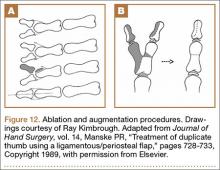
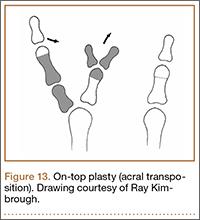
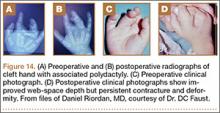
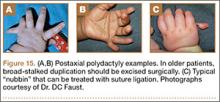
User login
Closed Rupture of the Flexor Profundus Tendon of Ring Finger: Case Report and Treatment Recommendations
Flexor tendons are considered the strongest component of the musculotendinous unit; they generally do not rupture unless weakened by an underlying pathologic condition.1 According to traditional teaching, when the musculotendinous unit is subjected to excessive forces, failure invariably occurs at the tendon insertion, at the musculotendinous junction, within the muscle substance, or at its origin from the bone before the tendon itself ruptures.1
Midsubstance tears in nonrheumatoid patients are less frequent and are typically attributable to an underlying cause.2 Possible pathologic conditions include, but are not limited to, osteoarthritis of the pisotriquetral joint,3 nonunion fracture of the hook of the hamate,4 lunate dislocation,5 accessory carpal bone,6 gouty infiltration of the flexor tendon,7 and tumor.8 In 1960, Boyes and colleagues9 presented a series of 80 flexor tendon ruptures in 78 patients over a 13-year period. Only 3 cases had no identifiable cause. The authors recommended using the term spontaneous for those ruptures that occur within the tendon substance without underlying or associated pathologic changes.
We describe a patient with spontaneous rupture of the flexor digitorum profundus (FDP) tendon at zone III, satisfying Boyes’ definition of the term spontaneous. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
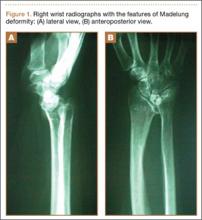
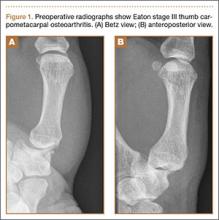
A 65-year-old, right-handed manual worker was assessed in our hand clinic 3 days after he felt a cramp in his left palm while lifting a heavy object. Shortly thereafter, he noted he could not flex his ring finger distal interphalangeal (DIP) joint. He could not recall any previous injury to his finger. No predisposing pathologic conditions or bone abnormalities were identified. Clinically, there was no tenderness, swelling, or ecchymosis evident. He had full passive range of motion (ROM) of his ring finger, and proximal interphalangeal (PIP) joint active ROM was 0/110º; however, he had no activity of the FDP of the ring finger. Preoperative radiographs were normal. The hook of the hamate was clinically and radiographically normal.
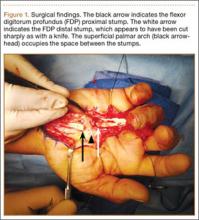
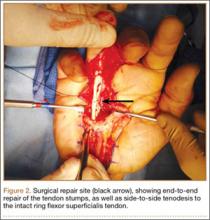
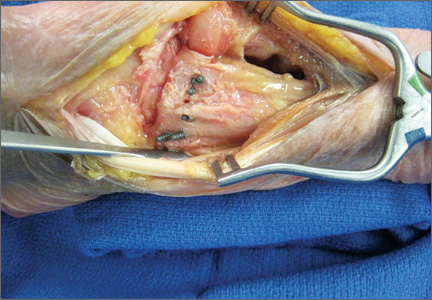
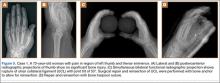
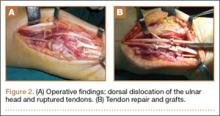
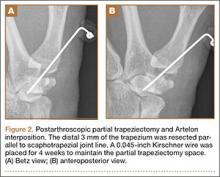
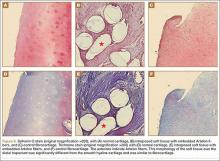
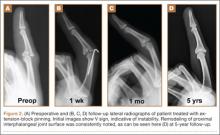
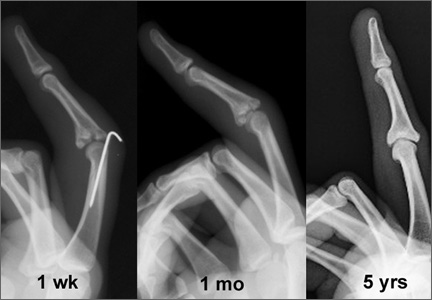
A preoperative diagnosis of FDP avulsion from the distal phalanx was made, and the operation was carried out 16 days after injury. Surgical exploration started in zone II and extended proximally into the distal palmar crease, but no stump was found in either location. Therefore, exploration was carried out to the midpalmar region, revealing the tendon rupture in zone III, in the region of the origin of the ring finger lumbrical muscle (Figure 1). The flexor digitorum superficialis tendon was intact. Macroscopically, both tendon and carpal tunnel appeared normal, with no evidence of tendon attrition; thus, the tendon was not sent for histologic examination. The ends of the ruptured FDP tendon to the ring finger were at the level of the superficial palmar arch, with the distal end appearing as though it had been cut sharply with a knife. Because of the short period of time from injury to exploration, delayed primary tendon repair was possible, along with side-to-side tenodesis to the intact ring finger flexor superficialis tendon in the palm (Figure 2). Two days after surgery, the patient started a controlled mobilization program using the Duran method.10
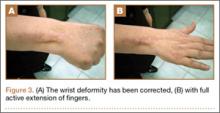
At final follow-up of 18 months, total active motion was 126°, which corresponds to a good outcome, according to the Strickland and Glogovac criteria.11 Grip strength was 50 kg, which was 84% of grip strength on the uninjured side. The patient was back to recreational activity but had not returned to work.
Discussion
Most flexor tendon ruptures result from avulsion of the FDP tendon at its distal phalanx insertion, commonly known as Jersey finger. However, true midsubstance spontaneous ruptures are infrequent. Reports of spontaneous tendon ruptures of all types, including those of the hand, have increased in incidence in most countries.12 Bois and colleagues,13 who have reviewed the literature over a 50-year period, found a total of 50 spontaneous ruptures of “normal” flexor tendon in 43 cases. The authors point to unique historical and physical examinaton findings that help differentiate spontaneous tendon ruptures from the more common FDP avulsions. Such findings include the sensation of a pop or snap, or a sudden sharp pain or cramp within the palmar region. In contrast, most avulsion ruptures cause discomfort within the region of the digit. In type I avulsion injuries of the FDP tendon, the proximal tendon stump usually retracts proximal to the digital tendon sheath, causing a tender mass in the palm.14 Flexor digitorum profundus tendon avulsions, however, are not typically associated with a snap or pop in the palm. When spontaneous ruptures of the hand occur, they typically involve the profundus tendon of the small finger, in the area of the lumbrical origin.13
In equivocal cases when the site of rupture is uncertain, ultrasound and magnetic resonance imaging may assist in making the diagnosis and provide important preoperative information for surgical decision-making and planning; this information may decrease postoperative morbidity by minimizing surgical dissection.
The etiology of spontaneous ruptures is incompletely understood. For any rupture of the ulnar flexor tendons, the hook of the hamate should be examined to rule out a previous fracture as a cause of tendon attrition.15 Tendon vascularization may be a cause for tendon rupture in the hand. When the blood supply of the lumbrical muscles was examined in 100 upper extremities from human cadavers using vascular injection studies,16 it was discovered that each lumbrical muscle received its arterial supply from 4 sources: the superficial palmar arch, the common palmar digital artery, the deep palmar arch, and the dorsal digital artery. There were no anastomoses between the networks supplying the lumbrical muscles and the FDP tendons within the palm, suggesting a possible watershed zone between the FDP tendon and lumbrical muscle origin. The patient described in this case had the tendon rupture in the area of potential hypovascularity at the lumbrical origin.
Important factors in the decision-making process for surgical treatment include the length of time between rupture and treatment, the site of rupture, and the condition of the ruptured tendon ends. Patients who present in the first 3 weeks of injury can be treated by primary tendon repair, provided that the ruptured tendon ends are not significantly frayed or attenuated. For patients presenting more than 3 weeks after injury, interposition tendon grafts or tendon transfers are suitable options for ruptures in zone III. Distal interphalangeal joint arthrodesis is another alternative in specific cases where reconstruction is not possible. In this case, direct end-to-end repair was possible, as well as tenodesis to the intact ring finger superficialis in order to prevent stretching of the repair.
Localizing the level of the tendon rupture clinically is difficult. When the site of the profundus tendon rupture is uncertain, and there is no tenderness in zone I or the PIP joint, the first incision should be made at the metacarpophalangeal joint level. This first incision will indicate if the rupture occurred in zone III. If the tendon is intact at that location, then the next incision should be at the level of the PIP joint.
Conclusion
We report a patient treated for spontaneous rupture of the flexor tendon in zone III. He was treated in the acute setting with direct tendon repair. It is important to consider spontaneous rupture of the tendon in patients presenting with a snap/pop and the sudden inability to flex a finger. A tendon rupture can be diagnosed as spontaneous in the absence of an underlying pathologic condition such as rheumatoid arthritis, gout, or occult carpal fractures. In the acute setting, these may be repaired primarily; however, if presenting after a few weeks, alternative surgical options, including interposition tendon grafts, tendon transfer, and DIP joint arthrodesis, should be considered.
1. McMaster PE. Tendon and muscle ruptures, clinical and experimental studies on the causes and location of subcutaneous ruptures. J Bone Joint Surg Am. 1933;15(3):705-722.
2. Folmar RC, Nelson CL, Phalen GS. Ruptures of the flexor tendons in hands of non-rheumatoid patients. J Bone Joint Surg Am. 1972;54(3):579-584.
3. Grant I, Berger AC, Ireland DC. Rupture of the flexor digitorum profundus tendon to the small finger within the carpal tunnel. Hand Surg. 2005;10(1):109-114.
4. Hartford JM, Murphy JM. Flexor digitorum profundus rupture of the small finger secondary to nonunion of the hook of the hamate: a case report. J Hand Surg Am. 1996;21(14):621-623.
5. Johnston GH, Bowen CV. Attritional flexor tendon ruptures by an old lunate dislocation. J Hand Surg Am. 1988;13(5):701-703.
6. Koizumi M, Kanda T, Satoh S, Yoshizu T, Maki Y, Tsubokawa N. Attritional rupture of the flexor digitorum profundus tendon to the index finger caused by accessory carpal bone in the carpal tunnel: a case report. J Hand Surg Am. 2005;30(1):142-146.
7. Wurapa RK, Zelouf DS. Flexor tendon rupture caused by gout: a case report. J Hand Surg Am. 2002;27(4):591-593.
8. Masada K, Kanazawa M, Fuji T. Flexor tendon ruptures caused by an intraosseous ganglion of the hook of the hamate. J Hand Surg Br. 1997;22(3)383-385.
9. Boyes JH, Wilson JN, Smith JW. Flexor-tendon ruptures in the forearm and hand. J Bone Joint Surg Am. 1960;42(4):637-646.
10. Duran R, Houser R, Coleman C, et al. A preliminary report in the use of controlled passive motion following flexor tendon repair in zones II and III [abstract]. J Hand Surg. 1976;1(1):79.
11. Strickland JW, Glogovac SV. Digital function following flexor tendon repair in Zone II: A comparison of immobilization and controlled passive motion techniques. J Hand Surg Am. 1980;5(6):537-543.
12. Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507-1525.
13. Bois AJ, Johnston G, Classen D. Spontaneous flexor tendon ruptures of the hand: case series and review of the literature. J Hand Surg Am. 2007;32(7):1061-1071.
14. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69.
15. Jebson PJ, Ferlic RJ, Engber WF. Spontaneous rupture of ulnar-sided digital flexor tendons: don’t forget the hamate. Iowa Orthop J. 1995;15:225-227.
16. Zbrodowski A, Mariéthoz E, Bednarkiewicz M, Gajisin S. The blood supply of the lumbrical muscles. J Hand Surg Br. 1998;23(3):384-388.
Flexor tendons are considered the strongest component of the musculotendinous unit; they generally do not rupture unless weakened by an underlying pathologic condition.1 According to traditional teaching, when the musculotendinous unit is subjected to excessive forces, failure invariably occurs at the tendon insertion, at the musculotendinous junction, within the muscle substance, or at its origin from the bone before the tendon itself ruptures.1
Midsubstance tears in nonrheumatoid patients are less frequent and are typically attributable to an underlying cause.2 Possible pathologic conditions include, but are not limited to, osteoarthritis of the pisotriquetral joint,3 nonunion fracture of the hook of the hamate,4 lunate dislocation,5 accessory carpal bone,6 gouty infiltration of the flexor tendon,7 and tumor.8 In 1960, Boyes and colleagues9 presented a series of 80 flexor tendon ruptures in 78 patients over a 13-year period. Only 3 cases had no identifiable cause. The authors recommended using the term spontaneous for those ruptures that occur within the tendon substance without underlying or associated pathologic changes.
We describe a patient with spontaneous rupture of the flexor digitorum profundus (FDP) tendon at zone III, satisfying Boyes’ definition of the term spontaneous. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 65-year-old, right-handed manual worker was assessed in our hand clinic 3 days after he felt a cramp in his left palm while lifting a heavy object. Shortly thereafter, he noted he could not flex his ring finger distal interphalangeal (DIP) joint. He could not recall any previous injury to his finger. No predisposing pathologic conditions or bone abnormalities were identified. Clinically, there was no tenderness, swelling, or ecchymosis evident. He had full passive range of motion (ROM) of his ring finger, and proximal interphalangeal (PIP) joint active ROM was 0/110º; however, he had no activity of the FDP of the ring finger. Preoperative radiographs were normal. The hook of the hamate was clinically and radiographically normal.
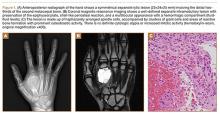
A preoperative diagnosis of FDP avulsion from the distal phalanx was made, and the operation was carried out 16 days after injury. Surgical exploration started in zone II and extended proximally into the distal palmar crease, but no stump was found in either location. Therefore, exploration was carried out to the midpalmar region, revealing the tendon rupture in zone III, in the region of the origin of the ring finger lumbrical muscle (Figure 1). The flexor digitorum superficialis tendon was intact. Macroscopically, both tendon and carpal tunnel appeared normal, with no evidence of tendon attrition; thus, the tendon was not sent for histologic examination. The ends of the ruptured FDP tendon to the ring finger were at the level of the superficial palmar arch, with the distal end appearing as though it had been cut sharply with a knife. Because of the short period of time from injury to exploration, delayed primary tendon repair was possible, along with side-to-side tenodesis to the intact ring finger flexor superficialis tendon in the palm (Figure 2). Two days after surgery, the patient started a controlled mobilization program using the Duran method.10
At final follow-up of 18 months, total active motion was 126°, which corresponds to a good outcome, according to the Strickland and Glogovac criteria.11 Grip strength was 50 kg, which was 84% of grip strength on the uninjured side. The patient was back to recreational activity but had not returned to work.
Discussion
Most flexor tendon ruptures result from avulsion of the FDP tendon at its distal phalanx insertion, commonly known as Jersey finger. However, true midsubstance spontaneous ruptures are infrequent. Reports of spontaneous tendon ruptures of all types, including those of the hand, have increased in incidence in most countries.12 Bois and colleagues,13 who have reviewed the literature over a 50-year period, found a total of 50 spontaneous ruptures of “normal” flexor tendon in 43 cases. The authors point to unique historical and physical examinaton findings that help differentiate spontaneous tendon ruptures from the more common FDP avulsions. Such findings include the sensation of a pop or snap, or a sudden sharp pain or cramp within the palmar region. In contrast, most avulsion ruptures cause discomfort within the region of the digit. In type I avulsion injuries of the FDP tendon, the proximal tendon stump usually retracts proximal to the digital tendon sheath, causing a tender mass in the palm.14 Flexor digitorum profundus tendon avulsions, however, are not typically associated with a snap or pop in the palm. When spontaneous ruptures of the hand occur, they typically involve the profundus tendon of the small finger, in the area of the lumbrical origin.13
In equivocal cases when the site of rupture is uncertain, ultrasound and magnetic resonance imaging may assist in making the diagnosis and provide important preoperative information for surgical decision-making and planning; this information may decrease postoperative morbidity by minimizing surgical dissection.
The etiology of spontaneous ruptures is incompletely understood. For any rupture of the ulnar flexor tendons, the hook of the hamate should be examined to rule out a previous fracture as a cause of tendon attrition.15 Tendon vascularization may be a cause for tendon rupture in the hand. When the blood supply of the lumbrical muscles was examined in 100 upper extremities from human cadavers using vascular injection studies,16 it was discovered that each lumbrical muscle received its arterial supply from 4 sources: the superficial palmar arch, the common palmar digital artery, the deep palmar arch, and the dorsal digital artery. There were no anastomoses between the networks supplying the lumbrical muscles and the FDP tendons within the palm, suggesting a possible watershed zone between the FDP tendon and lumbrical muscle origin. The patient described in this case had the tendon rupture in the area of potential hypovascularity at the lumbrical origin.
Important factors in the decision-making process for surgical treatment include the length of time between rupture and treatment, the site of rupture, and the condition of the ruptured tendon ends. Patients who present in the first 3 weeks of injury can be treated by primary tendon repair, provided that the ruptured tendon ends are not significantly frayed or attenuated. For patients presenting more than 3 weeks after injury, interposition tendon grafts or tendon transfers are suitable options for ruptures in zone III. Distal interphalangeal joint arthrodesis is another alternative in specific cases where reconstruction is not possible. In this case, direct end-to-end repair was possible, as well as tenodesis to the intact ring finger superficialis in order to prevent stretching of the repair.
Localizing the level of the tendon rupture clinically is difficult. When the site of the profundus tendon rupture is uncertain, and there is no tenderness in zone I or the PIP joint, the first incision should be made at the metacarpophalangeal joint level. This first incision will indicate if the rupture occurred in zone III. If the tendon is intact at that location, then the next incision should be at the level of the PIP joint.
Conclusion
We report a patient treated for spontaneous rupture of the flexor tendon in zone III. He was treated in the acute setting with direct tendon repair. It is important to consider spontaneous rupture of the tendon in patients presenting with a snap/pop and the sudden inability to flex a finger. A tendon rupture can be diagnosed as spontaneous in the absence of an underlying pathologic condition such as rheumatoid arthritis, gout, or occult carpal fractures. In the acute setting, these may be repaired primarily; however, if presenting after a few weeks, alternative surgical options, including interposition tendon grafts, tendon transfer, and DIP joint arthrodesis, should be considered.
Flexor tendons are considered the strongest component of the musculotendinous unit; they generally do not rupture unless weakened by an underlying pathologic condition.1 According to traditional teaching, when the musculotendinous unit is subjected to excessive forces, failure invariably occurs at the tendon insertion, at the musculotendinous junction, within the muscle substance, or at its origin from the bone before the tendon itself ruptures.1
Midsubstance tears in nonrheumatoid patients are less frequent and are typically attributable to an underlying cause.2 Possible pathologic conditions include, but are not limited to, osteoarthritis of the pisotriquetral joint,3 nonunion fracture of the hook of the hamate,4 lunate dislocation,5 accessory carpal bone,6 gouty infiltration of the flexor tendon,7 and tumor.8 In 1960, Boyes and colleagues9 presented a series of 80 flexor tendon ruptures in 78 patients over a 13-year period. Only 3 cases had no identifiable cause. The authors recommended using the term spontaneous for those ruptures that occur within the tendon substance without underlying or associated pathologic changes.
We describe a patient with spontaneous rupture of the flexor digitorum profundus (FDP) tendon at zone III, satisfying Boyes’ definition of the term spontaneous. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 65-year-old, right-handed manual worker was assessed in our hand clinic 3 days after he felt a cramp in his left palm while lifting a heavy object. Shortly thereafter, he noted he could not flex his ring finger distal interphalangeal (DIP) joint. He could not recall any previous injury to his finger. No predisposing pathologic conditions or bone abnormalities were identified. Clinically, there was no tenderness, swelling, or ecchymosis evident. He had full passive range of motion (ROM) of his ring finger, and proximal interphalangeal (PIP) joint active ROM was 0/110º; however, he had no activity of the FDP of the ring finger. Preoperative radiographs were normal. The hook of the hamate was clinically and radiographically normal.
A preoperative diagnosis of FDP avulsion from the distal phalanx was made, and the operation was carried out 16 days after injury. Surgical exploration started in zone II and extended proximally into the distal palmar crease, but no stump was found in either location. Therefore, exploration was carried out to the midpalmar region, revealing the tendon rupture in zone III, in the region of the origin of the ring finger lumbrical muscle (Figure 1). The flexor digitorum superficialis tendon was intact. Macroscopically, both tendon and carpal tunnel appeared normal, with no evidence of tendon attrition; thus, the tendon was not sent for histologic examination. The ends of the ruptured FDP tendon to the ring finger were at the level of the superficial palmar arch, with the distal end appearing as though it had been cut sharply with a knife. Because of the short period of time from injury to exploration, delayed primary tendon repair was possible, along with side-to-side tenodesis to the intact ring finger flexor superficialis tendon in the palm (Figure 2). Two days after surgery, the patient started a controlled mobilization program using the Duran method.10
At final follow-up of 18 months, total active motion was 126°, which corresponds to a good outcome, according to the Strickland and Glogovac criteria.11 Grip strength was 50 kg, which was 84% of grip strength on the uninjured side. The patient was back to recreational activity but had not returned to work.
Discussion
Most flexor tendon ruptures result from avulsion of the FDP tendon at its distal phalanx insertion, commonly known as Jersey finger. However, true midsubstance spontaneous ruptures are infrequent. Reports of spontaneous tendon ruptures of all types, including those of the hand, have increased in incidence in most countries.12 Bois and colleagues,13 who have reviewed the literature over a 50-year period, found a total of 50 spontaneous ruptures of “normal” flexor tendon in 43 cases. The authors point to unique historical and physical examinaton findings that help differentiate spontaneous tendon ruptures from the more common FDP avulsions. Such findings include the sensation of a pop or snap, or a sudden sharp pain or cramp within the palmar region. In contrast, most avulsion ruptures cause discomfort within the region of the digit. In type I avulsion injuries of the FDP tendon, the proximal tendon stump usually retracts proximal to the digital tendon sheath, causing a tender mass in the palm.14 Flexor digitorum profundus tendon avulsions, however, are not typically associated with a snap or pop in the palm. When spontaneous ruptures of the hand occur, they typically involve the profundus tendon of the small finger, in the area of the lumbrical origin.13
In equivocal cases when the site of rupture is uncertain, ultrasound and magnetic resonance imaging may assist in making the diagnosis and provide important preoperative information for surgical decision-making and planning; this information may decrease postoperative morbidity by minimizing surgical dissection.
The etiology of spontaneous ruptures is incompletely understood. For any rupture of the ulnar flexor tendons, the hook of the hamate should be examined to rule out a previous fracture as a cause of tendon attrition.15 Tendon vascularization may be a cause for tendon rupture in the hand. When the blood supply of the lumbrical muscles was examined in 100 upper extremities from human cadavers using vascular injection studies,16 it was discovered that each lumbrical muscle received its arterial supply from 4 sources: the superficial palmar arch, the common palmar digital artery, the deep palmar arch, and the dorsal digital artery. There were no anastomoses between the networks supplying the lumbrical muscles and the FDP tendons within the palm, suggesting a possible watershed zone between the FDP tendon and lumbrical muscle origin. The patient described in this case had the tendon rupture in the area of potential hypovascularity at the lumbrical origin.
Important factors in the decision-making process for surgical treatment include the length of time between rupture and treatment, the site of rupture, and the condition of the ruptured tendon ends. Patients who present in the first 3 weeks of injury can be treated by primary tendon repair, provided that the ruptured tendon ends are not significantly frayed or attenuated. For patients presenting more than 3 weeks after injury, interposition tendon grafts or tendon transfers are suitable options for ruptures in zone III. Distal interphalangeal joint arthrodesis is another alternative in specific cases where reconstruction is not possible. In this case, direct end-to-end repair was possible, as well as tenodesis to the intact ring finger superficialis in order to prevent stretching of the repair.
Localizing the level of the tendon rupture clinically is difficult. When the site of the profundus tendon rupture is uncertain, and there is no tenderness in zone I or the PIP joint, the first incision should be made at the metacarpophalangeal joint level. This first incision will indicate if the rupture occurred in zone III. If the tendon is intact at that location, then the next incision should be at the level of the PIP joint.
Conclusion
We report a patient treated for spontaneous rupture of the flexor tendon in zone III. He was treated in the acute setting with direct tendon repair. It is important to consider spontaneous rupture of the tendon in patients presenting with a snap/pop and the sudden inability to flex a finger. A tendon rupture can be diagnosed as spontaneous in the absence of an underlying pathologic condition such as rheumatoid arthritis, gout, or occult carpal fractures. In the acute setting, these may be repaired primarily; however, if presenting after a few weeks, alternative surgical options, including interposition tendon grafts, tendon transfer, and DIP joint arthrodesis, should be considered.
1. McMaster PE. Tendon and muscle ruptures, clinical and experimental studies on the causes and location of subcutaneous ruptures. J Bone Joint Surg Am. 1933;15(3):705-722.
2. Folmar RC, Nelson CL, Phalen GS. Ruptures of the flexor tendons in hands of non-rheumatoid patients. J Bone Joint Surg Am. 1972;54(3):579-584.
3. Grant I, Berger AC, Ireland DC. Rupture of the flexor digitorum profundus tendon to the small finger within the carpal tunnel. Hand Surg. 2005;10(1):109-114.
4. Hartford JM, Murphy JM. Flexor digitorum profundus rupture of the small finger secondary to nonunion of the hook of the hamate: a case report. J Hand Surg Am. 1996;21(14):621-623.
5. Johnston GH, Bowen CV. Attritional flexor tendon ruptures by an old lunate dislocation. J Hand Surg Am. 1988;13(5):701-703.
6. Koizumi M, Kanda T, Satoh S, Yoshizu T, Maki Y, Tsubokawa N. Attritional rupture of the flexor digitorum profundus tendon to the index finger caused by accessory carpal bone in the carpal tunnel: a case report. J Hand Surg Am. 2005;30(1):142-146.
7. Wurapa RK, Zelouf DS. Flexor tendon rupture caused by gout: a case report. J Hand Surg Am. 2002;27(4):591-593.
8. Masada K, Kanazawa M, Fuji T. Flexor tendon ruptures caused by an intraosseous ganglion of the hook of the hamate. J Hand Surg Br. 1997;22(3)383-385.
9. Boyes JH, Wilson JN, Smith JW. Flexor-tendon ruptures in the forearm and hand. J Bone Joint Surg Am. 1960;42(4):637-646.
10. Duran R, Houser R, Coleman C, et al. A preliminary report in the use of controlled passive motion following flexor tendon repair in zones II and III [abstract]. J Hand Surg. 1976;1(1):79.
11. Strickland JW, Glogovac SV. Digital function following flexor tendon repair in Zone II: A comparison of immobilization and controlled passive motion techniques. J Hand Surg Am. 1980;5(6):537-543.
12. Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507-1525.
13. Bois AJ, Johnston G, Classen D. Spontaneous flexor tendon ruptures of the hand: case series and review of the literature. J Hand Surg Am. 2007;32(7):1061-1071.
14. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69.
15. Jebson PJ, Ferlic RJ, Engber WF. Spontaneous rupture of ulnar-sided digital flexor tendons: don’t forget the hamate. Iowa Orthop J. 1995;15:225-227.
16. Zbrodowski A, Mariéthoz E, Bednarkiewicz M, Gajisin S. The blood supply of the lumbrical muscles. J Hand Surg Br. 1998;23(3):384-388.
1. McMaster PE. Tendon and muscle ruptures, clinical and experimental studies on the causes and location of subcutaneous ruptures. J Bone Joint Surg Am. 1933;15(3):705-722.
2. Folmar RC, Nelson CL, Phalen GS. Ruptures of the flexor tendons in hands of non-rheumatoid patients. J Bone Joint Surg Am. 1972;54(3):579-584.
3. Grant I, Berger AC, Ireland DC. Rupture of the flexor digitorum profundus tendon to the small finger within the carpal tunnel. Hand Surg. 2005;10(1):109-114.
4. Hartford JM, Murphy JM. Flexor digitorum profundus rupture of the small finger secondary to nonunion of the hook of the hamate: a case report. J Hand Surg Am. 1996;21(14):621-623.
5. Johnston GH, Bowen CV. Attritional flexor tendon ruptures by an old lunate dislocation. J Hand Surg Am. 1988;13(5):701-703.
6. Koizumi M, Kanda T, Satoh S, Yoshizu T, Maki Y, Tsubokawa N. Attritional rupture of the flexor digitorum profundus tendon to the index finger caused by accessory carpal bone in the carpal tunnel: a case report. J Hand Surg Am. 2005;30(1):142-146.
7. Wurapa RK, Zelouf DS. Flexor tendon rupture caused by gout: a case report. J Hand Surg Am. 2002;27(4):591-593.
8. Masada K, Kanazawa M, Fuji T. Flexor tendon ruptures caused by an intraosseous ganglion of the hook of the hamate. J Hand Surg Br. 1997;22(3)383-385.
9. Boyes JH, Wilson JN, Smith JW. Flexor-tendon ruptures in the forearm and hand. J Bone Joint Surg Am. 1960;42(4):637-646.
10. Duran R, Houser R, Coleman C, et al. A preliminary report in the use of controlled passive motion following flexor tendon repair in zones II and III [abstract]. J Hand Surg. 1976;1(1):79.
11. Strickland JW, Glogovac SV. Digital function following flexor tendon repair in Zone II: A comparison of immobilization and controlled passive motion techniques. J Hand Surg Am. 1980;5(6):537-543.
12. Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507-1525.
13. Bois AJ, Johnston G, Classen D. Spontaneous flexor tendon ruptures of the hand: case series and review of the literature. J Hand Surg Am. 2007;32(7):1061-1071.
14. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69.
15. Jebson PJ, Ferlic RJ, Engber WF. Spontaneous rupture of ulnar-sided digital flexor tendons: don’t forget the hamate. Iowa Orthop J. 1995;15:225-227.
16. Zbrodowski A, Mariéthoz E, Bednarkiewicz M, Gajisin S. The blood supply of the lumbrical muscles. J Hand Surg Br. 1998;23(3):384-388.
Cadaveric Study of Appropriate Screw Length for Distal Radius Stabilization Using Volar Plate Fixation
Distal radius fractures constitute 15% of all extremity fractures and are the most common upper extremity fractures.1-3 The incidence of distal radius fractures is continuing to escalate because of the expanding elderly population and concurrent increase in osteoporosis.3,4 In addition, open reduction and internal fixation with a volar locking plate for distal radius fractures are more commonly being performed by general orthopedists, who may not perform these surgeries frequently. Surgically treated patients experience less time immobilized and have a higher chance of regaining previous functional status.2 In a commonly used technique, volar fixed-angle plating is used to stabilize the distal radius. With the rising popularity of this method, more patients are having postoperative complications.1,3,5,6 Extensor tendon irritation and attritional rupture constitute up to 50% of all complications stemming from volar plating of the distal radius.1
Volar plate fixation of the distal radius was originally designed to decrease postoperative tendon complications by preventing the flexor and extensor tendons from coming into direct contact with the surgically placed plates and/or screws.1 This technique places the volar plate under the belly of the pronator quadratus muscle. Shielding the flexor tendons, the pronator quadratus can prevent the volar plate from causing flexor tendon attrition. This shielding does not occur on the dorsal side of the wrist because the extensor tendons are in full contact with the dorsal radius. As such, volar fixation gained in popularity on the premise of preventing extensor tendon complications by directly avoiding the dorsal compartment.1,7
The most common complication of volar plating ironically involves the dorsal compartment.1,7 The typical distal radius fracture occurs when a fall on an outstretched hand results in significant dorsal comminution. In these cases, it can be difficult to judge the appropriate screw length, as the depth gauge does not have an intact cortex to hook. There is the temptation to use intraoperative fluoroscopy and the depth gauge to estimate screw lengths at the distal radius, especially in cases in which a surgeon may not perform this type of surgery often. More specifically, use of a lateral image to gauge the appropriate length for screws may be tempting, but a false estimate is possible.
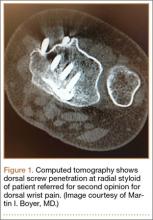
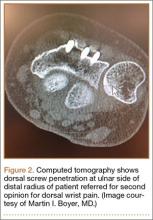
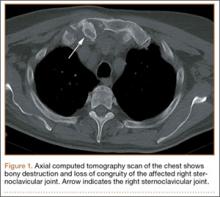
Screw prominence on the dorsal cortex may be caused by the complex geometry of the distal radius. This geometry is produced by the Lister tubercle and its adjacent groove for the extensor pollicis longus.7 The dorsal shape of the distal radius is a dome or dihedral with the thickest part at the Lister tubercle. The dihedral shape may hide possible dorsal screw prominence on a lateral radiograph, but screw prominence can be appreciated with computed tomography (CT) (Figures 1, 2).
We conducted a study to determine if and where screw prominence occurs, and in what amount, to establish general guidelines for screw depth based on lateral radiographs. We also wanted to be able to highlight the potential source of postoperative complications.
Materials and Methods
Twelve preserved cadaveric forearms were used for this study. Two sets of arms were paired, and the other arms came from different cadavers. In total, 5 male arms (3 left, 2 right) and 7 female arms (5 left, 2 right) were used.
The arms were harvested using a bone saw to cut through the humerus just proximal to the epicondyles, keeping the ulna and radius completely intact. Each arm was examined by the naked eye and by fluoroscopy to determine if any significant anatomical or traumatic variations in the distal radius were present. None showed any abnormal variation.
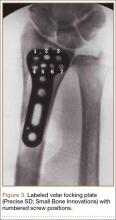
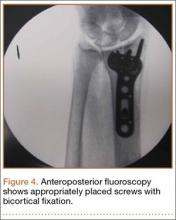
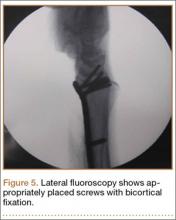
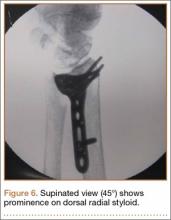
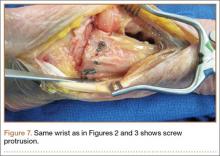
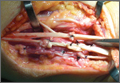
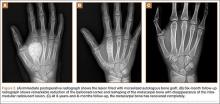
The flexor tendons and volar structures were removed to allow easy visualization and access to the distal radius. The volar locking plates (Precise SD; Small Bone Innovations) were positioned to the best anatomical and radiographic fit and secured with a proximal and distal Kirschner wire (Figure 3). A single cortical screw was placed through the shaft for compression. All 7 distal holes were drilled bicortically using an appropriately sized 2.0-mm drill and the standard block drill guide. A depth gauge was used in concordance with fluoroscopy to estimate the distance between cortices and appropriate screw lengths for each hole. A standard lateral view was used to determine the depth based on aligning the depth gauge at the dorsal cortex. The hook was not used to hook the dorsal cortex, as typically the dorsal cortex is severely comminuted and unavailable for measurement. Next, all 7 locking screws of premeasured length were secured into their respective holes. Anteroposterior, lateral, and oblique (forearm supinated and pronated 45°) radiographs were obtained to visualize screw placement and possible dorsal screw prominence (Figures 4-6).8 The extensor tendons and dorsal structures were then dissected away to expose any violation of the dorsal compartments, and calipers were used to measure absolute dorsal screw prominence and the depth of the Lister tubercle (Figure 7).
Mean (SD) dorsal prominence at each screw position was calculated. The screws were also categorized into radial (1,4), central (2,5), and ulnar (3,6,7) groups based on location within the plate (Figure 3). Equality of means testing was performed using a 1-way analysis of variance followed by a Bonferroni test.
Results
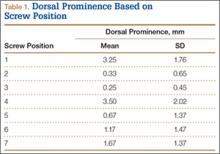
Mean (SD) dorsal prominence in millimeters is listed in Table 1. Positions 1 and 4 had significantly more dorsal prominence than the other 5 screw positions (P < .01 for all comparisons). Mean (SD) dorsal prominence based on grouped screw positions is listed in Table 2. There was significantly more dorsal prominence in the radial group that in the central group (P < .001) and ulnar group (P < .001). Mean depth of the Lister tubercle was 3.25 mm.
All prominent screws in the radial aspect of the radius were detected using a supinated 45° view. A 45° pronated view was not successful in demonstrating screw prominence on the ulnar side of the wrist because of overlap of the ulnar head.
Discussion
Extensor tendon irritation and extensor tendon rupture are frequent yet preventable complications of using volar plating systems to stabilize distal radius fractures. Many recent studies have investigated the intraoperative methodologies in order to identify real-time adjustments the surgeon can make to prevent negative outcomes. The first report of extensor tendon injury caused by volar plate fixation (published in 1989) was attributed to dorsal screw prominence.9,10 Even today, extensor tendon complications remain a challenge, as screw prominence is difficult to ascertain even with multiple intraoperative radiologic views.1,8
This study simulated real-time radiographic views to estimate if screws had extended into the dorsal compartment. These radiographic predictions were then correlated with the absolute dorsal screw prominence seen after dorsal compartment dissection. We determined that the supinated oblique view was the best imaging view for identifying radial styloid screw prominence.
Mean depth of the Lister tubercle was 3.25 mm (similar to previously reported 2 mm11). However, there was no correlation identified between depth of the Lister tubercle and amount of dorsal screw prominence.
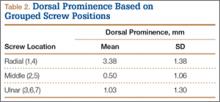
We wanted to identify high-risk areas and estimate expected dorsal screw prominence in order to make appropriate intraoperative screw length adjustments. The radius is divided into radial, central, and ulnar columns. The central screw positions had the least dorsal screw prominence (mean, 0.50 mm). This central position was considered low-risk. Both the radial and the ulnar screw positions had more dorsal screw prominence (means, 3.38 mm and 1.03 mm, respectively). Only the radial screws had significantly more prominence. However, this study was not powered to detect a difference as small as that between the central and ulnar screw positions. Despite the lack of statistical significance, it is clear from the data that the ulnar screws trend toward more dorsal prominence, and, therefore, screw measurements at both the radial and ulnar screw locations (using the depth gauge) require adjustments.
Extensor tendon contact was difficult to determine based on any specific screw length, as the extensor tendon had to be dissected to determine prominence. Based on observations, a prominence of 2 mm seemed to present a risk for tendon irritation. The periosteum and the rounded end of the screw may obviate the risk with 1 mm of prominence. However, this observation may not hold true in an in vivo situation.
This study had several limitations. First, only a single brand of plate was used, making these findings specific to this system. However, concepts and conclusions can be extrapolated to all systems. The radial side had the highest risk for prominence, and this factor should be accounted for when selecting screw lengths. In addition, the ulnar column also poses some risk, but not to the degree of the radial column. Another limitation is that fractures were not created in these radii; therefore, dorsal comminution was not recreated. In some cases, the dorsal cortex may be displaced dorsally and be somewhat protective. This study is not meant to be an exhaustive study on all volar plates or provide absolute recommendations. It is meant to suggest caution to surgeons who may not be familiar with the complex anatomy of the dorsal radius and to identify areas where the risk for screw penetration is highest.
Shortening screw lengths at the positions described may trigger surgeons’ concerns about stabilizing distal radius fractures. In a 2012 biomechanical study, Wall and colleagues12 found no difference between unicortical screws (placed at 75% of the distance to the dorsal cortex) and bicortical screws in effectiveness in stabilizing distal radius fractures.12 The proposed reduction will result in the desired bicortical screw lengths but limit prominence. In addition, in the setting of dorsal comminution, the increased stability gained by bicortical fixation is minimal.
In fractures with an intact dorsal cortex, standard depth gauges will likely produce appropriate screw length measurements. However, even in this situation, and based on the results reported by Wall and colleagues,12 subtraction of 1 to 2 mm may prove prudent. In cases in which the dorsal cortex is comminuted and screw estimates based on fluoroscopy are used, the lateral image may provide estimates that lead to screw prominence. A 45° supinated view should be used to check screw length for the radial side, the column most at risk. However, comminution may also obscure this view. We cannot comment on that, as the present study did not create comminuted fractures of the distal radius. In addition, the ulnar column posed a lesser but real risk of screw prominence, which must also be accounted for, and typically is not appreciated with alternate views.
Last, use of live fluoroscopy instead of standard anteroposterior and lateral views may prove valuable in assessing hardware placement and screw length in the setting of a comminuted distal radius fracture. Through use of live fluoroscopy, prominent screws, especially those on the radial side, may be identified, and potential tendon injury may be avoided. Keeping in mind the shape of the dorsal aspect of the distal radius should assist surgeons in preventing screw prominence dorsally and limit complications.
1. Maschke SD, Evans PJ, Schub D, Drake R, Lawton JN. Radiographic evaluation of dorsal screw penetration after volar fixed-angle plating of the distal radius: a cadaveric study. Hand. 2007;2(3):144-150.
2. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
3. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg. 2004;29(1):96-102.
4. Protopsaltis TS, Ruch DS. Volar approach to distal radius fractures. J Hand Surg. 2008;33(6):958-965.
5. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
6. Gruber G, Zacherl M, Giessauf C, et al. Quality of life after volar plate fixation of articular fractures of the distal part of the radius. J Bone Joint Surg Am. 2010;92(5):1170-1178.
7. Clement H, Pichler W, Nelson D, Hausleitner L, Tesch NP, Grechenig W. Morphometric analysis of Lister’s tubercle and its consequences on volar plate fixation of distal radius fractures. J Hand Surg. 2008;33(10):1716-1719.
8. Ozer K, Wolf JM, Watkins B, Hak DJ. Comparison of 4 fluoroscopic views for dorsal cortex screw penetration after volar plating of the distal radius. J Hand Surg. 2012;37(5):963-967.
9. Perry DC, Machin DM, Casaletto JA, Brown DJ. Minimising the risk of extensor pollicis longus rupture following volar plate fixation of distal radius fractures: a cadaveric study. Ann R Coll Surg Engl. 2011;93(1):57-60.
10. Wong-Chung J, Quinlan W. Rupture of extensor pollicis longus following fixation of a distal radius fracture. Injury. 1989;20(6):375-376.
11. Park DH, Goldie BS. Volar plating for distal radius fractures—do not trust the image intensifier when judging distal subchondral screw length. Tech Hand Up Extrem Surg. 2012;16(3):169-172.
12. Wall LB, Brodt MD, Silva MJ, Boyer MI, Calfee RP. The effects of screw length on stability of simulated osteoporotic distal radius fractures fixed with volar locking plates. J Hand Surg. 2012;37(3):446-453.
Distal radius fractures constitute 15% of all extremity fractures and are the most common upper extremity fractures.1-3 The incidence of distal radius fractures is continuing to escalate because of the expanding elderly population and concurrent increase in osteoporosis.3,4 In addition, open reduction and internal fixation with a volar locking plate for distal radius fractures are more commonly being performed by general orthopedists, who may not perform these surgeries frequently. Surgically treated patients experience less time immobilized and have a higher chance of regaining previous functional status.2 In a commonly used technique, volar fixed-angle plating is used to stabilize the distal radius. With the rising popularity of this method, more patients are having postoperative complications.1,3,5,6 Extensor tendon irritation and attritional rupture constitute up to 50% of all complications stemming from volar plating of the distal radius.1
Volar plate fixation of the distal radius was originally designed to decrease postoperative tendon complications by preventing the flexor and extensor tendons from coming into direct contact with the surgically placed plates and/or screws.1 This technique places the volar plate under the belly of the pronator quadratus muscle. Shielding the flexor tendons, the pronator quadratus can prevent the volar plate from causing flexor tendon attrition. This shielding does not occur on the dorsal side of the wrist because the extensor tendons are in full contact with the dorsal radius. As such, volar fixation gained in popularity on the premise of preventing extensor tendon complications by directly avoiding the dorsal compartment.1,7
The most common complication of volar plating ironically involves the dorsal compartment.1,7 The typical distal radius fracture occurs when a fall on an outstretched hand results in significant dorsal comminution. In these cases, it can be difficult to judge the appropriate screw length, as the depth gauge does not have an intact cortex to hook. There is the temptation to use intraoperative fluoroscopy and the depth gauge to estimate screw lengths at the distal radius, especially in cases in which a surgeon may not perform this type of surgery often. More specifically, use of a lateral image to gauge the appropriate length for screws may be tempting, but a false estimate is possible.
Screw prominence on the dorsal cortex may be caused by the complex geometry of the distal radius. This geometry is produced by the Lister tubercle and its adjacent groove for the extensor pollicis longus.7 The dorsal shape of the distal radius is a dome or dihedral with the thickest part at the Lister tubercle. The dihedral shape may hide possible dorsal screw prominence on a lateral radiograph, but screw prominence can be appreciated with computed tomography (CT) (Figures 1, 2).
We conducted a study to determine if and where screw prominence occurs, and in what amount, to establish general guidelines for screw depth based on lateral radiographs. We also wanted to be able to highlight the potential source of postoperative complications.
Materials and Methods
Twelve preserved cadaveric forearms were used for this study. Two sets of arms were paired, and the other arms came from different cadavers. In total, 5 male arms (3 left, 2 right) and 7 female arms (5 left, 2 right) were used.
The arms were harvested using a bone saw to cut through the humerus just proximal to the epicondyles, keeping the ulna and radius completely intact. Each arm was examined by the naked eye and by fluoroscopy to determine if any significant anatomical or traumatic variations in the distal radius were present. None showed any abnormal variation.
The flexor tendons and volar structures were removed to allow easy visualization and access to the distal radius. The volar locking plates (Precise SD; Small Bone Innovations) were positioned to the best anatomical and radiographic fit and secured with a proximal and distal Kirschner wire (Figure 3). A single cortical screw was placed through the shaft for compression. All 7 distal holes were drilled bicortically using an appropriately sized 2.0-mm drill and the standard block drill guide. A depth gauge was used in concordance with fluoroscopy to estimate the distance between cortices and appropriate screw lengths for each hole. A standard lateral view was used to determine the depth based on aligning the depth gauge at the dorsal cortex. The hook was not used to hook the dorsal cortex, as typically the dorsal cortex is severely comminuted and unavailable for measurement. Next, all 7 locking screws of premeasured length were secured into their respective holes. Anteroposterior, lateral, and oblique (forearm supinated and pronated 45°) radiographs were obtained to visualize screw placement and possible dorsal screw prominence (Figures 4-6).8 The extensor tendons and dorsal structures were then dissected away to expose any violation of the dorsal compartments, and calipers were used to measure absolute dorsal screw prominence and the depth of the Lister tubercle (Figure 7).
Mean (SD) dorsal prominence at each screw position was calculated. The screws were also categorized into radial (1,4), central (2,5), and ulnar (3,6,7) groups based on location within the plate (Figure 3). Equality of means testing was performed using a 1-way analysis of variance followed by a Bonferroni test.
Results
Mean (SD) dorsal prominence in millimeters is listed in Table 1. Positions 1 and 4 had significantly more dorsal prominence than the other 5 screw positions (P < .01 for all comparisons). Mean (SD) dorsal prominence based on grouped screw positions is listed in Table 2. There was significantly more dorsal prominence in the radial group that in the central group (P < .001) and ulnar group (P < .001). Mean depth of the Lister tubercle was 3.25 mm.
