User login
Genotype-guided warfarin dosing reduced adverse events in arthroplasty patients
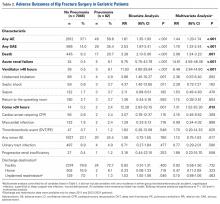
The difference in the composite endpoint (major bleeding within 30 days, international normalized ratio [INR] of 4 or greater within 30 days, venous thromboembolism within 60 days, or death within 30 days) in the Genetic Informatics Trial of Warfarin to Prevent Deep Vein Thrombosis (GIFT) trial was mainly driven by a significant difference in episodes of elevated INR, reported Brian F. Gage, MD, and his colleagues (JAMA 2017;318[12]:1115-1124. doi: 10.1001/jama.2017.11469).
A total of 1,597 patients completed the trial. Of 808 patients in the genotype-guided group, 10.8% met one of the endpoints. Of 789 in the clinically guided warfarin dosing group, 14.7% met at least 1 of the endpoints. There were no deaths in the study.
“Widespread use of genotype-guided dosing will depend on reimbursement, regulations, and logistics. Although several commercial platforms for warfarin-related genes have been approved by the Food and Drug Administration and the European Medicines Agency, routine genotyping is not yet recommended,” wrote Dr. Gage of Washington University, St. Louis, and his coauthors.
The Centers for Medicare and Medicaid Services used its Coverage with Evidence Development program to fund genotyping in this trial and will review the results to determine future coverage, the researchers added.
In GIFT, patients were randomized to an 11-day regimen of warfarin guided either by a clinical algorithm or by their individual genotype. The team tested for four polymorphisms known to affect warfarin metabolism: VKORC1-1639G>A, CYP2C9*2, CYP2C9*3, and CYP4F2 V433M. The treatment goal was an INR of 1.8-2. After 11 days, physicians could administer warfarin according to their own judgment.
The absolute difference of 3.9% in the composite endpoint was largely driven by a 2.8% absolute difference in the rate of an INR of 4 or greater. The rate difference between the two groups was 0.8% for major bleeding, and 0.7% for VTE.
About 41% of the cohort was considered to be at high risk of bleeding complications, and this group accrued the highest benefit from genotype-based dosing. Among them, the composite endpoint was 11.5% compared with 15.2% in the clinical algorithm group – an absolute difference of 3.76%.
The benefit was consistent among black patients, and those with CYP2C9.
By day 90, one VTE had occurred in each group. An intracranial hemorrhage occurred in one patient in the clinically guided group, 2 months after stopping warfarin.
The clinical benefit of genotype-based dosing influenced 90-day outcomes as well, with the composite endpoint occurring in 11% of the genotype group and 15% of the clinically guided group (absolute difference 3.9%).
Among the 1,588 patients who had their percentage of time in the therapeutic range (PTTR) calculated, genotyping improved PTTR time by 3.4% overall. The effect was especially strong from days 4 to 14, when it improved PTTR by 5.7% relative to clinical guidance.
Three other studies have examined the effect of a genotype-based warfarin dosing regimen, Dr. Gage and his coauthors noted: Two found no benefit, and a third found that such guidance improved INR control. GIFT has several advantages over those trials, which the authors said lend credence to its results.
“Compared with previous studies, this trial was larger, used genotype-guided dosing for a longer duration, and incorporated more genes into the dosing algorithm …The longer period of genotype-guided dosing likely prevented cases of supratherapeutic INR that were common in these trials,” they wrote.
Dr. Gage reported no financial disclosures, but several coauthors reported ties with pharmaceutical and imaging companies.
Warfarin is the most commonly used anticoagulant in the world, and a significant cause of emergency department visits and hospitalizations, especially among older patients. Walking the fine line between dosing too little and too much is not an easy task – especially since warfarin response is influenced by diet, comorbidities, interactions with other medications and – as studies over the last 20 years have confirmed – many genetic variants.
Also, the practicality of genotyping every patient who needs anticoagulation therapy must be questioned. Based on the results of GIFT, 26 patients would need to be genotyped to prevent one event, typically an INR of 4 or greater. Although the cost of genotyping continues to decline, health insurers and publicly funded health systems have not yet been convinced that genotype-guided warfarin prescribing is a cost-effective strategy.
The benefits of genotyping would likely be less in patients with atrial fibrillation, for example, as they run a lower risk of VTE than do arthroplasty patients. The GIFT surgeries were all elective, so there was plenty of time to get back genotyping results before starting warfarin. That is a luxury not afforded to many patients in need of anticoagulation.
It’s possible, however, that the benefits of genotyping might be larger in the real world. GIFT was conducted at academic medical centers and used a clinical dosing algorithm as comparator. As a result, adverse event rates were likely lower in the comparison group than would be expected in other clinical settings with less-intense INR monitoring or empirically based initiation regimens.
Still, GIFT’s results are gaining global attention. Based on prepublication results of the GIFT trial, the Clinical Pharmacogenetics Implementation Consortium (CPIC), an international research network that develops consensus recommendations about the use of pharmacogenomic test results, recently published guidelines about genotype-guided dosing for warfarin. The group now recommends using genotype-guided warfarin dosing based on CYP2C9*2, CYP2C9*3, and VKORC1 variants for adult patients of non-African ancestry. It also recommends that patients with combinations of high-risk variants would benefit from an alternative anticoagulant strategy, because of likely greater risks of poor INR control and bleeding.
A single pharmacogenomic test covering many common variants relevant to multiple prescribing decisions over time is far more likely to be a cost-effective approach; however, there is no evidence for this proposition. Until then, it might be simpler and less expensive to use clinical dosing algorithms to reduce the risks of anticoagulation.
Jon D. Emery, PhD, is the Herman Professor of Primary Care Cancer Research at the University of Melbourne and Western Health, Melbourne. He made these remarks in an accompanying editorial (JAMA 2017;318;110-2 doi: 10.1001/jama.2017.11465 ).
Warfarin is the most commonly used anticoagulant in the world, and a significant cause of emergency department visits and hospitalizations, especially among older patients. Walking the fine line between dosing too little and too much is not an easy task – especially since warfarin response is influenced by diet, comorbidities, interactions with other medications and – as studies over the last 20 years have confirmed – many genetic variants.
Also, the practicality of genotyping every patient who needs anticoagulation therapy must be questioned. Based on the results of GIFT, 26 patients would need to be genotyped to prevent one event, typically an INR of 4 or greater. Although the cost of genotyping continues to decline, health insurers and publicly funded health systems have not yet been convinced that genotype-guided warfarin prescribing is a cost-effective strategy.
The benefits of genotyping would likely be less in patients with atrial fibrillation, for example, as they run a lower risk of VTE than do arthroplasty patients. The GIFT surgeries were all elective, so there was plenty of time to get back genotyping results before starting warfarin. That is a luxury not afforded to many patients in need of anticoagulation.
It’s possible, however, that the benefits of genotyping might be larger in the real world. GIFT was conducted at academic medical centers and used a clinical dosing algorithm as comparator. As a result, adverse event rates were likely lower in the comparison group than would be expected in other clinical settings with less-intense INR monitoring or empirically based initiation regimens.
Still, GIFT’s results are gaining global attention. Based on prepublication results of the GIFT trial, the Clinical Pharmacogenetics Implementation Consortium (CPIC), an international research network that develops consensus recommendations about the use of pharmacogenomic test results, recently published guidelines about genotype-guided dosing for warfarin. The group now recommends using genotype-guided warfarin dosing based on CYP2C9*2, CYP2C9*3, and VKORC1 variants for adult patients of non-African ancestry. It also recommends that patients with combinations of high-risk variants would benefit from an alternative anticoagulant strategy, because of likely greater risks of poor INR control and bleeding.
A single pharmacogenomic test covering many common variants relevant to multiple prescribing decisions over time is far more likely to be a cost-effective approach; however, there is no evidence for this proposition. Until then, it might be simpler and less expensive to use clinical dosing algorithms to reduce the risks of anticoagulation.
Jon D. Emery, PhD, is the Herman Professor of Primary Care Cancer Research at the University of Melbourne and Western Health, Melbourne. He made these remarks in an accompanying editorial (JAMA 2017;318;110-2 doi: 10.1001/jama.2017.11465 ).
Warfarin is the most commonly used anticoagulant in the world, and a significant cause of emergency department visits and hospitalizations, especially among older patients. Walking the fine line between dosing too little and too much is not an easy task – especially since warfarin response is influenced by diet, comorbidities, interactions with other medications and – as studies over the last 20 years have confirmed – many genetic variants.
Also, the practicality of genotyping every patient who needs anticoagulation therapy must be questioned. Based on the results of GIFT, 26 patients would need to be genotyped to prevent one event, typically an INR of 4 or greater. Although the cost of genotyping continues to decline, health insurers and publicly funded health systems have not yet been convinced that genotype-guided warfarin prescribing is a cost-effective strategy.
The benefits of genotyping would likely be less in patients with atrial fibrillation, for example, as they run a lower risk of VTE than do arthroplasty patients. The GIFT surgeries were all elective, so there was plenty of time to get back genotyping results before starting warfarin. That is a luxury not afforded to many patients in need of anticoagulation.
It’s possible, however, that the benefits of genotyping might be larger in the real world. GIFT was conducted at academic medical centers and used a clinical dosing algorithm as comparator. As a result, adverse event rates were likely lower in the comparison group than would be expected in other clinical settings with less-intense INR monitoring or empirically based initiation regimens.
Still, GIFT’s results are gaining global attention. Based on prepublication results of the GIFT trial, the Clinical Pharmacogenetics Implementation Consortium (CPIC), an international research network that develops consensus recommendations about the use of pharmacogenomic test results, recently published guidelines about genotype-guided dosing for warfarin. The group now recommends using genotype-guided warfarin dosing based on CYP2C9*2, CYP2C9*3, and VKORC1 variants for adult patients of non-African ancestry. It also recommends that patients with combinations of high-risk variants would benefit from an alternative anticoagulant strategy, because of likely greater risks of poor INR control and bleeding.
A single pharmacogenomic test covering many common variants relevant to multiple prescribing decisions over time is far more likely to be a cost-effective approach; however, there is no evidence for this proposition. Until then, it might be simpler and less expensive to use clinical dosing algorithms to reduce the risks of anticoagulation.
Jon D. Emery, PhD, is the Herman Professor of Primary Care Cancer Research at the University of Melbourne and Western Health, Melbourne. He made these remarks in an accompanying editorial (JAMA 2017;318;110-2 doi: 10.1001/jama.2017.11465 ).
The difference in the composite endpoint (major bleeding within 30 days, international normalized ratio [INR] of 4 or greater within 30 days, venous thromboembolism within 60 days, or death within 30 days) in the Genetic Informatics Trial of Warfarin to Prevent Deep Vein Thrombosis (GIFT) trial was mainly driven by a significant difference in episodes of elevated INR, reported Brian F. Gage, MD, and his colleagues (JAMA 2017;318[12]:1115-1124. doi: 10.1001/jama.2017.11469).
A total of 1,597 patients completed the trial. Of 808 patients in the genotype-guided group, 10.8% met one of the endpoints. Of 789 in the clinically guided warfarin dosing group, 14.7% met at least 1 of the endpoints. There were no deaths in the study.
“Widespread use of genotype-guided dosing will depend on reimbursement, regulations, and logistics. Although several commercial platforms for warfarin-related genes have been approved by the Food and Drug Administration and the European Medicines Agency, routine genotyping is not yet recommended,” wrote Dr. Gage of Washington University, St. Louis, and his coauthors.
The Centers for Medicare and Medicaid Services used its Coverage with Evidence Development program to fund genotyping in this trial and will review the results to determine future coverage, the researchers added.
In GIFT, patients were randomized to an 11-day regimen of warfarin guided either by a clinical algorithm or by their individual genotype. The team tested for four polymorphisms known to affect warfarin metabolism: VKORC1-1639G>A, CYP2C9*2, CYP2C9*3, and CYP4F2 V433M. The treatment goal was an INR of 1.8-2. After 11 days, physicians could administer warfarin according to their own judgment.
The absolute difference of 3.9% in the composite endpoint was largely driven by a 2.8% absolute difference in the rate of an INR of 4 or greater. The rate difference between the two groups was 0.8% for major bleeding, and 0.7% for VTE.
About 41% of the cohort was considered to be at high risk of bleeding complications, and this group accrued the highest benefit from genotype-based dosing. Among them, the composite endpoint was 11.5% compared with 15.2% in the clinical algorithm group – an absolute difference of 3.76%.
The benefit was consistent among black patients, and those with CYP2C9.
By day 90, one VTE had occurred in each group. An intracranial hemorrhage occurred in one patient in the clinically guided group, 2 months after stopping warfarin.
The clinical benefit of genotype-based dosing influenced 90-day outcomes as well, with the composite endpoint occurring in 11% of the genotype group and 15% of the clinically guided group (absolute difference 3.9%).
Among the 1,588 patients who had their percentage of time in the therapeutic range (PTTR) calculated, genotyping improved PTTR time by 3.4% overall. The effect was especially strong from days 4 to 14, when it improved PTTR by 5.7% relative to clinical guidance.
Three other studies have examined the effect of a genotype-based warfarin dosing regimen, Dr. Gage and his coauthors noted: Two found no benefit, and a third found that such guidance improved INR control. GIFT has several advantages over those trials, which the authors said lend credence to its results.
“Compared with previous studies, this trial was larger, used genotype-guided dosing for a longer duration, and incorporated more genes into the dosing algorithm …The longer period of genotype-guided dosing likely prevented cases of supratherapeutic INR that were common in these trials,” they wrote.
Dr. Gage reported no financial disclosures, but several coauthors reported ties with pharmaceutical and imaging companies.
The difference in the composite endpoint (major bleeding within 30 days, international normalized ratio [INR] of 4 or greater within 30 days, venous thromboembolism within 60 days, or death within 30 days) in the Genetic Informatics Trial of Warfarin to Prevent Deep Vein Thrombosis (GIFT) trial was mainly driven by a significant difference in episodes of elevated INR, reported Brian F. Gage, MD, and his colleagues (JAMA 2017;318[12]:1115-1124. doi: 10.1001/jama.2017.11469).
A total of 1,597 patients completed the trial. Of 808 patients in the genotype-guided group, 10.8% met one of the endpoints. Of 789 in the clinically guided warfarin dosing group, 14.7% met at least 1 of the endpoints. There were no deaths in the study.
“Widespread use of genotype-guided dosing will depend on reimbursement, regulations, and logistics. Although several commercial platforms for warfarin-related genes have been approved by the Food and Drug Administration and the European Medicines Agency, routine genotyping is not yet recommended,” wrote Dr. Gage of Washington University, St. Louis, and his coauthors.
The Centers for Medicare and Medicaid Services used its Coverage with Evidence Development program to fund genotyping in this trial and will review the results to determine future coverage, the researchers added.
In GIFT, patients were randomized to an 11-day regimen of warfarin guided either by a clinical algorithm or by their individual genotype. The team tested for four polymorphisms known to affect warfarin metabolism: VKORC1-1639G>A, CYP2C9*2, CYP2C9*3, and CYP4F2 V433M. The treatment goal was an INR of 1.8-2. After 11 days, physicians could administer warfarin according to their own judgment.
The absolute difference of 3.9% in the composite endpoint was largely driven by a 2.8% absolute difference in the rate of an INR of 4 or greater. The rate difference between the two groups was 0.8% for major bleeding, and 0.7% for VTE.
About 41% of the cohort was considered to be at high risk of bleeding complications, and this group accrued the highest benefit from genotype-based dosing. Among them, the composite endpoint was 11.5% compared with 15.2% in the clinical algorithm group – an absolute difference of 3.76%.
The benefit was consistent among black patients, and those with CYP2C9.
By day 90, one VTE had occurred in each group. An intracranial hemorrhage occurred in one patient in the clinically guided group, 2 months after stopping warfarin.
The clinical benefit of genotype-based dosing influenced 90-day outcomes as well, with the composite endpoint occurring in 11% of the genotype group and 15% of the clinically guided group (absolute difference 3.9%).
Among the 1,588 patients who had their percentage of time in the therapeutic range (PTTR) calculated, genotyping improved PTTR time by 3.4% overall. The effect was especially strong from days 4 to 14, when it improved PTTR by 5.7% relative to clinical guidance.
Three other studies have examined the effect of a genotype-based warfarin dosing regimen, Dr. Gage and his coauthors noted: Two found no benefit, and a third found that such guidance improved INR control. GIFT has several advantages over those trials, which the authors said lend credence to its results.
“Compared with previous studies, this trial was larger, used genotype-guided dosing for a longer duration, and incorporated more genes into the dosing algorithm …The longer period of genotype-guided dosing likely prevented cases of supratherapeutic INR that were common in these trials,” they wrote.
Dr. Gage reported no financial disclosures, but several coauthors reported ties with pharmaceutical and imaging companies.
FROM JAMA
Key clinical point:
Major finding: Genotype-guided dosing reduced adverse events – primarily elevated INRs – by almost 4% compared to clinically based warfarin dosing.
Data source: A randomized trial comprising 1,650 elderly patients undergoing elective knee or hip arthroplasty.
Disclosures: Dr. Gage had no financial disclosures, but several of his coauthors noted relationships with pharmaceutical and imaging companies.
Use of Intravenous Tranexamic Acid Improves Early Ambulation After Total Knee Arthroplasty and Anterior and Posterior Total Hip Arthroplasty
Take-Home Points
- IV-TXA significantly reduces intraoperative blood loss following TJA.
- Early mobilization correlates with reduced incidence of postoperative complications.
- IV-TXA minimizes postoperative anemia, facilitating improved early ambulation following TJA.
- IV-TXA significantly reduces the need for postoperative transfusions.
- IV-TXA is safe to use with no adverse events noted.
By the year 2020, use of primary total knee arthroplasty (TKA) in the United States will increase an estimated 110%, to 1.375 million procedures annually, and use of primary total hip arthroplasty (THA) will increase an estimated 75%, to more than 500,000 procedures.1 Minimizing perioperative blood loss and improving early postoperative ambulation both correlate with reduced postoperative morbidity, allowing patients to return to their daily lives expeditiously.
Tranexamic acid (TXA), a fibrinolytic inhibitor, competitively blocks lysine receptor binding sites of plasminogen, sustaining and stabilizing the fibrin architecture.2 TXA must be present to occupy binding sites before plasminogen binds to fibrin, validating the need for preoperative administration so the drug is available early in the fibrinolytic cascade.3 Intravenous (IV) TXA diffuses rapidly into joint fluid and the synovial membrane.4 Drug concentration and elimination half-life in joint fluid are equivalent to those in serum. Elimination of TXA occurs by glomerular filtration, with about 30% of a 10-mg/kg dose removed in 1 hour, 55% over the first 3 hours, and 90% within 24 hours of IV administration.5
The efficacy of IV-TXA in minimizing total joint arthroplasty (TJA) perioperative blood loss has been proved in small studies and meta-analyses.6-9 TXA-induced blood conservation decreases or eliminates the need for postoperative transfusion, which can impede valuable, early ambulation.10 In addition, the positive clinical safety profile of TXA supports routine use of TXA in TJA.6,11-15
The benefits of early ambulation after TJA are well established. Getting patients to walk on the day of surgery is a key part of effective and rapid postoperative rehabilitation. Early mobilization correlates with reduced incidence of venous thrombosis and postoperative complications.16 In contrast to bed rest, sitting and standing promotes oxygen saturation, which improves tissue healing and minimizes adverse pulmonary events. Oxygen saturation also preserves muscle strength and blood flow, reducing the risk of venous thromboembolism and ulcers. Muscle strength must be maintained so normal gait can be regained.17 Compared with rehabilitation initiated 48 to 72 hours after TKA, rehabilitation initiated within 24 hours reduced the number of sessions needed to achieve independence and normal gait; in addition, early mobilization improved patient reports of pain after surgery.18 An evaluation of Denmark registry data revealed that mobilization to walking and use of crutches or canes was achieved earlier when ambulation was initiated on day of surgery.19 Finally, mobilization on day of surgery and during the immediate postoperative period improved long-term quality of life after TJA.20
We conducted a retrospective cohort study to determine if use of IV-TXA improves early ambulation and reduces blood loss after TKA and anterior and posterior THA. We hypothesized that IV-TXA use would reduce postoperative anemia and improve early ambulation and outcomes without producing adverse events during the immediate postoperative period. TXA reduces bleeding, and reduced incidence of hemarthrosis, wound swelling, and anemia could facilitate ambulation, reduce complications, and shorten recovery in patients who undergo TJA.
Patients and Methods
In February 2014, this retrospective cohort study received Institutional Review Board approval to compare the safety and efficacy of IV-TXA (vs no TXA) in patients who underwent TKA, anterior THA, and posterior THA.
In March 2012, multidisciplinary protocols were standardized to ensure a uniform hospital course for patients at our institution. All patients underwent preoperative testing and evaluation by a nurse practitioner and an anesthesiologist. In March 2013, IV-TXA became our standard of care. TXA use was contraindicated in patients with thromboembolic disease or with hypersensitivity to TXA. Patients without a contraindication were given two 10-mg/kg IV-TXA doses, each administered over 15 to 30 minutes; the first dose was administered before incision, and the second was infused at case close and/or at least 60 minutes after the first dose. Most TKA patients received regional (femoral) anesthesia and analgesia, and most THA patients received spinal or epidural anesthesia and analgesia. In a small percentage of cases, IV analgesia was patient-controlled, as determined by the pain service. There were no significant differences in anesthesia/analgesia modality between the 2 study groups—patients who received TXA and those who did not. Patients were then transitioned to oral opioids for pain management, unless otherwise contraindicated, and were ambulated 4 hours after end of surgery, unless medically unstable. Hematology and chemistry laboratory values were monitored daily during admission.
Patients underwent physical therapy (PT) after surgery and until hospital discharge. Physical therapists blinded to patients’ intraoperative use or no use of TXA measured ambulation. After initial evaluation on postoperative day 0 (POD-0), patients were ambulated twice daily. The daily ambulation distance used for the study was the larger of the 2 daily PT distances (occasionally, patients were unable to participate fully in both sessions). Patients received either enoxaparin or rivaroxaban for postoperative thromboprophylaxis (the anticoagulant used was based on surgeon preference). Enoxaparin was subcutaneously administered at 30 mg every 12 hours for TKA, 40 mg once daily for THA, 30 mg once daily for calculated creatinine clearance under 30 mL/min, or 40 mg every 12 hours for body mass index (BMI) 40 or above. With enoxaparin, therapy duration was 14 days. Oral rivaroxaban was administered at 10 mg once daily for 12 days for TKA and 35 days for THA unless contraindicated.
The primary outcome variables were ambulation measured on POD-1 and POD-2 and intraoperative blood loss. In addition, hemoglobin and hematocrit were measured on POD-0, POD-1, and POD-2. Ambulation was defined as number of feet walked during postoperative hospitalization. To calculate intraoperative blood loss, the anesthesiologist subtracted any saline irrigation volume from the total volume in the suction canister. Also noted were postoperative transfusions and any diagnosis of postoperative venous thromboembolism—specifically, deep vein thrombosis (DVT) or pulmonary embolism (PE).
Demographic and clinical characteristics of the TXA and no-TXA groups were compared using either 2-sample t test (for continuous variables) or χ2 test (for categorical variables).
The ambulation outcome was log-transformed to meet standard assumptions of Gaussian residuals and equality of variance. Means and 95% confidence intervals (CIs) were calculated on the log scale and were anti-logged so the results could be presented in their original units.
A linear mixed model was used to model intraoperative blood loss as a function of group (TXA, no TXA), procedure (TKA, anterior THA, posterior THA), and potential confounders (age, sex, BMI, operative time).
Linear mixed models for repeated measures were used to compare outcomes (hemoglobin, hematocrit) between groups (TXA, no TXA) and procedures (TKA, anterior THA, posterior THA) and to compare changes in outcomes over time. Group, procedure, and operative time interactions were explored. Potential confounders (age, sex, BMI, operative time) were included in the model as well.
A χ2 test was used to compare the groups (TXA, no TXA) on postoperative blood transfusion (yes, no). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used. Need for transfusion was clinically assessed case by case. Symptomatic anemia (dyspnea on exertion, headaches, tachycardia) was used as the primary indication for transfusion once hemoglobin fell below 8 g/dL or hematocrit below 24%. Number of patients with a postoperative thrombus formation was minimal. Therefore, this outcome was described with summary statistics and was not formally analyzed.
Results
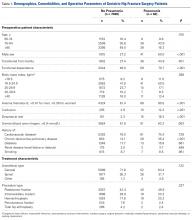
Of the 477 patients who underwent TJAs (275 TKAs, 98 anterior THAs, 104 posterior THAs; all unilateral), 111 did not receive TXA (June 2012-February 2013), and 366 received TXA (March 2013-January 2014). Other than for the addition of IV-TXA, the same standardized protocols instituted in March 2012 continued throughout the study period. The difference in sample size between the TXA and no-TXA groups was not statistically significant and did not influence the outcome measures.
Ambulation
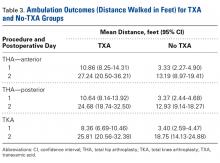
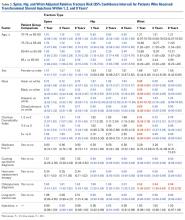
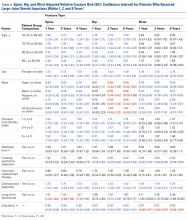
There was a significant (P = .0066) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P < .0001), sex (P < .0001), BMI (P < .0001), and operative time (P = .8308). Regarding TKA, mean ambulation was higher for the TXA group than for the no-TXA group at POD-1 (8.36 vs 3.40 feet; P < .0001) and POD-2 (25.81 vs 18.75 feet; P = .0054). The same was true for anterior THA at POD-1 (10.86 vs 3.33 feet; P < .0001) and POD-2 (27.24 vs 13.19 feet; P < .0001) and posterior THA at POD-1 (10.64 vs 3.37 feet; P < .0001) and POD-2 (24.68 vs 12.93 feet; P = .0002). See Table 3.
Intraoperative Blood Loss
There was a significant 3-way interaction of TXA, procedure (P < .0053), and operative time (P < .0001) after adjusting for age (P < .6136), sex (P = .1147), and BMI (P = .6180). Regarding TKA, mean intraoperative blood loss was significantly lower for the TXA group than for the no-TXA group (241.58 vs 287.81 mL; P = .0004). The same was true for anterior THA (352.91 vs 533.79 mL; P < .0001). Regarding posterior THA, there was no significant difference between the TXA and no-TXA groups (326.00 vs 350.16 mL; P = .3246). See Table 4.
Hemoglobin
There was a significant (P = .0008) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .0174), sex (P < .0001), BMI (P = .0007), and operative time (P = .0002). Regarding TKA, postoperative hemoglobin levels were higher for the TXA group than for the no-TXA group at POD-0 (12.10 vs 11.68 g/dL; P = .0135), POD-1 (11.62 vs 10.67 g/dL; P < .0001), and POD-2 (11.02 vs 10.11 g/dL; P < .0001). The same was true for anterior THA at POD-1 (11.03 vs 10.19 g/dL; P = .0034) and POD-2 (10.57 vs 9.64 g/dL; P = .0009) and posterior THA at POD-2 (11.04 vs 10.16 g/dL; P = .0003). See Table 5.
Hematocrit
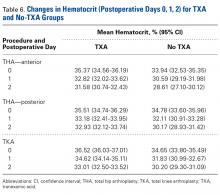
There was a significant (P < .0006) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .1597), sex (P < .0001), BMI (P < .0001), and operative time (P = .0003). Regarding TKA, postoperative hematocrit levels were higher for the TXA group than for the no-TXA group at POD-0 (36.52% vs 34.65%; P < .0001), POD-1 (34.62% vs 31.83%; P < .0001), and POD-2 (33.01% vs 30.20%; P < .0001). The same was true for anterior THA at POD-1 (32.82% vs 30.59%; P = .0037) and POD-2 (31.58% vs 28.61%; P = .0004) and posterior THA at POD-2 (32.93% vs 30.17%; P < .0001). See Table 6.
Postoperative Transfusions
Of the 477 patients, 25 (5.24%) required a postoperative transfusion. Postoperative transfusions were less likely (P < .0001) required in the TXA group (1.64%, 6/366) than in the no-TXA group (17.12%, 19/111). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used, and the different procedures were not evaluated separately.
Deep Vein Thrombosis and Pulmonary Embolism
Of the 477 patients, 2 developed a DVT, and 5 developed a PE. Both DVTs occurred in the TXA group (2/366, 0.55%; 95% CI, 0.07%-1.96%). Of the 5 PEs, 4 occurred in the TXA group (4/366, 1.09%; 95% CI, 0.30%-2.77%), and 1 occurred in the no-TXA group (1/111, 0.90%; 95% CI, 0.02%-4.92%). Given the exceedingly small number of events, no statistical significance was noted between groups.
Discussion
Orthopedic surgeons carefully balance patient expectations, societal needs, and regulatory mandates while providing excellent care and working under payers’ financial restrictions. The Centers for Medicare & Medicaid Services announced that, starting in 2016, TJAs will be reimbursed in total as a single bundled payment, adding to the need to provide optimal care in a fiscally responsible manner.21 Standardized protocols implementing multimodal therapies are pivotal in achieving favorable postoperative outcomes.
Our study results showed that IV-TXA use minimized hemoglobin and hematocrit reductions after TKA, anterior THA, and posterior THA. Postoperative anemia correlates with decreased ambulation ability and performance during the early postoperative period. In general, higher postoperative hemoglobin and hematocrit levels result in improved motor performance and shorter recovery.22 In addition, early ambulation is a validated predictor of favorable TJA outcomes. In our study, for TKA, anterior THA, and posterior THA, ambulation on POD-1 and POD-2 was significantly better for patients who received TXA than for patients who did not.
Transfusion rates were markedly lower for our TXA group than for our no-TXA group (1.64% vs 17.12%), confirming the findings of numerous other studies on outcomes of TJA with TXA.2,3,6-12,14,15 Transfusions impede physical therapy and affect hospitalization costs.
Although potential thrombosis-related adverse events remain an endpoint in studies involving TXA, we found a comparably low incidence of postoperative venous thrombosis in our TXA and no-TXA groups (1.09% and 0.90%, respectively). In addition, no patient in either group developed a postoperative arterial thrombosis.
This is the largest single-center study of TXA use in TKA, anterior THA, and posterior THA. The effect of TXA use on postoperative ambulation was not previously found with TJA.
This study had its limitations. First, it was not prospective, randomized, or double-blinded. However, the physical therapists who mobilized patients and recorded ambulation data were blinded to the study and its hypothesis and followed a standardized protocol for all patients. In addition, intraoperative blood loss was recorded by an anesthesiologist using a standardized protocol, and patients received TXA per orthopedic protocol and surgeon preference, without selection bias. Another limitation was that ambulation data were captured only for POD-1 and POD-2 (most patients were discharged by POD-3). However, a goal of the study was to capture immediate postoperative data in order to determine the efficacy of intraoperative TXA. Subsequent studies can determine if this early benefit leads to long-term clinical outcome improvements.
In reducing blood loss and transfusion rates, intra-articular TXA is as efficacious as IV-TXA.23-25 We anticipate that the improved clinical outcomes found with IV-TXA in our study will be similar with intra-articular TXA, but more study is needed to confirm this hypothesis.
Conclusion
This retrospective cohort study found that use of IV-TXA in TJA improved early ambulation and clinical outcomes (reduced anemia, fewer transfusions) in the initial postoperative period, without producing adverse events.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83(4):596-601.
3. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty. Acta Orthop Scand. 2001;72(5):442-448.
4. Tanaka N, Sakahashi, H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001;83(5):702-705.
5. Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol). 1980;14:41-47.
6. George DA, Sarraf KM, Nwaboku H. Single perioperative dose of tranexamic acid in primary hip and knee arthroplasty. Eur J Orthop Surg Traumatol. 2015;25(1):129-133.
7. Vigna-Taglianti F, Basso L, Rolfo P, et al. Tranexamic acid for reducing blood transfusions in arthroplasty interventions: a cost-effective practice. Eur J Orthop Surg Traumatol. 2014;24(4):545-551.
8. Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003;31(5):529-537.
9. Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829.
10. Sculco PK, Pagnano MW. Perioperative solutions for rapid recovery joint arthroplasty: get ahead and stay ahead. J Arthroplasty. 2015;30(4):518-520.
11. Lozano M, Basora M, Peidro L, et al. Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sang. 2008;95(1):39-44.
12. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783.
13. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement. A systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
14. Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA. Clin Orthop Relat Res. 2011;469(10):2874-2880.
15. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
16. Stowers M, Lemanu DP, Coleman B, Hill AG, Munro JT. Review article: perioperative care in enhanced recovery for total hip and knee arthroplasty. J Orthop Surg (Hong Kong). 2014;22(3):383-392.
17. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation. Acta Orthop. 2008;79(5):624-630.
18. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
19. Husted H, Hansen HC, Holm G, et al. What determines length of stay after total hip and knee arthroplasty? A nationwide study in Denmark. Arch Orthop Trauma Surg. 2010;130(2):263-268.
20. Husted H. Fast-track hip and knee arthroplasty: clinical and organizational aspects. Acta Orthop Suppl. 2012;83(346):1-39.
21. Comprehensive Care for Joint Replacement Model. CMS.gov. https://innovation.cms.gov/initiatives/cjr. Updated October 5, 2017.
