User login
Keeping laparoscopy safe for the obese patient
As I was writing my introduction to this current edition of the Master Class in Gynecologic Surgery, focusing on minimally invasive surgery for the obese female patient, I was listening to Chuck Todd, host of “Meet the Press.” Instantaneously, television and my thoughts became one; in the last segment of the program, Mr. Todd discussed what he was able to consume for $50 at the Iowa State Fair. I learned that his diet that day consisted of a pork chop on a stick, mac and cheese, a bacon-wrapped corn dog, cheese on a stick with jalapeños, a deep-fried Twinkie, and even fried apple pie with bacon. While Mr. Todd is thin and healthy, the array of foods at the fair reflects our nation’s penchant toward fast food that is fat laden and fried. Though our county is not alone in the world, obesity has reached epidemic proportion in the United States.
According to a May 2015 Department of Health & Human Services report on the health status of the nation, 69% of adults in the United States are overweight and 35% are obese. As a result, the minimally invasive gynecologic surgeon is dealing with an increasing population of women with comorbidities related to their obesity that can confound surgery outcomes. Moreover, anatomic landmarks that the young medical student learns in his or her first anatomy classes are modified due to the size of panniculus and the migration of the umbilicus relative to the bifurcation of the aorta.
I asked Dr. Amina Ahmed to join me in discussing the management of the obese patient undergoing minimally invasive gynecologic surgery. After completing her fellowship in gynecologic oncology, Dr. Ahmed has been on staff at both the University of Iowa Hospitals and Clinics, Iowa City, and Advocate Lutheran General Hospital, Park Ridge, Ill. She will soon join the gynecologic oncology faculty at Rush University Medical Center, Chicago. Given the increased rate of obesity in both Chicago and Iowa, Dr. Ahmed has become an expert in this area in a short period of time.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant and on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien.
As I was writing my introduction to this current edition of the Master Class in Gynecologic Surgery, focusing on minimally invasive surgery for the obese female patient, I was listening to Chuck Todd, host of “Meet the Press.” Instantaneously, television and my thoughts became one; in the last segment of the program, Mr. Todd discussed what he was able to consume for $50 at the Iowa State Fair. I learned that his diet that day consisted of a pork chop on a stick, mac and cheese, a bacon-wrapped corn dog, cheese on a stick with jalapeños, a deep-fried Twinkie, and even fried apple pie with bacon. While Mr. Todd is thin and healthy, the array of foods at the fair reflects our nation’s penchant toward fast food that is fat laden and fried. Though our county is not alone in the world, obesity has reached epidemic proportion in the United States.
According to a May 2015 Department of Health & Human Services report on the health status of the nation, 69% of adults in the United States are overweight and 35% are obese. As a result, the minimally invasive gynecologic surgeon is dealing with an increasing population of women with comorbidities related to their obesity that can confound surgery outcomes. Moreover, anatomic landmarks that the young medical student learns in his or her first anatomy classes are modified due to the size of panniculus and the migration of the umbilicus relative to the bifurcation of the aorta.
I asked Dr. Amina Ahmed to join me in discussing the management of the obese patient undergoing minimally invasive gynecologic surgery. After completing her fellowship in gynecologic oncology, Dr. Ahmed has been on staff at both the University of Iowa Hospitals and Clinics, Iowa City, and Advocate Lutheran General Hospital, Park Ridge, Ill. She will soon join the gynecologic oncology faculty at Rush University Medical Center, Chicago. Given the increased rate of obesity in both Chicago and Iowa, Dr. Ahmed has become an expert in this area in a short period of time.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant and on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien.
As I was writing my introduction to this current edition of the Master Class in Gynecologic Surgery, focusing on minimally invasive surgery for the obese female patient, I was listening to Chuck Todd, host of “Meet the Press.” Instantaneously, television and my thoughts became one; in the last segment of the program, Mr. Todd discussed what he was able to consume for $50 at the Iowa State Fair. I learned that his diet that day consisted of a pork chop on a stick, mac and cheese, a bacon-wrapped corn dog, cheese on a stick with jalapeños, a deep-fried Twinkie, and even fried apple pie with bacon. While Mr. Todd is thin and healthy, the array of foods at the fair reflects our nation’s penchant toward fast food that is fat laden and fried. Though our county is not alone in the world, obesity has reached epidemic proportion in the United States.
According to a May 2015 Department of Health & Human Services report on the health status of the nation, 69% of adults in the United States are overweight and 35% are obese. As a result, the minimally invasive gynecologic surgeon is dealing with an increasing population of women with comorbidities related to their obesity that can confound surgery outcomes. Moreover, anatomic landmarks that the young medical student learns in his or her first anatomy classes are modified due to the size of panniculus and the migration of the umbilicus relative to the bifurcation of the aorta.
I asked Dr. Amina Ahmed to join me in discussing the management of the obese patient undergoing minimally invasive gynecologic surgery. After completing her fellowship in gynecologic oncology, Dr. Ahmed has been on staff at both the University of Iowa Hospitals and Clinics, Iowa City, and Advocate Lutheran General Hospital, Park Ridge, Ill. She will soon join the gynecologic oncology faculty at Rush University Medical Center, Chicago. Given the increased rate of obesity in both Chicago and Iowa, Dr. Ahmed has become an expert in this area in a short period of time.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant and on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien.
Positioning obese patients for minimally invasive gynecologic surgery
The current epidemic of obesity presents gynecologic surgeons with the challenge of safely and successfully performing minimally invasive surgery in women who are morbidly or superobese.
In 2004, the prevalence of a body mass index greater than 40 kg/m2 was almost 7.0% in females in the United States (JAMA. 2006 Apr 5;295[13]:1549-55.). Most recently, 8.3% of women were reported to have a BMI greater than 40 (JAMA. 2014 Feb 26;311[8]:806-14.). This is a value that the World Health Organization defines as Class III obesity and that, according to further stratification reported in the surgical literature, includes the categories of morbid obesity (40-44.9), superobesity (greater than 45), and super-superobesity (greater than 60).
As a gynecologic oncologist, I see firsthand the impact of obesity on the risk of multiple gynecologic conditions and female cancers, including endometrial cancer, as well as the benefits of a minimally invasive approach. I frequently perform hysterectomies via the minimally invasive approach to treat precancer and cancer of the uterus in morbidly and superobese women who have significant central adiposity.
MIGS benefits in the obese
In the past 15 years, and particularly in the past decade, evidence that obese patients benefit from laparoscopic surgery compared with traditional laparotomy has increased. I consider minimally invasive surgery the standard of care for women with endometrial cancer, regardless of the BMI.
As Dr. Stacey A. Scheib and her colleagues wrote in a recent review on laparoscopy in the morbidly obese, most of the gynecologic literature comparing laparoscopic surgery with laparotomy in this population is focused on gynecologic oncology because obesity is so strongly associated with endometrial and other cancers in women (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:182-95.). In one prospective study of women with clinical stage I endometrial cancer and BMIs between 28 and 60, those who underwent laparoscopic surgery – 40 of 42 women over 2 years – had significantly longer operative times but less operative morbidity, shorter hospital stays, faster recovery and better postsurgical quality of life, compared with women who had undergone laparotomy in the previous 2 years. The control patients also had clinical stage I endometrial cancer and similar BMIs (Gynecol Oncol. 2000 Sep;78[3 Pt 1]:329-35.).
Research comparing robotics and conventional laparoscopy in obese gynecologic surgery patients is limited, and findings are inconsistent. It will remain difficult to compare the two approaches because few surgeons are equally skilled in both approaches and because the learning curve for conventional laparoscopy is so much steeper than for robotics.
I favor the robotic approach for morbidly and superobese patients for its superior visualization and ergonomics.
Patient positioning
It is important to use an operative bed that will accommodate the weight and width of obese patients and enable Trendelenburg positioning of up to 45 degrees. We use a bariatric bed with a 1,000-pound weight limit.
Obese patients are at greater risk for neuromuscular injuries and pressure sores, so careful patient positioning and padding of pressure points is critically important. We have found a surgical bean bag to be much more effective in preventing slippage for the morbidly or superobese patient than is egg-crate foam. The bean bag conforms nicely to the shape of the patient’s back, neck, and arms when it is appropriately desufflated. After desufflation, the bean bag must be well taped onto the operative bed.
I sometimes use shoulder blocks for extra assurance. When used, these braces must be attached to the bean bag and not to the patient.
We typically pad the arms completely with gel pads or foam before the bean bag is desufflated. We also often pad the knees and calves before the legs are placed and secured in stirrups made for the morbidly obese, with the buttocks slightly off the table.
In a review of literature on obesity and laparoscopy outcomes, Dr. Georgine Lamvu and her associates recommended that the arms be tucked in the “military” position, along the length of the body (Am J Obstet Gynecol. 2004 Aug;191[2]:669-74.). To ensure that both arms are properly tucked against the length of the body, we use bed extenders or sleds to widen the bed as necessary.
Abdominal access
I use the open Hasson technique in my obese patients and enter the peritoneum under direct visualization. In patients with high levels of morbid obesity, I have found it helpful to retract the adipose tissue using thin Breisky vaginal retractors. These retractors can hold the adipose tissue away from the fascia to facilitate entry into the abdominal cavity via the open technique.
Utilizing the umbilicus as the initial entry point – often desirable in minimally invasive surgery – is frequently not possible in morbidly obese patients because as BMI increases, the umbilicus migrates toward the pubic bone and away from the aortic bifurcation. In patients who were overweight (BMI greater than 25), Dr. W.W. Hurd and his associates noted a repositioning of the umbilicus below the aortic bifurcation of 2 cm or greater (Obstet Gynecol. 1992 Jul;80[1]:48-51.).
Instead, a supraumbilical or left upper quadrant site for initial entry enables optimal triangulation of trocars and visualization of disease. The trocars must then be placed more lateral and cephalad than in thinner women. In doing so, risk to the inferior epigastric is mitigated. Moreover, longer trocar lengths (150 mm) may be required.
To utilize an umbilical entry, it is imperative that the panniculus be placed cephalad to a position between the two anterior iliac spines (Obstet Gynecol. 1998 Nov;92[5]:869-72.). By doing this, the umbilicus is now repositioned relative to the bifurcation of the aorta similar to the thinner patient. This can either be accomplished using assistants to move the panniculus cephalad or taping the panniculus.
Alternatively, if the Hasson technique is not utilized, a Veress needle (50 mm in length) may be used. Based on MRI and CT visualization, Dr. Hurd has long recommended using a 90-degree angle in the obese population, compared with a 45-degree angle in nonobese women (J Reprod Med. 1991;36[7]:473-6.).
I usually place the patient into a moderate Trendelenburg position before docking the robot and observe the patient’s cardiac and respiratory responses to the induction of anesthesia. Adjustments in the degree of Trendelenburg positioning, the insufflation pressure level, and the ventilation settings can then be made if necessary. Occasionally I will decrease the insufflation pressure from 15 to 12 mm Hg, for instance, to accommodate ventilation needs.
A note from Dr. Charles E. Miller, Master Class Medical Editor
It must be recognized that not all physicians agree with the use of shoulder braces. In a review of literature on brachial plexus injuries in gynecologic surgery during 1980-2012, Dr. Nigel Pereira and his associates identified eight case reports, all of which involved Trendelenburg positioning and seven of which utilized shoulder braces. In their evaluation of the literature, the authors concluded that “the force of the shoulder braces on the clavicle and scapula opposes the force of gravity on the humerus, thereby stretching the brachial plexus and leading to nerve injury. This is particularly exaggerated when the arm is hyperabducted (less than 90 degrees), the head is laterally flexed to the opposite side, or the abducted arm is sagging.”
The authors also point out that longer times spent under general anesthesia (commensurate with increased operating times) increase the risk of brachial plexus injury “by increasing joint mobility (particularly when muscle relaxants are used) because the neighboring bony structure is more likely to compress or impinge on the brachial plexus” (CRSLS e2014.00077. [doi:10.4293/CRSLS.2014.00077]).
More pearls from Dr. Miller
Preoperative care. Prior to surgery it is important to examine a patient’s panniculus closely for evidence of infection. As the area underneath the panniculus receives little oxygen, it is at greater risk for both bacterial and fungal infections. If infection is noted, treatment prior to surgery is strongly recommended. Moreover, as the skin under the panniculus is often times “broken down,” which can compromise healing, lateral incisions should not be made in this area.
Since obese women have more severe comorbidities (such as metabolic syndrome, obstructed sleep apnea, coronary artery disease, poorly controlled hypertension, and a difficult airway) and a greater risk of perioperative complications than women who are not obese, they generally require a more-extensive preoperative work-up and additional perioperative considerations. If the minimally invasive gynecologic surgeon is uncomfortable with evaluation of cardiac and pulmonary status, medical clearance and perioperative consultation with an anesthesiologist prior to surgery is strongly recommended.
Perioperative care. There are no studies in the literature supporting the use of antibiotic prophylaxis prior to surgery despite the increased risk of postoperative wound infection in morbidly obese patients. Increased risk of surgical site infection post abdominal hysterectomy has been noted in women with a BMI greater than 35. Therefore, consideration should be given to the use of prophylactic antibiotics. For patients weighing more than 80 kg, I advise using 2 gm prophylactic cefazolin; increase this to 3 gm in patients that weigh more than 120 kg.
The morbidly obese patient is also at greater risk of deep venous thrombosis, especially when the procedure is lengthy. Sequential compression devices are essential. Moreover, use of such antithrombotic agents as Lovenox [enoxaparin] and heparin should be considered until the patient is ambulating.
Postoperative care. It is imperative to stress the need for extensive pulmonary toilet or hygiene (i.e., coughing and breathing deeply to clear mucus and secretions from the airways) as well as early ambulation. The patient should also be counseled to use pain medication judiciously. And until the patient is mobile, the use of antithrombotic agents, such as Lovenox and heparin, should be continued.
Dr. Ahmed reports that she has no disclosures related to this Master Class. Dr. Miller disclosed that he is a consultant and is on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien. Email Dr. Ahmed and Dr. Miller at obnews@frontlinemedcom.com.
The current epidemic of obesity presents gynecologic surgeons with the challenge of safely and successfully performing minimally invasive surgery in women who are morbidly or superobese.
In 2004, the prevalence of a body mass index greater than 40 kg/m2 was almost 7.0% in females in the United States (JAMA. 2006 Apr 5;295[13]:1549-55.). Most recently, 8.3% of women were reported to have a BMI greater than 40 (JAMA. 2014 Feb 26;311[8]:806-14.). This is a value that the World Health Organization defines as Class III obesity and that, according to further stratification reported in the surgical literature, includes the categories of morbid obesity (40-44.9), superobesity (greater than 45), and super-superobesity (greater than 60).
As a gynecologic oncologist, I see firsthand the impact of obesity on the risk of multiple gynecologic conditions and female cancers, including endometrial cancer, as well as the benefits of a minimally invasive approach. I frequently perform hysterectomies via the minimally invasive approach to treat precancer and cancer of the uterus in morbidly and superobese women who have significant central adiposity.
MIGS benefits in the obese
In the past 15 years, and particularly in the past decade, evidence that obese patients benefit from laparoscopic surgery compared with traditional laparotomy has increased. I consider minimally invasive surgery the standard of care for women with endometrial cancer, regardless of the BMI.
As Dr. Stacey A. Scheib and her colleagues wrote in a recent review on laparoscopy in the morbidly obese, most of the gynecologic literature comparing laparoscopic surgery with laparotomy in this population is focused on gynecologic oncology because obesity is so strongly associated with endometrial and other cancers in women (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:182-95.). In one prospective study of women with clinical stage I endometrial cancer and BMIs between 28 and 60, those who underwent laparoscopic surgery – 40 of 42 women over 2 years – had significantly longer operative times but less operative morbidity, shorter hospital stays, faster recovery and better postsurgical quality of life, compared with women who had undergone laparotomy in the previous 2 years. The control patients also had clinical stage I endometrial cancer and similar BMIs (Gynecol Oncol. 2000 Sep;78[3 Pt 1]:329-35.).
Research comparing robotics and conventional laparoscopy in obese gynecologic surgery patients is limited, and findings are inconsistent. It will remain difficult to compare the two approaches because few surgeons are equally skilled in both approaches and because the learning curve for conventional laparoscopy is so much steeper than for robotics.
I favor the robotic approach for morbidly and superobese patients for its superior visualization and ergonomics.
Patient positioning
It is important to use an operative bed that will accommodate the weight and width of obese patients and enable Trendelenburg positioning of up to 45 degrees. We use a bariatric bed with a 1,000-pound weight limit.
Obese patients are at greater risk for neuromuscular injuries and pressure sores, so careful patient positioning and padding of pressure points is critically important. We have found a surgical bean bag to be much more effective in preventing slippage for the morbidly or superobese patient than is egg-crate foam. The bean bag conforms nicely to the shape of the patient’s back, neck, and arms when it is appropriately desufflated. After desufflation, the bean bag must be well taped onto the operative bed.
I sometimes use shoulder blocks for extra assurance. When used, these braces must be attached to the bean bag and not to the patient.
We typically pad the arms completely with gel pads or foam before the bean bag is desufflated. We also often pad the knees and calves before the legs are placed and secured in stirrups made for the morbidly obese, with the buttocks slightly off the table.
In a review of literature on obesity and laparoscopy outcomes, Dr. Georgine Lamvu and her associates recommended that the arms be tucked in the “military” position, along the length of the body (Am J Obstet Gynecol. 2004 Aug;191[2]:669-74.). To ensure that both arms are properly tucked against the length of the body, we use bed extenders or sleds to widen the bed as necessary.
Abdominal access
I use the open Hasson technique in my obese patients and enter the peritoneum under direct visualization. In patients with high levels of morbid obesity, I have found it helpful to retract the adipose tissue using thin Breisky vaginal retractors. These retractors can hold the adipose tissue away from the fascia to facilitate entry into the abdominal cavity via the open technique.
Utilizing the umbilicus as the initial entry point – often desirable in minimally invasive surgery – is frequently not possible in morbidly obese patients because as BMI increases, the umbilicus migrates toward the pubic bone and away from the aortic bifurcation. In patients who were overweight (BMI greater than 25), Dr. W.W. Hurd and his associates noted a repositioning of the umbilicus below the aortic bifurcation of 2 cm or greater (Obstet Gynecol. 1992 Jul;80[1]:48-51.).
Instead, a supraumbilical or left upper quadrant site for initial entry enables optimal triangulation of trocars and visualization of disease. The trocars must then be placed more lateral and cephalad than in thinner women. In doing so, risk to the inferior epigastric is mitigated. Moreover, longer trocar lengths (150 mm) may be required.
To utilize an umbilical entry, it is imperative that the panniculus be placed cephalad to a position between the two anterior iliac spines (Obstet Gynecol. 1998 Nov;92[5]:869-72.). By doing this, the umbilicus is now repositioned relative to the bifurcation of the aorta similar to the thinner patient. This can either be accomplished using assistants to move the panniculus cephalad or taping the panniculus.
Alternatively, if the Hasson technique is not utilized, a Veress needle (50 mm in length) may be used. Based on MRI and CT visualization, Dr. Hurd has long recommended using a 90-degree angle in the obese population, compared with a 45-degree angle in nonobese women (J Reprod Med. 1991;36[7]:473-6.).
I usually place the patient into a moderate Trendelenburg position before docking the robot and observe the patient’s cardiac and respiratory responses to the induction of anesthesia. Adjustments in the degree of Trendelenburg positioning, the insufflation pressure level, and the ventilation settings can then be made if necessary. Occasionally I will decrease the insufflation pressure from 15 to 12 mm Hg, for instance, to accommodate ventilation needs.
A note from Dr. Charles E. Miller, Master Class Medical Editor
It must be recognized that not all physicians agree with the use of shoulder braces. In a review of literature on brachial plexus injuries in gynecologic surgery during 1980-2012, Dr. Nigel Pereira and his associates identified eight case reports, all of which involved Trendelenburg positioning and seven of which utilized shoulder braces. In their evaluation of the literature, the authors concluded that “the force of the shoulder braces on the clavicle and scapula opposes the force of gravity on the humerus, thereby stretching the brachial plexus and leading to nerve injury. This is particularly exaggerated when the arm is hyperabducted (less than 90 degrees), the head is laterally flexed to the opposite side, or the abducted arm is sagging.”
The authors also point out that longer times spent under general anesthesia (commensurate with increased operating times) increase the risk of brachial plexus injury “by increasing joint mobility (particularly when muscle relaxants are used) because the neighboring bony structure is more likely to compress or impinge on the brachial plexus” (CRSLS e2014.00077. [doi:10.4293/CRSLS.2014.00077]).
More pearls from Dr. Miller
Preoperative care. Prior to surgery it is important to examine a patient’s panniculus closely for evidence of infection. As the area underneath the panniculus receives little oxygen, it is at greater risk for both bacterial and fungal infections. If infection is noted, treatment prior to surgery is strongly recommended. Moreover, as the skin under the panniculus is often times “broken down,” which can compromise healing, lateral incisions should not be made in this area.
Since obese women have more severe comorbidities (such as metabolic syndrome, obstructed sleep apnea, coronary artery disease, poorly controlled hypertension, and a difficult airway) and a greater risk of perioperative complications than women who are not obese, they generally require a more-extensive preoperative work-up and additional perioperative considerations. If the minimally invasive gynecologic surgeon is uncomfortable with evaluation of cardiac and pulmonary status, medical clearance and perioperative consultation with an anesthesiologist prior to surgery is strongly recommended.
Perioperative care. There are no studies in the literature supporting the use of antibiotic prophylaxis prior to surgery despite the increased risk of postoperative wound infection in morbidly obese patients. Increased risk of surgical site infection post abdominal hysterectomy has been noted in women with a BMI greater than 35. Therefore, consideration should be given to the use of prophylactic antibiotics. For patients weighing more than 80 kg, I advise using 2 gm prophylactic cefazolin; increase this to 3 gm in patients that weigh more than 120 kg.
The morbidly obese patient is also at greater risk of deep venous thrombosis, especially when the procedure is lengthy. Sequential compression devices are essential. Moreover, use of such antithrombotic agents as Lovenox [enoxaparin] and heparin should be considered until the patient is ambulating.
Postoperative care. It is imperative to stress the need for extensive pulmonary toilet or hygiene (i.e., coughing and breathing deeply to clear mucus and secretions from the airways) as well as early ambulation. The patient should also be counseled to use pain medication judiciously. And until the patient is mobile, the use of antithrombotic agents, such as Lovenox and heparin, should be continued.
Dr. Ahmed reports that she has no disclosures related to this Master Class. Dr. Miller disclosed that he is a consultant and is on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien. Email Dr. Ahmed and Dr. Miller at obnews@frontlinemedcom.com.
The current epidemic of obesity presents gynecologic surgeons with the challenge of safely and successfully performing minimally invasive surgery in women who are morbidly or superobese.
