User login
Treat chronic endometritis to improve implantation rates
In a meta-analysis of five studies of chronic endometritis (CE), women cured of the condition had significantly higher rates of pregnancies, live births, and successful implantations compared with women who had persistent CE.
“These findings potentially suggest that CE is a reversible factor of infertility, whose recognition and therapy may provide better chances at subsequent [in vitro fertilization] attempts,” wrote Amerigo Vitagliano, MD, of the University of Padua (Italy), and his coauthors.
They sought to examine the effect of CE treatment on implantation for women with recurrent implantation failure. While CE is correlated with infertility, prior studies have not resolved the question of whether curing CE would restore fertility. The condition is cured in as many of 80% of cases with a single cycle of antibiotics.
The systematic review found five studies with a total of 796 patients with recurrent implantation failure in Argentina, China, Italy, Japan, and the United States. Two studies compared cured CE with persistent CE, and three studies compared cured CE with patients not affected by CE.
Only one of the studies evaluated CE patients receiving antibiotics with CE patients not receiving antibiotics. The study showed that there was no difference between those two groups in clinical pregnancy rate, ongoing (12 or more weeks’ gestation) pregnancy rate/live birth rate, or implantation rate.
The significant result was the difference between cured and persistent CE. Those numbers worked out to a higher ongoing pregnancy rate/live birth rate (odds ratio, 6.81; 95% confidence interval, 2.08-22.24; P = .001), clinical pregnancy rate (OR, 4.98; 95% CI, 1.72-14.43; P = .003), and implantation rate (OR, 3.24; 95% CI, 1.33-7.88; P = .01), with no difference in the miscarriage rate (P = .30).
The authors recommend further research in the form of randomized controlled trials to confirm whether completed CE treatment will improve in vitro fertilization success, and whether routine CE screening is advisable for all patients with recurrent implantation failure. At present, they recommend that diagnosed cases of CE be resolved before continuing with fertility treatment.
“If our results are confirmed, CE may represent a new therapeutic target for women suffering from [recurrent implantation failure], with affordable access (diagnosed through a simple endometrial biopsy and treated by oral antibiotics),” they wrote.
The authors reported having no financial disclosures.
SOURCE: Vitagliano A et al. Fertil Steril. 2018 Jun. doi: 10.1016/j.fertnstert.2018.03.017.
In a meta-analysis of five studies of chronic endometritis (CE), women cured of the condition had significantly higher rates of pregnancies, live births, and successful implantations compared with women who had persistent CE.
“These findings potentially suggest that CE is a reversible factor of infertility, whose recognition and therapy may provide better chances at subsequent [in vitro fertilization] attempts,” wrote Amerigo Vitagliano, MD, of the University of Padua (Italy), and his coauthors.
They sought to examine the effect of CE treatment on implantation for women with recurrent implantation failure. While CE is correlated with infertility, prior studies have not resolved the question of whether curing CE would restore fertility. The condition is cured in as many of 80% of cases with a single cycle of antibiotics.
The systematic review found five studies with a total of 796 patients with recurrent implantation failure in Argentina, China, Italy, Japan, and the United States. Two studies compared cured CE with persistent CE, and three studies compared cured CE with patients not affected by CE.
Only one of the studies evaluated CE patients receiving antibiotics with CE patients not receiving antibiotics. The study showed that there was no difference between those two groups in clinical pregnancy rate, ongoing (12 or more weeks’ gestation) pregnancy rate/live birth rate, or implantation rate.
The significant result was the difference between cured and persistent CE. Those numbers worked out to a higher ongoing pregnancy rate/live birth rate (odds ratio, 6.81; 95% confidence interval, 2.08-22.24; P = .001), clinical pregnancy rate (OR, 4.98; 95% CI, 1.72-14.43; P = .003), and implantation rate (OR, 3.24; 95% CI, 1.33-7.88; P = .01), with no difference in the miscarriage rate (P = .30).
The authors recommend further research in the form of randomized controlled trials to confirm whether completed CE treatment will improve in vitro fertilization success, and whether routine CE screening is advisable for all patients with recurrent implantation failure. At present, they recommend that diagnosed cases of CE be resolved before continuing with fertility treatment.
“If our results are confirmed, CE may represent a new therapeutic target for women suffering from [recurrent implantation failure], with affordable access (diagnosed through a simple endometrial biopsy and treated by oral antibiotics),” they wrote.
The authors reported having no financial disclosures.
SOURCE: Vitagliano A et al. Fertil Steril. 2018 Jun. doi: 10.1016/j.fertnstert.2018.03.017.
In a meta-analysis of five studies of chronic endometritis (CE), women cured of the condition had significantly higher rates of pregnancies, live births, and successful implantations compared with women who had persistent CE.
“These findings potentially suggest that CE is a reversible factor of infertility, whose recognition and therapy may provide better chances at subsequent [in vitro fertilization] attempts,” wrote Amerigo Vitagliano, MD, of the University of Padua (Italy), and his coauthors.
They sought to examine the effect of CE treatment on implantation for women with recurrent implantation failure. While CE is correlated with infertility, prior studies have not resolved the question of whether curing CE would restore fertility. The condition is cured in as many of 80% of cases with a single cycle of antibiotics.
The systematic review found five studies with a total of 796 patients with recurrent implantation failure in Argentina, China, Italy, Japan, and the United States. Two studies compared cured CE with persistent CE, and three studies compared cured CE with patients not affected by CE.
Only one of the studies evaluated CE patients receiving antibiotics with CE patients not receiving antibiotics. The study showed that there was no difference between those two groups in clinical pregnancy rate, ongoing (12 or more weeks’ gestation) pregnancy rate/live birth rate, or implantation rate.
The significant result was the difference between cured and persistent CE. Those numbers worked out to a higher ongoing pregnancy rate/live birth rate (odds ratio, 6.81; 95% confidence interval, 2.08-22.24; P = .001), clinical pregnancy rate (OR, 4.98; 95% CI, 1.72-14.43; P = .003), and implantation rate (OR, 3.24; 95% CI, 1.33-7.88; P = .01), with no difference in the miscarriage rate (P = .30).
The authors recommend further research in the form of randomized controlled trials to confirm whether completed CE treatment will improve in vitro fertilization success, and whether routine CE screening is advisable for all patients with recurrent implantation failure. At present, they recommend that diagnosed cases of CE be resolved before continuing with fertility treatment.
“If our results are confirmed, CE may represent a new therapeutic target for women suffering from [recurrent implantation failure], with affordable access (diagnosed through a simple endometrial biopsy and treated by oral antibiotics),” they wrote.
The authors reported having no financial disclosures.
SOURCE: Vitagliano A et al. Fertil Steril. 2018 Jun. doi: 10.1016/j.fertnstert.2018.03.017.
FROM FERTILITY & STERILITY
HPV testing detects cervical precancers earlier than cytology
Women who received only a primary human papillomavirus test were 58% less likely to develop grade 3 or worse cervical intraepithelial neoplasia (CIN3+) by 48 months than women who had the traditional Pap cytology screen.
The primary HPV test also reduced the 2-year risk of CIN2+ neoplasia, compared with Pap smear alone, Gina Suzanne Ogilvie, MD, and her colleagues reported in JAMA.
“These results have demonstrated that primary HPV testing detects cervical neoplasia earlier and more accurately than cytology,” wrote Dr. Ogilvie of the University of British Columbia, Vancouver, and her colleagues.
HPV FOCAL (the Human Papilloma Virus For Cervical Cancers Screening trial) enrolled 19,009 Canadian women aged 25-65 years and randomized them to two cervical cancer screening paradigms: Pap liquid-based cytology (LBC) or primary HPV testing.
The intervention group (9,552) had cervical cancer screening with a high-risk HPV DNA test that detects types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. If either test was positive, they were referred for colposcopy. If both tests were negative, they returned for their final screen with both tests at 48 months.
The control group underwent primary LBC testing, followed by HPV testing for women with atypical squamous cells of unknown significance (ASCUS). If these tests were both positive, they were referred for colposcopy. Women who were positive for ASCUS and HPV negative returned in 12 months and were referred for colposcopy if they had ASCUS or any higher-grade abnormality. At 48 months, they also returned and underwent both screening tests.
The primary outcome was the rate of CIN3+ at 48 months. Secondary endpoints included the 48-month rate of CIN2+, the threshold for colposcopy referral, and the effect of primary HPV testing on colposcopy.
In the first round of screening, HPV testing detected significantly more cases of CIN3+ than did LBC (risk ratio, 1.61). This was an absolute difference of 2.67 more cases per 1,000 screened women.
At 48 months, the rate of CIN3+ was significantly lower in the intervention group than in the control group (2.3 vs. 5.5 per 1,000; RR, 0.42). This represents an absolute difference of 3.2 fewer cases per 1,000, the investigators said.
Overall, however, the two methods detected about the same number of cases by 48 months, the investigators said.
“Cumulative CIN3+ incidence curves show no significantly different disease detection across trial groups in the intervention group. The cumulative incidence was higher earlier in the trial at 18 months and 42 months, compared with the control group. ... By the end of trial follow-up (72 months), incidence was similar across both groups.”
Women who were HPV negative at baseline reaped the biggest benefit. The 48-month HPV incidence rate among them was 1.4 per 1,000, compared with 5.4 per 1,000 in the control group. This 75% risk reduction (RR, 0.25) represents an absolute reduction of 4 cases per 1,000 women.
The intervention group was 61% more likely to have a CIN2+ result by 12 months (RR, 1.61), but 53% less likely to have it at 48 months (RR, 0.47).
“By 48 months, significantly fewer CIN2+ cases were detected overall and across all age groups in the intervention group, compared with the control group,” the team said. At 48 months, the CIN2+ rate was 5 per 1,000 vs. 10.6 per 1,000 – a 53% reduced risk (RR, 0.47) and an absolute reduction of 5.6 cases per 1,000.
Again, the benefit accrued early and mostly in women who were negative by HPV or cytology at baseline. Among these, the CIN2+ risk for the intervention, compared with the control group, was 64% lower (RR, 0.36), and the absolute difference in incidence was 6.38 per 1,000.
This early detection came at a cost, however. Colposcopies were significantly more common in the intervention group in the first screening round (57 vs. 30.8). However, by 48 months, colposcopy rates were lower in the intervention group, compared with the control group (49.2 vs. 70.5). By the end of the study, cumulative colposcopy referral rates were similar (106.2 vs. 101.5).
Dr. Ogilvie and her colleagues suggested that this ultimate similarity in colposcopy rate shows that fears about overdiagnosis with HPV testing are unfounded.
“One of the concerns for adopting HPV-based screening is the lower CIN2+ specificity of HPV testing, compared with cytology, leading to higher screen positive rates and the resulting need for more colposcopies and biopsies. Unnecessary colposcopies potentially cause unintended harm for women and increased costs to health care systems. In this trial, round 1 colposcopy rates in the HPV-tested group were significantly higher than the cytology-tested group. However, by 48 months, the colposcopy rate in the intervention group was reduced while the control group rate increased.
“This increase is partly a result of HPV and cytology co-testing at trial end. Of the 513 control-group women referred for colposcopy at exit, 304 (59%) were cytology negative and HPV positive. In the HPV-tested group, the colposcopy rate decreased in the second round of screening, which more accurately reflects the ongoing impact of HPV-based screening on a colposcopy program. The baseline colposcopy referral rate reflects what happens when HPV-based screening is first implemented, when both prevalent and incident infections will be detected,” the investigators said.
The Canadian Institute of Health Research funded the study. Dr. Ogilvie was a coinvestigator on adjunct studies funded by Hologic and Roche, designed to compare the performance of different HPV assays. Funding for the adjunct studies was not applied to the main HPV FOCAL trial.
SOURCE: Ogilvie GS et al. JAMA. 2018;320:43-52.
Primary HPV testing has been available since 2014 in the United States, but has yet to replace Pap smears, L. Stewart Massad, MD, said in an accompanying editorial (JAMA. 2018;320:35-37).
The reasons are complex and numerous, beginning with the probability that the more sensitive HPV test can run up alarming, but unnecessary, red flags, especially for younger women.
“Adoption of primary HPV screening has been delayed by the suboptimal specificity of this approach, estimated at 85%-95%, especially among populations of young women who often carry HPV infections that regress without oncogenic consequence. These HPV infections represent true-positive HPV assays, but are false-positive cancer screens.”
Lack of patient education is another factor.
“HPV is almost universally acquired by sexually active adults. The virus usually clears in response to immune recognition, although clearance of this intraepithelial virus may take more than a year, and yet HPV may recur and first be detected decades later in the context of long-term monogamy or abstinence.
“Communicating a positive HPV test result requires sensitivity by the clinician and may entail lengthy counseling about the natural history of HPV, the lack of curative therapy, and the low absolute risk of progression to cancer. The clinical implications of an HPV diagnosis for sexual partners and offspring are marginal yet may be quite distressing for an affected woman.”
The HPV vaccine is already affecting cervical cancer rates and will complicate the picture even more. HPV FOCAL completed recruitment in 2012. Since then, rates of HPV 16 and 18, the most cervically carcinogenic serotypes, have fallen in the wake of the 2006 vaccine approval.
This reduction is becoming more apparent as women who were adolescents at vaccination and at the time the study was launched are now aging into screening cohorts. Lower prevalence of cervical precancer has changed the accuracy of screening tests in ways that are only now being appreciated, but that will further favor adoption of primary HPV screening by lowering its false-positive rate.”
The HPV test used in the FOCAL study was also suboptimal, compared with newer versions. The test incorporated all carcinogenic HPV serotypes in a single positive or negative result.
“More recent assays provide HPV genotyping that allows nuanced risk stratification, especially immediate referral to colposcopy for women who screen positive for HPV 16 or HPV 18. Triage for women who test positive for HPV was by cytology, which may not be the optimal triage test because other assays that do not depend on cytotechnologists’ vigilance are becoming available for this purpose. However, these advances should result in fewer false-positive results, further favoring HPV screening over cytology.”
The future of the Pap test remains unclear. Organizations that develop cancer screening guidelines continue to debate the issue.
“A draft recommendation on cervical screening from the U.S. Preventive Services Task Force recommended either cytology testing at 3-year intervals or primary HPV testing at 5-year intervals for women 30-65 years of age, but the final recommendation statement has not yet been released. Fortunately for women, both modalities are so effective for cancer screening that an adequately powered comparative effectiveness trial is likely impossible.”
Dr. Massad is a gynecologic oncology surgeon at Washington University, St. Louis. He has consulted with malpractice attorneys in cases alleging missed cervical cancer but has no financial ties with pharmaceutical companies.
Primary HPV testing has been available since 2014 in the United States, but has yet to replace Pap smears, L. Stewart Massad, MD, said in an accompanying editorial (JAMA. 2018;320:35-37).
The reasons are complex and numerous, beginning with the probability that the more sensitive HPV test can run up alarming, but unnecessary, red flags, especially for younger women.
“Adoption of primary HPV screening has been delayed by the suboptimal specificity of this approach, estimated at 85%-95%, especially among populations of young women who often carry HPV infections that regress without oncogenic consequence. These HPV infections represent true-positive HPV assays, but are false-positive cancer screens.”
Lack of patient education is another factor.
“HPV is almost universally acquired by sexually active adults. The virus usually clears in response to immune recognition, although clearance of this intraepithelial virus may take more than a year, and yet HPV may recur and first be detected decades later in the context of long-term monogamy or abstinence.
“Communicating a positive HPV test result requires sensitivity by the clinician and may entail lengthy counseling about the natural history of HPV, the lack of curative therapy, and the low absolute risk of progression to cancer. The clinical implications of an HPV diagnosis for sexual partners and offspring are marginal yet may be quite distressing for an affected woman.”
The HPV vaccine is already affecting cervical cancer rates and will complicate the picture even more. HPV FOCAL completed recruitment in 2012. Since then, rates of HPV 16 and 18, the most cervically carcinogenic serotypes, have fallen in the wake of the 2006 vaccine approval.
This reduction is becoming more apparent as women who were adolescents at vaccination and at the time the study was launched are now aging into screening cohorts. Lower prevalence of cervical precancer has changed the accuracy of screening tests in ways that are only now being appreciated, but that will further favor adoption of primary HPV screening by lowering its false-positive rate.”
The HPV test used in the FOCAL study was also suboptimal, compared with newer versions. The test incorporated all carcinogenic HPV serotypes in a single positive or negative result.
“More recent assays provide HPV genotyping that allows nuanced risk stratification, especially immediate referral to colposcopy for women who screen positive for HPV 16 or HPV 18. Triage for women who test positive for HPV was by cytology, which may not be the optimal triage test because other assays that do not depend on cytotechnologists’ vigilance are becoming available for this purpose. However, these advances should result in fewer false-positive results, further favoring HPV screening over cytology.”
The future of the Pap test remains unclear. Organizations that develop cancer screening guidelines continue to debate the issue.
“A draft recommendation on cervical screening from the U.S. Preventive Services Task Force recommended either cytology testing at 3-year intervals or primary HPV testing at 5-year intervals for women 30-65 years of age, but the final recommendation statement has not yet been released. Fortunately for women, both modalities are so effective for cancer screening that an adequately powered comparative effectiveness trial is likely impossible.”
Dr. Massad is a gynecologic oncology surgeon at Washington University, St. Louis. He has consulted with malpractice attorneys in cases alleging missed cervical cancer but has no financial ties with pharmaceutical companies.
Primary HPV testing has been available since 2014 in the United States, but has yet to replace Pap smears, L. Stewart Massad, MD, said in an accompanying editorial (JAMA. 2018;320:35-37).
The reasons are complex and numerous, beginning with the probability that the more sensitive HPV test can run up alarming, but unnecessary, red flags, especially for younger women.
“Adoption of primary HPV screening has been delayed by the suboptimal specificity of this approach, estimated at 85%-95%, especially among populations of young women who often carry HPV infections that regress without oncogenic consequence. These HPV infections represent true-positive HPV assays, but are false-positive cancer screens.”
Lack of patient education is another factor.
“HPV is almost universally acquired by sexually active adults. The virus usually clears in response to immune recognition, although clearance of this intraepithelial virus may take more than a year, and yet HPV may recur and first be detected decades later in the context of long-term monogamy or abstinence.
“Communicating a positive HPV test result requires sensitivity by the clinician and may entail lengthy counseling about the natural history of HPV, the lack of curative therapy, and the low absolute risk of progression to cancer. The clinical implications of an HPV diagnosis for sexual partners and offspring are marginal yet may be quite distressing for an affected woman.”
The HPV vaccine is already affecting cervical cancer rates and will complicate the picture even more. HPV FOCAL completed recruitment in 2012. Since then, rates of HPV 16 and 18, the most cervically carcinogenic serotypes, have fallen in the wake of the 2006 vaccine approval.
This reduction is becoming more apparent as women who were adolescents at vaccination and at the time the study was launched are now aging into screening cohorts. Lower prevalence of cervical precancer has changed the accuracy of screening tests in ways that are only now being appreciated, but that will further favor adoption of primary HPV screening by lowering its false-positive rate.”
The HPV test used in the FOCAL study was also suboptimal, compared with newer versions. The test incorporated all carcinogenic HPV serotypes in a single positive or negative result.
“More recent assays provide HPV genotyping that allows nuanced risk stratification, especially immediate referral to colposcopy for women who screen positive for HPV 16 or HPV 18. Triage for women who test positive for HPV was by cytology, which may not be the optimal triage test because other assays that do not depend on cytotechnologists’ vigilance are becoming available for this purpose. However, these advances should result in fewer false-positive results, further favoring HPV screening over cytology.”
The future of the Pap test remains unclear. Organizations that develop cancer screening guidelines continue to debate the issue.
“A draft recommendation on cervical screening from the U.S. Preventive Services Task Force recommended either cytology testing at 3-year intervals or primary HPV testing at 5-year intervals for women 30-65 years of age, but the final recommendation statement has not yet been released. Fortunately for women, both modalities are so effective for cancer screening that an adequately powered comparative effectiveness trial is likely impossible.”
Dr. Massad is a gynecologic oncology surgeon at Washington University, St. Louis. He has consulted with malpractice attorneys in cases alleging missed cervical cancer but has no financial ties with pharmaceutical companies.
Women who received only a primary human papillomavirus test were 58% less likely to develop grade 3 or worse cervical intraepithelial neoplasia (CIN3+) by 48 months than women who had the traditional Pap cytology screen.
The primary HPV test also reduced the 2-year risk of CIN2+ neoplasia, compared with Pap smear alone, Gina Suzanne Ogilvie, MD, and her colleagues reported in JAMA.
“These results have demonstrated that primary HPV testing detects cervical neoplasia earlier and more accurately than cytology,” wrote Dr. Ogilvie of the University of British Columbia, Vancouver, and her colleagues.
HPV FOCAL (the Human Papilloma Virus For Cervical Cancers Screening trial) enrolled 19,009 Canadian women aged 25-65 years and randomized them to two cervical cancer screening paradigms: Pap liquid-based cytology (LBC) or primary HPV testing.
The intervention group (9,552) had cervical cancer screening with a high-risk HPV DNA test that detects types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. If either test was positive, they were referred for colposcopy. If both tests were negative, they returned for their final screen with both tests at 48 months.
The control group underwent primary LBC testing, followed by HPV testing for women with atypical squamous cells of unknown significance (ASCUS). If these tests were both positive, they were referred for colposcopy. Women who were positive for ASCUS and HPV negative returned in 12 months and were referred for colposcopy if they had ASCUS or any higher-grade abnormality. At 48 months, they also returned and underwent both screening tests.
The primary outcome was the rate of CIN3+ at 48 months. Secondary endpoints included the 48-month rate of CIN2+, the threshold for colposcopy referral, and the effect of primary HPV testing on colposcopy.
In the first round of screening, HPV testing detected significantly more cases of CIN3+ than did LBC (risk ratio, 1.61). This was an absolute difference of 2.67 more cases per 1,000 screened women.
At 48 months, the rate of CIN3+ was significantly lower in the intervention group than in the control group (2.3 vs. 5.5 per 1,000; RR, 0.42). This represents an absolute difference of 3.2 fewer cases per 1,000, the investigators said.
Overall, however, the two methods detected about the same number of cases by 48 months, the investigators said.
“Cumulative CIN3+ incidence curves show no significantly different disease detection across trial groups in the intervention group. The cumulative incidence was higher earlier in the trial at 18 months and 42 months, compared with the control group. ... By the end of trial follow-up (72 months), incidence was similar across both groups.”
Women who were HPV negative at baseline reaped the biggest benefit. The 48-month HPV incidence rate among them was 1.4 per 1,000, compared with 5.4 per 1,000 in the control group. This 75% risk reduction (RR, 0.25) represents an absolute reduction of 4 cases per 1,000 women.
The intervention group was 61% more likely to have a CIN2+ result by 12 months (RR, 1.61), but 53% less likely to have it at 48 months (RR, 0.47).
“By 48 months, significantly fewer CIN2+ cases were detected overall and across all age groups in the intervention group, compared with the control group,” the team said. At 48 months, the CIN2+ rate was 5 per 1,000 vs. 10.6 per 1,000 – a 53% reduced risk (RR, 0.47) and an absolute reduction of 5.6 cases per 1,000.
Again, the benefit accrued early and mostly in women who were negative by HPV or cytology at baseline. Among these, the CIN2+ risk for the intervention, compared with the control group, was 64% lower (RR, 0.36), and the absolute difference in incidence was 6.38 per 1,000.
This early detection came at a cost, however. Colposcopies were significantly more common in the intervention group in the first screening round (57 vs. 30.8). However, by 48 months, colposcopy rates were lower in the intervention group, compared with the control group (49.2 vs. 70.5). By the end of the study, cumulative colposcopy referral rates were similar (106.2 vs. 101.5).
Dr. Ogilvie and her colleagues suggested that this ultimate similarity in colposcopy rate shows that fears about overdiagnosis with HPV testing are unfounded.
“One of the concerns for adopting HPV-based screening is the lower CIN2+ specificity of HPV testing, compared with cytology, leading to higher screen positive rates and the resulting need for more colposcopies and biopsies. Unnecessary colposcopies potentially cause unintended harm for women and increased costs to health care systems. In this trial, round 1 colposcopy rates in the HPV-tested group were significantly higher than the cytology-tested group. However, by 48 months, the colposcopy rate in the intervention group was reduced while the control group rate increased.
“This increase is partly a result of HPV and cytology co-testing at trial end. Of the 513 control-group women referred for colposcopy at exit, 304 (59%) were cytology negative and HPV positive. In the HPV-tested group, the colposcopy rate decreased in the second round of screening, which more accurately reflects the ongoing impact of HPV-based screening on a colposcopy program. The baseline colposcopy referral rate reflects what happens when HPV-based screening is first implemented, when both prevalent and incident infections will be detected,” the investigators said.
The Canadian Institute of Health Research funded the study. Dr. Ogilvie was a coinvestigator on adjunct studies funded by Hologic and Roche, designed to compare the performance of different HPV assays. Funding for the adjunct studies was not applied to the main HPV FOCAL trial.
SOURCE: Ogilvie GS et al. JAMA. 2018;320:43-52.
Women who received only a primary human papillomavirus test were 58% less likely to develop grade 3 or worse cervical intraepithelial neoplasia (CIN3+) by 48 months than women who had the traditional Pap cytology screen.
The primary HPV test also reduced the 2-year risk of CIN2+ neoplasia, compared with Pap smear alone, Gina Suzanne Ogilvie, MD, and her colleagues reported in JAMA.
“These results have demonstrated that primary HPV testing detects cervical neoplasia earlier and more accurately than cytology,” wrote Dr. Ogilvie of the University of British Columbia, Vancouver, and her colleagues.
HPV FOCAL (the Human Papilloma Virus For Cervical Cancers Screening trial) enrolled 19,009 Canadian women aged 25-65 years and randomized them to two cervical cancer screening paradigms: Pap liquid-based cytology (LBC) or primary HPV testing.
The intervention group (9,552) had cervical cancer screening with a high-risk HPV DNA test that detects types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. If either test was positive, they were referred for colposcopy. If both tests were negative, they returned for their final screen with both tests at 48 months.
The control group underwent primary LBC testing, followed by HPV testing for women with atypical squamous cells of unknown significance (ASCUS). If these tests were both positive, they were referred for colposcopy. Women who were positive for ASCUS and HPV negative returned in 12 months and were referred for colposcopy if they had ASCUS or any higher-grade abnormality. At 48 months, they also returned and underwent both screening tests.
The primary outcome was the rate of CIN3+ at 48 months. Secondary endpoints included the 48-month rate of CIN2+, the threshold for colposcopy referral, and the effect of primary HPV testing on colposcopy.
In the first round of screening, HPV testing detected significantly more cases of CIN3+ than did LBC (risk ratio, 1.61). This was an absolute difference of 2.67 more cases per 1,000 screened women.
At 48 months, the rate of CIN3+ was significantly lower in the intervention group than in the control group (2.3 vs. 5.5 per 1,000; RR, 0.42). This represents an absolute difference of 3.2 fewer cases per 1,000, the investigators said.
