User login
Leptin expression may help differentiate renal lesions
Expression of leptin could help differentiate benign renal oncocytomas from chromophobe renal cell carcinoma (RCC), results of a recent pathology study suggest.
Renal oncocytomas had significantly increased nuclear expression of leptin, compared with the eosinophilic subtype of chromophobe RCC, according to the study.
If translated into clinical urology and pathology practice, quantification of leptin expression could help in the difficult task of differentiating between these renal lesions, according to the investigators, led by Glenda Gobe, PhD, of the Kidney Disease Research Group at the University of Queensland in Brisbane, Australia.
“This may have a major clinical impact in reducing unnecessary intervention and treatment-related harm,” Dr. Gobe and her colleagues reported in Pathology.
They obtained archived paraffin blocks from human renal tumor tissue obtained between 2009 and 2014. Paraffin sections were immunostained for leptin and leptin receptors.
The investigators evaluated 30 chromophobe RCC specimens – 15 eosinophilic and 15 noneosinophilic variants – and 15 renal oncocytomas. They also included 30 clear cell RCCs, the most common histological subtype of RCC, to verify immunohistochemistry (IHC) staining patterns.
Results showed that both leptin and leptin receptor IHC was significantly increased in tumor versus in matched, noncancerous kidney tissue.
Expression of both leptin and leptin receptor was highest for renal oncocytomas versus both the chromophobe and clear cell RCCs. On closer scrutiny, the investigators said, nuclear expression of leptin in renal oncocytomas had a significantly higher intensity versus chromophobe RCC.
“This important finding could prove to be helpful in the distinction between chromophobe RCC and renal oncocytoma,” Dr. Gobe and her coauthors wrote in their report.
In a subgroup analysis, they found a significantly increased nuclear leptin intensity for the renal oncocytomas as compared to the eosinophilic variants of chromophobe RCC (P = .016 by Dunn’s multiple comparisons test).
By contrast, testing did not reveal a significant difference for renal oncocytomas versus noneosinophilic chromophobe RCC (P = .0939) or versus clear cell RCC (P greater than .999).
Leptin, secreted by adipose cells that help regulate energy balance through inhibition of hunger, may play a role in carcinogenesis, according to investigators. Studies to date have shown that the hormone may impact carcinogenesis through cell proliferation, inhibition of apoptosis, and other potential mechanisms.
Applying this biomarker in clinical practice could more reliably characterize renal lesions with no or limited malignant potential, according to the investigators.
“The distinction of renal oncocytoma from chromophobe RCC will dictate different management pathways, as renal oncocytoma is benign while chromophobe RCC is a malignant subtype which will require further surveillance,” Dr. Gobe and her coinvestigators wrote.
The University of Malaya, Kuala Lumpur, Malaysia, funded the study. Dr. Gobe and her coauthors stated that they had no conflicts of interest.
SOURCE: Gobe G et al. Pathology. 2018 Aug;50(5):504-10.
Expression of leptin could help differentiate benign renal oncocytomas from chromophobe renal cell carcinoma (RCC), results of a recent pathology study suggest.
Renal oncocytomas had significantly increased nuclear expression of leptin, compared with the eosinophilic subtype of chromophobe RCC, according to the study.
If translated into clinical urology and pathology practice, quantification of leptin expression could help in the difficult task of differentiating between these renal lesions, according to the investigators, led by Glenda Gobe, PhD, of the Kidney Disease Research Group at the University of Queensland in Brisbane, Australia.
“This may have a major clinical impact in reducing unnecessary intervention and treatment-related harm,” Dr. Gobe and her colleagues reported in Pathology.
They obtained archived paraffin blocks from human renal tumor tissue obtained between 2009 and 2014. Paraffin sections were immunostained for leptin and leptin receptors.
The investigators evaluated 30 chromophobe RCC specimens – 15 eosinophilic and 15 noneosinophilic variants – and 15 renal oncocytomas. They also included 30 clear cell RCCs, the most common histological subtype of RCC, to verify immunohistochemistry (IHC) staining patterns.
Results showed that both leptin and leptin receptor IHC was significantly increased in tumor versus in matched, noncancerous kidney tissue.
Expression of both leptin and leptin receptor was highest for renal oncocytomas versus both the chromophobe and clear cell RCCs. On closer scrutiny, the investigators said, nuclear expression of leptin in renal oncocytomas had a significantly higher intensity versus chromophobe RCC.
“This important finding could prove to be helpful in the distinction between chromophobe RCC and renal oncocytoma,” Dr. Gobe and her coauthors wrote in their report.
In a subgroup analysis, they found a significantly increased nuclear leptin intensity for the renal oncocytomas as compared to the eosinophilic variants of chromophobe RCC (P = .016 by Dunn’s multiple comparisons test).
By contrast, testing did not reveal a significant difference for renal oncocytomas versus noneosinophilic chromophobe RCC (P = .0939) or versus clear cell RCC (P greater than .999).
Leptin, secreted by adipose cells that help regulate energy balance through inhibition of hunger, may play a role in carcinogenesis, according to investigators. Studies to date have shown that the hormone may impact carcinogenesis through cell proliferation, inhibition of apoptosis, and other potential mechanisms.
Applying this biomarker in clinical practice could more reliably characterize renal lesions with no or limited malignant potential, according to the investigators.
“The distinction of renal oncocytoma from chromophobe RCC will dictate different management pathways, as renal oncocytoma is benign while chromophobe RCC is a malignant subtype which will require further surveillance,” Dr. Gobe and her coinvestigators wrote.
The University of Malaya, Kuala Lumpur, Malaysia, funded the study. Dr. Gobe and her coauthors stated that they had no conflicts of interest.
SOURCE: Gobe G et al. Pathology. 2018 Aug;50(5):504-10.
Expression of leptin could help differentiate benign renal oncocytomas from chromophobe renal cell carcinoma (RCC), results of a recent pathology study suggest.
Renal oncocytomas had significantly increased nuclear expression of leptin, compared with the eosinophilic subtype of chromophobe RCC, according to the study.
If translated into clinical urology and pathology practice, quantification of leptin expression could help in the difficult task of differentiating between these renal lesions, according to the investigators, led by Glenda Gobe, PhD, of the Kidney Disease Research Group at the University of Queensland in Brisbane, Australia.
“This may have a major clinical impact in reducing unnecessary intervention and treatment-related harm,” Dr. Gobe and her colleagues reported in Pathology.
They obtained archived paraffin blocks from human renal tumor tissue obtained between 2009 and 2014. Paraffin sections were immunostained for leptin and leptin receptors.
The investigators evaluated 30 chromophobe RCC specimens – 15 eosinophilic and 15 noneosinophilic variants – and 15 renal oncocytomas. They also included 30 clear cell RCCs, the most common histological subtype of RCC, to verify immunohistochemistry (IHC) staining patterns.
Results showed that both leptin and leptin receptor IHC was significantly increased in tumor versus in matched, noncancerous kidney tissue.
Expression of both leptin and leptin receptor was highest for renal oncocytomas versus both the chromophobe and clear cell RCCs. On closer scrutiny, the investigators said, nuclear expression of leptin in renal oncocytomas had a significantly higher intensity versus chromophobe RCC.
“This important finding could prove to be helpful in the distinction between chromophobe RCC and renal oncocytoma,” Dr. Gobe and her coauthors wrote in their report.
In a subgroup analysis, they found a significantly increased nuclear leptin intensity for the renal oncocytomas as compared to the eosinophilic variants of chromophobe RCC (P = .016 by Dunn’s multiple comparisons test).
By contrast, testing did not reveal a significant difference for renal oncocytomas versus noneosinophilic chromophobe RCC (P = .0939) or versus clear cell RCC (P greater than .999).
Leptin, secreted by adipose cells that help regulate energy balance through inhibition of hunger, may play a role in carcinogenesis, according to investigators. Studies to date have shown that the hormone may impact carcinogenesis through cell proliferation, inhibition of apoptosis, and other potential mechanisms.
Applying this biomarker in clinical practice could more reliably characterize renal lesions with no or limited malignant potential, according to the investigators.
“The distinction of renal oncocytoma from chromophobe RCC will dictate different management pathways, as renal oncocytoma is benign while chromophobe RCC is a malignant subtype which will require further surveillance,” Dr. Gobe and her coinvestigators wrote.
The University of Malaya, Kuala Lumpur, Malaysia, funded the study. Dr. Gobe and her coauthors stated that they had no conflicts of interest.
SOURCE: Gobe G et al. Pathology. 2018 Aug;50(5):504-10.
FROM PATHOLOGY
Key clinical point: Measuring leptin expression could help differentiate benign renal oncocytomas from chromophobe renal cell carcinoma (RCC).
Major finding: Nuclear leptin intensity was significantly increased for renal oncocytomas versus eosinophilic variants of chromophobe RCC (P = 0.016).
Study details: Evaluation of 75 archived tissue samples, including 30 chromophobe RCC specimens, 15 renal oncocytomas, and 30 clear cell RCCs, along with matched, noncancerous kidney tissue specimens.
Disclosures: The University of Malaya, Kuala Lumpur, Malaysia, funded the study. The authors stated that they had no conflicts of interest.
Source: Gobe G et al. Pathology. 2018 Aug;50(5):504-10.
Renal hemangioma? Think again
What appears at first glance to be a renal vascular tumor may in fact be a rare type of renal cell carcinoma (RCC), authors of a case study cautioned.
A tumor recovered from a 62-year old woman who underwent a partial nephrectomy for an incidentally discovered asymptomatic left renal mass contained arborizing vessels that mimicked hemangioma. Immunohistochemical staining of the tumor highlighted the vascular component but masked epithelial cells, a situation that, in the absence of other clues, might cause a misdiagnosis, reported Kanika Taneja, MD, from the Henry Ford Cancer Institute in Detroit, and her colleagues.
The authors were tipped off to an unusual presentation, however, by mixed signals from immunohistochemical staining, leading them to an admittedly fuzzy diagnosis of “unclassified hemangioma-like RCC.”
“This case highlights that renal cell carcinoma must be strongly considered in the differential diagnosis of renal vascular tumors, and broadens the spectrum of histologies that may mimic hemangioma,” they wrote in Human Pathology.
Although there are a few recent reports in the medical literature of clear cell RCC tumors that mimic hemangiomas, the authors noted that, “to our knowledge, non–clear cell hemangioma-like renal cell carcinoma has not been previously reported.”
The tumor in question was removed from the patient with clear surgical margins during a partial nephrectomy.
Gross examination showed a 2.6 by 2.5 by 2.5 cm, well-circumscribed, tan-brown hemorrhagic mass. On microscopic examination the tumor had hemangioma-like features and lacked typical clear cell morphology, and immunohistochemical staining did little to clarify the picture.
Specifically, although staining highlighted the epithelial component of the tumor, the investigators saw what they described as “an abnormal combination of positive markers” that are normally used to distinguish clear cell from papillary histologies, effectively throwing a monkey wrench into the diagnostic works.
The marker profile in this case included cytokeratin 7, high molecular weight cytokeratin, and carbonic anhydrase IX, but only minimal labeling for alpha-methylacyl-CoA racemase and absence of GATA3.
To add to the confusion, fluorescent in situ hybridization “revealed negative studies for chromosome 3p, trisomy 7 or 17, and MITF family translocations, failing to further place this unique neoplasm into a definitive category,” Dr. Taneja and her colleagues wrote, adding that further study of the tumor may help to clarify whether it represents a distinct tumor type or is simply an unusual pattern caused by degeneration and involution of a known tumor type.
The authors did not disclose a study funding source, but reported having no conflicts of interest.
SOURCE: Taneja K et al. Hum Pathol. 2017 Nov 2. doi: 10.1016/j.humpath.2017.09.015.
What appears at first glance to be a renal vascular tumor may in fact be a rare type of renal cell carcinoma (RCC), authors of a case study cautioned.
A tumor recovered from a 62-year old woman who underwent a partial nephrectomy for an incidentally discovered asymptomatic left renal mass contained arborizing vessels that mimicked hemangioma. Immunohistochemical staining of the tumor highlighted the vascular component but masked epithelial cells, a situation that, in the absence of other clues, might cause a misdiagnosis, reported Kanika Taneja, MD, from the Henry Ford Cancer Institute in Detroit, and her colleagues.
The authors were tipped off to an unusual presentation, however, by mixed signals from immunohistochemical staining, leading them to an admittedly fuzzy diagnosis of “unclassified hemangioma-like RCC.”
“This case highlights that renal cell carcinoma must be strongly considered in the differential diagnosis of renal vascular tumors, and broadens the spectrum of histologies that may mimic hemangioma,” they wrote in Human Pathology.
Although there are a few recent reports in the medical literature of clear cell RCC tumors that mimic hemangiomas, the authors noted that, “to our knowledge, non–clear cell hemangioma-like renal cell carcinoma has not been previously reported.”
The tumor in question was removed from the patient with clear surgical margins during a partial nephrectomy.
Gross examination showed a 2.6 by 2.5 by 2.5 cm, well-circumscribed, tan-brown hemorrhagic mass. On microscopic examination the tumor had hemangioma-like features and lacked typical clear cell morphology, and immunohistochemical staining did little to clarify the picture.
Specifically, although staining highlighted the epithelial component of the tumor, the investigators saw what they described as “an abnormal combination of positive markers” that are normally used to distinguish clear cell from papillary histologies, effectively throwing a monkey wrench into the diagnostic works.
The marker profile in this case included cytokeratin 7, high molecular weight cytokeratin, and carbonic anhydrase IX, but only minimal labeling for alpha-methylacyl-CoA racemase and absence of GATA3.
To add to the confusion, fluorescent in situ hybridization “revealed negative studies for chromosome 3p, trisomy 7 or 17, and MITF family translocations, failing to further place this unique neoplasm into a definitive category,” Dr. Taneja and her colleagues wrote, adding that further study of the tumor may help to clarify whether it represents a distinct tumor type or is simply an unusual pattern caused by degeneration and involution of a known tumor type.
The authors did not disclose a study funding source, but reported having no conflicts of interest.
SOURCE: Taneja K et al. Hum Pathol. 2017 Nov 2. doi: 10.1016/j.humpath.2017.09.015.
What appears at first glance to be a renal vascular tumor may in fact be a rare type of renal cell carcinoma (RCC), authors of a case study cautioned.
A tumor recovered from a 62-year old woman who underwent a partial nephrectomy for an incidentally discovered asymptomatic left renal mass contained arborizing vessels that mimicked hemangioma. Immunohistochemical staining of the tumor highlighted the vascular component but masked epithelial cells, a situation that, in the absence of other clues, might cause a misdiagnosis, reported Kanika Taneja, MD, from the Henry Ford Cancer Institute in Detroit, and her colleagues.
The authors were tipped off to an unusual presentation, however, by mixed signals from immunohistochemical staining, leading them to an admittedly fuzzy diagnosis of “unclassified hemangioma-like RCC.”
“This case highlights that renal cell carcinoma must be strongly considered in the differential diagnosis of renal vascular tumors, and broadens the spectrum of histologies that may mimic hemangioma,” they wrote in Human Pathology.
Although there are a few recent reports in the medical literature of clear cell RCC tumors that mimic hemangiomas, the authors noted that, “to our knowledge, non–clear cell hemangioma-like renal cell carcinoma has not been previously reported.”
The tumor in question was removed from the patient with clear surgical margins during a partial nephrectomy.
Gross examination showed a 2.6 by 2.5 by 2.5 cm, well-circumscribed, tan-brown hemorrhagic mass. On microscopic examination the tumor had hemangioma-like features and lacked typical clear cell morphology, and immunohistochemical staining did little to clarify the picture.
Specifically, although staining highlighted the epithelial component of the tumor, the investigators saw what they described as “an abnormal combination of positive markers” that are normally used to distinguish clear cell from papillary histologies, effectively throwing a monkey wrench into the diagnostic works.
The marker profile in this case included cytokeratin 7, high molecular weight cytokeratin, and carbonic anhydrase IX, but only minimal labeling for alpha-methylacyl-CoA racemase and absence of GATA3.
To add to the confusion, fluorescent in situ hybridization “revealed negative studies for chromosome 3p, trisomy 7 or 17, and MITF family translocations, failing to further place this unique neoplasm into a definitive category,” Dr. Taneja and her colleagues wrote, adding that further study of the tumor may help to clarify whether it represents a distinct tumor type or is simply an unusual pattern caused by degeneration and involution of a known tumor type.
The authors did not disclose a study funding source, but reported having no conflicts of interest.
SOURCE: Taneja K et al. Hum Pathol. 2017 Nov 2. doi: 10.1016/j.humpath.2017.09.015.
FROM HUMAN PATHOLOGY
Key clinical point: Unusual morphology of renal cell carcinoma tumors may lead to a misdiagnosis of renal hemangioma.
Major finding: The unusual architecture and microscopic features of a specific tumor led to a diagnosis of unclassified hemangioma-like renal cell carcinoma.
Study details: A case report of a tumor removed from a 62-year-old woman.
Disclosures: The authors did not disclose a study funding source, but reported having no conflicts of interest.
Source: Taneja K et al. Hum Pathol. 2017 Nov 2. doi: 10.1016/j.humpath.2017.09.015.
Meeting the potential of immunotherapy: new targets provide rational combinations
The relationship between the immune system and tumors is complex and dynamic, and for immunotherapy to reach its full potential it will likely need to attack on multiple fronts. Here, we discuss some of the latest and most promising developments in the immuno-oncology field designed to build on the successes and address limitations.
The anti-tumor immune response
Cancer is a disease of genomic instability, whereby genetic alterations ranging from a single nucleotide to the whole chromosome level frequently occur. Although cancers derive from a patient’s own tissues, these genetic differences can mark the cancer cell as non-self, triggering an immune response to eliminate these cells.
The first hints of this anti-tumor immunity date back more than a century and a half and sparked the concept of mobilizing the immune system to treat patients.1-3 Although early pioneers achieved little progress in this regard, their efforts provided invaluable insights into the complex and dynamic relationship between a tumor and the immune system that are now translating into real clinical successes.
We now understand that the immune system has a dual role in both restraining and promoting cancer development and have translated this understanding into the theory of cancer immunoediting. Immunoediting has three stages: elimination, wherein the tumor is seemingly destroyed by the innate and adaptive immune response; equilibrium, in which cancer cells that were able to escape elimination are selected for growth; and escape, whereby these resistant cancer cells overwhelm the immune system and develop into a symptomatic lesion.4,5
Immuno-oncologists have also described the cancer immunity cycle to capture the steps that are required for an effective anti-tumor immune response and defects in this cycle form the basis of the most common mechanisms used by cancer cells to subvert the anti-tumor immune response. Much like the cancer hallmarks did for molecularly targeted cancer drugs, the cancer immunity cycle serves as the intellectual framework for cancer immunotherapy.6,7
Exploiting nature’s weapon of mass destruction
Initially, attempts at immunotherapy focused on boosting the immune response using adjuvants and cytokines. The characterization of subtle differences between tumor cells and normal cells led to the development of vaccines and cell-based therapies that exploited these tumor-associated antigens (TAAs).1-6
Despite the approval of a therapeutic vaccine, sipuleucel-T, in 2010 for the treatment of metastatic prostate cancer, in general the success of vaccines has been limited. Marketing authorization for sipuleucel-T was recently withdrawn in Europe, and although it is still available in the United States, it is not widely used because of issues with production and administration. Other vaccines, such as GVAX, which looked particularly promising in early-stage clinical trials, failed to show clinical efficacy in subsequent testing.8,9
Cell-based therapies, such as adoptive cellular therapy (ACT), in which immune cells are removed from the host, primed to attack cancer cells, and then reinfused back into the patient, have focused on T cells because they are the major effectors of the adaptive immune response. Clinical success with the most common approach, tumor-infiltrating lymphocyte (TIL)
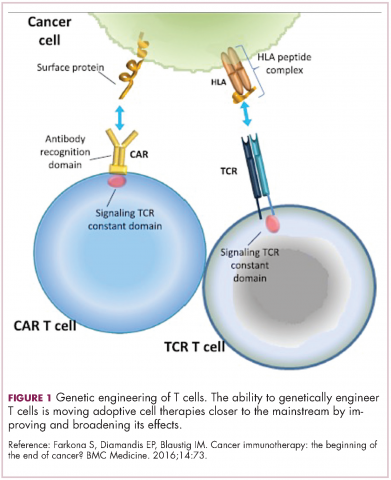
Two key techniques have been developed (Figure 1). T-cell receptor (TCR) therapy involves genetically modifying the receptor on the surface of T cells that is responsible for recognizing antigens bound to major histocompatibility complex (MHC) molecules on the surface of antigen-presenting cells (APCs). The TCR can be altered to recognize a specific TAA or modified to improve its antigen recognition and binding capabilities. This type of therapy is limited by the fact that the TCRs need to be genetically matched to the patient’s immune type.
Releasing the brakes
To ensure that it is only activated at the appropriate time and not in response to the antigens expressed on the surface of the host’s own tissues or harmless materials, the immune system has developed numerous mechanisms for immunological tolerance. Cancer cells are able to exploit these mechanisms to allow them to evade the anti-tumor immune response. One of the main ways in which they do this is by manipulating the signaling pathways involved in T-cell activation, which play a vital role in tolerance.12
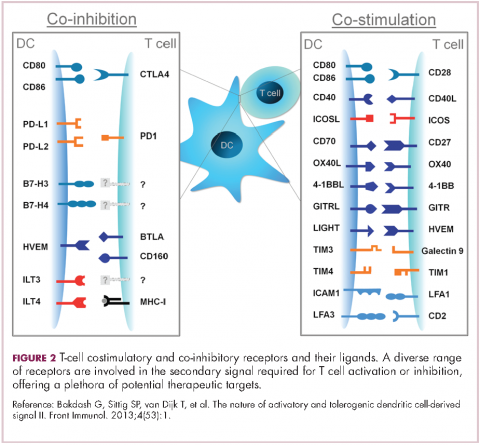
To become fully activated, T cells require a primary signal generated by an interaction between the TCR and the antigen-MHC complex on the surface of an APC, followed by secondary costimulatory signals generated by a range of different receptors present on the T-cell surface binding to their ligands on the APC.
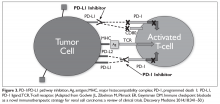
If the second signal is inhibitory rather than stimulatory, then the T cell is deactivated instead of becoming activated. Two key coinhibitory receptors are programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) and tumor cells are able to overcome the anti-tumor immune response in part by expressing the ligands that bind these receptors to dampen the activity of tumor-infiltrating T cells and induce tolerance.13
The development of inhibitors of CTLA-4 and PD-1 and their respective ligands has driven some of the most dramatic successes with cancer immunotherapy, particularly with PD-1-targeting drugs which have fewer side effects. Targeting of this pathway has resulted in durable responses, revolutionizing the treatment of metastatic melanoma, with recently published long-term survival data for pembrolizumab showing that 40% of patients were alive 3 years after initiating treatment and, in a separate study, 34% of nivolumab-treated patients were still alive after 5 years.14,15 More recently, PD-1 inhibitors have been slowly expanding into a range of other cancer types and 4 immune checkpoint inhibitors are now approved by the United States Food and Drug Administration (FDA): ipilimumab (Yervoy), nivolumab (Opdivo), pembrolizumab (Keytruda) and atezolizumab (Tecentriq).
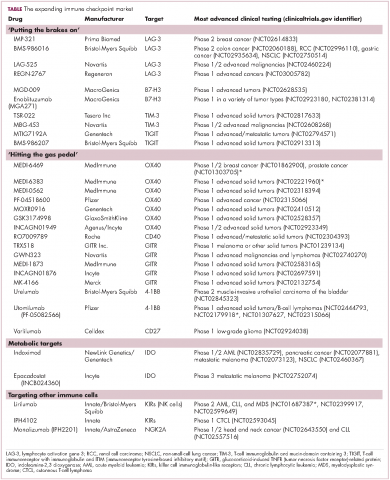
Six years on from the first approval in this drug class and an extensive network of coinhibitory receptors has been uncovered – so-called immune checkpoints – many of which are now also serving as therapeutic targets (Table, Figure 2).16 Lymphocyte activation gene 3 (LAG-3) is a member of the immunoglobulin superfamily of receptors that is expressed on a number of different types of immune cell. In addition to negatively regulating cytotoxic T-cell activation like PD-1 and CTLA-4, it is also thought to regulate the immunosuppressive functions of regulatory T cells and the maturation and activation of dendritic cells. T-cell immunoglobulin and mucin domain-containing 3 (TIM-3) is found on the surface of helper and cytotoxic T cells and regulates T-cell inhibition as well as macrophage activation. Inhibitors of both proteins have been developed that are being evaluated in phase 1 or 2 clinical trials in a variety of tumor types.17
Indeed, although T cells have commanded the most attention, there is growing appreciation of the potential for targeting other types of immune cell that play a role in the anti-tumor immune response or in fostering an immunosuppressive microenvironment. NK cells have been a particular focus, since they represent the body’s first line of immune defense and they appear to have analogous inhibitory and activating receptors expressed on their surface that regulate their cytotoxic activity.
The best-defined NK cell receptors are the killer cell immunoglobulin-like receptors (KIRs) that bind to the MHC class I proteins found on the surface of all cells that distinguish them as ‘self’ or ‘non-self’. KIRs can be either activating or inhibitory, depending upon their structure and the ligands to which they bind.19 To date, 2 antibodies targeting inhibitory KIRs have been developed. Though there has been some disappointment with these drugs, most recently a phase 2 trial of lirilumab in elderly patients with acute myeloid leukemia, which missed its primary endpoint, they continue to be evaluated in clinical trials.20
The inhibitory immune checkpoint field has also expanded to include molecules that regulate T-cell activity in other ways. Most prominently, this includes enzymes like indoleamine-2,3 dioxygenase (IDO), which is involved in the metabolism of the essential amino acid tryptophan. IDO-induced depletion of tryptophan and generation of tryptophan metabolites is toxic to cytotoxic T cells, and IDO is also thought to directly activate regulatory T cells, thus the net effect of IDO is immunosuppression. Two IDO inhibitors are currently being developed.21
Stepping on the gas
Despite their unprecedented success, immune checkpoint inhibitors are not effective in all patients or in all tumor types. Their efficacy is limited in large part by the requirement for a pre-existing anti-tumor immune response. If there are no T cells within the tumor microenvironment then releasing the brakes on the immune system won’t help.
More recently, researchers have returned to the idea of stimulating an anti-tumor immune response, this time by targeting the other side of the immune checkpoint coin, the costimulatory molecules. These drugs could prove more effective as they aren’t reliant on a pre-existing anti-tumor immune response. A number of agonist antibodies designed to target these receptors have now been developed and are undergoing clinical evaluation.22
Furthest along in development are those targeting OX40, a costimulatory molecule that is upregulated on the surface of T cells once they have been fully activated by the TCR signal and an initial costimulatory signal. OX40 is thought to be involved in a more long-term immune response and in the formation of a memory response. A mouse monoclonal antibody had a potent immune-stimulating effect accompanied by the regression of at least 1 metastatic lesion in 30% of patients treated in a phase 1 clinical trial, but was limited by the generation of anti-mouse antibodies. 7 OX40 agonists are now in clinical development, 6 fully human monoclonal antibodies and 1 OX40 ligand-Fc fusion protein, MEDI-6383.23
Combinations are key
Many researchers are now reaching the conclusion that combination therapy is likely to be key in expanding the scope of immunotherapy into currently unresponsive patient populations. Investigating rational combinations is already becoming a burgeoning area of the immuno-oncology field, with a variety of different strategies being tested.
