User login
Who was responsible for excessive oxytocin doses? $18.2M verdict
Who was responsible for excessive oxytocin doses? $18.2M verdict
Early in the morning, a woman at 40 weeks’ gestation presented to the hospital for induction of labor managed by her ObGyn. Labor was lengthy, and the mother was given increasing doses of 22, 24, and 26 mIU/min of oxytocin to stimulate labor. The baby was delivered in the evening. The child suffered a hypoxic birth injury and has cerebral palsy.
Parents’ claim Excessive oxytocin was administered, causing uterine hyperstimulation and excessive contractions. Nurses failed to inform the ObGyn of an abnormal fetal heart rate during the afternoon.
Defendants’ defense The parties disputed the oxytocin orders. The ObGyn claimed she has a standing order against oxytocin doses over 20 mIU/min. The nurses claimed that the dosage was based on the ObGyn’s verbal orders, which the ObGyn denied. The ObGyn denied negligence and maintained that if she’d known of the oxytocin administration greater than 20 mIU/min and the abnormal fetal heart rate, she immediately would have called for cesarean delivery. The hospital denied negligence and maintained that the oxytocin was administered 10 hours before delivery and played no role in fetal distress.
Verdict At trial, the ObGyn did not call expert witnesses and, in closing arguments, the physician’s attorney asked for exoneration of the ObGyn and a finding of fault solely against the hospital. An $18.2 million Washington verdict was returned against the hospital.
What caused the child’s Erb’s palsy?
A mother presented to the hospital for induction of labor. Oxytocin was administered and the first stage of labor progressed normally. When the mother began pushing, the ObGyn noted a turtle sign at crowning and called for assistance. The ObGyn attempted to deliver the fetus with downward guidance of the fetal head but encountered shoulder dystocia and a nuchal cord. He unwrapped the cord and instructed the nursing staff to place the mother in the McRobert’s position to help dislodge the right shoulder. When that did not work, the ObGyn performed a first-degree episiotomy and completed delivery. The child was found to have Erb’s palsy of the right arm. She underwent decompression and neurolysis of the brachial plexus using sural nerve grafts but still has reduced use of her right arm.
Parents’ claim Shoulder dystocia was improperly managed, causing the brachial plexus injury.
Defendants’ defense The ObGyn and hospital system denied negligence. The child’s injury occurred in utero due to natural forces of the mother’s uterine contractions.
Verdict An Ohio defense verdict was returned.
Woman claims lack of proper consent
A 47-year-old woman underwent endometrial ablation performed by her ObGyn. During the procedure, the uterus was perforated and the ObGyn performed a hysterectomy. Six days later, the patient was found to have peritonitis and underwent bowel repair surgery. The patient developed untreatable bowel adhesions that cause chronic pain.
Patient’s claim There were less expensive and invasive alternatives to the ablation that the ObGyn did not offer. The patient claimed lack of informed consent for the ablation and hysterectomy and negligence in perforating the bowel. The ObGyn was also negligent in failing to recognize the perforation and to diagnose peritonitis in a timely manner.
Texas state law requires consent for hysterectomies without documented evidence of immediate danger to life. Her husband did not have the authority to consent on her behalf.
Physician’s defense The husband gave informed consent. Failure to recognize the perforation was not negligent; it is a known risk of the surgery. The patient’s care was transferred to another physician after the second postoperative day.
Verdict A $200,000 Texas settlement was reached.
Bowel obstruction in pregnant woman
A 29-year-old woman at 27 weeks’ gestation had abdominal pain. She went to a community hospital where a hospitalist was assigned to her care. After a day, the patient was found to have a small bowel obstruction and necrosis of the bowel. The baby was delivered preterm. The mother underwent 12 operations; half of her intestines were resected. The mother is being treated for posttraumatic stress syndrome. The child is autistic.
Parents’ claim The hospitalist did not diagnose the mother’s intestinal blockage in a timely manner and did not obtain an obstetric consult or notify the patient’s ObGyn. The hospital staff did not follow protocol to notify the mother’s ObGyn. The child’s autism is a result of preterm delivery.
Defendants’ defense The hospital denied any duty to notify the ObGyn if the patient was admitted to the hospital for nonobstetric reasons. The case was settled during trial.
Verdict A $4.2 million Washington settlement was reached including $3 million from the hospital.
Fourth-degree perineal tear and continuing pain after delivery
A woman in her 30s went to the hospital for induction of labor. After many hours, the ObGyn used vacuum extraction due to maternal fatigue. The baby emerged in compound presentation, with her hand at the side of her head. She weighed 9 lb 12 oz at birth. A fourth-degree perineal tear occurred at birth. Postpartum, a rectovaginal fistula developed that required several repair operations. The mother is unable to have intercourse due to continuing vaginal pain and discomfort.
Patient’s Claim Knowing that the father’s head was overly large, the ObGyn should have better estimated the fetus’ size, and should have performed cesarean delivery.
Physician’s defense The ObGyn admitted that he knew the baby was large but maintained that a large fetus does not mandate a cesarean delivery. There were no indications that the baby’s head or body was too large to fit through the mother’s pelvis, so a vaginal delivery was appropriate. A perineal tear is a known complication of childbirth and could not be prevented. The patient’s current pain is unrelated to the perineal tear.
Verdict A Pennsylvania defense verdict was returned.
Breast cancer missed in woman with dense breasts
In 2003, a 44-year-old woman was told she had dense fibrocystic breasts. From 2003 through 2009 she regularly saw a breast surgeon due to concern that breast cancer might be difficult to detect.
In August 2009, her ObGyn identified a questionable mass in her left breast after ultrasonography and mammography. The patient saw the surgeon in late September 2009; no further imaging was ordered and she was told to return in a year.
The patient, concerned about the mass, returned to the surgeon in May 2010. Testing revealed cancer, and she underwent radical mastectomy and other treatment.
Patient’s claim Because the mass had not been treated in a timely manner, her 5-year survival rate in May 2010 was less than 50%. The surgeon was negligent in failing to order additional testing in September 2009. Magnetic resonance imaging (MRI) would have detected the cancer at a time when her survival rate could have been 80%.
Physician’s defense The cancer was diagnosed in a timely manner. An earlier diagnosis would not have changed the outcome.
Verdict A Tennessee defense verdict was returned.
Child stillborn, mother injured after vacuum extraction
When the mother’s labor slowed at a birthing center, she received several medications including castor oil, blue cohosh, and black cohosh to induce labor. The mother was later transferred by ambulance to a hospital. Ninety minutes after admission, the ObGyn used vacuum extraction to deliver a stillborn child. The mother sustained damage to her rectum, uterus, and vagina, had repair surgery, and has been unable to get pregnant again.
Parents’ claim While in labor at the birthing center, the castor oil, blue cohosh, and black cohosh caused the patient’s uterus to contract excessively and contributed to fetal death. The patient should have been transferred to the hospital earlier. Cesarean delivery should have been performed immediately upon her arrival at the hospital but the ObGyn did not arrive at the hospital for an hour after the patient’s admission.
Defendants’ defense The head midwife at the birthing center conceded negligence. The hospital claimed that the fetus was already dead before the mother arrived. The ObGyn denied negligence, arguing that he had no supervisory role or ownership in the birthing center and was not present during the mother’s labor. He also claimed that the fetus was dead in utero 12 or more hours before delivery and that an infectious process had developed in the mother during the 17 hours that she was at the birthing center.
Verdict A $4,095,000 Florida verdict was returned against the ObGyn. A directed verdict was granted for the hospital.
Patient still in pain after labia reduction
A 44-year-old woman underwent surgical reduction of her labia minora performed by a gynecologist. The procedure was intended to relieve discomfort during sexual activity. The patient continues to have pain.
Patient’s claim An excessive amount of the right labia minora was removed because proper presurgical demarcation of the operative area was not performed. Her pain during intercourse has worsened and she cannot properly urinate.
Physician’s defense Presurgical demarcation was correctly completed using clamps. Surgery was properly performed. The asymmetry is due to poor healing of the surgical wound. The patient’s clitoris was not scarred. The patient never reported complications related to urination to her gynecologist. Her ongoing pain is due to an estrogen deficiency.
Verdict A New York defense verdict was returned.
Uterine rupture after version for breech presentation: $7M
A woman went to the hospital for delivery of her baby. The fetus was in breech position, but the mother requested vaginal delivery. When the ObGyn attempted an external cephalic version to turn the baby, the uterus ruptured and the placenta was damaged. The baby sustained hypoxic-ischemic encephalopathy resulting in cerebral palsy (CP). He requires constant nursing care.
Parents’ claim The ObGyn failed to recognize fetal distress during the breech version. The ObGyn improperly performed the version, causing the uterine rupture. There was lack of informed consent for the version.
Defendants’ defense The case was settled during trial.
Verdict A $7 million New Jersey settlement was reached.
Sepsis following hysterectomy
An ObGyn performed total abdominal hysterectomy to treat uterine fibroids in a 26-year-old woman. Despite reporting abdominal pain, the patient was discharged on postsurgical day 4.
Three days later, she went to a different hospital with moderate diffuse abdominal pain, constipation, nausea, emesis, tachycardia, and low-grade fever. An abdominal radiograph was taken, the patient was given morphine and ketorolac, and she was sent home.
She returned to the first hospital 3 days later reporting fever, nausea, emesis, diarrhea, and severe abdominal pain. After an abdominal computed tomography (CT) scan revealed numerous fluid- and gas-filled collections, indicative of abscess, intravenous antibiotics were ordered and administered.
Six days later, an infectious disease physician was consulted. He made a diagnosis of sepsis secondary to abdominal infection.
The next day, an abdominal CT scan revealed enlargement of multiple abdominal and pelvic fluid collections.
At exploratory laparotomy, purulent fluid was found in the anterior fascial compartment, with gross pus in the abdomen. The entire bowel was dilated, inflamed, and matted. Necrotic rind and infection were noted on multiple surfaces of the colon and small intestine and the transverse colon was gangrenous and sealed to the right lower quadrant. The patient’s intestines were resected and an ileostomy was placed, which was reversed several months later.
Patient’s claim The ObGyn did not offer an alternative to hysterectomy. The ObGyn was negligent in injuring the small intestine during surgery and failing to recognize and treat it intraoperatively. The patient should not have been discharged based on her reported symptoms. Failure to recognize and treat the injury led to sepsis with severe complications and months of recuperation.
Physician’s defense There was no negligence; small bowel injury is a known risk of hysterectomy. Other caregivers at both hospitals were at fault for not properly diagnosing and treating the infection.
Verdict A $901,420 Nevada verdict was returned; the ObGyn was found 85% at fault and other parties 15% at fault. The court granted the physician’s motion to reduce the verdict to $436,954, which included $371,411 from the ObGyn.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements, & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Who was responsible for excessive oxytocin doses? $18.2M verdict
Early in the morning, a woman at 40 weeks’ gestation presented to the hospital for induction of labor managed by her ObGyn. Labor was lengthy, and the mother was given increasing doses of 22, 24, and 26 mIU/min of oxytocin to stimulate labor. The baby was delivered in the evening. The child suffered a hypoxic birth injury and has cerebral palsy.
Parents’ claim Excessive oxytocin was administered, causing uterine hyperstimulation and excessive contractions. Nurses failed to inform the ObGyn of an abnormal fetal heart rate during the afternoon.
Defendants’ defense The parties disputed the oxytocin orders. The ObGyn claimed she has a standing order against oxytocin doses over 20 mIU/min. The nurses claimed that the dosage was based on the ObGyn’s verbal orders, which the ObGyn denied. The ObGyn denied negligence and maintained that if she’d known of the oxytocin administration greater than 20 mIU/min and the abnormal fetal heart rate, she immediately would have called for cesarean delivery. The hospital denied negligence and maintained that the oxytocin was administered 10 hours before delivery and played no role in fetal distress.
Verdict At trial, the ObGyn did not call expert witnesses and, in closing arguments, the physician’s attorney asked for exoneration of the ObGyn and a finding of fault solely against the hospital. An $18.2 million Washington verdict was returned against the hospital.
What caused the child’s Erb’s palsy?
A mother presented to the hospital for induction of labor. Oxytocin was administered and the first stage of labor progressed normally. When the mother began pushing, the ObGyn noted a turtle sign at crowning and called for assistance. The ObGyn attempted to deliver the fetus with downward guidance of the fetal head but encountered shoulder dystocia and a nuchal cord. He unwrapped the cord and instructed the nursing staff to place the mother in the McRobert’s position to help dislodge the right shoulder. When that did not work, the ObGyn performed a first-degree episiotomy and completed delivery. The child was found to have Erb’s palsy of the right arm. She underwent decompression and neurolysis of the brachial plexus using sural nerve grafts but still has reduced use of her right arm.
Parents’ claim Shoulder dystocia was improperly managed, causing the brachial plexus injury.
Defendants’ defense The ObGyn and hospital system denied negligence. The child’s injury occurred in utero due to natural forces of the mother’s uterine contractions.
Verdict An Ohio defense verdict was returned.
Woman claims lack of proper consent
A 47-year-old woman underwent endometrial ablation performed by her ObGyn. During the procedure, the uterus was perforated and the ObGyn performed a hysterectomy. Six days later, the patient was found to have peritonitis and underwent bowel repair surgery. The patient developed untreatable bowel adhesions that cause chronic pain.
Patient’s claim There were less expensive and invasive alternatives to the ablation that the ObGyn did not offer. The patient claimed lack of informed consent for the ablation and hysterectomy and negligence in perforating the bowel. The ObGyn was also negligent in failing to recognize the perforation and to diagnose peritonitis in a timely manner.
Texas state law requires consent for hysterectomies without documented evidence of immediate danger to life. Her husband did not have the authority to consent on her behalf.
Physician’s defense The husband gave informed consent. Failure to recognize the perforation was not negligent; it is a known risk of the surgery. The patient’s care was transferred to another physician after the second postoperative day.
Verdict A $200,000 Texas settlement was reached.
Bowel obstruction in pregnant woman
A 29-year-old woman at 27 weeks’ gestation had abdominal pain. She went to a community hospital where a hospitalist was assigned to her care. After a day, the patient was found to have a small bowel obstruction and necrosis of the bowel. The baby was delivered preterm. The mother underwent 12 operations; half of her intestines were resected. The mother is being treated for posttraumatic stress syndrome. The child is autistic.
Parents’ claim The hospitalist did not diagnose the mother’s intestinal blockage in a timely manner and did not obtain an obstetric consult or notify the patient’s ObGyn. The hospital staff did not follow protocol to notify the mother’s ObGyn. The child’s autism is a result of preterm delivery.
Defendants’ defense The hospital denied any duty to notify the ObGyn if the patient was admitted to the hospital for nonobstetric reasons. The case was settled during trial.
Verdict A $4.2 million Washington settlement was reached including $3 million from the hospital.
Fourth-degree perineal tear and continuing pain after delivery
A woman in her 30s went to the hospital for induction of labor. After many hours, the ObGyn used vacuum extraction due to maternal fatigue. The baby emerged in compound presentation, with her hand at the side of her head. She weighed 9 lb 12 oz at birth. A fourth-degree perineal tear occurred at birth. Postpartum, a rectovaginal fistula developed that required several repair operations. The mother is unable to have intercourse due to continuing vaginal pain and discomfort.
Patient’s Claim Knowing that the father’s head was overly large, the ObGyn should have better estimated the fetus’ size, and should have performed cesarean delivery.
Physician’s defense The ObGyn admitted that he knew the baby was large but maintained that a large fetus does not mandate a cesarean delivery. There were no indications that the baby’s head or body was too large to fit through the mother’s pelvis, so a vaginal delivery was appropriate. A perineal tear is a known complication of childbirth and could not be prevented. The patient’s current pain is unrelated to the perineal tear.
Verdict A Pennsylvania defense verdict was returned.
Breast cancer missed in woman with dense breasts
In 2003, a 44-year-old woman was told she had dense fibrocystic breasts. From 2003 through 2009 she regularly saw a breast surgeon due to concern that breast cancer might be difficult to detect.
In August 2009, her ObGyn identified a questionable mass in her left breast after ultrasonography and mammography. The patient saw the surgeon in late September 2009; no further imaging was ordered and she was told to return in a year.
The patient, concerned about the mass, returned to the surgeon in May 2010. Testing revealed cancer, and she underwent radical mastectomy and other treatment.
Patient’s claim Because the mass had not been treated in a timely manner, her 5-year survival rate in May 2010 was less than 50%. The surgeon was negligent in failing to order additional testing in September 2009. Magnetic resonance imaging (MRI) would have detected the cancer at a time when her survival rate could have been 80%.
Physician’s defense The cancer was diagnosed in a timely manner. An earlier diagnosis would not have changed the outcome.
Verdict A Tennessee defense verdict was returned.
Child stillborn, mother injured after vacuum extraction
When the mother’s labor slowed at a birthing center, she received several medications including castor oil, blue cohosh, and black cohosh to induce labor. The mother was later transferred by ambulance to a hospital. Ninety minutes after admission, the ObGyn used vacuum extraction to deliver a stillborn child. The mother sustained damage to her rectum, uterus, and vagina, had repair surgery, and has been unable to get pregnant again.
