User login
I-125 brachytherapy for prostate cancer linked to small increase in bladder cancer risk
Second primary malignancies affected 10.8% of men who received I-125 brachytherapy as monotherapy for prostate cancer, researchers reported in the April issue of Clinical Oncology.
But only bladder cancer had a small increase in risk in these patients compared with the general population, with the highest risk occurring during the first 4 years of follow-up after implant, said Dr. Ann Henry and her associates at St. James’s University Hospital in Leeds, England (Clin. Oncol. 2014;26:210-5).
The investigators studied 1,805 consecutive patients who received I-125 brachytherapy as monotherapy for localized prostate cancer from 1995 to 2006 at a single public hospital. Their mean age at treatment was 63 years (interquartile range, 58-68). The researchers defined possible radiation-induced cancers as developing at least 5 years after primary radiotherapy, and with histologies distinct from prostate adenocarcinoma. The median follow-up was 8 years with 487 patients (31%) having 10 years or more.
In all, 170 patients (10.8%) were diagnosed with second primary malignancies at least 1 year after I-125 brachytherapy implant, and 77 (4.9%) were diagnosed at least 5 years after implant, the investigators said. Bladder and rectal cancers were the most common, with 10-year cumulative incidences of 1% and 0.84%, respectively.
Only bladder cancer had a standardized incidence rate that exceeded that of the general population (SIR, 1.54; 95% confidence interval, 0.96-2.46). But the increase was small, and the excess risk was slightly higher during the first 4 years of follow-up (1.69; 95% confidence interval, 0.87-3.34) than during subsequent years (SIR, 1.42; 95% CI, 0.75-2.70). For this reason, the result was probably an artifact of increased urologic surveillance not caused by brachytherapy, said Dr. Henry and her associates.
"This should not act as a deterrent to patients considering low-dose-rate brachytherapy as a treatment modality for early prostate cancer," the investigators said.
The research was supported by ONCURA, which is part of GE Healthcare. The authors did not disclose any conflicts of interest.
Second primary malignancies affected 10.8% of men who received I-125 brachytherapy as monotherapy for prostate cancer, researchers reported in the April issue of Clinical Oncology.
But only bladder cancer had a small increase in risk in these patients compared with the general population, with the highest risk occurring during the first 4 years of follow-up after implant, said Dr. Ann Henry and her associates at St. James’s University Hospital in Leeds, England (Clin. Oncol. 2014;26:210-5).
The investigators studied 1,805 consecutive patients who received I-125 brachytherapy as monotherapy for localized prostate cancer from 1995 to 2006 at a single public hospital. Their mean age at treatment was 63 years (interquartile range, 58-68). The researchers defined possible radiation-induced cancers as developing at least 5 years after primary radiotherapy, and with histologies distinct from prostate adenocarcinoma. The median follow-up was 8 years with 487 patients (31%) having 10 years or more.
In all, 170 patients (10.8%) were diagnosed with second primary malignancies at least 1 year after I-125 brachytherapy implant, and 77 (4.9%) were diagnosed at least 5 years after implant, the investigators said. Bladder and rectal cancers were the most common, with 10-year cumulative incidences of 1% and 0.84%, respectively.
Only bladder cancer had a standardized incidence rate that exceeded that of the general population (SIR, 1.54; 95% confidence interval, 0.96-2.46). But the increase was small, and the excess risk was slightly higher during the first 4 years of follow-up (1.69; 95% confidence interval, 0.87-3.34) than during subsequent years (SIR, 1.42; 95% CI, 0.75-2.70). For this reason, the result was probably an artifact of increased urologic surveillance not caused by brachytherapy, said Dr. Henry and her associates.
"This should not act as a deterrent to patients considering low-dose-rate brachytherapy as a treatment modality for early prostate cancer," the investigators said.
The research was supported by ONCURA, which is part of GE Healthcare. The authors did not disclose any conflicts of interest.
Second primary malignancies affected 10.8% of men who received I-125 brachytherapy as monotherapy for prostate cancer, researchers reported in the April issue of Clinical Oncology.
But only bladder cancer had a small increase in risk in these patients compared with the general population, with the highest risk occurring during the first 4 years of follow-up after implant, said Dr. Ann Henry and her associates at St. James’s University Hospital in Leeds, England (Clin. Oncol. 2014;26:210-5).
The investigators studied 1,805 consecutive patients who received I-125 brachytherapy as monotherapy for localized prostate cancer from 1995 to 2006 at a single public hospital. Their mean age at treatment was 63 years (interquartile range, 58-68). The researchers defined possible radiation-induced cancers as developing at least 5 years after primary radiotherapy, and with histologies distinct from prostate adenocarcinoma. The median follow-up was 8 years with 487 patients (31%) having 10 years or more.
In all, 170 patients (10.8%) were diagnosed with second primary malignancies at least 1 year after I-125 brachytherapy implant, and 77 (4.9%) were diagnosed at least 5 years after implant, the investigators said. Bladder and rectal cancers were the most common, with 10-year cumulative incidences of 1% and 0.84%, respectively.
Only bladder cancer had a standardized incidence rate that exceeded that of the general population (SIR, 1.54; 95% confidence interval, 0.96-2.46). But the increase was small, and the excess risk was slightly higher during the first 4 years of follow-up (1.69; 95% confidence interval, 0.87-3.34) than during subsequent years (SIR, 1.42; 95% CI, 0.75-2.70). For this reason, the result was probably an artifact of increased urologic surveillance not caused by brachytherapy, said Dr. Henry and her associates.
"This should not act as a deterrent to patients considering low-dose-rate brachytherapy as a treatment modality for early prostate cancer," the investigators said.
The research was supported by ONCURA, which is part of GE Healthcare. The authors did not disclose any conflicts of interest.
FROM CLINICAL ONCOLOGY
Major Finding: Of 1,805 consecutive patients, 170 (10.8%) had second primary malignancies diagnosed at least 1 year after I-125 brachytherapy implant, and 77 (4.9%) were diagnosed at least 5 years after implant. Only bladder cancer had a standardized incidence rate that was higher than that in the general population (SIR, 1.54; 95% confidence interval, 0.96-2.46).
Data Source: A prospective registry study of 1,805 consecutive patients who received I-125 brachytherapy as monotherapy for localized prostate cancer. Patients were treated from 1995 to 2006 at a single public hospital.
Disclosures: The research was supported by ONCURA, which is part of GE Healthcare. The authors did not disclose any conflicts of interest.
No benefit from androgen deprivation therapy in localized prostate cancer
For most men with localized prostate cancer who defer surgery or radiation, primary androgen deprivation therapy offers no mortality benefit, Dr. Arnold L. Potosky reported.
Dr. Potosky of Georgetown University Medical Center, Washington, D.C., and colleagues looked at 15,170 men with newly diagnosed, clinically localized prostate cancer who did not receive curative therapy, including radiation, radical prostatectomy, or chemotherapy (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.54.2043]).
Overall, 23% of the cohort had primary androgen deprivation therapy (PADT) initiated within the first year of diagnosis. After adjustment for factors including age, baseline prostate-specific antigen, Gleason score, and T stage, the authors found that there was no difference between PADT-treated men and their PADT-naive counterparts in all-cause mortality (hazard ratio, 1.04; 95% confidence interval, 0.97-1.11) or prostate-cancer specific mortality (HR, 1.03; 95% CI, 0.89-1.19).
Indeed, despite its widespread use among patients who do not undergo curative-intent treatments, "the risk of serious adverse events and the high costs associated with its use mitigate any clinical or policy rationale for PADT use in these men," the authors wrote.
They did report a small but significant decreased risk of all-cause mortality (HR, 0.88; 95% CI, 0.78-0.97) in men with high-risk cancer (pretreatment PSA greater than 20; Gleason score, 8-10; clinical stage, T2c-T3a), but "the observed benefit was relatively small and should not be taken as definitive, given the limitations of our data," they noted.
An increased risk of death was reported in men with low-risk cancer (HR, 1.41; 95% CI, 0.99-1.82) and no difference in risk for men with intermediate-risk cancer (HR, 1.12; 95% CI, 0.92-1.32). There were no differences in risk of prostate cancer mortality by risk group category.
The authors disclosed no conflicts of interest related to this study. The study was supported by grants from the National Cancer Institute.
For most men with localized prostate cancer who defer surgery or radiation, primary androgen deprivation therapy offers no mortality benefit, Dr. Arnold L. Potosky reported.
Dr. Potosky of Georgetown University Medical Center, Washington, D.C., and colleagues looked at 15,170 men with newly diagnosed, clinically localized prostate cancer who did not receive curative therapy, including radiation, radical prostatectomy, or chemotherapy (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.54.2043]).
Overall, 23% of the cohort had primary androgen deprivation therapy (PADT) initiated within the first year of diagnosis. After adjustment for factors including age, baseline prostate-specific antigen, Gleason score, and T stage, the authors found that there was no difference between PADT-treated men and their PADT-naive counterparts in all-cause mortality (hazard ratio, 1.04; 95% confidence interval, 0.97-1.11) or prostate-cancer specific mortality (HR, 1.03; 95% CI, 0.89-1.19).
Indeed, despite its widespread use among patients who do not undergo curative-intent treatments, "the risk of serious adverse events and the high costs associated with its use mitigate any clinical or policy rationale for PADT use in these men," the authors wrote.
They did report a small but significant decreased risk of all-cause mortality (HR, 0.88; 95% CI, 0.78-0.97) in men with high-risk cancer (pretreatment PSA greater than 20; Gleason score, 8-10; clinical stage, T2c-T3a), but "the observed benefit was relatively small and should not be taken as definitive, given the limitations of our data," they noted.
An increased risk of death was reported in men with low-risk cancer (HR, 1.41; 95% CI, 0.99-1.82) and no difference in risk for men with intermediate-risk cancer (HR, 1.12; 95% CI, 0.92-1.32). There were no differences in risk of prostate cancer mortality by risk group category.
The authors disclosed no conflicts of interest related to this study. The study was supported by grants from the National Cancer Institute.
For most men with localized prostate cancer who defer surgery or radiation, primary androgen deprivation therapy offers no mortality benefit, Dr. Arnold L. Potosky reported.
Dr. Potosky of Georgetown University Medical Center, Washington, D.C., and colleagues looked at 15,170 men with newly diagnosed, clinically localized prostate cancer who did not receive curative therapy, including radiation, radical prostatectomy, or chemotherapy (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.54.2043]).
Overall, 23% of the cohort had primary androgen deprivation therapy (PADT) initiated within the first year of diagnosis. After adjustment for factors including age, baseline prostate-specific antigen, Gleason score, and T stage, the authors found that there was no difference between PADT-treated men and their PADT-naive counterparts in all-cause mortality (hazard ratio, 1.04; 95% confidence interval, 0.97-1.11) or prostate-cancer specific mortality (HR, 1.03; 95% CI, 0.89-1.19).
Indeed, despite its widespread use among patients who do not undergo curative-intent treatments, "the risk of serious adverse events and the high costs associated with its use mitigate any clinical or policy rationale for PADT use in these men," the authors wrote.
