User login
Can patients with COPD or asthma take a beta-blocker?
Yes. Treatment with beta-adrenergic receptor blockers decreases the mortality rate in patients with coronary artery disease or heart failure, as well as during the perioperative period in selected patients (eg, those with a history of myocardial infarction, a positive stress test, or current chest pain due to myocardial ischemia). The current evidence supports giving beta-blockers to patients with coronary artery disease and chronic obstructive pulmonary disease (COPD) or asthma, which lowers the 1-year mortality rate to a degree similar to that in patients without COPD or asthma, and without worsening respiratory function.1 However, many clinicians still hesitate to start patients with COPD or asthma on a beta-blocker due to the fear of bronchoconstriction.2
THE RISKS
In patients with reversible airway disease, beta-blockers may increase airway reactivity and bronchospasm, as well as decrease the response to inhaled or oral beta-receptor agonists.3 Even topical ophthalmic nonselective beta-blockers for glaucoma can cause a worsening of pulmonary function.4 However, these data are from small trials in the 1970s and 1980s.
On the other hand, not giving beta-blockers can pose a risk of death. In a retrospective study of more than 200,000 patients with myocardial infarction, Gottlieb et al5 found that beta-blockers were associated with a 40% reduction in mortality rates in patients with conditions often considered a contraindication to beta-blocker therapy, such as congestive heart failure, pulmonary disease, and older age.5
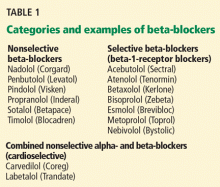
CARDIOSELECTIVE BETA-BLOCKERS
Cardioselective beta-blockers with an affinity for the beta-1 receptor theoretically result in fewer adverse effects on the lungs. They competitively block the response to beta-adrenergic stimulation and selectively block beta-1 receptors with little or no effect on beta-2 receptors, except perhaps at high doses. However, this possible high-dose effect requires further study.
The effect of cardioselective beta-blockers on respiratory function was evaluated in two meta-analyses,6,7 one in patients with mild to moderate reactive airway disease, the other in patients with mild to severe COPD. Patients with reactive airway disease who received a single dose of a beta-blocker had a 7.46% reduction in forced expiratory volume in the first second of expiration (FEV1), an effect that was completely reversed by treatment with a beta-agonist inhaler. The FEV1 increased by a statistically significantly greater amount in response to beta-agonists in patients who received beta-blockers (a single dose or continuous therapy) than in those who did not receive beta-blockers. Patients who received continuous cardioselective beta-blockers experienced no significant drop in FEV1, and no new symptoms developed. These results led the authors to conclude that cardioselective beta-blockers do not cause a significant reduction in pulmonary function in patients with mild to moderate reactive airway disease and COPD and are therefore safe to use. A single dose of a cardioselective beta-blocker may produce a small decrease in FEV1, especially in patients with reactive airway disease, but as therapy is continued over days to weeks, there is no significant change in symptoms or FEV1 and no increase in the need for beta-agonist inhalers.
A major limitation of the two meta-analyses was that the patients were younger than most patients who require beta-blockers: the average age was 40 in patients with reactive airway disease, and 54 in patients with COPD. Also important to consider is that only patients with mild to moderate reactive airway disease were included. Patients with severe asthma, especially those with active bronchospasm, may react differently to even cardioselective beta-blockers.
NONSELECTIVE BETA-BLOCKERS
Recent studies suggest that nonselective beta-blockers can affect respiratory function in patients with COPD, but they have failed to show any harm. For example, propranolol (Inderal) was shown to worsen pulmonary function and to decrease the sensitivity of the airway to the effects of long-acting beta-2-agonists, but the 15 patients included in this study had no increase in respiratory symptoms.8
It has also been suggested that combined nonselective beta- and alpha-receptor blockade—eg, with labetalol (Trandate) or carvedilol (Coreg)—might be better tolerated than nonselective beta-blockers in patients with COPD.9 However, from limited data, Kotlyar et al10 suggested that carvedilol may be less well tolerated in patients with asthma than with COPD. All current evidence on combined nonselective beta-and alpha-blockade is observational, and it is not yet clear whether this class of beta-blockers is better tolerated due to alpha-blockade or merely because nonselective beta-blockers themselves are well tolerated.
OUR RECOMMENDATIONS
- Chen J, Radford MJ, Wang Y, Marciniak TA, Krumholz HM. Effectiveness of beta-blocker therapy after acute myocardial infarction in elderly patients with chronic obstructive pulmonary disease or asthma. J Am Coll Cardiol 2001; 37:1950–1956.
- The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med 1997; 157:2413–2446.
- Benson MK, Berrill WT, Cruickshank JM, Sterling GS. A comparison of four beta-adrenoceptor antagonists in patients with asthma. Br J Clin Pharmacol 1978; 5:415–419.
- Fraunfelder FT, Barker AF. Respiratory effects of timolol. N Engl J Med 1984; 311:1441.
- Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med 1998; 339:489–497.
- Salpeter SR, Ormiston TM, Salpeter EE, Poole PJ, Cates CJ. Cardioselective beta-blockers for chronic obstructive pulmonary disease: a meta-analysis. Respir Med 2003; 97:1094–1101.
- Salpeter SR, Ormiston TM, Salpeter EE. Cardioselective beta-blockers in patients with reactive airway disease: a meta-analysis. Ann Intern Med 2002; 137:715–725.
- van der Woude HJ, Zaagsma J, Postma DS, Winter TH, van Hulst M, Aalbers R. Detrimental effects of beta-blockers in COPD: a concern for nonselective beta-blockers. Chest 2005; 127:818–824.
- Sirak TE, Jelic S, Le Jemtel TH. Therapeutic update: non-selective beta- and alpha-adrenergic blockade in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol 2004; 44:497–502.
- Kotlyar E, Keogh AM, Macdonald PS, Arnold RH, McCaffrey DJ, Glanville AR. Tolerability of carvedilol in patients with heart failure and concomitant chronic obstructive pulmonary disease or asthma. J Heart Lung Transplant 2002; 21:1290–1295.
Yes. Treatment with beta-adrenergic receptor blockers decreases the mortality rate in patients with coronary artery disease or heart failure, as well as during the perioperative period in selected patients (eg, those with a history of myocardial infarction, a positive stress test, or current chest pain due to myocardial ischemia). The current evidence supports giving beta-blockers to patients with coronary artery disease and chronic obstructive pulmonary disease (COPD) or asthma, which lowers the 1-year mortality rate to a degree similar to that in patients without COPD or asthma, and without worsening respiratory function.1 However, many clinicians still hesitate to start patients with COPD or asthma on a beta-blocker due to the fear of bronchoconstriction.2
THE RISKS
In patients with reversible airway disease, beta-blockers may increase airway reactivity and bronchospasm, as well as decrease the response to inhaled or oral beta-receptor agonists.3 Even topical ophthalmic nonselective beta-blockers for glaucoma can cause a worsening of pulmonary function.4 However, these data are from small trials in the 1970s and 1980s.
On the other hand, not giving beta-blockers can pose a risk of death. In a retrospective study of more than 200,000 patients with myocardial infarction, Gottlieb et al5 found that beta-blockers were associated with a 40% reduction in mortality rates in patients with conditions often considered a contraindication to beta-blocker therapy, such as congestive heart failure, pulmonary disease, and older age.5
CARDIOSELECTIVE BETA-BLOCKERS
Cardioselective beta-blockers with an affinity for the beta-1 receptor theoretically result in fewer adverse effects on the lungs. They competitively block the response to beta-adrenergic stimulation and selectively block beta-1 receptors with little or no effect on beta-2 receptors, except perhaps at high doses. However, this possible high-dose effect requires further study.
The effect of cardioselective beta-blockers on respiratory function was evaluated in two meta-analyses,6,7 one in patients with mild to moderate reactive airway disease, the other in patients with mild to severe COPD. Patients with reactive airway disease who received a single dose of a beta-blocker had a 7.46% reduction in forced expiratory volume in the first second of expiration (FEV1), an effect that was completely reversed by treatment with a beta-agonist inhaler. The FEV1 increased by a statistically significantly greater amount in response to beta-agonists in patients who received beta-blockers (a single dose or continuous therapy) than in those who did not receive beta-blockers. Patients who received continuous cardioselective beta-blockers experienced no significant drop in FEV1, and no new symptoms developed. These results led the authors to conclude that cardioselective beta-blockers do not cause a significant reduction in pulmonary function in patients with mild to moderate reactive airway disease and COPD and are therefore safe to use. A single dose of a cardioselective beta-blocker may produce a small decrease in FEV1, especially in patients with reactive airway disease, but as therapy is continued over days to weeks, there is no significant change in symptoms or FEV1 and no increase in the need for beta-agonist inhalers.
A major limitation of the two meta-analyses was that the patients were younger than most patients who require beta-blockers: the average age was 40 in patients with reactive airway disease, and 54 in patients with COPD. Also important to consider is that only patients with mild to moderate reactive airway disease were included. Patients with severe asthma, especially those with active bronchospasm, may react differently to even cardioselective beta-blockers.
NONSELECTIVE BETA-BLOCKERS
Recent studies suggest that nonselective beta-blockers can affect respiratory function in patients with COPD, but they have failed to show any harm. For example, propranolol (Inderal) was shown to worsen pulmonary function and to decrease the sensitivity of the airway to the effects of long-acting beta-2-agonists, but the 15 patients included in this study had no increase in respiratory symptoms.8
It has also been suggested that combined nonselective beta- and alpha-receptor blockade—eg, with labetalol (Trandate) or carvedilol (Coreg)—might be better tolerated than nonselective beta-blockers in patients with COPD.9 However, from limited data, Kotlyar et al10 suggested that carvedilol may be less well tolerated in patients with asthma than with COPD. All current evidence on combined nonselective beta-and alpha-blockade is observational, and it is not yet clear whether this class of beta-blockers is better tolerated due to alpha-blockade or merely because nonselective beta-blockers themselves are well tolerated.
OUR RECOMMENDATIONS
Yes. Treatment with beta-adrenergic receptor blockers decreases the mortality rate in patients with coronary artery disease or heart failure, as well as during the perioperative period in selected patients (eg, those with a history of myocardial infarction, a positive stress test, or current chest pain due to myocardial ischemia). The current evidence supports giving beta-blockers to patients with coronary artery disease and chronic obstructive pulmonary disease (COPD) or asthma, which lowers the 1-year mortality rate to a degree similar to that in patients without COPD or asthma, and without worsening respiratory function.1 However, many clinicians still hesitate to start patients with COPD or asthma on a beta-blocker due to the fear of bronchoconstriction.2
THE RISKS
In patients with reversible airway disease, beta-blockers may increase airway reactivity and bronchospasm, as well as decrease the response to inhaled or oral beta-receptor agonists.3 Even topical ophthalmic nonselective beta-blockers for glaucoma can cause a worsening of pulmonary function.4 However, these data are from small trials in the 1970s and 1980s.
On the other hand, not giving beta-blockers can pose a risk of death. In a retrospective study of more than 200,000 patients with myocardial infarction, Gottlieb et al5 found that beta-blockers were associated with a 40% reduction in mortality rates in patients with conditions often considered a contraindication to beta-blocker therapy, such as congestive heart failure, pulmonary disease, and older age.5
CARDIOSELECTIVE BETA-BLOCKERS
Cardioselective beta-blockers with an affinity for the beta-1 receptor theoretically result in fewer adverse effects on the lungs. They competitively block the response to beta-adrenergic stimulation and selectively block beta-1 receptors with little or no effect on beta-2 receptors, except perhaps at high doses. However, this possible high-dose effect requires further study.
The effect of cardioselective beta-blockers on respiratory function was evaluated in two meta-analyses,6,7 one in patients with mild to moderate reactive airway disease, the other in patients with mild to severe COPD. Patients with reactive airway disease who received a single dose of a beta-blocker had a 7.46% reduction in forced expiratory volume in the first second of expiration (FEV1), an effect that was completely reversed by treatment with a beta-agonist inhaler. The FEV1 increased by a statistically significantly greater amount in response to beta-agonists in patients who received beta-blockers (a single dose or continuous therapy) than in those who did not receive beta-blockers. Patients who received continuous cardioselective beta-blockers experienced no significant drop in FEV1, and no new symptoms developed. These results led the authors to conclude that cardioselective beta-blockers do not cause a significant reduction in pulmonary function in patients with mild to moderate reactive airway disease and COPD and are therefore safe to use. A single dose of a cardioselective beta-blocker may produce a small decrease in FEV1, especially in patients with reactive airway disease, but as therapy is continued over days to weeks, there is no significant change in symptoms or FEV1 and no increase in the need for beta-agonist inhalers.
A major limitation of the two meta-analyses was that the patients were younger than most patients who require beta-blockers: the average age was 40 in patients with reactive airway disease, and 54 in patients with COPD. Also important to consider is that only patients with mild to moderate reactive airway disease were included. Patients with severe asthma, especially those with active bronchospasm, may react differently to even cardioselective beta-blockers.
NONSELECTIVE BETA-BLOCKERS
Recent studies suggest that nonselective beta-blockers can affect respiratory function in patients with COPD, but they have failed to show any harm. For example, propranolol (Inderal) was shown to worsen pulmonary function and to decrease the sensitivity of the airway to the effects of long-acting beta-2-agonists, but the 15 patients included in this study had no increase in respiratory symptoms.8
It has also been suggested that combined nonselective beta- and alpha-receptor blockade—eg, with labetalol (Trandate) or carvedilol (Coreg)—might be better tolerated than nonselective beta-blockers in patients with COPD.9 However, from limited data, Kotlyar et al10 suggested that carvedilol may be less well tolerated in patients with asthma than with COPD. All current evidence on combined nonselective beta-and alpha-blockade is observational, and it is not yet clear whether this class of beta-blockers is better tolerated due to alpha-blockade or merely because nonselective beta-blockers themselves are well tolerated.
OUR RECOMMENDATIONS
- Chen J, Radford MJ, Wang Y, Marciniak TA, Krumholz HM. Effectiveness of beta-blocker therapy after acute myocardial infarction in elderly patients with chronic obstructive pulmonary disease or asthma. J Am Coll Cardiol 2001; 37:1950–1956.
- The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med 1997; 157:2413–2446.
- Benson MK, Berrill WT, Cruickshank JM, Sterling GS. A comparison of four beta-adrenoceptor antagonists in patients with asthma. Br J Clin Pharmacol 1978; 5:415–419.
- Fraunfelder FT, Barker AF. Respiratory effects of timolol. N Engl J Med 1984; 311:1441.
- Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med 1998; 339:489–497.
- Salpeter SR, Ormiston TM, Salpeter EE, Poole PJ, Cates CJ. Cardioselective beta-blockers for chronic obstructive pulmonary disease: a meta-analysis. Respir Med 2003; 97:1094–1101.
- Salpeter SR, Ormiston TM, Salpeter EE. Cardioselective beta-blockers in patients with reactive airway disease: a meta-analysis. Ann Intern Med 2002; 137:715–725.
- van der Woude HJ, Zaagsma J, Postma DS, Winter TH, van Hulst M, Aalbers R. Detrimental effects of beta-blockers in COPD: a concern for nonselective beta-blockers. Chest 2005; 127:818–824.
- Sirak TE, Jelic S, Le Jemtel TH. Therapeutic update: non-selective beta- and alpha-adrenergic blockade in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol 2004; 44:497–502.
- Kotlyar E, Keogh AM, Macdonald PS, Arnold RH, McCaffrey DJ, Glanville AR. Tolerability of carvedilol in patients with heart failure and concomitant chronic obstructive pulmonary disease or asthma. J Heart Lung Transplant 2002; 21:1290–1295.
- Chen J, Radford MJ, Wang Y, Marciniak TA, Krumholz HM. Effectiveness of beta-blocker therapy after acute myocardial infarction in elderly patients with chronic obstructive pulmonary disease or asthma. J Am Coll Cardiol 2001; 37:1950–1956.
- The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med 1997; 157:2413–2446.
- Benson MK, Berrill WT, Cruickshank JM, Sterling GS. A comparison of four beta-adrenoceptor antagonists in patients with asthma. Br J Clin Pharmacol 1978; 5:415–419.
- Fraunfelder FT, Barker AF. Respiratory effects of timolol. N Engl J Med 1984; 311:1441.
- Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med 1998; 339:489–497.
- Salpeter SR, Ormiston TM, Salpeter EE, Poole PJ, Cates CJ. Cardioselective beta-blockers for chronic obstructive pulmonary disease: a meta-analysis. Respir Med 2003; 97:1094–1101.
- Salpeter SR, Ormiston TM, Salpeter EE. Cardioselective beta-blockers in patients with reactive airway disease: a meta-analysis. Ann Intern Med 2002; 137:715–725.
- van der Woude HJ, Zaagsma J, Postma DS, Winter TH, van Hulst M, Aalbers R. Detrimental effects of beta-blockers in COPD: a concern for nonselective beta-blockers. Chest 2005; 127:818–824.
- Sirak TE, Jelic S, Le Jemtel TH. Therapeutic update: non-selective beta- and alpha-adrenergic blockade in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol 2004; 44:497–502.
- Kotlyar E, Keogh AM, Macdonald PS, Arnold RH, McCaffrey DJ, Glanville AR. Tolerability of carvedilol in patients with heart failure and concomitant chronic obstructive pulmonary disease or asthma. J Heart Lung Transplant 2002; 21:1290–1295.
Woman Thrown From Horse
ANSWER
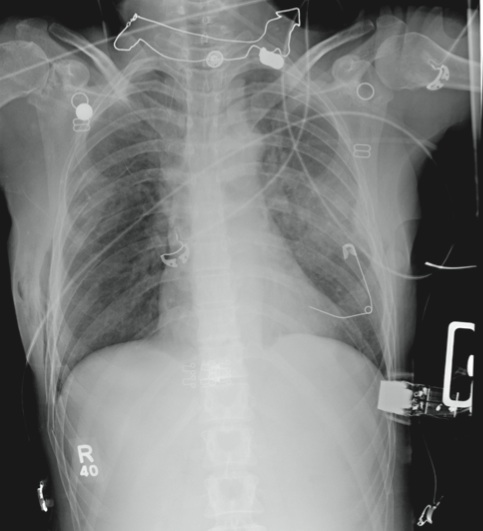
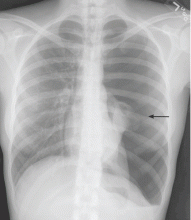
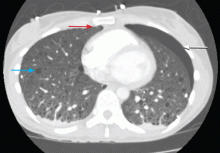
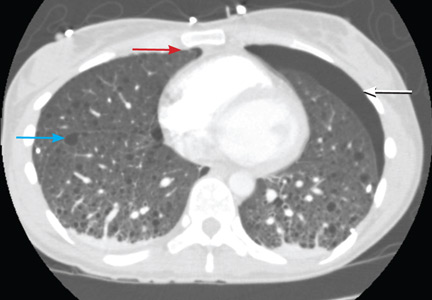
The radiograph demonstrates several findings. First, there are multiple bilateral rib fractures. Five to six ribs are broken and displaced on both sides. In addition, there are bilateral small apical pneumothoraces. Finally, there is evidence of bilateral pulmonary contusions beginning to form, more so on the left than the right side.
This patient was admitted to the ICU, where a chest tube was placed and a thoracic epidural was administered for pain control. As her contusions worsened, she was electively intubated and placed on mechanical ventilation for a few days. She subsequently underwent open reduction and internal fixation of her rib fractures. As her contusions improved, she was weaned off the ventilator and extubated, eventually making a full recovery.
ANSWER
The radiograph demonstrates several findings. First, there are multiple bilateral rib fractures. Five to six ribs are broken and displaced on both sides. In addition, there are bilateral small apical pneumothoraces. Finally, there is evidence of bilateral pulmonary contusions beginning to form, more so on the left than the right side.
This patient was admitted to the ICU, where a chest tube was placed and a thoracic epidural was administered for pain control. As her contusions worsened, she was electively intubated and placed on mechanical ventilation for a few days. She subsequently underwent open reduction and internal fixation of her rib fractures. As her contusions improved, she was weaned off the ventilator and extubated, eventually making a full recovery.
ANSWER
The radiograph demonstrates several findings. First, there are multiple bilateral rib fractures. Five to six ribs are broken and displaced on both sides. In addition, there are bilateral small apical pneumothoraces. Finally, there is evidence of bilateral pulmonary contusions beginning to form, more so on the left than the right side.
This patient was admitted to the ICU, where a chest tube was placed and a thoracic epidural was administered for pain control. As her contusions worsened, she was electively intubated and placed on mechanical ventilation for a few days. She subsequently underwent open reduction and internal fixation of her rib fractures. As her contusions improved, she was weaned off the ventilator and extubated, eventually making a full recovery.
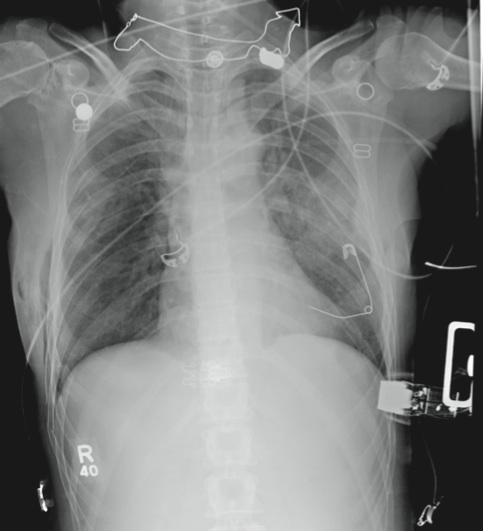
A 53-year-old woman is brought to your facility complaining of right-side chest pain. Earlier this evening, while riding her horse, she was thrown off; the horse then fell on top of her. She denies any loss of consciousness. Most of her pain occurs when she inhales. The patient’s medical history is unremarkable. Her vital signs are: blood pressure, 148/87 mm Hg; heart rate, 100 beats/min; respiratory rate, 14 breaths/min; and O2 saturation, 100% with oxygen via nasal cannula. Primary survey reveals moderate tenderness to palpation along the right side of the patient’s chest, with associated crepitus. Some decreased breath sounds are noted on the right, along with crackles. She is moving all of her extremities and otherwise appears neurologically intact. A stat portable chest radiograph is obtained in the trauma bay before the patient is transported for CT scans. What is your impression?
Grand Rounds: Woman, 30, Survives Near-Exsanguination
While waiting to cross a street, a 30-year-old woman was suddenly struck by an oncoming vehicle, which crushed her legs against a parked automobile. She sustained a life-threatening traumatic injury and nearly exsanguinated at the scene. Nearby pedestrians assisted her, including a man who applied his belt to the woman’s left thigh to prevent complete exsanguination following the crush. She was emergently transported to an adult regional trauma center and admitted to the ICU.
The patient was given multiple transfusions of packed red blood cells, platelets, and frozen plasma in attempts to restore hemostasis. She underwent emergent surgery for a complete washout, debridement, and compartment fasciotomy on the right leg. The left leg required an above-knee amputation. Following surgery, full-thickness and split-thickness wounds were present on both extremities.
Before the accident, the woman had a history of hypertension controlled with a single antihypertensive. She was obese, with a BMI of 31.9. She had no surgical history. She denied excessive alcohol consumption, illicit drug use, or smoking. She was unaware of having any food or drug allergies.
The woman was married and had a 6-month-old baby. Until her accident, she was employed full-time as an investment accountant. She expressed contentment regarding her home, family, work, and busy lifestyle.
Once the patient’s condition was stabilized and hemostasis achieved in the trauma ICU, the bilateral lower-extremity wounds were managed by application of foam dressings via negative-pressure therapy. The dressings were changed on the patient’s lower-extremity wounds three times per week for about three weeks. When the wounds’ depth decreased and granulation was achieved, split-thickness skin grafts (STSGs) harvested from the right anterior thigh were applied to the open wounds (see Figure 1) in the operating room.
Following application of the STSGs and hemostasis of the patient’s donor site, silver silicone foam dressings were applied directly over the right lower-extremity graft and the donor site in the operating room. The dressings remained in place for four days (see Figure 2). A nonadherent, petrolatum-based contact layer was then applied to the left lower-extremity amputation graft site, followed by a negative-pressure foam dressing.
The negative-pressure pump was programmed for 75 mm Hg continuous therapy for four days. The silver silicone foam and negative-pressure foam dressings were removed from the respective graft sites on the fourth postpostoperative day. The grafts were viable and intact (see Figures 3 and 4). The silver silicone foam was reapplied to the lower-extremity STSGs and donor site and changed every four days.
When a few pinpoint dehisced areas were noted on the grafts, a silver-coated absorbent antimicrobial dressing was applied. A nonadherent, petrolatum-based contact layer, followed by wide-mesh stretch gauze, was secured as an exterior dressing over the graft sites. Both lower-extremity dressings were layered with elastic wraps to prevent edema. The dressings were changed daily for two weeks.
On postoperative Day 4, the silver silicone foam was removed from the donor site. A nonadherent contact layer of bismuth tribromophenate petrolatum, followed by the silver silicone foam, was selected for placement over the donor site. Gauze and an elastic wrap were secured as an exterior dressing and removed three days later.
The donor site dressing was reduced to a layer of bismuth tribromophenate petrolatum and left open to air. As the edges of the nonadherent contact layer dried, they were trimmed with scissors (see Figure 5). A moisturizing cocoa butter–based lotion was applied daily to the exposed areas of the donor site.
During the patient’s third postoperative week at the trauma center, as she underwent a continuum of aggressive rehabilitation and wound care, the donor and STSG sites were pronounced healed (see Figures 6, 7, and 8). The donor site was left open to air, with daily use of cocoa butter lotion. Maintenance care of the graft sites included daily application of cocoa butter lotion, stretch gauze, and elastic wraps. The patient was discharged from the rehabilitation unit to home, where she awaited a prosthetic fitting.
Throughout the patient’s hospitalization and rehabilitation, surgical, medical, pain, and nutrition management were monitored on a continuum, as were laboratory values. Her vital signs remained within reasonable limits. The patient remained infection free and experienced neither medical nor surgical complications during the course of her hospital stay.
DISCUSSION
Traumatic injuries often result in bodily deformities, amputations, and death. They represent the leading cause of death among people in the US younger than 45.1,2
Compartment syndrome develops when increased pressure within a bodily cavity minimizes capillary perfusion, resulting in decreased tissue viability.3 Edema and hemorrhage are also precipitating factors for this condition.3 When it goes unrelieved, compromised circulation can lead to muscle devitalization. Amputation of the affected appendage may be necessary unless circulation is restored.
Surgical fasciotomy can help alleviate pressure within the musculofascial compartment to improve circulation.4 Typically, such a procedure leaves large open wounds—a challenge for the clinician who cares for the affected patient. Once the wound is stabilized and the tissue becomes viable with granulation, application of an STSG can be considered.5,6
STSGs provide effective closure for open wounds. The grafting procedure entails removing, processing, and placing a portion of skin, both epidermal and partial dermal layers, on an open wound. Successful grafting requires adequate circulation, but excess bacteria can impede graft viability.7 Graft sites must be kept clean and moist without edema.5 Immediate application of negative-pressure therapy directly on an STSG has been shown to result in an outstanding graft “take,” as compared with use of traditional dressings.5,6,8
Nutritional status must be optimal for a successful graft take, with adequate intake of protein, calories, fluids, vitamins, and minerals.9,10
Treatment
Since days of old when traumatic wounds were treated with goat dung and honey, an array of methods and products has been developed, including numerous agents for cutaneous injuries alone.4 Occlusive, semiocclusive, or bacteriostatic topical ointments, foam, silver, or a combination of products can be used to manage and heal surgical wounds, grafts (including STSGs), and donor sites.11,12
Currently, negative pressure is also used in STSGs to accelerate healing.7,11 When applied to a wound, negative-pressure therapy enhances granulation, removes excess exudate, and creates a moist environment for healing.5,13
Silver-coated absorbent antimicrobial dressings appear to reduce bacteria on the surface of the wound surfaces and postoperative surgical sites without inducing bacterial resistance or adversely affecting healthy tissue.12,14-16 Silver silicone foam reduces bacterial colonization on wound surfaces and absorbs exudate into the foam dressing.17,18 Wounds with devitalized tissue and excessive drainage are at risk for infection, inflammation, and chronic duration.19
Nutrition
It has been reported that about half of all persons admitted to US hospitals are malnourished, with increased risk for morbidity and mortality.20 After experiencing hemorrhage, even well-nourished patients require additional protein and iron for successful recovery.10 Obese patients appear more susceptible to infection and surgical wound dehiscence than are their thinner counterparts, but further research is needed to study the impact of wound development and healing in this population.14
Tissue regeneration is known to require the amino acids arginine and glutamine for the construction of protein; additional research is also needed to support the theory that supplemental glutamine promotes wound healing.9,21 Surgical patients and patients with wounds benefit from protein-enhanced diets; zinc and vitamins A and C can also help improve wound healing and clinical outcomes.22 Monitoring protein and prealbumin levels is helpful in evaluating nutritional status, allowing the clinician to modify the medical nutrition plan and optimize the patient’s health and wellness.22
Rehabilitation
Surgical amputations of the lower extremities impair balance and mobility, necessitating extensive physical therapy and rehabilitation for affected patients.23,24 Aggressive rehabilitation typically is exhausting for patients. It is important to initiate a supportive team approach (including physical and occupational therapists) soon after surgery, continuing beyond the acute hospitalization into rehabilitation. Individualized, patient-centered goals are targeted and amended as necessary. Physical and occupational therapy increase in intensity and duration to optimize the patient’s functionality. Prosthetic fitting takes place after edema diminishes and the limb is fully healed.24
Patient Outcome
An obese young woman who sustained traumatic lower-extremity injuries and amputation experienced an optimal clinical outcome after 54 days of management. Exceptional surgical and medical strategies were initiated in the adult regional trauma center’s ICU and concluded at the adjoining rehabilitation center.
Strategic selection of products and interventions—negative pressure, silver silicone foam, silver-coated absorbent antimicrobial dressings, nonadherent contact layers, stretch gauze, and elastic wraps—and constant monitoring, including that of the patient’s nutritional status, resulted in expedient resolution of her traumatic wounds, STSGs, and donor site.
CONCLUSION
Despite revolutionary advances and life-sustaining measures in the surgical, medical, and wound care arena, traumatic events remain potentially debilitating and life-threatening for young adults. Triage of the trauma patient for appropriate medical care and collaborative management involving a team of trauma specialists and clinicians of all disciplines can now provide life-sustaining opportunities for these patients.
1. WISQARS™ (Web-based Injury Statistics Query and Reporting System). Leading Causes of Death Reports, 1999–2007. webappa.cdc.gov/sasweb/ncipc/leadcaus10.html. June 18, 2010.
2. Sasser SM, Hunt RC, Sullivent EE, et al; CDC. Guidelines for field triage of injured patients: recommendations of the National Expert Panel on Field Triage. MMWR Morb Mortal Wkly Rep. 2009;58(RR01): 1-35.
3. Feliciano DV. The management of extremity compartment syndrome. In: Cameron JL, ed. Current Surgical Therapy. 9th ed. Philadelphia, PA: Elsevier; 2008:1032-1036.
4. Kaufmann CR. Initial assessment and management. In: Feliciano DV, Mattox KL, Moore EE, eds. Trauma. 6th ed. New York: McGraw-Hill; 2008:169-184.
5. Mendez-Eastman S. Guidelines for using negative pressure wound therapy. Adv Skin Wound Care. 2001;14(6):314-322.
6. Snyder RJ, Doyle H, Delbridge T. Applying split-thickness skin grafts: a step-by-step clinical guide and nursing implications. Ostomy Wound Manage. 2001;47(11):20-26.
7. Sood R. Achauer and Sood’s Burn Surgery, Reconstruction and Rehabilitation. Philadelphia: WB Saunders. 2006.
8. Hanasono MM, Skoracki RJ. Securing skin grafts to microvascular free flaps using the vacuum-assisted closure (VAC) device. Ann Plast Surg. 2007;58(5):573-576.
9. Dorner B, Posthauer ME, Thomas D; National Pressure Ulcer Advisory Panel. The role of nutrition in pressure ulcer prevention and treatment (2009). www.npuap.org/Nutrition%20White%20Paper%20Website%20Version.pdf. Accessed June 18, 2010.
10. Frankenfield D. Energy expenditure and protein requirements after traumatic injury. Nutr Clin Pract. 2006;21(5):430-437.
11. Greenhalgh D. Topical antimicrobial agents for burn wounds. Clin Plast Surg. 2009;36(4):597-606.
12. Castellano JJ, Shafii SM, Ko F, et al. Comparative evaluation of silver-containing antimicrobial dressings and drugs. Int Wound J. 2007;4(2):114-122.
13. Baharestani MM. Negative pressure wound therapy in the adjunctive management of necrotizing fasciitis: examining clinical outcomes. Ostomy Wound Manage. 2008;54(4):44-50.
14. Childress BB, Berceli SA, Nelson PR, et al. Impact of an absorbent silver-eluting dressing system on lower extremity revascularization wound complications. Ann Vasc Surg. 2007;21(5):598-602.
15. Sibbald RG, Contreras-Ruiz J, Coutts P, et al. Bacteriology, inflammation, and healing: a study of nanocrystalline silver dressings in chronic venous leg ulcers. Adv Skin Wound Care. 2007;20(10):549-558.
16. Brett DW. A discussion of silver as an antimicrobial agent: alleviating the confusion. Ostomy Wound Manage. 2006;52(1):34-41.
17. Barrett S. Mepilex Ag: an antimicrobial, absorbent foam dressing with Safetac technology. Br J Nurs. 2009;18(20):S28, S30-S36.
18. Barrows C. Enhancing patient outcomes—reducing the bottom line: the use of antimicrobial soft silicone foam dressing in home health. Home Healthc Nurse. 2009;27(5):279-284.
19. National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisory Panel. Prevention and treatment of pressure ulcers: clinical practice guideline. Washington, DC: National Pressure Ulcer Advisory Panel; 2009.
20. Naber TH, Schermer T, de Bree A, et al. Prevalence of malnutrition in nonsurgical hospitalized patients and its association with disease complications. Am J Clin Nutr. 1997;66(5):1232-1239.
21. Ziegler TR, Benfell K, Smith RJ, et al. Safety and metabolic effects of L-glutamine administration in humans. JPEN J Parenter Enteral Nutr. 1990;14(4 suppl):137S-146S.
22. Skin conditions, pressure ulcers, and vitamin deficiencies. In: Escott-Stump S. Nutrition and Diagnosis–Related Care. 6th ed. Baltimore: Lippincott Williams & Wilkins. 2007:108-117.
23. van Velzen JM, van Bennekom CA, Polomski W, et al. Physical capacity and walking ability after lower limb amputation: a systematic review. Clin Rehabil. 2006;20(11):999-1016.
24. Ehlers CF. Integumentary disease and disorders/wound management. In: Malone DJ, Lindsay KLB, eds. Physical Therapy in Acute Care: A Clinician’s Guide. Thorofare, NJ: Slack Inc US; 2006:585-616.
While waiting to cross a street, a 30-year-old woman was suddenly struck by an oncoming vehicle, which crushed her legs against a parked automobile. She sustained a life-threatening traumatic injury and nearly exsanguinated at the scene. Nearby pedestrians assisted her, including a man who applied his belt to the woman’s left thigh to prevent complete exsanguination following the crush. She was emergently transported to an adult regional trauma center and admitted to the ICU.
The patient was given multiple transfusions of packed red blood cells, platelets, and frozen plasma in attempts to restore hemostasis. She underwent emergent surgery for a complete washout, debridement, and compartment fasciotomy on the right leg. The left leg required an above-knee amputation. Following surgery, full-thickness and split-thickness wounds were present on both extremities.
Before the accident, the woman had a history of hypertension controlled with a single antihypertensive. She was obese, with a BMI of 31.9. She had no surgical history. She denied excessive alcohol consumption, illicit drug use, or smoking. She was unaware of having any food or drug allergies.
The woman was married and had a 6-month-old baby. Until her accident, she was employed full-time as an investment accountant. She expressed contentment regarding her home, family, work, and busy lifestyle.
Once the patient’s condition was stabilized and hemostasis achieved in the trauma ICU, the bilateral lower-extremity wounds were managed by application of foam dressings via negative-pressure therapy. The dressings were changed on the patient’s lower-extremity wounds three times per week for about three weeks. When the wounds’ depth decreased and granulation was achieved, split-thickness skin grafts (STSGs) harvested from the right anterior thigh were applied to the open wounds (see Figure 1) in the operating room.
Following application of the STSGs and hemostasis of the patient’s donor site, silver silicone foam dressings were applied directly over the right lower-extremity graft and the donor site in the operating room. The dressings remained in place for four days (see Figure 2). A nonadherent, petrolatum-based contact layer was then applied to the left lower-extremity amputation graft site, followed by a negative-pressure foam dressing.
The negative-pressure pump was programmed for 75 mm Hg continuous therapy for four days. The silver silicone foam and negative-pressure foam dressings were removed from the respective graft sites on the fourth postpostoperative day. The grafts were viable and intact (see Figures 3 and 4). The silver silicone foam was reapplied to the lower-extremity STSGs and donor site and changed every four days.
When a few pinpoint dehisced areas were noted on the grafts, a silver-coated absorbent antimicrobial dressing was applied. A nonadherent, petrolatum-based contact layer, followed by wide-mesh stretch gauze, was secured as an exterior dressing over the graft sites. Both lower-extremity dressings were layered with elastic wraps to prevent edema. The dressings were changed daily for two weeks.
On postoperative Day 4, the silver silicone foam was removed from the donor site. A nonadherent contact layer of bismuth tribromophenate petrolatum, followed by the silver silicone foam, was selected for placement over the donor site. Gauze and an elastic wrap were secured as an exterior dressing and removed three days later.
The donor site dressing was reduced to a layer of bismuth tribromophenate petrolatum and left open to air. As the edges of the nonadherent contact layer dried, they were trimmed with scissors (see Figure 5). A moisturizing cocoa butter–based lotion was applied daily to the exposed areas of the donor site.
During the patient’s third postoperative week at the trauma center, as she underwent a continuum of aggressive rehabilitation and wound care, the donor and STSG sites were pronounced healed (see Figures 6, 7, and 8). The donor site was left open to air, with daily use of cocoa butter lotion. Maintenance care of the graft sites included daily application of cocoa butter lotion, stretch gauze, and elastic wraps. The patient was discharged from the rehabilitation unit to home, where she awaited a prosthetic fitting.
Throughout the patient’s hospitalization and rehabilitation, surgical, medical, pain, and nutrition management were monitored on a continuum, as were laboratory values. Her vital signs remained within reasonable limits. The patient remained infection free and experienced neither medical nor surgical complications during the course of her hospital stay.
DISCUSSION
Traumatic injuries often result in bodily deformities, amputations, and death. They represent the leading cause of death among people in the US younger than 45.1,2
Compartment syndrome develops when increased pressure within a bodily cavity minimizes capillary perfusion, resulting in decreased tissue viability.3 Edema and hemorrhage are also precipitating factors for this condition.3 When it goes unrelieved, compromised circulation can lead to muscle devitalization. Amputation of the affected appendage may be necessary unless circulation is restored.
Surgical fasciotomy can help alleviate pressure within the musculofascial compartment to improve circulation.4 Typically, such a procedure leaves large open wounds—a challenge for the clinician who cares for the affected patient. Once the wound is stabilized and the tissue becomes viable with granulation, application of an STSG can be considered.5,6
STSGs provide effective closure for open wounds. The grafting procedure entails removing, processing, and placing a portion of skin, both epidermal and partial dermal layers, on an open wound. Successful grafting requires adequate circulation, but excess bacteria can impede graft viability.7 Graft sites must be kept clean and moist without edema.5 Immediate application of negative-pressure therapy directly on an STSG has been shown to result in an outstanding graft “take,” as compared with use of traditional dressings.5,6,8
Nutritional status must be optimal for a successful graft take, with adequate intake of protein, calories, fluids, vitamins, and minerals.9,10
Treatment
Since days of old when traumatic wounds were treated with goat dung and honey, an array of methods and products has been developed, including numerous agents for cutaneous injuries alone.4 Occlusive, semiocclusive, or bacteriostatic topical ointments, foam, silver, or a combination of products can be used to manage and heal surgical wounds, grafts (including STSGs), and donor sites.11,12
Currently, negative pressure is also used in STSGs to accelerate healing.7,11 When applied to a wound, negative-pressure therapy enhances granulation, removes excess exudate, and creates a moist environment for healing.5,13
Silver-coated absorbent antimicrobial dressings appear to reduce bacteria on the surface of the wound surfaces and postoperative surgical sites without inducing bacterial resistance or adversely affecting healthy tissue.12,14-16 Silver silicone foam reduces bacterial colonization on wound surfaces and absorbs exudate into the foam dressing.17,18 Wounds with devitalized tissue and excessive drainage are at risk for infection, inflammation, and chronic duration.19
Nutrition
It has been reported that about half of all persons admitted to US hospitals are malnourished, with increased risk for morbidity and mortality.20 After experiencing hemorrhage, even well-nourished patients require additional protein and iron for successful recovery.10 Obese patients appear more susceptible to infection and surgical wound dehiscence than are their thinner counterparts, but further research is needed to study the impact of wound development and healing in this population.14
Tissue regeneration is known to require the amino acids arginine and glutamine for the construction of protein; additional research is also needed to support the theory that supplemental glutamine promotes wound healing.9,21 Surgical patients and patients with wounds benefit from protein-enhanced diets; zinc and vitamins A and C can also help improve wound healing and clinical outcomes.22 Monitoring protein and prealbumin levels is helpful in evaluating nutritional status, allowing the clinician to modify the medical nutrition plan and optimize the patient’s health and wellness.22
Rehabilitation
Surgical amputations of the lower extremities impair balance and mobility, necessitating extensive physical therapy and rehabilitation for affected patients.23,24 Aggressive rehabilitation typically is exhausting for patients. It is important to initiate a supportive team approach (including physical and occupational therapists) soon after surgery, continuing beyond the acute hospitalization into rehabilitation. Individualized, patient-centered goals are targeted and amended as necessary. Physical and occupational therapy increase in intensity and duration to optimize the patient’s functionality. Prosthetic fitting takes place after edema diminishes and the limb is fully healed.24
Patient Outcome
An obese young woman who sustained traumatic lower-extremity injuries and amputation experienced an optimal clinical outcome after 54 days of management. Exceptional surgical and medical strategies were initiated in the adult regional trauma center’s ICU and concluded at the adjoining rehabilitation center.
Strategic selection of products and interventions—negative pressure, silver silicone foam, silver-coated absorbent antimicrobial dressings, nonadherent contact layers, stretch gauze, and elastic wraps—and constant monitoring, including that of the patient’s nutritional status, resulted in expedient resolution of her traumatic wounds, STSGs, and donor site.
CONCLUSION
Despite revolutionary advances and life-sustaining measures in the surgical, medical, and wound care arena, traumatic events remain potentially debilitating and life-threatening for young adults. Triage of the trauma patient for appropriate medical care and collaborative management involving a team of trauma specialists and clinicians of all disciplines can now provide life-sustaining opportunities for these patients.
While waiting to cross a street, a 30-year-old woman was suddenly struck by an oncoming vehicle, which crushed her legs against a parked automobile. She sustained a life-threatening traumatic injury and nearly exsanguinated at the scene. Nearby pedestrians assisted her, including a man who applied his belt to the woman’s left thigh to prevent complete exsanguination following the crush. She was emergently transported to an adult regional trauma center and admitted to the ICU.
The patient was given multiple transfusions of packed red blood cells, platelets, and frozen plasma in attempts to restore hemostasis. She underwent emergent surgery for a complete washout, debridement, and compartment fasciotomy on the right leg. The left leg required an above-knee amputation. Following surgery, full-thickness and split-thickness wounds were present on both extremities.
Before the accident, the woman had a history of hypertension controlled with a single antihypertensive. She was obese, with a BMI of 31.9. She had no surgical history. She denied excessive alcohol consumption, illicit drug use, or smoking. She was unaware of having any food or drug allergies.
The woman was married and had a 6-month-old baby. Until her accident, she was employed full-time as an investment accountant. She expressed contentment regarding her home, family, work, and busy lifestyle.
Once the patient’s condition was stabilized and hemostasis achieved in the trauma ICU, the bilateral lower-extremity wounds were managed by application of foam dressings via negative-pressure therapy. The dressings were changed on the patient’s lower-extremity wounds three times per week for about three weeks. When the wounds’ depth decreased and granulation was achieved, split-thickness skin grafts (STSGs) harvested from the right anterior thigh were applied to the open wounds (see Figure 1) in the operating room.
Following application of the STSGs and hemostasis of the patient’s donor site, silver silicone foam dressings were applied directly over the right lower-extremity graft and the donor site in the operating room. The dressings remained in place for four days (see Figure 2). A nonadherent, petrolatum-based contact layer was then applied to the left lower-extremity amputation graft site, followed by a negative-pressure foam dressing.
The negative-pressure pump was programmed for 75 mm Hg continuous therapy for four days. The silver silicone foam and negative-pressure foam dressings were removed from the respective graft sites on the fourth postpostoperative day. The grafts were viable and intact (see Figures 3 and 4). The silver silicone foam was reapplied to the lower-extremity STSGs and donor site and changed every four days.
When a few pinpoint dehisced areas were noted on the grafts, a silver-coated absorbent antimicrobial dressing was applied. A nonadherent, petrolatum-based contact layer, followed by wide-mesh stretch gauze, was secured as an exterior dressing over the graft sites. Both lower-extremity dressings were layered with elastic wraps to prevent edema. The dressings were changed daily for two weeks.
On postoperative Day 4, the silver silicone foam was removed from the donor site. A nonadherent contact layer of bismuth tribromophenate petrolatum, followed by the silver silicone foam, was selected for placement over the donor site. Gauze and an elastic wrap were secured as an exterior dressing and removed three days later.
The donor site dressing was reduced to a layer of bismuth tribromophenate petrolatum and left open to air. As the edges of the nonadherent contact layer dried, they were trimmed with scissors (see Figure 5). A moisturizing cocoa butter–based lotion was applied daily to the exposed areas of the donor site.
During the patient’s third postoperative week at the trauma center, as she underwent a continuum of aggressive rehabilitation and wound care, the donor and STSG sites were pronounced healed (see Figures 6, 7, and 8). The donor site was left open to air, with daily use of cocoa butter lotion. Maintenance care of the graft sites included daily application of cocoa butter lotion, stretch gauze, and elastic wraps. The patient was discharged from the rehabilitation unit to home, where she awaited a prosthetic fitting.
Throughout the patient’s hospitalization and rehabilitation, surgical, medical, pain, and nutrition management were monitored on a continuum, as were laboratory values. Her vital signs remained within reasonable limits. The patient remained infection free and experienced neither medical nor surgical complications during the course of her hospital stay.
DISCUSSION
Traumatic injuries often result in bodily deformities, amputations, and death. They represent the leading cause of death among people in the US younger than 45.1,2
Compartment syndrome develops when increased pressure within a bodily cavity minimizes capillary perfusion, resulting in decreased tissue viability.3 Edema and hemorrhage are also precipitating factors for this condition.3 When it goes unrelieved, compromised circulation can lead to muscle devitalization. Amputation of the affected appendage may be necessary unless circulation is restored.
