User login
Vaginal intraepithelial neoplasia: What to do when dysplasia persists after hysterectomy
Vaginal intraepithelial neoplasia (VAIN) is a condition that frequently poses therapeutic dilemmas for gynecologists. VAIN represents dysplastic changes to the epithelium of the vaginal mucosa, and like cervical neoplasia, the extent of disease is characterized as levels I, II, or III dependent upon the depth of involvement in the epithelial layer by dysplastic cells. While VAIN itself typically is asymptomatic and not a harmful condition, it carries a 12% risk of progression to invasive vaginal carcinoma, so accurate identification, thorough treatment, and ongoing surveillance are essential.1
VAIN is associated with high-risk human papillomavirus (HPV) infection, tobacco use, and prior cervical dysplasia. Of women with VAIN, 65% have undergone a prior hysterectomy for cervical dysplasia, which emphasizes the nondefinitive nature of such an intervention.2 These women should be very closely followed for at least 20 years with vaginal cytologic and/or HPV surveillance. High-risk HPV infection is present in 85% of women with VAIN, and the presence of high-risk HPV is a predictor for recurrent VAIN. Recurrent and persistent VAIN also is more common in postmenopausal women and those with multifocal disease.
The most common location for VAIN is at the upper third of the vagina (including the vaginal cuff). It commonly arises within the vaginal fornices, which may be difficult to fully visualize because of their puckered appearance, redundant vaginal tissues, and extensive vaginal rogation.
A diagnosis of VAIN is typically obtained from vaginal cytology which reveals atypical or dysplastic cells. Such a result should prompt the physician to perform vaginal colposcopy and directed biopsies. Comprehensive visualization of the vaginal cuff can be limited in cases where the vaginal fornices are tethered, deeply puckered, or when there is significant mucosal rogation.
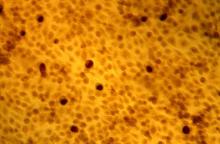
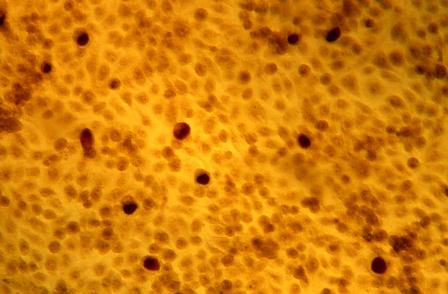
The application of 4% acetic acid or Lugol’s iodine are techniques that can enhance the detection of dysplastic vaginal mucosa. Lugol’s iodine selectively stains normal, glycogenated cells, and spares dysplastic glycogen-free cells. The sharp contrast between the brown iodine-stained tissues and the white dysplastic tissues aids in detection of dysplastic areas.
If colposcopic biopsy reveals low grade dysplasia (VAIN I) it does not require intervention, and has a very low rate of conversion to invasive vaginal carcinoma. However moderate- and high-grade vaginal dysplastic lesions should be treated because of the potential for malignant transformation.
Options for treatment of VAIN include topical, ablative, and excisional procedures. Observation also is an option but should be reserved for patients who are closely monitored with repeated colposcopic examinations, and probably should best be reserved for patients with VAIN I or II lesions.
Excisional procedures
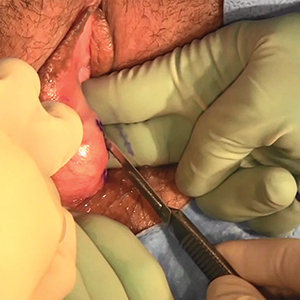
The most common excisional procedure employed for VAIN is upper vaginectomy. In this procedure, the surgeon grasps and tents up the vaginal mucosa, incises the mucosa without penetrating the subepithelial tissue layers such as bladder and rectum. The vaginal mucosa then is carefully separated from the underlying endopelvic fascial plane. The specimen should be oriented, ideally on a cork board, with pins or sutures to ascribe margins and borders. Excision is best utilized for women with unifocal disease, or those who fail or do not tolerate ablative or topical interventions.
The most significant risks of excision include the potential for damage to underlying pelvic visceral structures, which is particularly concerning in postmenopausal women with thin vaginal epithelium. Vaginectomy is commonly associated with vaginal shortening or narrowing, which can be deleterious for quality of life. Retrospective series have described a 30% incidence of recurrence after vaginectomy, likely secondary to incomplete excision of all affected tissue.3
Ablation
Ablation of dysplastic foci with a carbon dioxide (CO2) laser is a common method for treatment of VAIN. CO2 laser should ablate tissue to a 1.5 mm minimum depth.3 The benefit of using CO2 laser is its ability to treat multifocal disease in situ without an extensive excisional procedure.
It is technically more straightforward than upper vaginectomy with less blood loss and shorter surgical times, and it can be easily accomplished in an outpatient surgical or office setting. However, one of its greatest limitations is the difficulty in visualizing all lesions and therefore adequately treating all sites. The vaginal rogations also make adequate laser ablation challenging because laser only is able to effectively ablate tissue that is oriented perpendicular to the laser beam.
In addition, there is no pathologic confirmation of adequacy of excision or margin status. These features may contribute to the modestly higher rates of recurrence of dysplasia following laser ablation, compared with vaginectomy.3 It also has been associated with more vaginal scarring than vaginectomy, which can have a negative effect on sexual health.
Topical agents
The most commonly utilized topical therapy for VAIN is the antimetabolite chemotherapeutic agent 5-fluorouracil (5FU). A typical schedule for 5FU treatment is to apply vaginally, at night, once a week for 8 weeks.4 Because it can cause extensive irritation to the vulvar and urethral epithelium, patients are recommended to apply barrier creams or ointments before and following the use of 5FU for several days, wash hands thoroughly after application, and to rinse and shower in the morning after rising. Severe irritation occurs in up to 16% of patients, but in general it is very well tolerated.

Its virtue is that it is able to conform and travel to all parts of the vaginal mucosa, including those that are poorly visualized within the fornices or vaginal folds. 5FU does not require a hospitalization or surgical procedure, can be applied by the patient at home, and preserves vaginal length and function. In recent reports, 5FU is associated with the lowest rates of recurrence (10%-30%), compared with excision or ablation, and therefore is a very attractive option for primary therapy.3 However, it requires patients to have a degree of comfort with vaginal application of drug and adherence with perineal care strategies to minimize the likelihood of toxicity.
The immune response modifier, imiquimod, that is commonly used in the treatment of vulvar dysplasia also has been described in the treatment of VAIN. It appears to have high rates of clearance (greater than 75%) and be most effective in the treatment of VAIN I.5 It requires application under colposcopic guidance three times a week for 8 weeks, which is a laborious undertaking for both patient and physician. Like 5FU, imiquimod is associated with vulvar and perineal irritation.
Vaginal estrogens are an alternative topical therapy for moderate- and high-grade VAIN and particularly useful for postmenopausal patients. They have been associated with a high rate (up to 90%) of resolution on follow-up vaginal cytology testing and are not associated with toxicities of the above stated therapies.6 Vaginal estrogen can be used alone or in addition to other therapeutic strategies. For example, it can be added to the nontreatment days of 5FU or postoperatively prescribed following laser or excisional procedures.
Radiation
Intracavitary brachytherapy is a technique in which a radiation source is placed within a cylinder or ovoids and placed within the vagina.7 Typically 45 Gy is delivered to a depth 0.5mm below the vaginal mucosal surface (“point z”). Recurrence occurs is approximately 10%-15% of patients, and toxicities can be severe, including vaginal stenosis and ulceration. This aggressive therapy typically is best reserved for cases that are refractory to other therapies. Following radiation, subsequent treatments are more difficult because of radiation-induced changes to the vaginal mucosa that can affect healing.
Vaginal dysplasia is a relatively common sequelae of high-risk HPV, particularly among women who have had a prior hysterectomy for cervical dysplasia. Because of anatomic changes following hysterectomy, adequate visualization and comprehensive vaginal treatment is difficult. Therefore, surgeons should avoid utilization of hysterectomy as a routine strategy to “cure” dysplasia as it may fail to achieve this cure and make subsequent evaluations and treatments of persistent dysplasia more difficult. Women who have had a hysterectomy for dysplasia should be closely followed for several decades, and they should be counseled that they have a persistent risk for vaginal disease. When VAIN develops, clinicians should consider topical therapies as primary treatment options because they may minimize toxicity and have high rates of enduring response.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant conflicts of interest.
References
1. Gynecol Oncol. 2016 Jun;141(3):507-10.
2. Arch Gynecol Obstet. 2016 Feb;293(2):415-9.
3. Anticancer Res. 2013 Jan;33(1):29-38.
4. Obstet Gynecol. 2017 Dec;130(6):1237-43.
5. Eur J Obstet Gynecol Reprod Biol. 2017 Nov;218:129-36.
6. J Low Genit Tract Dis. 2014 Apr;18(2):115-21.
7. Gynecol Oncol. 2007 Jul;106(1):105-11.
Vaginal intraepithelial neoplasia (VAIN) is a condition that frequently poses therapeutic dilemmas for gynecologists. VAIN represents dysplastic changes to the epithelium of the vaginal mucosa, and like cervical neoplasia, the extent of disease is characterized as levels I, II, or III dependent upon the depth of involvement in the epithelial layer by dysplastic cells. While VAIN itself typically is asymptomatic and not a harmful condition, it carries a 12% risk of progression to invasive vaginal carcinoma, so accurate identification, thorough treatment, and ongoing surveillance are essential.1
VAIN is associated with high-risk human papillomavirus (HPV) infection, tobacco use, and prior cervical dysplasia. Of women with VAIN, 65% have undergone a prior hysterectomy for cervical dysplasia, which emphasizes the nondefinitive nature of such an intervention.2 These women should be very closely followed for at least 20 years with vaginal cytologic and/or HPV surveillance. High-risk HPV infection is present in 85% of women with VAIN, and the presence of high-risk HPV is a predictor for recurrent VAIN. Recurrent and persistent VAIN also is more common in postmenopausal women and those with multifocal disease.
The most common location for VAIN is at the upper third of the vagina (including the vaginal cuff). It commonly arises within the vaginal fornices, which may be difficult to fully visualize because of their puckered appearance, redundant vaginal tissues, and extensive vaginal rogation.
A diagnosis of VAIN is typically obtained from vaginal cytology which reveals atypical or dysplastic cells. Such a result should prompt the physician to perform vaginal colposcopy and directed biopsies. Comprehensive visualization of the vaginal cuff can be limited in cases where the vaginal fornices are tethered, deeply puckered, or when there is significant mucosal rogation.
The application of 4% acetic acid or Lugol’s iodine are techniques that can enhance the detection of dysplastic vaginal mucosa. Lugol’s iodine selectively stains normal, glycogenated cells, and spares dysplastic glycogen-free cells. The sharp contrast between the brown iodine-stained tissues and the white dysplastic tissues aids in detection of dysplastic areas.
If colposcopic biopsy reveals low grade dysplasia (VAIN I) it does not require intervention, and has a very low rate of conversion to invasive vaginal carcinoma. However moderate- and high-grade vaginal dysplastic lesions should be treated because of the potential for malignant transformation.
Options for treatment of VAIN include topical, ablative, and excisional procedures. Observation also is an option but should be reserved for patients who are closely monitored with repeated colposcopic examinations, and probably should best be reserved for patients with VAIN I or II lesions.
Excisional procedures
The most common excisional procedure employed for VAIN is upper vaginectomy. In this procedure, the surgeon grasps and tents up the vaginal mucosa, incises the mucosa without penetrating the subepithelial tissue layers such as bladder and rectum. The vaginal mucosa then is carefully separated from the underlying endopelvic fascial plane. The specimen should be oriented, ideally on a cork board, with pins or sutures to ascribe margins and borders. Excision is best utilized for women with unifocal disease, or those who fail or do not tolerate ablative or topical interventions.
The most significant risks of excision include the potential for damage to underlying pelvic visceral structures, which is particularly concerning in postmenopausal women with thin vaginal epithelium. Vaginectomy is commonly associated with vaginal shortening or narrowing, which can be deleterious for quality of life. Retrospective series have described a 30% incidence of recurrence after vaginectomy, likely secondary to incomplete excision of all affected tissue.3
Ablation
Ablation of dysplastic foci with a carbon dioxide (CO2) laser is a common method for treatment of VAIN. CO2 laser should ablate tissue to a 1.5 mm minimum depth.3 The benefit of using CO2 laser is its ability to treat multifocal disease in situ without an extensive excisional procedure.
It is technically more straightforward than upper vaginectomy with less blood loss and shorter surgical times, and it can be easily accomplished in an outpatient surgical or office setting. However, one of its greatest limitations is the difficulty in visualizing all lesions and therefore adequately treating all sites. The vaginal rogations also make adequate laser ablation challenging because laser only is able to effectively ablate tissue that is oriented perpendicular to the laser beam.
In addition, there is no pathologic confirmation of adequacy of excision or margin status. These features may contribute to the modestly higher rates of recurrence of dysplasia following laser ablation, compared with vaginectomy.3 It also has been associated with more vaginal scarring than vaginectomy, which can have a negative effect on sexual health.
Topical agents
The most commonly utilized topical therapy for VAIN is the antimetabolite chemotherapeutic agent 5-fluorouracil (5FU). A typical schedule for 5FU treatment is to apply vaginally, at night, once a week for 8 weeks.4 Because it can cause extensive irritation to the vulvar and urethral epithelium, patients are recommended to apply barrier creams or ointments before and following the use of 5FU for several days, wash hands thoroughly after application, and to rinse and shower in the morning after rising. Severe irritation occurs in up to 16% of patients, but in general it is very well tolerated.

Its virtue is that it is able to conform and travel to all parts of the vaginal mucosa, including those that are poorly visualized within the fornices or vaginal folds. 5FU does not require a hospitalization or surgical procedure, can be applied by the patient at home, and preserves vaginal length and function. In recent reports, 5FU is associated with the lowest rates of recurrence (10%-30%), compared with excision or ablation, and therefore is a very attractive option for primary therapy.3 However, it requires patients to have a degree of comfort with vaginal application of drug and adherence with perineal care strategies to minimize the likelihood of toxicity.
The immune response modifier, imiquimod, that is commonly used in the treatment of vulvar dysplasia also has been described in the treatment of VAIN. It appears to have high rates of clearance (greater than 75%) and be most effective in the treatment of VAIN I.5 It requires application under colposcopic guidance three times a week for 8 weeks, which is a laborious undertaking for both patient and physician. Like 5FU, imiquimod is associated with vulvar and perineal irritation.
Vaginal estrogens are an alternative topical therapy for moderate- and high-grade VAIN and particularly useful for postmenopausal patients. They have been associated with a high rate (up to 90%) of resolution on follow-up vaginal cytology testing and are not associated with toxicities of the above stated therapies.6 Vaginal estrogen can be used alone or in addition to other therapeutic strategies. For example, it can be added to the nontreatment days of 5FU or postoperatively prescribed following laser or excisional procedures.
Radiation
Intracavitary brachytherapy is a technique in which a radiation source is placed within a cylinder or ovoids and placed within the vagina.7 Typically 45 Gy is delivered to a depth 0.5mm below the vaginal mucosal surface (“point z”). Recurrence occurs is approximately 10%-15% of patients, and toxicities can be severe, including vaginal stenosis and ulceration. This aggressive therapy typically is best reserved for cases that are refractory to other therapies. Following radiation, subsequent treatments are more difficult because of radiation-induced changes to the vaginal mucosa that can affect healing.
Vaginal dysplasia is a relatively common sequelae of high-risk HPV, particularly among women who have had a prior hysterectomy for cervical dysplasia. Because of anatomic changes following hysterectomy, adequate visualization and comprehensive vaginal treatment is difficult. Therefore, surgeons should avoid utilization of hysterectomy as a routine strategy to “cure” dysplasia as it may fail to achieve this cure and make subsequent evaluations and treatments of persistent dysplasia more difficult. Women who have had a hysterectomy for dysplasia should be closely followed for several decades, and they should be counseled that they have a persistent risk for vaginal disease. When VAIN develops, clinicians should consider topical therapies as primary treatment options because they may minimize toxicity and have high rates of enduring response.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant conflicts of interest.
References
1. Gynecol Oncol. 2016 Jun;141(3):507-10.
2. Arch Gynecol Obstet. 2016 Feb;293(2):415-9.
3. Anticancer Res. 2013 Jan;33(1):29-38.
4. Obstet Gynecol. 2017 Dec;130(6):1237-43.
5. Eur J Obstet Gynecol Reprod Biol. 2017 Nov;218:129-36.
6. J Low Genit Tract Dis. 2014 Apr;18(2):115-21.
7. Gynecol Oncol. 2007 Jul;106(1):105-11.
Vaginal intraepithelial neoplasia (VAIN) is a condition that frequently poses therapeutic dilemmas for gynecologists. VAIN represents dysplastic changes to the epithelium of the vaginal mucosa, and like cervical neoplasia, the extent of disease is characterized as levels I, II, or III dependent upon the depth of involvement in the epithelial layer by dysplastic cells. While VAIN itself typically is asymptomatic and not a harmful condition, it carries a 12% risk of progression to invasive vaginal carcinoma, so accurate identification, thorough treatment, and ongoing surveillance are essential.1
VAIN is associated with high-risk human papillomavirus (HPV) infection, tobacco use, and prior cervical dysplasia. Of women with VAIN, 65% have undergone a prior hysterectomy for cervical dysplasia, which emphasizes the nondefinitive nature of such an intervention.2 These women should be very closely followed for at least 20 years with vaginal cytologic and/or HPV surveillance. High-risk HPV infection is present in 85% of women with VAIN, and the presence of high-risk HPV is a predictor for recurrent VAIN. Recurrent and persistent VAIN also is more common in postmenopausal women and those with multifocal disease.
The most common location for VAIN is at the upper third of the vagina (including the vaginal cuff). It commonly arises within the vaginal fornices, which may be difficult to fully visualize because of their puckered appearance, redundant vaginal tissues, and extensive vaginal rogation.
A diagnosis of VAIN is typically obtained from vaginal cytology which reveals atypical or dysplastic cells. Such a result should prompt the physician to perform vaginal colposcopy and directed biopsies. Comprehensive visualization of the vaginal cuff can be limited in cases where the vaginal fornices are tethered, deeply puckered, or when there is significant mucosal rogation.
The application of 4% acetic acid or Lugol’s iodine are techniques that can enhance the detection of dysplastic vaginal mucosa. Lugol’s iodine selectively stains normal, glycogenated cells, and spares dysplastic glycogen-free cells. The sharp contrast between the brown iodine-stained tissues and the white dysplastic tissues aids in detection of dysplastic areas.
If colposcopic biopsy reveals low grade dysplasia (VAIN I) it does not require intervention, and has a very low rate of conversion to invasive vaginal carcinoma. However moderate- and high-grade vaginal dysplastic lesions should be treated because of the potential for malignant transformation.
Options for treatment of VAIN include topical, ablative, and excisional procedures. Observation also is an option but should be reserved for patients who are closely monitored with repeated colposcopic examinations, and probably should best be reserved for patients with VAIN I or II lesions.
Excisional procedures
The most common excisional procedure employed for VAIN is upper vaginectomy. In this procedure, the surgeon grasps and tents up the vaginal mucosa, incises the mucosa without penetrating the subepithelial tissue layers such as bladder and rectum. The vaginal mucosa then is carefully separated from the underlying endopelvic fascial plane. The specimen should be oriented, ideally on a cork board, with pins or sutures to ascribe margins and borders. Excision is best utilized for women with unifocal disease, or those who fail or do not tolerate ablative or topical interventions.
The most significant risks of excision include the potential for damage to underlying pelvic visceral structures, which is particularly concerning in postmenopausal women with thin vaginal epithelium. Vaginectomy is commonly associated with vaginal shortening or narrowing, which can be deleterious for quality of life. Retrospective series have described a 30% incidence of recurrence after vaginectomy, likely secondary to incomplete excision of all affected tissue.3
Ablation
Ablation of dysplastic foci with a carbon dioxide (CO2) laser is a common method for treatment of VAIN. CO2 laser should ablate tissue to a 1.5 mm minimum depth.3 The benefit of using CO2 laser is its ability to treat multifocal disease in situ without an extensive excisional procedure.
