User login
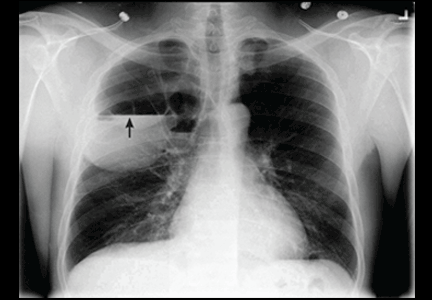
Construction Worker Falls From Scaffolding
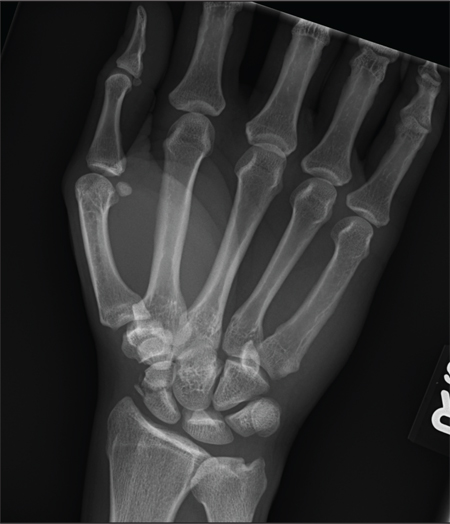
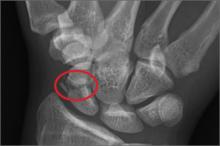
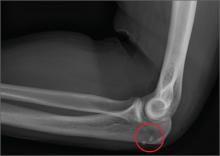
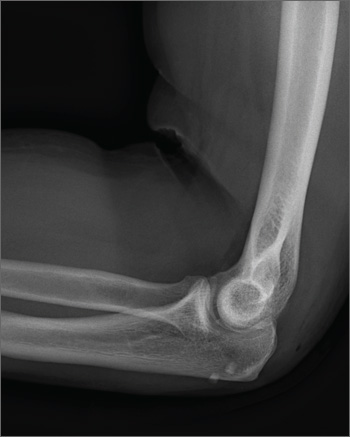
A 45-year-old construction worker is brought to your facility for evaluation following a fall. He was at a job site, standing on scaffolding approximately 20 feet above the ground, when he accidentally fell. He does not remember for sure, but he thinks he landed on his face. He did briefly lose consciousness. He is complaining of right-side facial pain and right wrist pain. His medical history is unremarkable. The physical exam reveals stable vital signs. The patient appears somewhat uncomfortable but is in no obvious distress. There is a moderate amount of periorbital soft-tissue swelling around his right eye, with moderate associated tenderness. Pupils are equal and react well bilaterally. Examination of the right wrist shows a moderate amount of soft-tissue swelling. The patient is unable to flex or extend his wrist due to pain. Good pulses and capillary refill of the nail beds are noted. There is also moderate tenderness along the base of the first metacarpal. Radiograph of the right wrist is shown. What is your impression?
Dyspnea after treatment of recurrent urinary tract infection
A 71-year-old woman came to the hospital because of generalized weakness, fatigue, and exertional dyspnea.
She had a history of anemia, recurrent urinary tract infections, and hyperactive bladder. She had been taking nitrofurantoin for a urinary tract infection and phenazopyridine for dysuria, and she noticed that her urine was dark-colored.
She was of northern European descent. She was unaware of any family history of blood-related disorders. She had been admitted to the hospital 6 weeks earlier for symptomatic anemia after taking nitrofurantoin for a urinary tract infection. At that time, she received 2 units of packed red blood cells and then was discharged. Follow-up blood work done 2 weeks later—including a glucose-6 phosphate dehydrogenase (G6PD) assay—was normal.
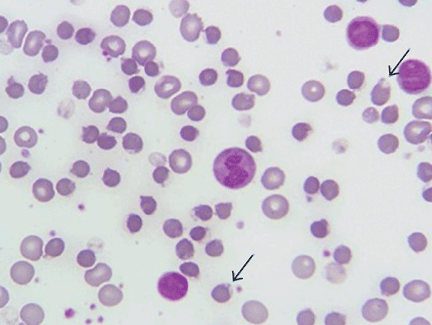
On physical examination, she was pale and weak. Her hemoglobin level was 5.5 g/dL (reference range 14.0–17.5), with normal white blood cell and platelet counts and an elevated reticulocyte count. A comprehensive metabolic panel showed elevated indirect bilirubin and lactate dehydrogenase levels. A direct Coombs test for autoimmune hemolytic anemia was negative, as was a haptoglobin assay to look for intravascular hemolytic anemia. G6PD levels were normal, yet a peripheral blood smear (Figure 1) showed features of G6PD deficiency.
What was the cause of her anemia?
GLUCOSE-6-PHOSPHATE DEHYDROGENASE DEFICIENCY
G6PD deficiency is an X-linked disorder1 that can present as hemolytic anemia. Symptoms of hemolysis can range from mild to severe on exposure to an inciting agent. Men are more commonly affected than women, and affected women are mostly heterozygous. The severity of hemolysis in heterozygous women depends on inactivation of the unaffected X chromosome in some cells.
When exposed to oxidizing agents, people with G6PD deficiency do not have enough nicotinamide adenine dinucleotide phosphate to protect red blood cells.2 This leads to oxidative denaturation of hemoglobin, formation of methemoglobin, and denaturation of globulin. These products are insoluble; they collect in red blood cells and are called Heinz bodies.3 When red blood cells containing Heinz bodies pass through the liver and spleen, the insoluble masses are taken up by macrophages, causing hemolysis and the formation of “bite cells”4 (so named because macrophages “bite” the Heinz bodies out of the red blood cells).
Patients with G6PD deficiency have all the clinical features of hemolytic anemia. On laboratory testing, the Coombs test is negative, the G6PD level is low, and the peripheral smear shows bite cells. The G6PD level is falsely normal or elevated during acute hemolysis because red blood cells deficient in G6PD are removed from circulation and replaced by young red blood cells. The G6PD level is also elevated after blood transfusion. Thus, the G6PD level should be tested 3 months after an acute event.
Hemolysis in G6PD is usually intermittent and self-limited. No treatment is needed except for avoidance of triggers and transfusion for symptomatic anemia. Of note, triggers include some of the drugs commonly used for urinary tract infections (sulfa drugs, nitrofurantoin, phenazopyridine) and antimalarials. Fava beans are also known to cause hemolytic crisis. A complete list of things to avoid can be found at www.g6pd.org/en/G6PDDeficiency/SafeUnsafe/DaEvitare_ISS-it.
There is no commercially available genetic testing kit for G6PD deficiency. Mutation analysis and G6PD gene sequencing are possible but are neither routinely done nor widely available.
BACK TO OUR PATIENT
Our patient’s hemolytic anemia was most likely drug-induced, secondary to a relative deficiency of G6PD. She had been taking nitrofurantoin and phenazopyridine; both of these are oxidizing agents and are known to cause acute hemolytic anemia in people with G6PD deficiency. The G6PD level can be normal after a recent blood transfusion and, as in our patient, during an acute episode of hemolysis.
Because of the strong suspicion of G6PD deficiency, both drugs were stopped when the patient was discharged from the hospital. She did not take either drug for 3 months. Her G6PD level was then retested and was found to be low, confirming the diagnosis. The patient was then advised not to take those drugs again. Since then, her hemoglobin level has remained stable and she has not needed any more blood transfusions.
- Mason PJ, Bautista JM, Gilsanz F. G6PD deficiency: the genotype-phenotype association. Blood Rev 2007; 21:267–283.
- Arese P, De Flora A. Pathophysiology of hemolysis in glucose-6-phosphate dehydrogenase deficiency. Semin Hematol 1990; 27:1–40.
- Jacob HS. Mechanisms of Heinz body formation and attachment to red cell membrane. Semin Hematol 1970; 7:341–354.
- Rifkind RA. Heinz body anemia: an ultrastructural study. II. Red cell sequestration and destruction. Blood 1965; 26:433–448.
A 71-year-old woman came to the hospital because of generalized weakness, fatigue, and exertional dyspnea.
She had a history of anemia, recurrent urinary tract infections, and hyperactive bladder. She had been taking nitrofurantoin for a urinary tract infection and phenazopyridine for dysuria, and she noticed that her urine was dark-colored.
She was of northern European descent. She was unaware of any family history of blood-related disorders. She had been admitted to the hospital 6 weeks earlier for symptomatic anemia after taking nitrofurantoin for a urinary tract infection. At that time, she received 2 units of packed red blood cells and then was discharged. Follow-up blood work done 2 weeks later—including a glucose-6 phosphate dehydrogenase (G6PD) assay—was normal.
On physical examination, she was pale and weak. Her hemoglobin level was 5.5 g/dL (reference range 14.0–17.5), with normal white blood cell and platelet counts and an elevated reticulocyte count. A comprehensive metabolic panel showed elevated indirect bilirubin and lactate dehydrogenase levels. A direct Coombs test for autoimmune hemolytic anemia was negative, as was a haptoglobin assay to look for intravascular hemolytic anemia. G6PD levels were normal, yet a peripheral blood smear (Figure 1) showed features of G6PD deficiency.
What was the cause of her anemia?

GLUCOSE-6-PHOSPHATE DEHYDROGENASE DEFICIENCY
G6PD deficiency is an X-linked disorder1 that can present as hemolytic anemia. Symptoms of hemolysis can range from mild to severe on exposure to an inciting agent. Men are more commonly affected than women, and affected women are mostly heterozygous. The severity of hemolysis in heterozygous women depends on inactivation of the unaffected X chromosome in some cells.
When exposed to oxidizing agents, people with G6PD deficiency do not have enough nicotinamide adenine dinucleotide phosphate to protect red blood cells.2 This leads to oxidative denaturation of hemoglobin, formation of methemoglobin, and denaturation of globulin. These products are insoluble; they collect in red blood cells and are called Heinz bodies.3 When red blood cells containing Heinz bodies pass through the liver and spleen, the insoluble masses are taken up by macrophages, causing hemolysis and the formation of “bite cells”4 (so named because macrophages “bite” the Heinz bodies out of the red blood cells).
Patients with G6PD deficiency have all the clinical features of hemolytic anemia. On laboratory testing, the Coombs test is negative, the G6PD level is low, and the peripheral smear shows bite cells. The G6PD level is falsely normal or elevated during acute hemolysis because red blood cells deficient in G6PD are removed from circulation and replaced by young red blood cells. The G6PD level is also elevated after blood transfusion. Thus, the G6PD level should be tested 3 months after an acute event.
Hemolysis in G6PD is usually intermittent and self-limited. No treatment is needed except for avoidance of triggers and transfusion for symptomatic anemia. Of note, triggers include some of the drugs commonly used for urinary tract infections (sulfa drugs, nitrofurantoin, phenazopyridine) and antimalarials. Fava beans are also known to cause hemolytic crisis. A complete list of things to avoid can be found at www.g6pd.org/en/G6PDDeficiency/SafeUnsafe/DaEvitare_ISS-it.
There is no commercially available genetic testing kit for G6PD deficiency. Mutation analysis and G6PD gene sequencing are possible but are neither routinely done nor widely available.
BACK TO OUR PATIENT
Our patient’s hemolytic anemia was most likely drug-induced, secondary to a relative deficiency of G6PD. She had been taking nitrofurantoin and phenazopyridine; both of these are oxidizing agents and are known to cause acute hemolytic anemia in people with G6PD deficiency. The G6PD level can be normal after a recent blood transfusion and, as in our patient, during an acute episode of hemolysis.
Because of the strong suspicion of G6PD deficiency, both drugs were stopped when the patient was discharged from the hospital. She did not take either drug for 3 months. Her G6PD level was then retested and was found to be low, confirming the diagnosis. The patient was then advised not to take those drugs again. Since then, her hemoglobin level has remained stable and she has not needed any more blood transfusions.
A 71-year-old woman came to the hospital because of generalized weakness, fatigue, and exertional dyspnea.
She had a history of anemia, recurrent urinary tract infections, and hyperactive bladder. She had been taking nitrofurantoin for a urinary tract infection and phenazopyridine for dysuria, and she noticed that her urine was dark-colored.
She was of northern European descent. She was unaware of any family history of blood-related disorders. She had been admitted to the hospital 6 weeks earlier for symptomatic anemia after taking nitrofurantoin for a urinary tract infection. At that time, she received 2 units of packed red blood cells and then was discharged. Follow-up blood work done 2 weeks later—including a glucose-6 phosphate dehydrogenase (G6PD) assay—was normal.
On physical examination, she was pale and weak. Her hemoglobin level was 5.5 g/dL (reference range 14.0–17.5), with normal white blood cell and platelet counts and an elevated reticulocyte count. A comprehensive metabolic panel showed elevated indirect bilirubin and lactate dehydrogenase levels. A direct Coombs test for autoimmune hemolytic anemia was negative, as was a haptoglobin assay to look for intravascular hemolytic anemia. G6PD levels were normal, yet a peripheral blood smear (Figure 1) showed features of G6PD deficiency.
What was the cause of her anemia?

GLUCOSE-6-PHOSPHATE DEHYDROGENASE DEFICIENCY
G6PD deficiency is an X-linked disorder1 that can present as hemolytic anemia. Symptoms of hemolysis can range from mild to severe on exposure to an inciting agent. Men are more commonly affected than women, and affected women are mostly heterozygous. The severity of hemolysis in heterozygous women depends on inactivation of the unaffected X chromosome in some cells.
When exposed to oxidizing agents, people with G6PD deficiency do not have enough nicotinamide adenine dinucleotide phosphate to protect red blood cells.2 This leads to oxidative denaturation of hemoglobin, formation of methemoglobin, and denaturation of globulin. These products are insoluble; they collect in red blood cells and are called Heinz bodies.3 When red blood cells containing Heinz bodies pass through the liver and spleen, the insoluble masses are taken up by macrophages, causing hemolysis and the formation of “bite cells”4 (so named because macrophages “bite” the Heinz bodies out of the red blood cells).
Patients with G6PD deficiency have all the clinical features of hemolytic anemia. On laboratory testing, the Coombs test is negative, the G6PD level is low, and the peripheral smear shows bite cells. The G6PD level is falsely normal or elevated during acute hemolysis because red blood cells deficient in G6PD are removed from circulation and replaced by young red blood cells. The G6PD level is also elevated after blood transfusion. Thus, the G6PD level should be tested 3 months after an acute event.
Hemolysis in G6PD is usually intermittent and self-limited. No treatment is needed except for avoidance of triggers and transfusion for symptomatic anemia. Of note, triggers include some of the drugs commonly used for urinary tract infections (sulfa drugs, nitrofurantoin, phenazopyridine) and antimalarials. Fava beans are also known to cause hemolytic crisis. A complete list of things to avoid can be found at www.g6pd.org/en/G6PDDeficiency/SafeUnsafe/DaEvitare_ISS-it.
There is no commercially available genetic testing kit for G6PD deficiency. Mutation analysis and G6PD gene sequencing are possible but are neither routinely done nor widely available.
BACK TO OUR PATIENT
Our patient’s hemolytic anemia was most likely drug-induced, secondary to a relative deficiency of G6PD. She had been taking nitrofurantoin and phenazopyridine; both of these are oxidizing agents and are known to cause acute hemolytic anemia in people with G6PD deficiency. The G6PD level can be normal after a recent blood transfusion and, as in our patient, during an acute episode of hemolysis.
Because of the strong suspicion of G6PD deficiency, both drugs were stopped when the patient was discharged from the hospital. She did not take either drug for 3 months. Her G6PD level was then retested and was found to be low, confirming the diagnosis. The patient was then advised not to take those drugs again. Since then, her hemoglobin level has remained stable and she has not needed any more blood transfusions.
- Mason PJ, Bautista JM, Gilsanz F. G6PD deficiency: the genotype-phenotype association. Blood Rev 2007; 21:267–283.
- Arese P, De Flora A. Pathophysiology of hemolysis in glucose-6-phosphate dehydrogenase deficiency. Semin Hematol 1990; 27:1–40.
- Jacob HS. Mechanisms of Heinz body formation and attachment to red cell membrane. Semin Hematol 1970; 7:341–354.
- Rifkind RA. Heinz body anemia: an ultrastructural study. II. Red cell sequestration and destruction. Blood 1965; 26:433–448.
- Mason PJ, Bautista JM, Gilsanz F. G6PD deficiency: the genotype-phenotype association. Blood Rev 2007; 21:267–283.
- Arese P, De Flora A. Pathophysiology of hemolysis in glucose-6-phosphate dehydrogenase deficiency. Semin Hematol 1990; 27:1–40.
- Jacob HS. Mechanisms of Heinz body formation and attachment to red cell membrane. Semin Hematol 1970; 7:341–354.
- Rifkind RA. Heinz body anemia: an ultrastructural study. II. Red cell sequestration and destruction. Blood 1965; 26:433–448.
Woman, 45, With Red, Scaly Nipple
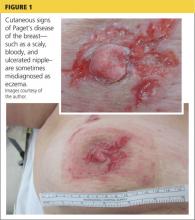
A 45-year-old woman noticed some redness and scaling around her right nipple. She applied peroxide and OTC antibiotic ointment for approximately seven months with mixed results. She sought medical attention when pain developed in the breast, along with some bloody discharge from the nipple (see Figure 1). Around that time, she also noticed three small nodules in the upper outer portion of the breast.
A mammogram and ultrasound revealed a 1.7 × 2.0–cm spiculated mass in the axillary tail, as well as two smaller breast lesions. A PET/CT scan ordered subsequently revealed intense uptake in the periareolar region and a suspicious axillary node. By then, the biopsy results had confirmed invasive ductal carcinoma, later determined to be Paget’s disease of the breast (PDB).
The patient’s previous medical history was significant for cystic breasts (never biopsied), chronic back pain, anxiety, and obesity. She was perimenopausal with irregular periods, the last one about 10 months ago. Her obstetric history included two pregnancies resulting in live births and no history of abortion; her menarche occurred at age 14 and her first pregnancy at 27. Family history was significant for leukemia in her maternal grandmother and niece. She did not use tobacco, alcohol, or illicit drugs. She lived at home with her husband and two daughters, who were all very supportive.
The patient elected to undergo a right modified radical mastectomy (MRM) and prophylactic left total mastectomy. MRM was performed on the right breast because sentinel lymph node identification was unsuccessful. This may have been due to involvement of the right subareolar plexus. Five of eight lymph nodes later tested positive for malignancy. The surgery was completed by placement of bilateral tissue expanders for eventual breast reconstruction.
Chemotherapy was started six weeks after surgery and included 15 weeks (five cycles) of docetaxel, carboplatin, and trastuzumab (a combination known as TCH), followed by 51 weeks (17 cycles) of trastuzumab, along with daily tamoxifen. The TCH regimen was followed by four weekly cycles of external beam radiation therapy (EBRT). Adverse effects of treatment have included chest wall dermatitis, right upper extremity lymphedema, nausea/vomiting, dyspnea, peripheral neuropathy, alopecia, and fatigue.
Discussion
Nearly 150 years ago, James Paget recognized a connection between skin changes around the nipple and deeper lesions in the breast.1 The disease that Paget identified is defined as the presence of intraepithelial adenocarcinoma cells (ie, Paget’s cells) within the epidermis of the nipple, with or without an underlying carcinoma.
An underlying breast cancer is present 85% to 95% of the time but is palpable in only approximately 50% of cases (see Figure 2). However, 25% of the time there is neither a palpable mass nor a mammographic abnormality. In these cases particularly, timely diagnosis depends on recognition of suspicious nipple changes, followed by a prompt and thorough diagnostic workup. Unfortunately, the accurate diagnosis of Paget’s disease still takes an average of several months.2
Paget’s disease is rare; it represents only 1% to 3% of new cases of female breast cancer, or about 2,250 cases a year.2-4 (The number of Paget’s disease cases per year was calculated by the author, based on the reported incidence of all breast cancers.) It is even more rare among men. For both genders, the peak age for this disease is between 50 and 60.2
Paget’s disease is an important entity for primary care PAs and NPs because it presents an opportunity to make a timely and life-changing diagnosis, and because it provides an elegant model for understanding current diagnostic and therapeutic approaches to breast cancer.
Clinical Presentation and Pathophysiology
The hallmark of PDB is scaly, vesicular, or ulcerated changes that begin in the nipple and spread to the areola. These changes are most often unilateral and may occur with pruritus, burning pain, and/or oozing from the nipple.5 This presentation is often mistaken for common skin conditions, such as eczema. Like eczema, changes in PDB may improve spontaneously and fluctuate over time, which is confusing for both the patient and clinician. A clinical pearl is that eczema is more likely to spread from the areola to the nipple, and will usually respond to topical corticosteroids. By contrast, changes in PDB tend to spread from the nipple to the areola, and corticosteroids do not provide a sustained response. Of note, Paget’s lesions may heal spontaneously even as the underlying malignancy progresses.6
PDB is unique because the underlying lesion and skin changes are not just coincidental. The cutaneous changes and the malignancy that lies beneath have a causal, not merely co-occurring, relationship. Paget himself believed that the nipple changes were both a precursor, and a promoter, of the underlying cancer.1 This transformation theory states that normal nipple epidermis turns into Paget’s cells spontaneously, before there is any underlying disease. This theory is supported by the fact that, occasionally (though rarely), no underlying breast cancer is ever found. Also, the concomitant tumor may be some distance (> 2 cm) from the nipple-areolar complex (NAC), suggesting a synchronous but causally unrelated lesion.6-8
Modern immunochemistry has turned PDB inside out. Today, PDB is believed to begin within the breast and then to spread “upward” to the NAC, called the epidermotrophic theory. This theory is supported by the fact that Paget’s cells share several molecular markers with their respective parenchymal tumors. Some researchers now propose that there is a single Paget’s progenitor cell with a motility factor that allows it to traverse the ductal system, resulting in nipple and skin changes that have come to be recognized as PDB.6-8
The invasive cancers that are associated with PDB are most likely to be both estrogen- and progesterone-receptor–negative and of a high histologic grade.3,7 Estrogen- and progesterone-sensitive tumors respond to hormonal manipulation therapy. Tumors that are receptor-negative and that have a more aggressive grade are more difficult to treat.
Differential Diagnosis
PDB may be confused with the early stages of inflammatory breast cancer (IBC), an aggressive malignant disease (see Table 1). Both conditions may present with erythema and skin thickening and may be mistaken for mastitis. However, IBC spreads rapidly through the entire breast, and clinical features may include tenderness, a feeling of heat or heaviness, breast enlargement, and significant lymphadenopathy. Current recommendations call for a biopsy of any area of breast inflammation that does not respond to antibiotics within seven days.9
PDB is not the only cutaneous manifestation of breast cancer. Others include carcinoma erysipeloides (inflammatory changes that resemble cellulitis), carcinoma telangiectaticum (vascularized plaques), and/or inflammatory papules or nodules appearing on the breast, back, neck, or scalp. Each of these non–Paget’s conditions involves lymphatic (versus ductal) spread and signifies advanced malignancy.10
Diagnosis and Staging
After biopsy of the nipple lesion(s), diagnosis proceeds to the assessment of the breast itself and ultimately to cancer staging. PDB may occur (in order of incidence):
• In conjunction with an invasive cancer
• With underlying ductal carcinoma in situ (DCIS)
• Without any underlying disease.7
Mammography is used to determine the extent and location of the underlying lesion(s), which is more likely to be peripheral and/or multicentric. However, in some cases, there are no mammographic changes, which is now recognized as an indication for performing a breast MRI.11 Once the lesion is located, direct or image-guided biopsy confirms whether it is invasive cancer or DCIS. Palpable masses that occur with PDB are usually invasive and signal advanced disease.2,6,12,13 Sentinel lymph node biopsy (SLNB), which is usually performed at the time of surgery, plays a critical role in cancer staging and treatment planning. SLNB reliably diagnoses axillary metastasis in approximately 98% of patients.14
Like other breast cancers, PDB is also categorized by the expression of molecular markers, including HER2 (human epidermal growth factor receptor 2). Cancer cells in which HER2 gene is overexpressed tend to proliferate more rapidly than others. HER-status can also provide a clue as to which chemotherapy agents are likely to be most effective.2
Treatment and Management
The primary treatment for breast cancer is surgery, which serves both diagnostic and therapeutic purposes. To be effective, surgical treatment of PDB requires excision of the NAC, also called central lumpectomy. This may be sufficient treatment in those rare cases in which the disease is confined to the NAC.11,12
For underlying tumors, partial mastectomy is an option when the tumor is small (< 2 cm) and located close enough to the NAC to achieve negative margins, while leaving a cosmetically acceptable breast. Partial mastectomy is usually followed by whole breast irradiation. A few centers offer intraoperative radiation therapy (IORT)—performed before the surgeon closes the incision—for patients who wish to avoid or limit the duration of postoperative radiation treatment.15-17
Complete mastectomy (including excision of the NAC) should be considered when:
• The distance between the NAC and the underlying tumor is significant
• Multicentric disease and/or diffuse calcifications exist
• Achieving negative margins would remove too much tissue to leave a cosmetically acceptable breast.
Evaluation of the axillary nodes is the same in PDB as with other breast cancers. Patients with disease localized to the NAC and no underlying carcinoma may choose to forego lymph node biopsy. The same is true for patients who have PDB with a single underlying DCIS. However, lymph node biopsy is always recommended in cases of multicentric DCIS or invasive disease, or if a mastectomy is planned.18,19
Sentinel node biopsy results determine whether the mastectomy should be simple (excision of the breast alone) or modified radical (breast and axillary nodes). Today, complete radical mastectomy (excision of the breast, axillary nodes, and pectoral muscle) is reserved for cases in which disease invades the chest wall.18,19
The use of adjuvant (postoperative) therapy in patients with DCIS (whether or not related to PDB) is still debated. For patients with invasive cancers, both radiation therapy and chemotherapy are usually indicated. The decision to use neoadjuvant (preoperative) chemotherapy is made on a case-by-case basis. All decisions are based on the nature of the underlying cancer, regardless of whether the diagnosis is PDB.