They did report a small but significant decreased risk of all-cause mortality (HR, 0.88; 95% CI, 0.78-0.97) in men with high-risk cancer (pretreatment PSA greater than 20; Gleason score, 8-10; clinical stage, T2c-T3a), but "the observed benefit was relatively small and should not be taken as definitive, given the limitations of our data," they noted.
An increased risk of death was reported in men with low-risk cancer (HR, 1.41; 95% CI, 0.99-1.82) and no difference in risk for men with intermediate-risk cancer (HR, 1.12; 95% CI, 0.92-1.32). There were no differences in risk of prostate cancer mortality by risk group category.
The authors disclosed no conflicts of interest related to this study. The study was supported by grants from the National Cancer Institute.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Major finding: Men with localized prostate cancer who deferred curative treatment but opted for primary androgen deprivation therapy saw no significant decrease in mortality (hazard ratio, 0.97; 95% confidence interval, 0.97-1.11).
Data source: A retrospective cohort study using comprehensive utilization and cancer registry data from three integrated health plans (n = 15,170).
Disclosures: The authors disclosed no conflicts of interest related to this study. The study was supported by grants from the National Cancer Institute.
The push for smaller, smarter cancer trials
The American Society of Clinical Oncology is pressing cancer researchers to rethink the design of future clinical trials to achieve larger gains in four common cancers.
The final recommendations, which come after months of deliberations and public comment, try to hit the sweet spot between proposing guidelines that are not obtainable, and thus ignored, and having ambitious yet realistic goals.
For pancreatic cancer, for example, the experts recommended that clinical trials seek to improve median overall survival by 50%, or 4-5 months, for patients eligible for FOLFIRINOX (leucovorin, fluorouracil, irinotecan, and oxaliplatin) and by 3-4 months for those eligible for gemcitabine (Gemzar) with or without nab-paclitaxel (Abraxane).
Overall survival (OS) was selected over progression-free survival as the primary endpoint, although it was acknowledged that OS poses challenges such as the need for longer follow-up, the potential confounding effect of post-study therapies, and use of second-line therapies for secondary mutations identified after progression during first-line targeted therapy.
Ultimately, an improvement in median OS of 2.5-6 months, depending on the setting, was identified as the minimum incremental improvement over standard therapy that would define a clinically meaningful outcome.
The recommendations, published March 17 in the Journal of Clinical Oncology (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.53.8009]), also note that incremental improvements should be accompanied by little to no added toxicity over current treatments, and that a highly toxic regimen should produce the greatest OS gains to be considered clinically meaningful.
"We expect that sponsors will appreciate the need for raising the bar with regard to clinical trial goals, but that they will be conservative in their adoption of the recommendations," Dr. Lee M. Ellis, committee chair and professor of surgery at the University of Texas M.D. Anderson Cancer Center, Houston, said in an interview. "Trials designed with less ambitious goals may still be of benefit to individual patients if trial endpoints are met and if we can develop methods to identify patients most likely to benefit from the intervention."
Achieving the "smaller and smarter" trials envisioned by the committee rests on the ability to select patients for targeted therapy based on the molecular drivers of their tumors, rather than enrolling all comers. Unfortunately, in many cases, targeted agents continue to be developed without complete understanding of the drug target and, therefore, companion diagnostics to aid in patient selection, the experts observed.
"It is difficult to hit a target when it is not certain where it is or if it is valid," agreed Dr. David M. Dilts, codirector of the Center for Management Research in Healthcare, Oregon Health & Science University, Portland, in an accompanying editorial (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.54.5277]). "This, not insubstantial risk, should be ameliorated in the near future as major clinical research organizations are banking specimens, some of which are highly annotated, and as technology to analyze such specimens becomes faster, better, and cheaper."
To further this goal, the expert committee calls on trial sponsors to develop comprehensive biospecimen banks for each trial.
"Obstacles to developing these banks include cost and the willingness and ability of trial sponsors to foot the bill," Dr. Ellis said. "However, we believe the investment will pay off in increasing our ability to understand the molecular drivers of cancer and, as a result, more appropriate targeted therapies for people with cancer."
QOL
Though quality of life was a common theme that arose in all working group discussions, the recommendations lack hard targets in this area. Instead, the working groups cited the 2011 approval of the Janus kinase 1 and 2 inhibitor ruxolitinib (Jakavi) for myelofibrosis as an example of how serial assessment of specific cancer-related symptoms can define a clinically meaningful outcome for patients.
"It is not enough to just mention how important quality of life is. A clinical trial must be designed with a suite of thoughtful, feasible, validated patient-reported outcome measures that capture clinical benefit," Ms. Musa Mayer, a long-time advocate for patients with metastatic breast cancer, said in an interview. "Observed adverse events can never fully account for the lived experience of a given treatment."
Breast cancer
For breast cancer, the committee selected metastatic triple-negative breast cancer that was previously untreated for metastatic disease. They recommend clinical trials aim for an increase in OS of 4.5-6 months, although it was noted that consensus was not achieved by the breast cancer group on the magnitude of the benefit that would be considered clinically meaningful. The current median overall survival in this poor-prognosis population is 18 months.
Lung cancer
The committee addressed two lung cancer populations: nonsquamous cell carcinoma and squamous cell carcinoma. They recommend clinical trials seek to improve OS by 3.25-4 months and by 2.5-3 months, respectively. Current baseline median OS in these groups is 13 and 10 months.
Colon cancer
The recommendations for colon cancer target patients with disease progression with all prior therapies, or who are not candidates for standard second- or third-line options. Here, the goal is to improve OS by 3-5 months over the current baseline median OS of 4-6 months.
Notably, the cost of delivering the recommended targets for all four cancers was not addressed by the committee. The ASCO Value of Cancer Care Task Force, however, is already tasked with evaluating the efficacy, toxicity, and cost of specific oncology treatments.
"The working group provided thoughtful recommendations for the topics considered, although the specific recommendations were limited," Ms. Patricia Haugen, breast cancer survivor and current member and previous chair of the Department of Defense Congressionally Directed Breast Cancer Research Program Integration Panel, said in an interview.
She is hopeful that the new recommendations will be followed, but said there needs to be broad support and commitment to changes that produce more meaningful clinical benefit. "That commitment must be real and must come from all parties involved in the clinical trials process, so that clinical trials that do not meet a high bar are not considered, funded, nor implemented," she said.
Editorialist Dr. Dilts agreed that advocates from many areas are needed if the recommended goals are to be reached and suggested what might be required is "a more DARPA [Defense Advanced Research Projects Agency] approach, where answering high-risk questions are fostered and supported."
Dr. Ellis reported a consultant/advisory role with Genentech, Roche, Imclone, Eli Lilly, and Amgen. Ms. Mayer, Ms. Haugen, and Dr. Dilts reported no potential conflicts of interest.
The American Society of Clinical Oncology is pressing cancer researchers to rethink the design of future clinical trials to achieve larger gains in four common cancers.
The final recommendations, which come after months of deliberations and public comment, try to hit the sweet spot between proposing guidelines that are not obtainable, and thus ignored, and having ambitious yet realistic goals.
For pancreatic cancer, for example, the experts recommended that clinical trials seek to improve median overall survival by 50%, or 4-5 months, for patients eligible for FOLFIRINOX (leucovorin, fluorouracil, irinotecan, and oxaliplatin) and by 3-4 months for those eligible for gemcitabine (Gemzar) with or without nab-paclitaxel (Abraxane).
Overall survival (OS) was selected over progression-free survival as the primary endpoint, although it was acknowledged that OS poses challenges such as the need for longer follow-up, the potential confounding effect of post-study therapies, and use of second-line therapies for secondary mutations identified after progression during first-line targeted therapy.
Ultimately, an improvement in median OS of 2.5-6 months, depending on the setting, was identified as the minimum incremental improvement over standard therapy that would define a clinically meaningful outcome.
The recommendations, published March 17 in the Journal of Clinical Oncology (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.53.8009]), also note that incremental improvements should be accompanied by little to no added toxicity over current treatments, and that a highly toxic regimen should produce the greatest OS gains to be considered clinically meaningful.
"We expect that sponsors will appreciate the need for raising the bar with regard to clinical trial goals, but that they will be conservative in their adoption of the recommendations," Dr. Lee M. Ellis, committee chair and professor of surgery at the University of Texas M.D. Anderson Cancer Center, Houston, said in an interview. "Trials designed with less ambitious goals may still be of benefit to individual patients if trial endpoints are met and if we can develop methods to identify patients most likely to benefit from the intervention."
Achieving the "smaller and smarter" trials envisioned by the committee rests on the ability to select patients for targeted therapy based on the molecular drivers of their tumors, rather than enrolling all comers. Unfortunately, in many cases, targeted agents continue to be developed without complete understanding of the drug target and, therefore, companion diagnostics to aid in patient selection, the experts observed.
"It is difficult to hit a target when it is not certain where it is or if it is valid," agreed Dr. David M. Dilts, codirector of the Center for Management Research in Healthcare, Oregon Health & Science University, Portland, in an accompanying editorial (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.54.5277]). "This, not insubstantial risk, should be ameliorated in the near future as major clinical research organizations are banking specimens, some of which are highly annotated, and as technology to analyze such specimens becomes faster, better, and cheaper."
To further this goal, the expert committee calls on trial sponsors to develop comprehensive biospecimen banks for each trial.
"Obstacles to developing these banks include cost and the willingness and ability of trial sponsors to foot the bill," Dr. Ellis said. "However, we believe the investment will pay off in increasing our ability to understand the molecular drivers of cancer and, as a result, more appropriate targeted therapies for people with cancer."
QOL
Though quality of life was a common theme that arose in all working group discussions, the recommendations lack hard targets in this area. Instead, the working groups cited the 2011 approval of the Janus kinase 1 and 2 inhibitor ruxolitinib (Jakavi) for myelofibrosis as an example of how serial assessment of specific cancer-related symptoms can define a clinically meaningful outcome for patients.
"It is not enough to just mention how important quality of life is. A clinical trial must be designed with a suite of thoughtful, feasible, validated patient-reported outcome measures that capture clinical benefit," Ms. Musa Mayer, a long-time advocate for patients with metastatic breast cancer, said in an interview. "Observed adverse events can never fully account for the lived experience of a given treatment."
Breast cancer
For breast cancer, the committee selected metastatic triple-negative breast cancer that was previously untreated for metastatic disease. They recommend clinical trials aim for an increase in OS of 4.5-6 months, although it was noted that consensus was not achieved by the breast cancer group on the magnitude of the benefit that would be considered clinically meaningful. The current median overall survival in this poor-prognosis population is 18 months.
Lung cancer
The committee addressed two lung cancer populations: nonsquamous cell carcinoma and squamous cell carcinoma. They recommend clinical trials seek to improve OS by 3.25-4 months and by 2.5-3 months, respectively. Current baseline median OS in these groups is 13 and 10 months.
Colon cancer
The recommendations for colon cancer target patients with disease progression with all prior therapies, or who are not candidates for standard second- or third-line options. Here, the goal is to improve OS by 3-5 months over the current baseline median OS of 4-6 months.