All prominent screws in the radial aspect of the radius were detected using a supinated 45° view. A 45° pronated view was not successful in demonstrating screw prominence on the ulnar side of the wrist because of overlap of the ulnar head.
Discussion
Extensor tendon irritation and extensor tendon rupture are frequent yet preventable complications of using volar plating systems to stabilize distal radius fractures. Many recent studies have investigated the intraoperative methodologies in order to identify real-time adjustments the surgeon can make to prevent negative outcomes. The first report of extensor tendon injury caused by volar plate fixation (published in 1989) was attributed to dorsal screw prominence.9,10 Even today, extensor tendon complications remain a challenge, as screw prominence is difficult to ascertain even with multiple intraoperative radiologic views.1,8
This study simulated real-time radiographic views to estimate if screws had extended into the dorsal compartment. These radiographic predictions were then correlated with the absolute dorsal screw prominence seen after dorsal compartment dissection. We determined that the supinated oblique view was the best imaging view for identifying radial styloid screw prominence.
Mean depth of the Lister tubercle was 3.25 mm (similar to previously reported 2 mm11). However, there was no correlation identified between depth of the Lister tubercle and amount of dorsal screw prominence.
We wanted to identify high-risk areas and estimate expected dorsal screw prominence in order to make appropriate intraoperative screw length adjustments. The radius is divided into radial, central, and ulnar columns. The central screw positions had the least dorsal screw prominence (mean, 0.50 mm). This central position was considered low-risk. Both the radial and the ulnar screw positions had more dorsal screw prominence (means, 3.38 mm and 1.03 mm, respectively). Only the radial screws had significantly more prominence. However, this study was not powered to detect a difference as small as that between the central and ulnar screw positions. Despite the lack of statistical significance, it is clear from the data that the ulnar screws trend toward more dorsal prominence, and, therefore, screw measurements at both the radial and ulnar screw locations (using the depth gauge) require adjustments.
Extensor tendon contact was difficult to determine based on any specific screw length, as the extensor tendon had to be dissected to determine prominence. Based on observations, a prominence of 2 mm seemed to present a risk for tendon irritation. The periosteum and the rounded end of the screw may obviate the risk with 1 mm of prominence. However, this observation may not hold true in an in vivo situation.
This study had several limitations. First, only a single brand of plate was used, making these findings specific to this system. However, concepts and conclusions can be extrapolated to all systems. The radial side had the highest risk for prominence, and this factor should be accounted for when selecting screw lengths. In addition, the ulnar column also poses some risk, but not to the degree of the radial column. Another limitation is that fractures were not created in these radii; therefore, dorsal comminution was not recreated. In some cases, the dorsal cortex may be displaced dorsally and be somewhat protective. This study is not meant to be an exhaustive study on all volar plates or provide absolute recommendations. It is meant to suggest caution to surgeons who may not be familiar with the complex anatomy of the dorsal radius and to identify areas where the risk for screw penetration is highest.
Shortening screw lengths at the positions described may trigger surgeons’ concerns about stabilizing distal radius fractures. In a 2012 biomechanical study, Wall and colleagues12 found no difference between unicortical screws (placed at 75% of the distance to the dorsal cortex) and bicortical screws in effectiveness in stabilizing distal radius fractures.12 The proposed reduction will result in the desired bicortical screw lengths but limit prominence. In addition, in the setting of dorsal comminution, the increased stability gained by bicortical fixation is minimal.
In fractures with an intact dorsal cortex, standard depth gauges will likely produce appropriate screw length measurements. However, even in this situation, and based on the results reported by Wall and colleagues,12 subtraction of 1 to 2 mm may prove prudent. In cases in which the dorsal cortex is comminuted and screw estimates based on fluoroscopy are used, the lateral image may provide estimates that lead to screw prominence. A 45° supinated view should be used to check screw length for the radial side, the column most at risk. However, comminution may also obscure this view. We cannot comment on that, as the present study did not create comminuted fractures of the distal radius. In addition, the ulnar column posed a lesser but real risk of screw prominence, which must also be accounted for, and typically is not appreciated with alternate views.
Last, use of live fluoroscopy instead of standard anteroposterior and lateral views may prove valuable in assessing hardware placement and screw length in the setting of a comminuted distal radius fracture. Through use of live fluoroscopy, prominent screws, especially those on the radial side, may be identified, and potential tendon injury may be avoided. Keeping in mind the shape of the dorsal aspect of the distal radius should assist surgeons in preventing screw prominence dorsally and limit complications.
Distal radius fractures constitute 15% of all extremity fractures and are the most common upper extremity fractures.1-3 The incidence of distal radius fractures is continuing to escalate because of the expanding elderly population and concurrent increase in osteoporosis.3,4 In addition, open reduction and internal fixation with a volar locking plate for distal radius fractures are more commonly being performed by general orthopedists, who may not perform these surgeries frequently. Surgically treated patients experience less time immobilized and have a higher chance of regaining previous functional status.2 In a commonly used technique, volar fixed-angle plating is used to stabilize the distal radius. With the rising popularity of this method, more patients are having postoperative complications.1,3,5,6 Extensor tendon irritation and attritional rupture constitute up to 50% of all complications stemming from volar plating of the distal radius.1
Volar plate fixation of the distal radius was originally designed to decrease postoperative tendon complications by preventing the flexor and extensor tendons from coming into direct contact with the surgically placed plates and/or screws.1 This technique places the volar plate under the belly of the pronator quadratus muscle. Shielding the flexor tendons, the pronator quadratus can prevent the volar plate from causing flexor tendon attrition. This shielding does not occur on the dorsal side of the wrist because the extensor tendons are in full contact with the dorsal radius. As such, volar fixation gained in popularity on the premise of preventing extensor tendon complications by directly avoiding the dorsal compartment.1,7
The most common complication of volar plating ironically involves the dorsal compartment.1,7 The typical distal radius fracture occurs when a fall on an outstretched hand results in significant dorsal comminution. In these cases, it can be difficult to judge the appropriate screw length, as the depth gauge does not have an intact cortex to hook. There is the temptation to use intraoperative fluoroscopy and the depth gauge to estimate screw lengths at the distal radius, especially in cases in which a surgeon may not perform this type of surgery often. More specifically, use of a lateral image to gauge the appropriate length for screws may be tempting, but a false estimate is possible.
Screw prominence on the dorsal cortex may be caused by the complex geometry of the distal radius. This geometry is produced by the Lister tubercle and its adjacent groove for the extensor pollicis longus.7 The dorsal shape of the distal radius is a dome or dihedral with the thickest part at the Lister tubercle. The dihedral shape may hide possible dorsal screw prominence on a lateral radiograph, but screw prominence can be appreciated with computed tomography (CT) (Figures 1, 2).
We conducted a study to determine if and where screw prominence occurs, and in what amount, to establish general guidelines for screw depth based on lateral radiographs. We also wanted to be able to highlight the potential source of postoperative complications.
Materials and Methods
Twelve preserved cadaveric forearms were used for this study. Two sets of arms were paired, and the other arms came from different cadavers. In total, 5 male arms (3 left, 2 right) and 7 female arms (5 left, 2 right) were used.
The arms were harvested using a bone saw to cut through the humerus just proximal to the epicondyles, keeping the ulna and radius completely intact. Each arm was examined by the naked eye and by fluoroscopy to determine if any significant anatomical or traumatic variations in the distal radius were present. None showed any abnormal variation.
The flexor tendons and volar structures were removed to allow easy visualization and access to the distal radius. The volar locking plates (Precise SD; Small Bone Innovations) were positioned to the best anatomical and radiographic fit and secured with a proximal and distal Kirschner wire (Figure 3). A single cortical screw was placed through the shaft for compression. All 7 distal holes were drilled bicortically using an appropriately sized 2.0-mm drill and the standard block drill guide. A depth gauge was used in concordance with fluoroscopy to estimate the distance between cortices and appropriate screw lengths for each hole. A standard lateral view was used to determine the depth based on aligning the depth gauge at the dorsal cortex. The hook was not used to hook the dorsal cortex, as typically the dorsal cortex is severely comminuted and unavailable for measurement. Next, all 7 locking screws of premeasured length were secured into their respective holes. Anteroposterior, lateral, and oblique (forearm supinated and pronated 45°) radiographs were obtained to visualize screw placement and possible dorsal screw prominence (Figures 4-6).8 The extensor tendons and dorsal structures were then dissected away to expose any violation of the dorsal compartments, and calipers were used to measure absolute dorsal screw prominence and the depth of the Lister tubercle (Figure 7).
Mean (SD) dorsal prominence at each screw position was calculated. The screws were also categorized into radial (1,4), central (2,5), and ulnar (3,6,7) groups based on location within the plate (Figure 3). Equality of means testing was performed using a 1-way analysis of variance followed by a Bonferroni test.
Results
Mean (SD) dorsal prominence in millimeters is listed in Table 1. Positions 1 and 4 had significantly more dorsal prominence than the other 5 screw positions (P < .01 for all comparisons). Mean (SD) dorsal prominence based on grouped screw positions is listed in Table 2. There was significantly more dorsal prominence in the radial group that in the central group (P < .001) and ulnar group (P < .001). Mean depth of the Lister tubercle was 3.25 mm.
All prominent screws in the radial aspect of the radius were detected using a supinated 45° view. A 45° pronated view was not successful in demonstrating screw prominence on the ulnar side of the wrist because of overlap of the ulnar head.
Discussion
Extensor tendon irritation and extensor tendon rupture are frequent yet preventable complications of using volar plating systems to stabilize distal radius fractures. Many recent studies have investigated the intraoperative methodologies in order to identify real-time adjustments the surgeon can make to prevent negative outcomes. The first report of extensor tendon injury caused by volar plate fixation (published in 1989) was attributed to dorsal screw prominence.9,10 Even today, extensor tendon complications remain a challenge, as screw prominence is difficult to ascertain even with multiple intraoperative radiologic views.1,8
This study simulated real-time radiographic views to estimate if screws had extended into the dorsal compartment. These radiographic predictions were then correlated with the absolute dorsal screw prominence seen after dorsal compartment dissection. We determined that the supinated oblique view was the best imaging view for identifying radial styloid screw prominence.
Mean depth of the Lister tubercle was 3.25 mm (similar to previously reported 2 mm11). However, there was no correlation identified between depth of the Lister tubercle and amount of dorsal screw prominence.
We wanted to identify high-risk areas and estimate expected dorsal screw prominence in order to make appropriate intraoperative screw length adjustments. The radius is divided into radial, central, and ulnar columns. The central screw positions had the least dorsal screw prominence (mean, 0.50 mm). This central position was considered low-risk. Both the radial and the ulnar screw positions had more dorsal screw prominence (means, 3.38 mm and 1.03 mm, respectively). Only the radial screws had significantly more prominence. However, this study was not powered to detect a difference as small as that between the central and ulnar screw positions. Despite the lack of statistical significance, it is clear from the data that the ulnar screws trend toward more dorsal prominence, and, therefore, screw measurements at both the radial and ulnar screw locations (using the depth gauge) require adjustments.
Extensor tendon contact was difficult to determine based on any specific screw length, as the extensor tendon had to be dissected to determine prominence. Based on observations, a prominence of 2 mm seemed to present a risk for tendon irritation. The periosteum and the rounded end of the screw may obviate the risk with 1 mm of prominence. However, this observation may not hold true in an in vivo situation.
This study had several limitations. First, only a single brand of plate was used, making these findings specific to this system. However, concepts and conclusions can be extrapolated to all systems. The radial side had the highest risk for prominence, and this factor should be accounted for when selecting screw lengths. In addition, the ulnar column also poses some risk, but not to the degree of the radial column. Another limitation is that fractures were not created in these radii; therefore, dorsal comminution was not recreated. In some cases, the dorsal cortex may be displaced dorsally and be somewhat protective. This study is not meant to be an exhaustive study on all volar plates or provide absolute recommendations. It is meant to suggest caution to surgeons who may not be familiar with the complex anatomy of the dorsal radius and to identify areas where the risk for screw penetration is highest.
Shortening screw lengths at the positions described may trigger surgeons’ concerns about stabilizing distal radius fractures. In a 2012 biomechanical study, Wall and colleagues12 found no difference between unicortical screws (placed at 75% of the distance to the dorsal cortex) and bicortical screws in effectiveness in stabilizing distal radius fractures.12 The proposed reduction will result in the desired bicortical screw lengths but limit prominence. In addition, in the setting of dorsal comminution, the increased stability gained by bicortical fixation is minimal.
In fractures with an intact dorsal cortex, standard depth gauges will likely produce appropriate screw length measurements. However, even in this situation, and based on the results reported by Wall and colleagues,12 subtraction of 1 to 2 mm may prove prudent. In cases in which the dorsal cortex is comminuted and screw estimates based on fluoroscopy are used, the lateral image may provide estimates that lead to screw prominence. A 45° supinated view should be used to check screw length for the radial side, the column most at risk. However, comminution may also obscure this view. We cannot comment on that, as the present study did not create comminuted fractures of the distal radius. In addition, the ulnar column posed a lesser but real risk of screw prominence, which must also be accounted for, and typically is not appreciated with alternate views.
Last, use of live fluoroscopy instead of standard anteroposterior and lateral views may prove valuable in assessing hardware placement and screw length in the setting of a comminuted distal radius fracture. Through use of live fluoroscopy, prominent screws, especially those on the radial side, may be identified, and potential tendon injury may be avoided. Keeping in mind the shape of the dorsal aspect of the distal radius should assist surgeons in preventing screw prominence dorsally and limit complications.
1. Maschke SD, Evans PJ, Schub D, Drake R, Lawton JN. Radiographic evaluation of dorsal screw penetration after volar fixed-angle plating of the distal radius: a cadaveric study. Hand. 2007;2(3):144-150.
2. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
3. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg. 2004;29(1):96-102.
4. Protopsaltis TS, Ruch DS. Volar approach to distal radius fractures. J Hand Surg. 2008;33(6):958-965.
5. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
6. Gruber G, Zacherl M, Giessauf C, et al. Quality of life after volar plate fixation of articular fractures of the distal part of the radius. J Bone Joint Surg Am. 2010;92(5):1170-1178.
7. Clement H, Pichler W, Nelson D, Hausleitner L, Tesch NP, Grechenig W. Morphometric analysis of Lister’s tubercle and its consequences on volar plate fixation of distal radius fractures. J Hand Surg. 2008;33(10):1716-1719.
8. Ozer K, Wolf JM, Watkins B, Hak DJ. Comparison of 4 fluoroscopic views for dorsal cortex screw penetration after volar plating of the distal radius. J Hand Surg. 2012;37(5):963-967.
9. Perry DC, Machin DM, Casaletto JA, Brown DJ. Minimising the risk of extensor pollicis longus rupture following volar plate fixation of distal radius fractures: a cadaveric study. Ann R Coll Surg Engl. 2011;93(1):57-60.
10. Wong-Chung J, Quinlan W. Rupture of extensor pollicis longus following fixation of a distal radius fracture. Injury. 1989;20(6):375-376.
11. Park DH, Goldie BS. Volar plating for distal radius fractures—do not trust the image intensifier when judging distal subchondral screw length. Tech Hand Up Extrem Surg. 2012;16(3):169-172.
12. Wall LB, Brodt MD, Silva MJ, Boyer MI, Calfee RP. The effects of screw length on stability of simulated osteoporotic distal radius fractures fixed with volar locking plates. J Hand Surg. 2012;37(3):446-453.
1. Maschke SD, Evans PJ, Schub D, Drake R, Lawton JN. Radiographic evaluation of dorsal screw penetration after volar fixed-angle plating of the distal radius: a cadaveric study. Hand. 2007;2(3):144-150.
2. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
3. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg. 2004;29(1):96-102.
4. Protopsaltis TS, Ruch DS. Volar approach to distal radius fractures. J Hand Surg. 2008;33(6):958-965.
5. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
6. Gruber G, Zacherl M, Giessauf C, et al. Quality of life after volar plate fixation of articular fractures of the distal part of the radius. J Bone Joint Surg Am. 2010;92(5):1170-1178.
7. Clement H, Pichler W, Nelson D, Hausleitner L, Tesch NP, Grechenig W. Morphometric analysis of Lister’s tubercle and its consequences on volar plate fixation of distal radius fractures. J Hand Surg. 2008;33(10):1716-1719.
8. Ozer K, Wolf JM, Watkins B, Hak DJ. Comparison of 4 fluoroscopic views for dorsal cortex screw penetration after volar plating of the distal radius. J Hand Surg. 2012;37(5):963-967.
9. Perry DC, Machin DM, Casaletto JA, Brown DJ. Minimising the risk of extensor pollicis longus rupture following volar plate fixation of distal radius fractures: a cadaveric study. Ann R Coll Surg Engl. 2011;93(1):57-60.
10. Wong-Chung J, Quinlan W. Rupture of extensor pollicis longus following fixation of a distal radius fracture. Injury. 1989;20(6):375-376.
11. Park DH, Goldie BS. Volar plating for distal radius fractures—do not trust the image intensifier when judging distal subchondral screw length. Tech Hand Up Extrem Surg. 2012;16(3):169-172.
12. Wall LB, Brodt MD, Silva MJ, Boyer MI, Calfee RP. The effects of screw length on stability of simulated osteoporotic distal radius fractures fixed with volar locking plates. J Hand Surg. 2012;37(3):446-453.
Trends in Thumb Carpometacarpal Interposition Arthroplasty in the United States, 2005–2011
A common entity, osteoarthritis (OA) at the base of the thumb is largely caused by the unique anatomy and biomechanics of the thumb carpometacarpal (CMC) joint.1 Radiographically evident CMC degeneration occurs in 40% of women and 25% of men over age 75 years, making the thumb CMC joint the most common site of surgical reconstruction for upper extremity OA.2,3
Over the past 40 years, numerous surgical techniques for managing thumb CMC-OA have been described. These include volar ligament reconstruction, first metacarpal osteotomy, CMC arthrodesis, CMC joint replacement, and trapeziectomy. Trapeziectomy can be performed in isolation or in combination with tendon interposition, ligament reconstruction, or ligament reconstruction and tendon interposition (LRTI).4-20 The authors of a recent systematic review concluded there is no evidence that any one surgical procedure for CMC-OA is superior to another in terms of pain, function, satisfaction, range of motion, or strength.4 Nevertheless, a recent survey found that 719 (62%) of 1156 US hand surgeons used LRTI as the treatment of choice for advanced CMC-OA.21
Our detailed literature search yielded no other database studies characterizing current trends in the practice patterns of US orthopedic surgeons who perform interposition arthroplasty for CMC arthritis. Analysis of these trends is important not only to patients but also to the broader orthopedic and health care community.22
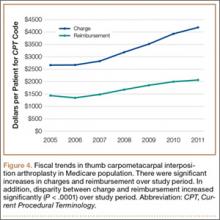
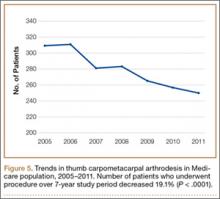
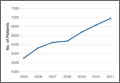
We conducted a study to investigate current trends in CMC interposition arthroplasty across time, sex, age, and region of the United States; per-patient charges and reimbursements; and the association between this procedure and concomitantly performed carpal tunnel syndrome (CTS) and carpal tunnel release (CTR). In addition, we compared incidence of CMC interposition arthroplasty with that of CMC arthrodesis.
Patients and Methods
All data were derived from the PearlDiver Patient Records Database (PearlDiver Technologies), a publicly available database of patients. The database stores procedure volumes, demographics, and average charge information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the present study were drawn from the Medicare database within the PearlDiver records, which has a total of 179,094,296 patient records covering the period 2005–2011. This study did not require institutional review board approval, as it used existing, publicly available data without identifiers linked to subjects.
PearlDiver Technologies granted us database access for academic research. The database was stored on a password-protected server maintained by PearlDiver. ICD-9 and CPT codes can be searched in isolation or in combination. Search results yield number of patients with a searched code (or combination of codes) in each year, age group, or region of the United States, as well as mean charge and mean reimbursement for the code or combination of codes.
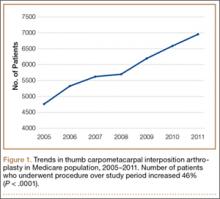
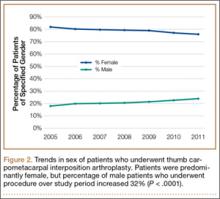
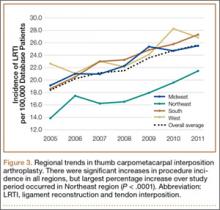
We used CPT code 25447 (arthroplasty, interposition, intercarpal, or CMC joints) to search the database for patients who underwent thumb CMC interposition arthroplasty. Although this code does not specify thumb, we are unaware of any procedure (other than thumb CMC interposition arthroplasty) typically given this code. Our search yielded procedure volumes, sex distribution, age distribution, region volumes, and mean per-patient charges and reimbursements for each CPT code. We then searched the resulting cohort for CTS (ICD-9 code 354.0), endoscopic CTR (CPT code 29848), and open CTR (CPT code 64721) to find CTR performed concomitantly with CMC interposition arthroplasty. Last, patients were tracked in the database past their surgery date to evaluate for postoperative physical or occupational therapy evaluations within 6 months (using CPT codes appearing in at least 1% of the cohort: 97001, 97003, 97004, 97110, 97112, 97124, 97140, 97150, 97350, 97535) and postoperative thumb, hand, or wrist radiographs within 6 months (using CPT codes appearing in at least 1% of the cohort: 73140, 73130, 73110). To ensure adequacy of 6-month postoperative data, we included in this portion of the study only those patients with surgery dates between 2005 and 2010.
For comparative purposes, we also searched the database for patients who underwent thumb CMC arthrodesis within the same period—using CPT codes 26841 and 26842 (arthrodesis CMC joint thumb, with or without internal fixation; with or without autograft) and CPT code 26820 (fusion in opposition, thumb, with autogenous graft).
Overall procedure volume data are reported as number of patients with the given CPT code in the database output in a given year. Age-group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who underwent the CPT code of interest that year. Mean charges and reimbursements are reported as results by the database for the code of interest (CPT 25447). Data for the region analysis are presented as an incidence, as there is an uneven distribution of patient volumes among regions. This incidence is calculated as number of patients in a particular region and year normalized to total number of patients in the database for that particular region or year. Regions are defined as Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-squared linear-by-linear association analysis was used to determine statistical significance with regard to trends over time in procedure volumes, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
In the database, we identified 41,171 unique patients who underwent CMC interposition arthroplasty between 2005 and 2011. Over the 7-year study period, number of patients who had CMC interposition arthroplasty increased 46.2%, from 4761 in 2005 to 6960 in 2011 (P < .0001) (Table 1, Figure 1). Throughout this period, females underwent CMC interposition arthroplasty more frequently than males at all time points (P < .0001). Overall ratio of female to male patients, however, changed significantly. In 2005, 18.1% of all CMC interposition arthroplasties were performed on male patients; this increased to 23.9% of all procedures by 2011 (P < .0001) (Figure 2). Table 1 presents an age-group analysis. There were no significant differences in relative percentage of patients in any given age group who underwent CMC interposition arthroplasty over the study period.
Analysis of overall procedure incidence by region revealed significant increases in all regions (P < .0001), ranging from 18.5% (West) to 54.5% (Northeast) (Figure 3). At all time points, the incidence of CMC interposition arthroplasty was significantly lower in the Northeast than in any other region and compared with the overall average.
Between 2005 and 2011, there were significant increases in both per-patient charges and reimbursements for CMC interposition arthroplasty (Figure 4). Mean per-patient charge increased from $2676 in 2005 to $4181 in 2011 (P < .0001), and mean per-patient reimbursement increased from $1445 in 2005 to $2061 in 2011 (P < .0001). The discrepancy between charge and reimbursement increased throughout the study period: Reimbursement in 2005 was 54.0% of the charge; this decreased to 49.3% by 2011 but was not statistically significant (P = .08).
Overall, 40.9% of patients who underwent CMC interposition arthroplasty also had a CTS diagnosis. Between 15.5% and 17.3% of these patients had concomitant open or endoscopic CTR at time of CMC interposition arthroplasty (Table 2). Percentage of patients who underwent concomitant CTR did not change significantly from 2005 to 2011 (P = .139). Use of postoperative occupational and/or physical therapy increased significantly over the study period, from 33.5% of patients in 2005 to 50.7% of patients in 2010 (P < .0001). Use of postoperative thumb, hand, and/or wrist radiography also increased throughout the study period, from 7.4% of patients in 2005 to 18.7% of patients in 2010 (P < .0001).
We identified 1916 unique patients who underwent thumb CMC arthrodesis between 2005 and 2011. Over the 7-year study period, there was a 19.1% decrease in number of patients who underwent CMC arthrodesis, from 309 in 2005 to 250 in 2011 (P < .0001) (Figure 5). Significantly fewer patients had CMC arthrodesis compared with CMC interposition arthroplasty at all time points, ranging from 6.5% (thumb CMC arthrodesis:CMC interposition arthroplasty) in 2005 to 3.6% in 2011 (P < .0001).
Discussion
Our results demonstrated a significant increase in use of thumb CMC interposition arthroplasty in a US Medicare population, with an increase of more than 46% from 2005 to 2011. This finding supports the findings of a recent cross-sectional survey-based study in which 719 (62%) of 1156 surveyed US hand surgeons reported performing trapeziectomy with LRTI for advanced thumb CMC-OA.21 A prior study had similar findings, with 692 (68%) of 1024 American Society for Surgery of the Hand (ASSH) members performing LRTI and 766 (75%) of 1024 performing some type of CMC interposition with trapeziectomy for advanced CMC-OA.23 This preference for CMC interposition arthroplasty prevails despite the fact that numerous studies have shown no superiority of any surgical procedure to another for CMC-OA in terms of pain, function, satisfaction, range of motion, and strength.7,15,18,19,24-34 Our data demonstrated that, not only does CMC interposition arthroplasty remain the most frequently used procedure for thumb CMC-OA, the incidence of CMC interposition arthroplasty continues to increase yearly.
The incidence of thumb CMC-OA is higher in women than in men, with more joint laxity a known contributor and subtle sex differences in trapezium geometry and congruence postulated as additional factors.3,35,36 This trend was confirmed in the present study, as females underwent significantly more CMC interposition arthroplasties at all time points. It is interesting that the overall ratio of female to male patients changed significantly over the study period, with the percentage of patients who were male increasing from 18.1% in 2005 to 23.9% in 2011. No previous studies have captured such a large cross section of the population to establish this trend. Although this trend is not necessarily intuitive, potential theories include increased acceptance of CMC interposition arthroplasty as a surgical option for male patients, and potentially a larger number of male patients seeking medical care for thumb CMC-OA in recent years.
Increases in procedure incidence were noted in all regions of the United States, but the largest percentage increase occurred in the Northeast. Despite this increase, the Northeast also had significantly lower CMC interposition arthroplasty incidence compared with all other regions and with the average procedure incidence throughout the study period—demonstrating some regional bias as to treatment of thumb CMC-OA. Unfortunately, because of database limitations and lack of specific CPT codes for other treatment options for thumb CMC-OA, we cannot ascertain if other types of surgery are more frequently used in the Northeast.
CTS and thumb CMC-OA often coexist.37 The estimated incidence of concomitant CTS in patients with CMC-OA is between 4% and 43%, but the rate of concomitant CTR and CMC interposition arthroplasty was not previously characterized in the literature.38,39 Results of the present study supported these findings; 41% of patients who underwent CMC interposition arthroplasty in our study also had a CTS diagnosis, compared with 43% in the 246-patient study by Florack and colleagues.38 We also found that 16% to 17% of patients who underwent CMC interposition arthroplasty underwent concomitant CTR; this rate remained consistent throughout the study period.
Our study demonstrated that, compared with CMC interposition arthroplasties, significantly fewer thumb CMC arthrodesis procedures were performed in the same Medicare population during the same period. Furthermore, the number of thumb CMC arthrodesis procedures declined yearly, with an overall decrease of 19% from 2005 to 2011. In a recent single-blinded, randomized trial, Vermeulen and colleagues40 compared thumb CMC arthrodesis and trapeziectomy with LRTI. They found superior patient satisfaction and significantly lower complication rates in women who underwent LRTI versus arthrodesis. The study was terminated prematurely because of these complications and thus was underpowered to determine differences in specific outcome measures. Previous studies comparing arthrodesis and interposition arthroplasties reported inconsistent outcomes. Hart and colleagues41 found no significant differences in pain or function between CMC arthrodesis and LRTI at a mean 7-year follow-up in a level II randomized controlled trial. Hartigan and colleagues15 reached similar conclusions in their retrospective comparison of the procedures. Without clear evidence supporting arthrodesis over interposition arthroplasty, the majority of surgeons favor interposition arthroplasty for thumb CMC-OA. Among Medicare patients, use of thumb CMC arthrodesis continues to fall.
This national database study had several limitations, which are common to all studies using the PearlDiver database22,42-47:
1. The power of the analysis depended on the quality of available data. Potential sources of error included accuracy of billing codes, and miscoding or noncoding by physicians.46
2. Although we used this database to try to accurately represent a large population of interest, we cannot guarantee the database represented a true cross section of the United States.
3. For the Medicare population, the PearlDiver database indexes data only in 7-year increments. Although the study period was long enough to detect significant trends, some data may not be accurately captured over a 7-year period.
4. Patients were not randomized to a treatment group.
5. The PearlDiver database does not include any clinical outcome data. Therefore, we cannot comment on the efficacy of the reported evaluations and interventions.
6. There is no specific CPT code for thumb CMC interposition arthroplasty. However, we are unaware of a CMC interposition arthroplasty performed for any area besides the thumb. Theoretically, the study population can include a negligible percentage of patients who had interposition arthroplasty of a CMC joint other than the thumb.
7. The database cannot be searched for use of thumb CMC-OA surgical techniques other than CMC interposition arthroplasty or arthrodesis, as isolated trapeziectomy, volar ligament reconstruction, implant arthroplasty, and metacarpal osteotomy lack specific CPT codes.
Conclusion
Thumb CMC-OA is a common entity among Medicare patients. There are numerous surgical options for cases that have failed conservative treatment. Despite the lack of evidence that thumb CMC interposition arthroplasty is superior to other surgical options, the number of patients who had this procedure increased 46% during the 2005–2011 study period. Although the majority of patients who undergo CMC interposition arthroplasty are female, the percentage of male patients has increased significantly. More than 40% of patients who have CMC interposition arthroplasty are also diagnosed with CTS, and 16% to 17% of patients who have CMC interposition arthroplasty will have a concomitant CTR. CMC arthrodesis is used in significantly fewer patients of Medicare age, and its use has been declining.
1. Hentz VR. Surgical treatment of trapeziometacarpal joint arthritis: a historical perspective. Clin Orthop Relat Res. 2014;472(4):1184-1189.
2. Armstrong AL, Hunter JB, Davis TR. The prevalence of degenerative arthritis of the base of the thumb in post-menopausal women. J Hand Surg Br. 1994;19(3):340-341.
3. Van Heest AE, Kallemeier P. Thumb carpal metacarpal arthritis. J Am Acad Orthop Surg. 2008;16(3):140-151.
4. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
5. Bodin ND, Spangler R, Thoder JJ. Interposition arthroplasty options for carpometacarpal arthritis of the thumb. Hand Clin. 2010;26(3):339-350, v-vi.
6. Cooney WP, Linscheid RL, Askew LJ. Total arthroplasty of the thumb trapeziometacarpal joint. Clin Orthop Relat Res. 1987;(220):35-45.
7. De Smet L, Vandenberghe L, Degreef I. Long-term outcome of trapeziectomy with ligament reconstruction and tendon interposition (LRTI) versus prosthesis arthroplasty for basal joint osteoarthritis of the thumb. Acta Orthop Belg. 2013;79(2):146-149.
8. Dell PC, Muniz RB. Interposition arthroplasty of the trapeziometacarpal joint for osteoarthritis. Clin Orthop Relat Res. 1987;(220):27-34.
9. Dhar S, Gray IC, Jones WA, Beddow FH. Simple excision of the trapezium for osteoarthritis of the carpometacarpal joint of the thumb. J Hand Surg Br. 1994;19(4):485-488.
10. Eaton RG, Littler JW. Ligament reconstruction for the painful thumb carpometacarpal joint. J Bone Joint Surg Am. 1973;55(8):1655-1666.
11. Eaton RG, Lane LB, Littler JW, Keyser JJ. Ligament reconstruction for the painful thumb carpometacarpal joint: a long-term assessment. J Hand Surg Am. 1984;9(5):692-699.
12. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg Am. 1985;10(5):645-654.
13. Elfar JC, Burton RI. Ligament reconstruction and tendon interposition for thumb basal arthritis. Hand Clin. 2013;29(1):15-25.
14. Froimson AI. Tendon arthroplasty of the trapeziometacarpal joint. Clin Orthop Relat Res. 1970;70:191-199.
15. Hartigan BJ, Stern PJ, Kiefhaber TR. Thumb carpometacarpal osteoarthritis: arthrodesis compared with ligament reconstruction and tendon interposition. J Bone Joint Surg Am. 2001;83(10):1470-1478.
16. Kenniston JA, Bozentka DJ. Treatment of advanced carpometacarpal joint disease: arthrodesis. Hand Clin. 2008;24(3):285-294, vi-vii.
17. Kokkalis ZT, Zanaros G, Weiser RW, Sotereanos DG. Trapezium resection with suspension and interposition arthroplasty using acellular dermal allograft for thumb carpometacarpal arthritis. J Hand Surg Am. 2009;34(6):1029-1036.
18. Kriegs-Au G, Petje G, Fojtl E, Ganger R, Zachs I. Ligament reconstruction with or without tendon interposition to treat primary thumb carpometacarpal osteoarthritis. Surgical technique. J Bone Joint Surg Am. 2005;87 suppl 1(Pt 1):78-85.
19. Park MJ, Lichtman G, Christian JB, et al. Surgical treatment of thumb carpometacarpal joint arthritis: a single institution experience from 1995–2005. Hand. 2008;3(4):304-310.
20. Park MJ, Lee AT, Yao J. Treatment of thumb carpometacarpal arthritis with arthroscopic hemitrapeziectomy and interposition arthroplasty. Orthopedics. 2012;35(12):e1759-e1764.
21. Wolf JM, Delaronde S. Current trends in nonoperative and operative treatment of trapeziometacarpal osteoarthritis: a survey of US hand surgeons. J Hand Surg Am. 2012;37(1):77-82.
22. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
23. Brunton LM, Wilgis EF. A survey to determine current practice patterns in the surgical treatment of advanced thumb carpometacarpal osteoarthrosis. Hand. 2010;5(4):415-422.
24. Belcher HJ, Nicholl JE. A comparison of trapeziectomy with and without ligament reconstruction and tendon interposition. J Hand Surg Br. 2000;25(4):350-356.
25. Davis TR, Pace A. Trapeziectomy for trapeziometacarpal joint osteoarthritis: is ligament reconstruction and temporary stabilisation of the pseudarthrosis with a Kirschner wire important? J Hand Surg Eur Vol. 2009;34(3):312-321.
26. Davis TR, Brady O, Dias JJ. Excision of the trapezium for osteoarthritis of the trapeziometacarpal joint: a study of the benefit of ligament reconstruction or tendon interposition. J Hand Surg Am. 2004;29(6):1069-1077.
27. De Smet L, Sioen W, Spaepen D, van Ransbeeck H. Treatment of basal joint arthritis of the thumb: trapeziectomy with or without tendon interposition/ligament reconstruction. Hand Surg. 2004;9(1):5-9.
28. Field J, Buchanan D. To suspend or not to suspend: a randomised single blind trial of simple trapeziectomy versus trapeziectomy and flexor carpi radialis suspension. J Hand Surg Eur Vol. 2007;32(4):462-466.
29. Gerwin M, Griffith A, Weiland AJ, Hotchkiss RN, McCormack RR. Ligament reconstruction basal joint arthroplasty without tendon interposition. Clin Orthop Relat Res. 1997;(342):42-45.
30. Jorheim M, Isaxon I, Flondell M, Kalen P, Atroshi I. Short-term outcomes of trapeziometacarpal Artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
31. Lehmann O, Herren DB, Simmen BR. Comparison of tendon suspension-interposition and silicon spacers in the treatment of degenerative osteoarthritis of the base of the thumb. Ann Chir Main Memb Super. 1998;17(1):25-30.
32. Nilsson A, Liljensten E, Bergstrom C, Sollerman C. Results from a degradable TMC joint spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
33. Schroder J, Kerkhoffs GM, Voerman HJ, Marti RK. Surgical treatment of basal joint disease of the thumb: comparison between resection-interposition arthroplasty and trapezio-metacarpal arthrodesis. Arch Orthop Trauma Surg. 2002;122(1):35-38.
34. Tagil M, Kopylov P. Swanson versus APL arthroplasty in the treatment of osteoarthritis of the trapeziometacarpal joint: a prospective and randomized study in 26 patients. J Hand Surg Br. 2002;27(5):452-456.
35. North ER, Rutledge WM. The trapezium-thumb metacarpal joint: the relationship of joint shape and degenerative joint disease. Hand. 1983;15(2):201-206.
36. Ateshian GA, Rosenwasser MP, Mow VC. Curvature characteristics and congruence of the thumb carpometacarpal joint: differences between female and male joints. J Biomech. 1992;25(6):591-607.
37. Sless Y, Sampson SP. Experience with transtrapezium approach for transverse carpal ligament release in patients with coexisted trapeziometacarpal joint osteoarthritis and carpal tunnel syndrome. Hand. 2007;2(3):151-154.
38. Florack TM, Miller RJ, Pellegrini VD, Burton RI, Dunn MG. The prevalence of carpal tunnel syndrome in patients with basal joint arthritis of the thumb. J Hand Surg Am. 1992;17(4):624-630.
39. Tsai TM, Laurentin-Perez LA, Wong MS, Tamai M. Ideas and innovations: radial approach to carpal tunnel release in conjunction with thumb carpometacarpal arthroplasty. Hand Surg. 2005;10(1):61-66.
40. Vermeulen GM, Brink SM, Slijper H, et al. Trapeziometacarpal arthrodesis or trapeziectomy with ligament reconstruction in primary trapeziometacarpal osteoarthritis: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(9):726-733.
41. Hart R, Janecek M, Siska V, Kucera B, Stipcak V. Interposition suspension arthroplasty according to Epping versus arthrodesis for trapeziometacarpal osteoarthritis. Eur Surg. 2006;38(6):433-438.
42. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005–2011. Am J Sports Med. 2013;41(10):2333-2339.
43. Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665.
44. Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443.
45. Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441.
46. Yeranosian MG, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. The costs associated with the evaluation of rotator cuff tears before surgical repair. J Shoulder Elbow Surg. 2013;22(12):1662-1666.
47. Daffner SD, Hymanson HJ, Wang JC. Cost and use of conservative management of lumbar disc herniation before surgical discectomy. Spine J. 2010;10(6):463-468.
A common entity, osteoarthritis (OA) at the base of the thumb is largely caused by the unique anatomy and biomechanics of the thumb carpometacarpal (CMC) joint.1 Radiographically evident CMC degeneration occurs in 40% of women and 25% of men over age 75 years, making the thumb CMC joint the most common site of surgical reconstruction for upper extremity OA.2,3
Over the past 40 years, numerous surgical techniques for managing thumb CMC-OA have been described. These include volar ligament reconstruction, first metacarpal osteotomy, CMC arthrodesis, CMC joint replacement, and trapeziectomy. Trapeziectomy can be performed in isolation or in combination with tendon interposition, ligament reconstruction, or ligament reconstruction and tendon interposition (LRTI).4-20 The authors of a recent systematic review concluded there is no evidence that any one surgical procedure for CMC-OA is superior to another in terms of pain, function, satisfaction, range of motion, or strength.4 Nevertheless, a recent survey found that 719 (62%) of 1156 US hand surgeons used LRTI as the treatment of choice for advanced CMC-OA.21
Our detailed literature search yielded no other database studies characterizing current trends in the practice patterns of US orthopedic surgeons who perform interposition arthroplasty for CMC arthritis. Analysis of these trends is important not only to patients but also to the broader orthopedic and health care community.22
We conducted a study to investigate current trends in CMC interposition arthroplasty across time, sex, age, and region of the United States; per-patient charges and reimbursements; and the association between this procedure and concomitantly performed carpal tunnel syndrome (CTS) and carpal tunnel release (CTR). In addition, we compared incidence of CMC interposition arthroplasty with that of CMC arthrodesis.
Patients and Methods
All data were derived from the PearlDiver Patient Records Database (PearlDiver Technologies), a publicly available database of patients. The database stores procedure volumes, demographics, and average charge information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the present study were drawn from the Medicare database within the PearlDiver records, which has a total of 179,094,296 patient records covering the period 2005–2011. This study did not require institutional review board approval, as it used existing, publicly available data without identifiers linked to subjects.
PearlDiver Technologies granted us database access for academic research. The database was stored on a password-protected server maintained by PearlDiver. ICD-9 and CPT codes can be searched in isolation or in combination. Search results yield number of patients with a searched code (or combination of codes) in each year, age group, or region of the United States, as well as mean charge and mean reimbursement for the code or combination of codes.
We used CPT code 25447 (arthroplasty, interposition, intercarpal, or CMC joints) to search the database for patients who underwent thumb CMC interposition arthroplasty. Although this code does not specify thumb, we are unaware of any procedure (other than thumb CMC interposition arthroplasty) typically given this code. Our search yielded procedure volumes, sex distribution, age distribution, region volumes, and mean per-patient charges and reimbursements for each CPT code. We then searched the resulting cohort for CTS (ICD-9 code 354.0), endoscopic CTR (CPT code 29848), and open CTR (CPT code 64721) to find CTR performed concomitantly with CMC interposition arthroplasty. Last, patients were tracked in the database past their surgery date to evaluate for postoperative physical or occupational therapy evaluations within 6 months (using CPT codes appearing in at least 1% of the cohort: 97001, 97003, 97004, 97110, 97112, 97124, 97140, 97150, 97350, 97535) and postoperative thumb, hand, or wrist radiographs within 6 months (using CPT codes appearing in at least 1% of the cohort: 73140, 73130, 73110). To ensure adequacy of 6-month postoperative data, we included in this portion of the study only those patients with surgery dates between 2005 and 2010.
For comparative purposes, we also searched the database for patients who underwent thumb CMC arthrodesis within the same period—using CPT codes 26841 and 26842 (arthrodesis CMC joint thumb, with or without internal fixation; with or without autograft) and CPT code 26820 (fusion in opposition, thumb, with autogenous graft).
Overall procedure volume data are reported as number of patients with the given CPT code in the database output in a given year. Age-group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who underwent the CPT code of interest that year. Mean charges and reimbursements are reported as results by the database for the code of interest (CPT 25447). Data for the region analysis are presented as an incidence, as there is an uneven distribution of patient volumes among regions. This incidence is calculated as number of patients in a particular region and year normalized to total number of patients in the database for that particular region or year. Regions are defined as Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-squared linear-by-linear association analysis was used to determine statistical significance with regard to trends over time in procedure volumes, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
In the database, we identified 41,171 unique patients who underwent CMC interposition arthroplasty between 2005 and 2011. Over the 7-year study period, number of patients who had CMC interposition arthroplasty increased 46.2%, from 4761 in 2005 to 6960 in 2011 (P < .0001) (Table 1, Figure 1). Throughout this period, females underwent CMC interposition arthroplasty more frequently than males at all time points (P < .0001). Overall ratio of female to male patients, however, changed significantly. In 2005, 18.1% of all CMC interposition arthroplasties were performed on male patients; this increased to 23.9% of all procedures by 2011 (P < .0001) (Figure 2). Table 1 presents an age-group analysis. There were no significant differences in relative percentage of patients in any given age group who underwent CMC interposition arthroplasty over the study period.
Analysis of overall procedure incidence by region revealed significant increases in all regions (P < .0001), ranging from 18.5% (West) to 54.5% (Northeast) (Figure 3). At all time points, the incidence of CMC interposition arthroplasty was significantly lower in the Northeast than in any other region and compared with the overall average.
Between 2005 and 2011, there were significant increases in both per-patient charges and reimbursements for CMC interposition arthroplasty (Figure 4). Mean per-patient charge increased from $2676 in 2005 to $4181 in 2011 (P < .0001), and mean per-patient reimbursement increased from $1445 in 2005 to $2061 in 2011 (P < .0001). The discrepancy between charge and reimbursement increased throughout the study period: Reimbursement in 2005 was 54.0% of the charge; this decreased to 49.3% by 2011 but was not statistically significant (P = .08).
Overall, 40.9% of patients who underwent CMC interposition arthroplasty also had a CTS diagnosis. Between 15.5% and 17.3% of these patients had concomitant open or endoscopic CTR at time of CMC interposition arthroplasty (Table 2). Percentage of patients who underwent concomitant CTR did not change significantly from 2005 to 2011 (P = .139). Use of postoperative occupational and/or physical therapy increased significantly over the study period, from 33.5% of patients in 2005 to 50.7% of patients in 2010 (P < .0001). Use of postoperative thumb, hand, and/or wrist radiography also increased throughout the study period, from 7.4% of patients in 2005 to 18.7% of patients in 2010 (P < .0001).
We identified 1916 unique patients who underwent thumb CMC arthrodesis between 2005 and 2011. Over the 7-year study period, there was a 19.1% decrease in number of patients who underwent CMC arthrodesis, from 309 in 2005 to 250 in 2011 (P < .0001) (Figure 5). Significantly fewer patients had CMC arthrodesis compared with CMC interposition arthroplasty at all time points, ranging from 6.5% (thumb CMC arthrodesis:CMC interposition arthroplasty) in 2005 to 3.6% in 2011 (P < .0001).
Discussion
Our results demonstrated a significant increase in use of thumb CMC interposition arthroplasty in a US Medicare population, with an increase of more than 46% from 2005 to 2011. This finding supports the findings of a recent cross-sectional survey-based study in which 719 (62%) of 1156 surveyed US hand surgeons reported performing trapeziectomy with LRTI for advanced thumb CMC-OA.21 A prior study had similar findings, with 692 (68%) of 1024 American Society for Surgery of the Hand (ASSH) members performing LRTI and 766 (75%) of 1024 performing some type of CMC interposition with trapeziectomy for advanced CMC-OA.23 This preference for CMC interposition arthroplasty prevails despite the fact that numerous studies have shown no superiority of any surgical procedure to another for CMC-OA in terms of pain, function, satisfaction, range of motion, and strength.7,15,18,19,24-34 Our data demonstrated that, not only does CMC interposition arthroplasty remain the most frequently used procedure for thumb CMC-OA, the incidence of CMC interposition arthroplasty continues to increase yearly.
The incidence of thumb CMC-OA is higher in women than in men, with more joint laxity a known contributor and subtle sex differences in trapezium geometry and congruence postulated as additional factors.3,35,36 This trend was confirmed in the present study, as females underwent significantly more CMC interposition arthroplasties at all time points. It is interesting that the overall ratio of female to male patients changed significantly over the study period, with the percentage of patients who were male increasing from 18.1% in 2005 to 23.9% in 2011. No previous studies have captured such a large cross section of the population to establish this trend. Although this trend is not necessarily intuitive, potential theories include increased acceptance of CMC interposition arthroplasty as a surgical option for male patients, and potentially a larger number of male patients seeking medical care for thumb CMC-OA in recent years.