Because PDB is categorized as invasive in at least 85% of cases, and because all invasive breast cancers carry about twice the risk for newly diagnosed contralateral disease, systematic follow-up is extremely important for patients with PDB. A clinical exam and updated history should be performed every four to six months during the first two years and at least annually after that. Screening recommendations, including a yearly mammogram, remain the same for asymptomatic patients. Patients with new or recurring symptoms—because they are at high risk for cancer recurrence—or who are undergoing treatment may have additional testing, including assessing for tumor markers, ultrasound, or MRI.2
PDB is treated with the same chemotherapy regimens as other breast cancers. In the early stages, chemotherapy reduces the risk for recurrence. In advanced breast cancer, the goal of chemotherapy is to reduce tumor size and achieve local control.
Prognosis
Patients with negative lymph node biopsy results have survival rates of 85% and 79% at five and 10 years, respectively. Patients with positive node results face survival rates of 32% at five years and 28% at 10 years. As with other cancers, anything that contributes to disease progression (including delayed diagnosis or treatment) decreases the patient’s survival rate.2,3 The overall prognosis for PDB is based on the nature of the underlying breast cancer, including its stage and other predictive factors—not on the fact that it is PDB.
Patient Outcome
Nearing the end of her treatment with trastuzumab, the patient became concerned about new-onset vaginal and left pelvic pain, along with some lower back discomfort. She mentioned these symptoms to her oncologist immediately. A transvaginal ultrasound could not rule out an ovarian neoplasm.
The patient elected to undergo total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH/BSO). This option allowed for removal of a mass discovered during the procedure, minimized the risk for subsequent endometrial cancer, and reduced the chance of recurrence of the patient’s estrogen/progesterone receptor–positive breast cancer. The mass itself turned out to be a benign pedunculated fibroid tumor.
The patient was relieved and continues to recover well. A follow-up PET/CT scan is scheduled for three months from now.
Conclusion
PDB is a complex disease that challenges our current understanding of breast cancer and its diagnosis and treatment. It depends uniquely upon ductal (versus blood or lymphatic) spread. Little did Paget and his contemporaries realize they had opened up such a porthole into modern histology. Nor did they appreciate the fact that they had identified an insidious breast cancer that declares itself through the skin.
Today, it is understood that by the time nipple changes of PDB appear, an underlying breast cancer most likely exists. In at least 25% of cases, there is neither a palpable mass nor a positive mammogram finding. For this reason, clinicians must maintain a high level of clinical suspicion and a low threshold for biopsy when there are skin changes at the nipple. This is especially true because the underlying lesions are more likely to be invasive cancers.
Surgical treatment will often mean complete mastectomy, whether simple, modified radical, or radical. This choice will be driven by the extent and location of the underlying disease. There is a role for partial mastectomy followed by radiation therapy in those rare cases in which PDB is confined to the NAC with no underlying tumor. Partial mastectomy is also a consideration when the underlying tumor is small and/or located close to the NAC. Patients with PDB may consider whole-breast or NAC reconstruction once radiation therapy and/or chemotherapy are completed.
PDB remains a poignant reminder for all clinicians of the importance of a thorough clinical exam and a well-focused history in all patients at risk for breast cancer. Moreover, it is an enduring example of the fact that common symptoms sometimes do signify something uncommon and potentially life- changing.
References
1. Paget J. On disease of the mammary areola preceding cancer of the mammary gland. In: Paget S, ed. Selected Essays and Addresses by Sir James Paget. London: Longmans, Green and Co.; 1902:145-148.
2. Sabel MS, Weaver DL. Paget disease of the breast. In: UpToDate. Chagpar AE, Hayes DF, Pierce LJ, eds. www.uptodate.com/contents/paget-disease-of-the-breast. Updated November 27, 2012. Accessed September 9, 2013.
3. Ortiz-Pagan S, Cunto-Amesty G, Narayan S. Effect of Paget’s disease on survival in breast cancer. Arch Surg. 2001;146:1267-1270.
4. American Cancer Society. Cancer facts & figures 2012. www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2012. Accessed September 9, 2013.
5. Ashikari R, Park K, Huvos AG, Urban JA. Paget’s disease of the breast. Cancer. 1970;3:680-685.
6. Sakorafas GH, Blanchard K, Sarr MG, Farley DR. Paget’s disease of the breast. Cancer Treatment Rev. 2001;27:9-18.
7. Chen C-Y, Sun L-M, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the U.S. Cancer. 2006;107:1448-1458.
8. Paone JF, Baker R. Pathogenesis and treatment of Paget’s disease of the breast. Cancer. 1981;48:825-829.
9. Nelson JA, Patel D, Mancuso P. Inflammatory breast cancer. ADVANCE for NPs and PAs. 2011;2(10):25-28.
10. Ngan V. Skin metastasis. DermNet NZ. New Zealand Dermatological Society. http://dermnetnz.org/lesions/metastasis.html. Accessed September 9, 2013.
11. Amano G, Yajima M, Moroboshi Y, et al. MRI accurately depicts underlying DCIS in a patient with Paget’s disease of the breast without palpable mass and mammography findings. Jpn J Clin Oncol. 2005;35:149-153.
12. Burrell HC, Evans AJ. Radiological assessment of the breast: what the surgical oncologist needs to know. Eur J Surg Oncol. 2001;27:689-691.
13. Muttarak M, Siriya B, Kongmebhol P. Paget’s disease of the breast: clinical, imaging and pathologic findings: a review of 16 patients. Biomed Imaging Interv J. 2001;7:e16, 1-7.
14. Laronga C, Hasson D, Hoover S, et al. Paget’s disease in the era of sentinel lymph node biopsy. Am J Surg. 2006;192:481-483.
15. Pezzi CM, Kukora JS, Audet IM. Breast conservation surgery using nipple-areolar resection for central breast cancers. Arch Surg. 2004;139:32-37.
16. Polgar C, Zsolt O, Tibor K, Janos F. Breast-conserving therapy for Paget disease of the nipple. Cancer. 2002;94:1904-1905.
17. Marshall JK, Griffith KA, Haffty BG, Solin LJ. Conservative management of Paget disease of the breast with radiotherapy. Cancer. 2003;97:2142-2149.
18. Vasquez B, Rousseau D, Hurd TC. Surgical management of breast cancer. Sem Oncol. 2007;34:234-240.
19. Mamounas EP. Continuing evolution in breast cancer surgical management. J Clin Oncol. 2005;23:1603-1606.
20. Nicholson BT, Harvey JA, Cohen MA. Nipple-areolar complex: normal anatomy and benign and malignant processes. Radiographics. 2009;29:509-523.
A 45-year-old woman noticed some redness and scaling around her right nipple. She applied peroxide and OTC antibiotic ointment for approximately seven months with mixed results. She sought medical attention when pain developed in the breast, along with some bloody discharge from the nipple (see Figure 1). Around that time, she also noticed three small nodules in the upper outer portion of the breast.
A mammogram and ultrasound revealed a 1.7 × 2.0–cm spiculated mass in the axillary tail, as well as two smaller breast lesions. A PET/CT scan ordered subsequently revealed intense uptake in the periareolar region and a suspicious axillary node. By then, the biopsy results had confirmed invasive ductal carcinoma, later determined to be Paget’s disease of the breast (PDB).
The patient’s previous medical history was significant for cystic breasts (never biopsied), chronic back pain, anxiety, and obesity. She was perimenopausal with irregular periods, the last one about 10 months ago. Her obstetric history included two pregnancies resulting in live births and no history of abortion; her menarche occurred at age 14 and her first pregnancy at 27. Family history was significant for leukemia in her maternal grandmother and niece. She did not use tobacco, alcohol, or illicit drugs. She lived at home with her husband and two daughters, who were all very supportive.
The patient elected to undergo a right modified radical mastectomy (MRM) and prophylactic left total mastectomy. MRM was performed on the right breast because sentinel lymph node identification was unsuccessful. This may have been due to involvement of the right subareolar plexus. Five of eight lymph nodes later tested positive for malignancy. The surgery was completed by placement of bilateral tissue expanders for eventual breast reconstruction.
Chemotherapy was started six weeks after surgery and included 15 weeks (five cycles) of docetaxel, carboplatin, and trastuzumab (a combination known as TCH), followed by 51 weeks (17 cycles) of trastuzumab, along with daily tamoxifen. The TCH regimen was followed by four weekly cycles of external beam radiation therapy (EBRT). Adverse effects of treatment have included chest wall dermatitis, right upper extremity lymphedema, nausea/vomiting, dyspnea, peripheral neuropathy, alopecia, and fatigue.
Discussion
Nearly 150 years ago, James Paget recognized a connection between skin changes around the nipple and deeper lesions in the breast.1 The disease that Paget identified is defined as the presence of intraepithelial adenocarcinoma cells (ie, Paget’s cells) within the epidermis of the nipple, with or without an underlying carcinoma.
An underlying breast cancer is present 85% to 95% of the time but is palpable in only approximately 50% of cases (see Figure 2). However, 25% of the time there is neither a palpable mass nor a mammographic abnormality. In these cases particularly, timely diagnosis depends on recognition of suspicious nipple changes, followed by a prompt and thorough diagnostic workup. Unfortunately, the accurate diagnosis of Paget’s disease still takes an average of several months.2
Paget’s disease is rare; it represents only 1% to 3% of new cases of female breast cancer, or about 2,250 cases a year.2-4 (The number of Paget’s disease cases per year was calculated by the author, based on the reported incidence of all breast cancers.) It is even more rare among men. For both genders, the peak age for this disease is between 50 and 60.2
Paget’s disease is an important entity for primary care PAs and NPs because it presents an opportunity to make a timely and life-changing diagnosis, and because it provides an elegant model for understanding current diagnostic and therapeutic approaches to breast cancer.
Clinical Presentation and Pathophysiology
The hallmark of PDB is scaly, vesicular, or ulcerated changes that begin in the nipple and spread to the areola. These changes are most often unilateral and may occur with pruritus, burning pain, and/or oozing from the nipple.5 This presentation is often mistaken for common skin conditions, such as eczema. Like eczema, changes in PDB may improve spontaneously and fluctuate over time, which is confusing for both the patient and clinician. A clinical pearl is that eczema is more likely to spread from the areola to the nipple, and will usually respond to topical corticosteroids. By contrast, changes in PDB tend to spread from the nipple to the areola, and corticosteroids do not provide a sustained response. Of note, Paget’s lesions may heal spontaneously even as the underlying malignancy progresses.6
PDB is unique because the underlying lesion and skin changes are not just coincidental. The cutaneous changes and the malignancy that lies beneath have a causal, not merely co-occurring, relationship. Paget himself believed that the nipple changes were both a precursor, and a promoter, of the underlying cancer.1 This transformation theory states that normal nipple epidermis turns into Paget’s cells spontaneously, before there is any underlying disease. This theory is supported by the fact that, occasionally (though rarely), no underlying breast cancer is ever found. Also, the concomitant tumor may be some distance (> 2 cm) from the nipple-areolar complex (NAC), suggesting a synchronous but causally unrelated lesion.6-8
Modern immunochemistry has turned PDB inside out. Today, PDB is believed to begin within the breast and then to spread “upward” to the NAC, called the epidermotrophic theory. This theory is supported by the fact that Paget’s cells share several molecular markers with their respective parenchymal tumors. Some researchers now propose that there is a single Paget’s progenitor cell with a motility factor that allows it to traverse the ductal system, resulting in nipple and skin changes that have come to be recognized as PDB.6-8
The invasive cancers that are associated with PDB are most likely to be both estrogen- and progesterone-receptor–negative and of a high histologic grade.3,7 Estrogen- and progesterone-sensitive tumors respond to hormonal manipulation therapy. Tumors that are receptor-negative and that have a more aggressive grade are more difficult to treat.
Differential Diagnosis
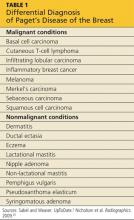
PDB may be confused with the early stages of inflammatory breast cancer (IBC), an aggressive malignant disease (see Table 1). Both conditions may present with erythema and skin thickening and may be mistaken for mastitis. However, IBC spreads rapidly through the entire breast, and clinical features may include tenderness, a feeling of heat or heaviness, breast enlargement, and significant lymphadenopathy. Current recommendations call for a biopsy of any area of breast inflammation that does not respond to antibiotics within seven days.9
PDB is not the only cutaneous manifestation of breast cancer. Others include carcinoma erysipeloides (inflammatory changes that resemble cellulitis), carcinoma telangiectaticum (vascularized plaques), and/or inflammatory papules or nodules appearing on the breast, back, neck, or scalp. Each of these non–Paget’s conditions involves lymphatic (versus ductal) spread and signifies advanced malignancy.10
Diagnosis and Staging
After biopsy of the nipple lesion(s), diagnosis proceeds to the assessment of the breast itself and ultimately to cancer staging. PDB may occur (in order of incidence):
• In conjunction with an invasive cancer
• With underlying ductal carcinoma in situ (DCIS)
• Without any underlying disease.7
Mammography is used to determine the extent and location of the underlying lesion(s), which is more likely to be peripheral and/or multicentric. However, in some cases, there are no mammographic changes, which is now recognized as an indication for performing a breast MRI.11 Once the lesion is located, direct or image-guided biopsy confirms whether it is invasive cancer or DCIS. Palpable masses that occur with PDB are usually invasive and signal advanced disease.2,6,12,13 Sentinel lymph node biopsy (SLNB), which is usually performed at the time of surgery, plays a critical role in cancer staging and treatment planning. SLNB reliably diagnoses axillary metastasis in approximately 98% of patients.14
Like other breast cancers, PDB is also categorized by the expression of molecular markers, including HER2 (human epidermal growth factor receptor 2). Cancer cells in which HER2 gene is overexpressed tend to proliferate more rapidly than others. HER-status can also provide a clue as to which chemotherapy agents are likely to be most effective.2
Treatment and Management
The primary treatment for breast cancer is surgery, which serves both diagnostic and therapeutic purposes. To be effective, surgical treatment of PDB requires excision of the NAC, also called central lumpectomy. This may be sufficient treatment in those rare cases in which the disease is confined to the NAC.11,12
For underlying tumors, partial mastectomy is an option when the tumor is small (< 2 cm) and located close enough to the NAC to achieve negative margins, while leaving a cosmetically acceptable breast. Partial mastectomy is usually followed by whole breast irradiation. A few centers offer intraoperative radiation therapy (IORT)—performed before the surgeon closes the incision—for patients who wish to avoid or limit the duration of postoperative radiation treatment.15-17
Complete mastectomy (including excision of the NAC) should be considered when:
• The distance between the NAC and the underlying tumor is significant
• Multicentric disease and/or diffuse calcifications exist
• Achieving negative margins would remove too much tissue to leave a cosmetically acceptable breast.
Evaluation of the axillary nodes is the same in PDB as with other breast cancers. Patients with disease localized to the NAC and no underlying carcinoma may choose to forego lymph node biopsy. The same is true for patients who have PDB with a single underlying DCIS. However, lymph node biopsy is always recommended in cases of multicentric DCIS or invasive disease, or if a mastectomy is planned.18,19
Sentinel node biopsy results determine whether the mastectomy should be simple (excision of the breast alone) or modified radical (breast and axillary nodes). Today, complete radical mastectomy (excision of the breast, axillary nodes, and pectoral muscle) is reserved for cases in which disease invades the chest wall.18,19
The use of adjuvant (postoperative) therapy in patients with DCIS (whether or not related to PDB) is still debated. For patients with invasive cancers, both radiation therapy and chemotherapy are usually indicated. The decision to use neoadjuvant (preoperative) chemotherapy is made on a case-by-case basis. All decisions are based on the nature of the underlying cancer, regardless of whether the diagnosis is PDB.
Because PDB is categorized as invasive in at least 85% of cases, and because all invasive breast cancers carry about twice the risk for newly diagnosed contralateral disease, systematic follow-up is extremely important for patients with PDB. A clinical exam and updated history should be performed every four to six months during the first two years and at least annually after that. Screening recommendations, including a yearly mammogram, remain the same for asymptomatic patients. Patients with new or recurring symptoms—because they are at high risk for cancer recurrence—or who are undergoing treatment may have additional testing, including assessing for tumor markers, ultrasound, or MRI.2
PDB is treated with the same chemotherapy regimens as other breast cancers. In the early stages, chemotherapy reduces the risk for recurrence. In advanced breast cancer, the goal of chemotherapy is to reduce tumor size and achieve local control.
Prognosis
Patients with negative lymph node biopsy results have survival rates of 85% and 79% at five and 10 years, respectively. Patients with positive node results face survival rates of 32% at five years and 28% at 10 years. As with other cancers, anything that contributes to disease progression (including delayed diagnosis or treatment) decreases the patient’s survival rate.2,3 The overall prognosis for PDB is based on the nature of the underlying breast cancer, including its stage and other predictive factors—not on the fact that it is PDB.
Patient Outcome
Nearing the end of her treatment with trastuzumab, the patient became concerned about new-onset vaginal and left pelvic pain, along with some lower back discomfort. She mentioned these symptoms to her oncologist immediately. A transvaginal ultrasound could not rule out an ovarian neoplasm.
The patient elected to undergo total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH/BSO). This option allowed for removal of a mass discovered during the procedure, minimized the risk for subsequent endometrial cancer, and reduced the chance of recurrence of the patient’s estrogen/progesterone receptor–positive breast cancer. The mass itself turned out to be a benign pedunculated fibroid tumor.
The patient was relieved and continues to recover well. A follow-up PET/CT scan is scheduled for three months from now.
Conclusion
PDB is a complex disease that challenges our current understanding of breast cancer and its diagnosis and treatment. It depends uniquely upon ductal (versus blood or lymphatic) spread. Little did Paget and his contemporaries realize they had opened up such a porthole into modern histology. Nor did they appreciate the fact that they had identified an insidious breast cancer that declares itself through the skin.
Today, it is understood that by the time nipple changes of PDB appear, an underlying breast cancer most likely exists. In at least 25% of cases, there is neither a palpable mass nor a positive mammogram finding. For this reason, clinicians must maintain a high level of clinical suspicion and a low threshold for biopsy when there are skin changes at the nipple. This is especially true because the underlying lesions are more likely to be invasive cancers.
Surgical treatment will often mean complete mastectomy, whether simple, modified radical, or radical. This choice will be driven by the extent and location of the underlying disease. There is a role for partial mastectomy followed by radiation therapy in those rare cases in which PDB is confined to the NAC with no underlying tumor. Partial mastectomy is also a consideration when the underlying tumor is small and/or located close to the NAC. Patients with PDB may consider whole-breast or NAC reconstruction once radiation therapy and/or chemotherapy are completed.
PDB remains a poignant reminder for all clinicians of the importance of a thorough clinical exam and a well-focused history in all patients at risk for breast cancer. Moreover, it is an enduring example of the fact that common symptoms sometimes do signify something uncommon and potentially life- changing.
References
1. Paget J. On disease of the mammary areola preceding cancer of the mammary gland. In: Paget S, ed. Selected Essays and Addresses by Sir James Paget. London: Longmans, Green and Co.; 1902:145-148.
2. Sabel MS, Weaver DL. Paget disease of the breast. In: UpToDate. Chagpar AE, Hayes DF, Pierce LJ, eds. www.uptodate.com/contents/paget-disease-of-the-breast. Updated November 27, 2012. Accessed September 9, 2013.
3. Ortiz-Pagan S, Cunto-Amesty G, Narayan S. Effect of Paget’s disease on survival in breast cancer. Arch Surg. 2001;146:1267-1270.
4. American Cancer Society. Cancer facts & figures 2012. www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2012. Accessed September 9, 2013.
5. Ashikari R, Park K, Huvos AG, Urban JA. Paget’s disease of the breast. Cancer. 1970;3:680-685.
6. Sakorafas GH, Blanchard K, Sarr MG, Farley DR. Paget’s disease of the breast. Cancer Treatment Rev. 2001;27:9-18.
7. Chen C-Y, Sun L-M, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the U.S. Cancer. 2006;107:1448-1458.
8. Paone JF, Baker R. Pathogenesis and treatment of Paget’s disease of the breast. Cancer. 1981;48:825-829.
9. Nelson JA, Patel D, Mancuso P. Inflammatory breast cancer. ADVANCE for NPs and PAs. 2011;2(10):25-28.
10. Ngan V. Skin metastasis. DermNet NZ. New Zealand Dermatological Society. http://dermnetnz.org/lesions/metastasis.html. Accessed September 9, 2013.
11. Amano G, Yajima M, Moroboshi Y, et al. MRI accurately depicts underlying DCIS in a patient with Paget’s disease of the breast without palpable mass and mammography findings. Jpn J Clin Oncol. 2005;35:149-153.
12. Burrell HC, Evans AJ. Radiological assessment of the breast: what the surgical oncologist needs to know. Eur J Surg Oncol. 2001;27:689-691.
13. Muttarak M, Siriya B, Kongmebhol P. Paget’s disease of the breast: clinical, imaging and pathologic findings: a review of 16 patients. Biomed Imaging Interv J. 2001;7:e16, 1-7.
14. Laronga C, Hasson D, Hoover S, et al. Paget’s disease in the era of sentinel lymph node biopsy. Am J Surg. 2006;192:481-483.
15. Pezzi CM, Kukora JS, Audet IM. Breast conservation surgery using nipple-areolar resection for central breast cancers. Arch Surg. 2004;139:32-37.
16. Polgar C, Zsolt O, Tibor K, Janos F. Breast-conserving therapy for Paget disease of the nipple. Cancer. 2002;94:1904-1905.
17. Marshall JK, Griffith KA, Haffty BG, Solin LJ. Conservative management of Paget disease of the breast with radiotherapy. Cancer. 2003;97:2142-2149.
18. Vasquez B, Rousseau D, Hurd TC. Surgical management of breast cancer. Sem Oncol. 2007;34:234-240.
19. Mamounas EP. Continuing evolution in breast cancer surgical management. J Clin Oncol. 2005;23:1603-1606.
20. Nicholson BT, Harvey JA, Cohen MA. Nipple-areolar complex: normal anatomy and benign and malignant processes. Radiographics. 2009;29:509-523.
A 45-year-old woman noticed some redness and scaling around her right nipple. She applied peroxide and OTC antibiotic ointment for approximately seven months with mixed results. She sought medical attention when pain developed in the breast, along with some bloody discharge from the nipple (see Figure 1). Around that time, she also noticed three small nodules in the upper outer portion of the breast.
A mammogram and ultrasound revealed a 1.7 × 2.0–cm spiculated mass in the axillary tail, as well as two smaller breast lesions. A PET/CT scan ordered subsequently revealed intense uptake in the periareolar region and a suspicious axillary node. By then, the biopsy results had confirmed invasive ductal carcinoma, later determined to be Paget’s disease of the breast (PDB).
The patient’s previous medical history was significant for cystic breasts (never biopsied), chronic back pain, anxiety, and obesity. She was perimenopausal with irregular periods, the last one about 10 months ago. Her obstetric history included two pregnancies resulting in live births and no history of abortion; her menarche occurred at age 14 and her first pregnancy at 27. Family history was significant for leukemia in her maternal grandmother and niece. She did not use tobacco, alcohol, or illicit drugs. She lived at home with her husband and two daughters, who were all very supportive.
The patient elected to undergo a right modified radical mastectomy (MRM) and prophylactic left total mastectomy. MRM was performed on the right breast because sentinel lymph node identification was unsuccessful. This may have been due to involvement of the right subareolar plexus. Five of eight lymph nodes later tested positive for malignancy. The surgery was completed by placement of bilateral tissue expanders for eventual breast reconstruction.
Chemotherapy was started six weeks after surgery and included 15 weeks (five cycles) of docetaxel, carboplatin, and trastuzumab (a combination known as TCH), followed by 51 weeks (17 cycles) of trastuzumab, along with daily tamoxifen. The TCH regimen was followed by four weekly cycles of external beam radiation therapy (EBRT). Adverse effects of treatment have included chest wall dermatitis, right upper extremity lymphedema, nausea/vomiting, dyspnea, peripheral neuropathy, alopecia, and fatigue.
Discussion
Nearly 150 years ago, James Paget recognized a connection between skin changes around the nipple and deeper lesions in the breast.1 The disease that Paget identified is defined as the presence of intraepithelial adenocarcinoma cells (ie, Paget’s cells) within the epidermis of the nipple, with or without an underlying carcinoma.
An underlying breast cancer is present 85% to 95% of the time but is palpable in only approximately 50% of cases (see Figure 2). However, 25% of the time there is neither a palpable mass nor a mammographic abnormality. In these cases particularly, timely diagnosis depends on recognition of suspicious nipple changes, followed by a prompt and thorough diagnostic workup. Unfortunately, the accurate diagnosis of Paget’s disease still takes an average of several months.2
Paget’s disease is rare; it represents only 1% to 3% of new cases of female breast cancer, or about 2,250 cases a year.2-4 (The number of Paget’s disease cases per year was calculated by the author, based on the reported incidence of all breast cancers.) It is even more rare among men. For both genders, the peak age for this disease is between 50 and 60.2
Paget’s disease is an important entity for primary care PAs and NPs because it presents an opportunity to make a timely and life-changing diagnosis, and because it provides an elegant model for understanding current diagnostic and therapeutic approaches to breast cancer.
