factors other than lack of diabetes education, education, or low socioeconomic status should be considered in explaining the nonadherence. They add that some research has shown that education programs have an “inconsistent relationship with patient adherence.” They suggest that such programs might be improved by placing greater emphasis on the importance of dosing insulin before meals.
Of the nearly 20% of patients who use insulin treatment, > 90% receive bolus insulin. The researchers note that respondents preferred a form of bolus insulin they can administer before, after, or during meals as they see fit. The respondents who injected postmeal were more likely than the premeal respondents to prefer this formulation.
“Time-specific” dosing of insulin can be an obstacle to adherence for patients with complicated, busy lives. More than half of patients with type 2 diabetes do not achieve their target HbA1c of 7% after insulin is added to their treatment regimen. Researchers from CAPHRI School for Public Health and Primary Care, and CARIM Institute in The Netherlands, who surveyed 1,483 adults with diabetes suggest that it may be time to rethink both prescribing and patient education, in part because of who fell into the nonadherent group.
The researchers conducted a web-based self-report survey. Of the respondents, 58% used bolus insulin before meals, 24% after meals, and 18% before, during, or after meals. The researchers excluded the “mixed” cohort, including 1,218 in the analysis.
Half the respondents in the postmeal cohort reported experiencing minor hypoglycemic events at least once a week compared with 35% of the premeal cohort. Similarly, more in the postmeal group had had major hypoglycemic events (38% vs 26%). The postmeal respondents also were more likely to have HbA1c ≥ 9% (40% vs 29%). And they were less likely to report always testing their blood glucose before injecting insulin (36% vs 54%).
Perhaps contrary to some expectations, the respondents who injected insulin postmeal were younger, had shorter duration of diabetes, had the highest level of college or university education, were more likely to be employed, and more frequently participated in diabetes education programs (including one-on-one programs).
The researchers say those data suggest that factors other than lack of diabetes education, education, or low socioeconomic status should be considered in explaining the nonadherence. They add that some research has shown that education programs have an “inconsistent relationship with patient adherence.” They suggest that such programs might be improved by placing greater emphasis on the importance of dosing insulin before meals.
Of the nearly 20% of patients who use insulin treatment, > 90% receive bolus insulin. The researchers note that respondents preferred a form of bolus insulin they can administer before, after, or during meals as they see fit. The respondents who injected postmeal were more likely than the premeal respondents to prefer this formulation.
Sudden-onset rash on the trunk and limbs • morbid obesity • family history of diabetes mellitus • Dx?
THE CASE
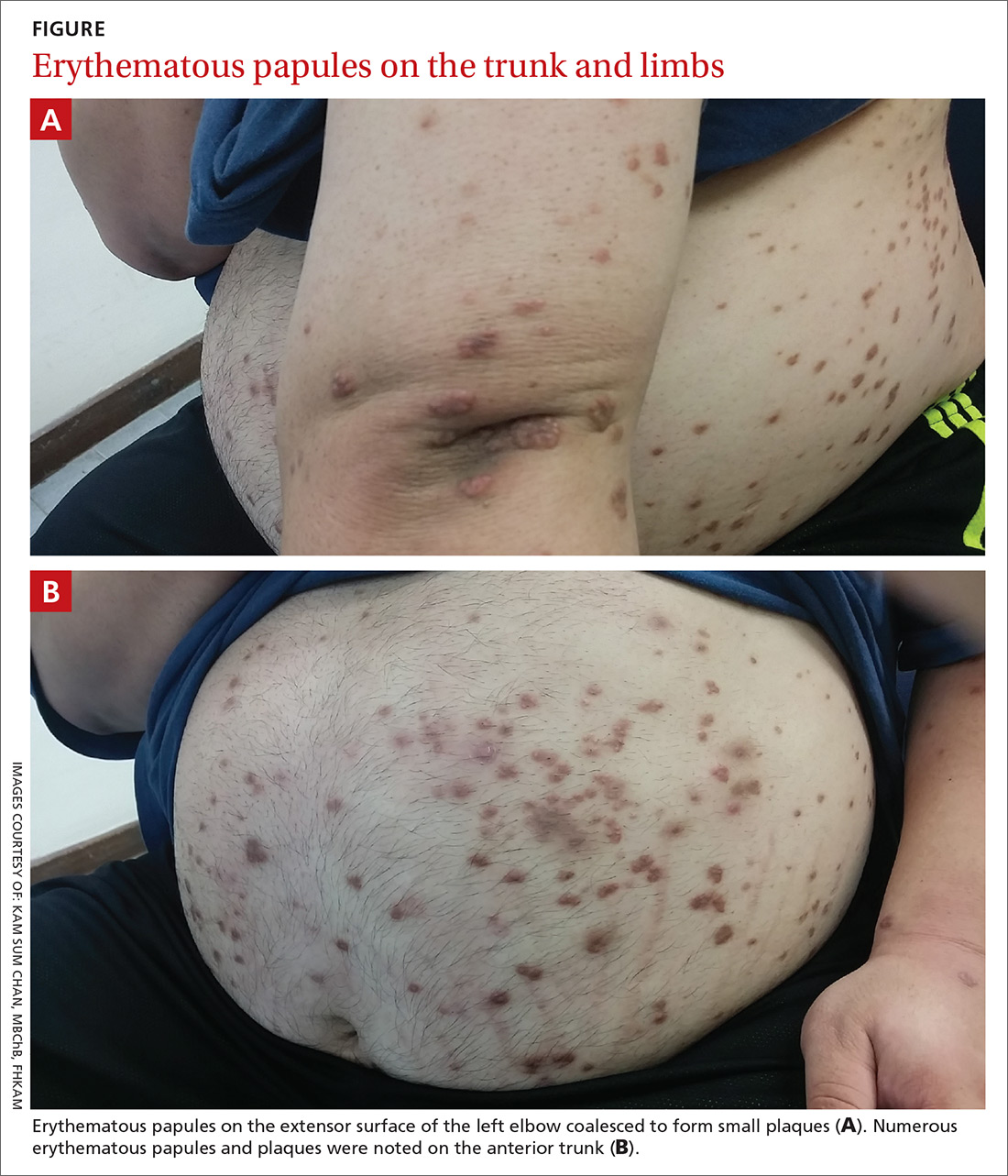
A 37-year-old man presented with a sudden-onset, nonpruritic, nonpainful, papular rash of 1 month’s duration on his trunk and both arms and legs. Two weeks prior to the current presentation, he consulted a general practitioner, who treated the rash with a course of unknown oral antibiotics; the patient showed no improvement. He recalled that on a few occasions, he used his fingers to express a creamy discharge from some of the lesions. This temporarily reduced the size of those papules.
His medical history was unremarkable except for morbid obesity. He did not drink alcohol regularly and was not taking any medications prior to the onset of the rash. He had no family history of hyperlipidemia, but his mother had a history of diabetes mellitus.
Physical examination showed numerous discrete erythematous papules with a creamy center on his trunk and his arms and legs. The lesions were more numerous on the extensor surfaces of the arms and legs. Some of the papules coalesced to form small plaques (FIGURE). There was no scaling, and the lesions were firm in texture. The patient’s face was spared, and there was no mucosal involvement. The patient was otherwise systemically well.