Overall, however, the two methods detected about the same number of cases by 48 months, the investigators said.
“Cumulative CIN3+ incidence curves show no significantly different disease detection across trial groups in the intervention group. The cumulative incidence was higher earlier in the trial at 18 months and 42 months, compared with the control group. ... By the end of trial follow-up (72 months), incidence was similar across both groups.”
Women who were HPV negative at baseline reaped the biggest benefit. The 48-month HPV incidence rate among them was 1.4 per 1,000, compared with 5.4 per 1,000 in the control group. This 75% risk reduction (RR, 0.25) represents an absolute reduction of 4 cases per 1,000 women.
The intervention group was 61% more likely to have a CIN2+ result by 12 months (RR, 1.61), but 53% less likely to have it at 48 months (RR, 0.47).
“By 48 months, significantly fewer CIN2+ cases were detected overall and across all age groups in the intervention group, compared with the control group,” the team said. At 48 months, the CIN2+ rate was 5 per 1,000 vs. 10.6 per 1,000 – a 53% reduced risk (RR, 0.47) and an absolute reduction of 5.6 cases per 1,000.
Again, the benefit accrued early and mostly in women who were negative by HPV or cytology at baseline. Among these, the CIN2+ risk for the intervention, compared with the control group, was 64% lower (RR, 0.36), and the absolute difference in incidence was 6.38 per 1,000.
This early detection came at a cost, however. Colposcopies were significantly more common in the intervention group in the first screening round (57 vs. 30.8). However, by 48 months, colposcopy rates were lower in the intervention group, compared with the control group (49.2 vs. 70.5). By the end of the study, cumulative colposcopy referral rates were similar (106.2 vs. 101.5).
Dr. Ogilvie and her colleagues suggested that this ultimate similarity in colposcopy rate shows that fears about overdiagnosis with HPV testing are unfounded.
“One of the concerns for adopting HPV-based screening is the lower CIN2+ specificity of HPV testing, compared with cytology, leading to higher screen positive rates and the resulting need for more colposcopies and biopsies. Unnecessary colposcopies potentially cause unintended harm for women and increased costs to health care systems. In this trial, round 1 colposcopy rates in the HPV-tested group were significantly higher than the cytology-tested group. However, by 48 months, the colposcopy rate in the intervention group was reduced while the control group rate increased.
“This increase is partly a result of HPV and cytology co-testing at trial end. Of the 513 control-group women referred for colposcopy at exit, 304 (59%) were cytology negative and HPV positive. In the HPV-tested group, the colposcopy rate decreased in the second round of screening, which more accurately reflects the ongoing impact of HPV-based screening on a colposcopy program. The baseline colposcopy referral rate reflects what happens when HPV-based screening is first implemented, when both prevalent and incident infections will be detected,” the investigators said.
The Canadian Institute of Health Research funded the study. Dr. Ogilvie was a coinvestigator on adjunct studies funded by Hologic and Roche, designed to compare the performance of different HPV assays. Funding for the adjunct studies was not applied to the main HPV FOCAL trial.
SOURCE: Ogilvie GS et al. JAMA. 2018;320:43-52.
FROM JAMA
Key clinical point: Compared with a Pap smear, HPV testing detected CIN3+ more often and earlier.
Major finding: Women who had HPV testing were 58% less likely to be CIN3+ 48 months later.
Study details: The prospective randomized trial comprised 19,009 women.
Disclosures: The Canadian Institute of Health Research funded the study, Dr. Ogilvie was a coinvestigator on adjunct studies funded by Hologic and Roche, designed to compare the performance of different HPV assays. Funding for the adjunct studies was not applied to the main HPV FOCAL trial.
Source: Ogilvie GS et al. JAMA. 2018;320:43-52.
Hands-on surgical training is incomparable
Hands-on surgical training is incomparable
I am not one to critique new technology or new technique. The article on use of virtual reality to not only teach technique but also to grade it caught my attention. I work in a small hospital without a million-dollar robot. Very complicated cases are sent out to larger hospitals. We have 2 new graduates who, like most new grads, have little experience with many surgical techniques. Dr. Lenihan and I were resident classmates, so I know he understands the rigors of a no-hour limit residency. Even with our residency, when we got out we relied on our partners to assist us until they knew we could do cases with a surgical assistant (SA) or a less experienced helper.
We are asking too much of our new graduates. It is up to us to provide the help and assistance with surgeries that they are not comfortable doing. While virtual reality training is great for teaching robotics and some laparoscopic techniques, it cannot teach things such as anterior and posterior repairs, tension-free vaginal tape procedures, and enterocoele repair. We can all watch YouTube tutorials, but actually doing surgery is very different. We owe it to our new graduates to provide mentoring and encouragement with their surgical cases. At our hospital, mentoring the first 10 cases performed by a new physician (new grad or otherwise) used to be required, but that requirement is gone. Our service is one of the few that still has 2 physicians at every major case. We have an SA available, but we prefer to assist each other. This makes our laparoscopic-assisted vaginal hysterectomy, bilateral salpingo-oophorectomy cases a 30- to 35-minute case. It allows us to teach anterior and posterior repair technique.
The involvement in surgical improvement is hands-on, and virtual reality training will never replace it.
Anthony J. Lemanski, MD
Kingman, Arizona
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Hands-on surgical training is incomparable
I am not one to critique new technology or new technique. The article on use of virtual reality to not only teach technique but also to grade it caught my attention. I work in a small hospital without a million-dollar robot. Very complicated cases are sent out to larger hospitals. We have 2 new graduates who, like most new grads, have little experience with many surgical techniques. Dr. Lenihan and I were resident classmates, so I know he understands the rigors of a no-hour limit residency. Even with our residency, when we got out we relied on our partners to assist us until they knew we could do cases with a surgical assistant (SA) or a less experienced helper.
We are asking too much of our new graduates. It is up to us to provide the help and assistance with surgeries that they are not comfortable doing. While virtual reality training is great for teaching robotics and some laparoscopic techniques, it cannot teach things such as anterior and posterior repairs, tension-free vaginal tape procedures, and enterocoele repair. We can all watch YouTube tutorials, but actually doing surgery is very different. We owe it to our new graduates to provide mentoring and encouragement with their surgical cases. At our hospital, mentoring the first 10 cases performed by a new physician (new grad or otherwise) used to be required, but that requirement is gone. Our service is one of the few that still has 2 physicians at every major case. We have an SA available, but we prefer to assist each other. This makes our laparoscopic-assisted vaginal hysterectomy, bilateral salpingo-oophorectomy cases a 30- to 35-minute case. It allows us to teach anterior and posterior repair technique.
The involvement in surgical improvement is hands-on, and virtual reality training will never replace it.
Anthony J. Lemanski, MD
Kingman, Arizona
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Hands-on surgical training is incomparable
I am not one to critique new technology or new technique. The article on use of virtual reality to not only teach technique but also to grade it caught my attention. I work in a small hospital without a million-dollar robot. Very complicated cases are sent out to larger hospitals. We have 2 new graduates who, like most new grads, have little experience with many surgical techniques. Dr. Lenihan and I were resident classmates, so I know he understands the rigors of a no-hour limit residency. Even with our residency, when we got out we relied on our partners to assist us until they knew we could do cases with a surgical assistant (SA) or a less experienced helper.
We are asking too much of our new graduates. It is up to us to provide the help and assistance with surgeries that they are not comfortable doing. While virtual reality training is great for teaching robotics and some laparoscopic techniques, it cannot teach things such as anterior and posterior repairs, tension-free vaginal tape procedures, and enterocoele repair. We can all watch YouTube tutorials, but actually doing surgery is very different. We owe it to our new graduates to provide mentoring and encouragement with their surgical cases. At our hospital, mentoring the first 10 cases performed by a new physician (new grad or otherwise) used to be required, but that requirement is gone. Our service is one of the few that still has 2 physicians at every major case. We have an SA available, but we prefer to assist each other. This makes our laparoscopic-assisted vaginal hysterectomy, bilateral salpingo-oophorectomy cases a 30- to 35-minute case. It allows us to teach anterior and posterior repair technique.
The involvement in surgical improvement is hands-on, and virtual reality training will never replace it.
Anthony J. Lemanski, MD
Kingman, Arizona
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Hypertensive crisis of pregnancy must be treated with all urgency
Hypertensive crisis of pregnancy must be treated with all urgency
The following happened approximately 27 years ago when I worked as an attending at a regional level 2 hospital in Puerto Rico. One afternoon I received a call from the emergency department that they had been managing a patient (G4P3) at 33 weeks of gestation for about 4 hours. The patient was consulted for hypertension when she went into a hypertensive encephalopathic coma. The patient was brought back to the birth center. Apresoline was given but did not bring the blood pressure down. Magnesium sulfate also was started at that time. I called a colleague from internal medicine and started to give nitroprusside.
Every time the patient’s blood pressure dropped from 120 mm Hg diastolic, she would become conscious and speak with us. As soon as her blood pressure went up, she would go into a coma. The patient was then transferred to a tertiary center in as stable a condition as possible. Cesarean delivery was performed, and the baby did not survive. The mother had an intracerebral hemorrhage. She was transferred to the supra-tertiary center in San Juan where she later passed away from complications of the hypertensive crisis. If the emergency physician had called me earlier, more could have been done.
This event is always fresh I my mind when I manage my patients in Ohio. Thank God for the newer medications we have available and the protocols to manage hypertensive crisis in pregnancy. I hope this experience heightens awareness of how deadly this condition can be.
David A. Rosado, MD
Celina, Ohio
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Hypertensive crisis of pregnancy must be treated with all urgency
The following happened approximately 27 years ago when I worked as an attending at a regional level 2 hospital in Puerto Rico. One afternoon I received a call from the emergency department that they had been managing a patient (G4P3) at 33 weeks of gestation for about 4 hours. The patient was consulted for hypertension when she went into a hypertensive encephalopathic coma. The patient was brought back to the birth center. Apresoline was given but did not bring the blood pressure down. Magnesium sulfate also was started at that time. I called a colleague from internal medicine and started to give nitroprusside.
Every time the patient’s blood pressure dropped from 120 mm Hg diastolic, she would become conscious and speak with us. As soon as her blood pressure went up, she would go into a coma. The patient was then transferred to a tertiary center in as stable a condition as possible. Cesarean delivery was performed, and the baby did not survive. The mother had an intracerebral hemorrhage. She was transferred to the supra-tertiary center in San Juan where she later passed away from complications of the hypertensive crisis. If the emergency physician had called me earlier, more could have been done.
This event is always fresh I my mind when I manage my patients in Ohio. Thank God for the newer medications we have available and the protocols to manage hypertensive crisis in pregnancy. I hope this experience heightens awareness of how deadly this condition can be.
David A. Rosado, MD
Celina, Ohio
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Hypertensive crisis of pregnancy must be treated with all urgency
The following happened approximately 27 years ago when I worked as an attending at a regional level 2 hospital in Puerto Rico. One afternoon I received a call from the emergency department that they had been managing a patient (G4P3) at 33 weeks of gestation for about 4 hours. The patient was consulted for hypertension when she went into a hypertensive encephalopathic coma. The patient was brought back to the birth center. Apresoline was given but did not bring the blood pressure down. Magnesium sulfate also was started at that time. I called a colleague from internal medicine and started to give nitroprusside.
Every time the patient’s blood pressure dropped from 120 mm Hg diastolic, she would become conscious and speak with us. As soon as her blood pressure went up, she would go into a coma. The patient was then transferred to a tertiary center in as stable a condition as possible. Cesarean delivery was performed, and the baby did not survive. The mother had an intracerebral hemorrhage. She was transferred to the supra-tertiary center in San Juan where she later passed away from complications of the hypertensive crisis. If the emergency physician had called me earlier, more could have been done.
This event is always fresh I my mind when I manage my patients in Ohio. Thank God for the newer medications we have available and the protocols to manage hypertensive crisis in pregnancy. I hope this experience heightens awareness of how deadly this condition can be.
David A. Rosado, MD
Celina, Ohio
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
2018 Update on infectious disease
In this Update I highlight 5 interesting investigations on infectious diseases. The first addresses the value of applying prophylactically a negative-pressure wound dressing to prevent surgical site infection (SSI) in obese women having cesarean delivery (CD). The second report assesses the effectiveness of a preoperative vaginal wash in reducing the frequency of postcesarean endometritis. The third investigation examines the role of systemic antibiotics, combined with surgical drainage, for patients who have subcutaneous abscesses ranging in size up to 5 cm. The fourth study presents new information about the major risk factors for Clostridium difficile infections in obstetric patients. The final study presents valuable sobering new data about the risks of congenital Zika virus infection.
Negative-pressure wound therapy after CD shows some benefit in preventing SSI
Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200-210.e1.
Yu and colleagues sought to determine if the prophylactic use of negative-pressure devices, compared with standard wound dressing, was effective in reducing the frequency of SSI after CD.
The authors searched multiple databases and initially identified 161 randomized controlled trials and cohort studies for further assessment. After applying rigorous exclusion criteria, they ultimately selected 9 studies for systematic review and meta-analysis. Six studies were randomized controlled trials (RCTs), 2 were retrospective cohort studies, and 1 was a prospective cohort study. Five studies were considered high quality; 4 were of low quality.
Details of the study
Several types of negative-pressure devices were used, but the 2 most common were the Prevena incision management system (KCI, San Antonio, Texas) and PICO negative- pressure wound therapy (Smith & Nephew, St. Petersburg, Florida). The majority of patients in all groups were at high risk for wound complications because of obesity.
The primary outcome of interest was the frequency of SSI. Secondary outcomes included dehiscence, seroma, endometritis, a composite measure for all wound complications, and hospital readmission.
The absolute risk of SSI in the intervention group was 5% (95% confidence interval [CI], 2.0%-7.0%) compared with 11% (95% CI, 7.0%-16.0%) in the standard dressing group. The pooled risk ratio was 0.45 (95% CI, 0.31-0.66). The absolute risk reduction was 6% (95% CI, -10.0% to -3.0%), and the number needed to treat was 17.
There were no significant differences in the rate of any of the secondary outcomes other than the composite of all wound complications. This difference was largely accounted for by the difference in the rate of SSI.
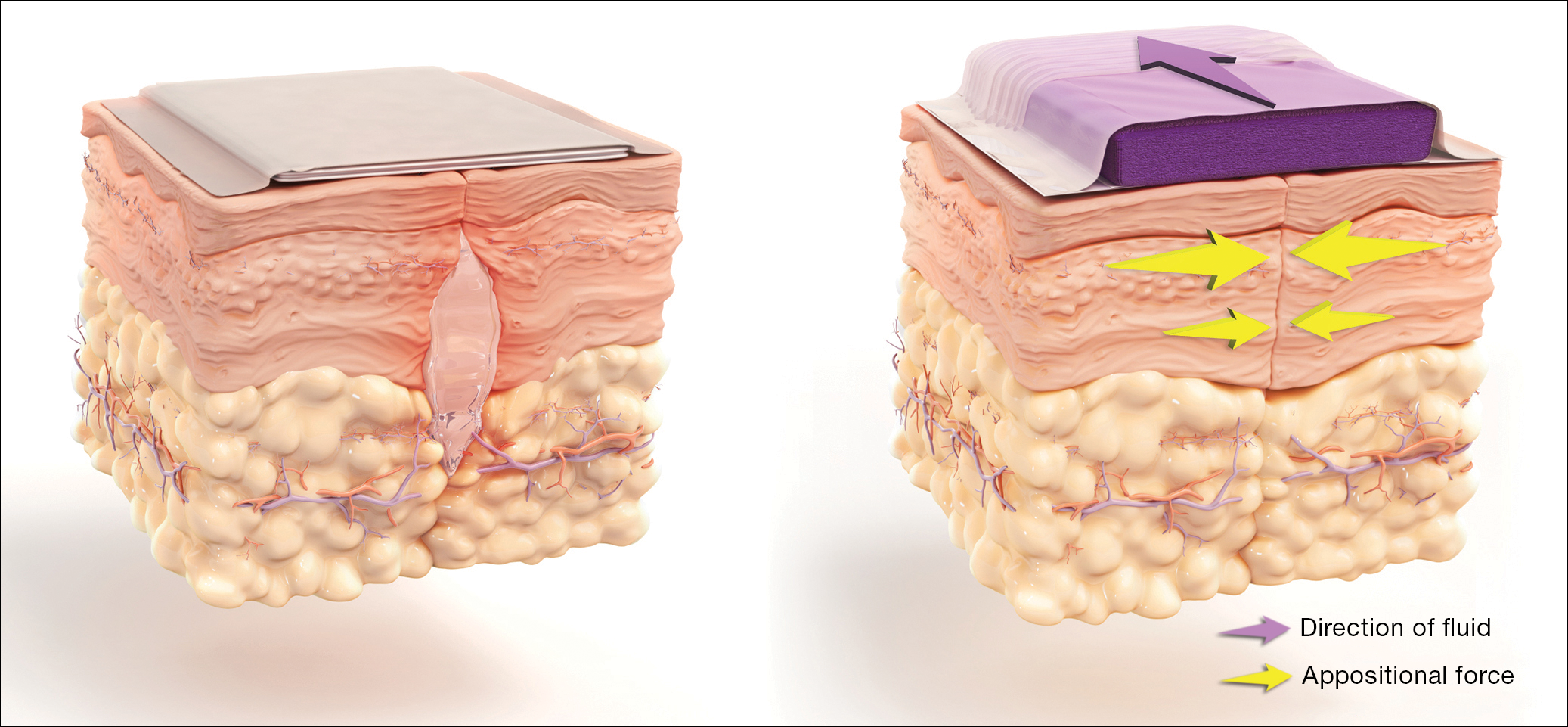
How negative-pressure devices aid wound healing
Yu and colleagues explained that negative-pressure devices exert their beneficial effects in various ways, including:
- shrinking the wound
- inducing cellular stretch
- removing extracellular fluids
- creating a favorable environment for healing
- promoting angiogenesis and neurogenesis.
Multiple studies in nonobstetric patients have shown that prophylactic use of negative-pressure devices is beneficial in reducing the rate of SSI.1 Yu and colleagues' systematic review and meta-analysis confirms those findings in a high-risk population of women having CD.
Study limitations
Before routinely adopting the use of negative-pressure devices for all women having CD, however, obstetricians should consider the following caveats:
- The investigations included in the study by Yu and colleagues did not consistently distinguish between scheduled versus unscheduled CDs.
- The reports did not systematically consider other major risk factors for wound complications besides obesity, and they did not control for these confounders in the statistical analyses.
- The studies included in the meta-analysis did not provide full descriptions of other measures that might influence the rate of SSIs, such as timing and selection of prophylactic antibiotics, selection of suture material, preoperative skin preparation, and closure techniques for the deep subcutaneous tissue and skin.
- None of the included studies systematically considered the cost-effectiveness of the negative-pressure devices. This is an important consideration given that the acquisition cost of these devices ranges from $200 to $500.
Results of the systematic review and meta-analysis by Yu and colleagues suggest that prophylactic negative-pressure wound therapy in high-risk mostly obese women after CD reduces SSI and overall wound complications. The study's limitations, however, must be kept in mind, and more data are needed. It would be most helpful if a large, well-designed RCT was conducted and included 2 groups with comparable multiple major risk factors for wound complications, and in which all women received the following important interventions2-4:
- removal of hair in the surgical site with a clipper, not a razor
- cleansing of the skin with a chlorhexidine rather than an iodophor solution
- closure of the deep subcutaneous tissue if the total subcutaneous layer exceeds 2 cm in depth
- closure of the skin with suture rather than staples
- administration of antibiotic prophylaxis, ideally with a combination of cefazolin plus azithromycin, prior to the surgical incision.
Read about vaginal cleansing’s effect on post-CD endometritis
Vaginal cleansing before CD lowers risk of postop endometritis
Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(3):527-538.
Caissutti and colleagues aimed to determine if cleansing the vagina with an antiseptic solution prior to surgery reduced the frequency of postcesarean endometritis. They included 16 RCTs (4,837 patients) in their systematic review and meta-analysis. The primary outcome was the frequency of postoperative endometritis.
Details of the study
The studies were conducted in several countries and included patients of various socioeconomic classes. Six trials included only patients having a scheduled CD; 9 included both scheduled and unscheduled cesareans; and 1 included only unscheduled cesareans. In 11 studies, povidone-iodine was the antiseptic solution used. Two trials used chlorhexidine diacetate 0.2%, and 1 used chlorhexidine diacetate 0.4%. One trial used metronidazole 0.5% gel, and another used the antiseptic cetrimide, which is a mixture of different quaternary ammonium salts, including cetrimonium bromide.
In all trials, patients received prophylactic antibiotics. The antibiotics were administered prior to the surgical incision in 6 trials; they were given after the umbilical cord was clamped in 6 trials. In 2 trials, the antibiotics were given at varying times, and in the final 2 trials, the timing of antibiotic administration was not reported. Of note, no trials described the method of placenta removal, a factor of considerable significance in influencing the rate of postoperative endometritis.5,6
Endometritis frequency reduced with vaginal cleansing; benefit greater in certain groups. Overall, in the 15 trials in which vaginal cleansing was compared with placebo or with no treatment, women in the treatment group had a significantly lower rate of endometritis (4.5% compared with 8.8%; relative risk [RR], 0.52; 95% CI, 0.37-0.72). When only women in labor were considered, the frequency of endometritis was 8.1% in the intervention group compared with 13.8% in the control group (RR, 0.52; 95% CI, 0.28-0.97). In the women who were not in labor, the difference in the incidence of endometritis was not statistically significant (3.5% vs 6.6%; RR, 0.62; 95% CI, 0.34-1.15).
In the subgroup analysis of women with ruptured membranes at the time of surgery, the incidence of endometritis was 4.3% in the treatment group compared with 20.1% in the control group (RR, 0.23; 95% CI, 0.10-0.52). In women with intact membranes at the time of surgery, the incidence of endometritis was not significantly reduced in the treatment group.
Interestingly, in the subgroup analysis of the 10 trials that used povidone-iodine, the reduction in the frequency of postcesarean endometritis was statistically significant (2.8% vs 6.3%; RR, 0.42; 95% CI, 0.25-0.71). However, this same protective effect was not observed in the women treated with chlorhexidine. In the 1 trial that directly compared povidone-iodine with chlorhexidine, there was no statistically significant difference in outcome.
Simple intervention, solid benefit
Endometritis is the most common complication following CD. The infection is polymicrobial, with mixed aerobic and anaerobic organisms. The principal risk factors for postcesarean endometritis are low socioeconomic status, extended duration of labor and ruptured membranes, multiple vaginal examinations, internal fetal monitoring, and pre-existing vaginal infections (principally, bacterial vaginosis and group B streptococcal colonization).
Two interventions are clearly of value in reducing the incidence of endometritis: administration of prophylactic antibiotics prior to the surgical incision and removal of the placenta by traction on the cord as opposed to manual extraction.5,6
The assessment by Caissutti and colleagues confirms that a third measure — preoperative vaginal cleansing — also helps reduce the incidence of postcesarean endometritis. The principal benefit is seen in women who have been in labor with ruptured membranes, although certainly it is not harmful in lower-risk patients. The intervention is simple and straightforward: a 30-second vaginal wash with a povidone-iodine solution just prior to surgery.
From my perspective, the interesting unanswered question is why a chlorhexidine solution with low alcohol content was not more effective than povidone-iodine, given that a chlorhexidine abdominal wash is superior to povidone-iodine in preventing wound infection after cesarean delivery.7 Until additional studies confirm the effectiveness of vaginal cleansing with chlorhexidine, I recommend the routine use of the povidone-iodine solution in all women having CD.
Read about management approaches for skin abscesses
Treat smaller skin abscesses with antibiotics after surgical drainage? Yes.
Daum RS, Miller LG, Immergluck L, et al; for the DMID 07-0051 Team. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017;376(26):2545-2555.
For treatment of subcutaneous abscesses that were 5 cm or smaller in diameter, investigators sought to determine if surgical drainage alone was equivalent to surgical drainage plus systemic antibiotics. After their abscess was drained, patients were randomly assigned to receive either clindamycin (300 mg 3 times daily) or trimethoprim-sulfamethoxazole (80 mg/400 mg twice daily) or placebo for 10 days. The primary outcome was clinical cure 7 to 10 days after treatment.
Details of the study
Daum and colleagues enrolled 786 participants (505 adults, 281 children) in the prospective double-blind study. Staphylococcus aureus was isolated from 527 patients (67.0%); methicillin-resistant S aureus (MRSA) was isolated from 388 (49.4%). The cure rate was similar in patients in the clindamycin group (83.1%) and the trimethoprim-sulfamethoxazole group (81.7%), and the cure rate in each antibiotic group was significantly higher than that in the placebo group (68.9%; P<.001 for both comparisons). The difference in treatment effect was specifically limited to patients who had S aureus isolated from their lesions.
Findings at follow-up. At 1 month of follow-up, new infections were less common in the clindamycin group (6.8%) than in the trimethoprim-sulfamethoxazole group (13.5%; P = .03) or the placebo group (12.4%; P = .06). However, the highest frequency of adverse effects occurred in the patients who received clindamycin (21.9% vs 11.1% vs 12.5%). No adverse effects were judged to be serious, and all resolved without sequela.
Controversy remains on antibiotic use after drainage
This study is important for 2 major reasons. First, soft tissue infections are quite commonand can evolve into serious problems, especially when the offending pathogen is MRSA. Second, controversy exists about whether systemic antibiotics are indicated if the subcutaneous abscess is relatively small and is adequately drained. For example, Talan and colleagues demonstrated that, in settings with a high prevalence of MRSA, surgical drainage combined with trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily) was superior to drainage plus placebo.8 However, Daum and Gold recently debated the issue of drainage plus antibiotics in a case vignette and reached opposite conclusions.9
In my opinion, this investigation by Daum and colleagues supports a role for consistent use of systemic antibiotics following surgical drainage of clinically significant subcutaneous abscesses that have a 5 cm or smaller diameter. Several oral antibiotics are effective against S aureus, including MRSA.10 These drugs include trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily), clindamycin (300-450 mg 3 times daily), doxycycline (100 mg twice daily), and minocycline (200 mg initially, then 100 mg every 12 hours).
Of these drugs, I prefer trimethoprim-sulfamethoxazole, provided that the patient does not have an allergy to sulfonamides. Trimethoprim-sulfamethoxazole is significantly less expensive than the other 3 drugs and usually is better tolerated. In particular, compared with clindamycin, trimethoprim-sulfamethoxazole is less likely to cause antibiotic-associated diarrhea, including Clostridium difficile infection. Trimethoprim-sulfamethoxazole should not be used in the first trimester of pregnancy because of concerns about fetal teratogenicity.
Read how to avoid C difficile infections in pregnant patients
Antibiotic use, common in the obstetric population, raises risk for C difficile infection
Ruiter-Ligeti J, Vincent S, Czuzoj-Shulman N, Abenhaim HA. Risk factors, incidence, and morbidity associated with obstetric Clostridium difficile infection. Obstet Gynecol. 2018;131(2):387-391.