Surgical fasciotomy can help alleviate pressure within the musculofascial compartment to improve circulation.4 Typically, such a procedure leaves large open wounds—a challenge for the clinician who cares for the affected patient. Once the wound is stabilized and the tissue becomes viable with granulation, application of an STSG can be considered.5,6
STSGs provide effective closure for open wounds. The grafting procedure entails removing, processing, and placing a portion of skin, both epidermal and partial dermal layers, on an open wound. Successful grafting requires adequate circulation, but excess bacteria can impede graft viability.7 Graft sites must be kept clean and moist without edema.5 Immediate application of negative-pressure therapy directly on an STSG has been shown to result in an outstanding graft “take,” as compared with use of traditional dressings.5,6,8
Nutritional status must be optimal for a successful graft take, with adequate intake of protein, calories, fluids, vitamins, and minerals.9,10
Treatment
Since days of old when traumatic wounds were treated with goat dung and honey, an array of methods and products has been developed, including numerous agents for cutaneous injuries alone.4 Occlusive, semiocclusive, or bacteriostatic topical ointments, foam, silver, or a combination of products can be used to manage and heal surgical wounds, grafts (including STSGs), and donor sites.11,12
Currently, negative pressure is also used in STSGs to accelerate healing.7,11 When applied to a wound, negative-pressure therapy enhances granulation, removes excess exudate, and creates a moist environment for healing.5,13
Silver-coated absorbent antimicrobial dressings appear to reduce bacteria on the surface of the wound surfaces and postoperative surgical sites without inducing bacterial resistance or adversely affecting healthy tissue.12,14-16 Silver silicone foam reduces bacterial colonization on wound surfaces and absorbs exudate into the foam dressing.17,18 Wounds with devitalized tissue and excessive drainage are at risk for infection, inflammation, and chronic duration.19
Nutrition
It has been reported that about half of all persons admitted to US hospitals are malnourished, with increased risk for morbidity and mortality.20 After experiencing hemorrhage, even well-nourished patients require additional protein and iron for successful recovery.10 Obese patients appear more susceptible to infection and surgical wound dehiscence than are their thinner counterparts, but further research is needed to study the impact of wound development and healing in this population.14
Tissue regeneration is known to require the amino acids arginine and glutamine for the construction of protein; additional research is also needed to support the theory that supplemental glutamine promotes wound healing.9,21 Surgical patients and patients with wounds benefit from protein-enhanced diets; zinc and vitamins A and C can also help improve wound healing and clinical outcomes.22 Monitoring protein and prealbumin levels is helpful in evaluating nutritional status, allowing the clinician to modify the medical nutrition plan and optimize the patient’s health and wellness.22
Rehabilitation
Surgical amputations of the lower extremities impair balance and mobility, necessitating extensive physical therapy and rehabilitation for affected patients.23,24 Aggressive rehabilitation typically is exhausting for patients. It is important to initiate a supportive team approach (including physical and occupational therapists) soon after surgery, continuing beyond the acute hospitalization into rehabilitation. Individualized, patient-centered goals are targeted and amended as necessary. Physical and occupational therapy increase in intensity and duration to optimize the patient’s functionality. Prosthetic fitting takes place after edema diminishes and the limb is fully healed.24
Patient Outcome
An obese young woman who sustained traumatic lower-extremity injuries and amputation experienced an optimal clinical outcome after 54 days of management. Exceptional surgical and medical strategies were initiated in the adult regional trauma center’s ICU and concluded at the adjoining rehabilitation center.
Strategic selection of products and interventions—negative pressure, silver silicone foam, silver-coated absorbent antimicrobial dressings, nonadherent contact layers, stretch gauze, and elastic wraps—and constant monitoring, including that of the patient’s nutritional status, resulted in expedient resolution of her traumatic wounds, STSGs, and donor site.
CONCLUSION
Despite revolutionary advances and life-sustaining measures in the surgical, medical, and wound care arena, traumatic events remain potentially debilitating and life-threatening for young adults. Triage of the trauma patient for appropriate medical care and collaborative management involving a team of trauma specialists and clinicians of all disciplines can now provide life-sustaining opportunities for these patients.
1. WISQARS™ (Web-based Injury Statistics Query and Reporting System). Leading Causes of Death Reports, 1999–2007. webappa.cdc.gov/sasweb/ncipc/leadcaus10.html. June 18, 2010.
2. Sasser SM, Hunt RC, Sullivent EE, et al; CDC. Guidelines for field triage of injured patients: recommendations of the National Expert Panel on Field Triage. MMWR Morb Mortal Wkly Rep. 2009;58(RR01): 1-35.
3. Feliciano DV. The management of extremity compartment syndrome. In: Cameron JL, ed. Current Surgical Therapy. 9th ed. Philadelphia, PA: Elsevier; 2008:1032-1036.
4. Kaufmann CR. Initial assessment and management. In: Feliciano DV, Mattox KL, Moore EE, eds. Trauma. 6th ed. New York: McGraw-Hill; 2008:169-184.
5. Mendez-Eastman S. Guidelines for using negative pressure wound therapy. Adv Skin Wound Care. 2001;14(6):314-322.
6. Snyder RJ, Doyle H, Delbridge T. Applying split-thickness skin grafts: a step-by-step clinical guide and nursing implications. Ostomy Wound Manage. 2001;47(11):20-26.
7. Sood R. Achauer and Sood’s Burn Surgery, Reconstruction and Rehabilitation. Philadelphia: WB Saunders. 2006.
8. Hanasono MM, Skoracki RJ. Securing skin grafts to microvascular free flaps using the vacuum-assisted closure (VAC) device. Ann Plast Surg. 2007;58(5):573-576.
9. Dorner B, Posthauer ME, Thomas D; National Pressure Ulcer Advisory Panel. The role of nutrition in pressure ulcer prevention and treatment (2009). www.npuap.org/Nutrition%20White%20Paper%20Website%20Version.pdf. Accessed June 18, 2010.
10. Frankenfield D. Energy expenditure and protein requirements after traumatic injury. Nutr Clin Pract. 2006;21(5):430-437.
11. Greenhalgh D. Topical antimicrobial agents for burn wounds. Clin Plast Surg. 2009;36(4):597-606.
12. Castellano JJ, Shafii SM, Ko F, et al. Comparative evaluation of silver-containing antimicrobial dressings and drugs. Int Wound J. 2007;4(2):114-122.
13. Baharestani MM. Negative pressure wound therapy in the adjunctive management of necrotizing fasciitis: examining clinical outcomes. Ostomy Wound Manage. 2008;54(4):44-50.
14. Childress BB, Berceli SA, Nelson PR, et al. Impact of an absorbent silver-eluting dressing system on lower extremity revascularization wound complications. Ann Vasc Surg. 2007;21(5):598-602.
15. Sibbald RG, Contreras-Ruiz J, Coutts P, et al. Bacteriology, inflammation, and healing: a study of nanocrystalline silver dressings in chronic venous leg ulcers. Adv Skin Wound Care. 2007;20(10):549-558.
16. Brett DW. A discussion of silver as an antimicrobial agent: alleviating the confusion. Ostomy Wound Manage. 2006;52(1):34-41.
17. Barrett S. Mepilex Ag: an antimicrobial, absorbent foam dressing with Safetac technology. Br J Nurs. 2009;18(20):S28, S30-S36.
18. Barrows C. Enhancing patient outcomes—reducing the bottom line: the use of antimicrobial soft silicone foam dressing in home health. Home Healthc Nurse. 2009;27(5):279-284.
19. National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisory Panel. Prevention and treatment of pressure ulcers: clinical practice guideline. Washington, DC: National Pressure Ulcer Advisory Panel; 2009.
20. Naber TH, Schermer T, de Bree A, et al. Prevalence of malnutrition in nonsurgical hospitalized patients and its association with disease complications. Am J Clin Nutr. 1997;66(5):1232-1239.
21. Ziegler TR, Benfell K, Smith RJ, et al. Safety and metabolic effects of L-glutamine administration in humans. JPEN J Parenter Enteral Nutr. 1990;14(4 suppl):137S-146S.
22. Skin conditions, pressure ulcers, and vitamin deficiencies. In: Escott-Stump S. Nutrition and Diagnosis–Related Care. 6th ed. Baltimore: Lippincott Williams & Wilkins. 2007:108-117.
23. van Velzen JM, van Bennekom CA, Polomski W, et al. Physical capacity and walking ability after lower limb amputation: a systematic review. Clin Rehabil. 2006;20(11):999-1016.
24. Ehlers CF. Integumentary disease and disorders/wound management. In: Malone DJ, Lindsay KLB, eds. Physical Therapy in Acute Care: A Clinician’s Guide. Thorofare, NJ: Slack Inc US; 2006:585-616.
1. WISQARS™ (Web-based Injury Statistics Query and Reporting System). Leading Causes of Death Reports, 1999–2007. webappa.cdc.gov/sasweb/ncipc/leadcaus10.html. June 18, 2010.
2. Sasser SM, Hunt RC, Sullivent EE, et al; CDC. Guidelines for field triage of injured patients: recommendations of the National Expert Panel on Field Triage. MMWR Morb Mortal Wkly Rep. 2009;58(RR01): 1-35.
3. Feliciano DV. The management of extremity compartment syndrome. In: Cameron JL, ed. Current Surgical Therapy. 9th ed. Philadelphia, PA: Elsevier; 2008:1032-1036.
4. Kaufmann CR. Initial assessment and management. In: Feliciano DV, Mattox KL, Moore EE, eds. Trauma. 6th ed. New York: McGraw-Hill; 2008:169-184.
5. Mendez-Eastman S. Guidelines for using negative pressure wound therapy. Adv Skin Wound Care. 2001;14(6):314-322.
6. Snyder RJ, Doyle H, Delbridge T. Applying split-thickness skin grafts: a step-by-step clinical guide and nursing implications. Ostomy Wound Manage. 2001;47(11):20-26.
7. Sood R. Achauer and Sood’s Burn Surgery, Reconstruction and Rehabilitation. Philadelphia: WB Saunders. 2006.
8. Hanasono MM, Skoracki RJ. Securing skin grafts to microvascular free flaps using the vacuum-assisted closure (VAC) device. Ann Plast Surg. 2007;58(5):573-576.
9. Dorner B, Posthauer ME, Thomas D; National Pressure Ulcer Advisory Panel. The role of nutrition in pressure ulcer prevention and treatment (2009). www.npuap.org/Nutrition%20White%20Paper%20Website%20Version.pdf. Accessed June 18, 2010.
10. Frankenfield D. Energy expenditure and protein requirements after traumatic injury. Nutr Clin Pract. 2006;21(5):430-437.
11. Greenhalgh D. Topical antimicrobial agents for burn wounds. Clin Plast Surg. 2009;36(4):597-606.
12. Castellano JJ, Shafii SM, Ko F, et al. Comparative evaluation of silver-containing antimicrobial dressings and drugs. Int Wound J. 2007;4(2):114-122.
13. Baharestani MM. Negative pressure wound therapy in the adjunctive management of necrotizing fasciitis: examining clinical outcomes. Ostomy Wound Manage. 2008;54(4):44-50.
14. Childress BB, Berceli SA, Nelson PR, et al. Impact of an absorbent silver-eluting dressing system on lower extremity revascularization wound complications. Ann Vasc Surg. 2007;21(5):598-602.
15. Sibbald RG, Contreras-Ruiz J, Coutts P, et al. Bacteriology, inflammation, and healing: a study of nanocrystalline silver dressings in chronic venous leg ulcers. Adv Skin Wound Care. 2007;20(10):549-558.
16. Brett DW. A discussion of silver as an antimicrobial agent: alleviating the confusion. Ostomy Wound Manage. 2006;52(1):34-41.
17. Barrett S. Mepilex Ag: an antimicrobial, absorbent foam dressing with Safetac technology. Br J Nurs. 2009;18(20):S28, S30-S36.
18. Barrows C. Enhancing patient outcomes—reducing the bottom line: the use of antimicrobial soft silicone foam dressing in home health. Home Healthc Nurse. 2009;27(5):279-284.
19. National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisory Panel. Prevention and treatment of pressure ulcers: clinical practice guideline. Washington, DC: National Pressure Ulcer Advisory Panel; 2009.
20. Naber TH, Schermer T, de Bree A, et al. Prevalence of malnutrition in nonsurgical hospitalized patients and its association with disease complications. Am J Clin Nutr. 1997;66(5):1232-1239.
21. Ziegler TR, Benfell K, Smith RJ, et al. Safety and metabolic effects of L-glutamine administration in humans. JPEN J Parenter Enteral Nutr. 1990;14(4 suppl):137S-146S.
22. Skin conditions, pressure ulcers, and vitamin deficiencies. In: Escott-Stump S. Nutrition and Diagnosis–Related Care. 6th ed. Baltimore: Lippincott Williams & Wilkins. 2007:108-117.
23. van Velzen JM, van Bennekom CA, Polomski W, et al. Physical capacity and walking ability after lower limb amputation: a systematic review. Clin Rehabil. 2006;20(11):999-1016.
24. Ehlers CF. Integumentary disease and disorders/wound management. In: Malone DJ, Lindsay KLB, eds. Physical Therapy in Acute Care: A Clinician’s Guide. Thorofare, NJ: Slack Inc US; 2006:585-616.
Incidence, outcomes, and management of bleeding in non-ST-elevation acute coronary syndromes
The medical management of non-ST-elevation acute coronary syndromes focuses on blocking the coagulation cascade and inhibiting platelets. This—plus diagnostic angiography followed, if needed, by revascularization—has reduced the rates of death and recurrent ischemic events.1 However, the combination of potent antithrombotic drugs and invasive procedures also increases the risk of bleeding.
This review discusses the incidence and complications associated with bleeding during the treatment of acute coronary syndromes and summarizes recommendations for preventing and managing bleeding in this setting.
THE TRUE INCIDENCE OF BLEEDING IS HARD TO DETERMINE
The optimal way to detect and analyze bleeding events in clinical trials and registries is highly debated. The reported incidences of bleeding during antithrombotic and antiplatelet therapy for non-ST-elevation acute coronary syndromes depend on how bleeding was defined, how the acute coronary syndromes were treated, and on other factors such as how the study was designed.
How was bleeding defined?
Since these classification schemes are based on different types of data, they yield different numbers when applied to the same study population. For instance, Rao et al4 pooled the data from the PURSUIT and PARAGON B trials (15,454 patients in all) and found that the incidence of severe bleeding (by the GUSTO criteria) was 1.2%, while the rate of major bleeding (by the TIMI criteria) was 8.2%.
What was the treatment strategy?
Another reason that the true incidence of bleeding is hard to determine is that different studies used treatment strategies that differed in the type, timing, and dose of antithrombotic agents and whether invasive procedures were used early. For example, if unfractionated heparin is used aggressively in regimens that are not adjusted for weight and with a higher target for the activated clotting time, the risk of bleeding is higher than with conservative dosing.5–7
Subherwal et al8 evaluated the effect of treatment strategy on the incidence of bleeding in patients with non-ST-elevation acute coronary syndromes who received two or more antithrombotic drugs in the CRUSADE Quality Improvement Initiative. The risk of bleeding was higher with an invasive approach (catheterization) than with a conservative approach (no catheterization), regardless of baseline bleeding risk.
What type of study was it?
Another source of variation is the design of the study. Registries differ from clinical trials in patient characteristics and in the way data are gathered (prospectively vs retrospectively).
In registries, data are often collected retrospectively, whereas in clinical trials the data are prospectively collected. For this reason, the definition of bleeding in registries is often based on events that are easily identified through chart review, such as transfusion. This may lead to a lower reported rate of bleeding, since other, less serious bleeding events such as access-site hematomas and epistaxis may not be documented in the medical record.
On the other hand, registries often include older and sicker patients, who may be more prone to bleeding and who are often excluded from clinical trials. This may lead to a higher rate of reported bleeding.9
Where the study was conducted makes a difference as well, owing to regional practice differences. For example, Moscucci et al10 reported that the incidence of major bleeding in 24,045 patients with non-ST-elevation acute coronary syndromes in the GRACE registry (in 14 countries worldwide) was 3.9%. In contrast, Yang et al11 reported that the rate of bleeding in the CRUSADE registry (in the United States) was 10.3%.
This difference was partly influenced by different definitions of bleeding. The GRACE registry defined major bleeding as life-threatening events requiring transfusion of two or more units of packed red blood cells, or resulting in an absolute decrease in the hematocrit of 10% or more or death, or hemorrhagic subdural hematoma. In contrast, the CRUSADE data reflect bleeding requiring transfusion. However, practice patterns such as greater use of invasive procedures in the United States may also be responsible.
Rao and colleagues12 examined international variation in blood transfusion rates among patients with acute coronary syndromes. Patients outside the United States were significantly less likely to receive transfusions, even after adjusting for patient and practice differences.
Taking these confounders into account, it is reasonable to estimate that the frequency of bleeding in patients with non-ST-elevation acute coronary syndromes ranges from less than 1% to 10%.13

BLEEDING IS ASSOCIATED WITH POOR OUTCOMES
Regardless of the definition or the data source, hemorrhagic complications are associated with a higher risk of death and nonfatal adverse events, both in the short term and in the long term.
Short-term outcomes
A higher risk of death. In the GRACE registry study by Moscucci et al10 discussed above, patients who had major bleeding were significantly more likely to die during their hospitalization than those who did not (odds ratio [OR] 1.64, 95% confidence interval [CI] 1.18–2.28).
Rao et al14 evaluated pooled data from the multicenter international GUSTO IIb, PURSUIT, and PARAGON A and B trials and found that the effects of bleeding in non-ST-elevation acute coronary syndromes extended beyond the hospital stay. The more severe the bleeding (by the GUSTO criteria), the greater the adjusted hazard ratio (HR) for death within 30 days:
- With mild bleeding—HR 1.6, 95% CI 1.3–1.9
- With moderate bleeding—HR 2.7, 95% CI 2.3–3.4
- With severe bleeding—HR 10.6, 95% CI 8.3–13.6.
The pattern was the same for death within 6 months:
- With mild bleeding—HR 1.4, 95% CI 1.2–1.6
- With moderate bleeding—HR 2.1, 95% CI 1.8–2.4
- With severe bleeding, HR 7.5, 95% CI 6.1–9.3.
These findings were confirmed by Eikelboom et al15 in 34,146 patients with acute coronary syndromes in the OASIS registry, the OASIS-2 trial, and the CURE randomized trial. In the first 30 days, five times as many patients died (12.8% vs 2.5%; P < .0009) among those who developed major bleeding compared with those who did not. These investigators defined major bleeding as bleeding that was life-threatening or significantly disabling or that required transfusion of two or more units of packed red blood cells.
A higher risk of nonfatal adverse events. Bleeding after antithrombotic therapy for non-ST-elevation acute coronary syndromes has also been associated with nonfatal adverse events such as stroke and stent thrombosis.
For example, in the study by Eikelboom et al,15 major bleeding was associated with a higher risk of recurrent ischemic events. Approximately 1 in 5 patients in the OASIS trials who developed major bleeding during the first 30 days died or had a myocardial infarction or stroke by 30 days, compared with 1 in 20 of those who did not develop major bleeding during the first 30 days. However, after events that occurred during the first 30 days were excluded, the association between major bleeding and both myocardial infarction and stroke was no longer evident between 30 days and 6 months.
Manoukian et al16 evaluated the impact of major bleeding in 13,819 patients with highrisk acute coronary syndromes undergoing treatment with an early invasive strategy in the ACUITY trial. At 30 days, patients with major bleeding had higher rates of the composite end point of death, myocardial infarction, or unplanned revascularization for ischemia (23.1% vs 6.8%, P < .0001) and of stent thrombosis (3.4% vs 0.6%, P < .0001).
Long-term outcomes
The association between bleeding and adverse outcomes persists in the long term as well, although the mechanisms underlying this association are not well studied.
Kinnaird et al17 examined the data from 10,974 unselected patients who underwent percutaneous coronary intervention. At 1 year, the following percentages of patients had died:
- After TIMI major bleeding—17.2%
- After TIMI minor bleeding—9.1%
- After no bleeding—5.5%.
However, after adjustment for potential confounders, only transfusion remained a significant predictor of 1-year mortality.
Mehran et al18 evaluated 1-year mortality data from the ACUITY trial. Compared with the rate in patients who had no major bleeding and no myocardial infarction, the hazard ratios for death were:
- After major bleeding—HR 3.5, 95% CI 2.7–4.4
- After myocardial infarction—HR 3.1, 95% CI 2.4–3.9.
Interestingly, the risk of death associated with myocardial infarction abated after 7 days, while the risk associated with bleeding persisted beyond 30 days and remained constant throughout the first year following the bleeding event.
Similarly, Ndrepepa and colleagues19 examined pooled data from four ISAR trials using the TIMI bleeding scale and found that myocardial infarction, target vessel revascularization, and major bleeding all had similar discriminatory ability at predicting 1-year mortality.
In patients undergoing elective or urgent percutaneous coronary intervention in the REPLACE-2 trial,20 independent predictors of death by 1 year were21:
- Major hemorrhage (OR 2.66, 95% CI 1.44–4.92)
- Periprocedural myocardial infarction (OR 2.46, 95% CI 1.44–4.20).
THEORIES OF HOW BLEEDING MAY CAUSE ADVERSE OUTCOMES
Several mechanisms have been proposed to explain the association between bleeding during treatment for acute coronary syndromes and adverse clinical outcomes.13,22
The immediate effects of bleeding are thought to be hypotension and a reflex hyperadrenergic state to compensate for the loss of intravascular volume.23 This physiologic response is believed to contribute to myocardial ischemia by further decreasing myocardial oxygen supply in obstructive coronary disease.
Trying to minimize blood loss, physicians may withhold anticoagulation and antiplatelet therapy, which in turn may lead to further ischemia.24 To compensate for blood loss, physicians may also resort to blood transfusion. However, depletion of 2,3-diphosphoglycerate and nitric oxide in stored donor red blood cells is postulated to reduce oxygen delivery by increasing hemoglobin’s affinity for oxygen, leading to induced microvascular obstruction and adverse inflammatory reactions.15,25
Recent data have also begun to elucidate the long-term effects of bleeding during acute coronary syndrome management. Patients with anemia during the acute phase of infarction have greater neurohormonal activation.26 These adaptive responses to anemia may lead to eccentric left ventricular remodeling that may lead to higher oxygen consumption, increased diastolic wall stress, interstitial fibrosis, and accelerated myocyte loss.27–30
Nevertheless, we must point out that although strong associations between bleeding and adverse outcomes have been established, direct causality has not.
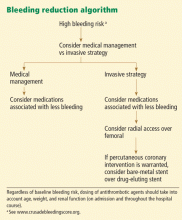
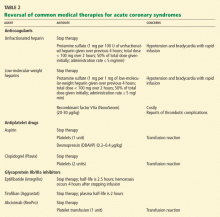
TO PREVENT BLEEDING, START BY ASSESSING RISK
The CRUSADE bleeding risk score
The CRUSADE bleeding score (calculator available at http://www.crusadebleedingscore.org/) was developed and validated in more than 89,000 community-treated patients with non-ST-elevation acute coronary syndromes.8 It is based on eight variables:
- Sex (higher risk in women)
- History of diabetes (higher risk)
- Prior vascular disease (higher risk)
- Heart rate (the higher the rate, the higher the risk)
- Systolic blood pressure (higher risk with pressures above or below the 121–180 mm Hg range)
- Signs of congestive heart failure (higher risk)
- Baseline hematocrit (the lower the hematocrit, the higher the risk)
- Creatinine clearance (by the Cockcroft-Gault formula; the lower the creatinine clearance, the higher the risk).
Patients who are found to have bleeding scores suggesting a moderate or higher risk of bleeding should be considered for medications associated with a favorable bleeding profile, and for radial access at the time of coronary angiography. Scores are graded as follows8:
- < 21: Very low risk
- 21–30: Low risk
- 31–40: Moderate risk
- 41–50: High risk
- > 50: Very high risk.
The CRUSADE bleeding score is unique in that, unlike earlier risk stratification tools, it was developed in a “real world” population, not in subgroups or in a clinical trial. It can be calculated at baseline to help guide the selection of treatment.8
Adjusting the heparin regimen in patients at risk of bleeding
Both the joint American College of Cardiology/American Heart Association1 and the European Society of Cardiology guidelines31 for the treatment of non-ST-elevation acute coronary syndromes recommend taking steps to prevent bleeding, such as adjusting the dosage of unfractionated heparin, using safer drugs, reducing the duration of antithrombotic treatment, and using combinations of antithrombotic and antiplatelet agents according to proven indications.31
In the CRUSADE registry, 42% of patients with non-ST-elevation acute coronary syndromes received at least one initial dose of antithrombotic drug outside the recommended range, resulting in an estimated 15% excess of bleeding events.32 Thus, proper dosing is a target for prevention.
Appropriate antithrombotic dosing takes into account the patient’s age, weight, and renal function. However, heparin dosage in the current guidelines1 is based on weight only: a loading dose of 60 U/kg (maximum 4,000 U) by intravenous bolus, then 12 U/kg/hour (maximum 1,000 U/hour) to maintain an activated partial thromboplastin time of 50 to 70 seconds.1
Renal dysfunction is particularly worrisome in patients with non-ST-elevation acute coronary syndromes because it is associated with higher rates of major bleeding and death. In the OASIS-5 trial,33 the overall risk of death was approximately five times higher in patients in the lowest quartile of renal function (glomerular filtration rate < 58 mL/min/1.73 m2) than in the highest quartile (glomerular filtration rate ≥ 86 mL/min/1.73 m2).
Renal function must be evaluated not only on admission but also throughout the hospital stay. Patients presenting with acute coronary syndromes often experience fluctuations in renal function that would call for adjustment of heparin dosing, either increasing the dose to maximize the drug’s efficacy if renal function is recovering or decreasing the dose to prevent bleeding if renal function is deteriorating.
Clopidogrel vs prasugrel
Certain medications should be avoided when the risk of bleeding outweighs any potential benefit in terms of ischemia.
For example, in a randomized trial,34 prasugrel (Effient), a potent thienopyridine, was associated with a significantly lower rate of the composite end point of stroke, myocardial infarction, or death than clopidogrel (Plavix) in patients with acute coronary syndromes undergoing percutaneous coronary interventions. However, it did not seem to offer any advantage in patients 75 years old and older, those with prior stroke or transient ischemic attack, or those weighing less than 60 kg, and it posed a substantially higher risk of bleeding.
With clopidogrel, the risk of acute bleeding is primarily in patients who undergo coronary artery bypass grafting within 5 days of receiving a dose.35,36 Therefore, clopidogrel should be stopped 5 to 7 days before bypass surgery.1 Importantly, there is no increased risk of recurrent ischemic events during this 5-day waiting period in patients who receive clopidogrel early. Therefore, the recommendation to stop clopidogrel before surgery does not negate the benefits of early treatment.36
Lower-risk drugs: Fondaparinux and bivalirudin
At this time, only two agents have been studied in clinical trials that have specifically focused on reducing bleeding risk: fondaparinux (Arixtra) and bivalirudin (Angiomax).20,37–39
Fondaparinux
OASIS-5 was a randomized, double-blind trial that compared fondaparinux and enoxaparin (Lovenox) in patients with acute coronary syndromes.38 Fondaparinux was similar to enoxaparin in terms of the combined end point of death, myocardial infarction, or refractory ischemia at 9 days, and fewer patients on fondaparinux developed bleeding (2.2% vs 4.1%, HR 0.52; 95% CI 0.44–0.61). This difference persisted during long-term follow-up.
Importantly, fewer patients died in the fondaparinux group. At 180 days, 638 (6.5%) of the patients in the enoxaparin group had died, compared with 574 (5.8%) in the fondaparinux group, a difference of 64 deaths (P = .05). The authors found that 41 fewer patients in the fondaparinux group than in the enoxaparin group died after major bleeding, and 20 fewer patients in the fondaparinux group died after minor bleeding.38 Thus, most of the difference in mortality rates between the two groups was attributed to a lower incidence of bleeding with fondaparinux.
Unfortunately, despite its safe bleeding profile, fondaparinux has fallen out of favor for use in acute coronary syndromes, owing to a higher risk of catheter thrombosis in the fondaparinux group (0.9%) than in those undergoing percutaneous coronary interventions with enoxaparin alone (0.4%) in the OASIS-5 trial.40
Bivalirudin
The direct thrombin inhibitor bivalirudin has been studied in three large randomized trials in patients undergoing percutaneous coronary interventions.20,37,41
The ACUITY trial37 was a prospective, open-label, randomized, multicenter trial that compared three regimens in patients with moderate or high-risk non-ST-elevation acute coronary syndromes:
- Heparin plus a glycoprotein IIb/IIIa inhibitor
- Bivalirudin plus a glycoprotein IIb/IIIa inhibitor
- Bivalirudin alone.
Bivalirudin alone was as effective as heparin plus a glycoprotein IIb/IIIa inhibitor with respect to the composite ischemia end point, which at 30 days had occurred in 7.8% vs 7.3% of the patients in these treatment groups (P = .32, RR 1.08; 95% CI 0.93–1.24), and it was superior with respect to major bleeding (3.0% vs 5.7%, P < .001, RR 0.53; 95% CI 0.43–0.65).
The HORIZONS-AMI study41 was a prospective, open-label, randomized, multicenter trial that compared bivalirudin alone vs heparin plus a glycoprotein IIb/IIIa inhibitor in patients with ST-elevation acute coronary syndromes who were undergoing primary percutaneous coronary interventions. The two primary end points were major bleeding and net adverse events.
At 1 year, patients assigned to bivalirudin had a lower rate of major bleeding than did controls (5.8% vs 9.2%, HR 0.61, 95% CI 0.48–0.78, P < .0001), with similar rates of major adverse cardiac events in both groups (11.9% vs 11.9%, HR 1.00, 95% CI 0.82– 1.21, P = .98).41
Both OASIS 5 and HORIZONS-AMI are examples of clinical trials in which strategies that reduced bleeding risk were also associated with improved survival.
For cardiac catheterization, inserting the catheter in the wrist poses less risk
Bleeding is currently the most common noncardiac complication in patients undergoing percutaneous coronary interventions, and it most often occurs at the vascular access site.17
Rao et al12 evaluated data from 593,094 procedures in the National Cardiovascular Data Registry and found that, compared with the femoral approach, the use of transradial percutaneous coronary intervention was associated with a similar rate of procedural success (OR 1.02, 95% CI 0.93–1.12) but a significantly lower risk of bleeding complications (OR 0.42, 95% CI 0.31–0.56) after multivariable adjustment.
The use of smaller sheath sizes (4F–6F) and preferential use of bivalirudin over unfractionated heparin and glycoprotein IIb/IIIa inhibitor therapy are other methods described to decrease the risk of bleeding after percutaneous coronary interventions.20,41–49
IF BLEEDING OCCURS
Once a bleeding complication occurs, cessation of therapy is a potential option. Stopping or reversing antithrombotic and antiplatelet therapy is warranted in the event of major bleeding (eg, gastrointestinal, retroperitoneal, intracranial).31
Stopping antithrombotic and antiplatelet therapy
Whether bleeding is minor or major, the risk of a recurrent thrombotic event must be considered, especially in patients who have undergone revascularization, stent implantation, or both. The risk of acute thrombotic events after interrupting antithrombotic or antiplatelet agents is considered greatest 4 to 5 days following revascularization or percutaneous coronary intervention.15 If bleeding can be controlled with local treatment such as pressure, packing, or dressing, antithrombotic and antiplatelet therapy need not be interrupted.50
Current guidelines recommend strict control of hemorrhage for at least 24 hours before reintroducing antiplatelet or antithrombotic agents.
It is also important to remember that in the setting of gastrointestinal bleeding due to peptic ulcer disease, adjunctive proton pump inhibitors are recommended after restarting antiplatelet or antithrombotic therapy or both.
Importantly, evidence-based antithrombotic medications (especially dual antiplatelet therapy) should be restarted once the acute bleeding event has resolved.31
Reversal of anticoagulant and antiplatelet therapies
Unfractionated heparin is reversed with infusion of protamine sulfate at a dose of 1 mg per 100 U of unfractionated heparin given over the previous 4 hours.51,52 The rate of protamine sulfate infusion should be less than 100 mg over 2 hours, with 50% of the dose given initially and subsequent doses titrated according to bleeding response.52,53 Protamine sulfate is associated with a risk of hypotension and bradycardia, and for this reason it should be given no faster than 5 mg/min.
Low-molecular-weight heparin (LMWH) can be inhibited by 1 mg of protamine sulfate for each 1 mg of LMWH given over the previous 4 hours.51,52
However, protamine sulfate only partially neutralizes the anticoagulant effect of LMWH. In cases in which protamine sulfate is unsuccessful in abating bleeding associated with LMWH use, guidelines allow for the use of recombinant factor VIIa (NovoSeven).31 In healthy volunteers given fondaparinux, recombinant factor VIIa normalized coagulation times and thrombin generation within 1.5 hours, with a sustained effect for 6 hours.52
It is important to note that the use of this agent has not been fully studied, it is very costly (a single dose of 40 μg/kg costs from $3,000 to $4,000), and it is linked to reports of increased risk of thrombotic complications.54,55
Antiplatelet agents are more complex to reverse. The antiplatelet actions of aspirin and clopidogrel wear off as new platelets are produced. Approximately 10% of a patient’s platelet count is produced daily; thus, the antiplatelet effects of aspirin and clopidogrel can persist for 5 to 10 days.31,56
If these agents need to be reversed quickly to stop bleeding, according to expert consensus the aspirin effect can be reversed by transfusion of one unit of platelets. The antiplatelet effect of clopidogrel is more significant than that of aspirin; thus, two units of platelets are recommended.56
Glycoprotein IIb/IIIa inhibitors. If a major bleeding event requires the reversal of glycoprotein IIb/IIIa inhibitor therapy, the treatment must take into consideration the pharmacodynamics of the target drug. Both eptifibatide (Integrilin) and tirofiban (Aggrastat) competitively inhibit glycoprotein IIb/IIIa receptors; thus, their effects depend on dosing, elimination, and time. Due to the stoichiometry of both drugs, transfusion of platelets is ineffective. Both eptifibatide and tirofiban are eliminated by the kidney; thus, normal renal function is key to the amount of time it takes for platelet function to return to baseline.57 Evidence suggests that fibrinogen-rich plasma can be administered to restore platelet function.31,58,59
Abciximab (ReoPro). Whereas reversal of eptifibatide and tirofiban focuses on overcoming competitive inhibition, neutralization of abciximab involves overcoming its high receptor affinity. At 24 hours after abciximab infusion is stopped, platelet aggregation may still be inhibited by up to 50%. Fortunately, owing to abciximab’s short plasma half-life and its dilution in serum, platelet transfusion is effective in reversing its antiplatelet effects.31,57
Blood transfusion
Long considered beneficial to critically ill patients, blood transfusion to maintain hematocrit levels during acute coronary syndromes has come under intense scrutiny. Randomized trials have shown that transfusion should not be given aggressively to critically ill patients.60 In acute coronary syndromes, there are only observational data.
Rao et al61 used detailed clinical data from 24,112 patients with acute coronary syndromes in the GUSTO IIb, PURSUIT, and PARAGON B trials to determine the association between blood transfusion and outcomes in patients who developed moderate to severe bleeding, anemia, or both during their hospitalization. The rates of death in the hospital and at 30 days were significantly higher in patients who received a transfusion (30-day mortality HR 3.94; 95% CI 3.36–4.75). However, there was no significant association between transfusion and the 30-day mortality rate if the nadir hematocrit was 25% or less.
Of note: no randomized clinical trial has evaluated transfusion strategies in acute coronary syndromes at this time. Until such data are available, it is reasonable to follow published guidelines and to avoid transfusion in stable patients with ischemic heart disease unless the hematocrit is 25% or less.31 The risks and benefits of blood transfusion should be carefully weighed. Routine use of transfusion to maintain predefined hemoglobin levels is not recommended in stable patients.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329:673–682.
- Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 1987; 76:142–154.
- Rao SV, O’Grady K, Pieper KS, et al. A comparison of the clinical impact of bleeding measured by two different classifications among patients with acute coronary syndromes. J Am Coll Cardiol 2006; 47:809–816.
- Granger CB, Hirsch J, Califf RM, et al. Activated partial thromboplastin time and outcome after thrombolytic therapy for acute myocardial infarction: results from the GUSTO-I trial. Circulation 1996; 93:870–878.
- Gilchrist IC, Berkowitz SD, Thompson TD, Califf RM, Granger CB. Heparin dosing and outcome in acute coronary syndromes: the GUSTO-IIb experience. Global Use of Strategies to Open Occluded Coronary Arteries. Am Heart J 2002; 144:73–80.
- Tolleson TR, O’Shea JC, Bittl JA, et al. Relationship between heparin anticoagulation and clinical outcomes in coronary stent intervention: observations from the ESPRIT trial. J Am Coll Cardiol 2003; 41:386–393.
- Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 2009; 119:1873–1882.
- Bassand JP. Bleeding and transfusion in acute coronary syndromes: a shift in the paradigm. Heart 2008; 94:661–666.
- Moscucci M, Fox KA, Cannon CP, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J 2003; 24:1815–1823.
- Yang X, Alexander KP, Chen AY, et al; CRUSADE Investigators. The implications of blood transfusions for patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE National Quality Improvement Initiative. J Am Coll Cardiol 2005; 46:1490–1495.
- Rao SV, Ou FS, Wang TY, et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv 2008; 1:379–386.
- Rao SV, Eikelboom JA, Granger CB, Harrington RA, Califf RM, Bassand JP. Bleeding and blood transfusion issues in patients with non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1193–1204.
- Rao SV, O’Grady K, Pieper KS, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol 2005; 96:1200–1206.
- Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006; 114:774–782.
- Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 2007; 49:1362–1368.
- Kinnaird TD, Stabile E, Mintz GS, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol 2003; 92:930–935.
- Mehran R, Pocock SJ, Stone GW, et al. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes: a risk model from the ACUITY trial. Eur Heart J 2009; 30:1457–1466.
- Ndrepepa G, Berger PB, Mehilli J, et al. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol 2008; 51:690–697.
- Lincoff AM, Bittl JA, Harrington RA, et al; REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003; 289:853–863.
- Feit F, Voeltz MD, Attubato MJ, et al. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. Am J Cardiol 2007; 100:1364–1369.
- Fitchett D. The impact of bleeding in patients with acute coronary syndromes: how to optimize the benefits of treatment and minimize the risk. Can J Cardiol 2007; 23:663–671.
- Bassand JP. Impact of anaemia, bleeding, and transfusions in acute coronary syndromes: a shift in the paradigm. Eur Heart J 2007; 28:1273–1274.
- Yan AT, Yan RT, Huynh T, et al; INTERACT Investigators. Bleeding and outcome in acute coronary syndrome: insights from continuous electrocardiogram monitoring in the Integrilin and Enoxaparin Randomized Assessment of Acute Coronary Syndrome Treatment (INTERACT) Trial. Am Heart J 2008; 156:769–775.
- Jolicoeur EM, O’Neill WW, Hellkamp A, et al; APEX-AMI Investigators. Transfusion and mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur Heart J 2009; 30:2575–2583.
- Gehi A, Ix J, Shlipak M, Pipkin SS, Whooley MA. Relation of anemia to low heart rate variability in patients with coronary heart disease (from the Heart and Soul study). Am J Cardiol 2005; 95:1474–1477.
- Anand I, McMurray JJ, Whitmore J, et al. Anemia and its relationship to clinical outcome in heart failure. Circulation 2004; 110:149–154.
- O’Riordan E, Foley RN. Effects of anaemia on cardiovascular status. Nephrol Dial Transplant 2000; 15(suppl 3):19–22.
- Olivetti G, Quaini F, Lagrasta C, et al. Myocyte cellular hypertrophy and hyperplasia contribute to ventricular wall remodeling in anemia-induced cardiac hypertrophy in rats. Am J Pathol 1992; 141:227–239.
- Aronson D, Suleiman M, Agmon Y, et al. Changes in haemoglobin levels during hospital course and long-term outcome after acute myocardial infarction. Eur Heart J 2007; 28:1289–1296.
- Task Force for Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of European Society of Cardiology; Bassand JP, Hamm CW, Ardissino D, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1598–1660.
- Alexander KP, Chen AY, Roe MT, et al; CRUSADE Investigators. Excess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromes. JAMA 2005; 294:3108–3116.
- Fox KA, Bassand JP, Mehta SR, et al; OASIS 5 Investigators. Influence of renal function on the efficacy and safety of fondaparinux relative to enoxaparin in non ST-segment elevation acute coronary syndromes. Ann Intern Med 2007; 147:304–310.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Berger JS, Frye CB, Harshaw Q, Edwards FH, Steinhubl SR, Becker RC. Impact of clopidogrel in patients with acute coronary syndromes requiring coronary artery bypass surgery: a multicenter analysis. J Am Coll Cardiol 2008; 52:1693–1701.
- Fox KA, Mehta SR, Peters R, et al; Clopidogrel in Unstable angina to prevent Recurrent ischemic Events Trial. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- Stone GW, McLaurin BT, Cox DA, et al; ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med 2006; 355:2203–2216.
- Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators; Yusuf S, Mehta SR, Chrolavicius S, et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med 2006; 354:1464–1476.
- Potsis TZ, Katsouras C, Goudevenos JA. Avoiding and managing bleeding complications in patients with non-ST-segment elevation acute coronary syndromes. Angiology 2009; 60:148–158.
- Mehta SR, Granger CB, Eikelboom JW, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol 2007; 50:1742–1751.
- Mehran R, Lansky AJ, Witzenbichler B, et al; HORIZONS-AMI Trial Investigators. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet 2009; 374:1149–1159.
- Stone GW, Ware JH, Bertrand ME, et al; ACUITY Investigators. Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one-year results from the ACUITY trial. JAMA 2007; 298:2497–2506.
- Cantor WJ, Mahaffey KW, Huang Z, et al. Bleeding complications in patients with acute coronary syndrome undergoing early invasive management can be reduced with radial access, smaller sheath sizes, and timely sheath removal. Catheter Cardiovasc Interv 2007; 69:73–83.
- Büchler JR, Ribeiro EE, Falcão JL, et al. A randomized trial of 5 versus 7 French guiding catheters for transfemoral percutaneous coronary stent implantation. J Interv Cardiol 2008; 21:50–55.
- Shammas NW, Allie D, Hall P, et al; APPROVE Investigators. Predictors of in-hospital and 30-day complications of peripheral vascular interventions using bivalirudin as the primary anticoagulant: results from the APPROVE Registry. J Invasive Cardiol 2005; 17:356–359.
- Doyle BJ, Ting HH, Bell MR, et al. Major femoral bleeding complications after percutaneous coronary intervention: incidence, predictors, and impact on long-term survival among 17,901 patients treated at the Mayo Clinic from 1994 to 2005. JACC Cardiovasc Interv 2008; 1:202–209.
- Stone GW, White HD, Ohman EM, et al; Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial investigators. Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial. Lancet 2007; 369:907–919.
- Stone GW, Bertrand ME, Moses JW, et al; ACUITY Investigators. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA 2007; 297:591–602.
- Lincoff AM, Bittl JA, Kleiman NS, et al; REPLACE-1 Investigators. Comparison of bivalirudin versus heparin during percutaneous coronary intervention (the Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events [REPLACE]-1 trial). Am J Cardiol 2004; 93:1092–1096.
- Barkun A, Bardou M, Marshall JK; Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2003; 139:843–857.
- Warkentin TE, Crowther MA. Reversing anticoagulants both old and new. Can J Anaesth 2002; 49:S11–S25.
- Crowther MA, Warkentin TE. Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: focus on new anticoagulant agents. Blood 2008; 111:4871–4879.
- Kessler CM. Current and future challenges of antithrombotic agents and anticoagulants: strategies for reversal of hemorrhagic complications. Semin Hematol 2004; 41(suppl 1):44–50.
- Ganguly S, Spengel K, Tilzer LL, O’Neal B, Simpson SQ. Recombinant factor VIIa: unregulated continuous use in patients with bleeding and coagulopathy does not alter mortality and outcome. Clin Lab Haematol 2006; 28:309–312.
- O’Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA 2006; 295:293–298.
- Beshay JE, Morgan H, Madden C, Yu W, Sarode R. Emergency reversal of anticoagulation and antiplatelet therapies in neurosurgical patients. J Neurosurg 2010; 112:307–318.
- Tcheng JE. Clinical challenges of platelet glycoprotein IIb/IIIa receptor inhibitor therapy: bleeding, reversal, thrombocytopenia, and retreatment. Am Heart J 2000; 139:S38–S45.
- Li YF, Spencer FA, Becker RC. Comparative efficacy of fibrinogen and platelet supplementation on the in vitro reversibility of competitive glycoprotein IIb/IIIa receptor-directed platelet inhibition. Am Heart J 2002; 143:725–732.
- Schroeder WS, Gandhi PJ. Emergency management of hemorrhagic complications in the era of glycoprotein IIb/IIIa receptor antagonists, clopidogrel, low molecular weight heparin, and third-generation fibrinolytic agents. Curr Cardiol Rep 2003; 5:310–317.
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340:409–417.
- Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 2004; 292:1555–1562.
The medical management of non-ST-elevation acute coronary syndromes focuses on blocking the coagulation cascade and inhibiting platelets. This—plus diagnostic angiography followed, if needed, by revascularization—has reduced the rates of death and recurrent ischemic events.1 However, the combination of potent antithrombotic drugs and invasive procedures also increases the risk of bleeding.
This review discusses the incidence and complications associated with bleeding during the treatment of acute coronary syndromes and summarizes recommendations for preventing and managing bleeding in this setting.
THE TRUE INCIDENCE OF BLEEDING IS HARD TO DETERMINE
The optimal way to detect and analyze bleeding events in clinical trials and registries is highly debated. The reported incidences of bleeding during antithrombotic and antiplatelet therapy for non-ST-elevation acute coronary syndromes depend on how bleeding was defined, how the acute coronary syndromes were treated, and on other factors such as how the study was designed.
How was bleeding defined?
Since these classification schemes are based on different types of data, they yield different numbers when applied to the same study population. For instance, Rao et al4 pooled the data from the PURSUIT and PARAGON B trials (15,454 patients in all) and found that the incidence of severe bleeding (by the GUSTO criteria) was 1.2%, while the rate of major bleeding (by the TIMI criteria) was 8.2%.
What was the treatment strategy?
Another reason that the true incidence of bleeding is hard to determine is that different studies used treatment strategies that differed in the type, timing, and dose of antithrombotic agents and whether invasive procedures were used early. For example, if unfractionated heparin is used aggressively in regimens that are not adjusted for weight and with a higher target for the activated clotting time, the risk of bleeding is higher than with conservative dosing.5–7
Subherwal et al8 evaluated the effect of treatment strategy on the incidence of bleeding in patients with non-ST-elevation acute coronary syndromes who received two or more antithrombotic drugs in the CRUSADE Quality Improvement Initiative. The risk of bleeding was higher with an invasive approach (catheterization) than with a conservative approach (no catheterization), regardless of baseline bleeding risk.
What type of study was it?
Another source of variation is the design of the study. Registries differ from clinical trials in patient characteristics and in the way data are gathered (prospectively vs retrospectively).
In registries, data are often collected retrospectively, whereas in clinical trials the data are prospectively collected. For this reason, the definition of bleeding in registries is often based on events that are easily identified through chart review, such as transfusion. This may lead to a lower reported rate of bleeding, since other, less serious bleeding events such as access-site hematomas and epistaxis may not be documented in the medical record.