THE DIAGNOSIS
Based on the morphology, distribution, and abrupt onset of the diffuse nonpruritic papules in this morbidly obese (but otherwise systemically well) middle-aged man, a clinical diagnosis of eruptive xanthoma was suspected. Subsequent blood testing revealed an elevated serum triglyceride level of 47.8 mmol/L (reference range, <1.7 mmol/L), elevated serum total cholesterol of 7.1 mmol/L (reference range, <6.2 mmol/L), and low serum high-density lipoprotein cholesterol of 0.7 mmol/L (reference range, >1 mmol/L in men). He also had an elevated fasting serum glucose level of 12.9 mmol/L (reference range, 3.9–5.6 mmol/L) and an elevated hemoglobin A1c (glycated hemoglobin) level of 10.9%.
Subsequent thyroid, liver, and renal function tests were normal, but the patient had heavy proteinuria, with an elevated urine albumin-to-creatinine ratio of 355.6 mg/mmol (reference range, ≤2.5 mg/mmol). The patient was referred to a dermatologist, who confirmed the clinical diagnosis without the need for a skin biopsy.
DISCUSSION
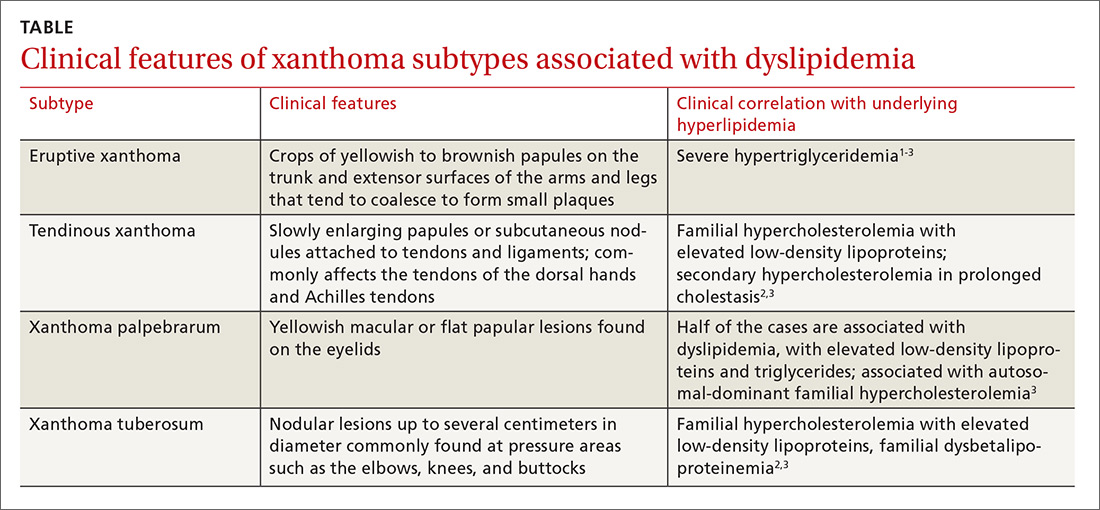
Eruptive xanthoma is characterized by an abrupt onset of crops of multiple yellowish to brownish papules that can coalesce into small plaques. The lesions can be generalized, but tend to be more densely distributed on the extensor surfaces of the arms and legs, buttocks, and thighs.5 Eruptive xanthoma often is associated with hypertriglyceridemia, which can be primary—as a result of a genetic defect caused by familial hypertriglyceridemia—or secondary, associated with poorly controlled diabetes mellitus, morbid obesity, excessive alcohol consumption, nephrotic syndrome, hypothyroidism, primary biliary cholangitis, and drugs like estrogen replacement therapies, corticosteroids, and isotretinoin.6 Pruritus and tenderness may or may not be present, and the Köbner phenomenon may occur.7
Continue to: The differential diagnosis
The differential diagnosis for eruptive xanthoma includes xanthoma disseminatum, non–Langerhans cell histiocytoses (eg, generalized eruptive histiocytosis), and cutaneous mastocytosis.1
Xanthoma disseminatum is an extremely rare, but benign, disorder of non–Langerhans cell origin. The average age of onset is older than 40 years. The rash consists of multiple red-yellow papules and nodules that most commonly present in flexural areas. Forty percent to 60% of patients have mucosal involvement, and rarely the central nervous system is involved.8
Generalized eruptive histiocytosis is another rare non–Langerhans cell histiocytosis that occurs mainly in adults and is characterized by widespread, symmetric, red-brown papules on the trunk, arms, and legs, and rarely the mucous membranes.9
Cutaneous mastocytosis, especially xanthelasmoid mastocytosis, consists of multiple pruritic, yellowish, papular or nodular lesions that may mimic eruptive xanthoma. It occurs mainly in children and rarely in adults.10
Confirming the diagnosis, initiating treatment
The diagnosis of eruptive xanthoma can be confirmed by skin biopsy if other differential diagnoses cannot be ruled out or the lesions do not resolve with treatment. Skin biopsy will reveal lipid-laden macrophages (known as foam cells) deposited in the dermis.7
Continue to: Treatment of eruptive xanthoma
Treatment of eruptive xanthoma involves management of the underlying causes of the condition. In most cases, dietary control, intensive triglyceride-lowering therapies, and treatment of other secondary causes of hypertriglyceridemia result in complete resolution of the lesions within several weeks.5
Our patient’s outcome
Our patient’s sudden-onset rash alerted us to the presence of type 2 diabetes mellitus, hypertriglyceridemia, and heavy proteinuria, which he was not aware of previously. We counselled him about stringent low-sugar, low-lipid diet control and exercise, and we started him on metformin and gemfibrozil. He was referred to an internal medicine specialist for further assessment and management of his severe hypertriglyceridemia and heavy proteinuria.
The rash started to wane 1 month after the patient started the metformin and gemfibrozil, and his drug regimen was changed to combination therapy with metformin/glimepiride and fenofibrate/simvastatin 6 weeks later when he was seen in the medical specialty clinic. Fundus photography performed 1 month after starting oral antidiabetic therapy showed no diabetic retinopathy or lipemia retinalis.
After 3 months of treatment, his serum triglycerides and hemoglobin A1c levels dropped to 3.8 mmol/L and 8.7%, respectively. The rash also resolved considerably, with only residual papules on the abdomen. This rapid clinical response to treatment of the underlying hypertriglyceridemia and diabetes further supported the clinical diagnosis of eruptive xanthoma.
THE TAKEAWAY
Eruptive xanthoma is relatively rare, but it is important for family physicians to recognize this clinical presentation as a potential indicator of severe hypertriglyceridemia. Recognizing hypertriglyceridemia early is important, as it can be associated with an increased risk for acute pancreatitis. Moreover, eruptive xanthoma might be the sole presenting symptom of underlying diabetes mellitus or familial hyperlipidemia, both of which can lead to a significant increase in cardiovascular risk if uncontrolled.
CORRESPONDENCE
Chan Kam Sum, MBChB, FRACGP, Tseung Kwan O Jockey Club General Out-patient Clinic, 99 Po Lam Road North, G/F, Tseung Kwan O, Kowloon, Hong Kong; cks048@ha.org.hk
1. Tang WK. Eruptive xanthoma. [case reports]. Hong Kong Dermatol Venereol Bull. 2001;9:172-175.
2. Frew J, Murrell D, Haber R. Fifty shades of yellow: a review of the xanthodermatoses. Int J Dermatol. 2015;54:1109-1123.
3. Zak A, Zeman M, Slaby A, et al. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:181-188.
4. Sandhu S, Al-Sarraf A, Taraboanta C, et al. Incidence of pancreatitis, secondary causes, and treatment of patients referred to specialty lipid clinic with severe hypertriglyceridemia: a retrospective cohort study. Lipids Health Dis. 2011;10:157.