The objective of this investigation was to identify risk factors for Clostridium difficile infection (previously termed pseudomembranous enterocolitis) in obstetric patients. The authors performed a retrospective cohort study using information from a large database maintained by the Agency for Healthcare Research and Quality. This database provides information about inpatient hospital stays in the United States, and it is the largest repository of its kind. It includes data from a sample of 1,000 US hospitals.
Details of the study
Ruiter-Ligeti and colleagues reviewed 13,881,592 births during 1999-2013 and identified 2,757 (0.02%) admissions for delivery complicated by C difficile infection, a rate of 20 admissions per 100,000 deliveries per year (95% CI, 19.13-20.62). The rate of admissions with this diagnosis doubled from 1999 (15 per 100,000) to 2013 (30 per 100,000, P<.001).
Among these obstetric patients, the principal risk factors for C difficile infection were older age, multiple gestation, long-term antibiotic use (not precisely defined), and concurrent diagnosis of inflammatory bowel disease. In addition, patients with pyelonephritis, perineal or cesarean wound infections, or pneumonia also were at increased risk, presumably because those patients required longer courses of broad-spectrum antibiotics.
Of additional note, when compared with women who did not have C difficile infection, patients with infection were more likely to develop a thromboembolic event (38.4 per 1,000), paralytic ileus (58.0 per 1,000), sepsis (46.4 per 1,000), and death (8.0 per 1,000).
Be on guard for C difficile infection in antibiotic-treated obstetric patients
C difficile infection is an uncommon but potentially very serious complication of antibiotic therapy. Given that approximately half of all women admitted for delivery are exposed to antibiotics because of prophylaxis for group B streptococcus infection, prophylaxis for CD, and treatment of chorioamnionitis and puerperal endometritis, clinicians constantly need to be vigilant for this complication.11
Affected patients typically present with frequent loose, watery stools and lower abdominal cramping. In severe cases, blood may be present in the stool, and signs of intestinal distention and even acute peritonitis may be evident. The diagnosis can be established by documenting a positive culture or polymerase chain reaction (PCR) assay for C difficile and a positive cytotoxin assay for toxins A and/or B. In addition, if endoscopy is performed, the characteristic gray membranous plaques can be visualized on the rectal and colonic mucosa.11
Discontinue antibiotic therapy. The first step in managing affected patients is to stop all antibiotics, if possible, or at least the one most likely to be the causative agent of C difficile infection. Patients with relatively mild clinical findings should be treated with oral metronidazole, 500 mg every 8 hours for 10 to 14 days. Patients with severe findings should be treated with oral vancomycin, 500 mg every 6 hours, plus IV metronidazole, 500 mg every 8 hours. The more seriously ill patient must be observed carefully for signs of bowel obstruction, intestinal perforation, peritonitis, and sepsis.
Clearly, clinicians should make every effort to prevent C difficile infection in the first place. The following preventive measures are essential:
- Avoid the use of extremely broad-spectrum antibiotics for prophylaxis for CD.
- When using therapeutic antibiotics, keep the spectrum as narrow as possible, consistent with adequately treating the pathogens causing the infection.
- Administer antibiotics for the shortest time possible, consistent with achieving a clinical cure or providing appropriate prophylaxis for surgical procedures (usually, a maximum of 3 doses).
- If a patient receiving antibiotics experiences more than 3 loose stools in 24 hours, either discontinue all antibiotics or substitute another drug for the most likely offending agent, depending on the clinical situation.
- If, after stopping or changing antibiotics, the clinical findings do not resolve promptly, perform a culture or PCR assay for C difficile and assays for the C difficile toxin. Treat as outlined above if these tests are positive.
Read about pregnancy outcomes and trimester of maternal Zika infection
Danger for birth defects with maternal Zika infection present in all trimesters, but greatest in first
Hoen B, Schaub B, Funk AL, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018;378(11):985-994.
To estimate the risk of congenital neurologic defects associated with Zika virus infection, Hoen and colleagues conducted a prospective cohort study of pregnant women with symptomatic Zika virus infection who were enrolled during March through November 2016 in French Guiana, Guadeloupe, and Martinique. All women had Zika virus infection confirmed by PCR assay.
Details of the study
The investigators reviewed 546 pregnancies, which resulted in the birth of 555 fetuses and infants. Thirty-nine fetuses and neonates (7%; 95% CI, 5.0-9.5) had neurologic and ocular findings known to be associated with Zika virus infection. Of these, 10 pregnancies were terminated, 1 fetus was stillborn, and 28 were live-born.
Microcephaly (defined as head circumference more than 2 SD below the mean) was present in 32 fetuses and infants (5.8%); 9 had severe microcephaly, defined as head circumference more than 3 SD below the mean. Neurologic and ocular abnormalities were more common when maternal infection occurred during the first trimester (24 of 189 fetuses and infants, 12.7%) compared with infection during the second trimester (9 of 252, 3.6%) or third trimester (6 of 114, 5.3%) (P = .001).
Studies report similar rates of fetal injury
Zika virus infection primarily is caused by a bite from the Aedes aegypti mosquito. The infection also can be transmitted by sexual contact, laboratory accident, and blood transfusion. Eighty percent of infected persons are asymptomatic. In symptomatic patients, the most common clinical manifestations are low-grade fever, a disseminated maculopapular rash, arthralgias, swelling of the hands and feet, and nonpurulent conjunctivitis.
The most ominous manifestation of congenital Zika virus infection is microcephaly. Other important manifestations include lissencephaly, pachygyria, cortical atrophy, ventriculomegaly, subcortical calcifications, ocular abnormalities, and arthrogryposis. Although most of these abnormalities are immediately visible in the neonate, some may not appear until the child is older.
The present study is an excellent complement to 2 recent reports that defined the risk of Zika virus-related fetal injury in patients in the United States and its territories. Based on an analysis of data from the US Zika Pregnancy Registry, Honein and colleagues reported an overall rate of congenital infection of 6%.12 The rate of fetal injury was 11% when the mother was infected in the first trimester and 0% when the infection occurred in the second or third trimester. The overall rate of infection and the first trimester rate of infection were similar to those reported by Hoen and colleagues.
Conversely, Shapiro-Mendoza and colleagues evaluated rates of infection in US territories (American Samoa, Puerto Rico, and the US Virgin Islands) and observed cases of fetal injury associated with second- and third-trimester maternal infection.13 These authors reported an overall rate of infection of 5% and an 8% rate of infection with first-trimester maternal infection. When maternal infection occurred in the second and third trimesters, the rates of fetal injury were 5% and 4%, respectively, figures almost identical to those reported by Hoen and colleagues. Of note, the investigations by Honein and Shapiro-Mendoza included women with both symptomatic and asymptomatic infection.
Taken together, the studies discussed provide 2 clear take-home messages:
- Both symptomatic and asymptomatic maternal infection pose a significant risk of injury to the fetus and neonate.
- Although the risk of fetal injury is greatest when maternal infection occurs in the first trimester, exposure in the second and third trimesters is still dangerous. The Zika virus is quite pathogenic and can cause debilitating injury to the developing fetus at any stage of gestation.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Hyldig N, Birke-Sorensen H, Kruse M, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103(5):477–486.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: evidence-based guidance. OBG Manag. 2016;28(11):41–47.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: 5 more evidence-based measures to consider. OBG Manag. 2016;28(12):18–22.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374(9):823–832.
- Wilbur MB, Daum RS, Gold HS. Skin abscess. N Engl J Med. 2016;374(9): 882–884.
- Singer AJ, Talan DA. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med. 2014;370(11):1039–1047.
- Unger JA, Whimbey E, Gravett MG, Eschenbach DA. The emergence of Clostridium difficile infection among peripartum women: a case-control study of a C difficile outbreak on an obstetrical service. Infect Dis Obstet Gynecol. 2011;267249. doi:10.1155/2011/267249.
- Honein MA, Dawson AL, Petersen EE, et al; US Zika Pregnancy Registry Collaboration. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317(1):59–68.
- Shapiro-Mendoza CK, Rice ME, Galang RR, et al; Zika Pregnancy and Infant Registries Working Group. Pregnancy outcomes after maternal Zika virus infection during pregnancy — US territories. January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(23):615–621.
In this Update I highlight 5 interesting investigations on infectious diseases. The first addresses the value of applying prophylactically a negative-pressure wound dressing to prevent surgical site infection (SSI) in obese women having cesarean delivery (CD). The second report assesses the effectiveness of a preoperative vaginal wash in reducing the frequency of postcesarean endometritis. The third investigation examines the role of systemic antibiotics, combined with surgical drainage, for patients who have subcutaneous abscesses ranging in size up to 5 cm. The fourth study presents new information about the major risk factors for Clostridium difficile infections in obstetric patients. The final study presents valuable sobering new data about the risks of congenital Zika virus infection.
Negative-pressure wound therapy after CD shows some benefit in preventing SSI
Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200-210.e1.
Yu and colleagues sought to determine if the prophylactic use of negative-pressure devices, compared with standard wound dressing, was effective in reducing the frequency of SSI after CD.
The authors searched multiple databases and initially identified 161 randomized controlled trials and cohort studies for further assessment. After applying rigorous exclusion criteria, they ultimately selected 9 studies for systematic review and meta-analysis. Six studies were randomized controlled trials (RCTs), 2 were retrospective cohort studies, and 1 was a prospective cohort study. Five studies were considered high quality; 4 were of low quality.
Details of the study
Several types of negative-pressure devices were used, but the 2 most common were the Prevena incision management system (KCI, San Antonio, Texas) and PICO negative- pressure wound therapy (Smith & Nephew, St. Petersburg, Florida). The majority of patients in all groups were at high risk for wound complications because of obesity.
The primary outcome of interest was the frequency of SSI. Secondary outcomes included dehiscence, seroma, endometritis, a composite measure for all wound complications, and hospital readmission.
The absolute risk of SSI in the intervention group was 5% (95% confidence interval [CI], 2.0%-7.0%) compared with 11% (95% CI, 7.0%-16.0%) in the standard dressing group. The pooled risk ratio was 0.45 (95% CI, 0.31-0.66). The absolute risk reduction was 6% (95% CI, -10.0% to -3.0%), and the number needed to treat was 17.
There were no significant differences in the rate of any of the secondary outcomes other than the composite of all wound complications. This difference was largely accounted for by the difference in the rate of SSI.

How negative-pressure devices aid wound healing
Yu and colleagues explained that negative-pressure devices exert their beneficial effects in various ways, including:
- shrinking the wound
- inducing cellular stretch
- removing extracellular fluids
- creating a favorable environment for healing
- promoting angiogenesis and neurogenesis.
Multiple studies in nonobstetric patients have shown that prophylactic use of negative-pressure devices is beneficial in reducing the rate of SSI.1 Yu and colleagues' systematic review and meta-analysis confirms those findings in a high-risk population of women having CD.
Study limitations
Before routinely adopting the use of negative-pressure devices for all women having CD, however, obstetricians should consider the following caveats:
- The investigations included in the study by Yu and colleagues did not consistently distinguish between scheduled versus unscheduled CDs.
- The reports did not systematically consider other major risk factors for wound complications besides obesity, and they did not control for these confounders in the statistical analyses.
- The studies included in the meta-analysis did not provide full descriptions of other measures that might influence the rate of SSIs, such as timing and selection of prophylactic antibiotics, selection of suture material, preoperative skin preparation, and closure techniques for the deep subcutaneous tissue and skin.
- None of the included studies systematically considered the cost-effectiveness of the negative-pressure devices. This is an important consideration given that the acquisition cost of these devices ranges from $200 to $500.
Results of the systematic review and meta-analysis by Yu and colleagues suggest that prophylactic negative-pressure wound therapy in high-risk mostly obese women after CD reduces SSI and overall wound complications. The study's limitations, however, must be kept in mind, and more data are needed. It would be most helpful if a large, well-designed RCT was conducted and included 2 groups with comparable multiple major risk factors for wound complications, and in which all women received the following important interventions2-4:
- removal of hair in the surgical site with a clipper, not a razor
- cleansing of the skin with a chlorhexidine rather than an iodophor solution
- closure of the deep subcutaneous tissue if the total subcutaneous layer exceeds 2 cm in depth
- closure of the skin with suture rather than staples
- administration of antibiotic prophylaxis, ideally with a combination of cefazolin plus azithromycin, prior to the surgical incision.
Read about vaginal cleansing’s effect on post-CD endometritis
Vaginal cleansing before CD lowers risk of postop endometritis
Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(3):527-538.
Caissutti and colleagues aimed to determine if cleansing the vagina with an antiseptic solution prior to surgery reduced the frequency of postcesarean endometritis. They included 16 RCTs (4,837 patients) in their systematic review and meta-analysis. The primary outcome was the frequency of postoperative endometritis.
Details of the study
The studies were conducted in several countries and included patients of various socioeconomic classes. Six trials included only patients having a scheduled CD; 9 included both scheduled and unscheduled cesareans; and 1 included only unscheduled cesareans. In 11 studies, povidone-iodine was the antiseptic solution used. Two trials used chlorhexidine diacetate 0.2%, and 1 used chlorhexidine diacetate 0.4%. One trial used metronidazole 0.5% gel, and another used the antiseptic cetrimide, which is a mixture of different quaternary ammonium salts, including cetrimonium bromide.
In all trials, patients received prophylactic antibiotics. The antibiotics were administered prior to the surgical incision in 6 trials; they were given after the umbilical cord was clamped in 6 trials. In 2 trials, the antibiotics were given at varying times, and in the final 2 trials, the timing of antibiotic administration was not reported. Of note, no trials described the method of placenta removal, a factor of considerable significance in influencing the rate of postoperative endometritis.5,6
Endometritis frequency reduced with vaginal cleansing; benefit greater in certain groups. Overall, in the 15 trials in which vaginal cleansing was compared with placebo or with no treatment, women in the treatment group had a significantly lower rate of endometritis (4.5% compared with 8.8%; relative risk [RR], 0.52; 95% CI, 0.37-0.72). When only women in labor were considered, the frequency of endometritis was 8.1% in the intervention group compared with 13.8% in the control group (RR, 0.52; 95% CI, 0.28-0.97). In the women who were not in labor, the difference in the incidence of endometritis was not statistically significant (3.5% vs 6.6%; RR, 0.62; 95% CI, 0.34-1.15).
In the subgroup analysis of women with ruptured membranes at the time of surgery, the incidence of endometritis was 4.3% in the treatment group compared with 20.1% in the control group (RR, 0.23; 95% CI, 0.10-0.52). In women with intact membranes at the time of surgery, the incidence of endometritis was not significantly reduced in the treatment group.
Interestingly, in the subgroup analysis of the 10 trials that used povidone-iodine, the reduction in the frequency of postcesarean endometritis was statistically significant (2.8% vs 6.3%; RR, 0.42; 95% CI, 0.25-0.71). However, this same protective effect was not observed in the women treated with chlorhexidine. In the 1 trial that directly compared povidone-iodine with chlorhexidine, there was no statistically significant difference in outcome.
Simple intervention, solid benefit
Endometritis is the most common complication following CD. The infection is polymicrobial, with mixed aerobic and anaerobic organisms. The principal risk factors for postcesarean endometritis are low socioeconomic status, extended duration of labor and ruptured membranes, multiple vaginal examinations, internal fetal monitoring, and pre-existing vaginal infections (principally, bacterial vaginosis and group B streptococcal colonization).
Two interventions are clearly of value in reducing the incidence of endometritis: administration of prophylactic antibiotics prior to the surgical incision and removal of the placenta by traction on the cord as opposed to manual extraction.5,6
The assessment by Caissutti and colleagues confirms that a third measure — preoperative vaginal cleansing — also helps reduce the incidence of postcesarean endometritis. The principal benefit is seen in women who have been in labor with ruptured membranes, although certainly it is not harmful in lower-risk patients. The intervention is simple and straightforward: a 30-second vaginal wash with a povidone-iodine solution just prior to surgery.
From my perspective, the interesting unanswered question is why a chlorhexidine solution with low alcohol content was not more effective than povidone-iodine, given that a chlorhexidine abdominal wash is superior to povidone-iodine in preventing wound infection after cesarean delivery.7 Until additional studies confirm the effectiveness of vaginal cleansing with chlorhexidine, I recommend the routine use of the povidone-iodine solution in all women having CD.
Read about management approaches for skin abscesses
Treat smaller skin abscesses with antibiotics after surgical drainage? Yes.
Daum RS, Miller LG, Immergluck L, et al; for the DMID 07-0051 Team. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017;376(26):2545-2555.
For treatment of subcutaneous abscesses that were 5 cm or smaller in diameter, investigators sought to determine if surgical drainage alone was equivalent to surgical drainage plus systemic antibiotics. After their abscess was drained, patients were randomly assigned to receive either clindamycin (300 mg 3 times daily) or trimethoprim-sulfamethoxazole (80 mg/400 mg twice daily) or placebo for 10 days. The primary outcome was clinical cure 7 to 10 days after treatment.
Details of the study
Daum and colleagues enrolled 786 participants (505 adults, 281 children) in the prospective double-blind study. Staphylococcus aureus was isolated from 527 patients (67.0%); methicillin-resistant S aureus (MRSA) was isolated from 388 (49.4%). The cure rate was similar in patients in the clindamycin group (83.1%) and the trimethoprim-sulfamethoxazole group (81.7%), and the cure rate in each antibiotic group was significantly higher than that in the placebo group (68.9%; P<.001 for both comparisons). The difference in treatment effect was specifically limited to patients who had S aureus isolated from their lesions.
Findings at follow-up. At 1 month of follow-up, new infections were less common in the clindamycin group (6.8%) than in the trimethoprim-sulfamethoxazole group (13.5%; P = .03) or the placebo group (12.4%; P = .06). However, the highest frequency of adverse effects occurred in the patients who received clindamycin (21.9% vs 11.1% vs 12.5%). No adverse effects were judged to be serious, and all resolved without sequela.
Controversy remains on antibiotic use after drainage
This study is important for 2 major reasons. First, soft tissue infections are quite commonand can evolve into serious problems, especially when the offending pathogen is MRSA. Second, controversy exists about whether systemic antibiotics are indicated if the subcutaneous abscess is relatively small and is adequately drained. For example, Talan and colleagues demonstrated that, in settings with a high prevalence of MRSA, surgical drainage combined with trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily) was superior to drainage plus placebo.8 However, Daum and Gold recently debated the issue of drainage plus antibiotics in a case vignette and reached opposite conclusions.9
In my opinion, this investigation by Daum and colleagues supports a role for consistent use of systemic antibiotics following surgical drainage of clinically significant subcutaneous abscesses that have a 5 cm or smaller diameter. Several oral antibiotics are effective against S aureus, including MRSA.10 These drugs include trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily), clindamycin (300-450 mg 3 times daily), doxycycline (100 mg twice daily), and minocycline (200 mg initially, then 100 mg every 12 hours).
Of these drugs, I prefer trimethoprim-sulfamethoxazole, provided that the patient does not have an allergy to sulfonamides. Trimethoprim-sulfamethoxazole is significantly less expensive than the other 3 drugs and usually is better tolerated. In particular, compared with clindamycin, trimethoprim-sulfamethoxazole is less likely to cause antibiotic-associated diarrhea, including Clostridium difficile infection. Trimethoprim-sulfamethoxazole should not be used in the first trimester of pregnancy because of concerns about fetal teratogenicity.
Read how to avoid C difficile infections in pregnant patients
Antibiotic use, common in the obstetric population, raises risk for C difficile infection
Ruiter-Ligeti J, Vincent S, Czuzoj-Shulman N, Abenhaim HA. Risk factors, incidence, and morbidity associated with obstetric Clostridium difficile infection. Obstet Gynecol. 2018;131(2):387-391.
The objective of this investigation was to identify risk factors for Clostridium difficile infection (previously termed pseudomembranous enterocolitis) in obstetric patients. The authors performed a retrospective cohort study using information from a large database maintained by the Agency for Healthcare Research and Quality. This database provides information about inpatient hospital stays in the United States, and it is the largest repository of its kind. It includes data from a sample of 1,000 US hospitals.
Details of the study
Ruiter-Ligeti and colleagues reviewed 13,881,592 births during 1999-2013 and identified 2,757 (0.02%) admissions for delivery complicated by C difficile infection, a rate of 20 admissions per 100,000 deliveries per year (95% CI, 19.13-20.62). The rate of admissions with this diagnosis doubled from 1999 (15 per 100,000) to 2013 (30 per 100,000, P<.001).
Among these obstetric patients, the principal risk factors for C difficile infection were older age, multiple gestation, long-term antibiotic use (not precisely defined), and concurrent diagnosis of inflammatory bowel disease. In addition, patients with pyelonephritis, perineal or cesarean wound infections, or pneumonia also were at increased risk, presumably because those patients required longer courses of broad-spectrum antibiotics.
Of additional note, when compared with women who did not have C difficile infection, patients with infection were more likely to develop a thromboembolic event (38.4 per 1,000), paralytic ileus (58.0 per 1,000), sepsis (46.4 per 1,000), and death (8.0 per 1,000).
Be on guard for C difficile infection in antibiotic-treated obstetric patients
C difficile infection is an uncommon but potentially very serious complication of antibiotic therapy. Given that approximately half of all women admitted for delivery are exposed to antibiotics because of prophylaxis for group B streptococcus infection, prophylaxis for CD, and treatment of chorioamnionitis and puerperal endometritis, clinicians constantly need to be vigilant for this complication.11
Affected patients typically present with frequent loose, watery stools and lower abdominal cramping. In severe cases, blood may be present in the stool, and signs of intestinal distention and even acute peritonitis may be evident. The diagnosis can be established by documenting a positive culture or polymerase chain reaction (PCR) assay for C difficile and a positive cytotoxin assay for toxins A and/or B. In addition, if endoscopy is performed, the characteristic gray membranous plaques can be visualized on the rectal and colonic mucosa.11
Discontinue antibiotic therapy. The first step in managing affected patients is to stop all antibiotics, if possible, or at least the one most likely to be the causative agent of C difficile infection. Patients with relatively mild clinical findings should be treated with oral metronidazole, 500 mg every 8 hours for 10 to 14 days. Patients with severe findings should be treated with oral vancomycin, 500 mg every 6 hours, plus IV metronidazole, 500 mg every 8 hours. The more seriously ill patient must be observed carefully for signs of bowel obstruction, intestinal perforation, peritonitis, and sepsis.
Clearly, clinicians should make every effort to prevent C difficile infection in the first place. The following preventive measures are essential:
- Avoid the use of extremely broad-spectrum antibiotics for prophylaxis for CD.
- When using therapeutic antibiotics, keep the spectrum as narrow as possible, consistent with adequately treating the pathogens causing the infection.
- Administer antibiotics for the shortest time possible, consistent with achieving a clinical cure or providing appropriate prophylaxis for surgical procedures (usually, a maximum of 3 doses).
- If a patient receiving antibiotics experiences more than 3 loose stools in 24 hours, either discontinue all antibiotics or substitute another drug for the most likely offending agent, depending on the clinical situation.
- If, after stopping or changing antibiotics, the clinical findings do not resolve promptly, perform a culture or PCR assay for C difficile and assays for the C difficile toxin. Treat as outlined above if these tests are positive.
Read about pregnancy outcomes and trimester of maternal Zika infection
Danger for birth defects with maternal Zika infection present in all trimesters, but greatest in first
Hoen B, Schaub B, Funk AL, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018;378(11):985-994.
To estimate the risk of congenital neurologic defects associated with Zika virus infection, Hoen and colleagues conducted a prospective cohort study of pregnant women with symptomatic Zika virus infection who were enrolled during March through November 2016 in French Guiana, Guadeloupe, and Martinique. All women had Zika virus infection confirmed by PCR assay.
Details of the study
The investigators reviewed 546 pregnancies, which resulted in the birth of 555 fetuses and infants. Thirty-nine fetuses and neonates (7%; 95% CI, 5.0-9.5) had neurologic and ocular findings known to be associated with Zika virus infection. Of these, 10 pregnancies were terminated, 1 fetus was stillborn, and 28 were live-born.
Microcephaly (defined as head circumference more than 2 SD below the mean) was present in 32 fetuses and infants (5.8%); 9 had severe microcephaly, defined as head circumference more than 3 SD below the mean. Neurologic and ocular abnormalities were more common when maternal infection occurred during the first trimester (24 of 189 fetuses and infants, 12.7%) compared with infection during the second trimester (9 of 252, 3.6%) or third trimester (6 of 114, 5.3%) (P = .001).
Studies report similar rates of fetal injury
Zika virus infection primarily is caused by a bite from the Aedes aegypti mosquito. The infection also can be transmitted by sexual contact, laboratory accident, and blood transfusion. Eighty percent of infected persons are asymptomatic. In symptomatic patients, the most common clinical manifestations are low-grade fever, a disseminated maculopapular rash, arthralgias, swelling of the hands and feet, and nonpurulent conjunctivitis.
The most ominous manifestation of congenital Zika virus infection is microcephaly. Other important manifestations include lissencephaly, pachygyria, cortical atrophy, ventriculomegaly, subcortical calcifications, ocular abnormalities, and arthrogryposis. Although most of these abnormalities are immediately visible in the neonate, some may not appear until the child is older.
The present study is an excellent complement to 2 recent reports that defined the risk of Zika virus-related fetal injury in patients in the United States and its territories. Based on an analysis of data from the US Zika Pregnancy Registry, Honein and colleagues reported an overall rate of congenital infection of 6%.12 The rate of fetal injury was 11% when the mother was infected in the first trimester and 0% when the infection occurred in the second or third trimester. The overall rate of infection and the first trimester rate of infection were similar to those reported by Hoen and colleagues.
Conversely, Shapiro-Mendoza and colleagues evaluated rates of infection in US territories (American Samoa, Puerto Rico, and the US Virgin Islands) and observed cases of fetal injury associated with second- and third-trimester maternal infection.13 These authors reported an overall rate of infection of 5% and an 8% rate of infection with first-trimester maternal infection. When maternal infection occurred in the second and third trimesters, the rates of fetal injury were 5% and 4%, respectively, figures almost identical to those reported by Hoen and colleagues. Of note, the investigations by Honein and Shapiro-Mendoza included women with both symptomatic and asymptomatic infection.
Taken together, the studies discussed provide 2 clear take-home messages:
- Both symptomatic and asymptomatic maternal infection pose a significant risk of injury to the fetus and neonate.
- Although the risk of fetal injury is greatest when maternal infection occurs in the first trimester, exposure in the second and third trimesters is still dangerous. The Zika virus is quite pathogenic and can cause debilitating injury to the developing fetus at any stage of gestation.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
In this Update I highlight 5 interesting investigations on infectious diseases. The first addresses the value of applying prophylactically a negative-pressure wound dressing to prevent surgical site infection (SSI) in obese women having cesarean delivery (CD). The second report assesses the effectiveness of a preoperative vaginal wash in reducing the frequency of postcesarean endometritis. The third investigation examines the role of systemic antibiotics, combined with surgical drainage, for patients who have subcutaneous abscesses ranging in size up to 5 cm. The fourth study presents new information about the major risk factors for Clostridium difficile infections in obstetric patients. The final study presents valuable sobering new data about the risks of congenital Zika virus infection.