In 2004, the prevalence of a body mass index greater than 40 kg/m2 was almost 7.0% in females in the United States (JAMA. 2006 Apr 5;295[13]:1549-55.). Most recently, 8.3% of women were reported to have a BMI greater than 40 (JAMA. 2014 Feb 26;311[8]:806-14.). This is a value that the World Health Organization defines as Class III obesity and that, according to further stratification reported in the surgical literature, includes the categories of morbid obesity (40-44.9), superobesity (greater than 45), and super-superobesity (greater than 60).
As a gynecologic oncologist, I see firsthand the impact of obesity on the risk of multiple gynecologic conditions and female cancers, including endometrial cancer, as well as the benefits of a minimally invasive approach. I frequently perform hysterectomies via the minimally invasive approach to treat precancer and cancer of the uterus in morbidly and superobese women who have significant central adiposity.
MIGS benefits in the obese
In the past 15 years, and particularly in the past decade, evidence that obese patients benefit from laparoscopic surgery compared with traditional laparotomy has increased. I consider minimally invasive surgery the standard of care for women with endometrial cancer, regardless of the BMI.
As Dr. Stacey A. Scheib and her colleagues wrote in a recent review on laparoscopy in the morbidly obese, most of the gynecologic literature comparing laparoscopic surgery with laparotomy in this population is focused on gynecologic oncology because obesity is so strongly associated with endometrial and other cancers in women (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:182-95.). In one prospective study of women with clinical stage I endometrial cancer and BMIs between 28 and 60, those who underwent laparoscopic surgery – 40 of 42 women over 2 years – had significantly longer operative times but less operative morbidity, shorter hospital stays, faster recovery and better postsurgical quality of life, compared with women who had undergone laparotomy in the previous 2 years. The control patients also had clinical stage I endometrial cancer and similar BMIs (Gynecol Oncol. 2000 Sep;78[3 Pt 1]:329-35.).
Research comparing robotics and conventional laparoscopy in obese gynecologic surgery patients is limited, and findings are inconsistent. It will remain difficult to compare the two approaches because few surgeons are equally skilled in both approaches and because the learning curve for conventional laparoscopy is so much steeper than for robotics.
I favor the robotic approach for morbidly and superobese patients for its superior visualization and ergonomics.
Patient positioning
It is important to use an operative bed that will accommodate the weight and width of obese patients and enable Trendelenburg positioning of up to 45 degrees. We use a bariatric bed with a 1,000-pound weight limit.
Obese patients are at greater risk for neuromuscular injuries and pressure sores, so careful patient positioning and padding of pressure points is critically important. We have found a surgical bean bag to be much more effective in preventing slippage for the morbidly or superobese patient than is egg-crate foam. The bean bag conforms nicely to the shape of the patient’s back, neck, and arms when it is appropriately desufflated. After desufflation, the bean bag must be well taped onto the operative bed.
I sometimes use shoulder blocks for extra assurance. When used, these braces must be attached to the bean bag and not to the patient.
We typically pad the arms completely with gel pads or foam before the bean bag is desufflated. We also often pad the knees and calves before the legs are placed and secured in stirrups made for the morbidly obese, with the buttocks slightly off the table.
In a review of literature on obesity and laparoscopy outcomes, Dr. Georgine Lamvu and her associates recommended that the arms be tucked in the “military” position, along the length of the body (Am J Obstet Gynecol. 2004 Aug;191[2]:669-74.). To ensure that both arms are properly tucked against the length of the body, we use bed extenders or sleds to widen the bed as necessary.
Abdominal access
I use the open Hasson technique in my obese patients and enter the peritoneum under direct visualization. In patients with high levels of morbid obesity, I have found it helpful to retract the adipose tissue using thin Breisky vaginal retractors. These retractors can hold the adipose tissue away from the fascia to facilitate entry into the abdominal cavity via the open technique.
Utilizing the umbilicus as the initial entry point – often desirable in minimally invasive surgery – is frequently not possible in morbidly obese patients because as BMI increases, the umbilicus migrates toward the pubic bone and away from the aortic bifurcation. In patients who were overweight (BMI greater than 25), Dr. W.W. Hurd and his associates noted a repositioning of the umbilicus below the aortic bifurcation of 2 cm or greater (Obstet Gynecol. 1992 Jul;80[1]:48-51.).
Instead, a supraumbilical or left upper quadrant site for initial entry enables optimal triangulation of trocars and visualization of disease. The trocars must then be placed more lateral and cephalad than in thinner women. In doing so, risk to the inferior epigastric is mitigated. Moreover, longer trocar lengths (150 mm) may be required.
To utilize an umbilical entry, it is imperative that the panniculus be placed cephalad to a position between the two anterior iliac spines (Obstet Gynecol. 1998 Nov;92[5]:869-72.). By doing this, the umbilicus is now repositioned relative to the bifurcation of the aorta similar to the thinner patient. This can either be accomplished using assistants to move the panniculus cephalad or taping the panniculus.
Alternatively, if the Hasson technique is not utilized, a Veress needle (50 mm in length) may be used. Based on MRI and CT visualization, Dr. Hurd has long recommended using a 90-degree angle in the obese population, compared with a 45-degree angle in nonobese women (J Reprod Med. 1991;36[7]:473-6.).
I usually place the patient into a moderate Trendelenburg position before docking the robot and observe the patient’s cardiac and respiratory responses to the induction of anesthesia. Adjustments in the degree of Trendelenburg positioning, the insufflation pressure level, and the ventilation settings can then be made if necessary. Occasionally I will decrease the insufflation pressure from 15 to 12 mm Hg, for instance, to accommodate ventilation needs.
A note from Dr. Charles E. Miller, Master Class Medical Editor
It must be recognized that not all physicians agree with the use of shoulder braces. In a review of literature on brachial plexus injuries in gynecologic surgery during 1980-2012, Dr. Nigel Pereira and his associates identified eight case reports, all of which involved Trendelenburg positioning and seven of which utilized shoulder braces. In their evaluation of the literature, the authors concluded that “the force of the shoulder braces on the clavicle and scapula opposes the force of gravity on the humerus, thereby stretching the brachial plexus and leading to nerve injury. This is particularly exaggerated when the arm is hyperabducted (less than 90 degrees), the head is laterally flexed to the opposite side, or the abducted arm is sagging.”
The authors also point out that longer times spent under general anesthesia (commensurate with increased operating times) increase the risk of brachial plexus injury “by increasing joint mobility (particularly when muscle relaxants are used) because the neighboring bony structure is more likely to compress or impinge on the brachial plexus” (CRSLS e2014.00077. [doi:10.4293/CRSLS.2014.00077]).
More pearls from Dr. Miller
Preoperative care. Prior to surgery it is important to examine a patient’s panniculus closely for evidence of infection. As the area underneath the panniculus receives little oxygen, it is at greater risk for both bacterial and fungal infections. If infection is noted, treatment prior to surgery is strongly recommended. Moreover, as the skin under the panniculus is often times “broken down,” which can compromise healing, lateral incisions should not be made in this area.
Since obese women have more severe comorbidities (such as metabolic syndrome, obstructed sleep apnea, coronary artery disease, poorly controlled hypertension, and a difficult airway) and a greater risk of perioperative complications than women who are not obese, they generally require a more-extensive preoperative work-up and additional perioperative considerations. If the minimally invasive gynecologic surgeon is uncomfortable with evaluation of cardiac and pulmonary status, medical clearance and perioperative consultation with an anesthesiologist prior to surgery is strongly recommended.
Perioperative care. There are no studies in the literature supporting the use of antibiotic prophylaxis prior to surgery despite the increased risk of postoperative wound infection in morbidly obese patients. Increased risk of surgical site infection post abdominal hysterectomy has been noted in women with a BMI greater than 35. Therefore, consideration should be given to the use of prophylactic antibiotics. For patients weighing more than 80 kg, I advise using 2 gm prophylactic cefazolin; increase this to 3 gm in patients that weigh more than 120 kg.
The morbidly obese patient is also at greater risk of deep venous thrombosis, especially when the procedure is lengthy. Sequential compression devices are essential. Moreover, use of such antithrombotic agents as Lovenox [enoxaparin] and heparin should be considered until the patient is ambulating.
Postoperative care. It is imperative to stress the need for extensive pulmonary toilet or hygiene (i.e., coughing and breathing deeply to clear mucus and secretions from the airways) as well as early ambulation. The patient should also be counseled to use pain medication judiciously. And until the patient is mobile, the use of antithrombotic agents, such as Lovenox and heparin, should be continued.
Dr. Ahmed reports that she has no disclosures related to this Master Class. Dr. Miller disclosed that he is a consultant and is on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien. Email Dr. Ahmed and Dr. Miller at obnews@frontlinemedcom.com.
Cervical cancer screening guidelines slow to gain traction
More than 3 years after the release of new cervical cancer screening guidelines, patients remain largely unaware of substantial changes in screening, and many physicians remain confused about or resistant to those changes.
The confusion is a matter of education, said Dr. Owen Montgomery, chairman of the department of obstetrics and gynecology at Drexel University, Philadelphia. As for the resistance, that’s less a reflection of concern about the science and more about the emotional and sociological processes involved in change, he said.
“It’s really good science,” Dr. Montgomery said of the data that formed the basis for the guidelines. “I was on the executive board of the [American College of Obstetricians and Gynecologists] when we signed off on the new recommendations. The science is valid.”
Two sets of guidelines were released in 2012, one by the U.S. Preventive Services Task Force (USPSTF) and one by the American Cancer Society (ACS) in conjunction with the American Society for Clinical Pathology (ASCP) and the American Society for Colposcopy and Cervical Pathology (ASCCP). Although the two guidelines differ in some respects, both recommend against a longstanding tradition: routine yearly Pap testing. The guidelines now call for screening intervals of 3-5 years depending on patient age and other factors, and recommend against screening those under age 21 years and over age 65 years in the absence of risk factors.
Knowledge gaps
Despite the widespread organizational support for the guidelines, data suggest that knowledge of them may be lacking among both patients and physicians. For instance, about 85% of 249 adult women who participated in a recent survey answered incorrectly when asked how often low-risk women aged 21-29 should be screened, and nearly 95% answered incorrectly when asked about the recommended frequency of screening in low-risk women over age 30 years.
According to Dr. Katherine O’Flynn O’Brien and her colleagues at George Washington University, Washington, who presented the results earlier this year at the American College of Obstetricians and Gynecologists annual scientific meeting, the findings suggest “that providers should focus more on educating women about changes to screening practices.”
But another survey presented at the same ACOG meeting showed that many providers may be unaware of the guideline changes or hesitant about embracing those changes.
Using a convenience sample of 165 medical and osteopathic physicians, physician assistants, and nurse practitioners, the investigators found that, in contrast with the current guidelines, about half do not perform cotesting in women aged 30-65 years every 5 years, and nearly 57% do not screen women aged 21-29 years with cytology alone every 3 years. More than 40% reported that they do not perform cytology screening in women aged 30-65 years every 3 years.
Addressing the ‘what ifs’
Confusion about the guidelines, and hesitation about implementing them, is not surprising as the changes – particularly the 3- to 5-year screening interval changes – are some of the most dramatic in recent memory, according to Dr. Constance Bohon, an ob.gyn. in Washington, D.C., and assistant clinical professor of ob.gyn. at George Washington University.
Certainly the guidelines have evolved with the science over the years, and some changes, such as screening only those over age 21 years, have been embraced, she said.
“But some changes are easier to accept than others,” she added, noting that “doing a Pap with cotesting in women after age 30 and repeating it again in 5 years is very difficult for many clinicians to accept ... part of the issue is that fine line in medicine between using the data and relying on clinical sense, clinical expertise.”
There is concern about the exceptions and the “what ifs,” she said.
What if a patient has a new partner? What if she develops an illness that results in a shift in immunity?
These concerns are valid, agreed Dr. Jill Rabin, professor of obstetrics and gynecology and cochief of the division of ambulatory care, Women’s Health Programs–PCAP Services at North Shore–Long Island Jewish Health System, New Hyde Park, N.Y.
But she stressed in an interview that the guidelines are just that – guidelines. “They aren’t written in stone,” she said, adding that the recommendations are based on good science, and that a careful reading shows there is plenty of room for clinical judgment.
“Guidelines evolve and we have to keep an open mind and make sure that we don’t use a one-size-fits-all model. That doesn’t really help our patients,” said Dr. Rabin, who also is head of the urogynecology department at Long Island Jewish Medical Center.
Patient expectations
Another hurdle to embracing the guidelines is that of patient expectations. One of the big misunderstandings among patients of all ages is that they will skip all visits for 3 years because of the interval in Pap testing, Dr. Montgomery said. It is important to stress that “the Pap is just one of the services done during the visit, and a relatively small one when you look at all the other things that are part of a comprehensive well woman visit,” he said.
He further noted that both patient and physician age may be an important factor in acceptance of the guidelines and the changes in screening frequency.
As a physician with a large population of patients in their postreproductive years, and as one who trains young residents, he has noticed a generational difference: Younger patients and physicians are more accepting of the changes, but women who have spent 30 or 40 years coming in for an annual Pap smear equate that with health; they feel that they have done their part in staying healthy, and they have a greater sense of the value of the visit, he said.
Dr. Bohon predicted that both the advancing science and the concerns of patients and physicians will be considered when new guidelines are developed.
“My hope is that we have a realistic view of what the Pap can do,” Dr. Bohon said, adding that she expects that human papillomavirus (HPV) testing will get more and more reliable, and that clinicians will gain experience with following the HPV test and using that as a determining factor in patient care.
Guidelines at a glance
The USPSTF guidelines recommend screening with cytology every 3 years in women aged 21-29 years, and either screening with cytology every 3 years or cotesting using cytology and HPV testing every 5 years in those aged 30-65.
They also recommend against screening after hysterectomy with removal of the cervix in women with no history of high-grade precancer or cervical cancer, and against screening with HPV testing in women younger than age 30, either alone or with cytology.
The ACS/ASCP/ASCCP guidelines recommend a Pap test every 3 years in women aged 21-29 years, with HPV testing only if needed after an abnormal Pap test result. Those aged 30-65 years should have both a Pap test and an HPV test every 5 years, although a Pap test alone every 3 years is also acceptable. Women over age 65 years who have been screened regularly with normal results do not require screening, but those over age 65 years who are diagnosed with cervical precancer should continue to be screened, the guidelines say.
In April, the American College of Physicians in April released its own set of guidelines for screening average-risk women; these guidelines adhere closely to the USPSTF guidelines, and have been endorsed by the American Congress of Obstetricians and Gynecologists and the ASCP (Ann Intern Med. 2015;162[12]:851-9).
More than 3 years after the release of new cervical cancer screening guidelines, patients remain largely unaware of substantial changes in screening, and many physicians remain confused about or resistant to those changes.
The confusion is a matter of education, said Dr. Owen Montgomery, chairman of the department of obstetrics and gynecology at Drexel University, Philadelphia. As for the resistance, that’s less a reflection of concern about the science and more about the emotional and sociological processes involved in change, he said.
“It’s really good science,” Dr. Montgomery said of the data that formed the basis for the guidelines. “I was on the executive board of the [American College of Obstetricians and Gynecologists] when we signed off on the new recommendations. The science is valid.”
Two sets of guidelines were released in 2012, one by the U.S. Preventive Services Task Force (USPSTF) and one by the American Cancer Society (ACS) in conjunction with the American Society for Clinical Pathology (ASCP) and the American Society for Colposcopy and Cervical Pathology (ASCCP). Although the two guidelines differ in some respects, both recommend against a longstanding tradition: routine yearly Pap testing. The guidelines now call for screening intervals of 3-5 years depending on patient age and other factors, and recommend against screening those under age 21 years and over age 65 years in the absence of risk factors.
Knowledge gaps
Despite the widespread organizational support for the guidelines, data suggest that knowledge of them may be lacking among both patients and physicians. For instance, about 85% of 249 adult women who participated in a recent survey answered incorrectly when asked how often low-risk women aged 21-29 should be screened, and nearly 95% answered incorrectly when asked about the recommended frequency of screening in low-risk women over age 30 years.
According to Dr. Katherine O’Flynn O’Brien and her colleagues at George Washington University, Washington, who presented the results earlier this year at the American College of Obstetricians and Gynecologists annual scientific meeting, the findings suggest “that providers should focus more on educating women about changes to screening practices.”
But another survey presented at the same ACOG meeting showed that many providers may be unaware of the guideline changes or hesitant about embracing those changes.
Using a convenience sample of 165 medical and osteopathic physicians, physician assistants, and nurse practitioners, the investigators found that, in contrast with the current guidelines, about half do not perform cotesting in women aged 30-65 years every 5 years, and nearly 57% do not screen women aged 21-29 years with cytology alone every 3 years. More than 40% reported that they do not perform cytology screening in women aged 30-65 years every 3 years.
Addressing the ‘what ifs’
Confusion about the guidelines, and hesitation about implementing them, is not surprising as the changes – particularly the 3- to 5-year screening interval changes – are some of the most dramatic in recent memory, according to Dr. Constance Bohon, an ob.gyn. in Washington, D.C., and assistant clinical professor of ob.gyn. at George Washington University.
Certainly the guidelines have evolved with the science over the years, and some changes, such as screening only those over age 21 years, have been embraced, she said.
“But some changes are easier to accept than others,” she added, noting that “doing a Pap with cotesting in women after age 30 and repeating it again in 5 years is very difficult for many clinicians to accept ... part of the issue is that fine line in medicine between using the data and relying on clinical sense, clinical expertise.”
There is concern about the exceptions and the “what ifs,” she said.
What if a patient has a new partner? What if she develops an illness that results in a shift in immunity?
These concerns are valid, agreed Dr. Jill Rabin, professor of obstetrics and gynecology and cochief of the division of ambulatory care, Women’s Health Programs–PCAP Services at North Shore–Long Island Jewish Health System, New Hyde Park, N.Y.
But she stressed in an interview that the guidelines are just that – guidelines. “They aren’t written in stone,” she said, adding that the recommendations are based on good science, and that a careful reading shows there is plenty of room for clinical judgment.
“Guidelines evolve and we have to keep an open mind and make sure that we don’t use a one-size-fits-all model. That doesn’t really help our patients,” said Dr. Rabin, who also is head of the urogynecology department at Long Island Jewish Medical Center.
Patient expectations
Another hurdle to embracing the guidelines is that of patient expectations. One of the big misunderstandings among patients of all ages is that they will skip all visits for 3 years because of the interval in Pap testing, Dr. Montgomery said. It is important to stress that “the Pap is just one of the services done during the visit, and a relatively small one when you look at all the other things that are part of a comprehensive well woman visit,” he said.
He further noted that both patient and physician age may be an important factor in acceptance of the guidelines and the changes in screening frequency.
As a physician with a large population of patients in their postreproductive years, and as one who trains young residents, he has noticed a generational difference: Younger patients and physicians are more accepting of the changes, but women who have spent 30 or 40 years coming in for an annual Pap smear equate that with health; they feel that they have done their part in staying healthy, and they have a greater sense of the value of the visit, he said.
Dr. Bohon predicted that both the advancing science and the concerns of patients and physicians will be considered when new guidelines are developed.
“My hope is that we have a realistic view of what the Pap can do,” Dr. Bohon said, adding that she expects that human papillomavirus (HPV) testing will get more and more reliable, and that clinicians will gain experience with following the HPV test and using that as a determining factor in patient care.
Guidelines at a glance
The USPSTF guidelines recommend screening with cytology every 3 years in women aged 21-29 years, and either screening with cytology every 3 years or cotesting using cytology and HPV testing every 5 years in those aged 30-65.
They also recommend against screening after hysterectomy with removal of the cervix in women with no history of high-grade precancer or cervical cancer, and against screening with HPV testing in women younger than age 30, either alone or with cytology.
The ACS/ASCP/ASCCP guidelines recommend a Pap test every 3 years in women aged 21-29 years, with HPV testing only if needed after an abnormal Pap test result. Those aged 30-65 years should have both a Pap test and an HPV test every 5 years, although a Pap test alone every 3 years is also acceptable. Women over age 65 years who have been screened regularly with normal results do not require screening, but those over age 65 years who are diagnosed with cervical precancer should continue to be screened, the guidelines say.
In April, the American College of Physicians in April released its own set of guidelines for screening average-risk women; these guidelines adhere closely to the USPSTF guidelines, and have been endorsed by the American Congress of Obstetricians and Gynecologists and the ASCP (Ann Intern Med. 2015;162[12]:851-9).
More than 3 years after the release of new cervical cancer screening guidelines, patients remain largely unaware of substantial changes in screening, and many physicians remain confused about or resistant to those changes.
The confusion is a matter of education, said Dr. Owen Montgomery, chairman of the department of obstetrics and gynecology at Drexel University, Philadelphia. As for the resistance, that’s less a reflection of concern about the science and more about the emotional and sociological processes involved in change, he said.
“It’s really good science,” Dr. Montgomery said of the data that formed the basis for the guidelines. “I was on the executive board of the [American College of Obstetricians and Gynecologists] when we signed off on the new recommendations. The science is valid.”
Two sets of guidelines were released in 2012, one by the U.S. Preventive Services Task Force (USPSTF) and one by the American Cancer Society (ACS) in conjunction with the American Society for Clinical Pathology (ASCP) and the American Society for Colposcopy and Cervical Pathology (ASCCP). Although the two guidelines differ in some respects, both recommend against a longstanding tradition: routine yearly Pap testing. The guidelines now call for screening intervals of 3-5 years depending on patient age and other factors, and recommend against screening those under age 21 years and over age 65 years in the absence of risk factors.
Knowledge gaps
Despite the widespread organizational support for the guidelines, data suggest that knowledge of them may be lacking among both patients and physicians. For instance, about 85% of 249 adult women who participated in a recent survey answered incorrectly when asked how often low-risk women aged 21-29 should be screened, and nearly 95% answered incorrectly when asked about the recommended frequency of screening in low-risk women over age 30 years.
According to Dr. Katherine O’Flynn O’Brien and her colleagues at George Washington University, Washington, who presented the results earlier this year at the American College of Obstetricians and Gynecologists annual scientific meeting, the findings suggest “that providers should focus more on educating women about changes to screening practices.”
But another survey presented at the same ACOG meeting showed that many providers may be unaware of the guideline changes or hesitant about embracing those changes.
Using a convenience sample of 165 medical and osteopathic physicians, physician assistants, and nurse practitioners, the investigators found that, in contrast with the current guidelines, about half do not perform cotesting in women aged 30-65 years every 5 years, and nearly 57% do not screen women aged 21-29 years with cytology alone every 3 years. More than 40% reported that they do not perform cytology screening in women aged 30-65 years every 3 years.
Addressing the ‘what ifs’
Confusion about the guidelines, and hesitation about implementing them, is not surprising as the changes – particularly the 3- to 5-year screening interval changes – are some of the most dramatic in recent memory, according to Dr. Constance Bohon, an ob.gyn. in Washington, D.C., and assistant clinical professor of ob.gyn. at George Washington University.
