User login
Obesity: American Association of Clinical Endocrinology (AACE)
Obesity rates in the United States have skyrocketed over the last 30 years, with rates for adults having doubled and rates for children tripled from 1980 to 2010. Approximately one-third of the U.S adult population is obese; that’s 72 million people. The consequences of obesity include an increased risk for stroke, hypertension, type 2 diabetes, liver and gallbladder disease, orthopedic complications, mental health conditions, cancers, elevated lipids, obstructive sleep apnea (OSA), and reproductive complications such as infertility.
There is now solid evidence that we can intervene to help patient lose weight and decrease the complications that result from obesity. To this end, the American Association of Clinical Endocrinology (AACE) has issued recommendations giving guidance for clinicians about how to approach this issue. Intensive approaches to lifestyle modification with diet and exercise can help patients lose 7% or more of their body weight and have been show to decrease progression from prediabetes to diabetes. Two new medications, lorcaserin and phentermine/topiramate ER, have received Food and Drug Administration approval over the past 2 years as an adjunct to diet for weight loss. Bariatric surgery has emerged as a safe and effective method of weight loss as well.
The AACE guidelines focus on a "complications-centric model" as opposed to a body mass index–driven approach. The guidelines recommend treating obesity to decrease the risk of developing adverse metabolic consequences such as diabetes and metabolic syndrome, and to decrease disability from mechanical comorbidities such as osteoarthritis and obstructive sleep apnea. The AACE guidelines place obese patients into two categories: those that have obesity-related comorbidities and those that do not. The guidelines recommend a graded approach to treatment. All overweight and obese patients should receive therapeutic lifestyle counseling focusing on diet and exercise. Medical or surgical treatment is then recommended for the patients who stand to benefit the most, those with obesity-related comorbidities and those with more severe obesity who have not been able to lose weight using lifestyle modification alone.
In the initial evaluation of overweight and obese patients, the patients should be assessed for cardiometabolic and mechanical complications of obesity, as well as the severity of those complications in order to determine the level of treatment that is appropriate. Patients with obesity-related comorbidities are classified into two groups. The first group includes those with insulin resistance and/or cardiovascular consequences. For this group, evaluation should include waist circumference, fasting, and 2-hour glucose tolerance testing, and lipids, blood pressure, and liver function testing. The second group is composed of people with mechanical consequences including OSA, stress incontinence, orthopedic complications, and chronic pulmonary diseases.
It is important to determine target goals for weight loss to improve mechanical and cardiometabolic complications. Weight loss of 5% or more is enough to affect improvement in metabolic parameters such as glucose and lipids, decrease progression to diabetes, and improve mechanical complications such as knee and hip pain in osteoarthritis. The next step in the approach to treatment is to determine the type and intensity. Therapeutic lifestyle changes (TLC) are important for all patients with diabetes and prediabetes, regardless of risk factors. TLC recommendations include smoking cessation, physical activity, weight management, and healthy eating. Exercise is recommended 5 days/week for more than 30 minutes of moderate intensity activity, to achieve a more than 60% age-related heart rate. The diet recommended reduced saturated fat to less than 7% of calories, reduced cholesterol intake to 200 mg/day, increased fiber to 10-25 g/day, increased plant sterols/stanol esters to 2 g/day, caloric restriction, reduced simple carbohydrates and sugars, increased intake of unsaturated fats, elimination of trans fats, increased marine-based omega-3 ethyl esters, and restriction of alcohol to 20-30 g/day.
For patients with comorbidities and with a BMI of 27 kg/m2 or more, consideration should be given to weight-loss medication in addition to lifestyle intervention. The currently approved medications for long-term weight loss include lorcaserin and phentermine/topiramate ER. In the FDA registration studies, the lorcaserin group had an average weight loss of 5.8% after 1 year vs. 2.2% in the placebo group. Phentermine/topiramate ER had an average weight loss of 10% at 1 year vs. 1.2% in the placebo group. These medications are FDA approved as adjuncts to lifestyle modification for the treatment of overweight patients with a BMI greater than 27 kg/m2 with comorbidities and for obese patients with a BMI greater than 30 kg/m2 regardless of comorbidities. Both medications improve blood pressure, triglycerides, and insulin sensitivity and prevent the progression to diabetes in patients with diabetes. Bariatric surgery should be considered for those with a BMI of 35 kg/m2 or morewith comorbidities, especially if they have failed using other methods.
Once goals are reached, reassess the patient to evaluate for more interventions, if needed. If the targets for improvement in complications were not reached, then the weight loss therapy should become more intense.
Bottom line
The AACE recommendations recognize obesity as a disease and have formulated guidelines using a "complications-centric model." Patients should be assessed for obesity and related complications. Lifestyle counseling should be provided for all overweight and obese individuals, with the addition of weight loss medications for individuals with a BMI of 27 kg/m2 or more who have obesity-related comorbidities, and the consideration of bariatric surgery for those with a BMI of 35 kg/m2 or more with comorbidities.
Reference
American Association of Clinical Endocrinologists’ Comprehensive Diabetes Management Algorithm 2013 Consensus Statement. Published May/June 2013, Endocrine Practice, Vol. 19 (Suppl. 2).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. McDonald is a second-year resident in the Family Medicine Residency Program at Abington Memorial Hospital.
Obesity rates in the United States have skyrocketed over the last 30 years, with rates for adults having doubled and rates for children tripled from 1980 to 2010. Approximately one-third of the U.S adult population is obese; that’s 72 million people. The consequences of obesity include an increased risk for stroke, hypertension, type 2 diabetes, liver and gallbladder disease, orthopedic complications, mental health conditions, cancers, elevated lipids, obstructive sleep apnea (OSA), and reproductive complications such as infertility.
There is now solid evidence that we can intervene to help patient lose weight and decrease the complications that result from obesity. To this end, the American Association of Clinical Endocrinology (AACE) has issued recommendations giving guidance for clinicians about how to approach this issue. Intensive approaches to lifestyle modification with diet and exercise can help patients lose 7% or more of their body weight and have been show to decrease progression from prediabetes to diabetes. Two new medications, lorcaserin and phentermine/topiramate ER, have received Food and Drug Administration approval over the past 2 years as an adjunct to diet for weight loss. Bariatric surgery has emerged as a safe and effective method of weight loss as well.
The AACE guidelines focus on a "complications-centric model" as opposed to a body mass index–driven approach. The guidelines recommend treating obesity to decrease the risk of developing adverse metabolic consequences such as diabetes and metabolic syndrome, and to decrease disability from mechanical comorbidities such as osteoarthritis and obstructive sleep apnea. The AACE guidelines place obese patients into two categories: those that have obesity-related comorbidities and those that do not. The guidelines recommend a graded approach to treatment. All overweight and obese patients should receive therapeutic lifestyle counseling focusing on diet and exercise. Medical or surgical treatment is then recommended for the patients who stand to benefit the most, those with obesity-related comorbidities and those with more severe obesity who have not been able to lose weight using lifestyle modification alone.
In the initial evaluation of overweight and obese patients, the patients should be assessed for cardiometabolic and mechanical complications of obesity, as well as the severity of those complications in order to determine the level of treatment that is appropriate. Patients with obesity-related comorbidities are classified into two groups. The first group includes those with insulin resistance and/or cardiovascular consequences. For this group, evaluation should include waist circumference, fasting, and 2-hour glucose tolerance testing, and lipids, blood pressure, and liver function testing. The second group is composed of people with mechanical consequences including OSA, stress incontinence, orthopedic complications, and chronic pulmonary diseases.
It is important to determine target goals for weight loss to improve mechanical and cardiometabolic complications. Weight loss of 5% or more is enough to affect improvement in metabolic parameters such as glucose and lipids, decrease progression to diabetes, and improve mechanical complications such as knee and hip pain in osteoarthritis. The next step in the approach to treatment is to determine the type and intensity. Therapeutic lifestyle changes (TLC) are important for all patients with diabetes and prediabetes, regardless of risk factors. TLC recommendations include smoking cessation, physical activity, weight management, and healthy eating. Exercise is recommended 5 days/week for more than 30 minutes of moderate intensity activity, to achieve a more than 60% age-related heart rate. The diet recommended reduced saturated fat to less than 7% of calories, reduced cholesterol intake to 200 mg/day, increased fiber to 10-25 g/day, increased plant sterols/stanol esters to 2 g/day, caloric restriction, reduced simple carbohydrates and sugars, increased intake of unsaturated fats, elimination of trans fats, increased marine-based omega-3 ethyl esters, and restriction of alcohol to 20-30 g/day.
For patients with comorbidities and with a BMI of 27 kg/m2 or more, consideration should be given to weight-loss medication in addition to lifestyle intervention. The currently approved medications for long-term weight loss include lorcaserin and phentermine/topiramate ER. In the FDA registration studies, the lorcaserin group had an average weight loss of 5.8% after 1 year vs. 2.2% in the placebo group. Phentermine/topiramate ER had an average weight loss of 10% at 1 year vs. 1.2% in the placebo group. These medications are FDA approved as adjuncts to lifestyle modification for the treatment of overweight patients with a BMI greater than 27 kg/m2 with comorbidities and for obese patients with a BMI greater than 30 kg/m2 regardless of comorbidities. Both medications improve blood pressure, triglycerides, and insulin sensitivity and prevent the progression to diabetes in patients with diabetes. Bariatric surgery should be considered for those with a BMI of 35 kg/m2 or morewith comorbidities, especially if they have failed using other methods.
Once goals are reached, reassess the patient to evaluate for more interventions, if needed. If the targets for improvement in complications were not reached, then the weight loss therapy should become more intense.
Bottom line
The AACE recommendations recognize obesity as a disease and have formulated guidelines using a "complications-centric model." Patients should be assessed for obesity and related complications. Lifestyle counseling should be provided for all overweight and obese individuals, with the addition of weight loss medications for individuals with a BMI of 27 kg/m2 or more who have obesity-related comorbidities, and the consideration of bariatric surgery for those with a BMI of 35 kg/m2 or more with comorbidities.
Reference
American Association of Clinical Endocrinologists’ Comprehensive Diabetes Management Algorithm 2013 Consensus Statement. Published May/June 2013, Endocrine Practice, Vol. 19 (Suppl. 2).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. McDonald is a second-year resident in the Family Medicine Residency Program at Abington Memorial Hospital.
Obesity rates in the United States have skyrocketed over the last 30 years, with rates for adults having doubled and rates for children tripled from 1980 to 2010. Approximately one-third of the U.S adult population is obese; that’s 72 million people. The consequences of obesity include an increased risk for stroke, hypertension, type 2 diabetes, liver and gallbladder disease, orthopedic complications, mental health conditions, cancers, elevated lipids, obstructive sleep apnea (OSA), and reproductive complications such as infertility.
There is now solid evidence that we can intervene to help patient lose weight and decrease the complications that result from obesity. To this end, the American Association of Clinical Endocrinology (AACE) has issued recommendations giving guidance for clinicians about how to approach this issue. Intensive approaches to lifestyle modification with diet and exercise can help patients lose 7% or more of their body weight and have been show to decrease progression from prediabetes to diabetes. Two new medications, lorcaserin and phentermine/topiramate ER, have received Food and Drug Administration approval over the past 2 years as an adjunct to diet for weight loss. Bariatric surgery has emerged as a safe and effective method of weight loss as well.
The AACE guidelines focus on a "complications-centric model" as opposed to a body mass index–driven approach. The guidelines recommend treating obesity to decrease the risk of developing adverse metabolic consequences such as diabetes and metabolic syndrome, and to decrease disability from mechanical comorbidities such as osteoarthritis and obstructive sleep apnea. The AACE guidelines place obese patients into two categories: those that have obesity-related comorbidities and those that do not. The guidelines recommend a graded approach to treatment. All overweight and obese patients should receive therapeutic lifestyle counseling focusing on diet and exercise. Medical or surgical treatment is then recommended for the patients who stand to benefit the most, those with obesity-related comorbidities and those with more severe obesity who have not been able to lose weight using lifestyle modification alone.
In the initial evaluation of overweight and obese patients, the patients should be assessed for cardiometabolic and mechanical complications of obesity, as well as the severity of those complications in order to determine the level of treatment that is appropriate. Patients with obesity-related comorbidities are classified into two groups. The first group includes those with insulin resistance and/or cardiovascular consequences. For this group, evaluation should include waist circumference, fasting, and 2-hour glucose tolerance testing, and lipids, blood pressure, and liver function testing. The second group is composed of people with mechanical consequences including OSA, stress incontinence, orthopedic complications, and chronic pulmonary diseases.
It is important to determine target goals for weight loss to improve mechanical and cardiometabolic complications. Weight loss of 5% or more is enough to affect improvement in metabolic parameters such as glucose and lipids, decrease progression to diabetes, and improve mechanical complications such as knee and hip pain in osteoarthritis. The next step in the approach to treatment is to determine the type and intensity. Therapeutic lifestyle changes (TLC) are important for all patients with diabetes and prediabetes, regardless of risk factors. TLC recommendations include smoking cessation, physical activity, weight management, and healthy eating. Exercise is recommended 5 days/week for more than 30 minutes of moderate intensity activity, to achieve a more than 60% age-related heart rate. The diet recommended reduced saturated fat to less than 7% of calories, reduced cholesterol intake to 200 mg/day, increased fiber to 10-25 g/day, increased plant sterols/stanol esters to 2 g/day, caloric restriction, reduced simple carbohydrates and sugars, increased intake of unsaturated fats, elimination of trans fats, increased marine-based omega-3 ethyl esters, and restriction of alcohol to 20-30 g/day.
For patients with comorbidities and with a BMI of 27 kg/m2 or more, consideration should be given to weight-loss medication in addition to lifestyle intervention. The currently approved medications for long-term weight loss include lorcaserin and phentermine/topiramate ER. In the FDA registration studies, the lorcaserin group had an average weight loss of 5.8% after 1 year vs. 2.2% in the placebo group. Phentermine/topiramate ER had an average weight loss of 10% at 1 year vs. 1.2% in the placebo group. These medications are FDA approved as adjuncts to lifestyle modification for the treatment of overweight patients with a BMI greater than 27 kg/m2 with comorbidities and for obese patients with a BMI greater than 30 kg/m2 regardless of comorbidities. Both medications improve blood pressure, triglycerides, and insulin sensitivity and prevent the progression to diabetes in patients with diabetes. Bariatric surgery should be considered for those with a BMI of 35 kg/m2 or morewith comorbidities, especially if they have failed using other methods.
Once goals are reached, reassess the patient to evaluate for more interventions, if needed. If the targets for improvement in complications were not reached, then the weight loss therapy should become more intense.
Bottom line
The AACE recommendations recognize obesity as a disease and have formulated guidelines using a "complications-centric model." Patients should be assessed for obesity and related complications. Lifestyle counseling should be provided for all overweight and obese individuals, with the addition of weight loss medications for individuals with a BMI of 27 kg/m2 or more who have obesity-related comorbidities, and the consideration of bariatric surgery for those with a BMI of 35 kg/m2 or more with comorbidities.
Reference
American Association of Clinical Endocrinologists’ Comprehensive Diabetes Management Algorithm 2013 Consensus Statement. Published May/June 2013, Endocrine Practice, Vol. 19 (Suppl. 2).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. McDonald is a second-year resident in the Family Medicine Residency Program at Abington Memorial Hospital.
FDA Advisory Committee recommends HPV test as primary screening tool for cervical cancer
The US Food and Drug Administration (FDA) Microbiology Devices Panel of the Medical Devices Advisory Committee has unanimously recommended that the cobas HPV (human papillomavirus) test be used as a first-line primary screening tool in women aged 25 years and older to assess their risk of cervical cancer based on the presence of clinically relevant high-risk HPV DNA. The committee’s recommendation indicates that the benefits outweigh the risks of the test, and that the cobas HPV test is safe and effective for the proposed indication for use.1
If approved by the FDA, the cobas HPV test would become the “first and only HPV test indicated as the first-line primary screen of cervical cancer in the United States.”2 Although the FDA is not required to follow the Advisory Committee’s recommendation, it takes the advice into consideration.
Data behind the recommendation
The Advisory Committee’s recommendation is supported by data from the ATHENA study, which included more than 47,000 women – the largest US-based registration study for cervical cancer screening. Data show that when the cobas HPV test was used as the primary test and Pap cytology as a secondary test, significantly more cervical disease was detected compared with Pap screening alone.2
“Through technological and scientific advancement, we now have a better screening tool for cervical cancer. Women around the world deserve the best tool to know their risk and reduce their chances of developing cervical cancer,” said Roland Diggelmann, COO for the Division of Roche Diagnostics, the company who developed and manufactures the test. “We look forward to working with the FDA and medical community to support the growing understanding and awareness of the role that HPV plays in cervical disease, and the importance of the cobas HPV test, which provides the necessary medical benefit to become the first-line test in a cervical cancer screening strategy.”2
How could current practice change as a result of final FDA approval?