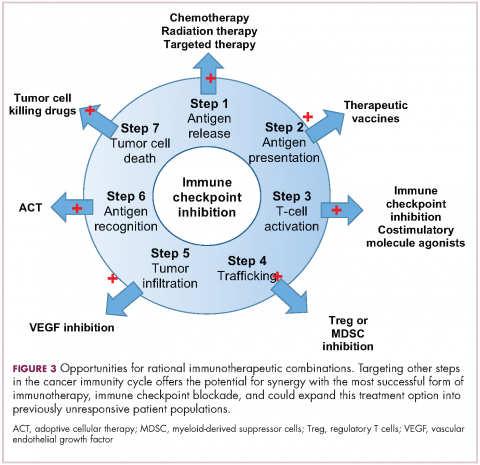
Now the question becomes what are the optimal combinations and the timing and sequencing of combination therapy is likely to be a paramount consideration. Developing combinations that have distinct mechanisms of action or target multiple steps in the cancer immunity cycle offers the greatest potential for therapeutic synergy since this is most likely to address potential mechanisms of resistance by blocking other paths to immune evasion for cancer cells (Figure 3).
Given the expanding network of immune-checkpoint inhibitors and agonists, the focal point of combination therapy has been combining immune checkpoint-targeting drugs with different mechanisms of action, including those that would simultaneously release the brakes and step on the gas pedal. The vast majority of ongoing clinical trials of approved checkpoint inhibitors and the drugs in development listed in the table are combination trials.
These efforts yielded the first FDA-approved combination immunotherapy regimen in 2015; nivolumab and ipilimumab for the treatment of metastatic melanoma. Approval was based on the demonstration of improved ORR, prolonged response duration, and improved progression-free survival among 142 patients treated with the combination, compared to either drug alone.24
The results of a phase 1/2 trial evaluating the combination of a 4-1BB receptor agonist urelumab with nivolumab in hematologic malignancies and solid tumors found the combination to be safe and particularly effective in patients with advanced/metastatic melanoma, with an ORR of 50%.25 Nivolumab was also combined with the CD27 agonist varlilumab in a phase 1/2 clinical trial of patients with solid tumors, for which data was also recently released. Among 46 patients enrolled, primarily those with colorectal and ovarian cancer the combination had an acceptable safety profile and favorable changes in intratumoral immune biomarkers were observed. The phase 2 portion of the trial is ongoing.26
Meanwhile, Incyte’s IDO inhibitor epacadostat has recently been making waves in combination with pembrolizumab in patients with advanced solid tumors. It demonstrated particularly promising clinical activity in patients with metastatic melanoma, with an overall response rate (ORR) of 57%, including 2 complete responses (CRs), prompting initiation of a phase 3 trial of this combination (NCT02752074).27
1. Adams JL, Smothers J, Srinivasan R, et al. Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Disc. 2015;14:603-622.
2. D’Errico G, Machado HL, Sainz Jr B. A current perspective on cancer immune therapy: step-by-step approach to constructing the magic bullet. Clin Trans Med. 2017;6:3.
3. Farkona S, Diamandis EP, Blaustig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73.
4. Meiliana A, Dewi NM, Wijaya A. Cancer immunotherapy: a review. Indones Biomed J. 2016;8(1):1-20.
5. Smyth MJ, Ngiow SF, Ribas A, et al. Combination cancer immunotherapies tailored to the tumor microenvironment. Nat Rev Clin Oncol. 2016;13:143-158.
6. de Charette M, Marabelle A, Houot R. Turning tumor cells into antigen presenting cells: The next step to improve cancer immunotherapy? Eur J Cancer 2016;68:134-147.
7. Chen DS and Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013;39:1-10.
8. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480-489.
9. Le DT, Wang-Gillam A, Picozzi V Jr, et al. A phase 2, randomized trial of GVAX Pancreas and CRS-207 immunotherapy versus GVAX alone in patients with metastatic pancreatic adenocarcinoma: Updated results. Presented at: the ASCO Gastrointestinal Cancers Symposium; January 16-18, 2014; San Francisco, CA. Abstract 177.
10. Sharpe M and Mount N. Genetically modified T cells in cancer therapy: opportunities and challenges. Dis Model Mech. 2015;8(4):337-350.
11. Perica K, Varela JC, Oelke M, et al. Adoptive T Cell Immunotherapy for Cancer. Ram Mai Med J. 2015;6(1):e0004.
12. Xing Y and Hogquist KA. T-Cell Tolerance: Central and Peripheral. Cold Spring Harb Perspect Biol. 2012;4:a006957.
13. Buchbinder EI and Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39(1):98-106.
14. Robert C, Ribas A, Hamid O, et al. 3-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J Clin Oncol. 2016(suppl;abstr 9503).
15. Hodi SF, Kluger HM, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Presented at the 2016 AACR Annual Meeting; April 16-20; New Orleans, LA. Abstract CT001.
16. Bakdash G, Sittig SP, van Dijk T, et al. The nature of activatory and tolerogenic dendritic cell-derived signal II. Front Immunol. 2013;4(53):1-18.
17. Sheridan C. Immuno-oncology moves beyond PD-1. Nat Biotechnol. 2015;33(7):673-675.
18. Blake SJ, Dougall WC, Miles JJ, et al. Molecular pathways: targeting CD96 and TIGIT for cancer immunotherapy. Clin Cancer Res. 2016;22(21):5183-5188.
19. Carotta S. Targeting NK cells for anticancer immunotherapy: clinical and preclinical approaches. Front Immunol. 2016;7:152.
20. Innate Pharma Web site. Innate Pharma Announces Top-Line Results from EFFIKIR Trial Evaluating the Efficacy of Lirilumab as a Single Agent in Elderly Patients with Acute Myeloid Leukemia. http://www.innate-pharma.com/en/news-events/press-releases/innate-pharma-announces-top-line-results-effikir-trial-evaluating-efficacy-lirilumab-single-agent-elderly-patients-acute-myeloid-leukemia. Last updated February 6, 2017. Accessed online February 22, 2017.
21. Sheridan C. IDO inhibitors move center stage in immuno-oncology. Nat Biotechnol. 2015;33(4):321-322.
22. Sanmamed MF, Pastor F, Rodriguez A, et al. Agonists of co-stimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol. 2015;42(4):640-655.
23. Linch SN, McNamara MJ, Redmond WL. OX40 agonists and combination immunotherapy: putting the pedal to the metal. Front Oncol. 2015;5:34.
24. U.S. Food and Drug Administration Web site. Nivolumab in combination with ipilimumab. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm465274.htm. Last updated October 1, 2015. Accessed online February 22, 2017.
25. Massarelli E. Clinical safety and efficacy assessment of the CD137 agonist urelumab alone and in combination with nivolumab in patients with hematologic and solid tumor malignancies. Presented at the 31st Annual Meeting of the Society for the Immunotherapy of Cancer; November 9-13, 2016; National Harbor, MD. Abstract 239.
26. Sanborn RE, Pishvain MJ, Callahan MK, et al. Phase I results from the combination of an immune-activating anti-CD27 antibody (varlilumab) in combination with PD-1 blockade (nivolumab): activation across multiple immune pathways without untoward immune-related adverse events. Clin Cancer Res. 2016;76(14):suppl. Abstract CT023.
27. Gangadhar T, Hamid O, Smith D.C, et al. Epacadostat plus pembrolizumab in patients with advanced melanoma and select solid tumors: updated phase 1 results from ECHO-202/KEYNOTE-037. Ann Oncol. 2016;27(6):379-400.
The relationship between the immune system and tumors is complex and dynamic, and for immunotherapy to reach its full potential it will likely need to attack on multiple fronts. Here, we discuss some of the latest and most promising developments in the immuno-oncology field designed to build on the successes and address limitations.
The anti-tumor immune response
Cancer is a disease of genomic instability, whereby genetic alterations ranging from a single nucleotide to the whole chromosome level frequently occur. Although cancers derive from a patient’s own tissues, these genetic differences can mark the cancer cell as non-self, triggering an immune response to eliminate these cells.
The first hints of this anti-tumor immunity date back more than a century and a half and sparked the concept of mobilizing the immune system to treat patients.1-3 Although early pioneers achieved little progress in this regard, their efforts provided invaluable insights into the complex and dynamic relationship between a tumor and the immune system that are now translating into real clinical successes.
We now understand that the immune system has a dual role in both restraining and promoting cancer development and have translated this understanding into the theory of cancer immunoediting. Immunoediting has three stages: elimination, wherein the tumor is seemingly destroyed by the innate and adaptive immune response; equilibrium, in which cancer cells that were able to escape elimination are selected for growth; and escape, whereby these resistant cancer cells overwhelm the immune system and develop into a symptomatic lesion.4,5
Immuno-oncologists have also described the cancer immunity cycle to capture the steps that are required for an effective anti-tumor immune response and defects in this cycle form the basis of the most common mechanisms used by cancer cells to subvert the anti-tumor immune response. Much like the cancer hallmarks did for molecularly targeted cancer drugs, the cancer immunity cycle serves as the intellectual framework for cancer immunotherapy.6,7
Exploiting nature’s weapon of mass destruction
Initially, attempts at immunotherapy focused on boosting the immune response using adjuvants and cytokines. The characterization of subtle differences between tumor cells and normal cells led to the development of vaccines and cell-based therapies that exploited these tumor-associated antigens (TAAs).1-6
Despite the approval of a therapeutic vaccine, sipuleucel-T, in 2010 for the treatment of metastatic prostate cancer, in general the success of vaccines has been limited. Marketing authorization for sipuleucel-T was recently withdrawn in Europe, and although it is still available in the United States, it is not widely used because of issues with production and administration. Other vaccines, such as GVAX, which looked particularly promising in early-stage clinical trials, failed to show clinical efficacy in subsequent testing.8,9
Cell-based therapies, such as adoptive cellular therapy (ACT), in which immune cells are removed from the host, primed to attack cancer cells, and then reinfused back into the patient, have focused on T cells because they are the major effectors of the adaptive immune response. Clinical success with the most common approach, tumor-infiltrating lymphocyte (TIL)
Two key techniques have been developed (Figure 1). T-cell receptor (TCR) therapy involves genetically modifying the receptor on the surface of T cells that is responsible for recognizing antigens bound to major histocompatibility complex (MHC) molecules on the surface of antigen-presenting cells (APCs). The TCR can be altered to recognize a specific TAA or modified to improve its antigen recognition and binding capabilities. This type of therapy is limited by the fact that the TCRs need to be genetically matched to the patient’s immune type.
Releasing the brakes
To ensure that it is only activated at the appropriate time and not in response to the antigens expressed on the surface of the host’s own tissues or harmless materials, the immune system has developed numerous mechanisms for immunological tolerance. Cancer cells are able to exploit these mechanisms to allow them to evade the anti-tumor immune response. One of the main ways in which they do this is by manipulating the signaling pathways involved in T-cell activation, which play a vital role in tolerance.12
To become fully activated, T cells require a primary signal generated by an interaction between the TCR and the antigen-MHC complex on the surface of an APC, followed by secondary costimulatory signals generated by a range of different receptors present on the T-cell surface binding to their ligands on the APC.
If the second signal is inhibitory rather than stimulatory, then the T cell is deactivated instead of becoming activated. Two key coinhibitory receptors are programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) and tumor cells are able to overcome the anti-tumor immune response in part by expressing the ligands that bind these receptors to dampen the activity of tumor-infiltrating T cells and induce tolerance.13
The development of inhibitors of CTLA-4 and PD-1 and their respective ligands has driven some of the most dramatic successes with cancer immunotherapy, particularly with PD-1-targeting drugs which have fewer side effects. Targeting of this pathway has resulted in durable responses, revolutionizing the treatment of metastatic melanoma, with recently published long-term survival data for pembrolizumab showing that 40% of patients were alive 3 years after initiating treatment and, in a separate study, 34% of nivolumab-treated patients were still alive after 5 years.14,15 More recently, PD-1 inhibitors have been slowly expanding into a range of other cancer types and 4 immune checkpoint inhibitors are now approved by the United States Food and Drug Administration (FDA): ipilimumab (Yervoy), nivolumab (Opdivo), pembrolizumab (Keytruda) and atezolizumab (Tecentriq).
Six years on from the first approval in this drug class and an extensive network of coinhibitory receptors has been uncovered – so-called immune checkpoints – many of which are now also serving as therapeutic targets (Table, Figure 2).16 Lymphocyte activation gene 3 (LAG-3) is a member of the immunoglobulin superfamily of receptors that is expressed on a number of different types of immune cell. In addition to negatively regulating cytotoxic T-cell activation like PD-1 and CTLA-4, it is also thought to regulate the immunosuppressive functions of regulatory T cells and the maturation and activation of dendritic cells. T-cell immunoglobulin and mucin domain-containing 3 (TIM-3) is found on the surface of helper and cytotoxic T cells and regulates T-cell inhibition as well as macrophage activation. Inhibitors of both proteins have been developed that are being evaluated in phase 1 or 2 clinical trials in a variety of tumor types.17
Indeed, although T cells have commanded the most attention, there is growing appreciation of the potential for targeting other types of immune cell that play a role in the anti-tumor immune response or in fostering an immunosuppressive microenvironment. NK cells have been a particular focus, since they represent the body’s first line of immune defense and they appear to have analogous inhibitory and activating receptors expressed on their surface that regulate their cytotoxic activity.
The best-defined NK cell receptors are the killer cell immunoglobulin-like receptors (KIRs) that bind to the MHC class I proteins found on the surface of all cells that distinguish them as ‘self’ or ‘non-self’. KIRs can be either activating or inhibitory, depending upon their structure and the ligands to which they bind.19 To date, 2 antibodies targeting inhibitory KIRs have been developed. Though there has been some disappointment with these drugs, most recently a phase 2 trial of lirilumab in elderly patients with acute myeloid leukemia, which missed its primary endpoint, they continue to be evaluated in clinical trials.20
The inhibitory immune checkpoint field has also expanded to include molecules that regulate T-cell activity in other ways. Most prominently, this includes enzymes like indoleamine-2,3 dioxygenase (IDO), which is involved in the metabolism of the essential amino acid tryptophan. IDO-induced depletion of tryptophan and generation of tryptophan metabolites is toxic to cytotoxic T cells, and IDO is also thought to directly activate regulatory T cells, thus the net effect of IDO is immunosuppression. Two IDO inhibitors are currently being developed.21
Stepping on the gas
Despite their unprecedented success, immune checkpoint inhibitors are not effective in all patients or in all tumor types. Their efficacy is limited in large part by the requirement for a pre-existing anti-tumor immune response. If there are no T cells within the tumor microenvironment then releasing the brakes on the immune system won’t help.
More recently, researchers have returned to the idea of stimulating an anti-tumor immune response, this time by targeting the other side of the immune checkpoint coin, the costimulatory molecules. These drugs could prove more effective as they aren’t reliant on a pre-existing anti-tumor immune response. A number of agonist antibodies designed to target these receptors have now been developed and are undergoing clinical evaluation.22
Furthest along in development are those targeting OX40, a costimulatory molecule that is upregulated on the surface of T cells once they have been fully activated by the TCR signal and an initial costimulatory signal. OX40 is thought to be involved in a more long-term immune response and in the formation of a memory response. A mouse monoclonal antibody had a potent immune-stimulating effect accompanied by the regression of at least 1 metastatic lesion in 30% of patients treated in a phase 1 clinical trial, but was limited by the generation of anti-mouse antibodies. 7 OX40 agonists are now in clinical development, 6 fully human monoclonal antibodies and 1 OX40 ligand-Fc fusion protein, MEDI-6383.23
Combinations are key
Many researchers are now reaching the conclusion that combination therapy is likely to be key in expanding the scope of immunotherapy into currently unresponsive patient populations. Investigating rational combinations is already becoming a burgeoning area of the immuno-oncology field, with a variety of different strategies being tested.
Now the question becomes what are the optimal combinations and the timing and sequencing of combination therapy is likely to be a paramount consideration. Developing combinations that have distinct mechanisms of action or target multiple steps in the cancer immunity cycle offers the greatest potential for therapeutic synergy since this is most likely to address potential mechanisms of resistance by blocking other paths to immune evasion for cancer cells (Figure 3).
Given the expanding network of immune-checkpoint inhibitors and agonists, the focal point of combination therapy has been combining immune checkpoint-targeting drugs with different mechanisms of action, including those that would simultaneously release the brakes and step on the gas pedal. The vast majority of ongoing clinical trials of approved checkpoint inhibitors and the drugs in development listed in the table are combination trials.
These efforts yielded the first FDA-approved combination immunotherapy regimen in 2015; nivolumab and ipilimumab for the treatment of metastatic melanoma. Approval was based on the demonstration of improved ORR, prolonged response duration, and improved progression-free survival among 142 patients treated with the combination, compared to either drug alone.24
The results of a phase 1/2 trial evaluating the combination of a 4-1BB receptor agonist urelumab with nivolumab in hematologic malignancies and solid tumors found the combination to be safe and particularly effective in patients with advanced/metastatic melanoma, with an ORR of 50%.25 Nivolumab was also combined with the CD27 agonist varlilumab in a phase 1/2 clinical trial of patients with solid tumors, for which data was also recently released. Among 46 patients enrolled, primarily those with colorectal and ovarian cancer the combination had an acceptable safety profile and favorable changes in intratumoral immune biomarkers were observed. The phase 2 portion of the trial is ongoing.26
Meanwhile, Incyte’s IDO inhibitor epacadostat has recently been making waves in combination with pembrolizumab in patients with advanced solid tumors. It demonstrated particularly promising clinical activity in patients with metastatic melanoma, with an overall response rate (ORR) of 57%, including 2 complete responses (CRs), prompting initiation of a phase 3 trial of this combination (NCT02752074).27
The relationship between the immune system and tumors is complex and dynamic, and for immunotherapy to reach its full potential it will likely need to attack on multiple fronts. Here, we discuss some of the latest and most promising developments in the immuno-oncology field designed to build on the successes and address limitations.
The anti-tumor immune response
Cancer is a disease of genomic instability, whereby genetic alterations ranging from a single nucleotide to the whole chromosome level frequently occur. Although cancers derive from a patient’s own tissues, these genetic differences can mark the cancer cell as non-self, triggering an immune response to eliminate these cells.
The first hints of this anti-tumor immunity date back more than a century and a half and sparked the concept of mobilizing the immune system to treat patients.1-3 Although early pioneers achieved little progress in this regard, their efforts provided invaluable insights into the complex and dynamic relationship between a tumor and the immune system that are now translating into real clinical successes.
We now understand that the immune system has a dual role in both restraining and promoting cancer development and have translated this understanding into the theory of cancer immunoediting. Immunoediting has three stages: elimination, wherein the tumor is seemingly destroyed by the innate and adaptive immune response; equilibrium, in which cancer cells that were able to escape elimination are selected for growth; and escape, whereby these resistant cancer cells overwhelm the immune system and develop into a symptomatic lesion.4,5
Immuno-oncologists have also described the cancer immunity cycle to capture the steps that are required for an effective anti-tumor immune response and defects in this cycle form the basis of the most common mechanisms used by cancer cells to subvert the anti-tumor immune response. Much like the cancer hallmarks did for molecularly targeted cancer drugs, the cancer immunity cycle serves as the intellectual framework for cancer immunotherapy.6,7
Exploiting nature’s weapon of mass destruction
Initially, attempts at immunotherapy focused on boosting the immune response using adjuvants and cytokines. The characterization of subtle differences between tumor cells and normal cells led to the development of vaccines and cell-based therapies that exploited these tumor-associated antigens (TAAs).1-6
Despite the approval of a therapeutic vaccine, sipuleucel-T, in 2010 for the treatment of metastatic prostate cancer, in general the success of vaccines has been limited. Marketing authorization for sipuleucel-T was recently withdrawn in Europe, and although it is still available in the United States, it is not widely used because of issues with production and administration. Other vaccines, such as GVAX, which looked particularly promising in early-stage clinical trials, failed to show clinical efficacy in subsequent testing.8,9
Cell-based therapies, such as adoptive cellular therapy (ACT), in which immune cells are removed from the host, primed to attack cancer cells, and then reinfused back into the patient, have focused on T cells because they are the major effectors of the adaptive immune response. Clinical success with the most common approach, tumor-infiltrating lymphocyte (TIL)
Two key techniques have been developed (Figure 1). T-cell receptor (TCR) therapy involves genetically modifying the receptor on the surface of T cells that is responsible for recognizing antigens bound to major histocompatibility complex (MHC) molecules on the surface of antigen-presenting cells (APCs). The TCR can be altered to recognize a specific TAA or modified to improve its antigen recognition and binding capabilities. This type of therapy is limited by the fact that the TCRs need to be genetically matched to the patient’s immune type.
Releasing the brakes
To ensure that it is only activated at the appropriate time and not in response to the antigens expressed on the surface of the host’s own tissues or harmless materials, the immune system has developed numerous mechanisms for immunological tolerance. Cancer cells are able to exploit these mechanisms to allow them to evade the anti-tumor immune response. One of the main ways in which they do this is by manipulating the signaling pathways involved in T-cell activation, which play a vital role in tolerance.12
To become fully activated, T cells require a primary signal generated by an interaction between the TCR and the antigen-MHC complex on the surface of an APC, followed by secondary costimulatory signals generated by a range of different receptors present on the T-cell surface binding to their ligands on the APC.
If the second signal is inhibitory rather than stimulatory, then the T cell is deactivated instead of becoming activated. Two key coinhibitory receptors are programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) and tumor cells are able to overcome the anti-tumor immune response in part by expressing the ligands that bind these receptors to dampen the activity of tumor-infiltrating T cells and induce tolerance.13
The development of inhibitors of CTLA-4 and PD-1 and their respective ligands has driven some of the most dramatic successes with cancer immunotherapy, particularly with PD-1-targeting drugs which have fewer side effects. Targeting of this pathway has resulted in durable responses, revolutionizing the treatment of metastatic melanoma, with recently published long-term survival data for pembrolizumab showing that 40% of patients were alive 3 years after initiating treatment and, in a separate study, 34% of nivolumab-treated patients were still alive after 5 years.14,15 More recently, PD-1 inhibitors have been slowly expanding into a range of other cancer types and 4 immune checkpoint inhibitors are now approved by the United States Food and Drug Administration (FDA): ipilimumab (Yervoy), nivolumab (Opdivo), pembrolizumab (Keytruda) and atezolizumab (Tecentriq).
Six years on from the first approval in this drug class and an extensive network of coinhibitory receptors has been uncovered – so-called immune checkpoints – many of which are now also serving as therapeutic targets (Table, Figure 2).16 Lymphocyte activation gene 3 (LAG-3) is a member of the immunoglobulin superfamily of receptors that is expressed on a number of different types of immune cell. In addition to negatively regulating cytotoxic T-cell activation like PD-1 and CTLA-4, it is also thought to regulate the immunosuppressive functions of regulatory T cells and the maturation and activation of dendritic cells. T-cell immunoglobulin and mucin domain-containing 3 (TIM-3) is found on the surface of helper and cytotoxic T cells and regulates T-cell inhibition as well as macrophage activation. Inhibitors of both proteins have been developed that are being evaluated in phase 1 or 2 clinical trials in a variety of tumor types.17
Indeed, although T cells have commanded the most attention, there is growing appreciation of the potential for targeting other types of immune cell that play a role in the anti-tumor immune response or in fostering an immunosuppressive microenvironment. NK cells have been a particular focus, since they represent the body’s first line of immune defense and they appear to have analogous inhibitory and activating receptors expressed on their surface that regulate their cytotoxic activity.
The best-defined NK cell receptors are the killer cell immunoglobulin-like receptors (KIRs) that bind to the MHC class I proteins found on the surface of all cells that distinguish them as ‘self’ or ‘non-self’. KIRs can be either activating or inhibitory, depending upon their structure and the ligands to which they bind.19 To date, 2 antibodies targeting inhibitory KIRs have been developed. Though there has been some disappointment with these drugs, most recently a phase 2 trial of lirilumab in elderly patients with acute myeloid leukemia, which missed its primary endpoint, they continue to be evaluated in clinical trials.20
The inhibitory immune checkpoint field has also expanded to include molecules that regulate T-cell activity in other ways. Most prominently, this includes enzymes like indoleamine-2,3 dioxygenase (IDO), which is involved in the metabolism of the essential amino acid tryptophan. IDO-induced depletion of tryptophan and generation of tryptophan metabolites is toxic to cytotoxic T cells, and IDO is also thought to directly activate regulatory T cells, thus the net effect of IDO is immunosuppression. Two IDO inhibitors are currently being developed.21
Stepping on the gas
Despite their unprecedented success, immune checkpoint inhibitors are not effective in all patients or in all tumor types. Their efficacy is limited in large part by the requirement for a pre-existing anti-tumor immune response. If there are no T cells within the tumor microenvironment then releasing the brakes on the immune system won’t help.
More recently, researchers have returned to the idea of stimulating an anti-tumor immune response, this time by targeting the other side of the immune checkpoint coin, the costimulatory molecules. These drugs could prove more effective as they aren’t reliant on a pre-existing anti-tumor immune response. A number of agonist antibodies designed to target these receptors have now been developed and are undergoing clinical evaluation.22
Furthest along in development are those targeting OX40, a costimulatory molecule that is upregulated on the surface of T cells once they have been fully activated by the TCR signal and an initial costimulatory signal. OX40 is thought to be involved in a more long-term immune response and in the formation of a memory response. A mouse monoclonal antibody had a potent immune-stimulating effect accompanied by the regression of at least 1 metastatic lesion in 30% of patients treated in a phase 1 clinical trial, but was limited by the generation of anti-mouse antibodies. 7 OX40 agonists are now in clinical development, 6 fully human monoclonal antibodies and 1 OX40 ligand-Fc fusion protein, MEDI-6383.23
Combinations are key
Many researchers are now reaching the conclusion that combination therapy is likely to be key in expanding the scope of immunotherapy into currently unresponsive patient populations. Investigating rational combinations is already becoming a burgeoning area of the immuno-oncology field, with a variety of different strategies being tested.
Now the question becomes what are the optimal combinations and the timing and sequencing of combination therapy is likely to be a paramount consideration. Developing combinations that have distinct mechanisms of action or target multiple steps in the cancer immunity cycle offers the greatest potential for therapeutic synergy since this is most likely to address potential mechanisms of resistance by blocking other paths to immune evasion for cancer cells (Figure 3).
Given the expanding network of immune-checkpoint inhibitors and agonists, the focal point of combination therapy has been combining immune checkpoint-targeting drugs with different mechanisms of action, including those that would simultaneously release the brakes and step on the gas pedal. The vast majority of ongoing clinical trials of approved checkpoint inhibitors and the drugs in development listed in the table are combination trials.
These efforts yielded the first FDA-approved combination immunotherapy regimen in 2015; nivolumab and ipilimumab for the treatment of metastatic melanoma. Approval was based on the demonstration of improved ORR, prolonged response duration, and improved progression-free survival among 142 patients treated with the combination, compared to either drug alone.24
The results of a phase 1/2 trial evaluating the combination of a 4-1BB receptor agonist urelumab with nivolumab in hematologic malignancies and solid tumors found the combination to be safe and particularly effective in patients with advanced/metastatic melanoma, with an ORR of 50%.25 Nivolumab was also combined with the CD27 agonist varlilumab in a phase 1/2 clinical trial of patients with solid tumors, for which data was also recently released. Among 46 patients enrolled, primarily those with colorectal and ovarian cancer the combination had an acceptable safety profile and favorable changes in intratumoral immune biomarkers were observed. The phase 2 portion of the trial is ongoing.26
Meanwhile, Incyte’s IDO inhibitor epacadostat has recently been making waves in combination with pembrolizumab in patients with advanced solid tumors. It demonstrated particularly promising clinical activity in patients with metastatic melanoma, with an overall response rate (ORR) of 57%, including 2 complete responses (CRs), prompting initiation of a phase 3 trial of this combination (NCT02752074).27
1. Adams JL, Smothers J, Srinivasan R, et al. Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Disc. 2015;14:603-622.
2. D’Errico G, Machado HL, Sainz Jr B. A current perspective on cancer immune therapy: step-by-step approach to constructing the magic bullet. Clin Trans Med. 2017;6:3.