On the other hand, registries often include older and sicker patients, who may be more prone to bleeding and who are often excluded from clinical trials. This may lead to a higher rate of reported bleeding.9
Where the study was conducted makes a difference as well, owing to regional practice differences. For example, Moscucci et al10 reported that the incidence of major bleeding in 24,045 patients with non-ST-elevation acute coronary syndromes in the GRACE registry (in 14 countries worldwide) was 3.9%. In contrast, Yang et al11 reported that the rate of bleeding in the CRUSADE registry (in the United States) was 10.3%.
This difference was partly influenced by different definitions of bleeding. The GRACE registry defined major bleeding as life-threatening events requiring transfusion of two or more units of packed red blood cells, or resulting in an absolute decrease in the hematocrit of 10% or more or death, or hemorrhagic subdural hematoma. In contrast, the CRUSADE data reflect bleeding requiring transfusion. However, practice patterns such as greater use of invasive procedures in the United States may also be responsible.
Rao and colleagues12 examined international variation in blood transfusion rates among patients with acute coronary syndromes. Patients outside the United States were significantly less likely to receive transfusions, even after adjusting for patient and practice differences.
Taking these confounders into account, it is reasonable to estimate that the frequency of bleeding in patients with non-ST-elevation acute coronary syndromes ranges from less than 1% to 10%.13

BLEEDING IS ASSOCIATED WITH POOR OUTCOMES
Regardless of the definition or the data source, hemorrhagic complications are associated with a higher risk of death and nonfatal adverse events, both in the short term and in the long term.
Short-term outcomes
A higher risk of death. In the GRACE registry study by Moscucci et al10 discussed above, patients who had major bleeding were significantly more likely to die during their hospitalization than those who did not (odds ratio [OR] 1.64, 95% confidence interval [CI] 1.18–2.28).
Rao et al14 evaluated pooled data from the multicenter international GUSTO IIb, PURSUIT, and PARAGON A and B trials and found that the effects of bleeding in non-ST-elevation acute coronary syndromes extended beyond the hospital stay. The more severe the bleeding (by the GUSTO criteria), the greater the adjusted hazard ratio (HR) for death within 30 days:
- With mild bleeding—HR 1.6, 95% CI 1.3–1.9
- With moderate bleeding—HR 2.7, 95% CI 2.3–3.4
- With severe bleeding—HR 10.6, 95% CI 8.3–13.6.
The pattern was the same for death within 6 months:
- With mild bleeding—HR 1.4, 95% CI 1.2–1.6
- With moderate bleeding—HR 2.1, 95% CI 1.8–2.4
- With severe bleeding, HR 7.5, 95% CI 6.1–9.3.
These findings were confirmed by Eikelboom et al15 in 34,146 patients with acute coronary syndromes in the OASIS registry, the OASIS-2 trial, and the CURE randomized trial. In the first 30 days, five times as many patients died (12.8% vs 2.5%; P < .0009) among those who developed major bleeding compared with those who did not. These investigators defined major bleeding as bleeding that was life-threatening or significantly disabling or that required transfusion of two or more units of packed red blood cells.
A higher risk of nonfatal adverse events. Bleeding after antithrombotic therapy for non-ST-elevation acute coronary syndromes has also been associated with nonfatal adverse events such as stroke and stent thrombosis.
For example, in the study by Eikelboom et al,15 major bleeding was associated with a higher risk of recurrent ischemic events. Approximately 1 in 5 patients in the OASIS trials who developed major bleeding during the first 30 days died or had a myocardial infarction or stroke by 30 days, compared with 1 in 20 of those who did not develop major bleeding during the first 30 days. However, after events that occurred during the first 30 days were excluded, the association between major bleeding and both myocardial infarction and stroke was no longer evident between 30 days and 6 months.
Manoukian et al16 evaluated the impact of major bleeding in 13,819 patients with highrisk acute coronary syndromes undergoing treatment with an early invasive strategy in the ACUITY trial. At 30 days, patients with major bleeding had higher rates of the composite end point of death, myocardial infarction, or unplanned revascularization for ischemia (23.1% vs 6.8%, P < .0001) and of stent thrombosis (3.4% vs 0.6%, P < .0001).
Long-term outcomes
The association between bleeding and adverse outcomes persists in the long term as well, although the mechanisms underlying this association are not well studied.
Kinnaird et al17 examined the data from 10,974 unselected patients who underwent percutaneous coronary intervention. At 1 year, the following percentages of patients had died:
- After TIMI major bleeding—17.2%
- After TIMI minor bleeding—9.1%
- After no bleeding—5.5%.
However, after adjustment for potential confounders, only transfusion remained a significant predictor of 1-year mortality.
Mehran et al18 evaluated 1-year mortality data from the ACUITY trial. Compared with the rate in patients who had no major bleeding and no myocardial infarction, the hazard ratios for death were:
- After major bleeding—HR 3.5, 95% CI 2.7–4.4
- After myocardial infarction—HR 3.1, 95% CI 2.4–3.9.
Interestingly, the risk of death associated with myocardial infarction abated after 7 days, while the risk associated with bleeding persisted beyond 30 days and remained constant throughout the first year following the bleeding event.
Similarly, Ndrepepa and colleagues19 examined pooled data from four ISAR trials using the TIMI bleeding scale and found that myocardial infarction, target vessel revascularization, and major bleeding all had similar discriminatory ability at predicting 1-year mortality.
In patients undergoing elective or urgent percutaneous coronary intervention in the REPLACE-2 trial,20 independent predictors of death by 1 year were21:
- Major hemorrhage (OR 2.66, 95% CI 1.44–4.92)
- Periprocedural myocardial infarction (OR 2.46, 95% CI 1.44–4.20).
THEORIES OF HOW BLEEDING MAY CAUSE ADVERSE OUTCOMES
Several mechanisms have been proposed to explain the association between bleeding during treatment for acute coronary syndromes and adverse clinical outcomes.13,22
The immediate effects of bleeding are thought to be hypotension and a reflex hyperadrenergic state to compensate for the loss of intravascular volume.23 This physiologic response is believed to contribute to myocardial ischemia by further decreasing myocardial oxygen supply in obstructive coronary disease.
Trying to minimize blood loss, physicians may withhold anticoagulation and antiplatelet therapy, which in turn may lead to further ischemia.24 To compensate for blood loss, physicians may also resort to blood transfusion. However, depletion of 2,3-diphosphoglycerate and nitric oxide in stored donor red blood cells is postulated to reduce oxygen delivery by increasing hemoglobin’s affinity for oxygen, leading to induced microvascular obstruction and adverse inflammatory reactions.15,25
Recent data have also begun to elucidate the long-term effects of bleeding during acute coronary syndrome management. Patients with anemia during the acute phase of infarction have greater neurohormonal activation.26 These adaptive responses to anemia may lead to eccentric left ventricular remodeling that may lead to higher oxygen consumption, increased diastolic wall stress, interstitial fibrosis, and accelerated myocyte loss.27–30
Nevertheless, we must point out that although strong associations between bleeding and adverse outcomes have been established, direct causality has not.
TO PREVENT BLEEDING, START BY ASSESSING RISK
The CRUSADE bleeding risk score
The CRUSADE bleeding score (calculator available at http://www.crusadebleedingscore.org/) was developed and validated in more than 89,000 community-treated patients with non-ST-elevation acute coronary syndromes.8 It is based on eight variables:
- Sex (higher risk in women)
- History of diabetes (higher risk)
- Prior vascular disease (higher risk)
- Heart rate (the higher the rate, the higher the risk)
- Systolic blood pressure (higher risk with pressures above or below the 121–180 mm Hg range)
- Signs of congestive heart failure (higher risk)
- Baseline hematocrit (the lower the hematocrit, the higher the risk)
- Creatinine clearance (by the Cockcroft-Gault formula; the lower the creatinine clearance, the higher the risk).
Patients who are found to have bleeding scores suggesting a moderate or higher risk of bleeding should be considered for medications associated with a favorable bleeding profile, and for radial access at the time of coronary angiography. Scores are graded as follows8:
- < 21: Very low risk
- 21–30: Low risk
- 31–40: Moderate risk
- 41–50: High risk
- > 50: Very high risk.
The CRUSADE bleeding score is unique in that, unlike earlier risk stratification tools, it was developed in a “real world” population, not in subgroups or in a clinical trial. It can be calculated at baseline to help guide the selection of treatment.8
Adjusting the heparin regimen in patients at risk of bleeding
Both the joint American College of Cardiology/American Heart Association1 and the European Society of Cardiology guidelines31 for the treatment of non-ST-elevation acute coronary syndromes recommend taking steps to prevent bleeding, such as adjusting the dosage of unfractionated heparin, using safer drugs, reducing the duration of antithrombotic treatment, and using combinations of antithrombotic and antiplatelet agents according to proven indications.31
In the CRUSADE registry, 42% of patients with non-ST-elevation acute coronary syndromes received at least one initial dose of antithrombotic drug outside the recommended range, resulting in an estimated 15% excess of bleeding events.32 Thus, proper dosing is a target for prevention.
Appropriate antithrombotic dosing takes into account the patient’s age, weight, and renal function. However, heparin dosage in the current guidelines1 is based on weight only: a loading dose of 60 U/kg (maximum 4,000 U) by intravenous bolus, then 12 U/kg/hour (maximum 1,000 U/hour) to maintain an activated partial thromboplastin time of 50 to 70 seconds.1
Renal dysfunction is particularly worrisome in patients with non-ST-elevation acute coronary syndromes because it is associated with higher rates of major bleeding and death. In the OASIS-5 trial,33 the overall risk of death was approximately five times higher in patients in the lowest quartile of renal function (glomerular filtration rate < 58 mL/min/1.73 m2) than in the highest quartile (glomerular filtration rate ≥ 86 mL/min/1.73 m2).
Renal function must be evaluated not only on admission but also throughout the hospital stay. Patients presenting with acute coronary syndromes often experience fluctuations in renal function that would call for adjustment of heparin dosing, either increasing the dose to maximize the drug’s efficacy if renal function is recovering or decreasing the dose to prevent bleeding if renal function is deteriorating.
Clopidogrel vs prasugrel
Certain medications should be avoided when the risk of bleeding outweighs any potential benefit in terms of ischemia.
For example, in a randomized trial,34 prasugrel (Effient), a potent thienopyridine, was associated with a significantly lower rate of the composite end point of stroke, myocardial infarction, or death than clopidogrel (Plavix) in patients with acute coronary syndromes undergoing percutaneous coronary interventions. However, it did not seem to offer any advantage in patients 75 years old and older, those with prior stroke or transient ischemic attack, or those weighing less than 60 kg, and it posed a substantially higher risk of bleeding.
With clopidogrel, the risk of acute bleeding is primarily in patients who undergo coronary artery bypass grafting within 5 days of receiving a dose.35,36 Therefore, clopidogrel should be stopped 5 to 7 days before bypass surgery.1 Importantly, there is no increased risk of recurrent ischemic events during this 5-day waiting period in patients who receive clopidogrel early. Therefore, the recommendation to stop clopidogrel before surgery does not negate the benefits of early treatment.36
Lower-risk drugs: Fondaparinux and bivalirudin
At this time, only two agents have been studied in clinical trials that have specifically focused on reducing bleeding risk: fondaparinux (Arixtra) and bivalirudin (Angiomax).20,37–39
Fondaparinux
OASIS-5 was a randomized, double-blind trial that compared fondaparinux and enoxaparin (Lovenox) in patients with acute coronary syndromes.38 Fondaparinux was similar to enoxaparin in terms of the combined end point of death, myocardial infarction, or refractory ischemia at 9 days, and fewer patients on fondaparinux developed bleeding (2.2% vs 4.1%, HR 0.52; 95% CI 0.44–0.61). This difference persisted during long-term follow-up.
Importantly, fewer patients died in the fondaparinux group. At 180 days, 638 (6.5%) of the patients in the enoxaparin group had died, compared with 574 (5.8%) in the fondaparinux group, a difference of 64 deaths (P = .05). The authors found that 41 fewer patients in the fondaparinux group than in the enoxaparin group died after major bleeding, and 20 fewer patients in the fondaparinux group died after minor bleeding.38 Thus, most of the difference in mortality rates between the two groups was attributed to a lower incidence of bleeding with fondaparinux.
Unfortunately, despite its safe bleeding profile, fondaparinux has fallen out of favor for use in acute coronary syndromes, owing to a higher risk of catheter thrombosis in the fondaparinux group (0.9%) than in those undergoing percutaneous coronary interventions with enoxaparin alone (0.4%) in the OASIS-5 trial.40
Bivalirudin
The direct thrombin inhibitor bivalirudin has been studied in three large randomized trials in patients undergoing percutaneous coronary interventions.20,37,41
The ACUITY trial37 was a prospective, open-label, randomized, multicenter trial that compared three regimens in patients with moderate or high-risk non-ST-elevation acute coronary syndromes:
- Heparin plus a glycoprotein IIb/IIIa inhibitor
- Bivalirudin plus a glycoprotein IIb/IIIa inhibitor
- Bivalirudin alone.
Bivalirudin alone was as effective as heparin plus a glycoprotein IIb/IIIa inhibitor with respect to the composite ischemia end point, which at 30 days had occurred in 7.8% vs 7.3% of the patients in these treatment groups (P = .32, RR 1.08; 95% CI 0.93–1.24), and it was superior with respect to major bleeding (3.0% vs 5.7%, P < .001, RR 0.53; 95% CI 0.43–0.65).
The HORIZONS-AMI study41 was a prospective, open-label, randomized, multicenter trial that compared bivalirudin alone vs heparin plus a glycoprotein IIb/IIIa inhibitor in patients with ST-elevation acute coronary syndromes who were undergoing primary percutaneous coronary interventions. The two primary end points were major bleeding and net adverse events.
At 1 year, patients assigned to bivalirudin had a lower rate of major bleeding than did controls (5.8% vs 9.2%, HR 0.61, 95% CI 0.48–0.78, P < .0001), with similar rates of major adverse cardiac events in both groups (11.9% vs 11.9%, HR 1.00, 95% CI 0.82– 1.21, P = .98).41
Both OASIS 5 and HORIZONS-AMI are examples of clinical trials in which strategies that reduced bleeding risk were also associated with improved survival.
For cardiac catheterization, inserting the catheter in the wrist poses less risk
Bleeding is currently the most common noncardiac complication in patients undergoing percutaneous coronary interventions, and it most often occurs at the vascular access site.17
Rao et al12 evaluated data from 593,094 procedures in the National Cardiovascular Data Registry and found that, compared with the femoral approach, the use of transradial percutaneous coronary intervention was associated with a similar rate of procedural success (OR 1.02, 95% CI 0.93–1.12) but a significantly lower risk of bleeding complications (OR 0.42, 95% CI 0.31–0.56) after multivariable adjustment.
The use of smaller sheath sizes (4F–6F) and preferential use of bivalirudin over unfractionated heparin and glycoprotein IIb/IIIa inhibitor therapy are other methods described to decrease the risk of bleeding after percutaneous coronary interventions.20,41–49
IF BLEEDING OCCURS
Once a bleeding complication occurs, cessation of therapy is a potential option. Stopping or reversing antithrombotic and antiplatelet therapy is warranted in the event of major bleeding (eg, gastrointestinal, retroperitoneal, intracranial).31
Stopping antithrombotic and antiplatelet therapy
Whether bleeding is minor or major, the risk of a recurrent thrombotic event must be considered, especially in patients who have undergone revascularization, stent implantation, or both. The risk of acute thrombotic events after interrupting antithrombotic or antiplatelet agents is considered greatest 4 to 5 days following revascularization or percutaneous coronary intervention.15 If bleeding can be controlled with local treatment such as pressure, packing, or dressing, antithrombotic and antiplatelet therapy need not be interrupted.50
Current guidelines recommend strict control of hemorrhage for at least 24 hours before reintroducing antiplatelet or antithrombotic agents.
It is also important to remember that in the setting of gastrointestinal bleeding due to peptic ulcer disease, adjunctive proton pump inhibitors are recommended after restarting antiplatelet or antithrombotic therapy or both.
Importantly, evidence-based antithrombotic medications (especially dual antiplatelet therapy) should be restarted once the acute bleeding event has resolved.31
Reversal of anticoagulant and antiplatelet therapies
Unfractionated heparin is reversed with infusion of protamine sulfate at a dose of 1 mg per 100 U of unfractionated heparin given over the previous 4 hours.51,52 The rate of protamine sulfate infusion should be less than 100 mg over 2 hours, with 50% of the dose given initially and subsequent doses titrated according to bleeding response.52,53 Protamine sulfate is associated with a risk of hypotension and bradycardia, and for this reason it should be given no faster than 5 mg/min.
Low-molecular-weight heparin (LMWH) can be inhibited by 1 mg of protamine sulfate for each 1 mg of LMWH given over the previous 4 hours.51,52
However, protamine sulfate only partially neutralizes the anticoagulant effect of LMWH. In cases in which protamine sulfate is unsuccessful in abating bleeding associated with LMWH use, guidelines allow for the use of recombinant factor VIIa (NovoSeven).31 In healthy volunteers given fondaparinux, recombinant factor VIIa normalized coagulation times and thrombin generation within 1.5 hours, with a sustained effect for 6 hours.52
It is important to note that the use of this agent has not been fully studied, it is very costly (a single dose of 40 μg/kg costs from $3,000 to $4,000), and it is linked to reports of increased risk of thrombotic complications.54,55
Antiplatelet agents are more complex to reverse. The antiplatelet actions of aspirin and clopidogrel wear off as new platelets are produced. Approximately 10% of a patient’s platelet count is produced daily; thus, the antiplatelet effects of aspirin and clopidogrel can persist for 5 to 10 days.31,56
If these agents need to be reversed quickly to stop bleeding, according to expert consensus the aspirin effect can be reversed by transfusion of one unit of platelets. The antiplatelet effect of clopidogrel is more significant than that of aspirin; thus, two units of platelets are recommended.56
Glycoprotein IIb/IIIa inhibitors. If a major bleeding event requires the reversal of glycoprotein IIb/IIIa inhibitor therapy, the treatment must take into consideration the pharmacodynamics of the target drug. Both eptifibatide (Integrilin) and tirofiban (Aggrastat) competitively inhibit glycoprotein IIb/IIIa receptors; thus, their effects depend on dosing, elimination, and time. Due to the stoichiometry of both drugs, transfusion of platelets is ineffective. Both eptifibatide and tirofiban are eliminated by the kidney; thus, normal renal function is key to the amount of time it takes for platelet function to return to baseline.57 Evidence suggests that fibrinogen-rich plasma can be administered to restore platelet function.31,58,59
Abciximab (ReoPro). Whereas reversal of eptifibatide and tirofiban focuses on overcoming competitive inhibition, neutralization of abciximab involves overcoming its high receptor affinity. At 24 hours after abciximab infusion is stopped, platelet aggregation may still be inhibited by up to 50%. Fortunately, owing to abciximab’s short plasma half-life and its dilution in serum, platelet transfusion is effective in reversing its antiplatelet effects.31,57
Blood transfusion
Long considered beneficial to critically ill patients, blood transfusion to maintain hematocrit levels during acute coronary syndromes has come under intense scrutiny. Randomized trials have shown that transfusion should not be given aggressively to critically ill patients.60 In acute coronary syndromes, there are only observational data.
Rao et al61 used detailed clinical data from 24,112 patients with acute coronary syndromes in the GUSTO IIb, PURSUIT, and PARAGON B trials to determine the association between blood transfusion and outcomes in patients who developed moderate to severe bleeding, anemia, or both during their hospitalization. The rates of death in the hospital and at 30 days were significantly higher in patients who received a transfusion (30-day mortality HR 3.94; 95% CI 3.36–4.75). However, there was no significant association between transfusion and the 30-day mortality rate if the nadir hematocrit was 25% or less.
Of note: no randomized clinical trial has evaluated transfusion strategies in acute coronary syndromes at this time. Until such data are available, it is reasonable to follow published guidelines and to avoid transfusion in stable patients with ischemic heart disease unless the hematocrit is 25% or less.31 The risks and benefits of blood transfusion should be carefully weighed. Routine use of transfusion to maintain predefined hemoglobin levels is not recommended in stable patients.
The medical management of non-ST-elevation acute coronary syndromes focuses on blocking the coagulation cascade and inhibiting platelets. This—plus diagnostic angiography followed, if needed, by revascularization—has reduced the rates of death and recurrent ischemic events.1 However, the combination of potent antithrombotic drugs and invasive procedures also increases the risk of bleeding.
This review discusses the incidence and complications associated with bleeding during the treatment of acute coronary syndromes and summarizes recommendations for preventing and managing bleeding in this setting.
THE TRUE INCIDENCE OF BLEEDING IS HARD TO DETERMINE
The optimal way to detect and analyze bleeding events in clinical trials and registries is highly debated. The reported incidences of bleeding during antithrombotic and antiplatelet therapy for non-ST-elevation acute coronary syndromes depend on how bleeding was defined, how the acute coronary syndromes were treated, and on other factors such as how the study was designed.
How was bleeding defined?
Since these classification schemes are based on different types of data, they yield different numbers when applied to the same study population. For instance, Rao et al4 pooled the data from the PURSUIT and PARAGON B trials (15,454 patients in all) and found that the incidence of severe bleeding (by the GUSTO criteria) was 1.2%, while the rate of major bleeding (by the TIMI criteria) was 8.2%.
What was the treatment strategy?
Another reason that the true incidence of bleeding is hard to determine is that different studies used treatment strategies that differed in the type, timing, and dose of antithrombotic agents and whether invasive procedures were used early. For example, if unfractionated heparin is used aggressively in regimens that are not adjusted for weight and with a higher target for the activated clotting time, the risk of bleeding is higher than with conservative dosing.5–7
Subherwal et al8 evaluated the effect of treatment strategy on the incidence of bleeding in patients with non-ST-elevation acute coronary syndromes who received two or more antithrombotic drugs in the CRUSADE Quality Improvement Initiative. The risk of bleeding was higher with an invasive approach (catheterization) than with a conservative approach (no catheterization), regardless of baseline bleeding risk.
What type of study was it?
Another source of variation is the design of the study. Registries differ from clinical trials in patient characteristics and in the way data are gathered (prospectively vs retrospectively).
In registries, data are often collected retrospectively, whereas in clinical trials the data are prospectively collected. For this reason, the definition of bleeding in registries is often based on events that are easily identified through chart review, such as transfusion. This may lead to a lower reported rate of bleeding, since other, less serious bleeding events such as access-site hematomas and epistaxis may not be documented in the medical record.
On the other hand, registries often include older and sicker patients, who may be more prone to bleeding and who are often excluded from clinical trials. This may lead to a higher rate of reported bleeding.9
Where the study was conducted makes a difference as well, owing to regional practice differences. For example, Moscucci et al10 reported that the incidence of major bleeding in 24,045 patients with non-ST-elevation acute coronary syndromes in the GRACE registry (in 14 countries worldwide) was 3.9%. In contrast, Yang et al11 reported that the rate of bleeding in the CRUSADE registry (in the United States) was 10.3%.
This difference was partly influenced by different definitions of bleeding. The GRACE registry defined major bleeding as life-threatening events requiring transfusion of two or more units of packed red blood cells, or resulting in an absolute decrease in the hematocrit of 10% or more or death, or hemorrhagic subdural hematoma. In contrast, the CRUSADE data reflect bleeding requiring transfusion. However, practice patterns such as greater use of invasive procedures in the United States may also be responsible.
Rao and colleagues12 examined international variation in blood transfusion rates among patients with acute coronary syndromes. Patients outside the United States were significantly less likely to receive transfusions, even after adjusting for patient and practice differences.
Taking these confounders into account, it is reasonable to estimate that the frequency of bleeding in patients with non-ST-elevation acute coronary syndromes ranges from less than 1% to 10%.13

BLEEDING IS ASSOCIATED WITH POOR OUTCOMES
Regardless of the definition or the data source, hemorrhagic complications are associated with a higher risk of death and nonfatal adverse events, both in the short term and in the long term.
Short-term outcomes
A higher risk of death. In the GRACE registry study by Moscucci et al10 discussed above, patients who had major bleeding were significantly more likely to die during their hospitalization than those who did not (odds ratio [OR] 1.64, 95% confidence interval [CI] 1.18–2.28).
Rao et al14 evaluated pooled data from the multicenter international GUSTO IIb, PURSUIT, and PARAGON A and B trials and found that the effects of bleeding in non-ST-elevation acute coronary syndromes extended beyond the hospital stay. The more severe the bleeding (by the GUSTO criteria), the greater the adjusted hazard ratio (HR) for death within 30 days:
- With mild bleeding—HR 1.6, 95% CI 1.3–1.9
- With moderate bleeding—HR 2.7, 95% CI 2.3–3.4
- With severe bleeding—HR 10.6, 95% CI 8.3–13.6.
The pattern was the same for death within 6 months:
- With mild bleeding—HR 1.4, 95% CI 1.2–1.6
- With moderate bleeding—HR 2.1, 95% CI 1.8–2.4
- With severe bleeding, HR 7.5, 95% CI 6.1–9.3.
These findings were confirmed by Eikelboom et al15 in 34,146 patients with acute coronary syndromes in the OASIS registry, the OASIS-2 trial, and the CURE randomized trial. In the first 30 days, five times as many patients died (12.8% vs 2.5%; P < .0009) among those who developed major bleeding compared with those who did not. These investigators defined major bleeding as bleeding that was life-threatening or significantly disabling or that required transfusion of two or more units of packed red blood cells.
A higher risk of nonfatal adverse events. Bleeding after antithrombotic therapy for non-ST-elevation acute coronary syndromes has also been associated with nonfatal adverse events such as stroke and stent thrombosis.
For example, in the study by Eikelboom et al,15 major bleeding was associated with a higher risk of recurrent ischemic events. Approximately 1 in 5 patients in the OASIS trials who developed major bleeding during the first 30 days died or had a myocardial infarction or stroke by 30 days, compared with 1 in 20 of those who did not develop major bleeding during the first 30 days. However, after events that occurred during the first 30 days were excluded, the association between major bleeding and both myocardial infarction and stroke was no longer evident between 30 days and 6 months.
Manoukian et al16 evaluated the impact of major bleeding in 13,819 patients with highrisk acute coronary syndromes undergoing treatment with an early invasive strategy in the ACUITY trial. At 30 days, patients with major bleeding had higher rates of the composite end point of death, myocardial infarction, or unplanned revascularization for ischemia (23.1% vs 6.8%, P < .0001) and of stent thrombosis (3.4% vs 0.6%, P < .0001).
Long-term outcomes
The association between bleeding and adverse outcomes persists in the long term as well, although the mechanisms underlying this association are not well studied.
Kinnaird et al17 examined the data from 10,974 unselected patients who underwent percutaneous coronary intervention. At 1 year, the following percentages of patients had died:
- After TIMI major bleeding—17.2%
- After TIMI minor bleeding—9.1%
- After no bleeding—5.5%.
However, after adjustment for potential confounders, only transfusion remained a significant predictor of 1-year mortality.
Mehran et al18 evaluated 1-year mortality data from the ACUITY trial. Compared with the rate in patients who had no major bleeding and no myocardial infarction, the hazard ratios for death were:
- After major bleeding—HR 3.5, 95% CI 2.7–4.4
- After myocardial infarction—HR 3.1, 95% CI 2.4–3.9.
Interestingly, the risk of death associated with myocardial infarction abated after 7 days, while the risk associated with bleeding persisted beyond 30 days and remained constant throughout the first year following the bleeding event.
Similarly, Ndrepepa and colleagues19 examined pooled data from four ISAR trials using the TIMI bleeding scale and found that myocardial infarction, target vessel revascularization, and major bleeding all had similar discriminatory ability at predicting 1-year mortality.
In patients undergoing elective or urgent percutaneous coronary intervention in the REPLACE-2 trial,20 independent predictors of death by 1 year were21:
- Major hemorrhage (OR 2.66, 95% CI 1.44–4.92)
- Periprocedural myocardial infarction (OR 2.46, 95% CI 1.44–4.20).
THEORIES OF HOW BLEEDING MAY CAUSE ADVERSE OUTCOMES
Several mechanisms have been proposed to explain the association between bleeding during treatment for acute coronary syndromes and adverse clinical outcomes.13,22
The immediate effects of bleeding are thought to be hypotension and a reflex hyperadrenergic state to compensate for the loss of intravascular volume.23 This physiologic response is believed to contribute to myocardial ischemia by further decreasing myocardial oxygen supply in obstructive coronary disease.
Trying to minimize blood loss, physicians may withhold anticoagulation and antiplatelet therapy, which in turn may lead to further ischemia.24 To compensate for blood loss, physicians may also resort to blood transfusion. However, depletion of 2,3-diphosphoglycerate and nitric oxide in stored donor red blood cells is postulated to reduce oxygen delivery by increasing hemoglobin’s affinity for oxygen, leading to induced microvascular obstruction and adverse inflammatory reactions.15,25
Recent data have also begun to elucidate the long-term effects of bleeding during acute coronary syndrome management. Patients with anemia during the acute phase of infarction have greater neurohormonal activation.26 These adaptive responses to anemia may lead to eccentric left ventricular remodeling that may lead to higher oxygen consumption, increased diastolic wall stress, interstitial fibrosis, and accelerated myocyte loss.27–30
Nevertheless, we must point out that although strong associations between bleeding and adverse outcomes have been established, direct causality has not.
TO PREVENT BLEEDING, START BY ASSESSING RISK
The CRUSADE bleeding risk score
The CRUSADE bleeding score (calculator available at http://www.crusadebleedingscore.org/) was developed and validated in more than 89,000 community-treated patients with non-ST-elevation acute coronary syndromes.8 It is based on eight variables:
- Sex (higher risk in women)
- History of diabetes (higher risk)
- Prior vascular disease (higher risk)
- Heart rate (the higher the rate, the higher the risk)
- Systolic blood pressure (higher risk with pressures above or below the 121–180 mm Hg range)
- Signs of congestive heart failure (higher risk)
- Baseline hematocrit (the lower the hematocrit, the higher the risk)
- Creatinine clearance (by the Cockcroft-Gault formula; the lower the creatinine clearance, the higher the risk).
Patients who are found to have bleeding scores suggesting a moderate or higher risk of bleeding should be considered for medications associated with a favorable bleeding profile, and for radial access at the time of coronary angiography. Scores are graded as follows8:
- < 21: Very low risk
- 21–30: Low risk
- 31–40: Moderate risk
- 41–50: High risk
- > 50: Very high risk.
The CRUSADE bleeding score is unique in that, unlike earlier risk stratification tools, it was developed in a “real world” population, not in subgroups or in a clinical trial. It can be calculated at baseline to help guide the selection of treatment.8
Adjusting the heparin regimen in patients at risk of bleeding
Both the joint American College of Cardiology/American Heart Association1 and the European Society of Cardiology guidelines31 for the treatment of non-ST-elevation acute coronary syndromes recommend taking steps to prevent bleeding, such as adjusting the dosage of unfractionated heparin, using safer drugs, reducing the duration of antithrombotic treatment, and using combinations of antithrombotic and antiplatelet agents according to proven indications.31
In the CRUSADE registry, 42% of patients with non-ST-elevation acute coronary syndromes received at least one initial dose of antithrombotic drug outside the recommended range, resulting in an estimated 15% excess of bleeding events.32 Thus, proper dosing is a target for prevention.
Appropriate antithrombotic dosing takes into account the patient’s age, weight, and renal function. However, heparin dosage in the current guidelines1 is based on weight only: a loading dose of 60 U/kg (maximum 4,000 U) by intravenous bolus, then 12 U/kg/hour (maximum 1,000 U/hour) to maintain an activated partial thromboplastin time of 50 to 70 seconds.1
Renal dysfunction is particularly worrisome in patients with non-ST-elevation acute coronary syndromes because it is associated with higher rates of major bleeding and death. In the OASIS-5 trial,33 the overall risk of death was approximately five times higher in patients in the lowest quartile of renal function (glomerular filtration rate < 58 mL/min/1.73 m2) than in the highest quartile (glomerular filtration rate ≥ 86 mL/min/1.73 m2).
Renal function must be evaluated not only on admission but also throughout the hospital stay. Patients presenting with acute coronary syndromes often experience fluctuations in renal function that would call for adjustment of heparin dosing, either increasing the dose to maximize the drug’s efficacy if renal function is recovering or decreasing the dose to prevent bleeding if renal function is deteriorating.
Clopidogrel vs prasugrel
Certain medications should be avoided when the risk of bleeding outweighs any potential benefit in terms of ischemia.
For example, in a randomized trial,34 prasugrel (Effient), a potent thienopyridine, was associated with a significantly lower rate of the composite end point of stroke, myocardial infarction, or death than clopidogrel (Plavix) in patients with acute coronary syndromes undergoing percutaneous coronary interventions. However, it did not seem to offer any advantage in patients 75 years old and older, those with prior stroke or transient ischemic attack, or those weighing less than 60 kg, and it posed a substantially higher risk of bleeding.
With clopidogrel, the risk of acute bleeding is primarily in patients who undergo coronary artery bypass grafting within 5 days of receiving a dose.35,36 Therefore, clopidogrel should be stopped 5 to 7 days before bypass surgery.1 Importantly, there is no increased risk of recurrent ischemic events during this 5-day waiting period in patients who receive clopidogrel early. Therefore, the recommendation to stop clopidogrel before surgery does not negate the benefits of early treatment.36
Lower-risk drugs: Fondaparinux and bivalirudin
At this time, only two agents have been studied in clinical trials that have specifically focused on reducing bleeding risk: fondaparinux (Arixtra) and bivalirudin (Angiomax).20,37–39
Fondaparinux
OASIS-5 was a randomized, double-blind trial that compared fondaparinux and enoxaparin (Lovenox) in patients with acute coronary syndromes.38 Fondaparinux was similar to enoxaparin in terms of the combined end point of death, myocardial infarction, or refractory ischemia at 9 days, and fewer patients on fondaparinux developed bleeding (2.2% vs 4.1%, HR 0.52; 95% CI 0.44–0.61). This difference persisted during long-term follow-up.
Importantly, fewer patients died in the fondaparinux group. At 180 days, 638 (6.5%) of the patients in the enoxaparin group had died, compared with 574 (5.8%) in the fondaparinux group, a difference of 64 deaths (P = .05). The authors found that 41 fewer patients in the fondaparinux group than in the enoxaparin group died after major bleeding, and 20 fewer patients in the fondaparinux group died after minor bleeding.38 Thus, most of the difference in mortality rates between the two groups was attributed to a lower incidence of bleeding with fondaparinux.
Unfortunately, despite its safe bleeding profile, fondaparinux has fallen out of favor for use in acute coronary syndromes, owing to a higher risk of catheter thrombosis in the fondaparinux group (0.9%) than in those undergoing percutaneous coronary interventions with enoxaparin alone (0.4%) in the OASIS-5 trial.40
Bivalirudin
The direct thrombin inhibitor bivalirudin has been studied in three large randomized trials in patients undergoing percutaneous coronary interventions.20,37,41
The ACUITY trial37 was a prospective, open-label, randomized, multicenter trial that compared three regimens in patients with moderate or high-risk non-ST-elevation acute coronary syndromes:
- Heparin plus a glycoprotein IIb/IIIa inhibitor
- Bivalirudin plus a glycoprotein IIb/IIIa inhibitor
- Bivalirudin alone.
Bivalirudin alone was as effective as heparin plus a glycoprotein IIb/IIIa inhibitor with respect to the composite ischemia end point, which at 30 days had occurred in 7.8% vs 7.3% of the patients in these treatment groups (P = .32, RR 1.08; 95% CI 0.93–1.24), and it was superior with respect to major bleeding (3.0% vs 5.7%, P < .001, RR 0.53; 95% CI 0.43–0.65).
The HORIZONS-AMI study41 was a prospective, open-label, randomized, multicenter trial that compared bivalirudin alone vs heparin plus a glycoprotein IIb/IIIa inhibitor in patients with ST-elevation acute coronary syndromes who were undergoing primary percutaneous coronary interventions. The two primary end points were major bleeding and net adverse events.
At 1 year, patients assigned to bivalirudin had a lower rate of major bleeding than did controls (5.8% vs 9.2%, HR 0.61, 95% CI 0.48–0.78, P < .0001), with similar rates of major adverse cardiac events in both groups (11.9% vs 11.9%, HR 1.00, 95% CI 0.82– 1.21, P = .98).41
Both OASIS 5 and HORIZONS-AMI are examples of clinical trials in which strategies that reduced bleeding risk were also associated with improved survival.
For cardiac catheterization, inserting the catheter in the wrist poses less risk
Bleeding is currently the most common noncardiac complication in patients undergoing percutaneous coronary interventions, and it most often occurs at the vascular access site.17
Rao et al12 evaluated data from 593,094 procedures in the National Cardiovascular Data Registry and found that, compared with the femoral approach, the use of transradial percutaneous coronary intervention was associated with a similar rate of procedural success (OR 1.02, 95% CI 0.93–1.12) but a significantly lower risk of bleeding complications (OR 0.42, 95% CI 0.31–0.56) after multivariable adjustment.
The use of smaller sheath sizes (4F–6F) and preferential use of bivalirudin over unfractionated heparin and glycoprotein IIb/IIIa inhibitor therapy are other methods described to decrease the risk of bleeding after percutaneous coronary interventions.20,41–49
IF BLEEDING OCCURS
Once a bleeding complication occurs, cessation of therapy is a potential option. Stopping or reversing antithrombotic and antiplatelet therapy is warranted in the event of major bleeding (eg, gastrointestinal, retroperitoneal, intracranial).31
Stopping antithrombotic and antiplatelet therapy
Whether bleeding is minor or major, the risk of a recurrent thrombotic event must be considered, especially in patients who have undergone revascularization, stent implantation, or both. The risk of acute thrombotic events after interrupting antithrombotic or antiplatelet agents is considered greatest 4 to 5 days following revascularization or percutaneous coronary intervention.15 If bleeding can be controlled with local treatment such as pressure, packing, or dressing, antithrombotic and antiplatelet therapy need not be interrupted.50
Current guidelines recommend strict control of hemorrhage for at least 24 hours before reintroducing antiplatelet or antithrombotic agents.
It is also important to remember that in the setting of gastrointestinal bleeding due to peptic ulcer disease, adjunctive proton pump inhibitors are recommended after restarting antiplatelet or antithrombotic therapy or both.
Importantly, evidence-based antithrombotic medications (especially dual antiplatelet therapy) should be restarted once the acute bleeding event has resolved.31
Reversal of anticoagulant and antiplatelet therapies
Unfractionated heparin is reversed with infusion of protamine sulfate at a dose of 1 mg per 100 U of unfractionated heparin given over the previous 4 hours.51,52 The rate of protamine sulfate infusion should be less than 100 mg over 2 hours, with 50% of the dose given initially and subsequent doses titrated according to bleeding response.52,53 Protamine sulfate is associated with a risk of hypotension and bradycardia, and for this reason it should be given no faster than 5 mg/min.
Low-molecular-weight heparin (LMWH) can be inhibited by 1 mg of protamine sulfate for each 1 mg of LMWH given over the previous 4 hours.51,52
However, protamine sulfate only partially neutralizes the anticoagulant effect of LMWH. In cases in which protamine sulfate is unsuccessful in abating bleeding associated with LMWH use, guidelines allow for the use of recombinant factor VIIa (NovoSeven).31 In healthy volunteers given fondaparinux, recombinant factor VIIa normalized coagulation times and thrombin generation within 1.5 hours, with a sustained effect for 6 hours.52
It is important to note that the use of this agent has not been fully studied, it is very costly (a single dose of 40 μg/kg costs from $3,000 to $4,000), and it is linked to reports of increased risk of thrombotic complications.54,55
Antiplatelet agents are more complex to reverse. The antiplatelet actions of aspirin and clopidogrel wear off as new platelets are produced. Approximately 10% of a patient’s platelet count is produced daily; thus, the antiplatelet effects of aspirin and clopidogrel can persist for 5 to 10 days.31,56
If these agents need to be reversed quickly to stop bleeding, according to expert consensus the aspirin effect can be reversed by transfusion of one unit of platelets. The antiplatelet effect of clopidogrel is more significant than that of aspirin; thus, two units of platelets are recommended.56
Glycoprotein IIb/IIIa inhibitors. If a major bleeding event requires the reversal of glycoprotein IIb/IIIa inhibitor therapy, the treatment must take into consideration the pharmacodynamics of the target drug. Both eptifibatide (Integrilin) and tirofiban (Aggrastat) competitively inhibit glycoprotein IIb/IIIa receptors; thus, their effects depend on dosing, elimination, and time. Due to the stoichiometry of both drugs, transfusion of platelets is ineffective. Both eptifibatide and tirofiban are eliminated by the kidney; thus, normal renal function is key to the amount of time it takes for platelet function to return to baseline.57 Evidence suggests that fibrinogen-rich plasma can be administered to restore platelet function.31,58,59
Abciximab (ReoPro). Whereas reversal of eptifibatide and tirofiban focuses on overcoming competitive inhibition, neutralization of abciximab involves overcoming its high receptor affinity. At 24 hours after abciximab infusion is stopped, platelet aggregation may still be inhibited by up to 50%. Fortunately, owing to abciximab’s short plasma half-life and its dilution in serum, platelet transfusion is effective in reversing its antiplatelet effects.31,57
Blood transfusion
Long considered beneficial to critically ill patients, blood transfusion to maintain hematocrit levels during acute coronary syndromes has come under intense scrutiny. Randomized trials have shown that transfusion should not be given aggressively to critically ill patients.60 In acute coronary syndromes, there are only observational data.
Rao et al61 used detailed clinical data from 24,112 patients with acute coronary syndromes in the GUSTO IIb, PURSUIT, and PARAGON B trials to determine the association between blood transfusion and outcomes in patients who developed moderate to severe bleeding, anemia, or both during their hospitalization. The rates of death in the hospital and at 30 days were significantly higher in patients who received a transfusion (30-day mortality HR 3.94; 95% CI 3.36–4.75). However, there was no significant association between transfusion and the 30-day mortality rate if the nadir hematocrit was 25% or less.
Of note: no randomized clinical trial has evaluated transfusion strategies in acute coronary syndromes at this time. Until such data are available, it is reasonable to follow published guidelines and to avoid transfusion in stable patients with ischemic heart disease unless the hematocrit is 25% or less.31 The risks and benefits of blood transfusion should be carefully weighed. Routine use of transfusion to maintain predefined hemoglobin levels is not recommended in stable patients.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329:673–682.
- Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 1987; 76:142–154.
- Rao SV, O’Grady K, Pieper KS, et al. A comparison of the clinical impact of bleeding measured by two different classifications among patients with acute coronary syndromes. J Am Coll Cardiol 2006; 47:809–816.
- Granger CB, Hirsch J, Califf RM, et al. Activated partial thromboplastin time and outcome after thrombolytic therapy for acute myocardial infarction: results from the GUSTO-I trial. Circulation 1996; 93:870–878.
- Gilchrist IC, Berkowitz SD, Thompson TD, Califf RM, Granger CB. Heparin dosing and outcome in acute coronary syndromes: the GUSTO-IIb experience. Global Use of Strategies to Open Occluded Coronary Arteries. Am Heart J 2002; 144:73–80.
- Tolleson TR, O’Shea JC, Bittl JA, et al. Relationship between heparin anticoagulation and clinical outcomes in coronary stent intervention: observations from the ESPRIT trial. J Am Coll Cardiol 2003; 41:386–393.
- Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 2009; 119:1873–1882.
- Bassand JP. Bleeding and transfusion in acute coronary syndromes: a shift in the paradigm. Heart 2008; 94:661–666.
- Moscucci M, Fox KA, Cannon CP, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J 2003; 24:1815–1823.
- Yang X, Alexander KP, Chen AY, et al; CRUSADE Investigators. The implications of blood transfusions for patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE National Quality Improvement Initiative. J Am Coll Cardiol 2005; 46:1490–1495.
- Rao SV, Ou FS, Wang TY, et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv 2008; 1:379–386.
- Rao SV, Eikelboom JA, Granger CB, Harrington RA, Califf RM, Bassand JP. Bleeding and blood transfusion issues in patients with non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1193–1204.
- Rao SV, O’Grady K, Pieper KS, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol 2005; 96:1200–1206.
- Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006; 114:774–782.
- Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 2007; 49:1362–1368.
- Kinnaird TD, Stabile E, Mintz GS, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol 2003; 92:930–935.
- Mehran R, Pocock SJ, Stone GW, et al. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes: a risk model from the ACUITY trial. Eur Heart J 2009; 30:1457–1466.
- Ndrepepa G, Berger PB, Mehilli J, et al. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol 2008; 51:690–697.
- Lincoff AM, Bittl JA, Harrington RA, et al; REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003; 289:853–863.
- Feit F, Voeltz MD, Attubato MJ, et al. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. Am J Cardiol 2007; 100:1364–1369.
- Fitchett D. The impact of bleeding in patients with acute coronary syndromes: how to optimize the benefits of treatment and minimize the risk. Can J Cardiol 2007; 23:663–671.
- Bassand JP. Impact of anaemia, bleeding, and transfusions in acute coronary syndromes: a shift in the paradigm. Eur Heart J 2007; 28:1273–1274.
- Yan AT, Yan RT, Huynh T, et al; INTERACT Investigators. Bleeding and outcome in acute coronary syndrome: insights from continuous electrocardiogram monitoring in the Integrilin and Enoxaparin Randomized Assessment of Acute Coronary Syndrome Treatment (INTERACT) Trial. Am Heart J 2008; 156:769–775.
- Jolicoeur EM, O’Neill WW, Hellkamp A, et al; APEX-AMI Investigators. Transfusion and mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur Heart J 2009; 30:2575–2583.
- Gehi A, Ix J, Shlipak M, Pipkin SS, Whooley MA. Relation of anemia to low heart rate variability in patients with coronary heart disease (from the Heart and Soul study). Am J Cardiol 2005; 95:1474–1477.
- Anand I, McMurray JJ, Whitmore J, et al. Anemia and its relationship to clinical outcome in heart failure. Circulation 2004; 110:149–154.
- O’Riordan E, Foley RN. Effects of anaemia on cardiovascular status. Nephrol Dial Transplant 2000; 15(suppl 3):19–22.
- Olivetti G, Quaini F, Lagrasta C, et al. Myocyte cellular hypertrophy and hyperplasia contribute to ventricular wall remodeling in anemia-induced cardiac hypertrophy in rats. Am J Pathol 1992; 141:227–239.
- Aronson D, Suleiman M, Agmon Y, et al. Changes in haemoglobin levels during hospital course and long-term outcome after acute myocardial infarction. Eur Heart J 2007; 28:1289–1296.
- Task Force for Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of European Society of Cardiology; Bassand JP, Hamm CW, Ardissino D, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1598–1660.
- Alexander KP, Chen AY, Roe MT, et al; CRUSADE Investigators. Excess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromes. JAMA 2005; 294:3108–3116.
- Fox KA, Bassand JP, Mehta SR, et al; OASIS 5 Investigators. Influence of renal function on the efficacy and safety of fondaparinux relative to enoxaparin in non ST-segment elevation acute coronary syndromes. Ann Intern Med 2007; 147:304–310.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Berger JS, Frye CB, Harshaw Q, Edwards FH, Steinhubl SR, Becker RC. Impact of clopidogrel in patients with acute coronary syndromes requiring coronary artery bypass surgery: a multicenter analysis. J Am Coll Cardiol 2008; 52:1693–1701.
