User login
FDA approves enzalutamide for non-metastatic CRPC
The Food and Drug Administration has expanded the prostate cancer indication for enzalutamide to include nonmetastatic castration-resistant prostate cancer (CRPC). The androgen-receptor inhibitor was first approved in 2012 for the treatment of patients with metastatic CRPC who had previously received chemotherapy and was granted approval in 2014 for men with metastatic CRPC who had not received chemotherapy.
The current approval was based on a statistically significant improvement in metastasis-free survival for patients receiving enzalutamide in the phase 3 PROSPER trial, a trial that randomized 1,401 patients (2:1) with nonmetastatic CRPC to 160 mg of oral enzalutamide daily or to placebo. Median metastasis-free survival was 36.6 months for those receiving enzalutamide versus 14.7 months for those receiving placebo (hazard ratio, 0.29; 95% confidence interval, 0.24-0.35; P less than .0001), the FDA said in a press statement.
The most common adverse events were asthenia/fatigue, hot flush, hypertension, dizziness, nausea, and falls.
The recommended dose for enzalutamide, marketed as Xtandi by Astellas Pharma US, is 160 mg (four 40-mg capsules) administered orally once daily.
The Food and Drug Administration has expanded the prostate cancer indication for enzalutamide to include nonmetastatic castration-resistant prostate cancer (CRPC). The androgen-receptor inhibitor was first approved in 2012 for the treatment of patients with metastatic CRPC who had previously received chemotherapy and was granted approval in 2014 for men with metastatic CRPC who had not received chemotherapy.
The current approval was based on a statistically significant improvement in metastasis-free survival for patients receiving enzalutamide in the phase 3 PROSPER trial, a trial that randomized 1,401 patients (2:1) with nonmetastatic CRPC to 160 mg of oral enzalutamide daily or to placebo. Median metastasis-free survival was 36.6 months for those receiving enzalutamide versus 14.7 months for those receiving placebo (hazard ratio, 0.29; 95% confidence interval, 0.24-0.35; P less than .0001), the FDA said in a press statement.
The most common adverse events were asthenia/fatigue, hot flush, hypertension, dizziness, nausea, and falls.
The recommended dose for enzalutamide, marketed as Xtandi by Astellas Pharma US, is 160 mg (four 40-mg capsules) administered orally once daily.
The Food and Drug Administration has expanded the prostate cancer indication for enzalutamide to include nonmetastatic castration-resistant prostate cancer (CRPC). The androgen-receptor inhibitor was first approved in 2012 for the treatment of patients with metastatic CRPC who had previously received chemotherapy and was granted approval in 2014 for men with metastatic CRPC who had not received chemotherapy.
The current approval was based on a statistically significant improvement in metastasis-free survival for patients receiving enzalutamide in the phase 3 PROSPER trial, a trial that randomized 1,401 patients (2:1) with nonmetastatic CRPC to 160 mg of oral enzalutamide daily or to placebo. Median metastasis-free survival was 36.6 months for those receiving enzalutamide versus 14.7 months for those receiving placebo (hazard ratio, 0.29; 95% confidence interval, 0.24-0.35; P less than .0001), the FDA said in a press statement.
The most common adverse events were asthenia/fatigue, hot flush, hypertension, dizziness, nausea, and falls.
The recommended dose for enzalutamide, marketed as Xtandi by Astellas Pharma US, is 160 mg (four 40-mg capsules) administered orally once daily.
Many actionable mutations may be missed in current testing of advanced RCC
Germline mutations in patients with advanced renal cell carcinoma may be more common than previously suspected.
In a single-center cohort of 254 patients with advanced renal cell carcinoma (RCC) who received matched tumor-germline DNA sequencing, over a third (35.7%) of patients who had mutations in genes associated with RCC had not met current clinical criteria for testing.
In all, pathogenic germline mutations were identified in 41 patients (16.1%), with 14 patients’ mutations (5.5%) in genes known to be associated with RCC. For the remaining 27 patients (10.5%), the mutations were in non–RCC-associated genes, investigators reported in JAMA Oncology.
Of the non–RCC-associated mutations, CHEK2 was particularly common among patients with clear cell RCC (ccRCC), occurring in eight patients with ccRCC and two with non ccRCC (nccRCC). The overall odds ratio for this mutation among the study cohort was 3.0, compared with the general population (95% confidence interval 1.3-5.8; P = .003). “Although there are currently no RCC-specific screening recommendations for individuals with CHEK2 mutations, there may be incremental screening for other cancers, justifying including this gene on RCC panel tests,” wrote Maria Carlo, MD, and her coauthors.
Germline FH mutations were seen in seven patients, all with nccRCC. This higher rate of hereditary leiomyomatosis and RCC (HLRCC) was higher than previously reported in the literature, and clinical cues to the diagnosis were few among the study patients. Even though clues pointing to HLRCC were seen when tumor samples were submitted for histopathology to the genitourinary specialists at the study site, “it is unclear whether nonspecialist pathologists would be able to draw the same conclusions,” wrote Dr. Carlo and her colleagues.
Renal cell cancer–associated mutations were significantly more common in patients with nccRCC than in the ccRCC group: 9/74 (11.7%) nccRCC patients had an RCC-associated mutation, compared with 3/177 of the ccRCC group (P = .001).
The patient’s course of therapy could be guided by the mutation identified in 10% (eight) of the nccRCC patients, “none of which would have been identified with somatic-only sequencing,” wrote Dr. Carlo and associates. “Our results suggest that germline mutations in cancer-associated genes in patients with advanced RCC may be prevalent, and many of these mutations can be used to guide therapy.”
The 254 patients (median age 56 years, 70.5% male, 83.1% non-Hispanic white) were drawn from 267 patients with American Joint Committee on Cancer (AJCC) stage III or IV RCC participating in clinical trials at Memorial Sloan Kettering Cancer Center, New York, where Dr. Carlo practices as an oncologist. The patients included in the cohort were those who consented to germline sequencing and results disclosure.
To determine which pathogenic variants were identified by the study protocol that would have been missed by current testing standards, the investigators assumed that for those who met guidelines, the multigene test panel would probe for VHL, VH, FLCN, MET, SDHB, SDHD, BAP1, TSC1, TSC2, TP53, and MITF. If another mutation was picked up by the next-generation sequencing used in the study, or if a mutation was found in an individual who otherwise would not have been tested, the finding was considered incremental and attributable to the study protocol.
Implications of the additional mutations picked up by the tumor-germline sequencing approach go beyond the patient, said the researchers, who have seen several of the study participants’ family members receive positive test results for cancer-associated mutations as well. “Relatives who are also found to carry FH mutations should be considered for RCC screening. Early detection may increase the likelihood of cure and survivorship,” wrote Dr. Carlo and her coinvestigators.
Dr. Carlo reported serving as a consultant for Pfizer. Other authors reported multiple associations with pharmaceutical companies. The study was funded by the National Institutes of Health, the J. Randall and Kathleen L. MacDonald Kidney Cancer Research Fund, and the Robert and Kate Niehaus Center for Inherited Cancer Genomics at Memorial Sloan Kettering Cancer Center.
SOURCE: Carlo M et al. JAMA Oncol. 2018 Jul 5. doi: 10.1001/jamaoncol.2018.1986.
In the present study, the number of patients who did not meet current criteria for genetic testing, but who had germline RCC-associated mutations, should prompt reevaluation of testing criteria for individuals with advanced RCC.
Particularly for patients with advanced nccRCC, a genetic referral should be considered to weigh germline testing as well as testing for an expanded set of mutations. Patients with advanced ccRCC may also benefit from a broader testing panel that may include some non-RCC related genes.
Further research is needed to elucidate the genotype-phenotype association in some of the non-RCC mutations seen in this cohort, particularly in CHEK2 mutations. There are currently no screening guidelines for CHEK2 in regard to RCC, and the risk for RCC among those with these mutations is not known. RCC patients who have biallelic loss of DNA damage repair genes such as CHEK2 may benefit from treatment that targets these pathways, though these therapies are not currently offered for RCC.
The study population were individuals with advanced RCC, and the increased numbers of pathogenic germline mutations seen in this population are consistent with other studies finding higher rates of these mutations in patients who have other cancer with advanced disease. As this body of knowledge accumulates, interdisciplinary teams will be able to give more accurate information about risk and prognosis to patients and families and, increasingly, offer optimized care.
Dr. Patrick Pilié is an oncologist at the University of Texas MD Anderson Cancer Center, Houston; Dr. Kathleen Cooney is chair of the department of internal medicine and H.A. and Edna Benning Presidential Endowed Chair at the University of Utah, Salt Lake City. These remarks are drawn from a jointly authored editorial accompanying the study’s publication.
In the present study, the number of patients who did not meet current criteria for genetic testing, but who had germline RCC-associated mutations, should prompt reevaluation of testing criteria for individuals with advanced RCC.
Particularly for patients with advanced nccRCC, a genetic referral should be considered to weigh germline testing as well as testing for an expanded set of mutations. Patients with advanced ccRCC may also benefit from a broader testing panel that may include some non-RCC related genes.
Further research is needed to elucidate the genotype-phenotype association in some of the non-RCC mutations seen in this cohort, particularly in CHEK2 mutations. There are currently no screening guidelines for CHEK2 in regard to RCC, and the risk for RCC among those with these mutations is not known. RCC patients who have biallelic loss of DNA damage repair genes such as CHEK2 may benefit from treatment that targets these pathways, though these therapies are not currently offered for RCC.
The study population were individuals with advanced RCC, and the increased numbers of pathogenic germline mutations seen in this population are consistent with other studies finding higher rates of these mutations in patients who have other cancer with advanced disease. As this body of knowledge accumulates, interdisciplinary teams will be able to give more accurate information about risk and prognosis to patients and families and, increasingly, offer optimized care.
Dr. Patrick Pilié is an oncologist at the University of Texas MD Anderson Cancer Center, Houston; Dr. Kathleen Cooney is chair of the department of internal medicine and H.A. and Edna Benning Presidential Endowed Chair at the University of Utah, Salt Lake City. These remarks are drawn from a jointly authored editorial accompanying the study’s publication.
In the present study, the number of patients who did not meet current criteria for genetic testing, but who had germline RCC-associated mutations, should prompt reevaluation of testing criteria for individuals with advanced RCC.
Particularly for patients with advanced nccRCC, a genetic referral should be considered to weigh germline testing as well as testing for an expanded set of mutations. Patients with advanced ccRCC may also benefit from a broader testing panel that may include some non-RCC related genes.
Further research is needed to elucidate the genotype-phenotype association in some of the non-RCC mutations seen in this cohort, particularly in CHEK2 mutations. There are currently no screening guidelines for CHEK2 in regard to RCC, and the risk for RCC among those with these mutations is not known. RCC patients who have biallelic loss of DNA damage repair genes such as CHEK2 may benefit from treatment that targets these pathways, though these therapies are not currently offered for RCC.
The study population were individuals with advanced RCC, and the increased numbers of pathogenic germline mutations seen in this population are consistent with other studies finding higher rates of these mutations in patients who have other cancer with advanced disease. As this body of knowledge accumulates, interdisciplinary teams will be able to give more accurate information about risk and prognosis to patients and families and, increasingly, offer optimized care.
Dr. Patrick Pilié is an oncologist at the University of Texas MD Anderson Cancer Center, Houston; Dr. Kathleen Cooney is chair of the department of internal medicine and H.A. and Edna Benning Presidential Endowed Chair at the University of Utah, Salt Lake City. These remarks are drawn from a jointly authored editorial accompanying the study’s publication.
Germline mutations in patients with advanced renal cell carcinoma may be more common than previously suspected.
In a single-center cohort of 254 patients with advanced renal cell carcinoma (RCC) who received matched tumor-germline DNA sequencing, over a third (35.7%) of patients who had mutations in genes associated with RCC had not met current clinical criteria for testing.
In all, pathogenic germline mutations were identified in 41 patients (16.1%), with 14 patients’ mutations (5.5%) in genes known to be associated with RCC. For the remaining 27 patients (10.5%), the mutations were in non–RCC-associated genes, investigators reported in JAMA Oncology.
Of the non–RCC-associated mutations, CHEK2 was particularly common among patients with clear cell RCC (ccRCC), occurring in eight patients with ccRCC and two with non ccRCC (nccRCC). The overall odds ratio for this mutation among the study cohort was 3.0, compared with the general population (95% confidence interval 1.3-5.8; P = .003). “Although there are currently no RCC-specific screening recommendations for individuals with CHEK2 mutations, there may be incremental screening for other cancers, justifying including this gene on RCC panel tests,” wrote Maria Carlo, MD, and her coauthors.
Germline FH mutations were seen in seven patients, all with nccRCC. This higher rate of hereditary leiomyomatosis and RCC (HLRCC) was higher than previously reported in the literature, and clinical cues to the diagnosis were few among the study patients. Even though clues pointing to HLRCC were seen when tumor samples were submitted for histopathology to the genitourinary specialists at the study site, “it is unclear whether nonspecialist pathologists would be able to draw the same conclusions,” wrote Dr. Carlo and her colleagues.
Renal cell cancer–associated mutations were significantly more common in patients with nccRCC than in the ccRCC group: 9/74 (11.7%) nccRCC patients had an RCC-associated mutation, compared with 3/177 of the ccRCC group (P = .001).
The patient’s course of therapy could be guided by the mutation identified in 10% (eight) of the nccRCC patients, “none of which would have been identified with somatic-only sequencing,” wrote Dr. Carlo and associates. “Our results suggest that germline mutations in cancer-associated genes in patients with advanced RCC may be prevalent, and many of these mutations can be used to guide therapy.”
The 254 patients (median age 56 years, 70.5% male, 83.1% non-Hispanic white) were drawn from 267 patients with American Joint Committee on Cancer (AJCC) stage III or IV RCC participating in clinical trials at Memorial Sloan Kettering Cancer Center, New York, where Dr. Carlo practices as an oncologist. The patients included in the cohort were those who consented to germline sequencing and results disclosure.
To determine which pathogenic variants were identified by the study protocol that would have been missed by current testing standards, the investigators assumed that for those who met guidelines, the multigene test panel would probe for VHL, VH, FLCN, MET, SDHB, SDHD, BAP1, TSC1, TSC2, TP53, and MITF. If another mutation was picked up by the next-generation sequencing used in the study, or if a mutation was found in an individual who otherwise would not have been tested, the finding was considered incremental and attributable to the study protocol.
Implications of the additional mutations picked up by the tumor-germline sequencing approach go beyond the patient, said the researchers, who have seen several of the study participants’ family members receive positive test results for cancer-associated mutations as well. “Relatives who are also found to carry FH mutations should be considered for RCC screening. Early detection may increase the likelihood of cure and survivorship,” wrote Dr. Carlo and her coinvestigators.
Dr. Carlo reported serving as a consultant for Pfizer. Other authors reported multiple associations with pharmaceutical companies. The study was funded by the National Institutes of Health, the J. Randall and Kathleen L. MacDonald Kidney Cancer Research Fund, and the Robert and Kate Niehaus Center for Inherited Cancer Genomics at Memorial Sloan Kettering Cancer Center.
SOURCE: Carlo M et al. JAMA Oncol. 2018 Jul 5. doi: 10.1001/jamaoncol.2018.1986.
Germline mutations in patients with advanced renal cell carcinoma may be more common than previously suspected.
In a single-center cohort of 254 patients with advanced renal cell carcinoma (RCC) who received matched tumor-germline DNA sequencing, over a third (35.7%) of patients who had mutations in genes associated with RCC had not met current clinical criteria for testing.
In all, pathogenic germline mutations were identified in 41 patients (16.1%), with 14 patients’ mutations (5.5%) in genes known to be associated with RCC. For the remaining 27 patients (10.5%), the mutations were in non–RCC-associated genes, investigators reported in JAMA Oncology.
Of the non–RCC-associated mutations, CHEK2 was particularly common among patients with clear cell RCC (ccRCC), occurring in eight patients with ccRCC and two with non ccRCC (nccRCC). The overall odds ratio for this mutation among the study cohort was 3.0, compared with the general population (95% confidence interval 1.3-5.8; P = .003). “Although there are currently no RCC-specific screening recommendations for individuals with CHEK2 mutations, there may be incremental screening for other cancers, justifying including this gene on RCC panel tests,” wrote Maria Carlo, MD, and her coauthors.
Germline FH mutations were seen in seven patients, all with nccRCC. This higher rate of hereditary leiomyomatosis and RCC (HLRCC) was higher than previously reported in the literature, and clinical cues to the diagnosis were few among the study patients. Even though clues pointing to HLRCC were seen when tumor samples were submitted for histopathology to the genitourinary specialists at the study site, “it is unclear whether nonspecialist pathologists would be able to draw the same conclusions,” wrote Dr. Carlo and her colleagues.
Renal cell cancer–associated mutations were significantly more common in patients with nccRCC than in the ccRCC group: 9/74 (11.7%) nccRCC patients had an RCC-associated mutation, compared with 3/177 of the ccRCC group (P = .001).
The patient’s course of therapy could be guided by the mutation identified in 10% (eight) of the nccRCC patients, “none of which would have been identified with somatic-only sequencing,” wrote Dr. Carlo and associates. “Our results suggest that germline mutations in cancer-associated genes in patients with advanced RCC may be prevalent, and many of these mutations can be used to guide therapy.”
The 254 patients (median age 56 years, 70.5% male, 83.1% non-Hispanic white) were drawn from 267 patients with American Joint Committee on Cancer (AJCC) stage III or IV RCC participating in clinical trials at Memorial Sloan Kettering Cancer Center, New York, where Dr. Carlo practices as an oncologist. The patients included in the cohort were those who consented to germline sequencing and results disclosure.
To determine which pathogenic variants were identified by the study protocol that would have been missed by current testing standards, the investigators assumed that for those who met guidelines, the multigene test panel would probe for VHL, VH, FLCN, MET, SDHB, SDHD, BAP1, TSC1, TSC2, TP53, and MITF. If another mutation was picked up by the next-generation sequencing used in the study, or if a mutation was found in an individual who otherwise would not have been tested, the finding was considered incremental and attributable to the study protocol.
Implications of the additional mutations picked up by the tumor-germline sequencing approach go beyond the patient, said the researchers, who have seen several of the study participants’ family members receive positive test results for cancer-associated mutations as well. “Relatives who are also found to carry FH mutations should be considered for RCC screening. Early detection may increase the likelihood of cure and survivorship,” wrote Dr. Carlo and her coinvestigators.
