User login
A 51-year-old woman with dyspnea
A 51-year-old woman presents to the emergency department with dyspnea, which began 4 days ago. She reports no chest pain, palpitations, hemoptysis, fevers, chills, weight loss, recent travel, immobility, or surgery. One week ago she noticed cramping in her right calf, but that has since resolved.
Her history includes hypertension, hypothyroidism, and immune-mediated glomerulonephritis with proteinuria. She is premenopausal. She takes losartan and levothyroxine; she is not taking oral contraceptives or herbal supplements. She is up to date with her cancer screening and has had negative findings on colonoscopy and mammography within the past year.
She has never smoked and she does not drink alcohol or use illicit drugs. Her mother has a history of provoked deep vein thrombosis and colon cancer.
Her temperature is 36.2°C (97.2°F), heart rate 163 beats per minute, blood pressure 158/102 mm Hg, respiratory rate 40 breaths per minute, and oxygen saturation by pulse oximetry 80% while breathing room air, corrected to 94% with oxygen 6 L/min via nasal cannula.
On physical examination, she is sitting upright on a stretcher and appears uncomfortable and anxious. She is awake and able to communicate clearly. Examination of the head, ears, eyes, nose, and throat is unremarkable, with moist mucous membranes. Her lungs are clear to auscultation. Her heart beat is very rapid, with a regular rhythm and an accentuated P2 heart sound. A right parasternal heave can be palpated in addition to a rightwardly displaced point of maximal impulse. The abdomen is normal, with no tenderness or organomegaly. She has no pain, edema, or erythema in the legs or feet, and she has strong, symmetric pulses (2+) in all extremities. The neurologic examination is nonfocal.
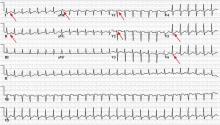
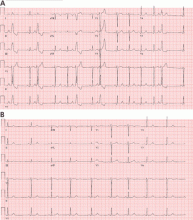
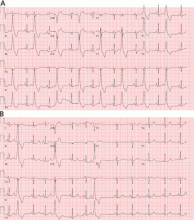
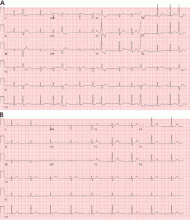
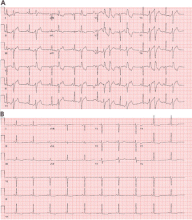
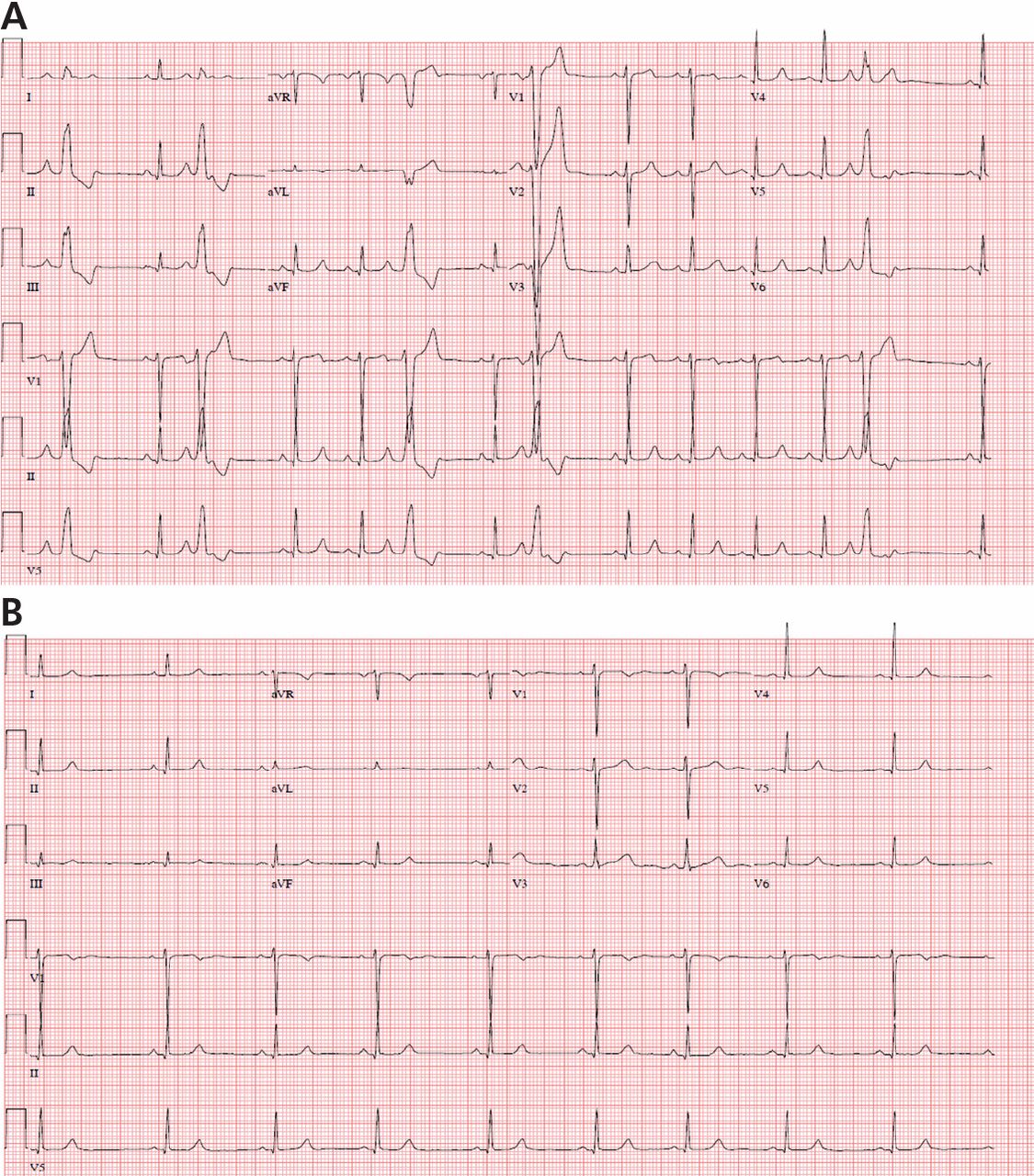
Electrocardiography (ECG) done on arrival (Figure 1) reveals supraventricular tachycardia, a normal axis, and nonspecific ST-segment and T-wave abnormalities, findings commonly seen in pulmonary embolism.1,2 On the other hand, her ECG does not show some of the other signs of right ventricular strain due to pulmonary embolism such as atrial arrhythmias, complete right bundle branch block, or inferior Q-waves.3,4
In view of her ECG findings and her symptoms of dyspnea, calf pain, tachypnea, tachycardia, and a pronounced P2 heart sound, her physician concludes that she very likely has a pulmonary embolism1 and orders an intravenous infusion of unfractionated heparin to be started immediately.
TESTING FOR PULMONARY EMBOLISM
1. Which of the following would be the best initial diagnostic imaging study to perform in this patient, who has a high pretest probability of pulmonary embolism?
- Multidetector computed tomographic (CT) pulmonary angiography
- Transthoracic echocardiography
- Magnetic resonance imaging
- Lower-extremity duplex ultrasonography
- Pulmonary angiography
- Ventilation-perfusion scintigraphy
Multidetector CT angiography is rapid, noninvasive, and highly sensitive (83%–90%) and specific (96%) for pulmonary embolism.5,6 In patients such as ours who have a high pretest probability of having the disease, its positive predictive value is 96%.5 Therefore, it would be the initial diagnostic study to perform in our patient.
Although transthoracic echocardiography is noninvasive and can detect right ventricular strain in the setting of pulmonary embolism, it may miss half of all pulmonary emboli detected by angiography.7,8
When technically adequate images are obtained, the combination of magnetic resonance angiography and magnetic resonance venography is very sensitive (92%) and specific (96%) for pulmonary embolism.9 However, one-fourth of patients undergoing these studies may have technically inadequate results, so this is not the best choice for diagnosis.9
As our patient complained of recent cramping in the right calf, lower-extremity duplex ultrasonography would be a reasonable test to screen for acute deep vein thrombosis as the source of pulmonary embolism. However, given her worrisome vital signs and impending hemodynamic collapse, CT pulmonary angiography would be a better initial test as it may guide more aggressive therapy. Furthermore, even if ultrasonography showed no evidence of deep vein thrombosis, clinical suspicion for pulmonary embolism would remain high enough that therapeutic anticoagulation would be continued until further testing ruled out this diagnosis.
Pulmonary angiography is the gold-standard test for pulmonary embolism. However, it is time-consuming, expensive, and invasive and so is not usually done unless the diagnosis cannot be made with other imaging studies.
Ventilation-perfusion scintigraphy is an established and safe diagnostic test for pulmonary embolism. It is particularly helpful in patients who have renal dysfunction or contrast allergy. The sensitivity of a high-probability scan is 78%, while the specificity of a very-low-probability scan is 97%.10 However, this study is often nondiagnostic (in 26.5% of cases),10 and further imaging may be required.
RESULTS OF CT ANGIOGRAPHY
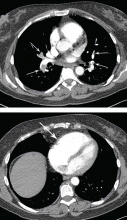
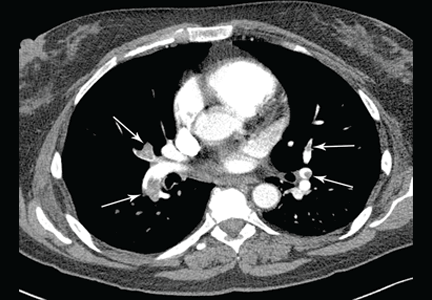
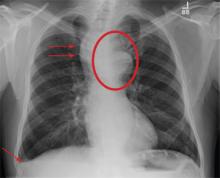
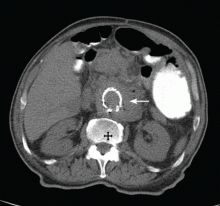
Our patient undergoes CT angiography, which reveals multiple bilateral pulmonary emboli and right ventricular enlargement (Figure 2). Transthoracic echocardiography shows dilation of the right ventricle, with severely reduced systolic function, an underfilled and hyperdynamic left ventricle (ejection fraction 75%), and moderate tricuspid valve regurgitation. Her right ventricular systolic pressure is estimated to be 47 mm Hg.
Doppler ultrasonography of the legs reveals an occlusive thrombus within the right small saphenous vein that bulges and extends into the right popliteal vein. Also noted is a nonocclusive thrombus in the upper right popliteal vein that likely originated from the thrombus in the small saphenous vein.
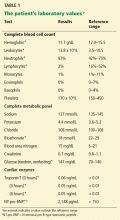
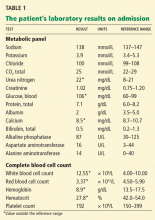
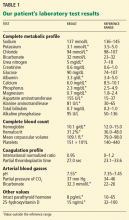
Initial laboratory testing (Table 1) shows elevations of the cardiac enzymes troponin T and N-terminal pro-B-type natriuretic peptide (NT-pro-BNP).
ESTIMATING PROGNOSIS IN PULMONARY EMBOLISM
2. Which of the following laboratory results at presentation is independently associated with a worse outcome in patients with pulmonary embolism?
- Elevated NT-pro-BNP
- Hypercalcemia
- Thrombocytosis
- Hypernatremia
- Elevated procalcitonin
The Pulmonary Embolism Severity Index11 and the Simplified Pulmonary Embolism Severity Index12 (Table 2) are clinical calculators that help predict 30-day risk of death in patients with pulmonary embolism. Our patient’s Pulmonary Embolism Severity Index score is 60, indicating a very low risk, but her simplified severity index score is 2, indicating a high risk.
A shock index score (the heart rate divided by the systolic blood pressure) greater than 1 is also a sensitive measure of risk.13 (Our patient’s shock index score is 1.03.) Although the simplified version is more accurate,14 the shock index is also helpful when deciding whether patients with suspected pulmonary embolism should receive early fibrinolysis.15
In a large registry of patients with confirmed pulmonary embolism, risk factors for death were age greater than 70, cancer, clinical congestive heart failure, chronic obstructive pulmonary disease, systolic blood pressure lower than 90 mm Hg, respiratory rate less than 20 per minute, and right ventricular hypokinesis.16 Right ventricular dysfunction progressing to right ventricular failure and cardiogenic shock is the most common cause of death in patients with pulmonary embolism.16–18
Post hoc analysis has also shown that elevations of the biomarkers BNP, NT-pro-BNP, and cardiac troponins I and T are associated with a prolonged hospital course and a higher risk of death within 30 days.19 Interestingly, a recent retrospective analysis found hyponatremia to be an independent risk factor for death in the short term.20
Thrombocytopenia, not thrombocytosis, is associated with worse outcomes in patients with pulmonary embolism.16 Procalcitonin is elevated in bacterial pneumonia but is normal in pulmonary embolism and so may be helpful in differentiating between the two.21,22 Hypernatremia, hypercalcemia, and elevated procalcitonin have not been shown to be independently associated with worse outcomes in acute pulmonary embolism.
Thus, of the answer choices shown above, elevated NT-pro-BNP is the correct answer.
Classified as massive, submassive, or low-risk
Pulmonary embolism is often stratified as massive, submassive, or low-risk, reflecting the severity and the degree of cardiovascular collapse. The treatment depends on the classification.
Pulmonary embolism is classified as massive if the patient has a cardiac arrest or a systolic blood pressure lower than 90 mm Hg for more than 15 minutes.23 Nearly half of patients in this category die.24
Pulmonary embolism is submassive if the patient has systolic pressure greater than 90 mm Hg but has right ventricular dysfunction, as evidenced by physical examination, elevated cardiac biomarkers, electrocardiography, transthoracic echocardiography, or computed tomography. The death rate is as high as 15%.24
Pulmonary embolism in a normotensive patient with no right ventricular dysfunction is defined as low-risk.
Our patient so far
Our patient has bilateral pulmonary emboli, most likely originating from a deep vein thrombosis in her right lower leg. Her pulmonary embolism would be classified as submassive, as her systolic pressure is greater than 90 mm Hg and right ventricular dysfunction—significant right ventricular strain—was noted on both transthoracic echocardiography and computed tomography. Also, cardiac biomarkers are elevated, and the physical examination revealed a prominent P2 sound and right parasternal heave, also suggestive of right ventricular dysfunction.
Now, 6 hours have passed, and even though she has been receiving intravenous heparin during this time, her shock index remains greater than 1, indicating hemodynamic instability. Her pulse rate is still markedly high—over 160 bpm—and she still appears quite anxious and uncomfortable.
HOW SHOULD THIS PATIENT BE TREATED?
3. Which of the following is the most appropriate treatment for this patient?
- Start warfarin immediately while bridging with unfractionated heparin, low-molecular-weight heparin, or fondaparinux
- Start fibrinolysis with alteplase
- Give metoprolol intravenously to control her heart rate
- Start dabigatran immediately while bridging with unfractionated heparin
- Place an inferior vena cava filter
- Consult cardiothoracic surgery for emergency pulmonary embolectomy
All patients with confirmed pulmonary embolism and no contraindications to anticoagulation should begin treatment with low-molecular-weight heparin, unfractionated heparin, or fondaparinux.23 In addition, this therapy should be started empirically while the patient is still undergoing diagnostic testing if the pretest probability of pulmonary embolism is intermediate or high.23
Warfarin is indicated for all patients with pulmonary embolism who do not have contraindications to it (Table 3). If unfractionated heparin, low-molecular-weight heparin, or fondaparinux has not already been started, it should be started at the same time as warfarin and should be continued until the international normalized ratio (INR) is within the therapeutic range.
Fibrinolysis. Treatment with a fibrinolytic agent in addition to heparin results in faster improvement of right ventricular function and pulmonary perfusion than with heparin alone.25 It may also decrease the incidence of pulmonary hypertension secondary to chronic thromboembolic disease.26 It should be considered in patients with massive pulmonary embolism.23
Whether fibrinolysis is appropriate for all patients with submassive pulmonary embolism remains controversial. Currently, it is not recommended for minor right ventricular dysfunction or myocardial necrosis if the patient has no signs of overt clinical decline.23 The Pulmonary Embolism Thrombolysis trial27 is an ongoing prospective randomized comparison of tenecteplase in a single bolus plus heparin vs heparin alone in normotensive patients with submassive pulmonary embolism, such as our patient. This trial may elucidate the benefit of fibrinolytic therapy in patients with submassive pulmonary embolism.
Patients at low risk are generally treated with heparin and warfarin anticoagulation alone. Fibrinolysis is not recommended in these patients, as the risk of bleeding outweighs the potential benefits.23
Metoprolol may not be advisable for our patient, as her tachycardia is likely compensatory, and beta-blocker therapy could blunt this compensatory response, leading to inadequate systemic perfusion.
Dabigatran is an oral direct thrombin inhibitor that does not require laboratory monitoring. It is currently approved for the prevention of stroke in patients with atrial fibrillation. It has been shown to be as effective as warfarin in the treatment of acute venous thromboembolism28 and may be a viable option in the future, but as of this writing it has not yet been approved in the United States for this indication. Furthermore, dabigatran inhibits thrombin immediately, so continued heparin bridging would not be necessary.
An inferior vena cava filter may prevent recurrent pulmonary embolism for patients who have absolute contraindications to anticoagulation, most significantly in the short term, ie, in the first few weeks after placement. However, these devices have not yet been shown to improve long-term mortality rates.
Embolectomy, percutaneous or surgical, is also an option. For patients in whom thrombolytic therapy is not effective, “rescue” surgical embolectomy has been associated with better outcomes compared with secondary thrombolysis and so should be considered.29
Back to our patient
An intravenous infusion of alteplase is started, and the patient’s tachycardia improves. Her oxygen requirements normalize, and she is transferred to the general medical floor the next day. She receives subcutaneous dalteparin as a bridge therapy, and warfarin is titrated to a goal INR of 2.0 to 3.0. Because of the acute deep vein thrombosis in her right lower leg, she is instructed to wear knee-high fitted compression hose for primary prevention of postphlebitic syndrome.
HOW LONG TO TREAT? IS GENETIC TESTING INDICATED?
Patients with a first episode of unprovoked venous thromboembolism should receive oral anticoagulants for 6 months, while those with recurrent unprovoked venous thromboembolism require lifelong oral anticoagulation.23
Whether to test for inherited thrombophilia after a first episode of venous thromboembolism to guide the duration of anticoagulation is controversial.30 Indiscriminate testing has not been recommended in these patients,31 but the American College of Medical Genetics recommends genetic screening for factor V Leiden in patients who have an unprovoked incident of venous thromboembolism before age 50.32
No randomized controlled trial has assessed whether thrombophilia testing decreases the recurrence rate of venous thromboembolism.33 One uncontrolled study suggested that testing for inherited thrombophilias in patients with a first episode does not affect the risk of recurrence.34 Testing is costly and may cause psychological distress for patients and family members.
Our patient is discharged home on warfarin for 6 months with subsequent follow-up evaluation in the thrombophilia clinic.
WHEN SHOULD WARFARIN BE RESTARTED?
4. If our patient were to discontinue oral anticoagulation in 6 months, which of the following, if present 1 month afterwards, would be a reason to restart oral anticoagulation?
- Elevated serum cotinine
- Positive pregnancy test
- Elevated follicle-stimulating hormone and luteinizing hormone and low estradiol levels
- Elevated D-dimer
Cotinine is a nicotine metabolite, and serum levels are elevated in smokers. Smoking and pregnancy both increase the risk of venous thromboembolism. However, smoking or pregnancy alone would not be a reason to increase the duration of anticoagulation.
Warfarin is contraindicated in pregnancy because of its teratogenic effects, particularly in the first trimester. Follicle-stimulating hormone and luteinizing hormone levels increase in response to decreased estradiol at menopause. Postmenopausal women are not at increased risk of venous thromboembolism unless they are taking oral estrogen hormone replacement therapy.
An elevated D-dimer level 1 month after stopping of oral anticoagulation has been associated with a higher rate of recurrence of venous thromboembolism, which is reduced by a resumption of anticoagulation.35 Therefore, consideration should be given to resuming anticoagulation in patients who have an elevated D-dimer level.
TAKE-HOME POINTS
It is important to distinguish between massive, submassive, and low-risk pulmonary embolism, since each type has a different treatment and prognosis.
Fibrinolytic therapy is indicated in patients with massive pulmonary embolism when no contraindication is present, whereas it is not indicated in those with low-risk pulmonary embolism.
Management of submassive pulmonary embolism continues to be an area of considerable debate. Current American Heart Association guidelines recommend consideration of fibrinolysis initially in patients with submassive acute pulmonary embolism if there is concurrent clinical evidence of adverse prognosis—eg, new hemodynamic instability, worsening respiratory insufficiency, severe right ventricular dysfunction, or major myocardial necrosis.23 On the other hand, the American College of Chest Physicians recommends against initial systemic fibrinolysis in submassive acute pulmonary embolism based on biomarkers or findings on ECG, transthoracic echocardiography, or CT, recommending it only in therapeutically anticoagulated patients deemed to be at high risk of hypotension.36
Since the optimal treatment of submassive pulmonary embolism is still not known, it is important that clinicians remain up to date on the evidence and guidelines.
- Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med 2007; 120:871–879.
- Stein PD, Saltzman HA, Weg JG. Clinical characteristics of patients with acute pulmonary embolism. Am J Cardiol 1991; 68:1723–1724.
- Ferrari E, Imbert A, Chevalier T, Mihoubi A, Morand P, Baudouy M. The ECG in pulmonary embolism. Predictive value of negative T waves in precordial leads—80 case reports. Chest 1997; 111:537–543.
- Geibel A, Zehender M, Kasper W, Olschewski M, Klima C, Konstantinides SV. Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J 2005; 25:843–848.
- Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med 2005; 352:1760–1768.
- Qanadli SD, Hajjam ME, Mesurolle B, et al. Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology 2000; 217:447–455.
- Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med 2001; 110:528–535.
- Bova C, Greco F, Misuraca G, et al. Diagnostic utility of echocardiography in patients with suspected pulmonary embolism. Am J Emerg Med 2003; 21:180–183.
- Stein PD, Chenevert TL, Fowler SE, et al; PIOPED III (Prospective Investigation of Pulmonary Embolism Diagnosis III) Investigators. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med 2010; 152:434–443,W142–W143.
- Sostman HD, Stein PD, Gottschalk A, Matta F, Hull R, Goodman L. Acute pulmonary embolism: sensitivity and specificity of ventilation-perfusion scintigraphy in PIOPED II study. Radiology 2008; 246:941–946.
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Otero R, Trujillo-Santos J, Cayuela A, et al; Registro Informatizado de la Enfermedad Tromboembólica (RIETE) Investigators. Haemodynamically unstable pulmonary embolism in the RIETE Registry: systolic blood pressure or shock index? Eur Respir J 2007; 30:1111–1116.
- Sam A, Sánchez D, Gómez V, et al. The shock index and the simplified PESI for identification of low-risk patients with acute pulmonary embolism. Eur Respir J 2011; 37:762–766.
- Kucher N, Luder CM, Dörnhöfer T, Windecker S, Meier B, Hess OM. Novel management strategy for patients with suspected pulmonary embolism. Eur Heart J 2003; 24:366–376.
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999; 353:1386–1389.
- ten Wolde M, Söhne M, Quak E, Mac Gillavry MR, Büller HR. Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med 2004; 164:1685–1689.
- Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J 2008; 29:1569–1577.
- Kucher N, Goldhaber SZ. Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism. Circulation 2003; 108:2191–2194.
- Scherz N, Labarère J, Méan M, Ibrahim SA, Fine MJ, Aujesky D. Prognostic importance of hyponatremia in patients with acute pulmonary embolism. Am J Respir Crit Care Med 2010; 182:1178–1183.
- Delèvaux I, André M, Aumaître O, Bègue RJ, Colombier M, Piette JC. Procalcitonin measurement for differential diagnosis between pulmonary embolism and pneumonia. Crit Care Med 2003; 31:661.
- Köktürk N, Kanbay A, Bukan N, Ekim N. The value of serum procalcitonin in differential diagnosis of pulmonary embolism and community-acquired pneumonia. Clin Appl Thromb Hemost 2011; 17:519–525.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Goldhaber SZ, Haire WD, Feldstein ML, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet 1993; 341:507–511.
- Kline JA, Steuerwald MT, Marchick MR, Hernandez-Nino J, Rose GA. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest 2009; 136:1202–1210.
- Steering Committee of PEITHO Investigators. Single-bolus tenecteplase plus heparin compared with heparin alone for normotensive patients with acute pulmonary embolism who have evidence of right ventricular dysfunction and myocardial injury: rationale and design of the Pulmonary Embolism Thrombolysis (PEITHO) trial. Am Heart J 2012; 163:33–38.e1.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Meneveau N, Séronde MF, Blonde MC, et al. Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest 2006; 129:1043–1050.
- Ho WK, Hankey GJ, Eikelboom JW. Should adult patients be routinely tested for heritable thrombophilia after an episode of venous thromboembolism? Med J Aust 2011; 195:139–142.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
- Cohn D, Vansenne F, de Borgie C, Middeldorp S. Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev 2009; ( 1):CD007069.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost 2008; 6:1474–1477.
- Palareti G, Cosmi B, Legnani C, et al; PROLONG Investigators. Ddimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006; 355:1780–1789.
- Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):7S–47S.
A 51-year-old woman presents to the emergency department with dyspnea, which began 4 days ago. She reports no chest pain, palpitations, hemoptysis, fevers, chills, weight loss, recent travel, immobility, or surgery. One week ago she noticed cramping in her right calf, but that has since resolved.
Her history includes hypertension, hypothyroidism, and immune-mediated glomerulonephritis with proteinuria. She is premenopausal. She takes losartan and levothyroxine; she is not taking oral contraceptives or herbal supplements. She is up to date with her cancer screening and has had negative findings on colonoscopy and mammography within the past year.
She has never smoked and she does not drink alcohol or use illicit drugs. Her mother has a history of provoked deep vein thrombosis and colon cancer.
Her temperature is 36.2°C (97.2°F), heart rate 163 beats per minute, blood pressure 158/102 mm Hg, respiratory rate 40 breaths per minute, and oxygen saturation by pulse oximetry 80% while breathing room air, corrected to 94% with oxygen 6 L/min via nasal cannula.
On physical examination, she is sitting upright on a stretcher and appears uncomfortable and anxious. She is awake and able to communicate clearly. Examination of the head, ears, eyes, nose, and throat is unremarkable, with moist mucous membranes. Her lungs are clear to auscultation. Her heart beat is very rapid, with a regular rhythm and an accentuated P2 heart sound. A right parasternal heave can be palpated in addition to a rightwardly displaced point of maximal impulse. The abdomen is normal, with no tenderness or organomegaly. She has no pain, edema, or erythema in the legs or feet, and she has strong, symmetric pulses (2+) in all extremities. The neurologic examination is nonfocal.
Electrocardiography (ECG) done on arrival (Figure 1) reveals supraventricular tachycardia, a normal axis, and nonspecific ST-segment and T-wave abnormalities, findings commonly seen in pulmonary embolism.1,2 On the other hand, her ECG does not show some of the other signs of right ventricular strain due to pulmonary embolism such as atrial arrhythmias, complete right bundle branch block, or inferior Q-waves.3,4
In view of her ECG findings and her symptoms of dyspnea, calf pain, tachypnea, tachycardia, and a pronounced P2 heart sound, her physician concludes that she very likely has a pulmonary embolism1 and orders an intravenous infusion of unfractionated heparin to be started immediately.
TESTING FOR PULMONARY EMBOLISM
1. Which of the following would be the best initial diagnostic imaging study to perform in this patient, who has a high pretest probability of pulmonary embolism?
- Multidetector computed tomographic (CT) pulmonary angiography
- Transthoracic echocardiography
- Magnetic resonance imaging
- Lower-extremity duplex ultrasonography
- Pulmonary angiography
- Ventilation-perfusion scintigraphy
Multidetector CT angiography is rapid, noninvasive, and highly sensitive (83%–90%) and specific (96%) for pulmonary embolism.5,6 In patients such as ours who have a high pretest probability of having the disease, its positive predictive value is 96%.5 Therefore, it would be the initial diagnostic study to perform in our patient.
Although transthoracic echocardiography is noninvasive and can detect right ventricular strain in the setting of pulmonary embolism, it may miss half of all pulmonary emboli detected by angiography.7,8
When technically adequate images are obtained, the combination of magnetic resonance angiography and magnetic resonance venography is very sensitive (92%) and specific (96%) for pulmonary embolism.9 However, one-fourth of patients undergoing these studies may have technically inadequate results, so this is not the best choice for diagnosis.9
As our patient complained of recent cramping in the right calf, lower-extremity duplex ultrasonography would be a reasonable test to screen for acute deep vein thrombosis as the source of pulmonary embolism. However, given her worrisome vital signs and impending hemodynamic collapse, CT pulmonary angiography would be a better initial test as it may guide more aggressive therapy. Furthermore, even if ultrasonography showed no evidence of deep vein thrombosis, clinical suspicion for pulmonary embolism would remain high enough that therapeutic anticoagulation would be continued until further testing ruled out this diagnosis.
Pulmonary angiography is the gold-standard test for pulmonary embolism. However, it is time-consuming, expensive, and invasive and so is not usually done unless the diagnosis cannot be made with other imaging studies.
Ventilation-perfusion scintigraphy is an established and safe diagnostic test for pulmonary embolism. It is particularly helpful in patients who have renal dysfunction or contrast allergy. The sensitivity of a high-probability scan is 78%, while the specificity of a very-low-probability scan is 97%.10 However, this study is often nondiagnostic (in 26.5% of cases),10 and further imaging may be required.
RESULTS OF CT ANGIOGRAPHY
Our patient undergoes CT angiography, which reveals multiple bilateral pulmonary emboli and right ventricular enlargement (Figure 2). Transthoracic echocardiography shows dilation of the right ventricle, with severely reduced systolic function, an underfilled and hyperdynamic left ventricle (ejection fraction 75%), and moderate tricuspid valve regurgitation. Her right ventricular systolic pressure is estimated to be 47 mm Hg.
Doppler ultrasonography of the legs reveals an occlusive thrombus within the right small saphenous vein that bulges and extends into the right popliteal vein. Also noted is a nonocclusive thrombus in the upper right popliteal vein that likely originated from the thrombus in the small saphenous vein.
Initial laboratory testing (Table 1) shows elevations of the cardiac enzymes troponin T and N-terminal pro-B-type natriuretic peptide (NT-pro-BNP).
ESTIMATING PROGNOSIS IN PULMONARY EMBOLISM
2. Which of the following laboratory results at presentation is independently associated with a worse outcome in patients with pulmonary embolism?
- Elevated NT-pro-BNP
- Hypercalcemia
- Thrombocytosis
- Hypernatremia
- Elevated procalcitonin
The Pulmonary Embolism Severity Index11 and the Simplified Pulmonary Embolism Severity Index12 (Table 2) are clinical calculators that help predict 30-day risk of death in patients with pulmonary embolism. Our patient’s Pulmonary Embolism Severity Index score is 60, indicating a very low risk, but her simplified severity index score is 2, indicating a high risk.
A shock index score (the heart rate divided by the systolic blood pressure) greater than 1 is also a sensitive measure of risk.13 (Our patient’s shock index score is 1.03.) Although the simplified version is more accurate,14 the shock index is also helpful when deciding whether patients with suspected pulmonary embolism should receive early fibrinolysis.15
In a large registry of patients with confirmed pulmonary embolism, risk factors for death were age greater than 70, cancer, clinical congestive heart failure, chronic obstructive pulmonary disease, systolic blood pressure lower than 90 mm Hg, respiratory rate less than 20 per minute, and right ventricular hypokinesis.16 Right ventricular dysfunction progressing to right ventricular failure and cardiogenic shock is the most common cause of death in patients with pulmonary embolism.16–18
Post hoc analysis has also shown that elevations of the biomarkers BNP, NT-pro-BNP, and cardiac troponins I and T are associated with a prolonged hospital course and a higher risk of death within 30 days.19 Interestingly, a recent retrospective analysis found hyponatremia to be an independent risk factor for death in the short term.20
Thrombocytopenia, not thrombocytosis, is associated with worse outcomes in patients with pulmonary embolism.16 Procalcitonin is elevated in bacterial pneumonia but is normal in pulmonary embolism and so may be helpful in differentiating between the two.21,22 Hypernatremia, hypercalcemia, and elevated procalcitonin have not been shown to be independently associated with worse outcomes in acute pulmonary embolism.
Thus, of the answer choices shown above, elevated NT-pro-BNP is the correct answer.
Classified as massive, submassive, or low-risk
Pulmonary embolism is often stratified as massive, submassive, or low-risk, reflecting the severity and the degree of cardiovascular collapse. The treatment depends on the classification.
Pulmonary embolism is classified as massive if the patient has a cardiac arrest or a systolic blood pressure lower than 90 mm Hg for more than 15 minutes.23 Nearly half of patients in this category die.24
Pulmonary embolism is submassive if the patient has systolic pressure greater than 90 mm Hg but has right ventricular dysfunction, as evidenced by physical examination, elevated cardiac biomarkers, electrocardiography, transthoracic echocardiography, or computed tomography. The death rate is as high as 15%.24
Pulmonary embolism in a normotensive patient with no right ventricular dysfunction is defined as low-risk.
Our patient so far
Our patient has bilateral pulmonary emboli, most likely originating from a deep vein thrombosis in her right lower leg. Her pulmonary embolism would be classified as submassive, as her systolic pressure is greater than 90 mm Hg and right ventricular dysfunction—significant right ventricular strain—was noted on both transthoracic echocardiography and computed tomography. Also, cardiac biomarkers are elevated, and the physical examination revealed a prominent P2 sound and right parasternal heave, also suggestive of right ventricular dysfunction.
Now, 6 hours have passed, and even though she has been receiving intravenous heparin during this time, her shock index remains greater than 1, indicating hemodynamic instability. Her pulse rate is still markedly high—over 160 bpm—and she still appears quite anxious and uncomfortable.
HOW SHOULD THIS PATIENT BE TREATED?
3. Which of the following is the most appropriate treatment for this patient?
- Start warfarin immediately while bridging with unfractionated heparin, low-molecular-weight heparin, or fondaparinux
- Start fibrinolysis with alteplase
- Give metoprolol intravenously to control her heart rate
- Start dabigatran immediately while bridging with unfractionated heparin
- Place an inferior vena cava filter
- Consult cardiothoracic surgery for emergency pulmonary embolectomy
All patients with confirmed pulmonary embolism and no contraindications to anticoagulation should begin treatment with low-molecular-weight heparin, unfractionated heparin, or fondaparinux.23 In addition, this therapy should be started empirically while the patient is still undergoing diagnostic testing if the pretest probability of pulmonary embolism is intermediate or high.23
Warfarin is indicated for all patients with pulmonary embolism who do not have contraindications to it (Table 3). If unfractionated heparin, low-molecular-weight heparin, or fondaparinux has not already been started, it should be started at the same time as warfarin and should be continued until the international normalized ratio (INR) is within the therapeutic range.
Fibrinolysis. Treatment with a fibrinolytic agent in addition to heparin results in faster improvement of right ventricular function and pulmonary perfusion than with heparin alone.25 It may also decrease the incidence of pulmonary hypertension secondary to chronic thromboembolic disease.26 It should be considered in patients with massive pulmonary embolism.23
Whether fibrinolysis is appropriate for all patients with submassive pulmonary embolism remains controversial. Currently, it is not recommended for minor right ventricular dysfunction or myocardial necrosis if the patient has no signs of overt clinical decline.23 The Pulmonary Embolism Thrombolysis trial27 is an ongoing prospective randomized comparison of tenecteplase in a single bolus plus heparin vs heparin alone in normotensive patients with submassive pulmonary embolism, such as our patient. This trial may elucidate the benefit of fibrinolytic therapy in patients with submassive pulmonary embolism.
Patients at low risk are generally treated with heparin and warfarin anticoagulation alone. Fibrinolysis is not recommended in these patients, as the risk of bleeding outweighs the potential benefits.23
Metoprolol may not be advisable for our patient, as her tachycardia is likely compensatory, and beta-blocker therapy could blunt this compensatory response, leading to inadequate systemic perfusion.
Dabigatran is an oral direct thrombin inhibitor that does not require laboratory monitoring. It is currently approved for the prevention of stroke in patients with atrial fibrillation. It has been shown to be as effective as warfarin in the treatment of acute venous thromboembolism28 and may be a viable option in the future, but as of this writing it has not yet been approved in the United States for this indication. Furthermore, dabigatran inhibits thrombin immediately, so continued heparin bridging would not be necessary.
An inferior vena cava filter may prevent recurrent pulmonary embolism for patients who have absolute contraindications to anticoagulation, most significantly in the short term, ie, in the first few weeks after placement. However, these devices have not yet been shown to improve long-term mortality rates.
Embolectomy, percutaneous or surgical, is also an option. For patients in whom thrombolytic therapy is not effective, “rescue” surgical embolectomy has been associated with better outcomes compared with secondary thrombolysis and so should be considered.29
Back to our patient
An intravenous infusion of alteplase is started, and the patient’s tachycardia improves. Her oxygen requirements normalize, and she is transferred to the general medical floor the next day. She receives subcutaneous dalteparin as a bridge therapy, and warfarin is titrated to a goal INR of 2.0 to 3.0. Because of the acute deep vein thrombosis in her right lower leg, she is instructed to wear knee-high fitted compression hose for primary prevention of postphlebitic syndrome.
HOW LONG TO TREAT? IS GENETIC TESTING INDICATED?
Patients with a first episode of unprovoked venous thromboembolism should receive oral anticoagulants for 6 months, while those with recurrent unprovoked venous thromboembolism require lifelong oral anticoagulation.23
Whether to test for inherited thrombophilia after a first episode of venous thromboembolism to guide the duration of anticoagulation is controversial.30 Indiscriminate testing has not been recommended in these patients,31 but the American College of Medical Genetics recommends genetic screening for factor V Leiden in patients who have an unprovoked incident of venous thromboembolism before age 50.32
No randomized controlled trial has assessed whether thrombophilia testing decreases the recurrence rate of venous thromboembolism.33 One uncontrolled study suggested that testing for inherited thrombophilias in patients with a first episode does not affect the risk of recurrence.34 Testing is costly and may cause psychological distress for patients and family members.
Our patient is discharged home on warfarin for 6 months with subsequent follow-up evaluation in the thrombophilia clinic.
WHEN SHOULD WARFARIN BE RESTARTED?
4. If our patient were to discontinue oral anticoagulation in 6 months, which of the following, if present 1 month afterwards, would be a reason to restart oral anticoagulation?
- Elevated serum cotinine
- Positive pregnancy test
- Elevated follicle-stimulating hormone and luteinizing hormone and low estradiol levels
- Elevated D-dimer
Cotinine is a nicotine metabolite, and serum levels are elevated in smokers. Smoking and pregnancy both increase the risk of venous thromboembolism. However, smoking or pregnancy alone would not be a reason to increase the duration of anticoagulation.
Warfarin is contraindicated in pregnancy because of its teratogenic effects, particularly in the first trimester. Follicle-stimulating hormone and luteinizing hormone levels increase in response to decreased estradiol at menopause. Postmenopausal women are not at increased risk of venous thromboembolism unless they are taking oral estrogen hormone replacement therapy.
An elevated D-dimer level 1 month after stopping of oral anticoagulation has been associated with a higher rate of recurrence of venous thromboembolism, which is reduced by a resumption of anticoagulation.35 Therefore, consideration should be given to resuming anticoagulation in patients who have an elevated D-dimer level.
TAKE-HOME POINTS
It is important to distinguish between massive, submassive, and low-risk pulmonary embolism, since each type has a different treatment and prognosis.
Fibrinolytic therapy is indicated in patients with massive pulmonary embolism when no contraindication is present, whereas it is not indicated in those with low-risk pulmonary embolism.
Management of submassive pulmonary embolism continues to be an area of considerable debate. Current American Heart Association guidelines recommend consideration of fibrinolysis initially in patients with submassive acute pulmonary embolism if there is concurrent clinical evidence of adverse prognosis—eg, new hemodynamic instability, worsening respiratory insufficiency, severe right ventricular dysfunction, or major myocardial necrosis.23 On the other hand, the American College of Chest Physicians recommends against initial systemic fibrinolysis in submassive acute pulmonary embolism based on biomarkers or findings on ECG, transthoracic echocardiography, or CT, recommending it only in therapeutically anticoagulated patients deemed to be at high risk of hypotension.36
Since the optimal treatment of submassive pulmonary embolism is still not known, it is important that clinicians remain up to date on the evidence and guidelines.
A 51-year-old woman presents to the emergency department with dyspnea, which began 4 days ago. She reports no chest pain, palpitations, hemoptysis, fevers, chills, weight loss, recent travel, immobility, or surgery. One week ago she noticed cramping in her right calf, but that has since resolved.
Her history includes hypertension, hypothyroidism, and immune-mediated glomerulonephritis with proteinuria. She is premenopausal. She takes losartan and levothyroxine; she is not taking oral contraceptives or herbal supplements. She is up to date with her cancer screening and has had negative findings on colonoscopy and mammography within the past year.
She has never smoked and she does not drink alcohol or use illicit drugs. Her mother has a history of provoked deep vein thrombosis and colon cancer.
Her temperature is 36.2°C (97.2°F), heart rate 163 beats per minute, blood pressure 158/102 mm Hg, respiratory rate 40 breaths per minute, and oxygen saturation by pulse oximetry 80% while breathing room air, corrected to 94% with oxygen 6 L/min via nasal cannula.
On physical examination, she is sitting upright on a stretcher and appears uncomfortable and anxious. She is awake and able to communicate clearly. Examination of the head, ears, eyes, nose, and throat is unremarkable, with moist mucous membranes. Her lungs are clear to auscultation. Her heart beat is very rapid, with a regular rhythm and an accentuated P2 heart sound. A right parasternal heave can be palpated in addition to a rightwardly displaced point of maximal impulse. The abdomen is normal, with no tenderness or organomegaly. She has no pain, edema, or erythema in the legs or feet, and she has strong, symmetric pulses (2+) in all extremities. The neurologic examination is nonfocal.
Electrocardiography (ECG) done on arrival (Figure 1) reveals supraventricular tachycardia, a normal axis, and nonspecific ST-segment and T-wave abnormalities, findings commonly seen in pulmonary embolism.1,2 On the other hand, her ECG does not show some of the other signs of right ventricular strain due to pulmonary embolism such as atrial arrhythmias, complete right bundle branch block, or inferior Q-waves.3,4
In view of her ECG findings and her symptoms of dyspnea, calf pain, tachypnea, tachycardia, and a pronounced P2 heart sound, her physician concludes that she very likely has a pulmonary embolism1 and orders an intravenous infusion of unfractionated heparin to be started immediately.
TESTING FOR PULMONARY EMBOLISM
1. Which of the following would be the best initial diagnostic imaging study to perform in this patient, who has a high pretest probability of pulmonary embolism?
- Multidetector computed tomographic (CT) pulmonary angiography
- Transthoracic echocardiography
- Magnetic resonance imaging
- Lower-extremity duplex ultrasonography
- Pulmonary angiography
- Ventilation-perfusion scintigraphy
Multidetector CT angiography is rapid, noninvasive, and highly sensitive (83%–90%) and specific (96%) for pulmonary embolism.5,6 In patients such as ours who have a high pretest probability of having the disease, its positive predictive value is 96%.5 Therefore, it would be the initial diagnostic study to perform in our patient.
Although transthoracic echocardiography is noninvasive and can detect right ventricular strain in the setting of pulmonary embolism, it may miss half of all pulmonary emboli detected by angiography.7,8
When technically adequate images are obtained, the combination of magnetic resonance angiography and magnetic resonance venography is very sensitive (92%) and specific (96%) for pulmonary embolism.9 However, one-fourth of patients undergoing these studies may have technically inadequate results, so this is not the best choice for diagnosis.9
As our patient complained of recent cramping in the right calf, lower-extremity duplex ultrasonography would be a reasonable test to screen for acute deep vein thrombosis as the source of pulmonary embolism. However, given her worrisome vital signs and impending hemodynamic collapse, CT pulmonary angiography would be a better initial test as it may guide more aggressive therapy. Furthermore, even if ultrasonography showed no evidence of deep vein thrombosis, clinical suspicion for pulmonary embolism would remain high enough that therapeutic anticoagulation would be continued until further testing ruled out this diagnosis.