22. Wang X, Rintala DH, Garber SL, Henson H. Association of hemoglobin levels, acute hemoglobin decrease, age, and co-morbidities with rehabilitation outcomes after total knee replacement. Am J Phys Med Rehabil. 2005;84(6):451-456.
23. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
Take-Home Points
- IV-TXA significantly reduces intraoperative blood loss following TJA.
- Early mobilization correlates with reduced incidence of postoperative complications.
- IV-TXA minimizes postoperative anemia, facilitating improved early ambulation following TJA.
- IV-TXA significantly reduces the need for postoperative transfusions.
- IV-TXA is safe to use with no adverse events noted.
By the year 2020, use of primary total knee arthroplasty (TKA) in the United States will increase an estimated 110%, to 1.375 million procedures annually, and use of primary total hip arthroplasty (THA) will increase an estimated 75%, to more than 500,000 procedures.1 Minimizing perioperative blood loss and improving early postoperative ambulation both correlate with reduced postoperative morbidity, allowing patients to return to their daily lives expeditiously.
Tranexamic acid (TXA), a fibrinolytic inhibitor, competitively blocks lysine receptor binding sites of plasminogen, sustaining and stabilizing the fibrin architecture.2 TXA must be present to occupy binding sites before plasminogen binds to fibrin, validating the need for preoperative administration so the drug is available early in the fibrinolytic cascade.3 Intravenous (IV) TXA diffuses rapidly into joint fluid and the synovial membrane.4 Drug concentration and elimination half-life in joint fluid are equivalent to those in serum. Elimination of TXA occurs by glomerular filtration, with about 30% of a 10-mg/kg dose removed in 1 hour, 55% over the first 3 hours, and 90% within 24 hours of IV administration.5
The efficacy of IV-TXA in minimizing total joint arthroplasty (TJA) perioperative blood loss has been proved in small studies and meta-analyses.6-9 TXA-induced blood conservation decreases or eliminates the need for postoperative transfusion, which can impede valuable, early ambulation.10 In addition, the positive clinical safety profile of TXA supports routine use of TXA in TJA.6,11-15
The benefits of early ambulation after TJA are well established. Getting patients to walk on the day of surgery is a key part of effective and rapid postoperative rehabilitation. Early mobilization correlates with reduced incidence of venous thrombosis and postoperative complications.16 In contrast to bed rest, sitting and standing promotes oxygen saturation, which improves tissue healing and minimizes adverse pulmonary events. Oxygen saturation also preserves muscle strength and blood flow, reducing the risk of venous thromboembolism and ulcers. Muscle strength must be maintained so normal gait can be regained.17 Compared with rehabilitation initiated 48 to 72 hours after TKA, rehabilitation initiated within 24 hours reduced the number of sessions needed to achieve independence and normal gait; in addition, early mobilization improved patient reports of pain after surgery.18 An evaluation of Denmark registry data revealed that mobilization to walking and use of crutches or canes was achieved earlier when ambulation was initiated on day of surgery.19 Finally, mobilization on day of surgery and during the immediate postoperative period improved long-term quality of life after TJA.20
We conducted a retrospective cohort study to determine if use of IV-TXA improves early ambulation and reduces blood loss after TKA and anterior and posterior THA. We hypothesized that IV-TXA use would reduce postoperative anemia and improve early ambulation and outcomes without producing adverse events during the immediate postoperative period. TXA reduces bleeding, and reduced incidence of hemarthrosis, wound swelling, and anemia could facilitate ambulation, reduce complications, and shorten recovery in patients who undergo TJA.
Patients and Methods
In February 2014, this retrospective cohort study received Institutional Review Board approval to compare the safety and efficacy of IV-TXA (vs no TXA) in patients who underwent TKA, anterior THA, and posterior THA.
In March 2012, multidisciplinary protocols were standardized to ensure a uniform hospital course for patients at our institution. All patients underwent preoperative testing and evaluation by a nurse practitioner and an anesthesiologist. In March 2013, IV-TXA became our standard of care. TXA use was contraindicated in patients with thromboembolic disease or with hypersensitivity to TXA. Patients without a contraindication were given two 10-mg/kg IV-TXA doses, each administered over 15 to 30 minutes; the first dose was administered before incision, and the second was infused at case close and/or at least 60 minutes after the first dose. Most TKA patients received regional (femoral) anesthesia and analgesia, and most THA patients received spinal or epidural anesthesia and analgesia. In a small percentage of cases, IV analgesia was patient-controlled, as determined by the pain service. There were no significant differences in anesthesia/analgesia modality between the 2 study groups—patients who received TXA and those who did not. Patients were then transitioned to oral opioids for pain management, unless otherwise contraindicated, and were ambulated 4 hours after end of surgery, unless medically unstable. Hematology and chemistry laboratory values were monitored daily during admission.
Patients underwent physical therapy (PT) after surgery and until hospital discharge. Physical therapists blinded to patients’ intraoperative use or no use of TXA measured ambulation. After initial evaluation on postoperative day 0 (POD-0), patients were ambulated twice daily. The daily ambulation distance used for the study was the larger of the 2 daily PT distances (occasionally, patients were unable to participate fully in both sessions). Patients received either enoxaparin or rivaroxaban for postoperative thromboprophylaxis (the anticoagulant used was based on surgeon preference). Enoxaparin was subcutaneously administered at 30 mg every 12 hours for TKA, 40 mg once daily for THA, 30 mg once daily for calculated creatinine clearance under 30 mL/min, or 40 mg every 12 hours for body mass index (BMI) 40 or above. With enoxaparin, therapy duration was 14 days. Oral rivaroxaban was administered at 10 mg once daily for 12 days for TKA and 35 days for THA unless contraindicated.
The primary outcome variables were ambulation measured on POD-1 and POD-2 and intraoperative blood loss. In addition, hemoglobin and hematocrit were measured on POD-0, POD-1, and POD-2. Ambulation was defined as number of feet walked during postoperative hospitalization. To calculate intraoperative blood loss, the anesthesiologist subtracted any saline irrigation volume from the total volume in the suction canister. Also noted were postoperative transfusions and any diagnosis of postoperative venous thromboembolism—specifically, deep vein thrombosis (DVT) or pulmonary embolism (PE).
Demographic and clinical characteristics of the TXA and no-TXA groups were compared using either 2-sample t test (for continuous variables) or χ2 test (for categorical variables).
The ambulation outcome was log-transformed to meet standard assumptions of Gaussian residuals and equality of variance. Means and 95% confidence intervals (CIs) were calculated on the log scale and were anti-logged so the results could be presented in their original units.
A linear mixed model was used to model intraoperative blood loss as a function of group (TXA, no TXA), procedure (TKA, anterior THA, posterior THA), and potential confounders (age, sex, BMI, operative time).
Linear mixed models for repeated measures were used to compare outcomes (hemoglobin, hematocrit) between groups (TXA, no TXA) and procedures (TKA, anterior THA, posterior THA) and to compare changes in outcomes over time. Group, procedure, and operative time interactions were explored. Potential confounders (age, sex, BMI, operative time) were included in the model as well.
A χ2 test was used to compare the groups (TXA, no TXA) on postoperative blood transfusion (yes, no). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used. Need for transfusion was clinically assessed case by case. Symptomatic anemia (dyspnea on exertion, headaches, tachycardia) was used as the primary indication for transfusion once hemoglobin fell below 8 g/dL or hematocrit below 24%. Number of patients with a postoperative thrombus formation was minimal. Therefore, this outcome was described with summary statistics and was not formally analyzed.
Results
Of the 477 patients who underwent TJAs (275 TKAs, 98 anterior THAs, 104 posterior THAs; all unilateral), 111 did not receive TXA (June 2012-February 2013), and 366 received TXA (March 2013-January 2014). Other than for the addition of IV-TXA, the same standardized protocols instituted in March 2012 continued throughout the study period. The difference in sample size between the TXA and no-TXA groups was not statistically significant and did not influence the outcome measures.
Ambulation
There was a significant (P = .0066) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P < .0001), sex (P < .0001), BMI (P < .0001), and operative time (P = .8308). Regarding TKA, mean ambulation was higher for the TXA group than for the no-TXA group at POD-1 (8.36 vs 3.40 feet; P < .0001) and POD-2 (25.81 vs 18.75 feet; P = .0054). The same was true for anterior THA at POD-1 (10.86 vs 3.33 feet; P < .0001) and POD-2 (27.24 vs 13.19 feet; P < .0001) and posterior THA at POD-1 (10.64 vs 3.37 feet; P < .0001) and POD-2 (24.68 vs 12.93 feet; P = .0002). See Table 3.
Intraoperative Blood Loss
There was a significant 3-way interaction of TXA, procedure (P < .0053), and operative time (P < .0001) after adjusting for age (P < .6136), sex (P = .1147), and BMI (P = .6180). Regarding TKA, mean intraoperative blood loss was significantly lower for the TXA group than for the no-TXA group (241.58 vs 287.81 mL; P = .0004). The same was true for anterior THA (352.91 vs 533.79 mL; P < .0001). Regarding posterior THA, there was no significant difference between the TXA and no-TXA groups (326.00 vs 350.16 mL; P = .3246). See Table 4.
Hemoglobin
There was a significant (P = .0008) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .0174), sex (P < .0001), BMI (P = .0007), and operative time (P = .0002). Regarding TKA, postoperative hemoglobin levels were higher for the TXA group than for the no-TXA group at POD-0 (12.10 vs 11.68 g/dL; P = .0135), POD-1 (11.62 vs 10.67 g/dL; P < .0001), and POD-2 (11.02 vs 10.11 g/dL; P < .0001). The same was true for anterior THA at POD-1 (11.03 vs 10.19 g/dL; P = .0034) and POD-2 (10.57 vs 9.64 g/dL; P = .0009) and posterior THA at POD-2 (11.04 vs 10.16 g/dL; P = .0003). See Table 5.
Hematocrit
There was a significant (P < .0006) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .1597), sex (P < .0001), BMI (P < .0001), and operative time (P = .0003). Regarding TKA, postoperative hematocrit levels were higher for the TXA group than for the no-TXA group at POD-0 (36.52% vs 34.65%; P < .0001), POD-1 (34.62% vs 31.83%; P < .0001), and POD-2 (33.01% vs 30.20%; P < .0001). The same was true for anterior THA at POD-1 (32.82% vs 30.59%; P = .0037) and POD-2 (31.58% vs 28.61%; P = .0004) and posterior THA at POD-2 (32.93% vs 30.17%; P < .0001). See Table 6.
Postoperative Transfusions
Of the 477 patients, 25 (5.24%) required a postoperative transfusion. Postoperative transfusions were less likely (P < .0001) required in the TXA group (1.64%, 6/366) than in the no-TXA group (17.12%, 19/111). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used, and the different procedures were not evaluated separately.
Deep Vein Thrombosis and Pulmonary Embolism
Of the 477 patients, 2 developed a DVT, and 5 developed a PE. Both DVTs occurred in the TXA group (2/366, 0.55%; 95% CI, 0.07%-1.96%). Of the 5 PEs, 4 occurred in the TXA group (4/366, 1.09%; 95% CI, 0.30%-2.77%), and 1 occurred in the no-TXA group (1/111, 0.90%; 95% CI, 0.02%-4.92%). Given the exceedingly small number of events, no statistical significance was noted between groups.
Discussion
Orthopedic surgeons carefully balance patient expectations, societal needs, and regulatory mandates while providing excellent care and working under payers’ financial restrictions. The Centers for Medicare & Medicaid Services announced that, starting in 2016, TJAs will be reimbursed in total as a single bundled payment, adding to the need to provide optimal care in a fiscally responsible manner.21 Standardized protocols implementing multimodal therapies are pivotal in achieving favorable postoperative outcomes.
Our study results showed that IV-TXA use minimized hemoglobin and hematocrit reductions after TKA, anterior THA, and posterior THA. Postoperative anemia correlates with decreased ambulation ability and performance during the early postoperative period. In general, higher postoperative hemoglobin and hematocrit levels result in improved motor performance and shorter recovery.22 In addition, early ambulation is a validated predictor of favorable TJA outcomes. In our study, for TKA, anterior THA, and posterior THA, ambulation on POD-1 and POD-2 was significantly better for patients who received TXA than for patients who did not.
Transfusion rates were markedly lower for our TXA group than for our no-TXA group (1.64% vs 17.12%), confirming the findings of numerous other studies on outcomes of TJA with TXA.2,3,6-12,14,15 Transfusions impede physical therapy and affect hospitalization costs.
Although potential thrombosis-related adverse events remain an endpoint in studies involving TXA, we found a comparably low incidence of postoperative venous thrombosis in our TXA and no-TXA groups (1.09% and 0.90%, respectively). In addition, no patient in either group developed a postoperative arterial thrombosis.
This is the largest single-center study of TXA use in TKA, anterior THA, and posterior THA. The effect of TXA use on postoperative ambulation was not previously found with TJA.
This study had its limitations. First, it was not prospective, randomized, or double-blinded. However, the physical therapists who mobilized patients and recorded ambulation data were blinded to the study and its hypothesis and followed a standardized protocol for all patients. In addition, intraoperative blood loss was recorded by an anesthesiologist using a standardized protocol, and patients received TXA per orthopedic protocol and surgeon preference, without selection bias. Another limitation was that ambulation data were captured only for POD-1 and POD-2 (most patients were discharged by POD-3). However, a goal of the study was to capture immediate postoperative data in order to determine the efficacy of intraoperative TXA. Subsequent studies can determine if this early benefit leads to long-term clinical outcome improvements.
In reducing blood loss and transfusion rates, intra-articular TXA is as efficacious as IV-TXA.23-25 We anticipate that the improved clinical outcomes found with IV-TXA in our study will be similar with intra-articular TXA, but more study is needed to confirm this hypothesis.
Conclusion
This retrospective cohort study found that use of IV-TXA in TJA improved early ambulation and clinical outcomes (reduced anemia, fewer transfusions) in the initial postoperative period, without producing adverse events.
Take-Home Points
- IV-TXA significantly reduces intraoperative blood loss following TJA.
- Early mobilization correlates with reduced incidence of postoperative complications.
- IV-TXA minimizes postoperative anemia, facilitating improved early ambulation following TJA.
- IV-TXA significantly reduces the need for postoperative transfusions.
- IV-TXA is safe to use with no adverse events noted.
By the year 2020, use of primary total knee arthroplasty (TKA) in the United States will increase an estimated 110%, to 1.375 million procedures annually, and use of primary total hip arthroplasty (THA) will increase an estimated 75%, to more than 500,000 procedures.1 Minimizing perioperative blood loss and improving early postoperative ambulation both correlate with reduced postoperative morbidity, allowing patients to return to their daily lives expeditiously.
Tranexamic acid (TXA), a fibrinolytic inhibitor, competitively blocks lysine receptor binding sites of plasminogen, sustaining and stabilizing the fibrin architecture.2 TXA must be present to occupy binding sites before plasminogen binds to fibrin, validating the need for preoperative administration so the drug is available early in the fibrinolytic cascade.3 Intravenous (IV) TXA diffuses rapidly into joint fluid and the synovial membrane.4 Drug concentration and elimination half-life in joint fluid are equivalent to those in serum. Elimination of TXA occurs by glomerular filtration, with about 30% of a 10-mg/kg dose removed in 1 hour, 55% over the first 3 hours, and 90% within 24 hours of IV administration.5
The efficacy of IV-TXA in minimizing total joint arthroplasty (TJA) perioperative blood loss has been proved in small studies and meta-analyses.6-9 TXA-induced blood conservation decreases or eliminates the need for postoperative transfusion, which can impede valuable, early ambulation.10 In addition, the positive clinical safety profile of TXA supports routine use of TXA in TJA.6,11-15
The benefits of early ambulation after TJA are well established. Getting patients to walk on the day of surgery is a key part of effective and rapid postoperative rehabilitation. Early mobilization correlates with reduced incidence of venous thrombosis and postoperative complications.16 In contrast to bed rest, sitting and standing promotes oxygen saturation, which improves tissue healing and minimizes adverse pulmonary events. Oxygen saturation also preserves muscle strength and blood flow, reducing the risk of venous thromboembolism and ulcers. Muscle strength must be maintained so normal gait can be regained.17 Compared with rehabilitation initiated 48 to 72 hours after TKA, rehabilitation initiated within 24 hours reduced the number of sessions needed to achieve independence and normal gait; in addition, early mobilization improved patient reports of pain after surgery.18 An evaluation of Denmark registry data revealed that mobilization to walking and use of crutches or canes was achieved earlier when ambulation was initiated on day of surgery.19 Finally, mobilization on day of surgery and during the immediate postoperative period improved long-term quality of life after TJA.20
We conducted a retrospective cohort study to determine if use of IV-TXA improves early ambulation and reduces blood loss after TKA and anterior and posterior THA. We hypothesized that IV-TXA use would reduce postoperative anemia and improve early ambulation and outcomes without producing adverse events during the immediate postoperative period. TXA reduces bleeding, and reduced incidence of hemarthrosis, wound swelling, and anemia could facilitate ambulation, reduce complications, and shorten recovery in patients who undergo TJA.
Patients and Methods
In February 2014, this retrospective cohort study received Institutional Review Board approval to compare the safety and efficacy of IV-TXA (vs no TXA) in patients who underwent TKA, anterior THA, and posterior THA.
In March 2012, multidisciplinary protocols were standardized to ensure a uniform hospital course for patients at our institution. All patients underwent preoperative testing and evaluation by a nurse practitioner and an anesthesiologist. In March 2013, IV-TXA became our standard of care. TXA use was contraindicated in patients with thromboembolic disease or with hypersensitivity to TXA. Patients without a contraindication were given two 10-mg/kg IV-TXA doses, each administered over 15 to 30 minutes; the first dose was administered before incision, and the second was infused at case close and/or at least 60 minutes after the first dose. Most TKA patients received regional (femoral) anesthesia and analgesia, and most THA patients received spinal or epidural anesthesia and analgesia. In a small percentage of cases, IV analgesia was patient-controlled, as determined by the pain service. There were no significant differences in anesthesia/analgesia modality between the 2 study groups—patients who received TXA and those who did not. Patients were then transitioned to oral opioids for pain management, unless otherwise contraindicated, and were ambulated 4 hours after end of surgery, unless medically unstable. Hematology and chemistry laboratory values were monitored daily during admission.
Patients underwent physical therapy (PT) after surgery and until hospital discharge. Physical therapists blinded to patients’ intraoperative use or no use of TXA measured ambulation. After initial evaluation on postoperative day 0 (POD-0), patients were ambulated twice daily. The daily ambulation distance used for the study was the larger of the 2 daily PT distances (occasionally, patients were unable to participate fully in both sessions). Patients received either enoxaparin or rivaroxaban for postoperative thromboprophylaxis (the anticoagulant used was based on surgeon preference). Enoxaparin was subcutaneously administered at 30 mg every 12 hours for TKA, 40 mg once daily for THA, 30 mg once daily for calculated creatinine clearance under 30 mL/min, or 40 mg every 12 hours for body mass index (BMI) 40 or above. With enoxaparin, therapy duration was 14 days. Oral rivaroxaban was administered at 10 mg once daily for 12 days for TKA and 35 days for THA unless contraindicated.
The primary outcome variables were ambulation measured on POD-1 and POD-2 and intraoperative blood loss. In addition, hemoglobin and hematocrit were measured on POD-0, POD-1, and POD-2. Ambulation was defined as number of feet walked during postoperative hospitalization. To calculate intraoperative blood loss, the anesthesiologist subtracted any saline irrigation volume from the total volume in the suction canister. Also noted were postoperative transfusions and any diagnosis of postoperative venous thromboembolism—specifically, deep vein thrombosis (DVT) or pulmonary embolism (PE).
Demographic and clinical characteristics of the TXA and no-TXA groups were compared using either 2-sample t test (for continuous variables) or χ2 test (for categorical variables).
The ambulation outcome was log-transformed to meet standard assumptions of Gaussian residuals and equality of variance. Means and 95% confidence intervals (CIs) were calculated on the log scale and were anti-logged so the results could be presented in their original units.
A linear mixed model was used to model intraoperative blood loss as a function of group (TXA, no TXA), procedure (TKA, anterior THA, posterior THA), and potential confounders (age, sex, BMI, operative time).
Linear mixed models for repeated measures were used to compare outcomes (hemoglobin, hematocrit) between groups (TXA, no TXA) and procedures (TKA, anterior THA, posterior THA) and to compare changes in outcomes over time. Group, procedure, and operative time interactions were explored. Potential confounders (age, sex, BMI, operative time) were included in the model as well.
A χ2 test was used to compare the groups (TXA, no TXA) on postoperative blood transfusion (yes, no). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used. Need for transfusion was clinically assessed case by case. Symptomatic anemia (dyspnea on exertion, headaches, tachycardia) was used as the primary indication for transfusion once hemoglobin fell below 8 g/dL or hematocrit below 24%. Number of patients with a postoperative thrombus formation was minimal. Therefore, this outcome was described with summary statistics and was not formally analyzed.
Results
Of the 477 patients who underwent TJAs (275 TKAs, 98 anterior THAs, 104 posterior THAs; all unilateral), 111 did not receive TXA (June 2012-February 2013), and 366 received TXA (March 2013-January 2014). Other than for the addition of IV-TXA, the same standardized protocols instituted in March 2012 continued throughout the study period. The difference in sample size between the TXA and no-TXA groups was not statistically significant and did not influence the outcome measures.
Ambulation
There was a significant (P = .0066) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P < .0001), sex (P < .0001), BMI (P < .0001), and operative time (P = .8308). Regarding TKA, mean ambulation was higher for the TXA group than for the no-TXA group at POD-1 (8.36 vs 3.40 feet; P < .0001) and POD-2 (25.81 vs 18.75 feet; P = .0054). The same was true for anterior THA at POD-1 (10.86 vs 3.33 feet; P < .0001) and POD-2 (27.24 vs 13.19 feet; P < .0001) and posterior THA at POD-1 (10.64 vs 3.37 feet; P < .0001) and POD-2 (24.68 vs 12.93 feet; P = .0002). See Table 3.
Intraoperative Blood Loss
There was a significant 3-way interaction of TXA, procedure (P < .0053), and operative time (P < .0001) after adjusting for age (P < .6136), sex (P = .1147), and BMI (P = .6180). Regarding TKA, mean intraoperative blood loss was significantly lower for the TXA group than for the no-TXA group (241.58 vs 287.81 mL; P = .0004). The same was true for anterior THA (352.91 vs 533.79 mL; P < .0001). Regarding posterior THA, there was no significant difference between the TXA and no-TXA groups (326.00 vs 350.16 mL; P = .3246). See Table 4.
Hemoglobin
There was a significant (P = .0008) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .0174), sex (P < .0001), BMI (P = .0007), and operative time (P = .0002). Regarding TKA, postoperative hemoglobin levels were higher for the TXA group than for the no-TXA group at POD-0 (12.10 vs 11.68 g/dL; P = .0135), POD-1 (11.62 vs 10.67 g/dL; P < .0001), and POD-2 (11.02 vs 10.11 g/dL; P < .0001). The same was true for anterior THA at POD-1 (11.03 vs 10.19 g/dL; P = .0034) and POD-2 (10.57 vs 9.64 g/dL; P = .0009) and posterior THA at POD-2 (11.04 vs 10.16 g/dL; P = .0003). See Table 5.
Hematocrit
There was a significant (P < .0006) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .1597), sex (P < .0001), BMI (P < .0001), and operative time (P = .0003). Regarding TKA, postoperative hematocrit levels were higher for the TXA group than for the no-TXA group at POD-0 (36.52% vs 34.65%; P < .0001), POD-1 (34.62% vs 31.83%; P < .0001), and POD-2 (33.01% vs 30.20%; P < .0001). The same was true for anterior THA at POD-1 (32.82% vs 30.59%; P = .0037) and POD-2 (31.58% vs 28.61%; P = .0004) and posterior THA at POD-2 (32.93% vs 30.17%; P < .0001). See Table 6.
Postoperative Transfusions
Of the 477 patients, 25 (5.24%) required a postoperative transfusion. Postoperative transfusions were less likely (P < .0001) required in the TXA group (1.64%, 6/366) than in the no-TXA group (17.12%, 19/111). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used, and the different procedures were not evaluated separately.
Deep Vein Thrombosis and Pulmonary Embolism
Of the 477 patients, 2 developed a DVT, and 5 developed a PE. Both DVTs occurred in the TXA group (2/366, 0.55%; 95% CI, 0.07%-1.96%). Of the 5 PEs, 4 occurred in the TXA group (4/366, 1.09%; 95% CI, 0.30%-2.77%), and 1 occurred in the no-TXA group (1/111, 0.90%; 95% CI, 0.02%-4.92%). Given the exceedingly small number of events, no statistical significance was noted between groups.
Discussion
Orthopedic surgeons carefully balance patient expectations, societal needs, and regulatory mandates while providing excellent care and working under payers’ financial restrictions. The Centers for Medicare & Medicaid Services announced that, starting in 2016, TJAs will be reimbursed in total as a single bundled payment, adding to the need to provide optimal care in a fiscally responsible manner.21 Standardized protocols implementing multimodal therapies are pivotal in achieving favorable postoperative outcomes.
Our study results showed that IV-TXA use minimized hemoglobin and hematocrit reductions after TKA, anterior THA, and posterior THA. Postoperative anemia correlates with decreased ambulation ability and performance during the early postoperative period. In general, higher postoperative hemoglobin and hematocrit levels result in improved motor performance and shorter recovery.22 In addition, early ambulation is a validated predictor of favorable TJA outcomes. In our study, for TKA, anterior THA, and posterior THA, ambulation on POD-1 and POD-2 was significantly better for patients who received TXA than for patients who did not.
Transfusion rates were markedly lower for our TXA group than for our no-TXA group (1.64% vs 17.12%), confirming the findings of numerous other studies on outcomes of TJA with TXA.2,3,6-12,14,15 Transfusions impede physical therapy and affect hospitalization costs.
Although potential thrombosis-related adverse events remain an endpoint in studies involving TXA, we found a comparably low incidence of postoperative venous thrombosis in our TXA and no-TXA groups (1.09% and 0.90%, respectively). In addition, no patient in either group developed a postoperative arterial thrombosis.
This is the largest single-center study of TXA use in TKA, anterior THA, and posterior THA. The effect of TXA use on postoperative ambulation was not previously found with TJA.
This study had its limitations. First, it was not prospective, randomized, or double-blinded. However, the physical therapists who mobilized patients and recorded ambulation data were blinded to the study and its hypothesis and followed a standardized protocol for all patients. In addition, intraoperative blood loss was recorded by an anesthesiologist using a standardized protocol, and patients received TXA per orthopedic protocol and surgeon preference, without selection bias. Another limitation was that ambulation data were captured only for POD-1 and POD-2 (most patients were discharged by POD-3). However, a goal of the study was to capture immediate postoperative data in order to determine the efficacy of intraoperative TXA. Subsequent studies can determine if this early benefit leads to long-term clinical outcome improvements.
In reducing blood loss and transfusion rates, intra-articular TXA is as efficacious as IV-TXA.23-25 We anticipate that the improved clinical outcomes found with IV-TXA in our study will be similar with intra-articular TXA, but more study is needed to confirm this hypothesis.
Conclusion
This retrospective cohort study found that use of IV-TXA in TJA improved early ambulation and clinical outcomes (reduced anemia, fewer transfusions) in the initial postoperative period, without producing adverse events.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83(4):596-601.
3. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty. Acta Orthop Scand. 2001;72(5):442-448.
4. Tanaka N, Sakahashi, H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001;83(5):702-705.
5. Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol). 1980;14:41-47.
6. George DA, Sarraf KM, Nwaboku H. Single perioperative dose of tranexamic acid in primary hip and knee arthroplasty. Eur J Orthop Surg Traumatol. 2015;25(1):129-133.
7. Vigna-Taglianti F, Basso L, Rolfo P, et al. Tranexamic acid for reducing blood transfusions in arthroplasty interventions: a cost-effective practice. Eur J Orthop Surg Traumatol. 2014;24(4):545-551.
8. Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003;31(5):529-537.
9. Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829.
10. Sculco PK, Pagnano MW. Perioperative solutions for rapid recovery joint arthroplasty: get ahead and stay ahead. J Arthroplasty. 2015;30(4):518-520.
11. Lozano M, Basora M, Peidro L, et al. Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sang. 2008;95(1):39-44.
12. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783.
13. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement. A systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
14. Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA. Clin Orthop Relat Res. 2011;469(10):2874-2880.
15. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
16. Stowers M, Lemanu DP, Coleman B, Hill AG, Munro JT. Review article: perioperative care in enhanced recovery for total hip and knee arthroplasty. J Orthop Surg (Hong Kong). 2014;22(3):383-392.
17. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation. Acta Orthop. 2008;79(5):624-630.
18. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
19. Husted H, Hansen HC, Holm G, et al. What determines length of stay after total hip and knee arthroplasty? A nationwide study in Denmark. Arch Orthop Trauma Surg. 2010;130(2):263-268.
20. Husted H. Fast-track hip and knee arthroplasty: clinical and organizational aspects. Acta Orthop Suppl. 2012;83(346):1-39.
21. Comprehensive Care for Joint Replacement Model. CMS.gov. https://innovation.cms.gov/initiatives/cjr. Updated October 5, 2017.
22. Wang X, Rintala DH, Garber SL, Henson H. Association of hemoglobin levels, acute hemoglobin decrease, age, and co-morbidities with rehabilitation outcomes after total knee replacement. Am J Phys Med Rehabil. 2005;84(6):451-456.
23. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83(4):596-601.
3. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty. Acta Orthop Scand. 2001;72(5):442-448.
4. Tanaka N, Sakahashi, H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001;83(5):702-705.
5. Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol). 1980;14:41-47.
6. George DA, Sarraf KM, Nwaboku H. Single perioperative dose of tranexamic acid in primary hip and knee arthroplasty. Eur J Orthop Surg Traumatol. 2015;25(1):129-133.
7. Vigna-Taglianti F, Basso L, Rolfo P, et al. Tranexamic acid for reducing blood transfusions in arthroplasty interventions: a cost-effective practice. Eur J Orthop Surg Traumatol. 2014;24(4):545-551.
8. Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003;31(5):529-537.
9. Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829.
10. Sculco PK, Pagnano MW. Perioperative solutions for rapid recovery joint arthroplasty: get ahead and stay ahead. J Arthroplasty. 2015;30(4):518-520.
11. Lozano M, Basora M, Peidro L, et al. Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sang. 2008;95(1):39-44.
12. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783.
13. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement. A systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
14. Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA. Clin Orthop Relat Res. 2011;469(10):2874-2880.
15. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
16. Stowers M, Lemanu DP, Coleman B, Hill AG, Munro JT. Review article: perioperative care in enhanced recovery for total hip and knee arthroplasty. J Orthop Surg (Hong Kong). 2014;22(3):383-392.
17. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation. Acta Orthop. 2008;79(5):624-630.
18. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
19. Husted H, Hansen HC, Holm G, et al. What determines length of stay after total hip and knee arthroplasty? A nationwide study in Denmark. Arch Orthop Trauma Surg. 2010;130(2):263-268.
20. Husted H. Fast-track hip and knee arthroplasty: clinical and organizational aspects. Acta Orthop Suppl. 2012;83(346):1-39.
21. Comprehensive Care for Joint Replacement Model. CMS.gov. https://innovation.cms.gov/initiatives/cjr. Updated October 5, 2017.
22. Wang X, Rintala DH, Garber SL, Henson H. Association of hemoglobin levels, acute hemoglobin decrease, age, and co-morbidities with rehabilitation outcomes after total knee replacement. Am J Phys Med Rehabil. 2005;84(6):451-456.
23. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
Risk of Osteoporotic Fracture After Steroid Injections in Patients With Medicare
Take-Home Points
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
Although statistically significant, this may not be clinically relevant.
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.
Take-Home Points
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
Although statistically significant, this may not be clinically relevant.
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
Take-Home Points
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
Although statistically significant, this may not be clinically relevant.
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.
Management and Prevention of Intraoperative Acetabular Fracture in Primary Total Hip Arthroplasty
Take Home Points
- IAF is an uncommon, but serious complication of primary THA.
- Small (<50 mm) cups are at higher risk for causing IAF.
- Prompt recognition is critical to prevent component migration and need for revision.
- Posterior column integrity is cirtical to a successful outcome when IAF occurs.
- Initial stable fixation, with or without intraoperative acetabular revision, is critical for successful outcome when IAF is identified.
Intraoperative acetabular fracture (IAF) is a rare complication of primary total hip arthroplasty (THA).1-3 IAFs commonly occur with impaction of the acetabular component. Studies have found that underreaming of the acetabulum and impaction of relatively large, elliptic, or monoblock components may increase the risk of IAFs.2-5 There is a paucity of literature on risk factors, treatment strategies, and outcomes of this potentially devastating complication.
In this article, we report on the incidence of IAF in primary THA at our high-volume institution and present strategies for managing and preventing this rare fracture.
