User login
Managing severe acute pancreatitis
Severe acute pancreatitis has been known since the time of Rembrandt, with Nicolaes Tulp—the physician credited as first describing it—immortalized in the famous painting, The Anatomy Lesson. However, progress in managing this disease has been disappointing. Treatment is mainly supportive, and we lack any true disease-modifying therapy. But we are learning to recognize the disease and treat it supportively better than in the past.
The early hours of severe acute pancreatitis are critical for instituting appropriate intervention. Prompt fluid resuscitation is key to preventing immediate and later morbidity and death. This article focuses on identifying and managing the most severe form of acute pancreatitis—necrotizing disease—and its complications.
NECROTIZING DISEASE ACCOUNTS FOR MOST PANCREATITIS DEATHS
The classification and definitions of acute pancreatitis were recently revised from the 1992 Atlanta system and published early in 2013.1 In addition, the American Pancreatic Association and the International Association of Pancreatology met in 2012 to develop evidence-based guidelines on managing severe pancreatitis.
An estimated 210,000 new cases of acute pancreatitis occur each year in the United States. About 20% of cases of severe acute pancreatitis are necrotizing disease, which accounts for nearly all the morbidity and death associated with acute pancreatitis.
The clinical spectrum of acute pancreatitis ranges from mild to life-threatening, reflecting interstitial (death rate < 1%) to necrotizing histology (the latter associated with a 25% risk of death if the pancreatitis becomes infected and a 10% risk if it is sterile). When death occurs early in the disease course, it tends to be from multiorgan failure; when death occurs later in the course, it tends to be from infection. Appropriate early treatment may prevent death in both categories.
DIAGNOSING ACUTE PANCREATITIS AND PREDICTING ITS SEVERITY
The diagnosis of acute pancreatitis requires two of the following three criteria:
- Clinical presentation—epigastric pain, nausea, vomiting
- Biochemical—amylase level more than three times the upper limit of normal, or lipase more than three times the upper limit of normal
- Evidence from computed tomography (CT), ultrasonography, or magnetic resonance imaging.
Although the biochemical criteria are variably sensitive for detecting acute pancreatitis (55%–100%), the specificity is very high (93% to 99%).
Recently, urinary trypsinogen-2, measured by dipstick, has also been used to aid diagnosis. It has a reasonable sensitivity (53%–96%) and specificity (85%) if positive (> 50 ng/mL).
Speed is critical
Over the years, many clinical prediction rules have been used for predicting the severity of acute pancreatitis. The Ranson criteria,2 from 1974, and the Acute Physiology and Chronic Health Evaluation (APACHE) II system3 are cumbersome and require waiting up to 48 hours after the onset of acute pancreatitis to obtain a complete score. The Imrie-Glasgow score is another predictor.
The systemic inflammatory response syndrome (SIRS) is currently the most important indicator of prognosis.4 Originally adopted for predicting the development of organ failure with sepsis, it requires at least two of the following criteria:
- Heart rate > 90 beats/min
- Core temperature < 36°C or > 38°C
- White blood cells < 4,000 or > 12,000/mm3
- Respirations > 20/min.
The advantages of this system are that it identifies risk very early in the course of the disease and can be assessed quickly in the emergency department.
The Bedside Index for Severity of Acute Pancreatitis (BISAP) score is another simple, easy-to-perform prognostic index,5,6 calculated by assigning 1 point for each of the following if present within the first 24 hours of presentation:
- Blood urea nitrogen > 25 mg/dL
- Abnormal mental status (Glasgow coma score < 15)
- Evidence of systemic inflammatory response syndrome
- Age > 60 years
- Pleural effusion seen on imaging study.
A score of 3 points is associated with a 5.3% rate of hospital death, 4 points with 12.7%, and 5 points with 22.5%.
At its most basic, severe acute pancreatitis is defined by organ failure (at least one organ from the respiratory, renal, or cardiovascular system) lasting for more than 48 hours. Failure for each organ is defined by the Marshall scoring system.1
EARLY MANAGEMENT IS KEY TO OUTCOME
The window of opportunity to make a significant difference in outcome is within the first 12 to 24 hours of presentation. Volume resuscitation is the cornerstone of early management. By the time of presentation for severe acute pancreatitis, the pancreas is already necrotic, so the aim is to minimize the systemic inflammatory response syndrome with the goals of reducing rates of organ failure, morbidity, and death. Necrotizing pancreatitis is essentially an ischemic event, and the goal of volume resuscitation is to maintain pancreatic and intestinal microcirculation to prevent intestinal ischemia and subsequent bacterial translocation.7
Early resuscitation with lactated Ringer’s solution recommended
The evidence supporting a specific protocol for fluid resuscitation in severe acute pancreatitis is not strong, but a few studies provide guidance.
Wu et al8 randomized 40 patients with acute pancreatitis to one of four arms: “goal-directed fluid resuscitation” with either lactated Ringer’s solution or normal saline, or standard therapy (by physician discretion) with either lactated Ringer’s solution or normal saline. Goal-directed therapy involved a bolus of 20 mL/kg given over 30 to 45 minutes at presentation followed by infusion with rates dependent on an algorithm based on change in blood urea nitrogen level at set times. Patients receiving either goal-directed or standard therapy had significantly lower rates of systemic inflammatory response syndrome at 24 hours than at admission. Most striking was that treatment with lactated Ringer’s solution was associated with dramatically improved rates, whereas normal saline showed no improvement.
In a retrospective study of patients with acute pancreatitis, Warndorf et al9 identified 340 patients who received early resuscitation (more than one-third of the total 72-hour fluid volume within 24 hours of presentation) and 90 patients who received late resuscitation (less than one-third of the total 72-hour fluid volume within 24 hours of presentation). Patients who received early resuscitation developed less systemic inflammatory response syndrome and organ failure, and required fewer interventions.
Monitoring for optimum fluid resuscitation
Fluid resuscitation should be carefully managed to avoid administering either inadequate or excessive amounts of fluid. Inadequate fluid resuscitation can result in renal failure, progression of necrosis, and possibly infectious complications. Excessive resuscitation—defined as more than 4 L in the first 24 hours—is associated with respiratory failure, pancreatic fluid collections, and abdominal compartment syndrome.
Optimum resuscitation is controlled fluid expansion averaging 5 to 10 mL/kg per hour, with 2,500 to 4,000 mL given in the first 24 hours.
Adequate volume resuscitation can be evaluated clinically with the following goals:
- Heart rate < 120 beats per minute
- Mean arterial pressure 65–85 mm Hg
- Urinary output > 1 mL/kg per hour
- Hematocrit 35%–44%.
EARLY CT IS JUSTIFIED ONLY IF DIAGNOSIS IS UNCLEAR
The normal pancreas takes up contrast in the same way as do the liver and spleen, so its enhancement on CT is similar. If there is interstitial pancreatitis, CT shows the pancreas with normal contrast uptake, but the organ appears “boggy” with indistinct outlines. With necrotizing pancreatitis, only small areas of tissue with normal contrast may be apparent.
Peripancreatic fat necrosis may also be visible on CT. Obese patients tend to have a worse clinical course of necrotizing pancreatitis, probably because of the associated peripancreatic fat that is incorporated into the pancreatic necrosis.
For clear-cut cases of acute pancreatitis, time is wasted waiting to obtain CT images, and this could delay fluid resuscitation. Results from immediate CT almost never change the clinical management during the first week of acute pancreatitis, and obtaining CT images is usually not recommended if the diagnosis of acute pancreatitis is clear. CT’s sensitivity for detecting necrosis is only 70% in the first 48 hours of presentation, so it is easy to be fooled by a false-negative scan: frequently, a scan does not show necrotizing pancreatitis until after 72 hours. In addition, evidence from animal studies indicates that contrast agents might worsen pancreatic necrosis.
Immediate CT is justified if the diagnosis is in doubt at presentation, such as to evaluate for other intra-abdominal conditions such as intestinal ischemia or a perforated duodenal ulcer.
Contrast-enhanced CT is recommended 72 to 96 hours after presentation, or earlier if the patient is worsening despite treatment. Specific CT protocols will be included in new management guidelines, expected to be published soon.
PREVENTING INFECTIOUS COMPLICATIONS
Risk of infection is associated with the degree of pancreatic necrosis. Patients with less than 30% necrosis have a 22.5% chance of infection, whereas those with more than 50% necrosis have a 46.5% risk of infection.10
Infection can develop from a variety of sources:
Bacterial translocation from the colon and small bowel is thought to be one of the major sources of infection in necrotic pancreatitis. Volume resuscitation and maintaining gut integrity with early enteral nutrition are believed to minimize the risk of bacterial translocation.
Hematogenous spread of bacteria is another suspected source of infection into the pancreas. Again, enteral nutrition also reduces the risk by minimizing the need for central catheters.
Biliary sources may also play a role. Bile duct stones or gall bladder infection can lead to infected pancreatic necrosis.
ANTIBIOTICS NOT ROUTINELY RECOMMENDED
Treating acute pancreatitis with antibiotics has fallen in and out of favor over the past decades. From being standard practice in the 1970s, it dropped off in the 1980s and 1990s and then became more common again.
Current recommendations from the American Pancreatic Association and the International Association of Pancreatology are not to routinely use intravenous antibiotics to prevent infection in necrotizing pancreatitis because of lack of evidence that it changes overall outcome. Antibiotic usage may be associated with more bacterial resistance and the introduction of fungal infections into the pancreas.
Selective gut decontamination, involving oral and rectal administration of neomycin and other antibiotics, was shown in a single randomized trial to reduce the incidence of infection, but it is very cumbersome and is not recommended for acute pancreatitis.
Treatment with probiotics is also not recommended and was shown in one study to lead to a worse outcome.11
ENTERAL BETTER THAN TOTAL PARENTERAL NUTRITION
Enteral tube feeding with either an elemental diet or a polymeric enteral formulation is the first-line therapy for necrotizing pancreatitis. Compared with total parenteral nutrition, it reduces infection, organ failure, hospital length of stay, the need for surgical intervention, and the risk of death. Total parenteral nutrition should be considered only for patients who do not tolerate enteral feeding because of severe ileus.
Conventional thinking for many years was to provide enteral feeding with a tube passed beyond the ligament of Treitz, thinking that it reduced stimulation to the pancreas. However, recent studies indicate that nasogastric feeding is equivalent to nasojejunal feeding in terms of nutrition, maintaining gut integrity, and outcome.
INTRA-ABDOMINAL HYPERTENSION AND ABDOMINAL COMPARTMENT SYNDROME
Movement of fluid into the intracellular space (“third-spacing”) occurs in acute pancreatitis and is exacerbated by fluid resuscitation. Intra-abdominal hypertension is associated with poor outcomes in patients with severe acute pancreatitis. Especially for patients with severe pancreatitis who are on mechanical ventilation, pressure should be monitored with transvesicular bladder measurements.
Intra-abdominal hypertension is defined as a sustained intra-abdominal pressure of more than 12 mm Hg, with the following grades:
- Grade 1: 12–15 mm Hg
- Grade 2: 16–20 mm Hg
- Grade 3: 21–25 mm Hg
- Grade 4: > 25 mm Hg.
Abdominal compartment syndrome is defined as a sustained intra-abdominal pressure of more than 20 mm Hg. It is associated with new organ dysfunction or failure. It should first be managed with ultrafiltration or diuretics to try to reduce the amount of fluid in the abdomen. Lumenal decompression can be tried with nasogastric or rectal tubes for the stomach and bowels. Ascites or retroperitoneal fluid can be drained percutaneously. In addition, analgesia and sedation to reduce abdominal muscle tone can help the patient become better ventilated. Neuromuscular blockade can also relax the abdomen.
Open abdominal decompression is the treatment of last resort to relieve abdominal compartment syndrome. The abdominal wall is not closed surgically but is allowed to heal by secondary intention (it “granulates in”).12
IDENTIFYING INFECTION
Fine-needle aspiration if clinical and imaging signs are not clear
Untreated infected pancreatitis is associated with a much higher risk of death than sterile pancreatic necrosis. Unfortunately, it can be difficult to determine if a patient with necrotizing pancreatitis has an infection because fever, tachycardia, and leukocytosis are usually present regardless. It is important to determine because mechanically intervening for sterile necrosis does not improve outcome.
Fine-needle aspiration, either guided by CT or done at the bedside with ultrasonography, with evaluation with Gram stain and culture, was widely used in the 1990s in cases of necrotizing pancreatitis to determine if infection was present. There has been a shift away from this because, although it can confirm the presence of infection, the false-negative rate is 15%. Clinical and imaging signs can be relied on in most cases to determine the presence of infection, and it is now recognized that fineneedle aspiration should be used only for select cases. Clinical studies have not shown that fine-needle aspiration improves outcomes.
Clinical scenarios typical of infected pancreatic necrosis include patients who have obvious signs of infection with no identifiable source, such as those who stabilize after acute severe acute pancreatitis, and then 10 to 14 days later become worse, with a dramatically higher white blood cell count and tachycardia. Such a patient likely needs an intervention regardless of the results of fine-needle aspiration.
On the other hand, a patient with a continually up-and-down course that never stabilizes over 3 weeks, with no identifiable source of infection, and with no peripancreatic gas apparent on imaging would be a good candidate for fine-needle aspiration.
If peripancreatic gas is seen on imaging, fine-needle aspiration is unnecessary. Peripancreatic gas is traditionally attributed to gasforming bacteria within the pancreas, but in my experience, it is usually from a fistula from the necrosis to the duodenum or the colon, the fistula being caused as the necrosis erodes at the hepatic flexure, the transverse colon, or the splenic flexure.
MECHANICAL INTERVENTIONS FOR INFECTIVE NECROSIS
Late, minimally invasive procedures preferred
Conventional management has shifted away from removing the necrosis with early surgical debridement of the pancreas. Experience with myocardial infarction shows that it is not necessary to remove a sterile necrotic organ, and studies with sterile pancreatic necrosis have found that surgical intervention is associated with a higher risk of death than medical management.
Documented infection has traditionally been considered a definite indication for debridement, but even that is being called into question as more studies are emerging of infected necrosis treated successfully with antibiotics alone.
Sterile necrosis with a fulminant course is a controversial indication for surgery. It was traditionally felt that surgery was worth trying for such patients, but this is no longer common practice.
For cases in which debridement was deemed advisable, surgery was done more frequently in the past. Now, a minimally invasive approach such as with endoscopy or percutaneous catheter is also used. Waiting until at least 4 weeks after the onset of acute pancreatitis is associated with a better outcome than intervening early.
WALLED-OFF NECROSIS
Watchful waiting or minimally invasive intervention
Patients who survive multiorgan failure but are still ill more than 4 weeks after the onset of pancreatitis should be suspected of having walled-off necrosis, formerly referred to as a pancreatic phlegmon. This term was abandoned after the 1992 Atlanta symposium.13 In the mid to late 1990s, the process was referred to as organized pancreatic necrosis. It is characterized by a mature, encapsulated collection of pancreatic or peripancreatic necrosis that contains variable amounts of amylase-rich fluid from pancreatic duct disruption.
Walled-off pancreatic necrosis (WOPN) is often confused with pancreatic pseudocyst; these may appear similar on CT, and higherdensity solid debris may be visible in walled-off necrosis within an otherwise homogenous-appearing collection. Magnetic resonance imaging defines liquid and solid much better than CT.
The best way to distinguish WOPN from pseudocyst is by clinical history: a patient with a preceding history of clinically severe acute pancreatitis almost always has necrotizing pancreatitis that evolves to walled-off necrosis, usually over 3 to 4 weeks.
Endoscopic removal and other minimally invasive approaches, such as aggressive percutaneous interventions, have replaced open necrosectomy for treatment, which was associated with high morbidity and mortality rates.14–16
Intervening for sterile walled-off necrosis is still a controversial topic: although systemically ill, the patient is no longer having life-threatening consequences, and watchful waiting might be just as expedient as intervention. Evidence to support either view is lacking. Most experts believe that intervention should be done if the patient has gastric outlet obstruction and intractable pain and is unable to eat 4 to 6 weeks after the onset of pancreatitis with WOPN. Infected WOPN is considered an indication for drainage.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974; 139:69–81.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–829.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20:864–874.
- Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008; 57:1698–1703.
- Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009; 104:966–971.
- Fisher JM, Gardner TB. The “golden hours” of management in acute pancreatitis. Am J Gastroenterol 2012; 107:1146–1150.
- Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:710–717.
- Warndorf MG, Kurtzman JT, Bartel MJ, et al. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:705–709.
- Beger HG, Rau BM. Severe acute pancreatitis: clinical course and management. World J Gastroenterol 2007; 13:5043–5051.
- Besselink MG, van Santvoort HC, Buskens E, et al; Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371:651–659.
- Fitzgerald JE, Gupta S, Masterson S, Sigurdsson HH. Laparostomy management using the ABThera open abdomen negative pressure therapy system in a grade IV open abdomen secondary to acute pancreatitis. Int Wound J 2012. doi: 1111/j.1742-481X2012.00953.x. [epub ahead of print]
- Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11–13, 1992. Arch Surg 1993; 128:586–590.
- Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology 1996; 111:755–764.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
Severe acute pancreatitis has been known since the time of Rembrandt, with Nicolaes Tulp—the physician credited as first describing it—immortalized in the famous painting, The Anatomy Lesson. However, progress in managing this disease has been disappointing. Treatment is mainly supportive, and we lack any true disease-modifying therapy. But we are learning to recognize the disease and treat it supportively better than in the past.
The early hours of severe acute pancreatitis are critical for instituting appropriate intervention. Prompt fluid resuscitation is key to preventing immediate and later morbidity and death. This article focuses on identifying and managing the most severe form of acute pancreatitis—necrotizing disease—and its complications.
NECROTIZING DISEASE ACCOUNTS FOR MOST PANCREATITIS DEATHS
The classification and definitions of acute pancreatitis were recently revised from the 1992 Atlanta system and published early in 2013.1 In addition, the American Pancreatic Association and the International Association of Pancreatology met in 2012 to develop evidence-based guidelines on managing severe pancreatitis.
An estimated 210,000 new cases of acute pancreatitis occur each year in the United States. About 20% of cases of severe acute pancreatitis are necrotizing disease, which accounts for nearly all the morbidity and death associated with acute pancreatitis.
The clinical spectrum of acute pancreatitis ranges from mild to life-threatening, reflecting interstitial (death rate < 1%) to necrotizing histology (the latter associated with a 25% risk of death if the pancreatitis becomes infected and a 10% risk if it is sterile). When death occurs early in the disease course, it tends to be from multiorgan failure; when death occurs later in the course, it tends to be from infection. Appropriate early treatment may prevent death in both categories.
DIAGNOSING ACUTE PANCREATITIS AND PREDICTING ITS SEVERITY
The diagnosis of acute pancreatitis requires two of the following three criteria:
- Clinical presentation—epigastric pain, nausea, vomiting
- Biochemical—amylase level more than three times the upper limit of normal, or lipase more than three times the upper limit of normal
- Evidence from computed tomography (CT), ultrasonography, or magnetic resonance imaging.
Although the biochemical criteria are variably sensitive for detecting acute pancreatitis (55%–100%), the specificity is very high (93% to 99%).
Recently, urinary trypsinogen-2, measured by dipstick, has also been used to aid diagnosis. It has a reasonable sensitivity (53%–96%) and specificity (85%) if positive (> 50 ng/mL).
Speed is critical
Over the years, many clinical prediction rules have been used for predicting the severity of acute pancreatitis. The Ranson criteria,2 from 1974, and the Acute Physiology and Chronic Health Evaluation (APACHE) II system3 are cumbersome and require waiting up to 48 hours after the onset of acute pancreatitis to obtain a complete score. The Imrie-Glasgow score is another predictor.
The systemic inflammatory response syndrome (SIRS) is currently the most important indicator of prognosis.4 Originally adopted for predicting the development of organ failure with sepsis, it requires at least two of the following criteria:
- Heart rate > 90 beats/min
- Core temperature < 36°C or > 38°C
- White blood cells < 4,000 or > 12,000/mm3
- Respirations > 20/min.
The advantages of this system are that it identifies risk very early in the course of the disease and can be assessed quickly in the emergency department.
The Bedside Index for Severity of Acute Pancreatitis (BISAP) score is another simple, easy-to-perform prognostic index,5,6 calculated by assigning 1 point for each of the following if present within the first 24 hours of presentation:
- Blood urea nitrogen > 25 mg/dL
- Abnormal mental status (Glasgow coma score < 15)
- Evidence of systemic inflammatory response syndrome
- Age > 60 years
- Pleural effusion seen on imaging study.
A score of 3 points is associated with a 5.3% rate of hospital death, 4 points with 12.7%, and 5 points with 22.5%.
At its most basic, severe acute pancreatitis is defined by organ failure (at least one organ from the respiratory, renal, or cardiovascular system) lasting for more than 48 hours. Failure for each organ is defined by the Marshall scoring system.1
EARLY MANAGEMENT IS KEY TO OUTCOME
The window of opportunity to make a significant difference in outcome is within the first 12 to 24 hours of presentation. Volume resuscitation is the cornerstone of early management. By the time of presentation for severe acute pancreatitis, the pancreas is already necrotic, so the aim is to minimize the systemic inflammatory response syndrome with the goals of reducing rates of organ failure, morbidity, and death. Necrotizing pancreatitis is essentially an ischemic event, and the goal of volume resuscitation is to maintain pancreatic and intestinal microcirculation to prevent intestinal ischemia and subsequent bacterial translocation.7
Early resuscitation with lactated Ringer’s solution recommended
The evidence supporting a specific protocol for fluid resuscitation in severe acute pancreatitis is not strong, but a few studies provide guidance.
Wu et al8 randomized 40 patients with acute pancreatitis to one of four arms: “goal-directed fluid resuscitation” with either lactated Ringer’s solution or normal saline, or standard therapy (by physician discretion) with either lactated Ringer’s solution or normal saline. Goal-directed therapy involved a bolus of 20 mL/kg given over 30 to 45 minutes at presentation followed by infusion with rates dependent on an algorithm based on change in blood urea nitrogen level at set times. Patients receiving either goal-directed or standard therapy had significantly lower rates of systemic inflammatory response syndrome at 24 hours than at admission. Most striking was that treatment with lactated Ringer’s solution was associated with dramatically improved rates, whereas normal saline showed no improvement.
In a retrospective study of patients with acute pancreatitis, Warndorf et al9 identified 340 patients who received early resuscitation (more than one-third of the total 72-hour fluid volume within 24 hours of presentation) and 90 patients who received late resuscitation (less than one-third of the total 72-hour fluid volume within 24 hours of presentation). Patients who received early resuscitation developed less systemic inflammatory response syndrome and organ failure, and required fewer interventions.
Monitoring for optimum fluid resuscitation
Fluid resuscitation should be carefully managed to avoid administering either inadequate or excessive amounts of fluid. Inadequate fluid resuscitation can result in renal failure, progression of necrosis, and possibly infectious complications. Excessive resuscitation—defined as more than 4 L in the first 24 hours—is associated with respiratory failure, pancreatic fluid collections, and abdominal compartment syndrome.
Optimum resuscitation is controlled fluid expansion averaging 5 to 10 mL/kg per hour, with 2,500 to 4,000 mL given in the first 24 hours.
Adequate volume resuscitation can be evaluated clinically with the following goals:
- Heart rate < 120 beats per minute
- Mean arterial pressure 65–85 mm Hg
- Urinary output > 1 mL/kg per hour
- Hematocrit 35%–44%.
EARLY CT IS JUSTIFIED ONLY IF DIAGNOSIS IS UNCLEAR
The normal pancreas takes up contrast in the same way as do the liver and spleen, so its enhancement on CT is similar. If there is interstitial pancreatitis, CT shows the pancreas with normal contrast uptake, but the organ appears “boggy” with indistinct outlines. With necrotizing pancreatitis, only small areas of tissue with normal contrast may be apparent.
Peripancreatic fat necrosis may also be visible on CT. Obese patients tend to have a worse clinical course of necrotizing pancreatitis, probably because of the associated peripancreatic fat that is incorporated into the pancreatic necrosis.
For clear-cut cases of acute pancreatitis, time is wasted waiting to obtain CT images, and this could delay fluid resuscitation. Results from immediate CT almost never change the clinical management during the first week of acute pancreatitis, and obtaining CT images is usually not recommended if the diagnosis of acute pancreatitis is clear. CT’s sensitivity for detecting necrosis is only 70% in the first 48 hours of presentation, so it is easy to be fooled by a false-negative scan: frequently, a scan does not show necrotizing pancreatitis until after 72 hours. In addition, evidence from animal studies indicates that contrast agents might worsen pancreatic necrosis.
Immediate CT is justified if the diagnosis is in doubt at presentation, such as to evaluate for other intra-abdominal conditions such as intestinal ischemia or a perforated duodenal ulcer.
Contrast-enhanced CT is recommended 72 to 96 hours after presentation, or earlier if the patient is worsening despite treatment. Specific CT protocols will be included in new management guidelines, expected to be published soon.
PREVENTING INFECTIOUS COMPLICATIONS
Risk of infection is associated with the degree of pancreatic necrosis. Patients with less than 30% necrosis have a 22.5% chance of infection, whereas those with more than 50% necrosis have a 46.5% risk of infection.10
Infection can develop from a variety of sources:
Bacterial translocation from the colon and small bowel is thought to be one of the major sources of infection in necrotic pancreatitis. Volume resuscitation and maintaining gut integrity with early enteral nutrition are believed to minimize the risk of bacterial translocation.
Hematogenous spread of bacteria is another suspected source of infection into the pancreas. Again, enteral nutrition also reduces the risk by minimizing the need for central catheters.
Biliary sources may also play a role. Bile duct stones or gall bladder infection can lead to infected pancreatic necrosis.
ANTIBIOTICS NOT ROUTINELY RECOMMENDED
Treating acute pancreatitis with antibiotics has fallen in and out of favor over the past decades. From being standard practice in the 1970s, it dropped off in the 1980s and 1990s and then became more common again.
Current recommendations from the American Pancreatic Association and the International Association of Pancreatology are not to routinely use intravenous antibiotics to prevent infection in necrotizing pancreatitis because of lack of evidence that it changes overall outcome. Antibiotic usage may be associated with more bacterial resistance and the introduction of fungal infections into the pancreas.
Selective gut decontamination, involving oral and rectal administration of neomycin and other antibiotics, was shown in a single randomized trial to reduce the incidence of infection, but it is very cumbersome and is not recommended for acute pancreatitis.
Treatment with probiotics is also not recommended and was shown in one study to lead to a worse outcome.11
ENTERAL BETTER THAN TOTAL PARENTERAL NUTRITION
Enteral tube feeding with either an elemental diet or a polymeric enteral formulation is the first-line therapy for necrotizing pancreatitis. Compared with total parenteral nutrition, it reduces infection, organ failure, hospital length of stay, the need for surgical intervention, and the risk of death. Total parenteral nutrition should be considered only for patients who do not tolerate enteral feeding because of severe ileus.
Conventional thinking for many years was to provide enteral feeding with a tube passed beyond the ligament of Treitz, thinking that it reduced stimulation to the pancreas. However, recent studies indicate that nasogastric feeding is equivalent to nasojejunal feeding in terms of nutrition, maintaining gut integrity, and outcome.
INTRA-ABDOMINAL HYPERTENSION AND ABDOMINAL COMPARTMENT SYNDROME
Movement of fluid into the intracellular space (“third-spacing”) occurs in acute pancreatitis and is exacerbated by fluid resuscitation. Intra-abdominal hypertension is associated with poor outcomes in patients with severe acute pancreatitis. Especially for patients with severe pancreatitis who are on mechanical ventilation, pressure should be monitored with transvesicular bladder measurements.
Intra-abdominal hypertension is defined as a sustained intra-abdominal pressure of more than 12 mm Hg, with the following grades:
- Grade 1: 12–15 mm Hg
- Grade 2: 16–20 mm Hg
- Grade 3: 21–25 mm Hg
- Grade 4: > 25 mm Hg.
Abdominal compartment syndrome is defined as a sustained intra-abdominal pressure of more than 20 mm Hg. It is associated with new organ dysfunction or failure. It should first be managed with ultrafiltration or diuretics to try to reduce the amount of fluid in the abdomen. Lumenal decompression can be tried with nasogastric or rectal tubes for the stomach and bowels. Ascites or retroperitoneal fluid can be drained percutaneously. In addition, analgesia and sedation to reduce abdominal muscle tone can help the patient become better ventilated. Neuromuscular blockade can also relax the abdomen.
Open abdominal decompression is the treatment of last resort to relieve abdominal compartment syndrome. The abdominal wall is not closed surgically but is allowed to heal by secondary intention (it “granulates in”).12
IDENTIFYING INFECTION
Fine-needle aspiration if clinical and imaging signs are not clear
Untreated infected pancreatitis is associated with a much higher risk of death than sterile pancreatic necrosis. Unfortunately, it can be difficult to determine if a patient with necrotizing pancreatitis has an infection because fever, tachycardia, and leukocytosis are usually present regardless. It is important to determine because mechanically intervening for sterile necrosis does not improve outcome.
Fine-needle aspiration, either guided by CT or done at the bedside with ultrasonography, with evaluation with Gram stain and culture, was widely used in the 1990s in cases of necrotizing pancreatitis to determine if infection was present. There has been a shift away from this because, although it can confirm the presence of infection, the false-negative rate is 15%. Clinical and imaging signs can be relied on in most cases to determine the presence of infection, and it is now recognized that fineneedle aspiration should be used only for select cases. Clinical studies have not shown that fine-needle aspiration improves outcomes.
Clinical scenarios typical of infected pancreatic necrosis include patients who have obvious signs of infection with no identifiable source, such as those who stabilize after acute severe acute pancreatitis, and then 10 to 14 days later become worse, with a dramatically higher white blood cell count and tachycardia. Such a patient likely needs an intervention regardless of the results of fine-needle aspiration.
On the other hand, a patient with a continually up-and-down course that never stabilizes over 3 weeks, with no identifiable source of infection, and with no peripancreatic gas apparent on imaging would be a good candidate for fine-needle aspiration.
If peripancreatic gas is seen on imaging, fine-needle aspiration is unnecessary. Peripancreatic gas is traditionally attributed to gasforming bacteria within the pancreas, but in my experience, it is usually from a fistula from the necrosis to the duodenum or the colon, the fistula being caused as the necrosis erodes at the hepatic flexure, the transverse colon, or the splenic flexure.
MECHANICAL INTERVENTIONS FOR INFECTIVE NECROSIS
Late, minimally invasive procedures preferred
Conventional management has shifted away from removing the necrosis with early surgical debridement of the pancreas. Experience with myocardial infarction shows that it is not necessary to remove a sterile necrotic organ, and studies with sterile pancreatic necrosis have found that surgical intervention is associated with a higher risk of death than medical management.
Documented infection has traditionally been considered a definite indication for debridement, but even that is being called into question as more studies are emerging of infected necrosis treated successfully with antibiotics alone.
Sterile necrosis with a fulminant course is a controversial indication for surgery. It was traditionally felt that surgery was worth trying for such patients, but this is no longer common practice.
For cases in which debridement was deemed advisable, surgery was done more frequently in the past. Now, a minimally invasive approach such as with endoscopy or percutaneous catheter is also used. Waiting until at least 4 weeks after the onset of acute pancreatitis is associated with a better outcome than intervening early.
WALLED-OFF NECROSIS
Watchful waiting or minimally invasive intervention
Patients who survive multiorgan failure but are still ill more than 4 weeks after the onset of pancreatitis should be suspected of having walled-off necrosis, formerly referred to as a pancreatic phlegmon. This term was abandoned after the 1992 Atlanta symposium.13 In the mid to late 1990s, the process was referred to as organized pancreatic necrosis. It is characterized by a mature, encapsulated collection of pancreatic or peripancreatic necrosis that contains variable amounts of amylase-rich fluid from pancreatic duct disruption.
Walled-off pancreatic necrosis (WOPN) is often confused with pancreatic pseudocyst; these may appear similar on CT, and higherdensity solid debris may be visible in walled-off necrosis within an otherwise homogenous-appearing collection. Magnetic resonance imaging defines liquid and solid much better than CT.
The best way to distinguish WOPN from pseudocyst is by clinical history: a patient with a preceding history of clinically severe acute pancreatitis almost always has necrotizing pancreatitis that evolves to walled-off necrosis, usually over 3 to 4 weeks.
Endoscopic removal and other minimally invasive approaches, such as aggressive percutaneous interventions, have replaced open necrosectomy for treatment, which was associated with high morbidity and mortality rates.14–16
Intervening for sterile walled-off necrosis is still a controversial topic: although systemically ill, the patient is no longer having life-threatening consequences, and watchful waiting might be just as expedient as intervention. Evidence to support either view is lacking. Most experts believe that intervention should be done if the patient has gastric outlet obstruction and intractable pain and is unable to eat 4 to 6 weeks after the onset of pancreatitis with WOPN. Infected WOPN is considered an indication for drainage.
Severe acute pancreatitis has been known since the time of Rembrandt, with Nicolaes Tulp—the physician credited as first describing it—immortalized in the famous painting, The Anatomy Lesson. However, progress in managing this disease has been disappointing. Treatment is mainly supportive, and we lack any true disease-modifying therapy. But we are learning to recognize the disease and treat it supportively better than in the past.
The early hours of severe acute pancreatitis are critical for instituting appropriate intervention. Prompt fluid resuscitation is key to preventing immediate and later morbidity and death. This article focuses on identifying and managing the most severe form of acute pancreatitis—necrotizing disease—and its complications.
NECROTIZING DISEASE ACCOUNTS FOR MOST PANCREATITIS DEATHS
The classification and definitions of acute pancreatitis were recently revised from the 1992 Atlanta system and published early in 2013.1 In addition, the American Pancreatic Association and the International Association of Pancreatology met in 2012 to develop evidence-based guidelines on managing severe pancreatitis.
An estimated 210,000 new cases of acute pancreatitis occur each year in the United States. About 20% of cases of severe acute pancreatitis are necrotizing disease, which accounts for nearly all the morbidity and death associated with acute pancreatitis.
The clinical spectrum of acute pancreatitis ranges from mild to life-threatening, reflecting interstitial (death rate < 1%) to necrotizing histology (the latter associated with a 25% risk of death if the pancreatitis becomes infected and a 10% risk if it is sterile). When death occurs early in the disease course, it tends to be from multiorgan failure; when death occurs later in the course, it tends to be from infection. Appropriate early treatment may prevent death in both categories.
DIAGNOSING ACUTE PANCREATITIS AND PREDICTING ITS SEVERITY
The diagnosis of acute pancreatitis requires two of the following three criteria:
- Clinical presentation—epigastric pain, nausea, vomiting
- Biochemical—amylase level more than three times the upper limit of normal, or lipase more than three times the upper limit of normal
- Evidence from computed tomography (CT), ultrasonography, or magnetic resonance imaging.
Although the biochemical criteria are variably sensitive for detecting acute pancreatitis (55%–100%), the specificity is very high (93% to 99%).
Recently, urinary trypsinogen-2, measured by dipstick, has also been used to aid diagnosis. It has a reasonable sensitivity (53%–96%) and specificity (85%) if positive (> 50 ng/mL).
Speed is critical
Over the years, many clinical prediction rules have been used for predicting the severity of acute pancreatitis. The Ranson criteria,2 from 1974, and the Acute Physiology and Chronic Health Evaluation (APACHE) II system3 are cumbersome and require waiting up to 48 hours after the onset of acute pancreatitis to obtain a complete score. The Imrie-Glasgow score is another predictor.
The systemic inflammatory response syndrome (SIRS) is currently the most important indicator of prognosis.4 Originally adopted for predicting the development of organ failure with sepsis, it requires at least two of the following criteria:
- Heart rate > 90 beats/min
- Core temperature < 36°C or > 38°C
- White blood cells < 4,000 or > 12,000/mm3
- Respirations > 20/min.
The advantages of this system are that it identifies risk very early in the course of the disease and can be assessed quickly in the emergency department.
The Bedside Index for Severity of Acute Pancreatitis (BISAP) score is another simple, easy-to-perform prognostic index,5,6 calculated by assigning 1 point for each of the following if present within the first 24 hours of presentation:
- Blood urea nitrogen > 25 mg/dL
- Abnormal mental status (Glasgow coma score < 15)
- Evidence of systemic inflammatory response syndrome
- Age > 60 years
- Pleural effusion seen on imaging study.
A score of 3 points is associated with a 5.3% rate of hospital death, 4 points with 12.7%, and 5 points with 22.5%.
At its most basic, severe acute pancreatitis is defined by organ failure (at least one organ from the respiratory, renal, or cardiovascular system) lasting for more than 48 hours. Failure for each organ is defined by the Marshall scoring system.1
EARLY MANAGEMENT IS KEY TO OUTCOME
The window of opportunity to make a significant difference in outcome is within the first 12 to 24 hours of presentation. Volume resuscitation is the cornerstone of early management. By the time of presentation for severe acute pancreatitis, the pancreas is already necrotic, so the aim is to minimize the systemic inflammatory response syndrome with the goals of reducing rates of organ failure, morbidity, and death. Necrotizing pancreatitis is essentially an ischemic event, and the goal of volume resuscitation is to maintain pancreatic and intestinal microcirculation to prevent intestinal ischemia and subsequent bacterial translocation.7
Early resuscitation with lactated Ringer’s solution recommended
The evidence supporting a specific protocol for fluid resuscitation in severe acute pancreatitis is not strong, but a few studies provide guidance.
Wu et al8 randomized 40 patients with acute pancreatitis to one of four arms: “goal-directed fluid resuscitation” with either lactated Ringer’s solution or normal saline, or standard therapy (by physician discretion) with either lactated Ringer’s solution or normal saline. Goal-directed therapy involved a bolus of 20 mL/kg given over 30 to 45 minutes at presentation followed by infusion with rates dependent on an algorithm based on change in blood urea nitrogen level at set times. Patients receiving either goal-directed or standard therapy had significantly lower rates of systemic inflammatory response syndrome at 24 hours than at admission. Most striking was that treatment with lactated Ringer’s solution was associated with dramatically improved rates, whereas normal saline showed no improvement.
In a retrospective study of patients with acute pancreatitis, Warndorf et al9 identified 340 patients who received early resuscitation (more than one-third of the total 72-hour fluid volume within 24 hours of presentation) and 90 patients who received late resuscitation (less than one-third of the total 72-hour fluid volume within 24 hours of presentation). Patients who received early resuscitation developed less systemic inflammatory response syndrome and organ failure, and required fewer interventions.
Monitoring for optimum fluid resuscitation
Fluid resuscitation should be carefully managed to avoid administering either inadequate or excessive amounts of fluid. Inadequate fluid resuscitation can result in renal failure, progression of necrosis, and possibly infectious complications. Excessive resuscitation—defined as more than 4 L in the first 24 hours—is associated with respiratory failure, pancreatic fluid collections, and abdominal compartment syndrome.
Optimum resuscitation is controlled fluid expansion averaging 5 to 10 mL/kg per hour, with 2,500 to 4,000 mL given in the first 24 hours.
Adequate volume resuscitation can be evaluated clinically with the following goals:
- Heart rate < 120 beats per minute
- Mean arterial pressure 65–85 mm Hg
- Urinary output > 1 mL/kg per hour
- Hematocrit 35%–44%.
EARLY CT IS JUSTIFIED ONLY IF DIAGNOSIS IS UNCLEAR
The normal pancreas takes up contrast in the same way as do the liver and spleen, so its enhancement on CT is similar. If there is interstitial pancreatitis, CT shows the pancreas with normal contrast uptake, but the organ appears “boggy” with indistinct outlines. With necrotizing pancreatitis, only small areas of tissue with normal contrast may be apparent.
Peripancreatic fat necrosis may also be visible on CT. Obese patients tend to have a worse clinical course of necrotizing pancreatitis, probably because of the associated peripancreatic fat that is incorporated into the pancreatic necrosis.
For clear-cut cases of acute pancreatitis, time is wasted waiting to obtain CT images, and this could delay fluid resuscitation. Results from immediate CT almost never change the clinical management during the first week of acute pancreatitis, and obtaining CT images is usually not recommended if the diagnosis of acute pancreatitis is clear. CT’s sensitivity for detecting necrosis is only 70% in the first 48 hours of presentation, so it is easy to be fooled by a false-negative scan: frequently, a scan does not show necrotizing pancreatitis until after 72 hours. In addition, evidence from animal studies indicates that contrast agents might worsen pancreatic necrosis.
Immediate CT is justified if the diagnosis is in doubt at presentation, such as to evaluate for other intra-abdominal conditions such as intestinal ischemia or a perforated duodenal ulcer.
Contrast-enhanced CT is recommended 72 to 96 hours after presentation, or earlier if the patient is worsening despite treatment. Specific CT protocols will be included in new management guidelines, expected to be published soon.
PREVENTING INFECTIOUS COMPLICATIONS
Risk of infection is associated with the degree of pancreatic necrosis. Patients with less than 30% necrosis have a 22.5% chance of infection, whereas those with more than 50% necrosis have a 46.5% risk of infection.10
Infection can develop from a variety of sources:
Bacterial translocation from the colon and small bowel is thought to be one of the major sources of infection in necrotic pancreatitis. Volume resuscitation and maintaining gut integrity with early enteral nutrition are believed to minimize the risk of bacterial translocation.
Hematogenous spread of bacteria is another suspected source of infection into the pancreas. Again, enteral nutrition also reduces the risk by minimizing the need for central catheters.
Biliary sources may also play a role. Bile duct stones or gall bladder infection can lead to infected pancreatic necrosis.
ANTIBIOTICS NOT ROUTINELY RECOMMENDED
Treating acute pancreatitis with antibiotics has fallen in and out of favor over the past decades. From being standard practice in the 1970s, it dropped off in the 1980s and 1990s and then became more common again.
Current recommendations from the American Pancreatic Association and the International Association of Pancreatology are not to routinely use intravenous antibiotics to prevent infection in necrotizing pancreatitis because of lack of evidence that it changes overall outcome. Antibiotic usage may be associated with more bacterial resistance and the introduction of fungal infections into the pancreas.
Selective gut decontamination, involving oral and rectal administration of neomycin and other antibiotics, was shown in a single randomized trial to reduce the incidence of infection, but it is very cumbersome and is not recommended for acute pancreatitis.
Treatment with probiotics is also not recommended and was shown in one study to lead to a worse outcome.11
ENTERAL BETTER THAN TOTAL PARENTERAL NUTRITION
Enteral tube feeding with either an elemental diet or a polymeric enteral formulation is the first-line therapy for necrotizing pancreatitis. Compared with total parenteral nutrition, it reduces infection, organ failure, hospital length of stay, the need for surgical intervention, and the risk of death. Total parenteral nutrition should be considered only for patients who do not tolerate enteral feeding because of severe ileus.
Conventional thinking for many years was to provide enteral feeding with a tube passed beyond the ligament of Treitz, thinking that it reduced stimulation to the pancreas. However, recent studies indicate that nasogastric feeding is equivalent to nasojejunal feeding in terms of nutrition, maintaining gut integrity, and outcome.
INTRA-ABDOMINAL HYPERTENSION AND ABDOMINAL COMPARTMENT SYNDROME
Movement of fluid into the intracellular space (“third-spacing”) occurs in acute pancreatitis and is exacerbated by fluid resuscitation. Intra-abdominal hypertension is associated with poor outcomes in patients with severe acute pancreatitis. Especially for patients with severe pancreatitis who are on mechanical ventilation, pressure should be monitored with transvesicular bladder measurements.
Intra-abdominal hypertension is defined as a sustained intra-abdominal pressure of more than 12 mm Hg, with the following grades:
- Grade 1: 12–15 mm Hg
- Grade 2: 16–20 mm Hg
- Grade 3: 21–25 mm Hg
- Grade 4: > 25 mm Hg.
Abdominal compartment syndrome is defined as a sustained intra-abdominal pressure of more than 20 mm Hg. It is associated with new organ dysfunction or failure. It should first be managed with ultrafiltration or diuretics to try to reduce the amount of fluid in the abdomen. Lumenal decompression can be tried with nasogastric or rectal tubes for the stomach and bowels. Ascites or retroperitoneal fluid can be drained percutaneously. In addition, analgesia and sedation to reduce abdominal muscle tone can help the patient become better ventilated. Neuromuscular blockade can also relax the abdomen.
Open abdominal decompression is the treatment of last resort to relieve abdominal compartment syndrome. The abdominal wall is not closed surgically but is allowed to heal by secondary intention (it “granulates in”).12
IDENTIFYING INFECTION
Fine-needle aspiration if clinical and imaging signs are not clear
Untreated infected pancreatitis is associated with a much higher risk of death than sterile pancreatic necrosis. Unfortunately, it can be difficult to determine if a patient with necrotizing pancreatitis has an infection because fever, tachycardia, and leukocytosis are usually present regardless. It is important to determine because mechanically intervening for sterile necrosis does not improve outcome.
Fine-needle aspiration, either guided by CT or done at the bedside with ultrasonography, with evaluation with Gram stain and culture, was widely used in the 1990s in cases of necrotizing pancreatitis to determine if infection was present. There has been a shift away from this because, although it can confirm the presence of infection, the false-negative rate is 15%. Clinical and imaging signs can be relied on in most cases to determine the presence of infection, and it is now recognized that fineneedle aspiration should be used only for select cases. Clinical studies have not shown that fine-needle aspiration improves outcomes.
Clinical scenarios typical of infected pancreatic necrosis include patients who have obvious signs of infection with no identifiable source, such as those who stabilize after acute severe acute pancreatitis, and then 10 to 14 days later become worse, with a dramatically higher white blood cell count and tachycardia. Such a patient likely needs an intervention regardless of the results of fine-needle aspiration.
On the other hand, a patient with a continually up-and-down course that never stabilizes over 3 weeks, with no identifiable source of infection, and with no peripancreatic gas apparent on imaging would be a good candidate for fine-needle aspiration.