Pulmonary angiography is the gold-standard test for pulmonary embolism. However, it is time-consuming, expensive, and invasive and so is not usually done unless the diagnosis cannot be made with other imaging studies.
Ventilation-perfusion scintigraphy is an established and safe diagnostic test for pulmonary embolism. It is particularly helpful in patients who have renal dysfunction or contrast allergy. The sensitivity of a high-probability scan is 78%, while the specificity of a very-low-probability scan is 97%.10 However, this study is often nondiagnostic (in 26.5% of cases),10 and further imaging may be required.
RESULTS OF CT ANGIOGRAPHY
Our patient undergoes CT angiography, which reveals multiple bilateral pulmonary emboli and right ventricular enlargement (Figure 2). Transthoracic echocardiography shows dilation of the right ventricle, with severely reduced systolic function, an underfilled and hyperdynamic left ventricle (ejection fraction 75%), and moderate tricuspid valve regurgitation. Her right ventricular systolic pressure is estimated to be 47 mm Hg.
Doppler ultrasonography of the legs reveals an occlusive thrombus within the right small saphenous vein that bulges and extends into the right popliteal vein. Also noted is a nonocclusive thrombus in the upper right popliteal vein that likely originated from the thrombus in the small saphenous vein.
Initial laboratory testing (Table 1) shows elevations of the cardiac enzymes troponin T and N-terminal pro-B-type natriuretic peptide (NT-pro-BNP).
ESTIMATING PROGNOSIS IN PULMONARY EMBOLISM
2. Which of the following laboratory results at presentation is independently associated with a worse outcome in patients with pulmonary embolism?
- Elevated NT-pro-BNP
- Hypercalcemia
- Thrombocytosis
- Hypernatremia
- Elevated procalcitonin
The Pulmonary Embolism Severity Index11 and the Simplified Pulmonary Embolism Severity Index12 (Table 2) are clinical calculators that help predict 30-day risk of death in patients with pulmonary embolism. Our patient’s Pulmonary Embolism Severity Index score is 60, indicating a very low risk, but her simplified severity index score is 2, indicating a high risk.
A shock index score (the heart rate divided by the systolic blood pressure) greater than 1 is also a sensitive measure of risk.13 (Our patient’s shock index score is 1.03.) Although the simplified version is more accurate,14 the shock index is also helpful when deciding whether patients with suspected pulmonary embolism should receive early fibrinolysis.15
In a large registry of patients with confirmed pulmonary embolism, risk factors for death were age greater than 70, cancer, clinical congestive heart failure, chronic obstructive pulmonary disease, systolic blood pressure lower than 90 mm Hg, respiratory rate less than 20 per minute, and right ventricular hypokinesis.16 Right ventricular dysfunction progressing to right ventricular failure and cardiogenic shock is the most common cause of death in patients with pulmonary embolism.16–18
Post hoc analysis has also shown that elevations of the biomarkers BNP, NT-pro-BNP, and cardiac troponins I and T are associated with a prolonged hospital course and a higher risk of death within 30 days.19 Interestingly, a recent retrospective analysis found hyponatremia to be an independent risk factor for death in the short term.20
Thrombocytopenia, not thrombocytosis, is associated with worse outcomes in patients with pulmonary embolism.16 Procalcitonin is elevated in bacterial pneumonia but is normal in pulmonary embolism and so may be helpful in differentiating between the two.21,22 Hypernatremia, hypercalcemia, and elevated procalcitonin have not been shown to be independently associated with worse outcomes in acute pulmonary embolism.
Thus, of the answer choices shown above, elevated NT-pro-BNP is the correct answer.
Classified as massive, submassive, or low-risk
Pulmonary embolism is often stratified as massive, submassive, or low-risk, reflecting the severity and the degree of cardiovascular collapse. The treatment depends on the classification.
Pulmonary embolism is classified as massive if the patient has a cardiac arrest or a systolic blood pressure lower than 90 mm Hg for more than 15 minutes.23 Nearly half of patients in this category die.24
Pulmonary embolism is submassive if the patient has systolic pressure greater than 90 mm Hg but has right ventricular dysfunction, as evidenced by physical examination, elevated cardiac biomarkers, electrocardiography, transthoracic echocardiography, or computed tomography. The death rate is as high as 15%.24
Pulmonary embolism in a normotensive patient with no right ventricular dysfunction is defined as low-risk.
Our patient so far
Our patient has bilateral pulmonary emboli, most likely originating from a deep vein thrombosis in her right lower leg. Her pulmonary embolism would be classified as submassive, as her systolic pressure is greater than 90 mm Hg and right ventricular dysfunction—significant right ventricular strain—was noted on both transthoracic echocardiography and computed tomography. Also, cardiac biomarkers are elevated, and the physical examination revealed a prominent P2 sound and right parasternal heave, also suggestive of right ventricular dysfunction.
Now, 6 hours have passed, and even though she has been receiving intravenous heparin during this time, her shock index remains greater than 1, indicating hemodynamic instability. Her pulse rate is still markedly high—over 160 bpm—and she still appears quite anxious and uncomfortable.
HOW SHOULD THIS PATIENT BE TREATED?
3. Which of the following is the most appropriate treatment for this patient?
- Start warfarin immediately while bridging with unfractionated heparin, low-molecular-weight heparin, or fondaparinux
- Start fibrinolysis with alteplase
- Give metoprolol intravenously to control her heart rate
- Start dabigatran immediately while bridging with unfractionated heparin
- Place an inferior vena cava filter
- Consult cardiothoracic surgery for emergency pulmonary embolectomy
All patients with confirmed pulmonary embolism and no contraindications to anticoagulation should begin treatment with low-molecular-weight heparin, unfractionated heparin, or fondaparinux.23 In addition, this therapy should be started empirically while the patient is still undergoing diagnostic testing if the pretest probability of pulmonary embolism is intermediate or high.23
Warfarin is indicated for all patients with pulmonary embolism who do not have contraindications to it (Table 3). If unfractionated heparin, low-molecular-weight heparin, or fondaparinux has not already been started, it should be started at the same time as warfarin and should be continued until the international normalized ratio (INR) is within the therapeutic range.
Fibrinolysis. Treatment with a fibrinolytic agent in addition to heparin results in faster improvement of right ventricular function and pulmonary perfusion than with heparin alone.25 It may also decrease the incidence of pulmonary hypertension secondary to chronic thromboembolic disease.26 It should be considered in patients with massive pulmonary embolism.23
Whether fibrinolysis is appropriate for all patients with submassive pulmonary embolism remains controversial. Currently, it is not recommended for minor right ventricular dysfunction or myocardial necrosis if the patient has no signs of overt clinical decline.23 The Pulmonary Embolism Thrombolysis trial27 is an ongoing prospective randomized comparison of tenecteplase in a single bolus plus heparin vs heparin alone in normotensive patients with submassive pulmonary embolism, such as our patient. This trial may elucidate the benefit of fibrinolytic therapy in patients with submassive pulmonary embolism.
Patients at low risk are generally treated with heparin and warfarin anticoagulation alone. Fibrinolysis is not recommended in these patients, as the risk of bleeding outweighs the potential benefits.23
Metoprolol may not be advisable for our patient, as her tachycardia is likely compensatory, and beta-blocker therapy could blunt this compensatory response, leading to inadequate systemic perfusion.
Dabigatran is an oral direct thrombin inhibitor that does not require laboratory monitoring. It is currently approved for the prevention of stroke in patients with atrial fibrillation. It has been shown to be as effective as warfarin in the treatment of acute venous thromboembolism28 and may be a viable option in the future, but as of this writing it has not yet been approved in the United States for this indication. Furthermore, dabigatran inhibits thrombin immediately, so continued heparin bridging would not be necessary.
An inferior vena cava filter may prevent recurrent pulmonary embolism for patients who have absolute contraindications to anticoagulation, most significantly in the short term, ie, in the first few weeks after placement. However, these devices have not yet been shown to improve long-term mortality rates.
Embolectomy, percutaneous or surgical, is also an option. For patients in whom thrombolytic therapy is not effective, “rescue” surgical embolectomy has been associated with better outcomes compared with secondary thrombolysis and so should be considered.29
Back to our patient
An intravenous infusion of alteplase is started, and the patient’s tachycardia improves. Her oxygen requirements normalize, and she is transferred to the general medical floor the next day. She receives subcutaneous dalteparin as a bridge therapy, and warfarin is titrated to a goal INR of 2.0 to 3.0. Because of the acute deep vein thrombosis in her right lower leg, she is instructed to wear knee-high fitted compression hose for primary prevention of postphlebitic syndrome.
HOW LONG TO TREAT? IS GENETIC TESTING INDICATED?
Patients with a first episode of unprovoked venous thromboembolism should receive oral anticoagulants for 6 months, while those with recurrent unprovoked venous thromboembolism require lifelong oral anticoagulation.23
Whether to test for inherited thrombophilia after a first episode of venous thromboembolism to guide the duration of anticoagulation is controversial.30 Indiscriminate testing has not been recommended in these patients,31 but the American College of Medical Genetics recommends genetic screening for factor V Leiden in patients who have an unprovoked incident of venous thromboembolism before age 50.32
No randomized controlled trial has assessed whether thrombophilia testing decreases the recurrence rate of venous thromboembolism.33 One uncontrolled study suggested that testing for inherited thrombophilias in patients with a first episode does not affect the risk of recurrence.34 Testing is costly and may cause psychological distress for patients and family members.
Our patient is discharged home on warfarin for 6 months with subsequent follow-up evaluation in the thrombophilia clinic.
WHEN SHOULD WARFARIN BE RESTARTED?
4. If our patient were to discontinue oral anticoagulation in 6 months, which of the following, if present 1 month afterwards, would be a reason to restart oral anticoagulation?
- Elevated serum cotinine
- Positive pregnancy test
- Elevated follicle-stimulating hormone and luteinizing hormone and low estradiol levels
- Elevated D-dimer
Cotinine is a nicotine metabolite, and serum levels are elevated in smokers. Smoking and pregnancy both increase the risk of venous thromboembolism. However, smoking or pregnancy alone would not be a reason to increase the duration of anticoagulation.
Warfarin is contraindicated in pregnancy because of its teratogenic effects, particularly in the first trimester. Follicle-stimulating hormone and luteinizing hormone levels increase in response to decreased estradiol at menopause. Postmenopausal women are not at increased risk of venous thromboembolism unless they are taking oral estrogen hormone replacement therapy.
An elevated D-dimer level 1 month after stopping of oral anticoagulation has been associated with a higher rate of recurrence of venous thromboembolism, which is reduced by a resumption of anticoagulation.35 Therefore, consideration should be given to resuming anticoagulation in patients who have an elevated D-dimer level.
TAKE-HOME POINTS
It is important to distinguish between massive, submassive, and low-risk pulmonary embolism, since each type has a different treatment and prognosis.
Fibrinolytic therapy is indicated in patients with massive pulmonary embolism when no contraindication is present, whereas it is not indicated in those with low-risk pulmonary embolism.
Management of submassive pulmonary embolism continues to be an area of considerable debate. Current American Heart Association guidelines recommend consideration of fibrinolysis initially in patients with submassive acute pulmonary embolism if there is concurrent clinical evidence of adverse prognosis—eg, new hemodynamic instability, worsening respiratory insufficiency, severe right ventricular dysfunction, or major myocardial necrosis.23 On the other hand, the American College of Chest Physicians recommends against initial systemic fibrinolysis in submassive acute pulmonary embolism based on biomarkers or findings on ECG, transthoracic echocardiography, or CT, recommending it only in therapeutically anticoagulated patients deemed to be at high risk of hypotension.36
Since the optimal treatment of submassive pulmonary embolism is still not known, it is important that clinicians remain up to date on the evidence and guidelines.
- Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med 2007; 120:871–879.
- Stein PD, Saltzman HA, Weg JG. Clinical characteristics of patients with acute pulmonary embolism. Am J Cardiol 1991; 68:1723–1724.
- Ferrari E, Imbert A, Chevalier T, Mihoubi A, Morand P, Baudouy M. The ECG in pulmonary embolism. Predictive value of negative T waves in precordial leads—80 case reports. Chest 1997; 111:537–543.
- Geibel A, Zehender M, Kasper W, Olschewski M, Klima C, Konstantinides SV. Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J 2005; 25:843–848.
- Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med 2005; 352:1760–1768.
- Qanadli SD, Hajjam ME, Mesurolle B, et al. Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology 2000; 217:447–455.
- Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med 2001; 110:528–535.
- Bova C, Greco F, Misuraca G, et al. Diagnostic utility of echocardiography in patients with suspected pulmonary embolism. Am J Emerg Med 2003; 21:180–183.
- Stein PD, Chenevert TL, Fowler SE, et al; PIOPED III (Prospective Investigation of Pulmonary Embolism Diagnosis III) Investigators. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med 2010; 152:434–443,W142–W143.
- Sostman HD, Stein PD, Gottschalk A, Matta F, Hull R, Goodman L. Acute pulmonary embolism: sensitivity and specificity of ventilation-perfusion scintigraphy in PIOPED II study. Radiology 2008; 246:941–946.
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Otero R, Trujillo-Santos J, Cayuela A, et al; Registro Informatizado de la Enfermedad Tromboembólica (RIETE) Investigators. Haemodynamically unstable pulmonary embolism in the RIETE Registry: systolic blood pressure or shock index? Eur Respir J 2007; 30:1111–1116.
- Sam A, Sánchez D, Gómez V, et al. The shock index and the simplified PESI for identification of low-risk patients with acute pulmonary embolism. Eur Respir J 2011; 37:762–766.
- Kucher N, Luder CM, Dörnhöfer T, Windecker S, Meier B, Hess OM. Novel management strategy for patients with suspected pulmonary embolism. Eur Heart J 2003; 24:366–376.
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999; 353:1386–1389.
- ten Wolde M, Söhne M, Quak E, Mac Gillavry MR, Büller HR. Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med 2004; 164:1685–1689.
- Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J 2008; 29:1569–1577.
- Kucher N, Goldhaber SZ. Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism. Circulation 2003; 108:2191–2194.
- Scherz N, Labarère J, Méan M, Ibrahim SA, Fine MJ, Aujesky D. Prognostic importance of hyponatremia in patients with acute pulmonary embolism. Am J Respir Crit Care Med 2010; 182:1178–1183.
- Delèvaux I, André M, Aumaître O, Bègue RJ, Colombier M, Piette JC. Procalcitonin measurement for differential diagnosis between pulmonary embolism and pneumonia. Crit Care Med 2003; 31:661.
- Köktürk N, Kanbay A, Bukan N, Ekim N. The value of serum procalcitonin in differential diagnosis of pulmonary embolism and community-acquired pneumonia. Clin Appl Thromb Hemost 2011; 17:519–525.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Goldhaber SZ, Haire WD, Feldstein ML, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet 1993; 341:507–511.
- Kline JA, Steuerwald MT, Marchick MR, Hernandez-Nino J, Rose GA. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest 2009; 136:1202–1210.
- Steering Committee of PEITHO Investigators. Single-bolus tenecteplase plus heparin compared with heparin alone for normotensive patients with acute pulmonary embolism who have evidence of right ventricular dysfunction and myocardial injury: rationale and design of the Pulmonary Embolism Thrombolysis (PEITHO) trial. Am Heart J 2012; 163:33–38.e1.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Meneveau N, Séronde MF, Blonde MC, et al. Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest 2006; 129:1043–1050.
- Ho WK, Hankey GJ, Eikelboom JW. Should adult patients be routinely tested for heritable thrombophilia after an episode of venous thromboembolism? Med J Aust 2011; 195:139–142.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
- Cohn D, Vansenne F, de Borgie C, Middeldorp S. Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev 2009; ( 1):CD007069.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost 2008; 6:1474–1477.
- Palareti G, Cosmi B, Legnani C, et al; PROLONG Investigators. Ddimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006; 355:1780–1789.
- Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):7S–47S.
- Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med 2007; 120:871–879.
- Stein PD, Saltzman HA, Weg JG. Clinical characteristics of patients with acute pulmonary embolism. Am J Cardiol 1991; 68:1723–1724.
- Ferrari E, Imbert A, Chevalier T, Mihoubi A, Morand P, Baudouy M. The ECG in pulmonary embolism. Predictive value of negative T waves in precordial leads—80 case reports. Chest 1997; 111:537–543.
- Geibel A, Zehender M, Kasper W, Olschewski M, Klima C, Konstantinides SV. Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J 2005; 25:843–848.
- Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med 2005; 352:1760–1768.
- Qanadli SD, Hajjam ME, Mesurolle B, et al. Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology 2000; 217:447–455.
- Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med 2001; 110:528–535.
- Bova C, Greco F, Misuraca G, et al. Diagnostic utility of echocardiography in patients with suspected pulmonary embolism. Am J Emerg Med 2003; 21:180–183.
- Stein PD, Chenevert TL, Fowler SE, et al; PIOPED III (Prospective Investigation of Pulmonary Embolism Diagnosis III) Investigators. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med 2010; 152:434–443,W142–W143.
- Sostman HD, Stein PD, Gottschalk A, Matta F, Hull R, Goodman L. Acute pulmonary embolism: sensitivity and specificity of ventilation-perfusion scintigraphy in PIOPED II study. Radiology 2008; 246:941–946.
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Otero R, Trujillo-Santos J, Cayuela A, et al; Registro Informatizado de la Enfermedad Tromboembólica (RIETE) Investigators. Haemodynamically unstable pulmonary embolism in the RIETE Registry: systolic blood pressure or shock index? Eur Respir J 2007; 30:1111–1116.
- Sam A, Sánchez D, Gómez V, et al. The shock index and the simplified PESI for identification of low-risk patients with acute pulmonary embolism. Eur Respir J 2011; 37:762–766.
- Kucher N, Luder CM, Dörnhöfer T, Windecker S, Meier B, Hess OM. Novel management strategy for patients with suspected pulmonary embolism. Eur Heart J 2003; 24:366–376.
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999; 353:1386–1389.
- ten Wolde M, Söhne M, Quak E, Mac Gillavry MR, Büller HR. Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med 2004; 164:1685–1689.
- Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J 2008; 29:1569–1577.
- Kucher N, Goldhaber SZ. Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism. Circulation 2003; 108:2191–2194.
- Scherz N, Labarère J, Méan M, Ibrahim SA, Fine MJ, Aujesky D. Prognostic importance of hyponatremia in patients with acute pulmonary embolism. Am J Respir Crit Care Med 2010; 182:1178–1183.
- Delèvaux I, André M, Aumaître O, Bègue RJ, Colombier M, Piette JC. Procalcitonin measurement for differential diagnosis between pulmonary embolism and pneumonia. Crit Care Med 2003; 31:661.
- Köktürk N, Kanbay A, Bukan N, Ekim N. The value of serum procalcitonin in differential diagnosis of pulmonary embolism and community-acquired pneumonia. Clin Appl Thromb Hemost 2011; 17:519–525.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Goldhaber SZ, Haire WD, Feldstein ML, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet 1993; 341:507–511.
- Kline JA, Steuerwald MT, Marchick MR, Hernandez-Nino J, Rose GA. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest 2009; 136:1202–1210.
- Steering Committee of PEITHO Investigators. Single-bolus tenecteplase plus heparin compared with heparin alone for normotensive patients with acute pulmonary embolism who have evidence of right ventricular dysfunction and myocardial injury: rationale and design of the Pulmonary Embolism Thrombolysis (PEITHO) trial. Am Heart J 2012; 163:33–38.e1.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Meneveau N, Séronde MF, Blonde MC, et al. Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest 2006; 129:1043–1050.
- Ho WK, Hankey GJ, Eikelboom JW. Should adult patients be routinely tested for heritable thrombophilia after an episode of venous thromboembolism? Med J Aust 2011; 195:139–142.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
- Cohn D, Vansenne F, de Borgie C, Middeldorp S. Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev 2009; ( 1):CD007069.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost 2008; 6:1474–1477.
- Palareti G, Cosmi B, Legnani C, et al; PROLONG Investigators. Ddimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006; 355:1780–1789.
- Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):7S–47S.
Woman With Hip Pain After Car Accident
ANSWER
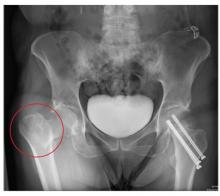
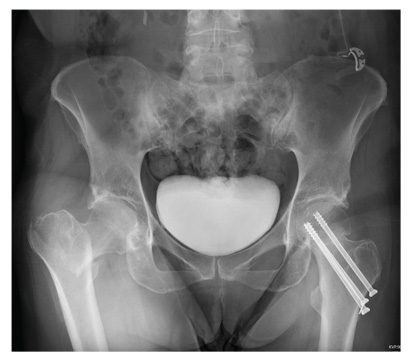
The radiograph demonstrates evidence of contrast material within the bladder. There is evidence of fixation of an old subcapital femoral neck fracture on the left.
There is an acute, mildly displaced right intertrochanteric fracture of the right hip. The orthopedic service was consulted, and plans were established to subsequently fix this fracture surgically.
ANSWER
The radiograph demonstrates evidence of contrast material within the bladder. There is evidence of fixation of an old subcapital femoral neck fracture on the left.
There is an acute, mildly displaced right intertrochanteric fracture of the right hip. The orthopedic service was consulted, and plans were established to subsequently fix this fracture surgically.
ANSWER
The radiograph demonstrates evidence of contrast material within the bladder. There is evidence of fixation of an old subcapital femoral neck fracture on the left.
There is an acute, mildly displaced right intertrochanteric fracture of the right hip. The orthopedic service was consulted, and plans were established to subsequently fix this fracture surgically.
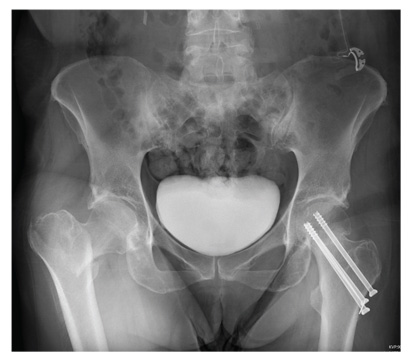
A 55-year-old woman is transferred to your facility with injuries sustained in a motor vehicle collision. She was an unrestrained front-seat passenger in a vehicle that rear-ended another vehicle. There was no airbag deployment, and the patient believes she struck her face on the windshield. At the outside facility, it was determined that she had a cervical fracture and facial fractures. Upon arrival at your facility, she is complaining of bilateral hip pain as well. Her medical history is significant for coronary artery disease, several myocardial infarctions, hypertension, and stroke. She has a pacemaker. Six months ago, she had an open reduction internal fixation of her left hip for a fracture she sustained in a fall. Primary survey reveals a female who is uncomfortable but alert and oriented. Vital signs are normal. She has some facial swelling and bruising. Her heart and lungs are clear; abdomen is benign. She is able to move her upper extremities with-out any problems. She has limited movement of her lower extremities due to pain in her pelvis. She is able to move both feet and toes, and distal pulses and sensation are intact. No obvious leg shortening is noted. A portable radiograph of the pelvis is obtained. What is your impression?
Child hit by car
ANSWER
The chest radiograph demonstrates no acute abnormalities within the lungs, ribs, or chest. Of note, there are two radiodensities consistent with teeth, which are presumed to be in the patient’s stomach (most likely secondary to being swallowed following trauma to his face). Upon reexamination, it is noted that the child’s two front incisors are missing, with minimally bleeding sockets. Other than reassurance, no specific intervention was required.
ANSWER
The chest radiograph demonstrates no acute abnormalities within the lungs, ribs, or chest. Of note, there are two radiodensities consistent with teeth, which are presumed to be in the patient’s stomach (most likely secondary to being swallowed following trauma to his face). Upon reexamination, it is noted that the child’s two front incisors are missing, with minimally bleeding sockets. Other than reassurance, no specific intervention was required.
ANSWER
The chest radiograph demonstrates no acute abnormalities within the lungs, ribs, or chest. Of note, there are two radiodensities consistent with teeth, which are presumed to be in the patient’s stomach (most likely secondary to being swallowed following trauma to his face). Upon reexamination, it is noted that the child’s two front incisors are missing, with minimally bleeding sockets. Other than reassurance, no specific intervention was required.
A 6-year-old boy is brought to your facility by ambulance after being hit by a car. The child was apparently riding his bike when a slow-moving vehicle turned onto the street and accidentally bumped him, knocking him to the ground. He was not wearing a helmet. The child is crying but somewhat consolable. His medical history is unremarkable. On initial assessment, he is awake, crying, and moving all of his extremities spontaneously. His vital signs include a temperature of 36.3°C; blood pressure, 149/72 mm Hg; pulse, 110 beats/min; and respiratory rate, 22 breaths/min. Physical examination reveals several abrasions to his face, nose, and lips. Otherwise, he is normocephalic. His pupils are equal and react appropriately. Heart and lung sounds are clear, and the abdomen appears benign. You order some preliminary labwork and CT of the head. In addition, a portable chest radiograph is obtained (shown). What is your impression?
Foot Pain Following a Car Crash
The radiograph demonstrates an acute fracture of the second, third, and fourth distal metatarsals. The third and fourth are mildly impacted.
In addition, there is a deformity noted within the medial cuneiform, strongly suggestive of a fracture. This was later confirmed by CT. Orthopedic consultation was obtained.
The radiograph demonstrates an acute fracture of the second, third, and fourth distal metatarsals. The third and fourth are mildly impacted.
In addition, there is a deformity noted within the medial cuneiform, strongly suggestive of a fracture. This was later confirmed by CT. Orthopedic consultation was obtained.
The radiograph demonstrates an acute fracture of the second, third, and fourth distal metatarsals. The third and fourth are mildly impacted.
In addition, there is a deformity noted within the medial cuneiform, strongly suggestive of a fracture. This was later confirmed by CT. Orthopedic consultation was obtained.
Following a motor vehicle collision, a 60-year-old woman is brought in by emergency medical transport. She was a restrained driver in a vehicle that went out of control, hit a tree, and ended up in a ditch. There was a prolonged extrication time (> 30 minutes) due to extensive damage to the front of the vehicle. On arrival, the patient is awake and alert, complaining primarily of pain in her left hip and right foot. Her medical history is unremarkable. She has an initial Glasgow Coma Scale score of 15. Her vital signs are: blood pressure, 154/100 mm Hg; pulse, 108 beats/min; respiratory rate, 16 breaths/min; and O2 saturation, 100% on room air. Primary survey is otherwise unremarkable. A series of radiographs are ordered; that of the right foot is shown. What is your impression?
Girl, 13, With a Bump on Her Leg
A girl, age 13 years, 4 months, presented to her primary care provider’s office for a well visit. Among her concerns, she mentioned a “bump” she had had on her right leg “for the past six months, maybe longer.” The area felt irritated when touched or when the patient “ran too much.” She had seen no change in the bump since she first noticed it. The patient knew of no trauma or other preceding factors. She denied any fever or warmth, redness, or ecchymosis to the area.
Medical history was unremarkable except for familial short stature and myopia. The patient was the fifth of eight children born to nonconsanguinous parents. She denied any surgical history or hospitalizations and was premenarcheal. She was up to date on all age-appropriate vaccines, with her meningococcal vaccine administered at that visit.
The patient’s blood pressure was 99/58 mm Hg with an apical pulse rate of 82 beats/min. Her growth parameters were following her curve. Her height was 55” (0.3 percentile); weight, 81 lb (7.5 percentile); and BMI, 18.8 (48.6 percentile).
The physical exam was normal with the exception of the musculoskeletal exam. Examination of the lower extremities revealed a palpable, 4 cm x 5 cm lesion at the right distal medial thigh just proximal to the knee. The lesion could not be visualized but on palpation was tender and firm. There was some question as to whether the lesion itself or inflamed soft tissue overlying the lesion was mobile. No overlying warmth, induration, erythema, or ecchymosis was noted.
Passive and active range of motion was intact at the hip and knee. No lesions to the upper extremities were evident, and no scoliosis was seen.
Blood work was done to rule out certain diagnoses. Results from a complete blood count with differential, lactate dehydrogenase (LDH), parathyroid hormone, lipid profiles, thyroid function, and a comprehensive metabolic profile were unremarkable. A low level of vitamin D 25-OH was detected: 21.7 ng/mL (normal range, 32 to 100 ng/mL).
Distal femur x-rays with posteroanterior, lateral, and oblique views were ordered. The imaging revealed a 3 cm x 3 cm lesion projecting from the “distal, somewhat medial” femur, which was diagnosed as a benign femoral osteochondroma. Significant inflammation to the surrounding soft-tissue structures was observed. A questionable old fracture of the osteochondroma was noted. The remaining bony structures and joints appeared normal.
An ultrasound of the lesion was also ordered to investigate soft-tissue swelling. This revealed a hypoechoic collar around the distal end of the osteochondroma, which could represent a fluid collection, hematoma from trauma, or bursitis. The soft tissues were deemed normal.
Because of the extent of inflammation, the radiologist recommended MRI without contrast to rule out bursitis or trauma to the osteochondroma.
DISCUSSION
Osteochondromas, which may be present in up to 3% of the general population, are the most common benign bone tumors.1-3 An osteochondroma is a cartilage-capped bony projection that arises on the external surface of the bone; it contains a marrow cavity that is continuous with the underlying bone.2,4 The majority of osteochondromas are solitary, accounting for perhaps 85% to 90% of all such lesions, and they are typically nonhereditary; the remaining 10% to 15% of osteochondromas are hereditary multiple osteochondromas or exostoses1,2 (see “Definition of Multiple Exostoses Syndrome”2,5,6,7).

Most lesions are painless and slow growing, and they usually occur in children and adolescents.2 They typically stop growing at skeletal maturity with the closure of the growth plates.3,8,9 There is no predilection for males or females in single lesions.2
Solitary osteochondromas typically appear in the lower extremities and at long tubular bone metaphyses,1-3,10 especially on the femur, humerus, tibia, spine, and hip. Any part of the skeleton can be affected, but 30% of lesions occur on the femur and 40% at either the proximal metaphysis of the tibia or the distal metaphysis of the femur.2,11
Most osteochondromas are asymptomatic and are found incidentally.1,3 However, some patients present with local pain as a result of irritation to adjacent structures, limitation of joint motion, growth disturbance, or fracture of the pedicle.3,4,9,11,12 A very small proportion of patients (no more than 1%) with solitary osteochondromas experience malignant transformation.2,3,6,7 No particular blood work is recommended for patients with solitary osteochondromas.2
Differential Diagnosis
In addition to osteochondromas, several other lesions should be considered in the patient with musculoskeletal lesions (see Table 15,6,13-19).
Cartilaginous tumors. Chondrosarcomas are malignant cartilaginous tumors.20-22 They commonly affect long bones, including the humerus and femur, and some flat bones, such as the pelvic bones.13,22 They are most commonly seen in adults, and have no predisposition by gender.13
Chondrosarcomas can be primary (ie, arising de novo) or secondary (developing on preexisting benign cartilaginous neoplasms, including osteochondromas). The majority of chondrosarcomas are slow growing, and they rarely metastasize. It is difficult to differentiate between a benign lesion (such as an osteochondroma) and a chondrosarcoma by either histology or radiology. However, reliable predictors for malignancy include size exceeding 5 cm and location in the axial skeleton.20
Bone tumors.Osteosarcomas are the most common malignant bone tumors in children and adolescents, with 400 to 560 US patients in this age-group diagnosed each year.14-16 Osteosarcomas are uncommon in children younger than 10; their incidence peaks during the early teenage years (median peak age, 16), then declines rapidly among older patients. They are more common in males than females.15
Osteosarcomas commonly develop during periods of rapid bone turnover, such as the adolescent growth spurt. Common sites include the distal femur, proximal humerus, and proximal tibia,15,16 particularly near the knee.13 Usually, osteosarcomas present with nonspecific symptoms, including strain-related pain of several months’ duration, which may disrupt sleep.16 Laboratory findings in affected patients may include elevations in LDH, alkaline phosphatase, and/or ESR.15,23
Physical exam reveals a visible or palpable mass in the affected area, along with decreased joint motion; localized warmth or erythema may also be present. Late signs of osteosarcoma include weight loss, general malaise, and fever. First-line imaging for the patient with a suspected osteosarcoma is x-ray, which will show ill-defined borders, osteoblastic and/or osteolytic features, and an associated soft tissue mass. Advanced imaging, such as MRI, is warranted.16
Ewing sarcoma, the second most common bone tumor in children and adolescents, is an aggressive form of childhood cancer.14,18 Approximately 25% of all Ewing sarcomas arise in soft tissues rather than bones.18 They are more common in whites than in other ethnic groups and have a slight male predominance.13,18 The median age at diagnosis is 15.13 The most common presenting symptoms are tumor related, such as pain or a noticeable mass. While x-rays are usually ordered first, MRI is preferred.18
Soft tissue tumors and masses.Rhabdomyosarcomas are malignancies that account for more than half of the soft tissue sarcomas in children and adolescents. Less than one-fifth of cases occur in the extremities, and most occur in children younger than 10. These lesions have a slight male predominance and are more common in whites than in other patients.14,17,24
Approximately 6% of childhood soft tissue tumors are adipose tissue tumors, which may be benign (eg, lipomas) or malignant (eg, liposarcomas). Lipomas in children account for nearly 4% of all soft tissue tumors and can be classified as superficial (which are often diagnosed clinically) or deep (frequently requiring imaging).25
Lymphomaaccounts for 7% of cancers in US children and adolescents and more than 25% of newly diagnosed cancers in patients between 15 and 19, making it the most common malignancy in adolescents and the third most common in children.26,27 Non-Hodgkin lymphoma is the fourth leading type of malignancy in US adolescents.27 Rarely, lymphomas present with primary event soft tissue involvement.28
Myositis ossificans (MO) is a rare benign disorder involving formation of heterotrophic bone in skeletal muscles and soft tissues.29 Though possible in patients of any age, MO is most commonly seen in adolescents and young adults. Often the result of soft tissue injury (in which case it is referred to as myositis ossificans circumscripta or traumatic), MO develops in areas that are exposed to trauma, such as the anterior thighs or arms. Lesions can be diagnosed via plain x-ray or CT, although MRI and ultrasound can also be useful evaluation tools.17,29,30
Because MO circumscripta typically presents as a painful soft tissue mass, it can be mistaken for a soft tissue sarcoma or an osteosarcoma; radiologic evaluation is required to make the proper diagnosis. Less common forms of MO are myositis ossificans progressiva and myositis ossificans without a history of trauma.29
Ollier diseaseis a rare, nonfamilial disorder characterized by multiple enchondromas (or enchondromatoses), which are distributed asymmetrically with areas of dysplastic cartilage. Enchondromas are benign cartilage tumors that frequently affect long tubular bones along the metaphyses in proximity to the growth plate. The enchondromas result in significant growth abnormalities. About one in 1 million people are diagnosed yearly.5,19 (Similarly, Maffucci syndrome is represented by multiple enchondromas in association with hemangiomas.5)
Ollier disease typically manifests during childhood5 with bone swelling, local pain, and palpable bony masses, which are often associated with bone deformities.19 Patients generally present with an asymmetric shortening of one extremity and the appearance of palpable bony masses on their fingers or toes, which may or may not be associated with pathologic fractures.5,19 In 20% to 50% of patients with Ollier disease, enchondromas are at risk for malignant transformation into chondrosarcomas.5
Vascular malformations. Certain abnormalities of vascular development cause birthmarks and abnormalities of varying degree in underlying tissues.31 They are usually present at birth and grow proportionally to the child’s growth.25,31 However, they can also be seen in later childhood and adolescence.
Radiologic Investigation
Plain radiography of the affected area is the first-line radiologic study to be performed.13 While most osteochondromas can be diagnosed by plain x-ray, cross-sectional imaging via CT or MRI is recommended in lesions with certain characteristics, such as a broad stalk or location in the axial skeleton. Because MRI involves no radiation exposure, it is a particularly good diagnostic tool for children.32
Ultrasound is a good imaging method for evaluating for complications of osteochondromas, including bursa formation or vascular compromise.32
Treatment and Management
Although usually asymptomatic, osteochondroma can trigger some significant symptoms. Osteochondromas are at risk for fracture and can cause body deformities, mechanical joint problems, weakness of the affected limb, numbness, vascular compression, aneurysm, arterial thrombosis, venous thrombosis, pain, acute ischemia, and nerve compression. Clinical signs of malignant transformation include pain, swelling, and increased lesion size.2
Surgical excision is recommended but should be delayed until after the patient has reached skeletal maturity in order to decrease the risk for recurrence.33
Patient Education and Follow-up
In addition to explaining appropriate pain management (eg, NSAIDs), it is especially important for the pediatric NP or PA to encourage the patient with a solitary osteochondroma to follow up with the pediatric orthopedic surgeon. Reasons include the need to monitor growth of the lesion (which is likely to continue in a patient who has not yet reached skeletal maturity) and assess for associated functional or joint problems. Patients should also be advised to seek the specialist’s attention if such problems develop or if pain increases.
Generally, the pediatric clinician should be sufficiently informed to answer questions about this condition from the patient or family. Any follow-up laboratory work recommended by the specialist can also be performed by the pediatric NP or PA.
OUTCOME FOR THE CASE PATIENT
MRI without contrast, as recommended by the radiologist to rule out a bursa or trauma to the osteochondroma, was considered an important part of the follow-up plan. As the patient had not yet reached skeletal maturity, she was referred to a pediatric orthopedic surgeon for possible excision of the lesion, due to its size and the pain associated with running or other exertion.
CONCLUSION
Solitary osteochondromas are the most common benign bone tumors. Although they are generally asymptomatic, pain and other symptoms can arise as a result of irritation to the adjacent structures. In this case, the patient’s chief complaint was an irritating “bump” that she had had on her right leg for at least six months.
Generally, follow-up monitoring of the osteochondroma and orthopedic follow-up care are warranted, at least until the patient reaches skeletal maturity. At that point, surgical excision of the lesion is recommended.
REFERENCES
1. Florez B, Mönckeberg J, Castillo G, Beguiristain J. Solitary osteochondroma long-term follow-up. J Pediatr Orthop B. 2008;17:91-94.
2. Kitsoulis P, Galani V, Stefanaki K, et al. Osteochondromas: review of the clinical, radiological and pathological features. In Vivo. 2008;22:633-646.
3. Ramos-Pascua LR, Sánchez-Herráez S, Alonso-Barrio JA, Alonso-León A. Solitary proximal end of femur osteochondroma: an indication and result of the en bloc resection without hip luxation [in Spanish]. Rev Esp Cir Ortop Traumatol. 2012;56:24-31.
4. Payne WT, Merrell G. Benign bony and soft tissue tumors of the hand. J Hand Surg. 2010;35:1901-1910.
5. Pannier S, Legeai-Mallet L. Hereditary multiple exostoses and enchondromatosis. Best Pract Res Clin Rheumatol. 2008;22:45-54.
6. Bovée JV. Multiple osteochondromas. Orphanet J Rare Dis. 2008;3(3).
7. Staals EL, Bacchini P, Mercuri M, Bertoni F. Dedifferentiated chondrosarcomas arising in preexisting osteochondromas. J Bone Joint Surg Am. 2007;89:987-993.
8. Singh R, Jain M, Siwach R, et al. Large para-articular osteochondroma of the knee joint: a case report. Acta Orthop Traumatol Turc. 2012;46:139-143.
9. Lee JY, Lee S, Joo KB, et al. Intraarticular osteochondroma of shoulder: a case report. Clin Imaging. 2013;37:379-381.
10. Kim Y-C, Ahn JH, Lee JW. Osteochondroma of the distal tibia complicated by a tibialis posterior tendon tear. J Foot Ankle Surg. 2012;51: 660-663.
11. Allagui M, Amara K, Aloui I, et al. Historical giant near-circumferential osteochondroma of the proximal humerus. J Shoulder Elbow Surg. 2010;19:e12-e15.
12. Li M, Luettringhaus T, Walker KR, Cole PA. Operative treatment of femoral neck osteochondroma through a digastric approach in a pediatric patient: a case report and review of the literature. J Pediatr Orthop B. 2012;21:230-234.
13. Hogendoorn PC, Athanasou N, Bielack S, et al; ESMO/EUROBONET Working Group. Bone sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 suppl 5:v204-v213.
14. Arndt CAS, Rose PS, Folpe AL, Laack NN. Common musculoskeletal tumors of childhood and adolescence. Mayo Clin Proc. 2012;87:475-487.
15. Ta HT, Dass CR, Choong PF, Dunstan DE. Osteosarcoma treatment: state of the art. Cancer Metastasis Rev. 2009;28:247-263.
16. Messerschmitt PJ, Garcia RM, Abdul-Karim FW, et al. Osteosarcoma. J Am Acad Orthop Surg. 2009;17:515-527.
17. Laffan EE, Ngan B-Y, Navarro OM. Pediatric soft-tissue tumors and pseudotumors: MR imaging features with pathologic correlation: Part 2. Tumors of fibroblastic/myofibroblastic, so-called fibrohistiocytic, muscular, lymphomatous, neurogenic, hair matrix, and uncertain origin. Radiographics. 2009;29:e36.
18. Balamuth NJ, Womer RB. Ewing’s sarcoma. Lancet Oncol. 2010;11(2):184.
19. D’Angelo L, Massimi L, Narducci A, Di Rocco C. Ollier disease. Childs Nerv Syst. 2009;25:647-653.
20. Gelderblom H, Hogendoorn PC, Dijkstra SD, et al. The clinical approach towards chondrosarcoma. Oncologist. 2008;13:320-329.
21. Nosratzehi T, Pakfetrat A. Chondrosarcoma. Zahedan J Res Med Sci. 2013;15:64-64.
22. Prado FO, Nishimoto IN, Perez DE, et al. Head and neck chondrosarcoma: analysis of 16 cases. Br J Oral Maxillofacial Surg. 2009;47:555-557.
23. Kim HJ, Chalmers PN, Morris CD. Pediatric osteogenic sarcoma. Curr Opin Pediatr. 2010;22:61-66.
24. Sultan I, Qaddoumi I, Yaser S, et al. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol. 2009;27:3391-3397.
25. Navarro OM, Laffan EE, Ngan B-Y. Pediatric soft-tissue tumors and pseudo-tumors: MR imaging features with pathologic correlation: Part 1. Imaging approach, pseudotumors, vascular lesions, and adipocytic tumors. Radiographics. 2009;29:887-906.
26. Gross TG, Termuhlen AM. Pediatric non-Hodgkin lymphoma. Curr Hematol Malig Rep. 2008;3:167-173.
27. Hochberg J, Waxman IM, Kelly KM, et al. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the science. Br J Haematol. 2009;144:24-40.
28. Derenzini E, Casadei B, Pellegrini C, et al. Non-Hodgkin lymphomas presenting as soft tissue masses: A single center experience and meta-analysis of the published series. Clin Lymphoma Myeloma Leuk. 2012 Dec 12. [Epub ahead of print]
29. Micheli A, Trapani S, Brizzi I, et al. Myositis ossificans circumscripta: a paediatric case and review of the literature. Eur J Pediatr. 2009;168:523-529.
30. McKenzie G, Raby N, Ritchie D. Non-neoplastic soft-tissue masses. Br J Radiol. 2009;82:775-785.
31. Buckmiller LM, Richter GT, Suen JY. Diagnosis and management of hemangiomas and vascular malformations of the head and neck. Oral Dis. 2010;16:405-418.
32. Khanna G, Bennett DL. Pediatric bone lesions: beyond the plain radiographic evaluation. Semin Roentgenol. 2012;47:90-99.
33. Rijal L, Nepal P, Baral S, et al. Solitary diaphyseal exostosis of femur, how common is it? Eur J Orthop Surg Traumatol. 2011;21:363-365.
A girl, age 13 years, 4 months, presented to her primary care provider’s office for a well visit. Among her concerns, she mentioned a “bump” she had had on her right leg “for the past six months, maybe longer.” The area felt irritated when touched or when the patient “ran too much.” She had seen no change in the bump since she first noticed it. The patient knew of no trauma or other preceding factors. She denied any fever or warmth, redness, or ecchymosis to the area.