Materials and Methods
Between 1997 and 2015, more than 20 fellowship-trained arthroplasty surgeons performed 21,519 primary THAs at our institution. After obtaining Institutional Review Board approval for this study, we retrospectively searched the hospital database and identified 16 patients (16 hips) who sustained an IAF in primary THA. Mean age of the cohort (13 women, 3 men) at time of surgery was 70 years (range, 42-89 years). Of the 16 patients, 13 had a preoperative diagnosis of osteoarthritis, 2 had posttraumatic arthritis, and 1 had rheumatoid arthritis. A posterolateral approach was used with 14 patients and a modified anterolateral approach with the other 2. Surgical technique and implant selection varied among surgeons. Thirteen THAs were performed with an all-press-fit technique and 3 with a hybrid technique (uncemented acetabular component, cemented femoral component). In 9 cases, the acetabular component underwent supplemental screw fixation. Whether to use acetabular component screws or cemented femoral components was decided intraoperatively by the surgeon.
The cohort’s acetabular components were either elliptic modular or hemispheric modular. The elliptic modular component used was the Peripheral Self-Locking (PSL) implant (Stryker Howmedica Osteonics), and the hemispheric modular components used were either the Trident implant (Stryker Howmedica Osteonics) or the ZTT-II implant (DePuy Synthes). Elliptic acetabular components have a peripheral flare, in contrast to true hemispheric acetabular components. Ten elliptic modular and 6 hemispheric modular components were implanted. In all cases, the difference between the final reamer used to prepare the acetabular bed and the true largest external diameter of the impacted shell was 2 mm or less.
The cohort’s 16 femoral components consisted of 8 Secur-Fit uncemented components (Stryker Howmedica Osteonics), 3 Accolade uncemented components (Stryker Howmedica Osteonics), 3 Omnifit EON cemented components (Stryker Howmedica Osteonics), and 2 S-ROM uncemented components (DePuy Synthes).
After surgery, all patients were followed up according to individual surgeon protocol for radiographic and physical examination.
Data on IAF incidence were obtained from a hospital database and were confirmed with electronic medical record (EMR) documentation. Also obtained were IAF causes and locations recorded in operative notes. For fractures identified after surgery, location was obtained from the immediate postoperative radiograph. Fracture management (eg, supplemental screw fixation, fracture reduction and fixation, bone grafting, acetabular component revision, protected weight-bearing) was determined from EMR documentation.
Results
Sixteen patients sustained an IAF in primary THA. All IAFs occurred in cases involving cementless acetabular components. The institution’s incidence of IAF with use of cementless components was 0.0007%.
Of the 5 IAFs (31%) identified during surgery, 4 were noted during impaction of the acetabular component, and 1 was noted during reaming. Eighty percent of these IAFs occurred directly posterior, and 60% were addressed at time of index procedure secondary to acetabular component instability. The other 11 fractures (69%) were identified on standard postoperative anteroposterior pelvis radiographs obtained in the postanesthesia care unit (PACU). Details of component characteristics, fracture location, immediate treatment, and weight-bearing precautions for all 16 patients are listed in the Table.
There were additional complications. One patient sustained an intraoperative proximal femur fracture, which was addressed at the index THA with application of a cerclage wire and reinsertion of the femoral component; no further surgical intervention was required, and the femur fracture healed uneventfully. Another patient had a postoperative ileus that required nasogastric tube decompression and monitoring in the intensive care unit; the ileus resolved spontaneously. A third patient, initially treated with bone grafting and cemented cup insertion, was diagnosed with a periprosthetic joint infection 3 weeks after the index THA and was treated with explantation of all components and girdlestone resection arthroplasty; 1 month after the resection arthroplasty, a persistently draining wound was treated with irrigation and débridement. There were no other medical complications, thromboembolic events, or dislocations.
One to 7 weeks after surgery, patients returned for initial follow-up, and radiographs were obtained for component stability assessment. Three patients presented with gross acetabular instability, and revisions were performed. Standard clinical follow-up continued for all patients per individual surgeon protocol. Mean follow-up was 4 years.
Discussion
IAF is an uncommon complication of THA. The rarity of IAFs makes it difficult to obtain a cohort large enough to study the problem. Given the increasing incidence of primary THAs and the almost ubiquitous use of press-fit acetabular components, surgeons who perform THAs undoubtedly will encounter IAFs in their own practice. In this article, we report our institution’s experience with periprosthetic IAFs and provide a framework for making decisions regarding these complications.
Anatomical locations of IAFs have been associated with variable outcomes. In a 2015 series, Laflamme and colleagues6 found posterior column stability a crucial factor in implant stability. Fractures with posterior column instability had a 67% failure rate, and patients with an intact posterior column reliably had osteointegration occur without further intervention.6 In our series, fractures that violated the posterior column had similar results. All these fractures required further operative intervention, either at the index procedure or in the early postoperative period. Loss of posterior column stability prevents secure fixation of the acetabular component, thereby preventing successful hip reconstruction. One posterior column fracture in our series was not recognized until after surgery, on a PACU radiograph, and 1 posterior column fracture was fully appreciated only after postoperative computed tomography (CT) was obtained during immediate hospitalization after the index procedure. In both cases, conservative management was unsuccessful. Revision arthroplasty (and in 1 case late posterior column fixation) was performed to achieve adequate reconstruction. There were no failures after posterior column fixation. In cases of posterior wall or column fracture, we recommend early aggressive treatment, preferably at the time of index arthroplasty, to prevent catastrophic failure.
Most commonly, periprosthetic IAFs go unnoticed until initial postoperative radiographs are examined.6 Eleven of the 16 IAFs in our series were first recognized on radiographs obtained in the PACU. Surgeons thus have difficult decisions to make. The literature has little discussion on managing early postoperative periprosthetic IAFs. Most recent studies, which consist of small series and case reports, have focused on late and often traumatic IAFs.7-9 These were initially classified by Peterson and Lewallen10 as type I, which are stable radiographically (no movement relative to previous radiographs) and do not produce pain with minor movement of the extremity, or type II, which are unstable radiographically (gross displacement of component) or produce pain with any hip motion. Type I fractures were more common and were often managed with protected weight-bearing and observation. The authors concluded that, in type I fractures, retaining the original acetabular component is difficult; however, when these fractures are treated appropriately, a functional prosthesis can be salvaged, and fracture union can be expected.
Less common are acetabular fractures detected during surgery, as in our study. In an outcome series, Haidukewych and colleagues3 reported on 21 periprosthetic acetabular fractures, all recognized during surgery and managed according to perceived stability of the component. All fractures healed uneventfully, and there were no other complications.
These studies provide a framework for addressing IAFs noticed in the early postoperative period. The diagnostic dilemma presented by these fractures was first discussed by Laflamme and colleagues.6 Nine of the 32 fractures in their series were classified as so-called type III fractures, recognized only after the early postoperative period. Additional radiographs (eg, Judet views) or CT scans were crucial in determining acetabular component stability, given the known poor outcomes associated with posterior column fracture. In our series, only 1 patient had CT performed after intraoperative recognition of fracture, and the extent of the fracture was not readily apparent on the patient’s postoperative radiograph. Given the successful recognition and treatment of these fractures in the early postoperative period in our series,
it is difficult to recommend advanced imaging for all periprosthetic IAFs. Perhaps this success is attributable to our almost universal use of screws for acetabular component fixation. Of the 11 patients with fractures recognized during the postoperative period, 8 had supplemental screw fixation at time of index surgery. If there is a question of fixation during component insertion, we recommend scrutinizing the acetabular rim for fracture and placing supplemental screw fixation. Screws placed for acetabular component fixation provide initial stability and may prevent early component failure in the setting of unrecognized medial or anterior fracture. In addition, when component stability is in question after impaction, we recommend using finger palpation to evaluate the sciatic notch for cortical step-off from an otherwise unrecognized fracture. Protected weight-bearing in the postoperative period may be left to the discretion of the surgeon, and the decision should be based on intraoperative stability of the acetabular component.
In our series, there was a disproportionate representation of fractures associated with elliptic acetabular components. All 5 of the fractures recognized during surgery and 5 of the 11 recognized after surgery occurred with elliptic components. The association between elliptic cup design and periprosthetic IAF was identified earlier, by Haidukewych and colleagues.3 Their series showed a statistically significant increase in fracture incidence with impaction of an elliptic cup into a bed prepared with a hemispheric reamer. In the present series, 75% of our acetabular components were impacted into a bed underreamed by 1 mm to 2 mm. It is typical of many surgeons at our institution to underream by 1 mm to 2 mm regardless of the type of component being implanted, though they show a growing trend to overream by only 1 mm with the PSL component, which has been both safe and reliable in preventing catastrophic posterior column fractures, especially with impaction of small (<50 mm) acetabular components. We have not observed early loosening or other evidence of failure with this technique. Cup impaction generates significant hoop stresses that can easily fracture sclerotic or otherwise poor-quality bone, and the dense bone around the acetabular rim experiences increased stress with impaction of elliptic components.2,11-15 Surgeons must understand the design traits of their components and be cognizant of the true difference between the diameter of the final reamer used and the real diameter of the acetabular component. We recommend having a difference of ≤1 mm to mitigate the risk of IAF occurring with cup insertion. With use of elliptic components, slight overreaming of the acetabular bed should be considered. More study is needed to better define these outcomes.
Study Limitations
Our study had several limitations, including the inherent biases of its retrospective design, small cohort size, and inclusion of multiple surgeons. Small cohort size is unavoidable given the low incidence of these injuries, and our study encompassed the experience of a high-volume hip arthroplasty service. There is the possibility that a subset of fractures may have persistently gone unrecognized, either during or after surgery, and the actual incidence of these complications may be higher. These outcomes represent our institutional experience addressing the complexities of these injuries. The lack of standardization in the management of these fractures in our series reflects the diagnostic dilemma they present, as well as the need for more study focused on their management and outcomes.
Conclusion
IAF, an uncommon complication of primary THA, most commonly occurs during component impaction. Acetabular component and surgical technique may influence the fracture rate. Intraoperative or prompt postoperative recognition of these fractures is crucial, as their location is associated with stability and outcome. Careful examination of postoperative radiographs, judicious use of advanced imaging, and close follow-up are needed to prevent early catastrophic failure. We argue against simply observing these unstable fractures and recommend early treatment with rigid fixation and, when necessary, acetabular component revision.
1. Sharkey PF, Hozack WJ, Callaghan JJ, et al. Acetabular fractures associated with cementless acetabular cup insertion: a report of 13 cases. J Arthroplasty.1999;14(4):426-431.
2. Kim YS, Callaghan JJ, Ahn PB, Brown TD. Fracture of the acetabulum during insertion of an oversized hemispherical component. J Bone Joint Surg Am. 1995;77(1):111-117.
3. Haidukewych GJ, Jacofsky DJ, Hanssen AD, Lewallen DG. Intraoperative fractures of the acetabulum during primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(9):1952-1956.
4. Curtis MJ, Jinnah RH, Wilson VD, Hungerford DS. The initial stability of uncemented acetabular components. J Bone Joint Surg Br. 1992;74(3):372-376.
5. Lachiewicz PF, Suh PB, Gilbert JA. In vitro initial fixation of porous-coated acetabular total hip components. A biomechanical and comparative study. J Arthroplasty. 1989;4(3):201-205.
6. Laflamme GY, Belzile EL, Fernandes JC, Vendittoli PA, Hébert-Davies J. Periprosthetic fractures of the acetabulum during component insertion: posterior column stability
is crucial. J Arthroplasty. 2015;30(2):265-269.
7. Desai G, Reis MD. Early postoperative acetabular discontinuity after total hip arthroplasty. J Arthroplasty. 2011;26(8):1570.e17-e19.
8. Gelalis ID, Politis AN, Arnaoutoglou CM, Georgakopoulos N, Mitsiou D, Xenakis TA. Traumatic periprosthetic acetabular fracture treated by acute one-stage revision arthroplasty. A case report and review of the literature. Injury. 2010;41(4):421-424.
9. Gras F, Marintschev I, Klos K, Fujak A, Mückley T, Hofmann GO. Navigated percutaneous screw fixation of a periprosthetic acetabular fracture. J Arthroplasty. 2010;25(7):1169.e1-e4.
10. Peterson CA, Lewallen DG. Periprosthetic fracture of the acetabulum after total hip arthroplasty. J Bone Joint Surg Am. 1996;78(8):1206-1213.
11. Hansen TM, Koenman JB, Headley AK. 3-D FEM analysis of interface fixation of acetabular implants. Trans Orthop Res Soc. 1992;17:400.
12. Yerby SA, Taylor JK, Murzic WJ. Acetabular component interface: press-fit fixation. Trans Orthop Res Soc. 1992;17:384.
13. Callaghan JJ. The clinical results and basic science of total hip arthroplasty with porous-coated prostheses. J Bone Joint Surg Am. 1993;75(2):299-310.
14. Cheng SL, Binnington AG, Bragdon CR, Jasty M, Harris WH, Davey JR. The effect of sizing mismatch on bone ingrowth into uncemented porous coated acetabular components: an in vivo canine study. Trans Orthop Res Soc. 1990;15:442.
15. Morscher E, Bereiter H, Lampert C, Cementless press-fit cup: principles, experimental data, and three-year follow-up study. Clin Orthop Relat Res. 1989;(249):12-20.
Take Home Points
- IAF is an uncommon, but serious complication of primary THA.
- Small (<50 mm) cups are at higher risk for causing IAF.
- Prompt recognition is critical to prevent component migration and need for revision.
- Posterior column integrity is cirtical to a successful outcome when IAF occurs.
- Initial stable fixation, with or without intraoperative acetabular revision, is critical for successful outcome when IAF is identified.
Intraoperative acetabular fracture (IAF) is a rare complication of primary total hip arthroplasty (THA).1-3 IAFs commonly occur with impaction of the acetabular component. Studies have found that underreaming of the acetabulum and impaction of relatively large, elliptic, or monoblock components may increase the risk of IAFs.2-5 There is a paucity of literature on risk factors, treatment strategies, and outcomes of this potentially devastating complication.
In this article, we report on the incidence of IAF in primary THA at our high-volume institution and present strategies for managing and preventing this rare fracture.
Materials and Methods
Between 1997 and 2015, more than 20 fellowship-trained arthroplasty surgeons performed 21,519 primary THAs at our institution. After obtaining Institutional Review Board approval for this study, we retrospectively searched the hospital database and identified 16 patients (16 hips) who sustained an IAF in primary THA. Mean age of the cohort (13 women, 3 men) at time of surgery was 70 years (range, 42-89 years). Of the 16 patients, 13 had a preoperative diagnosis of osteoarthritis, 2 had posttraumatic arthritis, and 1 had rheumatoid arthritis. A posterolateral approach was used with 14 patients and a modified anterolateral approach with the other 2. Surgical technique and implant selection varied among surgeons. Thirteen THAs were performed with an all-press-fit technique and 3 with a hybrid technique (uncemented acetabular component, cemented femoral component). In 9 cases, the acetabular component underwent supplemental screw fixation. Whether to use acetabular component screws or cemented femoral components was decided intraoperatively by the surgeon.
The cohort’s acetabular components were either elliptic modular or hemispheric modular. The elliptic modular component used was the Peripheral Self-Locking (PSL) implant (Stryker Howmedica Osteonics), and the hemispheric modular components used were either the Trident implant (Stryker Howmedica Osteonics) or the ZTT-II implant (DePuy Synthes). Elliptic acetabular components have a peripheral flare, in contrast to true hemispheric acetabular components. Ten elliptic modular and 6 hemispheric modular components were implanted. In all cases, the difference between the final reamer used to prepare the acetabular bed and the true largest external diameter of the impacted shell was 2 mm or less.
The cohort’s 16 femoral components consisted of 8 Secur-Fit uncemented components (Stryker Howmedica Osteonics), 3 Accolade uncemented components (Stryker Howmedica Osteonics), 3 Omnifit EON cemented components (Stryker Howmedica Osteonics), and 2 S-ROM uncemented components (DePuy Synthes).
After surgery, all patients were followed up according to individual surgeon protocol for radiographic and physical examination.
Data on IAF incidence were obtained from a hospital database and were confirmed with electronic medical record (EMR) documentation. Also obtained were IAF causes and locations recorded in operative notes. For fractures identified after surgery, location was obtained from the immediate postoperative radiograph. Fracture management (eg, supplemental screw fixation, fracture reduction and fixation, bone grafting, acetabular component revision, protected weight-bearing) was determined from EMR documentation.
Results
Sixteen patients sustained an IAF in primary THA. All IAFs occurred in cases involving cementless acetabular components. The institution’s incidence of IAF with use of cementless components was 0.0007%.
Of the 5 IAFs (31%) identified during surgery, 4 were noted during impaction of the acetabular component, and 1 was noted during reaming. Eighty percent of these IAFs occurred directly posterior, and 60% were addressed at time of index procedure secondary to acetabular component instability. The other 11 fractures (69%) were identified on standard postoperative anteroposterior pelvis radiographs obtained in the postanesthesia care unit (PACU). Details of component characteristics, fracture location, immediate treatment, and weight-bearing precautions for all 16 patients are listed in the Table.
There were additional complications. One patient sustained an intraoperative proximal femur fracture, which was addressed at the index THA with application of a cerclage wire and reinsertion of the femoral component; no further surgical intervention was required, and the femur fracture healed uneventfully. Another patient had a postoperative ileus that required nasogastric tube decompression and monitoring in the intensive care unit; the ileus resolved spontaneously. A third patient, initially treated with bone grafting and cemented cup insertion, was diagnosed with a periprosthetic joint infection 3 weeks after the index THA and was treated with explantation of all components and girdlestone resection arthroplasty; 1 month after the resection arthroplasty, a persistently draining wound was treated with irrigation and débridement. There were no other medical complications, thromboembolic events, or dislocations.
One to 7 weeks after surgery, patients returned for initial follow-up, and radiographs were obtained for component stability assessment. Three patients presented with gross acetabular instability, and revisions were performed. Standard clinical follow-up continued for all patients per individual surgeon protocol. Mean follow-up was 4 years.
Discussion
IAF is an uncommon complication of THA. The rarity of IAFs makes it difficult to obtain a cohort large enough to study the problem. Given the increasing incidence of primary THAs and the almost ubiquitous use of press-fit acetabular components, surgeons who perform THAs undoubtedly will encounter IAFs in their own practice. In this article, we report our institution’s experience with periprosthetic IAFs and provide a framework for making decisions regarding these complications.
Anatomical locations of IAFs have been associated with variable outcomes. In a 2015 series, Laflamme and colleagues6 found posterior column stability a crucial factor in implant stability. Fractures with posterior column instability had a 67% failure rate, and patients with an intact posterior column reliably had osteointegration occur without further intervention.6 In our series, fractures that violated the posterior column had similar results. All these fractures required further operative intervention, either at the index procedure or in the early postoperative period. Loss of posterior column stability prevents secure fixation of the acetabular component, thereby preventing successful hip reconstruction. One posterior column fracture in our series was not recognized until after surgery, on a PACU radiograph, and 1 posterior column fracture was fully appreciated only after postoperative computed tomography (CT) was obtained during immediate hospitalization after the index procedure. In both cases, conservative management was unsuccessful. Revision arthroplasty (and in 1 case late posterior column fixation) was performed to achieve adequate reconstruction. There were no failures after posterior column fixation. In cases of posterior wall or column fracture, we recommend early aggressive treatment, preferably at the time of index arthroplasty, to prevent catastrophic failure.
Most commonly, periprosthetic IAFs go unnoticed until initial postoperative radiographs are examined.6 Eleven of the 16 IAFs in our series were first recognized on radiographs obtained in the PACU. Surgeons thus have difficult decisions to make. The literature has little discussion on managing early postoperative periprosthetic IAFs. Most recent studies, which consist of small series and case reports, have focused on late and often traumatic IAFs.7-9 These were initially classified by Peterson and Lewallen10 as type I, which are stable radiographically (no movement relative to previous radiographs) and do not produce pain with minor movement of the extremity, or type II, which are unstable radiographically (gross displacement of component) or produce pain with any hip motion. Type I fractures were more common and were often managed with protected weight-bearing and observation. The authors concluded that, in type I fractures, retaining the original acetabular component is difficult; however, when these fractures are treated appropriately, a functional prosthesis can be salvaged, and fracture union can be expected.
Less common are acetabular fractures detected during surgery, as in our study. In an outcome series, Haidukewych and colleagues3 reported on 21 periprosthetic acetabular fractures, all recognized during surgery and managed according to perceived stability of the component. All fractures healed uneventfully, and there were no other complications.
These studies provide a framework for addressing IAFs noticed in the early postoperative period. The diagnostic dilemma presented by these fractures was first discussed by Laflamme and colleagues.6 Nine of the 32 fractures in their series were classified as so-called type III fractures, recognized only after the early postoperative period. Additional radiographs (eg, Judet views) or CT scans were crucial in determining acetabular component stability, given the known poor outcomes associated with posterior column fracture. In our series, only 1 patient had CT performed after intraoperative recognition of fracture, and the extent of the fracture was not readily apparent on the patient’s postoperative radiograph. Given the successful recognition and treatment of these fractures in the early postoperative period in our series,
it is difficult to recommend advanced imaging for all periprosthetic IAFs. Perhaps this success is attributable to our almost universal use of screws for acetabular component fixation. Of the 11 patients with fractures recognized during the postoperative period, 8 had supplemental screw fixation at time of index surgery. If there is a question of fixation during component insertion, we recommend scrutinizing the acetabular rim for fracture and placing supplemental screw fixation. Screws placed for acetabular component fixation provide initial stability and may prevent early component failure in the setting of unrecognized medial or anterior fracture. In addition, when component stability is in question after impaction, we recommend using finger palpation to evaluate the sciatic notch for cortical step-off from an otherwise unrecognized fracture. Protected weight-bearing in the postoperative period may be left to the discretion of the surgeon, and the decision should be based on intraoperative stability of the acetabular component.
In our series, there was a disproportionate representation of fractures associated with elliptic acetabular components. All 5 of the fractures recognized during surgery and 5 of the 11 recognized after surgery occurred with elliptic components. The association between elliptic cup design and periprosthetic IAF was identified earlier, by Haidukewych and colleagues.3 Their series showed a statistically significant increase in fracture incidence with impaction of an elliptic cup into a bed prepared with a hemispheric reamer. In the present series, 75% of our acetabular components were impacted into a bed underreamed by 1 mm to 2 mm. It is typical of many surgeons at our institution to underream by 1 mm to 2 mm regardless of the type of component being implanted, though they show a growing trend to overream by only 1 mm with the PSL component, which has been both safe and reliable in preventing catastrophic posterior column fractures, especially with impaction of small (<50 mm) acetabular components. We have not observed early loosening or other evidence of failure with this technique. Cup impaction generates significant hoop stresses that can easily fracture sclerotic or otherwise poor-quality bone, and the dense bone around the acetabular rim experiences increased stress with impaction of elliptic components.2,11-15 Surgeons must understand the design traits of their components and be cognizant of the true difference between the diameter of the final reamer used and the real diameter of the acetabular component. We recommend having a difference of ≤1 mm to mitigate the risk of IAF occurring with cup insertion. With use of elliptic components, slight overreaming of the acetabular bed should be considered. More study is needed to better define these outcomes.
Study Limitations
Our study had several limitations, including the inherent biases of its retrospective design, small cohort size, and inclusion of multiple surgeons. Small cohort size is unavoidable given the low incidence of these injuries, and our study encompassed the experience of a high-volume hip arthroplasty service. There is the possibility that a subset of fractures may have persistently gone unrecognized, either during or after surgery, and the actual incidence of these complications may be higher. These outcomes represent our institutional experience addressing the complexities of these injuries. The lack of standardization in the management of these fractures in our series reflects the diagnostic dilemma they present, as well as the need for more study focused on their management and outcomes.
Conclusion
IAF, an uncommon complication of primary THA, most commonly occurs during component impaction. Acetabular component and surgical technique may influence the fracture rate. Intraoperative or prompt postoperative recognition of these fractures is crucial, as their location is associated with stability and outcome. Careful examination of postoperative radiographs, judicious use of advanced imaging, and close follow-up are needed to prevent early catastrophic failure. We argue against simply observing these unstable fractures and recommend early treatment with rigid fixation and, when necessary, acetabular component revision.
Take Home Points
- IAF is an uncommon, but serious complication of primary THA.
- Small (<50 mm) cups are at higher risk for causing IAF.
- Prompt recognition is critical to prevent component migration and need for revision.
- Posterior column integrity is cirtical to a successful outcome when IAF occurs.
- Initial stable fixation, with or without intraoperative acetabular revision, is critical for successful outcome when IAF is identified.
Intraoperative acetabular fracture (IAF) is a rare complication of primary total hip arthroplasty (THA).1-3 IAFs commonly occur with impaction of the acetabular component. Studies have found that underreaming of the acetabulum and impaction of relatively large, elliptic, or monoblock components may increase the risk of IAFs.2-5 There is a paucity of literature on risk factors, treatment strategies, and outcomes of this potentially devastating complication.
In this article, we report on the incidence of IAF in primary THA at our high-volume institution and present strategies for managing and preventing this rare fracture.
Materials and Methods
Between 1997 and 2015, more than 20 fellowship-trained arthroplasty surgeons performed 21,519 primary THAs at our institution. After obtaining Institutional Review Board approval for this study, we retrospectively searched the hospital database and identified 16 patients (16 hips) who sustained an IAF in primary THA. Mean age of the cohort (13 women, 3 men) at time of surgery was 70 years (range, 42-89 years). Of the 16 patients, 13 had a preoperative diagnosis of osteoarthritis, 2 had posttraumatic arthritis, and 1 had rheumatoid arthritis. A posterolateral approach was used with 14 patients and a modified anterolateral approach with the other 2. Surgical technique and implant selection varied among surgeons. Thirteen THAs were performed with an all-press-fit technique and 3 with a hybrid technique (uncemented acetabular component, cemented femoral component). In 9 cases, the acetabular component underwent supplemental screw fixation. Whether to use acetabular component screws or cemented femoral components was decided intraoperatively by the surgeon.
The cohort’s acetabular components were either elliptic modular or hemispheric modular. The elliptic modular component used was the Peripheral Self-Locking (PSL) implant (Stryker Howmedica Osteonics), and the hemispheric modular components used were either the Trident implant (Stryker Howmedica Osteonics) or the ZTT-II implant (DePuy Synthes). Elliptic acetabular components have a peripheral flare, in contrast to true hemispheric acetabular components. Ten elliptic modular and 6 hemispheric modular components were implanted. In all cases, the difference between the final reamer used to prepare the acetabular bed and the true largest external diameter of the impacted shell was 2 mm or less.
The cohort’s 16 femoral components consisted of 8 Secur-Fit uncemented components (Stryker Howmedica Osteonics), 3 Accolade uncemented components (Stryker Howmedica Osteonics), 3 Omnifit EON cemented components (Stryker Howmedica Osteonics), and 2 S-ROM uncemented components (DePuy Synthes).
After surgery, all patients were followed up according to individual surgeon protocol for radiographic and physical examination.
Data on IAF incidence were obtained from a hospital database and were confirmed with electronic medical record (EMR) documentation. Also obtained were IAF causes and locations recorded in operative notes. For fractures identified after surgery, location was obtained from the immediate postoperative radiograph. Fracture management (eg, supplemental screw fixation, fracture reduction and fixation, bone grafting, acetabular component revision, protected weight-bearing) was determined from EMR documentation.
Results
Sixteen patients sustained an IAF in primary THA. All IAFs occurred in cases involving cementless acetabular components. The institution’s incidence of IAF with use of cementless components was 0.0007%.
Of the 5 IAFs (31%) identified during surgery, 4 were noted during impaction of the acetabular component, and 1 was noted during reaming. Eighty percent of these IAFs occurred directly posterior, and 60% were addressed at time of index procedure secondary to acetabular component instability. The other 11 fractures (69%) were identified on standard postoperative anteroposterior pelvis radiographs obtained in the postanesthesia care unit (PACU). Details of component characteristics, fracture location, immediate treatment, and weight-bearing precautions for all 16 patients are listed in the Table.
There were additional complications. One patient sustained an intraoperative proximal femur fracture, which was addressed at the index THA with application of a cerclage wire and reinsertion of the femoral component; no further surgical intervention was required, and the femur fracture healed uneventfully. Another patient had a postoperative ileus that required nasogastric tube decompression and monitoring in the intensive care unit; the ileus resolved spontaneously. A third patient, initially treated with bone grafting and cemented cup insertion, was diagnosed with a periprosthetic joint infection 3 weeks after the index THA and was treated with explantation of all components and girdlestone resection arthroplasty; 1 month after the resection arthroplasty, a persistently draining wound was treated with irrigation and débridement. There were no other medical complications, thromboembolic events, or dislocations.
One to 7 weeks after surgery, patients returned for initial follow-up, and radiographs were obtained for component stability assessment. Three patients presented with gross acetabular instability, and revisions were performed. Standard clinical follow-up continued for all patients per individual surgeon protocol. Mean follow-up was 4 years.
Discussion
IAF is an uncommon complication of THA. The rarity of IAFs makes it difficult to obtain a cohort large enough to study the problem. Given the increasing incidence of primary THAs and the almost ubiquitous use of press-fit acetabular components, surgeons who perform THAs undoubtedly will encounter IAFs in their own practice. In this article, we report our institution’s experience with periprosthetic IAFs and provide a framework for making decisions regarding these complications.
Anatomical locations of IAFs have been associated with variable outcomes. In a 2015 series, Laflamme and colleagues6 found posterior column stability a crucial factor in implant stability. Fractures with posterior column instability had a 67% failure rate, and patients with an intact posterior column reliably had osteointegration occur without further intervention.6 In our series, fractures that violated the posterior column had similar results. All these fractures required further operative intervention, either at the index procedure or in the early postoperative period. Loss of posterior column stability prevents secure fixation of the acetabular component, thereby preventing successful hip reconstruction. One posterior column fracture in our series was not recognized until after surgery, on a PACU radiograph, and 1 posterior column fracture was fully appreciated only after postoperative computed tomography (CT) was obtained during immediate hospitalization after the index procedure. In both cases, conservative management was unsuccessful. Revision arthroplasty (and in 1 case late posterior column fixation) was performed to achieve adequate reconstruction. There were no failures after posterior column fixation. In cases of posterior wall or column fracture, we recommend early aggressive treatment, preferably at the time of index arthroplasty, to prevent catastrophic failure.
Most commonly, periprosthetic IAFs go unnoticed until initial postoperative radiographs are examined.6 Eleven of the 16 IAFs in our series were first recognized on radiographs obtained in the PACU. Surgeons thus have difficult decisions to make. The literature has little discussion on managing early postoperative periprosthetic IAFs. Most recent studies, which consist of small series and case reports, have focused on late and often traumatic IAFs.7-9 These were initially classified by Peterson and Lewallen10 as type I, which are stable radiographically (no movement relative to previous radiographs) and do not produce pain with minor movement of the extremity, or type II, which are unstable radiographically (gross displacement of component) or produce pain with any hip motion. Type I fractures were more common and were often managed with protected weight-bearing and observation. The authors concluded that, in type I fractures, retaining the original acetabular component is difficult; however, when these fractures are treated appropriately, a functional prosthesis can be salvaged, and fracture union can be expected.
Less common are acetabular fractures detected during surgery, as in our study. In an outcome series, Haidukewych and colleagues3 reported on 21 periprosthetic acetabular fractures, all recognized during surgery and managed according to perceived stability of the component. All fractures healed uneventfully, and there were no other complications.
These studies provide a framework for addressing IAFs noticed in the early postoperative period. The diagnostic dilemma presented by these fractures was first discussed by Laflamme and colleagues.6 Nine of the 32 fractures in their series were classified as so-called type III fractures, recognized only after the early postoperative period. Additional radiographs (eg, Judet views) or CT scans were crucial in determining acetabular component stability, given the known poor outcomes associated with posterior column fracture. In our series, only 1 patient had CT performed after intraoperative recognition of fracture, and the extent of the fracture was not readily apparent on the patient’s postoperative radiograph. Given the successful recognition and treatment of these fractures in the early postoperative period in our series,
it is difficult to recommend advanced imaging for all periprosthetic IAFs. Perhaps this success is attributable to our almost universal use of screws for acetabular component fixation. Of the 11 patients with fractures recognized during the postoperative period, 8 had supplemental screw fixation at time of index surgery. If there is a question of fixation during component insertion, we recommend scrutinizing the acetabular rim for fracture and placing supplemental screw fixation. Screws placed for acetabular component fixation provide initial stability and may prevent early component failure in the setting of unrecognized medial or anterior fracture. In addition, when component stability is in question after impaction, we recommend using finger palpation to evaluate the sciatic notch for cortical step-off from an otherwise unrecognized fracture. Protected weight-bearing in the postoperative period may be left to the discretion of the surgeon, and the decision should be based on intraoperative stability of the acetabular component.
In our series, there was a disproportionate representation of fractures associated with elliptic acetabular components. All 5 of the fractures recognized during surgery and 5 of the 11 recognized after surgery occurred with elliptic components. The association between elliptic cup design and periprosthetic IAF was identified earlier, by Haidukewych and colleagues.3 Their series showed a statistically significant increase in fracture incidence with impaction of an elliptic cup into a bed prepared with a hemispheric reamer. In the present series, 75% of our acetabular components were impacted into a bed underreamed by 1 mm to 2 mm. It is typical of many surgeons at our institution to underream by 1 mm to 2 mm regardless of the type of component being implanted, though they show a growing trend to overream by only 1 mm with the PSL component, which has been both safe and reliable in preventing catastrophic posterior column fractures, especially with impaction of small (<50 mm) acetabular components. We have not observed early loosening or other evidence of failure with this technique. Cup impaction generates significant hoop stresses that can easily fracture sclerotic or otherwise poor-quality bone, and the dense bone around the acetabular rim experiences increased stress with impaction of elliptic components.2,11-15 Surgeons must understand the design traits of their components and be cognizant of the true difference between the diameter of the final reamer used and the real diameter of the acetabular component. We recommend having a difference of ≤1 mm to mitigate the risk of IAF occurring with cup insertion. With use of elliptic components, slight overreaming of the acetabular bed should be considered. More study is needed to better define these outcomes.