If peripancreatic gas is seen on imaging, fine-needle aspiration is unnecessary. Peripancreatic gas is traditionally attributed to gasforming bacteria within the pancreas, but in my experience, it is usually from a fistula from the necrosis to the duodenum or the colon, the fistula being caused as the necrosis erodes at the hepatic flexure, the transverse colon, or the splenic flexure.
MECHANICAL INTERVENTIONS FOR INFECTIVE NECROSIS
Late, minimally invasive procedures preferred
Conventional management has shifted away from removing the necrosis with early surgical debridement of the pancreas. Experience with myocardial infarction shows that it is not necessary to remove a sterile necrotic organ, and studies with sterile pancreatic necrosis have found that surgical intervention is associated with a higher risk of death than medical management.
Documented infection has traditionally been considered a definite indication for debridement, but even that is being called into question as more studies are emerging of infected necrosis treated successfully with antibiotics alone.
Sterile necrosis with a fulminant course is a controversial indication for surgery. It was traditionally felt that surgery was worth trying for such patients, but this is no longer common practice.
For cases in which debridement was deemed advisable, surgery was done more frequently in the past. Now, a minimally invasive approach such as with endoscopy or percutaneous catheter is also used. Waiting until at least 4 weeks after the onset of acute pancreatitis is associated with a better outcome than intervening early.
WALLED-OFF NECROSIS
Watchful waiting or minimally invasive intervention
Patients who survive multiorgan failure but are still ill more than 4 weeks after the onset of pancreatitis should be suspected of having walled-off necrosis, formerly referred to as a pancreatic phlegmon. This term was abandoned after the 1992 Atlanta symposium.13 In the mid to late 1990s, the process was referred to as organized pancreatic necrosis. It is characterized by a mature, encapsulated collection of pancreatic or peripancreatic necrosis that contains variable amounts of amylase-rich fluid from pancreatic duct disruption.
Walled-off pancreatic necrosis (WOPN) is often confused with pancreatic pseudocyst; these may appear similar on CT, and higherdensity solid debris may be visible in walled-off necrosis within an otherwise homogenous-appearing collection. Magnetic resonance imaging defines liquid and solid much better than CT.
The best way to distinguish WOPN from pseudocyst is by clinical history: a patient with a preceding history of clinically severe acute pancreatitis almost always has necrotizing pancreatitis that evolves to walled-off necrosis, usually over 3 to 4 weeks.
Endoscopic removal and other minimally invasive approaches, such as aggressive percutaneous interventions, have replaced open necrosectomy for treatment, which was associated with high morbidity and mortality rates.14–16
Intervening for sterile walled-off necrosis is still a controversial topic: although systemically ill, the patient is no longer having life-threatening consequences, and watchful waiting might be just as expedient as intervention. Evidence to support either view is lacking. Most experts believe that intervention should be done if the patient has gastric outlet obstruction and intractable pain and is unable to eat 4 to 6 weeks after the onset of pancreatitis with WOPN. Infected WOPN is considered an indication for drainage.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974; 139:69–81.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–829.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20:864–874.
- Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008; 57:1698–1703.
- Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009; 104:966–971.
- Fisher JM, Gardner TB. The “golden hours” of management in acute pancreatitis. Am J Gastroenterol 2012; 107:1146–1150.
- Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:710–717.
- Warndorf MG, Kurtzman JT, Bartel MJ, et al. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:705–709.
- Beger HG, Rau BM. Severe acute pancreatitis: clinical course and management. World J Gastroenterol 2007; 13:5043–5051.
- Besselink MG, van Santvoort HC, Buskens E, et al; Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371:651–659.
- Fitzgerald JE, Gupta S, Masterson S, Sigurdsson HH. Laparostomy management using the ABThera open abdomen negative pressure therapy system in a grade IV open abdomen secondary to acute pancreatitis. Int Wound J 2012. doi: 1111/j.1742-481X2012.00953.x. [epub ahead of print]
- Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11–13, 1992. Arch Surg 1993; 128:586–590.
- Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology 1996; 111:755–764.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974; 139:69–81.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–829.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20:864–874.
- Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008; 57:1698–1703.
- Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009; 104:966–971.
- Fisher JM, Gardner TB. The “golden hours” of management in acute pancreatitis. Am J Gastroenterol 2012; 107:1146–1150.
- Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:710–717.
- Warndorf MG, Kurtzman JT, Bartel MJ, et al. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:705–709.
- Beger HG, Rau BM. Severe acute pancreatitis: clinical course and management. World J Gastroenterol 2007; 13:5043–5051.
- Besselink MG, van Santvoort HC, Buskens E, et al; Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371:651–659.
- Fitzgerald JE, Gupta S, Masterson S, Sigurdsson HH. Laparostomy management using the ABThera open abdomen negative pressure therapy system in a grade IV open abdomen secondary to acute pancreatitis. Int Wound J 2012. doi: 1111/j.1742-481X2012.00953.x. [epub ahead of print]
- Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11–13, 1992. Arch Surg 1993; 128:586–590.
- Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology 1996; 111:755–764.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
KEY POINTS
- Routine early computed tomography to evaluate patients with severe acute pancreatitis wastes time and is necessary only if the diagnosis at presentation is not clearly consistent with acute pancreatitis.
- Optimum fluid resuscitation is now recommended, using lactated Ringer’s solution at a rate of 5 to 10 mL/kg per hour, with 2,500 to 4,000 mL given in the first 24 hours.
- Enteral feeding with either an elemental diet or a polymeric enteral formulation is first-line nutritional therapy.
- Antibiotics are no longer routinely used to prevent infection.
- Relief of abdominal compartment syndrome should be attempted by multiple means before resorting to open abdominal decompression.
Hand Slammed in Door
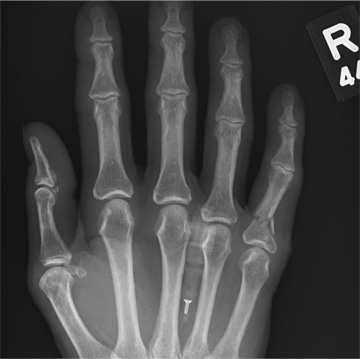
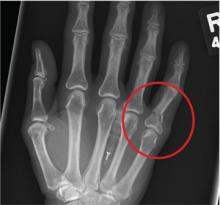
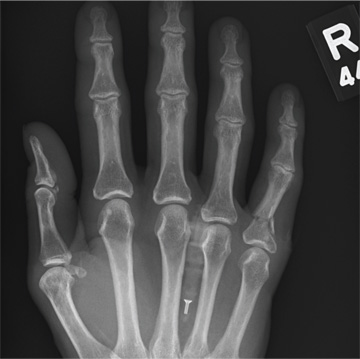
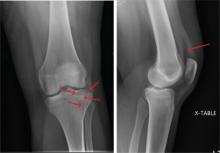
A 48-year-old woman presents to the urgent care center with complaints of right hand pain second-ary to an injury she sustained earlier in the day. Her hand was accidentally caught in a metal door as it was being shut by someone else. The door struck her in the middorsal aspect of her hand. She is now complaining of pain and swelling. She is healthy except for mild but well-controlled hypertension. Her vital signs are normal. Examina-tion of her right hand shows mild to moderate soft tissue swelling and some early bruising. There is extreme tenderness over the fourth and fifth metacarpal bones. Good capillary refill time is noted, and sensation is intact. She is able to flex her fingers somewhat, although this is limited by the swelling. Radiograph of the right hand is obtained. What is your impression?
Unresponsive Woman Extricated From Car
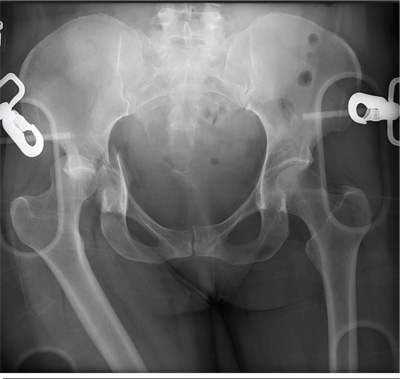
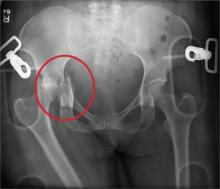
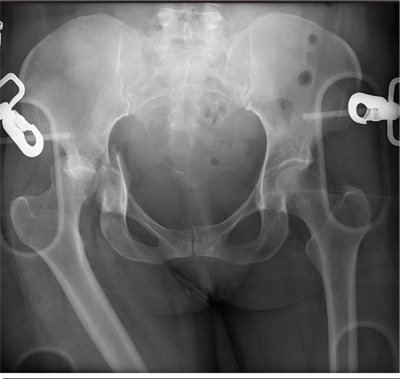
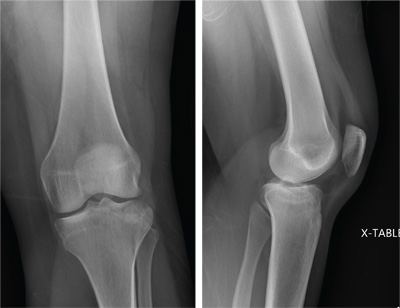
An unidentified female is brought in as a trauma code. She was apparently in a motor vehicle crash, the specific details of which are unclear. Emergency medical personnel describe extensive damage to her vehicle, which resulted in a pro-longed extrication time. Due to unresponsiveness at the scene, she was intubated in the field. The patient is probably in her late 30s to early 40s. She has two large-bore IV lines with normal saline infusing at a wide-open rate. Blood pressure is 90/60 mm Hg and heart rate, 140 beats/min. She has several superficial lacerations on her head, her pupils are fixed and dilated, and there is minimal withdrawal to pain in her extremities. No other trauma is im-mediately evident. Portable radiographs of her chest and pelvis are obtained prior to sending her for CT. Pelvis radiograph is shown. What is your impression?
Options for managing severe aortic stenosis: A case-based review
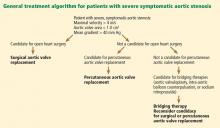
Surgical aortic valve replacement remains the gold standard treatment for symptomatic aortic valve stenosis in patients at low or moderate risk of surgical complications. But this is a disease of the elderly, many of whom are too frail or too sick to undergo surgery.
Now, patients who cannot undergo this surgery can be offered the less invasive option of transcatheter aortic valve replacement. Balloon valvuloplasty, sodium nitroprusside, and intra-aortic balloon counterpulsation can buy time for ill patients while more permanent mechanical interventions are being considered.
In this review, we will present several cases that highlight management choices for patients with severe aortic stenosis.
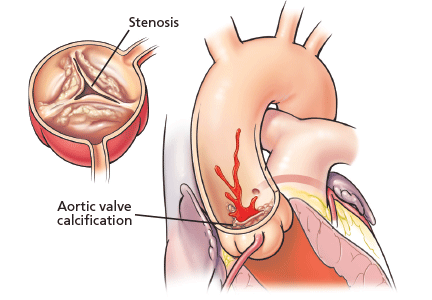
A PROGRESSIVE DISEASE OF THE ELDERLY
Aortic stenosis is the most common acquired valvular disease in the United States, and its incidence and prevalence are rising as the population ages. Epidemiologic studies suggest that 2% to 7% of all patients over age 65 have it.1,2
The natural history of the untreated disease is well established, with several case series showing an average decrease of 0.1 cm2 per year in aortic valve area and an increase of 7 mm Hg per year in the pressure gradient across the valve once the diagnosis is made.3,4 Development of angina, syncope, or heart failure is associated with adverse clinical outcomes, including death, and warrants prompt intervention with aortic valve replacement.5–7 Without intervention, the mortality rates reach as high as 75% in 3 years once symptoms develop.
Statins, bisphosphonates, and angiotensin-converting enzyme inhibitors have been used in attempts to slow or reverse the progression of aortic stenosis. However, studies of these drugs have had mixed results, and no definitive benefit has been shown.8–13 Surgical aortic valve replacement, on the other hand, normalizes the life expectancy of patients with aortic stenosis to that of age- and sex-matched controls and remains the gold standard therapy for patients who have symptoms.14
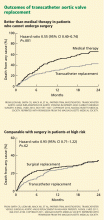
Traditionally, valve replacement has involved open heart surgery, since it requires direct visualization of the valve while the patient is on cardiopulmonary bypass. Unfortunately, many patients have multiple comorbid conditions and therefore are not candidates for open heart surgery. Options for these patients include aortic valvuloplasty and transcatheter aortic valve replacement. While there is considerable experience with aortic valvuloplasty, transcatheter aortic valve replacement is relatively new. In large randomized trials and registries, the transcatheter procedure has been shown to significantly improve long-term survival compared with medical management alone in inoperable patients and to have benefit similar to that of surgery in the high-risk population.15–17
CASE 1: SEVERE, SYMPTOMATIC STENOSIS IN A GOOD SURGICAL CANDIDATE
Mr. A, age 83, presents with shortness of breath and peripheral edema that have been worsening over the past several months. His pulse rate is 64 beats per minute and his blood pressure is 110/90 mm Hg. Auscultation reveals an absent aortic second heart sound with a late peaking systolic murmur that increases with expiration.
On echocardiography, his left ventricular ejection fraction is 55%, peak transaortic valve gradient 88 mm Hg, mean gradient 60 mm Hg, and effective valve area 0.6 cm2. He undergoes catheterization of the left side of his heart, which shows normal coronary arteries.
Mr. A also has hypertension and hyperlipidemia; his renal and pulmonary functions are normal.
How would you manage Mr. A’s aortic stenosis?
Symptomatic aortic stenosis leads to adverse clinical outcomes if managed medically without mechanical intervention,5–7 but patients who undergo aortic valve replacement have age-corrected postoperative survival rates that are nearly normal.14 Furthermore, thanks to improvements in surgical techniques and perioperative management, surgical mortality rates have decreased significantly in recent years and now range from 1% to 8%.18–20 The accumulated evidence showing clear superiority of a surgical approach over medical therapy has greatly simplified the therapeutic algorithm.21
Consequently, the current guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) give surgery a class I indication (evidence or general agreement that the procedure is beneficial, useful, and effective) for symptomatic severe aortic stenosis (Figure 1). This level of recommendation also applies to patients who have severe but asymptomatic aortic stenosis who are undergoing other types of cardiac surgery and also to patients with severe aortic stenosis and left ventricular dysfunction (defined as an ejection fraction < 50%).21
Mr. A was referred for surgical aortic valve replacement, given its clear survival benefit.
CASE 2: SYMPTOMS AND LEFT VENTRICULAR DYSFUNCTION
Ms. B, age 79, has hypertension and hyperlipidemia and now presents to the outpatient department with worsening shortness of breath and chest discomfort. Electrocardiography shows significant left ventricular hypertrophy and abnormal repolarization. Left heart catheterization reveals mild nonobstructive coronary artery disease.
Echocardiography reveals an ejection fraction of 25%, severe left ventricular hypertrophy, and global hypokinesis. The aortic valve leaflets appear heavily calcified, with restricted motion. The peak and mean gradients across the aortic valve are 40 and 28 mm Hg, and the valve area is 0.8 cm2. Right heart catheterization shows a cardiac output of 3.1 L/min.
Does this patient’s aortic stenosis account for her clinical presentation?
Managing patients who have suspected severe aortic stenosis, left ventricular dysfunction, and low aortic valve gradients can be challenging. Although data for surgical intervention are not as robust for these patient subsets as for patients like Mr. A, several case series have suggested that survival in these patients is significantly better with surgery than with medical therapy alone.22–27
Specific factors predict whether patients with ventricular dysfunction and low gradients will benefit from aortic valve replacement. Dobutamine stress echocardiography is helpful in distinguishing true severe aortic stenosis from “pseudostenosis,” in which leaflet motion is restricted due to primary cardiomyopathy and low flow. Distinguishing between true aortic stenosis and pseudostenosis is of paramount value, as surgery is associated with improved long-term outcomes in patients with true aortic stenosis (even though they are at higher surgical risk), whereas those with pseudostenosis will not benefit from surgery.28–31
Infusion of dobutamine increases the flow across the aortic valve (if the left ventricle has contractile reserve; more on this below), and an increasing valve area with increasing doses of dobutamine is consistent with pseudostenosis. In this situation, treatment of the underlying cardiomyopathy is indicated as opposed to replacement of the aortic valve (Figure 2).
Contractile reserve is defined as an increase in stroke volume (> 20%), valvular gradient (> 10 mm Hg), or peak velocity (> 0.6 m/s) with peak dobutamine infusion. The presence of contractile reserve in patients with aortic stenosis identifies a high-risk group that benefits from aortic valve replacement (Figure 2).
Treatment of patients who have inadequate reserve is controversial. In the absence of contractile reserve, an adjunct imaging study such as computed tomography may be of value in detecting calcified valve leaflets, as the presence of calcium is associated with true aortic stenosis. Comorbid conditions should be taken into account as well, given the higher surgical risk in this patient subset, as aortic valve replacement in this already high-risk group of patients might be futile in some cases.
The ACC/AHA guidelines now give dobutamine stress echocardiography a class IIa indication (meaning the weight of the evidence or opinion is in favor of usefulness or efficacy) for determination of contractile reserve and valvular stenosis for patients with an ejection fraction of 30% or less or a mean gradient of 40 mm Hg or less.21
Ms. B underwent dobutamine stress echocardiography. It showed increases in ejection fraction, stroke volume, and transvalvular gradients, indicating that she did have contractile reserve and true severe aortic stenosis. Consequently, she was referred for surgical aortic valve replacement.
CASE 3: MODERATE STENOSIS AND THREE-VESSEL CORONARY ARTERY DISEASE
Mr. C, age 81, has hypertension and hyperlipidemia. He now presents to the emergency department with chest discomfort that began suddenly, awakening him from sleep. His presenting electrocardiogram shows nonspecific changes, and he is diagnosed with non-ST-elevation myocardial infarction. He undergoes left heart catheterization, which reveals severe three-vessel coronary artery disease.
Echocardiography reveals an ejection fraction of 55% and aortic stenosis, with an aortic valve area of 1.2 cm2, a peak gradient of 44 mm Hg, and a mean gradient of 28 mm Hg.
How would you manage his aortic stenosis?
Moderate aortic stenosis in a patient who needs surgery for severe triple-vessel coronary artery disease, other valve diseases, or aortic disease raises the question of whether aortic valve replacement should be performed in conjunction with these surgeries. Although these patients would not otherwise qualify for aortic valve replacement, the fact that they will undergo a procedure that will expose them to the risks associated with open heart surgery makes them reasonable candidates. Even if the patient does not need aortic valve replacement right now, aortic stenosis progresses at a predictable rate—the valve area decreases by a mean of 0.1 cm2/year and the gradients increase by 7 mm Hg/year. Therefore, clinical judgment should be exercised so that the patient will not need to undergo open heart surgery again in the near future.
The ACC/AHA guidelines recommend aortic valve replacement for patients with moderate aortic stenosis undergoing coronary artery bypass grafting or surgery on the aorta or other heart valves, giving it a class IIa indication.21 This recommendation is based on several retrospective case series that evaluated survival, the need for reoperation for aortic valve replacement, or both in patients undergoing coronary artery bypass grafting.32–35
No data exist, however, on adding aortic valve replacement to coronary artery bypass grafting in cases of mild aortic stenosis. As a result, it is controversial and carries a class IIb recommendation (meaning that its usefulness or efficacy is less well established). The ACC/AHA guidelines state that aortic valve replacement “may be considered” in patients undergoing coronary artery bypass grafting who have mild aortic stenosis (mean gradient < 30 mm Hg or jet velocity < 3 m/s) when there is evidence, such as moderate or severe valve calcification, that progression may be rapid (level of evidence C: based only on consensus opinion of experts, case studies or standard of care).21
Mr. C, who has moderate aortic stenosis, underwent aortic valve replacement in conjunction with three-vessel bypass grafting.
CASE 4: ASYMPTOMATIC BUT SEVERE STENOSIS
Mr. D, age 74, has hypertension, hyperlipidemia, and aortic stenosis. He now presents to the outpatient department for his annual echocardiogram to follow his aortic stenosis. He has a sedentary lifestyle but feels well performing activities of daily living. He denies dyspnea on exertion, chest pain, or syncope.
His echocardiogram reveals an effective aortic valve area of 0.7 cm2, peak gradient 90 mm Hg, and mean gradient 70 mm Hg. There is evidence of severe left ventricular hypertrophy, and the valve leaflets show bulky calcification and severe restriction. An echocardiogram performed at the same institution a year earlier revealed gradients of 60 and 40 mm Hg.
Blood is drawn for laboratory tests, including N-terminal pro-brain natriuretic peptide, which is 350 pg/mL (reference range for his age < 125 pg/mL). He is referred for a treadmill stress test, which elicits symptoms at a moderate activity level.
How would you manage his aortic stenosis?
Aortic valve replacement can be considered in patients who have asymptomatic but severe aortic stenosis with preserved left ventricular function (class IIb indication).21
Clinical assessment of asymptomatic aortic stenosis can be challenging, however, as patients may underreport their symptoms or decrease their activity levels to avoid symptoms. Exercise testing in such patients can elicit symptoms, unmask diminished exercise capacity, and help determine if they should be referred for surgery.36,37 Natriuretic peptide levels have been shown to correlate with the severity of aortic stenosis,38,39 and more importantly, to help predict symptom onset, cardiac death, and need for aortic valve replacement.40–42
Some patients with asymptomatic but severe aortic stenosis are at higher risk of morbidity and death. High-risk subsets include patients with rapid progression of aortic stenosis and those with critical aortic stenosis characterized by an aortic valve area less than 0.60 cm2, mean gradient greater than 60 mm Hg, and jet velocity greater than 5.0 m/s. It is reasonable to offer these patients surgery if their expected operative mortality risk is less than 1.0%.21
Mr. D has evidence of rapid progression as defined by an increase in aortic jet velocity of more than 0.3 m/s/year. He is at low surgical risk and was referred for elective aortic valve replacement.
CASE 5: TOO FRAIL FOR SURGERY
Mr. E, age 84, has severe aortic stenosis (valve area 0.6 cm2, peak and mean gradients of 88 and 56 mm Hg), coronary artery disease status post coronary artery bypass grafting, moderate chronic obstructive pulmonary disease (forced expiratory volume in 1 second 0.8 L), chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, hyperlipidemia, and diabetes mellitus. He has preserved left ventricular function. He presents to the outpatient department with worsening shortness of breath and peripheral edema over the past several months. Your impression is that he is very frail. How would you manage Mr. E’s aortic stenosis?
Advances in surgical techniques and perioperative management over the years have enabled higher-risk patients to undergo surgical aortic valve replacement with excellent out-comes.18–20,43 Yet many patients still cannot undergo surgery because their risk is too high. Patients ineligible for surgery have traditionally been treated medically—with poor out-comes—or with balloon aortic valvuloplasty to palliate symptoms.
Transcatheter aortic valve replacement, approved by the US Food and Drug Administration (FDA) in 2011, now provides another option for these patients. In this procedure, a bioprosthetic valve mounted on a metal frame is implanted over the native stenotic valve.
Currently, the only FDA-approved and commercially available valve in the United States is the Edwards SAPIEN valve, which has bovine pericardial tissue leaflets fixed to a balloon-expandable stainless steel frame (Figure 3). In the Placement of Aortic Transcatheter Valves (PARTNER) trial,15 patients who could not undergo surgery who underwent transcatheter replacement with this valve had a significantly better survival rate than patients treated medically.15,17 Use of this valve has also been compared against conventional surgical aortic valve replacement in high-risk patients and was found to have similar long-term outcomes (Figure 4).16 It was on the basis of this trial that this valve was granted approval for patients who cannot undergo surgery.
The standard of care for high-risk patients remains surgical aortic valve replacement, although it remains to be seen whether transcatheter replacement will be made available as well to patients eligible for surgery in the near future. There are currently no randomized data for transcatheter aortic valve replacement in patients at moderate to low surgical risk, and these patients should not be considered for this procedure.
Although the initial studies are encouraging for patients who cannot undergo surgery and who are at high risk without it, several issues and concerns remain. Importantly, the long-term durability of the transcatheter valve and longer-term outcomes remain unknown. Furthermore, the risk of vascular complications remains high (10% to 15%), dictating the need for careful patient selection. There are also concerns about the risks of stroke and of paravalvular aortic insufficiency. These issues are being investigated and addressed, however, and we hope that with increasing operator experience and improvements in the technique, outcomes will be improved.
Which approach for transcatheter aortic valve replacement?
There are several considerations in determining a patient’s eligibility for transcatheter aortic valve replacement.
Initially, these valves were placed by a transvenous, transseptal approach, but now retrograde placement through the femoral artery has become standard. In this procedure, the device is advanced retrograde from the femoral artery through the aorta and placed across the native aortic valve under fluoroscopic and echocardiographic guidance.
Patients who are not eligible for transfemoral placement because of severe atherosclerosis, tortuosity, or ectasia of the iliofemoral artery or aorta can still undergo percutaneous treatment with a transapical approach. This is a hybrid surgical-transcatheter approach in which the valve is delivered through a sheath placed by left ventricular apical puncture.17,44
A newer approach gaining popularity is the transaortic technique, in which the ascending aorta is accessed directly through a ministernotomy and the delivery sheath is placed with a direct puncture. Other approaches are through the axillary and subclavian arteries.
Other valves are under development
Several other valves are under development and will likely change the landscape of transcatheter aortic valve replacement with improving outcomes. Valves that are available in the United States are shown in Figure 3. The CoreValve, consisting of porcine pericardial leaflets mounted on a self-expanding nitinol stent, is currently being studied in a trial in the United States, and the manufacturer (Medtronic) will seek approval when results are complete in the near future.
Mr. E was initially referred for surgery, but when deemed to be unable to undergo surgery was found to be a good candidate for transcatheter aortic valve replacement.
CASE 6: LIFE-LIMITING COMORBID ILLNESS
Mr. F, age 77, has multiple problems: severe aortic stenosis (aortic valve area 0.6 cm2; peak and mean gradients of 92 and 59 mm Hg), stage IV pancreatic cancer, coronary artery disease status post coronary artery bypass grafting, chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, and hyperlipidemia. He presents to the outpatient department with shortness of breath at rest, orthopnea, effort intolerance, and peripheral edema over the past several months.
On physical examination rales in both lung bases can be heard. Left heart catheterization shows patent bypass grafts.
How would you manage Mr. F’s aortic stenosis?
Aortic valve replacement is not considered an option in patients with noncardiac illnesses and comorbidities that are life-limiting in the near term. Under these circumstances, aortic valvuloplasty can be offered as a means of palliating symptoms or, if the comorbid conditions can be modified, as a bridge to more definitive treatment with aortic valve replacement.
Since first described in 1986,45 percutaneous aortic valvuloplasty has been studied in several case series and registries, with consistent findings. Acutely, it increases the valve area and lessens the gradients across the valve, relieving symptoms. The risk of death during the procedure ranged from 3% to 13.5% in several case series, with a 30-day survival rate greater than 85%.46 However, the hemodynamic and symptomatic improvement is only short-term, as valve area and gradients gradually worsen within several months.47,48 Consequently, balloon valvuloplasty is considered a palliative approach.
Mr. F has a potentially life-limiting illness, ie, cancer, which would make him a candidate for aortic valvuloplasty rather than replacement. He can be referred for evaluation for this procedure in hopes of palliating his symptoms by relieving his dyspnea and improving his quality of life.
CASE 7: HEMODYNAMIC INSTABILITY
Mr. G, age 87, is scheduled for surgical aortic valve replacement because of severe aortic stenosis (valve area 0.5 cm2, peak and mean gradients 89 and 45 mm Hg) with an ejection fraction of 30%.
Two weeks before his scheduled surgery he presents to the emergency department with worsening fluid overload and increasing shortness of breath. His initial laboratory work shows new-onset renal failure, and he has signs of hypoperfusion on physical examination. He is transferred to the cardiac intensive care unit for further care.
How would you manage his aortic stenosis?
Patients with decompensated aortic stenosis and hemodynamic instability are at extreme risk during surgery. Medical stabilization beforehand may mitigate the risks associated with surgical or transcatheter aortic valve replacement. Aortic valvuloplasty, treatment with sodium nitroprusside, and support with intra-aortic balloon counterpulsation may help stabilize patients in this “low-output” setting.
Sodium nitroprusside has long been used in low-output states. By relaxing vascular smooth muscle, it leads to increased venous capacitance, decreasing preload and congestion. It also decreases systemic vascular resistance with a subsequent decrease in afterload, which in turn improves systolic emptying. Together, these effects reduce systolic and diastolic wall stress, lower myocardial oxygen consumption, and ultimately increase cardiac output.49,50
These theoretical benefits translate to clinical improvement and increased cardiac output, as shown in a case series of 25 patients with severe aortic stenosis and left ventricular systolic dysfunction (ejection fraction 35%) presenting in a low-output state in the absence of hypotension.51 These findings have led to a ACC/AHA recommendation for the use of sodium nitroprusside in patients who have severe aortic stenosis presenting in low-output state with decompensated heart failure.21
Intra-aortic balloon counterpulsation, introduced in 1968, has been used in several clinical settings, including acute coronary syndromes, intractable ventricular arrhythmias, and refractory heart failure, and for support of hemodynamics in the perioperative setting. Its role in managing ventricular septal rupture and acute mitral regurgitation is well established. It reliably reduces afterload and improves coronary perfusion, augmenting the cardiac output. This in turn leads to improved systemic perfusion, which can buy time for a critically ill patient during which the primary disease process is addressed.
Recently, a case series in which intraaortic balloon counterpulsation devices were placed in patients with severe aortic stenosis and cardiogenic shock showed findings similar to those with sodium nitroprusside infusion. Specifically, their use was associated with improved cardiac indices and filling pressures with a decrease in systemic vascular resistance. These changes have led to increased cardiac performance, resulting in better systemic perfusion.52 Thus, intra-aortic balloon counterpulsation can be an option for stabilizing patients with severe aortic stenosis and cardiogenic shock.
Mr. G was treated with sodium nitroprusside and intravenous diuretics. He achieved symptomatic relief and his renal function returned to baseline. He subsequently underwent aortic valve replacement during the hospitalization.
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009; 373:956–966.
- Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993; 21:1220–1225.
- Otto CM, Pearlman AS, Gardner CL. Hemodynamic progression of aortic stenosis in adults assessed by Doppler echocardiography. J Am Coll Cardiol 1989; 13:545–550.
- Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997; 95:2262–2270.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Turina J, Hess O, Sepulcri F, Krayenbuehl HP. Spontaneous course of aortic valve disease. Eur Heart J 1987; 8:471–483.
- Horstkotte D, Loogen F. The natural history of aortic valve stenosis. Eur Heart J 1988; 9(suppl E):57–64.
- Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation 2001; 104:2205–2209.
- Cowell SJ, Newby DE, Prescott RJ, et al; Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005; 352:2389–2397.
- Rossebø AB, Pedersen TR, Boman K, et al; SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008; 359:1343–1356.
- Moura LM, Ramos SF, Zamorano JL, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol 2007; 49:554–561.
- Rosenhek R, Rader F, Loho N, et al. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation 2004; 110:1291–1295.
- O’Brien KD, Probstfield JL, Caulked MT, et al. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch Intern Med 2005; 165:858–862.
- Lindblom D, Lindblom U, Qvist J, Lundström H. Long-term relative survival rates after heart valve replacement. J Am Coll Cardiol 1990; 15:566–573.
- Makkar RR, Fontana G P, Jilaihawi H, et al; PARTNER Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012; 366:1696–1704.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Di Eusanio M, Fortuna D, Cristell D, et al; RERIC (Emilia Romagna Cardiac Surgery Registry) Investigators. Contemporary outcomes of conventional aortic valve replacement in 638 octogenarians: insights from an Italian Regional Cardiac Surgery Registry (RERIC). Eur J Cardiothorac Surg 2012; 41:1247–1252.
- Di Eusanio M, Fortuna D, De Palma R, et al. Aortic valve replacement: results and predictors of mortality from a contemporary series of 2256 patients. J Thorac Cardiovasc Surg 2011; 141:940–947.
- Jamieson WR, Edwards FH, Schwartz M, Bero JW, Clark RE, Grover FL. Risk stratification for cardiac valve replacement. National Cardiac Surgery Database. Database Committee of the Society of Thoracic Surgeons. Ann Thorac Surg 1999; 67:943–951.
- Bonow RO, Carabello BA, Chatterjee K, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 52:e1–e142.
- Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007; 115:2856–2864.
- Vaquette B, Corbineau H, Laurent M, et al. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart 2005; 91:1324–1329.
- Connolly HM, Oh JK, Orszulak TA, et al. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction. Prognostic indicators. Circulation 1997; 95:2395–2400.
- Connolly HM, Oh JK, Schaff HV, et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation 2000; 101:1940–1946.
- Pereira JJ, Lauer MS, Bashir M, et al. Survival after aortic valve replacement for severe aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am Coll Cardiol 2002; 39:1356–1363.
- Pai RG, Varadarajan P, Razzouk A. Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg 2008; 86:1781–1789.
- Monin JL, Monchi M, Gest V, Duval-Moulin AM, Dubois-Rande JL, Gueret P. Aortic stenosis with severe left ventricular dysfunction and low transvalvular pressure gradients: risk stratification by low-dose dobutamine echocardiography. J Am Coll Cardiol 2001; 37:2101–2107.
- Monin JL, Quéré J P, Monchi M, et al. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 2003; 108:319–324.
- Zuppiroli A, Mori F, Olivotto I, Castelli G, Favilli S, Dolara A. Therapeutic implications of contractile reserve elicited by dobutamine echocardiography in symptomatic, low-gradient aortic stenosis. Ital Heart J 2003; 4:264–270.
- Tribouilloy C, Lévy F, Rusinaru D, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol 2009; 53:1865–1873.
- Ahmed AA, Graham AN, Lovell D, O’Kane HO. Management of mild to moderate aortic valve disease during coronary artery bypass grafting. Eur J Cardiothorac Surg 2003; 24:535–539.
- Verhoye J P, Merlicco F, Sami IM, et al. Aortic valve replacement for aortic stenosis after previous coronary artery bypass grafting: could early reoperation be prevented? J Heart Valve Dis 2006; 15:474–478.
- Hochrein J, Lucke JC, Harrison JK, et al. Mortality and need for reoperation in patients with mild-to-moderate asymptomatic aortic valve disease undergoing coronary artery bypass graft alone. Am Heart J 1999; 138:791–797.
- Pereira JJ, Balaban K, Lauer MS, Lytle B, Thomas JD, Garcia MJ. Aortic valve replacement in patients with mild or moderate aortic stenosis and coronary bypass surgery. Am J Med 2005; 118:735–742.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Weber M, Hausen M, Arnold R, et al. Prognostic value of N-terminal pro-B-type natriuretic peptide for conservatively and surgically treated patients with aortic valve stenosis. Heart 2006; 92:1639–1644.
- Gerber IL, Stewart RA, Legget ME, et al. Increased plasma natriuretic peptide levels refect symptom onset in aortic stenosis. Circulation 2003; 107:1884–1890.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lancellotti P, Moonen M, Magne J, et al. Prognostic effect of long-axis left ventricular dysfunction and B-type natriuretic peptide levels in asymptomatic aortic stenosis. Am J Cardiol 2010; 105:383–388.
- Langanay T, Flécher E, Fouquet O, et al. Aortic valve replacement in the elderly: the real life. Ann Thorac Surg 2012; 93:70–77.
- Christofferson RD, Kapadia SR, Rajagopal V, Tuzcu EM. Emerging transcatheter therapies for aortic and mitral disease. Heart 2009; 95:148–155.
- Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1986; 1:63–67.
- Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 1991; 84:2383–2397.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Bernard Y, Etievent J, Mourand JL, et al. Long-term results of percutaneous aortic valvuloplasty compared with aortic valve replacement in patients more than 75 years old. J Am Coll Cardiol 1992; 20:796–801.
- Elkayam U, Janmohamed M, Habib M, Hatamizadeh P. Vasodilators in the management of acute heart failure. Crit Care Med 2008; 36(suppl 1):S95–S105.
- Popovic ZB, Khot UN, Novaro GM, et al. Effects of sodium nitroprusside in aortic stenosis associated with severe heart failure: pressure-volume loop analysis using a numerical model. Am J Physiol Heart Circ Physiol 2005; 288:H416–H423.
- Khot UN, Novaro GM, Popovic ZB, et al. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med 2003; 348:1756–1763.
- Aksoy O, Yousefzai R, Singh D, et al. Cardiogenic shock in the setting of severe aortic stenosis: role of intra-aortic balloon pump support. Heart 2011; 97:838–843.
Surgical aortic valve replacement remains the gold standard treatment for symptomatic aortic valve stenosis in patients at low or moderate risk of surgical complications. But this is a disease of the elderly, many of whom are too frail or too sick to undergo surgery.
Now, patients who cannot undergo this surgery can be offered the less invasive option of transcatheter aortic valve replacement. Balloon valvuloplasty, sodium nitroprusside, and intra-aortic balloon counterpulsation can buy time for ill patients while more permanent mechanical interventions are being considered.
In this review, we will present several cases that highlight management choices for patients with severe aortic stenosis.
A PROGRESSIVE DISEASE OF THE ELDERLY
Aortic stenosis is the most common acquired valvular disease in the United States, and its incidence and prevalence are rising as the population ages. Epidemiologic studies suggest that 2% to 7% of all patients over age 65 have it.1,2
The natural history of the untreated disease is well established, with several case series showing an average decrease of 0.1 cm2 per year in aortic valve area and an increase of 7 mm Hg per year in the pressure gradient across the valve once the diagnosis is made.3,4 Development of angina, syncope, or heart failure is associated with adverse clinical outcomes, including death, and warrants prompt intervention with aortic valve replacement.5–7 Without intervention, the mortality rates reach as high as 75% in 3 years once symptoms develop.
Statins, bisphosphonates, and angiotensin-converting enzyme inhibitors have been used in attempts to slow or reverse the progression of aortic stenosis. However, studies of these drugs have had mixed results, and no definitive benefit has been shown.8–13 Surgical aortic valve replacement, on the other hand, normalizes the life expectancy of patients with aortic stenosis to that of age- and sex-matched controls and remains the gold standard therapy for patients who have symptoms.14
Traditionally, valve replacement has involved open heart surgery, since it requires direct visualization of the valve while the patient is on cardiopulmonary bypass. Unfortunately, many patients have multiple comorbid conditions and therefore are not candidates for open heart surgery. Options for these patients include aortic valvuloplasty and transcatheter aortic valve replacement. While there is considerable experience with aortic valvuloplasty, transcatheter aortic valve replacement is relatively new. In large randomized trials and registries, the transcatheter procedure has been shown to significantly improve long-term survival compared with medical management alone in inoperable patients and to have benefit similar to that of surgery in the high-risk population.15–17
CASE 1: SEVERE, SYMPTOMATIC STENOSIS IN A GOOD SURGICAL CANDIDATE
Mr. A, age 83, presents with shortness of breath and peripheral edema that have been worsening over the past several months. His pulse rate is 64 beats per minute and his blood pressure is 110/90 mm Hg. Auscultation reveals an absent aortic second heart sound with a late peaking systolic murmur that increases with expiration.
On echocardiography, his left ventricular ejection fraction is 55%, peak transaortic valve gradient 88 mm Hg, mean gradient 60 mm Hg, and effective valve area 0.6 cm2. He undergoes catheterization of the left side of his heart, which shows normal coronary arteries.
Mr. A also has hypertension and hyperlipidemia; his renal and pulmonary functions are normal.
How would you manage Mr. A’s aortic stenosis?
Symptomatic aortic stenosis leads to adverse clinical outcomes if managed medically without mechanical intervention,5–7 but patients who undergo aortic valve replacement have age-corrected postoperative survival rates that are nearly normal.14 Furthermore, thanks to improvements in surgical techniques and perioperative management, surgical mortality rates have decreased significantly in recent years and now range from 1% to 8%.18–20 The accumulated evidence showing clear superiority of a surgical approach over medical therapy has greatly simplified the therapeutic algorithm.21
Consequently, the current guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) give surgery a class I indication (evidence or general agreement that the procedure is beneficial, useful, and effective) for symptomatic severe aortic stenosis (Figure 1). This level of recommendation also applies to patients who have severe but asymptomatic aortic stenosis who are undergoing other types of cardiac surgery and also to patients with severe aortic stenosis and left ventricular dysfunction (defined as an ejection fraction < 50%).21
Mr. A was referred for surgical aortic valve replacement, given its clear survival benefit.
CASE 2: SYMPTOMS AND LEFT VENTRICULAR DYSFUNCTION
Ms. B, age 79, has hypertension and hyperlipidemia and now presents to the outpatient department with worsening shortness of breath and chest discomfort. Electrocardiography shows significant left ventricular hypertrophy and abnormal repolarization. Left heart catheterization reveals mild nonobstructive coronary artery disease.
Echocardiography reveals an ejection fraction of 25%, severe left ventricular hypertrophy, and global hypokinesis. The aortic valve leaflets appear heavily calcified, with restricted motion. The peak and mean gradients across the aortic valve are 40 and 28 mm Hg, and the valve area is 0.8 cm2. Right heart catheterization shows a cardiac output of 3.1 L/min.
Does this patient’s aortic stenosis account for her clinical presentation?
Managing patients who have suspected severe aortic stenosis, left ventricular dysfunction, and low aortic valve gradients can be challenging. Although data for surgical intervention are not as robust for these patient subsets as for patients like Mr. A, several case series have suggested that survival in these patients is significantly better with surgery than with medical therapy alone.22–27
Specific factors predict whether patients with ventricular dysfunction and low gradients will benefit from aortic valve replacement. Dobutamine stress echocardiography is helpful in distinguishing true severe aortic stenosis from “pseudostenosis,” in which leaflet motion is restricted due to primary cardiomyopathy and low flow. Distinguishing between true aortic stenosis and pseudostenosis is of paramount value, as surgery is associated with improved long-term outcomes in patients with true aortic stenosis (even though they are at higher surgical risk), whereas those with pseudostenosis will not benefit from surgery.28–31
Infusion of dobutamine increases the flow across the aortic valve (if the left ventricle has contractile reserve; more on this below), and an increasing valve area with increasing doses of dobutamine is consistent with pseudostenosis. In this situation, treatment of the underlying cardiomyopathy is indicated as opposed to replacement of the aortic valve (Figure 2).
Contractile reserve is defined as an increase in stroke volume (> 20%), valvular gradient (> 10 mm Hg), or peak velocity (> 0.6 m/s) with peak dobutamine infusion. The presence of contractile reserve in patients with aortic stenosis identifies a high-risk group that benefits from aortic valve replacement (Figure 2).
Treatment of patients who have inadequate reserve is controversial. In the absence of contractile reserve, an adjunct imaging study such as computed tomography may be of value in detecting calcified valve leaflets, as the presence of calcium is associated with true aortic stenosis. Comorbid conditions should be taken into account as well, given the higher surgical risk in this patient subset, as aortic valve replacement in this already high-risk group of patients might be futile in some cases.
The ACC/AHA guidelines now give dobutamine stress echocardiography a class IIa indication (meaning the weight of the evidence or opinion is in favor of usefulness or efficacy) for determination of contractile reserve and valvular stenosis for patients with an ejection fraction of 30% or less or a mean gradient of 40 mm Hg or less.21
Ms. B underwent dobutamine stress echocardiography. It showed increases in ejection fraction, stroke volume, and transvalvular gradients, indicating that she did have contractile reserve and true severe aortic stenosis. Consequently, she was referred for surgical aortic valve replacement.
CASE 3: MODERATE STENOSIS AND THREE-VESSEL CORONARY ARTERY DISEASE
Mr. C, age 81, has hypertension and hyperlipidemia. He now presents to the emergency department with chest discomfort that began suddenly, awakening him from sleep. His presenting electrocardiogram shows nonspecific changes, and he is diagnosed with non-ST-elevation myocardial infarction. He undergoes left heart catheterization, which reveals severe three-vessel coronary artery disease.
Echocardiography reveals an ejection fraction of 55% and aortic stenosis, with an aortic valve area of 1.2 cm2, a peak gradient of 44 mm Hg, and a mean gradient of 28 mm Hg.
How would you manage his aortic stenosis?
Moderate aortic stenosis in a patient who needs surgery for severe triple-vessel coronary artery disease, other valve diseases, or aortic disease raises the question of whether aortic valve replacement should be performed in conjunction with these surgeries. Although these patients would not otherwise qualify for aortic valve replacement, the fact that they will undergo a procedure that will expose them to the risks associated with open heart surgery makes them reasonable candidates. Even if the patient does not need aortic valve replacement right now, aortic stenosis progresses at a predictable rate—the valve area decreases by a mean of 0.1 cm2/year and the gradients increase by 7 mm Hg/year. Therefore, clinical judgment should be exercised so that the patient will not need to undergo open heart surgery again in the near future.
The ACC/AHA guidelines recommend aortic valve replacement for patients with moderate aortic stenosis undergoing coronary artery bypass grafting or surgery on the aorta or other heart valves, giving it a class IIa indication.21 This recommendation is based on several retrospective case series that evaluated survival, the need for reoperation for aortic valve replacement, or both in patients undergoing coronary artery bypass grafting.32–35
No data exist, however, on adding aortic valve replacement to coronary artery bypass grafting in cases of mild aortic stenosis. As a result, it is controversial and carries a class IIb recommendation (meaning that its usefulness or efficacy is less well established). The ACC/AHA guidelines state that aortic valve replacement “may be considered” in patients undergoing coronary artery bypass grafting who have mild aortic stenosis (mean gradient < 30 mm Hg or jet velocity < 3 m/s) when there is evidence, such as moderate or severe valve calcification, that progression may be rapid (level of evidence C: based only on consensus opinion of experts, case studies or standard of care).21
Mr. C, who has moderate aortic stenosis, underwent aortic valve replacement in conjunction with three-vessel bypass grafting.
CASE 4: ASYMPTOMATIC BUT SEVERE STENOSIS
Mr. D, age 74, has hypertension, hyperlipidemia, and aortic stenosis. He now presents to the outpatient department for his annual echocardiogram to follow his aortic stenosis. He has a sedentary lifestyle but feels well performing activities of daily living. He denies dyspnea on exertion, chest pain, or syncope.