Clinical Presentation and Pathophysiology
The hallmark of PDB is scaly, vesicular, or ulcerated changes that begin in the nipple and spread to the areola. These changes are most often unilateral and may occur with pruritus, burning pain, and/or oozing from the nipple.5 This presentation is often mistaken for common skin conditions, such as eczema. Like eczema, changes in PDB may improve spontaneously and fluctuate over time, which is confusing for both the patient and clinician. A clinical pearl is that eczema is more likely to spread from the areola to the nipple, and will usually respond to topical corticosteroids. By contrast, changes in PDB tend to spread from the nipple to the areola, and corticosteroids do not provide a sustained response. Of note, Paget’s lesions may heal spontaneously even as the underlying malignancy progresses.6
PDB is unique because the underlying lesion and skin changes are not just coincidental. The cutaneous changes and the malignancy that lies beneath have a causal, not merely co-occurring, relationship. Paget himself believed that the nipple changes were both a precursor, and a promoter, of the underlying cancer.1 This transformation theory states that normal nipple epidermis turns into Paget’s cells spontaneously, before there is any underlying disease. This theory is supported by the fact that, occasionally (though rarely), no underlying breast cancer is ever found. Also, the concomitant tumor may be some distance (> 2 cm) from the nipple-areolar complex (NAC), suggesting a synchronous but causally unrelated lesion.6-8
Modern immunochemistry has turned PDB inside out. Today, PDB is believed to begin within the breast and then to spread “upward” to the NAC, called the epidermotrophic theory. This theory is supported by the fact that Paget’s cells share several molecular markers with their respective parenchymal tumors. Some researchers now propose that there is a single Paget’s progenitor cell with a motility factor that allows it to traverse the ductal system, resulting in nipple and skin changes that have come to be recognized as PDB.6-8
The invasive cancers that are associated with PDB are most likely to be both estrogen- and progesterone-receptor–negative and of a high histologic grade.3,7 Estrogen- and progesterone-sensitive tumors respond to hormonal manipulation therapy. Tumors that are receptor-negative and that have a more aggressive grade are more difficult to treat.
Differential Diagnosis
PDB may be confused with the early stages of inflammatory breast cancer (IBC), an aggressive malignant disease (see Table 1). Both conditions may present with erythema and skin thickening and may be mistaken for mastitis. However, IBC spreads rapidly through the entire breast, and clinical features may include tenderness, a feeling of heat or heaviness, breast enlargement, and significant lymphadenopathy. Current recommendations call for a biopsy of any area of breast inflammation that does not respond to antibiotics within seven days.9
PDB is not the only cutaneous manifestation of breast cancer. Others include carcinoma erysipeloides (inflammatory changes that resemble cellulitis), carcinoma telangiectaticum (vascularized plaques), and/or inflammatory papules or nodules appearing on the breast, back, neck, or scalp. Each of these non–Paget’s conditions involves lymphatic (versus ductal) spread and signifies advanced malignancy.10
Diagnosis and Staging
After biopsy of the nipple lesion(s), diagnosis proceeds to the assessment of the breast itself and ultimately to cancer staging. PDB may occur (in order of incidence):
• In conjunction with an invasive cancer
• With underlying ductal carcinoma in situ (DCIS)
• Without any underlying disease.7
Mammography is used to determine the extent and location of the underlying lesion(s), which is more likely to be peripheral and/or multicentric. However, in some cases, there are no mammographic changes, which is now recognized as an indication for performing a breast MRI.11 Once the lesion is located, direct or image-guided biopsy confirms whether it is invasive cancer or DCIS. Palpable masses that occur with PDB are usually invasive and signal advanced disease.2,6,12,13 Sentinel lymph node biopsy (SLNB), which is usually performed at the time of surgery, plays a critical role in cancer staging and treatment planning. SLNB reliably diagnoses axillary metastasis in approximately 98% of patients.14
Like other breast cancers, PDB is also categorized by the expression of molecular markers, including HER2 (human epidermal growth factor receptor 2). Cancer cells in which HER2 gene is overexpressed tend to proliferate more rapidly than others. HER-status can also provide a clue as to which chemotherapy agents are likely to be most effective.2
Treatment and Management
The primary treatment for breast cancer is surgery, which serves both diagnostic and therapeutic purposes. To be effective, surgical treatment of PDB requires excision of the NAC, also called central lumpectomy. This may be sufficient treatment in those rare cases in which the disease is confined to the NAC.11,12
For underlying tumors, partial mastectomy is an option when the tumor is small (< 2 cm) and located close enough to the NAC to achieve negative margins, while leaving a cosmetically acceptable breast. Partial mastectomy is usually followed by whole breast irradiation. A few centers offer intraoperative radiation therapy (IORT)—performed before the surgeon closes the incision—for patients who wish to avoid or limit the duration of postoperative radiation treatment.15-17
Complete mastectomy (including excision of the NAC) should be considered when:
• The distance between the NAC and the underlying tumor is significant
• Multicentric disease and/or diffuse calcifications exist
• Achieving negative margins would remove too much tissue to leave a cosmetically acceptable breast.
Evaluation of the axillary nodes is the same in PDB as with other breast cancers. Patients with disease localized to the NAC and no underlying carcinoma may choose to forego lymph node biopsy. The same is true for patients who have PDB with a single underlying DCIS. However, lymph node biopsy is always recommended in cases of multicentric DCIS or invasive disease, or if a mastectomy is planned.18,19
Sentinel node biopsy results determine whether the mastectomy should be simple (excision of the breast alone) or modified radical (breast and axillary nodes). Today, complete radical mastectomy (excision of the breast, axillary nodes, and pectoral muscle) is reserved for cases in which disease invades the chest wall.18,19
The use of adjuvant (postoperative) therapy in patients with DCIS (whether or not related to PDB) is still debated. For patients with invasive cancers, both radiation therapy and chemotherapy are usually indicated. The decision to use neoadjuvant (preoperative) chemotherapy is made on a case-by-case basis. All decisions are based on the nature of the underlying cancer, regardless of whether the diagnosis is PDB.
Because PDB is categorized as invasive in at least 85% of cases, and because all invasive breast cancers carry about twice the risk for newly diagnosed contralateral disease, systematic follow-up is extremely important for patients with PDB. A clinical exam and updated history should be performed every four to six months during the first two years and at least annually after that. Screening recommendations, including a yearly mammogram, remain the same for asymptomatic patients. Patients with new or recurring symptoms—because they are at high risk for cancer recurrence—or who are undergoing treatment may have additional testing, including assessing for tumor markers, ultrasound, or MRI.2
PDB is treated with the same chemotherapy regimens as other breast cancers. In the early stages, chemotherapy reduces the risk for recurrence. In advanced breast cancer, the goal of chemotherapy is to reduce tumor size and achieve local control.
Prognosis
Patients with negative lymph node biopsy results have survival rates of 85% and 79% at five and 10 years, respectively. Patients with positive node results face survival rates of 32% at five years and 28% at 10 years. As with other cancers, anything that contributes to disease progression (including delayed diagnosis or treatment) decreases the patient’s survival rate.2,3 The overall prognosis for PDB is based on the nature of the underlying breast cancer, including its stage and other predictive factors—not on the fact that it is PDB.
Patient Outcome
Nearing the end of her treatment with trastuzumab, the patient became concerned about new-onset vaginal and left pelvic pain, along with some lower back discomfort. She mentioned these symptoms to her oncologist immediately. A transvaginal ultrasound could not rule out an ovarian neoplasm.
The patient elected to undergo total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH/BSO). This option allowed for removal of a mass discovered during the procedure, minimized the risk for subsequent endometrial cancer, and reduced the chance of recurrence of the patient’s estrogen/progesterone receptor–positive breast cancer. The mass itself turned out to be a benign pedunculated fibroid tumor.
The patient was relieved and continues to recover well. A follow-up PET/CT scan is scheduled for three months from now.
Conclusion
PDB is a complex disease that challenges our current understanding of breast cancer and its diagnosis and treatment. It depends uniquely upon ductal (versus blood or lymphatic) spread. Little did Paget and his contemporaries realize they had opened up such a porthole into modern histology. Nor did they appreciate the fact that they had identified an insidious breast cancer that declares itself through the skin.
Today, it is understood that by the time nipple changes of PDB appear, an underlying breast cancer most likely exists. In at least 25% of cases, there is neither a palpable mass nor a positive mammogram finding. For this reason, clinicians must maintain a high level of clinical suspicion and a low threshold for biopsy when there are skin changes at the nipple. This is especially true because the underlying lesions are more likely to be invasive cancers.
Surgical treatment will often mean complete mastectomy, whether simple, modified radical, or radical. This choice will be driven by the extent and location of the underlying disease. There is a role for partial mastectomy followed by radiation therapy in those rare cases in which PDB is confined to the NAC with no underlying tumor. Partial mastectomy is also a consideration when the underlying tumor is small and/or located close to the NAC. Patients with PDB may consider whole-breast or NAC reconstruction once radiation therapy and/or chemotherapy are completed.
PDB remains a poignant reminder for all clinicians of the importance of a thorough clinical exam and a well-focused history in all patients at risk for breast cancer. Moreover, it is an enduring example of the fact that common symptoms sometimes do signify something uncommon and potentially life- changing.
References
1. Paget J. On disease of the mammary areola preceding cancer of the mammary gland. In: Paget S, ed. Selected Essays and Addresses by Sir James Paget. London: Longmans, Green and Co.; 1902:145-148.
2. Sabel MS, Weaver DL. Paget disease of the breast. In: UpToDate. Chagpar AE, Hayes DF, Pierce LJ, eds. www.uptodate.com/contents/paget-disease-of-the-breast. Updated November 27, 2012. Accessed September 9, 2013.
3. Ortiz-Pagan S, Cunto-Amesty G, Narayan S. Effect of Paget’s disease on survival in breast cancer. Arch Surg. 2001;146:1267-1270.
4. American Cancer Society. Cancer facts & figures 2012. www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2012. Accessed September 9, 2013.
5. Ashikari R, Park K, Huvos AG, Urban JA. Paget’s disease of the breast. Cancer. 1970;3:680-685.
6. Sakorafas GH, Blanchard K, Sarr MG, Farley DR. Paget’s disease of the breast. Cancer Treatment Rev. 2001;27:9-18.
7. Chen C-Y, Sun L-M, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the U.S. Cancer. 2006;107:1448-1458.
8. Paone JF, Baker R. Pathogenesis and treatment of Paget’s disease of the breast. Cancer. 1981;48:825-829.
9. Nelson JA, Patel D, Mancuso P. Inflammatory breast cancer. ADVANCE for NPs and PAs. 2011;2(10):25-28.
10. Ngan V. Skin metastasis. DermNet NZ. New Zealand Dermatological Society. http://dermnetnz.org/lesions/metastasis.html. Accessed September 9, 2013.
11. Amano G, Yajima M, Moroboshi Y, et al. MRI accurately depicts underlying DCIS in a patient with Paget’s disease of the breast without palpable mass and mammography findings. Jpn J Clin Oncol. 2005;35:149-153.
12. Burrell HC, Evans AJ. Radiological assessment of the breast: what the surgical oncologist needs to know. Eur J Surg Oncol. 2001;27:689-691.
13. Muttarak M, Siriya B, Kongmebhol P. Paget’s disease of the breast: clinical, imaging and pathologic findings: a review of 16 patients. Biomed Imaging Interv J. 2001;7:e16, 1-7.
14. Laronga C, Hasson D, Hoover S, et al. Paget’s disease in the era of sentinel lymph node biopsy. Am J Surg. 2006;192:481-483.
15. Pezzi CM, Kukora JS, Audet IM. Breast conservation surgery using nipple-areolar resection for central breast cancers. Arch Surg. 2004;139:32-37.
16. Polgar C, Zsolt O, Tibor K, Janos F. Breast-conserving therapy for Paget disease of the nipple. Cancer. 2002;94:1904-1905.
17. Marshall JK, Griffith KA, Haffty BG, Solin LJ. Conservative management of Paget disease of the breast with radiotherapy. Cancer. 2003;97:2142-2149.
18. Vasquez B, Rousseau D, Hurd TC. Surgical management of breast cancer. Sem Oncol. 2007;34:234-240.
19. Mamounas EP. Continuing evolution in breast cancer surgical management. J Clin Oncol. 2005;23:1603-1606.
20. Nicholson BT, Harvey JA, Cohen MA. Nipple-areolar complex: normal anatomy and benign and malignant processes. Radiographics. 2009;29:509-523.
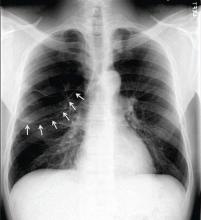
Woman Assaulted on Street
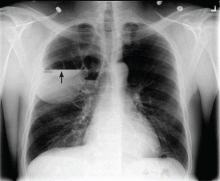
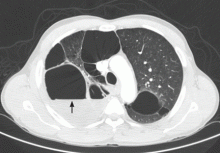
The chest radiograph demonstrates a massive amount of soft tissue and subcutaneous emphysema extending from the neck down through the chest and into the lower chest/upper abdomen. In addition, there are several fractured posterior ribs bilaterally. No large pneumothorax or pneumomediastinum is noted.
Because of the extent and mechanism of injury, CT of the chest, abdomen, and pelvis had already been ordered. Arrangements were also made for the patient to be admitted to the ICU for closer observation.
The chest radiograph demonstrates a massive amount of soft tissue and subcutaneous emphysema extending from the neck down through the chest and into the lower chest/upper abdomen. In addition, there are several fractured posterior ribs bilaterally. No large pneumothorax or pneumomediastinum is noted.
Because of the extent and mechanism of injury, CT of the chest, abdomen, and pelvis had already been ordered. Arrangements were also made for the patient to be admitted to the ICU for closer observation.
The chest radiograph demonstrates a massive amount of soft tissue and subcutaneous emphysema extending from the neck down through the chest and into the lower chest/upper abdomen. In addition, there are several fractured posterior ribs bilaterally. No large pneumothorax or pneumomediastinum is noted.
Because of the extent and mechanism of injury, CT of the chest, abdomen, and pelvis had already been ordered. Arrangements were also made for the patient to be admitted to the ICU for closer observation.
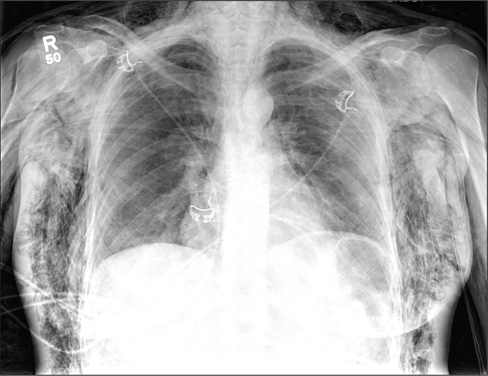
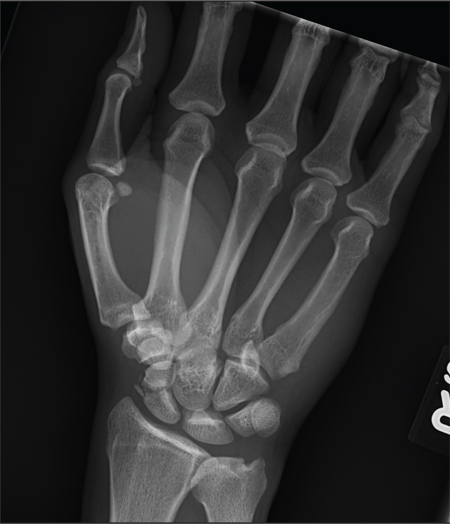
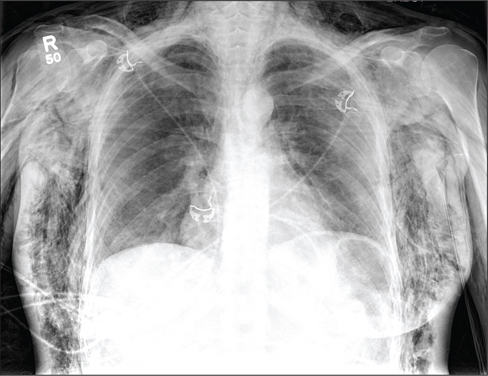
A 40-year-old woman is brought in by EMS for evaluation of injuries secondary to being assaulted. She was out walking late last night when she was approached by two men who pushed her to the ground and began punching and kicking her repeatedly on her face, chest, and back. She is primarily complaining of chest wall and back pain. Her medical history is significant for hypertension, diet-controlled diabetes, and “some sort of heart problem” for which she takes medication. Surgical history is significant for hysterectomy, cholecystectomy, and appendectomy. She smokes more than a pack of cigarettes per day and consumes at least a six-pack of beer daily. Initial exam shows an anxious female who appears somewhat uncomfortable but is in no obvious distress. Her vital signs are as follows: blood pressure, 130/88 mm Hg; pulse, 120 beats/min; respiratory rate, 22 breaths/min; and O2 saturation, 100% on room air. Physical exam reveals extensive facial/periorbital swelling, as well as swelling in the neck. Some splinting is noted. There is extensive crepitus noted within the soft tissue of the face, neck, and chest wall. Also, there is moderate tenderness bilaterally over the ribs. Chest radiograph is obtained (shown). What is your impression?
Biomarkers in the emergency workup of chest pain: Uses, limitations, and future
Each year in the United States, more than 8 million people come to the emergency department with chest pain, but only a minority are eventually diagnosed with a heart attack.1
Confronted with signs and symptoms that could represent an acute coronary syndrome, clinicians need to know whether the patient has a benign condition and can safely be sent home or is in urgent need of hospitalization—and they need to do so in a safe, timely, and cost-effective manner.2,3
Testing for biomarkers of cardiac injury, especially troponins I and T, is an accepted part of the assessment of chest pain. However, the interpretation of these cardiac biomarkers is complicated by the fact they can be elevated from noncoronary causes of chest pain such as pulmonary embolism or renal impairment, and thus should be considered only as part of the patient’s total clinical picture. This uncertainty can result in longer hospital stays and increased testing.
Thus, researchers are searching for new biomarkers that could allow for more rapid and accurate diagnosis and estimation of prognosis.
In this article we will examine the advantages and limitations of measuring cardiac biomarkers. We then discuss the emerging data on new biomarkers, including the very promising high-sensitivity troponin assays, cystatin C, and other markers, and the potential for biomarkers to be used instead of or in combination with stress testing in the evaluation of patients who have no initial evidence of ischemia.
SCENARIO 1: ELEVATED TROPONIN AND ST-SEGMENT ELEVATION
A 46-year-old woman presents to the emergency department with chest pain that started 2 hours earlier. Electrocardiography (ECG) initially shows sinus tachycardia with ST-segment depression and negative T waves in lead aVL. Her cardiac biomarker values (troponin I and creatine kinase MB) are normal. Repeated troponin I measurements show elevations of 250 ng/L, whereas her creatine kinase MB level is within the optimal range. Coronary angiography is unremarkable. Echocardiography shows right ventricular pressure overload in the pulmonary artery and the right ventricle. How should this patient be further evaluated?
SCENARIO 2: ELEVATED TROPONIN AND LEFT VENTRICULAR HYPERTROPHY
A 47-year-old man is admitted with worsening dyspnea and chest pain that worsens with coughing and inspiration. He has a history of end-stage renal disease secondary to poorly controlled hypertension and is being treated with hemodialysis, which he missed for the past 4 weeks while failing to take his hypertension medication. His blood pressure is 270/130 mm Hg. Chest auscultation reveals signs of pulmonary edema—ie, crackles at the end of inspiration. His troponin T level is 394 ng/L. ECG indicates left ventricular hypertrophy. How should this patient be further evaluated?
TROPONIN IS SPECIFIC FOR INJURY, BUT NOT FOR INFARCTION
American College of Cardiology and American Heart Association (ACC/AHA) guidelines4 recommend that clinicians ask themselves two questions: what is the likelihood that the patient is truly having an acute coronary syndrome secondary to coronary artery disease, and what is the likelihood of an adverse clinical outcome? Clues come from the initial measurements of biomarkers of cardiac injury, history, physical examination, and ECG (Table 1),5 and subsequent care is based on the estimated degree of risk.
Troponin revolutionized the diagnosis and risk stratification of chest pain. The ACC/AHA guidelines call for measuring biomarkers—preferably troponin—in all patients who present with chest discomfort consistent with an acute coronary syndrome.4,6
Cardiac troponins I and T have been the biomarkers of choice for detecting myocardial injury,4,6 since elevated concentrations are highly sensitive and tissue-specific.7 Moreover, they identify patients at short-term and long-term risk of cardiac events.4,8
The introduction of troponin testing led to a substantial increase in the rate of diagnosis of myocardial infarction (MI), with an increase in cardiac care unit admissions of more than 20%.9,10 This was partly because troponin is released into the blood with even minute myocardial damage, so that some patients who previously would have been diagnosed with unstable angina are now found to have non-ST-segment-elevation MI.10 However, the increase in admissions may also represent an increase in misdiagnoses, with many clinicians equating an elevated troponin level with acute MI.11
Although an elevated troponin level is 100% specific for myocardial injury, it is not synonymous with MI.12 Myocardial injury can be caused by a cardiac condition such as tachyarrhythmia, cardiac trauma, congestive heart failure, ventricular hypertrophy, myocarditis, or pericarditis, or by a noncardiac condition such as sepsis, respiratory failure, pulmonary embolism, pulmonary hypertension, cancer chemotherapy, or renal insufficiency.4,13 Therefore, to avoid a misdiagnosis of MI, the troponin level must be considered in the clinical context.
In fact, Alcalai et al11 noted that almost half of patients with elevated troponin did not really have an acute coronary syndrome. More importantly, in-hospital and long-term survival rates were significantly better for patients with an acute coronary syndrome than for those without, illustrating the importance of identifying and treating the true disease instead of mislabeling the problem as MI.
Bayesian theory predicts that patients with chest pain who have elevated troponin are less likely to truly have an acute coronary syndrome if the rest of their clinical presentation indicates a low probability for heart disease.14 Indeed, when McDonald et al15 used a risk-scoring index based on sex, a history of heart failure or coronary artery disease, the ECG, and use of aspirin, the positive predictive value of an abnormal troponin level was 83% at a risk score of 4 or greater, 63% at a score of 3, 52% at a score of 2, 32% at a score of 1, and 29% at a score of 0.
Thus, cardiac biomarkers are not a substitute for traditional clinical assessment, but rather should be used “in conjunction with the clinical history, physical examination, and interpretation of the ECG.”6 Consequently, diagnostic protocols that incorporate pretest clinical features to identify low-risk patients have a higher negative predictive value.
This was illustrated in a study by Than et al16 that aimed to prospectively validate the safety of an accelerated diagnostic protocol to assess chest pain suggestive of an acute coronary syndrome. The protocol included a structured pretest probability scoring method (ie, the Thrombolysis in Myocardial Infarction [TIMI] score), ECG, and a point-of-care biomarker panel of troponin, creatine kinase MB, and myoglobin. The protocol had a negative predictive value of 99.1%, whereas the use of biomarkers alone had a value of 96.1%.
HISTORY AND PHYSICAL EXAMINATION PROVIDE KEY INFORMATION
In a review, Heidenreich et al8 noted certain demographic characteristics associated with worse outcomes—ie, older age and male sex; a history of medical conditions such as diabetes, MI, and hypertension; and heart failure on presentation.
A careful assessment of chest pain and associated symptoms helps narrow the differential diagnosis. Features that increase the likelihood of a cardiac origin of chest pain are:
- Chest pain at the time of presentation (likelihood ratio [LR] = 2.0)
- Radiation of the pain to the right shoulder (LR = 2.9), the left arm (LR = 2.3), or both arms (LR = 7.1)
- Nausea or vomiting (LR = 1.9)
- Diaphoresis (LR = 2.0).17
The physical examination can detect highrisk features such as new murmurs, hypotension, diaphoresis, pulmonary edema, and rales. It is more specific than sensitive and is useful in identifying low-risk patients by targeting potential noncardiac causes of the patient’s symptoms.18
The efficacy of clinical assessment was studied in 2,271 patients with chest pain presenting to the emergency department.19 In this cohort, a low-risk group with a 30-day major cardiovascular event rate (death, MI, stroke, or revascularization) of 2.5% could be identified through the use of the US Agency for Health Care Policy and Research criteria.
Electrocardiography
ECG provides important diagnostic and prognostic information and independently predicts death or MI, even after adjustment for cardiac biomarker measurements,20,21 making it pivotal in the evaluation.4 The key features on ECG that increase the probability of MI are:
- New ST-segment elevation (LR 5.7–53.9)
- New Q waves (LR 5.3–24.8).17
One study20 found that while the troponin T level was a powerful independent marker in patients presenting with MI, its value for risk stratification was enhanced when it was combined with a standard measure such as ECG.20 While more than 90% of patients with STsegment elevation had an adverse outcome, only 31.7% of those patients had an elevated troponin T level.
No component is sufficient by itself
Thus, in spite of the proliferation of cardiac diagnostic tests, the initial bedside assessment of chest pain remains paramount. In fact, in patients presenting to the emergency department with chest pain, low risk (ie, those with a < 5% probability of MI) may be identified by presenting symptoms, medical history, and ECG alone.19
Furthermore, although clinical assessment, ECG, and cardiac biomarker testing each provide incremental benefit in assessing chest pain, no component is sufficient by itself. Sanchis et al22 found that even in patients with a normal troponin I level, the risk remained high in the case of ST-segment depression, and that even without signs of ischemia, the probability of cardiac events was 16% when the chest pain score was 11 points or higher.22 Consequently, a normal troponin level, ECG, or any other predictor alone would not ensure a good prognosis.
BIOMARKERS INSTEAD OF STRESS TESTING?
The ACC/AHA guidelines for the diagnosis of patients with unstable angina and non-STsegment elevation MI say that stable patients at low risk with no evidence of ischemia on initial assessment can be admitted to a chest pain unit for observation with serial cardiac biomarkers and ECG.4 At the end of the observation period, those who have reassuring results on ECG and normal cardiac biomarker measurements undergo functional cardiac testing or stress testing, or both.4
Exercise treadmill testing is a cornerstone of confirmatory testing in an accelerated diagnostic protocol because it is readily available, safe, and easy to do.18 A low-risk result was shown to have a high negative predictive value,23,24 so that the likelihood of an acute coronary syndrome is low enough for safe discharge.