Negative-pressure wound therapy after CD shows some benefit in preventing SSI
Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200-210.e1.
Yu and colleagues sought to determine if the prophylactic use of negative-pressure devices, compared with standard wound dressing, was effective in reducing the frequency of SSI after CD.
The authors searched multiple databases and initially identified 161 randomized controlled trials and cohort studies for further assessment. After applying rigorous exclusion criteria, they ultimately selected 9 studies for systematic review and meta-analysis. Six studies were randomized controlled trials (RCTs), 2 were retrospective cohort studies, and 1 was a prospective cohort study. Five studies were considered high quality; 4 were of low quality.
Details of the study
Several types of negative-pressure devices were used, but the 2 most common were the Prevena incision management system (KCI, San Antonio, Texas) and PICO negative- pressure wound therapy (Smith & Nephew, St. Petersburg, Florida). The majority of patients in all groups were at high risk for wound complications because of obesity.
The primary outcome of interest was the frequency of SSI. Secondary outcomes included dehiscence, seroma, endometritis, a composite measure for all wound complications, and hospital readmission.
The absolute risk of SSI in the intervention group was 5% (95% confidence interval [CI], 2.0%-7.0%) compared with 11% (95% CI, 7.0%-16.0%) in the standard dressing group. The pooled risk ratio was 0.45 (95% CI, 0.31-0.66). The absolute risk reduction was 6% (95% CI, -10.0% to -3.0%), and the number needed to treat was 17.
There were no significant differences in the rate of any of the secondary outcomes other than the composite of all wound complications. This difference was largely accounted for by the difference in the rate of SSI.

How negative-pressure devices aid wound healing
Yu and colleagues explained that negative-pressure devices exert their beneficial effects in various ways, including:
- shrinking the wound
- inducing cellular stretch
- removing extracellular fluids
- creating a favorable environment for healing
- promoting angiogenesis and neurogenesis.
Multiple studies in nonobstetric patients have shown that prophylactic use of negative-pressure devices is beneficial in reducing the rate of SSI.1 Yu and colleagues' systematic review and meta-analysis confirms those findings in a high-risk population of women having CD.
Study limitations
Before routinely adopting the use of negative-pressure devices for all women having CD, however, obstetricians should consider the following caveats:
- The investigations included in the study by Yu and colleagues did not consistently distinguish between scheduled versus unscheduled CDs.
- The reports did not systematically consider other major risk factors for wound complications besides obesity, and they did not control for these confounders in the statistical analyses.
- The studies included in the meta-analysis did not provide full descriptions of other measures that might influence the rate of SSIs, such as timing and selection of prophylactic antibiotics, selection of suture material, preoperative skin preparation, and closure techniques for the deep subcutaneous tissue and skin.
- None of the included studies systematically considered the cost-effectiveness of the negative-pressure devices. This is an important consideration given that the acquisition cost of these devices ranges from $200 to $500.
Results of the systematic review and meta-analysis by Yu and colleagues suggest that prophylactic negative-pressure wound therapy in high-risk mostly obese women after CD reduces SSI and overall wound complications. The study's limitations, however, must be kept in mind, and more data are needed. It would be most helpful if a large, well-designed RCT was conducted and included 2 groups with comparable multiple major risk factors for wound complications, and in which all women received the following important interventions2-4:
- removal of hair in the surgical site with a clipper, not a razor
- cleansing of the skin with a chlorhexidine rather than an iodophor solution
- closure of the deep subcutaneous tissue if the total subcutaneous layer exceeds 2 cm in depth
- closure of the skin with suture rather than staples
- administration of antibiotic prophylaxis, ideally with a combination of cefazolin plus azithromycin, prior to the surgical incision.
Read about vaginal cleansing’s effect on post-CD endometritis
Vaginal cleansing before CD lowers risk of postop endometritis
Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(3):527-538.
Caissutti and colleagues aimed to determine if cleansing the vagina with an antiseptic solution prior to surgery reduced the frequency of postcesarean endometritis. They included 16 RCTs (4,837 patients) in their systematic review and meta-analysis. The primary outcome was the frequency of postoperative endometritis.
Details of the study
The studies were conducted in several countries and included patients of various socioeconomic classes. Six trials included only patients having a scheduled CD; 9 included both scheduled and unscheduled cesareans; and 1 included only unscheduled cesareans. In 11 studies, povidone-iodine was the antiseptic solution used. Two trials used chlorhexidine diacetate 0.2%, and 1 used chlorhexidine diacetate 0.4%. One trial used metronidazole 0.5% gel, and another used the antiseptic cetrimide, which is a mixture of different quaternary ammonium salts, including cetrimonium bromide.
In all trials, patients received prophylactic antibiotics. The antibiotics were administered prior to the surgical incision in 6 trials; they were given after the umbilical cord was clamped in 6 trials. In 2 trials, the antibiotics were given at varying times, and in the final 2 trials, the timing of antibiotic administration was not reported. Of note, no trials described the method of placenta removal, a factor of considerable significance in influencing the rate of postoperative endometritis.5,6
Endometritis frequency reduced with vaginal cleansing; benefit greater in certain groups. Overall, in the 15 trials in which vaginal cleansing was compared with placebo or with no treatment, women in the treatment group had a significantly lower rate of endometritis (4.5% compared with 8.8%; relative risk [RR], 0.52; 95% CI, 0.37-0.72). When only women in labor were considered, the frequency of endometritis was 8.1% in the intervention group compared with 13.8% in the control group (RR, 0.52; 95% CI, 0.28-0.97). In the women who were not in labor, the difference in the incidence of endometritis was not statistically significant (3.5% vs 6.6%; RR, 0.62; 95% CI, 0.34-1.15).
In the subgroup analysis of women with ruptured membranes at the time of surgery, the incidence of endometritis was 4.3% in the treatment group compared with 20.1% in the control group (RR, 0.23; 95% CI, 0.10-0.52). In women with intact membranes at the time of surgery, the incidence of endometritis was not significantly reduced in the treatment group.
Interestingly, in the subgroup analysis of the 10 trials that used povidone-iodine, the reduction in the frequency of postcesarean endometritis was statistically significant (2.8% vs 6.3%; RR, 0.42; 95% CI, 0.25-0.71). However, this same protective effect was not observed in the women treated with chlorhexidine. In the 1 trial that directly compared povidone-iodine with chlorhexidine, there was no statistically significant difference in outcome.
Simple intervention, solid benefit
Endometritis is the most common complication following CD. The infection is polymicrobial, with mixed aerobic and anaerobic organisms. The principal risk factors for postcesarean endometritis are low socioeconomic status, extended duration of labor and ruptured membranes, multiple vaginal examinations, internal fetal monitoring, and pre-existing vaginal infections (principally, bacterial vaginosis and group B streptococcal colonization).
Two interventions are clearly of value in reducing the incidence of endometritis: administration of prophylactic antibiotics prior to the surgical incision and removal of the placenta by traction on the cord as opposed to manual extraction.5,6
The assessment by Caissutti and colleagues confirms that a third measure — preoperative vaginal cleansing — also helps reduce the incidence of postcesarean endometritis. The principal benefit is seen in women who have been in labor with ruptured membranes, although certainly it is not harmful in lower-risk patients. The intervention is simple and straightforward: a 30-second vaginal wash with a povidone-iodine solution just prior to surgery.
From my perspective, the interesting unanswered question is why a chlorhexidine solution with low alcohol content was not more effective than povidone-iodine, given that a chlorhexidine abdominal wash is superior to povidone-iodine in preventing wound infection after cesarean delivery.7 Until additional studies confirm the effectiveness of vaginal cleansing with chlorhexidine, I recommend the routine use of the povidone-iodine solution in all women having CD.
Read about management approaches for skin abscesses
Treat smaller skin abscesses with antibiotics after surgical drainage? Yes.
Daum RS, Miller LG, Immergluck L, et al; for the DMID 07-0051 Team. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017;376(26):2545-2555.
For treatment of subcutaneous abscesses that were 5 cm or smaller in diameter, investigators sought to determine if surgical drainage alone was equivalent to surgical drainage plus systemic antibiotics. After their abscess was drained, patients were randomly assigned to receive either clindamycin (300 mg 3 times daily) or trimethoprim-sulfamethoxazole (80 mg/400 mg twice daily) or placebo for 10 days. The primary outcome was clinical cure 7 to 10 days after treatment.
Details of the study
Daum and colleagues enrolled 786 participants (505 adults, 281 children) in the prospective double-blind study. Staphylococcus aureus was isolated from 527 patients (67.0%); methicillin-resistant S aureus (MRSA) was isolated from 388 (49.4%). The cure rate was similar in patients in the clindamycin group (83.1%) and the trimethoprim-sulfamethoxazole group (81.7%), and the cure rate in each antibiotic group was significantly higher than that in the placebo group (68.9%; P<.001 for both comparisons). The difference in treatment effect was specifically limited to patients who had S aureus isolated from their lesions.
Findings at follow-up. At 1 month of follow-up, new infections were less common in the clindamycin group (6.8%) than in the trimethoprim-sulfamethoxazole group (13.5%; P = .03) or the placebo group (12.4%; P = .06). However, the highest frequency of adverse effects occurred in the patients who received clindamycin (21.9% vs 11.1% vs 12.5%). No adverse effects were judged to be serious, and all resolved without sequela.
Controversy remains on antibiotic use after drainage
This study is important for 2 major reasons. First, soft tissue infections are quite commonand can evolve into serious problems, especially when the offending pathogen is MRSA. Second, controversy exists about whether systemic antibiotics are indicated if the subcutaneous abscess is relatively small and is adequately drained. For example, Talan and colleagues demonstrated that, in settings with a high prevalence of MRSA, surgical drainage combined with trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily) was superior to drainage plus placebo.8 However, Daum and Gold recently debated the issue of drainage plus antibiotics in a case vignette and reached opposite conclusions.9
In my opinion, this investigation by Daum and colleagues supports a role for consistent use of systemic antibiotics following surgical drainage of clinically significant subcutaneous abscesses that have a 5 cm or smaller diameter. Several oral antibiotics are effective against S aureus, including MRSA.10 These drugs include trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily), clindamycin (300-450 mg 3 times daily), doxycycline (100 mg twice daily), and minocycline (200 mg initially, then 100 mg every 12 hours).
Of these drugs, I prefer trimethoprim-sulfamethoxazole, provided that the patient does not have an allergy to sulfonamides. Trimethoprim-sulfamethoxazole is significantly less expensive than the other 3 drugs and usually is better tolerated. In particular, compared with clindamycin, trimethoprim-sulfamethoxazole is less likely to cause antibiotic-associated diarrhea, including Clostridium difficile infection. Trimethoprim-sulfamethoxazole should not be used in the first trimester of pregnancy because of concerns about fetal teratogenicity.
Read how to avoid C difficile infections in pregnant patients
Antibiotic use, common in the obstetric population, raises risk for C difficile infection
Ruiter-Ligeti J, Vincent S, Czuzoj-Shulman N, Abenhaim HA. Risk factors, incidence, and morbidity associated with obstetric Clostridium difficile infection. Obstet Gynecol. 2018;131(2):387-391.
The objective of this investigation was to identify risk factors for Clostridium difficile infection (previously termed pseudomembranous enterocolitis) in obstetric patients. The authors performed a retrospective cohort study using information from a large database maintained by the Agency for Healthcare Research and Quality. This database provides information about inpatient hospital stays in the United States, and it is the largest repository of its kind. It includes data from a sample of 1,000 US hospitals.
Details of the study
Ruiter-Ligeti and colleagues reviewed 13,881,592 births during 1999-2013 and identified 2,757 (0.02%) admissions for delivery complicated by C difficile infection, a rate of 20 admissions per 100,000 deliveries per year (95% CI, 19.13-20.62). The rate of admissions with this diagnosis doubled from 1999 (15 per 100,000) to 2013 (30 per 100,000, P<.001).
Among these obstetric patients, the principal risk factors for C difficile infection were older age, multiple gestation, long-term antibiotic use (not precisely defined), and concurrent diagnosis of inflammatory bowel disease. In addition, patients with pyelonephritis, perineal or cesarean wound infections, or pneumonia also were at increased risk, presumably because those patients required longer courses of broad-spectrum antibiotics.
Of additional note, when compared with women who did not have C difficile infection, patients with infection were more likely to develop a thromboembolic event (38.4 per 1,000), paralytic ileus (58.0 per 1,000), sepsis (46.4 per 1,000), and death (8.0 per 1,000).
Be on guard for C difficile infection in antibiotic-treated obstetric patients
C difficile infection is an uncommon but potentially very serious complication of antibiotic therapy. Given that approximately half of all women admitted for delivery are exposed to antibiotics because of prophylaxis for group B streptococcus infection, prophylaxis for CD, and treatment of chorioamnionitis and puerperal endometritis, clinicians constantly need to be vigilant for this complication.11
Affected patients typically present with frequent loose, watery stools and lower abdominal cramping. In severe cases, blood may be present in the stool, and signs of intestinal distention and even acute peritonitis may be evident. The diagnosis can be established by documenting a positive culture or polymerase chain reaction (PCR) assay for C difficile and a positive cytotoxin assay for toxins A and/or B. In addition, if endoscopy is performed, the characteristic gray membranous plaques can be visualized on the rectal and colonic mucosa.11
Discontinue antibiotic therapy. The first step in managing affected patients is to stop all antibiotics, if possible, or at least the one most likely to be the causative agent of C difficile infection. Patients with relatively mild clinical findings should be treated with oral metronidazole, 500 mg every 8 hours for 10 to 14 days. Patients with severe findings should be treated with oral vancomycin, 500 mg every 6 hours, plus IV metronidazole, 500 mg every 8 hours. The more seriously ill patient must be observed carefully for signs of bowel obstruction, intestinal perforation, peritonitis, and sepsis.
Clearly, clinicians should make every effort to prevent C difficile infection in the first place. The following preventive measures are essential:
- Avoid the use of extremely broad-spectrum antibiotics for prophylaxis for CD.
- When using therapeutic antibiotics, keep the spectrum as narrow as possible, consistent with adequately treating the pathogens causing the infection.
- Administer antibiotics for the shortest time possible, consistent with achieving a clinical cure or providing appropriate prophylaxis for surgical procedures (usually, a maximum of 3 doses).
- If a patient receiving antibiotics experiences more than 3 loose stools in 24 hours, either discontinue all antibiotics or substitute another drug for the most likely offending agent, depending on the clinical situation.
- If, after stopping or changing antibiotics, the clinical findings do not resolve promptly, perform a culture or PCR assay for C difficile and assays for the C difficile toxin. Treat as outlined above if these tests are positive.
Read about pregnancy outcomes and trimester of maternal Zika infection
Danger for birth defects with maternal Zika infection present in all trimesters, but greatest in first
Hoen B, Schaub B, Funk AL, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018;378(11):985-994.
To estimate the risk of congenital neurologic defects associated with Zika virus infection, Hoen and colleagues conducted a prospective cohort study of pregnant women with symptomatic Zika virus infection who were enrolled during March through November 2016 in French Guiana, Guadeloupe, and Martinique. All women had Zika virus infection confirmed by PCR assay.
Details of the study
The investigators reviewed 546 pregnancies, which resulted in the birth of 555 fetuses and infants. Thirty-nine fetuses and neonates (7%; 95% CI, 5.0-9.5) had neurologic and ocular findings known to be associated with Zika virus infection. Of these, 10 pregnancies were terminated, 1 fetus was stillborn, and 28 were live-born.
Microcephaly (defined as head circumference more than 2 SD below the mean) was present in 32 fetuses and infants (5.8%); 9 had severe microcephaly, defined as head circumference more than 3 SD below the mean. Neurologic and ocular abnormalities were more common when maternal infection occurred during the first trimester (24 of 189 fetuses and infants, 12.7%) compared with infection during the second trimester (9 of 252, 3.6%) or third trimester (6 of 114, 5.3%) (P = .001).
Studies report similar rates of fetal injury
Zika virus infection primarily is caused by a bite from the Aedes aegypti mosquito. The infection also can be transmitted by sexual contact, laboratory accident, and blood transfusion. Eighty percent of infected persons are asymptomatic. In symptomatic patients, the most common clinical manifestations are low-grade fever, a disseminated maculopapular rash, arthralgias, swelling of the hands and feet, and nonpurulent conjunctivitis.
The most ominous manifestation of congenital Zika virus infection is microcephaly. Other important manifestations include lissencephaly, pachygyria, cortical atrophy, ventriculomegaly, subcortical calcifications, ocular abnormalities, and arthrogryposis. Although most of these abnormalities are immediately visible in the neonate, some may not appear until the child is older.
The present study is an excellent complement to 2 recent reports that defined the risk of Zika virus-related fetal injury in patients in the United States and its territories. Based on an analysis of data from the US Zika Pregnancy Registry, Honein and colleagues reported an overall rate of congenital infection of 6%.12 The rate of fetal injury was 11% when the mother was infected in the first trimester and 0% when the infection occurred in the second or third trimester. The overall rate of infection and the first trimester rate of infection were similar to those reported by Hoen and colleagues.
Conversely, Shapiro-Mendoza and colleagues evaluated rates of infection in US territories (American Samoa, Puerto Rico, and the US Virgin Islands) and observed cases of fetal injury associated with second- and third-trimester maternal infection.13 These authors reported an overall rate of infection of 5% and an 8% rate of infection with first-trimester maternal infection. When maternal infection occurred in the second and third trimesters, the rates of fetal injury were 5% and 4%, respectively, figures almost identical to those reported by Hoen and colleagues. Of note, the investigations by Honein and Shapiro-Mendoza included women with both symptomatic and asymptomatic infection.
Taken together, the studies discussed provide 2 clear take-home messages:
- Both symptomatic and asymptomatic maternal infection pose a significant risk of injury to the fetus and neonate.
- Although the risk of fetal injury is greatest when maternal infection occurs in the first trimester, exposure in the second and third trimesters is still dangerous. The Zika virus is quite pathogenic and can cause debilitating injury to the developing fetus at any stage of gestation.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Hyldig N, Birke-Sorensen H, Kruse M, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103(5):477–486.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: evidence-based guidance. OBG Manag. 2016;28(11):41–47.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: 5 more evidence-based measures to consider. OBG Manag. 2016;28(12):18–22.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374(9):823–832.
- Wilbur MB, Daum RS, Gold HS. Skin abscess. N Engl J Med. 2016;374(9): 882–884.
- Singer AJ, Talan DA. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med. 2014;370(11):1039–1047.
- Unger JA, Whimbey E, Gravett MG, Eschenbach DA. The emergence of Clostridium difficile infection among peripartum women: a case-control study of a C difficile outbreak on an obstetrical service. Infect Dis Obstet Gynecol. 2011;267249. doi:10.1155/2011/267249.
- Honein MA, Dawson AL, Petersen EE, et al; US Zika Pregnancy Registry Collaboration. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317(1):59–68.
- Shapiro-Mendoza CK, Rice ME, Galang RR, et al; Zika Pregnancy and Infant Registries Working Group. Pregnancy outcomes after maternal Zika virus infection during pregnancy — US territories. January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(23):615–621.
- Hyldig N, Birke-Sorensen H, Kruse M, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103(5):477–486.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: evidence-based guidance. OBG Manag. 2016;28(11):41–47.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: 5 more evidence-based measures to consider. OBG Manag. 2016;28(12):18–22.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374(9):823–832.
- Wilbur MB, Daum RS, Gold HS. Skin abscess. N Engl J Med. 2016;374(9): 882–884.
- Singer AJ, Talan DA. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med. 2014;370(11):1039–1047.
- Unger JA, Whimbey E, Gravett MG, Eschenbach DA. The emergence of Clostridium difficile infection among peripartum women: a case-control study of a C difficile outbreak on an obstetrical service. Infect Dis Obstet Gynecol. 2011;267249. doi:10.1155/2011/267249.
- Honein MA, Dawson AL, Petersen EE, et al; US Zika Pregnancy Registry Collaboration. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317(1):59–68.
- Shapiro-Mendoza CK, Rice ME, Galang RR, et al; Zika Pregnancy and Infant Registries Working Group. Pregnancy outcomes after maternal Zika virus infection during pregnancy — US territories. January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(23):615–621.
IN THIS ARTICLE
- Vaginal cleansing before CD lowers risk of postop endometritis
- Treat smaller skin abscesses with antibiotics after surgical drainage? Yes.
- Antibiotic use, common in the obstetric population, raises risk for C difficile infection
- Danger for birth defects with maternal Zika infection present in all trimesters, but greatest in first
Human trafficking: How ObGyns can—and should—be helping survivors

Despite increasing media coverage of human trafficking and the gravity of its many ramifications, I am struck by how often trainees and other clinicians present to me patients for which trafficking is a real potential concern—yet who give me a blank expression when I ask if anyone has screened these patients for being victims of trafficking. I suspect that few of us anticipated, during medical training, that we would be providing care to women who are enslaved.
How large is the problem?
It is impossible to comprehend the true scope of human trafficking. Estimates are that 20.9 million men, women, and children globally are forced into work that they are not free to leave.1
Although human trafficking is recognized as a global phenomenon, its prevalence in the United States is significant enough that it should prompt the health care community to engage in helping identify and assist victims/survivors: From January until June of 2017, the National Human Trafficking Hotline received 13,807 telephone calls, resulting in reporting of 4,460 cases.2 Indeed, from 2015 to 2016 there was a 35.7% increase in the number of hotline cases reported, for a total of 7,572 (6,340—more than 80%—of which regarded females). California had the most cases reported (1,323), followed by Texas (670) and Florida (550); those 3 states also reported an increase in trafficking crime. Vermont (5), Rhode Island (9), and Alaska (10) reported the fewest calls.3
How is trafficking defined?
The United Nations Office on Drugs and Crime defines “trafficking in persons” as:
… recruitment, transportation, transfer, harbouring or receipt of persons, by means of the threat or use of force or other forms of coercion, of abduction, of fraud, of deception, of the abuse of power or of a position of vulnerability or of the giving or receiving of payments or benefits to achieve the consent of a person having control over another person, for the purpose of exploitation. Exploitation shall include, at a minimum, the exploitation of the prostitution of others or other forms of sexual exploitation, forced labour or services, slavery or practices similar to slavery, servitude or the removal of organs.4
Traffickers prey on potentially vulnerable people. Girls and young women who have experienced poverty, homelessness, childhood sexual abuse, substance abuse, gender nonconformity, mental illness, or developmental delay are at particular risk.5 Children who have had interactions with Child Protective Services, come from a dysfunctional family, or have lived in a community with high crime, political or social unrest, corruption, or gender bias and discrimination are also at increased risk.6
Read about clues that raise clinical suspicion
Clues that raise clinical suspicion
A number of potential signs should make providers suspicious about potential human trafficking. Some of those signs are similar to the red flags we see in intimate partner violence, such as:
- having a difficult time talking to the patient alone
- having the accompanying person answer the patient’s questions
- body language that suggests fear, anxiety, or distrust (eg, shifting positions, looking away, appearing withdrawn)
- physical examination inconsistent with the history
- physical injury (especially multiple injuries or injuries in various stages of healing)
- refusal of interpreter services.
Trafficked girls or women may appear overly familiar with sex, have unexpected material possessions, or appear to be giving scripted or memorized answers to queries.7 Traffickers often confiscate their victims’ personal identification. They try to prevent victims from knowing their geographic locales: Patients might not have any documentation or awareness of exact surroundings (eg, their home address). Patients may be wearing clothes considered inappropriate for the weather or venue. They may have tattoos that are marks of branding.8
Medical consequences of being trafficked are obvious, numerous, and serious
Many medical sequelae that result from trafficking are obvious, given the nature of work that victims are forced to do. For example, overcrowding can lead to infectious disease, such as tuberculosis.9 Inadequate access to preventive or basic medical services can result in weight loss, poor dentition, and untreated chronic medical conditions.
If victims are experiencing physical or sexual abuse, they can present with evidence of blunt trauma, ligature marks, skin burns, wounds inflicted by weapons, and vaginal lacerations.10 A study found that 63% of survivors reported at least 10 somatic symptoms, including headache, fatigue, dizziness, back pain, abdominal or pelvic pain, memory loss, and symptoms of genital infectious disease.11
Girls and women being trafficked for sex may experience many of the sequelae of unprotected intercourse: irregular bleeding, unintended pregnancy, unwanted or unsafe pregnancy termination, vaginal trauma, and sexually transmitted infection (STI).12 In a study of trafficking survivors, 38% were HIV-positive.13
Trafficking survivors can suffer myriad mental health conditions, with high rates of depression, anxiety, posttraumatic stress, and suicidal ideation.14 A study of 387 survivors found that 12% had attempted to harm themselves or commit suicide the month before they were interviewed.15
Substance abuse is also a common problem among trafficking victims.16 One survivor interviewed in a recent study said:
It was much more difficult to work sober because I was dealing with emotions or the pain that I was feeling during intercourse, because when you have sex with people 8, 9, 10 times a day, even more than that, it starts to hurt a lot. And being high made it easier to deal with that and also it made it easier for me to get away from my body while it was happening, place my brain somewhere else.17
Because of the substantial risk of mental health problems, including substance abuse, among trafficking survivors, the physical exam of a patient should include careful assessment of demeanor and mental health status. Of course, comprehensive inspection for signs of physical or blunt trauma is paramount.
Read about Patient and staff safety during the visit
Patient and staff safety during the visit
Providers should be aware of potential safety concerns, both for the patient and for the staff. Creative strategies should be utilized to screen the patient in private. The use of interpreter services—either in person or over the telephone—should be presented and facilitated as being a routine part of practice. Any person who accompanies the patient should be asked to leave the examining room, either as a statement of practice routine or under the guise of having him (or her) step out to obtain paperwork or provide documentation.
Care of victims
Trauma-informed care should be a guiding principle for trafficking survivors. This involves empowering the patient, who may feel victimized again if asked to undress and undergo multiple physical examinations. Macias-Konstantopoulos noted: “A trauma-informed approach to care acknowledges the pervasiveness and effect of trauma across the life span of the individual, recognizes the vulnerabilities and emotional triggers of trauma survivors, minimizes repeated traumatization and fosters physical, psychological, and emotional safety, recovery, health and well-being.”18
The patient should be counseled that she has control over her body and can guide different aspects of the examination. For example the provider should discuss: 1) the amount of clothing deemed optimal for an examination, 2) the availability of a support person during the exam (for instance, a nurse or a social worker) if the patient requests one, and 3) utilization of whatever strategies the patient deems optimal for her to be most comfortable during the exam (such as leaving the door slightly ajar or having a mutually agreed-on signal to interrupt the exam).
