User login
No increase in primary ovarian insufficiency with HPV vaccine
The human papillomavirus vaccine does not appear to be associated with an increased risk of ovarian insufficiency, according to researchers.
Allison L. Naleway, PhD, of the Center for Health Research at Kaiser Permanente Northwest, Portland, Ore., and her coauthors wrote that a previous case series had raised concerns about a possible link between the human papillomavirus (HPV) vaccine and primary ovarian insufficiency (POI) in six young women who developed the condition within 12 months of vaccination.
Using EHR data, researchers identified 46 women aged 11-34 years with idiopathic POI – 33 probable cases and 13 possible cases – after excluding cases with known causes. Eighteen of these cases also were excluded because they were diagnosed before the HPV vaccine was available.
They found that only 1 of the remaining 28 patients had been vaccinated against HPV before the symptom onset: a 16-year-old girl who was vaccinated about 23 months before the first clinical evaluation for delayed menarche. Their report was published in Pediatrics.
The adjusted hazard ratio for POI was therefore 0.30 after HPV vaccine, compared with 0.88 after Tdap, 1.42 after inactivated influenza vaccine, and 0.94 after meningococcal conjugate vaccine.
More than one-half of the 46 confirmed POI cases were diagnosed at age 27 years or older, and only one patient was diagnosed under 15 years of age.
“If POI is triggered by HPV or other adolescent vaccine exposure, we would have expected to see elevated incidence in the younger women who were most likely to be vaccinated, but instead we observed higher incidence in older women (greater than 26 years of age), which is consistent with 1 other population-based study of POI prevalence,” the authors wrote.
They acknowledged that studying POI as a vaccine-related adverse event was challenging because the time from symptom onset to diagnosis was variable. However, they said that 81% of their cohort was followed up for more than 2 years, and a mean of 5.14 years, so the potential for misclassification was “minimal.”
Dr. Naleway and her associates also noted that diagnoses of POI can be difficult to accurately identify, and symptoms may be masked by oral contraceptive use.
“Despite the challenges and limitations discussed above, we believe this study should lessen concern surrounding potential impact on fertility from HPV or other adolescent vaccination,” they wrote.
The Centers for Disease Control and Prevention supported the study. Three authors declared funding from pharmaceutical companies for unrelated studies. No other conflicts of interest were declared.
SOURCE: Naleway A et al. Pediatrics 2018;42(3):e20180943.
The human papillomavirus vaccine does not appear to be associated with an increased risk of ovarian insufficiency, according to researchers.
Allison L. Naleway, PhD, of the Center for Health Research at Kaiser Permanente Northwest, Portland, Ore., and her coauthors wrote that a previous case series had raised concerns about a possible link between the human papillomavirus (HPV) vaccine and primary ovarian insufficiency (POI) in six young women who developed the condition within 12 months of vaccination.
Using EHR data, researchers identified 46 women aged 11-34 years with idiopathic POI – 33 probable cases and 13 possible cases – after excluding cases with known causes. Eighteen of these cases also were excluded because they were diagnosed before the HPV vaccine was available.
They found that only 1 of the remaining 28 patients had been vaccinated against HPV before the symptom onset: a 16-year-old girl who was vaccinated about 23 months before the first clinical evaluation for delayed menarche. Their report was published in Pediatrics.
The adjusted hazard ratio for POI was therefore 0.30 after HPV vaccine, compared with 0.88 after Tdap, 1.42 after inactivated influenza vaccine, and 0.94 after meningococcal conjugate vaccine.
More than one-half of the 46 confirmed POI cases were diagnosed at age 27 years or older, and only one patient was diagnosed under 15 years of age.
“If POI is triggered by HPV or other adolescent vaccine exposure, we would have expected to see elevated incidence in the younger women who were most likely to be vaccinated, but instead we observed higher incidence in older women (greater than 26 years of age), which is consistent with 1 other population-based study of POI prevalence,” the authors wrote.
They acknowledged that studying POI as a vaccine-related adverse event was challenging because the time from symptom onset to diagnosis was variable. However, they said that 81% of their cohort was followed up for more than 2 years, and a mean of 5.14 years, so the potential for misclassification was “minimal.”
Dr. Naleway and her associates also noted that diagnoses of POI can be difficult to accurately identify, and symptoms may be masked by oral contraceptive use.
“Despite the challenges and limitations discussed above, we believe this study should lessen concern surrounding potential impact on fertility from HPV or other adolescent vaccination,” they wrote.
The Centers for Disease Control and Prevention supported the study. Three authors declared funding from pharmaceutical companies for unrelated studies. No other conflicts of interest were declared.
SOURCE: Naleway A et al. Pediatrics 2018;42(3):e20180943.
The human papillomavirus vaccine does not appear to be associated with an increased risk of ovarian insufficiency, according to researchers.
Allison L. Naleway, PhD, of the Center for Health Research at Kaiser Permanente Northwest, Portland, Ore., and her coauthors wrote that a previous case series had raised concerns about a possible link between the human papillomavirus (HPV) vaccine and primary ovarian insufficiency (POI) in six young women who developed the condition within 12 months of vaccination.
Using EHR data, researchers identified 46 women aged 11-34 years with idiopathic POI – 33 probable cases and 13 possible cases – after excluding cases with known causes. Eighteen of these cases also were excluded because they were diagnosed before the HPV vaccine was available.
They found that only 1 of the remaining 28 patients had been vaccinated against HPV before the symptom onset: a 16-year-old girl who was vaccinated about 23 months before the first clinical evaluation for delayed menarche. Their report was published in Pediatrics.
The adjusted hazard ratio for POI was therefore 0.30 after HPV vaccine, compared with 0.88 after Tdap, 1.42 after inactivated influenza vaccine, and 0.94 after meningococcal conjugate vaccine.
More than one-half of the 46 confirmed POI cases were diagnosed at age 27 years or older, and only one patient was diagnosed under 15 years of age.
“If POI is triggered by HPV or other adolescent vaccine exposure, we would have expected to see elevated incidence in the younger women who were most likely to be vaccinated, but instead we observed higher incidence in older women (greater than 26 years of age), which is consistent with 1 other population-based study of POI prevalence,” the authors wrote.
They acknowledged that studying POI as a vaccine-related adverse event was challenging because the time from symptom onset to diagnosis was variable. However, they said that 81% of their cohort was followed up for more than 2 years, and a mean of 5.14 years, so the potential for misclassification was “minimal.”
Dr. Naleway and her associates also noted that diagnoses of POI can be difficult to accurately identify, and symptoms may be masked by oral contraceptive use.
“Despite the challenges and limitations discussed above, we believe this study should lessen concern surrounding potential impact on fertility from HPV or other adolescent vaccination,” they wrote.
The Centers for Disease Control and Prevention supported the study. Three authors declared funding from pharmaceutical companies for unrelated studies. No other conflicts of interest were declared.
SOURCE: Naleway A et al. Pediatrics 2018;42(3):e20180943.
FROM PEDIATRICS
Key clinical point:
Major finding: The adjusted hazard ratio for POI was 0.30 after HPV vaccine, compared with 0.88 after Tdap, 1.42 after inactivated influenza vaccine, and 0.94 after meningococcal conjugate vaccine.
Study details: Analysis of medical records data for 46 women with confirmed iatrogenic primary ovarian failure.
Disclosures: The study was supported by the Centers for Disease Control and Prevention. Three authors declared funding from pharmaceutical companies for unrelated studies. No other conflicts of interest were declared.
Source: Naleway A et al. Pediatrics 2018;142(3):e20180943.
Sexual minorities seeking abortion report high levels of male violence
Pregnant lesbian and bisexual women who seek abortions are more likely than are their heterosexual counterparts to be the victims of violence by the men who impregnated them, a new study finds.
Rachel K. Jones, PhD, of the Guttmacher Institute, New York, and her associates also found that these sexual minority women, plus a group of individuals who described their sexual orientation as “something else,” were much more likely to report exposure to sexual and physical violence.
“No patient should be presumed to be heterosexual for any reason, including a pregnancy history. All pregnancies – like all patients – should be treated as unique and operating within the dynamic and interconnected circumstances of peoples’ lives, which may encompass differences in sexual orientation and exposure to violence,” the researchers wrote. Their report is in Obstetrics & Gynecology.
Previous research has suggested that nonheterosexual women are more likely than are straight women to become pregnant unintentionally. There also are signs suggesting that they have more abortions, too, although the findings are iffy, the study authors wrote.
For this study, Dr. Jones and her associates examined questionnaire answers of 8,380 women who responded to the Guttmacher Institute’s 2014 Abortion Patient Survey. All were undergoing abortions at 87 U.S. nonhospital facilities that performed 30 or more abortions each per year.
Of the sample, about 9% declined to describe their sexual orientation. Of the rest, 94% described themselves as heterosexual; of those, 41% were white, 28% were black, and 22% were Hispanic. Most were in their 20s, 47% were never married, and 48% had incomes below the federal poverty level.
Women also described themselves as bisexual (4%), “something else” (1%), and lesbian (0.4%). All these groups were more likely than were heterosexuals to be below the federal poverty level; more than half of the lesbian and bisexual respondents said they had previously given birth.
Fifteen percent of lesbians said their current pregnancy was caused by forced sex, compared with 1% of heterosexuals and 3% of bisexuals. (P less than .001).
Bisexuals (9%) and lesbians (33%) were more likely than were heterosexuals (4%) to say the men who impregnated them had physically abused them. The same was true for sexual abuse, which was reported by 7% of bisexuals, 35% of lesbians, and 2% of heterosexuals. After the researchers controlled for various factors including age and race, lesbians remained much more likely to report physical abuse, sexual abuse, and forced sex at the hands of the men who impregnated them (odds ratios = 15, 25, and 10, respectively, P less than .001).
“Exposure to physical and sexual violence was substantially higher among each of the sexual minority groups compared with their heterosexual counterparts, sometimes by a factor of 15 or more,” the study authors wrote. “We found that lesbian respondents had the highest levels of exposure to violence, perhaps because this population was more likely to have had sex with a man only in the context of forced sex.”
The researchers noted that their study has various limitations, such as low numbers of sexual minority women and the 4-year gap since the data were collected.
Still, Dr. Jones and her associates wrote, the study has strengths. “Health care providers, including those working in abortion settings, need to be aware that a proportion of their patient population identifies as something other than heterosexual,” they wrote.
The study was funded by the Susan Thompson Buffett Foundation with support from the National Institutes of Health via a grant to the Guttmacher Center for Population Research Innovation and Dissemination. The study authors reported no relevant financial disclosures.
SOURCE: Jones R et al. Obstet Gynecol. 2018 Sep;132(3):605-11.
Pregnant lesbian and bisexual women who seek abortions are more likely than are their heterosexual counterparts to be the victims of violence by the men who impregnated them, a new study finds.
Rachel K. Jones, PhD, of the Guttmacher Institute, New York, and her associates also found that these sexual minority women, plus a group of individuals who described their sexual orientation as “something else,” were much more likely to report exposure to sexual and physical violence.
“No patient should be presumed to be heterosexual for any reason, including a pregnancy history. All pregnancies – like all patients – should be treated as unique and operating within the dynamic and interconnected circumstances of peoples’ lives, which may encompass differences in sexual orientation and exposure to violence,” the researchers wrote. Their report is in Obstetrics & Gynecology.
Previous research has suggested that nonheterosexual women are more likely than are straight women to become pregnant unintentionally. There also are signs suggesting that they have more abortions, too, although the findings are iffy, the study authors wrote.
For this study, Dr. Jones and her associates examined questionnaire answers of 8,380 women who responded to the Guttmacher Institute’s 2014 Abortion Patient Survey. All were undergoing abortions at 87 U.S. nonhospital facilities that performed 30 or more abortions each per year.
Of the sample, about 9% declined to describe their sexual orientation. Of the rest, 94% described themselves as heterosexual; of those, 41% were white, 28% were black, and 22% were Hispanic. Most were in their 20s, 47% were never married, and 48% had incomes below the federal poverty level.
Women also described themselves as bisexual (4%), “something else” (1%), and lesbian (0.4%). All these groups were more likely than were heterosexuals to be below the federal poverty level; more than half of the lesbian and bisexual respondents said they had previously given birth.
Fifteen percent of lesbians said their current pregnancy was caused by forced sex, compared with 1% of heterosexuals and 3% of bisexuals. (P less than .001).
Bisexuals (9%) and lesbians (33%) were more likely than were heterosexuals (4%) to say the men who impregnated them had physically abused them. The same was true for sexual abuse, which was reported by 7% of bisexuals, 35% of lesbians, and 2% of heterosexuals. After the researchers controlled for various factors including age and race, lesbians remained much more likely to report physical abuse, sexual abuse, and forced sex at the hands of the men who impregnated them (odds ratios = 15, 25, and 10, respectively, P less than .001).
“Exposure to physical and sexual violence was substantially higher among each of the sexual minority groups compared with their heterosexual counterparts, sometimes by a factor of 15 or more,” the study authors wrote. “We found that lesbian respondents had the highest levels of exposure to violence, perhaps because this population was more likely to have had sex with a man only in the context of forced sex.”
The researchers noted that their study has various limitations, such as low numbers of sexual minority women and the 4-year gap since the data were collected.
Still, Dr. Jones and her associates wrote, the study has strengths. “Health care providers, including those working in abortion settings, need to be aware that a proportion of their patient population identifies as something other than heterosexual,” they wrote.
The study was funded by the Susan Thompson Buffett Foundation with support from the National Institutes of Health via a grant to the Guttmacher Center for Population Research Innovation and Dissemination. The study authors reported no relevant financial disclosures.
SOURCE: Jones R et al. Obstet Gynecol. 2018 Sep;132(3):605-11.
Pregnant lesbian and bisexual women who seek abortions are more likely than are their heterosexual counterparts to be the victims of violence by the men who impregnated them, a new study finds.
Rachel K. Jones, PhD, of the Guttmacher Institute, New York, and her associates also found that these sexual minority women, plus a group of individuals who described their sexual orientation as “something else,” were much more likely to report exposure to sexual and physical violence.
“No patient should be presumed to be heterosexual for any reason, including a pregnancy history. All pregnancies – like all patients – should be treated as unique and operating within the dynamic and interconnected circumstances of peoples’ lives, which may encompass differences in sexual orientation and exposure to violence,” the researchers wrote. Their report is in Obstetrics & Gynecology.
Previous research has suggested that nonheterosexual women are more likely than are straight women to become pregnant unintentionally. There also are signs suggesting that they have more abortions, too, although the findings are iffy, the study authors wrote.
For this study, Dr. Jones and her associates examined questionnaire answers of 8,380 women who responded to the Guttmacher Institute’s 2014 Abortion Patient Survey. All were undergoing abortions at 87 U.S. nonhospital facilities that performed 30 or more abortions each per year.
Of the sample, about 9% declined to describe their sexual orientation. Of the rest, 94% described themselves as heterosexual; of those, 41% were white, 28% were black, and 22% were Hispanic. Most were in their 20s, 47% were never married, and 48% had incomes below the federal poverty level.
Women also described themselves as bisexual (4%), “something else” (1%), and lesbian (0.4%). All these groups were more likely than were heterosexuals to be below the federal poverty level; more than half of the lesbian and bisexual respondents said they had previously given birth.
Fifteen percent of lesbians said their current pregnancy was caused by forced sex, compared with 1% of heterosexuals and 3% of bisexuals. (P less than .001).
Bisexuals (9%) and lesbians (33%) were more likely than were heterosexuals (4%) to say the men who impregnated them had physically abused them. The same was true for sexual abuse, which was reported by 7% of bisexuals, 35% of lesbians, and 2% of heterosexuals. After the researchers controlled for various factors including age and race, lesbians remained much more likely to report physical abuse, sexual abuse, and forced sex at the hands of the men who impregnated them (odds ratios = 15, 25, and 10, respectively, P less than .001).
“Exposure to physical and sexual violence was substantially higher among each of the sexual minority groups compared with their heterosexual counterparts, sometimes by a factor of 15 or more,” the study authors wrote. “We found that lesbian respondents had the highest levels of exposure to violence, perhaps because this population was more likely to have had sex with a man only in the context of forced sex.”
The researchers noted that their study has various limitations, such as low numbers of sexual minority women and the 4-year gap since the data were collected.
Still, Dr. Jones and her associates wrote, the study has strengths. “Health care providers, including those working in abortion settings, need to be aware that a proportion of their patient population identifies as something other than heterosexual,” they wrote.
The study was funded by the Susan Thompson Buffett Foundation with support from the National Institutes of Health via a grant to the Guttmacher Center for Population Research Innovation and Dissemination. The study authors reported no relevant financial disclosures.
SOURCE: Jones R et al. Obstet Gynecol. 2018 Sep;132(3):605-11.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: Fifteen percent of lesbians said their current pregnancy was caused by forced sex, compared with 1% of heterosexuals (P less than .001), and 3% of bisexuals. Lesbians (33%) were more likely than were heterosexuals (4%) to say the man who impregnated them had physically and/or sexually abused them.
Study details: A 2014 survey of 8,380 women seeking abortions at 87 U.S. nonhospital facilities.
Disclosures: The study was funded by the Susan Thompson Buffett Foundation with support from the National Institutes of Health via a grant to the Guttmacher Center for Population Research Innovation and Dissemination. The study authors reported no relevant financial disclosures.
Source: Jones R et al. Obstet Gynecol. 2018 Sep;132(3):605-11.
FDA approves first mobile app for contraceptive use
The Food and Drug Administration has approved marketing of the first medical mobile application that can be used as a contraceptive, the agency announced in a written statement.
The app, called Natural Cycles, contains an algorithm that calculates the days of the month women are likely to be fertile, based on daily body temperature readings and menstrual cycle information, and is intended for premenopausal women aged 18 years and older. App users are instructed to take their daily body temperatures with a basal body thermometer and enter the information into the app. The more-sensitive basal body thermometer can detect temperature elevations during ovulation, and the app will display a warning on days when users are most fertile. During these days, women should either abstain from sex or use protection.