Dr. Carlo reported serving as a consultant for Pfizer. Other authors reported multiple associations with pharmaceutical companies. The study was funded by the National Institutes of Health, the J. Randall and Kathleen L. MacDonald Kidney Cancer Research Fund, and the Robert and Kate Niehaus Center for Inherited Cancer Genomics at Memorial Sloan Kettering Cancer Center.
SOURCE: Carlo M et al. JAMA Oncol. 2018 Jul 5. doi: 10.1001/jamaoncol.2018.1986.
FROM JAMA ONCOLOGY
Key clinical point: A broader approach to sequencing of patients with advanced RCC may identify patients for targeted therapy.
Major finding: Pathogenic germline mutations were seen in 16% of patients with advanced RCC
Study details: Prospective single-center cohort study of 254 patients with advanced RCC.
Disclosures: Dr. Carlo reported serving as a consultant for Pfizer. Other authors reported multiple associations with pharmaceutical companies. The study was funded by the National Institutes of Health, the J.Randall and Kathleen L. MacDonald Kidney Cancer Research Fund, and the Robert and Kate Niehaus Center for Inherited Cancer Genomics at Memorial Sloan Kettering Cancer Center.
Source: Carlo M et al. JAMA Oncol. 2018 July 5. doi: 10.1001/jamaoncol.2018.1986.
PSMA-targeting docetaxel nanoparticles active, safe for mCRPC
For men with chemotherapy-naive metastatic castration-resistant prostate cancer (mCRPC), a docetaxel-containing nanoparticle directed against prostate-specific membrane antigen (PSMA) was both active and well tolerated.
Among 42 men with mCRPC that had progressed after treatment with abiraterone acetate (Zytiga) and/or enzalutamide (Xtandi), the 6-month radiographic progression-free survival (PFS) rate, the primary endpoint, was 65%, and approximately one-third of evaluable patients had a prostate-specific antigen (PSA) response and measurable disease response, reported Karen A. Autio, MD, MSc, of Memorial Sloan Kettering Cancer Center, New York, and her colleagues.
Treatment with the PSMA-directed docetaxel-containing nanoparticle, called BIND-014, was also associated in some patients with a rapid and robust decline in PSMA-positive circulating tumor cells (CTCs), the investigators noted. The report was published in JAMA Oncology.
“Many aspects of our study – reductions in PSA, radiographically confirmed disease control in bone and visceral metastatic disease, favorable CTC conversions, and an acceptable adverse effect profile – were promising for BIND-014. However, standard therapy with docetaxel is widely used and effective in treating this disease, and as a natural comparator for a phase 3 randomized clinical trial with BIND-014, it sets a high bar for efficacy in an unselected population,” they wrote.
Their findings suggest that patients with PSMA-positive CTCs might be good candidates for treatment with BIND-014, which is associated with lower toxicities than standard docetaxel, the investigators said.
In the open-label phase 2 trial, 42 men (median age 66) with chemotherapy-naive mCRPC that had progressed on abiraterone and/or enzalutamide were treated with intravenous BIND-014 at dosages of 60 mg/m2 on the first day of each 21-day cycle, plus prednisone 5 mg twice daily, until disease progression, intolerable toxicity, or treatment discontinuation at the treating physician’s discretion.
The median number of doses delivered was six.
Of 40 evaluable patients, 12 (30%) had a PSA response, defined as a decrease of 50% or greater from baseline. Among 19 patients with disease measurable according to Response Evaluation Criteria in Solid Tumors, version 1.1, six (32%) had responses, including one complete response and five partial responses. Nine other patients had stable disease.
The median PFS was 9.9 months. As noted, the radiographic PFS rate at 6 months was 65% (26 of 40 patients).
CTC enumeration was performed on samples from 39 patients, of whom 67% had unfavorable counts at baseline, defined as 5 or more CTCs per 7.5 mL of blood.
After treatment, 13 of the patients had a conversion to a favorable count, including 6 whose CTCs became undetectable.
The most common treatment-related adverse events were fatigue, occurring in 69% of patients, nausea in 55%, diarrhea in 45%, and patient-reported neuropathy in 33%. Most toxicities were grade 1 or 2.
Grade 3 or 4 hematological toxicities included lymphopenia in five patients, anemia in three, neutropenia in one, leukopenia in one, and febrile neutropenia in one.
Nonhematological grade 3 or 4 events included fatigue and nausea in two patients each, and dyspnea and decreased appetite in one patient each.
“In this trial, two principal lessons are worth highlighting: the reduction in both total CTCs, which is a marker of clinical benefit, and specifically PSMA-positive CTCs, suggests that the detection of PSMA-positive CTCs before treatment can be used to identify patients who are most likely to benefit in addition to serving as a pharmacodynamic measure,” the investigators wrote.
“Second, there was marked intrapatient and interpatient heterogeneity of PSMA expression on CTCs. This underscores the complexity of targeting tumors with single cell-surface markers,” they wrote.
SOURCE: Autio KA et al. JAMA Oncol. 2018 July 5 doi: 10.1001/jamaoncol.2018.2168.
For men with chemotherapy-naive metastatic castration-resistant prostate cancer (mCRPC), a docetaxel-containing nanoparticle directed against prostate-specific membrane antigen (PSMA) was both active and well tolerated.
Among 42 men with mCRPC that had progressed after treatment with abiraterone acetate (Zytiga) and/or enzalutamide (Xtandi), the 6-month radiographic progression-free survival (PFS) rate, the primary endpoint, was 65%, and approximately one-third of evaluable patients had a prostate-specific antigen (PSA) response and measurable disease response, reported Karen A. Autio, MD, MSc, of Memorial Sloan Kettering Cancer Center, New York, and her colleagues.
Treatment with the PSMA-directed docetaxel-containing nanoparticle, called BIND-014, was also associated in some patients with a rapid and robust decline in PSMA-positive circulating tumor cells (CTCs), the investigators noted. The report was published in JAMA Oncology.
“Many aspects of our study – reductions in PSA, radiographically confirmed disease control in bone and visceral metastatic disease, favorable CTC conversions, and an acceptable adverse effect profile – were promising for BIND-014. However, standard therapy with docetaxel is widely used and effective in treating this disease, and as a natural comparator for a phase 3 randomized clinical trial with BIND-014, it sets a high bar for efficacy in an unselected population,” they wrote.
Their findings suggest that patients with PSMA-positive CTCs might be good candidates for treatment with BIND-014, which is associated with lower toxicities than standard docetaxel, the investigators said.
In the open-label phase 2 trial, 42 men (median age 66) with chemotherapy-naive mCRPC that had progressed on abiraterone and/or enzalutamide were treated with intravenous BIND-014 at dosages of 60 mg/m2 on the first day of each 21-day cycle, plus prednisone 5 mg twice daily, until disease progression, intolerable toxicity, or treatment discontinuation at the treating physician’s discretion.
The median number of doses delivered was six.
Of 40 evaluable patients, 12 (30%) had a PSA response, defined as a decrease of 50% or greater from baseline. Among 19 patients with disease measurable according to Response Evaluation Criteria in Solid Tumors, version 1.1, six (32%) had responses, including one complete response and five partial responses. Nine other patients had stable disease.
The median PFS was 9.9 months. As noted, the radiographic PFS rate at 6 months was 65% (26 of 40 patients).
CTC enumeration was performed on samples from 39 patients, of whom 67% had unfavorable counts at baseline, defined as 5 or more CTCs per 7.5 mL of blood.
After treatment, 13 of the patients had a conversion to a favorable count, including 6 whose CTCs became undetectable.
The most common treatment-related adverse events were fatigue, occurring in 69% of patients, nausea in 55%, diarrhea in 45%, and patient-reported neuropathy in 33%. Most toxicities were grade 1 or 2.
Grade 3 or 4 hematological toxicities included lymphopenia in five patients, anemia in three, neutropenia in one, leukopenia in one, and febrile neutropenia in one.
Nonhematological grade 3 or 4 events included fatigue and nausea in two patients each, and dyspnea and decreased appetite in one patient each.
“In this trial, two principal lessons are worth highlighting: the reduction in both total CTCs, which is a marker of clinical benefit, and specifically PSMA-positive CTCs, suggests that the detection of PSMA-positive CTCs before treatment can be used to identify patients who are most likely to benefit in addition to serving as a pharmacodynamic measure,” the investigators wrote.
“Second, there was marked intrapatient and interpatient heterogeneity of PSMA expression on CTCs. This underscores the complexity of targeting tumors with single cell-surface markers,” they wrote.
SOURCE: Autio KA et al. JAMA Oncol. 2018 July 5 doi: 10.1001/jamaoncol.2018.2168.
For men with chemotherapy-naive metastatic castration-resistant prostate cancer (mCRPC), a docetaxel-containing nanoparticle directed against prostate-specific membrane antigen (PSMA) was both active and well tolerated.
Among 42 men with mCRPC that had progressed after treatment with abiraterone acetate (Zytiga) and/or enzalutamide (Xtandi), the 6-month radiographic progression-free survival (PFS) rate, the primary endpoint, was 65%, and approximately one-third of evaluable patients had a prostate-specific antigen (PSA) response and measurable disease response, reported Karen A. Autio, MD, MSc, of Memorial Sloan Kettering Cancer Center, New York, and her colleagues.
Treatment with the PSMA-directed docetaxel-containing nanoparticle, called BIND-014, was also associated in some patients with a rapid and robust decline in PSMA-positive circulating tumor cells (CTCs), the investigators noted. The report was published in JAMA Oncology.
“Many aspects of our study – reductions in PSA, radiographically confirmed disease control in bone and visceral metastatic disease, favorable CTC conversions, and an acceptable adverse effect profile – were promising for BIND-014. However, standard therapy with docetaxel is widely used and effective in treating this disease, and as a natural comparator for a phase 3 randomized clinical trial with BIND-014, it sets a high bar for efficacy in an unselected population,” they wrote.
Their findings suggest that patients with PSMA-positive CTCs might be good candidates for treatment with BIND-014, which is associated with lower toxicities than standard docetaxel, the investigators said.
In the open-label phase 2 trial, 42 men (median age 66) with chemotherapy-naive mCRPC that had progressed on abiraterone and/or enzalutamide were treated with intravenous BIND-014 at dosages of 60 mg/m2 on the first day of each 21-day cycle, plus prednisone 5 mg twice daily, until disease progression, intolerable toxicity, or treatment discontinuation at the treating physician’s discretion.
The median number of doses delivered was six.
Of 40 evaluable patients, 12 (30%) had a PSA response, defined as a decrease of 50% or greater from baseline. Among 19 patients with disease measurable according to Response Evaluation Criteria in Solid Tumors, version 1.1, six (32%) had responses, including one complete response and five partial responses. Nine other patients had stable disease.
The median PFS was 9.9 months. As noted, the radiographic PFS rate at 6 months was 65% (26 of 40 patients).
CTC enumeration was performed on samples from 39 patients, of whom 67% had unfavorable counts at baseline, defined as 5 or more CTCs per 7.5 mL of blood.
After treatment, 13 of the patients had a conversion to a favorable count, including 6 whose CTCs became undetectable.
The most common treatment-related adverse events were fatigue, occurring in 69% of patients, nausea in 55%, diarrhea in 45%, and patient-reported neuropathy in 33%. Most toxicities were grade 1 or 2.
Grade 3 or 4 hematological toxicities included lymphopenia in five patients, anemia in three, neutropenia in one, leukopenia in one, and febrile neutropenia in one.
Nonhematological grade 3 or 4 events included fatigue and nausea in two patients each, and dyspnea and decreased appetite in one patient each.
“In this trial, two principal lessons are worth highlighting: the reduction in both total CTCs, which is a marker of clinical benefit, and specifically PSMA-positive CTCs, suggests that the detection of PSMA-positive CTCs before treatment can be used to identify patients who are most likely to benefit in addition to serving as a pharmacodynamic measure,” the investigators wrote.
“Second, there was marked intrapatient and interpatient heterogeneity of PSMA expression on CTCs. This underscores the complexity of targeting tumors with single cell-surface markers,” they wrote.
SOURCE: Autio KA et al. JAMA Oncol. 2018 July 5 doi: 10.1001/jamaoncol.2018.2168.
FROM JAMA ONCOLOGY
Key clinical point: A docetaxel-encapsulating nanoparticle targeted against PSMA was safe and showed good activity.
Major finding: The 6-month PFS rate, the primary endpoint, was 65%.
Study details: Open label phase 2 trial of 42 men with mCRPC that progressed on abiraterone acetate and/or enzalutamide.
Disclosures: The study was funded by BIND Therapeutics and by grants from the National Institutes of Health, Sidney Kimmel Center for Prostate and Urologic Cancers, and the David H. Koch Prostate Cancer Research Fund. Dr. Autio had no disclosures. Several coauthors are employees of BIND Therapeutics, and others are employed by Epic Sciences, maker of the CTC assay used in the study. Other coauthors reported advising/consulting, research support, and speakers bureau activities for multiple companies.
Source: Autio KA et al. JAMA Oncol. 2018 July 5. doi: 10.1001/jamaoncol.2018.2168.
KEYNOTE-427: Pembrolizumab monotherapy shows promise in accRCC
CHICAGO – (accRCC), according to findings from the phase 2 KEYNOTE-427 study.
At a median follow-up of 12 months, the overall response rate in 110 study participants with at least one post-baseline assessment was 38%. Three patients (2.7%) achieved a complete response and 39 (35.5%) achieved a partial response, David F. McDermott, MD, reported at the annual meeting of the American Society of Clinical Oncology.
“The disease control rate was 59%,” he said.
Overall, 67% of the patients experienced a reduction in tumor burden, 14% experienced at least an 80% reduction, and 7% experienced a 100% reduction of their target lesion, said Dr. McDermott of Beth Israel Deaconess Medical Center, Boston.
“Most tumor responses occurred early in the course of therapy,” he noted.
The median time to response was 2.8 months, and the median duration of response was not reached at data cutoff, but 74.8% of responders had a response lasting at least 6 months.
An analysis by International Metastatic Renal Cell Carcinoma Database Criteria (IMDC) category showed a confirmed overall response rate (ORR) of 32% among 41 patients with favorable risk, and 42% in 69 patients with intermediate or poor risk.
“Nine of 17 patients in the poor risk group achieved a major response,” Dr. McDermott noted. “Complete and durable responders were seen in all IMDC subgroups.”
In 46 patients with increased PD-L1 expression or a combined positive score of at least 1 the confirmed ORR was 50.0%, and in 53 patients with low PD-L1 expression and a combined positive score less than 1 it was 26%. The ORR was 45% in the remaining patients in whom PD-L1testing could not be performed.
“Of note, all of the complete responses were seen in the PD-L1-high or CPS-greater-than-1 group,” he said.
Median progression-free survival was 8.7 months, and median overall survival has not been reached.
Tolerability of pembrolizumab in this study was acceptable and consistent with that seen with pembrolizumab monotherapy in other tumor types. Although 80% of patients experienced a treatment-related adverse event, the events mainly included fatigue, pruritus, diarrhea, rash, and arthralgia, occurring in 12.7% to 27.3% of patients, he said.
Grade 3/4 events occurred in 21.8% of patients and one patient experienced a fatal grade 5 case of pneumonitis, he added, noting that 11% of patients discontinued treatment because of a treatment-related adverse event.
Overall, 61 patients discontinued therapy, and 33 of those discontinued because of disease progression.
Programmed death-1 (PD-1) inhibitor-based combination therapies have been shown to have clinical benefit when used first-line in accRCC, but data with respect to the clinical impact of first-line PD-1 inhibitor monotherapy are lacking, Dr. McDermott explained.
KEYNOTE-427 was a single-arm, open-label, two-cohort study evaluating the efficacy and safety of pembrolizumab as first-line monotherapy in accRCC and advanced non–clear cell RCC (anccRCC). Patients had accRCC or anccRCC, measurable disease, no prior systemic therapy and Karnofsky Performance Status score of 70% or greater. They were treated with intravenous pembrolizumab at a dose of 200 mg every 3 weeks, and response was assessed at week 12, then every 6 weeks thereafter until week 54, then every 12 weeks.
The current analysis focused on the accRCC cohort and showed that in treatment-naive patients with histologically confirmed accRCC and measurable disease, pembrolizumab shows promising antitumor activity across IMDC risk groups, he said.
“Encouraging activity was also observed in key subgroups, such as the IMDC intermediate/poor risk group ... and patients with [programmed death-ligand 1]-positive tumors,” he said. “The findings ... provide support for the exploration of pembrolizumab in the adjuvant setting and will allow investigators to put the benefit of anti-PD-1-based combination therapies in better context,” he concluded, noting that KEYNOTE-564, a study of pembrolizumab in the adjuvant setting is currently enrolling, and the current study (KEYNOTE-427) cohort B exploring pembrolizumab monotherapy in anccRCC patients is ongoing.
Merck sponsored the study. Dr. McDermott reported consulting or advisory roles with Array BioPharma, Bristol-Myers Squibb, Exelixis, Genentech/Roche, Merck, Novartis, Pfizer, and X4 Pharma. His institution has received research funding from Prometheus Laboratories.
SOURCE: McDermott DF et al., ASCO 2018 Abstract 4500.
CHICAGO – (accRCC), according to findings from the phase 2 KEYNOTE-427 study.
At a median follow-up of 12 months, the overall response rate in 110 study participants with at least one post-baseline assessment was 38%. Three patients (2.7%) achieved a complete response and 39 (35.5%) achieved a partial response, David F. McDermott, MD, reported at the annual meeting of the American Society of Clinical Oncology.
“The disease control rate was 59%,” he said.
Overall, 67% of the patients experienced a reduction in tumor burden, 14% experienced at least an 80% reduction, and 7% experienced a 100% reduction of their target lesion, said Dr. McDermott of Beth Israel Deaconess Medical Center, Boston.
“Most tumor responses occurred early in the course of therapy,” he noted.
The median time to response was 2.8 months, and the median duration of response was not reached at data cutoff, but 74.8% of responders had a response lasting at least 6 months.
An analysis by International Metastatic Renal Cell Carcinoma Database Criteria (IMDC) category showed a confirmed overall response rate (ORR) of 32% among 41 patients with favorable risk, and 42% in 69 patients with intermediate or poor risk.
“Nine of 17 patients in the poor risk group achieved a major response,” Dr. McDermott noted. “Complete and durable responders were seen in all IMDC subgroups.”