5. Holsinger JM, Campbell SM, Witman P. Multiple erythematous-yellow, dome-shaped papules. Am Fam Physician. 2010;82:517.
6. Loeckermann S, Braun-Falco M. Eruptive xanthomas in association with metabolic syndrome. Clin Exp Dermatol. 2010;35:565-566.
7. Merola JF, Mengden SJ, Soldano A, et al. Eruptive xanthomas. Dermatol Online J. 2008;14:10.
8. Park M, Boone B, Devas S. Xanthoma disseminatum: case report and mini-review of the literature. Acta Dermatovenerol Croat. 2014;22:150-154.
9. Attia A, Seleit I, El Badawy N, et al. Photoletter to the editor: generalized eruptive histiocytoma. J Dermatol Case Rep. 2011;5:53-55.
10. Nabavi NS, Nejad MH, Feli S, et al. Adult onset of xanthelasmoid mastocytosis: report of a rare entity. Indian J Dermatol. 2016;61:468.
THE CASE
A 37-year-old man presented with a sudden-onset, nonpruritic, nonpainful, papular rash of 1 month’s duration on his trunk and both arms and legs. Two weeks prior to the current presentation, he consulted a general practitioner, who treated the rash with a course of unknown oral antibiotics; the patient showed no improvement. He recalled that on a few occasions, he used his fingers to express a creamy discharge from some of the lesions. This temporarily reduced the size of those papules.
His medical history was unremarkable except for morbid obesity. He did not drink alcohol regularly and was not taking any medications prior to the onset of the rash. He had no family history of hyperlipidemia, but his mother had a history of diabetes mellitus.
Physical examination showed numerous discrete erythematous papules with a creamy center on his trunk and his arms and legs. The lesions were more numerous on the extensor surfaces of the arms and legs. Some of the papules coalesced to form small plaques (FIGURE). There was no scaling, and the lesions were firm in texture. The patient’s face was spared, and there was no mucosal involvement. The patient was otherwise systemically well.
THE DIAGNOSIS
Based on the morphology, distribution, and abrupt onset of the diffuse nonpruritic papules in this morbidly obese (but otherwise systemically well) middle-aged man, a clinical diagnosis of eruptive xanthoma was suspected. Subsequent blood testing revealed an elevated serum triglyceride level of 47.8 mmol/L (reference range, <1.7 mmol/L), elevated serum total cholesterol of 7.1 mmol/L (reference range, <6.2 mmol/L), and low serum high-density lipoprotein cholesterol of 0.7 mmol/L (reference range, >1 mmol/L in men). He also had an elevated fasting serum glucose level of 12.9 mmol/L (reference range, 3.9–5.6 mmol/L) and an elevated hemoglobin A1c (glycated hemoglobin) level of 10.9%.
Subsequent thyroid, liver, and renal function tests were normal, but the patient had heavy proteinuria, with an elevated urine albumin-to-creatinine ratio of 355.6 mg/mmol (reference range, ≤2.5 mg/mmol). The patient was referred to a dermatologist, who confirmed the clinical diagnosis without the need for a skin biopsy.
DISCUSSION
Eruptive xanthoma is characterized by an abrupt onset of crops of multiple yellowish to brownish papules that can coalesce into small plaques. The lesions can be generalized, but tend to be more densely distributed on the extensor surfaces of the arms and legs, buttocks, and thighs.5 Eruptive xanthoma often is associated with hypertriglyceridemia, which can be primary—as a result of a genetic defect caused by familial hypertriglyceridemia—or secondary, associated with poorly controlled diabetes mellitus, morbid obesity, excessive alcohol consumption, nephrotic syndrome, hypothyroidism, primary biliary cholangitis, and drugs like estrogen replacement therapies, corticosteroids, and isotretinoin.6 Pruritus and tenderness may or may not be present, and the Köbner phenomenon may occur.7
Continue to: The differential diagnosis
The differential diagnosis for eruptive xanthoma includes xanthoma disseminatum, non–Langerhans cell histiocytoses (eg, generalized eruptive histiocytosis), and cutaneous mastocytosis.1
Xanthoma disseminatum is an extremely rare, but benign, disorder of non–Langerhans cell origin. The average age of onset is older than 40 years. The rash consists of multiple red-yellow papules and nodules that most commonly present in flexural areas. Forty percent to 60% of patients have mucosal involvement, and rarely the central nervous system is involved.8
Generalized eruptive histiocytosis is another rare non–Langerhans cell histiocytosis that occurs mainly in adults and is characterized by widespread, symmetric, red-brown papules on the trunk, arms, and legs, and rarely the mucous membranes.9
Cutaneous mastocytosis, especially xanthelasmoid mastocytosis, consists of multiple pruritic, yellowish, papular or nodular lesions that may mimic eruptive xanthoma. It occurs mainly in children and rarely in adults.10
Confirming the diagnosis, initiating treatment
The diagnosis of eruptive xanthoma can be confirmed by skin biopsy if other differential diagnoses cannot be ruled out or the lesions do not resolve with treatment. Skin biopsy will reveal lipid-laden macrophages (known as foam cells) deposited in the dermis.7
Continue to: Treatment of eruptive xanthoma
Treatment of eruptive xanthoma involves management of the underlying causes of the condition. In most cases, dietary control, intensive triglyceride-lowering therapies, and treatment of other secondary causes of hypertriglyceridemia result in complete resolution of the lesions within several weeks.5
Our patient’s outcome
Our patient’s sudden-onset rash alerted us to the presence of type 2 diabetes mellitus, hypertriglyceridemia, and heavy proteinuria, which he was not aware of previously. We counselled him about stringent low-sugar, low-lipid diet control and exercise, and we started him on metformin and gemfibrozil. He was referred to an internal medicine specialist for further assessment and management of his severe hypertriglyceridemia and heavy proteinuria.
The rash started to wane 1 month after the patient started the metformin and gemfibrozil, and his drug regimen was changed to combination therapy with metformin/glimepiride and fenofibrate/simvastatin 6 weeks later when he was seen in the medical specialty clinic. Fundus photography performed 1 month after starting oral antidiabetic therapy showed no diabetic retinopathy or lipemia retinalis.
After 3 months of treatment, his serum triglycerides and hemoglobin A1c levels dropped to 3.8 mmol/L and 8.7%, respectively. The rash also resolved considerably, with only residual papules on the abdomen. This rapid clinical response to treatment of the underlying hypertriglyceridemia and diabetes further supported the clinical diagnosis of eruptive xanthoma.
THE TAKEAWAY
Eruptive xanthoma is relatively rare, but it is important for family physicians to recognize this clinical presentation as a potential indicator of severe hypertriglyceridemia. Recognizing hypertriglyceridemia early is important, as it can be associated with an increased risk for acute pancreatitis. Moreover, eruptive xanthoma might be the sole presenting symptom of underlying diabetes mellitus or familial hyperlipidemia, both of which can lead to a significant increase in cardiovascular risk if uncontrolled.
CORRESPONDENCE
Chan Kam Sum, MBChB, FRACGP, Tseung Kwan O Jockey Club General Out-patient Clinic, 99 Po Lam Road North, G/F, Tseung Kwan O, Kowloon, Hong Kong; cks048@ha.org.hk
THE CASE
A 37-year-old man presented with a sudden-onset, nonpruritic, nonpainful, papular rash of 1 month’s duration on his trunk and both arms and legs. Two weeks prior to the current presentation, he consulted a general practitioner, who treated the rash with a course of unknown oral antibiotics; the patient showed no improvement. He recalled that on a few occasions, he used his fingers to express a creamy discharge from some of the lesions. This temporarily reduced the size of those papules.