Parents’ claim While in labor at the birthing center, the castor oil, blue cohosh, and black cohosh caused the patient’s uterus to contract excessively and contributed to fetal death. The patient should have been transferred to the hospital earlier. Cesarean delivery should have been performed immediately upon her arrival at the hospital but the ObGyn did not arrive at the hospital for an hour after the patient’s admission.
Defendants’ defense The head midwife at the birthing center conceded negligence. The hospital claimed that the fetus was already dead before the mother arrived. The ObGyn denied negligence, arguing that he had no supervisory role or ownership in the birthing center and was not present during the mother’s labor. He also claimed that the fetus was dead in utero 12 or more hours before delivery and that an infectious process had developed in the mother during the 17 hours that she was at the birthing center.
Verdict A $4,095,000 Florida verdict was returned against the ObGyn. A directed verdict was granted for the hospital.
Patient still in pain after labia reduction
A 44-year-old woman underwent surgical reduction of her labia minora performed by a gynecologist. The procedure was intended to relieve discomfort during sexual activity. The patient continues to have pain.
Patient’s claim An excessive amount of the right labia minora was removed because proper presurgical demarcation of the operative area was not performed. Her pain during intercourse has worsened and she cannot properly urinate.
Physician’s defense Presurgical demarcation was correctly completed using clamps. Surgery was properly performed. The asymmetry is due to poor healing of the surgical wound. The patient’s clitoris was not scarred. The patient never reported complications related to urination to her gynecologist. Her ongoing pain is due to an estrogen deficiency.
Verdict A New York defense verdict was returned.
Uterine rupture after version for breech presentation: $7M
A woman went to the hospital for delivery of her baby. The fetus was in breech position, but the mother requested vaginal delivery. When the ObGyn attempted an external cephalic version to turn the baby, the uterus ruptured and the placenta was damaged. The baby sustained hypoxic-ischemic encephalopathy resulting in cerebral palsy (CP). He requires constant nursing care.
Parents’ claim The ObGyn failed to recognize fetal distress during the breech version. The ObGyn improperly performed the version, causing the uterine rupture. There was lack of informed consent for the version.
Defendants’ defense The case was settled during trial.
Verdict A $7 million New Jersey settlement was reached.
Sepsis following hysterectomy
An ObGyn performed total abdominal hysterectomy to treat uterine fibroids in a 26-year-old woman. Despite reporting abdominal pain, the patient was discharged on postsurgical day 4.
Three days later, she went to a different hospital with moderate diffuse abdominal pain, constipation, nausea, emesis, tachycardia, and low-grade fever. An abdominal radiograph was taken, the patient was given morphine and ketorolac, and she was sent home.
She returned to the first hospital 3 days later reporting fever, nausea, emesis, diarrhea, and severe abdominal pain. After an abdominal computed tomography (CT) scan revealed numerous fluid- and gas-filled collections, indicative of abscess, intravenous antibiotics were ordered and administered.
Six days later, an infectious disease physician was consulted. He made a diagnosis of sepsis secondary to abdominal infection.
The next day, an abdominal CT scan revealed enlargement of multiple abdominal and pelvic fluid collections.
At exploratory laparotomy, purulent fluid was found in the anterior fascial compartment, with gross pus in the abdomen. The entire bowel was dilated, inflamed, and matted. Necrotic rind and infection were noted on multiple surfaces of the colon and small intestine and the transverse colon was gangrenous and sealed to the right lower quadrant. The patient’s intestines were resected and an ileostomy was placed, which was reversed several months later.
Patient’s claim The ObGyn did not offer an alternative to hysterectomy. The ObGyn was negligent in injuring the small intestine during surgery and failing to recognize and treat it intraoperatively. The patient should not have been discharged based on her reported symptoms. Failure to recognize and treat the injury led to sepsis with severe complications and months of recuperation.
Physician’s defense There was no negligence; small bowel injury is a known risk of hysterectomy. Other caregivers at both hospitals were at fault for not properly diagnosing and treating the infection.
Verdict A $901,420 Nevada verdict was returned; the ObGyn was found 85% at fault and other parties 15% at fault. The court granted the physician’s motion to reduce the verdict to $436,954, which included $371,411 from the ObGyn.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements, & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Who was responsible for excessive oxytocin doses? $18.2M verdict
Early in the morning, a woman at 40 weeks’ gestation presented to the hospital for induction of labor managed by her ObGyn. Labor was lengthy, and the mother was given increasing doses of 22, 24, and 26 mIU/min of oxytocin to stimulate labor. The baby was delivered in the evening. The child suffered a hypoxic birth injury and has cerebral palsy.
Parents’ claim Excessive oxytocin was administered, causing uterine hyperstimulation and excessive contractions. Nurses failed to inform the ObGyn of an abnormal fetal heart rate during the afternoon.
Defendants’ defense The parties disputed the oxytocin orders. The ObGyn claimed she has a standing order against oxytocin doses over 20 mIU/min. The nurses claimed that the dosage was based on the ObGyn’s verbal orders, which the ObGyn denied. The ObGyn denied negligence and maintained that if she’d known of the oxytocin administration greater than 20 mIU/min and the abnormal fetal heart rate, she immediately would have called for cesarean delivery. The hospital denied negligence and maintained that the oxytocin was administered 10 hours before delivery and played no role in fetal distress.
Verdict At trial, the ObGyn did not call expert witnesses and, in closing arguments, the physician’s attorney asked for exoneration of the ObGyn and a finding of fault solely against the hospital. An $18.2 million Washington verdict was returned against the hospital.
What caused the child’s Erb’s palsy?
A mother presented to the hospital for induction of labor. Oxytocin was administered and the first stage of labor progressed normally. When the mother began pushing, the ObGyn noted a turtle sign at crowning and called for assistance. The ObGyn attempted to deliver the fetus with downward guidance of the fetal head but encountered shoulder dystocia and a nuchal cord. He unwrapped the cord and instructed the nursing staff to place the mother in the McRobert’s position to help dislodge the right shoulder. When that did not work, the ObGyn performed a first-degree episiotomy and completed delivery. The child was found to have Erb’s palsy of the right arm. She underwent decompression and neurolysis of the brachial plexus using sural nerve grafts but still has reduced use of her right arm.
Parents’ claim Shoulder dystocia was improperly managed, causing the brachial plexus injury.
Defendants’ defense The ObGyn and hospital system denied negligence. The child’s injury occurred in utero due to natural forces of the mother’s uterine contractions.
Verdict An Ohio defense verdict was returned.
Woman claims lack of proper consent
A 47-year-old woman underwent endometrial ablation performed by her ObGyn. During the procedure, the uterus was perforated and the ObGyn performed a hysterectomy. Six days later, the patient was found to have peritonitis and underwent bowel repair surgery. The patient developed untreatable bowel adhesions that cause chronic pain.
Patient’s claim There were less expensive and invasive alternatives to the ablation that the ObGyn did not offer. The patient claimed lack of informed consent for the ablation and hysterectomy and negligence in perforating the bowel. The ObGyn was also negligent in failing to recognize the perforation and to diagnose peritonitis in a timely manner.
Texas state law requires consent for hysterectomies without documented evidence of immediate danger to life. Her husband did not have the authority to consent on her behalf.
Physician’s defense The husband gave informed consent. Failure to recognize the perforation was not negligent; it is a known risk of the surgery. The patient’s care was transferred to another physician after the second postoperative day.
Verdict A $200,000 Texas settlement was reached.
Bowel obstruction in pregnant woman
A 29-year-old woman at 27 weeks’ gestation had abdominal pain. She went to a community hospital where a hospitalist was assigned to her care. After a day, the patient was found to have a small bowel obstruction and necrosis of the bowel. The baby was delivered preterm. The mother underwent 12 operations; half of her intestines were resected. The mother is being treated for posttraumatic stress syndrome. The child is autistic.
Parents’ claim The hospitalist did not diagnose the mother’s intestinal blockage in a timely manner and did not obtain an obstetric consult or notify the patient’s ObGyn. The hospital staff did not follow protocol to notify the mother’s ObGyn. The child’s autism is a result of preterm delivery.
Defendants’ defense The hospital denied any duty to notify the ObGyn if the patient was admitted to the hospital for nonobstetric reasons. The case was settled during trial.
Verdict A $4.2 million Washington settlement was reached including $3 million from the hospital.
Fourth-degree perineal tear and continuing pain after delivery
A woman in her 30s went to the hospital for induction of labor. After many hours, the ObGyn used vacuum extraction due to maternal fatigue. The baby emerged in compound presentation, with her hand at the side of her head. She weighed 9 lb 12 oz at birth. A fourth-degree perineal tear occurred at birth. Postpartum, a rectovaginal fistula developed that required several repair operations. The mother is unable to have intercourse due to continuing vaginal pain and discomfort.
Patient’s Claim Knowing that the father’s head was overly large, the ObGyn should have better estimated the fetus’ size, and should have performed cesarean delivery.
Physician’s defense The ObGyn admitted that he knew the baby was large but maintained that a large fetus does not mandate a cesarean delivery. There were no indications that the baby’s head or body was too large to fit through the mother’s pelvis, so a vaginal delivery was appropriate. A perineal tear is a known complication of childbirth and could not be prevented. The patient’s current pain is unrelated to the perineal tear.
Verdict A Pennsylvania defense verdict was returned.
Breast cancer missed in woman with dense breasts
In 2003, a 44-year-old woman was told she had dense fibrocystic breasts. From 2003 through 2009 she regularly saw a breast surgeon due to concern that breast cancer might be difficult to detect.
In August 2009, her ObGyn identified a questionable mass in her left breast after ultrasonography and mammography. The patient saw the surgeon in late September 2009; no further imaging was ordered and she was told to return in a year.
The patient, concerned about the mass, returned to the surgeon in May 2010. Testing revealed cancer, and she underwent radical mastectomy and other treatment.
Patient’s claim Because the mass had not been treated in a timely manner, her 5-year survival rate in May 2010 was less than 50%. The surgeon was negligent in failing to order additional testing in September 2009. Magnetic resonance imaging (MRI) would have detected the cancer at a time when her survival rate could have been 80%.
Physician’s defense The cancer was diagnosed in a timely manner. An earlier diagnosis would not have changed the outcome.
Verdict A Tennessee defense verdict was returned.
Child stillborn, mother injured after vacuum extraction
When the mother’s labor slowed at a birthing center, she received several medications including castor oil, blue cohosh, and black cohosh to induce labor. The mother was later transferred by ambulance to a hospital. Ninety minutes after admission, the ObGyn used vacuum extraction to deliver a stillborn child. The mother sustained damage to her rectum, uterus, and vagina, had repair surgery, and has been unable to get pregnant again.
Parents’ claim While in labor at the birthing center, the castor oil, blue cohosh, and black cohosh caused the patient’s uterus to contract excessively and contributed to fetal death. The patient should have been transferred to the hospital earlier. Cesarean delivery should have been performed immediately upon her arrival at the hospital but the ObGyn did not arrive at the hospital for an hour after the patient’s admission.
Defendants’ defense The head midwife at the birthing center conceded negligence. The hospital claimed that the fetus was already dead before the mother arrived. The ObGyn denied negligence, arguing that he had no supervisory role or ownership in the birthing center and was not present during the mother’s labor. He also claimed that the fetus was dead in utero 12 or more hours before delivery and that an infectious process had developed in the mother during the 17 hours that she was at the birthing center.
Verdict A $4,095,000 Florida verdict was returned against the ObGyn. A directed verdict was granted for the hospital.
Patient still in pain after labia reduction
A 44-year-old woman underwent surgical reduction of her labia minora performed by a gynecologist. The procedure was intended to relieve discomfort during sexual activity. The patient continues to have pain.
Patient’s claim An excessive amount of the right labia minora was removed because proper presurgical demarcation of the operative area was not performed. Her pain during intercourse has worsened and she cannot properly urinate.
Physician’s defense Presurgical demarcation was correctly completed using clamps. Surgery was properly performed. The asymmetry is due to poor healing of the surgical wound. The patient’s clitoris was not scarred. The patient never reported complications related to urination to her gynecologist. Her ongoing pain is due to an estrogen deficiency.
Verdict A New York defense verdict was returned.
Uterine rupture after version for breech presentation: $7M
A woman went to the hospital for delivery of her baby. The fetus was in breech position, but the mother requested vaginal delivery. When the ObGyn attempted an external cephalic version to turn the baby, the uterus ruptured and the placenta was damaged. The baby sustained hypoxic-ischemic encephalopathy resulting in cerebral palsy (CP). He requires constant nursing care.
Parents’ claim The ObGyn failed to recognize fetal distress during the breech version. The ObGyn improperly performed the version, causing the uterine rupture. There was lack of informed consent for the version.
Defendants’ defense The case was settled during trial.
Verdict A $7 million New Jersey settlement was reached.
Sepsis following hysterectomy
An ObGyn performed total abdominal hysterectomy to treat uterine fibroids in a 26-year-old woman. Despite reporting abdominal pain, the patient was discharged on postsurgical day 4.
Three days later, she went to a different hospital with moderate diffuse abdominal pain, constipation, nausea, emesis, tachycardia, and low-grade fever. An abdominal radiograph was taken, the patient was given morphine and ketorolac, and she was sent home.
She returned to the first hospital 3 days later reporting fever, nausea, emesis, diarrhea, and severe abdominal pain. After an abdominal computed tomography (CT) scan revealed numerous fluid- and gas-filled collections, indicative of abscess, intravenous antibiotics were ordered and administered.
Six days later, an infectious disease physician was consulted. He made a diagnosis of sepsis secondary to abdominal infection.
The next day, an abdominal CT scan revealed enlargement of multiple abdominal and pelvic fluid collections.
At exploratory laparotomy, purulent fluid was found in the anterior fascial compartment, with gross pus in the abdomen. The entire bowel was dilated, inflamed, and matted. Necrotic rind and infection were noted on multiple surfaces of the colon and small intestine and the transverse colon was gangrenous and sealed to the right lower quadrant. The patient’s intestines were resected and an ileostomy was placed, which was reversed several months later.
Patient’s claim The ObGyn did not offer an alternative to hysterectomy. The ObGyn was negligent in injuring the small intestine during surgery and failing to recognize and treat it intraoperatively. The patient should not have been discharged based on her reported symptoms. Failure to recognize and treat the injury led to sepsis with severe complications and months of recuperation.
Physician’s defense There was no negligence; small bowel injury is a known risk of hysterectomy. Other caregivers at both hospitals were at fault for not properly diagnosing and treating the infection.
Verdict A $901,420 Nevada verdict was returned; the ObGyn was found 85% at fault and other parties 15% at fault. The court granted the physician’s motion to reduce the verdict to $436,954, which included $371,411 from the ObGyn.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements, & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In this article
- What caused the child’s Erb’s palsy?
- Woman claims lack of proper consent
- Bowel obstruction in pregnant woman
- Fourth-degree perineal tear and continuing pain after delivery
- Breast cancer missed in woman with dense breasts
- Child stillborn, mother injured after vacuum extraction
- Patient still in pain after labia reduction
- Uterine rupture after version for breech presentation: $7M
- Sepsis following hysterectomy
AUA: Long-term use of Botox may decrease urinary incontinence
NEW ORLEANS – Long-term treatment with onabotulinumtoxinA significantly decreased daily urinary incontinence episodes in patients with overactive bladder syndrome, with no increase in adverse effects tied to repeated treatment, according to Dr. Victor W. Nitti.
Dr. Nitti and his colleagues conducted a multicenter extension study evaluating the long-term efficacy and safety of repeated treatments with onabotulinumtoxinA (onabotA) in patients with overactive bladder (OAB).
After completion of either of two 24-week, randomized phase III trials, patients were eligible to enter a 3-year extension study in which they could receive multiple onabotA treatments at 100 units per dose, Dr Nitti reported at the annual meeting of the American Urological Association.
Patients were treated “as needed” based on their request, and their fulfillment of the prespecified qualification criteria. “Patients requesting treatment had to have at least two urgency incontinence episodes in a 3-day diary, at least 12 weeks since their last treatment, and their postvoid residual had to be less than 200 cc,” said Dr. Nitti, professor of urology at New York University, New York. Therefore, the total number of treatments delivered during the study differed among patients depending on need.
Coprimary endpoints included change from baseline in urinary incontinence episodes per day at week 12 and the proportion of patients reporting improvement or great improvement in their urinary incontinence (UI) at 12 weeks. Data were assessed for six subpopulations of patients based on the number of onabotA treatments (one to six) needed during the study; duration of effect (time to request for retreatment) in all six cycles also was evaluated in order to assess the consistency of response to repeated treatments.
Local anesthesia was administered to patients, and onabotA was delivered via injection into the muscle of the bladder. “Once the botulinum toxin gets into the terminal nerve, it will prevent the release of neurotransmitters, particularly acetylcholine; when acetylcholine is not released, there is less of a trigger for the bladder to contract,” he explained.