Notably, the cost of delivering the recommended targets for all four cancers was not addressed by the committee. The ASCO Value of Cancer Care Task Force, however, is already tasked with evaluating the efficacy, toxicity, and cost of specific oncology treatments.
"The working group provided thoughtful recommendations for the topics considered, although the specific recommendations were limited," Ms. Patricia Haugen, breast cancer survivor and current member and previous chair of the Department of Defense Congressionally Directed Breast Cancer Research Program Integration Panel, said in an interview.
She is hopeful that the new recommendations will be followed, but said there needs to be broad support and commitment to changes that produce more meaningful clinical benefit. "That commitment must be real and must come from all parties involved in the clinical trials process, so that clinical trials that do not meet a high bar are not considered, funded, nor implemented," she said.
Editorialist Dr. Dilts agreed that advocates from many areas are needed if the recommended goals are to be reached and suggested what might be required is "a more DARPA [Defense Advanced Research Projects Agency] approach, where answering high-risk questions are fostered and supported."
Dr. Ellis reported a consultant/advisory role with Genentech, Roche, Imclone, Eli Lilly, and Amgen. Ms. Mayer, Ms. Haugen, and Dr. Dilts reported no potential conflicts of interest.
The American Society of Clinical Oncology is pressing cancer researchers to rethink the design of future clinical trials to achieve larger gains in four common cancers.
The final recommendations, which come after months of deliberations and public comment, try to hit the sweet spot between proposing guidelines that are not obtainable, and thus ignored, and having ambitious yet realistic goals.
For pancreatic cancer, for example, the experts recommended that clinical trials seek to improve median overall survival by 50%, or 4-5 months, for patients eligible for FOLFIRINOX (leucovorin, fluorouracil, irinotecan, and oxaliplatin) and by 3-4 months for those eligible for gemcitabine (Gemzar) with or without nab-paclitaxel (Abraxane).
Overall survival (OS) was selected over progression-free survival as the primary endpoint, although it was acknowledged that OS poses challenges such as the need for longer follow-up, the potential confounding effect of post-study therapies, and use of second-line therapies for secondary mutations identified after progression during first-line targeted therapy.
Ultimately, an improvement in median OS of 2.5-6 months, depending on the setting, was identified as the minimum incremental improvement over standard therapy that would define a clinically meaningful outcome.
The recommendations, published March 17 in the Journal of Clinical Oncology (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.53.8009]), also note that incremental improvements should be accompanied by little to no added toxicity over current treatments, and that a highly toxic regimen should produce the greatest OS gains to be considered clinically meaningful.
"We expect that sponsors will appreciate the need for raising the bar with regard to clinical trial goals, but that they will be conservative in their adoption of the recommendations," Dr. Lee M. Ellis, committee chair and professor of surgery at the University of Texas M.D. Anderson Cancer Center, Houston, said in an interview. "Trials designed with less ambitious goals may still be of benefit to individual patients if trial endpoints are met and if we can develop methods to identify patients most likely to benefit from the intervention."
Achieving the "smaller and smarter" trials envisioned by the committee rests on the ability to select patients for targeted therapy based on the molecular drivers of their tumors, rather than enrolling all comers. Unfortunately, in many cases, targeted agents continue to be developed without complete understanding of the drug target and, therefore, companion diagnostics to aid in patient selection, the experts observed.
"It is difficult to hit a target when it is not certain where it is or if it is valid," agreed Dr. David M. Dilts, codirector of the Center for Management Research in Healthcare, Oregon Health & Science University, Portland, in an accompanying editorial (J. Clin. Oncol. 2014 Mar. 17 [doi:10.1200/JCO.2013.54.5277]). "This, not insubstantial risk, should be ameliorated in the near future as major clinical research organizations are banking specimens, some of which are highly annotated, and as technology to analyze such specimens becomes faster, better, and cheaper."
To further this goal, the expert committee calls on trial sponsors to develop comprehensive biospecimen banks for each trial.
"Obstacles to developing these banks include cost and the willingness and ability of trial sponsors to foot the bill," Dr. Ellis said. "However, we believe the investment will pay off in increasing our ability to understand the molecular drivers of cancer and, as a result, more appropriate targeted therapies for people with cancer."
QOL
Though quality of life was a common theme that arose in all working group discussions, the recommendations lack hard targets in this area. Instead, the working groups cited the 2011 approval of the Janus kinase 1 and 2 inhibitor ruxolitinib (Jakavi) for myelofibrosis as an example of how serial assessment of specific cancer-related symptoms can define a clinically meaningful outcome for patients.
"It is not enough to just mention how important quality of life is. A clinical trial must be designed with a suite of thoughtful, feasible, validated patient-reported outcome measures that capture clinical benefit," Ms. Musa Mayer, a long-time advocate for patients with metastatic breast cancer, said in an interview. "Observed adverse events can never fully account for the lived experience of a given treatment."
Breast cancer
For breast cancer, the committee selected metastatic triple-negative breast cancer that was previously untreated for metastatic disease. They recommend clinical trials aim for an increase in OS of 4.5-6 months, although it was noted that consensus was not achieved by the breast cancer group on the magnitude of the benefit that would be considered clinically meaningful. The current median overall survival in this poor-prognosis population is 18 months.
Lung cancer
The committee addressed two lung cancer populations: nonsquamous cell carcinoma and squamous cell carcinoma. They recommend clinical trials seek to improve OS by 3.25-4 months and by 2.5-3 months, respectively. Current baseline median OS in these groups is 13 and 10 months.
Colon cancer
The recommendations for colon cancer target patients with disease progression with all prior therapies, or who are not candidates for standard second- or third-line options. Here, the goal is to improve OS by 3-5 months over the current baseline median OS of 4-6 months.
Notably, the cost of delivering the recommended targets for all four cancers was not addressed by the committee. The ASCO Value of Cancer Care Task Force, however, is already tasked with evaluating the efficacy, toxicity, and cost of specific oncology treatments.
"The working group provided thoughtful recommendations for the topics considered, although the specific recommendations were limited," Ms. Patricia Haugen, breast cancer survivor and current member and previous chair of the Department of Defense Congressionally Directed Breast Cancer Research Program Integration Panel, said in an interview.
She is hopeful that the new recommendations will be followed, but said there needs to be broad support and commitment to changes that produce more meaningful clinical benefit. "That commitment must be real and must come from all parties involved in the clinical trials process, so that clinical trials that do not meet a high bar are not considered, funded, nor implemented," she said.
Editorialist Dr. Dilts agreed that advocates from many areas are needed if the recommended goals are to be reached and suggested what might be required is "a more DARPA [Defense Advanced Research Projects Agency] approach, where answering high-risk questions are fostered and supported."
Dr. Ellis reported a consultant/advisory role with Genentech, Roche, Imclone, Eli Lilly, and Amgen. Ms. Mayer, Ms. Haugen, and Dr. Dilts reported no potential conflicts of interest.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Positive surgical margins do not independently predict prostate cancer mortality
Positive surgical margins alone do not predict death from prostate cancer in men who undergo radical prostatectomy, investigators reported in the April issue of European Urology.
Positive surgical margins (PSMs) were not significantly associated with prostate cancer–specific mortality after adjustment for fixed covariates and postoperative radiotherapy, reported Dr. Andrew J. Stephenson, of the Cleveland Clinic’s Glickman Urological & Kidney Institute, and his associates.
Investigators analyzed data from 11,521 men with localized prostate cancer. Patients had undergone radical prostatectomy at four universities and cancer centers between 1987 and 2005.
At 15 years of follow-up, the prostate cancer–specific mortality for men with negative surgical margins was 6%, compared with 10% for men with PSMs (P less than .001).
But PSMs did not independently predict prostate cancer–specific mortality in regression models, the investigators reported (Eur. Urol. 2004;65:675-80).
That finding was true when researchers modeled only fixed covariates, such as age, Gleason score, seminal vesicle invasion, lymph node involvement, prostate-specific antigen (PSA), and extraprostatic extension (hazard ratio, 1.04; 95% confidence interval, 0.7-1.5), and also when they adjusted for postoperative radiotherapy, either as a single parameter (HR, 0.96; 95% CI, 0.7-1.4) or as early versus late treatment (HR, 1.01; 95% CI, 0.7-1.4).
The lack of an association called into question "the rationale for postoperative radiotherapy for PSMs in the absence of other adverse features," as well as "the relevance of PSM rates as a measure of surgical proficiency," the investigators said.
Even expert pathologists may not agree on PSMs and whether PSMs could be artifacts from surgery or pathologic processing, they noted. In addition, residual cancer from PSMs could lack biological characteristics needed for progression.
However, PSMs "should be avoided" because they worry patients and significantly increase the risks of biochemical recurrence and need for secondary treatment, Dr. Stephenson and associates said.
All patients in the study were treated at high-volume hospitals, and PSMs at low-volume hospitals could have a different prognosis. The study also lacked data on length and number of PSMs, the investigators noted.
Dr. Stephenson was partially supported by the Robert Wood Johnson Foundation Physician Faculty Scholars Program and the Astellas/American Urological Association Rising Stars in Urology Program. He reported no relevant financial conflicts of interest.
The "important and new aspect of this study," said Dr. Markus Graefen and Dr. Hartwig Huland, is that it accounted for postoperative radiotherapy. Previous studies modeled only fixed pathologic variables.
The data show that a positive surgical margin "is the product of a large cancer with a bad prognosis rather than an independent risk factor" for cancer-specific mortality, they said.
However, the study could not address whether or not to withhold early radiotherapy and wait for a PSA relapse to deliver early salvage radiotherapy. That answer requires results from the RADICALS (Radiotherapy and Androgen Deprivation in Combination after Local Surgery) study, which randomized patients to adjuvant radiotherapy or early salvage radiotherapy with and without additional hormonal therapy.
In the meantime, clinicians can help ease patients’ fears by explaining that positive surgical margins indicate the need for further treatment, but do not independently increase their risk of dying from prostate cancer.
Dr. Markus Graefen and Dr. Hartwig Huland are with the Martini-Klinik Prostate Cancer Center, University-Hospital Hamburg-Eppendorf, Germany. These remarks were taken from their editorial accompanying Dr. Stephenson’s report (Eur. Urol. 2014;65:681-2).
The "important and new aspect of this study," said Dr. Markus Graefen and Dr. Hartwig Huland, is that it accounted for postoperative radiotherapy. Previous studies modeled only fixed pathologic variables.
The data show that a positive surgical margin "is the product of a large cancer with a bad prognosis rather than an independent risk factor" for cancer-specific mortality, they said.
However, the study could not address whether or not to withhold early radiotherapy and wait for a PSA relapse to deliver early salvage radiotherapy. That answer requires results from the RADICALS (Radiotherapy and Androgen Deprivation in Combination after Local Surgery) study, which randomized patients to adjuvant radiotherapy or early salvage radiotherapy with and without additional hormonal therapy.
In the meantime, clinicians can help ease patients’ fears by explaining that positive surgical margins indicate the need for further treatment, but do not independently increase their risk of dying from prostate cancer.