Medical history was unremarkable except for familial short stature and myopia. The patient was the fifth of eight children born to nonconsanguinous parents. She denied any surgical history or hospitalizations and was premenarcheal. She was up to date on all age-appropriate vaccines, with her meningococcal vaccine administered at that visit.
The patient’s blood pressure was 99/58 mm Hg with an apical pulse rate of 82 beats/min. Her growth parameters were following her curve. Her height was 55” (0.3 percentile); weight, 81 lb (7.5 percentile); and BMI, 18.8 (48.6 percentile).
The physical exam was normal with the exception of the musculoskeletal exam. Examination of the lower extremities revealed a palpable, 4 cm x 5 cm lesion at the right distal medial thigh just proximal to the knee. The lesion could not be visualized but on palpation was tender and firm. There was some question as to whether the lesion itself or inflamed soft tissue overlying the lesion was mobile. No overlying warmth, induration, erythema, or ecchymosis was noted.
Passive and active range of motion was intact at the hip and knee. No lesions to the upper extremities were evident, and no scoliosis was seen.
Blood work was done to rule out certain diagnoses. Results from a complete blood count with differential, lactate dehydrogenase (LDH), parathyroid hormone, lipid profiles, thyroid function, and a comprehensive metabolic profile were unremarkable. A low level of vitamin D 25-OH was detected: 21.7 ng/mL (normal range, 32 to 100 ng/mL).
Distal femur x-rays with posteroanterior, lateral, and oblique views were ordered. The imaging revealed a 3 cm x 3 cm lesion projecting from the “distal, somewhat medial” femur, which was diagnosed as a benign femoral osteochondroma. Significant inflammation to the surrounding soft-tissue structures was observed. A questionable old fracture of the osteochondroma was noted. The remaining bony structures and joints appeared normal.
An ultrasound of the lesion was also ordered to investigate soft-tissue swelling. This revealed a hypoechoic collar around the distal end of the osteochondroma, which could represent a fluid collection, hematoma from trauma, or bursitis. The soft tissues were deemed normal.
Because of the extent of inflammation, the radiologist recommended MRI without contrast to rule out bursitis or trauma to the osteochondroma.
DISCUSSION
Osteochondromas, which may be present in up to 3% of the general population, are the most common benign bone tumors.1-3 An osteochondroma is a cartilage-capped bony projection that arises on the external surface of the bone; it contains a marrow cavity that is continuous with the underlying bone.2,4 The majority of osteochondromas are solitary, accounting for perhaps 85% to 90% of all such lesions, and they are typically nonhereditary; the remaining 10% to 15% of osteochondromas are hereditary multiple osteochondromas or exostoses1,2 (see “Definition of Multiple Exostoses Syndrome”2,5,6,7).

Most lesions are painless and slow growing, and they usually occur in children and adolescents.2 They typically stop growing at skeletal maturity with the closure of the growth plates.3,8,9 There is no predilection for males or females in single lesions.2
Solitary osteochondromas typically appear in the lower extremities and at long tubular bone metaphyses,1-3,10 especially on the femur, humerus, tibia, spine, and hip. Any part of the skeleton can be affected, but 30% of lesions occur on the femur and 40% at either the proximal metaphysis of the tibia or the distal metaphysis of the femur.2,11
Most osteochondromas are asymptomatic and are found incidentally.1,3 However, some patients present with local pain as a result of irritation to adjacent structures, limitation of joint motion, growth disturbance, or fracture of the pedicle.3,4,9,11,12 A very small proportion of patients (no more than 1%) with solitary osteochondromas experience malignant transformation.2,3,6,7 No particular blood work is recommended for patients with solitary osteochondromas.2
Differential Diagnosis
In addition to osteochondromas, several other lesions should be considered in the patient with musculoskeletal lesions (see Table 15,6,13-19).

Cartilaginous tumors. Chondrosarcomas are malignant cartilaginous tumors.20-22 They commonly affect long bones, including the humerus and femur, and some flat bones, such as the pelvic bones.13,22 They are most commonly seen in adults, and have no predisposition by gender.13
Chondrosarcomas can be primary (ie, arising de novo) or secondary (developing on preexisting benign cartilaginous neoplasms, including osteochondromas). The majority of chondrosarcomas are slow growing, and they rarely metastasize. It is difficult to differentiate between a benign lesion (such as an osteochondroma) and a chondrosarcoma by either histology or radiology. However, reliable predictors for malignancy include size exceeding 5 cm and location in the axial skeleton.20
Bone tumors.Osteosarcomas are the most common malignant bone tumors in children and adolescents, with 400 to 560 US patients in this age-group diagnosed each year.14-16 Osteosarcomas are uncommon in children younger than 10; their incidence peaks during the early teenage years (median peak age, 16), then declines rapidly among older patients. They are more common in males than females.15
Osteosarcomas commonly develop during periods of rapid bone turnover, such as the adolescent growth spurt. Common sites include the distal femur, proximal humerus, and proximal tibia,15,16 particularly near the knee.13 Usually, osteosarcomas present with nonspecific symptoms, including strain-related pain of several months’ duration, which may disrupt sleep.16 Laboratory findings in affected patients may include elevations in LDH, alkaline phosphatase, and/or ESR.15,23
Physical exam reveals a visible or palpable mass in the affected area, along with decreased joint motion; localized warmth or erythema may also be present. Late signs of osteosarcoma include weight loss, general malaise, and fever. First-line imaging for the patient with a suspected osteosarcoma is x-ray, which will show ill-defined borders, osteoblastic and/or osteolytic features, and an associated soft tissue mass. Advanced imaging, such as MRI, is warranted.16
Ewing sarcoma, the second most common bone tumor in children and adolescents, is an aggressive form of childhood cancer.14,18 Approximately 25% of all Ewing sarcomas arise in soft tissues rather than bones.18 They are more common in whites than in other ethnic groups and have a slight male predominance.13,18 The median age at diagnosis is 15.13 The most common presenting symptoms are tumor related, such as pain or a noticeable mass. While x-rays are usually ordered first, MRI is preferred.18
Soft tissue tumors and masses.Rhabdomyosarcomas are malignancies that account for more than half of the soft tissue sarcomas in children and adolescents. Less than one-fifth of cases occur in the extremities, and most occur in children younger than 10. These lesions have a slight male predominance and are more common in whites than in other patients.14,17,24
Approximately 6% of childhood soft tissue tumors are adipose tissue tumors, which may be benign (eg, lipomas) or malignant (eg, liposarcomas). Lipomas in children account for nearly 4% of all soft tissue tumors and can be classified as superficial (which are often diagnosed clinically) or deep (frequently requiring imaging).25
Lymphomaaccounts for 7% of cancers in US children and adolescents and more than 25% of newly diagnosed cancers in patients between 15 and 19, making it the most common malignancy in adolescents and the third most common in children.26,27 Non-Hodgkin lymphoma is the fourth leading type of malignancy in US adolescents.27 Rarely, lymphomas present with primary event soft tissue involvement.28
Myositis ossificans (MO) is a rare benign disorder involving formation of heterotrophic bone in skeletal muscles and soft tissues.29 Though possible in patients of any age, MO is most commonly seen in adolescents and young adults. Often the result of soft tissue injury (in which case it is referred to as myositis ossificans circumscripta or traumatic), MO develops in areas that are exposed to trauma, such as the anterior thighs or arms. Lesions can be diagnosed via plain x-ray or CT, although MRI and ultrasound can also be useful evaluation tools.17,29,30
Because MO circumscripta typically presents as a painful soft tissue mass, it can be mistaken for a soft tissue sarcoma or an osteosarcoma; radiologic evaluation is required to make the proper diagnosis. Less common forms of MO are myositis ossificans progressiva and myositis ossificans without a history of trauma.29
Ollier diseaseis a rare, nonfamilial disorder characterized by multiple enchondromas (or enchondromatoses), which are distributed asymmetrically with areas of dysplastic cartilage. Enchondromas are benign cartilage tumors that frequently affect long tubular bones along the metaphyses in proximity to the growth plate. The enchondromas result in significant growth abnormalities. About one in 1 million people are diagnosed yearly.5,19 (Similarly, Maffucci syndrome is represented by multiple enchondromas in association with hemangiomas.5)
Ollier disease typically manifests during childhood5 with bone swelling, local pain, and palpable bony masses, which are often associated with bone deformities.19 Patients generally present with an asymmetric shortening of one extremity and the appearance of palpable bony masses on their fingers or toes, which may or may not be associated with pathologic fractures.5,19 In 20% to 50% of patients with Ollier disease, enchondromas are at risk for malignant transformation into chondrosarcomas.5
Vascular malformations. Certain abnormalities of vascular development cause birthmarks and abnormalities of varying degree in underlying tissues.31 They are usually present at birth and grow proportionally to the child’s growth.25,31 However, they can also be seen in later childhood and adolescence.
Radiologic Investigation
Plain radiography of the affected area is the first-line radiologic study to be performed.13 While most osteochondromas can be diagnosed by plain x-ray, cross-sectional imaging via CT or MRI is recommended in lesions with certain characteristics, such as a broad stalk or location in the axial skeleton. Because MRI involves no radiation exposure, it is a particularly good diagnostic tool for children.32
Ultrasound is a good imaging method for evaluating for complications of osteochondromas, including bursa formation or vascular compromise.32
Treatment and Management
Although usually asymptomatic, osteochondroma can trigger some significant symptoms. Osteochondromas are at risk for fracture and can cause body deformities, mechanical joint problems, weakness of the affected limb, numbness, vascular compression, aneurysm, arterial thrombosis, venous thrombosis, pain, acute ischemia, and nerve compression. Clinical signs of malignant transformation include pain, swelling, and increased lesion size.2
Surgical excision is recommended but should be delayed until after the patient has reached skeletal maturity in order to decrease the risk for recurrence.33
Patient Education and Follow-up
In addition to explaining appropriate pain management (eg, NSAIDs), it is especially important for the pediatric NP or PA to encourage the patient with a solitary osteochondroma to follow up with the pediatric orthopedic surgeon. Reasons include the need to monitor growth of the lesion (which is likely to continue in a patient who has not yet reached skeletal maturity) and assess for associated functional or joint problems. Patients should also be advised to seek the specialist’s attention if such problems develop or if pain increases.
Generally, the pediatric clinician should be sufficiently informed to answer questions about this condition from the patient or family. Any follow-up laboratory work recommended by the specialist can also be performed by the pediatric NP or PA.
OUTCOME FOR THE CASE PATIENT
MRI without contrast, as recommended by the radiologist to rule out a bursa or trauma to the osteochondroma, was considered an important part of the follow-up plan. As the patient had not yet reached skeletal maturity, she was referred to a pediatric orthopedic surgeon for possible excision of the lesion, due to its size and the pain associated with running or other exertion.
CONCLUSION
Solitary osteochondromas are the most common benign bone tumors. Although they are generally asymptomatic, pain and other symptoms can arise as a result of irritation to the adjacent structures. In this case, the patient’s chief complaint was an irritating “bump” that she had had on her right leg for at least six months.
Generally, follow-up monitoring of the osteochondroma and orthopedic follow-up care are warranted, at least until the patient reaches skeletal maturity. At that point, surgical excision of the lesion is recommended.
REFERENCES
1. Florez B, Mönckeberg J, Castillo G, Beguiristain J. Solitary osteochondroma long-term follow-up. J Pediatr Orthop B. 2008;17:91-94.
2. Kitsoulis P, Galani V, Stefanaki K, et al. Osteochondromas: review of the clinical, radiological and pathological features. In Vivo. 2008;22:633-646.
3. Ramos-Pascua LR, Sánchez-Herráez S, Alonso-Barrio JA, Alonso-León A. Solitary proximal end of femur osteochondroma: an indication and result of the en bloc resection without hip luxation [in Spanish]. Rev Esp Cir Ortop Traumatol. 2012;56:24-31.
4. Payne WT, Merrell G. Benign bony and soft tissue tumors of the hand. J Hand Surg. 2010;35:1901-1910.
5. Pannier S, Legeai-Mallet L. Hereditary multiple exostoses and enchondromatosis. Best Pract Res Clin Rheumatol. 2008;22:45-54.
6. Bovée JV. Multiple osteochondromas. Orphanet J Rare Dis. 2008;3(3).
7. Staals EL, Bacchini P, Mercuri M, Bertoni F. Dedifferentiated chondrosarcomas arising in preexisting osteochondromas. J Bone Joint Surg Am. 2007;89:987-993.
8. Singh R, Jain M, Siwach R, et al. Large para-articular osteochondroma of the knee joint: a case report. Acta Orthop Traumatol Turc. 2012;46:139-143.
9. Lee JY, Lee S, Joo KB, et al. Intraarticular osteochondroma of shoulder: a case report. Clin Imaging. 2013;37:379-381.
10. Kim Y-C, Ahn JH, Lee JW. Osteochondroma of the distal tibia complicated by a tibialis posterior tendon tear. J Foot Ankle Surg. 2012;51: 660-663.
11. Allagui M, Amara K, Aloui I, et al. Historical giant near-circumferential osteochondroma of the proximal humerus. J Shoulder Elbow Surg. 2010;19:e12-e15.
12. Li M, Luettringhaus T, Walker KR, Cole PA. Operative treatment of femoral neck osteochondroma through a digastric approach in a pediatric patient: a case report and review of the literature. J Pediatr Orthop B. 2012;21:230-234.
13. Hogendoorn PC, Athanasou N, Bielack S, et al; ESMO/EUROBONET Working Group. Bone sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 suppl 5:v204-v213.
14. Arndt CAS, Rose PS, Folpe AL, Laack NN. Common musculoskeletal tumors of childhood and adolescence. Mayo Clin Proc. 2012;87:475-487.
15. Ta HT, Dass CR, Choong PF, Dunstan DE. Osteosarcoma treatment: state of the art. Cancer Metastasis Rev. 2009;28:247-263.
16. Messerschmitt PJ, Garcia RM, Abdul-Karim FW, et al. Osteosarcoma. J Am Acad Orthop Surg. 2009;17:515-527.
17. Laffan EE, Ngan B-Y, Navarro OM. Pediatric soft-tissue tumors and pseudotumors: MR imaging features with pathologic correlation: Part 2. Tumors of fibroblastic/myofibroblastic, so-called fibrohistiocytic, muscular, lymphomatous, neurogenic, hair matrix, and uncertain origin. Radiographics. 2009;29:e36.
18. Balamuth NJ, Womer RB. Ewing’s sarcoma. Lancet Oncol. 2010;11(2):184.
19. D’Angelo L, Massimi L, Narducci A, Di Rocco C. Ollier disease. Childs Nerv Syst. 2009;25:647-653.
20. Gelderblom H, Hogendoorn PC, Dijkstra SD, et al. The clinical approach towards chondrosarcoma. Oncologist. 2008;13:320-329.
21. Nosratzehi T, Pakfetrat A. Chondrosarcoma. Zahedan J Res Med Sci. 2013;15:64-64.
22. Prado FO, Nishimoto IN, Perez DE, et al. Head and neck chondrosarcoma: analysis of 16 cases. Br J Oral Maxillofacial Surg. 2009;47:555-557.
23. Kim HJ, Chalmers PN, Morris CD. Pediatric osteogenic sarcoma. Curr Opin Pediatr. 2010;22:61-66.
24. Sultan I, Qaddoumi I, Yaser S, et al. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol. 2009;27:3391-3397.
25. Navarro OM, Laffan EE, Ngan B-Y. Pediatric soft-tissue tumors and pseudo-tumors: MR imaging features with pathologic correlation: Part 1. Imaging approach, pseudotumors, vascular lesions, and adipocytic tumors. Radiographics. 2009;29:887-906.
26. Gross TG, Termuhlen AM. Pediatric non-Hodgkin lymphoma. Curr Hematol Malig Rep. 2008;3:167-173.
27. Hochberg J, Waxman IM, Kelly KM, et al. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the science. Br J Haematol. 2009;144:24-40.
28. Derenzini E, Casadei B, Pellegrini C, et al. Non-Hodgkin lymphomas presenting as soft tissue masses: A single center experience and meta-analysis of the published series. Clin Lymphoma Myeloma Leuk. 2012 Dec 12. [Epub ahead of print]
29. Micheli A, Trapani S, Brizzi I, et al. Myositis ossificans circumscripta: a paediatric case and review of the literature. Eur J Pediatr. 2009;168:523-529.
30. McKenzie G, Raby N, Ritchie D. Non-neoplastic soft-tissue masses. Br J Radiol. 2009;82:775-785.
31. Buckmiller LM, Richter GT, Suen JY. Diagnosis and management of hemangiomas and vascular malformations of the head and neck. Oral Dis. 2010;16:405-418.
32. Khanna G, Bennett DL. Pediatric bone lesions: beyond the plain radiographic evaluation. Semin Roentgenol. 2012;47:90-99.
33. Rijal L, Nepal P, Baral S, et al. Solitary diaphyseal exostosis of femur, how common is it? Eur J Orthop Surg Traumatol. 2011;21:363-365.
A girl, age 13 years, 4 months, presented to her primary care provider’s office for a well visit. Among her concerns, she mentioned a “bump” she had had on her right leg “for the past six months, maybe longer.” The area felt irritated when touched or when the patient “ran too much.” She had seen no change in the bump since she first noticed it. The patient knew of no trauma or other preceding factors. She denied any fever or warmth, redness, or ecchymosis to the area.
Medical history was unremarkable except for familial short stature and myopia. The patient was the fifth of eight children born to nonconsanguinous parents. She denied any surgical history or hospitalizations and was premenarcheal. She was up to date on all age-appropriate vaccines, with her meningococcal vaccine administered at that visit.
The patient’s blood pressure was 99/58 mm Hg with an apical pulse rate of 82 beats/min. Her growth parameters were following her curve. Her height was 55” (0.3 percentile); weight, 81 lb (7.5 percentile); and BMI, 18.8 (48.6 percentile).
The physical exam was normal with the exception of the musculoskeletal exam. Examination of the lower extremities revealed a palpable, 4 cm x 5 cm lesion at the right distal medial thigh just proximal to the knee. The lesion could not be visualized but on palpation was tender and firm. There was some question as to whether the lesion itself or inflamed soft tissue overlying the lesion was mobile. No overlying warmth, induration, erythema, or ecchymosis was noted.
Passive and active range of motion was intact at the hip and knee. No lesions to the upper extremities were evident, and no scoliosis was seen.
Blood work was done to rule out certain diagnoses. Results from a complete blood count with differential, lactate dehydrogenase (LDH), parathyroid hormone, lipid profiles, thyroid function, and a comprehensive metabolic profile were unremarkable. A low level of vitamin D 25-OH was detected: 21.7 ng/mL (normal range, 32 to 100 ng/mL).
Distal femur x-rays with posteroanterior, lateral, and oblique views were ordered. The imaging revealed a 3 cm x 3 cm lesion projecting from the “distal, somewhat medial” femur, which was diagnosed as a benign femoral osteochondroma. Significant inflammation to the surrounding soft-tissue structures was observed. A questionable old fracture of the osteochondroma was noted. The remaining bony structures and joints appeared normal.
An ultrasound of the lesion was also ordered to investigate soft-tissue swelling. This revealed a hypoechoic collar around the distal end of the osteochondroma, which could represent a fluid collection, hematoma from trauma, or bursitis. The soft tissues were deemed normal.
Because of the extent of inflammation, the radiologist recommended MRI without contrast to rule out bursitis or trauma to the osteochondroma.
DISCUSSION
Osteochondromas, which may be present in up to 3% of the general population, are the most common benign bone tumors.1-3 An osteochondroma is a cartilage-capped bony projection that arises on the external surface of the bone; it contains a marrow cavity that is continuous with the underlying bone.2,4 The majority of osteochondromas are solitary, accounting for perhaps 85% to 90% of all such lesions, and they are typically nonhereditary; the remaining 10% to 15% of osteochondromas are hereditary multiple osteochondromas or exostoses1,2 (see “Definition of Multiple Exostoses Syndrome”2,5,6,7).

Most lesions are painless and slow growing, and they usually occur in children and adolescents.2 They typically stop growing at skeletal maturity with the closure of the growth plates.3,8,9 There is no predilection for males or females in single lesions.2
Solitary osteochondromas typically appear in the lower extremities and at long tubular bone metaphyses,1-3,10 especially on the femur, humerus, tibia, spine, and hip. Any part of the skeleton can be affected, but 30% of lesions occur on the femur and 40% at either the proximal metaphysis of the tibia or the distal metaphysis of the femur.2,11
Most osteochondromas are asymptomatic and are found incidentally.1,3 However, some patients present with local pain as a result of irritation to adjacent structures, limitation of joint motion, growth disturbance, or fracture of the pedicle.3,4,9,11,12 A very small proportion of patients (no more than 1%) with solitary osteochondromas experience malignant transformation.2,3,6,7 No particular blood work is recommended for patients with solitary osteochondromas.2
Differential Diagnosis
In addition to osteochondromas, several other lesions should be considered in the patient with musculoskeletal lesions (see Table 15,6,13-19).

Cartilaginous tumors. Chondrosarcomas are malignant cartilaginous tumors.20-22 They commonly affect long bones, including the humerus and femur, and some flat bones, such as the pelvic bones.13,22 They are most commonly seen in adults, and have no predisposition by gender.13
Chondrosarcomas can be primary (ie, arising de novo) or secondary (developing on preexisting benign cartilaginous neoplasms, including osteochondromas). The majority of chondrosarcomas are slow growing, and they rarely metastasize. It is difficult to differentiate between a benign lesion (such as an osteochondroma) and a chondrosarcoma by either histology or radiology. However, reliable predictors for malignancy include size exceeding 5 cm and location in the axial skeleton.20
Bone tumors.Osteosarcomas are the most common malignant bone tumors in children and adolescents, with 400 to 560 US patients in this age-group diagnosed each year.14-16 Osteosarcomas are uncommon in children younger than 10; their incidence peaks during the early teenage years (median peak age, 16), then declines rapidly among older patients. They are more common in males than females.15
Osteosarcomas commonly develop during periods of rapid bone turnover, such as the adolescent growth spurt. Common sites include the distal femur, proximal humerus, and proximal tibia,15,16 particularly near the knee.13 Usually, osteosarcomas present with nonspecific symptoms, including strain-related pain of several months’ duration, which may disrupt sleep.16 Laboratory findings in affected patients may include elevations in LDH, alkaline phosphatase, and/or ESR.15,23
Physical exam reveals a visible or palpable mass in the affected area, along with decreased joint motion; localized warmth or erythema may also be present. Late signs of osteosarcoma include weight loss, general malaise, and fever. First-line imaging for the patient with a suspected osteosarcoma is x-ray, which will show ill-defined borders, osteoblastic and/or osteolytic features, and an associated soft tissue mass. Advanced imaging, such as MRI, is warranted.16
Ewing sarcoma, the second most common bone tumor in children and adolescents, is an aggressive form of childhood cancer.14,18 Approximately 25% of all Ewing sarcomas arise in soft tissues rather than bones.18 They are more common in whites than in other ethnic groups and have a slight male predominance.13,18 The median age at diagnosis is 15.13 The most common presenting symptoms are tumor related, such as pain or a noticeable mass. While x-rays are usually ordered first, MRI is preferred.18
Soft tissue tumors and masses.Rhabdomyosarcomas are malignancies that account for more than half of the soft tissue sarcomas in children and adolescents. Less than one-fifth of cases occur in the extremities, and most occur in children younger than 10. These lesions have a slight male predominance and are more common in whites than in other patients.14,17,24
Approximately 6% of childhood soft tissue tumors are adipose tissue tumors, which may be benign (eg, lipomas) or malignant (eg, liposarcomas). Lipomas in children account for nearly 4% of all soft tissue tumors and can be classified as superficial (which are often diagnosed clinically) or deep (frequently requiring imaging).25
Lymphomaaccounts for 7% of cancers in US children and adolescents and more than 25% of newly diagnosed cancers in patients between 15 and 19, making it the most common malignancy in adolescents and the third most common in children.26,27 Non-Hodgkin lymphoma is the fourth leading type of malignancy in US adolescents.27 Rarely, lymphomas present with primary event soft tissue involvement.28
Myositis ossificans (MO) is a rare benign disorder involving formation of heterotrophic bone in skeletal muscles and soft tissues.29 Though possible in patients of any age, MO is most commonly seen in adolescents and young adults. Often the result of soft tissue injury (in which case it is referred to as myositis ossificans circumscripta or traumatic), MO develops in areas that are exposed to trauma, such as the anterior thighs or arms. Lesions can be diagnosed via plain x-ray or CT, although MRI and ultrasound can also be useful evaluation tools.17,29,30
Because MO circumscripta typically presents as a painful soft tissue mass, it can be mistaken for a soft tissue sarcoma or an osteosarcoma; radiologic evaluation is required to make the proper diagnosis. Less common forms of MO are myositis ossificans progressiva and myositis ossificans without a history of trauma.29
Ollier diseaseis a rare, nonfamilial disorder characterized by multiple enchondromas (or enchondromatoses), which are distributed asymmetrically with areas of dysplastic cartilage. Enchondromas are benign cartilage tumors that frequently affect long tubular bones along the metaphyses in proximity to the growth plate. The enchondromas result in significant growth abnormalities. About one in 1 million people are diagnosed yearly.5,19 (Similarly, Maffucci syndrome is represented by multiple enchondromas in association with hemangiomas.5)
Ollier disease typically manifests during childhood5 with bone swelling, local pain, and palpable bony masses, which are often associated with bone deformities.19 Patients generally present with an asymmetric shortening of one extremity and the appearance of palpable bony masses on their fingers or toes, which may or may not be associated with pathologic fractures.5,19 In 20% to 50% of patients with Ollier disease, enchondromas are at risk for malignant transformation into chondrosarcomas.5
Vascular malformations. Certain abnormalities of vascular development cause birthmarks and abnormalities of varying degree in underlying tissues.31 They are usually present at birth and grow proportionally to the child’s growth.25,31 However, they can also be seen in later childhood and adolescence.
Radiologic Investigation
Plain radiography of the affected area is the first-line radiologic study to be performed.13 While most osteochondromas can be diagnosed by plain x-ray, cross-sectional imaging via CT or MRI is recommended in lesions with certain characteristics, such as a broad stalk or location in the axial skeleton. Because MRI involves no radiation exposure, it is a particularly good diagnostic tool for children.32
Ultrasound is a good imaging method for evaluating for complications of osteochondromas, including bursa formation or vascular compromise.32
Treatment and Management
Although usually asymptomatic, osteochondroma can trigger some significant symptoms. Osteochondromas are at risk for fracture and can cause body deformities, mechanical joint problems, weakness of the affected limb, numbness, vascular compression, aneurysm, arterial thrombosis, venous thrombosis, pain, acute ischemia, and nerve compression. Clinical signs of malignant transformation include pain, swelling, and increased lesion size.2
Surgical excision is recommended but should be delayed until after the patient has reached skeletal maturity in order to decrease the risk for recurrence.33
Patient Education and Follow-up
In addition to explaining appropriate pain management (eg, NSAIDs), it is especially important for the pediatric NP or PA to encourage the patient with a solitary osteochondroma to follow up with the pediatric orthopedic surgeon. Reasons include the need to monitor growth of the lesion (which is likely to continue in a patient who has not yet reached skeletal maturity) and assess for associated functional or joint problems. Patients should also be advised to seek the specialist’s attention if such problems develop or if pain increases.
Generally, the pediatric clinician should be sufficiently informed to answer questions about this condition from the patient or family. Any follow-up laboratory work recommended by the specialist can also be performed by the pediatric NP or PA.
OUTCOME FOR THE CASE PATIENT
MRI without contrast, as recommended by the radiologist to rule out a bursa or trauma to the osteochondroma, was considered an important part of the follow-up plan. As the patient had not yet reached skeletal maturity, she was referred to a pediatric orthopedic surgeon for possible excision of the lesion, due to its size and the pain associated with running or other exertion.
CONCLUSION
Solitary osteochondromas are the most common benign bone tumors. Although they are generally asymptomatic, pain and other symptoms can arise as a result of irritation to the adjacent structures. In this case, the patient’s chief complaint was an irritating “bump” that she had had on her right leg for at least six months.
Generally, follow-up monitoring of the osteochondroma and orthopedic follow-up care are warranted, at least until the patient reaches skeletal maturity. At that point, surgical excision of the lesion is recommended.
REFERENCES
1. Florez B, Mönckeberg J, Castillo G, Beguiristain J. Solitary osteochondroma long-term follow-up. J Pediatr Orthop B. 2008;17:91-94.
2. Kitsoulis P, Galani V, Stefanaki K, et al. Osteochondromas: review of the clinical, radiological and pathological features. In Vivo. 2008;22:633-646.
3. Ramos-Pascua LR, Sánchez-Herráez S, Alonso-Barrio JA, Alonso-León A. Solitary proximal end of femur osteochondroma: an indication and result of the en bloc resection without hip luxation [in Spanish]. Rev Esp Cir Ortop Traumatol. 2012;56:24-31.
4. Payne WT, Merrell G. Benign bony and soft tissue tumors of the hand. J Hand Surg. 2010;35:1901-1910.
5. Pannier S, Legeai-Mallet L. Hereditary multiple exostoses and enchondromatosis. Best Pract Res Clin Rheumatol. 2008;22:45-54.
6. Bovée JV. Multiple osteochondromas. Orphanet J Rare Dis. 2008;3(3).
7. Staals EL, Bacchini P, Mercuri M, Bertoni F. Dedifferentiated chondrosarcomas arising in preexisting osteochondromas. J Bone Joint Surg Am. 2007;89:987-993.
8. Singh R, Jain M, Siwach R, et al. Large para-articular osteochondroma of the knee joint: a case report. Acta Orthop Traumatol Turc. 2012;46:139-143.
9. Lee JY, Lee S, Joo KB, et al. Intraarticular osteochondroma of shoulder: a case report. Clin Imaging. 2013;37:379-381.
10. Kim Y-C, Ahn JH, Lee JW. Osteochondroma of the distal tibia complicated by a tibialis posterior tendon tear. J Foot Ankle Surg. 2012;51: 660-663.
11. Allagui M, Amara K, Aloui I, et al. Historical giant near-circumferential osteochondroma of the proximal humerus. J Shoulder Elbow Surg. 2010;19:e12-e15.
12. Li M, Luettringhaus T, Walker KR, Cole PA. Operative treatment of femoral neck osteochondroma through a digastric approach in a pediatric patient: a case report and review of the literature. J Pediatr Orthop B. 2012;21:230-234.
13. Hogendoorn PC, Athanasou N, Bielack S, et al; ESMO/EUROBONET Working Group. Bone sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 suppl 5:v204-v213.
14. Arndt CAS, Rose PS, Folpe AL, Laack NN. Common musculoskeletal tumors of childhood and adolescence. Mayo Clin Proc. 2012;87:475-487.
15. Ta HT, Dass CR, Choong PF, Dunstan DE. Osteosarcoma treatment: state of the art. Cancer Metastasis Rev. 2009;28:247-263.
16. Messerschmitt PJ, Garcia RM, Abdul-Karim FW, et al. Osteosarcoma. J Am Acad Orthop Surg. 2009;17:515-527.
17. Laffan EE, Ngan B-Y, Navarro OM. Pediatric soft-tissue tumors and pseudotumors: MR imaging features with pathologic correlation: Part 2. Tumors of fibroblastic/myofibroblastic, so-called fibrohistiocytic, muscular, lymphomatous, neurogenic, hair matrix, and uncertain origin. Radiographics. 2009;29:e36.
18. Balamuth NJ, Womer RB. Ewing’s sarcoma. Lancet Oncol. 2010;11(2):184.
19. D’Angelo L, Massimi L, Narducci A, Di Rocco C. Ollier disease. Childs Nerv Syst. 2009;25:647-653.
20. Gelderblom H, Hogendoorn PC, Dijkstra SD, et al. The clinical approach towards chondrosarcoma. Oncologist. 2008;13:320-329.
21. Nosratzehi T, Pakfetrat A. Chondrosarcoma. Zahedan J Res Med Sci. 2013;15:64-64.
22. Prado FO, Nishimoto IN, Perez DE, et al. Head and neck chondrosarcoma: analysis of 16 cases. Br J Oral Maxillofacial Surg. 2009;47:555-557.
23. Kim HJ, Chalmers PN, Morris CD. Pediatric osteogenic sarcoma. Curr Opin Pediatr. 2010;22:61-66.
24. Sultan I, Qaddoumi I, Yaser S, et al. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol. 2009;27:3391-3397.
25. Navarro OM, Laffan EE, Ngan B-Y. Pediatric soft-tissue tumors and pseudo-tumors: MR imaging features with pathologic correlation: Part 1. Imaging approach, pseudotumors, vascular lesions, and adipocytic tumors. Radiographics. 2009;29:887-906.
26. Gross TG, Termuhlen AM. Pediatric non-Hodgkin lymphoma. Curr Hematol Malig Rep. 2008;3:167-173.
27. Hochberg J, Waxman IM, Kelly KM, et al. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the science. Br J Haematol. 2009;144:24-40.
28. Derenzini E, Casadei B, Pellegrini C, et al. Non-Hodgkin lymphomas presenting as soft tissue masses: A single center experience and meta-analysis of the published series. Clin Lymphoma Myeloma Leuk. 2012 Dec 12. [Epub ahead of print]
29. Micheli A, Trapani S, Brizzi I, et al. Myositis ossificans circumscripta: a paediatric case and review of the literature. Eur J Pediatr. 2009;168:523-529.
30. McKenzie G, Raby N, Ritchie D. Non-neoplastic soft-tissue masses. Br J Radiol. 2009;82:775-785.
31. Buckmiller LM, Richter GT, Suen JY. Diagnosis and management of hemangiomas and vascular malformations of the head and neck. Oral Dis. 2010;16:405-418.
32. Khanna G, Bennett DL. Pediatric bone lesions: beyond the plain radiographic evaluation. Semin Roentgenol. 2012;47:90-99.
33. Rijal L, Nepal P, Baral S, et al. Solitary diaphyseal exostosis of femur, how common is it? Eur J Orthop Surg Traumatol. 2011;21:363-365.
Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban
In the past several years, three new oral anticoagulants—dabigatran etexilate (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis)—have been approved for use in the United States. These long-awaited agents are appealing because they are easy to use, do not require laboratory monitoring, and have demonstrated equivalence, or in some cases, superiority to warfarin in preventing stroke or systemic embolism in at-risk populations.1–4 However, unlike warfarin, they have no specific reversal agents. How then should one manage spontaneous bleeding problems and those due to drug overdose, and how can we quickly reverse anticoagulation if emergency surgery is needed?
For these reasons, physicians and patients have been wary of these agents. However, with a systematic approach based on an understanding of the properties of these drugs, the appropriate use and interpretation of coagulation tests, and awareness of available therapeutic strategies, physicians can more confidently provide care for patients who require urgent reversal of anticoagulant effects.
Here, we review the available literature and suggest practical strategies for management based on an understanding of the pharmacokinetic and pharmacodynamic effects of these drugs and our current knowledge of the coagulation tests.
NEED FOR ANTICOAGULANTS
Anticoagulants are important in preventing systemic embolization in patients with atrial fibrillation and preventing pulmonary embolism in patients with venous thromboembolism.
And the numbers are staggering. The estimated prevalence of atrial fibrillation in the United States was 3.03 million in 2005 and is projected to increase to 7.56 million by 2050.5 Ischemic stroke is the most serious complication of atrial fibrillation, which accounts for 23.5% of strokes in patients ages 80 through 89 according to Framingham data.6 Venous thromboembolism accounts for 900,000 incident or recurrent fatal and nonfatal events in the United States yearly.7
HOW THE NEW AGENTS BLOCK COAGULATION
Thrombin (factor IIa), a serine protease, is central to the process of clot formation during hemostasis. It activates factors V, VIII, and XI (thus generating more thrombin), catalyzes the conversion of fibrinogen to fibrin, and stimulates platelet aggregation. Its role in the final steps of the coagulation cascade has made it a target for new direct thrombin inhibitors such as dabigatran.
Factor Xa is a serine protease that plays a central role in the coagulation cascade. It is a desirable target for anticoagulation because it is the convergence point for the extrinsic and the intrinsic coagulation pathways. It converts prothrombin to thrombin. Rivaroxaban and apixaban are direct factor Xa inhibitors (Figure 1).
Dabigatran, a direct thrombin inhibitor
Dabigatran etexilate is a synthetic, orally available prodrug that is rapidly absorbed and converted by esterases to its active form, dabigatran, a potent direct inhibitor of both free thrombin and clot-bound thrombin.8
Plasma levels of dabigatran peak within 2 hours of administration, and its half-life is 14 to 17 hours.9 Dabigatran is eliminated mainly via the kidneys, with more that 80% of the drug excreted unchanged in the urine (Table 1).
Rivaroxaban, a factor Xa inhibitor
Rivaroxaban is a potent, selective, direct factor Xa inhibitor.
Plasma levels of rivaroxaban peak 2 to 3 hours after administration, and it is cleared with a terminal half-life of 7 to 11 hours.10,11
Rivaroxaban is eliminated by the kidneys and in the feces. The kidneys eliminate one-third of the active drug unchanged and another one-third as inactive metabolites. The remaining one-third is metabolized by the liver and then excreted in the feces. Rivaroxaban has a predictable and dose-dependent pharmacodynamic and pharmacokinetic profile that is not affected by age, sex, or body weight (Table 1).12
Apixaban, an oral factor Xa inhibitor
Apixaban is a selective, direct oral factor Xa inhibitor.
Plasma levels of apixaban peak about 3 hours after administration, and its terminal half-life is 8 to 14 hours.13 Apixaban is eliminated by oxidative metabolism, by the kidney, and in the feces. It has predictable pharmacodynamic and pharmacokinetic profiles and has the least renal dependence of the three agents (Table 1).
THE NEW ORAL ANTICOAGULANTS AND BLOOD COAGULATION ASSAYS
Assessment of the anticoagulant activity of the new oral anticoagulants is not necessary in routine clinical practice, but it may be useful in planning intervention in patients with major bleeding, those with drug overdose, or those who need emergency surgery.
The activated partial thromboplastin time
The activated partial thromboplastin time (aPTT) is a measure of the activity of the intrinsic pathway of the coagulation cascade.
Dabigatran. There is a curvilinear relationship between the aPTT and the plasma concentration of dabigatran and other direct thrombin inhibitors, although the aPTT prolongation appears to vary with different reagents and coagulometers.9,14,15 However, Stangier et al9 found a linear relationship between the aPTT and the square root of the dabigatran plasma concentration.
Rivaroxaban prolongs the aPTT in a dose-dependent manner, but there is no standard for calibration of this assay. Hence, the aPTT is not recommended for monitoring rivaroxaban in clinical practice.
Apixaban may also prolong the aPTT, but there are limited data on its reactivity with different reagents.
The prothrombin time and international normalized ratio
The prothrombin time and international normalized ratio (INR) are measures of the extrinsic pathway of the coagulation cascade.
Dabigatran. The INR has a linear response to the dabigatran concentration, but it is insensitive.9 Hence, it is not suitable for monitoring the anticoagulant effects of direct thrombin inhibitors.
Rivaroxaban. The prothrombin time correlates strongly with the plasma concentration of rivaroxaban in healthy trial participants11 and in patients undergoing total hip arthroplasty or total knee arthroplasty.16 Samama et al17 noted that, unlike with vitamin K antagonists, the INR cannot be used to monitor patients on rivaroxaban because the prothrombin time results varied with different reagents. They used a standard calibration curve to express the prothrombin time results in plasma concentrations of rivaroxaban rather than in seconds or the INR.
Apixaban increases the INR in a dose-dependent manner.18 Its effect on different reagents remains unknown.
The thrombin time
The thrombin time reflects the activity of thrombin in the plasma. The amount of thrombin and the concentration of thrombin inhibitors in the plasma sample determine the time to clot formation.
Dabigatran. The thrombin time displays a linear dose-response to dabigatran, but only over the range of therapeutic concentrations. At a dabigatran concentration greater than 600 ng/mL, the test often exceeds the maximum measurement time of coagulometers.9 Hence, this test is too sensitive for emergency monitoring, especially in cases of drug overdose. However, it is well suited for determining if any dabigatran is present.
Rivaroxaban and apixaban have no effect on the thrombin time.
The Hemoclot direct thrombin inhibitor assay and dabigatran
The Hemoclot direct thrombin inhibitor assay (Hyphen BioMed, France) is a sensitive diluted thrombin time assay that can be used for quantitative measurement of dabigatran activity in plasma. This test is based on inhibition of a constant amount of highly purified human alpha-thrombin by adding it to diluted test plasma (1:8 to 1:20) mixed with normal pooled human plasma.19,20
Stangier et al19 found that the Hemoclot assay was suitable for calculating a wide range of dabigatran concentrations up to 4,000 nmol/L (1,886 ng/mL). Although this finding has not been confirmed in larger studies, this test may provide a rapid and accurate assessment of dabigatran’s anticoagulant activity in cases of emergency surgery or overdose.
The ecarin clotting time and dabigatran
The ecarin clotting time is a measure of the activity of direct thrombin inhibitors, but not the factor Xa inhibitors.
Ecarin is a highly purified metalloprotease isolated from the venom of a snake, Echis carinatus, and it generates meizothrombin from prothrombin.21 Meizothrombin facilitates clot formation by converting fibrinogen to fibrin and, like thrombin, it can be inactivated by direct thrombin inhibitors, thereby prolonging the clotting time.
The limitations of the ecarin clotting time include dependence on the plasma levels of fibrinogen and prothrombin.
The ecarin chromogenic assay and dabigatran
The ecarin chromogenic assay is an improvement on the principle of the ecarin clotting time that can be used to measure the activity of direct thrombin inhibitors.22 In this test, ecarin is added to a plasma sample to generate meizothrombin, and the amidolytic activity of meizothrombin towards a chromogenic substrate is then determined.
Results of the ecarin chromogenic assay are not influenced by the levels of fibrinogen or prothrombin. Another advantage is that this assay can be used in automated and manual analyzers, thus enabling its use at the bedside. However, to our knowledge, it is not being regularly used to monitor direct thrombin inhibitors in the clinical setting, and there is no standard calibration of the ecarin clotting time method.
Assays of factor Xa activity
A variety of assays to monitor the anticoagulant activity of factor Xa inhibitors have been proposed.23–25 All measure inhibition of the activity of factor Xa using methods similar to those used in monitoring heparin levels. All require calibrators with a known concentration of the Xa inhibitor; many are easily adapted for laboratories currently providing measurement of factor Xa inhibition from heparin.23 These assays have been suggested as a better indicator of plasma concentration of factor Xa inhibitor drugs than the prothrombin time.25
CONTROLLING BLEEDING IN PATIENTS ON THE NEW ORAL ANTICOAGULANTS
Bleeding is an anticipated adverse event in patients taking anticoagulants. It is associated with significant morbidity and risk of death.26,27
Many physicians still have limited experience with using the new oral anticoagulants and managing the attendant bleeding risks. Hence, we recommend that every health institution have a treatment policy or algorithm to guide all clinical staff in the management of such emergencies.
Prevention of bleeding
Management of bleeding from these agents should begin with preventing bleeding in the first place.
The physician should adhere to the recommended dosages of these medications. Studies have shown that the plasma concentration of these drugs and the risk of bleeding increase with increasing dosage.1,28,29
In addition, these medications should be used for the shortest time for which anticoagulation is required, especially when used for preventing deep vein thrombosis. Prolonged use increases the risk of bleeding.30,31
Most patients who need anticoagulation have comorbidities such as heart failure, renal failure, diabetes mellitus, and hypertension. Although the kidneys play a major role in the excretion of dabigatran and, to some extent, rivaroxaban and apixaban, patients with severe renal impairment were excluded from the major trials of all three drugs.1–3 Hence, to avoid excessive drug accumulation and bleeding, these medications should not be used in such patients pending further studies. Further, patients taking these medications should be closely followed to detect new clinical situations, such as acute renal failure, that will necessitate their discontinuation or dose adjustment.
If surgery is needed
If a patient taking a new oral anticoagulant needs to undergo elective surgery, it is important to temporarily discontinue the drug, assess the risk of bleeding, and test for renal impairment.