His echocardiogram reveals an effective aortic valve area of 0.7 cm2, peak gradient 90 mm Hg, and mean gradient 70 mm Hg. There is evidence of severe left ventricular hypertrophy, and the valve leaflets show bulky calcification and severe restriction. An echocardiogram performed at the same institution a year earlier revealed gradients of 60 and 40 mm Hg.
Blood is drawn for laboratory tests, including N-terminal pro-brain natriuretic peptide, which is 350 pg/mL (reference range for his age < 125 pg/mL). He is referred for a treadmill stress test, which elicits symptoms at a moderate activity level.
How would you manage his aortic stenosis?
Aortic valve replacement can be considered in patients who have asymptomatic but severe aortic stenosis with preserved left ventricular function (class IIb indication).21
Clinical assessment of asymptomatic aortic stenosis can be challenging, however, as patients may underreport their symptoms or decrease their activity levels to avoid symptoms. Exercise testing in such patients can elicit symptoms, unmask diminished exercise capacity, and help determine if they should be referred for surgery.36,37 Natriuretic peptide levels have been shown to correlate with the severity of aortic stenosis,38,39 and more importantly, to help predict symptom onset, cardiac death, and need for aortic valve replacement.40–42
Some patients with asymptomatic but severe aortic stenosis are at higher risk of morbidity and death. High-risk subsets include patients with rapid progression of aortic stenosis and those with critical aortic stenosis characterized by an aortic valve area less than 0.60 cm2, mean gradient greater than 60 mm Hg, and jet velocity greater than 5.0 m/s. It is reasonable to offer these patients surgery if their expected operative mortality risk is less than 1.0%.21
Mr. D has evidence of rapid progression as defined by an increase in aortic jet velocity of more than 0.3 m/s/year. He is at low surgical risk and was referred for elective aortic valve replacement.
CASE 5: TOO FRAIL FOR SURGERY
Mr. E, age 84, has severe aortic stenosis (valve area 0.6 cm2, peak and mean gradients of 88 and 56 mm Hg), coronary artery disease status post coronary artery bypass grafting, moderate chronic obstructive pulmonary disease (forced expiratory volume in 1 second 0.8 L), chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, hyperlipidemia, and diabetes mellitus. He has preserved left ventricular function. He presents to the outpatient department with worsening shortness of breath and peripheral edema over the past several months. Your impression is that he is very frail. How would you manage Mr. E’s aortic stenosis?
Advances in surgical techniques and perioperative management over the years have enabled higher-risk patients to undergo surgical aortic valve replacement with excellent out-comes.18–20,43 Yet many patients still cannot undergo surgery because their risk is too high. Patients ineligible for surgery have traditionally been treated medically—with poor out-comes—or with balloon aortic valvuloplasty to palliate symptoms.
Transcatheter aortic valve replacement, approved by the US Food and Drug Administration (FDA) in 2011, now provides another option for these patients. In this procedure, a bioprosthetic valve mounted on a metal frame is implanted over the native stenotic valve.
Currently, the only FDA-approved and commercially available valve in the United States is the Edwards SAPIEN valve, which has bovine pericardial tissue leaflets fixed to a balloon-expandable stainless steel frame (Figure 3). In the Placement of Aortic Transcatheter Valves (PARTNER) trial,15 patients who could not undergo surgery who underwent transcatheter replacement with this valve had a significantly better survival rate than patients treated medically.15,17 Use of this valve has also been compared against conventional surgical aortic valve replacement in high-risk patients and was found to have similar long-term outcomes (Figure 4).16 It was on the basis of this trial that this valve was granted approval for patients who cannot undergo surgery.
The standard of care for high-risk patients remains surgical aortic valve replacement, although it remains to be seen whether transcatheter replacement will be made available as well to patients eligible for surgery in the near future. There are currently no randomized data for transcatheter aortic valve replacement in patients at moderate to low surgical risk, and these patients should not be considered for this procedure.
Although the initial studies are encouraging for patients who cannot undergo surgery and who are at high risk without it, several issues and concerns remain. Importantly, the long-term durability of the transcatheter valve and longer-term outcomes remain unknown. Furthermore, the risk of vascular complications remains high (10% to 15%), dictating the need for careful patient selection. There are also concerns about the risks of stroke and of paravalvular aortic insufficiency. These issues are being investigated and addressed, however, and we hope that with increasing operator experience and improvements in the technique, outcomes will be improved.
Which approach for transcatheter aortic valve replacement?
There are several considerations in determining a patient’s eligibility for transcatheter aortic valve replacement.
Initially, these valves were placed by a transvenous, transseptal approach, but now retrograde placement through the femoral artery has become standard. In this procedure, the device is advanced retrograde from the femoral artery through the aorta and placed across the native aortic valve under fluoroscopic and echocardiographic guidance.
Patients who are not eligible for transfemoral placement because of severe atherosclerosis, tortuosity, or ectasia of the iliofemoral artery or aorta can still undergo percutaneous treatment with a transapical approach. This is a hybrid surgical-transcatheter approach in which the valve is delivered through a sheath placed by left ventricular apical puncture.17,44
A newer approach gaining popularity is the transaortic technique, in which the ascending aorta is accessed directly through a ministernotomy and the delivery sheath is placed with a direct puncture. Other approaches are through the axillary and subclavian arteries.
Other valves are under development
Several other valves are under development and will likely change the landscape of transcatheter aortic valve replacement with improving outcomes. Valves that are available in the United States are shown in Figure 3. The CoreValve, consisting of porcine pericardial leaflets mounted on a self-expanding nitinol stent, is currently being studied in a trial in the United States, and the manufacturer (Medtronic) will seek approval when results are complete in the near future.
Mr. E was initially referred for surgery, but when deemed to be unable to undergo surgery was found to be a good candidate for transcatheter aortic valve replacement.
CASE 6: LIFE-LIMITING COMORBID ILLNESS
Mr. F, age 77, has multiple problems: severe aortic stenosis (aortic valve area 0.6 cm2; peak and mean gradients of 92 and 59 mm Hg), stage IV pancreatic cancer, coronary artery disease status post coronary artery bypass grafting, chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, and hyperlipidemia. He presents to the outpatient department with shortness of breath at rest, orthopnea, effort intolerance, and peripheral edema over the past several months.
On physical examination rales in both lung bases can be heard. Left heart catheterization shows patent bypass grafts.
How would you manage Mr. F’s aortic stenosis?
Aortic valve replacement is not considered an option in patients with noncardiac illnesses and comorbidities that are life-limiting in the near term. Under these circumstances, aortic valvuloplasty can be offered as a means of palliating symptoms or, if the comorbid conditions can be modified, as a bridge to more definitive treatment with aortic valve replacement.
Since first described in 1986,45 percutaneous aortic valvuloplasty has been studied in several case series and registries, with consistent findings. Acutely, it increases the valve area and lessens the gradients across the valve, relieving symptoms. The risk of death during the procedure ranged from 3% to 13.5% in several case series, with a 30-day survival rate greater than 85%.46 However, the hemodynamic and symptomatic improvement is only short-term, as valve area and gradients gradually worsen within several months.47,48 Consequently, balloon valvuloplasty is considered a palliative approach.
Mr. F has a potentially life-limiting illness, ie, cancer, which would make him a candidate for aortic valvuloplasty rather than replacement. He can be referred for evaluation for this procedure in hopes of palliating his symptoms by relieving his dyspnea and improving his quality of life.
CASE 7: HEMODYNAMIC INSTABILITY
Mr. G, age 87, is scheduled for surgical aortic valve replacement because of severe aortic stenosis (valve area 0.5 cm2, peak and mean gradients 89 and 45 mm Hg) with an ejection fraction of 30%.
Two weeks before his scheduled surgery he presents to the emergency department with worsening fluid overload and increasing shortness of breath. His initial laboratory work shows new-onset renal failure, and he has signs of hypoperfusion on physical examination. He is transferred to the cardiac intensive care unit for further care.
How would you manage his aortic stenosis?
Patients with decompensated aortic stenosis and hemodynamic instability are at extreme risk during surgery. Medical stabilization beforehand may mitigate the risks associated with surgical or transcatheter aortic valve replacement. Aortic valvuloplasty, treatment with sodium nitroprusside, and support with intra-aortic balloon counterpulsation may help stabilize patients in this “low-output” setting.
Sodium nitroprusside has long been used in low-output states. By relaxing vascular smooth muscle, it leads to increased venous capacitance, decreasing preload and congestion. It also decreases systemic vascular resistance with a subsequent decrease in afterload, which in turn improves systolic emptying. Together, these effects reduce systolic and diastolic wall stress, lower myocardial oxygen consumption, and ultimately increase cardiac output.49,50
These theoretical benefits translate to clinical improvement and increased cardiac output, as shown in a case series of 25 patients with severe aortic stenosis and left ventricular systolic dysfunction (ejection fraction 35%) presenting in a low-output state in the absence of hypotension.51 These findings have led to a ACC/AHA recommendation for the use of sodium nitroprusside in patients who have severe aortic stenosis presenting in low-output state with decompensated heart failure.21
Intra-aortic balloon counterpulsation, introduced in 1968, has been used in several clinical settings, including acute coronary syndromes, intractable ventricular arrhythmias, and refractory heart failure, and for support of hemodynamics in the perioperative setting. Its role in managing ventricular septal rupture and acute mitral regurgitation is well established. It reliably reduces afterload and improves coronary perfusion, augmenting the cardiac output. This in turn leads to improved systemic perfusion, which can buy time for a critically ill patient during which the primary disease process is addressed.
Recently, a case series in which intraaortic balloon counterpulsation devices were placed in patients with severe aortic stenosis and cardiogenic shock showed findings similar to those with sodium nitroprusside infusion. Specifically, their use was associated with improved cardiac indices and filling pressures with a decrease in systemic vascular resistance. These changes have led to increased cardiac performance, resulting in better systemic perfusion.52 Thus, intra-aortic balloon counterpulsation can be an option for stabilizing patients with severe aortic stenosis and cardiogenic shock.
Mr. G was treated with sodium nitroprusside and intravenous diuretics. He achieved symptomatic relief and his renal function returned to baseline. He subsequently underwent aortic valve replacement during the hospitalization.
Surgical aortic valve replacement remains the gold standard treatment for symptomatic aortic valve stenosis in patients at low or moderate risk of surgical complications. But this is a disease of the elderly, many of whom are too frail or too sick to undergo surgery.
Now, patients who cannot undergo this surgery can be offered the less invasive option of transcatheter aortic valve replacement. Balloon valvuloplasty, sodium nitroprusside, and intra-aortic balloon counterpulsation can buy time for ill patients while more permanent mechanical interventions are being considered.
In this review, we will present several cases that highlight management choices for patients with severe aortic stenosis.
A PROGRESSIVE DISEASE OF THE ELDERLY
Aortic stenosis is the most common acquired valvular disease in the United States, and its incidence and prevalence are rising as the population ages. Epidemiologic studies suggest that 2% to 7% of all patients over age 65 have it.1,2
The natural history of the untreated disease is well established, with several case series showing an average decrease of 0.1 cm2 per year in aortic valve area and an increase of 7 mm Hg per year in the pressure gradient across the valve once the diagnosis is made.3,4 Development of angina, syncope, or heart failure is associated with adverse clinical outcomes, including death, and warrants prompt intervention with aortic valve replacement.5–7 Without intervention, the mortality rates reach as high as 75% in 3 years once symptoms develop.
Statins, bisphosphonates, and angiotensin-converting enzyme inhibitors have been used in attempts to slow or reverse the progression of aortic stenosis. However, studies of these drugs have had mixed results, and no definitive benefit has been shown.8–13 Surgical aortic valve replacement, on the other hand, normalizes the life expectancy of patients with aortic stenosis to that of age- and sex-matched controls and remains the gold standard therapy for patients who have symptoms.14
Traditionally, valve replacement has involved open heart surgery, since it requires direct visualization of the valve while the patient is on cardiopulmonary bypass. Unfortunately, many patients have multiple comorbid conditions and therefore are not candidates for open heart surgery. Options for these patients include aortic valvuloplasty and transcatheter aortic valve replacement. While there is considerable experience with aortic valvuloplasty, transcatheter aortic valve replacement is relatively new. In large randomized trials and registries, the transcatheter procedure has been shown to significantly improve long-term survival compared with medical management alone in inoperable patients and to have benefit similar to that of surgery in the high-risk population.15–17
CASE 1: SEVERE, SYMPTOMATIC STENOSIS IN A GOOD SURGICAL CANDIDATE
Mr. A, age 83, presents with shortness of breath and peripheral edema that have been worsening over the past several months. His pulse rate is 64 beats per minute and his blood pressure is 110/90 mm Hg. Auscultation reveals an absent aortic second heart sound with a late peaking systolic murmur that increases with expiration.
On echocardiography, his left ventricular ejection fraction is 55%, peak transaortic valve gradient 88 mm Hg, mean gradient 60 mm Hg, and effective valve area 0.6 cm2. He undergoes catheterization of the left side of his heart, which shows normal coronary arteries.
Mr. A also has hypertension and hyperlipidemia; his renal and pulmonary functions are normal.
How would you manage Mr. A’s aortic stenosis?
Symptomatic aortic stenosis leads to adverse clinical outcomes if managed medically without mechanical intervention,5–7 but patients who undergo aortic valve replacement have age-corrected postoperative survival rates that are nearly normal.14 Furthermore, thanks to improvements in surgical techniques and perioperative management, surgical mortality rates have decreased significantly in recent years and now range from 1% to 8%.18–20 The accumulated evidence showing clear superiority of a surgical approach over medical therapy has greatly simplified the therapeutic algorithm.21
Consequently, the current guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) give surgery a class I indication (evidence or general agreement that the procedure is beneficial, useful, and effective) for symptomatic severe aortic stenosis (Figure 1). This level of recommendation also applies to patients who have severe but asymptomatic aortic stenosis who are undergoing other types of cardiac surgery and also to patients with severe aortic stenosis and left ventricular dysfunction (defined as an ejection fraction < 50%).21
Mr. A was referred for surgical aortic valve replacement, given its clear survival benefit.
CASE 2: SYMPTOMS AND LEFT VENTRICULAR DYSFUNCTION
Ms. B, age 79, has hypertension and hyperlipidemia and now presents to the outpatient department with worsening shortness of breath and chest discomfort. Electrocardiography shows significant left ventricular hypertrophy and abnormal repolarization. Left heart catheterization reveals mild nonobstructive coronary artery disease.
Echocardiography reveals an ejection fraction of 25%, severe left ventricular hypertrophy, and global hypokinesis. The aortic valve leaflets appear heavily calcified, with restricted motion. The peak and mean gradients across the aortic valve are 40 and 28 mm Hg, and the valve area is 0.8 cm2. Right heart catheterization shows a cardiac output of 3.1 L/min.
Does this patient’s aortic stenosis account for her clinical presentation?
Managing patients who have suspected severe aortic stenosis, left ventricular dysfunction, and low aortic valve gradients can be challenging. Although data for surgical intervention are not as robust for these patient subsets as for patients like Mr. A, several case series have suggested that survival in these patients is significantly better with surgery than with medical therapy alone.22–27
Specific factors predict whether patients with ventricular dysfunction and low gradients will benefit from aortic valve replacement. Dobutamine stress echocardiography is helpful in distinguishing true severe aortic stenosis from “pseudostenosis,” in which leaflet motion is restricted due to primary cardiomyopathy and low flow. Distinguishing between true aortic stenosis and pseudostenosis is of paramount value, as surgery is associated with improved long-term outcomes in patients with true aortic stenosis (even though they are at higher surgical risk), whereas those with pseudostenosis will not benefit from surgery.28–31
Infusion of dobutamine increases the flow across the aortic valve (if the left ventricle has contractile reserve; more on this below), and an increasing valve area with increasing doses of dobutamine is consistent with pseudostenosis. In this situation, treatment of the underlying cardiomyopathy is indicated as opposed to replacement of the aortic valve (Figure 2).
Contractile reserve is defined as an increase in stroke volume (> 20%), valvular gradient (> 10 mm Hg), or peak velocity (> 0.6 m/s) with peak dobutamine infusion. The presence of contractile reserve in patients with aortic stenosis identifies a high-risk group that benefits from aortic valve replacement (Figure 2).
Treatment of patients who have inadequate reserve is controversial. In the absence of contractile reserve, an adjunct imaging study such as computed tomography may be of value in detecting calcified valve leaflets, as the presence of calcium is associated with true aortic stenosis. Comorbid conditions should be taken into account as well, given the higher surgical risk in this patient subset, as aortic valve replacement in this already high-risk group of patients might be futile in some cases.
The ACC/AHA guidelines now give dobutamine stress echocardiography a class IIa indication (meaning the weight of the evidence or opinion is in favor of usefulness or efficacy) for determination of contractile reserve and valvular stenosis for patients with an ejection fraction of 30% or less or a mean gradient of 40 mm Hg or less.21
Ms. B underwent dobutamine stress echocardiography. It showed increases in ejection fraction, stroke volume, and transvalvular gradients, indicating that she did have contractile reserve and true severe aortic stenosis. Consequently, she was referred for surgical aortic valve replacement.
CASE 3: MODERATE STENOSIS AND THREE-VESSEL CORONARY ARTERY DISEASE
Mr. C, age 81, has hypertension and hyperlipidemia. He now presents to the emergency department with chest discomfort that began suddenly, awakening him from sleep. His presenting electrocardiogram shows nonspecific changes, and he is diagnosed with non-ST-elevation myocardial infarction. He undergoes left heart catheterization, which reveals severe three-vessel coronary artery disease.
Echocardiography reveals an ejection fraction of 55% and aortic stenosis, with an aortic valve area of 1.2 cm2, a peak gradient of 44 mm Hg, and a mean gradient of 28 mm Hg.
How would you manage his aortic stenosis?
Moderate aortic stenosis in a patient who needs surgery for severe triple-vessel coronary artery disease, other valve diseases, or aortic disease raises the question of whether aortic valve replacement should be performed in conjunction with these surgeries. Although these patients would not otherwise qualify for aortic valve replacement, the fact that they will undergo a procedure that will expose them to the risks associated with open heart surgery makes them reasonable candidates. Even if the patient does not need aortic valve replacement right now, aortic stenosis progresses at a predictable rate—the valve area decreases by a mean of 0.1 cm2/year and the gradients increase by 7 mm Hg/year. Therefore, clinical judgment should be exercised so that the patient will not need to undergo open heart surgery again in the near future.
The ACC/AHA guidelines recommend aortic valve replacement for patients with moderate aortic stenosis undergoing coronary artery bypass grafting or surgery on the aorta or other heart valves, giving it a class IIa indication.21 This recommendation is based on several retrospective case series that evaluated survival, the need for reoperation for aortic valve replacement, or both in patients undergoing coronary artery bypass grafting.32–35
No data exist, however, on adding aortic valve replacement to coronary artery bypass grafting in cases of mild aortic stenosis. As a result, it is controversial and carries a class IIb recommendation (meaning that its usefulness or efficacy is less well established). The ACC/AHA guidelines state that aortic valve replacement “may be considered” in patients undergoing coronary artery bypass grafting who have mild aortic stenosis (mean gradient < 30 mm Hg or jet velocity < 3 m/s) when there is evidence, such as moderate or severe valve calcification, that progression may be rapid (level of evidence C: based only on consensus opinion of experts, case studies or standard of care).21
Mr. C, who has moderate aortic stenosis, underwent aortic valve replacement in conjunction with three-vessel bypass grafting.
CASE 4: ASYMPTOMATIC BUT SEVERE STENOSIS
Mr. D, age 74, has hypertension, hyperlipidemia, and aortic stenosis. He now presents to the outpatient department for his annual echocardiogram to follow his aortic stenosis. He has a sedentary lifestyle but feels well performing activities of daily living. He denies dyspnea on exertion, chest pain, or syncope.
His echocardiogram reveals an effective aortic valve area of 0.7 cm2, peak gradient 90 mm Hg, and mean gradient 70 mm Hg. There is evidence of severe left ventricular hypertrophy, and the valve leaflets show bulky calcification and severe restriction. An echocardiogram performed at the same institution a year earlier revealed gradients of 60 and 40 mm Hg.
Blood is drawn for laboratory tests, including N-terminal pro-brain natriuretic peptide, which is 350 pg/mL (reference range for his age < 125 pg/mL). He is referred for a treadmill stress test, which elicits symptoms at a moderate activity level.
How would you manage his aortic stenosis?
Aortic valve replacement can be considered in patients who have asymptomatic but severe aortic stenosis with preserved left ventricular function (class IIb indication).21
Clinical assessment of asymptomatic aortic stenosis can be challenging, however, as patients may underreport their symptoms or decrease their activity levels to avoid symptoms. Exercise testing in such patients can elicit symptoms, unmask diminished exercise capacity, and help determine if they should be referred for surgery.36,37 Natriuretic peptide levels have been shown to correlate with the severity of aortic stenosis,38,39 and more importantly, to help predict symptom onset, cardiac death, and need for aortic valve replacement.40–42
Some patients with asymptomatic but severe aortic stenosis are at higher risk of morbidity and death. High-risk subsets include patients with rapid progression of aortic stenosis and those with critical aortic stenosis characterized by an aortic valve area less than 0.60 cm2, mean gradient greater than 60 mm Hg, and jet velocity greater than 5.0 m/s. It is reasonable to offer these patients surgery if their expected operative mortality risk is less than 1.0%.21
Mr. D has evidence of rapid progression as defined by an increase in aortic jet velocity of more than 0.3 m/s/year. He is at low surgical risk and was referred for elective aortic valve replacement.
CASE 5: TOO FRAIL FOR SURGERY
Mr. E, age 84, has severe aortic stenosis (valve area 0.6 cm2, peak and mean gradients of 88 and 56 mm Hg), coronary artery disease status post coronary artery bypass grafting, moderate chronic obstructive pulmonary disease (forced expiratory volume in 1 second 0.8 L), chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, hyperlipidemia, and diabetes mellitus. He has preserved left ventricular function. He presents to the outpatient department with worsening shortness of breath and peripheral edema over the past several months. Your impression is that he is very frail. How would you manage Mr. E’s aortic stenosis?
Advances in surgical techniques and perioperative management over the years have enabled higher-risk patients to undergo surgical aortic valve replacement with excellent out-comes.18–20,43 Yet many patients still cannot undergo surgery because their risk is too high. Patients ineligible for surgery have traditionally been treated medically—with poor out-comes—or with balloon aortic valvuloplasty to palliate symptoms.
Transcatheter aortic valve replacement, approved by the US Food and Drug Administration (FDA) in 2011, now provides another option for these patients. In this procedure, a bioprosthetic valve mounted on a metal frame is implanted over the native stenotic valve.
Currently, the only FDA-approved and commercially available valve in the United States is the Edwards SAPIEN valve, which has bovine pericardial tissue leaflets fixed to a balloon-expandable stainless steel frame (Figure 3). In the Placement of Aortic Transcatheter Valves (PARTNER) trial,15 patients who could not undergo surgery who underwent transcatheter replacement with this valve had a significantly better survival rate than patients treated medically.15,17 Use of this valve has also been compared against conventional surgical aortic valve replacement in high-risk patients and was found to have similar long-term outcomes (Figure 4).16 It was on the basis of this trial that this valve was granted approval for patients who cannot undergo surgery.
The standard of care for high-risk patients remains surgical aortic valve replacement, although it remains to be seen whether transcatheter replacement will be made available as well to patients eligible for surgery in the near future. There are currently no randomized data for transcatheter aortic valve replacement in patients at moderate to low surgical risk, and these patients should not be considered for this procedure.
Although the initial studies are encouraging for patients who cannot undergo surgery and who are at high risk without it, several issues and concerns remain. Importantly, the long-term durability of the transcatheter valve and longer-term outcomes remain unknown. Furthermore, the risk of vascular complications remains high (10% to 15%), dictating the need for careful patient selection. There are also concerns about the risks of stroke and of paravalvular aortic insufficiency. These issues are being investigated and addressed, however, and we hope that with increasing operator experience and improvements in the technique, outcomes will be improved.
Which approach for transcatheter aortic valve replacement?
There are several considerations in determining a patient’s eligibility for transcatheter aortic valve replacement.
Initially, these valves were placed by a transvenous, transseptal approach, but now retrograde placement through the femoral artery has become standard. In this procedure, the device is advanced retrograde from the femoral artery through the aorta and placed across the native aortic valve under fluoroscopic and echocardiographic guidance.
Patients who are not eligible for transfemoral placement because of severe atherosclerosis, tortuosity, or ectasia of the iliofemoral artery or aorta can still undergo percutaneous treatment with a transapical approach. This is a hybrid surgical-transcatheter approach in which the valve is delivered through a sheath placed by left ventricular apical puncture.17,44
A newer approach gaining popularity is the transaortic technique, in which the ascending aorta is accessed directly through a ministernotomy and the delivery sheath is placed with a direct puncture. Other approaches are through the axillary and subclavian arteries.
Other valves are under development
Several other valves are under development and will likely change the landscape of transcatheter aortic valve replacement with improving outcomes. Valves that are available in the United States are shown in Figure 3. The CoreValve, consisting of porcine pericardial leaflets mounted on a self-expanding nitinol stent, is currently being studied in a trial in the United States, and the manufacturer (Medtronic) will seek approval when results are complete in the near future.
Mr. E was initially referred for surgery, but when deemed to be unable to undergo surgery was found to be a good candidate for transcatheter aortic valve replacement.
CASE 6: LIFE-LIMITING COMORBID ILLNESS
Mr. F, age 77, has multiple problems: severe aortic stenosis (aortic valve area 0.6 cm2; peak and mean gradients of 92 and 59 mm Hg), stage IV pancreatic cancer, coronary artery disease status post coronary artery bypass grafting, chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, and hyperlipidemia. He presents to the outpatient department with shortness of breath at rest, orthopnea, effort intolerance, and peripheral edema over the past several months.
On physical examination rales in both lung bases can be heard. Left heart catheterization shows patent bypass grafts.
How would you manage Mr. F’s aortic stenosis?
Aortic valve replacement is not considered an option in patients with noncardiac illnesses and comorbidities that are life-limiting in the near term. Under these circumstances, aortic valvuloplasty can be offered as a means of palliating symptoms or, if the comorbid conditions can be modified, as a bridge to more definitive treatment with aortic valve replacement.
Since first described in 1986,45 percutaneous aortic valvuloplasty has been studied in several case series and registries, with consistent findings. Acutely, it increases the valve area and lessens the gradients across the valve, relieving symptoms. The risk of death during the procedure ranged from 3% to 13.5% in several case series, with a 30-day survival rate greater than 85%.46 However, the hemodynamic and symptomatic improvement is only short-term, as valve area and gradients gradually worsen within several months.47,48 Consequently, balloon valvuloplasty is considered a palliative approach.
Mr. F has a potentially life-limiting illness, ie, cancer, which would make him a candidate for aortic valvuloplasty rather than replacement. He can be referred for evaluation for this procedure in hopes of palliating his symptoms by relieving his dyspnea and improving his quality of life.
CASE 7: HEMODYNAMIC INSTABILITY
Mr. G, age 87, is scheduled for surgical aortic valve replacement because of severe aortic stenosis (valve area 0.5 cm2, peak and mean gradients 89 and 45 mm Hg) with an ejection fraction of 30%.
Two weeks before his scheduled surgery he presents to the emergency department with worsening fluid overload and increasing shortness of breath. His initial laboratory work shows new-onset renal failure, and he has signs of hypoperfusion on physical examination. He is transferred to the cardiac intensive care unit for further care.
How would you manage his aortic stenosis?
Patients with decompensated aortic stenosis and hemodynamic instability are at extreme risk during surgery. Medical stabilization beforehand may mitigate the risks associated with surgical or transcatheter aortic valve replacement. Aortic valvuloplasty, treatment with sodium nitroprusside, and support with intra-aortic balloon counterpulsation may help stabilize patients in this “low-output” setting.
Sodium nitroprusside has long been used in low-output states. By relaxing vascular smooth muscle, it leads to increased venous capacitance, decreasing preload and congestion. It also decreases systemic vascular resistance with a subsequent decrease in afterload, which in turn improves systolic emptying. Together, these effects reduce systolic and diastolic wall stress, lower myocardial oxygen consumption, and ultimately increase cardiac output.49,50
These theoretical benefits translate to clinical improvement and increased cardiac output, as shown in a case series of 25 patients with severe aortic stenosis and left ventricular systolic dysfunction (ejection fraction 35%) presenting in a low-output state in the absence of hypotension.51 These findings have led to a ACC/AHA recommendation for the use of sodium nitroprusside in patients who have severe aortic stenosis presenting in low-output state with decompensated heart failure.21
Intra-aortic balloon counterpulsation, introduced in 1968, has been used in several clinical settings, including acute coronary syndromes, intractable ventricular arrhythmias, and refractory heart failure, and for support of hemodynamics in the perioperative setting. Its role in managing ventricular septal rupture and acute mitral regurgitation is well established. It reliably reduces afterload and improves coronary perfusion, augmenting the cardiac output. This in turn leads to improved systemic perfusion, which can buy time for a critically ill patient during which the primary disease process is addressed.
Recently, a case series in which intraaortic balloon counterpulsation devices were placed in patients with severe aortic stenosis and cardiogenic shock showed findings similar to those with sodium nitroprusside infusion. Specifically, their use was associated with improved cardiac indices and filling pressures with a decrease in systemic vascular resistance. These changes have led to increased cardiac performance, resulting in better systemic perfusion.52 Thus, intra-aortic balloon counterpulsation can be an option for stabilizing patients with severe aortic stenosis and cardiogenic shock.
Mr. G was treated with sodium nitroprusside and intravenous diuretics. He achieved symptomatic relief and his renal function returned to baseline. He subsequently underwent aortic valve replacement during the hospitalization.
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009; 373:956–966.
- Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993; 21:1220–1225.
- Otto CM, Pearlman AS, Gardner CL. Hemodynamic progression of aortic stenosis in adults assessed by Doppler echocardiography. J Am Coll Cardiol 1989; 13:545–550.
- Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997; 95:2262–2270.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Turina J, Hess O, Sepulcri F, Krayenbuehl HP. Spontaneous course of aortic valve disease. Eur Heart J 1987; 8:471–483.
- Horstkotte D, Loogen F. The natural history of aortic valve stenosis. Eur Heart J 1988; 9(suppl E):57–64.
- Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation 2001; 104:2205–2209.
- Cowell SJ, Newby DE, Prescott RJ, et al; Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005; 352:2389–2397.
- Rossebø AB, Pedersen TR, Boman K, et al; SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008; 359:1343–1356.
- Moura LM, Ramos SF, Zamorano JL, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol 2007; 49:554–561.
- Rosenhek R, Rader F, Loho N, et al. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation 2004; 110:1291–1295.
- O’Brien KD, Probstfield JL, Caulked MT, et al. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch Intern Med 2005; 165:858–862.
- Lindblom D, Lindblom U, Qvist J, Lundström H. Long-term relative survival rates after heart valve replacement. J Am Coll Cardiol 1990; 15:566–573.
- Makkar RR, Fontana G P, Jilaihawi H, et al; PARTNER Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012; 366:1696–1704.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Di Eusanio M, Fortuna D, Cristell D, et al; RERIC (Emilia Romagna Cardiac Surgery Registry) Investigators. Contemporary outcomes of conventional aortic valve replacement in 638 octogenarians: insights from an Italian Regional Cardiac Surgery Registry (RERIC). Eur J Cardiothorac Surg 2012; 41:1247–1252.
- Di Eusanio M, Fortuna D, De Palma R, et al. Aortic valve replacement: results and predictors of mortality from a contemporary series of 2256 patients. J Thorac Cardiovasc Surg 2011; 141:940–947.
- Jamieson WR, Edwards FH, Schwartz M, Bero JW, Clark RE, Grover FL. Risk stratification for cardiac valve replacement. National Cardiac Surgery Database. Database Committee of the Society of Thoracic Surgeons. Ann Thorac Surg 1999; 67:943–951.
- Bonow RO, Carabello BA, Chatterjee K, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 52:e1–e142.
- Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007; 115:2856–2864.
- Vaquette B, Corbineau H, Laurent M, et al. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart 2005; 91:1324–1329.
- Connolly HM, Oh JK, Orszulak TA, et al. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction. Prognostic indicators. Circulation 1997; 95:2395–2400.
- Connolly HM, Oh JK, Schaff HV, et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation 2000; 101:1940–1946.
- Pereira JJ, Lauer MS, Bashir M, et al. Survival after aortic valve replacement for severe aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am Coll Cardiol 2002; 39:1356–1363.
- Pai RG, Varadarajan P, Razzouk A. Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg 2008; 86:1781–1789.
- Monin JL, Monchi M, Gest V, Duval-Moulin AM, Dubois-Rande JL, Gueret P. Aortic stenosis with severe left ventricular dysfunction and low transvalvular pressure gradients: risk stratification by low-dose dobutamine echocardiography. J Am Coll Cardiol 2001; 37:2101–2107.
- Monin JL, Quéré J P, Monchi M, et al. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 2003; 108:319–324.
- Zuppiroli A, Mori F, Olivotto I, Castelli G, Favilli S, Dolara A. Therapeutic implications of contractile reserve elicited by dobutamine echocardiography in symptomatic, low-gradient aortic stenosis. Ital Heart J 2003; 4:264–270.
- Tribouilloy C, Lévy F, Rusinaru D, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol 2009; 53:1865–1873.
- Ahmed AA, Graham AN, Lovell D, O’Kane HO. Management of mild to moderate aortic valve disease during coronary artery bypass grafting. Eur J Cardiothorac Surg 2003; 24:535–539.
- Verhoye J P, Merlicco F, Sami IM, et al. Aortic valve replacement for aortic stenosis after previous coronary artery bypass grafting: could early reoperation be prevented? J Heart Valve Dis 2006; 15:474–478.
- Hochrein J, Lucke JC, Harrison JK, et al. Mortality and need for reoperation in patients with mild-to-moderate asymptomatic aortic valve disease undergoing coronary artery bypass graft alone. Am Heart J 1999; 138:791–797.
- Pereira JJ, Balaban K, Lauer MS, Lytle B, Thomas JD, Garcia MJ. Aortic valve replacement in patients with mild or moderate aortic stenosis and coronary bypass surgery. Am J Med 2005; 118:735–742.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Weber M, Hausen M, Arnold R, et al. Prognostic value of N-terminal pro-B-type natriuretic peptide for conservatively and surgically treated patients with aortic valve stenosis. Heart 2006; 92:1639–1644.
- Gerber IL, Stewart RA, Legget ME, et al. Increased plasma natriuretic peptide levels refect symptom onset in aortic stenosis. Circulation 2003; 107:1884–1890.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lancellotti P, Moonen M, Magne J, et al. Prognostic effect of long-axis left ventricular dysfunction and B-type natriuretic peptide levels in asymptomatic aortic stenosis. Am J Cardiol 2010; 105:383–388.
- Langanay T, Flécher E, Fouquet O, et al. Aortic valve replacement in the elderly: the real life. Ann Thorac Surg 2012; 93:70–77.
- Christofferson RD, Kapadia SR, Rajagopal V, Tuzcu EM. Emerging transcatheter therapies for aortic and mitral disease. Heart 2009; 95:148–155.
- Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1986; 1:63–67.
- Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 1991; 84:2383–2397.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Bernard Y, Etievent J, Mourand JL, et al. Long-term results of percutaneous aortic valvuloplasty compared with aortic valve replacement in patients more than 75 years old. J Am Coll Cardiol 1992; 20:796–801.
- Elkayam U, Janmohamed M, Habib M, Hatamizadeh P. Vasodilators in the management of acute heart failure. Crit Care Med 2008; 36(suppl 1):S95–S105.
- Popovic ZB, Khot UN, Novaro GM, et al. Effects of sodium nitroprusside in aortic stenosis associated with severe heart failure: pressure-volume loop analysis using a numerical model. Am J Physiol Heart Circ Physiol 2005; 288:H416–H423.
- Khot UN, Novaro GM, Popovic ZB, et al. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med 2003; 348:1756–1763.
- Aksoy O, Yousefzai R, Singh D, et al. Cardiogenic shock in the setting of severe aortic stenosis: role of intra-aortic balloon pump support. Heart 2011; 97:838–843.
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009; 373:956–966.
- Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993; 21:1220–1225.
- Otto CM, Pearlman AS, Gardner CL. Hemodynamic progression of aortic stenosis in adults assessed by Doppler echocardiography. J Am Coll Cardiol 1989; 13:545–550.
- Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997; 95:2262–2270.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Turina J, Hess O, Sepulcri F, Krayenbuehl HP. Spontaneous course of aortic valve disease. Eur Heart J 1987; 8:471–483.
- Horstkotte D, Loogen F. The natural history of aortic valve stenosis. Eur Heart J 1988; 9(suppl E):57–64.
- Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation 2001; 104:2205–2209.
- Cowell SJ, Newby DE, Prescott RJ, et al; Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005; 352:2389–2397.
- Rossebø AB, Pedersen TR, Boman K, et al; SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008; 359:1343–1356.
- Moura LM, Ramos SF, Zamorano JL, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol 2007; 49:554–561.
- Rosenhek R, Rader F, Loho N, et al. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation 2004; 110:1291–1295.
- O’Brien KD, Probstfield JL, Caulked MT, et al. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch Intern Med 2005; 165:858–862.
- Lindblom D, Lindblom U, Qvist J, Lundström H. Long-term relative survival rates after heart valve replacement. J Am Coll Cardiol 1990; 15:566–573.
- Makkar RR, Fontana G P, Jilaihawi H, et al; PARTNER Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012; 366:1696–1704.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Di Eusanio M, Fortuna D, Cristell D, et al; RERIC (Emilia Romagna Cardiac Surgery Registry) Investigators. Contemporary outcomes of conventional aortic valve replacement in 638 octogenarians: insights from an Italian Regional Cardiac Surgery Registry (RERIC). Eur J Cardiothorac Surg 2012; 41:1247–1252.
- Di Eusanio M, Fortuna D, De Palma R, et al. Aortic valve replacement: results and predictors of mortality from a contemporary series of 2256 patients. J Thorac Cardiovasc Surg 2011; 141:940–947.
- Jamieson WR, Edwards FH, Schwartz M, Bero JW, Clark RE, Grover FL. Risk stratification for cardiac valve replacement. National Cardiac Surgery Database. Database Committee of the Society of Thoracic Surgeons. Ann Thorac Surg 1999; 67:943–951.
- Bonow RO, Carabello BA, Chatterjee K, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 52:e1–e142.
- Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007; 115:2856–2864.
- Vaquette B, Corbineau H, Laurent M, et al. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart 2005; 91:1324–1329.
- Connolly HM, Oh JK, Orszulak TA, et al. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction. Prognostic indicators. Circulation 1997; 95:2395–2400.
- Connolly HM, Oh JK, Schaff HV, et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation 2000; 101:1940–1946.
- Pereira JJ, Lauer MS, Bashir M, et al. Survival after aortic valve replacement for severe aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am Coll Cardiol 2002; 39:1356–1363.
- Pai RG, Varadarajan P, Razzouk A. Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg 2008; 86:1781–1789.
- Monin JL, Monchi M, Gest V, Duval-Moulin AM, Dubois-Rande JL, Gueret P. Aortic stenosis with severe left ventricular dysfunction and low transvalvular pressure gradients: risk stratification by low-dose dobutamine echocardiography. J Am Coll Cardiol 2001; 37:2101–2107.
- Monin JL, Quéré J P, Monchi M, et al. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 2003; 108:319–324.
- Zuppiroli A, Mori F, Olivotto I, Castelli G, Favilli S, Dolara A. Therapeutic implications of contractile reserve elicited by dobutamine echocardiography in symptomatic, low-gradient aortic stenosis. Ital Heart J 2003; 4:264–270.
- Tribouilloy C, Lévy F, Rusinaru D, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol 2009; 53:1865–1873.
- Ahmed AA, Graham AN, Lovell D, O’Kane HO. Management of mild to moderate aortic valve disease during coronary artery bypass grafting. Eur J Cardiothorac Surg 2003; 24:535–539.
- Verhoye J P, Merlicco F, Sami IM, et al. Aortic valve replacement for aortic stenosis after previous coronary artery bypass grafting: could early reoperation be prevented? J Heart Valve Dis 2006; 15:474–478.
- Hochrein J, Lucke JC, Harrison JK, et al. Mortality and need for reoperation in patients with mild-to-moderate asymptomatic aortic valve disease undergoing coronary artery bypass graft alone. Am Heart J 1999; 138:791–797.
- Pereira JJ, Balaban K, Lauer MS, Lytle B, Thomas JD, Garcia MJ. Aortic valve replacement in patients with mild or moderate aortic stenosis and coronary bypass surgery. Am J Med 2005; 118:735–742.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Weber M, Hausen M, Arnold R, et al. Prognostic value of N-terminal pro-B-type natriuretic peptide for conservatively and surgically treated patients with aortic valve stenosis. Heart 2006; 92:1639–1644.
- Gerber IL, Stewart RA, Legget ME, et al. Increased plasma natriuretic peptide levels refect symptom onset in aortic stenosis. Circulation 2003; 107:1884–1890.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lancellotti P, Moonen M, Magne J, et al. Prognostic effect of long-axis left ventricular dysfunction and B-type natriuretic peptide levels in asymptomatic aortic stenosis. Am J Cardiol 2010; 105:383–388.
- Langanay T, Flécher E, Fouquet O, et al. Aortic valve replacement in the elderly: the real life. Ann Thorac Surg 2012; 93:70–77.
- Christofferson RD, Kapadia SR, Rajagopal V, Tuzcu EM. Emerging transcatheter therapies for aortic and mitral disease. Heart 2009; 95:148–155.
- Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1986; 1:63–67.
- Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 1991; 84:2383–2397.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Bernard Y, Etievent J, Mourand JL, et al. Long-term results of percutaneous aortic valvuloplasty compared with aortic valve replacement in patients more than 75 years old. J Am Coll Cardiol 1992; 20:796–801.
- Elkayam U, Janmohamed M, Habib M, Hatamizadeh P. Vasodilators in the management of acute heart failure. Crit Care Med 2008; 36(suppl 1):S95–S105.
- Popovic ZB, Khot UN, Novaro GM, et al. Effects of sodium nitroprusside in aortic stenosis associated with severe heart failure: pressure-volume loop analysis using a numerical model. Am J Physiol Heart Circ Physiol 2005; 288:H416–H423.
- Khot UN, Novaro GM, Popovic ZB, et al. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med 2003; 348:1756–1763.
- Aksoy O, Yousefzai R, Singh D, et al. Cardiogenic shock in the setting of severe aortic stenosis: role of intra-aortic balloon pump support. Heart 2011; 97:838–843.
KEY POINTS
- Calcific aortic stenosis is the most common acquired valvular disease, and its prevalence is increasing as the population ages.
- Patients who have symptoms should be referred for aortic valve replacement. Patients who are not candidates for open heart surgery may be eligible for transcatheter aortic valve replacement.
- For high-risk patients with multiple comorbidities, “bridging” therapies such as aortic valvuloplasty are an option.
- In patients with aortic stenosis who present with hemodynamic instability and circulatory collapse, time can be gained with the use of intravenous sodium nitroprusside (in the absence of hypotension) or intra-aortic balloon counterpulsation while more definitive treatment decisions are being made.
Aortic valve replacement: Options, improvements, and costs
How aortic valve disease is managed continues to evolve, with novel approaches for both aortic valve stenosis and regurgitation.1–8 Indeed, because of the spectrum of procedures, a multispecialty committee was formed to provide a detailed guideline to help physicians work through the various options.4
The paper by Aksoy and colleagues in this issue of the Journal gives further insight into the complexities of decision-making.
As a rule, the indications for a procedure to treat aortic valvular disease continue to be based on whether the patient develops certain symptoms (fatigue, exertional dyspnea, shortness of breath, syncope, chest pain), myocardial deterioration, reduced ejection fraction, or ventricular dilatation.4 Furthermore, the options depend on whether the patient has comorbid disease and is a candidate for surgical aortic valve replacement.
OPEN SURGERY: THE MAINSTAY OF TREATMENT
Open surgery—including in recent years minimally invasive J-incision “keyhole” repair or replacement—has been the mainstay of treatment. The results of surgical aortic valve repair have been excellent, so that 10 years after surgery 95% of patients who have undergone a modified David reimplantation operation have not needed a repeat operation.3 The results are comparable for repair of bicuspid aortic valves.2,3
Furthermore, surgical aortic valve replacement has become very safe. At Cleveland Clinic in 2011, only 3 (0.6%) of 479 patients died during isolated aortic valve replacement, and in 2012 the mortality rate was even better, with only 1 death (0.2%) among 495 patients as of November 2012.
GOOD RESULTS WITH TRANSCATHETER AORTIC VALVE REPLACEMENT
For a new valve procedure to be accepted into practice, it must be easy to do, safe, and consistently good in performance measures such as producing low gradients, eliminating aortic regurgitation, and leading to high rates of long-term freedom from reoperation and of survival. To see if percutaneous aortic valve replacement meets these criteria, it was evaluated by both us at Cleveland Clinic and our colleagues at other institutions in the laboratory and also in feasibility trials in the United States.
The subsequent Placement of Transcatheter Aortic Valves (PARTNER) trial established the benefit of this procedure in terms of superior survival for patients who could not undergo surgery.8 Hence, the transcatheter device was approved for patients who cannot undergo surgery who meet certain criteria (valve area < 0.8 cm2; mean gradient > 40 mm Hg or peak gradient > 64 mm Hg). Of note, the cost per procedure was $78,000, or approximately $50,000 per year of life saved.