It is technically more straightforward than upper vaginectomy with less blood loss and shorter surgical times, and it can be easily accomplished in an outpatient surgical or office setting. However, one of its greatest limitations is the difficulty in visualizing all lesions and therefore adequately treating all sites. The vaginal rogations also make adequate laser ablation challenging because laser only is able to effectively ablate tissue that is oriented perpendicular to the laser beam.
In addition, there is no pathologic confirmation of adequacy of excision or margin status. These features may contribute to the modestly higher rates of recurrence of dysplasia following laser ablation, compared with vaginectomy.3 It also has been associated with more vaginal scarring than vaginectomy, which can have a negative effect on sexual health.
Topical agents
The most commonly utilized topical therapy for VAIN is the antimetabolite chemotherapeutic agent 5-fluorouracil (5FU). A typical schedule for 5FU treatment is to apply vaginally, at night, once a week for 8 weeks.4 Because it can cause extensive irritation to the vulvar and urethral epithelium, patients are recommended to apply barrier creams or ointments before and following the use of 5FU for several days, wash hands thoroughly after application, and to rinse and shower in the morning after rising. Severe irritation occurs in up to 16% of patients, but in general it is very well tolerated.

Its virtue is that it is able to conform and travel to all parts of the vaginal mucosa, including those that are poorly visualized within the fornices or vaginal folds. 5FU does not require a hospitalization or surgical procedure, can be applied by the patient at home, and preserves vaginal length and function. In recent reports, 5FU is associated with the lowest rates of recurrence (10%-30%), compared with excision or ablation, and therefore is a very attractive option for primary therapy.3 However, it requires patients to have a degree of comfort with vaginal application of drug and adherence with perineal care strategies to minimize the likelihood of toxicity.
The immune response modifier, imiquimod, that is commonly used in the treatment of vulvar dysplasia also has been described in the treatment of VAIN. It appears to have high rates of clearance (greater than 75%) and be most effective in the treatment of VAIN I.5 It requires application under colposcopic guidance three times a week for 8 weeks, which is a laborious undertaking for both patient and physician. Like 5FU, imiquimod is associated with vulvar and perineal irritation.
Vaginal estrogens are an alternative topical therapy for moderate- and high-grade VAIN and particularly useful for postmenopausal patients. They have been associated with a high rate (up to 90%) of resolution on follow-up vaginal cytology testing and are not associated with toxicities of the above stated therapies.6 Vaginal estrogen can be used alone or in addition to other therapeutic strategies. For example, it can be added to the nontreatment days of 5FU or postoperatively prescribed following laser or excisional procedures.
Radiation
Intracavitary brachytherapy is a technique in which a radiation source is placed within a cylinder or ovoids and placed within the vagina.7 Typically 45 Gy is delivered to a depth 0.5mm below the vaginal mucosal surface (“point z”). Recurrence occurs is approximately 10%-15% of patients, and toxicities can be severe, including vaginal stenosis and ulceration. This aggressive therapy typically is best reserved for cases that are refractory to other therapies. Following radiation, subsequent treatments are more difficult because of radiation-induced changes to the vaginal mucosa that can affect healing.
Vaginal dysplasia is a relatively common sequelae of high-risk HPV, particularly among women who have had a prior hysterectomy for cervical dysplasia. Because of anatomic changes following hysterectomy, adequate visualization and comprehensive vaginal treatment is difficult. Therefore, surgeons should avoid utilization of hysterectomy as a routine strategy to “cure” dysplasia as it may fail to achieve this cure and make subsequent evaluations and treatments of persistent dysplasia more difficult. Women who have had a hysterectomy for dysplasia should be closely followed for several decades, and they should be counseled that they have a persistent risk for vaginal disease. When VAIN develops, clinicians should consider topical therapies as primary treatment options because they may minimize toxicity and have high rates of enduring response.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant conflicts of interest.
References
1. Gynecol Oncol. 2016 Jun;141(3):507-10.
2. Arch Gynecol Obstet. 2016 Feb;293(2):415-9.
3. Anticancer Res. 2013 Jan;33(1):29-38.
4. Obstet Gynecol. 2017 Dec;130(6):1237-43.
5. Eur J Obstet Gynecol Reprod Biol. 2017 Nov;218:129-36.
6. J Low Genit Tract Dis. 2014 Apr;18(2):115-21.
7. Gynecol Oncol. 2007 Jul;106(1):105-11.
The opioid crisis: Treating pregnant women with addiction
Age cutoff suggested for STI screening in HIV patients
WASHINGTON – Current guidelines recommend a minimum of annual screening for gonorrhea and chlamydia in all sexually active individuals with HIV infection. However, this goal, which is frequently not attained in HIV clinics, may be excessive in certain populations with HIV infection.
In particular, women as well as men who have sex exclusively with women (MSW) may best be served by targeted, age-based screening rather than universal screening, according to a presentation by Susan A. Tuddenham, MD, an assistant professor of medicine at Johns Hopkins University, Baltimore.
“Detection and treatment of gonorrhea and chlamydia in HIV-positive patients in the United States is a priority both because of patient morbidity and because of the potential for these infections to enhance transmission of HIV,” said Dr. Tuddenham.
She and her colleagues assessed data from 16,864 gonorrhea and chlamydia tests of all adults in care at three HIV Research Network sites during 2011-2014. She presented the data at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
They assessed the number needed to screen (NNS) in order to identify a single infection across three risk populations of individuals with HIV infection: 1,123 women, 1,236 men who have sex only with women (MSW), and 3,501 men who have sex with men (MSM). NNS was defined as the number of persons tested divided by the number who tested positive and was calculated for the three risk groups for urogenital and extragenital (rectal and pharyngeal) sampling and by age.
Dr. Tuddenham and her colleagues found that NNS based on urogenital screening was similar in all three groups for those individuals aged younger than or equal to 25 years: 15 for women (95% confidence interval, 9-71); 21 for MSW (95% CI, 6-171); and 20 for MSM (95% CI, 12-36). However at ages greater than 25 years, the picture changed, with urogenital NNS increasing to 363 for women (95% CI, 167-1000); 160 for MSW (95% CI, 100-333). For MSM over the age of 25 years, however, the NNS only increased to 46 (95% CI, 38-56).
There were insufficient numbers of extragenital screenings of women and MSW for analysis. But for MSM, rectal NNS was 5 and 10 for those men aged 25 years and younger and those aged over age 25 years, respectively, and pharyngeal NNS was 8 and 20 for the two groups, respectively.
“Our results provide some support for age-based screening cutoffs for women and MSW, with universal screening appropriate for those less than or equal to 25 years of age, and targeted screening for those over 25,” said Dr. Tuddenham. She emphasized the importance of continued universal screening of MSM of all ages for gonorrhea/chlamydia, in particular using extragenital screening as well in order to capture those missed by urogenital screening alone.
Dr. Tuddenham reported that she had no disclosures.
mlesney@mdedge.com
WASHINGTON – Current guidelines recommend a minimum of annual screening for gonorrhea and chlamydia in all sexually active individuals with HIV infection. However, this goal, which is frequently not attained in HIV clinics, may be excessive in certain populations with HIV infection.
In particular, women as well as men who have sex exclusively with women (MSW) may best be served by targeted, age-based screening rather than universal screening, according to a presentation by Susan A. Tuddenham, MD, an assistant professor of medicine at Johns Hopkins University, Baltimore.
“Detection and treatment of gonorrhea and chlamydia in HIV-positive patients in the United States is a priority both because of patient morbidity and because of the potential for these infections to enhance transmission of HIV,” said Dr. Tuddenham.
She and her colleagues assessed data from 16,864 gonorrhea and chlamydia tests of all adults in care at three HIV Research Network sites during 2011-2014. She presented the data at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
They assessed the number needed to screen (NNS) in order to identify a single infection across three risk populations of individuals with HIV infection: 1,123 women, 1,236 men who have sex only with women (MSW), and 3,501 men who have sex with men (MSM). NNS was defined as the number of persons tested divided by the number who tested positive and was calculated for the three risk groups for urogenital and extragenital (rectal and pharyngeal) sampling and by age.
Dr. Tuddenham and her colleagues found that NNS based on urogenital screening was similar in all three groups for those individuals aged younger than or equal to 25 years: 15 for women (95% confidence interval, 9-71); 21 for MSW (95% CI, 6-171); and 20 for MSM (95% CI, 12-36). However at ages greater than 25 years, the picture changed, with urogenital NNS increasing to 363 for women (95% CI, 167-1000); 160 for MSW (95% CI, 100-333). For MSM over the age of 25 years, however, the NNS only increased to 46 (95% CI, 38-56).
There were insufficient numbers of extragenital screenings of women and MSW for analysis. But for MSM, rectal NNS was 5 and 10 for those men aged 25 years and younger and those aged over age 25 years, respectively, and pharyngeal NNS was 8 and 20 for the two groups, respectively.
“Our results provide some support for age-based screening cutoffs for women and MSW, with universal screening appropriate for those less than or equal to 25 years of age, and targeted screening for those over 25,” said Dr. Tuddenham. She emphasized the importance of continued universal screening of MSM of all ages for gonorrhea/chlamydia, in particular using extragenital screening as well in order to capture those missed by urogenital screening alone.
Dr. Tuddenham reported that she had no disclosures.
mlesney@mdedge.com
WASHINGTON – Current guidelines recommend a minimum of annual screening for gonorrhea and chlamydia in all sexually active individuals with HIV infection. However, this goal, which is frequently not attained in HIV clinics, may be excessive in certain populations with HIV infection.
In particular, women as well as men who have sex exclusively with women (MSW) may best be served by targeted, age-based screening rather than universal screening, according to a presentation by Susan A. Tuddenham, MD, an assistant professor of medicine at Johns Hopkins University, Baltimore.
“Detection and treatment of gonorrhea and chlamydia in HIV-positive patients in the United States is a priority both because of patient morbidity and because of the potential for these infections to enhance transmission of HIV,” said Dr. Tuddenham.
She and her colleagues assessed data from 16,864 gonorrhea and chlamydia tests of all adults in care at three HIV Research Network sites during 2011-2014. She presented the data at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
They assessed the number needed to screen (NNS) in order to identify a single infection across three risk populations of individuals with HIV infection: 1,123 women, 1,236 men who have sex only with women (MSW), and 3,501 men who have sex with men (MSM). NNS was defined as the number of persons tested divided by the number who tested positive and was calculated for the three risk groups for urogenital and extragenital (rectal and pharyngeal) sampling and by age.
Dr. Tuddenham and her colleagues found that NNS based on urogenital screening was similar in all three groups for those individuals aged younger than or equal to 25 years: 15 for women (95% confidence interval, 9-71); 21 for MSW (95% CI, 6-171); and 20 for MSM (95% CI, 12-36). However at ages greater than 25 years, the picture changed, with urogenital NNS increasing to 363 for women (95% CI, 167-1000); 160 for MSW (95% CI, 100-333). For MSM over the age of 25 years, however, the NNS only increased to 46 (95% CI, 38-56).
There were insufficient numbers of extragenital screenings of women and MSW for analysis. But for MSM, rectal NNS was 5 and 10 for those men aged 25 years and younger and those aged over age 25 years, respectively, and pharyngeal NNS was 8 and 20 for the two groups, respectively.
“Our results provide some support for age-based screening cutoffs for women and MSW, with universal screening appropriate for those less than or equal to 25 years of age, and targeted screening for those over 25,” said Dr. Tuddenham. She emphasized the importance of continued universal screening of MSM of all ages for gonorrhea/chlamydia, in particular using extragenital screening as well in order to capture those missed by urogenital screening alone.
Dr. Tuddenham reported that she had no disclosures.
mlesney@mdedge.com
REPORTING FROM THE 2018 STD PREVENTION CONFERENCE
Key clinical point: Men who have sex with men with HIV should be regularly screened for STIs regardless of age.
Major finding: The number needed to screen to detect an STI in individuals infected with HIV aged over 25 years were 363 (women); 160 (men who have sex exclusively with women); and 46 (men who have sex with men).
Study details: Gonorrhea/chlamydia tests were assessed from 16,864 individuals infected with HIV and number needed to screen calculated.
Disclosures: Dr. Tuddenham reported that she had no disclosures.
Product Update: PICO NPWT; Encision; TimerCap; AMA
SURGICAL SITE WOUND THERAPY
PICO NPWT is a negative-pressure wound therapy device to treat surgical site infection (SSI). According to Smith & Nephew, a new meta-analysis demonstrates that the prophylactic application of PICO with AIRLOCK™ Technology significantly reduces surgical site complications by 58%, the rate of dehiscence by 26%, and length of stay by one-half day when compared with standard care.
The PICO System is canister-free and disposable. Patients can be discharged safely with PICO in place. Seven days of therapy are provided in each kit, with 1 pump, 2 dressings, and fixation strips to allow for a dressing change.
PICO uses a 4-layer multifunction dressing design in which the layers work together to ensure that negative pressure is delivered to the wound bed and exudate is removed through absorption and evaporation. Approximately 20% of fluid still remains in the dressing. The top film layer has a high-moisture vapor transmission rate to transpire as much as 80% of the exudate, says Smith & Nephew.
FOR MORE INFORMATION, VISIT: http://www.smith-nephew.com/
SHIELDED LAPAROSCOPIC INSTRUMENTS PREVENT BURNS
Encision’s patented Active Electrode Monitoring (AEM®) Shielded Laparoscopic Instruments eliminate patient burns and the associated complications.
Every 90 minutes in the United States, a patient is severely injured from a stray energy burn during laparoscopic surgery, according to Encision. The AEM® Shielded Instruments are designed to eliminate burns caused by monopolar energy insulation failure and capacitive coupling, reducing complications and re-admissions.
In addition to helping health care professionals improve patient safety in line with a recent FDA safety communication, Active Electrode Monitoring is a recommended practice of AORN and AAGL.
Encision offers a complete line of premium laparoscopic monopolar surgical instruments with integrated AEM® technology as well as complimentary products to improve clinical effectiveness and patient safety, including bipolar and cold instrumentation.
FOR MORE INFORMATION, VISIT: https://www.encision.com/
iSORT: 7-DAY BLUETOOTH PILLBOX
TimerCap has a new Bluetooth-enabled 7-day pill box called the iSort that sends reminders to take medication to a patient’s phone using a free TimerCap App found at the AppStore and Android Market.
The iSort automatically records and stores the times when each door/slot is opened and closed. It knows which door has been used and seamlessly updates the TimerCap App. The app will notify the patient and, if designated, a caregiver, whenever a dose is due or missed using pictures to show what and how many meds are scheduled. More than one iSort box can be used with the app.
iSort provides reminders that help improve adherence to medication dosing instructions and eliminates annoying false alarms, double entries, and unnecessary reminders when pills already have been taken. The portable iSort uses 2 AA batteries that need to be changed about once per year.
FOR MORE INFORMATION, VISIT: https://www.timercap.com/isort
PLATFORM TO COORDINATE HEALTH AND TECHNOLOGY
The American Medical Association (AMA) recently has established a new initiative that introduces a solution to improve, organize, and share health care information. The Integrated Health Model Initiative (IHMI) is a platform that coordinates the health and technology sectors around a common data model. IHMI fills the national imperative to pioneer a shared framework for organizing health data, emphasizing patient-centric information, and refining data elements to those most predictive of better outcomes. The AMA says that evolving available health data to depict a complete picture of a patient’s journey from wellness to illness to treatment and beyond allows health care delivery to fully focus on patient outcomes, goals, and wellness. Participation in IHMI is open to all health care and technology stakeholders.
FOR MORE INFORMATION, VISIT: www.ama-assn.org/ihmi
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
SURGICAL SITE WOUND THERAPY
PICO NPWT is a negative-pressure wound therapy device to treat surgical site infection (SSI). According to Smith & Nephew, a new meta-analysis demonstrates that the prophylactic application of PICO with AIRLOCK™ Technology significantly reduces surgical site complications by 58%, the rate of dehiscence by 26%, and length of stay by one-half day when compared with standard care.
The PICO System is canister-free and disposable. Patients can be discharged safely with PICO in place. Seven days of therapy are provided in each kit, with 1 pump, 2 dressings, and fixation strips to allow for a dressing change.
PICO uses a 4-layer multifunction dressing design in which the layers work together to ensure that negative pressure is delivered to the wound bed and exudate is removed through absorption and evaporation. Approximately 20% of fluid still remains in the dressing. The top film layer has a high-moisture vapor transmission rate to transpire as much as 80% of the exudate, says Smith & Nephew.
FOR MORE INFORMATION, VISIT: http://www.smith-nephew.com/
SHIELDED LAPAROSCOPIC INSTRUMENTS PREVENT BURNS
Encision’s patented Active Electrode Monitoring (AEM®) Shielded Laparoscopic Instruments eliminate patient burns and the associated complications.
Every 90 minutes in the United States, a patient is severely injured from a stray energy burn during laparoscopic surgery, according to Encision. The AEM® Shielded Instruments are designed to eliminate burns caused by monopolar energy insulation failure and capacitive coupling, reducing complications and re-admissions.
In addition to helping health care professionals improve patient safety in line with a recent FDA safety communication, Active Electrode Monitoring is a recommended practice of AORN and AAGL.
Encision offers a complete line of premium laparoscopic monopolar surgical instruments with integrated AEM® technology as well as complimentary products to improve clinical effectiveness and patient safety, including bipolar and cold instrumentation.
FOR MORE INFORMATION, VISIT: https://www.encision.com/
iSORT: 7-DAY BLUETOOTH PILLBOX
TimerCap has a new Bluetooth-enabled 7-day pill box called the iSort that sends reminders to take medication to a patient’s phone using a free TimerCap App found at the AppStore and Android Market.
The iSort automatically records and stores the times when each door/slot is opened and closed. It knows which door has been used and seamlessly updates the TimerCap App. The app will notify the patient and, if designated, a caregiver, whenever a dose is due or missed using pictures to show what and how many meds are scheduled. More than one iSort box can be used with the app.
iSort provides reminders that help improve adherence to medication dosing instructions and eliminates annoying false alarms, double entries, and unnecessary reminders when pills already have been taken. The portable iSort uses 2 AA batteries that need to be changed about once per year.
FOR MORE INFORMATION, VISIT: https://www.timercap.com/isort
PLATFORM TO COORDINATE HEALTH AND TECHNOLOGY
The American Medical Association (AMA) recently has established a new initiative that introduces a solution to improve, organize, and share health care information. The Integrated Health Model Initiative (IHMI) is a platform that coordinates the health and technology sectors around a common data model. IHMI fills the national imperative to pioneer a shared framework for organizing health data, emphasizing patient-centric information, and refining data elements to those most predictive of better outcomes. The AMA says that evolving available health data to depict a complete picture of a patient’s journey from wellness to illness to treatment and beyond allows health care delivery to fully focus on patient outcomes, goals, and wellness. Participation in IHMI is open to all health care and technology stakeholders.
FOR MORE INFORMATION, VISIT: www.ama-assn.org/ihmi
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
SURGICAL SITE WOUND THERAPY
PICO NPWT is a negative-pressure wound therapy device to treat surgical site infection (SSI). According to Smith & Nephew, a new meta-analysis demonstrates that the prophylactic application of PICO with AIRLOCK™ Technology significantly reduces surgical site complications by 58%, the rate of dehiscence by 26%, and length of stay by one-half day when compared with standard care.
The PICO System is canister-free and disposable. Patients can be discharged safely with PICO in place. Seven days of therapy are provided in each kit, with 1 pump, 2 dressings, and fixation strips to allow for a dressing change.
PICO uses a 4-layer multifunction dressing design in which the layers work together to ensure that negative pressure is delivered to the wound bed and exudate is removed through absorption and evaporation. Approximately 20% of fluid still remains in the dressing. The top film layer has a high-moisture vapor transmission rate to transpire as much as 80% of the exudate, says Smith & Nephew.
FOR MORE INFORMATION, VISIT: http://www.smith-nephew.com/
SHIELDED LAPAROSCOPIC INSTRUMENTS PREVENT BURNS
Encision’s patented Active Electrode Monitoring (AEM®) Shielded Laparoscopic Instruments eliminate patient burns and the associated complications.
Every 90 minutes in the United States, a patient is severely injured from a stray energy burn during laparoscopic surgery, according to Encision. The AEM® Shielded Instruments are designed to eliminate burns caused by monopolar energy insulation failure and capacitive coupling, reducing complications and re-admissions.