3. Farkona S, Diamandis EP, Blaustig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73.
4. Meiliana A, Dewi NM, Wijaya A. Cancer immunotherapy: a review. Indones Biomed J. 2016;8(1):1-20.
5. Smyth MJ, Ngiow SF, Ribas A, et al. Combination cancer immunotherapies tailored to the tumor microenvironment. Nat Rev Clin Oncol. 2016;13:143-158.
6. de Charette M, Marabelle A, Houot R. Turning tumor cells into antigen presenting cells: The next step to improve cancer immunotherapy? Eur J Cancer 2016;68:134-147.
7. Chen DS and Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013;39:1-10.
8. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480-489.
9. Le DT, Wang-Gillam A, Picozzi V Jr, et al. A phase 2, randomized trial of GVAX Pancreas and CRS-207 immunotherapy versus GVAX alone in patients with metastatic pancreatic adenocarcinoma: Updated results. Presented at: the ASCO Gastrointestinal Cancers Symposium; January 16-18, 2014; San Francisco, CA. Abstract 177.
10. Sharpe M and Mount N. Genetically modified T cells in cancer therapy: opportunities and challenges. Dis Model Mech. 2015;8(4):337-350.
11. Perica K, Varela JC, Oelke M, et al. Adoptive T Cell Immunotherapy for Cancer. Ram Mai Med J. 2015;6(1):e0004.
12. Xing Y and Hogquist KA. T-Cell Tolerance: Central and Peripheral. Cold Spring Harb Perspect Biol. 2012;4:a006957.
13. Buchbinder EI and Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39(1):98-106.
14. Robert C, Ribas A, Hamid O, et al. 3-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J Clin Oncol. 2016(suppl;abstr 9503).
15. Hodi SF, Kluger HM, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Presented at the 2016 AACR Annual Meeting; April 16-20; New Orleans, LA. Abstract CT001.
16. Bakdash G, Sittig SP, van Dijk T, et al. The nature of activatory and tolerogenic dendritic cell-derived signal II. Front Immunol. 2013;4(53):1-18.
17. Sheridan C. Immuno-oncology moves beyond PD-1. Nat Biotechnol. 2015;33(7):673-675.
18. Blake SJ, Dougall WC, Miles JJ, et al. Molecular pathways: targeting CD96 and TIGIT for cancer immunotherapy. Clin Cancer Res. 2016;22(21):5183-5188.
19. Carotta S. Targeting NK cells for anticancer immunotherapy: clinical and preclinical approaches. Front Immunol. 2016;7:152.
20. Innate Pharma Web site. Innate Pharma Announces Top-Line Results from EFFIKIR Trial Evaluating the Efficacy of Lirilumab as a Single Agent in Elderly Patients with Acute Myeloid Leukemia. http://www.innate-pharma.com/en/news-events/press-releases/innate-pharma-announces-top-line-results-effikir-trial-evaluating-efficacy-lirilumab-single-agent-elderly-patients-acute-myeloid-leukemia. Last updated February 6, 2017. Accessed online February 22, 2017.
21. Sheridan C. IDO inhibitors move center stage in immuno-oncology. Nat Biotechnol. 2015;33(4):321-322.
22. Sanmamed MF, Pastor F, Rodriguez A, et al. Agonists of co-stimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol. 2015;42(4):640-655.
23. Linch SN, McNamara MJ, Redmond WL. OX40 agonists and combination immunotherapy: putting the pedal to the metal. Front Oncol. 2015;5:34.
24. U.S. Food and Drug Administration Web site. Nivolumab in combination with ipilimumab. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm465274.htm. Last updated October 1, 2015. Accessed online February 22, 2017.
25. Massarelli E. Clinical safety and efficacy assessment of the CD137 agonist urelumab alone and in combination with nivolumab in patients with hematologic and solid tumor malignancies. Presented at the 31st Annual Meeting of the Society for the Immunotherapy of Cancer; November 9-13, 2016; National Harbor, MD. Abstract 239.
26. Sanborn RE, Pishvain MJ, Callahan MK, et al. Phase I results from the combination of an immune-activating anti-CD27 antibody (varlilumab) in combination with PD-1 blockade (nivolumab): activation across multiple immune pathways without untoward immune-related adverse events. Clin Cancer Res. 2016;76(14):suppl. Abstract CT023.
27. Gangadhar T, Hamid O, Smith D.C, et al. Epacadostat plus pembrolizumab in patients with advanced melanoma and select solid tumors: updated phase 1 results from ECHO-202/KEYNOTE-037. Ann Oncol. 2016;27(6):379-400.
1. Adams JL, Smothers J, Srinivasan R, et al. Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Disc. 2015;14:603-622.
2. D’Errico G, Machado HL, Sainz Jr B. A current perspective on cancer immune therapy: step-by-step approach to constructing the magic bullet. Clin Trans Med. 2017;6:3.
3. Farkona S, Diamandis EP, Blaustig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73.
4. Meiliana A, Dewi NM, Wijaya A. Cancer immunotherapy: a review. Indones Biomed J. 2016;8(1):1-20.
5. Smyth MJ, Ngiow SF, Ribas A, et al. Combination cancer immunotherapies tailored to the tumor microenvironment. Nat Rev Clin Oncol. 2016;13:143-158.
6. de Charette M, Marabelle A, Houot R. Turning tumor cells into antigen presenting cells: The next step to improve cancer immunotherapy? Eur J Cancer 2016;68:134-147.
7. Chen DS and Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013;39:1-10.
8. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480-489.
9. Le DT, Wang-Gillam A, Picozzi V Jr, et al. A phase 2, randomized trial of GVAX Pancreas and CRS-207 immunotherapy versus GVAX alone in patients with metastatic pancreatic adenocarcinoma: Updated results. Presented at: the ASCO Gastrointestinal Cancers Symposium; January 16-18, 2014; San Francisco, CA. Abstract 177.
10. Sharpe M and Mount N. Genetically modified T cells in cancer therapy: opportunities and challenges. Dis Model Mech. 2015;8(4):337-350.
11. Perica K, Varela JC, Oelke M, et al. Adoptive T Cell Immunotherapy for Cancer. Ram Mai Med J. 2015;6(1):e0004.
12. Xing Y and Hogquist KA. T-Cell Tolerance: Central and Peripheral. Cold Spring Harb Perspect Biol. 2012;4:a006957.
13. Buchbinder EI and Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39(1):98-106.
14. Robert C, Ribas A, Hamid O, et al. 3-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J Clin Oncol. 2016(suppl;abstr 9503).
15. Hodi SF, Kluger HM, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Presented at the 2016 AACR Annual Meeting; April 16-20; New Orleans, LA. Abstract CT001.
16. Bakdash G, Sittig SP, van Dijk T, et al. The nature of activatory and tolerogenic dendritic cell-derived signal II. Front Immunol. 2013;4(53):1-18.
17. Sheridan C. Immuno-oncology moves beyond PD-1. Nat Biotechnol. 2015;33(7):673-675.
18. Blake SJ, Dougall WC, Miles JJ, et al. Molecular pathways: targeting CD96 and TIGIT for cancer immunotherapy. Clin Cancer Res. 2016;22(21):5183-5188.
19. Carotta S. Targeting NK cells for anticancer immunotherapy: clinical and preclinical approaches. Front Immunol. 2016;7:152.
20. Innate Pharma Web site. Innate Pharma Announces Top-Line Results from EFFIKIR Trial Evaluating the Efficacy of Lirilumab as a Single Agent in Elderly Patients with Acute Myeloid Leukemia. http://www.innate-pharma.com/en/news-events/press-releases/innate-pharma-announces-top-line-results-effikir-trial-evaluating-efficacy-lirilumab-single-agent-elderly-patients-acute-myeloid-leukemia. Last updated February 6, 2017. Accessed online February 22, 2017.
21. Sheridan C. IDO inhibitors move center stage in immuno-oncology. Nat Biotechnol. 2015;33(4):321-322.
22. Sanmamed MF, Pastor F, Rodriguez A, et al. Agonists of co-stimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol. 2015;42(4):640-655.
23. Linch SN, McNamara MJ, Redmond WL. OX40 agonists and combination immunotherapy: putting the pedal to the metal. Front Oncol. 2015;5:34.
24. U.S. Food and Drug Administration Web site. Nivolumab in combination with ipilimumab. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm465274.htm. Last updated October 1, 2015. Accessed online February 22, 2017.
25. Massarelli E. Clinical safety and efficacy assessment of the CD137 agonist urelumab alone and in combination with nivolumab in patients with hematologic and solid tumor malignancies. Presented at the 31st Annual Meeting of the Society for the Immunotherapy of Cancer; November 9-13, 2016; National Harbor, MD. Abstract 239.
26. Sanborn RE, Pishvain MJ, Callahan MK, et al. Phase I results from the combination of an immune-activating anti-CD27 antibody (varlilumab) in combination with PD-1 blockade (nivolumab): activation across multiple immune pathways without untoward immune-related adverse events. Clin Cancer Res. 2016;76(14):suppl. Abstract CT023.
27. Gangadhar T, Hamid O, Smith D.C, et al. Epacadostat plus pembrolizumab in patients with advanced melanoma and select solid tumors: updated phase 1 results from ECHO-202/KEYNOTE-037. Ann Oncol. 2016;27(6):379-400.
Plasma GAG provides prognostic info for RCC
Plasma glycosaminoglycan (GAG) measurements can accurately distinguish metastatic clear-cell renal cell carcinoma (ccRCC) from healthy samples, and can provide accurate diagnostic and prognostic information that may be of value in managing the disease, according to new findings.
A new GAG score had 93.5% sensitivity and 94.7% specificity (discovery set) for differentiating RCC from healthy samples, and the sensitivity estimate was independently validated. The score remained independent and uncorrelated to tumor stage, grade, size, and histology, or confounders such as age or gender, according to investigators. The report is in European Oncology Urology.
The authors note that in both retrospective and prospective studies of metastatic ccRCC cases, the composition and levels of plasma and urine GAGs are significantly different when compared with healthy specimens, and GAG scores have correlated with patient outcomes including progression free and overall survival in some cohorts. But it remains unclear if the alterations in plasma and urine GAGs are limited to just metastatic cases of ccRCC or if they correlate with other histopathologic characteristics in RCC. It is also unclear if the correlation between GAG scores and prognosis is limited to patients who receive systemic therapy or if it is applicable to those who are surgically treated RCC as well.
“These results expand our knowledge on the diagnostic and prognostic potential of plasma GAGs in RCC, which was so far limited to metastatic ccRCC in our previous studies,” wrote Francesco Gatto, MD, of Chalmers University of Technology, Göteborg, Sweden, and his colleagues. “Plasma GAG alterations appear to originate as a response to the tumor and occur early if not concomitantly with tumor formation, and probably independent of its progression.”
To investigate the sensitivity and specificity of plasma GAGs for detection of early-stage RCC as well as its utility in predicting recurrence and death after RCC surgery, Dr. Gatto and his team conducted a retrospective case-control study that included 175 RCC patients who underwent surgery between May 2011 and February 2014 and 19 healthy controls.
Plasma GAGs were measured in both preoperative and postoperative RCC cases and the control group, and a discovery set was analyzed to update the historical GAG score. The sensitivity of the new GAG score that was developed for detecting RCC versus controls was then validated using the remaining samples.
In the first discovery set, which included 67 participants, the new GAG score distinguished RCC from controls with an area under the receiver operating characteristic curve (AUC) of 0.999. In their validation cohort (n = 108), the new GAG score achieved an AUC of 0.991 (95% CI 0.977-1) and at the prespecified cutoff, the validated sensitivity was 93.5%. Specificity could not be validated because the same control group was used in both sets.
Factors including tumor size, grade, and stage, radical nephrectomy, and positive surgical margins were significantly associated with overall survival as were three of five GAG properties in the new scoring system, although the new GAG score did not reach significance by itself (hazard ratio, 1.25; P = 0.08). When looking at whether the new GAG score changed after surgery, the authors found that it was quite variable across patients, and an increased score was observed for 53% of cases and a decrease for 47% after surgery. This change did not appear correlated with outcomes as shown by the recurrence rate within 2 years of surgery.
SOURCE: Gatto F et al. Eur Urol Oncol. 2018 Jun 13. doi: 10.1016/j.euo.2018.04.015.
Plasma glycosaminoglycan (GAG) measurements can accurately distinguish metastatic clear-cell renal cell carcinoma (ccRCC) from healthy samples, and can provide accurate diagnostic and prognostic information that may be of value in managing the disease, according to new findings.
A new GAG score had 93.5% sensitivity and 94.7% specificity (discovery set) for differentiating RCC from healthy samples, and the sensitivity estimate was independently validated. The score remained independent and uncorrelated to tumor stage, grade, size, and histology, or confounders such as age or gender, according to investigators. The report is in European Oncology Urology.
The authors note that in both retrospective and prospective studies of metastatic ccRCC cases, the composition and levels of plasma and urine GAGs are significantly different when compared with healthy specimens, and GAG scores have correlated with patient outcomes including progression free and overall survival in some cohorts. But it remains unclear if the alterations in plasma and urine GAGs are limited to just metastatic cases of ccRCC or if they correlate with other histopathologic characteristics in RCC. It is also unclear if the correlation between GAG scores and prognosis is limited to patients who receive systemic therapy or if it is applicable to those who are surgically treated RCC as well.
“These results expand our knowledge on the diagnostic and prognostic potential of plasma GAGs in RCC, which was so far limited to metastatic ccRCC in our previous studies,” wrote Francesco Gatto, MD, of Chalmers University of Technology, Göteborg, Sweden, and his colleagues. “Plasma GAG alterations appear to originate as a response to the tumor and occur early if not concomitantly with tumor formation, and probably independent of its progression.”
To investigate the sensitivity and specificity of plasma GAGs for detection of early-stage RCC as well as its utility in predicting recurrence and death after RCC surgery, Dr. Gatto and his team conducted a retrospective case-control study that included 175 RCC patients who underwent surgery between May 2011 and February 2014 and 19 healthy controls.
Plasma GAGs were measured in both preoperative and postoperative RCC cases and the control group, and a discovery set was analyzed to update the historical GAG score. The sensitivity of the new GAG score that was developed for detecting RCC versus controls was then validated using the remaining samples.
In the first discovery set, which included 67 participants, the new GAG score distinguished RCC from controls with an area under the receiver operating characteristic curve (AUC) of 0.999. In their validation cohort (n = 108), the new GAG score achieved an AUC of 0.991 (95% CI 0.977-1) and at the prespecified cutoff, the validated sensitivity was 93.5%. Specificity could not be validated because the same control group was used in both sets.
Factors including tumor size, grade, and stage, radical nephrectomy, and positive surgical margins were significantly associated with overall survival as were three of five GAG properties in the new scoring system, although the new GAG score did not reach significance by itself (hazard ratio, 1.25; P = 0.08). When looking at whether the new GAG score changed after surgery, the authors found that it was quite variable across patients, and an increased score was observed for 53% of cases and a decrease for 47% after surgery. This change did not appear correlated with outcomes as shown by the recurrence rate within 2 years of surgery.
SOURCE: Gatto F et al. Eur Urol Oncol. 2018 Jun 13. doi: 10.1016/j.euo.2018.04.015.
Plasma glycosaminoglycan (GAG) measurements can accurately distinguish metastatic clear-cell renal cell carcinoma (ccRCC) from healthy samples, and can provide accurate diagnostic and prognostic information that may be of value in managing the disease, according to new findings.
A new GAG score had 93.5% sensitivity and 94.7% specificity (discovery set) for differentiating RCC from healthy samples, and the sensitivity estimate was independently validated. The score remained independent and uncorrelated to tumor stage, grade, size, and histology, or confounders such as age or gender, according to investigators. The report is in European Oncology Urology.
The authors note that in both retrospective and prospective studies of metastatic ccRCC cases, the composition and levels of plasma and urine GAGs are significantly different when compared with healthy specimens, and GAG scores have correlated with patient outcomes including progression free and overall survival in some cohorts. But it remains unclear if the alterations in plasma and urine GAGs are limited to just metastatic cases of ccRCC or if they correlate with other histopathologic characteristics in RCC. It is also unclear if the correlation between GAG scores and prognosis is limited to patients who receive systemic therapy or if it is applicable to those who are surgically treated RCC as well.
“These results expand our knowledge on the diagnostic and prognostic potential of plasma GAGs in RCC, which was so far limited to metastatic ccRCC in our previous studies,” wrote Francesco Gatto, MD, of Chalmers University of Technology, Göteborg, Sweden, and his colleagues. “Plasma GAG alterations appear to originate as a response to the tumor and occur early if not concomitantly with tumor formation, and probably independent of its progression.”
To investigate the sensitivity and specificity of plasma GAGs for detection of early-stage RCC as well as its utility in predicting recurrence and death after RCC surgery, Dr. Gatto and his team conducted a retrospective case-control study that included 175 RCC patients who underwent surgery between May 2011 and February 2014 and 19 healthy controls.
Plasma GAGs were measured in both preoperative and postoperative RCC cases and the control group, and a discovery set was analyzed to update the historical GAG score. The sensitivity of the new GAG score that was developed for detecting RCC versus controls was then validated using the remaining samples.
In the first discovery set, which included 67 participants, the new GAG score distinguished RCC from controls with an area under the receiver operating characteristic curve (AUC) of 0.999. In their validation cohort (n = 108), the new GAG score achieved an AUC of 0.991 (95% CI 0.977-1) and at the prespecified cutoff, the validated sensitivity was 93.5%. Specificity could not be validated because the same control group was used in both sets.
Factors including tumor size, grade, and stage, radical nephrectomy, and positive surgical margins were significantly associated with overall survival as were three of five GAG properties in the new scoring system, although the new GAG score did not reach significance by itself (hazard ratio, 1.25; P = 0.08). When looking at whether the new GAG score changed after surgery, the authors found that it was quite variable across patients, and an increased score was observed for 53% of cases and a decrease for 47% after surgery. This change did not appear correlated with outcomes as shown by the recurrence rate within 2 years of surgery.
SOURCE: Gatto F et al. Eur Urol Oncol. 2018 Jun 13. doi: 10.1016/j.euo.2018.04.015.
FROM EUROPEAN ONCOLOGY UROLOGY
Key clinical point: GAG measurements can provide accurate diagnostic and prognostic information for surgically treated renal cell carcinoma.
Major finding: A new GAG score had 93.5% sensitivity and 94.7% specificity for differentiating RCC from controls.
Study details: Retrospective case-control study that included 175 RCC patients and 19 controls.
Disclosures: The study was funded by the Knut and Alice Wallenberg Foundation to Chalmers University of Technology and MSK Cancer Center Support Grant P30-CA008748 to the Memorial Sloan Kettering Cancer Center. Dr. Francesco Gatto and Dr. Jens Nielsen were listed as coinventors on patent applications related to the biomarkers described in this study and both are shareholders in Elypta AB, which owned the above-mentioned patent applications. There are no other disclosures.
Source: Gatto F et al. Eur Urol Oncol. 2018 Jun 13. doi: 10.1016/j.euo.2018.04.015.
CT features associated with (some) ccRCC subtypes
In patients with clear cell renal cell carcinoma (ccRCC), radiogenomic analysis can reveal associations between specific features on CT imaging and messenger RNA-based tumor subtyping, authors of a preliminary analysis contend.
Among 177 patients with ccRCC for whom CT data and molecular subtyping information were available, well-defined vs. poorly-defined tumor margins were significantly associated with messenger RNA (mRNA) subtype m1 tumors, and a combination of margin definition and other qualitative tumor features was significantly associated with m3 subtype, reported Lan Bowen, MD, and Li Xiaojing, MD, from Huizhou (China) Central People’s Hospital.
“We demonstrated m1-subtype is positively associated with well-defined margin while m3-subtype is positively associated with ill-defined margin, renal vein invasion, and collecting-system invasion. These findings should be further investigated and validated in other cohorts,” they wrote. The report was published in Academic Radiology.
Radiogenomic analysis is a method for identifying associations between imaging features and gene expression profiles to provide information that can be used for clinical decision making.
The investigators used the technique to retrospectively explore associations between ccRCC mRNA-based subtyping and CT features.
They identified data on a total of 177 patients with ccRCC from The Cancer Genome Atlas for whom complete CT imaging, including contrast-enhanced images, and mRNA-based subtyping were available.
CT features studied included calcifications, margin definition, renal vein invasion, collecting-system invasion, multicystic tumors, nodular tumor enhancement, and intratumoral vasculature.
In univariate logistic regression analysis, well-defined tumor margins (vs. poorly-defined margins) were significant associated with m1 subtype (odd ratio [OR] 2.104, P = .041).
Similarly, a combination of well-defined tumor margins (OR 2.104, P = .013), no collecting system invasion (OR 0.421, P = .028), and renal vein invasion (OR 2.164, P = .026) were significantly associated with the m3 subtype.
They were no significant associations between CT imaging features and either the m2 or m4 subtypes, the authors found.
The authors did not report study funding sources or potential conflicts of interest.
SOURCE: Bowen L, Xiaojing L. Acad Radiol. doi: 10.1016/j.acra.2018.05.002.
In patients with clear cell renal cell carcinoma (ccRCC), radiogenomic analysis can reveal associations between specific features on CT imaging and messenger RNA-based tumor subtyping, authors of a preliminary analysis contend.
Among 177 patients with ccRCC for whom CT data and molecular subtyping information were available, well-defined vs. poorly-defined tumor margins were significantly associated with messenger RNA (mRNA) subtype m1 tumors, and a combination of margin definition and other qualitative tumor features was significantly associated with m3 subtype, reported Lan Bowen, MD, and Li Xiaojing, MD, from Huizhou (China) Central People’s Hospital.
“We demonstrated m1-subtype is positively associated with well-defined margin while m3-subtype is positively associated with ill-defined margin, renal vein invasion, and collecting-system invasion. These findings should be further investigated and validated in other cohorts,” they wrote. The report was published in Academic Radiology.
Radiogenomic analysis is a method for identifying associations between imaging features and gene expression profiles to provide information that can be used for clinical decision making.
The investigators used the technique to retrospectively explore associations between ccRCC mRNA-based subtyping and CT features.
They identified data on a total of 177 patients with ccRCC from The Cancer Genome Atlas for whom complete CT imaging, including contrast-enhanced images, and mRNA-based subtyping were available.
CT features studied included calcifications, margin definition, renal vein invasion, collecting-system invasion, multicystic tumors, nodular tumor enhancement, and intratumoral vasculature.
In univariate logistic regression analysis, well-defined tumor margins (vs. poorly-defined margins) were significant associated with m1 subtype (odd ratio [OR] 2.104, P = .041).
Similarly, a combination of well-defined tumor margins (OR 2.104, P = .013), no collecting system invasion (OR 0.421, P = .028), and renal vein invasion (OR 2.164, P = .026) were significantly associated with the m3 subtype.
They were no significant associations between CT imaging features and either the m2 or m4 subtypes, the authors found.
The authors did not report study funding sources or potential conflicts of interest.
SOURCE: Bowen L, Xiaojing L. Acad Radiol. doi: 10.1016/j.acra.2018.05.002.
In patients with clear cell renal cell carcinoma (ccRCC), radiogenomic analysis can reveal associations between specific features on CT imaging and messenger RNA-based tumor subtyping, authors of a preliminary analysis contend.
Among 177 patients with ccRCC for whom CT data and molecular subtyping information were available, well-defined vs. poorly-defined tumor margins were significantly associated with messenger RNA (mRNA) subtype m1 tumors, and a combination of margin definition and other qualitative tumor features was significantly associated with m3 subtype, reported Lan Bowen, MD, and Li Xiaojing, MD, from Huizhou (China) Central People’s Hospital.
“We demonstrated m1-subtype is positively associated with well-defined margin while m3-subtype is positively associated with ill-defined margin, renal vein invasion, and collecting-system invasion. These findings should be further investigated and validated in other cohorts,” they wrote. The report was published in Academic Radiology.
Radiogenomic analysis is a method for identifying associations between imaging features and gene expression profiles to provide information that can be used for clinical decision making.
The investigators used the technique to retrospectively explore associations between ccRCC mRNA-based subtyping and CT features.
They identified data on a total of 177 patients with ccRCC from The Cancer Genome Atlas for whom complete CT imaging, including contrast-enhanced images, and mRNA-based subtyping were available.
CT features studied included calcifications, margin definition, renal vein invasion, collecting-system invasion, multicystic tumors, nodular tumor enhancement, and intratumoral vasculature.
In univariate logistic regression analysis, well-defined tumor margins (vs. poorly-defined margins) were significant associated with m1 subtype (odd ratio [OR] 2.104, P = .041).
Similarly, a combination of well-defined tumor margins (OR 2.104, P = .013), no collecting system invasion (OR 0.421, P = .028), and renal vein invasion (OR 2.164, P = .026) were significantly associated with the m3 subtype.
They were no significant associations between CT imaging features and either the m2 or m4 subtypes, the authors found.
The authors did not report study funding sources or potential conflicts of interest.
SOURCE: Bowen L, Xiaojing L. Acad Radiol. doi: 10.1016/j.acra.2018.05.002.
FROM ACADEMIC RADIOLOGY
Key clinical point: Radiogenomic analysis may allow detection of clear cell renal cell carcinoma (ccRCC) subtypes.
Major finding: Well-defined tumor margins on CT were significantly associated with the ccRCC m1 subtype.
Study details: Retrospective analysis of the association between CT features and molecular subtype in 177 patients with ccRCC.
Disclosures: The authors did not report study funding sources or potential conflicts of interest.
Source: Bowen L, Xiaojing L. Acad Radiol. 2018 Jul 29. doi: 10.1016/j.acra.2018.05.002.
Rare clear cell papillary RCC has indolent course
Clear cell papillary renal cell carcinoma (CCPRCC), a recently identified, rare type of renal tumor, appears to have an indolent course with low risk of either local invasion or distant metastasis, results of a small retrospective study suggest.
Among 25 patients with CCPRCC followed for as long as 119 months, there were no cases of local recurrence or distant metastasis, reported Wei-Jen Chen, MD, of Tapei (Taiwan) Veterans General Hospital, and colleagues.
“Based on our findings, CCPRCC has an indolent behavior even if the patients are immunosuppressed or if they receive less invasive therapy. Microscopically, CCPRCC is considered to be a tumor of low malignant potential, as all tumors in our series were of low nuclear grade,” they wrote in a study published online in the Journal of the Chinese Medical Association.
“Whenever the diagnosis is made in a high grade renal tumor, it should be carefully re-confirmed by either cytogenetic or molecular genetic methods,” the authors reported.
CCPRCC was newly recognized as a distinct renal malignancy in the 2016 World Health Organization Classification of Tumors of the Urinary System and Male Genital Organs. The classification describes CCPRCC as “a renal epithelial neoplasm composed of low-grade clear epithelial cells arranged in tubules and papillae with a predominantly linear nuclear alignment away from the basement membrane.”