However, the overall process is not ideal since it is time-consuming, generates additional costs, and can have false-positive results in patients who are otherwise deemed not to be at high risk. While some studies provided an optimistic view about discharging low-risk patients with negative biomarkers without stress testing,7,25 others have discouraged omitting exercise treadmill testing from protocols.22,26
Others have proposed combining a biomarker with an imaging study such as coronary computed tomographic (CT) angiography.27 Normal findings on this study have been shown to have a negative predictive value of up to 100% for ruling out an acute coronary syndrome and the occurrence of major adverse cardiovascular events in the long term.28,29 Furthermore, it allows more-inclusive assessments of chest pain and can exclude other life-threatening causes such as pulmonary embolism and aortic dissection (referred to as the “triple rule-out”).30
However, 25% to 50% of patients presenting to the emergency department with chest pain may not be candidates for CT angiography because of obesity, contrast allergy, intolerance to beta-blockade, arrhythmia, renal insufficiency, or a history of coronary artery disease.18 Moreover, it may be more efficient and less costly to discharge some patients without coronary CT angiography31 with the help of novel biomarkers without routine additional testing. This may spare patients the additional radiation exposure from CT angiography or nuclear imaging.27,32
New biomarkers may, it is hoped, better distinguish patients at low risk from those at high risk without resorting to stress testing. Several of these markers are moving toward mainstream clinical use. For a biomarker to be prognostically equivalent to stress testing, it must be able to tell us if the likelihood of an acute coronary syndrome is low enough for safe discharge—ie, it must have a significantly high negative predictive value. Also, it must be an independent predictor of adverse outcomes, particularly in patients deemed at low risk by initial low troponin measurements. Biomarkers that have shown promise in this regard include high-sensitivity troponin, brain-type natriuretic peptide (BNP), cystatin C, and ischemia-modified albumin.
HIGH-SENSITIVITY CARDIAC TROPONIN ASSAYS
Although we speak of “high-sensitivity troponin,” these new assays detect the same molecule as do traditional troponin assays. The difference is that high-sensitivity assays can detect and measure troponin at concentrations much lower than the traditional assays can. In fact, high-sensitivity troponin assays can detect and measure troponin at very low levels in almost all healthy people.
Studies have shown that the high-sensitivity assays have better analytical accuracy and sensitivity than older assays.12
Aldous et al33 reported that, in patients who presented to the emergency department within 4 hours of the onset of chest pain, an elevation in troponin T on a high-sensitivity assay had a positive predictive value of 53.8% and a negative predictive value of 98.3%.
Weber et al34 found the diagnostic value of the high-sensitivity troponin T assay to be superior to that of a contemporary troponin T assay (area under the receiver-operating-characteristics curve [AUC] of 0.949 vs 0.929). Even when the contemporary troponin T assay was negative, the high-sensitivity assay provided strong diagnostic information (AUC 0.81). Furthermore, the high-sensitivity assay provided superior independent prognostic power for death within 6 months.
Hochholzer et al35 reported a prognostic accuracy for death significantly higher (AUC 0.79) than that of contemporary troponin T (AUC 0.69). A concentration of high-sensitivity troponin T above 14 ng/L improved the prediction of death (hazard ratio 2.60) but not of subsequent acute MI in patients with acute chest pain. Therefore, a negative high-sensitivity troponin T assay identifies patients with a good prognosis and who may be discharged without further testing if their clinical presentation and ECG are also reassuring.
Keller et al36 compared the diagnostic performance of the high-sensitivity cardiac troponin I assay against 11 other biomarkers, including a contemporary cardiac troponin I assay. The contemporary troponin I and the high-sensitivity troponin I assays performed best. The high-sensitivity troponin I assay at admission had a sensitivity of 82.3% and a negative predictive value of 94.7% for ruling out acute MI, whereas the contemporary troponin I assay had a sensitivity of 79.4% and a negative predictive value of 94.0%.
Using levels obtained at 3 hours after admission, the sensitivity was 98.2% and the negative predictive value was 99.4% for both troponin I assays. Combining the 99th percentile cutoff at admission with the serial change in troponin concentration within 3 hours, the positive predictive value for ruling in acute MI for high-sensitivity cardiac troponin I increased from 75.1% at admission to 95.8% after 3 hours; for the contemporary assay, it increased from 80.9% at admission to 96.1%.36
The authors concluded that performing either of the cardiac troponin I assays 3 hours after admission may help in ruling out MI early on, with a negative predictive value greater than 99%. Moreover, the relative change in concentration within the 3 hours after admission, combined with the 99th percentile diagnostic cutoff value on admission, improves specificity, allowing acute MI to be accurately ruled in.36
Of note, though studies have confirmed that a measurement at 3 hours identifies most cases of MI early, they have not used the recommended maximal sensitivity interval for troponin measurements (6 hours or more).6
A proposed algorithm for diagnosing acute MI with a high-sensitivity assay
While high-sensitivity troponin T assays can improve the early diagnosis of acute MI, how best to use them is yet to be defined. They still lack specificity for acute coronary syndromes, with positive predictive values as low as 50%.37
Reichlin et al38 developed and validated an algorithm for rapidly ruling out or ruling in acute MI using a high-sensitivity cardiac troponin T assay, incorporating baseline values and absolute changes within the first hour. Using a baseline threshold of 12 ng/L or less and an absolute change of 3 ng/L or less, they found a sensitivity and negative predictive value of 100%, making these good criteria for ruling out acute MI.
Using a baseline threshold of 60 ng/L or greater and a change from baseline to 1 hour of at least 15 ng/L, the specificity was 97% and the positive predictive value was 84%, making these good criteria for ruling in acute MI.
Patients whose values were in between were classified as being in an “observationalzone group,” in which the prevalence of acute MI was 8%. The cumulative 30-day survival rate was 99.8% in patients in whom the test ruled out MI, 98.6% in the observational-zone patients, and 95.3% in patients in whom the test ruled in MI.38 Using this simple algorithm allowed a safe rule-out as well as an accurate rule-in of acute MI within 1 hour in 77% of unselected patients with acute chest pain; thus, it may obviate the need for prolonged monitoring and serial measurements in three out of four patients.”
Newby39 stated that such an algorithmic approach must be validated in a prospective study that assesses not only sensitivity, negative predictive value, specificity, and positive predictive value, but also the implications for clinical outcomes and the cost of widespread implementation.
In the meantime, clinicians must keep in mind that patient populations in clinical practice are less selected, the prevalence of MI may broadly vary, and confounding comorbidities such as heart failure and renal insufficiency are more common. Studies are also needed to verify whether other factors such as age, sex, and time from symptom onset should be considered.
BRAIN-TYPE NATRIURETIC PEPTIDE
BNP is a 32-amino-acid natriuretic peptide that is released from myocytes. The amount released depends on wall stress brought on by heart failure, ischemic heart disease, or other conditions.
In a study of the diagnostic utility of BNP in the workup of acute chest pain, Haaf et al40 found that BNP levels at presentation were significantly higher in patients with acute MI than in patients with other diagnoses. However, the diagnostic accuracy of BNP was lower than that of cardiac troponin T at presentation, though its independent predictive value for all-cause mortality was more accurate than that of troponin T.
Elevation of the BNP 41 or the N-terminal pro-BNP 42,43 level was shown to also provide unique prognostic information in patients with suspected and confirmed acute coronary syndrome and was associated with higher rates of short-term and long-term mortality. Therefore, BNP appears useful for the prognosis but not the diagnosis of acute coronary syndromes.
CYSTATIN C
The protein cystatin C, widely used as a biomarker for kidney disease, has more recently been touted as a prognostic marker in acute coronary syndromes.
Jernberg et al44 reported that, in patients with a suspected or confirmed acute coronary syndrome, a single measurement of cystatin C significantly improved the early stratification of risk.44 Specifically, the cystatin C level was independently associated with mortality risk but not with the risk of subsequent MI.
In another study,45 the cystatin C concentration independently predicted the risk of cardiovascular death or MI in non-ST-segment elevation acute coronary syndrome. However, the additive predictive value of cystatin C in these patients was found to be small when clinical risk factors and biomarkers of MI were used in the prediction model. Therefore, cystatin C may predict global risk but does not appear to be useful in diagnosing MI.
ISCHEMIA-MODIFIED ALBUMIN
A major limitation of troponin is that it cannot detect reversible myocardial ischemia in the absence of cardiac necrosis, making stress testing necessary to unmask potential reversible ischemia.
Ischemia-modified albumin has been proposed as a means of detecting cardiac ischemia even if necrosis is absent. It is a product of the N-terminus alteration of albumin caused by myocardial ischemia, which reduces the ability of cobalt to bind to albumin and can be detected with the albumin cobalt binding test. This marker might have a high negative predictive value, ruling out acute coronary syndromes in conditions of low pretest probability with negative necrosis markers and ECG.13,46
Although ischemia-modified albumin does show promise, doubt remains as to its validity as a biomarker, as its mechanism of generation is not known. Some have suggested that it is in fact a marker of oxidative stress.47
PANELS OF MARKERS
The individual biomarkers we have discussed here have advantages and limitations in the emergency workup of chest pain. The concept of using a multimarker panel has been raised as a way of amplifying the positive attributes of individual biomarkers and compensating for their shortcomings.
Sabatine et al48 tested this approach in patients with acute coronary syndromes who were at high risk of an adverse outcome. When patients were categorized at presentation on the basis of the number of elevated biomarkers such as cardiac troponin I, C-reactive protein, and BNP, the risk of death nearly doubled with each additional biomarker that was elevated.
The relationship was similar for the end points of MI, heart failure, and the composite at 30 days and 10 months. In a cohort of 1,635 patients, the number of elevated biomarkers remained a predictor of the composite end point after adjustment for known clinical predictors. The risk of death, MI, or heart failure by 6 months was 2.1 times higher in patients with one elevated biomarker, 3.1 times higher in those with two, and 3.7 times higher in those with three.
The authors concluded that a multimarker strategy that categorizes patients on the basis of the number of elevated biomarkers at presentation allows risk-stratification of short- and long-term cardiac events.
Tello-Montoliu et al49 tested this idea in patients with non-ST-segment elevation acute coronary syndromes using a panel consisting of cardiac troponin T, C-reactive protein, N-terminal pro-BNP, and fibrin D-dimer. The risk of a major event (death, new acute coronary syndrome, revascularization, or heart failure) at 6 months was associated with abnormal biomarker levels, especially with the presence of three positive biomarkers, even after adjustment for clinical characteristics and ECG findings.
van der Zee et al43 showed that a positive biomarker panel consisting of C-reactive protein and N-terminal pro-BNP identified patients with chest pain and a normal or nondiagnostic ECG who have a high long-term risk of cardiovascular death.
Glaser et al50 evaluated the combination of cardiac troponin I, BNP, homocysteine, C-reactive protein, placental growth factor, myeloperoxidase, choline, soluble CD40 ligand, ischemia-modified albumin, and lipoprotein-associated phospholipase A2 in patients with a suspected acute coronary syndrome. The combination of BNP, placental growth factor, and estimated glomerular filtration rate was the most accurate predictor of major adverse cardiovascular events compared with any other biomarker or clinical factor. With appropriate cutoff values, the negative predictive value for a major adverse cardiovascular event at 1 year was as high as 99.1%.
This study highlighted the importance of combining biomarkers, showing that with a negative predictive value of 97% for 30-day events, the combination of placental growth factor, BNP, and cardiac troponin I may help surmount the delay from symptom onset to cardiac troponin increase, thus permitting a more timely diagnosis and safe discharge within 12 hours.
Comment. These studies raise the promise that panels of biomarkers can be used in patients deemed to be at low risk after clinical assessment and troponin evaluation to enable them to be safely discharged early and to obviate the need for stress testing.
If we assume that unstable cardiac disease requiring hospitalization accounts for 35% of patients with chest pain, a hypothetical panel of biomarkers with a sensitivity and specificity of 95% for adverse cardiac outcomes would have a positive predictive value of 91% and a negative predictive value of 97%. The negative likelihood ratio of this hypothetical biomarker panel would be 0.05, while the positive likelihood ratio would be 19. This performance level means that in patients with a pretest probability less than 50%, the posttest probability can be reduced to below 10%, so that such patients can be safely discharged without further hospital evaluation.
Conversely, a positive test result in patients with pretest probability of 30% or greater raises the posttest probability to nearly 90%, meaning that such patients should be considered for aggressive intervention without the need for stress testing.
RETURN TO OUR SCENARIOS
Chest pain remains a nonspecific complaint, and the interpretation of biomarkers to find the cause presents clinicians with challenges, as illustrated by the cases introduced at the beginning of this article.
The cardiac troponin I elevation in scenario 1 led to an initial diagnosis of unstable angina. However, coronary angiography showed lesion-free coronary arteries, thus excluding ischemic heart disease. When other diseases that could cause elevated cardiac troponin I were considered and investigated with further diagnostic tests such as D-dimer, pulmonary embolism became the new working diagnosis, and this was confirmed by CT angiography.
Similarly, given the laboratory values for the patient in scenario 2, the condition could have been mistaken for an acute coronary syndrome. However, the absence of evidence on ECG to support this diagnosis would indicate an erroneously elevated biomarker secondary to his background of chronic renal insufficiency.
- Pitts SR, Niska RW, Xu J, Burt CW. National hospital ambulatory medical care survey: 2006 emergency department summary. Natl Health Stat Report 2008;1–38.
- Vucic R, Knezevic S, Lazic Z, et al. Elevation of troponin values in differential diagnosis of chest pain in view of pulmonary thromboembolism. Vojnosanit Pregl 2012; 69:913–916.
- Croitoru M, Taegtmeyer H. Spurious rises in troponin T in end-stage renal disease. Lancet 1995; 346:974.
- Anderson JL, Adams CD, Antman EM, et al; 2011 Writing Group Members; ACCF/AHA Task Force Members. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 123:e426–e579.
- Unstable angina: diagnosis and management. Guideline overview. Agency for Health Care Policy and Research. J Natl Med Assoc 1994; 86:649,710–712.
- Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation 2007; 115:e356–e375.
- Hamm CW, Goldmann BU, Heeschen C, Kreymann G, Berger J, Meinertz T. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N Engl J Med 1997; 337:1648–1653.
- Heidenreich PA, Go A, Melsop KA, et al. Prediction of risk for patients with unstable angina. Evid Rep Technol Assess (Summ) 2000; August:1–3.
- Kontos MC, Fritz LM, Anderson FP, Tatum JL, Ornato JP, Jesse RL. Impact of the troponin standard on the prevalence of acute myocardial infarction. Am Heart J 2003; 146:446–452.
- Amit G, Gilutz H, Cafri C, Wolak A, Ilia R, Zahger D. What have the new definition of acute myocardial infarction and the introduction of troponin measurement done to the coronary care unit? Impacts on admission rate, length of stay, case mix and mortality. Cardiology 2004; 102:171–176.
- Alcalai R, Planer D, Culhaoglu A, Osman A, Pollak A, Lotan C. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med 2007; 167:276–281.
- Thygesen K, Mair J, Katus H, et al. Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J 2010; 31:2197–2204.
- Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol 2006; 48:1–11.
- Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA 1994; 271:703–707.
- McDonald MA, Holroyd B, Comeau A, Hervas-Malo M, Welsh RC. Clinical risk scoring beyond initial troponin values: results from a large, prospective, unselected acute chest pain population. Can J Cardiol 2007; 23:287–292.
- Than M, Cullen L, Reid CM, et al. A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): a prospective observational validation study. Lancet 2011; 377:1077–1084.
- Panju AA, Hemmelgarn BR, Guyatt GH, Simel DL. The rational clinical examination. Is this patient having a myocardial infarction? JAMA 1998; 280:1256–1263.
- Amsterdam EA, Kirk JD, Bluemke DA, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation 2010; 122:1756–1776.
- Farkouh ME, Aneja A, Reeder GS, et al. Clinical risk stratification in the emergency department predicts long-term cardiovascular outcomes in a population-based cohort presenting with acute chest pain: primary results of the Olmsted county chest pain study. Medicine (Baltimore) 2009; 88:307–313.
- Ohman EM, Armstrong PW, Christenson RH, et al. Cardiac troponin T levels for risk stratification in acute myocardial ischemia. GUSTO IIA Investigators. N Engl J Med 1996; 335:1333–1341.
- Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med 1996; 335:1342–1349.
- Sanchis J, Bodí V, Llácer A, et al. Predictors of short-term outcome in acute chest pain without ST-segment elevation. Int J Cardiol 2003; 92:193–199.
- Gomez MA, Anderson JL, Karagounis LA, Muhlestein JB, Mooers FB. An emergency department-based protocol for rapidly ruling out myocardial ischemia reduces hospital time and expense: results of a randomized study (ROMIO). J Am Coll Cardiol 1996; 28:25–33.
- Diercks DB, Gibler WB, Liu T, Sayre MR, Storrow AB. Identification of patients at risk by graded exercise testing in an emergency department chest pain center. Am J Cardiol 2000; 86:289–292.
- Rahman F, Mitra B, Cameron PA, Coleridge J. Stress testing before discharge is not required for patients with low and intermediate risk of acute coronary syndrome after emergency department short stay assessment. Emerg Med Australas 2010; 22:449–456.
- Kontos MC, Anderson FP, Alimard R, Ornato JP, Tatum JL, Jesse RL. Ability of troponin I to predict cardiac events in patients admitted from the emergency department. J Am Coll Cardiol 2000; 36:1818–1823.
- Hoffmann U, Truong QA, Schoenfeld DA, et al; ROMICAT-II Investigators. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012; 367:299–308.
- Hoffmann U, Bamberg F, Chae CU, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol 2009; 53:1642–1650.
- Goldstein JA, Chinnaiyan KM, Abidov A, et al; CT-STAT Investigators. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol 2011; 58:1414–1422.
- White CS, Kuo D, Kelemen M, et al. Chest pain evaluation in the emergency department: can MDCT provide a comprehensive evaluation? AJR Am J Roentgenol 2005; 185:533–540.
- Redberg RF. Coronary CT angiography for acute chest pain. N Engl J Med 2012; 367:375–376.
- Shreibati JB, Baker LC, Hlatky MA. Association of coronary CT angiography or stress testing with subsequent utilization and spending among Medicare beneficiaries. JAMA 2011; 306:2128–2136.
- Aldous S, Pemberton C, Richards AM, Troughton R, Than M. High-sensitivity troponin T for early rule-out of myocardial infarction in recent onset chest pain. Emerg Med J 2012; 29:805–810.
- Weber M, Bazzino O, Navarro Estrada JL, et al. Improved diagnostic and prognostic performance of a new high-sensitive troponin T assay in patients with acute coronary syndrome. Am Heart J 2011; 162:81–88.
- Hochholzer W, Reichlin T, Twerenbold R, et al. Incremental value of high-sensitivity cardiac troponin T for risk prediction in patients with suspected acute myocardial infarction. Clin Chem 2011; 57:1318–1326.
- Keller T, Zeller T, Ojeda F, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA 2011; 306:2684–2693.
- Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009; 361:858–867.
- Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012; 172:1211–1218.
- Newby LK. Myocardial infarction rule-out in the emergency department: are high-sensitivity troponins the answer?: Comment on “One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T”. Arch Intern Med 2012; 172:1218–1219.
- Haaf P, Reichlin T, Corson N, et al. B-type natriuretic peptide in the early diagnosis and risk stratification of acute chest pain. Am J Med 2011; 124:444–445.
- Sun T, Wang L, Zhang Y. Prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. Arch Med Res 2006; 37:502–505.
- Galvani M, Ottani F, Oltrona L, et al; Italian Working Group on Atherosclerosis, Thrombosis, and Vascular Biology and the Associazione Nazionale Medici Cardiologi Ospedalieri (ANMCO). N-terminal pro-brain natriuretic peptide on admission has prognostic value across the whole spectrum of acute coronary syndromes. Circulation 2004; 110:128–134.
- van der Zee PM, Cornel JH, Bholasingh R, Fischer JC, van Straalen JP, De Winter RJ. N-terminal pro B-type natriuretic peptide identifies patients with chest pain at high long-term cardiovascular risk. Am J Med 2011; 124:961–969.
- Jernberg T, Lindahl B, James S, Larsson A, Hansson LO, Wallentin L. Cystatin C: a novel predictor of outcome in suspected or confirmed non-ST-elevation acute coronary syndrome. Circulation 2004; 110:2342–2348.
- Akerblom Å, Wallentin L, Siegbahn A, et al. Cystatin C and estimated glomerular filtration rate as predictors for adverse outcome in patients with ST-elevation and non-ST-elevation acute coronary syndromes: results from the Platelet Inhibition and Patient Outcomes study. Clin Chem 2012; 58:190–199.
- Anwaruddin S, Januzzi JL, Baggish AL, Lewandrowski EL, Lewandrowski KB. Ischemia-modified albumin improves the usefulness of standard cardiac biomarkers for the diagnosis of myocardial ischemia in the emergency department setting. Am J Clin Pathol 2005; 123:140–145.
- Senes M, Kazan N, Coskun O, Zengi O, Inan L, Yücel D. Oxidative and nitrosative stress in acute ischaemic stroke. Ann Clin Biochem 2007; 44:43–47.
- Sabatine MS, Morrow DA, de Lemos JA, et al. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation 2002; 105:1760–1763.
- Tello-Montoliu A, Marín F, Roldán V, et al. A multimarker risk stratification approach to non-ST elevation acute coronary syndrome: implications of troponin T, CRP, NT pro-BNP and fibrin D-dimer levels. J Intern Med 2007; 262:651–658.
- Glaser R, Peacock WF, Wu AH, Muller R, Möckel M, Apple FS. Placental growth factor and B-type natriuretic peptide as independent predictors of risk from a multibiomarker panel in suspected acute coronary syndrome (Acute Risk and Related Outcomes Assessed With Cardiac Biomarkers [ARROW]) study. Am J Cardiol 2011; 107:821–826.
Each year in the United States, more than 8 million people come to the emergency department with chest pain, but only a minority are eventually diagnosed with a heart attack.1
Confronted with signs and symptoms that could represent an acute coronary syndrome, clinicians need to know whether the patient has a benign condition and can safely be sent home or is in urgent need of hospitalization—and they need to do so in a safe, timely, and cost-effective manner.2,3
Testing for biomarkers of cardiac injury, especially troponins I and T, is an accepted part of the assessment of chest pain. However, the interpretation of these cardiac biomarkers is complicated by the fact they can be elevated from noncoronary causes of chest pain such as pulmonary embolism or renal impairment, and thus should be considered only as part of the patient’s total clinical picture. This uncertainty can result in longer hospital stays and increased testing.
Thus, researchers are searching for new biomarkers that could allow for more rapid and accurate diagnosis and estimation of prognosis.
In this article we will examine the advantages and limitations of measuring cardiac biomarkers. We then discuss the emerging data on new biomarkers, including the very promising high-sensitivity troponin assays, cystatin C, and other markers, and the potential for biomarkers to be used instead of or in combination with stress testing in the evaluation of patients who have no initial evidence of ischemia.
SCENARIO 1: ELEVATED TROPONIN AND ST-SEGMENT ELEVATION
A 46-year-old woman presents to the emergency department with chest pain that started 2 hours earlier. Electrocardiography (ECG) initially shows sinus tachycardia with ST-segment depression and negative T waves in lead aVL. Her cardiac biomarker values (troponin I and creatine kinase MB) are normal. Repeated troponin I measurements show elevations of 250 ng/L, whereas her creatine kinase MB level is within the optimal range. Coronary angiography is unremarkable. Echocardiography shows right ventricular pressure overload in the pulmonary artery and the right ventricle. How should this patient be further evaluated?
SCENARIO 2: ELEVATED TROPONIN AND LEFT VENTRICULAR HYPERTROPHY
A 47-year-old man is admitted with worsening dyspnea and chest pain that worsens with coughing and inspiration. He has a history of end-stage renal disease secondary to poorly controlled hypertension and is being treated with hemodialysis, which he missed for the past 4 weeks while failing to take his hypertension medication. His blood pressure is 270/130 mm Hg. Chest auscultation reveals signs of pulmonary edema—ie, crackles at the end of inspiration. His troponin T level is 394 ng/L. ECG indicates left ventricular hypertrophy. How should this patient be further evaluated?
TROPONIN IS SPECIFIC FOR INJURY, BUT NOT FOR INFARCTION
American College of Cardiology and American Heart Association (ACC/AHA) guidelines4 recommend that clinicians ask themselves two questions: what is the likelihood that the patient is truly having an acute coronary syndrome secondary to coronary artery disease, and what is the likelihood of an adverse clinical outcome? Clues come from the initial measurements of biomarkers of cardiac injury, history, physical examination, and ECG (Table 1),5 and subsequent care is based on the estimated degree of risk.
Troponin revolutionized the diagnosis and risk stratification of chest pain. The ACC/AHA guidelines call for measuring biomarkers—preferably troponin—in all patients who present with chest discomfort consistent with an acute coronary syndrome.4,6
Cardiac troponins I and T have been the biomarkers of choice for detecting myocardial injury,4,6 since elevated concentrations are highly sensitive and tissue-specific.7 Moreover, they identify patients at short-term and long-term risk of cardiac events.4,8
The introduction of troponin testing led to a substantial increase in the rate of diagnosis of myocardial infarction (MI), with an increase in cardiac care unit admissions of more than 20%.9,10 This was partly because troponin is released into the blood with even minute myocardial damage, so that some patients who previously would have been diagnosed with unstable angina are now found to have non-ST-segment-elevation MI.10 However, the increase in admissions may also represent an increase in misdiagnoses, with many clinicians equating an elevated troponin level with acute MI.11
Although an elevated troponin level is 100% specific for myocardial injury, it is not synonymous with MI.12 Myocardial injury can be caused by a cardiac condition such as tachyarrhythmia, cardiac trauma, congestive heart failure, ventricular hypertrophy, myocarditis, or pericarditis, or by a noncardiac condition such as sepsis, respiratory failure, pulmonary embolism, pulmonary hypertension, cancer chemotherapy, or renal insufficiency.4,13 Therefore, to avoid a misdiagnosis of MI, the troponin level must be considered in the clinical context.