In addition to helping health care professionals improve patient safety in line with a recent FDA safety communication, Active Electrode Monitoring is a recommended practice of AORN and AAGL.
Encision offers a complete line of premium laparoscopic monopolar surgical instruments with integrated AEM® technology as well as complimentary products to improve clinical effectiveness and patient safety, including bipolar and cold instrumentation.
FOR MORE INFORMATION, VISIT: https://www.encision.com/
iSORT: 7-DAY BLUETOOTH PILLBOX
TimerCap has a new Bluetooth-enabled 7-day pill box called the iSort that sends reminders to take medication to a patient’s phone using a free TimerCap App found at the AppStore and Android Market.
The iSort automatically records and stores the times when each door/slot is opened and closed. It knows which door has been used and seamlessly updates the TimerCap App. The app will notify the patient and, if designated, a caregiver, whenever a dose is due or missed using pictures to show what and how many meds are scheduled. More than one iSort box can be used with the app.
iSort provides reminders that help improve adherence to medication dosing instructions and eliminates annoying false alarms, double entries, and unnecessary reminders when pills already have been taken. The portable iSort uses 2 AA batteries that need to be changed about once per year.
FOR MORE INFORMATION, VISIT: https://www.timercap.com/isort
PLATFORM TO COORDINATE HEALTH AND TECHNOLOGY
The American Medical Association (AMA) recently has established a new initiative that introduces a solution to improve, organize, and share health care information. The Integrated Health Model Initiative (IHMI) is a platform that coordinates the health and technology sectors around a common data model. IHMI fills the national imperative to pioneer a shared framework for organizing health data, emphasizing patient-centric information, and refining data elements to those most predictive of better outcomes. The AMA says that evolving available health data to depict a complete picture of a patient’s journey from wellness to illness to treatment and beyond allows health care delivery to fully focus on patient outcomes, goals, and wellness. Participation in IHMI is open to all health care and technology stakeholders.
FOR MORE INFORMATION, VISIT: www.ama-assn.org/ihmi
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
The techno vagina: The laser and radiofrequency device boom in gynecology
In recent years, an increasing number of laser and radiofrequency device outpatient treatments have been heralded as safe and effective interventions for various gynecologic conditions. Laser devices and radiofrequency technology rapidly have been incorporated into certain clinical settings, including medical practices specializing in dermatology, plastic surgery, and gynecology. While this developing technology has excellent promise, many clinical and research questions remain unanswered.

Concerns about energy-based vaginal treatments
Although marketing material often suggests otherwise, most laser and radiofrequency devices are cleared by the US Food and Drug Administration (FDA) only for nonspecific gynecologic and hematologic interventions. However, both laser and radiofrequency device treatments, performed as outpatient procedures, have been touted as appropriate interventions for many conditions, including female sexual dysfunction, arousal and orgasmic concerns, vaginal laxity, vaginismus, lichen sclerosus, urinary incontinence, and vulvar vestibulitis.
Well-designed studies are needed. Prospective, randomized sham-controlled trials of energy-based devices are rare, and most data in the public domain are derived from case series. Many studies are of short duration with limited follow-up. Randomized controlled trials therefore are warranted and should have stringent inclusion and exclusion criteria. Body dysmorphic syndrome, for example, should be a trial exclusion. Study design for research should include the use of standardized, validated scales and long-term follow-up of participants.
Which specialists have the expertise to offer treatment? Important ethical and medical concerns regarding the technology need to be addressed. A prime concern is determining which health care professional specialist is best qualified to assess and treat underlying gynecologic conditions. It is not uncommon to see internists, emergency medicine providers, family physicians, plastic surgeons, psychiatrists, and dermatologists self-proclaiming their gynecologic “vaginal rejuvenation” expertise.
In my experience, some ObGyns have voiced concern about the diverse medical specialties involved in performing these procedures. Currently, no standard level of training is required to perform them. In addition, those providers lack the training needed to adequately and accurately assess the potential for confounding, underlying gynecologic pathology, and they are inadequately trained to offer patients the full gamut of therapeutic interventions. Many may be unfamiliar with female pelvic anatomy and sexual function and a multidisciplinary treatment paradigm.
We need established standards. A common vernacular, nosology, classification, and decision-tree assessment paradigm for genitopelvic laxity (related to the condition of the pelvic floor and not simply a loose feeling in the vagina) is lacking, which may make research and peer-to-peer discussions difficult.
Which patients are appropriate candidates? Proper patient selection criteria for energy-based vaginal treatment have not been standardized, yet this remains a paramount need. A comprehensive patient evaluation should be performed and include a discussion on the difference between an aesthetic complaint and a functional medical problem. Assessment should include the patient’s level of concern or distress and the impact of her symptoms on her overall quality of life. Patients should be evaluated for body dysmorphic syndrome and relationship discord. A complete physical examination, including a detailed pelvic assessment, often is indicated. A treatment algorithm that incorporates conservative therapies coupled with medical, technologic, and psychologic interventions also should be developed.
Various energy-based devices are available for outpatient procedures
Although the number of procedures performed (such as vaginal rejuvenation, labiaplasty, vulvar liposculpturing, hymenoplasty, G-spot amplification, and O-Shot treatment) for both cosmetic and functional problems has increased, the published scientific data on the procedures’ short- and long-term efficacy and safety are limited. The American College of Obstetricians and Gynecologists (ACOG) published a committee opinion stating that many of these procedures, including “vaginal rejuvenation,” may not be considered medically indicated and may lack scientific merit or ample supportive data to confirm their efficacy and safety.1 ObGyns should proceed with caution before incorporating these technologic treatments into their medical practice.
Much diversity exists within the device-technology space. The underpinnings of each device vary regarding their proposed mechanism of action and theoretical therapeutic and tissue effect. In device marketing materials, many devices have been claimed to have effects on multiple tissue types (for example, both vaginal mucosa and vulvar tissue), whereas others are said to have more focal and localized effects (that is, targeted behind the hymenal ring). Some are marketed as a one-time treatment, while others require multiple repeated treatments over an extended period. When it comes to published data, adverse effect reporting remains limited and follow-up data often are short term.
Radiofrequency and laser devices are separate and very distinct technologies with similar and differing proposed utilizations. Combining radiofrequency and laser treatments in tandem or sequentially may have clinical utility, but long-term safety may be a concern for lasers.
Radiofrequency-based devices
Typically, radiofrequency device treatments:
- are used for outpatient procedures
- do not require topical anesthesia
- are constructed to emit focused electromagnetic waves
- are applied to vaginal, vulvar, or vaginal introital or vestibular tissue
- deliver energy to the deeper connective tissue of the vaginal wall architecture.
Radiofrequency device energy can be monopolar, unipolar, bipolar, or multipolar depending on design. Design also dictates current and the number of electrodes that pass from the device to the grounding pad. Monopolar is the only type of radiofrequency that has a grounding pad; bipolar and multipolar energy returns to the treatment tip.
Radiofrequency devices typically are FDA 510(k)-cleared devices for nonspecific electrocoagulation and hemostasis for surgical procedures. None are currently FDA cleared in the United States for the treatment of vaginal or vulvar laxity or genitourinary syndrome of menopause (GSM). These energy-based devices aim to induce collagen contraction, neocollagenesis, vascularization, and growth factor infiltration to restore the elasticity and moisture of the underlying vaginal mucosa. Heat shock protein activation and inflammation activation are thought to be the underlying mechanisms of action.2–5
Treatment outcomes with 2 radiofrequency devices
Multiple prospective small case series studies have reported outcomes of women treated with the ThermiVa (ThermiAesthetics LLC) radiofrequency system.3,4 Typically, 3 treatments (with a between-treatment interval of 4 to 6 weeks) were applied. The clinical end point temperature had a range of 40°C to 45°C, which was maintained for 3 to 5 minutes per treated zone during 30 minutes’ total treatment time.
Some participants self-reported improvement in vaginal laxity symptoms with the 3 treatments. In addition, women reported subjective improvements in both vaginal atrophy symptoms and sexual function, including positive effect in multiple domains. No serious adverse events were reported in these case series. However, there was no placebo-controlled arm, and validated questionnaires were not used in much of this research.3,4
In another trial, the ThermiVa system was studied in a cohort of 25 sexually active women with self-reported anorgasmia or increased latency to orgasmic response.6 Participants received 3 treatments 4 weeks apart. Approximately three-quarters of the participants reported improved orgasmic responsivity, vaginal lubrication, and clitoral sensitivity. Notably, this research did not use validated questionnaires or a placebo or sham-controlled design. The authors suggested sustained treatment benefits at 9 to 12 months. While repeat treatment was advocated, data were lacking to support the optimal time for repeat treatment efficacy.6
A cryogen-cooled monopolar radiofrequency device, the Viveve system (Viveve Medical, Inc) differs from other radiofrequency procedures because it systematically cryogen cools and protects the surface of the vaginal mucosal tissue while heating the underlying structures.
The Viveve system was evaluated in 2 small pilot studies (24 and 30 participants) and in a large, randomized, sham-controlled, prospective trial that included 108 participants (VIVEVE I trial).5,7,8 Results from both preliminary small studies indicated that participants experienced significant improvement in overall sexual function at 6 months. In one of the small studies (in Japanese women), sustained efficacy at 12 months posttreatment was reported.7 Neither small study included a placebo-control arm, but they did include the use of validated questionnaires.
In the VIVEVE I trial (a multicenter international study), treatment in the active group consisted of a single, 30-minute outpatient procedure that delivered 90 J/cm2 of radiofrequency energy at the level just behind the hymenal ring behind the vaginal introitus. The sham-treated group received ≤1 J/cm2 of energy with a similar machine tip.8
Statistically significant improvements were reported in the arousal and orgasm domains of the validated Female Sexual Function Index (FSFI) for the active-treatment group compared with the sham-treated group. In addition, there were statistically significant differences in the FSFI and the Female Sexual Distress Scale–Revised total scores in favor of active treatment. Participants in the active-treatment arm reported statistically significant improvement in overall sexual satisfaction coupled with lowered overall sexual distress.8
These data are provocative, since the Viveve treatment demonstrated superior efficacy compared with the sham treatment, and prior evidence demonstrated that medical device trials employing a sham arm often demonstrate particularly large placebo/sham effects.9 A confirmatory randomized, sham-controlled multicenter US-based trial is currently underway. At present, the VIVEVE I trial remains the only published, large-scale, randomized, sham-controlled, blinded study of a radiofrequency-based treatment.
New emerging data support the efficacy and safety of this specific radiofrequency treatment in patients with mild to moderate urinary stress incontinence; further studies confirming these outcomes are anticipated. The Viveve system is approved in many countries for various conditions, including urinary incontinence (1 country), sexual function (17 countries), vaginal laxity (41 countries), and electrocoagulation and hemostasis (4 countries, including the United States).
Laser technology devices
Laser (Light Amplification by Stimulated Emission of Radiation) therapy, which uses a carbon dioxide (CO2), argon, YAG, or erbium energy source, also is currently marketed as a method to improve various gynecologic conditions, including genital pelvic relaxation syndrome, vaginal laxity, GSM, lichen sclerosus, and sexual problems such as dyspareunia and arousal or orgasmic disorders.
The CO2 laser therapy device, such as the MonaLisa Touch (DEKA Laser), appears to be very popular and widely available. It delivers fractional CO2 laser energy to the vaginal wall, creating sequential micro traumas that subsequently undergo a healing reaction; the newly healed area has an improved underlying tissue architecture (but at a superficial level). The laser’s proposed mechanism of action is that it ablates only a minute fraction of the superficial lamina propria; it acts primarily to stimulate rapid healing of the tissue, creating new collagen and elastic fibers. There is no evidence of scarring.10
Treatment outcomes with laser device therapy
Authors of a 2017 study series of CO2 laser treatments in women with moderate to severe GSM found that 84% of participants experienced significant improvement in sexual function, dyspareunia, and otherwise unspecified sexual issues from pretreatment to 12 to 24 months posttreatment.11 These findings are consistent with several other case series and provide supportive evidence for the efficacy and safety of CO2 laser therapy. This technology may be appropriate for the treatment of GSM.
Laser technology shows excellent promise for the treatment of GSM symptoms by virtue of its superficial mechanism of action. In addition, several trials have demonstrated efficacy and safety in breast cancer patient populations.12 This is particularly interesting since breast cancer treatments, such as aromatase inhibitors (considered a mainstay of cancer treatment), can cause severe atrophic vaginitis. Breast cancer survivors often avoid minimally absorbed local vaginal hormonal products, and over-the-counter products (moisturizers and lubricants) are not widely accepted. Hence, a nonhormonal treatment for distressing GSM symptoms is welcomed in this population.
Pagano and colleagues recently studied 82 breast cancer survivors in whom treatment with vaginal moisturizers and lubricants failed.12 Participants underwent 3 laser treatment cycles approximately 30 to 40 days apart; they demonstrated improvements in vaginal dryness, vaginal itchiness, stinging, dyspareunia, and reduced sensitivity.
Microablative fractional CO2 laser may help reestablish a normative vaginal microbiome by altering the prevalence of lactobacillus species and reestablishing a normative postmenopausal vaginal flora.13
The tracking and reporting of adverse events associated with laser procedures has been less than optimal. In my personal clinical experience, consequences from both short- and long-term laser treatments have included vaginal canal agglutination, worsening dyspareunia, and constricture causing vaginal hemorrhage.
Cruz and colleagues recently conducted a randomized, double-blind, placebo-controlled clinical trial designed to evaluate the efficacy of fractional CO2 laser compared with topical estriol and laser plus estriol for the treatment of vaginal atrophy in 45 postmenopausal women.14 They found statistically significant differences in dyspareunia, dryness, and burning compared with baseline levels in all 3 treatment groups. Results with the fractional CO2 laser treatment were deemed to be similar to those of the topical estriol and the combined therapy.14
By contrast, an erbium (Er):YAG laser, such as the IntimaLase (Fotona, LLC) laser, functions by heating the pelvic tissue and collagen within the introitus and vaginal canal.15,16 When the underlying collagen is heated, the fibers are thought to thicken and shorten, which may result in immediate contracture of the treated tissue. Additionally, this process stimulates the existing collagen to undergo remodeling and it also may cause neocollagen deposition.15 In a general review of gynecologic procedures, after 1 to 4 treatment sessions (depending on the study), most patients reported improved sexual satisfaction or vaginal tightness.15
Although trials have included small numbers of patients, early evidence suggests some lasers with reportedly deeper penetration may be useful for treatment of vaginal laxity, but further studies are needed. In smaller studies, the Er:YAG laser has shown efficacy and safety in the treatment of stress urinary incontinence and improved lower urinary tract symptoms, quality of life, and sexual function.16,17
Insurance does not cover energy-based treatment costs
Currently, both laser and radiofrequency device treatments are considered fee-for-service interventions. Radiofrequency and laser treatments for gynecologic conditions are not covered by health insurance, and treatment costs can be prohibitive for many patients. In addition, the long-term safety of these treatments remains to be studied further, and the optimal time for a repeat procedure has yet to be elucidated.
The FDA cautions against energy-based procedures
In July 2018, the FDA released a statement of concern reiterating the need for research and randomized clinical trials before energy-based device treatments can be widely accepted, and that they are currently cleared only for general gynecologic indications and not for disorders and symptoms related to menopause, urinary incontinence, or sexual function.18
The FDA stated that “we have not cleared or approved for marketing any energy-based devices to treat these symptoms or conditions [vaginal laxity; vaginal atrophy, dryness, or itching; pain during sexual intercourse; pain during urination; decreased sexual sensation], or any symptoms related to menopause, urinary incontinence, or sexual function.” The FDA noted that serious complications have been reported, including vaginal burns, scarring, pain during sexual intercourse, and recurring, chronic pain. The FDA issued letters to 7 companies regarding concerns about the marketing of their devices for off-label use and promotion.
Several societies have responded. ACOG reaffirmed its 2016 position statement on fractional laser treatment of vulvovaginal atrophy.19 JoAnn Pinkerton, MD, Executive Director of The North American Menopause Society (NAMS), and Sheryl Kingsberg, PhD, President of NAMS, alerted their members that both health care professionals and consumers should tread cautiously, and they encouraged scrutiny of existing evidence as all energy-based treatments are not created equal.20 They noted that some research does exist and cited 2 randomized, sham-controlled clinical trials that have been published.
Looking forward
Various novel technologic therapies are entering the gynecologic market. ObGyns must critically evaluate these emerging technologies with a keen understanding of their underlying mechanism of action, the level of scientific evidence, and the treatment’s proposed therapeutic value.
Radiofrequency energy devices appear to be better positioned to treat urinary incontinence and vaginal relaxation syndrome because of their capability for deep tissue penetration. Current data show that laser technology has excellent promise for the treatment and management of GSM. Both technologies warrant further investigation in long-term randomized, sham-controlled trials that assess efficacy and safety with validated instruments over an extended period. In addition, should these technologies prove useful in the overall treatment armamentarium for gynecologic conditions, the question of affordability and insurance coverage needs to be addressed.
ObGyns must advocate for female sexual wellness and encourage a comprehensive multidisciplinary team approach for offering various therapies. Ultimately, responsible use of evidence-based innovative technology should be incorporated into the treatment paradigm.
Despite recent technologic advancements and applications in gynecologic care, minimally absorbed local vaginal hormonal products (creams, rings, intravaginal tablets) and estrogen agonists/antagonists remain the mainstay and frontline treatment for moderate to severe dyspareunia, a symptom of vulvovaginal atrophy due to menopause. Newer medications, such as intravaginal steroids1 and the recently approved bioidentical estradiol nonapplicator vaginal inserts,2 also offer excellent efficacy and safety in the treatment of this condition. These medications now are included under expanded insurance coverage, and they offer safe, simple, and cost-effective treatments for this underdiagnosed condition.
References
- Intrarosa [package insert]. Waltham, MA: AMAG Pharmaceuticals Inc; February 2018.
- Imvexxy [package insert]. Boca Raton, FL: TherapeuticsMD; 2018.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- ACOG Committee on Gynecologic Practice. ACOG Committee Opinion No. 378: Vaginal "rejuvenation" and cosmetic vaginal procedures. Obstet Gynecol. 2007;110(3):737-738.
- Dunbar SW, Goldberg DJ. Radiofrequency in cosmetic dermatology: an update. J Drugs Dermatol. 2015;14(11):1229-1238.
- Leibaschoff G, Izasa PG, Cardona JL, Miklos JR, Moore RD. Transcutaneous temperature-controlled radiofrequency (TTCRF) for the treatment of menopausal vaginal/genitourinary symptoms. Surg Technol Int. 2016;29:149-159.
- Alinsod RM. Temperature controlled radiofrequency for vulvovaginal laxity. Prime J. July 23, 2015. https://www.prime-journal.com/temperature-controlled-radiofrequency-for-vulvovaginal-laxity/. Accessed August 15, 2018.
- Millheiser LS, Pauls RN, Herbst SJ, Chen BH. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7(9):3088-3095.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48(7):641-645.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22 (9):775-781 .
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14(2):215-225.
- Kaptchuk TJ, Goldman P, Stone DA, Statson WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53(8): 786-792.
- Gotkin RH, Sarnoff SD, Cannarozzo G, Sadick NS, Alexiades-Armenakas M. Ablative skin resurfacing with a novel microablative CO2 laser. J Drugs Dermatol. 2009;8(2):138-144.
- Behnia-Willison F, Sarraf S, Miller J, et al. Safety and long-term efficacy of fractional CO2 laser treatment in women suffering from genitourinary syndrome of menopause. Eur J Obstet Gynecol Reprod Biol. 2017;213:39-44.
- Pagano T, De Rosa P, Vallone R, et al. Fractional microablative CO2 laser in breast cancer survivors affected by iatrogenic vulvovaginal atrophy after failure of nonestrogenic local treatments, a retrospective study. Menopause. 2018;25(6):657-662.
- Anthanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climateric. 2016;19(5):512-518.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25(1): 21-28.
- Vizintin Z, Rivera M, Fistonic I, et al. Novel minimally invasive VSP Er:YAG laser treatments in gynecology. J Laser Health Acad. 2012;2012(1):46-58.
- Tien YM, Hsio SM, Lee CN, Lin HH. Effects of laser procedure for female urodynamic stress incontinence on pad weight, urodynamics, and sexual function. Int Urogynecol J. 2017;28(3):469-476.
- Oginc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Laser Surg Med. 2015;47(9):689-697.