- Fox KA, Mehta SR, Peters R, et al; Clopidogrel in Unstable angina to prevent Recurrent ischemic Events Trial. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- Stone GW, McLaurin BT, Cox DA, et al; ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med 2006; 355:2203–2216.
- Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators; Yusuf S, Mehta SR, Chrolavicius S, et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med 2006; 354:1464–1476.
- Potsis TZ, Katsouras C, Goudevenos JA. Avoiding and managing bleeding complications in patients with non-ST-segment elevation acute coronary syndromes. Angiology 2009; 60:148–158.
- Mehta SR, Granger CB, Eikelboom JW, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol 2007; 50:1742–1751.
- Mehran R, Lansky AJ, Witzenbichler B, et al; HORIZONS-AMI Trial Investigators. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet 2009; 374:1149–1159.
- Stone GW, Ware JH, Bertrand ME, et al; ACUITY Investigators. Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one-year results from the ACUITY trial. JAMA 2007; 298:2497–2506.
- Cantor WJ, Mahaffey KW, Huang Z, et al. Bleeding complications in patients with acute coronary syndrome undergoing early invasive management can be reduced with radial access, smaller sheath sizes, and timely sheath removal. Catheter Cardiovasc Interv 2007; 69:73–83.
- Büchler JR, Ribeiro EE, Falcão JL, et al. A randomized trial of 5 versus 7 French guiding catheters for transfemoral percutaneous coronary stent implantation. J Interv Cardiol 2008; 21:50–55.
- Shammas NW, Allie D, Hall P, et al; APPROVE Investigators. Predictors of in-hospital and 30-day complications of peripheral vascular interventions using bivalirudin as the primary anticoagulant: results from the APPROVE Registry. J Invasive Cardiol 2005; 17:356–359.
- Doyle BJ, Ting HH, Bell MR, et al. Major femoral bleeding complications after percutaneous coronary intervention: incidence, predictors, and impact on long-term survival among 17,901 patients treated at the Mayo Clinic from 1994 to 2005. JACC Cardiovasc Interv 2008; 1:202–209.
- Stone GW, White HD, Ohman EM, et al; Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial investigators. Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial. Lancet 2007; 369:907–919.
- Stone GW, Bertrand ME, Moses JW, et al; ACUITY Investigators. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA 2007; 297:591–602.
- Lincoff AM, Bittl JA, Kleiman NS, et al; REPLACE-1 Investigators. Comparison of bivalirudin versus heparin during percutaneous coronary intervention (the Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events [REPLACE]-1 trial). Am J Cardiol 2004; 93:1092–1096.
- Barkun A, Bardou M, Marshall JK; Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2003; 139:843–857.
- Warkentin TE, Crowther MA. Reversing anticoagulants both old and new. Can J Anaesth 2002; 49:S11–S25.
- Crowther MA, Warkentin TE. Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: focus on new anticoagulant agents. Blood 2008; 111:4871–4879.
- Kessler CM. Current and future challenges of antithrombotic agents and anticoagulants: strategies for reversal of hemorrhagic complications. Semin Hematol 2004; 41(suppl 1):44–50.
- Ganguly S, Spengel K, Tilzer LL, O’Neal B, Simpson SQ. Recombinant factor VIIa: unregulated continuous use in patients with bleeding and coagulopathy does not alter mortality and outcome. Clin Lab Haematol 2006; 28:309–312.
- O’Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA 2006; 295:293–298.
- Beshay JE, Morgan H, Madden C, Yu W, Sarode R. Emergency reversal of anticoagulation and antiplatelet therapies in neurosurgical patients. J Neurosurg 2010; 112:307–318.
- Tcheng JE. Clinical challenges of platelet glycoprotein IIb/IIIa receptor inhibitor therapy: bleeding, reversal, thrombocytopenia, and retreatment. Am Heart J 2000; 139:S38–S45.
- Li YF, Spencer FA, Becker RC. Comparative efficacy of fibrinogen and platelet supplementation on the in vitro reversibility of competitive glycoprotein IIb/IIIa receptor-directed platelet inhibition. Am Heart J 2002; 143:725–732.
- Schroeder WS, Gandhi PJ. Emergency management of hemorrhagic complications in the era of glycoprotein IIb/IIIa receptor antagonists, clopidogrel, low molecular weight heparin, and third-generation fibrinolytic agents. Curr Cardiol Rep 2003; 5:310–317.
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340:409–417.
- Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 2004; 292:1555–1562.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329:673–682.
- Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 1987; 76:142–154.
- Rao SV, O’Grady K, Pieper KS, et al. A comparison of the clinical impact of bleeding measured by two different classifications among patients with acute coronary syndromes. J Am Coll Cardiol 2006; 47:809–816.
- Granger CB, Hirsch J, Califf RM, et al. Activated partial thromboplastin time and outcome after thrombolytic therapy for acute myocardial infarction: results from the GUSTO-I trial. Circulation 1996; 93:870–878.
- Gilchrist IC, Berkowitz SD, Thompson TD, Califf RM, Granger CB. Heparin dosing and outcome in acute coronary syndromes: the GUSTO-IIb experience. Global Use of Strategies to Open Occluded Coronary Arteries. Am Heart J 2002; 144:73–80.
- Tolleson TR, O’Shea JC, Bittl JA, et al. Relationship between heparin anticoagulation and clinical outcomes in coronary stent intervention: observations from the ESPRIT trial. J Am Coll Cardiol 2003; 41:386–393.
- Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 2009; 119:1873–1882.
- Bassand JP. Bleeding and transfusion in acute coronary syndromes: a shift in the paradigm. Heart 2008; 94:661–666.
- Moscucci M, Fox KA, Cannon CP, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J 2003; 24:1815–1823.
- Yang X, Alexander KP, Chen AY, et al; CRUSADE Investigators. The implications of blood transfusions for patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE National Quality Improvement Initiative. J Am Coll Cardiol 2005; 46:1490–1495.
- Rao SV, Ou FS, Wang TY, et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv 2008; 1:379–386.
- Rao SV, Eikelboom JA, Granger CB, Harrington RA, Califf RM, Bassand JP. Bleeding and blood transfusion issues in patients with non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1193–1204.
- Rao SV, O’Grady K, Pieper KS, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol 2005; 96:1200–1206.
- Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006; 114:774–782.
- Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 2007; 49:1362–1368.
- Kinnaird TD, Stabile E, Mintz GS, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol 2003; 92:930–935.
- Mehran R, Pocock SJ, Stone GW, et al. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes: a risk model from the ACUITY trial. Eur Heart J 2009; 30:1457–1466.
- Ndrepepa G, Berger PB, Mehilli J, et al. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol 2008; 51:690–697.
- Lincoff AM, Bittl JA, Harrington RA, et al; REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003; 289:853–863.
- Feit F, Voeltz MD, Attubato MJ, et al. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. Am J Cardiol 2007; 100:1364–1369.
- Fitchett D. The impact of bleeding in patients with acute coronary syndromes: how to optimize the benefits of treatment and minimize the risk. Can J Cardiol 2007; 23:663–671.
- Bassand JP. Impact of anaemia, bleeding, and transfusions in acute coronary syndromes: a shift in the paradigm. Eur Heart J 2007; 28:1273–1274.
- Yan AT, Yan RT, Huynh T, et al; INTERACT Investigators. Bleeding and outcome in acute coronary syndrome: insights from continuous electrocardiogram monitoring in the Integrilin and Enoxaparin Randomized Assessment of Acute Coronary Syndrome Treatment (INTERACT) Trial. Am Heart J 2008; 156:769–775.
- Jolicoeur EM, O’Neill WW, Hellkamp A, et al; APEX-AMI Investigators. Transfusion and mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur Heart J 2009; 30:2575–2583.
- Gehi A, Ix J, Shlipak M, Pipkin SS, Whooley MA. Relation of anemia to low heart rate variability in patients with coronary heart disease (from the Heart and Soul study). Am J Cardiol 2005; 95:1474–1477.
- Anand I, McMurray JJ, Whitmore J, et al. Anemia and its relationship to clinical outcome in heart failure. Circulation 2004; 110:149–154.
- O’Riordan E, Foley RN. Effects of anaemia on cardiovascular status. Nephrol Dial Transplant 2000; 15(suppl 3):19–22.
- Olivetti G, Quaini F, Lagrasta C, et al. Myocyte cellular hypertrophy and hyperplasia contribute to ventricular wall remodeling in anemia-induced cardiac hypertrophy in rats. Am J Pathol 1992; 141:227–239.
- Aronson D, Suleiman M, Agmon Y, et al. Changes in haemoglobin levels during hospital course and long-term outcome after acute myocardial infarction. Eur Heart J 2007; 28:1289–1296.
- Task Force for Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of European Society of Cardiology; Bassand JP, Hamm CW, Ardissino D, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1598–1660.
- Alexander KP, Chen AY, Roe MT, et al; CRUSADE Investigators. Excess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromes. JAMA 2005; 294:3108–3116.
- Fox KA, Bassand JP, Mehta SR, et al; OASIS 5 Investigators. Influence of renal function on the efficacy and safety of fondaparinux relative to enoxaparin in non ST-segment elevation acute coronary syndromes. Ann Intern Med 2007; 147:304–310.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Berger JS, Frye CB, Harshaw Q, Edwards FH, Steinhubl SR, Becker RC. Impact of clopidogrel in patients with acute coronary syndromes requiring coronary artery bypass surgery: a multicenter analysis. J Am Coll Cardiol 2008; 52:1693–1701.
- Fox KA, Mehta SR, Peters R, et al; Clopidogrel in Unstable angina to prevent Recurrent ischemic Events Trial. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- Stone GW, McLaurin BT, Cox DA, et al; ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med 2006; 355:2203–2216.
- Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators; Yusuf S, Mehta SR, Chrolavicius S, et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med 2006; 354:1464–1476.
- Potsis TZ, Katsouras C, Goudevenos JA. Avoiding and managing bleeding complications in patients with non-ST-segment elevation acute coronary syndromes. Angiology 2009; 60:148–158.
- Mehta SR, Granger CB, Eikelboom JW, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol 2007; 50:1742–1751.
- Mehran R, Lansky AJ, Witzenbichler B, et al; HORIZONS-AMI Trial Investigators. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet 2009; 374:1149–1159.
- Stone GW, Ware JH, Bertrand ME, et al; ACUITY Investigators. Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one-year results from the ACUITY trial. JAMA 2007; 298:2497–2506.
- Cantor WJ, Mahaffey KW, Huang Z, et al. Bleeding complications in patients with acute coronary syndrome undergoing early invasive management can be reduced with radial access, smaller sheath sizes, and timely sheath removal. Catheter Cardiovasc Interv 2007; 69:73–83.
- Büchler JR, Ribeiro EE, Falcão JL, et al. A randomized trial of 5 versus 7 French guiding catheters for transfemoral percutaneous coronary stent implantation. J Interv Cardiol 2008; 21:50–55.
- Shammas NW, Allie D, Hall P, et al; APPROVE Investigators. Predictors of in-hospital and 30-day complications of peripheral vascular interventions using bivalirudin as the primary anticoagulant: results from the APPROVE Registry. J Invasive Cardiol 2005; 17:356–359.
- Doyle BJ, Ting HH, Bell MR, et al. Major femoral bleeding complications after percutaneous coronary intervention: incidence, predictors, and impact on long-term survival among 17,901 patients treated at the Mayo Clinic from 1994 to 2005. JACC Cardiovasc Interv 2008; 1:202–209.
- Stone GW, White HD, Ohman EM, et al; Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial investigators. Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial. Lancet 2007; 369:907–919.
- Stone GW, Bertrand ME, Moses JW, et al; ACUITY Investigators. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA 2007; 297:591–602.
- Lincoff AM, Bittl JA, Kleiman NS, et al; REPLACE-1 Investigators. Comparison of bivalirudin versus heparin during percutaneous coronary intervention (the Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events [REPLACE]-1 trial). Am J Cardiol 2004; 93:1092–1096.
- Barkun A, Bardou M, Marshall JK; Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2003; 139:843–857.
- Warkentin TE, Crowther MA. Reversing anticoagulants both old and new. Can J Anaesth 2002; 49:S11–S25.
- Crowther MA, Warkentin TE. Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: focus on new anticoagulant agents. Blood 2008; 111:4871–4879.
- Kessler CM. Current and future challenges of antithrombotic agents and anticoagulants: strategies for reversal of hemorrhagic complications. Semin Hematol 2004; 41(suppl 1):44–50.
- Ganguly S, Spengel K, Tilzer LL, O’Neal B, Simpson SQ. Recombinant factor VIIa: unregulated continuous use in patients with bleeding and coagulopathy does not alter mortality and outcome. Clin Lab Haematol 2006; 28:309–312.
- O’Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA 2006; 295:293–298.
- Beshay JE, Morgan H, Madden C, Yu W, Sarode R. Emergency reversal of anticoagulation and antiplatelet therapies in neurosurgical patients. J Neurosurg 2010; 112:307–318.
- Tcheng JE. Clinical challenges of platelet glycoprotein IIb/IIIa receptor inhibitor therapy: bleeding, reversal, thrombocytopenia, and retreatment. Am Heart J 2000; 139:S38–S45.
- Li YF, Spencer FA, Becker RC. Comparative efficacy of fibrinogen and platelet supplementation on the in vitro reversibility of competitive glycoprotein IIb/IIIa receptor-directed platelet inhibition. Am Heart J 2002; 143:725–732.
- Schroeder WS, Gandhi PJ. Emergency management of hemorrhagic complications in the era of glycoprotein IIb/IIIa receptor antagonists, clopidogrel, low molecular weight heparin, and third-generation fibrinolytic agents. Curr Cardiol Rep 2003; 5:310–317.
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340:409–417.
- Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 2004; 292:1555–1562.
KEY POINTS
- The reported incidence of bleeding after treatment for non-ST-elevation acute coronary syndromes ranges from less than 1% to 10%, depending on a number of factors.
- Bleeding is strongly associated with adverse outcomes, although a causal relationship has not been established.
- Patients should be assessed for risk of bleeding so that the antithrombotic and antiplatelet regimen can be adjusted, safer alternatives can be considered, and percutaneous interventions can be used less aggressively for those at high risk.
- If bleeding develops and the risk of continued bleeding outweighs the risk of recurrent ischemia, antithrombotic and antiplatelet drug therapy can be interrupted and other agents given to reverse the effects of these drugs.
Should patients with mild asthma use inhaled steroids?
Yes. A number of large randomized controlled trials have shown inhaled corticosteroids to be beneficial in low doses for patients who have mild persistent asthma, and therefore these drugs are strongly recommended in this situation.1
Asthma care providers should, however, consider this “yes” in the context of asthma severity, the goals of therapy, and the benefits and risks associated with inhaled corticosteroids.
CLASSIFICATION OF ASTHMA SEVERITY
Although the studies of asthma prevalence had methodologic limitations and therefore the true prevalence of mild persistent asthma cannot be determined, it is common. Fuhlbrigge et al2 reported that most asthma patients have some form of persistent asthma. In contrast, Dusser et al3 reviewed available studies and concluded that most patients with asthma have either intermittent or mild persistent asthma.
GOALS: REDUCE IMPAIRMENT AND RISK
The goals of asthma management are to:
Reduce impairment by controlling symptoms so that normal activity levels can be maintained, by minimizing the need for short-acting bronchodilator use, and by maintaining normal pulmonary function; and to
Reduce risk by preventing progressive loss of lung function and recurrent exacerbations, and by optimizing pharmacotherapy while minimizing potential adverse effects.1
EVIDENCE OF BENEFIT
The OPTIMA trial4 (Low Dose Inhaled Budesonide and Formoterol in Mild Persistent Asthma) was a double-blind, randomized trial carried out in 198 centers in 17 countries. Compared with those randomized to receive placebo, patients who were randomized to receive an inhaled corticosteroid, ie, budesonide (Pulmicort) 100 μg twice daily, had 60% fewer severe exacerbations (relative risk [RR] 0.4, 95% confidence interval [CI] 0.27–0.59) and 48% fewer days when their asthma was poorly controlled (RR 0.52, 95% CI 0.4–0.67). Adding a long-acting beta-agonist did not change this outcome.
The START study5 (Inhaled Steroid Treatment as Regular Therapy in Early Asthma) showed that, compared with placebo, starting inhaled budesonide within the first 2 years of asthma symptoms in patients with mild persistent asthma was associated with better asthma control and less need for additional asthma medication.
The IMPACT study6 (Improving Asthma Control Trial) showed that inhaled steroids need to be taken daily, on a regular schedule, rather than intermittently as needed. Patients received either inhaled budesonide as needed, budesonide 200 μg twice daily every day, or zafirlukast (Accolate) 20 mg twice daily. Daily budesonide therapy resulted in better asthma control, less bronchial hyperresponsiveness, and less airway inflammation compared with intermittent use, zafirlukast therapy, or placebo. Daily zafirlukast and intermittent steroid treatment produced similar results for all outcomes measured.
Despite this strong evidence supporting regular use of inhaled corticosteroids in patients with mild persistent asthma, many patients choose to take them intermittently.
Suissa et al7 found, in a large observational cohort study, that fewer patients died of asthma if they were receiving low-dose inhaled corticosteroids than if they were not. The rate of death due to asthma was lower in patients who had used more inhaled corticosteroids over the previous year, and the death rate was higher in those who had discontinued inhaled corticosteroids in the previous 3 months than in those who continued using them.
STEROIDS DO NOT SLOW THE LOSS OF LUNG FUNCTION
Compared with people without asthma, asthma patients have substantially lower values of forced expiratory volume in the first second of expiration (FEV1). They also have a faster rate of functional decline: the average decrease in FEV1 in asthma patients is 38 mL per year, compared with 22 mL per year in nonasthmatic people.9
Although inhaled corticosteroids have been shown to increase lung function in asthma patients in the short term, there is little convincing evidence to suggest that they affect the rate of decline in the long term.10 In fact, airway inflammation and bronchial hyperresponsiveness return to baseline within 2 weeks after inhaled corticosteroids are discontinued.10
DO INHALED CORTICOSTEROIDS STUNT CHILDREN’S GROWTH?
The safety of long-term low-dose inhaled corticosteroids is well established in adults. However, two large randomized controlled trials found that children treated with low-dose inhaled steroids (budesonide 200–400 μg per day) grew 1 to 1.5 cm less over 3 to 5 years of treatment than children receiving placebo.11 However, this effect was primarily evident within the first year of therapy, and growth velocity was similar to that with placebo at the end of the treatment period (4 to 6 years).12
Agertoft and Pedersen13 found that taking inhaled corticosteroids long-term is unlikely to have an effect on final height. Children who took inhaled budesonide (up to an average daily dose of 500 μg) into adulthood ended up no shorter than those who did not.
Based on these and other data, inhaled corticosteroids are generally considered safe at recommended doses. However, the decision to prescribe them for long-term therapy should be based on the risks and benefits to the individual patient.1
ALTERNATIVE DRUGS FOR MILD PERSISTENT ASTHMA
Leukotriene-modifying drugs include the leukotriene receptor antagonists montelukast (Singulair) and zafirlukast and the 5-lipoxygenase inhibitor zileuton (Zyflo CR). These drugs have been associated with statistically significant improvement in FEV1 compared with placebo in patients with mild to moderate asthma, reductions in both blood and sputum eosinophils,14 and attenuation of bronchoconstriction with exercise.11
Large randomized trials comparing leukotriene modifier therapy with low-dose inhaled steroids in adults and children with mild persistent asthma have found that although outcomes improve with either therapy, the improvement is statistically superior with inhaled steroids for most asthma-control measures. 6,8 Low-dose inhaled steroid therapy in patients with mild persistent and moderate persistent asthma has been associated with superior clinical outcomes as well as greater improvement in pulmonary function than treatment with antileukotriene drugs (Table 2).8
Asthma is heterogeneous, and properly selected patients with mild persistent asthma may achieve good control with leukotrienemodifier monotherapy.15 Alternatives for patients with mild persistent asthma include the methylxanthine theophylline, but this drug is less desirable due to its narrow therapeutic index. 1 The inhaled cromones nedocromil (Tilade) and cromolyn (Intal) were other options in this patient population, but their short half-lives made them less practical, and US production has been discontinued.
THE BOTTOM LINE
Though leukotriene receptor antagonists can be effective, the daily use of inhaled corticosteroids results in higher asthma control test scores, more symptom-free days, greater pre-bronchodilator FEV1, and decreased percentage of sputum eosinophils6 in patients with mild persistent asthma, and the addition of a long-acting beta agonist does not provide additional benefit.4 Furthermore, daily use of inhaled corticosteroids in these patients has also been associated with a lower rate of asthma-related deaths and with less need for systemic corticosteroid therapy,7,8 even though inhaled corticosteroids have not yet been shown to alter the progressive loss of lung function.10
- National Heart, Lung, and Blood Institute. Guidelines for the Diagnosis and Management of Asthma (EPR-3). www.nhlbi.nih.gov/guidelines/asthma/. Accessed March 26, 2010.
- Fuhlbrigge AL, Adams RJ, Guilbert TW, et al. The burden of asthma in the United States: level and distribution are dependent on interpretation of the National Asthma Education and Prevention Program. Am J Respir Crit Care Med 2002; 166:1044–1049.
- Dusser D, Montani D, Chanez P, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy 2007; 62:591–604.
- O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001; 164:1392–1397.
- Busse WW, Pedersen S, Pauwels RA, et al; START Investigators Group. The Inhaled Steroid Treatment As Regular Therapy in Early Asthma (START) study 5-year follow-up: effectiveness of early intervention with budesonide in mild persistent asthma. J Allergy Clin Immunol 2008; 121:1167–1174.
- Boushey HA, Sorkness CA, King TS, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med 2005; 352:1519–1528.
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000; 343:332–356.
- Busse W, Wolfe J, Storms W, et al. Fluticasone propionate compared with zafirlukast in controlling persistent asthma: a randomized double-blind, placebo-controlled trial. J Fam Pract 2001; 50:595–602.
- Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med 1998; 339:1194–1200.
- Fanta CH. Asthma. N Engl J Med 2009; 360:1002–1014.
- O’Byrne PM, Parameswaran K. Pharmacological management of mild or moderate persistent asthma. Lancet 2006; 368:794–803.
- The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000; 343:1054–1063.
- Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000; 343:1064–1069.
- Pizzichini E, Leff JA, Reiss TF, et al. Montelukast reduces airway eosinophilic inflammation in asthma: a randomized, controlled trial. Eur Respir J 1999; 14:12–18.
- Kraft M, Israel E, O’Connor GT. Clinical decisions. Treatment of mild persistent asthma. N Engl J Med 2007; 356:2096–2100.
Yes. A number of large randomized controlled trials have shown inhaled corticosteroids to be beneficial in low doses for patients who have mild persistent asthma, and therefore these drugs are strongly recommended in this situation.1
Asthma care providers should, however, consider this “yes” in the context of asthma severity, the goals of therapy, and the benefits and risks associated with inhaled corticosteroids.
CLASSIFICATION OF ASTHMA SEVERITY
Although the studies of asthma prevalence had methodologic limitations and therefore the true prevalence of mild persistent asthma cannot be determined, it is common. Fuhlbrigge et al2 reported that most asthma patients have some form of persistent asthma. In contrast, Dusser et al3 reviewed available studies and concluded that most patients with asthma have either intermittent or mild persistent asthma.
GOALS: REDUCE IMPAIRMENT AND RISK
The goals of asthma management are to:
Reduce impairment by controlling symptoms so that normal activity levels can be maintained, by minimizing the need for short-acting bronchodilator use, and by maintaining normal pulmonary function; and to
Reduce risk by preventing progressive loss of lung function and recurrent exacerbations, and by optimizing pharmacotherapy while minimizing potential adverse effects.1
EVIDENCE OF BENEFIT
The OPTIMA trial4 (Low Dose Inhaled Budesonide and Formoterol in Mild Persistent Asthma) was a double-blind, randomized trial carried out in 198 centers in 17 countries. Compared with those randomized to receive placebo, patients who were randomized to receive an inhaled corticosteroid, ie, budesonide (Pulmicort) 100 μg twice daily, had 60% fewer severe exacerbations (relative risk [RR] 0.4, 95% confidence interval [CI] 0.27–0.59) and 48% fewer days when their asthma was poorly controlled (RR 0.52, 95% CI 0.4–0.67). Adding a long-acting beta-agonist did not change this outcome.
The START study5 (Inhaled Steroid Treatment as Regular Therapy in Early Asthma) showed that, compared with placebo, starting inhaled budesonide within the first 2 years of asthma symptoms in patients with mild persistent asthma was associated with better asthma control and less need for additional asthma medication.
The IMPACT study6 (Improving Asthma Control Trial) showed that inhaled steroids need to be taken daily, on a regular schedule, rather than intermittently as needed. Patients received either inhaled budesonide as needed, budesonide 200 μg twice daily every day, or zafirlukast (Accolate) 20 mg twice daily. Daily budesonide therapy resulted in better asthma control, less bronchial hyperresponsiveness, and less airway inflammation compared with intermittent use, zafirlukast therapy, or placebo. Daily zafirlukast and intermittent steroid treatment produced similar results for all outcomes measured.
Despite this strong evidence supporting regular use of inhaled corticosteroids in patients with mild persistent asthma, many patients choose to take them intermittently.
Suissa et al7 found, in a large observational cohort study, that fewer patients died of asthma if they were receiving low-dose inhaled corticosteroids than if they were not. The rate of death due to asthma was lower in patients who had used more inhaled corticosteroids over the previous year, and the death rate was higher in those who had discontinued inhaled corticosteroids in the previous 3 months than in those who continued using them.
STEROIDS DO NOT SLOW THE LOSS OF LUNG FUNCTION
Compared with people without asthma, asthma patients have substantially lower values of forced expiratory volume in the first second of expiration (FEV1). They also have a faster rate of functional decline: the average decrease in FEV1 in asthma patients is 38 mL per year, compared with 22 mL per year in nonasthmatic people.9
Although inhaled corticosteroids have been shown to increase lung function in asthma patients in the short term, there is little convincing evidence to suggest that they affect the rate of decline in the long term.10 In fact, airway inflammation and bronchial hyperresponsiveness return to baseline within 2 weeks after inhaled corticosteroids are discontinued.10
DO INHALED CORTICOSTEROIDS STUNT CHILDREN’S GROWTH?
The safety of long-term low-dose inhaled corticosteroids is well established in adults. However, two large randomized controlled trials found that children treated with low-dose inhaled steroids (budesonide 200–400 μg per day) grew 1 to 1.5 cm less over 3 to 5 years of treatment than children receiving placebo.11 However, this effect was primarily evident within the first year of therapy, and growth velocity was similar to that with placebo at the end of the treatment period (4 to 6 years).12
Agertoft and Pedersen13 found that taking inhaled corticosteroids long-term is unlikely to have an effect on final height. Children who took inhaled budesonide (up to an average daily dose of 500 μg) into adulthood ended up no shorter than those who did not.
Based on these and other data, inhaled corticosteroids are generally considered safe at recommended doses. However, the decision to prescribe them for long-term therapy should be based on the risks and benefits to the individual patient.1
ALTERNATIVE DRUGS FOR MILD PERSISTENT ASTHMA
Leukotriene-modifying drugs include the leukotriene receptor antagonists montelukast (Singulair) and zafirlukast and the 5-lipoxygenase inhibitor zileuton (Zyflo CR). These drugs have been associated with statistically significant improvement in FEV1 compared with placebo in patients with mild to moderate asthma, reductions in both blood and sputum eosinophils,14 and attenuation of bronchoconstriction with exercise.11
Large randomized trials comparing leukotriene modifier therapy with low-dose inhaled steroids in adults and children with mild persistent asthma have found that although outcomes improve with either therapy, the improvement is statistically superior with inhaled steroids for most asthma-control measures. 6,8 Low-dose inhaled steroid therapy in patients with mild persistent and moderate persistent asthma has been associated with superior clinical outcomes as well as greater improvement in pulmonary function than treatment with antileukotriene drugs (Table 2).8
Asthma is heterogeneous, and properly selected patients with mild persistent asthma may achieve good control with leukotrienemodifier monotherapy.15 Alternatives for patients with mild persistent asthma include the methylxanthine theophylline, but this drug is less desirable due to its narrow therapeutic index. 1 The inhaled cromones nedocromil (Tilade) and cromolyn (Intal) were other options in this patient population, but their short half-lives made them less practical, and US production has been discontinued.
THE BOTTOM LINE
Though leukotriene receptor antagonists can be effective, the daily use of inhaled corticosteroids results in higher asthma control test scores, more symptom-free days, greater pre-bronchodilator FEV1, and decreased percentage of sputum eosinophils6 in patients with mild persistent asthma, and the addition of a long-acting beta agonist does not provide additional benefit.4 Furthermore, daily use of inhaled corticosteroids in these patients has also been associated with a lower rate of asthma-related deaths and with less need for systemic corticosteroid therapy,7,8 even though inhaled corticosteroids have not yet been shown to alter the progressive loss of lung function.10
Yes. A number of large randomized controlled trials have shown inhaled corticosteroids to be beneficial in low doses for patients who have mild persistent asthma, and therefore these drugs are strongly recommended in this situation.1
Asthma care providers should, however, consider this “yes” in the context of asthma severity, the goals of therapy, and the benefits and risks associated with inhaled corticosteroids.
CLASSIFICATION OF ASTHMA SEVERITY
Although the studies of asthma prevalence had methodologic limitations and therefore the true prevalence of mild persistent asthma cannot be determined, it is common. Fuhlbrigge et al2 reported that most asthma patients have some form of persistent asthma. In contrast, Dusser et al3 reviewed available studies and concluded that most patients with asthma have either intermittent or mild persistent asthma.
GOALS: REDUCE IMPAIRMENT AND RISK
The goals of asthma management are to:
Reduce impairment by controlling symptoms so that normal activity levels can be maintained, by minimizing the need for short-acting bronchodilator use, and by maintaining normal pulmonary function; and to
Reduce risk by preventing progressive loss of lung function and recurrent exacerbations, and by optimizing pharmacotherapy while minimizing potential adverse effects.1
EVIDENCE OF BENEFIT
The OPTIMA trial4 (Low Dose Inhaled Budesonide and Formoterol in Mild Persistent Asthma) was a double-blind, randomized trial carried out in 198 centers in 17 countries. Compared with those randomized to receive placebo, patients who were randomized to receive an inhaled corticosteroid, ie, budesonide (Pulmicort) 100 μg twice daily, had 60% fewer severe exacerbations (relative risk [RR] 0.4, 95% confidence interval [CI] 0.27–0.59) and 48% fewer days when their asthma was poorly controlled (RR 0.52, 95% CI 0.4–0.67). Adding a long-acting beta-agonist did not change this outcome.
The START study5 (Inhaled Steroid Treatment as Regular Therapy in Early Asthma) showed that, compared with placebo, starting inhaled budesonide within the first 2 years of asthma symptoms in patients with mild persistent asthma was associated with better asthma control and less need for additional asthma medication.
The IMPACT study6 (Improving Asthma Control Trial) showed that inhaled steroids need to be taken daily, on a regular schedule, rather than intermittently as needed. Patients received either inhaled budesonide as needed, budesonide 200 μg twice daily every day, or zafirlukast (Accolate) 20 mg twice daily. Daily budesonide therapy resulted in better asthma control, less bronchial hyperresponsiveness, and less airway inflammation compared with intermittent use, zafirlukast therapy, or placebo. Daily zafirlukast and intermittent steroid treatment produced similar results for all outcomes measured.
Despite this strong evidence supporting regular use of inhaled corticosteroids in patients with mild persistent asthma, many patients choose to take them intermittently.
Suissa et al7 found, in a large observational cohort study, that fewer patients died of asthma if they were receiving low-dose inhaled corticosteroids than if they were not. The rate of death due to asthma was lower in patients who had used more inhaled corticosteroids over the previous year, and the death rate was higher in those who had discontinued inhaled corticosteroids in the previous 3 months than in those who continued using them.
STEROIDS DO NOT SLOW THE LOSS OF LUNG FUNCTION
Compared with people without asthma, asthma patients have substantially lower values of forced expiratory volume in the first second of expiration (FEV1). They also have a faster rate of functional decline: the average decrease in FEV1 in asthma patients is 38 mL per year, compared with 22 mL per year in nonasthmatic people.9
Although inhaled corticosteroids have been shown to increase lung function in asthma patients in the short term, there is little convincing evidence to suggest that they affect the rate of decline in the long term.10 In fact, airway inflammation and bronchial hyperresponsiveness return to baseline within 2 weeks after inhaled corticosteroids are discontinued.10
DO INHALED CORTICOSTEROIDS STUNT CHILDREN’S GROWTH?
The safety of long-term low-dose inhaled corticosteroids is well established in adults. However, two large randomized controlled trials found that children treated with low-dose inhaled steroids (budesonide 200–400 μg per day) grew 1 to 1.5 cm less over 3 to 5 years of treatment than children receiving placebo.11 However, this effect was primarily evident within the first year of therapy, and growth velocity was similar to that with placebo at the end of the treatment period (4 to 6 years).12
Agertoft and Pedersen13 found that taking inhaled corticosteroids long-term is unlikely to have an effect on final height. Children who took inhaled budesonide (up to an average daily dose of 500 μg) into adulthood ended up no shorter than those who did not.
Based on these and other data, inhaled corticosteroids are generally considered safe at recommended doses. However, the decision to prescribe them for long-term therapy should be based on the risks and benefits to the individual patient.1
ALTERNATIVE DRUGS FOR MILD PERSISTENT ASTHMA
Leukotriene-modifying drugs include the leukotriene receptor antagonists montelukast (Singulair) and zafirlukast and the 5-lipoxygenase inhibitor zileuton (Zyflo CR). These drugs have been associated with statistically significant improvement in FEV1 compared with placebo in patients with mild to moderate asthma, reductions in both blood and sputum eosinophils,14 and attenuation of bronchoconstriction with exercise.11
Large randomized trials comparing leukotriene modifier therapy with low-dose inhaled steroids in adults and children with mild persistent asthma have found that although outcomes improve with either therapy, the improvement is statistically superior with inhaled steroids for most asthma-control measures. 6,8 Low-dose inhaled steroid therapy in patients with mild persistent and moderate persistent asthma has been associated with superior clinical outcomes as well as greater improvement in pulmonary function than treatment with antileukotriene drugs (Table 2).8
Asthma is heterogeneous, and properly selected patients with mild persistent asthma may achieve good control with leukotrienemodifier monotherapy.15 Alternatives for patients with mild persistent asthma include the methylxanthine theophylline, but this drug is less desirable due to its narrow therapeutic index. 1 The inhaled cromones nedocromil (Tilade) and cromolyn (Intal) were other options in this patient population, but their short half-lives made them less practical, and US production has been discontinued.
THE BOTTOM LINE
Though leukotriene receptor antagonists can be effective, the daily use of inhaled corticosteroids results in higher asthma control test scores, more symptom-free days, greater pre-bronchodilator FEV1, and decreased percentage of sputum eosinophils6 in patients with mild persistent asthma, and the addition of a long-acting beta agonist does not provide additional benefit.4 Furthermore, daily use of inhaled corticosteroids in these patients has also been associated with a lower rate of asthma-related deaths and with less need for systemic corticosteroid therapy,7,8 even though inhaled corticosteroids have not yet been shown to alter the progressive loss of lung function.10
- National Heart, Lung, and Blood Institute. Guidelines for the Diagnosis and Management of Asthma (EPR-3). www.nhlbi.nih.gov/guidelines/asthma/. Accessed March 26, 2010.
- Fuhlbrigge AL, Adams RJ, Guilbert TW, et al. The burden of asthma in the United States: level and distribution are dependent on interpretation of the National Asthma Education and Prevention Program. Am J Respir Crit Care Med 2002; 166:1044–1049.
- Dusser D, Montani D, Chanez P, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy 2007; 62:591–604.
- O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001; 164:1392–1397.
- Busse WW, Pedersen S, Pauwels RA, et al; START Investigators Group. The Inhaled Steroid Treatment As Regular Therapy in Early Asthma (START) study 5-year follow-up: effectiveness of early intervention with budesonide in mild persistent asthma. J Allergy Clin Immunol 2008; 121:1167–1174.
- Boushey HA, Sorkness CA, King TS, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med 2005; 352:1519–1528.
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000; 343:332–356.
- Busse W, Wolfe J, Storms W, et al. Fluticasone propionate compared with zafirlukast in controlling persistent asthma: a randomized double-blind, placebo-controlled trial. J Fam Pract 2001; 50:595–602.
- Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med 1998; 339:1194–1200.
- Fanta CH. Asthma. N Engl J Med 2009; 360:1002–1014.
- O’Byrne PM, Parameswaran K. Pharmacological management of mild or moderate persistent asthma. Lancet 2006; 368:794–803.
- The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000; 343:1054–1063.
- Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000; 343:1064–1069.
- Pizzichini E, Leff JA, Reiss TF, et al. Montelukast reduces airway eosinophilic inflammation in asthma: a randomized, controlled trial. Eur Respir J 1999; 14:12–18.
- Kraft M, Israel E, O’Connor GT. Clinical decisions. Treatment of mild persistent asthma. N Engl J Med 2007; 356:2096–2100.
- National Heart, Lung, and Blood Institute. Guidelines for the Diagnosis and Management of Asthma (EPR-3). www.nhlbi.nih.gov/guidelines/asthma/. Accessed March 26, 2010.
- Fuhlbrigge AL, Adams RJ, Guilbert TW, et al. The burden of asthma in the United States: level and distribution are dependent on interpretation of the National Asthma Education and Prevention Program. Am J Respir Crit Care Med 2002; 166:1044–1049.
- Dusser D, Montani D, Chanez P, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy 2007; 62:591–604.
- O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001; 164:1392–1397.
- Busse WW, Pedersen S, Pauwels RA, et al; START Investigators Group. The Inhaled Steroid Treatment As Regular Therapy in Early Asthma (START) study 5-year follow-up: effectiveness of early intervention with budesonide in mild persistent asthma. J Allergy Clin Immunol 2008; 121:1167–1174.
- Boushey HA, Sorkness CA, King TS, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med 2005; 352:1519–1528.
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000; 343:332–356.
- Busse W, Wolfe J, Storms W, et al. Fluticasone propionate compared with zafirlukast in controlling persistent asthma: a randomized double-blind, placebo-controlled trial. J Fam Pract 2001; 50:595–602.
- Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med 1998; 339:1194–1200.
- Fanta CH. Asthma. N Engl J Med 2009; 360:1002–1014.
- O’Byrne PM, Parameswaran K. Pharmacological management of mild or moderate persistent asthma. Lancet 2006; 368:794–803.
- The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000; 343:1054–1063.
- Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000; 343:1064–1069.
- Pizzichini E, Leff JA, Reiss TF, et al. Montelukast reduces airway eosinophilic inflammation in asthma: a randomized, controlled trial. Eur Respir J 1999; 14:12–18.
- Kraft M, Israel E, O’Connor GT. Clinical decisions. Treatment of mild persistent asthma. N Engl J Med 2007; 356:2096–2100.
Recurrent spontaneous pneumothorax
Q: Which is the most likely diagnosis?
- Pulmonary Langerhans cell histiocytosis
- Cystic fibrosis
- Pneumocystis jirovecii pneumonia
- Alpha-1 antitrypsin deficiency
- Tuberous sclerosis complex with lymphangioleiomyomatosis
A: The correct diagnosis is tuberous sclerosis complex with lymphangioleiomyomatosis. The lymphangioleiomyomatosis was suggested by the CT findings, by the recurrence of pneumothorax, and, later, by biopsy results. Lymphangioleiomyomatosis occurs in about 30% of women with the tuberous sclerosis complex.1 However, 10% to 15% of women with lymphangioleiomyomatosis do not have tuberous sclerosis complex, 2 in which case the condition is called sporadic lymphangioleiomyomatosis.
Tuberous sclerosis complex can involve the nerves (seizures, brain tumors), the lungs (lymphangioleiomyomatosis, causing pneumothorax or chylothorax), and the skin; skin lesions include facial angiofibromas (Figure 1), ash-leaf spot (Figure 2), and shagreen patch (Figure 3). It is also associated with abdominal involvement (lymphangiomyomas, renal angiomyolipomas).3
Lymphangioleiomyomatosis usually presents as spontaneous pneumothorax in women of childbearing age. After initial stabilization of pneumothorax with simple aspiration or thoracostomy, the patient should undergo ipsilateral chemical or surgical pleurodesis, as the risk of recurrent pneumothorax is greater than 70%.4 Single or bilateral lung transplantation has been accepted as therapy for end-stage pulmonary lymphangioleiomyomatosis, characterized by recurrent pneumothoraces and chylous pleural fluid collections causing respiratory failure (marked dyspnea, hypoxemia, and reductions in forced expiratory volume in the first second of expiration and in diffusing capacity for carbon monoxide. Recurrence of lymphangioleiomyomatosis in the allograft lung is rare.5 Hormone therapies such as intramuscular progesterone, oral progestins, or gonadotropin-releasing hormone agonists have been used for lymphangioleiomyomatosis (not pneumothorax), but they are no longer recommended.
Pulmonary Langerhans cell histiocytosis, cystic fibrosis, Pneumocystis jirovecii pneumonia, and alpha-1 antitrypsin deficiency have all been associated with spontaneous pneumothorax.
Pulmonary Langerhans cell histiocytosis can affect multiple systems, including lung, skin, bone, and the pituitary gland. More than 90% of patients have a history of smoking.6 Chest radiography reveals a reticulonodular pattern with involvement of the middle and upper lobes. Later, the nodules tend to cavitate and form contiguous cysts that may mimic lymphangioleiomyomatosis on a high-resolution chest CT.
Cystic fibrosis is most often diagnosed before the age of 3.7 In adults, it can present as sinus and pulmonary disease (chronic cough with sputum production, chronic sinusitis with nasal polyposis, radiographic evidence of bronchiectasis and, less commonly, pneumothorax); as a gastrointestinal tract and nutritional abnormality (pancreatic insufficiency, distal intestinal obstruction, focal biliary cirrhosis); and as male infertility.
Pneumocystis jiroveciipneumonia occurs mainly in patients on chronic immunosuppressive drugs or with immune deficiency due to HIV infection. Typical radiographic features are bilateral perihilar interstitial infiltrates that become increasingly homogeneous and diffuse as the disease progresses. Less common findings include solitary or multiple nodules, upper-lobe infiltrates in patients receiving aerosolized pentamidine (NebuPent), pneumatoceles, and pneumothorax.8
Alpha-1 antitrypsin is an inhibitor of neutrophil elastase. Deficiency is associated with severe, early-onset panacinar emphysema with a basilar predominance, with chronic liver disease including cirrhosis, and less commonly with panniculitis and vasculitis associated with antineutrophil cytoplasmic antibody.9 Coalescence of panacinar emphysema leads to the formation of bullae and is important in the development of spontaneous pneumothorax.10
The patient underwent bilateral talc pleurodesis. Lung biopsy at the same time confirmed lymphangioleiomyomatosis. One month later, the right pneumothorax recurred, and she underwent pleurodesis in the right hemithorax with tetracycline. Six months after the second pleurodesis, she was asymptomatic.
- Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc 2000; 75:591–594.
- Strizheva GD, Carsillo T, Kruger WD, Sullivan EJ, Ryu JH, Henske EP. The spectrum of mutations in TSC1 and TSC2 in women with tuberous sclerosis and lymphangiomyomatosis. Am J Respir Crit Care Med 2001; 163:253–258.
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008; 133:507–516.
- Almoosa KF, Ryu JH, Mendez J, et al. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest 2006; 129:1274–1281.
- Benden C, Rea F, Behr J, et al. Lung transplantation for lymphangioleiomyomatosis: the European Experience. J Heart Lung Transplant 2009; 28:1–7.
- Vassallo R, Ryu JH, Colby TV, Hartman T, Limper AH. Pulmonary Langerhans’-cell histiocytosis. N Engl J Med 2000; 342:1969–1978.
- Boyle MP. Adult cystic fibrosis. JAMA 2007; 298:1787–1793.
- Thomas CF, Limper AH. Pneumocystis pneumonia. N Engl J Med 2004; 350:2487–2498.
- Silverman EK, Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med 2009; 360:2749–2757.
- Anderson AE, Furlaneto JA, Foraker AG. Bronchopulmonary derangements in nonsmokers. Am Rev Respir Dis 1970; 101:518–527.
Q: Which is the most likely diagnosis?
- Pulmonary Langerhans cell histiocytosis
- Cystic fibrosis
- Pneumocystis jirovecii pneumonia
- Alpha-1 antitrypsin deficiency
- Tuberous sclerosis complex with lymphangioleiomyomatosis
A: The correct diagnosis is tuberous sclerosis complex with lymphangioleiomyomatosis. The lymphangioleiomyomatosis was suggested by the CT findings, by the recurrence of pneumothorax, and, later, by biopsy results. Lymphangioleiomyomatosis occurs in about 30% of women with the tuberous sclerosis complex.1 However, 10% to 15% of women with lymphangioleiomyomatosis do not have tuberous sclerosis complex, 2 in which case the condition is called sporadic lymphangioleiomyomatosis.
Tuberous sclerosis complex can involve the nerves (seizures, brain tumors), the lungs (lymphangioleiomyomatosis, causing pneumothorax or chylothorax), and the skin; skin lesions include facial angiofibromas (Figure 1), ash-leaf spot (Figure 2), and shagreen patch (Figure 3). It is also associated with abdominal involvement (lymphangiomyomas, renal angiomyolipomas).3
Lymphangioleiomyomatosis usually presents as spontaneous pneumothorax in women of childbearing age. After initial stabilization of pneumothorax with simple aspiration or thoracostomy, the patient should undergo ipsilateral chemical or surgical pleurodesis, as the risk of recurrent pneumothorax is greater than 70%.4 Single or bilateral lung transplantation has been accepted as therapy for end-stage pulmonary lymphangioleiomyomatosis, characterized by recurrent pneumothoraces and chylous pleural fluid collections causing respiratory failure (marked dyspnea, hypoxemia, and reductions in forced expiratory volume in the first second of expiration and in diffusing capacity for carbon monoxide. Recurrence of lymphangioleiomyomatosis in the allograft lung is rare.5 Hormone therapies such as intramuscular progesterone, oral progestins, or gonadotropin-releasing hormone agonists have been used for lymphangioleiomyomatosis (not pneumothorax), but they are no longer recommended.
Pulmonary Langerhans cell histiocytosis, cystic fibrosis, Pneumocystis jirovecii pneumonia, and alpha-1 antitrypsin deficiency have all been associated with spontaneous pneumothorax.
Pulmonary Langerhans cell histiocytosis can affect multiple systems, including lung, skin, bone, and the pituitary gland. More than 90% of patients have a history of smoking.6 Chest radiography reveals a reticulonodular pattern with involvement of the middle and upper lobes. Later, the nodules tend to cavitate and form contiguous cysts that may mimic lymphangioleiomyomatosis on a high-resolution chest CT.
Cystic fibrosis is most often diagnosed before the age of 3.7 In adults, it can present as sinus and pulmonary disease (chronic cough with sputum production, chronic sinusitis with nasal polyposis, radiographic evidence of bronchiectasis and, less commonly, pneumothorax); as a gastrointestinal tract and nutritional abnormality (pancreatic insufficiency, distal intestinal obstruction, focal biliary cirrhosis); and as male infertility.