Study Limitations
Our study had several limitations, including the inherent biases of its retrospective design, small cohort size, and inclusion of multiple surgeons. Small cohort size is unavoidable given the low incidence of these injuries, and our study encompassed the experience of a high-volume hip arthroplasty service. There is the possibility that a subset of fractures may have persistently gone unrecognized, either during or after surgery, and the actual incidence of these complications may be higher. These outcomes represent our institutional experience addressing the complexities of these injuries. The lack of standardization in the management of these fractures in our series reflects the diagnostic dilemma they present, as well as the need for more study focused on their management and outcomes.
Conclusion
IAF, an uncommon complication of primary THA, most commonly occurs during component impaction. Acetabular component and surgical technique may influence the fracture rate. Intraoperative or prompt postoperative recognition of these fractures is crucial, as their location is associated with stability and outcome. Careful examination of postoperative radiographs, judicious use of advanced imaging, and close follow-up are needed to prevent early catastrophic failure. We argue against simply observing these unstable fractures and recommend early treatment with rigid fixation and, when necessary, acetabular component revision.
1. Sharkey PF, Hozack WJ, Callaghan JJ, et al. Acetabular fractures associated with cementless acetabular cup insertion: a report of 13 cases. J Arthroplasty.1999;14(4):426-431.
2. Kim YS, Callaghan JJ, Ahn PB, Brown TD. Fracture of the acetabulum during insertion of an oversized hemispherical component. J Bone Joint Surg Am. 1995;77(1):111-117.
3. Haidukewych GJ, Jacofsky DJ, Hanssen AD, Lewallen DG. Intraoperative fractures of the acetabulum during primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(9):1952-1956.
4. Curtis MJ, Jinnah RH, Wilson VD, Hungerford DS. The initial stability of uncemented acetabular components. J Bone Joint Surg Br. 1992;74(3):372-376.
5. Lachiewicz PF, Suh PB, Gilbert JA. In vitro initial fixation of porous-coated acetabular total hip components. A biomechanical and comparative study. J Arthroplasty. 1989;4(3):201-205.
6. Laflamme GY, Belzile EL, Fernandes JC, Vendittoli PA, Hébert-Davies J. Periprosthetic fractures of the acetabulum during component insertion: posterior column stability
is crucial. J Arthroplasty. 2015;30(2):265-269.
7. Desai G, Reis MD. Early postoperative acetabular discontinuity after total hip arthroplasty. J Arthroplasty. 2011;26(8):1570.e17-e19.
8. Gelalis ID, Politis AN, Arnaoutoglou CM, Georgakopoulos N, Mitsiou D, Xenakis TA. Traumatic periprosthetic acetabular fracture treated by acute one-stage revision arthroplasty. A case report and review of the literature. Injury. 2010;41(4):421-424.
9. Gras F, Marintschev I, Klos K, Fujak A, Mückley T, Hofmann GO. Navigated percutaneous screw fixation of a periprosthetic acetabular fracture. J Arthroplasty. 2010;25(7):1169.e1-e4.
10. Peterson CA, Lewallen DG. Periprosthetic fracture of the acetabulum after total hip arthroplasty. J Bone Joint Surg Am. 1996;78(8):1206-1213.
11. Hansen TM, Koenman JB, Headley AK. 3-D FEM analysis of interface fixation of acetabular implants. Trans Orthop Res Soc. 1992;17:400.
12. Yerby SA, Taylor JK, Murzic WJ. Acetabular component interface: press-fit fixation. Trans Orthop Res Soc. 1992;17:384.
13. Callaghan JJ. The clinical results and basic science of total hip arthroplasty with porous-coated prostheses. J Bone Joint Surg Am. 1993;75(2):299-310.
14. Cheng SL, Binnington AG, Bragdon CR, Jasty M, Harris WH, Davey JR. The effect of sizing mismatch on bone ingrowth into uncemented porous coated acetabular components: an in vivo canine study. Trans Orthop Res Soc. 1990;15:442.
15. Morscher E, Bereiter H, Lampert C, Cementless press-fit cup: principles, experimental data, and three-year follow-up study. Clin Orthop Relat Res. 1989;(249):12-20.
1. Sharkey PF, Hozack WJ, Callaghan JJ, et al. Acetabular fractures associated with cementless acetabular cup insertion: a report of 13 cases. J Arthroplasty.1999;14(4):426-431.
2. Kim YS, Callaghan JJ, Ahn PB, Brown TD. Fracture of the acetabulum during insertion of an oversized hemispherical component. J Bone Joint Surg Am. 1995;77(1):111-117.
3. Haidukewych GJ, Jacofsky DJ, Hanssen AD, Lewallen DG. Intraoperative fractures of the acetabulum during primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(9):1952-1956.
4. Curtis MJ, Jinnah RH, Wilson VD, Hungerford DS. The initial stability of uncemented acetabular components. J Bone Joint Surg Br. 1992;74(3):372-376.
5. Lachiewicz PF, Suh PB, Gilbert JA. In vitro initial fixation of porous-coated acetabular total hip components. A biomechanical and comparative study. J Arthroplasty. 1989;4(3):201-205.
6. Laflamme GY, Belzile EL, Fernandes JC, Vendittoli PA, Hébert-Davies J. Periprosthetic fractures of the acetabulum during component insertion: posterior column stability
is crucial. J Arthroplasty. 2015;30(2):265-269.
7. Desai G, Reis MD. Early postoperative acetabular discontinuity after total hip arthroplasty. J Arthroplasty. 2011;26(8):1570.e17-e19.
8. Gelalis ID, Politis AN, Arnaoutoglou CM, Georgakopoulos N, Mitsiou D, Xenakis TA. Traumatic periprosthetic acetabular fracture treated by acute one-stage revision arthroplasty. A case report and review of the literature. Injury. 2010;41(4):421-424.
9. Gras F, Marintschev I, Klos K, Fujak A, Mückley T, Hofmann GO. Navigated percutaneous screw fixation of a periprosthetic acetabular fracture. J Arthroplasty. 2010;25(7):1169.e1-e4.
10. Peterson CA, Lewallen DG. Periprosthetic fracture of the acetabulum after total hip arthroplasty. J Bone Joint Surg Am. 1996;78(8):1206-1213.
11. Hansen TM, Koenman JB, Headley AK. 3-D FEM analysis of interface fixation of acetabular implants. Trans Orthop Res Soc. 1992;17:400.
12. Yerby SA, Taylor JK, Murzic WJ. Acetabular component interface: press-fit fixation. Trans Orthop Res Soc. 1992;17:384.
13. Callaghan JJ. The clinical results and basic science of total hip arthroplasty with porous-coated prostheses. J Bone Joint Surg Am. 1993;75(2):299-310.
14. Cheng SL, Binnington AG, Bragdon CR, Jasty M, Harris WH, Davey JR. The effect of sizing mismatch on bone ingrowth into uncemented porous coated acetabular components: an in vivo canine study. Trans Orthop Res Soc. 1990;15:442.
15. Morscher E, Bereiter H, Lampert C, Cementless press-fit cup: principles, experimental data, and three-year follow-up study. Clin Orthop Relat Res. 1989;(249):12-20.
VIDEO: Hip, knee replacements fall in Danish RA patients
MADRID – The rates of both total hip and total knee replacement surgeries dropped among Danish patients with rheumatoid arthritis since the mid-1990s, reductions that were coincident with more widespread use of biologic drugs as well as with other improvements in care, according to analyses of Danish national health records.
“The introduction of guidelines [on biologic drug use] in 2002 and increasing use of biologic drugs [as a result] may have contributed to this positive development,” Lene Dreyer, MD, said at the European Congress of Rheumatology. Other factors that may have also contributed include widespread use of conventional disease-modifying antirheumatic drugs (DMARDs) and adoption of a treat-to-target strategy by many clinicians.
In 1996, the first year studied and before any biologic DMARDs were routinely used for rheumatoid arthritis, the rate of total knee replacement was nearly 6/1,000 person-years among RA patients, compared with a 0.42/1,000 person-years rate in the general adult Danish population, a roughly 14-fold excess among the RA patients, Dr. Dreyer reported. But by 2016, ”this gap had almost disappeared,” she said in a video interview. “It seems like rheumatologists in Denmark are doing a good job” treating RA patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That may have been especially true subsequent to 2002, when the Danish Institute for Rational Pharmacotherapy issued recommendations that opened the door to wider use of biologic DMARDs, such as tumor necrosis factor inhibitors, to treat RA patients, noted Dr. Dreyer of Gentofte University Hospital, Copenhagen. During 2003-2011, use of total knee replacement surgery in RA patients fell by an average annualized rate of 0.2 surgeries/1,000 person-years. But among the general Danish population the average annualized rate of knee surgeries rose by 0.08/1,000 person-years.
“This is a very important finding,” commented Robert Landewé, MD, PhD, professor of rheumatology at the Academic Medical Center in Amsterdam. “It is extremely difficult to test the effect of the introduction of the [biologic DMARD] guidelines,” he cautioned. But he highlighted the positive finding that the excess of hip and knee replacement surgeries in patients with RA, compared with the general population, had recently narrowed.
Dr. Dreyer and her associates used records from the Danish National Patient Register to compare 29,427 patients with incident RA during 1996-2011 with more than 290,000 matched control individuals. All people studied had not undergone knee or hip replacement surgery prior to their entry into the study. The researchers used an “interrupted time series analysis” to examine the possible impact of the introduction of widespread access to biologic DMARDs starting in 2003.
The analysis showed that the rate of total hip replacements in 1996 was nearly 9 surgeries/1,000 person-years among RA patients and nearly 3/1,000 person-years in the general population, a threefold excess for RA patients. This rate fell by an average annual rate of 0.38/1,000 person-years among RA patients both before and after 2002, so that by 2011 the rate was roughly half the 1996 rate, about 4.5/1,000 patient-years. The rate in the general population rose during 1996-2011, and by 2011 was nearly 4/1,000 person-years and so nearly the same as RA patients. Wider availability of biologic DMARDs for RA patients starting in 2003 did not have an apparent impact on the rate of total hip replacement.
In contrast, wider use of biologic DMARDs appeared to have an effect on the rate of total knee surgeries among RA patients. During 1996-2001, the rate rose by an annual average of 0.19/1,000 person-years, very similar to the 0.21/1,000 person-years annual rise in the general Danish population. However, during 2003-2011, the average annual rate of total knee surgery fell by 0.20/1,000 person-years in the RA patients but continued to rise at an annual average rate of 0.08/1,000 person-years in the general population, Dr. Dreyer reported.
Additional Danish registry data exist for patients who received biologic DMARDs, and Dr. Dreyer said that she and her associates hope to use this to further examine the impact of these drugs on patient outcomes.
Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – The rates of both total hip and total knee replacement surgeries dropped among Danish patients with rheumatoid arthritis since the mid-1990s, reductions that were coincident with more widespread use of biologic drugs as well as with other improvements in care, according to analyses of Danish national health records.
“The introduction of guidelines [on biologic drug use] in 2002 and increasing use of biologic drugs [as a result] may have contributed to this positive development,” Lene Dreyer, MD, said at the European Congress of Rheumatology. Other factors that may have also contributed include widespread use of conventional disease-modifying antirheumatic drugs (DMARDs) and adoption of a treat-to-target strategy by many clinicians.
In 1996, the first year studied and before any biologic DMARDs were routinely used for rheumatoid arthritis, the rate of total knee replacement was nearly 6/1,000 person-years among RA patients, compared with a 0.42/1,000 person-years rate in the general adult Danish population, a roughly 14-fold excess among the RA patients, Dr. Dreyer reported. But by 2016, ”this gap had almost disappeared,” she said in a video interview. “It seems like rheumatologists in Denmark are doing a good job” treating RA patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That may have been especially true subsequent to 2002, when the Danish Institute for Rational Pharmacotherapy issued recommendations that opened the door to wider use of biologic DMARDs, such as tumor necrosis factor inhibitors, to treat RA patients, noted Dr. Dreyer of Gentofte University Hospital, Copenhagen. During 2003-2011, use of total knee replacement surgery in RA patients fell by an average annualized rate of 0.2 surgeries/1,000 person-years. But among the general Danish population the average annualized rate of knee surgeries rose by 0.08/1,000 person-years.
“This is a very important finding,” commented Robert Landewé, MD, PhD, professor of rheumatology at the Academic Medical Center in Amsterdam. “It is extremely difficult to test the effect of the introduction of the [biologic DMARD] guidelines,” he cautioned. But he highlighted the positive finding that the excess of hip and knee replacement surgeries in patients with RA, compared with the general population, had recently narrowed.
Dr. Dreyer and her associates used records from the Danish National Patient Register to compare 29,427 patients with incident RA during 1996-2011 with more than 290,000 matched control individuals. All people studied had not undergone knee or hip replacement surgery prior to their entry into the study. The researchers used an “interrupted time series analysis” to examine the possible impact of the introduction of widespread access to biologic DMARDs starting in 2003.
The analysis showed that the rate of total hip replacements in 1996 was nearly 9 surgeries/1,000 person-years among RA patients and nearly 3/1,000 person-years in the general population, a threefold excess for RA patients. This rate fell by an average annual rate of 0.38/1,000 person-years among RA patients both before and after 2002, so that by 2011 the rate was roughly half the 1996 rate, about 4.5/1,000 patient-years. The rate in the general population rose during 1996-2011, and by 2011 was nearly 4/1,000 person-years and so nearly the same as RA patients. Wider availability of biologic DMARDs for RA patients starting in 2003 did not have an apparent impact on the rate of total hip replacement.
In contrast, wider use of biologic DMARDs appeared to have an effect on the rate of total knee surgeries among RA patients. During 1996-2001, the rate rose by an annual average of 0.19/1,000 person-years, very similar to the 0.21/1,000 person-years annual rise in the general Danish population. However, during 2003-2011, the average annual rate of total knee surgery fell by 0.20/1,000 person-years in the RA patients but continued to rise at an annual average rate of 0.08/1,000 person-years in the general population, Dr. Dreyer reported.
Additional Danish registry data exist for patients who received biologic DMARDs, and Dr. Dreyer said that she and her associates hope to use this to further examine the impact of these drugs on patient outcomes.
Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – The rates of both total hip and total knee replacement surgeries dropped among Danish patients with rheumatoid arthritis since the mid-1990s, reductions that were coincident with more widespread use of biologic drugs as well as with other improvements in care, according to analyses of Danish national health records.
“The introduction of guidelines [on biologic drug use] in 2002 and increasing use of biologic drugs [as a result] may have contributed to this positive development,” Lene Dreyer, MD, said at the European Congress of Rheumatology. Other factors that may have also contributed include widespread use of conventional disease-modifying antirheumatic drugs (DMARDs) and adoption of a treat-to-target strategy by many clinicians.
In 1996, the first year studied and before any biologic DMARDs were routinely used for rheumatoid arthritis, the rate of total knee replacement was nearly 6/1,000 person-years among RA patients, compared with a 0.42/1,000 person-years rate in the general adult Danish population, a roughly 14-fold excess among the RA patients, Dr. Dreyer reported. But by 2016, ”this gap had almost disappeared,” she said in a video interview. “It seems like rheumatologists in Denmark are doing a good job” treating RA patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That may have been especially true subsequent to 2002, when the Danish Institute for Rational Pharmacotherapy issued recommendations that opened the door to wider use of biologic DMARDs, such as tumor necrosis factor inhibitors, to treat RA patients, noted Dr. Dreyer of Gentofte University Hospital, Copenhagen. During 2003-2011, use of total knee replacement surgery in RA patients fell by an average annualized rate of 0.2 surgeries/1,000 person-years. But among the general Danish population the average annualized rate of knee surgeries rose by 0.08/1,000 person-years.
“This is a very important finding,” commented Robert Landewé, MD, PhD, professor of rheumatology at the Academic Medical Center in Amsterdam. “It is extremely difficult to test the effect of the introduction of the [biologic DMARD] guidelines,” he cautioned. But he highlighted the positive finding that the excess of hip and knee replacement surgeries in patients with RA, compared with the general population, had recently narrowed.
Dr. Dreyer and her associates used records from the Danish National Patient Register to compare 29,427 patients with incident RA during 1996-2011 with more than 290,000 matched control individuals. All people studied had not undergone knee or hip replacement surgery prior to their entry into the study. The researchers used an “interrupted time series analysis” to examine the possible impact of the introduction of widespread access to biologic DMARDs starting in 2003.
The analysis showed that the rate of total hip replacements in 1996 was nearly 9 surgeries/1,000 person-years among RA patients and nearly 3/1,000 person-years in the general population, a threefold excess for RA patients. This rate fell by an average annual rate of 0.38/1,000 person-years among RA patients both before and after 2002, so that by 2011 the rate was roughly half the 1996 rate, about 4.5/1,000 patient-years. The rate in the general population rose during 1996-2011, and by 2011 was nearly 4/1,000 person-years and so nearly the same as RA patients. Wider availability of biologic DMARDs for RA patients starting in 2003 did not have an apparent impact on the rate of total hip replacement.
In contrast, wider use of biologic DMARDs appeared to have an effect on the rate of total knee surgeries among RA patients. During 1996-2001, the rate rose by an annual average of 0.19/1,000 person-years, very similar to the 0.21/1,000 person-years annual rise in the general Danish population. However, during 2003-2011, the average annual rate of total knee surgery fell by 0.20/1,000 person-years in the RA patients but continued to rise at an annual average rate of 0.08/1,000 person-years in the general population, Dr. Dreyer reported.
Additional Danish registry data exist for patients who received biologic DMARDs, and Dr. Dreyer said that she and her associates hope to use this to further examine the impact of these drugs on patient outcomes.
Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: RA patient hip replacements fell from nearly 9/1,000 person-years in 1996 to about 4.5/1,000 person-years in 2011.
Data source: Records from more than 300,000 people in the Danish National Patient Register.
Disclosures: Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
Does Preoperative Pneumonia Affect Complications of Geriatric Hip Fracture Surgery?
Take-Home Points
- The prevalence of preoperative pneumonia is 1.2% among hip fracture patients aged >65 years.
- Preoperative pneumonia is an independent risk factor for mortality and adverse events including renal failure, prolonged ventilator dependence, and prolonged altered mental status after geriatric hip fracture surgery.
- Underweight BMI (<18.5 kg/m2) was associated with higher mortality within 30 days among hip fracture patients admitted with pneumonia.
- The mortality rate normalized to that of patients without pneumonia within 2 weeks of hip fracture surgery.
- Time from admission to surgery was not associated with adverse events or mortality among hip fracture patients admitted with pneumonia.
Preoperative pneumonia remains relatively unexplored as a risk factor for adverse outcomes in geriatric hip fracture surgery. Dated studies report a 0.3% to 3.2% prevalence of “recent pneumonia” in patients presenting with hip fracture but provide neither a definition of pneumonia based on clinical criteria nor a subset analysis of outcomes in the pneumonia group.1-3 Although active pneumonia has been identified as a preoperative optimization target in the management guidelines for geriatric hip fracture,4 we are unaware of any studies that have reported on differences in demographics, comorbidities, delay to surgery, or adverse outcomes between hip fracture patients with and without preoperative pneumonia.
This paucity of information on the effect of preoperative pneumonia in the hip fracture population may be related to low prevalence of preoperative pneumonia and a cadre of variable definitions, which limit identification of a cohort of patients with preoperative pneumonia large enough from which to draw meaningful results. Database studies, especially those using surgical registries rather than administrative or reimbursement data, offer particular advantages for investigation of such rare clinical entities.5Medical care of patients with pneumonia alone is known to be facilitated by assessments of mortality risk from clinical and laboratory data. The modified British Thoracic Society rule/CURB-65 (confusion, urea, respiratory rate, blood pressure) score is strongly predictive of mortality in hospitalized adults with pneumonia (odds ratio [OR], 4.59; 95% confidence interval [CI], 1.42-14.85; P = .011) and may guide antibiotic therapy, laboratory investigations, and the decision to intubate in a patient with pneumonia.6-8 This score is predictive of adverse events (AEs), hospital length of stay, and use of intensive care services.6,7,9-13 We hypothesized that preoperative clinical indicators assessed by pneumonia severity scores as well as patient demographics and baseline comorbidities may also have prognostic value for risk of AEs in a cohort of geriatric hip fracture surgery patients with preoperative pneumonia.
In this article, we first describe the prevalence of preoperative pneumonia in geriatric hip fracture surgery patients as well as demographic and operative differences between patients with and without the disease. We then ask 3 questions: Is preoperative pneumonia an independent risk factor for mortality and adverse outcomes in geriatric hip fracture surgery? Is there a postoperative interval during which the unadjusted mortality rate is higher among patients with preoperative pneumonia? In patients with preoperative pneumonia, what are the predictors of morbidity and mortality?
Methods
Yale University’s Human Investigations Committee approved this retrospective cohort study, which used the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database for the period 2005 to 2012. ACS-NSQIP is a prospective, multi-institutional outcomes program that collects data on preoperative comorbidities, intraoperative variables, and 30-day postoperative outcomes for patients undergoing surgical procedures in inpatient and outpatient settings.14
Unlike administrative databases, which are based on reimbursement data, ACS-NSQIP data are collected by trained surgical clinical reviewers for the purposes of quality improvement and clinical research, and data quality is ensured with routine auditing.15 The program has gained a high degree of respect as a powerful and valid data source in both general16 and orthopedic17 surgery literature. The database offers a particular advantage with respect to the study of preoperative pneumonia: Only patients with new or recently diagnosed pneumonia on antibiotic therapy who meet strict criteria for characteristic findings on chest radiography, clinical signs and symptoms of respiratory illness, and positive cultures are coded as having actively treated pneumonia at time of surgery.15
To identify hip fracture patients over the age of 65 years who underwent operative fixation of a hip fracture, we used Current Procedural Terminology (CPT) hip fracture codes, including 27235 (percutaneous screw fixation), 27236 or 27244 (plate-and-screw fixation), and 27245 (intramedullary device), as well as 27125 (hemiarthroplasty) and 27130 (arthroplasty) for patients with a postoperative International Classification of Disease, Ninth Revision (ICD-9) diagnosis code (820.x, 820.2x, or 820.8) consistent with acute hip fracture.18,19 Procedure type, anesthesia type, and delay from admission to surgery were captured for all procedures.
Preoperative demographics included age, sex, transfer origin, functional status, and body mass index (BMI) category. Binary comorbidities were classified as preoperative anemia (hematocrit, <0.41 for men, <0.36 for women), confusion, dyspnea at rest, uremia (blood urea nitrogen, >6.8 mmol/L), history of cardiovascular disease (congestive heart failure, myocardial infarction, percutaneous coronary intervention, angina pectoris, medically treated hypertension, peripheral vascular disease, or resting claudication), chronic obstructive pulmonary disease, diabetes, renal disease (renal failure or dialysis), and cigarette use in preceding 12 months.20,21 Although preoperative hypotension and respiratory rate are often considered in patients with pneumonia, these variables were not available from the ACS-NSQIP data.6,22Pearson χ2 test for categorical variables was used to compare baseline demographics and operative characteristics between patients with and without pneumonia, and Student t test was used to compare intervals from hospital admission to hip fracture surgery, surgery start to surgery stop, and surgery to discharge between patients with and without preoperative pneumonia.
Binary outcome measures were compared between patients with and without preoperative pneumonia. “Any AE” included any serious AE (SAE) or any minor AE. SAEs included death, acute renal failure, ventilator use >48 hours, unplanned intubation, septic shock, sepsis, return to operating room, coma >24 hours, cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction, thromboembolic event (deep vein thrombosis or pulmonary embolism), and stroke/cerebrovascular accident. Minor AEs included progressive renal insufficiency, urinary tract infection, organ/space infection, superficial surgical-site infection, deep surgical-site infection, and wound dehiscence. Other binary outcome measures included discharge destination and unplanned readmission within 30 days after hip fracture surgery.23Poisson regression with robust error variance as described by Zou24 was used to compare the rates of any, minor, and individual AEs, and any SAEs, between patients with and without pneumonia. Multivariate analysis accounted for the baseline variables in Table 1. AEs that occurred more than once in each group were included in the analyses.
Kaplan-Meier survival analysis was performed for postoperative mortality within 30 days. Within the preoperative pneumonia group, covariates from Table 1 were identified as predictors of any AE, SAE, or death within 30 days after hip fracture surgery by stepwise multivariate Poisson regression with robust error variance. When interval from admission to surgery was longer than 24, 48, 72, or 96 hours, it was also included as a covariate. Variables that did not show an association with AEs at the P < .20 level were not included in the final regression model. All analyses were performed with Stata/SE Version 12.0 statistical software (StataCorp).
Results
Of the 7128 geriatric hip fracture patients in this study, 82 (1.2%) had active pneumonia at time of surgery (Table 1). Age, BMI, preoperative uremia, history of cardiovascular disease, diabetes, renal disease, and smoking were similar between groups. In addition, there was no difference in anesthesia type or fixation procedure between the pneumonia and no-pneumonia groups. Patients with preoperative pneumonia differed significantly with respect to sex, transfer from facility, preoperative functional dependence, anemia, confusion, dyspnea at rest, and history of chronic obstructive pulmonary disease (Table 1).
Interval from admission to surgery was longer (P < .001) for geriatric hip fracture patients with preoperative pneumonia (mean, 6.8 days; 95% CI, 2.5-11.1 days) than for those without pneumonia (mean, 1.5 days; CI, 1.4-1.5 days). There was no difference (P = .124) in operative time between the pneumonia group (mean, 72.8 min; CI, 64.0-81.5 min) and the no-pneumonia group (mean, 66.1 min; CI, 61.2-67.0 min). Interval from surgery to discharge was longer (P < .001) for patients with preoperative pneumonia (mean, 10.1 days; CI, 6.9-13.4 days) than for those without pneumonia (mean, 6.3 days; CI, 6.1-6.4 days).
Adverse outcomes of geriatric hip fracture surgery are listed in Table 2. In the multivariate analysis, preoperative pneumonia was significantly associated with any AE (relative risk [RR]) = 1.44) and any SAE (RR = 1.79).
Survival patterns diverged between patients with and without preoperative pneumonia (Figure). The unadjusted mortality rate was qualitatively higher in patients with preoperative pneumonia than in patients without pneumonia during the first days after hip fracture (slopes of unadjusted mortality curves in Figure). Of note, no patient under age 75 years with pneumonia at time of surgery died within the 30-day study period.
Among geriatric hip fracture patients with preoperative pneumonia, multivariate analyses revealed no significant association of any preoperative comorbidity with any AE or any SAE. Given the gravity of the death complication, however, death within 30 days after surgery was analyzed separately, and was found to be significantly associated (RR = 4.67) with being underweight (BMI, <18.5 kg/m2) (Table 3). Admission-to-surgery interval longer than 24, 48, 72, or 96 hours did not reach significance at the P < 0.2 level in the stepwise regressions and therefore was not associated with a higher or lower risk of any AE, SAE, or death.
Discussion
In the general US population, pneumonia accounts for 1.4% of deaths in people 65 years to 74 years old, 2.1% in people 75 years to 84 years, and 3.1% in people 85 years or older. In total, 3.4% of hospital inpatient deaths are attributed to pneumonia.25 In hospitalized general orthopedic surgical patients as well as hip fracture patients, pneumonia is strongly associated with increased mortality.26,27
We identified a preoperative pneumonia prevalence of 1.2%, which is comparable to the rates reported in the literature (0.3%-3.2%).1-3 To our knowledge, our study represents the largest series of patients with preoperative pneumonia at time of hip fracture repair, and the first to independently associate preoperative pneumonia with increased incidence of AEs, including death.
This study had its limitations. First, the ACS-NSQIP morbidity and mortality data, which are limited to the first 30 postoperative days, may be skewed because AEs that occurred after that interval are not captured. Second, coding of pneumonia in ACS-NSQIP does not convey specific information about the disease and its severity—infectious organism(s) responsible; acquisition setting (healthcare or community); treatment given, including antibiotic(s) selection, steroid use, dosing, and duration; and measures of treatment efficacy—limiting interpretation of the difference in delay to surgery. We cannot say whether the longer interval in patients with pneumonia reflects medical optimization, or whether the delay itself or any interventions during that time positively or negatively affected outcomes. In addition, despite using a large national database, we obtained a relatively small sample of patients (82) who had pneumonia before surgical hip fracture repair.
Multivariate analysis controlling for baseline demographics and comorbidities revealed that multiple SAEs were independently associated with preoperative pneumonia (overall SAE, RR = 1.79). Postoperative use of ventilator support for longer than 48 hours (RR = 6.48) and coma longer than 24 hours (RR = 7.31) are expected given the severity of pulmonary compromise in the study cohort.28,29 Acute renal failure (RR = 14.61) can occur in both hip fracture patients and community-acquired pneumonia patients and may be a multifactorial complication of the pulmonary infection, of the anesthesia, or of the surgical intervention in this cohort.30-32Unadjusted mortality in hip fracture takes months to a year to normalize to that of age-matched controls.32-34 In our series, the unadjusted death rate in the pneumonia cohort (Figure) was transiently elevated during the first weeks after surgery but then drew nearer the rate in the nondiseased hip fracture cohort by the end of the first month. Early death in the pneumonia group likely was multifactorial, potentially influenced by the increased burden of comorbidities in the pneumonia group at baseline, and the longer delay to surgery,35-38 as well as by the natural history of treated pneumonia in hospital patients, who, compared with age-matched hospitalized controls, also exhibit higher mortality during only the first 2 to 4 months of hospitalization for pneumonia.39 We regret that quality improvement strategies in the treatment of geriatric hip fracture surgery with pneumonia cannot be extrapolated from these results.
Similarly, the utility of BMI <18.5 kg/m2 as an actionable preoperative finding cannot be assessed from these results. However, we propose that underweight geriatric hip fracture patients with pneumonia may benefit from more aggressive preoperative optimization that does not delay surgery. Higher acuity of postoperative care, including more intensive nursing care and early coordination of care with respiratory therapists and medical comanagement teams, may also be beneficial.
Anesthesia type did not differ between patients with and without preoperative pneumonia and was not associated with AEs in patients with preoperative pneumonia. Consistent with our findings, multiple studies have reported no significant differences in short-term outcomes of hip fracture repair between general and spinal anesthesia, though no other study has compared the benefits of general and spinal anesthesia for patients with preoperative pneumonia.40-44 Although spinal anesthesia (relative to general anesthesia) has been reported to have benefits in hip and knee arthroplasty, these benefits appear not to translate to hip fracture repair.45-50 The results of the present study suggest that general and spinal anesthesia may be equivalent in terms of risk for the geriatric hip fracture patient with preoperative pneumonia.43,44Our attempt to evaluate the CURB-65 pneumonia severity score as a prognosticator of AEs was thwarted by the absence of required variables in the ACS-NSQIP dataset (confusion, uremia, dyspnea, and age were available; hypotension and blood pressure were not). In our analysis, we did include, individually, variables previously found to predict AEs in the medical pneumonia population (confusion, uremia, dyspnea at rest, anemia).9-11,32 However, these clinical findings are nonspecific in hip fracture patients, who may become anemic, confused, dyspneic, or uremic from a multitude of factors related to their injury and unrelated to pneumonia, including but not limited to hemorrhage, muscle damage, renal injury, and pulmonary embolism. It is not surprising that confusion, uremia, dyspnea at rest, and anemia were not individually predictive of AEs or death within 30 days after surgery in the cohort of geriatric hip fracture patients with pneumonia.
There is no literature that argues for or against delaying hip fracture surgery in geriatric hip fracture patients with pneumonia. The surgical delay observed in this population is ostensibly related to medical optimization of the pneumonia and/or underlying comorbidities. However, we did not find a morbidity or mortality detriment or benefit in delaying surgery by 1 to 4 days in this population. Delay of surgery is a poor covariate, given extensive confounding by medical management and preoperative optimizing of comorbid conditions (reflected in our independent variable and covariates) as well as institutional and surgeon variations in policy and behavior and other unaccounted influences. Although some authors have found no difference in mortality or major AEs between hip fracture patients who had a surgical delay and those who did not,31,51-53 other series and meta-analyses have suggested a mortality detriment in a surgical delay of more than 2 days36,54 or 4 days55 from admission. Given our data, we cannot recommend against immediate hip fracture repair in the subpopulation of geriatric hip fracture patients with pneumonia.
Our study findings suggest that preoperative pneumonia is a rare independent risk factor for AEs after hip fracture surgery in geriatric patients. Underweight BMI is predictive of death in geriatric hip fracture surgery patients who present with pneumonia, whereas early surgical repair appears not to be associated with adverse outcomes. Further investigation is warranted to determine if such patients benefit from specific preoperative and postoperative strategies for optimizing medical and surgical care based on these findings.
Am J Orthop. 2017;46(3):E177-E185. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Sexson SB, Lehner JT. Factors affecting hip fracture mortality. J Orthop Trauma. 1987;1(4):298-305.