Routine health care maintenance should be offered, including an assessment of overall physical and dental health and screening for STI and mental health. Screening for substances of abuse should be considered. If indicated, emergency contraception, postexposure HIV prophylaxis, immunizations, and empiric antibiotics for STI should be offered.19
Screening when indicated by evidence, suspicion, or concern
Unlike the case with intimate partner violence, experts do not recommend universal screening for human trafficking. Clinicians should be comfortable, however, trying to elicit that history when a concern arises, either because of identified risk factors, red flags, or concerns that arise from the findings of the history or physical. Ideally, clinicians should consider becoming comfortable choosing a few screening questions to regularly incorporate into their assessment. The US Department of Health & Human Services (HHS) offers a list of questions that can be utilized (TABLE).20
In January 2018, the Office on Trafficking in Persons, a unit of the HHS Administration for Children and Families, released an “Adult Human Trafficking Screening Tool and Guide.” The document includes 2 excellent tools21 that clinicians can utilize to identify patients who should be screened and how to identify and assist survivors (FIGURE 1 and FIGURE 2).
Clinicians, in their encounters with patients, are particularly well-positioned to intersect with, and identify, survivors. Regrettably, such opportunities are often missed—and victims thus remain unidentified and trapped in their circumstances. A study revealed that one-half of survivors who were interviewed reported seeing a physician while they were being trafficked.22 Even more alarming, another study showed that 87.8% of survivors had received health care during their captivity.23 It is dismaying to know that these patients left those health care settings without receiving the assistance they truly need and with their true circumstances remaining unidentified.
Read about Finding assistance and support
Finding assistance and support
Centers in the United States now provide trauma-informed care for trafficking survivors in a confidential setting (see “Specialized care is increasingly available”).24 A physician who works at a center in New York City noted: “Our survivors told us that more than fear or pain, the feelings that sat with them most often were worthlessness and invisibility. We can do better as physicians and as educators to expose this epidemic and care for its victims.”24
Here is a sampling of the growing number of centers in the United States that provide trauma-centered care for survivors of human trafficking:
- Survivor Clinic at New York Presbyterian Hospital-Weill Cornell Medical College, New York, New York
- EMPOWER Clinic for Survivors of Sex Trafficking and Sexual Violence at NYU Langone Health, New York, New York
- Freedom Clinic at Massachusetts General Hospital, Boston
- The Hope Through Health Clinic, Austin, Texas
- Pacific Survivor Center, Honolulu, Hawaii
Most clinicians practice in settings that do not have easy access to such subspecialized centers, however. For them, the National Human Trafficking Hotline can be an invaluable resource (see “Hotline is a valuable resource”).25 Law enforcement and social services colleagues also can be useful allies.
Uncertain how you can help a patient who is a victim of human trafficking? For assistance and support, contact the National Human Trafficking Hotline--24 hours a day, 7 days a week, and in 200 languages--in any of 3 ways:
- By telephone: (888) 373-7888
- By text: 233733
- On the web: https://humantraffickinghotline.orga
aIncludes a search field that clinicians can use to look up the nearest resources for additional assistance.
Let’s turn our concern and awareness into results
We, as providers of women’s health care, are uniquely positioned to help these most vulnerable of people, many of whom have been stripped of personal documents and denied access to financial resources and community support. As a medical community, we should strive to combat this tragic epidemic, 1 patient at a time.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- International Labour Organization. New ILO Global Estimate of Forced Labour: 20.9 million victims. http://www.ilo.org/global/about-the-ilo/newsroom/news/WCMS_182109/lang--en/index.htm. Published June 2012. Accessed May 30, 2018.
- National Human Trafficking Hotline. Hotline statistics. https://humantraffickinghotline.org/states. Accessed May 30, 2018.
- Cone A. Report: Human trafficking in U.S. rose 35.7 percent in one year. United Press International (UPI). https://www.upi.com/Report-Human-trafficking-in-US-rose-357-percent-in-one-year/5571486328579. Published February 5, 2017. Accessed May 30, 2018.
- United Nations Office on Drugs and Crime. Human trafficking. http://www.unodc.org/unodc/en/human-trafficking/what-is-human-trafficking.html. Accessed May 30, 2018.
- Risk factors for and consequences of commercial sexual exploitation and sex trafficking of minors. In Clayton E, Krugman R, Simon P, eds; Committee on the Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States; Board on Children, Youth, and Families; Committee on Law and Justice; Institute of Medicine; National Research Council. Confronting Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States. Washington, DC: The National Academies Press; 2013.
- Greenbaum J, Crawford-Jakubiak JE. Committee on Child Abuse and Neglect. Child sex trafficking and commercial sexual exploitation: health care needs of victims. Pediatrics. 2015:135(3);566–574.
- Alpert E, Ahn R, Albright E, Purcell G, Burke T, Macias-Konstantanopoulos W. Human Trafficking: Guidebook on Identification, Assessment, and Response in the Health Care Setting. Waltham, MA: Massachusetts General Hospital and Massachusetts Medical Society; 2014. http://www.massmed.org/Patient-Care/Health-Topics/Violence-Prevention-and-Intervention/Human-Trafficking-(pdf). Accessed May 30, 2018.
- National Human Trafficking Training and Technical Assistance Center. Adult human trafficking screening tool and guide. http://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Published January 2018. Accessed May 30, 2018.
- Steele S. Human trafficking, labor brokering, and mining in southern Africa: responding to a decentralized and hidden public health disaster. Int J Health Serv. 2013;43(4):665–680.
- Becker HJ, Bechtel K. Recognizing victims of human trafficking in the pediatric emergency department. Pediatr Emerg Care. 2015;31(2):144–147.
- Zimmerman C, Hossain M, Yun K, et al. The health of trafficked women: a survey of women entering postrafficking services in Europe. Am J Public Health. 2008;98(1):55–59.
- Tracy EE, Macias-Konstantopoulos W. Identifying and assisting sexually exploited and trafficked patients seeking women’s health care services. Obstet Gynecol. 2017;130(2):443–453.
- Silverman JG, Decker MR, Gupta J, Maheshwari A, Willis BM, Raj A. HIV prevalence and predictors of infection in sex-trafficked Nepalese girls and women. JAMA. 2007;298(5):536–542.
- Rafferty Y. Child trafficking and commercial sexual exploitation: a review of promising prevention policies and programs. Am J Orthopsychiatry. 2013;83(4):559–575.
- Kiss L, Yun K, Pocock N, Zimmerman C. Exploitation, violence, and suicide risk among child and adolescent survivors of human trafficking in the Greater Mekong Subregion. JAMA Pediatr. 2015;169(9):e152278.
- Stoklosa H, MacGibbon M, Stoklosa J. Human trafficking, mental illness, and addiction: avoiding diagnostic overshadowing. AMA J Ethics. 2017;19(1):23–34.
- Ravi A, Pfeiffer MR, Rosner Z, Shea JA. Trafficking and trauma: insight and advice for the healthcare system from sex-trafficked women incarcerated on Rikers Island. Med Care. 2017;55(12):1017–1022.
- Macias-Konstantopoulos W. Human trafficking: the role of medicine in interrupting the cycle of abuse and violence. Ann Intern Med. 2016:165(8):582–588.
- Chung RJ, English A. Commercial sexual exploitation and sex trafficking of adolescents. Curr Opin Pediatr. 2015;27(4):427–433.
- Resources: Screening tool for victims of human trafficking. Washington, DC: US Department of Health and Human Services. https://www.justice.gov/sites/default/files/usao-ndia/legacy/2011/10/14/health_screen_questions.pdf. Accessed May 30, 2018.
- US Department of Health and Human Services. Adult human trafficking screening tool and guide. January 2018. https://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Accessed May 30, 2018.
- Baldwin SB, Eisenman DP, Sayles JN, Ryan G, Chuang KS. Identification of human trafficking victims in health care settings. Health Hum Rights. 2011;13(1):e36–e49.
- Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in health-care facilities. Ann Health Law. 2014;23:61–91.
- Geynisman-Tan JM, Taylor JS, Edersheim T, Taubel D. All the darkness we don’t see. Am J Obstet Gynecol. 2017;216(2):135.e1–e5.
- National Human Trafficking Hotline. https://humantraffickinghotline.org. Accessed May 30, 2018.

Despite increasing media coverage of human trafficking and the gravity of its many ramifications, I am struck by how often trainees and other clinicians present to me patients for which trafficking is a real potential concern—yet who give me a blank expression when I ask if anyone has screened these patients for being victims of trafficking. I suspect that few of us anticipated, during medical training, that we would be providing care to women who are enslaved.
How large is the problem?
It is impossible to comprehend the true scope of human trafficking. Estimates are that 20.9 million men, women, and children globally are forced into work that they are not free to leave.1
Although human trafficking is recognized as a global phenomenon, its prevalence in the United States is significant enough that it should prompt the health care community to engage in helping identify and assist victims/survivors: From January until June of 2017, the National Human Trafficking Hotline received 13,807 telephone calls, resulting in reporting of 4,460 cases.2 Indeed, from 2015 to 2016 there was a 35.7% increase in the number of hotline cases reported, for a total of 7,572 (6,340—more than 80%—of which regarded females). California had the most cases reported (1,323), followed by Texas (670) and Florida (550); those 3 states also reported an increase in trafficking crime. Vermont (5), Rhode Island (9), and Alaska (10) reported the fewest calls.3
How is trafficking defined?
The United Nations Office on Drugs and Crime defines “trafficking in persons” as:
… recruitment, transportation, transfer, harbouring or receipt of persons, by means of the threat or use of force or other forms of coercion, of abduction, of fraud, of deception, of the abuse of power or of a position of vulnerability or of the giving or receiving of payments or benefits to achieve the consent of a person having control over another person, for the purpose of exploitation. Exploitation shall include, at a minimum, the exploitation of the prostitution of others or other forms of sexual exploitation, forced labour or services, slavery or practices similar to slavery, servitude or the removal of organs.4
Traffickers prey on potentially vulnerable people. Girls and young women who have experienced poverty, homelessness, childhood sexual abuse, substance abuse, gender nonconformity, mental illness, or developmental delay are at particular risk.5 Children who have had interactions with Child Protective Services, come from a dysfunctional family, or have lived in a community with high crime, political or social unrest, corruption, or gender bias and discrimination are also at increased risk.6
Read about clues that raise clinical suspicion
Clues that raise clinical suspicion
A number of potential signs should make providers suspicious about potential human trafficking. Some of those signs are similar to the red flags we see in intimate partner violence, such as:
- having a difficult time talking to the patient alone
- having the accompanying person answer the patient’s questions
- body language that suggests fear, anxiety, or distrust (eg, shifting positions, looking away, appearing withdrawn)
- physical examination inconsistent with the history
- physical injury (especially multiple injuries or injuries in various stages of healing)
- refusal of interpreter services.
Trafficked girls or women may appear overly familiar with sex, have unexpected material possessions, or appear to be giving scripted or memorized answers to queries.7 Traffickers often confiscate their victims’ personal identification. They try to prevent victims from knowing their geographic locales: Patients might not have any documentation or awareness of exact surroundings (eg, their home address). Patients may be wearing clothes considered inappropriate for the weather or venue. They may have tattoos that are marks of branding.8
Medical consequences of being trafficked are obvious, numerous, and serious
Many medical sequelae that result from trafficking are obvious, given the nature of work that victims are forced to do. For example, overcrowding can lead to infectious disease, such as tuberculosis.9 Inadequate access to preventive or basic medical services can result in weight loss, poor dentition, and untreated chronic medical conditions.
If victims are experiencing physical or sexual abuse, they can present with evidence of blunt trauma, ligature marks, skin burns, wounds inflicted by weapons, and vaginal lacerations.10 A study found that 63% of survivors reported at least 10 somatic symptoms, including headache, fatigue, dizziness, back pain, abdominal or pelvic pain, memory loss, and symptoms of genital infectious disease.11
Girls and women being trafficked for sex may experience many of the sequelae of unprotected intercourse: irregular bleeding, unintended pregnancy, unwanted or unsafe pregnancy termination, vaginal trauma, and sexually transmitted infection (STI).12 In a study of trafficking survivors, 38% were HIV-positive.13
Trafficking survivors can suffer myriad mental health conditions, with high rates of depression, anxiety, posttraumatic stress, and suicidal ideation.14 A study of 387 survivors found that 12% had attempted to harm themselves or commit suicide the month before they were interviewed.15
Substance abuse is also a common problem among trafficking victims.16 One survivor interviewed in a recent study said:
It was much more difficult to work sober because I was dealing with emotions or the pain that I was feeling during intercourse, because when you have sex with people 8, 9, 10 times a day, even more than that, it starts to hurt a lot. And being high made it easier to deal with that and also it made it easier for me to get away from my body while it was happening, place my brain somewhere else.17
Because of the substantial risk of mental health problems, including substance abuse, among trafficking survivors, the physical exam of a patient should include careful assessment of demeanor and mental health status. Of course, comprehensive inspection for signs of physical or blunt trauma is paramount.
Read about Patient and staff safety during the visit
Patient and staff safety during the visit
Providers should be aware of potential safety concerns, both for the patient and for the staff. Creative strategies should be utilized to screen the patient in private. The use of interpreter services—either in person or over the telephone—should be presented and facilitated as being a routine part of practice. Any person who accompanies the patient should be asked to leave the examining room, either as a statement of practice routine or under the guise of having him (or her) step out to obtain paperwork or provide documentation.
Care of victims
Trauma-informed care should be a guiding principle for trafficking survivors. This involves empowering the patient, who may feel victimized again if asked to undress and undergo multiple physical examinations. Macias-Konstantopoulos noted: “A trauma-informed approach to care acknowledges the pervasiveness and effect of trauma across the life span of the individual, recognizes the vulnerabilities and emotional triggers of trauma survivors, minimizes repeated traumatization and fosters physical, psychological, and emotional safety, recovery, health and well-being.”18
The patient should be counseled that she has control over her body and can guide different aspects of the examination. For example the provider should discuss: 1) the amount of clothing deemed optimal for an examination, 2) the availability of a support person during the exam (for instance, a nurse or a social worker) if the patient requests one, and 3) utilization of whatever strategies the patient deems optimal for her to be most comfortable during the exam (such as leaving the door slightly ajar or having a mutually agreed-on signal to interrupt the exam).
Routine health care maintenance should be offered, including an assessment of overall physical and dental health and screening for STI and mental health. Screening for substances of abuse should be considered. If indicated, emergency contraception, postexposure HIV prophylaxis, immunizations, and empiric antibiotics for STI should be offered.19
Screening when indicated by evidence, suspicion, or concern
Unlike the case with intimate partner violence, experts do not recommend universal screening for human trafficking. Clinicians should be comfortable, however, trying to elicit that history when a concern arises, either because of identified risk factors, red flags, or concerns that arise from the findings of the history or physical. Ideally, clinicians should consider becoming comfortable choosing a few screening questions to regularly incorporate into their assessment. The US Department of Health & Human Services (HHS) offers a list of questions that can be utilized (TABLE).20
In January 2018, the Office on Trafficking in Persons, a unit of the HHS Administration for Children and Families, released an “Adult Human Trafficking Screening Tool and Guide.” The document includes 2 excellent tools21 that clinicians can utilize to identify patients who should be screened and how to identify and assist survivors (FIGURE 1 and FIGURE 2).
Clinicians, in their encounters with patients, are particularly well-positioned to intersect with, and identify, survivors. Regrettably, such opportunities are often missed—and victims thus remain unidentified and trapped in their circumstances. A study revealed that one-half of survivors who were interviewed reported seeing a physician while they were being trafficked.22 Even more alarming, another study showed that 87.8% of survivors had received health care during their captivity.23 It is dismaying to know that these patients left those health care settings without receiving the assistance they truly need and with their true circumstances remaining unidentified.
Read about Finding assistance and support
Finding assistance and support
Centers in the United States now provide trauma-informed care for trafficking survivors in a confidential setting (see “Specialized care is increasingly available”).24 A physician who works at a center in New York City noted: “Our survivors told us that more than fear or pain, the feelings that sat with them most often were worthlessness and invisibility. We can do better as physicians and as educators to expose this epidemic and care for its victims.”24
Here is a sampling of the growing number of centers in the United States that provide trauma-centered care for survivors of human trafficking:
- Survivor Clinic at New York Presbyterian Hospital-Weill Cornell Medical College, New York, New York
- EMPOWER Clinic for Survivors of Sex Trafficking and Sexual Violence at NYU Langone Health, New York, New York
- Freedom Clinic at Massachusetts General Hospital, Boston
- The Hope Through Health Clinic, Austin, Texas
- Pacific Survivor Center, Honolulu, Hawaii
Most clinicians practice in settings that do not have easy access to such subspecialized centers, however. For them, the National Human Trafficking Hotline can be an invaluable resource (see “Hotline is a valuable resource”).25 Law enforcement and social services colleagues also can be useful allies.
Uncertain how you can help a patient who is a victim of human trafficking? For assistance and support, contact the National Human Trafficking Hotline--24 hours a day, 7 days a week, and in 200 languages--in any of 3 ways:
- By telephone: (888) 373-7888
- By text: 233733
- On the web: https://humantraffickinghotline.orga
aIncludes a search field that clinicians can use to look up the nearest resources for additional assistance.
Let’s turn our concern and awareness into results
We, as providers of women’s health care, are uniquely positioned to help these most vulnerable of people, many of whom have been stripped of personal documents and denied access to financial resources and community support. As a medical community, we should strive to combat this tragic epidemic, 1 patient at a time.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.

Despite increasing media coverage of human trafficking and the gravity of its many ramifications, I am struck by how often trainees and other clinicians present to me patients for which trafficking is a real potential concern—yet who give me a blank expression when I ask if anyone has screened these patients for being victims of trafficking. I suspect that few of us anticipated, during medical training, that we would be providing care to women who are enslaved.
How large is the problem?
It is impossible to comprehend the true scope of human trafficking. Estimates are that 20.9 million men, women, and children globally are forced into work that they are not free to leave.1
Although human trafficking is recognized as a global phenomenon, its prevalence in the United States is significant enough that it should prompt the health care community to engage in helping identify and assist victims/survivors: From January until June of 2017, the National Human Trafficking Hotline received 13,807 telephone calls, resulting in reporting of 4,460 cases.2 Indeed, from 2015 to 2016 there was a 35.7% increase in the number of hotline cases reported, for a total of 7,572 (6,340—more than 80%—of which regarded females). California had the most cases reported (1,323), followed by Texas (670) and Florida (550); those 3 states also reported an increase in trafficking crime. Vermont (5), Rhode Island (9), and Alaska (10) reported the fewest calls.3
How is trafficking defined?
The United Nations Office on Drugs and Crime defines “trafficking in persons” as:
… recruitment, transportation, transfer, harbouring or receipt of persons, by means of the threat or use of force or other forms of coercion, of abduction, of fraud, of deception, of the abuse of power or of a position of vulnerability or of the giving or receiving of payments or benefits to achieve the consent of a person having control over another person, for the purpose of exploitation. Exploitation shall include, at a minimum, the exploitation of the prostitution of others or other forms of sexual exploitation, forced labour or services, slavery or practices similar to slavery, servitude or the removal of organs.4
Traffickers prey on potentially vulnerable people. Girls and young women who have experienced poverty, homelessness, childhood sexual abuse, substance abuse, gender nonconformity, mental illness, or developmental delay are at particular risk.5 Children who have had interactions with Child Protective Services, come from a dysfunctional family, or have lived in a community with high crime, political or social unrest, corruption, or gender bias and discrimination are also at increased risk.6
Read about clues that raise clinical suspicion
Clues that raise clinical suspicion
A number of potential signs should make providers suspicious about potential human trafficking. Some of those signs are similar to the red flags we see in intimate partner violence, such as:
- having a difficult time talking to the patient alone
- having the accompanying person answer the patient’s questions
- body language that suggests fear, anxiety, or distrust (eg, shifting positions, looking away, appearing withdrawn)
- physical examination inconsistent with the history
- physical injury (especially multiple injuries or injuries in various stages of healing)
- refusal of interpreter services.
Trafficked girls or women may appear overly familiar with sex, have unexpected material possessions, or appear to be giving scripted or memorized answers to queries.7 Traffickers often confiscate their victims’ personal identification. They try to prevent victims from knowing their geographic locales: Patients might not have any documentation or awareness of exact surroundings (eg, their home address). Patients may be wearing clothes considered inappropriate for the weather or venue. They may have tattoos that are marks of branding.8
Medical consequences of being trafficked are obvious, numerous, and serious
Many medical sequelae that result from trafficking are obvious, given the nature of work that victims are forced to do. For example, overcrowding can lead to infectious disease, such as tuberculosis.9 Inadequate access to preventive or basic medical services can result in weight loss, poor dentition, and untreated chronic medical conditions.
If victims are experiencing physical or sexual abuse, they can present with evidence of blunt trauma, ligature marks, skin burns, wounds inflicted by weapons, and vaginal lacerations.10 A study found that 63% of survivors reported at least 10 somatic symptoms, including headache, fatigue, dizziness, back pain, abdominal or pelvic pain, memory loss, and symptoms of genital infectious disease.11
Girls and women being trafficked for sex may experience many of the sequelae of unprotected intercourse: irregular bleeding, unintended pregnancy, unwanted or unsafe pregnancy termination, vaginal trauma, and sexually transmitted infection (STI).12 In a study of trafficking survivors, 38% were HIV-positive.13
Trafficking survivors can suffer myriad mental health conditions, with high rates of depression, anxiety, posttraumatic stress, and suicidal ideation.14 A study of 387 survivors found that 12% had attempted to harm themselves or commit suicide the month before they were interviewed.15
Substance abuse is also a common problem among trafficking victims.16 One survivor interviewed in a recent study said:
It was much more difficult to work sober because I was dealing with emotions or the pain that I was feeling during intercourse, because when you have sex with people 8, 9, 10 times a day, even more than that, it starts to hurt a lot. And being high made it easier to deal with that and also it made it easier for me to get away from my body while it was happening, place my brain somewhere else.17
Because of the substantial risk of mental health problems, including substance abuse, among trafficking survivors, the physical exam of a patient should include careful assessment of demeanor and mental health status. Of course, comprehensive inspection for signs of physical or blunt trauma is paramount.
Read about Patient and staff safety during the visit
Patient and staff safety during the visit
Providers should be aware of potential safety concerns, both for the patient and for the staff. Creative strategies should be utilized to screen the patient in private. The use of interpreter services—either in person or over the telephone—should be presented and facilitated as being a routine part of practice. Any person who accompanies the patient should be asked to leave the examining room, either as a statement of practice routine or under the guise of having him (or her) step out to obtain paperwork or provide documentation.
Care of victims
Trauma-informed care should be a guiding principle for trafficking survivors. This involves empowering the patient, who may feel victimized again if asked to undress and undergo multiple physical examinations. Macias-Konstantopoulos noted: “A trauma-informed approach to care acknowledges the pervasiveness and effect of trauma across the life span of the individual, recognizes the vulnerabilities and emotional triggers of trauma survivors, minimizes repeated traumatization and fosters physical, psychological, and emotional safety, recovery, health and well-being.”18
The patient should be counseled that she has control over her body and can guide different aspects of the examination. For example the provider should discuss: 1) the amount of clothing deemed optimal for an examination, 2) the availability of a support person during the exam (for instance, a nurse or a social worker) if the patient requests one, and 3) utilization of whatever strategies the patient deems optimal for her to be most comfortable during the exam (such as leaving the door slightly ajar or having a mutually agreed-on signal to interrupt the exam).
Routine health care maintenance should be offered, including an assessment of overall physical and dental health and screening for STI and mental health. Screening for substances of abuse should be considered. If indicated, emergency contraception, postexposure HIV prophylaxis, immunizations, and empiric antibiotics for STI should be offered.19
Screening when indicated by evidence, suspicion, or concern
Unlike the case with intimate partner violence, experts do not recommend universal screening for human trafficking. Clinicians should be comfortable, however, trying to elicit that history when a concern arises, either because of identified risk factors, red flags, or concerns that arise from the findings of the history or physical. Ideally, clinicians should consider becoming comfortable choosing a few screening questions to regularly incorporate into their assessment. The US Department of Health & Human Services (HHS) offers a list of questions that can be utilized (TABLE).20
In January 2018, the Office on Trafficking in Persons, a unit of the HHS Administration for Children and Families, released an “Adult Human Trafficking Screening Tool and Guide.” The document includes 2 excellent tools21 that clinicians can utilize to identify patients who should be screened and how to identify and assist survivors (FIGURE 1 and FIGURE 2).
Clinicians, in their encounters with patients, are particularly well-positioned to intersect with, and identify, survivors. Regrettably, such opportunities are often missed—and victims thus remain unidentified and trapped in their circumstances. A study revealed that one-half of survivors who were interviewed reported seeing a physician while they were being trafficked.22 Even more alarming, another study showed that 87.8% of survivors had received health care during their captivity.23 It is dismaying to know that these patients left those health care settings without receiving the assistance they truly need and with their true circumstances remaining unidentified.
Read about Finding assistance and support
Finding assistance and support
Centers in the United States now provide trauma-informed care for trafficking survivors in a confidential setting (see “Specialized care is increasingly available”).24 A physician who works at a center in New York City noted: “Our survivors told us that more than fear or pain, the feelings that sat with them most often were worthlessness and invisibility. We can do better as physicians and as educators to expose this epidemic and care for its victims.”24
Here is a sampling of the growing number of centers in the United States that provide trauma-centered care for survivors of human trafficking:
- Survivor Clinic at New York Presbyterian Hospital-Weill Cornell Medical College, New York, New York
- EMPOWER Clinic for Survivors of Sex Trafficking and Sexual Violence at NYU Langone Health, New York, New York
- Freedom Clinic at Massachusetts General Hospital, Boston
- The Hope Through Health Clinic, Austin, Texas
- Pacific Survivor Center, Honolulu, Hawaii
Most clinicians practice in settings that do not have easy access to such subspecialized centers, however. For them, the National Human Trafficking Hotline can be an invaluable resource (see “Hotline is a valuable resource”).25 Law enforcement and social services colleagues also can be useful allies.
Uncertain how you can help a patient who is a victim of human trafficking? For assistance and support, contact the National Human Trafficking Hotline--24 hours a day, 7 days a week, and in 200 languages--in any of 3 ways:
- By telephone: (888) 373-7888
- By text: 233733
- On the web: https://humantraffickinghotline.orga
aIncludes a search field that clinicians can use to look up the nearest resources for additional assistance.
Let’s turn our concern and awareness into results
We, as providers of women’s health care, are uniquely positioned to help these most vulnerable of people, many of whom have been stripped of personal documents and denied access to financial resources and community support. As a medical community, we should strive to combat this tragic epidemic, 1 patient at a time.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- International Labour Organization. New ILO Global Estimate of Forced Labour: 20.9 million victims. http://www.ilo.org/global/about-the-ilo/newsroom/news/WCMS_182109/lang--en/index.htm. Published June 2012. Accessed May 30, 2018.