His medical history was unremarkable except for morbid obesity. He did not drink alcohol regularly and was not taking any medications prior to the onset of the rash. He had no family history of hyperlipidemia, but his mother had a history of diabetes mellitus.
Physical examination showed numerous discrete erythematous papules with a creamy center on his trunk and his arms and legs. The lesions were more numerous on the extensor surfaces of the arms and legs. Some of the papules coalesced to form small plaques (FIGURE). There was no scaling, and the lesions were firm in texture. The patient’s face was spared, and there was no mucosal involvement. The patient was otherwise systemically well.
THE DIAGNOSIS
Based on the morphology, distribution, and abrupt onset of the diffuse nonpruritic papules in this morbidly obese (but otherwise systemically well) middle-aged man, a clinical diagnosis of eruptive xanthoma was suspected. Subsequent blood testing revealed an elevated serum triglyceride level of 47.8 mmol/L (reference range, <1.7 mmol/L), elevated serum total cholesterol of 7.1 mmol/L (reference range, <6.2 mmol/L), and low serum high-density lipoprotein cholesterol of 0.7 mmol/L (reference range, >1 mmol/L in men). He also had an elevated fasting serum glucose level of 12.9 mmol/L (reference range, 3.9–5.6 mmol/L) and an elevated hemoglobin A1c (glycated hemoglobin) level of 10.9%.
Subsequent thyroid, liver, and renal function tests were normal, but the patient had heavy proteinuria, with an elevated urine albumin-to-creatinine ratio of 355.6 mg/mmol (reference range, ≤2.5 mg/mmol). The patient was referred to a dermatologist, who confirmed the clinical diagnosis without the need for a skin biopsy.
DISCUSSION
Eruptive xanthoma is characterized by an abrupt onset of crops of multiple yellowish to brownish papules that can coalesce into small plaques. The lesions can be generalized, but tend to be more densely distributed on the extensor surfaces of the arms and legs, buttocks, and thighs.5 Eruptive xanthoma often is associated with hypertriglyceridemia, which can be primary—as a result of a genetic defect caused by familial hypertriglyceridemia—or secondary, associated with poorly controlled diabetes mellitus, morbid obesity, excessive alcohol consumption, nephrotic syndrome, hypothyroidism, primary biliary cholangitis, and drugs like estrogen replacement therapies, corticosteroids, and isotretinoin.6 Pruritus and tenderness may or may not be present, and the Köbner phenomenon may occur.7
Continue to: The differential diagnosis
The differential diagnosis for eruptive xanthoma includes xanthoma disseminatum, non–Langerhans cell histiocytoses (eg, generalized eruptive histiocytosis), and cutaneous mastocytosis.1
Xanthoma disseminatum is an extremely rare, but benign, disorder of non–Langerhans cell origin. The average age of onset is older than 40 years. The rash consists of multiple red-yellow papules and nodules that most commonly present in flexural areas. Forty percent to 60% of patients have mucosal involvement, and rarely the central nervous system is involved.8
Generalized eruptive histiocytosis is another rare non–Langerhans cell histiocytosis that occurs mainly in adults and is characterized by widespread, symmetric, red-brown papules on the trunk, arms, and legs, and rarely the mucous membranes.9
Cutaneous mastocytosis, especially xanthelasmoid mastocytosis, consists of multiple pruritic, yellowish, papular or nodular lesions that may mimic eruptive xanthoma. It occurs mainly in children and rarely in adults.10
Confirming the diagnosis, initiating treatment
The diagnosis of eruptive xanthoma can be confirmed by skin biopsy if other differential diagnoses cannot be ruled out or the lesions do not resolve with treatment. Skin biopsy will reveal lipid-laden macrophages (known as foam cells) deposited in the dermis.7
Continue to: Treatment of eruptive xanthoma
Treatment of eruptive xanthoma involves management of the underlying causes of the condition. In most cases, dietary control, intensive triglyceride-lowering therapies, and treatment of other secondary causes of hypertriglyceridemia result in complete resolution of the lesions within several weeks.5
Our patient’s outcome
Our patient’s sudden-onset rash alerted us to the presence of type 2 diabetes mellitus, hypertriglyceridemia, and heavy proteinuria, which he was not aware of previously. We counselled him about stringent low-sugar, low-lipid diet control and exercise, and we started him on metformin and gemfibrozil. He was referred to an internal medicine specialist for further assessment and management of his severe hypertriglyceridemia and heavy proteinuria.
The rash started to wane 1 month after the patient started the metformin and gemfibrozil, and his drug regimen was changed to combination therapy with metformin/glimepiride and fenofibrate/simvastatin 6 weeks later when he was seen in the medical specialty clinic. Fundus photography performed 1 month after starting oral antidiabetic therapy showed no diabetic retinopathy or lipemia retinalis.
After 3 months of treatment, his serum triglycerides and hemoglobin A1c levels dropped to 3.8 mmol/L and 8.7%, respectively. The rash also resolved considerably, with only residual papules on the abdomen. This rapid clinical response to treatment of the underlying hypertriglyceridemia and diabetes further supported the clinical diagnosis of eruptive xanthoma.
THE TAKEAWAY
Eruptive xanthoma is relatively rare, but it is important for family physicians to recognize this clinical presentation as a potential indicator of severe hypertriglyceridemia. Recognizing hypertriglyceridemia early is important, as it can be associated with an increased risk for acute pancreatitis. Moreover, eruptive xanthoma might be the sole presenting symptom of underlying diabetes mellitus or familial hyperlipidemia, both of which can lead to a significant increase in cardiovascular risk if uncontrolled.
CORRESPONDENCE
Chan Kam Sum, MBChB, FRACGP, Tseung Kwan O Jockey Club General Out-patient Clinic, 99 Po Lam Road North, G/F, Tseung Kwan O, Kowloon, Hong Kong; cks048@ha.org.hk
1. Tang WK. Eruptive xanthoma. [case reports]. Hong Kong Dermatol Venereol Bull. 2001;9:172-175.
2. Frew J, Murrell D, Haber R. Fifty shades of yellow: a review of the xanthodermatoses. Int J Dermatol. 2015;54:1109-1123.
3. Zak A, Zeman M, Slaby A, et al. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:181-188.
4. Sandhu S, Al-Sarraf A, Taraboanta C, et al. Incidence of pancreatitis, secondary causes, and treatment of patients referred to specialty lipid clinic with severe hypertriglyceridemia: a retrospective cohort study. Lipids Health Dis. 2011;10:157.
5. Holsinger JM, Campbell SM, Witman P. Multiple erythematous-yellow, dome-shaped papules. Am Fam Physician. 2010;82:517.
6. Loeckermann S, Braun-Falco M. Eruptive xanthomas in association with metabolic syndrome. Clin Exp Dermatol. 2010;35:565-566.
7. Merola JF, Mengden SJ, Soldano A, et al. Eruptive xanthomas. Dermatol Online J. 2008;14:10.
8. Park M, Boone B, Devas S. Xanthoma disseminatum: case report and mini-review of the literature. Acta Dermatovenerol Croat. 2014;22:150-154.