2. Mullen JO, Mullen NL. Hip fracture mortality: a prospective, multifactorial study to predict and minimize death risk. Clin Orthop Relat Res. 1992;(280):214-222.
3. Kenzora JE, McCarthy RE, Lowell JD, Sledge CB. Hip fracture mortality. Relation to age, treatment, preoperative illness, time of surgery, and complications. Clin Orthop Relat Res. 1984;(186):45-56.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24(4):701-719.
5. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
6. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377-382.
7. Myint PK, Kamath AV, Vowler SL, Maisey DN, Harrison BDW. The CURB (confusion, urea, respiratory rate and blood pressure) criteria in community-acquired pneumonia (CAP) in hospitalised elderly patients aged 65 years and over: a prospective observational cohort study. Age Ageing. 2005;34(1):75-77.
8. Wilkinson M, Woodhead MA. Guidelines for community-acquired pneumonia in the ICU. Curr Opin Crit Care. 2004;10(1):59-64.
9. Buising K, Thursky K, Black J, et al. A prospective comparison of severity scores for identifying patients with severe community acquired pneumonia: reconsidering what is meant by severe pneumonia. Thorax. 2006;61(5):419-424.
10. Ewig S, De Roux A, Bauer T, et al. Validation of predictive rules and indices of severity for community acquired pneumonia. Thorax. 2004;59(5):421-427.
11. Yandiola PP, Capelastegui A, Quintana J, et al. Prospective comparison of severity scores for predicting clinically relevant outcomes for patients hospitalized with community-acquired pneumonia. Chest. 2009;135(6):1572-1579.
12. Lim WS, Lewis S, Macfarlane JT. Severity prediction rules in community acquired pneumonia: a validation study. Thorax. 2000;55(3):219-223.
13. Bauer TT, Ewig S, Marre R, Suttorp N, Welte T; CAPNETZ Study Group. CRB‐65 predicts death from community‐acquired pneumonia. J Intern Med. 2006;260(1):93-101.
14. Khuri SF. The NSQIP: a new frontier in surgery. Surgery. 2005;138(5):837-843.
15. American College of Surgeons. User Guide for the 2012 ACS NSQIP Participant Use Data File: American College of Surgeons National Surgical Quality Improvement Program. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug12.ashx. Published October 2013. Accessed October 8, 2014.
16. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267.
17. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889.
18. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42.
19. Katzan I, Cebul R, Husak S, Dawson N, Baker D. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60(4):620-625.
20. Fisher MA, Matthei JD, Obirieze A, et al. Open reduction internal fixation versus hemiarthroplasty versus total hip arthroplasty in the elderly: a review of the National Surgical Quality Improvement Program database. J Surg Res. 2013;181(2):193-198.
21. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma. 2014;28(2):63-69.
22. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia: a meta-analysis. JAMA. 1996;275(2):134-141.
23. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
24. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004:159(7):702-706.
25. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 20138;61(4):1-117.
26. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84(4):562-572.
27. Myers AH, Robinson EG, Van Natta ML, Michelson JD, Collins K, Baker SP. Hip fractures among the elderly: factors associated with in-hospital mortality. Am J Epidemiol. 1991;134(10):1128-1137.
28. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
29. Leroy O, Santre C, Beuscart C, et al. A five-year study of severe community-acquired pneumonia with emphasis on prognosis in patients admitted to an intensive care unit. Intensive Care Med. 1995;21(1):24-31.
30. Urwin S, Parker M, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455.
31. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743.
32. Niederman MS, Mandell LA, Anzueto A, et al; American Thoracic Society. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730-1754.
33. Koval KJ, Skovron ML, Aharonoff GB, Zuckerman JD. Predictors of functional recovery after hip fracture in the elderly. Clin Orthop Relat Res. 1998;(348):22-28.
34. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
35. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology. 2000;39(4):346-349.
36. Bottle A, Aylin P. Mortality associated with delay in operation after hip fracture: observational study. BMJ. 2006;332(7547):947-951.
37. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709.
38. Simunovic N, Devereaux P, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616.
39. Kaplan V, Clermont G, Griffin MF, et al. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163(3):317-323.
40. Parker MJ, Handoll HH, Griffiths R. Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev. 2004;(4):CD000521.
41. Chakladar A, White SM. Cost estimates of spinal versus general anaesthesia for fractured neck of femur surgery. Anaesthesia. 2010;65(8):810-814.
42. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
43. Gilbert TB, Hawkes WG, Hebel JR, et al. Spinal anesthesia versus general anesthesia for hip fracture repair: a longitudinal observation of 741 elderly patients during 2-year follow-up. Am J Orthop. 2000;29(1):25-35.
44. O’Hara DA, Duff A, Berlin JA, et al. The effect of anesthetic technique on postoperative outcomes in hip fracture repair. Anesthesiology. 2000;92(4):947-957.
45. Hole A, Terjesen T, Breivik H. Epidural versus general anaesthesia for total hip arthroplasty in elderly patients. Acta Anaesthesiol Scand. 1980;24(4):279-287.
46. Rashiq S, Finegan BA. The effect of spinal anesthesia on blood transfusion rate in total joint arthroplasty. Can J Surg. 2006;49(6):391-396.
47. Chang CC, Lin HC, Lin HW, Lin HC. Anesthetic management and surgical site infections in total hip or knee replacement: a population-based study. Anesthesiology. 2010;113(2):279-284.
48. Mauermann WJ, Shilling AM, Zuo Z. A comparison of neuraxial block versus general anesthesia for elective total hip replacement: a meta-analysis. Anesth Analg. 2006;103(4):1018-1025.
49. Hu S, Zhang ZY, Hua YQ, Li J, Cai ZD. A comparison of regional and general anaesthesia for total replacement of the hip or knee: a meta-analysis. J Bone Joint Surg Br. 2009;91(7):935-942.
50. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
51. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697.
52. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559.
53. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489.
54. Shiga T, Wajima Zi, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anesth. 2008;55(3):146-154.
55. Streubel P, Ricci W, Wong A, Gardner M. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
Take-Home Points
- The prevalence of preoperative pneumonia is 1.2% among hip fracture patients aged >65 years.
- Preoperative pneumonia is an independent risk factor for mortality and adverse events including renal failure, prolonged ventilator dependence, and prolonged altered mental status after geriatric hip fracture surgery.
- Underweight BMI (<18.5 kg/m2) was associated with higher mortality within 30 days among hip fracture patients admitted with pneumonia.
- The mortality rate normalized to that of patients without pneumonia within 2 weeks of hip fracture surgery.
- Time from admission to surgery was not associated with adverse events or mortality among hip fracture patients admitted with pneumonia.
Preoperative pneumonia remains relatively unexplored as a risk factor for adverse outcomes in geriatric hip fracture surgery. Dated studies report a 0.3% to 3.2% prevalence of “recent pneumonia” in patients presenting with hip fracture but provide neither a definition of pneumonia based on clinical criteria nor a subset analysis of outcomes in the pneumonia group.1-3 Although active pneumonia has been identified as a preoperative optimization target in the management guidelines for geriatric hip fracture,4 we are unaware of any studies that have reported on differences in demographics, comorbidities, delay to surgery, or adverse outcomes between hip fracture patients with and without preoperative pneumonia.
This paucity of information on the effect of preoperative pneumonia in the hip fracture population may be related to low prevalence of preoperative pneumonia and a cadre of variable definitions, which limit identification of a cohort of patients with preoperative pneumonia large enough from which to draw meaningful results. Database studies, especially those using surgical registries rather than administrative or reimbursement data, offer particular advantages for investigation of such rare clinical entities.5Medical care of patients with pneumonia alone is known to be facilitated by assessments of mortality risk from clinical and laboratory data. The modified British Thoracic Society rule/CURB-65 (confusion, urea, respiratory rate, blood pressure) score is strongly predictive of mortality in hospitalized adults with pneumonia (odds ratio [OR], 4.59; 95% confidence interval [CI], 1.42-14.85; P = .011) and may guide antibiotic therapy, laboratory investigations, and the decision to intubate in a patient with pneumonia.6-8 This score is predictive of adverse events (AEs), hospital length of stay, and use of intensive care services.6,7,9-13 We hypothesized that preoperative clinical indicators assessed by pneumonia severity scores as well as patient demographics and baseline comorbidities may also have prognostic value for risk of AEs in a cohort of geriatric hip fracture surgery patients with preoperative pneumonia.
In this article, we first describe the prevalence of preoperative pneumonia in geriatric hip fracture surgery patients as well as demographic and operative differences between patients with and without the disease. We then ask 3 questions: Is preoperative pneumonia an independent risk factor for mortality and adverse outcomes in geriatric hip fracture surgery? Is there a postoperative interval during which the unadjusted mortality rate is higher among patients with preoperative pneumonia? In patients with preoperative pneumonia, what are the predictors of morbidity and mortality?
Methods
Yale University’s Human Investigations Committee approved this retrospective cohort study, which used the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database for the period 2005 to 2012. ACS-NSQIP is a prospective, multi-institutional outcomes program that collects data on preoperative comorbidities, intraoperative variables, and 30-day postoperative outcomes for patients undergoing surgical procedures in inpatient and outpatient settings.14
Unlike administrative databases, which are based on reimbursement data, ACS-NSQIP data are collected by trained surgical clinical reviewers for the purposes of quality improvement and clinical research, and data quality is ensured with routine auditing.15 The program has gained a high degree of respect as a powerful and valid data source in both general16 and orthopedic17 surgery literature. The database offers a particular advantage with respect to the study of preoperative pneumonia: Only patients with new or recently diagnosed pneumonia on antibiotic therapy who meet strict criteria for characteristic findings on chest radiography, clinical signs and symptoms of respiratory illness, and positive cultures are coded as having actively treated pneumonia at time of surgery.15
To identify hip fracture patients over the age of 65 years who underwent operative fixation of a hip fracture, we used Current Procedural Terminology (CPT) hip fracture codes, including 27235 (percutaneous screw fixation), 27236 or 27244 (plate-and-screw fixation), and 27245 (intramedullary device), as well as 27125 (hemiarthroplasty) and 27130 (arthroplasty) for patients with a postoperative International Classification of Disease, Ninth Revision (ICD-9) diagnosis code (820.x, 820.2x, or 820.8) consistent with acute hip fracture.18,19 Procedure type, anesthesia type, and delay from admission to surgery were captured for all procedures.
Preoperative demographics included age, sex, transfer origin, functional status, and body mass index (BMI) category. Binary comorbidities were classified as preoperative anemia (hematocrit, <0.41 for men, <0.36 for women), confusion, dyspnea at rest, uremia (blood urea nitrogen, >6.8 mmol/L), history of cardiovascular disease (congestive heart failure, myocardial infarction, percutaneous coronary intervention, angina pectoris, medically treated hypertension, peripheral vascular disease, or resting claudication), chronic obstructive pulmonary disease, diabetes, renal disease (renal failure or dialysis), and cigarette use in preceding 12 months.20,21 Although preoperative hypotension and respiratory rate are often considered in patients with pneumonia, these variables were not available from the ACS-NSQIP data.6,22Pearson χ2 test for categorical variables was used to compare baseline demographics and operative characteristics between patients with and without pneumonia, and Student t test was used to compare intervals from hospital admission to hip fracture surgery, surgery start to surgery stop, and surgery to discharge between patients with and without preoperative pneumonia.
Binary outcome measures were compared between patients with and without preoperative pneumonia. “Any AE” included any serious AE (SAE) or any minor AE. SAEs included death, acute renal failure, ventilator use >48 hours, unplanned intubation, septic shock, sepsis, return to operating room, coma >24 hours, cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction, thromboembolic event (deep vein thrombosis or pulmonary embolism), and stroke/cerebrovascular accident. Minor AEs included progressive renal insufficiency, urinary tract infection, organ/space infection, superficial surgical-site infection, deep surgical-site infection, and wound dehiscence. Other binary outcome measures included discharge destination and unplanned readmission within 30 days after hip fracture surgery.23Poisson regression with robust error variance as described by Zou24 was used to compare the rates of any, minor, and individual AEs, and any SAEs, between patients with and without pneumonia. Multivariate analysis accounted for the baseline variables in Table 1. AEs that occurred more than once in each group were included in the analyses.
Kaplan-Meier survival analysis was performed for postoperative mortality within 30 days. Within the preoperative pneumonia group, covariates from Table 1 were identified as predictors of any AE, SAE, or death within 30 days after hip fracture surgery by stepwise multivariate Poisson regression with robust error variance. When interval from admission to surgery was longer than 24, 48, 72, or 96 hours, it was also included as a covariate. Variables that did not show an association with AEs at the P < .20 level were not included in the final regression model. All analyses were performed with Stata/SE Version 12.0 statistical software (StataCorp).
Results
Of the 7128 geriatric hip fracture patients in this study, 82 (1.2%) had active pneumonia at time of surgery (Table 1). Age, BMI, preoperative uremia, history of cardiovascular disease, diabetes, renal disease, and smoking were similar between groups. In addition, there was no difference in anesthesia type or fixation procedure between the pneumonia and no-pneumonia groups. Patients with preoperative pneumonia differed significantly with respect to sex, transfer from facility, preoperative functional dependence, anemia, confusion, dyspnea at rest, and history of chronic obstructive pulmonary disease (Table 1).
Interval from admission to surgery was longer (P < .001) for geriatric hip fracture patients with preoperative pneumonia (mean, 6.8 days; 95% CI, 2.5-11.1 days) than for those without pneumonia (mean, 1.5 days; CI, 1.4-1.5 days). There was no difference (P = .124) in operative time between the pneumonia group (mean, 72.8 min; CI, 64.0-81.5 min) and the no-pneumonia group (mean, 66.1 min; CI, 61.2-67.0 min). Interval from surgery to discharge was longer (P < .001) for patients with preoperative pneumonia (mean, 10.1 days; CI, 6.9-13.4 days) than for those without pneumonia (mean, 6.3 days; CI, 6.1-6.4 days).
Adverse outcomes of geriatric hip fracture surgery are listed in Table 2. In the multivariate analysis, preoperative pneumonia was significantly associated with any AE (relative risk [RR]) = 1.44) and any SAE (RR = 1.79).
Survival patterns diverged between patients with and without preoperative pneumonia (Figure). The unadjusted mortality rate was qualitatively higher in patients with preoperative pneumonia than in patients without pneumonia during the first days after hip fracture (slopes of unadjusted mortality curves in Figure). Of note, no patient under age 75 years with pneumonia at time of surgery died within the 30-day study period.
Among geriatric hip fracture patients with preoperative pneumonia, multivariate analyses revealed no significant association of any preoperative comorbidity with any AE or any SAE. Given the gravity of the death complication, however, death within 30 days after surgery was analyzed separately, and was found to be significantly associated (RR = 4.67) with being underweight (BMI, <18.5 kg/m2) (Table 3). Admission-to-surgery interval longer than 24, 48, 72, or 96 hours did not reach significance at the P < 0.2 level in the stepwise regressions and therefore was not associated with a higher or lower risk of any AE, SAE, or death.
Discussion
In the general US population, pneumonia accounts for 1.4% of deaths in people 65 years to 74 years old, 2.1% in people 75 years to 84 years, and 3.1% in people 85 years or older. In total, 3.4% of hospital inpatient deaths are attributed to pneumonia.25 In hospitalized general orthopedic surgical patients as well as hip fracture patients, pneumonia is strongly associated with increased mortality.26,27
We identified a preoperative pneumonia prevalence of 1.2%, which is comparable to the rates reported in the literature (0.3%-3.2%).1-3 To our knowledge, our study represents the largest series of patients with preoperative pneumonia at time of hip fracture repair, and the first to independently associate preoperative pneumonia with increased incidence of AEs, including death.
This study had its limitations. First, the ACS-NSQIP morbidity and mortality data, which are limited to the first 30 postoperative days, may be skewed because AEs that occurred after that interval are not captured. Second, coding of pneumonia in ACS-NSQIP does not convey specific information about the disease and its severity—infectious organism(s) responsible; acquisition setting (healthcare or community); treatment given, including antibiotic(s) selection, steroid use, dosing, and duration; and measures of treatment efficacy—limiting interpretation of the difference in delay to surgery. We cannot say whether the longer interval in patients with pneumonia reflects medical optimization, or whether the delay itself or any interventions during that time positively or negatively affected outcomes. In addition, despite using a large national database, we obtained a relatively small sample of patients (82) who had pneumonia before surgical hip fracture repair.
Multivariate analysis controlling for baseline demographics and comorbidities revealed that multiple SAEs were independently associated with preoperative pneumonia (overall SAE, RR = 1.79). Postoperative use of ventilator support for longer than 48 hours (RR = 6.48) and coma longer than 24 hours (RR = 7.31) are expected given the severity of pulmonary compromise in the study cohort.28,29 Acute renal failure (RR = 14.61) can occur in both hip fracture patients and community-acquired pneumonia patients and may be a multifactorial complication of the pulmonary infection, of the anesthesia, or of the surgical intervention in this cohort.30-32Unadjusted mortality in hip fracture takes months to a year to normalize to that of age-matched controls.32-34 In our series, the unadjusted death rate in the pneumonia cohort (Figure) was transiently elevated during the first weeks after surgery but then drew nearer the rate in the nondiseased hip fracture cohort by the end of the first month. Early death in the pneumonia group likely was multifactorial, potentially influenced by the increased burden of comorbidities in the pneumonia group at baseline, and the longer delay to surgery,35-38 as well as by the natural history of treated pneumonia in hospital patients, who, compared with age-matched hospitalized controls, also exhibit higher mortality during only the first 2 to 4 months of hospitalization for pneumonia.39 We regret that quality improvement strategies in the treatment of geriatric hip fracture surgery with pneumonia cannot be extrapolated from these results.
Similarly, the utility of BMI <18.5 kg/m2 as an actionable preoperative finding cannot be assessed from these results. However, we propose that underweight geriatric hip fracture patients with pneumonia may benefit from more aggressive preoperative optimization that does not delay surgery. Higher acuity of postoperative care, including more intensive nursing care and early coordination of care with respiratory therapists and medical comanagement teams, may also be beneficial.
Anesthesia type did not differ between patients with and without preoperative pneumonia and was not associated with AEs in patients with preoperative pneumonia. Consistent with our findings, multiple studies have reported no significant differences in short-term outcomes of hip fracture repair between general and spinal anesthesia, though no other study has compared the benefits of general and spinal anesthesia for patients with preoperative pneumonia.40-44 Although spinal anesthesia (relative to general anesthesia) has been reported to have benefits in hip and knee arthroplasty, these benefits appear not to translate to hip fracture repair.45-50 The results of the present study suggest that general and spinal anesthesia may be equivalent in terms of risk for the geriatric hip fracture patient with preoperative pneumonia.43,44Our attempt to evaluate the CURB-65 pneumonia severity score as a prognosticator of AEs was thwarted by the absence of required variables in the ACS-NSQIP dataset (confusion, uremia, dyspnea, and age were available; hypotension and blood pressure were not). In our analysis, we did include, individually, variables previously found to predict AEs in the medical pneumonia population (confusion, uremia, dyspnea at rest, anemia).9-11,32 However, these clinical findings are nonspecific in hip fracture patients, who may become anemic, confused, dyspneic, or uremic from a multitude of factors related to their injury and unrelated to pneumonia, including but not limited to hemorrhage, muscle damage, renal injury, and pulmonary embolism. It is not surprising that confusion, uremia, dyspnea at rest, and anemia were not individually predictive of AEs or death within 30 days after surgery in the cohort of geriatric hip fracture patients with pneumonia.
There is no literature that argues for or against delaying hip fracture surgery in geriatric hip fracture patients with pneumonia. The surgical delay observed in this population is ostensibly related to medical optimization of the pneumonia and/or underlying comorbidities. However, we did not find a morbidity or mortality detriment or benefit in delaying surgery by 1 to 4 days in this population. Delay of surgery is a poor covariate, given extensive confounding by medical management and preoperative optimizing of comorbid conditions (reflected in our independent variable and covariates) as well as institutional and surgeon variations in policy and behavior and other unaccounted influences. Although some authors have found no difference in mortality or major AEs between hip fracture patients who had a surgical delay and those who did not,31,51-53 other series and meta-analyses have suggested a mortality detriment in a surgical delay of more than 2 days36,54 or 4 days55 from admission. Given our data, we cannot recommend against immediate hip fracture repair in the subpopulation of geriatric hip fracture patients with pneumonia.
Our study findings suggest that preoperative pneumonia is a rare independent risk factor for AEs after hip fracture surgery in geriatric patients. Underweight BMI is predictive of death in geriatric hip fracture surgery patients who present with pneumonia, whereas early surgical repair appears not to be associated with adverse outcomes. Further investigation is warranted to determine if such patients benefit from specific preoperative and postoperative strategies for optimizing medical and surgical care based on these findings.
Am J Orthop. 2017;46(3):E177-E185. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- The prevalence of preoperative pneumonia is 1.2% among hip fracture patients aged >65 years.
- Preoperative pneumonia is an independent risk factor for mortality and adverse events including renal failure, prolonged ventilator dependence, and prolonged altered mental status after geriatric hip fracture surgery.
- Underweight BMI (<18.5 kg/m2) was associated with higher mortality within 30 days among hip fracture patients admitted with pneumonia.
- The mortality rate normalized to that of patients without pneumonia within 2 weeks of hip fracture surgery.
- Time from admission to surgery was not associated with adverse events or mortality among hip fracture patients admitted with pneumonia.
Preoperative pneumonia remains relatively unexplored as a risk factor for adverse outcomes in geriatric hip fracture surgery. Dated studies report a 0.3% to 3.2% prevalence of “recent pneumonia” in patients presenting with hip fracture but provide neither a definition of pneumonia based on clinical criteria nor a subset analysis of outcomes in the pneumonia group.1-3 Although active pneumonia has been identified as a preoperative optimization target in the management guidelines for geriatric hip fracture,4 we are unaware of any studies that have reported on differences in demographics, comorbidities, delay to surgery, or adverse outcomes between hip fracture patients with and without preoperative pneumonia.
This paucity of information on the effect of preoperative pneumonia in the hip fracture population may be related to low prevalence of preoperative pneumonia and a cadre of variable definitions, which limit identification of a cohort of patients with preoperative pneumonia large enough from which to draw meaningful results. Database studies, especially those using surgical registries rather than administrative or reimbursement data, offer particular advantages for investigation of such rare clinical entities.5Medical care of patients with pneumonia alone is known to be facilitated by assessments of mortality risk from clinical and laboratory data. The modified British Thoracic Society rule/CURB-65 (confusion, urea, respiratory rate, blood pressure) score is strongly predictive of mortality in hospitalized adults with pneumonia (odds ratio [OR], 4.59; 95% confidence interval [CI], 1.42-14.85; P = .011) and may guide antibiotic therapy, laboratory investigations, and the decision to intubate in a patient with pneumonia.6-8 This score is predictive of adverse events (AEs), hospital length of stay, and use of intensive care services.6,7,9-13 We hypothesized that preoperative clinical indicators assessed by pneumonia severity scores as well as patient demographics and baseline comorbidities may also have prognostic value for risk of AEs in a cohort of geriatric hip fracture surgery patients with preoperative pneumonia.
In this article, we first describe the prevalence of preoperative pneumonia in geriatric hip fracture surgery patients as well as demographic and operative differences between patients with and without the disease. We then ask 3 questions: Is preoperative pneumonia an independent risk factor for mortality and adverse outcomes in geriatric hip fracture surgery? Is there a postoperative interval during which the unadjusted mortality rate is higher among patients with preoperative pneumonia? In patients with preoperative pneumonia, what are the predictors of morbidity and mortality?
Methods
Yale University’s Human Investigations Committee approved this retrospective cohort study, which used the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database for the period 2005 to 2012. ACS-NSQIP is a prospective, multi-institutional outcomes program that collects data on preoperative comorbidities, intraoperative variables, and 30-day postoperative outcomes for patients undergoing surgical procedures in inpatient and outpatient settings.14
Unlike administrative databases, which are based on reimbursement data, ACS-NSQIP data are collected by trained surgical clinical reviewers for the purposes of quality improvement and clinical research, and data quality is ensured with routine auditing.15 The program has gained a high degree of respect as a powerful and valid data source in both general16 and orthopedic17 surgery literature. The database offers a particular advantage with respect to the study of preoperative pneumonia: Only patients with new or recently diagnosed pneumonia on antibiotic therapy who meet strict criteria for characteristic findings on chest radiography, clinical signs and symptoms of respiratory illness, and positive cultures are coded as having actively treated pneumonia at time of surgery.15
To identify hip fracture patients over the age of 65 years who underwent operative fixation of a hip fracture, we used Current Procedural Terminology (CPT) hip fracture codes, including 27235 (percutaneous screw fixation), 27236 or 27244 (plate-and-screw fixation), and 27245 (intramedullary device), as well as 27125 (hemiarthroplasty) and 27130 (arthroplasty) for patients with a postoperative International Classification of Disease, Ninth Revision (ICD-9) diagnosis code (820.x, 820.2x, or 820.8) consistent with acute hip fracture.18,19 Procedure type, anesthesia type, and delay from admission to surgery were captured for all procedures.
Preoperative demographics included age, sex, transfer origin, functional status, and body mass index (BMI) category. Binary comorbidities were classified as preoperative anemia (hematocrit, <0.41 for men, <0.36 for women), confusion, dyspnea at rest, uremia (blood urea nitrogen, >6.8 mmol/L), history of cardiovascular disease (congestive heart failure, myocardial infarction, percutaneous coronary intervention, angina pectoris, medically treated hypertension, peripheral vascular disease, or resting claudication), chronic obstructive pulmonary disease, diabetes, renal disease (renal failure or dialysis), and cigarette use in preceding 12 months.20,21 Although preoperative hypotension and respiratory rate are often considered in patients with pneumonia, these variables were not available from the ACS-NSQIP data.6,22Pearson χ2 test for categorical variables was used to compare baseline demographics and operative characteristics between patients with and without pneumonia, and Student t test was used to compare intervals from hospital admission to hip fracture surgery, surgery start to surgery stop, and surgery to discharge between patients with and without preoperative pneumonia.
Binary outcome measures were compared between patients with and without preoperative pneumonia. “Any AE” included any serious AE (SAE) or any minor AE. SAEs included death, acute renal failure, ventilator use >48 hours, unplanned intubation, septic shock, sepsis, return to operating room, coma >24 hours, cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction, thromboembolic event (deep vein thrombosis or pulmonary embolism), and stroke/cerebrovascular accident. Minor AEs included progressive renal insufficiency, urinary tract infection, organ/space infection, superficial surgical-site infection, deep surgical-site infection, and wound dehiscence. Other binary outcome measures included discharge destination and unplanned readmission within 30 days after hip fracture surgery.23Poisson regression with robust error variance as described by Zou24 was used to compare the rates of any, minor, and individual AEs, and any SAEs, between patients with and without pneumonia. Multivariate analysis accounted for the baseline variables in Table 1. AEs that occurred more than once in each group were included in the analyses.
Kaplan-Meier survival analysis was performed for postoperative mortality within 30 days. Within the preoperative pneumonia group, covariates from Table 1 were identified as predictors of any AE, SAE, or death within 30 days after hip fracture surgery by stepwise multivariate Poisson regression with robust error variance. When interval from admission to surgery was longer than 24, 48, 72, or 96 hours, it was also included as a covariate. Variables that did not show an association with AEs at the P < .20 level were not included in the final regression model. All analyses were performed with Stata/SE Version 12.0 statistical software (StataCorp).
Results
Of the 7128 geriatric hip fracture patients in this study, 82 (1.2%) had active pneumonia at time of surgery (Table 1). Age, BMI, preoperative uremia, history of cardiovascular disease, diabetes, renal disease, and smoking were similar between groups. In addition, there was no difference in anesthesia type or fixation procedure between the pneumonia and no-pneumonia groups. Patients with preoperative pneumonia differed significantly with respect to sex, transfer from facility, preoperative functional dependence, anemia, confusion, dyspnea at rest, and history of chronic obstructive pulmonary disease (Table 1).
Interval from admission to surgery was longer (P < .001) for geriatric hip fracture patients with preoperative pneumonia (mean, 6.8 days; 95% CI, 2.5-11.1 days) than for those without pneumonia (mean, 1.5 days; CI, 1.4-1.5 days). There was no difference (P = .124) in operative time between the pneumonia group (mean, 72.8 min; CI, 64.0-81.5 min) and the no-pneumonia group (mean, 66.1 min; CI, 61.2-67.0 min). Interval from surgery to discharge was longer (P < .001) for patients with preoperative pneumonia (mean, 10.1 days; CI, 6.9-13.4 days) than for those without pneumonia (mean, 6.3 days; CI, 6.1-6.4 days).
Adverse outcomes of geriatric hip fracture surgery are listed in Table 2. In the multivariate analysis, preoperative pneumonia was significantly associated with any AE (relative risk [RR]) = 1.44) and any SAE (RR = 1.79).
Survival patterns diverged between patients with and without preoperative pneumonia (Figure). The unadjusted mortality rate was qualitatively higher in patients with preoperative pneumonia than in patients without pneumonia during the first days after hip fracture (slopes of unadjusted mortality curves in Figure). Of note, no patient under age 75 years with pneumonia at time of surgery died within the 30-day study period.
Among geriatric hip fracture patients with preoperative pneumonia, multivariate analyses revealed no significant association of any preoperative comorbidity with any AE or any SAE. Given the gravity of the death complication, however, death within 30 days after surgery was analyzed separately, and was found to be significantly associated (RR = 4.67) with being underweight (BMI, <18.5 kg/m2) (Table 3). Admission-to-surgery interval longer than 24, 48, 72, or 96 hours did not reach significance at the P < 0.2 level in the stepwise regressions and therefore was not associated with a higher or lower risk of any AE, SAE, or death.
Discussion
In the general US population, pneumonia accounts for 1.4% of deaths in people 65 years to 74 years old, 2.1% in people 75 years to 84 years, and 3.1% in people 85 years or older. In total, 3.4% of hospital inpatient deaths are attributed to pneumonia.25 In hospitalized general orthopedic surgical patients as well as hip fracture patients, pneumonia is strongly associated with increased mortality.26,27
We identified a preoperative pneumonia prevalence of 1.2%, which is comparable to the rates reported in the literature (0.3%-3.2%).1-3 To our knowledge, our study represents the largest series of patients with preoperative pneumonia at time of hip fracture repair, and the first to independently associate preoperative pneumonia with increased incidence of AEs, including death.
This study had its limitations. First, the ACS-NSQIP morbidity and mortality data, which are limited to the first 30 postoperative days, may be skewed because AEs that occurred after that interval are not captured. Second, coding of pneumonia in ACS-NSQIP does not convey specific information about the disease and its severity—infectious organism(s) responsible; acquisition setting (healthcare or community); treatment given, including antibiotic(s) selection, steroid use, dosing, and duration; and measures of treatment efficacy—limiting interpretation of the difference in delay to surgery. We cannot say whether the longer interval in patients with pneumonia reflects medical optimization, or whether the delay itself or any interventions during that time positively or negatively affected outcomes. In addition, despite using a large national database, we obtained a relatively small sample of patients (82) who had pneumonia before surgical hip fracture repair.
Multivariate analysis controlling for baseline demographics and comorbidities revealed that multiple SAEs were independently associated with preoperative pneumonia (overall SAE, RR = 1.79). Postoperative use of ventilator support for longer than 48 hours (RR = 6.48) and coma longer than 24 hours (RR = 7.31) are expected given the severity of pulmonary compromise in the study cohort.28,29 Acute renal failure (RR = 14.61) can occur in both hip fracture patients and community-acquired pneumonia patients and may be a multifactorial complication of the pulmonary infection, of the anesthesia, or of the surgical intervention in this cohort.30-32Unadjusted mortality in hip fracture takes months to a year to normalize to that of age-matched controls.32-34 In our series, the unadjusted death rate in the pneumonia cohort (Figure) was transiently elevated during the first weeks after surgery but then drew nearer the rate in the nondiseased hip fracture cohort by the end of the first month. Early death in the pneumonia group likely was multifactorial, potentially influenced by the increased burden of comorbidities in the pneumonia group at baseline, and the longer delay to surgery,35-38 as well as by the natural history of treated pneumonia in hospital patients, who, compared with age-matched hospitalized controls, also exhibit higher mortality during only the first 2 to 4 months of hospitalization for pneumonia.39 We regret that quality improvement strategies in the treatment of geriatric hip fracture surgery with pneumonia cannot be extrapolated from these results.
Similarly, the utility of BMI <18.5 kg/m2 as an actionable preoperative finding cannot be assessed from these results. However, we propose that underweight geriatric hip fracture patients with pneumonia may benefit from more aggressive preoperative optimization that does not delay surgery. Higher acuity of postoperative care, including more intensive nursing care and early coordination of care with respiratory therapists and medical comanagement teams, may also be beneficial.