Renal impairment is particularly relevant in the case of dabigatran, since more than 80% of the unchanged drug is cleared by the kidneys. Decreasing the dose, prolonging the dosing interval, or both have been suggested as means to reduce the risk of bleeding in patients with renal impairment who are taking dabigatran.32,33 Patients with normal renal function undergoing low-risk surgery should discontinue dabigatran at least 24 hours before the surgery. If the creatinine clearance is 31 to 50 mL/min, inclusively, the last dose should be at least 48 hours before the procedure for low-risk surgery, and 4 days before a procedure that poses a high risk of bleeding.32–34 Some experts have given the same recommendations for rivaroxaban and apixaban (Table 2).34
The aPTT and prothrombin time are readily available tests, but they cannot determine the residual anticoagulant effects of dabigatran, rivaroxaban, or apixaban. However, in many (but not all) cases, a normal aPTT suggests that the hemostatic function is not impaired by dabigatran, and a normal prothrombin time or an absence of anti-factor Xa activity would similarly exclude hemostatic dysfunction caused by rivaroxaban or apixaban. These tests are potentially useful as adjuncts before surgical procedures that require complete hemostasis.
Furthermore, a normal thrombin time rules out the presence of a significant amount of dabigatran. Therefore, a normal thrombin time might be particularly useful in a patient undergoing a high-risk intervention such as epidural cannulation or neurosurgery and who is normally receiving dabigatran.
Managing overdose and bleeding complications
Assessing the severity of bleeding is the key to managing bleeding complications (Table 3).
Minor bleeding such as epistaxis and ecchymosis can be managed symptomatically (eg, with nasal packing), perhaps with short-term withdrawal of the anticoagulant. Moderate bleeding such as upper or lower gastrointestinal bleeding can be managed by withdrawal of the anticoagulant, clinical monitoring, blood transfusion if needed, and treatment directed at the etiology.
Major and life-threatening bleeding (eg, intracerebral hemorrhage) requires aggressive treatment in the intensive care unit, withdrawal of the anticoagulant, mechanical compression of the bleeding site if accessible, fluid replacement and blood transfusion as appropriate, and interventional procedures. Nonspecific reversal agents might be considered in patients with major or life-threatening bleeding.
The half-life of dabigatran after multiple doses is approximately 14 to 17 hours and is not dose-dependent.9 Hence, if there is no active bleeding after a dabigatran overdose, stopping the drug may be sufficient. Since the pharmacodynamic effect of dabigatran declines in parallel to its plasma concentration, urgent but not emergency surgery may need to be delayed for only about 12 hours from the last dose of dabigatran.
The 2011 American College of Cardiology Foundation/American Heart Association guidelines recommend that patients with severe hemorrhage resulting from dabigatran should receive supportive therapy, including transfusion of fresh-frozen plasma, transfusion of packed red blood cells, or surgical intervention if appropriate.35 However, transfusion of fresh-frozen plasma is debatable because there is no evidence to support its use in this situation. While fresh-frozen plasma may be useful in cases of coagulation factor depletion, it does not effectively reverse inhibition of coagulation factors.36
Off-label use of nonspecific hemostatic agents
To date, no specific agent has been demonstrated to reverse excessive bleeding in patients taking the new oral anticoagulants. However, in view of their procoagulant capabilities, nonspecific hemostatic agents have been suggested for use in reversal of major bleeding resulting from these drugs.37–39 Examples are:
Recombinant factor VIIa (NovoSeven) initiates thrombin generation by activating factor X.
Four-factor prothrombin complex concentrate (Beriplex, recently approved in the United States) contains relatively large amounts of four nonactive vitamin K-dependent procoagulant factors (factors II, VII, IX, and X) that stimulate thrombin formation.
Three-factor prothrombin complex concentrate (Bebulin VH and Profilnine SD) contains low amounts of nonactive factor VII relative to factors II, IX, and X. In some centers a four-factor equivalent is produced by transfusion of a three-factor product with the addition of small amounts of recombinant factor VIIa or fresh-frozen plasma to replace the missing factor VII.40
Activated prothrombin complex concentrate (FEIBA NF) contains activated factor VII and factors II, IX, and X, mainly in nonactivated form.36 Therefore, it combines the effect of both recombinant factor VIIa and four-factor prothrombin complex concentrate.37
Studies of nonspecific hemostatic agents
In a study of rats infused with high doses of dabigatran, van Ryn et al38 observed that activated prothrombin complex concentrate at a dose of 50 or 100 U/kg and recombinant factor VIIa at a dose of 0.1 or 0.5 mg/kg reduced the rat-tail bleeding time in a dose-dependent manner but not the blood loss, compared with controls, even with a higher dose of recombinant factor VIIa (1 mg/kg). Recombinant factor VIIa also reversed the prolonged aPTT induced by dabigatran, whereas activated prothrombin complex concentrate did not. They suggested that recombinant factor VIIa and activated prothrombin complex concentrate may be potential antidotes for dabigatran-induced severe bleeding in humans.
In an ex vivo study of healthy people who took a single dose of dabigatran 150 mg or rivaroxaban 20 mg, Marlu et al37 found that activated prothrombin complex concentrate and four-factor prothrombin complex concentrate could be reasonable antidotes to these drugs.
Dabigatran-associated bleeding after cardiac surgery in humans has been successfully managed with hemodialysis and recombinant factor VIIa, although the efficacy of the latter cannot be individually assessed in the study.41
In a randomized placebo-controlled trial aimed at reversing rivaroxaban and dabigatran in healthy participants, Eerenberg et al39 showed that four-factor prothrombin complex concentrate at a dose of 50 IU/kg reversed prolongation of the prothrombin time and decreased the endogenous thrombin potential in those who received rivaroxaban, but it failed to reverse the aPTT, the endogenous thrombin potential, and thrombin time in those who received dabigatran.
However, Marlu et al reported that four-factor prothrombin complex concentrate at three doses (12.5 U/kg, 25 U/kg, and 50 U/kg)—or better still, activated prothrombin complex concentrate (40–80 U/kg)—could be a useful antidote to dabigatran.37
It is important to note that the healthy participants in the Eerenberg et al study39 took dabigatran 150 mg twice daily and rivaroxaban 20 mg daily for 2.5 days, whereas those in the Marlu et al study37 took the same dose of each medication, but only once.
The three-factor prothrombin complex concentrate products have been shown to be less effective than four-factor ones in reversing supratherapeutic INRs in patients with warfarin overdose, but whether this will be true with the new oral anticoagulants remains unknown. Furthermore, the four-factor concentrates effectively reversed warfarin-induced coagulopathy and bleeding in patients,42 but to our knowledge, the same is yet to be demonstrated in bleeding related to the newer agents.
Other measures
Gastric lavage or the administration of activated charcoal (or in some cases both) may reduce drug absorption if done within 2 or 3 hours of drug ingestion (Table 1). Because it is lipophilic, more than 99.9% of dabigatran etexilate was adsorbed by activated charcoal from water prepared to simulate gastric fluid in an in vitro experiment by van Ryn et al.43 This has not been tested in patients, and no similar study has been done for rivaroxaban or apixaban. However, use of charcoal in cases of recent ingestion, particularly with intentional overdose of these agents, seems reasonable.
Hemodialysis may reverse the anticoagulant effects of dabigatran overdose or severe bleeding because only about 35% of dabigatran is bound to plasma proteins (Table 1). In a single-center study, 50 mg of dabigatran etexilate was given orally to six patients with end-stage renal disease before dialysis, and the mean fraction of the drug removed by the dialyzer was 62% at 2 hours and 68% at 4 hours.32 This study suggests that hemodialysis may be useful to accelerate the removal of the drug in cases of life-threatening bleeding.
Rivaroxaban and apixaban are not dialyzable: the plasma protein binding of rivaroxaban is 95% and that of apixaban is 87%.
FUTURE DIRECTIONS
Because the new oral anticoagulants, unlike warfarin, have a wide therapeutic window, routine anticoagulant monitoring is not needed and might be misleading. However, there are times when monitoring might be useful; at such times, a validated, widely available, easily understood test would be good to have—but we don’t have it—at least not yet.
Therapeutic ranges for the aPTT have been established empirically for heparin in various indications.44 Additional study is needed to determine if an appropriate aPTT range can be determined for the new oral anticoagulants, particularly dabigatran.
Similarly, as with low-molecular-weight heparins, anti-factor Xa activity monitoring may become a more available validated means of testing for exposure to rivaroxaban and apixaban. More promising, using concepts derived from the development of the INR for warfarin monitoring,45 Tripodi et al46 have derived normalized INR-like assays to report rivaroxaban levels. A standardized schema for reporting results is being developed.46 Studies are required to determine if and how this assay may be useful. Initial trials in this regard are encouraging.47
Finally, the thrombotic risk associated with the use of nonspecific prohemostatic agents is unknown.37,48 Additional studies are required to standardize their dosages, frequency of administration, and duration of action, as well as to quantify their complications in bleeding patients.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009; 104:1534–1539.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991; 22:983–988.
- Heit JA, Cohen AT, Anderson FA; on behalf of the VTE Impact Assessment Group. Estimated annual number of incident and recurrent, non-fatal and fatal venous thromboembolism (VTE) events in the US. Blood (ASH Annual Meeting Abstracts) 2005; 106:abstract 910.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
- Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007; 64:292–303.
- Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939—an oral, direct factor Xa inhibitor—after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 2005; 61:873–880.
- Mueck W, Becka M, Kubitza D, Voith B, Zuehlsdorf M. Population model of the pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor—in healthy subjects. Int J Clin Pharmacol Ther 2007; 45:335–344.
- Weitz JI, Eikelboom JW, Samama MM; American College of Chest Physicians. New antithrombotic drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e120S–e151S.
- Raghavan N, Frost CE, Yu Z, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos 2009; 37:74–81.
- Cullberg M, Eriksson UG, Larsson M, Karlsson MO. Population modelling of the effect of inogatran, at thrombin inhibitor, on ex vivo coagulation time (APTT) in healthy subjects and patients with coronary artery disease. Br J Clin Pharmacol 2001; 51:71–79.
- Carlsson SC, Mattsson C, Eriksson UG, et al. A review of the effects of the oral direct thrombin inhibitor ximelagatran on coagulation assays. Thromb Res 2005; 115:9–18.
- Mueck W, Eriksson BI, Bauer KA, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor—in patients undergoing major orthopaedic surgery. Clin Pharmacokinet 2008; 47:203–216.
- Samama MM, Martinoli JL, LeFlem L, et al. Assessment of laboratory assays to measure rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2010; 103:815–825.
- Wong PC, Crain EJ, Xin B, et al. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J Thromb Haemost 2008; 6:820–829.
- Stangier J, Feuring M. Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis 2012; 23:138–143.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Nowak G. The ecarin clotting time, a universal method to quantify direct thrombin inhibitors. Pathophysiol Haemost Thromb 2003–2004; 33:173–183.
- Lange U, Nowak G, Bucha E. Ecarin chromogenic assay—a new method for quantitative determination of direct thrombin inhibitors like hirudin. Pathophysiol Haemost Thromb 2003–2004; 33:184–191.
- Samama MM, Contant G, Spiro TE, et al; Rivaroxaban Anti-Factor Xa Chromogenic Assay Field Trial Laboratories. Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost 2012; 107:379–387.
- Miyares MA, Davis K. Newer oral anticoagulants: a review of laboratory monitoring options and reversal agents in the hemorrhagic patient. Am J Health Syst Pharm 2012; 69:1473–1484.
- Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost 2010; 104:1263–1271.
- Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006; 114:774–782.
- Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 2007; 49:1362–1368.
- Perzborn E, Strassburger J, Wilmen A, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939—an oral, direct factor Xa inhibitor. J Thromb Haemost 2005; 3:514–521.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358:2765–2775.
- Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358:2776–2786.
- Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010; 49:259–268.
- US Food and Drug Administration (FDA). Medication Guide: Pradaxa (dabigatran etexilate mesylate) capsules. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM231720.pdf. Accessed June 5, 2013.
- Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood 2012; 119:3016–3023.
- Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/ AHA/ HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2011; 57:1330–1337.
- Crowther MA, Warkentin TE. Managing bleeding in anticoagulated patients with a focus on novel therapeutic agents. J Thromb Haemost 2009; 7(suppl 1):107–110.
- Marlu R, Hodaj E, Paris A, Albaladejo P, Cracowski JL, Pernod G. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Haemost 2012; 108:217–224.
- van Ryn J, Ruehl D, Priepke H, Hauel N, Wienen W. Reversibility of the anticoagulant effect of high doses of the direct thrombin inhibitor dabigatran, by recombinant factor VIIa or activated prothrombin complex concentrate. 13th Congress of the European Hematology Association, June 12–15, 2008. Hematologica 2008; 93( s1):148Abs.0370.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Holland L, Warkentin TE, Refaai M, Crowther MA, Johnston MA, Sarode R. Suboptimal effect of a three-factor prothrombin complex concentrate (Profilnine-SD) in correcting supratherapeutic international normalized ratio due to warfarin overdose. Transfusion 2009; 49:1171–1177.
- Warkentin TE, Margetts P, Connolly SJ, Lamy A, Ricci C, Eikelboom JW. Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood 2012; 119:2172–2174.
- Song MM, Warne CP, Crowther MA. Prothrombin complex concentrate (PCC, Octaplex) in patients requiring immediate reversal of vitamin K antagonist anticoagulation. Thromb Res 2012; 129:526–529.
- van Ryn J, Sieger P, Kink-Eiband M, Gansser D, Clemens A. Adsorption of dabigatran etexilate in water or dabigatran in pooled human plasma by activated charcoal in vitro. 51st ASH Annual Meeting and Exposition. Abstract no. 1065. http://ash.confex.com/ash/2009/webprogram/Paper21383.html. Accessed June 5, 2013.
- Hirsh J. Heparin. N Engl J Med 1991; 324:1565–1574.
- van den Besselaar AMHP, Poller L, Tripodi A. Guidelines for thromboplastins and plasmas used to control for oral anticoagulant therapy. WHO Technical Report Series 1999; 889:64–93.
- Tripodi A, Chantarangkul V, Guinet C, Samama MM. The international normalized ratio calibrated for rivaroxaban has the potential to normalize prothrombin time results for rivaroxaban-treated patients: Results of an in vitro study. J Thromb Haemost 2011; 9:226–228.
- Samama MM, Contant G, Spiro TE, et al; Rivaroxaban Prothrombin Time Field Trial Laboratories. Evaluation of the prothrombin time for measuring rivaroxaban plasma concentrations using calibrators and controls: results of a multicenter field trial. Clin Appl Thromb Hemost 2012; 18:150–158.
- Ehrlich HJ, Henzl MJ, Gomperts ED. Safety of factor VIII inhibitor bypass activity (FEIBA): 10-year compilation of thrombotic adverse events. Haemophilia 2002; 8:83–90.
In the past several years, three new oral anticoagulants—dabigatran etexilate (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis)—have been approved for use in the United States. These long-awaited agents are appealing because they are easy to use, do not require laboratory monitoring, and have demonstrated equivalence, or in some cases, superiority to warfarin in preventing stroke or systemic embolism in at-risk populations.1–4 However, unlike warfarin, they have no specific reversal agents. How then should one manage spontaneous bleeding problems and those due to drug overdose, and how can we quickly reverse anticoagulation if emergency surgery is needed?
For these reasons, physicians and patients have been wary of these agents. However, with a systematic approach based on an understanding of the properties of these drugs, the appropriate use and interpretation of coagulation tests, and awareness of available therapeutic strategies, physicians can more confidently provide care for patients who require urgent reversal of anticoagulant effects.
Here, we review the available literature and suggest practical strategies for management based on an understanding of the pharmacokinetic and pharmacodynamic effects of these drugs and our current knowledge of the coagulation tests.
NEED FOR ANTICOAGULANTS
Anticoagulants are important in preventing systemic embolization in patients with atrial fibrillation and preventing pulmonary embolism in patients with venous thromboembolism.
And the numbers are staggering. The estimated prevalence of atrial fibrillation in the United States was 3.03 million in 2005 and is projected to increase to 7.56 million by 2050.5 Ischemic stroke is the most serious complication of atrial fibrillation, which accounts for 23.5% of strokes in patients ages 80 through 89 according to Framingham data.6 Venous thromboembolism accounts for 900,000 incident or recurrent fatal and nonfatal events in the United States yearly.7
HOW THE NEW AGENTS BLOCK COAGULATION
Thrombin (factor IIa), a serine protease, is central to the process of clot formation during hemostasis. It activates factors V, VIII, and XI (thus generating more thrombin), catalyzes the conversion of fibrinogen to fibrin, and stimulates platelet aggregation. Its role in the final steps of the coagulation cascade has made it a target for new direct thrombin inhibitors such as dabigatran.
Factor Xa is a serine protease that plays a central role in the coagulation cascade. It is a desirable target for anticoagulation because it is the convergence point for the extrinsic and the intrinsic coagulation pathways. It converts prothrombin to thrombin. Rivaroxaban and apixaban are direct factor Xa inhibitors (Figure 1).
Dabigatran, a direct thrombin inhibitor
Dabigatran etexilate is a synthetic, orally available prodrug that is rapidly absorbed and converted by esterases to its active form, dabigatran, a potent direct inhibitor of both free thrombin and clot-bound thrombin.8
Plasma levels of dabigatran peak within 2 hours of administration, and its half-life is 14 to 17 hours.9 Dabigatran is eliminated mainly via the kidneys, with more that 80% of the drug excreted unchanged in the urine (Table 1).
Rivaroxaban, a factor Xa inhibitor
Rivaroxaban is a potent, selective, direct factor Xa inhibitor.
Plasma levels of rivaroxaban peak 2 to 3 hours after administration, and it is cleared with a terminal half-life of 7 to 11 hours.10,11
Rivaroxaban is eliminated by the kidneys and in the feces. The kidneys eliminate one-third of the active drug unchanged and another one-third as inactive metabolites. The remaining one-third is metabolized by the liver and then excreted in the feces. Rivaroxaban has a predictable and dose-dependent pharmacodynamic and pharmacokinetic profile that is not affected by age, sex, or body weight (Table 1).12
Apixaban, an oral factor Xa inhibitor
Apixaban is a selective, direct oral factor Xa inhibitor.
Plasma levels of apixaban peak about 3 hours after administration, and its terminal half-life is 8 to 14 hours.13 Apixaban is eliminated by oxidative metabolism, by the kidney, and in the feces. It has predictable pharmacodynamic and pharmacokinetic profiles and has the least renal dependence of the three agents (Table 1).
THE NEW ORAL ANTICOAGULANTS AND BLOOD COAGULATION ASSAYS
Assessment of the anticoagulant activity of the new oral anticoagulants is not necessary in routine clinical practice, but it may be useful in planning intervention in patients with major bleeding, those with drug overdose, or those who need emergency surgery.
The activated partial thromboplastin time
The activated partial thromboplastin time (aPTT) is a measure of the activity of the intrinsic pathway of the coagulation cascade.
Dabigatran. There is a curvilinear relationship between the aPTT and the plasma concentration of dabigatran and other direct thrombin inhibitors, although the aPTT prolongation appears to vary with different reagents and coagulometers.9,14,15 However, Stangier et al9 found a linear relationship between the aPTT and the square root of the dabigatran plasma concentration.
Rivaroxaban prolongs the aPTT in a dose-dependent manner, but there is no standard for calibration of this assay. Hence, the aPTT is not recommended for monitoring rivaroxaban in clinical practice.
Apixaban may also prolong the aPTT, but there are limited data on its reactivity with different reagents.
The prothrombin time and international normalized ratio
The prothrombin time and international normalized ratio (INR) are measures of the extrinsic pathway of the coagulation cascade.
Dabigatran. The INR has a linear response to the dabigatran concentration, but it is insensitive.9 Hence, it is not suitable for monitoring the anticoagulant effects of direct thrombin inhibitors.
Rivaroxaban. The prothrombin time correlates strongly with the plasma concentration of rivaroxaban in healthy trial participants11 and in patients undergoing total hip arthroplasty or total knee arthroplasty.16 Samama et al17 noted that, unlike with vitamin K antagonists, the INR cannot be used to monitor patients on rivaroxaban because the prothrombin time results varied with different reagents. They used a standard calibration curve to express the prothrombin time results in plasma concentrations of rivaroxaban rather than in seconds or the INR.
Apixaban increases the INR in a dose-dependent manner.18 Its effect on different reagents remains unknown.
The thrombin time
The thrombin time reflects the activity of thrombin in the plasma. The amount of thrombin and the concentration of thrombin inhibitors in the plasma sample determine the time to clot formation.
Dabigatran. The thrombin time displays a linear dose-response to dabigatran, but only over the range of therapeutic concentrations. At a dabigatran concentration greater than 600 ng/mL, the test often exceeds the maximum measurement time of coagulometers.9 Hence, this test is too sensitive for emergency monitoring, especially in cases of drug overdose. However, it is well suited for determining if any dabigatran is present.
Rivaroxaban and apixaban have no effect on the thrombin time.
The Hemoclot direct thrombin inhibitor assay and dabigatran
The Hemoclot direct thrombin inhibitor assay (Hyphen BioMed, France) is a sensitive diluted thrombin time assay that can be used for quantitative measurement of dabigatran activity in plasma. This test is based on inhibition of a constant amount of highly purified human alpha-thrombin by adding it to diluted test plasma (1:8 to 1:20) mixed with normal pooled human plasma.19,20
Stangier et al19 found that the Hemoclot assay was suitable for calculating a wide range of dabigatran concentrations up to 4,000 nmol/L (1,886 ng/mL). Although this finding has not been confirmed in larger studies, this test may provide a rapid and accurate assessment of dabigatran’s anticoagulant activity in cases of emergency surgery or overdose.
The ecarin clotting time and dabigatran
The ecarin clotting time is a measure of the activity of direct thrombin inhibitors, but not the factor Xa inhibitors.
Ecarin is a highly purified metalloprotease isolated from the venom of a snake, Echis carinatus, and it generates meizothrombin from prothrombin.21 Meizothrombin facilitates clot formation by converting fibrinogen to fibrin and, like thrombin, it can be inactivated by direct thrombin inhibitors, thereby prolonging the clotting time.
The limitations of the ecarin clotting time include dependence on the plasma levels of fibrinogen and prothrombin.
The ecarin chromogenic assay and dabigatran
The ecarin chromogenic assay is an improvement on the principle of the ecarin clotting time that can be used to measure the activity of direct thrombin inhibitors.22 In this test, ecarin is added to a plasma sample to generate meizothrombin, and the amidolytic activity of meizothrombin towards a chromogenic substrate is then determined.
Results of the ecarin chromogenic assay are not influenced by the levels of fibrinogen or prothrombin. Another advantage is that this assay can be used in automated and manual analyzers, thus enabling its use at the bedside. However, to our knowledge, it is not being regularly used to monitor direct thrombin inhibitors in the clinical setting, and there is no standard calibration of the ecarin clotting time method.
Assays of factor Xa activity
A variety of assays to monitor the anticoagulant activity of factor Xa inhibitors have been proposed.23–25 All measure inhibition of the activity of factor Xa using methods similar to those used in monitoring heparin levels. All require calibrators with a known concentration of the Xa inhibitor; many are easily adapted for laboratories currently providing measurement of factor Xa inhibition from heparin.23 These assays have been suggested as a better indicator of plasma concentration of factor Xa inhibitor drugs than the prothrombin time.25
CONTROLLING BLEEDING IN PATIENTS ON THE NEW ORAL ANTICOAGULANTS
Bleeding is an anticipated adverse event in patients taking anticoagulants. It is associated with significant morbidity and risk of death.26,27
Many physicians still have limited experience with using the new oral anticoagulants and managing the attendant bleeding risks. Hence, we recommend that every health institution have a treatment policy or algorithm to guide all clinical staff in the management of such emergencies.
Prevention of bleeding
Management of bleeding from these agents should begin with preventing bleeding in the first place.
The physician should adhere to the recommended dosages of these medications. Studies have shown that the plasma concentration of these drugs and the risk of bleeding increase with increasing dosage.1,28,29
In addition, these medications should be used for the shortest time for which anticoagulation is required, especially when used for preventing deep vein thrombosis. Prolonged use increases the risk of bleeding.30,31
Most patients who need anticoagulation have comorbidities such as heart failure, renal failure, diabetes mellitus, and hypertension. Although the kidneys play a major role in the excretion of dabigatran and, to some extent, rivaroxaban and apixaban, patients with severe renal impairment were excluded from the major trials of all three drugs.1–3 Hence, to avoid excessive drug accumulation and bleeding, these medications should not be used in such patients pending further studies. Further, patients taking these medications should be closely followed to detect new clinical situations, such as acute renal failure, that will necessitate their discontinuation or dose adjustment.
If surgery is needed
If a patient taking a new oral anticoagulant needs to undergo elective surgery, it is important to temporarily discontinue the drug, assess the risk of bleeding, and test for renal impairment.
Renal impairment is particularly relevant in the case of dabigatran, since more than 80% of the unchanged drug is cleared by the kidneys. Decreasing the dose, prolonging the dosing interval, or both have been suggested as means to reduce the risk of bleeding in patients with renal impairment who are taking dabigatran.32,33 Patients with normal renal function undergoing low-risk surgery should discontinue dabigatran at least 24 hours before the surgery. If the creatinine clearance is 31 to 50 mL/min, inclusively, the last dose should be at least 48 hours before the procedure for low-risk surgery, and 4 days before a procedure that poses a high risk of bleeding.32–34 Some experts have given the same recommendations for rivaroxaban and apixaban (Table 2).34
The aPTT and prothrombin time are readily available tests, but they cannot determine the residual anticoagulant effects of dabigatran, rivaroxaban, or apixaban. However, in many (but not all) cases, a normal aPTT suggests that the hemostatic function is not impaired by dabigatran, and a normal prothrombin time or an absence of anti-factor Xa activity would similarly exclude hemostatic dysfunction caused by rivaroxaban or apixaban. These tests are potentially useful as adjuncts before surgical procedures that require complete hemostasis.
Furthermore, a normal thrombin time rules out the presence of a significant amount of dabigatran. Therefore, a normal thrombin time might be particularly useful in a patient undergoing a high-risk intervention such as epidural cannulation or neurosurgery and who is normally receiving dabigatran.
Managing overdose and bleeding complications
Assessing the severity of bleeding is the key to managing bleeding complications (Table 3).
Minor bleeding such as epistaxis and ecchymosis can be managed symptomatically (eg, with nasal packing), perhaps with short-term withdrawal of the anticoagulant. Moderate bleeding such as upper or lower gastrointestinal bleeding can be managed by withdrawal of the anticoagulant, clinical monitoring, blood transfusion if needed, and treatment directed at the etiology.
Major and life-threatening bleeding (eg, intracerebral hemorrhage) requires aggressive treatment in the intensive care unit, withdrawal of the anticoagulant, mechanical compression of the bleeding site if accessible, fluid replacement and blood transfusion as appropriate, and interventional procedures. Nonspecific reversal agents might be considered in patients with major or life-threatening bleeding.
The half-life of dabigatran after multiple doses is approximately 14 to 17 hours and is not dose-dependent.9 Hence, if there is no active bleeding after a dabigatran overdose, stopping the drug may be sufficient. Since the pharmacodynamic effect of dabigatran declines in parallel to its plasma concentration, urgent but not emergency surgery may need to be delayed for only about 12 hours from the last dose of dabigatran.
The 2011 American College of Cardiology Foundation/American Heart Association guidelines recommend that patients with severe hemorrhage resulting from dabigatran should receive supportive therapy, including transfusion of fresh-frozen plasma, transfusion of packed red blood cells, or surgical intervention if appropriate.35 However, transfusion of fresh-frozen plasma is debatable because there is no evidence to support its use in this situation. While fresh-frozen plasma may be useful in cases of coagulation factor depletion, it does not effectively reverse inhibition of coagulation factors.36
Off-label use of nonspecific hemostatic agents
To date, no specific agent has been demonstrated to reverse excessive bleeding in patients taking the new oral anticoagulants. However, in view of their procoagulant capabilities, nonspecific hemostatic agents have been suggested for use in reversal of major bleeding resulting from these drugs.37–39 Examples are:
Recombinant factor VIIa (NovoSeven) initiates thrombin generation by activating factor X.
Four-factor prothrombin complex concentrate (Beriplex, recently approved in the United States) contains relatively large amounts of four nonactive vitamin K-dependent procoagulant factors (factors II, VII, IX, and X) that stimulate thrombin formation.
Three-factor prothrombin complex concentrate (Bebulin VH and Profilnine SD) contains low amounts of nonactive factor VII relative to factors II, IX, and X. In some centers a four-factor equivalent is produced by transfusion of a three-factor product with the addition of small amounts of recombinant factor VIIa or fresh-frozen plasma to replace the missing factor VII.40
Activated prothrombin complex concentrate (FEIBA NF) contains activated factor VII and factors II, IX, and X, mainly in nonactivated form.36 Therefore, it combines the effect of both recombinant factor VIIa and four-factor prothrombin complex concentrate.37
Studies of nonspecific hemostatic agents
In a study of rats infused with high doses of dabigatran, van Ryn et al38 observed that activated prothrombin complex concentrate at a dose of 50 or 100 U/kg and recombinant factor VIIa at a dose of 0.1 or 0.5 mg/kg reduced the rat-tail bleeding time in a dose-dependent manner but not the blood loss, compared with controls, even with a higher dose of recombinant factor VIIa (1 mg/kg). Recombinant factor VIIa also reversed the prolonged aPTT induced by dabigatran, whereas activated prothrombin complex concentrate did not. They suggested that recombinant factor VIIa and activated prothrombin complex concentrate may be potential antidotes for dabigatran-induced severe bleeding in humans.
In an ex vivo study of healthy people who took a single dose of dabigatran 150 mg or rivaroxaban 20 mg, Marlu et al37 found that activated prothrombin complex concentrate and four-factor prothrombin complex concentrate could be reasonable antidotes to these drugs.
Dabigatran-associated bleeding after cardiac surgery in humans has been successfully managed with hemodialysis and recombinant factor VIIa, although the efficacy of the latter cannot be individually assessed in the study.41
In a randomized placebo-controlled trial aimed at reversing rivaroxaban and dabigatran in healthy participants, Eerenberg et al39 showed that four-factor prothrombin complex concentrate at a dose of 50 IU/kg reversed prolongation of the prothrombin time and decreased the endogenous thrombin potential in those who received rivaroxaban, but it failed to reverse the aPTT, the endogenous thrombin potential, and thrombin time in those who received dabigatran.
However, Marlu et al reported that four-factor prothrombin complex concentrate at three doses (12.5 U/kg, 25 U/kg, and 50 U/kg)—or better still, activated prothrombin complex concentrate (40–80 U/kg)—could be a useful antidote to dabigatran.37
It is important to note that the healthy participants in the Eerenberg et al study39 took dabigatran 150 mg twice daily and rivaroxaban 20 mg daily for 2.5 days, whereas those in the Marlu et al study37 took the same dose of each medication, but only once.
The three-factor prothrombin complex concentrate products have been shown to be less effective than four-factor ones in reversing supratherapeutic INRs in patients with warfarin overdose, but whether this will be true with the new oral anticoagulants remains unknown. Furthermore, the four-factor concentrates effectively reversed warfarin-induced coagulopathy and bleeding in patients,42 but to our knowledge, the same is yet to be demonstrated in bleeding related to the newer agents.
Other measures
Gastric lavage or the administration of activated charcoal (or in some cases both) may reduce drug absorption if done within 2 or 3 hours of drug ingestion (Table 1). Because it is lipophilic, more than 99.9% of dabigatran etexilate was adsorbed by activated charcoal from water prepared to simulate gastric fluid in an in vitro experiment by van Ryn et al.43 This has not been tested in patients, and no similar study has been done for rivaroxaban or apixaban. However, use of charcoal in cases of recent ingestion, particularly with intentional overdose of these agents, seems reasonable.
Hemodialysis may reverse the anticoagulant effects of dabigatran overdose or severe bleeding because only about 35% of dabigatran is bound to plasma proteins (Table 1). In a single-center study, 50 mg of dabigatran etexilate was given orally to six patients with end-stage renal disease before dialysis, and the mean fraction of the drug removed by the dialyzer was 62% at 2 hours and 68% at 4 hours.32 This study suggests that hemodialysis may be useful to accelerate the removal of the drug in cases of life-threatening bleeding.
Rivaroxaban and apixaban are not dialyzable: the plasma protein binding of rivaroxaban is 95% and that of apixaban is 87%.
FUTURE DIRECTIONS
Because the new oral anticoagulants, unlike warfarin, have a wide therapeutic window, routine anticoagulant monitoring is not needed and might be misleading. However, there are times when monitoring might be useful; at such times, a validated, widely available, easily understood test would be good to have—but we don’t have it—at least not yet.
Therapeutic ranges for the aPTT have been established empirically for heparin in various indications.44 Additional study is needed to determine if an appropriate aPTT range can be determined for the new oral anticoagulants, particularly dabigatran.
Similarly, as with low-molecular-weight heparins, anti-factor Xa activity monitoring may become a more available validated means of testing for exposure to rivaroxaban and apixaban. More promising, using concepts derived from the development of the INR for warfarin monitoring,45 Tripodi et al46 have derived normalized INR-like assays to report rivaroxaban levels. A standardized schema for reporting results is being developed.46 Studies are required to determine if and how this assay may be useful. Initial trials in this regard are encouraging.47
Finally, the thrombotic risk associated with the use of nonspecific prohemostatic agents is unknown.37,48 Additional studies are required to standardize their dosages, frequency of administration, and duration of action, as well as to quantify their complications in bleeding patients.
In the past several years, three new oral anticoagulants—dabigatran etexilate (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis)—have been approved for use in the United States. These long-awaited agents are appealing because they are easy to use, do not require laboratory monitoring, and have demonstrated equivalence, or in some cases, superiority to warfarin in preventing stroke or systemic embolism in at-risk populations.1–4 However, unlike warfarin, they have no specific reversal agents. How then should one manage spontaneous bleeding problems and those due to drug overdose, and how can we quickly reverse anticoagulation if emergency surgery is needed?
For these reasons, physicians and patients have been wary of these agents. However, with a systematic approach based on an understanding of the properties of these drugs, the appropriate use and interpretation of coagulation tests, and awareness of available therapeutic strategies, physicians can more confidently provide care for patients who require urgent reversal of anticoagulant effects.
Here, we review the available literature and suggest practical strategies for management based on an understanding of the pharmacokinetic and pharmacodynamic effects of these drugs and our current knowledge of the coagulation tests.
NEED FOR ANTICOAGULANTS
Anticoagulants are important in preventing systemic embolization in patients with atrial fibrillation and preventing pulmonary embolism in patients with venous thromboembolism.
And the numbers are staggering. The estimated prevalence of atrial fibrillation in the United States was 3.03 million in 2005 and is projected to increase to 7.56 million by 2050.5 Ischemic stroke is the most serious complication of atrial fibrillation, which accounts for 23.5% of strokes in patients ages 80 through 89 according to Framingham data.6 Venous thromboembolism accounts for 900,000 incident or recurrent fatal and nonfatal events in the United States yearly.7
HOW THE NEW AGENTS BLOCK COAGULATION
Thrombin (factor IIa), a serine protease, is central to the process of clot formation during hemostasis. It activates factors V, VIII, and XI (thus generating more thrombin), catalyzes the conversion of fibrinogen to fibrin, and stimulates platelet aggregation. Its role in the final steps of the coagulation cascade has made it a target for new direct thrombin inhibitors such as dabigatran.
Factor Xa is a serine protease that plays a central role in the coagulation cascade. It is a desirable target for anticoagulation because it is the convergence point for the extrinsic and the intrinsic coagulation pathways. It converts prothrombin to thrombin. Rivaroxaban and apixaban are direct factor Xa inhibitors (Figure 1).
Dabigatran, a direct thrombin inhibitor
Dabigatran etexilate is a synthetic, orally available prodrug that is rapidly absorbed and converted by esterases to its active form, dabigatran, a potent direct inhibitor of both free thrombin and clot-bound thrombin.8
Plasma levels of dabigatran peak within 2 hours of administration, and its half-life is 14 to 17 hours.9 Dabigatran is eliminated mainly via the kidneys, with more that 80% of the drug excreted unchanged in the urine (Table 1).
Rivaroxaban, a factor Xa inhibitor
Rivaroxaban is a potent, selective, direct factor Xa inhibitor.
Plasma levels of rivaroxaban peak 2 to 3 hours after administration, and it is cleared with a terminal half-life of 7 to 11 hours.10,11
Rivaroxaban is eliminated by the kidneys and in the feces. The kidneys eliminate one-third of the active drug unchanged and another one-third as inactive metabolites. The remaining one-third is metabolized by the liver and then excreted in the feces. Rivaroxaban has a predictable and dose-dependent pharmacodynamic and pharmacokinetic profile that is not affected by age, sex, or body weight (Table 1).12
Apixaban, an oral factor Xa inhibitor
Apixaban is a selective, direct oral factor Xa inhibitor.
Plasma levels of apixaban peak about 3 hours after administration, and its terminal half-life is 8 to 14 hours.13 Apixaban is eliminated by oxidative metabolism, by the kidney, and in the feces. It has predictable pharmacodynamic and pharmacokinetic profiles and has the least renal dependence of the three agents (Table 1).
THE NEW ORAL ANTICOAGULANTS AND BLOOD COAGULATION ASSAYS
Assessment of the anticoagulant activity of the new oral anticoagulants is not necessary in routine clinical practice, but it may be useful in planning intervention in patients with major bleeding, those with drug overdose, or those who need emergency surgery.
The activated partial thromboplastin time
The activated partial thromboplastin time (aPTT) is a measure of the activity of the intrinsic pathway of the coagulation cascade.
Dabigatran. There is a curvilinear relationship between the aPTT and the plasma concentration of dabigatran and other direct thrombin inhibitors, although the aPTT prolongation appears to vary with different reagents and coagulometers.9,14,15 However, Stangier et al9 found a linear relationship between the aPTT and the square root of the dabigatran plasma concentration.
Rivaroxaban prolongs the aPTT in a dose-dependent manner, but there is no standard for calibration of this assay. Hence, the aPTT is not recommended for monitoring rivaroxaban in clinical practice.
Apixaban may also prolong the aPTT, but there are limited data on its reactivity with different reagents.
The prothrombin time and international normalized ratio
The prothrombin time and international normalized ratio (INR) are measures of the extrinsic pathway of the coagulation cascade.
Dabigatran. The INR has a linear response to the dabigatran concentration, but it is insensitive.9 Hence, it is not suitable for monitoring the anticoagulant effects of direct thrombin inhibitors.
Rivaroxaban. The prothrombin time correlates strongly with the plasma concentration of rivaroxaban in healthy trial participants11 and in patients undergoing total hip arthroplasty or total knee arthroplasty.16 Samama et al17 noted that, unlike with vitamin K antagonists, the INR cannot be used to monitor patients on rivaroxaban because the prothrombin time results varied with different reagents. They used a standard calibration curve to express the prothrombin time results in plasma concentrations of rivaroxaban rather than in seconds or the INR.
Apixaban increases the INR in a dose-dependent manner.18 Its effect on different reagents remains unknown.
The thrombin time
The thrombin time reflects the activity of thrombin in the plasma. The amount of thrombin and the concentration of thrombin inhibitors in the plasma sample determine the time to clot formation.
Dabigatran. The thrombin time displays a linear dose-response to dabigatran, but only over the range of therapeutic concentrations. At a dabigatran concentration greater than 600 ng/mL, the test often exceeds the maximum measurement time of coagulometers.9 Hence, this test is too sensitive for emergency monitoring, especially in cases of drug overdose. However, it is well suited for determining if any dabigatran is present.
Rivaroxaban and apixaban have no effect on the thrombin time.
The Hemoclot direct thrombin inhibitor assay and dabigatran
The Hemoclot direct thrombin inhibitor assay (Hyphen BioMed, France) is a sensitive diluted thrombin time assay that can be used for quantitative measurement of dabigatran activity in plasma. This test is based on inhibition of a constant amount of highly purified human alpha-thrombin by adding it to diluted test plasma (1:8 to 1:20) mixed with normal pooled human plasma.19,20
Stangier et al19 found that the Hemoclot assay was suitable for calculating a wide range of dabigatran concentrations up to 4,000 nmol/L (1,886 ng/mL). Although this finding has not been confirmed in larger studies, this test may provide a rapid and accurate assessment of dabigatran’s anticoagulant activity in cases of emergency surgery or overdose.
The ecarin clotting time and dabigatran
The ecarin clotting time is a measure of the activity of direct thrombin inhibitors, but not the factor Xa inhibitors.
Ecarin is a highly purified metalloprotease isolated from the venom of a snake, Echis carinatus, and it generates meizothrombin from prothrombin.21 Meizothrombin facilitates clot formation by converting fibrinogen to fibrin and, like thrombin, it can be inactivated by direct thrombin inhibitors, thereby prolonging the clotting time.
The limitations of the ecarin clotting time include dependence on the plasma levels of fibrinogen and prothrombin.
The ecarin chromogenic assay and dabigatran
The ecarin chromogenic assay is an improvement on the principle of the ecarin clotting time that can be used to measure the activity of direct thrombin inhibitors.22 In this test, ecarin is added to a plasma sample to generate meizothrombin, and the amidolytic activity of meizothrombin towards a chromogenic substrate is then determined.
Results of the ecarin chromogenic assay are not influenced by the levels of fibrinogen or prothrombin. Another advantage is that this assay can be used in automated and manual analyzers, thus enabling its use at the bedside. However, to our knowledge, it is not being regularly used to monitor direct thrombin inhibitors in the clinical setting, and there is no standard calibration of the ecarin clotting time method.
Assays of factor Xa activity
A variety of assays to monitor the anticoagulant activity of factor Xa inhibitors have been proposed.23–25 All measure inhibition of the activity of factor Xa using methods similar to those used in monitoring heparin levels. All require calibrators with a known concentration of the Xa inhibitor; many are easily adapted for laboratories currently providing measurement of factor Xa inhibition from heparin.23 These assays have been suggested as a better indicator of plasma concentration of factor Xa inhibitor drugs than the prothrombin time.25
CONTROLLING BLEEDING IN PATIENTS ON THE NEW ORAL ANTICOAGULANTS
Bleeding is an anticipated adverse event in patients taking anticoagulants. It is associated with significant morbidity and risk of death.26,27
Many physicians still have limited experience with using the new oral anticoagulants and managing the attendant bleeding risks. Hence, we recommend that every health institution have a treatment policy or algorithm to guide all clinical staff in the management of such emergencies.
Prevention of bleeding
Management of bleeding from these agents should begin with preventing bleeding in the first place.
The physician should adhere to the recommended dosages of these medications. Studies have shown that the plasma concentration of these drugs and the risk of bleeding increase with increasing dosage.1,28,29
In addition, these medications should be used for the shortest time for which anticoagulation is required, especially when used for preventing deep vein thrombosis. Prolonged use increases the risk of bleeding.30,31
Most patients who need anticoagulation have comorbidities such as heart failure, renal failure, diabetes mellitus, and hypertension. Although the kidneys play a major role in the excretion of dabigatran and, to some extent, rivaroxaban and apixaban, patients with severe renal impairment were excluded from the major trials of all three drugs.1–3 Hence, to avoid excessive drug accumulation and bleeding, these medications should not be used in such patients pending further studies. Further, patients taking these medications should be closely followed to detect new clinical situations, such as acute renal failure, that will necessitate their discontinuation or dose adjustment.
If surgery is needed
If a patient taking a new oral anticoagulant needs to undergo elective surgery, it is important to temporarily discontinue the drug, assess the risk of bleeding, and test for renal impairment.
Renal impairment is particularly relevant in the case of dabigatran, since more than 80% of the unchanged drug is cleared by the kidneys. Decreasing the dose, prolonging the dosing interval, or both have been suggested as means to reduce the risk of bleeding in patients with renal impairment who are taking dabigatran.32,33 Patients with normal renal function undergoing low-risk surgery should discontinue dabigatran at least 24 hours before the surgery. If the creatinine clearance is 31 to 50 mL/min, inclusively, the last dose should be at least 48 hours before the procedure for low-risk surgery, and 4 days before a procedure that poses a high risk of bleeding.32–34 Some experts have given the same recommendations for rivaroxaban and apixaban (Table 2).34
The aPTT and prothrombin time are readily available tests, but they cannot determine the residual anticoagulant effects of dabigatran, rivaroxaban, or apixaban. However, in many (but not all) cases, a normal aPTT suggests that the hemostatic function is not impaired by dabigatran, and a normal prothrombin time or an absence of anti-factor Xa activity would similarly exclude hemostatic dysfunction caused by rivaroxaban or apixaban. These tests are potentially useful as adjuncts before surgical procedures that require complete hemostasis.