9. Attia A, Seleit I, El Badawy N, et al. Photoletter to the editor: generalized eruptive histiocytoma. J Dermatol Case Rep. 2011;5:53-55.
10. Nabavi NS, Nejad MH, Feli S, et al. Adult onset of xanthelasmoid mastocytosis: report of a rare entity. Indian J Dermatol. 2016;61:468.
1. Tang WK. Eruptive xanthoma. [case reports]. Hong Kong Dermatol Venereol Bull. 2001;9:172-175.
2. Frew J, Murrell D, Haber R. Fifty shades of yellow: a review of the xanthodermatoses. Int J Dermatol. 2015;54:1109-1123.
3. Zak A, Zeman M, Slaby A, et al. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:181-188.
4. Sandhu S, Al-Sarraf A, Taraboanta C, et al. Incidence of pancreatitis, secondary causes, and treatment of patients referred to specialty lipid clinic with severe hypertriglyceridemia: a retrospective cohort study. Lipids Health Dis. 2011;10:157.
5. Holsinger JM, Campbell SM, Witman P. Multiple erythematous-yellow, dome-shaped papules. Am Fam Physician. 2010;82:517.
6. Loeckermann S, Braun-Falco M. Eruptive xanthomas in association with metabolic syndrome. Clin Exp Dermatol. 2010;35:565-566.
7. Merola JF, Mengden SJ, Soldano A, et al. Eruptive xanthomas. Dermatol Online J. 2008;14:10.
8. Park M, Boone B, Devas S. Xanthoma disseminatum: case report and mini-review of the literature. Acta Dermatovenerol Croat. 2014;22:150-154.
9. Attia A, Seleit I, El Badawy N, et al. Photoletter to the editor: generalized eruptive histiocytoma. J Dermatol Case Rep. 2011;5:53-55.
10. Nabavi NS, Nejad MH, Feli S, et al. Adult onset of xanthelasmoid mastocytosis: report of a rare entity. Indian J Dermatol. 2016;61:468.
Bariatric surgery + medical therapy: Effective Tx for T2DM?
ILLUSTRATIVE CASE
A 46-year-old woman presents with a body mass index (BMI) of 28 kg/m2, a 4-year history of type 2 diabetes mellitus (T2DM), and a glycated hemoglobin (HgbA1c) of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 units/d, with minimal change in HgbA1c. Should you recommend bariatric surgery as an option for the treatment of diabetes?
One in 11 Americans has diabetes and at least 95% of those have type 2.2,3 The treatment of T2DM is generally multimodal in order to target the various mechanisms that cause hyperglycemia. Treatment strategies may include lifestyle modifications, decreasing insulin resistance, increasing secretion of insulin, insulin replacement, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) currently recommends diet, exercise, and behavioral modifications as first-line therapy for the management of diabetes,2 but these by themselves are often inadequate. In addition to various pharmacotherapeutic strategies for other populations with T2DM (see the PURL, “How do these 3 diabetes agents compare in reducing mortality?”), the ADA recommends bariatric surgery for the treatment of patients with T2DM, a BMI ≥35 kg/m2, and uncontrolled hyperglycemia.2,4 However, this recommendation from the ADA supporting bariatric surgery is based only on short-term studies.
For example, one single-center nonblinded randomized controlled trial (RCT) involving 60 patients with a BMI ≥35 kg/m2 found reductions in HgbA1C levels from the average baseline of 8.65±1.45% to 7.7±0.6% in the IMT group and to 6.4±1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35 kg/m2), gastric bypass had better outcomes than sleeve gastrectomy, with 93% of patients in the gastric bypass group achieving remission of T2DM vs 47% of patients in the sleeve gastrectomy group (P=.02) over a 12-month period.6
The current study sought to examine the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up shows surgery + intensive medical therapy works
This study by Schauer et al was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were 20 to 60 years of age, had a BMI of 27 to 43 kg/m2, and had an HgbA1C >7%. Patients with previous bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Each patient was randomly placed in a 1:1:1 fashion into 3 groups: IMT only, IMT and gastric bypass, or IMT and sleeve gastrectomy. All patients underwent IMT as defined by the ADA. The primary outcome was the number of patients with an HgbA1c ≤6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period leaving 149; 134 completed the 5-year follow-up; 8 patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment; an additional 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an HgbA1c of ≤6% compared with the IMT group (14 of 49 gastric bypass patients vs 2 of 38 IMT patients; P=.01; 11 of 47 sleeve gastrectomy patients vs 2 of 38 IMT patients; P=.03). Compared with those in the IMT group, the patients in the bariatric surgery and sleeve gastrectomy groups showed greater reductions from baseline in body weight and triglyceride levels, and greater increases from baseline in high-density lipoprotein (HDL) cholesterol levels; they also required less diabetic medication for glycemic control (see TABLE 11). However, when data were imputed for the intention-to-treat analysis, P-values were P=0.08 for gastric bypass and P=0.17 for sleeve gastrectomy compared with the IMT group for lowering HgbA1c.
WHAT’S NEW?
Adding surgery has big benefits with minimal adverse effects
Prior studies that evaluated the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates bariatric surgery plus IMT has long-term benefits with minimal adverse events compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM for patients with a BMI ≥27 kg/m2, which is below the starting BMI (35 kg/m2) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications, such as gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis, in this patient population is significant.1 Complications can include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling regarding the possible complications is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1c, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size of the study...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who received gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up compared with the patients who received sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to that with IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and the cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Asssociation. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (suppl 1):S81-S89.
3. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 1, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
ILLUSTRATIVE CASE
A 46-year-old woman presents with a body mass index (BMI) of 28 kg/m2, a 4-year history of type 2 diabetes mellitus (T2DM), and a glycated hemoglobin (HgbA1c) of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 units/d, with minimal change in HgbA1c. Should you recommend bariatric surgery as an option for the treatment of diabetes?
One in 11 Americans has diabetes and at least 95% of those have type 2.2,3 The treatment of T2DM is generally multimodal in order to target the various mechanisms that cause hyperglycemia. Treatment strategies may include lifestyle modifications, decreasing insulin resistance, increasing secretion of insulin, insulin replacement, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) currently recommends diet, exercise, and behavioral modifications as first-line therapy for the management of diabetes,2 but these by themselves are often inadequate. In addition to various pharmacotherapeutic strategies for other populations with T2DM (see the PURL, “How do these 3 diabetes agents compare in reducing mortality?”), the ADA recommends bariatric surgery for the treatment of patients with T2DM, a BMI ≥35 kg/m2, and uncontrolled hyperglycemia.2,4 However, this recommendation from the ADA supporting bariatric surgery is based only on short-term studies.