In 46 patients with increased PD-L1 expression or a combined positive score of at least 1 the confirmed ORR was 50.0%, and in 53 patients with low PD-L1 expression and a combined positive score less than 1 it was 26%. The ORR was 45% in the remaining patients in whom PD-L1testing could not be performed.
“Of note, all of the complete responses were seen in the PD-L1-high or CPS-greater-than-1 group,” he said.
Median progression-free survival was 8.7 months, and median overall survival has not been reached.
Tolerability of pembrolizumab in this study was acceptable and consistent with that seen with pembrolizumab monotherapy in other tumor types. Although 80% of patients experienced a treatment-related adverse event, the events mainly included fatigue, pruritus, diarrhea, rash, and arthralgia, occurring in 12.7% to 27.3% of patients, he said.
Grade 3/4 events occurred in 21.8% of patients and one patient experienced a fatal grade 5 case of pneumonitis, he added, noting that 11% of patients discontinued treatment because of a treatment-related adverse event.
Overall, 61 patients discontinued therapy, and 33 of those discontinued because of disease progression.
Programmed death-1 (PD-1) inhibitor-based combination therapies have been shown to have clinical benefit when used first-line in accRCC, but data with respect to the clinical impact of first-line PD-1 inhibitor monotherapy are lacking, Dr. McDermott explained.
KEYNOTE-427 was a single-arm, open-label, two-cohort study evaluating the efficacy and safety of pembrolizumab as first-line monotherapy in accRCC and advanced non–clear cell RCC (anccRCC). Patients had accRCC or anccRCC, measurable disease, no prior systemic therapy and Karnofsky Performance Status score of 70% or greater. They were treated with intravenous pembrolizumab at a dose of 200 mg every 3 weeks, and response was assessed at week 12, then every 6 weeks thereafter until week 54, then every 12 weeks.
The current analysis focused on the accRCC cohort and showed that in treatment-naive patients with histologically confirmed accRCC and measurable disease, pembrolizumab shows promising antitumor activity across IMDC risk groups, he said.
“Encouraging activity was also observed in key subgroups, such as the IMDC intermediate/poor risk group ... and patients with [programmed death-ligand 1]-positive tumors,” he said. “The findings ... provide support for the exploration of pembrolizumab in the adjuvant setting and will allow investigators to put the benefit of anti-PD-1-based combination therapies in better context,” he concluded, noting that KEYNOTE-564, a study of pembrolizumab in the adjuvant setting is currently enrolling, and the current study (KEYNOTE-427) cohort B exploring pembrolizumab monotherapy in anccRCC patients is ongoing.
Merck sponsored the study. Dr. McDermott reported consulting or advisory roles with Array BioPharma, Bristol-Myers Squibb, Exelixis, Genentech/Roche, Merck, Novartis, Pfizer, and X4 Pharma. His institution has received research funding from Prometheus Laboratories.
SOURCE: McDermott DF et al., ASCO 2018 Abstract 4500.
CHICAGO – (accRCC), according to findings from the phase 2 KEYNOTE-427 study.
At a median follow-up of 12 months, the overall response rate in 110 study participants with at least one post-baseline assessment was 38%. Three patients (2.7%) achieved a complete response and 39 (35.5%) achieved a partial response, David F. McDermott, MD, reported at the annual meeting of the American Society of Clinical Oncology.
“The disease control rate was 59%,” he said.
Overall, 67% of the patients experienced a reduction in tumor burden, 14% experienced at least an 80% reduction, and 7% experienced a 100% reduction of their target lesion, said Dr. McDermott of Beth Israel Deaconess Medical Center, Boston.
“Most tumor responses occurred early in the course of therapy,” he noted.
The median time to response was 2.8 months, and the median duration of response was not reached at data cutoff, but 74.8% of responders had a response lasting at least 6 months.
An analysis by International Metastatic Renal Cell Carcinoma Database Criteria (IMDC) category showed a confirmed overall response rate (ORR) of 32% among 41 patients with favorable risk, and 42% in 69 patients with intermediate or poor risk.
“Nine of 17 patients in the poor risk group achieved a major response,” Dr. McDermott noted. “Complete and durable responders were seen in all IMDC subgroups.”
In 46 patients with increased PD-L1 expression or a combined positive score of at least 1 the confirmed ORR was 50.0%, and in 53 patients with low PD-L1 expression and a combined positive score less than 1 it was 26%. The ORR was 45% in the remaining patients in whom PD-L1testing could not be performed.
“Of note, all of the complete responses were seen in the PD-L1-high or CPS-greater-than-1 group,” he said.
Median progression-free survival was 8.7 months, and median overall survival has not been reached.
Tolerability of pembrolizumab in this study was acceptable and consistent with that seen with pembrolizumab monotherapy in other tumor types. Although 80% of patients experienced a treatment-related adverse event, the events mainly included fatigue, pruritus, diarrhea, rash, and arthralgia, occurring in 12.7% to 27.3% of patients, he said.
Grade 3/4 events occurred in 21.8% of patients and one patient experienced a fatal grade 5 case of pneumonitis, he added, noting that 11% of patients discontinued treatment because of a treatment-related adverse event.
Overall, 61 patients discontinued therapy, and 33 of those discontinued because of disease progression.
Programmed death-1 (PD-1) inhibitor-based combination therapies have been shown to have clinical benefit when used first-line in accRCC, but data with respect to the clinical impact of first-line PD-1 inhibitor monotherapy are lacking, Dr. McDermott explained.
KEYNOTE-427 was a single-arm, open-label, two-cohort study evaluating the efficacy and safety of pembrolizumab as first-line monotherapy in accRCC and advanced non–clear cell RCC (anccRCC). Patients had accRCC or anccRCC, measurable disease, no prior systemic therapy and Karnofsky Performance Status score of 70% or greater. They were treated with intravenous pembrolizumab at a dose of 200 mg every 3 weeks, and response was assessed at week 12, then every 6 weeks thereafter until week 54, then every 12 weeks.
The current analysis focused on the accRCC cohort and showed that in treatment-naive patients with histologically confirmed accRCC and measurable disease, pembrolizumab shows promising antitumor activity across IMDC risk groups, he said.
“Encouraging activity was also observed in key subgroups, such as the IMDC intermediate/poor risk group ... and patients with [programmed death-ligand 1]-positive tumors,” he said. “The findings ... provide support for the exploration of pembrolizumab in the adjuvant setting and will allow investigators to put the benefit of anti-PD-1-based combination therapies in better context,” he concluded, noting that KEYNOTE-564, a study of pembrolizumab in the adjuvant setting is currently enrolling, and the current study (KEYNOTE-427) cohort B exploring pembrolizumab monotherapy in anccRCC patients is ongoing.
Merck sponsored the study. Dr. McDermott reported consulting or advisory roles with Array BioPharma, Bristol-Myers Squibb, Exelixis, Genentech/Roche, Merck, Novartis, Pfizer, and X4 Pharma. His institution has received research funding from Prometheus Laboratories.
SOURCE: McDermott DF et al., ASCO 2018 Abstract 4500.
REPORTING FROM ASCO 2018
Key clinical point: Pembrolizumab monotherapy shows promising efficacy and tolerability in accRCC.
Major finding: Overall response rate was 38%.
Study details: The phase 2 KEYNOTE-427 trial of 110 patients from one of two study cohorts.
Disclosures: Merck sponsored the study. Dr. McDermott reported consulting or advisory roles with Array BioPharma, Bristol-Myers Squibb, Exelixis, Genentech/Roche, Merck, Novartis, Pfizer, and X4 Pharma. His institution has received research funding from Prometheus Laboratories.
Source: McDermott DF et al. ASCO 2018, Abstract 4500.
ASCO 2018: Less is more as ‘tailoring’ takes on new meaning
A record-setting 40,000-plus oncology professionals attended this year’s annual meeting of the American Society of Clinical Oncology (ASCO) in Chicago. The outstanding education and scientific program, with the theme of Delivering Discoveries: Expanding the Reach of Precision Medicine, was planned and led by ASCO President Dr Bruce Johnson, professor and director of Thoracic Oncology at the Dana Farber Cancer Institute in Boston, and chaired by Sarah Cannon’s Dr David Spigel and Harvard’s Dr Ann Partridge. A recurring finding throughout the meeting was that “less is more” in several key areas of cancer therapy. From small molecules targeting driver mutations across various tumors to the application of immunotherapy in subsets of common cancers, it is clear that more patients are experiencing dramatic results from novel approaches.
A featured plenary session trial was TAILORx, a study of 10,273 women with hormone-receptor–positive, surgically resected breast cancer that had not spread to the lymph nodes, was less than 5 cm, and was not positive for the HER2 gene amplification. This clinical trial was sponsored by the NCI and initiated in 2006. It used the OncotypeDX genetic test to stratify patients into groups of low, intermediate, or high risk for recurrence. The low-risk patients received only hormonal therapy, and the high-risk patients were treated with hormonal therapy plus chemotherapy.
Dr Joseph Sparano, professor of Medicine and Women’s Health at the Albert Einstein College of Medicine in New York, presented the results from the group of 6,700 intermediate risk women who were randomized to receive hormonal therapy alone or in combination with chemotherapy. After 9 years of follow-up, 83.3% of the volunteers, as Dr Sparano appropriately referred to them, who were treated with hormonal therapy were still cancer free, compared with 84.3% of those who also received chemotherapy, demonstrating no statistical benefit for the addition of chemotherapy. Of note, breast cancer experts discussing the trial, including Dr Lisa Carey, professor of Breast Cancer Research at the UNC Lineberger Cancer Institute in Chapel Hill, urged that younger women, under the age of 50, with recurrence scores (RS) toward the higher end of the intermediate risk group (RS, 16-25) should still discuss and consider chemotherapy with their physicians. In summary, all patients fitting the study criteria with low (
These landmark and practice changing results mean that each year about 60,000 women in the United States will be spared the side effects of toxic drugs. These 10,273 study volunteers are true heroes to the women who will be diagnosed with breast cancer in coming years.
In the field of lung cancer, many new trial results using immunotherapy were presented, with the most talked about being single-agent pembrolizumab, a PD1 inhibitor, improving survival over traditional chemotherapy in patients with PD-L1 positive tumors, which comprise the majority of squamous cell and adenocarcinomas of the lung. Also in the plenary, Dr Gilberto Lopes of the Sylvester Cancer Center at the University of Miami, presented these results from the KEYNOTE-042 study. In patients with PD-L1 tumor proportion score (TPS) of >1%, the benefit in overall survival (OS) of pembrolizumab compared with chemotherapy was 16.7 versus 12.1 months, respectively (HR, 0.81). In those patients with a TPS of >20%, the OS benefit was 17.7 versus 1.0 months (HR, 0.77), and in the group with a TPS of >50%, the benefit was 20.0 versus 12.2 months (HR, 0.69). Overall, the quality of life and the occurrence of side effects were substantially better for those patients receiving immunotherapy alone. Other findings presented at the meeting demonstrated the benefit of adding immunotherapy to chemotherapy and of treating with combination immunotherapy (PD-1 and CTLA-4 inhibitors). Many options now exist, much work remains to be done, and accrual to clinical trials is more important than ever.
Another plenary session trial evaluated the benefit of performing a nephrectomy in patients with advanced or metastatic renal cell carcinoma (RCC), a long-held and practiced standard of care. Dr Arnaud Mejean of Paris Descartes University presented findings from the CARMENA trial, which randomized 450 patients with metastatic clear cell RCC to receive cytoreductive nephrectomy followed by sunitinib, or sunitinib alone. The OS results of 18.4 versus 13.9 months, respectively (HR, 0.89) favored sunitinib alone in this noninferiority analysis. Other endpoints lined up in favor of not removing the cancerous kidney, and the presenter and discussants were united in their opinion of the results and the resulting change in doing less surgery in these patients.
In a step away from less therapy, the European Pediatric Soft Tissue Sarcoma Study showed that adding 6 months of low-dose maintenance chemotherapy after standard intensive therapy improves survival in children with high-risk rhabdomyosarcoma. The addition of a vinorelbine and cyclophosphamide low-dose regimen improved 5-year disease-free survival from 69.8% to 77.6% (HR, 0.68) and OS from 73.7% to 86.5% (HR, 0.52) as presented by Dr Gianni Bisogno, University of Padovani, Italy. The maintenance regimen showed no increase in toxicity and actually fewer infections were noted.
In the area of molecular profiling, multiple studies at the meeting demonstrated the importance of assessing cancers for mutations as outstanding results were seen with therapies for NTRK, RET, ROS, and MSI-high driven tumors. In a debate on the role of molecular profiling, I had the opportunity to declare and support our position at Sarah Cannon that all patients with relapsed or metastatic cancers should have this testing performed. It will be through better understanding of the biology of these cancers that we will advance the field for all patients while sometimes finding a target or mutation that will dramatically change the life of a patient.
In keeping with the meeting’s theme, Delivering Discoveries: Expanding the Reach of Precision Medicine, the presentations and the discussions clearly demonstrated that through the use of precision medicine techniques such as prognostic gene assays and molecular profiling, patients can receive the best therapy, even “tailored” therapy, which may often actually be less therapy. It is an exciting time in cancer research, and I have never been more optimistic about the future of cancer treatment for our patients.
A record-setting 40,000-plus oncology professionals attended this year’s annual meeting of the American Society of Clinical Oncology (ASCO) in Chicago. The outstanding education and scientific program, with the theme of Delivering Discoveries: Expanding the Reach of Precision Medicine, was planned and led by ASCO President Dr Bruce Johnson, professor and director of Thoracic Oncology at the Dana Farber Cancer Institute in Boston, and chaired by Sarah Cannon’s Dr David Spigel and Harvard’s Dr Ann Partridge. A recurring finding throughout the meeting was that “less is more” in several key areas of cancer therapy. From small molecules targeting driver mutations across various tumors to the application of immunotherapy in subsets of common cancers, it is clear that more patients are experiencing dramatic results from novel approaches.
A featured plenary session trial was TAILORx, a study of 10,273 women with hormone-receptor–positive, surgically resected breast cancer that had not spread to the lymph nodes, was less than 5 cm, and was not positive for the HER2 gene amplification. This clinical trial was sponsored by the NCI and initiated in 2006. It used the OncotypeDX genetic test to stratify patients into groups of low, intermediate, or high risk for recurrence. The low-risk patients received only hormonal therapy, and the high-risk patients were treated with hormonal therapy plus chemotherapy.
Dr Joseph Sparano, professor of Medicine and Women’s Health at the Albert Einstein College of Medicine in New York, presented the results from the group of 6,700 intermediate risk women who were randomized to receive hormonal therapy alone or in combination with chemotherapy. After 9 years of follow-up, 83.3% of the volunteers, as Dr Sparano appropriately referred to them, who were treated with hormonal therapy were still cancer free, compared with 84.3% of those who also received chemotherapy, demonstrating no statistical benefit for the addition of chemotherapy. Of note, breast cancer experts discussing the trial, including Dr Lisa Carey, professor of Breast Cancer Research at the UNC Lineberger Cancer Institute in Chapel Hill, urged that younger women, under the age of 50, with recurrence scores (RS) toward the higher end of the intermediate risk group (RS, 16-25) should still discuss and consider chemotherapy with their physicians. In summary, all patients fitting the study criteria with low (
These landmark and practice changing results mean that each year about 60,000 women in the United States will be spared the side effects of toxic drugs. These 10,273 study volunteers are true heroes to the women who will be diagnosed with breast cancer in coming years.
In the field of lung cancer, many new trial results using immunotherapy were presented, with the most talked about being single-agent pembrolizumab, a PD1 inhibitor, improving survival over traditional chemotherapy in patients with PD-L1 positive tumors, which comprise the majority of squamous cell and adenocarcinomas of the lung. Also in the plenary, Dr Gilberto Lopes of the Sylvester Cancer Center at the University of Miami, presented these results from the KEYNOTE-042 study. In patients with PD-L1 tumor proportion score (TPS) of >1%, the benefit in overall survival (OS) of pembrolizumab compared with chemotherapy was 16.7 versus 12.1 months, respectively (HR, 0.81). In those patients with a TPS of >20%, the OS benefit was 17.7 versus 1.0 months (HR, 0.77), and in the group with a TPS of >50%, the benefit was 20.0 versus 12.2 months (HR, 0.69). Overall, the quality of life and the occurrence of side effects were substantially better for those patients receiving immunotherapy alone. Other findings presented at the meeting demonstrated the benefit of adding immunotherapy to chemotherapy and of treating with combination immunotherapy (PD-1 and CTLA-4 inhibitors). Many options now exist, much work remains to be done, and accrual to clinical trials is more important than ever.
Another plenary session trial evaluated the benefit of performing a nephrectomy in patients with advanced or metastatic renal cell carcinoma (RCC), a long-held and practiced standard of care. Dr Arnaud Mejean of Paris Descartes University presented findings from the CARMENA trial, which randomized 450 patients with metastatic clear cell RCC to receive cytoreductive nephrectomy followed by sunitinib, or sunitinib alone. The OS results of 18.4 versus 13.9 months, respectively (HR, 0.89) favored sunitinib alone in this noninferiority analysis. Other endpoints lined up in favor of not removing the cancerous kidney, and the presenter and discussants were united in their opinion of the results and the resulting change in doing less surgery in these patients.
In a step away from less therapy, the European Pediatric Soft Tissue Sarcoma Study showed that adding 6 months of low-dose maintenance chemotherapy after standard intensive therapy improves survival in children with high-risk rhabdomyosarcoma. The addition of a vinorelbine and cyclophosphamide low-dose regimen improved 5-year disease-free survival from 69.8% to 77.6% (HR, 0.68) and OS from 73.7% to 86.5% (HR, 0.52) as presented by Dr Gianni Bisogno, University of Padovani, Italy. The maintenance regimen showed no increase in toxicity and actually fewer infections were noted.
In the area of molecular profiling, multiple studies at the meeting demonstrated the importance of assessing cancers for mutations as outstanding results were seen with therapies for NTRK, RET, ROS, and MSI-high driven tumors. In a debate on the role of molecular profiling, I had the opportunity to declare and support our position at Sarah Cannon that all patients with relapsed or metastatic cancers should have this testing performed. It will be through better understanding of the biology of these cancers that we will advance the field for all patients while sometimes finding a target or mutation that will dramatically change the life of a patient.
In keeping with the meeting’s theme, Delivering Discoveries: Expanding the Reach of Precision Medicine, the presentations and the discussions clearly demonstrated that through the use of precision medicine techniques such as prognostic gene assays and molecular profiling, patients can receive the best therapy, even “tailored” therapy, which may often actually be less therapy. It is an exciting time in cancer research, and I have never been more optimistic about the future of cancer treatment for our patients.