Increases in procedure incidence were noted in all regions of the United States, but the largest percentage increase occurred in the Northeast. Despite this increase, the Northeast also had significantly lower CMC interposition arthroplasty incidence compared with all other regions and with the average procedure incidence throughout the study period—demonstrating some regional bias as to treatment of thumb CMC-OA. Unfortunately, because of database limitations and lack of specific CPT codes for other treatment options for thumb CMC-OA, we cannot ascertain if other types of surgery are more frequently used in the Northeast.
CTS and thumb CMC-OA often coexist.37 The estimated incidence of concomitant CTS in patients with CMC-OA is between 4% and 43%, but the rate of concomitant CTR and CMC interposition arthroplasty was not previously characterized in the literature.38,39 Results of the present study supported these findings; 41% of patients who underwent CMC interposition arthroplasty in our study also had a CTS diagnosis, compared with 43% in the 246-patient study by Florack and colleagues.38 We also found that 16% to 17% of patients who underwent CMC interposition arthroplasty underwent concomitant CTR; this rate remained consistent throughout the study period.
Our study demonstrated that, compared with CMC interposition arthroplasties, significantly fewer thumb CMC arthrodesis procedures were performed in the same Medicare population during the same period. Furthermore, the number of thumb CMC arthrodesis procedures declined yearly, with an overall decrease of 19% from 2005 to 2011. In a recent single-blinded, randomized trial, Vermeulen and colleagues40 compared thumb CMC arthrodesis and trapeziectomy with LRTI. They found superior patient satisfaction and significantly lower complication rates in women who underwent LRTI versus arthrodesis. The study was terminated prematurely because of these complications and thus was underpowered to determine differences in specific outcome measures. Previous studies comparing arthrodesis and interposition arthroplasties reported inconsistent outcomes. Hart and colleagues41 found no significant differences in pain or function between CMC arthrodesis and LRTI at a mean 7-year follow-up in a level II randomized controlled trial. Hartigan and colleagues15 reached similar conclusions in their retrospective comparison of the procedures. Without clear evidence supporting arthrodesis over interposition arthroplasty, the majority of surgeons favor interposition arthroplasty for thumb CMC-OA. Among Medicare patients, use of thumb CMC arthrodesis continues to fall.
This national database study had several limitations, which are common to all studies using the PearlDiver database22,42-47:
1. The power of the analysis depended on the quality of available data. Potential sources of error included accuracy of billing codes, and miscoding or noncoding by physicians.46
2. Although we used this database to try to accurately represent a large population of interest, we cannot guarantee the database represented a true cross section of the United States.
3. For the Medicare population, the PearlDiver database indexes data only in 7-year increments. Although the study period was long enough to detect significant trends, some data may not be accurately captured over a 7-year period.
4. Patients were not randomized to a treatment group.
5. The PearlDiver database does not include any clinical outcome data. Therefore, we cannot comment on the efficacy of the reported evaluations and interventions.
6. There is no specific CPT code for thumb CMC interposition arthroplasty. However, we are unaware of a CMC interposition arthroplasty performed for any area besides the thumb. Theoretically, the study population can include a negligible percentage of patients who had interposition arthroplasty of a CMC joint other than the thumb.
7. The database cannot be searched for use of thumb CMC-OA surgical techniques other than CMC interposition arthroplasty or arthrodesis, as isolated trapeziectomy, volar ligament reconstruction, implant arthroplasty, and metacarpal osteotomy lack specific CPT codes.
Conclusion
Thumb CMC-OA is a common entity among Medicare patients. There are numerous surgical options for cases that have failed conservative treatment. Despite the lack of evidence that thumb CMC interposition arthroplasty is superior to other surgical options, the number of patients who had this procedure increased 46% during the 2005–2011 study period. Although the majority of patients who undergo CMC interposition arthroplasty are female, the percentage of male patients has increased significantly. More than 40% of patients who have CMC interposition arthroplasty are also diagnosed with CTS, and 16% to 17% of patients who have CMC interposition arthroplasty will have a concomitant CTR. CMC arthrodesis is used in significantly fewer patients of Medicare age, and its use has been declining.
A common entity, osteoarthritis (OA) at the base of the thumb is largely caused by the unique anatomy and biomechanics of the thumb carpometacarpal (CMC) joint.1 Radiographically evident CMC degeneration occurs in 40% of women and 25% of men over age 75 years, making the thumb CMC joint the most common site of surgical reconstruction for upper extremity OA.2,3
Over the past 40 years, numerous surgical techniques for managing thumb CMC-OA have been described. These include volar ligament reconstruction, first metacarpal osteotomy, CMC arthrodesis, CMC joint replacement, and trapeziectomy. Trapeziectomy can be performed in isolation or in combination with tendon interposition, ligament reconstruction, or ligament reconstruction and tendon interposition (LRTI).4-20 The authors of a recent systematic review concluded there is no evidence that any one surgical procedure for CMC-OA is superior to another in terms of pain, function, satisfaction, range of motion, or strength.4 Nevertheless, a recent survey found that 719 (62%) of 1156 US hand surgeons used LRTI as the treatment of choice for advanced CMC-OA.21
Our detailed literature search yielded no other database studies characterizing current trends in the practice patterns of US orthopedic surgeons who perform interposition arthroplasty for CMC arthritis. Analysis of these trends is important not only to patients but also to the broader orthopedic and health care community.22
We conducted a study to investigate current trends in CMC interposition arthroplasty across time, sex, age, and region of the United States; per-patient charges and reimbursements; and the association between this procedure and concomitantly performed carpal tunnel syndrome (CTS) and carpal tunnel release (CTR). In addition, we compared incidence of CMC interposition arthroplasty with that of CMC arthrodesis.
Patients and Methods
All data were derived from the PearlDiver Patient Records Database (PearlDiver Technologies), a publicly available database of patients. The database stores procedure volumes, demographics, and average charge information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the present study were drawn from the Medicare database within the PearlDiver records, which has a total of 179,094,296 patient records covering the period 2005–2011. This study did not require institutional review board approval, as it used existing, publicly available data without identifiers linked to subjects.
PearlDiver Technologies granted us database access for academic research. The database was stored on a password-protected server maintained by PearlDiver. ICD-9 and CPT codes can be searched in isolation or in combination. Search results yield number of patients with a searched code (or combination of codes) in each year, age group, or region of the United States, as well as mean charge and mean reimbursement for the code or combination of codes.
We used CPT code 25447 (arthroplasty, interposition, intercarpal, or CMC joints) to search the database for patients who underwent thumb CMC interposition arthroplasty. Although this code does not specify thumb, we are unaware of any procedure (other than thumb CMC interposition arthroplasty) typically given this code. Our search yielded procedure volumes, sex distribution, age distribution, region volumes, and mean per-patient charges and reimbursements for each CPT code. We then searched the resulting cohort for CTS (ICD-9 code 354.0), endoscopic CTR (CPT code 29848), and open CTR (CPT code 64721) to find CTR performed concomitantly with CMC interposition arthroplasty. Last, patients were tracked in the database past their surgery date to evaluate for postoperative physical or occupational therapy evaluations within 6 months (using CPT codes appearing in at least 1% of the cohort: 97001, 97003, 97004, 97110, 97112, 97124, 97140, 97150, 97350, 97535) and postoperative thumb, hand, or wrist radiographs within 6 months (using CPT codes appearing in at least 1% of the cohort: 73140, 73130, 73110). To ensure adequacy of 6-month postoperative data, we included in this portion of the study only those patients with surgery dates between 2005 and 2010.
For comparative purposes, we also searched the database for patients who underwent thumb CMC arthrodesis within the same period—using CPT codes 26841 and 26842 (arthrodesis CMC joint thumb, with or without internal fixation; with or without autograft) and CPT code 26820 (fusion in opposition, thumb, with autogenous graft).
Overall procedure volume data are reported as number of patients with the given CPT code in the database output in a given year. Age-group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who underwent the CPT code of interest that year. Mean charges and reimbursements are reported as results by the database for the code of interest (CPT 25447). Data for the region analysis are presented as an incidence, as there is an uneven distribution of patient volumes among regions. This incidence is calculated as number of patients in a particular region and year normalized to total number of patients in the database for that particular region or year. Regions are defined as Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-squared linear-by-linear association analysis was used to determine statistical significance with regard to trends over time in procedure volumes, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
In the database, we identified 41,171 unique patients who underwent CMC interposition arthroplasty between 2005 and 2011. Over the 7-year study period, number of patients who had CMC interposition arthroplasty increased 46.2%, from 4761 in 2005 to 6960 in 2011 (P < .0001) (Table 1, Figure 1). Throughout this period, females underwent CMC interposition arthroplasty more frequently than males at all time points (P < .0001). Overall ratio of female to male patients, however, changed significantly. In 2005, 18.1% of all CMC interposition arthroplasties were performed on male patients; this increased to 23.9% of all procedures by 2011 (P < .0001) (Figure 2). Table 1 presents an age-group analysis. There were no significant differences in relative percentage of patients in any given age group who underwent CMC interposition arthroplasty over the study period.
Analysis of overall procedure incidence by region revealed significant increases in all regions (P < .0001), ranging from 18.5% (West) to 54.5% (Northeast) (Figure 3). At all time points, the incidence of CMC interposition arthroplasty was significantly lower in the Northeast than in any other region and compared with the overall average.
Between 2005 and 2011, there were significant increases in both per-patient charges and reimbursements for CMC interposition arthroplasty (Figure 4). Mean per-patient charge increased from $2676 in 2005 to $4181 in 2011 (P < .0001), and mean per-patient reimbursement increased from $1445 in 2005 to $2061 in 2011 (P < .0001). The discrepancy between charge and reimbursement increased throughout the study period: Reimbursement in 2005 was 54.0% of the charge; this decreased to 49.3% by 2011 but was not statistically significant (P = .08).
Overall, 40.9% of patients who underwent CMC interposition arthroplasty also had a CTS diagnosis. Between 15.5% and 17.3% of these patients had concomitant open or endoscopic CTR at time of CMC interposition arthroplasty (Table 2). Percentage of patients who underwent concomitant CTR did not change significantly from 2005 to 2011 (P = .139). Use of postoperative occupational and/or physical therapy increased significantly over the study period, from 33.5% of patients in 2005 to 50.7% of patients in 2010 (P < .0001). Use of postoperative thumb, hand, and/or wrist radiography also increased throughout the study period, from 7.4% of patients in 2005 to 18.7% of patients in 2010 (P < .0001).
We identified 1916 unique patients who underwent thumb CMC arthrodesis between 2005 and 2011. Over the 7-year study period, there was a 19.1% decrease in number of patients who underwent CMC arthrodesis, from 309 in 2005 to 250 in 2011 (P < .0001) (Figure 5). Significantly fewer patients had CMC arthrodesis compared with CMC interposition arthroplasty at all time points, ranging from 6.5% (thumb CMC arthrodesis:CMC interposition arthroplasty) in 2005 to 3.6% in 2011 (P < .0001).
Discussion
Our results demonstrated a significant increase in use of thumb CMC interposition arthroplasty in a US Medicare population, with an increase of more than 46% from 2005 to 2011. This finding supports the findings of a recent cross-sectional survey-based study in which 719 (62%) of 1156 surveyed US hand surgeons reported performing trapeziectomy with LRTI for advanced thumb CMC-OA.21 A prior study had similar findings, with 692 (68%) of 1024 American Society for Surgery of the Hand (ASSH) members performing LRTI and 766 (75%) of 1024 performing some type of CMC interposition with trapeziectomy for advanced CMC-OA.23 This preference for CMC interposition arthroplasty prevails despite the fact that numerous studies have shown no superiority of any surgical procedure to another for CMC-OA in terms of pain, function, satisfaction, range of motion, and strength.7,15,18,19,24-34 Our data demonstrated that, not only does CMC interposition arthroplasty remain the most frequently used procedure for thumb CMC-OA, the incidence of CMC interposition arthroplasty continues to increase yearly.
The incidence of thumb CMC-OA is higher in women than in men, with more joint laxity a known contributor and subtle sex differences in trapezium geometry and congruence postulated as additional factors.3,35,36 This trend was confirmed in the present study, as females underwent significantly more CMC interposition arthroplasties at all time points. It is interesting that the overall ratio of female to male patients changed significantly over the study period, with the percentage of patients who were male increasing from 18.1% in 2005 to 23.9% in 2011. No previous studies have captured such a large cross section of the population to establish this trend. Although this trend is not necessarily intuitive, potential theories include increased acceptance of CMC interposition arthroplasty as a surgical option for male patients, and potentially a larger number of male patients seeking medical care for thumb CMC-OA in recent years.
Increases in procedure incidence were noted in all regions of the United States, but the largest percentage increase occurred in the Northeast. Despite this increase, the Northeast also had significantly lower CMC interposition arthroplasty incidence compared with all other regions and with the average procedure incidence throughout the study period—demonstrating some regional bias as to treatment of thumb CMC-OA. Unfortunately, because of database limitations and lack of specific CPT codes for other treatment options for thumb CMC-OA, we cannot ascertain if other types of surgery are more frequently used in the Northeast.
CTS and thumb CMC-OA often coexist.37 The estimated incidence of concomitant CTS in patients with CMC-OA is between 4% and 43%, but the rate of concomitant CTR and CMC interposition arthroplasty was not previously characterized in the literature.38,39 Results of the present study supported these findings; 41% of patients who underwent CMC interposition arthroplasty in our study also had a CTS diagnosis, compared with 43% in the 246-patient study by Florack and colleagues.38 We also found that 16% to 17% of patients who underwent CMC interposition arthroplasty underwent concomitant CTR; this rate remained consistent throughout the study period.
Our study demonstrated that, compared with CMC interposition arthroplasties, significantly fewer thumb CMC arthrodesis procedures were performed in the same Medicare population during the same period. Furthermore, the number of thumb CMC arthrodesis procedures declined yearly, with an overall decrease of 19% from 2005 to 2011. In a recent single-blinded, randomized trial, Vermeulen and colleagues40 compared thumb CMC arthrodesis and trapeziectomy with LRTI. They found superior patient satisfaction and significantly lower complication rates in women who underwent LRTI versus arthrodesis. The study was terminated prematurely because of these complications and thus was underpowered to determine differences in specific outcome measures. Previous studies comparing arthrodesis and interposition arthroplasties reported inconsistent outcomes. Hart and colleagues41 found no significant differences in pain or function between CMC arthrodesis and LRTI at a mean 7-year follow-up in a level II randomized controlled trial. Hartigan and colleagues15 reached similar conclusions in their retrospective comparison of the procedures. Without clear evidence supporting arthrodesis over interposition arthroplasty, the majority of surgeons favor interposition arthroplasty for thumb CMC-OA. Among Medicare patients, use of thumb CMC arthrodesis continues to fall.
This national database study had several limitations, which are common to all studies using the PearlDiver database22,42-47:
1. The power of the analysis depended on the quality of available data. Potential sources of error included accuracy of billing codes, and miscoding or noncoding by physicians.46
2. Although we used this database to try to accurately represent a large population of interest, we cannot guarantee the database represented a true cross section of the United States.
3. For the Medicare population, the PearlDiver database indexes data only in 7-year increments. Although the study period was long enough to detect significant trends, some data may not be accurately captured over a 7-year period.
4. Patients were not randomized to a treatment group.
5. The PearlDiver database does not include any clinical outcome data. Therefore, we cannot comment on the efficacy of the reported evaluations and interventions.
6. There is no specific CPT code for thumb CMC interposition arthroplasty. However, we are unaware of a CMC interposition arthroplasty performed for any area besides the thumb. Theoretically, the study population can include a negligible percentage of patients who had interposition arthroplasty of a CMC joint other than the thumb.
7. The database cannot be searched for use of thumb CMC-OA surgical techniques other than CMC interposition arthroplasty or arthrodesis, as isolated trapeziectomy, volar ligament reconstruction, implant arthroplasty, and metacarpal osteotomy lack specific CPT codes.
Conclusion
Thumb CMC-OA is a common entity among Medicare patients. There are numerous surgical options for cases that have failed conservative treatment. Despite the lack of evidence that thumb CMC interposition arthroplasty is superior to other surgical options, the number of patients who had this procedure increased 46% during the 2005–2011 study period. Although the majority of patients who undergo CMC interposition arthroplasty are female, the percentage of male patients has increased significantly. More than 40% of patients who have CMC interposition arthroplasty are also diagnosed with CTS, and 16% to 17% of patients who have CMC interposition arthroplasty will have a concomitant CTR. CMC arthrodesis is used in significantly fewer patients of Medicare age, and its use has been declining.
1. Hentz VR. Surgical treatment of trapeziometacarpal joint arthritis: a historical perspective. Clin Orthop Relat Res. 2014;472(4):1184-1189.
2. Armstrong AL, Hunter JB, Davis TR. The prevalence of degenerative arthritis of the base of the thumb in post-menopausal women. J Hand Surg Br. 1994;19(3):340-341.
3. Van Heest AE, Kallemeier P. Thumb carpal metacarpal arthritis. J Am Acad Orthop Surg. 2008;16(3):140-151.
4. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
5. Bodin ND, Spangler R, Thoder JJ. Interposition arthroplasty options for carpometacarpal arthritis of the thumb. Hand Clin. 2010;26(3):339-350, v-vi.
6. Cooney WP, Linscheid RL, Askew LJ. Total arthroplasty of the thumb trapeziometacarpal joint. Clin Orthop Relat Res. 1987;(220):35-45.
7. De Smet L, Vandenberghe L, Degreef I. Long-term outcome of trapeziectomy with ligament reconstruction and tendon interposition (LRTI) versus prosthesis arthroplasty for basal joint osteoarthritis of the thumb. Acta Orthop Belg. 2013;79(2):146-149.
8. Dell PC, Muniz RB. Interposition arthroplasty of the trapeziometacarpal joint for osteoarthritis. Clin Orthop Relat Res. 1987;(220):27-34.
9. Dhar S, Gray IC, Jones WA, Beddow FH. Simple excision of the trapezium for osteoarthritis of the carpometacarpal joint of the thumb. J Hand Surg Br. 1994;19(4):485-488.
10. Eaton RG, Littler JW. Ligament reconstruction for the painful thumb carpometacarpal joint. J Bone Joint Surg Am. 1973;55(8):1655-1666.
11. Eaton RG, Lane LB, Littler JW, Keyser JJ. Ligament reconstruction for the painful thumb carpometacarpal joint: a long-term assessment. J Hand Surg Am. 1984;9(5):692-699.
12. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg Am. 1985;10(5):645-654.
13. Elfar JC, Burton RI. Ligament reconstruction and tendon interposition for thumb basal arthritis. Hand Clin. 2013;29(1):15-25.
14. Froimson AI. Tendon arthroplasty of the trapeziometacarpal joint. Clin Orthop Relat Res. 1970;70:191-199.
15. Hartigan BJ, Stern PJ, Kiefhaber TR. Thumb carpometacarpal osteoarthritis: arthrodesis compared with ligament reconstruction and tendon interposition. J Bone Joint Surg Am. 2001;83(10):1470-1478.
16. Kenniston JA, Bozentka DJ. Treatment of advanced carpometacarpal joint disease: arthrodesis. Hand Clin. 2008;24(3):285-294, vi-vii.
17. Kokkalis ZT, Zanaros G, Weiser RW, Sotereanos DG. Trapezium resection with suspension and interposition arthroplasty using acellular dermal allograft for thumb carpometacarpal arthritis. J Hand Surg Am. 2009;34(6):1029-1036.
18. Kriegs-Au G, Petje G, Fojtl E, Ganger R, Zachs I. Ligament reconstruction with or without tendon interposition to treat primary thumb carpometacarpal osteoarthritis. Surgical technique. J Bone Joint Surg Am. 2005;87 suppl 1(Pt 1):78-85.
19. Park MJ, Lichtman G, Christian JB, et al. Surgical treatment of thumb carpometacarpal joint arthritis: a single institution experience from 1995–2005. Hand. 2008;3(4):304-310.
20. Park MJ, Lee AT, Yao J. Treatment of thumb carpometacarpal arthritis with arthroscopic hemitrapeziectomy and interposition arthroplasty. Orthopedics. 2012;35(12):e1759-e1764.
21. Wolf JM, Delaronde S. Current trends in nonoperative and operative treatment of trapeziometacarpal osteoarthritis: a survey of US hand surgeons. J Hand Surg Am. 2012;37(1):77-82.
22. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
23. Brunton LM, Wilgis EF. A survey to determine current practice patterns in the surgical treatment of advanced thumb carpometacarpal osteoarthrosis. Hand. 2010;5(4):415-422.
24. Belcher HJ, Nicholl JE. A comparison of trapeziectomy with and without ligament reconstruction and tendon interposition. J Hand Surg Br. 2000;25(4):350-356.
25. Davis TR, Pace A. Trapeziectomy for trapeziometacarpal joint osteoarthritis: is ligament reconstruction and temporary stabilisation of the pseudarthrosis with a Kirschner wire important? J Hand Surg Eur Vol. 2009;34(3):312-321.
26. Davis TR, Brady O, Dias JJ. Excision of the trapezium for osteoarthritis of the trapeziometacarpal joint: a study of the benefit of ligament reconstruction or tendon interposition. J Hand Surg Am. 2004;29(6):1069-1077.
27. De Smet L, Sioen W, Spaepen D, van Ransbeeck H. Treatment of basal joint arthritis of the thumb: trapeziectomy with or without tendon interposition/ligament reconstruction. Hand Surg. 2004;9(1):5-9.
28. Field J, Buchanan D. To suspend or not to suspend: a randomised single blind trial of simple trapeziectomy versus trapeziectomy and flexor carpi radialis suspension. J Hand Surg Eur Vol. 2007;32(4):462-466.
29. Gerwin M, Griffith A, Weiland AJ, Hotchkiss RN, McCormack RR. Ligament reconstruction basal joint arthroplasty without tendon interposition. Clin Orthop Relat Res. 1997;(342):42-45.
30. Jorheim M, Isaxon I, Flondell M, Kalen P, Atroshi I. Short-term outcomes of trapeziometacarpal Artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
31. Lehmann O, Herren DB, Simmen BR. Comparison of tendon suspension-interposition and silicon spacers in the treatment of degenerative osteoarthritis of the base of the thumb. Ann Chir Main Memb Super. 1998;17(1):25-30.
32. Nilsson A, Liljensten E, Bergstrom C, Sollerman C. Results from a degradable TMC joint spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
33. Schroder J, Kerkhoffs GM, Voerman HJ, Marti RK. Surgical treatment of basal joint disease of the thumb: comparison between resection-interposition arthroplasty and trapezio-metacarpal arthrodesis. Arch Orthop Trauma Surg. 2002;122(1):35-38.
34. Tagil M, Kopylov P. Swanson versus APL arthroplasty in the treatment of osteoarthritis of the trapeziometacarpal joint: a prospective and randomized study in 26 patients. J Hand Surg Br. 2002;27(5):452-456.
35. North ER, Rutledge WM. The trapezium-thumb metacarpal joint: the relationship of joint shape and degenerative joint disease. Hand. 1983;15(2):201-206.
36. Ateshian GA, Rosenwasser MP, Mow VC. Curvature characteristics and congruence of the thumb carpometacarpal joint: differences between female and male joints. J Biomech. 1992;25(6):591-607.
37. Sless Y, Sampson SP. Experience with transtrapezium approach for transverse carpal ligament release in patients with coexisted trapeziometacarpal joint osteoarthritis and carpal tunnel syndrome. Hand. 2007;2(3):151-154.
38. Florack TM, Miller RJ, Pellegrini VD, Burton RI, Dunn MG. The prevalence of carpal tunnel syndrome in patients with basal joint arthritis of the thumb. J Hand Surg Am. 1992;17(4):624-630.
39. Tsai TM, Laurentin-Perez LA, Wong MS, Tamai M. Ideas and innovations: radial approach to carpal tunnel release in conjunction with thumb carpometacarpal arthroplasty. Hand Surg. 2005;10(1):61-66.
40. Vermeulen GM, Brink SM, Slijper H, et al. Trapeziometacarpal arthrodesis or trapeziectomy with ligament reconstruction in primary trapeziometacarpal osteoarthritis: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(9):726-733.
41. Hart R, Janecek M, Siska V, Kucera B, Stipcak V. Interposition suspension arthroplasty according to Epping versus arthrodesis for trapeziometacarpal osteoarthritis. Eur Surg. 2006;38(6):433-438.
42. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005–2011. Am J Sports Med. 2013;41(10):2333-2339.
43. Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665.
44. Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443.
45. Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441.
46. Yeranosian MG, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. The costs associated with the evaluation of rotator cuff tears before surgical repair. J Shoulder Elbow Surg. 2013;22(12):1662-1666.
47. Daffner SD, Hymanson HJ, Wang JC. Cost and use of conservative management of lumbar disc herniation before surgical discectomy. Spine J. 2010;10(6):463-468.
1. Hentz VR. Surgical treatment of trapeziometacarpal joint arthritis: a historical perspective. Clin Orthop Relat Res. 2014;472(4):1184-1189.
2. Armstrong AL, Hunter JB, Davis TR. The prevalence of degenerative arthritis of the base of the thumb in post-menopausal women. J Hand Surg Br. 1994;19(3):340-341.
3. Van Heest AE, Kallemeier P. Thumb carpal metacarpal arthritis. J Am Acad Orthop Surg. 2008;16(3):140-151.
4. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
5. Bodin ND, Spangler R, Thoder JJ. Interposition arthroplasty options for carpometacarpal arthritis of the thumb. Hand Clin. 2010;26(3):339-350, v-vi.
6. Cooney WP, Linscheid RL, Askew LJ. Total arthroplasty of the thumb trapeziometacarpal joint. Clin Orthop Relat Res. 1987;(220):35-45.
7. De Smet L, Vandenberghe L, Degreef I. Long-term outcome of trapeziectomy with ligament reconstruction and tendon interposition (LRTI) versus prosthesis arthroplasty for basal joint osteoarthritis of the thumb. Acta Orthop Belg. 2013;79(2):146-149.
8. Dell PC, Muniz RB. Interposition arthroplasty of the trapeziometacarpal joint for osteoarthritis. Clin Orthop Relat Res. 1987;(220):27-34.
9. Dhar S, Gray IC, Jones WA, Beddow FH. Simple excision of the trapezium for osteoarthritis of the carpometacarpal joint of the thumb. J Hand Surg Br. 1994;19(4):485-488.
10. Eaton RG, Littler JW. Ligament reconstruction for the painful thumb carpometacarpal joint. J Bone Joint Surg Am. 1973;55(8):1655-1666.
11. Eaton RG, Lane LB, Littler JW, Keyser JJ. Ligament reconstruction for the painful thumb carpometacarpal joint: a long-term assessment. J Hand Surg Am. 1984;9(5):692-699.
12. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg Am. 1985;10(5):645-654.
13. Elfar JC, Burton RI. Ligament reconstruction and tendon interposition for thumb basal arthritis. Hand Clin. 2013;29(1):15-25.
14. Froimson AI. Tendon arthroplasty of the trapeziometacarpal joint. Clin Orthop Relat Res. 1970;70:191-199.
15. Hartigan BJ, Stern PJ, Kiefhaber TR. Thumb carpometacarpal osteoarthritis: arthrodesis compared with ligament reconstruction and tendon interposition. J Bone Joint Surg Am. 2001;83(10):1470-1478.
16. Kenniston JA, Bozentka DJ. Treatment of advanced carpometacarpal joint disease: arthrodesis. Hand Clin. 2008;24(3):285-294, vi-vii.
17. Kokkalis ZT, Zanaros G, Weiser RW, Sotereanos DG. Trapezium resection with suspension and interposition arthroplasty using acellular dermal allograft for thumb carpometacarpal arthritis. J Hand Surg Am. 2009;34(6):1029-1036.
18. Kriegs-Au G, Petje G, Fojtl E, Ganger R, Zachs I. Ligament reconstruction with or without tendon interposition to treat primary thumb carpometacarpal osteoarthritis. Surgical technique. J Bone Joint Surg Am. 2005;87 suppl 1(Pt 1):78-85.
19. Park MJ, Lichtman G, Christian JB, et al. Surgical treatment of thumb carpometacarpal joint arthritis: a single institution experience from 1995–2005. Hand. 2008;3(4):304-310.
20. Park MJ, Lee AT, Yao J. Treatment of thumb carpometacarpal arthritis with arthroscopic hemitrapeziectomy and interposition arthroplasty. Orthopedics. 2012;35(12):e1759-e1764.
21. Wolf JM, Delaronde S. Current trends in nonoperative and operative treatment of trapeziometacarpal osteoarthritis: a survey of US hand surgeons. J Hand Surg Am. 2012;37(1):77-82.
22. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
23. Brunton LM, Wilgis EF. A survey to determine current practice patterns in the surgical treatment of advanced thumb carpometacarpal osteoarthrosis. Hand. 2010;5(4):415-422.
24. Belcher HJ, Nicholl JE. A comparison of trapeziectomy with and without ligament reconstruction and tendon interposition. J Hand Surg Br. 2000;25(4):350-356.
25. Davis TR, Pace A. Trapeziectomy for trapeziometacarpal joint osteoarthritis: is ligament reconstruction and temporary stabilisation of the pseudarthrosis with a Kirschner wire important? J Hand Surg Eur Vol. 2009;34(3):312-321.
26. Davis TR, Brady O, Dias JJ. Excision of the trapezium for osteoarthritis of the trapeziometacarpal joint: a study of the benefit of ligament reconstruction or tendon interposition. J Hand Surg Am. 2004;29(6):1069-1077.
27. De Smet L, Sioen W, Spaepen D, van Ransbeeck H. Treatment of basal joint arthritis of the thumb: trapeziectomy with or without tendon interposition/ligament reconstruction. Hand Surg. 2004;9(1):5-9.
28. Field J, Buchanan D. To suspend or not to suspend: a randomised single blind trial of simple trapeziectomy versus trapeziectomy and flexor carpi radialis suspension. J Hand Surg Eur Vol. 2007;32(4):462-466.
29. Gerwin M, Griffith A, Weiland AJ, Hotchkiss RN, McCormack RR. Ligament reconstruction basal joint arthroplasty without tendon interposition. Clin Orthop Relat Res. 1997;(342):42-45.
30. Jorheim M, Isaxon I, Flondell M, Kalen P, Atroshi I. Short-term outcomes of trapeziometacarpal Artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
31. Lehmann O, Herren DB, Simmen BR. Comparison of tendon suspension-interposition and silicon spacers in the treatment of degenerative osteoarthritis of the base of the thumb. Ann Chir Main Memb Super. 1998;17(1):25-30.
32. Nilsson A, Liljensten E, Bergstrom C, Sollerman C. Results from a degradable TMC joint spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
33. Schroder J, Kerkhoffs GM, Voerman HJ, Marti RK. Surgical treatment of basal joint disease of the thumb: comparison between resection-interposition arthroplasty and trapezio-metacarpal arthrodesis. Arch Orthop Trauma Surg. 2002;122(1):35-38.
34. Tagil M, Kopylov P. Swanson versus APL arthroplasty in the treatment of osteoarthritis of the trapeziometacarpal joint: a prospective and randomized study in 26 patients. J Hand Surg Br. 2002;27(5):452-456.
35. North ER, Rutledge WM. The trapezium-thumb metacarpal joint: the relationship of joint shape and degenerative joint disease. Hand. 1983;15(2):201-206.
36. Ateshian GA, Rosenwasser MP, Mow VC. Curvature characteristics and congruence of the thumb carpometacarpal joint: differences between female and male joints. J Biomech. 1992;25(6):591-607.
37. Sless Y, Sampson SP. Experience with transtrapezium approach for transverse carpal ligament release in patients with coexisted trapeziometacarpal joint osteoarthritis and carpal tunnel syndrome. Hand. 2007;2(3):151-154.
38. Florack TM, Miller RJ, Pellegrini VD, Burton RI, Dunn MG. The prevalence of carpal tunnel syndrome in patients with basal joint arthritis of the thumb. J Hand Surg Am. 1992;17(4):624-630.
39. Tsai TM, Laurentin-Perez LA, Wong MS, Tamai M. Ideas and innovations: radial approach to carpal tunnel release in conjunction with thumb carpometacarpal arthroplasty. Hand Surg. 2005;10(1):61-66.
40. Vermeulen GM, Brink SM, Slijper H, et al. Trapeziometacarpal arthrodesis or trapeziectomy with ligament reconstruction in primary trapeziometacarpal osteoarthritis: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(9):726-733.
41. Hart R, Janecek M, Siska V, Kucera B, Stipcak V. Interposition suspension arthroplasty according to Epping versus arthrodesis for trapeziometacarpal osteoarthritis. Eur Surg. 2006;38(6):433-438.
42. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005–2011. Am J Sports Med. 2013;41(10):2333-2339.
43. Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665.
44. Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443.
45. Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441.
46. Yeranosian MG, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. The costs associated with the evaluation of rotator cuff tears before surgical repair. J Shoulder Elbow Surg. 2013;22(12):1662-1666.
47. Daffner SD, Hymanson HJ, Wang JC. Cost and use of conservative management of lumbar disc herniation before surgical discectomy. Spine J. 2010;10(6):463-468.
Simultaneous Bilateral Functional Radiography in Ulnar Collateral Ligament Lesion of the Thumb: An Original Technique
Gamekeeper’s or skier’s thumb is caused by an injury to the ulnar collateral ligament (UCL) of the metacarpophalangeal (MCP) joint of the thumb. The mechanism of injury is forced radial and palmar abduction and hyperextension.
This lesion was initially described in 1955 by Campbell.1 It occurred in gamekeepers who worked in preserves in Scotland. The UCL was injured because of the way they killed rabbits—hence, gamekeeper’s thumb. Now these injuries are more common in skiers—skier’s thumb. In skiers, the mechanism of injury is the force exerted by the ski pole strap on the thumb during a fall. This injury is also seen in breakdancers.1,2
Different lesions can result, the most common being that of the UCL. The UCL lesion may be partial, with no joint instability,3,4 or total, with instability and subdislocation of the proximal phalanx.5-9 Rupture of the thumb adductor aponeurosis and displacement of the long extensor have been described as the cause of thumb instability.6-8
UCL rupture can occur in its extension or can cause a fracture-tearing in the proximal phalanx.9-12 Intra-articular fractures are sometimes found. The essential problem in UCL injuries is the impossibility of spontaneous healing once the rupture is complete, because of the Stener effect. (When the UCL ruptures, its proximal part retracts and runs above the fibrous expansion of the adductor muscle, which is interposed between the 2 parts of the ruptured UCL and prevents healing, even if the thumb is immobilized.) In these cases, only surgery can repair the lesion.2
In any thumb injury, particularly one caused by hyperabduction, a UCL lesion should be considered. The main problem is diagnosing sprain severity, which is evidenced by the degree of joint hypermobility. Radiologic examination should be performed in all cases to rule out fracture with tear, posterior capsular tear, palmar plate tear, and palmar subdislocation of the proximal phalanx, all of which are associated with UCL tearing.7-9
If the diagnosis is suspected, and radiographs show no fracture, comparative radiographs should be obtained in forced valgus.
Technique
We report on a simple, reliable, reproducible method that allows the patient’s thumbs to be compared, under the same force application conditions, on a single radiograph. This technique reduces the patient’s and examiner’s exposure to x-rays and is well tolerated by the patient. Anesthesia for the thumb is usually not necessary.
In each hand, the patient holds a cylindrical object, such as a drinking glass (standard diameter, 7.5-8.5 cm). We use an elastic crepe bandage roll (diameter, 7.5 cm; width, 10 cm). This roll is common in emergency departments (EDs) and easily accessible. The patient holds the rolls in his or her hands with the thumbs in the posteroanterior position (Figures 1–3) and places himself or herself on a 18×24-cm frame or directly on the radiography table.
Both thumbs are captured on a single functional radiograph for comparison of forced valgus of the MCP joints, as in our example cases. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Control Case
The single functional radiograph of both thumbs showed no evidence of joint laxity on the valgus stress test (Figure 4).
Case 1
A 72-year-old woman landed on her left hand when she fell backward while supporting the hand on a piece of furniture. She presented to the ED with pain in the region of the thumb and thenar eminence. Posteroanterior and lateral radiograph projections showed no significant bone injury (Figure 5). Given the patient’s persistent pain, the traumatologist suspected damage to the thumb UCL, so a simultaneous bilateral functional radiographic projection was obtained. The projection showed joint laxity, implying damage to the thumb UCL. Repair and reinsertion of the UCL were performed using a bone harpoon suture.
Case 2
A 58-year-old man sustained a left hand injury when, using both hands, he tried to catch hold of a falling wooden plank. When he presented to the ED the following week, he was given a diagnosis of thumb contusion and forced hyperabduction and was wearing a metal strap for immobilization. Radiographs showed no bone damage (Figure 6). Thumb UCL injury was suspected on the basis of the physical examination findings and the mechanism of injury. A bilateral simultaneous functional radiographic projection showed significant joint laxity. Surgical treatment with the pull-out technique was performed.
Case 3
A 44-year-old woman experienced forced traction from a dog leash and presented to the ED with pain in the right thumb region. Radiographs showed no bone damage (Figure 7). Thumb UCL injury was suspected. A bilateral simultaneous functional radiographic projection showed slight joint laxity, a sprain was diagnosed, and plaster bandaging was applied. Figures 8A–8D show the accurate thumb positions for performing the functional radiograph in forced valgus. We call the technique J.J.’s thumb radiographic projection.
Discussion
Examination using the stress test to cause joint tilt is crucial in making an accurate diagnosis and deciding on the most appropriate therapeutic approach.10 Most authors accept that surgical management is required in joint tilts over 30º, as these involve complete UCL rupture.10-12
The MCP joint must be examined in flexion, when the main fascicle of the UCL is tight, and not in extension, when the main fascicle of the UCL is relaxed. If we examine the thumb in extension, radial deviations may occur that are not caused by joint instability. Tilt here must be compared with that of the healthy side.11
Early diagnosis and adequate management are essential, as unnoticed or undervalued injuries can progress to painful sequelae, associated with stiffness, instability, and osteoarthritis, with evident harm to the grip and pinch functions of the hand. In many cases, clinical evidence of MCP joint instability is difficult. The radiologic diagnosis is usually obtained with comparative radiographs in forced valgus of both thumbs.
The forced valgus maneuver typically is performed by the examiner, who must stay with the patient in the radiography room and wear radiologic protection. Incredibly, some patients must force the valgus themselves.
The maneuver we have described clearly has complications, as it is painful, and some patients are uncooperative. Usually the thumb is anesthetized, and the examiner assumes the exposure to x-rays. The valgus deviation force that can be applied during stability testing may lead to further disruption of a partially torn ligament or displacement of a ruptured ligament if the overforced maneuver is performed.13,14 That does not occur with our technique. On the other hand, the forces applied to the thumbs must be symmetrical for comparison purposes. The way to prevent these inconveniences is to perform the forced valgus maneuver over both thumbs simultaneously, under the same force application conditions and on a single radiograph, without requiring the examiner to remain with the patient in the radiography room.
Heim15 designed a system for simultaneous functional radiographs, but an apparatus must be built to adapt it to the frame of the radiography table, and the technique involves hyperpronating both hands and bandaging them to the forearm—which is uncomfortable and bothersome for patients and, in our opinion, has a poor application in high-volume EDs.
The technique of having the patient hold a bandage roll (J.J.’s thumb radiographic projection) offers several advantages:
1. The thumb can be placed in flexion, tightening the main fascicle of the UCL, which is how the UCL must be examined.
2. Forced valgus is allowed. Holding a water glass involves opening the thumb and the necessary stability of the MCP joint of the thumb (grip function of thumb); this radiographic technique is functional.
3. The examiner need not stay with the patient in the radiography room or be exposed to x-rays.
4. The bandage roll is thick enough to generate forced valgus in a patient with large hands. The nonrigid roll makes the examination more tolerable and avoids overforced valgus, eliminating the need for anesthetic blockade.
5. The technique is accessible and simple. In fact, there is no need to remove the roll from its wrapping.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb: a clinical and anatomic study. J Bone Joint Surg Br. 1962;44(4):869-879.
3. Stener B. Hyperextension injuries to the metacarpophalangeal joint of the thumb: rupture of ligaments, fracture of sesamoid bones, rupture of flexor pollicis brevis. An anatomical and clinical study. Acta Chir Scand. 1963;125:275-293.
4. Coonrad RW, Goldner JL. A study of the pathological findings and treatment in soft-tissue injury of the thumb metacarpophalangeal joint. With a clinical study of the normal range of motion in one thousand thumbs and a study of post mortem findings of ligamentous structures in relation to function. J Bone Joint Surg Am. 1968;50(3):439-451.
5. Parikh M, Nahigian S, Froimson A. Gamekeeper’s thumb. Plast Reconstr Surg. 1976;58(1):24-31.
6. Kaplan EB. The pathology and treatment of radial subluxation of the thumb with ulnar displacement of the head of the first metacarpal. J Bone Joint Surg Am. 1961;43:541-546.
7. Yamanaka K, Yoshida K, Inoue H, Inoue A, Miyagi T. Locking of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1985;67(5):782-787.
8. Sennwald G, Segmüller G, Egli A. The late reconstruction of the ligament of the metacarpo-phalangeal joint of the thumb [in English, French]. Ann Chir Main. 1987;6(1):15-24.
9. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
10. Louis DS, Huebner JJ Jr, Hankin FM. Rupture and displacement of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. Preoperative diagnosis. J Bone Joint Surg Am. 1986;68(9):1320-1326.
11. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
12. Ritting AW, Baldwin PC, Rodner CM. Ulnar collateral ligament injury of the thumb metacarpophalangeal joint. Clin J Sport Med. 2010;20(2):106-112.
13. Cooper JG, Johnstone AJ, Hider P, Ardagh MW. Local anaesthetic infiltration increases the accuracy of assessment of ulnar collateral ligament injuries. Emerg Med Australas. 2005;17(2):132-136.
14. Noszian IM, Dinkhauser LM, Straub GM, Orthner E. Ulnar collateral ligament injuries of the thumb. Dislocation caused by stress radiography in 2 cases. Acta Orthop Scand. 1995;66(2):156-157.
15. Heim U. Simultaneous functional bilateral radiographies of the metacarpophalangeal joint of the thumb in hyper-pronation [in French]. Ann Chir Main. 1982;1(2):183-186.
Gamekeeper’s or skier’s thumb is caused by an injury to the ulnar collateral ligament (UCL) of the metacarpophalangeal (MCP) joint of the thumb. The mechanism of injury is forced radial and palmar abduction and hyperextension.
This lesion was initially described in 1955 by Campbell.1 It occurred in gamekeepers who worked in preserves in Scotland. The UCL was injured because of the way they killed rabbits—hence, gamekeeper’s thumb. Now these injuries are more common in skiers—skier’s thumb. In skiers, the mechanism of injury is the force exerted by the ski pole strap on the thumb during a fall. This injury is also seen in breakdancers.1,2
Different lesions can result, the most common being that of the UCL. The UCL lesion may be partial, with no joint instability,3,4 or total, with instability and subdislocation of the proximal phalanx.5-9 Rupture of the thumb adductor aponeurosis and displacement of the long extensor have been described as the cause of thumb instability.6-8
UCL rupture can occur in its extension or can cause a fracture-tearing in the proximal phalanx.9-12 Intra-articular fractures are sometimes found. The essential problem in UCL injuries is the impossibility of spontaneous healing once the rupture is complete, because of the Stener effect. (When the UCL ruptures, its proximal part retracts and runs above the fibrous expansion of the adductor muscle, which is interposed between the 2 parts of the ruptured UCL and prevents healing, even if the thumb is immobilized.) In these cases, only surgery can repair the lesion.2
In any thumb injury, particularly one caused by hyperabduction, a UCL lesion should be considered. The main problem is diagnosing sprain severity, which is evidenced by the degree of joint hypermobility. Radiologic examination should be performed in all cases to rule out fracture with tear, posterior capsular tear, palmar plate tear, and palmar subdislocation of the proximal phalanx, all of which are associated with UCL tearing.7-9
If the diagnosis is suspected, and radiographs show no fracture, comparative radiographs should be obtained in forced valgus.
Technique
We report on a simple, reliable, reproducible method that allows the patient’s thumbs to be compared, under the same force application conditions, on a single radiograph. This technique reduces the patient’s and examiner’s exposure to x-rays and is well tolerated by the patient. Anesthesia for the thumb is usually not necessary.
In each hand, the patient holds a cylindrical object, such as a drinking glass (standard diameter, 7.5-8.5 cm). We use an elastic crepe bandage roll (diameter, 7.5 cm; width, 10 cm). This roll is common in emergency departments (EDs) and easily accessible. The patient holds the rolls in his or her hands with the thumbs in the posteroanterior position (Figures 1–3) and places himself or herself on a 18×24-cm frame or directly on the radiography table.
Both thumbs are captured on a single functional radiograph for comparison of forced valgus of the MCP joints, as in our example cases. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Control Case
The single functional radiograph of both thumbs showed no evidence of joint laxity on the valgus stress test (Figure 4).
Case 1
A 72-year-old woman landed on her left hand when she fell backward while supporting the hand on a piece of furniture. She presented to the ED with pain in the region of the thumb and thenar eminence. Posteroanterior and lateral radiograph projections showed no significant bone injury (Figure 5). Given the patient’s persistent pain, the traumatologist suspected damage to the thumb UCL, so a simultaneous bilateral functional radiographic projection was obtained. The projection showed joint laxity, implying damage to the thumb UCL. Repair and reinsertion of the UCL were performed using a bone harpoon suture.
Case 2
A 58-year-old man sustained a left hand injury when, using both hands, he tried to catch hold of a falling wooden plank. When he presented to the ED the following week, he was given a diagnosis of thumb contusion and forced hyperabduction and was wearing a metal strap for immobilization. Radiographs showed no bone damage (Figure 6). Thumb UCL injury was suspected on the basis of the physical examination findings and the mechanism of injury. A bilateral simultaneous functional radiographic projection showed significant joint laxity. Surgical treatment with the pull-out technique was performed.
Case 3
A 44-year-old woman experienced forced traction from a dog leash and presented to the ED with pain in the right thumb region. Radiographs showed no bone damage (Figure 7). Thumb UCL injury was suspected. A bilateral simultaneous functional radiographic projection showed slight joint laxity, a sprain was diagnosed, and plaster bandaging was applied. Figures 8A–8D show the accurate thumb positions for performing the functional radiograph in forced valgus. We call the technique J.J.’s thumb radiographic projection.
Discussion
Examination using the stress test to cause joint tilt is crucial in making an accurate diagnosis and deciding on the most appropriate therapeutic approach.10 Most authors accept that surgical management is required in joint tilts over 30º, as these involve complete UCL rupture.10-12
The MCP joint must be examined in flexion, when the main fascicle of the UCL is tight, and not in extension, when the main fascicle of the UCL is relaxed. If we examine the thumb in extension, radial deviations may occur that are not caused by joint instability. Tilt here must be compared with that of the healthy side.11
Early diagnosis and adequate management are essential, as unnoticed or undervalued injuries can progress to painful sequelae, associated with stiffness, instability, and osteoarthritis, with evident harm to the grip and pinch functions of the hand. In many cases, clinical evidence of MCP joint instability is difficult. The radiologic diagnosis is usually obtained with comparative radiographs in forced valgus of both thumbs.