In fact, Alcalai et al11 noted that almost half of patients with elevated troponin did not really have an acute coronary syndrome. More importantly, in-hospital and long-term survival rates were significantly better for patients with an acute coronary syndrome than for those without, illustrating the importance of identifying and treating the true disease instead of mislabeling the problem as MI.
Bayesian theory predicts that patients with chest pain who have elevated troponin are less likely to truly have an acute coronary syndrome if the rest of their clinical presentation indicates a low probability for heart disease.14 Indeed, when McDonald et al15 used a risk-scoring index based on sex, a history of heart failure or coronary artery disease, the ECG, and use of aspirin, the positive predictive value of an abnormal troponin level was 83% at a risk score of 4 or greater, 63% at a score of 3, 52% at a score of 2, 32% at a score of 1, and 29% at a score of 0.
Thus, cardiac biomarkers are not a substitute for traditional clinical assessment, but rather should be used “in conjunction with the clinical history, physical examination, and interpretation of the ECG.”6 Consequently, diagnostic protocols that incorporate pretest clinical features to identify low-risk patients have a higher negative predictive value.
This was illustrated in a study by Than et al16 that aimed to prospectively validate the safety of an accelerated diagnostic protocol to assess chest pain suggestive of an acute coronary syndrome. The protocol included a structured pretest probability scoring method (ie, the Thrombolysis in Myocardial Infarction [TIMI] score), ECG, and a point-of-care biomarker panel of troponin, creatine kinase MB, and myoglobin. The protocol had a negative predictive value of 99.1%, whereas the use of biomarkers alone had a value of 96.1%.
HISTORY AND PHYSICAL EXAMINATION PROVIDE KEY INFORMATION
In a review, Heidenreich et al8 noted certain demographic characteristics associated with worse outcomes—ie, older age and male sex; a history of medical conditions such as diabetes, MI, and hypertension; and heart failure on presentation.
A careful assessment of chest pain and associated symptoms helps narrow the differential diagnosis. Features that increase the likelihood of a cardiac origin of chest pain are:
- Chest pain at the time of presentation (likelihood ratio [LR] = 2.0)
- Radiation of the pain to the right shoulder (LR = 2.9), the left arm (LR = 2.3), or both arms (LR = 7.1)
- Nausea or vomiting (LR = 1.9)
- Diaphoresis (LR = 2.0).17
The physical examination can detect highrisk features such as new murmurs, hypotension, diaphoresis, pulmonary edema, and rales. It is more specific than sensitive and is useful in identifying low-risk patients by targeting potential noncardiac causes of the patient’s symptoms.18
The efficacy of clinical assessment was studied in 2,271 patients with chest pain presenting to the emergency department.19 In this cohort, a low-risk group with a 30-day major cardiovascular event rate (death, MI, stroke, or revascularization) of 2.5% could be identified through the use of the US Agency for Health Care Policy and Research criteria.
Electrocardiography
ECG provides important diagnostic and prognostic information and independently predicts death or MI, even after adjustment for cardiac biomarker measurements,20,21 making it pivotal in the evaluation.4 The key features on ECG that increase the probability of MI are:
- New ST-segment elevation (LR 5.7–53.9)
- New Q waves (LR 5.3–24.8).17
One study20 found that while the troponin T level was a powerful independent marker in patients presenting with MI, its value for risk stratification was enhanced when it was combined with a standard measure such as ECG.20 While more than 90% of patients with STsegment elevation had an adverse outcome, only 31.7% of those patients had an elevated troponin T level.
No component is sufficient by itself
Thus, in spite of the proliferation of cardiac diagnostic tests, the initial bedside assessment of chest pain remains paramount. In fact, in patients presenting to the emergency department with chest pain, low risk (ie, those with a < 5% probability of MI) may be identified by presenting symptoms, medical history, and ECG alone.19
Furthermore, although clinical assessment, ECG, and cardiac biomarker testing each provide incremental benefit in assessing chest pain, no component is sufficient by itself. Sanchis et al22 found that even in patients with a normal troponin I level, the risk remained high in the case of ST-segment depression, and that even without signs of ischemia, the probability of cardiac events was 16% when the chest pain score was 11 points or higher.22 Consequently, a normal troponin level, ECG, or any other predictor alone would not ensure a good prognosis.
BIOMARKERS INSTEAD OF STRESS TESTING?
The ACC/AHA guidelines for the diagnosis of patients with unstable angina and non-STsegment elevation MI say that stable patients at low risk with no evidence of ischemia on initial assessment can be admitted to a chest pain unit for observation with serial cardiac biomarkers and ECG.4 At the end of the observation period, those who have reassuring results on ECG and normal cardiac biomarker measurements undergo functional cardiac testing or stress testing, or both.4
Exercise treadmill testing is a cornerstone of confirmatory testing in an accelerated diagnostic protocol because it is readily available, safe, and easy to do.18 A low-risk result was shown to have a high negative predictive value,23,24 so that the likelihood of an acute coronary syndrome is low enough for safe discharge.
However, the overall process is not ideal since it is time-consuming, generates additional costs, and can have false-positive results in patients who are otherwise deemed not to be at high risk. While some studies provided an optimistic view about discharging low-risk patients with negative biomarkers without stress testing,7,25 others have discouraged omitting exercise treadmill testing from protocols.22,26
Others have proposed combining a biomarker with an imaging study such as coronary computed tomographic (CT) angiography.27 Normal findings on this study have been shown to have a negative predictive value of up to 100% for ruling out an acute coronary syndrome and the occurrence of major adverse cardiovascular events in the long term.28,29 Furthermore, it allows more-inclusive assessments of chest pain and can exclude other life-threatening causes such as pulmonary embolism and aortic dissection (referred to as the “triple rule-out”).30
However, 25% to 50% of patients presenting to the emergency department with chest pain may not be candidates for CT angiography because of obesity, contrast allergy, intolerance to beta-blockade, arrhythmia, renal insufficiency, or a history of coronary artery disease.18 Moreover, it may be more efficient and less costly to discharge some patients without coronary CT angiography31 with the help of novel biomarkers without routine additional testing. This may spare patients the additional radiation exposure from CT angiography or nuclear imaging.27,32
New biomarkers may, it is hoped, better distinguish patients at low risk from those at high risk without resorting to stress testing. Several of these markers are moving toward mainstream clinical use. For a biomarker to be prognostically equivalent to stress testing, it must be able to tell us if the likelihood of an acute coronary syndrome is low enough for safe discharge—ie, it must have a significantly high negative predictive value. Also, it must be an independent predictor of adverse outcomes, particularly in patients deemed at low risk by initial low troponin measurements. Biomarkers that have shown promise in this regard include high-sensitivity troponin, brain-type natriuretic peptide (BNP), cystatin C, and ischemia-modified albumin.
HIGH-SENSITIVITY CARDIAC TROPONIN ASSAYS
Although we speak of “high-sensitivity troponin,” these new assays detect the same molecule as do traditional troponin assays. The difference is that high-sensitivity assays can detect and measure troponin at concentrations much lower than the traditional assays can. In fact, high-sensitivity troponin assays can detect and measure troponin at very low levels in almost all healthy people.
Studies have shown that the high-sensitivity assays have better analytical accuracy and sensitivity than older assays.12
Aldous et al33 reported that, in patients who presented to the emergency department within 4 hours of the onset of chest pain, an elevation in troponin T on a high-sensitivity assay had a positive predictive value of 53.8% and a negative predictive value of 98.3%.
Weber et al34 found the diagnostic value of the high-sensitivity troponin T assay to be superior to that of a contemporary troponin T assay (area under the receiver-operating-characteristics curve [AUC] of 0.949 vs 0.929). Even when the contemporary troponin T assay was negative, the high-sensitivity assay provided strong diagnostic information (AUC 0.81). Furthermore, the high-sensitivity assay provided superior independent prognostic power for death within 6 months.
Hochholzer et al35 reported a prognostic accuracy for death significantly higher (AUC 0.79) than that of contemporary troponin T (AUC 0.69). A concentration of high-sensitivity troponin T above 14 ng/L improved the prediction of death (hazard ratio 2.60) but not of subsequent acute MI in patients with acute chest pain. Therefore, a negative high-sensitivity troponin T assay identifies patients with a good prognosis and who may be discharged without further testing if their clinical presentation and ECG are also reassuring.
Keller et al36 compared the diagnostic performance of the high-sensitivity cardiac troponin I assay against 11 other biomarkers, including a contemporary cardiac troponin I assay. The contemporary troponin I and the high-sensitivity troponin I assays performed best. The high-sensitivity troponin I assay at admission had a sensitivity of 82.3% and a negative predictive value of 94.7% for ruling out acute MI, whereas the contemporary troponin I assay had a sensitivity of 79.4% and a negative predictive value of 94.0%.
Using levels obtained at 3 hours after admission, the sensitivity was 98.2% and the negative predictive value was 99.4% for both troponin I assays. Combining the 99th percentile cutoff at admission with the serial change in troponin concentration within 3 hours, the positive predictive value for ruling in acute MI for high-sensitivity cardiac troponin I increased from 75.1% at admission to 95.8% after 3 hours; for the contemporary assay, it increased from 80.9% at admission to 96.1%.36
The authors concluded that performing either of the cardiac troponin I assays 3 hours after admission may help in ruling out MI early on, with a negative predictive value greater than 99%. Moreover, the relative change in concentration within the 3 hours after admission, combined with the 99th percentile diagnostic cutoff value on admission, improves specificity, allowing acute MI to be accurately ruled in.36
Of note, though studies have confirmed that a measurement at 3 hours identifies most cases of MI early, they have not used the recommended maximal sensitivity interval for troponin measurements (6 hours or more).6
A proposed algorithm for diagnosing acute MI with a high-sensitivity assay
While high-sensitivity troponin T assays can improve the early diagnosis of acute MI, how best to use them is yet to be defined. They still lack specificity for acute coronary syndromes, with positive predictive values as low as 50%.37
Reichlin et al38 developed and validated an algorithm for rapidly ruling out or ruling in acute MI using a high-sensitivity cardiac troponin T assay, incorporating baseline values and absolute changes within the first hour. Using a baseline threshold of 12 ng/L or less and an absolute change of 3 ng/L or less, they found a sensitivity and negative predictive value of 100%, making these good criteria for ruling out acute MI.
Using a baseline threshold of 60 ng/L or greater and a change from baseline to 1 hour of at least 15 ng/L, the specificity was 97% and the positive predictive value was 84%, making these good criteria for ruling in acute MI.
Patients whose values were in between were classified as being in an “observationalzone group,” in which the prevalence of acute MI was 8%. The cumulative 30-day survival rate was 99.8% in patients in whom the test ruled out MI, 98.6% in the observational-zone patients, and 95.3% in patients in whom the test ruled in MI.38 Using this simple algorithm allowed a safe rule-out as well as an accurate rule-in of acute MI within 1 hour in 77% of unselected patients with acute chest pain; thus, it may obviate the need for prolonged monitoring and serial measurements in three out of four patients.”
Newby39 stated that such an algorithmic approach must be validated in a prospective study that assesses not only sensitivity, negative predictive value, specificity, and positive predictive value, but also the implications for clinical outcomes and the cost of widespread implementation.
In the meantime, clinicians must keep in mind that patient populations in clinical practice are less selected, the prevalence of MI may broadly vary, and confounding comorbidities such as heart failure and renal insufficiency are more common. Studies are also needed to verify whether other factors such as age, sex, and time from symptom onset should be considered.
BRAIN-TYPE NATRIURETIC PEPTIDE
BNP is a 32-amino-acid natriuretic peptide that is released from myocytes. The amount released depends on wall stress brought on by heart failure, ischemic heart disease, or other conditions.
In a study of the diagnostic utility of BNP in the workup of acute chest pain, Haaf et al40 found that BNP levels at presentation were significantly higher in patients with acute MI than in patients with other diagnoses. However, the diagnostic accuracy of BNP was lower than that of cardiac troponin T at presentation, though its independent predictive value for all-cause mortality was more accurate than that of troponin T.
Elevation of the BNP 41 or the N-terminal pro-BNP 42,43 level was shown to also provide unique prognostic information in patients with suspected and confirmed acute coronary syndrome and was associated with higher rates of short-term and long-term mortality. Therefore, BNP appears useful for the prognosis but not the diagnosis of acute coronary syndromes.
CYSTATIN C
The protein cystatin C, widely used as a biomarker for kidney disease, has more recently been touted as a prognostic marker in acute coronary syndromes.
Jernberg et al44 reported that, in patients with a suspected or confirmed acute coronary syndrome, a single measurement of cystatin C significantly improved the early stratification of risk.44 Specifically, the cystatin C level was independently associated with mortality risk but not with the risk of subsequent MI.
In another study,45 the cystatin C concentration independently predicted the risk of cardiovascular death or MI in non-ST-segment elevation acute coronary syndrome. However, the additive predictive value of cystatin C in these patients was found to be small when clinical risk factors and biomarkers of MI were used in the prediction model. Therefore, cystatin C may predict global risk but does not appear to be useful in diagnosing MI.
ISCHEMIA-MODIFIED ALBUMIN
A major limitation of troponin is that it cannot detect reversible myocardial ischemia in the absence of cardiac necrosis, making stress testing necessary to unmask potential reversible ischemia.
Ischemia-modified albumin has been proposed as a means of detecting cardiac ischemia even if necrosis is absent. It is a product of the N-terminus alteration of albumin caused by myocardial ischemia, which reduces the ability of cobalt to bind to albumin and can be detected with the albumin cobalt binding test. This marker might have a high negative predictive value, ruling out acute coronary syndromes in conditions of low pretest probability with negative necrosis markers and ECG.13,46
Although ischemia-modified albumin does show promise, doubt remains as to its validity as a biomarker, as its mechanism of generation is not known. Some have suggested that it is in fact a marker of oxidative stress.47
PANELS OF MARKERS
The individual biomarkers we have discussed here have advantages and limitations in the emergency workup of chest pain. The concept of using a multimarker panel has been raised as a way of amplifying the positive attributes of individual biomarkers and compensating for their shortcomings.
Sabatine et al48 tested this approach in patients with acute coronary syndromes who were at high risk of an adverse outcome. When patients were categorized at presentation on the basis of the number of elevated biomarkers such as cardiac troponin I, C-reactive protein, and BNP, the risk of death nearly doubled with each additional biomarker that was elevated.
The relationship was similar for the end points of MI, heart failure, and the composite at 30 days and 10 months. In a cohort of 1,635 patients, the number of elevated biomarkers remained a predictor of the composite end point after adjustment for known clinical predictors. The risk of death, MI, or heart failure by 6 months was 2.1 times higher in patients with one elevated biomarker, 3.1 times higher in those with two, and 3.7 times higher in those with three.
The authors concluded that a multimarker strategy that categorizes patients on the basis of the number of elevated biomarkers at presentation allows risk-stratification of short- and long-term cardiac events.
Tello-Montoliu et al49 tested this idea in patients with non-ST-segment elevation acute coronary syndromes using a panel consisting of cardiac troponin T, C-reactive protein, N-terminal pro-BNP, and fibrin D-dimer. The risk of a major event (death, new acute coronary syndrome, revascularization, or heart failure) at 6 months was associated with abnormal biomarker levels, especially with the presence of three positive biomarkers, even after adjustment for clinical characteristics and ECG findings.
van der Zee et al43 showed that a positive biomarker panel consisting of C-reactive protein and N-terminal pro-BNP identified patients with chest pain and a normal or nondiagnostic ECG who have a high long-term risk of cardiovascular death.
Glaser et al50 evaluated the combination of cardiac troponin I, BNP, homocysteine, C-reactive protein, placental growth factor, myeloperoxidase, choline, soluble CD40 ligand, ischemia-modified albumin, and lipoprotein-associated phospholipase A2 in patients with a suspected acute coronary syndrome. The combination of BNP, placental growth factor, and estimated glomerular filtration rate was the most accurate predictor of major adverse cardiovascular events compared with any other biomarker or clinical factor. With appropriate cutoff values, the negative predictive value for a major adverse cardiovascular event at 1 year was as high as 99.1%.
This study highlighted the importance of combining biomarkers, showing that with a negative predictive value of 97% for 30-day events, the combination of placental growth factor, BNP, and cardiac troponin I may help surmount the delay from symptom onset to cardiac troponin increase, thus permitting a more timely diagnosis and safe discharge within 12 hours.
Comment. These studies raise the promise that panels of biomarkers can be used in patients deemed to be at low risk after clinical assessment and troponin evaluation to enable them to be safely discharged early and to obviate the need for stress testing.
If we assume that unstable cardiac disease requiring hospitalization accounts for 35% of patients with chest pain, a hypothetical panel of biomarkers with a sensitivity and specificity of 95% for adverse cardiac outcomes would have a positive predictive value of 91% and a negative predictive value of 97%. The negative likelihood ratio of this hypothetical biomarker panel would be 0.05, while the positive likelihood ratio would be 19. This performance level means that in patients with a pretest probability less than 50%, the posttest probability can be reduced to below 10%, so that such patients can be safely discharged without further hospital evaluation.
Conversely, a positive test result in patients with pretest probability of 30% or greater raises the posttest probability to nearly 90%, meaning that such patients should be considered for aggressive intervention without the need for stress testing.
RETURN TO OUR SCENARIOS
Chest pain remains a nonspecific complaint, and the interpretation of biomarkers to find the cause presents clinicians with challenges, as illustrated by the cases introduced at the beginning of this article.
The cardiac troponin I elevation in scenario 1 led to an initial diagnosis of unstable angina. However, coronary angiography showed lesion-free coronary arteries, thus excluding ischemic heart disease. When other diseases that could cause elevated cardiac troponin I were considered and investigated with further diagnostic tests such as D-dimer, pulmonary embolism became the new working diagnosis, and this was confirmed by CT angiography.
Similarly, given the laboratory values for the patient in scenario 2, the condition could have been mistaken for an acute coronary syndrome. However, the absence of evidence on ECG to support this diagnosis would indicate an erroneously elevated biomarker secondary to his background of chronic renal insufficiency.
Each year in the United States, more than 8 million people come to the emergency department with chest pain, but only a minority are eventually diagnosed with a heart attack.1
Confronted with signs and symptoms that could represent an acute coronary syndrome, clinicians need to know whether the patient has a benign condition and can safely be sent home or is in urgent need of hospitalization—and they need to do so in a safe, timely, and cost-effective manner.2,3
Testing for biomarkers of cardiac injury, especially troponins I and T, is an accepted part of the assessment of chest pain. However, the interpretation of these cardiac biomarkers is complicated by the fact they can be elevated from noncoronary causes of chest pain such as pulmonary embolism or renal impairment, and thus should be considered only as part of the patient’s total clinical picture. This uncertainty can result in longer hospital stays and increased testing.
Thus, researchers are searching for new biomarkers that could allow for more rapid and accurate diagnosis and estimation of prognosis.
In this article we will examine the advantages and limitations of measuring cardiac biomarkers. We then discuss the emerging data on new biomarkers, including the very promising high-sensitivity troponin assays, cystatin C, and other markers, and the potential for biomarkers to be used instead of or in combination with stress testing in the evaluation of patients who have no initial evidence of ischemia.
SCENARIO 1: ELEVATED TROPONIN AND ST-SEGMENT ELEVATION
A 46-year-old woman presents to the emergency department with chest pain that started 2 hours earlier. Electrocardiography (ECG) initially shows sinus tachycardia with ST-segment depression and negative T waves in lead aVL. Her cardiac biomarker values (troponin I and creatine kinase MB) are normal. Repeated troponin I measurements show elevations of 250 ng/L, whereas her creatine kinase MB level is within the optimal range. Coronary angiography is unremarkable. Echocardiography shows right ventricular pressure overload in the pulmonary artery and the right ventricle. How should this patient be further evaluated?
SCENARIO 2: ELEVATED TROPONIN AND LEFT VENTRICULAR HYPERTROPHY
A 47-year-old man is admitted with worsening dyspnea and chest pain that worsens with coughing and inspiration. He has a history of end-stage renal disease secondary to poorly controlled hypertension and is being treated with hemodialysis, which he missed for the past 4 weeks while failing to take his hypertension medication. His blood pressure is 270/130 mm Hg. Chest auscultation reveals signs of pulmonary edema—ie, crackles at the end of inspiration. His troponin T level is 394 ng/L. ECG indicates left ventricular hypertrophy. How should this patient be further evaluated?
TROPONIN IS SPECIFIC FOR INJURY, BUT NOT FOR INFARCTION
American College of Cardiology and American Heart Association (ACC/AHA) guidelines4 recommend that clinicians ask themselves two questions: what is the likelihood that the patient is truly having an acute coronary syndrome secondary to coronary artery disease, and what is the likelihood of an adverse clinical outcome? Clues come from the initial measurements of biomarkers of cardiac injury, history, physical examination, and ECG (Table 1),5 and subsequent care is based on the estimated degree of risk.
Troponin revolutionized the diagnosis and risk stratification of chest pain. The ACC/AHA guidelines call for measuring biomarkers—preferably troponin—in all patients who present with chest discomfort consistent with an acute coronary syndrome.4,6
Cardiac troponins I and T have been the biomarkers of choice for detecting myocardial injury,4,6 since elevated concentrations are highly sensitive and tissue-specific.7 Moreover, they identify patients at short-term and long-term risk of cardiac events.4,8
The introduction of troponin testing led to a substantial increase in the rate of diagnosis of myocardial infarction (MI), with an increase in cardiac care unit admissions of more than 20%.9,10 This was partly because troponin is released into the blood with even minute myocardial damage, so that some patients who previously would have been diagnosed with unstable angina are now found to have non-ST-segment-elevation MI.10 However, the increase in admissions may also represent an increase in misdiagnoses, with many clinicians equating an elevated troponin level with acute MI.11
Although an elevated troponin level is 100% specific for myocardial injury, it is not synonymous with MI.12 Myocardial injury can be caused by a cardiac condition such as tachyarrhythmia, cardiac trauma, congestive heart failure, ventricular hypertrophy, myocarditis, or pericarditis, or by a noncardiac condition such as sepsis, respiratory failure, pulmonary embolism, pulmonary hypertension, cancer chemotherapy, or renal insufficiency.4,13 Therefore, to avoid a misdiagnosis of MI, the troponin level must be considered in the clinical context.
In fact, Alcalai et al11 noted that almost half of patients with elevated troponin did not really have an acute coronary syndrome. More importantly, in-hospital and long-term survival rates were significantly better for patients with an acute coronary syndrome than for those without, illustrating the importance of identifying and treating the true disease instead of mislabeling the problem as MI.
Bayesian theory predicts that patients with chest pain who have elevated troponin are less likely to truly have an acute coronary syndrome if the rest of their clinical presentation indicates a low probability for heart disease.14 Indeed, when McDonald et al15 used a risk-scoring index based on sex, a history of heart failure or coronary artery disease, the ECG, and use of aspirin, the positive predictive value of an abnormal troponin level was 83% at a risk score of 4 or greater, 63% at a score of 3, 52% at a score of 2, 32% at a score of 1, and 29% at a score of 0.
Thus, cardiac biomarkers are not a substitute for traditional clinical assessment, but rather should be used “in conjunction with the clinical history, physical examination, and interpretation of the ECG.”6 Consequently, diagnostic protocols that incorporate pretest clinical features to identify low-risk patients have a higher negative predictive value.
This was illustrated in a study by Than et al16 that aimed to prospectively validate the safety of an accelerated diagnostic protocol to assess chest pain suggestive of an acute coronary syndrome. The protocol included a structured pretest probability scoring method (ie, the Thrombolysis in Myocardial Infarction [TIMI] score), ECG, and a point-of-care biomarker panel of troponin, creatine kinase MB, and myoglobin. The protocol had a negative predictive value of 99.1%, whereas the use of biomarkers alone had a value of 96.1%.
HISTORY AND PHYSICAL EXAMINATION PROVIDE KEY INFORMATION
In a review, Heidenreich et al8 noted certain demographic characteristics associated with worse outcomes—ie, older age and male sex; a history of medical conditions such as diabetes, MI, and hypertension; and heart failure on presentation.
A careful assessment of chest pain and associated symptoms helps narrow the differential diagnosis. Features that increase the likelihood of a cardiac origin of chest pain are:
- Chest pain at the time of presentation (likelihood ratio [LR] = 2.0)
- Radiation of the pain to the right shoulder (LR = 2.9), the left arm (LR = 2.3), or both arms (LR = 7.1)
- Nausea or vomiting (LR = 1.9)
- Diaphoresis (LR = 2.0).17
The physical examination can detect highrisk features such as new murmurs, hypotension, diaphoresis, pulmonary edema, and rales. It is more specific than sensitive and is useful in identifying low-risk patients by targeting potential noncardiac causes of the patient’s symptoms.18
The efficacy of clinical assessment was studied in 2,271 patients with chest pain presenting to the emergency department.19 In this cohort, a low-risk group with a 30-day major cardiovascular event rate (death, MI, stroke, or revascularization) of 2.5% could be identified through the use of the US Agency for Health Care Policy and Research criteria.
Electrocardiography
ECG provides important diagnostic and prognostic information and independently predicts death or MI, even after adjustment for cardiac biomarker measurements,20,21 making it pivotal in the evaluation.4 The key features on ECG that increase the probability of MI are:
- New ST-segment elevation (LR 5.7–53.9)
- New Q waves (LR 5.3–24.8).17
One study20 found that while the troponin T level was a powerful independent marker in patients presenting with MI, its value for risk stratification was enhanced when it was combined with a standard measure such as ECG.20 While more than 90% of patients with STsegment elevation had an adverse outcome, only 31.7% of those patients had an elevated troponin T level.