- US Food and Drug Administration. FDA warns against use of energy based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed August 16, 2018.
- The American College of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and US Food and Drug Administration clearance: position statement. May 2016. https://www.acog.org/Clinical-Guidance-and-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Accessed August 16, 2018.
- The North American Menopause Society. FDA mandating vaginal laser manufacturers present valid data before marketing. August 1, 2018. https://www.menopause.org/docs/default-source/default-document-library/nams-responds-to-fda-mandate-on-vaginal-laser-manufacturers-08-01-2018.pdf. Accessed August 16, 2018.
In recent years, an increasing number of laser and radiofrequency device outpatient treatments have been heralded as safe and effective interventions for various gynecologic conditions. Laser devices and radiofrequency technology rapidly have been incorporated into certain clinical settings, including medical practices specializing in dermatology, plastic surgery, and gynecology. While this developing technology has excellent promise, many clinical and research questions remain unanswered.

Concerns about energy-based vaginal treatments
Although marketing material often suggests otherwise, most laser and radiofrequency devices are cleared by the US Food and Drug Administration (FDA) only for nonspecific gynecologic and hematologic interventions. However, both laser and radiofrequency device treatments, performed as outpatient procedures, have been touted as appropriate interventions for many conditions, including female sexual dysfunction, arousal and orgasmic concerns, vaginal laxity, vaginismus, lichen sclerosus, urinary incontinence, and vulvar vestibulitis.
Well-designed studies are needed. Prospective, randomized sham-controlled trials of energy-based devices are rare, and most data in the public domain are derived from case series. Many studies are of short duration with limited follow-up. Randomized controlled trials therefore are warranted and should have stringent inclusion and exclusion criteria. Body dysmorphic syndrome, for example, should be a trial exclusion. Study design for research should include the use of standardized, validated scales and long-term follow-up of participants.
Which specialists have the expertise to offer treatment? Important ethical and medical concerns regarding the technology need to be addressed. A prime concern is determining which health care professional specialist is best qualified to assess and treat underlying gynecologic conditions. It is not uncommon to see internists, emergency medicine providers, family physicians, plastic surgeons, psychiatrists, and dermatologists self-proclaiming their gynecologic “vaginal rejuvenation” expertise.
In my experience, some ObGyns have voiced concern about the diverse medical specialties involved in performing these procedures. Currently, no standard level of training is required to perform them. In addition, those providers lack the training needed to adequately and accurately assess the potential for confounding, underlying gynecologic pathology, and they are inadequately trained to offer patients the full gamut of therapeutic interventions. Many may be unfamiliar with female pelvic anatomy and sexual function and a multidisciplinary treatment paradigm.
We need established standards. A common vernacular, nosology, classification, and decision-tree assessment paradigm for genitopelvic laxity (related to the condition of the pelvic floor and not simply a loose feeling in the vagina) is lacking, which may make research and peer-to-peer discussions difficult.
Which patients are appropriate candidates? Proper patient selection criteria for energy-based vaginal treatment have not been standardized, yet this remains a paramount need. A comprehensive patient evaluation should be performed and include a discussion on the difference between an aesthetic complaint and a functional medical problem. Assessment should include the patient’s level of concern or distress and the impact of her symptoms on her overall quality of life. Patients should be evaluated for body dysmorphic syndrome and relationship discord. A complete physical examination, including a detailed pelvic assessment, often is indicated. A treatment algorithm that incorporates conservative therapies coupled with medical, technologic, and psychologic interventions also should be developed.
Various energy-based devices are available for outpatient procedures
Although the number of procedures performed (such as vaginal rejuvenation, labiaplasty, vulvar liposculpturing, hymenoplasty, G-spot amplification, and O-Shot treatment) for both cosmetic and functional problems has increased, the published scientific data on the procedures’ short- and long-term efficacy and safety are limited. The American College of Obstetricians and Gynecologists (ACOG) published a committee opinion stating that many of these procedures, including “vaginal rejuvenation,” may not be considered medically indicated and may lack scientific merit or ample supportive data to confirm their efficacy and safety.1 ObGyns should proceed with caution before incorporating these technologic treatments into their medical practice.
Much diversity exists within the device-technology space. The underpinnings of each device vary regarding their proposed mechanism of action and theoretical therapeutic and tissue effect. In device marketing materials, many devices have been claimed to have effects on multiple tissue types (for example, both vaginal mucosa and vulvar tissue), whereas others are said to have more focal and localized effects (that is, targeted behind the hymenal ring). Some are marketed as a one-time treatment, while others require multiple repeated treatments over an extended period. When it comes to published data, adverse effect reporting remains limited and follow-up data often are short term.
Radiofrequency and laser devices are separate and very distinct technologies with similar and differing proposed utilizations. Combining radiofrequency and laser treatments in tandem or sequentially may have clinical utility, but long-term safety may be a concern for lasers.
Radiofrequency-based devices
Typically, radiofrequency device treatments:
- are used for outpatient procedures
- do not require topical anesthesia
- are constructed to emit focused electromagnetic waves
- are applied to vaginal, vulvar, or vaginal introital or vestibular tissue
- deliver energy to the deeper connective tissue of the vaginal wall architecture.
Radiofrequency device energy can be monopolar, unipolar, bipolar, or multipolar depending on design. Design also dictates current and the number of electrodes that pass from the device to the grounding pad. Monopolar is the only type of radiofrequency that has a grounding pad; bipolar and multipolar energy returns to the treatment tip.
Radiofrequency devices typically are FDA 510(k)-cleared devices for nonspecific electrocoagulation and hemostasis for surgical procedures. None are currently FDA cleared in the United States for the treatment of vaginal or vulvar laxity or genitourinary syndrome of menopause (GSM). These energy-based devices aim to induce collagen contraction, neocollagenesis, vascularization, and growth factor infiltration to restore the elasticity and moisture of the underlying vaginal mucosa. Heat shock protein activation and inflammation activation are thought to be the underlying mechanisms of action.2–5
Treatment outcomes with 2 radiofrequency devices
Multiple prospective small case series studies have reported outcomes of women treated with the ThermiVa (ThermiAesthetics LLC) radiofrequency system.3,4 Typically, 3 treatments (with a between-treatment interval of 4 to 6 weeks) were applied. The clinical end point temperature had a range of 40°C to 45°C, which was maintained for 3 to 5 minutes per treated zone during 30 minutes’ total treatment time.
Some participants self-reported improvement in vaginal laxity symptoms with the 3 treatments. In addition, women reported subjective improvements in both vaginal atrophy symptoms and sexual function, including positive effect in multiple domains. No serious adverse events were reported in these case series. However, there was no placebo-controlled arm, and validated questionnaires were not used in much of this research.3,4
In another trial, the ThermiVa system was studied in a cohort of 25 sexually active women with self-reported anorgasmia or increased latency to orgasmic response.6 Participants received 3 treatments 4 weeks apart. Approximately three-quarters of the participants reported improved orgasmic responsivity, vaginal lubrication, and clitoral sensitivity. Notably, this research did not use validated questionnaires or a placebo or sham-controlled design. The authors suggested sustained treatment benefits at 9 to 12 months. While repeat treatment was advocated, data were lacking to support the optimal time for repeat treatment efficacy.6
A cryogen-cooled monopolar radiofrequency device, the Viveve system (Viveve Medical, Inc) differs from other radiofrequency procedures because it systematically cryogen cools and protects the surface of the vaginal mucosal tissue while heating the underlying structures.
The Viveve system was evaluated in 2 small pilot studies (24 and 30 participants) and in a large, randomized, sham-controlled, prospective trial that included 108 participants (VIVEVE I trial).5,7,8 Results from both preliminary small studies indicated that participants experienced significant improvement in overall sexual function at 6 months. In one of the small studies (in Japanese women), sustained efficacy at 12 months posttreatment was reported.7 Neither small study included a placebo-control arm, but they did include the use of validated questionnaires.
In the VIVEVE I trial (a multicenter international study), treatment in the active group consisted of a single, 30-minute outpatient procedure that delivered 90 J/cm2 of radiofrequency energy at the level just behind the hymenal ring behind the vaginal introitus. The sham-treated group received ≤1 J/cm2 of energy with a similar machine tip.8
Statistically significant improvements were reported in the arousal and orgasm domains of the validated Female Sexual Function Index (FSFI) for the active-treatment group compared with the sham-treated group. In addition, there were statistically significant differences in the FSFI and the Female Sexual Distress Scale–Revised total scores in favor of active treatment. Participants in the active-treatment arm reported statistically significant improvement in overall sexual satisfaction coupled with lowered overall sexual distress.8
These data are provocative, since the Viveve treatment demonstrated superior efficacy compared with the sham treatment, and prior evidence demonstrated that medical device trials employing a sham arm often demonstrate particularly large placebo/sham effects.9 A confirmatory randomized, sham-controlled multicenter US-based trial is currently underway. At present, the VIVEVE I trial remains the only published, large-scale, randomized, sham-controlled, blinded study of a radiofrequency-based treatment.
New emerging data support the efficacy and safety of this specific radiofrequency treatment in patients with mild to moderate urinary stress incontinence; further studies confirming these outcomes are anticipated. The Viveve system is approved in many countries for various conditions, including urinary incontinence (1 country), sexual function (17 countries), vaginal laxity (41 countries), and electrocoagulation and hemostasis (4 countries, including the United States).
Laser technology devices
Laser (Light Amplification by Stimulated Emission of Radiation) therapy, which uses a carbon dioxide (CO2), argon, YAG, or erbium energy source, also is currently marketed as a method to improve various gynecologic conditions, including genital pelvic relaxation syndrome, vaginal laxity, GSM, lichen sclerosus, and sexual problems such as dyspareunia and arousal or orgasmic disorders.
The CO2 laser therapy device, such as the MonaLisa Touch (DEKA Laser), appears to be very popular and widely available. It delivers fractional CO2 laser energy to the vaginal wall, creating sequential micro traumas that subsequently undergo a healing reaction; the newly healed area has an improved underlying tissue architecture (but at a superficial level). The laser’s proposed mechanism of action is that it ablates only a minute fraction of the superficial lamina propria; it acts primarily to stimulate rapid healing of the tissue, creating new collagen and elastic fibers. There is no evidence of scarring.10
Treatment outcomes with laser device therapy
Authors of a 2017 study series of CO2 laser treatments in women with moderate to severe GSM found that 84% of participants experienced significant improvement in sexual function, dyspareunia, and otherwise unspecified sexual issues from pretreatment to 12 to 24 months posttreatment.11 These findings are consistent with several other case series and provide supportive evidence for the efficacy and safety of CO2 laser therapy. This technology may be appropriate for the treatment of GSM.
Laser technology shows excellent promise for the treatment of GSM symptoms by virtue of its superficial mechanism of action. In addition, several trials have demonstrated efficacy and safety in breast cancer patient populations.12 This is particularly interesting since breast cancer treatments, such as aromatase inhibitors (considered a mainstay of cancer treatment), can cause severe atrophic vaginitis. Breast cancer survivors often avoid minimally absorbed local vaginal hormonal products, and over-the-counter products (moisturizers and lubricants) are not widely accepted. Hence, a nonhormonal treatment for distressing GSM symptoms is welcomed in this population.
Pagano and colleagues recently studied 82 breast cancer survivors in whom treatment with vaginal moisturizers and lubricants failed.12 Participants underwent 3 laser treatment cycles approximately 30 to 40 days apart; they demonstrated improvements in vaginal dryness, vaginal itchiness, stinging, dyspareunia, and reduced sensitivity.
Microablative fractional CO2 laser may help reestablish a normative vaginal microbiome by altering the prevalence of lactobacillus species and reestablishing a normative postmenopausal vaginal flora.13
The tracking and reporting of adverse events associated with laser procedures has been less than optimal. In my personal clinical experience, consequences from both short- and long-term laser treatments have included vaginal canal agglutination, worsening dyspareunia, and constricture causing vaginal hemorrhage.
Cruz and colleagues recently conducted a randomized, double-blind, placebo-controlled clinical trial designed to evaluate the efficacy of fractional CO2 laser compared with topical estriol and laser plus estriol for the treatment of vaginal atrophy in 45 postmenopausal women.14 They found statistically significant differences in dyspareunia, dryness, and burning compared with baseline levels in all 3 treatment groups. Results with the fractional CO2 laser treatment were deemed to be similar to those of the topical estriol and the combined therapy.14
By contrast, an erbium (Er):YAG laser, such as the IntimaLase (Fotona, LLC) laser, functions by heating the pelvic tissue and collagen within the introitus and vaginal canal.15,16 When the underlying collagen is heated, the fibers are thought to thicken and shorten, which may result in immediate contracture of the treated tissue. Additionally, this process stimulates the existing collagen to undergo remodeling and it also may cause neocollagen deposition.15 In a general review of gynecologic procedures, after 1 to 4 treatment sessions (depending on the study), most patients reported improved sexual satisfaction or vaginal tightness.15
Although trials have included small numbers of patients, early evidence suggests some lasers with reportedly deeper penetration may be useful for treatment of vaginal laxity, but further studies are needed. In smaller studies, the Er:YAG laser has shown efficacy and safety in the treatment of stress urinary incontinence and improved lower urinary tract symptoms, quality of life, and sexual function.16,17
Insurance does not cover energy-based treatment costs
Currently, both laser and radiofrequency device treatments are considered fee-for-service interventions. Radiofrequency and laser treatments for gynecologic conditions are not covered by health insurance, and treatment costs can be prohibitive for many patients. In addition, the long-term safety of these treatments remains to be studied further, and the optimal time for a repeat procedure has yet to be elucidated.
The FDA cautions against energy-based procedures
In July 2018, the FDA released a statement of concern reiterating the need for research and randomized clinical trials before energy-based device treatments can be widely accepted, and that they are currently cleared only for general gynecologic indications and not for disorders and symptoms related to menopause, urinary incontinence, or sexual function.18
The FDA stated that “we have not cleared or approved for marketing any energy-based devices to treat these symptoms or conditions [vaginal laxity; vaginal atrophy, dryness, or itching; pain during sexual intercourse; pain during urination; decreased sexual sensation], or any symptoms related to menopause, urinary incontinence, or sexual function.” The FDA noted that serious complications have been reported, including vaginal burns, scarring, pain during sexual intercourse, and recurring, chronic pain. The FDA issued letters to 7 companies regarding concerns about the marketing of their devices for off-label use and promotion.
Several societies have responded. ACOG reaffirmed its 2016 position statement on fractional laser treatment of vulvovaginal atrophy.19 JoAnn Pinkerton, MD, Executive Director of The North American Menopause Society (NAMS), and Sheryl Kingsberg, PhD, President of NAMS, alerted their members that both health care professionals and consumers should tread cautiously, and they encouraged scrutiny of existing evidence as all energy-based treatments are not created equal.20 They noted that some research does exist and cited 2 randomized, sham-controlled clinical trials that have been published.
Looking forward
Various novel technologic therapies are entering the gynecologic market. ObGyns must critically evaluate these emerging technologies with a keen understanding of their underlying mechanism of action, the level of scientific evidence, and the treatment’s proposed therapeutic value.
Radiofrequency energy devices appear to be better positioned to treat urinary incontinence and vaginal relaxation syndrome because of their capability for deep tissue penetration. Current data show that laser technology has excellent promise for the treatment and management of GSM. Both technologies warrant further investigation in long-term randomized, sham-controlled trials that assess efficacy and safety with validated instruments over an extended period. In addition, should these technologies prove useful in the overall treatment armamentarium for gynecologic conditions, the question of affordability and insurance coverage needs to be addressed.
ObGyns must advocate for female sexual wellness and encourage a comprehensive multidisciplinary team approach for offering various therapies. Ultimately, responsible use of evidence-based innovative technology should be incorporated into the treatment paradigm.
Despite recent technologic advancements and applications in gynecologic care, minimally absorbed local vaginal hormonal products (creams, rings, intravaginal tablets) and estrogen agonists/antagonists remain the mainstay and frontline treatment for moderate to severe dyspareunia, a symptom of vulvovaginal atrophy due to menopause. Newer medications, such as intravaginal steroids1 and the recently approved bioidentical estradiol nonapplicator vaginal inserts,2 also offer excellent efficacy and safety in the treatment of this condition. These medications now are included under expanded insurance coverage, and they offer safe, simple, and cost-effective treatments for this underdiagnosed condition.
References
- Intrarosa [package insert]. Waltham, MA: AMAG Pharmaceuticals Inc; February 2018.
- Imvexxy [package insert]. Boca Raton, FL: TherapeuticsMD; 2018.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
In recent years, an increasing number of laser and radiofrequency device outpatient treatments have been heralded as safe and effective interventions for various gynecologic conditions. Laser devices and radiofrequency technology rapidly have been incorporated into certain clinical settings, including medical practices specializing in dermatology, plastic surgery, and gynecology. While this developing technology has excellent promise, many clinical and research questions remain unanswered.

Concerns about energy-based vaginal treatments
Although marketing material often suggests otherwise, most laser and radiofrequency devices are cleared by the US Food and Drug Administration (FDA) only for nonspecific gynecologic and hematologic interventions. However, both laser and radiofrequency device treatments, performed as outpatient procedures, have been touted as appropriate interventions for many conditions, including female sexual dysfunction, arousal and orgasmic concerns, vaginal laxity, vaginismus, lichen sclerosus, urinary incontinence, and vulvar vestibulitis.
Well-designed studies are needed. Prospective, randomized sham-controlled trials of energy-based devices are rare, and most data in the public domain are derived from case series. Many studies are of short duration with limited follow-up. Randomized controlled trials therefore are warranted and should have stringent inclusion and exclusion criteria. Body dysmorphic syndrome, for example, should be a trial exclusion. Study design for research should include the use of standardized, validated scales and long-term follow-up of participants.
Which specialists have the expertise to offer treatment? Important ethical and medical concerns regarding the technology need to be addressed. A prime concern is determining which health care professional specialist is best qualified to assess and treat underlying gynecologic conditions. It is not uncommon to see internists, emergency medicine providers, family physicians, plastic surgeons, psychiatrists, and dermatologists self-proclaiming their gynecologic “vaginal rejuvenation” expertise.
In my experience, some ObGyns have voiced concern about the diverse medical specialties involved in performing these procedures. Currently, no standard level of training is required to perform them. In addition, those providers lack the training needed to adequately and accurately assess the potential for confounding, underlying gynecologic pathology, and they are inadequately trained to offer patients the full gamut of therapeutic interventions. Many may be unfamiliar with female pelvic anatomy and sexual function and a multidisciplinary treatment paradigm.
We need established standards. A common vernacular, nosology, classification, and decision-tree assessment paradigm for genitopelvic laxity (related to the condition of the pelvic floor and not simply a loose feeling in the vagina) is lacking, which may make research and peer-to-peer discussions difficult.
Which patients are appropriate candidates? Proper patient selection criteria for energy-based vaginal treatment have not been standardized, yet this remains a paramount need. A comprehensive patient evaluation should be performed and include a discussion on the difference between an aesthetic complaint and a functional medical problem. Assessment should include the patient’s level of concern or distress and the impact of her symptoms on her overall quality of life. Patients should be evaluated for body dysmorphic syndrome and relationship discord. A complete physical examination, including a detailed pelvic assessment, often is indicated. A treatment algorithm that incorporates conservative therapies coupled with medical, technologic, and psychologic interventions also should be developed.
Various energy-based devices are available for outpatient procedures
Although the number of procedures performed (such as vaginal rejuvenation, labiaplasty, vulvar liposculpturing, hymenoplasty, G-spot amplification, and O-Shot treatment) for both cosmetic and functional problems has increased, the published scientific data on the procedures’ short- and long-term efficacy and safety are limited. The American College of Obstetricians and Gynecologists (ACOG) published a committee opinion stating that many of these procedures, including “vaginal rejuvenation,” may not be considered medically indicated and may lack scientific merit or ample supportive data to confirm their efficacy and safety.1 ObGyns should proceed with caution before incorporating these technologic treatments into their medical practice.
Much diversity exists within the device-technology space. The underpinnings of each device vary regarding their proposed mechanism of action and theoretical therapeutic and tissue effect. In device marketing materials, many devices have been claimed to have effects on multiple tissue types (for example, both vaginal mucosa and vulvar tissue), whereas others are said to have more focal and localized effects (that is, targeted behind the hymenal ring). Some are marketed as a one-time treatment, while others require multiple repeated treatments over an extended period. When it comes to published data, adverse effect reporting remains limited and follow-up data often are short term.