Although rare, these tumors account for up to 5% of all resected renal tumors, and arise sporadically in patients with end-stage renal disease (ESRD) and von Hippel-Lindau syndrome, the classification states, adding that “according to current knowledge, these tumors have indolent behavior.”
To see if they could verify that last statement, Dr. Chen and associates collected data on all patients diagnosed at their institution with CCPRCC from January 2008 through September 2016.
They identified a total of 25 patients (11 men and 14 women) with a mean age at diagnosis of 62.8 years. Of this group, three patients with poor general condition underwent cryotherapy after a biopsy-confirmed diagnosis of CCPRCC.
All of the remaining 22 patients underwent surgical resection. Of this group, four had ESRD; three of these patients had received a kidney transplant prior to diagnosis of CCPRCC in the native kidneys, and one had three tumors over both kidneys.
“Three patients had other types of synchronous RCC; one with acquired cystic kidney disease-associated RCC, and the others with ccRCC. All CCPRCCs were localized and low grade (pT1a- pT1b, Fuhrman grade 2), and all of the patients are currently alive with no evidence of disease,” the investigators wrote.
Mean follow-up was 49.7 months (range 12 to 119 months).
One additional patient who was not included in the series was initially diagnosed with CCPRCC with lung metastasis. This patient, who died 3 years and 8 months after cytoreductive nephrectomy, had a clinical course distinct from that of all the other patients, leading the investigators to reexamine his kidney specimen with whole-exome sequencing. The sequencing led to a revision of the diagnosis to clear cell RCC.
The investigators noted that clear cell RCC, papillary RCC, and translocation RCC are three RCC subtypes that should be considered in the differential diagnosis of CCPRCC.
The investigators did not report a study funding source. They reported having no conflicts of interest relevant to the subject matter or materials discussed in the article.
SOURCE: Chen W-J et al. J Chinese Med Assoc. 2018 July 20. doi: 10.1016/j.jcma.2018.04.005.
Clear cell papillary renal cell carcinoma (CCPRCC), a recently identified, rare type of renal tumor, appears to have an indolent course with low risk of either local invasion or distant metastasis, results of a small retrospective study suggest.
Among 25 patients with CCPRCC followed for as long as 119 months, there were no cases of local recurrence or distant metastasis, reported Wei-Jen Chen, MD, of Tapei (Taiwan) Veterans General Hospital, and colleagues.
“Based on our findings, CCPRCC has an indolent behavior even if the patients are immunosuppressed or if they receive less invasive therapy. Microscopically, CCPRCC is considered to be a tumor of low malignant potential, as all tumors in our series were of low nuclear grade,” they wrote in a study published online in the Journal of the Chinese Medical Association.
“Whenever the diagnosis is made in a high grade renal tumor, it should be carefully re-confirmed by either cytogenetic or molecular genetic methods,” the authors reported.
CCPRCC was newly recognized as a distinct renal malignancy in the 2016 World Health Organization Classification of Tumors of the Urinary System and Male Genital Organs. The classification describes CCPRCC as “a renal epithelial neoplasm composed of low-grade clear epithelial cells arranged in tubules and papillae with a predominantly linear nuclear alignment away from the basement membrane.”
Although rare, these tumors account for up to 5% of all resected renal tumors, and arise sporadically in patients with end-stage renal disease (ESRD) and von Hippel-Lindau syndrome, the classification states, adding that “according to current knowledge, these tumors have indolent behavior.”
To see if they could verify that last statement, Dr. Chen and associates collected data on all patients diagnosed at their institution with CCPRCC from January 2008 through September 2016.
They identified a total of 25 patients (11 men and 14 women) with a mean age at diagnosis of 62.8 years. Of this group, three patients with poor general condition underwent cryotherapy after a biopsy-confirmed diagnosis of CCPRCC.
All of the remaining 22 patients underwent surgical resection. Of this group, four had ESRD; three of these patients had received a kidney transplant prior to diagnosis of CCPRCC in the native kidneys, and one had three tumors over both kidneys.
“Three patients had other types of synchronous RCC; one with acquired cystic kidney disease-associated RCC, and the others with ccRCC. All CCPRCCs were localized and low grade (pT1a- pT1b, Fuhrman grade 2), and all of the patients are currently alive with no evidence of disease,” the investigators wrote.
Mean follow-up was 49.7 months (range 12 to 119 months).
One additional patient who was not included in the series was initially diagnosed with CCPRCC with lung metastasis. This patient, who died 3 years and 8 months after cytoreductive nephrectomy, had a clinical course distinct from that of all the other patients, leading the investigators to reexamine his kidney specimen with whole-exome sequencing. The sequencing led to a revision of the diagnosis to clear cell RCC.
The investigators noted that clear cell RCC, papillary RCC, and translocation RCC are three RCC subtypes that should be considered in the differential diagnosis of CCPRCC.
The investigators did not report a study funding source. They reported having no conflicts of interest relevant to the subject matter or materials discussed in the article.
SOURCE: Chen W-J et al. J Chinese Med Assoc. 2018 July 20. doi: 10.1016/j.jcma.2018.04.005.
Clear cell papillary renal cell carcinoma (CCPRCC), a recently identified, rare type of renal tumor, appears to have an indolent course with low risk of either local invasion or distant metastasis, results of a small retrospective study suggest.
Among 25 patients with CCPRCC followed for as long as 119 months, there were no cases of local recurrence or distant metastasis, reported Wei-Jen Chen, MD, of Tapei (Taiwan) Veterans General Hospital, and colleagues.
“Based on our findings, CCPRCC has an indolent behavior even if the patients are immunosuppressed or if they receive less invasive therapy. Microscopically, CCPRCC is considered to be a tumor of low malignant potential, as all tumors in our series were of low nuclear grade,” they wrote in a study published online in the Journal of the Chinese Medical Association.
“Whenever the diagnosis is made in a high grade renal tumor, it should be carefully re-confirmed by either cytogenetic or molecular genetic methods,” the authors reported.
CCPRCC was newly recognized as a distinct renal malignancy in the 2016 World Health Organization Classification of Tumors of the Urinary System and Male Genital Organs. The classification describes CCPRCC as “a renal epithelial neoplasm composed of low-grade clear epithelial cells arranged in tubules and papillae with a predominantly linear nuclear alignment away from the basement membrane.”
Although rare, these tumors account for up to 5% of all resected renal tumors, and arise sporadically in patients with end-stage renal disease (ESRD) and von Hippel-Lindau syndrome, the classification states, adding that “according to current knowledge, these tumors have indolent behavior.”
To see if they could verify that last statement, Dr. Chen and associates collected data on all patients diagnosed at their institution with CCPRCC from January 2008 through September 2016.
They identified a total of 25 patients (11 men and 14 women) with a mean age at diagnosis of 62.8 years. Of this group, three patients with poor general condition underwent cryotherapy after a biopsy-confirmed diagnosis of CCPRCC.
All of the remaining 22 patients underwent surgical resection. Of this group, four had ESRD; three of these patients had received a kidney transplant prior to diagnosis of CCPRCC in the native kidneys, and one had three tumors over both kidneys.
“Three patients had other types of synchronous RCC; one with acquired cystic kidney disease-associated RCC, and the others with ccRCC. All CCPRCCs were localized and low grade (pT1a- pT1b, Fuhrman grade 2), and all of the patients are currently alive with no evidence of disease,” the investigators wrote.
Mean follow-up was 49.7 months (range 12 to 119 months).
One additional patient who was not included in the series was initially diagnosed with CCPRCC with lung metastasis. This patient, who died 3 years and 8 months after cytoreductive nephrectomy, had a clinical course distinct from that of all the other patients, leading the investigators to reexamine his kidney specimen with whole-exome sequencing. The sequencing led to a revision of the diagnosis to clear cell RCC.
The investigators noted that clear cell RCC, papillary RCC, and translocation RCC are three RCC subtypes that should be considered in the differential diagnosis of CCPRCC.
The investigators did not report a study funding source. They reported having no conflicts of interest relevant to the subject matter or materials discussed in the article.
SOURCE: Chen W-J et al. J Chinese Med Assoc. 2018 July 20. doi: 10.1016/j.jcma.2018.04.005.
FROM JOURNAL OF THE CHINESE MEDICAL SOCIETY
Key clinical point: Clear cell papillary renal cell carcinoma (CCPRCC) is a distinct renal tumor type with an apparently indolent clinical course.
Major finding: There were no cases of local recurrence or distant metastasis of CCPRCC after a mean of 49.7 months of follow-up (range 12-119 months).
Study details: Retrospective case study of 25 patients diagnosed and treated at a single center.
Disclosures: The investigators did not report a study funding source. They reported having no conflicts of interest relevant to the subject matter or materials discussed in the article.
Source: Chen W-J et al. J Chinese Med Assoc. 2018 July 20 doi: 10.1016/j.jcma.2018.04.005.
Conditional OS estimates show upfront TKI benefit in mRCC
An analysis of conditional survival outcomes for patients with metastatic renal cell carcinoma (mRCC) has suggested that first-line therapy with a tyrosine kinase inhibitor (TKI) can result in improved survival odds over time.
Patients with mRCC treated with a TKI upfront had gradual increases over time in conditional overall survival estimates when compared with baseline survival predictions, a retrospective review has indicated.
Patients who survived at least 36 months after the start of therapy had an estimated 36-month conditional overall survival (OS) rate that was 7.3% higher than the predicted survival at the initiation of therapy, reported Seong Il Seo, MD, PhD, from Samsung Medical Center in Seoul, North Korea, and his colleagues.
The investigators also found that, while predictors of survival changed over time, previous metastasectomy was a key prognosticator of conditional overall survival throughout 36 months of follow-up, they reported in The Journal of Urology.
“To our knowledge, our data are the first to reveal the beneficial role of metastasectomy on conditional OS probabilities with time since an initial survival estimation, particularly in patients at intermediate and poor risk. [Conditional survival] estimates can be beneficial to counsel patients with mRCC about more practical prognoses and helpful to continuously adjust surveillance planning in these patients,” they wrote.
Conditional survival is an analytical method for providing more accurate estimates of how prognoses change over time when patients with aggressive metastatic disease, such as mRCC, are exposed to therapies such as nephrectomy or TKIs.
The investigators retrospectively reviewed records for 1,131 patients with mRCC in the Korean Renal Cancer Study Group database. They calculated conditional OS using a nomogram that indicated the likelihood that a patient would survive an additional number of years given that he or she had already survived a certain number of years. They also created a multivariate regression model to identify predictors of conditional survival over time.
They found that, at all survival times after the start of TKI therapy (6, 12, 18, 24, and 36 months), conditional overall survival gradually increased when compared with baseline survival estimates.
“While the actual overall survival rate decreased with time, the 36-month conditional overall survival rate was calculated as 7.3% higher in patients who had already survived 36 months compared to baseline estimations at the time of initial tyrosine kinase inhibitor treatment,” they wrote.
In the multivariate model, prognostic factors such as gender, pathologic T stage, and Heng risk classification became nonsignificant over time, but previous metastasectomy remained a significant independent predictor of survival after TKI therapy at all time points except for 18 months.
“This study largely corroborates previous data from the IMDC (International Metastatic Renal Cell Carcinoma Database Consortium), and it provides useful information on prognostication,” commented Adam B. Weiner, MD, of Northwestern University in Chicago, in a brief accompanying editorial.
The study was supported by a National Research Foundation of Korea research grant funded by the Ministry of Science and Information and Communications Technology and by a Korea Health Technology R&D Project grant through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare in South Korea. No conflicts of interest were reported.
SOURCE: Kang M et al. J Urol. 2018 June 22. doi: 10.1016/j.juro.2018.06.030.
An analysis of conditional survival outcomes for patients with metastatic renal cell carcinoma (mRCC) has suggested that first-line therapy with a tyrosine kinase inhibitor (TKI) can result in improved survival odds over time.
Patients with mRCC treated with a TKI upfront had gradual increases over time in conditional overall survival estimates when compared with baseline survival predictions, a retrospective review has indicated.
Patients who survived at least 36 months after the start of therapy had an estimated 36-month conditional overall survival (OS) rate that was 7.3% higher than the predicted survival at the initiation of therapy, reported Seong Il Seo, MD, PhD, from Samsung Medical Center in Seoul, North Korea, and his colleagues.
The investigators also found that, while predictors of survival changed over time, previous metastasectomy was a key prognosticator of conditional overall survival throughout 36 months of follow-up, they reported in The Journal of Urology.
“To our knowledge, our data are the first to reveal the beneficial role of metastasectomy on conditional OS probabilities with time since an initial survival estimation, particularly in patients at intermediate and poor risk. [Conditional survival] estimates can be beneficial to counsel patients with mRCC about more practical prognoses and helpful to continuously adjust surveillance planning in these patients,” they wrote.
Conditional survival is an analytical method for providing more accurate estimates of how prognoses change over time when patients with aggressive metastatic disease, such as mRCC, are exposed to therapies such as nephrectomy or TKIs.
The investigators retrospectively reviewed records for 1,131 patients with mRCC in the Korean Renal Cancer Study Group database. They calculated conditional OS using a nomogram that indicated the likelihood that a patient would survive an additional number of years given that he or she had already survived a certain number of years. They also created a multivariate regression model to identify predictors of conditional survival over time.
They found that, at all survival times after the start of TKI therapy (6, 12, 18, 24, and 36 months), conditional overall survival gradually increased when compared with baseline survival estimates.
“While the actual overall survival rate decreased with time, the 36-month conditional overall survival rate was calculated as 7.3% higher in patients who had already survived 36 months compared to baseline estimations at the time of initial tyrosine kinase inhibitor treatment,” they wrote.
In the multivariate model, prognostic factors such as gender, pathologic T stage, and Heng risk classification became nonsignificant over time, but previous metastasectomy remained a significant independent predictor of survival after TKI therapy at all time points except for 18 months.
“This study largely corroborates previous data from the IMDC (International Metastatic Renal Cell Carcinoma Database Consortium), and it provides useful information on prognostication,” commented Adam B. Weiner, MD, of Northwestern University in Chicago, in a brief accompanying editorial.
The study was supported by a National Research Foundation of Korea research grant funded by the Ministry of Science and Information and Communications Technology and by a Korea Health Technology R&D Project grant through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare in South Korea. No conflicts of interest were reported.
SOURCE: Kang M et al. J Urol. 2018 June 22. doi: 10.1016/j.juro.2018.06.030.
An analysis of conditional survival outcomes for patients with metastatic renal cell carcinoma (mRCC) has suggested that first-line therapy with a tyrosine kinase inhibitor (TKI) can result in improved survival odds over time.
Patients with mRCC treated with a TKI upfront had gradual increases over time in conditional overall survival estimates when compared with baseline survival predictions, a retrospective review has indicated.
Patients who survived at least 36 months after the start of therapy had an estimated 36-month conditional overall survival (OS) rate that was 7.3% higher than the predicted survival at the initiation of therapy, reported Seong Il Seo, MD, PhD, from Samsung Medical Center in Seoul, North Korea, and his colleagues.
The investigators also found that, while predictors of survival changed over time, previous metastasectomy was a key prognosticator of conditional overall survival throughout 36 months of follow-up, they reported in The Journal of Urology.
“To our knowledge, our data are the first to reveal the beneficial role of metastasectomy on conditional OS probabilities with time since an initial survival estimation, particularly in patients at intermediate and poor risk. [Conditional survival] estimates can be beneficial to counsel patients with mRCC about more practical prognoses and helpful to continuously adjust surveillance planning in these patients,” they wrote.
Conditional survival is an analytical method for providing more accurate estimates of how prognoses change over time when patients with aggressive metastatic disease, such as mRCC, are exposed to therapies such as nephrectomy or TKIs.
The investigators retrospectively reviewed records for 1,131 patients with mRCC in the Korean Renal Cancer Study Group database. They calculated conditional OS using a nomogram that indicated the likelihood that a patient would survive an additional number of years given that he or she had already survived a certain number of years. They also created a multivariate regression model to identify predictors of conditional survival over time.
They found that, at all survival times after the start of TKI therapy (6, 12, 18, 24, and 36 months), conditional overall survival gradually increased when compared with baseline survival estimates.
“While the actual overall survival rate decreased with time, the 36-month conditional overall survival rate was calculated as 7.3% higher in patients who had already survived 36 months compared to baseline estimations at the time of initial tyrosine kinase inhibitor treatment,” they wrote.
In the multivariate model, prognostic factors such as gender, pathologic T stage, and Heng risk classification became nonsignificant over time, but previous metastasectomy remained a significant independent predictor of survival after TKI therapy at all time points except for 18 months.
“This study largely corroborates previous data from the IMDC (International Metastatic Renal Cell Carcinoma Database Consortium), and it provides useful information on prognostication,” commented Adam B. Weiner, MD, of Northwestern University in Chicago, in a brief accompanying editorial.
The study was supported by a National Research Foundation of Korea research grant funded by the Ministry of Science and Information and Communications Technology and by a Korea Health Technology R&D Project grant through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare in South Korea. No conflicts of interest were reported.
SOURCE: Kang M et al. J Urol. 2018 June 22. doi: 10.1016/j.juro.2018.06.030.
FROM THE JOURNAL OF UROLOGY
Key clinical point: Conditional overall survival estimates may help clinicians adjust surveillance planning in patients with mRCC.
Major finding: At all survival times after the start of TKI therapy, conditional overall survival gradually increased when compared with baseline survival estimates.
Study details: Retrospective review of records on 1,131 patients with mRCC.
Disclosures: The study was supported by a National Research Foundation of Korea research grant funded by the Ministry of Science and Information and Communications Technology and by a Korea Health Technology R&D Project grant through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare in South Korea. No conflicts of interest were reported.
Source: Kang M et al. J Urol. 2018 June 22. doi: 10.1016/j.juro.2018.06.030.
Urothelial Carcinoma: Muscle-Invasive and Metastatic Disease
Introduction
Bladder cancer is by far the most common cancer of the urinary system. Worldwide, approximately 450,000 new cases are diagnosed and 165,000 deaths are caused by bladder cancer each year.1 In the United States and in Europe, the most common type of bladder cancer is urothelial carcinoma (also referred to as transitional cell carcinoma), which accounts for more than 90% of all bladder cancers in these regions of the world. The remainder of bladder cancers are divided among squamous cell carcinomas, adenocarcinomas, small cell carcinomas, and, even more rarely, between various other nonepithelial tumors (eg, sarcoma).
Bladder cancer is classically thought of as a disease of the elderly, with a median age at diagnosis of 69 years in men and 71 years in women.2 The incidence of bladder cancer increases with age: in persons aged 65 to 69 years, incidence is 142 per 100,000 men and 33 per 100,000 women, and in those older than 85 years the rate doubles to 296 per 100,000 men and 74 per 100,000 women.3 The incidence is 3 times greater in men than in women.4
Urothelial carcinoma is traditionally categorized by its degree of invasion into the bladder wall: superficial (non-muscle-invasive), muscle-invasive, or metastatic disease. At the time of diagnosis, most patients have non-muscle-invasive disease (~60%); about 4% of all patients present initially with metastatic disease.5 This article focuses on metastatic bladder cancer, but muscle-invasive disease is discussed as well.
The most important factor contributing to the development of urothelial carcinoma is tobacco smoking. The risk of developing bladder cancer is 4 to 5 times higher in smokers as compared to nonsmokers, with some variation according to sex.6 Quantity of smoking exposure also plays a role, with heavy smokers demonstrating a higher likelihood for high-grade tumors with muscle invasion (or beyond) when compared to light smokers.7 Another important risk factor is occupational exposure to industrial materials, such as carpets, paints, plastics, and industrial chemicals. This type of exposure may be responsible for, or at least contribute to, the development of approximately 20% of urothelial carcinomas. Other risk factors for urothelial carcinoma include but are not limited to prior radiation to the pelvis, prior upper tract urothelial malignancy, human papillomavirus infection, and prior bladder augmentation.
Diagnosis and Staging
Case Presentation
A 63-year-old man with a past medical history of diabetes, deep vein thrombosis, occasional alcohol use, and regular pipe tobacco use presents to his primary care physician with complaints of hematuria. He reports that his urine was a dark red color that morning, which had never happened before. The patient is hemodynamically stable upon evaluation in the office, and a point-of-care urinalysis dipstick is strongly positive for blood. He is referred to a urologist for further evaluation.
In the urology office, urine microscopy is notable for more than 50 red blood cells (RBCs) per high-power field with normal RBC morphology. Flexible cystoscopy performed in the office reveals a single 2-cm, sessile, verrucous, nodular lesion located on the anterior bladder wall. A urine sample and a bladder wash specimen are sent for cytology evaluation. The patient is scheduled to undergo a complete transurethral resection of bladder tumor (TURBT) later that week with samples sent to pathology for evaluation.
- What are the clinical features of bladder cancer?
Hematuria is the most common presentation of bladder cancer, although its specificity is far lower than traditionally thought. In fact, only about 2% to 20% of cases that present with hematuria are found to be caused by malignancy. However, the incidence of genitourinary tract malignancy is much higher in patients presenting with gross hematuria (10%–20%)8–10 than in patients with microscopic hematuria alone.8,10–14 Typically, hematuria associated with malignancy is painless. Multiple studies have shown, however, that hematuria can be a normal variant, with one study demonstrating that up to 61% of patients with hematuria had no identifiable abnormality.8,10,11,13
Abdominal pain, flank pain, dysuria, urinary frequency/urgency, or other irritative voiding symptoms in the absence of hematuria can be presenting symptoms of bladder cancer as well. In these settings, discomfort typically suggests more advanced malignancy with at least local involvement or obstruction. Suprapubic pain may herald invasion into perivesical tissues and nerves, while involvement of the obturator fossa, perirectal fat, urogenital diaphragm, or presacral nerves can often present with perineal or rectal pain. Similarly, lower abdominal pain may represent involvement of lymph nodes, and right upper quadrant pain may signal liver metastasis. Cough or shortness of breath may signify metastatic disease in the lung. Finally, back, rib, or other boney pain may suggest distant metastasis.
- What next steps are required to complete this patient’s staging?
White light cystoscopy remains the gold standard for diagnosis and initial staging of bladder cancer. Additional tools include urine cytology and upper tract studies, including renal computed tomography (CT) urograms. Full urologic evaluation with all 3 modalities (cystourethroscopy, urinary cytology, and upper tract evaluation) is warranted for patients with a high suspicion for malignant etiology of hematuria. CT urograms are particularly useful for upper tract evaluation because they can be used to visualize kidney parenchyma, both renal pelvises and ureters, and pertinent abdominal and pelvic lymph nodes. Initial staging is completed through TURBT, which should ideally contain a segment of muscularis propria to distinguish between Ta (noninvasive), T1, and T2 tumors (Figure 1).
Regarding staging, T1 tumors are distinguished from Ta malignancies by their involvement in the urothelial basement membrane. Tumor invasion into the muscularis propria indicates T2 tumors, while T3 tumors extend through the muscle into the serosa and involve the complete thickness of the bladder wall. Involvement of nearby structures defines T4 bladder cancers, with T4a malignancies involving adjacent organs (prostate, vagina, uterus, or bowel) and T4b tumors involving the abdominal wall, pelvic wall, or other more distant organs. According to the American Joint Committee on Cancer’s most recent TNM staging system (Table 1),16 lymph node involvement in the true pelvis (that is, N1–N3) with T1 to T4a disease is now classified as stage III disease.
Bladder cancer is often broadly categorized as either non-muscle-invasive or muscle-invasive (which can include metastatic disease). This classification has important implications for treatment. As such, all diagnostic biopsies should be performed with the goal of reaching at least the depth of the muscularis propria in order to accurately detect potential muscularis invasion. If no muscle is detected in the initial specimen, re-resection is recommended if safe and feasible. In cases where muscle cannot be obtained, imaging evidence of T3 disease from CT or magnetic resonance imaging may be used as a surrogate indicator. Once muscle-invasive disease is confirmed, CT evaluation of the chest is also recommended, as bladder cancer can metastasize to the lungs; furthermore, patients are often at risk for secondary concomitant lung cancers given that smoking is the most prevalent risk factor for both. However, patients with small, indeterminate lung nodules not amenable to biopsy should not be denied curative intent treatment given the high likelihood that they represent benign findings.17
Pathogenesis
Because non-muscle-invasive and muscle-invasive tumors behave so differently, they are thought to arise from 2 distinct mechanisms. Although there is overlap and non-muscle-invasive cancer can certainly progress to a high-grade, invasive type of malignancy over time, current theory proposes that non-muscle-invasive bladder cancer predominantly develops just from urothelial hyperplasia, which then recruits branching vasculature to grow slowly. More aggressive urothelial carcinomas, including muscle-invasive and metastatic disease, are instead thought to arise directly from flat dysplasia that progresses to carcinoma in situ, and is much more prone to invasive growth and distant spread.18
Regardless of grade and stage, the most commonly identified genomic alterations in urothelial carcinoma are mutations in the promoter region of the telomerase reverse transcriptase (TERT) gene, which have been identified in approximately 70% of cases.19 Mutations in TERT can be readily detected in urine sediments and may ultimately have implications for diagnosis and early detection.20,21 In current practice, however, the clinical relevance of these observations remains under development. Other genomic alterations that may contribute to the development of urothelial carcinoma, and also provide new potential therapeutic targets, include alterations in the TP53 gene, the RB (retinoblastoma) gene, and the FGFR3 (fibroblast growth factor receptor) gene. FGFR3 has particular significance as it appears to be relatively common in non-muscle invasive disease (up to 60%–70%) and is likely an actionable driver mutation that may define a particular molecular subset of urothelial carcinoma; thus, it may have important implications for treatment decisions.22
Treatment
Case Continued
Pathologic evaluation of the specimen reveals a high-grade urothelial carcinoma with tumor invasion into the muscularis propria. A CT urogram is performed and does not reveal any notably enlarged pelvic nodes or suspicious lesions in the upper urinary tract. CT chest does not reveal any evidence of distant metastatic disease. Given the presence of muscle-invasive disease, the patient agrees to proceed with neoadjuvant chemotherapy and radical cystoprostatectomy with pelvic node dissection. He undergoes treatment with dose-dense (accelerated) MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) for 3 cycles, followed by surgery with cystoprostatectomy. Overall, he tolerates the procedure well and recovers quickly. Pathology reveals the presence of disease in 2 regional nodes, consistent with T4a (stage III) disease, and a small degree of residual disease in the bladder. He is followed closely in the oncology clinic, returning for urine cytology, liver and renal function tests, and imaging with CT of chest, abdomen, and pelvis every 3 months.
- What is the first-line approach to management in patients with muscle-invasive disease?
- How would the treatment strategy differ if the patient had presented with metastatic disease (stage IV)?
First-Line Management for Curative Intent: Muscle-Invasive Disease
Muscle-invasive urothelial carcinoma (including T2, T3, or T4 disease) is typically treated in a multidisciplinary fashion with neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy. This approach is recommended over radical cystectomy alone because of high relapse rates following cystectomy alone, even in the setting of bilateral pelvic lymphadenectomy.23 However, because of the associated short- and long-term toxicity of cisplatin-based regimens, this optimal treatment paradigm is reserved for patients deemed cisplatin-eligible.