The cobas HPV test is currently FDA-approved for co-testing with the Pap smear in women older than age 30 for cervical cancer screening, and for screening patients aged 21 and older with abnormal cervical cytology results.
Mark H. Einstein, MD, MS, chair of the cervical cancer education efforts of the Foundation for Women’s Cancer and professor of obstetrics and gynecology at Albert Einstein Cancer Center and Montefiore Medical Center in Bronx, New York, says final approval of this testing as a primary screening tool represents significant changes to clinical practice. However, “similar to what happened when co-testing [with the cobas HPV test] was approved, it took time for scientific stakeholding groups to update clinical guidelines, then years before clinicians adopted it into routine practice.”
“Unlike a new prescription, clinical algorithms tend to be 'hard-wired' into clinicians heads, and adopting significant change is a process,” Einstein says. “It’s likely that a new cervical cancer screening testing clinical algorithm would be adopted by some clinicians early and by many clinicians over time.” He added that the Society of Gynecologic Oncologists and the American Society for Colposcopy and Cervical Pathology have an interim clinical guidance document currently drafted, and those guidelines will be released soon after any decisions by the FDA.
When that time comes (assuming final FDA approval is received), Einstein says, “some clinical settings will be able to start with the more sensitive HPV test. For some patients, this will be followed by genotyping or cytology. This has been shown to be an effective strategy for honing in on the most at-risk women in a screening population.”
- FDA Executive Summary: March 12, 2014. 2014 Meeting Materials of the Microbiology Devices Panel. FDA Web site. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/MicrobiologyDevicesPanel/UCM388564.pdf. Accessed March 14, 2014.
- FDA Advisory Committee unanimously recommends Roche's HPV Test as primary screening tool for detection of women at high risk for cervical cancer [media release]. http://www.roche.com/media/media_releases/med-cor-2014-03-13.htm. Published March 13, 2014. Accessed March 14, 2014.
The US Food and Drug Administration (FDA) Microbiology Devices Panel of the Medical Devices Advisory Committee has unanimously recommended that the cobas HPV (human papillomavirus) test be used as a first-line primary screening tool in women aged 25 years and older to assess their risk of cervical cancer based on the presence of clinically relevant high-risk HPV DNA. The committee’s recommendation indicates that the benefits outweigh the risks of the test, and that the cobas HPV test is safe and effective for the proposed indication for use.1
If approved by the FDA, the cobas HPV test would become the “first and only HPV test indicated as the first-line primary screen of cervical cancer in the United States.”2 Although the FDA is not required to follow the Advisory Committee’s recommendation, it takes the advice into consideration.
Data behind the recommendation
The Advisory Committee’s recommendation is supported by data from the ATHENA study, which included more than 47,000 women – the largest US-based registration study for cervical cancer screening. Data show that when the cobas HPV test was used as the primary test and Pap cytology as a secondary test, significantly more cervical disease was detected compared with Pap screening alone.2
“Through technological and scientific advancement, we now have a better screening tool for cervical cancer. Women around the world deserve the best tool to know their risk and reduce their chances of developing cervical cancer,” said Roland Diggelmann, COO for the Division of Roche Diagnostics, the company who developed and manufactures the test. “We look forward to working with the FDA and medical community to support the growing understanding and awareness of the role that HPV plays in cervical disease, and the importance of the cobas HPV test, which provides the necessary medical benefit to become the first-line test in a cervical cancer screening strategy.”2
How could current practice change as a result of final FDA approval?
The cobas HPV test is currently FDA-approved for co-testing with the Pap smear in women older than age 30 for cervical cancer screening, and for screening patients aged 21 and older with abnormal cervical cytology results.
Mark H. Einstein, MD, MS, chair of the cervical cancer education efforts of the Foundation for Women’s Cancer and professor of obstetrics and gynecology at Albert Einstein Cancer Center and Montefiore Medical Center in Bronx, New York, says final approval of this testing as a primary screening tool represents significant changes to clinical practice. However, “similar to what happened when co-testing [with the cobas HPV test] was approved, it took time for scientific stakeholding groups to update clinical guidelines, then years before clinicians adopted it into routine practice.”
“Unlike a new prescription, clinical algorithms tend to be 'hard-wired' into clinicians heads, and adopting significant change is a process,” Einstein says. “It’s likely that a new cervical cancer screening testing clinical algorithm would be adopted by some clinicians early and by many clinicians over time.” He added that the Society of Gynecologic Oncologists and the American Society for Colposcopy and Cervical Pathology have an interim clinical guidance document currently drafted, and those guidelines will be released soon after any decisions by the FDA.
When that time comes (assuming final FDA approval is received), Einstein says, “some clinical settings will be able to start with the more sensitive HPV test. For some patients, this will be followed by genotyping or cytology. This has been shown to be an effective strategy for honing in on the most at-risk women in a screening population.”
The US Food and Drug Administration (FDA) Microbiology Devices Panel of the Medical Devices Advisory Committee has unanimously recommended that the cobas HPV (human papillomavirus) test be used as a first-line primary screening tool in women aged 25 years and older to assess their risk of cervical cancer based on the presence of clinically relevant high-risk HPV DNA. The committee’s recommendation indicates that the benefits outweigh the risks of the test, and that the cobas HPV test is safe and effective for the proposed indication for use.1
If approved by the FDA, the cobas HPV test would become the “first and only HPV test indicated as the first-line primary screen of cervical cancer in the United States.”2 Although the FDA is not required to follow the Advisory Committee’s recommendation, it takes the advice into consideration.
Data behind the recommendation
The Advisory Committee’s recommendation is supported by data from the ATHENA study, which included more than 47,000 women – the largest US-based registration study for cervical cancer screening. Data show that when the cobas HPV test was used as the primary test and Pap cytology as a secondary test, significantly more cervical disease was detected compared with Pap screening alone.2
“Through technological and scientific advancement, we now have a better screening tool for cervical cancer. Women around the world deserve the best tool to know their risk and reduce their chances of developing cervical cancer,” said Roland Diggelmann, COO for the Division of Roche Diagnostics, the company who developed and manufactures the test. “We look forward to working with the FDA and medical community to support the growing understanding and awareness of the role that HPV plays in cervical disease, and the importance of the cobas HPV test, which provides the necessary medical benefit to become the first-line test in a cervical cancer screening strategy.”2
How could current practice change as a result of final FDA approval?
The cobas HPV test is currently FDA-approved for co-testing with the Pap smear in women older than age 30 for cervical cancer screening, and for screening patients aged 21 and older with abnormal cervical cytology results.
Mark H. Einstein, MD, MS, chair of the cervical cancer education efforts of the Foundation for Women’s Cancer and professor of obstetrics and gynecology at Albert Einstein Cancer Center and Montefiore Medical Center in Bronx, New York, says final approval of this testing as a primary screening tool represents significant changes to clinical practice. However, “similar to what happened when co-testing [with the cobas HPV test] was approved, it took time for scientific stakeholding groups to update clinical guidelines, then years before clinicians adopted it into routine practice.”
“Unlike a new prescription, clinical algorithms tend to be 'hard-wired' into clinicians heads, and adopting significant change is a process,” Einstein says. “It’s likely that a new cervical cancer screening testing clinical algorithm would be adopted by some clinicians early and by many clinicians over time.” He added that the Society of Gynecologic Oncologists and the American Society for Colposcopy and Cervical Pathology have an interim clinical guidance document currently drafted, and those guidelines will be released soon after any decisions by the FDA.
When that time comes (assuming final FDA approval is received), Einstein says, “some clinical settings will be able to start with the more sensitive HPV test. For some patients, this will be followed by genotyping or cytology. This has been shown to be an effective strategy for honing in on the most at-risk women in a screening population.”
- FDA Executive Summary: March 12, 2014. 2014 Meeting Materials of the Microbiology Devices Panel. FDA Web site. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/MicrobiologyDevicesPanel/UCM388564.pdf. Accessed March 14, 2014.
- FDA Advisory Committee unanimously recommends Roche's HPV Test as primary screening tool for detection of women at high risk for cervical cancer [media release]. http://www.roche.com/media/media_releases/med-cor-2014-03-13.htm. Published March 13, 2014. Accessed March 14, 2014.
- FDA Executive Summary: March 12, 2014. 2014 Meeting Materials of the Microbiology Devices Panel. FDA Web site. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/MicrobiologyDevicesPanel/UCM388564.pdf. Accessed March 14, 2014.
- FDA Advisory Committee unanimously recommends Roche's HPV Test as primary screening tool for detection of women at high risk for cervical cancer [media release]. http://www.roche.com/media/media_releases/med-cor-2014-03-13.htm. Published March 13, 2014. Accessed March 14, 2014.
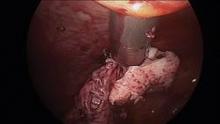
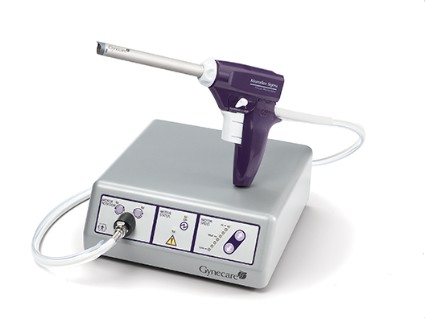
Putting morcellation into perspective – ‘Just the facts, Ma’am, nothing but the facts’
Intra-abdominal (intracorporeal) morcellation, especially electronically powered morcellation, has recently come under scrutiny. Generally performed at the time of conventional laparoscopic or robotic supracervical hysterectomy, total hysterectomy for the large uterus, or myomectomy, both power and cold-knife morcellation may splatter tissue fragments in the pelvis and abdomen, leading to potential parasitizing of the tissue and ectopic growth. Recent evidence indicates inadvertent morcellation of a leiomyosarcoma may negatively affect the patient’s subsequent disease-free survival and overall survival.
Concerns about morcellation heightened after Dr. Amy J. Reed, an anesthesiologist at Beth Israel Deaconess Medical Center, Boston, and a mother of 6, underwent presumed fibroid surgery and was diagnosed, post morcellation, with leiomyosarcoma. Dr. Reed’s husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon at Brigham and Women’s Hospital, Boston, where his wife’s surgery was performed, is calling for a moratorium on intra-abdominal morcellation, whether it involves the use of a power morcellator, or for that matter, the cold knife.
It is imperative and incumbent upon our specialty to have a detailed evaluation of the risks and benefits of morcellation. While morcellation of the rare leiomyosarcoma is a risk, banning intraabdominal/intrapelvic morcellation will certainly have a profound negative impact on patients who are able to undergo a minimally invasive gynecologic procedure. Banning morcellation would increase intraoperative risk and subsequent concern of postoperative pelvic adhesions and thus, potential impact on fertility (post myomectomy), dyspareunia, and pelvic pain. Further, a ban would incur higher costs and more loss of patient productivity (Hum. Reprod. 1998 13:2102-6). These concerns were the basis for the AAGL position statement touting a minimally invasive approach to hysterectomy (J. Minim. Invasive Gynecol. 2011;18:1-3).
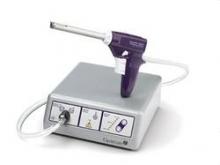
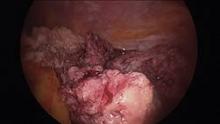
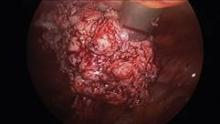
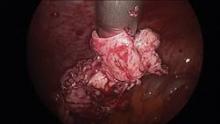
Since their introduction in the mid-1990s, electronically powered morcellators have been used to remove the uterus, fibroid(s), spleen, or kidney. Varying in size from 12-20 mm, electronic morcellators generally consist of a rotating circular blade at the end of a hollow tube. A tenaculum or multitoothed grasper is placed through the tube and blade to grasp the tissue to the revolving blade. The specimen is then removed in strips. Tissue splatter is inevitable, at least until the technique evolves to allow morcellation to be performed within the confines of a bag.
Benign uterine fibroids are the most common pelvic tumor in women. Literature reviews indicate the lifetime risk is 70% for white women and 80% in women of African ancestry. Uterine sarcomas occur in 3-7 women per 100,000 (Am. J. Obstet. Gynecol. 2011;205:492.e1-5). Further, Dr. Kimberly A. Kho of the University of Texas Southwestern Medical Center, Dallas, and Dr. Ceana H. Dr. Nezhat of Atlanta Center for Minimally Invasive Surgery and Reproductive Medicine, conducted a meta-analysis of 5,666 uterine procedures, and found 13 unsuspected uterine sarcomas, for a prevalence of 0.23% (JAMA 2014 [doi:10.1001/jama.2014.1093]).
This finding is consistent with that of a previous study by Dr. W.H. Parker who also noted a 0.23% risk, based on data from 1,332 women undergoing surgery secondary to uterine fibroids. Interestingly, in Dr. Parker’s study, the risk was 0.27% among women with rapidly growing leiomyoma, often thought to be a risk factor for sarcoma development (Obstet. Gynecol. 1994;83:414-8).
Because of the difficulty of making a preoperative diagnosis of leiomyosarcoma, it is doubtful that this risk will be decreased in the near future. Risk factors have not been well established, although a twofold higher incidence of leiomyosarcomas has been observed in black women (Gynecol. Oncol. 2004;93:204-8). Increasing age would appear to increase uterine sarcoma risk, as the majority of cases are diagnosed in postmenopausal women. Tamoxifen, when used for 5 or more years, appears to be associated with higher sarcoma rates (J. Clin. Oncol. 2002;20:2758-60) as is a history of pelvic irradiation or childhood retinoblastoma.
Unless metastatic disease is present, symptoms are similar for leiomyomas and leiomyosarcomas. A rapidly growing mass, a finding associated with an increased risk of uterine sarcoma, was not seen in Parker’s study of 1,332 women undergoing hysterectomy or myomectomy for uterine leiomyoma. Similarly, size does not count; a large uterine mass or increased uterine size did not appear to be associated with a greater risk of sarcoma (Gynecol. Oncol. 2003;89:460-9).
Some contend that failed response with such therapies as gonadotropin-releasing hormone agonists and uterine artery embolization are associated with increased incidence of leiomyosarcoma, but the data are not convincing (Eur. J. Obstet. Gynecol. Reprod. Biol. 1998;76:237-40).
Physical examination and imaging may be helpful in finding enlarged lymph nodes, but imaging methods have not been reliably shown to enable a preoperative diagnosis of uterine leiomyosarcoma (Lancet Oncol. 2009;10:1188-98; AJR Am. J. Roentgenol. 2003;181:1369-74). Further, while some physicians point out that an ill-defined margin may increase leiomyosarcoma risk, this finding is certainly noted as well with benign adenomyomas.
Finally, data are scant in support of preoperative endometrial sampling to establish a diagnosis of leiomyosarcoma. In two studies comparing a total of 14 patients, 7 were correctly diagnosed with leiomyosarcoma prior to surgery (Am. J. Obstet. Gynecol. 1990;162:968-74; Gynecol. Oncol. 2008;110:43-8).
With little differentiation in clinical presentation and the inability to distinguish leiomyoma from leiomyosarcoma based on imaging or sampling, it is not surprising that patients undergoing morcellation for an expected benign condition would subsequently be diagnosed with uterine leiomyosarcoma. With this in mind, it is important to review the current body of literature to further evaluate the risks and benefits of morcellation, and what place minimally invasive gynecologic surgery will have for the treatment of uterine masses.
Tumor morcellation of unrecognized leiomyosarcomas was significantly associated with poorer disease free survival (odds ratio, 2.59, P = 1.43), higher stage (I vs. II; [OR, 19.12, P = .037]) and poorer overall survival (OR, 3.07, P =.040) in a 2011 study. Park et al. assessed 56 consecutive patients, 25 with morcellation and 31 without tumor morcellation, who had stage I and stage II uterine leiomyosarcomas and were treated between 1989 and 2010. The percentage of patients with dissemination also was noted to be greater in patients with tumor morcellation (44% vs. 12.9%, P =.032). Interestingly, ovarian tissue was more frequently preserved in the morcellation group (38.7% vs. 72%, P =.013) (Gynecol. Oncol. 2011;122:255-9)
In response to a subsequent Letter to the Editor about these risks, the study’s author put the findings in perspective. "The frequency of incidental uterine leiomyosarcoma in patients who undergo surgery for presumed uterine leiomyoma is extremely rare. At our medical center, only 49 of 22,825 patients (0.21%) who underwent surgery for presumed uterine leiomyoma had incidental uterine leiomyosarcoma. Therefore, we believe that surgeons need not avoid non-laparotomic* surgical routes because of the rare possibility of an incidental diagnosis of leiomyosarcoma, even when tumor morcellation is required" (Gynecol. Oncol. 2012;124:172-3).