For example, one single-center nonblinded randomized controlled trial (RCT) involving 60 patients with a BMI ≥35 kg/m2 found reductions in HgbA1C levels from the average baseline of 8.65±1.45% to 7.7±0.6% in the IMT group and to 6.4±1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35 kg/m2), gastric bypass had better outcomes than sleeve gastrectomy, with 93% of patients in the gastric bypass group achieving remission of T2DM vs 47% of patients in the sleeve gastrectomy group (P=.02) over a 12-month period.6
The current study sought to examine the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up shows surgery + intensive medical therapy works
This study by Schauer et al was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were 20 to 60 years of age, had a BMI of 27 to 43 kg/m2, and had an HgbA1C >7%. Patients with previous bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Each patient was randomly placed in a 1:1:1 fashion into 3 groups: IMT only, IMT and gastric bypass, or IMT and sleeve gastrectomy. All patients underwent IMT as defined by the ADA. The primary outcome was the number of patients with an HgbA1c ≤6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period leaving 149; 134 completed the 5-year follow-up; 8 patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment; an additional 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an HgbA1c of ≤6% compared with the IMT group (14 of 49 gastric bypass patients vs 2 of 38 IMT patients; P=.01; 11 of 47 sleeve gastrectomy patients vs 2 of 38 IMT patients; P=.03). Compared with those in the IMT group, the patients in the bariatric surgery and sleeve gastrectomy groups showed greater reductions from baseline in body weight and triglyceride levels, and greater increases from baseline in high-density lipoprotein (HDL) cholesterol levels; they also required less diabetic medication for glycemic control (see TABLE 11). However, when data were imputed for the intention-to-treat analysis, P-values were P=0.08 for gastric bypass and P=0.17 for sleeve gastrectomy compared with the IMT group for lowering HgbA1c.
WHAT’S NEW?
Adding surgery has big benefits with minimal adverse effects
Prior studies that evaluated the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates bariatric surgery plus IMT has long-term benefits with minimal adverse events compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM for patients with a BMI ≥27 kg/m2, which is below the starting BMI (35 kg/m2) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications, such as gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis, in this patient population is significant.1 Complications can include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling regarding the possible complications is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1c, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size of the study...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who received gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up compared with the patients who received sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to that with IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and the cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 46-year-old woman presents with a body mass index (BMI) of 28 kg/m2, a 4-year history of type 2 diabetes mellitus (T2DM), and a glycated hemoglobin (HgbA1c) of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 units/d, with minimal change in HgbA1c. Should you recommend bariatric surgery as an option for the treatment of diabetes?
One in 11 Americans has diabetes and at least 95% of those have type 2.2,3 The treatment of T2DM is generally multimodal in order to target the various mechanisms that cause hyperglycemia. Treatment strategies may include lifestyle modifications, decreasing insulin resistance, increasing secretion of insulin, insulin replacement, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) currently recommends diet, exercise, and behavioral modifications as first-line therapy for the management of diabetes,2 but these by themselves are often inadequate. In addition to various pharmacotherapeutic strategies for other populations with T2DM (see the PURL, “How do these 3 diabetes agents compare in reducing mortality?”), the ADA recommends bariatric surgery for the treatment of patients with T2DM, a BMI ≥35 kg/m2, and uncontrolled hyperglycemia.2,4 However, this recommendation from the ADA supporting bariatric surgery is based only on short-term studies.
For example, one single-center nonblinded randomized controlled trial (RCT) involving 60 patients with a BMI ≥35 kg/m2 found reductions in HgbA1C levels from the average baseline of 8.65±1.45% to 7.7±0.6% in the IMT group and to 6.4±1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35 kg/m2), gastric bypass had better outcomes than sleeve gastrectomy, with 93% of patients in the gastric bypass group achieving remission of T2DM vs 47% of patients in the sleeve gastrectomy group (P=.02) over a 12-month period.6
The current study sought to examine the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up shows surgery + intensive medical therapy works
This study by Schauer et al was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were 20 to 60 years of age, had a BMI of 27 to 43 kg/m2, and had an HgbA1C >7%. Patients with previous bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Each patient was randomly placed in a 1:1:1 fashion into 3 groups: IMT only, IMT and gastric bypass, or IMT and sleeve gastrectomy. All patients underwent IMT as defined by the ADA. The primary outcome was the number of patients with an HgbA1c ≤6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period leaving 149; 134 completed the 5-year follow-up; 8 patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment; an additional 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an HgbA1c of ≤6% compared with the IMT group (14 of 49 gastric bypass patients vs 2 of 38 IMT patients; P=.01; 11 of 47 sleeve gastrectomy patients vs 2 of 38 IMT patients; P=.03). Compared with those in the IMT group, the patients in the bariatric surgery and sleeve gastrectomy groups showed greater reductions from baseline in body weight and triglyceride levels, and greater increases from baseline in high-density lipoprotein (HDL) cholesterol levels; they also required less diabetic medication for glycemic control (see TABLE 11). However, when data were imputed for the intention-to-treat analysis, P-values were P=0.08 for gastric bypass and P=0.17 for sleeve gastrectomy compared with the IMT group for lowering HgbA1c.
WHAT’S NEW?
Adding surgery has big benefits with minimal adverse effects
Prior studies that evaluated the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates bariatric surgery plus IMT has long-term benefits with minimal adverse events compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM for patients with a BMI ≥27 kg/m2, which is below the starting BMI (35 kg/m2) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications, such as gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis, in this patient population is significant.1 Complications can include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling regarding the possible complications is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1c, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size of the study...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who received gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up compared with the patients who received sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to that with IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and the cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Asssociation. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (suppl 1):S81-S89.
3. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 1, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Asssociation. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (suppl 1):S81-S89.
3. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 1, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
PRACTICE CHANGER
Consider bariatric surgery with medical therapy as a treatment option for adults with uncontrolled type 2 diabetes and a body mass index ≥27 kg/m2.1
STRENGTH OF RECOMMENDATION
B: Based on a nonblinded, single-center, randomized controlled trial.
Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
How do these 3 diabetes agents compare in reducing mortality?
ILLUSTRATIVE CASE
A 64-year-old man with taype 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care hemoglobin A1c is 9.5%, and he is currently taking only metformin 1000 mg bid. You are considering adding an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and cardiovascular (CV) mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people, or 1 of every 11, now struggles to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of medications available that are aimed at lowering blood sugar and improving diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The “American Diabetes Association Standards of Medical Care in Diabetes” points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin, and the GLP-1 agonist liraglutide, as agents that should be added to metformin and lifestyle modification in patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists and the American College of Endocrinology guidelines do include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in strength of recommendations for these classes is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s are associated with better mortality outcomes than DPP-4s
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176,310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to reduce all-cause mortality and CV endpoints in patients with T2DM. The authors analyzed English-language RCTs that followed patients with T2DM for at least 12 weeks and compared SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to one another, to placebo, or to no treatment.
A majority of the patients in both the intervention and control groups were taking additional diabetes medications, such as metformin, prior to enrollment and during the trials. About half of the patients analyzed were enrolled in trials that specifically evaluated patients at elevated CV risk, which is notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group, hazard ratio [HR]=0.80; 95% credible interval [CrI], 0.71-0.89; absolute risk difference [RD]= –1%; number needed to treat [NNT]=100; GLP-1 agonist group, HR=0.88; 95% CrI, 0.81-0.94; absolute RD= -0.6%; NNT=167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR=1.02; 95% CrI, 0.94-1.11; absolute RD=0.1%). Both the SGLT-2 inhibitor (HR=0.78; 95% CrI, 0.68-0.90; absolute RD= –0.9%; NNT=111) and GLP-1 agonist (HR=0.86; 95% CrI, 0.77-0.96; absolute RD= –0.5%; NNT=200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR=0.79; 95% Crl, 0.69-0.91; absolute RD= –0.8%; NNT=125) and GLP-1 agonist (HR=0.85; Crl, 95% 0.77-0.94; absolute RD= –0.5%; NNT=200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR=0.62; 95% CrI, 0.54-0.72; absolute RD= –1.1%; NNT=91) and MIs (HR=0.86; 95% CrI, 0.77–0.97; absolute RD= –0.6%; NNT=167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR=0.67; 95% CrI, 0.57 to 0.80; absolute RD= –0.9; NNT=111) or DPP-4 inhibitors (HR=0.55; 95% CrI, 0.46-0.67; absolute RD= –1.1%; NNT=91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups saw lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists, HR=2; 95% CrI, 1.70-2.37; absolute RD=4.7%; number needed to harm [NNH]=21; SGLT-2 inhibitors, HR=1.8; 95% CrI, 1.44-2.25; absolute RD=5.8%; NNH=17; and DPP-4 inhibitors, HR=1.93; 95% CrI, 1.59-2.35; absolute RD=3.1%; NNH=32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR]=4.19; 95% confidence interval [CI], 3.45-5.09; absolute RD=6%; NNH=16), but not of urinary tract infection or lower limb amputation, although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR=1.58; 95% CI, 1.04-2.39; absolute RD=0.1%; NNH=1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than is the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 classes of medication.