Medical fitness to receive cisplatin-based chemotherapy is assessed by a number of factors and varies by institution, but most frequently consider functional status (Eastern Cooperative Oncology Group [ECOG] performance status or Karnofsky Performance Status), creatinine clearance, hearing preservation, peripheral neuropathy, and cardiac function.24 Many programs will elect to defer cisplatin-based chemotherapy in patients with low performance status (ie, < 60–70 on Karnofsky scale or > 2 on ECOG scale), creatinine clearance below 60 mL/min, or significant heart failure (NHYA class III or worse). Cisplatin-based chemotherapy may worsen hearing loss in those with hearing loss of 25 dB from baseline at 2 continuous frequencies and also may worsen neuropathy in those with baseline grade 1 peripheral neuropathy. However, these adverse outcomes must be balanced against the curative intent of the multimodality systemic approach.
In patients with renal insufficiency, caution must be taken with regard to cisplatin. Percutaneous nephrostomy placement or ureteral stenting should be attempted to relieve any ureteral outlet obstruction and restore kidney function if a patient’s renal insufficiency has resulted from this obstruction. If medical renal disease or long-term renal insufficiency is present, however, patients should instead be referred for immediate cystectomy or for a bladder-preserving approach. Generally, a creatinine clearance of 60 mL/min is required to safely receive cisplatin-based chemotherapy, although some advocate for treatment with a creatinine clearance as low as 50 mL/min. When this extended criterion is used, the dose of cisplatin may be split over 2 days to minimize renal toxicity and maximize hydration. Analysis of renal function utilizing a 24-hour urine collection should be incorporated whenever possible, as estimates of creatinine clearance have been demonstrated to be inaccurate in some instances.25
For cisplatin-eligible patients, neoadjuvant chemotherapy with a cisplatin base has consistently demonstrated a survival benefit when given prior to surgery.26,27 Historically, several different platinum-based regimens have been studied, with none showing superior effectiveness in a randomized trial over the others in the neoadjuvant setting. These regimens have included classic MVAC, dose-dense MVAC (MVAC with pegfilgrastim), GC (gemcitabine and cisplatin), and CMV (methotrexate, vinblastine, cisplatin, and leucovorin).
While classic MVAC was preferred in the 1990s and early 2000s,28,29 the availability of growth factor, such as pegfilgrastim, has made dose-dense MVAC (otherwise referred to as accelerated MVAC or ddMVAC) widely preferred and universally recommended over classic MVAC. The ddMVAC regimen with the addition of a synthetic granulocyte colony-stimulating factor (G-CSF) is substantially better tolerated than classic MVAC, as the G-CSF support minimizes the severe toxicities of classic MVAC, such as myelosuppression and mucositis, and allows for the administration of drugs in a dose-dense fashion.30,31
Both ddMVAC and GC are considered reasonable options for neoadjuvant chemotherapy and are the predominant choices for cisplatin-eligible patients (Table 2).
Prospective data defining the role of adjuvant chemotherapy for patients after cystectomy has been fraught by a variety of factors, including the known benefit of neoadjuvant chemotherapy, the high complication rate of cystectomy making chemotherapy infeasible, and clinician bias that has hampered accrual in prior trials. Thus, no level 1 evidence exists defining the benefit of adjuvant chemotherapy in patients who did not receive neoadjuvant therapy. In a report of the largest study performed in this setting, there was a statistically significant benefit in PFS but not in OS.36 Criticisms of this trial include its lack of statistical power due to a failure to accrue the targeted goal and the preponderance of node-positive patients. Regardless, for patients who have pT2–4, N1 disease after radical cystectomy and remain cisplatin-eligible after not receiving neoadjuvant chemotherapy, this remains an option.
Despite the established clinical dogma surrounding neoadjuvant chemotherapy followed by surgery, some patients are either not eligible for or decline to receive radical cystectomy, while others are not candidates for neoadjuvant cisplatin-based chemotherapy for the reasons outlined above. For patients who are surgical candidates but unable to receive neoadjuvant chemotherapy due to renal or cardiac function, they may proceed directly to surgery. For patients unable or unwilling to proceed to radical cystectomy regardless, bladder preservation strategies exist. Maximal TURBT may be an option for some patients, but, as outlined above, used alone this would be likely to lead to a high degree of local and distant failure. Combined modality chemoradiotherapy as consolidation after maximal TURBT is an established option for patients unable to undergo surgery or seeking bladder preservation. Several trials have demonstrated encouraging outcomes with this approach and were highlighted in a large meta-analysis.37 Various chemosensitizing chemotherapeutic regimens have been evaluated, including cisplatin alone or as a doublet, gemcitabine alone, and 5-fluouracil plus mitomycin C, but no randomized studies have compared these regimens to each other, nor have they been compared to surgical approaches. However, this strategy remains an option as an alternative to surgery.
First-Line Management: Metastatic Disease
The approach to therapy in patients who present with metastatic urothelial carcinoma is very similar to that used in neoadjuvant perioperative chemotherapy. The consensus first-line treatment in medically appropriate patients is cisplatin-based chemotherapy with either GC or ddMVAC (both category 1 National Comprehensive Cancer Network [NCCN] recommendations; Figure 2).30,31,38–40
Head-to-head studies specifically comparing ddMVAC and GC have been limited. GC has been compared to classic MVAC, with results showing equivalent efficacy but improved tolerability, as expected.38,40 ddMVAC was compared with a modified version of GC (termed “dose-dense GC”) in a phase 3 study from Greece, which demonstrated similar outcomes.41
Surgical intervention with radical cystectomy and regional lymph node dissection is typically deferred for patients who present with distant metastatic disease, unlike those who present with locally advanced disease. Radical cystectomy has traditionally been thought of as overly aggressive without sufficient benefit, although evidence to guide this approach remains sparse.42 As such, most expert recommendations and consensus statements simply recommend against surgical intervention and leave the decision between ddMVAC and GC up to the individual clinician.
In patients who are not eligible for cisplatin therapy, it is reasonable to consider chemotherapy with a combination of gemcitabine and carboplatin. This combination has been shown to be equivalent to MCAVI (methotrexate, carboplatin, vinblastine) in terms of overall survival (OS; 9 months versus 8 months) and progression-free survival (PFS; 6 months versus 4 months) with significantly fewer serious toxicities (9% versus 21%).43
The advent of immunotherapy in recent years has provided several new alternatives for cisplatin-ineligible patients. While immunotherapies such as pembrolizumab or atezolizumab are not yet recommended as first-line therapy for cisplatin-eligible patients, these 2 drugs are approved as options for first-line therapy in cisplatin-ineligible patients with metastatic disease. In a recent phase 2 trial (IMvigor210) involving 119 patients who were given atezolizumab as first-line therapy, median PFS was 2.7 months and median OS was 15.9 months.44 Another trial using data from patients in the KEYNOTE-052 study who received pembrolizumab as first-line therapy demonstrated antitumor activity with pembrolizumab and acceptable tolerability in cisplatin-ineligible patients with advanced urothelial carcinoma.45 The primary endpoint was objective response (either complete or partial response), which was achieved in 24% of the intention-to-treat population. Median PFS was 2 months, and 6-month OS was observed in 67% of patients. Both atezolizumab and pembrolizumab were given accelerated approval based on these single-arm studies in this setting. However, due to inferior outcomes in subsequent trials that included single-agent immunotherapy arms for patients in the first-line setting, the US Food and Drug Administration (FDA) has clarified the approval. In the subsequent trials, patients with a low PD-L1 biomarker based on the individual assay used for each drug did worse on immunotherapy alone (compared to chemotherapy or both combined), and the single-therapy arms were stopped early. Thus, the FDA now recommends that pembrolizumab or atezolizumab be used in the first line only for cisplatin-ineligible patients who have PD-L1 expression on tumor cells above the threshold studied on each individual assay, or are unfit for any platinum-based chemotherapy. Further study regarding the optimal role of biomarkers and chemotherapy-immunotherapy combinations is ongoing.
Case Continued
Ten months after his procedure, the patient is found to have prominent retroperitoneal lymphadenopathy and a 1.0-cm liver nodule suspicious for malignancy is noted on surveillance imaging. CT-guided biopsy of the liver reveals high-grade urothelial carcinoma, consistent with both recurrence and distant metastasis. The patient is informed that he needs to resume systemic therapy for recurrent metastatic disease. The options discussed include salvage single-agent chemotherapy with gemcitabine or immunotherapy with pembrolizumab. He elects to move forward with immunotherapy and is scheduled to begin pembrolizumab.
- What other immunotherapies might this patient consider for second-line therapy?
- Is chemotherapy a second-line option for this patient?
Second-Line Therapies and Management of Progressive Disease
Disease progression is unfortunately seen in the majority of cases of advanced urothelial carcinoma.46 New second-line therapies have recently been approved by the FDA in the form of monoclonal antibodies targeting programmed death 1 (PD-1) and a PD-1 ligand (PD-L1) (Figure 3).
Approval of pembrolizumab, a PD-1 inhibitor, was largely supported by the Keynote-045 trial,47,48 which looked at 542 patients who had progressed or recurred after platinum-based chemotherapy. These patients were randomly assigned to either pembrolizumab or investigator’s choice of chemotherapy (paclitaxel, docetaxel, or vinflunine). Patients treated with pembrolizumab had a significantly improved OS (median of 10.3 months versus 7.4 months), but no statistically significant difference in PFS (2.1 months versus 3.3 months). Interestingly, the rate of responses of 12 months or longer was higher with pembrolizumab than with more traditional second-line chemotherapy (68% versus 35%). The strength of this data has led to a category 1 recommendation in the most recent NCCN guidelines.39
The approval of atezolizumab, a PD-L1 inhibitor, as a second-line therapy for advanced urothelial carcinoma is largely supported by data from IMvigor211, a phase 3 trial that studied 931 patients randomly assigned to atezolizumab or investigator’s choice chemotherapy. OS did not differ significantly between patients in the atezolizumab group who had ≥ 5% expression of PD-L1 on tumor-infiltrating immune cells and patients in the chemotherapy group (11.1 months versus 10.6 months), but mean duration of response was longer (15.9 months versus 8.3 months).49 Therapy with atezolizumab had significantly fewer toxicities than chemotherapy (grade 3 or 4 toxicities of 20% versus 43%).
Phase 3 studies of nivolumab (PD-1 inhibitor), avelumab (PD-L1 inhibitor), and durvalumab (PD-L1 inhibitor) have not yet been published. These agents have received accelerated approval, however, as second-line treatment of advanced urothelial carcinoma based on promising data from phase 1 and phase 2 studies.50–52
Second-line chemotherapy is also an option for patients who do not qualify for immunotherapy or who progress during or after immunotherapy. Although there has been a great deal of excitement about new developments with immunotherapy and the survival benefit seen compared to investigator’s choice chemotherapy, the fact remains that most patients do not respond to immunotherapy. Still, some patients do derive benefit from single-agent chemotherapy in the platinum-refractory setting. Options based on primarily single-arm studies include gemcitabine, paclitaxel, docetaxel, pemetrexed, ifosfamide, oxaliplatin, and eribulin (Figure 2). In a randomized phase 3 trial, vinflunine demonstrated an OS benefit in platinum-refractory patients compared to best supportive care; it subsequently received approval by the European Medicines Agency.53 More recently in the phase 3 RANGE trial, docetaxel plus ramucirumab (a monoclonal antibody targeting vascular endothelial growth factor receptor 2) was compared to docetaxel plus placebo and met its primary endpoint of an improvement in PFS (median 4.07 months versus 2.76 months, P = 0.0118).54 OS has not been reported and this regimen has not yet received regulatory approval, however. Unfortunately, trials comparing these regimens are lacking, and response rates and survival remain modest. Clearly, better therapies and biomarkers to help personalize treatment options are needed.
Further investigations are underway with alternative regimens, including but not limited to targeted therapy in the setting of specific genetic and epigenetic alterations. These include mutations affecting tyrosine kinase receptors (eg, RAS/RAF, PI3K, AKT, and mTOR), cell cycle regulators (eg, TP53 or RB1), FGFR3 mutations, PTEN deletions, gene amplifications (eg, FGFR1, CCND1, and MDM2), or changes in genes responsible for chromatin remodeling (eg, UTX, CHD6, or ARID1A). As noted, there is particular excitement regarding FGFR3 inhibitors, which have shown compelling efficacy in phase 1 and 2 single-arm trials. Several agents are being evaluated in randomized trials and represent a potential path to the first targeted therapeutic class with a role in urothelial malignancies.
Surgical resection of metastases may be considered in very select cases.55 Surgery may have a role in limiting metastatic complications and improving cancer control, but this should be discussed at length with the patient using a multidisciplinary approach with careful restaging prior to surgery.
Case Continued
The patient remains on pembrolizumab every 3 weeks as per protocol with regular surveillance imaging. His disease stabilizes as the nodule in his liver and the retroperitoneal lymph nodes, all representing metastatic disease, became slightly smaller in size without evidence of any new disease. He continues to follow up closely with his genitourinary oncologist, undergoing regular surveillance and imaging every 3 months without evidence of disease progression.
Approximately 12 months into therapy, the patient notices a nonproductive cough with progressive and rapidly worsening shortness of breath. He is noted to be hypoxic with oxygen saturation levels to 79% in clinic and is sent immediately to the emergency department by his oncologist. Diffuse bilateral reticular opacities are noted on chest radiograph. Non-contrast CT scan demonstrates diffuse ground-glass opacities consistent with acute respiratory distress syndrome–pattern pneumonitis. He is admitted to the intensive care unit.
The patient is aggressively treated with high-flow nasal oxygen supplementation, intravenous steroids, and empiric antibiotics. He slowly improves on high-dose steroids (methylprednisolone 1 mg/kg/day) without requiring intubation or infliximab therapy and is discharged home in stable condition after 10 days. Oral steroid therapy is continued with a long taper over 6 weeks. In the setting of his grade 3 pneumonitis, pembrolizumab is discontinued and the patient is scheduled for a follow-up appointment with his oncologist to discuss next steps.
- In addition to pneumonitis, what other toxicities should you monitor for in patients treated with an immune checkpoint inhibitor?
- Is this patient a candidate to receive immunotherapy again in the future?
Treatment Toxicities
As use of immune checkpoint inhibitors has become more prevalent, the medical community has become increasingly aware of various immune-related adverse effects (irAE) associated with these drugs. These toxicities can be seen in virtually any organ system, and even vague complaints that arise years after therapy initiation should be treated with a high level of suspicion. The most commonly affected organ systems include the skin, gastrointestinal (GI) tract, lungs, liver, and endocrine system, although all other organ systems can be involved (Table 3) and toxicities appear to be similar across individual drugs.
The American Society of Clinical Oncology recently published a complete set of recommendations to guide clinicians on appropriate treatment strategies for each manifestation of immunotherapy-related toxicity.56 The details of these recommendations largely fall outside the purview of this article, but the mainstays of management are worth noting. These include high-dose systemic glucocorticoids, along with supportive care and cessation of immunotherapy in grade 3 or 4 toxicities. Infliximab is frequently recommended as an adjunct in severe or refractory cases.
Chemotherapy-related toxicities, on the other hand, are well-described and tend to be more familiar to patients and clinicians (Table 3). Classic MVAC, which has now been largely replaced by ddMVAC, was notoriously difficult to tolerate. It was known for a high rate of serious (grade 3 or 4) myelosuppressive complications as well as frequent GI toxicities. These complications include neutropenia (57%), stomatitis (10%), and nausea and vomiting (6%).23 ddMVAC with growth factor support is much better tolerated than classic MVAC. Prominent complaints with ddMVAC still can include nausea, GI distress, mucositis, and fatigue, but the incidence of myelosuppressive complications in particular has markedly decreased. GC is largely well tolerated, with minimal nausea and manageable myelotoxicity, but it is associated with an increased risk of venous thromboembolism.38
Prognosis
Case Conclusion
After returning home, the patient discusses his complicated medical course with his oncologist. Given his continued high quality of life with good functional status, he requests to continue with therapy for his metastatic bladder cancer and is interested in joining a clinical trial. He is referred to a nearby academic center with openings in a clinical trial for which he would be eligible. In the meantime, his oncologist guides him through filling out an advance directive and recommends that he make an appointment with palliative care services to ensure adequate home support for any future needs he may have.
- What is the estimated 5-year survival rate for patients with metastatic bladder cancer?
Overall, prognosis in patients with metastatic bladder cancer remains poor. Median survival in patients being treated with multi-agent chemotherapy is approximately 15 months,38,40 with an expected 5-year survival of just 15%. This is much improved, however, as prior to the advent of modern chemotherapy estimated survival was just 6 months with metastatic bladder cancer. Importantly, these figures do not take into account the recent advancements with immunotherapy, and thus it is reasonable to assume survival rates may continue to improve. In light of these recent advances, it is strongly recommended that whenever possible patients and clinicians consider participation in clinical trials to continue uncovering new and better therapies moving forward.
A number of tools have been developed to help risk stratify patients based on comorbidity, performance status, and other characteristics, but none have been universally adopted.57–60 As with many other malignancies, performance status is an important predictor of clinical outcomes in these patients.61–63 Sites of metastasis also may serve to suggest the course of disease. Patients with visceral metastases typically exhibit significantly worse disease with a shortened survival. The role of molecular factors as prognostic markers in bladder cancer is still under investigation. Many biomarkers are being considered (including mutations and polymorphisms in p53, ERCC1, and ERCC2), and evidence suggests some may have a role in prognosis; thus far, none have been validated as prognostic or predictive tools in urothelial carcinoma.
Conclusion
Bladder cancer includes an aggressive group of genitourinary tract malignancies, of which urothelial carcinoma is by far the most common in the Western world. Cisplatin-based therapy remains a mainstay of treatment for eligible patients with both localized and metastatic disease, but immunotherapies have provided a new and promising tool to use in the setting of progressing malignancy. The individual impact of these agents on OS is still being examined. Further studies and ongoing participation in clinical trials whenever possible continue to be essential to the discovery of future treatment options for this highly aggressive disease.
1. Stewart BW, Kleihues P. World cancer report. IARCPress. 2003.
2. Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 2009;115:68–74.
3. Hinotsu S, Akaza H, Miki T, et al. Bladder cancer develops 6 years earlier in current smokers: Analysis of bladder cancer registry data collected by the cancer registration committee of the Japanese Urological Association. Int J Urol 2009;16:64–9.
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
5. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: bladder cancer. 2018. https://seer.cancer.gov/statfacts/html/urinb.html. Accessed May 5, 2018.
6. Freedman ND, Silverman DT, Hollenbeck AR, et al. Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306:737–45.
7. Pietzak EJ, Mucksavage P, Guzzo TJ, Malkowicz SB. Heavy cigarette smoking and aggressive bladder cancer at initial presentation. Urology 2015;86:968–73.
8. Khadra MH, Pickard RS, Charlton M, et al. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol 2000;163:524–7.
9. Grossman HB, Messing E, Soloway M, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA 2005;293:810–16.
10. Mariani AJ, Mariani MC, Macchioni C, et al. The significance of adult hematuria: 1,000 hematuria evaluations including a risk-benefit and cost-effectiveness analysis. J Urol 1989;141:350–5.
11. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part II: patient evaluation, cytology, voided markers, imaging, cystoscopy, nephrology evaluation, and follow-up. Urology 2001;57:604–10.
12. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part I: definition, detection, prevalence, and etiology. Urology 2001;57:599–603.
13. Mohr DN, Offord KP, Owen RA, Melton LJ. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986;256:224–9.
14. Messing EM, Young TB, Hunt VB, et al. Home screening for hematuria: results of a multiclinic study. J Urol 1992;148:289–92.
15. Gray PJ, Lin CC, Jemal A, et al. Clinical–pathologic stage discrepancy in bladder cancer patients treated with radical cystectomy: results from the National Cancer Data Base. Int J Radiat Oncol 2014;88:1048–56.
16. Bochner B, Hansel D, Efstathiou J, et al. Urinary bladder. In: Amin M, ed. AJCC cancer staging manual. 8th. New York: Springer; 2017:757.
17. Cahn DB, McGreen B, Lee A, et al. Clinical destiny of indeterminate pulmonary nodules in patients undergoing radical cystectomy for urothelial carcinoma of the bladder [abstract]. J Clin Oncol 2017;35(6 suppl):297-297.
18. Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15:25–41.
19. Kurtis B, Zhuge J, Ojaimi C, et al. Recurrent TERT promoter mutations in urothelial carcinoma and potential clinical applications. Ann Diagn Pathol 2016;21:7–11.
20. Ito H, Kyo S, Kanaya T, et al. Detection of human telomerase reverse transcriptase messenger RNA in voided urine samples as a useful diagnostic tool for bladder cancer. Clin Cancer Res 1998;4:2807–10.
21. Utting M, Werner W, Dahse R, et al. Microsatellite analysis of free tumor DNA in urine, serum, and plasma of patients: a minimally invasive method for the detection of bladder cancer. Clin Cancer Res 2002;8:35–40.
22. Sethakorn N, O’Donnell PH. Spectrum of genomic alterations in FGFR3: current appraisal of the potential role of FGFR3 in advanced urothelial carcinoma. BJU Int 2016;118:681–91.
23. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66.
24. Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer ‘unfit’ for cisplatin-based chemotherapy. J Clin Oncol 2011;29:2432–8.
25. Raj GV, Iasonos A, Herr H, Donat SM. Formulas calculating creatinine clearance are inadequate for determining eligibility for cisplatin-based chemotherapy in bladder cancer. J Clin Oncol 2006;24:3095–100.26. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 2003;361:1927–34.
27. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data. Eur Urol 2005;48:202–6.
28. Sternberg CN. A critical review of the management of bladder cancer. Crit Rev Oncol Hematol 1999;31:193–207.
29. Sternberg CN, Yagoda A, Scher HI, et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol 1985;133:403–7.
30. Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 2006;42:50–4.
31. Sternberg CN, de Mulder PHM, Schornagel JH, et al. Randomized phase III trial of high–dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol No. 30924. J Clin Oncol 2001;19:2638–46.
32. Soloway MS, Einstein A, Corder MP, et al. A comparison of cisplatin and the combination of cisplatin and cyclophosphamide in advanced urothelial cancer. A National Bladder Cancer Collaborative Group A Study. Cancer 1983;52:767–72.
33. Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 2014;32:1895–901.
34. Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 2014;4:1140–53.
35. Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol 2015;68:959–67.
36. Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol 2015;16:76–86.
37. Mak RH, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 2014;32:3801–9.
38. von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18:3068–77.
39. Flaig T, Spiess P, Agarwal N, et al. National Comprehensive Cancer Network. Bladder cancer (version 3.2018). 2018. www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed May 5, 2018.
40. von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005;23:4602–8.
41. Bamias A, Dafni U, Karadimou A, et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: a Hellenic Cooperative Oncology Group study (HE 16/03). Ann Oncol 2013;24:1011–7.
42. Li R, Metcalfe M, Kukreja J, Navai N. Role of radical cystectomy in non-organ confined bladder cancer: a systematic review. Bladder Cancer 2018;4:31–40.
43. De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012;30:191–9.
44. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67–76.
45. Balar A V, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:1483–92.
46. Manoharan M, Ayyathurai R, Soloway MS. Radical cystectomy for urothelial carcinoma of the bladder: an analysis of perioperative and survival outcome. BJU Int 2009;104:1227–32.
47. Bellmunt J, de Wit R, Vaughn D, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26.
48. Bajorin D, de Wit R, Vaughn D, et al. Planned survival analysis from KEYNOTE-045: Phase 3, open-label study of pembrolizumab (pembro) versus paclitaxel, docetaxel, or vinflunine in recurrent, advanced urothelial cancer (UC). (Abstract 4501). J Clin Oncol 2017;35(15_suppl):4501-4501.
49. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391:748–57.
50. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312–22.
51. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018;19:51–64.
52. Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma. JAMA Oncol 2017;3:e172411.
53. Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009;27:4454–61.
54. Petrylak DP, de Wit R, Chi KN, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): a randomised, double-blind, phase 3 trial. Lancet 2017;390:2266–77.
55. Abufaraj M, Dalbagni G, Daneshmand S, et al. The role of surgery in metastatic bladder cancer: a systematic review. Eur Urol 2018;73:543–57.
56. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68.
57. Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999;17:3173–81.
58. Mayr R, May M, Martini T, et al. Comorbidity and performance indices as predictors of cancer-independent mortality but not of cancer-specific mortality after radical cystectomy for urothelial carcinoma of the bladder. Eur Urol 2012;62:662–70.
59. Nakagawa T, Hara T, Kawahara T, et al. Prognostic risk stratification of patients with urothelial carcinoma of the bladder with recurrence after radical cystectomy. J Urol 2013;189:1275–81.
60. Ploeg M, Kums AC, Aben KK, et al. Prognostic factors for survival in patients with recurrence of muscle invasive bladder cancer after treatment with curative intent. Clin Genitourin Cancer 2011;9:14–21.
61. Saxman SB, Propert KJ, Einhorn LH, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 1997;15:2564–9.
62. Lin CC, Hsu CH, Huang CY, et al. Prognostic factors for metastatic urothelial carcinoma treated with cisplatin and 5-fluorouracil-based regimens. Urology 2007;69:479–84.
63. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2:187–93.
Introduction
Bladder cancer is by far the most common cancer of the urinary system. Worldwide, approximately 450,000 new cases are diagnosed and 165,000 deaths are caused by bladder cancer each year.1 In the United States and in Europe, the most common type of bladder cancer is urothelial carcinoma (also referred to as transitional cell carcinoma), which accounts for more than 90% of all bladder cancers in these regions of the world. The remainder of bladder cancers are divided among squamous cell carcinomas, adenocarcinomas, small cell carcinomas, and, even more rarely, between various other nonepithelial tumors (eg, sarcoma).
Bladder cancer is classically thought of as a disease of the elderly, with a median age at diagnosis of 69 years in men and 71 years in women.2 The incidence of bladder cancer increases with age: in persons aged 65 to 69 years, incidence is 142 per 100,000 men and 33 per 100,000 women, and in those older than 85 years the rate doubles to 296 per 100,000 men and 74 per 100,000 women.3 The incidence is 3 times greater in men than in women.4
Urothelial carcinoma is traditionally categorized by its degree of invasion into the bladder wall: superficial (non-muscle-invasive), muscle-invasive, or metastatic disease. At the time of diagnosis, most patients have non-muscle-invasive disease (~60%); about 4% of all patients present initially with metastatic disease.5 This article focuses on metastatic bladder cancer, but muscle-invasive disease is discussed as well.
The most important factor contributing to the development of urothelial carcinoma is tobacco smoking. The risk of developing bladder cancer is 4 to 5 times higher in smokers as compared to nonsmokers, with some variation according to sex.6 Quantity of smoking exposure also plays a role, with heavy smokers demonstrating a higher likelihood for high-grade tumors with muscle invasion (or beyond) when compared to light smokers.7 Another important risk factor is occupational exposure to industrial materials, such as carpets, paints, plastics, and industrial chemicals. This type of exposure may be responsible for, or at least contribute to, the development of approximately 20% of urothelial carcinomas. Other risk factors for urothelial carcinoma include but are not limited to prior radiation to the pelvis, prior upper tract urothelial malignancy, human papillomavirus infection, and prior bladder augmentation.
Diagnosis and Staging
Case Presentation
A 63-year-old man with a past medical history of diabetes, deep vein thrombosis, occasional alcohol use, and regular pipe tobacco use presents to his primary care physician with complaints of hematuria. He reports that his urine was a dark red color that morning, which had never happened before. The patient is hemodynamically stable upon evaluation in the office, and a point-of-care urinalysis dipstick is strongly positive for blood. He is referred to a urologist for further evaluation.