Of the 829 patients enrolled, 51.7% completed the 3-year study. About 5% did not complete the study because of an adverse event, and only 5.7% dropped out because of a lack of efficacy. “Over the 31/2-year period, patients were lost to follow-up, had protocol violations, and sites closed. So most of the reasons for discontinuation weren’t due to lack of efficacy or adverse events,” Dr. Nitti said.
The baseline mean UI episodes per day was a little over 5.5 for all treatment cycles; consistent reductions in UI episodes were observed in the overall population results regardless of the number of treatments received, with overall reduction between 3.1 and 3.8 episodes per day.
“Also consistent was the number of patients who reported being greatly improved or improved on the treatment benefit scale, which remained at right around 80% regardless of treatment cycle,” he added.
Patients who received fewer treatments had a longer duration of effect than those who received more treatments. The overall median duration of effect was 7.6 months, and 34.2% of the patients reported control of their urinary incontinence symptoms for at least 6 months. The median time to request retreatment was >6 to ≤12 months for 37.2%, and >12 months for 28.5% of patients. Urinary tract infection was the most common adverse event observed.
Based on these data, Dr. Nitti and his colleagues concluded that long-term treatment of OAB with onabotA resulted in decreased daily urinary incontinence episodes, with no increase in adverse events tied to recurrent treatment.
Dr. Nitti disclosed financial relationships with Allergan, and numerous other pharmaceutical and device companies.
NEW ORLEANS – Long-term treatment with onabotulinumtoxinA significantly decreased daily urinary incontinence episodes in patients with overactive bladder syndrome, with no increase in adverse effects tied to repeated treatment, according to Dr. Victor W. Nitti.
Dr. Nitti and his colleagues conducted a multicenter extension study evaluating the long-term efficacy and safety of repeated treatments with onabotulinumtoxinA (onabotA) in patients with overactive bladder (OAB).
After completion of either of two 24-week, randomized phase III trials, patients were eligible to enter a 3-year extension study in which they could receive multiple onabotA treatments at 100 units per dose, Dr Nitti reported at the annual meeting of the American Urological Association.
Patients were treated “as needed” based on their request, and their fulfillment of the prespecified qualification criteria. “Patients requesting treatment had to have at least two urgency incontinence episodes in a 3-day diary, at least 12 weeks since their last treatment, and their postvoid residual had to be less than 200 cc,” said Dr. Nitti, professor of urology at New York University, New York. Therefore, the total number of treatments delivered during the study differed among patients depending on need.
Coprimary endpoints included change from baseline in urinary incontinence episodes per day at week 12 and the proportion of patients reporting improvement or great improvement in their urinary incontinence (UI) at 12 weeks. Data were assessed for six subpopulations of patients based on the number of onabotA treatments (one to six) needed during the study; duration of effect (time to request for retreatment) in all six cycles also was evaluated in order to assess the consistency of response to repeated treatments.
Local anesthesia was administered to patients, and onabotA was delivered via injection into the muscle of the bladder. “Once the botulinum toxin gets into the terminal nerve, it will prevent the release of neurotransmitters, particularly acetylcholine; when acetylcholine is not released, there is less of a trigger for the bladder to contract,” he explained.
Of the 829 patients enrolled, 51.7% completed the 3-year study. About 5% did not complete the study because of an adverse event, and only 5.7% dropped out because of a lack of efficacy. “Over the 31/2-year period, patients were lost to follow-up, had protocol violations, and sites closed. So most of the reasons for discontinuation weren’t due to lack of efficacy or adverse events,” Dr. Nitti said.
The baseline mean UI episodes per day was a little over 5.5 for all treatment cycles; consistent reductions in UI episodes were observed in the overall population results regardless of the number of treatments received, with overall reduction between 3.1 and 3.8 episodes per day.
“Also consistent was the number of patients who reported being greatly improved or improved on the treatment benefit scale, which remained at right around 80% regardless of treatment cycle,” he added.
Patients who received fewer treatments had a longer duration of effect than those who received more treatments. The overall median duration of effect was 7.6 months, and 34.2% of the patients reported control of their urinary incontinence symptoms for at least 6 months. The median time to request retreatment was >6 to ≤12 months for 37.2%, and >12 months for 28.5% of patients. Urinary tract infection was the most common adverse event observed.
Based on these data, Dr. Nitti and his colleagues concluded that long-term treatment of OAB with onabotA resulted in decreased daily urinary incontinence episodes, with no increase in adverse events tied to recurrent treatment.
Dr. Nitti disclosed financial relationships with Allergan, and numerous other pharmaceutical and device companies.
NEW ORLEANS – Long-term treatment with onabotulinumtoxinA significantly decreased daily urinary incontinence episodes in patients with overactive bladder syndrome, with no increase in adverse effects tied to repeated treatment, according to Dr. Victor W. Nitti.
Dr. Nitti and his colleagues conducted a multicenter extension study evaluating the long-term efficacy and safety of repeated treatments with onabotulinumtoxinA (onabotA) in patients with overactive bladder (OAB).
After completion of either of two 24-week, randomized phase III trials, patients were eligible to enter a 3-year extension study in which they could receive multiple onabotA treatments at 100 units per dose, Dr Nitti reported at the annual meeting of the American Urological Association.
Patients were treated “as needed” based on their request, and their fulfillment of the prespecified qualification criteria. “Patients requesting treatment had to have at least two urgency incontinence episodes in a 3-day diary, at least 12 weeks since their last treatment, and their postvoid residual had to be less than 200 cc,” said Dr. Nitti, professor of urology at New York University, New York. Therefore, the total number of treatments delivered during the study differed among patients depending on need.
Coprimary endpoints included change from baseline in urinary incontinence episodes per day at week 12 and the proportion of patients reporting improvement or great improvement in their urinary incontinence (UI) at 12 weeks. Data were assessed for six subpopulations of patients based on the number of onabotA treatments (one to six) needed during the study; duration of effect (time to request for retreatment) in all six cycles also was evaluated in order to assess the consistency of response to repeated treatments.
Local anesthesia was administered to patients, and onabotA was delivered via injection into the muscle of the bladder. “Once the botulinum toxin gets into the terminal nerve, it will prevent the release of neurotransmitters, particularly acetylcholine; when acetylcholine is not released, there is less of a trigger for the bladder to contract,” he explained.
Of the 829 patients enrolled, 51.7% completed the 3-year study. About 5% did not complete the study because of an adverse event, and only 5.7% dropped out because of a lack of efficacy. “Over the 31/2-year period, patients were lost to follow-up, had protocol violations, and sites closed. So most of the reasons for discontinuation weren’t due to lack of efficacy or adverse events,” Dr. Nitti said.
The baseline mean UI episodes per day was a little over 5.5 for all treatment cycles; consistent reductions in UI episodes were observed in the overall population results regardless of the number of treatments received, with overall reduction between 3.1 and 3.8 episodes per day.
“Also consistent was the number of patients who reported being greatly improved or improved on the treatment benefit scale, which remained at right around 80% regardless of treatment cycle,” he added.
Patients who received fewer treatments had a longer duration of effect than those who received more treatments. The overall median duration of effect was 7.6 months, and 34.2% of the patients reported control of their urinary incontinence symptoms for at least 6 months. The median time to request retreatment was >6 to ≤12 months for 37.2%, and >12 months for 28.5% of patients. Urinary tract infection was the most common adverse event observed.
Based on these data, Dr. Nitti and his colleagues concluded that long-term treatment of OAB with onabotA resulted in decreased daily urinary incontinence episodes, with no increase in adverse events tied to recurrent treatment.
Dr. Nitti disclosed financial relationships with Allergan, and numerous other pharmaceutical and device companies.
AT THE AUA ANNUAL MEETING
Key clinical point: OnabotulinumtoxinA delivered via injection into the muscle of the bladder appears to be a good option for patients with overactive bladder syndrome (OAB) experiencing daily urinary incontinence (UI) episodes.
Major finding: Consistent reductions in UI episodes were observed in the overall population results, regardless of the number of treatments received. Overall, reductions were between 3.1 and 3.8 episodes per day.
Data source: A multicenter extension study of more than 400 patients with OAB experiencing daily UI episodes.
Disclosures: Dr. Nitti disclosed financial relationships with Allergan, and numerous other pharmaceutical and device companies.
Sexually transmitted infections missed as UTIs are overdiagnosed
Women may be receiving unnecessary antibiotics for overdiagnosed urinary tract infections while their sexually transmitted infections go undetected, according to a recent study in an urban academic emergency department.
“Our study is a reflection of what happens in current clinical practice in an ED setting including adult women 18-65 years of age for whom UTI diagnoses and empiric therapy for UTI are often given even in the absence of any UTI-related symptoms and without a urine culture,” Dr. Michelle T. Hecker of MetroHealth Medical Center, Cleveland, and her colleagues wrote in the Journal of Clinical Microbiology (J. Clin. Microbiol. 2015 [doi:10.1128/JCM.00670-15]).
Overdiagnosis of UTI was not only a common cause of unnecessary antibiotic use, it also contributed to underdiagnosis of STI since 64% of the patients with a missed STI were diagnosed as having a UTI instead, they reported.
The researchers compared urinalysis, culture, and nucleic acid amplification testing for gonorrhea, chlamydia, and trichomoniasis among 264 women, aged 18-65 years, who presented to an urban academic emergency department over a 2-month period. Although providers diagnosed 66% of these women with UTIs, less than half these women (48%) had a positive urine culture and more than half (57%) received treatment without a urine culture.
Among the 23% of women overall who had at least one positive STI test, 37% (22 of 60 women) did not receive treatment for their STI within 7 days of their visit, and 14 of those 22 women (64%) received a UTI diagnosis instead of an STI diagnosis.
Urinalysis was abnormal for 92% of all the women in the study and did not predict positive urine cultures. The researchers determined the positive predictive value of abnormal urinalysis to be 41% and the negative predictive value to be 76%.
“Based on our data and others, we believe that alternative test and treat strategies for managing women with [genitourinary] and nonspecific abdominal pain in the ED should be evaluated,” Dr. Hecker and her associates wrote.
They specifically recommended decreasing urinalysis testing and increasing urine culture and STI testing.
The research was supported by a grant from the Centers for Disease Control and Prevention. One of the researchers reported that he is an R&D scientist employed by Hologic.
Women may be receiving unnecessary antibiotics for overdiagnosed urinary tract infections while their sexually transmitted infections go undetected, according to a recent study in an urban academic emergency department.
“Our study is a reflection of what happens in current clinical practice in an ED setting including adult women 18-65 years of age for whom UTI diagnoses and empiric therapy for UTI are often given even in the absence of any UTI-related symptoms and without a urine culture,” Dr. Michelle T. Hecker of MetroHealth Medical Center, Cleveland, and her colleagues wrote in the Journal of Clinical Microbiology (J. Clin. Microbiol. 2015 [doi:10.1128/JCM.00670-15]).
Overdiagnosis of UTI was not only a common cause of unnecessary antibiotic use, it also contributed to underdiagnosis of STI since 64% of the patients with a missed STI were diagnosed as having a UTI instead, they reported.
The researchers compared urinalysis, culture, and nucleic acid amplification testing for gonorrhea, chlamydia, and trichomoniasis among 264 women, aged 18-65 years, who presented to an urban academic emergency department over a 2-month period. Although providers diagnosed 66% of these women with UTIs, less than half these women (48%) had a positive urine culture and more than half (57%) received treatment without a urine culture.
Among the 23% of women overall who had at least one positive STI test, 37% (22 of 60 women) did not receive treatment for their STI within 7 days of their visit, and 14 of those 22 women (64%) received a UTI diagnosis instead of an STI diagnosis.
Urinalysis was abnormal for 92% of all the women in the study and did not predict positive urine cultures. The researchers determined the positive predictive value of abnormal urinalysis to be 41% and the negative predictive value to be 76%.
“Based on our data and others, we believe that alternative test and treat strategies for managing women with [genitourinary] and nonspecific abdominal pain in the ED should be evaluated,” Dr. Hecker and her associates wrote.
They specifically recommended decreasing urinalysis testing and increasing urine culture and STI testing.
The research was supported by a grant from the Centers for Disease Control and Prevention. One of the researchers reported that he is an R&D scientist employed by Hologic.
Women may be receiving unnecessary antibiotics for overdiagnosed urinary tract infections while their sexually transmitted infections go undetected, according to a recent study in an urban academic emergency department.
“Our study is a reflection of what happens in current clinical practice in an ED setting including adult women 18-65 years of age for whom UTI diagnoses and empiric therapy for UTI are often given even in the absence of any UTI-related symptoms and without a urine culture,” Dr. Michelle T. Hecker of MetroHealth Medical Center, Cleveland, and her colleagues wrote in the Journal of Clinical Microbiology (J. Clin. Microbiol. 2015 [doi:10.1128/JCM.00670-15]).
Overdiagnosis of UTI was not only a common cause of unnecessary antibiotic use, it also contributed to underdiagnosis of STI since 64% of the patients with a missed STI were diagnosed as having a UTI instead, they reported.
The researchers compared urinalysis, culture, and nucleic acid amplification testing for gonorrhea, chlamydia, and trichomoniasis among 264 women, aged 18-65 years, who presented to an urban academic emergency department over a 2-month period. Although providers diagnosed 66% of these women with UTIs, less than half these women (48%) had a positive urine culture and more than half (57%) received treatment without a urine culture.
Among the 23% of women overall who had at least one positive STI test, 37% (22 of 60 women) did not receive treatment for their STI within 7 days of their visit, and 14 of those 22 women (64%) received a UTI diagnosis instead of an STI diagnosis.
Urinalysis was abnormal for 92% of all the women in the study and did not predict positive urine cultures. The researchers determined the positive predictive value of abnormal urinalysis to be 41% and the negative predictive value to be 76%.
“Based on our data and others, we believe that alternative test and treat strategies for managing women with [genitourinary] and nonspecific abdominal pain in the ED should be evaluated,” Dr. Hecker and her associates wrote.
They specifically recommended decreasing urinalysis testing and increasing urine culture and STI testing.
The research was supported by a grant from the Centers for Disease Control and Prevention. One of the researchers reported that he is an R&D scientist employed by Hologic.
FROM THE JOURNAL OF CLINICAL MICROBIOLOGY
Key clinical point: Overdiagnosis of urinary tract infections and underdiagnosis of sexually transmitted infections are common in women presenting to the emergency department.
Major finding: About 52% of women were overdiagnosed with a UTI; STI underdiagnosis was 37%.
Data source: The findings are based on a 2-month observational cohort of 264 women presenting at an urban academic emergency department with genitourinary symptoms or diagnosed infections.
Disclosures: The research was supported by a grant from the Centers for Disease Control and Prevention. One of the researchers reported that he is an R&D scientist employed by Hologic.
Does adjuvant oophorectomy improve survival in BRCA1 or BRCA2 mutation carriers with breast cancer?
Although bilateral salpingo-oophorectomy is known to prevent breast and ovarian cancer in BRCA mutation carriers,1 published reports also have suggested that, among mutation carriers with breast cancer, oophorectomy improves survival. In this retrospective analysis, investigators focused on women with stage I or II breast cancer and a BRCA1 or BRCA2 mutation, observing them for as long as 20 years after diagnosis. Survival rates were compared between women who did and did not undergo oophorectomy.
Details of the trial
Metcalfe and colleagues followed women with a BRCA1 or BRCA2 mutation and intact ovaries who were diagnosed with breast cancer at age 65 or younger between 1975 and 2008, tracking them for a mean of 12.5 years. Of 676 women, 345 underwent oophorectomy, usually with the intent of preventing ovarian cancer.
Overall, oophorectomy was associated with a 56% reduction in the risk of breast cancer-specific mortality (P = .005). Among breast cancer survivors with a BRCA1 mutation, oophorectomy was associated with a significant 62% reduction in breast cancer mortality. Among BRCA2 carriers, the observed 43% reduction in breast cancer mortality did not achieve statistical significance (P = .23).
Full impact of oophorectomy may be difficult to tease out
As Metcalfe and colleagues point out, recent improvements in breast imaging that have led to earlier diagnosis, as well as improvements in the treatment of breast cancer, might attenuate the mortality benefits observed with oophorectomy.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This important report underscores the importance of testing all women with early-stage breast cancer for BRCA mutations, and informs the management of known BRCA1 carriers with breast cancer.
—Andrew M. Kaunitz, MD
Reference
1. Finch APM, Lubinski J, Moller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. JCO. 2014;32(15):1547–1553.
Although bilateral salpingo-oophorectomy is known to prevent breast and ovarian cancer in BRCA mutation carriers,1 published reports also have suggested that, among mutation carriers with breast cancer, oophorectomy improves survival. In this retrospective analysis, investigators focused on women with stage I or II breast cancer and a BRCA1 or BRCA2 mutation, observing them for as long as 20 years after diagnosis. Survival rates were compared between women who did and did not undergo oophorectomy.