Radiofrequency and laser devices are separate and very distinct technologies with similar and differing proposed utilizations. Combining radiofrequency and laser treatments in tandem or sequentially may have clinical utility, but long-term safety may be a concern for lasers.
Radiofrequency-based devices
Typically, radiofrequency device treatments:
- are used for outpatient procedures
- do not require topical anesthesia
- are constructed to emit focused electromagnetic waves
- are applied to vaginal, vulvar, or vaginal introital or vestibular tissue
- deliver energy to the deeper connective tissue of the vaginal wall architecture.
Radiofrequency device energy can be monopolar, unipolar, bipolar, or multipolar depending on design. Design also dictates current and the number of electrodes that pass from the device to the grounding pad. Monopolar is the only type of radiofrequency that has a grounding pad; bipolar and multipolar energy returns to the treatment tip.
Radiofrequency devices typically are FDA 510(k)-cleared devices for nonspecific electrocoagulation and hemostasis for surgical procedures. None are currently FDA cleared in the United States for the treatment of vaginal or vulvar laxity or genitourinary syndrome of menopause (GSM). These energy-based devices aim to induce collagen contraction, neocollagenesis, vascularization, and growth factor infiltration to restore the elasticity and moisture of the underlying vaginal mucosa. Heat shock protein activation and inflammation activation are thought to be the underlying mechanisms of action.2–5
Treatment outcomes with 2 radiofrequency devices
Multiple prospective small case series studies have reported outcomes of women treated with the ThermiVa (ThermiAesthetics LLC) radiofrequency system.3,4 Typically, 3 treatments (with a between-treatment interval of 4 to 6 weeks) were applied. The clinical end point temperature had a range of 40°C to 45°C, which was maintained for 3 to 5 minutes per treated zone during 30 minutes’ total treatment time.
Some participants self-reported improvement in vaginal laxity symptoms with the 3 treatments. In addition, women reported subjective improvements in both vaginal atrophy symptoms and sexual function, including positive effect in multiple domains. No serious adverse events were reported in these case series. However, there was no placebo-controlled arm, and validated questionnaires were not used in much of this research.3,4
In another trial, the ThermiVa system was studied in a cohort of 25 sexually active women with self-reported anorgasmia or increased latency to orgasmic response.6 Participants received 3 treatments 4 weeks apart. Approximately three-quarters of the participants reported improved orgasmic responsivity, vaginal lubrication, and clitoral sensitivity. Notably, this research did not use validated questionnaires or a placebo or sham-controlled design. The authors suggested sustained treatment benefits at 9 to 12 months. While repeat treatment was advocated, data were lacking to support the optimal time for repeat treatment efficacy.6
A cryogen-cooled monopolar radiofrequency device, the Viveve system (Viveve Medical, Inc) differs from other radiofrequency procedures because it systematically cryogen cools and protects the surface of the vaginal mucosal tissue while heating the underlying structures.
The Viveve system was evaluated in 2 small pilot studies (24 and 30 participants) and in a large, randomized, sham-controlled, prospective trial that included 108 participants (VIVEVE I trial).5,7,8 Results from both preliminary small studies indicated that participants experienced significant improvement in overall sexual function at 6 months. In one of the small studies (in Japanese women), sustained efficacy at 12 months posttreatment was reported.7 Neither small study included a placebo-control arm, but they did include the use of validated questionnaires.
In the VIVEVE I trial (a multicenter international study), treatment in the active group consisted of a single, 30-minute outpatient procedure that delivered 90 J/cm2 of radiofrequency energy at the level just behind the hymenal ring behind the vaginal introitus. The sham-treated group received ≤1 J/cm2 of energy with a similar machine tip.8
Statistically significant improvements were reported in the arousal and orgasm domains of the validated Female Sexual Function Index (FSFI) for the active-treatment group compared with the sham-treated group. In addition, there were statistically significant differences in the FSFI and the Female Sexual Distress Scale–Revised total scores in favor of active treatment. Participants in the active-treatment arm reported statistically significant improvement in overall sexual satisfaction coupled with lowered overall sexual distress.8
These data are provocative, since the Viveve treatment demonstrated superior efficacy compared with the sham treatment, and prior evidence demonstrated that medical device trials employing a sham arm often demonstrate particularly large placebo/sham effects.9 A confirmatory randomized, sham-controlled multicenter US-based trial is currently underway. At present, the VIVEVE I trial remains the only published, large-scale, randomized, sham-controlled, blinded study of a radiofrequency-based treatment.
New emerging data support the efficacy and safety of this specific radiofrequency treatment in patients with mild to moderate urinary stress incontinence; further studies confirming these outcomes are anticipated. The Viveve system is approved in many countries for various conditions, including urinary incontinence (1 country), sexual function (17 countries), vaginal laxity (41 countries), and electrocoagulation and hemostasis (4 countries, including the United States).
Laser technology devices
Laser (Light Amplification by Stimulated Emission of Radiation) therapy, which uses a carbon dioxide (CO2), argon, YAG, or erbium energy source, also is currently marketed as a method to improve various gynecologic conditions, including genital pelvic relaxation syndrome, vaginal laxity, GSM, lichen sclerosus, and sexual problems such as dyspareunia and arousal or orgasmic disorders.
The CO2 laser therapy device, such as the MonaLisa Touch (DEKA Laser), appears to be very popular and widely available. It delivers fractional CO2 laser energy to the vaginal wall, creating sequential micro traumas that subsequently undergo a healing reaction; the newly healed area has an improved underlying tissue architecture (but at a superficial level). The laser’s proposed mechanism of action is that it ablates only a minute fraction of the superficial lamina propria; it acts primarily to stimulate rapid healing of the tissue, creating new collagen and elastic fibers. There is no evidence of scarring.10
Treatment outcomes with laser device therapy
Authors of a 2017 study series of CO2 laser treatments in women with moderate to severe GSM found that 84% of participants experienced significant improvement in sexual function, dyspareunia, and otherwise unspecified sexual issues from pretreatment to 12 to 24 months posttreatment.11 These findings are consistent with several other case series and provide supportive evidence for the efficacy and safety of CO2 laser therapy. This technology may be appropriate for the treatment of GSM.
Laser technology shows excellent promise for the treatment of GSM symptoms by virtue of its superficial mechanism of action. In addition, several trials have demonstrated efficacy and safety in breast cancer patient populations.12 This is particularly interesting since breast cancer treatments, such as aromatase inhibitors (considered a mainstay of cancer treatment), can cause severe atrophic vaginitis. Breast cancer survivors often avoid minimally absorbed local vaginal hormonal products, and over-the-counter products (moisturizers and lubricants) are not widely accepted. Hence, a nonhormonal treatment for distressing GSM symptoms is welcomed in this population.
Pagano and colleagues recently studied 82 breast cancer survivors in whom treatment with vaginal moisturizers and lubricants failed.12 Participants underwent 3 laser treatment cycles approximately 30 to 40 days apart; they demonstrated improvements in vaginal dryness, vaginal itchiness, stinging, dyspareunia, and reduced sensitivity.
Microablative fractional CO2 laser may help reestablish a normative vaginal microbiome by altering the prevalence of lactobacillus species and reestablishing a normative postmenopausal vaginal flora.13
The tracking and reporting of adverse events associated with laser procedures has been less than optimal. In my personal clinical experience, consequences from both short- and long-term laser treatments have included vaginal canal agglutination, worsening dyspareunia, and constricture causing vaginal hemorrhage.
Cruz and colleagues recently conducted a randomized, double-blind, placebo-controlled clinical trial designed to evaluate the efficacy of fractional CO2 laser compared with topical estriol and laser plus estriol for the treatment of vaginal atrophy in 45 postmenopausal women.14 They found statistically significant differences in dyspareunia, dryness, and burning compared with baseline levels in all 3 treatment groups. Results with the fractional CO2 laser treatment were deemed to be similar to those of the topical estriol and the combined therapy.14
By contrast, an erbium (Er):YAG laser, such as the IntimaLase (Fotona, LLC) laser, functions by heating the pelvic tissue and collagen within the introitus and vaginal canal.15,16 When the underlying collagen is heated, the fibers are thought to thicken and shorten, which may result in immediate contracture of the treated tissue. Additionally, this process stimulates the existing collagen to undergo remodeling and it also may cause neocollagen deposition.15 In a general review of gynecologic procedures, after 1 to 4 treatment sessions (depending on the study), most patients reported improved sexual satisfaction or vaginal tightness.15
Although trials have included small numbers of patients, early evidence suggests some lasers with reportedly deeper penetration may be useful for treatment of vaginal laxity, but further studies are needed. In smaller studies, the Er:YAG laser has shown efficacy and safety in the treatment of stress urinary incontinence and improved lower urinary tract symptoms, quality of life, and sexual function.16,17
Insurance does not cover energy-based treatment costs
Currently, both laser and radiofrequency device treatments are considered fee-for-service interventions. Radiofrequency and laser treatments for gynecologic conditions are not covered by health insurance, and treatment costs can be prohibitive for many patients. In addition, the long-term safety of these treatments remains to be studied further, and the optimal time for a repeat procedure has yet to be elucidated.
The FDA cautions against energy-based procedures
In July 2018, the FDA released a statement of concern reiterating the need for research and randomized clinical trials before energy-based device treatments can be widely accepted, and that they are currently cleared only for general gynecologic indications and not for disorders and symptoms related to menopause, urinary incontinence, or sexual function.18
The FDA stated that “we have not cleared or approved for marketing any energy-based devices to treat these symptoms or conditions [vaginal laxity; vaginal atrophy, dryness, or itching; pain during sexual intercourse; pain during urination; decreased sexual sensation], or any symptoms related to menopause, urinary incontinence, or sexual function.” The FDA noted that serious complications have been reported, including vaginal burns, scarring, pain during sexual intercourse, and recurring, chronic pain. The FDA issued letters to 7 companies regarding concerns about the marketing of their devices for off-label use and promotion.
Several societies have responded. ACOG reaffirmed its 2016 position statement on fractional laser treatment of vulvovaginal atrophy.19 JoAnn Pinkerton, MD, Executive Director of The North American Menopause Society (NAMS), and Sheryl Kingsberg, PhD, President of NAMS, alerted their members that both health care professionals and consumers should tread cautiously, and they encouraged scrutiny of existing evidence as all energy-based treatments are not created equal.20 They noted that some research does exist and cited 2 randomized, sham-controlled clinical trials that have been published.
Looking forward
Various novel technologic therapies are entering the gynecologic market. ObGyns must critically evaluate these emerging technologies with a keen understanding of their underlying mechanism of action, the level of scientific evidence, and the treatment’s proposed therapeutic value.
Radiofrequency energy devices appear to be better positioned to treat urinary incontinence and vaginal relaxation syndrome because of their capability for deep tissue penetration. Current data show that laser technology has excellent promise for the treatment and management of GSM. Both technologies warrant further investigation in long-term randomized, sham-controlled trials that assess efficacy and safety with validated instruments over an extended period. In addition, should these technologies prove useful in the overall treatment armamentarium for gynecologic conditions, the question of affordability and insurance coverage needs to be addressed.
ObGyns must advocate for female sexual wellness and encourage a comprehensive multidisciplinary team approach for offering various therapies. Ultimately, responsible use of evidence-based innovative technology should be incorporated into the treatment paradigm.
Despite recent technologic advancements and applications in gynecologic care, minimally absorbed local vaginal hormonal products (creams, rings, intravaginal tablets) and estrogen agonists/antagonists remain the mainstay and frontline treatment for moderate to severe dyspareunia, a symptom of vulvovaginal atrophy due to menopause. Newer medications, such as intravaginal steroids1 and the recently approved bioidentical estradiol nonapplicator vaginal inserts,2 also offer excellent efficacy and safety in the treatment of this condition. These medications now are included under expanded insurance coverage, and they offer safe, simple, and cost-effective treatments for this underdiagnosed condition.
References
- Intrarosa [package insert]. Waltham, MA: AMAG Pharmaceuticals Inc; February 2018.
- Imvexxy [package insert]. Boca Raton, FL: TherapeuticsMD; 2018.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- ACOG Committee on Gynecologic Practice. ACOG Committee Opinion No. 378: Vaginal "rejuvenation" and cosmetic vaginal procedures. Obstet Gynecol. 2007;110(3):737-738.
- Dunbar SW, Goldberg DJ. Radiofrequency in cosmetic dermatology: an update. J Drugs Dermatol. 2015;14(11):1229-1238.
- Leibaschoff G, Izasa PG, Cardona JL, Miklos JR, Moore RD. Transcutaneous temperature-controlled radiofrequency (TTCRF) for the treatment of menopausal vaginal/genitourinary symptoms. Surg Technol Int. 2016;29:149-159.
- Alinsod RM. Temperature controlled radiofrequency for vulvovaginal laxity. Prime J. July 23, 2015. https://www.prime-journal.com/temperature-controlled-radiofrequency-for-vulvovaginal-laxity/. Accessed August 15, 2018.
- Millheiser LS, Pauls RN, Herbst SJ, Chen BH. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7(9):3088-3095.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48(7):641-645.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22 (9):775-781 .
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14(2):215-225.
- Kaptchuk TJ, Goldman P, Stone DA, Statson WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53(8): 786-792.
- Gotkin RH, Sarnoff SD, Cannarozzo G, Sadick NS, Alexiades-Armenakas M. Ablative skin resurfacing with a novel microablative CO2 laser. J Drugs Dermatol. 2009;8(2):138-144.
- Behnia-Willison F, Sarraf S, Miller J, et al. Safety and long-term efficacy of fractional CO2 laser treatment in women suffering from genitourinary syndrome of menopause. Eur J Obstet Gynecol Reprod Biol. 2017;213:39-44.
- Pagano T, De Rosa P, Vallone R, et al. Fractional microablative CO2 laser in breast cancer survivors affected by iatrogenic vulvovaginal atrophy after failure of nonestrogenic local treatments, a retrospective study. Menopause. 2018;25(6):657-662.
- Anthanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climateric. 2016;19(5):512-518.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25(1): 21-28.
- Vizintin Z, Rivera M, Fistonic I, et al. Novel minimally invasive VSP Er:YAG laser treatments in gynecology. J Laser Health Acad. 2012;2012(1):46-58.
- Tien YM, Hsio SM, Lee CN, Lin HH. Effects of laser procedure for female urodynamic stress incontinence on pad weight, urodynamics, and sexual function. Int Urogynecol J. 2017;28(3):469-476.
- Oginc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Laser Surg Med. 2015;47(9):689-697.
- US Food and Drug Administration. FDA warns against use of energy based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed August 16, 2018.
- The American College of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and US Food and Drug Administration clearance: position statement. May 2016. https://www.acog.org/Clinical-Guidance-and-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Accessed August 16, 2018.
- The North American Menopause Society. FDA mandating vaginal laser manufacturers present valid data before marketing. August 1, 2018. https://www.menopause.org/docs/default-source/default-document-library/nams-responds-to-fda-mandate-on-vaginal-laser-manufacturers-08-01-2018.pdf. Accessed August 16, 2018.
- ACOG Committee on Gynecologic Practice. ACOG Committee Opinion No. 378: Vaginal "rejuvenation" and cosmetic vaginal procedures. Obstet Gynecol. 2007;110(3):737-738.
- Dunbar SW, Goldberg DJ. Radiofrequency in cosmetic dermatology: an update. J Drugs Dermatol. 2015;14(11):1229-1238.
- Leibaschoff G, Izasa PG, Cardona JL, Miklos JR, Moore RD. Transcutaneous temperature-controlled radiofrequency (TTCRF) for the treatment of menopausal vaginal/genitourinary symptoms. Surg Technol Int. 2016;29:149-159.
- Alinsod RM. Temperature controlled radiofrequency for vulvovaginal laxity. Prime J. July 23, 2015. https://www.prime-journal.com/temperature-controlled-radiofrequency-for-vulvovaginal-laxity/. Accessed August 15, 2018.
- Millheiser LS, Pauls RN, Herbst SJ, Chen BH. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7(9):3088-3095.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48(7):641-645.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22 (9):775-781 .
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14(2):215-225.
- Kaptchuk TJ, Goldman P, Stone DA, Statson WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53(8): 786-792.
- Gotkin RH, Sarnoff SD, Cannarozzo G, Sadick NS, Alexiades-Armenakas M. Ablative skin resurfacing with a novel microablative CO2 laser. J Drugs Dermatol. 2009;8(2):138-144.
- Behnia-Willison F, Sarraf S, Miller J, et al. Safety and long-term efficacy of fractional CO2 laser treatment in women suffering from genitourinary syndrome of menopause. Eur J Obstet Gynecol Reprod Biol. 2017;213:39-44.
- Pagano T, De Rosa P, Vallone R, et al. Fractional microablative CO2 laser in breast cancer survivors affected by iatrogenic vulvovaginal atrophy after failure of nonestrogenic local treatments, a retrospective study. Menopause. 2018;25(6):657-662.
- Anthanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climateric. 2016;19(5):512-518.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25(1): 21-28.
- Vizintin Z, Rivera M, Fistonic I, et al. Novel minimally invasive VSP Er:YAG laser treatments in gynecology. J Laser Health Acad. 2012;2012(1):46-58.
- Tien YM, Hsio SM, Lee CN, Lin HH. Effects of laser procedure for female urodynamic stress incontinence on pad weight, urodynamics, and sexual function. Int Urogynecol J. 2017;28(3):469-476.
- Oginc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Laser Surg Med. 2015;47(9):689-697.
- US Food and Drug Administration. FDA warns against use of energy based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed August 16, 2018.
- The American College of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and US Food and Drug Administration clearance: position statement. May 2016. https://www.acog.org/Clinical-Guidance-and-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Accessed August 16, 2018.
- The North American Menopause Society. FDA mandating vaginal laser manufacturers present valid data before marketing. August 1, 2018. https://www.menopause.org/docs/default-source/default-document-library/nams-responds-to-fda-mandate-on-vaginal-laser-manufacturers-08-01-2018.pdf. Accessed August 16, 2018.
An oath to save lives against a backdrop of growing disparities
Practicing in the field of obstetrics and gynecology affords us a special privilege: we are part of the most important and unforgettable events in our patients’ lives, both in sickness and in health. Along with the great joys we share comes profound responsibility and the recognition that we are only as effective as the team with whom we work. Although we live in a country that is home to some of the best health care systems in the world, the maternal mortality rates and disease burden among women in underserved communities belie this fact. A University of Washington study demonstrated a more than 20-year gap in life expectancy between wealthy and poor communities in the United States from 1980 to 2014.1 Not surprisingly, access to medical care was a contributing factor.
Poverty only partly explains this disparity. Racial differences are at play as well. In 1992, a seminal study by Schoendorf and colleagues2 demonstrated that the death rates of babies born to educated African American parents were higher due to lower birth weights. Concern recently has been amplified, and many lay publications have publicly raised the alarm.3 Several states have started investigating the causes, and the American College of Obstetrics and Gynecology, as well as other organizations, are studying possible solutions.
With nearly 50% of US births financed by Medicaid,5 there was great hope that the Patient Protection and Affordable Care Act and expansion of Medicaid would result in improved access and quality of health care for underserved patients; however, it has become apparent that coverage did not confer improved access to quality care, especially for medical specialties.
Urban and rural poor populations generally seek medical services from safety net clinics staffed by midlevel and physician primary care providers whose tight schedules, documentation demands, and low reimbursement rates are coupled with complex medical and socioeconomic patient populations. While these providers may be skilled in basic primary care, their patients often present with conditions outside their scope of practice. Our country’s growing physician shortage, along with patient location and personal logistics, adds to the challenges for patients and providers alike. And who among us is not asked several times a week, even by our well-insured patients, for a primary care or specialist physician recommendation? The barriers for seeking medical care in rural populations are even greater, as local hospitals and clinics are closing at an alarming rate.
Alumni at work
Communities of physicians across the country recognize both the access problem and the potential to create solutions. Organizations such as Project ECHO, launched in 2003 through the University of New Mexico, connect rural providers with university physicians to aid in treatment of hepatitis C and other illnesses.
As the date for implementation of the Patient Protection and Affordable Care Act approached, a group of medical school alumni leaders recognized that we could come together and offer our services to address growing health care disparities. Galvanized by the challenge, the Medical Alumni Volunteer Expert Network, or MAVEN Project, was, in our parlance, “born.”