In clinical studies comprising more than 15,000 women, Natural Cycles had a “perfect use” failure rate of 1.8% and a “normal use” failure rate of 6.5%. This compares favorably with other forms of contraception and birth control: Male condoms have a perfect use failure rate of 2.0% and a normal use failure rate of 18.0%, and combined oral contraceptives have a perfect use failure rate of 0.3% and a normal use failure rate of 9.0%, according to the Association of Reproductive Care Professionals.
“Consumers are increasingly using digital health technologies to inform their everyday health decisions, and this new app can provide an effective method of contraception if it’s used carefully and correctly,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in the statement.
The app should not be used by women with a condition in which a pregnancy would present risk to the mother or fetus or by women who are using a birth control or hormonal treatment that inhibits ovulation. Natural Cycles does not protect against sexually transmitted infections.
The Food and Drug Administration has approved marketing of the first medical mobile application that can be used as a contraceptive, the agency announced in a written statement.
The app, called Natural Cycles, contains an algorithm that calculates the days of the month women are likely to be fertile, based on daily body temperature readings and menstrual cycle information, and is intended for premenopausal women aged 18 years and older. App users are instructed to take their daily body temperatures with a basal body thermometer and enter the information into the app. The more-sensitive basal body thermometer can detect temperature elevations during ovulation, and the app will display a warning on days when users are most fertile. During these days, women should either abstain from sex or use protection.
In clinical studies comprising more than 15,000 women, Natural Cycles had a “perfect use” failure rate of 1.8% and a “normal use” failure rate of 6.5%. This compares favorably with other forms of contraception and birth control: Male condoms have a perfect use failure rate of 2.0% and a normal use failure rate of 18.0%, and combined oral contraceptives have a perfect use failure rate of 0.3% and a normal use failure rate of 9.0%, according to the Association of Reproductive Care Professionals.
“Consumers are increasingly using digital health technologies to inform their everyday health decisions, and this new app can provide an effective method of contraception if it’s used carefully and correctly,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in the statement.
The app should not be used by women with a condition in which a pregnancy would present risk to the mother or fetus or by women who are using a birth control or hormonal treatment that inhibits ovulation. Natural Cycles does not protect against sexually transmitted infections.
The Food and Drug Administration has approved marketing of the first medical mobile application that can be used as a contraceptive, the agency announced in a written statement.
The app, called Natural Cycles, contains an algorithm that calculates the days of the month women are likely to be fertile, based on daily body temperature readings and menstrual cycle information, and is intended for premenopausal women aged 18 years and older. App users are instructed to take their daily body temperatures with a basal body thermometer and enter the information into the app. The more-sensitive basal body thermometer can detect temperature elevations during ovulation, and the app will display a warning on days when users are most fertile. During these days, women should either abstain from sex or use protection.
In clinical studies comprising more than 15,000 women, Natural Cycles had a “perfect use” failure rate of 1.8% and a “normal use” failure rate of 6.5%. This compares favorably with other forms of contraception and birth control: Male condoms have a perfect use failure rate of 2.0% and a normal use failure rate of 18.0%, and combined oral contraceptives have a perfect use failure rate of 0.3% and a normal use failure rate of 9.0%, according to the Association of Reproductive Care Professionals.
“Consumers are increasingly using digital health technologies to inform their everyday health decisions, and this new app can provide an effective method of contraception if it’s used carefully and correctly,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in the statement.
The app should not be used by women with a condition in which a pregnancy would present risk to the mother or fetus or by women who are using a birth control or hormonal treatment that inhibits ovulation. Natural Cycles does not protect against sexually transmitted infections.
FDA warning shines light on vaginal rejuvenation
The Food and Drug Administration has issued a stern warning to several manufacturers and a statement of caution to the public concerning “vaginal rejuvenation,” an umbrella term for a host of procedures to alter vaginal tissue for therapeutic or cosmetic purposes.
Lasers and other energy-based devices have been approved to treat abnormal or precancerous cervical or vaginal tissue and general warts, but the FDA has not approved any to treat vaginal atrophy, urinary incontinence, or reduced sexual function.
Device manufacturers claim lasers can address these conditions despite limited scientific evidence for their safety or efficacy. Insurers do not reimburse the procedures, considering them to be cosmetic.
In a July 30 statement, FDA Commissioner Scott Gottlieb, MD, slammed “deceptive” marketing practices on the part of manufacturers.
The FDA has reviewed 12 complaints since December 2015 of adverse effects related to vaginal procedures using the devices. Two were from manufacturers reporting pain and bleeding in patients following treatment, FDA spokeswoman Deborah Kotz said in an interview. “The FDA has also received voluntary MedWatch reports from individual patients who experienced significant pain and discomfort from procedures performed with these devices.”
The agency has targeted seven firms: Alma Lasers, BTL Aesthetics, BTL Industries, Cynosure, InMode, Sciton, and ThermiGen, with letters demanding evidence of FDA approval, clearance, or intent to seek clearance for use of their products on female genitalia. They also asked for evidence backing specific claims.
In a July 26 letter to BTL Industries, for example, the FDA demanded to know why the firm was marketing its Exilis laser device, approved for the treatment of facial wrinkles, as “Ultra Femme 360,” which it called “a whole new approach to women’s intimate health.” The device, according to the manufacturer, “provides the shortest noninvasive radio-frequency treatment available for female intimate parts” and “is proven to increase elastin and collagen in the treatment area.”
The FDA asked Cynosure, the maker of the Mona Lisa Touch, a system marketed as an FDA-approved treatment for vaginal atrophy, for evidence to support its claims that Mona Lisa Touch “is the only technology for vaginal and vulvar health with over 18+ published clinical studies” and is clinically proven to treat “painful symptoms of menopause, including intimacy.” They also asked for information about a modification to the originally approved device that was not brought to the FDA’s attention.
In a letter to Alma Lasers, whose Pixel CO2 Laser System was approved for use in a broad use of surgical applications including gynecologic surgery, the FDA noted that the device was being marketed as “FEMILIFT,” a laser procedure designed to “improve vaginal irregularities” and to assist “in vaginal mucosa revitalization.” The FDA demanded evidence for those claims.
The manufacturers have 30 days to respond to the FDA, which has not ruled out seeking enforcement action against firms with unsatisfactory responses.
For more than a decade, researchers have shown that healthy vaginal morphology is exceptionally wide ranging, including a recent study in more than 650 women, the largest to date (BJOG. 2018 Jun 25. doi: 10.1111/1471-0528.15387). Nonetheless, interest in elective vaginal procedures has only increased, with an industry report from the International Society of Aesthetic and Plastic Surgery describing a 45% increase in the use of one surgery, vaginal labiaplasty, between 2014 and 2016. Most procedures were performed in Brazil and the United States.
While plastic surgery societies support vaginal rejuvenation procedures, the American College of Obstetricians and Gynecologists has long frowned on them, with its first critique issued in a 2007 committee opinion. “No adequate studies have been published assessing the long-term satisfaction, safety, and complication rates for these procedures,” the association said last year in its most recent update on the subject.
Gynecologist David M. Jaspan, DO, of the Einstein Healthcare Network in Philadelphia, echoed ACOG’s views and said he welcomed FDA interest in vaginal rejuvenation.
The practice has “never been endorsed by the College or a board. It’s been considered a cosmetic procedure and it’s been under scrutiny for at least a decade,” Dr. Jaspan said. “I have reservations about the clinical outcomes and the training surrounds these procedures and I anxiously await randomized controlled trials to further evaluate them.”
Gynecologists who offer the procedures caution that they may have a role, and that randomized trials are underway to determine which groups of women might be best helped.
Marie Paraiso, MD, professor of obstetrics and gynecology at the Cleveland Clinic, said she uses the Mona Lisa Touch, a CO2 fractionated laser, to treat patients with genitourinary syndrome of menopause (GSM). These patients, Dr. Paraiso said, “complain of vaginal dryness and are unable to have intercourse or experience significant pain during or after intercourse. Some of them also may have irritative voiding, urinary frequency and urgency, or mild stress incontinence.”
Dr. Paraiso’s group has performed some 300 treatments with the laser and “we have fortunately not had patients complaining of persistent vaginal pain or scarring.” About 80%-90% of patients respond, she said, with some 20%-25% seeking retreatment within a year. “I believe for women who have contraindications to hormonal therapy or do not tolerate or cannot afford prolonged hormonal therapy, the CO2 fractional vaginal laser has been effective.”
Dr. Paraiso is also leading a multisite clinical trial randomizing about 200 patients with GSM to the laser treatment or estrogen-based vaginal creams, and following them for 6 months; thus far, she said, 6 of 89 patients, half in the laser arm, have reported mild to moderate adverse events.
Dr. Paraiso said she does not have a financial relationship with the manufacturer of Mona Lisa Touch, and that the trial was funded by the Foundation for Female Health Awareness, which receives unrestricted research grants from some device makers. “Our institute owns the laser and I have never been paid to train anyone to perform these procedures,” Dr. Paraiso added. “Our onus was to study the laser in order to improve the lives of our patients.”
Other trials comparing vaginal lasers with sham treatment are currently underway or in planning.
The North American Menopause Society struck a cautious note in response to the FDA criticism. In a statement issued August 1, JoAnn Pinkerton, MD, the society’s executive director, said the field needed prospective, randomized, sham-controlled trials of the laser and energy therapies. The therapies “may turn out to be an appropriate choice for many women, particularly for those concerned about breast cancer risk” associated with hormonal treatments. But until more robust data are available, doctors should “discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, and FDA-approved vaginal therapies such as vaginal estrogen and intravaginal dehydroepiandrosterone and oral therapies such as hormone therapy and ospemifene to determine the best treatment for women with GSM.”
Any discussion of vaginal energy-based therapies, should include the disclosure that these have not been approved for the specific indication, Dr. Pinkerton cautioned.
The term “vaginal rejuvenation,” coined by cosmetic gynecologists, incorporates surgeries designed to modify the appearance of the vulva, reduce the redundancy of vaginal tissue, and improve vaginal tone.
Endorsed by some well-known academic gynecologists, these devices have been promoted as “safe and effective” without any prospective, randomized studies and without accountability for conflicts of interest including “educational stipends” from device manufacturers and clinicians’ need to recoup the high cost of the devices themselves.
Studies have generally been limited to fewer than 100 patients followed for 12 weeks or less. Companies are not informing doctors that the devices may not be FDA approved for the purposes advertised, nor are they providing adverse effects reports. Laser and radio-frequency procedures at best demonstrate temporary, marginal improvement in vaginal tone and dyspareunia, and at worst are associated with increased pelvic pain and dyspareunia, as well as vaginal, rectal, and bladder thermal burns. For those of us who specialize in cosmetic surgery, they have very limited benefit with a significant risk of injury to the patient even when properly used.
Julio Cesar Novoa, MD, is a private practice ob.gyn from El Paso, Tex. He reported no relevant conflicts of interest.
The term “vaginal rejuvenation,” coined by cosmetic gynecologists, incorporates surgeries designed to modify the appearance of the vulva, reduce the redundancy of vaginal tissue, and improve vaginal tone.
Endorsed by some well-known academic gynecologists, these devices have been promoted as “safe and effective” without any prospective, randomized studies and without accountability for conflicts of interest including “educational stipends” from device manufacturers and clinicians’ need to recoup the high cost of the devices themselves.
Studies have generally been limited to fewer than 100 patients followed for 12 weeks or less. Companies are not informing doctors that the devices may not be FDA approved for the purposes advertised, nor are they providing adverse effects reports. Laser and radio-frequency procedures at best demonstrate temporary, marginal improvement in vaginal tone and dyspareunia, and at worst are associated with increased pelvic pain and dyspareunia, as well as vaginal, rectal, and bladder thermal burns. For those of us who specialize in cosmetic surgery, they have very limited benefit with a significant risk of injury to the patient even when properly used.
Julio Cesar Novoa, MD, is a private practice ob.gyn from El Paso, Tex. He reported no relevant conflicts of interest.
The term “vaginal rejuvenation,” coined by cosmetic gynecologists, incorporates surgeries designed to modify the appearance of the vulva, reduce the redundancy of vaginal tissue, and improve vaginal tone.
Endorsed by some well-known academic gynecologists, these devices have been promoted as “safe and effective” without any prospective, randomized studies and without accountability for conflicts of interest including “educational stipends” from device manufacturers and clinicians’ need to recoup the high cost of the devices themselves.
Studies have generally been limited to fewer than 100 patients followed for 12 weeks or less. Companies are not informing doctors that the devices may not be FDA approved for the purposes advertised, nor are they providing adverse effects reports. Laser and radio-frequency procedures at best demonstrate temporary, marginal improvement in vaginal tone and dyspareunia, and at worst are associated with increased pelvic pain and dyspareunia, as well as vaginal, rectal, and bladder thermal burns. For those of us who specialize in cosmetic surgery, they have very limited benefit with a significant risk of injury to the patient even when properly used.
Julio Cesar Novoa, MD, is a private practice ob.gyn from El Paso, Tex. He reported no relevant conflicts of interest.
The Food and Drug Administration has issued a stern warning to several manufacturers and a statement of caution to the public concerning “vaginal rejuvenation,” an umbrella term for a host of procedures to alter vaginal tissue for therapeutic or cosmetic purposes.
Lasers and other energy-based devices have been approved to treat abnormal or precancerous cervical or vaginal tissue and general warts, but the FDA has not approved any to treat vaginal atrophy, urinary incontinence, or reduced sexual function.
Device manufacturers claim lasers can address these conditions despite limited scientific evidence for their safety or efficacy. Insurers do not reimburse the procedures, considering them to be cosmetic.
In a July 30 statement, FDA Commissioner Scott Gottlieb, MD, slammed “deceptive” marketing practices on the part of manufacturers.
The FDA has reviewed 12 complaints since December 2015 of adverse effects related to vaginal procedures using the devices. Two were from manufacturers reporting pain and bleeding in patients following treatment, FDA spokeswoman Deborah Kotz said in an interview. “The FDA has also received voluntary MedWatch reports from individual patients who experienced significant pain and discomfort from procedures performed with these devices.”
The agency has targeted seven firms: Alma Lasers, BTL Aesthetics, BTL Industries, Cynosure, InMode, Sciton, and ThermiGen, with letters demanding evidence of FDA approval, clearance, or intent to seek clearance for use of their products on female genitalia. They also asked for evidence backing specific claims.
In a July 26 letter to BTL Industries, for example, the FDA demanded to know why the firm was marketing its Exilis laser device, approved for the treatment of facial wrinkles, as “Ultra Femme 360,” which it called “a whole new approach to women’s intimate health.” The device, according to the manufacturer, “provides the shortest noninvasive radio-frequency treatment available for female intimate parts” and “is proven to increase elastin and collagen in the treatment area.”
The FDA asked Cynosure, the maker of the Mona Lisa Touch, a system marketed as an FDA-approved treatment for vaginal atrophy, for evidence to support its claims that Mona Lisa Touch “is the only technology for vaginal and vulvar health with over 18+ published clinical studies” and is clinically proven to treat “painful symptoms of menopause, including intimacy.” They also asked for information about a modification to the originally approved device that was not brought to the FDA’s attention.
In a letter to Alma Lasers, whose Pixel CO2 Laser System was approved for use in a broad use of surgical applications including gynecologic surgery, the FDA noted that the device was being marketed as “FEMILIFT,” a laser procedure designed to “improve vaginal irregularities” and to assist “in vaginal mucosa revitalization.” The FDA demanded evidence for those claims.
The manufacturers have 30 days to respond to the FDA, which has not ruled out seeking enforcement action against firms with unsatisfactory responses.
For more than a decade, researchers have shown that healthy vaginal morphology is exceptionally wide ranging, including a recent study in more than 650 women, the largest to date (BJOG. 2018 Jun 25. doi: 10.1111/1471-0528.15387). Nonetheless, interest in elective vaginal procedures has only increased, with an industry report from the International Society of Aesthetic and Plastic Surgery describing a 45% increase in the use of one surgery, vaginal labiaplasty, between 2014 and 2016. Most procedures were performed in Brazil and the United States.
While plastic surgery societies support vaginal rejuvenation procedures, the American College of Obstetricians and Gynecologists has long frowned on them, with its first critique issued in a 2007 committee opinion. “No adequate studies have been published assessing the long-term satisfaction, safety, and complication rates for these procedures,” the association said last year in its most recent update on the subject.
Gynecologist David M. Jaspan, DO, of the Einstein Healthcare Network in Philadelphia, echoed ACOG’s views and said he welcomed FDA interest in vaginal rejuvenation.
The practice has “never been endorsed by the College or a board. It’s been considered a cosmetic procedure and it’s been under scrutiny for at least a decade,” Dr. Jaspan said. “I have reservations about the clinical outcomes and the training surrounds these procedures and I anxiously await randomized controlled trials to further evaluate them.”
Gynecologists who offer the procedures caution that they may have a role, and that randomized trials are underway to determine which groups of women might be best helped.
Marie Paraiso, MD, professor of obstetrics and gynecology at the Cleveland Clinic, said she uses the Mona Lisa Touch, a CO2 fractionated laser, to treat patients with genitourinary syndrome of menopause (GSM). These patients, Dr. Paraiso said, “complain of vaginal dryness and are unable to have intercourse or experience significant pain during or after intercourse. Some of them also may have irritative voiding, urinary frequency and urgency, or mild stress incontinence.”
Dr. Paraiso’s group has performed some 300 treatments with the laser and “we have fortunately not had patients complaining of persistent vaginal pain or scarring.” About 80%-90% of patients respond, she said, with some 20%-25% seeking retreatment within a year. “I believe for women who have contraindications to hormonal therapy or do not tolerate or cannot afford prolonged hormonal therapy, the CO2 fractional vaginal laser has been effective.”
Dr. Paraiso is also leading a multisite clinical trial randomizing about 200 patients with GSM to the laser treatment or estrogen-based vaginal creams, and following them for 6 months; thus far, she said, 6 of 89 patients, half in the laser arm, have reported mild to moderate adverse events.