Certainly the guidelines have evolved with the science over the years, and some changes, such as screening only those over age 21 years, have been embraced, she said.
“But some changes are easier to accept than others,” she added, noting that “doing a Pap with cotesting in women after age 30 and repeating it again in 5 years is very difficult for many clinicians to accept ... part of the issue is that fine line in medicine between using the data and relying on clinical sense, clinical expertise.”
There is concern about the exceptions and the “what ifs,” she said.
What if a patient has a new partner? What if she develops an illness that results in a shift in immunity?
These concerns are valid, agreed Dr. Jill Rabin, professor of obstetrics and gynecology and cochief of the division of ambulatory care, Women’s Health Programs–PCAP Services at North Shore–Long Island Jewish Health System, New Hyde Park, N.Y.
But she stressed in an interview that the guidelines are just that – guidelines. “They aren’t written in stone,” she said, adding that the recommendations are based on good science, and that a careful reading shows there is plenty of room for clinical judgment.
“Guidelines evolve and we have to keep an open mind and make sure that we don’t use a one-size-fits-all model. That doesn’t really help our patients,” said Dr. Rabin, who also is head of the urogynecology department at Long Island Jewish Medical Center.
Patient expectations
Another hurdle to embracing the guidelines is that of patient expectations. One of the big misunderstandings among patients of all ages is that they will skip all visits for 3 years because of the interval in Pap testing, Dr. Montgomery said. It is important to stress that “the Pap is just one of the services done during the visit, and a relatively small one when you look at all the other things that are part of a comprehensive well woman visit,” he said.
He further noted that both patient and physician age may be an important factor in acceptance of the guidelines and the changes in screening frequency.
As a physician with a large population of patients in their postreproductive years, and as one who trains young residents, he has noticed a generational difference: Younger patients and physicians are more accepting of the changes, but women who have spent 30 or 40 years coming in for an annual Pap smear equate that with health; they feel that they have done their part in staying healthy, and they have a greater sense of the value of the visit, he said.
Dr. Bohon predicted that both the advancing science and the concerns of patients and physicians will be considered when new guidelines are developed.
“My hope is that we have a realistic view of what the Pap can do,” Dr. Bohon said, adding that she expects that human papillomavirus (HPV) testing will get more and more reliable, and that clinicians will gain experience with following the HPV test and using that as a determining factor in patient care.
Guidelines at a glance
The USPSTF guidelines recommend screening with cytology every 3 years in women aged 21-29 years, and either screening with cytology every 3 years or cotesting using cytology and HPV testing every 5 years in those aged 30-65.
They also recommend against screening after hysterectomy with removal of the cervix in women with no history of high-grade precancer or cervical cancer, and against screening with HPV testing in women younger than age 30, either alone or with cytology.
The ACS/ASCP/ASCCP guidelines recommend a Pap test every 3 years in women aged 21-29 years, with HPV testing only if needed after an abnormal Pap test result. Those aged 30-65 years should have both a Pap test and an HPV test every 5 years, although a Pap test alone every 3 years is also acceptable. Women over age 65 years who have been screened regularly with normal results do not require screening, but those over age 65 years who are diagnosed with cervical precancer should continue to be screened, the guidelines say.
In April, the American College of Physicians in April released its own set of guidelines for screening average-risk women; these guidelines adhere closely to the USPSTF guidelines, and have been endorsed by the American Congress of Obstetricians and Gynecologists and the ASCP (Ann Intern Med. 2015;162[12]:851-9).
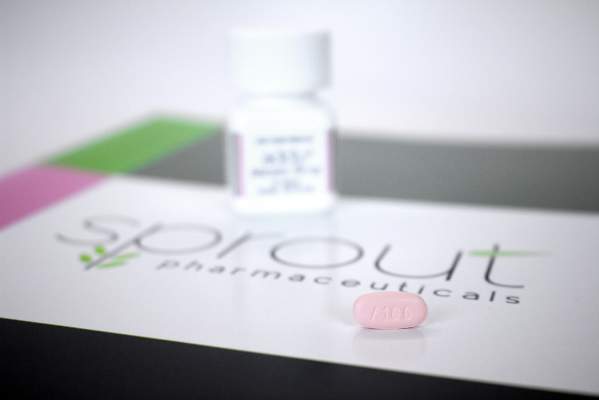
FDA approves flibanserin for low female sexual desire
The Food and Drug Administration has approved flibanserin as the first drug treatment for acquired, generalized hypoactive sexual desire disorder in premenopausal women. The drug – marketed as Addyi – is expected to be available by Oct. 17.
Conditions of the drug’s third – and successful – bid for approval, however, include a risk evaluation and mitigation strategy (REMS), post-marketing research, and a boxed warning to highlight the risk of severe hypotension and syncope for some patients.
The decision adheres to the recommendations of two FDA advisory panels – the Bone, Reproductive and Urologic Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee – which met jointly on June 4 and voted 18-6 for approval of the non-hormonal, centrally-acting drug. The panels advised that additional measures beyond labeling language be put in place to address concerns about serious adverse events associated with flibanserin. Unpredictable episodes of syncope have been reported, and significant interactions with alcohol can worsen the hypotension and syncope flibanserin can cause.
Flibanserin is a mixed agonist/antagonist for serotonin and dopamine receptors. It is meant to be taken orally at bedtime on a chronic basis; the dose is 100mg. Previous applications for approval in 2009 by then-manufacturer Boehringer Ingelheim, and in 2013 by current manufacturer Sprout Pharmaceuticals, were denied.
The REMS requires that prescribers of flibanserin complete training about the risks of severe hypotension and syncope when the drug is taken with alcohol, and that a patient-provider agreement form about these risks be signed. Pharmacies must also certify with the REMS program.
The black box warning will state that the use of alcohol is contraindicated when taking flibanserin, that it should not be taken with moderate or strong CYP3A4 inhibitors, and that it is contraindicated for those with liver impairment. The FDA is also requiring more study of flibanserin and alcohol in women.
In a series of three phase III clinical trials in North America, premenopausal women who met the DSM-IV diagnostic criteria for hypoactive sexual desire disorder (HSDD) and who were in a stable monogamous relationship took flibanserin or placebo. Of the more than 1,200 women in each study arm, those taking flibanserin had a statistically significant improvement in the number of satisfying sexual events (SSEs) per month, and also showed a significant increase in sexual desire, though overall effect sizes were modest. Women on placebo experienced an increase of 1.5 SSEs per month, compared to 2.5 SSEs per month in those taking flibanserin.
The primary endpoint for sexual desire in the first two studies was a response on an electronic diary reporting the highest level of desire over the last 24 hours. For the final study, the primary desire endpoint was the desire domain of the Female Sexual Function Index, which asked respondents to reflect on their desire over the previous 28 days. In the third clinical trial, women taking flibanserin showed a significantly greater increase in desire than those taking placebo.
Side effects and adverse events associated with flibanserin included drowsiness, hypotension, and syncope. Since the drug is metabolized through the CYP3A4 system, potential for drug-drug and drug-alcohol interaction exists.
Dr. Walid Gellad, co-director of the Center for Pharmaceutical Policy and Prescribing at the University of Pittsburgh, was on the FDA panel that voted in favor of flibanserin’s approval. In an interview, he explained that he attempted to balance the need for the medication against both the very real safety concerns and the relatively modest effect size.
As flibanserin hits the market, he said, “there is no doubt that the adverse events are going to be worse in real life than we saw in the trials.” Off-label use and drinking while taking the drug in spite of warnings are the likely contributors, he said.
On the other hand, “biology is important,” said Deborah Arrindell, vice president for health policy for the American Sexual Health Association, a supporter of Even the Score, a coalition of groups that includes Sprout Pharmaceuticals and that has pushed for approval of the drug. Having a medical treatment for women with HSDD, she said, represents real progress.
“We recognize that medical and biological factors are at play in human sexuality,” she said.
Women who have concerns about their level of sexual desire will be able to work with their providers to determine if flibanserin is a medication that could help them. Approval of flibanserin, Ms. Arrindell said, signals further acknowledgement that “women have a right to sexual pleasure, and that sexual health is part of one’s overall health and well-being.”
Dr. Lisa Larkin, scientific co-chair of Even the Score, and director of the UC Women’s Center in Cincinnati, applauded the FDA’s decision, saying that she hopes the approval will open the pipeline to research and development for more medications for women’s sexual health. Dr. Larkin, an internist with a special interest in women’s health, does not receive compensation for her position with Even the Score.
“This is a landmark day,” she said, even with the physician education and post-marketing requirements imposed by the FDA. “The patients I see are very distressed. These are real women suffering from a real medical condition.”
But Leonore Tiefer, Ph.D., clinical associate professor of psychiatry at New York University, expressed her disappointment in what she characterized as the FDA’s failure to adhere to its core mission. The FDA, she said, has become “too porous to external influences. To yield to pressure from industry was a mistake.”
She explained that as a sexologist, she has many non-pharmacologic treatments in her toolkit to help women with low sexual desire.
“I don’t think this is going to help women,” Dr. Tiefer said.
On Twitter @karioakes
The Food and Drug Administration has approved flibanserin as the first drug treatment for acquired, generalized hypoactive sexual desire disorder in premenopausal women. The drug – marketed as Addyi – is expected to be available by Oct. 17.
Conditions of the drug’s third – and successful – bid for approval, however, include a risk evaluation and mitigation strategy (REMS), post-marketing research, and a boxed warning to highlight the risk of severe hypotension and syncope for some patients.
The decision adheres to the recommendations of two FDA advisory panels – the Bone, Reproductive and Urologic Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee – which met jointly on June 4 and voted 18-6 for approval of the non-hormonal, centrally-acting drug. The panels advised that additional measures beyond labeling language be put in place to address concerns about serious adverse events associated with flibanserin. Unpredictable episodes of syncope have been reported, and significant interactions with alcohol can worsen the hypotension and syncope flibanserin can cause.
Flibanserin is a mixed agonist/antagonist for serotonin and dopamine receptors. It is meant to be taken orally at bedtime on a chronic basis; the dose is 100mg. Previous applications for approval in 2009 by then-manufacturer Boehringer Ingelheim, and in 2013 by current manufacturer Sprout Pharmaceuticals, were denied.
The REMS requires that prescribers of flibanserin complete training about the risks of severe hypotension and syncope when the drug is taken with alcohol, and that a patient-provider agreement form about these risks be signed. Pharmacies must also certify with the REMS program.
The black box warning will state that the use of alcohol is contraindicated when taking flibanserin, that it should not be taken with moderate or strong CYP3A4 inhibitors, and that it is contraindicated for those with liver impairment. The FDA is also requiring more study of flibanserin and alcohol in women.
In a series of three phase III clinical trials in North America, premenopausal women who met the DSM-IV diagnostic criteria for hypoactive sexual desire disorder (HSDD) and who were in a stable monogamous relationship took flibanserin or placebo. Of the more than 1,200 women in each study arm, those taking flibanserin had a statistically significant improvement in the number of satisfying sexual events (SSEs) per month, and also showed a significant increase in sexual desire, though overall effect sizes were modest. Women on placebo experienced an increase of 1.5 SSEs per month, compared to 2.5 SSEs per month in those taking flibanserin.
The primary endpoint for sexual desire in the first two studies was a response on an electronic diary reporting the highest level of desire over the last 24 hours. For the final study, the primary desire endpoint was the desire domain of the Female Sexual Function Index, which asked respondents to reflect on their desire over the previous 28 days. In the third clinical trial, women taking flibanserin showed a significantly greater increase in desire than those taking placebo.
Side effects and adverse events associated with flibanserin included drowsiness, hypotension, and syncope. Since the drug is metabolized through the CYP3A4 system, potential for drug-drug and drug-alcohol interaction exists.
Dr. Walid Gellad, co-director of the Center for Pharmaceutical Policy and Prescribing at the University of Pittsburgh, was on the FDA panel that voted in favor of flibanserin’s approval. In an interview, he explained that he attempted to balance the need for the medication against both the very real safety concerns and the relatively modest effect size.
As flibanserin hits the market, he said, “there is no doubt that the adverse events are going to be worse in real life than we saw in the trials.” Off-label use and drinking while taking the drug in spite of warnings are the likely contributors, he said.
On the other hand, “biology is important,” said Deborah Arrindell, vice president for health policy for the American Sexual Health Association, a supporter of Even the Score, a coalition of groups that includes Sprout Pharmaceuticals and that has pushed for approval of the drug. Having a medical treatment for women with HSDD, she said, represents real progress.
“We recognize that medical and biological factors are at play in human sexuality,” she said.
Women who have concerns about their level of sexual desire will be able to work with their providers to determine if flibanserin is a medication that could help them. Approval of flibanserin, Ms. Arrindell said, signals further acknowledgement that “women have a right to sexual pleasure, and that sexual health is part of one’s overall health and well-being.”
Dr. Lisa Larkin, scientific co-chair of Even the Score, and director of the UC Women’s Center in Cincinnati, applauded the FDA’s decision, saying that she hopes the approval will open the pipeline to research and development for more medications for women’s sexual health. Dr. Larkin, an internist with a special interest in women’s health, does not receive compensation for her position with Even the Score.
“This is a landmark day,” she said, even with the physician education and post-marketing requirements imposed by the FDA. “The patients I see are very distressed. These are real women suffering from a real medical condition.”
But Leonore Tiefer, Ph.D., clinical associate professor of psychiatry at New York University, expressed her disappointment in what she characterized as the FDA’s failure to adhere to its core mission. The FDA, she said, has become “too porous to external influences. To yield to pressure from industry was a mistake.”
She explained that as a sexologist, she has many non-pharmacologic treatments in her toolkit to help women with low sexual desire.
“I don’t think this is going to help women,” Dr. Tiefer said.
On Twitter @karioakes
The Food and Drug Administration has approved flibanserin as the first drug treatment for acquired, generalized hypoactive sexual desire disorder in premenopausal women. The drug – marketed as Addyi – is expected to be available by Oct. 17.
Conditions of the drug’s third – and successful – bid for approval, however, include a risk evaluation and mitigation strategy (REMS), post-marketing research, and a boxed warning to highlight the risk of severe hypotension and syncope for some patients.
The decision adheres to the recommendations of two FDA advisory panels – the Bone, Reproductive and Urologic Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee – which met jointly on June 4 and voted 18-6 for approval of the non-hormonal, centrally-acting drug. The panels advised that additional measures beyond labeling language be put in place to address concerns about serious adverse events associated with flibanserin. Unpredictable episodes of syncope have been reported, and significant interactions with alcohol can worsen the hypotension and syncope flibanserin can cause.
Flibanserin is a mixed agonist/antagonist for serotonin and dopamine receptors. It is meant to be taken orally at bedtime on a chronic basis; the dose is 100mg. Previous applications for approval in 2009 by then-manufacturer Boehringer Ingelheim, and in 2013 by current manufacturer Sprout Pharmaceuticals, were denied.
The REMS requires that prescribers of flibanserin complete training about the risks of severe hypotension and syncope when the drug is taken with alcohol, and that a patient-provider agreement form about these risks be signed. Pharmacies must also certify with the REMS program.
The black box warning will state that the use of alcohol is contraindicated when taking flibanserin, that it should not be taken with moderate or strong CYP3A4 inhibitors, and that it is contraindicated for those with liver impairment. The FDA is also requiring more study of flibanserin and alcohol in women.
In a series of three phase III clinical trials in North America, premenopausal women who met the DSM-IV diagnostic criteria for hypoactive sexual desire disorder (HSDD) and who were in a stable monogamous relationship took flibanserin or placebo. Of the more than 1,200 women in each study arm, those taking flibanserin had a statistically significant improvement in the number of satisfying sexual events (SSEs) per month, and also showed a significant increase in sexual desire, though overall effect sizes were modest. Women on placebo experienced an increase of 1.5 SSEs per month, compared to 2.5 SSEs per month in those taking flibanserin.
The primary endpoint for sexual desire in the first two studies was a response on an electronic diary reporting the highest level of desire over the last 24 hours. For the final study, the primary desire endpoint was the desire domain of the Female Sexual Function Index, which asked respondents to reflect on their desire over the previous 28 days. In the third clinical trial, women taking flibanserin showed a significantly greater increase in desire than those taking placebo.
Side effects and adverse events associated with flibanserin included drowsiness, hypotension, and syncope. Since the drug is metabolized through the CYP3A4 system, potential for drug-drug and drug-alcohol interaction exists.
Dr. Walid Gellad, co-director of the Center for Pharmaceutical Policy and Prescribing at the University of Pittsburgh, was on the FDA panel that voted in favor of flibanserin’s approval. In an interview, he explained that he attempted to balance the need for the medication against both the very real safety concerns and the relatively modest effect size.
As flibanserin hits the market, he said, “there is no doubt that the adverse events are going to be worse in real life than we saw in the trials.” Off-label use and drinking while taking the drug in spite of warnings are the likely contributors, he said.
On the other hand, “biology is important,” said Deborah Arrindell, vice president for health policy for the American Sexual Health Association, a supporter of Even the Score, a coalition of groups that includes Sprout Pharmaceuticals and that has pushed for approval of the drug. Having a medical treatment for women with HSDD, she said, represents real progress.
“We recognize that medical and biological factors are at play in human sexuality,” she said.
Women who have concerns about their level of sexual desire will be able to work with their providers to determine if flibanserin is a medication that could help them. Approval of flibanserin, Ms. Arrindell said, signals further acknowledgement that “women have a right to sexual pleasure, and that sexual health is part of one’s overall health and well-being.”
Dr. Lisa Larkin, scientific co-chair of Even the Score, and director of the UC Women’s Center in Cincinnati, applauded the FDA’s decision, saying that she hopes the approval will open the pipeline to research and development for more medications for women’s sexual health. Dr. Larkin, an internist with a special interest in women’s health, does not receive compensation for her position with Even the Score.
“This is a landmark day,” she said, even with the physician education and post-marketing requirements imposed by the FDA. “The patients I see are very distressed. These are real women suffering from a real medical condition.”
But Leonore Tiefer, Ph.D., clinical associate professor of psychiatry at New York University, expressed her disappointment in what she characterized as the FDA’s failure to adhere to its core mission. The FDA, she said, has become “too porous to external influences. To yield to pressure from industry was a mistake.”
She explained that as a sexologist, she has many non-pharmacologic treatments in her toolkit to help women with low sexual desire.
“I don’t think this is going to help women,” Dr. Tiefer said.
On Twitter @karioakes
“Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts
Ultrasonography is the preferred imaging method to evaluate most adnexal cysts. Most types of pelvic cyst pathology have characteristic findings that, when identified, can guide counseling and management decisions. For instance, simple cysts have thin walls, are uniformly hypoechoic, and show no blood flow on color Doppler. Endometriomas, on the other hand, demonstrate diffuse, low-level internal echoes on ultrasonography.
In parts 1 and 2 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- and mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma).
In this part 3, we detail imaging for hydrosalpinx and pelvic inclusion cysts. In part 4 we will consider cystadenomas and ovarian neoplasias.
hydrosalpinx
These cysts are caused by fimbrial obstruction and result in tubal distention with serous fluid. A hydrosalpinx may occur following an episode of salpingitis or pelvic surgery.
Sonographic features diagnostic for hydrosalpinx include a tubular or S-shaped cystic mass separate from the ovary, with:
- “beads on a string” or “cogwheel” appearance (small round nodules less than 3 mm in size that represent endosalpingeal folds when viewed in cross section)
- “waist sign” (indentations on opposite sides)
- incomplete septations, which result from segments of distended tube folding over/adhering to other tubal segments
Levine and colleagues noted that 3-dimensional imaging may be helpful when the diagnosis is uncertain.1
When a mass is noted that has features classic for hydrosalpinx, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommends1:
- no further imaging is necessary to establish the diagnosis
- frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 1 through 6 below (slides of image collections), we present 5 cases, including one of a 45-year-old patient presenting with chronic pelvic pain who was found to have bilateral hydrosalginges and right-sided tubo-ovarian complex.
pelvic inclusion cysts
Pelvic/peritoneal inclusion cysts, or peritoneal pseudocysts, are typically associated with factors that increase the risk for pelvic adhesive disease (including endometriosis, pelvic inflammatory disease, or prior pelvic surgery).
Classic sonographic features of pelvic inclusion cysts are:
- cystic mass, usually with septations/loculations
- the mass follows the contour of adjacent organs
- ovary at edge of the mass or sometimes suspended within it
- with or without flow in septation on color Doppler
When a mass is noted that has features classic for a peritoneal inclusion cyst, the US Society of Radiologists in Ultrasound recommends that1:
- no further imaging is necessary to establish the diagnosis (although further imaging may be needed if the diagnosis is uncertain)
- the frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 7 through 22 below (slides of image collections), we present several cases that demonstrate pelvic inclusion cysts on imaging. One case involves a 25-year-old patient presenting for 2- and 3-dimensional pelvic imaging due to infertility. She had a history of laparoscopic left ovarian cystectomy, right paratubal cystectomy, and lysis of adhesions. She was found to have a pelvic inclusion cyst and an endometrioma in the left ovary.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Figure 12
Figure 13
Figure 14
Figure 15
Figure 16
Figure 17
Figure 18
Figure 19
Figure 20
Figure 21
Figure 22
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Ultrasound Q. 2010;26(3):121−131.
Ultrasonography is the preferred imaging method to evaluate most adnexal cysts. Most types of pelvic cyst pathology have characteristic findings that, when identified, can guide counseling and management decisions. For instance, simple cysts have thin walls, are uniformly hypoechoic, and show no blood flow on color Doppler. Endometriomas, on the other hand, demonstrate diffuse, low-level internal echoes on ultrasonography.
In parts 1 and 2 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- and mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma).
In this part 3, we detail imaging for hydrosalpinx and pelvic inclusion cysts. In part 4 we will consider cystadenomas and ovarian neoplasias.
hydrosalpinx
These cysts are caused by fimbrial obstruction and result in tubal distention with serous fluid. A hydrosalpinx may occur following an episode of salpingitis or pelvic surgery.
Sonographic features diagnostic for hydrosalpinx include a tubular or S-shaped cystic mass separate from the ovary, with:
- “beads on a string” or “cogwheel” appearance (small round nodules less than 3 mm in size that represent endosalpingeal folds when viewed in cross section)
- “waist sign” (indentations on opposite sides)
- incomplete septations, which result from segments of distended tube folding over/adhering to other tubal segments
Levine and colleagues noted that 3-dimensional imaging may be helpful when the diagnosis is uncertain.1
When a mass is noted that has features classic for hydrosalpinx, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommends1:
- no further imaging is necessary to establish the diagnosis
- frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 1 through 6 below (slides of image collections), we present 5 cases, including one of a 45-year-old patient presenting with chronic pelvic pain who was found to have bilateral hydrosalginges and right-sided tubo-ovarian complex.
pelvic inclusion cysts
Pelvic/peritoneal inclusion cysts, or peritoneal pseudocysts, are typically associated with factors that increase the risk for pelvic adhesive disease (including endometriosis, pelvic inflammatory disease, or prior pelvic surgery).