The forced valgus maneuver typically is performed by the examiner, who must stay with the patient in the radiography room and wear radiologic protection. Incredibly, some patients must force the valgus themselves.
The maneuver we have described clearly has complications, as it is painful, and some patients are uncooperative. Usually the thumb is anesthetized, and the examiner assumes the exposure to x-rays. The valgus deviation force that can be applied during stability testing may lead to further disruption of a partially torn ligament or displacement of a ruptured ligament if the overforced maneuver is performed.13,14 That does not occur with our technique. On the other hand, the forces applied to the thumbs must be symmetrical for comparison purposes. The way to prevent these inconveniences is to perform the forced valgus maneuver over both thumbs simultaneously, under the same force application conditions and on a single radiograph, without requiring the examiner to remain with the patient in the radiography room.
Heim15 designed a system for simultaneous functional radiographs, but an apparatus must be built to adapt it to the frame of the radiography table, and the technique involves hyperpronating both hands and bandaging them to the forearm—which is uncomfortable and bothersome for patients and, in our opinion, has a poor application in high-volume EDs.
The technique of having the patient hold a bandage roll (J.J.’s thumb radiographic projection) offers several advantages:
1. The thumb can be placed in flexion, tightening the main fascicle of the UCL, which is how the UCL must be examined.
2. Forced valgus is allowed. Holding a water glass involves opening the thumb and the necessary stability of the MCP joint of the thumb (grip function of thumb); this radiographic technique is functional.
3. The examiner need not stay with the patient in the radiography room or be exposed to x-rays.
4. The bandage roll is thick enough to generate forced valgus in a patient with large hands. The nonrigid roll makes the examination more tolerable and avoids overforced valgus, eliminating the need for anesthetic blockade.
5. The technique is accessible and simple. In fact, there is no need to remove the roll from its wrapping.
Gamekeeper’s or skier’s thumb is caused by an injury to the ulnar collateral ligament (UCL) of the metacarpophalangeal (MCP) joint of the thumb. The mechanism of injury is forced radial and palmar abduction and hyperextension.
This lesion was initially described in 1955 by Campbell.1 It occurred in gamekeepers who worked in preserves in Scotland. The UCL was injured because of the way they killed rabbits—hence, gamekeeper’s thumb. Now these injuries are more common in skiers—skier’s thumb. In skiers, the mechanism of injury is the force exerted by the ski pole strap on the thumb during a fall. This injury is also seen in breakdancers.1,2
Different lesions can result, the most common being that of the UCL. The UCL lesion may be partial, with no joint instability,3,4 or total, with instability and subdislocation of the proximal phalanx.5-9 Rupture of the thumb adductor aponeurosis and displacement of the long extensor have been described as the cause of thumb instability.6-8
UCL rupture can occur in its extension or can cause a fracture-tearing in the proximal phalanx.9-12 Intra-articular fractures are sometimes found. The essential problem in UCL injuries is the impossibility of spontaneous healing once the rupture is complete, because of the Stener effect. (When the UCL ruptures, its proximal part retracts and runs above the fibrous expansion of the adductor muscle, which is interposed between the 2 parts of the ruptured UCL and prevents healing, even if the thumb is immobilized.) In these cases, only surgery can repair the lesion.2
In any thumb injury, particularly one caused by hyperabduction, a UCL lesion should be considered. The main problem is diagnosing sprain severity, which is evidenced by the degree of joint hypermobility. Radiologic examination should be performed in all cases to rule out fracture with tear, posterior capsular tear, palmar plate tear, and palmar subdislocation of the proximal phalanx, all of which are associated with UCL tearing.7-9
If the diagnosis is suspected, and radiographs show no fracture, comparative radiographs should be obtained in forced valgus.
Technique
We report on a simple, reliable, reproducible method that allows the patient’s thumbs to be compared, under the same force application conditions, on a single radiograph. This technique reduces the patient’s and examiner’s exposure to x-rays and is well tolerated by the patient. Anesthesia for the thumb is usually not necessary.
In each hand, the patient holds a cylindrical object, such as a drinking glass (standard diameter, 7.5-8.5 cm). We use an elastic crepe bandage roll (diameter, 7.5 cm; width, 10 cm). This roll is common in emergency departments (EDs) and easily accessible. The patient holds the rolls in his or her hands with the thumbs in the posteroanterior position (Figures 1–3) and places himself or herself on a 18×24-cm frame or directly on the radiography table.
Both thumbs are captured on a single functional radiograph for comparison of forced valgus of the MCP joints, as in our example cases. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Control Case
The single functional radiograph of both thumbs showed no evidence of joint laxity on the valgus stress test (Figure 4).
Case 1
A 72-year-old woman landed on her left hand when she fell backward while supporting the hand on a piece of furniture. She presented to the ED with pain in the region of the thumb and thenar eminence. Posteroanterior and lateral radiograph projections showed no significant bone injury (Figure 5). Given the patient’s persistent pain, the traumatologist suspected damage to the thumb UCL, so a simultaneous bilateral functional radiographic projection was obtained. The projection showed joint laxity, implying damage to the thumb UCL. Repair and reinsertion of the UCL were performed using a bone harpoon suture.
Case 2
A 58-year-old man sustained a left hand injury when, using both hands, he tried to catch hold of a falling wooden plank. When he presented to the ED the following week, he was given a diagnosis of thumb contusion and forced hyperabduction and was wearing a metal strap for immobilization. Radiographs showed no bone damage (Figure 6). Thumb UCL injury was suspected on the basis of the physical examination findings and the mechanism of injury. A bilateral simultaneous functional radiographic projection showed significant joint laxity. Surgical treatment with the pull-out technique was performed.
Case 3
A 44-year-old woman experienced forced traction from a dog leash and presented to the ED with pain in the right thumb region. Radiographs showed no bone damage (Figure 7). Thumb UCL injury was suspected. A bilateral simultaneous functional radiographic projection showed slight joint laxity, a sprain was diagnosed, and plaster bandaging was applied. Figures 8A–8D show the accurate thumb positions for performing the functional radiograph in forced valgus. We call the technique J.J.’s thumb radiographic projection.
Discussion
Examination using the stress test to cause joint tilt is crucial in making an accurate diagnosis and deciding on the most appropriate therapeutic approach.10 Most authors accept that surgical management is required in joint tilts over 30º, as these involve complete UCL rupture.10-12
The MCP joint must be examined in flexion, when the main fascicle of the UCL is tight, and not in extension, when the main fascicle of the UCL is relaxed. If we examine the thumb in extension, radial deviations may occur that are not caused by joint instability. Tilt here must be compared with that of the healthy side.11
Early diagnosis and adequate management are essential, as unnoticed or undervalued injuries can progress to painful sequelae, associated with stiffness, instability, and osteoarthritis, with evident harm to the grip and pinch functions of the hand. In many cases, clinical evidence of MCP joint instability is difficult. The radiologic diagnosis is usually obtained with comparative radiographs in forced valgus of both thumbs.
The forced valgus maneuver typically is performed by the examiner, who must stay with the patient in the radiography room and wear radiologic protection. Incredibly, some patients must force the valgus themselves.
The maneuver we have described clearly has complications, as it is painful, and some patients are uncooperative. Usually the thumb is anesthetized, and the examiner assumes the exposure to x-rays. The valgus deviation force that can be applied during stability testing may lead to further disruption of a partially torn ligament or displacement of a ruptured ligament if the overforced maneuver is performed.13,14 That does not occur with our technique. On the other hand, the forces applied to the thumbs must be symmetrical for comparison purposes. The way to prevent these inconveniences is to perform the forced valgus maneuver over both thumbs simultaneously, under the same force application conditions and on a single radiograph, without requiring the examiner to remain with the patient in the radiography room.
Heim15 designed a system for simultaneous functional radiographs, but an apparatus must be built to adapt it to the frame of the radiography table, and the technique involves hyperpronating both hands and bandaging them to the forearm—which is uncomfortable and bothersome for patients and, in our opinion, has a poor application in high-volume EDs.
The technique of having the patient hold a bandage roll (J.J.’s thumb radiographic projection) offers several advantages:
1. The thumb can be placed in flexion, tightening the main fascicle of the UCL, which is how the UCL must be examined.
2. Forced valgus is allowed. Holding a water glass involves opening the thumb and the necessary stability of the MCP joint of the thumb (grip function of thumb); this radiographic technique is functional.
3. The examiner need not stay with the patient in the radiography room or be exposed to x-rays.
4. The bandage roll is thick enough to generate forced valgus in a patient with large hands. The nonrigid roll makes the examination more tolerable and avoids overforced valgus, eliminating the need for anesthetic blockade.
5. The technique is accessible and simple. In fact, there is no need to remove the roll from its wrapping.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb: a clinical and anatomic study. J Bone Joint Surg Br. 1962;44(4):869-879.
3. Stener B. Hyperextension injuries to the metacarpophalangeal joint of the thumb: rupture of ligaments, fracture of sesamoid bones, rupture of flexor pollicis brevis. An anatomical and clinical study. Acta Chir Scand. 1963;125:275-293.
4. Coonrad RW, Goldner JL. A study of the pathological findings and treatment in soft-tissue injury of the thumb metacarpophalangeal joint. With a clinical study of the normal range of motion in one thousand thumbs and a study of post mortem findings of ligamentous structures in relation to function. J Bone Joint Surg Am. 1968;50(3):439-451.
5. Parikh M, Nahigian S, Froimson A. Gamekeeper’s thumb. Plast Reconstr Surg. 1976;58(1):24-31.
6. Kaplan EB. The pathology and treatment of radial subluxation of the thumb with ulnar displacement of the head of the first metacarpal. J Bone Joint Surg Am. 1961;43:541-546.
7. Yamanaka K, Yoshida K, Inoue H, Inoue A, Miyagi T. Locking of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1985;67(5):782-787.
8. Sennwald G, Segmüller G, Egli A. The late reconstruction of the ligament of the metacarpo-phalangeal joint of the thumb [in English, French]. Ann Chir Main. 1987;6(1):15-24.
9. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
10. Louis DS, Huebner JJ Jr, Hankin FM. Rupture and displacement of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. Preoperative diagnosis. J Bone Joint Surg Am. 1986;68(9):1320-1326.
11. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
12. Ritting AW, Baldwin PC, Rodner CM. Ulnar collateral ligament injury of the thumb metacarpophalangeal joint. Clin J Sport Med. 2010;20(2):106-112.
13. Cooper JG, Johnstone AJ, Hider P, Ardagh MW. Local anaesthetic infiltration increases the accuracy of assessment of ulnar collateral ligament injuries. Emerg Med Australas. 2005;17(2):132-136.
14. Noszian IM, Dinkhauser LM, Straub GM, Orthner E. Ulnar collateral ligament injuries of the thumb. Dislocation caused by stress radiography in 2 cases. Acta Orthop Scand. 1995;66(2):156-157.
15. Heim U. Simultaneous functional bilateral radiographies of the metacarpophalangeal joint of the thumb in hyper-pronation [in French]. Ann Chir Main. 1982;1(2):183-186.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb: a clinical and anatomic study. J Bone Joint Surg Br. 1962;44(4):869-879.
3. Stener B. Hyperextension injuries to the metacarpophalangeal joint of the thumb: rupture of ligaments, fracture of sesamoid bones, rupture of flexor pollicis brevis. An anatomical and clinical study. Acta Chir Scand. 1963;125:275-293.
4. Coonrad RW, Goldner JL. A study of the pathological findings and treatment in soft-tissue injury of the thumb metacarpophalangeal joint. With a clinical study of the normal range of motion in one thousand thumbs and a study of post mortem findings of ligamentous structures in relation to function. J Bone Joint Surg Am. 1968;50(3):439-451.
5. Parikh M, Nahigian S, Froimson A. Gamekeeper’s thumb. Plast Reconstr Surg. 1976;58(1):24-31.
6. Kaplan EB. The pathology and treatment of radial subluxation of the thumb with ulnar displacement of the head of the first metacarpal. J Bone Joint Surg Am. 1961;43:541-546.
7. Yamanaka K, Yoshida K, Inoue H, Inoue A, Miyagi T. Locking of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1985;67(5):782-787.
8. Sennwald G, Segmüller G, Egli A. The late reconstruction of the ligament of the metacarpo-phalangeal joint of the thumb [in English, French]. Ann Chir Main. 1987;6(1):15-24.
9. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
10. Louis DS, Huebner JJ Jr, Hankin FM. Rupture and displacement of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. Preoperative diagnosis. J Bone Joint Surg Am. 1986;68(9):1320-1326.
11. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
12. Ritting AW, Baldwin PC, Rodner CM. Ulnar collateral ligament injury of the thumb metacarpophalangeal joint. Clin J Sport Med. 2010;20(2):106-112.
13. Cooper JG, Johnstone AJ, Hider P, Ardagh MW. Local anaesthetic infiltration increases the accuracy of assessment of ulnar collateral ligament injuries. Emerg Med Australas. 2005;17(2):132-136.
14. Noszian IM, Dinkhauser LM, Straub GM, Orthner E. Ulnar collateral ligament injuries of the thumb. Dislocation caused by stress radiography in 2 cases. Acta Orthop Scand. 1995;66(2):156-157.
15. Heim U. Simultaneous functional bilateral radiographies of the metacarpophalangeal joint of the thumb in hyper-pronation [in French]. Ann Chir Main. 1982;1(2):183-186.
Madelung Deformity and Extensor Tendon Rupture
Extensor tendon rupture in chronic Madelung deformity, as a result of tendon attrition on the dislocated distal ulna, occurs infrequently. However, it is often seen in patients with rheumatoid arthritis. This issue has been reported in only a few English-language case reports. Here we report a case of multiple tendon ruptures in a previously undiagnosed Madelung deformity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old active woman presented with 50 days’ inability to extend the fourth and fifth fingers of her dominant right hand. The loss of finger extension progressed, over several weeks, to involve the third finger as well. The first 2 tendon ruptures had been triggered by lifting a light grocery bag, when she noticed a sharp sudden pain and “pop.” The third rupture occurred spontaneously with a snapping sound the night before surgery.
The patient had observed some prominence on the ulnar side of her right wrist since childhood but had never experienced any pain or functional disability. There was neither history of trauma, inflammatory disease, diabetes mellitus, or infection, nor positive family history of similar wrist deformity.
The physical examination showed a dorsally subluxated distal radioulnar joint, prominent ulnar styloid, and mild ulnar and volar deviation of the wrist along with limitation of wrist dorsiflexion. Complete loss of active extension of the 3 ulnar fingers was demonstrated, while neurovascular status and all other hand evaluations were normal. The wrist radiographs confirmed the typical findings of Madelung deformity (Figure 1).
Repair of the ruptured tendons and resection of the prominent distal ulna (Darrach procedure) was planned. (Given the patient’s age and evidence of degenerative changes in the radiocarpal joint, correction of the Madelung deformity did not seem necessary). At time of surgery, the recently ruptured third finger extensor tendon was easily found and approximated, and end-to-end repair was performed. The fourth and fifth fingers, however, had to be fished out more proximally from dense granulation tissue. After the distal ulna was resected for a distance of 1.5 cm, meticulous repair of the ulnar collateral ligament and the capsule and periosteum over the end of the ulna was performed. Then, for grafting of the ruptured tendons, the extensor indicis proprius tendon was isolated and transected at the second metacarpophalangeal joint level. A piece of this tendon was used as interpositional graft for the fourth extensor tendon, and the main tendon unit was transferred to the fifth finger extensor. The extensor digiti quinti tendon, which was about to rupture, was further reinforced by suturing it side to side to the muscle and tendon of the extensor indicis proprius (Figure 2).
Postoperatively, the wrist was kept in extension in a cast for 3 weeks while the fingers were free for active movement. A removable wrist splint was used for an additional month. At 3-month follow-up, the patient had regained full and strong finger extension and wrist motion.
At 3-year follow-up, the patient was pain-free, and had full extension of all fingers, full forearm rotation, and near-normal motion (better than her preoperative motion). The grip power on the operated right hand was 215 N, and pinch power was 93 N. (The values for the left side were 254 N and 83 N, respectively, using the Jamar hydraulic hand dynamometer [Patterson Medical].) The patient has had no additional tendon rupture (Figure 3).
Discussion
Madelung deformity was first described by Madelung in 1878 and several cases have reported this deformity. However, extensor tendon rupture caused by Madelung deformity is very rare, reported in few cases.1
Extensor tendon rupture caused by chronic Madelung deformity has been reported few times in the English literature. Goodwin1 apparently published the first report of such an occurrence in 1979. Ducloyer and colleagues2 from France reported 6 cases of extensor tendon rupture as a result of inferior distal radioulnar joint deformity of Madelung. Jebson and colleagues3 reported bilateral spontaneous extensor tendon ruptures in Madelung deformity in 1992.
The mechanism of tendon rupture seems to be mechanical, resulting from continuous rubbing and erosion of tendons over the deformed ulnar head, which has a rough irregular surface4 and leads to fraying of the tendons and eventual rupture and retraction of the severed tendon ends. This rupture usually progresses stepwise from more medial to the lateral tendons.2 Older patients are, therefore, subject to chronic repetitive attritional trauma leading to tendon rupture.
Tendons may rupture as a result of a variety of conditions, such as chronic synovitis in rheumatoid arthritis, systemic lupus erythematosus, mixed connective tissue disease, or crystal deposition in gout.5-8 Some other metabolic or endocrine conditions that involve tendon ruptures include diabetes mellitus, chronic renal failure, and hyperparathyroidism. Steroid injection into the tendons also has a detrimental effect on tendon integrity and may cause tendon tear.9 Mechanical factors, such as erosion on bony prominences, are well-known etiologies for tendon rupture, as commonly seen in rheumatoid arthritis, and have been reported in Kienböck disease,10 thumb carpometacarpal arthritis,11 Colles fracture, scaphoid fracture nonunion,12 and Madelung deformity.
Conclusion
Our case reflects the usual middle-aged female presentation of such a tendon rupture. The tendon ruptures were spontaneous in the reported order of ulnar to radial, beginning with the little and ring fingers, and progressed radially. The patient had isolated Madelung deformity with no other sign of dyschondrosteosis13 or dwarfism, conditions commonly mentioned in association with Madelung deformity. This case report should raise awareness about possible tendon rupture in any chronic case of Madelung deformity.
1. Goodwin DR, Michels CH, Weissman SL. Spontaneous rupture of extensor tendons in Madelung’s deformity. Hand. 1979;11(1):72-75.
2. Ducloyer P, Leclercq C, Lisfrance R, Saffar P. Spontaneous rupture of the extensor tendons of the fingers in Madelung’s deformity. J Hand Surg Br. 1991;16(3):329-333.
3. Jebson PJ, Blair WF. Bilateral spontaneous extensor tendon ruptures in Madelung’s deformity. J Hand Surg Am. 1992;17(2):277-280.
4. Schulstad I. Madelung’s deformity with extensor tendon rupture. Case report. Scand J Plast Reconstr Surg. 1971;5(2):153-155.
5. Gong HS, Lee JO, Baek GH, et al. Extensor tendon rupture in rheumatoid arthritis: a survey of patients between 2005 and 2010 at five Korean hospitals. Hand Surg. 2012;17(1):43-47.
6. Oishi H, Oda R, Morisaki S, Fujiwara H, Tokunaga D, Kubo T. Spontaneous tendon rupture of the extensor digitrum communis in systemic lupus erythematosus. Mod Rheumatol. 2013;23(3);608-610.
7. Kobayashi A, Futami T, Tadano I, Fujita M. Spontaneous rupture of extensor tendons at the wrist in a patient with mixed connective tissue disease. Mod Rheumatol. 2002;12(3):256-258.
8. Iwamoto T, Toki H, Ikari K, Yamanaka H, Momohara S. Multiple extensor tendon ruptures caused by tophaceous gout. Mod Rheumatol. 2010;20(2):210-212.
9. Nquyen ML, Jones NF. Rupture of both abductor pollicis longus and extensor pollicis brevis tendon after steroid injection for de quervain tenosynovitis. Plast Reconstr Surg. 2012;129(5):883e-886e.
10. Hernández-Cortés P, Pajares-López M, Gómez-Sánchez R, Garrido-Gómez, Lara-Garcia F. Rupture of extensor tendon secondary to previously undiagnosed Kienböck disease. J Plast Surg Hand Surg. 2012;46(3-4):291-293.
11. Apard T, Marcucci L, Jarriges J. Spontaneous rupture of extensor pollicis longus in isolated trapeziometacarpal arthritis. Chir Main. 2011;30(5):349-351.
12. Harvey FJ, Harvey PM. Three rare causes of extensor tendon rupture. J Hand Surg Am. 1989;14(6):957-962.
13. Duro EA, Prado GS. Clinical variations in Léri-Weill dyschondrosteosis. An Esp Pediatr. 1990;33(5):461-463.
Extensor tendon rupture in chronic Madelung deformity, as a result of tendon attrition on the dislocated distal ulna, occurs infrequently. However, it is often seen in patients with rheumatoid arthritis. This issue has been reported in only a few English-language case reports. Here we report a case of multiple tendon ruptures in a previously undiagnosed Madelung deformity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old active woman presented with 50 days’ inability to extend the fourth and fifth fingers of her dominant right hand. The loss of finger extension progressed, over several weeks, to involve the third finger as well. The first 2 tendon ruptures had been triggered by lifting a light grocery bag, when she noticed a sharp sudden pain and “pop.” The third rupture occurred spontaneously with a snapping sound the night before surgery.
The patient had observed some prominence on the ulnar side of her right wrist since childhood but had never experienced any pain or functional disability. There was neither history of trauma, inflammatory disease, diabetes mellitus, or infection, nor positive family history of similar wrist deformity.
The physical examination showed a dorsally subluxated distal radioulnar joint, prominent ulnar styloid, and mild ulnar and volar deviation of the wrist along with limitation of wrist dorsiflexion. Complete loss of active extension of the 3 ulnar fingers was demonstrated, while neurovascular status and all other hand evaluations were normal. The wrist radiographs confirmed the typical findings of Madelung deformity (Figure 1).
Repair of the ruptured tendons and resection of the prominent distal ulna (Darrach procedure) was planned. (Given the patient’s age and evidence of degenerative changes in the radiocarpal joint, correction of the Madelung deformity did not seem necessary). At time of surgery, the recently ruptured third finger extensor tendon was easily found and approximated, and end-to-end repair was performed. The fourth and fifth fingers, however, had to be fished out more proximally from dense granulation tissue. After the distal ulna was resected for a distance of 1.5 cm, meticulous repair of the ulnar collateral ligament and the capsule and periosteum over the end of the ulna was performed. Then, for grafting of the ruptured tendons, the extensor indicis proprius tendon was isolated and transected at the second metacarpophalangeal joint level. A piece of this tendon was used as interpositional graft for the fourth extensor tendon, and the main tendon unit was transferred to the fifth finger extensor. The extensor digiti quinti tendon, which was about to rupture, was further reinforced by suturing it side to side to the muscle and tendon of the extensor indicis proprius (Figure 2).
Postoperatively, the wrist was kept in extension in a cast for 3 weeks while the fingers were free for active movement. A removable wrist splint was used for an additional month. At 3-month follow-up, the patient had regained full and strong finger extension and wrist motion.
At 3-year follow-up, the patient was pain-free, and had full extension of all fingers, full forearm rotation, and near-normal motion (better than her preoperative motion). The grip power on the operated right hand was 215 N, and pinch power was 93 N. (The values for the left side were 254 N and 83 N, respectively, using the Jamar hydraulic hand dynamometer [Patterson Medical].) The patient has had no additional tendon rupture (Figure 3).
Discussion
Madelung deformity was first described by Madelung in 1878 and several cases have reported this deformity. However, extensor tendon rupture caused by Madelung deformity is very rare, reported in few cases.1
Extensor tendon rupture caused by chronic Madelung deformity has been reported few times in the English literature. Goodwin1 apparently published the first report of such an occurrence in 1979. Ducloyer and colleagues2 from France reported 6 cases of extensor tendon rupture as a result of inferior distal radioulnar joint deformity of Madelung. Jebson and colleagues3 reported bilateral spontaneous extensor tendon ruptures in Madelung deformity in 1992.
The mechanism of tendon rupture seems to be mechanical, resulting from continuous rubbing and erosion of tendons over the deformed ulnar head, which has a rough irregular surface4 and leads to fraying of the tendons and eventual rupture and retraction of the severed tendon ends. This rupture usually progresses stepwise from more medial to the lateral tendons.2 Older patients are, therefore, subject to chronic repetitive attritional trauma leading to tendon rupture.
Tendons may rupture as a result of a variety of conditions, such as chronic synovitis in rheumatoid arthritis, systemic lupus erythematosus, mixed connective tissue disease, or crystal deposition in gout.5-8 Some other metabolic or endocrine conditions that involve tendon ruptures include diabetes mellitus, chronic renal failure, and hyperparathyroidism. Steroid injection into the tendons also has a detrimental effect on tendon integrity and may cause tendon tear.9 Mechanical factors, such as erosion on bony prominences, are well-known etiologies for tendon rupture, as commonly seen in rheumatoid arthritis, and have been reported in Kienböck disease,10 thumb carpometacarpal arthritis,11 Colles fracture, scaphoid fracture nonunion,12 and Madelung deformity.
Conclusion
Our case reflects the usual middle-aged female presentation of such a tendon rupture. The tendon ruptures were spontaneous in the reported order of ulnar to radial, beginning with the little and ring fingers, and progressed radially. The patient had isolated Madelung deformity with no other sign of dyschondrosteosis13 or dwarfism, conditions commonly mentioned in association with Madelung deformity. This case report should raise awareness about possible tendon rupture in any chronic case of Madelung deformity.
Extensor tendon rupture in chronic Madelung deformity, as a result of tendon attrition on the dislocated distal ulna, occurs infrequently. However, it is often seen in patients with rheumatoid arthritis. This issue has been reported in only a few English-language case reports. Here we report a case of multiple tendon ruptures in a previously undiagnosed Madelung deformity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old active woman presented with 50 days’ inability to extend the fourth and fifth fingers of her dominant right hand. The loss of finger extension progressed, over several weeks, to involve the third finger as well. The first 2 tendon ruptures had been triggered by lifting a light grocery bag, when she noticed a sharp sudden pain and “pop.” The third rupture occurred spontaneously with a snapping sound the night before surgery.
The patient had observed some prominence on the ulnar side of her right wrist since childhood but had never experienced any pain or functional disability. There was neither history of trauma, inflammatory disease, diabetes mellitus, or infection, nor positive family history of similar wrist deformity.
The physical examination showed a dorsally subluxated distal radioulnar joint, prominent ulnar styloid, and mild ulnar and volar deviation of the wrist along with limitation of wrist dorsiflexion. Complete loss of active extension of the 3 ulnar fingers was demonstrated, while neurovascular status and all other hand evaluations were normal. The wrist radiographs confirmed the typical findings of Madelung deformity (Figure 1).
Repair of the ruptured tendons and resection of the prominent distal ulna (Darrach procedure) was planned. (Given the patient’s age and evidence of degenerative changes in the radiocarpal joint, correction of the Madelung deformity did not seem necessary). At time of surgery, the recently ruptured third finger extensor tendon was easily found and approximated, and end-to-end repair was performed. The fourth and fifth fingers, however, had to be fished out more proximally from dense granulation tissue. After the distal ulna was resected for a distance of 1.5 cm, meticulous repair of the ulnar collateral ligament and the capsule and periosteum over the end of the ulna was performed. Then, for grafting of the ruptured tendons, the extensor indicis proprius tendon was isolated and transected at the second metacarpophalangeal joint level. A piece of this tendon was used as interpositional graft for the fourth extensor tendon, and the main tendon unit was transferred to the fifth finger extensor. The extensor digiti quinti tendon, which was about to rupture, was further reinforced by suturing it side to side to the muscle and tendon of the extensor indicis proprius (Figure 2).
Postoperatively, the wrist was kept in extension in a cast for 3 weeks while the fingers were free for active movement. A removable wrist splint was used for an additional month. At 3-month follow-up, the patient had regained full and strong finger extension and wrist motion.
At 3-year follow-up, the patient was pain-free, and had full extension of all fingers, full forearm rotation, and near-normal motion (better than her preoperative motion). The grip power on the operated right hand was 215 N, and pinch power was 93 N. (The values for the left side were 254 N and 83 N, respectively, using the Jamar hydraulic hand dynamometer [Patterson Medical].) The patient has had no additional tendon rupture (Figure 3).
Discussion
Madelung deformity was first described by Madelung in 1878 and several cases have reported this deformity. However, extensor tendon rupture caused by Madelung deformity is very rare, reported in few cases.1
Extensor tendon rupture caused by chronic Madelung deformity has been reported few times in the English literature. Goodwin1 apparently published the first report of such an occurrence in 1979. Ducloyer and colleagues2 from France reported 6 cases of extensor tendon rupture as a result of inferior distal radioulnar joint deformity of Madelung. Jebson and colleagues3 reported bilateral spontaneous extensor tendon ruptures in Madelung deformity in 1992.
The mechanism of tendon rupture seems to be mechanical, resulting from continuous rubbing and erosion of tendons over the deformed ulnar head, which has a rough irregular surface4 and leads to fraying of the tendons and eventual rupture and retraction of the severed tendon ends. This rupture usually progresses stepwise from more medial to the lateral tendons.2 Older patients are, therefore, subject to chronic repetitive attritional trauma leading to tendon rupture.
Tendons may rupture as a result of a variety of conditions, such as chronic synovitis in rheumatoid arthritis, systemic lupus erythematosus, mixed connective tissue disease, or crystal deposition in gout.5-8 Some other metabolic or endocrine conditions that involve tendon ruptures include diabetes mellitus, chronic renal failure, and hyperparathyroidism. Steroid injection into the tendons also has a detrimental effect on tendon integrity and may cause tendon tear.9 Mechanical factors, such as erosion on bony prominences, are well-known etiologies for tendon rupture, as commonly seen in rheumatoid arthritis, and have been reported in Kienböck disease,10 thumb carpometacarpal arthritis,11 Colles fracture, scaphoid fracture nonunion,12 and Madelung deformity.
Conclusion
Our case reflects the usual middle-aged female presentation of such a tendon rupture. The tendon ruptures were spontaneous in the reported order of ulnar to radial, beginning with the little and ring fingers, and progressed radially. The patient had isolated Madelung deformity with no other sign of dyschondrosteosis13 or dwarfism, conditions commonly mentioned in association with Madelung deformity. This case report should raise awareness about possible tendon rupture in any chronic case of Madelung deformity.
1. Goodwin DR, Michels CH, Weissman SL. Spontaneous rupture of extensor tendons in Madelung’s deformity. Hand. 1979;11(1):72-75.
2. Ducloyer P, Leclercq C, Lisfrance R, Saffar P. Spontaneous rupture of the extensor tendons of the fingers in Madelung’s deformity. J Hand Surg Br. 1991;16(3):329-333.
3. Jebson PJ, Blair WF. Bilateral spontaneous extensor tendon ruptures in Madelung’s deformity. J Hand Surg Am. 1992;17(2):277-280.
4. Schulstad I. Madelung’s deformity with extensor tendon rupture. Case report. Scand J Plast Reconstr Surg. 1971;5(2):153-155.
5. Gong HS, Lee JO, Baek GH, et al. Extensor tendon rupture in rheumatoid arthritis: a survey of patients between 2005 and 2010 at five Korean hospitals. Hand Surg. 2012;17(1):43-47.
6. Oishi H, Oda R, Morisaki S, Fujiwara H, Tokunaga D, Kubo T. Spontaneous tendon rupture of the extensor digitrum communis in systemic lupus erythematosus. Mod Rheumatol. 2013;23(3);608-610.
7. Kobayashi A, Futami T, Tadano I, Fujita M. Spontaneous rupture of extensor tendons at the wrist in a patient with mixed connective tissue disease. Mod Rheumatol. 2002;12(3):256-258.
8. Iwamoto T, Toki H, Ikari K, Yamanaka H, Momohara S. Multiple extensor tendon ruptures caused by tophaceous gout. Mod Rheumatol. 2010;20(2):210-212.
9. Nquyen ML, Jones NF. Rupture of both abductor pollicis longus and extensor pollicis brevis tendon after steroid injection for de quervain tenosynovitis. Plast Reconstr Surg. 2012;129(5):883e-886e.
10. Hernández-Cortés P, Pajares-López M, Gómez-Sánchez R, Garrido-Gómez, Lara-Garcia F. Rupture of extensor tendon secondary to previously undiagnosed Kienböck disease. J Plast Surg Hand Surg. 2012;46(3-4):291-293.
11. Apard T, Marcucci L, Jarriges J. Spontaneous rupture of extensor pollicis longus in isolated trapeziometacarpal arthritis. Chir Main. 2011;30(5):349-351.
12. Harvey FJ, Harvey PM. Three rare causes of extensor tendon rupture. J Hand Surg Am. 1989;14(6):957-962.
13. Duro EA, Prado GS. Clinical variations in Léri-Weill dyschondrosteosis. An Esp Pediatr. 1990;33(5):461-463.
1. Goodwin DR, Michels CH, Weissman SL. Spontaneous rupture of extensor tendons in Madelung’s deformity. Hand. 1979;11(1):72-75.
2. Ducloyer P, Leclercq C, Lisfrance R, Saffar P. Spontaneous rupture of the extensor tendons of the fingers in Madelung’s deformity. J Hand Surg Br. 1991;16(3):329-333.
3. Jebson PJ, Blair WF. Bilateral spontaneous extensor tendon ruptures in Madelung’s deformity. J Hand Surg Am. 1992;17(2):277-280.
4. Schulstad I. Madelung’s deformity with extensor tendon rupture. Case report. Scand J Plast Reconstr Surg. 1971;5(2):153-155.
5. Gong HS, Lee JO, Baek GH, et al. Extensor tendon rupture in rheumatoid arthritis: a survey of patients between 2005 and 2010 at five Korean hospitals. Hand Surg. 2012;17(1):43-47.
6. Oishi H, Oda R, Morisaki S, Fujiwara H, Tokunaga D, Kubo T. Spontaneous tendon rupture of the extensor digitrum communis in systemic lupus erythematosus. Mod Rheumatol. 2013;23(3);608-610.
7. Kobayashi A, Futami T, Tadano I, Fujita M. Spontaneous rupture of extensor tendons at the wrist in a patient with mixed connective tissue disease. Mod Rheumatol. 2002;12(3):256-258.
8. Iwamoto T, Toki H, Ikari K, Yamanaka H, Momohara S. Multiple extensor tendon ruptures caused by tophaceous gout. Mod Rheumatol. 2010;20(2):210-212.
9. Nquyen ML, Jones NF. Rupture of both abductor pollicis longus and extensor pollicis brevis tendon after steroid injection for de quervain tenosynovitis. Plast Reconstr Surg. 2012;129(5):883e-886e.
10. Hernández-Cortés P, Pajares-López M, Gómez-Sánchez R, Garrido-Gómez, Lara-Garcia F. Rupture of extensor tendon secondary to previously undiagnosed Kienböck disease. J Plast Surg Hand Surg. 2012;46(3-4):291-293.
11. Apard T, Marcucci L, Jarriges J. Spontaneous rupture of extensor pollicis longus in isolated trapeziometacarpal arthritis. Chir Main. 2011;30(5):349-351.
12. Harvey FJ, Harvey PM. Three rare causes of extensor tendon rupture. J Hand Surg Am. 1989;14(6):957-962.
13. Duro EA, Prado GS. Clinical variations in Léri-Weill dyschondrosteosis. An Esp Pediatr. 1990;33(5):461-463.
Septic Arthritis and Osteomyelitis Caused by Pasteurella multocida
A few days after an incidental cat bite, a patient presented to the emergency department for treatment of poison sumac exposure. He was discharged with oral methylprednisolone for the dermatitis and returned 1 week later with symptoms, examination findings, and laboratory results consistent with sepsis and bilateral upper extremity necrotizing soft-tissue infections. After administering multiple irrigation and débridement procedures, hyperbaric oxygen treatments, and an antibiotic regimen, the patient’s status greatly improved. However, the patient returned 1 month later with a new sternoclavicular joint prominence that was associated with painful crepitus. Additionally, he noted that his wrists were gradually becoming more swollen and painful. Imaging studies showed a lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint bilaterally, consistent with osteomyelitis. The patient was treated with ertapenem for 6 weeks, and his polyarthropathy resolved. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 73-year-old, right-hand–dominant man with no notable medical history presented to the emergency department for treatment of poison sumac exposure, incidentally, a few days after being bitten by a cat on the bilateral distal upper extremities. He was prescribed a course of oral methylprednisolone for dermatitis. A week later, the patient returned to the emergency department with altered mental status, fevers, diaphoresis, lethargy, and polyarthralgia. At the time of presentation, the patient’s vital signs were labile, and he was found to have extensive bilateral upper extremity erythema, blistering, petechiae, purpuric lesions, and exquisite pain with passive range of motion of his fingers and wrists. His leukocyte count was 25.1 × 109/L, and he had elevated C-reactive protein level and erythrocyte sedimentation rate of 150 mg/L and 120 mm/h, respectively. He was admitted for management of sepsis and presumed bilateral upper extremity necrotizing soft-tissue infection.
Broad-spectrum intravenous (IV) antibiotics (vancomycin, piperacillin, tazobactam) were initiated after blood cultures were obtained, and the patient was taken emergently to the operating theatre for irrigation and débridement of his hands and wrists bilaterally. Arthrotomy of the wrist and débridement of the distal extensor compartment and its tenosynovium were performed on the right forearm, in addition to a decompressive fasciotomy of the left forearm. Postoperatively, the patient’s mental status improved and his vital signs gradually normalized. He received multiple hyperbaric oxygen treatments and underwent several additional operative débridement procedures with eventual closure of his wounds. At initial presentation, the differential diagnosis for the severe soft-tissue infection included necrotizing fasciitis or myositis caused by any of a variety of bacterial pathogens. Most notably, it was important to elicit the history of a cat bite to include and consider Pasteurella multocida as a potential pathogen. Initial cultures supported the diagnosis of acute P multocida necrotizing skin and soft-tissue infection, in addition to septic arthritis. The patient’s blood and intraoperative wound cultures grew P multocida. The antibiotic treatment was tailored initially to ampicillin and sulbactam and then to a final regimen of orally administered ciprofloxacin (750 mg twice a day), once susceptibility testing was performed on the cultures. On hospital day 10, the patient was discharged home, receiving a 6-week course of ciprofloxacin to complete the 8-week course of treatment.
At follow-up, approximately 1 month after discharge, the patient noted that he had developed a new right sternoclavicular joint prominence that was associated with painful crepitus. He also noted that his wrists were gradually becoming more swollen and painful bilaterally. Computed tomography scans of the chest were obtained to evaluate the sternoclavicular joint (Figure 1). Repeat radiographs of the wrists were also obtained (Figure 2). Imaging showed lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint, consistent with osteomyelitis. The C-reactive protein level and erythrocyte sedimentation rate at this time were 34 mg/L and 124 mm/h, respectively.
The patient returned to the operating room for débridement and biopsy of the right sternoclavicular joint and left wrist. This patient’s delayed presentation was characterized by a subacute worsening of isolated musculoskeletal complaints. The differential diagnosis then included infection with the same bacterial pathogen versus reactive or inflammatory arthritis. Several intraoperative cultures failed to grow any bacteria, including P multocida, although P multocida was the presumptive cause of the erosive polyarthropathy, considering that symptoms eventually resolved with a repeated course of IV-administered ertapenem for 6 weeks. The patient experienced complete resolution of his joint pain and swelling. He was able to resume his activities of daily living and had no further recurrence of symptoms at follow-up 3 months later.
Discussion
Cat bites often are the source of Pasteurella species infections because the bacteria are carried by more than 90% of cats.1 These types of infections can cause septic arthritis, osteomyelitis, and deep subcutaneous and myofascial infections because of the sharp and narrow morphology of cat teeth. The infections can progress to necrotizing fasciitis and myositis if not recognized early, as was the case with our patient. Prophylactic antibiotic administration for animal bites is controversial and is not a universal practice.1,2Pasteurella bacteremia is an atypical progression that occurs more often in patients with pneumonia, septic arthritis, or meningitis. Cases of Pasteurella sepsis, necrotizing fasciitis, and septic arthritis have been reported.3-7 However, associated progressive septic arthritis and osteomyelitis, despite initial clinical improvement, have not been reported. Severe infection (ie, sepsis and septic shock) can occur in infants, pregnant women, and other immunocompromised patients.7 Immune suppression of our patient with steroid medication for poison sumac dermatitis likely contributed to the progression and systemic spread of an initially benign cat bite. Before prescribing steroids, it is imperative to ask about exposures and encourage patients to seek prompt medical attention with worsening or new symptoms. Healthy individuals rarely develop bacteremia; however, in these cases, mortality remains high at approximately 25%.4,6
The clinical course of this case emphasizes the need for vigilance and thoroughness in obtaining histories from patients presenting with seemingly benign complaints, especially in vulnerable populations, such as infants, pregnant women, and immunocompromised adults. In this case, the progression of symptoms might have been avoided if the patient’s dermatitis had been treated conservatively or with topical rather than systemic steroids.
1. Esposito S, Picciolli I, Semino M, Principi N. Dog and cat bite-associated infections in children. Eur J Clin Microbiol Infect Dis. 2013;32(8):971-976.
2. Medeiros I, Saconato H. Antibiotic prophylaxis for mammalian bites. Cochrane Database Syst Rev. 2001;(2):CD001738.
3. Haybaeck J, Schindler C, Braza P, Willinger B, Drlicek M. Rapidly progressive and lethal septicemia due to infection with Pasteurella multocida in an infant. Wien Klin Wochenschr. 2009;121(5-6):216-219.
4. Migliore E, Serraino C, Brignone C, et al. Pasteurella multocida infection in a cirrhotic patient: case report, microbiological aspects and a review of the literature. Adv Med Sci. 2009;54(1):109-112.
5. Mugambi SM, Ullian ME. Bacteremia, sepsis, and peritonitis with Pasteurella multocida in a peritoneal dialysis patient. Perit Dial Int. 2010;30(3):381-383.
6. Weber DJ, Wolfson JS, Swartz MN, Hooper DC. Pasteurella multocida infections. Report of 34 cases and review of the literature. Medicine (Baltimore). 1984;63(3):133-154.
7. Oehler RL, Velez AP, Mizrachi M, Lamarche J, Gompf S. Bite-related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439-447.
A few days after an incidental cat bite, a patient presented to the emergency department for treatment of poison sumac exposure. He was discharged with oral methylprednisolone for the dermatitis and returned 1 week later with symptoms, examination findings, and laboratory results consistent with sepsis and bilateral upper extremity necrotizing soft-tissue infections. After administering multiple irrigation and débridement procedures, hyperbaric oxygen treatments, and an antibiotic regimen, the patient’s status greatly improved. However, the patient returned 1 month later with a new sternoclavicular joint prominence that was associated with painful crepitus. Additionally, he noted that his wrists were gradually becoming more swollen and painful. Imaging studies showed a lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint bilaterally, consistent with osteomyelitis. The patient was treated with ertapenem for 6 weeks, and his polyarthropathy resolved. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 73-year-old, right-hand–dominant man with no notable medical history presented to the emergency department for treatment of poison sumac exposure, incidentally, a few days after being bitten by a cat on the bilateral distal upper extremities. He was prescribed a course of oral methylprednisolone for dermatitis. A week later, the patient returned to the emergency department with altered mental status, fevers, diaphoresis, lethargy, and polyarthralgia. At the time of presentation, the patient’s vital signs were labile, and he was found to have extensive bilateral upper extremity erythema, blistering, petechiae, purpuric lesions, and exquisite pain with passive range of motion of his fingers and wrists. His leukocyte count was 25.1 × 109/L, and he had elevated C-reactive protein level and erythrocyte sedimentation rate of 150 mg/L and 120 mm/h, respectively. He was admitted for management of sepsis and presumed bilateral upper extremity necrotizing soft-tissue infection.
Broad-spectrum intravenous (IV) antibiotics (vancomycin, piperacillin, tazobactam) were initiated after blood cultures were obtained, and the patient was taken emergently to the operating theatre for irrigation and débridement of his hands and wrists bilaterally. Arthrotomy of the wrist and débridement of the distal extensor compartment and its tenosynovium were performed on the right forearm, in addition to a decompressive fasciotomy of the left forearm. Postoperatively, the patient’s mental status improved and his vital signs gradually normalized. He received multiple hyperbaric oxygen treatments and underwent several additional operative débridement procedures with eventual closure of his wounds. At initial presentation, the differential diagnosis for the severe soft-tissue infection included necrotizing fasciitis or myositis caused by any of a variety of bacterial pathogens. Most notably, it was important to elicit the history of a cat bite to include and consider Pasteurella multocida as a potential pathogen. Initial cultures supported the diagnosis of acute P multocida necrotizing skin and soft-tissue infection, in addition to septic arthritis. The patient’s blood and intraoperative wound cultures grew P multocida. The antibiotic treatment was tailored initially to ampicillin and sulbactam and then to a final regimen of orally administered ciprofloxacin (750 mg twice a day), once susceptibility testing was performed on the cultures. On hospital day 10, the patient was discharged home, receiving a 6-week course of ciprofloxacin to complete the 8-week course of treatment.
At follow-up, approximately 1 month after discharge, the patient noted that he had developed a new right sternoclavicular joint prominence that was associated with painful crepitus. He also noted that his wrists were gradually becoming more swollen and painful bilaterally. Computed tomography scans of the chest were obtained to evaluate the sternoclavicular joint (Figure 1). Repeat radiographs of the wrists were also obtained (Figure 2). Imaging showed lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint, consistent with osteomyelitis. The C-reactive protein level and erythrocyte sedimentation rate at this time were 34 mg/L and 124 mm/h, respectively.
The patient returned to the operating room for débridement and biopsy of the right sternoclavicular joint and left wrist. This patient’s delayed presentation was characterized by a subacute worsening of isolated musculoskeletal complaints. The differential diagnosis then included infection with the same bacterial pathogen versus reactive or inflammatory arthritis. Several intraoperative cultures failed to grow any bacteria, including P multocida, although P multocida was the presumptive cause of the erosive polyarthropathy, considering that symptoms eventually resolved with a repeated course of IV-administered ertapenem for 6 weeks. The patient experienced complete resolution of his joint pain and swelling. He was able to resume his activities of daily living and had no further recurrence of symptoms at follow-up 3 months later.
Discussion
Cat bites often are the source of Pasteurella species infections because the bacteria are carried by more than 90% of cats.1 These types of infections can cause septic arthritis, osteomyelitis, and deep subcutaneous and myofascial infections because of the sharp and narrow morphology of cat teeth. The infections can progress to necrotizing fasciitis and myositis if not recognized early, as was the case with our patient. Prophylactic antibiotic administration for animal bites is controversial and is not a universal practice.1,2Pasteurella bacteremia is an atypical progression that occurs more often in patients with pneumonia, septic arthritis, or meningitis. Cases of Pasteurella sepsis, necrotizing fasciitis, and septic arthritis have been reported.3-7 However, associated progressive septic arthritis and osteomyelitis, despite initial clinical improvement, have not been reported. Severe infection (ie, sepsis and septic shock) can occur in infants, pregnant women, and other immunocompromised patients.7 Immune suppression of our patient with steroid medication for poison sumac dermatitis likely contributed to the progression and systemic spread of an initially benign cat bite. Before prescribing steroids, it is imperative to ask about exposures and encourage patients to seek prompt medical attention with worsening or new symptoms. Healthy individuals rarely develop bacteremia; however, in these cases, mortality remains high at approximately 25%.4,6
The clinical course of this case emphasizes the need for vigilance and thoroughness in obtaining histories from patients presenting with seemingly benign complaints, especially in vulnerable populations, such as infants, pregnant women, and immunocompromised adults. In this case, the progression of symptoms might have been avoided if the patient’s dermatitis had been treated conservatively or with topical rather than systemic steroids.