Pneumocystis jiroveciipneumonia occurs mainly in patients on chronic immunosuppressive drugs or with immune deficiency due to HIV infection. Typical radiographic features are bilateral perihilar interstitial infiltrates that become increasingly homogeneous and diffuse as the disease progresses. Less common findings include solitary or multiple nodules, upper-lobe infiltrates in patients receiving aerosolized pentamidine (NebuPent), pneumatoceles, and pneumothorax.8
Alpha-1 antitrypsin is an inhibitor of neutrophil elastase. Deficiency is associated with severe, early-onset panacinar emphysema with a basilar predominance, with chronic liver disease including cirrhosis, and less commonly with panniculitis and vasculitis associated with antineutrophil cytoplasmic antibody.9 Coalescence of panacinar emphysema leads to the formation of bullae and is important in the development of spontaneous pneumothorax.10
The patient underwent bilateral talc pleurodesis. Lung biopsy at the same time confirmed lymphangioleiomyomatosis. One month later, the right pneumothorax recurred, and she underwent pleurodesis in the right hemithorax with tetracycline. Six months after the second pleurodesis, she was asymptomatic.
Q: Which is the most likely diagnosis?
- Pulmonary Langerhans cell histiocytosis
- Cystic fibrosis
- Pneumocystis jirovecii pneumonia
- Alpha-1 antitrypsin deficiency
- Tuberous sclerosis complex with lymphangioleiomyomatosis
A: The correct diagnosis is tuberous sclerosis complex with lymphangioleiomyomatosis. The lymphangioleiomyomatosis was suggested by the CT findings, by the recurrence of pneumothorax, and, later, by biopsy results. Lymphangioleiomyomatosis occurs in about 30% of women with the tuberous sclerosis complex.1 However, 10% to 15% of women with lymphangioleiomyomatosis do not have tuberous sclerosis complex, 2 in which case the condition is called sporadic lymphangioleiomyomatosis.
Tuberous sclerosis complex can involve the nerves (seizures, brain tumors), the lungs (lymphangioleiomyomatosis, causing pneumothorax or chylothorax), and the skin; skin lesions include facial angiofibromas (Figure 1), ash-leaf spot (Figure 2), and shagreen patch (Figure 3). It is also associated with abdominal involvement (lymphangiomyomas, renal angiomyolipomas).3
Lymphangioleiomyomatosis usually presents as spontaneous pneumothorax in women of childbearing age. After initial stabilization of pneumothorax with simple aspiration or thoracostomy, the patient should undergo ipsilateral chemical or surgical pleurodesis, as the risk of recurrent pneumothorax is greater than 70%.4 Single or bilateral lung transplantation has been accepted as therapy for end-stage pulmonary lymphangioleiomyomatosis, characterized by recurrent pneumothoraces and chylous pleural fluid collections causing respiratory failure (marked dyspnea, hypoxemia, and reductions in forced expiratory volume in the first second of expiration and in diffusing capacity for carbon monoxide. Recurrence of lymphangioleiomyomatosis in the allograft lung is rare.5 Hormone therapies such as intramuscular progesterone, oral progestins, or gonadotropin-releasing hormone agonists have been used for lymphangioleiomyomatosis (not pneumothorax), but they are no longer recommended.
Pulmonary Langerhans cell histiocytosis, cystic fibrosis, Pneumocystis jirovecii pneumonia, and alpha-1 antitrypsin deficiency have all been associated with spontaneous pneumothorax.
Pulmonary Langerhans cell histiocytosis can affect multiple systems, including lung, skin, bone, and the pituitary gland. More than 90% of patients have a history of smoking.6 Chest radiography reveals a reticulonodular pattern with involvement of the middle and upper lobes. Later, the nodules tend to cavitate and form contiguous cysts that may mimic lymphangioleiomyomatosis on a high-resolution chest CT.
Cystic fibrosis is most often diagnosed before the age of 3.7 In adults, it can present as sinus and pulmonary disease (chronic cough with sputum production, chronic sinusitis with nasal polyposis, radiographic evidence of bronchiectasis and, less commonly, pneumothorax); as a gastrointestinal tract and nutritional abnormality (pancreatic insufficiency, distal intestinal obstruction, focal biliary cirrhosis); and as male infertility.
Pneumocystis jiroveciipneumonia occurs mainly in patients on chronic immunosuppressive drugs or with immune deficiency due to HIV infection. Typical radiographic features are bilateral perihilar interstitial infiltrates that become increasingly homogeneous and diffuse as the disease progresses. Less common findings include solitary or multiple nodules, upper-lobe infiltrates in patients receiving aerosolized pentamidine (NebuPent), pneumatoceles, and pneumothorax.8
Alpha-1 antitrypsin is an inhibitor of neutrophil elastase. Deficiency is associated with severe, early-onset panacinar emphysema with a basilar predominance, with chronic liver disease including cirrhosis, and less commonly with panniculitis and vasculitis associated with antineutrophil cytoplasmic antibody.9 Coalescence of panacinar emphysema leads to the formation of bullae and is important in the development of spontaneous pneumothorax.10
The patient underwent bilateral talc pleurodesis. Lung biopsy at the same time confirmed lymphangioleiomyomatosis. One month later, the right pneumothorax recurred, and she underwent pleurodesis in the right hemithorax with tetracycline. Six months after the second pleurodesis, she was asymptomatic.
- Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc 2000; 75:591–594.
- Strizheva GD, Carsillo T, Kruger WD, Sullivan EJ, Ryu JH, Henske EP. The spectrum of mutations in TSC1 and TSC2 in women with tuberous sclerosis and lymphangiomyomatosis. Am J Respir Crit Care Med 2001; 163:253–258.
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008; 133:507–516.
- Almoosa KF, Ryu JH, Mendez J, et al. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest 2006; 129:1274–1281.
- Benden C, Rea F, Behr J, et al. Lung transplantation for lymphangioleiomyomatosis: the European Experience. J Heart Lung Transplant 2009; 28:1–7.
- Vassallo R, Ryu JH, Colby TV, Hartman T, Limper AH. Pulmonary Langerhans’-cell histiocytosis. N Engl J Med 2000; 342:1969–1978.
- Boyle MP. Adult cystic fibrosis. JAMA 2007; 298:1787–1793.
- Thomas CF, Limper AH. Pneumocystis pneumonia. N Engl J Med 2004; 350:2487–2498.
- Silverman EK, Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med 2009; 360:2749–2757.
- Anderson AE, Furlaneto JA, Foraker AG. Bronchopulmonary derangements in nonsmokers. Am Rev Respir Dis 1970; 101:518–527.
- Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc 2000; 75:591–594.
- Strizheva GD, Carsillo T, Kruger WD, Sullivan EJ, Ryu JH, Henske EP. The spectrum of mutations in TSC1 and TSC2 in women with tuberous sclerosis and lymphangiomyomatosis. Am J Respir Crit Care Med 2001; 163:253–258.
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008; 133:507–516.
- Almoosa KF, Ryu JH, Mendez J, et al. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest 2006; 129:1274–1281.
- Benden C, Rea F, Behr J, et al. Lung transplantation for lymphangioleiomyomatosis: the European Experience. J Heart Lung Transplant 2009; 28:1–7.
- Vassallo R, Ryu JH, Colby TV, Hartman T, Limper AH. Pulmonary Langerhans’-cell histiocytosis. N Engl J Med 2000; 342:1969–1978.
- Boyle MP. Adult cystic fibrosis. JAMA 2007; 298:1787–1793.
- Thomas CF, Limper AH. Pneumocystis pneumonia. N Engl J Med 2004; 350:2487–2498.
- Silverman EK, Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med 2009; 360:2749–2757.
- Anderson AE, Furlaneto JA, Foraker AG. Bronchopulmonary derangements in nonsmokers. Am Rev Respir Dis 1970; 101:518–527.
Grand Rounds: Woman, 80, With Hallucinations and Tremors
An 80-year-old Mandarin-speaking Chinese woman was referred to a mental health outpatient clinic for evaluation and treatment. The patient had a history of mild depression, for which she had been treated for many years with sertraline.
Five years earlier at age 75, the patient had been evaluated by a psychiatrist after she began to experience psychotic symptoms, including frequent repetitive auditory hallucinations of people counting, alternating with music from her childhood. At that time, she also had persecutory paranoid thoughts and delusional thinking that she was receiving messages in Mandarin while watching American TV programs. Initially, her only cognitive disturbance was an inability to differentiate among numbers on a calendar or a telephone keypad. No reports of memory problems were noted. Although the patient acknowledged auditory hallucinations, she denied experiencing command auditory hallucinations or hallucinations of other forms. The patient had no history of suicide attempts and denied suicidal or homicidal ideation. She had no history of psychiatric hospitalization.
The psychiatrist made a diagnosis of major depressive disorder with psychotic features, not otherwise specified1 and prescribed sertraline 50 mg/d. The patient was also started on risperidone 0.25 mg/d for management of her psychotic symptoms, with the dosage gradually increased to 2.0 mg/d over five years. While taking this combination, the patient experienced stable mood and fewer paranoid thoughts, although her auditory hallucinations continued.
Two months before the current visit, the patient moved into a retirement living facility, and she reported having adapted well to the new setting. She was sleeping well and had a good appetite. Her BMI was within normal range.
The patient described herself as a single parent for nearly 40 years, raising one daughter. Formerly high functioning, she had held a full-time clerical job until age 70. She appeared well-groomed, polite but anxious, and oriented to time, person, and place. Her speech was normal, her thought processes were coherent, and her mood was stable. However, her affect was constricted; she acknowledged auditory hallucinations, which impaired her thought content. The patient reported feeling increased anxiety prior to any nonroutine activity, such as a doctor’s appointment; this, she said, would cause insomnia, leaving her to pace in her room.
During the examination, fine tremors on upper and lower extremities were noted. The patient’s Abnormal Involuntary Movement Scale (AIMS) score2 was 13, which placed her in the highest risk category for antipsychotic-induced dopamine-blockade extrapyramidal symptoms (EPS). The patient was found to be negative for tardive dyskinesia, with no abnormal facial movements. She was aware of the tremors in her limbs and said she felt bothered by them.
The patient had an unsteady gait and used a four-point walker. Her Mini-Mental State Exam (MMSE) score3 was 28/30, which was normal for her age and education level (high school completed).
Apart from the described symptoms, the patient was healthy for her age and had no other medical diagnosis. Her vital signs were within normal range. The medical work-up to rule out other causes of dementia yielded negative results. Lab values were normal, including electrolyte levels and thyroid tests. The patient’s hearing test showed age-related hearing loss of full range, not limited to high pitch. She was able to engage in a meaningful conversation at a normal volume. Clinically, however, it was concerning to observe the possible signs of EPS and the relatively high risperidone dosage, considering the patient’s advanced age.
After the meeting with the patient, a treatment plan was created to 1) gradually reduce the dosage of antipsychotic medication, and 2) refer her to a neurologist for a complete work-up to rule out underlying neurologic disorders, such as dementia. Risperidone was tapered by increments of 0.25 mg/d every three to four weeks; throughout this process, the patient was closely monitored by the nursing staff at the retirement living facility. Monthly appointments were scheduled at the outpatient mental health clinic for evaluation and medication management.
Two months after the initial mental health clinic visit, the patient’s condition was pronounced stable on the current regimen of sertraline 50 mg/d and risperidone 1.0 mg/d. She was later seen by a neurologist, who made a diagnosis of Parkinson’s disease and placed her on carbidopa-levodopa (1 1/2 tablets, 25/100 mg, tid). The patient’s auditory hallucinations continued with the same intensity as at baseline, but fewer tremors were noted in her extremities. By six months into the tapering process (with risperidone reduced at that time to 0.25 mg/d and carbidopa-levodopa to 25/100 mg tid), the patient had begun to experience dissipation of the tremors, and her AIMS score2 was 0. She was able to replace her four-point walker with a cane.
One year after her initial visit to the mental health clinic, the patient’s neurologist suggested replacing risperidone with quetiapine (12.5 mg/d) for its improved tolerability and lower adverse effect profile.4 She continued to take sertraline and carbidopa-levodopa.
Improvement of symptoms was noted following the switch. After one month on the revised regimen, the patient reported that the number of auditory hallucinations persisted, but that their intensity had decreased dramatically. She had a brighter affect and appeared to feel uplifted and more energetic. She became involved in the social activities offered at the retirement living facility and the mental health clinic. She also maintained a steady gait without her cane. According to the patient’s daughter, her mother was at her best psychological state since the onset of psychotic symptoms six years earlier. The pharmacologic regimen had reached its maximum benefit.
At a mental health appointment at the outpatient clinic 18 months after her initial visit there, it was evident that the patient’s auditory hallucinations persisted as a major stressor. She began to complain about other residents in her facility. She said she disliked the resident with whom she shared meals, and she claimed that other residents often spit on the floor in front of her room. The nursing staff did not confirm these incidents, which they considered a delusion despite the patient’s “evidence” (the tissues she said she had used to clean up).
Additionally, a new theme had emerged in the patient’s auditory hallucinations. She reported hearing a male voice that announced changes in meal times. Although she knew there was no public address system in her room or in the hallway, the “announcement” was so convincing that she would go to the dining room and once there, realize that nothing had changed. She seemed to drift between reality and her hallucinations/delusions. According to her daughter, the patient’s independent and reserved personality forced her to internalize her stressors—in this case, her frustration about the other residents—which fed into her hallucinations and delusions.
In response to her worsening psychotic symptoms, the patient’s provider increased her quetiapine dosage from 12.5 mg/d to 25 mg/d. Her MMSE score3 at this visit was 25/30.
Two months later, the patient exhibited increasing symptoms of paranoia, delusions, and auditory hallucinations. She continued to respond to the “broadcast” messages about meal times, and she voiced her frustrations to others who spoke Mandarin. She became agitated in response to out-of-the-ordinary events. When her alarm clock battery ran out, for example, she insisted that “a man’s voice” kept reminding her to replace the battery; in response, she placed the alarm clock in the refrigerator, later explaining, “Now I don’t need to worry about it.”
Her cognitive status began to show obvious, progressive deterioration, with an MMSE score3 of 22/30 at this visit—a significant reduction from previous scores. Worsening of her short-term memory became apparent when she had difficulty playing bingo and was unable to remember her appointment or the current date. She became upset when others corrected her.
In a review of the trends in this patient’s clinical presentation, it became increasingly evident to the patient’s mental health care providers that she had Lewy body dementia.
DISCUSSION
Dementia with Lewy bodies (DLB), a progressive disease, is the second most common cause of neurodegenerative dementia after Alzheimer’s disease.5-7 It is estimated that DLB accounts for 20% of US cases of dementia (ie, about 800,000 patients).8,9 Although public awareness of DLB is on the rise, the disorder is still underrecognized and underdiagnosed because its clinical manifestations so closely resemble those of Alzheimer’s disease, Parkinson’s disease, and psychosis.10,11
Clinical symptoms of DLB include progressive cognitive decline, cognitive fluctuation, EPS, and parkinsonism; hallucinations involving all five senses, particularly sight; delusions; REM sleep disturbance, with or without vivid and frightening dreams; changes in mood and behavior; impaired judgment and insight; and autonomic dysfunction, such as orthostatic hypotension and carotid-sinus hypersensitivity.5,11-15
The symptoms of DLB are caused by the accumulations of Lewy bodies, that is, deposits of alpha-synuclein protein in the nuclei of neurons. Lewy bodies destroy neurons over time, resulting in the destruction of dopaminergic and acetylcholinergic pathways from the brain stem to areas of the cerebral cortex associated with cognition and motor functions.4,5,16
DLB is a spectrum disorder; it often coexists with Parkinson’s disease or Alzheimer’s disease, as Lewy bodies are also found in patients with these illnesses.7 This poses a challenge for formulating a differential diagnosis, particularly in patients with fluctuating cognition,10 and for attempting to establish disease prevalence.
Diagnosis
Currently, a conclusive diagnosis of DLB can be confirmed only through postmortem autopsy, although use of medial temporal lobe volume (via structural MRI) and regional blood flow (via single photon emission CT [SPECT] tracers) is being investigated.17
The diagnosis of DLB is currently based on the presenting clinical symptoms and the exclusion of other medical conditions whose symptoms mimic those of DLB.7 The screening assessment may include a neurologic/psychiatric assessment (MMSE, psychiatric evaluation, and interviews with family members or caretakers), neuroimaging such as MRI to rule out other organic causes, and laboratory evaluation to rule out potentially reversible causes of dementia, including electrolyte imbalance, vitamin deficiency (specifically vitamin B12), anemia, thyroid dysfunction, and kidney or liver impairment.18
The American Psychiatric Assocation1 categorizes DLB under “Dementia Due to Other General Medical Conditions” (294.1x). The World Health Organization19 includes it among “Other specified degenerative diseases of the nervous system” (G31.8).
Treatment
Lewy body dementia is an irreversible neurologic degenerative disorder. Treatment for DLB comprises symptom management, primarily through pharmacology; however, the response to medication is highly individualized. Treatment includes management of the following symptoms:
Cognitive impairment. Cholinesterase inhibitors, such as rivastigmine (3 to 12 mg/d), donepezil (10 mg/d), or galantamine (titrated up to 12 mg bid),20-23 improve attention and behavior and reduce apathy, anxiety, delusions, and hallucinations. As cognitive impairment worsens, memantine (10 mg bid) may be effective.24 The potential for anticholinergic adverse effects requires close monitoring in patients taking these agents.
Parkinsonian symptoms. Medications indicated for Parkinson’s disease and syndrome, such as carbidopa-levodopa (25/100 mg tid), can be effective; dosage may be slowly titrated upward as tolerated and if needed for symptom management.25,26 The dopaminergic effect of antiparkinson medications may intensify the psychotic symptoms and worsen the REM sleep pattern. In this case, a low-dose atypical antipsychotic is suggested27,28 (see below).
Psychotic symptoms. An atypical antipsychotic agent, such as quetiapine (12.5 mg), risperidone (0.25 mg), olanzapine (2.5 mg), ziprasidone (20 mg), aripiprazole (2 mg), or paliperidone (1.5 mg), may be used. Because of the DLB-associated risk of neuroleptic sensitivity, atypical antipsychotic agents should be initiated at a low dose with slow upward titration17,26,29; quetiapine appears less likely than risperidone or olanzapine to cause neuroleptic sensitivity or to trigger EPS.4 For Asian patients, who often respond to lower doses of these medications (and are more easily affected by associated adverse effects), Chen et al30 recommend a starting dose of about one-half the recommended dose.
Depression. An SSRI antidepressant with relatively simple pharmacologic properties and moderate half-life may be used to manage symptoms of depression.26,31,32 Long–half-life SSRIs (eg, fluoxetine) should be avoided in elderly patients; in response to SNRIs (serotonin-norepinephrine reuptake inhibitors), these patients may experience elevated blood pressures and pulses, with subsequent morbidity.33
REM sleep disturbances. Clonazepam (0.25 mg), melatonin (3.0 mg), or quetiapine (12.5 mg) may be administered at bedtime.34
Important Lessons
In general, providers should consider the benefits and risks of any pharmacologic treatment and avoid polypharmacy, if possible. Family and caretakers should be included in the treatment planning, with a focus on prioritizing and managing the most debilitating symptoms or dysfunctions that prompt concerns for safety.
For optimal homeostasis, some DLB patients may require joint pharmacologic modalities that appear counterintuitive—for example, an antiparkinsonism (dopaminergic) agent for parkinsonian symptoms or neuroleptic-induced EPS, versus an antipsychotic (eg, a dopamine antagonist) to treat profound hallucinations.26
As the response to treatment for DLB is highly individualized, it is essential to titrate and augment with care.
CONCLUSION
In DLB, as with other dementing illnesses, the onset of symptoms can be gradual and insidious, posing a great challenge to the clinician who seeks to confirm the diagnosis. In the illness’s early stages, the clinician may have to treat targeted symptoms and adjust the treatment plan once signs of the pathologic origins emerge.
It is critical to understand the mechanisms of psychotropic medications and targeted neurotransmitters when evaluating treatment for DLB. Titrating or augmenting these medications in elderly patients requires the clinician to follow a principle of start low and go slow, making only one change at a time.
It is always helpful to include family members in the patient’s care and to gather information on previous history, personality traits, family history, and cultural components. It is also important to communicate with other specialists to implement collaborative care.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed (text revision). Washington, DC: American Psychiatric Association; 2000:167.
2. National Institute of Mental Health. Abnormal Involuntary Movement Scale (AIMS). www.atlantapsychia try.com/forms/AIMS.pdf. Accessed May 20, 2010.
3. Mini–Mental State Examination. www.nmaging .state.nm.us/pdf_files/Mini_Mental_Status_Exam.pdf. Accessed May 20, 2010.
4. Baskys A. Lewy body dementia: the litmus test for neuroleptic sensitivity and extrapyramidal symptoms. J Clin Psychiatry. 2004;65 suppl 11:16-22.
5. Weisman D, McKeith I. Dementia with Lewy bodies. Semin Neurol. 2007;27(1):42-47.
6. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(3 suppl):417-423.
7. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Neurology. 1996;47(5):1113-1124.
8. Hill C, Reiss N. Lewy body dementia (2008). www.mentalhelp.net/poc/view_doc.php?type=doc& id=13151&cn=231. Accessed May 20, 2010.
9. Lewy Body Dementia Association, Inc. Lewy body dementia: current issues in diagnosis and treatment. www.lewybodydementia.org. Accessed May 20, 2010.
10. Varanese S, Perfetti B, Monaco D, et al. Fluctuating cognition and different cognitive and behavioural profiles in Parkinson’s disease with dementia: comparison of dementia with Lewy bodies and Alzheimer’s disease. J Neurol. 2010 Jan 22. [Epub ahead of print]
11. Kurita A, Murakami M, Takagi S, et al. Visual hallucinations and altered visual information processing in Parkinson disease and dementia with Lewy bodies. Mov Disorder. 2010;25(2):167-171.
12. Gagnon JF, Postuma RB, Mazza S, et al. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5(5):424-432.
13. Dodel R, Csoti I, Ebersbach G, et al. Lewy body dementia and Parkinson’s disease with dementia. J Neurol. 2008;255 suppl 5:39-47.
14. Sonnesyn H, Nilsen DW, Rongve A, et al. High prevalence of orthostatic hypotension in mild dementia. Dement Geriatr Cogn Disord. 2009;28(4):307-313.
15. Kenny RA, Shaw FE, O’Brien JT, et al. Carotid sinus syndrome is common in dementia with Lewy bodies and correlates with deep white matter lesions. J Neurol Neurosurg Psychiatry. 2004;75(7):966-971.
16. Hickey C, Chisholm T, Passmore MJ, et al. Differentiating the dementias: revisiting synucleinopathies and tauopathies. Curr Alzheimer Res. 2008;5(1):52-60.
17. McKeith IG, Burn DJ, Ballard CG, et al. Dementia with Lewy bodies. Semin Clin Neuropsychiatry. 2003; 8(1):46-57.
18. Bird TD, Miller BL. Dementia. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw Hill Medical; 2008:2536-2549.
19. World Health Organization. International Classification of Diseases (ICD), Version 2007. Chapter VI: Diseases of the Central Nervous System. http://apps.who.int/classifications/apps/icd/icd10online/index.htm?kg00.htm+. Accessed May 20, 2010.
20. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
21. Emre M, Cummings JL, Lane RM. Rivastigmine in dementia associated with Parkinson’s disease and Alzheimer’s disease: similarities and differences. J Alzheimers Dis. 2007;11(4):509-519.
22. Lam B, Hollingdrake E, Kennedy JL, et al. Cholinesterase inhibitors in Alzheimer’s disease and Lewy body spectrum disorders: the emerging pharmacogenetic story. Hum Genomics. 2009;4(2):91-106.
23. Wild R, Pettit T, Burns A. Cholinesterase inhibitors for dementia with Lewy bodies. Cochrane Database Syst Rev. 2003;(3):CD003672.
24. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
25. Merck & Co., Inc. Sinemet® CR (carbidopa-levodopa) sustained-release tablets. http://packageinserts.bms.com/pi/pi_sinemet_cr.pdf. Accessed May 20, 2010.
26. Fernandez HH, Wu CK, Ott BR. Pharmacotherapy of dementia with Lewy bodies. Expert Opin Pharmacother. 2003;4(11):2027-2037.
27. Kato K, Wada T, Kawakatsu S, Otani K. Improvement of both psychotic symptoms and Parkinsonism in a case of dementia with Lewy bodies by the combination therapy of risperidone and L-DOPA. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(1):201-203.
28. Yamauchi K, Takehisa M, Tsuno M, et al. Levodopa improved rapid eye movement sleep behavior disorder with diffuse Lewy body disease. Gen Hosp Psychiatry. 2003;25(2):140-142.
29. Stahl SM. The Prescriber’s Guide: Stahl’s Essential Psychopharmacology. Cambridge University Press; 2006:459.
30. Chen JP, Barron C, Lin KM, Chung H. Prescribing medication for Asians with mental disorders. West J Med. 2002;176(4):271-275.
31. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
32. Pollock BG, Mulsant BH, Rosen J, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry. 2002;159(3):460-465.
33. Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med. 2009;76(3):167-174.
34. Gagnon JF, Postuma RB, Montplaisir J. Update on the pharmacology of REM sleep behavior disorder. Neurology. 2006;67(5):742-747
An 80-year-old Mandarin-speaking Chinese woman was referred to a mental health outpatient clinic for evaluation and treatment. The patient had a history of mild depression, for which she had been treated for many years with sertraline.
Five years earlier at age 75, the patient had been evaluated by a psychiatrist after she began to experience psychotic symptoms, including frequent repetitive auditory hallucinations of people counting, alternating with music from her childhood. At that time, she also had persecutory paranoid thoughts and delusional thinking that she was receiving messages in Mandarin while watching American TV programs. Initially, her only cognitive disturbance was an inability to differentiate among numbers on a calendar or a telephone keypad. No reports of memory problems were noted. Although the patient acknowledged auditory hallucinations, she denied experiencing command auditory hallucinations or hallucinations of other forms. The patient had no history of suicide attempts and denied suicidal or homicidal ideation. She had no history of psychiatric hospitalization.
The psychiatrist made a diagnosis of major depressive disorder with psychotic features, not otherwise specified1 and prescribed sertraline 50 mg/d. The patient was also started on risperidone 0.25 mg/d for management of her psychotic symptoms, with the dosage gradually increased to 2.0 mg/d over five years. While taking this combination, the patient experienced stable mood and fewer paranoid thoughts, although her auditory hallucinations continued.
Two months before the current visit, the patient moved into a retirement living facility, and she reported having adapted well to the new setting. She was sleeping well and had a good appetite. Her BMI was within normal range.
The patient described herself as a single parent for nearly 40 years, raising one daughter. Formerly high functioning, she had held a full-time clerical job until age 70. She appeared well-groomed, polite but anxious, and oriented to time, person, and place. Her speech was normal, her thought processes were coherent, and her mood was stable. However, her affect was constricted; she acknowledged auditory hallucinations, which impaired her thought content. The patient reported feeling increased anxiety prior to any nonroutine activity, such as a doctor’s appointment; this, she said, would cause insomnia, leaving her to pace in her room.
During the examination, fine tremors on upper and lower extremities were noted. The patient’s Abnormal Involuntary Movement Scale (AIMS) score2 was 13, which placed her in the highest risk category for antipsychotic-induced dopamine-blockade extrapyramidal symptoms (EPS). The patient was found to be negative for tardive dyskinesia, with no abnormal facial movements. She was aware of the tremors in her limbs and said she felt bothered by them.
The patient had an unsteady gait and used a four-point walker. Her Mini-Mental State Exam (MMSE) score3 was 28/30, which was normal for her age and education level (high school completed).
Apart from the described symptoms, the patient was healthy for her age and had no other medical diagnosis. Her vital signs were within normal range. The medical work-up to rule out other causes of dementia yielded negative results. Lab values were normal, including electrolyte levels and thyroid tests. The patient’s hearing test showed age-related hearing loss of full range, not limited to high pitch. She was able to engage in a meaningful conversation at a normal volume. Clinically, however, it was concerning to observe the possible signs of EPS and the relatively high risperidone dosage, considering the patient’s advanced age.
After the meeting with the patient, a treatment plan was created to 1) gradually reduce the dosage of antipsychotic medication, and 2) refer her to a neurologist for a complete work-up to rule out underlying neurologic disorders, such as dementia. Risperidone was tapered by increments of 0.25 mg/d every three to four weeks; throughout this process, the patient was closely monitored by the nursing staff at the retirement living facility. Monthly appointments were scheduled at the outpatient mental health clinic for evaluation and medication management.
Two months after the initial mental health clinic visit, the patient’s condition was pronounced stable on the current regimen of sertraline 50 mg/d and risperidone 1.0 mg/d. She was later seen by a neurologist, who made a diagnosis of Parkinson’s disease and placed her on carbidopa-levodopa (1 1/2 tablets, 25/100 mg, tid). The patient’s auditory hallucinations continued with the same intensity as at baseline, but fewer tremors were noted in her extremities. By six months into the tapering process (with risperidone reduced at that time to 0.25 mg/d and carbidopa-levodopa to 25/100 mg tid), the patient had begun to experience dissipation of the tremors, and her AIMS score2 was 0. She was able to replace her four-point walker with a cane.
One year after her initial visit to the mental health clinic, the patient’s neurologist suggested replacing risperidone with quetiapine (12.5 mg/d) for its improved tolerability and lower adverse effect profile.4 She continued to take sertraline and carbidopa-levodopa.
Improvement of symptoms was noted following the switch. After one month on the revised regimen, the patient reported that the number of auditory hallucinations persisted, but that their intensity had decreased dramatically. She had a brighter affect and appeared to feel uplifted and more energetic. She became involved in the social activities offered at the retirement living facility and the mental health clinic. She also maintained a steady gait without her cane. According to the patient’s daughter, her mother was at her best psychological state since the onset of psychotic symptoms six years earlier. The pharmacologic regimen had reached its maximum benefit.
At a mental health appointment at the outpatient clinic 18 months after her initial visit there, it was evident that the patient’s auditory hallucinations persisted as a major stressor. She began to complain about other residents in her facility. She said she disliked the resident with whom she shared meals, and she claimed that other residents often spit on the floor in front of her room. The nursing staff did not confirm these incidents, which they considered a delusion despite the patient’s “evidence” (the tissues she said she had used to clean up).
Additionally, a new theme had emerged in the patient’s auditory hallucinations. She reported hearing a male voice that announced changes in meal times. Although she knew there was no public address system in her room or in the hallway, the “announcement” was so convincing that she would go to the dining room and once there, realize that nothing had changed. She seemed to drift between reality and her hallucinations/delusions. According to her daughter, the patient’s independent and reserved personality forced her to internalize her stressors—in this case, her frustration about the other residents—which fed into her hallucinations and delusions.
In response to her worsening psychotic symptoms, the patient’s provider increased her quetiapine dosage from 12.5 mg/d to 25 mg/d. Her MMSE score3 at this visit was 25/30.
Two months later, the patient exhibited increasing symptoms of paranoia, delusions, and auditory hallucinations. She continued to respond to the “broadcast” messages about meal times, and she voiced her frustrations to others who spoke Mandarin. She became agitated in response to out-of-the-ordinary events. When her alarm clock battery ran out, for example, she insisted that “a man’s voice” kept reminding her to replace the battery; in response, she placed the alarm clock in the refrigerator, later explaining, “Now I don’t need to worry about it.”
Her cognitive status began to show obvious, progressive deterioration, with an MMSE score3 of 22/30 at this visit—a significant reduction from previous scores. Worsening of her short-term memory became apparent when she had difficulty playing bingo and was unable to remember her appointment or the current date. She became upset when others corrected her.
In a review of the trends in this patient’s clinical presentation, it became increasingly evident to the patient’s mental health care providers that she had Lewy body dementia.
DISCUSSION
Dementia with Lewy bodies (DLB), a progressive disease, is the second most common cause of neurodegenerative dementia after Alzheimer’s disease.5-7 It is estimated that DLB accounts for 20% of US cases of dementia (ie, about 800,000 patients).8,9 Although public awareness of DLB is on the rise, the disorder is still underrecognized and underdiagnosed because its clinical manifestations so closely resemble those of Alzheimer’s disease, Parkinson’s disease, and psychosis.10,11
Clinical symptoms of DLB include progressive cognitive decline, cognitive fluctuation, EPS, and parkinsonism; hallucinations involving all five senses, particularly sight; delusions; REM sleep disturbance, with or without vivid and frightening dreams; changes in mood and behavior; impaired judgment and insight; and autonomic dysfunction, such as orthostatic hypotension and carotid-sinus hypersensitivity.5,11-15
The symptoms of DLB are caused by the accumulations of Lewy bodies, that is, deposits of alpha-synuclein protein in the nuclei of neurons. Lewy bodies destroy neurons over time, resulting in the destruction of dopaminergic and acetylcholinergic pathways from the brain stem to areas of the cerebral cortex associated with cognition and motor functions.4,5,16
DLB is a spectrum disorder; it often coexists with Parkinson’s disease or Alzheimer’s disease, as Lewy bodies are also found in patients with these illnesses.7 This poses a challenge for formulating a differential diagnosis, particularly in patients with fluctuating cognition,10 and for attempting to establish disease prevalence.
Diagnosis
Currently, a conclusive diagnosis of DLB can be confirmed only through postmortem autopsy, although use of medial temporal lobe volume (via structural MRI) and regional blood flow (via single photon emission CT [SPECT] tracers) is being investigated.17
The diagnosis of DLB is currently based on the presenting clinical symptoms and the exclusion of other medical conditions whose symptoms mimic those of DLB.7 The screening assessment may include a neurologic/psychiatric assessment (MMSE, psychiatric evaluation, and interviews with family members or caretakers), neuroimaging such as MRI to rule out other organic causes, and laboratory evaluation to rule out potentially reversible causes of dementia, including electrolyte imbalance, vitamin deficiency (specifically vitamin B12), anemia, thyroid dysfunction, and kidney or liver impairment.18
The American Psychiatric Assocation1 categorizes DLB under “Dementia Due to Other General Medical Conditions” (294.1x). The World Health Organization19 includes it among “Other specified degenerative diseases of the nervous system” (G31.8).
Treatment
Lewy body dementia is an irreversible neurologic degenerative disorder. Treatment for DLB comprises symptom management, primarily through pharmacology; however, the response to medication is highly individualized. Treatment includes management of the following symptoms:
Cognitive impairment. Cholinesterase inhibitors, such as rivastigmine (3 to 12 mg/d), donepezil (10 mg/d), or galantamine (titrated up to 12 mg bid),20-23 improve attention and behavior and reduce apathy, anxiety, delusions, and hallucinations. As cognitive impairment worsens, memantine (10 mg bid) may be effective.24 The potential for anticholinergic adverse effects requires close monitoring in patients taking these agents.
Parkinsonian symptoms. Medications indicated for Parkinson’s disease and syndrome, such as carbidopa-levodopa (25/100 mg tid), can be effective; dosage may be slowly titrated upward as tolerated and if needed for symptom management.25,26 The dopaminergic effect of antiparkinson medications may intensify the psychotic symptoms and worsen the REM sleep pattern. In this case, a low-dose atypical antipsychotic is suggested27,28 (see below).
Psychotic symptoms. An atypical antipsychotic agent, such as quetiapine (12.5 mg), risperidone (0.25 mg), olanzapine (2.5 mg), ziprasidone (20 mg), aripiprazole (2 mg), or paliperidone (1.5 mg), may be used. Because of the DLB-associated risk of neuroleptic sensitivity, atypical antipsychotic agents should be initiated at a low dose with slow upward titration17,26,29; quetiapine appears less likely than risperidone or olanzapine to cause neuroleptic sensitivity or to trigger EPS.4 For Asian patients, who often respond to lower doses of these medications (and are more easily affected by associated adverse effects), Chen et al30 recommend a starting dose of about one-half the recommended dose.
Depression. An SSRI antidepressant with relatively simple pharmacologic properties and moderate half-life may be used to manage symptoms of depression.26,31,32 Long–half-life SSRIs (eg, fluoxetine) should be avoided in elderly patients; in response to SNRIs (serotonin-norepinephrine reuptake inhibitors), these patients may experience elevated blood pressures and pulses, with subsequent morbidity.33
REM sleep disturbances. Clonazepam (0.25 mg), melatonin (3.0 mg), or quetiapine (12.5 mg) may be administered at bedtime.34
Important Lessons
In general, providers should consider the benefits and risks of any pharmacologic treatment and avoid polypharmacy, if possible. Family and caretakers should be included in the treatment planning, with a focus on prioritizing and managing the most debilitating symptoms or dysfunctions that prompt concerns for safety.
For optimal homeostasis, some DLB patients may require joint pharmacologic modalities that appear counterintuitive—for example, an antiparkinsonism (dopaminergic) agent for parkinsonian symptoms or neuroleptic-induced EPS, versus an antipsychotic (eg, a dopamine antagonist) to treat profound hallucinations.26
As the response to treatment for DLB is highly individualized, it is essential to titrate and augment with care.
CONCLUSION
In DLB, as with other dementing illnesses, the onset of symptoms can be gradual and insidious, posing a great challenge to the clinician who seeks to confirm the diagnosis. In the illness’s early stages, the clinician may have to treat targeted symptoms and adjust the treatment plan once signs of the pathologic origins emerge.
It is critical to understand the mechanisms of psychotropic medications and targeted neurotransmitters when evaluating treatment for DLB. Titrating or augmenting these medications in elderly patients requires the clinician to follow a principle of start low and go slow, making only one change at a time.
It is always helpful to include family members in the patient’s care and to gather information on previous history, personality traits, family history, and cultural components. It is also important to communicate with other specialists to implement collaborative care.
An 80-year-old Mandarin-speaking Chinese woman was referred to a mental health outpatient clinic for evaluation and treatment. The patient had a history of mild depression, for which she had been treated for many years with sertraline.
Five years earlier at age 75, the patient had been evaluated by a psychiatrist after she began to experience psychotic symptoms, including frequent repetitive auditory hallucinations of people counting, alternating with music from her childhood. At that time, she also had persecutory paranoid thoughts and delusional thinking that she was receiving messages in Mandarin while watching American TV programs. Initially, her only cognitive disturbance was an inability to differentiate among numbers on a calendar or a telephone keypad. No reports of memory problems were noted. Although the patient acknowledged auditory hallucinations, she denied experiencing command auditory hallucinations or hallucinations of other forms. The patient had no history of suicide attempts and denied suicidal or homicidal ideation. She had no history of psychiatric hospitalization.
The psychiatrist made a diagnosis of major depressive disorder with psychotic features, not otherwise specified1 and prescribed sertraline 50 mg/d. The patient was also started on risperidone 0.25 mg/d for management of her psychotic symptoms, with the dosage gradually increased to 2.0 mg/d over five years. While taking this combination, the patient experienced stable mood and fewer paranoid thoughts, although her auditory hallucinations continued.
Two months before the current visit, the patient moved into a retirement living facility, and she reported having adapted well to the new setting. She was sleeping well and had a good appetite. Her BMI was within normal range.
The patient described herself as a single parent for nearly 40 years, raising one daughter. Formerly high functioning, she had held a full-time clerical job until age 70. She appeared well-groomed, polite but anxious, and oriented to time, person, and place. Her speech was normal, her thought processes were coherent, and her mood was stable. However, her affect was constricted; she acknowledged auditory hallucinations, which impaired her thought content. The patient reported feeling increased anxiety prior to any nonroutine activity, such as a doctor’s appointment; this, she said, would cause insomnia, leaving her to pace in her room.
During the examination, fine tremors on upper and lower extremities were noted. The patient’s Abnormal Involuntary Movement Scale (AIMS) score2 was 13, which placed her in the highest risk category for antipsychotic-induced dopamine-blockade extrapyramidal symptoms (EPS). The patient was found to be negative for tardive dyskinesia, with no abnormal facial movements. She was aware of the tremors in her limbs and said she felt bothered by them.
The patient had an unsteady gait and used a four-point walker. Her Mini-Mental State Exam (MMSE) score3 was 28/30, which was normal for her age and education level (high school completed).
Apart from the described symptoms, the patient was healthy for her age and had no other medical diagnosis. Her vital signs were within normal range. The medical work-up to rule out other causes of dementia yielded negative results. Lab values were normal, including electrolyte levels and thyroid tests. The patient’s hearing test showed age-related hearing loss of full range, not limited to high pitch. She was able to engage in a meaningful conversation at a normal volume. Clinically, however, it was concerning to observe the possible signs of EPS and the relatively high risperidone dosage, considering the patient’s advanced age.
After the meeting with the patient, a treatment plan was created to 1) gradually reduce the dosage of antipsychotic medication, and 2) refer her to a neurologist for a complete work-up to rule out underlying neurologic disorders, such as dementia. Risperidone was tapered by increments of 0.25 mg/d every three to four weeks; throughout this process, the patient was closely monitored by the nursing staff at the retirement living facility. Monthly appointments were scheduled at the outpatient mental health clinic for evaluation and medication management.
Two months after the initial mental health clinic visit, the patient’s condition was pronounced stable on the current regimen of sertraline 50 mg/d and risperidone 1.0 mg/d. She was later seen by a neurologist, who made a diagnosis of Parkinson’s disease and placed her on carbidopa-levodopa (1 1/2 tablets, 25/100 mg, tid). The patient’s auditory hallucinations continued with the same intensity as at baseline, but fewer tremors were noted in her extremities. By six months into the tapering process (with risperidone reduced at that time to 0.25 mg/d and carbidopa-levodopa to 25/100 mg tid), the patient had begun to experience dissipation of the tremors, and her AIMS score2 was 0. She was able to replace her four-point walker with a cane.
One year after her initial visit to the mental health clinic, the patient’s neurologist suggested replacing risperidone with quetiapine (12.5 mg/d) for its improved tolerability and lower adverse effect profile.4 She continued to take sertraline and carbidopa-levodopa.
Improvement of symptoms was noted following the switch. After one month on the revised regimen, the patient reported that the number of auditory hallucinations persisted, but that their intensity had decreased dramatically. She had a brighter affect and appeared to feel uplifted and more energetic. She became involved in the social activities offered at the retirement living facility and the mental health clinic. She also maintained a steady gait without her cane. According to the patient’s daughter, her mother was at her best psychological state since the onset of psychotic symptoms six years earlier. The pharmacologic regimen had reached its maximum benefit.
At a mental health appointment at the outpatient clinic 18 months after her initial visit there, it was evident that the patient’s auditory hallucinations persisted as a major stressor. She began to complain about other residents in her facility. She said she disliked the resident with whom she shared meals, and she claimed that other residents often spit on the floor in front of her room. The nursing staff did not confirm these incidents, which they considered a delusion despite the patient’s “evidence” (the tissues she said she had used to clean up).
Additionally, a new theme had emerged in the patient’s auditory hallucinations. She reported hearing a male voice that announced changes in meal times. Although she knew there was no public address system in her room or in the hallway, the “announcement” was so convincing that she would go to the dining room and once there, realize that nothing had changed. She seemed to drift between reality and her hallucinations/delusions. According to her daughter, the patient’s independent and reserved personality forced her to internalize her stressors—in this case, her frustration about the other residents—which fed into her hallucinations and delusions.
In response to her worsening psychotic symptoms, the patient’s provider increased her quetiapine dosage from 12.5 mg/d to 25 mg/d. Her MMSE score3 at this visit was 25/30.
Two months later, the patient exhibited increasing symptoms of paranoia, delusions, and auditory hallucinations. She continued to respond to the “broadcast” messages about meal times, and she voiced her frustrations to others who spoke Mandarin. She became agitated in response to out-of-the-ordinary events. When her alarm clock battery ran out, for example, she insisted that “a man’s voice” kept reminding her to replace the battery; in response, she placed the alarm clock in the refrigerator, later explaining, “Now I don’t need to worry about it.”
Her cognitive status began to show obvious, progressive deterioration, with an MMSE score3 of 22/30 at this visit—a significant reduction from previous scores. Worsening of her short-term memory became apparent when she had difficulty playing bingo and was unable to remember her appointment or the current date. She became upset when others corrected her.
In a review of the trends in this patient’s clinical presentation, it became increasingly evident to the patient’s mental health care providers that she had Lewy body dementia.
DISCUSSION
Dementia with Lewy bodies (DLB), a progressive disease, is the second most common cause of neurodegenerative dementia after Alzheimer’s disease.5-7 It is estimated that DLB accounts for 20% of US cases of dementia (ie, about 800,000 patients).8,9 Although public awareness of DLB is on the rise, the disorder is still underrecognized and underdiagnosed because its clinical manifestations so closely resemble those of Alzheimer’s disease, Parkinson’s disease, and psychosis.10,11
Clinical symptoms of DLB include progressive cognitive decline, cognitive fluctuation, EPS, and parkinsonism; hallucinations involving all five senses, particularly sight; delusions; REM sleep disturbance, with or without vivid and frightening dreams; changes in mood and behavior; impaired judgment and insight; and autonomic dysfunction, such as orthostatic hypotension and carotid-sinus hypersensitivity.5,11-15
The symptoms of DLB are caused by the accumulations of Lewy bodies, that is, deposits of alpha-synuclein protein in the nuclei of neurons. Lewy bodies destroy neurons over time, resulting in the destruction of dopaminergic and acetylcholinergic pathways from the brain stem to areas of the cerebral cortex associated with cognition and motor functions.4,5,16
DLB is a spectrum disorder; it often coexists with Parkinson’s disease or Alzheimer’s disease, as Lewy bodies are also found in patients with these illnesses.7 This poses a challenge for formulating a differential diagnosis, particularly in patients with fluctuating cognition,10 and for attempting to establish disease prevalence.
Diagnosis
Currently, a conclusive diagnosis of DLB can be confirmed only through postmortem autopsy, although use of medial temporal lobe volume (via structural MRI) and regional blood flow (via single photon emission CT [SPECT] tracers) is being investigated.17
The diagnosis of DLB is currently based on the presenting clinical symptoms and the exclusion of other medical conditions whose symptoms mimic those of DLB.7 The screening assessment may include a neurologic/psychiatric assessment (MMSE, psychiatric evaluation, and interviews with family members or caretakers), neuroimaging such as MRI to rule out other organic causes, and laboratory evaluation to rule out potentially reversible causes of dementia, including electrolyte imbalance, vitamin deficiency (specifically vitamin B12), anemia, thyroid dysfunction, and kidney or liver impairment.18
The American Psychiatric Assocation1 categorizes DLB under “Dementia Due to Other General Medical Conditions” (294.1x). The World Health Organization19 includes it among “Other specified degenerative diseases of the nervous system” (G31.8).
Treatment
Lewy body dementia is an irreversible neurologic degenerative disorder. Treatment for DLB comprises symptom management, primarily through pharmacology; however, the response to medication is highly individualized. Treatment includes management of the following symptoms:
Cognitive impairment. Cholinesterase inhibitors, such as rivastigmine (3 to 12 mg/d), donepezil (10 mg/d), or galantamine (titrated up to 12 mg bid),20-23 improve attention and behavior and reduce apathy, anxiety, delusions, and hallucinations. As cognitive impairment worsens, memantine (10 mg bid) may be effective.24 The potential for anticholinergic adverse effects requires close monitoring in patients taking these agents.