However, there was relatively low heterogeneity among the studies included (I2=12), which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation may represent challenges
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors, and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists, must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes–2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Published December 2015. Updated May 2017. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 Executive Summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
ILLUSTRATIVE CASE
A 64-year-old man with taype 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care hemoglobin A1c is 9.5%, and he is currently taking only metformin 1000 mg bid. You are considering adding an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and cardiovascular (CV) mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people, or 1 of every 11, now struggles to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of medications available that are aimed at lowering blood sugar and improving diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The “American Diabetes Association Standards of Medical Care in Diabetes” points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin, and the GLP-1 agonist liraglutide, as agents that should be added to metformin and lifestyle modification in patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists and the American College of Endocrinology guidelines do include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in strength of recommendations for these classes is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s are associated with better mortality outcomes than DPP-4s
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176,310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to reduce all-cause mortality and CV endpoints in patients with T2DM. The authors analyzed English-language RCTs that followed patients with T2DM for at least 12 weeks and compared SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to one another, to placebo, or to no treatment.
A majority of the patients in both the intervention and control groups were taking additional diabetes medications, such as metformin, prior to enrollment and during the trials. About half of the patients analyzed were enrolled in trials that specifically evaluated patients at elevated CV risk, which is notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group, hazard ratio [HR]=0.80; 95% credible interval [CrI], 0.71-0.89; absolute risk difference [RD]= –1%; number needed to treat [NNT]=100; GLP-1 agonist group, HR=0.88; 95% CrI, 0.81-0.94; absolute RD= -0.6%; NNT=167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR=1.02; 95% CrI, 0.94-1.11; absolute RD=0.1%). Both the SGLT-2 inhibitor (HR=0.78; 95% CrI, 0.68-0.90; absolute RD= –0.9%; NNT=111) and GLP-1 agonist (HR=0.86; 95% CrI, 0.77-0.96; absolute RD= –0.5%; NNT=200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR=0.79; 95% Crl, 0.69-0.91; absolute RD= –0.8%; NNT=125) and GLP-1 agonist (HR=0.85; Crl, 95% 0.77-0.94; absolute RD= –0.5%; NNT=200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR=0.62; 95% CrI, 0.54-0.72; absolute RD= –1.1%; NNT=91) and MIs (HR=0.86; 95% CrI, 0.77–0.97; absolute RD= –0.6%; NNT=167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR=0.67; 95% CrI, 0.57 to 0.80; absolute RD= –0.9; NNT=111) or DPP-4 inhibitors (HR=0.55; 95% CrI, 0.46-0.67; absolute RD= –1.1%; NNT=91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups saw lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists, HR=2; 95% CrI, 1.70-2.37; absolute RD=4.7%; number needed to harm [NNH]=21; SGLT-2 inhibitors, HR=1.8; 95% CrI, 1.44-2.25; absolute RD=5.8%; NNH=17; and DPP-4 inhibitors, HR=1.93; 95% CrI, 1.59-2.35; absolute RD=3.1%; NNH=32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR]=4.19; 95% confidence interval [CI], 3.45-5.09; absolute RD=6%; NNH=16), but not of urinary tract infection or lower limb amputation, although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR=1.58; 95% CI, 1.04-2.39; absolute RD=0.1%; NNH=1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than is the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 classes of medication.
However, there was relatively low heterogeneity among the studies included (I2=12), which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation may represent challenges
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors, and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists, must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 64-year-old man with taype 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care hemoglobin A1c is 9.5%, and he is currently taking only metformin 1000 mg bid. You are considering adding an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and cardiovascular (CV) mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people, or 1 of every 11, now struggles to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of medications available that are aimed at lowering blood sugar and improving diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The “American Diabetes Association Standards of Medical Care in Diabetes” points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin, and the GLP-1 agonist liraglutide, as agents that should be added to metformin and lifestyle modification in patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists and the American College of Endocrinology guidelines do include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in strength of recommendations for these classes is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s are associated with better mortality outcomes than DPP-4s
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176,310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to reduce all-cause mortality and CV endpoints in patients with T2DM. The authors analyzed English-language RCTs that followed patients with T2DM for at least 12 weeks and compared SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to one another, to placebo, or to no treatment.
A majority of the patients in both the intervention and control groups were taking additional diabetes medications, such as metformin, prior to enrollment and during the trials. About half of the patients analyzed were enrolled in trials that specifically evaluated patients at elevated CV risk, which is notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group, hazard ratio [HR]=0.80; 95% credible interval [CrI], 0.71-0.89; absolute risk difference [RD]= –1%; number needed to treat [NNT]=100; GLP-1 agonist group, HR=0.88; 95% CrI, 0.81-0.94; absolute RD= -0.6%; NNT=167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR=1.02; 95% CrI, 0.94-1.11; absolute RD=0.1%). Both the SGLT-2 inhibitor (HR=0.78; 95% CrI, 0.68-0.90; absolute RD= –0.9%; NNT=111) and GLP-1 agonist (HR=0.86; 95% CrI, 0.77-0.96; absolute RD= –0.5%; NNT=200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR=0.79; 95% Crl, 0.69-0.91; absolute RD= –0.8%; NNT=125) and GLP-1 agonist (HR=0.85; Crl, 95% 0.77-0.94; absolute RD= –0.5%; NNT=200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR=0.62; 95% CrI, 0.54-0.72; absolute RD= –1.1%; NNT=91) and MIs (HR=0.86; 95% CrI, 0.77–0.97; absolute RD= –0.6%; NNT=167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR=0.67; 95% CrI, 0.57 to 0.80; absolute RD= –0.9; NNT=111) or DPP-4 inhibitors (HR=0.55; 95% CrI, 0.46-0.67; absolute RD= –1.1%; NNT=91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups saw lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists, HR=2; 95% CrI, 1.70-2.37; absolute RD=4.7%; number needed to harm [NNH]=21; SGLT-2 inhibitors, HR=1.8; 95% CrI, 1.44-2.25; absolute RD=5.8%; NNH=17; and DPP-4 inhibitors, HR=1.93; 95% CrI, 1.59-2.35; absolute RD=3.1%; NNH=32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR]=4.19; 95% confidence interval [CI], 3.45-5.09; absolute RD=6%; NNH=16), but not of urinary tract infection or lower limb amputation, although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR=1.58; 95% CI, 1.04-2.39; absolute RD=0.1%; NNH=1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than is the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 classes of medication.