Anesthesia type did not differ between patients with and without preoperative pneumonia and was not associated with AEs in patients with preoperative pneumonia. Consistent with our findings, multiple studies have reported no significant differences in short-term outcomes of hip fracture repair between general and spinal anesthesia, though no other study has compared the benefits of general and spinal anesthesia for patients with preoperative pneumonia.40-44 Although spinal anesthesia (relative to general anesthesia) has been reported to have benefits in hip and knee arthroplasty, these benefits appear not to translate to hip fracture repair.45-50 The results of the present study suggest that general and spinal anesthesia may be equivalent in terms of risk for the geriatric hip fracture patient with preoperative pneumonia.43,44Our attempt to evaluate the CURB-65 pneumonia severity score as a prognosticator of AEs was thwarted by the absence of required variables in the ACS-NSQIP dataset (confusion, uremia, dyspnea, and age were available; hypotension and blood pressure were not). In our analysis, we did include, individually, variables previously found to predict AEs in the medical pneumonia population (confusion, uremia, dyspnea at rest, anemia).9-11,32 However, these clinical findings are nonspecific in hip fracture patients, who may become anemic, confused, dyspneic, or uremic from a multitude of factors related to their injury and unrelated to pneumonia, including but not limited to hemorrhage, muscle damage, renal injury, and pulmonary embolism. It is not surprising that confusion, uremia, dyspnea at rest, and anemia were not individually predictive of AEs or death within 30 days after surgery in the cohort of geriatric hip fracture patients with pneumonia.
There is no literature that argues for or against delaying hip fracture surgery in geriatric hip fracture patients with pneumonia. The surgical delay observed in this population is ostensibly related to medical optimization of the pneumonia and/or underlying comorbidities. However, we did not find a morbidity or mortality detriment or benefit in delaying surgery by 1 to 4 days in this population. Delay of surgery is a poor covariate, given extensive confounding by medical management and preoperative optimizing of comorbid conditions (reflected in our independent variable and covariates) as well as institutional and surgeon variations in policy and behavior and other unaccounted influences. Although some authors have found no difference in mortality or major AEs between hip fracture patients who had a surgical delay and those who did not,31,51-53 other series and meta-analyses have suggested a mortality detriment in a surgical delay of more than 2 days36,54 or 4 days55 from admission. Given our data, we cannot recommend against immediate hip fracture repair in the subpopulation of geriatric hip fracture patients with pneumonia.
Our study findings suggest that preoperative pneumonia is a rare independent risk factor for AEs after hip fracture surgery in geriatric patients. Underweight BMI is predictive of death in geriatric hip fracture surgery patients who present with pneumonia, whereas early surgical repair appears not to be associated with adverse outcomes. Further investigation is warranted to determine if such patients benefit from specific preoperative and postoperative strategies for optimizing medical and surgical care based on these findings.
Am J Orthop. 2017;46(3):E177-E185. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Sexson SB, Lehner JT. Factors affecting hip fracture mortality. J Orthop Trauma. 1987;1(4):298-305.
2. Mullen JO, Mullen NL. Hip fracture mortality: a prospective, multifactorial study to predict and minimize death risk. Clin Orthop Relat Res. 1992;(280):214-222.
3. Kenzora JE, McCarthy RE, Lowell JD, Sledge CB. Hip fracture mortality. Relation to age, treatment, preoperative illness, time of surgery, and complications. Clin Orthop Relat Res. 1984;(186):45-56.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24(4):701-719.
5. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
6. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377-382.
7. Myint PK, Kamath AV, Vowler SL, Maisey DN, Harrison BDW. The CURB (confusion, urea, respiratory rate and blood pressure) criteria in community-acquired pneumonia (CAP) in hospitalised elderly patients aged 65 years and over: a prospective observational cohort study. Age Ageing. 2005;34(1):75-77.
8. Wilkinson M, Woodhead MA. Guidelines for community-acquired pneumonia in the ICU. Curr Opin Crit Care. 2004;10(1):59-64.
9. Buising K, Thursky K, Black J, et al. A prospective comparison of severity scores for identifying patients with severe community acquired pneumonia: reconsidering what is meant by severe pneumonia. Thorax. 2006;61(5):419-424.
10. Ewig S, De Roux A, Bauer T, et al. Validation of predictive rules and indices of severity for community acquired pneumonia. Thorax. 2004;59(5):421-427.
11. Yandiola PP, Capelastegui A, Quintana J, et al. Prospective comparison of severity scores for predicting clinically relevant outcomes for patients hospitalized with community-acquired pneumonia. Chest. 2009;135(6):1572-1579.
12. Lim WS, Lewis S, Macfarlane JT. Severity prediction rules in community acquired pneumonia: a validation study. Thorax. 2000;55(3):219-223.
13. Bauer TT, Ewig S, Marre R, Suttorp N, Welte T; CAPNETZ Study Group. CRB‐65 predicts death from community‐acquired pneumonia. J Intern Med. 2006;260(1):93-101.
14. Khuri SF. The NSQIP: a new frontier in surgery. Surgery. 2005;138(5):837-843.
15. American College of Surgeons. User Guide for the 2012 ACS NSQIP Participant Use Data File: American College of Surgeons National Surgical Quality Improvement Program. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug12.ashx. Published October 2013. Accessed October 8, 2014.
16. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267.
17. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889.
18. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42.
19. Katzan I, Cebul R, Husak S, Dawson N, Baker D. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60(4):620-625.
20. Fisher MA, Matthei JD, Obirieze A, et al. Open reduction internal fixation versus hemiarthroplasty versus total hip arthroplasty in the elderly: a review of the National Surgical Quality Improvement Program database. J Surg Res. 2013;181(2):193-198.
21. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma. 2014;28(2):63-69.
22. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia: a meta-analysis. JAMA. 1996;275(2):134-141.
23. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
24. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004:159(7):702-706.
25. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 20138;61(4):1-117.
26. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84(4):562-572.
27. Myers AH, Robinson EG, Van Natta ML, Michelson JD, Collins K, Baker SP. Hip fractures among the elderly: factors associated with in-hospital mortality. Am J Epidemiol. 1991;134(10):1128-1137.
28. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
29. Leroy O, Santre C, Beuscart C, et al. A five-year study of severe community-acquired pneumonia with emphasis on prognosis in patients admitted to an intensive care unit. Intensive Care Med. 1995;21(1):24-31.
30. Urwin S, Parker M, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455.
31. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743.
32. Niederman MS, Mandell LA, Anzueto A, et al; American Thoracic Society. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730-1754.
33. Koval KJ, Skovron ML, Aharonoff GB, Zuckerman JD. Predictors of functional recovery after hip fracture in the elderly. Clin Orthop Relat Res. 1998;(348):22-28.
34. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
35. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology. 2000;39(4):346-349.
36. Bottle A, Aylin P. Mortality associated with delay in operation after hip fracture: observational study. BMJ. 2006;332(7547):947-951.
37. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709.
38. Simunovic N, Devereaux P, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616.
39. Kaplan V, Clermont G, Griffin MF, et al. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163(3):317-323.
40. Parker MJ, Handoll HH, Griffiths R. Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev. 2004;(4):CD000521.
41. Chakladar A, White SM. Cost estimates of spinal versus general anaesthesia for fractured neck of femur surgery. Anaesthesia. 2010;65(8):810-814.
42. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
43. Gilbert TB, Hawkes WG, Hebel JR, et al. Spinal anesthesia versus general anesthesia for hip fracture repair: a longitudinal observation of 741 elderly patients during 2-year follow-up. Am J Orthop. 2000;29(1):25-35.
44. O’Hara DA, Duff A, Berlin JA, et al. The effect of anesthetic technique on postoperative outcomes in hip fracture repair. Anesthesiology. 2000;92(4):947-957.
45. Hole A, Terjesen T, Breivik H. Epidural versus general anaesthesia for total hip arthroplasty in elderly patients. Acta Anaesthesiol Scand. 1980;24(4):279-287.
46. Rashiq S, Finegan BA. The effect of spinal anesthesia on blood transfusion rate in total joint arthroplasty. Can J Surg. 2006;49(6):391-396.
47. Chang CC, Lin HC, Lin HW, Lin HC. Anesthetic management and surgical site infections in total hip or knee replacement: a population-based study. Anesthesiology. 2010;113(2):279-284.
48. Mauermann WJ, Shilling AM, Zuo Z. A comparison of neuraxial block versus general anesthesia for elective total hip replacement: a meta-analysis. Anesth Analg. 2006;103(4):1018-1025.
49. Hu S, Zhang ZY, Hua YQ, Li J, Cai ZD. A comparison of regional and general anaesthesia for total replacement of the hip or knee: a meta-analysis. J Bone Joint Surg Br. 2009;91(7):935-942.
50. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
51. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697.
52. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559.
53. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489.
54. Shiga T, Wajima Zi, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anesth. 2008;55(3):146-154.
55. Streubel P, Ricci W, Wong A, Gardner M. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
1. Sexson SB, Lehner JT. Factors affecting hip fracture mortality. J Orthop Trauma. 1987;1(4):298-305.
2. Mullen JO, Mullen NL. Hip fracture mortality: a prospective, multifactorial study to predict and minimize death risk. Clin Orthop Relat Res. 1992;(280):214-222.
3. Kenzora JE, McCarthy RE, Lowell JD, Sledge CB. Hip fracture mortality. Relation to age, treatment, preoperative illness, time of surgery, and complications. Clin Orthop Relat Res. 1984;(186):45-56.
4. Auron-Gomez M, Michota F. Medical management of hip fracture. Clin Geriatr Med. 2008;24(4):701-719.
5. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
6. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377-382.
7. Myint PK, Kamath AV, Vowler SL, Maisey DN, Harrison BDW. The CURB (confusion, urea, respiratory rate and blood pressure) criteria in community-acquired pneumonia (CAP) in hospitalised elderly patients aged 65 years and over: a prospective observational cohort study. Age Ageing. 2005;34(1):75-77.
8. Wilkinson M, Woodhead MA. Guidelines for community-acquired pneumonia in the ICU. Curr Opin Crit Care. 2004;10(1):59-64.
9. Buising K, Thursky K, Black J, et al. A prospective comparison of severity scores for identifying patients with severe community acquired pneumonia: reconsidering what is meant by severe pneumonia. Thorax. 2006;61(5):419-424.
10. Ewig S, De Roux A, Bauer T, et al. Validation of predictive rules and indices of severity for community acquired pneumonia. Thorax. 2004;59(5):421-427.
11. Yandiola PP, Capelastegui A, Quintana J, et al. Prospective comparison of severity scores for predicting clinically relevant outcomes for patients hospitalized with community-acquired pneumonia. Chest. 2009;135(6):1572-1579.
12. Lim WS, Lewis S, Macfarlane JT. Severity prediction rules in community acquired pneumonia: a validation study. Thorax. 2000;55(3):219-223.
13. Bauer TT, Ewig S, Marre R, Suttorp N, Welte T; CAPNETZ Study Group. CRB‐65 predicts death from community‐acquired pneumonia. J Intern Med. 2006;260(1):93-101.
14. Khuri SF. The NSQIP: a new frontier in surgery. Surgery. 2005;138(5):837-843.
15. American College of Surgeons. User Guide for the 2012 ACS NSQIP Participant Use Data File: American College of Surgeons National Surgical Quality Improvement Program. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug12.ashx. Published October 2013. Accessed October 8, 2014.
16. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267.
17. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889.
18. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42.
19. Katzan I, Cebul R, Husak S, Dawson N, Baker D. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60(4):620-625.
20. Fisher MA, Matthei JD, Obirieze A, et al. Open reduction internal fixation versus hemiarthroplasty versus total hip arthroplasty in the elderly: a review of the National Surgical Quality Improvement Program database. J Surg Res. 2013;181(2):193-198.
21. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma. 2014;28(2):63-69.
22. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia: a meta-analysis. JAMA. 1996;275(2):134-141.
23. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
24. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004:159(7):702-706.
25. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 20138;61(4):1-117.
26. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84(4):562-572.
27. Myers AH, Robinson EG, Van Natta ML, Michelson JD, Collins K, Baker SP. Hip fractures among the elderly: factors associated with in-hospital mortality. Am J Epidemiol. 1991;134(10):1128-1137.
28. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
29. Leroy O, Santre C, Beuscart C, et al. A five-year study of severe community-acquired pneumonia with emphasis on prognosis in patients admitted to an intensive care unit. Intensive Care Med. 1995;21(1):24-31.
30. Urwin S, Parker M, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455.
31. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743.
32. Niederman MS, Mandell LA, Anzueto A, et al; American Thoracic Society. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730-1754.
33. Koval KJ, Skovron ML, Aharonoff GB, Zuckerman JD. Predictors of functional recovery after hip fracture in the elderly. Clin Orthop Relat Res. 1998;(348):22-28.
34. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
35. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology. 2000;39(4):346-349.
36. Bottle A, Aylin P. Mortality associated with delay in operation after hip fracture: observational study. BMJ. 2006;332(7547):947-951.
37. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709.
38. Simunovic N, Devereaux P, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616.
39. Kaplan V, Clermont G, Griffin MF, et al. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163(3):317-323.
40. Parker MJ, Handoll HH, Griffiths R. Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev. 2004;(4):CD000521.
41. Chakladar A, White SM. Cost estimates of spinal versus general anaesthesia for fractured neck of femur surgery. Anaesthesia. 2010;65(8):810-814.
42. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
43. Gilbert TB, Hawkes WG, Hebel JR, et al. Spinal anesthesia versus general anesthesia for hip fracture repair: a longitudinal observation of 741 elderly patients during 2-year follow-up. Am J Orthop. 2000;29(1):25-35.
44. O’Hara DA, Duff A, Berlin JA, et al. The effect of anesthetic technique on postoperative outcomes in hip fracture repair. Anesthesiology. 2000;92(4):947-957.
45. Hole A, Terjesen T, Breivik H. Epidural versus general anaesthesia for total hip arthroplasty in elderly patients. Acta Anaesthesiol Scand. 1980;24(4):279-287.
46. Rashiq S, Finegan BA. The effect of spinal anesthesia on blood transfusion rate in total joint arthroplasty. Can J Surg. 2006;49(6):391-396.
47. Chang CC, Lin HC, Lin HW, Lin HC. Anesthetic management and surgical site infections in total hip or knee replacement: a population-based study. Anesthesiology. 2010;113(2):279-284.
48. Mauermann WJ, Shilling AM, Zuo Z. A comparison of neuraxial block versus general anesthesia for elective total hip replacement: a meta-analysis. Anesth Analg. 2006;103(4):1018-1025.
49. Hu S, Zhang ZY, Hua YQ, Li J, Cai ZD. A comparison of regional and general anaesthesia for total replacement of the hip or knee: a meta-analysis. J Bone Joint Surg Br. 2009;91(7):935-942.
50. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
51. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697.
52. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559.
53. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489.
54. Shiga T, Wajima Zi, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anesth. 2008;55(3):146-154.
55. Streubel P, Ricci W, Wong A, Gardner M. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
Acute Intraprosthetic Dissociation of a Dual-Mobility Hip in the United States
Take-Home Points
- AIPD of DM-THA is defined by dissociation within 1 year of implantation resulting from component impingement or closed reduction maneuvers.
- This is a distinct entity from “late” IPD (>1 year) from implantation as this is associated most often with polyethylene wear, component loosening, and arthrofibrosis.
- A history of DM dislocation followed by subjective “clunking,” instability, and a series of more frequent dislocations should raise concern for AIPD.
- Classic radiographic findings of AIPD include eccentric hip reduction and soft tissue radiolucency (ie, halo sign) from dissociated polyethylene component.
- Treating practitioners of AIPD should consider closed reduction with general anesthesia and sedation in the operating room to limit risk of dissociation.
Dual-mobility (DM) components were invented in the 1970s and have been used in primary and revision total hip arthroplasty (THA) in Europe ever since.1 However, DM components are most commonly used in the treatment of recurrent hip instability, and early results have been promising.2 In DM-THAs, a smaller (22-mm or 28-mm) metal femoral head snap-fits into a larger polyethylene ball (inner articulation), which articulates with a highly polished metal shell (outer articulation), which is either implanted directly in the acetabulum or placed in an uncemented acetabular cup. The 2 articulations used in these devices theoretically increase hip range of motion (ROM) and increase the inferior head displacement distance (jump distance) required for dislocation.3
However, this DM articulation with increased ROM may also cause chronic impingement of the femoral component neck or Morse taper against the outer polyethylene bearing, resulting in polyethylene wear and late intraprosthetic dissociation (IPD) (separation of inner articulation between femoral head and polyethylene liner). In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation during the period 1989 to 1997. In 2013, Philippot and colleagues5 reported that 81 of 1960 primary THAs developed IPD a mean of 9 years after implantation. These IPD cases were attributed to polyethylene wear or outer articulation blockage caused by arthrofibrosis or heterotopic ossification. Reports of acute IPD (AIPD), however, are rare. In 2011, Stigbrand and Ullmark6 reported 3 cases in which the DM prosthesis dislocated within 1 year after implantation. It was suggested that the inner metal head dissociated from the larger polyethylene component after attempted closed reduction for dislocation (separation of larger polyethylene component from acetabulum or acetabular liner).
DM components were unavailable to surgeons in the United States until 2011. The first US Food and Drug Administration (FDA)-approved DM device was the MDM (Modular Dual Mobility, Stryker). To our knowledge, 2 cases of AIPD with this prosthesis have been reported.7, 8 As with the cases in Europe, closed reduction was the suspected cause, but there was no explanation for the initial dislocation event.
In this article, we present the case of a nondemented man who developed AIPD of a THA with the MDM component and a 28-mm femoral head with a skirted neck (StelKast). His operative findings suggest a poor head-to-neck ratio caused by a larger diameter femoral neck or a skirted prosthesis, or a forceful reduction maneuver, may predispose DM components to AIPD. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In 2012, a 63-year-old man with a history of drug abuse underwent left primary THA. Seven posterior dislocations and 3 years later, the acetabular component was revised to the MDM prosthesis; the well-fixed StelKast femoral component was retained (Figure 1).
Within 3 months after revision surgery, the left hip dislocated 3 times in 1 week, when the patient bent over to retrieve an object on the ground. The first 2 dislocations were treated with closed reduction under conscious sedation at an outside emergency department.
With the patient’s erythrocyte sedimentation rate and C-reactive protein level both normal, a second revision was performed. During surgery, the polyethylene head was found beneath the gluteus maximus (Figure 4).
Discussion
Recurrent dislocation and instability accounts for 22.5% of THA revisions in the United States.9 Until 2011, options for managing recurrent dislocation in the United States included modular component exchange, component revision for malposition, and use of constrained components.10
In 1974, Bousquet first reported use of the DM prosthesis in primary THA; the prosthesis allowed increased stability without sacrificing motion or fixation.1 However, longer-term studies of DM components disclosed a new complication, IPD. In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation of the Bousquet prosthesis.
AIPD, which occurs within 1 year after implantation, has been reported much less often than late IPD. Stigbrand and Ullmark6 reported 3 cases of AIPD that developed within 7 months after implantation of Amplitude and Advantage (Zimmer Biomet) DM prostheses.
This unusual complication apparently is not confined to a specific implant or region. Since the MDM component was introduced in the United States, 2 more cases of AIPD have been identified (Table). Banzhof and colleagues7 reported the case of a 68-year-old woman who, 2 months after the MDM was placed for recurrent instability, dislocated the component while rising from a seated position. Her IPD most likely resulted from a closed reduction. The affected hip eventually required closed reduction in the operating room. Postreduction radiographs showed the characteristic eccentric appearance; a halo, also visible in the soft tissues, corresponded with the dissociated radiolucent polyethylene liner. The authors attributed the early failure to an eccentrically seated metal liner that separated the locking mechanism. The MDM component was revised to a conventional THA, with the femoral head upsized and length added.
Ward and colleagues8 reported the case of an 87-year-old woman who had a conventional THA revised to an MDM component for recurrent instability. Two months after surgery, this patient, who had dementia, experienced 2 posterior dislocations while rising from a chair. Closed reduction in the emergency department seemed successful, but later she presented to the surgeon’s office with symptoms of instability and clunking, complaints similar to our patient’s. Radiographs showed an eccentric reduction caused by IPD, and the MDM component was revised to a constrained liner. Adding a MDM component to a retained DePuy (DePuy Synthes) femoral stem and head is considered “off-label use,” which, the authors proposed, may have been related to the AIPD in their patient’s case. However, one manufacturer’s femoral component and head are often mated with another manufacturer’s acetabular component to allow for a less complex revision. Our recommendation for surgeons is that, before proceeding with this treatment option, they investigate each component’s exact dimensions to ensure there are no subtle size differences that could cause problems. For example, a 28-mm head diameter that is actually 28.2 mm may affect mating properties, with the inner polyethylene articulation causing AIPD to develop.
Other cases of earlier IPD have been described, but they do not fit the APID definition given in this article. Riviere and colleagues14 reported the case of a 42-year-old man who, because of a previous adverse reaction to metal debris, underwent revision to a DM polyethylene ball in a retained BHR (Birmingham Hip Resurfacing) acetabular shell (Birmingham Hip, Smith & Nephew). Unfortunately, IPD occurred 14 months after surgery. Banka and colleagues15 reported the case of a 70-year-old woman who underwent revision to a DM cup for recurrent instability, but they did not specify the length of time between implantation and IPD and did not offer an explanation for the complication. Finally, Odland and Sierra16 reported the case of a 77-year-old man, with previous intertrochanteric and pelvic fractures, who underwent revision to a DM cup with retention of a Waldemar femoral component (Waldemar Link). He spontaneously developed IPD with ambulation 2 years after surgery.
Certainly, our patient’s presentation course is similar to other patients’. Within 3 months after revision to the MDM component, his left hip dislocated 3 times in 1 week. We contend his AIPD resulted from closed reduction, with the polyethylene dislodged from the femoral head with contact on the acetabulum. A larger or skirted neck may increase impingement during normal activity and thereby widen the polyethylene opening excessively and/or reduce the polyethylene ball ROM to impinge during the relocation maneuver. In this case, dissociation was noted only after the third dislocation. Pathognomonic eccentric positioning of the head in the acetabulum and, less commonly, the halo sign were evident on postreduction radiographs. Optimal treatment for AIPD of a DM component is controversial. Choices are limited to a constrained liner or, if possible, repeat DM with larger components. For recurrent dislocation, our patient underwent revision to an MDM component, but a femoral head with a skirted neck was used in an attempt to increase soft-tissue tension. During the second revision, minor eccentric wear of the inner articulation of the polyethylene component (consistent with impingement) was noted, and wear was visible on inspection of the outer articulation. We think his AIPD resulted from femoral neck impingement of the skirted head against the polyethylene ball.
AIPD is a discrete entity, with sudden failure of a DM component within 1 year after implantation. AIPD is characterized by dissociation of the femoral head from the inner articulation, resulting from impingement or closed reduction. More studies are needed to determine which patients with DM components are at highest risk and which treatment is most appropriate. We recommend taking extra care when reducing hips with this articulation and adopting a low threshold for general anesthesia use in the presence of paralysis.
Am J Orthop. 2017;46(3):E154-E159. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
2. Lachiewicz PF, Watters TS. The use of dual-mobility components in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(8):481-486.
3. De Martino I, Triantafyllopoulos GK, Sculco PK, Sculco TP. Dual mobility cups in total hip arthroplasty. World J Orthop. 2014;5(3):180-187.
4. Lecuire F, Benareau I, Rubini J, Basso M. Intra-prosthetic dislocation of the Bousquet dual mobility socket [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2004;90(3):249-255.
5. Philippot R, Boyer B, Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471(3):965-970.
6. Stigbrand H, Ullmark G. Component dissociation after closed reduction of dual mobility sockets—a report of three cases. Hip Int. 2011;21(2):263-266.
7. Banzhof JA, Robbins CE, Ven AV, Talmo CT, Bono JV. Femoral head dislodgement complicating use of a dual mobility prosthesis for recurrent instability. J Arthroplasty. 2013;28(3):543.e1-e3.
8. Ward JP, McCardel BR, Hallstrom BR. Complete dissociation of the polyethylene component in a newly available dual-mobility bearing used in total hip arthroplasty: a case report. JBJS Case Connect. 2013;3(3):e94.
9. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
10. Parvizi J, Picinic E, Sharkey PF. Revision total hip arthroplasty for instability: surgical techniques and principles. J Bone Joint Surg Am. 2008;90(5):1134-1142.
11. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
12. Lachiewicz PF, Kelley SS. The use of constrained components in total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(4):233-238.
13. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
14. Riviere C, Lavigne M, Alghamdi A, Vendittoli PA. Early failure of metal-on-metal large-diameter head total hip arthroplasty revised with a dual-mobility bearing: a case report. JBJS Case Connect. 2013;3(3):e95.
15. Banka TR, Ast MP, Parks ML. Early intraprosthetic dislocation in a revision dual-mobility hip prosthesis. Orthopedics. 2014;37(4):e395-e397.
16. Odland AN, Sierra RJ. Intraprosthetic dislocation of a contemporary dual-mobility design used during conversion THA. Orthopedics. 2014;37(12):e1124-e1128.
Take-Home Points
- AIPD of DM-THA is defined by dissociation within 1 year of implantation resulting from component impingement or closed reduction maneuvers.
- This is a distinct entity from “late” IPD (>1 year) from implantation as this is associated most often with polyethylene wear, component loosening, and arthrofibrosis.
- A history of DM dislocation followed by subjective “clunking,” instability, and a series of more frequent dislocations should raise concern for AIPD.
- Classic radiographic findings of AIPD include eccentric hip reduction and soft tissue radiolucency (ie, halo sign) from dissociated polyethylene component.
- Treating practitioners of AIPD should consider closed reduction with general anesthesia and sedation in the operating room to limit risk of dissociation.
Dual-mobility (DM) components were invented in the 1970s and have been used in primary and revision total hip arthroplasty (THA) in Europe ever since.1 However, DM components are most commonly used in the treatment of recurrent hip instability, and early results have been promising.2 In DM-THAs, a smaller (22-mm or 28-mm) metal femoral head snap-fits into a larger polyethylene ball (inner articulation), which articulates with a highly polished metal shell (outer articulation), which is either implanted directly in the acetabulum or placed in an uncemented acetabular cup. The 2 articulations used in these devices theoretically increase hip range of motion (ROM) and increase the inferior head displacement distance (jump distance) required for dislocation.3
However, this DM articulation with increased ROM may also cause chronic impingement of the femoral component neck or Morse taper against the outer polyethylene bearing, resulting in polyethylene wear and late intraprosthetic dissociation (IPD) (separation of inner articulation between femoral head and polyethylene liner). In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation during the period 1989 to 1997. In 2013, Philippot and colleagues5 reported that 81 of 1960 primary THAs developed IPD a mean of 9 years after implantation. These IPD cases were attributed to polyethylene wear or outer articulation blockage caused by arthrofibrosis or heterotopic ossification. Reports of acute IPD (AIPD), however, are rare. In 2011, Stigbrand and Ullmark6 reported 3 cases in which the DM prosthesis dislocated within 1 year after implantation. It was suggested that the inner metal head dissociated from the larger polyethylene component after attempted closed reduction for dislocation (separation of larger polyethylene component from acetabulum or acetabular liner).
DM components were unavailable to surgeons in the United States until 2011. The first US Food and Drug Administration (FDA)-approved DM device was the MDM (Modular Dual Mobility, Stryker). To our knowledge, 2 cases of AIPD with this prosthesis have been reported.7, 8 As with the cases in Europe, closed reduction was the suspected cause, but there was no explanation for the initial dislocation event.
In this article, we present the case of a nondemented man who developed AIPD of a THA with the MDM component and a 28-mm femoral head with a skirted neck (StelKast). His operative findings suggest a poor head-to-neck ratio caused by a larger diameter femoral neck or a skirted prosthesis, or a forceful reduction maneuver, may predispose DM components to AIPD. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In 2012, a 63-year-old man with a history of drug abuse underwent left primary THA. Seven posterior dislocations and 3 years later, the acetabular component was revised to the MDM prosthesis; the well-fixed StelKast femoral component was retained (Figure 1).
Within 3 months after revision surgery, the left hip dislocated 3 times in 1 week, when the patient bent over to retrieve an object on the ground. The first 2 dislocations were treated with closed reduction under conscious sedation at an outside emergency department.
With the patient’s erythrocyte sedimentation rate and C-reactive protein level both normal, a second revision was performed. During surgery, the polyethylene head was found beneath the gluteus maximus (Figure 4).
Discussion
Recurrent dislocation and instability accounts for 22.5% of THA revisions in the United States.9 Until 2011, options for managing recurrent dislocation in the United States included modular component exchange, component revision for malposition, and use of constrained components.10
In 1974, Bousquet first reported use of the DM prosthesis in primary THA; the prosthesis allowed increased stability without sacrificing motion or fixation.1 However, longer-term studies of DM components disclosed a new complication, IPD. In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation of the Bousquet prosthesis.
AIPD, which occurs within 1 year after implantation, has been reported much less often than late IPD. Stigbrand and Ullmark6 reported 3 cases of AIPD that developed within 7 months after implantation of Amplitude and Advantage (Zimmer Biomet) DM prostheses.
This unusual complication apparently is not confined to a specific implant or region. Since the MDM component was introduced in the United States, 2 more cases of AIPD have been identified (Table). Banzhof and colleagues7 reported the case of a 68-year-old woman who, 2 months after the MDM was placed for recurrent instability, dislocated the component while rising from a seated position. Her IPD most likely resulted from a closed reduction. The affected hip eventually required closed reduction in the operating room. Postreduction radiographs showed the characteristic eccentric appearance; a halo, also visible in the soft tissues, corresponded with the dissociated radiolucent polyethylene liner. The authors attributed the early failure to an eccentrically seated metal liner that separated the locking mechanism. The MDM component was revised to a conventional THA, with the femoral head upsized and length added.
Ward and colleagues8 reported the case of an 87-year-old woman who had a conventional THA revised to an MDM component for recurrent instability. Two months after surgery, this patient, who had dementia, experienced 2 posterior dislocations while rising from a chair. Closed reduction in the emergency department seemed successful, but later she presented to the surgeon’s office with symptoms of instability and clunking, complaints similar to our patient’s. Radiographs showed an eccentric reduction caused by IPD, and the MDM component was revised to a constrained liner. Adding a MDM component to a retained DePuy (DePuy Synthes) femoral stem and head is considered “off-label use,” which, the authors proposed, may have been related to the AIPD in their patient’s case. However, one manufacturer’s femoral component and head are often mated with another manufacturer’s acetabular component to allow for a less complex revision. Our recommendation for surgeons is that, before proceeding with this treatment option, they investigate each component’s exact dimensions to ensure there are no subtle size differences that could cause problems. For example, a 28-mm head diameter that is actually 28.2 mm may affect mating properties, with the inner polyethylene articulation causing AIPD to develop.
Other cases of earlier IPD have been described, but they do not fit the APID definition given in this article. Riviere and colleagues14 reported the case of a 42-year-old man who, because of a previous adverse reaction to metal debris, underwent revision to a DM polyethylene ball in a retained BHR (Birmingham Hip Resurfacing) acetabular shell (Birmingham Hip, Smith & Nephew). Unfortunately, IPD occurred 14 months after surgery. Banka and colleagues15 reported the case of a 70-year-old woman who underwent revision to a DM cup for recurrent instability, but they did not specify the length of time between implantation and IPD and did not offer an explanation for the complication. Finally, Odland and Sierra16 reported the case of a 77-year-old man, with previous intertrochanteric and pelvic fractures, who underwent revision to a DM cup with retention of a Waldemar femoral component (Waldemar Link). He spontaneously developed IPD with ambulation 2 years after surgery.
Certainly, our patient’s presentation course is similar to other patients’. Within 3 months after revision to the MDM component, his left hip dislocated 3 times in 1 week. We contend his AIPD resulted from closed reduction, with the polyethylene dislodged from the femoral head with contact on the acetabulum. A larger or skirted neck may increase impingement during normal activity and thereby widen the polyethylene opening excessively and/or reduce the polyethylene ball ROM to impinge during the relocation maneuver. In this case, dissociation was noted only after the third dislocation. Pathognomonic eccentric positioning of the head in the acetabulum and, less commonly, the halo sign were evident on postreduction radiographs. Optimal treatment for AIPD of a DM component is controversial. Choices are limited to a constrained liner or, if possible, repeat DM with larger components. For recurrent dislocation, our patient underwent revision to an MDM component, but a femoral head with a skirted neck was used in an attempt to increase soft-tissue tension. During the second revision, minor eccentric wear of the inner articulation of the polyethylene component (consistent with impingement) was noted, and wear was visible on inspection of the outer articulation. We think his AIPD resulted from femoral neck impingement of the skirted head against the polyethylene ball.
AIPD is a discrete entity, with sudden failure of a DM component within 1 year after implantation. AIPD is characterized by dissociation of the femoral head from the inner articulation, resulting from impingement or closed reduction. More studies are needed to determine which patients with DM components are at highest risk and which treatment is most appropriate. We recommend taking extra care when reducing hips with this articulation and adopting a low threshold for general anesthesia use in the presence of paralysis.
Am J Orthop. 2017;46(3):E154-E159. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- AIPD of DM-THA is defined by dissociation within 1 year of implantation resulting from component impingement or closed reduction maneuvers.
- This is a distinct entity from “late” IPD (>1 year) from implantation as this is associated most often with polyethylene wear, component loosening, and arthrofibrosis.
- A history of DM dislocation followed by subjective “clunking,” instability, and a series of more frequent dislocations should raise concern for AIPD.
- Classic radiographic findings of AIPD include eccentric hip reduction and soft tissue radiolucency (ie, halo sign) from dissociated polyethylene component.
- Treating practitioners of AIPD should consider closed reduction with general anesthesia and sedation in the operating room to limit risk of dissociation.