Details of the trial
Metcalfe and colleagues followed women with a BRCA1 or BRCA2 mutation and intact ovaries who were diagnosed with breast cancer at age 65 or younger between 1975 and 2008, tracking them for a mean of 12.5 years. Of 676 women, 345 underwent oophorectomy, usually with the intent of preventing ovarian cancer.
Overall, oophorectomy was associated with a 56% reduction in the risk of breast cancer-specific mortality (P = .005). Among breast cancer survivors with a BRCA1 mutation, oophorectomy was associated with a significant 62% reduction in breast cancer mortality. Among BRCA2 carriers, the observed 43% reduction in breast cancer mortality did not achieve statistical significance (P = .23).
Full impact of oophorectomy may be difficult to tease out
As Metcalfe and colleagues point out, recent improvements in breast imaging that have led to earlier diagnosis, as well as improvements in the treatment of breast cancer, might attenuate the mortality benefits observed with oophorectomy.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This important report underscores the importance of testing all women with early-stage breast cancer for BRCA mutations, and informs the management of known BRCA1 carriers with breast cancer.
—Andrew M. Kaunitz, MD
Although bilateral salpingo-oophorectomy is known to prevent breast and ovarian cancer in BRCA mutation carriers,1 published reports also have suggested that, among mutation carriers with breast cancer, oophorectomy improves survival. In this retrospective analysis, investigators focused on women with stage I or II breast cancer and a BRCA1 or BRCA2 mutation, observing them for as long as 20 years after diagnosis. Survival rates were compared between women who did and did not undergo oophorectomy.
Details of the trial
Metcalfe and colleagues followed women with a BRCA1 or BRCA2 mutation and intact ovaries who were diagnosed with breast cancer at age 65 or younger between 1975 and 2008, tracking them for a mean of 12.5 years. Of 676 women, 345 underwent oophorectomy, usually with the intent of preventing ovarian cancer.
Overall, oophorectomy was associated with a 56% reduction in the risk of breast cancer-specific mortality (P = .005). Among breast cancer survivors with a BRCA1 mutation, oophorectomy was associated with a significant 62% reduction in breast cancer mortality. Among BRCA2 carriers, the observed 43% reduction in breast cancer mortality did not achieve statistical significance (P = .23).
Full impact of oophorectomy may be difficult to tease out
As Metcalfe and colleagues point out, recent improvements in breast imaging that have led to earlier diagnosis, as well as improvements in the treatment of breast cancer, might attenuate the mortality benefits observed with oophorectomy.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This important report underscores the importance of testing all women with early-stage breast cancer for BRCA mutations, and informs the management of known BRCA1 carriers with breast cancer.
—Andrew M. Kaunitz, MD
Reference
1. Finch APM, Lubinski J, Moller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. JCO. 2014;32(15):1547–1553.
Reference
1. Finch APM, Lubinski J, Moller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. JCO. 2014;32(15):1547–1553.
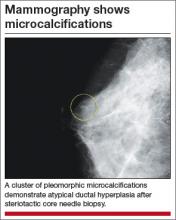
Atypical hyperplasia of the breast: Cancer risk-reduction strategies
Of the approximately 1 million benign breast biopsies obtained annually from US women, some 10% yield a diagnosis of atypical hyperplasia, microscopically classified as ductal or lobular. Atypical hyperplasia represents a “proliferation of dysplastic, monotonous epithelial-cell populations that include clonal subpopulations. In models of breast carcinogenesis, atypical hyperplasia occupies a transitional zone between benign and malignant disease,” write Hartmann and colleagues, the authors of a recent special report in the New England Journal of Medicine.1
Long-term follow-up studies have found atypical hyperplasia to confer a relative risk for breast cancer of 4.0. Although these findings are well established, the cumulative absolute risk for breast cancer conferred by a diagnosis of atypical hyperplasia only recently has been described. Hartmann and colleagues note that it approaches 30% over 25 years.1
Recommendations for clinical practice
The authors of this special report do a service to women and their clinicians by pointing out the high long-term risk of malignancy faced by women with atypical hyperplasia of the breast. They also make a number of important recommendations for practice:
- When counseling patients with this diagnosis, it is preferable to use cumulative incidence data because the most commonly used breast cancer risk-prediction models do not accurately estimate the risk for breast malignancy in women with atypical hyperplasia.
- When atypical hyperplasia of the breast is found after core-needle biopsy (FIGURE), surgical excision of the site is recommended to ensure that cancer was not missed as a result of a sampling error. This recommendation derives from National Comprehensive Cancer Network (NCCN) guidelines.2 “In the case of atypical ductal hyperplasia, the frequency of finding breast cancer (‘upgrading’) with surgical excision is 15% to 30% or even higher, despite the use of large-gauge (9- or 11-gauge) core-needle biopsy with vacuum-assisted devices,” Hartmann and colleagues note.
- Women with atypical hyperplasia clearly should receive annual mammographic screening. Although screening magnetic resonance imaging (MRI) may play a role in assessing women with this diagnosis, no prospective trial data have evaluated its utility in this setting. Screening MRI’s low specificity may lead to many unnecessary biopsies with benign findings. This in turn can generate so much anxiety that women may pursue prophylactic bilateral mastectomy to avoid a lifetime of stress related to breast cancer concerns. Women with atypical hyperplasia should be included in future trials of new breast imaging technologies.
- As with other high-risk women, those who have been diagnosed with atypical hyperplasia are well served by being referred to and followed by a physician with special expertise in breast disease who can arrange appropriate screening and follow-up. (See the sidebar, “Here’s how I counsel women with atypical hyperplasia about their management options.”)
- Women with a history of atypical hyperplasia who are considering initiation of systemic menopausal hormone therapyshould be aware that they have a higher baseline risk for invasive breast cancer than other women. Accordingly, the absolute risk of invasive breast cancer associated with use of estrogen-progestin menopausal hormone therapy (EPT) is also likely substantially higher than in average-risk women. Therefore, among women with a history of atypical hyperplasia of the breast who have an intact uterus, use of EPT should be minimized.
- Selective estrogen receptor modulators such as tamoxifen and raloxifene should be more widely used by women with atypical hyperplasia because of their ability to reduce breast cancer risk. Aromatase inhibitors also should be prescribed more widely in this population. (Again, see the sidebar, “Here’s how I counsel women with atypical hyperplasia about their management options.”)
When chemoprevention may be in order
If the 5-year risk of breast cancer by the Gail model is greater than 1.7%, and the patient is older than 35 years, I counsel her that she qualifies for chemoprevention with prophylactic endocrine therapy with the selective estrogen receptor modulators tamoxifen or raloxifene, or the aromatase inhibitor exemestane.1 The choice of drug depends on her menopausal status, bone mineral density, and presence of other comorbidities.
Although tamoxifen is indicated for breast cancer chemoprophylaxis in premenopausal and postmenopausal women, raloxifene is only approved for risk reduction in postmenopausal women. Likewise, aromatase inhibitors (which have shown high efficacy in chemoprophylaxis but are not FDA-approved for this indication) should be used only in postmenopausal women.
Who might gain the most from tamoxifen? The tamoxifen risk/benefit calculator2,3 can be used to weigh the benefit of breast cancer prevention against the risk of the drug’s adverse effects. Life-threatening adverse effects can include thromboembolic events and endometrial malignancy.2,3 Based on recommendations from the US Preventive Services Task Force, women with a 5-year risk of breast cancer equal to or greater than 3% are most likely to benefit from 5 years of prophylactic endocrine therapy.2 In women who are posthysterectomy, the benefit/risk ratio associated with tamoxifen use is higher.
When is annual MRI appropriate?
The decision to perform annual screening breast MRI should be based on a strong family history rather than strictly a biopsy diagnosis of atypia. The Claus and BRCAPRO models are more appropriate here, as they use only family history information and do not incorporate biopsy results. There are no data to support the use of screening breast MRI in patients with atypia who do not have a strong family history or a deleterious genetic mutation.4,5
Patients with proliferative breast disease tend to have a substantial amount of vague glandular enhancement on breast MRI. Screening MRI in patients with atypia is more likely to lead to frequent false-positive results and unnecessary benign biopsies and cause significant patient anxiety. Without endocrine blockade, breast MRI in this population tends to be nondiagnostic, with a very low yield for breast cancer diagnosis (positive predictive value, 20%).6 Repeated false-positive results of screening MRI in this population can cause patient anxiety, culminating in unnecessary mastectomies. If the Claus or BRCAPRO models yield a lifetime risk for breast cancer above 20%, or the breasts are extremely dense, I discuss with my patient the possibility of adding screening breast MRI.
When ordering breast MRI, it’s important to be aware that this imaging requires gadolinium intravenous contrast, which is excreted through the kidney and requires adequate renal function. This contrast agent can lead to nephrosclerosis in patients with renal insufficiency. In patients with hypertension, diabetes, age over 60, or prior chemotherapy, a recent serum blood urea nitrogen/creatinine level is required. Therefore, the decision to perform annual breast MRI for the rest of a woman’s life should not be taken lightly.
As a part of comprehensive risk assessment, it is important to identify patients who qualify for genetic testing. The addition of screening breast MRI should be heavily dependent on family history, results of BRCA testing and, possibly, mammographic breast density.
Make sure your patient knows that her condition places her at elevated risk, and refer her to a breast specialist
It’s also important to involve the patient in decision making to help ensure that she is proactive and adherent when choosing the best way to manage her risk. The key is to educate her about the importance of atypia.
Many women are told that their follow-up surgical excision was “benign,” and the subject of “atypia” or risk reduction is never addressed. It’s important that the right diagnostic terminology and coding are documented in the medical record so that the finding of atypia is not downgraded to a “benign breast biopsy.”
Finally, due to the complexities of this issue, evaluation by a qualified breast specialist or high-risk cancer program is recommended.
—Laila Samiian, MD
References
1. Cuzick J, Sestak I, Bonanni B, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381(9880):1827–1834.
2. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29(17):2327–2333.
3. Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91(21):1829–1846.
4. Port ER, Park A, Borgen PI, Morris E, Montgomery LL. Results of MRI screening for breast cancer in high-risk patients with LCIS and atypical hyperplasia. Ann Surg Oncol. 2007;14(3):1051–1057.
5. Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. Special report: atypical hyperplasia of the breast—risk assessment and management options. N Eng J Med. 2015;372(1):78–89.
6. Schwartz T, Cyr A, Margenthaler J. Screening breast magnetic resonance imaging in women with atypia or lobular carcinoma in situ. J Surg Res. 2015;193(2):519–522.
Most women will not develop breast malignancy
As Hartmann and colleagues point out, all is not dire once a woman is diagnosed with atypical hyperplasia of the breast. In most of these women, breast cancer will not develop—and if it does develop, it may occur at an age when mortality from other causes is more likely than from breast cancer. In this respect, women with atypical hyperplasia of the breast are different from carriers of BRCA mutations. Although women with atypical hyperplasia as well as mutation carriers are both at high lifetime risk for breast cancer, breast malignancies occur at an earlier age in mutation carriers. Accordingly, as the authors of this special report advise, in general, a diagnosis of atypical hyperplasia should not be considered an indication for risk-reducing bilateral mastectomy.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Hartman LC, Degnim AC Santen RJ, Dupont WD, Ghosh K. Special report: atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med. 2015;372(1):78–89.
2. National Comprehensive Cancer Network. Clinical practice guidelines: breast cancer screening and diagnosis, version 1. 2014. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#detection. Accessed March 24, 2015.
Of the approximately 1 million benign breast biopsies obtained annually from US women, some 10% yield a diagnosis of atypical hyperplasia, microscopically classified as ductal or lobular. Atypical hyperplasia represents a “proliferation of dysplastic, monotonous epithelial-cell populations that include clonal subpopulations. In models of breast carcinogenesis, atypical hyperplasia occupies a transitional zone between benign and malignant disease,” write Hartmann and colleagues, the authors of a recent special report in the New England Journal of Medicine.1
Long-term follow-up studies have found atypical hyperplasia to confer a relative risk for breast cancer of 4.0. Although these findings are well established, the cumulative absolute risk for breast cancer conferred by a diagnosis of atypical hyperplasia only recently has been described. Hartmann and colleagues note that it approaches 30% over 25 years.1
Recommendations for clinical practice
The authors of this special report do a service to women and their clinicians by pointing out the high long-term risk of malignancy faced by women with atypical hyperplasia of the breast. They also make a number of important recommendations for practice:
- When counseling patients with this diagnosis, it is preferable to use cumulative incidence data because the most commonly used breast cancer risk-prediction models do not accurately estimate the risk for breast malignancy in women with atypical hyperplasia.
- When atypical hyperplasia of the breast is found after core-needle biopsy (FIGURE), surgical excision of the site is recommended to ensure that cancer was not missed as a result of a sampling error. This recommendation derives from National Comprehensive Cancer Network (NCCN) guidelines.2 “In the case of atypical ductal hyperplasia, the frequency of finding breast cancer (‘upgrading’) with surgical excision is 15% to 30% or even higher, despite the use of large-gauge (9- or 11-gauge) core-needle biopsy with vacuum-assisted devices,” Hartmann and colleagues note.
- Women with atypical hyperplasia clearly should receive annual mammographic screening. Although screening magnetic resonance imaging (MRI) may play a role in assessing women with this diagnosis, no prospective trial data have evaluated its utility in this setting. Screening MRI’s low specificity may lead to many unnecessary biopsies with benign findings. This in turn can generate so much anxiety that women may pursue prophylactic bilateral mastectomy to avoid a lifetime of stress related to breast cancer concerns. Women with atypical hyperplasia should be included in future trials of new breast imaging technologies.
- As with other high-risk women, those who have been diagnosed with atypical hyperplasia are well served by being referred to and followed by a physician with special expertise in breast disease who can arrange appropriate screening and follow-up. (See the sidebar, “Here’s how I counsel women with atypical hyperplasia about their management options.”)
- Women with a history of atypical hyperplasia who are considering initiation of systemic menopausal hormone therapyshould be aware that they have a higher baseline risk for invasive breast cancer than other women. Accordingly, the absolute risk of invasive breast cancer associated with use of estrogen-progestin menopausal hormone therapy (EPT) is also likely substantially higher than in average-risk women. Therefore, among women with a history of atypical hyperplasia of the breast who have an intact uterus, use of EPT should be minimized.
- Selective estrogen receptor modulators such as tamoxifen and raloxifene should be more widely used by women with atypical hyperplasia because of their ability to reduce breast cancer risk. Aromatase inhibitors also should be prescribed more widely in this population. (Again, see the sidebar, “Here’s how I counsel women with atypical hyperplasia about their management options.”)
When chemoprevention may be in order
If the 5-year risk of breast cancer by the Gail model is greater than 1.7%, and the patient is older than 35 years, I counsel her that she qualifies for chemoprevention with prophylactic endocrine therapy with the selective estrogen receptor modulators tamoxifen or raloxifene, or the aromatase inhibitor exemestane.1 The choice of drug depends on her menopausal status, bone mineral density, and presence of other comorbidities.
Although tamoxifen is indicated for breast cancer chemoprophylaxis in premenopausal and postmenopausal women, raloxifene is only approved for risk reduction in postmenopausal women. Likewise, aromatase inhibitors (which have shown high efficacy in chemoprophylaxis but are not FDA-approved for this indication) should be used only in postmenopausal women.
Who might gain the most from tamoxifen? The tamoxifen risk/benefit calculator2,3 can be used to weigh the benefit of breast cancer prevention against the risk of the drug’s adverse effects. Life-threatening adverse effects can include thromboembolic events and endometrial malignancy.2,3 Based on recommendations from the US Preventive Services Task Force, women with a 5-year risk of breast cancer equal to or greater than 3% are most likely to benefit from 5 years of prophylactic endocrine therapy.2 In women who are posthysterectomy, the benefit/risk ratio associated with tamoxifen use is higher.
When is annual MRI appropriate?
The decision to perform annual screening breast MRI should be based on a strong family history rather than strictly a biopsy diagnosis of atypia. The Claus and BRCAPRO models are more appropriate here, as they use only family history information and do not incorporate biopsy results. There are no data to support the use of screening breast MRI in patients with atypia who do not have a strong family history or a deleterious genetic mutation.4,5
Patients with proliferative breast disease tend to have a substantial amount of vague glandular enhancement on breast MRI. Screening MRI in patients with atypia is more likely to lead to frequent false-positive results and unnecessary benign biopsies and cause significant patient anxiety. Without endocrine blockade, breast MRI in this population tends to be nondiagnostic, with a very low yield for breast cancer diagnosis (positive predictive value, 20%).6 Repeated false-positive results of screening MRI in this population can cause patient anxiety, culminating in unnecessary mastectomies. If the Claus or BRCAPRO models yield a lifetime risk for breast cancer above 20%, or the breasts are extremely dense, I discuss with my patient the possibility of adding screening breast MRI.