Additionally, a retrospective study from Brigham & Women’s Hospital found that disease was often already disseminated before morcellation procedures. In 21 patients with a median age of 46 years and no documented evidence of extrauterine disease, 15 had uterine leiomyosarcomas and 6 had smooth muscle tumors of uncertain malignant potential that were inadvertently morcellated; data was incorporated from January 2005 to January 2012. While most patients underwent power morcellation with laparoscopy, two underwent laparoscopically assisted vaginal hysterectomy with hand morcellation, and one patient had a vaginal hysterectomy with hand morcellation.
Immediate surgical reexploration was performed for staging in 12 patients. Significant findings of disseminated intraperitoneal disease were detected in two of seven patients with presumed stage I uterine leiomyosarcoma and in one of four patients with presumed stage I smooth muscle tumors of uncertain malignant potential. Moreover, of the eight patients who did not have disseminated disease at the time of the staging procedure, one subsequently had a recurrence. The remaining patients had no recurrences and remain disease free.
One patient was already FIGO stage IV at the original surgery, two more patients were upstaged at the original surgery and underwent re-exploration at 18 and 20 months respectively (certainly, a long period prior to second look). Moreover, the authors note various reasons why a significant number of patients were upstaged; including incorrect staging after initial surgery, progression of disease during the time interval, or secondary to direct seeding of morcellated tumor fragments. Five of the 15 leiomyosarcoma patients were deceased at the time of the publication. The authors also point out that their study is limited by the fact that it is retrospective, and access to information regarding care received from non-affiliated institutions is limited (Gynecol. Oncol. 2014;132:360-5).
In summary, morcellation of an unsuspected uterine sarcoma, whether using an electrically powered morcellator at the time of laparoscopy or cold knife at time of vaginal surgery, appears to have a negative impact; however, the studies to date are merely retrospective case studies. By no means do they provide the evidence required to place a moratorium on morcellation.
Further, if such a ban is imposed, would it then not be equally justifiable to pose similar regulations on use of oral contraceptives for symptom relief, endometrial ablation when fibroids are involved, or for that matter, uterine artery embolization? All these potential treatment regimens delay diagnosis and treatment and leave the potential uterine sarcoma in situ.
In the end, while the disease-free survival as well as overall survival appears to be hindered by dissemination of leiomyosarcoma at time of both electronic and cold-knife morcellation, the diagnosis is fortunately rare. A moratorium on the technique, however, would increase the number of concomitant laparotomies that would be required, and along with it, the increased inherent risk as well as prolonged recovery. At the present time, without better diagnostic tools or safer morcellation techniques, it is imperative to have an open dialogue of the risks and benefits of morcellation and minimally invasive surgery with patients presenting with anticipated fibroids. Additionally, our industry partners must be empowered to create safer morcellation techniques. This would appear to be morcellation within a bag.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. Dr. Miller said he is a consultant for Ethicon, which manufactures a morcellator.
*Correction, 3/19/2014: An earlier version of this story misstated the type of surgical route.
Intra-abdominal (intracorporeal) morcellation, especially electronically powered morcellation, has recently come under scrutiny. Generally performed at the time of conventional laparoscopic or robotic supracervical hysterectomy, total hysterectomy for the large uterus, or myomectomy, both power and cold-knife morcellation may splatter tissue fragments in the pelvis and abdomen, leading to potential parasitizing of the tissue and ectopic growth. Recent evidence indicates inadvertent morcellation of a leiomyosarcoma may negatively affect the patient’s subsequent disease-free survival and overall survival.
Concerns about morcellation heightened after Dr. Amy J. Reed, an anesthesiologist at Beth Israel Deaconess Medical Center, Boston, and a mother of 6, underwent presumed fibroid surgery and was diagnosed, post morcellation, with leiomyosarcoma. Dr. Reed’s husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon at Brigham and Women’s Hospital, Boston, where his wife’s surgery was performed, is calling for a moratorium on intra-abdominal morcellation, whether it involves the use of a power morcellator, or for that matter, the cold knife.
It is imperative and incumbent upon our specialty to have a detailed evaluation of the risks and benefits of morcellation. While morcellation of the rare leiomyosarcoma is a risk, banning intraabdominal/intrapelvic morcellation will certainly have a profound negative impact on patients who are able to undergo a minimally invasive gynecologic procedure. Banning morcellation would increase intraoperative risk and subsequent concern of postoperative pelvic adhesions and thus, potential impact on fertility (post myomectomy), dyspareunia, and pelvic pain. Further, a ban would incur higher costs and more loss of patient productivity (Hum. Reprod. 1998 13:2102-6). These concerns were the basis for the AAGL position statement touting a minimally invasive approach to hysterectomy (J. Minim. Invasive Gynecol. 2011;18:1-3).
Since their introduction in the mid-1990s, electronically powered morcellators have been used to remove the uterus, fibroid(s), spleen, or kidney. Varying in size from 12-20 mm, electronic morcellators generally consist of a rotating circular blade at the end of a hollow tube. A tenaculum or multitoothed grasper is placed through the tube and blade to grasp the tissue to the revolving blade. The specimen is then removed in strips. Tissue splatter is inevitable, at least until the technique evolves to allow morcellation to be performed within the confines of a bag.
Benign uterine fibroids are the most common pelvic tumor in women. Literature reviews indicate the lifetime risk is 70% for white women and 80% in women of African ancestry. Uterine sarcomas occur in 3-7 women per 100,000 (Am. J. Obstet. Gynecol. 2011;205:492.e1-5). Further, Dr. Kimberly A. Kho of the University of Texas Southwestern Medical Center, Dallas, and Dr. Ceana H. Dr. Nezhat of Atlanta Center for Minimally Invasive Surgery and Reproductive Medicine, conducted a meta-analysis of 5,666 uterine procedures, and found 13 unsuspected uterine sarcomas, for a prevalence of 0.23% (JAMA 2014 [doi:10.1001/jama.2014.1093]).
This finding is consistent with that of a previous study by Dr. W.H. Parker who also noted a 0.23% risk, based on data from 1,332 women undergoing surgery secondary to uterine fibroids. Interestingly, in Dr. Parker’s study, the risk was 0.27% among women with rapidly growing leiomyoma, often thought to be a risk factor for sarcoma development (Obstet. Gynecol. 1994;83:414-8).
Because of the difficulty of making a preoperative diagnosis of leiomyosarcoma, it is doubtful that this risk will be decreased in the near future. Risk factors have not been well established, although a twofold higher incidence of leiomyosarcomas has been observed in black women (Gynecol. Oncol. 2004;93:204-8). Increasing age would appear to increase uterine sarcoma risk, as the majority of cases are diagnosed in postmenopausal women. Tamoxifen, when used for 5 or more years, appears to be associated with higher sarcoma rates (J. Clin. Oncol. 2002;20:2758-60) as is a history of pelvic irradiation or childhood retinoblastoma.
Unless metastatic disease is present, symptoms are similar for leiomyomas and leiomyosarcomas. A rapidly growing mass, a finding associated with an increased risk of uterine sarcoma, was not seen in Parker’s study of 1,332 women undergoing hysterectomy or myomectomy for uterine leiomyoma. Similarly, size does not count; a large uterine mass or increased uterine size did not appear to be associated with a greater risk of sarcoma (Gynecol. Oncol. 2003;89:460-9).
Some contend that failed response with such therapies as gonadotropin-releasing hormone agonists and uterine artery embolization are associated with increased incidence of leiomyosarcoma, but the data are not convincing (Eur. J. Obstet. Gynecol. Reprod. Biol. 1998;76:237-40).
Physical examination and imaging may be helpful in finding enlarged lymph nodes, but imaging methods have not been reliably shown to enable a preoperative diagnosis of uterine leiomyosarcoma (Lancet Oncol. 2009;10:1188-98; AJR Am. J. Roentgenol. 2003;181:1369-74). Further, while some physicians point out that an ill-defined margin may increase leiomyosarcoma risk, this finding is certainly noted as well with benign adenomyomas.
Finally, data are scant in support of preoperative endometrial sampling to establish a diagnosis of leiomyosarcoma. In two studies comparing a total of 14 patients, 7 were correctly diagnosed with leiomyosarcoma prior to surgery (Am. J. Obstet. Gynecol. 1990;162:968-74; Gynecol. Oncol. 2008;110:43-8).
With little differentiation in clinical presentation and the inability to distinguish leiomyoma from leiomyosarcoma based on imaging or sampling, it is not surprising that patients undergoing morcellation for an expected benign condition would subsequently be diagnosed with uterine leiomyosarcoma. With this in mind, it is important to review the current body of literature to further evaluate the risks and benefits of morcellation, and what place minimally invasive gynecologic surgery will have for the treatment of uterine masses.
Tumor morcellation of unrecognized leiomyosarcomas was significantly associated with poorer disease free survival (odds ratio, 2.59, P = 1.43), higher stage (I vs. II; [OR, 19.12, P = .037]) and poorer overall survival (OR, 3.07, P =.040) in a 2011 study. Park et al. assessed 56 consecutive patients, 25 with morcellation and 31 without tumor morcellation, who had stage I and stage II uterine leiomyosarcomas and were treated between 1989 and 2010. The percentage of patients with dissemination also was noted to be greater in patients with tumor morcellation (44% vs. 12.9%, P =.032). Interestingly, ovarian tissue was more frequently preserved in the morcellation group (38.7% vs. 72%, P =.013) (Gynecol. Oncol. 2011;122:255-9)
In response to a subsequent Letter to the Editor about these risks, the study’s author put the findings in perspective. "The frequency of incidental uterine leiomyosarcoma in patients who undergo surgery for presumed uterine leiomyoma is extremely rare. At our medical center, only 49 of 22,825 patients (0.21%) who underwent surgery for presumed uterine leiomyoma had incidental uterine leiomyosarcoma. Therefore, we believe that surgeons need not avoid non-laparotomic* surgical routes because of the rare possibility of an incidental diagnosis of leiomyosarcoma, even when tumor morcellation is required" (Gynecol. Oncol. 2012;124:172-3).
Additionally, a retrospective study from Brigham & Women’s Hospital found that disease was often already disseminated before morcellation procedures. In 21 patients with a median age of 46 years and no documented evidence of extrauterine disease, 15 had uterine leiomyosarcomas and 6 had smooth muscle tumors of uncertain malignant potential that were inadvertently morcellated; data was incorporated from January 2005 to January 2012. While most patients underwent power morcellation with laparoscopy, two underwent laparoscopically assisted vaginal hysterectomy with hand morcellation, and one patient had a vaginal hysterectomy with hand morcellation.
Immediate surgical reexploration was performed for staging in 12 patients. Significant findings of disseminated intraperitoneal disease were detected in two of seven patients with presumed stage I uterine leiomyosarcoma and in one of four patients with presumed stage I smooth muscle tumors of uncertain malignant potential. Moreover, of the eight patients who did not have disseminated disease at the time of the staging procedure, one subsequently had a recurrence. The remaining patients had no recurrences and remain disease free.
One patient was already FIGO stage IV at the original surgery, two more patients were upstaged at the original surgery and underwent re-exploration at 18 and 20 months respectively (certainly, a long period prior to second look). Moreover, the authors note various reasons why a significant number of patients were upstaged; including incorrect staging after initial surgery, progression of disease during the time interval, or secondary to direct seeding of morcellated tumor fragments. Five of the 15 leiomyosarcoma patients were deceased at the time of the publication. The authors also point out that their study is limited by the fact that it is retrospective, and access to information regarding care received from non-affiliated institutions is limited (Gynecol. Oncol. 2014;132:360-5).
In summary, morcellation of an unsuspected uterine sarcoma, whether using an electrically powered morcellator at the time of laparoscopy or cold knife at time of vaginal surgery, appears to have a negative impact; however, the studies to date are merely retrospective case studies. By no means do they provide the evidence required to place a moratorium on morcellation.
Further, if such a ban is imposed, would it then not be equally justifiable to pose similar regulations on use of oral contraceptives for symptom relief, endometrial ablation when fibroids are involved, or for that matter, uterine artery embolization? All these potential treatment regimens delay diagnosis and treatment and leave the potential uterine sarcoma in situ.
In the end, while the disease-free survival as well as overall survival appears to be hindered by dissemination of leiomyosarcoma at time of both electronic and cold-knife morcellation, the diagnosis is fortunately rare. A moratorium on the technique, however, would increase the number of concomitant laparotomies that would be required, and along with it, the increased inherent risk as well as prolonged recovery. At the present time, without better diagnostic tools or safer morcellation techniques, it is imperative to have an open dialogue of the risks and benefits of morcellation and minimally invasive surgery with patients presenting with anticipated fibroids. Additionally, our industry partners must be empowered to create safer morcellation techniques. This would appear to be morcellation within a bag.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. Dr. Miller said he is a consultant for Ethicon, which manufactures a morcellator.
*Correction, 3/19/2014: An earlier version of this story misstated the type of surgical route.
Intra-abdominal (intracorporeal) morcellation, especially electronically powered morcellation, has recently come under scrutiny. Generally performed at the time of conventional laparoscopic or robotic supracervical hysterectomy, total hysterectomy for the large uterus, or myomectomy, both power and cold-knife morcellation may splatter tissue fragments in the pelvis and abdomen, leading to potential parasitizing of the tissue and ectopic growth. Recent evidence indicates inadvertent morcellation of a leiomyosarcoma may negatively affect the patient’s subsequent disease-free survival and overall survival.
Concerns about morcellation heightened after Dr. Amy J. Reed, an anesthesiologist at Beth Israel Deaconess Medical Center, Boston, and a mother of 6, underwent presumed fibroid surgery and was diagnosed, post morcellation, with leiomyosarcoma. Dr. Reed’s husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon at Brigham and Women’s Hospital, Boston, where his wife’s surgery was performed, is calling for a moratorium on intra-abdominal morcellation, whether it involves the use of a power morcellator, or for that matter, the cold knife.
It is imperative and incumbent upon our specialty to have a detailed evaluation of the risks and benefits of morcellation. While morcellation of the rare leiomyosarcoma is a risk, banning intraabdominal/intrapelvic morcellation will certainly have a profound negative impact on patients who are able to undergo a minimally invasive gynecologic procedure. Banning morcellation would increase intraoperative risk and subsequent concern of postoperative pelvic adhesions and thus, potential impact on fertility (post myomectomy), dyspareunia, and pelvic pain. Further, a ban would incur higher costs and more loss of patient productivity (Hum. Reprod. 1998 13:2102-6). These concerns were the basis for the AAGL position statement touting a minimally invasive approach to hysterectomy (J. Minim. Invasive Gynecol. 2011;18:1-3).
Since their introduction in the mid-1990s, electronically powered morcellators have been used to remove the uterus, fibroid(s), spleen, or kidney. Varying in size from 12-20 mm, electronic morcellators generally consist of a rotating circular blade at the end of a hollow tube. A tenaculum or multitoothed grasper is placed through the tube and blade to grasp the tissue to the revolving blade. The specimen is then removed in strips. Tissue splatter is inevitable, at least until the technique evolves to allow morcellation to be performed within the confines of a bag.
Benign uterine fibroids are the most common pelvic tumor in women. Literature reviews indicate the lifetime risk is 70% for white women and 80% in women of African ancestry. Uterine sarcomas occur in 3-7 women per 100,000 (Am. J. Obstet. Gynecol. 2011;205:492.e1-5). Further, Dr. Kimberly A. Kho of the University of Texas Southwestern Medical Center, Dallas, and Dr. Ceana H. Dr. Nezhat of Atlanta Center for Minimally Invasive Surgery and Reproductive Medicine, conducted a meta-analysis of 5,666 uterine procedures, and found 13 unsuspected uterine sarcomas, for a prevalence of 0.23% (JAMA 2014 [doi:10.1001/jama.2014.1093]).
This finding is consistent with that of a previous study by Dr. W.H. Parker who also noted a 0.23% risk, based on data from 1,332 women undergoing surgery secondary to uterine fibroids. Interestingly, in Dr. Parker’s study, the risk was 0.27% among women with rapidly growing leiomyoma, often thought to be a risk factor for sarcoma development (Obstet. Gynecol. 1994;83:414-8).
Because of the difficulty of making a preoperative diagnosis of leiomyosarcoma, it is doubtful that this risk will be decreased in the near future. Risk factors have not been well established, although a twofold higher incidence of leiomyosarcomas has been observed in black women (Gynecol. Oncol. 2004;93:204-8). Increasing age would appear to increase uterine sarcoma risk, as the majority of cases are diagnosed in postmenopausal women. Tamoxifen, when used for 5 or more years, appears to be associated with higher sarcoma rates (J. Clin. Oncol. 2002;20:2758-60) as is a history of pelvic irradiation or childhood retinoblastoma.
Unless metastatic disease is present, symptoms are similar for leiomyomas and leiomyosarcomas. A rapidly growing mass, a finding associated with an increased risk of uterine sarcoma, was not seen in Parker’s study of 1,332 women undergoing hysterectomy or myomectomy for uterine leiomyoma. Similarly, size does not count; a large uterine mass or increased uterine size did not appear to be associated with a greater risk of sarcoma (Gynecol. Oncol. 2003;89:460-9).