Dr. Markus Graefen and Dr. Hartwig Huland are with the Martini-Klinik Prostate Cancer Center, University-Hospital Hamburg-Eppendorf, Germany. These remarks were taken from their editorial accompanying Dr. Stephenson’s report (Eur. Urol. 2014;65:681-2).
The "important and new aspect of this study," said Dr. Markus Graefen and Dr. Hartwig Huland, is that it accounted for postoperative radiotherapy. Previous studies modeled only fixed pathologic variables.
The data show that a positive surgical margin "is the product of a large cancer with a bad prognosis rather than an independent risk factor" for cancer-specific mortality, they said.
However, the study could not address whether or not to withhold early radiotherapy and wait for a PSA relapse to deliver early salvage radiotherapy. That answer requires results from the RADICALS (Radiotherapy and Androgen Deprivation in Combination after Local Surgery) study, which randomized patients to adjuvant radiotherapy or early salvage radiotherapy with and without additional hormonal therapy.
In the meantime, clinicians can help ease patients’ fears by explaining that positive surgical margins indicate the need for further treatment, but do not independently increase their risk of dying from prostate cancer.
Dr. Markus Graefen and Dr. Hartwig Huland are with the Martini-Klinik Prostate Cancer Center, University-Hospital Hamburg-Eppendorf, Germany. These remarks were taken from their editorial accompanying Dr. Stephenson’s report (Eur. Urol. 2014;65:681-2).
Positive surgical margins alone do not predict death from prostate cancer in men who undergo radical prostatectomy, investigators reported in the April issue of European Urology.
Positive surgical margins (PSMs) were not significantly associated with prostate cancer–specific mortality after adjustment for fixed covariates and postoperative radiotherapy, reported Dr. Andrew J. Stephenson, of the Cleveland Clinic’s Glickman Urological & Kidney Institute, and his associates.
Investigators analyzed data from 11,521 men with localized prostate cancer. Patients had undergone radical prostatectomy at four universities and cancer centers between 1987 and 2005.
At 15 years of follow-up, the prostate cancer–specific mortality for men with negative surgical margins was 6%, compared with 10% for men with PSMs (P less than .001).
But PSMs did not independently predict prostate cancer–specific mortality in regression models, the investigators reported (Eur. Urol. 2004;65:675-80).
That finding was true when researchers modeled only fixed covariates, such as age, Gleason score, seminal vesicle invasion, lymph node involvement, prostate-specific antigen (PSA), and extraprostatic extension (hazard ratio, 1.04; 95% confidence interval, 0.7-1.5), and also when they adjusted for postoperative radiotherapy, either as a single parameter (HR, 0.96; 95% CI, 0.7-1.4) or as early versus late treatment (HR, 1.01; 95% CI, 0.7-1.4).
The lack of an association called into question "the rationale for postoperative radiotherapy for PSMs in the absence of other adverse features," as well as "the relevance of PSM rates as a measure of surgical proficiency," the investigators said.
Even expert pathologists may not agree on PSMs and whether PSMs could be artifacts from surgery or pathologic processing, they noted. In addition, residual cancer from PSMs could lack biological characteristics needed for progression.
However, PSMs "should be avoided" because they worry patients and significantly increase the risks of biochemical recurrence and need for secondary treatment, Dr. Stephenson and associates said.
All patients in the study were treated at high-volume hospitals, and PSMs at low-volume hospitals could have a different prognosis. The study also lacked data on length and number of PSMs, the investigators noted.
Dr. Stephenson was partially supported by the Robert Wood Johnson Foundation Physician Faculty Scholars Program and the Astellas/American Urological Association Rising Stars in Urology Program. He reported no relevant financial conflicts of interest.
Positive surgical margins alone do not predict death from prostate cancer in men who undergo radical prostatectomy, investigators reported in the April issue of European Urology.
Positive surgical margins (PSMs) were not significantly associated with prostate cancer–specific mortality after adjustment for fixed covariates and postoperative radiotherapy, reported Dr. Andrew J. Stephenson, of the Cleveland Clinic’s Glickman Urological & Kidney Institute, and his associates.
Investigators analyzed data from 11,521 men with localized prostate cancer. Patients had undergone radical prostatectomy at four universities and cancer centers between 1987 and 2005.
At 15 years of follow-up, the prostate cancer–specific mortality for men with negative surgical margins was 6%, compared with 10% for men with PSMs (P less than .001).
But PSMs did not independently predict prostate cancer–specific mortality in regression models, the investigators reported (Eur. Urol. 2004;65:675-80).
That finding was true when researchers modeled only fixed covariates, such as age, Gleason score, seminal vesicle invasion, lymph node involvement, prostate-specific antigen (PSA), and extraprostatic extension (hazard ratio, 1.04; 95% confidence interval, 0.7-1.5), and also when they adjusted for postoperative radiotherapy, either as a single parameter (HR, 0.96; 95% CI, 0.7-1.4) or as early versus late treatment (HR, 1.01; 95% CI, 0.7-1.4).
The lack of an association called into question "the rationale for postoperative radiotherapy for PSMs in the absence of other adverse features," as well as "the relevance of PSM rates as a measure of surgical proficiency," the investigators said.
Even expert pathologists may not agree on PSMs and whether PSMs could be artifacts from surgery or pathologic processing, they noted. In addition, residual cancer from PSMs could lack biological characteristics needed for progression.
However, PSMs "should be avoided" because they worry patients and significantly increase the risks of biochemical recurrence and need for secondary treatment, Dr. Stephenson and associates said.
All patients in the study were treated at high-volume hospitals, and PSMs at low-volume hospitals could have a different prognosis. The study also lacked data on length and number of PSMs, the investigators noted.
Dr. Stephenson was partially supported by the Robert Wood Johnson Foundation Physician Faculty Scholars Program and the Astellas/American Urological Association Rising Stars in Urology Program. He reported no relevant financial conflicts of interest.
FROM EUROPEAN UROLOGY
Major finding: Positive surgical margins were not significantly associated with prostate cancer–specific mortality within 15 years of radical prostatectomy after adjustment for fixed covariates and postoperative radiotherapy.
Data source: Multicenter cohort study of 11,521 men with localized prostate cancer who underwent radical prostatectomy between 1987 and 2005.
Disclosures: Dr. Stephenson was partially supported by the Robert Wood Johnson Foundation Physician Faculty Scholars Program and the Astellas/American Urological Association Rising Stars in Urology Program. Dr. Stephenson, Dr. Graefen, and Dr. Huland reported no relevant financial conflicts of interest.
Prostatectomy found superior to watchful waiting over long term
Extended follow-up confirms that radical prostatectomy continues to substantially reduce mortality from any cause, mortality from prostate cancer, and the risk of developing metastases over the long term, compared with watchful waiting, investigators reported March 5 in the New England Journal of Medicine.
These protective effects were most pronounced in men who were under age 65 at diagnosis, but older men had the additional benefit from radical prostatectomy of improved quality of life, because of a significantly lower risk of palliative care, including androgen-deprivation therapy. "The life experience after the diagnosis of prostate cancer differs substantially between the two study groups and evolves over decades," said Dr. Anna Bill-Axelson of Uppsala (Sweden) University Hospital and her associates.
Between 1989 and 1999, men with localized prostate cancer who were under age 75 and had life expectancies beyond 10 years, were randomly assigned to undergo radical prostatectomy (347 patients) or watchful waiting (348 patients) at 14 medical centers in Sweden, Finland, and Iceland. A total of 447 men (64%) died during extended follow-up lasting until 2012 (median follow-up, 13.4 years).
All-cause mortality was 56.1% in the prostatectomy group, significantly lower than the 68.9% all-cause mortality in the watchful waiting group; the number needed to treat to prevent 1 death at 18 years of follow-up was 8. Similarly, prostate cancer–specific mortality was 17.7% with prostatectomy vs. 28.7% with watchful waiting, and the difference between the two study groups continued to increase over time, the investigators reported (N. Engl. J. Med. 2014 March 5 [doi: 10.1056/NEMoa1311593]).
Among men under age 65 at diagnosis, all-cause mortality was 25.5 percentage points lower, prostate cancer–specific mortality was 15.8 percentage points lower, and risk of metastases was 15.8 percentage points lower with prostatectomy than with watchful waiting. "In this age group, the number needed to treat to prevent 1 death from prostate cancer was 4," Dr. Bill-Axelson and her associates said.
This study was supported by the Swedish Cancer Society, the National Institutes of Health, the Karolinska Institute, the Prostate Cancer Foundation, and the Percy Falk Foundation. The authors reported that they had no financial conflicts of interest.
Extended follow-up confirms that radical prostatectomy continues to substantially reduce mortality from any cause, mortality from prostate cancer, and the risk of developing metastases over the long term, compared with watchful waiting, investigators reported March 5 in the New England Journal of Medicine.
These protective effects were most pronounced in men who were under age 65 at diagnosis, but older men had the additional benefit from radical prostatectomy of improved quality of life, because of a significantly lower risk of palliative care, including androgen-deprivation therapy. "The life experience after the diagnosis of prostate cancer differs substantially between the two study groups and evolves over decades," said Dr. Anna Bill-Axelson of Uppsala (Sweden) University Hospital and her associates.
Between 1989 and 1999, men with localized prostate cancer who were under age 75 and had life expectancies beyond 10 years, were randomly assigned to undergo radical prostatectomy (347 patients) or watchful waiting (348 patients) at 14 medical centers in Sweden, Finland, and Iceland. A total of 447 men (64%) died during extended follow-up lasting until 2012 (median follow-up, 13.4 years).
All-cause mortality was 56.1% in the prostatectomy group, significantly lower than the 68.9% all-cause mortality in the watchful waiting group; the number needed to treat to prevent 1 death at 18 years of follow-up was 8. Similarly, prostate cancer–specific mortality was 17.7% with prostatectomy vs. 28.7% with watchful waiting, and the difference between the two study groups continued to increase over time, the investigators reported (N. Engl. J. Med. 2014 March 5 [doi: 10.1056/NEMoa1311593]).
Among men under age 65 at diagnosis, all-cause mortality was 25.5 percentage points lower, prostate cancer–specific mortality was 15.8 percentage points lower, and risk of metastases was 15.8 percentage points lower with prostatectomy than with watchful waiting. "In this age group, the number needed to treat to prevent 1 death from prostate cancer was 4," Dr. Bill-Axelson and her associates said.
This study was supported by the Swedish Cancer Society, the National Institutes of Health, the Karolinska Institute, the Prostate Cancer Foundation, and the Percy Falk Foundation. The authors reported that they had no financial conflicts of interest.
Extended follow-up confirms that radical prostatectomy continues to substantially reduce mortality from any cause, mortality from prostate cancer, and the risk of developing metastases over the long term, compared with watchful waiting, investigators reported March 5 in the New England Journal of Medicine.
These protective effects were most pronounced in men who were under age 65 at diagnosis, but older men had the additional benefit from radical prostatectomy of improved quality of life, because of a significantly lower risk of palliative care, including androgen-deprivation therapy. "The life experience after the diagnosis of prostate cancer differs substantially between the two study groups and evolves over decades," said Dr. Anna Bill-Axelson of Uppsala (Sweden) University Hospital and her associates.