In the urology office, urine microscopy is notable for more than 50 red blood cells (RBCs) per high-power field with normal RBC morphology. Flexible cystoscopy performed in the office reveals a single 2-cm, sessile, verrucous, nodular lesion located on the anterior bladder wall. A urine sample and a bladder wash specimen are sent for cytology evaluation. The patient is scheduled to undergo a complete transurethral resection of bladder tumor (TURBT) later that week with samples sent to pathology for evaluation.
- What are the clinical features of bladder cancer?
Hematuria is the most common presentation of bladder cancer, although its specificity is far lower than traditionally thought. In fact, only about 2% to 20% of cases that present with hematuria are found to be caused by malignancy. However, the incidence of genitourinary tract malignancy is much higher in patients presenting with gross hematuria (10%–20%)8–10 than in patients with microscopic hematuria alone.8,10–14 Typically, hematuria associated with malignancy is painless. Multiple studies have shown, however, that hematuria can be a normal variant, with one study demonstrating that up to 61% of patients with hematuria had no identifiable abnormality.8,10,11,13
Abdominal pain, flank pain, dysuria, urinary frequency/urgency, or other irritative voiding symptoms in the absence of hematuria can be presenting symptoms of bladder cancer as well. In these settings, discomfort typically suggests more advanced malignancy with at least local involvement or obstruction. Suprapubic pain may herald invasion into perivesical tissues and nerves, while involvement of the obturator fossa, perirectal fat, urogenital diaphragm, or presacral nerves can often present with perineal or rectal pain. Similarly, lower abdominal pain may represent involvement of lymph nodes, and right upper quadrant pain may signal liver metastasis. Cough or shortness of breath may signify metastatic disease in the lung. Finally, back, rib, or other boney pain may suggest distant metastasis.
- What next steps are required to complete this patient’s staging?
White light cystoscopy remains the gold standard for diagnosis and initial staging of bladder cancer. Additional tools include urine cytology and upper tract studies, including renal computed tomography (CT) urograms. Full urologic evaluation with all 3 modalities (cystourethroscopy, urinary cytology, and upper tract evaluation) is warranted for patients with a high suspicion for malignant etiology of hematuria. CT urograms are particularly useful for upper tract evaluation because they can be used to visualize kidney parenchyma, both renal pelvises and ureters, and pertinent abdominal and pelvic lymph nodes. Initial staging is completed through TURBT, which should ideally contain a segment of muscularis propria to distinguish between Ta (noninvasive), T1, and T2 tumors (Figure 1).
Regarding staging, T1 tumors are distinguished from Ta malignancies by their involvement in the urothelial basement membrane. Tumor invasion into the muscularis propria indicates T2 tumors, while T3 tumors extend through the muscle into the serosa and involve the complete thickness of the bladder wall. Involvement of nearby structures defines T4 bladder cancers, with T4a malignancies involving adjacent organs (prostate, vagina, uterus, or bowel) and T4b tumors involving the abdominal wall, pelvic wall, or other more distant organs. According to the American Joint Committee on Cancer’s most recent TNM staging system (Table 1),16 lymph node involvement in the true pelvis (that is, N1–N3) with T1 to T4a disease is now classified as stage III disease.
Bladder cancer is often broadly categorized as either non-muscle-invasive or muscle-invasive (which can include metastatic disease). This classification has important implications for treatment. As such, all diagnostic biopsies should be performed with the goal of reaching at least the depth of the muscularis propria in order to accurately detect potential muscularis invasion. If no muscle is detected in the initial specimen, re-resection is recommended if safe and feasible. In cases where muscle cannot be obtained, imaging evidence of T3 disease from CT or magnetic resonance imaging may be used as a surrogate indicator. Once muscle-invasive disease is confirmed, CT evaluation of the chest is also recommended, as bladder cancer can metastasize to the lungs; furthermore, patients are often at risk for secondary concomitant lung cancers given that smoking is the most prevalent risk factor for both. However, patients with small, indeterminate lung nodules not amenable to biopsy should not be denied curative intent treatment given the high likelihood that they represent benign findings.17
Pathogenesis
Because non-muscle-invasive and muscle-invasive tumors behave so differently, they are thought to arise from 2 distinct mechanisms. Although there is overlap and non-muscle-invasive cancer can certainly progress to a high-grade, invasive type of malignancy over time, current theory proposes that non-muscle-invasive bladder cancer predominantly develops just from urothelial hyperplasia, which then recruits branching vasculature to grow slowly. More aggressive urothelial carcinomas, including muscle-invasive and metastatic disease, are instead thought to arise directly from flat dysplasia that progresses to carcinoma in situ, and is much more prone to invasive growth and distant spread.18
Regardless of grade and stage, the most commonly identified genomic alterations in urothelial carcinoma are mutations in the promoter region of the telomerase reverse transcriptase (TERT) gene, which have been identified in approximately 70% of cases.19 Mutations in TERT can be readily detected in urine sediments and may ultimately have implications for diagnosis and early detection.20,21 In current practice, however, the clinical relevance of these observations remains under development. Other genomic alterations that may contribute to the development of urothelial carcinoma, and also provide new potential therapeutic targets, include alterations in the TP53 gene, the RB (retinoblastoma) gene, and the FGFR3 (fibroblast growth factor receptor) gene. FGFR3 has particular significance as it appears to be relatively common in non-muscle invasive disease (up to 60%–70%) and is likely an actionable driver mutation that may define a particular molecular subset of urothelial carcinoma; thus, it may have important implications for treatment decisions.22
Treatment
Case Continued
Pathologic evaluation of the specimen reveals a high-grade urothelial carcinoma with tumor invasion into the muscularis propria. A CT urogram is performed and does not reveal any notably enlarged pelvic nodes or suspicious lesions in the upper urinary tract. CT chest does not reveal any evidence of distant metastatic disease. Given the presence of muscle-invasive disease, the patient agrees to proceed with neoadjuvant chemotherapy and radical cystoprostatectomy with pelvic node dissection. He undergoes treatment with dose-dense (accelerated) MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) for 3 cycles, followed by surgery with cystoprostatectomy. Overall, he tolerates the procedure well and recovers quickly. Pathology reveals the presence of disease in 2 regional nodes, consistent with T4a (stage III) disease, and a small degree of residual disease in the bladder. He is followed closely in the oncology clinic, returning for urine cytology, liver and renal function tests, and imaging with CT of chest, abdomen, and pelvis every 3 months.
- What is the first-line approach to management in patients with muscle-invasive disease?
- How would the treatment strategy differ if the patient had presented with metastatic disease (stage IV)?
First-Line Management for Curative Intent: Muscle-Invasive Disease
Muscle-invasive urothelial carcinoma (including T2, T3, or T4 disease) is typically treated in a multidisciplinary fashion with neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy. This approach is recommended over radical cystectomy alone because of high relapse rates following cystectomy alone, even in the setting of bilateral pelvic lymphadenectomy.23 However, because of the associated short- and long-term toxicity of cisplatin-based regimens, this optimal treatment paradigm is reserved for patients deemed cisplatin-eligible.
Medical fitness to receive cisplatin-based chemotherapy is assessed by a number of factors and varies by institution, but most frequently consider functional status (Eastern Cooperative Oncology Group [ECOG] performance status or Karnofsky Performance Status), creatinine clearance, hearing preservation, peripheral neuropathy, and cardiac function.24 Many programs will elect to defer cisplatin-based chemotherapy in patients with low performance status (ie, < 60–70 on Karnofsky scale or > 2 on ECOG scale), creatinine clearance below 60 mL/min, or significant heart failure (NHYA class III or worse). Cisplatin-based chemotherapy may worsen hearing loss in those with hearing loss of 25 dB from baseline at 2 continuous frequencies and also may worsen neuropathy in those with baseline grade 1 peripheral neuropathy. However, these adverse outcomes must be balanced against the curative intent of the multimodality systemic approach.
In patients with renal insufficiency, caution must be taken with regard to cisplatin. Percutaneous nephrostomy placement or ureteral stenting should be attempted to relieve any ureteral outlet obstruction and restore kidney function if a patient’s renal insufficiency has resulted from this obstruction. If medical renal disease or long-term renal insufficiency is present, however, patients should instead be referred for immediate cystectomy or for a bladder-preserving approach. Generally, a creatinine clearance of 60 mL/min is required to safely receive cisplatin-based chemotherapy, although some advocate for treatment with a creatinine clearance as low as 50 mL/min. When this extended criterion is used, the dose of cisplatin may be split over 2 days to minimize renal toxicity and maximize hydration. Analysis of renal function utilizing a 24-hour urine collection should be incorporated whenever possible, as estimates of creatinine clearance have been demonstrated to be inaccurate in some instances.25
For cisplatin-eligible patients, neoadjuvant chemotherapy with a cisplatin base has consistently demonstrated a survival benefit when given prior to surgery.26,27 Historically, several different platinum-based regimens have been studied, with none showing superior effectiveness in a randomized trial over the others in the neoadjuvant setting. These regimens have included classic MVAC, dose-dense MVAC (MVAC with pegfilgrastim), GC (gemcitabine and cisplatin), and CMV (methotrexate, vinblastine, cisplatin, and leucovorin).
While classic MVAC was preferred in the 1990s and early 2000s,28,29 the availability of growth factor, such as pegfilgrastim, has made dose-dense MVAC (otherwise referred to as accelerated MVAC or ddMVAC) widely preferred and universally recommended over classic MVAC. The ddMVAC regimen with the addition of a synthetic granulocyte colony-stimulating factor (G-CSF) is substantially better tolerated than classic MVAC, as the G-CSF support minimizes the severe toxicities of classic MVAC, such as myelosuppression and mucositis, and allows for the administration of drugs in a dose-dense fashion.30,31
Both ddMVAC and GC are considered reasonable options for neoadjuvant chemotherapy and are the predominant choices for cisplatin-eligible patients (Table 2).
Prospective data defining the role of adjuvant chemotherapy for patients after cystectomy has been fraught by a variety of factors, including the known benefit of neoadjuvant chemotherapy, the high complication rate of cystectomy making chemotherapy infeasible, and clinician bias that has hampered accrual in prior trials. Thus, no level 1 evidence exists defining the benefit of adjuvant chemotherapy in patients who did not receive neoadjuvant therapy. In a report of the largest study performed in this setting, there was a statistically significant benefit in PFS but not in OS.36 Criticisms of this trial include its lack of statistical power due to a failure to accrue the targeted goal and the preponderance of node-positive patients. Regardless, for patients who have pT2–4, N1 disease after radical cystectomy and remain cisplatin-eligible after not receiving neoadjuvant chemotherapy, this remains an option.
Despite the established clinical dogma surrounding neoadjuvant chemotherapy followed by surgery, some patients are either not eligible for or decline to receive radical cystectomy, while others are not candidates for neoadjuvant cisplatin-based chemotherapy for the reasons outlined above. For patients who are surgical candidates but unable to receive neoadjuvant chemotherapy due to renal or cardiac function, they may proceed directly to surgery. For patients unable or unwilling to proceed to radical cystectomy regardless, bladder preservation strategies exist. Maximal TURBT may be an option for some patients, but, as outlined above, used alone this would be likely to lead to a high degree of local and distant failure. Combined modality chemoradiotherapy as consolidation after maximal TURBT is an established option for patients unable to undergo surgery or seeking bladder preservation. Several trials have demonstrated encouraging outcomes with this approach and were highlighted in a large meta-analysis.37 Various chemosensitizing chemotherapeutic regimens have been evaluated, including cisplatin alone or as a doublet, gemcitabine alone, and 5-fluouracil plus mitomycin C, but no randomized studies have compared these regimens to each other, nor have they been compared to surgical approaches. However, this strategy remains an option as an alternative to surgery.
First-Line Management: Metastatic Disease
The approach to therapy in patients who present with metastatic urothelial carcinoma is very similar to that used in neoadjuvant perioperative chemotherapy. The consensus first-line treatment in medically appropriate patients is cisplatin-based chemotherapy with either GC or ddMVAC (both category 1 National Comprehensive Cancer Network [NCCN] recommendations; Figure 2).30,31,38–40
Head-to-head studies specifically comparing ddMVAC and GC have been limited. GC has been compared to classic MVAC, with results showing equivalent efficacy but improved tolerability, as expected.38,40 ddMVAC was compared with a modified version of GC (termed “dose-dense GC”) in a phase 3 study from Greece, which demonstrated similar outcomes.41
Surgical intervention with radical cystectomy and regional lymph node dissection is typically deferred for patients who present with distant metastatic disease, unlike those who present with locally advanced disease. Radical cystectomy has traditionally been thought of as overly aggressive without sufficient benefit, although evidence to guide this approach remains sparse.42 As such, most expert recommendations and consensus statements simply recommend against surgical intervention and leave the decision between ddMVAC and GC up to the individual clinician.
In patients who are not eligible for cisplatin therapy, it is reasonable to consider chemotherapy with a combination of gemcitabine and carboplatin. This combination has been shown to be equivalent to MCAVI (methotrexate, carboplatin, vinblastine) in terms of overall survival (OS; 9 months versus 8 months) and progression-free survival (PFS; 6 months versus 4 months) with significantly fewer serious toxicities (9% versus 21%).43
The advent of immunotherapy in recent years has provided several new alternatives for cisplatin-ineligible patients. While immunotherapies such as pembrolizumab or atezolizumab are not yet recommended as first-line therapy for cisplatin-eligible patients, these 2 drugs are approved as options for first-line therapy in cisplatin-ineligible patients with metastatic disease. In a recent phase 2 trial (IMvigor210) involving 119 patients who were given atezolizumab as first-line therapy, median PFS was 2.7 months and median OS was 15.9 months.44 Another trial using data from patients in the KEYNOTE-052 study who received pembrolizumab as first-line therapy demonstrated antitumor activity with pembrolizumab and acceptable tolerability in cisplatin-ineligible patients with advanced urothelial carcinoma.45 The primary endpoint was objective response (either complete or partial response), which was achieved in 24% of the intention-to-treat population. Median PFS was 2 months, and 6-month OS was observed in 67% of patients. Both atezolizumab and pembrolizumab were given accelerated approval based on these single-arm studies in this setting. However, due to inferior outcomes in subsequent trials that included single-agent immunotherapy arms for patients in the first-line setting, the US Food and Drug Administration (FDA) has clarified the approval. In the subsequent trials, patients with a low PD-L1 biomarker based on the individual assay used for each drug did worse on immunotherapy alone (compared to chemotherapy or both combined), and the single-therapy arms were stopped early. Thus, the FDA now recommends that pembrolizumab or atezolizumab be used in the first line only for cisplatin-ineligible patients who have PD-L1 expression on tumor cells above the threshold studied on each individual assay, or are unfit for any platinum-based chemotherapy. Further study regarding the optimal role of biomarkers and chemotherapy-immunotherapy combinations is ongoing.
Case Continued
Ten months after his procedure, the patient is found to have prominent retroperitoneal lymphadenopathy and a 1.0-cm liver nodule suspicious for malignancy is noted on surveillance imaging. CT-guided biopsy of the liver reveals high-grade urothelial carcinoma, consistent with both recurrence and distant metastasis. The patient is informed that he needs to resume systemic therapy for recurrent metastatic disease. The options discussed include salvage single-agent chemotherapy with gemcitabine or immunotherapy with pembrolizumab. He elects to move forward with immunotherapy and is scheduled to begin pembrolizumab.
- What other immunotherapies might this patient consider for second-line therapy?
- Is chemotherapy a second-line option for this patient?
Second-Line Therapies and Management of Progressive Disease
Disease progression is unfortunately seen in the majority of cases of advanced urothelial carcinoma.46 New second-line therapies have recently been approved by the FDA in the form of monoclonal antibodies targeting programmed death 1 (PD-1) and a PD-1 ligand (PD-L1) (Figure 3).
Approval of pembrolizumab, a PD-1 inhibitor, was largely supported by the Keynote-045 trial,47,48 which looked at 542 patients who had progressed or recurred after platinum-based chemotherapy. These patients were randomly assigned to either pembrolizumab or investigator’s choice of chemotherapy (paclitaxel, docetaxel, or vinflunine). Patients treated with pembrolizumab had a significantly improved OS (median of 10.3 months versus 7.4 months), but no statistically significant difference in PFS (2.1 months versus 3.3 months). Interestingly, the rate of responses of 12 months or longer was higher with pembrolizumab than with more traditional second-line chemotherapy (68% versus 35%). The strength of this data has led to a category 1 recommendation in the most recent NCCN guidelines.39
The approval of atezolizumab, a PD-L1 inhibitor, as a second-line therapy for advanced urothelial carcinoma is largely supported by data from IMvigor211, a phase 3 trial that studied 931 patients randomly assigned to atezolizumab or investigator’s choice chemotherapy. OS did not differ significantly between patients in the atezolizumab group who had ≥ 5% expression of PD-L1 on tumor-infiltrating immune cells and patients in the chemotherapy group (11.1 months versus 10.6 months), but mean duration of response was longer (15.9 months versus 8.3 months).49 Therapy with atezolizumab had significantly fewer toxicities than chemotherapy (grade 3 or 4 toxicities of 20% versus 43%).
Phase 3 studies of nivolumab (PD-1 inhibitor), avelumab (PD-L1 inhibitor), and durvalumab (PD-L1 inhibitor) have not yet been published. These agents have received accelerated approval, however, as second-line treatment of advanced urothelial carcinoma based on promising data from phase 1 and phase 2 studies.50–52
Second-line chemotherapy is also an option for patients who do not qualify for immunotherapy or who progress during or after immunotherapy. Although there has been a great deal of excitement about new developments with immunotherapy and the survival benefit seen compared to investigator’s choice chemotherapy, the fact remains that most patients do not respond to immunotherapy. Still, some patients do derive benefit from single-agent chemotherapy in the platinum-refractory setting. Options based on primarily single-arm studies include gemcitabine, paclitaxel, docetaxel, pemetrexed, ifosfamide, oxaliplatin, and eribulin (Figure 2). In a randomized phase 3 trial, vinflunine demonstrated an OS benefit in platinum-refractory patients compared to best supportive care; it subsequently received approval by the European Medicines Agency.53 More recently in the phase 3 RANGE trial, docetaxel plus ramucirumab (a monoclonal antibody targeting vascular endothelial growth factor receptor 2) was compared to docetaxel plus placebo and met its primary endpoint of an improvement in PFS (median 4.07 months versus 2.76 months, P = 0.0118).54 OS has not been reported and this regimen has not yet received regulatory approval, however. Unfortunately, trials comparing these regimens are lacking, and response rates and survival remain modest. Clearly, better therapies and biomarkers to help personalize treatment options are needed.
Further investigations are underway with alternative regimens, including but not limited to targeted therapy in the setting of specific genetic and epigenetic alterations. These include mutations affecting tyrosine kinase receptors (eg, RAS/RAF, PI3K, AKT, and mTOR), cell cycle regulators (eg, TP53 or RB1), FGFR3 mutations, PTEN deletions, gene amplifications (eg, FGFR1, CCND1, and MDM2), or changes in genes responsible for chromatin remodeling (eg, UTX, CHD6, or ARID1A). As noted, there is particular excitement regarding FGFR3 inhibitors, which have shown compelling efficacy in phase 1 and 2 single-arm trials. Several agents are being evaluated in randomized trials and represent a potential path to the first targeted therapeutic class with a role in urothelial malignancies.
Surgical resection of metastases may be considered in very select cases.55 Surgery may have a role in limiting metastatic complications and improving cancer control, but this should be discussed at length with the patient using a multidisciplinary approach with careful restaging prior to surgery.
Case Continued
The patient remains on pembrolizumab every 3 weeks as per protocol with regular surveillance imaging. His disease stabilizes as the nodule in his liver and the retroperitoneal lymph nodes, all representing metastatic disease, became slightly smaller in size without evidence of any new disease. He continues to follow up closely with his genitourinary oncologist, undergoing regular surveillance and imaging every 3 months without evidence of disease progression.
Approximately 12 months into therapy, the patient notices a nonproductive cough with progressive and rapidly worsening shortness of breath. He is noted to be hypoxic with oxygen saturation levels to 79% in clinic and is sent immediately to the emergency department by his oncologist. Diffuse bilateral reticular opacities are noted on chest radiograph. Non-contrast CT scan demonstrates diffuse ground-glass opacities consistent with acute respiratory distress syndrome–pattern pneumonitis. He is admitted to the intensive care unit.
The patient is aggressively treated with high-flow nasal oxygen supplementation, intravenous steroids, and empiric antibiotics. He slowly improves on high-dose steroids (methylprednisolone 1 mg/kg/day) without requiring intubation or infliximab therapy and is discharged home in stable condition after 10 days. Oral steroid therapy is continued with a long taper over 6 weeks. In the setting of his grade 3 pneumonitis, pembrolizumab is discontinued and the patient is scheduled for a follow-up appointment with his oncologist to discuss next steps.
- In addition to pneumonitis, what other toxicities should you monitor for in patients treated with an immune checkpoint inhibitor?
- Is this patient a candidate to receive immunotherapy again in the future?
Treatment Toxicities
As use of immune checkpoint inhibitors has become more prevalent, the medical community has become increasingly aware of various immune-related adverse effects (irAE) associated with these drugs. These toxicities can be seen in virtually any organ system, and even vague complaints that arise years after therapy initiation should be treated with a high level of suspicion. The most commonly affected organ systems include the skin, gastrointestinal (GI) tract, lungs, liver, and endocrine system, although all other organ systems can be involved (Table 3) and toxicities appear to be similar across individual drugs.
The American Society of Clinical Oncology recently published a complete set of recommendations to guide clinicians on appropriate treatment strategies for each manifestation of immunotherapy-related toxicity.56 The details of these recommendations largely fall outside the purview of this article, but the mainstays of management are worth noting. These include high-dose systemic glucocorticoids, along with supportive care and cessation of immunotherapy in grade 3 or 4 toxicities. Infliximab is frequently recommended as an adjunct in severe or refractory cases.
Chemotherapy-related toxicities, on the other hand, are well-described and tend to be more familiar to patients and clinicians (Table 3). Classic MVAC, which has now been largely replaced by ddMVAC, was notoriously difficult to tolerate. It was known for a high rate of serious (grade 3 or 4) myelosuppressive complications as well as frequent GI toxicities. These complications include neutropenia (57%), stomatitis (10%), and nausea and vomiting (6%).23 ddMVAC with growth factor support is much better tolerated than classic MVAC. Prominent complaints with ddMVAC still can include nausea, GI distress, mucositis, and fatigue, but the incidence of myelosuppressive complications in particular has markedly decreased. GC is largely well tolerated, with minimal nausea and manageable myelotoxicity, but it is associated with an increased risk of venous thromboembolism.38
Prognosis
Case Conclusion
After returning home, the patient discusses his complicated medical course with his oncologist. Given his continued high quality of life with good functional status, he requests to continue with therapy for his metastatic bladder cancer and is interested in joining a clinical trial. He is referred to a nearby academic center with openings in a clinical trial for which he would be eligible. In the meantime, his oncologist guides him through filling out an advance directive and recommends that he make an appointment with palliative care services to ensure adequate home support for any future needs he may have.
- What is the estimated 5-year survival rate for patients with metastatic bladder cancer?
Overall, prognosis in patients with metastatic bladder cancer remains poor. Median survival in patients being treated with multi-agent chemotherapy is approximately 15 months,38,40 with an expected 5-year survival of just 15%. This is much improved, however, as prior to the advent of modern chemotherapy estimated survival was just 6 months with metastatic bladder cancer. Importantly, these figures do not take into account the recent advancements with immunotherapy, and thus it is reasonable to assume survival rates may continue to improve. In light of these recent advances, it is strongly recommended that whenever possible patients and clinicians consider participation in clinical trials to continue uncovering new and better therapies moving forward.
A number of tools have been developed to help risk stratify patients based on comorbidity, performance status, and other characteristics, but none have been universally adopted.57–60 As with many other malignancies, performance status is an important predictor of clinical outcomes in these patients.61–63 Sites of metastasis also may serve to suggest the course of disease. Patients with visceral metastases typically exhibit significantly worse disease with a shortened survival. The role of molecular factors as prognostic markers in bladder cancer is still under investigation. Many biomarkers are being considered (including mutations and polymorphisms in p53, ERCC1, and ERCC2), and evidence suggests some may have a role in prognosis; thus far, none have been validated as prognostic or predictive tools in urothelial carcinoma.
Conclusion
Bladder cancer includes an aggressive group of genitourinary tract malignancies, of which urothelial carcinoma is by far the most common in the Western world. Cisplatin-based therapy remains a mainstay of treatment for eligible patients with both localized and metastatic disease, but immunotherapies have provided a new and promising tool to use in the setting of progressing malignancy. The individual impact of these agents on OS is still being examined. Further studies and ongoing participation in clinical trials whenever possible continue to be essential to the discovery of future treatment options for this highly aggressive disease.
Introduction
Bladder cancer is by far the most common cancer of the urinary system. Worldwide, approximately 450,000 new cases are diagnosed and 165,000 deaths are caused by bladder cancer each year.1 In the United States and in Europe, the most common type of bladder cancer is urothelial carcinoma (also referred to as transitional cell carcinoma), which accounts for more than 90% of all bladder cancers in these regions of the world. The remainder of bladder cancers are divided among squamous cell carcinomas, adenocarcinomas, small cell carcinomas, and, even more rarely, between various other nonepithelial tumors (eg, sarcoma).
Bladder cancer is classically thought of as a disease of the elderly, with a median age at diagnosis of 69 years in men and 71 years in women.2 The incidence of bladder cancer increases with age: in persons aged 65 to 69 years, incidence is 142 per 100,000 men and 33 per 100,000 women, and in those older than 85 years the rate doubles to 296 per 100,000 men and 74 per 100,000 women.3 The incidence is 3 times greater in men than in women.4
Urothelial carcinoma is traditionally categorized by its degree of invasion into the bladder wall: superficial (non-muscle-invasive), muscle-invasive, or metastatic disease. At the time of diagnosis, most patients have non-muscle-invasive disease (~60%); about 4% of all patients present initially with metastatic disease.5 This article focuses on metastatic bladder cancer, but muscle-invasive disease is discussed as well.
The most important factor contributing to the development of urothelial carcinoma is tobacco smoking. The risk of developing bladder cancer is 4 to 5 times higher in smokers as compared to nonsmokers, with some variation according to sex.6 Quantity of smoking exposure also plays a role, with heavy smokers demonstrating a higher likelihood for high-grade tumors with muscle invasion (or beyond) when compared to light smokers.7 Another important risk factor is occupational exposure to industrial materials, such as carpets, paints, plastics, and industrial chemicals. This type of exposure may be responsible for, or at least contribute to, the development of approximately 20% of urothelial carcinomas. Other risk factors for urothelial carcinoma include but are not limited to prior radiation to the pelvis, prior upper tract urothelial malignancy, human papillomavirus infection, and prior bladder augmentation.
Diagnosis and Staging
Case Presentation
A 63-year-old man with a past medical history of diabetes, deep vein thrombosis, occasional alcohol use, and regular pipe tobacco use presents to his primary care physician with complaints of hematuria. He reports that his urine was a dark red color that morning, which had never happened before. The patient is hemodynamically stable upon evaluation in the office, and a point-of-care urinalysis dipstick is strongly positive for blood. He is referred to a urologist for further evaluation.
In the urology office, urine microscopy is notable for more than 50 red blood cells (RBCs) per high-power field with normal RBC morphology. Flexible cystoscopy performed in the office reveals a single 2-cm, sessile, verrucous, nodular lesion located on the anterior bladder wall. A urine sample and a bladder wash specimen are sent for cytology evaluation. The patient is scheduled to undergo a complete transurethral resection of bladder tumor (TURBT) later that week with samples sent to pathology for evaluation.