Dual-mobility (DM) components were invented in the 1970s and have been used in primary and revision total hip arthroplasty (THA) in Europe ever since.1 However, DM components are most commonly used in the treatment of recurrent hip instability, and early results have been promising.2 In DM-THAs, a smaller (22-mm or 28-mm) metal femoral head snap-fits into a larger polyethylene ball (inner articulation), which articulates with a highly polished metal shell (outer articulation), which is either implanted directly in the acetabulum or placed in an uncemented acetabular cup. The 2 articulations used in these devices theoretically increase hip range of motion (ROM) and increase the inferior head displacement distance (jump distance) required for dislocation.3
However, this DM articulation with increased ROM may also cause chronic impingement of the femoral component neck or Morse taper against the outer polyethylene bearing, resulting in polyethylene wear and late intraprosthetic dissociation (IPD) (separation of inner articulation between femoral head and polyethylene liner). In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation during the period 1989 to 1997. In 2013, Philippot and colleagues5 reported that 81 of 1960 primary THAs developed IPD a mean of 9 years after implantation. These IPD cases were attributed to polyethylene wear or outer articulation blockage caused by arthrofibrosis or heterotopic ossification. Reports of acute IPD (AIPD), however, are rare. In 2011, Stigbrand and Ullmark6 reported 3 cases in which the DM prosthesis dislocated within 1 year after implantation. It was suggested that the inner metal head dissociated from the larger polyethylene component after attempted closed reduction for dislocation (separation of larger polyethylene component from acetabulum or acetabular liner).
DM components were unavailable to surgeons in the United States until 2011. The first US Food and Drug Administration (FDA)-approved DM device was the MDM (Modular Dual Mobility, Stryker). To our knowledge, 2 cases of AIPD with this prosthesis have been reported.7, 8 As with the cases in Europe, closed reduction was the suspected cause, but there was no explanation for the initial dislocation event.
In this article, we present the case of a nondemented man who developed AIPD of a THA with the MDM component and a 28-mm femoral head with a skirted neck (StelKast). His operative findings suggest a poor head-to-neck ratio caused by a larger diameter femoral neck or a skirted prosthesis, or a forceful reduction maneuver, may predispose DM components to AIPD. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In 2012, a 63-year-old man with a history of drug abuse underwent left primary THA. Seven posterior dislocations and 3 years later, the acetabular component was revised to the MDM prosthesis; the well-fixed StelKast femoral component was retained (Figure 1).
Within 3 months after revision surgery, the left hip dislocated 3 times in 1 week, when the patient bent over to retrieve an object on the ground. The first 2 dislocations were treated with closed reduction under conscious sedation at an outside emergency department.
With the patient’s erythrocyte sedimentation rate and C-reactive protein level both normal, a second revision was performed. During surgery, the polyethylene head was found beneath the gluteus maximus (Figure 4).
Discussion
Recurrent dislocation and instability accounts for 22.5% of THA revisions in the United States.9 Until 2011, options for managing recurrent dislocation in the United States included modular component exchange, component revision for malposition, and use of constrained components.10
In 1974, Bousquet first reported use of the DM prosthesis in primary THA; the prosthesis allowed increased stability without sacrificing motion or fixation.1 However, longer-term studies of DM components disclosed a new complication, IPD. In 2004, Lecuire and colleagues4 reported 7 cases of IPD occurring a mean of 10 years after implantation of the Bousquet prosthesis.
AIPD, which occurs within 1 year after implantation, has been reported much less often than late IPD. Stigbrand and Ullmark6 reported 3 cases of AIPD that developed within 7 months after implantation of Amplitude and Advantage (Zimmer Biomet) DM prostheses.
This unusual complication apparently is not confined to a specific implant or region. Since the MDM component was introduced in the United States, 2 more cases of AIPD have been identified (Table). Banzhof and colleagues7 reported the case of a 68-year-old woman who, 2 months after the MDM was placed for recurrent instability, dislocated the component while rising from a seated position. Her IPD most likely resulted from a closed reduction. The affected hip eventually required closed reduction in the operating room. Postreduction radiographs showed the characteristic eccentric appearance; a halo, also visible in the soft tissues, corresponded with the dissociated radiolucent polyethylene liner. The authors attributed the early failure to an eccentrically seated metal liner that separated the locking mechanism. The MDM component was revised to a conventional THA, with the femoral head upsized and length added.
Ward and colleagues8 reported the case of an 87-year-old woman who had a conventional THA revised to an MDM component for recurrent instability. Two months after surgery, this patient, who had dementia, experienced 2 posterior dislocations while rising from a chair. Closed reduction in the emergency department seemed successful, but later she presented to the surgeon’s office with symptoms of instability and clunking, complaints similar to our patient’s. Radiographs showed an eccentric reduction caused by IPD, and the MDM component was revised to a constrained liner. Adding a MDM component to a retained DePuy (DePuy Synthes) femoral stem and head is considered “off-label use,” which, the authors proposed, may have been related to the AIPD in their patient’s case. However, one manufacturer’s femoral component and head are often mated with another manufacturer’s acetabular component to allow for a less complex revision. Our recommendation for surgeons is that, before proceeding with this treatment option, they investigate each component’s exact dimensions to ensure there are no subtle size differences that could cause problems. For example, a 28-mm head diameter that is actually 28.2 mm may affect mating properties, with the inner polyethylene articulation causing AIPD to develop.
Other cases of earlier IPD have been described, but they do not fit the APID definition given in this article. Riviere and colleagues14 reported the case of a 42-year-old man who, because of a previous adverse reaction to metal debris, underwent revision to a DM polyethylene ball in a retained BHR (Birmingham Hip Resurfacing) acetabular shell (Birmingham Hip, Smith & Nephew). Unfortunately, IPD occurred 14 months after surgery. Banka and colleagues15 reported the case of a 70-year-old woman who underwent revision to a DM cup for recurrent instability, but they did not specify the length of time between implantation and IPD and did not offer an explanation for the complication. Finally, Odland and Sierra16 reported the case of a 77-year-old man, with previous intertrochanteric and pelvic fractures, who underwent revision to a DM cup with retention of a Waldemar femoral component (Waldemar Link). He spontaneously developed IPD with ambulation 2 years after surgery.
Certainly, our patient’s presentation course is similar to other patients’. Within 3 months after revision to the MDM component, his left hip dislocated 3 times in 1 week. We contend his AIPD resulted from closed reduction, with the polyethylene dislodged from the femoral head with contact on the acetabulum. A larger or skirted neck may increase impingement during normal activity and thereby widen the polyethylene opening excessively and/or reduce the polyethylene ball ROM to impinge during the relocation maneuver. In this case, dissociation was noted only after the third dislocation. Pathognomonic eccentric positioning of the head in the acetabulum and, less commonly, the halo sign were evident on postreduction radiographs. Optimal treatment for AIPD of a DM component is controversial. Choices are limited to a constrained liner or, if possible, repeat DM with larger components. For recurrent dislocation, our patient underwent revision to an MDM component, but a femoral head with a skirted neck was used in an attempt to increase soft-tissue tension. During the second revision, minor eccentric wear of the inner articulation of the polyethylene component (consistent with impingement) was noted, and wear was visible on inspection of the outer articulation. We think his AIPD resulted from femoral neck impingement of the skirted head against the polyethylene ball.
AIPD is a discrete entity, with sudden failure of a DM component within 1 year after implantation. AIPD is characterized by dissociation of the femoral head from the inner articulation, resulting from impingement or closed reduction. More studies are needed to determine which patients with DM components are at highest risk and which treatment is most appropriate. We recommend taking extra care when reducing hips with this articulation and adopting a low threshold for general anesthesia use in the presence of paralysis.
Am J Orthop. 2017;46(3):E154-E159. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
2. Lachiewicz PF, Watters TS. The use of dual-mobility components in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(8):481-486.
3. De Martino I, Triantafyllopoulos GK, Sculco PK, Sculco TP. Dual mobility cups in total hip arthroplasty. World J Orthop. 2014;5(3):180-187.
4. Lecuire F, Benareau I, Rubini J, Basso M. Intra-prosthetic dislocation of the Bousquet dual mobility socket [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2004;90(3):249-255.
5. Philippot R, Boyer B, Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471(3):965-970.
6. Stigbrand H, Ullmark G. Component dissociation after closed reduction of dual mobility sockets—a report of three cases. Hip Int. 2011;21(2):263-266.
7. Banzhof JA, Robbins CE, Ven AV, Talmo CT, Bono JV. Femoral head dislodgement complicating use of a dual mobility prosthesis for recurrent instability. J Arthroplasty. 2013;28(3):543.e1-e3.
8. Ward JP, McCardel BR, Hallstrom BR. Complete dissociation of the polyethylene component in a newly available dual-mobility bearing used in total hip arthroplasty: a case report. JBJS Case Connect. 2013;3(3):e94.
9. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
10. Parvizi J, Picinic E, Sharkey PF. Revision total hip arthroplasty for instability: surgical techniques and principles. J Bone Joint Surg Am. 2008;90(5):1134-1142.
11. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
12. Lachiewicz PF, Kelley SS. The use of constrained components in total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(4):233-238.
13. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
14. Riviere C, Lavigne M, Alghamdi A, Vendittoli PA. Early failure of metal-on-metal large-diameter head total hip arthroplasty revised with a dual-mobility bearing: a case report. JBJS Case Connect. 2013;3(3):e95.
15. Banka TR, Ast MP, Parks ML. Early intraprosthetic dislocation in a revision dual-mobility hip prosthesis. Orthopedics. 2014;37(4):e395-e397.
16. Odland AN, Sierra RJ. Intraprosthetic dislocation of a contemporary dual-mobility design used during conversion THA. Orthopedics. 2014;37(12):e1124-e1128.
1. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
2. Lachiewicz PF, Watters TS. The use of dual-mobility components in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(8):481-486.
3. De Martino I, Triantafyllopoulos GK, Sculco PK, Sculco TP. Dual mobility cups in total hip arthroplasty. World J Orthop. 2014;5(3):180-187.
4. Lecuire F, Benareau I, Rubini J, Basso M. Intra-prosthetic dislocation of the Bousquet dual mobility socket [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2004;90(3):249-255.
5. Philippot R, Boyer B, Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471(3):965-970.
6. Stigbrand H, Ullmark G. Component dissociation after closed reduction of dual mobility sockets—a report of three cases. Hip Int. 2011;21(2):263-266.
7. Banzhof JA, Robbins CE, Ven AV, Talmo CT, Bono JV. Femoral head dislodgement complicating use of a dual mobility prosthesis for recurrent instability. J Arthroplasty. 2013;28(3):543.e1-e3.
8. Ward JP, McCardel BR, Hallstrom BR. Complete dissociation of the polyethylene component in a newly available dual-mobility bearing used in total hip arthroplasty: a case report. JBJS Case Connect. 2013;3(3):e94.
9. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
10. Parvizi J, Picinic E, Sharkey PF. Revision total hip arthroplasty for instability: surgical techniques and principles. J Bone Joint Surg Am. 2008;90(5):1134-1142.
11. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
12. Lachiewicz PF, Kelley SS. The use of constrained components in total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(4):233-238.
13. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
14. Riviere C, Lavigne M, Alghamdi A, Vendittoli PA. Early failure of metal-on-metal large-diameter head total hip arthroplasty revised with a dual-mobility bearing: a case report. JBJS Case Connect. 2013;3(3):e95.
15. Banka TR, Ast MP, Parks ML. Early intraprosthetic dislocation in a revision dual-mobility hip prosthesis. Orthopedics. 2014;37(4):e395-e397.
16. Odland AN, Sierra RJ. Intraprosthetic dislocation of a contemporary dual-mobility design used during conversion THA. Orthopedics. 2014;37(12):e1124-e1128.
For bone and joint infections, oral antibiotics match IV, cost less
VIENNA – Oral antibiotic therapy is just as effective as intravenous treatment in curing bone and joint infections, but costs about $3,500 less.
Treating these infections with oral agents also “improves patient autonomy, as it’s not necessary to have IV lines at home,” and represents a generally wiser use of powerful antibiotics, Matthew Scarborough, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
OVIVA (Oral vs. Intravenous Antibiotics for Bone and Joint Infection) was conducted at 26 sites in the United Kingdom. It randomized 1,054 adults with bone or joint infections to 6 weeks of either oral or intravenous treatment.
An important aspect of the trial was that both oral and IV treatment choices were made before randomization, Dr. Scarborough said. However, the decisions on what drug to use were left up to the treating physician and depended on the infection site and pathogen.
The primary outcome was definite treatment failure (bacteriologic, histologic, and clinical). Patients were followed for 1 year.
Patients were a median of 60 years old. All had surgical treatment before antibiotic therapy, including debridement and, in those with implants, removal of infected devices. The lower limb was involved in 81%, including hip, knee, and foot. The infection was in an upper limb in 10% and in the spine in 7%.
Staphylococcus aureus was present in 38% of cases, coagulase-negative staphylococci in 27%, and streptococci in 15%. Gram-negative bacteria were found in 22%.
For those patients randomized to IV therapy, glycopeptides and cephalosporins were most commonly employed (41% and 33%, respectively). For oral therapy, quinolones and penicillins were most common (37% and 16%). Most patients (74%) continued antibiotic treatment for more than 6 weeks. Forty patients were lost to follow-up.
In the primary intent-to-treat analysis, the failure rate was 13% for oral therapy and 14% for IV therapy, not a significant difference. Results were similar in the other analyses, including a modified intent to treat with only patients who had complete 1-year data, and a per-protocol analysis. All of the point prevalence numbers favored oral therapy, but crossed the null. Curves in the time-to-treatment-failure analysis were virtually superimposable, as were curves in time to discontinuation of therapy.
Another subgroup analysis examined treatment failure by infective organism; again, there were no significant treatment differences in any of the pathogen subgroups examined (S. aureus, coagulase-negative staph, streptococci species, and other gram-negative bacteria).
Nor did the type of antibiotic significantly affect failure rate, Dr. Scarborough noted. The median length of stay was 14 days for patients on IV treatment and 11 days for those taking oral medications. The incidence of serious adverse events was very similar – about 86% in each group.
On a visual analog scale that assessed health-related quality of life, patients taking oral treatment reported better mobility, self-care, and activity level, and less pain, discomfort, anxiety, and depression than those taking IV medications.
Cost represented the other significant difference between the groups. Over 1 year, the mean IV treatment cost was the equivalent of $17,152, and the mean oral treatment cost was $13,611 – a significant difference of $3,541.
“This represents a potential savings to the National Health Service of 16-25 million pounds sterling ($20.6 million-$32.3 million) per year,” Dr. Scarborough said. “All coming at no expense of good clinical outcomes.”
OVIVA was sponsored by the U.K. National Institute of Health Research. Dr. Scarborough had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
VIENNA – Oral antibiotic therapy is just as effective as intravenous treatment in curing bone and joint infections, but costs about $3,500 less.
Treating these infections with oral agents also “improves patient autonomy, as it’s not necessary to have IV lines at home,” and represents a generally wiser use of powerful antibiotics, Matthew Scarborough, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
OVIVA (Oral vs. Intravenous Antibiotics for Bone and Joint Infection) was conducted at 26 sites in the United Kingdom. It randomized 1,054 adults with bone or joint infections to 6 weeks of either oral or intravenous treatment.
An important aspect of the trial was that both oral and IV treatment choices were made before randomization, Dr. Scarborough said. However, the decisions on what drug to use were left up to the treating physician and depended on the infection site and pathogen.
The primary outcome was definite treatment failure (bacteriologic, histologic, and clinical). Patients were followed for 1 year.
Patients were a median of 60 years old. All had surgical treatment before antibiotic therapy, including debridement and, in those with implants, removal of infected devices. The lower limb was involved in 81%, including hip, knee, and foot. The infection was in an upper limb in 10% and in the spine in 7%.
Staphylococcus aureus was present in 38% of cases, coagulase-negative staphylococci in 27%, and streptococci in 15%. Gram-negative bacteria were found in 22%.
For those patients randomized to IV therapy, glycopeptides and cephalosporins were most commonly employed (41% and 33%, respectively). For oral therapy, quinolones and penicillins were most common (37% and 16%). Most patients (74%) continued antibiotic treatment for more than 6 weeks. Forty patients were lost to follow-up.
In the primary intent-to-treat analysis, the failure rate was 13% for oral therapy and 14% for IV therapy, not a significant difference. Results were similar in the other analyses, including a modified intent to treat with only patients who had complete 1-year data, and a per-protocol analysis. All of the point prevalence numbers favored oral therapy, but crossed the null. Curves in the time-to-treatment-failure analysis were virtually superimposable, as were curves in time to discontinuation of therapy.
Another subgroup analysis examined treatment failure by infective organism; again, there were no significant treatment differences in any of the pathogen subgroups examined (S. aureus, coagulase-negative staph, streptococci species, and other gram-negative bacteria).
Nor did the type of antibiotic significantly affect failure rate, Dr. Scarborough noted. The median length of stay was 14 days for patients on IV treatment and 11 days for those taking oral medications. The incidence of serious adverse events was very similar – about 86% in each group.
On a visual analog scale that assessed health-related quality of life, patients taking oral treatment reported better mobility, self-care, and activity level, and less pain, discomfort, anxiety, and depression than those taking IV medications.
Cost represented the other significant difference between the groups. Over 1 year, the mean IV treatment cost was the equivalent of $17,152, and the mean oral treatment cost was $13,611 – a significant difference of $3,541.
“This represents a potential savings to the National Health Service of 16-25 million pounds sterling ($20.6 million-$32.3 million) per year,” Dr. Scarborough said. “All coming at no expense of good clinical outcomes.”
OVIVA was sponsored by the U.K. National Institute of Health Research. Dr. Scarborough had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
VIENNA – Oral antibiotic therapy is just as effective as intravenous treatment in curing bone and joint infections, but costs about $3,500 less.
Treating these infections with oral agents also “improves patient autonomy, as it’s not necessary to have IV lines at home,” and represents a generally wiser use of powerful antibiotics, Matthew Scarborough, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
OVIVA (Oral vs. Intravenous Antibiotics for Bone and Joint Infection) was conducted at 26 sites in the United Kingdom. It randomized 1,054 adults with bone or joint infections to 6 weeks of either oral or intravenous treatment.
An important aspect of the trial was that both oral and IV treatment choices were made before randomization, Dr. Scarborough said. However, the decisions on what drug to use were left up to the treating physician and depended on the infection site and pathogen.
The primary outcome was definite treatment failure (bacteriologic, histologic, and clinical). Patients were followed for 1 year.
Patients were a median of 60 years old. All had surgical treatment before antibiotic therapy, including debridement and, in those with implants, removal of infected devices. The lower limb was involved in 81%, including hip, knee, and foot. The infection was in an upper limb in 10% and in the spine in 7%.
Staphylococcus aureus was present in 38% of cases, coagulase-negative staphylococci in 27%, and streptococci in 15%. Gram-negative bacteria were found in 22%.
For those patients randomized to IV therapy, glycopeptides and cephalosporins were most commonly employed (41% and 33%, respectively). For oral therapy, quinolones and penicillins were most common (37% and 16%). Most patients (74%) continued antibiotic treatment for more than 6 weeks. Forty patients were lost to follow-up.
In the primary intent-to-treat analysis, the failure rate was 13% for oral therapy and 14% for IV therapy, not a significant difference. Results were similar in the other analyses, including a modified intent to treat with only patients who had complete 1-year data, and a per-protocol analysis. All of the point prevalence numbers favored oral therapy, but crossed the null. Curves in the time-to-treatment-failure analysis were virtually superimposable, as were curves in time to discontinuation of therapy.
Another subgroup analysis examined treatment failure by infective organism; again, there were no significant treatment differences in any of the pathogen subgroups examined (S. aureus, coagulase-negative staph, streptococci species, and other gram-negative bacteria).
Nor did the type of antibiotic significantly affect failure rate, Dr. Scarborough noted. The median length of stay was 14 days for patients on IV treatment and 11 days for those taking oral medications. The incidence of serious adverse events was very similar – about 86% in each group.
On a visual analog scale that assessed health-related quality of life, patients taking oral treatment reported better mobility, self-care, and activity level, and less pain, discomfort, anxiety, and depression than those taking IV medications.
Cost represented the other significant difference between the groups. Over 1 year, the mean IV treatment cost was the equivalent of $17,152, and the mean oral treatment cost was $13,611 – a significant difference of $3,541.
“This represents a potential savings to the National Health Service of 16-25 million pounds sterling ($20.6 million-$32.3 million) per year,” Dr. Scarborough said. “All coming at no expense of good clinical outcomes.”
OVIVA was sponsored by the U.K. National Institute of Health Research. Dr. Scarborough had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT ECCMID 2017
Key clinical point:
Major finding: At 1 year, cure rates were identical, but oral treatment cost about $3,500 less than IV treatment.
Data source: The study randomized 1,054 patients.
Disclosures: OVIVA was sponsored by the U.K. National Institute of Health Research. Dr. Scarborough had no financial disclosures.
Antibiotic prophylaxis for artificial joints
A 66-year-old woman 3 years status post hip replacement is seen for dental work. The dentist contacts the clinic for an antibiotic prescription. The patient has a penicillin allergy (rash). What do you recommend?
A. Clindamycin one dose before dental work.
B. Amoxicillin one dose before dental work.
C. Amoxicillin one dose before, one dose 4 hours after dental work.
D. Clindamycin one dose before dental work, one dose 4 hours after dental work.
E. No antibiotics.
Many patients with prosthetic joints will request antibiotics to take prior to dental procedures. Sometimes this request comes from the dental office.
When I ask patients why they feel they need antibiotics, they often reply that they were told by their orthopedic surgeons or their dentist that they would need to take antibiotics before dental procedures.
In an era when Clostridium difficile infection is a common and dangerous complication in the elderly, avoidance of unnecessary antibiotics is critical. In the United States, it is estimated that there are 240,000 patients infected with C. difficile annually, with 24,000 deaths at a cost of $6 billion.1
Is there compelling evidence to justify giving antibiotic prophylaxis for dental procedures to patients with prosthetic joints?
This information has called into question the wisdom of giving antibiotic prophylaxis for dental procedures when the same patients have transient bacteremias as a regular part of day-to-day life, and mouth organisms were infrequent causes of prosthetic joint infections.
The American Academy of Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) released an advisory statement 20 years ago on antibiotic prophylaxis for patients with dental replacements, which concluded: “Antibiotic prophylaxis is not indicated for dental patients with pins, plates, and screws, nor is it routinely indicated for most dental patients with total joint replacements.”4
In 2003, the AAOS and the ADA released updated guidelines that stated: “Presently, no scientific evidence supports the position that antibiotic prophylaxis to prevent hematogenous infections is required prior to dental treatment in patients with total joint prostheses. The risk/benefit and cost/effectiveness ratios fail to justify the administration of routine antibiotics.”5
Great confusion arose in 2009 when the AAOS published a position paper on its website that reversed this position.6 Interestingly, the statement was done by the AAOS alone, and not done in conjunction with the ADA.
In this position paper, the AAOS recommended that health care providers consider antibiotic prophylaxis prior to invasive procedures on all patients who had prosthetic joints, regardless of how long those joints have been in place. This major change in recommendations was not based on any new evidence that had been reviewed since the 2003 guidelines.
There are two studies that address outcome of patients with prosthetic joints who have and have not received prophylactic antibiotics.
Elie Berbari, MD, and colleagues reported on the results of a prospective case-control study comparing patients with prosthetic joints hospitalized with hip or knee infections with patients who had prosthetic joints hospitalized at the same time who did not have hip or knee infections.7
There was no increased risk of prosthetic hip or knee infection for patients undergoing a dental procedure who were not receiving antibiotic prophylaxis (odds ratio, 0.8; 95% confidence interval, 0.4-1.6), compared with the risk for patients not undergoing a dental procedure (OR, 0.6; 95% CI, 0.4-1.1). Antibiotic prophylaxis in patients undergoing high and low risk dental procedures did not decrease the risk of prosthetic joint infections.
In 2012, the AAOS and the ADA published updated guidelines with the following summary recommendation: “The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.”8 They referenced the Berbari study as the best available evidence.
Feng-Chen Kao, MD, and colleagues published a study this year with a design very similar to the Berbari study, with similar results.9 All Taiwanese residents who had received hip or knee replacements over a 12-year period were screened. Those who had received dental procedures were matched with individuals who had not had dental procedures. The dental procedure group was subdivided into a group that received antibiotics and one that didn’t.
There was no difference in infection rates between the group that had received dental procedures and the group that did not, and no difference in infection rates between those who received prophylactic antibiotics and those who didn’t.
I think this myth can be put to rest. There is no evidence to give patients with joint prostheses prophylactic antibiotics before dental procedures.
References
1. Steckelberg J.M., Osmon D.R. Prosthetic joint infections. In: Bisno A.L., Waldvogel F.A., eds. Infections associated with indwelling medical devices. Third ed., Washington, D.C.: American Society of Microbiology Press, 2000:173-209.
2. J Dent Res. 2004 Feb;83(2):170-4.
3. J Clin Periodontol. 2006 Feb;33(6):401-7.
4. J Am Dent Assoc. 1997 Jul;128(7):1004-8.
5. J Am Dent Assoc. 2003 Jul;134(7):895-9.
6. Spec Care Dentist. 2009 Nov-Dec;29(6):229-31.
7. Clin Infect Dis. 2010 Jan 1;50(1):8-16.
8. J Dent (Shiraz). 2013 Mar;14(1):49-52.
9. Infect Control Hosp Epidemiol. 2017 Feb;38(2):154-61.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
A 66-year-old woman 3 years status post hip replacement is seen for dental work. The dentist contacts the clinic for an antibiotic prescription. The patient has a penicillin allergy (rash). What do you recommend?
A. Clindamycin one dose before dental work.
B. Amoxicillin one dose before dental work.
C. Amoxicillin one dose before, one dose 4 hours after dental work.
D. Clindamycin one dose before dental work, one dose 4 hours after dental work.
E. No antibiotics.
Many patients with prosthetic joints will request antibiotics to take prior to dental procedures. Sometimes this request comes from the dental office.
When I ask patients why they feel they need antibiotics, they often reply that they were told by their orthopedic surgeons or their dentist that they would need to take antibiotics before dental procedures.
In an era when Clostridium difficile infection is a common and dangerous complication in the elderly, avoidance of unnecessary antibiotics is critical. In the United States, it is estimated that there are 240,000 patients infected with C. difficile annually, with 24,000 deaths at a cost of $6 billion.1
Is there compelling evidence to justify giving antibiotic prophylaxis for dental procedures to patients with prosthetic joints?
This information has called into question the wisdom of giving antibiotic prophylaxis for dental procedures when the same patients have transient bacteremias as a regular part of day-to-day life, and mouth organisms were infrequent causes of prosthetic joint infections.
The American Academy of Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) released an advisory statement 20 years ago on antibiotic prophylaxis for patients with dental replacements, which concluded: “Antibiotic prophylaxis is not indicated for dental patients with pins, plates, and screws, nor is it routinely indicated for most dental patients with total joint replacements.”4
In 2003, the AAOS and the ADA released updated guidelines that stated: “Presently, no scientific evidence supports the position that antibiotic prophylaxis to prevent hematogenous infections is required prior to dental treatment in patients with total joint prostheses. The risk/benefit and cost/effectiveness ratios fail to justify the administration of routine antibiotics.”5
Great confusion arose in 2009 when the AAOS published a position paper on its website that reversed this position.6 Interestingly, the statement was done by the AAOS alone, and not done in conjunction with the ADA.
In this position paper, the AAOS recommended that health care providers consider antibiotic prophylaxis prior to invasive procedures on all patients who had prosthetic joints, regardless of how long those joints have been in place. This major change in recommendations was not based on any new evidence that had been reviewed since the 2003 guidelines.
There are two studies that address outcome of patients with prosthetic joints who have and have not received prophylactic antibiotics.
Elie Berbari, MD, and colleagues reported on the results of a prospective case-control study comparing patients with prosthetic joints hospitalized with hip or knee infections with patients who had prosthetic joints hospitalized at the same time who did not have hip or knee infections.7
There was no increased risk of prosthetic hip or knee infection for patients undergoing a dental procedure who were not receiving antibiotic prophylaxis (odds ratio, 0.8; 95% confidence interval, 0.4-1.6), compared with the risk for patients not undergoing a dental procedure (OR, 0.6; 95% CI, 0.4-1.1). Antibiotic prophylaxis in patients undergoing high and low risk dental procedures did not decrease the risk of prosthetic joint infections.
In 2012, the AAOS and the ADA published updated guidelines with the following summary recommendation: “The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.”8 They referenced the Berbari study as the best available evidence.
Feng-Chen Kao, MD, and colleagues published a study this year with a design very similar to the Berbari study, with similar results.9 All Taiwanese residents who had received hip or knee replacements over a 12-year period were screened. Those who had received dental procedures were matched with individuals who had not had dental procedures. The dental procedure group was subdivided into a group that received antibiotics and one that didn’t.
There was no difference in infection rates between the group that had received dental procedures and the group that did not, and no difference in infection rates between those who received prophylactic antibiotics and those who didn’t.
I think this myth can be put to rest. There is no evidence to give patients with joint prostheses prophylactic antibiotics before dental procedures.
References
1. Steckelberg J.M., Osmon D.R. Prosthetic joint infections. In: Bisno A.L., Waldvogel F.A., eds. Infections associated with indwelling medical devices. Third ed., Washington, D.C.: American Society of Microbiology Press, 2000:173-209.
2. J Dent Res. 2004 Feb;83(2):170-4.
3. J Clin Periodontol. 2006 Feb;33(6):401-7.
4. J Am Dent Assoc. 1997 Jul;128(7):1004-8.
5. J Am Dent Assoc. 2003 Jul;134(7):895-9.
6. Spec Care Dentist. 2009 Nov-Dec;29(6):229-31.
7. Clin Infect Dis. 2010 Jan 1;50(1):8-16.
8. J Dent (Shiraz). 2013 Mar;14(1):49-52.
9. Infect Control Hosp Epidemiol. 2017 Feb;38(2):154-61.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
A 66-year-old woman 3 years status post hip replacement is seen for dental work. The dentist contacts the clinic for an antibiotic prescription. The patient has a penicillin allergy (rash). What do you recommend?
A. Clindamycin one dose before dental work.
B. Amoxicillin one dose before dental work.
C. Amoxicillin one dose before, one dose 4 hours after dental work.
D. Clindamycin one dose before dental work, one dose 4 hours after dental work.
E. No antibiotics.
Many patients with prosthetic joints will request antibiotics to take prior to dental procedures. Sometimes this request comes from the dental office.
When I ask patients why they feel they need antibiotics, they often reply that they were told by their orthopedic surgeons or their dentist that they would need to take antibiotics before dental procedures.
In an era when Clostridium difficile infection is a common and dangerous complication in the elderly, avoidance of unnecessary antibiotics is critical. In the United States, it is estimated that there are 240,000 patients infected with C. difficile annually, with 24,000 deaths at a cost of $6 billion.1
Is there compelling evidence to justify giving antibiotic prophylaxis for dental procedures to patients with prosthetic joints?
This information has called into question the wisdom of giving antibiotic prophylaxis for dental procedures when the same patients have transient bacteremias as a regular part of day-to-day life, and mouth organisms were infrequent causes of prosthetic joint infections.
The American Academy of Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) released an advisory statement 20 years ago on antibiotic prophylaxis for patients with dental replacements, which concluded: “Antibiotic prophylaxis is not indicated for dental patients with pins, plates, and screws, nor is it routinely indicated for most dental patients with total joint replacements.”4
In 2003, the AAOS and the ADA released updated guidelines that stated: “Presently, no scientific evidence supports the position that antibiotic prophylaxis to prevent hematogenous infections is required prior to dental treatment in patients with total joint prostheses. The risk/benefit and cost/effectiveness ratios fail to justify the administration of routine antibiotics.”5
Great confusion arose in 2009 when the AAOS published a position paper on its website that reversed this position.6 Interestingly, the statement was done by the AAOS alone, and not done in conjunction with the ADA.
In this position paper, the AAOS recommended that health care providers consider antibiotic prophylaxis prior to invasive procedures on all patients who had prosthetic joints, regardless of how long those joints have been in place. This major change in recommendations was not based on any new evidence that had been reviewed since the 2003 guidelines.
There are two studies that address outcome of patients with prosthetic joints who have and have not received prophylactic antibiotics.
Elie Berbari, MD, and colleagues reported on the results of a prospective case-control study comparing patients with prosthetic joints hospitalized with hip or knee infections with patients who had prosthetic joints hospitalized at the same time who did not have hip or knee infections.7
There was no increased risk of prosthetic hip or knee infection for patients undergoing a dental procedure who were not receiving antibiotic prophylaxis (odds ratio, 0.8; 95% confidence interval, 0.4-1.6), compared with the risk for patients not undergoing a dental procedure (OR, 0.6; 95% CI, 0.4-1.1). Antibiotic prophylaxis in patients undergoing high and low risk dental procedures did not decrease the risk of prosthetic joint infections.
In 2012, the AAOS and the ADA published updated guidelines with the following summary recommendation: “The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.”8 They referenced the Berbari study as the best available evidence.
Feng-Chen Kao, MD, and colleagues published a study this year with a design very similar to the Berbari study, with similar results.9 All Taiwanese residents who had received hip or knee replacements over a 12-year period were screened. Those who had received dental procedures were matched with individuals who had not had dental procedures. The dental procedure group was subdivided into a group that received antibiotics and one that didn’t.