Dr. Paraiso said she does not have a financial relationship with the manufacturer of Mona Lisa Touch, and that the trial was funded by the Foundation for Female Health Awareness, which receives unrestricted research grants from some device makers. “Our institute owns the laser and I have never been paid to train anyone to perform these procedures,” Dr. Paraiso added. “Our onus was to study the laser in order to improve the lives of our patients.”
Other trials comparing vaginal lasers with sham treatment are currently underway or in planning.
The North American Menopause Society struck a cautious note in response to the FDA criticism. In a statement issued August 1, JoAnn Pinkerton, MD, the society’s executive director, said the field needed prospective, randomized, sham-controlled trials of the laser and energy therapies. The therapies “may turn out to be an appropriate choice for many women, particularly for those concerned about breast cancer risk” associated with hormonal treatments. But until more robust data are available, doctors should “discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, and FDA-approved vaginal therapies such as vaginal estrogen and intravaginal dehydroepiandrosterone and oral therapies such as hormone therapy and ospemifene to determine the best treatment for women with GSM.”
Any discussion of vaginal energy-based therapies, should include the disclosure that these have not been approved for the specific indication, Dr. Pinkerton cautioned.
The Food and Drug Administration has issued a stern warning to several manufacturers and a statement of caution to the public concerning “vaginal rejuvenation,” an umbrella term for a host of procedures to alter vaginal tissue for therapeutic or cosmetic purposes.
Lasers and other energy-based devices have been approved to treat abnormal or precancerous cervical or vaginal tissue and general warts, but the FDA has not approved any to treat vaginal atrophy, urinary incontinence, or reduced sexual function.
Device manufacturers claim lasers can address these conditions despite limited scientific evidence for their safety or efficacy. Insurers do not reimburse the procedures, considering them to be cosmetic.
In a July 30 statement, FDA Commissioner Scott Gottlieb, MD, slammed “deceptive” marketing practices on the part of manufacturers.
The FDA has reviewed 12 complaints since December 2015 of adverse effects related to vaginal procedures using the devices. Two were from manufacturers reporting pain and bleeding in patients following treatment, FDA spokeswoman Deborah Kotz said in an interview. “The FDA has also received voluntary MedWatch reports from individual patients who experienced significant pain and discomfort from procedures performed with these devices.”
The agency has targeted seven firms: Alma Lasers, BTL Aesthetics, BTL Industries, Cynosure, InMode, Sciton, and ThermiGen, with letters demanding evidence of FDA approval, clearance, or intent to seek clearance for use of their products on female genitalia. They also asked for evidence backing specific claims.
In a July 26 letter to BTL Industries, for example, the FDA demanded to know why the firm was marketing its Exilis laser device, approved for the treatment of facial wrinkles, as “Ultra Femme 360,” which it called “a whole new approach to women’s intimate health.” The device, according to the manufacturer, “provides the shortest noninvasive radio-frequency treatment available for female intimate parts” and “is proven to increase elastin and collagen in the treatment area.”
The FDA asked Cynosure, the maker of the Mona Lisa Touch, a system marketed as an FDA-approved treatment for vaginal atrophy, for evidence to support its claims that Mona Lisa Touch “is the only technology for vaginal and vulvar health with over 18+ published clinical studies” and is clinically proven to treat “painful symptoms of menopause, including intimacy.” They also asked for information about a modification to the originally approved device that was not brought to the FDA’s attention.
In a letter to Alma Lasers, whose Pixel CO2 Laser System was approved for use in a broad use of surgical applications including gynecologic surgery, the FDA noted that the device was being marketed as “FEMILIFT,” a laser procedure designed to “improve vaginal irregularities” and to assist “in vaginal mucosa revitalization.” The FDA demanded evidence for those claims.
The manufacturers have 30 days to respond to the FDA, which has not ruled out seeking enforcement action against firms with unsatisfactory responses.
For more than a decade, researchers have shown that healthy vaginal morphology is exceptionally wide ranging, including a recent study in more than 650 women, the largest to date (BJOG. 2018 Jun 25. doi: 10.1111/1471-0528.15387). Nonetheless, interest in elective vaginal procedures has only increased, with an industry report from the International Society of Aesthetic and Plastic Surgery describing a 45% increase in the use of one surgery, vaginal labiaplasty, between 2014 and 2016. Most procedures were performed in Brazil and the United States.
While plastic surgery societies support vaginal rejuvenation procedures, the American College of Obstetricians and Gynecologists has long frowned on them, with its first critique issued in a 2007 committee opinion. “No adequate studies have been published assessing the long-term satisfaction, safety, and complication rates for these procedures,” the association said last year in its most recent update on the subject.
Gynecologist David M. Jaspan, DO, of the Einstein Healthcare Network in Philadelphia, echoed ACOG’s views and said he welcomed FDA interest in vaginal rejuvenation.
The practice has “never been endorsed by the College or a board. It’s been considered a cosmetic procedure and it’s been under scrutiny for at least a decade,” Dr. Jaspan said. “I have reservations about the clinical outcomes and the training surrounds these procedures and I anxiously await randomized controlled trials to further evaluate them.”
Gynecologists who offer the procedures caution that they may have a role, and that randomized trials are underway to determine which groups of women might be best helped.
Marie Paraiso, MD, professor of obstetrics and gynecology at the Cleveland Clinic, said she uses the Mona Lisa Touch, a CO2 fractionated laser, to treat patients with genitourinary syndrome of menopause (GSM). These patients, Dr. Paraiso said, “complain of vaginal dryness and are unable to have intercourse or experience significant pain during or after intercourse. Some of them also may have irritative voiding, urinary frequency and urgency, or mild stress incontinence.”
Dr. Paraiso’s group has performed some 300 treatments with the laser and “we have fortunately not had patients complaining of persistent vaginal pain or scarring.” About 80%-90% of patients respond, she said, with some 20%-25% seeking retreatment within a year. “I believe for women who have contraindications to hormonal therapy or do not tolerate or cannot afford prolonged hormonal therapy, the CO2 fractional vaginal laser has been effective.”
Dr. Paraiso is also leading a multisite clinical trial randomizing about 200 patients with GSM to the laser treatment or estrogen-based vaginal creams, and following them for 6 months; thus far, she said, 6 of 89 patients, half in the laser arm, have reported mild to moderate adverse events.
Dr. Paraiso said she does not have a financial relationship with the manufacturer of Mona Lisa Touch, and that the trial was funded by the Foundation for Female Health Awareness, which receives unrestricted research grants from some device makers. “Our institute owns the laser and I have never been paid to train anyone to perform these procedures,” Dr. Paraiso added. “Our onus was to study the laser in order to improve the lives of our patients.”
Other trials comparing vaginal lasers with sham treatment are currently underway or in planning.
The North American Menopause Society struck a cautious note in response to the FDA criticism. In a statement issued August 1, JoAnn Pinkerton, MD, the society’s executive director, said the field needed prospective, randomized, sham-controlled trials of the laser and energy therapies. The therapies “may turn out to be an appropriate choice for many women, particularly for those concerned about breast cancer risk” associated with hormonal treatments. But until more robust data are available, doctors should “discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, and FDA-approved vaginal therapies such as vaginal estrogen and intravaginal dehydroepiandrosterone and oral therapies such as hormone therapy and ospemifene to determine the best treatment for women with GSM.”
Any discussion of vaginal energy-based therapies, should include the disclosure that these have not been approved for the specific indication, Dr. Pinkerton cautioned.
2018 Update on abnormal uterine bleeding
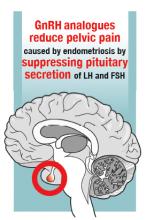
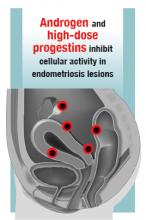
Over the past year, a few gems have been published to help us manage and treat abnormal uterine bleeding (AUB). One study suggests an order of performing hysteroscopy and endometrial biopsy, another emphasizes the continued cost-effectiveness of the levonorgestrel-releasing intrauterine system (LNG-IUS), while a third provides more evidence that ulipristal acetate is effective in the management of leiomyomas.
Optimal order of office hysteroscopy and endometrial biopsy?
Sarkar P, Mikhail E, Schickler R, Plosker S, Imudia AN. Optimal order of successive office hysteroscopy and endometrial biopsy for the evaluation of abnormal uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2017;130(3):565-572.
Office hysteroscopy and endometrial biopsy are frequently used in the evaluation of women presenting with AUB. Sarkar and colleagues conducted a study aimed at estimating the optimal order of office hysteroscopy and endometrial biopsy when performed successively among premenopausal women.
Pain perception, procedure duration, and other outcomes
This prospective single-blind randomized trial included 78 consecutive patients. The primary outcome was detection of any difference in patients' global pain perception based on the order of the procedures. Secondary outcome measures included determining whether the procedure order affected the duration of the procedures, the adequacy of the endometrial biopsy sample, the number of attempts to obtain an adequate tissue sample, and optimal visualization of the endometrial cavity during office hysteroscopy.
Order not important, but other factors may be
Not surprisingly, the results showed that the order in which the procedures were performed had no effect on patients' pain perception or on the overall procedure duration. Assessed using a visual analog scale scored from 1 to 10, global pain perception in the hysteroscopy-first patients (group A, n = 40) compared with the biopsy-first patients (group B, n = 38) was similar (7 vs 7, P = .57; 95% confidence interval [CI], 5.8-7.1). Procedure duration also was similar in group A and group B (3 vs 3, P = .32; 95% CI, 3.3-4.1).
However, when hysteroscopy was performed first, the quality of endometrial cavity images was superior compared with images from patients in whom biopsy was performed first. The number of endometrial biopsy curette passes required to obtain an adequate tissue sample was lower in the biopsy-first patients. The endometrial biopsy specimen was adequate for histologic evaluation regardless of whether hysteroscopy or biopsy was performed first.
Sarkar and colleagues suggested that their study findings emphasize the importance of individualizing the order of successive procedures to achieve the most clinically relevant result with maximum ease and comfort. They proposed that patients who have a high index of suspicion for occult malignancy or endometrial hyperplasia should have a biopsy procedure first so that adequate tissue samples can be obtained with fewer attempts. In patients with underlying uterine anatomic defects, performing hysteroscopy first would be clinically relevant to obtain the best images for optimal surgical planning.
Read next: Which treatment for AUB is most cost-effective?
Which treatment for AUB is most cost-effective?
Spencer JC, Louie M, Moulder JK, et al. Cost-effectiveness of treatments for heavy menstrual bleeding. Am J Obstet Gynecol. 2017;217(5):574.e1-574e.9.
The costs associated with heavy menstrual bleeding are significant. Spencer and colleagues sought to evaluate the relative cost-effectiveness of 4 treatment options for heavy menstrual bleeding: hysterectomy, resectoscopic endometrial ablation, nonresectoscopic endometrial ablation, and the LNG-IUS in a hypothetical cohort of 100,000 premenopausal women. No previous studies have examined the cost-effectiveness of these options in the context of the US health care setting.
Decision tree used for analysis
The authors formulated a decision tree to evaluate private payer costs and quality-adjusted life-years over a 5-year time horizon for premenopausal women with heavy menstrual bleeding and no suspected malignancy. For each treatment option, the authors used probabilities to estimate frequencies of complications and treatment failure leading to additional therapies. They compared the treatments in terms of total average costs, quality-adjusted life years, and incremental cost-effectiveness ratios.
Comparing costs, quality of life, and complications
Quality of life was fairly high for all treatment options; however, the estimated costs and the complications of each treatment were markedly different between treatment options. The LNG-IUS was superior to all alternatives in terms of both cost and quality, making it the dominant strategy. The 5-year cost for the LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500). When examined over a range of possible values, the LNG-IUS was cost-effective compared with hysterectomy in the large majority of scenarios (90%).
If the LNG-IUS is removed from consideration because of either patient preference or clinical judgment, the decision between hysterectomy and ablation is more complex. Hysterectomy results in better quality of life in the majority of simulations, but it is cost-effective in just more than half of the simulations compared with either resectoscopic or nonresectoscopic ablation. Therefore, consideration of cost, procedure-specific complications, and patient preferences may guide the therapeutic decision between hysterectomy and endometrial ablation.
The 52-mg LNG-IUS was superior to all treatment alternatives in both cost and quality, making it the dominant strategy for the treatment of heavy menstrual bleeding.
Ulipristal may be useful for managing AUB associated with uterine leiomyomas
Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;131(3):431-439.
Managing uterine leiomyomas is a common issue for gynecologists, as up to 70% of white women and more than 80% of black women of reproductive age in the United States have leiomyomas.
Ulipristal acetate is an orally administered selective progesterone-receptor modulator that decreases bleeding and reduces leiomyoma size. Although trials conducted in Europe found ulipristal to be superior to placebo and noninferior to leuprolide acetate in controlling bleeding and reducing leiomyoma size, those initial trials were conducted in a predominantly white population.
Study assessed efficacy and safety
Simon and colleagues recently conducted a randomized double-blind, placebo-controlled trial designed to assess the safety and efficacy of ulipristal in a more diverse population, such as patients in the United States. The 148 participants included in the study were randomly assigned on a 1:1:1 basis to once-daily oral ulipristal 5 mg, ulipristal 10 mg, or placebo for 12 weeks, with a 12-week drug-free follow-up.
Amenorrhea achieved and quality of life improved
The investigators found that ulipristal in 5-mg and 10-mg doses was well tolerated and superior to placebo in both the rate of and the time to amenorrhea (the coprimary end points) in women with symptomatic leiomyomas. In women treated with ulipristal 5 mg, amenorrhea was achieved in 25 of 53 (47.2%; 97.5% CI, 31.6-63.2), and of those treated with the 10-mg dose, 28 of 48 (58.3%; 97.5% CI, 41.2-74.1) achieved amenorrhea (P<.001 for both groups), compared with 1 of 56 (1.8%; 97.5% CI, 0.0-10.9) in the placebo group.
AUB continues to be a significant issue for many women. As women's health care providers, it is important that we deliver care with high value (Quality ÷ Cost). Therefore, consider these takeaway points:
- The LNG-IUS consistently delivers high value by affecting both sides of this equation. We should use it more.
- Although we do not yet know what ulipristal acetate will cost in the United States, effective medical treatments usually affect both sides of the Quality ÷ Cost equation, and new medications on the horizon are worth knowing about.
- Last, efficiency with office-based hysteroscopy is also an opportunity to increase value by improving biopsy and visualization quality.
Ulipristal treatment also was shown to improve health-related quality of life, including physical and social activities. No patient discontinued ulipristal because of lack of efficacy, and 1 patient in the placebo group stopped taking the drug because of an adverse event. Estradiol levels were maintained at midfollicular levels during ulipristal treatment, and endometrial biopsies did not show any atypical or malignant changes. These results are consistent with those of the studies conducted in Europe in a predominantly white, nonobese population.
Results of this study help to define a niche for ulipristal when hysterectomy is not an option for women who wish to preserve fertility. Further, although leuprolide is used for preoperative hematologic improvement of anemia, its use results in hypoestrogenic adverse effects.
The findings from this and other studies suggest that ulipristal may be useful for the medical management of AUB associated with uterine leiomyomas, especially for patients desiring uterine- and fertility-sparing treatment. Hopefully, this treatment will be available soon in the United States.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Over the past year, a few gems have been published to help us manage and treat abnormal uterine bleeding (AUB). One study suggests an order of performing hysteroscopy and endometrial biopsy, another emphasizes the continued cost-effectiveness of the levonorgestrel-releasing intrauterine system (LNG-IUS), while a third provides more evidence that ulipristal acetate is effective in the management of leiomyomas.
Optimal order of office hysteroscopy and endometrial biopsy?
Sarkar P, Mikhail E, Schickler R, Plosker S, Imudia AN. Optimal order of successive office hysteroscopy and endometrial biopsy for the evaluation of abnormal uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2017;130(3):565-572.
Office hysteroscopy and endometrial biopsy are frequently used in the evaluation of women presenting with AUB. Sarkar and colleagues conducted a study aimed at estimating the optimal order of office hysteroscopy and endometrial biopsy when performed successively among premenopausal women.
Pain perception, procedure duration, and other outcomes
This prospective single-blind randomized trial included 78 consecutive patients. The primary outcome was detection of any difference in patients' global pain perception based on the order of the procedures. Secondary outcome measures included determining whether the procedure order affected the duration of the procedures, the adequacy of the endometrial biopsy sample, the number of attempts to obtain an adequate tissue sample, and optimal visualization of the endometrial cavity during office hysteroscopy.
Order not important, but other factors may be
Not surprisingly, the results showed that the order in which the procedures were performed had no effect on patients' pain perception or on the overall procedure duration. Assessed using a visual analog scale scored from 1 to 10, global pain perception in the hysteroscopy-first patients (group A, n = 40) compared with the biopsy-first patients (group B, n = 38) was similar (7 vs 7, P = .57; 95% confidence interval [CI], 5.8-7.1). Procedure duration also was similar in group A and group B (3 vs 3, P = .32; 95% CI, 3.3-4.1).
However, when hysteroscopy was performed first, the quality of endometrial cavity images was superior compared with images from patients in whom biopsy was performed first. The number of endometrial biopsy curette passes required to obtain an adequate tissue sample was lower in the biopsy-first patients. The endometrial biopsy specimen was adequate for histologic evaluation regardless of whether hysteroscopy or biopsy was performed first.
Sarkar and colleagues suggested that their study findings emphasize the importance of individualizing the order of successive procedures to achieve the most clinically relevant result with maximum ease and comfort. They proposed that patients who have a high index of suspicion for occult malignancy or endometrial hyperplasia should have a biopsy procedure first so that adequate tissue samples can be obtained with fewer attempts. In patients with underlying uterine anatomic defects, performing hysteroscopy first would be clinically relevant to obtain the best images for optimal surgical planning.
Read next: Which treatment for AUB is most cost-effective?
Which treatment for AUB is most cost-effective?
Spencer JC, Louie M, Moulder JK, et al. Cost-effectiveness of treatments for heavy menstrual bleeding. Am J Obstet Gynecol. 2017;217(5):574.e1-574e.9.