A record-setting 40,000-plus oncology professionals attended this year’s annual meeting of the American Society of Clinical Oncology (ASCO) in Chicago. The outstanding education and scientific program, with the theme of Delivering Discoveries: Expanding the Reach of Precision Medicine, was planned and led by ASCO President Dr Bruce Johnson, professor and director of Thoracic Oncology at the Dana Farber Cancer Institute in Boston, and chaired by Sarah Cannon’s Dr David Spigel and Harvard’s Dr Ann Partridge. A recurring finding throughout the meeting was that “less is more” in several key areas of cancer therapy. From small molecules targeting driver mutations across various tumors to the application of immunotherapy in subsets of common cancers, it is clear that more patients are experiencing dramatic results from novel approaches.
A featured plenary session trial was TAILORx, a study of 10,273 women with hormone-receptor–positive, surgically resected breast cancer that had not spread to the lymph nodes, was less than 5 cm, and was not positive for the HER2 gene amplification. This clinical trial was sponsored by the NCI and initiated in 2006. It used the OncotypeDX genetic test to stratify patients into groups of low, intermediate, or high risk for recurrence. The low-risk patients received only hormonal therapy, and the high-risk patients were treated with hormonal therapy plus chemotherapy.
Dr Joseph Sparano, professor of Medicine and Women’s Health at the Albert Einstein College of Medicine in New York, presented the results from the group of 6,700 intermediate risk women who were randomized to receive hormonal therapy alone or in combination with chemotherapy. After 9 years of follow-up, 83.3% of the volunteers, as Dr Sparano appropriately referred to them, who were treated with hormonal therapy were still cancer free, compared with 84.3% of those who also received chemotherapy, demonstrating no statistical benefit for the addition of chemotherapy. Of note, breast cancer experts discussing the trial, including Dr Lisa Carey, professor of Breast Cancer Research at the UNC Lineberger Cancer Institute in Chapel Hill, urged that younger women, under the age of 50, with recurrence scores (RS) toward the higher end of the intermediate risk group (RS, 16-25) should still discuss and consider chemotherapy with their physicians. In summary, all patients fitting the study criteria with low (
These landmark and practice changing results mean that each year about 60,000 women in the United States will be spared the side effects of toxic drugs. These 10,273 study volunteers are true heroes to the women who will be diagnosed with breast cancer in coming years.
In the field of lung cancer, many new trial results using immunotherapy were presented, with the most talked about being single-agent pembrolizumab, a PD1 inhibitor, improving survival over traditional chemotherapy in patients with PD-L1 positive tumors, which comprise the majority of squamous cell and adenocarcinomas of the lung. Also in the plenary, Dr Gilberto Lopes of the Sylvester Cancer Center at the University of Miami, presented these results from the KEYNOTE-042 study. In patients with PD-L1 tumor proportion score (TPS) of >1%, the benefit in overall survival (OS) of pembrolizumab compared with chemotherapy was 16.7 versus 12.1 months, respectively (HR, 0.81). In those patients with a TPS of >20%, the OS benefit was 17.7 versus 1.0 months (HR, 0.77), and in the group with a TPS of >50%, the benefit was 20.0 versus 12.2 months (HR, 0.69). Overall, the quality of life and the occurrence of side effects were substantially better for those patients receiving immunotherapy alone. Other findings presented at the meeting demonstrated the benefit of adding immunotherapy to chemotherapy and of treating with combination immunotherapy (PD-1 and CTLA-4 inhibitors). Many options now exist, much work remains to be done, and accrual to clinical trials is more important than ever.
Another plenary session trial evaluated the benefit of performing a nephrectomy in patients with advanced or metastatic renal cell carcinoma (RCC), a long-held and practiced standard of care. Dr Arnaud Mejean of Paris Descartes University presented findings from the CARMENA trial, which randomized 450 patients with metastatic clear cell RCC to receive cytoreductive nephrectomy followed by sunitinib, or sunitinib alone. The OS results of 18.4 versus 13.9 months, respectively (HR, 0.89) favored sunitinib alone in this noninferiority analysis. Other endpoints lined up in favor of not removing the cancerous kidney, and the presenter and discussants were united in their opinion of the results and the resulting change in doing less surgery in these patients.
In a step away from less therapy, the European Pediatric Soft Tissue Sarcoma Study showed that adding 6 months of low-dose maintenance chemotherapy after standard intensive therapy improves survival in children with high-risk rhabdomyosarcoma. The addition of a vinorelbine and cyclophosphamide low-dose regimen improved 5-year disease-free survival from 69.8% to 77.6% (HR, 0.68) and OS from 73.7% to 86.5% (HR, 0.52) as presented by Dr Gianni Bisogno, University of Padovani, Italy. The maintenance regimen showed no increase in toxicity and actually fewer infections were noted.
In the area of molecular profiling, multiple studies at the meeting demonstrated the importance of assessing cancers for mutations as outstanding results were seen with therapies for NTRK, RET, ROS, and MSI-high driven tumors. In a debate on the role of molecular profiling, I had the opportunity to declare and support our position at Sarah Cannon that all patients with relapsed or metastatic cancers should have this testing performed. It will be through better understanding of the biology of these cancers that we will advance the field for all patients while sometimes finding a target or mutation that will dramatically change the life of a patient.
In keeping with the meeting’s theme, Delivering Discoveries: Expanding the Reach of Precision Medicine, the presentations and the discussions clearly demonstrated that through the use of precision medicine techniques such as prognostic gene assays and molecular profiling, patients can receive the best therapy, even “tailored” therapy, which may often actually be less therapy. It is an exciting time in cancer research, and I have never been more optimistic about the future of cancer treatment for our patients.
Psychosocial factors and treatment satisfaction after radical prostatectomy
More than 164,690 men are expected to be diagnosed with prostate cancer in the United States in 2018.1 Men with prostate cancer face not only stress associated with the diagnosis but also decisional conflict regarding different treatment options.2 Most men diagnosed with clinically localized prostate cancer receive 1 or more of the following treatments: radical prostatectomy, external-beam radiation therapy, and/or brachytherapy, all of which are associated with posttreatment urological or sexual side effects including bowel, urinary, or erectile dysfunction.3-5 Men who choose active surveillance may experience increased anxiety associated with the constant vigilance and monitoring of their tumor status along with the uncertainty of not definitively removing or radiating their prostate.6 In addition to direct functional limitations of sexual and urological side effects, treatment can also lead to secondary psychosocial effects, including depression, self-blame, embarrassment, guilt, lower masculine self-esteem, increased reticence to participate socially or engage in sexual activity, and relationship distress.7-9 Therefore, health-related quality of life (HRQoL) and treatment satisfaction are important for this population.
Urological and sexual side effects of prostate cancer treatments are often a primary focus during treatment decision making between patients and providers. However, little prospective empirical data exist regarding the role of HRQoL and other nonurological physical and psychosocial outcomes on overall treatment satisfaction. The purpose of this study was to prospectively evaluate the role of both urological and nonurological outcomes on overall treatment satisfaction in men diagnosed with prostate cancer. We hypothesize that such an understanding can help describe changes in physical and psychosocial factors that are important to men beyond traditional urological outcomes, including their association with overall treatment satisfaction.
Methods
This was a prospective longitudinal assessment of patients from the Department of Urology at Northwestern University’s Feinberg School of Medicine in Chicago. Patients were eligible if they met the following inclusion criteria: they had been diagnosed with clinically localized or locally advanced prostate cancer; they had not yet received a primary treatment (eg, surgery, radiation, active surveillance) before their baseline assessment; they were 18 years or older; and they were able to read, write, speak, and understand English. Patients were excluded if they had a physical debilitation that would make participation not feasible or would create undue hardship, or if they had a history of diagnosed severe mental illness or hospitalization for chronic psychiatric reasons, as identified by referring physicians.
Eligible participants were approached before their treatment decision (if any). Patient enrollment occurred in 2 ways. For patients invited to participate during their clinic visit, the research assistant explained the study and obtained written informed consent for interested patients. A unique user identification and password was created for each patient, and they practiced using the touch screen computer while the research assistant observed and provided guidance as needed. When the patients were ready to start their pretreatment online interview, they completed the questionnaires by themselves. For patients who were invited to participate but were not scheduled to return in the foreseeable future, enrollment was carried out differently. In those cases, participating physicians contacted eligible patients who were not scheduled for a visit and informed them of the study opportunity. Interested patients were contacted by the research assistant who provided them with the study website address, which directed them to the online consent form. After a patient had completed the consent form, he was prompted to self-register. He received a unique user identification and password that could be used to complete the baseline assessment and subsequent assessments. However, for interested patients who did not have access to a computer or Internet connection, the research assistant provided them with paper consent forms and paper versions of all study assessments. After participants had completed the baseline assessment, the research assistant provided them with a written schedule of future assessments, which were expected to occur at 1 month posttreatment, 3 months posttreatment, 6 months posttreatment, and 12 months posttreatment.
For all follow-up appointments, participants could complete assessments either at clinic visits or from home using a secure online assessment platform called Assessment Center.10 The research assistant used a patient log to track participants and their progress in the study, which included study number, patient name (or initials), registration date, date of birth, sex, and timeline of completed or future assessments. The research assistant called or emailed participants (depending on patient preference) about a week before each of their follow-up assessments to facilitate adherence. If the participant did not log into the system by the target day, the research assistant contacted him the following day (target day +1) with a phone or email reminder to log into the system and complete the assessments. If the participant did not log in by midnight 1 day after the target day, the research assistant attempted to contact him one last time (target day +2) with either a reminder to log into the system or to ascertain his status that might be related to his noncompletion. Overall, a participant was called or e-mailed 1 to 3 times to remind him of his assessment. If he was unresponsive after 3 attempts, he was recorded as having withdrawn for an unknown reason.
At baseline and each follow-up time point, study participants completed a battery of patient-reported outcome measures, with most coming from the Patient-Reported Outcomes Measurement Information System (PROMIS)11 and the Surgical Outcomes Measurement System (SOMS).12 PROMIS is a National Institutes of Health (NIH) funded measurement system that has helped standardize and improve self-reported assessment of health status, symptoms, side effects, and different aspects of HRQoL, including physical, emotional, cognitive, and social health. SOMS is a suite of patient-reported outcome measures assessing important aspects of HRQoL after surgery. It was developed with feedback from surgeons, postoperative patients, and surgical nurses. PROMIS items were directly incorporated into numerous SOMS measures to facilitate easier comparisons and score crosswalks across measures and patient populations. In addition to PROMIS and SOMS measures, we also administered several well-known instruments of urological and sexual function, including the International Index of Erectile Function (IIEF) and American Urological Association Symptom Score Index (AUASS).13,14
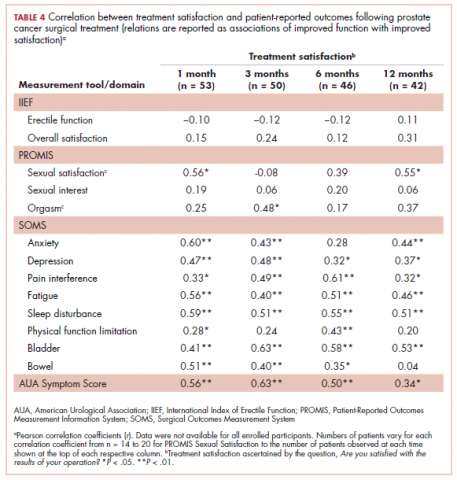
Outcome measures were compared across sociodemographic and clinical variables at each time point using t tests for numerical variables (age) and with chi-square or Fisher exact tests for categorical variables; those variables with significant differences were used as covariates in statistical models. To examine differences in patient-reported scores over time, we used repeated measures analysis of covariance with general linear modeling methods. We used Pearson correlation coefficients to evaluate for correlations between quality-of-life outcomes and treatment satisfaction.
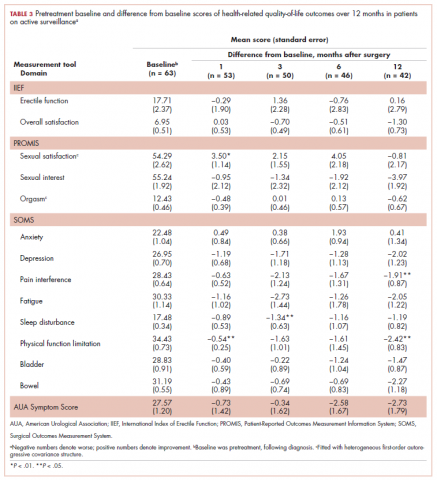
Not all participants completed each of the follow-up surveys, and reasons for dropout were prospectively documented. Most participants elected surgical resection as their primary treatment compared with the fewer than 10% of patients who chose radiation or chemotherapy as their primary treatment and about 20% of men who chose active surveillance after their initial diagnosis. Therefore, our analysis focused on patients who elected surgical resection. For comparison purposes, we included the HRQoL results from active surveillance patients.
Results
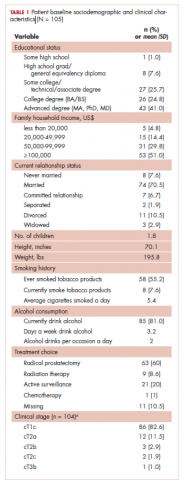
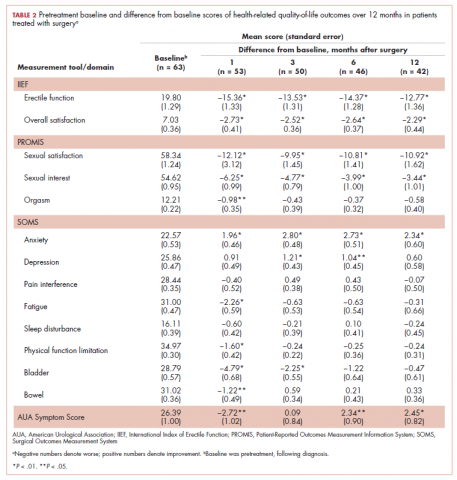
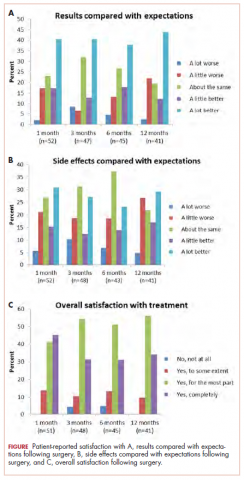
A total of 105 patients diagnosed with prostate cancer were enrolled in the study. Response rates decreased throughout the study (n = 75 at 1 month; n = 71 at 3 months; n = 64 at 6 months; n = 54 at 12 months). Sociodemographic and clinical characteristics of participants are shown in Table 1. The mean change from pretreatment (baseline) scores for each measure in patients treated with surgery is shown in Table 2, and the mean change from pretreatment scores in patients who elected active surveillance is shown in Table 3 (in both tables, a negative score denotes worsened function, and a positive change denotes improvement).
After surgery, patients reported significantly lower erectile function and sexual satisfaction scores. These included statistically significant decreases for IIEF Erectile Function, IIEF Overall Satisfaction, PROMIS Sexual Satisfaction, PROMIS Sexual Interest, and PROMIS Orgasm. In patients treated with surgery, there were significant improvements in anxiety observed for patients at each follow-up time, whereas significantly worse bladder problems were observed on SOMS Bladder at 1 and 3 months but returned to baseline by 12 months after surgery. AUASS was worse at 1 month but significantly improved at 6 and 12 months. Fatigue scores significantly worsened at 1 month but were no longer significant at 6 and 12 months. Physical Function was worsened at 1 month but not throughout the rest of the study. Bowel Problems (SOMS) were significantly worse at 1 month, but changes became nonsignificant on subsequent assessments. The only 2 domains that did not demonstrate any significant changes o
In active surveillance patients, sexual function domains were generally unchanged over the course of the study. However, unlike treated patients, there was no significant improvement in anxiety, depression, pain, fatigue, or sleep. In fact, most of these domains demonstrated worsened functioning, although these were not statistically significant. Urinary domains generally remained unchanged.
Pearson correlation coefficients between HRQoL measures and overall treatment satisfaction (assessed by the question, Are you satisfied with the results of your operation?) at each follow-up time point in patients treated with surgery are shown in Table 4. Relations between treatment satisfaction and sexual outcomes were generally statistically insignificant (r, .08-.56). However, sleep disturbance, depression, pain interference, fatigue, embarrassment, and bladder problems all demonstrated statistically significant positive associations with treatment satisfaction, with coefficients ranging from small to medium in magnitude (r, .32-.61). Other outcomes such as anxiety, physical function, and bowel problems demonstrated small to medium statistically significant associations with treatment satisfaction (r, .04-.60) but not at every time point. We performed t tests to examine treatment satisfaction in patients with detectable initial posttreatment prostate-specific antigen (PSA; >0.01 ng/mL). We found no difference in treatment satisfaction between patients with detectable PSA values and those with undetectable PSA at each time point.
When the patients were asked, Compared with what you expected, how do you rate the results of your operation?, most of those treated with surgery reported that the results of their operation were better than they had expected (Figure 1A; p. e137). More than 75% of the patients had results that were as expected or better than expected. When asked, Compared with what you expected, how do you rate your side effects of the operation?, almost 70% of patients reported side effects no worse than expected (Figure 1B). When asked, Are you satisfied with the results of your operation?, most patients reported that overall, they were satisfied with the results of their operation (Figure 1C).
At 12 months, none of the patients reported overall dissatisfaction with their treatment choice. More than 90% of patients were mostly or completely satisfied with the results of their operation.
Discussion
This prospective study assessed the HRQoL from pretreatment through 12 months posttreatment in men diagnosed with clinically localized prostate cancer that had been treated with surgery. Although the indicators of sexual function significantly decreased over time, they were not meaningfully associated with overall treatment satisfaction. Instead, a host of other factors, including psychosocial (eg, anxiety, depression, body image dissatisfaction, embarrassment), nonurological physical symptoms (pain interference, physical function, sleep disturbance, fatigue), and bladder problems, were significantly related to overall treatment satisfaction. Although this may not be surprising in other clinical oncology paradigms, the sheer surfeit of focus and attention on sexual function has overshadowed aspects of HRQoL that many men report are important to them, despite worsened sexual function outcomes.