The PARTNER A trial showed that the risk of death after transcatheter aortic valve replacement was as low as after open surgery, although the risk of stroke or transient ischemic attack risk was higher—indeed, with the transfemoral approach it was 3 times higher (4.6% vs 1.4%, P < .05).9,10 Furthermore, half the patients had perivalvular leakage after the new procedure, and even mild leakage reduced the survival rate at 2 years.11
Nevertheless, we have now done nearly 400 transcatheter aortic valve replacement procedures in patients who could not undergo open surgery or who would have been at extreme risk during surgery. With the transfemoral approach, in 267 patients, 1 patient died (0.4%), and 2 had strokes (0.7%). (In the rest of the patients, we used alternatives to the transfemoral approach, such as the transaortic, transapical, and transaxillary approaches, also with good results.)
Thus, transcatheter aortic valve replacement in properly selected patients can meet the above criteria.
COSTS AND THE FUTURE
Based on the PARTNER trial results, the Centers for Medicare and Medicaid Services (CMS) agreed to pay for this procedure at the same rate as for surgical aortic valve replacement for patients who cannot or should not undergo surgery, with the approval of two surgeons and within the context of a national registry.10
The reimbursement is adjusted for geographic area. In the United States, for example, hospitals on the East Coast or West Coast receive $88,000 to $94,000 per case, while most other areas receive $32,000 to $62,000.
The surgeon and cardiologist share the professional fee of approximately $2,500, although typically we have a team of eight to 10 physicians (representing the fields of anesthesia, echocardiography, surgery, and cardiology) in the operating room for every procedure, in addition to nursing and technical staff. The challenge for institutions and providers, however, is that the device costs $32,500, and CMS reimbursement does not cover the cost of both the valve and the procedure in many localities. This may affect how widely the valve is eventually used.
While many more options are available now for management of aortic valve disease (minimally invasive repair or replacement, and newer devices), the future usage of transcatheter aortic valve replacement may become dependent on costs, newer devices, cheaper iterations, competition, and CMS reimbursement.
There are now two additional trials, SURTAVI and PARTNER A2, evaluating transcatheter vs open aortic valve replacement in lower-risk patients. The issues that will have to be addressed with new iterations are the risk of stroke and transient ischemic attack, perivalvular leakage, and the costs of the devices.
Newer reports would suggest that the results with transcatheter aortic valve replacement in inoperable and high-risk patients continue to improve as experience evolves.
- Svensson LG, Blackstone EH, Cosgrove DM. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Svensson LG, Kim KH, Blackstone EH, et al. Bicuspid aortic valve surgery with proactive ascending aorta repair. J Thorac Cardiovasc Surg 2011; 142:622–629.e1–e3.
- Svensson LG, Batizy LH, Blackstone EH, et al. Results of matching valve and root repair to aortic valve and root pathology. J Thorac Cardiovasc Surg 2011; 142:1491–1498.e7.
- Svensson LG, Adams DH, Bonow RO, et al. Aortic valve and ascending aorta guidelines for management and quality measures: executive summary. Ann Thorac Surg 2013; 10.1016/j.athoracsur.2012.12.027, Epub ahead of print
- Svensson LG, D’Agostino RS. “J” incision minimal-access valve operations”. Ann Thorac Surg 1998; 66:1110–1112.
- Johnston DR, Atik FA, Rajeswaran J, et al. Outcomes of less invasive J-incision approach to aortic valve surgery. J Thorac Cardiovasc Surg 2012; 144:852–858.e3.
- Albacker TB, Blackstone EH, Williams SJ, et al. Should less-invasive aortic valve replacement be avoided in patients with pulmonary dysfunction? J Thorac Cardiovasc Surg 2013; Epub ahead of print.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Svensson LG, Tuzcu M, Kapadia S, et al. A comprehensive review of the PARTNER trial. J Thorac Cardiovasc Surg 2013; 145(suppl):S11–S16.
- Kodali SK, Williams MR, Smith CR, et al; PARTNER Trial Investigators. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012; 366:1686–1695.
How aortic valve disease is managed continues to evolve, with novel approaches for both aortic valve stenosis and regurgitation.1–8 Indeed, because of the spectrum of procedures, a multispecialty committee was formed to provide a detailed guideline to help physicians work through the various options.4
The paper by Aksoy and colleagues in this issue of the Journal gives further insight into the complexities of decision-making.
As a rule, the indications for a procedure to treat aortic valvular disease continue to be based on whether the patient develops certain symptoms (fatigue, exertional dyspnea, shortness of breath, syncope, chest pain), myocardial deterioration, reduced ejection fraction, or ventricular dilatation.4 Furthermore, the options depend on whether the patient has comorbid disease and is a candidate for surgical aortic valve replacement.
OPEN SURGERY: THE MAINSTAY OF TREATMENT
Open surgery—including in recent years minimally invasive J-incision “keyhole” repair or replacement—has been the mainstay of treatment. The results of surgical aortic valve repair have been excellent, so that 10 years after surgery 95% of patients who have undergone a modified David reimplantation operation have not needed a repeat operation.3 The results are comparable for repair of bicuspid aortic valves.2,3
Furthermore, surgical aortic valve replacement has become very safe. At Cleveland Clinic in 2011, only 3 (0.6%) of 479 patients died during isolated aortic valve replacement, and in 2012 the mortality rate was even better, with only 1 death (0.2%) among 495 patients as of November 2012.
GOOD RESULTS WITH TRANSCATHETER AORTIC VALVE REPLACEMENT
For a new valve procedure to be accepted into practice, it must be easy to do, safe, and consistently good in performance measures such as producing low gradients, eliminating aortic regurgitation, and leading to high rates of long-term freedom from reoperation and of survival. To see if percutaneous aortic valve replacement meets these criteria, it was evaluated by both us at Cleveland Clinic and our colleagues at other institutions in the laboratory and also in feasibility trials in the United States.
The subsequent Placement of Transcatheter Aortic Valves (PARTNER) trial established the benefit of this procedure in terms of superior survival for patients who could not undergo surgery.8 Hence, the transcatheter device was approved for patients who cannot undergo surgery who meet certain criteria (valve area < 0.8 cm2; mean gradient > 40 mm Hg or peak gradient > 64 mm Hg). Of note, the cost per procedure was $78,000, or approximately $50,000 per year of life saved.
The PARTNER A trial showed that the risk of death after transcatheter aortic valve replacement was as low as after open surgery, although the risk of stroke or transient ischemic attack risk was higher—indeed, with the transfemoral approach it was 3 times higher (4.6% vs 1.4%, P < .05).9,10 Furthermore, half the patients had perivalvular leakage after the new procedure, and even mild leakage reduced the survival rate at 2 years.11
Nevertheless, we have now done nearly 400 transcatheter aortic valve replacement procedures in patients who could not undergo open surgery or who would have been at extreme risk during surgery. With the transfemoral approach, in 267 patients, 1 patient died (0.4%), and 2 had strokes (0.7%). (In the rest of the patients, we used alternatives to the transfemoral approach, such as the transaortic, transapical, and transaxillary approaches, also with good results.)
Thus, transcatheter aortic valve replacement in properly selected patients can meet the above criteria.
COSTS AND THE FUTURE
Based on the PARTNER trial results, the Centers for Medicare and Medicaid Services (CMS) agreed to pay for this procedure at the same rate as for surgical aortic valve replacement for patients who cannot or should not undergo surgery, with the approval of two surgeons and within the context of a national registry.10
The reimbursement is adjusted for geographic area. In the United States, for example, hospitals on the East Coast or West Coast receive $88,000 to $94,000 per case, while most other areas receive $32,000 to $62,000.
The surgeon and cardiologist share the professional fee of approximately $2,500, although typically we have a team of eight to 10 physicians (representing the fields of anesthesia, echocardiography, surgery, and cardiology) in the operating room for every procedure, in addition to nursing and technical staff. The challenge for institutions and providers, however, is that the device costs $32,500, and CMS reimbursement does not cover the cost of both the valve and the procedure in many localities. This may affect how widely the valve is eventually used.
While many more options are available now for management of aortic valve disease (minimally invasive repair or replacement, and newer devices), the future usage of transcatheter aortic valve replacement may become dependent on costs, newer devices, cheaper iterations, competition, and CMS reimbursement.
There are now two additional trials, SURTAVI and PARTNER A2, evaluating transcatheter vs open aortic valve replacement in lower-risk patients. The issues that will have to be addressed with new iterations are the risk of stroke and transient ischemic attack, perivalvular leakage, and the costs of the devices.
Newer reports would suggest that the results with transcatheter aortic valve replacement in inoperable and high-risk patients continue to improve as experience evolves.
How aortic valve disease is managed continues to evolve, with novel approaches for both aortic valve stenosis and regurgitation.1–8 Indeed, because of the spectrum of procedures, a multispecialty committee was formed to provide a detailed guideline to help physicians work through the various options.4
The paper by Aksoy and colleagues in this issue of the Journal gives further insight into the complexities of decision-making.
As a rule, the indications for a procedure to treat aortic valvular disease continue to be based on whether the patient develops certain symptoms (fatigue, exertional dyspnea, shortness of breath, syncope, chest pain), myocardial deterioration, reduced ejection fraction, or ventricular dilatation.4 Furthermore, the options depend on whether the patient has comorbid disease and is a candidate for surgical aortic valve replacement.
OPEN SURGERY: THE MAINSTAY OF TREATMENT
Open surgery—including in recent years minimally invasive J-incision “keyhole” repair or replacement—has been the mainstay of treatment. The results of surgical aortic valve repair have been excellent, so that 10 years after surgery 95% of patients who have undergone a modified David reimplantation operation have not needed a repeat operation.3 The results are comparable for repair of bicuspid aortic valves.2,3
Furthermore, surgical aortic valve replacement has become very safe. At Cleveland Clinic in 2011, only 3 (0.6%) of 479 patients died during isolated aortic valve replacement, and in 2012 the mortality rate was even better, with only 1 death (0.2%) among 495 patients as of November 2012.
GOOD RESULTS WITH TRANSCATHETER AORTIC VALVE REPLACEMENT
For a new valve procedure to be accepted into practice, it must be easy to do, safe, and consistently good in performance measures such as producing low gradients, eliminating aortic regurgitation, and leading to high rates of long-term freedom from reoperation and of survival. To see if percutaneous aortic valve replacement meets these criteria, it was evaluated by both us at Cleveland Clinic and our colleagues at other institutions in the laboratory and also in feasibility trials in the United States.
The subsequent Placement of Transcatheter Aortic Valves (PARTNER) trial established the benefit of this procedure in terms of superior survival for patients who could not undergo surgery.8 Hence, the transcatheter device was approved for patients who cannot undergo surgery who meet certain criteria (valve area < 0.8 cm2; mean gradient > 40 mm Hg or peak gradient > 64 mm Hg). Of note, the cost per procedure was $78,000, or approximately $50,000 per year of life saved.
The PARTNER A trial showed that the risk of death after transcatheter aortic valve replacement was as low as after open surgery, although the risk of stroke or transient ischemic attack risk was higher—indeed, with the transfemoral approach it was 3 times higher (4.6% vs 1.4%, P < .05).9,10 Furthermore, half the patients had perivalvular leakage after the new procedure, and even mild leakage reduced the survival rate at 2 years.11
Nevertheless, we have now done nearly 400 transcatheter aortic valve replacement procedures in patients who could not undergo open surgery or who would have been at extreme risk during surgery. With the transfemoral approach, in 267 patients, 1 patient died (0.4%), and 2 had strokes (0.7%). (In the rest of the patients, we used alternatives to the transfemoral approach, such as the transaortic, transapical, and transaxillary approaches, also with good results.)
Thus, transcatheter aortic valve replacement in properly selected patients can meet the above criteria.
COSTS AND THE FUTURE
Based on the PARTNER trial results, the Centers for Medicare and Medicaid Services (CMS) agreed to pay for this procedure at the same rate as for surgical aortic valve replacement for patients who cannot or should not undergo surgery, with the approval of two surgeons and within the context of a national registry.10
The reimbursement is adjusted for geographic area. In the United States, for example, hospitals on the East Coast or West Coast receive $88,000 to $94,000 per case, while most other areas receive $32,000 to $62,000.
The surgeon and cardiologist share the professional fee of approximately $2,500, although typically we have a team of eight to 10 physicians (representing the fields of anesthesia, echocardiography, surgery, and cardiology) in the operating room for every procedure, in addition to nursing and technical staff. The challenge for institutions and providers, however, is that the device costs $32,500, and CMS reimbursement does not cover the cost of both the valve and the procedure in many localities. This may affect how widely the valve is eventually used.
While many more options are available now for management of aortic valve disease (minimally invasive repair or replacement, and newer devices), the future usage of transcatheter aortic valve replacement may become dependent on costs, newer devices, cheaper iterations, competition, and CMS reimbursement.
There are now two additional trials, SURTAVI and PARTNER A2, evaluating transcatheter vs open aortic valve replacement in lower-risk patients. The issues that will have to be addressed with new iterations are the risk of stroke and transient ischemic attack, perivalvular leakage, and the costs of the devices.
Newer reports would suggest that the results with transcatheter aortic valve replacement in inoperable and high-risk patients continue to improve as experience evolves.
- Svensson LG, Blackstone EH, Cosgrove DM. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Svensson LG, Kim KH, Blackstone EH, et al. Bicuspid aortic valve surgery with proactive ascending aorta repair. J Thorac Cardiovasc Surg 2011; 142:622–629.e1–e3.
- Svensson LG, Batizy LH, Blackstone EH, et al. Results of matching valve and root repair to aortic valve and root pathology. J Thorac Cardiovasc Surg 2011; 142:1491–1498.e7.
- Svensson LG, Adams DH, Bonow RO, et al. Aortic valve and ascending aorta guidelines for management and quality measures: executive summary. Ann Thorac Surg 2013; 10.1016/j.athoracsur.2012.12.027, Epub ahead of print
- Svensson LG, D’Agostino RS. “J” incision minimal-access valve operations”. Ann Thorac Surg 1998; 66:1110–1112.
- Johnston DR, Atik FA, Rajeswaran J, et al. Outcomes of less invasive J-incision approach to aortic valve surgery. J Thorac Cardiovasc Surg 2012; 144:852–858.e3.
- Albacker TB, Blackstone EH, Williams SJ, et al. Should less-invasive aortic valve replacement be avoided in patients with pulmonary dysfunction? J Thorac Cardiovasc Surg 2013; Epub ahead of print.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Svensson LG, Tuzcu M, Kapadia S, et al. A comprehensive review of the PARTNER trial. J Thorac Cardiovasc Surg 2013; 145(suppl):S11–S16.
- Kodali SK, Williams MR, Smith CR, et al; PARTNER Trial Investigators. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012; 366:1686–1695.
- Svensson LG, Blackstone EH, Cosgrove DM. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Svensson LG, Kim KH, Blackstone EH, et al. Bicuspid aortic valve surgery with proactive ascending aorta repair. J Thorac Cardiovasc Surg 2011; 142:622–629.e1–e3.
- Svensson LG, Batizy LH, Blackstone EH, et al. Results of matching valve and root repair to aortic valve and root pathology. J Thorac Cardiovasc Surg 2011; 142:1491–1498.e7.
- Svensson LG, Adams DH, Bonow RO, et al. Aortic valve and ascending aorta guidelines for management and quality measures: executive summary. Ann Thorac Surg 2013; 10.1016/j.athoracsur.2012.12.027, Epub ahead of print
- Svensson LG, D’Agostino RS. “J” incision minimal-access valve operations”. Ann Thorac Surg 1998; 66:1110–1112.
- Johnston DR, Atik FA, Rajeswaran J, et al. Outcomes of less invasive J-incision approach to aortic valve surgery. J Thorac Cardiovasc Surg 2012; 144:852–858.e3.
- Albacker TB, Blackstone EH, Williams SJ, et al. Should less-invasive aortic valve replacement be avoided in patients with pulmonary dysfunction? J Thorac Cardiovasc Surg 2013; Epub ahead of print.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Svensson LG, Tuzcu M, Kapadia S, et al. A comprehensive review of the PARTNER trial. J Thorac Cardiovasc Surg 2013; 145(suppl):S11–S16.
- Kodali SK, Williams MR, Smith CR, et al; PARTNER Trial Investigators. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012; 366:1686–1695.
Man, 57, With Dyspnea After Chiropractic Manipulation
A 57-year-old man presented to the emergency department (ED) with a two-day history of worsening shortness of breath, light-headedness, and back pain. The patient, who had a history of ankylosing spondylitis, had been receiving weekly therapy from a chiropractor for about 10 years. One week before presenting to the ED, he had begun to undergo daily manipulations under anesthesia (MUA)—an aggressive chiropractic procedure that is administered while the patient is under monitored, procedural sedation. After the second day of treatment, the patient began to experience worsening back pain and progressive light-headedness and shortness of breath.
At a follow-up visit with his chiropractor, he was found to have decreased O2 saturation and was directed to go to the hospital for evaluation. On arrival at the ED, the patient was awake and alert. He had intact motor strength in all extremities, no sensory abnormalities, intact symmetric reflexes, and no bladder or bowel dysfunction, with a negative Babinski sign. His O2 saturation was 92% on 5 L of oxygen. An absence of breath sounds was noted on the left side.
Chest x-ray (see Figure 1) was performed, which demonstrated complete opacification of the left hemithorax, consistent with a large pleural effusion or hemothorax. CT scan of the thoracic spine showed diffuse ankylosis. A complex oblique coronal and transversely oriented fracture with 7 mm of displacement was identified, beginning at the right anterior inferior lateral margin of the T8 vertebral body and extending centrally and inferiorly to the left and right into the T9 vertebral body. The fracture continued through the right T9-10 neural foramen and what was probably the right fused T9-10 facet joint. The fracture exited through the left superior and lateral margin of the T10 vertebral body and the left T10-11 neural foramen (see Figures 2, 3, and 4).
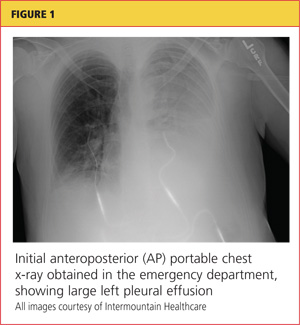
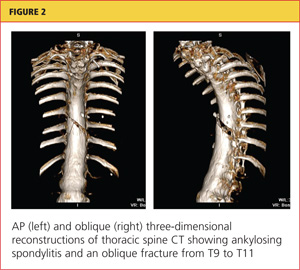
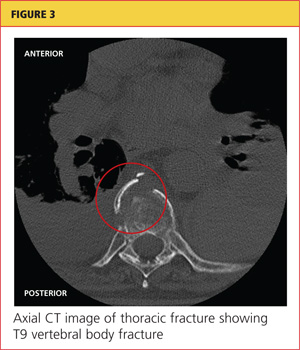
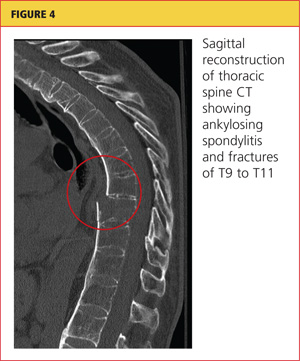
A chest tube was inserted in the ED, and 1,600 mL of old blood was immediately drained. The patient was admitted to the ICU on the trauma service. He was taken to surgery for open reduction and internal fixation of his unstable thoracic spine fracture on day 3 of hospitalization, after his pulmonary condition stabilized. Pedicle screws were placed from T7 through T12 during the spinal fusion. Good reduction of the fracture was observed following the spine surgery (see Figures 5 and 6). At the conclusion of surgery, an epidural catheter was placed in the thoracic spine to administer pain control.
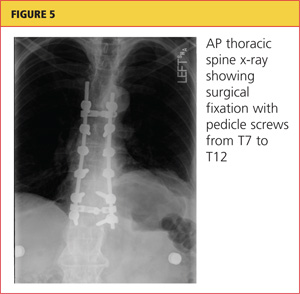
After the spine portion of the procedure, the patient was repositioned and underwent video-assisted thoracoscopic surgery of the left hemithorax for evacuation of retained hemothorax. The patient tolerated the procedure well and was taken to the ICU for recovery.
On postoperative day 2, the patient complained of chest pain and experienced hypoxemia with activity. CT angiography of the chest demonstrated bilateral segmental and subsegmental pulmonary emboli. The epidural catheter was discontinued. Six hours later, a heparin drip was started, and the patient was transitioned to therapeutic enoxaparin and warfarin. When methicillin-sensitive Staphylococcus aureus (MSSA) was detected in his hemothorax fluid, he was treated with a course of nafcillin.
The patient was discharged to home on postoperative day 12. He has remained neurologically intact and has returned to his former work activities. He is not taking narcotic pain medications.
Discussion
Chiropractic care is a popular alternative health care modality in the United States. Researchers for the 2007 National Health Interview Study1 reported an annual use of chiropractic manipulation of 8.6%, while the Medical Expenditure Panel Survey2 data yielded an estimate of 12.6 million adults using chiropractic manipulation in 2006—translating to a prevalence of 5.6%. Despite the popularity of chiropractic medicine, few well-designed studies have been conducted to support its use.3,4 Because of its designation as an alternative therapy, however, chiropractic manipulation has not been subjected to rigorous efficacy and safety evaluations.5
Given the inconsistency of the evidence to support chiropractic manipulation, the practice's safety profile is a concern. The risks associated with spinal manipulation are generally described in case reports and small series. Most serious adverse events described in the literature are cerebrovascular in nature and tend to occur after cervical manipulation.6,7 Fractures after spine manipulation are exceedingly rare, and published literature on this topic consists of a few isolated case reports, with all fractures occurring in the cervical spine in patients with an underlying pathologic condition.8-10
In 2009, Gouveia et al5 reviewed the published literature regarding all adverse events resulting from chiropractic manipulation. The authors found one randomized controlled trial, two case-control studies, six prospective studies, 12 surveys, three retrospective studies, and 100 case reports. The spectrum of complications identified ranged from benign and transient, such as local discomfort, to far more serious: stroke, myelopathy, radiculopathy, subdural hematoma, spinal fluid leakage, cauda equina syndrome, herniated disc, diaphragmatic palsy, and vertebral fractures. The authors were unable to perform a true meta-analysis because of the heterogeneity of the data, but they concluded that complications associated with chiropractic procedures are "frequent."5
Manipulations Under Anesthesia
MUA is a procedure that combines chiropractic adjustments and manipulations with general anesthesia or procedural sedation.11 The theory behind this strategy is that the anesthesia or sedation reduces pain and muscle spasm that may hinder the manipulation, allowing the practitioner to more effectively break up joint adhesions and reduce segmental dysfunction than if the patient had not undergone anesthesia.11
MUA is generally indicated in patients who have not responded to a 4- to 8-week trial of traditional manipulation therapy.12 It is also considered in patients who have "painful and restricting muscular guarding [that] interferes with the performance of spinal adjustments, mobilizations, and soft tissue release techniques."13
In the chiropractic literature, between 3% and 10% of patients are estimated to be candidates for MUA.12,14 It is not completely clear, however, what diagnoses are most likely to be treated successfully with this technique. Contraindications to MUA are generally the same as those for manipulation in conscious patients. A published list of contraindications from the Committee for Manipulation under Anesthesia (2003)15 included malignancy with bony metastasis, tuberculosis of the bone, recent fracture, acute arthritis, acute gout, diabetic neuropathy, syphilitic articular lesions, excessive spinal osteoporosis, disk fragmentation, direct nerve root impingement, and evidence of cord or caudal compression by tumor, ankylosis, or other space-occupying lesions.
MUA generally begins with deep procedural sedation, managed by an anesthesiologist. Once an adequate level of sedation is achieved, the manipulations are performed. Both high- and low-velocity thrusts are used, but it is recommended that the force exerted should be much less, and the manipulations performed with more caution, than in patients who are not anesthetized.12
For the thoracic spine, the patient is manipulated in the supine position with the arms crossed over the chest. The practitioner places one hand in a fist under the spine with the other hand on the patient's crossed arms, then delivers an anterior-to-posterior thrust. This is repeated until all affected segments have been treated.11,12
Literature to support the use of MUA for various indications is largely anecdotal. The largest published series13 is of 177 patients with chronic spinal pain who each underwent three MUA sessions followed by four to six weeks of traditional manipulations. The authors found that pain, as measured by visual analog scale, was reduced by 62% in patients with cervical spine pain, and by 60% in patients with lumbar pain. No adverse events were reported in the study.
Kohlbeck and Haldeman12 reviewed the reported complications of MUA across all published literature. They found that in 17 published papers, the overall complication rate was 0.7%, mainly represented by transitory increased pain. No spinal fractures were reported.
This case demonstrates a rare but serious complication of chiropractic MUA. It is unclear exactly what mechanism of injury led to an unstable thoracic spine fracture with massive hemothorax, and the precise cause will probably never be known. The clinicians who treated the case patient find it curious that the reported rate of adverse events following this procedure is so low, but they suspect an element of reporting bias in the chiropractic literature.
Conclusion
Iatrogenic injury after chiropractic manipulation is uncommon, but it can be devastating. Few serious complications of chiropractic MUA have been reported, but the literature is lacking in well-designed research studies. Despite the dearth of clinical trials to support its safety and efficacy, use of MUA has continued in the chiropractic community. This case demonstrates that serious adverse outcomes can occur, and more rigorous studies are needed to delineate the true benefits and risks of this set of chiropractic procedures.
References
1. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-9.
2. Davis MA, Sirovich BE, Weeks WB. Utilization and expenditures on chiropractic care in the United States from 1997 to 2006. Health Serv Res. 2009;45:748-761.
3. Canadian Chiropractic Association; Canadian Federation of Chiropractic Regulatory Boards; Clinical Practice Guidelines Development Initiative; Guidelines Development Committee. Chiropractic clinical practice guideline: evidence-based treatment of adult neck pain not due to whiplash. J Can Chiropr Assoc. 2005;49:417-421.
4.
Hurwitz EL, Aker PD, Adams AH, et al. Manipulation and mobilization of the cervical spine: a systematic review of the literature. Spine (Phila Pa 1976). 1996;21:1746-1760.
5.Gouveia LO, Castanho P, Ferreira JJ. Safety of chiropractic interventions: a systematic review. Spine (Phila Pa 1976). 2009;34:E405-E413.
6. Di Fabio RP. Manipulation of the cervical spine: risks and benefits. Phys Ther. 1999;79:50-65.
7. Nadareishvili Z, Norris JW. Stroke from traumatic arterial dissection. Lancet. 1999;354:159-160.
8. Austin RT. Pathological vertebral fractures after spinal manipulation. Br Med J (Clin Res Ed). 1985;291:1114-1115.
9. Ea HK, Weber AJ, Yon F, Lioté F. Osteoporotic fracture of the dens revealed by cervical manipulation. Joint Bone Spine. 2004;71:246-250.
10. Schmitz A, Lutterbey G, von Engelhardt L, et al. Pathological cervical fracture after spinal manipulation in a pregnant patient. J Manipulative Physiol Ther. 2005;28:633-636.
11. Cremata E, Collins S, Clauson W, et al. Manipulation under anesthesia: a report of four cases. J Manipulative Physiol Ther. 2005;28:526-533.
12. Kohlbeck FJ, Haldeman S. Medication-assisted spinal manipulation. Spine J. 2002;2:288-302.
13. West DT, Mathews RS, Miller MR, Kent GM. Effective management of spinal pain in one hundred seventy-seven patients evaluated for manipulation under anesthesia. J Manipulative Physiol Ther. 1999;22:299-308.
14. Morey LW Jr. Osteopathic manipulation under general anesthesia. J Am Osteopath Assoc. 1973;73:116-127.
15. Tain L, Gunderson C, Cremata E, et al; Committee for Manipulation Under Anesthesia. Recommendations to the Industrial Medical Council Work Group of California for manipulation under anesthesia use for injured workers. Sacramento, CA: Industrial Medical Council; 2003.
A 57-year-old man presented to the emergency department (ED) with a two-day history of worsening shortness of breath, light-headedness, and back pain. The patient, who had a history of ankylosing spondylitis, had been receiving weekly therapy from a chiropractor for about 10 years. One week before presenting to the ED, he had begun to undergo daily manipulations under anesthesia (MUA)—an aggressive chiropractic procedure that is administered while the patient is under monitored, procedural sedation. After the second day of treatment, the patient began to experience worsening back pain and progressive light-headedness and shortness of breath.
At a follow-up visit with his chiropractor, he was found to have decreased O2 saturation and was directed to go to the hospital for evaluation. On arrival at the ED, the patient was awake and alert. He had intact motor strength in all extremities, no sensory abnormalities, intact symmetric reflexes, and no bladder or bowel dysfunction, with a negative Babinski sign. His O2 saturation was 92% on 5 L of oxygen. An absence of breath sounds was noted on the left side.
Chest x-ray (see Figure 1) was performed, which demonstrated complete opacification of the left hemithorax, consistent with a large pleural effusion or hemothorax. CT scan of the thoracic spine showed diffuse ankylosis. A complex oblique coronal and transversely oriented fracture with 7 mm of displacement was identified, beginning at the right anterior inferior lateral margin of the T8 vertebral body and extending centrally and inferiorly to the left and right into the T9 vertebral body. The fracture continued through the right T9-10 neural foramen and what was probably the right fused T9-10 facet joint. The fracture exited through the left superior and lateral margin of the T10 vertebral body and the left T10-11 neural foramen (see Figures 2, 3, and 4).




A chest tube was inserted in the ED, and 1,600 mL of old blood was immediately drained. The patient was admitted to the ICU on the trauma service. He was taken to surgery for open reduction and internal fixation of his unstable thoracic spine fracture on day 3 of hospitalization, after his pulmonary condition stabilized. Pedicle screws were placed from T7 through T12 during the spinal fusion. Good reduction of the fracture was observed following the spine surgery (see Figures 5 and 6). At the conclusion of surgery, an epidural catheter was placed in the thoracic spine to administer pain control.


After the spine portion of the procedure, the patient was repositioned and underwent video-assisted thoracoscopic surgery of the left hemithorax for evacuation of retained hemothorax. The patient tolerated the procedure well and was taken to the ICU for recovery.
On postoperative day 2, the patient complained of chest pain and experienced hypoxemia with activity. CT angiography of the chest demonstrated bilateral segmental and subsegmental pulmonary emboli. The epidural catheter was discontinued. Six hours later, a heparin drip was started, and the patient was transitioned to therapeutic enoxaparin and warfarin. When methicillin-sensitive Staphylococcus aureus (MSSA) was detected in his hemothorax fluid, he was treated with a course of nafcillin.
The patient was discharged to home on postoperative day 12. He has remained neurologically intact and has returned to his former work activities. He is not taking narcotic pain medications.
Discussion
Chiropractic care is a popular alternative health care modality in the United States. Researchers for the 2007 National Health Interview Study1 reported an annual use of chiropractic manipulation of 8.6%, while the Medical Expenditure Panel Survey2 data yielded an estimate of 12.6 million adults using chiropractic manipulation in 2006—translating to a prevalence of 5.6%. Despite the popularity of chiropractic medicine, few well-designed studies have been conducted to support its use.3,4 Because of its designation as an alternative therapy, however, chiropractic manipulation has not been subjected to rigorous efficacy and safety evaluations.5
Given the inconsistency of the evidence to support chiropractic manipulation, the practice's safety profile is a concern. The risks associated with spinal manipulation are generally described in case reports and small series. Most serious adverse events described in the literature are cerebrovascular in nature and tend to occur after cervical manipulation.6,7 Fractures after spine manipulation are exceedingly rare, and published literature on this topic consists of a few isolated case reports, with all fractures occurring in the cervical spine in patients with an underlying pathologic condition.8-10
In 2009, Gouveia et al5 reviewed the published literature regarding all adverse events resulting from chiropractic manipulation. The authors found one randomized controlled trial, two case-control studies, six prospective studies, 12 surveys, three retrospective studies, and 100 case reports. The spectrum of complications identified ranged from benign and transient, such as local discomfort, to far more serious: stroke, myelopathy, radiculopathy, subdural hematoma, spinal fluid leakage, cauda equina syndrome, herniated disc, diaphragmatic palsy, and vertebral fractures. The authors were unable to perform a true meta-analysis because of the heterogeneity of the data, but they concluded that complications associated with chiropractic procedures are "frequent."5
Manipulations Under Anesthesia
MUA is a procedure that combines chiropractic adjustments and manipulations with general anesthesia or procedural sedation.11 The theory behind this strategy is that the anesthesia or sedation reduces pain and muscle spasm that may hinder the manipulation, allowing the practitioner to more effectively break up joint adhesions and reduce segmental dysfunction than if the patient had not undergone anesthesia.11
MUA is generally indicated in patients who have not responded to a 4- to 8-week trial of traditional manipulation therapy.12 It is also considered in patients who have "painful and restricting muscular guarding [that] interferes with the performance of spinal adjustments, mobilizations, and soft tissue release techniques."13
In the chiropractic literature, between 3% and 10% of patients are estimated to be candidates for MUA.12,14 It is not completely clear, however, what diagnoses are most likely to be treated successfully with this technique. Contraindications to MUA are generally the same as those for manipulation in conscious patients. A published list of contraindications from the Committee for Manipulation under Anesthesia (2003)15 included malignancy with bony metastasis, tuberculosis of the bone, recent fracture, acute arthritis, acute gout, diabetic neuropathy, syphilitic articular lesions, excessive spinal osteoporosis, disk fragmentation, direct nerve root impingement, and evidence of cord or caudal compression by tumor, ankylosis, or other space-occupying lesions.
MUA generally begins with deep procedural sedation, managed by an anesthesiologist. Once an adequate level of sedation is achieved, the manipulations are performed. Both high- and low-velocity thrusts are used, but it is recommended that the force exerted should be much less, and the manipulations performed with more caution, than in patients who are not anesthetized.12
For the thoracic spine, the patient is manipulated in the supine position with the arms crossed over the chest. The practitioner places one hand in a fist under the spine with the other hand on the patient's crossed arms, then delivers an anterior-to-posterior thrust. This is repeated until all affected segments have been treated.11,12
Literature to support the use of MUA for various indications is largely anecdotal. The largest published series13 is of 177 patients with chronic spinal pain who each underwent three MUA sessions followed by four to six weeks of traditional manipulations. The authors found that pain, as measured by visual analog scale, was reduced by 62% in patients with cervical spine pain, and by 60% in patients with lumbar pain. No adverse events were reported in the study.
Kohlbeck and Haldeman12 reviewed the reported complications of MUA across all published literature. They found that in 17 published papers, the overall complication rate was 0.7%, mainly represented by transitory increased pain. No spinal fractures were reported.
This case demonstrates a rare but serious complication of chiropractic MUA. It is unclear exactly what mechanism of injury led to an unstable thoracic spine fracture with massive hemothorax, and the precise cause will probably never be known. The clinicians who treated the case patient find it curious that the reported rate of adverse events following this procedure is so low, but they suspect an element of reporting bias in the chiropractic literature.
Conclusion
Iatrogenic injury after chiropractic manipulation is uncommon, but it can be devastating. Few serious complications of chiropractic MUA have been reported, but the literature is lacking in well-designed research studies. Despite the dearth of clinical trials to support its safety and efficacy, use of MUA has continued in the chiropractic community. This case demonstrates that serious adverse outcomes can occur, and more rigorous studies are needed to delineate the true benefits and risks of this set of chiropractic procedures.
References
1. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-9.
2. Davis MA, Sirovich BE, Weeks WB. Utilization and expenditures on chiropractic care in the United States from 1997 to 2006. Health Serv Res. 2009;45:748-761.
3. Canadian Chiropractic Association; Canadian Federation of Chiropractic Regulatory Boards; Clinical Practice Guidelines Development Initiative; Guidelines Development Committee. Chiropractic clinical practice guideline: evidence-based treatment of adult neck pain not due to whiplash. J Can Chiropr Assoc. 2005;49:417-421.
4.
Hurwitz EL, Aker PD, Adams AH, et al. Manipulation and mobilization of the cervical spine: a systematic review of the literature. Spine (Phila Pa 1976). 1996;21:1746-1760.
5.Gouveia LO, Castanho P, Ferreira JJ. Safety of chiropractic interventions: a systematic review. Spine (Phila Pa 1976). 2009;34:E405-E413.
6. Di Fabio RP. Manipulation of the cervical spine: risks and benefits. Phys Ther. 1999;79:50-65.
7. Nadareishvili Z, Norris JW. Stroke from traumatic arterial dissection. Lancet. 1999;354:159-160.
8. Austin RT. Pathological vertebral fractures after spinal manipulation. Br Med J (Clin Res Ed). 1985;291:1114-1115.
9. Ea HK, Weber AJ, Yon F, Lioté F. Osteoporotic fracture of the dens revealed by cervical manipulation. Joint Bone Spine. 2004;71:246-250.
10. Schmitz A, Lutterbey G, von Engelhardt L, et al. Pathological cervical fracture after spinal manipulation in a pregnant patient. J Manipulative Physiol Ther. 2005;28:633-636.
11. Cremata E, Collins S, Clauson W, et al. Manipulation under anesthesia: a report of four cases. J Manipulative Physiol Ther. 2005;28:526-533.
12. Kohlbeck FJ, Haldeman S. Medication-assisted spinal manipulation. Spine J. 2002;2:288-302.
13. West DT, Mathews RS, Miller MR, Kent GM. Effective management of spinal pain in one hundred seventy-seven patients evaluated for manipulation under anesthesia. J Manipulative Physiol Ther. 1999;22:299-308.
14. Morey LW Jr. Osteopathic manipulation under general anesthesia. J Am Osteopath Assoc. 1973;73:116-127.
15. Tain L, Gunderson C, Cremata E, et al; Committee for Manipulation Under Anesthesia. Recommendations to the Industrial Medical Council Work Group of California for manipulation under anesthesia use for injured workers. Sacramento, CA: Industrial Medical Council; 2003.
A 57-year-old man presented to the emergency department (ED) with a two-day history of worsening shortness of breath, light-headedness, and back pain. The patient, who had a history of ankylosing spondylitis, had been receiving weekly therapy from a chiropractor for about 10 years. One week before presenting to the ED, he had begun to undergo daily manipulations under anesthesia (MUA)—an aggressive chiropractic procedure that is administered while the patient is under monitored, procedural sedation. After the second day of treatment, the patient began to experience worsening back pain and progressive light-headedness and shortness of breath.
At a follow-up visit with his chiropractor, he was found to have decreased O2 saturation and was directed to go to the hospital for evaluation. On arrival at the ED, the patient was awake and alert. He had intact motor strength in all extremities, no sensory abnormalities, intact symmetric reflexes, and no bladder or bowel dysfunction, with a negative Babinski sign. His O2 saturation was 92% on 5 L of oxygen. An absence of breath sounds was noted on the left side.
Chest x-ray (see Figure 1) was performed, which demonstrated complete opacification of the left hemithorax, consistent with a large pleural effusion or hemothorax. CT scan of the thoracic spine showed diffuse ankylosis. A complex oblique coronal and transversely oriented fracture with 7 mm of displacement was identified, beginning at the right anterior inferior lateral margin of the T8 vertebral body and extending centrally and inferiorly to the left and right into the T9 vertebral body. The fracture continued through the right T9-10 neural foramen and what was probably the right fused T9-10 facet joint. The fracture exited through the left superior and lateral margin of the T10 vertebral body and the left T10-11 neural foramen (see Figures 2, 3, and 4).




A chest tube was inserted in the ED, and 1,600 mL of old blood was immediately drained. The patient was admitted to the ICU on the trauma service. He was taken to surgery for open reduction and internal fixation of his unstable thoracic spine fracture on day 3 of hospitalization, after his pulmonary condition stabilized. Pedicle screws were placed from T7 through T12 during the spinal fusion. Good reduction of the fracture was observed following the spine surgery (see Figures 5 and 6). At the conclusion of surgery, an epidural catheter was placed in the thoracic spine to administer pain control.


After the spine portion of the procedure, the patient was repositioned and underwent video-assisted thoracoscopic surgery of the left hemithorax for evacuation of retained hemothorax. The patient tolerated the procedure well and was taken to the ICU for recovery.
On postoperative day 2, the patient complained of chest pain and experienced hypoxemia with activity. CT angiography of the chest demonstrated bilateral segmental and subsegmental pulmonary emboli. The epidural catheter was discontinued. Six hours later, a heparin drip was started, and the patient was transitioned to therapeutic enoxaparin and warfarin. When methicillin-sensitive Staphylococcus aureus (MSSA) was detected in his hemothorax fluid, he was treated with a course of nafcillin.
The patient was discharged to home on postoperative day 12. He has remained neurologically intact and has returned to his former work activities. He is not taking narcotic pain medications.
Discussion
Chiropractic care is a popular alternative health care modality in the United States. Researchers for the 2007 National Health Interview Study1 reported an annual use of chiropractic manipulation of 8.6%, while the Medical Expenditure Panel Survey2 data yielded an estimate of 12.6 million adults using chiropractic manipulation in 2006—translating to a prevalence of 5.6%. Despite the popularity of chiropractic medicine, few well-designed studies have been conducted to support its use.3,4 Because of its designation as an alternative therapy, however, chiropractic manipulation has not been subjected to rigorous efficacy and safety evaluations.5
Given the inconsistency of the evidence to support chiropractic manipulation, the practice's safety profile is a concern. The risks associated with spinal manipulation are generally described in case reports and small series. Most serious adverse events described in the literature are cerebrovascular in nature and tend to occur after cervical manipulation.6,7 Fractures after spine manipulation are exceedingly rare, and published literature on this topic consists of a few isolated case reports, with all fractures occurring in the cervical spine in patients with an underlying pathologic condition.8-10
In 2009, Gouveia et al5 reviewed the published literature regarding all adverse events resulting from chiropractic manipulation. The authors found one randomized controlled trial, two case-control studies, six prospective studies, 12 surveys, three retrospective studies, and 100 case reports. The spectrum of complications identified ranged from benign and transient, such as local discomfort, to far more serious: stroke, myelopathy, radiculopathy, subdural hematoma, spinal fluid leakage, cauda equina syndrome, herniated disc, diaphragmatic palsy, and vertebral fractures. The authors were unable to perform a true meta-analysis because of the heterogeneity of the data, but they concluded that complications associated with chiropractic procedures are "frequent."5
Manipulations Under Anesthesia
MUA is a procedure that combines chiropractic adjustments and manipulations with general anesthesia or procedural sedation.11 The theory behind this strategy is that the anesthesia or sedation reduces pain and muscle spasm that may hinder the manipulation, allowing the practitioner to more effectively break up joint adhesions and reduce segmental dysfunction than if the patient had not undergone anesthesia.11
MUA is generally indicated in patients who have not responded to a 4- to 8-week trial of traditional manipulation therapy.12 It is also considered in patients who have "painful and restricting muscular guarding [that] interferes with the performance of spinal adjustments, mobilizations, and soft tissue release techniques."13
In the chiropractic literature, between 3% and 10% of patients are estimated to be candidates for MUA.12,14 It is not completely clear, however, what diagnoses are most likely to be treated successfully with this technique. Contraindications to MUA are generally the same as those for manipulation in conscious patients. A published list of contraindications from the Committee for Manipulation under Anesthesia (2003)15 included malignancy with bony metastasis, tuberculosis of the bone, recent fracture, acute arthritis, acute gout, diabetic neuropathy, syphilitic articular lesions, excessive spinal osteoporosis, disk fragmentation, direct nerve root impingement, and evidence of cord or caudal compression by tumor, ankylosis, or other space-occupying lesions.
MUA generally begins with deep procedural sedation, managed by an anesthesiologist. Once an adequate level of sedation is achieved, the manipulations are performed. Both high- and low-velocity thrusts are used, but it is recommended that the force exerted should be much less, and the manipulations performed with more caution, than in patients who are not anesthetized.12
For the thoracic spine, the patient is manipulated in the supine position with the arms crossed over the chest. The practitioner places one hand in a fist under the spine with the other hand on the patient's crossed arms, then delivers an anterior-to-posterior thrust. This is repeated until all affected segments have been treated.11,12
Literature to support the use of MUA for various indications is largely anecdotal. The largest published series13 is of 177 patients with chronic spinal pain who each underwent three MUA sessions followed by four to six weeks of traditional manipulations. The authors found that pain, as measured by visual analog scale, was reduced by 62% in patients with cervical spine pain, and by 60% in patients with lumbar pain. No adverse events were reported in the study.
Kohlbeck and Haldeman12 reviewed the reported complications of MUA across all published literature. They found that in 17 published papers, the overall complication rate was 0.7%, mainly represented by transitory increased pain. No spinal fractures were reported.
This case demonstrates a rare but serious complication of chiropractic MUA. It is unclear exactly what mechanism of injury led to an unstable thoracic spine fracture with massive hemothorax, and the precise cause will probably never be known. The clinicians who treated the case patient find it curious that the reported rate of adverse events following this procedure is so low, but they suspect an element of reporting bias in the chiropractic literature.
Conclusion
Iatrogenic injury after chiropractic manipulation is uncommon, but it can be devastating. Few serious complications of chiropractic MUA have been reported, but the literature is lacking in well-designed research studies. Despite the dearth of clinical trials to support its safety and efficacy, use of MUA has continued in the chiropractic community. This case demonstrates that serious adverse outcomes can occur, and more rigorous studies are needed to delineate the true benefits and risks of this set of chiropractic procedures.
References
1. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-9.
2. Davis MA, Sirovich BE, Weeks WB. Utilization and expenditures on chiropractic care in the United States from 1997 to 2006. Health Serv Res. 2009;45:748-761.
3. Canadian Chiropractic Association; Canadian Federation of Chiropractic Regulatory Boards; Clinical Practice Guidelines Development Initiative; Guidelines Development Committee. Chiropractic clinical practice guideline: evidence-based treatment of adult neck pain not due to whiplash. J Can Chiropr Assoc. 2005;49:417-421.
4.
Hurwitz EL, Aker PD, Adams AH, et al. Manipulation and mobilization of the cervical spine: a systematic review of the literature. Spine (Phila Pa 1976). 1996;21:1746-1760.