Furthermore, a normal thrombin time rules out the presence of a significant amount of dabigatran. Therefore, a normal thrombin time might be particularly useful in a patient undergoing a high-risk intervention such as epidural cannulation or neurosurgery and who is normally receiving dabigatran.
Managing overdose and bleeding complications
Assessing the severity of bleeding is the key to managing bleeding complications (Table 3).
Minor bleeding such as epistaxis and ecchymosis can be managed symptomatically (eg, with nasal packing), perhaps with short-term withdrawal of the anticoagulant. Moderate bleeding such as upper or lower gastrointestinal bleeding can be managed by withdrawal of the anticoagulant, clinical monitoring, blood transfusion if needed, and treatment directed at the etiology.
Major and life-threatening bleeding (eg, intracerebral hemorrhage) requires aggressive treatment in the intensive care unit, withdrawal of the anticoagulant, mechanical compression of the bleeding site if accessible, fluid replacement and blood transfusion as appropriate, and interventional procedures. Nonspecific reversal agents might be considered in patients with major or life-threatening bleeding.
The half-life of dabigatran after multiple doses is approximately 14 to 17 hours and is not dose-dependent.9 Hence, if there is no active bleeding after a dabigatran overdose, stopping the drug may be sufficient. Since the pharmacodynamic effect of dabigatran declines in parallel to its plasma concentration, urgent but not emergency surgery may need to be delayed for only about 12 hours from the last dose of dabigatran.
The 2011 American College of Cardiology Foundation/American Heart Association guidelines recommend that patients with severe hemorrhage resulting from dabigatran should receive supportive therapy, including transfusion of fresh-frozen plasma, transfusion of packed red blood cells, or surgical intervention if appropriate.35 However, transfusion of fresh-frozen plasma is debatable because there is no evidence to support its use in this situation. While fresh-frozen plasma may be useful in cases of coagulation factor depletion, it does not effectively reverse inhibition of coagulation factors.36
Off-label use of nonspecific hemostatic agents
To date, no specific agent has been demonstrated to reverse excessive bleeding in patients taking the new oral anticoagulants. However, in view of their procoagulant capabilities, nonspecific hemostatic agents have been suggested for use in reversal of major bleeding resulting from these drugs.37–39 Examples are:
Recombinant factor VIIa (NovoSeven) initiates thrombin generation by activating factor X.
Four-factor prothrombin complex concentrate (Beriplex, recently approved in the United States) contains relatively large amounts of four nonactive vitamin K-dependent procoagulant factors (factors II, VII, IX, and X) that stimulate thrombin formation.
Three-factor prothrombin complex concentrate (Bebulin VH and Profilnine SD) contains low amounts of nonactive factor VII relative to factors II, IX, and X. In some centers a four-factor equivalent is produced by transfusion of a three-factor product with the addition of small amounts of recombinant factor VIIa or fresh-frozen plasma to replace the missing factor VII.40
Activated prothrombin complex concentrate (FEIBA NF) contains activated factor VII and factors II, IX, and X, mainly in nonactivated form.36 Therefore, it combines the effect of both recombinant factor VIIa and four-factor prothrombin complex concentrate.37
Studies of nonspecific hemostatic agents
In a study of rats infused with high doses of dabigatran, van Ryn et al38 observed that activated prothrombin complex concentrate at a dose of 50 or 100 U/kg and recombinant factor VIIa at a dose of 0.1 or 0.5 mg/kg reduced the rat-tail bleeding time in a dose-dependent manner but not the blood loss, compared with controls, even with a higher dose of recombinant factor VIIa (1 mg/kg). Recombinant factor VIIa also reversed the prolonged aPTT induced by dabigatran, whereas activated prothrombin complex concentrate did not. They suggested that recombinant factor VIIa and activated prothrombin complex concentrate may be potential antidotes for dabigatran-induced severe bleeding in humans.
In an ex vivo study of healthy people who took a single dose of dabigatran 150 mg or rivaroxaban 20 mg, Marlu et al37 found that activated prothrombin complex concentrate and four-factor prothrombin complex concentrate could be reasonable antidotes to these drugs.
Dabigatran-associated bleeding after cardiac surgery in humans has been successfully managed with hemodialysis and recombinant factor VIIa, although the efficacy of the latter cannot be individually assessed in the study.41
In a randomized placebo-controlled trial aimed at reversing rivaroxaban and dabigatran in healthy participants, Eerenberg et al39 showed that four-factor prothrombin complex concentrate at a dose of 50 IU/kg reversed prolongation of the prothrombin time and decreased the endogenous thrombin potential in those who received rivaroxaban, but it failed to reverse the aPTT, the endogenous thrombin potential, and thrombin time in those who received dabigatran.
However, Marlu et al reported that four-factor prothrombin complex concentrate at three doses (12.5 U/kg, 25 U/kg, and 50 U/kg)—or better still, activated prothrombin complex concentrate (40–80 U/kg)—could be a useful antidote to dabigatran.37
It is important to note that the healthy participants in the Eerenberg et al study39 took dabigatran 150 mg twice daily and rivaroxaban 20 mg daily for 2.5 days, whereas those in the Marlu et al study37 took the same dose of each medication, but only once.
The three-factor prothrombin complex concentrate products have been shown to be less effective than four-factor ones in reversing supratherapeutic INRs in patients with warfarin overdose, but whether this will be true with the new oral anticoagulants remains unknown. Furthermore, the four-factor concentrates effectively reversed warfarin-induced coagulopathy and bleeding in patients,42 but to our knowledge, the same is yet to be demonstrated in bleeding related to the newer agents.
Other measures
Gastric lavage or the administration of activated charcoal (or in some cases both) may reduce drug absorption if done within 2 or 3 hours of drug ingestion (Table 1). Because it is lipophilic, more than 99.9% of dabigatran etexilate was adsorbed by activated charcoal from water prepared to simulate gastric fluid in an in vitro experiment by van Ryn et al.43 This has not been tested in patients, and no similar study has been done for rivaroxaban or apixaban. However, use of charcoal in cases of recent ingestion, particularly with intentional overdose of these agents, seems reasonable.
Hemodialysis may reverse the anticoagulant effects of dabigatran overdose or severe bleeding because only about 35% of dabigatran is bound to plasma proteins (Table 1). In a single-center study, 50 mg of dabigatran etexilate was given orally to six patients with end-stage renal disease before dialysis, and the mean fraction of the drug removed by the dialyzer was 62% at 2 hours and 68% at 4 hours.32 This study suggests that hemodialysis may be useful to accelerate the removal of the drug in cases of life-threatening bleeding.
Rivaroxaban and apixaban are not dialyzable: the plasma protein binding of rivaroxaban is 95% and that of apixaban is 87%.
FUTURE DIRECTIONS
Because the new oral anticoagulants, unlike warfarin, have a wide therapeutic window, routine anticoagulant monitoring is not needed and might be misleading. However, there are times when monitoring might be useful; at such times, a validated, widely available, easily understood test would be good to have—but we don’t have it—at least not yet.
Therapeutic ranges for the aPTT have been established empirically for heparin in various indications.44 Additional study is needed to determine if an appropriate aPTT range can be determined for the new oral anticoagulants, particularly dabigatran.
Similarly, as with low-molecular-weight heparins, anti-factor Xa activity monitoring may become a more available validated means of testing for exposure to rivaroxaban and apixaban. More promising, using concepts derived from the development of the INR for warfarin monitoring,45 Tripodi et al46 have derived normalized INR-like assays to report rivaroxaban levels. A standardized schema for reporting results is being developed.46 Studies are required to determine if and how this assay may be useful. Initial trials in this regard are encouraging.47
Finally, the thrombotic risk associated with the use of nonspecific prohemostatic agents is unknown.37,48 Additional studies are required to standardize their dosages, frequency of administration, and duration of action, as well as to quantify their complications in bleeding patients.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009; 104:1534–1539.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991; 22:983–988.
- Heit JA, Cohen AT, Anderson FA; on behalf of the VTE Impact Assessment Group. Estimated annual number of incident and recurrent, non-fatal and fatal venous thromboembolism (VTE) events in the US. Blood (ASH Annual Meeting Abstracts) 2005; 106:abstract 910.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
- Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007; 64:292–303.
- Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939—an oral, direct factor Xa inhibitor—after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 2005; 61:873–880.
- Mueck W, Becka M, Kubitza D, Voith B, Zuehlsdorf M. Population model of the pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor—in healthy subjects. Int J Clin Pharmacol Ther 2007; 45:335–344.
- Weitz JI, Eikelboom JW, Samama MM; American College of Chest Physicians. New antithrombotic drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e120S–e151S.
- Raghavan N, Frost CE, Yu Z, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos 2009; 37:74–81.
- Cullberg M, Eriksson UG, Larsson M, Karlsson MO. Population modelling of the effect of inogatran, at thrombin inhibitor, on ex vivo coagulation time (APTT) in healthy subjects and patients with coronary artery disease. Br J Clin Pharmacol 2001; 51:71–79.
- Carlsson SC, Mattsson C, Eriksson UG, et al. A review of the effects of the oral direct thrombin inhibitor ximelagatran on coagulation assays. Thromb Res 2005; 115:9–18.
- Mueck W, Eriksson BI, Bauer KA, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor—in patients undergoing major orthopaedic surgery. Clin Pharmacokinet 2008; 47:203–216.
- Samama MM, Martinoli JL, LeFlem L, et al. Assessment of laboratory assays to measure rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2010; 103:815–825.
- Wong PC, Crain EJ, Xin B, et al. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J Thromb Haemost 2008; 6:820–829.
- Stangier J, Feuring M. Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis 2012; 23:138–143.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Nowak G. The ecarin clotting time, a universal method to quantify direct thrombin inhibitors. Pathophysiol Haemost Thromb 2003–2004; 33:173–183.
- Lange U, Nowak G, Bucha E. Ecarin chromogenic assay—a new method for quantitative determination of direct thrombin inhibitors like hirudin. Pathophysiol Haemost Thromb 2003–2004; 33:184–191.
- Samama MM, Contant G, Spiro TE, et al; Rivaroxaban Anti-Factor Xa Chromogenic Assay Field Trial Laboratories. Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost 2012; 107:379–387.
- Miyares MA, Davis K. Newer oral anticoagulants: a review of laboratory monitoring options and reversal agents in the hemorrhagic patient. Am J Health Syst Pharm 2012; 69:1473–1484.
- Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost 2010; 104:1263–1271.
- Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006; 114:774–782.
- Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 2007; 49:1362–1368.
- Perzborn E, Strassburger J, Wilmen A, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939—an oral, direct factor Xa inhibitor. J Thromb Haemost 2005; 3:514–521.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358:2765–2775.
- Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358:2776–2786.
- Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010; 49:259–268.
- US Food and Drug Administration (FDA). Medication Guide: Pradaxa (dabigatran etexilate mesylate) capsules. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM231720.pdf. Accessed June 5, 2013.
- Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood 2012; 119:3016–3023.
- Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/ AHA/ HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2011; 57:1330–1337.
- Crowther MA, Warkentin TE. Managing bleeding in anticoagulated patients with a focus on novel therapeutic agents. J Thromb Haemost 2009; 7(suppl 1):107–110.
- Marlu R, Hodaj E, Paris A, Albaladejo P, Cracowski JL, Pernod G. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Haemost 2012; 108:217–224.
- van Ryn J, Ruehl D, Priepke H, Hauel N, Wienen W. Reversibility of the anticoagulant effect of high doses of the direct thrombin inhibitor dabigatran, by recombinant factor VIIa or activated prothrombin complex concentrate. 13th Congress of the European Hematology Association, June 12–15, 2008. Hematologica 2008; 93( s1):148Abs.0370.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Holland L, Warkentin TE, Refaai M, Crowther MA, Johnston MA, Sarode R. Suboptimal effect of a three-factor prothrombin complex concentrate (Profilnine-SD) in correcting supratherapeutic international normalized ratio due to warfarin overdose. Transfusion 2009; 49:1171–1177.
- Warkentin TE, Margetts P, Connolly SJ, Lamy A, Ricci C, Eikelboom JW. Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood 2012; 119:2172–2174.
- Song MM, Warne CP, Crowther MA. Prothrombin complex concentrate (PCC, Octaplex) in patients requiring immediate reversal of vitamin K antagonist anticoagulation. Thromb Res 2012; 129:526–529.
- van Ryn J, Sieger P, Kink-Eiband M, Gansser D, Clemens A. Adsorption of dabigatran etexilate in water or dabigatran in pooled human plasma by activated charcoal in vitro. 51st ASH Annual Meeting and Exposition. Abstract no. 1065. http://ash.confex.com/ash/2009/webprogram/Paper21383.html. Accessed June 5, 2013.
- Hirsh J. Heparin. N Engl J Med 1991; 324:1565–1574.
- van den Besselaar AMHP, Poller L, Tripodi A. Guidelines for thromboplastins and plasmas used to control for oral anticoagulant therapy. WHO Technical Report Series 1999; 889:64–93.
- Tripodi A, Chantarangkul V, Guinet C, Samama MM. The international normalized ratio calibrated for rivaroxaban has the potential to normalize prothrombin time results for rivaroxaban-treated patients: Results of an in vitro study. J Thromb Haemost 2011; 9:226–228.
- Samama MM, Contant G, Spiro TE, et al; Rivaroxaban Prothrombin Time Field Trial Laboratories. Evaluation of the prothrombin time for measuring rivaroxaban plasma concentrations using calibrators and controls: results of a multicenter field trial. Clin Appl Thromb Hemost 2012; 18:150–158.
- Ehrlich HJ, Henzl MJ, Gomperts ED. Safety of factor VIII inhibitor bypass activity (FEIBA): 10-year compilation of thrombotic adverse events. Haemophilia 2002; 8:83–90.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009; 104:1534–1539.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991; 22:983–988.
- Heit JA, Cohen AT, Anderson FA; on behalf of the VTE Impact Assessment Group. Estimated annual number of incident and recurrent, non-fatal and fatal venous thromboembolism (VTE) events in the US. Blood (ASH Annual Meeting Abstracts) 2005; 106:abstract 910.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
- Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007; 64:292–303.
- Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939—an oral, direct factor Xa inhibitor—after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 2005; 61:873–880.
- Mueck W, Becka M, Kubitza D, Voith B, Zuehlsdorf M. Population model of the pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor—in healthy subjects. Int J Clin Pharmacol Ther 2007; 45:335–344.
- Weitz JI, Eikelboom JW, Samama MM; American College of Chest Physicians. New antithrombotic drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e120S–e151S.
- Raghavan N, Frost CE, Yu Z, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos 2009; 37:74–81.
- Cullberg M, Eriksson UG, Larsson M, Karlsson MO. Population modelling of the effect of inogatran, at thrombin inhibitor, on ex vivo coagulation time (APTT) in healthy subjects and patients with coronary artery disease. Br J Clin Pharmacol 2001; 51:71–79.
- Carlsson SC, Mattsson C, Eriksson UG, et al. A review of the effects of the oral direct thrombin inhibitor ximelagatran on coagulation assays. Thromb Res 2005; 115:9–18.
- Mueck W, Eriksson BI, Bauer KA, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor—in patients undergoing major orthopaedic surgery. Clin Pharmacokinet 2008; 47:203–216.
- Samama MM, Martinoli JL, LeFlem L, et al. Assessment of laboratory assays to measure rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2010; 103:815–825.
- Wong PC, Crain EJ, Xin B, et al. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J Thromb Haemost 2008; 6:820–829.
- Stangier J, Feuring M. Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis 2012; 23:138–143.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Nowak G. The ecarin clotting time, a universal method to quantify direct thrombin inhibitors. Pathophysiol Haemost Thromb 2003–2004; 33:173–183.
- Lange U, Nowak G, Bucha E. Ecarin chromogenic assay—a new method for quantitative determination of direct thrombin inhibitors like hirudin. Pathophysiol Haemost Thromb 2003–2004; 33:184–191.
- Samama MM, Contant G, Spiro TE, et al; Rivaroxaban Anti-Factor Xa Chromogenic Assay Field Trial Laboratories. Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost 2012; 107:379–387.
- Miyares MA, Davis K. Newer oral anticoagulants: a review of laboratory monitoring options and reversal agents in the hemorrhagic patient. Am J Health Syst Pharm 2012; 69:1473–1484.
- Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost 2010; 104:1263–1271.
- Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006; 114:774–782.
- Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 2007; 49:1362–1368.
- Perzborn E, Strassburger J, Wilmen A, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939—an oral, direct factor Xa inhibitor. J Thromb Haemost 2005; 3:514–521.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358:2765–2775.
- Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358:2776–2786.
- Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010; 49:259–268.
- US Food and Drug Administration (FDA). Medication Guide: Pradaxa (dabigatran etexilate mesylate) capsules. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM231720.pdf. Accessed June 5, 2013.
- Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood 2012; 119:3016–3023.
- Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/ AHA/ HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2011; 57:1330–1337.
- Crowther MA, Warkentin TE. Managing bleeding in anticoagulated patients with a focus on novel therapeutic agents. J Thromb Haemost 2009; 7(suppl 1):107–110.
- Marlu R, Hodaj E, Paris A, Albaladejo P, Cracowski JL, Pernod G. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Haemost 2012; 108:217–224.
- van Ryn J, Ruehl D, Priepke H, Hauel N, Wienen W. Reversibility of the anticoagulant effect of high doses of the direct thrombin inhibitor dabigatran, by recombinant factor VIIa or activated prothrombin complex concentrate. 13th Congress of the European Hematology Association, June 12–15, 2008. Hematologica 2008; 93( s1):148Abs.0370.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Holland L, Warkentin TE, Refaai M, Crowther MA, Johnston MA, Sarode R. Suboptimal effect of a three-factor prothrombin complex concentrate (Profilnine-SD) in correcting supratherapeutic international normalized ratio due to warfarin overdose. Transfusion 2009; 49:1171–1177.
- Warkentin TE, Margetts P, Connolly SJ, Lamy A, Ricci C, Eikelboom JW. Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood 2012; 119:2172–2174.
- Song MM, Warne CP, Crowther MA. Prothrombin complex concentrate (PCC, Octaplex) in patients requiring immediate reversal of vitamin K antagonist anticoagulation. Thromb Res 2012; 129:526–529.
- van Ryn J, Sieger P, Kink-Eiband M, Gansser D, Clemens A. Adsorption of dabigatran etexilate in water or dabigatran in pooled human plasma by activated charcoal in vitro. 51st ASH Annual Meeting and Exposition. Abstract no. 1065. http://ash.confex.com/ash/2009/webprogram/Paper21383.html. Accessed June 5, 2013.
- Hirsh J. Heparin. N Engl J Med 1991; 324:1565–1574.
- van den Besselaar AMHP, Poller L, Tripodi A. Guidelines for thromboplastins and plasmas used to control for oral anticoagulant therapy. WHO Technical Report Series 1999; 889:64–93.
- Tripodi A, Chantarangkul V, Guinet C, Samama MM. The international normalized ratio calibrated for rivaroxaban has the potential to normalize prothrombin time results for rivaroxaban-treated patients: Results of an in vitro study. J Thromb Haemost 2011; 9:226–228.
- Samama MM, Contant G, Spiro TE, et al; Rivaroxaban Prothrombin Time Field Trial Laboratories. Evaluation of the prothrombin time for measuring rivaroxaban plasma concentrations using calibrators and controls: results of a multicenter field trial. Clin Appl Thromb Hemost 2012; 18:150–158.
- Ehrlich HJ, Henzl MJ, Gomperts ED. Safety of factor VIII inhibitor bypass activity (FEIBA): 10-year compilation of thrombotic adverse events. Haemophilia 2002; 8:83–90.
KEY POINTS
- Thromboprophylaxis with anticoagulants is an important aspect of managing patients at risk of systemic or pulmonary embolization.
- Dabigatran is a direct inhibitor of thrombin (factor IIa); rivaroxaban and apixaban inhibit factor Xa.
- Monitoring of coagulation function is not routinely necessary with the new drugs but may be useful in emergencies.
- Nonspecific hemostatic agents that have been suggested for off-label use in reversing excessive bleeding in patients taking the new oral anticoagulants include recombinant factor VIIa, three-factor and four-factor prothrombin complex concentrate, and activated prothrombin complex concentrate.
Cough and Back Pain in a Man With COPD
ANSWER
The radiograph shows some evidence of hyperinflated lungs, consistent with COPD. There is a small right effusion evident.
Of note is a superior mediastinal mass, which is causing right-sided and anterior displacement of the intrathoracic trachea. The differential includes possible adenopathy related to a carcinoma or a substernal goiter. Further diagnostic studies and surgical evaluation are warranted.
In this particular case, review of the patient’s imaging history showed he had a chest radiograph two years ago, at which time these findings were present. This favors substernal goiter as the diagnosis. Multinodular goiter was later confirmed with a thyroid ultrasound, and referral to general surgery was made.
ANSWER
The radiograph shows some evidence of hyperinflated lungs, consistent with COPD. There is a small right effusion evident.
Of note is a superior mediastinal mass, which is causing right-sided and anterior displacement of the intrathoracic trachea. The differential includes possible adenopathy related to a carcinoma or a substernal goiter. Further diagnostic studies and surgical evaluation are warranted.
In this particular case, review of the patient’s imaging history showed he had a chest radiograph two years ago, at which time these findings were present. This favors substernal goiter as the diagnosis. Multinodular goiter was later confirmed with a thyroid ultrasound, and referral to general surgery was made.
ANSWER
The radiograph shows some evidence of hyperinflated lungs, consistent with COPD. There is a small right effusion evident.
Of note is a superior mediastinal mass, which is causing right-sided and anterior displacement of the intrathoracic trachea. The differential includes possible adenopathy related to a carcinoma or a substernal goiter. Further diagnostic studies and surgical evaluation are warranted.
In this particular case, review of the patient’s imaging history showed he had a chest radiograph two years ago, at which time these findings were present. This favors substernal goiter as the diagnosis. Multinodular goiter was later confirmed with a thyroid ultrasound, and referral to general surgery was made.
A 60-year-old man presents for evaluation of fever, cough, and back pain. His symptoms have been intermittent but have worsened over the past month or so. He has had no treatment prior to today’s visit. His medical history is significant for hypertension, COPD, and chronic renal insufficiency. He denies any history of tobacco use. On physical exam, you see an older man in no obvious distress. His vital signs are stable. He is afe-brile, with a blood pressure of 150/90 mm Hg, a heart rate of 66 beats/min, and a respiratory rate of 18 breaths/min. His O2 saturation is 98% on room air. His neck is supple, with no evidence of ade-nopathy. Lung sounds are slightly decreased bilaterally, with a few crackles heard. The rest of his physical exam, overall, is normal. You order preliminary lab work as well as a chest radiograph (shown). What is your impression?
Evaluation and management of premature ventricular complexes
Premature ventricular complexes (PVCs) are a common cause of palpitations, and are also often detected incidentally on electrocardiography (ECG), ambulatory monitoring, or inpatient telemetry. At the cellular level, ventricular myocytes spontaneously depolarize to create an extra systole that is “out of sync” with the cardiac cycle.
Although nearly everyone has some PVCs from time to time, people vary widely in their frequency of PVCs and their sensitivity to them.1,2 Some patients are exquisitely sensitive to even a small number of PVCs, while others are completely unaware of PVCs in a bigeminal pattern (ie, every other heartbeat). This article will review the evaluation and management of PVCs with a focus on clinical aspects.
DIAGNOSTIC EVALUATION
Personal and family history
Symptoms. The initial history should establish the presence, extent, timing, and duration of symptoms. Patients may use the word “palpitations” to describe their symptoms, but they also describe them as “hard” heartbeats, “chest-thumping,” or as a “catch” or “skipped” heartbeat. Related symptoms may include difficulty breathing, chest pain, fatigue, and dizziness.
The interview should determine whether the symptoms represent a minor nuisance or a major quality-of-life issue to the patient, and whether there are any specific associations or triggers. For example, it is very common for patients to become aware of PVCs at night, particularly in certain positions, such as lying on the left side. Patients often associate PVC symptoms with emotional stress, exercise, or caffeine or stimulant use.
Medication use. An accurate and up-to-date list of prescription medications should be screened for alpha-, beta-, or dopamine-receptor agonist drugs. Similarly, any use of over-the-counter sympathomimetic medications and nonprescription supplements should be elicited, including compounded elixirs or beverages. Many commercially available products designed to treat fatigue or increase alertness contain large doses of caffeine or other stimulants. It is also important to consider the use of illicit substances such as cocaine, amphetamine, methamphetamine, and their derivatives.
The patient’s medical and surgical history should be queried for any known structural heart disease, including coronary artery disease, myocardial infarction, congestive heart failure, valvular heart disease, congenital heart disease, and heritable conditions such as hypertrophic cardiomyopathy, prolonged QT syndromes, or other channel disorders. Pulmonary disorders such as sarcoidosis, pulmonary hypertension, or obstructive sleep apnea are also relevant. Similarly, it is important to identify endocrine disorders, including thyroid problems, sex hormone abnormalities, or adrenal gland conditions.
A careful family history should include any instance of sudden death in first-degree relatives, any heritable cardiac conditions, or coronary artery disease at an early age.
Physical examination
The physical examination should focus on findings that suggest underlying structural heart disease. Findings suggestive of congestive heart failure include elevated jugular venous pressures, abnormal cardiac sounds, pulmonary rales, abnormal arterial pulses, or peripheral edema. A murmur or a pathologic heart sound should raise suspicion of valvular or congenital heart disease when present in a young patient.
Inspection and palpation of the thyroid can reveal a related disorder. Obvious skin changes or neurologic findings can similarly reveal a systemic and possibly related clinical disorder that can have cardiac manifestations (eg, muscular dystrophy).
Electrocardiography, Holter monitoring, and other monitoring
Assessment of the cardiac rhythm includes 12-lead ECG and ambulatory Holter monitoring, typically for 24 or 48 hours.
Holter monitoring provides a continuous recording, usually in at least two or three leads. Patients are given a symptom journal or are asked to keep a diary of symptoms experienced during the monitoring period. The monitor is worn underneath clothing and is returned for download upon completion. Technicians process the data with the aid of computer software, and the final output is reviewed and interpreted by a cardiologist or cardiac electrophysiologist.
Holter monitoring for at least 24 hours is a critical step in assessing any patient with known or suspected PVCs, as it can both quantify the total burden of ventricular ectopy and identify the presence of any related ventricular tachycardia. In addition, it can detect additional supraventricular arrhythmias or bradycardia during the monitoring period. The PVC burden is an important measurement; it is expressed as the percentage of heartbeats that were ventricular extrasystoles during the monitoring period.
Both ECG and Holter monitoring are limited in that they are only snapshots of the rhythm during the period when a patient is actually hooked up. Many patients experience PVCs in clusters every very few days or weeks. Such a pattern is unlikely to be detected by a single ECG or 24- or 48-hour Holter monitoring.
A 30-day ambulatory event monitor (also known as a wearable loop recorder) is an important diagnostic tool in these scenarios. The concept is very similar to that of Holter monitoring, except that the device provides a continuous loop recording of the cardiac rhythm that is digitally stored in clips when the patient activates the device. Some wearable loop recorders also have auto-save features for heart rates falling outside of a programmed range.
Mobile outpatient cardiac telemetry is the most comprehensive form of noninvasive rhythm monitoring available. This is essentially the equivalent of continuous inpatient cardiac telemetry, but in a patient who is not hospitalized. It is a wearable ambulatory device providing continuous recordings, real-time automatic detections, and patient-activated symptom recordings. It can be used for up to 6 weeks. Advantages include detection and quantification of asymptomatic events, and real-time transmissions that the physician can act upon. The major disadvantage is cost, including coverage denial by many third-party payers.
This test is rarely indicated as part of a PVC evaluation and is typically ordered only by a cardiologist or cardiac electrophysiologist.
Noninvasive cardiac evaluation
Surface echocardiography is indicated to look for overt structural heart disease and can reliably detect abnormalities in cardiac chamber size, wall thickness, and function. Valvular heart disease is concomitantly identified by two-dimensional imaging as well as by color Doppler. The finding of significant structural heart disease in conjunction with PVCs should prompt a cardiology referral, as this carries significant prognostic implications.3–5
Exercise treadmill stress testing is appropriate for patients who experience PVCs with exercise or for whom an evaluation for coronary artery disease is indicated. The expected finding would be an increase in PVCs or ventricular tachycardia with exercise or in the subsequent recovery period. Exercise testing can be combined with either echocardiographic or nuclear perfusion imaging to evaluate the possibility of myocardial ischemia. For patients unable to exercise, pharmacologic stress testing with dobutamine or a vasodilator agent can be performed.
Advanced noninvasive cardiac imaging— such as computed tomography, magnetic resonance imaging, or positron-emission tomography—should be reserved for specific clinical indications such as congenital heart disease, suspected cardiac sarcoidosis, and infiltrative heart disease, and for specific cardiomyopathies, such as hypertrophic cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy. For example, frequent PVCs with a left bundle branch block morphology and superior axis raise the concern for a right ventricular disorder and may prompt cardiac magnetic resonance imaging for either arrhythmogenic right ventricular cardiomyopathy or sarcoidosis.
PVCs WITHOUT STRUCTURAL HEART DISEASE
Outflow tract PVCs and ventricular tachycardia
The right or left ventricular outflow tracts, or the epicardial tissue immediately adjacent to the aortic sinuses of Valsalva are the most common sites of origin for ventricular ectopy in the absence of structural heart disease.6–9 Affected cells often demonstrate a triggered activity mechanism due to cyclic adenosine monophosphate-mediated and calcium-dependent delayed after-depolarizations.7,8
Most of these foci are in the right ventricular outflow tract, producing a left bundle branch block morphology with an inferior axis (positive R waves in limb leads II, III, and aVF) and typical precordial R-wave transition in V3 and V4 (Figure 1). A minority are in the left ventricular outflow tract, producing a right bundle branch block with an inferior axis pattern, or in the aortic sinuses with a left bundle branch block pattern but with early precordial R transition in V2 and V3.
A study in 122 patients showed that right and left outflow tract arrhythmias had similar electrophysiologic properties and pharmacologic sensitivities, providing evidence for shared mechanisms possibly due to the common embryologic origin of these structures.9
Such arrhythmias are typically catecholamine-sensitive and are sometimes inducible with burst pacing in the electrophysiology laboratory. The short ventricular coupling intervals can promote intracellular calcium overload in the affected cells, leading to triggered activity.
Therefore, outflow tract PVCs and ventricular tachycardia are commonly encountered clinically during exercise and, to an even greater extent, in the postexercise cool-down period. Similarly, they can be worse during periods of emotional stress or fatigue, when the body’s endogenous catecholamine production is elevated. However, it is worthwhile to note that there are exceptions to this principle in which faster sinus rates seem to overdrive the PVCs in some patients, causing them to become paradoxically more frequent at rest, or even during sleep.
Outflow tract PVCs can be managed medically with beta-blockers, nondihydropyridine calcium channel blockers (verapamil or diltiazem), or, less commonly, class IC drugs such as flecainide. They are also highly curable by catheter ablation (Figure 2), with procedure success rates greater than 90%.9.10
However, a subset of outflow tract PVCs nested deep in a triangle of epicardial tissue between the right and left endocardial surface and underneath the left main coronary artery can be challenging. This region has been labeled the left ventricular summit, and is shielded from ablation by an epicardial fat pad in the adjacent pericardial space.11 Ablation attempts made from the right and left endocardial surfaces as well as the epicardial surface (pericardial space) sometimes cannot adequately penetrate the tissue deep enough to reach the originating focus deep within this triangle. While ablation cannot always fully eliminate the PVC, ablation from more than one of the sites listed can generally reduce its burden, often in combination with suppressive medical therapy (Figure 3).
Fascicular PVCs
Fascicular PVCs originate from within the left ventricular His-Purkinje system12 and produce a right bundle branch block morphology with either an anterior or posterior hemiblock pattern (Figure 4). Exit from the posterior fascicle causes an anterior hemiblock pattern, and exit from the anterior fascicle a posterior hemiblock pattern. Utilization of the rapidly conducting His-Purkinje system gives these PVCs a very narrow QRS duration, sometimes approaching 120 milliseconds or shorter. This occasionally causes them to be mistaken for aberrantly conducted supraventricular beats. Such spontaneous PVCs are commonly associated with both sustained and nonsustained ventricular tachycardia and are usually sensitive to verapamil.13
Special issues relating to mapping and catheter ablation of fascicular arrhythmias involve the identification of Purkinje fiber potentials and associated procedural diagnostic maneuvers during tachycardia.14
Other sites for PVCs
Other sites of origin for PVCs in the absence of structural heart disease include ventricular tissue adjacent to the aortomitral continuity,15 the tricuspid annulus,16 the mitral valve annulus, 17 papillary muscles,18 and other Purkinje-adjacent structures such as left ventricular false tendons.19 An example of a papillary muscle PVC is shown in Figures 5 and 6.
Curable by catheter ablation
Any of these PVCs can potentially be cured by catheter ablation when present at a sufficient burden to allow for activation mapping in the electrophysiology laboratory. The threshold for offering ablation varies among operators, but is generally around 10% or greater. Pacemapping is a technique applied in the electrophysiology laboratory when medically refractory symptomatic PVCs occurring at a lower burden require ablation.
PVCs WITH AN UNDERLYING CARDIAC CONDITION
Coronary artery disease
Tissue injury and death caused by acute myocardial infarction has long been recognized as a common cause of spontaneous ventricular ectopy attributed to infarct border zones of ischemic or hibernating myocardium.20,21
Suppression has not been associated with improved outcomes, as shown for class IC drugs in the landmark Cardiac Arrhythmia Suppression Trial (CAST),22 or in the amiodarone treatment arm of the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II).23 Therefore, treatment of ventricular ectopy in this patient population is usually symptom-driven unless there is hemodynamic intolerance, tachycardia-related cardiomyopathy, or a very high burden of PVCs in a patient who may be at risk of developing tachycardia-related cardiomyopathy. Antiarrhythmic drug treatment, when required, usually involves beta-blockers or class III medications such as sotalol or amiodarone.
Nonischemic dilated cardiomyopathy
This category includes patients with a wide variety of disease states including valvular heart disease, lymphocytic and other viral myocarditis, cardiac sarcoidosis, amyloidosis and other infiltrative diseases, familial conditions, and idiopathic dilated cardiomyopathy (ie, etiology unknown). Although it is a heterogeneous group, a common theme is that PVCs in this patient cohort may require epicardial mapping and ablation.24 Similarly, epicardial PVCs and ventricular tachycardia cluster at the basal posterolateral left ventricle near the mitral annulus, for unclear reasons.25
While specific criteria have been published, an epicardial focus is suggested by slowing of the initial QRS segment, pseudo-delta waves, a wider overall QRS, and Q waves in limb lead I.26
Treatment is symptom-driven unless the patient has a tachycardia-related cardiomyopathy or a high burden associated with the risk for its development. Antiarrhythmic drug therapy, when required, typically involves a beta-blocker or a class III drug such as sotalol or amiodarone. Sotalol is used in this population but has limited safety data and should be used cautiously in patients without an implantable cardioverter-defibrillator.
Arrhythmogenic right ventricular cardiomyopathy
Spontaneous ventricular ectopy and tachycardia are common, if not expected, in patients with this heritable autosomal dominant disorder. This condition is progressive and associated with the risk of sudden cardiac death. Criteria for diagnosis were established in 2010, and patients with suspected arrhythmogenic right ventricular cardiomyopathy often undergo cardiac magnetic resonance imaging.27 Diagnostic findings include fibro-fatty tissue replacement, which usually starts in the right ventricle but can progress to involve the left ventricle. PVCs and ventricular tachycardia can involve the right ventricular free wall and are often epicardial.
Catheter ablation is usually palliative, as future arrhythmias are expected. Many patients with this condition require an implantable cardioverter-defibrillator for prevention of sudden cardiac death, and some go on to cardiac transplantation as the disease progresses and ventricular arrhythmias become incessant.
Other conditions
Spontaneous ventricular ectopy is common in other heritable and acquired cardiomyopathies including hypertrophic cardiomyopathy and in infiltrative or inflammatory disorders such as cardiac amyloidosis and sarcoidosis. While technically falling under the rubric of nonischemic heart disease, the presence of spontaneous ventricular ectopy carries specific prognostic implications depending on the underlying diagnosis. Therefore, an appropriate referral for complete cardiac evaluation should be considered when a heritable disorder or other acquired structural heart disease is suspected.
TACHYCARDIA-RELATED CARDIOMYOPATHY
Tachycardia-related cardiomyopathy refers to left ventricular systolic dysfunction that is primarily caused by arrhythmias. This includes frequent PVCs or ventricular tachycardia but also atrial arrhythmias occurring at a high burden that directly weaken myocardial function over time. Although much research has been devoted to this condition, our understanding of its etiology and pathology is incomplete.
PVCs and ventricular ectopy burdens in excess of 15% to 20% have been associated with the development of this condition.28,29 However, it is important to note that cardiomyopathy can also develop at lower burdens.30 One study found that a burden greater than 24% was 79% sensitive and 78% specific for development of tachycardia-related cardiomyopathy.31 Additional studies have demonstrated specific PVC morphologic features such as slurring in the initial QRS segment and also PVCs occurring at shorter coupling intervals as being associated with cardiomyopathy.32–34
For these reasons, both quantification of the total burden and careful evaluation of available electrocardiograms and rhythm strips are important even in asymptomatic patients with frequent PVCs. Similarly, unexplained left ventricular dysfunction in patients with PVC burdens in these discussed ranges should raise suspicion for this diagnosis. Patients with tachycardia-related cardiomyopathy usually have at least partially reversible left ventricular dysfunction when identified or treated early.29,35
MEDICAL AND ABLATIVE TREATMENT
Available treatments include medical suppression and catheter ablation. One needs to exercise clinical judgment and incorporate all of the PVC-related data to make treatment decisions.
Little data for trigger avoidance and behavioral modification
Some patients report a strong association between palpitations related to PVCs and caffeine intake, other stimulants, or other dietary triggers. However, few data exist to support the role of trigger avoidance and behavioral modification in treatment. In fact, an older randomized trial in 81 men found no benefit in a program of total abstinence from caffeine and smoking, moderation of alcohol intake, and physical conditioning.36
Nonetheless, some argue in favor of advising patients to make these dietary and lifestyle changes, given the overall health benefits of aggressive risk-factor modification for cardiovascular disease.37 Certainly, a trial of trigger avoidance and behavioral modification seems reasonable for patients who have strongly associated historical triggers in the absence of structural heart disease and PVCs occurring at a low to modest burden.
Beta-blockers are the mainstay
Beta-blockers are the mainstay of medical suppression of PVCs, primarily through their effect on beta-1 adrenergic receptors to reduce intracellular cyclic adenosine monophosphate and thus decrease automaticity. Blocking beta-1 receptors also causes a negative chronotropic effect, reducing the resting sinus rate in addition to slowing atrioventricular nodal conduction.
Cardioselective beta-blockers include atenolol, betaxolol, metoprolol, and nadolol. These drugs are effective in suppressing PVCs, or at least in reducing the burden to more tolerable levels.
Beta-blockers are most strongly indicated in patients who require PVC suppression and who have concomitant coronary artery disease, prior myocardial infarction, or other cardiomyopathy, as this drug class favorably affects long-term prognosis in these conditions.
Common side effects of beta-blockers include fatigue, shortness of breath, depressed mood, and loss of libido. Side effects can present a significant challenge, particularly for younger patients. Noncardioselective beta-blockers are less commonly prescribed, with the exception of propranolol, which is an effective sympatholytic drug that blocks both beta-1 and beta-2 receptors.
Many patients with asthma or peripheral arterial disease can tolerate these drugs well despite concerns about provoked bronchospasm or claudication, respectively, and neither of these conditions is considered an absolute contraindication. Excessive bradycardia with beta-blocker therapy can lead to dizziness, lightheadedness, or overt syncope, and these drugs should be used with caution in patients with baseline sinus node dysfunction or atrioventricular nodal disease.
Nondihydropyridine calcium channel blockers
Nondihydropyridine calcium channel blockers are particularly effective for PVC suppression in patients without structural heart disease by the mechanisms previously described involving intracellular calcium channels. In particular, they are highly effective and are considered the drugs of choice in treating fascicular PVCs.
Verapamil is a potent drug in this class, but it also commonly causes constipation as a side effect. Diltiazem is less constipating but can cause fatigue, drowsiness, and headaches. Both drugs reduce the resting heart rate and slow atrioventricular nodal conduction. Patients predisposed to bradycardia or atrioventricular block can develop dizziness or overt syncope. Calcium channel blockers are also used cautiously in patients with congestive heart failure, given their potential negative inotropic effects.
Overall, calcium channel blockers are a very reasonable choice for young patients without structural heart disease who need PVC suppression.
Other antiarrhythmic drugs
Sotalol merits special consideration because it has both beta-blocker and class III antiarrhythmic properties, blocking potassium channels and prolonging cardiac repolarization. It can be very effective in PVC suppression but also creates some degree of QT prolongation. The QT-prolonging effect is accentuated in patients with baseline QT prolongation or abnormal renal function. Rarely, this can lead to torsades de pointes. As a safety precaution, some patients are admitted to the hospital when they start sotalol therapy so that they can be monitored with continuous telemetry and ECG to detect excessive QT prolongation.
Amiodarone is a versatile drug with mixed pharmacologic properties that include a predominantly potassium channel-blocking class III drug effect. However, this effect is balanced by its other pharmacologic properties that make QT prolongation less of a clinical concern. Excessive QT prolongation may still occur when used concomitantly with other QT-prolonging drugs.
Amiodarone is very effective in suppressing PVCs and ventricular arrhythmias but has considerable short-term and long-term side effects. Cumulative toxicity risks include damage to the thyroid gland, liver, skin, eyes, and lungs. Routine thyroid function testing, pulmonary function testing, and eye examinations are often considered for patients on long-term amiodarone therapy. Short-term use of this drug does not typically require such surveillance.
Catheter ablation
As mentioned in the previous sections, catheter ablation is a safe and effective treatment for PVCs. It is curative in most cases, and significantly reduces the PVC burden in others.
Procedure. Patients are brought to the electrophysiology laboratory in a fasted state and are partially sedated with an intravenous drug such as midazolam or fentanyl, or both. Steerable catheters are placed into appropriate cardiac chambers from femoral access sites, which are infiltrated with local anesthesia. Sometimes sedative or analgesic drugs must be limited if they are known to suppress PVCs.
Most operators prefer a technique called activation mapping, in which the catheter is maneuvered to home in on the precise PVC origin within the heart, which is subsequently ablated. This technique has very high success rates, but having enough spontaneous PVCs to map during the procedure is essential for the technique to succeed. Conversely, not having sufficient PVCs on the day of the procedure is a common reason that ablation fails or cannot be performed at all.
Pace-mapping is an alternate technique that does not require a continuous stream of PVCs. This involves pacing from different candidate locations inside the heart in an effort to precisely match the ECG appearance of the clinical PVC and to ablate at this site. Although activation mapping generally yields higher success rates and is preferred by most operators, pace-mapping can be successful when a perfect 12–12 match is elicited. In many cases, the two techniques are used together during the same procedure, particularly if the patient’s PVCs spontaneously wax and wane, as they often do.
Risks. Like any medical procedure, catheter ablation carries some inherent risks, including rare but potentially serious events. Unstable arrhythmias may require pace-termination from the catheter or, rarely, shock-termination externally. Even more rare is cardiac arrest requiring cardiopulmonary resuscitation. Uncommon but life-threatening complications also include pericardial effusion or cardiac tamponade requiring percutaneous drainage or, rarely, emergency surgical correction. Although such events are life-threatening, death is extremely rare.
Complications causing permanent disability are also very uncommon but include the risk of collateral injury to the conduction system requiring permanent pacemaker placement, injury to the coronary vessels requiring urgent treatment, or diaphragmatic injury affecting breathing. Left-sided cardiac ablation also carries a small risk of stroke, which is mitigated by giving intravenous heparin during the procedure.