Some contend that failed response with such therapies as gonadotropin-releasing hormone agonists and uterine artery embolization are associated with increased incidence of leiomyosarcoma, but the data are not convincing (Eur. J. Obstet. Gynecol. Reprod. Biol. 1998;76:237-40).
Physical examination and imaging may be helpful in finding enlarged lymph nodes, but imaging methods have not been reliably shown to enable a preoperative diagnosis of uterine leiomyosarcoma (Lancet Oncol. 2009;10:1188-98; AJR Am. J. Roentgenol. 2003;181:1369-74). Further, while some physicians point out that an ill-defined margin may increase leiomyosarcoma risk, this finding is certainly noted as well with benign adenomyomas.
Finally, data are scant in support of preoperative endometrial sampling to establish a diagnosis of leiomyosarcoma. In two studies comparing a total of 14 patients, 7 were correctly diagnosed with leiomyosarcoma prior to surgery (Am. J. Obstet. Gynecol. 1990;162:968-74; Gynecol. Oncol. 2008;110:43-8).
With little differentiation in clinical presentation and the inability to distinguish leiomyoma from leiomyosarcoma based on imaging or sampling, it is not surprising that patients undergoing morcellation for an expected benign condition would subsequently be diagnosed with uterine leiomyosarcoma. With this in mind, it is important to review the current body of literature to further evaluate the risks and benefits of morcellation, and what place minimally invasive gynecologic surgery will have for the treatment of uterine masses.
Tumor morcellation of unrecognized leiomyosarcomas was significantly associated with poorer disease free survival (odds ratio, 2.59, P = 1.43), higher stage (I vs. II; [OR, 19.12, P = .037]) and poorer overall survival (OR, 3.07, P =.040) in a 2011 study. Park et al. assessed 56 consecutive patients, 25 with morcellation and 31 without tumor morcellation, who had stage I and stage II uterine leiomyosarcomas and were treated between 1989 and 2010. The percentage of patients with dissemination also was noted to be greater in patients with tumor morcellation (44% vs. 12.9%, P =.032). Interestingly, ovarian tissue was more frequently preserved in the morcellation group (38.7% vs. 72%, P =.013) (Gynecol. Oncol. 2011;122:255-9)
In response to a subsequent Letter to the Editor about these risks, the study’s author put the findings in perspective. "The frequency of incidental uterine leiomyosarcoma in patients who undergo surgery for presumed uterine leiomyoma is extremely rare. At our medical center, only 49 of 22,825 patients (0.21%) who underwent surgery for presumed uterine leiomyoma had incidental uterine leiomyosarcoma. Therefore, we believe that surgeons need not avoid non-laparotomic* surgical routes because of the rare possibility of an incidental diagnosis of leiomyosarcoma, even when tumor morcellation is required" (Gynecol. Oncol. 2012;124:172-3).
Additionally, a retrospective study from Brigham & Women’s Hospital found that disease was often already disseminated before morcellation procedures. In 21 patients with a median age of 46 years and no documented evidence of extrauterine disease, 15 had uterine leiomyosarcomas and 6 had smooth muscle tumors of uncertain malignant potential that were inadvertently morcellated; data was incorporated from January 2005 to January 2012. While most patients underwent power morcellation with laparoscopy, two underwent laparoscopically assisted vaginal hysterectomy with hand morcellation, and one patient had a vaginal hysterectomy with hand morcellation.
Immediate surgical reexploration was performed for staging in 12 patients. Significant findings of disseminated intraperitoneal disease were detected in two of seven patients with presumed stage I uterine leiomyosarcoma and in one of four patients with presumed stage I smooth muscle tumors of uncertain malignant potential. Moreover, of the eight patients who did not have disseminated disease at the time of the staging procedure, one subsequently had a recurrence. The remaining patients had no recurrences and remain disease free.
One patient was already FIGO stage IV at the original surgery, two more patients were upstaged at the original surgery and underwent re-exploration at 18 and 20 months respectively (certainly, a long period prior to second look). Moreover, the authors note various reasons why a significant number of patients were upstaged; including incorrect staging after initial surgery, progression of disease during the time interval, or secondary to direct seeding of morcellated tumor fragments. Five of the 15 leiomyosarcoma patients were deceased at the time of the publication. The authors also point out that their study is limited by the fact that it is retrospective, and access to information regarding care received from non-affiliated institutions is limited (Gynecol. Oncol. 2014;132:360-5).
In summary, morcellation of an unsuspected uterine sarcoma, whether using an electrically powered morcellator at the time of laparoscopy or cold knife at time of vaginal surgery, appears to have a negative impact; however, the studies to date are merely retrospective case studies. By no means do they provide the evidence required to place a moratorium on morcellation.
Further, if such a ban is imposed, would it then not be equally justifiable to pose similar regulations on use of oral contraceptives for symptom relief, endometrial ablation when fibroids are involved, or for that matter, uterine artery embolization? All these potential treatment regimens delay diagnosis and treatment and leave the potential uterine sarcoma in situ.
In the end, while the disease-free survival as well as overall survival appears to be hindered by dissemination of leiomyosarcoma at time of both electronic and cold-knife morcellation, the diagnosis is fortunately rare. A moratorium on the technique, however, would increase the number of concomitant laparotomies that would be required, and along with it, the increased inherent risk as well as prolonged recovery. At the present time, without better diagnostic tools or safer morcellation techniques, it is imperative to have an open dialogue of the risks and benefits of morcellation and minimally invasive surgery with patients presenting with anticipated fibroids. Additionally, our industry partners must be empowered to create safer morcellation techniques. This would appear to be morcellation within a bag.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy, and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. Dr. Miller said he is a consultant for Ethicon, which manufactures a morcellator.
*Correction, 3/19/2014: An earlier version of this story misstated the type of surgical route.
Whole-genome sequencing not ready for prime time
Clinicians and the public have held great hope for years that genomic analysis would transform the prevention and treatment of disease, but although costs may be plummeting, challenges persist, investigators reported March 12th in JAMA.
Sequencing the genome of individuals has become much less expensive, but translating those results into clinically useful information is labor intensive, and fraught with inconsistencies and uncertainties, reported Dr. Frederick E. Dewey of the Stanford (Calif.) Center for Inherited Cardiovascular Disease and his associates (JAMA 2014;311:1035-44).
Three academic primary care physicians and two academic medical geneticists separately reviewed whole-genome sequencing (WGS) results from 12 adult participants, and each proposed clinical follow-up based on the findings. The DNA from whole-blood samples obtained from five men and seven women without any known inherited disease risk was sequenced by commonly used platforms (all 12 at Illumina, with confirmatory sequencing performed for 9 of the participants by Complete Genomics). Two of the primary care physicians had no previous experience with genomic medicine or genetics training, the investigators reported.
Up to one-fifth of gene variants known to be associated with disease risk were not covered at a minimum threshold for discovery, meaning there could be significant gaps in predicting individual risk. For those genetic variants that met the threshold, concordance between the two sequencing platforms was high for common variants (99%), but was substantially lower for genetic variants that were candidates for inherited disease risk (median, 33%; range, 10%-75%), Dr. Dewey and his associates said.
For each participant, the WGS identified between 90 and 127 novel or rare genetic variations, and between 12 and 20 commonly reported variations, all candidates for curation with respect to personal risk and carrier status for inherited disease. The geneticists’ review of the evidence for pathogenicity for each variant required a median time of 54 minutes (range, 5-223 minutes). Based on this finding, investigators estimated the median cost for sequencing and variant interpretation for each participant was $14,815.
Review of the medical genomics report by the three primary care physicians prompted consideration of a median one to three initial follow-up referrals and diagnostic tests per participant. The agreement between physicians about suitability for follow-up of findings was fair, the investigators reported.
In the end, 1 of the 12 participants went on to take action as a result of the findings. A BRCA1 mutation was found in a woman with no family history of breast or ovarian cancer, and she subsequently underwent prophylactic bilateral salpingo-oophorectomy.
Overall, the results illustrate several challenges to clinical adoption of whole-genome sequencing, the authors suggested. Although the analytical validity of WGS is improving, technical challenges to sensitive and accurate assessment of individual genetic variation remain. The finding of only one-third of insertion/deletion genetic variants in inherited disease genes confirmed by the second sequencing platform suggests that variants likely to be pathogenic are more often inconsistently identified, Dr. Dewey and his associates said.
"In this exploratory study of 12 volunteer adults, the use of WGS was associated with incomplete coverage of inherited disease genes, low reproducibility of genetic variation with the highest potential clinical effects, and uncertainty about clinically reportable findings," they wrote.
The research was supported by grants from the National Institutes of Health, the Breetwor Family Foundation, and the LeDucq Foundation. Dr. Dewey reported being a stockholder and member of the scientific advisory board of Personalis, a privately held genome interpretation company, and receiving royalties for patented technology related to genome sequencing. Some of his associates were scientific advisers for companies involved in genomics or received royalties for patented technologies related to genome sequencing.
lnikolaides@frontlinemedcom.com
On Twitter @nikolaideslaura
Clinicians and the public have held great hope for years that genomic analysis would transform the prevention and treatment of disease, but although costs may be plummeting, challenges persist, investigators reported March 12th in JAMA.
Sequencing the genome of individuals has become much less expensive, but translating those results into clinically useful information is labor intensive, and fraught with inconsistencies and uncertainties, reported Dr. Frederick E. Dewey of the Stanford (Calif.) Center for Inherited Cardiovascular Disease and his associates (JAMA 2014;311:1035-44).
Three academic primary care physicians and two academic medical geneticists separately reviewed whole-genome sequencing (WGS) results from 12 adult participants, and each proposed clinical follow-up based on the findings. The DNA from whole-blood samples obtained from five men and seven women without any known inherited disease risk was sequenced by commonly used platforms (all 12 at Illumina, with confirmatory sequencing performed for 9 of the participants by Complete Genomics). Two of the primary care physicians had no previous experience with genomic medicine or genetics training, the investigators reported.
Up to one-fifth of gene variants known to be associated with disease risk were not covered at a minimum threshold for discovery, meaning there could be significant gaps in predicting individual risk. For those genetic variants that met the threshold, concordance between the two sequencing platforms was high for common variants (99%), but was substantially lower for genetic variants that were candidates for inherited disease risk (median, 33%; range, 10%-75%), Dr. Dewey and his associates said.
For each participant, the WGS identified between 90 and 127 novel or rare genetic variations, and between 12 and 20 commonly reported variations, all candidates for curation with respect to personal risk and carrier status for inherited disease. The geneticists’ review of the evidence for pathogenicity for each variant required a median time of 54 minutes (range, 5-223 minutes). Based on this finding, investigators estimated the median cost for sequencing and variant interpretation for each participant was $14,815.
Review of the medical genomics report by the three primary care physicians prompted consideration of a median one to three initial follow-up referrals and diagnostic tests per participant. The agreement between physicians about suitability for follow-up of findings was fair, the investigators reported.
In the end, 1 of the 12 participants went on to take action as a result of the findings. A BRCA1 mutation was found in a woman with no family history of breast or ovarian cancer, and she subsequently underwent prophylactic bilateral salpingo-oophorectomy.
Overall, the results illustrate several challenges to clinical adoption of whole-genome sequencing, the authors suggested. Although the analytical validity of WGS is improving, technical challenges to sensitive and accurate assessment of individual genetic variation remain. The finding of only one-third of insertion/deletion genetic variants in inherited disease genes confirmed by the second sequencing platform suggests that variants likely to be pathogenic are more often inconsistently identified, Dr. Dewey and his associates said.
"In this exploratory study of 12 volunteer adults, the use of WGS was associated with incomplete coverage of inherited disease genes, low reproducibility of genetic variation with the highest potential clinical effects, and uncertainty about clinically reportable findings," they wrote.
The research was supported by grants from the National Institutes of Health, the Breetwor Family Foundation, and the LeDucq Foundation. Dr. Dewey reported being a stockholder and member of the scientific advisory board of Personalis, a privately held genome interpretation company, and receiving royalties for patented technology related to genome sequencing. Some of his associates were scientific advisers for companies involved in genomics or received royalties for patented technologies related to genome sequencing.
lnikolaides@frontlinemedcom.com
On Twitter @nikolaideslaura
Clinicians and the public have held great hope for years that genomic analysis would transform the prevention and treatment of disease, but although costs may be plummeting, challenges persist, investigators reported March 12th in JAMA.
Sequencing the genome of individuals has become much less expensive, but translating those results into clinically useful information is labor intensive, and fraught with inconsistencies and uncertainties, reported Dr. Frederick E. Dewey of the Stanford (Calif.) Center for Inherited Cardiovascular Disease and his associates (JAMA 2014;311:1035-44).
Three academic primary care physicians and two academic medical geneticists separately reviewed whole-genome sequencing (WGS) results from 12 adult participants, and each proposed clinical follow-up based on the findings. The DNA from whole-blood samples obtained from five men and seven women without any known inherited disease risk was sequenced by commonly used platforms (all 12 at Illumina, with confirmatory sequencing performed for 9 of the participants by Complete Genomics). Two of the primary care physicians had no previous experience with genomic medicine or genetics training, the investigators reported.
Up to one-fifth of gene variants known to be associated with disease risk were not covered at a minimum threshold for discovery, meaning there could be significant gaps in predicting individual risk. For those genetic variants that met the threshold, concordance between the two sequencing platforms was high for common variants (99%), but was substantially lower for genetic variants that were candidates for inherited disease risk (median, 33%; range, 10%-75%), Dr. Dewey and his associates said.
For each participant, the WGS identified between 90 and 127 novel or rare genetic variations, and between 12 and 20 commonly reported variations, all candidates for curation with respect to personal risk and carrier status for inherited disease. The geneticists’ review of the evidence for pathogenicity for each variant required a median time of 54 minutes (range, 5-223 minutes). Based on this finding, investigators estimated the median cost for sequencing and variant interpretation for each participant was $14,815.
Review of the medical genomics report by the three primary care physicians prompted consideration of a median one to three initial follow-up referrals and diagnostic tests per participant. The agreement between physicians about suitability for follow-up of findings was fair, the investigators reported.
In the end, 1 of the 12 participants went on to take action as a result of the findings. A BRCA1 mutation was found in a woman with no family history of breast or ovarian cancer, and she subsequently underwent prophylactic bilateral salpingo-oophorectomy.
Overall, the results illustrate several challenges to clinical adoption of whole-genome sequencing, the authors suggested. Although the analytical validity of WGS is improving, technical challenges to sensitive and accurate assessment of individual genetic variation remain. The finding of only one-third of insertion/deletion genetic variants in inherited disease genes confirmed by the second sequencing platform suggests that variants likely to be pathogenic are more often inconsistently identified, Dr. Dewey and his associates said.
"In this exploratory study of 12 volunteer adults, the use of WGS was associated with incomplete coverage of inherited disease genes, low reproducibility of genetic variation with the highest potential clinical effects, and uncertainty about clinically reportable findings," they wrote.
The research was supported by grants from the National Institutes of Health, the Breetwor Family Foundation, and the LeDucq Foundation. Dr. Dewey reported being a stockholder and member of the scientific advisory board of Personalis, a privately held genome interpretation company, and receiving royalties for patented technology related to genome sequencing. Some of his associates were scientific advisers for companies involved in genomics or received royalties for patented technologies related to genome sequencing.
lnikolaides@frontlinemedcom.com
On Twitter @nikolaideslaura
FROM JAMA
Major finding: For each participant, whole-genome sequencing identified between 90 and 127 novel or rare genetic variations, and between 12 and 20 commonly reported variations.
Data source: Whole-genome sequencing was performed from whole-blood samples of 12 adult volunteers, and reports were analyzed by geneticists and primary care physicians for follow-up.
Disclosures: The research was supported by grants from the National Institutes of Health, the Breetwor Family Foundation, and the LeDucq Foundation. Dr. Dewey reported being a stockholder and member of the scientific advisory board of Personalis, a privately held genome interpretation company, and receiving royalties for patented technology related to genome sequencing. Some of his associates were scientific advisers for companies involved in genomics or received royalties for patented technologies related to genome sequencing.
U.S. tracking of HPV in cervical precancers confirms half due to 16/18
U.S. surveillance data show that half of high-grade cervical lesions are caused by human papillomavirus 16 and 18.
Another 25% are attributable to the five additional HPV types 31/33/45/52/58 included in the investigational 9-valent vaccine.
The analysis fills in some knowledge gaps regarding U.S. women and was based on 5,189 specimens drawn from women, aged 21-39 years, diagnosed with cervical intraepithelial neoplasia (CIN) 2 and 3 and adenocarcinoma in situ (AIS) (CIN2+) from 2008 through 2011 at five U.S. sites in the HPV-IMPACT sentinel surveillance project.