5.Gouveia LO, Castanho P, Ferreira JJ. Safety of chiropractic interventions: a systematic review. Spine (Phila Pa 1976). 2009;34:E405-E413.
6. Di Fabio RP. Manipulation of the cervical spine: risks and benefits. Phys Ther. 1999;79:50-65.
7. Nadareishvili Z, Norris JW. Stroke from traumatic arterial dissection. Lancet. 1999;354:159-160.
8. Austin RT. Pathological vertebral fractures after spinal manipulation. Br Med J (Clin Res Ed). 1985;291:1114-1115.
9. Ea HK, Weber AJ, Yon F, Lioté F. Osteoporotic fracture of the dens revealed by cervical manipulation. Joint Bone Spine. 2004;71:246-250.
10. Schmitz A, Lutterbey G, von Engelhardt L, et al. Pathological cervical fracture after spinal manipulation in a pregnant patient. J Manipulative Physiol Ther. 2005;28:633-636.
11. Cremata E, Collins S, Clauson W, et al. Manipulation under anesthesia: a report of four cases. J Manipulative Physiol Ther. 2005;28:526-533.
12. Kohlbeck FJ, Haldeman S. Medication-assisted spinal manipulation. Spine J. 2002;2:288-302.
13. West DT, Mathews RS, Miller MR, Kent GM. Effective management of spinal pain in one hundred seventy-seven patients evaluated for manipulation under anesthesia. J Manipulative Physiol Ther. 1999;22:299-308.
14. Morey LW Jr. Osteopathic manipulation under general anesthesia. J Am Osteopath Assoc. 1973;73:116-127.
15. Tain L, Gunderson C, Cremata E, et al; Committee for Manipulation Under Anesthesia. Recommendations to the Industrial Medical Council Work Group of California for manipulation under anesthesia use for injured workers. Sacramento, CA: Industrial Medical Council; 2003.
A 67-year old man with an abdominal aortic aneurysm
A 67-year-old man presented for evaluation of an abdominal aortic aneurysm, noted 1 month previously after his primary care physician ordered screening ultrasonography as part of a routine annual physical examination. The man was experiencing no symptoms.
He had type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, and hyperlipidemia. He smoked two packs of cigarettes a day. He had never had surgery. His current medications included diltiazem, fenofibrate, niacin, and aspirin; because he had chronic obstructive pulmonary disease, he was not on a beta-blocker.
His father had died suddenly at the age of 77; his death was attributed to a cardiac cause, but no formal autopsy was performed. Neither the patient’s siblings nor his children were screened for aneurysms.
On physical examination, he was comfortable and in no acute distress. His blood pressure was 156/71 mm Hg, pulse 60, temperature 36.1°C (97.0°F), and body mass index 30.15 kg/m2, which is in the obese range.
He had no jugular venous distention, no carotid bruits, and no lymphadenopathy. The cardiac examination was unremarkable, with regular rate, normal sinus rhythm, and no murmurs. On pulmonary examination, inspiratory and expiratory wheezes were noted in all lung fields.
His abdomen was obese but not tender to palpation. The aneurysm was not palpable. His pedal pulses were normal. The remainder of the examination was normal.
WHO SHOULD BE SCREENED?
1. For which of the following groups does the United States Preventive Services Task Force (USPSTF) strongly recommend screening for abdominal aortic aneurysms?
- Men and women over age 65
- Men and women who have ever smoked and are over age 65
- Men over age 75 and men over age 65 who smoke
- Men age 65 to 75 who have ever smoked
In 2005, the USPSTF recommended one-time screening ultrasonography for all men age 65 to 75 who have ever smoked. On the basis of evidence available at the time, it made no recommendation for men age 65 to 75 who have never smoked, and it recommended against screening women.1
ANEURYSMS ARE COMMON, OFTEN ASYMPTOMATIC, UNTIL THEY RUPTURE
Abdominal aortic aneurysms are relatively common in older adults, with a prevalence of 1.4% in the US population age 50 to 84 years.2 In four randomized controlled trials of aneurysm screening in Europe and Australia, the prevalence of any aneurysm (not just abdominal aortic aneurysms) in men was 6% (95% confidence interval 5–6).3–6
Fewer studies are available on the prevalence in women. One study found a prevalence of 0.7% in 10,012 US women, compared with 3.9% in men.7
In a recent report of the aneurysm screening program in the United Kingdom, the incidence of aneurysms had decreased from historically reported estimates.8,9
In the year 2000, abdominal aortic aneurysms caused 15,000 deaths in the United States and were the 10th leading cause of death in white men age 65 to 74.10 The actual number of deaths may be larger, since some people may die suddenly of an aneurysm with no evaluation for attributable cause.11
Aortic aneurysms are often asymptomatic until they rupture, making them difficult to detect without a focused screening program. The goal of treatment is to avoid spontaneous rupture and death. When aneurysms rupture, the estimated death rate is 80%.6
EVIDENCE IN FAVOR OF SCREENING
Ultrasonography is nearly 100% sensitive and specific in detecting abdominal aortic aneurysms in patients without symptoms.12 In comparison, abdominal palpation is 68% sensitive and 75% specific.13
The larger the aneurysm, the higher the risk of rupture.14–16 The annual risk of rupture is:
- 0.5% with aneurysms smaller than 4.0 cm
- 1.0% with aneurysms 4.0–4.9 cm
- 11% with aneurysms 5.0–5.9 cm
- 26% with aneurysms 6.0–6.9 cm.
Several large randomized controlled trials in men over age 65 evaluated the effect of screening programs for abdominal aortic aneurysms on the rate of deaths from this cause.3–6,17 A meta-analysis of these trials found a relative risk of 0.60 in favor of screening—ie, men over age 65 who were screened had a 40% lower risk of dying of an abdominal aortic aneurysm than men who were not screened.18 In long-term follow-up, the rate continued to be about 50% lower with screening than without.19,20 The absolute reduction in risk of death was 0.13%.21
Absolute risk reduction and number needed to screen
2. If screening offers an absolute risk reduction in the death rate of 0.13%, how many need to be screened to prevent one death?
- 769
- 856
- 1,300
- 13,000
The number of patients that need to be screened to prevent one death is called the number needed to screen.22 It is calculated as 1 divided by the absolute risk reduction. Therefore, in screening for abdominal aortic aneurysms, the number needed to screen is 1/0.0013, or 769. Recall that these numbers are from men over age 65, with no upper limit in age. If we consider only men age 65 to 75, the absolute risk reduction is 0.16%, which corresponds to a number needed to screen of 625.
To put this in perspective, the number of people who need to be screened using fecal occult blood testing to prevent one death from colon cancer is 808, and the number of women who need to undergo mammography to prevent one breast cancer death is 1,887.21,22
Criteria for a good screening test
3. Which of the following is not one of the World Health Organization’s guiding principles for adopting a screening test?
- The disease must be common, or it must have grave consequences if it is not detected
- The disease must be detectable in a latent or early stage
- A screening test must exist that is acceptable to patients
- A treatment must exist that affects the natural history of the disease and its prognosis
- The cost of screening must be reasonable
- The screening test must have high sensitivity and specificity
In 1968, the World Health Organization published guidelines that continue to be used to determine the acceptability of screening tests.23 These principles state that for a screening test to be acceptable, the disease must be highly prevalent or result in grave consequences if not detected. The disease must have a latent or early stage in which it can be detected, and treatment must be available at that stage that affects the natural history and prognosis of the illness. The test must also be acceptable to patients physically, and the cost of it should be balanced in relation to possible expenditure on medical care as a whole.
As discussed previously, abdominal aortic aneurysms are common, and the consequences of rupture are grave. If the condition is detected early, treatment is available that can be lifesaving. Additionally, abdominal ultrasonography is noninvasive and inexpensive (costing roughly a few hundred dollars).24 Therefore, all of the World Health Organization criteria are satisfied. Improved outcomes with newer endovascular techniques for repair23 will likely also improve the value of screening.
Although high sensitivity and specificity are not required to satisfy the criteria, abdominal ultrasonography is nearly 100% sensitive and specific for detecting abdominal aortic aneurysms in patients without symptoms.12
Given the prevalence of the disease, by one estimate, if current USPSTF guidelines are followed (ie, if we screen only men age 65 to 75 who have ever smoked), for every 20 men we screen, we would detect one abdominal aortic aneurysm, and we would detect 29.5% of all of these aneurysms.2 If we screen all patients age 50 to 84, 74 people would need to be screened to detect one abdominal aortic aneurysm, but a much greater percentage of all of these aneurysms would be detected.
SHOULD OTHER GROUPS BE SCREENED?
4. The patient has a 40-year-old daughter who has hypertension and a 20-pack-year history of smoking. Should she be screened for an abdominal aortic aneurysm?
- Yes
- No
The 2005 USPSTF report recommends onetime ultrasonographic screening for all men age 65 to 75 who have ever smoked.1
The American Heart Association made a similar recommendation in 2005 in conjunction with the Society for Vascular Surgery, the American Association of Vascular Surgery, the Society for Vascular Medicine and Biology, and others.25 However, these groups also support screening men age 60 and older who are siblings or children of patients with abdominal aortic aneurysms, using physical examination and abdominal ultrasonography.
Both of the guidelines exclude women (who account for 41% of all deaths from this disease by one estimate) and nonsmokers (who account for 22%).2
The USPSTF makes no recommendation about nonsmokers, but it specifically recommends against screening women, stating that women have a low prevalence of large abdominal aortic aneurysms and that few women die of this disease. Therefore, according to the USPSTF, the risks of early treatment in women—including morbidity and death with surgical treatment and associated psychological harms—are not worth the benefits.1
However, a study of 3.1 million Americans found that women who have multiple cardiovascular risk factors such as smoking, hypertension, hyperlipidemia, and a family history of abdominal aortic aneurysm are at as great or greater risk of abdominal aortic aneurysm as men who fit the USPSTF criteria.2 Additionally, a positive family history of abdominal aortic aneurysm was among the strongest predictors of a diagnosis of abdominal aortic aneurysm on screening.2
Since 2005, newer guidelines have been released that broaden the recommendations for who should be screened. The Society for Vascular Surgery12 recommends screening:
- All men age 65 and older
- Men age 55 and older and women age 65 and older who have a family history of abdominal aortic aneurysm
- Women age 65 and older who have ever smoked.
A recent Swedish study demonstrated that the prevalence of abdominal aortic aneurysms in siblings of patients known to have this condition is significantly higher than in the general population; of the siblings who were screened, 11% had an abdominal aortic aneurysm, as did 17% of brothers and 6% of sisters.26
Nevertheless, broadened screening remains controversial, and more investigations of family history-based screening are ongoing.
WHEN DOES AN ABDOMINAL AORTIC ANEURYSM NEED SURGERY ?
Our patient was diagnosed with an infrarenal abdominal aortic aneurysm 6.5 cm in diameter and with bilateral common iliac artery aneurysms measuring 3.8 cm on the left and 5.2 cm on the right.
Computed tomography (CT) was done for preoperative planning (Figures 1 and 2), as it can define the aneurysm better for surgical intervention. Ultrasonography, while nearly 99% sensitive and specific for finding abdominal aortic aneurysms,12 does not provide the view of the abdominal anatomy that may be needed in surgical planning. The patient was seen by a vascular surgeon, and appropriate preoperative testing was done; the results showed that his risk during an open surgical procedure would be slightly above average.
The decision that needed to be made in this case was whether the patient should undergo surgery (either open or endovascular) or only medical intervention. In two randomized controlled trials comparing immediate intervention vs ongoing surveillance, the best threshold for surgical intervention was an aneurysm larger than 5.5 cm.27–29 Both trials found no benefit in terms of survival with surgical repair of aneurysms 4.0 to 5.4 cm: there was no long-term difference in the rate of survival in patients who underwent early surgical intervention compared with surveillance until the aneurysm was larger than 5.5 cm.
But this was with open surgery. What about endovascular repair? More recent studies that evaluated endovascular repair of small aneurysms (4.0–5.0 cm) found no improvement in end points, including time to aneurysm rupture and rate of aneurysm-related death, compared with surveillance.30,31
Treat risk factors
Medical therapy currently focuses on reducing risk factors for aneurysm growth and rupture, including hypertension, hyperlipidemia, and smoking, but research is focusing on angiotensin-converting enzyme inhibitors and experimental agents such as metalloproteinase inhibitors.32,33
Smoking is a major risk factor in the development, growth, and rupture of abdominal aortic aneurysms,34 and the 2005 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) recommend that everyone with an abdominal aortic aneurysm or a family history of it be advised to stop smoking.25 This is especially important in light of data that show a higher risk of abdominal aortic aneurysm with a higher volume of smoking (total pack-years) and a decrease in risk with time since quitting.2
Medical management also includes treating other associated cardiovascular risk factors, including hypertension and dyslipidemia. The ACC/AHA guidelines recommend that patients with abdominal aortic aneurysms be treated similarly to patients with atherosclerotic disease or a coronary artery disease equivalent, including giving them a statin and an antiplatelet drug such as aspirin.
The ACC/AHA guidelines also recommend that patients who are managed medically and have an aneurysm of 3.0 to 4.0 cm undergo ultrasonographic monitoring every 2 to 3 years, and those with an aneurysm of 4.0 to 5.4 cm undergo monitoring with ultrasonography or CT every 6 to 12 months.25
5. Which of the following is the treatment of choice for our patient’s high blood pressure?
- Propranolol
- Lisinopril
- Hydralazine
- Hydrochlorothiazide
The recommended agents for blood pressure control in this patient population are betablockers, such as propranolol. In a small study of patients with infrarenal aortic aneurysms, beta-blockers reduced the mean expansion rate from 0.68 cm/year to 0.36 cm/year, although larger trials have not yet confirmed this benefit.35,36 The 2005 ACC/AHA guidelines recommend beta-blockers for patients who are being managed medically.25 Other antihypertensive drugs can be added to achieve optimal blood pressure control after the addition of a beta-blocker.
Open vs endovascular repair
If a patient has an abdominal aortic aneurysm larger than 5.5 cm or if the benefits of surgery are determined to outweigh the risks, a surgical plan should be developed. Patients should be evaluated for surgical risk factors, and this should guide the choice of surgical approach—ie, open repair or endovascular repair.
Compared with open repair, endovascular repair has been increasing in popularity. It has a lower rate of complications, including a significantly lower rate of perioperative death, even though patients who undergo endovascular repair are on average significantly older than those who undergo open repair.37–39
Endovascular repair is performed with open or percutaneous access of the common femoral artery. An endograft, which is packed into an introductory sheath, is introduced into the aorta and expands upon unsheathing. It is positioned to “land” in sealing zones of normal-caliber aorta, where it seals to exclude the aneurysm from circulatory flow (Figure 3).
This is different from the open approach in that it avoids the large incision and aortic cross-clamping necessary in open repair. In open repair, a large incision is made in the patient’s abdomen and the aorta is cross-clamped to stop blood flow. The aneurysm is then incised and a graft is sutured into place to protect the vessel wall from stress (Figure 4).
CASE CONCLUDED
Our patient elected to undergo endovascular repair of his aneurysm with a bifurcated graft (Figure 3). He was able to walk the day after his procedure, and he was sent home that same day. According to the guidelines of the Society for Vascular Surgery,40 he will have surveillance CT angiography at 1 and 12 months to detect “endoleak” or aneurysm enlargement. If these are not seen, he will then undergo routine surveillance with abdominal duplex ultrasonography.
- US Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med 2005; 142:198–202.
- Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 2010; 52:539–548.
- Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ 2005; 330:750.
- Ashton HA, Buxton MJ, Day NE, et al; Multicentre Aneurysm Screening Study Group. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002; 360:1531–1539.
- Norman PE, Jamrozik K, Lawrence-Brown MM, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 2004; 329:1259.
- Vardulaki KA, Walker NM, Couto E, et al. Late results concerning feasibility and compliance from a randomized trial of ultrasonographic screening for abdominal aortic aneurysm. Br J Surg 2002; 89:861–864.
- Derubertis BG, Trocciola SM, Ryer EJ, et al. Abdominal aortic aneurysm in women: prevalence, risk factors, and implications for screening. J Vasc Surg 2007; 46:630–635.
- Sandiford P, Mosquera D, Bramley D. Trends in incidence and mortality from abdominal aortic aneurysm in New Zealand. Br J Surg 2011; 98:645–651.
- Anjum A, Powell JT. Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg 2012; 43:161–166.
- Anderson RN. Deaths: leading causes for 2000. Natl Vital Stat Rep 2002; 50:1–85.
- Kent KC, Zwolak RM, Jaff MR, et al; Society for Vascular Surgery; American Association of Vascular Surgery; Society for Vascular Medicine and Biology. Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg 2004; 39:267–269.
- Chaikof EL, Brewster DC, Dalman RL, et al; Society for Vascular Surgery. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg 2009; 50(suppl 4):S2–S49.
- Fink HA, Lederle FA, Roth CS, Bowles CA, Nelson DB, Haas MA. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med 2000; 160:833–836.
- Reed WW, Hallett JW, Damiano MA, Ballard DJ. Learning from the last ultrasound. A population-based study of patients with abdominal aortic aneurysm. Arch Intern Med 1997; 157:2064–2068.
- Bernstein EF, Dilley RB, Goldberger LE, Gosink BB, Leopold GR. Growth rates of small abdominal aortic aneurysms. Surgery 1976; 80:765–773.
- Cronenwett JL, Sargent SK, Wall MH, et al. Variables that affect the expansion rate and outcome of small abdominal aortic aneurysms. J Vasc Surg 1990; 11:260–268.
- Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg 2002; 89:283–285.
- Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med 2005; 142:203–211.
- Lindholt JS, Sørensen J, Søgaard R, Henneberg EW. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg 2010; 97:826–834.
- Thompson SG, Ashton HA, Gao L, Scott RA; Multicentre Aneurysm Screening Study Group. Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised Multicentre Aneurysm Screening Study. BMJ 2009; 338:b2307.
- Mastracci TM, Cina CS. Regarding Screening for abdominal aortic aneurysm reduces both aneurysm-related and all-cause mortality (letter). J Vasc Surg 2007; 46:1312.
- Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ 1998; 317:307–312.
- Wilson JMG, Jungner G. Principles and practice of screening for disease. World Health Organization. Public Health Papers #34.
- Lee TY, Korn P, Heller JA, et al. The cost-effectiveness of a “quickscreen” program for abdominal aortic aneurysms. Surgery 2002; 132:399–407.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
- Linné A, Lindström D, Hultgren R. High prevalence of abdominal aortic aneurysms in brothers and sisters of patients despite a low prevalence in the population. J Vasc Surg 2012; 56:305–310.
- The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet 1998; 352:1649–1655.
- Lederle FA, Johnson GR, Wilson SE, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med 1997; 126:441–449.
- Brewster DC, Cronenwett JL, Hallett JW, Johnston KW, Krupski WC, Matsumura JS; Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg 2003; 37:1106–117.
- Ouriel K, Clair DG, Kent KC, Zarins CK; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg 2010; 51:1081–1087.
- De Rango P, Verzini F, Parlani G; Comparison of surveillance vs Aortic Endografting for Small Aneurysm Repair (CAESAR) Investigators. Quality of life in patients with small abdominal aortic aneurysm: the effect of early endovascular repair versus surveillance in the CAESAR trial. Eur J Vasc Endovasc Surg 2011; 41:324–331.
- Antoniou GA, Lazarides MK, Patera S, et al. Assessment of insertion/deletion polymorphism of the angiotensin-converting enzyme gene in abdominal aortic aneurysm and inguinal hernia. Vascular 2012; Epub ahead of print.
- Ogata T, Shibamura H, Tromp G, et al. Genetic analysis of polymorphisms in biologically relevant candidate genes in patients with abdominal aortic aneurysms. J Vasc Surg 2005; 41:1036–1042.
- Powell JT, Greenhalgh RM. Clinical practice. Small abdominal aortic aneurysms. N Engl J Med 2003; 348:1895–1901.
- Gadowski GR, Pilcher DB, Ricci MA. Abdominal aortic aneurysm expansion rate: effect of size and beta-adrenergic blockade. J Vasc Surg 1994; 19:727–731.
- Propanolol Aneurysm Trial Investigators. Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg 2002; 35:72–79.
- Jackson RS, Chang DC, Freischlag JA. Comparison of long-term survival after open vs endovascular repair of intact abdominal aortic aneurysm among Medicare beneficiaries. JAMA 2012; 307:1621–1628.
- Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg 2006; 43:446–451.
- Giles KA, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn ML. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg 2009; 49:543–550.
- Chaikof EL, Blankensteijn JD, Harris PL, et al; Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2002; 35:1048–1060.
A 67-year-old man presented for evaluation of an abdominal aortic aneurysm, noted 1 month previously after his primary care physician ordered screening ultrasonography as part of a routine annual physical examination. The man was experiencing no symptoms.
He had type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, and hyperlipidemia. He smoked two packs of cigarettes a day. He had never had surgery. His current medications included diltiazem, fenofibrate, niacin, and aspirin; because he had chronic obstructive pulmonary disease, he was not on a beta-blocker.
His father had died suddenly at the age of 77; his death was attributed to a cardiac cause, but no formal autopsy was performed. Neither the patient’s siblings nor his children were screened for aneurysms.
On physical examination, he was comfortable and in no acute distress. His blood pressure was 156/71 mm Hg, pulse 60, temperature 36.1°C (97.0°F), and body mass index 30.15 kg/m2, which is in the obese range.
He had no jugular venous distention, no carotid bruits, and no lymphadenopathy. The cardiac examination was unremarkable, with regular rate, normal sinus rhythm, and no murmurs. On pulmonary examination, inspiratory and expiratory wheezes were noted in all lung fields.
His abdomen was obese but not tender to palpation. The aneurysm was not palpable. His pedal pulses were normal. The remainder of the examination was normal.
WHO SHOULD BE SCREENED?
1. For which of the following groups does the United States Preventive Services Task Force (USPSTF) strongly recommend screening for abdominal aortic aneurysms?
- Men and women over age 65
- Men and women who have ever smoked and are over age 65
- Men over age 75 and men over age 65 who smoke
- Men age 65 to 75 who have ever smoked
In 2005, the USPSTF recommended one-time screening ultrasonography for all men age 65 to 75 who have ever smoked. On the basis of evidence available at the time, it made no recommendation for men age 65 to 75 who have never smoked, and it recommended against screening women.1
ANEURYSMS ARE COMMON, OFTEN ASYMPTOMATIC, UNTIL THEY RUPTURE
Abdominal aortic aneurysms are relatively common in older adults, with a prevalence of 1.4% in the US population age 50 to 84 years.2 In four randomized controlled trials of aneurysm screening in Europe and Australia, the prevalence of any aneurysm (not just abdominal aortic aneurysms) in men was 6% (95% confidence interval 5–6).3–6
Fewer studies are available on the prevalence in women. One study found a prevalence of 0.7% in 10,012 US women, compared with 3.9% in men.7
In a recent report of the aneurysm screening program in the United Kingdom, the incidence of aneurysms had decreased from historically reported estimates.8,9
In the year 2000, abdominal aortic aneurysms caused 15,000 deaths in the United States and were the 10th leading cause of death in white men age 65 to 74.10 The actual number of deaths may be larger, since some people may die suddenly of an aneurysm with no evaluation for attributable cause.11
Aortic aneurysms are often asymptomatic until they rupture, making them difficult to detect without a focused screening program. The goal of treatment is to avoid spontaneous rupture and death. When aneurysms rupture, the estimated death rate is 80%.6
EVIDENCE IN FAVOR OF SCREENING
Ultrasonography is nearly 100% sensitive and specific in detecting abdominal aortic aneurysms in patients without symptoms.12 In comparison, abdominal palpation is 68% sensitive and 75% specific.13
The larger the aneurysm, the higher the risk of rupture.14–16 The annual risk of rupture is:
- 0.5% with aneurysms smaller than 4.0 cm
- 1.0% with aneurysms 4.0–4.9 cm
- 11% with aneurysms 5.0–5.9 cm
- 26% with aneurysms 6.0–6.9 cm.
Several large randomized controlled trials in men over age 65 evaluated the effect of screening programs for abdominal aortic aneurysms on the rate of deaths from this cause.3–6,17 A meta-analysis of these trials found a relative risk of 0.60 in favor of screening—ie, men over age 65 who were screened had a 40% lower risk of dying of an abdominal aortic aneurysm than men who were not screened.18 In long-term follow-up, the rate continued to be about 50% lower with screening than without.19,20 The absolute reduction in risk of death was 0.13%.21
Absolute risk reduction and number needed to screen
2. If screening offers an absolute risk reduction in the death rate of 0.13%, how many need to be screened to prevent one death?
- 769
- 856
- 1,300
- 13,000
The number of patients that need to be screened to prevent one death is called the number needed to screen.22 It is calculated as 1 divided by the absolute risk reduction. Therefore, in screening for abdominal aortic aneurysms, the number needed to screen is 1/0.0013, or 769. Recall that these numbers are from men over age 65, with no upper limit in age. If we consider only men age 65 to 75, the absolute risk reduction is 0.16%, which corresponds to a number needed to screen of 625.
To put this in perspective, the number of people who need to be screened using fecal occult blood testing to prevent one death from colon cancer is 808, and the number of women who need to undergo mammography to prevent one breast cancer death is 1,887.21,22
Criteria for a good screening test
3. Which of the following is not one of the World Health Organization’s guiding principles for adopting a screening test?
- The disease must be common, or it must have grave consequences if it is not detected
- The disease must be detectable in a latent or early stage
- A screening test must exist that is acceptable to patients
- A treatment must exist that affects the natural history of the disease and its prognosis
- The cost of screening must be reasonable
- The screening test must have high sensitivity and specificity
In 1968, the World Health Organization published guidelines that continue to be used to determine the acceptability of screening tests.23 These principles state that for a screening test to be acceptable, the disease must be highly prevalent or result in grave consequences if not detected. The disease must have a latent or early stage in which it can be detected, and treatment must be available at that stage that affects the natural history and prognosis of the illness. The test must also be acceptable to patients physically, and the cost of it should be balanced in relation to possible expenditure on medical care as a whole.
As discussed previously, abdominal aortic aneurysms are common, and the consequences of rupture are grave. If the condition is detected early, treatment is available that can be lifesaving. Additionally, abdominal ultrasonography is noninvasive and inexpensive (costing roughly a few hundred dollars).24 Therefore, all of the World Health Organization criteria are satisfied. Improved outcomes with newer endovascular techniques for repair23 will likely also improve the value of screening.
Although high sensitivity and specificity are not required to satisfy the criteria, abdominal ultrasonography is nearly 100% sensitive and specific for detecting abdominal aortic aneurysms in patients without symptoms.12
Given the prevalence of the disease, by one estimate, if current USPSTF guidelines are followed (ie, if we screen only men age 65 to 75 who have ever smoked), for every 20 men we screen, we would detect one abdominal aortic aneurysm, and we would detect 29.5% of all of these aneurysms.2 If we screen all patients age 50 to 84, 74 people would need to be screened to detect one abdominal aortic aneurysm, but a much greater percentage of all of these aneurysms would be detected.
SHOULD OTHER GROUPS BE SCREENED?
4. The patient has a 40-year-old daughter who has hypertension and a 20-pack-year history of smoking. Should she be screened for an abdominal aortic aneurysm?
- Yes
- No
The 2005 USPSTF report recommends onetime ultrasonographic screening for all men age 65 to 75 who have ever smoked.1
The American Heart Association made a similar recommendation in 2005 in conjunction with the Society for Vascular Surgery, the American Association of Vascular Surgery, the Society for Vascular Medicine and Biology, and others.25 However, these groups also support screening men age 60 and older who are siblings or children of patients with abdominal aortic aneurysms, using physical examination and abdominal ultrasonography.
Both of the guidelines exclude women (who account for 41% of all deaths from this disease by one estimate) and nonsmokers (who account for 22%).2
The USPSTF makes no recommendation about nonsmokers, but it specifically recommends against screening women, stating that women have a low prevalence of large abdominal aortic aneurysms and that few women die of this disease. Therefore, according to the USPSTF, the risks of early treatment in women—including morbidity and death with surgical treatment and associated psychological harms—are not worth the benefits.1
However, a study of 3.1 million Americans found that women who have multiple cardiovascular risk factors such as smoking, hypertension, hyperlipidemia, and a family history of abdominal aortic aneurysm are at as great or greater risk of abdominal aortic aneurysm as men who fit the USPSTF criteria.2 Additionally, a positive family history of abdominal aortic aneurysm was among the strongest predictors of a diagnosis of abdominal aortic aneurysm on screening.2
Since 2005, newer guidelines have been released that broaden the recommendations for who should be screened. The Society for Vascular Surgery12 recommends screening:
- All men age 65 and older
- Men age 55 and older and women age 65 and older who have a family history of abdominal aortic aneurysm
- Women age 65 and older who have ever smoked.
A recent Swedish study demonstrated that the prevalence of abdominal aortic aneurysms in siblings of patients known to have this condition is significantly higher than in the general population; of the siblings who were screened, 11% had an abdominal aortic aneurysm, as did 17% of brothers and 6% of sisters.26
Nevertheless, broadened screening remains controversial, and more investigations of family history-based screening are ongoing.
WHEN DOES AN ABDOMINAL AORTIC ANEURYSM NEED SURGERY ?
Our patient was diagnosed with an infrarenal abdominal aortic aneurysm 6.5 cm in diameter and with bilateral common iliac artery aneurysms measuring 3.8 cm on the left and 5.2 cm on the right.
Computed tomography (CT) was done for preoperative planning (Figures 1 and 2), as it can define the aneurysm better for surgical intervention. Ultrasonography, while nearly 99% sensitive and specific for finding abdominal aortic aneurysms,12 does not provide the view of the abdominal anatomy that may be needed in surgical planning. The patient was seen by a vascular surgeon, and appropriate preoperative testing was done; the results showed that his risk during an open surgical procedure would be slightly above average.
The decision that needed to be made in this case was whether the patient should undergo surgery (either open or endovascular) or only medical intervention. In two randomized controlled trials comparing immediate intervention vs ongoing surveillance, the best threshold for surgical intervention was an aneurysm larger than 5.5 cm.27–29 Both trials found no benefit in terms of survival with surgical repair of aneurysms 4.0 to 5.4 cm: there was no long-term difference in the rate of survival in patients who underwent early surgical intervention compared with surveillance until the aneurysm was larger than 5.5 cm.
But this was with open surgery. What about endovascular repair? More recent studies that evaluated endovascular repair of small aneurysms (4.0–5.0 cm) found no improvement in end points, including time to aneurysm rupture and rate of aneurysm-related death, compared with surveillance.30,31
Treat risk factors
Medical therapy currently focuses on reducing risk factors for aneurysm growth and rupture, including hypertension, hyperlipidemia, and smoking, but research is focusing on angiotensin-converting enzyme inhibitors and experimental agents such as metalloproteinase inhibitors.32,33
Smoking is a major risk factor in the development, growth, and rupture of abdominal aortic aneurysms,34 and the 2005 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) recommend that everyone with an abdominal aortic aneurysm or a family history of it be advised to stop smoking.25 This is especially important in light of data that show a higher risk of abdominal aortic aneurysm with a higher volume of smoking (total pack-years) and a decrease in risk with time since quitting.2
Medical management also includes treating other associated cardiovascular risk factors, including hypertension and dyslipidemia. The ACC/AHA guidelines recommend that patients with abdominal aortic aneurysms be treated similarly to patients with atherosclerotic disease or a coronary artery disease equivalent, including giving them a statin and an antiplatelet drug such as aspirin.
The ACC/AHA guidelines also recommend that patients who are managed medically and have an aneurysm of 3.0 to 4.0 cm undergo ultrasonographic monitoring every 2 to 3 years, and those with an aneurysm of 4.0 to 5.4 cm undergo monitoring with ultrasonography or CT every 6 to 12 months.25
5. Which of the following is the treatment of choice for our patient’s high blood pressure?
- Propranolol
- Lisinopril
- Hydralazine
- Hydrochlorothiazide
The recommended agents for blood pressure control in this patient population are betablockers, such as propranolol. In a small study of patients with infrarenal aortic aneurysms, beta-blockers reduced the mean expansion rate from 0.68 cm/year to 0.36 cm/year, although larger trials have not yet confirmed this benefit.35,36 The 2005 ACC/AHA guidelines recommend beta-blockers for patients who are being managed medically.25 Other antihypertensive drugs can be added to achieve optimal blood pressure control after the addition of a beta-blocker.
Open vs endovascular repair
If a patient has an abdominal aortic aneurysm larger than 5.5 cm or if the benefits of surgery are determined to outweigh the risks, a surgical plan should be developed. Patients should be evaluated for surgical risk factors, and this should guide the choice of surgical approach—ie, open repair or endovascular repair.
Compared with open repair, endovascular repair has been increasing in popularity. It has a lower rate of complications, including a significantly lower rate of perioperative death, even though patients who undergo endovascular repair are on average significantly older than those who undergo open repair.37–39
Endovascular repair is performed with open or percutaneous access of the common femoral artery. An endograft, which is packed into an introductory sheath, is introduced into the aorta and expands upon unsheathing. It is positioned to “land” in sealing zones of normal-caliber aorta, where it seals to exclude the aneurysm from circulatory flow (Figure 3).
This is different from the open approach in that it avoids the large incision and aortic cross-clamping necessary in open repair. In open repair, a large incision is made in the patient’s abdomen and the aorta is cross-clamped to stop blood flow. The aneurysm is then incised and a graft is sutured into place to protect the vessel wall from stress (Figure 4).
CASE CONCLUDED
Our patient elected to undergo endovascular repair of his aneurysm with a bifurcated graft (Figure 3). He was able to walk the day after his procedure, and he was sent home that same day. According to the guidelines of the Society for Vascular Surgery,40 he will have surveillance CT angiography at 1 and 12 months to detect “endoleak” or aneurysm enlargement. If these are not seen, he will then undergo routine surveillance with abdominal duplex ultrasonography.
A 67-year-old man presented for evaluation of an abdominal aortic aneurysm, noted 1 month previously after his primary care physician ordered screening ultrasonography as part of a routine annual physical examination. The man was experiencing no symptoms.
He had type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, and hyperlipidemia. He smoked two packs of cigarettes a day. He had never had surgery. His current medications included diltiazem, fenofibrate, niacin, and aspirin; because he had chronic obstructive pulmonary disease, he was not on a beta-blocker.
His father had died suddenly at the age of 77; his death was attributed to a cardiac cause, but no formal autopsy was performed. Neither the patient’s siblings nor his children were screened for aneurysms.
On physical examination, he was comfortable and in no acute distress. His blood pressure was 156/71 mm Hg, pulse 60, temperature 36.1°C (97.0°F), and body mass index 30.15 kg/m2, which is in the obese range.
He had no jugular venous distention, no carotid bruits, and no lymphadenopathy. The cardiac examination was unremarkable, with regular rate, normal sinus rhythm, and no murmurs. On pulmonary examination, inspiratory and expiratory wheezes were noted in all lung fields.
His abdomen was obese but not tender to palpation. The aneurysm was not palpable. His pedal pulses were normal. The remainder of the examination was normal.
WHO SHOULD BE SCREENED?
1. For which of the following groups does the United States Preventive Services Task Force (USPSTF) strongly recommend screening for abdominal aortic aneurysms?
- Men and women over age 65
- Men and women who have ever smoked and are over age 65
- Men over age 75 and men over age 65 who smoke
- Men age 65 to 75 who have ever smoked
In 2005, the USPSTF recommended one-time screening ultrasonography for all men age 65 to 75 who have ever smoked. On the basis of evidence available at the time, it made no recommendation for men age 65 to 75 who have never smoked, and it recommended against screening women.1
ANEURYSMS ARE COMMON, OFTEN ASYMPTOMATIC, UNTIL THEY RUPTURE
Abdominal aortic aneurysms are relatively common in older adults, with a prevalence of 1.4% in the US population age 50 to 84 years.2 In four randomized controlled trials of aneurysm screening in Europe and Australia, the prevalence of any aneurysm (not just abdominal aortic aneurysms) in men was 6% (95% confidence interval 5–6).3–6
Fewer studies are available on the prevalence in women. One study found a prevalence of 0.7% in 10,012 US women, compared with 3.9% in men.7
In a recent report of the aneurysm screening program in the United Kingdom, the incidence of aneurysms had decreased from historically reported estimates.8,9
In the year 2000, abdominal aortic aneurysms caused 15,000 deaths in the United States and were the 10th leading cause of death in white men age 65 to 74.10 The actual number of deaths may be larger, since some people may die suddenly of an aneurysm with no evaluation for attributable cause.11
Aortic aneurysms are often asymptomatic until they rupture, making them difficult to detect without a focused screening program. The goal of treatment is to avoid spontaneous rupture and death. When aneurysms rupture, the estimated death rate is 80%.6
EVIDENCE IN FAVOR OF SCREENING
Ultrasonography is nearly 100% sensitive and specific in detecting abdominal aortic aneurysms in patients without symptoms.12 In comparison, abdominal palpation is 68% sensitive and 75% specific.13
The larger the aneurysm, the higher the risk of rupture.14–16 The annual risk of rupture is:
- 0.5% with aneurysms smaller than 4.0 cm
- 1.0% with aneurysms 4.0–4.9 cm
- 11% with aneurysms 5.0–5.9 cm
- 26% with aneurysms 6.0–6.9 cm.
Several large randomized controlled trials in men over age 65 evaluated the effect of screening programs for abdominal aortic aneurysms on the rate of deaths from this cause.3–6,17 A meta-analysis of these trials found a relative risk of 0.60 in favor of screening—ie, men over age 65 who were screened had a 40% lower risk of dying of an abdominal aortic aneurysm than men who were not screened.18 In long-term follow-up, the rate continued to be about 50% lower with screening than without.19,20 The absolute reduction in risk of death was 0.13%.21
Absolute risk reduction and number needed to screen
2. If screening offers an absolute risk reduction in the death rate of 0.13%, how many need to be screened to prevent one death?
- 769
- 856
- 1,300
- 13,000
The number of patients that need to be screened to prevent one death is called the number needed to screen.22 It is calculated as 1 divided by the absolute risk reduction. Therefore, in screening for abdominal aortic aneurysms, the number needed to screen is 1/0.0013, or 769. Recall that these numbers are from men over age 65, with no upper limit in age. If we consider only men age 65 to 75, the absolute risk reduction is 0.16%, which corresponds to a number needed to screen of 625.
To put this in perspective, the number of people who need to be screened using fecal occult blood testing to prevent one death from colon cancer is 808, and the number of women who need to undergo mammography to prevent one breast cancer death is 1,887.21,22
Criteria for a good screening test
3. Which of the following is not one of the World Health Organization’s guiding principles for adopting a screening test?
- The disease must be common, or it must have grave consequences if it is not detected
- The disease must be detectable in a latent or early stage
- A screening test must exist that is acceptable to patients
- A treatment must exist that affects the natural history of the disease and its prognosis
- The cost of screening must be reasonable
- The screening test must have high sensitivity and specificity
In 1968, the World Health Organization published guidelines that continue to be used to determine the acceptability of screening tests.23 These principles state that for a screening test to be acceptable, the disease must be highly prevalent or result in grave consequences if not detected. The disease must have a latent or early stage in which it can be detected, and treatment must be available at that stage that affects the natural history and prognosis of the illness. The test must also be acceptable to patients physically, and the cost of it should be balanced in relation to possible expenditure on medical care as a whole.
As discussed previously, abdominal aortic aneurysms are common, and the consequences of rupture are grave. If the condition is detected early, treatment is available that can be lifesaving. Additionally, abdominal ultrasonography is noninvasive and inexpensive (costing roughly a few hundred dollars).24 Therefore, all of the World Health Organization criteria are satisfied. Improved outcomes with newer endovascular techniques for repair23 will likely also improve the value of screening.
Although high sensitivity and specificity are not required to satisfy the criteria, abdominal ultrasonography is nearly 100% sensitive and specific for detecting abdominal aortic aneurysms in patients without symptoms.12
Given the prevalence of the disease, by one estimate, if current USPSTF guidelines are followed (ie, if we screen only men age 65 to 75 who have ever smoked), for every 20 men we screen, we would detect one abdominal aortic aneurysm, and we would detect 29.5% of all of these aneurysms.2 If we screen all patients age 50 to 84, 74 people would need to be screened to detect one abdominal aortic aneurysm, but a much greater percentage of all of these aneurysms would be detected.
SHOULD OTHER GROUPS BE SCREENED?
4. The patient has a 40-year-old daughter who has hypertension and a 20-pack-year history of smoking. Should she be screened for an abdominal aortic aneurysm?
- Yes
- No
The 2005 USPSTF report recommends onetime ultrasonographic screening for all men age 65 to 75 who have ever smoked.1
The American Heart Association made a similar recommendation in 2005 in conjunction with the Society for Vascular Surgery, the American Association of Vascular Surgery, the Society for Vascular Medicine and Biology, and others.25 However, these groups also support screening men age 60 and older who are siblings or children of patients with abdominal aortic aneurysms, using physical examination and abdominal ultrasonography.
Both of the guidelines exclude women (who account for 41% of all deaths from this disease by one estimate) and nonsmokers (who account for 22%).2
The USPSTF makes no recommendation about nonsmokers, but it specifically recommends against screening women, stating that women have a low prevalence of large abdominal aortic aneurysms and that few women die of this disease. Therefore, according to the USPSTF, the risks of early treatment in women—including morbidity and death with surgical treatment and associated psychological harms—are not worth the benefits.1
However, a study of 3.1 million Americans found that women who have multiple cardiovascular risk factors such as smoking, hypertension, hyperlipidemia, and a family history of abdominal aortic aneurysm are at as great or greater risk of abdominal aortic aneurysm as men who fit the USPSTF criteria.2 Additionally, a positive family history of abdominal aortic aneurysm was among the strongest predictors of a diagnosis of abdominal aortic aneurysm on screening.2
Since 2005, newer guidelines have been released that broaden the recommendations for who should be screened. The Society for Vascular Surgery12 recommends screening:
- All men age 65 and older
- Men age 55 and older and women age 65 and older who have a family history of abdominal aortic aneurysm
- Women age 65 and older who have ever smoked.
A recent Swedish study demonstrated that the prevalence of abdominal aortic aneurysms in siblings of patients known to have this condition is significantly higher than in the general population; of the siblings who were screened, 11% had an abdominal aortic aneurysm, as did 17% of brothers and 6% of sisters.26
Nevertheless, broadened screening remains controversial, and more investigations of family history-based screening are ongoing.
WHEN DOES AN ABDOMINAL AORTIC ANEURYSM NEED SURGERY ?
Our patient was diagnosed with an infrarenal abdominal aortic aneurysm 6.5 cm in diameter and with bilateral common iliac artery aneurysms measuring 3.8 cm on the left and 5.2 cm on the right.
Computed tomography (CT) was done for preoperative planning (Figures 1 and 2), as it can define the aneurysm better for surgical intervention. Ultrasonography, while nearly 99% sensitive and specific for finding abdominal aortic aneurysms,12 does not provide the view of the abdominal anatomy that may be needed in surgical planning. The patient was seen by a vascular surgeon, and appropriate preoperative testing was done; the results showed that his risk during an open surgical procedure would be slightly above average.
The decision that needed to be made in this case was whether the patient should undergo surgery (either open or endovascular) or only medical intervention. In two randomized controlled trials comparing immediate intervention vs ongoing surveillance, the best threshold for surgical intervention was an aneurysm larger than 5.5 cm.27–29 Both trials found no benefit in terms of survival with surgical repair of aneurysms 4.0 to 5.4 cm: there was no long-term difference in the rate of survival in patients who underwent early surgical intervention compared with surveillance until the aneurysm was larger than 5.5 cm.
But this was with open surgery. What about endovascular repair? More recent studies that evaluated endovascular repair of small aneurysms (4.0–5.0 cm) found no improvement in end points, including time to aneurysm rupture and rate of aneurysm-related death, compared with surveillance.30,31
Treat risk factors
Medical therapy currently focuses on reducing risk factors for aneurysm growth and rupture, including hypertension, hyperlipidemia, and smoking, but research is focusing on angiotensin-converting enzyme inhibitors and experimental agents such as metalloproteinase inhibitors.32,33
Smoking is a major risk factor in the development, growth, and rupture of abdominal aortic aneurysms,34 and the 2005 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) recommend that everyone with an abdominal aortic aneurysm or a family history of it be advised to stop smoking.25 This is especially important in light of data that show a higher risk of abdominal aortic aneurysm with a higher volume of smoking (total pack-years) and a decrease in risk with time since quitting.2
Medical management also includes treating other associated cardiovascular risk factors, including hypertension and dyslipidemia. The ACC/AHA guidelines recommend that patients with abdominal aortic aneurysms be treated similarly to patients with atherosclerotic disease or a coronary artery disease equivalent, including giving them a statin and an antiplatelet drug such as aspirin.
The ACC/AHA guidelines also recommend that patients who are managed medically and have an aneurysm of 3.0 to 4.0 cm undergo ultrasonographic monitoring every 2 to 3 years, and those with an aneurysm of 4.0 to 5.4 cm undergo monitoring with ultrasonography or CT every 6 to 12 months.25
5. Which of the following is the treatment of choice for our patient’s high blood pressure?
- Propranolol
- Lisinopril
- Hydralazine
- Hydrochlorothiazide
The recommended agents for blood pressure control in this patient population are betablockers, such as propranolol. In a small study of patients with infrarenal aortic aneurysms, beta-blockers reduced the mean expansion rate from 0.68 cm/year to 0.36 cm/year, although larger trials have not yet confirmed this benefit.35,36 The 2005 ACC/AHA guidelines recommend beta-blockers for patients who are being managed medically.25 Other antihypertensive drugs can be added to achieve optimal blood pressure control after the addition of a beta-blocker.
Open vs endovascular repair
If a patient has an abdominal aortic aneurysm larger than 5.5 cm or if the benefits of surgery are determined to outweigh the risks, a surgical plan should be developed. Patients should be evaluated for surgical risk factors, and this should guide the choice of surgical approach—ie, open repair or endovascular repair.