More common but generally non-life-threatening complications include femoral vascular events such as hematomas, pseudoaneurysms, or fistulas that sometimes require subsequent treatment. These complications are generally treatable but can significantly prolong the recovery period.
Catheter ablation procedures are typically 2 to 6 hours in duration, depending on the chambers involved, PVC frequency, and other considerations. Postprocedure bed rest is required for a number of hours. A Foley catheter is sometimes used for patient comfort when a prolonged procedure is anticipated. This carries a small risk of urinary tract infection. Epicardial catheter ablation that requires access to the surface of the heart (ie, the pericardial space) is uncommon but carries some unique risks, including rare injury to coronary vessels or adjacent organs such as the liver or stomach.
Overall, both endocardial and epicardial catheter ablation can be performed safely and effectively in the overwhelming majority of patients, but understanding and explaining the potential risks remains a crucial part of the informed consent process.
TAKE-HOME POINTS
- PVCs are a common cause of palpitations but are also noted as incidental findings by ECG, Holter monitoring, and inpatient telemetry.
- The diagnostic evaluation includes an assessment for underlying structural heart disease and quantification of the total PVC burden.
- Patients without structural heart disease and with low-to-modest PVC burdens may not require specific treatment. PVCs at greater burdens, typically 15% to 20%, or with specific high-risk features carry a risk of tachycardia-related cardiomyopathy and may require treatment even if they are asymptomatic. These high-risk features include initial QRS slurring and PVCs occurring at shorter coupling intervals.
- Treatment involves medical therapy with a beta-blocker, a calcium channel blocker, or another antiarrhythmic drug, and catheter ablation in selected cases.
- Catheter ablation can be curative but is typically reserved for drug-intolerant or medically refractory patients with a high PVC burden.
- Kostis JB, McCrone K, Moreyra AE, et al. Premature ventricular complexes in the absence of identifiable heart disease. Circulation 1981; 63:1351–1356.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009; 95:1230–1237.
- Simpson RJ, Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2002; 143:535–540.
- Chakko CS, Gheorghiade M. Ventricular arrhythmias in severe heart failure: incidence, significance, and effectiveness of antiarrhythmic therapy. Am Heart J 1985; 109:497–504.
- Gami AS, Noheria A, Lachman N, et al. Anatomical correlates relevant to ablation above the semilunar valves for the cardiac electrophysiologist: a study of 603 hearts. J Interv Card Electrophysiol 2011; 30:5–15.
- Lerman BB, Belardinelli L, West GA, Berne RM, DiMarco JP. Adenosine-sensitive ventricular tachycardia: evidence suggesting cyclic AMP-mediated triggered activity. Circulation 1986; 74:270–280.
- Lerman BB, Stein K, Engelstein ED, et al. Mechanism of repetitive monomorphic ventricular tachycardia. Circulation 1995; 92:421–429.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. J Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Kim RJ, Iwai S, Markowitz SM, Shah BK, Stein KM, Lerman BB. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J Am Coll Cardiol 2007; 49:2035–2043.
- Yamada T, McElderry HT, Doppalapudi H, et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol 2010; 3:616–623.
- Ouyang F, Cappato R, Ernst S, et al. Electroanatomic substrate of idiopathic left ventricular tachycardia: unidirectional block and macro-reentry within the Purkinje network. Circulation 2002; 105:462–469.
- Iwai S, Lerman BB. Management of ventricular tachycardia in patients with clinically normal hearts. Curr Cardiol Rep 2000; 2:515–521.
- Nogami A. Purkinje-related arrhythmias part I: monomorphic ventricular tachycardias. Pacing Clin Electrophysiol 2011; 34:624–650.
- Letsas KP, Efremidis M, Kollias G, Xydonas S, Sideris A. Electrocardiographic and electrophysiologic characteristics of ventricular extrasystoles arising from the aortomitral continuity. Cardiol Res Pract 2011; 2011:864964.
- Tada H, Tadokoro K, Ito S, et al. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm 2007; 4:7–16.
- Tada H, Ito S, Naito S, et al. Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol 2005; 45:877–886.
- Doppalapudi H, Yamada T, McElderry HT, Plumb VJ, Epstein AE, Kay GN. Ventricular tachycardia originating from the posterior papillary muscle in the left ventricle: a distinct clinical syndrome. Circ Arrhythm Electrophysiol 2008; 1:23–29.
- Scheinman MM. Role of the His-Purkinje system in the genesis of cardiac arrhythmia. Heart Rhythm 2009; 6:1050–1058.
- Bigger JT, Dresdale FJ, Heissenbuttel RH, Weld FM, Wit AL. Ventricular arrhythmias in ischemic heart disease: mechanism, prevalence, significance, and management. Prog Cardiovasc Dis 1977; 19:255–300.
- Eldar M, Sievner Z, Goldbourt U, Reicher-Reiss H, Kaplinsky E, Behar S. Primary ventricular tachycardia in acute myocardial infarction: clinical characteristics and mortality. The SPRINT Study Group. Ann Intern Med 1992; 117:31–36.
- Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med 1989; 321:406–412.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol 2009; 54:799–808.
- Marchlinski FE. Perivalvular fibrosis and monomorphic ventricular tachycardia: toward a unifying hypothesis in nonischemic cardiomyopathy. Circulation 2007; 116:1998–2001.
- Vallès E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol 2010; 3:63–71.
- Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010; 121:1533–1541.
- Lee GK, Klarich KW, Grogan M, Cha YM. Premature ventricular contraction-induced cardiomyopathy: a treatable condition. Circ Arrhythm Electrophysiol 2012; 5:229–236.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Kanei Y, Friedman M, Ogawa N, Hanon S, Lam P, Schweitzer P. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol 2008; 13:81–85.
- Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010; 7:865–869.
- Moulton KP, Medcalf T, Lazzara R. Premature ventricular complex morphology. A marker for left ventricular structure and function. Circulation 1990; 81:1245–1251.
- Olgun H, Yokokawa M, Baman T, et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm 2011; 8:1046–1049.
- Sun Y, Blom NA, Yu Y, et al. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: an echocardiographic evaluation. Int J Cardiovasc Imaging 2003; 19:295–299.
- Sarrazin JF, Labounty T, Kuhne M, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm 2009; 6:1543–1549.
- DeBacker G, Jacobs D, Prineas R, et al. Ventricular premature contractions: a randomized non-drug intervention trial in normal men. Circulation 1979; 59:762–769.
- Glatter KA, Myers R, Chiamvimonvat N. Recommendations regarding dietary intake and caffeine and alcohol consumption in patients with cardiac arrhythmias: what do you tell your patients to do or not to do? Curr Treat Options Cardiovasc Med 2012; 14:529–535.
Premature ventricular complexes (PVCs) are a common cause of palpitations, and are also often detected incidentally on electrocardiography (ECG), ambulatory monitoring, or inpatient telemetry. At the cellular level, ventricular myocytes spontaneously depolarize to create an extra systole that is “out of sync” with the cardiac cycle.
Although nearly everyone has some PVCs from time to time, people vary widely in their frequency of PVCs and their sensitivity to them.1,2 Some patients are exquisitely sensitive to even a small number of PVCs, while others are completely unaware of PVCs in a bigeminal pattern (ie, every other heartbeat). This article will review the evaluation and management of PVCs with a focus on clinical aspects.
DIAGNOSTIC EVALUATION
Personal and family history
Symptoms. The initial history should establish the presence, extent, timing, and duration of symptoms. Patients may use the word “palpitations” to describe their symptoms, but they also describe them as “hard” heartbeats, “chest-thumping,” or as a “catch” or “skipped” heartbeat. Related symptoms may include difficulty breathing, chest pain, fatigue, and dizziness.
The interview should determine whether the symptoms represent a minor nuisance or a major quality-of-life issue to the patient, and whether there are any specific associations or triggers. For example, it is very common for patients to become aware of PVCs at night, particularly in certain positions, such as lying on the left side. Patients often associate PVC symptoms with emotional stress, exercise, or caffeine or stimulant use.
Medication use. An accurate and up-to-date list of prescription medications should be screened for alpha-, beta-, or dopamine-receptor agonist drugs. Similarly, any use of over-the-counter sympathomimetic medications and nonprescription supplements should be elicited, including compounded elixirs or beverages. Many commercially available products designed to treat fatigue or increase alertness contain large doses of caffeine or other stimulants. It is also important to consider the use of illicit substances such as cocaine, amphetamine, methamphetamine, and their derivatives.
The patient’s medical and surgical history should be queried for any known structural heart disease, including coronary artery disease, myocardial infarction, congestive heart failure, valvular heart disease, congenital heart disease, and heritable conditions such as hypertrophic cardiomyopathy, prolonged QT syndromes, or other channel disorders. Pulmonary disorders such as sarcoidosis, pulmonary hypertension, or obstructive sleep apnea are also relevant. Similarly, it is important to identify endocrine disorders, including thyroid problems, sex hormone abnormalities, or adrenal gland conditions.
A careful family history should include any instance of sudden death in first-degree relatives, any heritable cardiac conditions, or coronary artery disease at an early age.
Physical examination
The physical examination should focus on findings that suggest underlying structural heart disease. Findings suggestive of congestive heart failure include elevated jugular venous pressures, abnormal cardiac sounds, pulmonary rales, abnormal arterial pulses, or peripheral edema. A murmur or a pathologic heart sound should raise suspicion of valvular or congenital heart disease when present in a young patient.
Inspection and palpation of the thyroid can reveal a related disorder. Obvious skin changes or neurologic findings can similarly reveal a systemic and possibly related clinical disorder that can have cardiac manifestations (eg, muscular dystrophy).
Electrocardiography, Holter monitoring, and other monitoring
Assessment of the cardiac rhythm includes 12-lead ECG and ambulatory Holter monitoring, typically for 24 or 48 hours.
Holter monitoring provides a continuous recording, usually in at least two or three leads. Patients are given a symptom journal or are asked to keep a diary of symptoms experienced during the monitoring period. The monitor is worn underneath clothing and is returned for download upon completion. Technicians process the data with the aid of computer software, and the final output is reviewed and interpreted by a cardiologist or cardiac electrophysiologist.
Holter monitoring for at least 24 hours is a critical step in assessing any patient with known or suspected PVCs, as it can both quantify the total burden of ventricular ectopy and identify the presence of any related ventricular tachycardia. In addition, it can detect additional supraventricular arrhythmias or bradycardia during the monitoring period. The PVC burden is an important measurement; it is expressed as the percentage of heartbeats that were ventricular extrasystoles during the monitoring period.
Both ECG and Holter monitoring are limited in that they are only snapshots of the rhythm during the period when a patient is actually hooked up. Many patients experience PVCs in clusters every very few days or weeks. Such a pattern is unlikely to be detected by a single ECG or 24- or 48-hour Holter monitoring.
A 30-day ambulatory event monitor (also known as a wearable loop recorder) is an important diagnostic tool in these scenarios. The concept is very similar to that of Holter monitoring, except that the device provides a continuous loop recording of the cardiac rhythm that is digitally stored in clips when the patient activates the device. Some wearable loop recorders also have auto-save features for heart rates falling outside of a programmed range.
Mobile outpatient cardiac telemetry is the most comprehensive form of noninvasive rhythm monitoring available. This is essentially the equivalent of continuous inpatient cardiac telemetry, but in a patient who is not hospitalized. It is a wearable ambulatory device providing continuous recordings, real-time automatic detections, and patient-activated symptom recordings. It can be used for up to 6 weeks. Advantages include detection and quantification of asymptomatic events, and real-time transmissions that the physician can act upon. The major disadvantage is cost, including coverage denial by many third-party payers.
This test is rarely indicated as part of a PVC evaluation and is typically ordered only by a cardiologist or cardiac electrophysiologist.
Noninvasive cardiac evaluation
Surface echocardiography is indicated to look for overt structural heart disease and can reliably detect abnormalities in cardiac chamber size, wall thickness, and function. Valvular heart disease is concomitantly identified by two-dimensional imaging as well as by color Doppler. The finding of significant structural heart disease in conjunction with PVCs should prompt a cardiology referral, as this carries significant prognostic implications.3–5
Exercise treadmill stress testing is appropriate for patients who experience PVCs with exercise or for whom an evaluation for coronary artery disease is indicated. The expected finding would be an increase in PVCs or ventricular tachycardia with exercise or in the subsequent recovery period. Exercise testing can be combined with either echocardiographic or nuclear perfusion imaging to evaluate the possibility of myocardial ischemia. For patients unable to exercise, pharmacologic stress testing with dobutamine or a vasodilator agent can be performed.
Advanced noninvasive cardiac imaging— such as computed tomography, magnetic resonance imaging, or positron-emission tomography—should be reserved for specific clinical indications such as congenital heart disease, suspected cardiac sarcoidosis, and infiltrative heart disease, and for specific cardiomyopathies, such as hypertrophic cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy. For example, frequent PVCs with a left bundle branch block morphology and superior axis raise the concern for a right ventricular disorder and may prompt cardiac magnetic resonance imaging for either arrhythmogenic right ventricular cardiomyopathy or sarcoidosis.
PVCs WITHOUT STRUCTURAL HEART DISEASE
Outflow tract PVCs and ventricular tachycardia
The right or left ventricular outflow tracts, or the epicardial tissue immediately adjacent to the aortic sinuses of Valsalva are the most common sites of origin for ventricular ectopy in the absence of structural heart disease.6–9 Affected cells often demonstrate a triggered activity mechanism due to cyclic adenosine monophosphate-mediated and calcium-dependent delayed after-depolarizations.7,8
Most of these foci are in the right ventricular outflow tract, producing a left bundle branch block morphology with an inferior axis (positive R waves in limb leads II, III, and aVF) and typical precordial R-wave transition in V3 and V4 (Figure 1). A minority are in the left ventricular outflow tract, producing a right bundle branch block with an inferior axis pattern, or in the aortic sinuses with a left bundle branch block pattern but with early precordial R transition in V2 and V3.
A study in 122 patients showed that right and left outflow tract arrhythmias had similar electrophysiologic properties and pharmacologic sensitivities, providing evidence for shared mechanisms possibly due to the common embryologic origin of these structures.9
Such arrhythmias are typically catecholamine-sensitive and are sometimes inducible with burst pacing in the electrophysiology laboratory. The short ventricular coupling intervals can promote intracellular calcium overload in the affected cells, leading to triggered activity.
Therefore, outflow tract PVCs and ventricular tachycardia are commonly encountered clinically during exercise and, to an even greater extent, in the postexercise cool-down period. Similarly, they can be worse during periods of emotional stress or fatigue, when the body’s endogenous catecholamine production is elevated. However, it is worthwhile to note that there are exceptions to this principle in which faster sinus rates seem to overdrive the PVCs in some patients, causing them to become paradoxically more frequent at rest, or even during sleep.
Outflow tract PVCs can be managed medically with beta-blockers, nondihydropyridine calcium channel blockers (verapamil or diltiazem), or, less commonly, class IC drugs such as flecainide. They are also highly curable by catheter ablation (Figure 2), with procedure success rates greater than 90%.9.10
However, a subset of outflow tract PVCs nested deep in a triangle of epicardial tissue between the right and left endocardial surface and underneath the left main coronary artery can be challenging. This region has been labeled the left ventricular summit, and is shielded from ablation by an epicardial fat pad in the adjacent pericardial space.11 Ablation attempts made from the right and left endocardial surfaces as well as the epicardial surface (pericardial space) sometimes cannot adequately penetrate the tissue deep enough to reach the originating focus deep within this triangle. While ablation cannot always fully eliminate the PVC, ablation from more than one of the sites listed can generally reduce its burden, often in combination with suppressive medical therapy (Figure 3).
Fascicular PVCs
Fascicular PVCs originate from within the left ventricular His-Purkinje system12 and produce a right bundle branch block morphology with either an anterior or posterior hemiblock pattern (Figure 4). Exit from the posterior fascicle causes an anterior hemiblock pattern, and exit from the anterior fascicle a posterior hemiblock pattern. Utilization of the rapidly conducting His-Purkinje system gives these PVCs a very narrow QRS duration, sometimes approaching 120 milliseconds or shorter. This occasionally causes them to be mistaken for aberrantly conducted supraventricular beats. Such spontaneous PVCs are commonly associated with both sustained and nonsustained ventricular tachycardia and are usually sensitive to verapamil.13
Special issues relating to mapping and catheter ablation of fascicular arrhythmias involve the identification of Purkinje fiber potentials and associated procedural diagnostic maneuvers during tachycardia.14
Other sites for PVCs
Other sites of origin for PVCs in the absence of structural heart disease include ventricular tissue adjacent to the aortomitral continuity,15 the tricuspid annulus,16 the mitral valve annulus, 17 papillary muscles,18 and other Purkinje-adjacent structures such as left ventricular false tendons.19 An example of a papillary muscle PVC is shown in Figures 5 and 6.
Curable by catheter ablation
Any of these PVCs can potentially be cured by catheter ablation when present at a sufficient burden to allow for activation mapping in the electrophysiology laboratory. The threshold for offering ablation varies among operators, but is generally around 10% or greater. Pacemapping is a technique applied in the electrophysiology laboratory when medically refractory symptomatic PVCs occurring at a lower burden require ablation.
PVCs WITH AN UNDERLYING CARDIAC CONDITION
Coronary artery disease
Tissue injury and death caused by acute myocardial infarction has long been recognized as a common cause of spontaneous ventricular ectopy attributed to infarct border zones of ischemic or hibernating myocardium.20,21
Suppression has not been associated with improved outcomes, as shown for class IC drugs in the landmark Cardiac Arrhythmia Suppression Trial (CAST),22 or in the amiodarone treatment arm of the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II).23 Therefore, treatment of ventricular ectopy in this patient population is usually symptom-driven unless there is hemodynamic intolerance, tachycardia-related cardiomyopathy, or a very high burden of PVCs in a patient who may be at risk of developing tachycardia-related cardiomyopathy. Antiarrhythmic drug treatment, when required, usually involves beta-blockers or class III medications such as sotalol or amiodarone.
Nonischemic dilated cardiomyopathy
This category includes patients with a wide variety of disease states including valvular heart disease, lymphocytic and other viral myocarditis, cardiac sarcoidosis, amyloidosis and other infiltrative diseases, familial conditions, and idiopathic dilated cardiomyopathy (ie, etiology unknown). Although it is a heterogeneous group, a common theme is that PVCs in this patient cohort may require epicardial mapping and ablation.24 Similarly, epicardial PVCs and ventricular tachycardia cluster at the basal posterolateral left ventricle near the mitral annulus, for unclear reasons.25
While specific criteria have been published, an epicardial focus is suggested by slowing of the initial QRS segment, pseudo-delta waves, a wider overall QRS, and Q waves in limb lead I.26
Treatment is symptom-driven unless the patient has a tachycardia-related cardiomyopathy or a high burden associated with the risk for its development. Antiarrhythmic drug therapy, when required, typically involves a beta-blocker or a class III drug such as sotalol or amiodarone. Sotalol is used in this population but has limited safety data and should be used cautiously in patients without an implantable cardioverter-defibrillator.
Arrhythmogenic right ventricular cardiomyopathy
Spontaneous ventricular ectopy and tachycardia are common, if not expected, in patients with this heritable autosomal dominant disorder. This condition is progressive and associated with the risk of sudden cardiac death. Criteria for diagnosis were established in 2010, and patients with suspected arrhythmogenic right ventricular cardiomyopathy often undergo cardiac magnetic resonance imaging.27 Diagnostic findings include fibro-fatty tissue replacement, which usually starts in the right ventricle but can progress to involve the left ventricle. PVCs and ventricular tachycardia can involve the right ventricular free wall and are often epicardial.
Catheter ablation is usually palliative, as future arrhythmias are expected. Many patients with this condition require an implantable cardioverter-defibrillator for prevention of sudden cardiac death, and some go on to cardiac transplantation as the disease progresses and ventricular arrhythmias become incessant.
Other conditions
Spontaneous ventricular ectopy is common in other heritable and acquired cardiomyopathies including hypertrophic cardiomyopathy and in infiltrative or inflammatory disorders such as cardiac amyloidosis and sarcoidosis. While technically falling under the rubric of nonischemic heart disease, the presence of spontaneous ventricular ectopy carries specific prognostic implications depending on the underlying diagnosis. Therefore, an appropriate referral for complete cardiac evaluation should be considered when a heritable disorder or other acquired structural heart disease is suspected.
TACHYCARDIA-RELATED CARDIOMYOPATHY
Tachycardia-related cardiomyopathy refers to left ventricular systolic dysfunction that is primarily caused by arrhythmias. This includes frequent PVCs or ventricular tachycardia but also atrial arrhythmias occurring at a high burden that directly weaken myocardial function over time. Although much research has been devoted to this condition, our understanding of its etiology and pathology is incomplete.
PVCs and ventricular ectopy burdens in excess of 15% to 20% have been associated with the development of this condition.28,29 However, it is important to note that cardiomyopathy can also develop at lower burdens.30 One study found that a burden greater than 24% was 79% sensitive and 78% specific for development of tachycardia-related cardiomyopathy.31 Additional studies have demonstrated specific PVC morphologic features such as slurring in the initial QRS segment and also PVCs occurring at shorter coupling intervals as being associated with cardiomyopathy.32–34
For these reasons, both quantification of the total burden and careful evaluation of available electrocardiograms and rhythm strips are important even in asymptomatic patients with frequent PVCs. Similarly, unexplained left ventricular dysfunction in patients with PVC burdens in these discussed ranges should raise suspicion for this diagnosis. Patients with tachycardia-related cardiomyopathy usually have at least partially reversible left ventricular dysfunction when identified or treated early.29,35
MEDICAL AND ABLATIVE TREATMENT
Available treatments include medical suppression and catheter ablation. One needs to exercise clinical judgment and incorporate all of the PVC-related data to make treatment decisions.
Little data for trigger avoidance and behavioral modification
Some patients report a strong association between palpitations related to PVCs and caffeine intake, other stimulants, or other dietary triggers. However, few data exist to support the role of trigger avoidance and behavioral modification in treatment. In fact, an older randomized trial in 81 men found no benefit in a program of total abstinence from caffeine and smoking, moderation of alcohol intake, and physical conditioning.36
Nonetheless, some argue in favor of advising patients to make these dietary and lifestyle changes, given the overall health benefits of aggressive risk-factor modification for cardiovascular disease.37 Certainly, a trial of trigger avoidance and behavioral modification seems reasonable for patients who have strongly associated historical triggers in the absence of structural heart disease and PVCs occurring at a low to modest burden.
Beta-blockers are the mainstay
Beta-blockers are the mainstay of medical suppression of PVCs, primarily through their effect on beta-1 adrenergic receptors to reduce intracellular cyclic adenosine monophosphate and thus decrease automaticity. Blocking beta-1 receptors also causes a negative chronotropic effect, reducing the resting sinus rate in addition to slowing atrioventricular nodal conduction.
Cardioselective beta-blockers include atenolol, betaxolol, metoprolol, and nadolol. These drugs are effective in suppressing PVCs, or at least in reducing the burden to more tolerable levels.
Beta-blockers are most strongly indicated in patients who require PVC suppression and who have concomitant coronary artery disease, prior myocardial infarction, or other cardiomyopathy, as this drug class favorably affects long-term prognosis in these conditions.
Common side effects of beta-blockers include fatigue, shortness of breath, depressed mood, and loss of libido. Side effects can present a significant challenge, particularly for younger patients. Noncardioselective beta-blockers are less commonly prescribed, with the exception of propranolol, which is an effective sympatholytic drug that blocks both beta-1 and beta-2 receptors.
Many patients with asthma or peripheral arterial disease can tolerate these drugs well despite concerns about provoked bronchospasm or claudication, respectively, and neither of these conditions is considered an absolute contraindication. Excessive bradycardia with beta-blocker therapy can lead to dizziness, lightheadedness, or overt syncope, and these drugs should be used with caution in patients with baseline sinus node dysfunction or atrioventricular nodal disease.
Nondihydropyridine calcium channel blockers
Nondihydropyridine calcium channel blockers are particularly effective for PVC suppression in patients without structural heart disease by the mechanisms previously described involving intracellular calcium channels. In particular, they are highly effective and are considered the drugs of choice in treating fascicular PVCs.
Verapamil is a potent drug in this class, but it also commonly causes constipation as a side effect. Diltiazem is less constipating but can cause fatigue, drowsiness, and headaches. Both drugs reduce the resting heart rate and slow atrioventricular nodal conduction. Patients predisposed to bradycardia or atrioventricular block can develop dizziness or overt syncope. Calcium channel blockers are also used cautiously in patients with congestive heart failure, given their potential negative inotropic effects.
Overall, calcium channel blockers are a very reasonable choice for young patients without structural heart disease who need PVC suppression.
Other antiarrhythmic drugs
Sotalol merits special consideration because it has both beta-blocker and class III antiarrhythmic properties, blocking potassium channels and prolonging cardiac repolarization. It can be very effective in PVC suppression but also creates some degree of QT prolongation. The QT-prolonging effect is accentuated in patients with baseline QT prolongation or abnormal renal function. Rarely, this can lead to torsades de pointes. As a safety precaution, some patients are admitted to the hospital when they start sotalol therapy so that they can be monitored with continuous telemetry and ECG to detect excessive QT prolongation.
Amiodarone is a versatile drug with mixed pharmacologic properties that include a predominantly potassium channel-blocking class III drug effect. However, this effect is balanced by its other pharmacologic properties that make QT prolongation less of a clinical concern. Excessive QT prolongation may still occur when used concomitantly with other QT-prolonging drugs.
Amiodarone is very effective in suppressing PVCs and ventricular arrhythmias but has considerable short-term and long-term side effects. Cumulative toxicity risks include damage to the thyroid gland, liver, skin, eyes, and lungs. Routine thyroid function testing, pulmonary function testing, and eye examinations are often considered for patients on long-term amiodarone therapy. Short-term use of this drug does not typically require such surveillance.
Catheter ablation
As mentioned in the previous sections, catheter ablation is a safe and effective treatment for PVCs. It is curative in most cases, and significantly reduces the PVC burden in others.
Procedure. Patients are brought to the electrophysiology laboratory in a fasted state and are partially sedated with an intravenous drug such as midazolam or fentanyl, or both. Steerable catheters are placed into appropriate cardiac chambers from femoral access sites, which are infiltrated with local anesthesia. Sometimes sedative or analgesic drugs must be limited if they are known to suppress PVCs.
Most operators prefer a technique called activation mapping, in which the catheter is maneuvered to home in on the precise PVC origin within the heart, which is subsequently ablated. This technique has very high success rates, but having enough spontaneous PVCs to map during the procedure is essential for the technique to succeed. Conversely, not having sufficient PVCs on the day of the procedure is a common reason that ablation fails or cannot be performed at all.
Pace-mapping is an alternate technique that does not require a continuous stream of PVCs. This involves pacing from different candidate locations inside the heart in an effort to precisely match the ECG appearance of the clinical PVC and to ablate at this site. Although activation mapping generally yields higher success rates and is preferred by most operators, pace-mapping can be successful when a perfect 12–12 match is elicited. In many cases, the two techniques are used together during the same procedure, particularly if the patient’s PVCs spontaneously wax and wane, as they often do.
Risks. Like any medical procedure, catheter ablation carries some inherent risks, including rare but potentially serious events. Unstable arrhythmias may require pace-termination from the catheter or, rarely, shock-termination externally. Even more rare is cardiac arrest requiring cardiopulmonary resuscitation. Uncommon but life-threatening complications also include pericardial effusion or cardiac tamponade requiring percutaneous drainage or, rarely, emergency surgical correction. Although such events are life-threatening, death is extremely rare.
Complications causing permanent disability are also very uncommon but include the risk of collateral injury to the conduction system requiring permanent pacemaker placement, injury to the coronary vessels requiring urgent treatment, or diaphragmatic injury affecting breathing. Left-sided cardiac ablation also carries a small risk of stroke, which is mitigated by giving intravenous heparin during the procedure.
More common but generally non-life-threatening complications include femoral vascular events such as hematomas, pseudoaneurysms, or fistulas that sometimes require subsequent treatment. These complications are generally treatable but can significantly prolong the recovery period.
Catheter ablation procedures are typically 2 to 6 hours in duration, depending on the chambers involved, PVC frequency, and other considerations. Postprocedure bed rest is required for a number of hours. A Foley catheter is sometimes used for patient comfort when a prolonged procedure is anticipated. This carries a small risk of urinary tract infection. Epicardial catheter ablation that requires access to the surface of the heart (ie, the pericardial space) is uncommon but carries some unique risks, including rare injury to coronary vessels or adjacent organs such as the liver or stomach.
Overall, both endocardial and epicardial catheter ablation can be performed safely and effectively in the overwhelming majority of patients, but understanding and explaining the potential risks remains a crucial part of the informed consent process.
TAKE-HOME POINTS
- PVCs are a common cause of palpitations but are also noted as incidental findings by ECG, Holter monitoring, and inpatient telemetry.
- The diagnostic evaluation includes an assessment for underlying structural heart disease and quantification of the total PVC burden.
- Patients without structural heart disease and with low-to-modest PVC burdens may not require specific treatment. PVCs at greater burdens, typically 15% to 20%, or with specific high-risk features carry a risk of tachycardia-related cardiomyopathy and may require treatment even if they are asymptomatic. These high-risk features include initial QRS slurring and PVCs occurring at shorter coupling intervals.
- Treatment involves medical therapy with a beta-blocker, a calcium channel blocker, or another antiarrhythmic drug, and catheter ablation in selected cases.
- Catheter ablation can be curative but is typically reserved for drug-intolerant or medically refractory patients with a high PVC burden.
Premature ventricular complexes (PVCs) are a common cause of palpitations, and are also often detected incidentally on electrocardiography (ECG), ambulatory monitoring, or inpatient telemetry. At the cellular level, ventricular myocytes spontaneously depolarize to create an extra systole that is “out of sync” with the cardiac cycle.
Although nearly everyone has some PVCs from time to time, people vary widely in their frequency of PVCs and their sensitivity to them.1,2 Some patients are exquisitely sensitive to even a small number of PVCs, while others are completely unaware of PVCs in a bigeminal pattern (ie, every other heartbeat). This article will review the evaluation and management of PVCs with a focus on clinical aspects.
DIAGNOSTIC EVALUATION
Personal and family history
Symptoms. The initial history should establish the presence, extent, timing, and duration of symptoms. Patients may use the word “palpitations” to describe their symptoms, but they also describe them as “hard” heartbeats, “chest-thumping,” or as a “catch” or “skipped” heartbeat. Related symptoms may include difficulty breathing, chest pain, fatigue, and dizziness.
The interview should determine whether the symptoms represent a minor nuisance or a major quality-of-life issue to the patient, and whether there are any specific associations or triggers. For example, it is very common for patients to become aware of PVCs at night, particularly in certain positions, such as lying on the left side. Patients often associate PVC symptoms with emotional stress, exercise, or caffeine or stimulant use.
Medication use. An accurate and up-to-date list of prescription medications should be screened for alpha-, beta-, or dopamine-receptor agonist drugs. Similarly, any use of over-the-counter sympathomimetic medications and nonprescription supplements should be elicited, including compounded elixirs or beverages. Many commercially available products designed to treat fatigue or increase alertness contain large doses of caffeine or other stimulants. It is also important to consider the use of illicit substances such as cocaine, amphetamine, methamphetamine, and their derivatives.
The patient’s medical and surgical history should be queried for any known structural heart disease, including coronary artery disease, myocardial infarction, congestive heart failure, valvular heart disease, congenital heart disease, and heritable conditions such as hypertrophic cardiomyopathy, prolonged QT syndromes, or other channel disorders. Pulmonary disorders such as sarcoidosis, pulmonary hypertension, or obstructive sleep apnea are also relevant. Similarly, it is important to identify endocrine disorders, including thyroid problems, sex hormone abnormalities, or adrenal gland conditions.
A careful family history should include any instance of sudden death in first-degree relatives, any heritable cardiac conditions, or coronary artery disease at an early age.
Physical examination
The physical examination should focus on findings that suggest underlying structural heart disease. Findings suggestive of congestive heart failure include elevated jugular venous pressures, abnormal cardiac sounds, pulmonary rales, abnormal arterial pulses, or peripheral edema. A murmur or a pathologic heart sound should raise suspicion of valvular or congenital heart disease when present in a young patient.
Inspection and palpation of the thyroid can reveal a related disorder. Obvious skin changes or neurologic findings can similarly reveal a systemic and possibly related clinical disorder that can have cardiac manifestations (eg, muscular dystrophy).
Electrocardiography, Holter monitoring, and other monitoring
Assessment of the cardiac rhythm includes 12-lead ECG and ambulatory Holter monitoring, typically for 24 or 48 hours.
Holter monitoring provides a continuous recording, usually in at least two or three leads. Patients are given a symptom journal or are asked to keep a diary of symptoms experienced during the monitoring period. The monitor is worn underneath clothing and is returned for download upon completion. Technicians process the data with the aid of computer software, and the final output is reviewed and interpreted by a cardiologist or cardiac electrophysiologist.
Holter monitoring for at least 24 hours is a critical step in assessing any patient with known or suspected PVCs, as it can both quantify the total burden of ventricular ectopy and identify the presence of any related ventricular tachycardia. In addition, it can detect additional supraventricular arrhythmias or bradycardia during the monitoring period. The PVC burden is an important measurement; it is expressed as the percentage of heartbeats that were ventricular extrasystoles during the monitoring period.
Both ECG and Holter monitoring are limited in that they are only snapshots of the rhythm during the period when a patient is actually hooked up. Many patients experience PVCs in clusters every very few days or weeks. Such a pattern is unlikely to be detected by a single ECG or 24- or 48-hour Holter monitoring.
A 30-day ambulatory event monitor (also known as a wearable loop recorder) is an important diagnostic tool in these scenarios. The concept is very similar to that of Holter monitoring, except that the device provides a continuous loop recording of the cardiac rhythm that is digitally stored in clips when the patient activates the device. Some wearable loop recorders also have auto-save features for heart rates falling outside of a programmed range.
Mobile outpatient cardiac telemetry is the most comprehensive form of noninvasive rhythm monitoring available. This is essentially the equivalent of continuous inpatient cardiac telemetry, but in a patient who is not hospitalized. It is a wearable ambulatory device providing continuous recordings, real-time automatic detections, and patient-activated symptom recordings. It can be used for up to 6 weeks. Advantages include detection and quantification of asymptomatic events, and real-time transmissions that the physician can act upon. The major disadvantage is cost, including coverage denial by many third-party payers.
This test is rarely indicated as part of a PVC evaluation and is typically ordered only by a cardiologist or cardiac electrophysiologist.
Noninvasive cardiac evaluation
Surface echocardiography is indicated to look for overt structural heart disease and can reliably detect abnormalities in cardiac chamber size, wall thickness, and function. Valvular heart disease is concomitantly identified by two-dimensional imaging as well as by color Doppler. The finding of significant structural heart disease in conjunction with PVCs should prompt a cardiology referral, as this carries significant prognostic implications.3–5
Exercise treadmill stress testing is appropriate for patients who experience PVCs with exercise or for whom an evaluation for coronary artery disease is indicated. The expected finding would be an increase in PVCs or ventricular tachycardia with exercise or in the subsequent recovery period. Exercise testing can be combined with either echocardiographic or nuclear perfusion imaging to evaluate the possibility of myocardial ischemia. For patients unable to exercise, pharmacologic stress testing with dobutamine or a vasodilator agent can be performed.
Advanced noninvasive cardiac imaging— such as computed tomography, magnetic resonance imaging, or positron-emission tomography—should be reserved for specific clinical indications such as congenital heart disease, suspected cardiac sarcoidosis, and infiltrative heart disease, and for specific cardiomyopathies, such as hypertrophic cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy. For example, frequent PVCs with a left bundle branch block morphology and superior axis raise the concern for a right ventricular disorder and may prompt cardiac magnetic resonance imaging for either arrhythmogenic right ventricular cardiomyopathy or sarcoidosis.
PVCs WITHOUT STRUCTURAL HEART DISEASE
Outflow tract PVCs and ventricular tachycardia
The right or left ventricular outflow tracts, or the epicardial tissue immediately adjacent to the aortic sinuses of Valsalva are the most common sites of origin for ventricular ectopy in the absence of structural heart disease.6–9 Affected cells often demonstrate a triggered activity mechanism due to cyclic adenosine monophosphate-mediated and calcium-dependent delayed after-depolarizations.7,8
Most of these foci are in the right ventricular outflow tract, producing a left bundle branch block morphology with an inferior axis (positive R waves in limb leads II, III, and aVF) and typical precordial R-wave transition in V3 and V4 (Figure 1). A minority are in the left ventricular outflow tract, producing a right bundle branch block with an inferior axis pattern, or in the aortic sinuses with a left bundle branch block pattern but with early precordial R transition in V2 and V3.
A study in 122 patients showed that right and left outflow tract arrhythmias had similar electrophysiologic properties and pharmacologic sensitivities, providing evidence for shared mechanisms possibly due to the common embryologic origin of these structures.9
Such arrhythmias are typically catecholamine-sensitive and are sometimes inducible with burst pacing in the electrophysiology laboratory. The short ventricular coupling intervals can promote intracellular calcium overload in the affected cells, leading to triggered activity.
Therefore, outflow tract PVCs and ventricular tachycardia are commonly encountered clinically during exercise and, to an even greater extent, in the postexercise cool-down period. Similarly, they can be worse during periods of emotional stress or fatigue, when the body’s endogenous catecholamine production is elevated. However, it is worthwhile to note that there are exceptions to this principle in which faster sinus rates seem to overdrive the PVCs in some patients, causing them to become paradoxically more frequent at rest, or even during sleep.
Outflow tract PVCs can be managed medically with beta-blockers, nondihydropyridine calcium channel blockers (verapamil or diltiazem), or, less commonly, class IC drugs such as flecainide. They are also highly curable by catheter ablation (Figure 2), with procedure success rates greater than 90%.9.10
However, a subset of outflow tract PVCs nested deep in a triangle of epicardial tissue between the right and left endocardial surface and underneath the left main coronary artery can be challenging. This region has been labeled the left ventricular summit, and is shielded from ablation by an epicardial fat pad in the adjacent pericardial space.11 Ablation attempts made from the right and left endocardial surfaces as well as the epicardial surface (pericardial space) sometimes cannot adequately penetrate the tissue deep enough to reach the originating focus deep within this triangle. While ablation cannot always fully eliminate the PVC, ablation from more than one of the sites listed can generally reduce its burden, often in combination with suppressive medical therapy (Figure 3).
Fascicular PVCs
Fascicular PVCs originate from within the left ventricular His-Purkinje system12 and produce a right bundle branch block morphology with either an anterior or posterior hemiblock pattern (Figure 4). Exit from the posterior fascicle causes an anterior hemiblock pattern, and exit from the anterior fascicle a posterior hemiblock pattern. Utilization of the rapidly conducting His-Purkinje system gives these PVCs a very narrow QRS duration, sometimes approaching 120 milliseconds or shorter. This occasionally causes them to be mistaken for aberrantly conducted supraventricular beats. Such spontaneous PVCs are commonly associated with both sustained and nonsustained ventricular tachycardia and are usually sensitive to verapamil.13
Special issues relating to mapping and catheter ablation of fascicular arrhythmias involve the identification of Purkinje fiber potentials and associated procedural diagnostic maneuvers during tachycardia.14
Other sites for PVCs
Other sites of origin for PVCs in the absence of structural heart disease include ventricular tissue adjacent to the aortomitral continuity,15 the tricuspid annulus,16 the mitral valve annulus, 17 papillary muscles,18 and other Purkinje-adjacent structures such as left ventricular false tendons.19 An example of a papillary muscle PVC is shown in Figures 5 and 6.
Curable by catheter ablation
Any of these PVCs can potentially be cured by catheter ablation when present at a sufficient burden to allow for activation mapping in the electrophysiology laboratory. The threshold for offering ablation varies among operators, but is generally around 10% or greater. Pacemapping is a technique applied in the electrophysiology laboratory when medically refractory symptomatic PVCs occurring at a lower burden require ablation.
PVCs WITH AN UNDERLYING CARDIAC CONDITION
Coronary artery disease
Tissue injury and death caused by acute myocardial infarction has long been recognized as a common cause of spontaneous ventricular ectopy attributed to infarct border zones of ischemic or hibernating myocardium.20,21
Suppression has not been associated with improved outcomes, as shown for class IC drugs in the landmark Cardiac Arrhythmia Suppression Trial (CAST),22 or in the amiodarone treatment arm of the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II).23 Therefore, treatment of ventricular ectopy in this patient population is usually symptom-driven unless there is hemodynamic intolerance, tachycardia-related cardiomyopathy, or a very high burden of PVCs in a patient who may be at risk of developing tachycardia-related cardiomyopathy. Antiarrhythmic drug treatment, when required, usually involves beta-blockers or class III medications such as sotalol or amiodarone.
Nonischemic dilated cardiomyopathy
This category includes patients with a wide variety of disease states including valvular heart disease, lymphocytic and other viral myocarditis, cardiac sarcoidosis, amyloidosis and other infiltrative diseases, familial conditions, and idiopathic dilated cardiomyopathy (ie, etiology unknown). Although it is a heterogeneous group, a common theme is that PVCs in this patient cohort may require epicardial mapping and ablation.24 Similarly, epicardial PVCs and ventricular tachycardia cluster at the basal posterolateral left ventricle near the mitral annulus, for unclear reasons.25
While specific criteria have been published, an epicardial focus is suggested by slowing of the initial QRS segment, pseudo-delta waves, a wider overall QRS, and Q waves in limb lead I.26
Treatment is symptom-driven unless the patient has a tachycardia-related cardiomyopathy or a high burden associated with the risk for its development. Antiarrhythmic drug therapy, when required, typically involves a beta-blocker or a class III drug such as sotalol or amiodarone. Sotalol is used in this population but has limited safety data and should be used cautiously in patients without an implantable cardioverter-defibrillator.
Arrhythmogenic right ventricular cardiomyopathy
Spontaneous ventricular ectopy and tachycardia are common, if not expected, in patients with this heritable autosomal dominant disorder. This condition is progressive and associated with the risk of sudden cardiac death. Criteria for diagnosis were established in 2010, and patients with suspected arrhythmogenic right ventricular cardiomyopathy often undergo cardiac magnetic resonance imaging.27 Diagnostic findings include fibro-fatty tissue replacement, which usually starts in the right ventricle but can progress to involve the left ventricle. PVCs and ventricular tachycardia can involve the right ventricular free wall and are often epicardial.
Catheter ablation is usually palliative, as future arrhythmias are expected. Many patients with this condition require an implantable cardioverter-defibrillator for prevention of sudden cardiac death, and some go on to cardiac transplantation as the disease progresses and ventricular arrhythmias become incessant.
Other conditions
Spontaneous ventricular ectopy is common in other heritable and acquired cardiomyopathies including hypertrophic cardiomyopathy and in infiltrative or inflammatory disorders such as cardiac amyloidosis and sarcoidosis. While technically falling under the rubric of nonischemic heart disease, the presence of spontaneous ventricular ectopy carries specific prognostic implications depending on the underlying diagnosis. Therefore, an appropriate referral for complete cardiac evaluation should be considered when a heritable disorder or other acquired structural heart disease is suspected.
TACHYCARDIA-RELATED CARDIOMYOPATHY
Tachycardia-related cardiomyopathy refers to left ventricular systolic dysfunction that is primarily caused by arrhythmias. This includes frequent PVCs or ventricular tachycardia but also atrial arrhythmias occurring at a high burden that directly weaken myocardial function over time. Although much research has been devoted to this condition, our understanding of its etiology and pathology is incomplete.