Classic sonographic features of pelvic inclusion cysts are:
- cystic mass, usually with septations/loculations
- the mass follows the contour of adjacent organs
- ovary at edge of the mass or sometimes suspended within it
- with or without flow in septation on color Doppler
When a mass is noted that has features classic for a peritoneal inclusion cyst, the US Society of Radiologists in Ultrasound recommends that1:
- no further imaging is necessary to establish the diagnosis (although further imaging may be needed if the diagnosis is uncertain)
- the frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 7 through 22 below (slides of image collections), we present several cases that demonstrate pelvic inclusion cysts on imaging. One case involves a 25-year-old patient presenting for 2- and 3-dimensional pelvic imaging due to infertility. She had a history of laparoscopic left ovarian cystectomy, right paratubal cystectomy, and lysis of adhesions. She was found to have a pelvic inclusion cyst and an endometrioma in the left ovary.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Figure 12
Figure 13
Figure 14
Figure 15
Figure 16
Figure 17
Figure 18
Figure 19
Figure 20
Figure 21
Figure 22
Ultrasonography is the preferred imaging method to evaluate most adnexal cysts. Most types of pelvic cyst pathology have characteristic findings that, when identified, can guide counseling and management decisions. For instance, simple cysts have thin walls, are uniformly hypoechoic, and show no blood flow on color Doppler. Endometriomas, on the other hand, demonstrate diffuse, low-level internal echoes on ultrasonography.
In parts 1 and 2 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- and mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma).
In this part 3, we detail imaging for hydrosalpinx and pelvic inclusion cysts. In part 4 we will consider cystadenomas and ovarian neoplasias.
hydrosalpinx
These cysts are caused by fimbrial obstruction and result in tubal distention with serous fluid. A hydrosalpinx may occur following an episode of salpingitis or pelvic surgery.
Sonographic features diagnostic for hydrosalpinx include a tubular or S-shaped cystic mass separate from the ovary, with:
- “beads on a string” or “cogwheel” appearance (small round nodules less than 3 mm in size that represent endosalpingeal folds when viewed in cross section)
- “waist sign” (indentations on opposite sides)
- incomplete septations, which result from segments of distended tube folding over/adhering to other tubal segments
Levine and colleagues noted that 3-dimensional imaging may be helpful when the diagnosis is uncertain.1
When a mass is noted that has features classic for hydrosalpinx, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement recommends1:
- no further imaging is necessary to establish the diagnosis
- frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 1 through 6 below (slides of image collections), we present 5 cases, including one of a 45-year-old patient presenting with chronic pelvic pain who was found to have bilateral hydrosalginges and right-sided tubo-ovarian complex.
pelvic inclusion cysts
Pelvic/peritoneal inclusion cysts, or peritoneal pseudocysts, are typically associated with factors that increase the risk for pelvic adhesive disease (including endometriosis, pelvic inflammatory disease, or prior pelvic surgery).
Classic sonographic features of pelvic inclusion cysts are:
- cystic mass, usually with septations/loculations
- the mass follows the contour of adjacent organs
- ovary at edge of the mass or sometimes suspended within it
- with or without flow in septation on color Doppler
When a mass is noted that has features classic for a peritoneal inclusion cyst, the US Society of Radiologists in Ultrasound recommends that1:
- no further imaging is necessary to establish the diagnosis (although further imaging may be needed if the diagnosis is uncertain)
- the frequency of follow-up imaging should be based on the patient’s age and clinical symptoms
In FIGURES 7 through 22 below (slides of image collections), we present several cases that demonstrate pelvic inclusion cysts on imaging. One case involves a 25-year-old patient presenting for 2- and 3-dimensional pelvic imaging due to infertility. She had a history of laparoscopic left ovarian cystectomy, right paratubal cystectomy, and lysis of adhesions. She was found to have a pelvic inclusion cyst and an endometrioma in the left ovary.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Figure 12
Figure 13
Figure 14
Figure 15
Figure 16
Figure 17
Figure 18
Figure 19
Figure 20
Figure 21
Figure 22
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Ultrasound Q. 2010;26(3):121−131.
Reference
1. Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Ultrasound Q. 2010;26(3):121−131.
9-valent HPV vaccine equally effective in adolescents, young women
Response to the 9-valent human papillomavirus vaccine was noninferior in boys and girls aged 9-15 years, compared with women aged 16-26 years, according to Dr. Pierre Van Damme of the University of Antwerp, Belgium, and his associates.
In all three groups, a seroconversion rate of greater than 99% was achieved for all HPV vaccine types in this study of 3,074 subjects. After 2.5 years, anti-HPV responses remained strong at over 90% for the boys and girls. The delivery of the 9-valent HPV vaccine was tolerated well in all groups, with boys and girls reporting injection-site adverse event rates of 72.8% and 81.9%, respectively, compared with 85.4% of young women.
The study findings support “bridging the efficacy findings in young women 16 to 26 years of age to girls and boys 9 to 15 years of age. The 9vHPV vaccine appears to be generally well tolerated in all groups,” the investigators wrote.
Find the full study in Pediatrics (doi: 10.1542/peds.2014-3745).
Response to the 9-valent human papillomavirus vaccine was noninferior in boys and girls aged 9-15 years, compared with women aged 16-26 years, according to Dr. Pierre Van Damme of the University of Antwerp, Belgium, and his associates.
In all three groups, a seroconversion rate of greater than 99% was achieved for all HPV vaccine types in this study of 3,074 subjects. After 2.5 years, anti-HPV responses remained strong at over 90% for the boys and girls. The delivery of the 9-valent HPV vaccine was tolerated well in all groups, with boys and girls reporting injection-site adverse event rates of 72.8% and 81.9%, respectively, compared with 85.4% of young women.
The study findings support “bridging the efficacy findings in young women 16 to 26 years of age to girls and boys 9 to 15 years of age. The 9vHPV vaccine appears to be generally well tolerated in all groups,” the investigators wrote.
Find the full study in Pediatrics (doi: 10.1542/peds.2014-3745).
Response to the 9-valent human papillomavirus vaccine was noninferior in boys and girls aged 9-15 years, compared with women aged 16-26 years, according to Dr. Pierre Van Damme of the University of Antwerp, Belgium, and his associates.
In all three groups, a seroconversion rate of greater than 99% was achieved for all HPV vaccine types in this study of 3,074 subjects. After 2.5 years, anti-HPV responses remained strong at over 90% for the boys and girls. The delivery of the 9-valent HPV vaccine was tolerated well in all groups, with boys and girls reporting injection-site adverse event rates of 72.8% and 81.9%, respectively, compared with 85.4% of young women.
The study findings support “bridging the efficacy findings in young women 16 to 26 years of age to girls and boys 9 to 15 years of age. The 9vHPV vaccine appears to be generally well tolerated in all groups,” the investigators wrote.
Find the full study in Pediatrics (doi: 10.1542/peds.2014-3745).
ICD-10-CM documentation and coding for GYN procedures
In 2 months, the new coding set will become the only accepted format for diagnostic coding on medical claims. By now, most clinicians and their staffs should have begun the training process, including the examination of current documentation patterns, to ensure that the more specific International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes can be reported.
In 2014, I informed you about the more general changes that are to come in the format and ideas for preparation.1 But now it is time to get down to the nitty-gritty (or granularity, if you prefer) of this coding format to ensure correct coding every time for your gynecology services. A separate article will appear in the September 2015 issue of OBG Management to describe diagnostic coding for obstetric care.
No wheel reinvention necessary
Many of the guidelines for ICD-9-CM will transfer over to ICD-10-CM, so it will not be necessary to reinvent the wheel—but there are important changes that will affect both your documentation and payers’ requirements for the highest level of specificity. There also will be some instructions in the tabular section of ICD-10-CM that will let you know whether a combination of codes can or cannot be reported together (called “excludes” notes). In the beginning, this process may require additional communication between practice staff and clinicians.
However, if your practice has prepared a teaching document that outlines currently used codes and compares them with ICD-10-CM code choices and provides comments in regard to issues such as code combinations, conversion to the new system should be almost seamless.
Remember, the documentation of the clinician drives the selection of the code. The less information provided, the less specificity—and the result may be increased denials due to medical necessity for procedures and treatments.
Most reported codes will begin with “N”
Although the format of the codes will change under ICD-10-CM, diagnostic reporting will remain the same for most of the gynecologic conditions reported, and clinicians should be aware that the codes they will be reporting mainly will come from those that begin with “N.” One advantage: None of these codes require a 7th character or utilize the “x” placeholder code. In fact, the majority of codes from this chapter will have a one-to-one counterpart in the ICD-9-CM codes. A few exceptions are outlined below.
In addition to the core of “N” codes, a handful of codes will come from other chapters to capture reasons for a gynecologic encounter or surgery. For instance, “Z” codes will be reported for encounters for reasons other than illness and include codes for contraceptive and procreative management, general counseling, history of diseases, preventive gynecologic examinations, and screening scenarios, to name just a few. “R” codes will be used most often for general signs and symptoms, such as abdominal pain or nausea and vomiting.
Your documentation will need to change in some important areas
When you see a patient for an injury to the urinary or pelvic organs that is not a complication of a procedure, or for a complication of a genitourinary prosthetic device, implant, or graft, you will need to document whether this is an initial or subsequent encounter or a sequela. This information is added as a 7th alpha character (a = initial, d = subsequent, s = sequela).
ICD-10-CM defines an initial encounter as the time period in which the patient is actively being treated. A subsequent encounter would be reported after the patient’s active treatment, while she is receiving routine care during the healing or recovery phase. For instance, you would report the encounter as subsequent when the patient is seen after her surgery for an injury to the ovary due to an automobile accident, but you would report an initial encounter for all visits through the surgical date of service when a patient presents with symptoms of mesh erosion requiring surgery. Sequela refers to a condition that developed as a result of another condition. For instance, if the patient’s intrauterine device (IUD) becomes embedded in the ostium due to an undetected uterine fibroid, that is a sequela.
The requirement to indicate laterality also will affect documentation, but this concept is limited to a few codes that might be reported by ObGyns. A designation of the right versus left organ will be required for reported cases of primary, secondary, borderline, or benign tumors of the breast, ovary, fallopian tube, broad ligament, and round ligament, as well as cancer in situ of the breast. However, the terms “bilateral” and “unilateral” are applied only to codes that describe hernias, acquired absence of the ovaries, and injuries to the ovaries and fallopian tubes that are not due to a surgical complication.
Unspecified codes still play a role
Unspecified ICD-10-CM codes still come into play when the clinician does not have enough information to assign a more specific code—that is, when, by the end of an encounter, no further information is available to assign a more specific diagnosis. For example, if a patient has signs of a fibroid upon examination, only the unspecified code can be reported until the clinician can discover whether it is intramural, submucosal, or subserosal. However, it would be equally incorrect to assign an unspecified code to an encounter once the nature of the fibroid has been determined.
Take note of these differences in coding
Here is a list of important new gynecologic coding requirements, which are presented in alphabetical order.
Amenorrhea, oligomenorrhea (N91.0–N91.5) and dysmenorrhea (N94.4–N94.5) will require documentation to indicate whether the condition is primary or secondary. Although an unspecified code is available, once treatment is begun the cause should be known and documented.
Artificial insemination problems will have a section:
- N98.0 Infection associated with artificial insemination
- N98.1 Hyperstimulation of ovaries
- N98.2 Complications of attempted introduction of fertilized ovum following in vitro fertilization
- N98.3 Complications of attempted introduction of embryo in embryo transfer
- N98.8 Other complications associated with artificial fertilization
- N98.9 Complication associated with artificial fertilization, unspecified.
Breast cancer codes will require documentation of which breast and what part of the breast is affected.
Contraceptive management highlights:
- Injectable contraceptives will have new codes for the initial prescription (Z30.013) and subsequent surveillance (Z30.42)
- IUD encounter for the prescription will have a new code (Z30.014), which is reported when the IUD is not being inserted on the same day
- Subdermal contraceptive implant surveillance will no longer have a specific code but will be included in the “other” contraceptive code Z30.49.
Conversion of a laparoscopic procedure to an open procedure will not have a code.
Cystocele, unspecified, will have code N81.10.
Dysplasia of vagina will be expanded into 3 codes based on mild, moderate, or unspecified: N89.0–N89.3.
Female genitourinary cancer codes:
- Documentation of right or left organs and which part of the uterus is affected will be required
- Cancer in situ of cervix will be expanded by site on the cervix: D06.0–D06.7
- Cancer in situ of the endometrium will have a specific code: D07.0.
Genuine stress urinary incontinence will only be referred to as stress incontinence (male or female). The code is now located in the urinary section of Chapter N: N39.3.
Genitourinary complications due to procedures and surgery will be organized in 1 section: N99
- Some conditions have more than 1 code based on cause:
- N99.2 Stricture of vagina due to surgical complication
- N89.5 Stricture of vagina not due to surgical complication
- N99.4 Pelvic adhesions due to surgical complication
- N73.6 Pelvic adhesions not due to surgical complication - Other codes will differentiate between intraoperative or postprocedure complications and whether the surgery is on the genitourinary system or a different surgery:
- N99.61 Intraoperative hemorrhage and hematoma of a genitourinary system organ or structure complicating a genitourinary system procedure
- N99.62 Intraoperative hemorrhage and hematoma of a genitourinary system organ or structure complicating other procedure
- N99.820 Postprocedural hemorrhage and hematoma of a genitourinary system organ or structure following a genitourinary system procedure
- N99.821 Postprocedural hemorrhage and hematoma of a genitourinary system organ or structure following other procedure.
Gynecologic examinations will have to include information on whether or not there were genitourinary abnormal findings on the exam. If so, an additional secondary code will be required to identify the abnormality: Z01.411 and finding code. (Without abnormal findings: Z01.419.) For instance, a diagnosis of bacterial vaginosis is made during the examination. The abnormal findings are not those from other areas such as the breast or thyroid.
Hematuria documentation must differentiate between gross: R31.0, benign essential:R31.1, or other forms: R31.2.
High-risk sexual behavior problems must be documented by heterosexual, bisexual, or homosexual behavior: Z72.51–Z72.53.
Hormonal contraceptives, long-term use, will have a specific code: Z79.3.
Hyperplasia without atypia (simple, complex, or benign) will be rolled into a single code: N85.01.
Immunizations, prophylactic, will not have specific codes as to type. An encounter for any type of immunization is Z23.
Pelvic pain will have its own symptom code: R10.2.
Personal history for cancer has been expanded:
- Personal history of cancer in situ:
- Z86.000 of breast
- Z86.001 of cervix uteri
- Z86.008 of other site - Personal history of benign neoplasm:
- Z86.012 of other benign neoplasm
- Z86.03 of uncertain behavior (borderline malignancies).
Procedures not carried out will be expanded in ICD-10 to include 2 new codes:
- Z53.01 Procedure contraindicated due to patient smoking
- Z53.21 Procedure not carried out because patient left before seeing physician.
Procreative management changes:
- Artificial insemination will not have a specific code
- New code for male factor infertility: Z31.81
- New code for Rh incompatibility: Z31.82. This code would be used when the patient presents for prophylactic rho(D) immune globulin in addition to the Z23 code for immunization. This code also would be reported for the patient being tested for isoimmunization with no test result at the time of the visit.
Uterine prolapse without vaginal wall prolapse (618.1) will not have a code replacement.
Vaginal conditions such as vaginal lacerations (old), leukorrhea not specified as infective, and vaginal hematoma will be represented by an “other” code: N89.8.
Vulvar cyst will have its own code: N90.7.
Vulvovaginitis has been expanded into category codes for acute, subacute/chronic conditions of both the vagina and the vulva, which changes the documentation requirements in order to code correctly: N76.0–N76.3.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Reference
1. Witt M. Moving forward with ICD-10: capitalize on this extra time. OBG Manag. 2014;26(7):17, 18, 20.
In 2 months, the new coding set will become the only accepted format for diagnostic coding on medical claims. By now, most clinicians and their staffs should have begun the training process, including the examination of current documentation patterns, to ensure that the more specific International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes can be reported.
In 2014, I informed you about the more general changes that are to come in the format and ideas for preparation.1 But now it is time to get down to the nitty-gritty (or granularity, if you prefer) of this coding format to ensure correct coding every time for your gynecology services. A separate article will appear in the September 2015 issue of OBG Management to describe diagnostic coding for obstetric care.
No wheel reinvention necessary
Many of the guidelines for ICD-9-CM will transfer over to ICD-10-CM, so it will not be necessary to reinvent the wheel—but there are important changes that will affect both your documentation and payers’ requirements for the highest level of specificity. There also will be some instructions in the tabular section of ICD-10-CM that will let you know whether a combination of codes can or cannot be reported together (called “excludes” notes). In the beginning, this process may require additional communication between practice staff and clinicians.
However, if your practice has prepared a teaching document that outlines currently used codes and compares them with ICD-10-CM code choices and provides comments in regard to issues such as code combinations, conversion to the new system should be almost seamless.
Remember, the documentation of the clinician drives the selection of the code. The less information provided, the less specificity—and the result may be increased denials due to medical necessity for procedures and treatments.
Most reported codes will begin with “N”
Although the format of the codes will change under ICD-10-CM, diagnostic reporting will remain the same for most of the gynecologic conditions reported, and clinicians should be aware that the codes they will be reporting mainly will come from those that begin with “N.” One advantage: None of these codes require a 7th character or utilize the “x” placeholder code. In fact, the majority of codes from this chapter will have a one-to-one counterpart in the ICD-9-CM codes. A few exceptions are outlined below.
In addition to the core of “N” codes, a handful of codes will come from other chapters to capture reasons for a gynecologic encounter or surgery. For instance, “Z” codes will be reported for encounters for reasons other than illness and include codes for contraceptive and procreative management, general counseling, history of diseases, preventive gynecologic examinations, and screening scenarios, to name just a few. “R” codes will be used most often for general signs and symptoms, such as abdominal pain or nausea and vomiting.
Your documentation will need to change in some important areas
When you see a patient for an injury to the urinary or pelvic organs that is not a complication of a procedure, or for a complication of a genitourinary prosthetic device, implant, or graft, you will need to document whether this is an initial or subsequent encounter or a sequela. This information is added as a 7th alpha character (a = initial, d = subsequent, s = sequela).
ICD-10-CM defines an initial encounter as the time period in which the patient is actively being treated. A subsequent encounter would be reported after the patient’s active treatment, while she is receiving routine care during the healing or recovery phase. For instance, you would report the encounter as subsequent when the patient is seen after her surgery for an injury to the ovary due to an automobile accident, but you would report an initial encounter for all visits through the surgical date of service when a patient presents with symptoms of mesh erosion requiring surgery. Sequela refers to a condition that developed as a result of another condition. For instance, if the patient’s intrauterine device (IUD) becomes embedded in the ostium due to an undetected uterine fibroid, that is a sequela.
The requirement to indicate laterality also will affect documentation, but this concept is limited to a few codes that might be reported by ObGyns. A designation of the right versus left organ will be required for reported cases of primary, secondary, borderline, or benign tumors of the breast, ovary, fallopian tube, broad ligament, and round ligament, as well as cancer in situ of the breast. However, the terms “bilateral” and “unilateral” are applied only to codes that describe hernias, acquired absence of the ovaries, and injuries to the ovaries and fallopian tubes that are not due to a surgical complication.
Unspecified codes still play a role
Unspecified ICD-10-CM codes still come into play when the clinician does not have enough information to assign a more specific code—that is, when, by the end of an encounter, no further information is available to assign a more specific diagnosis. For example, if a patient has signs of a fibroid upon examination, only the unspecified code can be reported until the clinician can discover whether it is intramural, submucosal, or subserosal. However, it would be equally incorrect to assign an unspecified code to an encounter once the nature of the fibroid has been determined.
Take note of these differences in coding
Here is a list of important new gynecologic coding requirements, which are presented in alphabetical order.
Amenorrhea, oligomenorrhea (N91.0–N91.5) and dysmenorrhea (N94.4–N94.5) will require documentation to indicate whether the condition is primary or secondary. Although an unspecified code is available, once treatment is begun the cause should be known and documented.
Artificial insemination problems will have a section:
- N98.0 Infection associated with artificial insemination
- N98.1 Hyperstimulation of ovaries
- N98.2 Complications of attempted introduction of fertilized ovum following in vitro fertilization
- N98.3 Complications of attempted introduction of embryo in embryo transfer
- N98.8 Other complications associated with artificial fertilization
- N98.9 Complication associated with artificial fertilization, unspecified.
Breast cancer codes will require documentation of which breast and what part of the breast is affected.
Contraceptive management highlights:
- Injectable contraceptives will have new codes for the initial prescription (Z30.013) and subsequent surveillance (Z30.42)
- IUD encounter for the prescription will have a new code (Z30.014), which is reported when the IUD is not being inserted on the same day
- Subdermal contraceptive implant surveillance will no longer have a specific code but will be included in the “other” contraceptive code Z30.49.
Conversion of a laparoscopic procedure to an open procedure will not have a code.
Cystocele, unspecified, will have code N81.10.
Dysplasia of vagina will be expanded into 3 codes based on mild, moderate, or unspecified: N89.0–N89.3.
Female genitourinary cancer codes:
- Documentation of right or left organs and which part of the uterus is affected will be required
- Cancer in situ of cervix will be expanded by site on the cervix: D06.0–D06.7
- Cancer in situ of the endometrium will have a specific code: D07.0.
Genuine stress urinary incontinence will only be referred to as stress incontinence (male or female). The code is now located in the urinary section of Chapter N: N39.3.
Genitourinary complications due to procedures and surgery will be organized in 1 section: N99
- Some conditions have more than 1 code based on cause:
- N99.2 Stricture of vagina due to surgical complication
- N89.5 Stricture of vagina not due to surgical complication
- N99.4 Pelvic adhesions due to surgical complication
- N73.6 Pelvic adhesions not due to surgical complication - Other codes will differentiate between intraoperative or postprocedure complications and whether the surgery is on the genitourinary system or a different surgery:
- N99.61 Intraoperative hemorrhage and hematoma of a genitourinary system organ or structure complicating a genitourinary system procedure
- N99.62 Intraoperative hemorrhage and hematoma of a genitourinary system organ or structure complicating other procedure
- N99.820 Postprocedural hemorrhage and hematoma of a genitourinary system organ or structure following a genitourinary system procedure
- N99.821 Postprocedural hemorrhage and hematoma of a genitourinary system organ or structure following other procedure.
Gynecologic examinations will have to include information on whether or not there were genitourinary abnormal findings on the exam. If so, an additional secondary code will be required to identify the abnormality: Z01.411 and finding code. (Without abnormal findings: Z01.419.) For instance, a diagnosis of bacterial vaginosis is made during the examination. The abnormal findings are not those from other areas such as the breast or thyroid.
Hematuria documentation must differentiate between gross: R31.0, benign essential:R31.1, or other forms: R31.2.