Compared with open repair, endovascular repair has been increasing in popularity. It has a lower rate of complications, including a significantly lower rate of perioperative death, even though patients who undergo endovascular repair are on average significantly older than those who undergo open repair.37–39
Endovascular repair is performed with open or percutaneous access of the common femoral artery. An endograft, which is packed into an introductory sheath, is introduced into the aorta and expands upon unsheathing. It is positioned to “land” in sealing zones of normal-caliber aorta, where it seals to exclude the aneurysm from circulatory flow (Figure 3).
This is different from the open approach in that it avoids the large incision and aortic cross-clamping necessary in open repair. In open repair, a large incision is made in the patient’s abdomen and the aorta is cross-clamped to stop blood flow. The aneurysm is then incised and a graft is sutured into place to protect the vessel wall from stress (Figure 4).
CASE CONCLUDED
Our patient elected to undergo endovascular repair of his aneurysm with a bifurcated graft (Figure 3). He was able to walk the day after his procedure, and he was sent home that same day. According to the guidelines of the Society for Vascular Surgery,40 he will have surveillance CT angiography at 1 and 12 months to detect “endoleak” or aneurysm enlargement. If these are not seen, he will then undergo routine surveillance with abdominal duplex ultrasonography.
- US Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med 2005; 142:198–202.
- Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 2010; 52:539–548.
- Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ 2005; 330:750.
- Ashton HA, Buxton MJ, Day NE, et al; Multicentre Aneurysm Screening Study Group. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002; 360:1531–1539.
- Norman PE, Jamrozik K, Lawrence-Brown MM, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 2004; 329:1259.
- Vardulaki KA, Walker NM, Couto E, et al. Late results concerning feasibility and compliance from a randomized trial of ultrasonographic screening for abdominal aortic aneurysm. Br J Surg 2002; 89:861–864.
- Derubertis BG, Trocciola SM, Ryer EJ, et al. Abdominal aortic aneurysm in women: prevalence, risk factors, and implications for screening. J Vasc Surg 2007; 46:630–635.
- Sandiford P, Mosquera D, Bramley D. Trends in incidence and mortality from abdominal aortic aneurysm in New Zealand. Br J Surg 2011; 98:645–651.
- Anjum A, Powell JT. Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg 2012; 43:161–166.
- Anderson RN. Deaths: leading causes for 2000. Natl Vital Stat Rep 2002; 50:1–85.
- Kent KC, Zwolak RM, Jaff MR, et al; Society for Vascular Surgery; American Association of Vascular Surgery; Society for Vascular Medicine and Biology. Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg 2004; 39:267–269.
- Chaikof EL, Brewster DC, Dalman RL, et al; Society for Vascular Surgery. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg 2009; 50(suppl 4):S2–S49.
- Fink HA, Lederle FA, Roth CS, Bowles CA, Nelson DB, Haas MA. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med 2000; 160:833–836.
- Reed WW, Hallett JW, Damiano MA, Ballard DJ. Learning from the last ultrasound. A population-based study of patients with abdominal aortic aneurysm. Arch Intern Med 1997; 157:2064–2068.
- Bernstein EF, Dilley RB, Goldberger LE, Gosink BB, Leopold GR. Growth rates of small abdominal aortic aneurysms. Surgery 1976; 80:765–773.
- Cronenwett JL, Sargent SK, Wall MH, et al. Variables that affect the expansion rate and outcome of small abdominal aortic aneurysms. J Vasc Surg 1990; 11:260–268.
- Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg 2002; 89:283–285.
- Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med 2005; 142:203–211.
- Lindholt JS, Sørensen J, Søgaard R, Henneberg EW. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg 2010; 97:826–834.
- Thompson SG, Ashton HA, Gao L, Scott RA; Multicentre Aneurysm Screening Study Group. Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised Multicentre Aneurysm Screening Study. BMJ 2009; 338:b2307.
- Mastracci TM, Cina CS. Regarding Screening for abdominal aortic aneurysm reduces both aneurysm-related and all-cause mortality (letter). J Vasc Surg 2007; 46:1312.
- Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ 1998; 317:307–312.
- Wilson JMG, Jungner G. Principles and practice of screening for disease. World Health Organization. Public Health Papers #34.
- Lee TY, Korn P, Heller JA, et al. The cost-effectiveness of a “quickscreen” program for abdominal aortic aneurysms. Surgery 2002; 132:399–407.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
- Linné A, Lindström D, Hultgren R. High prevalence of abdominal aortic aneurysms in brothers and sisters of patients despite a low prevalence in the population. J Vasc Surg 2012; 56:305–310.
- The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet 1998; 352:1649–1655.
- Lederle FA, Johnson GR, Wilson SE, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med 1997; 126:441–449.
- Brewster DC, Cronenwett JL, Hallett JW, Johnston KW, Krupski WC, Matsumura JS; Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg 2003; 37:1106–117.
- Ouriel K, Clair DG, Kent KC, Zarins CK; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg 2010; 51:1081–1087.
- De Rango P, Verzini F, Parlani G; Comparison of surveillance vs Aortic Endografting for Small Aneurysm Repair (CAESAR) Investigators. Quality of life in patients with small abdominal aortic aneurysm: the effect of early endovascular repair versus surveillance in the CAESAR trial. Eur J Vasc Endovasc Surg 2011; 41:324–331.
- Antoniou GA, Lazarides MK, Patera S, et al. Assessment of insertion/deletion polymorphism of the angiotensin-converting enzyme gene in abdominal aortic aneurysm and inguinal hernia. Vascular 2012; Epub ahead of print.
- Ogata T, Shibamura H, Tromp G, et al. Genetic analysis of polymorphisms in biologically relevant candidate genes in patients with abdominal aortic aneurysms. J Vasc Surg 2005; 41:1036–1042.
- Powell JT, Greenhalgh RM. Clinical practice. Small abdominal aortic aneurysms. N Engl J Med 2003; 348:1895–1901.
- Gadowski GR, Pilcher DB, Ricci MA. Abdominal aortic aneurysm expansion rate: effect of size and beta-adrenergic blockade. J Vasc Surg 1994; 19:727–731.
- Propanolol Aneurysm Trial Investigators. Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg 2002; 35:72–79.
- Jackson RS, Chang DC, Freischlag JA. Comparison of long-term survival after open vs endovascular repair of intact abdominal aortic aneurysm among Medicare beneficiaries. JAMA 2012; 307:1621–1628.
- Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg 2006; 43:446–451.
- Giles KA, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn ML. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg 2009; 49:543–550.
- Chaikof EL, Blankensteijn JD, Harris PL, et al; Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2002; 35:1048–1060.
- US Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med 2005; 142:198–202.
- Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 2010; 52:539–548.
- Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ 2005; 330:750.
- Ashton HA, Buxton MJ, Day NE, et al; Multicentre Aneurysm Screening Study Group. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002; 360:1531–1539.
- Norman PE, Jamrozik K, Lawrence-Brown MM, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 2004; 329:1259.
- Vardulaki KA, Walker NM, Couto E, et al. Late results concerning feasibility and compliance from a randomized trial of ultrasonographic screening for abdominal aortic aneurysm. Br J Surg 2002; 89:861–864.
- Derubertis BG, Trocciola SM, Ryer EJ, et al. Abdominal aortic aneurysm in women: prevalence, risk factors, and implications for screening. J Vasc Surg 2007; 46:630–635.
- Sandiford P, Mosquera D, Bramley D. Trends in incidence and mortality from abdominal aortic aneurysm in New Zealand. Br J Surg 2011; 98:645–651.
- Anjum A, Powell JT. Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg 2012; 43:161–166.
- Anderson RN. Deaths: leading causes for 2000. Natl Vital Stat Rep 2002; 50:1–85.
- Kent KC, Zwolak RM, Jaff MR, et al; Society for Vascular Surgery; American Association of Vascular Surgery; Society for Vascular Medicine and Biology. Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg 2004; 39:267–269.
- Chaikof EL, Brewster DC, Dalman RL, et al; Society for Vascular Surgery. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg 2009; 50(suppl 4):S2–S49.
- Fink HA, Lederle FA, Roth CS, Bowles CA, Nelson DB, Haas MA. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med 2000; 160:833–836.
- Reed WW, Hallett JW, Damiano MA, Ballard DJ. Learning from the last ultrasound. A population-based study of patients with abdominal aortic aneurysm. Arch Intern Med 1997; 157:2064–2068.
- Bernstein EF, Dilley RB, Goldberger LE, Gosink BB, Leopold GR. Growth rates of small abdominal aortic aneurysms. Surgery 1976; 80:765–773.
- Cronenwett JL, Sargent SK, Wall MH, et al. Variables that affect the expansion rate and outcome of small abdominal aortic aneurysms. J Vasc Surg 1990; 11:260–268.
- Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg 2002; 89:283–285.
- Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med 2005; 142:203–211.
- Lindholt JS, Sørensen J, Søgaard R, Henneberg EW. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg 2010; 97:826–834.
- Thompson SG, Ashton HA, Gao L, Scott RA; Multicentre Aneurysm Screening Study Group. Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised Multicentre Aneurysm Screening Study. BMJ 2009; 338:b2307.
- Mastracci TM, Cina CS. Regarding Screening for abdominal aortic aneurysm reduces both aneurysm-related and all-cause mortality (letter). J Vasc Surg 2007; 46:1312.
- Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ 1998; 317:307–312.
- Wilson JMG, Jungner G. Principles and practice of screening for disease. World Health Organization. Public Health Papers #34.
- Lee TY, Korn P, Heller JA, et al. The cost-effectiveness of a “quickscreen” program for abdominal aortic aneurysms. Surgery 2002; 132:399–407.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
- Linné A, Lindström D, Hultgren R. High prevalence of abdominal aortic aneurysms in brothers and sisters of patients despite a low prevalence in the population. J Vasc Surg 2012; 56:305–310.
- The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet 1998; 352:1649–1655.
- Lederle FA, Johnson GR, Wilson SE, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med 1997; 126:441–449.
- Brewster DC, Cronenwett JL, Hallett JW, Johnston KW, Krupski WC, Matsumura JS; Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg 2003; 37:1106–117.
- Ouriel K, Clair DG, Kent KC, Zarins CK; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg 2010; 51:1081–1087.
- De Rango P, Verzini F, Parlani G; Comparison of surveillance vs Aortic Endografting for Small Aneurysm Repair (CAESAR) Investigators. Quality of life in patients with small abdominal aortic aneurysm: the effect of early endovascular repair versus surveillance in the CAESAR trial. Eur J Vasc Endovasc Surg 2011; 41:324–331.
- Antoniou GA, Lazarides MK, Patera S, et al. Assessment of insertion/deletion polymorphism of the angiotensin-converting enzyme gene in abdominal aortic aneurysm and inguinal hernia. Vascular 2012; Epub ahead of print.
- Ogata T, Shibamura H, Tromp G, et al. Genetic analysis of polymorphisms in biologically relevant candidate genes in patients with abdominal aortic aneurysms. J Vasc Surg 2005; 41:1036–1042.
- Powell JT, Greenhalgh RM. Clinical practice. Small abdominal aortic aneurysms. N Engl J Med 2003; 348:1895–1901.
- Gadowski GR, Pilcher DB, Ricci MA. Abdominal aortic aneurysm expansion rate: effect of size and beta-adrenergic blockade. J Vasc Surg 1994; 19:727–731.
- Propanolol Aneurysm Trial Investigators. Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg 2002; 35:72–79.
- Jackson RS, Chang DC, Freischlag JA. Comparison of long-term survival after open vs endovascular repair of intact abdominal aortic aneurysm among Medicare beneficiaries. JAMA 2012; 307:1621–1628.
- Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg 2006; 43:446–451.
- Giles KA, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn ML. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg 2009; 49:543–550.
- Chaikof EL, Blankensteijn JD, Harris PL, et al; Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2002; 35:1048–1060.
Knee Pain After Falling Off Ladder
ANSWER
The radiograph shows a lucency within the lateral tibial plateau and tibial metaphysis, consistent with a fracture. It is mildly depressed and slightly comminuted.
Fluid collection is also evident on the lateral view, likely reflecting a lipohemarthrosis. The patient was placed in a knee immobilizer and made non–weight-bearing. She was instructed to follow up with an orthopedist when she returned home (as she was visiting from out of town).
ANSWER
The radiograph shows a lucency within the lateral tibial plateau and tibial metaphysis, consistent with a fracture. It is mildly depressed and slightly comminuted.
Fluid collection is also evident on the lateral view, likely reflecting a lipohemarthrosis. The patient was placed in a knee immobilizer and made non–weight-bearing. She was instructed to follow up with an orthopedist when she returned home (as she was visiting from out of town).
ANSWER
The radiograph shows a lucency within the lateral tibial plateau and tibial metaphysis, consistent with a fracture. It is mildly depressed and slightly comminuted.
Fluid collection is also evident on the lateral view, likely reflecting a lipohemarthrosis. The patient was placed in a knee immobilizer and made non–weight-bearing. She was instructed to follow up with an orthopedist when she returned home (as she was visiting from out of town).
A 25-year-old woman presents for evaluation of left knee pain secondary to a fall. She states she was descending a ladder when she missed a step while still several feet above the ground. She landed on her left foot, awkwardly twisting her leg. She now has swelling and pain in her knee and difficulty bearing weight on that leg. Her medical history is unremarkable. Examination reveals a moderate amount of swelling that limits her ability to flex her left knee. She has diffuse tenderness throughout the knee. Because of the swelling and the patient’s severe discomfort, instability tests are not performed. She has good distal pulses and sensation. Radiographs of the knee are obtained. What is your impression?
Cardiac tamponade: 12 pearls in diagnosis and management
Cardiac tamponade is a life-threatening condition that can be palliated or cured, depending on its cause and on the timeliness of treatment. Making a timely diagnosis and providing the appropriate treatment can be gratifying for both patient and physician.
Cardiac tamponade occurs when fluid in the pericardial space reaches a pressure exceeding central venous pressure. This leads to jugular venous distention, visceral organ engorgement, edema, and elevated pulmonary venous pressure that causes dyspnea. Despite compensatory tachycardia, the decrease in cardiac filling leads to a fall in cardiac output and to arterial hypoperfusion of vital organs.
PEARL 1: SLOW ACCUMULATION LEADS TO EDEMA
The rate at which pericardial fluid accumulates influences the clinical presentation of cardiac tamponade, in particular whether or not there is edema. Whereas rapid accumulation is characterized more by hypotension than by edema, the slow accumulation of pericardial fluid affords the patient time to drink enough liquid to keep the central venous pressure higher than the rising pericardial pressure. Thus, edema and dyspnea are more prominent features of cardiac tamponade when there is a slow rise in pericardial pressure.
PEARL 2: EDEMA IS NOT ALWAYS TREATED WITH A DIURETIC
Edema is not always treated with a diuretic. In a patient who has a pericardial effusion that has developed slowly and who has been drinking enough fluid to keep the central venous pressure higher than the pericardial pressure, a diuretic can remove enough volume from the circulation to lower the central venous pressure below the intrapericardial pressure and thus convert a benign pericardial effusion to potentially lethal cardiac tamponade.
One must understand the cause of edema or low urine output before treating it. This underscores the importance of the history and the physical examination. All of the following must be assessed:
- Symptoms and time course of the illness
- Concurrent medical illnesses
- Neck veins
- Blood pressure and its response to inspiration
- Heart sounds
- Heart rate and rhythm
- Abdominal organ engorgement
- Edema (or its absence).
PEARL 3: UNDERSTANDING THE CAUSE IS ESSENTIAL
Understanding the cause of cardiac tamponade is essential.
A trauma patient first encountered in the emergency department may have an underlying disease, but the focus is squarely on the effects of trauma or violent injury. In a patient with multiple trauma, hypotension and tachycardia that do not respond to intravenous volume replacement when there is an obvious rise in central venous pressure should be clues to cardiac tamponade.1
If the patient has recently undergone a cardiac procedure (for example, cardiac surgery, myocardial biopsy, coronary intervention, electrophysiologic study with intracardiac electrodes, transvenous pacemaker placement, pacemaker lead extraction, or radiofrequency ablation), knowing about the procedure narrows the differential diagnosis when hypotension, tachycardia, and jugular venous distention develop.
PEARL 4: CARDIAC OR AORTIC RUPTURE REQUIRES SURGERY
When the etiology of cardiac tamponade is cardiac or aortic rupture, the treatment is surgical.
Painful sudden causes of cardiac tamponade include hemopericardium due to rupture of the free wall after myocardial infarction, and spontaneous or posttraumatic dissection and rupture of the ascending aorta. Prompt diagnosis is necessary, but since these lesions will not close and heal spontaneously, the definitive treatment should be surgery. Moreover, needle removal of intrapericardial blood that has been opposing further bleeding is sure to permit bleeding to recur, often with lethal consequences.2
Causes of cardiac tamponade that have a less-acute onset are likely to be complications of medical problems. Medical illnesses known to be associated with cardiac tamponade include:
- Infectious disease (idiopathic or viral, associated with smallpox vaccination, mycobacterial, purulent bacterial, fungal)
- Metastatic cancer (lung, breast, esophagus, lymphoma, pancreas, liver, leukemia, stomach, melanoma)3
- Connective tissue disease (rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis, scleroderma, Wegener granulomatosis, acute rheumatic fever)
- Endocrine disease (hypothyroidism)
- Drug side effects (procainamide, isoniazid, hydralazine, minoxidil, phenytoin, anticoagulants, methysergide)
- Inflammatory bowel disease (Crohn disease, ulcerative colitis)
- Congestive heart failure
- Uremia
- Radiation therapy
- Postmyocardial infarction syndrome (Dressler syndrome)
- Postpericardiotomy syndrome.
PEARL 5: REVIEW IMAGING BEFORE DIAGNOSING
What often brings a patient with cardiac tamponade to the attention of the physician is a finding on echocardiography, computed tomography, or magnetic resonance imaging of the chest.
Always review the imaging studies before making the diagnosis of cardiac tamponade. These tests must be reviewed to assess the anatomy and the size and location of the effusion. Particularly, one must look for atrial and right ventricular collapse and inferior vena caval plethora, which are echocardiographic signs of cardiac tamponade.4 Figures 1, 2, and 3 show imaging studies in a patient who presented with worsening cough 2 weeks after undergoing a cardiac procedure and who was found to have cardiac tamponade.
When the history and these imaging studies place cardiac tamponade high in the differential diagnosis as the cause of edema or dyspnea, it is time to reexamine the patient. The best first step is to measure pulsus paradoxus.
HOW PULSUS PARADOXUS OCCURS
To fully appreciate the subtleties of the next pearls, it is necessary to understand the pathophysiology of cardiac tamponade.
When pericardial fluid accumulation raises the pericardial pressure above the central venous pressure and pulmonary venous pressure (intravascular pressure), blood will not passively return to the right side of the heart from the venae cavae nor to the left side of the heart from the pulmonary veins unless it is influenced by the effects of respiration on intrathoracic pressure. During respiration, the right and left sides of the heart are alternately filled and deprived of their respective venous return.
During inspiration, as the intrathoracic pressure decreases, blood in the venae cavae empties into the right side of the heart, while blood in the pulmonary veins preferentially remains in the pulmonary veins, underfilling the left side of the heart. Since the right ventricle is more filled than the left ventricle during inspiration, the ventricular septum shifts from right to left, further opposing pulmonary venous return. As a result, during cardiac tamponade, the systemic blood pressure falls with inspiration.
During expiration the opposite occurs. Expiration decreases the intrathoracic volume, so the intrathoracic pressure rises. This tends to oppose vena caval return to the right side of the heart and to favor pulmonary venous return to the left side of the heart. The ventricular septum shifts from left to right, further accommodating left ventricular filling, raising stroke volume, and increasing blood pressure. This exaggerated alternate filling of the right and left sides of the heart during cardiac tamponade is what accounts for pulsus paradoxus, an inspiratory fall in systolic blood pressure of greater than 10 mm Hg.
If intravascular pressure is low (due to hemorrhage, dehydration, or diuretic therapy), the pressure in the pericardial space needed to oppose venous return is much less. In this low-pressure scenario, the results are low cardiac output and hypotension, which are treated by giving intravenous fluids to maintain intravascular volume.
PEARL 6: MEASURE PULSUS PARADOXUS
When cardiac tamponade is considered, one must always measure the pulsus paradoxus.
The term pulsus paradoxus was coined by Adolph Kussmaul in 1873, before physicians could even measure blood pressure. All they could do at that time was palpate the pulse and listen to the heart. Kussmaul described his observation as a conspicuous discrepancy between the cardiac action and the arterial pulse.
Although not described by Kussmaul, another explanation for this finding might be more suited to the use of the word “paradoxical.” When the pulse is palpated in a normal patient, with inspiration the pulse rate will increase via the Bainbridge reflex, and with expiration it will decrease. But in a patient with cardiac tamponade, there is a paradoxical inspiratory slowing of the pulse (because the decreased magnitude of the pulse at times makes it imperceptible) and an expiratory increase in pulse rate as the magnitude of the pulse again makes it palpable.
The magnitude of the fall in systolic blood pressure during inspiration has been used to estimate the level of hemodynamic impairment resulting from pericardial effusion.5 A rapidly accumulating pericardial effusion can have more hemodynamic impact than a much larger one that accumulates slowly. Thus, the intrapericardial pressure must be considered more than the volume of pericardial fluid.
When there is severe cardiac tamponade and overt pulsus paradoxus, simple palpation of a proximal arterial pulse can detect a marked inspiratory decrease or loss of the pulse, which returns with expiration. Tachycardia is almost always present, unless the cause is hypothyroidism.6
How to measure pulsus paradoxus with a manual sphygmomanometer
A stethoscope and manual sphygmomanometer are all that is needed to measure pulsus paradoxus. A noninvasive blood pressure monitor that averages multiple measurements cannot detect or quantify pulsus paradoxus.
The patient should be supine with the head elevated 30° to 45°, and the examiner should be seated comfortably at the patient’s side. The manometer should be on the opposite side of the patient in plain view of the examiner. Position the cuff on the arm above the elbow and place your stethoscope on the antecubital fossa. Then:
- Inflate the cuff 20 mm Hg above the highest systolic pressure previously auscultated.
- Slowly decrease the manometer pressure by 5 mm Hg and hold it there through two or three respiratory cycles while listening for the first Korotkoff (systolic) sound. Repeat this until you can hear the systolic sound (but only during expiration) and mentally note the pressure.
- Continue to decrease the manometer pressure by 5-mm Hg increments while listening. When the Korotkoff sounds no longer disappear with inspiration, mentally note this second value as well. The pulsus paradoxus is the difference between these values.
- When the Korotkoff sounds disappear as the manometer pressure is decreased, note this final value. This is the diastolic blood pressure.
PEARL 7: THE PLETHYSMOGRAM WAVE-FORM PARALLELS PULSUS PARADOXUS
Manual measurement of blood pressure and pulsus paradoxus can be difficult, especially in an obese patient or one with a fat-distorted arm on which the cuff does not maintain its position. In such patients, increased girth of the neck and abdomen also make it difficult to assess the jugular venous distention and visceral organ engorgement that characterize cardiac tamponade.
When the use of a sphygmomanometer is not possible, an arterial catheter can be inserted to demonstrate pulsus paradoxus. Simpler, however, is the novel use of another noninvasive instrument to detect and coarsely quantify pulsus paradoxus.7 The waveform on finger pulse oximetry can demonstrate pulsus paradoxus. The plethysmogram of the finger pulse oximeter can demonstrate the decrease in magnitude of the waveform with each inspiration (Figure 4).
Caution must be taken when interpreting this waveform, as with any measurement of pulsus paradoxus, to exclude a concomitant arrhythmia.
PEARL 8: PULSUS PARADOXUS WITHOUT CARDIAC TAMPONADE
Pulsus paradoxus can be present in the absence of cardiac tamponade. Once pulsus paradoxus of more than 10 mm Hg is measured, one must be sure the patient does not have a condition that can cause pulsus paradoxus without cardiac tamponade. Most of these are pulmonary conditions that necessitate an exaggerated inspiratory effort that can lower intrathoracic pressure sufficiently to oppose pulmonary venous return and cause a fall in systemic blood pressure:
- Chronic bronchitis
- Emphysema
- Mucus plug
- Pneumothorax
- Pulmonary embolism
- Stridor.
In these, there may be pulsus paradoxus, but not due to cardiac tamponade.
PEARL 9: CARDIAC TAMPONADE CAN BE PRESENT WITHOUT PULSUS PARADOXUS
Cardiac tamponade can be present without pulsus paradoxus. This occurs when certain conditions prevent inspiratory underfilling of the left ventricle relative to the filling of the right ventricle.8
How does this work? In cardiac tamponade, factors that drive the exaggerated fall in arterial pressure with inspiration (pulsus paradoxus) are the augmented right ventricular filling and the decreased left ventricular filling, both due to the lowering of the intrathoracic pressure. As the vena caval emptying is augmented, the right ventricular filling is increased, the ventricular septum shifts to the left, and pulmonary venous return to the heart is decreased.
Factors that can oppose pulsus paradoxus:
- Positive pressure ventilation prevents pulsus paradoxus by preventing the fall in intrathoracic pressure.
- Severe aortic regurgitation does not permit underfilling of the left ventricle during inspiration.
- An atrial septal defect will always equalize the right and left atrial pressures, preventing differential right ventricular and left ventricular filling with inspiration.
- Severe left ventricular hypertrophy does not permit the inspiratory shift of the ventricular septum from right to left that would otherwise lead to decreased left ventricular filling.
- Severe left ventricular dysfunction, with its low stroke volume and severe elevation of left ventricular end-diastolic pressure, never permits underfilling of the left ventricle, despite cardiac tamponade and an inspiratory decrease in intrathoracic pressure.
- Intravascular volume depletion due to hemorrhage, hemodialysis, or mistaken use of diuretics to treat edema can cause marked hypotension, making pulsus paradoxus impossible to detect.
Knowledge of underlying medical conditions, the likelihood of their causing cardiac tamponade, and the appearance of the echocardiogram prompt the physician to look further when the presence or absence of pulsus paradoxus does not fit with the working diagnosis.
The echocardiogram can give hints to the etiology of a pericardial effusion, such as clotted blood after trauma or a cardiac-perforating procedure, tumor studding of the epicardium,9 or fibrin strands indicating chronicity or an inflammatory process.10 Diastolic collapse of the right ventricle, more than collapse of the right atrium or left atrium, speaks for the severity of cardiac tamponade. With hemodynamically significant pericardial effusion and cardiac tamponade, the inferior vena cava is distended and does not decrease in size with inspiration unless there is severe intravascular volume depletion, at which time the inferior vena cava is underfilled throughout the respiratory cycle.
PEARL 10: PLAN HOW TO DRAIN
The size and location of the pericardial effusion and the patient’s hemodynamics must be integrated when deciding how to relieve cardiac tamponade. When cardiac tamponade is indeed severe and the patient and physician agree that it must be drained, the options are percutaneous needle aspiration (pericardiocentesis) and surgical pericardiostomy (creation of a pericardial window). Here again, as assessed by echocardiography, the access to the pericardial fluid should influence the choice.
Pericardiocentesis can be safely done if certain criteria are met. The patient must be able to lie still in the supine position, perhaps with the head of the bed elevated 30 degrees. Anticoagulation must be reversed or allowed time to resolve if drainage is not an emergency.
Pericardiocentesis can be risky or unsuccessful if there is not enough pericardial fluid to permit respiratory cardiac motion without perforating the heart with the needle; if the effusion is loculated (confined to a pocket) posteriorly; or if it is too far from the skin to permit precise control and placement of a spinal needle into the pericardial space. In cases of cardiac tamponade in which the anatomy indicates surgical pericardiostomy but severe hypotension prevents the induction of anesthesia and positive-pressure ventilation—which can result in profound, irreversible hypotension—percutaneous needle drainage (pericardiocentesis) should be performed in the operating room to relieve the tamponade before the induction of anesthesia and the surgical drainage.11
To reiterate, a suspected cardiac or aortic rupture that causes cardiac tamponade is usually large and not apt to self-seal. In such cases, the halt in the accumulation of pericardial blood is due to hypotension and not due to spontaneous resolution. Open surgical drainage is required from the outset because an initial success of pericardiocentesis yields to the recurrence of cardiac tamponade.
PEARL 11: ANTICIPATE WHAT THE FLUID SHOULD LOOK LIKE
Before performing pericardiocentesis, anticipate the appearance of the pericardial fluid on the basis of the presumed etiology, ie:
- Sanguinous—trauma, heart surgery, cardiac perforation from a procedure, anticoagulation, uremia, or malignancy
- Serous—congestive heart failure, acute radiation therapy
- Purulent—infections (natural or postoperative)
- Turbid (like gold paint)—mycobacterial infection, rheumatoid arthritis, myxedema
- Chylous—pericardium fistulized to the thoracic duct by a natural or postsurgical cause.
Sanguinous pericardial effusion encountered during a pericardiocentesis, if not anticipated, can be daunting and can cause the operator to question if it is the result of inadvertent needle placement in a cardiac chamber. If the needle is indeed in the heart, blood often surges out under pressure in pulses, which strongly suggests that the needle is not in the pericardial space and should be removed; but if confirmation of the location is needed before removing the needle, it can be done by injecting 2 mL of agitated sterile saline through the pericardiocentesis needle during echocardiographic imaging.12
Before inserting the needle, the ideal access location and needle angle must be determined by the operator with echocardiographic transducer in hand. The distance from skin to a point just through the parietal pericardium can also be measured at this time.
Once the needle is in the pericardial fluid (and you are confident of its placement), removal of 50 to 100 mL of the fluid with a large syringe can be enough to afford the patient easier breathing, higher blood pressure, and lower pulsus paradoxus—and even the physician will breathe easier. The same syringe can be filled and emptied multiple times. Less traumatic and more complete removal of pericardial fluid requires insertion of a multihole pigtail catheter over a J-tipped guidewire that is introduced through the needle.
PEARL 12: DRAIN SLOWLY TO AVOID PULMONARY EDEMA
Pulmonary edema is an uncommon complication of pericardiocentesis that might be avoidable. Heralded by sudden coughing and pink, frothy sputum, it can rapidly deteriorate into respiratory failure. The mechanism has been attributed to a sudden increase in right ventricular stroke volume and resultant left ventricular filling after the excess pericardial fluid has been removed, before the systemic arteries, which constrict to keep the systemic blood pressure up during cardiac tamponade, have had time to relax.13
To avoid this complication, if the volume of pericardial fluid responsible for cardiac tamponade is large, it should be removed slowly,14 stopping for a several-minute rest after each 250 mL. Catheter removal of pericardial fluid by gravity drainage over 24 hours has been suggested.15 A drawback to this approach is catheter clotting or sludging before all the fluid has been removed. It is helpful to keep the drainage catheter close to the patient’s body temperature to make the fluid less viscous. Output should be monitored hourly.
When the pericardial fluid has been completely drained, one must decide how long to leave the catheter in. One reason to remove the catheter at this time is that it causes pleuritic pain; another is to avoid introducing infection. A reason to leave the catheter in is to observe the effect of medical treatment on the hourly pericardial fluid output. Nonsteroidal anti-inflammatory drugs are the drugs of first choice when treating pericardial inflammation and suppressing production of pericardial fluid.16 In most cases the catheter should not be left in place for more than 3 days.
Laboratory analysis of the pericardial fluid should shed light on its suspected cause. Analysis usually involves chemistry testing, microscopic inspection of blood cell smears, cytology, microbiologic stains and cultures, and immunologic tests. Results often take days. Meyers and colleagues17 expound on this subject.
- Schiavone WA, Ghumrawi BK, Catalano DR, et al. The use of echocardiography in the emergency management of nonpenetrating traumatic cardiac rupture. Ann Emerg Med 1991; 20:1248–1250.
- Manuchehry A, Fontana GP, Gurudevan S, Marchevsky AM, Siegel RJ. Missed diagnosis of limited ascending aortic dissection by multiple imaging modalities leading to fatal cardiac tamponade and aortic rupture. Echocardiography 2011; 28:E187–E190.
- Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med 1993; 117:1027–1031.
- Tsang TS, Oh JK, Seward JB, Tajik AJ. Diagnostic value of echocardiography in cardiac tamponade. Herz 2000; 25:734–740.
- Curtiss EI, Reddy PS, Uretsky BF, Cecchetti AA. Pulsus paradoxus: definition and relation to the severity of cardiac tamponade. Am Heart J 1988; 115:391–398.
- Wang JL, Hsieh MJ, Lee CH, et al. Hypothyroid cardiac tamponade: clinical features, electrocardiography, pericardial fluid and management. Am J Med Sci 2010; 340:276–281.
- Tamburro RF, Ring JC, Womback K. Detection of pulsus paradoxus associated with large pericardial effusions in pediatric patients by analysis of the pulse-oximetry waveform. Pediatrics 2002; 109:673–677.
- Spodick DH. Pulsus paradoxus. In:Spodick DH, editor. The Pericardium: A Comprehensive Textbook. New York, NY: Marcel Dekker; 1997:191–199.
- Burke A, Jeudy J, Virmani R. Cardiac tumors. In:Topol EJ, editor. Textbook of Cardiovascular Medicine. 3rd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2007:710–720.
- Roberts WC. Pericardial heart disease: Its morphologic features and its causes. Proc (Bayl Univ Med Cent) 2005; 18:38–55.
- Stoelting RK, Miller RD, editors. Basics of Anesthesia. 4th ed. New York, NY: Churchill Livingstone; 2000:264–265.
- Ainsworth CD, Salehian O. Echo-guided pericardiocentesis: let the bubbles show the way. Circulation 2011; 123:e210–e211.
- Maisch B, Seferovic PM, Ristic AD, et al; Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J 2004; 25:587–610.
- Vandyke WH, Cure J, Chakko CS, Gheorghiade M. Pulmonary edema after pericardiocentesis for cardiac tamponade. N Engl J Med 1983; 309:595–596.
- Bernal JM, Pradhan J, Li T, Tchokonte R, Afonso L. Acute pulmonary edema following pericardiocentesis for cardiac tamponade. Can J Cardiol 2007; 23:1155–1156.
- Sagristà-Sauleda J, Mercé AS, Soler-Soler J. Diagnosis and management of pericardial effusion. World J Cardiol 2011; 3:135–143.
- Meyers DG, Meyers RE, Prendergast TW. The usefulness of diagnostic tests on pericardial fluid. Chest 1997; 111:1213–1221.
Cardiac tamponade is a life-threatening condition that can be palliated or cured, depending on its cause and on the timeliness of treatment. Making a timely diagnosis and providing the appropriate treatment can be gratifying for both patient and physician.
Cardiac tamponade occurs when fluid in the pericardial space reaches a pressure exceeding central venous pressure. This leads to jugular venous distention, visceral organ engorgement, edema, and elevated pulmonary venous pressure that causes dyspnea. Despite compensatory tachycardia, the decrease in cardiac filling leads to a fall in cardiac output and to arterial hypoperfusion of vital organs.
PEARL 1: SLOW ACCUMULATION LEADS TO EDEMA
The rate at which pericardial fluid accumulates influences the clinical presentation of cardiac tamponade, in particular whether or not there is edema. Whereas rapid accumulation is characterized more by hypotension than by edema, the slow accumulation of pericardial fluid affords the patient time to drink enough liquid to keep the central venous pressure higher than the rising pericardial pressure. Thus, edema and dyspnea are more prominent features of cardiac tamponade when there is a slow rise in pericardial pressure.
PEARL 2: EDEMA IS NOT ALWAYS TREATED WITH A DIURETIC
Edema is not always treated with a diuretic. In a patient who has a pericardial effusion that has developed slowly and who has been drinking enough fluid to keep the central venous pressure higher than the pericardial pressure, a diuretic can remove enough volume from the circulation to lower the central venous pressure below the intrapericardial pressure and thus convert a benign pericardial effusion to potentially lethal cardiac tamponade.
One must understand the cause of edema or low urine output before treating it. This underscores the importance of the history and the physical examination. All of the following must be assessed:
- Symptoms and time course of the illness
- Concurrent medical illnesses
- Neck veins
- Blood pressure and its response to inspiration
- Heart sounds
- Heart rate and rhythm
- Abdominal organ engorgement
- Edema (or its absence).
PEARL 3: UNDERSTANDING THE CAUSE IS ESSENTIAL
Understanding the cause of cardiac tamponade is essential.
A trauma patient first encountered in the emergency department may have an underlying disease, but the focus is squarely on the effects of trauma or violent injury. In a patient with multiple trauma, hypotension and tachycardia that do not respond to intravenous volume replacement when there is an obvious rise in central venous pressure should be clues to cardiac tamponade.1
If the patient has recently undergone a cardiac procedure (for example, cardiac surgery, myocardial biopsy, coronary intervention, electrophysiologic study with intracardiac electrodes, transvenous pacemaker placement, pacemaker lead extraction, or radiofrequency ablation), knowing about the procedure narrows the differential diagnosis when hypotension, tachycardia, and jugular venous distention develop.
PEARL 4: CARDIAC OR AORTIC RUPTURE REQUIRES SURGERY
When the etiology of cardiac tamponade is cardiac or aortic rupture, the treatment is surgical.
Painful sudden causes of cardiac tamponade include hemopericardium due to rupture of the free wall after myocardial infarction, and spontaneous or posttraumatic dissection and rupture of the ascending aorta. Prompt diagnosis is necessary, but since these lesions will not close and heal spontaneously, the definitive treatment should be surgery. Moreover, needle removal of intrapericardial blood that has been opposing further bleeding is sure to permit bleeding to recur, often with lethal consequences.2
Causes of cardiac tamponade that have a less-acute onset are likely to be complications of medical problems. Medical illnesses known to be associated with cardiac tamponade include:
- Infectious disease (idiopathic or viral, associated with smallpox vaccination, mycobacterial, purulent bacterial, fungal)
- Metastatic cancer (lung, breast, esophagus, lymphoma, pancreas, liver, leukemia, stomach, melanoma)3
- Connective tissue disease (rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis, scleroderma, Wegener granulomatosis, acute rheumatic fever)
- Endocrine disease (hypothyroidism)
- Drug side effects (procainamide, isoniazid, hydralazine, minoxidil, phenytoin, anticoagulants, methysergide)
- Inflammatory bowel disease (Crohn disease, ulcerative colitis)
- Congestive heart failure
- Uremia
- Radiation therapy
- Postmyocardial infarction syndrome (Dressler syndrome)
- Postpericardiotomy syndrome.
PEARL 5: REVIEW IMAGING BEFORE DIAGNOSING
What often brings a patient with cardiac tamponade to the attention of the physician is a finding on echocardiography, computed tomography, or magnetic resonance imaging of the chest.
Always review the imaging studies before making the diagnosis of cardiac tamponade. These tests must be reviewed to assess the anatomy and the size and location of the effusion. Particularly, one must look for atrial and right ventricular collapse and inferior vena caval plethora, which are echocardiographic signs of cardiac tamponade.4 Figures 1, 2, and 3 show imaging studies in a patient who presented with worsening cough 2 weeks after undergoing a cardiac procedure and who was found to have cardiac tamponade.
When the history and these imaging studies place cardiac tamponade high in the differential diagnosis as the cause of edema or dyspnea, it is time to reexamine the patient. The best first step is to measure pulsus paradoxus.
HOW PULSUS PARADOXUS OCCURS
To fully appreciate the subtleties of the next pearls, it is necessary to understand the pathophysiology of cardiac tamponade.
When pericardial fluid accumulation raises the pericardial pressure above the central venous pressure and pulmonary venous pressure (intravascular pressure), blood will not passively return to the right side of the heart from the venae cavae nor to the left side of the heart from the pulmonary veins unless it is influenced by the effects of respiration on intrathoracic pressure. During respiration, the right and left sides of the heart are alternately filled and deprived of their respective venous return.
During inspiration, as the intrathoracic pressure decreases, blood in the venae cavae empties into the right side of the heart, while blood in the pulmonary veins preferentially remains in the pulmonary veins, underfilling the left side of the heart. Since the right ventricle is more filled than the left ventricle during inspiration, the ventricular septum shifts from right to left, further opposing pulmonary venous return. As a result, during cardiac tamponade, the systemic blood pressure falls with inspiration.
During expiration the opposite occurs. Expiration decreases the intrathoracic volume, so the intrathoracic pressure rises. This tends to oppose vena caval return to the right side of the heart and to favor pulmonary venous return to the left side of the heart. The ventricular septum shifts from left to right, further accommodating left ventricular filling, raising stroke volume, and increasing blood pressure. This exaggerated alternate filling of the right and left sides of the heart during cardiac tamponade is what accounts for pulsus paradoxus, an inspiratory fall in systolic blood pressure of greater than 10 mm Hg.
If intravascular pressure is low (due to hemorrhage, dehydration, or diuretic therapy), the pressure in the pericardial space needed to oppose venous return is much less. In this low-pressure scenario, the results are low cardiac output and hypotension, which are treated by giving intravenous fluids to maintain intravascular volume.
PEARL 6: MEASURE PULSUS PARADOXUS
When cardiac tamponade is considered, one must always measure the pulsus paradoxus.
The term pulsus paradoxus was coined by Adolph Kussmaul in 1873, before physicians could even measure blood pressure. All they could do at that time was palpate the pulse and listen to the heart. Kussmaul described his observation as a conspicuous discrepancy between the cardiac action and the arterial pulse.
Although not described by Kussmaul, another explanation for this finding might be more suited to the use of the word “paradoxical.” When the pulse is palpated in a normal patient, with inspiration the pulse rate will increase via the Bainbridge reflex, and with expiration it will decrease. But in a patient with cardiac tamponade, there is a paradoxical inspiratory slowing of the pulse (because the decreased magnitude of the pulse at times makes it imperceptible) and an expiratory increase in pulse rate as the magnitude of the pulse again makes it palpable.
The magnitude of the fall in systolic blood pressure during inspiration has been used to estimate the level of hemodynamic impairment resulting from pericardial effusion.5 A rapidly accumulating pericardial effusion can have more hemodynamic impact than a much larger one that accumulates slowly. Thus, the intrapericardial pressure must be considered more than the volume of pericardial fluid.
When there is severe cardiac tamponade and overt pulsus paradoxus, simple palpation of a proximal arterial pulse can detect a marked inspiratory decrease or loss of the pulse, which returns with expiration. Tachycardia is almost always present, unless the cause is hypothyroidism.6
How to measure pulsus paradoxus with a manual sphygmomanometer
A stethoscope and manual sphygmomanometer are all that is needed to measure pulsus paradoxus. A noninvasive blood pressure monitor that averages multiple measurements cannot detect or quantify pulsus paradoxus.
The patient should be supine with the head elevated 30° to 45°, and the examiner should be seated comfortably at the patient’s side. The manometer should be on the opposite side of the patient in plain view of the examiner. Position the cuff on the arm above the elbow and place your stethoscope on the antecubital fossa. Then:
- Inflate the cuff 20 mm Hg above the highest systolic pressure previously auscultated.
- Slowly decrease the manometer pressure by 5 mm Hg and hold it there through two or three respiratory cycles while listening for the first Korotkoff (systolic) sound. Repeat this until you can hear the systolic sound (but only during expiration) and mentally note the pressure.
- Continue to decrease the manometer pressure by 5-mm Hg increments while listening. When the Korotkoff sounds no longer disappear with inspiration, mentally note this second value as well. The pulsus paradoxus is the difference between these values.
- When the Korotkoff sounds disappear as the manometer pressure is decreased, note this final value. This is the diastolic blood pressure.
PEARL 7: THE PLETHYSMOGRAM WAVE-FORM PARALLELS PULSUS PARADOXUS
Manual measurement of blood pressure and pulsus paradoxus can be difficult, especially in an obese patient or one with a fat-distorted arm on which the cuff does not maintain its position. In such patients, increased girth of the neck and abdomen also make it difficult to assess the jugular venous distention and visceral organ engorgement that characterize cardiac tamponade.
When the use of a sphygmomanometer is not possible, an arterial catheter can be inserted to demonstrate pulsus paradoxus. Simpler, however, is the novel use of another noninvasive instrument to detect and coarsely quantify pulsus paradoxus.7 The waveform on finger pulse oximetry can demonstrate pulsus paradoxus. The plethysmogram of the finger pulse oximeter can demonstrate the decrease in magnitude of the waveform with each inspiration (Figure 4).
Caution must be taken when interpreting this waveform, as with any measurement of pulsus paradoxus, to exclude a concomitant arrhythmia.
PEARL 8: PULSUS PARADOXUS WITHOUT CARDIAC TAMPONADE
Pulsus paradoxus can be present in the absence of cardiac tamponade. Once pulsus paradoxus of more than 10 mm Hg is measured, one must be sure the patient does not have a condition that can cause pulsus paradoxus without cardiac tamponade. Most of these are pulmonary conditions that necessitate an exaggerated inspiratory effort that can lower intrathoracic pressure sufficiently to oppose pulmonary venous return and cause a fall in systemic blood pressure:
- Chronic bronchitis
- Emphysema
- Mucus plug
- Pneumothorax
- Pulmonary embolism
- Stridor.
In these, there may be pulsus paradoxus, but not due to cardiac tamponade.
PEARL 9: CARDIAC TAMPONADE CAN BE PRESENT WITHOUT PULSUS PARADOXUS
Cardiac tamponade can be present without pulsus paradoxus. This occurs when certain conditions prevent inspiratory underfilling of the left ventricle relative to the filling of the right ventricle.8
How does this work? In cardiac tamponade, factors that drive the exaggerated fall in arterial pressure with inspiration (pulsus paradoxus) are the augmented right ventricular filling and the decreased left ventricular filling, both due to the lowering of the intrathoracic pressure. As the vena caval emptying is augmented, the right ventricular filling is increased, the ventricular septum shifts to the left, and pulmonary venous return to the heart is decreased.
Factors that can oppose pulsus paradoxus:
- Positive pressure ventilation prevents pulsus paradoxus by preventing the fall in intrathoracic pressure.