HPV 16 was the most commonly detected type among all lesions, while type 18 was relatively uncommon, accounting for only about 5% of lesions, Dr. Susan Hariri said at a meeting of the Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices (ACIP).
HPV 16 and 18 increased with lesion severity from 40% in CIN2 to 66% in CIN3/AIS.
Type 31 was the most common of the five additional HPV types in the 9-valent vaccine, followed by types 52 and 58, both of which are more frequent in CIN2 than CIN3. Types 33 and 45 were detected in less than 5% of specimens across all histologic types.
The most common other high-risk, oncogenic types not found in any vaccine were 35 and 51, identified in 7% and 9% of CIN2 lesions, said Dr. Hariri of the CDC’s National Center for HIV, Viral Hepatitis, STD, and TB Prevention.
American Nurses Association representative Carol Hayes, M.P.H., a certified nurse-midwife, questioned this finding and asked why types 33 and 45 were included in the 9-valent vaccine, but 35 and 51 were not.
A representative from Merck responded that the five additional types in the 9-valent vaccine were selected based on their attribution to cervical cancer lesions, not precancers.
Proportional type attribution data analyses by race and age revealed HPV 16 and 18 were most common across all age and racial/ethnic groups, although some differences were identified. Types 16 and 18 declined from 50% for all groups to about 44% among the oldest age group (55-99 years), where the five additional HPV vaccine types were on the rise.
Types 31/33/45/52/58 were more common in racial and ethnic groups (28%-32%) than in whites (22%), while other high-risk HPV types were more common in blacks (20%) than other racial groups (14%-16%).
"The reasons for these differences are not clear and are probably multifactorial, but may be due to differences in the underlying prevalence of HPV in the subpopulations or to differences in screening and treatment," Dr. Hariri said.
Dr. Alain Luxembourg of Merck, the vaccine developer, said the 9-valent vaccine has the potential not only to prevent cancer, but to prevent a lot of "precancerous lesions, which in countries with cervical cancer screening programs, means a lower need for invasive procedures."
He presented a summary of 9-valent clinical data including the pivotal efficacy trial among females, aged 16-26 years, which showed noninferior immunogenicity for HPV 16 and 18 compared with the quadrivalent vaccine, and about 97% protection against disease related to the five additional strains. An immunobridging study also showed noninferior immunogenicity in adolescents compared with adults.
Regulatory action is expected by the Food and Drug Administration on the 9-valent vaccine within the next 3 months, he said.
ACIP is not expected to vote on whether to recommend the 9-valent vaccine until February 2015 at the earliest, following further review of clinical and health economics data and regulatory approval, said Dr. Lauri Markowitz of the CDC’s National Center for HIV, Viral Hepatitis, STD, and TB Prevention.
Considerations for the candidate vaccine include routine vaccination at age 11 or 12 years, vaccination of older females and males who were not vaccinated at the recommended age, vaccination of persons fully or partially vaccinated with quadrivalent vaccine, and the timing for consideration of males, aged 16-26 years, because it’s anticipated that the vaccine will not be licensed in this age group at the time of first licensure.
ACIP’s HPV work group also plans to review reduced-dose vaccination schedules in more detail. Some jurisdictions are already using two-dose schedules in their national or provincial immunization programs, and GlaxoSmithKline’s Cervarix received European marketing approval in December 2013 as a two-dose schedule (0, 6 months) for girls aged 9-14 years, Dr. Markowitz said.
As ACIP members met, Sanofi Pasteur announced that its quadrivalent HPV vaccine, Gardasil, was one step closer to receiving European approval for a two-dose schedule (0, 6 months) in girls and boys aged 9-13 years.
Finally, ACIP’s members unanimously affirmed efforts to update its 2007 HPV vaccine statement. The new statement, which still needs to go through editing before a final vote, consolidates and clarifies existing recommendations and updates background and clinical trial data. It also adds a variety of new sections including postlicensure safety data, areas of ongoing research, and future priorities, including the 9-valent vaccine and reduced-dose schedules, she said.
Dr. Hariri and Dr. Markowitz reported having no financial disclosures. Dr. Luxembourg is an employee of Merck.
U.S. surveillance data show that half of high-grade cervical lesions are caused by human papillomavirus 16 and 18.
Another 25% are attributable to the five additional HPV types 31/33/45/52/58 included in the investigational 9-valent vaccine.
The analysis fills in some knowledge gaps regarding U.S. women and was based on 5,189 specimens drawn from women, aged 21-39 years, diagnosed with cervical intraepithelial neoplasia (CIN) 2 and 3 and adenocarcinoma in situ (AIS) (CIN2+) from 2008 through 2011 at five U.S. sites in the HPV-IMPACT sentinel surveillance project.
HPV 16 was the most commonly detected type among all lesions, while type 18 was relatively uncommon, accounting for only about 5% of lesions, Dr. Susan Hariri said at a meeting of the Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices (ACIP).
HPV 16 and 18 increased with lesion severity from 40% in CIN2 to 66% in CIN3/AIS.
Type 31 was the most common of the five additional HPV types in the 9-valent vaccine, followed by types 52 and 58, both of which are more frequent in CIN2 than CIN3. Types 33 and 45 were detected in less than 5% of specimens across all histologic types.
The most common other high-risk, oncogenic types not found in any vaccine were 35 and 51, identified in 7% and 9% of CIN2 lesions, said Dr. Hariri of the CDC’s National Center for HIV, Viral Hepatitis, STD, and TB Prevention.
American Nurses Association representative Carol Hayes, M.P.H., a certified nurse-midwife, questioned this finding and asked why types 33 and 45 were included in the 9-valent vaccine, but 35 and 51 were not.
A representative from Merck responded that the five additional types in the 9-valent vaccine were selected based on their attribution to cervical cancer lesions, not precancers.
Proportional type attribution data analyses by race and age revealed HPV 16 and 18 were most common across all age and racial/ethnic groups, although some differences were identified. Types 16 and 18 declined from 50% for all groups to about 44% among the oldest age group (55-99 years), where the five additional HPV vaccine types were on the rise.
Types 31/33/45/52/58 were more common in racial and ethnic groups (28%-32%) than in whites (22%), while other high-risk HPV types were more common in blacks (20%) than other racial groups (14%-16%).
"The reasons for these differences are not clear and are probably multifactorial, but may be due to differences in the underlying prevalence of HPV in the subpopulations or to differences in screening and treatment," Dr. Hariri said.
Dr. Alain Luxembourg of Merck, the vaccine developer, said the 9-valent vaccine has the potential not only to prevent cancer, but to prevent a lot of "precancerous lesions, which in countries with cervical cancer screening programs, means a lower need for invasive procedures."
He presented a summary of 9-valent clinical data including the pivotal efficacy trial among females, aged 16-26 years, which showed noninferior immunogenicity for HPV 16 and 18 compared with the quadrivalent vaccine, and about 97% protection against disease related to the five additional strains. An immunobridging study also showed noninferior immunogenicity in adolescents compared with adults.
Regulatory action is expected by the Food and Drug Administration on the 9-valent vaccine within the next 3 months, he said.
ACIP is not expected to vote on whether to recommend the 9-valent vaccine until February 2015 at the earliest, following further review of clinical and health economics data and regulatory approval, said Dr. Lauri Markowitz of the CDC’s National Center for HIV, Viral Hepatitis, STD, and TB Prevention.
Considerations for the candidate vaccine include routine vaccination at age 11 or 12 years, vaccination of older females and males who were not vaccinated at the recommended age, vaccination of persons fully or partially vaccinated with quadrivalent vaccine, and the timing for consideration of males, aged 16-26 years, because it’s anticipated that the vaccine will not be licensed in this age group at the time of first licensure.
ACIP’s HPV work group also plans to review reduced-dose vaccination schedules in more detail. Some jurisdictions are already using two-dose schedules in their national or provincial immunization programs, and GlaxoSmithKline’s Cervarix received European marketing approval in December 2013 as a two-dose schedule (0, 6 months) for girls aged 9-14 years, Dr. Markowitz said.
As ACIP members met, Sanofi Pasteur announced that its quadrivalent HPV vaccine, Gardasil, was one step closer to receiving European approval for a two-dose schedule (0, 6 months) in girls and boys aged 9-13 years.
Finally, ACIP’s members unanimously affirmed efforts to update its 2007 HPV vaccine statement. The new statement, which still needs to go through editing before a final vote, consolidates and clarifies existing recommendations and updates background and clinical trial data. It also adds a variety of new sections including postlicensure safety data, areas of ongoing research, and future priorities, including the 9-valent vaccine and reduced-dose schedules, she said.
Dr. Hariri and Dr. Markowitz reported having no financial disclosures. Dr. Luxembourg is an employee of Merck.
U.S. surveillance data show that half of high-grade cervical lesions are caused by human papillomavirus 16 and 18.
Another 25% are attributable to the five additional HPV types 31/33/45/52/58 included in the investigational 9-valent vaccine.
The analysis fills in some knowledge gaps regarding U.S. women and was based on 5,189 specimens drawn from women, aged 21-39 years, diagnosed with cervical intraepithelial neoplasia (CIN) 2 and 3 and adenocarcinoma in situ (AIS) (CIN2+) from 2008 through 2011 at five U.S. sites in the HPV-IMPACT sentinel surveillance project.
HPV 16 was the most commonly detected type among all lesions, while type 18 was relatively uncommon, accounting for only about 5% of lesions, Dr. Susan Hariri said at a meeting of the Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices (ACIP).
HPV 16 and 18 increased with lesion severity from 40% in CIN2 to 66% in CIN3/AIS.
Type 31 was the most common of the five additional HPV types in the 9-valent vaccine, followed by types 52 and 58, both of which are more frequent in CIN2 than CIN3. Types 33 and 45 were detected in less than 5% of specimens across all histologic types.
The most common other high-risk, oncogenic types not found in any vaccine were 35 and 51, identified in 7% and 9% of CIN2 lesions, said Dr. Hariri of the CDC’s National Center for HIV, Viral Hepatitis, STD, and TB Prevention.
American Nurses Association representative Carol Hayes, M.P.H., a certified nurse-midwife, questioned this finding and asked why types 33 and 45 were included in the 9-valent vaccine, but 35 and 51 were not.
A representative from Merck responded that the five additional types in the 9-valent vaccine were selected based on their attribution to cervical cancer lesions, not precancers.
Proportional type attribution data analyses by race and age revealed HPV 16 and 18 were most common across all age and racial/ethnic groups, although some differences were identified. Types 16 and 18 declined from 50% for all groups to about 44% among the oldest age group (55-99 years), where the five additional HPV vaccine types were on the rise.
Types 31/33/45/52/58 were more common in racial and ethnic groups (28%-32%) than in whites (22%), while other high-risk HPV types were more common in blacks (20%) than other racial groups (14%-16%).
"The reasons for these differences are not clear and are probably multifactorial, but may be due to differences in the underlying prevalence of HPV in the subpopulations or to differences in screening and treatment," Dr. Hariri said.
Dr. Alain Luxembourg of Merck, the vaccine developer, said the 9-valent vaccine has the potential not only to prevent cancer, but to prevent a lot of "precancerous lesions, which in countries with cervical cancer screening programs, means a lower need for invasive procedures."
He presented a summary of 9-valent clinical data including the pivotal efficacy trial among females, aged 16-26 years, which showed noninferior immunogenicity for HPV 16 and 18 compared with the quadrivalent vaccine, and about 97% protection against disease related to the five additional strains. An immunobridging study also showed noninferior immunogenicity in adolescents compared with adults.
Regulatory action is expected by the Food and Drug Administration on the 9-valent vaccine within the next 3 months, he said.
ACIP is not expected to vote on whether to recommend the 9-valent vaccine until February 2015 at the earliest, following further review of clinical and health economics data and regulatory approval, said Dr. Lauri Markowitz of the CDC’s National Center for HIV, Viral Hepatitis, STD, and TB Prevention.
Considerations for the candidate vaccine include routine vaccination at age 11 or 12 years, vaccination of older females and males who were not vaccinated at the recommended age, vaccination of persons fully or partially vaccinated with quadrivalent vaccine, and the timing for consideration of males, aged 16-26 years, because it’s anticipated that the vaccine will not be licensed in this age group at the time of first licensure.
ACIP’s HPV work group also plans to review reduced-dose vaccination schedules in more detail. Some jurisdictions are already using two-dose schedules in their national or provincial immunization programs, and GlaxoSmithKline’s Cervarix received European marketing approval in December 2013 as a two-dose schedule (0, 6 months) for girls aged 9-14 years, Dr. Markowitz said.
As ACIP members met, Sanofi Pasteur announced that its quadrivalent HPV vaccine, Gardasil, was one step closer to receiving European approval for a two-dose schedule (0, 6 months) in girls and boys aged 9-13 years.
Finally, ACIP’s members unanimously affirmed efforts to update its 2007 HPV vaccine statement. The new statement, which still needs to go through editing before a final vote, consolidates and clarifies existing recommendations and updates background and clinical trial data. It also adds a variety of new sections including postlicensure safety data, areas of ongoing research, and future priorities, including the 9-valent vaccine and reduced-dose schedules, she said.
Dr. Hariri and Dr. Markowitz reported having no financial disclosures. Dr. Luxembourg is an employee of Merck.
FROM AN ACIP MEETING
Major finding: HPV 16 was the most commonly detected type among all lesions, while type 18 was relatively uncommon, accounting for only about 5% of lesions.
Data source: A retrospective analysis of 5,189 cervical specimens in the HPV-IMPACT project.
Disclosures: Dr. Hariri and Dr. Markowitz reported having no financial disclosures. Dr. Luxembourg is an employee of Merck.
Woman-to-woman HIV transmission likely in Texas case
Female-to-female transmission probably caused an HIV infection in a Texas woman, according to a report from the Centers for Disease Control and Prevention.
The 46-year-old woman was in a monogamous relationship with an HIV-positive 43-year-old woman. The patient reported no sexual contact with a man for 10 years prior to infection. She regularly sold plasma to supplement her income, and learned of her HIV status in April 2012*, when routine lab work required before every donation came back positive. The labs from her last donation 6 months earlier were clean, Shirley K. Chan and her colleagues wrote in the March 14 issue of the Morbidity and Mortality Weekly Report (2014; 63:209-12).
The patient* did not have any of the classic HIV risk factors, including intravenous drug use, tattoos, acupuncture, transfusions, or transplants. The strains in both women were nearly 98% genetically identical. Therefore, wrote Ms. Chan of the Houston Department of Health and Human Services and her coauthors, fluid exchange with the partner was the most likely source of transmission. The women reported sharing sex toys, engaging in sex during menstruation, and having sex rough enough to cause bleeding in either.
The HIV-positive partner had been diagnosed in 2008. At that time, her HIV-1 viral load was 82,000 copies/mL, and her CD4+ T-lymphocyte count was 372 cells/mm3 (25%). She began antiretroviral treatment in early 2009, but stopped in November 2010. When she was lost to follow-up in January 2011, her HIV-1 viral load had dropped to 178 copies/mL, and her CD4+ T-lymphocyte count had increased to 554 cells/mm3 (44%).
At the time of diagnosis, the patient’s HIV-1 viral load was 23,600 copies/mL. When the partner was tested at that point, her HIV-1 viral load was much lower than the last measurement (69,000 copies/mL).
Woman-to-woman HIV transmission is considered very rare, but the true rate is difficult to determine because confounding risk factors – most often intravenous drug use and sex with men – are usually present, the authors wrote in an editorial note. This finding underscores the need for preemptive counseling for all HIV patients, regardless of their sexual orientation and relationship status, the investigators noted.
"All persons at risk for HIV, including all discordant couples, should receive information regarding the prevention of HIV and sexually transmitted infections to prevent the HIV-negative partner from acquiring the infection. Furthermore, all persons identified as infected with HIV should be linked to and retained in medical care. Control of HIV infection with suppression of viral load can result in better health outcomes and a reduced chance of transmitting HIV to partners," Dr. Chan and her associates said.
As public health care workers, none of the authors had any financial disclosures.
On Twitter @alz_gal
*Correction, 3/13/2014: An earlier version of this article misstated the time of the patient's diagnosis. In addition, only that the patient did not have any of the classic HIV risk factors.
Female-to-female transmission probably caused an HIV infection in a Texas woman, according to a report from the Centers for Disease Control and Prevention.
The 46-year-old woman was in a monogamous relationship with an HIV-positive 43-year-old woman. The patient reported no sexual contact with a man for 10 years prior to infection. She regularly sold plasma to supplement her income, and learned of her HIV status in April 2012*, when routine lab work required before every donation came back positive. The labs from her last donation 6 months earlier were clean, Shirley K. Chan and her colleagues wrote in the March 14 issue of the Morbidity and Mortality Weekly Report (2014; 63:209-12).