High-risk sexual behavior problems must be documented by heterosexual, bisexual, or homosexual behavior: Z72.51–Z72.53.
Hormonal contraceptives, long-term use, will have a specific code: Z79.3.
Hyperplasia without atypia (simple, complex, or benign) will be rolled into a single code: N85.01.
Immunizations, prophylactic, will not have specific codes as to type. An encounter for any type of immunization is Z23.
Pelvic pain will have its own symptom code: R10.2.
Personal history for cancer has been expanded:
- Personal history of cancer in situ:
- Z86.000 of breast
- Z86.001 of cervix uteri
- Z86.008 of other site - Personal history of benign neoplasm:
- Z86.012 of other benign neoplasm
- Z86.03 of uncertain behavior (borderline malignancies).
Procedures not carried out will be expanded in ICD-10 to include 2 new codes:
- Z53.01 Procedure contraindicated due to patient smoking
- Z53.21 Procedure not carried out because patient left before seeing physician.
Procreative management changes:
- Artificial insemination will not have a specific code
- New code for male factor infertility: Z31.81
- New code for Rh incompatibility: Z31.82. This code would be used when the patient presents for prophylactic rho(D) immune globulin in addition to the Z23 code for immunization. This code also would be reported for the patient being tested for isoimmunization with no test result at the time of the visit.
Uterine prolapse without vaginal wall prolapse (618.1) will not have a code replacement.
Vaginal conditions such as vaginal lacerations (old), leukorrhea not specified as infective, and vaginal hematoma will be represented by an “other” code: N89.8.
Vulvar cyst will have its own code: N90.7.
Vulvovaginitis has been expanded into category codes for acute, subacute/chronic conditions of both the vagina and the vulva, which changes the documentation requirements in order to code correctly: N76.0–N76.3.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In 2 months, the new coding set will become the only accepted format for diagnostic coding on medical claims. By now, most clinicians and their staffs should have begun the training process, including the examination of current documentation patterns, to ensure that the more specific International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes can be reported.
In 2014, I informed you about the more general changes that are to come in the format and ideas for preparation.1 But now it is time to get down to the nitty-gritty (or granularity, if you prefer) of this coding format to ensure correct coding every time for your gynecology services. A separate article will appear in the September 2015 issue of OBG Management to describe diagnostic coding for obstetric care.
No wheel reinvention necessary
Many of the guidelines for ICD-9-CM will transfer over to ICD-10-CM, so it will not be necessary to reinvent the wheel—but there are important changes that will affect both your documentation and payers’ requirements for the highest level of specificity. There also will be some instructions in the tabular section of ICD-10-CM that will let you know whether a combination of codes can or cannot be reported together (called “excludes” notes). In the beginning, this process may require additional communication between practice staff and clinicians.
However, if your practice has prepared a teaching document that outlines currently used codes and compares them with ICD-10-CM code choices and provides comments in regard to issues such as code combinations, conversion to the new system should be almost seamless.
Remember, the documentation of the clinician drives the selection of the code. The less information provided, the less specificity—and the result may be increased denials due to medical necessity for procedures and treatments.
Most reported codes will begin with “N”
Although the format of the codes will change under ICD-10-CM, diagnostic reporting will remain the same for most of the gynecologic conditions reported, and clinicians should be aware that the codes they will be reporting mainly will come from those that begin with “N.” One advantage: None of these codes require a 7th character or utilize the “x” placeholder code. In fact, the majority of codes from this chapter will have a one-to-one counterpart in the ICD-9-CM codes. A few exceptions are outlined below.
In addition to the core of “N” codes, a handful of codes will come from other chapters to capture reasons for a gynecologic encounter or surgery. For instance, “Z” codes will be reported for encounters for reasons other than illness and include codes for contraceptive and procreative management, general counseling, history of diseases, preventive gynecologic examinations, and screening scenarios, to name just a few. “R” codes will be used most often for general signs and symptoms, such as abdominal pain or nausea and vomiting.
Your documentation will need to change in some important areas
When you see a patient for an injury to the urinary or pelvic organs that is not a complication of a procedure, or for a complication of a genitourinary prosthetic device, implant, or graft, you will need to document whether this is an initial or subsequent encounter or a sequela. This information is added as a 7th alpha character (a = initial, d = subsequent, s = sequela).
ICD-10-CM defines an initial encounter as the time period in which the patient is actively being treated. A subsequent encounter would be reported after the patient’s active treatment, while she is receiving routine care during the healing or recovery phase. For instance, you would report the encounter as subsequent when the patient is seen after her surgery for an injury to the ovary due to an automobile accident, but you would report an initial encounter for all visits through the surgical date of service when a patient presents with symptoms of mesh erosion requiring surgery. Sequela refers to a condition that developed as a result of another condition. For instance, if the patient’s intrauterine device (IUD) becomes embedded in the ostium due to an undetected uterine fibroid, that is a sequela.
The requirement to indicate laterality also will affect documentation, but this concept is limited to a few codes that might be reported by ObGyns. A designation of the right versus left organ will be required for reported cases of primary, secondary, borderline, or benign tumors of the breast, ovary, fallopian tube, broad ligament, and round ligament, as well as cancer in situ of the breast. However, the terms “bilateral” and “unilateral” are applied only to codes that describe hernias, acquired absence of the ovaries, and injuries to the ovaries and fallopian tubes that are not due to a surgical complication.
Unspecified codes still play a role
Unspecified ICD-10-CM codes still come into play when the clinician does not have enough information to assign a more specific code—that is, when, by the end of an encounter, no further information is available to assign a more specific diagnosis. For example, if a patient has signs of a fibroid upon examination, only the unspecified code can be reported until the clinician can discover whether it is intramural, submucosal, or subserosal. However, it would be equally incorrect to assign an unspecified code to an encounter once the nature of the fibroid has been determined.
Take note of these differences in coding
Here is a list of important new gynecologic coding requirements, which are presented in alphabetical order.
Amenorrhea, oligomenorrhea (N91.0–N91.5) and dysmenorrhea (N94.4–N94.5) will require documentation to indicate whether the condition is primary or secondary. Although an unspecified code is available, once treatment is begun the cause should be known and documented.
Artificial insemination problems will have a section:
- N98.0 Infection associated with artificial insemination
- N98.1 Hyperstimulation of ovaries
- N98.2 Complications of attempted introduction of fertilized ovum following in vitro fertilization
- N98.3 Complications of attempted introduction of embryo in embryo transfer
- N98.8 Other complications associated with artificial fertilization
- N98.9 Complication associated with artificial fertilization, unspecified.
Breast cancer codes will require documentation of which breast and what part of the breast is affected.
Contraceptive management highlights:
- Injectable contraceptives will have new codes for the initial prescription (Z30.013) and subsequent surveillance (Z30.42)
- IUD encounter for the prescription will have a new code (Z30.014), which is reported when the IUD is not being inserted on the same day
- Subdermal contraceptive implant surveillance will no longer have a specific code but will be included in the “other” contraceptive code Z30.49.
Conversion of a laparoscopic procedure to an open procedure will not have a code.
Cystocele, unspecified, will have code N81.10.
Dysplasia of vagina will be expanded into 3 codes based on mild, moderate, or unspecified: N89.0–N89.3.
Female genitourinary cancer codes:
- Documentation of right or left organs and which part of the uterus is affected will be required
- Cancer in situ of cervix will be expanded by site on the cervix: D06.0–D06.7
- Cancer in situ of the endometrium will have a specific code: D07.0.
Genuine stress urinary incontinence will only be referred to as stress incontinence (male or female). The code is now located in the urinary section of Chapter N: N39.3.
Genitourinary complications due to procedures and surgery will be organized in 1 section: N99
- Some conditions have more than 1 code based on cause:
- N99.2 Stricture of vagina due to surgical complication
- N89.5 Stricture of vagina not due to surgical complication
- N99.4 Pelvic adhesions due to surgical complication
- N73.6 Pelvic adhesions not due to surgical complication - Other codes will differentiate between intraoperative or postprocedure complications and whether the surgery is on the genitourinary system or a different surgery:
- N99.61 Intraoperative hemorrhage and hematoma of a genitourinary system organ or structure complicating a genitourinary system procedure
- N99.62 Intraoperative hemorrhage and hematoma of a genitourinary system organ or structure complicating other procedure
- N99.820 Postprocedural hemorrhage and hematoma of a genitourinary system organ or structure following a genitourinary system procedure
- N99.821 Postprocedural hemorrhage and hematoma of a genitourinary system organ or structure following other procedure.
Gynecologic examinations will have to include information on whether or not there were genitourinary abnormal findings on the exam. If so, an additional secondary code will be required to identify the abnormality: Z01.411 and finding code. (Without abnormal findings: Z01.419.) For instance, a diagnosis of bacterial vaginosis is made during the examination. The abnormal findings are not those from other areas such as the breast or thyroid.
Hematuria documentation must differentiate between gross: R31.0, benign essential:R31.1, or other forms: R31.2.
High-risk sexual behavior problems must be documented by heterosexual, bisexual, or homosexual behavior: Z72.51–Z72.53.
Hormonal contraceptives, long-term use, will have a specific code: Z79.3.
Hyperplasia without atypia (simple, complex, or benign) will be rolled into a single code: N85.01.
Immunizations, prophylactic, will not have specific codes as to type. An encounter for any type of immunization is Z23.
Pelvic pain will have its own symptom code: R10.2.
Personal history for cancer has been expanded:
- Personal history of cancer in situ:
- Z86.000 of breast
- Z86.001 of cervix uteri
- Z86.008 of other site - Personal history of benign neoplasm:
- Z86.012 of other benign neoplasm
- Z86.03 of uncertain behavior (borderline malignancies).
Procedures not carried out will be expanded in ICD-10 to include 2 new codes:
- Z53.01 Procedure contraindicated due to patient smoking
- Z53.21 Procedure not carried out because patient left before seeing physician.
Procreative management changes:
- Artificial insemination will not have a specific code
- New code for male factor infertility: Z31.81
- New code for Rh incompatibility: Z31.82. This code would be used when the patient presents for prophylactic rho(D) immune globulin in addition to the Z23 code for immunization. This code also would be reported for the patient being tested for isoimmunization with no test result at the time of the visit.
Uterine prolapse without vaginal wall prolapse (618.1) will not have a code replacement.
Vaginal conditions such as vaginal lacerations (old), leukorrhea not specified as infective, and vaginal hematoma will be represented by an “other” code: N89.8.
Vulvar cyst will have its own code: N90.7.
Vulvovaginitis has been expanded into category codes for acute, subacute/chronic conditions of both the vagina and the vulva, which changes the documentation requirements in order to code correctly: N76.0–N76.3.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Reference
1. Witt M. Moving forward with ICD-10: capitalize on this extra time. OBG Manag. 2014;26(7):17, 18, 20.
Reference
1. Witt M. Moving forward with ICD-10: capitalize on this extra time. OBG Manag. 2014;26(7):17, 18, 20.
Do ACOG guidelines protect us from liability?
“THE SGR IS ABOLISHED! WHAT COMES NEXT?”
LUCIA DIVENERE, MA (PRACTICE MANAGEMENT; JUNE 2015)
Do ACOG guidelines protect us from liability?
I read Ms. DiVenere’s June article with interest, but I found this point she quoted confusing: "The law protects physicians from liability from federal or state standards of care. No health care guideline or other standard developed under federal or state requirements associated with this law may be used as a standard of care or duty of care owed by a health care professional to a patient in a medical liability lawsuit."
I have 2 questions: How do you interpret the use of guidelines by the American College of Obstetricians and Gynecologists (ACOG), since they are developed independently by a specialty society rather than by federal or state “requirements”? Does this only pertain to liability lawsuits concerning billing of fees, or does it pertain to medical malpractice civil lawsuits?
In the Medicare Access and CHIP Reauthorization Act, I find this section that seems to contradict the protection1:
What is the bottom line? No law can protect and provide immunity to a physician for true medical malpractice. This federal law says “no preemption.”
Arnold D. Wharton, MD
Tyler, Texas
Reference
1. Pub L No. 114–10. Medicare Access and CHIP Reauthorization Act of 2015. 114th Congress. Title 1—SGR repeal and Medicare Provider Payment Modernization. §106. Reducing administrative burden and other provisions. 129 STAT.143. http://www.gpo.gov/fdsys/pkg/PLAW-114publ10/pdf/PLAW-114publ10.pdf. Accessed June 10, 2015.
Ms. DiVenere responds
I thank Dr. Wharton for his interesting perspective. To answer the first questions, this section of the law only applies to guidelines and standards created by a federal or state entity, not to ACOG guidelines, and is intended to provide one area of protection from medical malpractice lawsuits. Interestingly, legislation has been introduced in the US House by Congressman Andy Barr (R-KY), with ACOG’s support, to create liability safe harbors for physicians who follow care guidelines developed by their relevant specialty society.
As for the question about preemption, this section of the law allows stronger state laws to stand; this federal law would not preempt state laws.
Statute of limitations still in effect; contact your insurer
While the end result to dismiss the patient was achieved, the statute of limitations for a possible malpractice suit had not fully run. I would suggest that the physician contact his/her insurer so that they can open a file and be alerted for a possible suit. Insurers generally require physicians to notify them of any potential suits.
Lynn Frame, MD, JD
Tulsa, Oklahoma
Dr. Sanfilippo and Mr. Smith respond
Our thanks to Dr. Frame for the good reminder that physicians should always remember the obligation to inform malpractice insurance carriers when a malpractice claim is being, or may be, filed. Insurance contracts vary somewhat regarding when notice must be given.
In the hypothetical case, there was an angry patient but no formal threat of legal action. Some lawyers take the sensible position that “when in doubt, notify.” Others are reluctant to “over notify” carriers. Our view is that this is one of the areas in which it may be beneficial for a physician to have an ongoing professional relationship with an attorney to allow for advice on when to provide insurance carrier notification.
Videos show very useful techniques for malpositioned IUDs
I have placed somewhere in the ballpark of 2,000 intrauterine devices (IUDs) and have had 2 perforations that I am aware of (and probably many more malpositioned IUDs that I am unaware of). Some of those were likely the cause of a patient’s pain and were either removed or hysteroscopically repositioned. Dr. Advincula’s edited video from several cases demonstrates very useful techniques in the surgical management of these problems.
Philip Ivey, MD
Casa Grande, Arizona
The IUD might not stay where I put it
For the past several years I have performed the majority (more than 95%) of IUD insertions with ultrasound guidance and have been very thankful at times for the assistance of my sonographer. Despite my knowledge of accurate placement, there are still patients who return months or years later with a malpositioned IUD. I have come to realize that the uterus is a dynamic organ—not a piece of concrete. Just because I put the IUD in the right place does not ensure that it will stay there. Fortunately, I have not yet had a perforation into the abdominal cavity.
I really enjoyed the videos and advice, as always!
Elizabeth Street, MD
Marietta, Georgia
Improved care for pregnant women during Ebola crisis
The article on Ebola in pregnancy noted how little we actually know about the Ebola virus. The Ebola virus was first documented in 1976 in Sudan and the Democratic Republic of the Congo,1 not in 1967 as the article stated. The Marburg virus outbreak occurred in 1967. Closely related, both viruses are filo viruses that cause hemorrhagic fever. A significant difference between the 2 is that the natural reservoir for the Marburg virus was identified. The outbreak in Marburg, Germany, which the virus is named for, was linked to African green monkeys imported from Uganda, East Africa.2 Bats also have been identified as a reservoir for the Marburg virus.3 However, there is only speculation as to whether the natural reservoir for the Ebola virus is fruit bats. A 3-month research study following the 1995 outbreak of Ebola virus in Kikwit, Democratic Republic of the Congo, tested more than 3,000 vertebrate species and was still unable to identify a natural carrier for the virus.4
The Ebola virus was first documented nearly 40 years ago and yet we know so little about it. This demonstrates the ongoing disparity in funding and research devoted to disease conditions that most often affect only third-world nations.
Also, I’d like to point out that the article’s comment that pregnant patients are triaged “last” during the current Ebola virus outbreak may not be completely accurate. Yes, pregnant women have a significantly higher rate of mortality with Ebola viral infection. I spoke with a nurse (name and location withheld for confidentiality) who is currently the Clinical Lead at an Ebola Holding Unit for pregnant and lactating women in a West African nation. According to her, improved resources were quickly mobilized by nongovernment organizations and other foreign health care volunteers following the initial reports of disease, a factor that significantly increased access to care for pregnant women and improved outcomes. Erin Kiser, DNP, FNP-BC, WHNP-BC
Fayetteville, North Carolina
References
1. World Health Organization. Ebola virus disease. Fact sheet No. 103. http://www.who.int/mediacentre/factsheets/fs103/en/. Updated April 2015. Accessed July 6, 2015.
2. World Health Organization. Marburg haemorrhagic fever. Fact sheet. http://www.who.int/mediacentre/factsheets/fs_marburg/en/. Published November 2012. Accessed July 8, 2015.
3. Towner JS, Pourrut X, Albariño CG, et al. Marburg virus infection detected in a common African bat. PLoS One. 2007;2(8):e764. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0000764.
4. Leirs H, Mills JN, Krebs JW, et al. Search for the Ebola virus reservoir in Kikwit, Democratic Republic of the Congo: Reflections on a vertebrate collection. J Infect Dis. 1999;179(suppl 1):S155–S163.
Had the chance to change my specialty, but didn’t
I trained in Mexico, where I was a board certified ObGyn and a maternal-fetal medicine specialist. When I came to the United States I had the opportunity to change my specialty, and I didn’t. As a “free agent” international medical graduate, I had to go through many hurdles. My gate to enter the American medical world was through a family practice residency. After a year, I realized my love was still obstetrics and gynecology. In 1996, I finished an ObGyn residency at Loma Linda University Medical Center in California, and have been board certified since 1998.
There are many things I like about this specialty. Mainly, it’s the diversity. A well-rounded ObGyn has to know internal medicine, pediatrics, and surgery and apply this knowledge to the pregnant patient—a feat somehow exclusive to ObGyns.
I have enjoyed a wonderful career and many rewards. I never stop thanking all those professors and colleagues who helped me develop the set of skills that I now possess.
Tomas A. Hernandez, MD
Pasco, Washington
Not again!
I would not go into obstetrics and gynecology again because of many reasons:
- It is a very difficult life, with no family time and calls 24 hours per day.
- The specialty is the bread and butter of malpractice attorneys, causing a lot of stress.
- Insurance companies, health maintenance organizations (HMOs), etc, pay ridiculously low reimbursement for obstetric and gynecologic procedures.
- Malpractice insurance premiums are so high that you can be forced to be without malpractice and therefore more exposed.
- Patients are extremely demanding. Because pregnancy is not a disease but a natural process, they expect perfect results every time (as if congenital malformations, chromosomal abnormalities, and pregnancy complications are your fault).
- There is no patient loyalty, or very little. If a patient changes HMOs she changes obstetricians. If a woman has to wait 20 minutes in the waiting room, she changes doctors—to one who doesn’t do obstetrics (too many pregnant women!).
I would like to say that ObGyn is a beautiful specialty, most likely the best of all medical specialties, if it was not for the attorneys’ greed and patients’ lack of understanding that we are not God. We are only doctors, working within a system that contributes to all of the above.
Manuel S. Mendizabal, MD
Miami, Florida
Are men discouraged from entering the ObGyn field?
Dr. Barbieri asks, “Why is obstetrics and gynecology a popular choice for medical students?” The unaddressed question is why is it unpopular for half of medical students? Ninety-three percent of resident graduates in the field are women, while women account for half of medical student graduates. Men rarely go into the specialty today. Perhaps job advertisements touting physician opportunities in “all female groups” discourage males. Perhaps hospitals’ “women’s health centers,” with “women taking care of women,” discourage males. Perhaps receptionists’ asking patients whether they prefer a male or female physician discourages male ObGyns. In the United States, two-thirds of outpatient office visits are made by women, and academic centers and hospitals focus on this demographic in their marketing. The business ends justify the unethical means.
The result of discouraging half your medical students from the field is a lower quality field. If male and female medical students are equally qualified for any field, and I believe this is true, then discouraging half the candidates from a field lowers the quality of the resulting field. This has been the product of all discrimination throughout the ages.
Joe Walsh, MD
Philadelphia, Pennsylvania
Dr. Barbieri responds
Drs. Hernandez and Mendizabal provide 2 divergent perspectives on our field. Dr. Hernandez cherishes the diversity of the clinical work in the field, and Dr. Mendizabal warns that night call and medical malpractice take a toll on a physician. Both perspectives are valid and important, and medical students entering the field should be alerted to these rewards and challenges.
I agree with Dr. Walsh that the majority of residents in obstetrics and gynecology are women. On December 31, 2013, of the 4,942 residents in obstetrics and gynecology in the United States, 82.5% were women.1 In the fields of orthopedic surgery, neurosurgery, and urology, male residents dominate the resident complement, constituting 86.3%, 84.1%, and 77.3% of the residents, respectively.1 It is interesting that the fields of obstetrics and gynecology, orthopedic surgery, neurosurgery, and urology are among the most competitive fields in the resident match. Based on personal observation, medical student clerkship directors and obstetrics and gynecology residency programs encourage both women and men to consider a career in obstetrics and gynecology and warmly welcome male applicants. Medical students select their preferred future specialty based on many factors. It is clear that in the past few years the medical students applying to obstetrics and gynecology are extremely capable, and I am confident that the future of women’s health is in the hands ofexcellent clinicians.
Reference
1. Brotherton SE, Etzel SI. Graduate medical education, 2013–2014. JAMA. 2014;312(22):2427–2445.
Who will teach this dying art to a new generation?
The article on rotational forceps has what I consider one glaring defect—who will teach this dying art to a new generation?
Now retired, I was military-residency trained in the 1970s when you had to do your own regional and conduction anesthesia as well as operative forceps delivery—and that did not mean a silastic cup vacuum extractor, though we had just started using the Malstrom vacuum. Breech forceps, Kielland rotations, occipito-transverse forceps application—you name it and we did it as we had to keep our cesarean delivery rate down. All of us were well skilled in operative vaginal delivery.
When I stopped practicing obstetrics, the fresh-out-of-residency people coming into our practice couldn’t do a low forceps delivery. If there is to be a reteaching of rotational forceps, they’d better catch us old codgers fast before we die off (I am 72) and grant us malpractice relief (I no longer have insurance). This is an art, not a science, and can’t be taught from a book or a computer model. Set up a crash course to teach this dying art, pay us well, and perhaps we will be able to pass this skill along. Otherwise it will be gone forever.
I have always said that forceps are like a shoehorn—used correctly, they make things so much easier.
Robert Frischer, MD
Wichita Falls, Texas
Why I now recommend 3D ultrasonography to my high-risk patients
In 2012, I attended a medical staff meeting where Dr. Ruby Chang spoke about a newly available modality at our hospital: 3D ultrasonography. Her slideshow included some impressive images of cancers that were not seen on mammogram but were unmistakable on sonography.