Between 1989 and 1999, men with localized prostate cancer who were under age 75 and had life expectancies beyond 10 years, were randomly assigned to undergo radical prostatectomy (347 patients) or watchful waiting (348 patients) at 14 medical centers in Sweden, Finland, and Iceland. A total of 447 men (64%) died during extended follow-up lasting until 2012 (median follow-up, 13.4 years).
All-cause mortality was 56.1% in the prostatectomy group, significantly lower than the 68.9% all-cause mortality in the watchful waiting group; the number needed to treat to prevent 1 death at 18 years of follow-up was 8. Similarly, prostate cancer–specific mortality was 17.7% with prostatectomy vs. 28.7% with watchful waiting, and the difference between the two study groups continued to increase over time, the investigators reported (N. Engl. J. Med. 2014 March 5 [doi: 10.1056/NEMoa1311593]).
Among men under age 65 at diagnosis, all-cause mortality was 25.5 percentage points lower, prostate cancer–specific mortality was 15.8 percentage points lower, and risk of metastases was 15.8 percentage points lower with prostatectomy than with watchful waiting. "In this age group, the number needed to treat to prevent 1 death from prostate cancer was 4," Dr. Bill-Axelson and her associates said.
This study was supported by the Swedish Cancer Society, the National Institutes of Health, the Karolinska Institute, the Prostate Cancer Foundation, and the Percy Falk Foundation. The authors reported that they had no financial conflicts of interest.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major finding: In the prostatectomy group, 18-year all-cause mortality was 56.1% and prostate cancer–specific mortality was 17.7%, which are significantly lower than the 68.9% all-cause mortality and 28.7% prostate cancer–specific mortality in the watchful waiting group.
Data source: Extended (23-year) follow-up of an international, randomized clinical trial involving 695 men with localized prostate cancer who underwent either radical prostatectomy or watchful waiting.
Disclosures: This study was supported by the Swedish Cancer Society, the National Institutes of Health, the Karolinska Institute, the Prostate Cancer Foundation, and the Percy Falk Foundation. No financial conflicts of interest were reported.
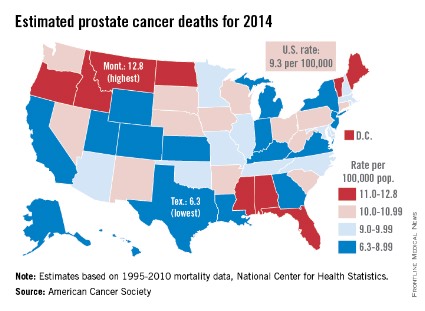
Prostate cancer death rate highest in Montana
Montana is expected to have the highest rate of prostate cancer deaths in the United States for 2014, and it will be more than twice as high as that of Texas, which should have the lowest rate, the American Cancer Society reported.
Based on mortality data for 1995-2010 from the National Center for Health Statistics, the ACS estimates put the death rate at 12.8 per 100,000 population for Montana and 6.3 per 100,000 for Texas.
After Texas, Wyoming has the lowest estimated prostate cancer rate for 2014 at 6.9 per 100,000, followed by Utah at 7.2. The District of Columbia has the second-highest rate at 12.4 per 100,000, with Maine third at 12.0, according to the ACS estimates.
Although the number of prostate cancer deaths has been decreasing – dropping by an average of 3.1% per year from 2006 to 2010 – it is still the second-leading cause of cancer death in men. With almost 29,500 deaths expected in the United States this year, the national death rate from prostate cancer for 2014 is estimated to be 9.3 per 100,000, according to the ACS report.
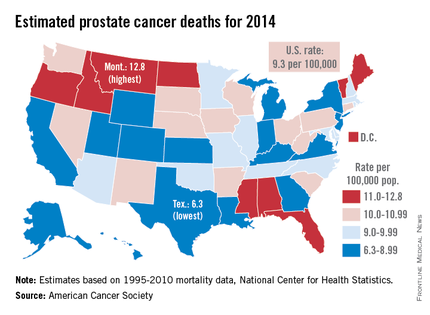
Montana is expected to have the highest rate of prostate cancer deaths in the United States for 2014, and it will be more than twice as high as that of Texas, which should have the lowest rate, the American Cancer Society reported.
Based on mortality data for 1995-2010 from the National Center for Health Statistics, the ACS estimates put the death rate at 12.8 per 100,000 population for Montana and 6.3 per 100,000 for Texas.
After Texas, Wyoming has the lowest estimated prostate cancer rate for 2014 at 6.9 per 100,000, followed by Utah at 7.2. The District of Columbia has the second-highest rate at 12.4 per 100,000, with Maine third at 12.0, according to the ACS estimates.
Although the number of prostate cancer deaths has been decreasing – dropping by an average of 3.1% per year from 2006 to 2010 – it is still the second-leading cause of cancer death in men. With almost 29,500 deaths expected in the United States this year, the national death rate from prostate cancer for 2014 is estimated to be 9.3 per 100,000, according to the ACS report.

Montana is expected to have the highest rate of prostate cancer deaths in the United States for 2014, and it will be more than twice as high as that of Texas, which should have the lowest rate, the American Cancer Society reported.
Based on mortality data for 1995-2010 from the National Center for Health Statistics, the ACS estimates put the death rate at 12.8 per 100,000 population for Montana and 6.3 per 100,000 for Texas.
After Texas, Wyoming has the lowest estimated prostate cancer rate for 2014 at 6.9 per 100,000, followed by Utah at 7.2. The District of Columbia has the second-highest rate at 12.4 per 100,000, with Maine third at 12.0, according to the ACS estimates.
Although the number of prostate cancer deaths has been decreasing – dropping by an average of 3.1% per year from 2006 to 2010 – it is still the second-leading cause of cancer death in men. With almost 29,500 deaths expected in the United States this year, the national death rate from prostate cancer for 2014 is estimated to be 9.3 per 100,000, according to the ACS report.

Beta-blockers linked to lower mortality in high-risk prostate cancer
The use of beta-blockers in men with metastatic and high-risk prostate cancer was associated with a reduced risk of prostate cancer–specific mortality independent of statin or acetylsalicylic acid use, according to a study published in European Urology.
Given these results, beta-blocker use should be further investigated where large registries are available, wrote Dr. Helene Hartvedt Grytli of the Institute of Cancer Research at Oslo University Hospital and her coinvestigators. "This is the first study to assess the association between beta-blocker use and prostate cancer–specific survival in a large cohort of men with known disease aggressiveness at diagnosis," they wrote.
The researchers obtained data from the Cancer Registry of Norway for men diagnosed with prostate cancer between 2004 and 2009. They coupled these data, using participants’ national identification numbers, with information from the Norwegian Prescription Database on filled prescriptions for beta-blockers, statins, and acetylsalicylic acid (ASA). Patients were considered users of these agents if they had filled at least one prescription of the respective drug both before and after diagnosis.
Mortality data were obtained from the Cancer Registry of Norway. A total of 3,561 men were eligible for analysis after exclusion criteria were accounted for; the criteria included having low- or intermediate-risk disease and planned treatment with either radical prostatectomy or radiation therapy.
Beta-blocker use was independently associated with a reduced risk of prostate cancer–specific mortality in a multivariable model (adjusted subhazard ratio, 0.79; 95% confidence interval, 0.68-0.91; P = .001). The median follow-up time was 39 months (Eur. Urol. 2014;635-41).
Among beta-blocker users, 72% also used either ASA or statins. The investigators performed a survival analysis, adjusting for the use of these drugs to study any possible confounding effect. Statin use and ASA use were both associated with a reduction in prostate cancer–specific mortality when each drug was analyzed separately. Adjustment for the use of these drug classes did not substantially change the subhazard ratio for beta-blocker use.
The researchers did acknowledge a possible limitation of their study: "Unknown patient characteristics of this patient group might have confounded our results, and additional observation studies are warranted to establish whether beta-blocker use has an independent effect on prostate cancer progression and survival."
The study was funded by the South-Eastern Norway Regional Health Authority, Oslo University Hospital, and the University of Oslo.
The use of beta-blockers in men with metastatic and high-risk prostate cancer was associated with a reduced risk of prostate cancer–specific mortality independent of statin or acetylsalicylic acid use, according to a study published in European Urology.
Given these results, beta-blocker use should be further investigated where large registries are available, wrote Dr. Helene Hartvedt Grytli of the Institute of Cancer Research at Oslo University Hospital and her coinvestigators. "This is the first study to assess the association between beta-blocker use and prostate cancer–specific survival in a large cohort of men with known disease aggressiveness at diagnosis," they wrote.
The researchers obtained data from the Cancer Registry of Norway for men diagnosed with prostate cancer between 2004 and 2009. They coupled these data, using participants’ national identification numbers, with information from the Norwegian Prescription Database on filled prescriptions for beta-blockers, statins, and acetylsalicylic acid (ASA). Patients were considered users of these agents if they had filled at least one prescription of the respective drug both before and after diagnosis.
Mortality data were obtained from the Cancer Registry of Norway. A total of 3,561 men were eligible for analysis after exclusion criteria were accounted for; the criteria included having low- or intermediate-risk disease and planned treatment with either radical prostatectomy or radiation therapy.
Beta-blocker use was independently associated with a reduced risk of prostate cancer–specific mortality in a multivariable model (adjusted subhazard ratio, 0.79; 95% confidence interval, 0.68-0.91; P = .001). The median follow-up time was 39 months (Eur. Urol. 2014;635-41).
Among beta-blocker users, 72% also used either ASA or statins. The investigators performed a survival analysis, adjusting for the use of these drugs to study any possible confounding effect. Statin use and ASA use were both associated with a reduction in prostate cancer–specific mortality when each drug was analyzed separately. Adjustment for the use of these drug classes did not substantially change the subhazard ratio for beta-blocker use.
The researchers did acknowledge a possible limitation of their study: "Unknown patient characteristics of this patient group might have confounded our results, and additional observation studies are warranted to establish whether beta-blocker use has an independent effect on prostate cancer progression and survival."
The study was funded by the South-Eastern Norway Regional Health Authority, Oslo University Hospital, and the University of Oslo.
The use of beta-blockers in men with metastatic and high-risk prostate cancer was associated with a reduced risk of prostate cancer–specific mortality independent of statin or acetylsalicylic acid use, according to a study published in European Urology.
Given these results, beta-blocker use should be further investigated where large registries are available, wrote Dr. Helene Hartvedt Grytli of the Institute of Cancer Research at Oslo University Hospital and her coinvestigators. "This is the first study to assess the association between beta-blocker use and prostate cancer–specific survival in a large cohort of men with known disease aggressiveness at diagnosis," they wrote.
The researchers obtained data from the Cancer Registry of Norway for men diagnosed with prostate cancer between 2004 and 2009. They coupled these data, using participants’ national identification numbers, with information from the Norwegian Prescription Database on filled prescriptions for beta-blockers, statins, and acetylsalicylic acid (ASA). Patients were considered users of these agents if they had filled at least one prescription of the respective drug both before and after diagnosis.