- What are the clinical features of bladder cancer?
Hematuria is the most common presentation of bladder cancer, although its specificity is far lower than traditionally thought. In fact, only about 2% to 20% of cases that present with hematuria are found to be caused by malignancy. However, the incidence of genitourinary tract malignancy is much higher in patients presenting with gross hematuria (10%–20%)8–10 than in patients with microscopic hematuria alone.8,10–14 Typically, hematuria associated with malignancy is painless. Multiple studies have shown, however, that hematuria can be a normal variant, with one study demonstrating that up to 61% of patients with hematuria had no identifiable abnormality.8,10,11,13
Abdominal pain, flank pain, dysuria, urinary frequency/urgency, or other irritative voiding symptoms in the absence of hematuria can be presenting symptoms of bladder cancer as well. In these settings, discomfort typically suggests more advanced malignancy with at least local involvement or obstruction. Suprapubic pain may herald invasion into perivesical tissues and nerves, while involvement of the obturator fossa, perirectal fat, urogenital diaphragm, or presacral nerves can often present with perineal or rectal pain. Similarly, lower abdominal pain may represent involvement of lymph nodes, and right upper quadrant pain may signal liver metastasis. Cough or shortness of breath may signify metastatic disease in the lung. Finally, back, rib, or other boney pain may suggest distant metastasis.
- What next steps are required to complete this patient’s staging?
White light cystoscopy remains the gold standard for diagnosis and initial staging of bladder cancer. Additional tools include urine cytology and upper tract studies, including renal computed tomography (CT) urograms. Full urologic evaluation with all 3 modalities (cystourethroscopy, urinary cytology, and upper tract evaluation) is warranted for patients with a high suspicion for malignant etiology of hematuria. CT urograms are particularly useful for upper tract evaluation because they can be used to visualize kidney parenchyma, both renal pelvises and ureters, and pertinent abdominal and pelvic lymph nodes. Initial staging is completed through TURBT, which should ideally contain a segment of muscularis propria to distinguish between Ta (noninvasive), T1, and T2 tumors (Figure 1).
Regarding staging, T1 tumors are distinguished from Ta malignancies by their involvement in the urothelial basement membrane. Tumor invasion into the muscularis propria indicates T2 tumors, while T3 tumors extend through the muscle into the serosa and involve the complete thickness of the bladder wall. Involvement of nearby structures defines T4 bladder cancers, with T4a malignancies involving adjacent organs (prostate, vagina, uterus, or bowel) and T4b tumors involving the abdominal wall, pelvic wall, or other more distant organs. According to the American Joint Committee on Cancer’s most recent TNM staging system (Table 1),16 lymph node involvement in the true pelvis (that is, N1–N3) with T1 to T4a disease is now classified as stage III disease.
Bladder cancer is often broadly categorized as either non-muscle-invasive or muscle-invasive (which can include metastatic disease). This classification has important implications for treatment. As such, all diagnostic biopsies should be performed with the goal of reaching at least the depth of the muscularis propria in order to accurately detect potential muscularis invasion. If no muscle is detected in the initial specimen, re-resection is recommended if safe and feasible. In cases where muscle cannot be obtained, imaging evidence of T3 disease from CT or magnetic resonance imaging may be used as a surrogate indicator. Once muscle-invasive disease is confirmed, CT evaluation of the chest is also recommended, as bladder cancer can metastasize to the lungs; furthermore, patients are often at risk for secondary concomitant lung cancers given that smoking is the most prevalent risk factor for both. However, patients with small, indeterminate lung nodules not amenable to biopsy should not be denied curative intent treatment given the high likelihood that they represent benign findings.17
Pathogenesis
Because non-muscle-invasive and muscle-invasive tumors behave so differently, they are thought to arise from 2 distinct mechanisms. Although there is overlap and non-muscle-invasive cancer can certainly progress to a high-grade, invasive type of malignancy over time, current theory proposes that non-muscle-invasive bladder cancer predominantly develops just from urothelial hyperplasia, which then recruits branching vasculature to grow slowly. More aggressive urothelial carcinomas, including muscle-invasive and metastatic disease, are instead thought to arise directly from flat dysplasia that progresses to carcinoma in situ, and is much more prone to invasive growth and distant spread.18
Regardless of grade and stage, the most commonly identified genomic alterations in urothelial carcinoma are mutations in the promoter region of the telomerase reverse transcriptase (TERT) gene, which have been identified in approximately 70% of cases.19 Mutations in TERT can be readily detected in urine sediments and may ultimately have implications for diagnosis and early detection.20,21 In current practice, however, the clinical relevance of these observations remains under development. Other genomic alterations that may contribute to the development of urothelial carcinoma, and also provide new potential therapeutic targets, include alterations in the TP53 gene, the RB (retinoblastoma) gene, and the FGFR3 (fibroblast growth factor receptor) gene. FGFR3 has particular significance as it appears to be relatively common in non-muscle invasive disease (up to 60%–70%) and is likely an actionable driver mutation that may define a particular molecular subset of urothelial carcinoma; thus, it may have important implications for treatment decisions.22
Treatment
Case Continued
Pathologic evaluation of the specimen reveals a high-grade urothelial carcinoma with tumor invasion into the muscularis propria. A CT urogram is performed and does not reveal any notably enlarged pelvic nodes or suspicious lesions in the upper urinary tract. CT chest does not reveal any evidence of distant metastatic disease. Given the presence of muscle-invasive disease, the patient agrees to proceed with neoadjuvant chemotherapy and radical cystoprostatectomy with pelvic node dissection. He undergoes treatment with dose-dense (accelerated) MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) for 3 cycles, followed by surgery with cystoprostatectomy. Overall, he tolerates the procedure well and recovers quickly. Pathology reveals the presence of disease in 2 regional nodes, consistent with T4a (stage III) disease, and a small degree of residual disease in the bladder. He is followed closely in the oncology clinic, returning for urine cytology, liver and renal function tests, and imaging with CT of chest, abdomen, and pelvis every 3 months.
- What is the first-line approach to management in patients with muscle-invasive disease?
- How would the treatment strategy differ if the patient had presented with metastatic disease (stage IV)?
First-Line Management for Curative Intent: Muscle-Invasive Disease
Muscle-invasive urothelial carcinoma (including T2, T3, or T4 disease) is typically treated in a multidisciplinary fashion with neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy. This approach is recommended over radical cystectomy alone because of high relapse rates following cystectomy alone, even in the setting of bilateral pelvic lymphadenectomy.23 However, because of the associated short- and long-term toxicity of cisplatin-based regimens, this optimal treatment paradigm is reserved for patients deemed cisplatin-eligible.
Medical fitness to receive cisplatin-based chemotherapy is assessed by a number of factors and varies by institution, but most frequently consider functional status (Eastern Cooperative Oncology Group [ECOG] performance status or Karnofsky Performance Status), creatinine clearance, hearing preservation, peripheral neuropathy, and cardiac function.24 Many programs will elect to defer cisplatin-based chemotherapy in patients with low performance status (ie, < 60–70 on Karnofsky scale or > 2 on ECOG scale), creatinine clearance below 60 mL/min, or significant heart failure (NHYA class III or worse). Cisplatin-based chemotherapy may worsen hearing loss in those with hearing loss of 25 dB from baseline at 2 continuous frequencies and also may worsen neuropathy in those with baseline grade 1 peripheral neuropathy. However, these adverse outcomes must be balanced against the curative intent of the multimodality systemic approach.
In patients with renal insufficiency, caution must be taken with regard to cisplatin. Percutaneous nephrostomy placement or ureteral stenting should be attempted to relieve any ureteral outlet obstruction and restore kidney function if a patient’s renal insufficiency has resulted from this obstruction. If medical renal disease or long-term renal insufficiency is present, however, patients should instead be referred for immediate cystectomy or for a bladder-preserving approach. Generally, a creatinine clearance of 60 mL/min is required to safely receive cisplatin-based chemotherapy, although some advocate for treatment with a creatinine clearance as low as 50 mL/min. When this extended criterion is used, the dose of cisplatin may be split over 2 days to minimize renal toxicity and maximize hydration. Analysis of renal function utilizing a 24-hour urine collection should be incorporated whenever possible, as estimates of creatinine clearance have been demonstrated to be inaccurate in some instances.25
For cisplatin-eligible patients, neoadjuvant chemotherapy with a cisplatin base has consistently demonstrated a survival benefit when given prior to surgery.26,27 Historically, several different platinum-based regimens have been studied, with none showing superior effectiveness in a randomized trial over the others in the neoadjuvant setting. These regimens have included classic MVAC, dose-dense MVAC (MVAC with pegfilgrastim), GC (gemcitabine and cisplatin), and CMV (methotrexate, vinblastine, cisplatin, and leucovorin).
While classic MVAC was preferred in the 1990s and early 2000s,28,29 the availability of growth factor, such as pegfilgrastim, has made dose-dense MVAC (otherwise referred to as accelerated MVAC or ddMVAC) widely preferred and universally recommended over classic MVAC. The ddMVAC regimen with the addition of a synthetic granulocyte colony-stimulating factor (G-CSF) is substantially better tolerated than classic MVAC, as the G-CSF support minimizes the severe toxicities of classic MVAC, such as myelosuppression and mucositis, and allows for the administration of drugs in a dose-dense fashion.30,31
Both ddMVAC and GC are considered reasonable options for neoadjuvant chemotherapy and are the predominant choices for cisplatin-eligible patients (Table 2).
Prospective data defining the role of adjuvant chemotherapy for patients after cystectomy has been fraught by a variety of factors, including the known benefit of neoadjuvant chemotherapy, the high complication rate of cystectomy making chemotherapy infeasible, and clinician bias that has hampered accrual in prior trials. Thus, no level 1 evidence exists defining the benefit of adjuvant chemotherapy in patients who did not receive neoadjuvant therapy. In a report of the largest study performed in this setting, there was a statistically significant benefit in PFS but not in OS.36 Criticisms of this trial include its lack of statistical power due to a failure to accrue the targeted goal and the preponderance of node-positive patients. Regardless, for patients who have pT2–4, N1 disease after radical cystectomy and remain cisplatin-eligible after not receiving neoadjuvant chemotherapy, this remains an option.
Despite the established clinical dogma surrounding neoadjuvant chemotherapy followed by surgery, some patients are either not eligible for or decline to receive radical cystectomy, while others are not candidates for neoadjuvant cisplatin-based chemotherapy for the reasons outlined above. For patients who are surgical candidates but unable to receive neoadjuvant chemotherapy due to renal or cardiac function, they may proceed directly to surgery. For patients unable or unwilling to proceed to radical cystectomy regardless, bladder preservation strategies exist. Maximal TURBT may be an option for some patients, but, as outlined above, used alone this would be likely to lead to a high degree of local and distant failure. Combined modality chemoradiotherapy as consolidation after maximal TURBT is an established option for patients unable to undergo surgery or seeking bladder preservation. Several trials have demonstrated encouraging outcomes with this approach and were highlighted in a large meta-analysis.37 Various chemosensitizing chemotherapeutic regimens have been evaluated, including cisplatin alone or as a doublet, gemcitabine alone, and 5-fluouracil plus mitomycin C, but no randomized studies have compared these regimens to each other, nor have they been compared to surgical approaches. However, this strategy remains an option as an alternative to surgery.
First-Line Management: Metastatic Disease
The approach to therapy in patients who present with metastatic urothelial carcinoma is very similar to that used in neoadjuvant perioperative chemotherapy. The consensus first-line treatment in medically appropriate patients is cisplatin-based chemotherapy with either GC or ddMVAC (both category 1 National Comprehensive Cancer Network [NCCN] recommendations; Figure 2).30,31,38–40
Head-to-head studies specifically comparing ddMVAC and GC have been limited. GC has been compared to classic MVAC, with results showing equivalent efficacy but improved tolerability, as expected.38,40 ddMVAC was compared with a modified version of GC (termed “dose-dense GC”) in a phase 3 study from Greece, which demonstrated similar outcomes.41
Surgical intervention with radical cystectomy and regional lymph node dissection is typically deferred for patients who present with distant metastatic disease, unlike those who present with locally advanced disease. Radical cystectomy has traditionally been thought of as overly aggressive without sufficient benefit, although evidence to guide this approach remains sparse.42 As such, most expert recommendations and consensus statements simply recommend against surgical intervention and leave the decision between ddMVAC and GC up to the individual clinician.
In patients who are not eligible for cisplatin therapy, it is reasonable to consider chemotherapy with a combination of gemcitabine and carboplatin. This combination has been shown to be equivalent to MCAVI (methotrexate, carboplatin, vinblastine) in terms of overall survival (OS; 9 months versus 8 months) and progression-free survival (PFS; 6 months versus 4 months) with significantly fewer serious toxicities (9% versus 21%).43
The advent of immunotherapy in recent years has provided several new alternatives for cisplatin-ineligible patients. While immunotherapies such as pembrolizumab or atezolizumab are not yet recommended as first-line therapy for cisplatin-eligible patients, these 2 drugs are approved as options for first-line therapy in cisplatin-ineligible patients with metastatic disease. In a recent phase 2 trial (IMvigor210) involving 119 patients who were given atezolizumab as first-line therapy, median PFS was 2.7 months and median OS was 15.9 months.44 Another trial using data from patients in the KEYNOTE-052 study who received pembrolizumab as first-line therapy demonstrated antitumor activity with pembrolizumab and acceptable tolerability in cisplatin-ineligible patients with advanced urothelial carcinoma.45 The primary endpoint was objective response (either complete or partial response), which was achieved in 24% of the intention-to-treat population. Median PFS was 2 months, and 6-month OS was observed in 67% of patients. Both atezolizumab and pembrolizumab were given accelerated approval based on these single-arm studies in this setting. However, due to inferior outcomes in subsequent trials that included single-agent immunotherapy arms for patients in the first-line setting, the US Food and Drug Administration (FDA) has clarified the approval. In the subsequent trials, patients with a low PD-L1 biomarker based on the individual assay used for each drug did worse on immunotherapy alone (compared to chemotherapy or both combined), and the single-therapy arms were stopped early. Thus, the FDA now recommends that pembrolizumab or atezolizumab be used in the first line only for cisplatin-ineligible patients who have PD-L1 expression on tumor cells above the threshold studied on each individual assay, or are unfit for any platinum-based chemotherapy. Further study regarding the optimal role of biomarkers and chemotherapy-immunotherapy combinations is ongoing.
Case Continued
Ten months after his procedure, the patient is found to have prominent retroperitoneal lymphadenopathy and a 1.0-cm liver nodule suspicious for malignancy is noted on surveillance imaging. CT-guided biopsy of the liver reveals high-grade urothelial carcinoma, consistent with both recurrence and distant metastasis. The patient is informed that he needs to resume systemic therapy for recurrent metastatic disease. The options discussed include salvage single-agent chemotherapy with gemcitabine or immunotherapy with pembrolizumab. He elects to move forward with immunotherapy and is scheduled to begin pembrolizumab.
- What other immunotherapies might this patient consider for second-line therapy?
- Is chemotherapy a second-line option for this patient?
Second-Line Therapies and Management of Progressive Disease
Disease progression is unfortunately seen in the majority of cases of advanced urothelial carcinoma.46 New second-line therapies have recently been approved by the FDA in the form of monoclonal antibodies targeting programmed death 1 (PD-1) and a PD-1 ligand (PD-L1) (Figure 3).
Approval of pembrolizumab, a PD-1 inhibitor, was largely supported by the Keynote-045 trial,47,48 which looked at 542 patients who had progressed or recurred after platinum-based chemotherapy. These patients were randomly assigned to either pembrolizumab or investigator’s choice of chemotherapy (paclitaxel, docetaxel, or vinflunine). Patients treated with pembrolizumab had a significantly improved OS (median of 10.3 months versus 7.4 months), but no statistically significant difference in PFS (2.1 months versus 3.3 months). Interestingly, the rate of responses of 12 months or longer was higher with pembrolizumab than with more traditional second-line chemotherapy (68% versus 35%). The strength of this data has led to a category 1 recommendation in the most recent NCCN guidelines.39
The approval of atezolizumab, a PD-L1 inhibitor, as a second-line therapy for advanced urothelial carcinoma is largely supported by data from IMvigor211, a phase 3 trial that studied 931 patients randomly assigned to atezolizumab or investigator’s choice chemotherapy. OS did not differ significantly between patients in the atezolizumab group who had ≥ 5% expression of PD-L1 on tumor-infiltrating immune cells and patients in the chemotherapy group (11.1 months versus 10.6 months), but mean duration of response was longer (15.9 months versus 8.3 months).49 Therapy with atezolizumab had significantly fewer toxicities than chemotherapy (grade 3 or 4 toxicities of 20% versus 43%).
Phase 3 studies of nivolumab (PD-1 inhibitor), avelumab (PD-L1 inhibitor), and durvalumab (PD-L1 inhibitor) have not yet been published. These agents have received accelerated approval, however, as second-line treatment of advanced urothelial carcinoma based on promising data from phase 1 and phase 2 studies.50–52
Second-line chemotherapy is also an option for patients who do not qualify for immunotherapy or who progress during or after immunotherapy. Although there has been a great deal of excitement about new developments with immunotherapy and the survival benefit seen compared to investigator’s choice chemotherapy, the fact remains that most patients do not respond to immunotherapy. Still, some patients do derive benefit from single-agent chemotherapy in the platinum-refractory setting. Options based on primarily single-arm studies include gemcitabine, paclitaxel, docetaxel, pemetrexed, ifosfamide, oxaliplatin, and eribulin (Figure 2). In a randomized phase 3 trial, vinflunine demonstrated an OS benefit in platinum-refractory patients compared to best supportive care; it subsequently received approval by the European Medicines Agency.53 More recently in the phase 3 RANGE trial, docetaxel plus ramucirumab (a monoclonal antibody targeting vascular endothelial growth factor receptor 2) was compared to docetaxel plus placebo and met its primary endpoint of an improvement in PFS (median 4.07 months versus 2.76 months, P = 0.0118).54 OS has not been reported and this regimen has not yet received regulatory approval, however. Unfortunately, trials comparing these regimens are lacking, and response rates and survival remain modest. Clearly, better therapies and biomarkers to help personalize treatment options are needed.
Further investigations are underway with alternative regimens, including but not limited to targeted therapy in the setting of specific genetic and epigenetic alterations. These include mutations affecting tyrosine kinase receptors (eg, RAS/RAF, PI3K, AKT, and mTOR), cell cycle regulators (eg, TP53 or RB1), FGFR3 mutations, PTEN deletions, gene amplifications (eg, FGFR1, CCND1, and MDM2), or changes in genes responsible for chromatin remodeling (eg, UTX, CHD6, or ARID1A). As noted, there is particular excitement regarding FGFR3 inhibitors, which have shown compelling efficacy in phase 1 and 2 single-arm trials. Several agents are being evaluated in randomized trials and represent a potential path to the first targeted therapeutic class with a role in urothelial malignancies.
Surgical resection of metastases may be considered in very select cases.55 Surgery may have a role in limiting metastatic complications and improving cancer control, but this should be discussed at length with the patient using a multidisciplinary approach with careful restaging prior to surgery.
Case Continued
The patient remains on pembrolizumab every 3 weeks as per protocol with regular surveillance imaging. His disease stabilizes as the nodule in his liver and the retroperitoneal lymph nodes, all representing metastatic disease, became slightly smaller in size without evidence of any new disease. He continues to follow up closely with his genitourinary oncologist, undergoing regular surveillance and imaging every 3 months without evidence of disease progression.
Approximately 12 months into therapy, the patient notices a nonproductive cough with progressive and rapidly worsening shortness of breath. He is noted to be hypoxic with oxygen saturation levels to 79% in clinic and is sent immediately to the emergency department by his oncologist. Diffuse bilateral reticular opacities are noted on chest radiograph. Non-contrast CT scan demonstrates diffuse ground-glass opacities consistent with acute respiratory distress syndrome–pattern pneumonitis. He is admitted to the intensive care unit.
The patient is aggressively treated with high-flow nasal oxygen supplementation, intravenous steroids, and empiric antibiotics. He slowly improves on high-dose steroids (methylprednisolone 1 mg/kg/day) without requiring intubation or infliximab therapy and is discharged home in stable condition after 10 days. Oral steroid therapy is continued with a long taper over 6 weeks. In the setting of his grade 3 pneumonitis, pembrolizumab is discontinued and the patient is scheduled for a follow-up appointment with his oncologist to discuss next steps.
- In addition to pneumonitis, what other toxicities should you monitor for in patients treated with an immune checkpoint inhibitor?
- Is this patient a candidate to receive immunotherapy again in the future?
Treatment Toxicities
As use of immune checkpoint inhibitors has become more prevalent, the medical community has become increasingly aware of various immune-related adverse effects (irAE) associated with these drugs. These toxicities can be seen in virtually any organ system, and even vague complaints that arise years after therapy initiation should be treated with a high level of suspicion. The most commonly affected organ systems include the skin, gastrointestinal (GI) tract, lungs, liver, and endocrine system, although all other organ systems can be involved (Table 3) and toxicities appear to be similar across individual drugs.
The American Society of Clinical Oncology recently published a complete set of recommendations to guide clinicians on appropriate treatment strategies for each manifestation of immunotherapy-related toxicity.56 The details of these recommendations largely fall outside the purview of this article, but the mainstays of management are worth noting. These include high-dose systemic glucocorticoids, along with supportive care and cessation of immunotherapy in grade 3 or 4 toxicities. Infliximab is frequently recommended as an adjunct in severe or refractory cases.
Chemotherapy-related toxicities, on the other hand, are well-described and tend to be more familiar to patients and clinicians (Table 3). Classic MVAC, which has now been largely replaced by ddMVAC, was notoriously difficult to tolerate. It was known for a high rate of serious (grade 3 or 4) myelosuppressive complications as well as frequent GI toxicities. These complications include neutropenia (57%), stomatitis (10%), and nausea and vomiting (6%).23 ddMVAC with growth factor support is much better tolerated than classic MVAC. Prominent complaints with ddMVAC still can include nausea, GI distress, mucositis, and fatigue, but the incidence of myelosuppressive complications in particular has markedly decreased. GC is largely well tolerated, with minimal nausea and manageable myelotoxicity, but it is associated with an increased risk of venous thromboembolism.38
Prognosis
Case Conclusion
After returning home, the patient discusses his complicated medical course with his oncologist. Given his continued high quality of life with good functional status, he requests to continue with therapy for his metastatic bladder cancer and is interested in joining a clinical trial. He is referred to a nearby academic center with openings in a clinical trial for which he would be eligible. In the meantime, his oncologist guides him through filling out an advance directive and recommends that he make an appointment with palliative care services to ensure adequate home support for any future needs he may have.
- What is the estimated 5-year survival rate for patients with metastatic bladder cancer?
Overall, prognosis in patients with metastatic bladder cancer remains poor. Median survival in patients being treated with multi-agent chemotherapy is approximately 15 months,38,40 with an expected 5-year survival of just 15%. This is much improved, however, as prior to the advent of modern chemotherapy estimated survival was just 6 months with metastatic bladder cancer. Importantly, these figures do not take into account the recent advancements with immunotherapy, and thus it is reasonable to assume survival rates may continue to improve. In light of these recent advances, it is strongly recommended that whenever possible patients and clinicians consider participation in clinical trials to continue uncovering new and better therapies moving forward.
A number of tools have been developed to help risk stratify patients based on comorbidity, performance status, and other characteristics, but none have been universally adopted.57–60 As with many other malignancies, performance status is an important predictor of clinical outcomes in these patients.61–63 Sites of metastasis also may serve to suggest the course of disease. Patients with visceral metastases typically exhibit significantly worse disease with a shortened survival. The role of molecular factors as prognostic markers in bladder cancer is still under investigation. Many biomarkers are being considered (including mutations and polymorphisms in p53, ERCC1, and ERCC2), and evidence suggests some may have a role in prognosis; thus far, none have been validated as prognostic or predictive tools in urothelial carcinoma.
Conclusion
Bladder cancer includes an aggressive group of genitourinary tract malignancies, of which urothelial carcinoma is by far the most common in the Western world. Cisplatin-based therapy remains a mainstay of treatment for eligible patients with both localized and metastatic disease, but immunotherapies have provided a new and promising tool to use in the setting of progressing malignancy. The individual impact of these agents on OS is still being examined. Further studies and ongoing participation in clinical trials whenever possible continue to be essential to the discovery of future treatment options for this highly aggressive disease.
1. Stewart BW, Kleihues P. World cancer report. IARCPress. 2003.
2. Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 2009;115:68–74.
3. Hinotsu S, Akaza H, Miki T, et al. Bladder cancer develops 6 years earlier in current smokers: Analysis of bladder cancer registry data collected by the cancer registration committee of the Japanese Urological Association. Int J Urol 2009;16:64–9.
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
5. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: bladder cancer. 2018. https://seer.cancer.gov/statfacts/html/urinb.html. Accessed May 5, 2018.
6. Freedman ND, Silverman DT, Hollenbeck AR, et al. Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306:737–45.
7. Pietzak EJ, Mucksavage P, Guzzo TJ, Malkowicz SB. Heavy cigarette smoking and aggressive bladder cancer at initial presentation. Urology 2015;86:968–73.
8. Khadra MH, Pickard RS, Charlton M, et al. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol 2000;163:524–7.
9. Grossman HB, Messing E, Soloway M, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA 2005;293:810–16.
10. Mariani AJ, Mariani MC, Macchioni C, et al. The significance of adult hematuria: 1,000 hematuria evaluations including a risk-benefit and cost-effectiveness analysis. J Urol 1989;141:350–5.
11. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part II: patient evaluation, cytology, voided markers, imaging, cystoscopy, nephrology evaluation, and follow-up. Urology 2001;57:604–10.
12. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part I: definition, detection, prevalence, and etiology. Urology 2001;57:599–603.
13. Mohr DN, Offord KP, Owen RA, Melton LJ. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986;256:224–9.
14. Messing EM, Young TB, Hunt VB, et al. Home screening for hematuria: results of a multiclinic study. J Urol 1992;148:289–92.
15. Gray PJ, Lin CC, Jemal A, et al. Clinical–pathologic stage discrepancy in bladder cancer patients treated with radical cystectomy: results from the National Cancer Data Base. Int J Radiat Oncol 2014;88:1048–56.
16. Bochner B, Hansel D, Efstathiou J, et al. Urinary bladder. In: Amin M, ed. AJCC cancer staging manual. 8th. New York: Springer; 2017:757.
17. Cahn DB, McGreen B, Lee A, et al. Clinical destiny of indeterminate pulmonary nodules in patients undergoing radical cystectomy for urothelial carcinoma of the bladder [abstract]. J Clin Oncol 2017;35(6 suppl):297-297.
18. Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15:25–41.
19. Kurtis B, Zhuge J, Ojaimi C, et al. Recurrent TERT promoter mutations in urothelial carcinoma and potential clinical applications. Ann Diagn Pathol 2016;21:7–11.
20. Ito H, Kyo S, Kanaya T, et al. Detection of human telomerase reverse transcriptase messenger RNA in voided urine samples as a useful diagnostic tool for bladder cancer. Clin Cancer Res 1998;4:2807–10.
21. Utting M, Werner W, Dahse R, et al. Microsatellite analysis of free tumor DNA in urine, serum, and plasma of patients: a minimally invasive method for the detection of bladder cancer. Clin Cancer Res 2002;8:35–40.