I decided to have a 3D ultrasound for myself in order to tell my patients what it was like. I also have “heterogeneously dense breasts” on mammogram. For the previous 10 years, my annual screening mammograms had all been negative. The 3D ultrasound showed an 8-mm cancer in my left breast—not palpable to me. A subsequent mammogram was still negative for cancer.
Luckily, the breast cancer was Stage 1 at surgery, and I did not need chemotherapy or radiation, opting for skin- and nipple-sparing double mastectomy. I had a double mastectomy because I believed that I could no longer trust screening mammography for a timely diagnosis.
To this day, I explain breast density to all of my higher-risk patients who have either heterogeneously or extremely dense breasts. I tell them that their mammograms may miss a cancer and that there is another test that might help detect cancer early. It’s a good thing to have another way to evaluate the breast, especially when our patients are being sent letters about their “dense breasts.” (The majority of my patients do not understand what this means.)
I realize that data may show that this modality isn’t the perfect solution and may lead to more testing and procedures, but in my case, it was worth it!
Strangely, to this day, I have not had one patient who had breast cancer diagnosed in this way.
It’s a shame that insurance companies don’t cover even partial cost for eligible patients.
Bettina Zatuchni, MD
Pleasanton, California
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
“THE SGR IS ABOLISHED! WHAT COMES NEXT?”
LUCIA DIVENERE, MA (PRACTICE MANAGEMENT; JUNE 2015)
Do ACOG guidelines protect us from liability?
I read Ms. DiVenere’s June article with interest, but I found this point she quoted confusing: "The law protects physicians from liability from federal or state standards of care. No health care guideline or other standard developed under federal or state requirements associated with this law may be used as a standard of care or duty of care owed by a health care professional to a patient in a medical liability lawsuit."
I have 2 questions: How do you interpret the use of guidelines by the American College of Obstetricians and Gynecologists (ACOG), since they are developed independently by a specialty society rather than by federal or state “requirements”? Does this only pertain to liability lawsuits concerning billing of fees, or does it pertain to medical malpractice civil lawsuits?
In the Medicare Access and CHIP Reauthorization Act, I find this section that seems to contradict the protection1:
What is the bottom line? No law can protect and provide immunity to a physician for true medical malpractice. This federal law says “no preemption.”
Arnold D. Wharton, MD
Tyler, Texas
Reference
1. Pub L No. 114–10. Medicare Access and CHIP Reauthorization Act of 2015. 114th Congress. Title 1—SGR repeal and Medicare Provider Payment Modernization. §106. Reducing administrative burden and other provisions. 129 STAT.143. http://www.gpo.gov/fdsys/pkg/PLAW-114publ10/pdf/PLAW-114publ10.pdf. Accessed June 10, 2015.
Ms. DiVenere responds
I thank Dr. Wharton for his interesting perspective. To answer the first questions, this section of the law only applies to guidelines and standards created by a federal or state entity, not to ACOG guidelines, and is intended to provide one area of protection from medical malpractice lawsuits. Interestingly, legislation has been introduced in the US House by Congressman Andy Barr (R-KY), with ACOG’s support, to create liability safe harbors for physicians who follow care guidelines developed by their relevant specialty society.
As for the question about preemption, this section of the law allows stronger state laws to stand; this federal law would not preempt state laws.
Statute of limitations still in effect; contact your insurer
While the end result to dismiss the patient was achieved, the statute of limitations for a possible malpractice suit had not fully run. I would suggest that the physician contact his/her insurer so that they can open a file and be alerted for a possible suit. Insurers generally require physicians to notify them of any potential suits.
Lynn Frame, MD, JD
Tulsa, Oklahoma
Dr. Sanfilippo and Mr. Smith respond
Our thanks to Dr. Frame for the good reminder that physicians should always remember the obligation to inform malpractice insurance carriers when a malpractice claim is being, or may be, filed. Insurance contracts vary somewhat regarding when notice must be given.
In the hypothetical case, there was an angry patient but no formal threat of legal action. Some lawyers take the sensible position that “when in doubt, notify.” Others are reluctant to “over notify” carriers. Our view is that this is one of the areas in which it may be beneficial for a physician to have an ongoing professional relationship with an attorney to allow for advice on when to provide insurance carrier notification.
Videos show very useful techniques for malpositioned IUDs
I have placed somewhere in the ballpark of 2,000 intrauterine devices (IUDs) and have had 2 perforations that I am aware of (and probably many more malpositioned IUDs that I am unaware of). Some of those were likely the cause of a patient’s pain and were either removed or hysteroscopically repositioned. Dr. Advincula’s edited video from several cases demonstrates very useful techniques in the surgical management of these problems.
Philip Ivey, MD
Casa Grande, Arizona
The IUD might not stay where I put it
For the past several years I have performed the majority (more than 95%) of IUD insertions with ultrasound guidance and have been very thankful at times for the assistance of my sonographer. Despite my knowledge of accurate placement, there are still patients who return months or years later with a malpositioned IUD. I have come to realize that the uterus is a dynamic organ—not a piece of concrete. Just because I put the IUD in the right place does not ensure that it will stay there. Fortunately, I have not yet had a perforation into the abdominal cavity.
I really enjoyed the videos and advice, as always!
Elizabeth Street, MD
Marietta, Georgia
Improved care for pregnant women during Ebola crisis
The article on Ebola in pregnancy noted how little we actually know about the Ebola virus. The Ebola virus was first documented in 1976 in Sudan and the Democratic Republic of the Congo,1 not in 1967 as the article stated. The Marburg virus outbreak occurred in 1967. Closely related, both viruses are filo viruses that cause hemorrhagic fever. A significant difference between the 2 is that the natural reservoir for the Marburg virus was identified. The outbreak in Marburg, Germany, which the virus is named for, was linked to African green monkeys imported from Uganda, East Africa.2 Bats also have been identified as a reservoir for the Marburg virus.3 However, there is only speculation as to whether the natural reservoir for the Ebola virus is fruit bats. A 3-month research study following the 1995 outbreak of Ebola virus in Kikwit, Democratic Republic of the Congo, tested more than 3,000 vertebrate species and was still unable to identify a natural carrier for the virus.4
The Ebola virus was first documented nearly 40 years ago and yet we know so little about it. This demonstrates the ongoing disparity in funding and research devoted to disease conditions that most often affect only third-world nations.
Also, I’d like to point out that the article’s comment that pregnant patients are triaged “last” during the current Ebola virus outbreak may not be completely accurate. Yes, pregnant women have a significantly higher rate of mortality with Ebola viral infection. I spoke with a nurse (name and location withheld for confidentiality) who is currently the Clinical Lead at an Ebola Holding Unit for pregnant and lactating women in a West African nation. According to her, improved resources were quickly mobilized by nongovernment organizations and other foreign health care volunteers following the initial reports of disease, a factor that significantly increased access to care for pregnant women and improved outcomes. Erin Kiser, DNP, FNP-BC, WHNP-BC
Fayetteville, North Carolina
References
1. World Health Organization. Ebola virus disease. Fact sheet No. 103. http://www.who.int/mediacentre/factsheets/fs103/en/. Updated April 2015. Accessed July 6, 2015.
2. World Health Organization. Marburg haemorrhagic fever. Fact sheet. http://www.who.int/mediacentre/factsheets/fs_marburg/en/. Published November 2012. Accessed July 8, 2015.
3. Towner JS, Pourrut X, Albariño CG, et al. Marburg virus infection detected in a common African bat. PLoS One. 2007;2(8):e764. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0000764.
4. Leirs H, Mills JN, Krebs JW, et al. Search for the Ebola virus reservoir in Kikwit, Democratic Republic of the Congo: Reflections on a vertebrate collection. J Infect Dis. 1999;179(suppl 1):S155–S163.
Had the chance to change my specialty, but didn’t
I trained in Mexico, where I was a board certified ObGyn and a maternal-fetal medicine specialist. When I came to the United States I had the opportunity to change my specialty, and I didn’t. As a “free agent” international medical graduate, I had to go through many hurdles. My gate to enter the American medical world was through a family practice residency. After a year, I realized my love was still obstetrics and gynecology. In 1996, I finished an ObGyn residency at Loma Linda University Medical Center in California, and have been board certified since 1998.
There are many things I like about this specialty. Mainly, it’s the diversity. A well-rounded ObGyn has to know internal medicine, pediatrics, and surgery and apply this knowledge to the pregnant patient—a feat somehow exclusive to ObGyns.
I have enjoyed a wonderful career and many rewards. I never stop thanking all those professors and colleagues who helped me develop the set of skills that I now possess.
Tomas A. Hernandez, MD
Pasco, Washington
Not again!
I would not go into obstetrics and gynecology again because of many reasons:
- It is a very difficult life, with no family time and calls 24 hours per day.
- The specialty is the bread and butter of malpractice attorneys, causing a lot of stress.
- Insurance companies, health maintenance organizations (HMOs), etc, pay ridiculously low reimbursement for obstetric and gynecologic procedures.
- Malpractice insurance premiums are so high that you can be forced to be without malpractice and therefore more exposed.
- Patients are extremely demanding. Because pregnancy is not a disease but a natural process, they expect perfect results every time (as if congenital malformations, chromosomal abnormalities, and pregnancy complications are your fault).
- There is no patient loyalty, or very little. If a patient changes HMOs she changes obstetricians. If a woman has to wait 20 minutes in the waiting room, she changes doctors—to one who doesn’t do obstetrics (too many pregnant women!).
I would like to say that ObGyn is a beautiful specialty, most likely the best of all medical specialties, if it was not for the attorneys’ greed and patients’ lack of understanding that we are not God. We are only doctors, working within a system that contributes to all of the above.
Manuel S. Mendizabal, MD
Miami, Florida
Are men discouraged from entering the ObGyn field?
Dr. Barbieri asks, “Why is obstetrics and gynecology a popular choice for medical students?” The unaddressed question is why is it unpopular for half of medical students? Ninety-three percent of resident graduates in the field are women, while women account for half of medical student graduates. Men rarely go into the specialty today. Perhaps job advertisements touting physician opportunities in “all female groups” discourage males. Perhaps hospitals’ “women’s health centers,” with “women taking care of women,” discourage males. Perhaps receptionists’ asking patients whether they prefer a male or female physician discourages male ObGyns. In the United States, two-thirds of outpatient office visits are made by women, and academic centers and hospitals focus on this demographic in their marketing. The business ends justify the unethical means.
The result of discouraging half your medical students from the field is a lower quality field. If male and female medical students are equally qualified for any field, and I believe this is true, then discouraging half the candidates from a field lowers the quality of the resulting field. This has been the product of all discrimination throughout the ages.
Joe Walsh, MD
Philadelphia, Pennsylvania
Dr. Barbieri responds
Drs. Hernandez and Mendizabal provide 2 divergent perspectives on our field. Dr. Hernandez cherishes the diversity of the clinical work in the field, and Dr. Mendizabal warns that night call and medical malpractice take a toll on a physician. Both perspectives are valid and important, and medical students entering the field should be alerted to these rewards and challenges.
I agree with Dr. Walsh that the majority of residents in obstetrics and gynecology are women. On December 31, 2013, of the 4,942 residents in obstetrics and gynecology in the United States, 82.5% were women.1 In the fields of orthopedic surgery, neurosurgery, and urology, male residents dominate the resident complement, constituting 86.3%, 84.1%, and 77.3% of the residents, respectively.1 It is interesting that the fields of obstetrics and gynecology, orthopedic surgery, neurosurgery, and urology are among the most competitive fields in the resident match. Based on personal observation, medical student clerkship directors and obstetrics and gynecology residency programs encourage both women and men to consider a career in obstetrics and gynecology and warmly welcome male applicants. Medical students select their preferred future specialty based on many factors. It is clear that in the past few years the medical students applying to obstetrics and gynecology are extremely capable, and I am confident that the future of women’s health is in the hands ofexcellent clinicians.
Reference
1. Brotherton SE, Etzel SI. Graduate medical education, 2013–2014. JAMA. 2014;312(22):2427–2445.
Who will teach this dying art to a new generation?
The article on rotational forceps has what I consider one glaring defect—who will teach this dying art to a new generation?
Now retired, I was military-residency trained in the 1970s when you had to do your own regional and conduction anesthesia as well as operative forceps delivery—and that did not mean a silastic cup vacuum extractor, though we had just started using the Malstrom vacuum. Breech forceps, Kielland rotations, occipito-transverse forceps application—you name it and we did it as we had to keep our cesarean delivery rate down. All of us were well skilled in operative vaginal delivery.
When I stopped practicing obstetrics, the fresh-out-of-residency people coming into our practice couldn’t do a low forceps delivery. If there is to be a reteaching of rotational forceps, they’d better catch us old codgers fast before we die off (I am 72) and grant us malpractice relief (I no longer have insurance). This is an art, not a science, and can’t be taught from a book or a computer model. Set up a crash course to teach this dying art, pay us well, and perhaps we will be able to pass this skill along. Otherwise it will be gone forever.
I have always said that forceps are like a shoehorn—used correctly, they make things so much easier.
Robert Frischer, MD
Wichita Falls, Texas
Why I now recommend 3D ultrasonography to my high-risk patients
In 2012, I attended a medical staff meeting where Dr. Ruby Chang spoke about a newly available modality at our hospital: 3D ultrasonography. Her slideshow included some impressive images of cancers that were not seen on mammogram but were unmistakable on sonography.
I decided to have a 3D ultrasound for myself in order to tell my patients what it was like. I also have “heterogeneously dense breasts” on mammogram. For the previous 10 years, my annual screening mammograms had all been negative. The 3D ultrasound showed an 8-mm cancer in my left breast—not palpable to me. A subsequent mammogram was still negative for cancer.
Luckily, the breast cancer was Stage 1 at surgery, and I did not need chemotherapy or radiation, opting for skin- and nipple-sparing double mastectomy. I had a double mastectomy because I believed that I could no longer trust screening mammography for a timely diagnosis.
To this day, I explain breast density to all of my higher-risk patients who have either heterogeneously or extremely dense breasts. I tell them that their mammograms may miss a cancer and that there is another test that might help detect cancer early. It’s a good thing to have another way to evaluate the breast, especially when our patients are being sent letters about their “dense breasts.” (The majority of my patients do not understand what this means.)
I realize that data may show that this modality isn’t the perfect solution and may lead to more testing and procedures, but in my case, it was worth it!
Strangely, to this day, I have not had one patient who had breast cancer diagnosed in this way.
It’s a shame that insurance companies don’t cover even partial cost for eligible patients.
Bettina Zatuchni, MD
Pleasanton, California
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
“THE SGR IS ABOLISHED! WHAT COMES NEXT?”
LUCIA DIVENERE, MA (PRACTICE MANAGEMENT; JUNE 2015)
Do ACOG guidelines protect us from liability?
I read Ms. DiVenere’s June article with interest, but I found this point she quoted confusing: "The law protects physicians from liability from federal or state standards of care. No health care guideline or other standard developed under federal or state requirements associated with this law may be used as a standard of care or duty of care owed by a health care professional to a patient in a medical liability lawsuit."
I have 2 questions: How do you interpret the use of guidelines by the American College of Obstetricians and Gynecologists (ACOG), since they are developed independently by a specialty society rather than by federal or state “requirements”? Does this only pertain to liability lawsuits concerning billing of fees, or does it pertain to medical malpractice civil lawsuits?
In the Medicare Access and CHIP Reauthorization Act, I find this section that seems to contradict the protection1:
What is the bottom line? No law can protect and provide immunity to a physician for true medical malpractice. This federal law says “no preemption.”
Arnold D. Wharton, MD
Tyler, Texas
Reference
1. Pub L No. 114–10. Medicare Access and CHIP Reauthorization Act of 2015. 114th Congress. Title 1—SGR repeal and Medicare Provider Payment Modernization. §106. Reducing administrative burden and other provisions. 129 STAT.143. http://www.gpo.gov/fdsys/pkg/PLAW-114publ10/pdf/PLAW-114publ10.pdf. Accessed June 10, 2015.
Ms. DiVenere responds
I thank Dr. Wharton for his interesting perspective. To answer the first questions, this section of the law only applies to guidelines and standards created by a federal or state entity, not to ACOG guidelines, and is intended to provide one area of protection from medical malpractice lawsuits. Interestingly, legislation has been introduced in the US House by Congressman Andy Barr (R-KY), with ACOG’s support, to create liability safe harbors for physicians who follow care guidelines developed by their relevant specialty society.
As for the question about preemption, this section of the law allows stronger state laws to stand; this federal law would not preempt state laws.
Statute of limitations still in effect; contact your insurer
While the end result to dismiss the patient was achieved, the statute of limitations for a possible malpractice suit had not fully run. I would suggest that the physician contact his/her insurer so that they can open a file and be alerted for a possible suit. Insurers generally require physicians to notify them of any potential suits.
Lynn Frame, MD, JD
Tulsa, Oklahoma
Dr. Sanfilippo and Mr. Smith respond
Our thanks to Dr. Frame for the good reminder that physicians should always remember the obligation to inform malpractice insurance carriers when a malpractice claim is being, or may be, filed. Insurance contracts vary somewhat regarding when notice must be given.
In the hypothetical case, there was an angry patient but no formal threat of legal action. Some lawyers take the sensible position that “when in doubt, notify.” Others are reluctant to “over notify” carriers. Our view is that this is one of the areas in which it may be beneficial for a physician to have an ongoing professional relationship with an attorney to allow for advice on when to provide insurance carrier notification.
Videos show very useful techniques for malpositioned IUDs
I have placed somewhere in the ballpark of 2,000 intrauterine devices (IUDs) and have had 2 perforations that I am aware of (and probably many more malpositioned IUDs that I am unaware of). Some of those were likely the cause of a patient’s pain and were either removed or hysteroscopically repositioned. Dr. Advincula’s edited video from several cases demonstrates very useful techniques in the surgical management of these problems.
Philip Ivey, MD
Casa Grande, Arizona
The IUD might not stay where I put it
For the past several years I have performed the majority (more than 95%) of IUD insertions with ultrasound guidance and have been very thankful at times for the assistance of my sonographer. Despite my knowledge of accurate placement, there are still patients who return months or years later with a malpositioned IUD. I have come to realize that the uterus is a dynamic organ—not a piece of concrete. Just because I put the IUD in the right place does not ensure that it will stay there. Fortunately, I have not yet had a perforation into the abdominal cavity.
I really enjoyed the videos and advice, as always!
Elizabeth Street, MD
Marietta, Georgia
Improved care for pregnant women during Ebola crisis
The article on Ebola in pregnancy noted how little we actually know about the Ebola virus. The Ebola virus was first documented in 1976 in Sudan and the Democratic Republic of the Congo,1 not in 1967 as the article stated. The Marburg virus outbreak occurred in 1967. Closely related, both viruses are filo viruses that cause hemorrhagic fever. A significant difference between the 2 is that the natural reservoir for the Marburg virus was identified. The outbreak in Marburg, Germany, which the virus is named for, was linked to African green monkeys imported from Uganda, East Africa.2 Bats also have been identified as a reservoir for the Marburg virus.3 However, there is only speculation as to whether the natural reservoir for the Ebola virus is fruit bats. A 3-month research study following the 1995 outbreak of Ebola virus in Kikwit, Democratic Republic of the Congo, tested more than 3,000 vertebrate species and was still unable to identify a natural carrier for the virus.4
The Ebola virus was first documented nearly 40 years ago and yet we know so little about it. This demonstrates the ongoing disparity in funding and research devoted to disease conditions that most often affect only third-world nations.
Also, I’d like to point out that the article’s comment that pregnant patients are triaged “last” during the current Ebola virus outbreak may not be completely accurate. Yes, pregnant women have a significantly higher rate of mortality with Ebola viral infection. I spoke with a nurse (name and location withheld for confidentiality) who is currently the Clinical Lead at an Ebola Holding Unit for pregnant and lactating women in a West African nation. According to her, improved resources were quickly mobilized by nongovernment organizations and other foreign health care volunteers following the initial reports of disease, a factor that significantly increased access to care for pregnant women and improved outcomes. Erin Kiser, DNP, FNP-BC, WHNP-BC
Fayetteville, North Carolina
References
1. World Health Organization. Ebola virus disease. Fact sheet No. 103. http://www.who.int/mediacentre/factsheets/fs103/en/. Updated April 2015. Accessed July 6, 2015.
2. World Health Organization. Marburg haemorrhagic fever. Fact sheet. http://www.who.int/mediacentre/factsheets/fs_marburg/en/. Published November 2012. Accessed July 8, 2015.
3. Towner JS, Pourrut X, Albariño CG, et al. Marburg virus infection detected in a common African bat. PLoS One. 2007;2(8):e764. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0000764.
4. Leirs H, Mills JN, Krebs JW, et al. Search for the Ebola virus reservoir in Kikwit, Democratic Republic of the Congo: Reflections on a vertebrate collection. J Infect Dis. 1999;179(suppl 1):S155–S163.
Had the chance to change my specialty, but didn’t
I trained in Mexico, where I was a board certified ObGyn and a maternal-fetal medicine specialist. When I came to the United States I had the opportunity to change my specialty, and I didn’t. As a “free agent” international medical graduate, I had to go through many hurdles. My gate to enter the American medical world was through a family practice residency. After a year, I realized my love was still obstetrics and gynecology. In 1996, I finished an ObGyn residency at Loma Linda University Medical Center in California, and have been board certified since 1998.
There are many things I like about this specialty. Mainly, it’s the diversity. A well-rounded ObGyn has to know internal medicine, pediatrics, and surgery and apply this knowledge to the pregnant patient—a feat somehow exclusive to ObGyns.
I have enjoyed a wonderful career and many rewards. I never stop thanking all those professors and colleagues who helped me develop the set of skills that I now possess.
Tomas A. Hernandez, MD
Pasco, Washington
Not again!
I would not go into obstetrics and gynecology again because of many reasons:
- It is a very difficult life, with no family time and calls 24 hours per day.
- The specialty is the bread and butter of malpractice attorneys, causing a lot of stress.
- Insurance companies, health maintenance organizations (HMOs), etc, pay ridiculously low reimbursement for obstetric and gynecologic procedures.
- Malpractice insurance premiums are so high that you can be forced to be without malpractice and therefore more exposed.
- Patients are extremely demanding. Because pregnancy is not a disease but a natural process, they expect perfect results every time (as if congenital malformations, chromosomal abnormalities, and pregnancy complications are your fault).
- There is no patient loyalty, or very little. If a patient changes HMOs she changes obstetricians. If a woman has to wait 20 minutes in the waiting room, she changes doctors—to one who doesn’t do obstetrics (too many pregnant women!).
I would like to say that ObGyn is a beautiful specialty, most likely the best of all medical specialties, if it was not for the attorneys’ greed and patients’ lack of understanding that we are not God. We are only doctors, working within a system that contributes to all of the above.
Manuel S. Mendizabal, MD
Miami, Florida
Are men discouraged from entering the ObGyn field?