When ordering breast MRI, it’s important to be aware that this imaging requires gadolinium intravenous contrast, which is excreted through the kidney and requires adequate renal function. This contrast agent can lead to nephrosclerosis in patients with renal insufficiency. In patients with hypertension, diabetes, age over 60, or prior chemotherapy, a recent serum blood urea nitrogen/creatinine level is required. Therefore, the decision to perform annual breast MRI for the rest of a woman’s life should not be taken lightly.
As a part of comprehensive risk assessment, it is important to identify patients who qualify for genetic testing. The addition of screening breast MRI should be heavily dependent on family history, results of BRCA testing and, possibly, mammographic breast density.
Make sure your patient knows that her condition places her at elevated risk, and refer her to a breast specialist
It’s also important to involve the patient in decision making to help ensure that she is proactive and adherent when choosing the best way to manage her risk. The key is to educate her about the importance of atypia.
Many women are told that their follow-up surgical excision was “benign,” and the subject of “atypia” or risk reduction is never addressed. It’s important that the right diagnostic terminology and coding are documented in the medical record so that the finding of atypia is not downgraded to a “benign breast biopsy.”
Finally, due to the complexities of this issue, evaluation by a qualified breast specialist or high-risk cancer program is recommended.
—Laila Samiian, MD
References
1. Cuzick J, Sestak I, Bonanni B, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381(9880):1827–1834.
2. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29(17):2327–2333.
3. Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91(21):1829–1846.
4. Port ER, Park A, Borgen PI, Morris E, Montgomery LL. Results of MRI screening for breast cancer in high-risk patients with LCIS and atypical hyperplasia. Ann Surg Oncol. 2007;14(3):1051–1057.
5. Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. Special report: atypical hyperplasia of the breast—risk assessment and management options. N Eng J Med. 2015;372(1):78–89.
6. Schwartz T, Cyr A, Margenthaler J. Screening breast magnetic resonance imaging in women with atypia or lobular carcinoma in situ. J Surg Res. 2015;193(2):519–522.
Most women will not develop breast malignancy
As Hartmann and colleagues point out, all is not dire once a woman is diagnosed with atypical hyperplasia of the breast. In most of these women, breast cancer will not develop—and if it does develop, it may occur at an age when mortality from other causes is more likely than from breast cancer. In this respect, women with atypical hyperplasia of the breast are different from carriers of BRCA mutations. Although women with atypical hyperplasia as well as mutation carriers are both at high lifetime risk for breast cancer, breast malignancies occur at an earlier age in mutation carriers. Accordingly, as the authors of this special report advise, in general, a diagnosis of atypical hyperplasia should not be considered an indication for risk-reducing bilateral mastectomy.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Of the approximately 1 million benign breast biopsies obtained annually from US women, some 10% yield a diagnosis of atypical hyperplasia, microscopically classified as ductal or lobular. Atypical hyperplasia represents a “proliferation of dysplastic, monotonous epithelial-cell populations that include clonal subpopulations. In models of breast carcinogenesis, atypical hyperplasia occupies a transitional zone between benign and malignant disease,” write Hartmann and colleagues, the authors of a recent special report in the New England Journal of Medicine.1
Long-term follow-up studies have found atypical hyperplasia to confer a relative risk for breast cancer of 4.0. Although these findings are well established, the cumulative absolute risk for breast cancer conferred by a diagnosis of atypical hyperplasia only recently has been described. Hartmann and colleagues note that it approaches 30% over 25 years.1
Recommendations for clinical practice
The authors of this special report do a service to women and their clinicians by pointing out the high long-term risk of malignancy faced by women with atypical hyperplasia of the breast. They also make a number of important recommendations for practice:
- When counseling patients with this diagnosis, it is preferable to use cumulative incidence data because the most commonly used breast cancer risk-prediction models do not accurately estimate the risk for breast malignancy in women with atypical hyperplasia.
- When atypical hyperplasia of the breast is found after core-needle biopsy (FIGURE), surgical excision of the site is recommended to ensure that cancer was not missed as a result of a sampling error. This recommendation derives from National Comprehensive Cancer Network (NCCN) guidelines.2 “In the case of atypical ductal hyperplasia, the frequency of finding breast cancer (‘upgrading’) with surgical excision is 15% to 30% or even higher, despite the use of large-gauge (9- or 11-gauge) core-needle biopsy with vacuum-assisted devices,” Hartmann and colleagues note.
- Women with atypical hyperplasia clearly should receive annual mammographic screening. Although screening magnetic resonance imaging (MRI) may play a role in assessing women with this diagnosis, no prospective trial data have evaluated its utility in this setting. Screening MRI’s low specificity may lead to many unnecessary biopsies with benign findings. This in turn can generate so much anxiety that women may pursue prophylactic bilateral mastectomy to avoid a lifetime of stress related to breast cancer concerns. Women with atypical hyperplasia should be included in future trials of new breast imaging technologies.
- As with other high-risk women, those who have been diagnosed with atypical hyperplasia are well served by being referred to and followed by a physician with special expertise in breast disease who can arrange appropriate screening and follow-up. (See the sidebar, “Here’s how I counsel women with atypical hyperplasia about their management options.”)
- Women with a history of atypical hyperplasia who are considering initiation of systemic menopausal hormone therapyshould be aware that they have a higher baseline risk for invasive breast cancer than other women. Accordingly, the absolute risk of invasive breast cancer associated with use of estrogen-progestin menopausal hormone therapy (EPT) is also likely substantially higher than in average-risk women. Therefore, among women with a history of atypical hyperplasia of the breast who have an intact uterus, use of EPT should be minimized.
- Selective estrogen receptor modulators such as tamoxifen and raloxifene should be more widely used by women with atypical hyperplasia because of their ability to reduce breast cancer risk. Aromatase inhibitors also should be prescribed more widely in this population. (Again, see the sidebar, “Here’s how I counsel women with atypical hyperplasia about their management options.”)
When chemoprevention may be in order
If the 5-year risk of breast cancer by the Gail model is greater than 1.7%, and the patient is older than 35 years, I counsel her that she qualifies for chemoprevention with prophylactic endocrine therapy with the selective estrogen receptor modulators tamoxifen or raloxifene, or the aromatase inhibitor exemestane.1 The choice of drug depends on her menopausal status, bone mineral density, and presence of other comorbidities.
Although tamoxifen is indicated for breast cancer chemoprophylaxis in premenopausal and postmenopausal women, raloxifene is only approved for risk reduction in postmenopausal women. Likewise, aromatase inhibitors (which have shown high efficacy in chemoprophylaxis but are not FDA-approved for this indication) should be used only in postmenopausal women.
Who might gain the most from tamoxifen? The tamoxifen risk/benefit calculator2,3 can be used to weigh the benefit of breast cancer prevention against the risk of the drug’s adverse effects. Life-threatening adverse effects can include thromboembolic events and endometrial malignancy.2,3 Based on recommendations from the US Preventive Services Task Force, women with a 5-year risk of breast cancer equal to or greater than 3% are most likely to benefit from 5 years of prophylactic endocrine therapy.2 In women who are posthysterectomy, the benefit/risk ratio associated with tamoxifen use is higher.
When is annual MRI appropriate?
The decision to perform annual screening breast MRI should be based on a strong family history rather than strictly a biopsy diagnosis of atypia. The Claus and BRCAPRO models are more appropriate here, as they use only family history information and do not incorporate biopsy results. There are no data to support the use of screening breast MRI in patients with atypia who do not have a strong family history or a deleterious genetic mutation.4,5
Patients with proliferative breast disease tend to have a substantial amount of vague glandular enhancement on breast MRI. Screening MRI in patients with atypia is more likely to lead to frequent false-positive results and unnecessary benign biopsies and cause significant patient anxiety. Without endocrine blockade, breast MRI in this population tends to be nondiagnostic, with a very low yield for breast cancer diagnosis (positive predictive value, 20%).6 Repeated false-positive results of screening MRI in this population can cause patient anxiety, culminating in unnecessary mastectomies. If the Claus or BRCAPRO models yield a lifetime risk for breast cancer above 20%, or the breasts are extremely dense, I discuss with my patient the possibility of adding screening breast MRI.
When ordering breast MRI, it’s important to be aware that this imaging requires gadolinium intravenous contrast, which is excreted through the kidney and requires adequate renal function. This contrast agent can lead to nephrosclerosis in patients with renal insufficiency. In patients with hypertension, diabetes, age over 60, or prior chemotherapy, a recent serum blood urea nitrogen/creatinine level is required. Therefore, the decision to perform annual breast MRI for the rest of a woman’s life should not be taken lightly.
As a part of comprehensive risk assessment, it is important to identify patients who qualify for genetic testing. The addition of screening breast MRI should be heavily dependent on family history, results of BRCA testing and, possibly, mammographic breast density.
Make sure your patient knows that her condition places her at elevated risk, and refer her to a breast specialist
It’s also important to involve the patient in decision making to help ensure that she is proactive and adherent when choosing the best way to manage her risk. The key is to educate her about the importance of atypia.
Many women are told that their follow-up surgical excision was “benign,” and the subject of “atypia” or risk reduction is never addressed. It’s important that the right diagnostic terminology and coding are documented in the medical record so that the finding of atypia is not downgraded to a “benign breast biopsy.”
Finally, due to the complexities of this issue, evaluation by a qualified breast specialist or high-risk cancer program is recommended.
—Laila Samiian, MD
References
1. Cuzick J, Sestak I, Bonanni B, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381(9880):1827–1834.
2. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29(17):2327–2333.
3. Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91(21):1829–1846.
4. Port ER, Park A, Borgen PI, Morris E, Montgomery LL. Results of MRI screening for breast cancer in high-risk patients with LCIS and atypical hyperplasia. Ann Surg Oncol. 2007;14(3):1051–1057.
5. Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. Special report: atypical hyperplasia of the breast—risk assessment and management options. N Eng J Med. 2015;372(1):78–89.
6. Schwartz T, Cyr A, Margenthaler J. Screening breast magnetic resonance imaging in women with atypia or lobular carcinoma in situ. J Surg Res. 2015;193(2):519–522.
Most women will not develop breast malignancy
As Hartmann and colleagues point out, all is not dire once a woman is diagnosed with atypical hyperplasia of the breast. In most of these women, breast cancer will not develop—and if it does develop, it may occur at an age when mortality from other causes is more likely than from breast cancer. In this respect, women with atypical hyperplasia of the breast are different from carriers of BRCA mutations. Although women with atypical hyperplasia as well as mutation carriers are both at high lifetime risk for breast cancer, breast malignancies occur at an earlier age in mutation carriers. Accordingly, as the authors of this special report advise, in general, a diagnosis of atypical hyperplasia should not be considered an indication for risk-reducing bilateral mastectomy.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Hartman LC, Degnim AC Santen RJ, Dupont WD, Ghosh K. Special report: atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med. 2015;372(1):78–89.
2. National Comprehensive Cancer Network. Clinical practice guidelines: breast cancer screening and diagnosis, version 1. 2014. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#detection. Accessed March 24, 2015.
1. Hartman LC, Degnim AC Santen RJ, Dupont WD, Ghosh K. Special report: atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med. 2015;372(1):78–89.
2. National Comprehensive Cancer Network. Clinical practice guidelines: breast cancer screening and diagnosis, version 1. 2014. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#detection. Accessed March 24, 2015.
In this Article
- Dr. Samiian: How I counsel patients about their management options
- Mammography shows microcalcifications
Court: Telemedicine abortions cannot be banned in Iowa
Iowa physicians cannot be prohibited from using telemedicine to prescribe abortion-inducing medication to patients, according to a recent ruling by the state Supreme Court.
The Iowa Supreme Court overturned a lower court ruling that upheld a 2013 ban of the practice by the Iowa Board of Medicine. The ban places an “undue burden” on a woman’s right to an abortion and violates the state Constitution, the Supreme Court justices said.
Suzanna M. de Baca, president and CEO of Planned Parenthood of the Heartland, said the ruling protects women’s access to a safe, legal abortion.
“Medical experts opposed this law because it harms women by blocking access to safe medical care,” Ms. de Baca said in a statement. “When it comes to health care, politics should never trump medicine.”
Iowa Board of Medicine officials argued that a physical examination by a physician before the abortion and a follow-up examination after the abortion are essential, given the health risks associated with abortion-inducing drugs.
“The rule was adopted to address what the board saw as the unsafe practice of medicine, and not to place an undue burden on women who choose to terminate their pregnancies,” Mark Bowden, the board’s executive director, said in a statement. “The board will discuss the opinion at its meeting to determine its full application.”
The legal dispute began in 2013 when the Iowa Board of Medicine passed a rule that established standards of practice for physicians who prescribe or administer abortion-inducing drugs. The rules required that physicians personally perform a physical examination and be physically present when the abortion-inducing drug is provided. The regulation effectively prevented doctors from providing abortion treatment through telemedicine, a practice that Planned Parenthood clinics had used since 2008, according to court documents.
The rule immediately drew criticism, including from the Iowa Medical Society (IMS). In an October 2013 letter to the Board of Medicine, Jeanine Freeman, then-IMS legal counsel, wrote that board members failed to show that physicians – particularly those who care for pregnant women – agreed that the rule’s criteria were necessary and appropriate to assure patient safety and health. The board also made no effort to seek expertise, to consider other protocols, or to come to reasonable consensus on medical practice and patient safety, Ms. Freeman wrote.
And despite the Board of Medicine’s statement that the criteria in the rule would relate only to drug-induced telemedicine abortions, the medical society raised concerns about the impact of the rule on other areas of telemedicine.
“The criteria adopted here essentially eliminate telemedicine as a mechanism for the delivery of this medical service with little explanation and inadequate grounding in medical practice standards,” Ms. Freeman wrote.
Through its executive vice president, the Iowa Medical Society declined to comment for this article.
Planned Parenthood of the Heartland, which includes Iowa, sued over the rule in 2013. The regulation was temporarily halted pending the case’s outcome. In 2014, a district court ruled in favor of the Board of Medicine, and Planned Parenthood appealed. In the June 19 decision, the Iowa Supreme Court reversed the lower court decision, ruling that the burden placed on women seeking to terminate a pregnancy outweighed the Board of Medicine’s justification for its rule.
“An issue of equal protection of the laws is lurking in this case,” the justices wrote. “The board appears to hold abortion to a different medical standard than other procedures.”
Iowa is not the first state to institute a prohibition against medication abortions via telemedicine. At least 16 states require that a clinician providing a medication abortion be physically present during the procedure, thereby prohibiting the use of telemedicine to prescribe medication for abortion remotely, according to a June 2015 report by the Guttmacher Institute, a nonprofit research and education group focused on advancing reproductive health.
On Twitter@legal_med
Iowa physicians cannot be prohibited from using telemedicine to prescribe abortion-inducing medication to patients, according to a recent ruling by the state Supreme Court.
The Iowa Supreme Court overturned a lower court ruling that upheld a 2013 ban of the practice by the Iowa Board of Medicine. The ban places an “undue burden” on a woman’s right to an abortion and violates the state Constitution, the Supreme Court justices said.
Suzanna M. de Baca, president and CEO of Planned Parenthood of the Heartland, said the ruling protects women’s access to a safe, legal abortion.
“Medical experts opposed this law because it harms women by blocking access to safe medical care,” Ms. de Baca said in a statement. “When it comes to health care, politics should never trump medicine.”
Iowa Board of Medicine officials argued that a physical examination by a physician before the abortion and a follow-up examination after the abortion are essential, given the health risks associated with abortion-inducing drugs.
“The rule was adopted to address what the board saw as the unsafe practice of medicine, and not to place an undue burden on women who choose to terminate their pregnancies,” Mark Bowden, the board’s executive director, said in a statement. “The board will discuss the opinion at its meeting to determine its full application.”
The legal dispute began in 2013 when the Iowa Board of Medicine passed a rule that established standards of practice for physicians who prescribe or administer abortion-inducing drugs. The rules required that physicians personally perform a physical examination and be physically present when the abortion-inducing drug is provided. The regulation effectively prevented doctors from providing abortion treatment through telemedicine, a practice that Planned Parenthood clinics had used since 2008, according to court documents.
The rule immediately drew criticism, including from the Iowa Medical Society (IMS). In an October 2013 letter to the Board of Medicine, Jeanine Freeman, then-IMS legal counsel, wrote that board members failed to show that physicians – particularly those who care for pregnant women – agreed that the rule’s criteria were necessary and appropriate to assure patient safety and health. The board also made no effort to seek expertise, to consider other protocols, or to come to reasonable consensus on medical practice and patient safety, Ms. Freeman wrote.
And despite the Board of Medicine’s statement that the criteria in the rule would relate only to drug-induced telemedicine abortions, the medical society raised concerns about the impact of the rule on other areas of telemedicine.
“The criteria adopted here essentially eliminate telemedicine as a mechanism for the delivery of this medical service with little explanation and inadequate grounding in medical practice standards,” Ms. Freeman wrote.
Through its executive vice president, the Iowa Medical Society declined to comment for this article.