Understanding potential treatment-related changes in HRQoL can be challenging for men when choosing providers and different therapeutic options. The increasing complexity of treatment in prostate cancer has created an opportunity to not only understand efficacy on cancer control but also focus on meaningful patient-reported outcomes. Hospitals and medical groups are increasingly aware of the importance of improving the patient care experience. Objective measures of patient satisfaction for health care providers, such as the Press-Ganey and Net Promoter score, exist to measure and improve patient experience. In prostate cancer, clinicians and large groups, including governmental agencies such as the US Preventive Services Task Force, have often focused on declines in urinary and erectile function15 without considering the full impact of prostate cancer treatment on global HRQoL. Our study was a prospective, longitudinal, self-reported examination of the impact, positive and negative, of prostate cancer treatment over a 12-month period.
Numerous studies have documented the treatment-related side effects of erectile, urinary, and bowel dysfunction in patients treated for prostate cancer, which may occur after definitive local therapies.5,16-18 The present study shows a similar impact on urinary, bowel, and erectile domains after treatment. Although erectile function scores remained lower through the course of the 12-month study, bowel and bladder domains returned to baseline by month 12. Unlike other studies, we also examined psychosocial and nonurological aspects of prostate cancer treatment. We found that there was a measurable and significant positive impact on other HRQoL measurements such as decreased anxiety. Despite a variety of declines across HRQoL domains, most patients reported that their results were largely as they had expected, and their side effects were the same or better than they had expected. No patient in the cohort reported being dissatisfied with his overall treatment, and more than 90% of patients were mostly or completely satisfied with their treatment choice. This highlights the point that while sexual and other urological domains of HRQoL are important, impairments in these areas do not necessarily reflect how many patients perceive success or satisfaction with their treatment choice. We also showed correlations between treatment satisfaction and improvement in sleep, anxiety, depression, and fatigue. It is worth noting that although there were decreases in the erectile and sexual function domains after treatment, those factors were not correlated with overall treatment satisfaction. Those factors may not routinely be assessed before, during, and after treatment for prostate cancer in most clinical encounters. However, because they were strongly associated with satisfaction with treatment outcomes in this study, identification in impairments may lead to opportunities to intervene and improve the patient experience. Therefore, important “teachable moments” may be missed (for both patients and providers) during treatment decision-making encounters if other factors beyond sexual and urological outcomes are not adequately considered and addressed. Furthermore, the results of our study may help clinicians counsel patients on their expectations for their recovery after surgery and identify particular issues related to HRQoL to pay close attention to in follow-up visits.
Strengths of our study include its prospective nature, which allowed evaluation of HRQoL outcomes at multiple time points throughout the first year after treatment. In addition, we used existing patient-reported outcome tools validated by the NIH to assess changes in HRQoL. PROMIS is an NIH-supported tool that can be leveraged in the pre- and posttreatment periods to identify patients who have impairments with HRQoL. It can provide clinicians with a unique opportunity to detect and intervene in setbacks and side effects to improve patient satisfaction and HRQoL.
Limitations of the current study include that most patients selected surgery for their treatment choice and that not all patients completed all longitudinal questionnaires, although this is expected in longitudinal studies of this nature. Although all the patients were approached and encouraged to participate, many did not participate and were not captured. In addition, not all patients completed end-of-study surveys. These factors may have biased our results because of unmeasurable factors related to nonparticipation or dropout. Our study encompassed the preoperative period up to 12 months postoperatively, which may fail to identify improvements or declines in HRQoL that may occur more than 12 months postoperatively, particularly related to continence and erectile function. The participants were enrolled by 6 surgeons, and we were not able to standardize the preoperative counseling either preoperatively or postoperatively, which may have biased our results. Finally, our study population consisted of predominantly white, married men of higher socioeconomic status; therefore, our results may not be generalizable to newly diagnosed prostate cancer patients overall.
Conclusions
By using validated self-administered questionnaires, we found that despite decreased sexual and urinary function, patients treated for prostate cancer were satisfied with their treatment choice. Correlates to higher patient satisfaction included decreased anxiety, depression, fatigue, and sleep disturbances.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7‐30.
2. Berry DL, Ellis WJ, Woods NF, Schwien C, Mullen KH, Yang C. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21(2):93-100.
3. Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50(4):711-718; discussion 718-720.
4. McCullough AR. Sexual dysfunction after radical prostatectomy. Rev Urol. 2005;7(2 suppl):S3-S10.
5. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261.
6. Latini DM, Hart SL, Knight SJ, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol. 2007;178(3, pt 1):826-831; discussion 831-832.
7. Meyer JP, Gillatt DA, Lockyer R, Macdonagh R. The effect of erectile dysfunction on the quality of life of men after radical prostatectomy. BJU Int. 2003;92(9):929-931.
8. Casey RG, Corcoran NM, Goldenberg SL. Quality of life issues in men undergoing androgen deprivation therapy: a review. Asian J Androl. 2012;14(2):226-231.
9. Segrin C, Badger TA, Harrington J. Interdependent psychological quality of life in dyads adjusting to prostate cancer. Health Psychol. 2012;31(1):70-79.
10. Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;11(3):304-314.
11. Cella D, Yount S, Rothrock N, et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3-S11.
12. Zapf M, Denham W, Barrera E, et al. Patient-centered outcomes after laparoscopic cholecystectomy. Surg Endosc. 2013;27(12):4491-4498.
13. Barry MJ, Fowler FJ Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549-1557; discussion 1564.
14. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830.
15. United States Preventive Services Task Force. Final update summary: prostate cancer: screening. http:// www.uspreventiveservicestaskforce.org/Page/ Document/UpdateSummaryFinal/prostate-cancer-screening. Updated July 2015. Accessed April 14, 2017
16. Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109(11):2239-2247.
17. Miwa S, Mizokami A, Konaka H, et al. Prospective longitudinal comparative study of health-related quality of life and treatment satisfaction in patients treated with hormone therapy, radical retropubic prostatectomy, and high or low dose rate brachytherapy for prostate cancer. Prostate Int. 2013;1(3):117-124.
18. Miller DC, Sanda MG, Dunn RL et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772-2780.
More than 164,690 men are expected to be diagnosed with prostate cancer in the United States in 2018.1 Men with prostate cancer face not only stress associated with the diagnosis but also decisional conflict regarding different treatment options.2 Most men diagnosed with clinically localized prostate cancer receive 1 or more of the following treatments: radical prostatectomy, external-beam radiation therapy, and/or brachytherapy, all of which are associated with posttreatment urological or sexual side effects including bowel, urinary, or erectile dysfunction.3-5 Men who choose active surveillance may experience increased anxiety associated with the constant vigilance and monitoring of their tumor status along with the uncertainty of not definitively removing or radiating their prostate.6 In addition to direct functional limitations of sexual and urological side effects, treatment can also lead to secondary psychosocial effects, including depression, self-blame, embarrassment, guilt, lower masculine self-esteem, increased reticence to participate socially or engage in sexual activity, and relationship distress.7-9 Therefore, health-related quality of life (HRQoL) and treatment satisfaction are important for this population.
Urological and sexual side effects of prostate cancer treatments are often a primary focus during treatment decision making between patients and providers. However, little prospective empirical data exist regarding the role of HRQoL and other nonurological physical and psychosocial outcomes on overall treatment satisfaction. The purpose of this study was to prospectively evaluate the role of both urological and nonurological outcomes on overall treatment satisfaction in men diagnosed with prostate cancer. We hypothesize that such an understanding can help describe changes in physical and psychosocial factors that are important to men beyond traditional urological outcomes, including their association with overall treatment satisfaction.
Methods
This was a prospective longitudinal assessment of patients from the Department of Urology at Northwestern University’s Feinberg School of Medicine in Chicago. Patients were eligible if they met the following inclusion criteria: they had been diagnosed with clinically localized or locally advanced prostate cancer; they had not yet received a primary treatment (eg, surgery, radiation, active surveillance) before their baseline assessment; they were 18 years or older; and they were able to read, write, speak, and understand English. Patients were excluded if they had a physical debilitation that would make participation not feasible or would create undue hardship, or if they had a history of diagnosed severe mental illness or hospitalization for chronic psychiatric reasons, as identified by referring physicians.
Eligible participants were approached before their treatment decision (if any). Patient enrollment occurred in 2 ways. For patients invited to participate during their clinic visit, the research assistant explained the study and obtained written informed consent for interested patients. A unique user identification and password was created for each patient, and they practiced using the touch screen computer while the research assistant observed and provided guidance as needed. When the patients were ready to start their pretreatment online interview, they completed the questionnaires by themselves. For patients who were invited to participate but were not scheduled to return in the foreseeable future, enrollment was carried out differently. In those cases, participating physicians contacted eligible patients who were not scheduled for a visit and informed them of the study opportunity. Interested patients were contacted by the research assistant who provided them with the study website address, which directed them to the online consent form. After a patient had completed the consent form, he was prompted to self-register. He received a unique user identification and password that could be used to complete the baseline assessment and subsequent assessments. However, for interested patients who did not have access to a computer or Internet connection, the research assistant provided them with paper consent forms and paper versions of all study assessments. After participants had completed the baseline assessment, the research assistant provided them with a written schedule of future assessments, which were expected to occur at 1 month posttreatment, 3 months posttreatment, 6 months posttreatment, and 12 months posttreatment.
For all follow-up appointments, participants could complete assessments either at clinic visits or from home using a secure online assessment platform called Assessment Center.10 The research assistant used a patient log to track participants and their progress in the study, which included study number, patient name (or initials), registration date, date of birth, sex, and timeline of completed or future assessments. The research assistant called or emailed participants (depending on patient preference) about a week before each of their follow-up assessments to facilitate adherence. If the participant did not log into the system by the target day, the research assistant contacted him the following day (target day +1) with a phone or email reminder to log into the system and complete the assessments. If the participant did not log in by midnight 1 day after the target day, the research assistant attempted to contact him one last time (target day +2) with either a reminder to log into the system or to ascertain his status that might be related to his noncompletion. Overall, a participant was called or e-mailed 1 to 3 times to remind him of his assessment. If he was unresponsive after 3 attempts, he was recorded as having withdrawn for an unknown reason.
At baseline and each follow-up time point, study participants completed a battery of patient-reported outcome measures, with most coming from the Patient-Reported Outcomes Measurement Information System (PROMIS)11 and the Surgical Outcomes Measurement System (SOMS).12 PROMIS is a National Institutes of Health (NIH) funded measurement system that has helped standardize and improve self-reported assessment of health status, symptoms, side effects, and different aspects of HRQoL, including physical, emotional, cognitive, and social health. SOMS is a suite of patient-reported outcome measures assessing important aspects of HRQoL after surgery. It was developed with feedback from surgeons, postoperative patients, and surgical nurses. PROMIS items were directly incorporated into numerous SOMS measures to facilitate easier comparisons and score crosswalks across measures and patient populations. In addition to PROMIS and SOMS measures, we also administered several well-known instruments of urological and sexual function, including the International Index of Erectile Function (IIEF) and American Urological Association Symptom Score Index (AUASS).13,14
Outcome measures were compared across sociodemographic and clinical variables at each time point using t tests for numerical variables (age) and with chi-square or Fisher exact tests for categorical variables; those variables with significant differences were used as covariates in statistical models. To examine differences in patient-reported scores over time, we used repeated measures analysis of covariance with general linear modeling methods. We used Pearson correlation coefficients to evaluate for correlations between quality-of-life outcomes and treatment satisfaction.
Not all participants completed each of the follow-up surveys, and reasons for dropout were prospectively documented. Most participants elected surgical resection as their primary treatment compared with the fewer than 10% of patients who chose radiation or chemotherapy as their primary treatment and about 20% of men who chose active surveillance after their initial diagnosis. Therefore, our analysis focused on patients who elected surgical resection. For comparison purposes, we included the HRQoL results from active surveillance patients.
Results
A total of 105 patients diagnosed with prostate cancer were enrolled in the study. Response rates decreased throughout the study (n = 75 at 1 month; n = 71 at 3 months; n = 64 at 6 months; n = 54 at 12 months). Sociodemographic and clinical characteristics of participants are shown in Table 1. The mean change from pretreatment (baseline) scores for each measure in patients treated with surgery is shown in Table 2, and the mean change from pretreatment scores in patients who elected active surveillance is shown in Table 3 (in both tables, a negative score denotes worsened function, and a positive change denotes improvement).
After surgery, patients reported significantly lower erectile function and sexual satisfaction scores. These included statistically significant decreases for IIEF Erectile Function, IIEF Overall Satisfaction, PROMIS Sexual Satisfaction, PROMIS Sexual Interest, and PROMIS Orgasm. In patients treated with surgery, there were significant improvements in anxiety observed for patients at each follow-up time, whereas significantly worse bladder problems were observed on SOMS Bladder at 1 and 3 months but returned to baseline by 12 months after surgery. AUASS was worse at 1 month but significantly improved at 6 and 12 months. Fatigue scores significantly worsened at 1 month but were no longer significant at 6 and 12 months. Physical Function was worsened at 1 month but not throughout the rest of the study. Bowel Problems (SOMS) were significantly worse at 1 month, but changes became nonsignificant on subsequent assessments. The only 2 domains that did not demonstrate any significant changes o
In active surveillance patients, sexual function domains were generally unchanged over the course of the study. However, unlike treated patients, there was no significant improvement in anxiety, depression, pain, fatigue, or sleep. In fact, most of these domains demonstrated worsened functioning, although these were not statistically significant. Urinary domains generally remained unchanged.
Pearson correlation coefficients between HRQoL measures and overall treatment satisfaction (assessed by the question, Are you satisfied with the results of your operation?) at each follow-up time point in patients treated with surgery are shown in Table 4. Relations between treatment satisfaction and sexual outcomes were generally statistically insignificant (r, .08-.56). However, sleep disturbance, depression, pain interference, fatigue, embarrassment, and bladder problems all demonstrated statistically significant positive associations with treatment satisfaction, with coefficients ranging from small to medium in magnitude (r, .32-.61). Other outcomes such as anxiety, physical function, and bowel problems demonstrated small to medium statistically significant associations with treatment satisfaction (r, .04-.60) but not at every time point. We performed t tests to examine treatment satisfaction in patients with detectable initial posttreatment prostate-specific antigen (PSA; >0.01 ng/mL). We found no difference in treatment satisfaction between patients with detectable PSA values and those with undetectable PSA at each time point.
When the patients were asked, Compared with what you expected, how do you rate the results of your operation?, most of those treated with surgery reported that the results of their operation were better than they had expected (Figure 1A; p. e137). More than 75% of the patients had results that were as expected or better than expected. When asked, Compared with what you expected, how do you rate your side effects of the operation?, almost 70% of patients reported side effects no worse than expected (Figure 1B). When asked, Are you satisfied with the results of your operation?, most patients reported that overall, they were satisfied with the results of their operation (Figure 1C).
At 12 months, none of the patients reported overall dissatisfaction with their treatment choice. More than 90% of patients were mostly or completely satisfied with the results of their operation.
Discussion
This prospective study assessed the HRQoL from pretreatment through 12 months posttreatment in men diagnosed with clinically localized prostate cancer that had been treated with surgery. Although the indicators of sexual function significantly decreased over time, they were not meaningfully associated with overall treatment satisfaction. Instead, a host of other factors, including psychosocial (eg, anxiety, depression, body image dissatisfaction, embarrassment), nonurological physical symptoms (pain interference, physical function, sleep disturbance, fatigue), and bladder problems, were significantly related to overall treatment satisfaction. Although this may not be surprising in other clinical oncology paradigms, the sheer surfeit of focus and attention on sexual function has overshadowed aspects of HRQoL that many men report are important to them, despite worsened sexual function outcomes.
Understanding potential treatment-related changes in HRQoL can be challenging for men when choosing providers and different therapeutic options. The increasing complexity of treatment in prostate cancer has created an opportunity to not only understand efficacy on cancer control but also focus on meaningful patient-reported outcomes. Hospitals and medical groups are increasingly aware of the importance of improving the patient care experience. Objective measures of patient satisfaction for health care providers, such as the Press-Ganey and Net Promoter score, exist to measure and improve patient experience. In prostate cancer, clinicians and large groups, including governmental agencies such as the US Preventive Services Task Force, have often focused on declines in urinary and erectile function15 without considering the full impact of prostate cancer treatment on global HRQoL. Our study was a prospective, longitudinal, self-reported examination of the impact, positive and negative, of prostate cancer treatment over a 12-month period.
Numerous studies have documented the treatment-related side effects of erectile, urinary, and bowel dysfunction in patients treated for prostate cancer, which may occur after definitive local therapies.5,16-18 The present study shows a similar impact on urinary, bowel, and erectile domains after treatment. Although erectile function scores remained lower through the course of the 12-month study, bowel and bladder domains returned to baseline by month 12. Unlike other studies, we also examined psychosocial and nonurological aspects of prostate cancer treatment. We found that there was a measurable and significant positive impact on other HRQoL measurements such as decreased anxiety. Despite a variety of declines across HRQoL domains, most patients reported that their results were largely as they had expected, and their side effects were the same or better than they had expected. No patient in the cohort reported being dissatisfied with his overall treatment, and more than 90% of patients were mostly or completely satisfied with their treatment choice. This highlights the point that while sexual and other urological domains of HRQoL are important, impairments in these areas do not necessarily reflect how many patients perceive success or satisfaction with their treatment choice. We also showed correlations between treatment satisfaction and improvement in sleep, anxiety, depression, and fatigue. It is worth noting that although there were decreases in the erectile and sexual function domains after treatment, those factors were not correlated with overall treatment satisfaction. Those factors may not routinely be assessed before, during, and after treatment for prostate cancer in most clinical encounters. However, because they were strongly associated with satisfaction with treatment outcomes in this study, identification in impairments may lead to opportunities to intervene and improve the patient experience. Therefore, important “teachable moments” may be missed (for both patients and providers) during treatment decision-making encounters if other factors beyond sexual and urological outcomes are not adequately considered and addressed. Furthermore, the results of our study may help clinicians counsel patients on their expectations for their recovery after surgery and identify particular issues related to HRQoL to pay close attention to in follow-up visits.
Strengths of our study include its prospective nature, which allowed evaluation of HRQoL outcomes at multiple time points throughout the first year after treatment. In addition, we used existing patient-reported outcome tools validated by the NIH to assess changes in HRQoL. PROMIS is an NIH-supported tool that can be leveraged in the pre- and posttreatment periods to identify patients who have impairments with HRQoL. It can provide clinicians with a unique opportunity to detect and intervene in setbacks and side effects to improve patient satisfaction and HRQoL.