- Severe aortic regurgitation does not permit underfilling of the left ventricle during inspiration.
- An atrial septal defect will always equalize the right and left atrial pressures, preventing differential right ventricular and left ventricular filling with inspiration.
- Severe left ventricular hypertrophy does not permit the inspiratory shift of the ventricular septum from right to left that would otherwise lead to decreased left ventricular filling.
- Severe left ventricular dysfunction, with its low stroke volume and severe elevation of left ventricular end-diastolic pressure, never permits underfilling of the left ventricle, despite cardiac tamponade and an inspiratory decrease in intrathoracic pressure.
- Intravascular volume depletion due to hemorrhage, hemodialysis, or mistaken use of diuretics to treat edema can cause marked hypotension, making pulsus paradoxus impossible to detect.
Knowledge of underlying medical conditions, the likelihood of their causing cardiac tamponade, and the appearance of the echocardiogram prompt the physician to look further when the presence or absence of pulsus paradoxus does not fit with the working diagnosis.
The echocardiogram can give hints to the etiology of a pericardial effusion, such as clotted blood after trauma or a cardiac-perforating procedure, tumor studding of the epicardium,9 or fibrin strands indicating chronicity or an inflammatory process.10 Diastolic collapse of the right ventricle, more than collapse of the right atrium or left atrium, speaks for the severity of cardiac tamponade. With hemodynamically significant pericardial effusion and cardiac tamponade, the inferior vena cava is distended and does not decrease in size with inspiration unless there is severe intravascular volume depletion, at which time the inferior vena cava is underfilled throughout the respiratory cycle.
PEARL 10: PLAN HOW TO DRAIN
The size and location of the pericardial effusion and the patient’s hemodynamics must be integrated when deciding how to relieve cardiac tamponade. When cardiac tamponade is indeed severe and the patient and physician agree that it must be drained, the options are percutaneous needle aspiration (pericardiocentesis) and surgical pericardiostomy (creation of a pericardial window). Here again, as assessed by echocardiography, the access to the pericardial fluid should influence the choice.
Pericardiocentesis can be safely done if certain criteria are met. The patient must be able to lie still in the supine position, perhaps with the head of the bed elevated 30 degrees. Anticoagulation must be reversed or allowed time to resolve if drainage is not an emergency.
Pericardiocentesis can be risky or unsuccessful if there is not enough pericardial fluid to permit respiratory cardiac motion without perforating the heart with the needle; if the effusion is loculated (confined to a pocket) posteriorly; or if it is too far from the skin to permit precise control and placement of a spinal needle into the pericardial space. In cases of cardiac tamponade in which the anatomy indicates surgical pericardiostomy but severe hypotension prevents the induction of anesthesia and positive-pressure ventilation—which can result in profound, irreversible hypotension—percutaneous needle drainage (pericardiocentesis) should be performed in the operating room to relieve the tamponade before the induction of anesthesia and the surgical drainage.11
To reiterate, a suspected cardiac or aortic rupture that causes cardiac tamponade is usually large and not apt to self-seal. In such cases, the halt in the accumulation of pericardial blood is due to hypotension and not due to spontaneous resolution. Open surgical drainage is required from the outset because an initial success of pericardiocentesis yields to the recurrence of cardiac tamponade.
PEARL 11: ANTICIPATE WHAT THE FLUID SHOULD LOOK LIKE
Before performing pericardiocentesis, anticipate the appearance of the pericardial fluid on the basis of the presumed etiology, ie:
- Sanguinous—trauma, heart surgery, cardiac perforation from a procedure, anticoagulation, uremia, or malignancy
- Serous—congestive heart failure, acute radiation therapy
- Purulent—infections (natural or postoperative)
- Turbid (like gold paint)—mycobacterial infection, rheumatoid arthritis, myxedema
- Chylous—pericardium fistulized to the thoracic duct by a natural or postsurgical cause.
Sanguinous pericardial effusion encountered during a pericardiocentesis, if not anticipated, can be daunting and can cause the operator to question if it is the result of inadvertent needle placement in a cardiac chamber. If the needle is indeed in the heart, blood often surges out under pressure in pulses, which strongly suggests that the needle is not in the pericardial space and should be removed; but if confirmation of the location is needed before removing the needle, it can be done by injecting 2 mL of agitated sterile saline through the pericardiocentesis needle during echocardiographic imaging.12
Before inserting the needle, the ideal access location and needle angle must be determined by the operator with echocardiographic transducer in hand. The distance from skin to a point just through the parietal pericardium can also be measured at this time.
Once the needle is in the pericardial fluid (and you are confident of its placement), removal of 50 to 100 mL of the fluid with a large syringe can be enough to afford the patient easier breathing, higher blood pressure, and lower pulsus paradoxus—and even the physician will breathe easier. The same syringe can be filled and emptied multiple times. Less traumatic and more complete removal of pericardial fluid requires insertion of a multihole pigtail catheter over a J-tipped guidewire that is introduced through the needle.
PEARL 12: DRAIN SLOWLY TO AVOID PULMONARY EDEMA
Pulmonary edema is an uncommon complication of pericardiocentesis that might be avoidable. Heralded by sudden coughing and pink, frothy sputum, it can rapidly deteriorate into respiratory failure. The mechanism has been attributed to a sudden increase in right ventricular stroke volume and resultant left ventricular filling after the excess pericardial fluid has been removed, before the systemic arteries, which constrict to keep the systemic blood pressure up during cardiac tamponade, have had time to relax.13
To avoid this complication, if the volume of pericardial fluid responsible for cardiac tamponade is large, it should be removed slowly,14 stopping for a several-minute rest after each 250 mL. Catheter removal of pericardial fluid by gravity drainage over 24 hours has been suggested.15 A drawback to this approach is catheter clotting or sludging before all the fluid has been removed. It is helpful to keep the drainage catheter close to the patient’s body temperature to make the fluid less viscous. Output should be monitored hourly.
When the pericardial fluid has been completely drained, one must decide how long to leave the catheter in. One reason to remove the catheter at this time is that it causes pleuritic pain; another is to avoid introducing infection. A reason to leave the catheter in is to observe the effect of medical treatment on the hourly pericardial fluid output. Nonsteroidal anti-inflammatory drugs are the drugs of first choice when treating pericardial inflammation and suppressing production of pericardial fluid.16 In most cases the catheter should not be left in place for more than 3 days.
Laboratory analysis of the pericardial fluid should shed light on its suspected cause. Analysis usually involves chemistry testing, microscopic inspection of blood cell smears, cytology, microbiologic stains and cultures, and immunologic tests. Results often take days. Meyers and colleagues17 expound on this subject.
Cardiac tamponade is a life-threatening condition that can be palliated or cured, depending on its cause and on the timeliness of treatment. Making a timely diagnosis and providing the appropriate treatment can be gratifying for both patient and physician.
Cardiac tamponade occurs when fluid in the pericardial space reaches a pressure exceeding central venous pressure. This leads to jugular venous distention, visceral organ engorgement, edema, and elevated pulmonary venous pressure that causes dyspnea. Despite compensatory tachycardia, the decrease in cardiac filling leads to a fall in cardiac output and to arterial hypoperfusion of vital organs.
PEARL 1: SLOW ACCUMULATION LEADS TO EDEMA
The rate at which pericardial fluid accumulates influences the clinical presentation of cardiac tamponade, in particular whether or not there is edema. Whereas rapid accumulation is characterized more by hypotension than by edema, the slow accumulation of pericardial fluid affords the patient time to drink enough liquid to keep the central venous pressure higher than the rising pericardial pressure. Thus, edema and dyspnea are more prominent features of cardiac tamponade when there is a slow rise in pericardial pressure.
PEARL 2: EDEMA IS NOT ALWAYS TREATED WITH A DIURETIC
Edema is not always treated with a diuretic. In a patient who has a pericardial effusion that has developed slowly and who has been drinking enough fluid to keep the central venous pressure higher than the pericardial pressure, a diuretic can remove enough volume from the circulation to lower the central venous pressure below the intrapericardial pressure and thus convert a benign pericardial effusion to potentially lethal cardiac tamponade.
One must understand the cause of edema or low urine output before treating it. This underscores the importance of the history and the physical examination. All of the following must be assessed:
- Symptoms and time course of the illness
- Concurrent medical illnesses
- Neck veins
- Blood pressure and its response to inspiration
- Heart sounds
- Heart rate and rhythm
- Abdominal organ engorgement
- Edema (or its absence).
PEARL 3: UNDERSTANDING THE CAUSE IS ESSENTIAL
Understanding the cause of cardiac tamponade is essential.
A trauma patient first encountered in the emergency department may have an underlying disease, but the focus is squarely on the effects of trauma or violent injury. In a patient with multiple trauma, hypotension and tachycardia that do not respond to intravenous volume replacement when there is an obvious rise in central venous pressure should be clues to cardiac tamponade.1
If the patient has recently undergone a cardiac procedure (for example, cardiac surgery, myocardial biopsy, coronary intervention, electrophysiologic study with intracardiac electrodes, transvenous pacemaker placement, pacemaker lead extraction, or radiofrequency ablation), knowing about the procedure narrows the differential diagnosis when hypotension, tachycardia, and jugular venous distention develop.
PEARL 4: CARDIAC OR AORTIC RUPTURE REQUIRES SURGERY
When the etiology of cardiac tamponade is cardiac or aortic rupture, the treatment is surgical.
Painful sudden causes of cardiac tamponade include hemopericardium due to rupture of the free wall after myocardial infarction, and spontaneous or posttraumatic dissection and rupture of the ascending aorta. Prompt diagnosis is necessary, but since these lesions will not close and heal spontaneously, the definitive treatment should be surgery. Moreover, needle removal of intrapericardial blood that has been opposing further bleeding is sure to permit bleeding to recur, often with lethal consequences.2
Causes of cardiac tamponade that have a less-acute onset are likely to be complications of medical problems. Medical illnesses known to be associated with cardiac tamponade include:
- Infectious disease (idiopathic or viral, associated with smallpox vaccination, mycobacterial, purulent bacterial, fungal)
- Metastatic cancer (lung, breast, esophagus, lymphoma, pancreas, liver, leukemia, stomach, melanoma)3
- Connective tissue disease (rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis, scleroderma, Wegener granulomatosis, acute rheumatic fever)
- Endocrine disease (hypothyroidism)
- Drug side effects (procainamide, isoniazid, hydralazine, minoxidil, phenytoin, anticoagulants, methysergide)
- Inflammatory bowel disease (Crohn disease, ulcerative colitis)
- Congestive heart failure
- Uremia
- Radiation therapy
- Postmyocardial infarction syndrome (Dressler syndrome)
- Postpericardiotomy syndrome.
PEARL 5: REVIEW IMAGING BEFORE DIAGNOSING
What often brings a patient with cardiac tamponade to the attention of the physician is a finding on echocardiography, computed tomography, or magnetic resonance imaging of the chest.
Always review the imaging studies before making the diagnosis of cardiac tamponade. These tests must be reviewed to assess the anatomy and the size and location of the effusion. Particularly, one must look for atrial and right ventricular collapse and inferior vena caval plethora, which are echocardiographic signs of cardiac tamponade.4 Figures 1, 2, and 3 show imaging studies in a patient who presented with worsening cough 2 weeks after undergoing a cardiac procedure and who was found to have cardiac tamponade.
When the history and these imaging studies place cardiac tamponade high in the differential diagnosis as the cause of edema or dyspnea, it is time to reexamine the patient. The best first step is to measure pulsus paradoxus.
HOW PULSUS PARADOXUS OCCURS
To fully appreciate the subtleties of the next pearls, it is necessary to understand the pathophysiology of cardiac tamponade.
When pericardial fluid accumulation raises the pericardial pressure above the central venous pressure and pulmonary venous pressure (intravascular pressure), blood will not passively return to the right side of the heart from the venae cavae nor to the left side of the heart from the pulmonary veins unless it is influenced by the effects of respiration on intrathoracic pressure. During respiration, the right and left sides of the heart are alternately filled and deprived of their respective venous return.
During inspiration, as the intrathoracic pressure decreases, blood in the venae cavae empties into the right side of the heart, while blood in the pulmonary veins preferentially remains in the pulmonary veins, underfilling the left side of the heart. Since the right ventricle is more filled than the left ventricle during inspiration, the ventricular septum shifts from right to left, further opposing pulmonary venous return. As a result, during cardiac tamponade, the systemic blood pressure falls with inspiration.
During expiration the opposite occurs. Expiration decreases the intrathoracic volume, so the intrathoracic pressure rises. This tends to oppose vena caval return to the right side of the heart and to favor pulmonary venous return to the left side of the heart. The ventricular septum shifts from left to right, further accommodating left ventricular filling, raising stroke volume, and increasing blood pressure. This exaggerated alternate filling of the right and left sides of the heart during cardiac tamponade is what accounts for pulsus paradoxus, an inspiratory fall in systolic blood pressure of greater than 10 mm Hg.
If intravascular pressure is low (due to hemorrhage, dehydration, or diuretic therapy), the pressure in the pericardial space needed to oppose venous return is much less. In this low-pressure scenario, the results are low cardiac output and hypotension, which are treated by giving intravenous fluids to maintain intravascular volume.
PEARL 6: MEASURE PULSUS PARADOXUS
When cardiac tamponade is considered, one must always measure the pulsus paradoxus.
The term pulsus paradoxus was coined by Adolph Kussmaul in 1873, before physicians could even measure blood pressure. All they could do at that time was palpate the pulse and listen to the heart. Kussmaul described his observation as a conspicuous discrepancy between the cardiac action and the arterial pulse.
Although not described by Kussmaul, another explanation for this finding might be more suited to the use of the word “paradoxical.” When the pulse is palpated in a normal patient, with inspiration the pulse rate will increase via the Bainbridge reflex, and with expiration it will decrease. But in a patient with cardiac tamponade, there is a paradoxical inspiratory slowing of the pulse (because the decreased magnitude of the pulse at times makes it imperceptible) and an expiratory increase in pulse rate as the magnitude of the pulse again makes it palpable.
The magnitude of the fall in systolic blood pressure during inspiration has been used to estimate the level of hemodynamic impairment resulting from pericardial effusion.5 A rapidly accumulating pericardial effusion can have more hemodynamic impact than a much larger one that accumulates slowly. Thus, the intrapericardial pressure must be considered more than the volume of pericardial fluid.
When there is severe cardiac tamponade and overt pulsus paradoxus, simple palpation of a proximal arterial pulse can detect a marked inspiratory decrease or loss of the pulse, which returns with expiration. Tachycardia is almost always present, unless the cause is hypothyroidism.6
How to measure pulsus paradoxus with a manual sphygmomanometer
A stethoscope and manual sphygmomanometer are all that is needed to measure pulsus paradoxus. A noninvasive blood pressure monitor that averages multiple measurements cannot detect or quantify pulsus paradoxus.
The patient should be supine with the head elevated 30° to 45°, and the examiner should be seated comfortably at the patient’s side. The manometer should be on the opposite side of the patient in plain view of the examiner. Position the cuff on the arm above the elbow and place your stethoscope on the antecubital fossa. Then:
- Inflate the cuff 20 mm Hg above the highest systolic pressure previously auscultated.
- Slowly decrease the manometer pressure by 5 mm Hg and hold it there through two or three respiratory cycles while listening for the first Korotkoff (systolic) sound. Repeat this until you can hear the systolic sound (but only during expiration) and mentally note the pressure.
- Continue to decrease the manometer pressure by 5-mm Hg increments while listening. When the Korotkoff sounds no longer disappear with inspiration, mentally note this second value as well. The pulsus paradoxus is the difference between these values.
- When the Korotkoff sounds disappear as the manometer pressure is decreased, note this final value. This is the diastolic blood pressure.
PEARL 7: THE PLETHYSMOGRAM WAVE-FORM PARALLELS PULSUS PARADOXUS
Manual measurement of blood pressure and pulsus paradoxus can be difficult, especially in an obese patient or one with a fat-distorted arm on which the cuff does not maintain its position. In such patients, increased girth of the neck and abdomen also make it difficult to assess the jugular venous distention and visceral organ engorgement that characterize cardiac tamponade.
When the use of a sphygmomanometer is not possible, an arterial catheter can be inserted to demonstrate pulsus paradoxus. Simpler, however, is the novel use of another noninvasive instrument to detect and coarsely quantify pulsus paradoxus.7 The waveform on finger pulse oximetry can demonstrate pulsus paradoxus. The plethysmogram of the finger pulse oximeter can demonstrate the decrease in magnitude of the waveform with each inspiration (Figure 4).
Caution must be taken when interpreting this waveform, as with any measurement of pulsus paradoxus, to exclude a concomitant arrhythmia.
PEARL 8: PULSUS PARADOXUS WITHOUT CARDIAC TAMPONADE
Pulsus paradoxus can be present in the absence of cardiac tamponade. Once pulsus paradoxus of more than 10 mm Hg is measured, one must be sure the patient does not have a condition that can cause pulsus paradoxus without cardiac tamponade. Most of these are pulmonary conditions that necessitate an exaggerated inspiratory effort that can lower intrathoracic pressure sufficiently to oppose pulmonary venous return and cause a fall in systemic blood pressure:
- Chronic bronchitis
- Emphysema
- Mucus plug
- Pneumothorax
- Pulmonary embolism
- Stridor.
In these, there may be pulsus paradoxus, but not due to cardiac tamponade.
PEARL 9: CARDIAC TAMPONADE CAN BE PRESENT WITHOUT PULSUS PARADOXUS
Cardiac tamponade can be present without pulsus paradoxus. This occurs when certain conditions prevent inspiratory underfilling of the left ventricle relative to the filling of the right ventricle.8
How does this work? In cardiac tamponade, factors that drive the exaggerated fall in arterial pressure with inspiration (pulsus paradoxus) are the augmented right ventricular filling and the decreased left ventricular filling, both due to the lowering of the intrathoracic pressure. As the vena caval emptying is augmented, the right ventricular filling is increased, the ventricular septum shifts to the left, and pulmonary venous return to the heart is decreased.
Factors that can oppose pulsus paradoxus:
- Positive pressure ventilation prevents pulsus paradoxus by preventing the fall in intrathoracic pressure.
- Severe aortic regurgitation does not permit underfilling of the left ventricle during inspiration.
- An atrial septal defect will always equalize the right and left atrial pressures, preventing differential right ventricular and left ventricular filling with inspiration.
- Severe left ventricular hypertrophy does not permit the inspiratory shift of the ventricular septum from right to left that would otherwise lead to decreased left ventricular filling.
- Severe left ventricular dysfunction, with its low stroke volume and severe elevation of left ventricular end-diastolic pressure, never permits underfilling of the left ventricle, despite cardiac tamponade and an inspiratory decrease in intrathoracic pressure.
- Intravascular volume depletion due to hemorrhage, hemodialysis, or mistaken use of diuretics to treat edema can cause marked hypotension, making pulsus paradoxus impossible to detect.
Knowledge of underlying medical conditions, the likelihood of their causing cardiac tamponade, and the appearance of the echocardiogram prompt the physician to look further when the presence or absence of pulsus paradoxus does not fit with the working diagnosis.
The echocardiogram can give hints to the etiology of a pericardial effusion, such as clotted blood after trauma or a cardiac-perforating procedure, tumor studding of the epicardium,9 or fibrin strands indicating chronicity or an inflammatory process.10 Diastolic collapse of the right ventricle, more than collapse of the right atrium or left atrium, speaks for the severity of cardiac tamponade. With hemodynamically significant pericardial effusion and cardiac tamponade, the inferior vena cava is distended and does not decrease in size with inspiration unless there is severe intravascular volume depletion, at which time the inferior vena cava is underfilled throughout the respiratory cycle.
PEARL 10: PLAN HOW TO DRAIN
The size and location of the pericardial effusion and the patient’s hemodynamics must be integrated when deciding how to relieve cardiac tamponade. When cardiac tamponade is indeed severe and the patient and physician agree that it must be drained, the options are percutaneous needle aspiration (pericardiocentesis) and surgical pericardiostomy (creation of a pericardial window). Here again, as assessed by echocardiography, the access to the pericardial fluid should influence the choice.
Pericardiocentesis can be safely done if certain criteria are met. The patient must be able to lie still in the supine position, perhaps with the head of the bed elevated 30 degrees. Anticoagulation must be reversed or allowed time to resolve if drainage is not an emergency.
Pericardiocentesis can be risky or unsuccessful if there is not enough pericardial fluid to permit respiratory cardiac motion without perforating the heart with the needle; if the effusion is loculated (confined to a pocket) posteriorly; or if it is too far from the skin to permit precise control and placement of a spinal needle into the pericardial space. In cases of cardiac tamponade in which the anatomy indicates surgical pericardiostomy but severe hypotension prevents the induction of anesthesia and positive-pressure ventilation—which can result in profound, irreversible hypotension—percutaneous needle drainage (pericardiocentesis) should be performed in the operating room to relieve the tamponade before the induction of anesthesia and the surgical drainage.11
To reiterate, a suspected cardiac or aortic rupture that causes cardiac tamponade is usually large and not apt to self-seal. In such cases, the halt in the accumulation of pericardial blood is due to hypotension and not due to spontaneous resolution. Open surgical drainage is required from the outset because an initial success of pericardiocentesis yields to the recurrence of cardiac tamponade.
PEARL 11: ANTICIPATE WHAT THE FLUID SHOULD LOOK LIKE
Before performing pericardiocentesis, anticipate the appearance of the pericardial fluid on the basis of the presumed etiology, ie:
- Sanguinous—trauma, heart surgery, cardiac perforation from a procedure, anticoagulation, uremia, or malignancy
- Serous—congestive heart failure, acute radiation therapy
- Purulent—infections (natural or postoperative)
- Turbid (like gold paint)—mycobacterial infection, rheumatoid arthritis, myxedema
- Chylous—pericardium fistulized to the thoracic duct by a natural or postsurgical cause.
Sanguinous pericardial effusion encountered during a pericardiocentesis, if not anticipated, can be daunting and can cause the operator to question if it is the result of inadvertent needle placement in a cardiac chamber. If the needle is indeed in the heart, blood often surges out under pressure in pulses, which strongly suggests that the needle is not in the pericardial space and should be removed; but if confirmation of the location is needed before removing the needle, it can be done by injecting 2 mL of agitated sterile saline through the pericardiocentesis needle during echocardiographic imaging.12
Before inserting the needle, the ideal access location and needle angle must be determined by the operator with echocardiographic transducer in hand. The distance from skin to a point just through the parietal pericardium can also be measured at this time.
Once the needle is in the pericardial fluid (and you are confident of its placement), removal of 50 to 100 mL of the fluid with a large syringe can be enough to afford the patient easier breathing, higher blood pressure, and lower pulsus paradoxus—and even the physician will breathe easier. The same syringe can be filled and emptied multiple times. Less traumatic and more complete removal of pericardial fluid requires insertion of a multihole pigtail catheter over a J-tipped guidewire that is introduced through the needle.
PEARL 12: DRAIN SLOWLY TO AVOID PULMONARY EDEMA
Pulmonary edema is an uncommon complication of pericardiocentesis that might be avoidable. Heralded by sudden coughing and pink, frothy sputum, it can rapidly deteriorate into respiratory failure. The mechanism has been attributed to a sudden increase in right ventricular stroke volume and resultant left ventricular filling after the excess pericardial fluid has been removed, before the systemic arteries, which constrict to keep the systemic blood pressure up during cardiac tamponade, have had time to relax.13
To avoid this complication, if the volume of pericardial fluid responsible for cardiac tamponade is large, it should be removed slowly,14 stopping for a several-minute rest after each 250 mL. Catheter removal of pericardial fluid by gravity drainage over 24 hours has been suggested.15 A drawback to this approach is catheter clotting or sludging before all the fluid has been removed. It is helpful to keep the drainage catheter close to the patient’s body temperature to make the fluid less viscous. Output should be monitored hourly.
When the pericardial fluid has been completely drained, one must decide how long to leave the catheter in. One reason to remove the catheter at this time is that it causes pleuritic pain; another is to avoid introducing infection. A reason to leave the catheter in is to observe the effect of medical treatment on the hourly pericardial fluid output. Nonsteroidal anti-inflammatory drugs are the drugs of first choice when treating pericardial inflammation and suppressing production of pericardial fluid.16 In most cases the catheter should not be left in place for more than 3 days.
Laboratory analysis of the pericardial fluid should shed light on its suspected cause. Analysis usually involves chemistry testing, microscopic inspection of blood cell smears, cytology, microbiologic stains and cultures, and immunologic tests. Results often take days. Meyers and colleagues17 expound on this subject.
- Schiavone WA, Ghumrawi BK, Catalano DR, et al. The use of echocardiography in the emergency management of nonpenetrating traumatic cardiac rupture. Ann Emerg Med 1991; 20:1248–1250.
- Manuchehry A, Fontana GP, Gurudevan S, Marchevsky AM, Siegel RJ. Missed diagnosis of limited ascending aortic dissection by multiple imaging modalities leading to fatal cardiac tamponade and aortic rupture. Echocardiography 2011; 28:E187–E190.
- Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med 1993; 117:1027–1031.
- Tsang TS, Oh JK, Seward JB, Tajik AJ. Diagnostic value of echocardiography in cardiac tamponade. Herz 2000; 25:734–740.
- Curtiss EI, Reddy PS, Uretsky BF, Cecchetti AA. Pulsus paradoxus: definition and relation to the severity of cardiac tamponade. Am Heart J 1988; 115:391–398.
- Wang JL, Hsieh MJ, Lee CH, et al. Hypothyroid cardiac tamponade: clinical features, electrocardiography, pericardial fluid and management. Am J Med Sci 2010; 340:276–281.
- Tamburro RF, Ring JC, Womback K. Detection of pulsus paradoxus associated with large pericardial effusions in pediatric patients by analysis of the pulse-oximetry waveform. Pediatrics 2002; 109:673–677.
- Spodick DH. Pulsus paradoxus. In:Spodick DH, editor. The Pericardium: A Comprehensive Textbook. New York, NY: Marcel Dekker; 1997:191–199.
- Burke A, Jeudy J, Virmani R. Cardiac tumors. In:Topol EJ, editor. Textbook of Cardiovascular Medicine. 3rd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2007:710–720.
- Roberts WC. Pericardial heart disease: Its morphologic features and its causes. Proc (Bayl Univ Med Cent) 2005; 18:38–55.
- Stoelting RK, Miller RD, editors. Basics of Anesthesia. 4th ed. New York, NY: Churchill Livingstone; 2000:264–265.
- Ainsworth CD, Salehian O. Echo-guided pericardiocentesis: let the bubbles show the way. Circulation 2011; 123:e210–e211.
- Maisch B, Seferovic PM, Ristic AD, et al; Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J 2004; 25:587–610.
- Vandyke WH, Cure J, Chakko CS, Gheorghiade M. Pulmonary edema after pericardiocentesis for cardiac tamponade. N Engl J Med 1983; 309:595–596.
- Bernal JM, Pradhan J, Li T, Tchokonte R, Afonso L. Acute pulmonary edema following pericardiocentesis for cardiac tamponade. Can J Cardiol 2007; 23:1155–1156.
- Sagristà-Sauleda J, Mercé AS, Soler-Soler J. Diagnosis and management of pericardial effusion. World J Cardiol 2011; 3:135–143.
- Meyers DG, Meyers RE, Prendergast TW. The usefulness of diagnostic tests on pericardial fluid. Chest 1997; 111:1213–1221.
- Schiavone WA, Ghumrawi BK, Catalano DR, et al. The use of echocardiography in the emergency management of nonpenetrating traumatic cardiac rupture. Ann Emerg Med 1991; 20:1248–1250.
- Manuchehry A, Fontana GP, Gurudevan S, Marchevsky AM, Siegel RJ. Missed diagnosis of limited ascending aortic dissection by multiple imaging modalities leading to fatal cardiac tamponade and aortic rupture. Echocardiography 2011; 28:E187–E190.
- Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med 1993; 117:1027–1031.
- Tsang TS, Oh JK, Seward JB, Tajik AJ. Diagnostic value of echocardiography in cardiac tamponade. Herz 2000; 25:734–740.
- Curtiss EI, Reddy PS, Uretsky BF, Cecchetti AA. Pulsus paradoxus: definition and relation to the severity of cardiac tamponade. Am Heart J 1988; 115:391–398.
- Wang JL, Hsieh MJ, Lee CH, et al. Hypothyroid cardiac tamponade: clinical features, electrocardiography, pericardial fluid and management. Am J Med Sci 2010; 340:276–281.
- Tamburro RF, Ring JC, Womback K. Detection of pulsus paradoxus associated with large pericardial effusions in pediatric patients by analysis of the pulse-oximetry waveform. Pediatrics 2002; 109:673–677.
- Spodick DH. Pulsus paradoxus. In:Spodick DH, editor. The Pericardium: A Comprehensive Textbook. New York, NY: Marcel Dekker; 1997:191–199.
- Burke A, Jeudy J, Virmani R. Cardiac tumors. In:Topol EJ, editor. Textbook of Cardiovascular Medicine. 3rd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2007:710–720.
- Roberts WC. Pericardial heart disease: Its morphologic features and its causes. Proc (Bayl Univ Med Cent) 2005; 18:38–55.
- Stoelting RK, Miller RD, editors. Basics of Anesthesia. 4th ed. New York, NY: Churchill Livingstone; 2000:264–265.
- Ainsworth CD, Salehian O. Echo-guided pericardiocentesis: let the bubbles show the way. Circulation 2011; 123:e210–e211.
- Maisch B, Seferovic PM, Ristic AD, et al; Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J 2004; 25:587–610.
- Vandyke WH, Cure J, Chakko CS, Gheorghiade M. Pulmonary edema after pericardiocentesis for cardiac tamponade. N Engl J Med 1983; 309:595–596.
- Bernal JM, Pradhan J, Li T, Tchokonte R, Afonso L. Acute pulmonary edema following pericardiocentesis for cardiac tamponade. Can J Cardiol 2007; 23:1155–1156.
- Sagristà-Sauleda J, Mercé AS, Soler-Soler J. Diagnosis and management of pericardial effusion. World J Cardiol 2011; 3:135–143.
- Meyers DG, Meyers RE, Prendergast TW. The usefulness of diagnostic tests on pericardial fluid. Chest 1997; 111:1213–1221.
KEY POINTS
- Slow accumulation of pericardial fluid can result in edema, whereas rapid accumulation leads to hypotension.
- Diuretics can worsen tamponade by removing enough volume from the circulation to lower the central venous pressure below the intrapericardial pressure.
- Try to determine why cardiac tamponade has occurred. Cardiac or aortic rupture requires surgery. If the gross appearance of the pericardial fluid does not match the presumed etiology, reconsider your diagnosis.
- Always review imaging studies before making the diagnosis of cardiac tamponade.
- When cardiac tamponade is considered, pulsus paradoxus must be measured, and if present, integrated with other physical findings and the echocardiogram. However, pulsus paradoxus can be present in the absence of cardiac tamponade, and vice versa.
- Consider the size and location of the pericardial effusion and the patient’s hemodynamic status when deciding between surgery and needle aspiration.
Pregnant Woman, 39, With Hypertension and New-Onset Proteinuria
A 39-year-old black woman, gravida 1, para 0, with an intrauterine pregnancy of 34 weeks and three days (according to last menstrual period and nine-week ultrasound) presented to her Ob-Gyn office for a routine prenatal visit. She was found to have an elevated blood pressure with new onset of 2+ proteinuria. The patient was sent to the labor and delivery unit at the adjoining hospital for serial blood pressure readings, laboratory work, and fetal monitoring.
The patient’s previous medical history was limited to sinusitis. She was taking no prescription medications, and her only listed allergy was to pineapple. Initial lab studies revealed elevations in liver enzymes, lactate dehydrogenase (LDH), uric acid, and serum creatinine, as well as thrombocytopenia (see Table 11-5). She also had a critically low blood glucose level, which conflicted with a normal follow-up reading.
At this point, the patient was thought to have HELLP syndrome6 (ie, hemolysis, elevated liver enzymes, low platelet count), or possibly acute fatty liver of pregnancy (AFLP).2,4,7-11 Additional labs were drawn immediately to confirm or rule out AFLP. These included repeat serum glucose (following a second reading with normal results), a serum ammonia level, prothrombin time (PT), and partial thromboplastin time (PTT). The most reliable values to distinguish AFLP from HELLP are profound hypoglycemia (found in 94% of women with AFLP12) and an elevated serum ammonia level.4
Given the serious nature of either diagnosis, immediate delivery of the infant was deemed necessary. Because the patient’s cervix was not found favorable for induction, she underwent low-transverse cesarean delivery without complications. She was noted to have essentially normal anatomy with the exception of a small subserosal fibroid posteriorly. Meconium-stained amniotic fluid was present. A male infant was delivered, weighing 5 lb with 1-minute and 5-minute Apgar scores of 8 and 9, respectively.
Postoperatively, the patient remained in the recovery area, where she received intensive monitoring. She experienced fluctuations in blood glucose, ranging from 33 to 144 mg/dL; she was started on 5% dextrose in lactated Ringer’s solution and treated with IV dextrose 50 g. While the patient was in surgical recovery, results from the second set of labs, drawn before surgery, were returned; findings included an elevated ammonia level and an abnormal coagulation panel, including PT of 25.3 sec, PTT of 48.4 sec, and a fibrinogen level of 116 mg/dL, confirming the suspected diagnosis of AFLP.
Magnesium sulfate, which had been started immediately postop, was discontinued on confirmation of the diagnosis of AFLP. The patient was initially somnolent as a result of general anesthesia but gradually returned to a fully normal sensorium by early morning on postop day 1. Postoperatively, the patient’s hemoglobin was found to be low (8.6 g/dL; reference range, 13.5 to 18.5 g/dL), so she was transfused with two units of packed red blood cells (PRBCs) and given fresh frozen plasma (FFP) to correct this coagulopathy. The patient’s platelets were also low at 82,000/mm3 (reference range, 140,000 to 340,000/mm3).
On postop day 1, the patient’s serum creatinine rose to 4.2 mg/dL and her total bilirubin increased to 14.4 mg/dL (reference ranges, 0.6 to 1.2 mg/dL and < 1.0 mg/dL, respectively). Given the multiple systems affected by AFLP and the need for intensive supportive care, the patient was transferred to the ICU.
On her arrival at the ICU, the patient’s vital signs were initially stable, and she was alert and oriented. However, within the next few hours, she became hypotensive and encephalopathic. She required aggressive fluid resuscitation and multiple transfusions of PRBCs and FFP due to persistent anemia and coagulopathy. Her vital signs were stabilized, but she continued to need blood transfusions.
Postop day 2, the patient became less responsive and was soon unable to follow commands or speak clearly. Her breathing remained stable with just 3 L of oxygen by nasal cannula, but in order to prevent aspiration and in consideration of a postoperative ileus, it was necessary to place a nasogastric tube with low intermittent suction. This produced a bloody return, but no intervention other than close monitoring and transfusion was performed at that time.
Abdominal ultrasound showed ascites and mild left-sided hydronephrosis with no gallstones. The common bile duct measured 3 mm in diameter.
Although liver biopsy is considered the gold standard for a confirmed diagnosis of AFLP,13,14 this procedure was contraindicated by the patient’s coagulopathy. Concern was also expressed by one consultant that the patient might have thrombotic thrombocytopenic purpura (TTP) in addition to AFLP. TTP can manifest with similar findings, such as anemia, thrombocytopenia, neurologic symptoms, and renal abnormalities, but usually fever is involved, and the patient was afebrile. A catheter was placed for hemodialysis and therapeutic plasma exchange (TPE). Given that TTP-associated mortality is significantly decreased by use of TPE,15 this intervention was deemed prudent. The patient underwent TPE on three consecutive days, postop days 2 through 4.
The patient’s mental status began to improve, and by postop day 6, she was able to follow commands and engage in brief conversations. By postop day 9, she had returned almost completely to her baseline mental status.
The patient’s liver function test results and total bilirubin, ammonia, and creatinine levels all improved over the first few postoperative days but began to rise again by day 6. In response to worsening renal and hepatic functioning, the decision was made on postop day 9 to transfer the patient to a hospital with liver transplantation capabilities, should this procedure become necessary.
Discussion
AFLP is a rare condition specific to pregnancy, affecting 1/7,000 to 1/20,000 pregnancies. Due to the low incidence of this disease, randomized controlled trials to study it are not possible. Instead, clinicians must learn either from individual case studies or from retrospective syntheses of cases reported over time.1,2,7 Fortunately, the wealth of information gleaned over the past 30 years has significantly reduced AFLP-associated maternal and fetal mortality and morbidity rates. In the 1980s, maternal and fetal mortality rates as high as 85% were reported.3 Worldwide, maternal mortality associated with AFLP has decreased significantly to 7% to 18%, whereas the fetal mortality rate has fallen to between 9% and 23%.1,16,17
Common trends among women who have developed AFLP include nulliparity, multiparity, and advanced maternal age. One retrospective study of 57 women who had developed AFLP revealed that 35 cases (61%) involved first-time pregnancies. It also showed that 10 (18%) of the women had twins, and 14 (25%) were older than 35.2 In another study of 35 cases of AFLP, 40% of the women were nulliparous, and 11.4% were multiparous, including one triplet gestation.12 In a third, smaller study, 80% of women affected by AFLP were multiparous.10 Currently, there is no known evidence linking any maternal behavior to development of AFLP.
Presentation
Women who present with AFLP often experience vague, nonspecific symptoms, leading to misdiagnosis or delayed diagnosis. Objective measurements, including physical exam findings, laboratory studies, and other diagnostic tests, will help with a diagnosis. The most frequent initial symptoms are nausea and vomiting (in 70% of patients) and abdominal pain (50% to 80%), epigastric or right upper-quadrant.3 Other common symptoms include fatigue, malaise, anorexia, weight gain, polyuria, and polydipsia.2,3,9,18,19
Because the presenting symptoms in AFLP can be vague, clinicians should complete a thorough physical exam to differentiate accurately among conditions associated with pregnancy. Physical signs present in women with AFLP can include jaundice, ascites, edema, confusion, abdominal tenderness, and fever. More severe cases can present with multisystem involvement, including acute renal failure, gastrointestinal bleeding, pancreatitis, coagulopathy, and hepatic encephalopathy.3,4,9,18
Diagnostic Tests
Relevant laboratory tests include a complete blood count (CBC), liver studies, chemistry, coagulation studies, and urinalysis (see Table 1). Viral causes should be ruled out by way of a hepatitis panel.3 In AFLP, the CBC may show elevated white blood cells, decreased hemoglobin and hematocrit, and decreased platelets. Liver studies show elevated hepatic aminotransferase, bilirubin, LDH, and ammonia levels. Chemistry results show elevated blood urea nitrogen and creatinine, and decreased blood glucose. Coagulation factors are affected, and prolonged PTT, decreased fibrinogen, and proteinurea may also be found.9
Though invasive and not often necessary4,13 (and not possible for the case patient), the definitive diagnostic test for AFLP is liver biopsy.13,14 Biopsy reveals a microvesicular fatty infiltration of the hepatocytes as minute fat droplets surrounding a centrally located nucleus. These fatty infiltrates stain with oil red O, specific for fat. Inflammation is present in 50% of cases. There may also be a picture similar to cholestasis with bile thrombi or deposits within the hepatocytes.20
Due to the risk for hemorrhage and the critical status of women with AFLP, biopsy is often not possible. Ultrasonography may show increased echogenicity; CT may show decreased or diffuse attenuation in the liver. These imaging studies, though possibly helpful in severe cases, often yield false-negative results.3,20
In the absence of another explanation for the patient’s symptoms, the Swansea criteria are used for diagnosis of AFLP.1 Six or more of the following criteria must be present to confirm this diagnosis: vomiting, abdominal pain, polydipsia or polyuria, encephalopathy, leukocytosis, elevated bilirubin, elevated liver enzymes, elevated ammonia, hypoglycemia, renal dysfunction, coagulopathy, elevated uric acid, ascites on ultrasound, and microvesicular steatosis on liver biopsy.1,2,5
Pathophysiology
Normal functions of the liver include metabolism, protein synthesis, and manufacturing of blood coagulation proteins. These functions are disturbed in the presence of AFLP. Thus, women with this disease experience signs and symptoms related directly to the dysfunction of these processes.20-22
Disturbances in the hepatocytes due to excess fatty acids impair the liver’s ability to convert unconjugated bilirubin into conjugated bilirubin, causing plasma levels of unconjugated bilirubin to rise. This increase in bilirubin explains the jaundiced appearance of women with AFLP. AFLP is often thought to occur in conjunction with preeclampsia in many, but not all, patients. Thrombocytopenia in these patients is felt to be secondary to peripheral vascular consumption. Conjugated bilirubin levels may also be increased due to decreased flow of conjugated bilirubin into the common bile duct.21
Another liver function that is disrupted is that of glycogen storage and conversion to glucose, and the liver’s ability to convert nutrients into glycogen is also impaired. Decreased storage of glycogen, along with the liver’s inability to break down previously stored glycogen, causes a decrease in serum glucose levels. Women with AFLP often require treatment with IV dextrose in response to marked hypoglycemia.16,21,23
The liver dysfunction associated with AFLP reduces adequate production of clotting factors and coagulation proteins. Thrombocytopenia, elevated clotting times, and bleeding are all problems seen in AFLP. Mild to moderate elevations in serum aminotransferases and elevated LDH also occur in patients with AFLP.23,24
Genetic Factor
There is little known about the etiology of AFLP, although recent data point to a genetic component that was found in as many as 62% of mothers in one study and in 25% of infants in another study.20-22 Fatty acid oxidation (FAO) is one of the processes of hepatic mitochondria, a process that relies on several enzymes. When FAO is interrupted, fatty acids are deposited in the liver cells, as seen in histologic studies of AFLP.25,26 The common thread in women with this disease is a mutation in one of the enzymes needed for FAO. This enzyme is the long-chain 3-hydroxyacyl-CoA dehydrogenase. Deficiencies in this enzyme are common in mothers with AFLP and their infants.3,16,20,23,27
Differential Diagnosis
Several complications of pregnancy that involve the liver may, on presentation, mimic AFLP.16,20,23,24,28 The most common are hyperemesis gravidarum and intrahepatic cholestasis of pregnancy23 (see Table 216,20,23,24,28); others are preeclampsia/eclampsia and HELLP syndrome. It is important to distinguish between the signs and symptoms associated with each of these disorders in order to provide the most effective treatment. Hepatitis serologies are important in the differential diagnosis when liver enzyme levels are exceptionally high.4,16,22,28
Treatment
The most effective treatment for AFLP is delivery of the infant; often, this alone causes the signs and symptoms of AFLP to resolve.8,21,27,29 In two of three cases in a small study by Aso et al,8 early delivery of the fetus led to complete resolution of symptoms and return to normal liver function. One of these patients was sent home four days after delivery; the other, 14 days later. Other patients may require more invasive treatment and support.8
Management in the ICU is often required to provide appropriate supportive care to the mother after delivery. Acute respiratory distress syndrome, pancreatitis, hemorrhage, encephalopathy, renal failure, and continual liver failure are among the severe complications associated with AFLP.4,8,10 Many women require intubation, dialysis, fluid resuscitation, blood product transfusion, and vasopressor therapy.3,8,11 Prophylactic antibiotics, IV steroids, and glucose may all be required in the supportive care and recovery of a mother with AFLP.3,8,11
TPE has also been useful in instances of severe complications.1,3,6 In one retrospective study, Martin et al1 recommended administration of TPE in patients with AFLP under the following circumstances:
(1) Deteriorating central nervous system abnormalities, such as sensorium changes or coma;
(2) Persistent coagulopathy requiring continued and aggressive blood product support with plasma, red cells, and/or cryoprecipitate;
(3) Advanced renal dysfunction that compromised fluid management;
(4) Progressive cardiopulmonary compromise; and/or
(5) Ongoing fluid management concerns, including significant ascites, edema, anuria/oliguria, and/or fluid overload.1
In rare cases, liver transplantation is needed in patients with AFLP. Westbrook et al18 reviewed 54 cases of liver disease in pregnancy in one UK hospital between 1997 and 2008. Of these, six patients with encephalopathy or elevated lactate were listed for liver transplant, including just one with a diagnosis of AFLP. This woman never actually underwent transplant but recovered in response to medical management alone.18 According to data reported in June 2011 by the Organ Procurement and Transplantation Network,30 liver transplantation was needed in only three US patients with AFLP between 2000 and 2011. Further retrospective studies on outcomes from transplant versus medical management should be considered to guide future decision making involving this invasive therapy.
The Case Patient
This 39-year-old patient presented during a routine prenatal visit with proteinuria and hypertension, possibly indicative of preeclampsia. Because of the serious nature of this potential diagnosis in pregnancy, she was admitted for monitoring and further testing. Although the diagnosis of AFLP was not confirmed until later, the patient’s preliminary lab studies showed elevated liver enzymes and low platelet counts, signifying the need for prompt intervention and delivery of the infant. At this point, the patient met criteria for HELLP syndrome, but AFLP was suspected after the initial finding of profound hypoglycemia led to further testing.
As an older mother experiencing pregnancy for the first time, this patient fit the profile for AFLP. She initially responded well after delivery of her infant but continued to experience complications. On the days that the patient was treated with TPE, her total bilirubin and liver enzymes were at their lowest. Perhaps this treatment should be considered in more cases of AFLP.
The patient was transferred to a hospital with liver transplantation capabilities, but she ultimately recovered without undergoing transplant.
Conclusion
For the primary obstetric care provider, being aware of the possible complications associated with pregnancy is important. Though uncommon, AFLP is a serious complication that should be ruled out in women who present with vague symptoms such as nausea, vomiting, and abdominal pain in the third trimester of pregnancy. The reduction in AFLP-associated morbidity and mortality during the past 20 years is a direct result of increased early recognition and therapeutic delivery.
Referral to a maternal fetal medicine specialist, gastroenterologist, hematologist, and/or nephrologist may be necessary and appropriate in the management of a woman with AFLP. Further study is indicated for use of TPE in more severe cases of AFLP, particularly in women affected by persistent thrombocytopenia and anemia.