Mortality data were obtained from the Cancer Registry of Norway. A total of 3,561 men were eligible for analysis after exclusion criteria were accounted for; the criteria included having low- or intermediate-risk disease and planned treatment with either radical prostatectomy or radiation therapy.
Beta-blocker use was independently associated with a reduced risk of prostate cancer–specific mortality in a multivariable model (adjusted subhazard ratio, 0.79; 95% confidence interval, 0.68-0.91; P = .001). The median follow-up time was 39 months (Eur. Urol. 2014;635-41).
Among beta-blocker users, 72% also used either ASA or statins. The investigators performed a survival analysis, adjusting for the use of these drugs to study any possible confounding effect. Statin use and ASA use were both associated with a reduction in prostate cancer–specific mortality when each drug was analyzed separately. Adjustment for the use of these drug classes did not substantially change the subhazard ratio for beta-blocker use.
The researchers did acknowledge a possible limitation of their study: "Unknown patient characteristics of this patient group might have confounded our results, and additional observation studies are warranted to establish whether beta-blocker use has an independent effect on prostate cancer progression and survival."
The study was funded by the South-Eastern Norway Regional Health Authority, Oslo University Hospital, and the University of Oslo.
FROM EUROPEAN UROLOGY
Major finding: Beta-blocker use was associated with reduced prostate cancer mortality (adjusted subhazard ratio, 0.79; 95% confidence interval, 0.68-0.91; P = .001).
Data source: An analysis of 3,561 men with metastatic or high-risk prostate cancer, sampled from data obtained from the Cancer Registry of Norway and the Norwegian Prescription Database.
Disclosures: The study was funded by the South-Eastern Norway Regional Health Authority, Oslo University Hospital, and the University of Oslo.
IMDC model is validated in setting of second-line targeted therapy
SAN FRANCISCO – The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) model is a valid tool for predicting survival in patients starting second-line targeted therapy for this disease, new data show.
In fact, it outperforms the leading model derived a decade ago, researchers reported at the 2014 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology.
The new model uses six clinical and laboratory factors to stratify patients into prognostic risk groups. It builds on the widely used three-factor Memorial Sloan-Kettering Cancer Center (MSKCC) second-line prognostic model (J. Clin. Oncol. 2004;22:454-63).
Results of the retrospective cohort study showed that the IMDC model performed well for risk stratification in this setting, with patients in the intermediate- and high-risk groups having roughly two and six times the risk of death, respectively, compared with peers in the favorable risk group, reported Dr. Jenny J. Ko of the British Columbia Cancer Agency, Vancouver. Also, it was superior to the MSKCC model for predicting survival as assessed by three statistical tests.
"The six-factor IMDC prognostic model has been validated in and can be applied to patients previously treated with targeted therapy, in addition to previously validated populations in the first-line targeted therapy and non–clear cell setting," she commented.
"The model fits better into the context of the contemporary treatment era, where targeted therapies are used in a sequential fashion," Dr. Ko added.
The study population was heterogeneous, she acknowledged. But "this can also mean that the cohort was generalizable, as our cohort includes trial and off-trial patients, and it also includes community and academic centers."
Invited discussant Dr. Daniel Canter of the Fox Chase Cancer Center, Philadelphia, noted that the durations of second-line therapy and survival after starting this therapy were fairly short, highlighting the need for better therapies.
The choice of first-line therapy for metastatic renal cell carcinoma (mRCC) may shift in the future away from sunitinib (Sutent) and sorafenib (Nexavar), used in the vast majority of the patients studied, he noted.
"My observation is that the medical oncologists that I work with seem to be using pazopanib (Votrient) more now as a first-line therapy because of the improved toxicity," Dr. Canter noted, and doing so may ultimately affect survival. "Obviously, a future question is the role of pazopanib. And ... an algorithm to define second-line choices is clearly something where there is an opportunity for work to be done."
Dr. Ko and her team studied 1,021 consecutive patients with mRCC from 19 centers who were starting second-line targeted therapy. (About a fifth had received immunotherapy before their first-line targeted therapy and were therefore on third-line therapy of any type.)
Eighty-five percent of patients had undergone nephrectomy. Their first-line targeted therapy had most often been sunitinib (67%) or sorafenib (20%).
The second-line therapies given included sorafenib (29%), sunitinib (22%), everolimus (Afinitor) (22%), and temsirolimus (Torisel) (12%), among others.
In a multivariate analysis, five of the six IMDC factors (Karnofsky performance status less than 80%; interval between diagnosis and treatment of less than 1 year; anemia; thrombocytosis; and neutrophilia) individually predicted an elevated risk of death after starting second-line therapy, reported Dr. Ko, who disclosed no conflicts of interest related to the research. Hypercalcemia did not, but few patients had this risk factor.
The median time on second-line targeted therapy was 3.9 months, and the median overall survival after starting this therapy was 12.5 months.
Compared with patients in the favorable IMDC risk group (having none of the risk factors), patients in the intermediate-risk group (having one or two) and patients in the high-risk group (having three or more) had significantly elevated risks of death (hazard ratios, 1.89 and 5.73).
In a likelihood ratio test, comparing the two models, the chi-square value was highly significant, suggesting that inclusion of the three additional factors in the IMDC model enhanced predictive performance over that with the MSKCC model.
Finally, in a reclassification calibration analysis, the chi-square value was much lower for the IMDC model than for the MSKCC model (6.2 vs. 54.2), indicating that the observed vs. predicted number of deaths at 1 year was better for the former.
SAN FRANCISCO – The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) model is a valid tool for predicting survival in patients starting second-line targeted therapy for this disease, new data show.
In fact, it outperforms the leading model derived a decade ago, researchers reported at the 2014 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology.
The new model uses six clinical and laboratory factors to stratify patients into prognostic risk groups. It builds on the widely used three-factor Memorial Sloan-Kettering Cancer Center (MSKCC) second-line prognostic model (J. Clin. Oncol. 2004;22:454-63).
Results of the retrospective cohort study showed that the IMDC model performed well for risk stratification in this setting, with patients in the intermediate- and high-risk groups having roughly two and six times the risk of death, respectively, compared with peers in the favorable risk group, reported Dr. Jenny J. Ko of the British Columbia Cancer Agency, Vancouver. Also, it was superior to the MSKCC model for predicting survival as assessed by three statistical tests.
"The six-factor IMDC prognostic model has been validated in and can be applied to patients previously treated with targeted therapy, in addition to previously validated populations in the first-line targeted therapy and non–clear cell setting," she commented.
"The model fits better into the context of the contemporary treatment era, where targeted therapies are used in a sequential fashion," Dr. Ko added.
The study population was heterogeneous, she acknowledged. But "this can also mean that the cohort was generalizable, as our cohort includes trial and off-trial patients, and it also includes community and academic centers."
Invited discussant Dr. Daniel Canter of the Fox Chase Cancer Center, Philadelphia, noted that the durations of second-line therapy and survival after starting this therapy were fairly short, highlighting the need for better therapies.
The choice of first-line therapy for metastatic renal cell carcinoma (mRCC) may shift in the future away from sunitinib (Sutent) and sorafenib (Nexavar), used in the vast majority of the patients studied, he noted.
"My observation is that the medical oncologists that I work with seem to be using pazopanib (Votrient) more now as a first-line therapy because of the improved toxicity," Dr. Canter noted, and doing so may ultimately affect survival. "Obviously, a future question is the role of pazopanib. And ... an algorithm to define second-line choices is clearly something where there is an opportunity for work to be done."
Dr. Ko and her team studied 1,021 consecutive patients with mRCC from 19 centers who were starting second-line targeted therapy. (About a fifth had received immunotherapy before their first-line targeted therapy and were therefore on third-line therapy of any type.)
Eighty-five percent of patients had undergone nephrectomy. Their first-line targeted therapy had most often been sunitinib (67%) or sorafenib (20%).
The second-line therapies given included sorafenib (29%), sunitinib (22%), everolimus (Afinitor) (22%), and temsirolimus (Torisel) (12%), among others.
In a multivariate analysis, five of the six IMDC factors (Karnofsky performance status less than 80%; interval between diagnosis and treatment of less than 1 year; anemia; thrombocytosis; and neutrophilia) individually predicted an elevated risk of death after starting second-line therapy, reported Dr. Ko, who disclosed no conflicts of interest related to the research. Hypercalcemia did not, but few patients had this risk factor.
The median time on second-line targeted therapy was 3.9 months, and the median overall survival after starting this therapy was 12.5 months.
Compared with patients in the favorable IMDC risk group (having none of the risk factors), patients in the intermediate-risk group (having one or two) and patients in the high-risk group (having three or more) had significantly elevated risks of death (hazard ratios, 1.89 and 5.73).
In a likelihood ratio test, comparing the two models, the chi-square value was highly significant, suggesting that inclusion of the three additional factors in the IMDC model enhanced predictive performance over that with the MSKCC model.
Finally, in a reclassification calibration analysis, the chi-square value was much lower for the IMDC model than for the MSKCC model (6.2 vs. 54.2), indicating that the observed vs. predicted number of deaths at 1 year was better for the former.
SAN FRANCISCO – The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) model is a valid tool for predicting survival in patients starting second-line targeted therapy for this disease, new data show.
In fact, it outperforms the leading model derived a decade ago, researchers reported at the 2014 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology.
The new model uses six clinical and laboratory factors to stratify patients into prognostic risk groups. It builds on the widely used three-factor Memorial Sloan-Kettering Cancer Center (MSKCC) second-line prognostic model (J. Clin. Oncol. 2004;22:454-63).
Results of the retrospective cohort study showed that the IMDC model performed well for risk stratification in this setting, with patients in the intermediate- and high-risk groups having roughly two and six times the risk of death, respectively, compared with peers in the favorable risk group, reported Dr. Jenny J. Ko of the British Columbia Cancer Agency, Vancouver. Also, it was superior to the MSKCC model for predicting survival as assessed by three statistical tests.
"The six-factor IMDC prognostic model has been validated in and can be applied to patients previously treated with targeted therapy, in addition to previously validated populations in the first-line targeted therapy and non–clear cell setting," she commented.
"The model fits better into the context of the contemporary treatment era, where targeted therapies are used in a sequential fashion," Dr. Ko added.
The study population was heterogeneous, she acknowledged. But "this can also mean that the cohort was generalizable, as our cohort includes trial and off-trial patients, and it also includes community and academic centers."
Invited discussant Dr. Daniel Canter of the Fox Chase Cancer Center, Philadelphia, noted that the durations of second-line therapy and survival after starting this therapy were fairly short, highlighting the need for better therapies.
The choice of first-line therapy for metastatic renal cell carcinoma (mRCC) may shift in the future away from sunitinib (Sutent) and sorafenib (Nexavar), used in the vast majority of the patients studied, he noted.