Limitations of the current study include that most patients selected surgery for their treatment choice and that not all patients completed all longitudinal questionnaires, although this is expected in longitudinal studies of this nature. Although all the patients were approached and encouraged to participate, many did not participate and were not captured. In addition, not all patients completed end-of-study surveys. These factors may have biased our results because of unmeasurable factors related to nonparticipation or dropout. Our study encompassed the preoperative period up to 12 months postoperatively, which may fail to identify improvements or declines in HRQoL that may occur more than 12 months postoperatively, particularly related to continence and erectile function. The participants were enrolled by 6 surgeons, and we were not able to standardize the preoperative counseling either preoperatively or postoperatively, which may have biased our results. Finally, our study population consisted of predominantly white, married men of higher socioeconomic status; therefore, our results may not be generalizable to newly diagnosed prostate cancer patients overall.
Conclusions
By using validated self-administered questionnaires, we found that despite decreased sexual and urinary function, patients treated for prostate cancer were satisfied with their treatment choice. Correlates to higher patient satisfaction included decreased anxiety, depression, fatigue, and sleep disturbances.
More than 164,690 men are expected to be diagnosed with prostate cancer in the United States in 2018.1 Men with prostate cancer face not only stress associated with the diagnosis but also decisional conflict regarding different treatment options.2 Most men diagnosed with clinically localized prostate cancer receive 1 or more of the following treatments: radical prostatectomy, external-beam radiation therapy, and/or brachytherapy, all of which are associated with posttreatment urological or sexual side effects including bowel, urinary, or erectile dysfunction.3-5 Men who choose active surveillance may experience increased anxiety associated with the constant vigilance and monitoring of their tumor status along with the uncertainty of not definitively removing or radiating their prostate.6 In addition to direct functional limitations of sexual and urological side effects, treatment can also lead to secondary psychosocial effects, including depression, self-blame, embarrassment, guilt, lower masculine self-esteem, increased reticence to participate socially or engage in sexual activity, and relationship distress.7-9 Therefore, health-related quality of life (HRQoL) and treatment satisfaction are important for this population.
Urological and sexual side effects of prostate cancer treatments are often a primary focus during treatment decision making between patients and providers. However, little prospective empirical data exist regarding the role of HRQoL and other nonurological physical and psychosocial outcomes on overall treatment satisfaction. The purpose of this study was to prospectively evaluate the role of both urological and nonurological outcomes on overall treatment satisfaction in men diagnosed with prostate cancer. We hypothesize that such an understanding can help describe changes in physical and psychosocial factors that are important to men beyond traditional urological outcomes, including their association with overall treatment satisfaction.
Methods
This was a prospective longitudinal assessment of patients from the Department of Urology at Northwestern University’s Feinberg School of Medicine in Chicago. Patients were eligible if they met the following inclusion criteria: they had been diagnosed with clinically localized or locally advanced prostate cancer; they had not yet received a primary treatment (eg, surgery, radiation, active surveillance) before their baseline assessment; they were 18 years or older; and they were able to read, write, speak, and understand English. Patients were excluded if they had a physical debilitation that would make participation not feasible or would create undue hardship, or if they had a history of diagnosed severe mental illness or hospitalization for chronic psychiatric reasons, as identified by referring physicians.
Eligible participants were approached before their treatment decision (if any). Patient enrollment occurred in 2 ways. For patients invited to participate during their clinic visit, the research assistant explained the study and obtained written informed consent for interested patients. A unique user identification and password was created for each patient, and they practiced using the touch screen computer while the research assistant observed and provided guidance as needed. When the patients were ready to start their pretreatment online interview, they completed the questionnaires by themselves. For patients who were invited to participate but were not scheduled to return in the foreseeable future, enrollment was carried out differently. In those cases, participating physicians contacted eligible patients who were not scheduled for a visit and informed them of the study opportunity. Interested patients were contacted by the research assistant who provided them with the study website address, which directed them to the online consent form. After a patient had completed the consent form, he was prompted to self-register. He received a unique user identification and password that could be used to complete the baseline assessment and subsequent assessments. However, for interested patients who did not have access to a computer or Internet connection, the research assistant provided them with paper consent forms and paper versions of all study assessments. After participants had completed the baseline assessment, the research assistant provided them with a written schedule of future assessments, which were expected to occur at 1 month posttreatment, 3 months posttreatment, 6 months posttreatment, and 12 months posttreatment.
For all follow-up appointments, participants could complete assessments either at clinic visits or from home using a secure online assessment platform called Assessment Center.10 The research assistant used a patient log to track participants and their progress in the study, which included study number, patient name (or initials), registration date, date of birth, sex, and timeline of completed or future assessments. The research assistant called or emailed participants (depending on patient preference) about a week before each of their follow-up assessments to facilitate adherence. If the participant did not log into the system by the target day, the research assistant contacted him the following day (target day +1) with a phone or email reminder to log into the system and complete the assessments. If the participant did not log in by midnight 1 day after the target day, the research assistant attempted to contact him one last time (target day +2) with either a reminder to log into the system or to ascertain his status that might be related to his noncompletion. Overall, a participant was called or e-mailed 1 to 3 times to remind him of his assessment. If he was unresponsive after 3 attempts, he was recorded as having withdrawn for an unknown reason.
At baseline and each follow-up time point, study participants completed a battery of patient-reported outcome measures, with most coming from the Patient-Reported Outcomes Measurement Information System (PROMIS)11 and the Surgical Outcomes Measurement System (SOMS).12 PROMIS is a National Institutes of Health (NIH) funded measurement system that has helped standardize and improve self-reported assessment of health status, symptoms, side effects, and different aspects of HRQoL, including physical, emotional, cognitive, and social health. SOMS is a suite of patient-reported outcome measures assessing important aspects of HRQoL after surgery. It was developed with feedback from surgeons, postoperative patients, and surgical nurses. PROMIS items were directly incorporated into numerous SOMS measures to facilitate easier comparisons and score crosswalks across measures and patient populations. In addition to PROMIS and SOMS measures, we also administered several well-known instruments of urological and sexual function, including the International Index of Erectile Function (IIEF) and American Urological Association Symptom Score Index (AUASS).13,14
Outcome measures were compared across sociodemographic and clinical variables at each time point using t tests for numerical variables (age) and with chi-square or Fisher exact tests for categorical variables; those variables with significant differences were used as covariates in statistical models. To examine differences in patient-reported scores over time, we used repeated measures analysis of covariance with general linear modeling methods. We used Pearson correlation coefficients to evaluate for correlations between quality-of-life outcomes and treatment satisfaction.
Not all participants completed each of the follow-up surveys, and reasons for dropout were prospectively documented. Most participants elected surgical resection as their primary treatment compared with the fewer than 10% of patients who chose radiation or chemotherapy as their primary treatment and about 20% of men who chose active surveillance after their initial diagnosis. Therefore, our analysis focused on patients who elected surgical resection. For comparison purposes, we included the HRQoL results from active surveillance patients.
Results
A total of 105 patients diagnosed with prostate cancer were enrolled in the study. Response rates decreased throughout the study (n = 75 at 1 month; n = 71 at 3 months; n = 64 at 6 months; n = 54 at 12 months). Sociodemographic and clinical characteristics of participants are shown in Table 1. The mean change from pretreatment (baseline) scores for each measure in patients treated with surgery is shown in Table 2, and the mean change from pretreatment scores in patients who elected active surveillance is shown in Table 3 (in both tables, a negative score denotes worsened function, and a positive change denotes improvement).
After surgery, patients reported significantly lower erectile function and sexual satisfaction scores. These included statistically significant decreases for IIEF Erectile Function, IIEF Overall Satisfaction, PROMIS Sexual Satisfaction, PROMIS Sexual Interest, and PROMIS Orgasm. In patients treated with surgery, there were significant improvements in anxiety observed for patients at each follow-up time, whereas significantly worse bladder problems were observed on SOMS Bladder at 1 and 3 months but returned to baseline by 12 months after surgery. AUASS was worse at 1 month but significantly improved at 6 and 12 months. Fatigue scores significantly worsened at 1 month but were no longer significant at 6 and 12 months. Physical Function was worsened at 1 month but not throughout the rest of the study. Bowel Problems (SOMS) were significantly worse at 1 month, but changes became nonsignificant on subsequent assessments. The only 2 domains that did not demonstrate any significant changes o
In active surveillance patients, sexual function domains were generally unchanged over the course of the study. However, unlike treated patients, there was no significant improvement in anxiety, depression, pain, fatigue, or sleep. In fact, most of these domains demonstrated worsened functioning, although these were not statistically significant. Urinary domains generally remained unchanged.
Pearson correlation coefficients between HRQoL measures and overall treatment satisfaction (assessed by the question, Are you satisfied with the results of your operation?) at each follow-up time point in patients treated with surgery are shown in Table 4. Relations between treatment satisfaction and sexual outcomes were generally statistically insignificant (r, .08-.56). However, sleep disturbance, depression, pain interference, fatigue, embarrassment, and bladder problems all demonstrated statistically significant positive associations with treatment satisfaction, with coefficients ranging from small to medium in magnitude (r, .32-.61). Other outcomes such as anxiety, physical function, and bowel problems demonstrated small to medium statistically significant associations with treatment satisfaction (r, .04-.60) but not at every time point. We performed t tests to examine treatment satisfaction in patients with detectable initial posttreatment prostate-specific antigen (PSA; >0.01 ng/mL). We found no difference in treatment satisfaction between patients with detectable PSA values and those with undetectable PSA at each time point.
When the patients were asked, Compared with what you expected, how do you rate the results of your operation?, most of those treated with surgery reported that the results of their operation were better than they had expected (Figure 1A; p. e137). More than 75% of the patients had results that were as expected or better than expected. When asked, Compared with what you expected, how do you rate your side effects of the operation?, almost 70% of patients reported side effects no worse than expected (Figure 1B). When asked, Are you satisfied with the results of your operation?, most patients reported that overall, they were satisfied with the results of their operation (Figure 1C).
At 12 months, none of the patients reported overall dissatisfaction with their treatment choice. More than 90% of patients were mostly or completely satisfied with the results of their operation.
Discussion
This prospective study assessed the HRQoL from pretreatment through 12 months posttreatment in men diagnosed with clinically localized prostate cancer that had been treated with surgery. Although the indicators of sexual function significantly decreased over time, they were not meaningfully associated with overall treatment satisfaction. Instead, a host of other factors, including psychosocial (eg, anxiety, depression, body image dissatisfaction, embarrassment), nonurological physical symptoms (pain interference, physical function, sleep disturbance, fatigue), and bladder problems, were significantly related to overall treatment satisfaction. Although this may not be surprising in other clinical oncology paradigms, the sheer surfeit of focus and attention on sexual function has overshadowed aspects of HRQoL that many men report are important to them, despite worsened sexual function outcomes.
Understanding potential treatment-related changes in HRQoL can be challenging for men when choosing providers and different therapeutic options. The increasing complexity of treatment in prostate cancer has created an opportunity to not only understand efficacy on cancer control but also focus on meaningful patient-reported outcomes. Hospitals and medical groups are increasingly aware of the importance of improving the patient care experience. Objective measures of patient satisfaction for health care providers, such as the Press-Ganey and Net Promoter score, exist to measure and improve patient experience. In prostate cancer, clinicians and large groups, including governmental agencies such as the US Preventive Services Task Force, have often focused on declines in urinary and erectile function15 without considering the full impact of prostate cancer treatment on global HRQoL. Our study was a prospective, longitudinal, self-reported examination of the impact, positive and negative, of prostate cancer treatment over a 12-month period.
Numerous studies have documented the treatment-related side effects of erectile, urinary, and bowel dysfunction in patients treated for prostate cancer, which may occur after definitive local therapies.5,16-18 The present study shows a similar impact on urinary, bowel, and erectile domains after treatment. Although erectile function scores remained lower through the course of the 12-month study, bowel and bladder domains returned to baseline by month 12. Unlike other studies, we also examined psychosocial and nonurological aspects of prostate cancer treatment. We found that there was a measurable and significant positive impact on other HRQoL measurements such as decreased anxiety. Despite a variety of declines across HRQoL domains, most patients reported that their results were largely as they had expected, and their side effects were the same or better than they had expected. No patient in the cohort reported being dissatisfied with his overall treatment, and more than 90% of patients were mostly or completely satisfied with their treatment choice. This highlights the point that while sexual and other urological domains of HRQoL are important, impairments in these areas do not necessarily reflect how many patients perceive success or satisfaction with their treatment choice. We also showed correlations between treatment satisfaction and improvement in sleep, anxiety, depression, and fatigue. It is worth noting that although there were decreases in the erectile and sexual function domains after treatment, those factors were not correlated with overall treatment satisfaction. Those factors may not routinely be assessed before, during, and after treatment for prostate cancer in most clinical encounters. However, because they were strongly associated with satisfaction with treatment outcomes in this study, identification in impairments may lead to opportunities to intervene and improve the patient experience. Therefore, important “teachable moments” may be missed (for both patients and providers) during treatment decision-making encounters if other factors beyond sexual and urological outcomes are not adequately considered and addressed. Furthermore, the results of our study may help clinicians counsel patients on their expectations for their recovery after surgery and identify particular issues related to HRQoL to pay close attention to in follow-up visits.
Strengths of our study include its prospective nature, which allowed evaluation of HRQoL outcomes at multiple time points throughout the first year after treatment. In addition, we used existing patient-reported outcome tools validated by the NIH to assess changes in HRQoL. PROMIS is an NIH-supported tool that can be leveraged in the pre- and posttreatment periods to identify patients who have impairments with HRQoL. It can provide clinicians with a unique opportunity to detect and intervene in setbacks and side effects to improve patient satisfaction and HRQoL.
Limitations of the current study include that most patients selected surgery for their treatment choice and that not all patients completed all longitudinal questionnaires, although this is expected in longitudinal studies of this nature. Although all the patients were approached and encouraged to participate, many did not participate and were not captured. In addition, not all patients completed end-of-study surveys. These factors may have biased our results because of unmeasurable factors related to nonparticipation or dropout. Our study encompassed the preoperative period up to 12 months postoperatively, which may fail to identify improvements or declines in HRQoL that may occur more than 12 months postoperatively, particularly related to continence and erectile function. The participants were enrolled by 6 surgeons, and we were not able to standardize the preoperative counseling either preoperatively or postoperatively, which may have biased our results. Finally, our study population consisted of predominantly white, married men of higher socioeconomic status; therefore, our results may not be generalizable to newly diagnosed prostate cancer patients overall.
Conclusions
By using validated self-administered questionnaires, we found that despite decreased sexual and urinary function, patients treated for prostate cancer were satisfied with their treatment choice. Correlates to higher patient satisfaction included decreased anxiety, depression, fatigue, and sleep disturbances.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7‐30.
2. Berry DL, Ellis WJ, Woods NF, Schwien C, Mullen KH, Yang C. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21(2):93-100.
3. Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50(4):711-718; discussion 718-720.
4. McCullough AR. Sexual dysfunction after radical prostatectomy. Rev Urol. 2005;7(2 suppl):S3-S10.
5. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261.
6. Latini DM, Hart SL, Knight SJ, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol. 2007;178(3, pt 1):826-831; discussion 831-832.
7. Meyer JP, Gillatt DA, Lockyer R, Macdonagh R. The effect of erectile dysfunction on the quality of life of men after radical prostatectomy. BJU Int. 2003;92(9):929-931.
8. Casey RG, Corcoran NM, Goldenberg SL. Quality of life issues in men undergoing androgen deprivation therapy: a review. Asian J Androl. 2012;14(2):226-231.
9. Segrin C, Badger TA, Harrington J. Interdependent psychological quality of life in dyads adjusting to prostate cancer. Health Psychol. 2012;31(1):70-79.
10. Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;11(3):304-314.
11. Cella D, Yount S, Rothrock N, et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3-S11.
12. Zapf M, Denham W, Barrera E, et al. Patient-centered outcomes after laparoscopic cholecystectomy. Surg Endosc. 2013;27(12):4491-4498.
13. Barry MJ, Fowler FJ Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549-1557; discussion 1564.
14. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830.
15. United States Preventive Services Task Force. Final update summary: prostate cancer: screening. http:// www.uspreventiveservicestaskforce.org/Page/ Document/UpdateSummaryFinal/prostate-cancer-screening. Updated July 2015. Accessed April 14, 2017
16. Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109(11):2239-2247.
17. Miwa S, Mizokami A, Konaka H, et al. Prospective longitudinal comparative study of health-related quality of life and treatment satisfaction in patients treated with hormone therapy, radical retropubic prostatectomy, and high or low dose rate brachytherapy for prostate cancer. Prostate Int. 2013;1(3):117-124.
18. Miller DC, Sanda MG, Dunn RL et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772-2780.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7‐30.
2. Berry DL, Ellis WJ, Woods NF, Schwien C, Mullen KH, Yang C. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21(2):93-100.
3. Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50(4):711-718; discussion 718-720.
4. McCullough AR. Sexual dysfunction after radical prostatectomy. Rev Urol. 2005;7(2 suppl):S3-S10.
5. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261.
6. Latini DM, Hart SL, Knight SJ, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol. 2007;178(3, pt 1):826-831; discussion 831-832.
7. Meyer JP, Gillatt DA, Lockyer R, Macdonagh R. The effect of erectile dysfunction on the quality of life of men after radical prostatectomy. BJU Int. 2003;92(9):929-931.
8. Casey RG, Corcoran NM, Goldenberg SL. Quality of life issues in men undergoing androgen deprivation therapy: a review. Asian J Androl. 2012;14(2):226-231.
9. Segrin C, Badger TA, Harrington J. Interdependent psychological quality of life in dyads adjusting to prostate cancer. Health Psychol. 2012;31(1):70-79.
10. Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;11(3):304-314.
11. Cella D, Yount S, Rothrock N, et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3-S11.
12. Zapf M, Denham W, Barrera E, et al. Patient-centered outcomes after laparoscopic cholecystectomy. Surg Endosc. 2013;27(12):4491-4498.
13. Barry MJ, Fowler FJ Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549-1557; discussion 1564.
14. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830.
15. United States Preventive Services Task Force. Final update summary: prostate cancer: screening. http:// www.uspreventiveservicestaskforce.org/Page/ Document/UpdateSummaryFinal/prostate-cancer-screening. Updated July 2015. Accessed April 14, 2017
16. Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109(11):2239-2247.