- National Human Trafficking Hotline. Hotline statistics. https://humantraffickinghotline.org/states. Accessed May 30, 2018.
- Cone A. Report: Human trafficking in U.S. rose 35.7 percent in one year. United Press International (UPI). https://www.upi.com/Report-Human-trafficking-in-US-rose-357-percent-in-one-year/5571486328579. Published February 5, 2017. Accessed May 30, 2018.
- United Nations Office on Drugs and Crime. Human trafficking. http://www.unodc.org/unodc/en/human-trafficking/what-is-human-trafficking.html. Accessed May 30, 2018.
- Risk factors for and consequences of commercial sexual exploitation and sex trafficking of minors. In Clayton E, Krugman R, Simon P, eds; Committee on the Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States; Board on Children, Youth, and Families; Committee on Law and Justice; Institute of Medicine; National Research Council. Confronting Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States. Washington, DC: The National Academies Press; 2013.
- Greenbaum J, Crawford-Jakubiak JE. Committee on Child Abuse and Neglect. Child sex trafficking and commercial sexual exploitation: health care needs of victims. Pediatrics. 2015:135(3);566–574.
- Alpert E, Ahn R, Albright E, Purcell G, Burke T, Macias-Konstantanopoulos W. Human Trafficking: Guidebook on Identification, Assessment, and Response in the Health Care Setting. Waltham, MA: Massachusetts General Hospital and Massachusetts Medical Society; 2014. http://www.massmed.org/Patient-Care/Health-Topics/Violence-Prevention-and-Intervention/Human-Trafficking-(pdf). Accessed May 30, 2018.
- National Human Trafficking Training and Technical Assistance Center. Adult human trafficking screening tool and guide. http://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Published January 2018. Accessed May 30, 2018.
- Steele S. Human trafficking, labor brokering, and mining in southern Africa: responding to a decentralized and hidden public health disaster. Int J Health Serv. 2013;43(4):665–680.
- Becker HJ, Bechtel K. Recognizing victims of human trafficking in the pediatric emergency department. Pediatr Emerg Care. 2015;31(2):144–147.
- Zimmerman C, Hossain M, Yun K, et al. The health of trafficked women: a survey of women entering postrafficking services in Europe. Am J Public Health. 2008;98(1):55–59.
- Tracy EE, Macias-Konstantopoulos W. Identifying and assisting sexually exploited and trafficked patients seeking women’s health care services. Obstet Gynecol. 2017;130(2):443–453.
- Silverman JG, Decker MR, Gupta J, Maheshwari A, Willis BM, Raj A. HIV prevalence and predictors of infection in sex-trafficked Nepalese girls and women. JAMA. 2007;298(5):536–542.
- Rafferty Y. Child trafficking and commercial sexual exploitation: a review of promising prevention policies and programs. Am J Orthopsychiatry. 2013;83(4):559–575.
- Kiss L, Yun K, Pocock N, Zimmerman C. Exploitation, violence, and suicide risk among child and adolescent survivors of human trafficking in the Greater Mekong Subregion. JAMA Pediatr. 2015;169(9):e152278.
- Stoklosa H, MacGibbon M, Stoklosa J. Human trafficking, mental illness, and addiction: avoiding diagnostic overshadowing. AMA J Ethics. 2017;19(1):23–34.
- Ravi A, Pfeiffer MR, Rosner Z, Shea JA. Trafficking and trauma: insight and advice for the healthcare system from sex-trafficked women incarcerated on Rikers Island. Med Care. 2017;55(12):1017–1022.
- Macias-Konstantopoulos W. Human trafficking: the role of medicine in interrupting the cycle of abuse and violence. Ann Intern Med. 2016:165(8):582–588.
- Chung RJ, English A. Commercial sexual exploitation and sex trafficking of adolescents. Curr Opin Pediatr. 2015;27(4):427–433.
- Resources: Screening tool for victims of human trafficking. Washington, DC: US Department of Health and Human Services. https://www.justice.gov/sites/default/files/usao-ndia/legacy/2011/10/14/health_screen_questions.pdf. Accessed May 30, 2018.
- US Department of Health and Human Services. Adult human trafficking screening tool and guide. January 2018. https://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Accessed May 30, 2018.
- Baldwin SB, Eisenman DP, Sayles JN, Ryan G, Chuang KS. Identification of human trafficking victims in health care settings. Health Hum Rights. 2011;13(1):e36–e49.
- Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in health-care facilities. Ann Health Law. 2014;23:61–91.
- Geynisman-Tan JM, Taylor JS, Edersheim T, Taubel D. All the darkness we don’t see. Am J Obstet Gynecol. 2017;216(2):135.e1–e5.
- National Human Trafficking Hotline. https://humantraffickinghotline.org. Accessed May 30, 2018.
- International Labour Organization. New ILO Global Estimate of Forced Labour: 20.9 million victims. http://www.ilo.org/global/about-the-ilo/newsroom/news/WCMS_182109/lang--en/index.htm. Published June 2012. Accessed May 30, 2018.
- National Human Trafficking Hotline. Hotline statistics. https://humantraffickinghotline.org/states. Accessed May 30, 2018.
- Cone A. Report: Human trafficking in U.S. rose 35.7 percent in one year. United Press International (UPI). https://www.upi.com/Report-Human-trafficking-in-US-rose-357-percent-in-one-year/5571486328579. Published February 5, 2017. Accessed May 30, 2018.
- United Nations Office on Drugs and Crime. Human trafficking. http://www.unodc.org/unodc/en/human-trafficking/what-is-human-trafficking.html. Accessed May 30, 2018.
- Risk factors for and consequences of commercial sexual exploitation and sex trafficking of minors. In Clayton E, Krugman R, Simon P, eds; Committee on the Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States; Board on Children, Youth, and Families; Committee on Law and Justice; Institute of Medicine; National Research Council. Confronting Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States. Washington, DC: The National Academies Press; 2013.
- Greenbaum J, Crawford-Jakubiak JE. Committee on Child Abuse and Neglect. Child sex trafficking and commercial sexual exploitation: health care needs of victims. Pediatrics. 2015:135(3);566–574.
- Alpert E, Ahn R, Albright E, Purcell G, Burke T, Macias-Konstantanopoulos W. Human Trafficking: Guidebook on Identification, Assessment, and Response in the Health Care Setting. Waltham, MA: Massachusetts General Hospital and Massachusetts Medical Society; 2014. http://www.massmed.org/Patient-Care/Health-Topics/Violence-Prevention-and-Intervention/Human-Trafficking-(pdf). Accessed May 30, 2018.
- National Human Trafficking Training and Technical Assistance Center. Adult human trafficking screening tool and guide. http://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Published January 2018. Accessed May 30, 2018.
- Steele S. Human trafficking, labor brokering, and mining in southern Africa: responding to a decentralized and hidden public health disaster. Int J Health Serv. 2013;43(4):665–680.
- Becker HJ, Bechtel K. Recognizing victims of human trafficking in the pediatric emergency department. Pediatr Emerg Care. 2015;31(2):144–147.
- Zimmerman C, Hossain M, Yun K, et al. The health of trafficked women: a survey of women entering postrafficking services in Europe. Am J Public Health. 2008;98(1):55–59.
- Tracy EE, Macias-Konstantopoulos W. Identifying and assisting sexually exploited and trafficked patients seeking women’s health care services. Obstet Gynecol. 2017;130(2):443–453.
- Silverman JG, Decker MR, Gupta J, Maheshwari A, Willis BM, Raj A. HIV prevalence and predictors of infection in sex-trafficked Nepalese girls and women. JAMA. 2007;298(5):536–542.
- Rafferty Y. Child trafficking and commercial sexual exploitation: a review of promising prevention policies and programs. Am J Orthopsychiatry. 2013;83(4):559–575.
- Kiss L, Yun K, Pocock N, Zimmerman C. Exploitation, violence, and suicide risk among child and adolescent survivors of human trafficking in the Greater Mekong Subregion. JAMA Pediatr. 2015;169(9):e152278.
- Stoklosa H, MacGibbon M, Stoklosa J. Human trafficking, mental illness, and addiction: avoiding diagnostic overshadowing. AMA J Ethics. 2017;19(1):23–34.
- Ravi A, Pfeiffer MR, Rosner Z, Shea JA. Trafficking and trauma: insight and advice for the healthcare system from sex-trafficked women incarcerated on Rikers Island. Med Care. 2017;55(12):1017–1022.
- Macias-Konstantopoulos W. Human trafficking: the role of medicine in interrupting the cycle of abuse and violence. Ann Intern Med. 2016:165(8):582–588.
- Chung RJ, English A. Commercial sexual exploitation and sex trafficking of adolescents. Curr Opin Pediatr. 2015;27(4):427–433.
- Resources: Screening tool for victims of human trafficking. Washington, DC: US Department of Health and Human Services. https://www.justice.gov/sites/default/files/usao-ndia/legacy/2011/10/14/health_screen_questions.pdf. Accessed May 30, 2018.
- US Department of Health and Human Services. Adult human trafficking screening tool and guide. January 2018. https://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Accessed May 30, 2018.
- Baldwin SB, Eisenman DP, Sayles JN, Ryan G, Chuang KS. Identification of human trafficking victims in health care settings. Health Hum Rights. 2011;13(1):e36–e49.
- Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in health-care facilities. Ann Health Law. 2014;23:61–91.
- Geynisman-Tan JM, Taylor JS, Edersheim T, Taubel D. All the darkness we don’t see. Am J Obstet Gynecol. 2017;216(2):135.e1–e5.
- National Human Trafficking Hotline. https://humantraffickinghotline.org. Accessed May 30, 2018.
IN THIS ARTICLE
- Clues to raise suspicion
- Medical consequences of trafficking
- Screening algorithm
Supreme Court supports anti-abortion centers in free speech case
The ruling protects people from being forced to express a message that violates their beliefs, said Michael Farris, president for Alliance Defending Freedom and counsel for the National Institute of Family and Life Advocates (NIFLA), the lead plaintiff.
“In this case, the government used its power to force pro-life pregnancy centers to provide free advertising for abortion,” Mr. Farris said in a statement. “The Supreme Court said that the government can’t do that and that it must respect pro-life beliefs. Tolerance and respect for good-faith differences of opinion are essential in a diverse society like ours. They enable us to coexist peacefully with one another. If we want to have freedom for ourselves, we have to extend it to others.”
California Attorney General Xavier Becerra expressed disappointment at the Supreme Court’s decision, saying the opinion complicates the state’s efforts to empower women with information about their health care.
“When it comes to making their health decisions, all California women – regardless of their economic background or zip code – deserve access to critical and nonbiased information to make their own informed decisions,” Mr. Becerra said in a statement. The “ruling is unfortunate, but our work to ensure that Californians receive accurate information about their health care options will continue.”
The legal challenge began after California passed its 2016 Reproductive Freedom, Accountability, Comprehensive Care, and Transparency (FACT) Act, which requires that pregnancy centers – many of which are anti-abortion – post notices about where patients can find free or low-cost abortion services. Another part of the law requires unlicensed pregnancy centers to disclose to women that they are not a licensed medical facility and have no medical professionals on staff.
The plaintiffs, led by the nonprofit NIFLA, argued that the law violated the First Amendment by requiring speech and because the measure unfairly targets centers that discourage abortions. California officials contended that the law was needed to address concerns that nonprofit organizations, often religious, were misrepresenting themselves as full-service reproductive health clinics and misleading women about their options.
However, in the court’s majority opinion, Associate Justice Clarence Thomas wrote that California can inform women about abortion services through other means, such as advertising, rather than burdening the plaintiffs with compelled speech.
“California cannot co-opt the licensed facilities to deliver its message for it,” he wrote. The “First Amendment does not permit the state to sacrifice speech for efficiency.”
In a concurring opinion, Associate Justice Anthony Kennedy wrote that the California law is a paradigmatic example of the serious threat presented when government seeks to impose its own message in the place of individual speech, thought, and expression.
“Governments must not be allowed to force persons to express a message contrary to their deepest convictions,” Justice Kennedy wrote. “Freedom of speech secures freedom of thought and belief. This law imperils those liberties.”
In a dissenting opinion, Associate Justice Stephen Breyer wrote that the high court’s majority stance contradicts a previous decision in which justices required physicians who performed abortions to give information about adoption services.
“If a state can lawfully require a doctor to tell a woman seeking an abortion about adoption services, why should it not be able, as here, to require a medical counselor to tell a woman seeking prenatal care or other reproductive health care about childbirth and abortion services?” he asked. “As the question suggests, there is no convincing reason to distinguish between information about adoption and information about abortion in this context.”
The Supreme Court’s decision has broad implications in the health care setting and other sectors that physicians and other professionals should celebrate, said Robert McNamara, a senior attorney for the Institute for Justice in Arlington, Va. The professional-speech doctrine that the Supreme Court rejected in the case posed a serious danger to the free-speech rights of health providers, Mr. McNamara said in an interview.
However, Heather Shumaker, senior counsel for reproductive rights and health at the National Women’s Law Center, said the ruling is detrimental to patients’ health care and chills their access to truthful, accurate medical information.
“Throughout the country, anti-abortion counseling centers provide false, misleading, or incomplete information, and frighten and coerce women to make certain decisions about their health care options,” Ms. Shumaker said in an interview. “This deception endangers women’s health and future fertility, and particularly burdens women of color and women struggling to make ends meet. It is devastating that [the] decision will make access to full reproductive health care more difficult.”
The American College of Obstetricians and Gynecologists (ACOG) also expressed disappointment at the Supreme Court’s ruling.
“Pregnant women who seek medical guidance must be able to trust that information being provided to them is truthful, medically accurate, and enables them to make informed decisions about their care,” ACOG President Lisa Hollier, MD, said in a statement. “Inaccurate and untruthful information can delay care and increase risk of medical complications.”
The ruling protects people from being forced to express a message that violates their beliefs, said Michael Farris, president for Alliance Defending Freedom and counsel for the National Institute of Family and Life Advocates (NIFLA), the lead plaintiff.
“In this case, the government used its power to force pro-life pregnancy centers to provide free advertising for abortion,” Mr. Farris said in a statement. “The Supreme Court said that the government can’t do that and that it must respect pro-life beliefs. Tolerance and respect for good-faith differences of opinion are essential in a diverse society like ours. They enable us to coexist peacefully with one another. If we want to have freedom for ourselves, we have to extend it to others.”
California Attorney General Xavier Becerra expressed disappointment at the Supreme Court’s decision, saying the opinion complicates the state’s efforts to empower women with information about their health care.
“When it comes to making their health decisions, all California women – regardless of their economic background or zip code – deserve access to critical and nonbiased information to make their own informed decisions,” Mr. Becerra said in a statement. The “ruling is unfortunate, but our work to ensure that Californians receive accurate information about their health care options will continue.”
The legal challenge began after California passed its 2016 Reproductive Freedom, Accountability, Comprehensive Care, and Transparency (FACT) Act, which requires that pregnancy centers – many of which are anti-abortion – post notices about where patients can find free or low-cost abortion services. Another part of the law requires unlicensed pregnancy centers to disclose to women that they are not a licensed medical facility and have no medical professionals on staff.
The plaintiffs, led by the nonprofit NIFLA, argued that the law violated the First Amendment by requiring speech and because the measure unfairly targets centers that discourage abortions. California officials contended that the law was needed to address concerns that nonprofit organizations, often religious, were misrepresenting themselves as full-service reproductive health clinics and misleading women about their options.
However, in the court’s majority opinion, Associate Justice Clarence Thomas wrote that California can inform women about abortion services through other means, such as advertising, rather than burdening the plaintiffs with compelled speech.
“California cannot co-opt the licensed facilities to deliver its message for it,” he wrote. The “First Amendment does not permit the state to sacrifice speech for efficiency.”
In a concurring opinion, Associate Justice Anthony Kennedy wrote that the California law is a paradigmatic example of the serious threat presented when government seeks to impose its own message in the place of individual speech, thought, and expression.
“Governments must not be allowed to force persons to express a message contrary to their deepest convictions,” Justice Kennedy wrote. “Freedom of speech secures freedom of thought and belief. This law imperils those liberties.”
In a dissenting opinion, Associate Justice Stephen Breyer wrote that the high court’s majority stance contradicts a previous decision in which justices required physicians who performed abortions to give information about adoption services.
“If a state can lawfully require a doctor to tell a woman seeking an abortion about adoption services, why should it not be able, as here, to require a medical counselor to tell a woman seeking prenatal care or other reproductive health care about childbirth and abortion services?” he asked. “As the question suggests, there is no convincing reason to distinguish between information about adoption and information about abortion in this context.”
The Supreme Court’s decision has broad implications in the health care setting and other sectors that physicians and other professionals should celebrate, said Robert McNamara, a senior attorney for the Institute for Justice in Arlington, Va. The professional-speech doctrine that the Supreme Court rejected in the case posed a serious danger to the free-speech rights of health providers, Mr. McNamara said in an interview.
However, Heather Shumaker, senior counsel for reproductive rights and health at the National Women’s Law Center, said the ruling is detrimental to patients’ health care and chills their access to truthful, accurate medical information.
“Throughout the country, anti-abortion counseling centers provide false, misleading, or incomplete information, and frighten and coerce women to make certain decisions about their health care options,” Ms. Shumaker said in an interview. “This deception endangers women’s health and future fertility, and particularly burdens women of color and women struggling to make ends meet. It is devastating that [the] decision will make access to full reproductive health care more difficult.”
The American College of Obstetricians and Gynecologists (ACOG) also expressed disappointment at the Supreme Court’s ruling.
“Pregnant women who seek medical guidance must be able to trust that information being provided to them is truthful, medically accurate, and enables them to make informed decisions about their care,” ACOG President Lisa Hollier, MD, said in a statement. “Inaccurate and untruthful information can delay care and increase risk of medical complications.”
The ruling protects people from being forced to express a message that violates their beliefs, said Michael Farris, president for Alliance Defending Freedom and counsel for the National Institute of Family and Life Advocates (NIFLA), the lead plaintiff.
“In this case, the government used its power to force pro-life pregnancy centers to provide free advertising for abortion,” Mr. Farris said in a statement. “The Supreme Court said that the government can’t do that and that it must respect pro-life beliefs. Tolerance and respect for good-faith differences of opinion are essential in a diverse society like ours. They enable us to coexist peacefully with one another. If we want to have freedom for ourselves, we have to extend it to others.”
California Attorney General Xavier Becerra expressed disappointment at the Supreme Court’s decision, saying the opinion complicates the state’s efforts to empower women with information about their health care.
“When it comes to making their health decisions, all California women – regardless of their economic background or zip code – deserve access to critical and nonbiased information to make their own informed decisions,” Mr. Becerra said in a statement. The “ruling is unfortunate, but our work to ensure that Californians receive accurate information about their health care options will continue.”
The legal challenge began after California passed its 2016 Reproductive Freedom, Accountability, Comprehensive Care, and Transparency (FACT) Act, which requires that pregnancy centers – many of which are anti-abortion – post notices about where patients can find free or low-cost abortion services. Another part of the law requires unlicensed pregnancy centers to disclose to women that they are not a licensed medical facility and have no medical professionals on staff.
The plaintiffs, led by the nonprofit NIFLA, argued that the law violated the First Amendment by requiring speech and because the measure unfairly targets centers that discourage abortions. California officials contended that the law was needed to address concerns that nonprofit organizations, often religious, were misrepresenting themselves as full-service reproductive health clinics and misleading women about their options.
However, in the court’s majority opinion, Associate Justice Clarence Thomas wrote that California can inform women about abortion services through other means, such as advertising, rather than burdening the plaintiffs with compelled speech.
“California cannot co-opt the licensed facilities to deliver its message for it,” he wrote. The “First Amendment does not permit the state to sacrifice speech for efficiency.”
In a concurring opinion, Associate Justice Anthony Kennedy wrote that the California law is a paradigmatic example of the serious threat presented when government seeks to impose its own message in the place of individual speech, thought, and expression.
“Governments must not be allowed to force persons to express a message contrary to their deepest convictions,” Justice Kennedy wrote. “Freedom of speech secures freedom of thought and belief. This law imperils those liberties.”
In a dissenting opinion, Associate Justice Stephen Breyer wrote that the high court’s majority stance contradicts a previous decision in which justices required physicians who performed abortions to give information about adoption services.
“If a state can lawfully require a doctor to tell a woman seeking an abortion about adoption services, why should it not be able, as here, to require a medical counselor to tell a woman seeking prenatal care or other reproductive health care about childbirth and abortion services?” he asked. “As the question suggests, there is no convincing reason to distinguish between information about adoption and information about abortion in this context.”
The Supreme Court’s decision has broad implications in the health care setting and other sectors that physicians and other professionals should celebrate, said Robert McNamara, a senior attorney for the Institute for Justice in Arlington, Va. The professional-speech doctrine that the Supreme Court rejected in the case posed a serious danger to the free-speech rights of health providers, Mr. McNamara said in an interview.
However, Heather Shumaker, senior counsel for reproductive rights and health at the National Women’s Law Center, said the ruling is detrimental to patients’ health care and chills their access to truthful, accurate medical information.
“Throughout the country, anti-abortion counseling centers provide false, misleading, or incomplete information, and frighten and coerce women to make certain decisions about their health care options,” Ms. Shumaker said in an interview. “This deception endangers women’s health and future fertility, and particularly burdens women of color and women struggling to make ends meet. It is devastating that [the] decision will make access to full reproductive health care more difficult.”
The American College of Obstetricians and Gynecologists (ACOG) also expressed disappointment at the Supreme Court’s ruling.
“Pregnant women who seek medical guidance must be able to trust that information being provided to them is truthful, medically accurate, and enables them to make informed decisions about their care,” ACOG President Lisa Hollier, MD, said in a statement. “Inaccurate and untruthful information can delay care and increase risk of medical complications.”
Are we ready for primary HPV testing for the prevention of cervical cancer?
Cervical cancer screening represents one of the great public health successes of the 20th Century. Two physician-scientists, George Papanicolaou, MD, PhD (1883–1962), and Harald zur Hausen, MD (1936–), made extraordinary contributions to the evolution of effective cervical cancer screening programs. Dr. Papanicolaou led development of the iconic Pap smear, creating techniques for collecting specimens and using cytologic techniques to identify cervical cancer and its precursors, and Dr. zur Hausen discovered the association of human papillomavirus (HPV) infection with cervical cancer.1,2
Although it is but a distant memory, in the 1930s cervical and uterine cancer caused more deaths among women than breast, lung, or ovarian cancer. The successful deployment of Pap smear screening resulted in a decrease in cervical cancer rates in developed countries. Cervical cancer deaths remain common in many parts of the world, however. Cervical cancer screening programs can reduce cervical cancer incidence by greater than 80%.3 In the United States between 1973 and 2006, the invasive cervical cancer age-adjusted incidence rates dropped from 10.28 to 3.97 per 100,000 women.4
HPV causes cervical cancer
Dr. zur Hausen dedicated his career to identifying viral causes of human cancer. In his Nobel Laureate autobiography, he reported that during his 2-year rotating residency, he loved his obstetrics and gynecology experience, but found it “physically highly demanding” and decided to focus his career in microbiology and immunology.5 After proving that herpes simplex virus did not cause cervical cancer he began to explore the role of HPV in the disease process. He first identified HPV types 6 and 11 and showed that these agents caused genital warts. He then used low-stringency hybridization techniques to identify HPV types 16 and 18 in specimens of cervical cancer. Later, he and his colleagues proved that two HPV proteins, E6 and E7, interfere with the function of cell cycle control proteins p53 and retinoblastoma protein, resulting in dysregulated cell growth and cancer.2 These findings permitted the development of both HPV vaccines and nucleic acid–based tests to identify high-risk oncogenic HPV (hrHPV) in cells and tissue specimens.
HPV vaccination
Dr. zur Hausen was an energetic and vocal advocate for the development and widescale deployment of HPV vaccines, including vaccination of males and females.6 Initially his ideas were rejected by the pharmaceutical industry, but eventually, with advances in virology and vaccine development, multiple companies pursued the development of HPV vaccines, the first cancer prevention vaccines. The best approach to cervical cancer prevention is intensive population-wide HPV vaccination of both boys and girls before exposure to the HPV virus. Beyond its beneficial effect on the incidence of cervical cancer, HPV vaccination also reduces the population incidence of anal, vulvar, and oropharyngeal cancer.7 Prevention of oropharyngeal cancer is especially important for men, supporting the recommendation for vaccination of all boys.8
Population-wide HPV vaccination will result in a lower prevalence of cervical cancer precursors and reduce the sensitivity of cytology, thereby making primary HPV screening more attractive.9 Based on one modelling study, universal HPV vaccination can reduce cervical cancer rates by greater than 50% over current levels, and introduction of primary HPV screening will reduce cervical cancer rates by an additional 20%.10 In an era of widespread vaccination for HPV, screening for cervical cancer should be intensified for nonvaccinated women.10
Read about Primary cervical cancer screening with cytology
Primary cervical cancer screening with cytology
Primary screening with cervical cytology alone remains an option supported by many authorities and professional society guidelines.11 Most studies report that HPV testing has greater sensitivity than cervical cytology alone, especially for the detection of adenocarcinoma of the cervix.12 In one Canadian study, 10,154 women were randomly assigned to HPV or cervical cytology testing. The sensitivity of HPV testing and cervical cytology for detecting cervical intraepithelial neoplasia grade 2 or 3 was 95% and 55%, respectively, with a specificity of 94% and 97%, respectively.13 When used together the sensitivity and specificity of cotesting was 100% and 93%, respectively, but resulted in an increased number of colposcopies, which may be costly and add stress for the patient. Many countries are beginning to move away from cervical cancer screening with cytology or cotesting to programs built upon a foundation of primary HPV testing.
Primary cervical cancer screening with HPV testing
The knowledge that hrHPV is a more sensitive test for cervical cancer and its precursors, as well as the relatively lower sensitivity of cytology, is the foundation for transitioning from primary screening with cervical cytology to primary screening with HPV testing. In the Netherlands14 and Australia15,16 HPV testing with reflex cytology is the nationwide approach to cervical cancer screening. The basic components of the Dutch primary HPV screening program, as explained by Dr. Lai van Zulyan Mandres, are14:
- Samples are collected by a general practitioner and sent to one of 5 central testing facilities for DNA testing for hrHPV.
- If all previous samples tested negative, the screening occurs at ages 30, 35, 40, 50, and 60 years, a minimum of 5 screens per woman.
- If there is a history of a previously positive hrHPV, the screening is intensified, with additional specimens collected at ages 45, 55, and 60 years.
- If the sample is hrHPV negative, the patient continues screening at the standard intervals. No cytology testing is performed.
- If the sample is hrHPV positive, reflex cytology is performed using the original collected sample. If the cytology shows no intraepithelial lesion or malignancy (NILM), another specimen is obtained for cytology within 6 months. If the second cytology specimen shows atypical squamous cells of undetermined significance (ASCUS) or a more worrisome cytology finding, the patient is sent for colposcopy. If two NILM cytology specimens have been obtained, the patient resumes primary hrHPV screening every 5 years.