Dr. Barbieri asks, “Why is obstetrics and gynecology a popular choice for medical students?” The unaddressed question is why is it unpopular for half of medical students? Ninety-three percent of resident graduates in the field are women, while women account for half of medical student graduates. Men rarely go into the specialty today. Perhaps job advertisements touting physician opportunities in “all female groups” discourage males. Perhaps hospitals’ “women’s health centers,” with “women taking care of women,” discourage males. Perhaps receptionists’ asking patients whether they prefer a male or female physician discourages male ObGyns. In the United States, two-thirds of outpatient office visits are made by women, and academic centers and hospitals focus on this demographic in their marketing. The business ends justify the unethical means.
The result of discouraging half your medical students from the field is a lower quality field. If male and female medical students are equally qualified for any field, and I believe this is true, then discouraging half the candidates from a field lowers the quality of the resulting field. This has been the product of all discrimination throughout the ages.
Joe Walsh, MD
Philadelphia, Pennsylvania
Dr. Barbieri responds
Drs. Hernandez and Mendizabal provide 2 divergent perspectives on our field. Dr. Hernandez cherishes the diversity of the clinical work in the field, and Dr. Mendizabal warns that night call and medical malpractice take a toll on a physician. Both perspectives are valid and important, and medical students entering the field should be alerted to these rewards and challenges.
I agree with Dr. Walsh that the majority of residents in obstetrics and gynecology are women. On December 31, 2013, of the 4,942 residents in obstetrics and gynecology in the United States, 82.5% were women.1 In the fields of orthopedic surgery, neurosurgery, and urology, male residents dominate the resident complement, constituting 86.3%, 84.1%, and 77.3% of the residents, respectively.1 It is interesting that the fields of obstetrics and gynecology, orthopedic surgery, neurosurgery, and urology are among the most competitive fields in the resident match. Based on personal observation, medical student clerkship directors and obstetrics and gynecology residency programs encourage both women and men to consider a career in obstetrics and gynecology and warmly welcome male applicants. Medical students select their preferred future specialty based on many factors. It is clear that in the past few years the medical students applying to obstetrics and gynecology are extremely capable, and I am confident that the future of women’s health is in the hands ofexcellent clinicians.
Reference
1. Brotherton SE, Etzel SI. Graduate medical education, 2013–2014. JAMA. 2014;312(22):2427–2445.
Who will teach this dying art to a new generation?
The article on rotational forceps has what I consider one glaring defect—who will teach this dying art to a new generation?
Now retired, I was military-residency trained in the 1970s when you had to do your own regional and conduction anesthesia as well as operative forceps delivery—and that did not mean a silastic cup vacuum extractor, though we had just started using the Malstrom vacuum. Breech forceps, Kielland rotations, occipito-transverse forceps application—you name it and we did it as we had to keep our cesarean delivery rate down. All of us were well skilled in operative vaginal delivery.
When I stopped practicing obstetrics, the fresh-out-of-residency people coming into our practice couldn’t do a low forceps delivery. If there is to be a reteaching of rotational forceps, they’d better catch us old codgers fast before we die off (I am 72) and grant us malpractice relief (I no longer have insurance). This is an art, not a science, and can’t be taught from a book or a computer model. Set up a crash course to teach this dying art, pay us well, and perhaps we will be able to pass this skill along. Otherwise it will be gone forever.
I have always said that forceps are like a shoehorn—used correctly, they make things so much easier.
Robert Frischer, MD
Wichita Falls, Texas
Why I now recommend 3D ultrasonography to my high-risk patients
In 2012, I attended a medical staff meeting where Dr. Ruby Chang spoke about a newly available modality at our hospital: 3D ultrasonography. Her slideshow included some impressive images of cancers that were not seen on mammogram but were unmistakable on sonography.
I decided to have a 3D ultrasound for myself in order to tell my patients what it was like. I also have “heterogeneously dense breasts” on mammogram. For the previous 10 years, my annual screening mammograms had all been negative. The 3D ultrasound showed an 8-mm cancer in my left breast—not palpable to me. A subsequent mammogram was still negative for cancer.
Luckily, the breast cancer was Stage 1 at surgery, and I did not need chemotherapy or radiation, opting for skin- and nipple-sparing double mastectomy. I had a double mastectomy because I believed that I could no longer trust screening mammography for a timely diagnosis.
To this day, I explain breast density to all of my higher-risk patients who have either heterogeneously or extremely dense breasts. I tell them that their mammograms may miss a cancer and that there is another test that might help detect cancer early. It’s a good thing to have another way to evaluate the breast, especially when our patients are being sent letters about their “dense breasts.” (The majority of my patients do not understand what this means.)
I realize that data may show that this modality isn’t the perfect solution and may lead to more testing and procedures, but in my case, it was worth it!
Strangely, to this day, I have not had one patient who had breast cancer diagnosed in this way.
It’s a shame that insurance companies don’t cover even partial cost for eligible patients.
Bettina Zatuchni, MD
Pleasanton, California
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Was the ObGyn’s dexterity compromised?
Was the ObGyn’s dexterity compromised?
A woman underwent a hysterectomy. During surgery, the patient’s bladder was injured; the ObGyn called in a urologist to make the repair.
Patient’s claim The ObGyn failed to inform the patient about the possible complications from hysterectomy. The patient also claimed fraudulent concealment because the ObGyn had suffered a serious injury 3 years earlier that affected his dexterity. At the time of surgery, the ObGyn had a pending lawsuit against the owner of the premises where he fell in which he claimed that he was unable to continue his surgical practice because of the injury. The ObGyn never informed the patient of the extent of his injury or any associated risks related to his injury.
Defendants’ defense The patient was fully informed that bladder injury is a known risk of the procedure. The ObGyn maintained that his injury only affected his ability to stand for many hours while operating. The hospital settled during trial.
Verdict A $12,000 Louisiana settlement was reached with the hospital. Summary judgment was granted to the ObGyn on the informed consent claim. A $30,000 verdict was returned on the fraud count.
Placental abruption: Was child dead?
At 32 weeks’ gestation, a woman was found to have placental abruption. At the hospital, her ObGyn could not find a fetal heartbeat or detectable fetal movement on ultrasonography. A radiologist performed another ultrasound 30 minutes later and detected a fetal heart rate of 47 bpm. An emergency cesarean delivery was performed. Soon after birth, the child had seizures and was found to have hypoxic ischemic encephalopathy and diffuse brain injury. The child is profoundly disabled.
Parents’ claim The ObGyn was negligent for failing to detect the fetal heart rate and in failing to respond properly to placental abruption. Cesarean delivery should have been performed immediately after placental abruption was identified.
Defendant’s defense The case was settled at trial.
Verdict A $13 million Illinois settlement was reached, including $5 million in cash and $8 million placed in trust for the child.
Large fetus, shoulder dystocia: Erb’s palsy
Labor was induced at 39 weeks’ gestation because the fetus was anticipated to be large. During vaginal delivery, shoulder dystocia was encountered. At birth, the baby weighed 9 lb 2 oz. She sustained a brachial plexus injury to the posterior shoulder with permanent nerve root damage and Erb’s palsy. The child continues to have limited use of her left arm and hand even after 3 corrective operations.
Parents’ claim While performing maneuvers to relieve shoulder dystocia, the ObGyn exerted excessive traction on the baby’s head, causing a C-5 nerve root injury and complete avulsion at C-8. A cesarean delivery should have been performed.
Physician’s defense There was no negligence. The nerve injury was caused by the natural forces of labor and the mother’s pushing while the posterior shoulder was wedged behind the mother’s sa-
cral promontory.
Verdict A $1 million Illinois verdict was returned.
Woman dies from toxemia
A 22-year-old woman was seen by her ObGyn 4 days after vaginal delivery. Early the next day, the patient had a seizure at home and was transported by ambulance to the hospital. She could not be resuscitated and died. At autopsy, the cause of death was determined to be toxemia from pregnancy.
Estate’s claim The ObGyn failed to properly diagnose and treat the patient’s hypertension.
Defendant’s defense The case was settled during trial.
Verdict A $775,000 New York settlement was reached.
Blood transfusion delayed for hours: $14.75M net award
After emergency cesarean delivery, the baby was extremely anemic. The physicians determined that a fetal-maternal hemorrhage had started days before, causing the fetus to lose most of her blood.
An hour after birth, the attending neonatologist ordered blood from the hospital’s blood bank and arranged for emergency transport to a neonatal intensive care unit (NICU). Blood transfusion did not occur prior to transport. The child has severe cerebral palsy (CP) and cannot walk or talk at age 8 years.
Parents’ claim The neonatologist ordered cross-matched blood, which, because it is tested for compatibility, takes longer to supply. Universal donor blood could have been delivered in 20 minutes or less because it is readily available. The ambulance from the receiving hospital took an hour to drive 9 miles between the facilities, a trip that should have taken 12 minutes. The ambulance staff did not call ahead to the medical center to have blood ready for the baby. It took 4.5 hours before the newborn received a blood transfusion, a delay that caused severe injury to the child.
Defendants’ defense The matter went to trial against the neonatologist and his employer after the other defendants settled.
Verdict Before trial, an ObGyn and the hospital settled for a combined $750,000, and the county agreed to a $12 million settlement. During trial, a $2 million Illinois settlement was reached.
Pregnant woman has a massive stroke: $10.9M
Pregnant with her third child and at 26 weeks’ gestation, a 35-year-old woman had a massive intracerebral hemorrhage at home.
The day before, she had contacted her ObGyn’s office to report severe headache and abdominal pain. The call was taken by an associate of her ObGyn, who told her there was no need to go to the hospital and suggested that she had a gastrointestinal virus.
The stroke caused severe cognitive impairment, loss of memory, partial vision loss, dysphasia, and partial paralysis on her right side. At trial, she was still undergoing therapy to regain mobility, speech, and memory. She uses a wheelchair.
Patient’s claim The covering ObGyn was negligent for not sending the patient to the hospital when she reported severe headache.
Defendants’ defense The ObGyn and medical practice denied negligence, contending that the patient’s pregnancy was normal and that there was no indication that she was at risk for a stroke.
Verdict A $10,928,188 Ohio verdict was returned.
Was the fetus properly monitored?
One month before her due date, a woman was found to have premature rupture of membranes. She had gestational diabetes controlled by diet. She was admitted for induction of labor.
For more than 12 hours, external fetal monitor heart-rate tracings were reassuring. Then tracings began to show variable decelerations. For a period of 90 minutes, it was impossible to evaluate the fetal heart rate because the monitor was not working. An internal monitor was not placed. Just prior to birth, the tracings showed a 15-minute period of fetal tachycardia with the heart rate at 180 bpm. The physician’s notes indicated that the baby’s head had crowned for a prolonged period of time.
The baby was floppy at birth with Apgar scores of 2, 4, and 6 at 1, 5, and 10 minutes, respectively. The child was resuscitated and transferred to the NICU. She was found to have perinatal asphyxia, severe metabolic acidosis, multiorgan injury, hypoxic ischemic encephalopathy, and seizures. She stayed in the NICU for 1 month. At age 9 years, she has developmental delays and memory problems, but no motor injuries.
Parents’ claim During the 90 minutes in which the fetal heart-rate monitor was not working properly, the fetus was in distress. An emergency cesarean delivery should have been performed when variable decelerations were seen on tracings.
Physician’s defense The lack of motor injury indicates that the injury was not related to birth.
Verdict A $2 million Michigan settlement was reached.
Rectal tear after vacuum extraction
Vacuum extraction was used to deliver a 47-year-old woman’s child. Later, the mother developed a rectovaginal fistula that became inflamed and involved vaginal passage of stool. The patient required 2 operations and still has residual complications.
Patient’s claim The ObGyn should have found and repaired the rectal tear at delivery. Vacuum extraction was used after only 2 pushes. The mother did not consent to the use of the vacuum extractor.
Physician’s defense The ObGyn admitted that he did not specifically remember this delivery. He claimed that there was informed consent and that the rectal injury was small and easy to overlook.
Verdict A $1.02 million New York verdict was returned.
Preeclamptic mother dies after giving birth
A 24-year-old woman developed preeclampsia when under prenatal care at a hospital clinic. At 36 weeks’ gestation, she presented to the clinic with a headache, “seeing spots,” and feeling ill; her blood pressure (BP) was 169/89 mm Hg. She was admitted for induction of labor and treated for preeclampsia with magnesium sulfate. A healthy baby was born 2 days later. The mother continued to have high BP and was prescribed nifedipine.
Her BP was 148/88 mm Hg at discharge. No antihypertensive medications were prescribed. She was given standard postpartum instructions and told to schedule a follow-up appointment in 6 to 8 weeks.
Five days after discharge, she experienced shortness of breath and swelling in her extremities, but did not seek medical attention until the next day, when breathing became labored. When emergency medical services arrived, she was in cardiac arrest. Prolonged resuscitation was required with intubation and artificial respiration. A computed tomog-raphy (CT) scan revealed cerebral edema from prolonged hypoxia. She was transferred to another hospital where a neurologist determined that she had suffered a profound anoxic brain injury. She died 3 days later.
Estate’s claim The hospital staff was negligent for failing to inform the patient of the signs and symptoms of continuing preeclampsia and for not prescribing antihypertensive medication at discharge. Her follow-up appointment should have been scheduled for 1 week.
Defendant’s defense The patient was given oral instructions regarding postpartum preeclampsia. The case was settled during trial.
Verdict A $50,000 North Carolina settlement was reached.
Was delivery properly managed?
When a 16-year-old woman was found to have preeclampsia, she was admitted and labor was induced using oxytocin. An external fetal heart-rate monitor was placed.
Three hours later, her ObGyn took over her care from the attending physician. He saw the patient once in the evening, then left to deliver a baby at another hospital. He maintained telephone contact with labor and delivery nurses, who told him that the mother’s labor was progressing as planned. Early the next morning, the nurse called the ObGyn to report that the mother was fully dilated and ready to deliver. The ObGyn was at the patient’s bedside within 30 minutes. After the mother pushed once, the ObGyn determined that a cesarean delivery was necessary.
After birth, the child suffered seizures in the NICU and was transferred to another facility. With CP and microcephaly, he cannot speak, is incontinent, has motor difficulties, and will require 24-hour care for life.
Parent’s claim Labor was not properly monitored. Oxytocin doses were too large and continued for too long.
Defendants’ defense The mother’s treatment was appropriate and timely. There was no negligence.
Verdict A confidential Kansas settlement was reached with another defendant during the trial. A defense verdict was returned for the ObGyn.
Evidence of CMV on ultrasonography
During her pregnancy in 2012, a woman contracted congenital cytomegalovirus (CMV), although she did not have any symptoms. The child has CP, a hearing deficiency, and other complications caused by the virus.
Parents’ claim The ObGyn failed to identify CMV, despite ultrasound evidence that the virus was affecting the fetus. Studies available at the time of the pregnancy show considerable success in treating the condition in utero with hyperimmune globulin antiviral agents.
Defendant’s defense The case was settled during trial.
Verdict A confidential Idaho settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements, & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Was the ObGyn’s dexterity compromised?
A woman underwent a hysterectomy. During surgery, the patient’s bladder was injured; the ObGyn called in a urologist to make the repair.
Patient’s claim The ObGyn failed to inform the patient about the possible complications from hysterectomy. The patient also claimed fraudulent concealment because the ObGyn had suffered a serious injury 3 years earlier that affected his dexterity. At the time of surgery, the ObGyn had a pending lawsuit against the owner of the premises where he fell in which he claimed that he was unable to continue his surgical practice because of the injury. The ObGyn never informed the patient of the extent of his injury or any associated risks related to his injury.
Defendants’ defense The patient was fully informed that bladder injury is a known risk of the procedure. The ObGyn maintained that his injury only affected his ability to stand for many hours while operating. The hospital settled during trial.
Verdict A $12,000 Louisiana settlement was reached with the hospital. Summary judgment was granted to the ObGyn on the informed consent claim. A $30,000 verdict was returned on the fraud count.
Placental abruption: Was child dead?
At 32 weeks’ gestation, a woman was found to have placental abruption. At the hospital, her ObGyn could not find a fetal heartbeat or detectable fetal movement on ultrasonography. A radiologist performed another ultrasound 30 minutes later and detected a fetal heart rate of 47 bpm. An emergency cesarean delivery was performed. Soon after birth, the child had seizures and was found to have hypoxic ischemic encephalopathy and diffuse brain injury. The child is profoundly disabled.
Parents’ claim The ObGyn was negligent for failing to detect the fetal heart rate and in failing to respond properly to placental abruption. Cesarean delivery should have been performed immediately after placental abruption was identified.
Defendant’s defense The case was settled at trial.
Verdict A $13 million Illinois settlement was reached, including $5 million in cash and $8 million placed in trust for the child.
Large fetus, shoulder dystocia: Erb’s palsy
Labor was induced at 39 weeks’ gestation because the fetus was anticipated to be large. During vaginal delivery, shoulder dystocia was encountered. At birth, the baby weighed 9 lb 2 oz. She sustained a brachial plexus injury to the posterior shoulder with permanent nerve root damage and Erb’s palsy. The child continues to have limited use of her left arm and hand even after 3 corrective operations.
Parents’ claim While performing maneuvers to relieve shoulder dystocia, the ObGyn exerted excessive traction on the baby’s head, causing a C-5 nerve root injury and complete avulsion at C-8. A cesarean delivery should have been performed.
Physician’s defense There was no negligence. The nerve injury was caused by the natural forces of labor and the mother’s pushing while the posterior shoulder was wedged behind the mother’s sa-
cral promontory.
Verdict A $1 million Illinois verdict was returned.
Woman dies from toxemia
A 22-year-old woman was seen by her ObGyn 4 days after vaginal delivery. Early the next day, the patient had a seizure at home and was transported by ambulance to the hospital. She could not be resuscitated and died. At autopsy, the cause of death was determined to be toxemia from pregnancy.
Estate’s claim The ObGyn failed to properly diagnose and treat the patient’s hypertension.
Defendant’s defense The case was settled during trial.
Verdict A $775,000 New York settlement was reached.
Blood transfusion delayed for hours: $14.75M net award
After emergency cesarean delivery, the baby was extremely anemic. The physicians determined that a fetal-maternal hemorrhage had started days before, causing the fetus to lose most of her blood.
An hour after birth, the attending neonatologist ordered blood from the hospital’s blood bank and arranged for emergency transport to a neonatal intensive care unit (NICU). Blood transfusion did not occur prior to transport. The child has severe cerebral palsy (CP) and cannot walk or talk at age 8 years.
Parents’ claim The neonatologist ordered cross-matched blood, which, because it is tested for compatibility, takes longer to supply. Universal donor blood could have been delivered in 20 minutes or less because it is readily available. The ambulance from the receiving hospital took an hour to drive 9 miles between the facilities, a trip that should have taken 12 minutes. The ambulance staff did not call ahead to the medical center to have blood ready for the baby. It took 4.5 hours before the newborn received a blood transfusion, a delay that caused severe injury to the child.
Defendants’ defense The matter went to trial against the neonatologist and his employer after the other defendants settled.
Verdict Before trial, an ObGyn and the hospital settled for a combined $750,000, and the county agreed to a $12 million settlement. During trial, a $2 million Illinois settlement was reached.
Pregnant woman has a massive stroke: $10.9M
Pregnant with her third child and at 26 weeks’ gestation, a 35-year-old woman had a massive intracerebral hemorrhage at home.
The day before, she had contacted her ObGyn’s office to report severe headache and abdominal pain. The call was taken by an associate of her ObGyn, who told her there was no need to go to the hospital and suggested that she had a gastrointestinal virus.
The stroke caused severe cognitive impairment, loss of memory, partial vision loss, dysphasia, and partial paralysis on her right side. At trial, she was still undergoing therapy to regain mobility, speech, and memory. She uses a wheelchair.
Patient’s claim The covering ObGyn was negligent for not sending the patient to the hospital when she reported severe headache.
Defendants’ defense The ObGyn and medical practice denied negligence, contending that the patient’s pregnancy was normal and that there was no indication that she was at risk for a stroke.
Verdict A $10,928,188 Ohio verdict was returned.
Was the fetus properly monitored?
One month before her due date, a woman was found to have premature rupture of membranes. She had gestational diabetes controlled by diet. She was admitted for induction of labor.
For more than 12 hours, external fetal monitor heart-rate tracings were reassuring. Then tracings began to show variable decelerations. For a period of 90 minutes, it was impossible to evaluate the fetal heart rate because the monitor was not working. An internal monitor was not placed. Just prior to birth, the tracings showed a 15-minute period of fetal tachycardia with the heart rate at 180 bpm. The physician’s notes indicated that the baby’s head had crowned for a prolonged period of time.
The baby was floppy at birth with Apgar scores of 2, 4, and 6 at 1, 5, and 10 minutes, respectively. The child was resuscitated and transferred to the NICU. She was found to have perinatal asphyxia, severe metabolic acidosis, multiorgan injury, hypoxic ischemic encephalopathy, and seizures. She stayed in the NICU for 1 month. At age 9 years, she has developmental delays and memory problems, but no motor injuries.
Parents’ claim During the 90 minutes in which the fetal heart-rate monitor was not working properly, the fetus was in distress. An emergency cesarean delivery should have been performed when variable decelerations were seen on tracings.
Physician’s defense The lack of motor injury indicates that the injury was not related to birth.
Verdict A $2 million Michigan settlement was reached.
Rectal tear after vacuum extraction
Vacuum extraction was used to deliver a 47-year-old woman’s child. Later, the mother developed a rectovaginal fistula that became inflamed and involved vaginal passage of stool. The patient required 2 operations and still has residual complications.
Patient’s claim The ObGyn should have found and repaired the rectal tear at delivery. Vacuum extraction was used after only 2 pushes. The mother did not consent to the use of the vacuum extractor.
Physician’s defense The ObGyn admitted that he did not specifically remember this delivery. He claimed that there was informed consent and that the rectal injury was small and easy to overlook.
Verdict A $1.02 million New York verdict was returned.
Preeclamptic mother dies after giving birth
A 24-year-old woman developed preeclampsia when under prenatal care at a hospital clinic. At 36 weeks’ gestation, she presented to the clinic with a headache, “seeing spots,” and feeling ill; her blood pressure (BP) was 169/89 mm Hg. She was admitted for induction of labor and treated for preeclampsia with magnesium sulfate. A healthy baby was born 2 days later. The mother continued to have high BP and was prescribed nifedipine.
Her BP was 148/88 mm Hg at discharge. No antihypertensive medications were prescribed. She was given standard postpartum instructions and told to schedule a follow-up appointment in 6 to 8 weeks.
Five days after discharge, she experienced shortness of breath and swelling in her extremities, but did not seek medical attention until the next day, when breathing became labored. When emergency medical services arrived, she was in cardiac arrest. Prolonged resuscitation was required with intubation and artificial respiration. A computed tomog-raphy (CT) scan revealed cerebral edema from prolonged hypoxia. She was transferred to another hospital where a neurologist determined that she had suffered a profound anoxic brain injury. She died 3 days later.