While the concept of the MAVEN Project was germinating, we interviewed numerous colleagues for advice and input and were struck by their desire—especially among the newly retired—to continue to give back. Medicine is a calling, not just a job, and for many of us the joy of helping—the exhilaration of that first birth that sold us on our specialty—gives us meaning and purpose. Many physicians who had left full-time clinical medicine missed the collegiality of the “doctors’ lounge.” Throughout our careers, we are part of a cohort: our medical school class, our residency partners, our hospital staff—we all crave community. With 36% of US physicians older than age 55 and 240,000 retired doctors in the country, we realized a motivated, previously untapped workforce could be marshaled to form a community to serve the most vulnerable among us.5
At the same time, telemedicine had come into its own. Simple technology could enable us to see each other on smartphones and computers and even perform portions of a physical examination from afar.
We realized we could marry opportunity (the workforce), need (underserved populations across the country), and technology. The Harvard Medical School Center Primary Care supported a feasibility study, and the MAVEN Project began “seeing” patients in 2016.
What happens when a safety net clinic receives a donation of life-altering oral diabetes medications but their providers lack the expertise to use them appropriately? A closet full of drugs. That is what the MAVEN Project discovered at one of our partner clinics. Enter our volunteer endocrinologist. She consulted with the medical team, reviewed how each medication should be prescribed and monitored, and gave instructions on which patients with diabetes would benefit the most from them.
The closet is emptying, the clinic providers are confidently prescribing the newest therapies, and patients are enjoying improved blood sugars and quality of life!
A model of hope
The MAVEN Project matches physician volunteers with safety net clinics serving patients in need and provides malpractice insurance and a Health Information Portability and Accountability Act–compliant technology platform to facilitate remote communication. Our volunteers mentor and educate primary care providers in the field and offer both immediate and asynchronous advisory consults. Clinic providers can group cases for discussion, ask urgent questions, or receive advice and support for the day-to-day challenges facing clinicians today. Clinics choose educational topics, focusing on tools needed for patient care rather than esoteric mechanisms of disease. Patients receive best-in-class care conveniently and locally, and by making volunteering easy, we build partnerships that augment patient and provider satisfaction, support long-term capacity building, and improve service delivery.
Our volunteer physicians now represent more than 30 medical specialties and 25 medical schools, and we have completed more than 2,000 consultations to date. Our clinics are located in 6 states (California, Florida, Massachusetts, New York, South Dakota, and Washington), and thanks to our model, physician state of licensure is not an impediment to volunteering. Several colleagues in our specialty are providing advice in women’s health.
Driving innovative solutions
Elizabeth Kopin, MD, an ObGyn who practiced for 28 years in Worcester, Massachusetts, and volunteers for the MAVEN Project, eloquently described in correspondence with Project coordinators the spirit that embodies the pursuit of medicine and the organization’s mission. As Dr. Kopin stated, “The driving force behind my entering medicine was to help people in an essential and meaningful way. I was especially driven to participate in the care of women. I wanted to gain knowledge and skills to help women with health care throughout their lives.”
Dr. Kopin’s capacity to care for patients in the clinic and hospital was progressively reduced as her multiple sclerosis advanced. As a result, she retired from clinical practice, but her desire to participate and contribute to medicine with the passion with which she entered it remained.
Her father was an internist who started a charitable clinic in Georgia. Like her father, Dr. Kopin began her medical career in academic medicine. Her father felt that his last 15 years in medicine were the most meaningful of his career because of his work with underserved populations. Dr. Kopin is following in his footsteps. For her, “Looking for a telehealth vehicle helping communities in need gives me the opportunity to use my abilities in the best way possible.” Dr. Kopin also stated, “Helping the underserved was something I wanted to devote my time to and The MAVEN Project has given me that possibility.”
We like to think of ourselves as Match. com meets the Peace Corps, with the goal to reach underserved patients in all 50 states in both rural and urban communities. We ask for as little as 4 hours of your time per month, and all you need is a computer or smartphone and a medical license. We welcome volunteers in active or part-time practice, academics, and industry: your years of wisdom are invaluable.
The vast complexities of the US health care system are by no measure easy to address, but standing by and allowing a fractured system to rupture is not an option. Each of us has an expertise and an opportunity to make incremental steps to ensure that those who need health care do not slip through the cracks. Dr. Kopin and I are fortunate to have a skill to help others and, in the MAVEN Project, a robust, dedicated network of individuals who share our vision.
There are many who have and continue to inspire a guiding conscience to serve beyond oneself. George H.W. Bush said it best when explaining why he founded the Points of Light organization nearly 3 decades ago6:
I have pursued life itself over many years now and with varying degrees of happiness. Some of my happiness still comes from trying to be in my own small way a true “point of light.” I believe I was right when I said, as President, there can be no definition of a successful life that does not include service to others. So I do that now, and I gain happiness. I do not seek a Pulitzer Prize. I do not want press attention…. I have found happiness. I no longer pursue it, for it is mine.
Please join us on our mission!
How to join
We are actively seeking specialty and primary care physicians to provide advisory consultations, mentorship, and education via telehealth technology. We welcome physician volunteers who:
- are newly retired, semi-retired, in industry, or in clinical practice
- have a minimum of 2 years of clinical practice experience
- have been active in the medical community in the past 3 years
- have an active or volunteer US medical license (any state)
- are able to provide 3 professional references
- are willing to commit a minimum of 4 hours per month for 6 months.
Visit us online to complete our physician volunteer inquiry form (https://www.mavenproject.org/work-with-us/#wwu-volunteer-as-a-physician-lightblue).
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. Inequalities in life expectancy among US counties, 1980 to 2014: temporal trends and key drivers. JAMA Intern Med. 2017;177:1003-1011.
- Schoendorf KC, Hogue CJ, Kleinman JC, et al. Mortality among infants of black as compared with white college-educated parents. N Engl J Med. 1992;326:1522-1526.
- Villarosa L. Why America's black mothers and babies are in a life-or-death crisis. New York Times. April 11, 2018. https://www.nytimes.com/2018/04/11/magazine/black-mothers-babies-death-maternal-mortality.html. Accessed August 14, 2018.
- Smith VK, Gifford K, Ellis E, et al; The Henry J. Kaiser Family Foundation; The National Association of Medical Directors. Implementing coverage and payment initiatives: results from a 50-state Medicaid budget survey for state fiscal years 2016 and 2017. http://files.kff.org/attachment/Report-Implementing-Coverage-and-Payment-Initiatives. Published October 2006. Accessed August 14, 2018.
- Association of American Medical Colleges. 2016 Physician Specialty Data Report: Executive Summary. https://www.aamc.org/download/471786/data/2016physicianspecialtydatareportexecutivesummary.pdf. Accessed August 23, 2018.
- Miller RW. Jenna Bush Hager shares George H.W. Bush 'point of light' letter after Trump jab. USA TODAY. July 7, 2018. https://www.usatoday.com/story/news/politics/onpolitics/2018/07/07/jenna-bush-hager-shares-george-h-w-bush-point-light-letter-donald-trump/765248002/. Accessed August 14, 2018.
Practicing in the field of obstetrics and gynecology affords us a special privilege: we are part of the most important and unforgettable events in our patients’ lives, both in sickness and in health. Along with the great joys we share comes profound responsibility and the recognition that we are only as effective as the team with whom we work. Although we live in a country that is home to some of the best health care systems in the world, the maternal mortality rates and disease burden among women in underserved communities belie this fact. A University of Washington study demonstrated a more than 20-year gap in life expectancy between wealthy and poor communities in the United States from 1980 to 2014.1 Not surprisingly, access to medical care was a contributing factor.
Poverty only partly explains this disparity. Racial differences are at play as well. In 1992, a seminal study by Schoendorf and colleagues2 demonstrated that the death rates of babies born to educated African American parents were higher due to lower birth weights. Concern recently has been amplified, and many lay publications have publicly raised the alarm.3 Several states have started investigating the causes, and the American College of Obstetrics and Gynecology, as well as other organizations, are studying possible solutions.
With nearly 50% of US births financed by Medicaid,5 there was great hope that the Patient Protection and Affordable Care Act and expansion of Medicaid would result in improved access and quality of health care for underserved patients; however, it has become apparent that coverage did not confer improved access to quality care, especially for medical specialties.
Urban and rural poor populations generally seek medical services from safety net clinics staffed by midlevel and physician primary care providers whose tight schedules, documentation demands, and low reimbursement rates are coupled with complex medical and socioeconomic patient populations. While these providers may be skilled in basic primary care, their patients often present with conditions outside their scope of practice. Our country’s growing physician shortage, along with patient location and personal logistics, adds to the challenges for patients and providers alike. And who among us is not asked several times a week, even by our well-insured patients, for a primary care or specialist physician recommendation? The barriers for seeking medical care in rural populations are even greater, as local hospitals and clinics are closing at an alarming rate.
Alumni at work
Communities of physicians across the country recognize both the access problem and the potential to create solutions. Organizations such as Project ECHO, launched in 2003 through the University of New Mexico, connect rural providers with university physicians to aid in treatment of hepatitis C and other illnesses.
As the date for implementation of the Patient Protection and Affordable Care Act approached, a group of medical school alumni leaders recognized that we could come together and offer our services to address growing health care disparities. Galvanized by the challenge, the Medical Alumni Volunteer Expert Network, or MAVEN Project, was, in our parlance, “born.”
While the concept of the MAVEN Project was germinating, we interviewed numerous colleagues for advice and input and were struck by their desire—especially among the newly retired—to continue to give back. Medicine is a calling, not just a job, and for many of us the joy of helping—the exhilaration of that first birth that sold us on our specialty—gives us meaning and purpose. Many physicians who had left full-time clinical medicine missed the collegiality of the “doctors’ lounge.” Throughout our careers, we are part of a cohort: our medical school class, our residency partners, our hospital staff—we all crave community. With 36% of US physicians older than age 55 and 240,000 retired doctors in the country, we realized a motivated, previously untapped workforce could be marshaled to form a community to serve the most vulnerable among us.5
At the same time, telemedicine had come into its own. Simple technology could enable us to see each other on smartphones and computers and even perform portions of a physical examination from afar.
We realized we could marry opportunity (the workforce), need (underserved populations across the country), and technology. The Harvard Medical School Center Primary Care supported a feasibility study, and the MAVEN Project began “seeing” patients in 2016.
What happens when a safety net clinic receives a donation of life-altering oral diabetes medications but their providers lack the expertise to use them appropriately? A closet full of drugs. That is what the MAVEN Project discovered at one of our partner clinics. Enter our volunteer endocrinologist. She consulted with the medical team, reviewed how each medication should be prescribed and monitored, and gave instructions on which patients with diabetes would benefit the most from them.
The closet is emptying, the clinic providers are confidently prescribing the newest therapies, and patients are enjoying improved blood sugars and quality of life!
A model of hope
The MAVEN Project matches physician volunteers with safety net clinics serving patients in need and provides malpractice insurance and a Health Information Portability and Accountability Act–compliant technology platform to facilitate remote communication. Our volunteers mentor and educate primary care providers in the field and offer both immediate and asynchronous advisory consults. Clinic providers can group cases for discussion, ask urgent questions, or receive advice and support for the day-to-day challenges facing clinicians today. Clinics choose educational topics, focusing on tools needed for patient care rather than esoteric mechanisms of disease. Patients receive best-in-class care conveniently and locally, and by making volunteering easy, we build partnerships that augment patient and provider satisfaction, support long-term capacity building, and improve service delivery.
Our volunteer physicians now represent more than 30 medical specialties and 25 medical schools, and we have completed more than 2,000 consultations to date. Our clinics are located in 6 states (California, Florida, Massachusetts, New York, South Dakota, and Washington), and thanks to our model, physician state of licensure is not an impediment to volunteering. Several colleagues in our specialty are providing advice in women’s health.
Driving innovative solutions
Elizabeth Kopin, MD, an ObGyn who practiced for 28 years in Worcester, Massachusetts, and volunteers for the MAVEN Project, eloquently described in correspondence with Project coordinators the spirit that embodies the pursuit of medicine and the organization’s mission. As Dr. Kopin stated, “The driving force behind my entering medicine was to help people in an essential and meaningful way. I was especially driven to participate in the care of women. I wanted to gain knowledge and skills to help women with health care throughout their lives.”
Dr. Kopin’s capacity to care for patients in the clinic and hospital was progressively reduced as her multiple sclerosis advanced. As a result, she retired from clinical practice, but her desire to participate and contribute to medicine with the passion with which she entered it remained.
Her father was an internist who started a charitable clinic in Georgia. Like her father, Dr. Kopin began her medical career in academic medicine. Her father felt that his last 15 years in medicine were the most meaningful of his career because of his work with underserved populations. Dr. Kopin is following in his footsteps. For her, “Looking for a telehealth vehicle helping communities in need gives me the opportunity to use my abilities in the best way possible.” Dr. Kopin also stated, “Helping the underserved was something I wanted to devote my time to and The MAVEN Project has given me that possibility.”
We like to think of ourselves as Match. com meets the Peace Corps, with the goal to reach underserved patients in all 50 states in both rural and urban communities. We ask for as little as 4 hours of your time per month, and all you need is a computer or smartphone and a medical license. We welcome volunteers in active or part-time practice, academics, and industry: your years of wisdom are invaluable.
The vast complexities of the US health care system are by no measure easy to address, but standing by and allowing a fractured system to rupture is not an option. Each of us has an expertise and an opportunity to make incremental steps to ensure that those who need health care do not slip through the cracks. Dr. Kopin and I are fortunate to have a skill to help others and, in the MAVEN Project, a robust, dedicated network of individuals who share our vision.
There are many who have and continue to inspire a guiding conscience to serve beyond oneself. George H.W. Bush said it best when explaining why he founded the Points of Light organization nearly 3 decades ago6:
I have pursued life itself over many years now and with varying degrees of happiness. Some of my happiness still comes from trying to be in my own small way a true “point of light.” I believe I was right when I said, as President, there can be no definition of a successful life that does not include service to others. So I do that now, and I gain happiness. I do not seek a Pulitzer Prize. I do not want press attention…. I have found happiness. I no longer pursue it, for it is mine.
Please join us on our mission!
How to join
We are actively seeking specialty and primary care physicians to provide advisory consultations, mentorship, and education via telehealth technology. We welcome physician volunteers who:
- are newly retired, semi-retired, in industry, or in clinical practice
- have a minimum of 2 years of clinical practice experience
- have been active in the medical community in the past 3 years
- have an active or volunteer US medical license (any state)
- are able to provide 3 professional references
- are willing to commit a minimum of 4 hours per month for 6 months.
Visit us online to complete our physician volunteer inquiry form (https://www.mavenproject.org/work-with-us/#wwu-volunteer-as-a-physician-lightblue).
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Practicing in the field of obstetrics and gynecology affords us a special privilege: we are part of the most important and unforgettable events in our patients’ lives, both in sickness and in health. Along with the great joys we share comes profound responsibility and the recognition that we are only as effective as the team with whom we work. Although we live in a country that is home to some of the best health care systems in the world, the maternal mortality rates and disease burden among women in underserved communities belie this fact. A University of Washington study demonstrated a more than 20-year gap in life expectancy between wealthy and poor communities in the United States from 1980 to 2014.1 Not surprisingly, access to medical care was a contributing factor.
Poverty only partly explains this disparity. Racial differences are at play as well. In 1992, a seminal study by Schoendorf and colleagues2 demonstrated that the death rates of babies born to educated African American parents were higher due to lower birth weights. Concern recently has been amplified, and many lay publications have publicly raised the alarm.3 Several states have started investigating the causes, and the American College of Obstetrics and Gynecology, as well as other organizations, are studying possible solutions.
With nearly 50% of US births financed by Medicaid,5 there was great hope that the Patient Protection and Affordable Care Act and expansion of Medicaid would result in improved access and quality of health care for underserved patients; however, it has become apparent that coverage did not confer improved access to quality care, especially for medical specialties.
Urban and rural poor populations generally seek medical services from safety net clinics staffed by midlevel and physician primary care providers whose tight schedules, documentation demands, and low reimbursement rates are coupled with complex medical and socioeconomic patient populations. While these providers may be skilled in basic primary care, their patients often present with conditions outside their scope of practice. Our country’s growing physician shortage, along with patient location and personal logistics, adds to the challenges for patients and providers alike. And who among us is not asked several times a week, even by our well-insured patients, for a primary care or specialist physician recommendation? The barriers for seeking medical care in rural populations are even greater, as local hospitals and clinics are closing at an alarming rate.
Alumni at work
Communities of physicians across the country recognize both the access problem and the potential to create solutions. Organizations such as Project ECHO, launched in 2003 through the University of New Mexico, connect rural providers with university physicians to aid in treatment of hepatitis C and other illnesses.
As the date for implementation of the Patient Protection and Affordable Care Act approached, a group of medical school alumni leaders recognized that we could come together and offer our services to address growing health care disparities. Galvanized by the challenge, the Medical Alumni Volunteer Expert Network, or MAVEN Project, was, in our parlance, “born.”
While the concept of the MAVEN Project was germinating, we interviewed numerous colleagues for advice and input and were struck by their desire—especially among the newly retired—to continue to give back. Medicine is a calling, not just a job, and for many of us the joy of helping—the exhilaration of that first birth that sold us on our specialty—gives us meaning and purpose. Many physicians who had left full-time clinical medicine missed the collegiality of the “doctors’ lounge.” Throughout our careers, we are part of a cohort: our medical school class, our residency partners, our hospital staff—we all crave community. With 36% of US physicians older than age 55 and 240,000 retired doctors in the country, we realized a motivated, previously untapped workforce could be marshaled to form a community to serve the most vulnerable among us.5
At the same time, telemedicine had come into its own. Simple technology could enable us to see each other on smartphones and computers and even perform portions of a physical examination from afar.
We realized we could marry opportunity (the workforce), need (underserved populations across the country), and technology. The Harvard Medical School Center Primary Care supported a feasibility study, and the MAVEN Project began “seeing” patients in 2016.
What happens when a safety net clinic receives a donation of life-altering oral diabetes medications but their providers lack the expertise to use them appropriately? A closet full of drugs. That is what the MAVEN Project discovered at one of our partner clinics. Enter our volunteer endocrinologist. She consulted with the medical team, reviewed how each medication should be prescribed and monitored, and gave instructions on which patients with diabetes would benefit the most from them.
The closet is emptying, the clinic providers are confidently prescribing the newest therapies, and patients are enjoying improved blood sugars and quality of life!
A model of hope
The MAVEN Project matches physician volunteers with safety net clinics serving patients in need and provides malpractice insurance and a Health Information Portability and Accountability Act–compliant technology platform to facilitate remote communication. Our volunteers mentor and educate primary care providers in the field and offer both immediate and asynchronous advisory consults. Clinic providers can group cases for discussion, ask urgent questions, or receive advice and support for the day-to-day challenges facing clinicians today. Clinics choose educational topics, focusing on tools needed for patient care rather than esoteric mechanisms of disease. Patients receive best-in-class care conveniently and locally, and by making volunteering easy, we build partnerships that augment patient and provider satisfaction, support long-term capacity building, and improve service delivery.
Our volunteer physicians now represent more than 30 medical specialties and 25 medical schools, and we have completed more than 2,000 consultations to date. Our clinics are located in 6 states (California, Florida, Massachusetts, New York, South Dakota, and Washington), and thanks to our model, physician state of licensure is not an impediment to volunteering. Several colleagues in our specialty are providing advice in women’s health.
Driving innovative solutions
Elizabeth Kopin, MD, an ObGyn who practiced for 28 years in Worcester, Massachusetts, and volunteers for the MAVEN Project, eloquently described in correspondence with Project coordinators the spirit that embodies the pursuit of medicine and the organization’s mission. As Dr. Kopin stated, “The driving force behind my entering medicine was to help people in an essential and meaningful way. I was especially driven to participate in the care of women. I wanted to gain knowledge and skills to help women with health care throughout their lives.”
Dr. Kopin’s capacity to care for patients in the clinic and hospital was progressively reduced as her multiple sclerosis advanced. As a result, she retired from clinical practice, but her desire to participate and contribute to medicine with the passion with which she entered it remained.