The costs associated with heavy menstrual bleeding are significant. Spencer and colleagues sought to evaluate the relative cost-effectiveness of 4 treatment options for heavy menstrual bleeding: hysterectomy, resectoscopic endometrial ablation, nonresectoscopic endometrial ablation, and the LNG-IUS in a hypothetical cohort of 100,000 premenopausal women. No previous studies have examined the cost-effectiveness of these options in the context of the US health care setting.
Decision tree used for analysis
The authors formulated a decision tree to evaluate private payer costs and quality-adjusted life-years over a 5-year time horizon for premenopausal women with heavy menstrual bleeding and no suspected malignancy. For each treatment option, the authors used probabilities to estimate frequencies of complications and treatment failure leading to additional therapies. They compared the treatments in terms of total average costs, quality-adjusted life years, and incremental cost-effectiveness ratios.
Comparing costs, quality of life, and complications
Quality of life was fairly high for all treatment options; however, the estimated costs and the complications of each treatment were markedly different between treatment options. The LNG-IUS was superior to all alternatives in terms of both cost and quality, making it the dominant strategy. The 5-year cost for the LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500). When examined over a range of possible values, the LNG-IUS was cost-effective compared with hysterectomy in the large majority of scenarios (90%).
If the LNG-IUS is removed from consideration because of either patient preference or clinical judgment, the decision between hysterectomy and ablation is more complex. Hysterectomy results in better quality of life in the majority of simulations, but it is cost-effective in just more than half of the simulations compared with either resectoscopic or nonresectoscopic ablation. Therefore, consideration of cost, procedure-specific complications, and patient preferences may guide the therapeutic decision between hysterectomy and endometrial ablation.
The 52-mg LNG-IUS was superior to all treatment alternatives in both cost and quality, making it the dominant strategy for the treatment of heavy menstrual bleeding.
Ulipristal may be useful for managing AUB associated with uterine leiomyomas
Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;131(3):431-439.
Managing uterine leiomyomas is a common issue for gynecologists, as up to 70% of white women and more than 80% of black women of reproductive age in the United States have leiomyomas.
Ulipristal acetate is an orally administered selective progesterone-receptor modulator that decreases bleeding and reduces leiomyoma size. Although trials conducted in Europe found ulipristal to be superior to placebo and noninferior to leuprolide acetate in controlling bleeding and reducing leiomyoma size, those initial trials were conducted in a predominantly white population.
Study assessed efficacy and safety
Simon and colleagues recently conducted a randomized double-blind, placebo-controlled trial designed to assess the safety and efficacy of ulipristal in a more diverse population, such as patients in the United States. The 148 participants included in the study were randomly assigned on a 1:1:1 basis to once-daily oral ulipristal 5 mg, ulipristal 10 mg, or placebo for 12 weeks, with a 12-week drug-free follow-up.
Amenorrhea achieved and quality of life improved
The investigators found that ulipristal in 5-mg and 10-mg doses was well tolerated and superior to placebo in both the rate of and the time to amenorrhea (the coprimary end points) in women with symptomatic leiomyomas. In women treated with ulipristal 5 mg, amenorrhea was achieved in 25 of 53 (47.2%; 97.5% CI, 31.6-63.2), and of those treated with the 10-mg dose, 28 of 48 (58.3%; 97.5% CI, 41.2-74.1) achieved amenorrhea (P<.001 for both groups), compared with 1 of 56 (1.8%; 97.5% CI, 0.0-10.9) in the placebo group.
AUB continues to be a significant issue for many women. As women's health care providers, it is important that we deliver care with high value (Quality ÷ Cost). Therefore, consider these takeaway points:
- The LNG-IUS consistently delivers high value by affecting both sides of this equation. We should use it more.
- Although we do not yet know what ulipristal acetate will cost in the United States, effective medical treatments usually affect both sides of the Quality ÷ Cost equation, and new medications on the horizon are worth knowing about.
- Last, efficiency with office-based hysteroscopy is also an opportunity to increase value by improving biopsy and visualization quality.
Ulipristal treatment also was shown to improve health-related quality of life, including physical and social activities. No patient discontinued ulipristal because of lack of efficacy, and 1 patient in the placebo group stopped taking the drug because of an adverse event. Estradiol levels were maintained at midfollicular levels during ulipristal treatment, and endometrial biopsies did not show any atypical or malignant changes. These results are consistent with those of the studies conducted in Europe in a predominantly white, nonobese population.
Results of this study help to define a niche for ulipristal when hysterectomy is not an option for women who wish to preserve fertility. Further, although leuprolide is used for preoperative hematologic improvement of anemia, its use results in hypoestrogenic adverse effects.
The findings from this and other studies suggest that ulipristal may be useful for the medical management of AUB associated with uterine leiomyomas, especially for patients desiring uterine- and fertility-sparing treatment. Hopefully, this treatment will be available soon in the United States.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Over the past year, a few gems have been published to help us manage and treat abnormal uterine bleeding (AUB). One study suggests an order of performing hysteroscopy and endometrial biopsy, another emphasizes the continued cost-effectiveness of the levonorgestrel-releasing intrauterine system (LNG-IUS), while a third provides more evidence that ulipristal acetate is effective in the management of leiomyomas.
Optimal order of office hysteroscopy and endometrial biopsy?
Sarkar P, Mikhail E, Schickler R, Plosker S, Imudia AN. Optimal order of successive office hysteroscopy and endometrial biopsy for the evaluation of abnormal uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2017;130(3):565-572.
Office hysteroscopy and endometrial biopsy are frequently used in the evaluation of women presenting with AUB. Sarkar and colleagues conducted a study aimed at estimating the optimal order of office hysteroscopy and endometrial biopsy when performed successively among premenopausal women.
Pain perception, procedure duration, and other outcomes
This prospective single-blind randomized trial included 78 consecutive patients. The primary outcome was detection of any difference in patients' global pain perception based on the order of the procedures. Secondary outcome measures included determining whether the procedure order affected the duration of the procedures, the adequacy of the endometrial biopsy sample, the number of attempts to obtain an adequate tissue sample, and optimal visualization of the endometrial cavity during office hysteroscopy.
Order not important, but other factors may be
Not surprisingly, the results showed that the order in which the procedures were performed had no effect on patients' pain perception or on the overall procedure duration. Assessed using a visual analog scale scored from 1 to 10, global pain perception in the hysteroscopy-first patients (group A, n = 40) compared with the biopsy-first patients (group B, n = 38) was similar (7 vs 7, P = .57; 95% confidence interval [CI], 5.8-7.1). Procedure duration also was similar in group A and group B (3 vs 3, P = .32; 95% CI, 3.3-4.1).
However, when hysteroscopy was performed first, the quality of endometrial cavity images was superior compared with images from patients in whom biopsy was performed first. The number of endometrial biopsy curette passes required to obtain an adequate tissue sample was lower in the biopsy-first patients. The endometrial biopsy specimen was adequate for histologic evaluation regardless of whether hysteroscopy or biopsy was performed first.
Sarkar and colleagues suggested that their study findings emphasize the importance of individualizing the order of successive procedures to achieve the most clinically relevant result with maximum ease and comfort. They proposed that patients who have a high index of suspicion for occult malignancy or endometrial hyperplasia should have a biopsy procedure first so that adequate tissue samples can be obtained with fewer attempts. In patients with underlying uterine anatomic defects, performing hysteroscopy first would be clinically relevant to obtain the best images for optimal surgical planning.
Read next: Which treatment for AUB is most cost-effective?
Which treatment for AUB is most cost-effective?
Spencer JC, Louie M, Moulder JK, et al. Cost-effectiveness of treatments for heavy menstrual bleeding. Am J Obstet Gynecol. 2017;217(5):574.e1-574e.9.
The costs associated with heavy menstrual bleeding are significant. Spencer and colleagues sought to evaluate the relative cost-effectiveness of 4 treatment options for heavy menstrual bleeding: hysterectomy, resectoscopic endometrial ablation, nonresectoscopic endometrial ablation, and the LNG-IUS in a hypothetical cohort of 100,000 premenopausal women. No previous studies have examined the cost-effectiveness of these options in the context of the US health care setting.
Decision tree used for analysis
The authors formulated a decision tree to evaluate private payer costs and quality-adjusted life-years over a 5-year time horizon for premenopausal women with heavy menstrual bleeding and no suspected malignancy. For each treatment option, the authors used probabilities to estimate frequencies of complications and treatment failure leading to additional therapies. They compared the treatments in terms of total average costs, quality-adjusted life years, and incremental cost-effectiveness ratios.
Comparing costs, quality of life, and complications
Quality of life was fairly high for all treatment options; however, the estimated costs and the complications of each treatment were markedly different between treatment options. The LNG-IUS was superior to all alternatives in terms of both cost and quality, making it the dominant strategy. The 5-year cost for the LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500). When examined over a range of possible values, the LNG-IUS was cost-effective compared with hysterectomy in the large majority of scenarios (90%).
If the LNG-IUS is removed from consideration because of either patient preference or clinical judgment, the decision between hysterectomy and ablation is more complex. Hysterectomy results in better quality of life in the majority of simulations, but it is cost-effective in just more than half of the simulations compared with either resectoscopic or nonresectoscopic ablation. Therefore, consideration of cost, procedure-specific complications, and patient preferences may guide the therapeutic decision between hysterectomy and endometrial ablation.
The 52-mg LNG-IUS was superior to all treatment alternatives in both cost and quality, making it the dominant strategy for the treatment of heavy menstrual bleeding.
Ulipristal may be useful for managing AUB associated with uterine leiomyomas
Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;131(3):431-439.
Managing uterine leiomyomas is a common issue for gynecologists, as up to 70% of white women and more than 80% of black women of reproductive age in the United States have leiomyomas.
Ulipristal acetate is an orally administered selective progesterone-receptor modulator that decreases bleeding and reduces leiomyoma size. Although trials conducted in Europe found ulipristal to be superior to placebo and noninferior to leuprolide acetate in controlling bleeding and reducing leiomyoma size, those initial trials were conducted in a predominantly white population.
Study assessed efficacy and safety
Simon and colleagues recently conducted a randomized double-blind, placebo-controlled trial designed to assess the safety and efficacy of ulipristal in a more diverse population, such as patients in the United States. The 148 participants included in the study were randomly assigned on a 1:1:1 basis to once-daily oral ulipristal 5 mg, ulipristal 10 mg, or placebo for 12 weeks, with a 12-week drug-free follow-up.
Amenorrhea achieved and quality of life improved
The investigators found that ulipristal in 5-mg and 10-mg doses was well tolerated and superior to placebo in both the rate of and the time to amenorrhea (the coprimary end points) in women with symptomatic leiomyomas. In women treated with ulipristal 5 mg, amenorrhea was achieved in 25 of 53 (47.2%; 97.5% CI, 31.6-63.2), and of those treated with the 10-mg dose, 28 of 48 (58.3%; 97.5% CI, 41.2-74.1) achieved amenorrhea (P<.001 for both groups), compared with 1 of 56 (1.8%; 97.5% CI, 0.0-10.9) in the placebo group.
AUB continues to be a significant issue for many women. As women's health care providers, it is important that we deliver care with high value (Quality ÷ Cost). Therefore, consider these takeaway points:
- The LNG-IUS consistently delivers high value by affecting both sides of this equation. We should use it more.
- Although we do not yet know what ulipristal acetate will cost in the United States, effective medical treatments usually affect both sides of the Quality ÷ Cost equation, and new medications on the horizon are worth knowing about.
- Last, efficiency with office-based hysteroscopy is also an opportunity to increase value by improving biopsy and visualization quality.
Ulipristal treatment also was shown to improve health-related quality of life, including physical and social activities. No patient discontinued ulipristal because of lack of efficacy, and 1 patient in the placebo group stopped taking the drug because of an adverse event. Estradiol levels were maintained at midfollicular levels during ulipristal treatment, and endometrial biopsies did not show any atypical or malignant changes. These results are consistent with those of the studies conducted in Europe in a predominantly white, nonobese population.
Results of this study help to define a niche for ulipristal when hysterectomy is not an option for women who wish to preserve fertility. Further, although leuprolide is used for preoperative hematologic improvement of anemia, its use results in hypoestrogenic adverse effects.
The findings from this and other studies suggest that ulipristal may be useful for the medical management of AUB associated with uterine leiomyomas, especially for patients desiring uterine- and fertility-sparing treatment. Hopefully, this treatment will be available soon in the United States.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Contraceptive considerations for women with headache and migraine
The use of hormonal contraception in women with headaches, especially migraine headaches, is an important topic. Approximately 43% of women in the United States report migraines.1 Roughly the same percentage of reproductive-aged women use hormonal contraception.2 Data suggest that all migraineurs have some increased risk of stroke. Therefore, can women with migraine headaches use combination hormonal contraception? And can women with severe headaches that are nonmigrainous use combination hormonal contraception? Let’s examine available data to help us answer these questions.
Risk factors for stroke
Migraine without aura is the most common subset, but migraine with aura is more problematic relative to the increased incidence of stroke.1
A migraine aura is visual 90% of the time.1 Symptoms can include flickering lights, spots, zigzag lines, a sense of pins and needles, or dysphasic speech. Aura precedes the headache and usually resolves within 1 hour after the aura begins.
In addition to migraine headaches, risk factors for stroke include increasing age, hypertension, the use of combination oral contraceptives (COCs), the contraceptive patch and ring, and smoking.1
Data indicate that the risk for ischemic stroke is increased in women with migraines even without the presence of other risk factors. In a meta-analysis of 14 observational studies, the risk of ischemic stroke among all migraineurs was about 2-fold (relative risk [RR], 2.2; 95% confidence interval [CI], 1.9–2.5) compared with the risk of ischemic stroke in women of the same age group who did not have migraine headaches. When there is migraine without aura, it was slightly less than 2-fold (RR, 1.8; 95% CI, 1.1–3.2). The risk of ischemic stroke among migraineurs with aura is increased more than 2 times compared with women without migraine (RR, 2.27; 95% CI, 1.61–3.19).3 However, the absolute risk of ischemic stroke among reproductive-aged women is 11 per 100,000 women years.4
Two observational studies show how additional risk factors increase that risk (TABLE).5,6 There are similar trends in terms of overall risk of stroke among women with all types of migraine. However, when you add smoking as an additional risk factor for women with migraine headaches, there is a substantial increase in the risk of stroke. When a woman who has migraines uses COCs, there is increased risk varying from 2-fold to almost 4-fold. When you combine migraine, smoking, and COCs, a very, very large risk factor (odds ratio [OR], 34.4; 95% CI, 3.27–3.61) was reported by Chang and colleagues.6
Although these risks are impressive, it is important to keep in mind that even with a 10-fold increase, we are only talking about 1 case per 1,000 migraineurs.4 Unfortunately, stroke often leads to major disability and even death, such that any reduction in risk is still important.
Preventing estrogen withdrawal or menstrual migraines
How should we treat a woman who uses hormonal contraception and reports estrogen withdrawal or menstrual migraines? Based on clinical evidence, there are 2 ways to reduce her symptoms:
- COCs. Reduce the hormone-free interval by having her take COCs for 3 to 4 days instead of 7 days, or eliminate the hormone-free interval altogether by continuous use of COCs, usually 3 months at a time.7
- NSAIDs. For those who do not want to alter how they take their hormonal product, use nonsteroidal anti-inflammatory drugs (NSAIDs) starting 7 days before the onset of menses and continuing for 13 days. In a clinical trial by Sances and colleagues, this plan reduced the frequency, duration, and severity of menstrual migraines.8
Probably altering how she takes the COC would make the most sense for most individuals instead of taking NSAIDs for 75% of each month.
Recommendations from the US MEC
The US Medical Eligibility Criteria (US MEC) from the Centers for Disease Control and Prevention (CDC) offers recommendations for contraceptive use9:
- For nonmigrainous headache, the CDC suggests that the benefits of using COCs outweigh the risks unless the headaches persist after 3 months of COC use.
- For migraine without aura, the benefits outweigh the risks in starting women who are younger than age 35 years on oral contraceptives. However, the risks of COCs outweigh the benefits in women who are age 35 years and older who develop migraine headache while on COCs, or who have risk factors for stroke.
- For migraine with aura, COCs are contraindicated.
- Progestin-only contraceptives. The CDC considers that the benefits of COC use outweigh any theoretical risk of stroke, even in women with risk factors or in women who have migraine with aura. Progestin-only contraceptives do not alter one’s risk of stroke, unlike contraceptives that contain estrogen.
My bottom line
Can women with migraine headaches begin the use of combination hormonal methods? Yes, if there is no aura in their migraines and they are not older than age 35.
Can women with severe headaches that are nonmigrainous use combination hormonal methods? Possibly, but you should discontinue COCs if headache severity persists or worsens, using a 3-month time period for evaluation.
How do you manage women with migraines during the hormone-free interval? Consider the continuous method or shorten the hormone-free interval.
Recommendations for complicated patients. Consulting the CDC’s US MEC database7 can provide assistance in your care of more complicated patients requesting contraception. I also recommend the book, “Contraception for the Medically Challenging Patient,” edited by Rebecca Allen and Carrie Cwiak.10 It links nicely with the CDC guidelines and presents more detail on each subject.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Stewart WF, Wood C, Reed MD, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28(11):1170–1178.
- Finer LB, Frohwirth LF, Dauphinee LA, Singh S, Moore AM. Reasons U.S. women have abortions: quantitative and qualitative perspectives. Perspect Sex Reprod Health. 2005;37(3):110–118.
- Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63–66.
- Petitti DB, Sydney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stoke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8–15.
- Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischemic stroke in young women. BMJ. 1995;310:830–833.
- Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318(7175):13–18.
- Edelman A, Gallo MF, Nichols MD, Jensen JT, Schulz KF, Grimes DA. Continuous versus cyclic use of combined oral contraceptives for contraception: systematic Cochrane review of randomized controlled trials. Hum Reprod. 2006;21(3):573–578.
- Sances G, Martignoni E, Fioroni L, Blandini F, Facchinetti F, Nappi G. Naproxen sodium in menstrual migraine prophylaxis: a double-blind placebo controlled study. Headache. 1990;30(11):705–709.