- If the specimen is hrHPV positive and cytology is ASCUS or more worrisome the patient is referred for colposcopy (FIGURE).14 The Dutch estimate that primary hrHPV screening will reduce the number of cervical cytology specimens by 90% annually.
Australia also has implemented nationwide primary HPV testing for cervical cancer screening. This change was implemented following a 10-year program of universal school-based vaccination of girls and boys, and biennial cytology screening for all women. The Australian screening program initiates hrHPV testing at age 25 years and thereafter every 5 years until age 74. If the hrHPV test is positive, reflex testing for HPV types 16 and 18 are performed on the original specimen along with cervical cytology. Women who test positive for HPV 16 or 18 are immediately referred for colposcopy. If the hrHPV test is positive and reflex testing for HPV 16 and 18 is negative, cervical cytology demonstrating ASCUS, low- or high-grade squamous intraepithelial lesions, or more worrisome results trigger a referral for colposcopy. The Australian program supports testing of self-collected vaginal samples for women who are underscreened or have never been screened.15,16
Read about Pros and cons of switching approaches
Pros and cons of switching approaches
Deployment of new technology often yields benefits and challenges. A putative benefit of primary HPV screening is a reduction in health care costs without an increase in cervical cancer deaths. Another benefit of primary HPV screening is that it may enable self-collection of specimens for analysis, thereby increasing access to cervical cancer screening for underserved and marginalized populations of women who are not currently participating in cervical cancer screening programs.17 One challenge is that many women are unaware that hrHPV is the cause of most invasive cervical cancers. The detection of hrHPV in a woman in a long-term relationship who was previously negative for hrHPV may cause the emotions of surprise, fear, anxiety, and anger, thereby stressing the relationship.18
Another concern is that many women are worried about no longer receiving the familiar “Pap smear” cancer screening test in which they have tremendous faith. When Australia transitioned to primary HPV screening, more than 70,000 women signed a petition to “save women’s lives” by permitting continued access to the cervical cytology testing.19 Primary HPV testing may result in a transient increase in the number of women referred for colposcopy, potentially overwhelming the capacity of the health care system to deliver this vital service.20,21 The HPV types that most often cause cervical cancer may vary among countries. For example, in Thailand, HPV 52 and 58 are frequently detected in women with high-grade squamous lesions, and including these subtypes in reflex genotyping may be of regional benefit.22
Primary cervical cancer screening with HPV testing: When will it be used widely in the United States?
In contrast to the United States, the Netherlands is a small, densely populated country that has a highly integrated health system with centralized laboratory centers, a nationwide electronic health record, and clinicians organized to perform as an integrated team. These features ensure that all lifetime tests results are available in one record, that HPV testing is highly standardized, and that clinicians will follow a prescribed care pathway. The Netherlands’ health system is organized to support the successful transition, in a single step, to primary HPV testing. The United States is the third most populous country in the world, following China and India, with a diverse approach to health care, a highly mobile population, no single interoperable electronic health record, and minimal central control of clinical practice. The United States is not organized to make a “big bang” transition to primary HPV cervical cancer screening. It is likely that the introduction of primary HPV screening will occur first in highly integrated health systems that control the clinical, laboratory, and electronic records of a large population.
The results of the ATHENA study provide a clear clinical algorithm for implementing a primary HPV screening program for cervical cancer in the United States.23–25 Samples are collected for hrHPV testing at a specified interval, 3 or 5 years, beginning at age 25 years. Women younger than age 25 years should be screened with cytology alone. Detection of hrHPV results in reflex viral typing for HPV 16 and 18. Women with samples positive for HPV 16 and 18 are immediately referred for colposcopy. Samples positive for hrHPV and negative for HPV 16 and 18 have reflex cytology testing performed on the original HPV specimen. If cytology testing reports NILM, repeat cotesting is performed in one year. If cytology testing reports ASCUS or a more concerning result, the woman is referred for colposcopy.
Malcolm Gladwell, in his book The Tipping Point, identified 3 processes that help push an innovative new approach from obscurity into widespread use.26 First, authoritative voices that can catalyze change need to consistently communicate their shared vision for the future. Second, there must be a clear message that galvanizes the many to change their approach. Third, the historical context must be supportive of the change. Over the next decade we are likely to hit a tipping point and transition from cervical cancer screening that relies on cervical cytology to an approach that prioritizes hrHPV testing. When that change will occur in the United States is unclear. But our colleagues in other countries already have transitioned to primary hrHPV testing for cervical cancer screening.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Hinsey JC. George Nicholas Papanicolaou, May 13, 1883–February 19, 1962. Acta Cytol. 1962;6:483–486.
- zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Physicians. 1999;111(6):581–587.
- International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention, Vol 10: Cervix Cancer Screening. Lyon, France: IARC Press; 2005.
- Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Women’s Health (Larchmt). 2012;21(10):1031–1037.
- Harold zur Hausen-Biographical. Nobelprize.org website. https://www.nobelprize.org/nobel_prizes/medicine/laureates/2008/hausen-bio.html. Accessed June 19, 2018.
- Michels KB, zur Hausen H. HPV vaccine for all. Lancet. 2009;374(9686):268–270.
- Hansen BT, Campbell S, Nygaard M. Long-term incidence of HPV-related cancers, and cases preventable by HPV vaccination: a registry-based study in Norway. BMJ Open. 2018;8(2):e019005.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27(5):6–8.
- Massad LS. Anticipating the impact of human papillomavirus vaccination on US cervical cancer prevention strategies. J Low Genit Tract Dis. 2018;22(2):123–125.
- Castanon A, Landy R, Pesola F, Windridge P, Sasieni P. Prediction of cervical cancer incidence in England, UK, up to 2040, under four scenarios: a modeling study. Lancet Public Health. 2018;3(1):e34–e43.
- Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891.
- Moukarzel LA, Angarita AM, VandenBussche C, et al. Preinvasive and invasive cervical adenocarcinoma: preceding low-risk or negative Pap result increases time to diagnosis. J Low Genital Tract Dis. 2017;21(2):91–96.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588.
- van Zuylen-Manders L. Primary HPV screening: The Dutch experience. http://www.britishcytology.org.uk/resources/Primary_HPV_screening_The_Dutch_experience.pdf. Accessed June 19, 2018.
- Hammond I, Canfell K, Saville M. A new era for cervical cancer screening in Australia: watch this space! Aust N Z J Obstet Gynaecol. 2017;57(5):499–501.
- Canfell K, Saville M, Caruana M, et al. Protocol for Compass: a randomised controlled trial of primary HPV testing versus cytology screening for cervical cancer in HPV-unvaccinated and vaccinated women aged 25-69 years living in Australia. BMJ Open. 2018;8(1):e016700.
- Wood B, Lofters A, Vahabi M. Strategies to reach marginalized women for cervical cancer screening: a qualitative study of stakeholder perspectives. Curr Oncol. 2018;25(1):e8–e16.
- Patel H, Moss EL, Sherman SM. HPV primary cervical cancer screening in England: women’s awareness and attitudes. Psychooncology. 2018;27(6):1559–1564.
- Obermair HM, Dodd RH, Bonner C, Jansen J, McCaffery K. “It has saved thousands of lives, so why change it?” Content analysis of objections to cervical cancer screening programme changes in Australia. BMJ Open. 2018;8(2):e019171.
- Hall MT, Simms KT, Lew JB, Smith MA, Saville M, Canfell K. Projected future impact of HPV vaccination and primary HPV screening on cervical cancer rates from 2017-2035: Example from Australia. PLoS One. 2018;13(2):e0185332.
- Rebolj M, Bonde J, Preisler S, Ejegod D, Rygaard C, Lynge E. Human papillomavirus assays and cytology in primary cervical screening of women aged 30 years and above. PLoS One. 2016;11(1):e0147326.
- Khunamornpong S, Settakorn J, Sukpan K, Suprasert P, Srisomboon J, Intaraphet S, Siriaunkgul S. Genotyping for human papillomavirus (HPV) 16/18/52/58 has a higher performance than HPV16/18 genotyping in triaging women with positive high-risk HPV test in Northern Thailand. PLoS One. 2016;11(6):e0158184.
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–337.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Gladwell M. The Tipping Point: How Little Things Can Make a Big Difference. New York, New York: Little Brown; 2000.
Cervical cancer screening represents one of the great public health successes of the 20th Century. Two physician-scientists, George Papanicolaou, MD, PhD (1883–1962), and Harald zur Hausen, MD (1936–), made extraordinary contributions to the evolution of effective cervical cancer screening programs. Dr. Papanicolaou led development of the iconic Pap smear, creating techniques for collecting specimens and using cytologic techniques to identify cervical cancer and its precursors, and Dr. zur Hausen discovered the association of human papillomavirus (HPV) infection with cervical cancer.1,2
Although it is but a distant memory, in the 1930s cervical and uterine cancer caused more deaths among women than breast, lung, or ovarian cancer. The successful deployment of Pap smear screening resulted in a decrease in cervical cancer rates in developed countries. Cervical cancer deaths remain common in many parts of the world, however. Cervical cancer screening programs can reduce cervical cancer incidence by greater than 80%.3 In the United States between 1973 and 2006, the invasive cervical cancer age-adjusted incidence rates dropped from 10.28 to 3.97 per 100,000 women.4
HPV causes cervical cancer
Dr. zur Hausen dedicated his career to identifying viral causes of human cancer. In his Nobel Laureate autobiography, he reported that during his 2-year rotating residency, he loved his obstetrics and gynecology experience, but found it “physically highly demanding” and decided to focus his career in microbiology and immunology.5 After proving that herpes simplex virus did not cause cervical cancer he began to explore the role of HPV in the disease process. He first identified HPV types 6 and 11 and showed that these agents caused genital warts. He then used low-stringency hybridization techniques to identify HPV types 16 and 18 in specimens of cervical cancer. Later, he and his colleagues proved that two HPV proteins, E6 and E7, interfere with the function of cell cycle control proteins p53 and retinoblastoma protein, resulting in dysregulated cell growth and cancer.2 These findings permitted the development of both HPV vaccines and nucleic acid–based tests to identify high-risk oncogenic HPV (hrHPV) in cells and tissue specimens.
HPV vaccination
Dr. zur Hausen was an energetic and vocal advocate for the development and widescale deployment of HPV vaccines, including vaccination of males and females.6 Initially his ideas were rejected by the pharmaceutical industry, but eventually, with advances in virology and vaccine development, multiple companies pursued the development of HPV vaccines, the first cancer prevention vaccines. The best approach to cervical cancer prevention is intensive population-wide HPV vaccination of both boys and girls before exposure to the HPV virus. Beyond its beneficial effect on the incidence of cervical cancer, HPV vaccination also reduces the population incidence of anal, vulvar, and oropharyngeal cancer.7 Prevention of oropharyngeal cancer is especially important for men, supporting the recommendation for vaccination of all boys.8
Population-wide HPV vaccination will result in a lower prevalence of cervical cancer precursors and reduce the sensitivity of cytology, thereby making primary HPV screening more attractive.9 Based on one modelling study, universal HPV vaccination can reduce cervical cancer rates by greater than 50% over current levels, and introduction of primary HPV screening will reduce cervical cancer rates by an additional 20%.10 In an era of widespread vaccination for HPV, screening for cervical cancer should be intensified for nonvaccinated women.10
Read about Primary cervical cancer screening with cytology
Primary cervical cancer screening with cytology
Primary screening with cervical cytology alone remains an option supported by many authorities and professional society guidelines.11 Most studies report that HPV testing has greater sensitivity than cervical cytology alone, especially for the detection of adenocarcinoma of the cervix.12 In one Canadian study, 10,154 women were randomly assigned to HPV or cervical cytology testing. The sensitivity of HPV testing and cervical cytology for detecting cervical intraepithelial neoplasia grade 2 or 3 was 95% and 55%, respectively, with a specificity of 94% and 97%, respectively.13 When used together the sensitivity and specificity of cotesting was 100% and 93%, respectively, but resulted in an increased number of colposcopies, which may be costly and add stress for the patient. Many countries are beginning to move away from cervical cancer screening with cytology or cotesting to programs built upon a foundation of primary HPV testing.
Primary cervical cancer screening with HPV testing
The knowledge that hrHPV is a more sensitive test for cervical cancer and its precursors, as well as the relatively lower sensitivity of cytology, is the foundation for transitioning from primary screening with cervical cytology to primary screening with HPV testing. In the Netherlands14 and Australia15,16 HPV testing with reflex cytology is the nationwide approach to cervical cancer screening. The basic components of the Dutch primary HPV screening program, as explained by Dr. Lai van Zulyan Mandres, are14:
- Samples are collected by a general practitioner and sent to one of 5 central testing facilities for DNA testing for hrHPV.
- If all previous samples tested negative, the screening occurs at ages 30, 35, 40, 50, and 60 years, a minimum of 5 screens per woman.
- If there is a history of a previously positive hrHPV, the screening is intensified, with additional specimens collected at ages 45, 55, and 60 years.
- If the sample is hrHPV negative, the patient continues screening at the standard intervals. No cytology testing is performed.
- If the sample is hrHPV positive, reflex cytology is performed using the original collected sample. If the cytology shows no intraepithelial lesion or malignancy (NILM), another specimen is obtained for cytology within 6 months. If the second cytology specimen shows atypical squamous cells of undetermined significance (ASCUS) or a more worrisome cytology finding, the patient is sent for colposcopy. If two NILM cytology specimens have been obtained, the patient resumes primary hrHPV screening every 5 years.
- If the specimen is hrHPV positive and cytology is ASCUS or more worrisome the patient is referred for colposcopy (FIGURE).14 The Dutch estimate that primary hrHPV screening will reduce the number of cervical cytology specimens by 90% annually.
Australia also has implemented nationwide primary HPV testing for cervical cancer screening. This change was implemented following a 10-year program of universal school-based vaccination of girls and boys, and biennial cytology screening for all women. The Australian screening program initiates hrHPV testing at age 25 years and thereafter every 5 years until age 74. If the hrHPV test is positive, reflex testing for HPV types 16 and 18 are performed on the original specimen along with cervical cytology. Women who test positive for HPV 16 or 18 are immediately referred for colposcopy. If the hrHPV test is positive and reflex testing for HPV 16 and 18 is negative, cervical cytology demonstrating ASCUS, low- or high-grade squamous intraepithelial lesions, or more worrisome results trigger a referral for colposcopy. The Australian program supports testing of self-collected vaginal samples for women who are underscreened or have never been screened.15,16
Read about Pros and cons of switching approaches
Pros and cons of switching approaches
Deployment of new technology often yields benefits and challenges. A putative benefit of primary HPV screening is a reduction in health care costs without an increase in cervical cancer deaths. Another benefit of primary HPV screening is that it may enable self-collection of specimens for analysis, thereby increasing access to cervical cancer screening for underserved and marginalized populations of women who are not currently participating in cervical cancer screening programs.17 One challenge is that many women are unaware that hrHPV is the cause of most invasive cervical cancers. The detection of hrHPV in a woman in a long-term relationship who was previously negative for hrHPV may cause the emotions of surprise, fear, anxiety, and anger, thereby stressing the relationship.18
Another concern is that many women are worried about no longer receiving the familiar “Pap smear” cancer screening test in which they have tremendous faith. When Australia transitioned to primary HPV screening, more than 70,000 women signed a petition to “save women’s lives” by permitting continued access to the cervical cytology testing.19 Primary HPV testing may result in a transient increase in the number of women referred for colposcopy, potentially overwhelming the capacity of the health care system to deliver this vital service.20,21 The HPV types that most often cause cervical cancer may vary among countries. For example, in Thailand, HPV 52 and 58 are frequently detected in women with high-grade squamous lesions, and including these subtypes in reflex genotyping may be of regional benefit.22
Primary cervical cancer screening with HPV testing: When will it be used widely in the United States?
In contrast to the United States, the Netherlands is a small, densely populated country that has a highly integrated health system with centralized laboratory centers, a nationwide electronic health record, and clinicians organized to perform as an integrated team. These features ensure that all lifetime tests results are available in one record, that HPV testing is highly standardized, and that clinicians will follow a prescribed care pathway. The Netherlands’ health system is organized to support the successful transition, in a single step, to primary HPV testing. The United States is the third most populous country in the world, following China and India, with a diverse approach to health care, a highly mobile population, no single interoperable electronic health record, and minimal central control of clinical practice. The United States is not organized to make a “big bang” transition to primary HPV cervical cancer screening. It is likely that the introduction of primary HPV screening will occur first in highly integrated health systems that control the clinical, laboratory, and electronic records of a large population.
The results of the ATHENA study provide a clear clinical algorithm for implementing a primary HPV screening program for cervical cancer in the United States.23–25 Samples are collected for hrHPV testing at a specified interval, 3 or 5 years, beginning at age 25 years. Women younger than age 25 years should be screened with cytology alone. Detection of hrHPV results in reflex viral typing for HPV 16 and 18. Women with samples positive for HPV 16 and 18 are immediately referred for colposcopy. Samples positive for hrHPV and negative for HPV 16 and 18 have reflex cytology testing performed on the original HPV specimen. If cytology testing reports NILM, repeat cotesting is performed in one year. If cytology testing reports ASCUS or a more concerning result, the woman is referred for colposcopy.
Malcolm Gladwell, in his book The Tipping Point, identified 3 processes that help push an innovative new approach from obscurity into widespread use.26 First, authoritative voices that can catalyze change need to consistently communicate their shared vision for the future. Second, there must be a clear message that galvanizes the many to change their approach. Third, the historical context must be supportive of the change. Over the next decade we are likely to hit a tipping point and transition from cervical cancer screening that relies on cervical cytology to an approach that prioritizes hrHPV testing. When that change will occur in the United States is unclear. But our colleagues in other countries already have transitioned to primary hrHPV testing for cervical cancer screening.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Cervical cancer screening represents one of the great public health successes of the 20th Century. Two physician-scientists, George Papanicolaou, MD, PhD (1883–1962), and Harald zur Hausen, MD (1936–), made extraordinary contributions to the evolution of effective cervical cancer screening programs. Dr. Papanicolaou led development of the iconic Pap smear, creating techniques for collecting specimens and using cytologic techniques to identify cervical cancer and its precursors, and Dr. zur Hausen discovered the association of human papillomavirus (HPV) infection with cervical cancer.1,2
Although it is but a distant memory, in the 1930s cervical and uterine cancer caused more deaths among women than breast, lung, or ovarian cancer. The successful deployment of Pap smear screening resulted in a decrease in cervical cancer rates in developed countries. Cervical cancer deaths remain common in many parts of the world, however. Cervical cancer screening programs can reduce cervical cancer incidence by greater than 80%.3 In the United States between 1973 and 2006, the invasive cervical cancer age-adjusted incidence rates dropped from 10.28 to 3.97 per 100,000 women.4
HPV causes cervical cancer
Dr. zur Hausen dedicated his career to identifying viral causes of human cancer. In his Nobel Laureate autobiography, he reported that during his 2-year rotating residency, he loved his obstetrics and gynecology experience, but found it “physically highly demanding” and decided to focus his career in microbiology and immunology.5 After proving that herpes simplex virus did not cause cervical cancer he began to explore the role of HPV in the disease process. He first identified HPV types 6 and 11 and showed that these agents caused genital warts. He then used low-stringency hybridization techniques to identify HPV types 16 and 18 in specimens of cervical cancer. Later, he and his colleagues proved that two HPV proteins, E6 and E7, interfere with the function of cell cycle control proteins p53 and retinoblastoma protein, resulting in dysregulated cell growth and cancer.2 These findings permitted the development of both HPV vaccines and nucleic acid–based tests to identify high-risk oncogenic HPV (hrHPV) in cells and tissue specimens.
HPV vaccination
Dr. zur Hausen was an energetic and vocal advocate for the development and widescale deployment of HPV vaccines, including vaccination of males and females.6 Initially his ideas were rejected by the pharmaceutical industry, but eventually, with advances in virology and vaccine development, multiple companies pursued the development of HPV vaccines, the first cancer prevention vaccines. The best approach to cervical cancer prevention is intensive population-wide HPV vaccination of both boys and girls before exposure to the HPV virus. Beyond its beneficial effect on the incidence of cervical cancer, HPV vaccination also reduces the population incidence of anal, vulvar, and oropharyngeal cancer.7 Prevention of oropharyngeal cancer is especially important for men, supporting the recommendation for vaccination of all boys.8
Population-wide HPV vaccination will result in a lower prevalence of cervical cancer precursors and reduce the sensitivity of cytology, thereby making primary HPV screening more attractive.9 Based on one modelling study, universal HPV vaccination can reduce cervical cancer rates by greater than 50% over current levels, and introduction of primary HPV screening will reduce cervical cancer rates by an additional 20%.10 In an era of widespread vaccination for HPV, screening for cervical cancer should be intensified for nonvaccinated women.10
Read about Primary cervical cancer screening with cytology
Primary cervical cancer screening with cytology
Primary screening with cervical cytology alone remains an option supported by many authorities and professional society guidelines.11 Most studies report that HPV testing has greater sensitivity than cervical cytology alone, especially for the detection of adenocarcinoma of the cervix.12 In one Canadian study, 10,154 women were randomly assigned to HPV or cervical cytology testing. The sensitivity of HPV testing and cervical cytology for detecting cervical intraepithelial neoplasia grade 2 or 3 was 95% and 55%, respectively, with a specificity of 94% and 97%, respectively.13 When used together the sensitivity and specificity of cotesting was 100% and 93%, respectively, but resulted in an increased number of colposcopies, which may be costly and add stress for the patient. Many countries are beginning to move away from cervical cancer screening with cytology or cotesting to programs built upon a foundation of primary HPV testing.
Primary cervical cancer screening with HPV testing
The knowledge that hrHPV is a more sensitive test for cervical cancer and its precursors, as well as the relatively lower sensitivity of cytology, is the foundation for transitioning from primary screening with cervical cytology to primary screening with HPV testing. In the Netherlands14 and Australia15,16 HPV testing with reflex cytology is the nationwide approach to cervical cancer screening. The basic components of the Dutch primary HPV screening program, as explained by Dr. Lai van Zulyan Mandres, are14:
- Samples are collected by a general practitioner and sent to one of 5 central testing facilities for DNA testing for hrHPV.
- If all previous samples tested negative, the screening occurs at ages 30, 35, 40, 50, and 60 years, a minimum of 5 screens per woman.
- If there is a history of a previously positive hrHPV, the screening is intensified, with additional specimens collected at ages 45, 55, and 60 years.
- If the sample is hrHPV negative, the patient continues screening at the standard intervals. No cytology testing is performed.
- If the sample is hrHPV positive, reflex cytology is performed using the original collected sample. If the cytology shows no intraepithelial lesion or malignancy (NILM), another specimen is obtained for cytology within 6 months. If the second cytology specimen shows atypical squamous cells of undetermined significance (ASCUS) or a more worrisome cytology finding, the patient is sent for colposcopy. If two NILM cytology specimens have been obtained, the patient resumes primary hrHPV screening every 5 years.
- If the specimen is hrHPV positive and cytology is ASCUS or more worrisome the patient is referred for colposcopy (FIGURE).14 The Dutch estimate that primary hrHPV screening will reduce the number of cervical cytology specimens by 90% annually.
Australia also has implemented nationwide primary HPV testing for cervical cancer screening. This change was implemented following a 10-year program of universal school-based vaccination of girls and boys, and biennial cytology screening for all women. The Australian screening program initiates hrHPV testing at age 25 years and thereafter every 5 years until age 74. If the hrHPV test is positive, reflex testing for HPV types 16 and 18 are performed on the original specimen along with cervical cytology. Women who test positive for HPV 16 or 18 are immediately referred for colposcopy. If the hrHPV test is positive and reflex testing for HPV 16 and 18 is negative, cervical cytology demonstrating ASCUS, low- or high-grade squamous intraepithelial lesions, or more worrisome results trigger a referral for colposcopy. The Australian program supports testing of self-collected vaginal samples for women who are underscreened or have never been screened.15,16
Read about Pros and cons of switching approaches
Pros and cons of switching approaches
Deployment of new technology often yields benefits and challenges. A putative benefit of primary HPV screening is a reduction in health care costs without an increase in cervical cancer deaths. Another benefit of primary HPV screening is that it may enable self-collection of specimens for analysis, thereby increasing access to cervical cancer screening for underserved and marginalized populations of women who are not currently participating in cervical cancer screening programs.17 One challenge is that many women are unaware that hrHPV is the cause of most invasive cervical cancers. The detection of hrHPV in a woman in a long-term relationship who was previously negative for hrHPV may cause the emotions of surprise, fear, anxiety, and anger, thereby stressing the relationship.18
Another concern is that many women are worried about no longer receiving the familiar “Pap smear” cancer screening test in which they have tremendous faith. When Australia transitioned to primary HPV screening, more than 70,000 women signed a petition to “save women’s lives” by permitting continued access to the cervical cytology testing.19 Primary HPV testing may result in a transient increase in the number of women referred for colposcopy, potentially overwhelming the capacity of the health care system to deliver this vital service.20,21 The HPV types that most often cause cervical cancer may vary among countries. For example, in Thailand, HPV 52 and 58 are frequently detected in women with high-grade squamous lesions, and including these subtypes in reflex genotyping may be of regional benefit.22
Primary cervical cancer screening with HPV testing: When will it be used widely in the United States?
In contrast to the United States, the Netherlands is a small, densely populated country that has a highly integrated health system with centralized laboratory centers, a nationwide electronic health record, and clinicians organized to perform as an integrated team. These features ensure that all lifetime tests results are available in one record, that HPV testing is highly standardized, and that clinicians will follow a prescribed care pathway. The Netherlands’ health system is organized to support the successful transition, in a single step, to primary HPV testing. The United States is the third most populous country in the world, following China and India, with a diverse approach to health care, a highly mobile population, no single interoperable electronic health record, and minimal central control of clinical practice. The United States is not organized to make a “big bang” transition to primary HPV cervical cancer screening. It is likely that the introduction of primary HPV screening will occur first in highly integrated health systems that control the clinical, laboratory, and electronic records of a large population.
The results of the ATHENA study provide a clear clinical algorithm for implementing a primary HPV screening program for cervical cancer in the United States.23–25 Samples are collected for hrHPV testing at a specified interval, 3 or 5 years, beginning at age 25 years. Women younger than age 25 years should be screened with cytology alone. Detection of hrHPV results in reflex viral typing for HPV 16 and 18. Women with samples positive for HPV 16 and 18 are immediately referred for colposcopy. Samples positive for hrHPV and negative for HPV 16 and 18 have reflex cytology testing performed on the original HPV specimen. If cytology testing reports NILM, repeat cotesting is performed in one year. If cytology testing reports ASCUS or a more concerning result, the woman is referred for colposcopy.