The patient* did not have any of the classic HIV risk factors, including intravenous drug use, tattoos, acupuncture, transfusions, or transplants. The strains in both women were nearly 98% genetically identical. Therefore, wrote Ms. Chan of the Houston Department of Health and Human Services and her coauthors, fluid exchange with the partner was the most likely source of transmission. The women reported sharing sex toys, engaging in sex during menstruation, and having sex rough enough to cause bleeding in either.
The HIV-positive partner had been diagnosed in 2008. At that time, her HIV-1 viral load was 82,000 copies/mL, and her CD4+ T-lymphocyte count was 372 cells/mm3 (25%). She began antiretroviral treatment in early 2009, but stopped in November 2010. When she was lost to follow-up in January 2011, her HIV-1 viral load had dropped to 178 copies/mL, and her CD4+ T-lymphocyte count had increased to 554 cells/mm3 (44%).
At the time of diagnosis, the patient’s HIV-1 viral load was 23,600 copies/mL. When the partner was tested at that point, her HIV-1 viral load was much lower than the last measurement (69,000 copies/mL).
Woman-to-woman HIV transmission is considered very rare, but the true rate is difficult to determine because confounding risk factors – most often intravenous drug use and sex with men – are usually present, the authors wrote in an editorial note. This finding underscores the need for preemptive counseling for all HIV patients, regardless of their sexual orientation and relationship status, the investigators noted.
"All persons at risk for HIV, including all discordant couples, should receive information regarding the prevention of HIV and sexually transmitted infections to prevent the HIV-negative partner from acquiring the infection. Furthermore, all persons identified as infected with HIV should be linked to and retained in medical care. Control of HIV infection with suppression of viral load can result in better health outcomes and a reduced chance of transmitting HIV to partners," Dr. Chan and her associates said.
As public health care workers, none of the authors had any financial disclosures.
On Twitter @alz_gal
*Correction, 3/13/2014: An earlier version of this article misstated the time of the patient's diagnosis. In addition, only that the patient did not have any of the classic HIV risk factors.
Female-to-female transmission probably caused an HIV infection in a Texas woman, according to a report from the Centers for Disease Control and Prevention.
The 46-year-old woman was in a monogamous relationship with an HIV-positive 43-year-old woman. The patient reported no sexual contact with a man for 10 years prior to infection. She regularly sold plasma to supplement her income, and learned of her HIV status in April 2012*, when routine lab work required before every donation came back positive. The labs from her last donation 6 months earlier were clean, Shirley K. Chan and her colleagues wrote in the March 14 issue of the Morbidity and Mortality Weekly Report (2014; 63:209-12).
The patient* did not have any of the classic HIV risk factors, including intravenous drug use, tattoos, acupuncture, transfusions, or transplants. The strains in both women were nearly 98% genetically identical. Therefore, wrote Ms. Chan of the Houston Department of Health and Human Services and her coauthors, fluid exchange with the partner was the most likely source of transmission. The women reported sharing sex toys, engaging in sex during menstruation, and having sex rough enough to cause bleeding in either.
The HIV-positive partner had been diagnosed in 2008. At that time, her HIV-1 viral load was 82,000 copies/mL, and her CD4+ T-lymphocyte count was 372 cells/mm3 (25%). She began antiretroviral treatment in early 2009, but stopped in November 2010. When she was lost to follow-up in January 2011, her HIV-1 viral load had dropped to 178 copies/mL, and her CD4+ T-lymphocyte count had increased to 554 cells/mm3 (44%).
At the time of diagnosis, the patient’s HIV-1 viral load was 23,600 copies/mL. When the partner was tested at that point, her HIV-1 viral load was much lower than the last measurement (69,000 copies/mL).
Woman-to-woman HIV transmission is considered very rare, but the true rate is difficult to determine because confounding risk factors – most often intravenous drug use and sex with men – are usually present, the authors wrote in an editorial note. This finding underscores the need for preemptive counseling for all HIV patients, regardless of their sexual orientation and relationship status, the investigators noted.
"All persons at risk for HIV, including all discordant couples, should receive information regarding the prevention of HIV and sexually transmitted infections to prevent the HIV-negative partner from acquiring the infection. Furthermore, all persons identified as infected with HIV should be linked to and retained in medical care. Control of HIV infection with suppression of viral load can result in better health outcomes and a reduced chance of transmitting HIV to partners," Dr. Chan and her associates said.
As public health care workers, none of the authors had any financial disclosures.
On Twitter @alz_gal
*Correction, 3/13/2014: An earlier version of this article misstated the time of the patient's diagnosis. In addition, only that the patient did not have any of the classic HIV risk factors.
FROM MMWR
Major finding: The Centers for Disease Control and Prevention has identified a probable case of female-to-female HIV transmission.
Data source: A single case report involving a 46-year-old in a monogamous relationship with a female partner who had a confirmed HIV infection; neither woman had any other risk factors.
Disclosures: As public health care workers, neither Ms. Chan nor any of her coauthors had any financial disclosures.
FDA panel unanimously backs cobas HPV test as primary screening tool
COLLEGE PARK, MD. – A Food and Drug Administration advisory panel has unanimously supported expanding the approval of the cobas human papilloma virus test to include its use as a first-line primary cervical cancer screening test in women aged 25 years and older.
At a meeting on March 12, the FDA’s Microbiology Devices Advisory Committee voted 13-0 that the benefits of the cobas human papillomavirus (HPV) test, manufactured by Roche, outweighed the risks for use in women aged 25 years and older, for use as "a first-line primary cervical screening test to detect high-risk HPV, including genotyping for [HPV] 16 and 18." The proposed indication also states that women who test negative for high-risk HPV types "should be followed-up in accordance with the physician’s assessment of screening and medical history, other risk factors, and professional guidelines," and that women who test positive for HPV genotypes 16 and/or 18 "should be referred to colposcopy." Those who are positive for the other high-risk HPV types, but negative for 16/18 with this test, "should be evaluated by cervical cytology to determine the need for referral to colposcopy."
Despite some concerns about the age and the potential for confusion and off-label use of the test, panelists also voted 13-0 that there was reasonable assurance that the test was safe and effective for these proposed indications, with several panelists commenting on the task ahead for professional societies once the FDA approves the test for this use.
The cobas HPV test is an in vitro diagnostic test that detects 14 high-risk HPV types, including types 16 and 18, which are associated with about 70% of invasive cervical cancers. It was approved by the FDA in 2011 for screening women aged 21 years and older with abnormal cervical cytology results and for use adjunctively with normal cervical cytology in women aged 30 years and older to assess the presence or absence of high-risk HPV genotypes.
For approval, Roche submitted results of the ATHENA trial, a prospective study of 47,208 women in the United States, which was the basis of the 2011 approval, including 3-year follow-up data. The results show that HPV testing alone provides greater protection against CIN3 (cervical intraepithelial neoplasia grade 3) and invasive cervical cancer than does cytology alone, and that primary HPV testing provides a similar level of protection against CIN3 and invasive cervical cancer as cotesting with HPV testing and cytology, according to Roche.
Based on the proposed use, in women aged 25 years and older, the sensitivity of HPV testing for detecting CIN3 or higher was 58.26% vs. 42.63% for the comparator cytology alone, according to the FDA summary of the data. The positive predictive value for detecting CIN3 or greater among women referred to colposcopy with HPV testing was 12.25% vs. 6.47% for women referred to colposcopy with the cytology, a statistically significant difference. The risk of CIN3 or greater among women not referred to colposcopy with HPV testing was 0.42% vs. 0.59% among the women not referred to colposcopy with cytology. The false positive rate for CIN3 or greater was 4.09% among those in the HPV testing group, vs. 6.04% among those in the comparator.
Panelist Alan Waxman, professor in the department of obstetrics and gynecology, University of New Mexico, Albuquerque, commended the way the study was conducted, the results of which left the panel little choice on how to vote, and said that women will be "well served by having more choices." Clinicians will have different options for screening women, with potentially different intervals and triage trees, and the challenge will be for the professional societies "to put together data-driven, evidence-based algorithms," and then provide education for health care providers and patients, Dr. Waxman said.
"The balance of safety and effectiveness depends critically on the screening interval," added another panelist, Dr. L. Stewart Massad, professor of obstetrics and gynecology in the division of gynecologic oncology, Washington University, St. Louis. "This is a powerful test with a very strong negative predictive value, and the likelihood of disease will be substantially less than in the data presented today in women who are screened at too short an interval after an initial negative screen," which he said he hoped would be considered by professional societies in the development of guidelines.
About 1% of the women in the ATHENA study had been vaccinated against HPV, and panelists noted that as HPV vaccination increases, the effectiveness of screening would be lower because there will be less disease to detect. The oncologist on the panel, Dr. Joanna Cain, professor of obstetrics and gynecology at the University of Massachusetts, Worcester, said that patient registries could help understand how these proposals will need to modified over time as a result of HPV vaccination.
The FDA usually follows the recommendations of its advisory panels.
Advisory panel members have been cleared of potential conflicts related to the meeting topic. Occasionally, a panelist is given a waiver, but no waivers were given at this meeting.
COLLEGE PARK, MD. – A Food and Drug Administration advisory panel has unanimously supported expanding the approval of the cobas human papilloma virus test to include its use as a first-line primary cervical cancer screening test in women aged 25 years and older.
At a meeting on March 12, the FDA’s Microbiology Devices Advisory Committee voted 13-0 that the benefits of the cobas human papillomavirus (HPV) test, manufactured by Roche, outweighed the risks for use in women aged 25 years and older, for use as "a first-line primary cervical screening test to detect high-risk HPV, including genotyping for [HPV] 16 and 18." The proposed indication also states that women who test negative for high-risk HPV types "should be followed-up in accordance with the physician’s assessment of screening and medical history, other risk factors, and professional guidelines," and that women who test positive for HPV genotypes 16 and/or 18 "should be referred to colposcopy." Those who are positive for the other high-risk HPV types, but negative for 16/18 with this test, "should be evaluated by cervical cytology to determine the need for referral to colposcopy."
Despite some concerns about the age and the potential for confusion and off-label use of the test, panelists also voted 13-0 that there was reasonable assurance that the test was safe and effective for these proposed indications, with several panelists commenting on the task ahead for professional societies once the FDA approves the test for this use.
The cobas HPV test is an in vitro diagnostic test that detects 14 high-risk HPV types, including types 16 and 18, which are associated with about 70% of invasive cervical cancers. It was approved by the FDA in 2011 for screening women aged 21 years and older with abnormal cervical cytology results and for use adjunctively with normal cervical cytology in women aged 30 years and older to assess the presence or absence of high-risk HPV genotypes.
For approval, Roche submitted results of the ATHENA trial, a prospective study of 47,208 women in the United States, which was the basis of the 2011 approval, including 3-year follow-up data. The results show that HPV testing alone provides greater protection against CIN3 (cervical intraepithelial neoplasia grade 3) and invasive cervical cancer than does cytology alone, and that primary HPV testing provides a similar level of protection against CIN3 and invasive cervical cancer as cotesting with HPV testing and cytology, according to Roche.
Based on the proposed use, in women aged 25 years and older, the sensitivity of HPV testing for detecting CIN3 or higher was 58.26% vs. 42.63% for the comparator cytology alone, according to the FDA summary of the data. The positive predictive value for detecting CIN3 or greater among women referred to colposcopy with HPV testing was 12.25% vs. 6.47% for women referred to colposcopy with the cytology, a statistically significant difference. The risk of CIN3 or greater among women not referred to colposcopy with HPV testing was 0.42% vs. 0.59% among the women not referred to colposcopy with cytology. The false positive rate for CIN3 or greater was 4.09% among those in the HPV testing group, vs. 6.04% among those in the comparator.
Panelist Alan Waxman, professor in the department of obstetrics and gynecology, University of New Mexico, Albuquerque, commended the way the study was conducted, the results of which left the panel little choice on how to vote, and said that women will be "well served by having more choices." Clinicians will have different options for screening women, with potentially different intervals and triage trees, and the challenge will be for the professional societies "to put together data-driven, evidence-based algorithms," and then provide education for health care providers and patients, Dr. Waxman said.
"The balance of safety and effectiveness depends critically on the screening interval," added another panelist, Dr. L. Stewart Massad, professor of obstetrics and gynecology in the division of gynecologic oncology, Washington University, St. Louis. "This is a powerful test with a very strong negative predictive value, and the likelihood of disease will be substantially less than in the data presented today in women who are screened at too short an interval after an initial negative screen," which he said he hoped would be considered by professional societies in the development of guidelines.
About 1% of the women in the ATHENA study had been vaccinated against HPV, and panelists noted that as HPV vaccination increases, the effectiveness of screening would be lower because there will be less disease to detect. The oncologist on the panel, Dr. Joanna Cain, professor of obstetrics and gynecology at the University of Massachusetts, Worcester, said that patient registries could help understand how these proposals will need to modified over time as a result of HPV vaccination.
The FDA usually follows the recommendations of its advisory panels.
Advisory panel members have been cleared of potential conflicts related to the meeting topic. Occasionally, a panelist is given a waiver, but no waivers were given at this meeting.
COLLEGE PARK, MD. – A Food and Drug Administration advisory panel has unanimously supported expanding the approval of the cobas human papilloma virus test to include its use as a first-line primary cervical cancer screening test in women aged 25 years and older.
At a meeting on March 12, the FDA’s Microbiology Devices Advisory Committee voted 13-0 that the benefits of the cobas human papillomavirus (HPV) test, manufactured by Roche, outweighed the risks for use in women aged 25 years and older, for use as "a first-line primary cervical screening test to detect high-risk HPV, including genotyping for [HPV] 16 and 18." The proposed indication also states that women who test negative for high-risk HPV types "should be followed-up in accordance with the physician’s assessment of screening and medical history, other risk factors, and professional guidelines," and that women who test positive for HPV genotypes 16 and/or 18 "should be referred to colposcopy." Those who are positive for the other high-risk HPV types, but negative for 16/18 with this test, "should be evaluated by cervical cytology to determine the need for referral to colposcopy."
Despite some concerns about the age and the potential for confusion and off-label use of the test, panelists also voted 13-0 that there was reasonable assurance that the test was safe and effective for these proposed indications, with several panelists commenting on the task ahead for professional societies once the FDA approves the test for this use.
The cobas HPV test is an in vitro diagnostic test that detects 14 high-risk HPV types, including types 16 and 18, which are associated with about 70% of invasive cervical cancers. It was approved by the FDA in 2011 for screening women aged 21 years and older with abnormal cervical cytology results and for use adjunctively with normal cervical cytology in women aged 30 years and older to assess the presence or absence of high-risk HPV genotypes.
For approval, Roche submitted results of the ATHENA trial, a prospective study of 47,208 women in the United States, which was the basis of the 2011 approval, including 3-year follow-up data. The results show that HPV testing alone provides greater protection against CIN3 (cervical intraepithelial neoplasia grade 3) and invasive cervical cancer than does cytology alone, and that primary HPV testing provides a similar level of protection against CIN3 and invasive cervical cancer as cotesting with HPV testing and cytology, according to Roche.
Based on the proposed use, in women aged 25 years and older, the sensitivity of HPV testing for detecting CIN3 or higher was 58.26% vs. 42.63% for the comparator cytology alone, according to the FDA summary of the data. The positive predictive value for detecting CIN3 or greater among women referred to colposcopy with HPV testing was 12.25% vs. 6.47% for women referred to colposcopy with the cytology, a statistically significant difference. The risk of CIN3 or greater among women not referred to colposcopy with HPV testing was 0.42% vs. 0.59% among the women not referred to colposcopy with cytology. The false positive rate for CIN3 or greater was 4.09% among those in the HPV testing group, vs. 6.04% among those in the comparator.
Panelist Alan Waxman, professor in the department of obstetrics and gynecology, University of New Mexico, Albuquerque, commended the way the study was conducted, the results of which left the panel little choice on how to vote, and said that women will be "well served by having more choices." Clinicians will have different options for screening women, with potentially different intervals and triage trees, and the challenge will be for the professional societies "to put together data-driven, evidence-based algorithms," and then provide education for health care providers and patients, Dr. Waxman said.
"The balance of safety and effectiveness depends critically on the screening interval," added another panelist, Dr. L. Stewart Massad, professor of obstetrics and gynecology in the division of gynecologic oncology, Washington University, St. Louis. "This is a powerful test with a very strong negative predictive value, and the likelihood of disease will be substantially less than in the data presented today in women who are screened at too short an interval after an initial negative screen," which he said he hoped would be considered by professional societies in the development of guidelines.
About 1% of the women in the ATHENA study had been vaccinated against HPV, and panelists noted that as HPV vaccination increases, the effectiveness of screening would be lower because there will be less disease to detect. The oncologist on the panel, Dr. Joanna Cain, professor of obstetrics and gynecology at the University of Massachusetts, Worcester, said that patient registries could help understand how these proposals will need to modified over time as a result of HPV vaccination.
The FDA usually follows the recommendations of its advisory panels.