- US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1–86. https://www.cdc .gov/mmwr/pdf/rr/rr59e0528.pdf. Accessed October 4, 2016.
- Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
The use of hormonal contraception in women with headaches, especially migraine headaches, is an important topic. Approximately 43% of women in the United States report migraines.1 Roughly the same percentage of reproductive-aged women use hormonal contraception.2 Data suggest that all migraineurs have some increased risk of stroke. Therefore, can women with migraine headaches use combination hormonal contraception? And can women with severe headaches that are nonmigrainous use combination hormonal contraception? Let’s examine available data to help us answer these questions.
Risk factors for stroke
Migraine without aura is the most common subset, but migraine with aura is more problematic relative to the increased incidence of stroke.1
A migraine aura is visual 90% of the time.1 Symptoms can include flickering lights, spots, zigzag lines, a sense of pins and needles, or dysphasic speech. Aura precedes the headache and usually resolves within 1 hour after the aura begins.
In addition to migraine headaches, risk factors for stroke include increasing age, hypertension, the use of combination oral contraceptives (COCs), the contraceptive patch and ring, and smoking.1
Data indicate that the risk for ischemic stroke is increased in women with migraines even without the presence of other risk factors. In a meta-analysis of 14 observational studies, the risk of ischemic stroke among all migraineurs was about 2-fold (relative risk [RR], 2.2; 95% confidence interval [CI], 1.9–2.5) compared with the risk of ischemic stroke in women of the same age group who did not have migraine headaches. When there is migraine without aura, it was slightly less than 2-fold (RR, 1.8; 95% CI, 1.1–3.2). The risk of ischemic stroke among migraineurs with aura is increased more than 2 times compared with women without migraine (RR, 2.27; 95% CI, 1.61–3.19).3 However, the absolute risk of ischemic stroke among reproductive-aged women is 11 per 100,000 women years.4
Two observational studies show how additional risk factors increase that risk (TABLE).5,6 There are similar trends in terms of overall risk of stroke among women with all types of migraine. However, when you add smoking as an additional risk factor for women with migraine headaches, there is a substantial increase in the risk of stroke. When a woman who has migraines uses COCs, there is increased risk varying from 2-fold to almost 4-fold. When you combine migraine, smoking, and COCs, a very, very large risk factor (odds ratio [OR], 34.4; 95% CI, 3.27–3.61) was reported by Chang and colleagues.6
Although these risks are impressive, it is important to keep in mind that even with a 10-fold increase, we are only talking about 1 case per 1,000 migraineurs.4 Unfortunately, stroke often leads to major disability and even death, such that any reduction in risk is still important.
Preventing estrogen withdrawal or menstrual migraines
How should we treat a woman who uses hormonal contraception and reports estrogen withdrawal or menstrual migraines? Based on clinical evidence, there are 2 ways to reduce her symptoms:
- COCs. Reduce the hormone-free interval by having her take COCs for 3 to 4 days instead of 7 days, or eliminate the hormone-free interval altogether by continuous use of COCs, usually 3 months at a time.7
- NSAIDs. For those who do not want to alter how they take their hormonal product, use nonsteroidal anti-inflammatory drugs (NSAIDs) starting 7 days before the onset of menses and continuing for 13 days. In a clinical trial by Sances and colleagues, this plan reduced the frequency, duration, and severity of menstrual migraines.8
Probably altering how she takes the COC would make the most sense for most individuals instead of taking NSAIDs for 75% of each month.
Recommendations from the US MEC
The US Medical Eligibility Criteria (US MEC) from the Centers for Disease Control and Prevention (CDC) offers recommendations for contraceptive use9:
- For nonmigrainous headache, the CDC suggests that the benefits of using COCs outweigh the risks unless the headaches persist after 3 months of COC use.
- For migraine without aura, the benefits outweigh the risks in starting women who are younger than age 35 years on oral contraceptives. However, the risks of COCs outweigh the benefits in women who are age 35 years and older who develop migraine headache while on COCs, or who have risk factors for stroke.
- For migraine with aura, COCs are contraindicated.
- Progestin-only contraceptives. The CDC considers that the benefits of COC use outweigh any theoretical risk of stroke, even in women with risk factors or in women who have migraine with aura. Progestin-only contraceptives do not alter one’s risk of stroke, unlike contraceptives that contain estrogen.
My bottom line
Can women with migraine headaches begin the use of combination hormonal methods? Yes, if there is no aura in their migraines and they are not older than age 35.
Can women with severe headaches that are nonmigrainous use combination hormonal methods? Possibly, but you should discontinue COCs if headache severity persists or worsens, using a 3-month time period for evaluation.
How do you manage women with migraines during the hormone-free interval? Consider the continuous method or shorten the hormone-free interval.
Recommendations for complicated patients. Consulting the CDC’s US MEC database7 can provide assistance in your care of more complicated patients requesting contraception. I also recommend the book, “Contraception for the Medically Challenging Patient,” edited by Rebecca Allen and Carrie Cwiak.10 It links nicely with the CDC guidelines and presents more detail on each subject.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
The use of hormonal contraception in women with headaches, especially migraine headaches, is an important topic. Approximately 43% of women in the United States report migraines.1 Roughly the same percentage of reproductive-aged women use hormonal contraception.2 Data suggest that all migraineurs have some increased risk of stroke. Therefore, can women with migraine headaches use combination hormonal contraception? And can women with severe headaches that are nonmigrainous use combination hormonal contraception? Let’s examine available data to help us answer these questions.
Risk factors for stroke
Migraine without aura is the most common subset, but migraine with aura is more problematic relative to the increased incidence of stroke.1
A migraine aura is visual 90% of the time.1 Symptoms can include flickering lights, spots, zigzag lines, a sense of pins and needles, or dysphasic speech. Aura precedes the headache and usually resolves within 1 hour after the aura begins.
In addition to migraine headaches, risk factors for stroke include increasing age, hypertension, the use of combination oral contraceptives (COCs), the contraceptive patch and ring, and smoking.1
Data indicate that the risk for ischemic stroke is increased in women with migraines even without the presence of other risk factors. In a meta-analysis of 14 observational studies, the risk of ischemic stroke among all migraineurs was about 2-fold (relative risk [RR], 2.2; 95% confidence interval [CI], 1.9–2.5) compared with the risk of ischemic stroke in women of the same age group who did not have migraine headaches. When there is migraine without aura, it was slightly less than 2-fold (RR, 1.8; 95% CI, 1.1–3.2). The risk of ischemic stroke among migraineurs with aura is increased more than 2 times compared with women without migraine (RR, 2.27; 95% CI, 1.61–3.19).3 However, the absolute risk of ischemic stroke among reproductive-aged women is 11 per 100,000 women years.4
Two observational studies show how additional risk factors increase that risk (TABLE).5,6 There are similar trends in terms of overall risk of stroke among women with all types of migraine. However, when you add smoking as an additional risk factor for women with migraine headaches, there is a substantial increase in the risk of stroke. When a woman who has migraines uses COCs, there is increased risk varying from 2-fold to almost 4-fold. When you combine migraine, smoking, and COCs, a very, very large risk factor (odds ratio [OR], 34.4; 95% CI, 3.27–3.61) was reported by Chang and colleagues.6
Although these risks are impressive, it is important to keep in mind that even with a 10-fold increase, we are only talking about 1 case per 1,000 migraineurs.4 Unfortunately, stroke often leads to major disability and even death, such that any reduction in risk is still important.
Preventing estrogen withdrawal or menstrual migraines
How should we treat a woman who uses hormonal contraception and reports estrogen withdrawal or menstrual migraines? Based on clinical evidence, there are 2 ways to reduce her symptoms:
- COCs. Reduce the hormone-free interval by having her take COCs for 3 to 4 days instead of 7 days, or eliminate the hormone-free interval altogether by continuous use of COCs, usually 3 months at a time.7
- NSAIDs. For those who do not want to alter how they take their hormonal product, use nonsteroidal anti-inflammatory drugs (NSAIDs) starting 7 days before the onset of menses and continuing for 13 days. In a clinical trial by Sances and colleagues, this plan reduced the frequency, duration, and severity of menstrual migraines.8
Probably altering how she takes the COC would make the most sense for most individuals instead of taking NSAIDs for 75% of each month.
Recommendations from the US MEC
The US Medical Eligibility Criteria (US MEC) from the Centers for Disease Control and Prevention (CDC) offers recommendations for contraceptive use9:
- For nonmigrainous headache, the CDC suggests that the benefits of using COCs outweigh the risks unless the headaches persist after 3 months of COC use.
- For migraine without aura, the benefits outweigh the risks in starting women who are younger than age 35 years on oral contraceptives. However, the risks of COCs outweigh the benefits in women who are age 35 years and older who develop migraine headache while on COCs, or who have risk factors for stroke.
- For migraine with aura, COCs are contraindicated.
- Progestin-only contraceptives. The CDC considers that the benefits of COC use outweigh any theoretical risk of stroke, even in women with risk factors or in women who have migraine with aura. Progestin-only contraceptives do not alter one’s risk of stroke, unlike contraceptives that contain estrogen.
My bottom line
Can women with migraine headaches begin the use of combination hormonal methods? Yes, if there is no aura in their migraines and they are not older than age 35.
Can women with severe headaches that are nonmigrainous use combination hormonal methods? Possibly, but you should discontinue COCs if headache severity persists or worsens, using a 3-month time period for evaluation.
How do you manage women with migraines during the hormone-free interval? Consider the continuous method or shorten the hormone-free interval.
Recommendations for complicated patients. Consulting the CDC’s US MEC database7 can provide assistance in your care of more complicated patients requesting contraception. I also recommend the book, “Contraception for the Medically Challenging Patient,” edited by Rebecca Allen and Carrie Cwiak.10 It links nicely with the CDC guidelines and presents more detail on each subject.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Stewart WF, Wood C, Reed MD, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28(11):1170–1178.
- Finer LB, Frohwirth LF, Dauphinee LA, Singh S, Moore AM. Reasons U.S. women have abortions: quantitative and qualitative perspectives. Perspect Sex Reprod Health. 2005;37(3):110–118.
- Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63–66.
- Petitti DB, Sydney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stoke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8–15.
- Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischemic stroke in young women. BMJ. 1995;310:830–833.
- Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318(7175):13–18.
- Edelman A, Gallo MF, Nichols MD, Jensen JT, Schulz KF, Grimes DA. Continuous versus cyclic use of combined oral contraceptives for contraception: systematic Cochrane review of randomized controlled trials. Hum Reprod. 2006;21(3):573–578.
- Sances G, Martignoni E, Fioroni L, Blandini F, Facchinetti F, Nappi G. Naproxen sodium in menstrual migraine prophylaxis: a double-blind placebo controlled study. Headache. 1990;30(11):705–709.
- US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1–86. https://www.cdc .gov/mmwr/pdf/rr/rr59e0528.pdf. Accessed October 4, 2016.
- Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
- Stewart WF, Wood C, Reed MD, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28(11):1170–1178.
- Finer LB, Frohwirth LF, Dauphinee LA, Singh S, Moore AM. Reasons U.S. women have abortions: quantitative and qualitative perspectives. Perspect Sex Reprod Health. 2005;37(3):110–118.
- Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63–66.
- Petitti DB, Sydney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stoke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8–15.
- Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischemic stroke in young women. BMJ. 1995;310:830–833.
- Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318(7175):13–18.
- Edelman A, Gallo MF, Nichols MD, Jensen JT, Schulz KF, Grimes DA. Continuous versus cyclic use of combined oral contraceptives for contraception: systematic Cochrane review of randomized controlled trials. Hum Reprod. 2006;21(3):573–578.
- Sances G, Martignoni E, Fioroni L, Blandini F, Facchinetti F, Nappi G. Naproxen sodium in menstrual migraine prophylaxis: a double-blind placebo controlled study. Headache. 1990;30(11):705–709.
- US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1–86. https://www.cdc .gov/mmwr/pdf/rr/rr59e0528.pdf. Accessed October 4, 2016.
- Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
Optimize the medical treatment of endometriosis—Use all available medications
CASE Endometriosis pain increases despite hormonal treatment
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy of a cul-de-sac lesion showed endometriosis on histopathology. The patient was treated with a continuous low-dose estrogen-progestin contraceptive. Initially, the treatment helped relieve her pain symptoms. Over the next year, while on that treatment, her pain gradually increased in severity until it was disabling. At an office visit, the primary clinician renewed the estrogen-progestin contraceptive for another year, even though it was not relieving the patient’s pain. The patient sought a second opinion.
We are the experts in the management of pelvic pain caused by endometriosis
Women’s health clinicians are the specialists best trained to care for patients with severe pain caused by endometriosis. Low-dose continuous estrogen-progestin contraceptives are commonly prescribed as a first-line hormonal treatment for pain caused by endometriosis. My observation is that estrogen-progestincontraceptives are often effective when initially prescribed, but with continued use over years, pain often recurs. Estrogen is known to stimulate endometriosis disease activity. Progestins at high doses suppress endometriosis disease activity. However, endometriosis implants often manifest decreased responsiveness to progestins, permitting the estrogen in the combination contraceptive to exert its disease-stimulating effect.1,2 I frequently see women with pelvic pain caused by endometriosis, who initially had a significant decrease in pain with continuous estrogen-progestin contraceptive treatment but who develop increasing pain with continued use of the medication. In this clinical situation, it is useful to consider stopping the estrogen-progestin therapy and to prescribe a hormone with a different mechanism of action (TABLE).
Progestin-only medications
Progestin-only medications are often effective in the treatment of pain caused by endometriosis. High-dose progestin-only medications suppress pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby suppressing ovarian synthesis of estrogen, resulting in low circulating levels of estrogen. This removes the estrogen stimulus that exacerbates endometriosis disease activity. High-dose progestins also directly suppress cellular activity in endometriosis implants. High-dose progestins often overcome the relative resistance of endometriosis lesions to progestin suppression of disease activity. Hence, high-dose progestin-only medications have two mechanisms of action: suppression of estrogen synthesis through pituitary suppression of LH and FSH, and direct inhibition of cellular activity in the endometriosis lesions. High-dose progestin-only treatments include:
- oral norethindrone acetate 5 mg daily
- oral medroxyprogesterone acetate (MPA) 20 to 40 mg daily
- subcutaneous, or depot MPA
- levonorgestrel-releasing intrauterine device (LNG-IUD).
In my practice, I frequently use oral norethindrone acetate 5 mg daily to treat pelvic pain caused by endometriosis. In one randomized trial, 90 women with pelvic pain and rectovaginal endometriosis were randomly assigned to treatment with norethindrone acetate 2.5 mg daily or an estrogen-progestin contraceptive. After 12 months of treatment, satisfaction with treatment was reported by 73% and 62% of the women in the norethindrone acetate and estrogen-progestin groups, respectively.3 The most common adverse effects reported by women taking norethindrone acetate were weight gain (27%) and decreased libido (9%).
Oral MPA at doses of 30 mg to 100 mg daily has been reported to be effective for the treatment of pelvic pain caused by endometriosis. MPA treatment can induce atrophy and pseudodecidualization in endometrium and endometriosis implants. In my practice I typically prescribe doses in the range of 20 mg to 40 mg daily. With oral MPA treatment, continued uterine bleeding may occur in up to 30% of women, somewhat limiting its efficacy.4–7
Subcutaneous and depot MPA have been reported to be effective in the treatment of pelvic pain caused by endometriosis.4,8 In some resource-limited countries, depot MPA may be the most available progestin for the treatment of pelvic pain caused by endometriosis.
The LNG-IUD, inserted after surgery for endometriosis, has been reported to result in decreased pelvic pain in studies with a modest number of participants.9–11
GnRH analogue medications
Gonadotropin-releasing hormone (GnRH) analogues, including both GnRH agonists (nafarelin, leuprolide, and goserelin) and GnRH antagonists (elagolix) reduce pelvic pain caused by endometriosis by suppressing pituitary secretion of LH and FSH, thereby reducing ovarian synthesis of estradiol. In the absence of estradiol stimulation, cellular activity in endometriosis lesions decreases and pain symptoms improve. In my practice, I frequently use either nafarelin12 or leuprolide acetate depot plus norethindrone add-back.13 I generally avoid the use of leuprolide depot monotherapy because in many women it causes severe vasomotor symptoms.
At standard doses, nafarelin therapy generally results in serum estradiol levels in the range of 20 to 30 pg/mL, a “sweet spot” associated with modest vasomotor symptoms and reduced cellular activity in endometriosis implants.12,14 In many women who become amenorrheic on nafarelin two sprays daily, the dose can be reduced with maintenance of pain control and ovarian suppression.15 Leuprolide acetate depot monotherapy results in serum estradiol levels in the range of 5 to 10 pg/mL, causing severe vasomotor symptoms and reduction in cellular activity in endometriosis lesions. To reduce the adverse effects of leuprolide acetate depot monotherapy, I generally initiate concomitant add-back therapy with norethindrone acetate.13 A little recognized pharmacokinetic observation is that a very small amount of norethindrone acetate, generally less than 1%, is metabolized to ethinyl estradiol.16
The oral GnRH antagonist, elagolix, 150 mg daily for up to 24 months or 200 mg twice daily for 6 months, was approved by the US Food and Drug Administration (FDA) in July 2018. It is now available in pharmacies. Elagolix treatment results in significant reduction in pain caused by endometriosis, but only moderately bothersome vasomotor symptoms.17,18 Elagolix likely will become a widely used medication because of the simplicity of oral administration, efficacy against endometriosis, and acceptable adverse-effect profile. A major disadvantage of the GnRH analogue-class of medications is that they are more expensive than the progestin medications mentioned above. Among the GnRH analogue class of medications, elagolix and goserelin are the least expensive.
Androgens
Estrogen stimulates cellular activity in endometriosis lesions. Androgen and high-dose progestins inhibit cellular activity in endometriosis lesions. Danazol, an attenuated androgen and a progestin is effectivein treating pelvic pain caused by endometriosis.19,20 However, many women decline to use danazol because it is often associated with weight gain. As an androgen, danazol can permanently change a woman’s voice pitch and should not be used by professional singers or speech therapists.