Malcolm Gladwell, in his book The Tipping Point, identified 3 processes that help push an innovative new approach from obscurity into widespread use.26 First, authoritative voices that can catalyze change need to consistently communicate their shared vision for the future. Second, there must be a clear message that galvanizes the many to change their approach. Third, the historical context must be supportive of the change. Over the next decade we are likely to hit a tipping point and transition from cervical cancer screening that relies on cervical cytology to an approach that prioritizes hrHPV testing. When that change will occur in the United States is unclear. But our colleagues in other countries already have transitioned to primary hrHPV testing for cervical cancer screening.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Hinsey JC. George Nicholas Papanicolaou, May 13, 1883–February 19, 1962. Acta Cytol. 1962;6:483–486.
- zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Physicians. 1999;111(6):581–587.
- International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention, Vol 10: Cervix Cancer Screening. Lyon, France: IARC Press; 2005.
- Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Women’s Health (Larchmt). 2012;21(10):1031–1037.
- Harold zur Hausen-Biographical. Nobelprize.org website. https://www.nobelprize.org/nobel_prizes/medicine/laureates/2008/hausen-bio.html. Accessed June 19, 2018.
- Michels KB, zur Hausen H. HPV vaccine for all. Lancet. 2009;374(9686):268–270.
- Hansen BT, Campbell S, Nygaard M. Long-term incidence of HPV-related cancers, and cases preventable by HPV vaccination: a registry-based study in Norway. BMJ Open. 2018;8(2):e019005.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27(5):6–8.
- Massad LS. Anticipating the impact of human papillomavirus vaccination on US cervical cancer prevention strategies. J Low Genit Tract Dis. 2018;22(2):123–125.
- Castanon A, Landy R, Pesola F, Windridge P, Sasieni P. Prediction of cervical cancer incidence in England, UK, up to 2040, under four scenarios: a modeling study. Lancet Public Health. 2018;3(1):e34–e43.
- Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891.
- Moukarzel LA, Angarita AM, VandenBussche C, et al. Preinvasive and invasive cervical adenocarcinoma: preceding low-risk or negative Pap result increases time to diagnosis. J Low Genital Tract Dis. 2017;21(2):91–96.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588.
- van Zuylen-Manders L. Primary HPV screening: The Dutch experience. http://www.britishcytology.org.uk/resources/Primary_HPV_screening_The_Dutch_experience.pdf. Accessed June 19, 2018.
- Hammond I, Canfell K, Saville M. A new era for cervical cancer screening in Australia: watch this space! Aust N Z J Obstet Gynaecol. 2017;57(5):499–501.
- Canfell K, Saville M, Caruana M, et al. Protocol for Compass: a randomised controlled trial of primary HPV testing versus cytology screening for cervical cancer in HPV-unvaccinated and vaccinated women aged 25-69 years living in Australia. BMJ Open. 2018;8(1):e016700.
- Wood B, Lofters A, Vahabi M. Strategies to reach marginalized women for cervical cancer screening: a qualitative study of stakeholder perspectives. Curr Oncol. 2018;25(1):e8–e16.
- Patel H, Moss EL, Sherman SM. HPV primary cervical cancer screening in England: women’s awareness and attitudes. Psychooncology. 2018;27(6):1559–1564.
- Obermair HM, Dodd RH, Bonner C, Jansen J, McCaffery K. “It has saved thousands of lives, so why change it?” Content analysis of objections to cervical cancer screening programme changes in Australia. BMJ Open. 2018;8(2):e019171.
- Hall MT, Simms KT, Lew JB, Smith MA, Saville M, Canfell K. Projected future impact of HPV vaccination and primary HPV screening on cervical cancer rates from 2017-2035: Example from Australia. PLoS One. 2018;13(2):e0185332.
- Rebolj M, Bonde J, Preisler S, Ejegod D, Rygaard C, Lynge E. Human papillomavirus assays and cytology in primary cervical screening of women aged 30 years and above. PLoS One. 2016;11(1):e0147326.
- Khunamornpong S, Settakorn J, Sukpan K, Suprasert P, Srisomboon J, Intaraphet S, Siriaunkgul S. Genotyping for human papillomavirus (HPV) 16/18/52/58 has a higher performance than HPV16/18 genotyping in triaging women with positive high-risk HPV test in Northern Thailand. PLoS One. 2016;11(6):e0158184.
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–337.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Gladwell M. The Tipping Point: How Little Things Can Make a Big Difference. New York, New York: Little Brown; 2000.
- Hinsey JC. George Nicholas Papanicolaou, May 13, 1883–February 19, 1962. Acta Cytol. 1962;6:483–486.
- zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Physicians. 1999;111(6):581–587.
- International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention, Vol 10: Cervix Cancer Screening. Lyon, France: IARC Press; 2005.
- Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Women’s Health (Larchmt). 2012;21(10):1031–1037.
- Harold zur Hausen-Biographical. Nobelprize.org website. https://www.nobelprize.org/nobel_prizes/medicine/laureates/2008/hausen-bio.html. Accessed June 19, 2018.
- Michels KB, zur Hausen H. HPV vaccine for all. Lancet. 2009;374(9686):268–270.
- Hansen BT, Campbell S, Nygaard M. Long-term incidence of HPV-related cancers, and cases preventable by HPV vaccination: a registry-based study in Norway. BMJ Open. 2018;8(2):e019005.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27(5):6–8.
- Massad LS. Anticipating the impact of human papillomavirus vaccination on US cervical cancer prevention strategies. J Low Genit Tract Dis. 2018;22(2):123–125.
- Castanon A, Landy R, Pesola F, Windridge P, Sasieni P. Prediction of cervical cancer incidence in England, UK, up to 2040, under four scenarios: a modeling study. Lancet Public Health. 2018;3(1):e34–e43.
- Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891.
- Moukarzel LA, Angarita AM, VandenBussche C, et al. Preinvasive and invasive cervical adenocarcinoma: preceding low-risk or negative Pap result increases time to diagnosis. J Low Genital Tract Dis. 2017;21(2):91–96.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588.
- van Zuylen-Manders L. Primary HPV screening: The Dutch experience. http://www.britishcytology.org.uk/resources/Primary_HPV_screening_The_Dutch_experience.pdf. Accessed June 19, 2018.
- Hammond I, Canfell K, Saville M. A new era for cervical cancer screening in Australia: watch this space! Aust N Z J Obstet Gynaecol. 2017;57(5):499–501.
- Canfell K, Saville M, Caruana M, et al. Protocol for Compass: a randomised controlled trial of primary HPV testing versus cytology screening for cervical cancer in HPV-unvaccinated and vaccinated women aged 25-69 years living in Australia. BMJ Open. 2018;8(1):e016700.
- Wood B, Lofters A, Vahabi M. Strategies to reach marginalized women for cervical cancer screening: a qualitative study of stakeholder perspectives. Curr Oncol. 2018;25(1):e8–e16.
- Patel H, Moss EL, Sherman SM. HPV primary cervical cancer screening in England: women’s awareness and attitudes. Psychooncology. 2018;27(6):1559–1564.
- Obermair HM, Dodd RH, Bonner C, Jansen J, McCaffery K. “It has saved thousands of lives, so why change it?” Content analysis of objections to cervical cancer screening programme changes in Australia. BMJ Open. 2018;8(2):e019171.
- Hall MT, Simms KT, Lew JB, Smith MA, Saville M, Canfell K. Projected future impact of HPV vaccination and primary HPV screening on cervical cancer rates from 2017-2035: Example from Australia. PLoS One. 2018;13(2):e0185332.
- Rebolj M, Bonde J, Preisler S, Ejegod D, Rygaard C, Lynge E. Human papillomavirus assays and cytology in primary cervical screening of women aged 30 years and above. PLoS One. 2016;11(1):e0147326.
- Khunamornpong S, Settakorn J, Sukpan K, Suprasert P, Srisomboon J, Intaraphet S, Siriaunkgul S. Genotyping for human papillomavirus (HPV) 16/18/52/58 has a higher performance than HPV16/18 genotyping in triaging women with positive high-risk HPV test in Northern Thailand. PLoS One. 2016;11(6):e0158184.
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–337.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Gladwell M. The Tipping Point: How Little Things Can Make a Big Difference. New York, New York: Little Brown; 2000.
Abortion not safer at an ambulatory surgical center
Abortion performed in an ambulatory surgery center (ASC) was not associated with a significant difference in abortion-related complications, compared with procedures performed in an office-based setting, according to results of a retrospective cohort study.
These findings might help inform decisions about the type of facility where induced abortions are performed, according to Sarah C. M. Roberts, DrPH, of the University of California, San Francisco, and her coauthors.
The U.S. Supreme Court ruled in 2016 that a Texas law requiring abortion facilities to meet ASC standards was unconstitutional, Dr. Roberts and her coauthors wrote in JAMA.
“Despite this ruling, 13 states currently have laws that require abortions to be provided in ASCs,” the authors wrote, noting that supporters of the laws argue that these requirements make abortions safer.
The laws have requirements such as separate procedure and recovery rooms, and specified hall and door widths. “Many of these apply only at a specific gestational week or gestational duration, typically in the second trimester,” they noted, adding that over 95% of induced abortions are performed in outpatient settings such as clinics or physician offices.
Their retrospective cohort study included a total of 50,311 induced abortions, of which 89% took place in office based settings and 11% in ASCs. Nearly half (47%) were first-trimester aspiration procedures, while 27% were first-trimester medication and 26% were second trimester or later.
Abortion-related morbidity or adverse events were reported for 3.33% of procedures overall. The adjusted incidence rate was 3.25% for ASC-based procedures, and similarly, 3.33% for office-based procedures.
The overall complication rate was higher than previous estimates based on insurance claims data, they said, but the estimate of major events was similar at 0.32%, breaking down to 0.26% for ASCs and 0.33% for office-based settings. The rate of infections was 0.58% for ASCs and 0.77% for office-based settings.
This is not the first study looking at the association between abortion-related events and the procedure setting, though the literature is limited, according to Dr. Roberts and her coauthors. One previous study showed fewer abortion-related events in clinics than in hospitals, while a recent review found similar abortion-related events following first-trimester abortions in hospitals, ASCs, and office-based settings.
One limitation of the current study is that the database included only abortions that were paid for by private insurance, which represents about 15% of the nearly 1 million procedures done each year in the United States.
“Thus, findings may not be generalizable to all abortions in the United States,” Dr. Roberts and her coauthors wrote.
The study was supported by a grant from the Society of Family Planning Research Fund. Study authors reported no conflicts of interest.
SOURCE: Roberts SCM et al. JAMA. 2018 Jun 26;319(24):2497-2506.
This new analysis provides further support that office settings are appropriate for abortion care and that office-based abortion care is appropriately safe, effective, and patient centered.
Results of this study support the safety of office-based abortions, including a low risk of infection, they added.
This comparison study of office-based abortion to abortion provided in an ambulatory surgery center (ASC) is important because 16 states impose restrictions that require abortion facilities adhere to ASC or ASC-equivalent standards.
Converting an office to an ASC is slow, complex, and although the cost of retrofitting a facility is moderately less, building an ASC costs an estimated $5 million, according to industry experts.
Requiring an office to meet an ASC-equivalent standard with no medical justification is too high a hurdle in many areas and serves to restrict women’s access to abortion.
Carolyn L. Westhoff, MD, and Anne R. Davis, MD, are with the department of obstetrics and gynecology at Columbia University, New York. These comments are based on their editorial in JAMA (2018 Jun 26;319[24]:2481-2483). Dr. Westhoff is the editor of Contraception and a senior medical advisor at Planned Parenthood Federation of America. Dr. Davis is consulting medical director for Physicians for Reproductive Health, a consultant for the New York City Department of Health, and an expert for the American Civil Liberties Union.
This new analysis provides further support that office settings are appropriate for abortion care and that office-based abortion care is appropriately safe, effective, and patient centered.
Results of this study support the safety of office-based abortions, including a low risk of infection, they added.
This comparison study of office-based abortion to abortion provided in an ambulatory surgery center (ASC) is important because 16 states impose restrictions that require abortion facilities adhere to ASC or ASC-equivalent standards.
Converting an office to an ASC is slow, complex, and although the cost of retrofitting a facility is moderately less, building an ASC costs an estimated $5 million, according to industry experts.
Requiring an office to meet an ASC-equivalent standard with no medical justification is too high a hurdle in many areas and serves to restrict women’s access to abortion.
Carolyn L. Westhoff, MD, and Anne R. Davis, MD, are with the department of obstetrics and gynecology at Columbia University, New York. These comments are based on their editorial in JAMA (2018 Jun 26;319[24]:2481-2483). Dr. Westhoff is the editor of Contraception and a senior medical advisor at Planned Parenthood Federation of America. Dr. Davis is consulting medical director for Physicians for Reproductive Health, a consultant for the New York City Department of Health, and an expert for the American Civil Liberties Union.
This new analysis provides further support that office settings are appropriate for abortion care and that office-based abortion care is appropriately safe, effective, and patient centered.
Results of this study support the safety of office-based abortions, including a low risk of infection, they added.
This comparison study of office-based abortion to abortion provided in an ambulatory surgery center (ASC) is important because 16 states impose restrictions that require abortion facilities adhere to ASC or ASC-equivalent standards.
Converting an office to an ASC is slow, complex, and although the cost of retrofitting a facility is moderately less, building an ASC costs an estimated $5 million, according to industry experts.
Requiring an office to meet an ASC-equivalent standard with no medical justification is too high a hurdle in many areas and serves to restrict women’s access to abortion.
Carolyn L. Westhoff, MD, and Anne R. Davis, MD, are with the department of obstetrics and gynecology at Columbia University, New York. These comments are based on their editorial in JAMA (2018 Jun 26;319[24]:2481-2483). Dr. Westhoff is the editor of Contraception and a senior medical advisor at Planned Parenthood Federation of America. Dr. Davis is consulting medical director for Physicians for Reproductive Health, a consultant for the New York City Department of Health, and an expert for the American Civil Liberties Union.
Abortion performed in an ambulatory surgery center (ASC) was not associated with a significant difference in abortion-related complications, compared with procedures performed in an office-based setting, according to results of a retrospective cohort study.
These findings might help inform decisions about the type of facility where induced abortions are performed, according to Sarah C. M. Roberts, DrPH, of the University of California, San Francisco, and her coauthors.
The U.S. Supreme Court ruled in 2016 that a Texas law requiring abortion facilities to meet ASC standards was unconstitutional, Dr. Roberts and her coauthors wrote in JAMA.
“Despite this ruling, 13 states currently have laws that require abortions to be provided in ASCs,” the authors wrote, noting that supporters of the laws argue that these requirements make abortions safer.
The laws have requirements such as separate procedure and recovery rooms, and specified hall and door widths. “Many of these apply only at a specific gestational week or gestational duration, typically in the second trimester,” they noted, adding that over 95% of induced abortions are performed in outpatient settings such as clinics or physician offices.
Their retrospective cohort study included a total of 50,311 induced abortions, of which 89% took place in office based settings and 11% in ASCs. Nearly half (47%) were first-trimester aspiration procedures, while 27% were first-trimester medication and 26% were second trimester or later.
Abortion-related morbidity or adverse events were reported for 3.33% of procedures overall. The adjusted incidence rate was 3.25% for ASC-based procedures, and similarly, 3.33% for office-based procedures.
The overall complication rate was higher than previous estimates based on insurance claims data, they said, but the estimate of major events was similar at 0.32%, breaking down to 0.26% for ASCs and 0.33% for office-based settings. The rate of infections was 0.58% for ASCs and 0.77% for office-based settings.
This is not the first study looking at the association between abortion-related events and the procedure setting, though the literature is limited, according to Dr. Roberts and her coauthors. One previous study showed fewer abortion-related events in clinics than in hospitals, while a recent review found similar abortion-related events following first-trimester abortions in hospitals, ASCs, and office-based settings.
One limitation of the current study is that the database included only abortions that were paid for by private insurance, which represents about 15% of the nearly 1 million procedures done each year in the United States.
“Thus, findings may not be generalizable to all abortions in the United States,” Dr. Roberts and her coauthors wrote.
The study was supported by a grant from the Society of Family Planning Research Fund. Study authors reported no conflicts of interest.
SOURCE: Roberts SCM et al. JAMA. 2018 Jun 26;319(24):2497-2506.
Abortion performed in an ambulatory surgery center (ASC) was not associated with a significant difference in abortion-related complications, compared with procedures performed in an office-based setting, according to results of a retrospective cohort study.
These findings might help inform decisions about the type of facility where induced abortions are performed, according to Sarah C. M. Roberts, DrPH, of the University of California, San Francisco, and her coauthors.
The U.S. Supreme Court ruled in 2016 that a Texas law requiring abortion facilities to meet ASC standards was unconstitutional, Dr. Roberts and her coauthors wrote in JAMA.
“Despite this ruling, 13 states currently have laws that require abortions to be provided in ASCs,” the authors wrote, noting that supporters of the laws argue that these requirements make abortions safer.
The laws have requirements such as separate procedure and recovery rooms, and specified hall and door widths. “Many of these apply only at a specific gestational week or gestational duration, typically in the second trimester,” they noted, adding that over 95% of induced abortions are performed in outpatient settings such as clinics or physician offices.
Their retrospective cohort study included a total of 50,311 induced abortions, of which 89% took place in office based settings and 11% in ASCs. Nearly half (47%) were first-trimester aspiration procedures, while 27% were first-trimester medication and 26% were second trimester or later.
Abortion-related morbidity or adverse events were reported for 3.33% of procedures overall. The adjusted incidence rate was 3.25% for ASC-based procedures, and similarly, 3.33% for office-based procedures.
The overall complication rate was higher than previous estimates based on insurance claims data, they said, but the estimate of major events was similar at 0.32%, breaking down to 0.26% for ASCs and 0.33% for office-based settings. The rate of infections was 0.58% for ASCs and 0.77% for office-based settings.
This is not the first study looking at the association between abortion-related events and the procedure setting, though the literature is limited, according to Dr. Roberts and her coauthors. One previous study showed fewer abortion-related events in clinics than in hospitals, while a recent review found similar abortion-related events following first-trimester abortions in hospitals, ASCs, and office-based settings.
One limitation of the current study is that the database included only abortions that were paid for by private insurance, which represents about 15% of the nearly 1 million procedures done each year in the United States.
“Thus, findings may not be generalizable to all abortions in the United States,” Dr. Roberts and her coauthors wrote.
The study was supported by a grant from the Society of Family Planning Research Fund. Study authors reported no conflicts of interest.
SOURCE: Roberts SCM et al. JAMA. 2018 Jun 26;319(24):2497-2506.
FROM JAMA
Key clinical point: Abortions in an ambulatory surgical center were not associated with a significant difference in abortion-related complications versus abortions in an office-based setting.
Major finding: The adjusted incidence rate of complications was 3.25% for ambulatory surgery centers and 3.33% for office-based settings.
Study details: A retrospective cohort study including 49,287 women with U.S. private health insurance who had 50,311 induced abortions.
Disclosures: The study was supported by a grant from the Society of Family Planning Research Fund. Study authors reported no conflicts of interest.
Source: Roberts SCM et al. JAMA 2018 Jun 26;319(24):2497-2506.
Clomiphene citrate improves pregnancy outcomes for PCOS patients
Clomiphene citrate significantly improved markers of polycystic ovarian syndrome (PCOS) and improved ovulation and pregnancy outcomes in women with PCOS, according to data from 72 women.
Nitric oxide (NO), interleukin-10 (IL-10), and matrix metalloproteinase–9 (MMP-9) “are known to be involved in the pathogenesis as well as the complications of PCOS,” wrote Angel Mercy Sylus, MD, of the Jawaharlal Institute of Postgraduate Medical Education & Research, Puducherry, India, and colleagues.
Clomiphene citrate is used to treat infertility, including infertile women with PCOS, but its mechanism of action remains unclear, the researchers wrote.
In a study published in the European Journal of Obstetrics & Gynecology and Reproductive Biology, the researchers enrolled 72 women with PCOS. The women received 50 mg of oral clomiphene citrate daily on days 3-7 of their cycles to induce ovulation. Levels of NO, IL-10, and MMP-9 were measured at baseline and after 3 weeks. The average age of the women was 25 years, and the average body mass index was 26.4 kg/m2.
After the participants took clomiphene citrate, their levels of NO and IL-10 were significantly higher, compared with baseline (P = .03 and P less than .001, respectively), and MMP-9 levels were significantly lower, compared with baseline (P less than .001).
The ovulation rate in the study population was 52.8%, and the clinical pregnancy rate was 19.4%. Levels of MMP-9 were significantly reduced (P less than .001) in the ovulatory group, compared with the nonovulatory group, the researchers noted. “Although the mechanism through which CC [clomiphene citrate] reduces MMP-9 and increases IL-10 is not clear, our findings indicate that CC therapy improves ovulation by reducing inflammation and reducing MMP-9 levels,” they wrote.
The findings were limited by several factors, mainly by the timing of the 4-week assessment of NO, IL-10, and MPP-9 for ethical reasons, the researchers wrote. They did not get study approval to conduct a separate blood collection. In addition, the study did not measure the effect of increasing doses of clomiphene citrate.
However, the results have suggested that clomiphene citrate can help promote ovulation and pregnancy for infertile women with PCOS, and further studies are needed to assess the mechanism of action and the effect of higher doses on NO, IL-10, and MPP-9, the researchers wrote.
The study was supported by a grant from the Jawaharlal Institute of Postgraduate Medical Education and Research intramural fund. The researchers had no financial conflicts to disclose.
SOURCE: Sylus AM et al. Eur J Obstet Gynecol Reprod Biol. 2018 Sept; 228:27-31.
Clomiphene citrate significantly improved markers of polycystic ovarian syndrome (PCOS) and improved ovulation and pregnancy outcomes in women with PCOS, according to data from 72 women.
Nitric oxide (NO), interleukin-10 (IL-10), and matrix metalloproteinase–9 (MMP-9) “are known to be involved in the pathogenesis as well as the complications of PCOS,” wrote Angel Mercy Sylus, MD, of the Jawaharlal Institute of Postgraduate Medical Education & Research, Puducherry, India, and colleagues.
Clomiphene citrate is used to treat infertility, including infertile women with PCOS, but its mechanism of action remains unclear, the researchers wrote.
In a study published in the European Journal of Obstetrics & Gynecology and Reproductive Biology, the researchers enrolled 72 women with PCOS. The women received 50 mg of oral clomiphene citrate daily on days 3-7 of their cycles to induce ovulation. Levels of NO, IL-10, and MMP-9 were measured at baseline and after 3 weeks. The average age of the women was 25 years, and the average body mass index was 26.4 kg/m2.
After the participants took clomiphene citrate, their levels of NO and IL-10 were significantly higher, compared with baseline (P = .03 and P less than .001, respectively), and MMP-9 levels were significantly lower, compared with baseline (P less than .001).
The ovulation rate in the study population was 52.8%, and the clinical pregnancy rate was 19.4%. Levels of MMP-9 were significantly reduced (P less than .001) in the ovulatory group, compared with the nonovulatory group, the researchers noted. “Although the mechanism through which CC [clomiphene citrate] reduces MMP-9 and increases IL-10 is not clear, our findings indicate that CC therapy improves ovulation by reducing inflammation and reducing MMP-9 levels,” they wrote.
The findings were limited by several factors, mainly by the timing of the 4-week assessment of NO, IL-10, and MPP-9 for ethical reasons, the researchers wrote. They did not get study approval to conduct a separate blood collection. In addition, the study did not measure the effect of increasing doses of clomiphene citrate.
However, the results have suggested that clomiphene citrate can help promote ovulation and pregnancy for infertile women with PCOS, and further studies are needed to assess the mechanism of action and the effect of higher doses on NO, IL-10, and MPP-9, the researchers wrote.
The study was supported by a grant from the Jawaharlal Institute of Postgraduate Medical Education and Research intramural fund. The researchers had no financial conflicts to disclose.
SOURCE: Sylus AM et al. Eur J Obstet Gynecol Reprod Biol. 2018 Sept; 228:27-31.
Clomiphene citrate significantly improved markers of polycystic ovarian syndrome (PCOS) and improved ovulation and pregnancy outcomes in women with PCOS, according to data from 72 women.
Nitric oxide (NO), interleukin-10 (IL-10), and matrix metalloproteinase–9 (MMP-9) “are known to be involved in the pathogenesis as well as the complications of PCOS,” wrote Angel Mercy Sylus, MD, of the Jawaharlal Institute of Postgraduate Medical Education & Research, Puducherry, India, and colleagues.
Clomiphene citrate is used to treat infertility, including infertile women with PCOS, but its mechanism of action remains unclear, the researchers wrote.
In a study published in the European Journal of Obstetrics & Gynecology and Reproductive Biology, the researchers enrolled 72 women with PCOS. The women received 50 mg of oral clomiphene citrate daily on days 3-7 of their cycles to induce ovulation. Levels of NO, IL-10, and MMP-9 were measured at baseline and after 3 weeks. The average age of the women was 25 years, and the average body mass index was 26.4 kg/m2.
After the participants took clomiphene citrate, their levels of NO and IL-10 were significantly higher, compared with baseline (P = .03 and P less than .001, respectively), and MMP-9 levels were significantly lower, compared with baseline (P less than .001).
The ovulation rate in the study population was 52.8%, and the clinical pregnancy rate was 19.4%. Levels of MMP-9 were significantly reduced (P less than .001) in the ovulatory group, compared with the nonovulatory group, the researchers noted. “Although the mechanism through which CC [clomiphene citrate] reduces MMP-9 and increases IL-10 is not clear, our findings indicate that CC therapy improves ovulation by reducing inflammation and reducing MMP-9 levels,” they wrote.
The findings were limited by several factors, mainly by the timing of the 4-week assessment of NO, IL-10, and MPP-9 for ethical reasons, the researchers wrote. They did not get study approval to conduct a separate blood collection. In addition, the study did not measure the effect of increasing doses of clomiphene citrate.
However, the results have suggested that clomiphene citrate can help promote ovulation and pregnancy for infertile women with PCOS, and further studies are needed to assess the mechanism of action and the effect of higher doses on NO, IL-10, and MPP-9, the researchers wrote.
The study was supported by a grant from the Jawaharlal Institute of Postgraduate Medical Education and Research intramural fund. The researchers had no financial conflicts to disclose.
SOURCE: Sylus AM et al. Eur J Obstet Gynecol Reprod Biol. 2018 Sept; 228:27-31.
FROM THE EUROPEAN JOURNAL OF OBSTETRICS & GYNECOLOGY AND REPRODUCTIVE BIOLOGY
Key clinical point: Clomiphene citrate increased the levels of both nitric oxide and interleukin-10 and reduced levels of matrix metalloproteinase–9.
Major finding: The ovulation rate was 53%, and the clinical pregnancy rate was 19% in PCOS women given clomiphene citrate.
Study details: The data come from 72 women with PCOS.
Disclosures: The study was supported by a grant from the Jawaharlal Institute of Postgraduate Medical Education and Research intramural fund. The researchers had no financial conflicts to disclose.
Source: Sylus A et al. Eur J Obstet Gynecol Reprod Biol. 2018 Sep;228:27-31.