Her father was an internist who started a charitable clinic in Georgia. Like her father, Dr. Kopin began her medical career in academic medicine. Her father felt that his last 15 years in medicine were the most meaningful of his career because of his work with underserved populations. Dr. Kopin is following in his footsteps. For her, “Looking for a telehealth vehicle helping communities in need gives me the opportunity to use my abilities in the best way possible.” Dr. Kopin also stated, “Helping the underserved was something I wanted to devote my time to and The MAVEN Project has given me that possibility.”
We like to think of ourselves as Match. com meets the Peace Corps, with the goal to reach underserved patients in all 50 states in both rural and urban communities. We ask for as little as 4 hours of your time per month, and all you need is a computer or smartphone and a medical license. We welcome volunteers in active or part-time practice, academics, and industry: your years of wisdom are invaluable.
The vast complexities of the US health care system are by no measure easy to address, but standing by and allowing a fractured system to rupture is not an option. Each of us has an expertise and an opportunity to make incremental steps to ensure that those who need health care do not slip through the cracks. Dr. Kopin and I are fortunate to have a skill to help others and, in the MAVEN Project, a robust, dedicated network of individuals who share our vision.
There are many who have and continue to inspire a guiding conscience to serve beyond oneself. George H.W. Bush said it best when explaining why he founded the Points of Light organization nearly 3 decades ago6:
I have pursued life itself over many years now and with varying degrees of happiness. Some of my happiness still comes from trying to be in my own small way a true “point of light.” I believe I was right when I said, as President, there can be no definition of a successful life that does not include service to others. So I do that now, and I gain happiness. I do not seek a Pulitzer Prize. I do not want press attention…. I have found happiness. I no longer pursue it, for it is mine.
Please join us on our mission!
How to join
We are actively seeking specialty and primary care physicians to provide advisory consultations, mentorship, and education via telehealth technology. We welcome physician volunteers who:
- are newly retired, semi-retired, in industry, or in clinical practice
- have a minimum of 2 years of clinical practice experience
- have been active in the medical community in the past 3 years
- have an active or volunteer US medical license (any state)
- are able to provide 3 professional references
- are willing to commit a minimum of 4 hours per month for 6 months.
Visit us online to complete our physician volunteer inquiry form (https://www.mavenproject.org/work-with-us/#wwu-volunteer-as-a-physician-lightblue).
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. Inequalities in life expectancy among US counties, 1980 to 2014: temporal trends and key drivers. JAMA Intern Med. 2017;177:1003-1011.
- Schoendorf KC, Hogue CJ, Kleinman JC, et al. Mortality among infants of black as compared with white college-educated parents. N Engl J Med. 1992;326:1522-1526.
- Villarosa L. Why America's black mothers and babies are in a life-or-death crisis. New York Times. April 11, 2018. https://www.nytimes.com/2018/04/11/magazine/black-mothers-babies-death-maternal-mortality.html. Accessed August 14, 2018.
- Smith VK, Gifford K, Ellis E, et al; The Henry J. Kaiser Family Foundation; The National Association of Medical Directors. Implementing coverage and payment initiatives: results from a 50-state Medicaid budget survey for state fiscal years 2016 and 2017. http://files.kff.org/attachment/Report-Implementing-Coverage-and-Payment-Initiatives. Published October 2006. Accessed August 14, 2018.
- Association of American Medical Colleges. 2016 Physician Specialty Data Report: Executive Summary. https://www.aamc.org/download/471786/data/2016physicianspecialtydatareportexecutivesummary.pdf. Accessed August 23, 2018.
- Miller RW. Jenna Bush Hager shares George H.W. Bush 'point of light' letter after Trump jab. USA TODAY. July 7, 2018. https://www.usatoday.com/story/news/politics/onpolitics/2018/07/07/jenna-bush-hager-shares-george-h-w-bush-point-light-letter-donald-trump/765248002/. Accessed August 14, 2018.
- Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. Inequalities in life expectancy among US counties, 1980 to 2014: temporal trends and key drivers. JAMA Intern Med. 2017;177:1003-1011.
- Schoendorf KC, Hogue CJ, Kleinman JC, et al. Mortality among infants of black as compared with white college-educated parents. N Engl J Med. 1992;326:1522-1526.
- Villarosa L. Why America's black mothers and babies are in a life-or-death crisis. New York Times. April 11, 2018. https://www.nytimes.com/2018/04/11/magazine/black-mothers-babies-death-maternal-mortality.html. Accessed August 14, 2018.
- Smith VK, Gifford K, Ellis E, et al; The Henry J. Kaiser Family Foundation; The National Association of Medical Directors. Implementing coverage and payment initiatives: results from a 50-state Medicaid budget survey for state fiscal years 2016 and 2017. http://files.kff.org/attachment/Report-Implementing-Coverage-and-Payment-Initiatives. Published October 2006. Accessed August 14, 2018.
- Association of American Medical Colleges. 2016 Physician Specialty Data Report: Executive Summary. https://www.aamc.org/download/471786/data/2016physicianspecialtydatareportexecutivesummary.pdf. Accessed August 23, 2018.
- Miller RW. Jenna Bush Hager shares George H.W. Bush 'point of light' letter after Trump jab. USA TODAY. July 7, 2018. https://www.usatoday.com/story/news/politics/onpolitics/2018/07/07/jenna-bush-hager-shares-george-h-w-bush-point-light-letter-donald-trump/765248002/. Accessed August 14, 2018.
Delayed diagnosis of breast cancer: $15M award
Delayed diagnosis of breast cancer: $15M award
A woman in her mid-50s had been seen by a breast surgeon for 16 years for regular mammograms and sonograms. In May 2009, the breast surgeon misinterpreted a mammogram as negative, as did a radiologist who re-read the mammogram weeks later. In December 2010, the patient returned to the breast surgeon with nipple discharge. No further testing was conducted. In October 2011, the patient was found to have Stage IIIA breast cancer involving 4 lymph nodes. She underwent left radical mastectomy, chemotherapy, radiation therapy, and breast reconstruction. At time of trial, the cancer had invaded her vertebrae, was Stage IV, and most likely incurable.
PATIENT'S CLAIM: Although the surgeon admittedly did not possess the qualifications required under the Mammography Quality Standards Act, he interpreted about 5,000 mammograms per year in his office. In this case, he failed to detect a small breast tumor in May 2009. He also failed to perform testing when the patient reported nipple discharge. A more timely diagnosis of breast cancer at Stage I would have provided a 90% chance of long-term survival.
DEFENDANTS' DEFENSE: The defense held the radiologist fully liable because the surgeon was not a qualified interpreter of mammography, therefore relying on the radiologist’s interpretation. The radiologist was legally responsible for the missed diagnosis.
VERDICT: A $15M New York verdict was reached, finding the breast surgeon 75% at fault and the radiologist 25%. The radiologist settled before the trial (the jury was not informed of this). The breast surgeon was responsible for $11.25M. The defense indicated intent to appeal.
Alleged failure to evacuate uterus after cesarean delivery
A 37-year-old woman underwent cesarean delivery (CD) performed by 2 ObGyns. After delivery, she began to hemorrhage and the uterus became atonic. Hysterectomy was performed but the bleeding did not stop. The ObGyns called in 3 other ObGyns. During exploratory laparotomy, the bleeding was halted.
PATIENT'S CLAIM: She and her husband had hoped to have more children but the hysterectomy precluded that. She sued all 5 ObGyns, alleging that the delivering ObGyns failed to properly perform the CD and that each physician failed to properly perform the laparotomy, causing a large scar. The claim was discontinued against the 3 surgical ObGyns; trial addressed the 2 delivering ObGyns.
The patient’s expert ObGyn remarked that the hemorrhage was caused by a small placental remnant that remained in the uterus as a result of inadequate evacuation following delivery. The presence of the remnant was indicated by the uterine atony and should have prompted immediate investigation. The physicians’ notes did not document exploration of the uterus prior to closure.
PHYSICIAN'S DEFENSE: The defense’s expert contended that atony would not be a result of a small remnant of placenta. The patient’s uterus was properly evacuated, the hemorrhage was an unforeseeable complication, and the ObGyns properly addressed the hemorrhage.
VERDICT: A New York defense verdict was returned.
Alleged bowel injury during hysterectomy
Two days after a woman underwent a hysterectomy performed by her ObGyn, she went to the emergency department with increasing pain. Her ObGyn admitted her to the hospital. A general surgeon performed an exploratory laparotomy the next day that revealed an abscess; a 1-cm perforation of the patient’s bowel was surgically repaired. The patient had a difficult recovery. She developed pneumonia and respiratory failure. She underwent multiple repair surgeries for recurrent abscesses and fistulas because the wound was slow to heal.
PATIENT'S CLAIM: The ObGyn’s surgical technique was negligent. He injured the bowel when inserting a trocar and did not identify the injury in a timely manner. The expert witness commented that such an injury can sometimes be a surgical complication, but not in this case: the ObGyn rushed the procedure because he had another patient waiting for CD at another hospital.
PHYSICIAN'S DEFENSE: The ObGyn denied negligence and contended that the trocar used in surgery was too blunt to have caused a perforation. It would have been obvious to the ObGyn during surgery if a perforation had occurred. The perforation developed days after surgery within an abscess.
VERDICT: A Mississippi defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Delayed diagnosis of breast cancer: $15M award
A woman in her mid-50s had been seen by a breast surgeon for 16 years for regular mammograms and sonograms. In May 2009, the breast surgeon misinterpreted a mammogram as negative, as did a radiologist who re-read the mammogram weeks later. In December 2010, the patient returned to the breast surgeon with nipple discharge. No further testing was conducted. In October 2011, the patient was found to have Stage IIIA breast cancer involving 4 lymph nodes. She underwent left radical mastectomy, chemotherapy, radiation therapy, and breast reconstruction. At time of trial, the cancer had invaded her vertebrae, was Stage IV, and most likely incurable.
PATIENT'S CLAIM: Although the surgeon admittedly did not possess the qualifications required under the Mammography Quality Standards Act, he interpreted about 5,000 mammograms per year in his office. In this case, he failed to detect a small breast tumor in May 2009. He also failed to perform testing when the patient reported nipple discharge. A more timely diagnosis of breast cancer at Stage I would have provided a 90% chance of long-term survival.
DEFENDANTS' DEFENSE: The defense held the radiologist fully liable because the surgeon was not a qualified interpreter of mammography, therefore relying on the radiologist’s interpretation. The radiologist was legally responsible for the missed diagnosis.
VERDICT: A $15M New York verdict was reached, finding the breast surgeon 75% at fault and the radiologist 25%. The radiologist settled before the trial (the jury was not informed of this). The breast surgeon was responsible for $11.25M. The defense indicated intent to appeal.
Alleged failure to evacuate uterus after cesarean delivery
A 37-year-old woman underwent cesarean delivery (CD) performed by 2 ObGyns. After delivery, she began to hemorrhage and the uterus became atonic. Hysterectomy was performed but the bleeding did not stop. The ObGyns called in 3 other ObGyns. During exploratory laparotomy, the bleeding was halted.
PATIENT'S CLAIM: She and her husband had hoped to have more children but the hysterectomy precluded that. She sued all 5 ObGyns, alleging that the delivering ObGyns failed to properly perform the CD and that each physician failed to properly perform the laparotomy, causing a large scar. The claim was discontinued against the 3 surgical ObGyns; trial addressed the 2 delivering ObGyns.
The patient’s expert ObGyn remarked that the hemorrhage was caused by a small placental remnant that remained in the uterus as a result of inadequate evacuation following delivery. The presence of the remnant was indicated by the uterine atony and should have prompted immediate investigation. The physicians’ notes did not document exploration of the uterus prior to closure.
PHYSICIAN'S DEFENSE: The defense’s expert contended that atony would not be a result of a small remnant of placenta. The patient’s uterus was properly evacuated, the hemorrhage was an unforeseeable complication, and the ObGyns properly addressed the hemorrhage.
VERDICT: A New York defense verdict was returned.
Alleged bowel injury during hysterectomy
Two days after a woman underwent a hysterectomy performed by her ObGyn, she went to the emergency department with increasing pain. Her ObGyn admitted her to the hospital. A general surgeon performed an exploratory laparotomy the next day that revealed an abscess; a 1-cm perforation of the patient’s bowel was surgically repaired. The patient had a difficult recovery. She developed pneumonia and respiratory failure. She underwent multiple repair surgeries for recurrent abscesses and fistulas because the wound was slow to heal.
PATIENT'S CLAIM: The ObGyn’s surgical technique was negligent. He injured the bowel when inserting a trocar and did not identify the injury in a timely manner. The expert witness commented that such an injury can sometimes be a surgical complication, but not in this case: the ObGyn rushed the procedure because he had another patient waiting for CD at another hospital.
PHYSICIAN'S DEFENSE: The ObGyn denied negligence and contended that the trocar used in surgery was too blunt to have caused a perforation. It would have been obvious to the ObGyn during surgery if a perforation had occurred. The perforation developed days after surgery within an abscess.
VERDICT: A Mississippi defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Delayed diagnosis of breast cancer: $15M award
A woman in her mid-50s had been seen by a breast surgeon for 16 years for regular mammograms and sonograms. In May 2009, the breast surgeon misinterpreted a mammogram as negative, as did a radiologist who re-read the mammogram weeks later. In December 2010, the patient returned to the breast surgeon with nipple discharge. No further testing was conducted. In October 2011, the patient was found to have Stage IIIA breast cancer involving 4 lymph nodes. She underwent left radical mastectomy, chemotherapy, radiation therapy, and breast reconstruction. At time of trial, the cancer had invaded her vertebrae, was Stage IV, and most likely incurable.
PATIENT'S CLAIM: Although the surgeon admittedly did not possess the qualifications required under the Mammography Quality Standards Act, he interpreted about 5,000 mammograms per year in his office. In this case, he failed to detect a small breast tumor in May 2009. He also failed to perform testing when the patient reported nipple discharge. A more timely diagnosis of breast cancer at Stage I would have provided a 90% chance of long-term survival.
DEFENDANTS' DEFENSE: The defense held the radiologist fully liable because the surgeon was not a qualified interpreter of mammography, therefore relying on the radiologist’s interpretation. The radiologist was legally responsible for the missed diagnosis.
VERDICT: A $15M New York verdict was reached, finding the breast surgeon 75% at fault and the radiologist 25%. The radiologist settled before the trial (the jury was not informed of this). The breast surgeon was responsible for $11.25M. The defense indicated intent to appeal.
Alleged failure to evacuate uterus after cesarean delivery
A 37-year-old woman underwent cesarean delivery (CD) performed by 2 ObGyns. After delivery, she began to hemorrhage and the uterus became atonic. Hysterectomy was performed but the bleeding did not stop. The ObGyns called in 3 other ObGyns. During exploratory laparotomy, the bleeding was halted.
PATIENT'S CLAIM: She and her husband had hoped to have more children but the hysterectomy precluded that. She sued all 5 ObGyns, alleging that the delivering ObGyns failed to properly perform the CD and that each physician failed to properly perform the laparotomy, causing a large scar. The claim was discontinued against the 3 surgical ObGyns; trial addressed the 2 delivering ObGyns.
The patient’s expert ObGyn remarked that the hemorrhage was caused by a small placental remnant that remained in the uterus as a result of inadequate evacuation following delivery. The presence of the remnant was indicated by the uterine atony and should have prompted immediate investigation. The physicians’ notes did not document exploration of the uterus prior to closure.
PHYSICIAN'S DEFENSE: The defense’s expert contended that atony would not be a result of a small remnant of placenta. The patient’s uterus was properly evacuated, the hemorrhage was an unforeseeable complication, and the ObGyns properly addressed the hemorrhage.
VERDICT: A New York defense verdict was returned.
Alleged bowel injury during hysterectomy
Two days after a woman underwent a hysterectomy performed by her ObGyn, she went to the emergency department with increasing pain. Her ObGyn admitted her to the hospital. A general surgeon performed an exploratory laparotomy the next day that revealed an abscess; a 1-cm perforation of the patient’s bowel was surgically repaired. The patient had a difficult recovery. She developed pneumonia and respiratory failure. She underwent multiple repair surgeries for recurrent abscesses and fistulas because the wound was slow to heal.
PATIENT'S CLAIM: The ObGyn’s surgical technique was negligent. He injured the bowel when inserting a trocar and did not identify the injury in a timely manner. The expert witness commented that such an injury can sometimes be a surgical complication, but not in this case: the ObGyn rushed the procedure because he had another patient waiting for CD at another hospital.
PHYSICIAN'S DEFENSE: The ObGyn denied negligence and contended that the trocar used in surgery was too blunt to have caused a perforation. It would have been obvious to the ObGyn during surgery if a perforation had occurred. The perforation developed days after surgery within an abscess.
VERDICT: A Mississippi defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Excision of a Bartholin gland cyst
Bartholin gland cysts comprise up to 2% of all outpatient gynecology visits each year1 and are a common consult for trainees in obstetrics and gynecology. Although excision of a Bartholin gland cyst is a procedure performed infrequently, knowledge of its anatomy and physiology is important for ObGyn trainees and practicing gynecologists, especially when attempts at conservative management have been exhausted.
Before proceeding with surgical excision, it is important to understand the basics of Bartholin gland anatomy, pathologies, and treatment options. This video demonstrates the excisional technique for a 46-year-old woman with a recurrent, symptomatic Bartholin gland cyst who failed prior conservative management. I hope that you will find this video from my colleagues beneficial to your clinical practice.

- Marzano DA, Haefner HK. The bartholin gland cyst: past, present, and future. J Low Genit Tract Dis. 2004;8(3):195–204.
Bartholin gland cysts comprise up to 2% of all outpatient gynecology visits each year1 and are a common consult for trainees in obstetrics and gynecology. Although excision of a Bartholin gland cyst is a procedure performed infrequently, knowledge of its anatomy and physiology is important for ObGyn trainees and practicing gynecologists, especially when attempts at conservative management have been exhausted.
Before proceeding with surgical excision, it is important to understand the basics of Bartholin gland anatomy, pathologies, and treatment options. This video demonstrates the excisional technique for a 46-year-old woman with a recurrent, symptomatic Bartholin gland cyst who failed prior conservative management. I hope that you will find this video from my colleagues beneficial to your clinical practice.

Bartholin gland cysts comprise up to 2% of all outpatient gynecology visits each year1 and are a common consult for trainees in obstetrics and gynecology. Although excision of a Bartholin gland cyst is a procedure performed infrequently, knowledge of its anatomy and physiology is important for ObGyn trainees and practicing gynecologists, especially when attempts at conservative management have been exhausted.
Before proceeding with surgical excision, it is important to understand the basics of Bartholin gland anatomy, pathologies, and treatment options. This video demonstrates the excisional technique for a 46-year-old woman with a recurrent, symptomatic Bartholin gland cyst who failed prior conservative management. I hope that you will find this video from my colleagues beneficial to your clinical practice.

- Marzano DA, Haefner HK. The bartholin gland cyst: past, present, and future. J Low Genit Tract Dis. 2004;8(3):195–204.
- Marzano DA, Haefner HK. The bartholin gland cyst: past, present, and future. J Low Genit Tract Dis. 2004;8(3):195–204.
No good reason to not use ultrasound during embryo transfer, expert says
CORONADO, CALIF. – according to William Schoolcraft, MD, HCLD.
At a meeting on IVF and embryo transfer sponsored by the University of California, San Diego, Dr. Schoolcraft said that ultrasound-guided embryo transfer helps clinicians avoid difficult transfers, minimizes contamination with blood, facilitates proper placement of the catheter tip, and minimizes the stimulation of uterine contractions. “We know that contaminating the catheter either with blood or mucous or endometrial tissue lowers clinical pregnancy rates, compared to a clean catheter,” said Dr. Schoolcraft, founder and medical director of the Colorado Center for Reproductive Medicine, Denver.
“Ultrasound guidance can help you follow the contour of the cervix and avoid touching the fundus. Your catheter should be free of blood, mucous, or endometrial cells when the embryologist examines it,” he said. In his clinical opinion, it’s hard to argue against using ultrasound guidance for embryo transfer. “It’s also very popular with IVF patients, because they get to visualize the transfer and have some reassurance that the embryo is delivered to their uterus,” he said.
The potential benefit of using three-dimensional ultrasound for embryo transfer is less clear. “It does require more expensive equipment and it’s a little more skill dependent, but in a randomized trial it didn’t lead to any difference in outcomes,” Dr. Schoolcraft said. “I think if you’re good with two-dimensional ultrasound, three-dimensional ultrasound doesn’t seem to have much benefit in terms of pregnancy outcomes.”