17. Miwa S, Mizokami A, Konaka H, et al. Prospective longitudinal comparative study of health-related quality of life and treatment satisfaction in patients treated with hormone therapy, radical retropubic prostatectomy, and high or low dose rate brachytherapy for prostate cancer. Prostate Int. 2013;1(3):117-124.
18. Miller DC, Sanda MG, Dunn RL et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772-2780.
Rural cancer patients report faster care than urban counterparts
in a survey of 6,826 Medicare beneficiaries.
Taken as a whole, a similar quality of care was reported between the two groups, but the picture changed when racial/ethnic subgroups were considered. Non-Hispanic black and Hispanic patients in rural locations reported inferior care to their urban counterparts, investigators wrote in Cancer.
“Cancer patients living in rural areas are vulnerable and have unique health care needs,” wrote lead author Michelle A. Mollica, PhD, of the National Cancer Institute, and her colleagues. “To our knowledge, this is the first study to explore the patient’s perception of the timeliness of care in such a large, multiregion sample of cancer patients.”
In 2003, the National Academy of Medicine concluded that living in a rural environment was associated with poorer health. Existing research surrounding cancer has echoed this concern, showing that rural patients have higher rates of cancer and mortality, longer delays in diagnosis, and limited access to care.
The current, retrospective study involved 6,140 urban and 686 rural Medicare beneficiaries who were aged at least 65 years when diagnosed with either breast, lung, colorectal, or prostate cancer. Consumer Assessment of Healthcare Providers and Systems surveys were conducted between 1998 and 2013, then linked with data from the Surveillance, Epidemiology, and End Results registry program.
Surveys were conducted within 12 months of diagnosis, during which time patients were asked about their access to care as defined by two composites: “Getting Needed Care” and “Getting Care Quickly.” Getting Needed Care included ease of making appointments and receiving treatments and Getting Care Quickly questions asked about appointment delays and time spent waiting at the doctor’s office. Answers were converted to a numerical score from 0 to 100, with 0 being the worst and 100 being the best.
For both composites, mean scores for urban and rural locations were greater than 85 out of 100.
In contrast to previous studies, urban patients reported longer delays in care, scoring Getting Care Quickly 2.27 points lower than rural patients (P = .02). Pacific Islanders and non-Hispanic Asian patients from rural places reported even faster care, ranking about 8 points higher than urban patients of the same race/ethnicity.
Locality did not have a significant impact on Getting Needed Care unless race/ethnicity was also considered (P = .04). Non-Hispanic white patients from rural locations scored Getting Needed Care about 2 points higher than urban white patients, while Hispanic and non-Hispanic black patients had an opposite trend, with this rural cohort ranking Getting Needed Care lower than urban patients of the same race/ethnicity.
“Geographic residence is but one important factor in cancer care delivery,” the authors noted. “There is a need for fine-grained research looking at specific barriers for urban residents, experiences of racial/ethnic minority survivors residing in rural areas, and rural-urban differences in the clinic settings in which medical care is delivered.”
The authors had no disclosures to report.
SOURCE: Mollica MA et al. Cancer. 2018 Jun 7. doi: 10.1002/cncr.31541.
in a survey of 6,826 Medicare beneficiaries.
Taken as a whole, a similar quality of care was reported between the two groups, but the picture changed when racial/ethnic subgroups were considered. Non-Hispanic black and Hispanic patients in rural locations reported inferior care to their urban counterparts, investigators wrote in Cancer.
“Cancer patients living in rural areas are vulnerable and have unique health care needs,” wrote lead author Michelle A. Mollica, PhD, of the National Cancer Institute, and her colleagues. “To our knowledge, this is the first study to explore the patient’s perception of the timeliness of care in such a large, multiregion sample of cancer patients.”
In 2003, the National Academy of Medicine concluded that living in a rural environment was associated with poorer health. Existing research surrounding cancer has echoed this concern, showing that rural patients have higher rates of cancer and mortality, longer delays in diagnosis, and limited access to care.
The current, retrospective study involved 6,140 urban and 686 rural Medicare beneficiaries who were aged at least 65 years when diagnosed with either breast, lung, colorectal, or prostate cancer. Consumer Assessment of Healthcare Providers and Systems surveys were conducted between 1998 and 2013, then linked with data from the Surveillance, Epidemiology, and End Results registry program.
Surveys were conducted within 12 months of diagnosis, during which time patients were asked about their access to care as defined by two composites: “Getting Needed Care” and “Getting Care Quickly.” Getting Needed Care included ease of making appointments and receiving treatments and Getting Care Quickly questions asked about appointment delays and time spent waiting at the doctor’s office. Answers were converted to a numerical score from 0 to 100, with 0 being the worst and 100 being the best.
For both composites, mean scores for urban and rural locations were greater than 85 out of 100.
In contrast to previous studies, urban patients reported longer delays in care, scoring Getting Care Quickly 2.27 points lower than rural patients (P = .02). Pacific Islanders and non-Hispanic Asian patients from rural places reported even faster care, ranking about 8 points higher than urban patients of the same race/ethnicity.
Locality did not have a significant impact on Getting Needed Care unless race/ethnicity was also considered (P = .04). Non-Hispanic white patients from rural locations scored Getting Needed Care about 2 points higher than urban white patients, while Hispanic and non-Hispanic black patients had an opposite trend, with this rural cohort ranking Getting Needed Care lower than urban patients of the same race/ethnicity.
“Geographic residence is but one important factor in cancer care delivery,” the authors noted. “There is a need for fine-grained research looking at specific barriers for urban residents, experiences of racial/ethnic minority survivors residing in rural areas, and rural-urban differences in the clinic settings in which medical care is delivered.”
The authors had no disclosures to report.
SOURCE: Mollica MA et al. Cancer. 2018 Jun 7. doi: 10.1002/cncr.31541.
in a survey of 6,826 Medicare beneficiaries.
Taken as a whole, a similar quality of care was reported between the two groups, but the picture changed when racial/ethnic subgroups were considered. Non-Hispanic black and Hispanic patients in rural locations reported inferior care to their urban counterparts, investigators wrote in Cancer.
“Cancer patients living in rural areas are vulnerable and have unique health care needs,” wrote lead author Michelle A. Mollica, PhD, of the National Cancer Institute, and her colleagues. “To our knowledge, this is the first study to explore the patient’s perception of the timeliness of care in such a large, multiregion sample of cancer patients.”
In 2003, the National Academy of Medicine concluded that living in a rural environment was associated with poorer health. Existing research surrounding cancer has echoed this concern, showing that rural patients have higher rates of cancer and mortality, longer delays in diagnosis, and limited access to care.
The current, retrospective study involved 6,140 urban and 686 rural Medicare beneficiaries who were aged at least 65 years when diagnosed with either breast, lung, colorectal, or prostate cancer. Consumer Assessment of Healthcare Providers and Systems surveys were conducted between 1998 and 2013, then linked with data from the Surveillance, Epidemiology, and End Results registry program.
Surveys were conducted within 12 months of diagnosis, during which time patients were asked about their access to care as defined by two composites: “Getting Needed Care” and “Getting Care Quickly.” Getting Needed Care included ease of making appointments and receiving treatments and Getting Care Quickly questions asked about appointment delays and time spent waiting at the doctor’s office. Answers were converted to a numerical score from 0 to 100, with 0 being the worst and 100 being the best.
For both composites, mean scores for urban and rural locations were greater than 85 out of 100.
In contrast to previous studies, urban patients reported longer delays in care, scoring Getting Care Quickly 2.27 points lower than rural patients (P = .02). Pacific Islanders and non-Hispanic Asian patients from rural places reported even faster care, ranking about 8 points higher than urban patients of the same race/ethnicity.
Locality did not have a significant impact on Getting Needed Care unless race/ethnicity was also considered (P = .04). Non-Hispanic white patients from rural locations scored Getting Needed Care about 2 points higher than urban white patients, while Hispanic and non-Hispanic black patients had an opposite trend, with this rural cohort ranking Getting Needed Care lower than urban patients of the same race/ethnicity.
“Geographic residence is but one important factor in cancer care delivery,” the authors noted. “There is a need for fine-grained research looking at specific barriers for urban residents, experiences of racial/ethnic minority survivors residing in rural areas, and rural-urban differences in the clinic settings in which medical care is delivered.”
The authors had no disclosures to report.
SOURCE: Mollica MA et al. Cancer. 2018 Jun 7. doi: 10.1002/cncr.31541.
FROM CANCER
Key clinical point: Cancer patients living in rural areas reported more timely care than urban patients.
Major finding: In a Consumer Assessment of Healthcare Providers and Systems (CAHPS) survey, urban patients rated “Getting Care Quickly” 2.27 points lower than rural patients (P = .02).
Study details: A retrospective study of 6,140 urban and 686 rural Medicare beneficiaries who were aged at least 65 years when diagnosed with either breast, lung, colorectal, or prostate cancer. CAHPS patient experience surveys were conducted between 1998 and 2013, then linked with Surveillance, Epidemiology, and End Results data.
Disclosures: The authors had no disclosures to report.
Source: Mollica MA et al. Cancer. 2018 Jun 7. doi: 10.1002/cncr.31541.
Protein activation could predict renal cell carcinoma recurrence
Levels of activity of a protein linked to malignancy could help predict if and when patients with renal cell carcinoma are likely to experience a recurrence, investigators report.
In a retrospective cohort study, the researchers looked at surgical specimens from 303 patients with localized clear cell renal cell carcinoma who had surgery between 1993 and 2011. The specimens were stained for antibodies against three key proteins; eukaryotic initiation factor 4E (eIF4E), eukaryotic initiation factor 4E binding protein 1 (4EBP1), and phospho eukaryotic initiation factor 4E binding protein 1 (p-4EBP1), reported Osamu Ichiyanagi, MD, of Yamagata (Japan) University, and colleagues. The study was published in Clinical Genitourinary Cancer.
The 4EBP1/eIF4E axis is known to regulate protein synthesis associated with malignant behavior. Researchers found that while the expression levels for the three proteins were similar between the recurrence-free and recurrence groups, patients who did not experience a recurrence had significantly lower activity in the 4EBP1/eIF4E axis.
The analysis showed that having intermediate or strong activation of the 4EBP1/eIF4E axis was an independent predictor of the risk of recurrence, as was Fuhrman grade 3/4 and pathological T stage of pT1b or above.
Only two patients who had weak activation of the 4EBP1/eIF4E axis experienced a recurrence (one early, one late).
Overall, 31 patients experienced an early recurrence – defined as recurrence within 5 years – and 16 patients experienced a recurrence after 5 years. Strong activation of the 4EBP1/eIF4E axis was an independent predictor of early recurrence.
About one-third of patients with nonmetastatic renal cell carcinoma who are curatively treated with nephrectomy experience a tumor recurrence, most commonly a distant metastasis. Late recurrence is known to occur in 6%-12% of patients.
“We show here for the first time that activation level of the 4EBP1/eIF4E axis in localised ccRCC may contribute not only to tumour recurrence but also to the timing of recurrence following curative nephrectomy,” wrote Dr. Ichiyanagi and coauthors. “Our data indicate that this activation may impact ER [early recurrence] and LR [late recurrence] events differentially.”
The study also found that more patients experienced recurrence after curative nephrectomy, suggesting that the 4EBP1/eIF4E axis became strongly activated after this.
The study was supported by Yamagata University Faculty of Medicine and the Japan Society for Promotion of Science. No conflicts of interest were declared.
SOURCE: Ichiyanagi O et al. Clin Genitourin Cancer. 2018 June 8. doi: 10.1016/j.clgc.2018.06.002.
Levels of activity of a protein linked to malignancy could help predict if and when patients with renal cell carcinoma are likely to experience a recurrence, investigators report.
In a retrospective cohort study, the researchers looked at surgical specimens from 303 patients with localized clear cell renal cell carcinoma who had surgery between 1993 and 2011. The specimens were stained for antibodies against three key proteins; eukaryotic initiation factor 4E (eIF4E), eukaryotic initiation factor 4E binding protein 1 (4EBP1), and phospho eukaryotic initiation factor 4E binding protein 1 (p-4EBP1), reported Osamu Ichiyanagi, MD, of Yamagata (Japan) University, and colleagues. The study was published in Clinical Genitourinary Cancer.
The 4EBP1/eIF4E axis is known to regulate protein synthesis associated with malignant behavior. Researchers found that while the expression levels for the three proteins were similar between the recurrence-free and recurrence groups, patients who did not experience a recurrence had significantly lower activity in the 4EBP1/eIF4E axis.
The analysis showed that having intermediate or strong activation of the 4EBP1/eIF4E axis was an independent predictor of the risk of recurrence, as was Fuhrman grade 3/4 and pathological T stage of pT1b or above.
Only two patients who had weak activation of the 4EBP1/eIF4E axis experienced a recurrence (one early, one late).
Overall, 31 patients experienced an early recurrence – defined as recurrence within 5 years – and 16 patients experienced a recurrence after 5 years. Strong activation of the 4EBP1/eIF4E axis was an independent predictor of early recurrence.
About one-third of patients with nonmetastatic renal cell carcinoma who are curatively treated with nephrectomy experience a tumor recurrence, most commonly a distant metastasis. Late recurrence is known to occur in 6%-12% of patients.
“We show here for the first time that activation level of the 4EBP1/eIF4E axis in localised ccRCC may contribute not only to tumour recurrence but also to the timing of recurrence following curative nephrectomy,” wrote Dr. Ichiyanagi and coauthors. “Our data indicate that this activation may impact ER [early recurrence] and LR [late recurrence] events differentially.”
The study also found that more patients experienced recurrence after curative nephrectomy, suggesting that the 4EBP1/eIF4E axis became strongly activated after this.
The study was supported by Yamagata University Faculty of Medicine and the Japan Society for Promotion of Science. No conflicts of interest were declared.
SOURCE: Ichiyanagi O et al. Clin Genitourin Cancer. 2018 June 8. doi: 10.1016/j.clgc.2018.06.002.
Levels of activity of a protein linked to malignancy could help predict if and when patients with renal cell carcinoma are likely to experience a recurrence, investigators report.
In a retrospective cohort study, the researchers looked at surgical specimens from 303 patients with localized clear cell renal cell carcinoma who had surgery between 1993 and 2011. The specimens were stained for antibodies against three key proteins; eukaryotic initiation factor 4E (eIF4E), eukaryotic initiation factor 4E binding protein 1 (4EBP1), and phospho eukaryotic initiation factor 4E binding protein 1 (p-4EBP1), reported Osamu Ichiyanagi, MD, of Yamagata (Japan) University, and colleagues. The study was published in Clinical Genitourinary Cancer.
The 4EBP1/eIF4E axis is known to regulate protein synthesis associated with malignant behavior. Researchers found that while the expression levels for the three proteins were similar between the recurrence-free and recurrence groups, patients who did not experience a recurrence had significantly lower activity in the 4EBP1/eIF4E axis.
The analysis showed that having intermediate or strong activation of the 4EBP1/eIF4E axis was an independent predictor of the risk of recurrence, as was Fuhrman grade 3/4 and pathological T stage of pT1b or above.
Only two patients who had weak activation of the 4EBP1/eIF4E axis experienced a recurrence (one early, one late).
Overall, 31 patients experienced an early recurrence – defined as recurrence within 5 years – and 16 patients experienced a recurrence after 5 years. Strong activation of the 4EBP1/eIF4E axis was an independent predictor of early recurrence.
About one-third of patients with nonmetastatic renal cell carcinoma who are curatively treated with nephrectomy experience a tumor recurrence, most commonly a distant metastasis. Late recurrence is known to occur in 6%-12% of patients.
“We show here for the first time that activation level of the 4EBP1/eIF4E axis in localised ccRCC may contribute not only to tumour recurrence but also to the timing of recurrence following curative nephrectomy,” wrote Dr. Ichiyanagi and coauthors. “Our data indicate that this activation may impact ER [early recurrence] and LR [late recurrence] events differentially.”
The study also found that more patients experienced recurrence after curative nephrectomy, suggesting that the 4EBP1/eIF4E axis became strongly activated after this.
The study was supported by Yamagata University Faculty of Medicine and the Japan Society for Promotion of Science. No conflicts of interest were declared.
SOURCE: Ichiyanagi O et al. Clin Genitourin Cancer. 2018 June 8. doi: 10.1016/j.clgc.2018.06.002.
FROM CLINICAL GENITOURINARY CANCER
Key clinical point: Protein activation levels of the 4EBP1/eIF4E axis may predict renal cell carcinoma recurrence.
Major finding: Strong protein activation of the 4EBP1/eIF4E axis may be linked to early recurrence of renal cell carcinoma.
Study details: Retrospective cohort study in 303 patients with localized clear cell renal cell carcinoma.
Disclosures: The study was supported by Yamagata University Faculty of Medicine and the Japan Society for Promotion of Science. No conflicts of interest were declared.
Source: Ichiyanagi O et al. Clin Genitourin Cancer. 2018 June 8. doi: 10.1016/j.clgc.2018.06.002.
Smoking tied to localized prostate cancer recurrence, metastasis, death
Patients with localized prostate cancer who were smokers at the time of local therapy had a higher risk of experiencing adverse outcomes and death related to the disease, a systematic review and meta-analysis has shown.
Current smokers in the study had a higher risk of biochemical recurrence, metastasis, and cancer-specific mortality after undergoing primary radical prostatectomy or radiotherapy.
“Our findings encourage radiation oncologists and urologists to counsel patients to stop smoking, using primary prostate cancer treatment as a teachable moment,” wrote Dr. Foerster and coauthors. The report was published in JAMA Oncology.
The investigators performed a database search of studies published from January 2000 to March 2017 and selected 11 articles for quantitative analysis. Those studies, which were all observational and not randomized, comprised 22,549 patients with prostate cancer undergoing radical prostatectomy or radiotherapy. Of those patients, 4,202 (18.6%) were current smokers.
Current smokers had a higher risk of cancer-specific mortality, the investigators found based on analysis of five studies (hazard ratio, 1.89; 95% confidence interval, 1.37-2.69; P less than .001).
They also had a significantly higher risk of biochemical recurrence, based on 10 studies (HR, 1.40; 95% CI, 1.18-1.66; P less than .001), and high risk of metastasis based on 3 studies (HR, 2.51; 95% CI, 1.80-3.51; P less than .001), the report shows.
Future studies need to more closely examine the link between smoking cessation and longer-term oncologic outcomes, as well as a more precise assessment of smoking exposure, the researchers concluded.
In the current study, results were inconclusive as to whether former smoking and time to cessation had any relationship to outcomes after radical prostatectomy or radiotherapy.