Aromatase Inhibitors
Estrogen is a critically important stimulus of cell activity in endometriosis lesions. Aromatase inhibitors, which block the synthesis of estrogen, have been explored in the treatment of endometriosis that has proven to be resistant to other therapies. Although the combination of an aromatase inhibitor plus a high-dose progestin or GnRH analogue may be effective, more data are needed before widely using the aromatase inhibitors in clinical practice.21
Don’t get stuck in a rut
When treating pelvic pain caused by endometriosis, if the patient’s hormone regimen is not working, prescribe a medication from another class of hormones. In the case presented above, a woman with pelvic pain and surgically proven endometriosis reported inadequate control of her pain symptoms with a continuous estrogen-progestin medication. Her physician prescribed another year of the same estrogen-progestin medication. Instead of renewing the medication, the physician could have offered the patient a hormone medication from another drug class: 1) progestin only, 2) GnRH analogue, or 3) danazol. By using every available hormonal agent, physicians will improve the treatment of pelvic pain caused by endometriosis. Millions of women in our country have pelvic pain caused by endometriosis. They are counting on us, women’s health specialists, to effectively treat their disease.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96(6):623–632.
- Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43.
- Vercellini P, Pietropaolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
- Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database of Syst Rev. 2012;(3):CD002122.
- Moghissi KS, Boyce CR. Management of endometriosis with oral medroxyprogesterone acetate. Obstet Gynecol. 1976;47(3):265–267.
- Telimaa S, Puolakka J, Rönnberg L, Kauppila A. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis. Gynecol Endocrinol. 1987;1(1):13–23.
- Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 pt 1):323–327.
- Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leu-prolide acetate in the treatment of endometriosis-associated pain. Fertil Steril. 2006;85(2):314–325.
- Abou-Setta AM, Houston B, Al-Inany HG, Farquhar C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database of Syst Rev. 2013;(1):CD005072.
- Tanmahasamut P, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Indhavivadhana S, Leerasiri P. Postoperative levonorgestrel-releasing intrauterine system for pelvic endometriosis-pain: a randomized controlled trial. Obstet Gynecol. 2012;119(3):519–526.
- Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and Depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273–279.
- Henzl MR, Corson SL, Moghissi K, Buttram VC, Berqvist C, Jacobsen J. Administration of nasal nafarelin as compared with oral danazol for endo-metriosis. A multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318(8):485–489.
- Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998; 91(1):16–24.
- Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166(2):740–745.
- Hull ME, Barbieri RL. Nafarelin in the treatment of endometriosis. Dose management. Gynecol Obstet Invest. 1994;37(4):263–264.
- Barbieri RL, Petro Z, Canick JA, Ryan KJ. Aromatization of norethindrone to ethinyl estradiol by human placental microsomes. J Clin Endocrinol Metab. 1983;57(2):299–303.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132(1):147–160.
- Selak V, Farquhar C, Prentice A, Singla A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2007;(4):CD000068.
- Barbieri RL, Ryan KJ. Danazol: endocrine pharmacology and therapeutic applications. Am J Obstet Gynecol. 1981;141(4):453–463.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
CASE Endometriosis pain increases despite hormonal treatment
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy of a cul-de-sac lesion showed endometriosis on histopathology. The patient was treated with a continuous low-dose estrogen-progestin contraceptive. Initially, the treatment helped relieve her pain symptoms. Over the next year, while on that treatment, her pain gradually increased in severity until it was disabling. At an office visit, the primary clinician renewed the estrogen-progestin contraceptive for another year, even though it was not relieving the patient’s pain. The patient sought a second opinion.
We are the experts in the management of pelvic pain caused by endometriosis
Women’s health clinicians are the specialists best trained to care for patients with severe pain caused by endometriosis. Low-dose continuous estrogen-progestin contraceptives are commonly prescribed as a first-line hormonal treatment for pain caused by endometriosis. My observation is that estrogen-progestincontraceptives are often effective when initially prescribed, but with continued use over years, pain often recurs. Estrogen is known to stimulate endometriosis disease activity. Progestins at high doses suppress endometriosis disease activity. However, endometriosis implants often manifest decreased responsiveness to progestins, permitting the estrogen in the combination contraceptive to exert its disease-stimulating effect.1,2 I frequently see women with pelvic pain caused by endometriosis, who initially had a significant decrease in pain with continuous estrogen-progestin contraceptive treatment but who develop increasing pain with continued use of the medication. In this clinical situation, it is useful to consider stopping the estrogen-progestin therapy and to prescribe a hormone with a different mechanism of action (TABLE).
Progestin-only medications
Progestin-only medications are often effective in the treatment of pain caused by endometriosis. High-dose progestin-only medications suppress pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby suppressing ovarian synthesis of estrogen, resulting in low circulating levels of estrogen. This removes the estrogen stimulus that exacerbates endometriosis disease activity. High-dose progestins also directly suppress cellular activity in endometriosis implants. High-dose progestins often overcome the relative resistance of endometriosis lesions to progestin suppression of disease activity. Hence, high-dose progestin-only medications have two mechanisms of action: suppression of estrogen synthesis through pituitary suppression of LH and FSH, and direct inhibition of cellular activity in the endometriosis lesions. High-dose progestin-only treatments include:
- oral norethindrone acetate 5 mg daily
- oral medroxyprogesterone acetate (MPA) 20 to 40 mg daily
- subcutaneous, or depot MPA
- levonorgestrel-releasing intrauterine device (LNG-IUD).
In my practice, I frequently use oral norethindrone acetate 5 mg daily to treat pelvic pain caused by endometriosis. In one randomized trial, 90 women with pelvic pain and rectovaginal endometriosis were randomly assigned to treatment with norethindrone acetate 2.5 mg daily or an estrogen-progestin contraceptive. After 12 months of treatment, satisfaction with treatment was reported by 73% and 62% of the women in the norethindrone acetate and estrogen-progestin groups, respectively.3 The most common adverse effects reported by women taking norethindrone acetate were weight gain (27%) and decreased libido (9%).
Oral MPA at doses of 30 mg to 100 mg daily has been reported to be effective for the treatment of pelvic pain caused by endometriosis. MPA treatment can induce atrophy and pseudodecidualization in endometrium and endometriosis implants. In my practice I typically prescribe doses in the range of 20 mg to 40 mg daily. With oral MPA treatment, continued uterine bleeding may occur in up to 30% of women, somewhat limiting its efficacy.4–7
Subcutaneous and depot MPA have been reported to be effective in the treatment of pelvic pain caused by endometriosis.4,8 In some resource-limited countries, depot MPA may be the most available progestin for the treatment of pelvic pain caused by endometriosis.
The LNG-IUD, inserted after surgery for endometriosis, has been reported to result in decreased pelvic pain in studies with a modest number of participants.9–11
GnRH analogue medications
Gonadotropin-releasing hormone (GnRH) analogues, including both GnRH agonists (nafarelin, leuprolide, and goserelin) and GnRH antagonists (elagolix) reduce pelvic pain caused by endometriosis by suppressing pituitary secretion of LH and FSH, thereby reducing ovarian synthesis of estradiol. In the absence of estradiol stimulation, cellular activity in endometriosis lesions decreases and pain symptoms improve. In my practice, I frequently use either nafarelin12 or leuprolide acetate depot plus norethindrone add-back.13 I generally avoid the use of leuprolide depot monotherapy because in many women it causes severe vasomotor symptoms.
At standard doses, nafarelin therapy generally results in serum estradiol levels in the range of 20 to 30 pg/mL, a “sweet spot” associated with modest vasomotor symptoms and reduced cellular activity in endometriosis implants.12,14 In many women who become amenorrheic on nafarelin two sprays daily, the dose can be reduced with maintenance of pain control and ovarian suppression.15 Leuprolide acetate depot monotherapy results in serum estradiol levels in the range of 5 to 10 pg/mL, causing severe vasomotor symptoms and reduction in cellular activity in endometriosis lesions. To reduce the adverse effects of leuprolide acetate depot monotherapy, I generally initiate concomitant add-back therapy with norethindrone acetate.13 A little recognized pharmacokinetic observation is that a very small amount of norethindrone acetate, generally less than 1%, is metabolized to ethinyl estradiol.16
The oral GnRH antagonist, elagolix, 150 mg daily for up to 24 months or 200 mg twice daily for 6 months, was approved by the US Food and Drug Administration (FDA) in July 2018. It is now available in pharmacies. Elagolix treatment results in significant reduction in pain caused by endometriosis, but only moderately bothersome vasomotor symptoms.17,18 Elagolix likely will become a widely used medication because of the simplicity of oral administration, efficacy against endometriosis, and acceptable adverse-effect profile. A major disadvantage of the GnRH analogue-class of medications is that they are more expensive than the progestin medications mentioned above. Among the GnRH analogue class of medications, elagolix and goserelin are the least expensive.
Androgens
Estrogen stimulates cellular activity in endometriosis lesions. Androgen and high-dose progestins inhibit cellular activity in endometriosis lesions. Danazol, an attenuated androgen and a progestin is effectivein treating pelvic pain caused by endometriosis.19,20 However, many women decline to use danazol because it is often associated with weight gain. As an androgen, danazol can permanently change a woman’s voice pitch and should not be used by professional singers or speech therapists.
Aromatase Inhibitors
Estrogen is a critically important stimulus of cell activity in endometriosis lesions. Aromatase inhibitors, which block the synthesis of estrogen, have been explored in the treatment of endometriosis that has proven to be resistant to other therapies. Although the combination of an aromatase inhibitor plus a high-dose progestin or GnRH analogue may be effective, more data are needed before widely using the aromatase inhibitors in clinical practice.21
Don’t get stuck in a rut
When treating pelvic pain caused by endometriosis, if the patient’s hormone regimen is not working, prescribe a medication from another class of hormones. In the case presented above, a woman with pelvic pain and surgically proven endometriosis reported inadequate control of her pain symptoms with a continuous estrogen-progestin medication. Her physician prescribed another year of the same estrogen-progestin medication. Instead of renewing the medication, the physician could have offered the patient a hormone medication from another drug class: 1) progestin only, 2) GnRH analogue, or 3) danazol. By using every available hormonal agent, physicians will improve the treatment of pelvic pain caused by endometriosis. Millions of women in our country have pelvic pain caused by endometriosis. They are counting on us, women’s health specialists, to effectively treat their disease.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
CASE Endometriosis pain increases despite hormonal treatment
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy of a cul-de-sac lesion showed endometriosis on histopathology. The patient was treated with a continuous low-dose estrogen-progestin contraceptive. Initially, the treatment helped relieve her pain symptoms. Over the next year, while on that treatment, her pain gradually increased in severity until it was disabling. At an office visit, the primary clinician renewed the estrogen-progestin contraceptive for another year, even though it was not relieving the patient’s pain. The patient sought a second opinion.
We are the experts in the management of pelvic pain caused by endometriosis
Women’s health clinicians are the specialists best trained to care for patients with severe pain caused by endometriosis. Low-dose continuous estrogen-progestin contraceptives are commonly prescribed as a first-line hormonal treatment for pain caused by endometriosis. My observation is that estrogen-progestincontraceptives are often effective when initially prescribed, but with continued use over years, pain often recurs. Estrogen is known to stimulate endometriosis disease activity. Progestins at high doses suppress endometriosis disease activity. However, endometriosis implants often manifest decreased responsiveness to progestins, permitting the estrogen in the combination contraceptive to exert its disease-stimulating effect.1,2 I frequently see women with pelvic pain caused by endometriosis, who initially had a significant decrease in pain with continuous estrogen-progestin contraceptive treatment but who develop increasing pain with continued use of the medication. In this clinical situation, it is useful to consider stopping the estrogen-progestin therapy and to prescribe a hormone with a different mechanism of action (TABLE).
Progestin-only medications
Progestin-only medications are often effective in the treatment of pain caused by endometriosis. High-dose progestin-only medications suppress pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby suppressing ovarian synthesis of estrogen, resulting in low circulating levels of estrogen. This removes the estrogen stimulus that exacerbates endometriosis disease activity. High-dose progestins also directly suppress cellular activity in endometriosis implants. High-dose progestins often overcome the relative resistance of endometriosis lesions to progestin suppression of disease activity. Hence, high-dose progestin-only medications have two mechanisms of action: suppression of estrogen synthesis through pituitary suppression of LH and FSH, and direct inhibition of cellular activity in the endometriosis lesions. High-dose progestin-only treatments include:
- oral norethindrone acetate 5 mg daily
- oral medroxyprogesterone acetate (MPA) 20 to 40 mg daily
- subcutaneous, or depot MPA
- levonorgestrel-releasing intrauterine device (LNG-IUD).
In my practice, I frequently use oral norethindrone acetate 5 mg daily to treat pelvic pain caused by endometriosis. In one randomized trial, 90 women with pelvic pain and rectovaginal endometriosis were randomly assigned to treatment with norethindrone acetate 2.5 mg daily or an estrogen-progestin contraceptive. After 12 months of treatment, satisfaction with treatment was reported by 73% and 62% of the women in the norethindrone acetate and estrogen-progestin groups, respectively.3 The most common adverse effects reported by women taking norethindrone acetate were weight gain (27%) and decreased libido (9%).
Oral MPA at doses of 30 mg to 100 mg daily has been reported to be effective for the treatment of pelvic pain caused by endometriosis. MPA treatment can induce atrophy and pseudodecidualization in endometrium and endometriosis implants. In my practice I typically prescribe doses in the range of 20 mg to 40 mg daily. With oral MPA treatment, continued uterine bleeding may occur in up to 30% of women, somewhat limiting its efficacy.4–7
Subcutaneous and depot MPA have been reported to be effective in the treatment of pelvic pain caused by endometriosis.4,8 In some resource-limited countries, depot MPA may be the most available progestin for the treatment of pelvic pain caused by endometriosis.
The LNG-IUD, inserted after surgery for endometriosis, has been reported to result in decreased pelvic pain in studies with a modest number of participants.9–11
GnRH analogue medications
Gonadotropin-releasing hormone (GnRH) analogues, including both GnRH agonists (nafarelin, leuprolide, and goserelin) and GnRH antagonists (elagolix) reduce pelvic pain caused by endometriosis by suppressing pituitary secretion of LH and FSH, thereby reducing ovarian synthesis of estradiol. In the absence of estradiol stimulation, cellular activity in endometriosis lesions decreases and pain symptoms improve. In my practice, I frequently use either nafarelin12 or leuprolide acetate depot plus norethindrone add-back.13 I generally avoid the use of leuprolide depot monotherapy because in many women it causes severe vasomotor symptoms.
At standard doses, nafarelin therapy generally results in serum estradiol levels in the range of 20 to 30 pg/mL, a “sweet spot” associated with modest vasomotor symptoms and reduced cellular activity in endometriosis implants.12,14 In many women who become amenorrheic on nafarelin two sprays daily, the dose can be reduced with maintenance of pain control and ovarian suppression.15 Leuprolide acetate depot monotherapy results in serum estradiol levels in the range of 5 to 10 pg/mL, causing severe vasomotor symptoms and reduction in cellular activity in endometriosis lesions. To reduce the adverse effects of leuprolide acetate depot monotherapy, I generally initiate concomitant add-back therapy with norethindrone acetate.13 A little recognized pharmacokinetic observation is that a very small amount of norethindrone acetate, generally less than 1%, is metabolized to ethinyl estradiol.16
The oral GnRH antagonist, elagolix, 150 mg daily for up to 24 months or 200 mg twice daily for 6 months, was approved by the US Food and Drug Administration (FDA) in July 2018. It is now available in pharmacies. Elagolix treatment results in significant reduction in pain caused by endometriosis, but only moderately bothersome vasomotor symptoms.17,18 Elagolix likely will become a widely used medication because of the simplicity of oral administration, efficacy against endometriosis, and acceptable adverse-effect profile. A major disadvantage of the GnRH analogue-class of medications is that they are more expensive than the progestin medications mentioned above. Among the GnRH analogue class of medications, elagolix and goserelin are the least expensive.
Androgens
Estrogen stimulates cellular activity in endometriosis lesions. Androgen and high-dose progestins inhibit cellular activity in endometriosis lesions. Danazol, an attenuated androgen and a progestin is effectivein treating pelvic pain caused by endometriosis.19,20 However, many women decline to use danazol because it is often associated with weight gain. As an androgen, danazol can permanently change a woman’s voice pitch and should not be used by professional singers or speech therapists.
Aromatase Inhibitors
Estrogen is a critically important stimulus of cell activity in endometriosis lesions. Aromatase inhibitors, which block the synthesis of estrogen, have been explored in the treatment of endometriosis that has proven to be resistant to other therapies. Although the combination of an aromatase inhibitor plus a high-dose progestin or GnRH analogue may be effective, more data are needed before widely using the aromatase inhibitors in clinical practice.21
Don’t get stuck in a rut
When treating pelvic pain caused by endometriosis, if the patient’s hormone regimen is not working, prescribe a medication from another class of hormones. In the case presented above, a woman with pelvic pain and surgically proven endometriosis reported inadequate control of her pain symptoms with a continuous estrogen-progestin medication. Her physician prescribed another year of the same estrogen-progestin medication. Instead of renewing the medication, the physician could have offered the patient a hormone medication from another drug class: 1) progestin only, 2) GnRH analogue, or 3) danazol. By using every available hormonal agent, physicians will improve the treatment of pelvic pain caused by endometriosis. Millions of women in our country have pelvic pain caused by endometriosis. They are counting on us, women’s health specialists, to effectively treat their disease.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96(6):623–632.
- Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43.
- Vercellini P, Pietropaolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
- Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database of Syst Rev. 2012;(3):CD002122.
- Moghissi KS, Boyce CR. Management of endometriosis with oral medroxyprogesterone acetate. Obstet Gynecol. 1976;47(3):265–267.
- Telimaa S, Puolakka J, Rönnberg L, Kauppila A. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis. Gynecol Endocrinol. 1987;1(1):13–23.
- Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 pt 1):323–327.
- Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leu-prolide acetate in the treatment of endometriosis-associated pain. Fertil Steril. 2006;85(2):314–325.