Advisory panel members have been cleared of potential conflicts related to the meeting topic. Occasionally, a panelist is given a waiver, but no waivers were given at this meeting.
AT AN FDA ADVISORY COMMITTEE MEETING
Ruling out malignancy in setting of an adnexal mass
An adnexal mass is a common gynecological presentation that can affect women of all ages. Typically, the mass is identified during an annual pelvic exam or incidentally when patients undergo pelvic imaging for evaluation of gastrointestinal or gynecological complaints.
The main goal of evaluating an adnexal mass in the nonacute setting is to rule out malignancy. A careful evaluation is needed to accurately distinguish benign from malignant masses, but often a definitive diagnosis only can be achieved with surgery. Hence, in the United States, women have a 5%-10% chance of undergoing surgery to evaluate a mass, but only 13%-21% of these patients are diagnosed with an ovarian cancer (Obstet. Gynecol. 2007;110:201-14).
We will review a stepwise approach for the evaluation of a newly diagnosed mass. As part of our review, we will discuss imaging findings that should prompt surgical evaluation or continued observation, as well as the correct use of the currently available serum biomarkers.
The majority of adnexal masses are benign and present most commonly in premenopausal women. However, in the outpatient setting, the evaluation approach should be aimed at ruling out malignancy regardless of age or reproductive status. The patient’s age should be considered clinically, as the suspicion for ovarian cancer should be heightened in postmenopausal women.
The evaluation should start with a detailed history because it may help in determining the etiology of the mass. Pelvic pain and pressure are very common but nonspecific symptoms in women with adnexal masses. However, if the pain is of sudden onset, urgent evaluation is warranted to rule out adnexal torsion or a ruptured hemorrhagic cyst. A history of dysmenorrhea and/or dyspareunia may suggest endometriosis and coexisting endometrioma, whereas a patient with fever and a vaginal discharge should be evaluated for a tubo-ovarian abscess.
Patients also should be asked about symptoms associated with ovarian cancer including early satiety, constipation, and bloating, as well as their duration. In addition, abnormal uterine bleeding or virilization may suggest the presence of estrogen- or testosterone-secreting tumors. Lastly, a detailed family history is important, as the presence of ovarian, breast, or colon cancer in the family would increase suspicion for hereditary ovarian cancer syndromes.
A thorough physical exam should include a speculum exam as well as bimanual and rectovaginal exams. The focus of the pelvic exam should be determining the size, mobility, and consistency of the mass, as well as other findings that may help discriminate benign versus malignant neoplasms. Malignant masses are usually solid, irregular in shape, and tend to be fixed. Nodularity in the posterior cul-de-sac also is associated with malignancy. The abdominal exam should focus on the presence or absence of ascites (fluid wave), an omental mass, or inguinal adenopathy. However, none of the findings on exam are specific for an ovarian or fallopian tube malignancy, and imaging should be obtained for further evaluation.
Ultrasound is the imaging study of choice for the evaluation of an adnexal mass because it is less expensive than and diagnostically equivalent to other imaging modalities. A pelvic ultrasound can help delineate the anatomic origin of the mass, but it also can detect characteristics of the mass that may help with the diagnosis, and the decision of whether or not to proceed to surgery. Endometriomas, mature teratomas (dermoid cysts), simple ovarian cysts, and hemorrhagic cysts have sonographic features that are highly predictive of the histology. Depending on whether or not the patient is symptomatic, the patient’s age, and comorbidities, these masses might be followed expectantly.
Ultrasound features that are suggestive of malignancy include solid components, septations greater than 2-3 mm, and vascular flow. The presence of ascites or peritoneal nodules detected at the time of ultrasound also is highly suspicious of malignancy in patients with a pelvic mass. If a pelvic ultrasound is equivocal, pelvic magnetic resonance imaging (MRI) is the second study of choice. Computed tomography (CT scan) should be used to evaluate for metastatic disease in patients with suspected ovarian carcinoma (ascites, adenopathy, peritoneal thickening or nodularity, omental thickening).
Serum biomarkers also may aid in the evaluation. The most well-studied and commonly used biomarker in the evaluation of an adnexal mass is CA-125. In general, the utility of CA-125 is limited mainly because of its low specificity, especially in premenopausal women. However, it can be used as adjunct when an ovarian malignancy is suspected based on the patient’s history, risk factors, and imaging findings. The American College of Obstetricians and Gynecologists (ACOG) and the Society of Gynecologic Oncologists (SGO) advise referral to a gynecologic oncologist for postmenopausal women with an elevated CA-125. Meanwhile, for premenopausal women, the recommendation is for referral of those with a "very elevated" CA-125. However, a specific value has not been established (Obstet. Gynecol. 2011;117:742-6).
CA-125 by itself should not be used to decide whether or not to take a patient to surgery. Nevertheless, once the decision to operate has been made, CA-125 can be used in conjunction with HE4 to calculate a Risk of Malignancy Algorithm (ROMA) score. The score is based on menopausal status, and if the calculated risk is elevated, patient referral to a gynecologic oncologist for her surgery should be strongly considered.
Similarly, the OVA1 test is currently approved by the Food and Drug Administration to assess the likelihood of malignancy in patients who are having surgery for an adnexal mass. The test is also based on menopausal status, and if elevated, a referral to a gynecologic oncologist is recommended. In young women with adnexal masses, germ cell tumor markers may be more helpful (lactate dehydrogenase [LDH], human chorionic gonadotropin [hCG], alpha-fetoprotein [AFP]), while in patients with signs or symptoms of estrogen or androgen excess, sex cord-stromal tumor markers (inhibin B, anti-Müllerian hormone [AMH], testosterone, dehydroepiandrosterone [DHEA], estradiol) would be appropriate to obtain. While no tumor marker is "diagnostic," the results may assist in the decision to perform surgery and consider referral to a gynecologic oncologist.
In summary, the workup for an adnexal mass should include a detailed medical and family history, a thorough physical exam, and imaging with pelvic ultrasound. For premenopausal women, there is a higher incidence of adnexal masses, and, in fact, most of them are benign. In these women, one must weigh the risk/benefit of close monitoring with pelvic ultrasound versus surgical intervention. A serum CA-125 can be helpful, but only if it is significantly elevated.
If uncertainty remains after a complete evaluation has been performed, it is appropriate to refer to a gynecologic oncologist. In postmenopausal women, serum biomarkers should be used in conjunction with the history, physical, and ultrasound because of the higher risk of malignancy. In addition, surgical intervention should be offered to these patients regardless of serum marker values in the setting of a complex mass. If there is high suspicion for malignancy by history and imaging or elevated ROMA or OVA1, referral to a gynecologic oncologist is prudent.
Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology, and a professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. Dr. Roque is a fellow in the gynecologic oncology program at the University of North Carolina. Neither Dr. Clarke-Pearson nor Dr. Roque has any relevant financial disclosures. E-mail them at obnews@frontlinemedcom.com.
An adnexal mass is a common gynecological presentation that can affect women of all ages. Typically, the mass is identified during an annual pelvic exam or incidentally when patients undergo pelvic imaging for evaluation of gastrointestinal or gynecological complaints.
The main goal of evaluating an adnexal mass in the nonacute setting is to rule out malignancy. A careful evaluation is needed to accurately distinguish benign from malignant masses, but often a definitive diagnosis only can be achieved with surgery. Hence, in the United States, women have a 5%-10% chance of undergoing surgery to evaluate a mass, but only 13%-21% of these patients are diagnosed with an ovarian cancer (Obstet. Gynecol. 2007;110:201-14).
We will review a stepwise approach for the evaluation of a newly diagnosed mass. As part of our review, we will discuss imaging findings that should prompt surgical evaluation or continued observation, as well as the correct use of the currently available serum biomarkers.
The majority of adnexal masses are benign and present most commonly in premenopausal women. However, in the outpatient setting, the evaluation approach should be aimed at ruling out malignancy regardless of age or reproductive status. The patient’s age should be considered clinically, as the suspicion for ovarian cancer should be heightened in postmenopausal women.
The evaluation should start with a detailed history because it may help in determining the etiology of the mass. Pelvic pain and pressure are very common but nonspecific symptoms in women with adnexal masses. However, if the pain is of sudden onset, urgent evaluation is warranted to rule out adnexal torsion or a ruptured hemorrhagic cyst. A history of dysmenorrhea and/or dyspareunia may suggest endometriosis and coexisting endometrioma, whereas a patient with fever and a vaginal discharge should be evaluated for a tubo-ovarian abscess.
Patients also should be asked about symptoms associated with ovarian cancer including early satiety, constipation, and bloating, as well as their duration. In addition, abnormal uterine bleeding or virilization may suggest the presence of estrogen- or testosterone-secreting tumors. Lastly, a detailed family history is important, as the presence of ovarian, breast, or colon cancer in the family would increase suspicion for hereditary ovarian cancer syndromes.
A thorough physical exam should include a speculum exam as well as bimanual and rectovaginal exams. The focus of the pelvic exam should be determining the size, mobility, and consistency of the mass, as well as other findings that may help discriminate benign versus malignant neoplasms. Malignant masses are usually solid, irregular in shape, and tend to be fixed. Nodularity in the posterior cul-de-sac also is associated with malignancy. The abdominal exam should focus on the presence or absence of ascites (fluid wave), an omental mass, or inguinal adenopathy. However, none of the findings on exam are specific for an ovarian or fallopian tube malignancy, and imaging should be obtained for further evaluation.
Ultrasound is the imaging study of choice for the evaluation of an adnexal mass because it is less expensive than and diagnostically equivalent to other imaging modalities. A pelvic ultrasound can help delineate the anatomic origin of the mass, but it also can detect characteristics of the mass that may help with the diagnosis, and the decision of whether or not to proceed to surgery. Endometriomas, mature teratomas (dermoid cysts), simple ovarian cysts, and hemorrhagic cysts have sonographic features that are highly predictive of the histology. Depending on whether or not the patient is symptomatic, the patient’s age, and comorbidities, these masses might be followed expectantly.
Ultrasound features that are suggestive of malignancy include solid components, septations greater than 2-3 mm, and vascular flow. The presence of ascites or peritoneal nodules detected at the time of ultrasound also is highly suspicious of malignancy in patients with a pelvic mass. If a pelvic ultrasound is equivocal, pelvic magnetic resonance imaging (MRI) is the second study of choice. Computed tomography (CT scan) should be used to evaluate for metastatic disease in patients with suspected ovarian carcinoma (ascites, adenopathy, peritoneal thickening or nodularity, omental thickening).
Serum biomarkers also may aid in the evaluation. The most well-studied and commonly used biomarker in the evaluation of an adnexal mass is CA-125. In general, the utility of CA-125 is limited mainly because of its low specificity, especially in premenopausal women. However, it can be used as adjunct when an ovarian malignancy is suspected based on the patient’s history, risk factors, and imaging findings. The American College of Obstetricians and Gynecologists (ACOG) and the Society of Gynecologic Oncologists (SGO) advise referral to a gynecologic oncologist for postmenopausal women with an elevated CA-125. Meanwhile, for premenopausal women, the recommendation is for referral of those with a "very elevated" CA-125. However, a specific value has not been established (Obstet. Gynecol. 2011;117:742-6).
CA-125 by itself should not be used to decide whether or not to take a patient to surgery. Nevertheless, once the decision to operate has been made, CA-125 can be used in conjunction with HE4 to calculate a Risk of Malignancy Algorithm (ROMA) score. The score is based on menopausal status, and if the calculated risk is elevated, patient referral to a gynecologic oncologist for her surgery should be strongly considered.
Similarly, the OVA1 test is currently approved by the Food and Drug Administration to assess the likelihood of malignancy in patients who are having surgery for an adnexal mass. The test is also based on menopausal status, and if elevated, a referral to a gynecologic oncologist is recommended. In young women with adnexal masses, germ cell tumor markers may be more helpful (lactate dehydrogenase [LDH], human chorionic gonadotropin [hCG], alpha-fetoprotein [AFP]), while in patients with signs or symptoms of estrogen or androgen excess, sex cord-stromal tumor markers (inhibin B, anti-Müllerian hormone [AMH], testosterone, dehydroepiandrosterone [DHEA], estradiol) would be appropriate to obtain. While no tumor marker is "diagnostic," the results may assist in the decision to perform surgery and consider referral to a gynecologic oncologist.
In summary, the workup for an adnexal mass should include a detailed medical and family history, a thorough physical exam, and imaging with pelvic ultrasound. For premenopausal women, there is a higher incidence of adnexal masses, and, in fact, most of them are benign. In these women, one must weigh the risk/benefit of close monitoring with pelvic ultrasound versus surgical intervention. A serum CA-125 can be helpful, but only if it is significantly elevated.
If uncertainty remains after a complete evaluation has been performed, it is appropriate to refer to a gynecologic oncologist. In postmenopausal women, serum biomarkers should be used in conjunction with the history, physical, and ultrasound because of the higher risk of malignancy. In addition, surgical intervention should be offered to these patients regardless of serum marker values in the setting of a complex mass. If there is high suspicion for malignancy by history and imaging or elevated ROMA or OVA1, referral to a gynecologic oncologist is prudent.
Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology, and a professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. Dr. Roque is a fellow in the gynecologic oncology program at the University of North Carolina. Neither Dr. Clarke-Pearson nor Dr. Roque has any relevant financial disclosures. E-mail them at obnews@frontlinemedcom.com.
An adnexal mass is a common gynecological presentation that can affect women of all ages. Typically, the mass is identified during an annual pelvic exam or incidentally when patients undergo pelvic imaging for evaluation of gastrointestinal or gynecological complaints.
The main goal of evaluating an adnexal mass in the nonacute setting is to rule out malignancy. A careful evaluation is needed to accurately distinguish benign from malignant masses, but often a definitive diagnosis only can be achieved with surgery. Hence, in the United States, women have a 5%-10% chance of undergoing surgery to evaluate a mass, but only 13%-21% of these patients are diagnosed with an ovarian cancer (Obstet. Gynecol. 2007;110:201-14).
We will review a stepwise approach for the evaluation of a newly diagnosed mass. As part of our review, we will discuss imaging findings that should prompt surgical evaluation or continued observation, as well as the correct use of the currently available serum biomarkers.
The majority of adnexal masses are benign and present most commonly in premenopausal women. However, in the outpatient setting, the evaluation approach should be aimed at ruling out malignancy regardless of age or reproductive status. The patient’s age should be considered clinically, as the suspicion for ovarian cancer should be heightened in postmenopausal women.
The evaluation should start with a detailed history because it may help in determining the etiology of the mass. Pelvic pain and pressure are very common but nonspecific symptoms in women with adnexal masses. However, if the pain is of sudden onset, urgent evaluation is warranted to rule out adnexal torsion or a ruptured hemorrhagic cyst. A history of dysmenorrhea and/or dyspareunia may suggest endometriosis and coexisting endometrioma, whereas a patient with fever and a vaginal discharge should be evaluated for a tubo-ovarian abscess.
Patients also should be asked about symptoms associated with ovarian cancer including early satiety, constipation, and bloating, as well as their duration. In addition, abnormal uterine bleeding or virilization may suggest the presence of estrogen- or testosterone-secreting tumors. Lastly, a detailed family history is important, as the presence of ovarian, breast, or colon cancer in the family would increase suspicion for hereditary ovarian cancer syndromes.
A thorough physical exam should include a speculum exam as well as bimanual and rectovaginal exams. The focus of the pelvic exam should be determining the size, mobility, and consistency of the mass, as well as other findings that may help discriminate benign versus malignant neoplasms. Malignant masses are usually solid, irregular in shape, and tend to be fixed. Nodularity in the posterior cul-de-sac also is associated with malignancy. The abdominal exam should focus on the presence or absence of ascites (fluid wave), an omental mass, or inguinal adenopathy. However, none of the findings on exam are specific for an ovarian or fallopian tube malignancy, and imaging should be obtained for further evaluation.
Ultrasound is the imaging study of choice for the evaluation of an adnexal mass because it is less expensive than and diagnostically equivalent to other imaging modalities. A pelvic ultrasound can help delineate the anatomic origin of the mass, but it also can detect characteristics of the mass that may help with the diagnosis, and the decision of whether or not to proceed to surgery. Endometriomas, mature teratomas (dermoid cysts), simple ovarian cysts, and hemorrhagic cysts have sonographic features that are highly predictive of the histology. Depending on whether or not the patient is symptomatic, the patient’s age, and comorbidities, these masses might be followed expectantly.
Ultrasound features that are suggestive of malignancy include solid components, septations greater than 2-3 mm, and vascular flow. The presence of ascites or peritoneal nodules detected at the time of ultrasound also is highly suspicious of malignancy in patients with a pelvic mass. If a pelvic ultrasound is equivocal, pelvic magnetic resonance imaging (MRI) is the second study of choice. Computed tomography (CT scan) should be used to evaluate for metastatic disease in patients with suspected ovarian carcinoma (ascites, adenopathy, peritoneal thickening or nodularity, omental thickening).