Estate’s claim The hospital staff was negligent for failing to inform the patient of the signs and symptoms of continuing preeclampsia and for not prescribing antihypertensive medication at discharge. Her follow-up appointment should have been scheduled for 1 week.
Defendant’s defense The patient was given oral instructions regarding postpartum preeclampsia. The case was settled during trial.
Verdict A $50,000 North Carolina settlement was reached.
Was delivery properly managed?
When a 16-year-old woman was found to have preeclampsia, she was admitted and labor was induced using oxytocin. An external fetal heart-rate monitor was placed.
Three hours later, her ObGyn took over her care from the attending physician. He saw the patient once in the evening, then left to deliver a baby at another hospital. He maintained telephone contact with labor and delivery nurses, who told him that the mother’s labor was progressing as planned. Early the next morning, the nurse called the ObGyn to report that the mother was fully dilated and ready to deliver. The ObGyn was at the patient’s bedside within 30 minutes. After the mother pushed once, the ObGyn determined that a cesarean delivery was necessary.
After birth, the child suffered seizures in the NICU and was transferred to another facility. With CP and microcephaly, he cannot speak, is incontinent, has motor difficulties, and will require 24-hour care for life.
Parent’s claim Labor was not properly monitored. Oxytocin doses were too large and continued for too long.
Defendants’ defense The mother’s treatment was appropriate and timely. There was no negligence.
Verdict A confidential Kansas settlement was reached with another defendant during the trial. A defense verdict was returned for the ObGyn.
Evidence of CMV on ultrasonography
During her pregnancy in 2012, a woman contracted congenital cytomegalovirus (CMV), although she did not have any symptoms. The child has CP, a hearing deficiency, and other complications caused by the virus.
Parents’ claim The ObGyn failed to identify CMV, despite ultrasound evidence that the virus was affecting the fetus. Studies available at the time of the pregnancy show considerable success in treating the condition in utero with hyperimmune globulin antiviral agents.
Defendant’s defense The case was settled during trial.
Verdict A confidential Idaho settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements, & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Was the ObGyn’s dexterity compromised?
A woman underwent a hysterectomy. During surgery, the patient’s bladder was injured; the ObGyn called in a urologist to make the repair.
Patient’s claim The ObGyn failed to inform the patient about the possible complications from hysterectomy. The patient also claimed fraudulent concealment because the ObGyn had suffered a serious injury 3 years earlier that affected his dexterity. At the time of surgery, the ObGyn had a pending lawsuit against the owner of the premises where he fell in which he claimed that he was unable to continue his surgical practice because of the injury. The ObGyn never informed the patient of the extent of his injury or any associated risks related to his injury.
Defendants’ defense The patient was fully informed that bladder injury is a known risk of the procedure. The ObGyn maintained that his injury only affected his ability to stand for many hours while operating. The hospital settled during trial.
Verdict A $12,000 Louisiana settlement was reached with the hospital. Summary judgment was granted to the ObGyn on the informed consent claim. A $30,000 verdict was returned on the fraud count.
Placental abruption: Was child dead?
At 32 weeks’ gestation, a woman was found to have placental abruption. At the hospital, her ObGyn could not find a fetal heartbeat or detectable fetal movement on ultrasonography. A radiologist performed another ultrasound 30 minutes later and detected a fetal heart rate of 47 bpm. An emergency cesarean delivery was performed. Soon after birth, the child had seizures and was found to have hypoxic ischemic encephalopathy and diffuse brain injury. The child is profoundly disabled.
Parents’ claim The ObGyn was negligent for failing to detect the fetal heart rate and in failing to respond properly to placental abruption. Cesarean delivery should have been performed immediately after placental abruption was identified.
Defendant’s defense The case was settled at trial.
Verdict A $13 million Illinois settlement was reached, including $5 million in cash and $8 million placed in trust for the child.
Large fetus, shoulder dystocia: Erb’s palsy
Labor was induced at 39 weeks’ gestation because the fetus was anticipated to be large. During vaginal delivery, shoulder dystocia was encountered. At birth, the baby weighed 9 lb 2 oz. She sustained a brachial plexus injury to the posterior shoulder with permanent nerve root damage and Erb’s palsy. The child continues to have limited use of her left arm and hand even after 3 corrective operations.
Parents’ claim While performing maneuvers to relieve shoulder dystocia, the ObGyn exerted excessive traction on the baby’s head, causing a C-5 nerve root injury and complete avulsion at C-8. A cesarean delivery should have been performed.
Physician’s defense There was no negligence. The nerve injury was caused by the natural forces of labor and the mother’s pushing while the posterior shoulder was wedged behind the mother’s sa-
cral promontory.
Verdict A $1 million Illinois verdict was returned.
Woman dies from toxemia
A 22-year-old woman was seen by her ObGyn 4 days after vaginal delivery. Early the next day, the patient had a seizure at home and was transported by ambulance to the hospital. She could not be resuscitated and died. At autopsy, the cause of death was determined to be toxemia from pregnancy.
Estate’s claim The ObGyn failed to properly diagnose and treat the patient’s hypertension.
Defendant’s defense The case was settled during trial.
Verdict A $775,000 New York settlement was reached.
Blood transfusion delayed for hours: $14.75M net award
After emergency cesarean delivery, the baby was extremely anemic. The physicians determined that a fetal-maternal hemorrhage had started days before, causing the fetus to lose most of her blood.
An hour after birth, the attending neonatologist ordered blood from the hospital’s blood bank and arranged for emergency transport to a neonatal intensive care unit (NICU). Blood transfusion did not occur prior to transport. The child has severe cerebral palsy (CP) and cannot walk or talk at age 8 years.
Parents’ claim The neonatologist ordered cross-matched blood, which, because it is tested for compatibility, takes longer to supply. Universal donor blood could have been delivered in 20 minutes or less because it is readily available. The ambulance from the receiving hospital took an hour to drive 9 miles between the facilities, a trip that should have taken 12 minutes. The ambulance staff did not call ahead to the medical center to have blood ready for the baby. It took 4.5 hours before the newborn received a blood transfusion, a delay that caused severe injury to the child.
Defendants’ defense The matter went to trial against the neonatologist and his employer after the other defendants settled.
Verdict Before trial, an ObGyn and the hospital settled for a combined $750,000, and the county agreed to a $12 million settlement. During trial, a $2 million Illinois settlement was reached.
Pregnant woman has a massive stroke: $10.9M
Pregnant with her third child and at 26 weeks’ gestation, a 35-year-old woman had a massive intracerebral hemorrhage at home.
The day before, she had contacted her ObGyn’s office to report severe headache and abdominal pain. The call was taken by an associate of her ObGyn, who told her there was no need to go to the hospital and suggested that she had a gastrointestinal virus.
The stroke caused severe cognitive impairment, loss of memory, partial vision loss, dysphasia, and partial paralysis on her right side. At trial, she was still undergoing therapy to regain mobility, speech, and memory. She uses a wheelchair.
Patient’s claim The covering ObGyn was negligent for not sending the patient to the hospital when she reported severe headache.
Defendants’ defense The ObGyn and medical practice denied negligence, contending that the patient’s pregnancy was normal and that there was no indication that she was at risk for a stroke.
Verdict A $10,928,188 Ohio verdict was returned.
Was the fetus properly monitored?
One month before her due date, a woman was found to have premature rupture of membranes. She had gestational diabetes controlled by diet. She was admitted for induction of labor.
For more than 12 hours, external fetal monitor heart-rate tracings were reassuring. Then tracings began to show variable decelerations. For a period of 90 minutes, it was impossible to evaluate the fetal heart rate because the monitor was not working. An internal monitor was not placed. Just prior to birth, the tracings showed a 15-minute period of fetal tachycardia with the heart rate at 180 bpm. The physician’s notes indicated that the baby’s head had crowned for a prolonged period of time.
The baby was floppy at birth with Apgar scores of 2, 4, and 6 at 1, 5, and 10 minutes, respectively. The child was resuscitated and transferred to the NICU. She was found to have perinatal asphyxia, severe metabolic acidosis, multiorgan injury, hypoxic ischemic encephalopathy, and seizures. She stayed in the NICU for 1 month. At age 9 years, she has developmental delays and memory problems, but no motor injuries.
Parents’ claim During the 90 minutes in which the fetal heart-rate monitor was not working properly, the fetus was in distress. An emergency cesarean delivery should have been performed when variable decelerations were seen on tracings.
Physician’s defense The lack of motor injury indicates that the injury was not related to birth.
Verdict A $2 million Michigan settlement was reached.
Rectal tear after vacuum extraction
Vacuum extraction was used to deliver a 47-year-old woman’s child. Later, the mother developed a rectovaginal fistula that became inflamed and involved vaginal passage of stool. The patient required 2 operations and still has residual complications.
Patient’s claim The ObGyn should have found and repaired the rectal tear at delivery. Vacuum extraction was used after only 2 pushes. The mother did not consent to the use of the vacuum extractor.
Physician’s defense The ObGyn admitted that he did not specifically remember this delivery. He claimed that there was informed consent and that the rectal injury was small and easy to overlook.
Verdict A $1.02 million New York verdict was returned.
Preeclamptic mother dies after giving birth
A 24-year-old woman developed preeclampsia when under prenatal care at a hospital clinic. At 36 weeks’ gestation, she presented to the clinic with a headache, “seeing spots,” and feeling ill; her blood pressure (BP) was 169/89 mm Hg. She was admitted for induction of labor and treated for preeclampsia with magnesium sulfate. A healthy baby was born 2 days later. The mother continued to have high BP and was prescribed nifedipine.
Her BP was 148/88 mm Hg at discharge. No antihypertensive medications were prescribed. She was given standard postpartum instructions and told to schedule a follow-up appointment in 6 to 8 weeks.
Five days after discharge, she experienced shortness of breath and swelling in her extremities, but did not seek medical attention until the next day, when breathing became labored. When emergency medical services arrived, she was in cardiac arrest. Prolonged resuscitation was required with intubation and artificial respiration. A computed tomog-raphy (CT) scan revealed cerebral edema from prolonged hypoxia. She was transferred to another hospital where a neurologist determined that she had suffered a profound anoxic brain injury. She died 3 days later.
Estate’s claim The hospital staff was negligent for failing to inform the patient of the signs and symptoms of continuing preeclampsia and for not prescribing antihypertensive medication at discharge. Her follow-up appointment should have been scheduled for 1 week.
Defendant’s defense The patient was given oral instructions regarding postpartum preeclampsia. The case was settled during trial.
Verdict A $50,000 North Carolina settlement was reached.
Was delivery properly managed?
When a 16-year-old woman was found to have preeclampsia, she was admitted and labor was induced using oxytocin. An external fetal heart-rate monitor was placed.
Three hours later, her ObGyn took over her care from the attending physician. He saw the patient once in the evening, then left to deliver a baby at another hospital. He maintained telephone contact with labor and delivery nurses, who told him that the mother’s labor was progressing as planned. Early the next morning, the nurse called the ObGyn to report that the mother was fully dilated and ready to deliver. The ObGyn was at the patient’s bedside within 30 minutes. After the mother pushed once, the ObGyn determined that a cesarean delivery was necessary.
After birth, the child suffered seizures in the NICU and was transferred to another facility. With CP and microcephaly, he cannot speak, is incontinent, has motor difficulties, and will require 24-hour care for life.
Parent’s claim Labor was not properly monitored. Oxytocin doses were too large and continued for too long.
Defendants’ defense The mother’s treatment was appropriate and timely. There was no negligence.
Verdict A confidential Kansas settlement was reached with another defendant during the trial. A defense verdict was returned for the ObGyn.
Evidence of CMV on ultrasonography
During her pregnancy in 2012, a woman contracted congenital cytomegalovirus (CMV), although she did not have any symptoms. The child has CP, a hearing deficiency, and other complications caused by the virus.
Parents’ claim The ObGyn failed to identify CMV, despite ultrasound evidence that the virus was affecting the fetus. Studies available at the time of the pregnancy show considerable success in treating the condition in utero with hyperimmune globulin antiviral agents.
Defendant’s defense The case was settled during trial.
Verdict A confidential Idaho settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements, & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In this Article
- Placental abruption: Was child dead?
- Large fetus, shoulder dystocia: Erb’s palsy
- Woman dies from toxemia
- Blood transfusion delayed for hours: $14.75M net award
- Pregnant woman has a massive stroke: $10.9M
- Was the fetus properly monitored?
- Rectal tear after vacuum extraction
- Preeclamptic mother dies after giving birth
- Was delivery properly managed?
- Evidence of CMV on ultrasonography
Frozen embryo transfer tips scales toward LGA babies
LISBON – Singletons born after frozen embryo transfer are at significantly increased risk for being born large for gestational age, results of a retrospective, case-matched cohort study confirm.
Frozen embryo transfer (FET) was found to be a significant independent risk factor for LGA among FET singletons (odds ratio, 1.697; P = .032) on par with multiparity (OR, 1.615; P = .039) and maternal body mass index (BMI) between 25 kg/m2 and 30 kg/m2 (OR, 1.85; P = .026).
BMI greater than 30 kg/m2 tripled LGA risk (OR, 3.12; P = .002).Fresh embryo transfer (ET) and intra-cytoplasmic sperm injection had no effect on LGA occurrence, whereas double embryo transfer insignificantly reduced the LGA risk, study author Sara Korosec, Ph.D., of University Medical Centre Ljubljana (Slovenia), reported.
The logistic regression model included smoking, hypertension, multiparity, BMI, gestational diabetes, and in vitro fertilization (IVF)/intra-cytoplasmic sperm injection.
“It appears we are running out of the most obvious maternal and IVF reasons for this phenomenon,” Dr. Korosec said at the annual meeting of the European Society of Human Reproduction and Embryology.
The findings are consistent with prior research demonstrating an increased risk for LGA in FET singletons, suggesting that the freezing and thawing procedures per se are partly to blame.
A recent Danish national cohort study (Hum. Reprod. 2014;29:618-27) reported adjusted odds ratios of 1.34 and 1.91 for LGA and macrosomia in FET singletons vs. singletons conceived after fresh ET. This increased risk was confirmed in a sibling cohort, where one singleton was born after FET and the next after fresh ET, or vice versa.
Danish study author Dr. Anja Pinborg of the University of Copenhagen rose from the audience to acknowledge that adjustment for BMI as a possible confounder was not possible in their study and applauded the inclusion of BMI and diabetes in the current analysis.
“I really congratulate you on your findings and it’s nice to see that after controlling for these factors, it’s still there,” she said.
Several audience members agreed with the investigators that further research is needed to identify possible reasons for higher LGA rates in FET babies now that the “low-hanging fruit,” including maternal diabetes and BMI, are being excluded. Other factors to consider include placental differences between fresh and frozen embryo pregnancies, embryo selection using vitrification vs. slow freezing, and “the thing we are all most afraid of, underlying epigenetic disturbances,” Dr. Korosec said.
Dr. Korosec and her associates identified 4,508 singleton pregnancies and births (211 after FET and 916 after fresh ET) conceived as a result of IVF at the university between 2004 and 2011 and 3,381 naturally conceived controls in the National Perinatal Data System. There were 3 controls for each IVF pregnancy, matched by maternal age, parity, and maternity hospital.
LGA, defined as a birth weight above the 90th percentile, occurred in 14.7% of FET singletons vs. 8.7% of FET controls (P = .017) and in 9.4% of fresh ET singletons vs. 9.1% of fresh controls (P = .791), Dr. Korosec reported.
LGA above the 95th percentile was reported in 10% of FET singletons vs. 4.9% of FET controls (P = .012) and in 4.9% of fresh ET vs. 5.1% of fresh controls (P = .862).
Significant LGA risk factors among fresh ET singletons were multiparity (OR, 1.61; P = .001), BMI 25-30 kg/m2 (OR 1.70; P = .000), and BMI greater than 30 kg/m2 (OR, 1.93; P = .0001), she said.
Dr. Korosec reported no conflicting interests.
LISBON – Singletons born after frozen embryo transfer are at significantly increased risk for being born large for gestational age, results of a retrospective, case-matched cohort study confirm.
Frozen embryo transfer (FET) was found to be a significant independent risk factor for LGA among FET singletons (odds ratio, 1.697; P = .032) on par with multiparity (OR, 1.615; P = .039) and maternal body mass index (BMI) between 25 kg/m2 and 30 kg/m2 (OR, 1.85; P = .026).
BMI greater than 30 kg/m2 tripled LGA risk (OR, 3.12; P = .002).Fresh embryo transfer (ET) and intra-cytoplasmic sperm injection had no effect on LGA occurrence, whereas double embryo transfer insignificantly reduced the LGA risk, study author Sara Korosec, Ph.D., of University Medical Centre Ljubljana (Slovenia), reported.
The logistic regression model included smoking, hypertension, multiparity, BMI, gestational diabetes, and in vitro fertilization (IVF)/intra-cytoplasmic sperm injection.
“It appears we are running out of the most obvious maternal and IVF reasons for this phenomenon,” Dr. Korosec said at the annual meeting of the European Society of Human Reproduction and Embryology.
The findings are consistent with prior research demonstrating an increased risk for LGA in FET singletons, suggesting that the freezing and thawing procedures per se are partly to blame.
A recent Danish national cohort study (Hum. Reprod. 2014;29:618-27) reported adjusted odds ratios of 1.34 and 1.91 for LGA and macrosomia in FET singletons vs. singletons conceived after fresh ET. This increased risk was confirmed in a sibling cohort, where one singleton was born after FET and the next after fresh ET, or vice versa.
Danish study author Dr. Anja Pinborg of the University of Copenhagen rose from the audience to acknowledge that adjustment for BMI as a possible confounder was not possible in their study and applauded the inclusion of BMI and diabetes in the current analysis.
“I really congratulate you on your findings and it’s nice to see that after controlling for these factors, it’s still there,” she said.
Several audience members agreed with the investigators that further research is needed to identify possible reasons for higher LGA rates in FET babies now that the “low-hanging fruit,” including maternal diabetes and BMI, are being excluded. Other factors to consider include placental differences between fresh and frozen embryo pregnancies, embryo selection using vitrification vs. slow freezing, and “the thing we are all most afraid of, underlying epigenetic disturbances,” Dr. Korosec said.
Dr. Korosec and her associates identified 4,508 singleton pregnancies and births (211 after FET and 916 after fresh ET) conceived as a result of IVF at the university between 2004 and 2011 and 3,381 naturally conceived controls in the National Perinatal Data System. There were 3 controls for each IVF pregnancy, matched by maternal age, parity, and maternity hospital.
LGA, defined as a birth weight above the 90th percentile, occurred in 14.7% of FET singletons vs. 8.7% of FET controls (P = .017) and in 9.4% of fresh ET singletons vs. 9.1% of fresh controls (P = .791), Dr. Korosec reported.
LGA above the 95th percentile was reported in 10% of FET singletons vs. 4.9% of FET controls (P = .012) and in 4.9% of fresh ET vs. 5.1% of fresh controls (P = .862).
Significant LGA risk factors among fresh ET singletons were multiparity (OR, 1.61; P = .001), BMI 25-30 kg/m2 (OR 1.70; P = .000), and BMI greater than 30 kg/m2 (OR, 1.93; P = .0001), she said.
Dr. Korosec reported no conflicting interests.
LISBON – Singletons born after frozen embryo transfer are at significantly increased risk for being born large for gestational age, results of a retrospective, case-matched cohort study confirm.
Frozen embryo transfer (FET) was found to be a significant independent risk factor for LGA among FET singletons (odds ratio, 1.697; P = .032) on par with multiparity (OR, 1.615; P = .039) and maternal body mass index (BMI) between 25 kg/m2 and 30 kg/m2 (OR, 1.85; P = .026).
BMI greater than 30 kg/m2 tripled LGA risk (OR, 3.12; P = .002).Fresh embryo transfer (ET) and intra-cytoplasmic sperm injection had no effect on LGA occurrence, whereas double embryo transfer insignificantly reduced the LGA risk, study author Sara Korosec, Ph.D., of University Medical Centre Ljubljana (Slovenia), reported.
The logistic regression model included smoking, hypertension, multiparity, BMI, gestational diabetes, and in vitro fertilization (IVF)/intra-cytoplasmic sperm injection.
“It appears we are running out of the most obvious maternal and IVF reasons for this phenomenon,” Dr. Korosec said at the annual meeting of the European Society of Human Reproduction and Embryology.
The findings are consistent with prior research demonstrating an increased risk for LGA in FET singletons, suggesting that the freezing and thawing procedures per se are partly to blame.
A recent Danish national cohort study (Hum. Reprod. 2014;29:618-27) reported adjusted odds ratios of 1.34 and 1.91 for LGA and macrosomia in FET singletons vs. singletons conceived after fresh ET. This increased risk was confirmed in a sibling cohort, where one singleton was born after FET and the next after fresh ET, or vice versa.
Danish study author Dr. Anja Pinborg of the University of Copenhagen rose from the audience to acknowledge that adjustment for BMI as a possible confounder was not possible in their study and applauded the inclusion of BMI and diabetes in the current analysis.
“I really congratulate you on your findings and it’s nice to see that after controlling for these factors, it’s still there,” she said.
Several audience members agreed with the investigators that further research is needed to identify possible reasons for higher LGA rates in FET babies now that the “low-hanging fruit,” including maternal diabetes and BMI, are being excluded. Other factors to consider include placental differences between fresh and frozen embryo pregnancies, embryo selection using vitrification vs. slow freezing, and “the thing we are all most afraid of, underlying epigenetic disturbances,” Dr. Korosec said.
Dr. Korosec and her associates identified 4,508 singleton pregnancies and births (211 after FET and 916 after fresh ET) conceived as a result of IVF at the university between 2004 and 2011 and 3,381 naturally conceived controls in the National Perinatal Data System. There were 3 controls for each IVF pregnancy, matched by maternal age, parity, and maternity hospital.
LGA, defined as a birth weight above the 90th percentile, occurred in 14.7% of FET singletons vs. 8.7% of FET controls (P = .017) and in 9.4% of fresh ET singletons vs. 9.1% of fresh controls (P = .791), Dr. Korosec reported.
LGA above the 95th percentile was reported in 10% of FET singletons vs. 4.9% of FET controls (P = .012) and in 4.9% of fresh ET vs. 5.1% of fresh controls (P = .862).
Significant LGA risk factors among fresh ET singletons were multiparity (OR, 1.61; P = .001), BMI 25-30 kg/m2 (OR 1.70; P = .000), and BMI greater than 30 kg/m2 (OR, 1.93; P = .0001), she said.
Dr. Korosec reported no conflicting interests.
AT ESHRE 2015
Key clinical point: Frozen embryo transfer is as significant a risk factor as multiparity and BMI for large for gestational age birth among FET singletons.
Major finding: Frozen embryo transfer was a risk factor for LGA birth (odds ratio, 1.697; P = .032).
Data source: Retrospective, case-matched cohort study of 4,508 singletons.
Disclosures: Dr. Korosec reported no conflicting interests.