- Abou-Setta AM, Houston B, Al-Inany HG, Farquhar C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database of Syst Rev. 2013;(1):CD005072.
- Tanmahasamut P, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Indhavivadhana S, Leerasiri P. Postoperative levonorgestrel-releasing intrauterine system for pelvic endometriosis-pain: a randomized controlled trial. Obstet Gynecol. 2012;119(3):519–526.
- Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and Depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273–279.
- Henzl MR, Corson SL, Moghissi K, Buttram VC, Berqvist C, Jacobsen J. Administration of nasal nafarelin as compared with oral danazol for endo-metriosis. A multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318(8):485–489.
- Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998; 91(1):16–24.
- Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166(2):740–745.
- Hull ME, Barbieri RL. Nafarelin in the treatment of endometriosis. Dose management. Gynecol Obstet Invest. 1994;37(4):263–264.
- Barbieri RL, Petro Z, Canick JA, Ryan KJ. Aromatization of norethindrone to ethinyl estradiol by human placental microsomes. J Clin Endocrinol Metab. 1983;57(2):299–303.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132(1):147–160.
- Selak V, Farquhar C, Prentice A, Singla A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2007;(4):CD000068.
- Barbieri RL, Ryan KJ. Danazol: endocrine pharmacology and therapeutic applications. Am J Obstet Gynecol. 1981;141(4):453–463.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96(6):623–632.
- Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43.
- Vercellini P, Pietropaolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
- Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database of Syst Rev. 2012;(3):CD002122.
- Moghissi KS, Boyce CR. Management of endometriosis with oral medroxyprogesterone acetate. Obstet Gynecol. 1976;47(3):265–267.
- Telimaa S, Puolakka J, Rönnberg L, Kauppila A. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis. Gynecol Endocrinol. 1987;1(1):13–23.
- Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 pt 1):323–327.
- Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leu-prolide acetate in the treatment of endometriosis-associated pain. Fertil Steril. 2006;85(2):314–325.
- Abou-Setta AM, Houston B, Al-Inany HG, Farquhar C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database of Syst Rev. 2013;(1):CD005072.
- Tanmahasamut P, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Indhavivadhana S, Leerasiri P. Postoperative levonorgestrel-releasing intrauterine system for pelvic endometriosis-pain: a randomized controlled trial. Obstet Gynecol. 2012;119(3):519–526.
- Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and Depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273–279.
- Henzl MR, Corson SL, Moghissi K, Buttram VC, Berqvist C, Jacobsen J. Administration of nasal nafarelin as compared with oral danazol for endo-metriosis. A multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318(8):485–489.
- Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998; 91(1):16–24.
- Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166(2):740–745.
- Hull ME, Barbieri RL. Nafarelin in the treatment of endometriosis. Dose management. Gynecol Obstet Invest. 1994;37(4):263–264.
- Barbieri RL, Petro Z, Canick JA, Ryan KJ. Aromatization of norethindrone to ethinyl estradiol by human placental microsomes. J Clin Endocrinol Metab. 1983;57(2):299–303.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132(1):147–160.
- Selak V, Farquhar C, Prentice A, Singla A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2007;(4):CD000068.
- Barbieri RL, Ryan KJ. Danazol: endocrine pharmacology and therapeutic applications. Am J Obstet Gynecol. 1981;141(4):453–463.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
MOC: ACOG’s role in developing a solution to the heated controversy
The American Board of Medical Specialties (ABMS) has decided to trade the phrase “maintenance of certification” (MOC) for “continuing board certification,” a seemingly minor change that has an important backstory. This is the story of how the physician community flexed its collective muscle and how the American College of Obstetricians and Gynecologists (ACOG) helped broker an important détente and pathway in a highly contentious issue.
Founded in 1933 as a nonprofit organization dedicated to maintaining high uniform standards among physicians, the ABMS and many of its specialty boards have found themselves, for more than a decade, under heavy fire from physicians (especially family physicians, internists, and surgeons), their 24 subspecialties, and the state medical societies representing them.
The ObGyn experience with the American Board of Obstetrics and Gynecology (ABOG), however, is better for a number of reasons. Historically, ABOG and ACOG have worked closely together, which is an anomaly among boards as many boards have an arms-length or even an antagonistic relationship with their specialty society.
The discussion below outlines physician concerns with the ABMS and related boards and describes efforts to address and rebuild the continuing board certification process.
Direct and indirect costs
Physicians are very concerned with the costs involved in MOC. Measurable costs include testing fees, while indirect costs include time, stress, travel to test centers, and threats to livelihood for failing a high-stakes examination. Physicians want the high-stakes exam eliminated.
Relevance to practice
Physicians often feel that the MOC has little relevance to their practice, which fuels a sense of resentment toward boards that they believe are dominated by physicians who no longer practice. Subspecialists feel farther away from general practice and the base exams. Generalists feel that the exams miss the points of their daily practice.
Lack of data to show improved quality of care
Physicians want to know that the MOC is worth their time, effort, and money because it improves patient care. To date, however, empirical or clinical data on patient outcomes are absent or ambiguous; most studies lack high-level data or do not investigate the MOC requirements. Physicians want to know what the best MOC practices are, what improves care, and that practices that make no difference will be discarded. In addition, they want timely knowledge alerts when evidence changes.
Relationship to licensing, employment, privileging, credentialing, and reimbursement
Hospitals, insurers, and states increasingly—and inappropriately—use board certification as the primary (sometimes only) default measure of a physician’s fitness for patient care. Physicians without board certification often are denied hospital privileges, inclusion in insurance panels, and even medical licenses. This changes certification from a voluntary physician self-improvement exercise into a can’t-earn-a-living-without-it cudgel.
Variation
Boards vary significantly in their MOC requirements and costs. The importance of an equal standard across all boards is a clear theme among physician concerns.
Role and authority of the ABMS and related boards
Many physicians are frustrated with the perceived autocratic nature of their boards—boards that lack transparency, do not solicit or allow input from practicing physicians, and are unresponsive to physician concerns.
According to Susan Ramin, MD, ABOG Associate Executive Director, ABOG is leading in a number of these areas, including:
- rapidly disseminating clinical information on emerging topics, such as Zika virus infection and opioid misuse
- offering physician choice of testing categories
- exempting high scorers from the secured written exam, which saved physicians a total of $881,000 in exam fees
- crediting physicians for what they already are doing, including serving on maternal mortality review committees, participating in registries, and participating in the Alliance for Innovation on Maternal Health (AIM)
- providing Lifelong Learning and Self-Assessment (LLSA) articles that, according to 90% of diplomates surveyed, are beneficial to their clinical practice (FIGURE).1,2
Our colleague physicians are not so lucky. In a 2015 New England Journal of Medicine Perspective, one physician called out the American Board of Internal Medicine as “a private, self-appointed certifying organization,” a not-for-profit organization that has “grown into a $55-million-per-year business.”3 He concluded that “many physicians are waking up to the fact that our profession is increasingly controlled by people not directly involved in patient care who have lost contact with the realities of day-to-day clinical practice.”3
State and society responses to MOC requirements
Frustration with an inability to resolve these concerns has grown steadily, bubbling over into state governments. The American Medical Association developed “model state legislation intended to prohibit hospitals, health care insurers, and state boards of medicine and osteopathic medicine from requiring participation in MOC processes as a condition of credentialing, privileging, insurance panel participation, licensure, or licensure renewal.”4
Some states are proposing or have enacted legislation that prohibits the use of MOC as a criterion for licensure, privileging, employment, reimbursement, and/or insurance panel participation. Eight states (Arizona, Georgia, Kentucky, Maryland, Maine, Missouri, Oklahoma, Tennessee) have enacted laws to prohibit the use of MOC for initial and renewal licensure decisions. Many states are actively considering MOC-related legislation, including Alaska, Florida, Iowa, Indiana, Maryland, Massachusetts, Michigan, Missouri, New Hampshire, New York, Ohio, Oklahoma, Rhode Island, South Carolina, Tennessee, Utah, Washington, and Wisconsin.
Legislation is not the only outlet for physician frustration. Some medical specialty societies are considering dropping board certification as a membership requirement; physicians are exploring developing alternative boards; and some physicians are defying the board certification requirement altogether, with thousands signing anti-MOC petitions.
ACOG asserts importance of maintaining self-regulation
While other specialties are actively advocating state legislation, ACOG and ABOG have worked together to oppose state legislation, believing that physician self-regulation is paramount. In fact, in 2017, ACOG and ABOG issued a joint statement urging state lawmakers to “not interfere with our decades of successful self-regulation and to realize that each medical society has its own experience with its MOC program.”5
Negotiations lead to new initiative
This brings us to an interesting situation. ACOG’s Executive Vice President and CEO Hal Lawrence III, MD, was tapped (in his position as Chair of the Specialty Society CEO Consortium) to represent physician specialties in negotiations and discussions with the boards, which were represented by Lois Nora, MD, JD, President and CEO of the ABMS, and state medical societies, represented by Donald Palmisano Jr, JD, Executive Director and CEO of the Medical Association of Georgia. Many state medical societies, boards, and physician specialty organizations participated in these meetings.
Throughout months of debate, Dr. Lawrence urged his colleagues to stay at the table and do the hard work of reaching an agreement, rather than ask politicians to solve medicine’s problems. This approach was leveraged by the serious efforts and threats of state legislation, which brought the boards to the table. In August 2017, 41 state medical societies and 33 national medical specialty societies wrote to Dr. Nora expressing their concerns that “professional self-regulation is under attack. Concerns regarding the usefulness of the high-stakes exam, the exorbitant costs of the MOC process, and the lack of transparent communication from the certifying boards have led to damaging the MOC brand, and creating state-based attacks on the MOC process.”6
In December 2017, Dr. Lawrence and Mr. Palmisano led a meeting of principals from the national medical specialty societies and state medical societies with leaders of ABMS and 8 specialty boards, including ABOG, an opportunity to secure meaningful change. Dr. Lawrence began by stressing that the interests of physicians and patients would be best served by all parties coming together and collaborating on a meaningful solution, to repair trust and preserve physician self-regulation.
Dr. Ramin presented ABOG’s approach to continuous certification, lifelong learning, and self-assessment. The American Board of Urology and the American Board of Psychiatry and Neurology indicated that they were basing important changes in their MOC process on ABOG’s work, including using 5 modules (1 general and 4 specific to the physician’s practice) and multiple open-book mini-exams based on selected journal articles as an alternative to the 10-year MOC exam.
The Vision Initiative. At that meeting and others, the ABMS and other boards heard physicians’ candid and sometimes blunt concerns. Dr. Nora spoke to the recently announced Continuing Board Certification: Vision for the Future program, also known as the “Vision Initiative,” a process designed to fundamentally rebuild the continuing certification process with input and guidance from practicing physicians. Physician response seemed uniform: Seeing is believing.
Importantly, all participants at the December meeting agreed to work together to rebuild trust and ensure professionalism and professional self-regulation, reflected in this Statement of Shared Purpose:
ABMS certifying boards and national medical specialty societies will collaborate to resolve differences in the process of ongoing certification and to fulfill the principles of professional self-regulation, achieving appropriate standardization, and assuring that ongoing certification is relevant to the practices of physicians without undue burden. Furthermore, the boards and societies, and their organizations (ABMS and CMSS [Council of Medical Specialty Societies]), will undertake necessary changes in a timely manner, and will commit to ongoing communication with state medical associations to solicit their input.4
Two ObGyns participating in the Vision Initiative are Haywood Brown, MD, ACOG’s Immediate Past President, and George Wendel, MD, ABOG’s Executive Director. The Vision Initiative is composed of 3 parts. Part 1, Organization, is complete. The committee is currently working on part 2, Envisioning the Future, an information-gathering component that includes physician surveys, hearings, open solicited input, and identifying new and better approaches. After the final report is delivered to the ABMS in February 2019, part 3, Implementation, will begin.
The Vision Initiative offers physicians an important opportunity to help shape the future of continuing education and certification. ObGyns and other physicians should consider reviewing and commenting on the draft report, due in November, during the public comment period. Visit https://visioninitiative.org for more information and to sign up for email updates.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- American Board of Obstetrics and Gynecology. From pilot to permanent: ABOG's program offering an innovative pathway integrating lifelong learning and self-assessment and external assessment is approved. https://www.abog.org/new/ABOG_PilotToPermanent.aspx. Accessed July 6, 2018.
- Ramin S. American Board of Obstetrics and Gynecology MOC program. PowerPoint presentation; December 4, 2017.
- Teirstein PS. Boarded to death--why maintenance of certification is bad for doctors and patients. N Engl J Med. 2015;372(2):106-108.
- AMA Council on Medical Education. Executive summary. 2017. https://www.ama-assn.org/sites/default/files/media-browser/public/council-on-med-ed/a18-cme-02.pdf. Accessed July 6, 2018.
- American College of Obstetricians and Gynecologists. ACOG-ABOG joint statement: political interference in physician maintenance of skills threatens women's health care. https://www.acog.org/-/media/Departments/State-Legislative-Activities/2017ACOG-ABMS-MOC-Statement.pdf?dmc=1&ts=20180706T1615538746. Accessed July 6, 2018.
- Letter to Lois Nora, MD, JD. August 18, 2017. https://www.mainemed.com/sites/default/files/content/MOC%20Letter%20082117.pdf. Accessed July 6, 2018.
The American Board of Medical Specialties (ABMS) has decided to trade the phrase “maintenance of certification” (MOC) for “continuing board certification,” a seemingly minor change that has an important backstory. This is the story of how the physician community flexed its collective muscle and how the American College of Obstetricians and Gynecologists (ACOG) helped broker an important détente and pathway in a highly contentious issue.
Founded in 1933 as a nonprofit organization dedicated to maintaining high uniform standards among physicians, the ABMS and many of its specialty boards have found themselves, for more than a decade, under heavy fire from physicians (especially family physicians, internists, and surgeons), their 24 subspecialties, and the state medical societies representing them.
The ObGyn experience with the American Board of Obstetrics and Gynecology (ABOG), however, is better for a number of reasons. Historically, ABOG and ACOG have worked closely together, which is an anomaly among boards as many boards have an arms-length or even an antagonistic relationship with their specialty society.
The discussion below outlines physician concerns with the ABMS and related boards and describes efforts to address and rebuild the continuing board certification process.
Direct and indirect costs
Physicians are very concerned with the costs involved in MOC. Measurable costs include testing fees, while indirect costs include time, stress, travel to test centers, and threats to livelihood for failing a high-stakes examination. Physicians want the high-stakes exam eliminated.
Relevance to practice
Physicians often feel that the MOC has little relevance to their practice, which fuels a sense of resentment toward boards that they believe are dominated by physicians who no longer practice. Subspecialists feel farther away from general practice and the base exams. Generalists feel that the exams miss the points of their daily practice.
Lack of data to show improved quality of care
Physicians want to know that the MOC is worth their time, effort, and money because it improves patient care. To date, however, empirical or clinical data on patient outcomes are absent or ambiguous; most studies lack high-level data or do not investigate the MOC requirements. Physicians want to know what the best MOC practices are, what improves care, and that practices that make no difference will be discarded. In addition, they want timely knowledge alerts when evidence changes.
Relationship to licensing, employment, privileging, credentialing, and reimbursement
Hospitals, insurers, and states increasingly—and inappropriately—use board certification as the primary (sometimes only) default measure of a physician’s fitness for patient care. Physicians without board certification often are denied hospital privileges, inclusion in insurance panels, and even medical licenses. This changes certification from a voluntary physician self-improvement exercise into a can’t-earn-a-living-without-it cudgel.
Variation
Boards vary significantly in their MOC requirements and costs. The importance of an equal standard across all boards is a clear theme among physician concerns.
Role and authority of the ABMS and related boards
Many physicians are frustrated with the perceived autocratic nature of their boards—boards that lack transparency, do not solicit or allow input from practicing physicians, and are unresponsive to physician concerns.
According to Susan Ramin, MD, ABOG Associate Executive Director, ABOG is leading in a number of these areas, including:
- rapidly disseminating clinical information on emerging topics, such as Zika virus infection and opioid misuse
- offering physician choice of testing categories
- exempting high scorers from the secured written exam, which saved physicians a total of $881,000 in exam fees
- crediting physicians for what they already are doing, including serving on maternal mortality review committees, participating in registries, and participating in the Alliance for Innovation on Maternal Health (AIM)
- providing Lifelong Learning and Self-Assessment (LLSA) articles that, according to 90% of diplomates surveyed, are beneficial to their clinical practice (FIGURE).1,2
Our colleague physicians are not so lucky. In a 2015 New England Journal of Medicine Perspective, one physician called out the American Board of Internal Medicine as “a private, self-appointed certifying organization,” a not-for-profit organization that has “grown into a $55-million-per-year business.”3 He concluded that “many physicians are waking up to the fact that our profession is increasingly controlled by people not directly involved in patient care who have lost contact with the realities of day-to-day clinical practice.”3
State and society responses to MOC requirements
Frustration with an inability to resolve these concerns has grown steadily, bubbling over into state governments. The American Medical Association developed “model state legislation intended to prohibit hospitals, health care insurers, and state boards of medicine and osteopathic medicine from requiring participation in MOC processes as a condition of credentialing, privileging, insurance panel participation, licensure, or licensure renewal.”4
Some states are proposing or have enacted legislation that prohibits the use of MOC as a criterion for licensure, privileging, employment, reimbursement, and/or insurance panel participation. Eight states (Arizona, Georgia, Kentucky, Maryland, Maine, Missouri, Oklahoma, Tennessee) have enacted laws to prohibit the use of MOC for initial and renewal licensure decisions. Many states are actively considering MOC-related legislation, including Alaska, Florida, Iowa, Indiana, Maryland, Massachusetts, Michigan, Missouri, New Hampshire, New York, Ohio, Oklahoma, Rhode Island, South Carolina, Tennessee, Utah, Washington, and Wisconsin.
Legislation is not the only outlet for physician frustration. Some medical specialty societies are considering dropping board certification as a membership requirement; physicians are exploring developing alternative boards; and some physicians are defying the board certification requirement altogether, with thousands signing anti-MOC petitions.