22. Sethakorn N, O’Donnell PH. Spectrum of genomic alterations in FGFR3: current appraisal of the potential role of FGFR3 in advanced urothelial carcinoma. BJU Int 2016;118:681–91.
23. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66.
24. Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer ‘unfit’ for cisplatin-based chemotherapy. J Clin Oncol 2011;29:2432–8.
25. Raj GV, Iasonos A, Herr H, Donat SM. Formulas calculating creatinine clearance are inadequate for determining eligibility for cisplatin-based chemotherapy in bladder cancer. J Clin Oncol 2006;24:3095–100.26. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 2003;361:1927–34.
27. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data. Eur Urol 2005;48:202–6.
28. Sternberg CN. A critical review of the management of bladder cancer. Crit Rev Oncol Hematol 1999;31:193–207.
29. Sternberg CN, Yagoda A, Scher HI, et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol 1985;133:403–7.
30. Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 2006;42:50–4.
31. Sternberg CN, de Mulder PHM, Schornagel JH, et al. Randomized phase III trial of high–dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol No. 30924. J Clin Oncol 2001;19:2638–46.
32. Soloway MS, Einstein A, Corder MP, et al. A comparison of cisplatin and the combination of cisplatin and cyclophosphamide in advanced urothelial cancer. A National Bladder Cancer Collaborative Group A Study. Cancer 1983;52:767–72.
33. Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 2014;32:1895–901.
34. Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 2014;4:1140–53.
35. Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol 2015;68:959–67.
36. Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol 2015;16:76–86.
37. Mak RH, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 2014;32:3801–9.
38. von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18:3068–77.
39. Flaig T, Spiess P, Agarwal N, et al. National Comprehensive Cancer Network. Bladder cancer (version 3.2018). 2018. www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed May 5, 2018.
40. von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005;23:4602–8.
41. Bamias A, Dafni U, Karadimou A, et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: a Hellenic Cooperative Oncology Group study (HE 16/03). Ann Oncol 2013;24:1011–7.
42. Li R, Metcalfe M, Kukreja J, Navai N. Role of radical cystectomy in non-organ confined bladder cancer: a systematic review. Bladder Cancer 2018;4:31–40.
43. De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012;30:191–9.
44. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67–76.
45. Balar A V, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:1483–92.
46. Manoharan M, Ayyathurai R, Soloway MS. Radical cystectomy for urothelial carcinoma of the bladder: an analysis of perioperative and survival outcome. BJU Int 2009;104:1227–32.
47. Bellmunt J, de Wit R, Vaughn D, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26.
48. Bajorin D, de Wit R, Vaughn D, et al. Planned survival analysis from KEYNOTE-045: Phase 3, open-label study of pembrolizumab (pembro) versus paclitaxel, docetaxel, or vinflunine in recurrent, advanced urothelial cancer (UC). (Abstract 4501). J Clin Oncol 2017;35(15_suppl):4501-4501.
49. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391:748–57.
50. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312–22.
51. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018;19:51–64.
52. Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma. JAMA Oncol 2017;3:e172411.
53. Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009;27:4454–61.
54. Petrylak DP, de Wit R, Chi KN, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): a randomised, double-blind, phase 3 trial. Lancet 2017;390:2266–77.
55. Abufaraj M, Dalbagni G, Daneshmand S, et al. The role of surgery in metastatic bladder cancer: a systematic review. Eur Urol 2018;73:543–57.
56. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68.
57. Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999;17:3173–81.
58. Mayr R, May M, Martini T, et al. Comorbidity and performance indices as predictors of cancer-independent mortality but not of cancer-specific mortality after radical cystectomy for urothelial carcinoma of the bladder. Eur Urol 2012;62:662–70.
59. Nakagawa T, Hara T, Kawahara T, et al. Prognostic risk stratification of patients with urothelial carcinoma of the bladder with recurrence after radical cystectomy. J Urol 2013;189:1275–81.
60. Ploeg M, Kums AC, Aben KK, et al. Prognostic factors for survival in patients with recurrence of muscle invasive bladder cancer after treatment with curative intent. Clin Genitourin Cancer 2011;9:14–21.
61. Saxman SB, Propert KJ, Einhorn LH, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 1997;15:2564–9.
62. Lin CC, Hsu CH, Huang CY, et al. Prognostic factors for metastatic urothelial carcinoma treated with cisplatin and 5-fluorouracil-based regimens. Urology 2007;69:479–84.
63. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2:187–93.
1. Stewart BW, Kleihues P. World cancer report. IARCPress. 2003.
2. Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 2009;115:68–74.
3. Hinotsu S, Akaza H, Miki T, et al. Bladder cancer develops 6 years earlier in current smokers: Analysis of bladder cancer registry data collected by the cancer registration committee of the Japanese Urological Association. Int J Urol 2009;16:64–9.
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
5. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: bladder cancer. 2018. https://seer.cancer.gov/statfacts/html/urinb.html. Accessed May 5, 2018.
6. Freedman ND, Silverman DT, Hollenbeck AR, et al. Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306:737–45.
7. Pietzak EJ, Mucksavage P, Guzzo TJ, Malkowicz SB. Heavy cigarette smoking and aggressive bladder cancer at initial presentation. Urology 2015;86:968–73.
8. Khadra MH, Pickard RS, Charlton M, et al. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol 2000;163:524–7.
9. Grossman HB, Messing E, Soloway M, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA 2005;293:810–16.
10. Mariani AJ, Mariani MC, Macchioni C, et al. The significance of adult hematuria: 1,000 hematuria evaluations including a risk-benefit and cost-effectiveness analysis. J Urol 1989;141:350–5.
11. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part II: patient evaluation, cytology, voided markers, imaging, cystoscopy, nephrology evaluation, and follow-up. Urology 2001;57:604–10.
12. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part I: definition, detection, prevalence, and etiology. Urology 2001;57:599–603.
13. Mohr DN, Offord KP, Owen RA, Melton LJ. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986;256:224–9.
14. Messing EM, Young TB, Hunt VB, et al. Home screening for hematuria: results of a multiclinic study. J Urol 1992;148:289–92.
15. Gray PJ, Lin CC, Jemal A, et al. Clinical–pathologic stage discrepancy in bladder cancer patients treated with radical cystectomy: results from the National Cancer Data Base. Int J Radiat Oncol 2014;88:1048–56.
16. Bochner B, Hansel D, Efstathiou J, et al. Urinary bladder. In: Amin M, ed. AJCC cancer staging manual. 8th. New York: Springer; 2017:757.
17. Cahn DB, McGreen B, Lee A, et al. Clinical destiny of indeterminate pulmonary nodules in patients undergoing radical cystectomy for urothelial carcinoma of the bladder [abstract]. J Clin Oncol 2017;35(6 suppl):297-297.
18. Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15:25–41.
19. Kurtis B, Zhuge J, Ojaimi C, et al. Recurrent TERT promoter mutations in urothelial carcinoma and potential clinical applications. Ann Diagn Pathol 2016;21:7–11.
20. Ito H, Kyo S, Kanaya T, et al. Detection of human telomerase reverse transcriptase messenger RNA in voided urine samples as a useful diagnostic tool for bladder cancer. Clin Cancer Res 1998;4:2807–10.
21. Utting M, Werner W, Dahse R, et al. Microsatellite analysis of free tumor DNA in urine, serum, and plasma of patients: a minimally invasive method for the detection of bladder cancer. Clin Cancer Res 2002;8:35–40.
22. Sethakorn N, O’Donnell PH. Spectrum of genomic alterations in FGFR3: current appraisal of the potential role of FGFR3 in advanced urothelial carcinoma. BJU Int 2016;118:681–91.
23. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66.
24. Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer ‘unfit’ for cisplatin-based chemotherapy. J Clin Oncol 2011;29:2432–8.
25. Raj GV, Iasonos A, Herr H, Donat SM. Formulas calculating creatinine clearance are inadequate for determining eligibility for cisplatin-based chemotherapy in bladder cancer. J Clin Oncol 2006;24:3095–100.26. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 2003;361:1927–34.
27. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data. Eur Urol 2005;48:202–6.
28. Sternberg CN. A critical review of the management of bladder cancer. Crit Rev Oncol Hematol 1999;31:193–207.
29. Sternberg CN, Yagoda A, Scher HI, et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol 1985;133:403–7.
30. Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 2006;42:50–4.
31. Sternberg CN, de Mulder PHM, Schornagel JH, et al. Randomized phase III trial of high–dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol No. 30924. J Clin Oncol 2001;19:2638–46.
32. Soloway MS, Einstein A, Corder MP, et al. A comparison of cisplatin and the combination of cisplatin and cyclophosphamide in advanced urothelial cancer. A National Bladder Cancer Collaborative Group A Study. Cancer 1983;52:767–72.
33. Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 2014;32:1895–901.
34. Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 2014;4:1140–53.
35. Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol 2015;68:959–67.
36. Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol 2015;16:76–86.
37. Mak RH, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 2014;32:3801–9.
38. von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18:3068–77.
39. Flaig T, Spiess P, Agarwal N, et al. National Comprehensive Cancer Network. Bladder cancer (version 3.2018). 2018. www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed May 5, 2018.
40. von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005;23:4602–8.
41. Bamias A, Dafni U, Karadimou A, et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: a Hellenic Cooperative Oncology Group study (HE 16/03). Ann Oncol 2013;24:1011–7.
42. Li R, Metcalfe M, Kukreja J, Navai N. Role of radical cystectomy in non-organ confined bladder cancer: a systematic review. Bladder Cancer 2018;4:31–40.
43. De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012;30:191–9.
44. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67–76.
45. Balar A V, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:1483–92.
46. Manoharan M, Ayyathurai R, Soloway MS. Radical cystectomy for urothelial carcinoma of the bladder: an analysis of perioperative and survival outcome. BJU Int 2009;104:1227–32.
47. Bellmunt J, de Wit R, Vaughn D, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26.
48. Bajorin D, de Wit R, Vaughn D, et al. Planned survival analysis from KEYNOTE-045: Phase 3, open-label study of pembrolizumab (pembro) versus paclitaxel, docetaxel, or vinflunine in recurrent, advanced urothelial cancer (UC). (Abstract 4501). J Clin Oncol 2017;35(15_suppl):4501-4501.
49. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391:748–57.
50. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312–22.
51. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018;19:51–64.
52. Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma. JAMA Oncol 2017;3:e172411.
53. Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009;27:4454–61.
54. Petrylak DP, de Wit R, Chi KN, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): a randomised, double-blind, phase 3 trial. Lancet 2017;390:2266–77.
55. Abufaraj M, Dalbagni G, Daneshmand S, et al. The role of surgery in metastatic bladder cancer: a systematic review. Eur Urol 2018;73:543–57.
56. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68.
57. Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999;17:3173–81.
58. Mayr R, May M, Martini T, et al. Comorbidity and performance indices as predictors of cancer-independent mortality but not of cancer-specific mortality after radical cystectomy for urothelial carcinoma of the bladder. Eur Urol 2012;62:662–70.
59. Nakagawa T, Hara T, Kawahara T, et al. Prognostic risk stratification of patients with urothelial carcinoma of the bladder with recurrence after radical cystectomy. J Urol 2013;189:1275–81.
60. Ploeg M, Kums AC, Aben KK, et al. Prognostic factors for survival in patients with recurrence of muscle invasive bladder cancer after treatment with curative intent. Clin Genitourin Cancer 2011;9:14–21.
61. Saxman SB, Propert KJ, Einhorn LH, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 1997;15:2564–9.
62. Lin CC, Hsu CH, Huang CY, et al. Prognostic factors for metastatic urothelial carcinoma treated with cisplatin and 5-fluorouracil-based regimens. Urology 2007;69:479–84.
63. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2:187–93.
FDA approves radioactive agent for adrenal tumors
(pheochromocytoma or paraganglioma) that are unresectable, have metastasized, and require systemic therapy.
This is the first FDA-approved drug for this use, the FDA said in a press announcement.
Approval is based on a single-arm, open-label clinical trial that included 68 patients. The primary endpoint was the number or patients with a 50% or greater reduction of antihypertensive medications lasting at least 6 months; the secondary endpoint was overall tumor response according to traditional imaging criteria. The primary endpoint was met by 17 patients, and the secondary endpoint was achieved in 15.
The most common severe side effects were lymphopenia, neutropenia, thrombocytopenia, fatigue, anemia, increased international normalized ratio, nausea, dizziness, hypertension, and vomiting. Furthermore, because this is a radioactive therapeutic agent, there is a warning about radiation exposure for both patients and family members, a risk that is greatest in pediatric patients.
Other warnings and precautions include a risk of myelosuppression, underactive thyroid, elevations in blood pressure, renal failure or kidney injury, and pneumonitis. Myelodysplastic syndrome and acute leukemias were observed in patients who received the radioactive agent, and the magnitude of this risk will continue to be studied, the FDA said.
The approval was granted to Progenics Pharmaceuticals.
(pheochromocytoma or paraganglioma) that are unresectable, have metastasized, and require systemic therapy.
This is the first FDA-approved drug for this use, the FDA said in a press announcement.
Approval is based on a single-arm, open-label clinical trial that included 68 patients. The primary endpoint was the number or patients with a 50% or greater reduction of antihypertensive medications lasting at least 6 months; the secondary endpoint was overall tumor response according to traditional imaging criteria. The primary endpoint was met by 17 patients, and the secondary endpoint was achieved in 15.
The most common severe side effects were lymphopenia, neutropenia, thrombocytopenia, fatigue, anemia, increased international normalized ratio, nausea, dizziness, hypertension, and vomiting. Furthermore, because this is a radioactive therapeutic agent, there is a warning about radiation exposure for both patients and family members, a risk that is greatest in pediatric patients.
Other warnings and precautions include a risk of myelosuppression, underactive thyroid, elevations in blood pressure, renal failure or kidney injury, and pneumonitis. Myelodysplastic syndrome and acute leukemias were observed in patients who received the radioactive agent, and the magnitude of this risk will continue to be studied, the FDA said.
The approval was granted to Progenics Pharmaceuticals.
(pheochromocytoma or paraganglioma) that are unresectable, have metastasized, and require systemic therapy.
This is the first FDA-approved drug for this use, the FDA said in a press announcement.
Approval is based on a single-arm, open-label clinical trial that included 68 patients. The primary endpoint was the number or patients with a 50% or greater reduction of antihypertensive medications lasting at least 6 months; the secondary endpoint was overall tumor response according to traditional imaging criteria. The primary endpoint was met by 17 patients, and the secondary endpoint was achieved in 15.
The most common severe side effects were lymphopenia, neutropenia, thrombocytopenia, fatigue, anemia, increased international normalized ratio, nausea, dizziness, hypertension, and vomiting. Furthermore, because this is a radioactive therapeutic agent, there is a warning about radiation exposure for both patients and family members, a risk that is greatest in pediatric patients.
Other warnings and precautions include a risk of myelosuppression, underactive thyroid, elevations in blood pressure, renal failure or kidney injury, and pneumonitis. Myelodysplastic syndrome and acute leukemias were observed in patients who received the radioactive agent, and the magnitude of this risk will continue to be studied, the FDA said.
The approval was granted to Progenics Pharmaceuticals.
Gene assays reveal some ‘unknown primary’ cancers as RCC
Gene expression profiling and/or immunohistochemistry can identify occult renal cell carcinoma (RCC) in a subset of patients diagnosed with carcinoma of unknown primary (CUP), suggesting that these patients could benefit from RCC-specific targeted therapy or immunotherapy, investigators contend.
Of 539 patients presenting at a single center with CUP, a 92-gene reverse transcription polymerase chain reaction molecular cancer classifier assay (MCCA) performed on biopsy specimens identified 24 as having RCC. All of the patients had clinical characteristics typical of advanced RCC, but none had suspicious renal lesions on CT scans, reported F. Anthony Greco, MD and John D. Hainsworth, MD, of the Sarah Cannon Cancer Center and Research Institute in Nashville, Tenn.
“Although further experience is necessary, these patients responded to RCC-specific therapy in a manner consistent with advanced RCC. These patients are unlikely to benefit from treatment with empiric chemotherapy. The reliable identification of RCC patients within the heterogeneous CUP population is possible using MCCA, and has potentially important therapeutic implications,” they wrote. The report was published in Clinical Genitourinary Cancer.
They noted that previously the only therapeutic option for patients with CUP suspected of being renal in origin was ineffective systemic chemotherapy, making a specific diagnosis of more academic interest than clinical importance.
“This situation has now changed because of the introduction of several targeted agents and immune checkpoint blockers that improve survival in patients with advanced RCC. It is likely that these new RCC treatments are also more effective than empiric chemotherapy for patients with CUP who have an occult renal primary site. Therefore, recognition of the RCC subset of patients within the CUP population has practical therapeutic importance,” they wrote.
Dr. Greco and Dr. Hainsworth conducted a retrospective review of patients at their center with CUP from 2008 through 2013 who had RCC predicted by MCCA.
A total of 539 patients presented with CUP during the study period, and of this group, 24 (4.4%) had RCC identified by MCCA.
The patients, 18 men and 6 women, with a median age of 61 years, all had abdominal CT scans that failed to show renal lesions suggestive of a primary RCC. Nine of the 24 patients had baseline MCCA performed as part of a prospective phase 2 clinical trial; the other 15 were patients treated at the center who had MCCA performed later in the clinical course, usually during or after first-line empiric chemotherapy.
Sixteen patients had metastases in the retroperitoneum, 10 in the mediastinum, 6 in bone, 5 in the liver, and 5 in lungs and/or pleura.
Pathologic studies using light microscopy showed poorly differentiated carcinomas in eight patients, poorly differentiated adenocarcinomas in nine, and well or moderately differentiated adenocarcinomas in seven patients.
A pathologist identified RCC as the possible primary in only 4 of the 24 patients. Immunohistochemistry tests in these patients were consistent with a diagnosis of RCC. Only 5 of the 24 had focal features suggestive of RCC, including one clear-cell and four papillary histologies.
Sixteen of the 24 patients received first-line treatment for advanced RCC, including sunitinib, temsirolimus, bevacizumab, and/or interleukin 1. Four other patients received RCC-specific therapy following empiric chemotherapy (three patients who received RCC-specific therapies in the first line also received it in the second line).
Among the 16 patients who received first-line RCC-specific therapies there were 3 partial responses (PR), 10 cases of stable disease (SD), 2 of progressive disease (PD), and 1 patient was not evaluable. The median duration of both PR and SD was 8 months.
Of the eight patients who received first-line empiric chemotherapy, one had a PR, two had SD, and five had PD.
For the seven patients who received second-line RCC-specific therapy after either first-line chemotherapy or site-specific therapy, responses included one PR, two SD, two PD, and two not evaluable.
Median survival for all 24 patients was 12 months (range 2 to more than 43 months). Median survival of the 16 patients who received first-line RCC-specific treatment was 14 months (range 2-25 months).
Median survival for all 20 patients who received RCC-specific treatment at some time during their course was 16 months (range, 2 to more than 43 months).
The authors called for further prospective studies of this subset of patients with CUP.
SOURCE: Greco FA, Hainsworth JD. Clin Genitourin Cancer. 2018 Aug;16(4):e893-8.
Gene expression profiling and/or immunohistochemistry can identify occult renal cell carcinoma (RCC) in a subset of patients diagnosed with carcinoma of unknown primary (CUP), suggesting that these patients could benefit from RCC-specific targeted therapy or immunotherapy, investigators contend.
Of 539 patients presenting at a single center with CUP, a 92-gene reverse transcription polymerase chain reaction molecular cancer classifier assay (MCCA) performed on biopsy specimens identified 24 as having RCC. All of the patients had clinical characteristics typical of advanced RCC, but none had suspicious renal lesions on CT scans, reported F. Anthony Greco, MD and John D. Hainsworth, MD, of the Sarah Cannon Cancer Center and Research Institute in Nashville, Tenn.
“Although further experience is necessary, these patients responded to RCC-specific therapy in a manner consistent with advanced RCC. These patients are unlikely to benefit from treatment with empiric chemotherapy. The reliable identification of RCC patients within the heterogeneous CUP population is possible using MCCA, and has potentially important therapeutic implications,” they wrote. The report was published in Clinical Genitourinary Cancer.
They noted that previously the only therapeutic option for patients with CUP suspected of being renal in origin was ineffective systemic chemotherapy, making a specific diagnosis of more academic interest than clinical importance.
“This situation has now changed because of the introduction of several targeted agents and immune checkpoint blockers that improve survival in patients with advanced RCC. It is likely that these new RCC treatments are also more effective than empiric chemotherapy for patients with CUP who have an occult renal primary site. Therefore, recognition of the RCC subset of patients within the CUP population has practical therapeutic importance,” they wrote.
Dr. Greco and Dr. Hainsworth conducted a retrospective review of patients at their center with CUP from 2008 through 2013 who had RCC predicted by MCCA.
A total of 539 patients presented with CUP during the study period, and of this group, 24 (4.4%) had RCC identified by MCCA.
The patients, 18 men and 6 women, with a median age of 61 years, all had abdominal CT scans that failed to show renal lesions suggestive of a primary RCC. Nine of the 24 patients had baseline MCCA performed as part of a prospective phase 2 clinical trial; the other 15 were patients treated at the center who had MCCA performed later in the clinical course, usually during or after first-line empiric chemotherapy.
Sixteen patients had metastases in the retroperitoneum, 10 in the mediastinum, 6 in bone, 5 in the liver, and 5 in lungs and/or pleura.
Pathologic studies using light microscopy showed poorly differentiated carcinomas in eight patients, poorly differentiated adenocarcinomas in nine, and well or moderately differentiated adenocarcinomas in seven patients.
A pathologist identified RCC as the possible primary in only 4 of the 24 patients. Immunohistochemistry tests in these patients were consistent with a diagnosis of RCC. Only 5 of the 24 had focal features suggestive of RCC, including one clear-cell and four papillary histologies.
Sixteen of the 24 patients received first-line treatment for advanced RCC, including sunitinib, temsirolimus, bevacizumab, and/or interleukin 1. Four other patients received RCC-specific therapy following empiric chemotherapy (three patients who received RCC-specific therapies in the first line also received it in the second line).
Among the 16 patients who received first-line RCC-specific therapies there were 3 partial responses (PR), 10 cases of stable disease (SD), 2 of progressive disease (PD), and 1 patient was not evaluable. The median duration of both PR and SD was 8 months.
Of the eight patients who received first-line empiric chemotherapy, one had a PR, two had SD, and five had PD.
For the seven patients who received second-line RCC-specific therapy after either first-line chemotherapy or site-specific therapy, responses included one PR, two SD, two PD, and two not evaluable.
Median survival for all 24 patients was 12 months (range 2 to more than 43 months). Median survival of the 16 patients who received first-line RCC-specific treatment was 14 months (range 2-25 months).
Median survival for all 20 patients who received RCC-specific treatment at some time during their course was 16 months (range, 2 to more than 43 months).
The authors called for further prospective studies of this subset of patients with CUP.
SOURCE: Greco FA, Hainsworth JD. Clin Genitourin Cancer. 2018 Aug;16(4):e893-8.
Gene expression profiling and/or immunohistochemistry can identify occult renal cell carcinoma (RCC) in a subset of patients diagnosed with carcinoma of unknown primary (CUP), suggesting that these patients could benefit from RCC-specific targeted therapy or immunotherapy, investigators contend.
Of 539 patients presenting at a single center with CUP, a 92-gene reverse transcription polymerase chain reaction molecular cancer classifier assay (MCCA) performed on biopsy specimens identified 24 as having RCC. All of the patients had clinical characteristics typical of advanced RCC, but none had suspicious renal lesions on CT scans, reported F. Anthony Greco, MD and John D. Hainsworth, MD, of the Sarah Cannon Cancer Center and Research Institute in Nashville, Tenn.
“Although further experience is necessary, these patients responded to RCC-specific therapy in a manner consistent with advanced RCC. These patients are unlikely to benefit from treatment with empiric chemotherapy. The reliable identification of RCC patients within the heterogeneous CUP population is possible using MCCA, and has potentially important therapeutic implications,” they wrote. The report was published in Clinical Genitourinary Cancer.
They noted that previously the only therapeutic option for patients with CUP suspected of being renal in origin was ineffective systemic chemotherapy, making a specific diagnosis of more academic interest than clinical importance.
“This situation has now changed because of the introduction of several targeted agents and immune checkpoint blockers that improve survival in patients with advanced RCC. It is likely that these new RCC treatments are also more effective than empiric chemotherapy for patients with CUP who have an occult renal primary site. Therefore, recognition of the RCC subset of patients within the CUP population has practical therapeutic importance,” they wrote.
Dr. Greco and Dr. Hainsworth conducted a retrospective review of patients at their center with CUP from 2008 through 2013 who had RCC predicted by MCCA.
A total of 539 patients presented with CUP during the study period, and of this group, 24 (4.4%) had RCC identified by MCCA.
The patients, 18 men and 6 women, with a median age of 61 years, all had abdominal CT scans that failed to show renal lesions suggestive of a primary RCC. Nine of the 24 patients had baseline MCCA performed as part of a prospective phase 2 clinical trial; the other 15 were patients treated at the center who had MCCA performed later in the clinical course, usually during or after first-line empiric chemotherapy.
Sixteen patients had metastases in the retroperitoneum, 10 in the mediastinum, 6 in bone, 5 in the liver, and 5 in lungs and/or pleura.
Pathologic studies using light microscopy showed poorly differentiated carcinomas in eight patients, poorly differentiated adenocarcinomas in nine, and well or moderately differentiated adenocarcinomas in seven patients.
A pathologist identified RCC as the possible primary in only 4 of the 24 patients. Immunohistochemistry tests in these patients were consistent with a diagnosis of RCC. Only 5 of the 24 had focal features suggestive of RCC, including one clear-cell and four papillary histologies.
Sixteen of the 24 patients received first-line treatment for advanced RCC, including sunitinib, temsirolimus, bevacizumab, and/or interleukin 1. Four other patients received RCC-specific therapy following empiric chemotherapy (three patients who received RCC-specific therapies in the first line also received it in the second line).
Among the 16 patients who received first-line RCC-specific therapies there were 3 partial responses (PR), 10 cases of stable disease (SD), 2 of progressive disease (PD), and 1 patient was not evaluable. The median duration of both PR and SD was 8 months.
Of the eight patients who received first-line empiric chemotherapy, one had a PR, two had SD, and five had PD.
For the seven patients who received second-line RCC-specific therapy after either first-line chemotherapy or site-specific therapy, responses included one PR, two SD, two PD, and two not evaluable.
Median survival for all 24 patients was 12 months (range 2 to more than 43 months). Median survival of the 16 patients who received first-line RCC-specific treatment was 14 months (range 2-25 months).
Median survival for all 20 patients who received RCC-specific treatment at some time during their course was 16 months (range, 2 to more than 43 months).
The authors called for further prospective studies of this subset of patients with CUP.
SOURCE: Greco FA, Hainsworth JD. Clin Genitourin Cancer. 2018 Aug;16(4):e893-8.
FROM CLINICAL GENITOURINARY CANCER
Key clinical point: Some carcinomas of unknown primary (CUP) can be identified as renal in origin by molecular assays and treated accordingly.
Major finding: Molecular assays identified RCC as the primary in 24 of 539 patients with CUP.
Study details: Retrospective review of patients with CUP presenting at a single center from 2008 through 2013.
Disclosures: The Minnie Pearl Cancer Research Foundation supported the study. Dr. Greco disclosed a consultant role and speakers bureau activities for bioTheranostics.
Source: Greco FA, Hainsworth JD. Clinical Genitourinary Cancer 16(4): e893-8.