A few days after an incidental cat bite, a patient presented to the emergency department for treatment of poison sumac exposure. He was discharged with oral methylprednisolone for the dermatitis and returned 1 week later with symptoms, examination findings, and laboratory results consistent with sepsis and bilateral upper extremity necrotizing soft-tissue infections. After administering multiple irrigation and débridement procedures, hyperbaric oxygen treatments, and an antibiotic regimen, the patient’s status greatly improved. However, the patient returned 1 month later with a new sternoclavicular joint prominence that was associated with painful crepitus. Additionally, he noted that his wrists were gradually becoming more swollen and painful. Imaging studies showed a lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint bilaterally, consistent with osteomyelitis. The patient was treated with ertapenem for 6 weeks, and his polyarthropathy resolved. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 73-year-old, right-hand–dominant man with no notable medical history presented to the emergency department for treatment of poison sumac exposure, incidentally, a few days after being bitten by a cat on the bilateral distal upper extremities. He was prescribed a course of oral methylprednisolone for dermatitis. A week later, the patient returned to the emergency department with altered mental status, fevers, diaphoresis, lethargy, and polyarthralgia. At the time of presentation, the patient’s vital signs were labile, and he was found to have extensive bilateral upper extremity erythema, blistering, petechiae, purpuric lesions, and exquisite pain with passive range of motion of his fingers and wrists. His leukocyte count was 25.1 × 109/L, and he had elevated C-reactive protein level and erythrocyte sedimentation rate of 150 mg/L and 120 mm/h, respectively. He was admitted for management of sepsis and presumed bilateral upper extremity necrotizing soft-tissue infection.
Broad-spectrum intravenous (IV) antibiotics (vancomycin, piperacillin, tazobactam) were initiated after blood cultures were obtained, and the patient was taken emergently to the operating theatre for irrigation and débridement of his hands and wrists bilaterally. Arthrotomy of the wrist and débridement of the distal extensor compartment and its tenosynovium were performed on the right forearm, in addition to a decompressive fasciotomy of the left forearm. Postoperatively, the patient’s mental status improved and his vital signs gradually normalized. He received multiple hyperbaric oxygen treatments and underwent several additional operative débridement procedures with eventual closure of his wounds. At initial presentation, the differential diagnosis for the severe soft-tissue infection included necrotizing fasciitis or myositis caused by any of a variety of bacterial pathogens. Most notably, it was important to elicit the history of a cat bite to include and consider Pasteurella multocida as a potential pathogen. Initial cultures supported the diagnosis of acute P multocida necrotizing skin and soft-tissue infection, in addition to septic arthritis. The patient’s blood and intraoperative wound cultures grew P multocida. The antibiotic treatment was tailored initially to ampicillin and sulbactam and then to a final regimen of orally administered ciprofloxacin (750 mg twice a day), once susceptibility testing was performed on the cultures. On hospital day 10, the patient was discharged home, receiving a 6-week course of ciprofloxacin to complete the 8-week course of treatment.
At follow-up, approximately 1 month after discharge, the patient noted that he had developed a new right sternoclavicular joint prominence that was associated with painful crepitus. He also noted that his wrists were gradually becoming more swollen and painful bilaterally. Computed tomography scans of the chest were obtained to evaluate the sternoclavicular joint (Figure 1). Repeat radiographs of the wrists were also obtained (Figure 2). Imaging showed lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint, consistent with osteomyelitis. The C-reactive protein level and erythrocyte sedimentation rate at this time were 34 mg/L and 124 mm/h, respectively.
The patient returned to the operating room for débridement and biopsy of the right sternoclavicular joint and left wrist. This patient’s delayed presentation was characterized by a subacute worsening of isolated musculoskeletal complaints. The differential diagnosis then included infection with the same bacterial pathogen versus reactive or inflammatory arthritis. Several intraoperative cultures failed to grow any bacteria, including P multocida, although P multocida was the presumptive cause of the erosive polyarthropathy, considering that symptoms eventually resolved with a repeated course of IV-administered ertapenem for 6 weeks. The patient experienced complete resolution of his joint pain and swelling. He was able to resume his activities of daily living and had no further recurrence of symptoms at follow-up 3 months later.
Discussion
Cat bites often are the source of Pasteurella species infections because the bacteria are carried by more than 90% of cats.1 These types of infections can cause septic arthritis, osteomyelitis, and deep subcutaneous and myofascial infections because of the sharp and narrow morphology of cat teeth. The infections can progress to necrotizing fasciitis and myositis if not recognized early, as was the case with our patient. Prophylactic antibiotic administration for animal bites is controversial and is not a universal practice.1,2Pasteurella bacteremia is an atypical progression that occurs more often in patients with pneumonia, septic arthritis, or meningitis. Cases of Pasteurella sepsis, necrotizing fasciitis, and septic arthritis have been reported.3-7 However, associated progressive septic arthritis and osteomyelitis, despite initial clinical improvement, have not been reported. Severe infection (ie, sepsis and septic shock) can occur in infants, pregnant women, and other immunocompromised patients.7 Immune suppression of our patient with steroid medication for poison sumac dermatitis likely contributed to the progression and systemic spread of an initially benign cat bite. Before prescribing steroids, it is imperative to ask about exposures and encourage patients to seek prompt medical attention with worsening or new symptoms. Healthy individuals rarely develop bacteremia; however, in these cases, mortality remains high at approximately 25%.4,6
The clinical course of this case emphasizes the need for vigilance and thoroughness in obtaining histories from patients presenting with seemingly benign complaints, especially in vulnerable populations, such as infants, pregnant women, and immunocompromised adults. In this case, the progression of symptoms might have been avoided if the patient’s dermatitis had been treated conservatively or with topical rather than systemic steroids.
1. Esposito S, Picciolli I, Semino M, Principi N. Dog and cat bite-associated infections in children. Eur J Clin Microbiol Infect Dis. 2013;32(8):971-976.
2. Medeiros I, Saconato H. Antibiotic prophylaxis for mammalian bites. Cochrane Database Syst Rev. 2001;(2):CD001738.
3. Haybaeck J, Schindler C, Braza P, Willinger B, Drlicek M. Rapidly progressive and lethal septicemia due to infection with Pasteurella multocida in an infant. Wien Klin Wochenschr. 2009;121(5-6):216-219.
4. Migliore E, Serraino C, Brignone C, et al. Pasteurella multocida infection in a cirrhotic patient: case report, microbiological aspects and a review of the literature. Adv Med Sci. 2009;54(1):109-112.
5. Mugambi SM, Ullian ME. Bacteremia, sepsis, and peritonitis with Pasteurella multocida in a peritoneal dialysis patient. Perit Dial Int. 2010;30(3):381-383.
6. Weber DJ, Wolfson JS, Swartz MN, Hooper DC. Pasteurella multocida infections. Report of 34 cases and review of the literature. Medicine (Baltimore). 1984;63(3):133-154.
7. Oehler RL, Velez AP, Mizrachi M, Lamarche J, Gompf S. Bite-related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439-447.
1. Esposito S, Picciolli I, Semino M, Principi N. Dog and cat bite-associated infections in children. Eur J Clin Microbiol Infect Dis. 2013;32(8):971-976.
2. Medeiros I, Saconato H. Antibiotic prophylaxis for mammalian bites. Cochrane Database Syst Rev. 2001;(2):CD001738.
3. Haybaeck J, Schindler C, Braza P, Willinger B, Drlicek M. Rapidly progressive and lethal septicemia due to infection with Pasteurella multocida in an infant. Wien Klin Wochenschr. 2009;121(5-6):216-219.
4. Migliore E, Serraino C, Brignone C, et al. Pasteurella multocida infection in a cirrhotic patient: case report, microbiological aspects and a review of the literature. Adv Med Sci. 2009;54(1):109-112.
5. Mugambi SM, Ullian ME. Bacteremia, sepsis, and peritonitis with Pasteurella multocida in a peritoneal dialysis patient. Perit Dial Int. 2010;30(3):381-383.
6. Weber DJ, Wolfson JS, Swartz MN, Hooper DC. Pasteurella multocida infections. Report of 34 cases and review of the literature. Medicine (Baltimore). 1984;63(3):133-154.
7. Oehler RL, Velez AP, Mizrachi M, Lamarche J, Gompf S. Bite-related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439-447.
Polydactyly of the Hand
Polydactyly is the presence of extra digits. Its incidence is likely underestimated because many practitioners treat simple “nubbins” without referring them to orthopedic specialists.1-3 Polydactyly can be detected by ultrasound as early as 14 weeks’ gestational age, with partial autoamputation seen in most isolated polydactylies.4 The thumb, responsible for 40% of hand function, must be able to oppose the other digits with a stable pinch.5 Polydactyly encumbers this motion when the duplicated digits deviate from normal alignment. Ezaki6 noted that the anatomy is better described as “split” than “duplicated.” There are many dichotomous ways to classify polydactyly: preaxial (radial) versus postaxial (ulnar), thumb versus triphalangeal, simple versus complex (Figure 1). Mixed polydactyly is defined as the presence of preaxial and postaxial polydactyly.7 Surgical management seeks to allow normal hand function and to restore cosmesis.
Epidemiology
Sun and colleagues8 reported the overall polydactyly incidence as 2 per 1000 live births in China from 1998 to 2009, with a slight male predominance; polydactyly was also 3 times more common than syndactyly in this population. Ivy,9 in a 5-year audit of Pennsylvania Department of Health records, found polydactyly to be the fourth most common congenital anomaly after clubfoot, cleft lip/palate, and spina bifida. Thumb duplication occurs in 0.08 to 1.4 per 1000 live births and is more common in American Indians and Asians than in other races.5,10 It occurs in a male-to-female ratio of 2.5 to 1 and is most often unilateral.5 Postaxial polydactyly is predominant in black infants; it is most often inherited in an autosomal dominant fashion, if isolated, or in an autosomal recessive pattern, if syndromic.1 A prospective San Diego study of 11,161 newborns found postaxial type B polydactyly in 1 per 531 live births (1 per 143 black infants, 1 per 1339 white infants); 76% of cases were bilateral, and 86% had a positive family history.3 In patients of non-African descent, it is associated with anomalies in other organs. Central duplication is rare and often autosomal dominant.5,10
Genetics and Development
As early as 1896, the heritability of polydactyly was noted.11 As of 2010, polydactyly has been associated with 310 diseases.12 Ninety-nine genes, most involved in regulation of anterior-posterior formation of the limb bud, have been implicated.12,13
The upper limb begins to form at day 26 in utero.14 Apoptosis in the interdigital necrotic zones results in the formation of individual digits. It is presumed that, in polydactyly, the involved tissue is hypoplastic because of an abnormal interaction between mesoderm and ectoderm.5 Presence of an apical ectodermal ridge determines the formation of a limb bud, and on it the zone of polarizing activity (ZPA) dictates preaxial and postaxial alignment.14,15 The ZPA is located on the posterior zone of the developing limb bud. The levels of GLI3, a zinc finger-containing DNA-binding protein, are highest in the anterior area, and HAND2, a basic helix-loop-helix DNA-binding protein, is found in the ZPA. This polarity promotes sonic hedgehog (Shh) gene expression in the posterior region, which in turn prevents GLI3 cleavage into its repressed form. GLI3R (repressed) and GLI3A (active) concentrations are highest, therefore, in the anterior and posterior portions of the bud, respectively. The GLI3A:GLI3R ratio is responsible for the identity and number of digits in the hand (ie, the thumb develops in regions of high GLI3R). GLI and Shh mutations lead to polydactylous hands with absent thumbs (Figure 2).16
Ciliopathies have also been shown to cause postaxial polydactyly, possibly because of the role that nonmotile cilia play in hedgehog signaling.17 Mutations in Shh genomic regulators cause preaxial polydactyly.18 HoxD activates Shh in the ZPA; HoxD13 mutations are associated with synpolydactyly.16,19 In each of these mutations, Shh production is altered, and some form of polydactyly results.
Associations
Many syndromes have been associated with polydactyly. Not all polydactyly is associated with other disorders, but the more complex the polydactyly, the more likely that other anomalies are present. Every patient who presents with polydactyly should have a full history taken and a physical examination performed (Figure 3). Any patient with syndromic findings or atypical presentations (eg, triphalangism, postaxial polydactyly in a patient of non-African descent, central and index polydactyly) should be referred to a geneticist.
Classifications
The Wassel20 classification describes the anatomical presentation of thumb duplication on the basis of 70 cases in Iowa (Figures 4, 5; Table 1). Because some duplications fall outside the Wassel classification, many researchers have proposed modifications (Figure 6).21-25
The Temtamy and McKusick10 classification, which is the product of geneticists, classifies duplications by grouping genetically related presentations (Table 2). It provides the most commonly used postaxial classification, with type A being a fully developed digit and type B a rudimentary and pedunculated digit, informally referred to as a nubbin. Type B is more common than type A. Given inheritance patterns, it is assumed that type A is likely multifactorial and type B autosomal dominant.10 Thumb polydactyly inheritance is still unclear. The other types of preaxial polydactyly and high degrees of polydactyly are rare but seem to be passed on in an autosomal dominant fashion on pedigree analysis.10
The Stelling and Turek classification presents the duplication from a tissue perspective: Type I duplication is a rudimentary mass devoid of other tissue elements; type II is a subtotal duplication with some normal structures; and type III is a duplication of the entire “osteoarticular column,” including the metacarpal.1 It is interesting to note that histology of type I duplications shows neuroma-like tissue.26-28 Again, normal is a relative term because, in polydactyly, the duplications are hypoplastic and deviated, with anomalous anatomy.
The Rayan classification describes ulnar polydactyly and was derived from a case study series of 148 patients in Oklahoma (Table 3).29
There are also some complex polydactylies that are not easily classified: ulnar dimelia, cleft hand, pentadactyly, and hyperphalangism. Ulnar dimelia, also known as “mirror hand,” is typically 7 digits with no thumb, but other variations are seen. The radius is often absent, and the elbow is abnormal. There is some debate about whether it is a fusion of 2 hands. Pentadactyly, or the 5-fingered hand, appears as 5 triphalangeal digits with no thumb (Figure 7).
Isolated thumb triphalangism might appear similar to pentadactyly. Miura30,31 pointed out that the radial digit in the pentadactylous hand may be opposable (thumb-like) or nonopposable; in his studies, the patients with the opposable thumb had a metacarpal with a proximal epiphysis (Figure 8). Consequently, the triphalangeal thumb metacarpal with a distal epiphysis is true pentadactyly, whereas that with a proximal epiphysis is hyperphalangism (Figure 9). Treatment of these complex polydactylies involves the same underlying principles as for preaxial and postaxial polydactyly, albeit with additional proximal upper extremity considerations.
When to Operate (Timing)
Ezaki6 recommended surgical intervention at age 6 to 9 months, before fine motor skills have developed with the abnormal anatomy. Cortical learning occurs as the child begins prehensile activities before 6 months, but the risks of anesthesia outweigh functional benefits until the child is older. Waiting until 1 year of age is not uncommon, though surgery at an earlier age may be beneficial if the polydactyly affects hand function.32 It is not uncommon to wait with the more balanced thumb polydactylies to assess thumb function. Hypoplasia might also delay surgical intervention until there is enough tissue inventory for reconstruction. Wassel20 noted that surgical intervention ideally occurs before the supernumerary elements displace the normal elements, as tends to happen with growth. Suture ligation is an option in the neonatal unit for some pedunculated digits.33 Studies have shown satisfactory results in adults treated for polydactyly, if the patient presents later than expected.34
Surgical Considerations
Knavel recommended simple excision, stating that “ablation requires no ingenuity and creates no problems.”5 This belief, though true for some duplications, will not lead to the best outcome for more complex polydactylies. The goal of surgery is a stable and well-aligned thumb for pinch and prehensile activity, as well as a cosmetically pleasing hand. Incisions should not be made linearly along the axis of the digit, as the scar will cause deviation with growth.24
Wassel type I polydactyly might appear incidentally as a broad thumb, in which case it requires no intervention (Figure 10). However, in Wassel types I and II polydactyly with deformity, the Bilhaut-Cloquet procedure is useful for both bifid and duplicated phalanges (Figure 11).5,6,30,32,35 Collateral ligaments may need to be released in type II because of difficulty in opposing the tissue. Cosmetic results with Bilhaut-Cloquet are unpredictable. The original technique required symmetrically sized digits; results today have been improved with microtechniques and preservation of an entire nail.36 Another option is ablation of the more hypoplastic osseous element and soft-tissue augmentation of the residual digit. The theme of ablation and augmentation is seen throughout the literature for the surgical treatment of polydactyly (Figure 12).1
For type III polydactyly, the bifid proximal phalanx is narrowed by resection and realigned with osteotomy of the remaining diaphysis. Type IV polydactyly, the most common thumb duplication, often requires advancement of the abductor pollicis brevis to the base of the proximal phalanx to aid in metacarpophalangeal (MCP) stabilization, abduction, and opposition. The metacarpal head, if broad and with 2 facets, can be shaped to form a single articulating surface. The metacarpal, occasionally with the proximal phalanx, often requires realignment by closing wedge osteotomy. Last, tendons on the resected bony elements should be rebalanced on the remaining digit, and anomalous slips must be released. For instance, given a radial insertion of the long flexor tendon on the distal phalanx, the tendon should be moved centrally. A strong flexor or extensor tendon on the amputated digit should be transferred to the remaining digit.24
Types V and VI are treated similarly to type IV, with the addition of a first web space Z-plasty or web widening if there is thenar eminence contracture. Acral transposition has also been described, with transposition of the tip of the ablated digit in place of the tip of the kept digit; this technique is ideal if one digit has a more normal proximal part while the other has a more normal distal part (Figure 13).35
Type VII thumb polydactyly, the type most likely inherited and associated with other disorders, should be treated like type VI. The nail should be preserved; amputation of the distal phalanx is not advised. Resection of the delta phalanx or 1 interphalangeal (IP) joint is an option. Articular surfaces will remodel if done before the age of 1 year. If the thenar eminence is hypoplastic, then Huber transfer of the abductor digiti minimi should be considered.37 Resection of the triphalangeal thumb is also advised, even if the biphalangeal thumb is more hypoplastic, with transfer of the ligaments and tendons, as described earlier.5,6,24,30,32,35
Thumb triphalangism, if isolated, and hyperphalangism in the other digits, can be treated with resection of the delta phalanx or one of the IP joints if it is affecting function or cosmesis.1,6 Wood and Flatt23 recommended early resection of a thumb delta phalanx because of the likelihood of deviation that impedes thumb function. For children, they recommended delta phalanx resection and Kirschner wire fixation for 6 weeks; for adults, they recommended resection or fusion of the joint, with osteotomy as needed for deviation.23,24 For thumb triphalangism, multiple surgeries are the norm, as Wood24 reported in his study of 21 patients who underwent 78 operations in total.
Index polydactyly may present as a simple pedunculated skin tag, which can be simply excised, or as a more complex musculoskeletal duplication. More complex presentations can be treated with procedures similar to those used for the thumb. Typically, the additional digit is radially deviated and angulated, eventually leading to impingement of thumb pinch and the first web space. Ray amputation is also an option if no reconstructive surgery will produce the stable, sensate radial pinch that is essential to hand function.32
Ring-finger polydactyly and long-finger polydactyly are often complicated by some element of syndactyly, resulting in a relative paucity of skin (Figure 14). There is failure of both formation (hypoplasia) and differentiation (syndactyly). The hypoplasia particularly affects the function of these digits by tethering them; multiple surgeries to restore proper hand function are the norm.1 Reconstructive surgery for these digits requires preoperative tissue inventory followed by resection and augmentation; as in syndactyly, skin for coverage is at a premium. Creation of a 3-fingered hand is an option.23
Temtamy and McKusick10 type A little-finger polydactyly is treated similarly to the thumb, with the caveat that hypothenar and intrinsic muscles that insert on the resected little finger are transferred to the remaining digit. In contrast to thumb polydactyly, the extrinsic musculature tends to be in good position. Suture ligation of type B polydactyly, as described by Flatt, is likely more common than orthopedists appreciate, as pediatricians and neonatal unit practitioners commonly perform this procedure in the nursery.1-3 It has been described with 2-0 Vicryl3 (Ethicon, Somerville, New Jersey) and 4-0 silk sutures,32 with the goal of necrosis and autoamputation. Parents should be told the finger generally falls off about 10 days (range, 4-21 days) after ligation.3 Multiple authors have cited a report of exsanguination from suture ligation, but we could not locate the primary source. It is advisable to wait until a patient is 6 months of age if planning to resect the nubbin in the operating room, given the anesthesia risk and the lack of functional impairment. Katz and Linder33 indicated they remove type B polydactyly in the nursery suite used for circumcisions; they use anesthetizing cream on the skin, and sharp excision with a scalpel, followed by direct pressure and Steri-Strip (3M, St. Paul, Minnesota) application. Suture ligation is recommended only if there is a narrow, thin (<2 mm) soft-tissue stalk; any broad or bony stalk necessitates surgical removal to avoid neuroma formation and failure of autonecrosis (Figure 15).27 Other options are a single swipe of a scalpel and elliptical excision; sharp transaction of the digital nerve with subsequent retraction is advised to avoid neuroma formation.2
Barton described ulnar dimelia operations as “spare parts surgery.”1 Extra digits are ablated and a thumb created (Figure 16). The hand might have a digit in relatively good rotational position for thumbplasty, or the principles of pollicization may need to be used. If the patient is already using the hand, the surgeon should note which finger the patient uses as a thumb.24 Any accompanying wrist flexion contracture must be corrected with careful attention to musculotendinous balancing. Because the forearm and elbow, and occasionally even the more proximal limb, will be abnormal in this disorder, multiple surgeries are again the norm.1
Pentadactyly is treated like thumb hypoplasia, with first web space creation.1
Complications
In polydactyly, a reoperation rate of up to 25% has been reported, with most reoperations performed because of residual or subsequent deformity.5,30,31,38 Risk factors for reoperation are type IV thumb duplication, preoperative “zigzag” deformity, and radially deviated thumb elements at presentation.5 The delta phalanx may not show on radiographs until the patient is 18 months old, but functional deformity will worsen as long as it is present. Zigzag deformity may be due to the delta phalanx or to musculotendinous imbalance, such as a radially inserted flexor pollicis longus (FPL) or lack of stable MCP abduction. Miura31 found that careful reconstruction of the joint capsule and thenar muscles from the ablated digit to the remnant digit is the key to a successful initial surgery. Lee and colleagues39 defined zigzag deformity as more than 20° MCP and IP angulation; for cases present before surgery, they recommended FPL relocation by the pullout technique in addition to osteotomies to prevent further interphalangeal deviation (Figures 17, 18).
Abnormal physeal growth, joint instability, and stiffness can all occur. Stiffness is particularly difficult to treat but seldom presents a functional problem. Joint enlargement, which is not uncommon, results from either broad articular surfaces or retained cartilage from the perichondral ring after resection that later ossifies.5,38 Nubbin-type duplications may not fall off after suture ligation, necessitating further excision, and a cosmetic bump is seen after 40% of suture ligations.3 Patillo and Rayan28 and Rayan and Frey29 warned against suture ligation unless the nubbin has a small stalk because of the possibility of infection and gangrene. The excised nubbin tissue is histologically nervous, and there have been reports of painful neuromas in the remaining scar of a ligated nubbin that respond well to excision.26,27,40 It is thought that these painful lesions form because the ligature prevents the digital nerves to the vestigial digit from retracting.27 Nail deformity and IP joint stiffness are seen with the Bilhaut-Cloquet procedure, though often finger function remains satisfactory.
Conclusion
Polydactyly is a common congenital hand abnormality. Its true incidence is unknown because of inconsistent documentation. Surgeons must strive for a functional, cosmetic hand, given a diverse set of possible anomalies. Hypoplasia is the rule; tissue should be ablated and augmented as necessary. Musculotendinous insertions may need to be centralized. Patients’ family members should always be counseled that more surgery may be needed in the future, as further deformity can occur with growth. Surgically corrected thumb duplications will be stiffer, shorter, and thinner than their normal counterparts. Nail ridges are common. However, it should be noted that 88% of these patients are satisfied with their results.41 Some amount of contracture and abnormal function should be expected with index-, long-, and ring-finger duplications. The only remnant of type B postaxial duplications may be a slight discoloration or bump, though stiffness and deformity can happen with a type A deformity. A “duplicated” digit that requires surgical correction will never be completely normal, but acceptable function is routinely achievable.
1. Graham TJ, Ress AM. Finger polydactyly. Hand Clin. 1998;14(1):49-64.
2. Abzug JM, Kozin SH. Treatment of postaxial polydactyly type B. J Hand Surg Am. 2013;38(6):1223-1225.
3. Watson BT, Hennrikus WL. Postaxial type-B polydactyly—prevalence and treatment. J Bone Joint Surg Am. 1997;79(1):65-68.
4. Zimmer EZ, Bronshtein M. Fetal polydactyly diagnosis during early pregnancy: clinical applications. Am J Obstet Gynecol. 2000;183(3):755-758.
5. Cohen MS. Thumb duplication. Hand Clin. 1998;14(1):17-27.
6. Ezaki M. Radial polydactyly. Hand Clin. 1990;6(4):577-588.
7. Nathan PA, Keniston RC. Crossed polydactyly: case report and review of the literature. J Bone Joint Surg Am. 1975;57(6):847-849.
8. Sun G, Xu ZM, Liang JF, Li L, Tang DX. Twelve-year prevalence of common neonatal congenital malformations in Zhejiang Province, China. World J Pediatr. 2011;7(4):331-336.
9. Ivy RH. Congenital anomalies as recorded on birth certificates in the Division of Vital Statistics of the Pennsylvania Department of Health, for the period of 1951–1955, inclusive. Plast Reconstr Surg. 1957;20(5):400-411.
10. Temtamy SA, McKusick VA. Polydactyly as a part of syndromes. In: Bergsma D, ed. Mudge JR, Paul NW, Conde Greene S, associate eds. The Genetics of Hand Malformations. New York, NY: Liss. Birth Defects Original Article Series. 1978;14(3):364-439.
11. Gould W, Pyle L. Anomalies and Curiosities of Medicine. New York, NY: Bell; 1896.
12. Biesecker LG. Polydactyly: how many disorders and how many genes: 2010 update. Dev Dyn. 2011;250(5):931-942.
13. Grzeschik K. Human limb malformations; an approach to the molecular basis of development. Int J Dev Biol. 2001;46(7):983-991.
14. Zaleske DJ. Development of the upper limb. Hand Clin. 1985;1(3):383-390.
15. Beatty E. Upper limb tissue differentiation in the human embryo. Hand Clin. 1985;1(3):391-404.
16. Anderson E, Peluso S, Lettice LA, Hill RE. Human limb abnormalities caused by disruption of hedgehog signaling. Trends Genet. 2012;28(8):364-373.
17. Ware SM, Aygun MG, Heldebrandt F. Spectrum of clinical diseases caused by disorders of primary cilia. Proc Am Thorac Soc. 2011;8(5):444-450.
18. Lettice LA, Hill RE. Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr Opin Genet Dev. 2005;15(3):294-300.
19. Al-Qattan MA. Type II familial synpolydactyly: report on two families with an emphasis on variations of expression. Eur J Hum Genet. 2011;19(1):112-114.
20. Wassel HD. The results of surgery for polydactyly of the thumb. Clin Orthop. 1969;(64):175-193.
21. Blauth W, Olason AT. Classification of polydactyly of the hands and feet. Arch Orthop Trauma Surg. 1988;107(6):334-344.
22. Wood VE. Super digit. Hand Clin. 1990;6(4):673-684.
23. Wood VE, Flatt AE. Congenital triangular bones in the hand. J Hand Surg Am. 1977;2(3):179-193.
24. Wood VE. Polydactyly and the triphalangeal thumb. J Hand Surg Am. 1978;3(5):436-444.
25. Zuidam JM, Selles RW, Ananta M, Runia J, Hovius SER. A classification system of radial polydactyly: inclusion of triphalangeal thumb and triplication. J Hand Surg Am. 2008;33(3):373-377.
26. Leber GE, Gosain AK. Surgical excision of pedunculated supernumerary digits prevents traumatic amputation neuromas. Pediatr Dermatol. 2003;20(2):108-112.
27. Mullick S, Borschel GH. A selective approach to treatment of ulnar polydactyly: preventing painful neuroma and incomplete excision. Pediatr Dermatol. 2001;27(1):39-42.
28. Patillo D, Rayan GM. Complications of suture ligation ablation for ulnar polydactyly: a report of two cases. Hand (N Y). 2011;6(1):102-105.
29. Rayan GM, Frey B. Ulnar polydactyly. Plastic Reconstr Surg. 2001;107(6):1449-1454.
30. Miura T. Triphalangeal thumb. Plastic Reconstr Surg. 1976;58(5):587-594.
31. Miura T. Duplicated thumb. Plastic Reconstr Surg. 1982;69(3):470-481.
32. Simmons BP. Polydactyly. Hand Clin. 1985;1(3):545-566.
33. Katz K, Linder N. Postaxial type B polydactyly treated by excision in the neonatal nursery. J Pediatr Orthop. 2011;31(4):448-449.
34. Manohar A, Beard AJ. Outcome of reconstruction for duplication of the thumb in adults aged over 40. Hand Surg. 2011;16(2):207-210.
35. Watt AJ, Chung KC. Duplication. Hand Clin. 2009;25(2):215-228.
36. Tonkin MA. Thumb duplication: concepts and techniques. Clin Orthop Surg. 2012;4(1):1-17.
37. Huber E. Relief operation in the case of paralysis of the median nerve. J Hand Surg Eur. 2004;29(1):35-37.
38. Mih AD. Complications of duplicate thumb reconstruction. Hand Clin. 1998;14(1):143-149.
39. Lee CC, Park HY, Yoon JO, Lee KW. Correction of Wassel type IV thumb duplication with zigzag deformity: results of a new method of flexor pollicis longus tendon relocation. J Hand Surg Eur. 2013;38(3):272-280.
40. Hare PJ. Rudimentary polydactyly. Br J Dermatol. 1954;66(11):402-408.
41. Yen CH, Chan WL, Leung HB, Mak KH. Thumb polydactyly: clinical outcome after reconstruction. J Orthop Surg (Hong Kong). 2006;14(3):295-302.
42. Edmunds JO. A tribute to Daniel C. Riordan, MD (1917–2012). Tulane University School of Medicine, Department of Orthopaedics website. http://tulane.edu/som/departments/orthopaedics/news-and-events/danriordantribute.cfm. Accessed March 31, 2015.
43. Faust DC, Herms R. Daniel C. Riordan, MD, 1917–2012. J Hand Surg Am. 2013;38(1):202-205.
Polydactyly is the presence of extra digits. Its incidence is likely underestimated because many practitioners treat simple “nubbins” without referring them to orthopedic specialists.1-3 Polydactyly can be detected by ultrasound as early as 14 weeks’ gestational age, with partial autoamputation seen in most isolated polydactylies.4 The thumb, responsible for 40% of hand function, must be able to oppose the other digits with a stable pinch.5 Polydactyly encumbers this motion when the duplicated digits deviate from normal alignment. Ezaki6 noted that the anatomy is better described as “split” than “duplicated.” There are many dichotomous ways to classify polydactyly: preaxial (radial) versus postaxial (ulnar), thumb versus triphalangeal, simple versus complex (Figure 1). Mixed polydactyly is defined as the presence of preaxial and postaxial polydactyly.7 Surgical management seeks to allow normal hand function and to restore cosmesis.
Epidemiology
Sun and colleagues8 reported the overall polydactyly incidence as 2 per 1000 live births in China from 1998 to 2009, with a slight male predominance; polydactyly was also 3 times more common than syndactyly in this population. Ivy,9 in a 5-year audit of Pennsylvania Department of Health records, found polydactyly to be the fourth most common congenital anomaly after clubfoot, cleft lip/palate, and spina bifida. Thumb duplication occurs in 0.08 to 1.4 per 1000 live births and is more common in American Indians and Asians than in other races.5,10 It occurs in a male-to-female ratio of 2.5 to 1 and is most often unilateral.5 Postaxial polydactyly is predominant in black infants; it is most often inherited in an autosomal dominant fashion, if isolated, or in an autosomal recessive pattern, if syndromic.1 A prospective San Diego study of 11,161 newborns found postaxial type B polydactyly in 1 per 531 live births (1 per 143 black infants, 1 per 1339 white infants); 76% of cases were bilateral, and 86% had a positive family history.3 In patients of non-African descent, it is associated with anomalies in other organs. Central duplication is rare and often autosomal dominant.5,10
Genetics and Development
As early as 1896, the heritability of polydactyly was noted.11 As of 2010, polydactyly has been associated with 310 diseases.12 Ninety-nine genes, most involved in regulation of anterior-posterior formation of the limb bud, have been implicated.12,13
The upper limb begins to form at day 26 in utero.14 Apoptosis in the interdigital necrotic zones results in the formation of individual digits. It is presumed that, in polydactyly, the involved tissue is hypoplastic because of an abnormal interaction between mesoderm and ectoderm.5 Presence of an apical ectodermal ridge determines the formation of a limb bud, and on it the zone of polarizing activity (ZPA) dictates preaxial and postaxial alignment.14,15 The ZPA is located on the posterior zone of the developing limb bud. The levels of GLI3, a zinc finger-containing DNA-binding protein, are highest in the anterior area, and HAND2, a basic helix-loop-helix DNA-binding protein, is found in the ZPA. This polarity promotes sonic hedgehog (Shh) gene expression in the posterior region, which in turn prevents GLI3 cleavage into its repressed form. GLI3R (repressed) and GLI3A (active) concentrations are highest, therefore, in the anterior and posterior portions of the bud, respectively. The GLI3A:GLI3R ratio is responsible for the identity and number of digits in the hand (ie, the thumb develops in regions of high GLI3R). GLI and Shh mutations lead to polydactylous hands with absent thumbs (Figure 2).16
Ciliopathies have also been shown to cause postaxial polydactyly, possibly because of the role that nonmotile cilia play in hedgehog signaling.17 Mutations in Shh genomic regulators cause preaxial polydactyly.18 HoxD activates Shh in the ZPA; HoxD13 mutations are associated with synpolydactyly.16,19 In each of these mutations, Shh production is altered, and some form of polydactyly results.
Associations
Many syndromes have been associated with polydactyly. Not all polydactyly is associated with other disorders, but the more complex the polydactyly, the more likely that other anomalies are present. Every patient who presents with polydactyly should have a full history taken and a physical examination performed (Figure 3). Any patient with syndromic findings or atypical presentations (eg, triphalangism, postaxial polydactyly in a patient of non-African descent, central and index polydactyly) should be referred to a geneticist.
Classifications
The Wassel20 classification describes the anatomical presentation of thumb duplication on the basis of 70 cases in Iowa (Figures 4, 5; Table 1). Because some duplications fall outside the Wassel classification, many researchers have proposed modifications (Figure 6).21-25
The Temtamy and McKusick10 classification, which is the product of geneticists, classifies duplications by grouping genetically related presentations (Table 2). It provides the most commonly used postaxial classification, with type A being a fully developed digit and type B a rudimentary and pedunculated digit, informally referred to as a nubbin. Type B is more common than type A. Given inheritance patterns, it is assumed that type A is likely multifactorial and type B autosomal dominant.10 Thumb polydactyly inheritance is still unclear. The other types of preaxial polydactyly and high degrees of polydactyly are rare but seem to be passed on in an autosomal dominant fashion on pedigree analysis.10
The Stelling and Turek classification presents the duplication from a tissue perspective: Type I duplication is a rudimentary mass devoid of other tissue elements; type II is a subtotal duplication with some normal structures; and type III is a duplication of the entire “osteoarticular column,” including the metacarpal.1 It is interesting to note that histology of type I duplications shows neuroma-like tissue.26-28 Again, normal is a relative term because, in polydactyly, the duplications are hypoplastic and deviated, with anomalous anatomy.
The Rayan classification describes ulnar polydactyly and was derived from a case study series of 148 patients in Oklahoma (Table 3).29
There are also some complex polydactylies that are not easily classified: ulnar dimelia, cleft hand, pentadactyly, and hyperphalangism. Ulnar dimelia, also known as “mirror hand,” is typically 7 digits with no thumb, but other variations are seen. The radius is often absent, and the elbow is abnormal. There is some debate about whether it is a fusion of 2 hands. Pentadactyly, or the 5-fingered hand, appears as 5 triphalangeal digits with no thumb (Figure 7).
Isolated thumb triphalangism might appear similar to pentadactyly. Miura30,31 pointed out that the radial digit in the pentadactylous hand may be opposable (thumb-like) or nonopposable; in his studies, the patients with the opposable thumb had a metacarpal with a proximal epiphysis (Figure 8). Consequently, the triphalangeal thumb metacarpal with a distal epiphysis is true pentadactyly, whereas that with a proximal epiphysis is hyperphalangism (Figure 9). Treatment of these complex polydactylies involves the same underlying principles as for preaxial and postaxial polydactyly, albeit with additional proximal upper extremity considerations.
When to Operate (Timing)
Ezaki6 recommended surgical intervention at age 6 to 9 months, before fine motor skills have developed with the abnormal anatomy. Cortical learning occurs as the child begins prehensile activities before 6 months, but the risks of anesthesia outweigh functional benefits until the child is older. Waiting until 1 year of age is not uncommon, though surgery at an earlier age may be beneficial if the polydactyly affects hand function.32 It is not uncommon to wait with the more balanced thumb polydactylies to assess thumb function. Hypoplasia might also delay surgical intervention until there is enough tissue inventory for reconstruction. Wassel20 noted that surgical intervention ideally occurs before the supernumerary elements displace the normal elements, as tends to happen with growth. Suture ligation is an option in the neonatal unit for some pedunculated digits.33 Studies have shown satisfactory results in adults treated for polydactyly, if the patient presents later than expected.34
Surgical Considerations
Knavel recommended simple excision, stating that “ablation requires no ingenuity and creates no problems.”5 This belief, though true for some duplications, will not lead to the best outcome for more complex polydactylies. The goal of surgery is a stable and well-aligned thumb for pinch and prehensile activity, as well as a cosmetically pleasing hand. Incisions should not be made linearly along the axis of the digit, as the scar will cause deviation with growth.24
Wassel type I polydactyly might appear incidentally as a broad thumb, in which case it requires no intervention (Figure 10). However, in Wassel types I and II polydactyly with deformity, the Bilhaut-Cloquet procedure is useful for both bifid and duplicated phalanges (Figure 11).5,6,30,32,35 Collateral ligaments may need to be released in type II because of difficulty in opposing the tissue. Cosmetic results with Bilhaut-Cloquet are unpredictable. The original technique required symmetrically sized digits; results today have been improved with microtechniques and preservation of an entire nail.36 Another option is ablation of the more hypoplastic osseous element and soft-tissue augmentation of the residual digit. The theme of ablation and augmentation is seen throughout the literature for the surgical treatment of polydactyly (Figure 12).1
For type III polydactyly, the bifid proximal phalanx is narrowed by resection and realigned with osteotomy of the remaining diaphysis. Type IV polydactyly, the most common thumb duplication, often requires advancement of the abductor pollicis brevis to the base of the proximal phalanx to aid in metacarpophalangeal (MCP) stabilization, abduction, and opposition. The metacarpal head, if broad and with 2 facets, can be shaped to form a single articulating surface. The metacarpal, occasionally with the proximal phalanx, often requires realignment by closing wedge osteotomy. Last, tendons on the resected bony elements should be rebalanced on the remaining digit, and anomalous slips must be released. For instance, given a radial insertion of the long flexor tendon on the distal phalanx, the tendon should be moved centrally. A strong flexor or extensor tendon on the amputated digit should be transferred to the remaining digit.24
Types V and VI are treated similarly to type IV, with the addition of a first web space Z-plasty or web widening if there is thenar eminence contracture. Acral transposition has also been described, with transposition of the tip of the ablated digit in place of the tip of the kept digit; this technique is ideal if one digit has a more normal proximal part while the other has a more normal distal part (Figure 13).35
Type VII thumb polydactyly, the type most likely inherited and associated with other disorders, should be treated like type VI. The nail should be preserved; amputation of the distal phalanx is not advised. Resection of the delta phalanx or 1 interphalangeal (IP) joint is an option. Articular surfaces will remodel if done before the age of 1 year. If the thenar eminence is hypoplastic, then Huber transfer of the abductor digiti minimi should be considered.37 Resection of the triphalangeal thumb is also advised, even if the biphalangeal thumb is more hypoplastic, with transfer of the ligaments and tendons, as described earlier.5,6,24,30,32,35
Thumb triphalangism, if isolated, and hyperphalangism in the other digits, can be treated with resection of the delta phalanx or one of the IP joints if it is affecting function or cosmesis.1,6 Wood and Flatt23 recommended early resection of a thumb delta phalanx because of the likelihood of deviation that impedes thumb function. For children, they recommended delta phalanx resection and Kirschner wire fixation for 6 weeks; for adults, they recommended resection or fusion of the joint, with osteotomy as needed for deviation.23,24 For thumb triphalangism, multiple surgeries are the norm, as Wood24 reported in his study of 21 patients who underwent 78 operations in total.
Index polydactyly may present as a simple pedunculated skin tag, which can be simply excised, or as a more complex musculoskeletal duplication. More complex presentations can be treated with procedures similar to those used for the thumb. Typically, the additional digit is radially deviated and angulated, eventually leading to impingement of thumb pinch and the first web space. Ray amputation is also an option if no reconstructive surgery will produce the stable, sensate radial pinch that is essential to hand function.32
Ring-finger polydactyly and long-finger polydactyly are often complicated by some element of syndactyly, resulting in a relative paucity of skin (Figure 14). There is failure of both formation (hypoplasia) and differentiation (syndactyly). The hypoplasia particularly affects the function of these digits by tethering them; multiple surgeries to restore proper hand function are the norm.1 Reconstructive surgery for these digits requires preoperative tissue inventory followed by resection and augmentation; as in syndactyly, skin for coverage is at a premium. Creation of a 3-fingered hand is an option.23
Temtamy and McKusick10 type A little-finger polydactyly is treated similarly to the thumb, with the caveat that hypothenar and intrinsic muscles that insert on the resected little finger are transferred to the remaining digit. In contrast to thumb polydactyly, the extrinsic musculature tends to be in good position. Suture ligation of type B polydactyly, as described by Flatt, is likely more common than orthopedists appreciate, as pediatricians and neonatal unit practitioners commonly perform this procedure in the nursery.1-3 It has been described with 2-0 Vicryl3 (Ethicon, Somerville, New Jersey) and 4-0 silk sutures,32 with the goal of necrosis and autoamputation. Parents should be told the finger generally falls off about 10 days (range, 4-21 days) after ligation.3 Multiple authors have cited a report of exsanguination from suture ligation, but we could not locate the primary source. It is advisable to wait until a patient is 6 months of age if planning to resect the nubbin in the operating room, given the anesthesia risk and the lack of functional impairment. Katz and Linder33 indicated they remove type B polydactyly in the nursery suite used for circumcisions; they use anesthetizing cream on the skin, and sharp excision with a scalpel, followed by direct pressure and Steri-Strip (3M, St. Paul, Minnesota) application. Suture ligation is recommended only if there is a narrow, thin (<2 mm) soft-tissue stalk; any broad or bony stalk necessitates surgical removal to avoid neuroma formation and failure of autonecrosis (Figure 15).27 Other options are a single swipe of a scalpel and elliptical excision; sharp transaction of the digital nerve with subsequent retraction is advised to avoid neuroma formation.2
Barton described ulnar dimelia operations as “spare parts surgery.”1 Extra digits are ablated and a thumb created (Figure 16). The hand might have a digit in relatively good rotational position for thumbplasty, or the principles of pollicization may need to be used. If the patient is already using the hand, the surgeon should note which finger the patient uses as a thumb.24 Any accompanying wrist flexion contracture must be corrected with careful attention to musculotendinous balancing. Because the forearm and elbow, and occasionally even the more proximal limb, will be abnormal in this disorder, multiple surgeries are again the norm.1
Pentadactyly is treated like thumb hypoplasia, with first web space creation.1
Complications
In polydactyly, a reoperation rate of up to 25% has been reported, with most reoperations performed because of residual or subsequent deformity.5,30,31,38 Risk factors for reoperation are type IV thumb duplication, preoperative “zigzag” deformity, and radially deviated thumb elements at presentation.5 The delta phalanx may not show on radiographs until the patient is 18 months old, but functional deformity will worsen as long as it is present. Zigzag deformity may be due to the delta phalanx or to musculotendinous imbalance, such as a radially inserted flexor pollicis longus (FPL) or lack of stable MCP abduction. Miura31 found that careful reconstruction of the joint capsule and thenar muscles from the ablated digit to the remnant digit is the key to a successful initial surgery. Lee and colleagues39 defined zigzag deformity as more than 20° MCP and IP angulation; for cases present before surgery, they recommended FPL relocation by the pullout technique in addition to osteotomies to prevent further interphalangeal deviation (Figures 17, 18).
Abnormal physeal growth, joint instability, and stiffness can all occur. Stiffness is particularly difficult to treat but seldom presents a functional problem. Joint enlargement, which is not uncommon, results from either broad articular surfaces or retained cartilage from the perichondral ring after resection that later ossifies.5,38 Nubbin-type duplications may not fall off after suture ligation, necessitating further excision, and a cosmetic bump is seen after 40% of suture ligations.3 Patillo and Rayan28 and Rayan and Frey29 warned against suture ligation unless the nubbin has a small stalk because of the possibility of infection and gangrene. The excised nubbin tissue is histologically nervous, and there have been reports of painful neuromas in the remaining scar of a ligated nubbin that respond well to excision.26,27,40 It is thought that these painful lesions form because the ligature prevents the digital nerves to the vestigial digit from retracting.27 Nail deformity and IP joint stiffness are seen with the Bilhaut-Cloquet procedure, though often finger function remains satisfactory.
Conclusion
Polydactyly is a common congenital hand abnormality. Its true incidence is unknown because of inconsistent documentation. Surgeons must strive for a functional, cosmetic hand, given a diverse set of possible anomalies. Hypoplasia is the rule; tissue should be ablated and augmented as necessary. Musculotendinous insertions may need to be centralized. Patients’ family members should always be counseled that more surgery may be needed in the future, as further deformity can occur with growth. Surgically corrected thumb duplications will be stiffer, shorter, and thinner than their normal counterparts. Nail ridges are common. However, it should be noted that 88% of these patients are satisfied with their results.41 Some amount of contracture and abnormal function should be expected with index-, long-, and ring-finger duplications. The only remnant of type B postaxial duplications may be a slight discoloration or bump, though stiffness and deformity can happen with a type A deformity. A “duplicated” digit that requires surgical correction will never be completely normal, but acceptable function is routinely achievable.