Planned Parenthood of the Heartland, which includes Iowa, sued over the rule in 2013. The regulation was temporarily halted pending the case’s outcome. In 2014, a district court ruled in favor of the Board of Medicine, and Planned Parenthood appealed. In the June 19 decision, the Iowa Supreme Court reversed the lower court decision, ruling that the burden placed on women seeking to terminate a pregnancy outweighed the Board of Medicine’s justification for its rule.
“An issue of equal protection of the laws is lurking in this case,” the justices wrote. “The board appears to hold abortion to a different medical standard than other procedures.”
Iowa is not the first state to institute a prohibition against medication abortions via telemedicine. At least 16 states require that a clinician providing a medication abortion be physically present during the procedure, thereby prohibiting the use of telemedicine to prescribe medication for abortion remotely, according to a June 2015 report by the Guttmacher Institute, a nonprofit research and education group focused on advancing reproductive health.
On Twitter@legal_med
Iowa physicians cannot be prohibited from using telemedicine to prescribe abortion-inducing medication to patients, according to a recent ruling by the state Supreme Court.
The Iowa Supreme Court overturned a lower court ruling that upheld a 2013 ban of the practice by the Iowa Board of Medicine. The ban places an “undue burden” on a woman’s right to an abortion and violates the state Constitution, the Supreme Court justices said.
Suzanna M. de Baca, president and CEO of Planned Parenthood of the Heartland, said the ruling protects women’s access to a safe, legal abortion.
“Medical experts opposed this law because it harms women by blocking access to safe medical care,” Ms. de Baca said in a statement. “When it comes to health care, politics should never trump medicine.”
Iowa Board of Medicine officials argued that a physical examination by a physician before the abortion and a follow-up examination after the abortion are essential, given the health risks associated with abortion-inducing drugs.
“The rule was adopted to address what the board saw as the unsafe practice of medicine, and not to place an undue burden on women who choose to terminate their pregnancies,” Mark Bowden, the board’s executive director, said in a statement. “The board will discuss the opinion at its meeting to determine its full application.”
The legal dispute began in 2013 when the Iowa Board of Medicine passed a rule that established standards of practice for physicians who prescribe or administer abortion-inducing drugs. The rules required that physicians personally perform a physical examination and be physically present when the abortion-inducing drug is provided. The regulation effectively prevented doctors from providing abortion treatment through telemedicine, a practice that Planned Parenthood clinics had used since 2008, according to court documents.
The rule immediately drew criticism, including from the Iowa Medical Society (IMS). In an October 2013 letter to the Board of Medicine, Jeanine Freeman, then-IMS legal counsel, wrote that board members failed to show that physicians – particularly those who care for pregnant women – agreed that the rule’s criteria were necessary and appropriate to assure patient safety and health. The board also made no effort to seek expertise, to consider other protocols, or to come to reasonable consensus on medical practice and patient safety, Ms. Freeman wrote.
And despite the Board of Medicine’s statement that the criteria in the rule would relate only to drug-induced telemedicine abortions, the medical society raised concerns about the impact of the rule on other areas of telemedicine.
“The criteria adopted here essentially eliminate telemedicine as a mechanism for the delivery of this medical service with little explanation and inadequate grounding in medical practice standards,” Ms. Freeman wrote.
Through its executive vice president, the Iowa Medical Society declined to comment for this article.
Planned Parenthood of the Heartland, which includes Iowa, sued over the rule in 2013. The regulation was temporarily halted pending the case’s outcome. In 2014, a district court ruled in favor of the Board of Medicine, and Planned Parenthood appealed. In the June 19 decision, the Iowa Supreme Court reversed the lower court decision, ruling that the burden placed on women seeking to terminate a pregnancy outweighed the Board of Medicine’s justification for its rule.
“An issue of equal protection of the laws is lurking in this case,” the justices wrote. “The board appears to hold abortion to a different medical standard than other procedures.”
Iowa is not the first state to institute a prohibition against medication abortions via telemedicine. At least 16 states require that a clinician providing a medication abortion be physically present during the procedure, thereby prohibiting the use of telemedicine to prescribe medication for abortion remotely, according to a June 2015 report by the Guttmacher Institute, a nonprofit research and education group focused on advancing reproductive health.
On Twitter@legal_med
Letrozole bests clomiphene again in infertile women with PCOS
LISBON – The aromatase inhibitor letrozole was associated with roughly a 42% increase in the pregnancy rate, compared with clomiphene citrate in infertile women with polycystic ovary syndrome in a double-blind, randomized study.
In an intention-to-treat analysis, the pregnancy rate was 61.2% with letrozole (Femara) versus 43% with clomiphene (P = .022). There also was a trend toward more live births with letrozole (48.8% vs. 35.4%; P = .089).
The per-protocol results were similar for pregnancy (61% vs. 43.2%; P = .029) and live births (48.1% vs. 35.1%; P = .108).
“We now have convincing evidence that letrozole is better than clomiphene and we should seriously consider moving on to letrozole,” principle investigator Dr. Saad Amer said at the annual meeting of the European Society of Human Reproduction and Embryology.
The results are consistent with the most recent Cochrane meta-analysis, which called for further research comparing letrozole with clomiphene as a primary ovulation induction agent in polycystic ovary syndrome (PCOS) because of low-quality evidence (Cochrane Database Syst. Rev. 2014 Feb. 24;2:CD010287).
A recent robust U.S. study (N. Engl. J. Med. 2014;371:119-29) showed higher live-birth and ovulation rates with letrozole vs. clomiphene in women with PCOS, but it included a markedly obese population with a mean body mass index (BMI) of 35 kg/m2 and thus does not reflect clinical practice worldwide, Dr. Amer said.
The current results are more generalizable, especially in Europe, because the patients fulfilled the widely accepted Rotterdam diagnostic criteria for PCOS and had a median BMI of 27.7 kg/m2 in the clomiphene group and 27.5 kg/m2 in the letrozole group, said Dr. Amer of the University of Nottingham in Derby, England.
The phase IV study evenly randomized 159 anovulatory women, aged 18-39 years, with a diagnosis of PCOS to one tablet of letrozole 2.5 mg or clomiphene 50 mg daily for 5 days, continuing until pregnancy or up to 6 cycles. If there was no response in the first cycle, the dose was increased to two tablets. If there was still no response, the patient was crossed over to the other treatment arm after a 6-week washout. Cycles were initially monitored with ultrasound follicle tracking, then mid-luteal serum progesterone measurements.
Among the 159 women in the intention-to-treat analysis, four conceived before treatment and four dropped out. The remaining 151 women included 77 given letrozole and 74 given clomiphene.
For the 60 women who crossed over, there was no significant difference in pregnancy and live birth rates between groups in either the intention-to-treat or per-protocol analyses.
Notably, however, 70.1% of women who started treatment with letrozole followed by clomiphene became pregnant vs. only 59.5% when the treatment strategy was reversed, while 56.2% started on letrozole and 49.4% started on clomiphene went on to a live birth.
“This tells us that if you take clomiphene first and then follow it with letrozole, you’re achieving almost the same result as just taking letrozole from the beginning,” Dr. Amer said.
The improved pregnancy rates, however, cannot be attributed to the effect of the endometrial factor, he said. Surprisingly, endometrial thickness was significantly greater in the clomiphene group than in the letrozole group (median, 9.0 mm vs. 8.4 mm; P = .002). Mono-follicular ovulation (83% vs. 85%) and multiple pregnancy (twins 0% vs. 6%) rates were similar.
No significant differences were observed between the clomiphene and letrozole groups in miscarriages (6 events vs. 9 events), ectopic pregnancies (0 vs. 1), or preterm births (2 vs. 4).
“Further research is required to investigate the mechanisms of increased pregnancy rates,” Dr. Amer said.
Serious adverse events included one hemorrhagic cyst in each group and a cholecystitis in the clomiphene group.
There was one fetal anomaly – a dilated left kidney – in the clomiphene group and none in the letrozole group, he said.
During a discussion of the results, reproductive medicine specialist Dr. Roy Homburg of Homerton University Hospital, London, said that it’s time for letrozole to recognized as the superior choice.
“Every study that has been done on the subject, every randomized controlled trial, every meta-analysis, every Cochrane database has shown exactly what you have shown – the superiority of letrozole over clomiphene,” Dr. Homburg said. “All this in addition to the fact that there are many more fetal abnormalities, congenital abnormalities with clomiphene rather than letrozole. I think it’s about time people start believing this and make sure letrozole is on-label rather than off-label.”
Dr. Amer agreed. “It’s now time for clomiphene to retire,” he said, receiving a round of applause from the audience.
The study was sponsored by Derby Hospitals NHS Foundation Trust. Dr. Amer reported having no financial disclosures.
On Twitter @pwendl
LISBON – The aromatase inhibitor letrozole was associated with roughly a 42% increase in the pregnancy rate, compared with clomiphene citrate in infertile women with polycystic ovary syndrome in a double-blind, randomized study.
In an intention-to-treat analysis, the pregnancy rate was 61.2% with letrozole (Femara) versus 43% with clomiphene (P = .022). There also was a trend toward more live births with letrozole (48.8% vs. 35.4%; P = .089).
The per-protocol results were similar for pregnancy (61% vs. 43.2%; P = .029) and live births (48.1% vs. 35.1%; P = .108).
“We now have convincing evidence that letrozole is better than clomiphene and we should seriously consider moving on to letrozole,” principle investigator Dr. Saad Amer said at the annual meeting of the European Society of Human Reproduction and Embryology.
The results are consistent with the most recent Cochrane meta-analysis, which called for further research comparing letrozole with clomiphene as a primary ovulation induction agent in polycystic ovary syndrome (PCOS) because of low-quality evidence (Cochrane Database Syst. Rev. 2014 Feb. 24;2:CD010287).
A recent robust U.S. study (N. Engl. J. Med. 2014;371:119-29) showed higher live-birth and ovulation rates with letrozole vs. clomiphene in women with PCOS, but it included a markedly obese population with a mean body mass index (BMI) of 35 kg/m2 and thus does not reflect clinical practice worldwide, Dr. Amer said.
The current results are more generalizable, especially in Europe, because the patients fulfilled the widely accepted Rotterdam diagnostic criteria for PCOS and had a median BMI of 27.7 kg/m2 in the clomiphene group and 27.5 kg/m2 in the letrozole group, said Dr. Amer of the University of Nottingham in Derby, England.
The phase IV study evenly randomized 159 anovulatory women, aged 18-39 years, with a diagnosis of PCOS to one tablet of letrozole 2.5 mg or clomiphene 50 mg daily for 5 days, continuing until pregnancy or up to 6 cycles. If there was no response in the first cycle, the dose was increased to two tablets. If there was still no response, the patient was crossed over to the other treatment arm after a 6-week washout. Cycles were initially monitored with ultrasound follicle tracking, then mid-luteal serum progesterone measurements.
Among the 159 women in the intention-to-treat analysis, four conceived before treatment and four dropped out. The remaining 151 women included 77 given letrozole and 74 given clomiphene.
For the 60 women who crossed over, there was no significant difference in pregnancy and live birth rates between groups in either the intention-to-treat or per-protocol analyses.
Notably, however, 70.1% of women who started treatment with letrozole followed by clomiphene became pregnant vs. only 59.5% when the treatment strategy was reversed, while 56.2% started on letrozole and 49.4% started on clomiphene went on to a live birth.
“This tells us that if you take clomiphene first and then follow it with letrozole, you’re achieving almost the same result as just taking letrozole from the beginning,” Dr. Amer said.
The improved pregnancy rates, however, cannot be attributed to the effect of the endometrial factor, he said. Surprisingly, endometrial thickness was significantly greater in the clomiphene group than in the letrozole group (median, 9.0 mm vs. 8.4 mm; P = .002). Mono-follicular ovulation (83% vs. 85%) and multiple pregnancy (twins 0% vs. 6%) rates were similar.
No significant differences were observed between the clomiphene and letrozole groups in miscarriages (6 events vs. 9 events), ectopic pregnancies (0 vs. 1), or preterm births (2 vs. 4).
“Further research is required to investigate the mechanisms of increased pregnancy rates,” Dr. Amer said.
Serious adverse events included one hemorrhagic cyst in each group and a cholecystitis in the clomiphene group.
There was one fetal anomaly – a dilated left kidney – in the clomiphene group and none in the letrozole group, he said.
During a discussion of the results, reproductive medicine specialist Dr. Roy Homburg of Homerton University Hospital, London, said that it’s time for letrozole to recognized as the superior choice.
“Every study that has been done on the subject, every randomized controlled trial, every meta-analysis, every Cochrane database has shown exactly what you have shown – the superiority of letrozole over clomiphene,” Dr. Homburg said. “All this in addition to the fact that there are many more fetal abnormalities, congenital abnormalities with clomiphene rather than letrozole. I think it’s about time people start believing this and make sure letrozole is on-label rather than off-label.”
Dr. Amer agreed. “It’s now time for clomiphene to retire,” he said, receiving a round of applause from the audience.
The study was sponsored by Derby Hospitals NHS Foundation Trust. Dr. Amer reported having no financial disclosures.
On Twitter @pwendl
LISBON – The aromatase inhibitor letrozole was associated with roughly a 42% increase in the pregnancy rate, compared with clomiphene citrate in infertile women with polycystic ovary syndrome in a double-blind, randomized study.
In an intention-to-treat analysis, the pregnancy rate was 61.2% with letrozole (Femara) versus 43% with clomiphene (P = .022). There also was a trend toward more live births with letrozole (48.8% vs. 35.4%; P = .089).
The per-protocol results were similar for pregnancy (61% vs. 43.2%; P = .029) and live births (48.1% vs. 35.1%; P = .108).
“We now have convincing evidence that letrozole is better than clomiphene and we should seriously consider moving on to letrozole,” principle investigator Dr. Saad Amer said at the annual meeting of the European Society of Human Reproduction and Embryology.
The results are consistent with the most recent Cochrane meta-analysis, which called for further research comparing letrozole with clomiphene as a primary ovulation induction agent in polycystic ovary syndrome (PCOS) because of low-quality evidence (Cochrane Database Syst. Rev. 2014 Feb. 24;2:CD010287).
A recent robust U.S. study (N. Engl. J. Med. 2014;371:119-29) showed higher live-birth and ovulation rates with letrozole vs. clomiphene in women with PCOS, but it included a markedly obese population with a mean body mass index (BMI) of 35 kg/m2 and thus does not reflect clinical practice worldwide, Dr. Amer said.
The current results are more generalizable, especially in Europe, because the patients fulfilled the widely accepted Rotterdam diagnostic criteria for PCOS and had a median BMI of 27.7 kg/m2 in the clomiphene group and 27.5 kg/m2 in the letrozole group, said Dr. Amer of the University of Nottingham in Derby, England.
The phase IV study evenly randomized 159 anovulatory women, aged 18-39 years, with a diagnosis of PCOS to one tablet of letrozole 2.5 mg or clomiphene 50 mg daily for 5 days, continuing until pregnancy or up to 6 cycles. If there was no response in the first cycle, the dose was increased to two tablets. If there was still no response, the patient was crossed over to the other treatment arm after a 6-week washout. Cycles were initially monitored with ultrasound follicle tracking, then mid-luteal serum progesterone measurements.
Among the 159 women in the intention-to-treat analysis, four conceived before treatment and four dropped out. The remaining 151 women included 77 given letrozole and 74 given clomiphene.
For the 60 women who crossed over, there was no significant difference in pregnancy and live birth rates between groups in either the intention-to-treat or per-protocol analyses.
Notably, however, 70.1% of women who started treatment with letrozole followed by clomiphene became pregnant vs. only 59.5% when the treatment strategy was reversed, while 56.2% started on letrozole and 49.4% started on clomiphene went on to a live birth.
“This tells us that if you take clomiphene first and then follow it with letrozole, you’re achieving almost the same result as just taking letrozole from the beginning,” Dr. Amer said.
The improved pregnancy rates, however, cannot be attributed to the effect of the endometrial factor, he said. Surprisingly, endometrial thickness was significantly greater in the clomiphene group than in the letrozole group (median, 9.0 mm vs. 8.4 mm; P = .002). Mono-follicular ovulation (83% vs. 85%) and multiple pregnancy (twins 0% vs. 6%) rates were similar.
No significant differences were observed between the clomiphene and letrozole groups in miscarriages (6 events vs. 9 events), ectopic pregnancies (0 vs. 1), or preterm births (2 vs. 4).
“Further research is required to investigate the mechanisms of increased pregnancy rates,” Dr. Amer said.
Serious adverse events included one hemorrhagic cyst in each group and a cholecystitis in the clomiphene group.
There was one fetal anomaly – a dilated left kidney – in the clomiphene group and none in the letrozole group, he said.
During a discussion of the results, reproductive medicine specialist Dr. Roy Homburg of Homerton University Hospital, London, said that it’s time for letrozole to recognized as the superior choice.