"My observation is that the medical oncologists that I work with seem to be using pazopanib (Votrient) more now as a first-line therapy because of the improved toxicity," Dr. Canter noted, and doing so may ultimately affect survival. "Obviously, a future question is the role of pazopanib. And ... an algorithm to define second-line choices is clearly something where there is an opportunity for work to be done."
Dr. Ko and her team studied 1,021 consecutive patients with mRCC from 19 centers who were starting second-line targeted therapy. (About a fifth had received immunotherapy before their first-line targeted therapy and were therefore on third-line therapy of any type.)
Eighty-five percent of patients had undergone nephrectomy. Their first-line targeted therapy had most often been sunitinib (67%) or sorafenib (20%).
The second-line therapies given included sorafenib (29%), sunitinib (22%), everolimus (Afinitor) (22%), and temsirolimus (Torisel) (12%), among others.
In a multivariate analysis, five of the six IMDC factors (Karnofsky performance status less than 80%; interval between diagnosis and treatment of less than 1 year; anemia; thrombocytosis; and neutrophilia) individually predicted an elevated risk of death after starting second-line therapy, reported Dr. Ko, who disclosed no conflicts of interest related to the research. Hypercalcemia did not, but few patients had this risk factor.
The median time on second-line targeted therapy was 3.9 months, and the median overall survival after starting this therapy was 12.5 months.
Compared with patients in the favorable IMDC risk group (having none of the risk factors), patients in the intermediate-risk group (having one or two) and patients in the high-risk group (having three or more) had significantly elevated risks of death (hazard ratios, 1.89 and 5.73).
In a likelihood ratio test, comparing the two models, the chi-square value was highly significant, suggesting that inclusion of the three additional factors in the IMDC model enhanced predictive performance over that with the MSKCC model.
Finally, in a reclassification calibration analysis, the chi-square value was much lower for the IMDC model than for the MSKCC model (6.2 vs. 54.2), indicating that the observed vs. predicted number of deaths at 1 year was better for the former.
AT THE GENITOURINARY CANCERS SYMPOSIUM
Major finding: Compared with patients in the favorable IMDC risk group, patients in the intermediate-risk group and patients in the high-risk group were significantly more likely to die (HR, 1.89 and 5.73).
Data source: A retrospective cohort study in 1,021 patients with mRCC starting second-line targeted therapy.
Disclosures: Dr. Ko disclosed no relevant conflicts of interest.
At least a third of low-risk cancers get upgraded at prostatectomy
Among prostate cancers that are judged to be low risk after physical exam and biopsy, and which thus are candidates for active surveillance, 33%-45% are upgraded or upstaged when the patient decides instead to undergo radical prostatectomy, according to a report in the February issue of Journal of Urology.
Researchers analyzed data regarding tumor and patient features for 4,500 cases in a national Swedish database of prostate cancers diagnosed during a 4-year period. All of these cases were judged to be low risk on the basis of Gleason scores, PSA findings, and biopsy results, but all of the men elected to undergo radical prostatectomy, said Dr. Annelies Vellekoop of New York University and Manhattan Veterans Affairs Medical Center, New York, and her associates.
Even though these cases met stringent criteria for active surveillance according to several different protocols, 33%-45% of them (depending on the protocol) were found at surgery to have adverse pathology signaling that the cancers were of higher risk than anticipated (J. Urol. 2014;191:350-7).
Three predictors of upstaging/upgrading – age over 60 years, high PSA level, and stage T2 cancer on biopsy – are already known to raise risk and are included in most risk-assessment criteria. But two other factors – PSA density greater than 0.15 ng/mL and the linear extent of cancer in mm within biopsy specimens – were found to be highly predictive in this study and should be considered as additional criteria to include in risk assessments, the investigators said.
This study was supported by the Swedish Research Council, the Swedish Cancer Foundation, Vasterbotten County Council, and Lion’s Cancer Research Foundation at Umea University. Dr. Vellekoop reported no financial conflicts of interest; one of her associates reported ties to Sanofi.
Among prostate cancers that are judged to be low risk after physical exam and biopsy, and which thus are candidates for active surveillance, 33%-45% are upgraded or upstaged when the patient decides instead to undergo radical prostatectomy, according to a report in the February issue of Journal of Urology.
Researchers analyzed data regarding tumor and patient features for 4,500 cases in a national Swedish database of prostate cancers diagnosed during a 4-year period. All of these cases were judged to be low risk on the basis of Gleason scores, PSA findings, and biopsy results, but all of the men elected to undergo radical prostatectomy, said Dr. Annelies Vellekoop of New York University and Manhattan Veterans Affairs Medical Center, New York, and her associates.
Even though these cases met stringent criteria for active surveillance according to several different protocols, 33%-45% of them (depending on the protocol) were found at surgery to have adverse pathology signaling that the cancers were of higher risk than anticipated (J. Urol. 2014;191:350-7).
Three predictors of upstaging/upgrading – age over 60 years, high PSA level, and stage T2 cancer on biopsy – are already known to raise risk and are included in most risk-assessment criteria. But two other factors – PSA density greater than 0.15 ng/mL and the linear extent of cancer in mm within biopsy specimens – were found to be highly predictive in this study and should be considered as additional criteria to include in risk assessments, the investigators said.
This study was supported by the Swedish Research Council, the Swedish Cancer Foundation, Vasterbotten County Council, and Lion’s Cancer Research Foundation at Umea University. Dr. Vellekoop reported no financial conflicts of interest; one of her associates reported ties to Sanofi.
Among prostate cancers that are judged to be low risk after physical exam and biopsy, and which thus are candidates for active surveillance, 33%-45% are upgraded or upstaged when the patient decides instead to undergo radical prostatectomy, according to a report in the February issue of Journal of Urology.
Researchers analyzed data regarding tumor and patient features for 4,500 cases in a national Swedish database of prostate cancers diagnosed during a 4-year period. All of these cases were judged to be low risk on the basis of Gleason scores, PSA findings, and biopsy results, but all of the men elected to undergo radical prostatectomy, said Dr. Annelies Vellekoop of New York University and Manhattan Veterans Affairs Medical Center, New York, and her associates.
Even though these cases met stringent criteria for active surveillance according to several different protocols, 33%-45% of them (depending on the protocol) were found at surgery to have adverse pathology signaling that the cancers were of higher risk than anticipated (J. Urol. 2014;191:350-7).
Three predictors of upstaging/upgrading – age over 60 years, high PSA level, and stage T2 cancer on biopsy – are already known to raise risk and are included in most risk-assessment criteria. But two other factors – PSA density greater than 0.15 ng/mL and the linear extent of cancer in mm within biopsy specimens – were found to be highly predictive in this study and should be considered as additional criteria to include in risk assessments, the investigators said.
This study was supported by the Swedish Research Council, the Swedish Cancer Foundation, Vasterbotten County Council, and Lion’s Cancer Research Foundation at Umea University. Dr. Vellekoop reported no financial conflicts of interest; one of her associates reported ties to Sanofi.
FROM JOURNAL OF UROLOGY
Major finding: Even though these "low-risk" cases met stringent criteria for active surveillance according to several different protocols, 33%-45% of them (depending on the protocol) were found at surgery to have adverse pathology signaling that the cancers were actually of higher risk.
Data source: An analysis of data regarding 4,500 men across Sweden diagnosed as having low-risk prostate cancer during a 4-year period but who underwent radical prostatectomy.
Disclosures: This study was supported by the Swedish Research Council, the Swedish Cancer Foundation, Vasterbotten County Council, and Lion’s Cancer Research Foundation at Umea University. Dr. Vellekoop reported no financial conflicts of interest; one of her associates reported ties to Sanofi.
Cytoreductive nephrectomy has selective survival benefit in mRCC
SAN FRANCISCO – Only selected patients with synchronous metastases from renal cell carcinoma derive a survival benefit from a cytoreductive nephrectomy, a retrospective cohort study showed.
Researchers studied more than 1,600 patients from the International mRCC (metastatic renal cell carcinoma) Database Consortium who developed metastases while their primary tumor was still in place.
The main analyses showed that as a whole, patients who underwent cytoreductive nephrectomy lived longer than their counterparts who did not have this surgery.
However, in stratified analyses, the surgery significantly prolonged survival only among patients who had a life expectancy exceeding 12 months and patients who had three or fewer risk factors by International mRCC Database Consortium (IMDC) criteria.
Risk factors include Karnofsky performance status less than 80%, an interval between diagnosis and treatment of less than 1 year, anemia, thrombocytosis, neutrophilia, and hypercalcemia
"Overall, I think we can say that cytoreductive nephrectomy may be beneficial in the age of targeted therapy. However, not all patients should have it," commented first author Dr. Daniel Y.C. Heng of the University of Calgary (Alta.).
"Patients with four or more IMDC risk factors and patients with limited life expectancy probably shouldn’t have a cytoreductive nephrectomy," he said. "Of course, there are probably exceptions to that rule; if patients are really symptomatic in the primary, for example, or have bleeding, maybe there are exceptions.
"But I think this is a very interesting and useful way to help select our patients for cytoreductive nephrectomy. Certainly, we are awaiting the CARMENA and SURTIME phase III randomized controlled trials to prospectively evaluate this, and hopefully, we will be able to look at the IMDC risk factors to see if they can aid in patient selection in a prospectively validated way," Dr. Heng said at the 2014 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology.
Cytoreductive nephrectomy carries risks of morbidity and mortality, and some patients may be unable to go on to receive systemic therapy, noted invited discussant Dr. Daniel Canter of the Fox Chase Cancer Center, Philadelphia.
"What I take home as the most important point of this talk is that we need to make better choices and risk stratify these patients before surgery," he said.
A key question arising from the new data is whether the IMDC risk groups should be redefined, according to Dr. Canter; for example, patients with three risk factors are currently placed in the poor risk group, yet they derived significant benefit from surgery in this study. "So is there room for further refinement in this model?" he asked.
"With such a large cohort, I think that adding to this data with complication rates and perioperative mortality would certainly be important in terms of future treatment decision making," he commented.
Session attendee Dr. Adam Metwalli of the National Cancer Institute asked, "Are there specific risk factors that seem to disproportionately cluster among those who have four or five that would help to distinguish them from the ones who have zero to three?"
"By far, the most important prognostic factor is Karnofsky performance status, and that makes sense," Dr. Heng replied. "It’s partially the ‘look test’ and how you think a patient will fare. So, that by far has the biggest contribution."
Introducing the study, he noted that cytoreductive nephrectomy has previously been found to prolong survival by about 6 months in patients with metastatic renal cell carcinoma treated in the immunotherapy era.
"But of course now we are in the age of targeted therapy, with VEGF [vascular endothelial growth factor] inhibitors and mTOR [mammalian target of rapamycin] inhibitors. These are drugs that are much more active than in the era of old immunotherapy. So it begs the question, is cytoreductive nephrectomy still relevant?" Dr. Heng said.
The researchers studied 1,658 patients from 20 institutions who had mRCC with synchronous metastases, of whom 59% underwent cytoreductive nephrectomy.
They had a median age of 59 years, and about half were in the IMDC intermediate-risk group. The most common prior targeted therapy was sunitinib (Sutent), received by about three-fourths, reported Dr. Heng.