Parkinsonian symptoms. Medications indicated for Parkinson’s disease and syndrome, such as carbidopa-levodopa (25/100 mg tid), can be effective; dosage may be slowly titrated upward as tolerated and if needed for symptom management.25,26 The dopaminergic effect of antiparkinson medications may intensify the psychotic symptoms and worsen the REM sleep pattern. In this case, a low-dose atypical antipsychotic is suggested27,28 (see below).
Psychotic symptoms. An atypical antipsychotic agent, such as quetiapine (12.5 mg), risperidone (0.25 mg), olanzapine (2.5 mg), ziprasidone (20 mg), aripiprazole (2 mg), or paliperidone (1.5 mg), may be used. Because of the DLB-associated risk of neuroleptic sensitivity, atypical antipsychotic agents should be initiated at a low dose with slow upward titration17,26,29; quetiapine appears less likely than risperidone or olanzapine to cause neuroleptic sensitivity or to trigger EPS.4 For Asian patients, who often respond to lower doses of these medications (and are more easily affected by associated adverse effects), Chen et al30 recommend a starting dose of about one-half the recommended dose.
Depression. An SSRI antidepressant with relatively simple pharmacologic properties and moderate half-life may be used to manage symptoms of depression.26,31,32 Long–half-life SSRIs (eg, fluoxetine) should be avoided in elderly patients; in response to SNRIs (serotonin-norepinephrine reuptake inhibitors), these patients may experience elevated blood pressures and pulses, with subsequent morbidity.33
REM sleep disturbances. Clonazepam (0.25 mg), melatonin (3.0 mg), or quetiapine (12.5 mg) may be administered at bedtime.34
Important Lessons
In general, providers should consider the benefits and risks of any pharmacologic treatment and avoid polypharmacy, if possible. Family and caretakers should be included in the treatment planning, with a focus on prioritizing and managing the most debilitating symptoms or dysfunctions that prompt concerns for safety.
For optimal homeostasis, some DLB patients may require joint pharmacologic modalities that appear counterintuitive—for example, an antiparkinsonism (dopaminergic) agent for parkinsonian symptoms or neuroleptic-induced EPS, versus an antipsychotic (eg, a dopamine antagonist) to treat profound hallucinations.26
As the response to treatment for DLB is highly individualized, it is essential to titrate and augment with care.
CONCLUSION
In DLB, as with other dementing illnesses, the onset of symptoms can be gradual and insidious, posing a great challenge to the clinician who seeks to confirm the diagnosis. In the illness’s early stages, the clinician may have to treat targeted symptoms and adjust the treatment plan once signs of the pathologic origins emerge.
It is critical to understand the mechanisms of psychotropic medications and targeted neurotransmitters when evaluating treatment for DLB. Titrating or augmenting these medications in elderly patients requires the clinician to follow a principle of start low and go slow, making only one change at a time.
It is always helpful to include family members in the patient’s care and to gather information on previous history, personality traits, family history, and cultural components. It is also important to communicate with other specialists to implement collaborative care.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed (text revision). Washington, DC: American Psychiatric Association; 2000:167.
2. National Institute of Mental Health. Abnormal Involuntary Movement Scale (AIMS). www.atlantapsychia try.com/forms/AIMS.pdf. Accessed May 20, 2010.
3. Mini–Mental State Examination. www.nmaging .state.nm.us/pdf_files/Mini_Mental_Status_Exam.pdf. Accessed May 20, 2010.
4. Baskys A. Lewy body dementia: the litmus test for neuroleptic sensitivity and extrapyramidal symptoms. J Clin Psychiatry. 2004;65 suppl 11:16-22.
5. Weisman D, McKeith I. Dementia with Lewy bodies. Semin Neurol. 2007;27(1):42-47.
6. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(3 suppl):417-423.
7. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Neurology. 1996;47(5):1113-1124.
8. Hill C, Reiss N. Lewy body dementia (2008). www.mentalhelp.net/poc/view_doc.php?type=doc& id=13151&cn=231. Accessed May 20, 2010.
9. Lewy Body Dementia Association, Inc. Lewy body dementia: current issues in diagnosis and treatment. www.lewybodydementia.org. Accessed May 20, 2010.
10. Varanese S, Perfetti B, Monaco D, et al. Fluctuating cognition and different cognitive and behavioural profiles in Parkinson’s disease with dementia: comparison of dementia with Lewy bodies and Alzheimer’s disease. J Neurol. 2010 Jan 22. [Epub ahead of print]
11. Kurita A, Murakami M, Takagi S, et al. Visual hallucinations and altered visual information processing in Parkinson disease and dementia with Lewy bodies. Mov Disorder. 2010;25(2):167-171.
12. Gagnon JF, Postuma RB, Mazza S, et al. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5(5):424-432.
13. Dodel R, Csoti I, Ebersbach G, et al. Lewy body dementia and Parkinson’s disease with dementia. J Neurol. 2008;255 suppl 5:39-47.
14. Sonnesyn H, Nilsen DW, Rongve A, et al. High prevalence of orthostatic hypotension in mild dementia. Dement Geriatr Cogn Disord. 2009;28(4):307-313.
15. Kenny RA, Shaw FE, O’Brien JT, et al. Carotid sinus syndrome is common in dementia with Lewy bodies and correlates with deep white matter lesions. J Neurol Neurosurg Psychiatry. 2004;75(7):966-971.
16. Hickey C, Chisholm T, Passmore MJ, et al. Differentiating the dementias: revisiting synucleinopathies and tauopathies. Curr Alzheimer Res. 2008;5(1):52-60.
17. McKeith IG, Burn DJ, Ballard CG, et al. Dementia with Lewy bodies. Semin Clin Neuropsychiatry. 2003; 8(1):46-57.
18. Bird TD, Miller BL. Dementia. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw Hill Medical; 2008:2536-2549.
19. World Health Organization. International Classification of Diseases (ICD), Version 2007. Chapter VI: Diseases of the Central Nervous System. http://apps.who.int/classifications/apps/icd/icd10online/index.htm?kg00.htm+. Accessed May 20, 2010.
20. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
21. Emre M, Cummings JL, Lane RM. Rivastigmine in dementia associated with Parkinson’s disease and Alzheimer’s disease: similarities and differences. J Alzheimers Dis. 2007;11(4):509-519.
22. Lam B, Hollingdrake E, Kennedy JL, et al. Cholinesterase inhibitors in Alzheimer’s disease and Lewy body spectrum disorders: the emerging pharmacogenetic story. Hum Genomics. 2009;4(2):91-106.
23. Wild R, Pettit T, Burns A. Cholinesterase inhibitors for dementia with Lewy bodies. Cochrane Database Syst Rev. 2003;(3):CD003672.
24. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
25. Merck & Co., Inc. Sinemet® CR (carbidopa-levodopa) sustained-release tablets. http://packageinserts.bms.com/pi/pi_sinemet_cr.pdf. Accessed May 20, 2010.
26. Fernandez HH, Wu CK, Ott BR. Pharmacotherapy of dementia with Lewy bodies. Expert Opin Pharmacother. 2003;4(11):2027-2037.
27. Kato K, Wada T, Kawakatsu S, Otani K. Improvement of both psychotic symptoms and Parkinsonism in a case of dementia with Lewy bodies by the combination therapy of risperidone and L-DOPA. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(1):201-203.
28. Yamauchi K, Takehisa M, Tsuno M, et al. Levodopa improved rapid eye movement sleep behavior disorder with diffuse Lewy body disease. Gen Hosp Psychiatry. 2003;25(2):140-142.
29. Stahl SM. The Prescriber’s Guide: Stahl’s Essential Psychopharmacology. Cambridge University Press; 2006:459.
30. Chen JP, Barron C, Lin KM, Chung H. Prescribing medication for Asians with mental disorders. West J Med. 2002;176(4):271-275.
31. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
32. Pollock BG, Mulsant BH, Rosen J, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry. 2002;159(3):460-465.
33. Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med. 2009;76(3):167-174.
34. Gagnon JF, Postuma RB, Montplaisir J. Update on the pharmacology of REM sleep behavior disorder. Neurology. 2006;67(5):742-747
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed (text revision). Washington, DC: American Psychiatric Association; 2000:167.
2. National Institute of Mental Health. Abnormal Involuntary Movement Scale (AIMS). www.atlantapsychia try.com/forms/AIMS.pdf. Accessed May 20, 2010.
3. Mini–Mental State Examination. www.nmaging .state.nm.us/pdf_files/Mini_Mental_Status_Exam.pdf. Accessed May 20, 2010.
4. Baskys A. Lewy body dementia: the litmus test for neuroleptic sensitivity and extrapyramidal symptoms. J Clin Psychiatry. 2004;65 suppl 11:16-22.
5. Weisman D, McKeith I. Dementia with Lewy bodies. Semin Neurol. 2007;27(1):42-47.
6. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(3 suppl):417-423.
7. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Neurology. 1996;47(5):1113-1124.
8. Hill C, Reiss N. Lewy body dementia (2008). www.mentalhelp.net/poc/view_doc.php?type=doc& id=13151&cn=231. Accessed May 20, 2010.
9. Lewy Body Dementia Association, Inc. Lewy body dementia: current issues in diagnosis and treatment. www.lewybodydementia.org. Accessed May 20, 2010.
10. Varanese S, Perfetti B, Monaco D, et al. Fluctuating cognition and different cognitive and behavioural profiles in Parkinson’s disease with dementia: comparison of dementia with Lewy bodies and Alzheimer’s disease. J Neurol. 2010 Jan 22. [Epub ahead of print]
11. Kurita A, Murakami M, Takagi S, et al. Visual hallucinations and altered visual information processing in Parkinson disease and dementia with Lewy bodies. Mov Disorder. 2010;25(2):167-171.
12. Gagnon JF, Postuma RB, Mazza S, et al. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5(5):424-432.
13. Dodel R, Csoti I, Ebersbach G, et al. Lewy body dementia and Parkinson’s disease with dementia. J Neurol. 2008;255 suppl 5:39-47.
14. Sonnesyn H, Nilsen DW, Rongve A, et al. High prevalence of orthostatic hypotension in mild dementia. Dement Geriatr Cogn Disord. 2009;28(4):307-313.
15. Kenny RA, Shaw FE, O’Brien JT, et al. Carotid sinus syndrome is common in dementia with Lewy bodies and correlates with deep white matter lesions. J Neurol Neurosurg Psychiatry. 2004;75(7):966-971.
16. Hickey C, Chisholm T, Passmore MJ, et al. Differentiating the dementias: revisiting synucleinopathies and tauopathies. Curr Alzheimer Res. 2008;5(1):52-60.
17. McKeith IG, Burn DJ, Ballard CG, et al. Dementia with Lewy bodies. Semin Clin Neuropsychiatry. 2003; 8(1):46-57.
18. Bird TD, Miller BL. Dementia. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw Hill Medical; 2008:2536-2549.
19. World Health Organization. International Classification of Diseases (ICD), Version 2007. Chapter VI: Diseases of the Central Nervous System. http://apps.who.int/classifications/apps/icd/icd10online/index.htm?kg00.htm+. Accessed May 20, 2010.
20. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
21. Emre M, Cummings JL, Lane RM. Rivastigmine in dementia associated with Parkinson’s disease and Alzheimer’s disease: similarities and differences. J Alzheimers Dis. 2007;11(4):509-519.
22. Lam B, Hollingdrake E, Kennedy JL, et al. Cholinesterase inhibitors in Alzheimer’s disease and Lewy body spectrum disorders: the emerging pharmacogenetic story. Hum Genomics. 2009;4(2):91-106.
23. Wild R, Pettit T, Burns A. Cholinesterase inhibitors for dementia with Lewy bodies. Cochrane Database Syst Rev. 2003;(3):CD003672.
24. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
25. Merck & Co., Inc. Sinemet® CR (carbidopa-levodopa) sustained-release tablets. http://packageinserts.bms.com/pi/pi_sinemet_cr.pdf. Accessed May 20, 2010.
26. Fernandez HH, Wu CK, Ott BR. Pharmacotherapy of dementia with Lewy bodies. Expert Opin Pharmacother. 2003;4(11):2027-2037.
27. Kato K, Wada T, Kawakatsu S, Otani K. Improvement of both psychotic symptoms and Parkinsonism in a case of dementia with Lewy bodies by the combination therapy of risperidone and L-DOPA. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(1):201-203.
28. Yamauchi K, Takehisa M, Tsuno M, et al. Levodopa improved rapid eye movement sleep behavior disorder with diffuse Lewy body disease. Gen Hosp Psychiatry. 2003;25(2):140-142.
29. Stahl SM. The Prescriber’s Guide: Stahl’s Essential Psychopharmacology. Cambridge University Press; 2006:459.
30. Chen JP, Barron C, Lin KM, Chung H. Prescribing medication for Asians with mental disorders. West J Med. 2002;176(4):271-275.
31. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
32. Pollock BG, Mulsant BH, Rosen J, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry. 2002;159(3):460-465.
33. Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med. 2009;76(3):167-174.
34. Gagnon JF, Postuma RB, Montplaisir J. Update on the pharmacology of REM sleep behavior disorder. Neurology. 2006;67(5):742-747
Noninvasive positive pressure ventilation: Increasing use in acute care
Noninvasive positive pressure ventilation (NIPPV)—delivered via a tight-fitting mask rather than via an endotracheal tube or tracheostomy—is one of the most important advances in the management of acute respiratory failure to emerge in the past 2 decades. It is now recommended as the first choice for ventilatory support in selected patients, such as those with exacerbations of chronic obstructive pulmonary disease (COPD) or with cardiogenic pulmonary edema.1–3 In fact, some authors suggest that using NIPPV in more than 20% of COPD patients is a characteristic of respiratory care departments that are “avid for change”4—change being a good thing.
However, NIPPV has not been universally accepted, with wide variations in its utilization. In a 2006 survey, it was being used in only 33% of patients with COPD or congestive heart failure, for which it might be indicated. 5 Some potential reasons for the low rate are that physicians do not know about it, respiratory therapists are not sufficiently trained in it, and hospitals lack the equipment to do it.5
Our goal in this review is to familiarize the reader with how NIPPV has evolved and with its indications and contraindications in specific acute care conditions.
FROM A VACUUM CLEANER TO THE INTENSIVE CARE UNIT
NIPPV appears to have been first tried in 1870 by Chaussier, who used a bag and face mask to resuscitate neonates.6
In 1936, Poulton and Oxon7 described their “pulmonary plus pressure machine,” which used a vacuum cleaner blower and a mask to increase the alveolar pressure and thus counteract the increased intrapulmonary pressure in patients with heart failure, pulmonary edema, Cheyne-Stokes breathing, and asthma.
In the 1940s, intermittent positive pressure breathing devices were developed for use in high-altitude aviation. Motley, Werko, and Cournand8,9 subsequently used these devices to treat acute respiratory failure in pneumonia, pulmonary edema, near-drowning, Guillain-Barré syndrome, and acute severe asthma.
Although NIPPV was shown to be effective for acute conditions, invasive ventilation became preferred, particularly as blood gas analysis and ventilator technologies simultaneously matured, spurred at least in part by the polio epidemics of the 1950s.10
NIPPV reemerged in the 1980s for use in chronic conditions. First, continuous positive airway pressure (CPAP) came into use for obstructive sleep apnea,11 followed by noninvasive positive-pressure volume ventilation in neuromuscular diseases.12 Bilevel positive pressure devices (ie, with separate inspiratory and expiratory pressures) soon followed, again initially for obstructive sleep apnea13 and then for diverse neuromuscular diseases.14
NIPPV is now a mainstream therapy for diverse conditions in acute and chronic care.3 One reason we now use it in acute conditions is to avoid the complications associated with intubation.
Some clinicians initially resisted using NIPPV, concerned that it demanded too much of the nurses’ time15 and was costly.16 However, in a 1997 study in patients with COPD and acute respiratory failure, Nava et al17 found that NIPPV was no more expensive and no more demanding of staff resources than invasive mechanical ventilation in the first 48 hours of ventilation. Further, after the first few days of ventilation, NIPPV put fewer time demands on physicians and nurses than did invasive mechanical ventilation.
THREE MODES: CPAP, PRESSURE-LIMITED, VOLUME-LIMITED
The term “noninvasive ventilation” generally encompasses various forms of positive pressure ventilation. However, negative pressure ventilation, in the form of diaphragm pacing, may regain a foothold in the devices used for respiratory support.18 We therefore favor the term “NIPPV” in this review.
NIPPV IN ACUTE RESPIRATORY FAILURE
The main reasons to use NIPPV instead of invasive ventilation in acute care are to avoid the complications of invasive ventilation, to improve outcomes (eg, reduce mortality rates, decrease hospital length of stay), and to decrease the cost of care.
NIPPV is the standard of care for acute exacerbations of COPD
In a meta-analysis of eight randomized controlled trials,24 the specific advantages of NIPPV compared with usual care in acute exacerbations of COPD included:
- A lower risk of treatment failure, defined as death, need for intubation, or inability to tolerate the treatment (relative risk [RR] 0.51, number needed to treat [NNT] to prevent one treatment failure = 5)
- A lower risk of intubation (RR 0.43, NNT = 5)
- A lower mortality rate (RR 0.41, NNT = 8)
- A lower risk of complications (RR 0.32, NNT = 3)
- A shorter hospital length of stay (by about 3 days).
Mechanisms by which NIPPV may impart these benefits include reducing the work of breathing, unloading the respiratory muscles, lessening diaphragmatic pressure swings, reducing the respiratory rate, eliminating diaphragmatic work, and counteracting the threshold loading effects of auto-positive end-expiratory pressure (auto-PEEP).24–26
Also, if a patient with COPD is intubated, NIPPV seems to help after the tube is removed, preventing postextubation respiratory failure and facilitating weaning from invasive ventilation.27 These topics are discussed below.
A Cochrane systematic review24 concluded that NIPPV should be tried early in the course of respiratory failure, before severe acidosis develops. The patients in the studies in this review all had partial pressure of arterial carbon dioxide (Paco2) levels greater than 45 mm Hg.
In patients with severe respiratory acidosis (pH < 7.25), NIPPV failure rates are greater than 50%. However, trying NIPPV may still be justified, even in the presence of hypercapnic encephalopathy, as long as no other indications for invasive support and facilities for prompt endotracheal intubation are available. 1
However, in another systematic review,26 in patients with mild COPD exacerbations (pH > 7.35), NIPPV was no more effective than standard medical therapy in preventing acute respiratory failure, preventing death, or reducing length of hospitalization. Moreover, nearly 50% of the patients could not tolerate NIPPV.
Rapid improvement in cardiogenic pulmonary edema, but possibly no lower mortality rate
The Three Interventions in Cardiogenic Pulmonary Oedema (3CPO) trial,28 with 1,156 patients, was the largest randomized trial to compare NIPPV and standard oxygen therapy for acute pulmonary edema. It found that NIPPV (either CPAP or noninvasive intermittent positive pressure ventilation) was significantly better than standard oxygen therapy (through a variable-delivery oxygen mask with a reservoir) in the first hour of treatment in terms of the dyspnea score, heart rate, acidosis, and hypercapnia. However, there were no significant differences between groups in the 7- or 30-day mortality rates, the rates of intubation, rates of admission to the critical care unit, or in the mean length of hospital stay.
In contrast, several smaller randomized trials and meta-analyses showed lower intubation and mortality rates with NIPPV.29,30 Factors that may account for those differences include a much lower intubation rate in the 3CPO trial (2.9% overall, compared with 20% with conventional therapy in other trials), a higher mortality rate in the 3CPO trial, and methodologic differences (eg, patients for whom standard therapy failed in the 3CPO trial received rescue NIPPV).
If NIPPV is beneficial in cardiogenic pulmonary edema, the mechanisms are probably its favorable hemodynamic effects and its positive end-expiratory pressure (PEEP) effect on flooded alveoli. Specifically, positive intrathoracic pressure can be expected to reduce both preload and afterload, with improvement in the cardiac index and reduced work of breathing. 31,32
Notwithstanding the possible lack of impact of NIPPV on death or intubation rates in this setting, the intervention rapidly improves dyspnea and respiratory and metabolic abnormalities and should be considered for treatment of cardiogenic pulmonary edema associated with severe respiratory distress. A subgroup in which the NIPPV may reduce intubation rates is those with hypercapnia.33 A concern that NIPPV may increase the rate of myocardial infarction34 was not confirmed in the 3CPO trial.28 Interestingly, there were no differences in outcomes between CPAP and noninvasive intermittent positive pressure ventilation in this setting.28,34,35
Immunocompromised patients with acute respiratory failure
A particular challenge of NIPPV in immunocompromised patients, particularly compared with its use in COPD exacerbation or cardiogenic pulmonary edema, is that the underlying pathophysiology of respiratory dysfunction in immunocompromised patients may not be readily reversible. Therefore, its application in this group may need to follow clearly defined indications.
In one trial,20 inclusion criteria were:
- Immune suppression (due to neutropenia after chemotherapy or bone marrow transplantation, immunosuppressive drugs for organ transplantation, corticosteroids, cytotoxic therapy for nonmalignant conditions, or the acquired immunodeficiency syndrome)
- Persistent pulmonary infiltrates
- Fever (temperature > 38.3°C; 100.9°F)
- A respiratory rate greater than 30 breaths per minute
- Severe dyspnea at rest
- Early hypoxemic acute respiratory failure, defined as a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (Pao2/Fio2 ratio) less than 200 while on oxygen.
Compared with patients who received conventional treatment, fewer of those randomized to additional intermittent noninvasive ventilation had to be intubated (46% vs 77%, P = .03), suffered serious complications (50% vs 81%, P = .02), or died in the intensive care unit (38% vs 69%, P = .03) or in the hospital (50% vs 81%, P = .02).
Similarly, in a randomized trial in 40 patients with acute respiratory failure after solid organ transplantation, more patients in the NIPPV group than in the control group had an improvement in the Pao2/Fio2 ratio within the first hour (70% vs 25%, P = .004) or a sustained improvement in the Pao2/Fio2 ratio (60% vs 25%, P = .03); fewer of them needed endotracheal intubation (20% vs 70%, P = .002); fewer of them died of complications (20% vs 50%, P = .05); they had a shorter length of stay in the intensive care unit (mean 5.5 vs 9 days, P = .03); and fewer of them died in the intensive care unit (20% vs 50%, P = .05). There was, however, no difference in the overall hospital mortality rate.36
MAY NOT HELP AFTER EXTUBATION, EXCEPT IN SPECIFIC CASES
NIPPV has been used to treat respiratory failure after extubation,22,37 to prevent acute respiratory failure after failure of weaning,38–41 and to support breathing in patients who failed a trial of spontaneous breathing.42–45
Unfortunately, the evidence for using NIPPV in respiratory failure after extubation, including unplanned extubation, appears to be unfavorable, except possibly in patients with chronic pulmonary disease (particularly COPD and possibly obesity) and hypercapnia. An international consensus report stated that NIPPV should be considered in patients with hypercapnic respiratory insufficiency, especially those with COPD, to shorten the duration of intubation, but that it should not be routinely used in extubation respiratory failure.46
Treatment of respiratory failure after extubation
Two recent randomized controlled trials compared NIPPV and standard care in patients who met the criteria for readiness for extubation but who developed respiratory failure after mechanical ventilation was discontinued. 22,37 Those two studies showed a longer time to reintubation for patients randomized to NIPPV but no differences in the rate of reintubation between the two groups and no difference in the lengths of stay in the intensive care unit.
Of greater concern, one study showed a higher rate of death in the intensive care unit in the NIPPV group than in the standard therapy group (25% vs 14%, respectively).22 This finding suggests that NIPPV delayed necessary reintubation in patients developing respiratory failure after extubation, with a consequent risk of fatal complications.
Prevention of respiratory failure after extubation
Other studies used NIPPV to prevent respiratory failure after extubation rather than wait to apply it after respiratory failure developed.38–41
Nava et al,40 in a trial in patients successfully weaned but considered to be at risk of reintubation, found that fewer of those randomized to NIPPV had to be reintubated than those who received standard care (8% vs 24%), and 10% fewer of them died in the intensive care unit. Risk factors for reintubation (and therefore eligibility criteria for this trial) included a Paco2 higher than 45 mm Hg, more than one consecutive failure of weaning, chronic heart failure, other comorbidity, weak cough, or stridor.
Extubated patients are a heterogeneous group, so if some subgroups benefit from a transition to NIPPV after extubation, it will be important to identify them. For instance, a subgroup analysis of a study by Ferrer et al38 indicated the survival benefit of NIPPV after extubation was limited to patients with chronic respiratory disorders and hypercapnia during a trial of spontaneous breathing.
In a subsequent successful test of this hypothesis, a randomized trial showed that the early use of noninvasive ventilation in patients with hypercapnia after a trial of spontaneous breathing and with chronic respiratory disorders (COPD, chronic bronchitis, bronchiectasis, obesity-hypoventilation, sequelae of tuberculosis, chest wall deformity, or chronic persistent asthma) reduced the risk of respiratory failure after extubation and the risk of death within the first 90 days.39
Others in which this approach may be helpful are obese patients who have high Paco2 levels. Compared with historical controls, 62 patients with a body mass index greater than 35 kg/m2 who received NIPPV in the 48 hours after extubation had a lower rate of respiratory failure, shorter lengths of stay in the intensive care unit and hospital, and, in the subgroup with hypercapnia, a lower hospital mortality rate.41
NIPPV to facilitate weaning
In several studies, mechanically ventilated patients who had failed a trial of spontaneous breathing were randomized to undergo either accelerated weaning, extubation, and NIPPV or conventional weaning with pressure support via mechanical ventilation.42–46 Most patients developed hypercapnia during the spontaneous breathing trials, and most of the patients had COPD.
A meta-analysis47 of the randomized trials of this approach concluded that, compared with continued invasive ventilation, NIPPV decreased the risk of death (relative risk 0.41) and of ventilator-associated pneumonia (relative risk 0.28) and reduced the total duration of mechanical ventilation by a weighted mean difference of 7.33 days. The benefits appeared to be most significant in patients with COPD.
NIPPV IN ASTHMA AND STATUS ASTHMATICUS
Noninvasive ventilation is an attractive alternative to intubation for patients with status asthmaticus, given the challenges and conflicting demands of maintaining ventilation despite severe airway obstruction.
In a 1996 prospective study of 17 episodes of asthma associated with acute respiratory failure, Meduri et al48 showed that NIPPV could progressively improve the pH and the Paco2 over 12 to 24 hours and reduce the respiratory rate.
In a subsequent controlled trial, Soroksky et al49 randomized 30 patients presenting to an emergency room with a severe asthma attack to NIPPV with conventional therapy vs conventional therapy only. The study group had a significantly greater increase in the forced expiratory volume in 1 second compared with the control group (54% vs 29%, respectively) and a lower hospitalization rate (18% vs 63%).
Another randomized trial of NIPPV, in patients with status asthmaticus presenting to an emergency room, was prematurely terminated due to a physician treatment bias that favored NIPPV.50 The preliminary results of that study showed a 7.3% higher intubation rate in the control group than in the NIPPV group, along with trends toward a lower intubation rate, a shorter length of hospital stay, and lower hospital charges in the NIPPV group.
Despite these initial favorable results, a Cochrane review concluded that the use of NIPPV in patients with status asthmaticus is controversial.51 NIPPV can be tried in selected patients such as those with mild to moderate respiratory distress (respiratory rate greater than 25 breaths per minute, use of accessory muscles to breathe, difficulty speaking), an arterial pH of 7.25 to 7.35, and a Paco2 of 45 to 55 mm Hg.52 Patients with impending respiratory failure or the inability to protect the airway should probably not be considered for NIPPV.52
IN ACUTE LUNG INJURY AND ACUTE RESPIRATORY DISTRESS SYNDROME
The most challenging application of NIPPV may be in patients with acute lung injury and the acute respiratory distress syndrome.
Initial trials of NIPPV in this setting have been disappointing, and a meta-analysis of the topic concluded that NIPPV was unlikely to have any significant benefit.53 An earlier study that used CPAP in patients with acute respiratory failure predominantly due to acute lung injury showed early physiologic improvements but no reduction in the need for intubation, no improvement in outcomes, and a higher rate of adverse events, including cardiac arrest, in those randomized to CPAP.54
A subsequent observational cohort specifically identified shock, metabolic acidosis, and severe hypoxemia as predictors of NIPPV failure.55
A more recent prospective study demonstrated that NIPPV improved gas exchange and obviated intubation in 54% of patients, with a consequent reduction in ventilator-associated pneumonia and a lower rate of death in the intensive care unit.56 A Simplified Acute Physiology Score (SAPS) II greater than 34 and a Pao2/Fio2 ratio less than 175 after 1 hour of NIPPV were identified as predicting that NIPPV would fail.56
MISCELLANEOUS APPLICATIONS
The more widespread use of NIPPV has encouraged its use in other acute situations, including during procedures such as percutaneous endoscopic gastrostomy (PEG)57,58 or bronchoscopy,59,60 for palliative use in patients listed as “do-not-intubate,”61–63 and for oxygenation before intubation.64
NIPPV during PEG tube insertion
NIPPV during PEG tube placement is particularly useful for patients with neuromuscular diseases who are at a combined risk of aspiration, poor oral intake, and respiratory failure during procedures. The experience with patients with amyotrophic lateral sclerosis58 and Duchenne muscular dystrophy57 indicates that even patients at high risk of respiratory failure during procedures can be successfully managed with NIPPV. The most recent practice parameters for patients with amyotrophic lateral sclerosis propose that patients with dysphagia may be exposed to less risk if the PEG procedure is performed when the forced vital capacity is greater than 50% of predicted.65
In randomized trials of CPAP59 or pressure-support NIPPV60 in high-risk hypoxemic patients who needed diagnostic bronchoscopy, patients in the intervention groups fared better than those who received oxygen alone, with better oxygenation during and after the procedure and a lower risk of postprocedure respiratory failure. Improved hemodynamics with a lower mean heart rate and a stable mean arterial pressure were also reported in one of those studies.60
Palliative use in ‘do-not-intubate’ patients
In patients who decline intubation, NIPPV appears to be most effective in reversing acute respiratory failure and improving mortality rates in those with COPD or with cardiogenic pulmonary edema.61,62 Controversy surrounding the use of NIPPV in “do-not-intubate” patients, particularly as a potentially uncomfortable life support technique, has been addressed by a task force of the Society of Critical Care Medicine, which recommends that it be applied only after careful discussion of goals of care and parameters of treatment with patients and their families.63
Oxygenation before intubation
In a prospective randomized study of oxygenation before rapid-sequence intubation via either a nonrebreather bag-valve mask or NIPPV, the NIPPV group had a higher oxygen saturation rate before, during, and after the intubation procedure.64
Acknowledgment: The authors wish to thank Jodith Janes of the Cleveland Clinic Alumni Library for her help with reference citations and with locating articles.
- Ambrosino N, Vagheggini G. Noninvasive positive pressure ventilation in the acute care setting: where are we? Eur Respir J 2008; 31:874–886.
- Hill NS, Brennan J, Garpestad E, Nava S. Noninvasive ventilation in acute respiratory failure. Crit Care Med 2007; 35:2402–2407.
- Nava S, Navalesi P, Conti G. Time of non-invasive ventilation. Intensive Care Med 2006; 32:361–370.
- Stoller JK, Kester L, Roberts VT, et al; An analysis of features of respiratory therapy departments that are avid for change. Respir Care 2008; 53:871–884.
- Maheshwari V, Paioli D, Rothaar R, Hill NS. Utilization of noninvasive ventilation in acute care hospitals: a regional survey. Chest 2006; 129:1226–1233.
- Obladen M. History of neonatal resuscitation. Part 1: Artificial ventilation. Neonatology 2008; 94:144–149.
- Poulton EP, Oxon DM. Left-sided heart failure with pulmonary oedema: its treatment with the “pulmonary plus” pressure machine. Lancet 1936; 228:981–983.
- Motley HL, Werko L. Observations on the clinical use of intermittent positive pressure. J Aviat Med 1947; 18:417–435.
- Cournand A, Motley HL. Physiological studies of the effects of intermittent positive pressure breathing on cardiac output in man. Am J Physiol 1948; 152:162–174.
- Severinghaus JW, Astrup P, Murray JF. Blood gas analysis and critical care medicine. Am J Respir Crit Care Med 1998; 157:S114–S122.
- Sullivan CE, Berthon-Jones M, Issa FG. Remission of severe obesity-hypoventilation syndrome after short-term treatment during sleep with nasal continuous positive airway pressure. Am Rev Respir Dis 1983; 128:177–181.
- Ellis ER, Bye PT, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with neuromuscular disease. Positive-pressure ventilation through a nose mask. Am Rev Respir Dis 1987; 135:148–152.
- Sanders MH, Kern N. Obstructive sleep apnea treated by independently adjusted inspiratory and expiratory positive airway pressures via nasal mask. Physiologic and clinical implications. Chest 1990; 98:317–324.
- Bach JR. Mechanical exsufflation, noninvasive ventilation, and new strategies for pulmonary rehabilitation and sleep disordered breathing. Bull N Y Acad Med 1992; 68:321–340.
- Chevrolet JC, Jolliet P, Abajo B, Toussi A, Louis M. Nasal positive pressure ventilation in patients with acute respiratory failure. Difficult and time-consuming procedure for nurses. Chest 1991; 100:775–782.
- Criner GJ, Kreimer DT, Tomaselli M, Pierson W, Evans D. Financial implications of noninvasive positive pressure ventilation (NPPV). Chest 1995; 108:475–481.
- Nava S, Evangelisti I, Rampulla C, Compagnoni ML, Fracchia C, Rubini F. Human and financial costs of noninvasive mechanical ventilation in patients affected by COPD and acute respiratory failure. Chest 1997; 111:1631–1638.
- DiMarco AF, Onders RP, Ignagni A, Kowalski KE, Mortimer JT. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest 2005; 127:671–678.
- Antonelli M, Conti G. Noninvasive positive pressure ventilation as treatment for acute respiratory failure in critically ill patients. Crit Care 2000; 4:15–22.
- Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med 2001; 344:481–487.
- L’Her E, Deye N, Lellouche F, et al. Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med 2005; 172:1112–1118.
- Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med 2004; 350:2452–2460.
- Hill NS. Noninvasive positive pressure ventilation for respiratory failure caused by exacerbations of chronic obstructive pulmonary disease: a standard of care? Crit Care 2003; 7:400–401.
- Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ 2003; 326:185–187.
- Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995; 333:817–822.
- Keenan SP, Sinuff T, Cook DJ, Hill NS. Which patients with acute exacerbation of chronic obstructive pulmonary disease benefit from noninvasive positive-pressure ventilation? A systematic review of the literature. Ann Intern Med 2003; 138:861–870.
- Epstein SK. Noninvasive ventilation to shorten the duration of mechanical ventilation. Respir Care 2009; 54:198–208.
- Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J; 3CPO Trialists. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med 2008; 359:142–151.
- Collins SP, Mielniczuk LM, Whittingham HA, Boseley ME, Schramm DR, Storrow AB. The use of noninvasive ventilation in emergency department patients with acute cardiogenic pulmonary edema: a systematic review. Ann Emerg Med 2006; 48:260–269.
- Masip J, Roque M, Sánchez B, Fernández R, Subirana M, Expósito JA. Noninvasive ventilation in acute cardiogenic pulmonary edema: systematic review and meta-analysis. JAMA 2005; 294:3124–3130.
- Baratz DM, Westbrook PR, Shah PK, Mohsenifar Z. Effect of nasal continuous positive airway pressure on cardiac output and oxygen delivery in patients with congestive heart failure. Chest 1992; 102:1397–1401.
- Naughton MT, Rahman MA, Hara K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation 1995; 91:1725–1731.
- Nava S, Carbone G, DiBattista N, et al. Noninvasive ventilation in cardiogenic pulmonary edema: a multicenter randomized trial. Am J Respir Crit Care Med 2003; 168:1432–1437.
- Mehta S, Jay GD, Woolard RH, et al. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med 1997; 25:620–628.
- Ho KM, Wong K. A comparison of continuous and bi-level positive airway pressure non-invasive ventilation in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Crit Care 2006; 10:R49.
- Antonelli M, Conti G, Bufi M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA 2000; 283:235–241.
- Keenan SP, Powers C, McCormack DG, Block G. Noninvasive positive-pressure ventilation for postextubation respiratory distress: a randomized controlled trial. JAMA 2002; 287:3238–3244.
- Ferrer M, Valencia M, Nicolas JM, Bernadich O, Badia JR, Torres A. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med 2006; 173:164–170.
- Ferrer M, Sellarés J, Valencia M, et al. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet 2009; 374:1082–1088.
- Nava S, Gregoretti C, Fanfulla F, et al. Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med 2005; 33:2465–2470.
- El-Solh AA, Aquilina A, Pineda L, Dhanvantri V, Grant B, Bouquin P. Noninvasive ventilation for prevention of post-extubation respiratory failure in obese patients. Eur Respir J 2006; 28:588–595.
- Ferrer M, Esquinas A, Arancibia F, et al. Noninvasive ventilation during persistent weaning failure: a randomized controlled trial. Am J Respir Crit Care Med 2003; 168:70–76.
- Girault C, Daudenthun I, Chevron V, Tamion F, Leroy J, Bonmarchand G. Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure: a prospective, randomized controlled study. Am J Respir Crit Care Med 1999; 160:86–92.
- Nava S, Ambrosino N, Clini E, et al. Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease. A randomized, controlled trial. Ann Intern Med 1998; 128:721–728.
- Trevisan CE, Vieira SR; Research Group in Mechanical Ventilation Weaning. Noninvasive mechanical ventilation may be useful in treating patients who fail weaning from invasive mechanical ventilation: a randomized clinical trial. Crit Care 2008; 12:R51.
- Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J 2007; 29:1033–1056.
- Burns KE, Adhikari NK, Meade MO. A meta-analysis of noninvasive weaning to facilitate liberation from mechanical ventilation. Can J Anaesth 2006; 53:305–315.
- Meduri GU, Cook TR, Turner RE, Cohen M, Leeper KV. Noninvasive positive pressure ventilation in status asthmaticus. Chest 1996; 110:767–774.
- Soroksky A, Stav D, Shpirer I. A pilot prospective, randomized, placebo-controlled trial of bilevel positive airway pressure in acute asthmatic attack. Chest 2003; 123:1018–1025.
- Holley MT, Morrissey TK, Seaberg DC, Afessa B, Wears RL. Ethical dilemmas in a randomized trial of asthma treatment: can Bayesian statistical analysis explain the results? Acad Emerg Med 2001; 8:1128–1135.
- Ram FS, Wellington S, Rowe BH, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev 2005;CD004360.
- Medoff BD. Invasive and noninvasive ventilation in patients with asthma. Respir Care 2008; 53:740–748.
- Agarwal R, Reddy C, Aggarwal AN, Gupta D. Is there a role for noninvasive ventilation in acute respiratory distress syndrome? A meta-analysis. Respir Med 2006; 100:2235–2238.
- Delclaux C, L’Her E, Alberti C, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA 2000; 284:2352–2360.
- Rana S, Jenad H, Gay PC, Buck CF, Hubmayr RD, Gajic O. Failure of non-invasive ventilation in patients with acute lung injury: observational cohort study. Crit Care 2006; 10:R79.
- Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med 2007; 35:18–25.
- Birnkrant DJ, Ferguson RD, Martin JE, Gordon GJ. Noninvasive ventilation during gastrostomy tube placement in patients with severe Duchenne muscular dystrophy: case reports and review of the literature. Pediatr Pulmonol 2006; 41:188–193.
- Boitano LJ, Jordan T, Benditt JO. Noninvasive ventilation allows gastrostomy tube placement in patients with advanced ALS. Neurology 2001; 56:413–414.
- Maitre B, Jaber S, Maggiore SM, et al. Continuous positive airway pressure during fiberoptic bronchoscopy in hypoxemic patients. A randomized double-blind study using a new device. Am J Respir Crit Care Med 2000; 162:1063–1067.
- Antonelli M, Conti G, Rocco M, et al. Noninvasive positive-pressure ventilation vs conventional oxygen supplementation in hypoxemic patients undergoing diagnostic bronchoscopy. Chest 2002; 121:1149–1154.
- Levy M, Tanios MA, Nelson D, et al. Outcomes of patients with do-not-intubate orders treated with noninvasive ventilation. Crit Care Med 2004; 32:2002–2007.
- Schettino G, Altobelli N, Kacmarek RM. Noninvasive positive pressure ventilation reverses acute respiratory failure in select “do-not-intubate” patients. Crit Care Med 2005; 33:1976–1982.
- Curtis JR, Cook DJ, Sinuff T, et al; Society of Critical Care Medicine Palliative Noninvasive Positive Ventilation Task Force. Noninvasive positive pressure ventilation in critical and palliative care settings: understanding the goals of therapy. Crit Care Med 2007; 35:932–939.
- Baillard C, Fosse JP, Sebbane M, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med 2006; 174:171–177.
- Miller RG, Jackson CE, Kasarskis EJ, et al; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009; 73:1218–1226.
Noninvasive positive pressure ventilation (NIPPV)—delivered via a tight-fitting mask rather than via an endotracheal tube or tracheostomy—is one of the most important advances in the management of acute respiratory failure to emerge in the past 2 decades. It is now recommended as the first choice for ventilatory support in selected patients, such as those with exacerbations of chronic obstructive pulmonary disease (COPD) or with cardiogenic pulmonary edema.1–3 In fact, some authors suggest that using NIPPV in more than 20% of COPD patients is a characteristic of respiratory care departments that are “avid for change”4—change being a good thing.
However, NIPPV has not been universally accepted, with wide variations in its utilization. In a 2006 survey, it was being used in only 33% of patients with COPD or congestive heart failure, for which it might be indicated. 5 Some potential reasons for the low rate are that physicians do not know about it, respiratory therapists are not sufficiently trained in it, and hospitals lack the equipment to do it.5
Our goal in this review is to familiarize the reader with how NIPPV has evolved and with its indications and contraindications in specific acute care conditions.
FROM A VACUUM CLEANER TO THE INTENSIVE CARE UNIT
NIPPV appears to have been first tried in 1870 by Chaussier, who used a bag and face mask to resuscitate neonates.6
In 1936, Poulton and Oxon7 described their “pulmonary plus pressure machine,” which used a vacuum cleaner blower and a mask to increase the alveolar pressure and thus counteract the increased intrapulmonary pressure in patients with heart failure, pulmonary edema, Cheyne-Stokes breathing, and asthma.
In the 1940s, intermittent positive pressure breathing devices were developed for use in high-altitude aviation. Motley, Werko, and Cournand8,9 subsequently used these devices to treat acute respiratory failure in pneumonia, pulmonary edema, near-drowning, Guillain-Barré syndrome, and acute severe asthma.
Although NIPPV was shown to be effective for acute conditions, invasive ventilation became preferred, particularly as blood gas analysis and ventilator technologies simultaneously matured, spurred at least in part by the polio epidemics of the 1950s.10
NIPPV reemerged in the 1980s for use in chronic conditions. First, continuous positive airway pressure (CPAP) came into use for obstructive sleep apnea,11 followed by noninvasive positive-pressure volume ventilation in neuromuscular diseases.12 Bilevel positive pressure devices (ie, with separate inspiratory and expiratory pressures) soon followed, again initially for obstructive sleep apnea13 and then for diverse neuromuscular diseases.14
NIPPV is now a mainstream therapy for diverse conditions in acute and chronic care.3 One reason we now use it in acute conditions is to avoid the complications associated with intubation.
Some clinicians initially resisted using NIPPV, concerned that it demanded too much of the nurses’ time15 and was costly.16 However, in a 1997 study in patients with COPD and acute respiratory failure, Nava et al17 found that NIPPV was no more expensive and no more demanding of staff resources than invasive mechanical ventilation in the first 48 hours of ventilation. Further, after the first few days of ventilation, NIPPV put fewer time demands on physicians and nurses than did invasive mechanical ventilation.
THREE MODES: CPAP, PRESSURE-LIMITED, VOLUME-LIMITED
The term “noninvasive ventilation” generally encompasses various forms of positive pressure ventilation. However, negative pressure ventilation, in the form of diaphragm pacing, may regain a foothold in the devices used for respiratory support.18 We therefore favor the term “NIPPV” in this review.
NIPPV IN ACUTE RESPIRATORY FAILURE
The main reasons to use NIPPV instead of invasive ventilation in acute care are to avoid the complications of invasive ventilation, to improve outcomes (eg, reduce mortality rates, decrease hospital length of stay), and to decrease the cost of care.
NIPPV is the standard of care for acute exacerbations of COPD
In a meta-analysis of eight randomized controlled trials,24 the specific advantages of NIPPV compared with usual care in acute exacerbations of COPD included:
- A lower risk of treatment failure, defined as death, need for intubation, or inability to tolerate the treatment (relative risk [RR] 0.51, number needed to treat [NNT] to prevent one treatment failure = 5)
- A lower risk of intubation (RR 0.43, NNT = 5)
- A lower mortality rate (RR 0.41, NNT = 8)
- A lower risk of complications (RR 0.32, NNT = 3)
- A shorter hospital length of stay (by about 3 days).
Mechanisms by which NIPPV may impart these benefits include reducing the work of breathing, unloading the respiratory muscles, lessening diaphragmatic pressure swings, reducing the respiratory rate, eliminating diaphragmatic work, and counteracting the threshold loading effects of auto-positive end-expiratory pressure (auto-PEEP).24–26
Also, if a patient with COPD is intubated, NIPPV seems to help after the tube is removed, preventing postextubation respiratory failure and facilitating weaning from invasive ventilation.27 These topics are discussed below.
A Cochrane systematic review24 concluded that NIPPV should be tried early in the course of respiratory failure, before severe acidosis develops. The patients in the studies in this review all had partial pressure of arterial carbon dioxide (Paco2) levels greater than 45 mm Hg.
In patients with severe respiratory acidosis (pH < 7.25), NIPPV failure rates are greater than 50%. However, trying NIPPV may still be justified, even in the presence of hypercapnic encephalopathy, as long as no other indications for invasive support and facilities for prompt endotracheal intubation are available. 1
However, in another systematic review,26 in patients with mild COPD exacerbations (pH > 7.35), NIPPV was no more effective than standard medical therapy in preventing acute respiratory failure, preventing death, or reducing length of hospitalization. Moreover, nearly 50% of the patients could not tolerate NIPPV.
Rapid improvement in cardiogenic pulmonary edema, but possibly no lower mortality rate
The Three Interventions in Cardiogenic Pulmonary Oedema (3CPO) trial,28 with 1,156 patients, was the largest randomized trial to compare NIPPV and standard oxygen therapy for acute pulmonary edema. It found that NIPPV (either CPAP or noninvasive intermittent positive pressure ventilation) was significantly better than standard oxygen therapy (through a variable-delivery oxygen mask with a reservoir) in the first hour of treatment in terms of the dyspnea score, heart rate, acidosis, and hypercapnia. However, there were no significant differences between groups in the 7- or 30-day mortality rates, the rates of intubation, rates of admission to the critical care unit, or in the mean length of hospital stay.
In contrast, several smaller randomized trials and meta-analyses showed lower intubation and mortality rates with NIPPV.29,30 Factors that may account for those differences include a much lower intubation rate in the 3CPO trial (2.9% overall, compared with 20% with conventional therapy in other trials), a higher mortality rate in the 3CPO trial, and methodologic differences (eg, patients for whom standard therapy failed in the 3CPO trial received rescue NIPPV).