Polydactyly is the presence of extra digits. Its incidence is likely underestimated because many practitioners treat simple “nubbins” without referring them to orthopedic specialists.1-3 Polydactyly can be detected by ultrasound as early as 14 weeks’ gestational age, with partial autoamputation seen in most isolated polydactylies.4 The thumb, responsible for 40% of hand function, must be able to oppose the other digits with a stable pinch.5 Polydactyly encumbers this motion when the duplicated digits deviate from normal alignment. Ezaki6 noted that the anatomy is better described as “split” than “duplicated.” There are many dichotomous ways to classify polydactyly: preaxial (radial) versus postaxial (ulnar), thumb versus triphalangeal, simple versus complex (Figure 1). Mixed polydactyly is defined as the presence of preaxial and postaxial polydactyly.7 Surgical management seeks to allow normal hand function and to restore cosmesis.
Epidemiology
Sun and colleagues8 reported the overall polydactyly incidence as 2 per 1000 live births in China from 1998 to 2009, with a slight male predominance; polydactyly was also 3 times more common than syndactyly in this population. Ivy,9 in a 5-year audit of Pennsylvania Department of Health records, found polydactyly to be the fourth most common congenital anomaly after clubfoot, cleft lip/palate, and spina bifida. Thumb duplication occurs in 0.08 to 1.4 per 1000 live births and is more common in American Indians and Asians than in other races.5,10 It occurs in a male-to-female ratio of 2.5 to 1 and is most often unilateral.5 Postaxial polydactyly is predominant in black infants; it is most often inherited in an autosomal dominant fashion, if isolated, or in an autosomal recessive pattern, if syndromic.1 A prospective San Diego study of 11,161 newborns found postaxial type B polydactyly in 1 per 531 live births (1 per 143 black infants, 1 per 1339 white infants); 76% of cases were bilateral, and 86% had a positive family history.3 In patients of non-African descent, it is associated with anomalies in other organs. Central duplication is rare and often autosomal dominant.5,10
Genetics and Development
As early as 1896, the heritability of polydactyly was noted.11 As of 2010, polydactyly has been associated with 310 diseases.12 Ninety-nine genes, most involved in regulation of anterior-posterior formation of the limb bud, have been implicated.12,13
The upper limb begins to form at day 26 in utero.14 Apoptosis in the interdigital necrotic zones results in the formation of individual digits. It is presumed that, in polydactyly, the involved tissue is hypoplastic because of an abnormal interaction between mesoderm and ectoderm.5 Presence of an apical ectodermal ridge determines the formation of a limb bud, and on it the zone of polarizing activity (ZPA) dictates preaxial and postaxial alignment.14,15 The ZPA is located on the posterior zone of the developing limb bud. The levels of GLI3, a zinc finger-containing DNA-binding protein, are highest in the anterior area, and HAND2, a basic helix-loop-helix DNA-binding protein, is found in the ZPA. This polarity promotes sonic hedgehog (Shh) gene expression in the posterior region, which in turn prevents GLI3 cleavage into its repressed form. GLI3R (repressed) and GLI3A (active) concentrations are highest, therefore, in the anterior and posterior portions of the bud, respectively. The GLI3A:GLI3R ratio is responsible for the identity and number of digits in the hand (ie, the thumb develops in regions of high GLI3R). GLI and Shh mutations lead to polydactylous hands with absent thumbs (Figure 2).16
Ciliopathies have also been shown to cause postaxial polydactyly, possibly because of the role that nonmotile cilia play in hedgehog signaling.17 Mutations in Shh genomic regulators cause preaxial polydactyly.18 HoxD activates Shh in the ZPA; HoxD13 mutations are associated with synpolydactyly.16,19 In each of these mutations, Shh production is altered, and some form of polydactyly results.
Associations
Many syndromes have been associated with polydactyly. Not all polydactyly is associated with other disorders, but the more complex the polydactyly, the more likely that other anomalies are present. Every patient who presents with polydactyly should have a full history taken and a physical examination performed (Figure 3). Any patient with syndromic findings or atypical presentations (eg, triphalangism, postaxial polydactyly in a patient of non-African descent, central and index polydactyly) should be referred to a geneticist.
Classifications
The Wassel20 classification describes the anatomical presentation of thumb duplication on the basis of 70 cases in Iowa (Figures 4, 5; Table 1). Because some duplications fall outside the Wassel classification, many researchers have proposed modifications (Figure 6).21-25
The Temtamy and McKusick10 classification, which is the product of geneticists, classifies duplications by grouping genetically related presentations (Table 2). It provides the most commonly used postaxial classification, with type A being a fully developed digit and type B a rudimentary and pedunculated digit, informally referred to as a nubbin. Type B is more common than type A. Given inheritance patterns, it is assumed that type A is likely multifactorial and type B autosomal dominant.10 Thumb polydactyly inheritance is still unclear. The other types of preaxial polydactyly and high degrees of polydactyly are rare but seem to be passed on in an autosomal dominant fashion on pedigree analysis.10
The Stelling and Turek classification presents the duplication from a tissue perspective: Type I duplication is a rudimentary mass devoid of other tissue elements; type II is a subtotal duplication with some normal structures; and type III is a duplication of the entire “osteoarticular column,” including the metacarpal.1 It is interesting to note that histology of type I duplications shows neuroma-like tissue.26-28 Again, normal is a relative term because, in polydactyly, the duplications are hypoplastic and deviated, with anomalous anatomy.
The Rayan classification describes ulnar polydactyly and was derived from a case study series of 148 patients in Oklahoma (Table 3).29
There are also some complex polydactylies that are not easily classified: ulnar dimelia, cleft hand, pentadactyly, and hyperphalangism. Ulnar dimelia, also known as “mirror hand,” is typically 7 digits with no thumb, but other variations are seen. The radius is often absent, and the elbow is abnormal. There is some debate about whether it is a fusion of 2 hands. Pentadactyly, or the 5-fingered hand, appears as 5 triphalangeal digits with no thumb (Figure 7).
Isolated thumb triphalangism might appear similar to pentadactyly. Miura30,31 pointed out that the radial digit in the pentadactylous hand may be opposable (thumb-like) or nonopposable; in his studies, the patients with the opposable thumb had a metacarpal with a proximal epiphysis (Figure 8). Consequently, the triphalangeal thumb metacarpal with a distal epiphysis is true pentadactyly, whereas that with a proximal epiphysis is hyperphalangism (Figure 9). Treatment of these complex polydactylies involves the same underlying principles as for preaxial and postaxial polydactyly, albeit with additional proximal upper extremity considerations.
When to Operate (Timing)
Ezaki6 recommended surgical intervention at age 6 to 9 months, before fine motor skills have developed with the abnormal anatomy. Cortical learning occurs as the child begins prehensile activities before 6 months, but the risks of anesthesia outweigh functional benefits until the child is older. Waiting until 1 year of age is not uncommon, though surgery at an earlier age may be beneficial if the polydactyly affects hand function.32 It is not uncommon to wait with the more balanced thumb polydactylies to assess thumb function. Hypoplasia might also delay surgical intervention until there is enough tissue inventory for reconstruction. Wassel20 noted that surgical intervention ideally occurs before the supernumerary elements displace the normal elements, as tends to happen with growth. Suture ligation is an option in the neonatal unit for some pedunculated digits.33 Studies have shown satisfactory results in adults treated for polydactyly, if the patient presents later than expected.34
Surgical Considerations
Knavel recommended simple excision, stating that “ablation requires no ingenuity and creates no problems.”5 This belief, though true for some duplications, will not lead to the best outcome for more complex polydactylies. The goal of surgery is a stable and well-aligned thumb for pinch and prehensile activity, as well as a cosmetically pleasing hand. Incisions should not be made linearly along the axis of the digit, as the scar will cause deviation with growth.24
Wassel type I polydactyly might appear incidentally as a broad thumb, in which case it requires no intervention (Figure 10). However, in Wassel types I and II polydactyly with deformity, the Bilhaut-Cloquet procedure is useful for both bifid and duplicated phalanges (Figure 11).5,6,30,32,35 Collateral ligaments may need to be released in type II because of difficulty in opposing the tissue. Cosmetic results with Bilhaut-Cloquet are unpredictable. The original technique required symmetrically sized digits; results today have been improved with microtechniques and preservation of an entire nail.36 Another option is ablation of the more hypoplastic osseous element and soft-tissue augmentation of the residual digit. The theme of ablation and augmentation is seen throughout the literature for the surgical treatment of polydactyly (Figure 12).1
For type III polydactyly, the bifid proximal phalanx is narrowed by resection and realigned with osteotomy of the remaining diaphysis. Type IV polydactyly, the most common thumb duplication, often requires advancement of the abductor pollicis brevis to the base of the proximal phalanx to aid in metacarpophalangeal (MCP) stabilization, abduction, and opposition. The metacarpal head, if broad and with 2 facets, can be shaped to form a single articulating surface. The metacarpal, occasionally with the proximal phalanx, often requires realignment by closing wedge osteotomy. Last, tendons on the resected bony elements should be rebalanced on the remaining digit, and anomalous slips must be released. For instance, given a radial insertion of the long flexor tendon on the distal phalanx, the tendon should be moved centrally. A strong flexor or extensor tendon on the amputated digit should be transferred to the remaining digit.24
Types V and VI are treated similarly to type IV, with the addition of a first web space Z-plasty or web widening if there is thenar eminence contracture. Acral transposition has also been described, with transposition of the tip of the ablated digit in place of the tip of the kept digit; this technique is ideal if one digit has a more normal proximal part while the other has a more normal distal part (Figure 13).35
Type VII thumb polydactyly, the type most likely inherited and associated with other disorders, should be treated like type VI. The nail should be preserved; amputation of the distal phalanx is not advised. Resection of the delta phalanx or 1 interphalangeal (IP) joint is an option. Articular surfaces will remodel if done before the age of 1 year. If the thenar eminence is hypoplastic, then Huber transfer of the abductor digiti minimi should be considered.37 Resection of the triphalangeal thumb is also advised, even if the biphalangeal thumb is more hypoplastic, with transfer of the ligaments and tendons, as described earlier.5,6,24,30,32,35
Thumb triphalangism, if isolated, and hyperphalangism in the other digits, can be treated with resection of the delta phalanx or one of the IP joints if it is affecting function or cosmesis.1,6 Wood and Flatt23 recommended early resection of a thumb delta phalanx because of the likelihood of deviation that impedes thumb function. For children, they recommended delta phalanx resection and Kirschner wire fixation for 6 weeks; for adults, they recommended resection or fusion of the joint, with osteotomy as needed for deviation.23,24 For thumb triphalangism, multiple surgeries are the norm, as Wood24 reported in his study of 21 patients who underwent 78 operations in total.
Index polydactyly may present as a simple pedunculated skin tag, which can be simply excised, or as a more complex musculoskeletal duplication. More complex presentations can be treated with procedures similar to those used for the thumb. Typically, the additional digit is radially deviated and angulated, eventually leading to impingement of thumb pinch and the first web space. Ray amputation is also an option if no reconstructive surgery will produce the stable, sensate radial pinch that is essential to hand function.32
Ring-finger polydactyly and long-finger polydactyly are often complicated by some element of syndactyly, resulting in a relative paucity of skin (Figure 14). There is failure of both formation (hypoplasia) and differentiation (syndactyly). The hypoplasia particularly affects the function of these digits by tethering them; multiple surgeries to restore proper hand function are the norm.1 Reconstructive surgery for these digits requires preoperative tissue inventory followed by resection and augmentation; as in syndactyly, skin for coverage is at a premium. Creation of a 3-fingered hand is an option.23
Temtamy and McKusick10 type A little-finger polydactyly is treated similarly to the thumb, with the caveat that hypothenar and intrinsic muscles that insert on the resected little finger are transferred to the remaining digit. In contrast to thumb polydactyly, the extrinsic musculature tends to be in good position. Suture ligation of type B polydactyly, as described by Flatt, is likely more common than orthopedists appreciate, as pediatricians and neonatal unit practitioners commonly perform this procedure in the nursery.1-3 It has been described with 2-0 Vicryl3 (Ethicon, Somerville, New Jersey) and 4-0 silk sutures,32 with the goal of necrosis and autoamputation. Parents should be told the finger generally falls off about 10 days (range, 4-21 days) after ligation.3 Multiple authors have cited a report of exsanguination from suture ligation, but we could not locate the primary source. It is advisable to wait until a patient is 6 months of age if planning to resect the nubbin in the operating room, given the anesthesia risk and the lack of functional impairment. Katz and Linder33 indicated they remove type B polydactyly in the nursery suite used for circumcisions; they use anesthetizing cream on the skin, and sharp excision with a scalpel, followed by direct pressure and Steri-Strip (3M, St. Paul, Minnesota) application. Suture ligation is recommended only if there is a narrow, thin (<2 mm) soft-tissue stalk; any broad or bony stalk necessitates surgical removal to avoid neuroma formation and failure of autonecrosis (Figure 15).27 Other options are a single swipe of a scalpel and elliptical excision; sharp transaction of the digital nerve with subsequent retraction is advised to avoid neuroma formation.2
Barton described ulnar dimelia operations as “spare parts surgery.”1 Extra digits are ablated and a thumb created (Figure 16). The hand might have a digit in relatively good rotational position for thumbplasty, or the principles of pollicization may need to be used. If the patient is already using the hand, the surgeon should note which finger the patient uses as a thumb.24 Any accompanying wrist flexion contracture must be corrected with careful attention to musculotendinous balancing. Because the forearm and elbow, and occasionally even the more proximal limb, will be abnormal in this disorder, multiple surgeries are again the norm.1
Pentadactyly is treated like thumb hypoplasia, with first web space creation.1
Complications
In polydactyly, a reoperation rate of up to 25% has been reported, with most reoperations performed because of residual or subsequent deformity.5,30,31,38 Risk factors for reoperation are type IV thumb duplication, preoperative “zigzag” deformity, and radially deviated thumb elements at presentation.5 The delta phalanx may not show on radiographs until the patient is 18 months old, but functional deformity will worsen as long as it is present. Zigzag deformity may be due to the delta phalanx or to musculotendinous imbalance, such as a radially inserted flexor pollicis longus (FPL) or lack of stable MCP abduction. Miura31 found that careful reconstruction of the joint capsule and thenar muscles from the ablated digit to the remnant digit is the key to a successful initial surgery. Lee and colleagues39 defined zigzag deformity as more than 20° MCP and IP angulation; for cases present before surgery, they recommended FPL relocation by the pullout technique in addition to osteotomies to prevent further interphalangeal deviation (Figures 17, 18).
Abnormal physeal growth, joint instability, and stiffness can all occur. Stiffness is particularly difficult to treat but seldom presents a functional problem. Joint enlargement, which is not uncommon, results from either broad articular surfaces or retained cartilage from the perichondral ring after resection that later ossifies.5,38 Nubbin-type duplications may not fall off after suture ligation, necessitating further excision, and a cosmetic bump is seen after 40% of suture ligations.3 Patillo and Rayan28 and Rayan and Frey29 warned against suture ligation unless the nubbin has a small stalk because of the possibility of infection and gangrene. The excised nubbin tissue is histologically nervous, and there have been reports of painful neuromas in the remaining scar of a ligated nubbin that respond well to excision.26,27,40 It is thought that these painful lesions form because the ligature prevents the digital nerves to the vestigial digit from retracting.27 Nail deformity and IP joint stiffness are seen with the Bilhaut-Cloquet procedure, though often finger function remains satisfactory.
Conclusion
Polydactyly is a common congenital hand abnormality. Its true incidence is unknown because of inconsistent documentation. Surgeons must strive for a functional, cosmetic hand, given a diverse set of possible anomalies. Hypoplasia is the rule; tissue should be ablated and augmented as necessary. Musculotendinous insertions may need to be centralized. Patients’ family members should always be counseled that more surgery may be needed in the future, as further deformity can occur with growth. Surgically corrected thumb duplications will be stiffer, shorter, and thinner than their normal counterparts. Nail ridges are common. However, it should be noted that 88% of these patients are satisfied with their results.41 Some amount of contracture and abnormal function should be expected with index-, long-, and ring-finger duplications. The only remnant of type B postaxial duplications may be a slight discoloration or bump, though stiffness and deformity can happen with a type A deformity. A “duplicated” digit that requires surgical correction will never be completely normal, but acceptable function is routinely achievable.
1. Graham TJ, Ress AM. Finger polydactyly. Hand Clin. 1998;14(1):49-64.
2. Abzug JM, Kozin SH. Treatment of postaxial polydactyly type B. J Hand Surg Am. 2013;38(6):1223-1225.
3. Watson BT, Hennrikus WL. Postaxial type-B polydactyly—prevalence and treatment. J Bone Joint Surg Am. 1997;79(1):65-68.
4. Zimmer EZ, Bronshtein M. Fetal polydactyly diagnosis during early pregnancy: clinical applications. Am J Obstet Gynecol. 2000;183(3):755-758.
5. Cohen MS. Thumb duplication. Hand Clin. 1998;14(1):17-27.
6. Ezaki M. Radial polydactyly. Hand Clin. 1990;6(4):577-588.
7. Nathan PA, Keniston RC. Crossed polydactyly: case report and review of the literature. J Bone Joint Surg Am. 1975;57(6):847-849.
8. Sun G, Xu ZM, Liang JF, Li L, Tang DX. Twelve-year prevalence of common neonatal congenital malformations in Zhejiang Province, China. World J Pediatr. 2011;7(4):331-336.
9. Ivy RH. Congenital anomalies as recorded on birth certificates in the Division of Vital Statistics of the Pennsylvania Department of Health, for the period of 1951–1955, inclusive. Plast Reconstr Surg. 1957;20(5):400-411.
10. Temtamy SA, McKusick VA. Polydactyly as a part of syndromes. In: Bergsma D, ed. Mudge JR, Paul NW, Conde Greene S, associate eds. The Genetics of Hand Malformations. New York, NY: Liss. Birth Defects Original Article Series. 1978;14(3):364-439.
11. Gould W, Pyle L. Anomalies and Curiosities of Medicine. New York, NY: Bell; 1896.
12. Biesecker LG. Polydactyly: how many disorders and how many genes: 2010 update. Dev Dyn. 2011;250(5):931-942.
13. Grzeschik K. Human limb malformations; an approach to the molecular basis of development. Int J Dev Biol. 2001;46(7):983-991.
14. Zaleske DJ. Development of the upper limb. Hand Clin. 1985;1(3):383-390.
15. Beatty E. Upper limb tissue differentiation in the human embryo. Hand Clin. 1985;1(3):391-404.
16. Anderson E, Peluso S, Lettice LA, Hill RE. Human limb abnormalities caused by disruption of hedgehog signaling. Trends Genet. 2012;28(8):364-373.
17. Ware SM, Aygun MG, Heldebrandt F. Spectrum of clinical diseases caused by disorders of primary cilia. Proc Am Thorac Soc. 2011;8(5):444-450.
18. Lettice LA, Hill RE. Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr Opin Genet Dev. 2005;15(3):294-300.
19. Al-Qattan MA. Type II familial synpolydactyly: report on two families with an emphasis on variations of expression. Eur J Hum Genet. 2011;19(1):112-114.
20. Wassel HD. The results of surgery for polydactyly of the thumb. Clin Orthop. 1969;(64):175-193.
21. Blauth W, Olason AT. Classification of polydactyly of the hands and feet. Arch Orthop Trauma Surg. 1988;107(6):334-344.
22. Wood VE. Super digit. Hand Clin. 1990;6(4):673-684.
23. Wood VE, Flatt AE. Congenital triangular bones in the hand. J Hand Surg Am. 1977;2(3):179-193.
24. Wood VE. Polydactyly and the triphalangeal thumb. J Hand Surg Am. 1978;3(5):436-444.
25. Zuidam JM, Selles RW, Ananta M, Runia J, Hovius SER. A classification system of radial polydactyly: inclusion of triphalangeal thumb and triplication. J Hand Surg Am. 2008;33(3):373-377.
26. Leber GE, Gosain AK. Surgical excision of pedunculated supernumerary digits prevents traumatic amputation neuromas. Pediatr Dermatol. 2003;20(2):108-112.
27. Mullick S, Borschel GH. A selective approach to treatment of ulnar polydactyly: preventing painful neuroma and incomplete excision. Pediatr Dermatol. 2001;27(1):39-42.
28. Patillo D, Rayan GM. Complications of suture ligation ablation for ulnar polydactyly: a report of two cases. Hand (N Y). 2011;6(1):102-105.
29. Rayan GM, Frey B. Ulnar polydactyly. Plastic Reconstr Surg. 2001;107(6):1449-1454.
30. Miura T. Triphalangeal thumb. Plastic Reconstr Surg. 1976;58(5):587-594.
31. Miura T. Duplicated thumb. Plastic Reconstr Surg. 1982;69(3):470-481.
32. Simmons BP. Polydactyly. Hand Clin. 1985;1(3):545-566.
33. Katz K, Linder N. Postaxial type B polydactyly treated by excision in the neonatal nursery. J Pediatr Orthop. 2011;31(4):448-449.
34. Manohar A, Beard AJ. Outcome of reconstruction for duplication of the thumb in adults aged over 40. Hand Surg. 2011;16(2):207-210.
35. Watt AJ, Chung KC. Duplication. Hand Clin. 2009;25(2):215-228.
36. Tonkin MA. Thumb duplication: concepts and techniques. Clin Orthop Surg. 2012;4(1):1-17.
37. Huber E. Relief operation in the case of paralysis of the median nerve. J Hand Surg Eur. 2004;29(1):35-37.
38. Mih AD. Complications of duplicate thumb reconstruction. Hand Clin. 1998;14(1):143-149.
39. Lee CC, Park HY, Yoon JO, Lee KW. Correction of Wassel type IV thumb duplication with zigzag deformity: results of a new method of flexor pollicis longus tendon relocation. J Hand Surg Eur. 2013;38(3):272-280.
40. Hare PJ. Rudimentary polydactyly. Br J Dermatol. 1954;66(11):402-408.
41. Yen CH, Chan WL, Leung HB, Mak KH. Thumb polydactyly: clinical outcome after reconstruction. J Orthop Surg (Hong Kong). 2006;14(3):295-302.
42. Edmunds JO. A tribute to Daniel C. Riordan, MD (1917–2012). Tulane University School of Medicine, Department of Orthopaedics website. http://tulane.edu/som/departments/orthopaedics/news-and-events/danriordantribute.cfm. Accessed March 31, 2015.
43. Faust DC, Herms R. Daniel C. Riordan, MD, 1917–2012. J Hand Surg Am. 2013;38(1):202-205.
1. Graham TJ, Ress AM. Finger polydactyly. Hand Clin. 1998;14(1):49-64.
2. Abzug JM, Kozin SH. Treatment of postaxial polydactyly type B. J Hand Surg Am. 2013;38(6):1223-1225.
3. Watson BT, Hennrikus WL. Postaxial type-B polydactyly—prevalence and treatment. J Bone Joint Surg Am. 1997;79(1):65-68.
4. Zimmer EZ, Bronshtein M. Fetal polydactyly diagnosis during early pregnancy: clinical applications. Am J Obstet Gynecol. 2000;183(3):755-758.
5. Cohen MS. Thumb duplication. Hand Clin. 1998;14(1):17-27.
6. Ezaki M. Radial polydactyly. Hand Clin. 1990;6(4):577-588.
7. Nathan PA, Keniston RC. Crossed polydactyly: case report and review of the literature. J Bone Joint Surg Am. 1975;57(6):847-849.
8. Sun G, Xu ZM, Liang JF, Li L, Tang DX. Twelve-year prevalence of common neonatal congenital malformations in Zhejiang Province, China. World J Pediatr. 2011;7(4):331-336.
9. Ivy RH. Congenital anomalies as recorded on birth certificates in the Division of Vital Statistics of the Pennsylvania Department of Health, for the period of 1951–1955, inclusive. Plast Reconstr Surg. 1957;20(5):400-411.
10. Temtamy SA, McKusick VA. Polydactyly as a part of syndromes. In: Bergsma D, ed. Mudge JR, Paul NW, Conde Greene S, associate eds. The Genetics of Hand Malformations. New York, NY: Liss. Birth Defects Original Article Series. 1978;14(3):364-439.
11. Gould W, Pyle L. Anomalies and Curiosities of Medicine. New York, NY: Bell; 1896.
12. Biesecker LG. Polydactyly: how many disorders and how many genes: 2010 update. Dev Dyn. 2011;250(5):931-942.
13. Grzeschik K. Human limb malformations; an approach to the molecular basis of development. Int J Dev Biol. 2001;46(7):983-991.
14. Zaleske DJ. Development of the upper limb. Hand Clin. 1985;1(3):383-390.
15. Beatty E. Upper limb tissue differentiation in the human embryo. Hand Clin. 1985;1(3):391-404.
16. Anderson E, Peluso S, Lettice LA, Hill RE. Human limb abnormalities caused by disruption of hedgehog signaling. Trends Genet. 2012;28(8):364-373.
17. Ware SM, Aygun MG, Heldebrandt F. Spectrum of clinical diseases caused by disorders of primary cilia. Proc Am Thorac Soc. 2011;8(5):444-450.
18. Lettice LA, Hill RE. Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr Opin Genet Dev. 2005;15(3):294-300.
19. Al-Qattan MA. Type II familial synpolydactyly: report on two families with an emphasis on variations of expression. Eur J Hum Genet. 2011;19(1):112-114.
20. Wassel HD. The results of surgery for polydactyly of the thumb. Clin Orthop. 1969;(64):175-193.
21. Blauth W, Olason AT. Classification of polydactyly of the hands and feet. Arch Orthop Trauma Surg. 1988;107(6):334-344.
22. Wood VE. Super digit. Hand Clin. 1990;6(4):673-684.
23. Wood VE, Flatt AE. Congenital triangular bones in the hand. J Hand Surg Am. 1977;2(3):179-193.
24. Wood VE. Polydactyly and the triphalangeal thumb. J Hand Surg Am. 1978;3(5):436-444.
25. Zuidam JM, Selles RW, Ananta M, Runia J, Hovius SER. A classification system of radial polydactyly: inclusion of triphalangeal thumb and triplication. J Hand Surg Am. 2008;33(3):373-377.
26. Leber GE, Gosain AK. Surgical excision of pedunculated supernumerary digits prevents traumatic amputation neuromas. Pediatr Dermatol. 2003;20(2):108-112.
27. Mullick S, Borschel GH. A selective approach to treatment of ulnar polydactyly: preventing painful neuroma and incomplete excision. Pediatr Dermatol. 2001;27(1):39-42.
28. Patillo D, Rayan GM. Complications of suture ligation ablation for ulnar polydactyly: a report of two cases. Hand (N Y). 2011;6(1):102-105.
29. Rayan GM, Frey B. Ulnar polydactyly. Plastic Reconstr Surg. 2001;107(6):1449-1454.
30. Miura T. Triphalangeal thumb. Plastic Reconstr Surg. 1976;58(5):587-594.
31. Miura T. Duplicated thumb. Plastic Reconstr Surg. 1982;69(3):470-481.
32. Simmons BP. Polydactyly. Hand Clin. 1985;1(3):545-566.
33. Katz K, Linder N. Postaxial type B polydactyly treated by excision in the neonatal nursery. J Pediatr Orthop. 2011;31(4):448-449.
34. Manohar A, Beard AJ. Outcome of reconstruction for duplication of the thumb in adults aged over 40. Hand Surg. 2011;16(2):207-210.
35. Watt AJ, Chung KC. Duplication. Hand Clin. 2009;25(2):215-228.
36. Tonkin MA. Thumb duplication: concepts and techniques. Clin Orthop Surg. 2012;4(1):1-17.
37. Huber E. Relief operation in the case of paralysis of the median nerve. J Hand Surg Eur. 2004;29(1):35-37.
38. Mih AD. Complications of duplicate thumb reconstruction. Hand Clin. 1998;14(1):143-149.
39. Lee CC, Park HY, Yoon JO, Lee KW. Correction of Wassel type IV thumb duplication with zigzag deformity: results of a new method of flexor pollicis longus tendon relocation. J Hand Surg Eur. 2013;38(3):272-280.
40. Hare PJ. Rudimentary polydactyly. Br J Dermatol. 1954;66(11):402-408.
41. Yen CH, Chan WL, Leung HB, Mak KH. Thumb polydactyly: clinical outcome after reconstruction. J Orthop Surg (Hong Kong). 2006;14(3):295-302.
42. Edmunds JO. A tribute to Daniel C. Riordan, MD (1917–2012). Tulane University School of Medicine, Department of Orthopaedics website. http://tulane.edu/som/departments/orthopaedics/news-and-events/danriordantribute.cfm. Accessed March 31, 2015.
43. Faust DC, Herms R. Daniel C. Riordan, MD, 1917–2012. J Hand Surg Am. 2013;38(1):202-205.
Failure of Artelon Interposition Arthroplasty After Partial Trapeziectomy: A Case Report With Histologic and Immunohistochemical Analysis
Osteoarthritis (OA) of the first carpometacarpal (CMC) joint is a common disabling condition that mostly affects women over 45 years of age.1 Surgical intervention is usually indicated in advanced stage OA of the first CMC joint that has failed conservative treatment. Several surgical techniques have been described, including partial or total trapeziectomy, interposition arthroplasty with or without ligament reconstruction,2,3 metacarpal osteotomy,4 hematoma and distraction arthroplasty,5 total joint arthroplasty, arthrodesis, and suspensionplasty.6 However, no single surgical procedure has proved to be superior.7
The Artelon implant (Artelon, Nashville, Tennessee) is a T-shaped spacer composed of a biocompatible and biodegradable polycaprolactone-based polyurethane urea polymer. The developers of the implant first presented its use in CMC OA in 2005.8 The device, an endoprosthetic replacement for the CMC joint, was designed to work through 2 modes of action: stabilization of the CMC joint by augmentation of the joint capsule and by formation of a new articular surface at the trapeziometacarpal interface. The interposed biomaterial has been described as preventing bony impingement and allowing time for replacement with a newly formed articular surface as it undergoes slow and controlled degradation.8
We present a patient with recurrent CMC pain and disability 4 years after arthroscopic hemitrapeziectomy and Artelon interposition and discuss the associated histologic findings. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 53-year-old man presented with painful disability of right thumb of several months’ duration. Clinical and radiographic evaluation supported the diagnosis of right thumb CMC joint Eaton stage III arthritis (Figures 1A, 1B). Surgical intervention was indicated after a failed course of conservative treatment, including splinting, nonsteroidal anti-inflammatory medications, activity modification, and corticosteroid injection. Preoperatively, the patient reported a visual analog scale (VAS) score of 8 with activity and 5 at rest, and a Disabilities of the Arm, Shoulder, and Hand (DASH) score of 72.5.
Arthroscopic débridement, hemitrapeziectomy, and interposition arthroplasty with the Artelon spacer were performed. Using standard thumb arthroscopy, 3 mm of the distal trapezium was excised and shaped parallel to scaphotrapezial joint. The wings of the standard-sized Artelon spacer were removed, and the central (articulating) portion was rolled into a tube and inserted through the 1R portal (directly radial to the abductor pollicis longus tendon) into the trapezial space. The Artelon spacer was unrolled within the joint to cover the remaining trapezium and was stabilized with the placement of a 0.045-inch Kirschner wire through the metacarpal, the spacer, and the remaining trapezium. The patient used a thumb spica splint for 4 weeks.
The postoperative radiographs showed a smooth and adequate hemitrapeziectomy with good alignment and implant position (Figures 2A, 2B). Four weeks after surgery, the Kirschner wire and cast were removed and physical therapy was initiated. The patient’s CMC pain gradually subsided. At the 3-month postoperative visit, the patient’s VAS score was 3 with activity and 1 at rest, with a DASH score of 28. His key pinch strength was 12 lb, compared with 20 lb on the contralateral side. At 6 months, the patient’s VAS score was 1 with activity and 0 at rest, with a DASH score of 12. His key pinch strength was 18 lb, compared with 22 lb on the contralateral side. At his 2-year postoperative visit, the patient was doing well with the exception of some mild residual pain when he opened tight jars. His VAS score was 1 with activity and 0 at rest, with a DASH score of 3. His key pinch strength was 20 lb, compared with 23 lb on the contralateral side. Radiographs showed good maintenance of the CMC space.
Four years postoperatively, the patient presented with worsening right CMC pain with decrease in pinch strength that interfered with his activities of daily living. His VAS score was 9 with activity and 6 at rest, with a DASH score of 70. On examination, pinch strength was 16 lb, compared with 22 lb on the contralateral side. Radiographs showed advancing arthritis with new osteophyte formation and irregular contour of distal trapezium (Figures 3A, 3B). The symptoms were refractory to conservative measures and continued to interfere with his activities of daily living. Revision surgical intervention was indicated and pursued in the form of an open CMC arthroplasty.
The intraoperative findings revealed degradation and disorganization of the Artelon implant within the central portion of the remaining distal trapezium. Rim osteophytes, especially along the ulnar aspect, were noted. Total trapeziectomy and débridement within the CMC space and suture-button suspensionplasty were performed.8 Slight degenerative changes of the distal scaphoid were also noted. The incision was irrigated, closed, and stabilized in a thumb spica splint (Figures 4A, 4B).
The harvested trapezium was immediately immersed in buffered formalin. The bone tissue was decalcified, dehydrated, embedded in paraffin, and sectioned in the coronal plane. The sections were stained with safranin O and trichrome, and light microscopic analysis was performed. Central erosion of distal trapezium without smooth resurfacing soft-tissue formation was noted grossly (Figure 5A) and microscopically (Figures 5B, 5C). The histologic morphology of the soft tissue over the distal trapezium was significantly different when compared with the smooth hyaline cartilage at the preserved trapezio-trapezoidal joint (Figures 6A-6F). Microscopic analysis also showed multinucleated giant cells within the soft tissue surrounding the degraded Artelon B (Figure 7).
Immunohistochemical analysis was performed to identify type I and type II collagen using the Histostain-Plus,3rd Gen IHC Detection Kit (Invitrogen Corporation, Camarillo, California) (Figures 8A-8F).9 The immunohistochemical stain was used to identify new hyaline cartilage formation that may have been induced by the Artelon as the resurfacing articulation. Hyaline cartilage contains mainly type II collagen, and collagen types VI, IX, X, XI, XII, and XIV all contribute to the mature matrix.10 Little type I collagen is found in hyaline cartilage. The results showed that the soft tissue over the distal trapezium with embedded Artelon fiber contained both type I and type II collagen. There was no visible hyaline cartilage formation induced by the Artelon. Both morphologic analysis and immunohistochemical staining revealed that the soft-tissue growth into the Artelon spacer on the distal trapezium consisted primarily of fibrocartilaginous tissue, which is composed mainly of type I collagen with some type II collagen.
Two weeks after total surgical excision of the Artelon implant, total trapeziectomy and suture-button suspensionplasty, the sutures were removed and physical therapy was initiated. Radiographs showed good alignment and position of thumb metacarpal with good maintenance of the implant and CMC space. Four months postoperatively, the patient reported that he was doing well without pain and without interference in his activities of daily living. On examination, the patient exhibited no pain with the CMC grind maneuver. Radial abduction of the right thumb was 85° and palmar abduction was 90° (compared with 100° and 90° of the left thumb), obtained by measuring the angle between thumb and index finger, respectively. Opposition was to the small finger metacarpophalangeal joint. Grip strength was 72 lb and pinch strength was 20 lb (compared with 70 lb and 24 lb, respectively, on the contralateral side).
Discussion
The use of Artelon as an endoprosthetic spacer to treat osteoarthritis in the CMC joint of the thumb appears to stabilize and resurface the joint while avoiding total trapeziectomy.8 Nilsson and colleagues8 presented a prospective study concluding that the Artelon CMC spacer provided better pinch strength when compared with a traditional abductor pollicis longus suspensionplasty procedure. This study also suggested incorporation of the device in the surface of the adjacent bone with no signs of foreign-body reaction. The synthetic material was shown to be safe and biocompatible in vitro and in animal studies.11-13
This case report describes the gross and histologic findings after continued pain led to explantation 4 years after arthroscopic partial trapeziectomy and insertion of the spacer. Intraoperative findings at this stage showed lack of incorporation of the Artelon material, central destruction of distal trapezium, and no evidence of smooth articular surface formation. Our histologic analysis showed only poorly organized fibrocartilage within the CMC space rather than a smooth articular surface. These histologic findings may correlate more with Jörheim and colleagues’14 matched cohort study, which showed that short-term outcomes after treatment with the Artelon implant were not clinically superior to those of tendon suspension-interposition arthroplasties. Multinucleated giant cells were also seen in our specimens. Choung and Tan15 presented a case report of foreign-body reaction to the Artelon spacer with histologic findings. The foreign body–type reactions associated with Artelon resulted in multinucleated giant cells in their specimens. Recently, several case reports have described similar foreign-body reactions.16 Nilsson and coauthors17 presented a randomized, controlled, multicenter study of 109 patients. They reported the Artelon CMC spacer did not result in superior results compared with tendon interposition arthroplasty. In a study by Gretzer and colleagues,18 the authors suggested that chronic inflammation may result from unstable Artelon fixation instead of the foreign-body reaction.
It is possible that the central erosion of the distal trapezium seen in our case may have resulted from chronic inflammation caused by foreign-body reaction and/or an unstably fixed spacer. The spacer was transfixed to the remaining trapezium in the CMC joint with a Kirschner wire followed by immobilization for 4 weeks. Poor soft-tissue integration of the Artelon spacer may have led to unintended motion and chronic inflammation, which may have also resulted in erosion between the Artelon spacer and the trapezium, leading to central destruction of the distal trapezium. Lastly, the byproducts formed by the degradation of the spacer may have resulted in erosion of the remaining trapezium.
Conclusion
The Artelon CMC spacer used in this patient provided comparable, but not superior, clinical results to other procedures. Histologically, the new articular surface in our patient was formed with rugged fibrocartilage instead of the expected smooth cartilaginous surface. The chronic inflammatory reaction may have resulted from foreign-body reaction, unstable implant fixation, or poor soft-tissue integration. This inflammatory reaction may have contributed to the patient’s recurrence of symptoms. These findings support recent clinical data that suggest the use of the Artelon spacer may not provide superior results to other surgical options for the treatment of CMC joint arthritis.
1. Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64(5):682-687.
2. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg. 1985;10(5):645-654.
3. Gibbons CE, Gosal HS, Choudri AH, Magnussen PA. Trapeziectomy for basal thumb joint osteoarthritis: 3- to 19-year follow-up. Int Orthop. 1999;23(4):216-218.
4. Gwynne-Jones DP, Penny ID, Sewell SA, Hughes TH. Basal thumb metacarpal osteotomy for trapeziometacarpal osteoarthritis. J Orthop Surg (Hong Kong). 2006;14(1):58-63.
5. Gray KV, Meals RA. Hematoma and distraction arthroplasty for thumb basal joint osteoarthritis: minimum 6.5-year follow-up evaluation. J Hand Surg Am. 2007;32(1):23-29.
6. Cox CA, Zlotolow DA, Yao J. Suture button suspensionplasty after arthroscopic hemitrapeziectomy for treatment of thumb carpometacarpal arthritis. Arthroscopy. 2010;26(10):1395-1403.
7. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
8. Nilsson A, Liljensten E, Bergström C, Sollerman C. Results from a degradable TMC joint Spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
9. Histostain®-Plus, 3rd Gen IHC Detection Kit [product information]. Invitrogen website. http://tools.invitrogen.com/content/sfs/manuals/859073_Rev1108.pdf. Revised November 2008. Accessed February 27, 2015.
10. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30-35.
11. Gisselfält K, Edberg B, Flodin P. Synthesis and properties of degradable poly(urethane urea)s to be used for ligament reconstructions. Biomacromolecules. 2002;3(5):951-958.
12. Liljensten E, Gisselfält K, Edberg B, et al. Studies of polyurethane urea bands for ACL reconstruction. J Mater Sci Mater Med. 2002;13(4):351-359.
13. Gretzer C, Gisselfält K, Liljensten E, Rydén L, Thomsen P. Adhesion, apoptosis and cytokine release of human mononuclear cells cultured on degradable poly(urethane urea), polystyrene and titanium in vitro. Biomaterials. 2003;24(17):2843-2852.
14. Jörheim M, Isaxon I, Flondell M, Kalén P, Atroshi I. Short-term outcomes of trapeziometacarpal artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
15. Choung EW, Tan V. Foreign-body reaction to the Artelon CMC joint spacer: case report. J Hand Surg Am. 2008;33(9):1617-1620.
16. Robinson PM, Muir LT. Foreign body reaction associated with Artelon: report of three cases. J Hand Surg Am. 2011;36(1):116-120.
17. Nilsson A, Wiig M, Alnehill H, et al. The Artelon CMC spacer compared with tendon interposition arthroplasty. Acta Orthop. 2010;81(2):237-244.
18. Gretzer C, Emanuelsson L, Liljensten E, Thomsen P. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed. 2006;17(6):669-687.
Osteoarthritis (OA) of the first carpometacarpal (CMC) joint is a common disabling condition that mostly affects women over 45 years of age.1 Surgical intervention is usually indicated in advanced stage OA of the first CMC joint that has failed conservative treatment. Several surgical techniques have been described, including partial or total trapeziectomy, interposition arthroplasty with or without ligament reconstruction,2,3 metacarpal osteotomy,4 hematoma and distraction arthroplasty,5 total joint arthroplasty, arthrodesis, and suspensionplasty.6 However, no single surgical procedure has proved to be superior.7
The Artelon implant (Artelon, Nashville, Tennessee) is a T-shaped spacer composed of a biocompatible and biodegradable polycaprolactone-based polyurethane urea polymer. The developers of the implant first presented its use in CMC OA in 2005.8 The device, an endoprosthetic replacement for the CMC joint, was designed to work through 2 modes of action: stabilization of the CMC joint by augmentation of the joint capsule and by formation of a new articular surface at the trapeziometacarpal interface. The interposed biomaterial has been described as preventing bony impingement and allowing time for replacement with a newly formed articular surface as it undergoes slow and controlled degradation.8
We present a patient with recurrent CMC pain and disability 4 years after arthroscopic hemitrapeziectomy and Artelon interposition and discuss the associated histologic findings. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 53-year-old man presented with painful disability of right thumb of several months’ duration. Clinical and radiographic evaluation supported the diagnosis of right thumb CMC joint Eaton stage III arthritis (Figures 1A, 1B). Surgical intervention was indicated after a failed course of conservative treatment, including splinting, nonsteroidal anti-inflammatory medications, activity modification, and corticosteroid injection. Preoperatively, the patient reported a visual analog scale (VAS) score of 8 with activity and 5 at rest, and a Disabilities of the Arm, Shoulder, and Hand (DASH) score of 72.5.
Arthroscopic débridement, hemitrapeziectomy, and interposition arthroplasty with the Artelon spacer were performed. Using standard thumb arthroscopy, 3 mm of the distal trapezium was excised and shaped parallel to scaphotrapezial joint. The wings of the standard-sized Artelon spacer were removed, and the central (articulating) portion was rolled into a tube and inserted through the 1R portal (directly radial to the abductor pollicis longus tendon) into the trapezial space. The Artelon spacer was unrolled within the joint to cover the remaining trapezium and was stabilized with the placement of a 0.045-inch Kirschner wire through the metacarpal, the spacer, and the remaining trapezium. The patient used a thumb spica splint for 4 weeks.
The postoperative radiographs showed a smooth and adequate hemitrapeziectomy with good alignment and implant position (Figures 2A, 2B). Four weeks after surgery, the Kirschner wire and cast were removed and physical therapy was initiated. The patient’s CMC pain gradually subsided. At the 3-month postoperative visit, the patient’s VAS score was 3 with activity and 1 at rest, with a DASH score of 28. His key pinch strength was 12 lb, compared with 20 lb on the contralateral side. At 6 months, the patient’s VAS score was 1 with activity and 0 at rest, with a DASH score of 12. His key pinch strength was 18 lb, compared with 22 lb on the contralateral side. At his 2-year postoperative visit, the patient was doing well with the exception of some mild residual pain when he opened tight jars. His VAS score was 1 with activity and 0 at rest, with a DASH score of 3. His key pinch strength was 20 lb, compared with 23 lb on the contralateral side. Radiographs showed good maintenance of the CMC space.
Four years postoperatively, the patient presented with worsening right CMC pain with decrease in pinch strength that interfered with his activities of daily living. His VAS score was 9 with activity and 6 at rest, with a DASH score of 70. On examination, pinch strength was 16 lb, compared with 22 lb on the contralateral side. Radiographs showed advancing arthritis with new osteophyte formation and irregular contour of distal trapezium (Figures 3A, 3B). The symptoms were refractory to conservative measures and continued to interfere with his activities of daily living. Revision surgical intervention was indicated and pursued in the form of an open CMC arthroplasty.
The intraoperative findings revealed degradation and disorganization of the Artelon implant within the central portion of the remaining distal trapezium. Rim osteophytes, especially along the ulnar aspect, were noted. Total trapeziectomy and débridement within the CMC space and suture-button suspensionplasty were performed.8 Slight degenerative changes of the distal scaphoid were also noted. The incision was irrigated, closed, and stabilized in a thumb spica splint (Figures 4A, 4B).
The harvested trapezium was immediately immersed in buffered formalin. The bone tissue was decalcified, dehydrated, embedded in paraffin, and sectioned in the coronal plane. The sections were stained with safranin O and trichrome, and light microscopic analysis was performed. Central erosion of distal trapezium without smooth resurfacing soft-tissue formation was noted grossly (Figure 5A) and microscopically (Figures 5B, 5C). The histologic morphology of the soft tissue over the distal trapezium was significantly different when compared with the smooth hyaline cartilage at the preserved trapezio-trapezoidal joint (Figures 6A-6F). Microscopic analysis also showed multinucleated giant cells within the soft tissue surrounding the degraded Artelon B (Figure 7).
Immunohistochemical analysis was performed to identify type I and type II collagen using the Histostain-Plus,3rd Gen IHC Detection Kit (Invitrogen Corporation, Camarillo, California) (Figures 8A-8F).9 The immunohistochemical stain was used to identify new hyaline cartilage formation that may have been induced by the Artelon as the resurfacing articulation. Hyaline cartilage contains mainly type II collagen, and collagen types VI, IX, X, XI, XII, and XIV all contribute to the mature matrix.10 Little type I collagen is found in hyaline cartilage. The results showed that the soft tissue over the distal trapezium with embedded Artelon fiber contained both type I and type II collagen. There was no visible hyaline cartilage formation induced by the Artelon. Both morphologic analysis and immunohistochemical staining revealed that the soft-tissue growth into the Artelon spacer on the distal trapezium consisted primarily of fibrocartilaginous tissue, which is composed mainly of type I collagen with some type II collagen.
Two weeks after total surgical excision of the Artelon implant, total trapeziectomy and suture-button suspensionplasty, the sutures were removed and physical therapy was initiated. Radiographs showed good alignment and position of thumb metacarpal with good maintenance of the implant and CMC space. Four months postoperatively, the patient reported that he was doing well without pain and without interference in his activities of daily living. On examination, the patient exhibited no pain with the CMC grind maneuver. Radial abduction of the right thumb was 85° and palmar abduction was 90° (compared with 100° and 90° of the left thumb), obtained by measuring the angle between thumb and index finger, respectively. Opposition was to the small finger metacarpophalangeal joint. Grip strength was 72 lb and pinch strength was 20 lb (compared with 70 lb and 24 lb, respectively, on the contralateral side).