No component is sufficient by itself
Thus, in spite of the proliferation of cardiac diagnostic tests, the initial bedside assessment of chest pain remains paramount. In fact, in patients presenting to the emergency department with chest pain, low risk (ie, those with a < 5% probability of MI) may be identified by presenting symptoms, medical history, and ECG alone.19
Furthermore, although clinical assessment, ECG, and cardiac biomarker testing each provide incremental benefit in assessing chest pain, no component is sufficient by itself. Sanchis et al22 found that even in patients with a normal troponin I level, the risk remained high in the case of ST-segment depression, and that even without signs of ischemia, the probability of cardiac events was 16% when the chest pain score was 11 points or higher.22 Consequently, a normal troponin level, ECG, or any other predictor alone would not ensure a good prognosis.
BIOMARKERS INSTEAD OF STRESS TESTING?
The ACC/AHA guidelines for the diagnosis of patients with unstable angina and non-STsegment elevation MI say that stable patients at low risk with no evidence of ischemia on initial assessment can be admitted to a chest pain unit for observation with serial cardiac biomarkers and ECG.4 At the end of the observation period, those who have reassuring results on ECG and normal cardiac biomarker measurements undergo functional cardiac testing or stress testing, or both.4
Exercise treadmill testing is a cornerstone of confirmatory testing in an accelerated diagnostic protocol because it is readily available, safe, and easy to do.18 A low-risk result was shown to have a high negative predictive value,23,24 so that the likelihood of an acute coronary syndrome is low enough for safe discharge.
However, the overall process is not ideal since it is time-consuming, generates additional costs, and can have false-positive results in patients who are otherwise deemed not to be at high risk. While some studies provided an optimistic view about discharging low-risk patients with negative biomarkers without stress testing,7,25 others have discouraged omitting exercise treadmill testing from protocols.22,26
Others have proposed combining a biomarker with an imaging study such as coronary computed tomographic (CT) angiography.27 Normal findings on this study have been shown to have a negative predictive value of up to 100% for ruling out an acute coronary syndrome and the occurrence of major adverse cardiovascular events in the long term.28,29 Furthermore, it allows more-inclusive assessments of chest pain and can exclude other life-threatening causes such as pulmonary embolism and aortic dissection (referred to as the “triple rule-out”).30
However, 25% to 50% of patients presenting to the emergency department with chest pain may not be candidates for CT angiography because of obesity, contrast allergy, intolerance to beta-blockade, arrhythmia, renal insufficiency, or a history of coronary artery disease.18 Moreover, it may be more efficient and less costly to discharge some patients without coronary CT angiography31 with the help of novel biomarkers without routine additional testing. This may spare patients the additional radiation exposure from CT angiography or nuclear imaging.27,32
New biomarkers may, it is hoped, better distinguish patients at low risk from those at high risk without resorting to stress testing. Several of these markers are moving toward mainstream clinical use. For a biomarker to be prognostically equivalent to stress testing, it must be able to tell us if the likelihood of an acute coronary syndrome is low enough for safe discharge—ie, it must have a significantly high negative predictive value. Also, it must be an independent predictor of adverse outcomes, particularly in patients deemed at low risk by initial low troponin measurements. Biomarkers that have shown promise in this regard include high-sensitivity troponin, brain-type natriuretic peptide (BNP), cystatin C, and ischemia-modified albumin.
HIGH-SENSITIVITY CARDIAC TROPONIN ASSAYS
Although we speak of “high-sensitivity troponin,” these new assays detect the same molecule as do traditional troponin assays. The difference is that high-sensitivity assays can detect and measure troponin at concentrations much lower than the traditional assays can. In fact, high-sensitivity troponin assays can detect and measure troponin at very low levels in almost all healthy people.
Studies have shown that the high-sensitivity assays have better analytical accuracy and sensitivity than older assays.12
Aldous et al33 reported that, in patients who presented to the emergency department within 4 hours of the onset of chest pain, an elevation in troponin T on a high-sensitivity assay had a positive predictive value of 53.8% and a negative predictive value of 98.3%.
Weber et al34 found the diagnostic value of the high-sensitivity troponin T assay to be superior to that of a contemporary troponin T assay (area under the receiver-operating-characteristics curve [AUC] of 0.949 vs 0.929). Even when the contemporary troponin T assay was negative, the high-sensitivity assay provided strong diagnostic information (AUC 0.81). Furthermore, the high-sensitivity assay provided superior independent prognostic power for death within 6 months.
Hochholzer et al35 reported a prognostic accuracy for death significantly higher (AUC 0.79) than that of contemporary troponin T (AUC 0.69). A concentration of high-sensitivity troponin T above 14 ng/L improved the prediction of death (hazard ratio 2.60) but not of subsequent acute MI in patients with acute chest pain. Therefore, a negative high-sensitivity troponin T assay identifies patients with a good prognosis and who may be discharged without further testing if their clinical presentation and ECG are also reassuring.
Keller et al36 compared the diagnostic performance of the high-sensitivity cardiac troponin I assay against 11 other biomarkers, including a contemporary cardiac troponin I assay. The contemporary troponin I and the high-sensitivity troponin I assays performed best. The high-sensitivity troponin I assay at admission had a sensitivity of 82.3% and a negative predictive value of 94.7% for ruling out acute MI, whereas the contemporary troponin I assay had a sensitivity of 79.4% and a negative predictive value of 94.0%.
Using levels obtained at 3 hours after admission, the sensitivity was 98.2% and the negative predictive value was 99.4% for both troponin I assays. Combining the 99th percentile cutoff at admission with the serial change in troponin concentration within 3 hours, the positive predictive value for ruling in acute MI for high-sensitivity cardiac troponin I increased from 75.1% at admission to 95.8% after 3 hours; for the contemporary assay, it increased from 80.9% at admission to 96.1%.36
The authors concluded that performing either of the cardiac troponin I assays 3 hours after admission may help in ruling out MI early on, with a negative predictive value greater than 99%. Moreover, the relative change in concentration within the 3 hours after admission, combined with the 99th percentile diagnostic cutoff value on admission, improves specificity, allowing acute MI to be accurately ruled in.36
Of note, though studies have confirmed that a measurement at 3 hours identifies most cases of MI early, they have not used the recommended maximal sensitivity interval for troponin measurements (6 hours or more).6
A proposed algorithm for diagnosing acute MI with a high-sensitivity assay
While high-sensitivity troponin T assays can improve the early diagnosis of acute MI, how best to use them is yet to be defined. They still lack specificity for acute coronary syndromes, with positive predictive values as low as 50%.37
Reichlin et al38 developed and validated an algorithm for rapidly ruling out or ruling in acute MI using a high-sensitivity cardiac troponin T assay, incorporating baseline values and absolute changes within the first hour. Using a baseline threshold of 12 ng/L or less and an absolute change of 3 ng/L or less, they found a sensitivity and negative predictive value of 100%, making these good criteria for ruling out acute MI.
Using a baseline threshold of 60 ng/L or greater and a change from baseline to 1 hour of at least 15 ng/L, the specificity was 97% and the positive predictive value was 84%, making these good criteria for ruling in acute MI.
Patients whose values were in between were classified as being in an “observationalzone group,” in which the prevalence of acute MI was 8%. The cumulative 30-day survival rate was 99.8% in patients in whom the test ruled out MI, 98.6% in the observational-zone patients, and 95.3% in patients in whom the test ruled in MI.38 Using this simple algorithm allowed a safe rule-out as well as an accurate rule-in of acute MI within 1 hour in 77% of unselected patients with acute chest pain; thus, it may obviate the need for prolonged monitoring and serial measurements in three out of four patients.”
Newby39 stated that such an algorithmic approach must be validated in a prospective study that assesses not only sensitivity, negative predictive value, specificity, and positive predictive value, but also the implications for clinical outcomes and the cost of widespread implementation.
In the meantime, clinicians must keep in mind that patient populations in clinical practice are less selected, the prevalence of MI may broadly vary, and confounding comorbidities such as heart failure and renal insufficiency are more common. Studies are also needed to verify whether other factors such as age, sex, and time from symptom onset should be considered.
BRAIN-TYPE NATRIURETIC PEPTIDE
BNP is a 32-amino-acid natriuretic peptide that is released from myocytes. The amount released depends on wall stress brought on by heart failure, ischemic heart disease, or other conditions.
In a study of the diagnostic utility of BNP in the workup of acute chest pain, Haaf et al40 found that BNP levels at presentation were significantly higher in patients with acute MI than in patients with other diagnoses. However, the diagnostic accuracy of BNP was lower than that of cardiac troponin T at presentation, though its independent predictive value for all-cause mortality was more accurate than that of troponin T.
Elevation of the BNP 41 or the N-terminal pro-BNP 42,43 level was shown to also provide unique prognostic information in patients with suspected and confirmed acute coronary syndrome and was associated with higher rates of short-term and long-term mortality. Therefore, BNP appears useful for the prognosis but not the diagnosis of acute coronary syndromes.
CYSTATIN C
The protein cystatin C, widely used as a biomarker for kidney disease, has more recently been touted as a prognostic marker in acute coronary syndromes.
Jernberg et al44 reported that, in patients with a suspected or confirmed acute coronary syndrome, a single measurement of cystatin C significantly improved the early stratification of risk.44 Specifically, the cystatin C level was independently associated with mortality risk but not with the risk of subsequent MI.
In another study,45 the cystatin C concentration independently predicted the risk of cardiovascular death or MI in non-ST-segment elevation acute coronary syndrome. However, the additive predictive value of cystatin C in these patients was found to be small when clinical risk factors and biomarkers of MI were used in the prediction model. Therefore, cystatin C may predict global risk but does not appear to be useful in diagnosing MI.
ISCHEMIA-MODIFIED ALBUMIN
A major limitation of troponin is that it cannot detect reversible myocardial ischemia in the absence of cardiac necrosis, making stress testing necessary to unmask potential reversible ischemia.
Ischemia-modified albumin has been proposed as a means of detecting cardiac ischemia even if necrosis is absent. It is a product of the N-terminus alteration of albumin caused by myocardial ischemia, which reduces the ability of cobalt to bind to albumin and can be detected with the albumin cobalt binding test. This marker might have a high negative predictive value, ruling out acute coronary syndromes in conditions of low pretest probability with negative necrosis markers and ECG.13,46
Although ischemia-modified albumin does show promise, doubt remains as to its validity as a biomarker, as its mechanism of generation is not known. Some have suggested that it is in fact a marker of oxidative stress.47
PANELS OF MARKERS
The individual biomarkers we have discussed here have advantages and limitations in the emergency workup of chest pain. The concept of using a multimarker panel has been raised as a way of amplifying the positive attributes of individual biomarkers and compensating for their shortcomings.
Sabatine et al48 tested this approach in patients with acute coronary syndromes who were at high risk of an adverse outcome. When patients were categorized at presentation on the basis of the number of elevated biomarkers such as cardiac troponin I, C-reactive protein, and BNP, the risk of death nearly doubled with each additional biomarker that was elevated.
The relationship was similar for the end points of MI, heart failure, and the composite at 30 days and 10 months. In a cohort of 1,635 patients, the number of elevated biomarkers remained a predictor of the composite end point after adjustment for known clinical predictors. The risk of death, MI, or heart failure by 6 months was 2.1 times higher in patients with one elevated biomarker, 3.1 times higher in those with two, and 3.7 times higher in those with three.
The authors concluded that a multimarker strategy that categorizes patients on the basis of the number of elevated biomarkers at presentation allows risk-stratification of short- and long-term cardiac events.
Tello-Montoliu et al49 tested this idea in patients with non-ST-segment elevation acute coronary syndromes using a panel consisting of cardiac troponin T, C-reactive protein, N-terminal pro-BNP, and fibrin D-dimer. The risk of a major event (death, new acute coronary syndrome, revascularization, or heart failure) at 6 months was associated with abnormal biomarker levels, especially with the presence of three positive biomarkers, even after adjustment for clinical characteristics and ECG findings.
van der Zee et al43 showed that a positive biomarker panel consisting of C-reactive protein and N-terminal pro-BNP identified patients with chest pain and a normal or nondiagnostic ECG who have a high long-term risk of cardiovascular death.
Glaser et al50 evaluated the combination of cardiac troponin I, BNP, homocysteine, C-reactive protein, placental growth factor, myeloperoxidase, choline, soluble CD40 ligand, ischemia-modified albumin, and lipoprotein-associated phospholipase A2 in patients with a suspected acute coronary syndrome. The combination of BNP, placental growth factor, and estimated glomerular filtration rate was the most accurate predictor of major adverse cardiovascular events compared with any other biomarker or clinical factor. With appropriate cutoff values, the negative predictive value for a major adverse cardiovascular event at 1 year was as high as 99.1%.
This study highlighted the importance of combining biomarkers, showing that with a negative predictive value of 97% for 30-day events, the combination of placental growth factor, BNP, and cardiac troponin I may help surmount the delay from symptom onset to cardiac troponin increase, thus permitting a more timely diagnosis and safe discharge within 12 hours.
Comment. These studies raise the promise that panels of biomarkers can be used in patients deemed to be at low risk after clinical assessment and troponin evaluation to enable them to be safely discharged early and to obviate the need for stress testing.
If we assume that unstable cardiac disease requiring hospitalization accounts for 35% of patients with chest pain, a hypothetical panel of biomarkers with a sensitivity and specificity of 95% for adverse cardiac outcomes would have a positive predictive value of 91% and a negative predictive value of 97%. The negative likelihood ratio of this hypothetical biomarker panel would be 0.05, while the positive likelihood ratio would be 19. This performance level means that in patients with a pretest probability less than 50%, the posttest probability can be reduced to below 10%, so that such patients can be safely discharged without further hospital evaluation.
Conversely, a positive test result in patients with pretest probability of 30% or greater raises the posttest probability to nearly 90%, meaning that such patients should be considered for aggressive intervention without the need for stress testing.
RETURN TO OUR SCENARIOS
Chest pain remains a nonspecific complaint, and the interpretation of biomarkers to find the cause presents clinicians with challenges, as illustrated by the cases introduced at the beginning of this article.
The cardiac troponin I elevation in scenario 1 led to an initial diagnosis of unstable angina. However, coronary angiography showed lesion-free coronary arteries, thus excluding ischemic heart disease. When other diseases that could cause elevated cardiac troponin I were considered and investigated with further diagnostic tests such as D-dimer, pulmonary embolism became the new working diagnosis, and this was confirmed by CT angiography.
Similarly, given the laboratory values for the patient in scenario 2, the condition could have been mistaken for an acute coronary syndrome. However, the absence of evidence on ECG to support this diagnosis would indicate an erroneously elevated biomarker secondary to his background of chronic renal insufficiency.
- Pitts SR, Niska RW, Xu J, Burt CW. National hospital ambulatory medical care survey: 2006 emergency department summary. Natl Health Stat Report 2008;1–38.
- Vucic R, Knezevic S, Lazic Z, et al. Elevation of troponin values in differential diagnosis of chest pain in view of pulmonary thromboembolism. Vojnosanit Pregl 2012; 69:913–916.
- Croitoru M, Taegtmeyer H. Spurious rises in troponin T in end-stage renal disease. Lancet 1995; 346:974.
- Anderson JL, Adams CD, Antman EM, et al; 2011 Writing Group Members; ACCF/AHA Task Force Members. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 123:e426–e579.
- Unstable angina: diagnosis and management. Guideline overview. Agency for Health Care Policy and Research. J Natl Med Assoc 1994; 86:649,710–712.
- Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation 2007; 115:e356–e375.
- Hamm CW, Goldmann BU, Heeschen C, Kreymann G, Berger J, Meinertz T. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N Engl J Med 1997; 337:1648–1653.
- Heidenreich PA, Go A, Melsop KA, et al. Prediction of risk for patients with unstable angina. Evid Rep Technol Assess (Summ) 2000; August:1–3.
- Kontos MC, Fritz LM, Anderson FP, Tatum JL, Ornato JP, Jesse RL. Impact of the troponin standard on the prevalence of acute myocardial infarction. Am Heart J 2003; 146:446–452.
- Amit G, Gilutz H, Cafri C, Wolak A, Ilia R, Zahger D. What have the new definition of acute myocardial infarction and the introduction of troponin measurement done to the coronary care unit? Impacts on admission rate, length of stay, case mix and mortality. Cardiology 2004; 102:171–176.
- Alcalai R, Planer D, Culhaoglu A, Osman A, Pollak A, Lotan C. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med 2007; 167:276–281.
- Thygesen K, Mair J, Katus H, et al. Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J 2010; 31:2197–2204.
- Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol 2006; 48:1–11.
- Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA 1994; 271:703–707.
- McDonald MA, Holroyd B, Comeau A, Hervas-Malo M, Welsh RC. Clinical risk scoring beyond initial troponin values: results from a large, prospective, unselected acute chest pain population. Can J Cardiol 2007; 23:287–292.
- Than M, Cullen L, Reid CM, et al. A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): a prospective observational validation study. Lancet 2011; 377:1077–1084.
- Panju AA, Hemmelgarn BR, Guyatt GH, Simel DL. The rational clinical examination. Is this patient having a myocardial infarction? JAMA 1998; 280:1256–1263.
- Amsterdam EA, Kirk JD, Bluemke DA, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation 2010; 122:1756–1776.
- Farkouh ME, Aneja A, Reeder GS, et al. Clinical risk stratification in the emergency department predicts long-term cardiovascular outcomes in a population-based cohort presenting with acute chest pain: primary results of the Olmsted county chest pain study. Medicine (Baltimore) 2009; 88:307–313.
- Ohman EM, Armstrong PW, Christenson RH, et al. Cardiac troponin T levels for risk stratification in acute myocardial ischemia. GUSTO IIA Investigators. N Engl J Med 1996; 335:1333–1341.
- Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med 1996; 335:1342–1349.
- Sanchis J, Bodí V, Llácer A, et al. Predictors of short-term outcome in acute chest pain without ST-segment elevation. Int J Cardiol 2003; 92:193–199.
- Gomez MA, Anderson JL, Karagounis LA, Muhlestein JB, Mooers FB. An emergency department-based protocol for rapidly ruling out myocardial ischemia reduces hospital time and expense: results of a randomized study (ROMIO). J Am Coll Cardiol 1996; 28:25–33.
- Diercks DB, Gibler WB, Liu T, Sayre MR, Storrow AB. Identification of patients at risk by graded exercise testing in an emergency department chest pain center. Am J Cardiol 2000; 86:289–292.
- Rahman F, Mitra B, Cameron PA, Coleridge J. Stress testing before discharge is not required for patients with low and intermediate risk of acute coronary syndrome after emergency department short stay assessment. Emerg Med Australas 2010; 22:449–456.
- Kontos MC, Anderson FP, Alimard R, Ornato JP, Tatum JL, Jesse RL. Ability of troponin I to predict cardiac events in patients admitted from the emergency department. J Am Coll Cardiol 2000; 36:1818–1823.
- Hoffmann U, Truong QA, Schoenfeld DA, et al; ROMICAT-II Investigators. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012; 367:299–308.
- Hoffmann U, Bamberg F, Chae CU, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol 2009; 53:1642–1650.
- Goldstein JA, Chinnaiyan KM, Abidov A, et al; CT-STAT Investigators. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol 2011; 58:1414–1422.
- White CS, Kuo D, Kelemen M, et al. Chest pain evaluation in the emergency department: can MDCT provide a comprehensive evaluation? AJR Am J Roentgenol 2005; 185:533–540.
- Redberg RF. Coronary CT angiography for acute chest pain. N Engl J Med 2012; 367:375–376.
- Shreibati JB, Baker LC, Hlatky MA. Association of coronary CT angiography or stress testing with subsequent utilization and spending among Medicare beneficiaries. JAMA 2011; 306:2128–2136.
- Aldous S, Pemberton C, Richards AM, Troughton R, Than M. High-sensitivity troponin T for early rule-out of myocardial infarction in recent onset chest pain. Emerg Med J 2012; 29:805–810.
- Weber M, Bazzino O, Navarro Estrada JL, et al. Improved diagnostic and prognostic performance of a new high-sensitive troponin T assay in patients with acute coronary syndrome. Am Heart J 2011; 162:81–88.
- Hochholzer W, Reichlin T, Twerenbold R, et al. Incremental value of high-sensitivity cardiac troponin T for risk prediction in patients with suspected acute myocardial infarction. Clin Chem 2011; 57:1318–1326.
- Keller T, Zeller T, Ojeda F, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA 2011; 306:2684–2693.
- Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009; 361:858–867.
- Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012; 172:1211–1218.
- Newby LK. Myocardial infarction rule-out in the emergency department: are high-sensitivity troponins the answer?: Comment on “One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T”. Arch Intern Med 2012; 172:1218–1219.
- Haaf P, Reichlin T, Corson N, et al. B-type natriuretic peptide in the early diagnosis and risk stratification of acute chest pain. Am J Med 2011; 124:444–445.
- Sun T, Wang L, Zhang Y. Prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. Arch Med Res 2006; 37:502–505.
- Galvani M, Ottani F, Oltrona L, et al; Italian Working Group on Atherosclerosis, Thrombosis, and Vascular Biology and the Associazione Nazionale Medici Cardiologi Ospedalieri (ANMCO). N-terminal pro-brain natriuretic peptide on admission has prognostic value across the whole spectrum of acute coronary syndromes. Circulation 2004; 110:128–134.
- van der Zee PM, Cornel JH, Bholasingh R, Fischer JC, van Straalen JP, De Winter RJ. N-terminal pro B-type natriuretic peptide identifies patients with chest pain at high long-term cardiovascular risk. Am J Med 2011; 124:961–969.
- Jernberg T, Lindahl B, James S, Larsson A, Hansson LO, Wallentin L. Cystatin C: a novel predictor of outcome in suspected or confirmed non-ST-elevation acute coronary syndrome. Circulation 2004; 110:2342–2348.
- Akerblom Å, Wallentin L, Siegbahn A, et al. Cystatin C and estimated glomerular filtration rate as predictors for adverse outcome in patients with ST-elevation and non-ST-elevation acute coronary syndromes: results from the Platelet Inhibition and Patient Outcomes study. Clin Chem 2012; 58:190–199.
- Anwaruddin S, Januzzi JL, Baggish AL, Lewandrowski EL, Lewandrowski KB. Ischemia-modified albumin improves the usefulness of standard cardiac biomarkers for the diagnosis of myocardial ischemia in the emergency department setting. Am J Clin Pathol 2005; 123:140–145.
- Senes M, Kazan N, Coskun O, Zengi O, Inan L, Yücel D. Oxidative and nitrosative stress in acute ischaemic stroke. Ann Clin Biochem 2007; 44:43–47.
- Sabatine MS, Morrow DA, de Lemos JA, et al. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation 2002; 105:1760–1763.
- Tello-Montoliu A, Marín F, Roldán V, et al. A multimarker risk stratification approach to non-ST elevation acute coronary syndrome: implications of troponin T, CRP, NT pro-BNP and fibrin D-dimer levels. J Intern Med 2007; 262:651–658.
- Glaser R, Peacock WF, Wu AH, Muller R, Möckel M, Apple FS. Placental growth factor and B-type natriuretic peptide as independent predictors of risk from a multibiomarker panel in suspected acute coronary syndrome (Acute Risk and Related Outcomes Assessed With Cardiac Biomarkers [ARROW]) study. Am J Cardiol 2011; 107:821–826.
- Pitts SR, Niska RW, Xu J, Burt CW. National hospital ambulatory medical care survey: 2006 emergency department summary. Natl Health Stat Report 2008;1–38.
- Vucic R, Knezevic S, Lazic Z, et al. Elevation of troponin values in differential diagnosis of chest pain in view of pulmonary thromboembolism. Vojnosanit Pregl 2012; 69:913–916.
- Croitoru M, Taegtmeyer H. Spurious rises in troponin T in end-stage renal disease. Lancet 1995; 346:974.
- Anderson JL, Adams CD, Antman EM, et al; 2011 Writing Group Members; ACCF/AHA Task Force Members. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 123:e426–e579.
- Unstable angina: diagnosis and management. Guideline overview. Agency for Health Care Policy and Research. J Natl Med Assoc 1994; 86:649,710–712.
- Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation 2007; 115:e356–e375.
- Hamm CW, Goldmann BU, Heeschen C, Kreymann G, Berger J, Meinertz T. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N Engl J Med 1997; 337:1648–1653.
- Heidenreich PA, Go A, Melsop KA, et al. Prediction of risk for patients with unstable angina. Evid Rep Technol Assess (Summ) 2000; August:1–3.
- Kontos MC, Fritz LM, Anderson FP, Tatum JL, Ornato JP, Jesse RL. Impact of the troponin standard on the prevalence of acute myocardial infarction. Am Heart J 2003; 146:446–452.
- Amit G, Gilutz H, Cafri C, Wolak A, Ilia R, Zahger D. What have the new definition of acute myocardial infarction and the introduction of troponin measurement done to the coronary care unit? Impacts on admission rate, length of stay, case mix and mortality. Cardiology 2004; 102:171–176.
- Alcalai R, Planer D, Culhaoglu A, Osman A, Pollak A, Lotan C. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med 2007; 167:276–281.
- Thygesen K, Mair J, Katus H, et al. Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J 2010; 31:2197–2204.
- Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol 2006; 48:1–11.
- Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA 1994; 271:703–707.
- McDonald MA, Holroyd B, Comeau A, Hervas-Malo M, Welsh RC. Clinical risk scoring beyond initial troponin values: results from a large, prospective, unselected acute chest pain population. Can J Cardiol 2007; 23:287–292.
- Than M, Cullen L, Reid CM, et al. A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): a prospective observational validation study. Lancet 2011; 377:1077–1084.
- Panju AA, Hemmelgarn BR, Guyatt GH, Simel DL. The rational clinical examination. Is this patient having a myocardial infarction? JAMA 1998; 280:1256–1263.
- Amsterdam EA, Kirk JD, Bluemke DA, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation 2010; 122:1756–1776.
- Farkouh ME, Aneja A, Reeder GS, et al. Clinical risk stratification in the emergency department predicts long-term cardiovascular outcomes in a population-based cohort presenting with acute chest pain: primary results of the Olmsted county chest pain study. Medicine (Baltimore) 2009; 88:307–313.