If NIPPV is beneficial in cardiogenic pulmonary edema, the mechanisms are probably its favorable hemodynamic effects and its positive end-expiratory pressure (PEEP) effect on flooded alveoli. Specifically, positive intrathoracic pressure can be expected to reduce both preload and afterload, with improvement in the cardiac index and reduced work of breathing. 31,32
Notwithstanding the possible lack of impact of NIPPV on death or intubation rates in this setting, the intervention rapidly improves dyspnea and respiratory and metabolic abnormalities and should be considered for treatment of cardiogenic pulmonary edema associated with severe respiratory distress. A subgroup in which the NIPPV may reduce intubation rates is those with hypercapnia.33 A concern that NIPPV may increase the rate of myocardial infarction34 was not confirmed in the 3CPO trial.28 Interestingly, there were no differences in outcomes between CPAP and noninvasive intermittent positive pressure ventilation in this setting.28,34,35
Immunocompromised patients with acute respiratory failure
A particular challenge of NIPPV in immunocompromised patients, particularly compared with its use in COPD exacerbation or cardiogenic pulmonary edema, is that the underlying pathophysiology of respiratory dysfunction in immunocompromised patients may not be readily reversible. Therefore, its application in this group may need to follow clearly defined indications.
In one trial,20 inclusion criteria were:
- Immune suppression (due to neutropenia after chemotherapy or bone marrow transplantation, immunosuppressive drugs for organ transplantation, corticosteroids, cytotoxic therapy for nonmalignant conditions, or the acquired immunodeficiency syndrome)
- Persistent pulmonary infiltrates
- Fever (temperature > 38.3°C; 100.9°F)
- A respiratory rate greater than 30 breaths per minute
- Severe dyspnea at rest
- Early hypoxemic acute respiratory failure, defined as a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (Pao2/Fio2 ratio) less than 200 while on oxygen.
Compared with patients who received conventional treatment, fewer of those randomized to additional intermittent noninvasive ventilation had to be intubated (46% vs 77%, P = .03), suffered serious complications (50% vs 81%, P = .02), or died in the intensive care unit (38% vs 69%, P = .03) or in the hospital (50% vs 81%, P = .02).
Similarly, in a randomized trial in 40 patients with acute respiratory failure after solid organ transplantation, more patients in the NIPPV group than in the control group had an improvement in the Pao2/Fio2 ratio within the first hour (70% vs 25%, P = .004) or a sustained improvement in the Pao2/Fio2 ratio (60% vs 25%, P = .03); fewer of them needed endotracheal intubation (20% vs 70%, P = .002); fewer of them died of complications (20% vs 50%, P = .05); they had a shorter length of stay in the intensive care unit (mean 5.5 vs 9 days, P = .03); and fewer of them died in the intensive care unit (20% vs 50%, P = .05). There was, however, no difference in the overall hospital mortality rate.36
MAY NOT HELP AFTER EXTUBATION, EXCEPT IN SPECIFIC CASES
NIPPV has been used to treat respiratory failure after extubation,22,37 to prevent acute respiratory failure after failure of weaning,38–41 and to support breathing in patients who failed a trial of spontaneous breathing.42–45
Unfortunately, the evidence for using NIPPV in respiratory failure after extubation, including unplanned extubation, appears to be unfavorable, except possibly in patients with chronic pulmonary disease (particularly COPD and possibly obesity) and hypercapnia. An international consensus report stated that NIPPV should be considered in patients with hypercapnic respiratory insufficiency, especially those with COPD, to shorten the duration of intubation, but that it should not be routinely used in extubation respiratory failure.46
Treatment of respiratory failure after extubation
Two recent randomized controlled trials compared NIPPV and standard care in patients who met the criteria for readiness for extubation but who developed respiratory failure after mechanical ventilation was discontinued. 22,37 Those two studies showed a longer time to reintubation for patients randomized to NIPPV but no differences in the rate of reintubation between the two groups and no difference in the lengths of stay in the intensive care unit.
Of greater concern, one study showed a higher rate of death in the intensive care unit in the NIPPV group than in the standard therapy group (25% vs 14%, respectively).22 This finding suggests that NIPPV delayed necessary reintubation in patients developing respiratory failure after extubation, with a consequent risk of fatal complications.
Prevention of respiratory failure after extubation
Other studies used NIPPV to prevent respiratory failure after extubation rather than wait to apply it after respiratory failure developed.38–41
Nava et al,40 in a trial in patients successfully weaned but considered to be at risk of reintubation, found that fewer of those randomized to NIPPV had to be reintubated than those who received standard care (8% vs 24%), and 10% fewer of them died in the intensive care unit. Risk factors for reintubation (and therefore eligibility criteria for this trial) included a Paco2 higher than 45 mm Hg, more than one consecutive failure of weaning, chronic heart failure, other comorbidity, weak cough, or stridor.
Extubated patients are a heterogeneous group, so if some subgroups benefit from a transition to NIPPV after extubation, it will be important to identify them. For instance, a subgroup analysis of a study by Ferrer et al38 indicated the survival benefit of NIPPV after extubation was limited to patients with chronic respiratory disorders and hypercapnia during a trial of spontaneous breathing.
In a subsequent successful test of this hypothesis, a randomized trial showed that the early use of noninvasive ventilation in patients with hypercapnia after a trial of spontaneous breathing and with chronic respiratory disorders (COPD, chronic bronchitis, bronchiectasis, obesity-hypoventilation, sequelae of tuberculosis, chest wall deformity, or chronic persistent asthma) reduced the risk of respiratory failure after extubation and the risk of death within the first 90 days.39
Others in which this approach may be helpful are obese patients who have high Paco2 levels. Compared with historical controls, 62 patients with a body mass index greater than 35 kg/m2 who received NIPPV in the 48 hours after extubation had a lower rate of respiratory failure, shorter lengths of stay in the intensive care unit and hospital, and, in the subgroup with hypercapnia, a lower hospital mortality rate.41
NIPPV to facilitate weaning
In several studies, mechanically ventilated patients who had failed a trial of spontaneous breathing were randomized to undergo either accelerated weaning, extubation, and NIPPV or conventional weaning with pressure support via mechanical ventilation.42–46 Most patients developed hypercapnia during the spontaneous breathing trials, and most of the patients had COPD.
A meta-analysis47 of the randomized trials of this approach concluded that, compared with continued invasive ventilation, NIPPV decreased the risk of death (relative risk 0.41) and of ventilator-associated pneumonia (relative risk 0.28) and reduced the total duration of mechanical ventilation by a weighted mean difference of 7.33 days. The benefits appeared to be most significant in patients with COPD.
NIPPV IN ASTHMA AND STATUS ASTHMATICUS
Noninvasive ventilation is an attractive alternative to intubation for patients with status asthmaticus, given the challenges and conflicting demands of maintaining ventilation despite severe airway obstruction.
In a 1996 prospective study of 17 episodes of asthma associated with acute respiratory failure, Meduri et al48 showed that NIPPV could progressively improve the pH and the Paco2 over 12 to 24 hours and reduce the respiratory rate.
In a subsequent controlled trial, Soroksky et al49 randomized 30 patients presenting to an emergency room with a severe asthma attack to NIPPV with conventional therapy vs conventional therapy only. The study group had a significantly greater increase in the forced expiratory volume in 1 second compared with the control group (54% vs 29%, respectively) and a lower hospitalization rate (18% vs 63%).
Another randomized trial of NIPPV, in patients with status asthmaticus presenting to an emergency room, was prematurely terminated due to a physician treatment bias that favored NIPPV.50 The preliminary results of that study showed a 7.3% higher intubation rate in the control group than in the NIPPV group, along with trends toward a lower intubation rate, a shorter length of hospital stay, and lower hospital charges in the NIPPV group.
Despite these initial favorable results, a Cochrane review concluded that the use of NIPPV in patients with status asthmaticus is controversial.51 NIPPV can be tried in selected patients such as those with mild to moderate respiratory distress (respiratory rate greater than 25 breaths per minute, use of accessory muscles to breathe, difficulty speaking), an arterial pH of 7.25 to 7.35, and a Paco2 of 45 to 55 mm Hg.52 Patients with impending respiratory failure or the inability to protect the airway should probably not be considered for NIPPV.52
IN ACUTE LUNG INJURY AND ACUTE RESPIRATORY DISTRESS SYNDROME
The most challenging application of NIPPV may be in patients with acute lung injury and the acute respiratory distress syndrome.
Initial trials of NIPPV in this setting have been disappointing, and a meta-analysis of the topic concluded that NIPPV was unlikely to have any significant benefit.53 An earlier study that used CPAP in patients with acute respiratory failure predominantly due to acute lung injury showed early physiologic improvements but no reduction in the need for intubation, no improvement in outcomes, and a higher rate of adverse events, including cardiac arrest, in those randomized to CPAP.54
A subsequent observational cohort specifically identified shock, metabolic acidosis, and severe hypoxemia as predictors of NIPPV failure.55
A more recent prospective study demonstrated that NIPPV improved gas exchange and obviated intubation in 54% of patients, with a consequent reduction in ventilator-associated pneumonia and a lower rate of death in the intensive care unit.56 A Simplified Acute Physiology Score (SAPS) II greater than 34 and a Pao2/Fio2 ratio less than 175 after 1 hour of NIPPV were identified as predicting that NIPPV would fail.56
MISCELLANEOUS APPLICATIONS
The more widespread use of NIPPV has encouraged its use in other acute situations, including during procedures such as percutaneous endoscopic gastrostomy (PEG)57,58 or bronchoscopy,59,60 for palliative use in patients listed as “do-not-intubate,”61–63 and for oxygenation before intubation.64
NIPPV during PEG tube insertion
NIPPV during PEG tube placement is particularly useful for patients with neuromuscular diseases who are at a combined risk of aspiration, poor oral intake, and respiratory failure during procedures. The experience with patients with amyotrophic lateral sclerosis58 and Duchenne muscular dystrophy57 indicates that even patients at high risk of respiratory failure during procedures can be successfully managed with NIPPV. The most recent practice parameters for patients with amyotrophic lateral sclerosis propose that patients with dysphagia may be exposed to less risk if the PEG procedure is performed when the forced vital capacity is greater than 50% of predicted.65
In randomized trials of CPAP59 or pressure-support NIPPV60 in high-risk hypoxemic patients who needed diagnostic bronchoscopy, patients in the intervention groups fared better than those who received oxygen alone, with better oxygenation during and after the procedure and a lower risk of postprocedure respiratory failure. Improved hemodynamics with a lower mean heart rate and a stable mean arterial pressure were also reported in one of those studies.60
Palliative use in ‘do-not-intubate’ patients
In patients who decline intubation, NIPPV appears to be most effective in reversing acute respiratory failure and improving mortality rates in those with COPD or with cardiogenic pulmonary edema.61,62 Controversy surrounding the use of NIPPV in “do-not-intubate” patients, particularly as a potentially uncomfortable life support technique, has been addressed by a task force of the Society of Critical Care Medicine, which recommends that it be applied only after careful discussion of goals of care and parameters of treatment with patients and their families.63
Oxygenation before intubation
In a prospective randomized study of oxygenation before rapid-sequence intubation via either a nonrebreather bag-valve mask or NIPPV, the NIPPV group had a higher oxygen saturation rate before, during, and after the intubation procedure.64
Acknowledgment: The authors wish to thank Jodith Janes of the Cleveland Clinic Alumni Library for her help with reference citations and with locating articles.
Noninvasive positive pressure ventilation (NIPPV)—delivered via a tight-fitting mask rather than via an endotracheal tube or tracheostomy—is one of the most important advances in the management of acute respiratory failure to emerge in the past 2 decades. It is now recommended as the first choice for ventilatory support in selected patients, such as those with exacerbations of chronic obstructive pulmonary disease (COPD) or with cardiogenic pulmonary edema.1–3 In fact, some authors suggest that using NIPPV in more than 20% of COPD patients is a characteristic of respiratory care departments that are “avid for change”4—change being a good thing.
However, NIPPV has not been universally accepted, with wide variations in its utilization. In a 2006 survey, it was being used in only 33% of patients with COPD or congestive heart failure, for which it might be indicated. 5 Some potential reasons for the low rate are that physicians do not know about it, respiratory therapists are not sufficiently trained in it, and hospitals lack the equipment to do it.5
Our goal in this review is to familiarize the reader with how NIPPV has evolved and with its indications and contraindications in specific acute care conditions.
FROM A VACUUM CLEANER TO THE INTENSIVE CARE UNIT
NIPPV appears to have been first tried in 1870 by Chaussier, who used a bag and face mask to resuscitate neonates.6
In 1936, Poulton and Oxon7 described their “pulmonary plus pressure machine,” which used a vacuum cleaner blower and a mask to increase the alveolar pressure and thus counteract the increased intrapulmonary pressure in patients with heart failure, pulmonary edema, Cheyne-Stokes breathing, and asthma.
In the 1940s, intermittent positive pressure breathing devices were developed for use in high-altitude aviation. Motley, Werko, and Cournand8,9 subsequently used these devices to treat acute respiratory failure in pneumonia, pulmonary edema, near-drowning, Guillain-Barré syndrome, and acute severe asthma.
Although NIPPV was shown to be effective for acute conditions, invasive ventilation became preferred, particularly as blood gas analysis and ventilator technologies simultaneously matured, spurred at least in part by the polio epidemics of the 1950s.10
NIPPV reemerged in the 1980s for use in chronic conditions. First, continuous positive airway pressure (CPAP) came into use for obstructive sleep apnea,11 followed by noninvasive positive-pressure volume ventilation in neuromuscular diseases.12 Bilevel positive pressure devices (ie, with separate inspiratory and expiratory pressures) soon followed, again initially for obstructive sleep apnea13 and then for diverse neuromuscular diseases.14
NIPPV is now a mainstream therapy for diverse conditions in acute and chronic care.3 One reason we now use it in acute conditions is to avoid the complications associated with intubation.
Some clinicians initially resisted using NIPPV, concerned that it demanded too much of the nurses’ time15 and was costly.16 However, in a 1997 study in patients with COPD and acute respiratory failure, Nava et al17 found that NIPPV was no more expensive and no more demanding of staff resources than invasive mechanical ventilation in the first 48 hours of ventilation. Further, after the first few days of ventilation, NIPPV put fewer time demands on physicians and nurses than did invasive mechanical ventilation.
THREE MODES: CPAP, PRESSURE-LIMITED, VOLUME-LIMITED
The term “noninvasive ventilation” generally encompasses various forms of positive pressure ventilation. However, negative pressure ventilation, in the form of diaphragm pacing, may regain a foothold in the devices used for respiratory support.18 We therefore favor the term “NIPPV” in this review.
NIPPV IN ACUTE RESPIRATORY FAILURE
The main reasons to use NIPPV instead of invasive ventilation in acute care are to avoid the complications of invasive ventilation, to improve outcomes (eg, reduce mortality rates, decrease hospital length of stay), and to decrease the cost of care.
NIPPV is the standard of care for acute exacerbations of COPD
In a meta-analysis of eight randomized controlled trials,24 the specific advantages of NIPPV compared with usual care in acute exacerbations of COPD included:
- A lower risk of treatment failure, defined as death, need for intubation, or inability to tolerate the treatment (relative risk [RR] 0.51, number needed to treat [NNT] to prevent one treatment failure = 5)
- A lower risk of intubation (RR 0.43, NNT = 5)
- A lower mortality rate (RR 0.41, NNT = 8)
- A lower risk of complications (RR 0.32, NNT = 3)
- A shorter hospital length of stay (by about 3 days).
Mechanisms by which NIPPV may impart these benefits include reducing the work of breathing, unloading the respiratory muscles, lessening diaphragmatic pressure swings, reducing the respiratory rate, eliminating diaphragmatic work, and counteracting the threshold loading effects of auto-positive end-expiratory pressure (auto-PEEP).24–26
Also, if a patient with COPD is intubated, NIPPV seems to help after the tube is removed, preventing postextubation respiratory failure and facilitating weaning from invasive ventilation.27 These topics are discussed below.
A Cochrane systematic review24 concluded that NIPPV should be tried early in the course of respiratory failure, before severe acidosis develops. The patients in the studies in this review all had partial pressure of arterial carbon dioxide (Paco2) levels greater than 45 mm Hg.
In patients with severe respiratory acidosis (pH < 7.25), NIPPV failure rates are greater than 50%. However, trying NIPPV may still be justified, even in the presence of hypercapnic encephalopathy, as long as no other indications for invasive support and facilities for prompt endotracheal intubation are available. 1
However, in another systematic review,26 in patients with mild COPD exacerbations (pH > 7.35), NIPPV was no more effective than standard medical therapy in preventing acute respiratory failure, preventing death, or reducing length of hospitalization. Moreover, nearly 50% of the patients could not tolerate NIPPV.
Rapid improvement in cardiogenic pulmonary edema, but possibly no lower mortality rate
The Three Interventions in Cardiogenic Pulmonary Oedema (3CPO) trial,28 with 1,156 patients, was the largest randomized trial to compare NIPPV and standard oxygen therapy for acute pulmonary edema. It found that NIPPV (either CPAP or noninvasive intermittent positive pressure ventilation) was significantly better than standard oxygen therapy (through a variable-delivery oxygen mask with a reservoir) in the first hour of treatment in terms of the dyspnea score, heart rate, acidosis, and hypercapnia. However, there were no significant differences between groups in the 7- or 30-day mortality rates, the rates of intubation, rates of admission to the critical care unit, or in the mean length of hospital stay.
In contrast, several smaller randomized trials and meta-analyses showed lower intubation and mortality rates with NIPPV.29,30 Factors that may account for those differences include a much lower intubation rate in the 3CPO trial (2.9% overall, compared with 20% with conventional therapy in other trials), a higher mortality rate in the 3CPO trial, and methodologic differences (eg, patients for whom standard therapy failed in the 3CPO trial received rescue NIPPV).
If NIPPV is beneficial in cardiogenic pulmonary edema, the mechanisms are probably its favorable hemodynamic effects and its positive end-expiratory pressure (PEEP) effect on flooded alveoli. Specifically, positive intrathoracic pressure can be expected to reduce both preload and afterload, with improvement in the cardiac index and reduced work of breathing. 31,32
Notwithstanding the possible lack of impact of NIPPV on death or intubation rates in this setting, the intervention rapidly improves dyspnea and respiratory and metabolic abnormalities and should be considered for treatment of cardiogenic pulmonary edema associated with severe respiratory distress. A subgroup in which the NIPPV may reduce intubation rates is those with hypercapnia.33 A concern that NIPPV may increase the rate of myocardial infarction34 was not confirmed in the 3CPO trial.28 Interestingly, there were no differences in outcomes between CPAP and noninvasive intermittent positive pressure ventilation in this setting.28,34,35
Immunocompromised patients with acute respiratory failure
A particular challenge of NIPPV in immunocompromised patients, particularly compared with its use in COPD exacerbation or cardiogenic pulmonary edema, is that the underlying pathophysiology of respiratory dysfunction in immunocompromised patients may not be readily reversible. Therefore, its application in this group may need to follow clearly defined indications.
In one trial,20 inclusion criteria were:
- Immune suppression (due to neutropenia after chemotherapy or bone marrow transplantation, immunosuppressive drugs for organ transplantation, corticosteroids, cytotoxic therapy for nonmalignant conditions, or the acquired immunodeficiency syndrome)
- Persistent pulmonary infiltrates
- Fever (temperature > 38.3°C; 100.9°F)
- A respiratory rate greater than 30 breaths per minute
- Severe dyspnea at rest
- Early hypoxemic acute respiratory failure, defined as a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (Pao2/Fio2 ratio) less than 200 while on oxygen.
Compared with patients who received conventional treatment, fewer of those randomized to additional intermittent noninvasive ventilation had to be intubated (46% vs 77%, P = .03), suffered serious complications (50% vs 81%, P = .02), or died in the intensive care unit (38% vs 69%, P = .03) or in the hospital (50% vs 81%, P = .02).
Similarly, in a randomized trial in 40 patients with acute respiratory failure after solid organ transplantation, more patients in the NIPPV group than in the control group had an improvement in the Pao2/Fio2 ratio within the first hour (70% vs 25%, P = .004) or a sustained improvement in the Pao2/Fio2 ratio (60% vs 25%, P = .03); fewer of them needed endotracheal intubation (20% vs 70%, P = .002); fewer of them died of complications (20% vs 50%, P = .05); they had a shorter length of stay in the intensive care unit (mean 5.5 vs 9 days, P = .03); and fewer of them died in the intensive care unit (20% vs 50%, P = .05). There was, however, no difference in the overall hospital mortality rate.36
MAY NOT HELP AFTER EXTUBATION, EXCEPT IN SPECIFIC CASES
NIPPV has been used to treat respiratory failure after extubation,22,37 to prevent acute respiratory failure after failure of weaning,38–41 and to support breathing in patients who failed a trial of spontaneous breathing.42–45
Unfortunately, the evidence for using NIPPV in respiratory failure after extubation, including unplanned extubation, appears to be unfavorable, except possibly in patients with chronic pulmonary disease (particularly COPD and possibly obesity) and hypercapnia. An international consensus report stated that NIPPV should be considered in patients with hypercapnic respiratory insufficiency, especially those with COPD, to shorten the duration of intubation, but that it should not be routinely used in extubation respiratory failure.46
Treatment of respiratory failure after extubation
Two recent randomized controlled trials compared NIPPV and standard care in patients who met the criteria for readiness for extubation but who developed respiratory failure after mechanical ventilation was discontinued. 22,37 Those two studies showed a longer time to reintubation for patients randomized to NIPPV but no differences in the rate of reintubation between the two groups and no difference in the lengths of stay in the intensive care unit.
Of greater concern, one study showed a higher rate of death in the intensive care unit in the NIPPV group than in the standard therapy group (25% vs 14%, respectively).22 This finding suggests that NIPPV delayed necessary reintubation in patients developing respiratory failure after extubation, with a consequent risk of fatal complications.
Prevention of respiratory failure after extubation
Other studies used NIPPV to prevent respiratory failure after extubation rather than wait to apply it after respiratory failure developed.38–41
Nava et al,40 in a trial in patients successfully weaned but considered to be at risk of reintubation, found that fewer of those randomized to NIPPV had to be reintubated than those who received standard care (8% vs 24%), and 10% fewer of them died in the intensive care unit. Risk factors for reintubation (and therefore eligibility criteria for this trial) included a Paco2 higher than 45 mm Hg, more than one consecutive failure of weaning, chronic heart failure, other comorbidity, weak cough, or stridor.
Extubated patients are a heterogeneous group, so if some subgroups benefit from a transition to NIPPV after extubation, it will be important to identify them. For instance, a subgroup analysis of a study by Ferrer et al38 indicated the survival benefit of NIPPV after extubation was limited to patients with chronic respiratory disorders and hypercapnia during a trial of spontaneous breathing.
In a subsequent successful test of this hypothesis, a randomized trial showed that the early use of noninvasive ventilation in patients with hypercapnia after a trial of spontaneous breathing and with chronic respiratory disorders (COPD, chronic bronchitis, bronchiectasis, obesity-hypoventilation, sequelae of tuberculosis, chest wall deformity, or chronic persistent asthma) reduced the risk of respiratory failure after extubation and the risk of death within the first 90 days.39
Others in which this approach may be helpful are obese patients who have high Paco2 levels. Compared with historical controls, 62 patients with a body mass index greater than 35 kg/m2 who received NIPPV in the 48 hours after extubation had a lower rate of respiratory failure, shorter lengths of stay in the intensive care unit and hospital, and, in the subgroup with hypercapnia, a lower hospital mortality rate.41
NIPPV to facilitate weaning
In several studies, mechanically ventilated patients who had failed a trial of spontaneous breathing were randomized to undergo either accelerated weaning, extubation, and NIPPV or conventional weaning with pressure support via mechanical ventilation.42–46 Most patients developed hypercapnia during the spontaneous breathing trials, and most of the patients had COPD.
A meta-analysis47 of the randomized trials of this approach concluded that, compared with continued invasive ventilation, NIPPV decreased the risk of death (relative risk 0.41) and of ventilator-associated pneumonia (relative risk 0.28) and reduced the total duration of mechanical ventilation by a weighted mean difference of 7.33 days. The benefits appeared to be most significant in patients with COPD.
NIPPV IN ASTHMA AND STATUS ASTHMATICUS
Noninvasive ventilation is an attractive alternative to intubation for patients with status asthmaticus, given the challenges and conflicting demands of maintaining ventilation despite severe airway obstruction.
In a 1996 prospective study of 17 episodes of asthma associated with acute respiratory failure, Meduri et al48 showed that NIPPV could progressively improve the pH and the Paco2 over 12 to 24 hours and reduce the respiratory rate.
In a subsequent controlled trial, Soroksky et al49 randomized 30 patients presenting to an emergency room with a severe asthma attack to NIPPV with conventional therapy vs conventional therapy only. The study group had a significantly greater increase in the forced expiratory volume in 1 second compared with the control group (54% vs 29%, respectively) and a lower hospitalization rate (18% vs 63%).
Another randomized trial of NIPPV, in patients with status asthmaticus presenting to an emergency room, was prematurely terminated due to a physician treatment bias that favored NIPPV.50 The preliminary results of that study showed a 7.3% higher intubation rate in the control group than in the NIPPV group, along with trends toward a lower intubation rate, a shorter length of hospital stay, and lower hospital charges in the NIPPV group.
Despite these initial favorable results, a Cochrane review concluded that the use of NIPPV in patients with status asthmaticus is controversial.51 NIPPV can be tried in selected patients such as those with mild to moderate respiratory distress (respiratory rate greater than 25 breaths per minute, use of accessory muscles to breathe, difficulty speaking), an arterial pH of 7.25 to 7.35, and a Paco2 of 45 to 55 mm Hg.52 Patients with impending respiratory failure or the inability to protect the airway should probably not be considered for NIPPV.52
IN ACUTE LUNG INJURY AND ACUTE RESPIRATORY DISTRESS SYNDROME
The most challenging application of NIPPV may be in patients with acute lung injury and the acute respiratory distress syndrome.
Initial trials of NIPPV in this setting have been disappointing, and a meta-analysis of the topic concluded that NIPPV was unlikely to have any significant benefit.53 An earlier study that used CPAP in patients with acute respiratory failure predominantly due to acute lung injury showed early physiologic improvements but no reduction in the need for intubation, no improvement in outcomes, and a higher rate of adverse events, including cardiac arrest, in those randomized to CPAP.54
A subsequent observational cohort specifically identified shock, metabolic acidosis, and severe hypoxemia as predictors of NIPPV failure.55
A more recent prospective study demonstrated that NIPPV improved gas exchange and obviated intubation in 54% of patients, with a consequent reduction in ventilator-associated pneumonia and a lower rate of death in the intensive care unit.56 A Simplified Acute Physiology Score (SAPS) II greater than 34 and a Pao2/Fio2 ratio less than 175 after 1 hour of NIPPV were identified as predicting that NIPPV would fail.56
MISCELLANEOUS APPLICATIONS
The more widespread use of NIPPV has encouraged its use in other acute situations, including during procedures such as percutaneous endoscopic gastrostomy (PEG)57,58 or bronchoscopy,59,60 for palliative use in patients listed as “do-not-intubate,”61–63 and for oxygenation before intubation.64
NIPPV during PEG tube insertion
NIPPV during PEG tube placement is particularly useful for patients with neuromuscular diseases who are at a combined risk of aspiration, poor oral intake, and respiratory failure during procedures. The experience with patients with amyotrophic lateral sclerosis58 and Duchenne muscular dystrophy57 indicates that even patients at high risk of respiratory failure during procedures can be successfully managed with NIPPV. The most recent practice parameters for patients with amyotrophic lateral sclerosis propose that patients with dysphagia may be exposed to less risk if the PEG procedure is performed when the forced vital capacity is greater than 50% of predicted.65
In randomized trials of CPAP59 or pressure-support NIPPV60 in high-risk hypoxemic patients who needed diagnostic bronchoscopy, patients in the intervention groups fared better than those who received oxygen alone, with better oxygenation during and after the procedure and a lower risk of postprocedure respiratory failure. Improved hemodynamics with a lower mean heart rate and a stable mean arterial pressure were also reported in one of those studies.60
Palliative use in ‘do-not-intubate’ patients
In patients who decline intubation, NIPPV appears to be most effective in reversing acute respiratory failure and improving mortality rates in those with COPD or with cardiogenic pulmonary edema.61,62 Controversy surrounding the use of NIPPV in “do-not-intubate” patients, particularly as a potentially uncomfortable life support technique, has been addressed by a task force of the Society of Critical Care Medicine, which recommends that it be applied only after careful discussion of goals of care and parameters of treatment with patients and their families.63
Oxygenation before intubation
In a prospective randomized study of oxygenation before rapid-sequence intubation via either a nonrebreather bag-valve mask or NIPPV, the NIPPV group had a higher oxygen saturation rate before, during, and after the intubation procedure.64
Acknowledgment: The authors wish to thank Jodith Janes of the Cleveland Clinic Alumni Library for her help with reference citations and with locating articles.
- Ambrosino N, Vagheggini G. Noninvasive positive pressure ventilation in the acute care setting: where are we? Eur Respir J 2008; 31:874–886.
- Hill NS, Brennan J, Garpestad E, Nava S. Noninvasive ventilation in acute respiratory failure. Crit Care Med 2007; 35:2402–2407.
- Nava S, Navalesi P, Conti G. Time of non-invasive ventilation. Intensive Care Med 2006; 32:361–370.
- Stoller JK, Kester L, Roberts VT, et al; An analysis of features of respiratory therapy departments that are avid for change. Respir Care 2008; 53:871–884.
- Maheshwari V, Paioli D, Rothaar R, Hill NS. Utilization of noninvasive ventilation in acute care hospitals: a regional survey. Chest 2006; 129:1226–1233.
- Obladen M. History of neonatal resuscitation. Part 1: Artificial ventilation. Neonatology 2008; 94:144–149.
- Poulton EP, Oxon DM. Left-sided heart failure with pulmonary oedema: its treatment with the “pulmonary plus” pressure machine. Lancet 1936; 228:981–983.
- Motley HL, Werko L. Observations on the clinical use of intermittent positive pressure. J Aviat Med 1947; 18:417–435.
- Cournand A, Motley HL. Physiological studies of the effects of intermittent positive pressure breathing on cardiac output in man. Am J Physiol 1948; 152:162–174.
- Severinghaus JW, Astrup P, Murray JF. Blood gas analysis and critical care medicine. Am J Respir Crit Care Med 1998; 157:S114–S122.
- Sullivan CE, Berthon-Jones M, Issa FG. Remission of severe obesity-hypoventilation syndrome after short-term treatment during sleep with nasal continuous positive airway pressure. Am Rev Respir Dis 1983; 128:177–181.
- Ellis ER, Bye PT, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with neuromuscular disease. Positive-pressure ventilation through a nose mask. Am Rev Respir Dis 1987; 135:148–152.
- Sanders MH, Kern N. Obstructive sleep apnea treated by independently adjusted inspiratory and expiratory positive airway pressures via nasal mask. Physiologic and clinical implications. Chest 1990; 98:317–324.
- Bach JR. Mechanical exsufflation, noninvasive ventilation, and new strategies for pulmonary rehabilitation and sleep disordered breathing. Bull N Y Acad Med 1992; 68:321–340.
- Chevrolet JC, Jolliet P, Abajo B, Toussi A, Louis M. Nasal positive pressure ventilation in patients with acute respiratory failure. Difficult and time-consuming procedure for nurses. Chest 1991; 100:775–782.
- Criner GJ, Kreimer DT, Tomaselli M, Pierson W, Evans D. Financial implications of noninvasive positive pressure ventilation (NPPV). Chest 1995; 108:475–481.
- Nava S, Evangelisti I, Rampulla C, Compagnoni ML, Fracchia C, Rubini F. Human and financial costs of noninvasive mechanical ventilation in patients affected by COPD and acute respiratory failure. Chest 1997; 111:1631–1638.
- DiMarco AF, Onders RP, Ignagni A, Kowalski KE, Mortimer JT. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest 2005; 127:671–678.
- Antonelli M, Conti G. Noninvasive positive pressure ventilation as treatment for acute respiratory failure in critically ill patients. Crit Care 2000; 4:15–22.
- Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med 2001; 344:481–487.
- L’Her E, Deye N, Lellouche F, et al. Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med 2005; 172:1112–1118.
- Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med 2004; 350:2452–2460.
- Hill NS. Noninvasive positive pressure ventilation for respiratory failure caused by exacerbations of chronic obstructive pulmonary disease: a standard of care? Crit Care 2003; 7:400–401.
- Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ 2003; 326:185–187.
- Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995; 333:817–822.
- Keenan SP, Sinuff T, Cook DJ, Hill NS. Which patients with acute exacerbation of chronic obstructive pulmonary disease benefit from noninvasive positive-pressure ventilation? A systematic review of the literature. Ann Intern Med 2003; 138:861–870.
- Epstein SK. Noninvasive ventilation to shorten the duration of mechanical ventilation. Respir Care 2009; 54:198–208.
- Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J; 3CPO Trialists. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med 2008; 359:142–151.
- Collins SP, Mielniczuk LM, Whittingham HA, Boseley ME, Schramm DR, Storrow AB. The use of noninvasive ventilation in emergency department patients with acute cardiogenic pulmonary edema: a systematic review. Ann Emerg Med 2006; 48:260–269.
- Masip J, Roque M, Sánchez B, Fernández R, Subirana M, Expósito JA. Noninvasive ventilation in acute cardiogenic pulmonary edema: systematic review and meta-analysis. JAMA 2005; 294:3124–3130.
- Baratz DM, Westbrook PR, Shah PK, Mohsenifar Z. Effect of nasal continuous positive airway pressure on cardiac output and oxygen delivery in patients with congestive heart failure. Chest 1992; 102:1397–1401.
- Naughton MT, Rahman MA, Hara K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation 1995; 91:1725–1731.
- Nava S, Carbone G, DiBattista N, et al. Noninvasive ventilation in cardiogenic pulmonary edema: a multicenter randomized trial. Am J Respir Crit Care Med 2003; 168:1432–1437.
- Mehta S, Jay GD, Woolard RH, et al. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med 1997; 25:620–628.
- Ho KM, Wong K. A comparison of continuous and bi-level positive airway pressure non-invasive ventilation in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Crit Care 2006; 10:R49.
- Antonelli M, Conti G, Bufi M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA 2000; 283:235–241.
- Keenan SP, Powers C, McCormack DG, Block G. Noninvasive positive-pressure ventilation for postextubation respiratory distress: a randomized controlled trial. JAMA 2002; 287:3238–3244.
- Ferrer M, Valencia M, Nicolas JM, Bernadich O, Badia JR, Torres A. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med 2006; 173:164–170.
- Ferrer M, Sellarés J, Valencia M, et al. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet 2009; 374:1082–1088.
- Nava S, Gregoretti C, Fanfulla F, et al. Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med 2005; 33:2465–2470.
- El-Solh AA, Aquilina A, Pineda L, Dhanvantri V, Grant B, Bouquin P. Noninvasive ventilation for prevention of post-extubation respiratory failure in obese patients. Eur Respir J 2006; 28:588–595.
- Ferrer M, Esquinas A, Arancibia F, et al. Noninvasive ventilation during persistent weaning failure: a randomized controlled trial. Am J Respir Crit Care Med 2003; 168:70–76.
- Girault C, Daudenthun I, Chevron V, Tamion F, Leroy J, Bonmarchand G. Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure: a prospective, randomized controlled study. Am J Respir Crit Care Med 1999; 160:86–92.
- Nava S, Ambrosino N, Clini E, et al. Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease. A randomized, controlled trial. Ann Intern Med 1998; 128:721–728.
- Trevisan CE, Vieira SR; Research Group in Mechanical Ventilation Weaning. Noninvasive mechanical ventilation may be useful in treating patients who fail weaning from invasive mechanical ventilation: a randomized clinical trial. Crit Care 2008; 12:R51.
- Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J 2007; 29:1033–1056.
- Burns KE, Adhikari NK, Meade MO. A meta-analysis of noninvasive weaning to facilitate liberation from mechanical ventilation. Can J Anaesth 2006; 53:305–315.
- Meduri GU, Cook TR, Turner RE, Cohen M, Leeper KV. Noninvasive positive pressure ventilation in status asthmaticus. Chest 1996; 110:767–774.
- Soroksky A, Stav D, Shpirer I. A pilot prospective, randomized, placebo-controlled trial of bilevel positive airway pressure in acute asthmatic attack. Chest 2003; 123:1018–1025.
- Holley MT, Morrissey TK, Seaberg DC, Afessa B, Wears RL. Ethical dilemmas in a randomized trial of asthma treatment: can Bayesian statistical analysis explain the results? Acad Emerg Med 2001; 8:1128–1135.
- Ram FS, Wellington S, Rowe BH, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev 2005;CD004360.
- Medoff BD. Invasive and noninvasive ventilation in patients with asthma. Respir Care 2008; 53:740–748.
- Agarwal R, Reddy C, Aggarwal AN, Gupta D. Is there a role for noninvasive ventilation in acute respiratory distress syndrome? A meta-analysis. Respir Med 2006; 100:2235–2238.
- Delclaux C, L’Her E, Alberti C, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA 2000; 284:2352–2360.
- Rana S, Jenad H, Gay PC, Buck CF, Hubmayr RD, Gajic O. Failure of non-invasive ventilation in patients with acute lung injury: observational cohort study. Crit Care 2006; 10:R79.
- Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med 2007; 35:18–25.
- Birnkrant DJ, Ferguson RD, Martin JE, Gordon GJ. Noninvasive ventilation during gastrostomy tube placement in patients with severe Duchenne muscular dystrophy: case reports and review of the literature. Pediatr Pulmonol 2006; 41:188–193.
- Boitano LJ, Jordan T, Benditt JO. Noninvasive ventilation allows gastrostomy tube placement in patients with advanced ALS. Neurology 2001; 56:413–414.
- Maitre B, Jaber S, Maggiore SM, et al. Continuous positive airway pressure during fiberoptic bronchoscopy in hypoxemic patients. A randomized double-blind study using a new device. Am J Respir Crit Care Med 2000; 162:1063–1067.
- Antonelli M, Conti G, Rocco M, et al. Noninvasive positive-pressure ventilation vs conventional oxygen supplementation in hypoxemic patients undergoing diagnostic bronchoscopy. Chest 2002; 121:1149–1154.
- Levy M, Tanios MA, Nelson D, et al. Outcomes of patients with do-not-intubate orders treated with noninvasive ventilation. Crit Care Med 2004; 32:2002–2007.
- Schettino G, Altobelli N, Kacmarek RM. Noninvasive positive pressure ventilation reverses acute respiratory failure in select “do-not-intubate” patients. Crit Care Med 2005; 33:1976–1982.
- Curtis JR, Cook DJ, Sinuff T, et al; Society of Critical Care Medicine Palliative Noninvasive Positive Ventilation Task Force. Noninvasive positive pressure ventilation in critical and palliative care settings: understanding the goals of therapy. Crit Care Med 2007; 35:932–939.
- Baillard C, Fosse JP, Sebbane M, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med 2006; 174:171–177.
- Miller RG, Jackson CE, Kasarskis EJ, et al; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009; 73:1218–1226.
- Ambrosino N, Vagheggini G. Noninvasive positive pressure ventilation in the acute care setting: where are we? Eur Respir J 2008; 31:874–886.
- Hill NS, Brennan J, Garpestad E, Nava S. Noninvasive ventilation in acute respiratory failure. Crit Care Med 2007; 35:2402–2407.
- Nava S, Navalesi P, Conti G. Time of non-invasive ventilation. Intensive Care Med 2006; 32:361–370.
- Stoller JK, Kester L, Roberts VT, et al; An analysis of features of respiratory therapy departments that are avid for change. Respir Care 2008; 53:871–884.
- Maheshwari V, Paioli D, Rothaar R, Hill NS. Utilization of noninvasive ventilation in acute care hospitals: a regional survey. Chest 2006; 129:1226–1233.
- Obladen M. History of neonatal resuscitation. Part 1: Artificial ventilation. Neonatology 2008; 94:144–149.
- Poulton EP, Oxon DM. Left-sided heart failure with pulmonary oedema: its treatment with the “pulmonary plus” pressure machine. Lancet 1936; 228:981–983.
- Motley HL, Werko L. Observations on the clinical use of intermittent positive pressure. J Aviat Med 1947; 18:417–435.
- Cournand A, Motley HL. Physiological studies of the effects of intermittent positive pressure breathing on cardiac output in man. Am J Physiol 1948; 152:162–174.
- Severinghaus JW, Astrup P, Murray JF. Blood gas analysis and critical care medicine. Am J Respir Crit Care Med 1998; 157:S114–S122.
- Sullivan CE, Berthon-Jones M, Issa FG. Remission of severe obesity-hypoventilation syndrome after short-term treatment during sleep with nasal continuous positive airway pressure. Am Rev Respir Dis 1983; 128:177–181.
- Ellis ER, Bye PT, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with neuromuscular disease. Positive-pressure ventilation through a nose mask. Am Rev Respir Dis 1987; 135:148–152.
- Sanders MH, Kern N. Obstructive sleep apnea treated by independently adjusted inspiratory and expiratory positive airway pressures via nasal mask. Physiologic and clinical implications. Chest 1990; 98:317–324.
- Bach JR. Mechanical exsufflation, noninvasive ventilation, and new strategies for pulmonary rehabilitation and sleep disordered breathing. Bull N Y Acad Med 1992; 68:321–340.
- Chevrolet JC, Jolliet P, Abajo B, Toussi A, Louis M. Nasal positive pressure ventilation in patients with acute respiratory failure. Difficult and time-consuming procedure for nurses. Chest 1991; 100:775–782.
- Criner GJ, Kreimer DT, Tomaselli M, Pierson W, Evans D. Financial implications of noninvasive positive pressure ventilation (NPPV). Chest 1995; 108:475–481.
- Nava S, Evangelisti I, Rampulla C, Compagnoni ML, Fracchia C, Rubini F. Human and financial costs of noninvasive mechanical ventilation in patients affected by COPD and acute respiratory failure. Chest 1997; 111:1631–1638.
- DiMarco AF, Onders RP, Ignagni A, Kowalski KE, Mortimer JT. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest 2005; 127:671–678.
- Antonelli M, Conti G. Noninvasive positive pressure ventilation as treatment for acute respiratory failure in critically ill patients. Crit Care 2000; 4:15–22.
- Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med 2001; 344:481–487.
- L’Her E, Deye N, Lellouche F, et al. Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med 2005; 172:1112–1118.
- Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med 2004; 350:2452–2460.
- Hill NS. Noninvasive positive pressure ventilation for respiratory failure caused by exacerbations of chronic obstructive pulmonary disease: a standard of care? Crit Care 2003; 7:400–401.
- Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ 2003; 326:185–187.
- Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995; 333:817–822.
- Keenan SP, Sinuff T, Cook DJ, Hill NS. Which patients with acute exacerbation of chronic obstructive pulmonary disease benefit from noninvasive positive-pressure ventilation? A systematic review of the literature. Ann Intern Med 2003; 138:861–870.
- Epstein SK. Noninvasive ventilation to shorten the duration of mechanical ventilation. Respir Care 2009; 54:198–208.
- Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J; 3CPO Trialists. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med 2008; 359:142–151.
- Collins SP, Mielniczuk LM, Whittingham HA, Boseley ME, Schramm DR, Storrow AB. The use of noninvasive ventilation in emergency department patients with acute cardiogenic pulmonary edema: a systematic review. Ann Emerg Med 2006; 48:260–269.
- Masip J, Roque M, Sánchez B, Fernández R, Subirana M, Expósito JA. Noninvasive ventilation in acute cardiogenic pulmonary edema: systematic review and meta-analysis. JAMA 2005; 294:3124–3130.
- Baratz DM, Westbrook PR, Shah PK, Mohsenifar Z. Effect of nasal continuous positive airway pressure on cardiac output and oxygen delivery in patients with congestive heart failure. Chest 1992; 102:1397–1401.
- Naughton MT, Rahman MA, Hara K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation 1995; 91:1725–1731.
- Nava S, Carbone G, DiBattista N, et al. Noninvasive ventilation in cardiogenic pulmonary edema: a multicenter randomized trial. Am J Respir Crit Care Med 2003; 168:1432–1437.
- Mehta S, Jay GD, Woolard RH, et al. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med 1997; 25:620–628.
- Ho KM, Wong K. A comparison of continuous and bi-level positive airway pressure non-invasive ventilation in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Crit Care 2006; 10:R49.
- Antonelli M, Conti G, Bufi M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA 2000; 283:235–241.
- Keenan SP, Powers C, McCormack DG, Block G. Noninvasive positive-pressure ventilation for postextubation respiratory distress: a randomized controlled trial. JAMA 2002; 287:3238–3244.
- Ferrer M, Valencia M, Nicolas JM, Bernadich O, Badia JR, Torres A. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med 2006; 173:164–170.
- Ferrer M, Sellarés J, Valencia M, et al. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet 2009; 374:1082–1088.
- Nava S, Gregoretti C, Fanfulla F, et al. Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med 2005; 33:2465–2470.
- El-Solh AA, Aquilina A, Pineda L, Dhanvantri V, Grant B, Bouquin P. Noninvasive ventilation for prevention of post-extubation respiratory failure in obese patients. Eur Respir J 2006; 28:588–595.
- Ferrer M, Esquinas A, Arancibia F, et al. Noninvasive ventilation during persistent weaning failure: a randomized controlled trial. Am J Respir Crit Care Med 2003; 168:70–76.
- Girault C, Daudenthun I, Chevron V, Tamion F, Leroy J, Bonmarchand G. Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure: a prospective, randomized controlled study. Am J Respir Crit Care Med 1999; 160:86–92.
- Nava S, Ambrosino N, Clini E, et al. Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease. A randomized, controlled trial. Ann Intern Med 1998; 128:721–728.
- Trevisan CE, Vieira SR; Research Group in Mechanical Ventilation Weaning. Noninvasive mechanical ventilation may be useful in treating patients who fail weaning from invasive mechanical ventilation: a randomized clinical trial. Crit Care 2008; 12:R51.
- Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J 2007; 29:1033–1056.
- Burns KE, Adhikari NK, Meade MO. A meta-analysis of noninvasive weaning to facilitate liberation from mechanical ventilation. Can J Anaesth 2006; 53:305–315.
- Meduri GU, Cook TR, Turner RE, Cohen M, Leeper KV. Noninvasive positive pressure ventilation in status asthmaticus. Chest 1996; 110:767–774.
- Soroksky A, Stav D, Shpirer I. A pilot prospective, randomized, placebo-controlled trial of bilevel positive airway pressure in acute asthmatic attack. Chest 2003; 123:1018–1025.
- Holley MT, Morrissey TK, Seaberg DC, Afessa B, Wears RL. Ethical dilemmas in a randomized trial of asthma treatment: can Bayesian statistical analysis explain the results? Acad Emerg Med 2001; 8:1128–1135.
- Ram FS, Wellington S, Rowe BH, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev 2005;CD004360.
- Medoff BD. Invasive and noninvasive ventilation in patients with asthma. Respir Care 2008; 53:740–748.
- Agarwal R, Reddy C, Aggarwal AN, Gupta D. Is there a role for noninvasive ventilation in acute respiratory distress syndrome? A meta-analysis. Respir Med 2006; 100:2235–2238.
- Delclaux C, L’Her E, Alberti C, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA 2000; 284:2352–2360.
- Rana S, Jenad H, Gay PC, Buck CF, Hubmayr RD, Gajic O. Failure of non-invasive ventilation in patients with acute lung injury: observational cohort study. Crit Care 2006; 10:R79.
- Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med 2007; 35:18–25.
- Birnkrant DJ, Ferguson RD, Martin JE, Gordon GJ. Noninvasive ventilation during gastrostomy tube placement in patients with severe Duchenne muscular dystrophy: case reports and review of the literature. Pediatr Pulmonol 2006; 41:188–193.
- Boitano LJ, Jordan T, Benditt JO. Noninvasive ventilation allows gastrostomy tube placement in patients with advanced ALS. Neurology 2001; 56:413–414.
- Maitre B, Jaber S, Maggiore SM, et al. Continuous positive airway pressure during fiberoptic bronchoscopy in hypoxemic patients. A randomized double-blind study using a new device. Am J Respir Crit Care Med 2000; 162:1063–1067.
- Antonelli M, Conti G, Rocco M, et al. Noninvasive positive-pressure ventilation vs conventional oxygen supplementation in hypoxemic patients undergoing diagnostic bronchoscopy. Chest 2002; 121:1149–1154.
- Levy M, Tanios MA, Nelson D, et al. Outcomes of patients with do-not-intubate orders treated with noninvasive ventilation. Crit Care Med 2004; 32:2002–2007.
- Schettino G, Altobelli N, Kacmarek RM. Noninvasive positive pressure ventilation reverses acute respiratory failure in select “do-not-intubate” patients. Crit Care Med 2005; 33:1976–1982.
- Curtis JR, Cook DJ, Sinuff T, et al; Society of Critical Care Medicine Palliative Noninvasive Positive Ventilation Task Force. Noninvasive positive pressure ventilation in critical and palliative care settings: understanding the goals of therapy. Crit Care Med 2007; 35:932–939.
- Baillard C, Fosse JP, Sebbane M, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med 2006; 174:171–177.
- Miller RG, Jackson CE, Kasarskis EJ, et al; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009; 73:1218–1226.
KEY POINTS
- The advantages of NIPPV over invasive ventilation are that it preserves normal physiologic functions such as coughing, swallowing, feeding, and speech and avoids the risks of tracheal and laryngeal injury and respiratory tract infections.
- The best level of evidence for the efficacy of NIPPV is in acute hypercarbic or hypoxemic respiratory failure during exacerbations of chronic obstructive pulmonary disease, in cardiogenic pulmonary edema, and in immunocompromised patients.
- NIPPV should not be applied indiscriminately for lessestablished indications (such as in unconscious patients, respiratory failure after extubation, acute lung injury, or acute respiratory distress syndrome), in severe hypoxemia or acidemia, or after failure to improve dyspnea or gas exchange. The use of NIPPV in these situations may delay a necessary intubation and increase the risks of such a delay, including death.
A rare complication of infective endocarditis
An 85-year-old woman presented to the emergency department with a 2-hour history of dyspnea, dizziness, generalized weakness, nausea, and diaphoresis. Her medical history included hypertension, end-stage renal disease with hemodialysis, and atrial fibrillation.
She had an arteriovenous fistula for dialysis access in her right upper arm, with erythema around the site.
Her creatine kinase level was 1,434 U/L (normal range 30–220), creatine kinase MB 143.4 ng/mL (0.0–8.8 ng/mL), and troponin T 0.1 ng/mL (0.0–0.1 ng/mL). She had ST elevation in leads I and aVL. She was taken for emergency cardiac catheterization.
Three blood cultures were drawn on the day of cardiac catheterization. Two grew gram-positive organisms: one grew coagulase-negative Staphylococcus, and the other grew gram-positive bacilli (anaerobic, non-sporeforming). On the basis of these findings, intravenous vancomycin (Vancocin) was started. Seventy-two hours later, one of two blood cultures again grew coagulase-negative Staphylococcus. Five days after the start of antibiotic treatment, blood cultures were negative, and the patient received intravenous vancomycin for 4 weeks (from the time the blood cultures became negative) for native mitral valve endocarditis.
EMBOLISM AND ENDOCARDITIS: KEY FEATURES
An embolic event occurs in 22% to 50% of cases of infective endocarditis and can involve the lungs, bowel, other organs, or extremities.1 The incidence of embolization of the coronary arteries in patients with infective endocarditis is unknown, but in one case series2 it occurred in 8 (7.5%) of 107 cases. The most common site of coronary embolism is the LAD.3
Myocardial infarction is a rare complication of coronary artery embolization.2 It was reported in 17 (2.9%) of 586 consecutive patients with infective endocarditis.4 In patients with infectious endocarditis complicated by myocardial infarction, the death rate was nearly double that seen in patients with infective endocarditis without myocardial infarction (64% vs 33%).4
TREATMENT
The best treatment for this complication of infective endocarditis is not known, as it has not been well studied. The high death rate in these patients makes restoration of coronary perfusion essential.
Thrombolytics are usually avoided in patients with septic embolization because of concerns about concurrent intracerebral mycotic aneurysms and the risk of hemorrhage.
Percutaneous transluminal angioplasty carries a risk of distal mobilization of emboli, development of mycotic aneurysm at the balloon dilation site, or reocclusion due to a mobile embolus.5 Stent placement may improve vessel patency but carries a theoretic risk of infection in bacteremic patients. Percutaneous embolectomy has also been used either prior to or instead of stent placement.6 Surgical options include embolectomy in patients who may require surgery, and coronary artery bypass grafting for patients with chronic embolization.7
- Baddour LM, Wilson WR, Bayer AS, et al; Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; Council on Cardiovascular Disease in the Young; Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia; American Heart Association; Infectious Diseases Society of America. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005; 111:e394–e434.
- Garvey GJ, Neu HC. Infective endocarditis—an evolving disease. A review of endocarditis at Columbia-Presbyterian Medical Center, 1968–1973. Medicine (Baltimore) 1978; 57:105–127.
- Glazier JJ. Interventional treatment of septic coronary embolism: sailing into uncharted and dangerous waters. J Interv Cardiol 2002; 15:305–307.
- Manzano MC, Vilacosta I, San Roman JA, et al. Acute cornary syndrome in infective endocarditis. Rev Esp Cardiol 2007; 60:24–31.
- Khan F, Khakoo R, Failinger C. Managing embolic myocardial infarction in infective endocarditis: current options. J Infect 2005; 51:e101–105.
- Glazier JJ, McGinnity JG, Spears JR. Coronary embolism complicating aortic valve endocarditis: treatment with placement of an intracoronary stent. Clin Cardiol 1997; 20:885–888.
- Baek MJ, Kim HK, Yu CW, Na CY. Surgery with surgical embolectomy for mitral valve endocarditis complicated by septic coronary embolism. Eur J Cardiothorac Surg 2008; 33:116–118.
An 85-year-old woman presented to the emergency department with a 2-hour history of dyspnea, dizziness, generalized weakness, nausea, and diaphoresis. Her medical history included hypertension, end-stage renal disease with hemodialysis, and atrial fibrillation.
She had an arteriovenous fistula for dialysis access in her right upper arm, with erythema around the site.
Her creatine kinase level was 1,434 U/L (normal range 30–220), creatine kinase MB 143.4 ng/mL (0.0–8.8 ng/mL), and troponin T 0.1 ng/mL (0.0–0.1 ng/mL). She had ST elevation in leads I and aVL. She was taken for emergency cardiac catheterization.
Three blood cultures were drawn on the day of cardiac catheterization. Two grew gram-positive organisms: one grew coagulase-negative Staphylococcus, and the other grew gram-positive bacilli (anaerobic, non-sporeforming). On the basis of these findings, intravenous vancomycin (Vancocin) was started. Seventy-two hours later, one of two blood cultures again grew coagulase-negative Staphylococcus. Five days after the start of antibiotic treatment, blood cultures were negative, and the patient received intravenous vancomycin for 4 weeks (from the time the blood cultures became negative) for native mitral valve endocarditis.
EMBOLISM AND ENDOCARDITIS: KEY FEATURES
An embolic event occurs in 22% to 50% of cases of infective endocarditis and can involve the lungs, bowel, other organs, or extremities.1 The incidence of embolization of the coronary arteries in patients with infective endocarditis is unknown, but in one case series2 it occurred in 8 (7.5%) of 107 cases. The most common site of coronary embolism is the LAD.3
Myocardial infarction is a rare complication of coronary artery embolization.2 It was reported in 17 (2.9%) of 586 consecutive patients with infective endocarditis.4 In patients with infectious endocarditis complicated by myocardial infarction, the death rate was nearly double that seen in patients with infective endocarditis without myocardial infarction (64% vs 33%).4
TREATMENT
The best treatment for this complication of infective endocarditis is not known, as it has not been well studied. The high death rate in these patients makes restoration of coronary perfusion essential.
Thrombolytics are usually avoided in patients with septic embolization because of concerns about concurrent intracerebral mycotic aneurysms and the risk of hemorrhage.
Percutaneous transluminal angioplasty carries a risk of distal mobilization of emboli, development of mycotic aneurysm at the balloon dilation site, or reocclusion due to a mobile embolus.5 Stent placement may improve vessel patency but carries a theoretic risk of infection in bacteremic patients. Percutaneous embolectomy has also been used either prior to or instead of stent placement.6 Surgical options include embolectomy in patients who may require surgery, and coronary artery bypass grafting for patients with chronic embolization.7
An 85-year-old woman presented to the emergency department with a 2-hour history of dyspnea, dizziness, generalized weakness, nausea, and diaphoresis. Her medical history included hypertension, end-stage renal disease with hemodialysis, and atrial fibrillation.
She had an arteriovenous fistula for dialysis access in her right upper arm, with erythema around the site.
Her creatine kinase level was 1,434 U/L (normal range 30–220), creatine kinase MB 143.4 ng/mL (0.0–8.8 ng/mL), and troponin T 0.1 ng/mL (0.0–0.1 ng/mL). She had ST elevation in leads I and aVL. She was taken for emergency cardiac catheterization.
Three blood cultures were drawn on the day of cardiac catheterization. Two grew gram-positive organisms: one grew coagulase-negative Staphylococcus, and the other grew gram-positive bacilli (anaerobic, non-sporeforming). On the basis of these findings, intravenous vancomycin (Vancocin) was started. Seventy-two hours later, one of two blood cultures again grew coagulase-negative Staphylococcus. Five days after the start of antibiotic treatment, blood cultures were negative, and the patient received intravenous vancomycin for 4 weeks (from the time the blood cultures became negative) for native mitral valve endocarditis.
EMBOLISM AND ENDOCARDITIS: KEY FEATURES
An embolic event occurs in 22% to 50% of cases of infective endocarditis and can involve the lungs, bowel, other organs, or extremities.1 The incidence of embolization of the coronary arteries in patients with infective endocarditis is unknown, but in one case series2 it occurred in 8 (7.5%) of 107 cases. The most common site of coronary embolism is the LAD.3
Myocardial infarction is a rare complication of coronary artery embolization.2 It was reported in 17 (2.9%) of 586 consecutive patients with infective endocarditis.4 In patients with infectious endocarditis complicated by myocardial infarction, the death rate was nearly double that seen in patients with infective endocarditis without myocardial infarction (64% vs 33%).4
TREATMENT
The best treatment for this complication of infective endocarditis is not known, as it has not been well studied. The high death rate in these patients makes restoration of coronary perfusion essential.
Thrombolytics are usually avoided in patients with septic embolization because of concerns about concurrent intracerebral mycotic aneurysms and the risk of hemorrhage.
Percutaneous transluminal angioplasty carries a risk of distal mobilization of emboli, development of mycotic aneurysm at the balloon dilation site, or reocclusion due to a mobile embolus.5 Stent placement may improve vessel patency but carries a theoretic risk of infection in bacteremic patients. Percutaneous embolectomy has also been used either prior to or instead of stent placement.6 Surgical options include embolectomy in patients who may require surgery, and coronary artery bypass grafting for patients with chronic embolization.7
- Baddour LM, Wilson WR, Bayer AS, et al; Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; Council on Cardiovascular Disease in the Young; Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia; American Heart Association; Infectious Diseases Society of America. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005; 111:e394–e434.
- Garvey GJ, Neu HC. Infective endocarditis—an evolving disease. A review of endocarditis at Columbia-Presbyterian Medical Center, 1968–1973. Medicine (Baltimore) 1978; 57:105–127.
- Glazier JJ. Interventional treatment of septic coronary embolism: sailing into uncharted and dangerous waters. J Interv Cardiol 2002; 15:305–307.
- Manzano MC, Vilacosta I, San Roman JA, et al. Acute cornary syndrome in infective endocarditis. Rev Esp Cardiol 2007; 60:24–31.
- Khan F, Khakoo R, Failinger C. Managing embolic myocardial infarction in infective endocarditis: current options. J Infect 2005; 51:e101–105.
- Glazier JJ, McGinnity JG, Spears JR. Coronary embolism complicating aortic valve endocarditis: treatment with placement of an intracoronary stent. Clin Cardiol 1997; 20:885–888.
- Baek MJ, Kim HK, Yu CW, Na CY. Surgery with surgical embolectomy for mitral valve endocarditis complicated by septic coronary embolism. Eur J Cardiothorac Surg 2008; 33:116–118.
- Baddour LM, Wilson WR, Bayer AS, et al; Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; Council on Cardiovascular Disease in the Young; Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia; American Heart Association; Infectious Diseases Society of America. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005; 111:e394–e434.
- Garvey GJ, Neu HC. Infective endocarditis—an evolving disease. A review of endocarditis at Columbia-Presbyterian Medical Center, 1968–1973. Medicine (Baltimore) 1978; 57:105–127.
- Glazier JJ. Interventional treatment of septic coronary embolism: sailing into uncharted and dangerous waters. J Interv Cardiol 2002; 15:305–307.
- Manzano MC, Vilacosta I, San Roman JA, et al. Acute cornary syndrome in infective endocarditis. Rev Esp Cardiol 2007; 60:24–31.
- Khan F, Khakoo R, Failinger C. Managing embolic myocardial infarction in infective endocarditis: current options. J Infect 2005; 51:e101–105.
- Glazier JJ, McGinnity JG, Spears JR. Coronary embolism complicating aortic valve endocarditis: treatment with placement of an intracoronary stent. Clin Cardiol 1997; 20:885–888.
- Baek MJ, Kim HK, Yu CW, Na CY. Surgery with surgical embolectomy for mitral valve endocarditis complicated by septic coronary embolism. Eur J Cardiothorac Surg 2008; 33:116–118.
Grand Rounds: Man, 65, With Delayed Pain After Hand Injury
A 65-year-old man presented to the emergency department (ED) with a two-week history of progressively severe pain in his right hand and difficulty moving his fingers. He reported that approximately two weeks earlier, while shoveling snow, he slipped and fell, landing on his right hand. Initially, he had no problems with his hand. He finished his shoveling and continued his normal daily activities.
Within two to three days he started to experience pain in his right hand, which grew progressively worse.
Because he did not have a primary care provider, the patient had a limited medical history. He reported having a mildly elevated prostate-specific antigen test years earlier. He underwent an appendectomy at age 15. He denied any other medical problems.
The patient was taking no medications and reported no known allergies to medications. He denied the use of tobacco, said he had one or two beers on an average day, and denied IV drug use. He was an artist and was married with one adult child. His family history was unremarkable with the exception of an alcoholic sister who died of cirrhosis at age 70.
During triage, vital signs were essentially normal: blood pressure, 142/74 mm Hg; heart rate, 78 beats/min; and respiratory rate, 20 breaths/min. The patient was afebrile at 37.2°C (98.9°F). Physical examination was remarkable for some edema and warmth of the right hand without any notable erythema. There was no evidence of any wound. Fingers all had good sensation; however, flexion of the index and long fingers elicited a significant increase in pain.
The remainder of the exam was unremarkable. The patient’s head was normocephalic and atraumatic. Pupils were equal, round, and reactive to light. Eyes were anicteric, and no rhinorrhea was evident. The neck was supple without palpable lymphadenopathy. Lungs were clear to auscultation bilaterally. No wheezes, rales, or rhonchi were appreciated. The heart had a regular rate and rhythm; no murmurs, rubs, or gallups were noted. The abdomen was soft and non-tender. The extremities, except as previously stated, were normal, with good pulses, sensation, and strength.
Initially, only radiographs of the right hand were ordered (see Figures 1 and 2). These demonstrated soft tissue swelling on the dorsum of the hand, and an area of hypodensity between the first and second metacarpals. There were no fractures, dislocations, or other bone or joint abnormalities.
After a review of the radiographs, it was clear that the patient’s diagnosis was not a simple answer of hand contusion or fracture; thus, the evaluation was expanded. Vital signs were repeated three hours after triage: blood pressure, 128/74 mm Hg; heart rate, 76 beats/min; and respiration, 20 breaths/min. The patient was now febrile at 37.6°C (99.7°F). Because of his fever and the anomaly on the patient’s hand radiograph, expansion of the evaluation continued.
Laboratory studies included a complete blood count: white blood cells (WBCs), 30,700/mcL (reference range,1 4,500 to 11,000/mcL); hemoglobin, 13.3 g/dL (13.8 to 17.2 g/dL for men); hematocrit, 40.0% (41% to 50% for men); platelets, 217,000/mcL (130 to 400 x 103/mcL). Initial chemistry panel results were normal except for serum glucose, 143 mg/dL (70 to 125 mg/dL).
Liver function test results were normal except for aspartate aminotransferase, 33 U/L (reference range,1 10 to 30 U/L) and albumin, 2.5 g/dL (3.5 to 5.0 g/dL). Once WBCs were found to exceed 30,000/mcL, the search for a cause was widened once more.
The continued studies included a chest radiograph with normal results, unremarkable CT of the abdomen and pelvis with IV contrast, blood cultures, and urinalysis. The urinalysis showed: blood, moderate; protein, trace; nitrites, positive; leukocytes, large; WBCs > 50/high-power field (reference range,1 5/high-power field or less); and numerous bacteria.
The final study performed in the ED evaluation of the patient was a CT of the right hand with IV contrast (see Figure 3). It demonstrated diffuse edema and a 9.0-mm area of low attenuation with some rim enhancement. The differential for these findings includes an abscess or a foreign body; the latter was deemed unlikely in light of the patient’s physical exam. In consideration of his elevated WBC count, the high number of WBCs in his urine, the fever, and the CT results, the patient was diagnosed with an abscess in his right hand that had been seeded, it was surmised, by an occult urosepsis after his fall.
Before the patient’s admission, a hand surgeon was consulted. The surgeon agreed with the diagnosis, and the patient was taken to the operating room (OR). He had been given piperacillin/tazobactam in the ED.
In the OR, the surgeon made a 3.0-cm incision, conducted an exploration, and identified a cavity that contained a small amount of purulence. He determined the lesion to be a resolving abscess. The wound was washed out, and the area was closed with a Penrose drain.
The patient was continued on the piperacillin/tazobactam. His blood culture was positive for gram-positive rods, and a low-grade fever persisted. An infectious disease specialist was consulted, and levofloxacin was added to the patient’s regimen.
After 24 hours of treatment, findings on urinalysis improved: blood, small; protein, trace; nitrites, negative; leukocytes, small; WBCs, 15 to 20/high-power field; and no bacteria. Over the next three days, the patient’s condition continued to improve. His hand drain was removed, and the pain and swelling subsided. He became afebrile, and his WBC count fell to 24,700/mcL. He was discharged to home with prescriptions for cephalexin and levofloxacin. Follow-up for postoperative care was arranged with the hand surgeon.
Discussion
Pyomyositis is defined as abscess formation deep within large striated muscles.1 Although this condition is uncommon, it is believed that an occult bacteremia can seed an area of damaged muscle (compared with healthy muscle, which ordinarily resists infection), allowing an abscess to form.1,2
Epidemiology
In a 2002 review involving 676 patients with primary pyomyositis, Bickels et al3 reported the condition in ages ranging from two months to 82 years (mean, 28.1 years). In a majority of cases, only a single muscle was involved; 112 patients (16.6%) were identified with multiple-site involvement. Only seven cases (0.1%) involved the hand.
In 452 cases (66.9%), a bacterial agent was identified. Among these, 350 (77%) had a positive culture for Staphylococcus aureus. Other isolates included Streptococcus pyogenes, Escherichia coli, Salmonella enteritidus, and Mycobacterium tuberculosis.1,3 It should be noted that community-acquired methicillin-resistant S aureus (CA-MRSA) is being implicated with increasing frequency in cases of pyomyositis.4-6
Because pyomyositis is not a reportable disease and has not been studied in large clinical trials, its incidence is uncertain, and proposed risk factors have not all been confirmed2 (see Table2,7).
Pathophysiology
While the etiology of primary pyomyositis is unclear, it is believed to be caused by a combination of bacteremia (chronic or transient) and damaged muscle. In a 1960 study published in the Lancet, Smith and Vickers8 performed autopsies on 327 patients who had died of culture-positive septicemia. Only two patients were found to have a muscle abscess. At that time, the investigators concluded that both muscle injury and bacteremia would need to be present in order for an abscess to form. In animal studies, bacteremia (eg, S aureus) does not appear to lead to pyomyositis except in cases of muscle abnormality or trauma (eg, electric shock, pinching injury).9,10
When a history of trauma can be identified in patients with pyomyositis, the condition typically develops near the affected muscle, and the infection appears within days to weeks.3 In cases in which an antecedent infection is identified and hematogenous spread of the bacteria to the skeletal muscle occurs, this is termed secondary pyomyositis.11
Disease Progression
Pyomyositis generally progresses in three stages, beginning with inflammation and advancing to a focal abscess, then to a septic state.3 The first stage develops between seven and 21 days after the initial incident, is typically subacute, involves mild pain and swelling with a “woody” texture, and is occasionally associated with fevers.2
Diagnosis of pyomyositis is usually made during the second stage, 10 to 21 days after the initial incident; by that time, the pain has increased, and the fever is more pronounced. Third-stage infection usually involves fluctuance and sepsis.2
Although MRI is considered most useful in the diagnosis of pyomyositis, CT and ultrasound allow for percutaneous needle aspiration and drainage.3
Treatment
The correct treatment for pyomyositis depends upon the stage at which the disease is identified. During the first stage (before formation of an abscess), antibiotic treatment alone may be sufficient.1 Once an abscess has formed, an incision and drainage will be required, in conjunction with or followed by appropriate antibiotic therapy.
When pyomyositis is properly treated during the first or second stage, a full recovery is likely.2,3 By the third stage, surgical debridement is required. Additionally, osteomyelitis may develop in the adjacent bones, followed by muscle scarring, residual weakness, and functional impairment.2,3 Reported pyomyositis-associated mortality ranges between less than 1% and 4%.2,12
The Case Patient
The case presented here was of particular interest for two reasons. First, the patient had a traumatic injury that initially caused him no concern but worsened progressively over 14 days. Although this is not the typical presentation of a traumatic injury, the ED staff could very easily have performed a radiograph, made a diagnosis of traumatic hand injury, and discharged the patient.
Second, men in their 60s do not commonly have urinary tract infections.13 The patient was questioned frequently by several providers about sexual behaviors, medical problems, and urinary symptoms. Repeatedly, he denied all of these issues. While a urinalysis may be omitted in the evaluation of an otherwise healthy, asymptomatic patient, its results in this case were a key piece of data.
It should be noted that the patient thought it inappropriate to be asked for urine samples. He repeatedly said, “It’s my hand!”
Conclusion
Even in patients presenting with the most routine complaint, a careful evaluation can reveal unexpected, serious problems. This patient complained of pain in his hand some time after a fall and ultimately was treated for an occult urosepsis and hand abscess—pyomyositis, which rarely occurs in small muscles, such as those of the hand. Either condition, left untreated, could have led to serious morbidity or even mortality.
1. Beers MH, Berkow R, eds. Merck Manual of Diagnosis and Therapy. 18th ed. Whitehouse Station, NJ: Merck Research Laboratories, 2006:1142-1143.
2. Crum-Cianflone NF. Bacterial, fungal, parasitic and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494.
3. Bickels J, Ben-Sira L, Kessler A, Wientroub S. Current concepts review: primary pyomyositis. J Bone Joint Surg Am. 2002;84-A(12):2277-2286.
4. Lo BM, Fickenscher BA. Primary pyomyositis caused by ca-MRSA. Int J Emerg Med. 2008;1(4):331-332.
5. Ruiz ME, Yohannes S, Wladyka CG. Pyomyositis caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2005;352(14):1488–1489.
6. Pannaraj PS, Hulten KG, Gonzalez BE, et al. Infective pyomyositis and myositis in children in the era of community-acquired, methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2006;43(8):953–960.
7. Ükinç K, Bayraktar M, Uzun O. A case of type 2 diabetes complicated with primary pyomyositis. Endocrinologist. 2009;19(3):129-130.
8. Smith IM, Vickers AB. Natural history of 338 treated and untreated patients with staphylococcal septicaemia (1936-1955). Lancet. 1960;1(7138):1318-1322.
9. Phoon E-S, Sebastin SJ, Tay S-C. Primary pyomyositis (bacterial myositis) of the pronator quadratus. J Hand Surg Eur Vol. 2009;34(4):549-551.
10. Christin L, Sarosi GA. Pyomyositis in North America: case reports and review. Clin Infect Dis. 1992; 15(4):668-677.
11. Sokolowski MJ, Koh JL. Pyomyositis of the shoulder girdle. Orthopedics. 2006;29(11):1030-1032.
12. Crum NF. Bacterial pyomyositis in the United States. Am J Med. 2004;117(6):420–428.
13. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113 suppl 1A:5S-13S.
A 65-year-old man presented to the emergency department (ED) with a two-week history of progressively severe pain in his right hand and difficulty moving his fingers. He reported that approximately two weeks earlier, while shoveling snow, he slipped and fell, landing on his right hand. Initially, he had no problems with his hand. He finished his shoveling and continued his normal daily activities.
Within two to three days he started to experience pain in his right hand, which grew progressively worse.
Because he did not have a primary care provider, the patient had a limited medical history. He reported having a mildly elevated prostate-specific antigen test years earlier. He underwent an appendectomy at age 15. He denied any other medical problems.
The patient was taking no medications and reported no known allergies to medications. He denied the use of tobacco, said he had one or two beers on an average day, and denied IV drug use. He was an artist and was married with one adult child. His family history was unremarkable with the exception of an alcoholic sister who died of cirrhosis at age 70.
During triage, vital signs were essentially normal: blood pressure, 142/74 mm Hg; heart rate, 78 beats/min; and respiratory rate, 20 breaths/min. The patient was afebrile at 37.2°C (98.9°F). Physical examination was remarkable for some edema and warmth of the right hand without any notable erythema. There was no evidence of any wound. Fingers all had good sensation; however, flexion of the index and long fingers elicited a significant increase in pain.
The remainder of the exam was unremarkable. The patient’s head was normocephalic and atraumatic. Pupils were equal, round, and reactive to light. Eyes were anicteric, and no rhinorrhea was evident. The neck was supple without palpable lymphadenopathy. Lungs were clear to auscultation bilaterally. No wheezes, rales, or rhonchi were appreciated. The heart had a regular rate and rhythm; no murmurs, rubs, or gallups were noted. The abdomen was soft and non-tender. The extremities, except as previously stated, were normal, with good pulses, sensation, and strength.
Initially, only radiographs of the right hand were ordered (see Figures 1 and 2). These demonstrated soft tissue swelling on the dorsum of the hand, and an area of hypodensity between the first and second metacarpals. There were no fractures, dislocations, or other bone or joint abnormalities.
After a review of the radiographs, it was clear that the patient’s diagnosis was not a simple answer of hand contusion or fracture; thus, the evaluation was expanded. Vital signs were repeated three hours after triage: blood pressure, 128/74 mm Hg; heart rate, 76 beats/min; and respiration, 20 breaths/min. The patient was now febrile at 37.6°C (99.7°F). Because of his fever and the anomaly on the patient’s hand radiograph, expansion of the evaluation continued.
Laboratory studies included a complete blood count: white blood cells (WBCs), 30,700/mcL (reference range,1 4,500 to 11,000/mcL); hemoglobin, 13.3 g/dL (13.8 to 17.2 g/dL for men); hematocrit, 40.0% (41% to 50% for men); platelets, 217,000/mcL (130 to 400 x 103/mcL). Initial chemistry panel results were normal except for serum glucose, 143 mg/dL (70 to 125 mg/dL).
Liver function test results were normal except for aspartate aminotransferase, 33 U/L (reference range,1 10 to 30 U/L) and albumin, 2.5 g/dL (3.5 to 5.0 g/dL). Once WBCs were found to exceed 30,000/mcL, the search for a cause was widened once more.
The continued studies included a chest radiograph with normal results, unremarkable CT of the abdomen and pelvis with IV contrast, blood cultures, and urinalysis. The urinalysis showed: blood, moderate; protein, trace; nitrites, positive; leukocytes, large; WBCs > 50/high-power field (reference range,1 5/high-power field or less); and numerous bacteria.
The final study performed in the ED evaluation of the patient was a CT of the right hand with IV contrast (see Figure 3). It demonstrated diffuse edema and a 9.0-mm area of low attenuation with some rim enhancement. The differential for these findings includes an abscess or a foreign body; the latter was deemed unlikely in light of the patient’s physical exam. In consideration of his elevated WBC count, the high number of WBCs in his urine, the fever, and the CT results, the patient was diagnosed with an abscess in his right hand that had been seeded, it was surmised, by an occult urosepsis after his fall.
Before the patient’s admission, a hand surgeon was consulted. The surgeon agreed with the diagnosis, and the patient was taken to the operating room (OR). He had been given piperacillin/tazobactam in the ED.
In the OR, the surgeon made a 3.0-cm incision, conducted an exploration, and identified a cavity that contained a small amount of purulence. He determined the lesion to be a resolving abscess. The wound was washed out, and the area was closed with a Penrose drain.
The patient was continued on the piperacillin/tazobactam. His blood culture was positive for gram-positive rods, and a low-grade fever persisted. An infectious disease specialist was consulted, and levofloxacin was added to the patient’s regimen.
After 24 hours of treatment, findings on urinalysis improved: blood, small; protein, trace; nitrites, negative; leukocytes, small; WBCs, 15 to 20/high-power field; and no bacteria. Over the next three days, the patient’s condition continued to improve. His hand drain was removed, and the pain and swelling subsided. He became afebrile, and his WBC count fell to 24,700/mcL. He was discharged to home with prescriptions for cephalexin and levofloxacin. Follow-up for postoperative care was arranged with the hand surgeon.
Discussion
Pyomyositis is defined as abscess formation deep within large striated muscles.1 Although this condition is uncommon, it is believed that an occult bacteremia can seed an area of damaged muscle (compared with healthy muscle, which ordinarily resists infection), allowing an abscess to form.1,2
Epidemiology
In a 2002 review involving 676 patients with primary pyomyositis, Bickels et al3 reported the condition in ages ranging from two months to 82 years (mean, 28.1 years). In a majority of cases, only a single muscle was involved; 112 patients (16.6%) were identified with multiple-site involvement. Only seven cases (0.1%) involved the hand.
In 452 cases (66.9%), a bacterial agent was identified. Among these, 350 (77%) had a positive culture for Staphylococcus aureus. Other isolates included Streptococcus pyogenes, Escherichia coli, Salmonella enteritidus, and Mycobacterium tuberculosis.1,3 It should be noted that community-acquired methicillin-resistant S aureus (CA-MRSA) is being implicated with increasing frequency in cases of pyomyositis.4-6
Because pyomyositis is not a reportable disease and has not been studied in large clinical trials, its incidence is uncertain, and proposed risk factors have not all been confirmed2 (see Table2,7).
Pathophysiology
While the etiology of primary pyomyositis is unclear, it is believed to be caused by a combination of bacteremia (chronic or transient) and damaged muscle. In a 1960 study published in the Lancet, Smith and Vickers8 performed autopsies on 327 patients who had died of culture-positive septicemia. Only two patients were found to have a muscle abscess. At that time, the investigators concluded that both muscle injury and bacteremia would need to be present in order for an abscess to form. In animal studies, bacteremia (eg, S aureus) does not appear to lead to pyomyositis except in cases of muscle abnormality or trauma (eg, electric shock, pinching injury).9,10
When a history of trauma can be identified in patients with pyomyositis, the condition typically develops near the affected muscle, and the infection appears within days to weeks.3 In cases in which an antecedent infection is identified and hematogenous spread of the bacteria to the skeletal muscle occurs, this is termed secondary pyomyositis.11
Disease Progression
Pyomyositis generally progresses in three stages, beginning with inflammation and advancing to a focal abscess, then to a septic state.3 The first stage develops between seven and 21 days after the initial incident, is typically subacute, involves mild pain and swelling with a “woody” texture, and is occasionally associated with fevers.2
Diagnosis of pyomyositis is usually made during the second stage, 10 to 21 days after the initial incident; by that time, the pain has increased, and the fever is more pronounced. Third-stage infection usually involves fluctuance and sepsis.2
Although MRI is considered most useful in the diagnosis of pyomyositis, CT and ultrasound allow for percutaneous needle aspiration and drainage.3
Treatment
The correct treatment for pyomyositis depends upon the stage at which the disease is identified. During the first stage (before formation of an abscess), antibiotic treatment alone may be sufficient.1 Once an abscess has formed, an incision and drainage will be required, in conjunction with or followed by appropriate antibiotic therapy.
When pyomyositis is properly treated during the first or second stage, a full recovery is likely.2,3 By the third stage, surgical debridement is required. Additionally, osteomyelitis may develop in the adjacent bones, followed by muscle scarring, residual weakness, and functional impairment.2,3 Reported pyomyositis-associated mortality ranges between less than 1% and 4%.2,12
The Case Patient
The case presented here was of particular interest for two reasons. First, the patient had a traumatic injury that initially caused him no concern but worsened progressively over 14 days. Although this is not the typical presentation of a traumatic injury, the ED staff could very easily have performed a radiograph, made a diagnosis of traumatic hand injury, and discharged the patient.
Second, men in their 60s do not commonly have urinary tract infections.13 The patient was questioned frequently by several providers about sexual behaviors, medical problems, and urinary symptoms. Repeatedly, he denied all of these issues. While a urinalysis may be omitted in the evaluation of an otherwise healthy, asymptomatic patient, its results in this case were a key piece of data.
It should be noted that the patient thought it inappropriate to be asked for urine samples. He repeatedly said, “It’s my hand!”
Conclusion
Even in patients presenting with the most routine complaint, a careful evaluation can reveal unexpected, serious problems. This patient complained of pain in his hand some time after a fall and ultimately was treated for an occult urosepsis and hand abscess—pyomyositis, which rarely occurs in small muscles, such as those of the hand. Either condition, left untreated, could have led to serious morbidity or even mortality.
A 65-year-old man presented to the emergency department (ED) with a two-week history of progressively severe pain in his right hand and difficulty moving his fingers. He reported that approximately two weeks earlier, while shoveling snow, he slipped and fell, landing on his right hand. Initially, he had no problems with his hand. He finished his shoveling and continued his normal daily activities.
Within two to three days he started to experience pain in his right hand, which grew progressively worse.
Because he did not have a primary care provider, the patient had a limited medical history. He reported having a mildly elevated prostate-specific antigen test years earlier. He underwent an appendectomy at age 15. He denied any other medical problems.
The patient was taking no medications and reported no known allergies to medications. He denied the use of tobacco, said he had one or two beers on an average day, and denied IV drug use. He was an artist and was married with one adult child. His family history was unremarkable with the exception of an alcoholic sister who died of cirrhosis at age 70.
During triage, vital signs were essentially normal: blood pressure, 142/74 mm Hg; heart rate, 78 beats/min; and respiratory rate, 20 breaths/min. The patient was afebrile at 37.2°C (98.9°F). Physical examination was remarkable for some edema and warmth of the right hand without any notable erythema. There was no evidence of any wound. Fingers all had good sensation; however, flexion of the index and long fingers elicited a significant increase in pain.
The remainder of the exam was unremarkable. The patient’s head was normocephalic and atraumatic. Pupils were equal, round, and reactive to light. Eyes were anicteric, and no rhinorrhea was evident. The neck was supple without palpable lymphadenopathy. Lungs were clear to auscultation bilaterally. No wheezes, rales, or rhonchi were appreciated. The heart had a regular rate and rhythm; no murmurs, rubs, or gallups were noted. The abdomen was soft and non-tender. The extremities, except as previously stated, were normal, with good pulses, sensation, and strength.
Initially, only radiographs of the right hand were ordered (see Figures 1 and 2). These demonstrated soft tissue swelling on the dorsum of the hand, and an area of hypodensity between the first and second metacarpals. There were no fractures, dislocations, or other bone or joint abnormalities.
After a review of the radiographs, it was clear that the patient’s diagnosis was not a simple answer of hand contusion or fracture; thus, the evaluation was expanded. Vital signs were repeated three hours after triage: blood pressure, 128/74 mm Hg; heart rate, 76 beats/min; and respiration, 20 breaths/min. The patient was now febrile at 37.6°C (99.7°F). Because of his fever and the anomaly on the patient’s hand radiograph, expansion of the evaluation continued.
Laboratory studies included a complete blood count: white blood cells (WBCs), 30,700/mcL (reference range,1 4,500 to 11,000/mcL); hemoglobin, 13.3 g/dL (13.8 to 17.2 g/dL for men); hematocrit, 40.0% (41% to 50% for men); platelets, 217,000/mcL (130 to 400 x 103/mcL). Initial chemistry panel results were normal except for serum glucose, 143 mg/dL (70 to 125 mg/dL).
Liver function test results were normal except for aspartate aminotransferase, 33 U/L (reference range,1 10 to 30 U/L) and albumin, 2.5 g/dL (3.5 to 5.0 g/dL). Once WBCs were found to exceed 30,000/mcL, the search for a cause was widened once more.
The continued studies included a chest radiograph with normal results, unremarkable CT of the abdomen and pelvis with IV contrast, blood cultures, and urinalysis. The urinalysis showed: blood, moderate; protein, trace; nitrites, positive; leukocytes, large; WBCs > 50/high-power field (reference range,1 5/high-power field or less); and numerous bacteria.
The final study performed in the ED evaluation of the patient was a CT of the right hand with IV contrast (see Figure 3). It demonstrated diffuse edema and a 9.0-mm area of low attenuation with some rim enhancement. The differential for these findings includes an abscess or a foreign body; the latter was deemed unlikely in light of the patient’s physical exam. In consideration of his elevated WBC count, the high number of WBCs in his urine, the fever, and the CT results, the patient was diagnosed with an abscess in his right hand that had been seeded, it was surmised, by an occult urosepsis after his fall.
Before the patient’s admission, a hand surgeon was consulted. The surgeon agreed with the diagnosis, and the patient was taken to the operating room (OR). He had been given piperacillin/tazobactam in the ED.
In the OR, the surgeon made a 3.0-cm incision, conducted an exploration, and identified a cavity that contained a small amount of purulence. He determined the lesion to be a resolving abscess. The wound was washed out, and the area was closed with a Penrose drain.
The patient was continued on the piperacillin/tazobactam. His blood culture was positive for gram-positive rods, and a low-grade fever persisted. An infectious disease specialist was consulted, and levofloxacin was added to the patient’s regimen.
After 24 hours of treatment, findings on urinalysis improved: blood, small; protein, trace; nitrites, negative; leukocytes, small; WBCs, 15 to 20/high-power field; and no bacteria. Over the next three days, the patient’s condition continued to improve. His hand drain was removed, and the pain and swelling subsided. He became afebrile, and his WBC count fell to 24,700/mcL. He was discharged to home with prescriptions for cephalexin and levofloxacin. Follow-up for postoperative care was arranged with the hand surgeon.
Discussion
Pyomyositis is defined as abscess formation deep within large striated muscles.1 Although this condition is uncommon, it is believed that an occult bacteremia can seed an area of damaged muscle (compared with healthy muscle, which ordinarily resists infection), allowing an abscess to form.1,2
Epidemiology
In a 2002 review involving 676 patients with primary pyomyositis, Bickels et al3 reported the condition in ages ranging from two months to 82 years (mean, 28.1 years). In a majority of cases, only a single muscle was involved; 112 patients (16.6%) were identified with multiple-site involvement. Only seven cases (0.1%) involved the hand.
In 452 cases (66.9%), a bacterial agent was identified. Among these, 350 (77%) had a positive culture for Staphylococcus aureus. Other isolates included Streptococcus pyogenes, Escherichia coli, Salmonella enteritidus, and Mycobacterium tuberculosis.1,3 It should be noted that community-acquired methicillin-resistant S aureus (CA-MRSA) is being implicated with increasing frequency in cases of pyomyositis.4-6
Because pyomyositis is not a reportable disease and has not been studied in large clinical trials, its incidence is uncertain, and proposed risk factors have not all been confirmed2 (see Table2,7).
Pathophysiology
While the etiology of primary pyomyositis is unclear, it is believed to be caused by a combination of bacteremia (chronic or transient) and damaged muscle. In a 1960 study published in the Lancet, Smith and Vickers8 performed autopsies on 327 patients who had died of culture-positive septicemia. Only two patients were found to have a muscle abscess. At that time, the investigators concluded that both muscle injury and bacteremia would need to be present in order for an abscess to form. In animal studies, bacteremia (eg, S aureus) does not appear to lead to pyomyositis except in cases of muscle abnormality or trauma (eg, electric shock, pinching injury).9,10
When a history of trauma can be identified in patients with pyomyositis, the condition typically develops near the affected muscle, and the infection appears within days to weeks.3 In cases in which an antecedent infection is identified and hematogenous spread of the bacteria to the skeletal muscle occurs, this is termed secondary pyomyositis.11
Disease Progression
Pyomyositis generally progresses in three stages, beginning with inflammation and advancing to a focal abscess, then to a septic state.3 The first stage develops between seven and 21 days after the initial incident, is typically subacute, involves mild pain and swelling with a “woody” texture, and is occasionally associated with fevers.2
Diagnosis of pyomyositis is usually made during the second stage, 10 to 21 days after the initial incident; by that time, the pain has increased, and the fever is more pronounced. Third-stage infection usually involves fluctuance and sepsis.2
Although MRI is considered most useful in the diagnosis of pyomyositis, CT and ultrasound allow for percutaneous needle aspiration and drainage.3
Treatment
The correct treatment for pyomyositis depends upon the stage at which the disease is identified. During the first stage (before formation of an abscess), antibiotic treatment alone may be sufficient.1 Once an abscess has formed, an incision and drainage will be required, in conjunction with or followed by appropriate antibiotic therapy.
When pyomyositis is properly treated during the first or second stage, a full recovery is likely.2,3 By the third stage, surgical debridement is required. Additionally, osteomyelitis may develop in the adjacent bones, followed by muscle scarring, residual weakness, and functional impairment.2,3 Reported pyomyositis-associated mortality ranges between less than 1% and 4%.2,12
The Case Patient
The case presented here was of particular interest for two reasons. First, the patient had a traumatic injury that initially caused him no concern but worsened progressively over 14 days. Although this is not the typical presentation of a traumatic injury, the ED staff could very easily have performed a radiograph, made a diagnosis of traumatic hand injury, and discharged the patient.
Second, men in their 60s do not commonly have urinary tract infections.13 The patient was questioned frequently by several providers about sexual behaviors, medical problems, and urinary symptoms. Repeatedly, he denied all of these issues. While a urinalysis may be omitted in the evaluation of an otherwise healthy, asymptomatic patient, its results in this case were a key piece of data.
It should be noted that the patient thought it inappropriate to be asked for urine samples. He repeatedly said, “It’s my hand!”
Conclusion
Even in patients presenting with the most routine complaint, a careful evaluation can reveal unexpected, serious problems. This patient complained of pain in his hand some time after a fall and ultimately was treated for an occult urosepsis and hand abscess—pyomyositis, which rarely occurs in small muscles, such as those of the hand. Either condition, left untreated, could have led to serious morbidity or even mortality.
1. Beers MH, Berkow R, eds. Merck Manual of Diagnosis and Therapy. 18th ed. Whitehouse Station, NJ: Merck Research Laboratories, 2006:1142-1143.
2. Crum-Cianflone NF. Bacterial, fungal, parasitic and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494.
3. Bickels J, Ben-Sira L, Kessler A, Wientroub S. Current concepts review: primary pyomyositis. J Bone Joint Surg Am. 2002;84-A(12):2277-2286.
4. Lo BM, Fickenscher BA. Primary pyomyositis caused by ca-MRSA. Int J Emerg Med. 2008;1(4):331-332.
5. Ruiz ME, Yohannes S, Wladyka CG. Pyomyositis caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2005;352(14):1488–1489.
6. Pannaraj PS, Hulten KG, Gonzalez BE, et al. Infective pyomyositis and myositis in children in the era of community-acquired, methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2006;43(8):953–960.
7. Ükinç K, Bayraktar M, Uzun O. A case of type 2 diabetes complicated with primary pyomyositis. Endocrinologist. 2009;19(3):129-130.
8. Smith IM, Vickers AB. Natural history of 338 treated and untreated patients with staphylococcal septicaemia (1936-1955). Lancet. 1960;1(7138):1318-1322.
9. Phoon E-S, Sebastin SJ, Tay S-C. Primary pyomyositis (bacterial myositis) of the pronator quadratus. J Hand Surg Eur Vol. 2009;34(4):549-551.
10. Christin L, Sarosi GA. Pyomyositis in North America: case reports and review. Clin Infect Dis. 1992; 15(4):668-677.
11. Sokolowski MJ, Koh JL. Pyomyositis of the shoulder girdle. Orthopedics. 2006;29(11):1030-1032.
12. Crum NF. Bacterial pyomyositis in the United States. Am J Med. 2004;117(6):420–428.
13. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113 suppl 1A:5S-13S.
1. Beers MH, Berkow R, eds. Merck Manual of Diagnosis and Therapy. 18th ed. Whitehouse Station, NJ: Merck Research Laboratories, 2006:1142-1143.
2. Crum-Cianflone NF. Bacterial, fungal, parasitic and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494.
3. Bickels J, Ben-Sira L, Kessler A, Wientroub S. Current concepts review: primary pyomyositis. J Bone Joint Surg Am. 2002;84-A(12):2277-2286.
4. Lo BM, Fickenscher BA. Primary pyomyositis caused by ca-MRSA. Int J Emerg Med. 2008;1(4):331-332.
5. Ruiz ME, Yohannes S, Wladyka CG. Pyomyositis caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2005;352(14):1488–1489.
6. Pannaraj PS, Hulten KG, Gonzalez BE, et al. Infective pyomyositis and myositis in children in the era of community-acquired, methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2006;43(8):953–960.
7. Ükinç K, Bayraktar M, Uzun O. A case of type 2 diabetes complicated with primary pyomyositis. Endocrinologist. 2009;19(3):129-130.
8. Smith IM, Vickers AB. Natural history of 338 treated and untreated patients with staphylococcal septicaemia (1936-1955). Lancet. 1960;1(7138):1318-1322.
9. Phoon E-S, Sebastin SJ, Tay S-C. Primary pyomyositis (bacterial myositis) of the pronator quadratus. J Hand Surg Eur Vol. 2009;34(4):549-551.
10. Christin L, Sarosi GA. Pyomyositis in North America: case reports and review. Clin Infect Dis. 1992; 15(4):668-677.
11. Sokolowski MJ, Koh JL. Pyomyositis of the shoulder girdle. Orthopedics. 2006;29(11):1030-1032.
12. Crum NF. Bacterial pyomyositis in the United States. Am J Med. 2004;117(6):420–428.
13. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113 suppl 1A:5S-13S.