Discussion
The use of Artelon as an endoprosthetic spacer to treat osteoarthritis in the CMC joint of the thumb appears to stabilize and resurface the joint while avoiding total trapeziectomy.8 Nilsson and colleagues8 presented a prospective study concluding that the Artelon CMC spacer provided better pinch strength when compared with a traditional abductor pollicis longus suspensionplasty procedure. This study also suggested incorporation of the device in the surface of the adjacent bone with no signs of foreign-body reaction. The synthetic material was shown to be safe and biocompatible in vitro and in animal studies.11-13
This case report describes the gross and histologic findings after continued pain led to explantation 4 years after arthroscopic partial trapeziectomy and insertion of the spacer. Intraoperative findings at this stage showed lack of incorporation of the Artelon material, central destruction of distal trapezium, and no evidence of smooth articular surface formation. Our histologic analysis showed only poorly organized fibrocartilage within the CMC space rather than a smooth articular surface. These histologic findings may correlate more with Jörheim and colleagues’14 matched cohort study, which showed that short-term outcomes after treatment with the Artelon implant were not clinically superior to those of tendon suspension-interposition arthroplasties. Multinucleated giant cells were also seen in our specimens. Choung and Tan15 presented a case report of foreign-body reaction to the Artelon spacer with histologic findings. The foreign body–type reactions associated with Artelon resulted in multinucleated giant cells in their specimens. Recently, several case reports have described similar foreign-body reactions.16 Nilsson and coauthors17 presented a randomized, controlled, multicenter study of 109 patients. They reported the Artelon CMC spacer did not result in superior results compared with tendon interposition arthroplasty. In a study by Gretzer and colleagues,18 the authors suggested that chronic inflammation may result from unstable Artelon fixation instead of the foreign-body reaction.
It is possible that the central erosion of the distal trapezium seen in our case may have resulted from chronic inflammation caused by foreign-body reaction and/or an unstably fixed spacer. The spacer was transfixed to the remaining trapezium in the CMC joint with a Kirschner wire followed by immobilization for 4 weeks. Poor soft-tissue integration of the Artelon spacer may have led to unintended motion and chronic inflammation, which may have also resulted in erosion between the Artelon spacer and the trapezium, leading to central destruction of the distal trapezium. Lastly, the byproducts formed by the degradation of the spacer may have resulted in erosion of the remaining trapezium.
Conclusion
The Artelon CMC spacer used in this patient provided comparable, but not superior, clinical results to other procedures. Histologically, the new articular surface in our patient was formed with rugged fibrocartilage instead of the expected smooth cartilaginous surface. The chronic inflammatory reaction may have resulted from foreign-body reaction, unstable implant fixation, or poor soft-tissue integration. This inflammatory reaction may have contributed to the patient’s recurrence of symptoms. These findings support recent clinical data that suggest the use of the Artelon spacer may not provide superior results to other surgical options for the treatment of CMC joint arthritis.
Osteoarthritis (OA) of the first carpometacarpal (CMC) joint is a common disabling condition that mostly affects women over 45 years of age.1 Surgical intervention is usually indicated in advanced stage OA of the first CMC joint that has failed conservative treatment. Several surgical techniques have been described, including partial or total trapeziectomy, interposition arthroplasty with or without ligament reconstruction,2,3 metacarpal osteotomy,4 hematoma and distraction arthroplasty,5 total joint arthroplasty, arthrodesis, and suspensionplasty.6 However, no single surgical procedure has proved to be superior.7
The Artelon implant (Artelon, Nashville, Tennessee) is a T-shaped spacer composed of a biocompatible and biodegradable polycaprolactone-based polyurethane urea polymer. The developers of the implant first presented its use in CMC OA in 2005.8 The device, an endoprosthetic replacement for the CMC joint, was designed to work through 2 modes of action: stabilization of the CMC joint by augmentation of the joint capsule and by formation of a new articular surface at the trapeziometacarpal interface. The interposed biomaterial has been described as preventing bony impingement and allowing time for replacement with a newly formed articular surface as it undergoes slow and controlled degradation.8
We present a patient with recurrent CMC pain and disability 4 years after arthroscopic hemitrapeziectomy and Artelon interposition and discuss the associated histologic findings. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 53-year-old man presented with painful disability of right thumb of several months’ duration. Clinical and radiographic evaluation supported the diagnosis of right thumb CMC joint Eaton stage III arthritis (Figures 1A, 1B). Surgical intervention was indicated after a failed course of conservative treatment, including splinting, nonsteroidal anti-inflammatory medications, activity modification, and corticosteroid injection. Preoperatively, the patient reported a visual analog scale (VAS) score of 8 with activity and 5 at rest, and a Disabilities of the Arm, Shoulder, and Hand (DASH) score of 72.5.
Arthroscopic débridement, hemitrapeziectomy, and interposition arthroplasty with the Artelon spacer were performed. Using standard thumb arthroscopy, 3 mm of the distal trapezium was excised and shaped parallel to scaphotrapezial joint. The wings of the standard-sized Artelon spacer were removed, and the central (articulating) portion was rolled into a tube and inserted through the 1R portal (directly radial to the abductor pollicis longus tendon) into the trapezial space. The Artelon spacer was unrolled within the joint to cover the remaining trapezium and was stabilized with the placement of a 0.045-inch Kirschner wire through the metacarpal, the spacer, and the remaining trapezium. The patient used a thumb spica splint for 4 weeks.
The postoperative radiographs showed a smooth and adequate hemitrapeziectomy with good alignment and implant position (Figures 2A, 2B). Four weeks after surgery, the Kirschner wire and cast were removed and physical therapy was initiated. The patient’s CMC pain gradually subsided. At the 3-month postoperative visit, the patient’s VAS score was 3 with activity and 1 at rest, with a DASH score of 28. His key pinch strength was 12 lb, compared with 20 lb on the contralateral side. At 6 months, the patient’s VAS score was 1 with activity and 0 at rest, with a DASH score of 12. His key pinch strength was 18 lb, compared with 22 lb on the contralateral side. At his 2-year postoperative visit, the patient was doing well with the exception of some mild residual pain when he opened tight jars. His VAS score was 1 with activity and 0 at rest, with a DASH score of 3. His key pinch strength was 20 lb, compared with 23 lb on the contralateral side. Radiographs showed good maintenance of the CMC space.
Four years postoperatively, the patient presented with worsening right CMC pain with decrease in pinch strength that interfered with his activities of daily living. His VAS score was 9 with activity and 6 at rest, with a DASH score of 70. On examination, pinch strength was 16 lb, compared with 22 lb on the contralateral side. Radiographs showed advancing arthritis with new osteophyte formation and irregular contour of distal trapezium (Figures 3A, 3B). The symptoms were refractory to conservative measures and continued to interfere with his activities of daily living. Revision surgical intervention was indicated and pursued in the form of an open CMC arthroplasty.
The intraoperative findings revealed degradation and disorganization of the Artelon implant within the central portion of the remaining distal trapezium. Rim osteophytes, especially along the ulnar aspect, were noted. Total trapeziectomy and débridement within the CMC space and suture-button suspensionplasty were performed.8 Slight degenerative changes of the distal scaphoid were also noted. The incision was irrigated, closed, and stabilized in a thumb spica splint (Figures 4A, 4B).
The harvested trapezium was immediately immersed in buffered formalin. The bone tissue was decalcified, dehydrated, embedded in paraffin, and sectioned in the coronal plane. The sections were stained with safranin O and trichrome, and light microscopic analysis was performed. Central erosion of distal trapezium without smooth resurfacing soft-tissue formation was noted grossly (Figure 5A) and microscopically (Figures 5B, 5C). The histologic morphology of the soft tissue over the distal trapezium was significantly different when compared with the smooth hyaline cartilage at the preserved trapezio-trapezoidal joint (Figures 6A-6F). Microscopic analysis also showed multinucleated giant cells within the soft tissue surrounding the degraded Artelon B (Figure 7).
Immunohistochemical analysis was performed to identify type I and type II collagen using the Histostain-Plus,3rd Gen IHC Detection Kit (Invitrogen Corporation, Camarillo, California) (Figures 8A-8F).9 The immunohistochemical stain was used to identify new hyaline cartilage formation that may have been induced by the Artelon as the resurfacing articulation. Hyaline cartilage contains mainly type II collagen, and collagen types VI, IX, X, XI, XII, and XIV all contribute to the mature matrix.10 Little type I collagen is found in hyaline cartilage. The results showed that the soft tissue over the distal trapezium with embedded Artelon fiber contained both type I and type II collagen. There was no visible hyaline cartilage formation induced by the Artelon. Both morphologic analysis and immunohistochemical staining revealed that the soft-tissue growth into the Artelon spacer on the distal trapezium consisted primarily of fibrocartilaginous tissue, which is composed mainly of type I collagen with some type II collagen.
Two weeks after total surgical excision of the Artelon implant, total trapeziectomy and suture-button suspensionplasty, the sutures were removed and physical therapy was initiated. Radiographs showed good alignment and position of thumb metacarpal with good maintenance of the implant and CMC space. Four months postoperatively, the patient reported that he was doing well without pain and without interference in his activities of daily living. On examination, the patient exhibited no pain with the CMC grind maneuver. Radial abduction of the right thumb was 85° and palmar abduction was 90° (compared with 100° and 90° of the left thumb), obtained by measuring the angle between thumb and index finger, respectively. Opposition was to the small finger metacarpophalangeal joint. Grip strength was 72 lb and pinch strength was 20 lb (compared with 70 lb and 24 lb, respectively, on the contralateral side).
Discussion
The use of Artelon as an endoprosthetic spacer to treat osteoarthritis in the CMC joint of the thumb appears to stabilize and resurface the joint while avoiding total trapeziectomy.8 Nilsson and colleagues8 presented a prospective study concluding that the Artelon CMC spacer provided better pinch strength when compared with a traditional abductor pollicis longus suspensionplasty procedure. This study also suggested incorporation of the device in the surface of the adjacent bone with no signs of foreign-body reaction. The synthetic material was shown to be safe and biocompatible in vitro and in animal studies.11-13
This case report describes the gross and histologic findings after continued pain led to explantation 4 years after arthroscopic partial trapeziectomy and insertion of the spacer. Intraoperative findings at this stage showed lack of incorporation of the Artelon material, central destruction of distal trapezium, and no evidence of smooth articular surface formation. Our histologic analysis showed only poorly organized fibrocartilage within the CMC space rather than a smooth articular surface. These histologic findings may correlate more with Jörheim and colleagues’14 matched cohort study, which showed that short-term outcomes after treatment with the Artelon implant were not clinically superior to those of tendon suspension-interposition arthroplasties. Multinucleated giant cells were also seen in our specimens. Choung and Tan15 presented a case report of foreign-body reaction to the Artelon spacer with histologic findings. The foreign body–type reactions associated with Artelon resulted in multinucleated giant cells in their specimens. Recently, several case reports have described similar foreign-body reactions.16 Nilsson and coauthors17 presented a randomized, controlled, multicenter study of 109 patients. They reported the Artelon CMC spacer did not result in superior results compared with tendon interposition arthroplasty. In a study by Gretzer and colleagues,18 the authors suggested that chronic inflammation may result from unstable Artelon fixation instead of the foreign-body reaction.
It is possible that the central erosion of the distal trapezium seen in our case may have resulted from chronic inflammation caused by foreign-body reaction and/or an unstably fixed spacer. The spacer was transfixed to the remaining trapezium in the CMC joint with a Kirschner wire followed by immobilization for 4 weeks. Poor soft-tissue integration of the Artelon spacer may have led to unintended motion and chronic inflammation, which may have also resulted in erosion between the Artelon spacer and the trapezium, leading to central destruction of the distal trapezium. Lastly, the byproducts formed by the degradation of the spacer may have resulted in erosion of the remaining trapezium.
Conclusion
The Artelon CMC spacer used in this patient provided comparable, but not superior, clinical results to other procedures. Histologically, the new articular surface in our patient was formed with rugged fibrocartilage instead of the expected smooth cartilaginous surface. The chronic inflammatory reaction may have resulted from foreign-body reaction, unstable implant fixation, or poor soft-tissue integration. This inflammatory reaction may have contributed to the patient’s recurrence of symptoms. These findings support recent clinical data that suggest the use of the Artelon spacer may not provide superior results to other surgical options for the treatment of CMC joint arthritis.
1. Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64(5):682-687.
2. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg. 1985;10(5):645-654.
3. Gibbons CE, Gosal HS, Choudri AH, Magnussen PA. Trapeziectomy for basal thumb joint osteoarthritis: 3- to 19-year follow-up. Int Orthop. 1999;23(4):216-218.
4. Gwynne-Jones DP, Penny ID, Sewell SA, Hughes TH. Basal thumb metacarpal osteotomy for trapeziometacarpal osteoarthritis. J Orthop Surg (Hong Kong). 2006;14(1):58-63.
5. Gray KV, Meals RA. Hematoma and distraction arthroplasty for thumb basal joint osteoarthritis: minimum 6.5-year follow-up evaluation. J Hand Surg Am. 2007;32(1):23-29.
6. Cox CA, Zlotolow DA, Yao J. Suture button suspensionplasty after arthroscopic hemitrapeziectomy for treatment of thumb carpometacarpal arthritis. Arthroscopy. 2010;26(10):1395-1403.
7. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
8. Nilsson A, Liljensten E, Bergström C, Sollerman C. Results from a degradable TMC joint Spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
9. Histostain®-Plus, 3rd Gen IHC Detection Kit [product information]. Invitrogen website. http://tools.invitrogen.com/content/sfs/manuals/859073_Rev1108.pdf. Revised November 2008. Accessed February 27, 2015.
10. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30-35.
11. Gisselfält K, Edberg B, Flodin P. Synthesis and properties of degradable poly(urethane urea)s to be used for ligament reconstructions. Biomacromolecules. 2002;3(5):951-958.
12. Liljensten E, Gisselfält K, Edberg B, et al. Studies of polyurethane urea bands for ACL reconstruction. J Mater Sci Mater Med. 2002;13(4):351-359.
13. Gretzer C, Gisselfält K, Liljensten E, Rydén L, Thomsen P. Adhesion, apoptosis and cytokine release of human mononuclear cells cultured on degradable poly(urethane urea), polystyrene and titanium in vitro. Biomaterials. 2003;24(17):2843-2852.
14. Jörheim M, Isaxon I, Flondell M, Kalén P, Atroshi I. Short-term outcomes of trapeziometacarpal artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
15. Choung EW, Tan V. Foreign-body reaction to the Artelon CMC joint spacer: case report. J Hand Surg Am. 2008;33(9):1617-1620.
16. Robinson PM, Muir LT. Foreign body reaction associated with Artelon: report of three cases. J Hand Surg Am. 2011;36(1):116-120.
17. Nilsson A, Wiig M, Alnehill H, et al. The Artelon CMC spacer compared with tendon interposition arthroplasty. Acta Orthop. 2010;81(2):237-244.
18. Gretzer C, Emanuelsson L, Liljensten E, Thomsen P. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed. 2006;17(6):669-687.
1. Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64(5):682-687.
2. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg. 1985;10(5):645-654.
3. Gibbons CE, Gosal HS, Choudri AH, Magnussen PA. Trapeziectomy for basal thumb joint osteoarthritis: 3- to 19-year follow-up. Int Orthop. 1999;23(4):216-218.
4. Gwynne-Jones DP, Penny ID, Sewell SA, Hughes TH. Basal thumb metacarpal osteotomy for trapeziometacarpal osteoarthritis. J Orthop Surg (Hong Kong). 2006;14(1):58-63.
5. Gray KV, Meals RA. Hematoma and distraction arthroplasty for thumb basal joint osteoarthritis: minimum 6.5-year follow-up evaluation. J Hand Surg Am. 2007;32(1):23-29.
6. Cox CA, Zlotolow DA, Yao J. Suture button suspensionplasty after arthroscopic hemitrapeziectomy for treatment of thumb carpometacarpal arthritis. Arthroscopy. 2010;26(10):1395-1403.
7. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
8. Nilsson A, Liljensten E, Bergström C, Sollerman C. Results from a degradable TMC joint Spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
9. Histostain®-Plus, 3rd Gen IHC Detection Kit [product information]. Invitrogen website. http://tools.invitrogen.com/content/sfs/manuals/859073_Rev1108.pdf. Revised November 2008. Accessed February 27, 2015.
10. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30-35.
11. Gisselfält K, Edberg B, Flodin P. Synthesis and properties of degradable poly(urethane urea)s to be used for ligament reconstructions. Biomacromolecules. 2002;3(5):951-958.
12. Liljensten E, Gisselfält K, Edberg B, et al. Studies of polyurethane urea bands for ACL reconstruction. J Mater Sci Mater Med. 2002;13(4):351-359.
13. Gretzer C, Gisselfält K, Liljensten E, Rydén L, Thomsen P. Adhesion, apoptosis and cytokine release of human mononuclear cells cultured on degradable poly(urethane urea), polystyrene and titanium in vitro. Biomaterials. 2003;24(17):2843-2852.
14. Jörheim M, Isaxon I, Flondell M, Kalén P, Atroshi I. Short-term outcomes of trapeziometacarpal artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
15. Choung EW, Tan V. Foreign-body reaction to the Artelon CMC joint spacer: case report. J Hand Surg Am. 2008;33(9):1617-1620.
16. Robinson PM, Muir LT. Foreign body reaction associated with Artelon: report of three cases. J Hand Surg Am. 2011;36(1):116-120.
17. Nilsson A, Wiig M, Alnehill H, et al. The Artelon CMC spacer compared with tendon interposition arthroplasty. Acta Orthop. 2010;81(2):237-244.
18. Gretzer C, Emanuelsson L, Liljensten E, Thomsen P. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed. 2006;17(6):669-687.
Aneurysmal Bone Cyst Involving the Metacarpal Bone in a Child
Less than 5% of aneurysmal bone cysts (ABCs) are located in the hand,1 and only a few cases have been reported in the literature.2-7 Unfortunately, it is impossible to predict when an ABC will exhibit aggressive behavior.4,8 Aneurysmal bone cysts and giant cell bone tumors have been considered benign9 lesions that can behave in a locally aggressive fashion.1 Optimal treatment has not been established because treatment is variable depending on the condition of the lesion. Several authors have recommended more radical treatment modalities, such as en bloc resection or excision diaphysectomy followed by strut bone grafting, which had a relatively low rate of recurrence. A relatively low rate of recurrence and other complications indicate that those techniques would serve as a good strategy for patients with expansile hand ABCs in terms of safety, simplicity, and reduced number of reoperations.3,7,10
This article reports a case of an ABC of the second metacarpal bone of the right hand in a 12-year-old boy treated with curettage and autologous morselized iliac bone grafting. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right hand–dominant 12-year-old-boy, who noticed the development of a lump in the dorsum of his right hand. On examination, we found a large, firm swelling of the dorsum of his right hand over the second metacarpal. Radiographic examination showed a symmetrical expansile lytic lesion (22×24×25 mm) involving the entire second metacarpal bone (Figure 1A). Magnetic resonance imaging (MRI) showed a well-defined expansile intramedullary lesion with preservation of the epiphyseal plate, shell-like periosteal reaction, and a multilocular appearance with a hemorrhagic compartment (fluid-fluid levels) (Figure 1B).
At surgery, we found a blood-filled cyst, and the cortex was very thin. The lesion extended to the distal two-thirds of the bone to the level of the physeal plate. We had considered using allograft or other bone substitutes. However, we did not have confidence in the bone-induction potential and power of osteogenesis of bone substitutes or allograft compared with autologous bone graft. Consequently, we performed autologous bone grafting, despite its being an invasive procedure, on the immature iliac crest. We performed thorough curettage of the intramedullary material without damaging the physeal plate, followed by impact morselized autologous bone grafting. Histologic examination confirmed that the final diagnosis was identical to the provisional diagnosis shown on MRI (Figure 1C). A thumb spica cast was applied for 4 weeks after surgery, and regular follow-up radiographs were taken for 3 years and 6 months until confirmation of complete normalization of the lesion without recurrence (Figures 2A-2C).
Discussion
Primary ABCs in the small tubular bones of the hands are rare. Less than 5% of aneurysmal cysts are located in the hand.1 Only a few small cases of this condition have been reported in the literature.2-7 Radiographic examination showed that, in all cases, the lesion was both expansile and completely lucent.7 Although radiographic finding of ABC in short tubular bone characteristically shows central symmetry with expansion into the diaphysis and subarticular bone, the appearance of an ABC on radiographs and angiograms is usually not diagnostic.8 Even though fluid-fluid levels are highly suggestive of ABC, only pathologic study confirms the diagnosis. MRI may be a good tool for postsurgery follow-up. On the basis of these ideas, we performed histological examination and confirmed the diagnosis of ABC of the metacarpus by radiograph and MRI.
The goals in the treatment of primary ABCs are preservation of function and avoidance of recurrence. Unfortunately, it is impossible to predict the possible aggressive behavior in ABCs. Active or aggressive character in certain localizations of ABC in children requires either curettage, which has a considerable recurrence rate, or radical segmental excision, which raises complex reconstructive challenges. Frassica and colleagues7 reported no recurrences in 3 patients treated by complete excision and bone grafting. Curettage and bone grafting in 7 cases were associated with 4 recurrences.7
Because optimal treatment has not been established,3 current recommendations vary, depending on the condition of the lesion. Several authors recommend more radical treatment modalities, such as en bloc resection, excision diaphysectomy, cryotherapy, and strut bone grafting, and a relatively low rate of recurrence and other complications indicates that those techniques would serve as a good strategy for patients with expansile ABCs in the hand.3,7,10 On the other hand, successful results with less aggressive procedures, such as curettage and autologous bone grafting, have been reported.4,5,8
In pediatric patients, surgery to preserve the growth plate is recommended.5 Ropars and colleagues4 suggested that aggressive treatment approaches, such as cryotherapy and resection with reconstruction, should be used only in cases when the articular surface is involved, when full-bone invasion of the phalanx or metacarpal has occurred, or in cases of more than 1 recurrence.
In conclusion, despite the high risk of recurrence of ABC treated with curettage with bone grafting, the findings of the present case show that ABC of the metacarpal bone in children can be treated successfully with curettage followed by morselized autologous bone grafting without recurrence.
1. Athanasian EA. Aneurysmal bone cyst and giant cell tumor of bone of the hand and distal radius. Hand Clin. 2004;20(3):269-281, vi.
2. Tarazona-Velutini P, Romo-Rodriguez R, Saleme-Cruz J. Aneurysmatic bone cyst in the proximal phalanx of a finger. Case report and literature review. Acta Ortop Mex. 2012;26(4):245-249.
3. Jafari D, Jamshidi K, Najdmazhar F, Shariatzade H, Liaghat O. Expansile aneurysmal bone cyst in the tubular bones of the hand treated with en bloc excision and autograft reconstruction: a report of 12 cases. J Hand Surg Eur Vol. 2011;36(8):648-655.
4. Ropars M, Kaila R, Briggs T, Cannon S. Aneurysmal bone cysts of the metacarpals and phalanges of the hand. A 6 case series and literature review. Chir Main. 2007;26(4-5):214-217.
5. Sproule JA, Salmo E, Mortimer G, O’Sullivan M. Aneursymal bone cyst of the proximal phalanx of the thumb in a child. Hand Surg. 2002;7(1):147-150.
6. Schwartz GB, Hammerman MZ. Aneurysmal bone cyst of the fifth metacarpal. Orthop Rev. 1989;18(12):1309-1314.
7. Frassica FJ, Amadio PC, Wold LE, Beabout JW. Aneurysmal bone cyst: clinicopathologic features and treatment of ten cases involving the hand. J Hand Surg Am. 1988;13(5):676-683.
8. Louahem D, Kouyoumdjian P, Ghanem I, et al. Active aneurysmal bone cysts in children: possible evolution after biopsy. J Child Orthop. 2012;6(4):333-338.
9. Lindfors NC. Treatment of a recurrent aneurysmal bone cyst with bioactive glass in a child allows for good bone remodelling and growth. Bone. 2009;45(2):398-400.
10. Salon A, Rémi J, Brunelle F, Drapé JL, Glorion Ch. Total replacement of a middle phalanx by free non-vascularized chondral graft, after failure of sclerotherapy for treatment of an aneurysmal bone cyst. Chir Main. 2005;24(3-4):187-192.
Less than 5% of aneurysmal bone cysts (ABCs) are located in the hand,1 and only a few cases have been reported in the literature.2-7 Unfortunately, it is impossible to predict when an ABC will exhibit aggressive behavior.4,8 Aneurysmal bone cysts and giant cell bone tumors have been considered benign9 lesions that can behave in a locally aggressive fashion.1 Optimal treatment has not been established because treatment is variable depending on the condition of the lesion. Several authors have recommended more radical treatment modalities, such as en bloc resection or excision diaphysectomy followed by strut bone grafting, which had a relatively low rate of recurrence. A relatively low rate of recurrence and other complications indicate that those techniques would serve as a good strategy for patients with expansile hand ABCs in terms of safety, simplicity, and reduced number of reoperations.3,7,10
This article reports a case of an ABC of the second metacarpal bone of the right hand in a 12-year-old boy treated with curettage and autologous morselized iliac bone grafting. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right hand–dominant 12-year-old-boy, who noticed the development of a lump in the dorsum of his right hand. On examination, we found a large, firm swelling of the dorsum of his right hand over the second metacarpal. Radiographic examination showed a symmetrical expansile lytic lesion (22×24×25 mm) involving the entire second metacarpal bone (Figure 1A). Magnetic resonance imaging (MRI) showed a well-defined expansile intramedullary lesion with preservation of the epiphyseal plate, shell-like periosteal reaction, and a multilocular appearance with a hemorrhagic compartment (fluid-fluid levels) (Figure 1B).
At surgery, we found a blood-filled cyst, and the cortex was very thin. The lesion extended to the distal two-thirds of the bone to the level of the physeal plate. We had considered using allograft or other bone substitutes. However, we did not have confidence in the bone-induction potential and power of osteogenesis of bone substitutes or allograft compared with autologous bone graft. Consequently, we performed autologous bone grafting, despite its being an invasive procedure, on the immature iliac crest. We performed thorough curettage of the intramedullary material without damaging the physeal plate, followed by impact morselized autologous bone grafting. Histologic examination confirmed that the final diagnosis was identical to the provisional diagnosis shown on MRI (Figure 1C). A thumb spica cast was applied for 4 weeks after surgery, and regular follow-up radiographs were taken for 3 years and 6 months until confirmation of complete normalization of the lesion without recurrence (Figures 2A-2C).
Discussion
Primary ABCs in the small tubular bones of the hands are rare. Less than 5% of aneurysmal cysts are located in the hand.1 Only a few small cases of this condition have been reported in the literature.2-7 Radiographic examination showed that, in all cases, the lesion was both expansile and completely lucent.7 Although radiographic finding of ABC in short tubular bone characteristically shows central symmetry with expansion into the diaphysis and subarticular bone, the appearance of an ABC on radiographs and angiograms is usually not diagnostic.8 Even though fluid-fluid levels are highly suggestive of ABC, only pathologic study confirms the diagnosis. MRI may be a good tool for postsurgery follow-up. On the basis of these ideas, we performed histological examination and confirmed the diagnosis of ABC of the metacarpus by radiograph and MRI.
The goals in the treatment of primary ABCs are preservation of function and avoidance of recurrence. Unfortunately, it is impossible to predict the possible aggressive behavior in ABCs. Active or aggressive character in certain localizations of ABC in children requires either curettage, which has a considerable recurrence rate, or radical segmental excision, which raises complex reconstructive challenges. Frassica and colleagues7 reported no recurrences in 3 patients treated by complete excision and bone grafting. Curettage and bone grafting in 7 cases were associated with 4 recurrences.7
Because optimal treatment has not been established,3 current recommendations vary, depending on the condition of the lesion. Several authors recommend more radical treatment modalities, such as en bloc resection, excision diaphysectomy, cryotherapy, and strut bone grafting, and a relatively low rate of recurrence and other complications indicates that those techniques would serve as a good strategy for patients with expansile ABCs in the hand.3,7,10 On the other hand, successful results with less aggressive procedures, such as curettage and autologous bone grafting, have been reported.4,5,8
In pediatric patients, surgery to preserve the growth plate is recommended.5 Ropars and colleagues4 suggested that aggressive treatment approaches, such as cryotherapy and resection with reconstruction, should be used only in cases when the articular surface is involved, when full-bone invasion of the phalanx or metacarpal has occurred, or in cases of more than 1 recurrence.
In conclusion, despite the high risk of recurrence of ABC treated with curettage with bone grafting, the findings of the present case show that ABC of the metacarpal bone in children can be treated successfully with curettage followed by morselized autologous bone grafting without recurrence.
Less than 5% of aneurysmal bone cysts (ABCs) are located in the hand,1 and only a few cases have been reported in the literature.2-7 Unfortunately, it is impossible to predict when an ABC will exhibit aggressive behavior.4,8 Aneurysmal bone cysts and giant cell bone tumors have been considered benign9 lesions that can behave in a locally aggressive fashion.1 Optimal treatment has not been established because treatment is variable depending on the condition of the lesion. Several authors have recommended more radical treatment modalities, such as en bloc resection or excision diaphysectomy followed by strut bone grafting, which had a relatively low rate of recurrence. A relatively low rate of recurrence and other complications indicate that those techniques would serve as a good strategy for patients with expansile hand ABCs in terms of safety, simplicity, and reduced number of reoperations.3,7,10
This article reports a case of an ABC of the second metacarpal bone of the right hand in a 12-year-old boy treated with curettage and autologous morselized iliac bone grafting. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right hand–dominant 12-year-old-boy, who noticed the development of a lump in the dorsum of his right hand. On examination, we found a large, firm swelling of the dorsum of his right hand over the second metacarpal. Radiographic examination showed a symmetrical expansile lytic lesion (22×24×25 mm) involving the entire second metacarpal bone (Figure 1A). Magnetic resonance imaging (MRI) showed a well-defined expansile intramedullary lesion with preservation of the epiphyseal plate, shell-like periosteal reaction, and a multilocular appearance with a hemorrhagic compartment (fluid-fluid levels) (Figure 1B).
At surgery, we found a blood-filled cyst, and the cortex was very thin. The lesion extended to the distal two-thirds of the bone to the level of the physeal plate. We had considered using allograft or other bone substitutes. However, we did not have confidence in the bone-induction potential and power of osteogenesis of bone substitutes or allograft compared with autologous bone graft. Consequently, we performed autologous bone grafting, despite its being an invasive procedure, on the immature iliac crest. We performed thorough curettage of the intramedullary material without damaging the physeal plate, followed by impact morselized autologous bone grafting. Histologic examination confirmed that the final diagnosis was identical to the provisional diagnosis shown on MRI (Figure 1C). A thumb spica cast was applied for 4 weeks after surgery, and regular follow-up radiographs were taken for 3 years and 6 months until confirmation of complete normalization of the lesion without recurrence (Figures 2A-2C).
Discussion
Primary ABCs in the small tubular bones of the hands are rare. Less than 5% of aneurysmal cysts are located in the hand.1 Only a few small cases of this condition have been reported in the literature.2-7 Radiographic examination showed that, in all cases, the lesion was both expansile and completely lucent.7 Although radiographic finding of ABC in short tubular bone characteristically shows central symmetry with expansion into the diaphysis and subarticular bone, the appearance of an ABC on radiographs and angiograms is usually not diagnostic.8 Even though fluid-fluid levels are highly suggestive of ABC, only pathologic study confirms the diagnosis. MRI may be a good tool for postsurgery follow-up. On the basis of these ideas, we performed histological examination and confirmed the diagnosis of ABC of the metacarpus by radiograph and MRI.
The goals in the treatment of primary ABCs are preservation of function and avoidance of recurrence. Unfortunately, it is impossible to predict the possible aggressive behavior in ABCs. Active or aggressive character in certain localizations of ABC in children requires either curettage, which has a considerable recurrence rate, or radical segmental excision, which raises complex reconstructive challenges. Frassica and colleagues7 reported no recurrences in 3 patients treated by complete excision and bone grafting. Curettage and bone grafting in 7 cases were associated with 4 recurrences.7
Because optimal treatment has not been established,3 current recommendations vary, depending on the condition of the lesion. Several authors recommend more radical treatment modalities, such as en bloc resection, excision diaphysectomy, cryotherapy, and strut bone grafting, and a relatively low rate of recurrence and other complications indicates that those techniques would serve as a good strategy for patients with expansile ABCs in the hand.3,7,10 On the other hand, successful results with less aggressive procedures, such as curettage and autologous bone grafting, have been reported.4,5,8
In pediatric patients, surgery to preserve the growth plate is recommended.5 Ropars and colleagues4 suggested that aggressive treatment approaches, such as cryotherapy and resection with reconstruction, should be used only in cases when the articular surface is involved, when full-bone invasion of the phalanx or metacarpal has occurred, or in cases of more than 1 recurrence.
In conclusion, despite the high risk of recurrence of ABC treated with curettage with bone grafting, the findings of the present case show that ABC of the metacarpal bone in children can be treated successfully with curettage followed by morselized autologous bone grafting without recurrence.
1. Athanasian EA. Aneurysmal bone cyst and giant cell tumor of bone of the hand and distal radius. Hand Clin. 2004;20(3):269-281, vi.
2. Tarazona-Velutini P, Romo-Rodriguez R, Saleme-Cruz J. Aneurysmatic bone cyst in the proximal phalanx of a finger. Case report and literature review. Acta Ortop Mex. 2012;26(4):245-249.
3. Jafari D, Jamshidi K, Najdmazhar F, Shariatzade H, Liaghat O. Expansile aneurysmal bone cyst in the tubular bones of the hand treated with en bloc excision and autograft reconstruction: a report of 12 cases. J Hand Surg Eur Vol. 2011;36(8):648-655.
4. Ropars M, Kaila R, Briggs T, Cannon S. Aneurysmal bone cysts of the metacarpals and phalanges of the hand. A 6 case series and literature review. Chir Main. 2007;26(4-5):214-217.
5. Sproule JA, Salmo E, Mortimer G, O’Sullivan M. Aneursymal bone cyst of the proximal phalanx of the thumb in a child. Hand Surg. 2002;7(1):147-150.
6. Schwartz GB, Hammerman MZ. Aneurysmal bone cyst of the fifth metacarpal. Orthop Rev. 1989;18(12):1309-1314.
7. Frassica FJ, Amadio PC, Wold LE, Beabout JW. Aneurysmal bone cyst: clinicopathologic features and treatment of ten cases involving the hand. J Hand Surg Am. 1988;13(5):676-683.
8. Louahem D, Kouyoumdjian P, Ghanem I, et al. Active aneurysmal bone cysts in children: possible evolution after biopsy. J Child Orthop. 2012;6(4):333-338.
9. Lindfors NC. Treatment of a recurrent aneurysmal bone cyst with bioactive glass in a child allows for good bone remodelling and growth. Bone. 2009;45(2):398-400.
10. Salon A, Rémi J, Brunelle F, Drapé JL, Glorion Ch. Total replacement of a middle phalanx by free non-vascularized chondral graft, after failure of sclerotherapy for treatment of an aneurysmal bone cyst. Chir Main. 2005;24(3-4):187-192.
1. Athanasian EA. Aneurysmal bone cyst and giant cell tumor of bone of the hand and distal radius. Hand Clin. 2004;20(3):269-281, vi.
2. Tarazona-Velutini P, Romo-Rodriguez R, Saleme-Cruz J. Aneurysmatic bone cyst in the proximal phalanx of a finger. Case report and literature review. Acta Ortop Mex. 2012;26(4):245-249.
3. Jafari D, Jamshidi K, Najdmazhar F, Shariatzade H, Liaghat O. Expansile aneurysmal bone cyst in the tubular bones of the hand treated with en bloc excision and autograft reconstruction: a report of 12 cases. J Hand Surg Eur Vol. 2011;36(8):648-655.
4. Ropars M, Kaila R, Briggs T, Cannon S. Aneurysmal bone cysts of the metacarpals and phalanges of the hand. A 6 case series and literature review. Chir Main. 2007;26(4-5):214-217.
5. Sproule JA, Salmo E, Mortimer G, O’Sullivan M. Aneursymal bone cyst of the proximal phalanx of the thumb in a child. Hand Surg. 2002;7(1):147-150.
6. Schwartz GB, Hammerman MZ. Aneurysmal bone cyst of the fifth metacarpal. Orthop Rev. 1989;18(12):1309-1314.
7. Frassica FJ, Amadio PC, Wold LE, Beabout JW. Aneurysmal bone cyst: clinicopathologic features and treatment of ten cases involving the hand. J Hand Surg Am. 1988;13(5):676-683.
8. Louahem D, Kouyoumdjian P, Ghanem I, et al. Active aneurysmal bone cysts in children: possible evolution after biopsy. J Child Orthop. 2012;6(4):333-338.
9. Lindfors NC. Treatment of a recurrent aneurysmal bone cyst with bioactive glass in a child allows for good bone remodelling and growth. Bone. 2009;45(2):398-400.
10. Salon A, Rémi J, Brunelle F, Drapé JL, Glorion Ch. Total replacement of a middle phalanx by free non-vascularized chondral graft, after failure of sclerotherapy for treatment of an aneurysmal bone cyst. Chir Main. 2005;24(3-4):187-192.
Unstable Dorsal Proximal Interphalangeal Joint Fracture-Dislocations Treated With Extension-Block Pinning
The proximal interphalangeal (PIP) joint plays a crucial role in hand function, accounting for an estimated 85% of the motion required to grasp an object.1 The anatomy and biomechanics of the PIP joint, however, make it particularly prone to injury.2,3 Dorsal PIP fracture-dislocations represent a subset of PIP injuries that often require surgical intervention.2 The stability of these fracture-dislocations largely depends on the extent of articular involvement of the base of the middle phalanx. Fractures that involve less than 30% of the joint surface typically remain stable after reduction.2,4,5 In cases in which involvement ranges from 30% to 50%, PIP joint stability is more tenuous, and more joint flexion is required to maintain concentric reduction. Fractures that involve more than 50% of the articular surface are unstable and require operative intervention.2,5,6 Fractures that require more than 30° of flexion for reduction maintenance are generally considered unstable and may benefit from surgical intervention.2
The goals of treatment for this injury are to restore a stable, concentrically reduced joint and initiate early joint mobilization to prevent stiffness, pain, recurrent instability, and posttraumatic arthritis.3,7 Numerous surgical interventions for unstable PIP fracture-dislocations have been proposed, including open reduction and internal fixation (ORIF),8-10 extension-block pinning (EBP),11-13 dynamic external fixation,14-17 volar plate arthroplasty,18,19 and hemi-hamate resurfacing arthroplasty.20,21 Many of these techniques can be technically demanding and may require prolonged immobilization. EBP can be performed easily and efficiently and allows for early joint motion.
Extension-block pinning—placing a Kirschner wire (K-wire) into the head of the proximal phalanx at an angle that blocks PIP extension and prevents joint subluxation—was first described by Sugawa and colleagues12 in 1979. In a study by Inoue and Tamura,11 patients treated with EBP had a mean PIP range of motion (ROM) of 94° at a mean follow-up of 14 months. In a series of 3 case reports, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM in patients treated with EBP.
We conducted a study to expand on previous research on pain, function, and satisfaction outcomes in addition to ROM. We hypothesized that percutaneous EBP is an effective treatment for unstable dorsal PIP fracture-dislocations and has efficacy similar to that of more complex and technically demanding methods of treatment.
Materials and Methods
We retrospectively reviewed patient charts to identify candidates for this study. Inclusion criteria were unstable dorsal PIP fracture-dislocations treated with EBP and minimum 4-month follow-up. (Fracture-dislocations were deemed unstable if they involved at least 30% of the articular surface or required more than 30° of flexion for reduction maintenance.) Exclusion criteria were open injury, neurovascular or tendon injury, or any prior injury to the PIP joint.
Twelve patients (5 females, 7 males) treated over a 4-year period (2002–2006) met the inclusion criteria. Mean age was 30 years (range, 15-64 years). Each surgery was performed by Dr. Hagberg or Dr. Balk. Half the cases involved the dominant hand. Two small fingers, 4 ring fingers, 2 long fingers, and 4 index fingers were injured. The injuries were sustained in an all-terrain vehicle accident (n = 1), in falls (n = 2), while swimming (n = 1), or while playing softball (n = 3), football (n = 4), or soccer (n = 1). Mean time from injury to surgery was 7.5 days (range, 4-27 days). Extent of articular surface involvement of the base of the fractured middle phalanx was calculated using preoperatively obtained lateral radiographs.
Surgical intervention was performed in a reproducible fashion. All patients were treated with closed reduction of the PIP joint under fluoroscopic guidance. Before pinning, joint stability was assessed fluoroscopically both at rest and through an arc of motion. A single smooth 0.045-in K-wire was then inserted percutaneously into the distal and dorsal aspects of the proximal phalanx in retrograde fashion (Figure 1). During wire insertion, the distal interphalangeal joint was flexed to relax the intrinsic mechanism, and the central slip tendon was pierced just proximal to its insertion. We have not noted significant adhesion formation about the central slip with this technique, likely because of limited tendon excursion in this location. Stable joint reduction was confirmed with fluoroscopy. No attempt was made to reduce the intra-articular fracture at the base of the middle phalanx.
A therapy program was initiated 2 to 9 days after surgery. At the first postoperative visit, patients were allowed to perform active ROM (AROM) with the pin in place (Figure 1). K-wires were removed a mean of 25 days (range, 17-31 days) after surgery. A static dorsal block splint was then applied, and patients were encouraged to remove it several times per day for AROM between 20° and full flexion until 6 weeks after surgery. At that time, formal occupational therapy was commenced for another 6 weeks. If there was residual flexion contracture of the PIP joint, dynamic extension splinting was initiated after fracture consolidation.
Mean follow-up was 35.5 months (range, 4-94 months). Postoperative anteroposterior and lateral radiographs were used to evaluate maintenance of joint congruity, fracture union, remodeling, and evidence of degenerative changes. At final follow-up, grip strength of injured and contralateral hands was measured with a dynamometer (Jamar; Patterson Medical, Warrenville, Illinois). AROM and passive ROM (PROM) of the PIP joint was documented at follow-up visits. In addition, patients rated their pain on a 0-to-10 visual analog scale (VAS), with 0 representing no pain and 10 representing excruciating pain. Patients also completed a questionnaire assessing satisfaction with surgical outcome. Physical function and disability were assessed with the Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH) questionnaire. Any complications, including the need for further surgeries, were documented. Pearson correlation coefficients and Student t tests (with significance set at P < .05) were used to compare outcomes.
Results
Radiographic reduction of joint dislocation was achieved and maintained in 11 of the 12 patients at a mean follow-up of 35.5 months (range, 4-94 months). Extent of joint surface involvement, based on preoperative lateral radiographs, averaged 43% (range, 25%-75%). Although no direct articular reduction was performed, remodeling of the joint surface was consistently noted at follow-up (Figure 2). Mild radiographic degenerative changes were noted at final follow-up in 4 patients, and moderate changes were noted in 1 patient. Radiographic union was achieved in all cases, and no pin-tract infections were noted.
Mean AROM of the PIP joint at final follow-up was 84° (range, 50°-110°), with patients lacking a mean of 7° of full extension and achieving mean flexion of 91°. Mean PROM was 93° (range, 75°-110°). There was no correlation between extent of articular surface involvement and ROM. Furthermore, no correlation was found between time from injury to surgery and ROM. Patients regained full grip strength in the operative hand. At final follow-up, mean grip strength was 79.4 pounds in the operative hand and 79.6 pounds in the contralateral hand, demonstrating equal grip strengths bilaterally.
Patients overall had very low levels of pain; mean VAS score was 0.64 (range, 0-3). Mean QuickDASH score was 5.7 (range, 0-30), suggesting minimal functional impairment. One patient developed a malunion of the middle phalanx fracture resulting in a rotational deformity and required corrective osteotomy. This patient’s VAS score (3) and QuickDASH score (30) were significantly higher than those of the other patients in the study. No other complications were noted by final follow-up.
A higher level of patient satisfaction was found to be directly related to length of follow-up (P < .05). Satisfaction was inversely related to higher VAS score (P < .05) and higher QuickDASH score (P < .001). Pain at work correlated with lower satisfaction level (P < .05). There was no correlation between patient satisfaction and AROM or PROM.
Discussion
The results of this study demonstrate the efficacy of EBP in the treatment of dorsal PIP joint fracture-dislocations. EBP maintained joint dislocation reduction and allowed for early mobilization, which resulted in good ROM, minimal pain, and good functional outcomes. Of note, postoperative patient satisfaction correlated with pain but not with ROM. It is possible that EBP yielded sufficient functional ROM in all patients such that improvement beyond this threshold did not lead to further improvement in satisfaction. Hume and colleagues23 found that mean PIP joint flexion of 60° is needed for activities of daily living. As mean PIP active flexion was 91° (range, 70°-105°) in the present study, it is possible that satisfaction did not correlate with ROM, as all 12 patients achieved active flexion of more than 60°. Despite the lack of correlation between ROM and satisfaction, early PIP joint mobilization is likely a key contributor to positive outcomes because of its significant role in cartilage healing.24
Postoperative ROM in the present study is consistent with that in other reports of patients with PIP joint fracture-dislocations treated with EBP.11,12,22 In a study by Inoue and Tamura,11 14 such patients had mean PIP ROM of 94° at a mean follow-up of 14 months. Viegas22 followed a series of 3 patients for a mean of 7 weeks. At final follow-up, their mean PIP arc of motion was 71°; they lacked 12° of full extension and achieved 83° of flexion. The larger PIP arc of motion (84°) found in the present study may be due to our significantly longer follow-up (35 months). Unlike us, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM. Our finding a lack of correlation may be a result of the significant amount of joint remodeling noted on follow-up radiographs.
Studies of transarticular pinning of PIP joints after dorsal PIP fracture-dislocations have reported outcomes similar to ours.25,26 Newington and colleagues25 evaluated 10 cases of transarticular pinning of the PIP joint and found mean arc of motion of 85° and equal grip strengths between injured and contralateral hands. In a series of 19 patients with PIP fracture-dislocations, Aladin and Davis26 noted similar outcomes of transarticular K-wire fixation and ORIF. In both of their treatment groups, however, there was evidence of PIP joint incongruity and subluxation. Of note, PIP arc motion was lower in their study than in ours.
Recent studies have evaluated unstable PIP fracture-dislocations treated with both EBP and percutaneous reduction and pinning with a second K-wire.13,27 At a mean follow-up of 18 months, Vitale and colleagues13 noted maintenance of concentric fracture reduction, good PIP ROM (mean range, 4°-93°), and low VAS and DASH scores (1.4 and 8, respectively). Waris and Alanen27 noted mean PIP AROM of 83° and low VAS and DASH scores (1 and 4, respectively). The EBP technique used in the present study did not involve percutaneous fracture reduction but achieved equally good ROM and VAS and QuickDASH scores.