The author would like to thank C. Leanne Browning, MD, obstetrics/gynecology, for her invaluable guidance and advice on this project.
References
1. Martin JN Jr, Briery CM, Rose CH, et al. Postpartum plasma exchange as adjunctive therapy for severe acute fatty liver of pregnancy. J Clin Apher. 2009;23(4):138-143.
2. Knight M, Nelson-Piercy C, Kurinczuk JJ; UK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57(7):951-956.
3. Barsoom MJ, Tierney BJ. Acute fatty liver of pregnancy (2011). http://emedicine.medscape.com/article/1562425-overview. Accessed January 21, 2013.
4. Ko HH, Yoshida E. Acute fatty liver of pregnancy. Can J Gastroenterol. 2006;20(1):25-30.
5. Rathi U, Bapat M, Rathi P, Abraham P. Effect of liver disease on maternal and fetal outcome: a prospective study. Indian J Gastroenterol. 2007;26(2):59-63.
6. Myers L. Postpartum plasma exchange in a woman with suspected thrombotic thrombocytopenic purpura (TTP) vs hemolysis, elevated liver enzymes, and low platelet syndrome (HELLP): a case study. Nephrol Nurs J. 2010;37(4):399-402.
7. Vigil-de Gracia P. Acute fatty liver and HELLP syndrome: two distinct pregnancy disorders. Int J Gynaecol Obstet. 2001;73(3):215-220.
8. Aso K, Hojo S, Yumoto Y, et al. Three cases of acute fatty liver of pregnancy: postpartum clinical course depends on interval between onset of symptoms and termination of pregnancy. J Matern Fetal Neonatal Med. 2010;23(9):1047-1049.
9. Wei Q, Zhang L, Liu X. Clinical diagnosis and treatment of acute fatty liver of pregnancy: a literature review and 11 new cases. J Obstet Gynaecol Res. 2010;36(4):751-756.
10. Barber MA, Eguiluz I, Martin A, et al. Acute fatty liver of pregnancy: analysis of five consecutive cases from a tertiary centre. J Obstet Gynaecol. 2010;30(3):241-243.
11. Ajayi AO, Alao MO. Case report: acute fatty liver of pregnancy in a 30-year-old Nigerian primigravida. Niger J Clin Pract. 2008;11(4):389-391.
12. Vigíl-de Gracia P, Montufar-Rueda C. Acute fatty liver of pregnancy: diagnosis, treatment, and outcome based on 35 consecutive cases. J Matern Fetal Neonatal Med. 2011;24(9):1143-1146.
13. Dey M, Reema K. Acute fatty liver of pregnancy. N Am J Med Sci. 2012;4(11):611-612.
14. Castro MA, Goodwin TM, Shaw KJ, et al. Disseminated intravascular coagulation and antithrombin III depression in acute fatty liver of pregnancy. Am J Obstet Gynecol. 1996;174(1 pt 1):211-216.
15. Altuntas F, Aydogdu I, Kabukcu S, et al. Therapeutic plasma exchange for the treatment of thrombotic thrombocytopenic purpura: a retrospective multicenter study. Transfus Apher Sci. 2007;36(1):57-67.
16. Hay JE. Liver disease in pregnancy. Hepatology. 2008;47(3):1067-1076.
17. Wand S, Waeschle RM, Von Ahsen N, et al. Acute fatty liver failure due to acute fatty liver of pregnancy. Minerva Anesthesiol. 2012;78(4):503-506.
18. Westbrook RH, Yeoman AD, Joshi D, et al. Outcomes of severe pregnancy-related liver disease: refining the role of transplantation. Am J Transplant. 2010;10(11):2520-2526.
19. Fesenmeier MF, Coppage KH, Lambers DS, et al. Acute fatty liver of pregnancy in 3 tertiary care centers. Am J Obstet Gynecol. 2005;192(5):1416-1419.
20. Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol. 2011;35(3):182-193.
21. Huether SE. Alterations of digestive function. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1452-1515.
22. Huether SE. Structure and function of the digestive system. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1420-1451.
23. Schutt VA, Minuk GY. Liver diseases unique to pregnancy. Best Pract Res Clin Gastroenterol. 2007;21(5):771-792.
24. Pan C, Perumalswami PV. Pregnancy-related liver diseases. Clin Liver Dis. 2011;15(1):199-208.
25. Ibdah JA. Acute fatty liver of pregnancy: an update on pathogenesis and clinical implications. World J Gastroenterol. 2006;12(46):7397-7404.
26. Browning MF, Levy HL, Wilkins-Haug LE, et al. Fetal fatty acid oxidation defects and maternal liver disease in pregnancy. Obstet Gynecol. 2006;107(1):115-120.
27. Dekker RR, Schutte JM, Stekelenburg J, et al. Maternal mortality and severe maternal morbidity from acute fatty liver of pregnancy in the Netherlands. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):27-31.
28. Lee NM, Brady CW. Liver disease in pregnancy. World J Gastroenterol. 2009;15(8):897-906.
29. Vora KS, Shah VR, Parikh GP. Acute fatty liver of pregnancy: a case report of an uncommon disease. Indian J Crit Care Med. 2009;13(1):34-36.
30. Organ Procurement and Transplantation Network, Scientific Registry of Transplant Recipients. OPTN/SRTR 2011 Annual Data Report: Liver. http://srtr.transplant.hrsa.gov/annual_reports/2011/pdf/03_%20liver_12.pdf. Accessed January 18, 2013.
A 39-year-old black woman, gravida 1, para 0, with an intrauterine pregnancy of 34 weeks and three days (according to last menstrual period and nine-week ultrasound) presented to her Ob-Gyn office for a routine prenatal visit. She was found to have an elevated blood pressure with new onset of 2+ proteinuria. The patient was sent to the labor and delivery unit at the adjoining hospital for serial blood pressure readings, laboratory work, and fetal monitoring.
The patient’s previous medical history was limited to sinusitis. She was taking no prescription medications, and her only listed allergy was to pineapple. Initial lab studies revealed elevations in liver enzymes, lactate dehydrogenase (LDH), uric acid, and serum creatinine, as well as thrombocytopenia (see Table 11-5). She also had a critically low blood glucose level, which conflicted with a normal follow-up reading.
At this point, the patient was thought to have HELLP syndrome6 (ie, hemolysis, elevated liver enzymes, low platelet count), or possibly acute fatty liver of pregnancy (AFLP).2,4,7-11 Additional labs were drawn immediately to confirm or rule out AFLP. These included repeat serum glucose (following a second reading with normal results), a serum ammonia level, prothrombin time (PT), and partial thromboplastin time (PTT). The most reliable values to distinguish AFLP from HELLP are profound hypoglycemia (found in 94% of women with AFLP12) and an elevated serum ammonia level.4
Given the serious nature of either diagnosis, immediate delivery of the infant was deemed necessary. Because the patient’s cervix was not found favorable for induction, she underwent low-transverse cesarean delivery without complications. She was noted to have essentially normal anatomy with the exception of a small subserosal fibroid posteriorly. Meconium-stained amniotic fluid was present. A male infant was delivered, weighing 5 lb with 1-minute and 5-minute Apgar scores of 8 and 9, respectively.
Postoperatively, the patient remained in the recovery area, where she received intensive monitoring. She experienced fluctuations in blood glucose, ranging from 33 to 144 mg/dL; she was started on 5% dextrose in lactated Ringer’s solution and treated with IV dextrose 50 g. While the patient was in surgical recovery, results from the second set of labs, drawn before surgery, were returned; findings included an elevated ammonia level and an abnormal coagulation panel, including PT of 25.3 sec, PTT of 48.4 sec, and a fibrinogen level of 116 mg/dL, confirming the suspected diagnosis of AFLP.
Magnesium sulfate, which had been started immediately postop, was discontinued on confirmation of the diagnosis of AFLP. The patient was initially somnolent as a result of general anesthesia but gradually returned to a fully normal sensorium by early morning on postop day 1. Postoperatively, the patient’s hemoglobin was found to be low (8.6 g/dL; reference range, 13.5 to 18.5 g/dL), so she was transfused with two units of packed red blood cells (PRBCs) and given fresh frozen plasma (FFP) to correct this coagulopathy. The patient’s platelets were also low at 82,000/mm3 (reference range, 140,000 to 340,000/mm3).
On postop day 1, the patient’s serum creatinine rose to 4.2 mg/dL and her total bilirubin increased to 14.4 mg/dL (reference ranges, 0.6 to 1.2 mg/dL and < 1.0 mg/dL, respectively). Given the multiple systems affected by AFLP and the need for intensive supportive care, the patient was transferred to the ICU.
On her arrival at the ICU, the patient’s vital signs were initially stable, and she was alert and oriented. However, within the next few hours, she became hypotensive and encephalopathic. She required aggressive fluid resuscitation and multiple transfusions of PRBCs and FFP due to persistent anemia and coagulopathy. Her vital signs were stabilized, but she continued to need blood transfusions.
Postop day 2, the patient became less responsive and was soon unable to follow commands or speak clearly. Her breathing remained stable with just 3 L of oxygen by nasal cannula, but in order to prevent aspiration and in consideration of a postoperative ileus, it was necessary to place a nasogastric tube with low intermittent suction. This produced a bloody return, but no intervention other than close monitoring and transfusion was performed at that time.
Abdominal ultrasound showed ascites and mild left-sided hydronephrosis with no gallstones. The common bile duct measured 3 mm in diameter.
Although liver biopsy is considered the gold standard for a confirmed diagnosis of AFLP,13,14 this procedure was contraindicated by the patient’s coagulopathy. Concern was also expressed by one consultant that the patient might have thrombotic thrombocytopenic purpura (TTP) in addition to AFLP. TTP can manifest with similar findings, such as anemia, thrombocytopenia, neurologic symptoms, and renal abnormalities, but usually fever is involved, and the patient was afebrile. A catheter was placed for hemodialysis and therapeutic plasma exchange (TPE). Given that TTP-associated mortality is significantly decreased by use of TPE,15 this intervention was deemed prudent. The patient underwent TPE on three consecutive days, postop days 2 through 4.
The patient’s mental status began to improve, and by postop day 6, she was able to follow commands and engage in brief conversations. By postop day 9, she had returned almost completely to her baseline mental status.
The patient’s liver function test results and total bilirubin, ammonia, and creatinine levels all improved over the first few postoperative days but began to rise again by day 6. In response to worsening renal and hepatic functioning, the decision was made on postop day 9 to transfer the patient to a hospital with liver transplantation capabilities, should this procedure become necessary.
Discussion
AFLP is a rare condition specific to pregnancy, affecting 1/7,000 to 1/20,000 pregnancies. Due to the low incidence of this disease, randomized controlled trials to study it are not possible. Instead, clinicians must learn either from individual case studies or from retrospective syntheses of cases reported over time.1,2,7 Fortunately, the wealth of information gleaned over the past 30 years has significantly reduced AFLP-associated maternal and fetal mortality and morbidity rates. In the 1980s, maternal and fetal mortality rates as high as 85% were reported.3 Worldwide, maternal mortality associated with AFLP has decreased significantly to 7% to 18%, whereas the fetal mortality rate has fallen to between 9% and 23%.1,16,17
Common trends among women who have developed AFLP include nulliparity, multiparity, and advanced maternal age. One retrospective study of 57 women who had developed AFLP revealed that 35 cases (61%) involved first-time pregnancies. It also showed that 10 (18%) of the women had twins, and 14 (25%) were older than 35.2 In another study of 35 cases of AFLP, 40% of the women were nulliparous, and 11.4% were multiparous, including one triplet gestation.12 In a third, smaller study, 80% of women affected by AFLP were multiparous.10 Currently, there is no known evidence linking any maternal behavior to development of AFLP.
Presentation
Women who present with AFLP often experience vague, nonspecific symptoms, leading to misdiagnosis or delayed diagnosis. Objective measurements, including physical exam findings, laboratory studies, and other diagnostic tests, will help with a diagnosis. The most frequent initial symptoms are nausea and vomiting (in 70% of patients) and abdominal pain (50% to 80%), epigastric or right upper-quadrant.3 Other common symptoms include fatigue, malaise, anorexia, weight gain, polyuria, and polydipsia.2,3,9,18,19
Because the presenting symptoms in AFLP can be vague, clinicians should complete a thorough physical exam to differentiate accurately among conditions associated with pregnancy. Physical signs present in women with AFLP can include jaundice, ascites, edema, confusion, abdominal tenderness, and fever. More severe cases can present with multisystem involvement, including acute renal failure, gastrointestinal bleeding, pancreatitis, coagulopathy, and hepatic encephalopathy.3,4,9,18
Diagnostic Tests
Relevant laboratory tests include a complete blood count (CBC), liver studies, chemistry, coagulation studies, and urinalysis (see Table 1). Viral causes should be ruled out by way of a hepatitis panel.3 In AFLP, the CBC may show elevated white blood cells, decreased hemoglobin and hematocrit, and decreased platelets. Liver studies show elevated hepatic aminotransferase, bilirubin, LDH, and ammonia levels. Chemistry results show elevated blood urea nitrogen and creatinine, and decreased blood glucose. Coagulation factors are affected, and prolonged PTT, decreased fibrinogen, and proteinurea may also be found.9
Though invasive and not often necessary4,13 (and not possible for the case patient), the definitive diagnostic test for AFLP is liver biopsy.13,14 Biopsy reveals a microvesicular fatty infiltration of the hepatocytes as minute fat droplets surrounding a centrally located nucleus. These fatty infiltrates stain with oil red O, specific for fat. Inflammation is present in 50% of cases. There may also be a picture similar to cholestasis with bile thrombi or deposits within the hepatocytes.20
Due to the risk for hemorrhage and the critical status of women with AFLP, biopsy is often not possible. Ultrasonography may show increased echogenicity; CT may show decreased or diffuse attenuation in the liver. These imaging studies, though possibly helpful in severe cases, often yield false-negative results.3,20
In the absence of another explanation for the patient’s symptoms, the Swansea criteria are used for diagnosis of AFLP.1 Six or more of the following criteria must be present to confirm this diagnosis: vomiting, abdominal pain, polydipsia or polyuria, encephalopathy, leukocytosis, elevated bilirubin, elevated liver enzymes, elevated ammonia, hypoglycemia, renal dysfunction, coagulopathy, elevated uric acid, ascites on ultrasound, and microvesicular steatosis on liver biopsy.1,2,5
Pathophysiology
Normal functions of the liver include metabolism, protein synthesis, and manufacturing of blood coagulation proteins. These functions are disturbed in the presence of AFLP. Thus, women with this disease experience signs and symptoms related directly to the dysfunction of these processes.20-22
Disturbances in the hepatocytes due to excess fatty acids impair the liver’s ability to convert unconjugated bilirubin into conjugated bilirubin, causing plasma levels of unconjugated bilirubin to rise. This increase in bilirubin explains the jaundiced appearance of women with AFLP. AFLP is often thought to occur in conjunction with preeclampsia in many, but not all, patients. Thrombocytopenia in these patients is felt to be secondary to peripheral vascular consumption. Conjugated bilirubin levels may also be increased due to decreased flow of conjugated bilirubin into the common bile duct.21
Another liver function that is disrupted is that of glycogen storage and conversion to glucose, and the liver’s ability to convert nutrients into glycogen is also impaired. Decreased storage of glycogen, along with the liver’s inability to break down previously stored glycogen, causes a decrease in serum glucose levels. Women with AFLP often require treatment with IV dextrose in response to marked hypoglycemia.16,21,23
The liver dysfunction associated with AFLP reduces adequate production of clotting factors and coagulation proteins. Thrombocytopenia, elevated clotting times, and bleeding are all problems seen in AFLP. Mild to moderate elevations in serum aminotransferases and elevated LDH also occur in patients with AFLP.23,24
Genetic Factor
There is little known about the etiology of AFLP, although recent data point to a genetic component that was found in as many as 62% of mothers in one study and in 25% of infants in another study.20-22 Fatty acid oxidation (FAO) is one of the processes of hepatic mitochondria, a process that relies on several enzymes. When FAO is interrupted, fatty acids are deposited in the liver cells, as seen in histologic studies of AFLP.25,26 The common thread in women with this disease is a mutation in one of the enzymes needed for FAO. This enzyme is the long-chain 3-hydroxyacyl-CoA dehydrogenase. Deficiencies in this enzyme are common in mothers with AFLP and their infants.3,16,20,23,27
Differential Diagnosis
Several complications of pregnancy that involve the liver may, on presentation, mimic AFLP.16,20,23,24,28 The most common are hyperemesis gravidarum and intrahepatic cholestasis of pregnancy23 (see Table 216,20,23,24,28); others are preeclampsia/eclampsia and HELLP syndrome. It is important to distinguish between the signs and symptoms associated with each of these disorders in order to provide the most effective treatment. Hepatitis serologies are important in the differential diagnosis when liver enzyme levels are exceptionally high.4,16,22,28
Treatment
The most effective treatment for AFLP is delivery of the infant; often, this alone causes the signs and symptoms of AFLP to resolve.8,21,27,29 In two of three cases in a small study by Aso et al,8 early delivery of the fetus led to complete resolution of symptoms and return to normal liver function. One of these patients was sent home four days after delivery; the other, 14 days later. Other patients may require more invasive treatment and support.8
Management in the ICU is often required to provide appropriate supportive care to the mother after delivery. Acute respiratory distress syndrome, pancreatitis, hemorrhage, encephalopathy, renal failure, and continual liver failure are among the severe complications associated with AFLP.4,8,10 Many women require intubation, dialysis, fluid resuscitation, blood product transfusion, and vasopressor therapy.3,8,11 Prophylactic antibiotics, IV steroids, and glucose may all be required in the supportive care and recovery of a mother with AFLP.3,8,11
TPE has also been useful in instances of severe complications.1,3,6 In one retrospective study, Martin et al1 recommended administration of TPE in patients with AFLP under the following circumstances:
(1) Deteriorating central nervous system abnormalities, such as sensorium changes or coma;
(2) Persistent coagulopathy requiring continued and aggressive blood product support with plasma, red cells, and/or cryoprecipitate;
(3) Advanced renal dysfunction that compromised fluid management;
(4) Progressive cardiopulmonary compromise; and/or
(5) Ongoing fluid management concerns, including significant ascites, edema, anuria/oliguria, and/or fluid overload.1
In rare cases, liver transplantation is needed in patients with AFLP. Westbrook et al18 reviewed 54 cases of liver disease in pregnancy in one UK hospital between 1997 and 2008. Of these, six patients with encephalopathy or elevated lactate were listed for liver transplant, including just one with a diagnosis of AFLP. This woman never actually underwent transplant but recovered in response to medical management alone.18 According to data reported in June 2011 by the Organ Procurement and Transplantation Network,30 liver transplantation was needed in only three US patients with AFLP between 2000 and 2011. Further retrospective studies on outcomes from transplant versus medical management should be considered to guide future decision making involving this invasive therapy.
The Case Patient
This 39-year-old patient presented during a routine prenatal visit with proteinuria and hypertension, possibly indicative of preeclampsia. Because of the serious nature of this potential diagnosis in pregnancy, she was admitted for monitoring and further testing. Although the diagnosis of AFLP was not confirmed until later, the patient’s preliminary lab studies showed elevated liver enzymes and low platelet counts, signifying the need for prompt intervention and delivery of the infant. At this point, the patient met criteria for HELLP syndrome, but AFLP was suspected after the initial finding of profound hypoglycemia led to further testing.
As an older mother experiencing pregnancy for the first time, this patient fit the profile for AFLP. She initially responded well after delivery of her infant but continued to experience complications. On the days that the patient was treated with TPE, her total bilirubin and liver enzymes were at their lowest. Perhaps this treatment should be considered in more cases of AFLP.
The patient was transferred to a hospital with liver transplantation capabilities, but she ultimately recovered without undergoing transplant.
Conclusion
For the primary obstetric care provider, being aware of the possible complications associated with pregnancy is important. Though uncommon, AFLP is a serious complication that should be ruled out in women who present with vague symptoms such as nausea, vomiting, and abdominal pain in the third trimester of pregnancy. The reduction in AFLP-associated morbidity and mortality during the past 20 years is a direct result of increased early recognition and therapeutic delivery.
Referral to a maternal fetal medicine specialist, gastroenterologist, hematologist, and/or nephrologist may be necessary and appropriate in the management of a woman with AFLP. Further study is indicated for use of TPE in more severe cases of AFLP, particularly in women affected by persistent thrombocytopenia and anemia.
The author would like to thank C. Leanne Browning, MD, obstetrics/gynecology, for her invaluable guidance and advice on this project.
References
1. Martin JN Jr, Briery CM, Rose CH, et al. Postpartum plasma exchange as adjunctive therapy for severe acute fatty liver of pregnancy. J Clin Apher. 2009;23(4):138-143.
2. Knight M, Nelson-Piercy C, Kurinczuk JJ; UK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57(7):951-956.
3. Barsoom MJ, Tierney BJ. Acute fatty liver of pregnancy (2011). http://emedicine.medscape.com/article/1562425-overview. Accessed January 21, 2013.
4. Ko HH, Yoshida E. Acute fatty liver of pregnancy. Can J Gastroenterol. 2006;20(1):25-30.
5. Rathi U, Bapat M, Rathi P, Abraham P. Effect of liver disease on maternal and fetal outcome: a prospective study. Indian J Gastroenterol. 2007;26(2):59-63.
6. Myers L. Postpartum plasma exchange in a woman with suspected thrombotic thrombocytopenic purpura (TTP) vs hemolysis, elevated liver enzymes, and low platelet syndrome (HELLP): a case study. Nephrol Nurs J. 2010;37(4):399-402.
7. Vigil-de Gracia P. Acute fatty liver and HELLP syndrome: two distinct pregnancy disorders. Int J Gynaecol Obstet. 2001;73(3):215-220.
8. Aso K, Hojo S, Yumoto Y, et al. Three cases of acute fatty liver of pregnancy: postpartum clinical course depends on interval between onset of symptoms and termination of pregnancy. J Matern Fetal Neonatal Med. 2010;23(9):1047-1049.
9. Wei Q, Zhang L, Liu X. Clinical diagnosis and treatment of acute fatty liver of pregnancy: a literature review and 11 new cases. J Obstet Gynaecol Res. 2010;36(4):751-756.
10. Barber MA, Eguiluz I, Martin A, et al. Acute fatty liver of pregnancy: analysis of five consecutive cases from a tertiary centre. J Obstet Gynaecol. 2010;30(3):241-243.
11. Ajayi AO, Alao MO. Case report: acute fatty liver of pregnancy in a 30-year-old Nigerian primigravida. Niger J Clin Pract. 2008;11(4):389-391.
12. Vigíl-de Gracia P, Montufar-Rueda C. Acute fatty liver of pregnancy: diagnosis, treatment, and outcome based on 35 consecutive cases. J Matern Fetal Neonatal Med. 2011;24(9):1143-1146.
13. Dey M, Reema K. Acute fatty liver of pregnancy. N Am J Med Sci. 2012;4(11):611-612.
14. Castro MA, Goodwin TM, Shaw KJ, et al. Disseminated intravascular coagulation and antithrombin III depression in acute fatty liver of pregnancy. Am J Obstet Gynecol. 1996;174(1 pt 1):211-216.
15. Altuntas F, Aydogdu I, Kabukcu S, et al. Therapeutic plasma exchange for the treatment of thrombotic thrombocytopenic purpura: a retrospective multicenter study. Transfus Apher Sci. 2007;36(1):57-67.
16. Hay JE. Liver disease in pregnancy. Hepatology. 2008;47(3):1067-1076.
17. Wand S, Waeschle RM, Von Ahsen N, et al. Acute fatty liver failure due to acute fatty liver of pregnancy. Minerva Anesthesiol. 2012;78(4):503-506.
18. Westbrook RH, Yeoman AD, Joshi D, et al. Outcomes of severe pregnancy-related liver disease: refining the role of transplantation. Am J Transplant. 2010;10(11):2520-2526.
19. Fesenmeier MF, Coppage KH, Lambers DS, et al. Acute fatty liver of pregnancy in 3 tertiary care centers. Am J Obstet Gynecol. 2005;192(5):1416-1419.
20. Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol. 2011;35(3):182-193.
21. Huether SE. Alterations of digestive function. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1452-1515.
22. Huether SE. Structure and function of the digestive system. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1420-1451.
23. Schutt VA, Minuk GY. Liver diseases unique to pregnancy. Best Pract Res Clin Gastroenterol. 2007;21(5):771-792.
24. Pan C, Perumalswami PV. Pregnancy-related liver diseases. Clin Liver Dis. 2011;15(1):199-208.
25. Ibdah JA. Acute fatty liver of pregnancy: an update on pathogenesis and clinical implications. World J Gastroenterol. 2006;12(46):7397-7404.
26. Browning MF, Levy HL, Wilkins-Haug LE, et al. Fetal fatty acid oxidation defects and maternal liver disease in pregnancy. Obstet Gynecol. 2006;107(1):115-120.
27. Dekker RR, Schutte JM, Stekelenburg J, et al. Maternal mortality and severe maternal morbidity from acute fatty liver of pregnancy in the Netherlands. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):27-31.
28. Lee NM, Brady CW. Liver disease in pregnancy. World J Gastroenterol. 2009;15(8):897-906.
29. Vora KS, Shah VR, Parikh GP. Acute fatty liver of pregnancy: a case report of an uncommon disease. Indian J Crit Care Med. 2009;13(1):34-36.
30. Organ Procurement and Transplantation Network, Scientific Registry of Transplant Recipients. OPTN/SRTR 2011 Annual Data Report: Liver. http://srtr.transplant.hrsa.gov/annual_reports/2011/pdf/03_%20liver_12.pdf. Accessed January 18, 2013.
A 39-year-old black woman, gravida 1, para 0, with an intrauterine pregnancy of 34 weeks and three days (according to last menstrual period and nine-week ultrasound) presented to her Ob-Gyn office for a routine prenatal visit. She was found to have an elevated blood pressure with new onset of 2+ proteinuria. The patient was sent to the labor and delivery unit at the adjoining hospital for serial blood pressure readings, laboratory work, and fetal monitoring.
The patient’s previous medical history was limited to sinusitis. She was taking no prescription medications, and her only listed allergy was to pineapple. Initial lab studies revealed elevations in liver enzymes, lactate dehydrogenase (LDH), uric acid, and serum creatinine, as well as thrombocytopenia (see Table 11-5). She also had a critically low blood glucose level, which conflicted with a normal follow-up reading.
At this point, the patient was thought to have HELLP syndrome6 (ie, hemolysis, elevated liver enzymes, low platelet count), or possibly acute fatty liver of pregnancy (AFLP).2,4,7-11 Additional labs were drawn immediately to confirm or rule out AFLP. These included repeat serum glucose (following a second reading with normal results), a serum ammonia level, prothrombin time (PT), and partial thromboplastin time (PTT). The most reliable values to distinguish AFLP from HELLP are profound hypoglycemia (found in 94% of women with AFLP12) and an elevated serum ammonia level.4
Given the serious nature of either diagnosis, immediate delivery of the infant was deemed necessary. Because the patient’s cervix was not found favorable for induction, she underwent low-transverse cesarean delivery without complications. She was noted to have essentially normal anatomy with the exception of a small subserosal fibroid posteriorly. Meconium-stained amniotic fluid was present. A male infant was delivered, weighing 5 lb with 1-minute and 5-minute Apgar scores of 8 and 9, respectively.
Postoperatively, the patient remained in the recovery area, where she received intensive monitoring. She experienced fluctuations in blood glucose, ranging from 33 to 144 mg/dL; she was started on 5% dextrose in lactated Ringer’s solution and treated with IV dextrose 50 g. While the patient was in surgical recovery, results from the second set of labs, drawn before surgery, were returned; findings included an elevated ammonia level and an abnormal coagulation panel, including PT of 25.3 sec, PTT of 48.4 sec, and a fibrinogen level of 116 mg/dL, confirming the suspected diagnosis of AFLP.
Magnesium sulfate, which had been started immediately postop, was discontinued on confirmation of the diagnosis of AFLP. The patient was initially somnolent as a result of general anesthesia but gradually returned to a fully normal sensorium by early morning on postop day 1. Postoperatively, the patient’s hemoglobin was found to be low (8.6 g/dL; reference range, 13.5 to 18.5 g/dL), so she was transfused with two units of packed red blood cells (PRBCs) and given fresh frozen plasma (FFP) to correct this coagulopathy. The patient’s platelets were also low at 82,000/mm3 (reference range, 140,000 to 340,000/mm3).
On postop day 1, the patient’s serum creatinine rose to 4.2 mg/dL and her total bilirubin increased to 14.4 mg/dL (reference ranges, 0.6 to 1.2 mg/dL and < 1.0 mg/dL, respectively). Given the multiple systems affected by AFLP and the need for intensive supportive care, the patient was transferred to the ICU.
On her arrival at the ICU, the patient’s vital signs were initially stable, and she was alert and oriented. However, within the next few hours, she became hypotensive and encephalopathic. She required aggressive fluid resuscitation and multiple transfusions of PRBCs and FFP due to persistent anemia and coagulopathy. Her vital signs were stabilized, but she continued to need blood transfusions.
Postop day 2, the patient became less responsive and was soon unable to follow commands or speak clearly. Her breathing remained stable with just 3 L of oxygen by nasal cannula, but in order to prevent aspiration and in consideration of a postoperative ileus, it was necessary to place a nasogastric tube with low intermittent suction. This produced a bloody return, but no intervention other than close monitoring and transfusion was performed at that time.
Abdominal ultrasound showed ascites and mild left-sided hydronephrosis with no gallstones. The common bile duct measured 3 mm in diameter.
Although liver biopsy is considered the gold standard for a confirmed diagnosis of AFLP,13,14 this procedure was contraindicated by the patient’s coagulopathy. Concern was also expressed by one consultant that the patient might have thrombotic thrombocytopenic purpura (TTP) in addition to AFLP. TTP can manifest with similar findings, such as anemia, thrombocytopenia, neurologic symptoms, and renal abnormalities, but usually fever is involved, and the patient was afebrile. A catheter was placed for hemodialysis and therapeutic plasma exchange (TPE). Given that TTP-associated mortality is significantly decreased by use of TPE,15 this intervention was deemed prudent. The patient underwent TPE on three consecutive days, postop days 2 through 4.
The patient’s mental status began to improve, and by postop day 6, she was able to follow commands and engage in brief conversations. By postop day 9, she had returned almost completely to her baseline mental status.
The patient’s liver function test results and total bilirubin, ammonia, and creatinine levels all improved over the first few postoperative days but began to rise again by day 6. In response to worsening renal and hepatic functioning, the decision was made on postop day 9 to transfer the patient to a hospital with liver transplantation capabilities, should this procedure become necessary.
Discussion
AFLP is a rare condition specific to pregnancy, affecting 1/7,000 to 1/20,000 pregnancies. Due to the low incidence of this disease, randomized controlled trials to study it are not possible. Instead, clinicians must learn either from individual case studies or from retrospective syntheses of cases reported over time.1,2,7 Fortunately, the wealth of information gleaned over the past 30 years has significantly reduced AFLP-associated maternal and fetal mortality and morbidity rates. In the 1980s, maternal and fetal mortality rates as high as 85% were reported.3 Worldwide, maternal mortality associated with AFLP has decreased significantly to 7% to 18%, whereas the fetal mortality rate has fallen to between 9% and 23%.1,16,17
Common trends among women who have developed AFLP include nulliparity, multiparity, and advanced maternal age. One retrospective study of 57 women who had developed AFLP revealed that 35 cases (61%) involved first-time pregnancies. It also showed that 10 (18%) of the women had twins, and 14 (25%) were older than 35.2 In another study of 35 cases of AFLP, 40% of the women were nulliparous, and 11.4% were multiparous, including one triplet gestation.12 In a third, smaller study, 80% of women affected by AFLP were multiparous.10 Currently, there is no known evidence linking any maternal behavior to development of AFLP.
Presentation
Women who present with AFLP often experience vague, nonspecific symptoms, leading to misdiagnosis or delayed diagnosis. Objective measurements, including physical exam findings, laboratory studies, and other diagnostic tests, will help with a diagnosis. The most frequent initial symptoms are nausea and vomiting (in 70% of patients) and abdominal pain (50% to 80%), epigastric or right upper-quadrant.3 Other common symptoms include fatigue, malaise, anorexia, weight gain, polyuria, and polydipsia.2,3,9,18,19
Because the presenting symptoms in AFLP can be vague, clinicians should complete a thorough physical exam to differentiate accurately among conditions associated with pregnancy. Physical signs present in women with AFLP can include jaundice, ascites, edema, confusion, abdominal tenderness, and fever. More severe cases can present with multisystem involvement, including acute renal failure, gastrointestinal bleeding, pancreatitis, coagulopathy, and hepatic encephalopathy.3,4,9,18
Diagnostic Tests
Relevant laboratory tests include a complete blood count (CBC), liver studies, chemistry, coagulation studies, and urinalysis (see Table 1). Viral causes should be ruled out by way of a hepatitis panel.3 In AFLP, the CBC may show elevated white blood cells, decreased hemoglobin and hematocrit, and decreased platelets. Liver studies show elevated hepatic aminotransferase, bilirubin, LDH, and ammonia levels. Chemistry results show elevated blood urea nitrogen and creatinine, and decreased blood glucose. Coagulation factors are affected, and prolonged PTT, decreased fibrinogen, and proteinurea may also be found.9
Though invasive and not often necessary4,13 (and not possible for the case patient), the definitive diagnostic test for AFLP is liver biopsy.13,14 Biopsy reveals a microvesicular fatty infiltration of the hepatocytes as minute fat droplets surrounding a centrally located nucleus. These fatty infiltrates stain with oil red O, specific for fat. Inflammation is present in 50% of cases. There may also be a picture similar to cholestasis with bile thrombi or deposits within the hepatocytes.20
Due to the risk for hemorrhage and the critical status of women with AFLP, biopsy is often not possible. Ultrasonography may show increased echogenicity; CT may show decreased or diffuse attenuation in the liver. These imaging studies, though possibly helpful in severe cases, often yield false-negative results.3,20
In the absence of another explanation for the patient’s symptoms, the Swansea criteria are used for diagnosis of AFLP.1 Six or more of the following criteria must be present to confirm this diagnosis: vomiting, abdominal pain, polydipsia or polyuria, encephalopathy, leukocytosis, elevated bilirubin, elevated liver enzymes, elevated ammonia, hypoglycemia, renal dysfunction, coagulopathy, elevated uric acid, ascites on ultrasound, and microvesicular steatosis on liver biopsy.1,2,5
Pathophysiology
Normal functions of the liver include metabolism, protein synthesis, and manufacturing of blood coagulation proteins. These functions are disturbed in the presence of AFLP. Thus, women with this disease experience signs and symptoms related directly to the dysfunction of these processes.20-22
Disturbances in the hepatocytes due to excess fatty acids impair the liver’s ability to convert unconjugated bilirubin into conjugated bilirubin, causing plasma levels of unconjugated bilirubin to rise. This increase in bilirubin explains the jaundiced appearance of women with AFLP. AFLP is often thought to occur in conjunction with preeclampsia in many, but not all, patients. Thrombocytopenia in these patients is felt to be secondary to peripheral vascular consumption. Conjugated bilirubin levels may also be increased due to decreased flow of conjugated bilirubin into the common bile duct.21
Another liver function that is disrupted is that of glycogen storage and conversion to glucose, and the liver’s ability to convert nutrients into glycogen is also impaired. Decreased storage of glycogen, along with the liver’s inability to break down previously stored glycogen, causes a decrease in serum glucose levels. Women with AFLP often require treatment with IV dextrose in response to marked hypoglycemia.16,21,23
The liver dysfunction associated with AFLP reduces adequate production of clotting factors and coagulation proteins. Thrombocytopenia, elevated clotting times, and bleeding are all problems seen in AFLP. Mild to moderate elevations in serum aminotransferases and elevated LDH also occur in patients with AFLP.23,24
Genetic Factor
There is little known about the etiology of AFLP, although recent data point to a genetic component that was found in as many as 62% of mothers in one study and in 25% of infants in another study.20-22 Fatty acid oxidation (FAO) is one of the processes of hepatic mitochondria, a process that relies on several enzymes. When FAO is interrupted, fatty acids are deposited in the liver cells, as seen in histologic studies of AFLP.25,26 The common thread in women with this disease is a mutation in one of the enzymes needed for FAO. This enzyme is the long-chain 3-hydroxyacyl-CoA dehydrogenase. Deficiencies in this enzyme are common in mothers with AFLP and their infants.3,16,20,23,27
Differential Diagnosis
Several complications of pregnancy that involve the liver may, on presentation, mimic AFLP.16,20,23,24,28 The most common are hyperemesis gravidarum and intrahepatic cholestasis of pregnancy23 (see Table 216,20,23,24,28); others are preeclampsia/eclampsia and HELLP syndrome. It is important to distinguish between the signs and symptoms associated with each of these disorders in order to provide the most effective treatment. Hepatitis serologies are important in the differential diagnosis when liver enzyme levels are exceptionally high.4,16,22,28
Treatment
The most effective treatment for AFLP is delivery of the infant; often, this alone causes the signs and symptoms of AFLP to resolve.8,21,27,29 In two of three cases in a small study by Aso et al,8 early delivery of the fetus led to complete resolution of symptoms and return to normal liver function. One of these patients was sent home four days after delivery; the other, 14 days later. Other patients may require more invasive treatment and support.8
Management in the ICU is often required to provide appropriate supportive care to the mother after delivery. Acute respiratory distress syndrome, pancreatitis, hemorrhage, encephalopathy, renal failure, and continual liver failure are among the severe complications associated with AFLP.4,8,10 Many women require intubation, dialysis, fluid resuscitation, blood product transfusion, and vasopressor therapy.3,8,11 Prophylactic antibiotics, IV steroids, and glucose may all be required in the supportive care and recovery of a mother with AFLP.3,8,11
TPE has also been useful in instances of severe complications.1,3,6 In one retrospective study, Martin et al1 recommended administration of TPE in patients with AFLP under the following circumstances:
(1) Deteriorating central nervous system abnormalities, such as sensorium changes or coma;
(2) Persistent coagulopathy requiring continued and aggressive blood product support with plasma, red cells, and/or cryoprecipitate;
(3) Advanced renal dysfunction that compromised fluid management;
(4) Progressive cardiopulmonary compromise; and/or
(5) Ongoing fluid management concerns, including significant ascites, edema, anuria/oliguria, and/or fluid overload.1
In rare cases, liver transplantation is needed in patients with AFLP. Westbrook et al18 reviewed 54 cases of liver disease in pregnancy in one UK hospital between 1997 and 2008. Of these, six patients with encephalopathy or elevated lactate were listed for liver transplant, including just one with a diagnosis of AFLP. This woman never actually underwent transplant but recovered in response to medical management alone.18 According to data reported in June 2011 by the Organ Procurement and Transplantation Network,30 liver transplantation was needed in only three US patients with AFLP between 2000 and 2011. Further retrospective studies on outcomes from transplant versus medical management should be considered to guide future decision making involving this invasive therapy.
The Case Patient
This 39-year-old patient presented during a routine prenatal visit with proteinuria and hypertension, possibly indicative of preeclampsia. Because of the serious nature of this potential diagnosis in pregnancy, she was admitted for monitoring and further testing. Although the diagnosis of AFLP was not confirmed until later, the patient’s preliminary lab studies showed elevated liver enzymes and low platelet counts, signifying the need for prompt intervention and delivery of the infant. At this point, the patient met criteria for HELLP syndrome, but AFLP was suspected after the initial finding of profound hypoglycemia led to further testing.
As an older mother experiencing pregnancy for the first time, this patient fit the profile for AFLP. She initially responded well after delivery of her infant but continued to experience complications. On the days that the patient was treated with TPE, her total bilirubin and liver enzymes were at their lowest. Perhaps this treatment should be considered in more cases of AFLP.
The patient was transferred to a hospital with liver transplantation capabilities, but she ultimately recovered without undergoing transplant.
Conclusion
For the primary obstetric care provider, being aware of the possible complications associated with pregnancy is important. Though uncommon, AFLP is a serious complication that should be ruled out in women who present with vague symptoms such as nausea, vomiting, and abdominal pain in the third trimester of pregnancy. The reduction in AFLP-associated morbidity and mortality during the past 20 years is a direct result of increased early recognition and therapeutic delivery.
Referral to a maternal fetal medicine specialist, gastroenterologist, hematologist, and/or nephrologist may be necessary and appropriate in the management of a woman with AFLP. Further study is indicated for use of TPE in more severe cases of AFLP, particularly in women affected by persistent thrombocytopenia and anemia.
The author would like to thank C. Leanne Browning, MD, obstetrics/gynecology, for her invaluable guidance and advice on this project.
References
1. Martin JN Jr, Briery CM, Rose CH, et al. Postpartum plasma exchange as adjunctive therapy for severe acute fatty liver of pregnancy. J Clin Apher. 2009;23(4):138-143.
2. Knight M, Nelson-Piercy C, Kurinczuk JJ; UK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57(7):951-956.
3. Barsoom MJ, Tierney BJ. Acute fatty liver of pregnancy (2011). http://emedicine.medscape.com/article/1562425-overview. Accessed January 21, 2013.
4. Ko HH, Yoshida E. Acute fatty liver of pregnancy. Can J Gastroenterol. 2006;20(1):25-30.
5. Rathi U, Bapat M, Rathi P, Abraham P. Effect of liver disease on maternal and fetal outcome: a prospective study. Indian J Gastroenterol. 2007;26(2):59-63.
6. Myers L. Postpartum plasma exchange in a woman with suspected thrombotic thrombocytopenic purpura (TTP) vs hemolysis, elevated liver enzymes, and low platelet syndrome (HELLP): a case study. Nephrol Nurs J. 2010;37(4):399-402.
7. Vigil-de Gracia P. Acute fatty liver and HELLP syndrome: two distinct pregnancy disorders. Int J Gynaecol Obstet. 2001;73(3):215-220.
8. Aso K, Hojo S, Yumoto Y, et al. Three cases of acute fatty liver of pregnancy: postpartum clinical course depends on interval between onset of symptoms and termination of pregnancy. J Matern Fetal Neonatal Med. 2010;23(9):1047-1049.
9. Wei Q, Zhang L, Liu X. Clinical diagnosis and treatment of acute fatty liver of pregnancy: a literature review and 11 new cases. J Obstet Gynaecol Res. 2010;36(4):751-756.
10. Barber MA, Eguiluz I, Martin A, et al. Acute fatty liver of pregnancy: analysis of five consecutive cases from a tertiary centre. J Obstet Gynaecol. 2010;30(3):241-243.
11. Ajayi AO, Alao MO. Case report: acute fatty liver of pregnancy in a 30-year-old Nigerian primigravida. Niger J Clin Pract. 2008;11(4):389-391.
12. Vigíl-de Gracia P, Montufar-Rueda C. Acute fatty liver of pregnancy: diagnosis, treatment, and outcome based on 35 consecutive cases. J Matern Fetal Neonatal Med. 2011;24(9):1143-1146.
13. Dey M, Reema K. Acute fatty liver of pregnancy. N Am J Med Sci. 2012;4(11):611-612.
14. Castro MA, Goodwin TM, Shaw KJ, et al. Disseminated intravascular coagulation and antithrombin III depression in acute fatty liver of pregnancy. Am J Obstet Gynecol. 1996;174(1 pt 1):211-216.
15. Altuntas F, Aydogdu I, Kabukcu S, et al. Therapeutic plasma exchange for the treatment of thrombotic thrombocytopenic purpura: a retrospective multicenter study. Transfus Apher Sci. 2007;36(1):57-67.
16. Hay JE. Liver disease in pregnancy. Hepatology. 2008;47(3):1067-1076.
17. Wand S, Waeschle RM, Von Ahsen N, et al. Acute fatty liver failure due to acute fatty liver of pregnancy. Minerva Anesthesiol. 2012;78(4):503-506.
18. Westbrook RH, Yeoman AD, Joshi D, et al. Outcomes of severe pregnancy-related liver disease: refining the role of transplantation. Am J Transplant. 2010;10(11):2520-2526.
19. Fesenmeier MF, Coppage KH, Lambers DS, et al. Acute fatty liver of pregnancy in 3 tertiary care centers. Am J Obstet Gynecol. 2005;192(5):1416-1419.
20. Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol. 2011;35(3):182-193.
21. Huether SE. Alterations of digestive function. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1452-1515.
22. Huether SE. Structure and function of the digestive system. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1420-1451.
23. Schutt VA, Minuk GY. Liver diseases unique to pregnancy. Best Pract Res Clin Gastroenterol. 2007;21(5):771-792.
24. Pan C, Perumalswami PV. Pregnancy-related liver diseases. Clin Liver Dis. 2011;15(1):199-208.
25. Ibdah JA. Acute fatty liver of pregnancy: an update on pathogenesis and clinical implications. World J Gastroenterol. 2006;12(46):7397-7404.
26. Browning MF, Levy HL, Wilkins-Haug LE, et al. Fetal fatty acid oxidation defects and maternal liver disease in pregnancy. Obstet Gynecol. 2006;107(1):115-120.
27. Dekker RR, Schutte JM, Stekelenburg J, et al. Maternal mortality and severe maternal morbidity from acute fatty liver of pregnancy in the Netherlands. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):27-31.
28. Lee NM, Brady CW. Liver disease in pregnancy. World J Gastroenterol. 2009;15(8):897-906.
29. Vora KS, Shah VR, Parikh GP. Acute fatty liver of pregnancy: a case report of an uncommon disease. Indian J Crit Care Med. 2009;13(1):34-36.
30. Organ Procurement and Transplantation Network, Scientific Registry of Transplant Recipients. OPTN/SRTR 2011 Annual Data Report: Liver. http://srtr.transplant.hrsa.gov/annual_reports/2011/pdf/03_%20liver_12.pdf. Accessed January 18, 2013.