In a study published in 2017, researchers from Barcelona analyzed 7,714 embryo transfers to determine the impact of maneuvers during embryo transfers on the pregnancy rate (Fertil Steril. 2017 Mar;107[3]:657-63.e1). Using the direct embryo transfer as a reference, each instrumentation needed to successfully deposit the embryos in the fundus served as an index of the difficulty of transfer. A difficult transfer occurred in 7.7% of cycles, and the researchers found that the clinical pregnancy rate decreased progressively with the use of additional maneuvers during embryo transfer. Specifically, the clinical pregnancy rate was 39.4% when no additional maneuvers were required, 36.9% when an outer catheter sheath was used (odds ratio, 0.89), 31.7% when a Wallace stylet was used (OR, 0.71), and 26.1% when a tenaculum was used (OR, 0.54). “I think without question, avoiding a difficult transfer is important and certainly a key to our success,” said Dr. Schoolcraft, who was not involved with the study.
The ideal depth of embryo transfer is “a bit complicated,” he said, but according to the best available evidence, a depth of 15-20 mm from the fundus by ultrasound guidance appears to optimize implantation by avoiding the lower cavity where implantation is compromised. This range of depth also avoids problems with upper cavity transfers, including trauma, contractions, and tubal pregnancy. “I think that transfers which are close to the fundus, and possibly in some cases touching the fundus, may lead to uterine contractions, plugging the catheter with endometrium and generating bleeding,” Dr. Schoolcraft said. He pointed out that during natural pregnancies, embryos implant in the upper fundus nearly 90% of the time, compared with 66% of the time during IVF pregnancies. “To mimic Mother Nature we don’t want to be too low, either,” he said. “We all know that placenta previa is increased with IVF. This may be due to placing the embryos too low.”
According to Dr. Schoolcraft, many published studies have found that significantly higher pregnancy rates occur with routine bladder distension prior to embryo transfer, probably because of the smooth and easy insertion of the embryo transfer. A Scandinavian meta-analysis found that the odds ratio favoring ultrasound guidance and a full bladder for ongoing pregnancy was 1.44 and clinical pregnancy was 1.55, which is similar to that seen during an earlier review from The Cochrane Collaborative, with an OR of 1.47 for ongoing pregnancy and OR 1.53 for live birth (Cochrane Database Syst Rev. 2016 Mar 17. doi: 10.1002/14651858.CD006107.pub4).
Dr. Schoolcraft reported having no financial disclosures.
CORONADO, CALIF. – according to William Schoolcraft, MD, HCLD.
At a meeting on IVF and embryo transfer sponsored by the University of California, San Diego, Dr. Schoolcraft said that ultrasound-guided embryo transfer helps clinicians avoid difficult transfers, minimizes contamination with blood, facilitates proper placement of the catheter tip, and minimizes the stimulation of uterine contractions. “We know that contaminating the catheter either with blood or mucous or endometrial tissue lowers clinical pregnancy rates, compared to a clean catheter,” said Dr. Schoolcraft, founder and medical director of the Colorado Center for Reproductive Medicine, Denver.
“Ultrasound guidance can help you follow the contour of the cervix and avoid touching the fundus. Your catheter should be free of blood, mucous, or endometrial cells when the embryologist examines it,” he said. In his clinical opinion, it’s hard to argue against using ultrasound guidance for embryo transfer. “It’s also very popular with IVF patients, because they get to visualize the transfer and have some reassurance that the embryo is delivered to their uterus,” he said.
The potential benefit of using three-dimensional ultrasound for embryo transfer is less clear. “It does require more expensive equipment and it’s a little more skill dependent, but in a randomized trial it didn’t lead to any difference in outcomes,” Dr. Schoolcraft said. “I think if you’re good with two-dimensional ultrasound, three-dimensional ultrasound doesn’t seem to have much benefit in terms of pregnancy outcomes.”
In a study published in 2017, researchers from Barcelona analyzed 7,714 embryo transfers to determine the impact of maneuvers during embryo transfers on the pregnancy rate (Fertil Steril. 2017 Mar;107[3]:657-63.e1). Using the direct embryo transfer as a reference, each instrumentation needed to successfully deposit the embryos in the fundus served as an index of the difficulty of transfer. A difficult transfer occurred in 7.7% of cycles, and the researchers found that the clinical pregnancy rate decreased progressively with the use of additional maneuvers during embryo transfer. Specifically, the clinical pregnancy rate was 39.4% when no additional maneuvers were required, 36.9% when an outer catheter sheath was used (odds ratio, 0.89), 31.7% when a Wallace stylet was used (OR, 0.71), and 26.1% when a tenaculum was used (OR, 0.54). “I think without question, avoiding a difficult transfer is important and certainly a key to our success,” said Dr. Schoolcraft, who was not involved with the study.
The ideal depth of embryo transfer is “a bit complicated,” he said, but according to the best available evidence, a depth of 15-20 mm from the fundus by ultrasound guidance appears to optimize implantation by avoiding the lower cavity where implantation is compromised. This range of depth also avoids problems with upper cavity transfers, including trauma, contractions, and tubal pregnancy. “I think that transfers which are close to the fundus, and possibly in some cases touching the fundus, may lead to uterine contractions, plugging the catheter with endometrium and generating bleeding,” Dr. Schoolcraft said. He pointed out that during natural pregnancies, embryos implant in the upper fundus nearly 90% of the time, compared with 66% of the time during IVF pregnancies. “To mimic Mother Nature we don’t want to be too low, either,” he said. “We all know that placenta previa is increased with IVF. This may be due to placing the embryos too low.”
According to Dr. Schoolcraft, many published studies have found that significantly higher pregnancy rates occur with routine bladder distension prior to embryo transfer, probably because of the smooth and easy insertion of the embryo transfer. A Scandinavian meta-analysis found that the odds ratio favoring ultrasound guidance and a full bladder for ongoing pregnancy was 1.44 and clinical pregnancy was 1.55, which is similar to that seen during an earlier review from The Cochrane Collaborative, with an OR of 1.47 for ongoing pregnancy and OR 1.53 for live birth (Cochrane Database Syst Rev. 2016 Mar 17. doi: 10.1002/14651858.CD006107.pub4).
Dr. Schoolcraft reported having no financial disclosures.
CORONADO, CALIF. – according to William Schoolcraft, MD, HCLD.
At a meeting on IVF and embryo transfer sponsored by the University of California, San Diego, Dr. Schoolcraft said that ultrasound-guided embryo transfer helps clinicians avoid difficult transfers, minimizes contamination with blood, facilitates proper placement of the catheter tip, and minimizes the stimulation of uterine contractions. “We know that contaminating the catheter either with blood or mucous or endometrial tissue lowers clinical pregnancy rates, compared to a clean catheter,” said Dr. Schoolcraft, founder and medical director of the Colorado Center for Reproductive Medicine, Denver.
“Ultrasound guidance can help you follow the contour of the cervix and avoid touching the fundus. Your catheter should be free of blood, mucous, or endometrial cells when the embryologist examines it,” he said. In his clinical opinion, it’s hard to argue against using ultrasound guidance for embryo transfer. “It’s also very popular with IVF patients, because they get to visualize the transfer and have some reassurance that the embryo is delivered to their uterus,” he said.
The potential benefit of using three-dimensional ultrasound for embryo transfer is less clear. “It does require more expensive equipment and it’s a little more skill dependent, but in a randomized trial it didn’t lead to any difference in outcomes,” Dr. Schoolcraft said. “I think if you’re good with two-dimensional ultrasound, three-dimensional ultrasound doesn’t seem to have much benefit in terms of pregnancy outcomes.”
In a study published in 2017, researchers from Barcelona analyzed 7,714 embryo transfers to determine the impact of maneuvers during embryo transfers on the pregnancy rate (Fertil Steril. 2017 Mar;107[3]:657-63.e1). Using the direct embryo transfer as a reference, each instrumentation needed to successfully deposit the embryos in the fundus served as an index of the difficulty of transfer. A difficult transfer occurred in 7.7% of cycles, and the researchers found that the clinical pregnancy rate decreased progressively with the use of additional maneuvers during embryo transfer. Specifically, the clinical pregnancy rate was 39.4% when no additional maneuvers were required, 36.9% when an outer catheter sheath was used (odds ratio, 0.89), 31.7% when a Wallace stylet was used (OR, 0.71), and 26.1% when a tenaculum was used (OR, 0.54). “I think without question, avoiding a difficult transfer is important and certainly a key to our success,” said Dr. Schoolcraft, who was not involved with the study.
The ideal depth of embryo transfer is “a bit complicated,” he said, but according to the best available evidence, a depth of 15-20 mm from the fundus by ultrasound guidance appears to optimize implantation by avoiding the lower cavity where implantation is compromised. This range of depth also avoids problems with upper cavity transfers, including trauma, contractions, and tubal pregnancy. “I think that transfers which are close to the fundus, and possibly in some cases touching the fundus, may lead to uterine contractions, plugging the catheter with endometrium and generating bleeding,” Dr. Schoolcraft said. He pointed out that during natural pregnancies, embryos implant in the upper fundus nearly 90% of the time, compared with 66% of the time during IVF pregnancies. “To mimic Mother Nature we don’t want to be too low, either,” he said. “We all know that placenta previa is increased with IVF. This may be due to placing the embryos too low.”
According to Dr. Schoolcraft, many published studies have found that significantly higher pregnancy rates occur with routine bladder distension prior to embryo transfer, probably because of the smooth and easy insertion of the embryo transfer. A Scandinavian meta-analysis found that the odds ratio favoring ultrasound guidance and a full bladder for ongoing pregnancy was 1.44 and clinical pregnancy was 1.55, which is similar to that seen during an earlier review from The Cochrane Collaborative, with an OR of 1.47 for ongoing pregnancy and OR 1.53 for live birth (Cochrane Database Syst Rev. 2016 Mar 17. doi: 10.1002/14651858.CD006107.pub4).
Dr. Schoolcraft reported having no financial disclosures.
EXPERT ANALYSIS FROM A CME MEETING SPONSORED BY UCSD
Is the most effective emergency contraception easily obtained at US pharmacies?
EXPERT COMMENTARY
Although it is available only by prescription, ulipristal acetate provides emergency contraception that is more effective than the emergency contraception provided by levonorgestrel (LNG), which is available without a prescription (TABLE). In addition, ulipristal acetate appears more effective than LNG in obese and overweight women.1,2 Package labeling for ulipristal acetate indicates that a single 30-mg tablet should be taken orally within 5 days of unprotected sex.
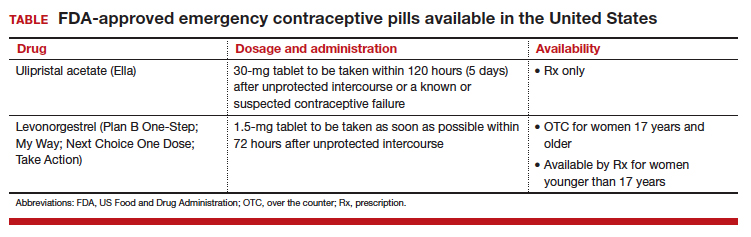
According to a survey of pharmacy availability of ulipristal acetate in Hawaii, 2.6% of retail pharmacies had the drug immediately available, compared with 82.4% for LNG, and 22.8% reported the ability to order it.3 To assess pharmacy availability of ulipristal acetate on a nationwide scale, Shigesato and colleagues conducted a national “secret shopper” telephone survey in 10 cities (each with a population of at least 500,000) in all major regions of the United States.
Details of the study
Independent pharmacies (defined as having fewer than 5 locations within the city) and chain pharmacies were included in the survey. The survey callers, representing themselves as uninsured 18-year-old women attempting to fill a prescription for ulipristal acetate, followed a semistructured questionnaire and recorded the responses. They asked about the immediate availability of ulipristal acetate and LNG, the pharmacy’s ability to order ulipristal acetate if not immediately available, out-of-pocket costs, instructions for use, and the differences between ulipristal acetate and LNG. Questions were directed to whichever pharmacy staff member answered the phone; callers did not specifically ask to speak to a pharmacist.
Of the 344 pharmacies included in this analysis, 10% (33) indicated that they could fill a prescription for ulipristal acetate immediately. While availability did not vary by region, there was a difference in immediate availability by city.
Almost three-quarters of pharmacies without immediate drug availability indicated that they could order ulipristal acetate, with a median predicted time for availability of 24 hours. Of the chain pharmacies, 81% (167 of 205) reported the ability to order ulipristal acetate, compared with 55% (57 of 106) of independent pharmacies.
When asked if ulipristal acetate was different from LNG, more than one-third of pharmacy personnel contacted stated either that there was no difference between ulipristal acetate and LNG or that they were not sure of a difference.
Study strengths and weaknesses
The authors noted that the secret shopper methodology, along with having callers speak to the pharmacy staff person who answered the call (rather than asking for the pharmacist), provided data that closely approximates real-world patient experiences.
Since more pharmacies than anticipated met exclusion criteria for the study, the estimate of ulipristal acetate immediate availability was less precise than the power analysis predicted. Further, results from the 10 large, geographically diverse cities may not be representative of all similarly sized cities nationally or all areas of the United States.
As the authors point out, a low prevalence of pharmacies stock ulipristal acetate, and more than 25% are not able to order this emergency contraception. This underscores the fact that access to the most effective oral emergency contraception is limited for US women. I agree with the authors’ speculation that access to ulipristal acetate may be even lower in rural areas. In many European countries, ulipristal acetate is available without a prescription. Clinicians caring for women who may benefit from emergency contraception, particularly those using short-acting or less effective contraceptives, may wish to prescribe ulipristal acetate in advance of need.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Kapp N, Abitbol JL, Mathé H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception. 2015;91(2):97–104.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Bullock H, Steele S, Kurata N, et al. Pharmacy access to ulipristal acetate in Hawaii: is a prescription enough? Contraception. 2016;93(5):452–454.
EXPERT COMMENTARY
Although it is available only by prescription, ulipristal acetate provides emergency contraception that is more effective than the emergency contraception provided by levonorgestrel (LNG), which is available without a prescription (TABLE). In addition, ulipristal acetate appears more effective than LNG in obese and overweight women.1,2 Package labeling for ulipristal acetate indicates that a single 30-mg tablet should be taken orally within 5 days of unprotected sex.

According to a survey of pharmacy availability of ulipristal acetate in Hawaii, 2.6% of retail pharmacies had the drug immediately available, compared with 82.4% for LNG, and 22.8% reported the ability to order it.3 To assess pharmacy availability of ulipristal acetate on a nationwide scale, Shigesato and colleagues conducted a national “secret shopper” telephone survey in 10 cities (each with a population of at least 500,000) in all major regions of the United States.
Details of the study
Independent pharmacies (defined as having fewer than 5 locations within the city) and chain pharmacies were included in the survey. The survey callers, representing themselves as uninsured 18-year-old women attempting to fill a prescription for ulipristal acetate, followed a semistructured questionnaire and recorded the responses. They asked about the immediate availability of ulipristal acetate and LNG, the pharmacy’s ability to order ulipristal acetate if not immediately available, out-of-pocket costs, instructions for use, and the differences between ulipristal acetate and LNG. Questions were directed to whichever pharmacy staff member answered the phone; callers did not specifically ask to speak to a pharmacist.
Of the 344 pharmacies included in this analysis, 10% (33) indicated that they could fill a prescription for ulipristal acetate immediately. While availability did not vary by region, there was a difference in immediate availability by city.
Almost three-quarters of pharmacies without immediate drug availability indicated that they could order ulipristal acetate, with a median predicted time for availability of 24 hours. Of the chain pharmacies, 81% (167 of 205) reported the ability to order ulipristal acetate, compared with 55% (57 of 106) of independent pharmacies.
When asked if ulipristal acetate was different from LNG, more than one-third of pharmacy personnel contacted stated either that there was no difference between ulipristal acetate and LNG or that they were not sure of a difference.
Study strengths and weaknesses
The authors noted that the secret shopper methodology, along with having callers speak to the pharmacy staff person who answered the call (rather than asking for the pharmacist), provided data that closely approximates real-world patient experiences.
Since more pharmacies than anticipated met exclusion criteria for the study, the estimate of ulipristal acetate immediate availability was less precise than the power analysis predicted. Further, results from the 10 large, geographically diverse cities may not be representative of all similarly sized cities nationally or all areas of the United States.
As the authors point out, a low prevalence of pharmacies stock ulipristal acetate, and more than 25% are not able to order this emergency contraception. This underscores the fact that access to the most effective oral emergency contraception is limited for US women. I agree with the authors’ speculation that access to ulipristal acetate may be even lower in rural areas. In many European countries, ulipristal acetate is available without a prescription. Clinicians caring for women who may benefit from emergency contraception, particularly those using short-acting or less effective contraceptives, may wish to prescribe ulipristal acetate in advance of need.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Although it is available only by prescription, ulipristal acetate provides emergency contraception that is more effective than the emergency contraception provided by levonorgestrel (LNG), which is available without a prescription (TABLE). In addition, ulipristal acetate appears more effective than LNG in obese and overweight women.1,2 Package labeling for ulipristal acetate indicates that a single 30-mg tablet should be taken orally within 5 days of unprotected sex.

According to a survey of pharmacy availability of ulipristal acetate in Hawaii, 2.6% of retail pharmacies had the drug immediately available, compared with 82.4% for LNG, and 22.8% reported the ability to order it.3 To assess pharmacy availability of ulipristal acetate on a nationwide scale, Shigesato and colleagues conducted a national “secret shopper” telephone survey in 10 cities (each with a population of at least 500,000) in all major regions of the United States.
Details of the study
Independent pharmacies (defined as having fewer than 5 locations within the city) and chain pharmacies were included in the survey. The survey callers, representing themselves as uninsured 18-year-old women attempting to fill a prescription for ulipristal acetate, followed a semistructured questionnaire and recorded the responses. They asked about the immediate availability of ulipristal acetate and LNG, the pharmacy’s ability to order ulipristal acetate if not immediately available, out-of-pocket costs, instructions for use, and the differences between ulipristal acetate and LNG. Questions were directed to whichever pharmacy staff member answered the phone; callers did not specifically ask to speak to a pharmacist.
Of the 344 pharmacies included in this analysis, 10% (33) indicated that they could fill a prescription for ulipristal acetate immediately. While availability did not vary by region, there was a difference in immediate availability by city.
Almost three-quarters of pharmacies without immediate drug availability indicated that they could order ulipristal acetate, with a median predicted time for availability of 24 hours. Of the chain pharmacies, 81% (167 of 205) reported the ability to order ulipristal acetate, compared with 55% (57 of 106) of independent pharmacies.
When asked if ulipristal acetate was different from LNG, more than one-third of pharmacy personnel contacted stated either that there was no difference between ulipristal acetate and LNG or that they were not sure of a difference.
Study strengths and weaknesses
The authors noted that the secret shopper methodology, along with having callers speak to the pharmacy staff person who answered the call (rather than asking for the pharmacist), provided data that closely approximates real-world patient experiences.
Since more pharmacies than anticipated met exclusion criteria for the study, the estimate of ulipristal acetate immediate availability was less precise than the power analysis predicted. Further, results from the 10 large, geographically diverse cities may not be representative of all similarly sized cities nationally or all areas of the United States.
As the authors point out, a low prevalence of pharmacies stock ulipristal acetate, and more than 25% are not able to order this emergency contraception. This underscores the fact that access to the most effective oral emergency contraception is limited for US women. I agree with the authors’ speculation that access to ulipristal acetate may be even lower in rural areas. In many European countries, ulipristal acetate is available without a prescription. Clinicians caring for women who may benefit from emergency contraception, particularly those using short-acting or less effective contraceptives, may wish to prescribe ulipristal acetate in advance of need.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Kapp N, Abitbol JL, Mathé H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception. 2015;91(2):97–104.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Bullock H, Steele S, Kurata N, et al. Pharmacy access to ulipristal acetate in Hawaii: is a prescription enough? Contraception. 2016;93(5):452–454.
- Kapp N, Abitbol JL, Mathé H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception. 2015;91(2):97–104.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Bullock H, Steele S, Kurata N, et al. Pharmacy access to ulipristal acetate in Hawaii: is a prescription enough? Contraception. 2016;93(5):452–454.