Clinical outcomes of EBP of PIP joint fracture-dislocations are also comparable to outcomes of more complex treatment methods.8-10,15-19,21,26,28-33 Dynamic distraction external fixation has led to equally good ROM (mean AROM, 80°-85°15,16) and VAS scores, but with a higher incidence of pin-site infection.14-17 ORIF of the intra-articular middle phalanx fracture has the advantage of obtaining a direct anatomical reduction, but clinical outcomes are similar to those in the present study (mean AROM, 70°; 78% pain-free9), and flexion contractures have been noted.8-10 Furthermore, reduction of the fractured PIP joint articular surface has not been shown to be necessary for good outcomes.16,34 This may be explained in part by PIP joint remodeling, which has been routinely observed on long-term follow-up by the senior authors of the present study. Hemi-hamate autografting and volar plate arthroplasty are other options that have had promising results in the treatment of acute and chronic unstable PIP fracture-dislocations.18-21 However, the postoperative ROM (mean AROM, 61°-85°18,21), VAS scores, and patient satisfaction (91% very satisfied21) of these operations are similar to those of EBP in the present study and may not justify the longer operative times and technical challenges associated with these techniques.
We believe that our study group’s 1 complication, a malunion that was treated with corrective osteotomy, resulted from lack of appreciation of the degree of injury. The teenaged female patient’s index finger PIP joint had a rotational malalignment that was not appreciated before or during surgery. After pinning and after ROM was restored, the index finger was observed crossing over the middle finger with digital flexion. The patient returned to the operating room for corrective osteotomy.
We recommend that surgeons assess alignment carefully, before and during surgery, when considering this technique. Although complications are rare, the technique is not for patients with rotational malalignment; ORIF may be more suitable in these cases. In addition, though EBP may be appropriate for pilon-type injuries, as it allows for early AROM, our procedure of choice for pilon fracture is dynamic external fixation, which in addition to allowing for AROM provides ligamentotaxis. In the event that a large volar articular fragment extends into the middle phalanx diaphysis, we typically proceed with ORIF through a volar shotgun approach. At our institution, injuries lasting more than 3 months are often treated with volar plate arthroplasty or hemi-hamate resurfacing. Finally, we believe that caution should be exercised when using this technique in patients with more than 50% articular involvement. In the present study, though we used this treatment in cases of up to 75% surface involvement, alternative techniques, such as hemi-hamate resurfacing arthroplasty, may provide a better volar bony buttress and limit the risk for recurrent instability. Despite its relative contraindications, our technique has been appropriate for more than 90% of the acute PIP fracture-dislocations we have seen.
This study expands on prior research by demonstrating good function, satisfaction, and pain outcomes of percutaneous EBP in the treatment of unstable dorsal PIP fracture-dislocations. In addition, this study demonstrated that the efficacy of EBP is similar to that of more complex and technically demanding methods of treatment. Our technique has the advantage of simplicity. It obviates the soft-tissue damage required for ORIF and more complex fixation techniques. Furthermore, use of this simple technique may save time and costs and lead to more reproducible outcomes.
One limitation of this study is its small sample size. It is possible that outcomes may have been different with a larger sample. Furthermore, we did not make a direct comparison with other treatment methods. To better determine the optimal treatment method for this fracture type, future studies should prospectively evaluate outcomes for multiple treatment modalities in a randomized fashion.
1. Leibovic SJ, Bowers WH. Anatomy of the proximal interphalangeal joint. Hand Clin. 1994;10(2):169-178.
2. Kiefhaber TR, Stern PJ. Fracture dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1998;23(3):368-380.
3. Ng CY, Oliver CW. Fractures of the proximal interphalangeal joints of the fingers. J Bone Joint Surg Br. 2009;91(6):705-712.
4. Isani A. Small joint injuries requiring surgical treatment. Orthop Clin North Am. 1986;17(3):407-419.
5. McElfresh EC, Dobyns JH, O’Brien ET. Management of fracture-dislocation of the proximal interphalangeal joints by extension-block splinting. J Bone Joint Surg Am. 1972;54(8):1705-1711.
6. Hastings H 2nd, Carroll C 4th. Treatment of closed articular fractures of the metacarpophalangeal and proximal interphalangeal joints. Hand Clin. 1988;4(3):503-527.
7. O’Rourke SK, Gaur S, Barton NJ. Long-term outcome of articular fractures of the phalanges: an eleven year follow up. J Hand Surg Br. 1989;14(2):183-193.
8. Grant I, Berger AC, Tham SK. Internal fixation of unstable fracture dislocations of the proximal interphalangeal joint. J Hand Surg Br. 2005;30(5):492-498.
9. Hamilton SC, Stern PJ, Fassler PR, Kiefhaber TR. Mini-screw fixation for the treatment of proximal interphalangeal joint dorsal fracture-dislocations. J Hand Surg Am. 2006;31(8):1349-1354.
10. Lee JY, Teoh LC. Dorsal fracture dislocations of the proximal interphalangeal joint treated by open reduction and interfragmentary screw fixation: indications, approaches and results. J Hand Surg Br. 2006;31(2):138-146.
11. Inoue G, Tamura Y. Treatment of fracture-dislocation of the proximal interphalangeal joint using extension-block Kirschner wire. Ann Chir Main Memb Super. 1991;10(6):564-568.
12. Sugawa I, Otani K, Kobayashi A. Treatment of fracture dislocation PIP-joint by Kirschner wire extension block method. Cent Jpn J Orthop Traumat. 1979;22:1409-1412.
13. Vitale MA, White NJ, Strauch RJ. A percutaneous technique to treat unstable dorsal fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2011;36(9):1453-1459.
14. Badia A, Riano F, Ravikoff J, Khouri R, Gonzalez-Hernandez E, Orbay JL. Dynamic intradigital external fixation for proximal interphalangeal joint fracture dislocations. J Hand Surg Am. 2005;30(1):154-160.
15. Ellis SJ, Cheng R, Prokopis P, et al. Treatment of proximal interphalangeal dorsal fracture-dislocation injuries with dynamic external fixation: a pins and rubber band system. J Hand Surg Am. 2007;32(8):1242-1250.
16. Morgan JP, Gordon DA, Klug MS, Perry PE, Barre PS. Dynamic digital traction for unstable comminuted intra-articular fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1995;20(4):565-573.
17. Ruland RT, Hogan CJ, Cannon DL, Slade JF. Use of dynamic distraction external fixation for unstable fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2008;33(1):19-25.
18. Dionysian E, Eaton RG. The long-term outcome of volar plate arthroplasty of the proximal interphalangeal joint. J Hand Surg Am. 2000;25(3):429-437.
19. Durham-Smith G, McCarten GM. Volar plate arthroplasty for closed proximal interphalangeal joint injuries. J Hand Surg Br. 1992;17(4):422-428.
20. Calfee RP, Kiefhaber TR, Sommerkamp TG, Stern PJ. Hemi-hamate arthroplasty provides functional reconstruction of acute and chronic proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2009;34(7):1232-1241.
21. Williams RM, Kiefhaber TR, Sommerkamp TG, Stern PJ. Treatment of unstable dorsal proximal interphalangeal fracture/dislocations using a hemi-hamate autograft. J Hand Surg Am. 2003;28(5):856-865.
22. Viegas SF. Extension block pinning for proximal interphalangeal joint fracture dislocations: preliminary report of a new technique. J Hand Surg Am. 1992;17(5):896-901.
23. Hume MC, Gellman H, McKellop H, Brumfield RH Jr. Functional range of motion of the joints of the hand. J Hand Surg Am. 1990;15(2):240-243.
24. Salter RB. The physiologic basis of continuous passive motion for articular cartilage healing and regeneration. Hand Clin. 1994;10(2):211-220.
25. Newington DP, Davis TR, Barton NJ. The treatment of dorsal fracture-dislocation of the proximal interphalangeal joint by closed reduction and Kirschner wire fixation: a 16-year follow up. J Hand Surg Br. 2001;26(6):537-540.
26. Aladin A, Davis TR. Dorsal fracture-dislocation of the proximal interphalangeal joint: a comparative study of percutaneous Kirschner wire fixation versus open reduction and internal fixation. J Hand Surg Br. 2005;30(2):120-128.
27. Waris E, Alanen V. Percutaneous, intramedullary fracture reduction and extension block pinning for dorsal proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2010;35(12):2046-2052.
28. Bain GI, Mehta JA, Heptinstall RJ, Bria M. Dynamic external fixation for injuries of the proximal interphalangeal joint. J Bone Joint Surg Br. 1998;80(6):1014-1019.
29. Eaton RG, Malerich MM. Volar plate arthroplasty of the proximal interphalangeal joint: a review of ten years’ experience. J Hand Surg Am. 1980;5(3):260-268.
30. Green A, Smith J, Redding M, Akelman E. Acute open reduction and rigid internal fixation of proximal interphalangeal joint fracture dislocation. J Hand Surg Am. 1992;17(3):512-517.
31. Inanami H, Ninomiya S, Okutsu I, Tarui T. Dynamic external finger fixator for fracture dislocation of the proximal interphalangeal joint. J Hand Surg Am. 1993;18(1):160-164.
32. Suzuki Y, Matsunaga T, Sato S, Yokoi T. The pins and rubbers traction system for treatment of comminuted intraarticular fractures and fracture-dislocations in the hand. J Hand Surg Br. 1994;19(1):98-107.
33. Weiss AP. Cerclage fixation for fracture dislocation of the proximal interphalangeal joint. Clin Orthop. 1996;(327):21-28.
34. Agee JM. Unstable fracture dislocations of the proximal interphalangeal joint. Treatment with the force couple splint. Clin Orthop. 1987;(214):101-112.
The proximal interphalangeal (PIP) joint plays a crucial role in hand function, accounting for an estimated 85% of the motion required to grasp an object.1 The anatomy and biomechanics of the PIP joint, however, make it particularly prone to injury.2,3 Dorsal PIP fracture-dislocations represent a subset of PIP injuries that often require surgical intervention.2 The stability of these fracture-dislocations largely depends on the extent of articular involvement of the base of the middle phalanx. Fractures that involve less than 30% of the joint surface typically remain stable after reduction.2,4,5 In cases in which involvement ranges from 30% to 50%, PIP joint stability is more tenuous, and more joint flexion is required to maintain concentric reduction. Fractures that involve more than 50% of the articular surface are unstable and require operative intervention.2,5,6 Fractures that require more than 30° of flexion for reduction maintenance are generally considered unstable and may benefit from surgical intervention.2
The goals of treatment for this injury are to restore a stable, concentrically reduced joint and initiate early joint mobilization to prevent stiffness, pain, recurrent instability, and posttraumatic arthritis.3,7 Numerous surgical interventions for unstable PIP fracture-dislocations have been proposed, including open reduction and internal fixation (ORIF),8-10 extension-block pinning (EBP),11-13 dynamic external fixation,14-17 volar plate arthroplasty,18,19 and hemi-hamate resurfacing arthroplasty.20,21 Many of these techniques can be technically demanding and may require prolonged immobilization. EBP can be performed easily and efficiently and allows for early joint motion.
Extension-block pinning—placing a Kirschner wire (K-wire) into the head of the proximal phalanx at an angle that blocks PIP extension and prevents joint subluxation—was first described by Sugawa and colleagues12 in 1979. In a study by Inoue and Tamura,11 patients treated with EBP had a mean PIP range of motion (ROM) of 94° at a mean follow-up of 14 months. In a series of 3 case reports, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM in patients treated with EBP.
We conducted a study to expand on previous research on pain, function, and satisfaction outcomes in addition to ROM. We hypothesized that percutaneous EBP is an effective treatment for unstable dorsal PIP fracture-dislocations and has efficacy similar to that of more complex and technically demanding methods of treatment.
Materials and Methods
We retrospectively reviewed patient charts to identify candidates for this study. Inclusion criteria were unstable dorsal PIP fracture-dislocations treated with EBP and minimum 4-month follow-up. (Fracture-dislocations were deemed unstable if they involved at least 30% of the articular surface or required more than 30° of flexion for reduction maintenance.) Exclusion criteria were open injury, neurovascular or tendon injury, or any prior injury to the PIP joint.
Twelve patients (5 females, 7 males) treated over a 4-year period (2002–2006) met the inclusion criteria. Mean age was 30 years (range, 15-64 years). Each surgery was performed by Dr. Hagberg or Dr. Balk. Half the cases involved the dominant hand. Two small fingers, 4 ring fingers, 2 long fingers, and 4 index fingers were injured. The injuries were sustained in an all-terrain vehicle accident (n = 1), in falls (n = 2), while swimming (n = 1), or while playing softball (n = 3), football (n = 4), or soccer (n = 1). Mean time from injury to surgery was 7.5 days (range, 4-27 days). Extent of articular surface involvement of the base of the fractured middle phalanx was calculated using preoperatively obtained lateral radiographs.
Surgical intervention was performed in a reproducible fashion. All patients were treated with closed reduction of the PIP joint under fluoroscopic guidance. Before pinning, joint stability was assessed fluoroscopically both at rest and through an arc of motion. A single smooth 0.045-in K-wire was then inserted percutaneously into the distal and dorsal aspects of the proximal phalanx in retrograde fashion (Figure 1). During wire insertion, the distal interphalangeal joint was flexed to relax the intrinsic mechanism, and the central slip tendon was pierced just proximal to its insertion. We have not noted significant adhesion formation about the central slip with this technique, likely because of limited tendon excursion in this location. Stable joint reduction was confirmed with fluoroscopy. No attempt was made to reduce the intra-articular fracture at the base of the middle phalanx.
A therapy program was initiated 2 to 9 days after surgery. At the first postoperative visit, patients were allowed to perform active ROM (AROM) with the pin in place (Figure 1). K-wires were removed a mean of 25 days (range, 17-31 days) after surgery. A static dorsal block splint was then applied, and patients were encouraged to remove it several times per day for AROM between 20° and full flexion until 6 weeks after surgery. At that time, formal occupational therapy was commenced for another 6 weeks. If there was residual flexion contracture of the PIP joint, dynamic extension splinting was initiated after fracture consolidation.
Mean follow-up was 35.5 months (range, 4-94 months). Postoperative anteroposterior and lateral radiographs were used to evaluate maintenance of joint congruity, fracture union, remodeling, and evidence of degenerative changes. At final follow-up, grip strength of injured and contralateral hands was measured with a dynamometer (Jamar; Patterson Medical, Warrenville, Illinois). AROM and passive ROM (PROM) of the PIP joint was documented at follow-up visits. In addition, patients rated their pain on a 0-to-10 visual analog scale (VAS), with 0 representing no pain and 10 representing excruciating pain. Patients also completed a questionnaire assessing satisfaction with surgical outcome. Physical function and disability were assessed with the Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH) questionnaire. Any complications, including the need for further surgeries, were documented. Pearson correlation coefficients and Student t tests (with significance set at P < .05) were used to compare outcomes.
Results
Radiographic reduction of joint dislocation was achieved and maintained in 11 of the 12 patients at a mean follow-up of 35.5 months (range, 4-94 months). Extent of joint surface involvement, based on preoperative lateral radiographs, averaged 43% (range, 25%-75%). Although no direct articular reduction was performed, remodeling of the joint surface was consistently noted at follow-up (Figure 2). Mild radiographic degenerative changes were noted at final follow-up in 4 patients, and moderate changes were noted in 1 patient. Radiographic union was achieved in all cases, and no pin-tract infections were noted.
Mean AROM of the PIP joint at final follow-up was 84° (range, 50°-110°), with patients lacking a mean of 7° of full extension and achieving mean flexion of 91°. Mean PROM was 93° (range, 75°-110°). There was no correlation between extent of articular surface involvement and ROM. Furthermore, no correlation was found between time from injury to surgery and ROM. Patients regained full grip strength in the operative hand. At final follow-up, mean grip strength was 79.4 pounds in the operative hand and 79.6 pounds in the contralateral hand, demonstrating equal grip strengths bilaterally.
Patients overall had very low levels of pain; mean VAS score was 0.64 (range, 0-3). Mean QuickDASH score was 5.7 (range, 0-30), suggesting minimal functional impairment. One patient developed a malunion of the middle phalanx fracture resulting in a rotational deformity and required corrective osteotomy. This patient’s VAS score (3) and QuickDASH score (30) were significantly higher than those of the other patients in the study. No other complications were noted by final follow-up.
A higher level of patient satisfaction was found to be directly related to length of follow-up (P < .05). Satisfaction was inversely related to higher VAS score (P < .05) and higher QuickDASH score (P < .001). Pain at work correlated with lower satisfaction level (P < .05). There was no correlation between patient satisfaction and AROM or PROM.
Discussion
The results of this study demonstrate the efficacy of EBP in the treatment of dorsal PIP joint fracture-dislocations. EBP maintained joint dislocation reduction and allowed for early mobilization, which resulted in good ROM, minimal pain, and good functional outcomes. Of note, postoperative patient satisfaction correlated with pain but not with ROM. It is possible that EBP yielded sufficient functional ROM in all patients such that improvement beyond this threshold did not lead to further improvement in satisfaction. Hume and colleagues23 found that mean PIP joint flexion of 60° is needed for activities of daily living. As mean PIP active flexion was 91° (range, 70°-105°) in the present study, it is possible that satisfaction did not correlate with ROM, as all 12 patients achieved active flexion of more than 60°. Despite the lack of correlation between ROM and satisfaction, early PIP joint mobilization is likely a key contributor to positive outcomes because of its significant role in cartilage healing.24
Postoperative ROM in the present study is consistent with that in other reports of patients with PIP joint fracture-dislocations treated with EBP.11,12,22 In a study by Inoue and Tamura,11 14 such patients had mean PIP ROM of 94° at a mean follow-up of 14 months. Viegas22 followed a series of 3 patients for a mean of 7 weeks. At final follow-up, their mean PIP arc of motion was 71°; they lacked 12° of full extension and achieved 83° of flexion. The larger PIP arc of motion (84°) found in the present study may be due to our significantly longer follow-up (35 months). Unlike us, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM. Our finding a lack of correlation may be a result of the significant amount of joint remodeling noted on follow-up radiographs.
Studies of transarticular pinning of PIP joints after dorsal PIP fracture-dislocations have reported outcomes similar to ours.25,26 Newington and colleagues25 evaluated 10 cases of transarticular pinning of the PIP joint and found mean arc of motion of 85° and equal grip strengths between injured and contralateral hands. In a series of 19 patients with PIP fracture-dislocations, Aladin and Davis26 noted similar outcomes of transarticular K-wire fixation and ORIF. In both of their treatment groups, however, there was evidence of PIP joint incongruity and subluxation. Of note, PIP arc motion was lower in their study than in ours.
Recent studies have evaluated unstable PIP fracture-dislocations treated with both EBP and percutaneous reduction and pinning with a second K-wire.13,27 At a mean follow-up of 18 months, Vitale and colleagues13 noted maintenance of concentric fracture reduction, good PIP ROM (mean range, 4°-93°), and low VAS and DASH scores (1.4 and 8, respectively). Waris and Alanen27 noted mean PIP AROM of 83° and low VAS and DASH scores (1 and 4, respectively). The EBP technique used in the present study did not involve percutaneous fracture reduction but achieved equally good ROM and VAS and QuickDASH scores.
Clinical outcomes of EBP of PIP joint fracture-dislocations are also comparable to outcomes of more complex treatment methods.8-10,15-19,21,26,28-33 Dynamic distraction external fixation has led to equally good ROM (mean AROM, 80°-85°15,16) and VAS scores, but with a higher incidence of pin-site infection.14-17 ORIF of the intra-articular middle phalanx fracture has the advantage of obtaining a direct anatomical reduction, but clinical outcomes are similar to those in the present study (mean AROM, 70°; 78% pain-free9), and flexion contractures have been noted.8-10 Furthermore, reduction of the fractured PIP joint articular surface has not been shown to be necessary for good outcomes.16,34 This may be explained in part by PIP joint remodeling, which has been routinely observed on long-term follow-up by the senior authors of the present study. Hemi-hamate autografting and volar plate arthroplasty are other options that have had promising results in the treatment of acute and chronic unstable PIP fracture-dislocations.18-21 However, the postoperative ROM (mean AROM, 61°-85°18,21), VAS scores, and patient satisfaction (91% very satisfied21) of these operations are similar to those of EBP in the present study and may not justify the longer operative times and technical challenges associated with these techniques.
We believe that our study group’s 1 complication, a malunion that was treated with corrective osteotomy, resulted from lack of appreciation of the degree of injury. The teenaged female patient’s index finger PIP joint had a rotational malalignment that was not appreciated before or during surgery. After pinning and after ROM was restored, the index finger was observed crossing over the middle finger with digital flexion. The patient returned to the operating room for corrective osteotomy.
We recommend that surgeons assess alignment carefully, before and during surgery, when considering this technique. Although complications are rare, the technique is not for patients with rotational malalignment; ORIF may be more suitable in these cases. In addition, though EBP may be appropriate for pilon-type injuries, as it allows for early AROM, our procedure of choice for pilon fracture is dynamic external fixation, which in addition to allowing for AROM provides ligamentotaxis. In the event that a large volar articular fragment extends into the middle phalanx diaphysis, we typically proceed with ORIF through a volar shotgun approach. At our institution, injuries lasting more than 3 months are often treated with volar plate arthroplasty or hemi-hamate resurfacing. Finally, we believe that caution should be exercised when using this technique in patients with more than 50% articular involvement. In the present study, though we used this treatment in cases of up to 75% surface involvement, alternative techniques, such as hemi-hamate resurfacing arthroplasty, may provide a better volar bony buttress and limit the risk for recurrent instability. Despite its relative contraindications, our technique has been appropriate for more than 90% of the acute PIP fracture-dislocations we have seen.
This study expands on prior research by demonstrating good function, satisfaction, and pain outcomes of percutaneous EBP in the treatment of unstable dorsal PIP fracture-dislocations. In addition, this study demonstrated that the efficacy of EBP is similar to that of more complex and technically demanding methods of treatment. Our technique has the advantage of simplicity. It obviates the soft-tissue damage required for ORIF and more complex fixation techniques. Furthermore, use of this simple technique may save time and costs and lead to more reproducible outcomes.
One limitation of this study is its small sample size. It is possible that outcomes may have been different with a larger sample. Furthermore, we did not make a direct comparison with other treatment methods. To better determine the optimal treatment method for this fracture type, future studies should prospectively evaluate outcomes for multiple treatment modalities in a randomized fashion.
The proximal interphalangeal (PIP) joint plays a crucial role in hand function, accounting for an estimated 85% of the motion required to grasp an object.1 The anatomy and biomechanics of the PIP joint, however, make it particularly prone to injury.2,3 Dorsal PIP fracture-dislocations represent a subset of PIP injuries that often require surgical intervention.2 The stability of these fracture-dislocations largely depends on the extent of articular involvement of the base of the middle phalanx. Fractures that involve less than 30% of the joint surface typically remain stable after reduction.2,4,5 In cases in which involvement ranges from 30% to 50%, PIP joint stability is more tenuous, and more joint flexion is required to maintain concentric reduction. Fractures that involve more than 50% of the articular surface are unstable and require operative intervention.2,5,6 Fractures that require more than 30° of flexion for reduction maintenance are generally considered unstable and may benefit from surgical intervention.2
The goals of treatment for this injury are to restore a stable, concentrically reduced joint and initiate early joint mobilization to prevent stiffness, pain, recurrent instability, and posttraumatic arthritis.3,7 Numerous surgical interventions for unstable PIP fracture-dislocations have been proposed, including open reduction and internal fixation (ORIF),8-10 extension-block pinning (EBP),11-13 dynamic external fixation,14-17 volar plate arthroplasty,18,19 and hemi-hamate resurfacing arthroplasty.20,21 Many of these techniques can be technically demanding and may require prolonged immobilization. EBP can be performed easily and efficiently and allows for early joint motion.
Extension-block pinning—placing a Kirschner wire (K-wire) into the head of the proximal phalanx at an angle that blocks PIP extension and prevents joint subluxation—was first described by Sugawa and colleagues12 in 1979. In a study by Inoue and Tamura,11 patients treated with EBP had a mean PIP range of motion (ROM) of 94° at a mean follow-up of 14 months. In a series of 3 case reports, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM in patients treated with EBP.
We conducted a study to expand on previous research on pain, function, and satisfaction outcomes in addition to ROM. We hypothesized that percutaneous EBP is an effective treatment for unstable dorsal PIP fracture-dislocations and has efficacy similar to that of more complex and technically demanding methods of treatment.
Materials and Methods
We retrospectively reviewed patient charts to identify candidates for this study. Inclusion criteria were unstable dorsal PIP fracture-dislocations treated with EBP and minimum 4-month follow-up. (Fracture-dislocations were deemed unstable if they involved at least 30% of the articular surface or required more than 30° of flexion for reduction maintenance.) Exclusion criteria were open injury, neurovascular or tendon injury, or any prior injury to the PIP joint.
Twelve patients (5 females, 7 males) treated over a 4-year period (2002–2006) met the inclusion criteria. Mean age was 30 years (range, 15-64 years). Each surgery was performed by Dr. Hagberg or Dr. Balk. Half the cases involved the dominant hand. Two small fingers, 4 ring fingers, 2 long fingers, and 4 index fingers were injured. The injuries were sustained in an all-terrain vehicle accident (n = 1), in falls (n = 2), while swimming (n = 1), or while playing softball (n = 3), football (n = 4), or soccer (n = 1). Mean time from injury to surgery was 7.5 days (range, 4-27 days). Extent of articular surface involvement of the base of the fractured middle phalanx was calculated using preoperatively obtained lateral radiographs.
Surgical intervention was performed in a reproducible fashion. All patients were treated with closed reduction of the PIP joint under fluoroscopic guidance. Before pinning, joint stability was assessed fluoroscopically both at rest and through an arc of motion. A single smooth 0.045-in K-wire was then inserted percutaneously into the distal and dorsal aspects of the proximal phalanx in retrograde fashion (Figure 1). During wire insertion, the distal interphalangeal joint was flexed to relax the intrinsic mechanism, and the central slip tendon was pierced just proximal to its insertion. We have not noted significant adhesion formation about the central slip with this technique, likely because of limited tendon excursion in this location. Stable joint reduction was confirmed with fluoroscopy. No attempt was made to reduce the intra-articular fracture at the base of the middle phalanx.
A therapy program was initiated 2 to 9 days after surgery. At the first postoperative visit, patients were allowed to perform active ROM (AROM) with the pin in place (Figure 1). K-wires were removed a mean of 25 days (range, 17-31 days) after surgery. A static dorsal block splint was then applied, and patients were encouraged to remove it several times per day for AROM between 20° and full flexion until 6 weeks after surgery. At that time, formal occupational therapy was commenced for another 6 weeks. If there was residual flexion contracture of the PIP joint, dynamic extension splinting was initiated after fracture consolidation.
Mean follow-up was 35.5 months (range, 4-94 months). Postoperative anteroposterior and lateral radiographs were used to evaluate maintenance of joint congruity, fracture union, remodeling, and evidence of degenerative changes. At final follow-up, grip strength of injured and contralateral hands was measured with a dynamometer (Jamar; Patterson Medical, Warrenville, Illinois). AROM and passive ROM (PROM) of the PIP joint was documented at follow-up visits. In addition, patients rated their pain on a 0-to-10 visual analog scale (VAS), with 0 representing no pain and 10 representing excruciating pain. Patients also completed a questionnaire assessing satisfaction with surgical outcome. Physical function and disability were assessed with the Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH) questionnaire. Any complications, including the need for further surgeries, were documented. Pearson correlation coefficients and Student t tests (with significance set at P < .05) were used to compare outcomes.
Results
Radiographic reduction of joint dislocation was achieved and maintained in 11 of the 12 patients at a mean follow-up of 35.5 months (range, 4-94 months). Extent of joint surface involvement, based on preoperative lateral radiographs, averaged 43% (range, 25%-75%). Although no direct articular reduction was performed, remodeling of the joint surface was consistently noted at follow-up (Figure 2). Mild radiographic degenerative changes were noted at final follow-up in 4 patients, and moderate changes were noted in 1 patient. Radiographic union was achieved in all cases, and no pin-tract infections were noted.
Mean AROM of the PIP joint at final follow-up was 84° (range, 50°-110°), with patients lacking a mean of 7° of full extension and achieving mean flexion of 91°. Mean PROM was 93° (range, 75°-110°). There was no correlation between extent of articular surface involvement and ROM. Furthermore, no correlation was found between time from injury to surgery and ROM. Patients regained full grip strength in the operative hand. At final follow-up, mean grip strength was 79.4 pounds in the operative hand and 79.6 pounds in the contralateral hand, demonstrating equal grip strengths bilaterally.
Patients overall had very low levels of pain; mean VAS score was 0.64 (range, 0-3). Mean QuickDASH score was 5.7 (range, 0-30), suggesting minimal functional impairment. One patient developed a malunion of the middle phalanx fracture resulting in a rotational deformity and required corrective osteotomy. This patient’s VAS score (3) and QuickDASH score (30) were significantly higher than those of the other patients in the study. No other complications were noted by final follow-up.
A higher level of patient satisfaction was found to be directly related to length of follow-up (P < .05). Satisfaction was inversely related to higher VAS score (P < .05) and higher QuickDASH score (P < .001). Pain at work correlated with lower satisfaction level (P < .05). There was no correlation between patient satisfaction and AROM or PROM.
Discussion
The results of this study demonstrate the efficacy of EBP in the treatment of dorsal PIP joint fracture-dislocations. EBP maintained joint dislocation reduction and allowed for early mobilization, which resulted in good ROM, minimal pain, and good functional outcomes. Of note, postoperative patient satisfaction correlated with pain but not with ROM. It is possible that EBP yielded sufficient functional ROM in all patients such that improvement beyond this threshold did not lead to further improvement in satisfaction. Hume and colleagues23 found that mean PIP joint flexion of 60° is needed for activities of daily living. As mean PIP active flexion was 91° (range, 70°-105°) in the present study, it is possible that satisfaction did not correlate with ROM, as all 12 patients achieved active flexion of more than 60°. Despite the lack of correlation between ROM and satisfaction, early PIP joint mobilization is likely a key contributor to positive outcomes because of its significant role in cartilage healing.24
Postoperative ROM in the present study is consistent with that in other reports of patients with PIP joint fracture-dislocations treated with EBP.11,12,22 In a study by Inoue and Tamura,11 14 such patients had mean PIP ROM of 94° at a mean follow-up of 14 months. Viegas22 followed a series of 3 patients for a mean of 7 weeks. At final follow-up, their mean PIP arc of motion was 71°; they lacked 12° of full extension and achieved 83° of flexion. The larger PIP arc of motion (84°) found in the present study may be due to our significantly longer follow-up (35 months). Unlike us, Viegas22 noted an inverse relationship between extent of articular surface involvement and postoperative ROM. Our finding a lack of correlation may be a result of the significant amount of joint remodeling noted on follow-up radiographs.
Studies of transarticular pinning of PIP joints after dorsal PIP fracture-dislocations have reported outcomes similar to ours.25,26 Newington and colleagues25 evaluated 10 cases of transarticular pinning of the PIP joint and found mean arc of motion of 85° and equal grip strengths between injured and contralateral hands. In a series of 19 patients with PIP fracture-dislocations, Aladin and Davis26 noted similar outcomes of transarticular K-wire fixation and ORIF. In both of their treatment groups, however, there was evidence of PIP joint incongruity and subluxation. Of note, PIP arc motion was lower in their study than in ours.
Recent studies have evaluated unstable PIP fracture-dislocations treated with both EBP and percutaneous reduction and pinning with a second K-wire.13,27 At a mean follow-up of 18 months, Vitale and colleagues13 noted maintenance of concentric fracture reduction, good PIP ROM (mean range, 4°-93°), and low VAS and DASH scores (1.4 and 8, respectively). Waris and Alanen27 noted mean PIP AROM of 83° and low VAS and DASH scores (1 and 4, respectively). The EBP technique used in the present study did not involve percutaneous fracture reduction but achieved equally good ROM and VAS and QuickDASH scores.
Clinical outcomes of EBP of PIP joint fracture-dislocations are also comparable to outcomes of more complex treatment methods.8-10,15-19,21,26,28-33 Dynamic distraction external fixation has led to equally good ROM (mean AROM, 80°-85°15,16) and VAS scores, but with a higher incidence of pin-site infection.14-17 ORIF of the intra-articular middle phalanx fracture has the advantage of obtaining a direct anatomical reduction, but clinical outcomes are similar to those in the present study (mean AROM, 70°; 78% pain-free9), and flexion contractures have been noted.8-10 Furthermore, reduction of the fractured PIP joint articular surface has not been shown to be necessary for good outcomes.16,34 This may be explained in part by PIP joint remodeling, which has been routinely observed on long-term follow-up by the senior authors of the present study. Hemi-hamate autografting and volar plate arthroplasty are other options that have had promising results in the treatment of acute and chronic unstable PIP fracture-dislocations.18-21 However, the postoperative ROM (mean AROM, 61°-85°18,21), VAS scores, and patient satisfaction (91% very satisfied21) of these operations are similar to those of EBP in the present study and may not justify the longer operative times and technical challenges associated with these techniques.
We believe that our study group’s 1 complication, a malunion that was treated with corrective osteotomy, resulted from lack of appreciation of the degree of injury. The teenaged female patient’s index finger PIP joint had a rotational malalignment that was not appreciated before or during surgery. After pinning and after ROM was restored, the index finger was observed crossing over the middle finger with digital flexion. The patient returned to the operating room for corrective osteotomy.
We recommend that surgeons assess alignment carefully, before and during surgery, when considering this technique. Although complications are rare, the technique is not for patients with rotational malalignment; ORIF may be more suitable in these cases. In addition, though EBP may be appropriate for pilon-type injuries, as it allows for early AROM, our procedure of choice for pilon fracture is dynamic external fixation, which in addition to allowing for AROM provides ligamentotaxis. In the event that a large volar articular fragment extends into the middle phalanx diaphysis, we typically proceed with ORIF through a volar shotgun approach. At our institution, injuries lasting more than 3 months are often treated with volar plate arthroplasty or hemi-hamate resurfacing. Finally, we believe that caution should be exercised when using this technique in patients with more than 50% articular involvement. In the present study, though we used this treatment in cases of up to 75% surface involvement, alternative techniques, such as hemi-hamate resurfacing arthroplasty, may provide a better volar bony buttress and limit the risk for recurrent instability. Despite its relative contraindications, our technique has been appropriate for more than 90% of the acute PIP fracture-dislocations we have seen.
This study expands on prior research by demonstrating good function, satisfaction, and pain outcomes of percutaneous EBP in the treatment of unstable dorsal PIP fracture-dislocations. In addition, this study demonstrated that the efficacy of EBP is similar to that of more complex and technically demanding methods of treatment. Our technique has the advantage of simplicity. It obviates the soft-tissue damage required for ORIF and more complex fixation techniques. Furthermore, use of this simple technique may save time and costs and lead to more reproducible outcomes.
One limitation of this study is its small sample size. It is possible that outcomes may have been different with a larger sample. Furthermore, we did not make a direct comparison with other treatment methods. To better determine the optimal treatment method for this fracture type, future studies should prospectively evaluate outcomes for multiple treatment modalities in a randomized fashion.
1. Leibovic SJ, Bowers WH. Anatomy of the proximal interphalangeal joint. Hand Clin. 1994;10(2):169-178.
2. Kiefhaber TR, Stern PJ. Fracture dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1998;23(3):368-380.
3. Ng CY, Oliver CW. Fractures of the proximal interphalangeal joints of the fingers. J Bone Joint Surg Br. 2009;91(6):705-712.
4. Isani A. Small joint injuries requiring surgical treatment. Orthop Clin North Am. 1986;17(3):407-419.
5. McElfresh EC, Dobyns JH, O’Brien ET. Management of fracture-dislocation of the proximal interphalangeal joints by extension-block splinting. J Bone Joint Surg Am. 1972;54(8):1705-1711.
6. Hastings H 2nd, Carroll C 4th. Treatment of closed articular fractures of the metacarpophalangeal and proximal interphalangeal joints. Hand Clin. 1988;4(3):503-527.
7. O’Rourke SK, Gaur S, Barton NJ. Long-term outcome of articular fractures of the phalanges: an eleven year follow up. J Hand Surg Br. 1989;14(2):183-193.
8. Grant I, Berger AC, Tham SK. Internal fixation of unstable fracture dislocations of the proximal interphalangeal joint. J Hand Surg Br. 2005;30(5):492-498.
9. Hamilton SC, Stern PJ, Fassler PR, Kiefhaber TR. Mini-screw fixation for the treatment of proximal interphalangeal joint dorsal fracture-dislocations. J Hand Surg Am. 2006;31(8):1349-1354.
10. Lee JY, Teoh LC. Dorsal fracture dislocations of the proximal interphalangeal joint treated by open reduction and interfragmentary screw fixation: indications, approaches and results. J Hand Surg Br. 2006;31(2):138-146.
11. Inoue G, Tamura Y. Treatment of fracture-dislocation of the proximal interphalangeal joint using extension-block Kirschner wire. Ann Chir Main Memb Super. 1991;10(6):564-568.
12. Sugawa I, Otani K, Kobayashi A. Treatment of fracture dislocation PIP-joint by Kirschner wire extension block method. Cent Jpn J Orthop Traumat. 1979;22:1409-1412.
13. Vitale MA, White NJ, Strauch RJ. A percutaneous technique to treat unstable dorsal fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2011;36(9):1453-1459.
14. Badia A, Riano F, Ravikoff J, Khouri R, Gonzalez-Hernandez E, Orbay JL. Dynamic intradigital external fixation for proximal interphalangeal joint fracture dislocations. J Hand Surg Am. 2005;30(1):154-160.
15. Ellis SJ, Cheng R, Prokopis P, et al. Treatment of proximal interphalangeal dorsal fracture-dislocation injuries with dynamic external fixation: a pins and rubber band system. J Hand Surg Am. 2007;32(8):1242-1250.
16. Morgan JP, Gordon DA, Klug MS, Perry PE, Barre PS. Dynamic digital traction for unstable comminuted intra-articular fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1995;20(4):565-573.
17. Ruland RT, Hogan CJ, Cannon DL, Slade JF. Use of dynamic distraction external fixation for unstable fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2008;33(1):19-25.
18. Dionysian E, Eaton RG. The long-term outcome of volar plate arthroplasty of the proximal interphalangeal joint. J Hand Surg Am. 2000;25(3):429-437.
19. Durham-Smith G, McCarten GM. Volar plate arthroplasty for closed proximal interphalangeal joint injuries. J Hand Surg Br. 1992;17(4):422-428.
20. Calfee RP, Kiefhaber TR, Sommerkamp TG, Stern PJ. Hemi-hamate arthroplasty provides functional reconstruction of acute and chronic proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2009;34(7):1232-1241.
21. Williams RM, Kiefhaber TR, Sommerkamp TG, Stern PJ. Treatment of unstable dorsal proximal interphalangeal fracture/dislocations using a hemi-hamate autograft. J Hand Surg Am. 2003;28(5):856-865.
22. Viegas SF. Extension block pinning for proximal interphalangeal joint fracture dislocations: preliminary report of a new technique. J Hand Surg Am. 1992;17(5):896-901.
23. Hume MC, Gellman H, McKellop H, Brumfield RH Jr. Functional range of motion of the joints of the hand. J Hand Surg Am. 1990;15(2):240-243.
24. Salter RB. The physiologic basis of continuous passive motion for articular cartilage healing and regeneration. Hand Clin. 1994;10(2):211-220.
25. Newington DP, Davis TR, Barton NJ. The treatment of dorsal fracture-dislocation of the proximal interphalangeal joint by closed reduction and Kirschner wire fixation: a 16-year follow up. J Hand Surg Br. 2001;26(6):537-540.
26. Aladin A, Davis TR. Dorsal fracture-dislocation of the proximal interphalangeal joint: a comparative study of percutaneous Kirschner wire fixation versus open reduction and internal fixation. J Hand Surg Br. 2005;30(2):120-128.
27. Waris E, Alanen V. Percutaneous, intramedullary fracture reduction and extension block pinning for dorsal proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2010;35(12):2046-2052.
28. Bain GI, Mehta JA, Heptinstall RJ, Bria M. Dynamic external fixation for injuries of the proximal interphalangeal joint. J Bone Joint Surg Br. 1998;80(6):1014-1019.
29. Eaton RG, Malerich MM. Volar plate arthroplasty of the proximal interphalangeal joint: a review of ten years’ experience. J Hand Surg Am. 1980;5(3):260-268.
30. Green A, Smith J, Redding M, Akelman E. Acute open reduction and rigid internal fixation of proximal interphalangeal joint fracture dislocation. J Hand Surg Am. 1992;17(3):512-517.
31. Inanami H, Ninomiya S, Okutsu I, Tarui T. Dynamic external finger fixator for fracture dislocation of the proximal interphalangeal joint. J Hand Surg Am. 1993;18(1):160-164.
32. Suzuki Y, Matsunaga T, Sato S, Yokoi T. The pins and rubbers traction system for treatment of comminuted intraarticular fractures and fracture-dislocations in the hand. J Hand Surg Br. 1994;19(1):98-107.
33. Weiss AP. Cerclage fixation for fracture dislocation of the proximal interphalangeal joint. Clin Orthop. 1996;(327):21-28.
34. Agee JM. Unstable fracture dislocations of the proximal interphalangeal joint. Treatment with the force couple splint. Clin Orthop. 1987;(214):101-112.
1. Leibovic SJ, Bowers WH. Anatomy of the proximal interphalangeal joint. Hand Clin. 1994;10(2):169-178.
2. Kiefhaber TR, Stern PJ. Fracture dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1998;23(3):368-380.
3. Ng CY, Oliver CW. Fractures of the proximal interphalangeal joints of the fingers. J Bone Joint Surg Br. 2009;91(6):705-712.
4. Isani A. Small joint injuries requiring surgical treatment. Orthop Clin North Am. 1986;17(3):407-419.
5. McElfresh EC, Dobyns JH, O’Brien ET. Management of fracture-dislocation of the proximal interphalangeal joints by extension-block splinting. J Bone Joint Surg Am. 1972;54(8):1705-1711.
6. Hastings H 2nd, Carroll C 4th. Treatment of closed articular fractures of the metacarpophalangeal and proximal interphalangeal joints. Hand Clin. 1988;4(3):503-527.
7. O’Rourke SK, Gaur S, Barton NJ. Long-term outcome of articular fractures of the phalanges: an eleven year follow up. J Hand Surg Br. 1989;14(2):183-193.
8. Grant I, Berger AC, Tham SK. Internal fixation of unstable fracture dislocations of the proximal interphalangeal joint. J Hand Surg Br. 2005;30(5):492-498.
9. Hamilton SC, Stern PJ, Fassler PR, Kiefhaber TR. Mini-screw fixation for the treatment of proximal interphalangeal joint dorsal fracture-dislocations. J Hand Surg Am. 2006;31(8):1349-1354.
10. Lee JY, Teoh LC. Dorsal fracture dislocations of the proximal interphalangeal joint treated by open reduction and interfragmentary screw fixation: indications, approaches and results. J Hand Surg Br. 2006;31(2):138-146.
11. Inoue G, Tamura Y. Treatment of fracture-dislocation of the proximal interphalangeal joint using extension-block Kirschner wire. Ann Chir Main Memb Super. 1991;10(6):564-568.
12. Sugawa I, Otani K, Kobayashi A. Treatment of fracture dislocation PIP-joint by Kirschner wire extension block method. Cent Jpn J Orthop Traumat. 1979;22:1409-1412.
13. Vitale MA, White NJ, Strauch RJ. A percutaneous technique to treat unstable dorsal fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2011;36(9):1453-1459.
14. Badia A, Riano F, Ravikoff J, Khouri R, Gonzalez-Hernandez E, Orbay JL. Dynamic intradigital external fixation for proximal interphalangeal joint fracture dislocations. J Hand Surg Am. 2005;30(1):154-160.
15. Ellis SJ, Cheng R, Prokopis P, et al. Treatment of proximal interphalangeal dorsal fracture-dislocation injuries with dynamic external fixation: a pins and rubber band system. J Hand Surg Am. 2007;32(8):1242-1250.
16. Morgan JP, Gordon DA, Klug MS, Perry PE, Barre PS. Dynamic digital traction for unstable comminuted intra-articular fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 1995;20(4):565-573.
17. Ruland RT, Hogan CJ, Cannon DL, Slade JF. Use of dynamic distraction external fixation for unstable fracture-dislocations of the proximal interphalangeal joint. J Hand Surg Am. 2008;33(1):19-25.
18. Dionysian E, Eaton RG. The long-term outcome of volar plate arthroplasty of the proximal interphalangeal joint. J Hand Surg Am. 2000;25(3):429-437.
19. Durham-Smith G, McCarten GM. Volar plate arthroplasty for closed proximal interphalangeal joint injuries. J Hand Surg Br. 1992;17(4):422-428.
20. Calfee RP, Kiefhaber TR, Sommerkamp TG, Stern PJ. Hemi-hamate arthroplasty provides functional reconstruction of acute and chronic proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2009;34(7):1232-1241.
21. Williams RM, Kiefhaber TR, Sommerkamp TG, Stern PJ. Treatment of unstable dorsal proximal interphalangeal fracture/dislocations using a hemi-hamate autograft. J Hand Surg Am. 2003;28(5):856-865.
22. Viegas SF. Extension block pinning for proximal interphalangeal joint fracture dislocations: preliminary report of a new technique. J Hand Surg Am. 1992;17(5):896-901.
23. Hume MC, Gellman H, McKellop H, Brumfield RH Jr. Functional range of motion of the joints of the hand. J Hand Surg Am. 1990;15(2):240-243.
24. Salter RB. The physiologic basis of continuous passive motion for articular cartilage healing and regeneration. Hand Clin. 1994;10(2):211-220.
25. Newington DP, Davis TR, Barton NJ. The treatment of dorsal fracture-dislocation of the proximal interphalangeal joint by closed reduction and Kirschner wire fixation: a 16-year follow up. J Hand Surg Br. 2001;26(6):537-540.
26. Aladin A, Davis TR. Dorsal fracture-dislocation of the proximal interphalangeal joint: a comparative study of percutaneous Kirschner wire fixation versus open reduction and internal fixation. J Hand Surg Br. 2005;30(2):120-128.
27. Waris E, Alanen V. Percutaneous, intramedullary fracture reduction and extension block pinning for dorsal proximal interphalangeal fracture-dislocations. J Hand Surg Am. 2010;35(12):2046-2052.
28. Bain GI, Mehta JA, Heptinstall RJ, Bria M. Dynamic external fixation for injuries of the proximal interphalangeal joint. J Bone Joint Surg Br. 1998;80(6):1014-1019.
29. Eaton RG, Malerich MM. Volar plate arthroplasty of the proximal interphalangeal joint: a review of ten years’ experience. J Hand Surg Am. 1980;5(3):260-268.
30. Green A, Smith J, Redding M, Akelman E. Acute open reduction and rigid internal fixation of proximal interphalangeal joint fracture dislocation. J Hand Surg Am. 1992;17(3):512-517.
31. Inanami H, Ninomiya S, Okutsu I, Tarui T. Dynamic external finger fixator for fracture dislocation of the proximal interphalangeal joint. J Hand Surg Am. 1993;18(1):160-164.
32. Suzuki Y, Matsunaga T, Sato S, Yokoi T. The pins and rubbers traction system for treatment of comminuted intraarticular fractures and fracture-dislocations in the hand. J Hand Surg Br. 1994;19(1):98-107.
33. Weiss AP. Cerclage fixation for fracture dislocation of the proximal interphalangeal joint. Clin Orthop. 1996;(327):21-28.
34. Agee JM. Unstable fracture dislocations of the proximal interphalangeal joint. Treatment with the force couple splint. Clin Orthop. 1987;(214):101-112.