Serum biomarkers also may aid in the evaluation. The most well-studied and commonly used biomarker in the evaluation of an adnexal mass is CA-125. In general, the utility of CA-125 is limited mainly because of its low specificity, especially in premenopausal women. However, it can be used as adjunct when an ovarian malignancy is suspected based on the patient’s history, risk factors, and imaging findings. The American College of Obstetricians and Gynecologists (ACOG) and the Society of Gynecologic Oncologists (SGO) advise referral to a gynecologic oncologist for postmenopausal women with an elevated CA-125. Meanwhile, for premenopausal women, the recommendation is for referral of those with a "very elevated" CA-125. However, a specific value has not been established (Obstet. Gynecol. 2011;117:742-6).
CA-125 by itself should not be used to decide whether or not to take a patient to surgery. Nevertheless, once the decision to operate has been made, CA-125 can be used in conjunction with HE4 to calculate a Risk of Malignancy Algorithm (ROMA) score. The score is based on menopausal status, and if the calculated risk is elevated, patient referral to a gynecologic oncologist for her surgery should be strongly considered.
Similarly, the OVA1 test is currently approved by the Food and Drug Administration to assess the likelihood of malignancy in patients who are having surgery for an adnexal mass. The test is also based on menopausal status, and if elevated, a referral to a gynecologic oncologist is recommended. In young women with adnexal masses, germ cell tumor markers may be more helpful (lactate dehydrogenase [LDH], human chorionic gonadotropin [hCG], alpha-fetoprotein [AFP]), while in patients with signs or symptoms of estrogen or androgen excess, sex cord-stromal tumor markers (inhibin B, anti-Müllerian hormone [AMH], testosterone, dehydroepiandrosterone [DHEA], estradiol) would be appropriate to obtain. While no tumor marker is "diagnostic," the results may assist in the decision to perform surgery and consider referral to a gynecologic oncologist.
In summary, the workup for an adnexal mass should include a detailed medical and family history, a thorough physical exam, and imaging with pelvic ultrasound. For premenopausal women, there is a higher incidence of adnexal masses, and, in fact, most of them are benign. In these women, one must weigh the risk/benefit of close monitoring with pelvic ultrasound versus surgical intervention. A serum CA-125 can be helpful, but only if it is significantly elevated.
If uncertainty remains after a complete evaluation has been performed, it is appropriate to refer to a gynecologic oncologist. In postmenopausal women, serum biomarkers should be used in conjunction with the history, physical, and ultrasound because of the higher risk of malignancy. In addition, surgical intervention should be offered to these patients regardless of serum marker values in the setting of a complex mass. If there is high suspicion for malignancy by history and imaging or elevated ROMA or OVA1, referral to a gynecologic oncologist is prudent.
Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology, and a professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. Dr. Roque is a fellow in the gynecologic oncology program at the University of North Carolina. Neither Dr. Clarke-Pearson nor Dr. Roque has any relevant financial disclosures. E-mail them at obnews@frontlinemedcom.com.
Two most common vaginal prolapse surgeries yield similar outcomes
Two-year outcomes were similar between women who underwent transvaginal sacrospinous ligament fixation and those who underwent transvaginal uterosacral ligament suspension to correct apical vaginal or uterine prolapse and stress incontinence in the first randomized trial to compare the two approaches.
The 5-year OPTIMAL (Operations and Pelvic Muscle Training in the Management of Apical Support Loss) trial at nine U.S. medical centers participating in the Pelvic Floor Disorders Network involved 374 women randomly assigned to transvaginal surgery for stage 2 through stage 4 prolapse. A total of 186 underwent sacrospinous ligament fixation (SSLF), while 188 patients underwent uterosacral ligament suspension (ULS).
The women also were separately randomized to receive perioperative behavioral therapy with pelvic floor muscle training or usual perioperative care, wrote Dr. Matthew D. Barber of the department of obstetrics and gynecology and the Women’s Health Institute at the Cleveland Clinic. The report appears in the March 11 issue of JAMA.
At 2-year follow-up, neither surgical approach was superior to the other in the composite primary outcome of the percentage of patients with surgical success: 60.5% for SSLF and 59.2% for ULS. The investigators defined successful surgery as the absence of descent of the vaginal apex more than one-third into the vaginal canal; anterior or posterior vaginal wall descent beyond the hymen; bothersome vaginal bulge symptoms; and further treatment for prolapse. The overall rates of failure were 18% for bothersome vaginal bulge, 17.5% for anterior and/or posterior prolapse, and 5.1% for further treatment such as more surgery or use of a pessary.
The two groups were no different in any secondary outcomes such as blood loss, duration of surgery, length of hospitalization, or postoperative treatment. Serious adverse events including bladder perforation and the formation of vaginal granulation tissue occurred in 16.7% of the SSLF group and 16.5% of the ULS group. There were no significant differences between women who received the behavioral intervention and those who did not, the investigators said (JAMA 2014;311:1023-34).
They noted that 4.3% of the women who underwent SSLF developed persistent pain, confirming previous reports that the procedure may cause acute neurologic pain, particularly buttock pain that may result from gluteal nerve entrapment. This highlights "the need to provide preoperative counseling to patients about this potential risk," Dr. Barber and his associates wrote.
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health. Some of Dr. Barber’s coauthors reported ties to Astellas, Pfizer, GlaxoSmithKline, Uromedica, IDEO, Xanodyne, Renew Medical, American Medical Systems, Ethicon/Johnson & Johnson, Boston Scientific, and Ferring Pharmaceuticals.
Two-year outcomes were similar between women who underwent transvaginal sacrospinous ligament fixation and those who underwent transvaginal uterosacral ligament suspension to correct apical vaginal or uterine prolapse and stress incontinence in the first randomized trial to compare the two approaches.
The 5-year OPTIMAL (Operations and Pelvic Muscle Training in the Management of Apical Support Loss) trial at nine U.S. medical centers participating in the Pelvic Floor Disorders Network involved 374 women randomly assigned to transvaginal surgery for stage 2 through stage 4 prolapse. A total of 186 underwent sacrospinous ligament fixation (SSLF), while 188 patients underwent uterosacral ligament suspension (ULS).
The women also were separately randomized to receive perioperative behavioral therapy with pelvic floor muscle training or usual perioperative care, wrote Dr. Matthew D. Barber of the department of obstetrics and gynecology and the Women’s Health Institute at the Cleveland Clinic. The report appears in the March 11 issue of JAMA.
At 2-year follow-up, neither surgical approach was superior to the other in the composite primary outcome of the percentage of patients with surgical success: 60.5% for SSLF and 59.2% for ULS. The investigators defined successful surgery as the absence of descent of the vaginal apex more than one-third into the vaginal canal; anterior or posterior vaginal wall descent beyond the hymen; bothersome vaginal bulge symptoms; and further treatment for prolapse. The overall rates of failure were 18% for bothersome vaginal bulge, 17.5% for anterior and/or posterior prolapse, and 5.1% for further treatment such as more surgery or use of a pessary.
The two groups were no different in any secondary outcomes such as blood loss, duration of surgery, length of hospitalization, or postoperative treatment. Serious adverse events including bladder perforation and the formation of vaginal granulation tissue occurred in 16.7% of the SSLF group and 16.5% of the ULS group. There were no significant differences between women who received the behavioral intervention and those who did not, the investigators said (JAMA 2014;311:1023-34).
They noted that 4.3% of the women who underwent SSLF developed persistent pain, confirming previous reports that the procedure may cause acute neurologic pain, particularly buttock pain that may result from gluteal nerve entrapment. This highlights "the need to provide preoperative counseling to patients about this potential risk," Dr. Barber and his associates wrote.
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health. Some of Dr. Barber’s coauthors reported ties to Astellas, Pfizer, GlaxoSmithKline, Uromedica, IDEO, Xanodyne, Renew Medical, American Medical Systems, Ethicon/Johnson & Johnson, Boston Scientific, and Ferring Pharmaceuticals.
Two-year outcomes were similar between women who underwent transvaginal sacrospinous ligament fixation and those who underwent transvaginal uterosacral ligament suspension to correct apical vaginal or uterine prolapse and stress incontinence in the first randomized trial to compare the two approaches.
The 5-year OPTIMAL (Operations and Pelvic Muscle Training in the Management of Apical Support Loss) trial at nine U.S. medical centers participating in the Pelvic Floor Disorders Network involved 374 women randomly assigned to transvaginal surgery for stage 2 through stage 4 prolapse. A total of 186 underwent sacrospinous ligament fixation (SSLF), while 188 patients underwent uterosacral ligament suspension (ULS).
The women also were separately randomized to receive perioperative behavioral therapy with pelvic floor muscle training or usual perioperative care, wrote Dr. Matthew D. Barber of the department of obstetrics and gynecology and the Women’s Health Institute at the Cleveland Clinic. The report appears in the March 11 issue of JAMA.
At 2-year follow-up, neither surgical approach was superior to the other in the composite primary outcome of the percentage of patients with surgical success: 60.5% for SSLF and 59.2% for ULS. The investigators defined successful surgery as the absence of descent of the vaginal apex more than one-third into the vaginal canal; anterior or posterior vaginal wall descent beyond the hymen; bothersome vaginal bulge symptoms; and further treatment for prolapse. The overall rates of failure were 18% for bothersome vaginal bulge, 17.5% for anterior and/or posterior prolapse, and 5.1% for further treatment such as more surgery or use of a pessary.
The two groups were no different in any secondary outcomes such as blood loss, duration of surgery, length of hospitalization, or postoperative treatment. Serious adverse events including bladder perforation and the formation of vaginal granulation tissue occurred in 16.7% of the SSLF group and 16.5% of the ULS group. There were no significant differences between women who received the behavioral intervention and those who did not, the investigators said (JAMA 2014;311:1023-34).
They noted that 4.3% of the women who underwent SSLF developed persistent pain, confirming previous reports that the procedure may cause acute neurologic pain, particularly buttock pain that may result from gluteal nerve entrapment. This highlights "the need to provide preoperative counseling to patients about this potential risk," Dr. Barber and his associates wrote.
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health. Some of Dr. Barber’s coauthors reported ties to Astellas, Pfizer, GlaxoSmithKline, Uromedica, IDEO, Xanodyne, Renew Medical, American Medical Systems, Ethicon/Johnson & Johnson, Boston Scientific, and Ferring Pharmaceuticals.
FROM JAMA
Major Finding: At 2-year follow-up, neither surgical approach was superior to the other in the composite primary outcome of the percentage of patients with surgical success: 60.5% for sacrospinous ligament fixation and 59.2% for uterosacral ligament suspension.
Data Source: A randomized trial comparing outcomes between 186 women who had SSLF and 188 who had ULS to correct apical vaginal or uterine prolapse.
Disclosures: This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health. Some of Dr. Barber’s coauthors reported ties to Astellas, Pfizer, GlaxoSmithKline, Uromedica, IDEO, Xanodyne, Renew Medical, American Medical Systems, Ethicon/Johnson & Johnson, Boston Scientific, and Ferring Pharmaceuticals.
Vaginal mesh found superior for vaginal vault prolapse with levator ani avulsion
Vaginal mesh surgery had a significantly lower failure rate than did sacrospinous vaginal colpopexy in women with vaginal vault prolapse and levator ani avulsion, investigators reported online March 11 in Ultrasound in Obstetrics & Gynecology.
At 1-year follow-up, 3% of patients treated with mesh had anatomic failure based on clinical and ultrasound evaluation, compared with 62%-65% of patients who underwent unilateral vaginal sacrospinous colpopexy with native tissue vaginal repair, reported Dr. Kamil Svabik and his associates at General University Hospital and the Charles University in Prague.
Vaginal mesh has been linked to adverse effects, so the researchers enrolled patients with post hysterectomy vaginal vault prolapse who also had levator ani avulsion only. "We considered it potentially unethical to offer mesh to all patients with post hysterectomy prolapse due to concerns about mesh complications, and since there is some evidence that reduction in recurrence due to mesh may be limited largely to patients with levator avulsion," they said (Ultrasound Obstet. Gynecol. 2014 March 11 [doi:10.1002/uog.13305]).
Among 70 women with ultrasound-diagnosed avulsions, 36 were randomized to the vaginal mesh procedure and 34 underwent sacrospinous fixation. At 1-year follow-up, there was one anatomic failure in the mesh group, while 22 (65%) patients in the sacrospinous fixation group had prolapses to the hymen or beyond, and 21 (63%) met ultrasound criteria for prolapse. The groups did not differ significantly in terms of incontinence, sexual function, or prolapse symptoms, which the researchers said might be because of the lack of power. No serious adverse effects were reported for either group.
The Gynecare Prolift mesh used in the study has been taken off the market, the investigators said, adding that other type-I polypropylene meshes might achieve similar results. They planned to assess long-term outcomes and complications.
The study was supported by the Grant Agency of the Ministry of Health of the Czech Republic and by Charles University in Prague. The authors disclosed no conflicts of interest except Dr. Martan, who reported having consulted for Bard, Gynecare, and AMS.
Vaginal mesh surgery had a significantly lower failure rate than did sacrospinous vaginal colpopexy in women with vaginal vault prolapse and levator ani avulsion, investigators reported online March 11 in Ultrasound in Obstetrics & Gynecology.
At 1-year follow-up, 3% of patients treated with mesh had anatomic failure based on clinical and ultrasound evaluation, compared with 62%-65% of patients who underwent unilateral vaginal sacrospinous colpopexy with native tissue vaginal repair, reported Dr. Kamil Svabik and his associates at General University Hospital and the Charles University in Prague.
Vaginal mesh has been linked to adverse effects, so the researchers enrolled patients with post hysterectomy vaginal vault prolapse who also had levator ani avulsion only. "We considered it potentially unethical to offer mesh to all patients with post hysterectomy prolapse due to concerns about mesh complications, and since there is some evidence that reduction in recurrence due to mesh may be limited largely to patients with levator avulsion," they said (Ultrasound Obstet. Gynecol. 2014 March 11 [doi:10.1002/uog.13305]).
Among 70 women with ultrasound-diagnosed avulsions, 36 were randomized to the vaginal mesh procedure and 34 underwent sacrospinous fixation. At 1-year follow-up, there was one anatomic failure in the mesh group, while 22 (65%) patients in the sacrospinous fixation group had prolapses to the hymen or beyond, and 21 (63%) met ultrasound criteria for prolapse. The groups did not differ significantly in terms of incontinence, sexual function, or prolapse symptoms, which the researchers said might be because of the lack of power. No serious adverse effects were reported for either group.
The Gynecare Prolift mesh used in the study has been taken off the market, the investigators said, adding that other type-I polypropylene meshes might achieve similar results. They planned to assess long-term outcomes and complications.
The study was supported by the Grant Agency of the Ministry of Health of the Czech Republic and by Charles University in Prague. The authors disclosed no conflicts of interest except Dr. Martan, who reported having consulted for Bard, Gynecare, and AMS.
Vaginal mesh surgery had a significantly lower failure rate than did sacrospinous vaginal colpopexy in women with vaginal vault prolapse and levator ani avulsion, investigators reported online March 11 in Ultrasound in Obstetrics & Gynecology.
At 1-year follow-up, 3% of patients treated with mesh had anatomic failure based on clinical and ultrasound evaluation, compared with 62%-65% of patients who underwent unilateral vaginal sacrospinous colpopexy with native tissue vaginal repair, reported Dr. Kamil Svabik and his associates at General University Hospital and the Charles University in Prague.
Vaginal mesh has been linked to adverse effects, so the researchers enrolled patients with post hysterectomy vaginal vault prolapse who also had levator ani avulsion only. "We considered it potentially unethical to offer mesh to all patients with post hysterectomy prolapse due to concerns about mesh complications, and since there is some evidence that reduction in recurrence due to mesh may be limited largely to patients with levator avulsion," they said (Ultrasound Obstet. Gynecol. 2014 March 11 [doi:10.1002/uog.13305]).
Among 70 women with ultrasound-diagnosed avulsions, 36 were randomized to the vaginal mesh procedure and 34 underwent sacrospinous fixation. At 1-year follow-up, there was one anatomic failure in the mesh group, while 22 (65%) patients in the sacrospinous fixation group had prolapses to the hymen or beyond, and 21 (63%) met ultrasound criteria for prolapse. The groups did not differ significantly in terms of incontinence, sexual function, or prolapse symptoms, which the researchers said might be because of the lack of power. No serious adverse effects were reported for either group.
The Gynecare Prolift mesh used in the study has been taken off the market, the investigators said, adding that other type-I polypropylene meshes might achieve similar results. They planned to assess long-term outcomes and complications.
The study was supported by the Grant Agency of the Ministry of Health of the Czech Republic and by Charles University in Prague. The authors disclosed no conflicts of interest except Dr. Martan, who reported having consulted for Bard, Gynecare, and AMS.
FROM ULTRASOUND IN OBSTETRICS & GYNECOLOGY