- Ohman EM, Armstrong PW, Christenson RH, et al. Cardiac troponin T levels for risk stratification in acute myocardial ischemia. GUSTO IIA Investigators. N Engl J Med 1996; 335:1333–1341.
- Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med 1996; 335:1342–1349.
- Sanchis J, Bodí V, Llácer A, et al. Predictors of short-term outcome in acute chest pain without ST-segment elevation. Int J Cardiol 2003; 92:193–199.
- Gomez MA, Anderson JL, Karagounis LA, Muhlestein JB, Mooers FB. An emergency department-based protocol for rapidly ruling out myocardial ischemia reduces hospital time and expense: results of a randomized study (ROMIO). J Am Coll Cardiol 1996; 28:25–33.
- Diercks DB, Gibler WB, Liu T, Sayre MR, Storrow AB. Identification of patients at risk by graded exercise testing in an emergency department chest pain center. Am J Cardiol 2000; 86:289–292.
- Rahman F, Mitra B, Cameron PA, Coleridge J. Stress testing before discharge is not required for patients with low and intermediate risk of acute coronary syndrome after emergency department short stay assessment. Emerg Med Australas 2010; 22:449–456.
- Kontos MC, Anderson FP, Alimard R, Ornato JP, Tatum JL, Jesse RL. Ability of troponin I to predict cardiac events in patients admitted from the emergency department. J Am Coll Cardiol 2000; 36:1818–1823.
- Hoffmann U, Truong QA, Schoenfeld DA, et al; ROMICAT-II Investigators. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012; 367:299–308.
- Hoffmann U, Bamberg F, Chae CU, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol 2009; 53:1642–1650.
- Goldstein JA, Chinnaiyan KM, Abidov A, et al; CT-STAT Investigators. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol 2011; 58:1414–1422.
- White CS, Kuo D, Kelemen M, et al. Chest pain evaluation in the emergency department: can MDCT provide a comprehensive evaluation? AJR Am J Roentgenol 2005; 185:533–540.
- Redberg RF. Coronary CT angiography for acute chest pain. N Engl J Med 2012; 367:375–376.
- Shreibati JB, Baker LC, Hlatky MA. Association of coronary CT angiography or stress testing with subsequent utilization and spending among Medicare beneficiaries. JAMA 2011; 306:2128–2136.
- Aldous S, Pemberton C, Richards AM, Troughton R, Than M. High-sensitivity troponin T for early rule-out of myocardial infarction in recent onset chest pain. Emerg Med J 2012; 29:805–810.
- Weber M, Bazzino O, Navarro Estrada JL, et al. Improved diagnostic and prognostic performance of a new high-sensitive troponin T assay in patients with acute coronary syndrome. Am Heart J 2011; 162:81–88.
- Hochholzer W, Reichlin T, Twerenbold R, et al. Incremental value of high-sensitivity cardiac troponin T for risk prediction in patients with suspected acute myocardial infarction. Clin Chem 2011; 57:1318–1326.
- Keller T, Zeller T, Ojeda F, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA 2011; 306:2684–2693.
- Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009; 361:858–867.
- Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012; 172:1211–1218.
- Newby LK. Myocardial infarction rule-out in the emergency department: are high-sensitivity troponins the answer?: Comment on “One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T”. Arch Intern Med 2012; 172:1218–1219.
- Haaf P, Reichlin T, Corson N, et al. B-type natriuretic peptide in the early diagnosis and risk stratification of acute chest pain. Am J Med 2011; 124:444–445.
- Sun T, Wang L, Zhang Y. Prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. Arch Med Res 2006; 37:502–505.
- Galvani M, Ottani F, Oltrona L, et al; Italian Working Group on Atherosclerosis, Thrombosis, and Vascular Biology and the Associazione Nazionale Medici Cardiologi Ospedalieri (ANMCO). N-terminal pro-brain natriuretic peptide on admission has prognostic value across the whole spectrum of acute coronary syndromes. Circulation 2004; 110:128–134.
- van der Zee PM, Cornel JH, Bholasingh R, Fischer JC, van Straalen JP, De Winter RJ. N-terminal pro B-type natriuretic peptide identifies patients with chest pain at high long-term cardiovascular risk. Am J Med 2011; 124:961–969.
- Jernberg T, Lindahl B, James S, Larsson A, Hansson LO, Wallentin L. Cystatin C: a novel predictor of outcome in suspected or confirmed non-ST-elevation acute coronary syndrome. Circulation 2004; 110:2342–2348.
- Akerblom Å, Wallentin L, Siegbahn A, et al. Cystatin C and estimated glomerular filtration rate as predictors for adverse outcome in patients with ST-elevation and non-ST-elevation acute coronary syndromes: results from the Platelet Inhibition and Patient Outcomes study. Clin Chem 2012; 58:190–199.
- Anwaruddin S, Januzzi JL, Baggish AL, Lewandrowski EL, Lewandrowski KB. Ischemia-modified albumin improves the usefulness of standard cardiac biomarkers for the diagnosis of myocardial ischemia in the emergency department setting. Am J Clin Pathol 2005; 123:140–145.
- Senes M, Kazan N, Coskun O, Zengi O, Inan L, Yücel D. Oxidative and nitrosative stress in acute ischaemic stroke. Ann Clin Biochem 2007; 44:43–47.
- Sabatine MS, Morrow DA, de Lemos JA, et al. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation 2002; 105:1760–1763.
- Tello-Montoliu A, Marín F, Roldán V, et al. A multimarker risk stratification approach to non-ST elevation acute coronary syndrome: implications of troponin T, CRP, NT pro-BNP and fibrin D-dimer levels. J Intern Med 2007; 262:651–658.
- Glaser R, Peacock WF, Wu AH, Muller R, Möckel M, Apple FS. Placental growth factor and B-type natriuretic peptide as independent predictors of risk from a multibiomarker panel in suspected acute coronary syndrome (Acute Risk and Related Outcomes Assessed With Cardiac Biomarkers [ARROW]) study. Am J Cardiol 2011; 107:821–826.
KEY POINTS
- Biomarkers of cardiac necrosis, particularly troponins I and T, can aid in risk assessment, but one must pay close attention to the underlying clinical context.
- Stable patients at low risk with no evidence of ischemia on initial assessment can be admitted to a chest pain unit for observation with serial biomarker testing and ECG.
- Highly sensitive troponin assays can improve the early diagnosis of acute myocardial infarction, but how best to use them is not yet defined.
- Biomarkers, used alone or in combination, have the potential to complement or replace stress testing, permitting more timely, accurate, and cost-effective diagnosis and earlier discharge of patients at low risk.
- Newer markers such as brain-type natriuretic peptide, cystatin C, and ischemia-modified albumin have shown promise but need to be thoroughly evaluated.
Fever, dyspnea, and a new heart murmur
A 35-year-old man presented to the emergency department because of night sweats, fever, chills, and shortness of breath. He also had an acute onset of blue discoloration of his right fourth finger. His symptoms (except for the finger discoloration) had begun about 6 months previously and had rapidly progressed despite several courses of different antibiotics of different types, given both intravenously in the hospital and orally at home. He had lost 20 lb during this time. Previously, he had been healthy.
About 1 month after his symptoms began, he had consulted his primary care physician, who detected a new grade 4/6 systolic and diastolic murmur. Transthoracic echocardiography about 2 months after that demonstrated mild aortic and mitral insufficiency but no echocardiographic features supporting infective endocarditis. Of note, the patient had no risk factors for endocarditis such as illicit drug use or poor dental health.
In the emergency department, his temperature was 99.4°F (37.4°C), pulse 109 beats per minute, and blood pressure 126/60 mm Hg. He had a grade 3/6 harsh holosystolic murmur best heard at the right upper sternal border, a grade 3/4 holodiastolic murmur audible across the precordium, and a grade 3/4 holosystolic blowing murmur best heard at the cardiac apex. Other findings included signs of aortic insufficiency—the Duroziez sign (a diastolic murmur heard over the femoral artery when compressed), Watson’s water-hammer pulse (indicating a wide pulse pressure), and the Müller sign (pulsation of the uvula)—and small Janeway lesions on the inner aspect of his right arm and palm.
Electrocardiography showed normal sinus rhythm, PR interval 128 ms, QRS complex 100 ms, QT interval 360 ms, and corrected QT interval 473 ms.
Blood cultures grew Streptococcus sanguinis. Both transthoracic and transesophageal echocardiography were done promptly and revealed multiple mobile echodensities attached to a trileaflet aortic valve, consistent with vegetations and valve leaflet destruction; severe (4+) aortic regurgitation with flow reversal in the abdominal aorta; mild mitral regurgitation; and a mitral valve aneurysm with mild mitral regurgitation (Figure 1).
INFECTIVE ENDOCARDITIS: WORTH CONSIDERING
S sanguinis is a member of the group of viridans streptococci. As a normal inhabitant of the healthy human mouth, it is found in dental plaque. It may enter the bloodstream during dental cleaning and may colonize the heart valves, particularly the mitral and aortic valves, where it is the most common cause of subacute bacterial endocarditis.
Infective endocarditis is often diagnosed clinically with the Duke criteria (www.med-calc.com/endocarditis.html).1 However, the variability of the clinical presentation and the nonspecific nature of the initial workup often create a diagnostic challenge for the evaluating physician.1,2
In cases of recurrent persistent fever and a new heart murmur, infective endocarditis must always be considered. Blood cultures should be ordered early and repeatedly. If blood cultures are positive, transesophageal echocardiography should be done without delay if transthoracic echocardiography was unremarkable. Prompt diagnosis and surgical intervention prevent complications.
MITRAL VALVE ANEURYSM IN AORTIC VALVE ENDOCARDITIS
Aortic valve endocarditis often also involves the mitral valve; mitral valve endocarditis is seen in 17% of patients undergoing surgery for aortic valve endocarditis.3 Proposed mechanisms for this association include jet lesions from aortic regurgitation, vegetation prolapse with direct contact between the aortic valve and anterior mitral leaflet (“kissing lesions”), and direct local spread of infection.4–7
One of every five patients with mitral valve involvement in aortic valve endocarditis has a mitral valve aneurysm.3 This is a serious finding, as it can lead to septic embolization. Also, the weakened lining of the mitral valve aneurysm can rupture, resulting in severe mitral regurgitation, acute pulmonary edema, and precipitous cardiopulmonary decompensation.5
Transesophageal echocardiography is more sensitive than transthoracic echocardiography for detecting mitral valve aneurysm.8 On two-dimensional echocardiography, the lesion appears as a narrow-necked, saccular echolucency with systolic protrusion into the left atrium. Color Doppler imaging often shows turbulent, high-velocity flow.
Differential diagnosis of mitral valve aneurysm
Differential diagnostic considerations include a valvular blood cyst, a congenital cardiac diverticulum, and mitral valve prolapse.
Valvular blood cysts are extremely rare in adults.9 These benign, congenital tumors are most often found on the atrioventricular valves in infants, in whom the reported incidence is between 25% and 100%. In almost all cases, these cysts are believed to regress spontaneously with time.
In almost all reported cases, the cyst involved the valvular apparatus or papillary muscle of the tricuspid, pulmonary, or mitral valve.10 Cysts consist of a benign diverticulum lined with flattened, cobblestone-shaped endothelium and are filled with blood. They can cause heart murmurs in otherwise asymptomatic patients.
On echocardiography, a blood cyst appears as an oval mass (often at the interatrial septum), often with normal cardiac function. In the rare case in which a blood cyst is found incidentally during echocardiography, the hemodynamic impact, if any, should be determined by Doppler techniques.
When benign, a valvular blood cyst can be safely monitored with echocardiographic follow-up.11 Treatment involves surgical resection of the mass in symptomatic patients in whom cardiac function is impaired by the presence of the cyst.
Congenital cardiac diverticuli are extremely rare, most often seen in children, and associated with a midline thoracoabdominal defect. Echocardiography can differentiate a ventricular diverticulum from an aneurysm or a pseudoaneurysm.
A ventricular diverticulum has a fibrous, narrow neck connecting with the ventricle, and a small circular echo-free space that communicates with the ventricle via this narrow neck.2 Doppler imaging shows systolic flow from the diverticulum to the ventricle, and systolic contractility may also be seen during cardiac catheterization. Congenital diverticulum is typically confused with ventricular aneurysm and, to a lesser degree, with mitral valve aneurysm.
Mitral valve prolapse is characterized by interchordal ballooning or hooding of the mitral valve leaflets that occurs when one or both floppy, enlarged leaflets prolapse into the left atrium during systole.
BACK TO OUR PATIENT
The patient underwent open heart surgery, with successful repair of the aortic root, replacement of the aortic valve, and repair of the mitral valve. An abscess was found within the aneurysmal cavity.
- Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994; 96:200–209.
- Prendergast BD. Diagnostic criteria and problems in infective endocarditis. Heart 2004; 90:611–613.
- Gonzalez-Lavin L, Lise M, Ross D. The importance of the ‘jet lesion’ in bacterial endocarditis involving the left heart. Surgical considerations. J Thorac Cardiovasc Surg 1970; 59:185–192.
- Silbiger JJ. Review: mitral valve aneurysms in infective endocarditis: mechanisms, clinical recognition, and treatment. J Heart Valve Dis 2009; 18:476–480.
- Reid CL, Chandraratna AN, Harrison E, et al. Mitral valve aneurysm: clinical features, echocardiographic-pathologic correlations. J Am Coll Cardiol 1983; 2:460–464.
- Rodbard S. Blood velocity and endocarditis. Circulation 1963; 27:18–28.
- Piper C, Hetzer R, Körfer R, Bergemann R, Horstkotte D. The importance of secondary mitral valve involvement in primary aortic valve endocarditis; the mitral kissing vegetation. Eur Heart J 2002; 23:79–86.
- Cziner DG, Rosenzweig BP, Katz ES, Keller AM, Daniel WG, Kronzon I. Transesophageal versus transthoracic echocardiography for diagnosing mitral valve perforation. Am J Cardiol 1992; 69:1495–1497.
- Roberts PF, Serra AJ, McNicholas KW, Shapira N, Lemole GM. Atrial blood cyst: a rare finding. Ann Thorac Surg 1996; 62:880–882.
- Grimaldi A, Capritti E, Pappalardo F, et al. Images in cardiovascular medicine: blood cyst of the mitral valve. J Cardiovasc Med 2012; 3:46.
- Boyd WC, Rosengart TK, Hartman GS. Isolated left ventricular diverticulum in an adult. J Cardiothorac Vasc Anesth 1999; 13:468–470.
A 35-year-old man presented to the emergency department because of night sweats, fever, chills, and shortness of breath. He also had an acute onset of blue discoloration of his right fourth finger. His symptoms (except for the finger discoloration) had begun about 6 months previously and had rapidly progressed despite several courses of different antibiotics of different types, given both intravenously in the hospital and orally at home. He had lost 20 lb during this time. Previously, he had been healthy.
About 1 month after his symptoms began, he had consulted his primary care physician, who detected a new grade 4/6 systolic and diastolic murmur. Transthoracic echocardiography about 2 months after that demonstrated mild aortic and mitral insufficiency but no echocardiographic features supporting infective endocarditis. Of note, the patient had no risk factors for endocarditis such as illicit drug use or poor dental health.
In the emergency department, his temperature was 99.4°F (37.4°C), pulse 109 beats per minute, and blood pressure 126/60 mm Hg. He had a grade 3/6 harsh holosystolic murmur best heard at the right upper sternal border, a grade 3/4 holodiastolic murmur audible across the precordium, and a grade 3/4 holosystolic blowing murmur best heard at the cardiac apex. Other findings included signs of aortic insufficiency—the Duroziez sign (a diastolic murmur heard over the femoral artery when compressed), Watson’s water-hammer pulse (indicating a wide pulse pressure), and the Müller sign (pulsation of the uvula)—and small Janeway lesions on the inner aspect of his right arm and palm.
Electrocardiography showed normal sinus rhythm, PR interval 128 ms, QRS complex 100 ms, QT interval 360 ms, and corrected QT interval 473 ms.
Blood cultures grew Streptococcus sanguinis. Both transthoracic and transesophageal echocardiography were done promptly and revealed multiple mobile echodensities attached to a trileaflet aortic valve, consistent with vegetations and valve leaflet destruction; severe (4+) aortic regurgitation with flow reversal in the abdominal aorta; mild mitral regurgitation; and a mitral valve aneurysm with mild mitral regurgitation (Figure 1).
INFECTIVE ENDOCARDITIS: WORTH CONSIDERING
S sanguinis is a member of the group of viridans streptococci. As a normal inhabitant of the healthy human mouth, it is found in dental plaque. It may enter the bloodstream during dental cleaning and may colonize the heart valves, particularly the mitral and aortic valves, where it is the most common cause of subacute bacterial endocarditis.
Infective endocarditis is often diagnosed clinically with the Duke criteria (www.med-calc.com/endocarditis.html).1 However, the variability of the clinical presentation and the nonspecific nature of the initial workup often create a diagnostic challenge for the evaluating physician.1,2
In cases of recurrent persistent fever and a new heart murmur, infective endocarditis must always be considered. Blood cultures should be ordered early and repeatedly. If blood cultures are positive, transesophageal echocardiography should be done without delay if transthoracic echocardiography was unremarkable. Prompt diagnosis and surgical intervention prevent complications.
MITRAL VALVE ANEURYSM IN AORTIC VALVE ENDOCARDITIS
Aortic valve endocarditis often also involves the mitral valve; mitral valve endocarditis is seen in 17% of patients undergoing surgery for aortic valve endocarditis.3 Proposed mechanisms for this association include jet lesions from aortic regurgitation, vegetation prolapse with direct contact between the aortic valve and anterior mitral leaflet (“kissing lesions”), and direct local spread of infection.4–7
One of every five patients with mitral valve involvement in aortic valve endocarditis has a mitral valve aneurysm.3 This is a serious finding, as it can lead to septic embolization. Also, the weakened lining of the mitral valve aneurysm can rupture, resulting in severe mitral regurgitation, acute pulmonary edema, and precipitous cardiopulmonary decompensation.5
Transesophageal echocardiography is more sensitive than transthoracic echocardiography for detecting mitral valve aneurysm.8 On two-dimensional echocardiography, the lesion appears as a narrow-necked, saccular echolucency with systolic protrusion into the left atrium. Color Doppler imaging often shows turbulent, high-velocity flow.
Differential diagnosis of mitral valve aneurysm
Differential diagnostic considerations include a valvular blood cyst, a congenital cardiac diverticulum, and mitral valve prolapse.
Valvular blood cysts are extremely rare in adults.9 These benign, congenital tumors are most often found on the atrioventricular valves in infants, in whom the reported incidence is between 25% and 100%. In almost all cases, these cysts are believed to regress spontaneously with time.
In almost all reported cases, the cyst involved the valvular apparatus or papillary muscle of the tricuspid, pulmonary, or mitral valve.10 Cysts consist of a benign diverticulum lined with flattened, cobblestone-shaped endothelium and are filled with blood. They can cause heart murmurs in otherwise asymptomatic patients.
On echocardiography, a blood cyst appears as an oval mass (often at the interatrial septum), often with normal cardiac function. In the rare case in which a blood cyst is found incidentally during echocardiography, the hemodynamic impact, if any, should be determined by Doppler techniques.
When benign, a valvular blood cyst can be safely monitored with echocardiographic follow-up.11 Treatment involves surgical resection of the mass in symptomatic patients in whom cardiac function is impaired by the presence of the cyst.
Congenital cardiac diverticuli are extremely rare, most often seen in children, and associated with a midline thoracoabdominal defect. Echocardiography can differentiate a ventricular diverticulum from an aneurysm or a pseudoaneurysm.
A ventricular diverticulum has a fibrous, narrow neck connecting with the ventricle, and a small circular echo-free space that communicates with the ventricle via this narrow neck.2 Doppler imaging shows systolic flow from the diverticulum to the ventricle, and systolic contractility may also be seen during cardiac catheterization. Congenital diverticulum is typically confused with ventricular aneurysm and, to a lesser degree, with mitral valve aneurysm.
Mitral valve prolapse is characterized by interchordal ballooning or hooding of the mitral valve leaflets that occurs when one or both floppy, enlarged leaflets prolapse into the left atrium during systole.
BACK TO OUR PATIENT
The patient underwent open heart surgery, with successful repair of the aortic root, replacement of the aortic valve, and repair of the mitral valve. An abscess was found within the aneurysmal cavity.
A 35-year-old man presented to the emergency department because of night sweats, fever, chills, and shortness of breath. He also had an acute onset of blue discoloration of his right fourth finger. His symptoms (except for the finger discoloration) had begun about 6 months previously and had rapidly progressed despite several courses of different antibiotics of different types, given both intravenously in the hospital and orally at home. He had lost 20 lb during this time. Previously, he had been healthy.
About 1 month after his symptoms began, he had consulted his primary care physician, who detected a new grade 4/6 systolic and diastolic murmur. Transthoracic echocardiography about 2 months after that demonstrated mild aortic and mitral insufficiency but no echocardiographic features supporting infective endocarditis. Of note, the patient had no risk factors for endocarditis such as illicit drug use or poor dental health.
In the emergency department, his temperature was 99.4°F (37.4°C), pulse 109 beats per minute, and blood pressure 126/60 mm Hg. He had a grade 3/6 harsh holosystolic murmur best heard at the right upper sternal border, a grade 3/4 holodiastolic murmur audible across the precordium, and a grade 3/4 holosystolic blowing murmur best heard at the cardiac apex. Other findings included signs of aortic insufficiency—the Duroziez sign (a diastolic murmur heard over the femoral artery when compressed), Watson’s water-hammer pulse (indicating a wide pulse pressure), and the Müller sign (pulsation of the uvula)—and small Janeway lesions on the inner aspect of his right arm and palm.
Electrocardiography showed normal sinus rhythm, PR interval 128 ms, QRS complex 100 ms, QT interval 360 ms, and corrected QT interval 473 ms.
Blood cultures grew Streptococcus sanguinis. Both transthoracic and transesophageal echocardiography were done promptly and revealed multiple mobile echodensities attached to a trileaflet aortic valve, consistent with vegetations and valve leaflet destruction; severe (4+) aortic regurgitation with flow reversal in the abdominal aorta; mild mitral regurgitation; and a mitral valve aneurysm with mild mitral regurgitation (Figure 1).
INFECTIVE ENDOCARDITIS: WORTH CONSIDERING
S sanguinis is a member of the group of viridans streptococci. As a normal inhabitant of the healthy human mouth, it is found in dental plaque. It may enter the bloodstream during dental cleaning and may colonize the heart valves, particularly the mitral and aortic valves, where it is the most common cause of subacute bacterial endocarditis.
Infective endocarditis is often diagnosed clinically with the Duke criteria (www.med-calc.com/endocarditis.html).1 However, the variability of the clinical presentation and the nonspecific nature of the initial workup often create a diagnostic challenge for the evaluating physician.1,2
In cases of recurrent persistent fever and a new heart murmur, infective endocarditis must always be considered. Blood cultures should be ordered early and repeatedly. If blood cultures are positive, transesophageal echocardiography should be done without delay if transthoracic echocardiography was unremarkable. Prompt diagnosis and surgical intervention prevent complications.
MITRAL VALVE ANEURYSM IN AORTIC VALVE ENDOCARDITIS
Aortic valve endocarditis often also involves the mitral valve; mitral valve endocarditis is seen in 17% of patients undergoing surgery for aortic valve endocarditis.3 Proposed mechanisms for this association include jet lesions from aortic regurgitation, vegetation prolapse with direct contact between the aortic valve and anterior mitral leaflet (“kissing lesions”), and direct local spread of infection.4–7
One of every five patients with mitral valve involvement in aortic valve endocarditis has a mitral valve aneurysm.3 This is a serious finding, as it can lead to septic embolization. Also, the weakened lining of the mitral valve aneurysm can rupture, resulting in severe mitral regurgitation, acute pulmonary edema, and precipitous cardiopulmonary decompensation.5
Transesophageal echocardiography is more sensitive than transthoracic echocardiography for detecting mitral valve aneurysm.8 On two-dimensional echocardiography, the lesion appears as a narrow-necked, saccular echolucency with systolic protrusion into the left atrium. Color Doppler imaging often shows turbulent, high-velocity flow.
Differential diagnosis of mitral valve aneurysm
Differential diagnostic considerations include a valvular blood cyst, a congenital cardiac diverticulum, and mitral valve prolapse.
Valvular blood cysts are extremely rare in adults.9 These benign, congenital tumors are most often found on the atrioventricular valves in infants, in whom the reported incidence is between 25% and 100%. In almost all cases, these cysts are believed to regress spontaneously with time.
In almost all reported cases, the cyst involved the valvular apparatus or papillary muscle of the tricuspid, pulmonary, or mitral valve.10 Cysts consist of a benign diverticulum lined with flattened, cobblestone-shaped endothelium and are filled with blood. They can cause heart murmurs in otherwise asymptomatic patients.
On echocardiography, a blood cyst appears as an oval mass (often at the interatrial septum), often with normal cardiac function. In the rare case in which a blood cyst is found incidentally during echocardiography, the hemodynamic impact, if any, should be determined by Doppler techniques.
When benign, a valvular blood cyst can be safely monitored with echocardiographic follow-up.11 Treatment involves surgical resection of the mass in symptomatic patients in whom cardiac function is impaired by the presence of the cyst.
Congenital cardiac diverticuli are extremely rare, most often seen in children, and associated with a midline thoracoabdominal defect. Echocardiography can differentiate a ventricular diverticulum from an aneurysm or a pseudoaneurysm.
A ventricular diverticulum has a fibrous, narrow neck connecting with the ventricle, and a small circular echo-free space that communicates with the ventricle via this narrow neck.2 Doppler imaging shows systolic flow from the diverticulum to the ventricle, and systolic contractility may also be seen during cardiac catheterization. Congenital diverticulum is typically confused with ventricular aneurysm and, to a lesser degree, with mitral valve aneurysm.