There was no difference in infection rates between the group that had received dental procedures and the group that did not, and no difference in infection rates between those who received prophylactic antibiotics and those who didn’t.
I think this myth can be put to rest. There is no evidence to give patients with joint prostheses prophylactic antibiotics before dental procedures.
References
1. Steckelberg J.M., Osmon D.R. Prosthetic joint infections. In: Bisno A.L., Waldvogel F.A., eds. Infections associated with indwelling medical devices. Third ed., Washington, D.C.: American Society of Microbiology Press, 2000:173-209.
2. J Dent Res. 2004 Feb;83(2):170-4.
3. J Clin Periodontol. 2006 Feb;33(6):401-7.
4. J Am Dent Assoc. 1997 Jul;128(7):1004-8.
5. J Am Dent Assoc. 2003 Jul;134(7):895-9.
6. Spec Care Dentist. 2009 Nov-Dec;29(6):229-31.
7. Clin Infect Dis. 2010 Jan 1;50(1):8-16.
8. J Dent (Shiraz). 2013 Mar;14(1):49-52.
9. Infect Control Hosp Epidemiol. 2017 Feb;38(2):154-61.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
Precision and Accuracy of Identification of Anatomical Surface Landmarks by 30 Expert Hip Arthroscopists
Take-Home Points
- Surface landmarks are routinely used for physical examination and surgical technique.
- Common surface landmarks used in establishing arthroscopic portals may be more difficult to accurately identify than previously thought.
- The greater trochanter was the surface landmark most precisely identified by expert examiners.
- Ultrasound examination identified landmarks varied from landmarks identified by palpation alone.
Anatomical surface landmarks about the hip and lower abdomen are often referenced when placing arthroscopic portals and office-based injections.1-3 However, the degree to which these landmarks can be reproducibly identified using only visual inspection and palpation is unknown.
Safe access to the hip joint and surrounding structures during hip arthroscopy has been a focus in the orthopedic literature. Authors have described anatomical relationships of recommended portals to neurovascular and other anatomical structures.4-6 This information has been reported in millimeters to centimeters of safety based on cadaver dissection studies.4-7We conducted a study to assess expert hip arthroscopists’ ability to identify, using only physical examination techniques, the anatomical structures used for reference when creating safe starting points for arthroscopic access. We hypothesized that variance in examiner-identified points would exceed safe distances from neurovascular structures for the most commonly used hip arthroscopic portals. The volunteer in this study provided written informed consent for print and electronic publication of this article.
Methods
In this study, we prospectively assessed 30 expert hip arthroscopic surgeons’ ability to identify commonly referenced surface landmarks on the adult male hip, using only inspection and manual palpation. Surgeons were defined as experts on the basis of their status as hip arthroscopy instructors at the Orthopaedic Learning Center (Rosemont, IL) for the Arthroscopy Association of North America and industry-sponsored hip arthroscopy education faculty (Arthrex). Five surface landmarks were selected for their relevance to publications on safe portal placement2-5: anterior superior iliac spine (ASIS), tip of greater trochanter (GT), rectus origin (RO), superficial inguinal ring (SIR), and psoas tendon (PT).
A healthy adult male volunteer was placed supine on an examination table and exposed distally from the mid abdomen, with the perineum and the genital area covered bikini-style. An expert musculoskeletal ultrasonographer used a handheld musculoskeletal ultrasound transducer (Sonosite) to identify the 5 landmarks. Short- and long-axis images of each structure were obtained. The examiner applied a round (1 cm in diameter), uniquely colored adhesive label to the skin over each location. A professional photographer using a Canon digital camera and fixed mounts made precise overhead and lateral images. The positional integrity and scale of these images were confirmed with referral to constant anatomical skin features. Images were archived for analysis (Figure 1A).
After the ultrasonographer’s labels were removed, each of the 30 expert hip arthroscopic surgeons identified the structures by static physical examination (inspection and palpation only) and applied the same colored labels to the skin.
Imaging software (Adobe Photoshop Creative Suite 5.1) was used to superimpose the digital images of the examiner labels on those of the ultrasound-verified anatomical labels (Figure 1C). Measurements were then taken with digital calipers to determine average distance from ultrasound label; accuracy within 10 mm of verified ultrasound label; true average location (TAL) determined by 95% confidence interval (CI); and interobserver variability calculated by 95% prediction interval, which determined the probability of where an additional examiner data point would lie.
In the second arm of the study, examiner data were compared with previously published data on arthroscopic portal safety.
Results
Average absolute distance from examiner labels to ultrasonographer labels was 31 mm for ASIS, 24 mm for GT, 26 mm for RO, 19 mm for SIR, and 35 mm for PT (Figure 2).
Of the 30 surgeons, 1 (3%) came within 10 mm of the ultrasound for ASIS, 1 (3%) for GT, 4 (13%) for RO, 5 (17%) for SIR, and 1 (3%) for PT (Table 1).
TAL as determined by CI was 16 mm medial and 29 mm inferior for ASIS; 8 mm anterior and 22 mm superior for GT; 10 mm medial and 25 mm inferior for RO; 5 mm lateral and 5 mm inferior for SIR; and 28 mm medial and 16 mm inferior for PT (Figure 3, Table 2). Interobserver variability determined by prediction interval had a range of 18 mm medial to lateral × 36 mm proximal to distal for ASIS; 33 mm anterior to posterior × 48 mm superior to inferior for GT; 41 mm medial to distal × 54 mm proximal to distal for RO; 51 mm medial to lateral × 74 mm proximal to distal for SIR; and 49 mm medial to distal × 61 mm proximal to distal for PT.
Given the difference between examiner data (direction and distance from ultrasound labels) and published data (distance to significant neurovascular structures), inaccurate identification of surface landmarks has the potential to lead to AP and MAP damage (Table 3). The examiner GT and ASIS surface landmarks used for AP overlapped directly with the safe distances for the lateral femoral cutaneous nerve and the terminal branch of the lateral circumflex femoral artery.
Discussion
Others have investigated examiners’ use of palpation, compared with ultrasound, to identify common shoulder and knee structures.8-10 In a 2011 systematic review, Gilliland and colleagues11 confirmed that accuracy was improved with use of ultrasound (vs palpation) for injections in the shoulder, hip, knee, wrist, and ankle. Given the scarcity of data in this setting, we conducted the present study to assess the precision and accuracy of expert arthroscopists in identifying common surface landmarks. We hypothesized that physical examination and ultrasound examination would differ significantly in precisely and accurately identifying these landmarks.
Working with a standard awake volunteer, our test group of examiners was consistently inaccurate when they accepted ultrasonographer-placed labels as the ideal. Precision within the group, however, trended toward close agreement; examiners consistently placed labels in the same direction and approximate magnitude away from ultrasonographer labels. This suggests that a discrepancy between the ultrasonographic surface structure definitions taught to ultrasonographers and the manually identified definitions taught to surgeons for arthroscopy (training bias) can generate differences in landmark identification.
Given reported low rates of complications in the creation of standard surface anatomy portals, more data is needed to correlate whether safe distance guidelines best apply to the points identified by hip experts or the points identified by ultrasonographers. In a 2013 systematic review, Harris and colleagues8 found a 7.5% overall complication rate, with temporary neuropraxia 1 of the 2 most common complications. Whether adding ultrasound to physical examination for the creation of some or all portals will reduce the incidence of these problems is unknown. Regardless of the anatomical area referenced by experts for portal creation, the tight grouping of examiner marks in our study supports a consensus regarding the location of the landmarks studied.
In our study of the use of surface anatomical landmarks for the creation of portals, we analyzed 4 previously described locations: ALP, AP, PLP, and MAP. ALP, AP, and PLP directly reference at least 1 surface anatomical structure; AP references 2 anatomical structures (ASIS, GT); and MAP indirectly references ASIS and GT and directly references ALP and AP. In cadaveric and radiographic studies, 7 neurovascular structures have been described in proximity to ALP, AP, MAP, and PLP: superior gluteal nerve, sciatic nerve, femoral nerve, lateral femoral cutaneous nerve, lateral circumflex femoral artery, and medial circumflex femoral artery.5,6 Our results showed that use of surface anatomy in AP and MAP creation most likely places structures at risk, given the overlap of examiner CIs and the previously published cadaveric5,6 and radiographic7 data.
Hua and colleagues12 confirmed the feasibility of using ultrasound for the creation of hip arthroscopy portals. More data is needed to assess how the standard palpation-and-fluoroscopy method described by Byrd3 compares with an ultrasound-guided technique in safety and cost. However, data from our study should not be used to justify a demand for ultrasound during arthroscopy portal establishment, as limitations do not permit such a recommendation.
With diagnostic injection remaining a mainstay of differential diagnosis and treatment about the hip,1 the data presented here suggest a potential for ultrasound in enhancing outcomes. There is evidence supporting the role of image guidance in improving palpation accuracy in the area of the biceps tendon in the forearm.10 Potentially, identification and treatment of specific extra-articular structures surrounding the hip could be made safer with more routine use of ultrasound.
Limitations
This study had several limitations. The surgeons were limited to palpation and static examination of a body in its natural state. Hip arthroscopic portals typically are created under traction and after a standard perineal post is placed for hip arthroscopy. In addition, in an awake injection setting, the clinician may receive patient feedback in the form of limb movement or speech. To what degree palpation or ultrasound will be affected in these scenarios is unknown.
Another limitation is the lack of serial examination by each examiner—intrarater variability could not be gauged. In addition, with only 1 ultrasonographic examination performed, there is the potential that adding ultrasonographic examinations, or having an examiner perform serial physical examinations, could better define the precision of each component. Given the practical limitations of our volunteer’s time and the schedules of 30 expert arthroscopists, we kept the chosen study design for its single setting.
Conclusion
Visual inspection and manual palpation are standard means of identifying common surface anatomical landmarks for the creation of arthroscopy portals and the placement of injections. Our study results showed variance in landmark identification between expert examiners and an ultrasonographer. The degree of variance exceeded established neurovascular safe zones, particularly for AP and MAP. This new evidence calls for further investigation into the best, safest means of performing hip arthroscopic techniques and injection-based interventions.
Am J Orthop. 2017;46(1):E65-E70. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46.
2. Dienst M, Seil R, Kohn DM. Safe arthroscopic access to the central compartment of the hip. Arthroscopy. 2005;21(12):1510-1514.
3. Byrd JW. Hip arthroscopy, the supine approach: technique and anatomy of the intraarticular and peripheral compartments. Tech Orthop. 2005;20(1):17-31.
4. Bond JL, Knutson ZA, Ebert A, Guanche CA. The 23-point arthroscopic examination of the hip: basic setup, portal placement, and surgical technique. Arthroscopy. 2009;25(4):416-429.
5. Roberson WJ, Kelly BT. The safe zone for hip arthroscopy: a cadaveric assessment of central, peripheral, and lateral compartment portal placement. Arthroscopy. 2008;24(9):1019-1026.
6. Byrd JW, Pappas JN, Pedley MJ. Hip arthroscopy: an anatomic study of portal placement and relationship to the extra-articular structures. Arthroscopy. 1995;11(4):418-423.
7. Watson JN, Bohnenkamp F, El-Bitar Y, Moretti V, Domb BG. Variability in locations of hip neurovascular structures and their proximity to hip arthroscopic portals. Arthroscopy. 2014;30(4):462-467.
8. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
9. Jacobson JA, Bedi A, Sekiya JK, Blankenbaker DG. Evaluation of the painful athletic hip: imaging options and imaging-guided injections. AJR Am J Roentgenol. 2012;199(3):516-524.
10. Gazzillo GP, Finnoff JT, Hall MM, Sayeed YA, Smith J. Accuracy of palpating the long head of the biceps tendon: an ultrasonographic study. PM R. 2011;3(11):1035-1040.
11. Gilliland CA, Salazar LD, Borchers JR. Ultrasound versus anatomic guidance for intra-articular and periarticular injection: a systematic review. Phys Sportsmed. 2011;39(3):121-131.
12. Hua Y, Yang Y, Chen S, et al. Ultrasound-guided establishment of hip arthroscopy portals. Arthroscopy. 2009;25(12):1491-1495.
Take-Home Points
- Surface landmarks are routinely used for physical examination and surgical technique.
- Common surface landmarks used in establishing arthroscopic portals may be more difficult to accurately identify than previously thought.
- The greater trochanter was the surface landmark most precisely identified by expert examiners.
- Ultrasound examination identified landmarks varied from landmarks identified by palpation alone.
Anatomical surface landmarks about the hip and lower abdomen are often referenced when placing arthroscopic portals and office-based injections.1-3 However, the degree to which these landmarks can be reproducibly identified using only visual inspection and palpation is unknown.
Safe access to the hip joint and surrounding structures during hip arthroscopy has been a focus in the orthopedic literature. Authors have described anatomical relationships of recommended portals to neurovascular and other anatomical structures.4-6 This information has been reported in millimeters to centimeters of safety based on cadaver dissection studies.4-7We conducted a study to assess expert hip arthroscopists’ ability to identify, using only physical examination techniques, the anatomical structures used for reference when creating safe starting points for arthroscopic access. We hypothesized that variance in examiner-identified points would exceed safe distances from neurovascular structures for the most commonly used hip arthroscopic portals. The volunteer in this study provided written informed consent for print and electronic publication of this article.
Methods
In this study, we prospectively assessed 30 expert hip arthroscopic surgeons’ ability to identify commonly referenced surface landmarks on the adult male hip, using only inspection and manual palpation. Surgeons were defined as experts on the basis of their status as hip arthroscopy instructors at the Orthopaedic Learning Center (Rosemont, IL) for the Arthroscopy Association of North America and industry-sponsored hip arthroscopy education faculty (Arthrex). Five surface landmarks were selected for their relevance to publications on safe portal placement2-5: anterior superior iliac spine (ASIS), tip of greater trochanter (GT), rectus origin (RO), superficial inguinal ring (SIR), and psoas tendon (PT).
A healthy adult male volunteer was placed supine on an examination table and exposed distally from the mid abdomen, with the perineum and the genital area covered bikini-style. An expert musculoskeletal ultrasonographer used a handheld musculoskeletal ultrasound transducer (Sonosite) to identify the 5 landmarks. Short- and long-axis images of each structure were obtained. The examiner applied a round (1 cm in diameter), uniquely colored adhesive label to the skin over each location. A professional photographer using a Canon digital camera and fixed mounts made precise overhead and lateral images. The positional integrity and scale of these images were confirmed with referral to constant anatomical skin features. Images were archived for analysis (Figure 1A).
After the ultrasonographer’s labels were removed, each of the 30 expert hip arthroscopic surgeons identified the structures by static physical examination (inspection and palpation only) and applied the same colored labels to the skin.
Imaging software (Adobe Photoshop Creative Suite 5.1) was used to superimpose the digital images of the examiner labels on those of the ultrasound-verified anatomical labels (Figure 1C). Measurements were then taken with digital calipers to determine average distance from ultrasound label; accuracy within 10 mm of verified ultrasound label; true average location (TAL) determined by 95% confidence interval (CI); and interobserver variability calculated by 95% prediction interval, which determined the probability of where an additional examiner data point would lie.
In the second arm of the study, examiner data were compared with previously published data on arthroscopic portal safety.
Results
Average absolute distance from examiner labels to ultrasonographer labels was 31 mm for ASIS, 24 mm for GT, 26 mm for RO, 19 mm for SIR, and 35 mm for PT (Figure 2).
Of the 30 surgeons, 1 (3%) came within 10 mm of the ultrasound for ASIS, 1 (3%) for GT, 4 (13%) for RO, 5 (17%) for SIR, and 1 (3%) for PT (Table 1).
TAL as determined by CI was 16 mm medial and 29 mm inferior for ASIS; 8 mm anterior and 22 mm superior for GT; 10 mm medial and 25 mm inferior for RO; 5 mm lateral and 5 mm inferior for SIR; and 28 mm medial and 16 mm inferior for PT (Figure 3, Table 2). Interobserver variability determined by prediction interval had a range of 18 mm medial to lateral × 36 mm proximal to distal for ASIS; 33 mm anterior to posterior × 48 mm superior to inferior for GT; 41 mm medial to distal × 54 mm proximal to distal for RO; 51 mm medial to lateral × 74 mm proximal to distal for SIR; and 49 mm medial to distal × 61 mm proximal to distal for PT.
Given the difference between examiner data (direction and distance from ultrasound labels) and published data (distance to significant neurovascular structures), inaccurate identification of surface landmarks has the potential to lead to AP and MAP damage (Table 3). The examiner GT and ASIS surface landmarks used for AP overlapped directly with the safe distances for the lateral femoral cutaneous nerve and the terminal branch of the lateral circumflex femoral artery.
Discussion
Others have investigated examiners’ use of palpation, compared with ultrasound, to identify common shoulder and knee structures.8-10 In a 2011 systematic review, Gilliland and colleagues11 confirmed that accuracy was improved with use of ultrasound (vs palpation) for injections in the shoulder, hip, knee, wrist, and ankle. Given the scarcity of data in this setting, we conducted the present study to assess the precision and accuracy of expert arthroscopists in identifying common surface landmarks. We hypothesized that physical examination and ultrasound examination would differ significantly in precisely and accurately identifying these landmarks.
Working with a standard awake volunteer, our test group of examiners was consistently inaccurate when they accepted ultrasonographer-placed labels as the ideal. Precision within the group, however, trended toward close agreement; examiners consistently placed labels in the same direction and approximate magnitude away from ultrasonographer labels. This suggests that a discrepancy between the ultrasonographic surface structure definitions taught to ultrasonographers and the manually identified definitions taught to surgeons for arthroscopy (training bias) can generate differences in landmark identification.
Given reported low rates of complications in the creation of standard surface anatomy portals, more data is needed to correlate whether safe distance guidelines best apply to the points identified by hip experts or the points identified by ultrasonographers. In a 2013 systematic review, Harris and colleagues8 found a 7.5% overall complication rate, with temporary neuropraxia 1 of the 2 most common complications. Whether adding ultrasound to physical examination for the creation of some or all portals will reduce the incidence of these problems is unknown. Regardless of the anatomical area referenced by experts for portal creation, the tight grouping of examiner marks in our study supports a consensus regarding the location of the landmarks studied.
In our study of the use of surface anatomical landmarks for the creation of portals, we analyzed 4 previously described locations: ALP, AP, PLP, and MAP. ALP, AP, and PLP directly reference at least 1 surface anatomical structure; AP references 2 anatomical structures (ASIS, GT); and MAP indirectly references ASIS and GT and directly references ALP and AP. In cadaveric and radiographic studies, 7 neurovascular structures have been described in proximity to ALP, AP, MAP, and PLP: superior gluteal nerve, sciatic nerve, femoral nerve, lateral femoral cutaneous nerve, lateral circumflex femoral artery, and medial circumflex femoral artery.5,6 Our results showed that use of surface anatomy in AP and MAP creation most likely places structures at risk, given the overlap of examiner CIs and the previously published cadaveric5,6 and radiographic7 data.
Hua and colleagues12 confirmed the feasibility of using ultrasound for the creation of hip arthroscopy portals. More data is needed to assess how the standard palpation-and-fluoroscopy method described by Byrd3 compares with an ultrasound-guided technique in safety and cost. However, data from our study should not be used to justify a demand for ultrasound during arthroscopy portal establishment, as limitations do not permit such a recommendation.
With diagnostic injection remaining a mainstay of differential diagnosis and treatment about the hip,1 the data presented here suggest a potential for ultrasound in enhancing outcomes. There is evidence supporting the role of image guidance in improving palpation accuracy in the area of the biceps tendon in the forearm.10 Potentially, identification and treatment of specific extra-articular structures surrounding the hip could be made safer with more routine use of ultrasound.
Limitations
This study had several limitations. The surgeons were limited to palpation and static examination of a body in its natural state. Hip arthroscopic portals typically are created under traction and after a standard perineal post is placed for hip arthroscopy. In addition, in an awake injection setting, the clinician may receive patient feedback in the form of limb movement or speech. To what degree palpation or ultrasound will be affected in these scenarios is unknown.
Another limitation is the lack of serial examination by each examiner—intrarater variability could not be gauged. In addition, with only 1 ultrasonographic examination performed, there is the potential that adding ultrasonographic examinations, or having an examiner perform serial physical examinations, could better define the precision of each component. Given the practical limitations of our volunteer’s time and the schedules of 30 expert arthroscopists, we kept the chosen study design for its single setting.
Conclusion
Visual inspection and manual palpation are standard means of identifying common surface anatomical landmarks for the creation of arthroscopy portals and the placement of injections. Our study results showed variance in landmark identification between expert examiners and an ultrasonographer. The degree of variance exceeded established neurovascular safe zones, particularly for AP and MAP. This new evidence calls for further investigation into the best, safest means of performing hip arthroscopic techniques and injection-based interventions.
Am J Orthop. 2017;46(1):E65-E70. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Surface landmarks are routinely used for physical examination and surgical technique.
- Common surface landmarks used in establishing arthroscopic portals may be more difficult to accurately identify than previously thought.
- The greater trochanter was the surface landmark most precisely identified by expert examiners.
- Ultrasound examination identified landmarks varied from landmarks identified by palpation alone.
Anatomical surface landmarks about the hip and lower abdomen are often referenced when placing arthroscopic portals and office-based injections.1-3 However, the degree to which these landmarks can be reproducibly identified using only visual inspection and palpation is unknown.
Safe access to the hip joint and surrounding structures during hip arthroscopy has been a focus in the orthopedic literature. Authors have described anatomical relationships of recommended portals to neurovascular and other anatomical structures.4-6 This information has been reported in millimeters to centimeters of safety based on cadaver dissection studies.4-7We conducted a study to assess expert hip arthroscopists’ ability to identify, using only physical examination techniques, the anatomical structures used for reference when creating safe starting points for arthroscopic access. We hypothesized that variance in examiner-identified points would exceed safe distances from neurovascular structures for the most commonly used hip arthroscopic portals. The volunteer in this study provided written informed consent for print and electronic publication of this article.
Methods
In this study, we prospectively assessed 30 expert hip arthroscopic surgeons’ ability to identify commonly referenced surface landmarks on the adult male hip, using only inspection and manual palpation. Surgeons were defined as experts on the basis of their status as hip arthroscopy instructors at the Orthopaedic Learning Center (Rosemont, IL) for the Arthroscopy Association of North America and industry-sponsored hip arthroscopy education faculty (Arthrex). Five surface landmarks were selected for their relevance to publications on safe portal placement2-5: anterior superior iliac spine (ASIS), tip of greater trochanter (GT), rectus origin (RO), superficial inguinal ring (SIR), and psoas tendon (PT).
A healthy adult male volunteer was placed supine on an examination table and exposed distally from the mid abdomen, with the perineum and the genital area covered bikini-style. An expert musculoskeletal ultrasonographer used a handheld musculoskeletal ultrasound transducer (Sonosite) to identify the 5 landmarks. Short- and long-axis images of each structure were obtained. The examiner applied a round (1 cm in diameter), uniquely colored adhesive label to the skin over each location. A professional photographer using a Canon digital camera and fixed mounts made precise overhead and lateral images. The positional integrity and scale of these images were confirmed with referral to constant anatomical skin features. Images were archived for analysis (Figure 1A).
After the ultrasonographer’s labels were removed, each of the 30 expert hip arthroscopic surgeons identified the structures by static physical examination (inspection and palpation only) and applied the same colored labels to the skin.
Imaging software (Adobe Photoshop Creative Suite 5.1) was used to superimpose the digital images of the examiner labels on those of the ultrasound-verified anatomical labels (Figure 1C). Measurements were then taken with digital calipers to determine average distance from ultrasound label; accuracy within 10 mm of verified ultrasound label; true average location (TAL) determined by 95% confidence interval (CI); and interobserver variability calculated by 95% prediction interval, which determined the probability of where an additional examiner data point would lie.
In the second arm of the study, examiner data were compared with previously published data on arthroscopic portal safety.
Results
Average absolute distance from examiner labels to ultrasonographer labels was 31 mm for ASIS, 24 mm for GT, 26 mm for RO, 19 mm for SIR, and 35 mm for PT (Figure 2).
Of the 30 surgeons, 1 (3%) came within 10 mm of the ultrasound for ASIS, 1 (3%) for GT, 4 (13%) for RO, 5 (17%) for SIR, and 1 (3%) for PT (Table 1).
TAL as determined by CI was 16 mm medial and 29 mm inferior for ASIS; 8 mm anterior and 22 mm superior for GT; 10 mm medial and 25 mm inferior for RO; 5 mm lateral and 5 mm inferior for SIR; and 28 mm medial and 16 mm inferior for PT (Figure 3, Table 2). Interobserver variability determined by prediction interval had a range of 18 mm medial to lateral × 36 mm proximal to distal for ASIS; 33 mm anterior to posterior × 48 mm superior to inferior for GT; 41 mm medial to distal × 54 mm proximal to distal for RO; 51 mm medial to lateral × 74 mm proximal to distal for SIR; and 49 mm medial to distal × 61 mm proximal to distal for PT.
Given the difference between examiner data (direction and distance from ultrasound labels) and published data (distance to significant neurovascular structures), inaccurate identification of surface landmarks has the potential to lead to AP and MAP damage (Table 3). The examiner GT and ASIS surface landmarks used for AP overlapped directly with the safe distances for the lateral femoral cutaneous nerve and the terminal branch of the lateral circumflex femoral artery.
Discussion
Others have investigated examiners’ use of palpation, compared with ultrasound, to identify common shoulder and knee structures.8-10 In a 2011 systematic review, Gilliland and colleagues11 confirmed that accuracy was improved with use of ultrasound (vs palpation) for injections in the shoulder, hip, knee, wrist, and ankle. Given the scarcity of data in this setting, we conducted the present study to assess the precision and accuracy of expert arthroscopists in identifying common surface landmarks. We hypothesized that physical examination and ultrasound examination would differ significantly in precisely and accurately identifying these landmarks.
Working with a standard awake volunteer, our test group of examiners was consistently inaccurate when they accepted ultrasonographer-placed labels as the ideal. Precision within the group, however, trended toward close agreement; examiners consistently placed labels in the same direction and approximate magnitude away from ultrasonographer labels. This suggests that a discrepancy between the ultrasonographic surface structure definitions taught to ultrasonographers and the manually identified definitions taught to surgeons for arthroscopy (training bias) can generate differences in landmark identification.
Given reported low rates of complications in the creation of standard surface anatomy portals, more data is needed to correlate whether safe distance guidelines best apply to the points identified by hip experts or the points identified by ultrasonographers. In a 2013 systematic review, Harris and colleagues8 found a 7.5% overall complication rate, with temporary neuropraxia 1 of the 2 most common complications. Whether adding ultrasound to physical examination for the creation of some or all portals will reduce the incidence of these problems is unknown. Regardless of the anatomical area referenced by experts for portal creation, the tight grouping of examiner marks in our study supports a consensus regarding the location of the landmarks studied.
In our study of the use of surface anatomical landmarks for the creation of portals, we analyzed 4 previously described locations: ALP, AP, PLP, and MAP. ALP, AP, and PLP directly reference at least 1 surface anatomical structure; AP references 2 anatomical structures (ASIS, GT); and MAP indirectly references ASIS and GT and directly references ALP and AP. In cadaveric and radiographic studies, 7 neurovascular structures have been described in proximity to ALP, AP, MAP, and PLP: superior gluteal nerve, sciatic nerve, femoral nerve, lateral femoral cutaneous nerve, lateral circumflex femoral artery, and medial circumflex femoral artery.5,6 Our results showed that use of surface anatomy in AP and MAP creation most likely places structures at risk, given the overlap of examiner CIs and the previously published cadaveric5,6 and radiographic7 data.
Hua and colleagues12 confirmed the feasibility of using ultrasound for the creation of hip arthroscopy portals. More data is needed to assess how the standard palpation-and-fluoroscopy method described by Byrd3 compares with an ultrasound-guided technique in safety and cost. However, data from our study should not be used to justify a demand for ultrasound during arthroscopy portal establishment, as limitations do not permit such a recommendation.
With diagnostic injection remaining a mainstay of differential diagnosis and treatment about the hip,1 the data presented here suggest a potential for ultrasound in enhancing outcomes. There is evidence supporting the role of image guidance in improving palpation accuracy in the area of the biceps tendon in the forearm.10 Potentially, identification and treatment of specific extra-articular structures surrounding the hip could be made safer with more routine use of ultrasound.
Limitations
This study had several limitations. The surgeons were limited to palpation and static examination of a body in its natural state. Hip arthroscopic portals typically are created under traction and after a standard perineal post is placed for hip arthroscopy. In addition, in an awake injection setting, the clinician may receive patient feedback in the form of limb movement or speech. To what degree palpation or ultrasound will be affected in these scenarios is unknown.
Another limitation is the lack of serial examination by each examiner—intrarater variability could not be gauged. In addition, with only 1 ultrasonographic examination performed, there is the potential that adding ultrasonographic examinations, or having an examiner perform serial physical examinations, could better define the precision of each component. Given the practical limitations of our volunteer’s time and the schedules of 30 expert arthroscopists, we kept the chosen study design for its single setting.
Conclusion
Visual inspection and manual palpation are standard means of identifying common surface anatomical landmarks for the creation of arthroscopy portals and the placement of injections. Our study results showed variance in landmark identification between expert examiners and an ultrasonographer. The degree of variance exceeded established neurovascular safe zones, particularly for AP and MAP. This new evidence calls for further investigation into the best, safest means of performing hip arthroscopic techniques and injection-based interventions.
Am J Orthop. 2017;46(1):E65-E70. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46.
2. Dienst M, Seil R, Kohn DM. Safe arthroscopic access to the central compartment of the hip. Arthroscopy. 2005;21(12):1510-1514.
3. Byrd JW. Hip arthroscopy, the supine approach: technique and anatomy of the intraarticular and peripheral compartments. Tech Orthop. 2005;20(1):17-31.
4. Bond JL, Knutson ZA, Ebert A, Guanche CA. The 23-point arthroscopic examination of the hip: basic setup, portal placement, and surgical technique. Arthroscopy. 2009;25(4):416-429.
5. Roberson WJ, Kelly BT. The safe zone for hip arthroscopy: a cadaveric assessment of central, peripheral, and lateral compartment portal placement. Arthroscopy. 2008;24(9):1019-1026.
6. Byrd JW, Pappas JN, Pedley MJ. Hip arthroscopy: an anatomic study of portal placement and relationship to the extra-articular structures. Arthroscopy. 1995;11(4):418-423.
7. Watson JN, Bohnenkamp F, El-Bitar Y, Moretti V, Domb BG. Variability in locations of hip neurovascular structures and their proximity to hip arthroscopic portals. Arthroscopy. 2014;30(4):462-467.
8. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
9. Jacobson JA, Bedi A, Sekiya JK, Blankenbaker DG. Evaluation of the painful athletic hip: imaging options and imaging-guided injections. AJR Am J Roentgenol. 2012;199(3):516-524.
10. Gazzillo GP, Finnoff JT, Hall MM, Sayeed YA, Smith J. Accuracy of palpating the long head of the biceps tendon: an ultrasonographic study. PM R. 2011;3(11):1035-1040.
11. Gilliland CA, Salazar LD, Borchers JR. Ultrasound versus anatomic guidance for intra-articular and periarticular injection: a systematic review. Phys Sportsmed. 2011;39(3):121-131.
12. Hua Y, Yang Y, Chen S, et al. Ultrasound-guided establishment of hip arthroscopy portals. Arthroscopy. 2009;25(12):1491-1495.
1. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46.
2. Dienst M, Seil R, Kohn DM. Safe arthroscopic access to the central compartment of the hip. Arthroscopy. 2005;21(12):1510-1514.
3. Byrd JW. Hip arthroscopy, the supine approach: technique and anatomy of the intraarticular and peripheral compartments. Tech Orthop. 2005;20(1):17-31.
4. Bond JL, Knutson ZA, Ebert A, Guanche CA. The 23-point arthroscopic examination of the hip: basic setup, portal placement, and surgical technique. Arthroscopy. 2009;25(4):416-429.
5. Roberson WJ, Kelly BT. The safe zone for hip arthroscopy: a cadaveric assessment of central, peripheral, and lateral compartment portal placement. Arthroscopy. 2008;24(9):1019-1026.
6. Byrd JW, Pappas JN, Pedley MJ. Hip arthroscopy: an anatomic study of portal placement and relationship to the extra-articular structures. Arthroscopy. 1995;11(4):418-423.
7. Watson JN, Bohnenkamp F, El-Bitar Y, Moretti V, Domb BG. Variability in locations of hip neurovascular structures and their proximity to hip arthroscopic portals. Arthroscopy. 2014;30(4):462-467.
8. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
9. Jacobson JA, Bedi A, Sekiya JK, Blankenbaker DG. Evaluation of the painful athletic hip: imaging options and imaging-guided injections. AJR Am J Roentgenol. 2012;199(3):516-524.
10. Gazzillo GP, Finnoff JT, Hall MM, Sayeed YA, Smith J. Accuracy of palpating the long head of the biceps tendon: an ultrasonographic study. PM R. 2011;3(11):1035-1040.
11. Gilliland CA, Salazar LD, Borchers JR. Ultrasound versus anatomic guidance for intra-articular and periarticular injection: a systematic review. Phys Sportsmed. 2011;39(3):121-131.
12. Hua Y, Yang Y, Chen S, et al. Ultrasound-guided establishment of hip arthroscopy portals. Arthroscopy. 2009;25(12):1491-1495.