“Available data were sparse and heterogenous,” they noted.
Dr. Foerster is supported by the Scholarship Foundation of Swiss Urology. One coauthor reported disclosures related to Astellas, Cepheid, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanochemia, Sanofi, and Wolff.
SOURCE: Foerster B et al. JAMA Oncol. 2018 May 24. doi: 10.1001/jamaoncol.2018.1071.
While previous studies have linked smoking to adverse outcomes in prostate cancer, including death, this study argues that the higher rate of prostate cancer–related death among smokers is a real effect with a biological cause, Stephen J. Freedland, MD, said in an editorial.
The current study included only men healthy enough to undergo aggressive treatment, which is an “important and necessary step to level the playing field,” Dr. Freedland wrote.
In that context, current smokers in this study had an 89% increased risk of prostate cancer–related death. “This finding shows that when we treat patients equally and minimize deaths from other causes, there is a stronger link between smoking and death from prostate cancer,” Dr. Freedland noted.
The finding also supports the notion that many smokers won’t live long enough to die from prostate cancer, given its slow-growing nature and the effects of smoking on competing causes of death, he added.
A scenario in which smokers did not live long enough to die of prostate cancer would predict a lower risk of prostate cancer–related death among smokers, he explained.
Because smoking has such clear adverse effects, physicians of all specialties should be hypervigilant about urging patients to quit smoking, Dr. Freedland concluded.
“If all of us remembered we are physicians first and oncologists and/or prostate cancer experts second, we will realize we are uniquely poised to help our patients, as the time of a new cancer diagnosis is a teachable moment when patients are very receptive to overall health advice,” he wrote.
Dr. Freedland is with the Center for Integrated Research on Cancer and Lifestyle, Cedars-Sinai Medical Center, Los Angeles. These comments are derived from his editorial in JAMA Oncology. Dr. Freeland had no reported conflict of interest disclosures.
While previous studies have linked smoking to adverse outcomes in prostate cancer, including death, this study argues that the higher rate of prostate cancer–related death among smokers is a real effect with a biological cause, Stephen J. Freedland, MD, said in an editorial.
The current study included only men healthy enough to undergo aggressive treatment, which is an “important and necessary step to level the playing field,” Dr. Freedland wrote.
In that context, current smokers in this study had an 89% increased risk of prostate cancer–related death. “This finding shows that when we treat patients equally and minimize deaths from other causes, there is a stronger link between smoking and death from prostate cancer,” Dr. Freedland noted.
The finding also supports the notion that many smokers won’t live long enough to die from prostate cancer, given its slow-growing nature and the effects of smoking on competing causes of death, he added.
A scenario in which smokers did not live long enough to die of prostate cancer would predict a lower risk of prostate cancer–related death among smokers, he explained.
Because smoking has such clear adverse effects, physicians of all specialties should be hypervigilant about urging patients to quit smoking, Dr. Freedland concluded.
“If all of us remembered we are physicians first and oncologists and/or prostate cancer experts second, we will realize we are uniquely poised to help our patients, as the time of a new cancer diagnosis is a teachable moment when patients are very receptive to overall health advice,” he wrote.
Dr. Freedland is with the Center for Integrated Research on Cancer and Lifestyle, Cedars-Sinai Medical Center, Los Angeles. These comments are derived from his editorial in JAMA Oncology. Dr. Freeland had no reported conflict of interest disclosures.
While previous studies have linked smoking to adverse outcomes in prostate cancer, including death, this study argues that the higher rate of prostate cancer–related death among smokers is a real effect with a biological cause, Stephen J. Freedland, MD, said in an editorial.
The current study included only men healthy enough to undergo aggressive treatment, which is an “important and necessary step to level the playing field,” Dr. Freedland wrote.
In that context, current smokers in this study had an 89% increased risk of prostate cancer–related death. “This finding shows that when we treat patients equally and minimize deaths from other causes, there is a stronger link between smoking and death from prostate cancer,” Dr. Freedland noted.
The finding also supports the notion that many smokers won’t live long enough to die from prostate cancer, given its slow-growing nature and the effects of smoking on competing causes of death, he added.
A scenario in which smokers did not live long enough to die of prostate cancer would predict a lower risk of prostate cancer–related death among smokers, he explained.
Because smoking has such clear adverse effects, physicians of all specialties should be hypervigilant about urging patients to quit smoking, Dr. Freedland concluded.
“If all of us remembered we are physicians first and oncologists and/or prostate cancer experts second, we will realize we are uniquely poised to help our patients, as the time of a new cancer diagnosis is a teachable moment when patients are very receptive to overall health advice,” he wrote.
Dr. Freedland is with the Center for Integrated Research on Cancer and Lifestyle, Cedars-Sinai Medical Center, Los Angeles. These comments are derived from his editorial in JAMA Oncology. Dr. Freeland had no reported conflict of interest disclosures.
Patients with localized prostate cancer who were smokers at the time of local therapy had a higher risk of experiencing adverse outcomes and death related to the disease, a systematic review and meta-analysis has shown.
Current smokers in the study had a higher risk of biochemical recurrence, metastasis, and cancer-specific mortality after undergoing primary radical prostatectomy or radiotherapy.
“Our findings encourage radiation oncologists and urologists to counsel patients to stop smoking, using primary prostate cancer treatment as a teachable moment,” wrote Dr. Foerster and coauthors. The report was published in JAMA Oncology.
The investigators performed a database search of studies published from January 2000 to March 2017 and selected 11 articles for quantitative analysis. Those studies, which were all observational and not randomized, comprised 22,549 patients with prostate cancer undergoing radical prostatectomy or radiotherapy. Of those patients, 4,202 (18.6%) were current smokers.
Current smokers had a higher risk of cancer-specific mortality, the investigators found based on analysis of five studies (hazard ratio, 1.89; 95% confidence interval, 1.37-2.69; P less than .001).
They also had a significantly higher risk of biochemical recurrence, based on 10 studies (HR, 1.40; 95% CI, 1.18-1.66; P less than .001), and high risk of metastasis based on 3 studies (HR, 2.51; 95% CI, 1.80-3.51; P less than .001), the report shows.
Future studies need to more closely examine the link between smoking cessation and longer-term oncologic outcomes, as well as a more precise assessment of smoking exposure, the researchers concluded.
In the current study, results were inconclusive as to whether former smoking and time to cessation had any relationship to outcomes after radical prostatectomy or radiotherapy.
“Available data were sparse and heterogenous,” they noted.
Dr. Foerster is supported by the Scholarship Foundation of Swiss Urology. One coauthor reported disclosures related to Astellas, Cepheid, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanochemia, Sanofi, and Wolff.
SOURCE: Foerster B et al. JAMA Oncol. 2018 May 24. doi: 10.1001/jamaoncol.2018.1071.
Patients with localized prostate cancer who were smokers at the time of local therapy had a higher risk of experiencing adverse outcomes and death related to the disease, a systematic review and meta-analysis has shown.
Current smokers in the study had a higher risk of biochemical recurrence, metastasis, and cancer-specific mortality after undergoing primary radical prostatectomy or radiotherapy.
“Our findings encourage radiation oncologists and urologists to counsel patients to stop smoking, using primary prostate cancer treatment as a teachable moment,” wrote Dr. Foerster and coauthors. The report was published in JAMA Oncology.
The investigators performed a database search of studies published from January 2000 to March 2017 and selected 11 articles for quantitative analysis. Those studies, which were all observational and not randomized, comprised 22,549 patients with prostate cancer undergoing radical prostatectomy or radiotherapy. Of those patients, 4,202 (18.6%) were current smokers.
Current smokers had a higher risk of cancer-specific mortality, the investigators found based on analysis of five studies (hazard ratio, 1.89; 95% confidence interval, 1.37-2.69; P less than .001).
They also had a significantly higher risk of biochemical recurrence, based on 10 studies (HR, 1.40; 95% CI, 1.18-1.66; P less than .001), and high risk of metastasis based on 3 studies (HR, 2.51; 95% CI, 1.80-3.51; P less than .001), the report shows.
Future studies need to more closely examine the link between smoking cessation and longer-term oncologic outcomes, as well as a more precise assessment of smoking exposure, the researchers concluded.
In the current study, results were inconclusive as to whether former smoking and time to cessation had any relationship to outcomes after radical prostatectomy or radiotherapy.
“Available data were sparse and heterogenous,” they noted.
Dr. Foerster is supported by the Scholarship Foundation of Swiss Urology. One coauthor reported disclosures related to Astellas, Cepheid, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanochemia, Sanofi, and Wolff.
SOURCE: Foerster B et al. JAMA Oncol. 2018 May 24. doi: 10.1001/jamaoncol.2018.1071.
FROM JAMA ONCOLOGY
Key clinical point: Patients with localized prostate cancer should be encouraged to quit smoking because of the risk of potentially worse oncologic outcomes.
Major finding: Current smokers had a higher risk of biochemical recurrence, metastasis, and cancer-specific mortality, with hazard ratios of 1.40, 2.51, and 1.89, respectively.
Study details: A systematic review and meta-analysis of 11 studies involving 22,549 patients with prostate cancer undergoing radical prostatectomy or radiotherapy.
Disclosures: The first author is supported by the Scholarship Foundation of Swiss Urology. One coauthor reported disclosures related to Astellas, Cepheid, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanochemia, Sanofi, and Wolff.
Source: Foerster B et al. JAMA Oncol. 2018 May 24. doi: 10.1001/jamaoncol.2018.1071.
Statin effect in prostate cancer may be caused by reduced inflammation
SAN FRANCISCO – according to the results of a new genetic analysis of prostate cancers in men in the Health Professionals Follow Up Study.
A previous analysis from the Health Professionals Follow Up Study published in 2015 showed no difference in the risk of lethal prostate cancer for those who began using statins after diagnosis of their tumors.
A genetic analysis of these lethal cases revealed that patients taking long-term statins had a lower incidence of phosphatase and tensin homolog (PTEN)–null cancers, which are associated with worse outcomes. Integrating molecular and epidemiologic data, PTEN and PI3K (phosphatidylinositol) signaling and inflammation and immune activation appear to be two potential mechanisms contributing to this association.
Genetic analysis of normal prostate tissue also showed unique traits among statin users. “We found that the top ten pathways that came up were almost all involved in inflammation or immune activation, and we found those differences only in tumor and adjacent normal prostate tissue. We didn’t see any pathways that were differentially expressed by statin use within the tumor tissue itself,” said Emma Allott, PhD, who presented the research at a poster session at the annual meeting of the American Urological Association.
An association between statin use and improved survival was first described in a 2006 study based on results from the Health Professionals Follow Up Study. Since then, “we’ve been generating molecular data on cancers that developed in statin users and nonusers,” said Dr. Allott of the University of North Carolina, Chapel Hill.
Of the 5,792 prostate cancer diagnoses, 17% were advanced cases, defined as stage T3b or more, having spread to lymph nodes or metastasized, or lethal; 13% were lethal, 46% were positive for the ERG oncogene, and 14% were PTEN-null. “Statin use was associated with a lower risk of PTEN-null prostate cancer, so that seems to drive some of the reduced association with lethal disease,” said Dr. Allott.
There was no association between lethality and ERG-positive or ERG-negative status. Those who used statins for more than 5 years were less likely to have a PTEN-null tumor (hazard ratio, 0.42; 95% confidence interval, 0.20-0.90) but not more likely to have a PTEN-positive tumor (HR, 1.18; 95% CI, 0.95-1.46).
Compared with never users, long-term statin users also were less likely to have advanced prostate cancer (multivariate analysis, HR, 0.62; 95% CI, 0.45-0.85) as well as lethal prostate cancer (HR, 0.52; 95% CI, 0.35-0.78).
The researchers conducted a gene set enrichment analysis in statin users and found an enrichment of T-cell, B-cell, and PI3K signaling in tumor-adjacent normal prostate tissue, as well as other changes. “We think maybe there’s a microenvironment inflammation component to the mechanism through which statins are associated with lower risk of lethal prostate cancer,” said Dr. Allott.
The molecular data could identify patient subgroups that could benefit from statins. Dr. Allott said that is the goal, but it will take time. “That’s more obviously translatable to the clinic, but we don’t yet have enough data in this cohort to look at that.”
SOURCE: Allott E et al. AUA 2018, Abstract MP21-01.
SAN FRANCISCO – according to the results of a new genetic analysis of prostate cancers in men in the Health Professionals Follow Up Study.
A previous analysis from the Health Professionals Follow Up Study published in 2015 showed no difference in the risk of lethal prostate cancer for those who began using statins after diagnosis of their tumors.
A genetic analysis of these lethal cases revealed that patients taking long-term statins had a lower incidence of phosphatase and tensin homolog (PTEN)–null cancers, which are associated with worse outcomes. Integrating molecular and epidemiologic data, PTEN and PI3K (phosphatidylinositol) signaling and inflammation and immune activation appear to be two potential mechanisms contributing to this association.
Genetic analysis of normal prostate tissue also showed unique traits among statin users. “We found that the top ten pathways that came up were almost all involved in inflammation or immune activation, and we found those differences only in tumor and adjacent normal prostate tissue. We didn’t see any pathways that were differentially expressed by statin use within the tumor tissue itself,” said Emma Allott, PhD, who presented the research at a poster session at the annual meeting of the American Urological Association.
An association between statin use and improved survival was first described in a 2006 study based on results from the Health Professionals Follow Up Study. Since then, “we’ve been generating molecular data on cancers that developed in statin users and nonusers,” said Dr. Allott of the University of North Carolina, Chapel Hill.
Of the 5,792 prostate cancer diagnoses, 17% were advanced cases, defined as stage T3b or more, having spread to lymph nodes or metastasized, or lethal; 13% were lethal, 46% were positive for the ERG oncogene, and 14% were PTEN-null. “Statin use was associated with a lower risk of PTEN-null prostate cancer, so that seems to drive some of the reduced association with lethal disease,” said Dr. Allott.
There was no association between lethality and ERG-positive or ERG-negative status. Those who used statins for more than 5 years were less likely to have a PTEN-null tumor (hazard ratio, 0.42; 95% confidence interval, 0.20-0.90) but not more likely to have a PTEN-positive tumor (HR, 1.18; 95% CI, 0.95-1.46).
Compared with never users, long-term statin users also were less likely to have advanced prostate cancer (multivariate analysis, HR, 0.62; 95% CI, 0.45-0.85) as well as lethal prostate cancer (HR, 0.52; 95% CI, 0.35-0.78).
The researchers conducted a gene set enrichment analysis in statin users and found an enrichment of T-cell, B-cell, and PI3K signaling in tumor-adjacent normal prostate tissue, as well as other changes. “We think maybe there’s a microenvironment inflammation component to the mechanism through which statins are associated with lower risk of lethal prostate cancer,” said Dr. Allott.
The molecular data could identify patient subgroups that could benefit from statins. Dr. Allott said that is the goal, but it will take time. “That’s more obviously translatable to the clinic, but we don’t yet have enough data in this cohort to look at that.”
SOURCE: Allott E et al. AUA 2018, Abstract MP21-01.
SAN FRANCISCO – according to the results of a new genetic analysis of prostate cancers in men in the Health Professionals Follow Up Study.
A previous analysis from the Health Professionals Follow Up Study published in 2015 showed no difference in the risk of lethal prostate cancer for those who began using statins after diagnosis of their tumors.
A genetic analysis of these lethal cases revealed that patients taking long-term statins had a lower incidence of phosphatase and tensin homolog (PTEN)–null cancers, which are associated with worse outcomes. Integrating molecular and epidemiologic data, PTEN and PI3K (phosphatidylinositol) signaling and inflammation and immune activation appear to be two potential mechanisms contributing to this association.
Genetic analysis of normal prostate tissue also showed unique traits among statin users. “We found that the top ten pathways that came up were almost all involved in inflammation or immune activation, and we found those differences only in tumor and adjacent normal prostate tissue. We didn’t see any pathways that were differentially expressed by statin use within the tumor tissue itself,” said Emma Allott, PhD, who presented the research at a poster session at the annual meeting of the American Urological Association.
An association between statin use and improved survival was first described in a 2006 study based on results from the Health Professionals Follow Up Study. Since then, “we’ve been generating molecular data on cancers that developed in statin users and nonusers,” said Dr. Allott of the University of North Carolina, Chapel Hill.
Of the 5,792 prostate cancer diagnoses, 17% were advanced cases, defined as stage T3b or more, having spread to lymph nodes or metastasized, or lethal; 13% were lethal, 46% were positive for the ERG oncogene, and 14% were PTEN-null. “Statin use was associated with a lower risk of PTEN-null prostate cancer, so that seems to drive some of the reduced association with lethal disease,” said Dr. Allott.
There was no association between lethality and ERG-positive or ERG-negative status. Those who used statins for more than 5 years were less likely to have a PTEN-null tumor (hazard ratio, 0.42; 95% confidence interval, 0.20-0.90) but not more likely to have a PTEN-positive tumor (HR, 1.18; 95% CI, 0.95-1.46).
Compared with never users, long-term statin users also were less likely to have advanced prostate cancer (multivariate analysis, HR, 0.62; 95% CI, 0.45-0.85) as well as lethal prostate cancer (HR, 0.52; 95% CI, 0.35-0.78).
The researchers conducted a gene set enrichment analysis in statin users and found an enrichment of T-cell, B-cell, and PI3K signaling in tumor-adjacent normal prostate tissue, as well as other changes. “We think maybe there’s a microenvironment inflammation component to the mechanism through which statins are associated with lower risk of lethal prostate cancer,” said Dr. Allott.
The molecular data could identify patient subgroups that could benefit from statins. Dr. Allott said that is the goal, but it will take time. “That’s more obviously translatable to the clinic, but we don’t yet have enough data in this cohort to look at that.”
SOURCE: Allott E et al. AUA 2018, Abstract MP21-01.
REPORTING FROM THE AUA ANNUAL MEETING
Key clinical point: Researchers hope that genetic analyses could eventually point to prostate cancer patients who might benefit from statin use.
Major finding: Long-term statin users had lower odds of having a pTEN-null tumor (hazard ratio, 0.42), which is associated with worse outcomes.
Study details: Retrospective analysis of 5,792 diagnoses of prostate cancer among 44,076 men.
Disclosures: The study was funded by the Irish Cancer Society, the John Fitzpatrick Fellowship, and the National Cancer Institute. Dr. Allott reported no relevant financial relationships.
Source: Allott E et al. AUA 2018, Abstract MP21-01.