However, there was relatively low heterogeneity among the studies included (I2=12), which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation may represent challenges
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors, and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists, must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes–2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Published December 2015. Updated May 2017. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 Executive Summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes–2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Published December 2015. Updated May 2017. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 Executive Summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
PRACTICE CHANGER
Consider adding a sodium-glucose cotransporter 2 (SGLT-2) inhibitor or a glucagon-like peptide 1 (GLP-1) agonist to the treatment regimen of patients with poorly controlled type 2 diabetes—especially those with higher CV risk. Doing so can reduce all-cause and cardiovascular (CV) mortality 1
STRENGTH OF RECOMMENDATION
B: Based on a network meta-analysis of 236 randomized controlled trials.
Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
More sleep can help youth manage type 1 diabetes
, according to a study of sleep duration and quality in young diabetes patients.
“This study adds to the growing body of literature that supports the cascading effects of sleep on multiple aspects of diabetes-related outcomes,” wrote lead author Sara S. Frye, PhD, of the University of Arizona, Tucson, and her coauthors, adding that the results “highlight the importance of assessing sleep in this population that appears to be at high risk for insufficient sleep duration.” The study was published in Sleep Medicine.
Dr. Frye and her colleagues recruited 111 children between the ages of 10 and 16 with type 1 diabetes mellitus to participate in their Glucose Regulation and Neurobehavioral Effects of Sleep (GRANES) study. The participants wore wrist actigraphs for an average of 5.5 nights to objectively measure sleep, including duration, quality, timing, and consistency. They completed self-reported sleep diaries each morning of the study. Glycemic control and diabetes management were assessed via hemoglobin A1c (HbA1c) levels and self-monitoring of blood glucose (SMBG) frequency, which were obtained via medical records. The participants and their parents also completed the Diabetes Management Scale.
Based on actigraphy data, the average total sleep time was 7.45 hours (standard deviation, 0.74), below the recommended duration of 9 hours for youths in this age group. All but one participant was recorded as sleeping less than the recommended amount. Average HbA1c of 9.11% (SD, 1.95) indicated poor diabetic control, and the average SMBG frequency was 4.90 (SD, 2.71) with a range of 1-14 checks per day. Per mediation analysis, for every additional hour of sleep, HbA1c was reduced by 0.33% and SMBG frequency went up by 0.88. In addition, SMBG frequency was related to HbA1c, supporting previous findings that “self-management behaviors play a critical role in maintaining diabetes control.”
The coauthors acknowledged the limitations of their study, including actigraphy data being logged over a 1-week period instead of the recommended 2 weeks. They also relied on medical records to determine HbA1c and SMBG rather than collecting that information along with the actigraphy data. However, they did note that HbA1c measures glucose levels over a 3-month period, which would have covered their participation in the study.
The study was supported by American Diabetes Association and cosponsored by the Order of the Amaranth Diabetes Foundation. The authors reported no conflicts of interest.
SOURCE: Frye SS et al. Sleep Med. 2019 Feb 16. doi: 10.1016/j.sleep.2019.01.043.
, according to a study of sleep duration and quality in young diabetes patients.
“This study adds to the growing body of literature that supports the cascading effects of sleep on multiple aspects of diabetes-related outcomes,” wrote lead author Sara S. Frye, PhD, of the University of Arizona, Tucson, and her coauthors, adding that the results “highlight the importance of assessing sleep in this population that appears to be at high risk for insufficient sleep duration.” The study was published in Sleep Medicine.
Dr. Frye and her colleagues recruited 111 children between the ages of 10 and 16 with type 1 diabetes mellitus to participate in their Glucose Regulation and Neurobehavioral Effects of Sleep (GRANES) study. The participants wore wrist actigraphs for an average of 5.5 nights to objectively measure sleep, including duration, quality, timing, and consistency. They completed self-reported sleep diaries each morning of the study. Glycemic control and diabetes management were assessed via hemoglobin A1c (HbA1c) levels and self-monitoring of blood glucose (SMBG) frequency, which were obtained via medical records. The participants and their parents also completed the Diabetes Management Scale.
Based on actigraphy data, the average total sleep time was 7.45 hours (standard deviation, 0.74), below the recommended duration of 9 hours for youths in this age group. All but one participant was recorded as sleeping less than the recommended amount. Average HbA1c of 9.11% (SD, 1.95) indicated poor diabetic control, and the average SMBG frequency was 4.90 (SD, 2.71) with a range of 1-14 checks per day. Per mediation analysis, for every additional hour of sleep, HbA1c was reduced by 0.33% and SMBG frequency went up by 0.88. In addition, SMBG frequency was related to HbA1c, supporting previous findings that “self-management behaviors play a critical role in maintaining diabetes control.”
The coauthors acknowledged the limitations of their study, including actigraphy data being logged over a 1-week period instead of the recommended 2 weeks. They also relied on medical records to determine HbA1c and SMBG rather than collecting that information along with the actigraphy data. However, they did note that HbA1c measures glucose levels over a 3-month period, which would have covered their participation in the study.
The study was supported by American Diabetes Association and cosponsored by the Order of the Amaranth Diabetes Foundation. The authors reported no conflicts of interest.
SOURCE: Frye SS et al. Sleep Med. 2019 Feb 16. doi: 10.1016/j.sleep.2019.01.043.
, according to a study of sleep duration and quality in young diabetes patients.
“This study adds to the growing body of literature that supports the cascading effects of sleep on multiple aspects of diabetes-related outcomes,” wrote lead author Sara S. Frye, PhD, of the University of Arizona, Tucson, and her coauthors, adding that the results “highlight the importance of assessing sleep in this population that appears to be at high risk for insufficient sleep duration.” The study was published in Sleep Medicine.
Dr. Frye and her colleagues recruited 111 children between the ages of 10 and 16 with type 1 diabetes mellitus to participate in their Glucose Regulation and Neurobehavioral Effects of Sleep (GRANES) study. The participants wore wrist actigraphs for an average of 5.5 nights to objectively measure sleep, including duration, quality, timing, and consistency. They completed self-reported sleep diaries each morning of the study. Glycemic control and diabetes management were assessed via hemoglobin A1c (HbA1c) levels and self-monitoring of blood glucose (SMBG) frequency, which were obtained via medical records. The participants and their parents also completed the Diabetes Management Scale.
Based on actigraphy data, the average total sleep time was 7.45 hours (standard deviation, 0.74), below the recommended duration of 9 hours for youths in this age group. All but one participant was recorded as sleeping less than the recommended amount. Average HbA1c of 9.11% (SD, 1.95) indicated poor diabetic control, and the average SMBG frequency was 4.90 (SD, 2.71) with a range of 1-14 checks per day. Per mediation analysis, for every additional hour of sleep, HbA1c was reduced by 0.33% and SMBG frequency went up by 0.88. In addition, SMBG frequency was related to HbA1c, supporting previous findings that “self-management behaviors play a critical role in maintaining diabetes control.”
The coauthors acknowledged the limitations of their study, including actigraphy data being logged over a 1-week period instead of the recommended 2 weeks. They also relied on medical records to determine HbA1c and SMBG rather than collecting that information along with the actigraphy data. However, they did note that HbA1c measures glucose levels over a 3-month period, which would have covered their participation in the study.
The study was supported by American Diabetes Association and cosponsored by the Order of the Amaranth Diabetes Foundation. The authors reported no conflicts of interest.
SOURCE: Frye SS et al. Sleep Med. 2019 Feb 16. doi: 10.1016/j.sleep.2019.01.043.
FROM SLEEP MEDICINE