PVCs and ventricular ectopy burdens in excess of 15% to 20% have been associated with the development of this condition.28,29 However, it is important to note that cardiomyopathy can also develop at lower burdens.30 One study found that a burden greater than 24% was 79% sensitive and 78% specific for development of tachycardia-related cardiomyopathy.31 Additional studies have demonstrated specific PVC morphologic features such as slurring in the initial QRS segment and also PVCs occurring at shorter coupling intervals as being associated with cardiomyopathy.32–34
For these reasons, both quantification of the total burden and careful evaluation of available electrocardiograms and rhythm strips are important even in asymptomatic patients with frequent PVCs. Similarly, unexplained left ventricular dysfunction in patients with PVC burdens in these discussed ranges should raise suspicion for this diagnosis. Patients with tachycardia-related cardiomyopathy usually have at least partially reversible left ventricular dysfunction when identified or treated early.29,35
MEDICAL AND ABLATIVE TREATMENT
Available treatments include medical suppression and catheter ablation. One needs to exercise clinical judgment and incorporate all of the PVC-related data to make treatment decisions.
Little data for trigger avoidance and behavioral modification
Some patients report a strong association between palpitations related to PVCs and caffeine intake, other stimulants, or other dietary triggers. However, few data exist to support the role of trigger avoidance and behavioral modification in treatment. In fact, an older randomized trial in 81 men found no benefit in a program of total abstinence from caffeine and smoking, moderation of alcohol intake, and physical conditioning.36
Nonetheless, some argue in favor of advising patients to make these dietary and lifestyle changes, given the overall health benefits of aggressive risk-factor modification for cardiovascular disease.37 Certainly, a trial of trigger avoidance and behavioral modification seems reasonable for patients who have strongly associated historical triggers in the absence of structural heart disease and PVCs occurring at a low to modest burden.
Beta-blockers are the mainstay
Beta-blockers are the mainstay of medical suppression of PVCs, primarily through their effect on beta-1 adrenergic receptors to reduce intracellular cyclic adenosine monophosphate and thus decrease automaticity. Blocking beta-1 receptors also causes a negative chronotropic effect, reducing the resting sinus rate in addition to slowing atrioventricular nodal conduction.
Cardioselective beta-blockers include atenolol, betaxolol, metoprolol, and nadolol. These drugs are effective in suppressing PVCs, or at least in reducing the burden to more tolerable levels.
Beta-blockers are most strongly indicated in patients who require PVC suppression and who have concomitant coronary artery disease, prior myocardial infarction, or other cardiomyopathy, as this drug class favorably affects long-term prognosis in these conditions.
Common side effects of beta-blockers include fatigue, shortness of breath, depressed mood, and loss of libido. Side effects can present a significant challenge, particularly for younger patients. Noncardioselective beta-blockers are less commonly prescribed, with the exception of propranolol, which is an effective sympatholytic drug that blocks both beta-1 and beta-2 receptors.
Many patients with asthma or peripheral arterial disease can tolerate these drugs well despite concerns about provoked bronchospasm or claudication, respectively, and neither of these conditions is considered an absolute contraindication. Excessive bradycardia with beta-blocker therapy can lead to dizziness, lightheadedness, or overt syncope, and these drugs should be used with caution in patients with baseline sinus node dysfunction or atrioventricular nodal disease.
Nondihydropyridine calcium channel blockers
Nondihydropyridine calcium channel blockers are particularly effective for PVC suppression in patients without structural heart disease by the mechanisms previously described involving intracellular calcium channels. In particular, they are highly effective and are considered the drugs of choice in treating fascicular PVCs.
Verapamil is a potent drug in this class, but it also commonly causes constipation as a side effect. Diltiazem is less constipating but can cause fatigue, drowsiness, and headaches. Both drugs reduce the resting heart rate and slow atrioventricular nodal conduction. Patients predisposed to bradycardia or atrioventricular block can develop dizziness or overt syncope. Calcium channel blockers are also used cautiously in patients with congestive heart failure, given their potential negative inotropic effects.
Overall, calcium channel blockers are a very reasonable choice for young patients without structural heart disease who need PVC suppression.
Other antiarrhythmic drugs
Sotalol merits special consideration because it has both beta-blocker and class III antiarrhythmic properties, blocking potassium channels and prolonging cardiac repolarization. It can be very effective in PVC suppression but also creates some degree of QT prolongation. The QT-prolonging effect is accentuated in patients with baseline QT prolongation or abnormal renal function. Rarely, this can lead to torsades de pointes. As a safety precaution, some patients are admitted to the hospital when they start sotalol therapy so that they can be monitored with continuous telemetry and ECG to detect excessive QT prolongation.
Amiodarone is a versatile drug with mixed pharmacologic properties that include a predominantly potassium channel-blocking class III drug effect. However, this effect is balanced by its other pharmacologic properties that make QT prolongation less of a clinical concern. Excessive QT prolongation may still occur when used concomitantly with other QT-prolonging drugs.
Amiodarone is very effective in suppressing PVCs and ventricular arrhythmias but has considerable short-term and long-term side effects. Cumulative toxicity risks include damage to the thyroid gland, liver, skin, eyes, and lungs. Routine thyroid function testing, pulmonary function testing, and eye examinations are often considered for patients on long-term amiodarone therapy. Short-term use of this drug does not typically require such surveillance.
Catheter ablation
As mentioned in the previous sections, catheter ablation is a safe and effective treatment for PVCs. It is curative in most cases, and significantly reduces the PVC burden in others.
Procedure. Patients are brought to the electrophysiology laboratory in a fasted state and are partially sedated with an intravenous drug such as midazolam or fentanyl, or both. Steerable catheters are placed into appropriate cardiac chambers from femoral access sites, which are infiltrated with local anesthesia. Sometimes sedative or analgesic drugs must be limited if they are known to suppress PVCs.
Most operators prefer a technique called activation mapping, in which the catheter is maneuvered to home in on the precise PVC origin within the heart, which is subsequently ablated. This technique has very high success rates, but having enough spontaneous PVCs to map during the procedure is essential for the technique to succeed. Conversely, not having sufficient PVCs on the day of the procedure is a common reason that ablation fails or cannot be performed at all.
Pace-mapping is an alternate technique that does not require a continuous stream of PVCs. This involves pacing from different candidate locations inside the heart in an effort to precisely match the ECG appearance of the clinical PVC and to ablate at this site. Although activation mapping generally yields higher success rates and is preferred by most operators, pace-mapping can be successful when a perfect 12–12 match is elicited. In many cases, the two techniques are used together during the same procedure, particularly if the patient’s PVCs spontaneously wax and wane, as they often do.
Risks. Like any medical procedure, catheter ablation carries some inherent risks, including rare but potentially serious events. Unstable arrhythmias may require pace-termination from the catheter or, rarely, shock-termination externally. Even more rare is cardiac arrest requiring cardiopulmonary resuscitation. Uncommon but life-threatening complications also include pericardial effusion or cardiac tamponade requiring percutaneous drainage or, rarely, emergency surgical correction. Although such events are life-threatening, death is extremely rare.
Complications causing permanent disability are also very uncommon but include the risk of collateral injury to the conduction system requiring permanent pacemaker placement, injury to the coronary vessels requiring urgent treatment, or diaphragmatic injury affecting breathing. Left-sided cardiac ablation also carries a small risk of stroke, which is mitigated by giving intravenous heparin during the procedure.
More common but generally non-life-threatening complications include femoral vascular events such as hematomas, pseudoaneurysms, or fistulas that sometimes require subsequent treatment. These complications are generally treatable but can significantly prolong the recovery period.
Catheter ablation procedures are typically 2 to 6 hours in duration, depending on the chambers involved, PVC frequency, and other considerations. Postprocedure bed rest is required for a number of hours. A Foley catheter is sometimes used for patient comfort when a prolonged procedure is anticipated. This carries a small risk of urinary tract infection. Epicardial catheter ablation that requires access to the surface of the heart (ie, the pericardial space) is uncommon but carries some unique risks, including rare injury to coronary vessels or adjacent organs such as the liver or stomach.
Overall, both endocardial and epicardial catheter ablation can be performed safely and effectively in the overwhelming majority of patients, but understanding and explaining the potential risks remains a crucial part of the informed consent process.
TAKE-HOME POINTS
- PVCs are a common cause of palpitations but are also noted as incidental findings by ECG, Holter monitoring, and inpatient telemetry.
- The diagnostic evaluation includes an assessment for underlying structural heart disease and quantification of the total PVC burden.
- Patients without structural heart disease and with low-to-modest PVC burdens may not require specific treatment. PVCs at greater burdens, typically 15% to 20%, or with specific high-risk features carry a risk of tachycardia-related cardiomyopathy and may require treatment even if they are asymptomatic. These high-risk features include initial QRS slurring and PVCs occurring at shorter coupling intervals.
- Treatment involves medical therapy with a beta-blocker, a calcium channel blocker, or another antiarrhythmic drug, and catheter ablation in selected cases.
- Catheter ablation can be curative but is typically reserved for drug-intolerant or medically refractory patients with a high PVC burden.
- Kostis JB, McCrone K, Moreyra AE, et al. Premature ventricular complexes in the absence of identifiable heart disease. Circulation 1981; 63:1351–1356.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009; 95:1230–1237.
- Simpson RJ, Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2002; 143:535–540.
- Chakko CS, Gheorghiade M. Ventricular arrhythmias in severe heart failure: incidence, significance, and effectiveness of antiarrhythmic therapy. Am Heart J 1985; 109:497–504.
- Gami AS, Noheria A, Lachman N, et al. Anatomical correlates relevant to ablation above the semilunar valves for the cardiac electrophysiologist: a study of 603 hearts. J Interv Card Electrophysiol 2011; 30:5–15.
- Lerman BB, Belardinelli L, West GA, Berne RM, DiMarco JP. Adenosine-sensitive ventricular tachycardia: evidence suggesting cyclic AMP-mediated triggered activity. Circulation 1986; 74:270–280.
- Lerman BB, Stein K, Engelstein ED, et al. Mechanism of repetitive monomorphic ventricular tachycardia. Circulation 1995; 92:421–429.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. J Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Kim RJ, Iwai S, Markowitz SM, Shah BK, Stein KM, Lerman BB. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J Am Coll Cardiol 2007; 49:2035–2043.
- Yamada T, McElderry HT, Doppalapudi H, et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol 2010; 3:616–623.
- Ouyang F, Cappato R, Ernst S, et al. Electroanatomic substrate of idiopathic left ventricular tachycardia: unidirectional block and macro-reentry within the Purkinje network. Circulation 2002; 105:462–469.
- Iwai S, Lerman BB. Management of ventricular tachycardia in patients with clinically normal hearts. Curr Cardiol Rep 2000; 2:515–521.
- Nogami A. Purkinje-related arrhythmias part I: monomorphic ventricular tachycardias. Pacing Clin Electrophysiol 2011; 34:624–650.
- Letsas KP, Efremidis M, Kollias G, Xydonas S, Sideris A. Electrocardiographic and electrophysiologic characteristics of ventricular extrasystoles arising from the aortomitral continuity. Cardiol Res Pract 2011; 2011:864964.
- Tada H, Tadokoro K, Ito S, et al. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm 2007; 4:7–16.
- Tada H, Ito S, Naito S, et al. Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol 2005; 45:877–886.
- Doppalapudi H, Yamada T, McElderry HT, Plumb VJ, Epstein AE, Kay GN. Ventricular tachycardia originating from the posterior papillary muscle in the left ventricle: a distinct clinical syndrome. Circ Arrhythm Electrophysiol 2008; 1:23–29.
- Scheinman MM. Role of the His-Purkinje system in the genesis of cardiac arrhythmia. Heart Rhythm 2009; 6:1050–1058.
- Bigger JT, Dresdale FJ, Heissenbuttel RH, Weld FM, Wit AL. Ventricular arrhythmias in ischemic heart disease: mechanism, prevalence, significance, and management. Prog Cardiovasc Dis 1977; 19:255–300.
- Eldar M, Sievner Z, Goldbourt U, Reicher-Reiss H, Kaplinsky E, Behar S. Primary ventricular tachycardia in acute myocardial infarction: clinical characteristics and mortality. The SPRINT Study Group. Ann Intern Med 1992; 117:31–36.
- Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med 1989; 321:406–412.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol 2009; 54:799–808.
- Marchlinski FE. Perivalvular fibrosis and monomorphic ventricular tachycardia: toward a unifying hypothesis in nonischemic cardiomyopathy. Circulation 2007; 116:1998–2001.
- Vallès E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol 2010; 3:63–71.
- Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010; 121:1533–1541.
- Lee GK, Klarich KW, Grogan M, Cha YM. Premature ventricular contraction-induced cardiomyopathy: a treatable condition. Circ Arrhythm Electrophysiol 2012; 5:229–236.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Kanei Y, Friedman M, Ogawa N, Hanon S, Lam P, Schweitzer P. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol 2008; 13:81–85.
- Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010; 7:865–869.
- Moulton KP, Medcalf T, Lazzara R. Premature ventricular complex morphology. A marker for left ventricular structure and function. Circulation 1990; 81:1245–1251.
- Olgun H, Yokokawa M, Baman T, et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm 2011; 8:1046–1049.
- Sun Y, Blom NA, Yu Y, et al. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: an echocardiographic evaluation. Int J Cardiovasc Imaging 2003; 19:295–299.
- Sarrazin JF, Labounty T, Kuhne M, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm 2009; 6:1543–1549.
- DeBacker G, Jacobs D, Prineas R, et al. Ventricular premature contractions: a randomized non-drug intervention trial in normal men. Circulation 1979; 59:762–769.
- Glatter KA, Myers R, Chiamvimonvat N. Recommendations regarding dietary intake and caffeine and alcohol consumption in patients with cardiac arrhythmias: what do you tell your patients to do or not to do? Curr Treat Options Cardiovasc Med 2012; 14:529–535.
- Kostis JB, McCrone K, Moreyra AE, et al. Premature ventricular complexes in the absence of identifiable heart disease. Circulation 1981; 63:1351–1356.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009; 95:1230–1237.
- Simpson RJ, Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2002; 143:535–540.
- Chakko CS, Gheorghiade M. Ventricular arrhythmias in severe heart failure: incidence, significance, and effectiveness of antiarrhythmic therapy. Am Heart J 1985; 109:497–504.
- Gami AS, Noheria A, Lachman N, et al. Anatomical correlates relevant to ablation above the semilunar valves for the cardiac electrophysiologist: a study of 603 hearts. J Interv Card Electrophysiol 2011; 30:5–15.
- Lerman BB, Belardinelli L, West GA, Berne RM, DiMarco JP. Adenosine-sensitive ventricular tachycardia: evidence suggesting cyclic AMP-mediated triggered activity. Circulation 1986; 74:270–280.
- Lerman BB, Stein K, Engelstein ED, et al. Mechanism of repetitive monomorphic ventricular tachycardia. Circulation 1995; 92:421–429.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. J Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Kim RJ, Iwai S, Markowitz SM, Shah BK, Stein KM, Lerman BB. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J Am Coll Cardiol 2007; 49:2035–2043.
- Yamada T, McElderry HT, Doppalapudi H, et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol 2010; 3:616–623.
- Ouyang F, Cappato R, Ernst S, et al. Electroanatomic substrate of idiopathic left ventricular tachycardia: unidirectional block and macro-reentry within the Purkinje network. Circulation 2002; 105:462–469.
- Iwai S, Lerman BB. Management of ventricular tachycardia in patients with clinically normal hearts. Curr Cardiol Rep 2000; 2:515–521.
- Nogami A. Purkinje-related arrhythmias part I: monomorphic ventricular tachycardias. Pacing Clin Electrophysiol 2011; 34:624–650.
- Letsas KP, Efremidis M, Kollias G, Xydonas S, Sideris A. Electrocardiographic and electrophysiologic characteristics of ventricular extrasystoles arising from the aortomitral continuity. Cardiol Res Pract 2011; 2011:864964.
- Tada H, Tadokoro K, Ito S, et al. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm 2007; 4:7–16.
- Tada H, Ito S, Naito S, et al. Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol 2005; 45:877–886.
- Doppalapudi H, Yamada T, McElderry HT, Plumb VJ, Epstein AE, Kay GN. Ventricular tachycardia originating from the posterior papillary muscle in the left ventricle: a distinct clinical syndrome. Circ Arrhythm Electrophysiol 2008; 1:23–29.
- Scheinman MM. Role of the His-Purkinje system in the genesis of cardiac arrhythmia. Heart Rhythm 2009; 6:1050–1058.
- Bigger JT, Dresdale FJ, Heissenbuttel RH, Weld FM, Wit AL. Ventricular arrhythmias in ischemic heart disease: mechanism, prevalence, significance, and management. Prog Cardiovasc Dis 1977; 19:255–300.
- Eldar M, Sievner Z, Goldbourt U, Reicher-Reiss H, Kaplinsky E, Behar S. Primary ventricular tachycardia in acute myocardial infarction: clinical characteristics and mortality. The SPRINT Study Group. Ann Intern Med 1992; 117:31–36.
- Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med 1989; 321:406–412.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol 2009; 54:799–808.
- Marchlinski FE. Perivalvular fibrosis and monomorphic ventricular tachycardia: toward a unifying hypothesis in nonischemic cardiomyopathy. Circulation 2007; 116:1998–2001.
- Vallès E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol 2010; 3:63–71.
- Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010; 121:1533–1541.
- Lee GK, Klarich KW, Grogan M, Cha YM. Premature ventricular contraction-induced cardiomyopathy: a treatable condition. Circ Arrhythm Electrophysiol 2012; 5:229–236.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Kanei Y, Friedman M, Ogawa N, Hanon S, Lam P, Schweitzer P. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol 2008; 13:81–85.
- Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010; 7:865–869.
- Moulton KP, Medcalf T, Lazzara R. Premature ventricular complex morphology. A marker for left ventricular structure and function. Circulation 1990; 81:1245–1251.
- Olgun H, Yokokawa M, Baman T, et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm 2011; 8:1046–1049.
- Sun Y, Blom NA, Yu Y, et al. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: an echocardiographic evaluation. Int J Cardiovasc Imaging 2003; 19:295–299.
- Sarrazin JF, Labounty T, Kuhne M, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm 2009; 6:1543–1549.
- DeBacker G, Jacobs D, Prineas R, et al. Ventricular premature contractions: a randomized non-drug intervention trial in normal men. Circulation 1979; 59:762–769.
- Glatter KA, Myers R, Chiamvimonvat N. Recommendations regarding dietary intake and caffeine and alcohol consumption in patients with cardiac arrhythmias: what do you tell your patients to do or not to do? Curr Treat Options Cardiovasc Med 2012; 14:529–535.
KEY POINTS
- Diagnostic evaluation should include an assessment for structural heart disease and quantification of the total PVC burden by ambulatory Holter monitoring.
- Patients without structural heart disease and low-to-modest PVC burdens do not always require treatment. PVCs at higher burdens (typically more than 15% to 20% of heartbeats) or strung together in runs of ventricular tachycardia pose a higher risk of tachycardia-related cardiomyopathy and heart failure, even if asymptomatic.
- When necessary, treatment for PVCs involves beta-blockers, calcium channel blockers, or other antiarrhythmic drugs and catheter ablation in selected cases.
- Catheter ablation can be curative, but it is typically reserved for drug-intolerant or medically refractory patients with a high PVC burden.
A 74-year-old man with abdominal pain
A 74-year-old man presented to the emergency department in December 2011 with a 1-week history of worsening abdominal pain, nausea with emesis, and decreased appetite. The pain was dull, diffuse, and not related to oral intake or bowel movements. He denied any bloody stools, melena, or hematemesis, but he had not had a bowel movement in the past week.
He was already known to have stage IV colon cancer with metastases to the lungs and liver. He had undergone a partial colectomy in 2009 and was receiving chemotherapy at the time of admission.
He also had an infrarenal abdominal aortic aneurysm that had been repaired in 2003 with endovascular placement of a Gore Excluder stent graft. This was complicated by a type II endoleak, treated with coil embolization. The same endoleak later recurred and was treated with injection of Onyx liquid embolic agent.
His medical history also included hypertension, type 2 diabetes mellitus, and hyperlipidemia. He had undergone a laparoscopic cholecystectomy in 2007.
He denied any fevers, chills, headache, lightheadedness, or change in vision. He had no respiratory, cardiac, or urinary symptoms. He had been constipated for the past few weeks and had recently been started on a bowel regimen, with mild relief. There had been no other changes to his medications.
His temperature on presentation was 97.5°F (36.4°C), blood pressure 120/64 mm Hg, pulse 96, respiratory rate 22, and oxygen saturation 95% on room air. He was awake, alert, oriented, and in no acute distress. His mucous membranes were dry. His lungs were clear to auscultation, and his heart sounds were normal. His bowel sounds were hyperactive and his abdomen was slightly tender diffusely, but there was no abdominal distention, rebound tenderness, guarding, or palpable masses. His joints, muscle strength, and muscle tone were normal. Table 1 shows his initial laboratory values.
Given the patient’s history of colon cancer, the emergency department physician ordered computed tomography (CT) of the abdomen to assess the state of his disease and to evaluate for bowel obstruction. The scan revealed a large abdominal aortic aneurysm with foci of gas within the aneurysmal sac. Metastases in the liver, lung, and retroperitoneum appeared stable; abundant colonic stool suggested constipation (Figure 1).
CAUSES OF PERIAORTIC GAS AFTER ANEURYSM REPAIR
1. What is the most common cause of periaortic ectopic gas in a patient with a repaired abdominal aortic aneurysm?
- Endoleak
- Stent graft infection
- Retroperitoneal fibrosis
- Aortoenteric fistula
Endoleak
Endoleak, a complication of endovascular abdominal aortic aneurysm repair, is defined as blood flow within the aneurysm sac but outside the endoluminal graft.1 It occurs in up to 15% of patients after endograft placement in the first month alone, and in up to 47% of patients eventually.2 It can lead to aneurysm enlargement and rupture. Endoleaks are classified into five types, each with different causes and management options.3,4 Contrast-enhanced CT is the most commonly used diagnostic tool.5
Endoleak cannot be ruled out in our patient, since CT was done without contrast. However, gas within the aneurysm is not consistent with this diagnosis.
Stent graft infection
Infection has been reported in 1% to 6% of patients receiving a stent graft for aortic aneurysm.6 They occur most commonly in the first year after placement; one study showed that 42% of patients diagnosed with graft infection presented within 3 months of endovascular repair.7
The leading cause of graft infection is contamination during the original procedure, but secondary infection from hematologic seeding and contamination from adjacent bowel are also possible.8 In our patient, who underwent graft placement followed by endovascular repairs of endoleaks, bacterial seeding of his aortic aneurysm from the procedures should be considered.9
The most common organisms are staphylococcal species, with Staphylococcus aureus more common in early infection and coagulase-negative staphylococci more common in late infection.10 Methicillin-resistant S aureus has been reported in as many as 25% of cases of graft infection. Diphtheroids and gram-negative enteric organisms should also be considered.11
CT is the most effective imaging test for graft infection. Perigraft soft tissue, fluid, and gas are the major CT findings.12
Given that our patient presented with abdominal pain, leukocytosis, and the CT finding of perigraft gas, graft infection should be high on our list differential diagnoses.
Retroperitoneal fibrosis
Retroperitoneal fibrosis is most often idiopathic, although many believe it is due to an exaggerated local inflammatory reaction to aortic atherosclerosis or is a manifestation of a systemic autoimmune disease.13 Secondary retroperitoneal fibrosis may be due to drugs, infection, or malignancy.
Pathologic findings include sclerotic plaques, typically around the abdominal vessels and ureters. Clinical presentations are often nonspecific, with early symptoms that include back or abdominal pain, malaise, anorexia, edema, and hematuria.14,15 Progressive ureteral obstruction can occur in later stages. CT with contrast is the imaging test of choice to visualize the extent of disease, with the fibrosis exhibiting attenuation similar to that of muscle.16
Initial treatment of idiopathic retroperitoneal fibrosis is with a glucocorticoid or other immunosuppressive agent, whereas treatment of secondary retroperitoneal fibrosis is aimed at the underlying cause.17 Late stages complicated by ureteral obstruction typically require surgery.18
Our patient did have some nonspecific complaints that could be due to retroperitoneal fibrosis. He also had an intra-abdominal malignancy, which could lead to secondary retroperitoneal fibrosis. However, his CT findings of periaortic gas are not consistent with this diagnosis.19
Aortoenteric fistula
Aortoenteric fistulas can be either primary or secondary.
Primary aortoenteric fistulas occur de novo in patients who have never undergone any surgery or procedure in the aorta. This type of fistula usually results from pressure erosion of an atherosclerotic abdominal aortic aneurysm into the gastrointestinal tract. They are rare, with an annual incidence of 0.04% to 0.07% in the general population.20,21
Secondary aortoenteric fistulas are complications of aortic reconstructive therapy. After open repair, a perianastomotic or pseudoaneurysmal fistula can develop into the gastrointestinal tract.4 Endovascular repair leaves the aortic wall intact with no exposed suture lines, but an aortoenteric fistula can still develop22 and in fact occur in 0.4% to 3.1% of recipients of stent grafts for abdominal aortic aneurysm repair.23 In such cases, it is commonly thought that graft infection can lead to formation of an aortoenteric fistula, but a penetrating gastrointestinal ulcer, tumor invasion, radiation therapy, and trauma have also been implicated.19,24–26 An aortoenteric fistula can present several months to several years after either open or endovascular abdominal aortic aneurysm repair.4,23
One of the main CT signs of an aortoenteric fistula is periaortic ectopic gas at least 3 to 4 weeks after surgery or endovascular repair.19 Gas around the stent graft is most commonly caused by infection, but an aortoenteric fistula must also be considered in our patient, as roughly one-third of graft infections present as aortoenteric fistula.27 Our patient denied having any gastrointestinal bleeding, but his hemoglobin concentration at presentation was 8.9 g/dL.
Highlight point. Perigraft gas after abdominal aortic aneurysm repair can be seen in graft infection and aortoenteric fistula.
SIGNS AND SYMPTOMS OF AORTOENTERIC FISTULA
2. What is the most common clinical sign or symptom of an aortoenteric fistula?
- Gastrointestinal bleeding
- Sepsis
- Abdominal pain
- Back pain
Gastrointestinal bleeding occurs in 80% of patients who have an aortoenteric fistula, sepsis in 40%, abdominal pain in 30%, and back pain in 15%.19 The classic triad of symptoms is gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass. However, symptoms can vary widely, and the classic triad is present in fewer than 25% of cases.28 Sepsis may be the predominant clinical manifestation, particularly in the early stages of fistula formation. Unexplained fever is an underrecognized early manifestation.24
Highlight point. The classic triad of symptoms of an aortoenteric fistula (gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass) is seen in fewer than 25% of cases.
Case continued: The patient develops frank bleeding
The vascular surgery service was consulted because of concern for an aortic graft infection, since surgical removal of the infected material is recommended.10 The patient was deemed to be a poor surgical candidate, given his stage IV colon cancer, so he was treated conservatively with broad-spectrum antibiotics.
Over the next 2 days, he had two episodes of dark, bloody bowel movements, but he remained hemodynamically stable. He subsequently developed frank bleeding per rectum with symptoms of lightheadedness, and his hemoglobin concentration fell to 6.9 g/dL. He was given a total of 3 units of packed red blood cells, which raised his hemoglobin level, but only to 8.3 g/dL. The gastroenterology service was consulted to evaluate for the source of the bleeding.
Comment. In a situation like this, an aortoenteric fistula is high on our list of differential diagnoses as the cause of bleeding, but other causes of frank bleeding per rectum such as diverticulosis, arteriovenous malformation, hemorrhoids, or a rapid upper-gastrointestinal bleed cannot be ruled out.
Upper-gastrointestinal endoscopy is the most commonly used diagnostic test for aortoenteric fistulas. It can also find other possible sources of gastrointestinal bleeding. CT with contrast is another option. It can depict the fistula itself or reveal signs of infection, such as gas or liquid surrounding the graft. In an emergency, when there is not enough time for diagnostic testing and an aortoenteric fistula is strongly suspected on clinical grounds, emergency surgical exploration is warranted.4,24
In our patient, the gastrointestinal service elected to first perform endoscopy to look for an aortoenteric fistula.
WHERE DO AORTOENTERIC FISTULAS OCCUR?
3. In which part of the gastrointestinal tract is an aortoenteric fistula most commonly located?
- Esophagus
- Stomach
- Duodenum
- Jejunum
Aortoenteric fistulas can occur at any of these locations, but 80% of cases of secondary aortoenteric fistula are in the duodenum, most often in the third or fourth (horizontal or ascending) part.19 Endoscopic visualization of a pulsatile bleeding mass in this area is diagnostic. However, even if no fistula is seen, upper endoscopy cannot rule out an aortoenteric fistula because the lesion can be located more distal than the scope can reach, which is typically no farther than the first or second parts.4,24
Case continued: What endoscopy showed
The esophagus was normal. There was old clotted blood in the stomach, but no lesions or ulcers. The duodenal bulb and second portion of the duodenum were normal. Three ulcers were noted in the third and fourth portions of the duodenum. The largest and deepest ulcer had an adherent blood clot, and the bowel wall was pulsatile in this region (Figure 2). These findings revealed the source of the gastrointestinal bleeding and were consistent with an aortoenteric fistula.
The patient’s initial bloody bowel movements were herald bleeds, ie, transient and self-limited episodes resulting from necrosis and mucosal ulceration. Herald bleeds can precede a massive gastrointestinal hemorrhage resulting from a true aortoenteric communication.19
Highlight point. Herald bleeds are self-limited and precede hemorrhage that results from a true aortoenteric communication.
TREATMENT OF AORTOENTERIC FISTULA
4. How are aortoenteric fistulas treated?
- Surgery
- Antibiotics
- Endoscopic intervention
Surgery is the definitive treatment. The traditional procedure is open surgical resection of the affected portion of the aorta followed by extra-anatomic (axillobifemoral) bypass or in situ aortic reconstruction using an antibiotic-impregnated prosthetic graft, autogenous femoral vein graft, or cryopreserved allograft.9,29 There have been cases of successful endovascular repair of aortoenteric fistulas, but this approach is generally used as a palliative bridge to definitive surgery.30
Antibiotics should be used if graft infection is suspected, ie, in most cases. However, surgery is still needed to repair the fistula and remove the source of infection. Cultures taken during surgical repair can help guide the choice of antibiotic after surgery.
Endoscopy can aid in diagnosing an aortoenteric fistula, as in the case of our patient. However, vascular surgery is necessary to close the communication between the aorta and the gastrointestinal tract.
Case continued: The patient declines treatment
In view of the patient’s enteroscopic findings, the vascular surgery service was again consulted for surgical correction of the aortoenteric fistula. Treatment was discussed with the patient and his family, but they declined any intervention in view of the high risk of morbidity and death that surgery would entail. Nearing the end of life with advanced cancer and a newly diagnosed aortoenteric fistula, the patient preferred comfort measures with hospice care.
Take-home points
Abdominal pain is the reason for 5% to 10% of emergency department visits, and between 35% to 41% of patients admitted to the hospital because of abdominal pain do not have a definitive diagnosis.31 It is crucial to think about an aortoenteric fistula in such patients who have a history of abdominal aortic aneurysm repair and gastrointestinal bleeding. Timely diagnosis and intervention are necessary to manage this otherwise-fatal condition.
- Hong C, Heiken JP, Sicard GA, Pilgram TK, Bae KT. Clinical significance of endoleak detected on follow-up CT after endovascular repair of abdominal aortic aneurysm. AJR Am J Roentgenol 2008; 191:808–813.
- Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg 2002; 35:1029–1035.
- Corriere MA, Feurer ID, Becker SY, et al. Endoleak following endovascular abdominal aortic aneurysm repair: implications for duration of screening. Ann Surg 2004; 239:800–805.
- Saratzis N, Saratzis A, Melas N, Ktenidis K, Kiskinis D. Aortoduodenal fistulas after endovascular stent-graft repair of abdominal aortic aneurysms: single-center experience and review of the literature. J Endovasc Ther 2008; 15:441–448.
- Demko TM, Diamond JR, Groff J. Obstructive nephropathy as a result of retroperitoneal fibrosis: a review of its pathogenesis and associations. J Am Soc Nephrol 1997; 8:684–688.
- Zetrenne E, McIntosh BC, McRae MH, Gusberg R, Evans GR, Narayan D. Prosthetic vascular graft infection: a multi-center review of surgical management. Yale J Biol Med 2007; 80:113–121.
- Vogel TR, Symons R, Flum DR. The incidence and factors associated with graft infection after aortic aneurysm repair. J Vasc Surg 2008; 47:264–269.
- Swain TW, Calligaro KD, Dougherty MD. Management of infected aortic prosthetic grafts. Vasc Endovascular Surg 2004; 38:75–82.
- Cernohorsky P, Reijnen MM, Tielliu IF, van Sterkenburg SM, van den Dungen JJ, Zeebregts CJ. The relevance of aortic endograft prosthetic infection. J Vasc Surg 2011; 54:327–333.
- FitzGerald SF, Kelly C, Humphreys H. Diagnosis and treatment of prosthetic aortic graft infections: confusion and inconsistency in the absence of evidence or consensus. J Antimicrob Chemother 2005; 56:996–999.
- Orton DF, LeVeen RF, Saigh JA, et al. Aortic prosthetic graft infections: radiologic manifestations and implications for management. Radiographics 2000; 20:977–993.
- Pacanowski JP, Dieter RS, Stevens SL, Freeman MB, Goldman MH. Endoleak: the achilles heel of endovascular abdominal aortic aneurysm exclusion—a case report. WMJ 2002; 101:57–58,63.
- van Bommel EF. Retroperitoneal fibrosis. Neth J Med 2002; 60:231–242.
- Utz DC, Henry JD. Retroperitoneal fibrosis. Med Clin North Am 1966; 50:1091–1099.
- Dalla-Palma L, Rocca-Rossetti S, Pozzi-Mucelli RS, Rizzatto G. Computed tomography in the diagnosis of retroperitoneal fibrosis. Urol Radiol 1981; 3:77–83.
- Harreby M, Bilde T, Helin P, Meyhoff HH, Vinterberg H, Nielsen VA. Retroperitoneal fibrosis treated with methylprednisolon pulse and disease-modifying antirheumatic drugs. Scand J Urol Nephrol 1994; 28:237–242.
- Jois RN, Gaffney K, Marshall T, Scott DG. Chronic periaortitis. Rheumatology (Oxford) 2004; 43:1441–1446.
- Saers SJ, Scheltinga MR. Primary aortoenteric fistula. Br J Surg 2005; 92:143–152.
- Baril DT, Carroccio A, Ellozy SH, et al. Evolving strategies for the treatment of aortoenteric fistulas. J Vasc Surg 2006; 44:250–257.
- Vu QD, Menias CO, Bhalla S, Peterson C, Wang LL, Balfe DM. Aortoenteric fistulas: CT features and potential mimics. Radiographics 2009; 29:197–209.
- Jayarajan S, Napolitano LM, Rectenwald JE, Upchurch GR. Primary aortoenteric fistula and endovascular repair. Vasc Endovascular Surg 2009; 43:592–596.
- Ruby BJ, Cogbill TH. Aortoduodenal fistula 5 years after endovascular abdominal aortic aneurysm repair with the Ancure stent graft. J Vasc Surg 2007; 45:834–836.
- Senadhi V, Brown JC, Arora D, Shaffer R, Shetty D, Mackrell P. A mysterious cause of gastrointestinal bleeding disguising itself as diverticulosis and peptic ulcer disease: a review of diagnostic modalities for aortoenteric fistula. Case Rep Gastroenterol 2010; 4:510–517.
- Simon T, Feller E. Diverse presentation of secondary aortoenteric fistulae. Case Report Med 2011; 2011:406730.
- Schwab CW, McMahon DJ, Phillips G, Pentecost MJ. Aortic balloon control of a traumatic aortoenteric fistula after damage control laparotomy: a case report. J Trauma 1996; 40:1021–1023.
- Napoli PJ, Meade PC, Adams CW. Primary aortoenteric fistula from a posttraumatic pseudoaneurysm. J Trauma 1996; 41:149–152.
- Laser A, Baker N, Rectenwald J, Eliason JL, Criado-Pallares E, Upchurch GR. Graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2011; 54:58–63.
- Luo CY, Lai CH, Wen JS, Lin BW. Secondary aortocolic fistula: case report and review of the literature. Ann Vasc Surg 2010; 24:256.e5–256.e12.
- Kim JY, Kim YW, Kim CJ, Lim HI, Kim DI, Huh S. Successful surgical treatment of aortoenteric fistula. J Korean Med Sci 2007; 22:846–850.
- Verhey P, Best A, Lakin P, Nachiondo J, Petersen B. Successful endovascular treatment of aortoenteric fistula secondary to eroding duodenal stent. J Vasc Interv Radiol 2006; 17:1345–1348.
- Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. In:Hockberger RS, editor: UpToDate. Waltham, MA: UpToDate, 2012.
A 74-year-old man presented to the emergency department in December 2011 with a 1-week history of worsening abdominal pain, nausea with emesis, and decreased appetite. The pain was dull, diffuse, and not related to oral intake or bowel movements. He denied any bloody stools, melena, or hematemesis, but he had not had a bowel movement in the past week.
He was already known to have stage IV colon cancer with metastases to the lungs and liver. He had undergone a partial colectomy in 2009 and was receiving chemotherapy at the time of admission.
He also had an infrarenal abdominal aortic aneurysm that had been repaired in 2003 with endovascular placement of a Gore Excluder stent graft. This was complicated by a type II endoleak, treated with coil embolization. The same endoleak later recurred and was treated with injection of Onyx liquid embolic agent.
His medical history also included hypertension, type 2 diabetes mellitus, and hyperlipidemia. He had undergone a laparoscopic cholecystectomy in 2007.
He denied any fevers, chills, headache, lightheadedness, or change in vision. He had no respiratory, cardiac, or urinary symptoms. He had been constipated for the past few weeks and had recently been started on a bowel regimen, with mild relief. There had been no other changes to his medications.
His temperature on presentation was 97.5°F (36.4°C), blood pressure 120/64 mm Hg, pulse 96, respiratory rate 22, and oxygen saturation 95% on room air. He was awake, alert, oriented, and in no acute distress. His mucous membranes were dry. His lungs were clear to auscultation, and his heart sounds were normal. His bowel sounds were hyperactive and his abdomen was slightly tender diffusely, but there was no abdominal distention, rebound tenderness, guarding, or palpable masses. His joints, muscle strength, and muscle tone were normal. Table 1 shows his initial laboratory values.
Given the patient’s history of colon cancer, the emergency department physician ordered computed tomography (CT) of the abdomen to assess the state of his disease and to evaluate for bowel obstruction. The scan revealed a large abdominal aortic aneurysm with foci of gas within the aneurysmal sac. Metastases in the liver, lung, and retroperitoneum appeared stable; abundant colonic stool suggested constipation (Figure 1).
CAUSES OF PERIAORTIC GAS AFTER ANEURYSM REPAIR
1. What is the most common cause of periaortic ectopic gas in a patient with a repaired abdominal aortic aneurysm?
- Endoleak
- Stent graft infection
- Retroperitoneal fibrosis
- Aortoenteric fistula
Endoleak
Endoleak, a complication of endovascular abdominal aortic aneurysm repair, is defined as blood flow within the aneurysm sac but outside the endoluminal graft.1 It occurs in up to 15% of patients after endograft placement in the first month alone, and in up to 47% of patients eventually.2 It can lead to aneurysm enlargement and rupture. Endoleaks are classified into five types, each with different causes and management options.3,4 Contrast-enhanced CT is the most commonly used diagnostic tool.5
Endoleak cannot be ruled out in our patient, since CT was done without contrast. However, gas within the aneurysm is not consistent with this diagnosis.
Stent graft infection
Infection has been reported in 1% to 6% of patients receiving a stent graft for aortic aneurysm.6 They occur most commonly in the first year after placement; one study showed that 42% of patients diagnosed with graft infection presented within 3 months of endovascular repair.7
The leading cause of graft infection is contamination during the original procedure, but secondary infection from hematologic seeding and contamination from adjacent bowel are also possible.8 In our patient, who underwent graft placement followed by endovascular repairs of endoleaks, bacterial seeding of his aortic aneurysm from the procedures should be considered.9
The most common organisms are staphylococcal species, with Staphylococcus aureus more common in early infection and coagulase-negative staphylococci more common in late infection.10 Methicillin-resistant S aureus has been reported in as many as 25% of cases of graft infection. Diphtheroids and gram-negative enteric organisms should also be considered.11
CT is the most effective imaging test for graft infection. Perigraft soft tissue, fluid, and gas are the major CT findings.12
Given that our patient presented with abdominal pain, leukocytosis, and the CT finding of perigraft gas, graft infection should be high on our list differential diagnoses.
Retroperitoneal fibrosis
Retroperitoneal fibrosis is most often idiopathic, although many believe it is due to an exaggerated local inflammatory reaction to aortic atherosclerosis or is a manifestation of a systemic autoimmune disease.13 Secondary retroperitoneal fibrosis may be due to drugs, infection, or malignancy.
Pathologic findings include sclerotic plaques, typically around the abdominal vessels and ureters. Clinical presentations are often nonspecific, with early symptoms that include back or abdominal pain, malaise, anorexia, edema, and hematuria.14,15 Progressive ureteral obstruction can occur in later stages. CT with contrast is the imaging test of choice to visualize the extent of disease, with the fibrosis exhibiting attenuation similar to that of muscle.16
Initial treatment of idiopathic retroperitoneal fibrosis is with a glucocorticoid or other immunosuppressive agent, whereas treatment of secondary retroperitoneal fibrosis is aimed at the underlying cause.17 Late stages complicated by ureteral obstruction typically require surgery.18
Our patient did have some nonspecific complaints that could be due to retroperitoneal fibrosis. He also had an intra-abdominal malignancy, which could lead to secondary retroperitoneal fibrosis. However, his CT findings of periaortic gas are not consistent with this diagnosis.19
Aortoenteric fistula
Aortoenteric fistulas can be either primary or secondary.
Primary aortoenteric fistulas occur de novo in patients who have never undergone any surgery or procedure in the aorta. This type of fistula usually results from pressure erosion of an atherosclerotic abdominal aortic aneurysm into the gastrointestinal tract. They are rare, with an annual incidence of 0.04% to 0.07% in the general population.20,21
Secondary aortoenteric fistulas are complications of aortic reconstructive therapy. After open repair, a perianastomotic or pseudoaneurysmal fistula can develop into the gastrointestinal tract.4 Endovascular repair leaves the aortic wall intact with no exposed suture lines, but an aortoenteric fistula can still develop22 and in fact occur in 0.4% to 3.1% of recipients of stent grafts for abdominal aortic aneurysm repair.23 In such cases, it is commonly thought that graft infection can lead to formation of an aortoenteric fistula, but a penetrating gastrointestinal ulcer, tumor invasion, radiation therapy, and trauma have also been implicated.19,24–26 An aortoenteric fistula can present several months to several years after either open or endovascular abdominal aortic aneurysm repair.4,23
One of the main CT signs of an aortoenteric fistula is periaortic ectopic gas at least 3 to 4 weeks after surgery or endovascular repair.19 Gas around the stent graft is most commonly caused by infection, but an aortoenteric fistula must also be considered in our patient, as roughly one-third of graft infections present as aortoenteric fistula.27 Our patient denied having any gastrointestinal bleeding, but his hemoglobin concentration at presentation was 8.9 g/dL.
Highlight point. Perigraft gas after abdominal aortic aneurysm repair can be seen in graft infection and aortoenteric fistula.
SIGNS AND SYMPTOMS OF AORTOENTERIC FISTULA
2. What is the most common clinical sign or symptom of an aortoenteric fistula?
- Gastrointestinal bleeding
- Sepsis
- Abdominal pain
- Back pain
Gastrointestinal bleeding occurs in 80% of patients who have an aortoenteric fistula, sepsis in 40%, abdominal pain in 30%, and back pain in 15%.19 The classic triad of symptoms is gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass. However, symptoms can vary widely, and the classic triad is present in fewer than 25% of cases.28 Sepsis may be the predominant clinical manifestation, particularly in the early stages of fistula formation. Unexplained fever is an underrecognized early manifestation.24
Highlight point. The classic triad of symptoms of an aortoenteric fistula (gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass) is seen in fewer than 25% of cases.
Case continued: The patient develops frank bleeding
The vascular surgery service was consulted because of concern for an aortic graft infection, since surgical removal of the infected material is recommended.10 The patient was deemed to be a poor surgical candidate, given his stage IV colon cancer, so he was treated conservatively with broad-spectrum antibiotics.