Mitral valve prolapse is characterized by interchordal ballooning or hooding of the mitral valve leaflets that occurs when one or both floppy, enlarged leaflets prolapse into the left atrium during systole.
BACK TO OUR PATIENT
The patient underwent open heart surgery, with successful repair of the aortic root, replacement of the aortic valve, and repair of the mitral valve. An abscess was found within the aneurysmal cavity.
- Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994; 96:200–209.
- Prendergast BD. Diagnostic criteria and problems in infective endocarditis. Heart 2004; 90:611–613.
- Gonzalez-Lavin L, Lise M, Ross D. The importance of the ‘jet lesion’ in bacterial endocarditis involving the left heart. Surgical considerations. J Thorac Cardiovasc Surg 1970; 59:185–192.
- Silbiger JJ. Review: mitral valve aneurysms in infective endocarditis: mechanisms, clinical recognition, and treatment. J Heart Valve Dis 2009; 18:476–480.
- Reid CL, Chandraratna AN, Harrison E, et al. Mitral valve aneurysm: clinical features, echocardiographic-pathologic correlations. J Am Coll Cardiol 1983; 2:460–464.
- Rodbard S. Blood velocity and endocarditis. Circulation 1963; 27:18–28.
- Piper C, Hetzer R, Körfer R, Bergemann R, Horstkotte D. The importance of secondary mitral valve involvement in primary aortic valve endocarditis; the mitral kissing vegetation. Eur Heart J 2002; 23:79–86.
- Cziner DG, Rosenzweig BP, Katz ES, Keller AM, Daniel WG, Kronzon I. Transesophageal versus transthoracic echocardiography for diagnosing mitral valve perforation. Am J Cardiol 1992; 69:1495–1497.
- Roberts PF, Serra AJ, McNicholas KW, Shapira N, Lemole GM. Atrial blood cyst: a rare finding. Ann Thorac Surg 1996; 62:880–882.
- Grimaldi A, Capritti E, Pappalardo F, et al. Images in cardiovascular medicine: blood cyst of the mitral valve. J Cardiovasc Med 2012; 3:46.
- Boyd WC, Rosengart TK, Hartman GS. Isolated left ventricular diverticulum in an adult. J Cardiothorac Vasc Anesth 1999; 13:468–470.
- Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994; 96:200–209.
- Prendergast BD. Diagnostic criteria and problems in infective endocarditis. Heart 2004; 90:611–613.
- Gonzalez-Lavin L, Lise M, Ross D. The importance of the ‘jet lesion’ in bacterial endocarditis involving the left heart. Surgical considerations. J Thorac Cardiovasc Surg 1970; 59:185–192.
- Silbiger JJ. Review: mitral valve aneurysms in infective endocarditis: mechanisms, clinical recognition, and treatment. J Heart Valve Dis 2009; 18:476–480.
- Reid CL, Chandraratna AN, Harrison E, et al. Mitral valve aneurysm: clinical features, echocardiographic-pathologic correlations. J Am Coll Cardiol 1983; 2:460–464.
- Rodbard S. Blood velocity and endocarditis. Circulation 1963; 27:18–28.
- Piper C, Hetzer R, Körfer R, Bergemann R, Horstkotte D. The importance of secondary mitral valve involvement in primary aortic valve endocarditis; the mitral kissing vegetation. Eur Heart J 2002; 23:79–86.
- Cziner DG, Rosenzweig BP, Katz ES, Keller AM, Daniel WG, Kronzon I. Transesophageal versus transthoracic echocardiography for diagnosing mitral valve perforation. Am J Cardiol 1992; 69:1495–1497.
- Roberts PF, Serra AJ, McNicholas KW, Shapira N, Lemole GM. Atrial blood cyst: a rare finding. Ann Thorac Surg 1996; 62:880–882.
- Grimaldi A, Capritti E, Pappalardo F, et al. Images in cardiovascular medicine: blood cyst of the mitral valve. J Cardiovasc Med 2012; 3:46.
- Boyd WC, Rosengart TK, Hartman GS. Isolated left ventricular diverticulum in an adult. J Cardiothorac Vasc Anesth 1999; 13:468–470.
It is not a ‘mini’-stroke, it is a call to action
When a patient tells a physician about a sudden episode of weakness, loss of vision, or loss of sensation that occurred but then quickly resolved, both the patient and the physician may feel a sense of relief. In many cases, the patient may not even seek medical evaluation. These events, when vascular in origin and not seizures or migraines, have been termed transient ischemic attacks (TIAs) by physicians, and are often called “mini-strokes” by patients. But as discussed by Drs. Shruti Sonni and David Thaler in this issue of the Journal, there is nothing “mini” about their significance.
In some ways, the perception of TIA (as opposed to stroke) has paralleled our understanding and initial misperception of non-ST-segment elevation myocardial infarction (NSTEMI). This type of acute coronary event was thought to be less severe than acute ST-elevation MI (STEMI), and patients with NSTEMI and unstable angina have historically not received the aggressive acute and preventive therapy received by patients with STEMI. But with the advent of more sensitive markers of myocardial necrosis, we now know that NSTEMI and unstable angina can be associated with significant tissue injury, and that the outcome after a year or so can be the same as or worse than if the initial injury was associated with ST-segment elevation.
A similar story has evolved with TIA. With sensitive diffusion-weighted magnetic resonance imaging, brain injury can often be detected even when it is not seen on computed tomography. Patients are often not evaluated as completely for reversible vascular lesions and may not receive aggressive secondary prevention. Yet shortly after suffering a TIA, a patient is even more likely to have another neurologic event than if the initial event had been a small stroke. And the neurologic event will more likely be a stroke with residual neurologic deficit.
All are reasons to educate our older patients—particularly those with diabetes, atrial fibrillation, peripheral vascular disease, and hypertension—about the significance of even apparently self-limited neurologic events. A TIA is a major warning signal.
When a patient tells a physician about a sudden episode of weakness, loss of vision, or loss of sensation that occurred but then quickly resolved, both the patient and the physician may feel a sense of relief. In many cases, the patient may not even seek medical evaluation. These events, when vascular in origin and not seizures or migraines, have been termed transient ischemic attacks (TIAs) by physicians, and are often called “mini-strokes” by patients. But as discussed by Drs. Shruti Sonni and David Thaler in this issue of the Journal, there is nothing “mini” about their significance.
In some ways, the perception of TIA (as opposed to stroke) has paralleled our understanding and initial misperception of non-ST-segment elevation myocardial infarction (NSTEMI). This type of acute coronary event was thought to be less severe than acute ST-elevation MI (STEMI), and patients with NSTEMI and unstable angina have historically not received the aggressive acute and preventive therapy received by patients with STEMI. But with the advent of more sensitive markers of myocardial necrosis, we now know that NSTEMI and unstable angina can be associated with significant tissue injury, and that the outcome after a year or so can be the same as or worse than if the initial injury was associated with ST-segment elevation.
A similar story has evolved with TIA. With sensitive diffusion-weighted magnetic resonance imaging, brain injury can often be detected even when it is not seen on computed tomography. Patients are often not evaluated as completely for reversible vascular lesions and may not receive aggressive secondary prevention. Yet shortly after suffering a TIA, a patient is even more likely to have another neurologic event than if the initial event had been a small stroke. And the neurologic event will more likely be a stroke with residual neurologic deficit.
All are reasons to educate our older patients—particularly those with diabetes, atrial fibrillation, peripheral vascular disease, and hypertension—about the significance of even apparently self-limited neurologic events. A TIA is a major warning signal.
When a patient tells a physician about a sudden episode of weakness, loss of vision, or loss of sensation that occurred but then quickly resolved, both the patient and the physician may feel a sense of relief. In many cases, the patient may not even seek medical evaluation. These events, when vascular in origin and not seizures or migraines, have been termed transient ischemic attacks (TIAs) by physicians, and are often called “mini-strokes” by patients. But as discussed by Drs. Shruti Sonni and David Thaler in this issue of the Journal, there is nothing “mini” about their significance.
In some ways, the perception of TIA (as opposed to stroke) has paralleled our understanding and initial misperception of non-ST-segment elevation myocardial infarction (NSTEMI). This type of acute coronary event was thought to be less severe than acute ST-elevation MI (STEMI), and patients with NSTEMI and unstable angina have historically not received the aggressive acute and preventive therapy received by patients with STEMI. But with the advent of more sensitive markers of myocardial necrosis, we now know that NSTEMI and unstable angina can be associated with significant tissue injury, and that the outcome after a year or so can be the same as or worse than if the initial injury was associated with ST-segment elevation.
A similar story has evolved with TIA. With sensitive diffusion-weighted magnetic resonance imaging, brain injury can often be detected even when it is not seen on computed tomography. Patients are often not evaluated as completely for reversible vascular lesions and may not receive aggressive secondary prevention. Yet shortly after suffering a TIA, a patient is even more likely to have another neurologic event than if the initial event had been a small stroke. And the neurologic event will more likely be a stroke with residual neurologic deficit.
All are reasons to educate our older patients—particularly those with diabetes, atrial fibrillation, peripheral vascular disease, and hypertension—about the significance of even apparently self-limited neurologic events. A TIA is a major warning signal.
Competitive Swimmer With Hip Pain
ANSWER
The radiograph demonstrates no evidence of an acute fracture or dislocation. Normal gas/stool pattern is present. Essentially, this radiograph is normal.
The patient most likely has an acute strain of her hip quadriceps or flexor. On occasion, severe enough strain injuries can cause a slight avulsion fracture within the hip at the muscle origination point. These can sometimes be evident on plain films.
ANSWER
The radiograph demonstrates no evidence of an acute fracture or dislocation. Normal gas/stool pattern is present. Essentially, this radiograph is normal.
The patient most likely has an acute strain of her hip quadriceps or flexor. On occasion, severe enough strain injuries can cause a slight avulsion fracture within the hip at the muscle origination point. These can sometimes be evident on plain films.
ANSWER
The radiograph demonstrates no evidence of an acute fracture or dislocation. Normal gas/stool pattern is present. Essentially, this radiograph is normal.
The patient most likely has an acute strain of her hip quadriceps or flexor. On occasion, severe enough strain injuries can cause a slight avulsion fracture within the hip at the muscle origination point. These can sometimes be evident on plain films.
A 17-year-old girl presents for evaluation of severe pain in her left hip. She is a competitive swimmer; earlier in the day, she was at practice doing dry land (out of the water) activities/exercises. Having completed a series of stretches and warm-up exercises, she and her teammates proceeded to do sprints. During one of these sprints, she immediately felt a “pop” in her left hip followed by severe, debilitating pain in that hip and thigh. Medical history is otherwise unremarkable. Physical exam reveals that it is extremely painful for the patient to bear weight on the affected leg. There is moderate-to-severe tenderness over the lateral hip. Some swelling is noted; no bruising is present. Distal pulses are good, and motor and sensation are intact. Radiograph of the pelvis is obtained (shown). What is your impression?
A Different Source of Elbow Pain
ANSWER
The radiograph shows no obvious fracture or dislocation. However, there are two small, radiopaque densities noted within the soft tissue. These are most likely consistent with broken glass pieces.
When the laceration was irrigated and the wound probed, the glass bits were found and removed prior to wound closure.
ANSWER
The radiograph shows no obvious fracture or dislocation. However, there are two small, radiopaque densities noted within the soft tissue. These are most likely consistent with broken glass pieces.
When the laceration was irrigated and the wound probed, the glass bits were found and removed prior to wound closure.
ANSWER
The radiograph shows no obvious fracture or dislocation. However, there are two small, radiopaque densities noted within the soft tissue. These are most likely consistent with broken glass pieces.
When the laceration was irrigated and the wound probed, the glass bits were found and removed prior to wound closure.
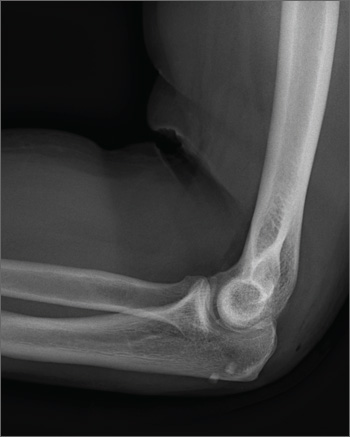
A 30-year-old man is brought to your facility after being in a motor vehicle collision. He was an unrestrained driver who lost control of his vehicle, went off the road, and hit a tree. Emergency personnel on the scene indicated there was moderate damage to his vehicle, including a broken windshield, and no air bag deployment. The patient is complaining of right shoulder, chest, hip, and left elbow pain. His medical history is unremarkable. His vital signs are normal. On physical examination, he has superficial lacerations on his forehead, face, and both forearms. Bleeding from all wounds is controlled. Palpation reveals some bruising of the right shoulder, chest, right hip, and left elbow; no obvious deformity or neurovascular compromise is noted. Multiple radiographs are ordered; the left elbow is shown. What is your impression?
Lung air-fluid level in a smoker
A 49-year-old man was referred for evaluation of an abnormal chest radiograph. A 25-pack-year smoker, he had a history of chronic shortness of breath on exertion with occasional coughing and whitish sputum production. He also had a history of hypertension. He had not had hemoptysis, fever, chills, weight loss, or other symptoms, and he had not traveled recently.
On examination, he appeared comfortable. His breath sounds were decreased bilaterally; the rest of his physical examination was normal. His medical history, social history, and review of systems were otherwise unremarkable.
His white blood cell count was 9.4 × 109/L (reference range 4.5–11.0), with a normal differential. His hemoglobin concentration was 166 g/L (140–175).
Pulmonary function testing demonstrated moderate obstruction, with the following values:
- Forced expiratory volume in the first second of expiration/ forced vital capacity 0.65
- Forced expiratory volume in the first second of expiration 2.40 L (72% of predicted)
- Total lung capacity 7.11 L (92% of predicted)
- Diffusing capacity of lung for carbon monoxide 58% of predicted.
He underwent radiography (Figure 1) and computed tomography of the chest (Figure 2).
DIAGNOSIS: INFECTED EMPHYSEMATOUS BULLAE
The patient had infected emphysematous bullae.
The diagnosis can typically be made by the new development of an air-fluid level in a patient known to have preexisting emphysematous bullae.1 If previous images are not available, the presence of other bullae in a patient with established chronic obstructive pulmonary disease, a thin-walled cavity, and a disproportionate presentation with impressive radiographic findings along with a subtle clinical picture can support the diagnosis.2 In most reported cases, patients are not significantly symptomatic or ill.3 The differential diagnosis includes loculated parapneumonic pleural effusion,4 lung abscess,5 tuberculosis,6 and infected pneumatocele.
Since percutaneous aspiration of the bullae has been discouraged,2 the causative organism is often not identified. Also, the role of bronchoscopy in the diagnostic evaluation and treatment of infected emphysematous bullae appears to be limited.7
Our patient had minimal symptoms and did not appear ill; he had a relatively unremarkable physical examination, no leukocytosis, and negative blood and sputum cultures, suggesting a benign presentation. In addition, chest radiography a few months before this presentation showed multiple large emphysematous bullae (Figure 3). The current chest radiograph suggested multiple thin-walled cavitary lesions with an air-fluid level, which was confirmed on computed tomography.
TREATMENT OF INFECTED EMPHYSEMATOUS BULLAE
Currently, there is no established therapy for infected emphysematous bullae. Because the presentation is usually relatively benign in most case series, conservative treatment with a prolonged course of antibiotics alone seems to be the most appropriate initial course of action. A follow-up evaluation with chest imaging is recommended. On the other hand, in patients with worse symptoms, percutaneous aspiration of the bullae should be considered, as it may guide antibiotic therapy.8
We started our patient on clindamycin and scheduled him for follow-up chest imaging in 6 weeks.
- Burgener FA. Pulmonary cavitary and cystic lesions. In:Burgener FA, Kormano M, Pudas T, editors. Differential Diagnosis in Conventional Radiology. 3rd ed. New York, Thieme; 2008: chap.24.
- Chandra D, Soubra SH, Musher DM. A 57-year-old man with a fluid-containing lung cavity: infection of an emphysematous bulla with methicillin-resistant Staphylococcus aureus. Chest 2006; 130:1942–1946.
- Leatherman JW, McDonald FM, Niewohner DE. Fluid-containing bullae in the lung. South Med J 1985; 78:708–710.
- Sahn SA. Diagnosis and management of parapneumonic effusions and empyema. Clin Infect Dis 2007; 45:1480–1486.
- Hammond JM, Potgieter PD, Hanslo D, Scott H, Roditi D. The etiology and antimicrobial susceptibility patterns of microorganisms in acute community-acquired lung abscess. Chest 1995; 108:937–941.
- Woodring JH, Vandiviere HM, Fried AM, Dillon ML, Williams TD, Melvin IG. Update: the radiographic features of pulmonary tuberculosis. AJR Am J Roentgenol 1986; 146:497–506.
- Chandra D, Rose SR, Carter RB, Musher DM, Hamill RJ. Fluid-containing emphysematous bullae: a spectrum of illness. Eur Respir J 2008; 32:303–306.
- Henao-Martinez AF, Fernandez JF, Adams SG, Restrepo C. Lung bullae with air-fluid levels: what is the appropriate therapeutic approach? Respir Care 2012; 57:642–645.
A 49-year-old man was referred for evaluation of an abnormal chest radiograph. A 25-pack-year smoker, he had a history of chronic shortness of breath on exertion with occasional coughing and whitish sputum production. He also had a history of hypertension. He had not had hemoptysis, fever, chills, weight loss, or other symptoms, and he had not traveled recently.
On examination, he appeared comfortable. His breath sounds were decreased bilaterally; the rest of his physical examination was normal. His medical history, social history, and review of systems were otherwise unremarkable.
His white blood cell count was 9.4 × 109/L (reference range 4.5–11.0), with a normal differential. His hemoglobin concentration was 166 g/L (140–175).
Pulmonary function testing demonstrated moderate obstruction, with the following values:
- Forced expiratory volume in the first second of expiration/ forced vital capacity 0.65
- Forced expiratory volume in the first second of expiration 2.40 L (72% of predicted)
- Total lung capacity 7.11 L (92% of predicted)
- Diffusing capacity of lung for carbon monoxide 58% of predicted.
He underwent radiography (Figure 1) and computed tomography of the chest (Figure 2).
DIAGNOSIS: INFECTED EMPHYSEMATOUS BULLAE
The patient had infected emphysematous bullae.
The diagnosis can typically be made by the new development of an air-fluid level in a patient known to have preexisting emphysematous bullae.1 If previous images are not available, the presence of other bullae in a patient with established chronic obstructive pulmonary disease, a thin-walled cavity, and a disproportionate presentation with impressive radiographic findings along with a subtle clinical picture can support the diagnosis.2 In most reported cases, patients are not significantly symptomatic or ill.3 The differential diagnosis includes loculated parapneumonic pleural effusion,4 lung abscess,5 tuberculosis,6 and infected pneumatocele.
Since percutaneous aspiration of the bullae has been discouraged,2 the causative organism is often not identified. Also, the role of bronchoscopy in the diagnostic evaluation and treatment of infected emphysematous bullae appears to be limited.7
Our patient had minimal symptoms and did not appear ill; he had a relatively unremarkable physical examination, no leukocytosis, and negative blood and sputum cultures, suggesting a benign presentation. In addition, chest radiography a few months before this presentation showed multiple large emphysematous bullae (Figure 3). The current chest radiograph suggested multiple thin-walled cavitary lesions with an air-fluid level, which was confirmed on computed tomography.
TREATMENT OF INFECTED EMPHYSEMATOUS BULLAE
Currently, there is no established therapy for infected emphysematous bullae. Because the presentation is usually relatively benign in most case series, conservative treatment with a prolonged course of antibiotics alone seems to be the most appropriate initial course of action. A follow-up evaluation with chest imaging is recommended. On the other hand, in patients with worse symptoms, percutaneous aspiration of the bullae should be considered, as it may guide antibiotic therapy.8
We started our patient on clindamycin and scheduled him for follow-up chest imaging in 6 weeks.
A 49-year-old man was referred for evaluation of an abnormal chest radiograph. A 25-pack-year smoker, he had a history of chronic shortness of breath on exertion with occasional coughing and whitish sputum production. He also had a history of hypertension. He had not had hemoptysis, fever, chills, weight loss, or other symptoms, and he had not traveled recently.
On examination, he appeared comfortable. His breath sounds were decreased bilaterally; the rest of his physical examination was normal. His medical history, social history, and review of systems were otherwise unremarkable.
His white blood cell count was 9.4 × 109/L (reference range 4.5–11.0), with a normal differential. His hemoglobin concentration was 166 g/L (140–175).
Pulmonary function testing demonstrated moderate obstruction, with the following values:
- Forced expiratory volume in the first second of expiration/ forced vital capacity 0.65
- Forced expiratory volume in the first second of expiration 2.40 L (72% of predicted)
- Total lung capacity 7.11 L (92% of predicted)
- Diffusing capacity of lung for carbon monoxide 58% of predicted.
He underwent radiography (Figure 1) and computed tomography of the chest (Figure 2).
DIAGNOSIS: INFECTED EMPHYSEMATOUS BULLAE
The patient had infected emphysematous bullae.
The diagnosis can typically be made by the new development of an air-fluid level in a patient known to have preexisting emphysematous bullae.1 If previous images are not available, the presence of other bullae in a patient with established chronic obstructive pulmonary disease, a thin-walled cavity, and a disproportionate presentation with impressive radiographic findings along with a subtle clinical picture can support the diagnosis.2 In most reported cases, patients are not significantly symptomatic or ill.3 The differential diagnosis includes loculated parapneumonic pleural effusion,4 lung abscess,5 tuberculosis,6 and infected pneumatocele.
Since percutaneous aspiration of the bullae has been discouraged,2 the causative organism is often not identified. Also, the role of bronchoscopy in the diagnostic evaluation and treatment of infected emphysematous bullae appears to be limited.7
Our patient had minimal symptoms and did not appear ill; he had a relatively unremarkable physical examination, no leukocytosis, and negative blood and sputum cultures, suggesting a benign presentation. In addition, chest radiography a few months before this presentation showed multiple large emphysematous bullae (Figure 3). The current chest radiograph suggested multiple thin-walled cavitary lesions with an air-fluid level, which was confirmed on computed tomography.
TREATMENT OF INFECTED EMPHYSEMATOUS BULLAE
Currently, there is no established therapy for infected emphysematous bullae. Because the presentation is usually relatively benign in most case series, conservative treatment with a prolonged course of antibiotics alone seems to be the most appropriate initial course of action. A follow-up evaluation with chest imaging is recommended. On the other hand, in patients with worse symptoms, percutaneous aspiration of the bullae should be considered, as it may guide antibiotic therapy.8
We started our patient on clindamycin and scheduled him for follow-up chest imaging in 6 weeks.
- Burgener FA. Pulmonary cavitary and cystic lesions. In:Burgener FA, Kormano M, Pudas T, editors. Differential Diagnosis in Conventional Radiology. 3rd ed. New York, Thieme; 2008: chap.24.
- Chandra D, Soubra SH, Musher DM. A 57-year-old man with a fluid-containing lung cavity: infection of an emphysematous bulla with methicillin-resistant Staphylococcus aureus. Chest 2006; 130:1942–1946.
- Leatherman JW, McDonald FM, Niewohner DE. Fluid-containing bullae in the lung. South Med J 1985; 78:708–710.
- Sahn SA. Diagnosis and management of parapneumonic effusions and empyema. Clin Infect Dis 2007; 45:1480–1486.
- Hammond JM, Potgieter PD, Hanslo D, Scott H, Roditi D. The etiology and antimicrobial susceptibility patterns of microorganisms in acute community-acquired lung abscess. Chest 1995; 108:937–941.
- Woodring JH, Vandiviere HM, Fried AM, Dillon ML, Williams TD, Melvin IG. Update: the radiographic features of pulmonary tuberculosis. AJR Am J Roentgenol 1986; 146:497–506.
- Chandra D, Rose SR, Carter RB, Musher DM, Hamill RJ. Fluid-containing emphysematous bullae: a spectrum of illness. Eur Respir J 2008; 32:303–306.
- Henao-Martinez AF, Fernandez JF, Adams SG, Restrepo C. Lung bullae with air-fluid levels: what is the appropriate therapeutic approach? Respir Care 2012; 57:642–645.
- Burgener FA. Pulmonary cavitary and cystic lesions. In:Burgener FA, Kormano M, Pudas T, editors. Differential Diagnosis in Conventional Radiology. 3rd ed. New York, Thieme; 2008: chap.24.
- Chandra D, Soubra SH, Musher DM. A 57-year-old man with a fluid-containing lung cavity: infection of an emphysematous bulla with methicillin-resistant Staphylococcus aureus. Chest 2006; 130:1942–1946.
- Leatherman JW, McDonald FM, Niewohner DE. Fluid-containing bullae in the lung. South Med J 1985; 78:708–710.
- Sahn SA. Diagnosis and management of parapneumonic effusions and empyema. Clin Infect Dis 2007; 45:1480–1486.
- Hammond JM, Potgieter PD, Hanslo D, Scott H, Roditi D. The etiology and antimicrobial susceptibility patterns of microorganisms in acute community-acquired lung abscess. Chest 1995; 108:937–941.
- Woodring JH, Vandiviere HM, Fried AM, Dillon ML, Williams TD, Melvin IG. Update: the radiographic features of pulmonary tuberculosis. AJR Am J Roentgenol 1986; 146:497–506.
- Chandra D, Rose SR, Carter RB, Musher DM, Hamill RJ. Fluid-containing emphysematous bullae: a spectrum of illness. Eur Respir J 2008; 32:303–306.
- Henao-Martinez AF, Fernandez JF, Adams SG, Restrepo C. Lung bullae with air-fluid levels: what is the appropriate therapeutic approach? Respir Care 2012; 57:642–645.