“Every study that has been done on the subject, every randomized controlled trial, every meta-analysis, every Cochrane database has shown exactly what you have shown – the superiority of letrozole over clomiphene,” Dr. Homburg said. “All this in addition to the fact that there are many more fetal abnormalities, congenital abnormalities with clomiphene rather than letrozole. I think it’s about time people start believing this and make sure letrozole is on-label rather than off-label.”
Dr. Amer agreed. “It’s now time for clomiphene to retire,” he said, receiving a round of applause from the audience.
The study was sponsored by Derby Hospitals NHS Foundation Trust. Dr. Amer reported having no financial disclosures.
On Twitter @pwendl
AT ESHRE 2015
Key clinical point: Letrozole is superior to clomiphene with regard to pregnancy rates in infertile women with PCOS.
Major finding: The pregnancy rate was 61.2% with letrozole versus 43% with clomiphene (P = .022).
Data source: A double-blind, randomized phase IV trial in 159 infertile women with polycystic ovarian syndrome.
Disclosures: The study was sponsored by Derby Hospitals NHS Foundation Trust. Dr. Amer reported having no financial disclosures.
EULAR: Women with RA have increased cervical neoplasia rates
ROME – Women with rheumatoid arthritis who have never been treated with a biologic drug had a modest but statistically significant increased rate of cervical intraepithelial neoplasia in a case-control study with more than 335,000 women.
The analysis showed an adjusted excess hazard for developing cervical intraepithelial neoplasia (CIN) I of 53%, compared with the general population, and an excess 39% rate of CIN II or III, both statistically significant differences, Dr. Hjalmar Wadstrom reported in a poster at the European Congress of Rheumatology.
The analysis also showed a small increase in the relative rate of invasive cervical cancers among the women with rheumatoid arthritis (RA) on treatment with conventional disease-modifying drugs, such as methotrexate, who never received a biologic disease-modifying drug. The 9% relative increase in the rate of invasive cancer, compared with the general population, did not achieve statistical significance, reported Dr. Wadstrom, a clinical epidemiology researcher at the Karolinska Institute in Stockholm.
The “moderately but not dramatically” increased rate of CIN “is not a reason for big concern or alarm,” but highlights that women with RA should comply with local cervical cancer–screening recommendations and programs, said Dr. Johan Askling, professor of rheumatology and clinical epidemiology at Karolinska and senior author of the study.
“It would be a shame if these RA patients with a small increased risk did not attend the [cervical screening] programs we have set up. Clinicians should make sure that women with RA attend to screening because nonattendance is their major risk factor,” he said in an interview. “Our findings don’t call for a change in screening recommendations, they just highlight the importance of attending to screening” in women with RA, Dr. Askling said.
The researchers ran a linkage analysis of Swedish registries and identified 34,984 adult women with RA and matched them with 300,331 women drawn from the general Swedish population. Age of the RA patients ranged from 18 to 97 years with a median age of 62 years, and they selected general-population controls who matched this group. In the regression analyses they ran to calculate hazard ratios they adjusted for age, education, prior cervical screening, comorbidities, marital status, and time hospitalized during prior 5 years. The analysis included CIN and invasive cancer cases during 14 years of follow-up, 1999-2012.
Both Dr. Wadstrom and Dr. Askling cited two potential factors behind the increased rates of CIN I, II, and III in women with RA: the inflammatory state of RA and treatment with immunosuppressive nonbiologic agents, such as methotrexate.
The increased CIN rates found in this analysis were also “seen in other patients treated with potent immunosuppressive drugs,” like organ transplant patients, Dr. Askling noted.
The current study serves as prelude to an analysis he and his associates are now running to assess cervical neoplasia and cancer rates among women with RA treated with biologic disease-modifying drugs.
On Twitter @mitchelzoler
ROME – Women with rheumatoid arthritis who have never been treated with a biologic drug had a modest but statistically significant increased rate of cervical intraepithelial neoplasia in a case-control study with more than 335,000 women.
The analysis showed an adjusted excess hazard for developing cervical intraepithelial neoplasia (CIN) I of 53%, compared with the general population, and an excess 39% rate of CIN II or III, both statistically significant differences, Dr. Hjalmar Wadstrom reported in a poster at the European Congress of Rheumatology.
The analysis also showed a small increase in the relative rate of invasive cervical cancers among the women with rheumatoid arthritis (RA) on treatment with conventional disease-modifying drugs, such as methotrexate, who never received a biologic disease-modifying drug. The 9% relative increase in the rate of invasive cancer, compared with the general population, did not achieve statistical significance, reported Dr. Wadstrom, a clinical epidemiology researcher at the Karolinska Institute in Stockholm.
The “moderately but not dramatically” increased rate of CIN “is not a reason for big concern or alarm,” but highlights that women with RA should comply with local cervical cancer–screening recommendations and programs, said Dr. Johan Askling, professor of rheumatology and clinical epidemiology at Karolinska and senior author of the study.
“It would be a shame if these RA patients with a small increased risk did not attend the [cervical screening] programs we have set up. Clinicians should make sure that women with RA attend to screening because nonattendance is their major risk factor,” he said in an interview. “Our findings don’t call for a change in screening recommendations, they just highlight the importance of attending to screening” in women with RA, Dr. Askling said.
The researchers ran a linkage analysis of Swedish registries and identified 34,984 adult women with RA and matched them with 300,331 women drawn from the general Swedish population. Age of the RA patients ranged from 18 to 97 years with a median age of 62 years, and they selected general-population controls who matched this group. In the regression analyses they ran to calculate hazard ratios they adjusted for age, education, prior cervical screening, comorbidities, marital status, and time hospitalized during prior 5 years. The analysis included CIN and invasive cancer cases during 14 years of follow-up, 1999-2012.
Both Dr. Wadstrom and Dr. Askling cited two potential factors behind the increased rates of CIN I, II, and III in women with RA: the inflammatory state of RA and treatment with immunosuppressive nonbiologic agents, such as methotrexate.
The increased CIN rates found in this analysis were also “seen in other patients treated with potent immunosuppressive drugs,” like organ transplant patients, Dr. Askling noted.
The current study serves as prelude to an analysis he and his associates are now running to assess cervical neoplasia and cancer rates among women with RA treated with biologic disease-modifying drugs.
On Twitter @mitchelzoler
ROME – Women with rheumatoid arthritis who have never been treated with a biologic drug had a modest but statistically significant increased rate of cervical intraepithelial neoplasia in a case-control study with more than 335,000 women.
The analysis showed an adjusted excess hazard for developing cervical intraepithelial neoplasia (CIN) I of 53%, compared with the general population, and an excess 39% rate of CIN II or III, both statistically significant differences, Dr. Hjalmar Wadstrom reported in a poster at the European Congress of Rheumatology.
The analysis also showed a small increase in the relative rate of invasive cervical cancers among the women with rheumatoid arthritis (RA) on treatment with conventional disease-modifying drugs, such as methotrexate, who never received a biologic disease-modifying drug. The 9% relative increase in the rate of invasive cancer, compared with the general population, did not achieve statistical significance, reported Dr. Wadstrom, a clinical epidemiology researcher at the Karolinska Institute in Stockholm.
The “moderately but not dramatically” increased rate of CIN “is not a reason for big concern or alarm,” but highlights that women with RA should comply with local cervical cancer–screening recommendations and programs, said Dr. Johan Askling, professor of rheumatology and clinical epidemiology at Karolinska and senior author of the study.
“It would be a shame if these RA patients with a small increased risk did not attend the [cervical screening] programs we have set up. Clinicians should make sure that women with RA attend to screening because nonattendance is their major risk factor,” he said in an interview. “Our findings don’t call for a change in screening recommendations, they just highlight the importance of attending to screening” in women with RA, Dr. Askling said.
The researchers ran a linkage analysis of Swedish registries and identified 34,984 adult women with RA and matched them with 300,331 women drawn from the general Swedish population. Age of the RA patients ranged from 18 to 97 years with a median age of 62 years, and they selected general-population controls who matched this group. In the regression analyses they ran to calculate hazard ratios they adjusted for age, education, prior cervical screening, comorbidities, marital status, and time hospitalized during prior 5 years. The analysis included CIN and invasive cancer cases during 14 years of follow-up, 1999-2012.
Both Dr. Wadstrom and Dr. Askling cited two potential factors behind the increased rates of CIN I, II, and III in women with RA: the inflammatory state of RA and treatment with immunosuppressive nonbiologic agents, such as methotrexate.
The increased CIN rates found in this analysis were also “seen in other patients treated with potent immunosuppressive drugs,” like organ transplant patients, Dr. Askling noted.
The current study serves as prelude to an analysis he and his associates are now running to assess cervical neoplasia and cancer rates among women with RA treated with biologic disease-modifying drugs.
On Twitter @mitchelzoler
AT THE EULAR 2015 CONGRESS
Key clinical point: Women with rheumatoid arthritis had significantly increased rates of CIN I, II, and III.
Major finding: Women with RA had a 53% increased rate of CIN I and a 39% increased rate of CIN II or III.
Data source: Case-control study of women drawn from Swedish national registries with 34,984 RA patients and 300,331 women from the general population.
Disclosures: Dr. Wadstrom had no disclosures. Dr. Askling has received research support from eight drug companies.
ESHRE: ART does not hurt academic performance in teens
LISBON – Conception by assisted reproductive technology is not associated with lower academic performance in adolescence, a large nationwide analysis showed.
In crude analyses, ART singletons had higher academic performance than spontaneously conceived singletons and ART twins performed as well as ART singletons. After adjustment for confounders, academic performance was similar between all singletons and between ART twins and ART singletons.
“These findings are very reassuring for the parents of ART children and for the ART society as a whole,” study author Anne Lærke Spangmose Pedersen said at the annual meeting of the European Society of Human Reproduction and Embryology.
ART children and twins in general have an increased risk of preterm delivery and low birth weight, but only a handful of studies have explored IQ in these children.
A recent study (BJOG 2014;121:1642-51) reported similar IQ, attention, and executive function in ART and non-ART children at age 5 years; however, no previous studies have included ninth-grade test scores in a complete national cohort of adolescents all conceived by ART, noted Ms. Pedersen, a medical student at Copenhagen University Hospital, Hvidovre, Denmark.
To do this, the investigators used compulsory national registers and the Danish IVF and Medical Birth Registry to identify 10,429 individuals born in Denmark from 1995 to 1998. This included all children conceived by ART (fresh embryo in-vitro fertilization and intracytoplasmic sperm injection), totaling 2,838 singletons and 1,930 twins, and a random sample of 5,661 non-ART singletons.
The primary outcome was the mean test score on the National Test, which is used for university entrance and completed by all ninth-grade students in Denmark at ages 15-16 years. Mandatory subjects include Danish, foreign languages, mathematics, and physics/chemistry, with scores ranging from –3 to +12 (mean 7). Scores were available for 2,544 ART singletons, 4,985 non-ART singletons, and 1,676 ART twins.
The mean test scores were 7.16 in ART singletons, 6.74 in non-ART singletons, and 7.21 in ART twins. The difference was statistically significant between ART and non-ART singletons (P < .001), but not between ART singletons and twins (P = .47), Ms. Pedersen said.
After adjustment for a variety of factors, including maternal age and socioeconomic status, which tend to be higher in ART families, the difference did not persist, she said.
“It’s not the final conclusion, but I think the data at this moment are reassuring,” session comoderator Dr. Willianne Nelenof Radboud University Nijmegen (the Netherlands) Medical Centre said in an interview. Like members of the audience, she said that data should be pooled from studies and that more data are needed from ART children and parents.
During the discussion of the results, Ms. Pedersen noted that preterm birth rates were significantly higher in ART singletons than non-ART singletons (4.6% vs. 2.7%; P < .001) and in ART twins, compared with ART singletons (22.8% vs. 4.6%; P < .001).
Ms. Pedersen reported having no financial disclosures.
On Twitter @pwendl
LISBON – Conception by assisted reproductive technology is not associated with lower academic performance in adolescence, a large nationwide analysis showed.
In crude analyses, ART singletons had higher academic performance than spontaneously conceived singletons and ART twins performed as well as ART singletons. After adjustment for confounders, academic performance was similar between all singletons and between ART twins and ART singletons.
“These findings are very reassuring for the parents of ART children and for the ART society as a whole,” study author Anne Lærke Spangmose Pedersen said at the annual meeting of the European Society of Human Reproduction and Embryology.
ART children and twins in general have an increased risk of preterm delivery and low birth weight, but only a handful of studies have explored IQ in these children.
A recent study (BJOG 2014;121:1642-51) reported similar IQ, attention, and executive function in ART and non-ART children at age 5 years; however, no previous studies have included ninth-grade test scores in a complete national cohort of adolescents all conceived by ART, noted Ms. Pedersen, a medical student at Copenhagen University Hospital, Hvidovre, Denmark.
To do this, the investigators used compulsory national registers and the Danish IVF and Medical Birth Registry to identify 10,429 individuals born in Denmark from 1995 to 1998. This included all children conceived by ART (fresh embryo in-vitro fertilization and intracytoplasmic sperm injection), totaling 2,838 singletons and 1,930 twins, and a random sample of 5,661 non-ART singletons.
The primary outcome was the mean test score on the National Test, which is used for university entrance and completed by all ninth-grade students in Denmark at ages 15-16 years. Mandatory subjects include Danish, foreign languages, mathematics, and physics/chemistry, with scores ranging from –3 to +12 (mean 7). Scores were available for 2,544 ART singletons, 4,985 non-ART singletons, and 1,676 ART twins.
The mean test scores were 7.16 in ART singletons, 6.74 in non-ART singletons, and 7.21 in ART twins. The difference was statistically significant between ART and non-ART singletons (P < .001), but not between ART singletons and twins (P = .47), Ms. Pedersen said.
After adjustment for a variety of factors, including maternal age and socioeconomic status, which tend to be higher in ART families, the difference did not persist, she said.
“It’s not the final conclusion, but I think the data at this moment are reassuring,” session comoderator Dr. Willianne Nelenof Radboud University Nijmegen (the Netherlands) Medical Centre said in an interview. Like members of the audience, she said that data should be pooled from studies and that more data are needed from ART children and parents.
During the discussion of the results, Ms. Pedersen noted that preterm birth rates were significantly higher in ART singletons than non-ART singletons (4.6% vs. 2.7%; P < .001) and in ART twins, compared with ART singletons (22.8% vs. 4.6%; P < .001).
Ms. Pedersen reported having no financial disclosures.
On Twitter @pwendl
LISBON – Conception by assisted reproductive technology is not associated with lower academic performance in adolescence, a large nationwide analysis showed.
In crude analyses, ART singletons had higher academic performance than spontaneously conceived singletons and ART twins performed as well as ART singletons. After adjustment for confounders, academic performance was similar between all singletons and between ART twins and ART singletons.
“These findings are very reassuring for the parents of ART children and for the ART society as a whole,” study author Anne Lærke Spangmose Pedersen said at the annual meeting of the European Society of Human Reproduction and Embryology.
ART children and twins in general have an increased risk of preterm delivery and low birth weight, but only a handful of studies have explored IQ in these children.
A recent study (BJOG 2014;121:1642-51) reported similar IQ, attention, and executive function in ART and non-ART children at age 5 years; however, no previous studies have included ninth-grade test scores in a complete national cohort of adolescents all conceived by ART, noted Ms. Pedersen, a medical student at Copenhagen University Hospital, Hvidovre, Denmark.
To do this, the investigators used compulsory national registers and the Danish IVF and Medical Birth Registry to identify 10,429 individuals born in Denmark from 1995 to 1998. This included all children conceived by ART (fresh embryo in-vitro fertilization and intracytoplasmic sperm injection), totaling 2,838 singletons and 1,930 twins, and a random sample of 5,661 non-ART singletons.
The primary outcome was the mean test score on the National Test, which is used for university entrance and completed by all ninth-grade students in Denmark at ages 15-16 years. Mandatory subjects include Danish, foreign languages, mathematics, and physics/chemistry, with scores ranging from –3 to +12 (mean 7). Scores were available for 2,544 ART singletons, 4,985 non-ART singletons, and 1,676 ART twins.
The mean test scores were 7.16 in ART singletons, 6.74 in non-ART singletons, and 7.21 in ART twins. The difference was statistically significant between ART and non-ART singletons (P < .001), but not between ART singletons and twins (P = .47), Ms. Pedersen said.
After adjustment for a variety of factors, including maternal age and socioeconomic status, which tend to be higher in ART families, the difference did not persist, she said.
“It’s not the final conclusion, but I think the data at this moment are reassuring,” session comoderator Dr. Willianne Nelenof Radboud University Nijmegen (the Netherlands) Medical Centre said in an interview. Like members of the audience, she said that data should be pooled from studies and that more data are needed from ART children and parents.
During the discussion of the results, Ms. Pedersen noted that preterm birth rates were significantly higher in ART singletons than non-ART singletons (4.6% vs. 2.7%; P < .001) and in ART twins, compared with ART singletons (22.8% vs. 4.6%; P < .001).
Ms. Pedersen reported having no financial disclosures.
On Twitter @pwendl
AT ESHRE 2015
Key clinical point: Conception by assisted reproductive technology is not associated with poorer academic performance in adolescence.