ACOG asserts importance of maintaining self-regulation
While other specialties are actively advocating state legislation, ACOG and ABOG have worked together to oppose state legislation, believing that physician self-regulation is paramount. In fact, in 2017, ACOG and ABOG issued a joint statement urging state lawmakers to “not interfere with our decades of successful self-regulation and to realize that each medical society has its own experience with its MOC program.”5
Negotiations lead to new initiative
This brings us to an interesting situation. ACOG’s Executive Vice President and CEO Hal Lawrence III, MD, was tapped (in his position as Chair of the Specialty Society CEO Consortium) to represent physician specialties in negotiations and discussions with the boards, which were represented by Lois Nora, MD, JD, President and CEO of the ABMS, and state medical societies, represented by Donald Palmisano Jr, JD, Executive Director and CEO of the Medical Association of Georgia. Many state medical societies, boards, and physician specialty organizations participated in these meetings.
Throughout months of debate, Dr. Lawrence urged his colleagues to stay at the table and do the hard work of reaching an agreement, rather than ask politicians to solve medicine’s problems. This approach was leveraged by the serious efforts and threats of state legislation, which brought the boards to the table. In August 2017, 41 state medical societies and 33 national medical specialty societies wrote to Dr. Nora expressing their concerns that “professional self-regulation is under attack. Concerns regarding the usefulness of the high-stakes exam, the exorbitant costs of the MOC process, and the lack of transparent communication from the certifying boards have led to damaging the MOC brand, and creating state-based attacks on the MOC process.”6
In December 2017, Dr. Lawrence and Mr. Palmisano led a meeting of principals from the national medical specialty societies and state medical societies with leaders of ABMS and 8 specialty boards, including ABOG, an opportunity to secure meaningful change. Dr. Lawrence began by stressing that the interests of physicians and patients would be best served by all parties coming together and collaborating on a meaningful solution, to repair trust and preserve physician self-regulation.
Dr. Ramin presented ABOG’s approach to continuous certification, lifelong learning, and self-assessment. The American Board of Urology and the American Board of Psychiatry and Neurology indicated that they were basing important changes in their MOC process on ABOG’s work, including using 5 modules (1 general and 4 specific to the physician’s practice) and multiple open-book mini-exams based on selected journal articles as an alternative to the 10-year MOC exam.
The Vision Initiative. At that meeting and others, the ABMS and other boards heard physicians’ candid and sometimes blunt concerns. Dr. Nora spoke to the recently announced Continuing Board Certification: Vision for the Future program, also known as the “Vision Initiative,” a process designed to fundamentally rebuild the continuing certification process with input and guidance from practicing physicians. Physician response seemed uniform: Seeing is believing.
Importantly, all participants at the December meeting agreed to work together to rebuild trust and ensure professionalism and professional self-regulation, reflected in this Statement of Shared Purpose:
ABMS certifying boards and national medical specialty societies will collaborate to resolve differences in the process of ongoing certification and to fulfill the principles of professional self-regulation, achieving appropriate standardization, and assuring that ongoing certification is relevant to the practices of physicians without undue burden. Furthermore, the boards and societies, and their organizations (ABMS and CMSS [Council of Medical Specialty Societies]), will undertake necessary changes in a timely manner, and will commit to ongoing communication with state medical associations to solicit their input.4
Two ObGyns participating in the Vision Initiative are Haywood Brown, MD, ACOG’s Immediate Past President, and George Wendel, MD, ABOG’s Executive Director. The Vision Initiative is composed of 3 parts. Part 1, Organization, is complete. The committee is currently working on part 2, Envisioning the Future, an information-gathering component that includes physician surveys, hearings, open solicited input, and identifying new and better approaches. After the final report is delivered to the ABMS in February 2019, part 3, Implementation, will begin.
The Vision Initiative offers physicians an important opportunity to help shape the future of continuing education and certification. ObGyns and other physicians should consider reviewing and commenting on the draft report, due in November, during the public comment period. Visit https://visioninitiative.org for more information and to sign up for email updates.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
The American Board of Medical Specialties (ABMS) has decided to trade the phrase “maintenance of certification” (MOC) for “continuing board certification,” a seemingly minor change that has an important backstory. This is the story of how the physician community flexed its collective muscle and how the American College of Obstetricians and Gynecologists (ACOG) helped broker an important détente and pathway in a highly contentious issue.
Founded in 1933 as a nonprofit organization dedicated to maintaining high uniform standards among physicians, the ABMS and many of its specialty boards have found themselves, for more than a decade, under heavy fire from physicians (especially family physicians, internists, and surgeons), their 24 subspecialties, and the state medical societies representing them.
The ObGyn experience with the American Board of Obstetrics and Gynecology (ABOG), however, is better for a number of reasons. Historically, ABOG and ACOG have worked closely together, which is an anomaly among boards as many boards have an arms-length or even an antagonistic relationship with their specialty society.
The discussion below outlines physician concerns with the ABMS and related boards and describes efforts to address and rebuild the continuing board certification process.
Direct and indirect costs
Physicians are very concerned with the costs involved in MOC. Measurable costs include testing fees, while indirect costs include time, stress, travel to test centers, and threats to livelihood for failing a high-stakes examination. Physicians want the high-stakes exam eliminated.
Relevance to practice
Physicians often feel that the MOC has little relevance to their practice, which fuels a sense of resentment toward boards that they believe are dominated by physicians who no longer practice. Subspecialists feel farther away from general practice and the base exams. Generalists feel that the exams miss the points of their daily practice.
Lack of data to show improved quality of care
Physicians want to know that the MOC is worth their time, effort, and money because it improves patient care. To date, however, empirical or clinical data on patient outcomes are absent or ambiguous; most studies lack high-level data or do not investigate the MOC requirements. Physicians want to know what the best MOC practices are, what improves care, and that practices that make no difference will be discarded. In addition, they want timely knowledge alerts when evidence changes.
Relationship to licensing, employment, privileging, credentialing, and reimbursement
Hospitals, insurers, and states increasingly—and inappropriately—use board certification as the primary (sometimes only) default measure of a physician’s fitness for patient care. Physicians without board certification often are denied hospital privileges, inclusion in insurance panels, and even medical licenses. This changes certification from a voluntary physician self-improvement exercise into a can’t-earn-a-living-without-it cudgel.
Variation
Boards vary significantly in their MOC requirements and costs. The importance of an equal standard across all boards is a clear theme among physician concerns.
Role and authority of the ABMS and related boards
Many physicians are frustrated with the perceived autocratic nature of their boards—boards that lack transparency, do not solicit or allow input from practicing physicians, and are unresponsive to physician concerns.
According to Susan Ramin, MD, ABOG Associate Executive Director, ABOG is leading in a number of these areas, including:
- rapidly disseminating clinical information on emerging topics, such as Zika virus infection and opioid misuse
- offering physician choice of testing categories
- exempting high scorers from the secured written exam, which saved physicians a total of $881,000 in exam fees
- crediting physicians for what they already are doing, including serving on maternal mortality review committees, participating in registries, and participating in the Alliance for Innovation on Maternal Health (AIM)
- providing Lifelong Learning and Self-Assessment (LLSA) articles that, according to 90% of diplomates surveyed, are beneficial to their clinical practice (FIGURE).1,2
Our colleague physicians are not so lucky. In a 2015 New England Journal of Medicine Perspective, one physician called out the American Board of Internal Medicine as “a private, self-appointed certifying organization,” a not-for-profit organization that has “grown into a $55-million-per-year business.”3 He concluded that “many physicians are waking up to the fact that our profession is increasingly controlled by people not directly involved in patient care who have lost contact with the realities of day-to-day clinical practice.”3
State and society responses to MOC requirements
Frustration with an inability to resolve these concerns has grown steadily, bubbling over into state governments. The American Medical Association developed “model state legislation intended to prohibit hospitals, health care insurers, and state boards of medicine and osteopathic medicine from requiring participation in MOC processes as a condition of credentialing, privileging, insurance panel participation, licensure, or licensure renewal.”4
Some states are proposing or have enacted legislation that prohibits the use of MOC as a criterion for licensure, privileging, employment, reimbursement, and/or insurance panel participation. Eight states (Arizona, Georgia, Kentucky, Maryland, Maine, Missouri, Oklahoma, Tennessee) have enacted laws to prohibit the use of MOC for initial and renewal licensure decisions. Many states are actively considering MOC-related legislation, including Alaska, Florida, Iowa, Indiana, Maryland, Massachusetts, Michigan, Missouri, New Hampshire, New York, Ohio, Oklahoma, Rhode Island, South Carolina, Tennessee, Utah, Washington, and Wisconsin.
Legislation is not the only outlet for physician frustration. Some medical specialty societies are considering dropping board certification as a membership requirement; physicians are exploring developing alternative boards; and some physicians are defying the board certification requirement altogether, with thousands signing anti-MOC petitions.
ACOG asserts importance of maintaining self-regulation
While other specialties are actively advocating state legislation, ACOG and ABOG have worked together to oppose state legislation, believing that physician self-regulation is paramount. In fact, in 2017, ACOG and ABOG issued a joint statement urging state lawmakers to “not interfere with our decades of successful self-regulation and to realize that each medical society has its own experience with its MOC program.”5
Negotiations lead to new initiative
This brings us to an interesting situation. ACOG’s Executive Vice President and CEO Hal Lawrence III, MD, was tapped (in his position as Chair of the Specialty Society CEO Consortium) to represent physician specialties in negotiations and discussions with the boards, which were represented by Lois Nora, MD, JD, President and CEO of the ABMS, and state medical societies, represented by Donald Palmisano Jr, JD, Executive Director and CEO of the Medical Association of Georgia. Many state medical societies, boards, and physician specialty organizations participated in these meetings.
Throughout months of debate, Dr. Lawrence urged his colleagues to stay at the table and do the hard work of reaching an agreement, rather than ask politicians to solve medicine’s problems. This approach was leveraged by the serious efforts and threats of state legislation, which brought the boards to the table. In August 2017, 41 state medical societies and 33 national medical specialty societies wrote to Dr. Nora expressing their concerns that “professional self-regulation is under attack. Concerns regarding the usefulness of the high-stakes exam, the exorbitant costs of the MOC process, and the lack of transparent communication from the certifying boards have led to damaging the MOC brand, and creating state-based attacks on the MOC process.”6
In December 2017, Dr. Lawrence and Mr. Palmisano led a meeting of principals from the national medical specialty societies and state medical societies with leaders of ABMS and 8 specialty boards, including ABOG, an opportunity to secure meaningful change. Dr. Lawrence began by stressing that the interests of physicians and patients would be best served by all parties coming together and collaborating on a meaningful solution, to repair trust and preserve physician self-regulation.
Dr. Ramin presented ABOG’s approach to continuous certification, lifelong learning, and self-assessment. The American Board of Urology and the American Board of Psychiatry and Neurology indicated that they were basing important changes in their MOC process on ABOG’s work, including using 5 modules (1 general and 4 specific to the physician’s practice) and multiple open-book mini-exams based on selected journal articles as an alternative to the 10-year MOC exam.
The Vision Initiative. At that meeting and others, the ABMS and other boards heard physicians’ candid and sometimes blunt concerns. Dr. Nora spoke to the recently announced Continuing Board Certification: Vision for the Future program, also known as the “Vision Initiative,” a process designed to fundamentally rebuild the continuing certification process with input and guidance from practicing physicians. Physician response seemed uniform: Seeing is believing.
Importantly, all participants at the December meeting agreed to work together to rebuild trust and ensure professionalism and professional self-regulation, reflected in this Statement of Shared Purpose:
ABMS certifying boards and national medical specialty societies will collaborate to resolve differences in the process of ongoing certification and to fulfill the principles of professional self-regulation, achieving appropriate standardization, and assuring that ongoing certification is relevant to the practices of physicians without undue burden. Furthermore, the boards and societies, and their organizations (ABMS and CMSS [Council of Medical Specialty Societies]), will undertake necessary changes in a timely manner, and will commit to ongoing communication with state medical associations to solicit their input.4
Two ObGyns participating in the Vision Initiative are Haywood Brown, MD, ACOG’s Immediate Past President, and George Wendel, MD, ABOG’s Executive Director. The Vision Initiative is composed of 3 parts. Part 1, Organization, is complete. The committee is currently working on part 2, Envisioning the Future, an information-gathering component that includes physician surveys, hearings, open solicited input, and identifying new and better approaches. After the final report is delivered to the ABMS in February 2019, part 3, Implementation, will begin.
The Vision Initiative offers physicians an important opportunity to help shape the future of continuing education and certification. ObGyns and other physicians should consider reviewing and commenting on the draft report, due in November, during the public comment period. Visit https://visioninitiative.org for more information and to sign up for email updates.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- American Board of Obstetrics and Gynecology. From pilot to permanent: ABOG's program offering an innovative pathway integrating lifelong learning and self-assessment and external assessment is approved. https://www.abog.org/new/ABOG_PilotToPermanent.aspx. Accessed July 6, 2018.
- Ramin S. American Board of Obstetrics and Gynecology MOC program. PowerPoint presentation; December 4, 2017.
- Teirstein PS. Boarded to death--why maintenance of certification is bad for doctors and patients. N Engl J Med. 2015;372(2):106-108.
- AMA Council on Medical Education. Executive summary. 2017. https://www.ama-assn.org/sites/default/files/media-browser/public/council-on-med-ed/a18-cme-02.pdf. Accessed July 6, 2018.
- American College of Obstetricians and Gynecologists. ACOG-ABOG joint statement: political interference in physician maintenance of skills threatens women's health care. https://www.acog.org/-/media/Departments/State-Legislative-Activities/2017ACOG-ABMS-MOC-Statement.pdf?dmc=1&ts=20180706T1615538746. Accessed July 6, 2018.
- Letter to Lois Nora, MD, JD. August 18, 2017. https://www.mainemed.com/sites/default/files/content/MOC%20Letter%20082117.pdf. Accessed July 6, 2018.
- American Board of Obstetrics and Gynecology. From pilot to permanent: ABOG's program offering an innovative pathway integrating lifelong learning and self-assessment and external assessment is approved. https://www.abog.org/new/ABOG_PilotToPermanent.aspx. Accessed July 6, 2018.
- Ramin S. American Board of Obstetrics and Gynecology MOC program. PowerPoint presentation; December 4, 2017.
- Teirstein PS. Boarded to death--why maintenance of certification is bad for doctors and patients. N Engl J Med. 2015;372(2):106-108.
- AMA Council on Medical Education. Executive summary. 2017. https://www.ama-assn.org/sites/default/files/media-browser/public/council-on-med-ed/a18-cme-02.pdf. Accessed July 6, 2018.
- American College of Obstetricians and Gynecologists. ACOG-ABOG joint statement: political interference in physician maintenance of skills threatens women's health care. https://www.acog.org/-/media/Departments/State-Legislative-Activities/2017ACOG-ABMS-MOC-Statement.pdf?dmc=1&ts=20180706T1615538746. Accessed July 6, 2018.
- Letter to Lois Nora, MD, JD. August 18, 2017. https://www.mainemed.com/sites/default/files/content/MOC%20Letter%20082117.pdf. Accessed July 6, 2018.
CDC apps specific for ObGyns
The Centers for Disease Control and Prevention (CDC) is a US federal agency under the Department of Health and Human Services. It is the nation’s leading public health institute. Its main goal is to save lives and protect people from health, safety, and security threats. The CDC website lists 25 no-cost applications that the agency has developed: https://www.cdc.gov/mobile/mobileapp.html.
This review will focus on 3 CDC apps (Table) that I feel are useful to ObGyn health care providers: Prevent Group B Strep (GBS), STD Tx Guide, and US Medical Eligibility Criteria for Contraceptive Use. In fact, in an evaluation of contraception apps for providers of family planning services, US Medical Eligibility Criteria for Contraceptive Use was one of the highest scoring apps.1 I will evaluate each app by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).2 I commend the CDC for developing these useful tools to assist health care providers.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Perry R, Lunde B, Chen KT. An evaluation of contraception mobile applications for providers of family planning services. Contraception. 2016;93(6):539-544.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
The Centers for Disease Control and Prevention (CDC) is a US federal agency under the Department of Health and Human Services. It is the nation’s leading public health institute. Its main goal is to save lives and protect people from health, safety, and security threats. The CDC website lists 25 no-cost applications that the agency has developed: https://www.cdc.gov/mobile/mobileapp.html.
This review will focus on 3 CDC apps (Table) that I feel are useful to ObGyn health care providers: Prevent Group B Strep (GBS), STD Tx Guide, and US Medical Eligibility Criteria for Contraceptive Use. In fact, in an evaluation of contraception apps for providers of family planning services, US Medical Eligibility Criteria for Contraceptive Use was one of the highest scoring apps.1 I will evaluate each app by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).2 I commend the CDC for developing these useful tools to assist health care providers.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
The Centers for Disease Control and Prevention (CDC) is a US federal agency under the Department of Health and Human Services. It is the nation’s leading public health institute. Its main goal is to save lives and protect people from health, safety, and security threats. The CDC website lists 25 no-cost applications that the agency has developed: https://www.cdc.gov/mobile/mobileapp.html.
This review will focus on 3 CDC apps (Table) that I feel are useful to ObGyn health care providers: Prevent Group B Strep (GBS), STD Tx Guide, and US Medical Eligibility Criteria for Contraceptive Use. In fact, in an evaluation of contraception apps for providers of family planning services, US Medical Eligibility Criteria for Contraceptive Use was one of the highest scoring apps.1 I will evaluate each app by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).2 I commend the CDC for developing these useful tools to assist health care providers.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Perry R, Lunde B, Chen KT. An evaluation of contraception mobile applications for providers of family planning services. Contraception. 2016;93(6):539-544.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
- Perry R, Lunde B, Chen KT. An evaluation of contraception mobile applications for providers of family planning services. Contraception. 2016;93(6):539-544.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
IN THIS ARTICLE
- Details on recommended apps
What works best for genitourinary syndrome of menopause: vaginal estrogen, vaginal laser, or combined laser and estrogen therapy?
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.
While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.
While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.
While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.