In unadjusted analyses, median overall survival was a significant 11 months longer for patients who underwent cytoreductive nephrectomy compared with their counterparts who did not (20.6 vs. 9.5 months). After adjustment for IMDC risk factors, the surgery was associated with a 40% reduction in the risk of death in the entire cohort (hazard ratio, 0.60; P less than .0001).
However, in analyses stratified by patient life expectancy, there was a significant survival benefit of cytoreductive nephrectomy only among patients expected to live 12-18 months (3.3-month incremental benefit; adjusted hazard ratio, 0.85; P = .049) or 18-24 months (5.2-month incremental benefit; adjusted hazard ratio, 0.72; P less than .0001).
Similarly, in analyses stratified by the number of risk factors present out of the six IMDC risk factors, there was a significant survival benefit of cytoreductive nephrectomy only among patients with three or fewer factors (incremental benefit of 6-10 months, P less than .005 for each).
The impact of overall tumor burden and the size of the primary tumor were not considered in this analysis but will be in subsequent analyses, according to Dr. Heng.
He disclosed that he is a consultant/adviser to Aveo, Bayer, Bristol-Myers Squibb, Novartis, and Pfizer.
SAN FRANCISCO – Only selected patients with synchronous metastases from renal cell carcinoma derive a survival benefit from a cytoreductive nephrectomy, a retrospective cohort study showed.
Researchers studied more than 1,600 patients from the International mRCC (metastatic renal cell carcinoma) Database Consortium who developed metastases while their primary tumor was still in place.
The main analyses showed that as a whole, patients who underwent cytoreductive nephrectomy lived longer than their counterparts who did not have this surgery.
However, in stratified analyses, the surgery significantly prolonged survival only among patients who had a life expectancy exceeding 12 months and patients who had three or fewer risk factors by International mRCC Database Consortium (IMDC) criteria.
Risk factors include Karnofsky performance status less than 80%, an interval between diagnosis and treatment of less than 1 year, anemia, thrombocytosis, neutrophilia, and hypercalcemia
"Overall, I think we can say that cytoreductive nephrectomy may be beneficial in the age of targeted therapy. However, not all patients should have it," commented first author Dr. Daniel Y.C. Heng of the University of Calgary (Alta.).
"Patients with four or more IMDC risk factors and patients with limited life expectancy probably shouldn’t have a cytoreductive nephrectomy," he said. "Of course, there are probably exceptions to that rule; if patients are really symptomatic in the primary, for example, or have bleeding, maybe there are exceptions.
"But I think this is a very interesting and useful way to help select our patients for cytoreductive nephrectomy. Certainly, we are awaiting the CARMENA and SURTIME phase III randomized controlled trials to prospectively evaluate this, and hopefully, we will be able to look at the IMDC risk factors to see if they can aid in patient selection in a prospectively validated way," Dr. Heng said at the 2014 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology.
Cytoreductive nephrectomy carries risks of morbidity and mortality, and some patients may be unable to go on to receive systemic therapy, noted invited discussant Dr. Daniel Canter of the Fox Chase Cancer Center, Philadelphia.
"What I take home as the most important point of this talk is that we need to make better choices and risk stratify these patients before surgery," he said.
A key question arising from the new data is whether the IMDC risk groups should be redefined, according to Dr. Canter; for example, patients with three risk factors are currently placed in the poor risk group, yet they derived significant benefit from surgery in this study. "So is there room for further refinement in this model?" he asked.
"With such a large cohort, I think that adding to this data with complication rates and perioperative mortality would certainly be important in terms of future treatment decision making," he commented.
Session attendee Dr. Adam Metwalli of the National Cancer Institute asked, "Are there specific risk factors that seem to disproportionately cluster among those who have four or five that would help to distinguish them from the ones who have zero to three?"
"By far, the most important prognostic factor is Karnofsky performance status, and that makes sense," Dr. Heng replied. "It’s partially the ‘look test’ and how you think a patient will fare. So, that by far has the biggest contribution."
Introducing the study, he noted that cytoreductive nephrectomy has previously been found to prolong survival by about 6 months in patients with metastatic renal cell carcinoma treated in the immunotherapy era.
"But of course now we are in the age of targeted therapy, with VEGF [vascular endothelial growth factor] inhibitors and mTOR [mammalian target of rapamycin] inhibitors. These are drugs that are much more active than in the era of old immunotherapy. So it begs the question, is cytoreductive nephrectomy still relevant?" Dr. Heng said.
The researchers studied 1,658 patients from 20 institutions who had mRCC with synchronous metastases, of whom 59% underwent cytoreductive nephrectomy.
They had a median age of 59 years, and about half were in the IMDC intermediate-risk group. The most common prior targeted therapy was sunitinib (Sutent), received by about three-fourths, reported Dr. Heng.
In unadjusted analyses, median overall survival was a significant 11 months longer for patients who underwent cytoreductive nephrectomy compared with their counterparts who did not (20.6 vs. 9.5 months). After adjustment for IMDC risk factors, the surgery was associated with a 40% reduction in the risk of death in the entire cohort (hazard ratio, 0.60; P less than .0001).
However, in analyses stratified by patient life expectancy, there was a significant survival benefit of cytoreductive nephrectomy only among patients expected to live 12-18 months (3.3-month incremental benefit; adjusted hazard ratio, 0.85; P = .049) or 18-24 months (5.2-month incremental benefit; adjusted hazard ratio, 0.72; P less than .0001).
Similarly, in analyses stratified by the number of risk factors present out of the six IMDC risk factors, there was a significant survival benefit of cytoreductive nephrectomy only among patients with three or fewer factors (incremental benefit of 6-10 months, P less than .005 for each).
The impact of overall tumor burden and the size of the primary tumor were not considered in this analysis but will be in subsequent analyses, according to Dr. Heng.
He disclosed that he is a consultant/adviser to Aveo, Bayer, Bristol-Myers Squibb, Novartis, and Pfizer.
SAN FRANCISCO – Only selected patients with synchronous metastases from renal cell carcinoma derive a survival benefit from a cytoreductive nephrectomy, a retrospective cohort study showed.
Researchers studied more than 1,600 patients from the International mRCC (metastatic renal cell carcinoma) Database Consortium who developed metastases while their primary tumor was still in place.
The main analyses showed that as a whole, patients who underwent cytoreductive nephrectomy lived longer than their counterparts who did not have this surgery.
However, in stratified analyses, the surgery significantly prolonged survival only among patients who had a life expectancy exceeding 12 months and patients who had three or fewer risk factors by International mRCC Database Consortium (IMDC) criteria.
Risk factors include Karnofsky performance status less than 80%, an interval between diagnosis and treatment of less than 1 year, anemia, thrombocytosis, neutrophilia, and hypercalcemia
"Overall, I think we can say that cytoreductive nephrectomy may be beneficial in the age of targeted therapy. However, not all patients should have it," commented first author Dr. Daniel Y.C. Heng of the University of Calgary (Alta.).
"Patients with four or more IMDC risk factors and patients with limited life expectancy probably shouldn’t have a cytoreductive nephrectomy," he said. "Of course, there are probably exceptions to that rule; if patients are really symptomatic in the primary, for example, or have bleeding, maybe there are exceptions.
"But I think this is a very interesting and useful way to help select our patients for cytoreductive nephrectomy. Certainly, we are awaiting the CARMENA and SURTIME phase III randomized controlled trials to prospectively evaluate this, and hopefully, we will be able to look at the IMDC risk factors to see if they can aid in patient selection in a prospectively validated way," Dr. Heng said at the 2014 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology.
Cytoreductive nephrectomy carries risks of morbidity and mortality, and some patients may be unable to go on to receive systemic therapy, noted invited discussant Dr. Daniel Canter of the Fox Chase Cancer Center, Philadelphia.
"What I take home as the most important point of this talk is that we need to make better choices and risk stratify these patients before surgery," he said.
A key question arising from the new data is whether the IMDC risk groups should be redefined, according to Dr. Canter; for example, patients with three risk factors are currently placed in the poor risk group, yet they derived significant benefit from surgery in this study. "So is there room for further refinement in this model?" he asked.
"With such a large cohort, I think that adding to this data with complication rates and perioperative mortality would certainly be important in terms of future treatment decision making," he commented.
Session attendee Dr. Adam Metwalli of the National Cancer Institute asked, "Are there specific risk factors that seem to disproportionately cluster among those who have four or five that would help to distinguish them from the ones who have zero to three?"
"By far, the most important prognostic factor is Karnofsky performance status, and that makes sense," Dr. Heng replied. "It’s partially the ‘look test’ and how you think a patient will fare. So, that by far has the biggest contribution."
Introducing the study, he noted that cytoreductive nephrectomy has previously been found to prolong survival by about 6 months in patients with metastatic renal cell carcinoma treated in the immunotherapy era.
"But of course now we are in the age of targeted therapy, with VEGF [vascular endothelial growth factor] inhibitors and mTOR [mammalian target of rapamycin] inhibitors. These are drugs that are much more active than in the era of old immunotherapy. So it begs the question, is cytoreductive nephrectomy still relevant?" Dr. Heng said.
The researchers studied 1,658 patients from 20 institutions who had mRCC with synchronous metastases, of whom 59% underwent cytoreductive nephrectomy.
They had a median age of 59 years, and about half were in the IMDC intermediate-risk group. The most common prior targeted therapy was sunitinib (Sutent), received by about three-fourths, reported Dr. Heng.
In unadjusted analyses, median overall survival was a significant 11 months longer for patients who underwent cytoreductive nephrectomy compared with their counterparts who did not (20.6 vs. 9.5 months). After adjustment for IMDC risk factors, the surgery was associated with a 40% reduction in the risk of death in the entire cohort (hazard ratio, 0.60; P less than .0001).
However, in analyses stratified by patient life expectancy, there was a significant survival benefit of cytoreductive nephrectomy only among patients expected to live 12-18 months (3.3-month incremental benefit; adjusted hazard ratio, 0.85; P = .049) or 18-24 months (5.2-month incremental benefit; adjusted hazard ratio, 0.72; P less than .0001).
Similarly, in analyses stratified by the number of risk factors present out of the six IMDC risk factors, there was a significant survival benefit of cytoreductive nephrectomy only among patients with three or fewer factors (incremental benefit of 6-10 months, P less than .005 for each).
The impact of overall tumor burden and the size of the primary tumor were not considered in this analysis but will be in subsequent analyses, according to Dr. Heng.
He disclosed that he is a consultant/adviser to Aveo, Bayer, Bristol-Myers Squibb, Novartis, and Pfizer.
AT THE GENITOURINARY CANCERS SYMPOSIUM
Major Finding: Cytoreductive nephrectomy significantly prolonged adjusted survival only among patients with a life expectancy exceeding 12 months and patients having three or fewer IMDC risk factors.
Data Source: A retrospective cohort study of 1,658 patients with synchronous metastases from renal cell carcinoma.
Disclosures: Dr. Heng disclosed that he is a consultant/adviser to Aveo, Bayer, Bristol-Myers Squibb, Novartis, and Pfizer.