Over the next 2 days, he had two episodes of dark, bloody bowel movements, but he remained hemodynamically stable. He subsequently developed frank bleeding per rectum with symptoms of lightheadedness, and his hemoglobin concentration fell to 6.9 g/dL. He was given a total of 3 units of packed red blood cells, which raised his hemoglobin level, but only to 8.3 g/dL. The gastroenterology service was consulted to evaluate for the source of the bleeding.
Comment. In a situation like this, an aortoenteric fistula is high on our list of differential diagnoses as the cause of bleeding, but other causes of frank bleeding per rectum such as diverticulosis, arteriovenous malformation, hemorrhoids, or a rapid upper-gastrointestinal bleed cannot be ruled out.
Upper-gastrointestinal endoscopy is the most commonly used diagnostic test for aortoenteric fistulas. It can also find other possible sources of gastrointestinal bleeding. CT with contrast is another option. It can depict the fistula itself or reveal signs of infection, such as gas or liquid surrounding the graft. In an emergency, when there is not enough time for diagnostic testing and an aortoenteric fistula is strongly suspected on clinical grounds, emergency surgical exploration is warranted.4,24
In our patient, the gastrointestinal service elected to first perform endoscopy to look for an aortoenteric fistula.
WHERE DO AORTOENTERIC FISTULAS OCCUR?
3. In which part of the gastrointestinal tract is an aortoenteric fistula most commonly located?
- Esophagus
- Stomach
- Duodenum
- Jejunum
Aortoenteric fistulas can occur at any of these locations, but 80% of cases of secondary aortoenteric fistula are in the duodenum, most often in the third or fourth (horizontal or ascending) part.19 Endoscopic visualization of a pulsatile bleeding mass in this area is diagnostic. However, even if no fistula is seen, upper endoscopy cannot rule out an aortoenteric fistula because the lesion can be located more distal than the scope can reach, which is typically no farther than the first or second parts.4,24
Case continued: What endoscopy showed
The esophagus was normal. There was old clotted blood in the stomach, but no lesions or ulcers. The duodenal bulb and second portion of the duodenum were normal. Three ulcers were noted in the third and fourth portions of the duodenum. The largest and deepest ulcer had an adherent blood clot, and the bowel wall was pulsatile in this region (Figure 2). These findings revealed the source of the gastrointestinal bleeding and were consistent with an aortoenteric fistula.
The patient’s initial bloody bowel movements were herald bleeds, ie, transient and self-limited episodes resulting from necrosis and mucosal ulceration. Herald bleeds can precede a massive gastrointestinal hemorrhage resulting from a true aortoenteric communication.19
Highlight point. Herald bleeds are self-limited and precede hemorrhage that results from a true aortoenteric communication.
TREATMENT OF AORTOENTERIC FISTULA
4. How are aortoenteric fistulas treated?
- Surgery
- Antibiotics
- Endoscopic intervention
Surgery is the definitive treatment. The traditional procedure is open surgical resection of the affected portion of the aorta followed by extra-anatomic (axillobifemoral) bypass or in situ aortic reconstruction using an antibiotic-impregnated prosthetic graft, autogenous femoral vein graft, or cryopreserved allograft.9,29 There have been cases of successful endovascular repair of aortoenteric fistulas, but this approach is generally used as a palliative bridge to definitive surgery.30
Antibiotics should be used if graft infection is suspected, ie, in most cases. However, surgery is still needed to repair the fistula and remove the source of infection. Cultures taken during surgical repair can help guide the choice of antibiotic after surgery.
Endoscopy can aid in diagnosing an aortoenteric fistula, as in the case of our patient. However, vascular surgery is necessary to close the communication between the aorta and the gastrointestinal tract.
Case continued: The patient declines treatment
In view of the patient’s enteroscopic findings, the vascular surgery service was again consulted for surgical correction of the aortoenteric fistula. Treatment was discussed with the patient and his family, but they declined any intervention in view of the high risk of morbidity and death that surgery would entail. Nearing the end of life with advanced cancer and a newly diagnosed aortoenteric fistula, the patient preferred comfort measures with hospice care.
Take-home points
Abdominal pain is the reason for 5% to 10% of emergency department visits, and between 35% to 41% of patients admitted to the hospital because of abdominal pain do not have a definitive diagnosis.31 It is crucial to think about an aortoenteric fistula in such patients who have a history of abdominal aortic aneurysm repair and gastrointestinal bleeding. Timely diagnosis and intervention are necessary to manage this otherwise-fatal condition.
A 74-year-old man presented to the emergency department in December 2011 with a 1-week history of worsening abdominal pain, nausea with emesis, and decreased appetite. The pain was dull, diffuse, and not related to oral intake or bowel movements. He denied any bloody stools, melena, or hematemesis, but he had not had a bowel movement in the past week.
He was already known to have stage IV colon cancer with metastases to the lungs and liver. He had undergone a partial colectomy in 2009 and was receiving chemotherapy at the time of admission.
He also had an infrarenal abdominal aortic aneurysm that had been repaired in 2003 with endovascular placement of a Gore Excluder stent graft. This was complicated by a type II endoleak, treated with coil embolization. The same endoleak later recurred and was treated with injection of Onyx liquid embolic agent.
His medical history also included hypertension, type 2 diabetes mellitus, and hyperlipidemia. He had undergone a laparoscopic cholecystectomy in 2007.
He denied any fevers, chills, headache, lightheadedness, or change in vision. He had no respiratory, cardiac, or urinary symptoms. He had been constipated for the past few weeks and had recently been started on a bowel regimen, with mild relief. There had been no other changes to his medications.
His temperature on presentation was 97.5°F (36.4°C), blood pressure 120/64 mm Hg, pulse 96, respiratory rate 22, and oxygen saturation 95% on room air. He was awake, alert, oriented, and in no acute distress. His mucous membranes were dry. His lungs were clear to auscultation, and his heart sounds were normal. His bowel sounds were hyperactive and his abdomen was slightly tender diffusely, but there was no abdominal distention, rebound tenderness, guarding, or palpable masses. His joints, muscle strength, and muscle tone were normal. Table 1 shows his initial laboratory values.
Given the patient’s history of colon cancer, the emergency department physician ordered computed tomography (CT) of the abdomen to assess the state of his disease and to evaluate for bowel obstruction. The scan revealed a large abdominal aortic aneurysm with foci of gas within the aneurysmal sac. Metastases in the liver, lung, and retroperitoneum appeared stable; abundant colonic stool suggested constipation (Figure 1).
CAUSES OF PERIAORTIC GAS AFTER ANEURYSM REPAIR
1. What is the most common cause of periaortic ectopic gas in a patient with a repaired abdominal aortic aneurysm?
- Endoleak
- Stent graft infection
- Retroperitoneal fibrosis
- Aortoenteric fistula
Endoleak
Endoleak, a complication of endovascular abdominal aortic aneurysm repair, is defined as blood flow within the aneurysm sac but outside the endoluminal graft.1 It occurs in up to 15% of patients after endograft placement in the first month alone, and in up to 47% of patients eventually.2 It can lead to aneurysm enlargement and rupture. Endoleaks are classified into five types, each with different causes and management options.3,4 Contrast-enhanced CT is the most commonly used diagnostic tool.5
Endoleak cannot be ruled out in our patient, since CT was done without contrast. However, gas within the aneurysm is not consistent with this diagnosis.
Stent graft infection
Infection has been reported in 1% to 6% of patients receiving a stent graft for aortic aneurysm.6 They occur most commonly in the first year after placement; one study showed that 42% of patients diagnosed with graft infection presented within 3 months of endovascular repair.7
The leading cause of graft infection is contamination during the original procedure, but secondary infection from hematologic seeding and contamination from adjacent bowel are also possible.8 In our patient, who underwent graft placement followed by endovascular repairs of endoleaks, bacterial seeding of his aortic aneurysm from the procedures should be considered.9
The most common organisms are staphylococcal species, with Staphylococcus aureus more common in early infection and coagulase-negative staphylococci more common in late infection.10 Methicillin-resistant S aureus has been reported in as many as 25% of cases of graft infection. Diphtheroids and gram-negative enteric organisms should also be considered.11
CT is the most effective imaging test for graft infection. Perigraft soft tissue, fluid, and gas are the major CT findings.12
Given that our patient presented with abdominal pain, leukocytosis, and the CT finding of perigraft gas, graft infection should be high on our list differential diagnoses.
Retroperitoneal fibrosis
Retroperitoneal fibrosis is most often idiopathic, although many believe it is due to an exaggerated local inflammatory reaction to aortic atherosclerosis or is a manifestation of a systemic autoimmune disease.13 Secondary retroperitoneal fibrosis may be due to drugs, infection, or malignancy.
Pathologic findings include sclerotic plaques, typically around the abdominal vessels and ureters. Clinical presentations are often nonspecific, with early symptoms that include back or abdominal pain, malaise, anorexia, edema, and hematuria.14,15 Progressive ureteral obstruction can occur in later stages. CT with contrast is the imaging test of choice to visualize the extent of disease, with the fibrosis exhibiting attenuation similar to that of muscle.16
Initial treatment of idiopathic retroperitoneal fibrosis is with a glucocorticoid or other immunosuppressive agent, whereas treatment of secondary retroperitoneal fibrosis is aimed at the underlying cause.17 Late stages complicated by ureteral obstruction typically require surgery.18
Our patient did have some nonspecific complaints that could be due to retroperitoneal fibrosis. He also had an intra-abdominal malignancy, which could lead to secondary retroperitoneal fibrosis. However, his CT findings of periaortic gas are not consistent with this diagnosis.19
Aortoenteric fistula
Aortoenteric fistulas can be either primary or secondary.
Primary aortoenteric fistulas occur de novo in patients who have never undergone any surgery or procedure in the aorta. This type of fistula usually results from pressure erosion of an atherosclerotic abdominal aortic aneurysm into the gastrointestinal tract. They are rare, with an annual incidence of 0.04% to 0.07% in the general population.20,21
Secondary aortoenteric fistulas are complications of aortic reconstructive therapy. After open repair, a perianastomotic or pseudoaneurysmal fistula can develop into the gastrointestinal tract.4 Endovascular repair leaves the aortic wall intact with no exposed suture lines, but an aortoenteric fistula can still develop22 and in fact occur in 0.4% to 3.1% of recipients of stent grafts for abdominal aortic aneurysm repair.23 In such cases, it is commonly thought that graft infection can lead to formation of an aortoenteric fistula, but a penetrating gastrointestinal ulcer, tumor invasion, radiation therapy, and trauma have also been implicated.19,24–26 An aortoenteric fistula can present several months to several years after either open or endovascular abdominal aortic aneurysm repair.4,23
One of the main CT signs of an aortoenteric fistula is periaortic ectopic gas at least 3 to 4 weeks after surgery or endovascular repair.19 Gas around the stent graft is most commonly caused by infection, but an aortoenteric fistula must also be considered in our patient, as roughly one-third of graft infections present as aortoenteric fistula.27 Our patient denied having any gastrointestinal bleeding, but his hemoglobin concentration at presentation was 8.9 g/dL.
Highlight point. Perigraft gas after abdominal aortic aneurysm repair can be seen in graft infection and aortoenteric fistula.
SIGNS AND SYMPTOMS OF AORTOENTERIC FISTULA
2. What is the most common clinical sign or symptom of an aortoenteric fistula?
- Gastrointestinal bleeding
- Sepsis
- Abdominal pain
- Back pain
Gastrointestinal bleeding occurs in 80% of patients who have an aortoenteric fistula, sepsis in 40%, abdominal pain in 30%, and back pain in 15%.19 The classic triad of symptoms is gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass. However, symptoms can vary widely, and the classic triad is present in fewer than 25% of cases.28 Sepsis may be the predominant clinical manifestation, particularly in the early stages of fistula formation. Unexplained fever is an underrecognized early manifestation.24
Highlight point. The classic triad of symptoms of an aortoenteric fistula (gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass) is seen in fewer than 25% of cases.
Case continued: The patient develops frank bleeding
The vascular surgery service was consulted because of concern for an aortic graft infection, since surgical removal of the infected material is recommended.10 The patient was deemed to be a poor surgical candidate, given his stage IV colon cancer, so he was treated conservatively with broad-spectrum antibiotics.
Over the next 2 days, he had two episodes of dark, bloody bowel movements, but he remained hemodynamically stable. He subsequently developed frank bleeding per rectum with symptoms of lightheadedness, and his hemoglobin concentration fell to 6.9 g/dL. He was given a total of 3 units of packed red blood cells, which raised his hemoglobin level, but only to 8.3 g/dL. The gastroenterology service was consulted to evaluate for the source of the bleeding.
Comment. In a situation like this, an aortoenteric fistula is high on our list of differential diagnoses as the cause of bleeding, but other causes of frank bleeding per rectum such as diverticulosis, arteriovenous malformation, hemorrhoids, or a rapid upper-gastrointestinal bleed cannot be ruled out.
Upper-gastrointestinal endoscopy is the most commonly used diagnostic test for aortoenteric fistulas. It can also find other possible sources of gastrointestinal bleeding. CT with contrast is another option. It can depict the fistula itself or reveal signs of infection, such as gas or liquid surrounding the graft. In an emergency, when there is not enough time for diagnostic testing and an aortoenteric fistula is strongly suspected on clinical grounds, emergency surgical exploration is warranted.4,24
In our patient, the gastrointestinal service elected to first perform endoscopy to look for an aortoenteric fistula.
WHERE DO AORTOENTERIC FISTULAS OCCUR?
3. In which part of the gastrointestinal tract is an aortoenteric fistula most commonly located?
- Esophagus
- Stomach
- Duodenum
- Jejunum
Aortoenteric fistulas can occur at any of these locations, but 80% of cases of secondary aortoenteric fistula are in the duodenum, most often in the third or fourth (horizontal or ascending) part.19 Endoscopic visualization of a pulsatile bleeding mass in this area is diagnostic. However, even if no fistula is seen, upper endoscopy cannot rule out an aortoenteric fistula because the lesion can be located more distal than the scope can reach, which is typically no farther than the first or second parts.4,24
Case continued: What endoscopy showed
The esophagus was normal. There was old clotted blood in the stomach, but no lesions or ulcers. The duodenal bulb and second portion of the duodenum were normal. Three ulcers were noted in the third and fourth portions of the duodenum. The largest and deepest ulcer had an adherent blood clot, and the bowel wall was pulsatile in this region (Figure 2). These findings revealed the source of the gastrointestinal bleeding and were consistent with an aortoenteric fistula.
The patient’s initial bloody bowel movements were herald bleeds, ie, transient and self-limited episodes resulting from necrosis and mucosal ulceration. Herald bleeds can precede a massive gastrointestinal hemorrhage resulting from a true aortoenteric communication.19
Highlight point. Herald bleeds are self-limited and precede hemorrhage that results from a true aortoenteric communication.
TREATMENT OF AORTOENTERIC FISTULA
4. How are aortoenteric fistulas treated?
- Surgery
- Antibiotics
- Endoscopic intervention
Surgery is the definitive treatment. The traditional procedure is open surgical resection of the affected portion of the aorta followed by extra-anatomic (axillobifemoral) bypass or in situ aortic reconstruction using an antibiotic-impregnated prosthetic graft, autogenous femoral vein graft, or cryopreserved allograft.9,29 There have been cases of successful endovascular repair of aortoenteric fistulas, but this approach is generally used as a palliative bridge to definitive surgery.30
Antibiotics should be used if graft infection is suspected, ie, in most cases. However, surgery is still needed to repair the fistula and remove the source of infection. Cultures taken during surgical repair can help guide the choice of antibiotic after surgery.
Endoscopy can aid in diagnosing an aortoenteric fistula, as in the case of our patient. However, vascular surgery is necessary to close the communication between the aorta and the gastrointestinal tract.
Case continued: The patient declines treatment
In view of the patient’s enteroscopic findings, the vascular surgery service was again consulted for surgical correction of the aortoenteric fistula. Treatment was discussed with the patient and his family, but they declined any intervention in view of the high risk of morbidity and death that surgery would entail. Nearing the end of life with advanced cancer and a newly diagnosed aortoenteric fistula, the patient preferred comfort measures with hospice care.
Take-home points
Abdominal pain is the reason for 5% to 10% of emergency department visits, and between 35% to 41% of patients admitted to the hospital because of abdominal pain do not have a definitive diagnosis.31 It is crucial to think about an aortoenteric fistula in such patients who have a history of abdominal aortic aneurysm repair and gastrointestinal bleeding. Timely diagnosis and intervention are necessary to manage this otherwise-fatal condition.
- Hong C, Heiken JP, Sicard GA, Pilgram TK, Bae KT. Clinical significance of endoleak detected on follow-up CT after endovascular repair of abdominal aortic aneurysm. AJR Am J Roentgenol 2008; 191:808–813.
- Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg 2002; 35:1029–1035.
- Corriere MA, Feurer ID, Becker SY, et al. Endoleak following endovascular abdominal aortic aneurysm repair: implications for duration of screening. Ann Surg 2004; 239:800–805.
- Saratzis N, Saratzis A, Melas N, Ktenidis K, Kiskinis D. Aortoduodenal fistulas after endovascular stent-graft repair of abdominal aortic aneurysms: single-center experience and review of the literature. J Endovasc Ther 2008; 15:441–448.
- Demko TM, Diamond JR, Groff J. Obstructive nephropathy as a result of retroperitoneal fibrosis: a review of its pathogenesis and associations. J Am Soc Nephrol 1997; 8:684–688.
- Zetrenne E, McIntosh BC, McRae MH, Gusberg R, Evans GR, Narayan D. Prosthetic vascular graft infection: a multi-center review of surgical management. Yale J Biol Med 2007; 80:113–121.
- Vogel TR, Symons R, Flum DR. The incidence and factors associated with graft infection after aortic aneurysm repair. J Vasc Surg 2008; 47:264–269.
- Swain TW, Calligaro KD, Dougherty MD. Management of infected aortic prosthetic grafts. Vasc Endovascular Surg 2004; 38:75–82.
- Cernohorsky P, Reijnen MM, Tielliu IF, van Sterkenburg SM, van den Dungen JJ, Zeebregts CJ. The relevance of aortic endograft prosthetic infection. J Vasc Surg 2011; 54:327–333.
- FitzGerald SF, Kelly C, Humphreys H. Diagnosis and treatment of prosthetic aortic graft infections: confusion and inconsistency in the absence of evidence or consensus. J Antimicrob Chemother 2005; 56:996–999.
- Orton DF, LeVeen RF, Saigh JA, et al. Aortic prosthetic graft infections: radiologic manifestations and implications for management. Radiographics 2000; 20:977–993.
- Pacanowski JP, Dieter RS, Stevens SL, Freeman MB, Goldman MH. Endoleak: the achilles heel of endovascular abdominal aortic aneurysm exclusion—a case report. WMJ 2002; 101:57–58,63.
- van Bommel EF. Retroperitoneal fibrosis. Neth J Med 2002; 60:231–242.
- Utz DC, Henry JD. Retroperitoneal fibrosis. Med Clin North Am 1966; 50:1091–1099.
- Dalla-Palma L, Rocca-Rossetti S, Pozzi-Mucelli RS, Rizzatto G. Computed tomography in the diagnosis of retroperitoneal fibrosis. Urol Radiol 1981; 3:77–83.
- Harreby M, Bilde T, Helin P, Meyhoff HH, Vinterberg H, Nielsen VA. Retroperitoneal fibrosis treated with methylprednisolon pulse and disease-modifying antirheumatic drugs. Scand J Urol Nephrol 1994; 28:237–242.
- Jois RN, Gaffney K, Marshall T, Scott DG. Chronic periaortitis. Rheumatology (Oxford) 2004; 43:1441–1446.
- Saers SJ, Scheltinga MR. Primary aortoenteric fistula. Br J Surg 2005; 92:143–152.
- Baril DT, Carroccio A, Ellozy SH, et al. Evolving strategies for the treatment of aortoenteric fistulas. J Vasc Surg 2006; 44:250–257.
- Vu QD, Menias CO, Bhalla S, Peterson C, Wang LL, Balfe DM. Aortoenteric fistulas: CT features and potential mimics. Radiographics 2009; 29:197–209.
- Jayarajan S, Napolitano LM, Rectenwald JE, Upchurch GR. Primary aortoenteric fistula and endovascular repair. Vasc Endovascular Surg 2009; 43:592–596.
- Ruby BJ, Cogbill TH. Aortoduodenal fistula 5 years after endovascular abdominal aortic aneurysm repair with the Ancure stent graft. J Vasc Surg 2007; 45:834–836.
- Senadhi V, Brown JC, Arora D, Shaffer R, Shetty D, Mackrell P. A mysterious cause of gastrointestinal bleeding disguising itself as diverticulosis and peptic ulcer disease: a review of diagnostic modalities for aortoenteric fistula. Case Rep Gastroenterol 2010; 4:510–517.
- Simon T, Feller E. Diverse presentation of secondary aortoenteric fistulae. Case Report Med 2011; 2011:406730.
- Schwab CW, McMahon DJ, Phillips G, Pentecost MJ. Aortic balloon control of a traumatic aortoenteric fistula after damage control laparotomy: a case report. J Trauma 1996; 40:1021–1023.
- Napoli PJ, Meade PC, Adams CW. Primary aortoenteric fistula from a posttraumatic pseudoaneurysm. J Trauma 1996; 41:149–152.
- Laser A, Baker N, Rectenwald J, Eliason JL, Criado-Pallares E, Upchurch GR. Graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2011; 54:58–63.
- Luo CY, Lai CH, Wen JS, Lin BW. Secondary aortocolic fistula: case report and review of the literature. Ann Vasc Surg 2010; 24:256.e5–256.e12.
- Kim JY, Kim YW, Kim CJ, Lim HI, Kim DI, Huh S. Successful surgical treatment of aortoenteric fistula. J Korean Med Sci 2007; 22:846–850.
- Verhey P, Best A, Lakin P, Nachiondo J, Petersen B. Successful endovascular treatment of aortoenteric fistula secondary to eroding duodenal stent. J Vasc Interv Radiol 2006; 17:1345–1348.
- Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. In:Hockberger RS, editor: UpToDate. Waltham, MA: UpToDate, 2012.
- Hong C, Heiken JP, Sicard GA, Pilgram TK, Bae KT. Clinical significance of endoleak detected on follow-up CT after endovascular repair of abdominal aortic aneurysm. AJR Am J Roentgenol 2008; 191:808–813.
- Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg 2002; 35:1029–1035.
- Corriere MA, Feurer ID, Becker SY, et al. Endoleak following endovascular abdominal aortic aneurysm repair: implications for duration of screening. Ann Surg 2004; 239:800–805.
- Saratzis N, Saratzis A, Melas N, Ktenidis K, Kiskinis D. Aortoduodenal fistulas after endovascular stent-graft repair of abdominal aortic aneurysms: single-center experience and review of the literature. J Endovasc Ther 2008; 15:441–448.
- Demko TM, Diamond JR, Groff J. Obstructive nephropathy as a result of retroperitoneal fibrosis: a review of its pathogenesis and associations. J Am Soc Nephrol 1997; 8:684–688.
- Zetrenne E, McIntosh BC, McRae MH, Gusberg R, Evans GR, Narayan D. Prosthetic vascular graft infection: a multi-center review of surgical management. Yale J Biol Med 2007; 80:113–121.
- Vogel TR, Symons R, Flum DR. The incidence and factors associated with graft infection after aortic aneurysm repair. J Vasc Surg 2008; 47:264–269.
- Swain TW, Calligaro KD, Dougherty MD. Management of infected aortic prosthetic grafts. Vasc Endovascular Surg 2004; 38:75–82.
- Cernohorsky P, Reijnen MM, Tielliu IF, van Sterkenburg SM, van den Dungen JJ, Zeebregts CJ. The relevance of aortic endograft prosthetic infection. J Vasc Surg 2011; 54:327–333.
- FitzGerald SF, Kelly C, Humphreys H. Diagnosis and treatment of prosthetic aortic graft infections: confusion and inconsistency in the absence of evidence or consensus. J Antimicrob Chemother 2005; 56:996–999.
- Orton DF, LeVeen RF, Saigh JA, et al. Aortic prosthetic graft infections: radiologic manifestations and implications for management. Radiographics 2000; 20:977–993.
- Pacanowski JP, Dieter RS, Stevens SL, Freeman MB, Goldman MH. Endoleak: the achilles heel of endovascular abdominal aortic aneurysm exclusion—a case report. WMJ 2002; 101:57–58,63.
- van Bommel EF. Retroperitoneal fibrosis. Neth J Med 2002; 60:231–242.
- Utz DC, Henry JD. Retroperitoneal fibrosis. Med Clin North Am 1966; 50:1091–1099.
- Dalla-Palma L, Rocca-Rossetti S, Pozzi-Mucelli RS, Rizzatto G. Computed tomography in the diagnosis of retroperitoneal fibrosis. Urol Radiol 1981; 3:77–83.
- Harreby M, Bilde T, Helin P, Meyhoff HH, Vinterberg H, Nielsen VA. Retroperitoneal fibrosis treated with methylprednisolon pulse and disease-modifying antirheumatic drugs. Scand J Urol Nephrol 1994; 28:237–242.
- Jois RN, Gaffney K, Marshall T, Scott DG. Chronic periaortitis. Rheumatology (Oxford) 2004; 43:1441–1446.
- Saers SJ, Scheltinga MR. Primary aortoenteric fistula. Br J Surg 2005; 92:143–152.
- Baril DT, Carroccio A, Ellozy SH, et al. Evolving strategies for the treatment of aortoenteric fistulas. J Vasc Surg 2006; 44:250–257.
- Vu QD, Menias CO, Bhalla S, Peterson C, Wang LL, Balfe DM. Aortoenteric fistulas: CT features and potential mimics. Radiographics 2009; 29:197–209.
- Jayarajan S, Napolitano LM, Rectenwald JE, Upchurch GR. Primary aortoenteric fistula and endovascular repair. Vasc Endovascular Surg 2009; 43:592–596.
- Ruby BJ, Cogbill TH. Aortoduodenal fistula 5 years after endovascular abdominal aortic aneurysm repair with the Ancure stent graft. J Vasc Surg 2007; 45:834–836.
- Senadhi V, Brown JC, Arora D, Shaffer R, Shetty D, Mackrell P. A mysterious cause of gastrointestinal bleeding disguising itself as diverticulosis and peptic ulcer disease: a review of diagnostic modalities for aortoenteric fistula. Case Rep Gastroenterol 2010; 4:510–517.
- Simon T, Feller E. Diverse presentation of secondary aortoenteric fistulae. Case Report Med 2011; 2011:406730.
- Schwab CW, McMahon DJ, Phillips G, Pentecost MJ. Aortic balloon control of a traumatic aortoenteric fistula after damage control laparotomy: a case report. J Trauma 1996; 40:1021–1023.
- Napoli PJ, Meade PC, Adams CW. Primary aortoenteric fistula from a posttraumatic pseudoaneurysm. J Trauma 1996; 41:149–152.
- Laser A, Baker N, Rectenwald J, Eliason JL, Criado-Pallares E, Upchurch GR. Graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2011; 54:58–63.
- Luo CY, Lai CH, Wen JS, Lin BW. Secondary aortocolic fistula: case report and review of the literature. Ann Vasc Surg 2010; 24:256.e5–256.e12.
- Kim JY, Kim YW, Kim CJ, Lim HI, Kim DI, Huh S. Successful surgical treatment of aortoenteric fistula. J Korean Med Sci 2007; 22:846–850.
- Verhey P, Best A, Lakin P, Nachiondo J, Petersen B. Successful endovascular treatment of aortoenteric fistula secondary to eroding duodenal stent. J Vasc Interv Radiol 2006; 17:1345–1348.
- Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. In:Hockberger RS, editor: UpToDate. Waltham, MA: UpToDate, 2012.
Stiff, numb hands
A 45-year-old woman with no chronic medical problems presented to the emergency room with a 1-day history of cramps and paresthesias in both hands and feet, mainly involving the fingers and toes. She said that after an argument with her daughter she began feeling anxious, and this was accompanied by shortness of breath and palpitations as well as generalized weakness, fatigue, and body aches. She also reported nausea and repeated vomiting but no abdominal pain, distention or change in bowel movements. She had had no loss of consciousness, confusion, incontinence, headache, dizziness, diplopia, or facial paresthesia.
She is a cigarette smoker, is alcohol-dependent, but does not use illicit drugs and is not on any medications.
Examination revealed a temperature of 37.1°C (98.8°F), blood pressure 150/75 mm Hg, heart rate 105 bpm, respiratory rate 24 breaths per minute, and oxygen saturation 97% on room air. She appeared very fatigued, thin, and in mild distress due to her cramps. Her mucous membranes were dry, but she had no orthostatic changes. She had noticeable carpopedal spasms (Figure 1), reproducible by inflating a blood-pressure cuff placed on her arm (Trousseau sign) (Figure 2). Also noted was the Chvostek sign—contraction of the ipsilateral facial muscles when the facial nerve is tapped just in front of the ear. The rest of the systemic evaluation was normal. Laboratory investigations were as listed in Table 1. Electrocardiography showed a prolonged QTc interval (0.5 sec). The chest radiograph was normal.
HYPERVENTILATION AND TETANY
The presumptive diagnosis was latent tetany caused by an electrolyte derangement, in this case a combination of hypocalcemia, hypomagnesemia, and hypokalemia as the result of alcohol abuse, repeated vomiting, and hyperventilation brought on by a severe attack of anxiety.
Tetany results from increased excitability of nerves and muscles, leading to painful muscle cramps.1,2 Typical symptoms include circumoral and distal paresthesias, stiffness, clumsiness, myalgia, carpopedal spasm, laryngospasm, bronchospasm, and generalized seizure. The Chvostek and Trousseau signs help to confirm the diagnosis of tetany.3,4
The differential diagnosis of carpopedal spasm includes other conditions of involuntary muscle contraction, such as myotonic disorders; myokymia from Isaac syndrome (writhing movements of the muscles under the skin visualized by continuous “rippling” movements of the muscle); stiff-man syndrome (an autoimmune-antiglutamic acid decarboxylase antibody-associated muscle rigidity that waxes and wanes with concurrent spasms); and snake envenomation.
In addition, our patient’s symptoms were probably brought on by hyperventilation. In general, patients with hyperventilation syndrome are young females who display various manifestations of underlying anxiety and can develop tetany even after a brief episode of hyperventilation. At the time of presentation, our patient was found to have mixed respiratory and metabolic alkalosis. The anxiety-induced hyperventilation likely contributed to the respiratory alkalosis. She had no other symptoms or signs to suggest an acute organic respiratory illness such as pulmonary embolism, pneumothorax, or infection. Vomiting likely caused the metabolic alkalosis and hypokalemia.
Tetany is usually triggered by acute hypocalcemia and is uncommon unless the serum ionized calcium concentration falls below 4.3 mg/dL (1.1 mmol/L), which usually corresponds to a serum total calcium concentration of 7.0 to 7.5 mg/dL (1.8 to 1.9 mmol/L). Patients with a gradual onset of hypocalcemia tend to have fewer symptoms.3,4
Although alkalosis alone can cause tetany, it also enhances tetany by reducing the level of ionized calcium in the serum. Alkalemia causes hypocalcemia by an intravascular chelative mechanism in which the decrease in concentration of hydrogen ions leaves the negatively charged binding sites on albumin available to bind ionized calcium.3
The same happens to the magnesium, a cation with the same size and valence. Significant hypomagnesemia is common in tetanic patients with hyperventilation attacks and may, by itself or in combination with hypocalcemia, cause tetany.2,5,6 Hypokalemia can develop in patients with hypomagnesemia or metabolic alkalosis and may lead to tetany.6,7 Furthermore, our patient was dependent on alcohol, and this is known to cause hypomagnesemia from the excessive urinary excretion of magnesium. This defect of alcohol-induced tubular dysfunction is reversible within 4 weeks of abstinence. Magnesium depletion can cause hypocalcemia by producing resistance to parathyroid hormone or by decreasing its secretion, and this occurs in severe hypomagnesemia, ie, when the serum magnesium concentration falls below 1.0 mg/dL (0.4 mmol/L).
IDENTIFY AND TREAT THE UNDERLYING CAUSE
The management of tetany consists of identifying and treating the underlying cause. Infusion of calcium or magnesium is effective as acute therapy for tetany, and, if appropriate, vitamin D supplementation should also be provided.3,4,7 However, if associated hyperventilation syndrome is present, patients benefit from reassurance and treatment for underlying psychological stress. The traditional treatment of rebreathing into a paper bag is no longer recommended because of the potential risk of hypoxia. Sedatives and antidepressants should be reserved for patients who have not responded to conservative treatment.
Our patient was given an explanation of the condition together with breathing exercises. She received lorazepam and was immediately treated with intravenous hydration, along with intravenous infusion of magnesium, calcium, and potassium. These interventions led to an immediate resolution of her symptoms.
Her low level of intact parathyroid hormone may also have been caused by hypomagnesemia. She was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption and to see us or her primary care doctor should the symptoms recur. As expected, all the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care 2008; 35:215–237.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Smets YF, Bokani N, de Meijer PH, Meinders AE. Tetany due to excessive use of alcohol: a possible magnesium deficiency [in Dutch]. Ned Tijdschr Geneeskd 2004; 148:641–644.
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18:2649–2652.
A 45-year-old woman with no chronic medical problems presented to the emergency room with a 1-day history of cramps and paresthesias in both hands and feet, mainly involving the fingers and toes. She said that after an argument with her daughter she began feeling anxious, and this was accompanied by shortness of breath and palpitations as well as generalized weakness, fatigue, and body aches. She also reported nausea and repeated vomiting but no abdominal pain, distention or change in bowel movements. She had had no loss of consciousness, confusion, incontinence, headache, dizziness, diplopia, or facial paresthesia.
She is a cigarette smoker, is alcohol-dependent, but does not use illicit drugs and is not on any medications.
Examination revealed a temperature of 37.1°C (98.8°F), blood pressure 150/75 mm Hg, heart rate 105 bpm, respiratory rate 24 breaths per minute, and oxygen saturation 97% on room air. She appeared very fatigued, thin, and in mild distress due to her cramps. Her mucous membranes were dry, but she had no orthostatic changes. She had noticeable carpopedal spasms (Figure 1), reproducible by inflating a blood-pressure cuff placed on her arm (Trousseau sign) (Figure 2). Also noted was the Chvostek sign—contraction of the ipsilateral facial muscles when the facial nerve is tapped just in front of the ear. The rest of the systemic evaluation was normal. Laboratory investigations were as listed in Table 1. Electrocardiography showed a prolonged QTc interval (0.5 sec). The chest radiograph was normal.
HYPERVENTILATION AND TETANY
The presumptive diagnosis was latent tetany caused by an electrolyte derangement, in this case a combination of hypocalcemia, hypomagnesemia, and hypokalemia as the result of alcohol abuse, repeated vomiting, and hyperventilation brought on by a severe attack of anxiety.
Tetany results from increased excitability of nerves and muscles, leading to painful muscle cramps.1,2 Typical symptoms include circumoral and distal paresthesias, stiffness, clumsiness, myalgia, carpopedal spasm, laryngospasm, bronchospasm, and generalized seizure. The Chvostek and Trousseau signs help to confirm the diagnosis of tetany.3,4
The differential diagnosis of carpopedal spasm includes other conditions of involuntary muscle contraction, such as myotonic disorders; myokymia from Isaac syndrome (writhing movements of the muscles under the skin visualized by continuous “rippling” movements of the muscle); stiff-man syndrome (an autoimmune-antiglutamic acid decarboxylase antibody-associated muscle rigidity that waxes and wanes with concurrent spasms); and snake envenomation.
In addition, our patient’s symptoms were probably brought on by hyperventilation. In general, patients with hyperventilation syndrome are young females who display various manifestations of underlying anxiety and can develop tetany even after a brief episode of hyperventilation. At the time of presentation, our patient was found to have mixed respiratory and metabolic alkalosis. The anxiety-induced hyperventilation likely contributed to the respiratory alkalosis. She had no other symptoms or signs to suggest an acute organic respiratory illness such as pulmonary embolism, pneumothorax, or infection. Vomiting likely caused the metabolic alkalosis and hypokalemia.
Tetany is usually triggered by acute hypocalcemia and is uncommon unless the serum ionized calcium concentration falls below 4.3 mg/dL (1.1 mmol/L), which usually corresponds to a serum total calcium concentration of 7.0 to 7.5 mg/dL (1.8 to 1.9 mmol/L). Patients with a gradual onset of hypocalcemia tend to have fewer symptoms.3,4
Although alkalosis alone can cause tetany, it also enhances tetany by reducing the level of ionized calcium in the serum. Alkalemia causes hypocalcemia by an intravascular chelative mechanism in which the decrease in concentration of hydrogen ions leaves the negatively charged binding sites on albumin available to bind ionized calcium.3
The same happens to the magnesium, a cation with the same size and valence. Significant hypomagnesemia is common in tetanic patients with hyperventilation attacks and may, by itself or in combination with hypocalcemia, cause tetany.2,5,6 Hypokalemia can develop in patients with hypomagnesemia or metabolic alkalosis and may lead to tetany.6,7 Furthermore, our patient was dependent on alcohol, and this is known to cause hypomagnesemia from the excessive urinary excretion of magnesium. This defect of alcohol-induced tubular dysfunction is reversible within 4 weeks of abstinence. Magnesium depletion can cause hypocalcemia by producing resistance to parathyroid hormone or by decreasing its secretion, and this occurs in severe hypomagnesemia, ie, when the serum magnesium concentration falls below 1.0 mg/dL (0.4 mmol/L).
IDENTIFY AND TREAT THE UNDERLYING CAUSE
The management of tetany consists of identifying and treating the underlying cause. Infusion of calcium or magnesium is effective as acute therapy for tetany, and, if appropriate, vitamin D supplementation should also be provided.3,4,7 However, if associated hyperventilation syndrome is present, patients benefit from reassurance and treatment for underlying psychological stress. The traditional treatment of rebreathing into a paper bag is no longer recommended because of the potential risk of hypoxia. Sedatives and antidepressants should be reserved for patients who have not responded to conservative treatment.
Our patient was given an explanation of the condition together with breathing exercises. She received lorazepam and was immediately treated with intravenous hydration, along with intravenous infusion of magnesium, calcium, and potassium. These interventions led to an immediate resolution of her symptoms.
Her low level of intact parathyroid hormone may also have been caused by hypomagnesemia. She was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption and to see us or her primary care doctor should the symptoms recur. As expected, all the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since.
A 45-year-old woman with no chronic medical problems presented to the emergency room with a 1-day history of cramps and paresthesias in both hands and feet, mainly involving the fingers and toes. She said that after an argument with her daughter she began feeling anxious, and this was accompanied by shortness of breath and palpitations as well as generalized weakness, fatigue, and body aches. She also reported nausea and repeated vomiting but no abdominal pain, distention or change in bowel movements. She had had no loss of consciousness, confusion, incontinence, headache, dizziness, diplopia, or facial paresthesia.
She is a cigarette smoker, is alcohol-dependent, but does not use illicit drugs and is not on any medications.
Examination revealed a temperature of 37.1°C (98.8°F), blood pressure 150/75 mm Hg, heart rate 105 bpm, respiratory rate 24 breaths per minute, and oxygen saturation 97% on room air. She appeared very fatigued, thin, and in mild distress due to her cramps. Her mucous membranes were dry, but she had no orthostatic changes. She had noticeable carpopedal spasms (Figure 1), reproducible by inflating a blood-pressure cuff placed on her arm (Trousseau sign) (Figure 2). Also noted was the Chvostek sign—contraction of the ipsilateral facial muscles when the facial nerve is tapped just in front of the ear. The rest of the systemic evaluation was normal. Laboratory investigations were as listed in Table 1. Electrocardiography showed a prolonged QTc interval (0.5 sec). The chest radiograph was normal.
HYPERVENTILATION AND TETANY
The presumptive diagnosis was latent tetany caused by an electrolyte derangement, in this case a combination of hypocalcemia, hypomagnesemia, and hypokalemia as the result of alcohol abuse, repeated vomiting, and hyperventilation brought on by a severe attack of anxiety.
Tetany results from increased excitability of nerves and muscles, leading to painful muscle cramps.1,2 Typical symptoms include circumoral and distal paresthesias, stiffness, clumsiness, myalgia, carpopedal spasm, laryngospasm, bronchospasm, and generalized seizure. The Chvostek and Trousseau signs help to confirm the diagnosis of tetany.3,4
The differential diagnosis of carpopedal spasm includes other conditions of involuntary muscle contraction, such as myotonic disorders; myokymia from Isaac syndrome (writhing movements of the muscles under the skin visualized by continuous “rippling” movements of the muscle); stiff-man syndrome (an autoimmune-antiglutamic acid decarboxylase antibody-associated muscle rigidity that waxes and wanes with concurrent spasms); and snake envenomation.
In addition, our patient’s symptoms were probably brought on by hyperventilation. In general, patients with hyperventilation syndrome are young females who display various manifestations of underlying anxiety and can develop tetany even after a brief episode of hyperventilation. At the time of presentation, our patient was found to have mixed respiratory and metabolic alkalosis. The anxiety-induced hyperventilation likely contributed to the respiratory alkalosis. She had no other symptoms or signs to suggest an acute organic respiratory illness such as pulmonary embolism, pneumothorax, or infection. Vomiting likely caused the metabolic alkalosis and hypokalemia.
Tetany is usually triggered by acute hypocalcemia and is uncommon unless the serum ionized calcium concentration falls below 4.3 mg/dL (1.1 mmol/L), which usually corresponds to a serum total calcium concentration of 7.0 to 7.5 mg/dL (1.8 to 1.9 mmol/L). Patients with a gradual onset of hypocalcemia tend to have fewer symptoms.3,4
Although alkalosis alone can cause tetany, it also enhances tetany by reducing the level of ionized calcium in the serum. Alkalemia causes hypocalcemia by an intravascular chelative mechanism in which the decrease in concentration of hydrogen ions leaves the negatively charged binding sites on albumin available to bind ionized calcium.3
The same happens to the magnesium, a cation with the same size and valence. Significant hypomagnesemia is common in tetanic patients with hyperventilation attacks and may, by itself or in combination with hypocalcemia, cause tetany.2,5,6 Hypokalemia can develop in patients with hypomagnesemia or metabolic alkalosis and may lead to tetany.6,7 Furthermore, our patient was dependent on alcohol, and this is known to cause hypomagnesemia from the excessive urinary excretion of magnesium. This defect of alcohol-induced tubular dysfunction is reversible within 4 weeks of abstinence. Magnesium depletion can cause hypocalcemia by producing resistance to parathyroid hormone or by decreasing its secretion, and this occurs in severe hypomagnesemia, ie, when the serum magnesium concentration falls below 1.0 mg/dL (0.4 mmol/L).
IDENTIFY AND TREAT THE UNDERLYING CAUSE
The management of tetany consists of identifying and treating the underlying cause. Infusion of calcium or magnesium is effective as acute therapy for tetany, and, if appropriate, vitamin D supplementation should also be provided.3,4,7 However, if associated hyperventilation syndrome is present, patients benefit from reassurance and treatment for underlying psychological stress. The traditional treatment of rebreathing into a paper bag is no longer recommended because of the potential risk of hypoxia. Sedatives and antidepressants should be reserved for patients who have not responded to conservative treatment.
Our patient was given an explanation of the condition together with breathing exercises. She received lorazepam and was immediately treated with intravenous hydration, along with intravenous infusion of magnesium, calcium, and potassium. These interventions led to an immediate resolution of her symptoms.
Her low level of intact parathyroid hormone may also have been caused by hypomagnesemia. She was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption and to see us or her primary care doctor should the symptoms recur. As expected, all the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care 2008; 35:215–237.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Smets YF, Bokani N, de Meijer PH, Meinders AE. Tetany due to excessive use of alcohol: a possible magnesium deficiency [in Dutch]. Ned Tijdschr Geneeskd 2004; 148:641–644.
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18:2649–2652.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care 2008; 35:215–237.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Smets YF, Bokani N, de Meijer PH, Meinders AE. Tetany due to excessive use of alcohol: a possible magnesium deficiency [in Dutch]. Ned Tijdschr Geneeskd 2004; 148:641–644.
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18:2649–2652.