Major finding: Mean test scores were 7.16 for ART singletons, 6.74 for non-ART singletons, and 7.21 for ART twins.
Data source: Danish national cohort study in 10,429 adolescents.
Disclosures: Ms. Pedersen reported having no financial disclosures.
Regular, outcomes-based treatment effective in vulvar lichen sclerosus
VANCOUVER, B.C. – Women with vulvar lichen sclerosus (VLS) who consistently used topical corticosteroids that had been titrated to normalize skin color and texture had significantly better outcomes than patients who sometimes skipped treatment, a prospective single-center cohort study found.
Most notably, none of the treatment-compliant patients developed vulvar carcinoma during an average of almost 5 years of follow-up, compared with 4.7% of the partially compliant group (P < .001), reported Dr. Jason Lee, a dermatologist with the University of Sydney, Australia.
“We are proposing a paradigm shift to change the way we manage this condition,” Dr. Lee said at the World Congress of Dermatology. “Clinicians should aim for individual regular treatment of patients with vulvar lichen sclerosus. The treatment is safe, and the course of the disease can be altered by treatment.”
The report appeared simultaneously in JAMA Dermatology (2015 June 12 [doi:10.1001/jamadermatol.2015.0643]).
Vulvar lichen sclerosus is an uncommon disease that causes vulvar itching, pain, and sexual dysfunction, and can significantly alter the vulvar architecture. Before clinicians began treating VLS with superpotent topical corticosteroids, about 5% of cases were thought to progress to vulvar intraepithelial neoplasia or invasive squamous cell carcinoma.
Furthermore, even fairly mild cases can become cancerous, Dr. Lee noted. “Until now, it was thought that cancer was inevitable in some women with VLS,” he added. “Short-term control is easy, but long-term management lacks data, and research has not addressed whether treatment can prevent scarring or cancer.”
To assess the impact of individualized topical corticosteroid therapy with regular follow-up, Dr. Lee and his associates studied 507 women with biopsy-proven VLS during 2008-2014. Patients averaged 55 years of age at presentation and had an average symptom history of 5 years.
The investigators graded cases as mild, moderate, severe, or very severe depending on degree of hyperkeratosis, and titrated treatment with the goal of normalizing skin color and texture. They classified patients as treatment-compliant if they said they followed treatment directions most or all of the time, and as partially compliant if they said they followed directions some, little, or none of the time. They saw patients at least once a year for follow-up.
Most patients initially needed superpotent or ultrapotent topical corticosteroids to control their disease and then switched to moderate or mild strength formulations to maintain results, Dr. Lee and his associates reported.
About 30% of patients were only partially compliant with treatment. These patients were significantly less likely to achieve symptom resolution (58% vs. 93%) and resolution of dyspareunia (66% vs. 94%), and were more likely to develop adhesions and scars during follow-up (40% vs. 3%), compared with fully compliant patients (P < .001 for all), the researchers said. Rates of steroid dermatitis and reversible steroid-induced cutaneous atrophy were similar between the fully and partially compliant groups (2.2% vs. 4%, and 1.1% vs. 2%, respectively).
Based on the findings, clinicians would need to treat 21 cases of VLS to prevent 1 case of vulvar cancer and 2.7 cases to prevent scarring, said Dr. Lee.
“This is the first study adequately powered to demonstrate that cancer and scarring can be prevented by regular treatment according to clinical outcome, not symptoms,” he added.
But Dr. Lee acknowledged that selection bias could have affected the results. “We did find that patients who were not complying with treatment were more likely to have severe disease at the start of the study,” he said. “They probably were at increased risk of cancer to start with.”
The dermatology department of Royal North Shore Hospital partially funded the work. The investigators reported having no relevant conflicts of interest.
We congratulate the authors on an article that has profound implications for patients with vulvar lichen sclerosus and their physicians worldwide. Until now, suppression of the disease has concerned physicians and has often been inadequate. The reduction in cancer among patients with VLS who were treated with [topical corticosteroids] is no longer speculative. This focus of concern can effectively communicate the message that long-term use of a TCS likely prevents the progression of VLS and the development of squamous cell carcinoma.
In the study, cohort treatment was tailored to the severity of disease, using the presence or absence and degree of hyperkeratosis as the principal marker of severity. The most severe cases with extensive hyperkeratosis were treated twice daily with a superpotent TCS and less severe cases with a reduced-potency TCS for the initial treatment period.
This use is in contrast to the recommendations of guidelines. Starting with a superpotent corticosteroid has been advocated for all patients either as an initial 3-month once-daily regimen or a tapered regimen for 3 months. Signs at presentation vary considerably, with some women displaying severe widespread genital pallor, hyperkeratosis, fissures, and erosions, whereas others show subtle pallor; there are yet others who are asymptomatic. Hyperkeratosis alone may not be a sufficient marker of active disease, because ecchymoses and erosions may also be important. Tailoring treatment to the severity of the disease makes sense, because in other inflammatory diseases, such as atopic eczema, dermatologists routinely tailor the potency of the corticosteroid used to the severity of the disease.
This article is an important contribution to our knowledge, providing the first evidence in women of the association between malignant neoplasms and poor compliance with treatment. This information will allow us to better inform our patients and encourage those who find it difficult to comply for various reasons.
Dr. Susan M. Cooper is with the department of dermatology, Oxford University Hospitals Trust, Oxford, England. Dr. Nina Madnani is with the department of dermatology, P.D. Hinduja National Hospital and Medical Research Center, Mumbai. Dr. Lynnette Margesson is with the Geisel School of Medicine, Dartmouth College, Hanover, N.H. They disclosed no conflicts of interest. These comments were excerpted from their accompanying editorial (JAMA Dermatol. 2015 June 12 [doi:10.1001/jamadermatol.2015.0644]).
We congratulate the authors on an article that has profound implications for patients with vulvar lichen sclerosus and their physicians worldwide. Until now, suppression of the disease has concerned physicians and has often been inadequate. The reduction in cancer among patients with VLS who were treated with [topical corticosteroids] is no longer speculative. This focus of concern can effectively communicate the message that long-term use of a TCS likely prevents the progression of VLS and the development of squamous cell carcinoma.
In the study, cohort treatment was tailored to the severity of disease, using the presence or absence and degree of hyperkeratosis as the principal marker of severity. The most severe cases with extensive hyperkeratosis were treated twice daily with a superpotent TCS and less severe cases with a reduced-potency TCS for the initial treatment period.
This use is in contrast to the recommendations of guidelines. Starting with a superpotent corticosteroid has been advocated for all patients either as an initial 3-month once-daily regimen or a tapered regimen for 3 months. Signs at presentation vary considerably, with some women displaying severe widespread genital pallor, hyperkeratosis, fissures, and erosions, whereas others show subtle pallor; there are yet others who are asymptomatic. Hyperkeratosis alone may not be a sufficient marker of active disease, because ecchymoses and erosions may also be important. Tailoring treatment to the severity of the disease makes sense, because in other inflammatory diseases, such as atopic eczema, dermatologists routinely tailor the potency of the corticosteroid used to the severity of the disease.
This article is an important contribution to our knowledge, providing the first evidence in women of the association between malignant neoplasms and poor compliance with treatment. This information will allow us to better inform our patients and encourage those who find it difficult to comply for various reasons.
Dr. Susan M. Cooper is with the department of dermatology, Oxford University Hospitals Trust, Oxford, England. Dr. Nina Madnani is with the department of dermatology, P.D. Hinduja National Hospital and Medical Research Center, Mumbai. Dr. Lynnette Margesson is with the Geisel School of Medicine, Dartmouth College, Hanover, N.H. They disclosed no conflicts of interest. These comments were excerpted from their accompanying editorial (JAMA Dermatol. 2015 June 12 [doi:10.1001/jamadermatol.2015.0644]).
We congratulate the authors on an article that has profound implications for patients with vulvar lichen sclerosus and their physicians worldwide. Until now, suppression of the disease has concerned physicians and has often been inadequate. The reduction in cancer among patients with VLS who were treated with [topical corticosteroids] is no longer speculative. This focus of concern can effectively communicate the message that long-term use of a TCS likely prevents the progression of VLS and the development of squamous cell carcinoma.
In the study, cohort treatment was tailored to the severity of disease, using the presence or absence and degree of hyperkeratosis as the principal marker of severity. The most severe cases with extensive hyperkeratosis were treated twice daily with a superpotent TCS and less severe cases with a reduced-potency TCS for the initial treatment period.
This use is in contrast to the recommendations of guidelines. Starting with a superpotent corticosteroid has been advocated for all patients either as an initial 3-month once-daily regimen or a tapered regimen for 3 months. Signs at presentation vary considerably, with some women displaying severe widespread genital pallor, hyperkeratosis, fissures, and erosions, whereas others show subtle pallor; there are yet others who are asymptomatic. Hyperkeratosis alone may not be a sufficient marker of active disease, because ecchymoses and erosions may also be important. Tailoring treatment to the severity of the disease makes sense, because in other inflammatory diseases, such as atopic eczema, dermatologists routinely tailor the potency of the corticosteroid used to the severity of the disease.
This article is an important contribution to our knowledge, providing the first evidence in women of the association between malignant neoplasms and poor compliance with treatment. This information will allow us to better inform our patients and encourage those who find it difficult to comply for various reasons.
Dr. Susan M. Cooper is with the department of dermatology, Oxford University Hospitals Trust, Oxford, England. Dr. Nina Madnani is with the department of dermatology, P.D. Hinduja National Hospital and Medical Research Center, Mumbai. Dr. Lynnette Margesson is with the Geisel School of Medicine, Dartmouth College, Hanover, N.H. They disclosed no conflicts of interest. These comments were excerpted from their accompanying editorial (JAMA Dermatol. 2015 June 12 [doi:10.1001/jamadermatol.2015.0644]).
VANCOUVER, B.C. – Women with vulvar lichen sclerosus (VLS) who consistently used topical corticosteroids that had been titrated to normalize skin color and texture had significantly better outcomes than patients who sometimes skipped treatment, a prospective single-center cohort study found.
Most notably, none of the treatment-compliant patients developed vulvar carcinoma during an average of almost 5 years of follow-up, compared with 4.7% of the partially compliant group (P < .001), reported Dr. Jason Lee, a dermatologist with the University of Sydney, Australia.
“We are proposing a paradigm shift to change the way we manage this condition,” Dr. Lee said at the World Congress of Dermatology. “Clinicians should aim for individual regular treatment of patients with vulvar lichen sclerosus. The treatment is safe, and the course of the disease can be altered by treatment.”
The report appeared simultaneously in JAMA Dermatology (2015 June 12 [doi:10.1001/jamadermatol.2015.0643]).
Vulvar lichen sclerosus is an uncommon disease that causes vulvar itching, pain, and sexual dysfunction, and can significantly alter the vulvar architecture. Before clinicians began treating VLS with superpotent topical corticosteroids, about 5% of cases were thought to progress to vulvar intraepithelial neoplasia or invasive squamous cell carcinoma.
Furthermore, even fairly mild cases can become cancerous, Dr. Lee noted. “Until now, it was thought that cancer was inevitable in some women with VLS,” he added. “Short-term control is easy, but long-term management lacks data, and research has not addressed whether treatment can prevent scarring or cancer.”
To assess the impact of individualized topical corticosteroid therapy with regular follow-up, Dr. Lee and his associates studied 507 women with biopsy-proven VLS during 2008-2014. Patients averaged 55 years of age at presentation and had an average symptom history of 5 years.
The investigators graded cases as mild, moderate, severe, or very severe depending on degree of hyperkeratosis, and titrated treatment with the goal of normalizing skin color and texture. They classified patients as treatment-compliant if they said they followed treatment directions most or all of the time, and as partially compliant if they said they followed directions some, little, or none of the time. They saw patients at least once a year for follow-up.
Most patients initially needed superpotent or ultrapotent topical corticosteroids to control their disease and then switched to moderate or mild strength formulations to maintain results, Dr. Lee and his associates reported.
About 30% of patients were only partially compliant with treatment. These patients were significantly less likely to achieve symptom resolution (58% vs. 93%) and resolution of dyspareunia (66% vs. 94%), and were more likely to develop adhesions and scars during follow-up (40% vs. 3%), compared with fully compliant patients (P < .001 for all), the researchers said. Rates of steroid dermatitis and reversible steroid-induced cutaneous atrophy were similar between the fully and partially compliant groups (2.2% vs. 4%, and 1.1% vs. 2%, respectively).
Based on the findings, clinicians would need to treat 21 cases of VLS to prevent 1 case of vulvar cancer and 2.7 cases to prevent scarring, said Dr. Lee.
“This is the first study adequately powered to demonstrate that cancer and scarring can be prevented by regular treatment according to clinical outcome, not symptoms,” he added.
But Dr. Lee acknowledged that selection bias could have affected the results. “We did find that patients who were not complying with treatment were more likely to have severe disease at the start of the study,” he said. “They probably were at increased risk of cancer to start with.”
The dermatology department of Royal North Shore Hospital partially funded the work. The investigators reported having no relevant conflicts of interest.
VANCOUVER, B.C. – Women with vulvar lichen sclerosus (VLS) who consistently used topical corticosteroids that had been titrated to normalize skin color and texture had significantly better outcomes than patients who sometimes skipped treatment, a prospective single-center cohort study found.
Most notably, none of the treatment-compliant patients developed vulvar carcinoma during an average of almost 5 years of follow-up, compared with 4.7% of the partially compliant group (P < .001), reported Dr. Jason Lee, a dermatologist with the University of Sydney, Australia.
“We are proposing a paradigm shift to change the way we manage this condition,” Dr. Lee said at the World Congress of Dermatology. “Clinicians should aim for individual regular treatment of patients with vulvar lichen sclerosus. The treatment is safe, and the course of the disease can be altered by treatment.”
The report appeared simultaneously in JAMA Dermatology (2015 June 12 [doi:10.1001/jamadermatol.2015.0643]).
Vulvar lichen sclerosus is an uncommon disease that causes vulvar itching, pain, and sexual dysfunction, and can significantly alter the vulvar architecture. Before clinicians began treating VLS with superpotent topical corticosteroids, about 5% of cases were thought to progress to vulvar intraepithelial neoplasia or invasive squamous cell carcinoma.
Furthermore, even fairly mild cases can become cancerous, Dr. Lee noted. “Until now, it was thought that cancer was inevitable in some women with VLS,” he added. “Short-term control is easy, but long-term management lacks data, and research has not addressed whether treatment can prevent scarring or cancer.”
To assess the impact of individualized topical corticosteroid therapy with regular follow-up, Dr. Lee and his associates studied 507 women with biopsy-proven VLS during 2008-2014. Patients averaged 55 years of age at presentation and had an average symptom history of 5 years.
The investigators graded cases as mild, moderate, severe, or very severe depending on degree of hyperkeratosis, and titrated treatment with the goal of normalizing skin color and texture. They classified patients as treatment-compliant if they said they followed treatment directions most or all of the time, and as partially compliant if they said they followed directions some, little, or none of the time. They saw patients at least once a year for follow-up.
Most patients initially needed superpotent or ultrapotent topical corticosteroids to control their disease and then switched to moderate or mild strength formulations to maintain results, Dr. Lee and his associates reported.
About 30% of patients were only partially compliant with treatment. These patients were significantly less likely to achieve symptom resolution (58% vs. 93%) and resolution of dyspareunia (66% vs. 94%), and were more likely to develop adhesions and scars during follow-up (40% vs. 3%), compared with fully compliant patients (P < .001 for all), the researchers said. Rates of steroid dermatitis and reversible steroid-induced cutaneous atrophy were similar between the fully and partially compliant groups (2.2% vs. 4%, and 1.1% vs. 2%, respectively).
Based on the findings, clinicians would need to treat 21 cases of VLS to prevent 1 case of vulvar cancer and 2.7 cases to prevent scarring, said Dr. Lee.
“This is the first study adequately powered to demonstrate that cancer and scarring can be prevented by regular treatment according to clinical outcome, not symptoms,” he added.
But Dr. Lee acknowledged that selection bias could have affected the results. “We did find that patients who were not complying with treatment were more likely to have severe disease at the start of the study,” he said. “They probably were at increased risk of cancer to start with.”
The dermatology department of Royal North Shore Hospital partially funded the work. The investigators reported having no relevant conflicts of interest.
AT WCD 2015
Key clinical point: Women with vulvar lichen sclerosus who consistently used topical corticosteroids titrated to normalize skin color and texture had significantly better symptoms, scarring, and rates of vulvar cancer compared with partially compliant patients.
Major finding: None of the women who consistently followed their individualized treatment plan developed vulvar carcinoma, compared with 4.7% of partially compliant patients (P < .001).
Data source: Single-center prospective, observational cohort study of 507 women with biopsy-proven vulvar lichen sclerosus.
Disclosures: The dermatology department of Royal North Shore Hospital partially funded the work. Dr. Lee reported having no relevant conflicts of interest.